
Concise International Chemical Assessment Document 22
ETHYLENE GLYCOL: Environmental aspects
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr Stuart Dobson, Institute of Terrestrial
Ecology, Natural Environment Research Council, Huntingdon, United
Kingdom
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2000
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall objectives
of the IPCS are to establish the scientific basis for assessment of
the risk to human health and the environment from exposure to
chemicals, through international peer review processes, as a
prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing-in-Publication Data
Ethylene glycol : environmental aspects.
(Concise international chemical assessment document ; 22)
1.Ethylene glycol - toxicity 2.Risk assessment
3.Environmental exposure
I.International Programme on Chemical Safety II.Series
ISBN 92 4 153022 7 (NLM Classification: QD 305.A4)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 2000
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS
7. EFFECTS ON ORGANISMS IN THE LABORATORY AND FIELD
7.1. Aquatic organisms
7.1.1. Toxicity of deicer formulations
7.1.2. Field effects
7.2. Terrestrial organisms
8. EFFECTS EVALUATION
8.1. Predicted environmental concentration
8.2. Predicted no-effect concentration
8.3. Environmental risk factors
INTERNATIONAL CHEMICAL SAFETY CARD
REFERENCES
APPENDIX 1 -- SOURCE DOCUMENTS
APPENDIX 2 -- CICAD PEER REVIEW
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACION
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) -- a cooperative programme of the
World Health Organization (WHO), the International Labour Organisation
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects of
chemicals upon human health and/or the environment. They are based on
selected national or regional evaluation documents or on existing
EHCs. Before acceptance for publication as CICADs by IPCS, these
documents undergo extensive peer review by internationally selected
experts to ensure their completeness, accuracy in the way in which the
original data are represented, and the validity of the conclusions
drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary of
all available data on a particular chemical; rather, they include only
that information considered critical for characterization of the risk
posed by the chemical. The critical studies are, however, presented in
sufficient detail to support the conclusions drawn. For additional
information, the reader should consult the identified source documents
upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based guidance values.
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact IPCS to inform it of the new information.
1 International Programme on Chemical Safety (1994)
Assessing human health risks of chemicals: deriviation of
guidance values for health-based exposure limits. Geneva, World
Health Organization (Environmental Health Criteria 170).
Procedures
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world -- expertise that is required to produce the
high-quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS to ensure that
it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments into
account and revise their draft, if necessary. The resulting second
draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions:
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board, the author has not adequately addressed all
comments of the reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards are
chosen according to the range of expertise required for a meeting and
the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
 1. EXECUTIVE SUMMARY
This CICAD on the environmental aspects of ethylene glycol was
prepared by the Institute of Terrestrial Ecology, United Kingdom,
based on the report Environmental hazard assessment: Ethylene glycol
(Nielsen et al., 1993). The report on ethylene glycol prepared by the
German Chemical Society Advisory Committee on Existing Chemicals of
Environmental Relevance (BUA, 1991) was also used as a source
document. In addition to these documents, a search of recent
literature was conducted up to 1998. Information on the nature of the
peer review process for the main source documents is presented in
Appendix 1. Information on the peer review of this CICAD is presented
in Appendix 2. This CICAD was approved as an international assessment
at a meeting of the Final Review Board, held in Washington, DC, USA,
on 8-11 December 1998. Participants at the Final Review Board meeting
are listed in Appendix 3. The International Chemical Safety Card (ICSC
0270) produced by the International Programme on Chemical Safety
(IPCS, 1993) has also been reproduced in this document.
Ethylene glycol (CAS No. 107-21-1) is a clear, colourless, syrupy
liquid with a sweet taste but no odour. It has low volatility. It is
miscible with water and some other solvents, slightly soluble in
ether, but practically insoluble in benzene, chlorinated hydrocarbons,
petroleum ethers, and oils. The log octanol/water partition
coefficient is -1.93 to -1.36.
Estimated world production capacity was 9.4 million tonnes in
1993. Release to the environment is mainly to the hydrosphere. The
largest local release to surface waters would follow ethylene glycol's
use as a deicer on airport runways and planes. On a worldwide basis,
approximately two-thirds of ethylene glycol is used as a chemical
intermediate, with a further one-quarter used as an antifreeze in
engine coolants.
Ethylene glycol released to the atmosphere will be degraded by
reaction with hydroxyl radicals; the half-life for the compound in
this reaction has been estimated at between 0.3 and 3.5 days.
No hydrolysis of ethylene glycol is expected in surface waters.
The compound has little or no capacity to bind to particulates
and will be mobile in soil.
The low octanol/water partition coefficient and measured
bioconcentration factors in a few organisms indicate low capacity for
bioaccumulation.
Ethylene glycol is readily biodegradable in standard tests using
sewage sludge. Many studies show biodegradation under both aerobic and
anaerobic conditions. Some studies suggest a lag phase before
degradation, but many do not. Degradation occurs in both adapted and
unadapted sludges. Rapid degradation has been reported in surface
waters (less in salt water than in fresh water), groundwater, and soil
inocula. Several strains of microorganisms capable of utilizing
ethylene glycol as a carbon source have been identified.
Limited data are available on measured concentrations of ethylene
glycol in environmental compartments. Levels measured in surface
waters have been generally low, at a few micrograms per litre.
Concentrations in wastewater from production plants, prior to
treatment, have averaged up to 1300 mg/litre. By far the highest
reported concentrations relate to runoff water from airports, with
levels up to 19 000 mg/litre.
Ethylene glycol has generally low toxicity to aquatic organisms.
Toxic thresholds for microorganisms are above 1000 mg/litre. EC50s
for growth in microalgae are 6500 mg/litre or higher. Acute toxicity
tests with aquatic invertebrates where a value could be determined
show LC50s above 20 000 mg/litre, and those with fish show LC50s
above 17 800 mg/litre. An amphibian test showed an LC50 for tadpoles
at 17 000 mg/litre. A no-observed-effect concentration (NOEC) for
chronic tests on daphnids of 8590 mg/litre (for reproductive
end-points) has been reported. A NOEC following short-term exposure of
fish has been reported at 15 380 mg/litre for growth.
Tests using deicer containing ethylene glycol showed greater
toxicity to aquatic organisms than observed with the pure compound,
indicating other toxic components of the formulations.
Laboratory tests exposing aquatic organisms to stream water
receiving runoff from airports have demonstrated toxic effects and
death. Field studies in the vicinity of an airport have reported toxic
signs consistent with ethylene glycol poisoning, fish kills, and
reduced biodiversity. These effects cannot definitively be ascribed to
ethylene glycol.
Terrestrial organisms are much less likely to be exposed to
ethylene glycol and generally show low sensitivity to the compound.
Concentrations above 100 000 mg/litre were needed to produce toxic
effects on yeasts and fungi from soil. Very high concentrations and
soaking of seeds produced inhibition of germination in some
experiments; these are not considered of environmental significance. A
no-observed-effect level (NOEL) for orally dosed ducks at 1221 mg/kg
body weight and reported lethal doses for poultry at around 8000 mg/kg
body weight indicate low toxicity to birds.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Ethylene glycol (C2H6O2; CAS No. 107-21-1) is also known as
1,2-ethanediol, 2-hydroxyethanol, 1,2-dihydroxyethane, glycol, glycol
alcohol, ethylene alcohol, and monoethylene glycol or MEG. Its
structure is illustrated below:
H H
' '
HO - C - C - OH
' '
H H
Ethylene glycol is a clear, colourless, syrupy liquid with a
sweet taste but no odour. The molecular mass is 62.07. It has low
volatility; its vapour pressure is 7.9 or 8.0 Pa at 20°C (Eisenreich
et al., 1981; ATSDR, 1997) and 12.2 Pa at 25°C (HSDB, 1998). It is
hygroscopic and absorbs twice its weight in water at 100% relative
humidity (Budavari, 1989). It is miscible with water, lower aliphatic
alcohols, glycerol, acetic acid, acetone and similar ketones,
aldehydes, pyridine, and similar coal tar bases. The compound is
slightly soluble in ether but practically insoluble in benzene and its
homologues, chlorinated hydrocarbons, petroleum ethers, and oils
(Budavari, 1989). The log octanol/water partition coefficient is -1.93
(Hansch & Leo, 1979) to -1.36.1 Other physical and chemical
properties can be found in the International Chemical Safety Card
(ICSC 0270) reproduced in this document.
1 Chou T, Hansch C (1986) Pomona College,
Claremont, CA, unpublished (cited in BUA, 1991).
3. ANALYTICAL METHODS
Ethylene glycol is measured in environmental samples by gas
chromatography, most commonly using flame ionization detection. Recent
methods have been described using high-resolution gas chromatography
coupled with mass spectrometry. Measurement in biological samples has
also used gas chromatography or high-resolution gas chromatography,
with additional methods employing high-performance liquid
chromatography or colorimetric determination. Detection limits were
not available for environmental media. Details of extraction and
concentration methods can be found in ATSDR (1997).
4. SOURCES OF ENVIRONMENTAL EXPOSURE
Although ethylene glycol can be prepared directly by alkaline
hydrolysis of chlorohydrin, hydrolysis of ethylene oxide is the more
usual method. The feed stream consists of ethylene oxide (from either
chlorohydrin or the direct oxidation of ethylene) and water. The
mixture is fed under pressure into a reaction vessel at a temperature
of about 100°C, which by the end of the reaction has risen to 170°C.
Some diethylene and triethylene glycol are produced by the reaction of
ethylene glycol with excess ethylene oxide. The crude glycol solution
is concentrated in a multiple-effect evaporator, and final separation
is achieved by distillation (Kent, 1974). Product proportions were
estimated by the US EPA (1980) as follows: ethylene glycol,
87.0-88.5%; diethylene glycol, 9.3-10.5%; and triethylene glycol,
2.2-2.5%; and by ICI Chemicals and Polymers Ltd. as 90%, 9%, and 1%,
respectively.1
Estimated world production capacity was 9.4 million tonnes in
1993.1 Total US production capacity was estimated at approximately 3
million tonnes in 1993 (SRI, 1993); this figure had been more or less
stable since 1989. United Kingdom production was estimated at 50 000 t
in 1993 based on a production capacity of 85 000 t/year.1 Production
volume in Germany was a maximum of 240 000 t in 1989; breakdown of
production capacity by region and country worldwide can be found in
BUA (1991). Production volume in Japan increased from 560 000 t in
1992 to 751 000 t in 1996 (Chemical Daily Company, 1997).
On a worldwide basis, approximately two-thirds of ethylene glycol
is used as a chemical intermediate in the manufacture of polyesters
for fibres, films, bottles, etc., with a further one-quarter used as
an antifreeze in engine coolants. In Western Europe, the pattern is
slightly different, with about half used in polyester manufacture and
a quarter in coolants. Ethylene glycol is also used for runway deicing
(the main source of high local concentrations in the environment), as
plasticizer for adhesives, as softener for cellulose film, as
glycoborates in electrolytic condensers, as glycol dinitrate in
explosives, for various heat transfer applications, as humectant in
inks, as antifreeze and plasticizer in paints, and to reduce gelling
of medium oil alkyds based on pentaerythritol.2 There are many
different formulations of ethylene glycol and propylene glycol for use
in runway deicing. In some locations, one or the other of the glycols
is used alone; more usually, however, they are used together. Other
components of the formulation differ widely between manufacturers, as
indicated by differing toxicity (see later sections). Details of
formulations are not available.
1 ICI Chemicals and Polymers Ltd. (1993) Personal
communication cited in Nielsen et al. (1993).
2 Chou T, Hansch C (1986) Pomona College,
Claremont, CA, unpublished (cited in BUA, 1991).
Release to the atmosphere from production and processing of
ethylene glycol and production of ethylene oxide was estimated at
<875 t in Germany in 1989; release to the hydrosphere was estimated
at <28 t from production and >2000 t from dispersed use as an
antifreeze (BUA, 1991). A maximum figure of 12 500 t of ethylene
glycol release from use as antifreeze in the United Kingdom, based on
production figures and proportion of use, was derived in Nielsen et
al. (1993); estimated release of total volatile organics to the
atmosphere from glycol production was 41-260 t/year. Industry
estimates of release to the environment from use in runway deicing in
the United Kingdom were 600-720 t in 19931; use of the compound in
runway deicers is declining. Details of releases reported through the
US Toxic Release Inventory by individual state can be found in ATSDR
(1997). Summary figures for the USA annually between 1990 and 1993
were as follows: 4600 t to air, 523 t to water, 577 t to soil, and
2675 t injected underground from production. Estimated figures of 6778
t released via publicly owned treatment works and 60 252 t released to
the environment away from production and industrial usage sites were
reported for the same period (ATSDR, 1997).
1 ICI Chemicals and Polymers Ltd. (1993) Personal
communication cited in Nielsen et al. (1993).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Ethylene glycol has a low vapour pressure (7.9 Pa at 20°C); it is
expected to exist almost entirely in the vapour phase if released to
the atmosphere (Eisenreich et al., 1981). The Henry's law constant for
ethylene glycol is 1.41 × 10-3 or 6.08 × 10-3 Pa.m3/mol, depending
on method of calculation (BUA, 1991), indicating a low capacity for
volatilization from water bodies or soil surfaces.
14C-labelled ethylene glycol adsorbed onto silica gel and
irradiated with light (wavelength >290 nm) degraded by 12.1% over 17
h (Freitag et al., 1985). Photodegradation is not expected, as the
molecule should not absorb at these wavelengths; the mechanism of this
breakdown is, therefore, unknown. Estimated half-life in the
atmosphere for reaction with hydroxyl radicals is 2.1 days (BUA,
1991), 8-84 h (Howard et al., 1991), or 1 day (Nielsen et al., 1993).
No hydrolysis of ethylene glycol is expected in the environment
(Lyman et al., 1982).
Lokke (1984) studied the adsorption of ethylene glycol to three
different soils in leaching experiments. There was effectively no
sorption, and soil partition coefficients (log Koc) of 0-0.62 were
determined. Migration rates in five soil types were measured by
Schramm et al. (1986) at between 4 and 27 cm per 12 h.
The low octanol/water partition coefficient of ethylene glycol
(log Kow -1.93 to -1.36) indicates a low potential for
bioaccumulation. Bioconcentration factors of 190 for the green alga
(Chlorella fusca) (Freitag et al., 1985), up to 0.27 in specific
tissues of the crayfish ( Procambarus sp.) (Khoury et al., 1993), and
10 for the golden orfe (Leuciscus idus melanotus) (Freitag et al.,
1985) confirm low bioaccumulation.
In standard biodegradation tests under Organisation for Economic
Co-operation and Development (OECD), US Environmental Protection
Agency (US EPA), and Japanese Ministry of International Trade and
Industry (MITI) guidelines, ethylene glycol was readily
biodegradable.1
Means & Anderson (1981) measured the biodegradation of ethylene
glycol under aerobic conditions in five different tests using various
aqueous media. Degradation was monitored using oxygen uptake,
dissolved organic carbon removal, or carbon dioxide production.
1 Unpublished reports from Dow Chemicals, Union Carbide, and ICI
Chemicals and Polymers Ltd.; cited in IUCLID (European Union
database), 1st ed., 1996.
Ethylene glycol was readily degraded in all tests with a lag period of
up to 3 days. Degradation to 10% or less of the starting concentration
was reported in all tests after between 1 and 21 days. Boatman et al.
(1986) used acclimated sewage sludge as inoculum and a concentration
of ethylene glycol equivalent to 20 mg carbon/litre. Significant
degradation, as measured by carbon dioxide production, did not occur
until day 14 of the test (an estimated lag period of 8-10 days was
reported). By day 21, 71% of the ethylene glycol was degraded. Using
activated sludge from a petrochemicals process, 92% chemical oxygen
demand (COD) removal and 93% total organic carbon removal over 24 h
were reported for ethylene glycol at an initial concentration of 172
mg/litre by Matsui et al. (1975). However, direct measurement using
gas chromatography showed 44% of ethylene glycol still present after
24 h; the authors explain the discrepancy as being due to poor
detection of the glycol by the analytical method used. Pitter (1976)
reported 96.8% removal of ethylene glycol within 120 h using adapted
activated sewage sludge based on COD measurements and an initial COD
of 200 mg/litre. A biodegradation rate of 41.7 mg COD/g per hour was
reported. Zahn & Wellens (1980) reported >90% degradation after 4
days' incubation of ethylene glycol in a batch biodegradability study;
no lag period was observed. Bridie et al. (1979) reported 36% of
theoretical oxygen demand (ThOD) after 5 days' incubation at 20°C
measured as biological oxygen demand (BOD) and 100% measured as COD;
using previously adapted sludge, 63% degradation as BOD was reported
after 5 days. Conway et al. (1983) reported 39% of theoretical BOD
after 5 days, rising to 73% by day 10 and 96% at day 20, using
domestic sewage sludge inoculum. Freitag et al. (1985) reported only
5.7% degradation of ethylene glycol at 0.05 mg/litre over 5 days using
municipal sewage sludge inoculum. McGahey & Bouwer (1992) studied
degradation of ethylene glycol using primary sewage treatment effluent
as the inoculum. After an initial lag period of 3 days, a typical
first-order kinetic rate constant of 1.13 ± 0.34/day at 25°C was
reported; the half-life for the reaction was calculated at between
11.5 and 21.5 h.
Evans & David (1974) studied the biodegradation of ethylene
glycol in four samples of river water under controlled laboratory
conditions. The samples were dosed with ethylene glycol at 0, 2, or 10
mg/litre and incubated at either 20°C or 8°C. At 20°C, primary
biodegradation was complete within 3 days in all four samples; at 8°C,
it was complete by day 14. Degradation rates were further reduced at
4°C. Price et al. (1974) assessed the biodegradation of ethylene
glycol in both fresh and salt water over a 20-day incubation period.
Concentrations of up to 10 mg ethylene glycol/litre were used. In
fresh water, 34% degradation was observed after 5 days, rising to 86%
by day 10 and 100% by day 20. Degradation was less in salt water
-- 20% after 5 days and 77% after 20 days.
McGahey & Bouwer (1992) studied the degradation of ethylene
glycol using natural groundwater and soil inocula. An initial glycol
concentration of 111 mg/litre was degraded in groundwater with a rate
constant of 0.76/day at 25°C; the lag period was less than 3 days, and
the half-life was estimated at 22 h. First-order degradation rate
constants for sandy loam soil and sandy silt soil were 1.01 and
2.90/day, respectively. A lag period of 3 days and a half-life of 16.5
h were reported for the sandy loam, and a lag period of 0 days and a
half-life of 6 h were reported for the sandy silt. Increasing the
ethylene glycol concentration to 10 000 mg/litre in the sandy loam
resulted in a greatly diminished rate constant of 0.05/day and minimal
degradation of the glycol. Reducing temperature in the sandy silt
inoculum from 25°C to 10°C resulted in a decrease in the rate constant
from 2.09 to 1.19/day and an increase in the half-life from 6 to 14 h;
however, nearly complete degradation was observed at both temperatures
within the incubation period. Biodegradation rates of ethylene
glycol-based aircraft deicing fluids were examined in soil microcosms
at 8°C. Initial concentrations of 390-4900 ethylene glycol/kg soil
were degraded at around 20 mg/kg per day (Klecka et al., 1993).
Haines & Alexander (1975) identified a soil bacterium
(Pseudomonas aeruginosa) capable of degrading ethylene glycol. The
bacterium had been originally grown on propylene glycol and was
capable of degrading 1 mg carbon per inoculum within 2 days (based on
oxygen consumption). Watson & Jones (1977) isolated bacteria from
sewage effluent and identified Acinetobacter and Pseudomonas
strains that degraded ethylene glycol. Flavobacterium isolates did
not degrade the compound. However, under strongly aerobic conditions,
Flavobacterium sp. converted ethylene glycol to glycolate and
eventually carbon dioxide (Willetts, 1981).
Dwyer & Tiedje (1983) assessed the degradation of ethylene glycol
in methanogenic enrichments of bacteria obtained from municipal sewage
sludge. The bacterial inoculum was dominated by two morphological
types of bacteria, Methanobacterium sp. and Desulfovibrio sp. A
concentration of 36 mmol ethylene glycol/litre (2.2 g/litre) was
incubated at 37°C, and, based on analysis of the compound, 100% of the
glycol was metabolized within 12 days. Products of degradation
included ethanol, acetate, and methane. Battersby & Wilson (1989)
assessed the degradation of ethylene glycol under methanogenic
conditions using primary digesting sludge from a sewage treatment
plant receiving both domestic and industrial wastewater. Degradation
was assessed as total gas production. The glycol at a concentration of
50 mg carbon/litre sludge was incubated at 35°C for 60 days. Total
degradation was achieved after 1-2 weeks (>80% of theoretical gas
production), and a short lag period of <1 day was reported. In
anaerobic conditions using an inoculum from a pretreatment lagoon for
petrochemical waste, ethylene glycol at a concentration of 135
mg/litre was degraded to 78% after 10 days; at 755 mg/litre,
degradation was 75-79% complete (Hovious et al., 1973). Under
anaerobic conditions, ethylene glycol was degraded by 89% within 7
days (Kameya et al., 1995). The anaerobic bacterium Clostridium
glycolicum isolated from pond ooze and adapted to ethylene glycol
could degrade 5.3 or 6.7 g ethylene glycol/litre under anaerobic
conditions (Gaston & Stadtman, 1963). Non-adapted Acetobacter
strains could degrade ethylene glycol at concentrations between 5 and
15 g/litre using the compound as sole carbon source under anaerobic
conditions (Kaushal & Walker, 1951; Hrotmatka & Polesofsky, 1962).
Following a spill of ethylene glycol in New Jersey, USA, in which
15 000 litres of coolant containing ethylene glycol as antifreeze at
275 g/litre were spilled, concentrations of the glycol in soil and
groundwater were measured at 4.9 and 2.1 g/litre, respectively. A
remediation procedure was initiated involving the pumping of nitrogen,
phosphate, and oxygen-saturated water into the contaminated ground;
after 26 days, 85-93% of the glycol had been degraded by naturally
occurring microorganisms. After 9 months, the concentration of
ethylene glycol was below the detection limit of 50 mg/litre (Flathman
et al., 1989).
6. ENVIRONMENTAL LEVELS
The Japan Environment Agency (1991) reported the results of two
environmental surveys of surface waters and sediments carried out in
1977 and 1986. In the earlier survey, ethylene glycol was not detected
in six samples of water and sediment (detection limits 0.1-0.4
mg/litre and 1-2 mg/kg, respectively). In the later survey, the
compound was not detected in 24 sediment samples (detection limit 0.06
mg/kg) but was found in 2 out of 24 water samples at levels of 1.3 and
2 µg/litre (detection limit 0.8 µg/litre).
Monitoring of ethylene glycol in runoff from airports has been
reviewed by Sills & Blakeslee (1992); levels in runoff water ranged up
to several thousand mg/litre. Concentrations up to 19 000 mg/litre
were reported for Salt Lake City International Airport, Salt Lake
City, UT, USA, up to 3100 mg/litre for Lester B. Pearson International
Airport in Toronto, Ontario, Canada, and up to 5050 mg/litre at
Stapleton International Airport in Denver, CO, USA. Concentrations of
up to 70 mg/litre were measured in stream water receiving runoff from
Lester B. Pearson International Airport. Ethylene glycol was not
detected in soil at the edge of runways in Denver, but levels of the
compound in groundwater below the sandy soil of Ottawa International
Airport, Ottawa, Ontario, Canada, were measured at up to 415 mg/litre;
concentrations peaked in June and declined to non-detectable in the
autumn.
Pitt et al. (1975) sampled the primary effluent from a municipal
sewage treatment plant. No details are given in the report, but levels
of ethylene glycol were reported at 3 µg/litre. Zeithoun & McIllhenny
(1971) identified ethylene glycol in the wastewater from glycol
production; in 51 samples from two production plants, concentrations
in wastewater ranged from 680 to 2300 mg/litre (average 1003-1306
mg/litre). In a similar number of samples from two 1,2-propanediol
production plants, concentrations of ethylene glycol in wastewater
ranged from 355 to 2550 mg/litre (average 960-1140 mg/litre).
Grabinska-Loniewska (1974) identified ethylene glycol as a constituent
of wastewater from a polyester fibre plant in Poland. Concentrations
ranged from 200 to 440 mg/litre (average 200 mg/litre, number of
samples unspecified).
Influent-contaminated groundwater to a bioremediation plant in
California, USA, contained ethylene glycol at up to 103 mg/litre (Ross
et al., 1988).
Lee et al. (1983) detected ethylene glycol in two samples of
Asiatic clams ( Corbicula sp.); no levels were reported.
Ethylene glycol was detected in ambient air at time-weighted
averages of <0.05-0.33 mg/m3 as aerosol and <0.05-10.4 mg/m3 as
vapour following spray application of deicer containing 50% of the
compound to bridges (LDOTD, 1990).
Ethylene glycol has been identified as a metabolite of the growth
regulator ethylene in a number of higher plants (Blomstrom & Beyer,
1980) and as naturally occurring in the edible fungus Tricholoma
matsutake (Ahn & Lee, 1986).
7. EFFECTS ON ORGANISMS IN THE LABORATORY AND FIELD
7.1 Aquatic organisms
Results of acute toxicity tests on aquatic organisms are
summarized in Table 1. Chronic toxicity tests were conducted on water
fleas (Ceriodaphnia dubia) over the period taken by 60% of the
controls to produce three broods. NOECs for mortality at 24 000
mg/litre and for reproduction at 8590 mg/litre were reported; an IC25
of 12 310 mg/litre was calculated. Seven-day toxicity tests conducted
on the fathead minnow (Pimephales promelas) gave NOECs for mortality
and growth at 32 000 mg/litre and 15 380 mg/litre, respectively, with
an IC25 at 22 520 mg/litre (Pillard, 1995). Masters et al. (1991)
exposed Ceriodaphnia dubia to ethylene glycol in the US EPA standard
7-day chronic toxicity test and also concurrently carried out 4-day
tests to compare results. Survival and production of young were
monitored. A "chronic index," the geometric mean of the NOEC and
lowest-observed-effect concentration (LOEC), was determined to be 4.2
mg/litre for production of young in both tests and >6.0 and 4.2
mg/litre for survival in the 4- and 7-day tests, respectively. Actual
NOECs and LOECs were not reported.
Mayes et al. (1983) compared the toxicity of ethylene glycol to
fathead minnows at three different ages (fry, 10-15 days old;
juveniles, 30-35 days old; and subadults, 60-94 days old) and found no
effect of age. However, Mayer & Ellersieck (1986) found older (1.1 g)
rainbow trout (Oncorhynchus mykiss) more sensitive than younger
(0.7 g) fish.
Ethylene glycol did not produce narcosis in tadpoles of the
common frog (Rana temporaria) at 28 550 mg/litre; tadpoles did
become sluggish after 5-6 h of exposure, but did not lose their
responsiveness to stimuli. However, death followed within 12-20 h. At
14 275 mg/litre, tadpoles kept moving for 24-30 h but died after about
36-48 h (Lipnick, 1991).
A reported 48-h LC50 value for tadpoles of the clawed toad
(Xenopus laevis) at 326 mg/litre (DeZwart & Slooff, 1987) is
considered invalid for the setting of standards following
correspondence from the authors. The study was part of a technicians'
training course, and no quality control was exercised.
7.1.1 Toxicity of deicer formulations
Pillard (1995) conducted acute and chronic tests on water fleas
(C. dubia) and fathead minnows using both pure ethylene glycol and
formulations of deicer based on the compound. For acute tests, 48-h
LC50s for the daphnid were 34 440 and 13 140 mg/litre for the pure
substance and formulation, respectively; chronic NOECs for survival
were 24 000 and 8400 mg/litre, respectively, and for reproduction,
8590 and <3330 mg/litre, respectively. For acute tests on the
minnows, 96-h LC50s were 72 860 and 8050 mg/litre, respectively;
chronic NOECs for survival were 32 000 and 6090 mg/litre and for
growth were 15 380 and <3330 mg/litre, respectively. The higher
toxicity of formulations was ascribed to other unknown constituents of
the formulations, including rust inhibitors, buffers, polymers, and
surfactants. Hartwell et al. (1995) conducted toxicity tests using
ethylene glycol-based deicer and determined 96-h LC50s for fathead
minnow, Daphnia magna, D. pulex, and C. dubia at 10 802, 4213,
4675, and 9845 mg glycol/litre, respectively. Seven-day exposure of
fathead minnows produced an identical LC50. A maximum acceptable
toxicant concentration (MATC) for reproduction of Ceriodaphnia was
calculated at 418 mg/litre. Gill and kidney lesions and calcium
oxalate crystals were found in exposed fish. The same species were
also cultured in stream water taken from an outflow stream from
stormwater basins at Baltimore Washington International Airport,
Maryland, USA, receiving runoff from deicing of runways. No fish
mortality was seen in either March or April water samples over 7 days.
However, oxalate crystals were seen after 7 days' exposure to the
March water. Significant reduction in survival of D. magna and D.
pulex was recorded over 96 h in the March water sample. Ceriodaphnia
dubia showed reduced survival only after 7 days, and production of
neonates was also reduced to 55% of controls. No significant adverse
effects on daphnids was seen with the April water (neonate production
was significantly increased in Ceriodaphnia).
Toxicity of formulations will vary considerably depending on the
particular constituents. For example, Union Carbide's UCAR 50/50
EG-Based Type I fluid for the 1997-98 aircraft deicing season has
lower aquatic toxicity figures than those quoted in the literature:
D. magna, 48-h EC50 88 000 mg/litre; fathead minnow, 96-h LC50
44 000 mg/litre; rainbow trout, 96-h LC50 34 200 mg/litre.1 For an
assessment of likely effects in the field, toxicity values for
particular formulations used will need to be determined.
7.1.2 Field effects
During early summer, 3 months after release of glycols into
streams draining from airport stormwater basins (Baltimore Washington
International Airport), fish were sampled from the stream. In
tesselated darters (Etheostoma olmstedi), oxalate crystals appeared
in the interstitial tissue of the kidneys and basal layers of tubules.
American eels (Aguilla rostrata) exhibited kidney lesions consistent
with oxalate damage, but no crystals were found.2 Pillard (1995)
cites his own unpublished report as showing fish kills in streams near
airports and aquatic community impairment in three streams receiving
runoff from airports.
1 Personal communication to IPCS.
2 Unpublished reports cited by Hartwell et al. (1995).
7.2 Terrestrial organisms
Incubation of yeast (Saccharomyces cerevisiae) in ethylene
glycol at a concentration of 150 g/litre produced a 1% reduction in
glucose utilization; a concentration of 172.5 g/litre produced <10%
inhibition (Gray & Sova, 1956). Concentrations of 200 g ethylene
glycol/litre prevented germination of conidia of the ascomycete fungus
Neurospora crassa; return to clean medium allowed germination.
Concentrations greater than 200 g/litre killed the spores (Bates &
Wilson, 1974). Using oxygen uptake and growth (turbidity) as
end-points, Khoury et al. (1990) reported an IC50 for heterotrophic
soil microorganisms at 114 300 mg/litre.
Bose & Bandyopadhyay (1975) soaked tomato seeds in ethylene
glycol solution at 5.5 g/litre. Only 50% of the soaked seeds
germinated, but those that did grew higher, bloomed earlier, and
carried twice the crop of untreated plants. Soaking of cluster bean
(Cyamopsis tetragonoloba) in aqueous ethylene glycol solutions at 10
or 20 g/litre for 8 h, following an initial 4-h soak in water, led to
some plants showing small leaves with shortened petioles, stunted
growth, and sterility (Bose & Naskar, 1975). Twenty-three percent of
rice seeds soaked in aqueous ethylene glycol at 10 g/litre for 24 h
germinated (compared with 48% of controls). Germinated plants showed
only marginal effects on growth, panicle length, grain weight, and
fertility, but tiller numbers were reduced by 40-50% compared with
controls (Bose & Bhattacharyya, 1975). Jute (Corchorus capsularis)
seeds soaked in ethylene glycol solution at 2 g/litre showed 84% of
control levels of germination. Plants that germinated following
treatment required 8 days longer to blossom, on average, showed a
higher degree of pollen sterility, and produced fewer and lighter
seeds than controls (Bose & Datta, 1973). Tobacco (Nicotiana xanthi)
plants sprayed with 5 ml of a solution of ethylene glycol at 34.
51.5, or 69 g/litre showed a dose-dependent 10-33% reduction in
terminal bud fresh weight, but no other overt effects were noted
(Steffens & Barer, 1984).
Toxic effects (unspecified) were noted in chickens fed a diet
containing 5% ethylene glycol for 27 days (Yoshida et al., 1969). An
LC50 for ethylene glycol in drinking-water at 75 100 mg/litre over 24
h was reported for chickens (Riddell et al., 1967). No deaths were
seen in chickens exposed through drinking-water at 27 800 mg/litre,
but renal oxalosis was observed. Chickens exposed at 14 500 mg/litre
drinking-water showed calcium oxalate crystals in renal tubules, but
no clinical signs were reported. Beasley & Buck (1980) reported lethal
doses for poultry to lie within the range of 7790-8900 mg/kg body
weight. A NOEL of 1221 mg/kg body weight and a lowest-observed-effect
level (LOEL) of 2553 mg/kg body weight were reported for orally dosed
mallard ducks (Anas platyrhynchos) (Stowe et al., 1981).
Table 1: Acute toxicity of ethylene glycol to aquatic organisms.
Organism End-point Concentration (mg/litre) Reference
Microorganisms
bacterium Pseudomonas Toxic threshold >10 000 Bringmann & Kuhn (1980a,b)
putida; protozoa (cell multiplication)
Entosiphon sulcatum,
Uronema parduczi
cyanobacterium Microcystis Toxic threshold 2000 Bringmann & Kuhn (1976)
aeruginosa (cell multiplication)
bacterium Pseudomonas EC0 (growth) 1000 Daugherty (1980)
aeruginosa EC100 (growth) 2000
bacterium Photobacterium 30-min EC50 (luminescence) 621 Kaiser & Palabrica (1991)
phosphoreum 5-min EC50 112 220 Calleja et al. (1993)
5-min EC50 166 000 Kahru et al. (1996)
bacteria from aquatic sediment EC50 (growth) 114 300 Khoury et al. (1990)
and sewage sludge
bacteria from sewage sludge EC50 (oxygen uptake) 224 600 Kilroy & Gray (1992)
anaerobic bacteria from sewage Toxic threshold 5000 Hoechst (1975)
sludge
flagellate euglenoid EC5 (growth in population) >10 000 AQUIREa
Algae
green alga Scenedesmus quadricauda Toxic threshold >10 000 Bringmann & Kuhn (1980a)
green alga Selenastrum capricornutum 96-h EC50 (growth, cell counts) 6500-7500 Dowb
96-h EC50 (growth, cell volume) 9500-13 000
168-h EC50 (growth, cell volume) 24 000
Table 1 (cont'd)
Organism End-point Concentration (mg/litre) Reference
Invertebrates
water flea Daphnia magna 48-h LC50 (immobilization) >10 000 Conway et al. (1983)
50 000 Hermens et al. (1984)
41 000-51 000 Gersich et al. (1986)
74 400 Calleja et al. (1994)
14 828c Hartwell et al. (1995)
24-h LC50 >10 000 Bringmann & Kuhn (1977)
24-h NOEC 2500
water flea Ceriodaphnia dubia 48-h LC50 25 800 Cowgill et al. (1985)
(22 600-29 900)
34 440 Pillard (1995)
crayfish Procambarus sp. 96-h LC50 91 430 Khoury et al. (1990)
common shrimp Crangon vulgaris 96-h LC50 50 000 AQUIREa
brine shrimp Artemia salina 24-h LC50 >20 000 Price et al. (1974)
180 420 Calleja et al. (1994)
brown shrimp Crangon crangon 96-h LC50 approx. 50 000 Blackman (1974)
Fish
rainbow trout Oncorhynchus mykiss 96-h LC50 >18 500 Jank et al. (1974)
17 800-45 600 Mayer & Ellersieck
(1986)
guppy Poecilia reticulata 168-h LC50 49 300 Konnemann (1981)
bluegill sunfish Lepomis macrochirus 96-h LC50 >111 300 Mayer & Ellersieck
(1986)
27 540 Khoury et al. (1990)
Table 1 (cont'd)
Organism End-point Concentration (mg/litre) Reference
fathead minnow Pimephales promelas 96-h LC50 >10 000 Conway et al. (1983)
49 000-57 000 Mayes et al. (1983)
72 860 Pillard (1995)
goldfish Carassius auratus 24-h LC50 >5000 Bridie et al. (1979)
Japanese killifish Oryzias latipes 48-h NOEC 900 Tsuji et al. (1986)
Amphibians
frog (tadpoles) Rana brevipoda 48-h LC50 17 000 Nishiushi (1984)
a AQUIRE (Aquatic Information Retrieval) Computerized database developed by the US Environmental Protection Agency.
b Dow (undated) Personal communication to IPCS.
c Value based on ethylene glycol content of a deicing product.
8. EFFECTS EVALUATION
Ethylene glycol released to the atmosphere will be degraded by
reaction with hydroxyl radicals; the half-life for this reaction has
been estimated at between 0.3 and 3.5 days.
No hydrolysis of ethylene glycol is expected in surface waters.
The compound has little or no capacity to bind to particulates
and will be mobile in soil.
The low octanol/water partition coefficient and measured
bioconcentration factors in a few organisms indicate low capacity for
bioaccumulation.
Ethylene glycol is readily biodegradable in standard tests using
sewage sludge. Many studies show biodegradation under both aerobic and
anaerobic conditions. Some studies suggest a lag phase before
degradation, but many do not. Degradation occurs in both adapted and
unadapted sludges. Rapid degradation has been reported in surface
waters (less in salt water than in fresh water), groundwater, and soil
inocula. Several strains of microorganisms capable of utilizing
ethylene glycol as a carbon source have been identified.
Limited data are available on measured concentrations of ethylene
glycol in environmental compartments. Levels measured in surface
waters have been generally low, at a few micrograms per litre.
Concentrations in wastewater from production plants, prior to
treatment, have averaged up to 1300 mg/litre. By far the highest
reported concentrations relate to runoff water from airports, with
levels up to 19 000 mg/litre.
Ethylene glycol has generally low toxicity to aquatic organisms.
Toxic thresholds for microorganisms are above 1000 mg/litre. EC50s
for growth in microalgae are 6500 mg/litre or higher. Acute toxicity
tests with aquatic invertebrates where a value could be determined
show LC50s above 20 000 mg/litre, and those with fish show LC50s
above 17 800 mg/litre. The only valid acute toxicity value for
amphibians is 17 000 mg/litre for Rana brevipoda tadpoles. A NOEC
for chronic tests on daphnids of 8590 mg/litre (for reproductive
end-points) has been reported. A NOEC following short-term exposure of
fish has been reported at 15 380 mg/litre for growth.
Tests using deicer containing ethylene glycol generally showed
greater toxicity to aquatic organisms than the pure compound,
indicating other toxic components of the formulations.
Laboratory tests exposing aquatic organisms to stream water
receiving runoff from airports have demonstrated toxic effects and
death. Field studies in the vicinity of an airport have reported toxic
signs consistent with ethylene glycol poisoning (oxalate crystal
formation), fish kills, and reduced biodiversity. These effects cannot
definitively be ascribed to ethylene glycol.
Terrestrial organisms are much less likely to be exposed to
ethylene glycol and generally show low sensitivity to the compound.
Concentrations above 100 000 mg/litre were needed to produce toxic
effects on yeasts and fungi from soil. Very high concentrations and
soaking of seeds produced inhibition of germination in some
experiments; these are not considered of environmental significance. A
NOEL for orally dosed ducks at 1221 mg/kg body weight and reported
lethal doses for poultry at around 8000 mg/kg body weight indicate low
toxicity to birds.
8.1 Predicted environmental concentration
There are reported measurements of ethylene glycol in the influx
wastewater to treatment plants at industrial sites manufacturing the
compound. These will be used as the basis for calculating a predicted
environmental concentration (PEC) after treatment. Average
concentrations up to 1306 mg/litre have been reported.
Based on this emission concentration, and using mainly default
values from the OECD Technical Guidance Manual, the initial
concentration in river water would be as follows:
PEClocal (water) = Ceffluent/[(1 + Kp(susp) × C(susp)) × D]
where:
* PEClocal (water) is the predicted environmental concentration (g/litre)
* Ceffluent is the concentration of the chemical in the wastewater
treatment plant effluent (g/litre), calculated as Ceffluent = I
× (100 - P)/100,
where:
I = input concentration to the wastewater treatment plant
(1.3 g/litre)
P = percent removal in the wastewater treatment plant (91%,
based on the "ready biodegradability" of the compound)
* Kp(susp) is the suspended matter/water adsorption coefficient,
calculated as Kp(susp) = foc(susp) × Koc, where:
foc(susp) = the fraction of organic carbon in suspended matter
(default 0.1)
Koc = 0.411 × Kow
where:
Kow = the octanol/water partition
coefficient (log Kow = -1.36)
* C(susp) is the concentration of suspended matter in the river
water in kg/litre (default concentration 15 mg/litre)
* D is the dilution factor for river flow (a conservative default
value of 10)
Under these very conservative conditions, PEClocal (water) = 11.7
mg/litre. This is substantially higher than reported concentrations in
surface water and represents a conservative estimate of initial
maximum concentration.
8.2 Predicted no-effect concentration
There is a substantial database on the toxicity of ethylene
glycol to aquatic organisms, representing acute and chronic test
results for two trophic levels and acute and short-term results for a
third. The distribution of test results is presented in Figure 1 for
different types of organism. The shaded points represent toxic
thresholds for microorganisms or algae, and these are not considered a
suitable basis for estimating a predicted no-effect concentration
(PNEC). It would be justifiable to apply an uncertainty factor of 10
to the chronic NOEC for daphnid reproduction at 8590 mg/litre given
the wide range of available data. This gives a PNEC of 859 mg/litre.
8.3 Environmental risk factors
It is clear from Figure 1 that risk to aquatic organisms from
production of ethylene glycol is very low, even based on conservative
assumptions; a risk factor of 0.013 is generated by comparing PEClocal
(water) against PNEC. Based on the few measured values in surface waters,
risk would be negligible (risk factor at 2.3 × 10-6).
It is also clear that concentrations in airport runoff would be
expected to cause severe field effects without mitigation. It is
difficult to estimate likely dilution of runoff in generalized terms;
however, dilution factors of at least 100-fold would be needed for the
reported concentrations. Concentrations may be substantially higher in
runoff water at particular airport sites. There is indication that
formulations might be significantly more toxic to aquatic organisms
than the pure ethylene glycol. It is also unlikely that only ethylene
glycol formulations would be used. The ready biodegradability of
glycols also increases risk to organisms from oxygen depletion in
surface waters. Risk assessment and field monitoring of overt effects
should be applied on a case-by-case basis to determine pollution
control measures needed.
1. EXECUTIVE SUMMARY
This CICAD on the environmental aspects of ethylene glycol was
prepared by the Institute of Terrestrial Ecology, United Kingdom,
based on the report Environmental hazard assessment: Ethylene glycol
(Nielsen et al., 1993). The report on ethylene glycol prepared by the
German Chemical Society Advisory Committee on Existing Chemicals of
Environmental Relevance (BUA, 1991) was also used as a source
document. In addition to these documents, a search of recent
literature was conducted up to 1998. Information on the nature of the
peer review process for the main source documents is presented in
Appendix 1. Information on the peer review of this CICAD is presented
in Appendix 2. This CICAD was approved as an international assessment
at a meeting of the Final Review Board, held in Washington, DC, USA,
on 8-11 December 1998. Participants at the Final Review Board meeting
are listed in Appendix 3. The International Chemical Safety Card (ICSC
0270) produced by the International Programme on Chemical Safety
(IPCS, 1993) has also been reproduced in this document.
Ethylene glycol (CAS No. 107-21-1) is a clear, colourless, syrupy
liquid with a sweet taste but no odour. It has low volatility. It is
miscible with water and some other solvents, slightly soluble in
ether, but practically insoluble in benzene, chlorinated hydrocarbons,
petroleum ethers, and oils. The log octanol/water partition
coefficient is -1.93 to -1.36.
Estimated world production capacity was 9.4 million tonnes in
1993. Release to the environment is mainly to the hydrosphere. The
largest local release to surface waters would follow ethylene glycol's
use as a deicer on airport runways and planes. On a worldwide basis,
approximately two-thirds of ethylene glycol is used as a chemical
intermediate, with a further one-quarter used as an antifreeze in
engine coolants.
Ethylene glycol released to the atmosphere will be degraded by
reaction with hydroxyl radicals; the half-life for the compound in
this reaction has been estimated at between 0.3 and 3.5 days.
No hydrolysis of ethylene glycol is expected in surface waters.
The compound has little or no capacity to bind to particulates
and will be mobile in soil.
The low octanol/water partition coefficient and measured
bioconcentration factors in a few organisms indicate low capacity for
bioaccumulation.
Ethylene glycol is readily biodegradable in standard tests using
sewage sludge. Many studies show biodegradation under both aerobic and
anaerobic conditions. Some studies suggest a lag phase before
degradation, but many do not. Degradation occurs in both adapted and
unadapted sludges. Rapid degradation has been reported in surface
waters (less in salt water than in fresh water), groundwater, and soil
inocula. Several strains of microorganisms capable of utilizing
ethylene glycol as a carbon source have been identified.
Limited data are available on measured concentrations of ethylene
glycol in environmental compartments. Levels measured in surface
waters have been generally low, at a few micrograms per litre.
Concentrations in wastewater from production plants, prior to
treatment, have averaged up to 1300 mg/litre. By far the highest
reported concentrations relate to runoff water from airports, with
levels up to 19 000 mg/litre.
Ethylene glycol has generally low toxicity to aquatic organisms.
Toxic thresholds for microorganisms are above 1000 mg/litre. EC50s
for growth in microalgae are 6500 mg/litre or higher. Acute toxicity
tests with aquatic invertebrates where a value could be determined
show LC50s above 20 000 mg/litre, and those with fish show LC50s
above 17 800 mg/litre. An amphibian test showed an LC50 for tadpoles
at 17 000 mg/litre. A no-observed-effect concentration (NOEC) for
chronic tests on daphnids of 8590 mg/litre (for reproductive
end-points) has been reported. A NOEC following short-term exposure of
fish has been reported at 15 380 mg/litre for growth.
Tests using deicer containing ethylene glycol showed greater
toxicity to aquatic organisms than observed with the pure compound,
indicating other toxic components of the formulations.
Laboratory tests exposing aquatic organisms to stream water
receiving runoff from airports have demonstrated toxic effects and
death. Field studies in the vicinity of an airport have reported toxic
signs consistent with ethylene glycol poisoning, fish kills, and
reduced biodiversity. These effects cannot definitively be ascribed to
ethylene glycol.
Terrestrial organisms are much less likely to be exposed to
ethylene glycol and generally show low sensitivity to the compound.
Concentrations above 100 000 mg/litre were needed to produce toxic
effects on yeasts and fungi from soil. Very high concentrations and
soaking of seeds produced inhibition of germination in some
experiments; these are not considered of environmental significance. A
no-observed-effect level (NOEL) for orally dosed ducks at 1221 mg/kg
body weight and reported lethal doses for poultry at around 8000 mg/kg
body weight indicate low toxicity to birds.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Ethylene glycol (C2H6O2; CAS No. 107-21-1) is also known as
1,2-ethanediol, 2-hydroxyethanol, 1,2-dihydroxyethane, glycol, glycol
alcohol, ethylene alcohol, and monoethylene glycol or MEG. Its
structure is illustrated below:
H H
' '
HO - C - C - OH
' '
H H
Ethylene glycol is a clear, colourless, syrupy liquid with a
sweet taste but no odour. The molecular mass is 62.07. It has low
volatility; its vapour pressure is 7.9 or 8.0 Pa at 20°C (Eisenreich
et al., 1981; ATSDR, 1997) and 12.2 Pa at 25°C (HSDB, 1998). It is
hygroscopic and absorbs twice its weight in water at 100% relative
humidity (Budavari, 1989). It is miscible with water, lower aliphatic
alcohols, glycerol, acetic acid, acetone and similar ketones,
aldehydes, pyridine, and similar coal tar bases. The compound is
slightly soluble in ether but practically insoluble in benzene and its
homologues, chlorinated hydrocarbons, petroleum ethers, and oils
(Budavari, 1989). The log octanol/water partition coefficient is -1.93
(Hansch & Leo, 1979) to -1.36.1 Other physical and chemical
properties can be found in the International Chemical Safety Card
(ICSC 0270) reproduced in this document.
1 Chou T, Hansch C (1986) Pomona College,
Claremont, CA, unpublished (cited in BUA, 1991).
3. ANALYTICAL METHODS
Ethylene glycol is measured in environmental samples by gas
chromatography, most commonly using flame ionization detection. Recent
methods have been described using high-resolution gas chromatography
coupled with mass spectrometry. Measurement in biological samples has
also used gas chromatography or high-resolution gas chromatography,
with additional methods employing high-performance liquid
chromatography or colorimetric determination. Detection limits were
not available for environmental media. Details of extraction and
concentration methods can be found in ATSDR (1997).
4. SOURCES OF ENVIRONMENTAL EXPOSURE
Although ethylene glycol can be prepared directly by alkaline
hydrolysis of chlorohydrin, hydrolysis of ethylene oxide is the more
usual method. The feed stream consists of ethylene oxide (from either
chlorohydrin or the direct oxidation of ethylene) and water. The
mixture is fed under pressure into a reaction vessel at a temperature
of about 100°C, which by the end of the reaction has risen to 170°C.
Some diethylene and triethylene glycol are produced by the reaction of
ethylene glycol with excess ethylene oxide. The crude glycol solution
is concentrated in a multiple-effect evaporator, and final separation
is achieved by distillation (Kent, 1974). Product proportions were
estimated by the US EPA (1980) as follows: ethylene glycol,
87.0-88.5%; diethylene glycol, 9.3-10.5%; and triethylene glycol,
2.2-2.5%; and by ICI Chemicals and Polymers Ltd. as 90%, 9%, and 1%,
respectively.1
Estimated world production capacity was 9.4 million tonnes in
1993.1 Total US production capacity was estimated at approximately 3
million tonnes in 1993 (SRI, 1993); this figure had been more or less
stable since 1989. United Kingdom production was estimated at 50 000 t
in 1993 based on a production capacity of 85 000 t/year.1 Production
volume in Germany was a maximum of 240 000 t in 1989; breakdown of
production capacity by region and country worldwide can be found in
BUA (1991). Production volume in Japan increased from 560 000 t in
1992 to 751 000 t in 1996 (Chemical Daily Company, 1997).
On a worldwide basis, approximately two-thirds of ethylene glycol
is used as a chemical intermediate in the manufacture of polyesters
for fibres, films, bottles, etc., with a further one-quarter used as
an antifreeze in engine coolants. In Western Europe, the pattern is
slightly different, with about half used in polyester manufacture and
a quarter in coolants. Ethylene glycol is also used for runway deicing
(the main source of high local concentrations in the environment), as
plasticizer for adhesives, as softener for cellulose film, as
glycoborates in electrolytic condensers, as glycol dinitrate in
explosives, for various heat transfer applications, as humectant in
inks, as antifreeze and plasticizer in paints, and to reduce gelling
of medium oil alkyds based on pentaerythritol.2 There are many
different formulations of ethylene glycol and propylene glycol for use
in runway deicing. In some locations, one or the other of the glycols
is used alone; more usually, however, they are used together. Other
components of the formulation differ widely between manufacturers, as
indicated by differing toxicity (see later sections). Details of
formulations are not available.
1 ICI Chemicals and Polymers Ltd. (1993) Personal
communication cited in Nielsen et al. (1993).
2 Chou T, Hansch C (1986) Pomona College,
Claremont, CA, unpublished (cited in BUA, 1991).
Release to the atmosphere from production and processing of
ethylene glycol and production of ethylene oxide was estimated at
<875 t in Germany in 1989; release to the hydrosphere was estimated
at <28 t from production and >2000 t from dispersed use as an
antifreeze (BUA, 1991). A maximum figure of 12 500 t of ethylene
glycol release from use as antifreeze in the United Kingdom, based on
production figures and proportion of use, was derived in Nielsen et
al. (1993); estimated release of total volatile organics to the
atmosphere from glycol production was 41-260 t/year. Industry
estimates of release to the environment from use in runway deicing in
the United Kingdom were 600-720 t in 19931; use of the compound in
runway deicers is declining. Details of releases reported through the
US Toxic Release Inventory by individual state can be found in ATSDR
(1997). Summary figures for the USA annually between 1990 and 1993
were as follows: 4600 t to air, 523 t to water, 577 t to soil, and
2675 t injected underground from production. Estimated figures of 6778
t released via publicly owned treatment works and 60 252 t released to
the environment away from production and industrial usage sites were
reported for the same period (ATSDR, 1997).
1 ICI Chemicals and Polymers Ltd. (1993) Personal
communication cited in Nielsen et al. (1993).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Ethylene glycol has a low vapour pressure (7.9 Pa at 20°C); it is
expected to exist almost entirely in the vapour phase if released to
the atmosphere (Eisenreich et al., 1981). The Henry's law constant for
ethylene glycol is 1.41 × 10-3 or 6.08 × 10-3 Pa.m3/mol, depending
on method of calculation (BUA, 1991), indicating a low capacity for
volatilization from water bodies or soil surfaces.
14C-labelled ethylene glycol adsorbed onto silica gel and
irradiated with light (wavelength >290 nm) degraded by 12.1% over 17
h (Freitag et al., 1985). Photodegradation is not expected, as the
molecule should not absorb at these wavelengths; the mechanism of this
breakdown is, therefore, unknown. Estimated half-life in the
atmosphere for reaction with hydroxyl radicals is 2.1 days (BUA,
1991), 8-84 h (Howard et al., 1991), or 1 day (Nielsen et al., 1993).
No hydrolysis of ethylene glycol is expected in the environment
(Lyman et al., 1982).
Lokke (1984) studied the adsorption of ethylene glycol to three
different soils in leaching experiments. There was effectively no
sorption, and soil partition coefficients (log Koc) of 0-0.62 were
determined. Migration rates in five soil types were measured by
Schramm et al. (1986) at between 4 and 27 cm per 12 h.
The low octanol/water partition coefficient of ethylene glycol
(log Kow -1.93 to -1.36) indicates a low potential for
bioaccumulation. Bioconcentration factors of 190 for the green alga
(Chlorella fusca) (Freitag et al., 1985), up to 0.27 in specific
tissues of the crayfish ( Procambarus sp.) (Khoury et al., 1993), and
10 for the golden orfe (Leuciscus idus melanotus) (Freitag et al.,
1985) confirm low bioaccumulation.
In standard biodegradation tests under Organisation for Economic
Co-operation and Development (OECD), US Environmental Protection
Agency (US EPA), and Japanese Ministry of International Trade and
Industry (MITI) guidelines, ethylene glycol was readily
biodegradable.1
Means & Anderson (1981) measured the biodegradation of ethylene
glycol under aerobic conditions in five different tests using various
aqueous media. Degradation was monitored using oxygen uptake,
dissolved organic carbon removal, or carbon dioxide production.
1 Unpublished reports from Dow Chemicals, Union Carbide, and ICI
Chemicals and Polymers Ltd.; cited in IUCLID (European Union
database), 1st ed., 1996.
Ethylene glycol was readily degraded in all tests with a lag period of
up to 3 days. Degradation to 10% or less of the starting concentration
was reported in all tests after between 1 and 21 days. Boatman et al.
(1986) used acclimated sewage sludge as inoculum and a concentration
of ethylene glycol equivalent to 20 mg carbon/litre. Significant
degradation, as measured by carbon dioxide production, did not occur
until day 14 of the test (an estimated lag period of 8-10 days was
reported). By day 21, 71% of the ethylene glycol was degraded. Using
activated sludge from a petrochemicals process, 92% chemical oxygen
demand (COD) removal and 93% total organic carbon removal over 24 h
were reported for ethylene glycol at an initial concentration of 172
mg/litre by Matsui et al. (1975). However, direct measurement using
gas chromatography showed 44% of ethylene glycol still present after
24 h; the authors explain the discrepancy as being due to poor
detection of the glycol by the analytical method used. Pitter (1976)
reported 96.8% removal of ethylene glycol within 120 h using adapted
activated sewage sludge based on COD measurements and an initial COD
of 200 mg/litre. A biodegradation rate of 41.7 mg COD/g per hour was
reported. Zahn & Wellens (1980) reported >90% degradation after 4
days' incubation of ethylene glycol in a batch biodegradability study;
no lag period was observed. Bridie et al. (1979) reported 36% of
theoretical oxygen demand (ThOD) after 5 days' incubation at 20°C
measured as biological oxygen demand (BOD) and 100% measured as COD;
using previously adapted sludge, 63% degradation as BOD was reported
after 5 days. Conway et al. (1983) reported 39% of theoretical BOD
after 5 days, rising to 73% by day 10 and 96% at day 20, using
domestic sewage sludge inoculum. Freitag et al. (1985) reported only
5.7% degradation of ethylene glycol at 0.05 mg/litre over 5 days using
municipal sewage sludge inoculum. McGahey & Bouwer (1992) studied
degradation of ethylene glycol using primary sewage treatment effluent
as the inoculum. After an initial lag period of 3 days, a typical
first-order kinetic rate constant of 1.13 ± 0.34/day at 25°C was
reported; the half-life for the reaction was calculated at between
11.5 and 21.5 h.
Evans & David (1974) studied the biodegradation of ethylene
glycol in four samples of river water under controlled laboratory
conditions. The samples were dosed with ethylene glycol at 0, 2, or 10
mg/litre and incubated at either 20°C or 8°C. At 20°C, primary
biodegradation was complete within 3 days in all four samples; at 8°C,
it was complete by day 14. Degradation rates were further reduced at
4°C. Price et al. (1974) assessed the biodegradation of ethylene
glycol in both fresh and salt water over a 20-day incubation period.
Concentrations of up to 10 mg ethylene glycol/litre were used. In
fresh water, 34% degradation was observed after 5 days, rising to 86%
by day 10 and 100% by day 20. Degradation was less in salt water
-- 20% after 5 days and 77% after 20 days.
McGahey & Bouwer (1992) studied the degradation of ethylene
glycol using natural groundwater and soil inocula. An initial glycol
concentration of 111 mg/litre was degraded in groundwater with a rate
constant of 0.76/day at 25°C; the lag period was less than 3 days, and
the half-life was estimated at 22 h. First-order degradation rate
constants for sandy loam soil and sandy silt soil were 1.01 and
2.90/day, respectively. A lag period of 3 days and a half-life of 16.5
h were reported for the sandy loam, and a lag period of 0 days and a
half-life of 6 h were reported for the sandy silt. Increasing the
ethylene glycol concentration to 10 000 mg/litre in the sandy loam
resulted in a greatly diminished rate constant of 0.05/day and minimal
degradation of the glycol. Reducing temperature in the sandy silt
inoculum from 25°C to 10°C resulted in a decrease in the rate constant
from 2.09 to 1.19/day and an increase in the half-life from 6 to 14 h;
however, nearly complete degradation was observed at both temperatures
within the incubation period. Biodegradation rates of ethylene
glycol-based aircraft deicing fluids were examined in soil microcosms
at 8°C. Initial concentrations of 390-4900 ethylene glycol/kg soil
were degraded at around 20 mg/kg per day (Klecka et al., 1993).
Haines & Alexander (1975) identified a soil bacterium
(Pseudomonas aeruginosa) capable of degrading ethylene glycol. The
bacterium had been originally grown on propylene glycol and was
capable of degrading 1 mg carbon per inoculum within 2 days (based on
oxygen consumption). Watson & Jones (1977) isolated bacteria from
sewage effluent and identified Acinetobacter and Pseudomonas
strains that degraded ethylene glycol. Flavobacterium isolates did
not degrade the compound. However, under strongly aerobic conditions,
Flavobacterium sp. converted ethylene glycol to glycolate and
eventually carbon dioxide (Willetts, 1981).
Dwyer & Tiedje (1983) assessed the degradation of ethylene glycol
in methanogenic enrichments of bacteria obtained from municipal sewage
sludge. The bacterial inoculum was dominated by two morphological
types of bacteria, Methanobacterium sp. and Desulfovibrio sp. A
concentration of 36 mmol ethylene glycol/litre (2.2 g/litre) was
incubated at 37°C, and, based on analysis of the compound, 100% of the
glycol was metabolized within 12 days. Products of degradation
included ethanol, acetate, and methane. Battersby & Wilson (1989)
assessed the degradation of ethylene glycol under methanogenic
conditions using primary digesting sludge from a sewage treatment
plant receiving both domestic and industrial wastewater. Degradation
was assessed as total gas production. The glycol at a concentration of
50 mg carbon/litre sludge was incubated at 35°C for 60 days. Total
degradation was achieved after 1-2 weeks (>80% of theoretical gas
production), and a short lag period of <1 day was reported. In
anaerobic conditions using an inoculum from a pretreatment lagoon for
petrochemical waste, ethylene glycol at a concentration of 135
mg/litre was degraded to 78% after 10 days; at 755 mg/litre,
degradation was 75-79% complete (Hovious et al., 1973). Under
anaerobic conditions, ethylene glycol was degraded by 89% within 7
days (Kameya et al., 1995). The anaerobic bacterium Clostridium
glycolicum isolated from pond ooze and adapted to ethylene glycol
could degrade 5.3 or 6.7 g ethylene glycol/litre under anaerobic
conditions (Gaston & Stadtman, 1963). Non-adapted Acetobacter
strains could degrade ethylene glycol at concentrations between 5 and
15 g/litre using the compound as sole carbon source under anaerobic
conditions (Kaushal & Walker, 1951; Hrotmatka & Polesofsky, 1962).
Following a spill of ethylene glycol in New Jersey, USA, in which
15 000 litres of coolant containing ethylene glycol as antifreeze at
275 g/litre were spilled, concentrations of the glycol in soil and
groundwater were measured at 4.9 and 2.1 g/litre, respectively. A
remediation procedure was initiated involving the pumping of nitrogen,
phosphate, and oxygen-saturated water into the contaminated ground;
after 26 days, 85-93% of the glycol had been degraded by naturally
occurring microorganisms. After 9 months, the concentration of
ethylene glycol was below the detection limit of 50 mg/litre (Flathman
et al., 1989).
6. ENVIRONMENTAL LEVELS
The Japan Environment Agency (1991) reported the results of two
environmental surveys of surface waters and sediments carried out in
1977 and 1986. In the earlier survey, ethylene glycol was not detected
in six samples of water and sediment (detection limits 0.1-0.4
mg/litre and 1-2 mg/kg, respectively). In the later survey, the
compound was not detected in 24 sediment samples (detection limit 0.06
mg/kg) but was found in 2 out of 24 water samples at levels of 1.3 and
2 µg/litre (detection limit 0.8 µg/litre).
Monitoring of ethylene glycol in runoff from airports has been
reviewed by Sills & Blakeslee (1992); levels in runoff water ranged up
to several thousand mg/litre. Concentrations up to 19 000 mg/litre
were reported for Salt Lake City International Airport, Salt Lake
City, UT, USA, up to 3100 mg/litre for Lester B. Pearson International
Airport in Toronto, Ontario, Canada, and up to 5050 mg/litre at
Stapleton International Airport in Denver, CO, USA. Concentrations of
up to 70 mg/litre were measured in stream water receiving runoff from
Lester B. Pearson International Airport. Ethylene glycol was not
detected in soil at the edge of runways in Denver, but levels of the
compound in groundwater below the sandy soil of Ottawa International
Airport, Ottawa, Ontario, Canada, were measured at up to 415 mg/litre;
concentrations peaked in June and declined to non-detectable in the
autumn.
Pitt et al. (1975) sampled the primary effluent from a municipal
sewage treatment plant. No details are given in the report, but levels
of ethylene glycol were reported at 3 µg/litre. Zeithoun & McIllhenny
(1971) identified ethylene glycol in the wastewater from glycol
production; in 51 samples from two production plants, concentrations
in wastewater ranged from 680 to 2300 mg/litre (average 1003-1306
mg/litre). In a similar number of samples from two 1,2-propanediol
production plants, concentrations of ethylene glycol in wastewater
ranged from 355 to 2550 mg/litre (average 960-1140 mg/litre).
Grabinska-Loniewska (1974) identified ethylene glycol as a constituent
of wastewater from a polyester fibre plant in Poland. Concentrations
ranged from 200 to 440 mg/litre (average 200 mg/litre, number of
samples unspecified).
Influent-contaminated groundwater to a bioremediation plant in
California, USA, contained ethylene glycol at up to 103 mg/litre (Ross
et al., 1988).
Lee et al. (1983) detected ethylene glycol in two samples of
Asiatic clams ( Corbicula sp.); no levels were reported.
Ethylene glycol was detected in ambient air at time-weighted
averages of <0.05-0.33 mg/m3 as aerosol and <0.05-10.4 mg/m3 as
vapour following spray application of deicer containing 50% of the
compound to bridges (LDOTD, 1990).
Ethylene glycol has been identified as a metabolite of the growth
regulator ethylene in a number of higher plants (Blomstrom & Beyer,
1980) and as naturally occurring in the edible fungus Tricholoma
matsutake (Ahn & Lee, 1986).
7. EFFECTS ON ORGANISMS IN THE LABORATORY AND FIELD
7.1 Aquatic organisms
Results of acute toxicity tests on aquatic organisms are
summarized in Table 1. Chronic toxicity tests were conducted on water
fleas (Ceriodaphnia dubia) over the period taken by 60% of the
controls to produce three broods. NOECs for mortality at 24 000
mg/litre and for reproduction at 8590 mg/litre were reported; an IC25
of 12 310 mg/litre was calculated. Seven-day toxicity tests conducted
on the fathead minnow (Pimephales promelas) gave NOECs for mortality
and growth at 32 000 mg/litre and 15 380 mg/litre, respectively, with
an IC25 at 22 520 mg/litre (Pillard, 1995). Masters et al. (1991)
exposed Ceriodaphnia dubia to ethylene glycol in the US EPA standard
7-day chronic toxicity test and also concurrently carried out 4-day
tests to compare results. Survival and production of young were
monitored. A "chronic index," the geometric mean of the NOEC and
lowest-observed-effect concentration (LOEC), was determined to be 4.2
mg/litre for production of young in both tests and >6.0 and 4.2
mg/litre for survival in the 4- and 7-day tests, respectively. Actual
NOECs and LOECs were not reported.
Mayes et al. (1983) compared the toxicity of ethylene glycol to
fathead minnows at three different ages (fry, 10-15 days old;
juveniles, 30-35 days old; and subadults, 60-94 days old) and found no
effect of age. However, Mayer & Ellersieck (1986) found older (1.1 g)
rainbow trout (Oncorhynchus mykiss) more sensitive than younger
(0.7 g) fish.
Ethylene glycol did not produce narcosis in tadpoles of the
common frog (Rana temporaria) at 28 550 mg/litre; tadpoles did
become sluggish after 5-6 h of exposure, but did not lose their
responsiveness to stimuli. However, death followed within 12-20 h. At
14 275 mg/litre, tadpoles kept moving for 24-30 h but died after about
36-48 h (Lipnick, 1991).
A reported 48-h LC50 value for tadpoles of the clawed toad
(Xenopus laevis) at 326 mg/litre (DeZwart & Slooff, 1987) is
considered invalid for the setting of standards following
correspondence from the authors. The study was part of a technicians'
training course, and no quality control was exercised.
7.1.1 Toxicity of deicer formulations
Pillard (1995) conducted acute and chronic tests on water fleas
(C. dubia) and fathead minnows using both pure ethylene glycol and
formulations of deicer based on the compound. For acute tests, 48-h
LC50s for the daphnid were 34 440 and 13 140 mg/litre for the pure
substance and formulation, respectively; chronic NOECs for survival
were 24 000 and 8400 mg/litre, respectively, and for reproduction,
8590 and <3330 mg/litre, respectively. For acute tests on the
minnows, 96-h LC50s were 72 860 and 8050 mg/litre, respectively;
chronic NOECs for survival were 32 000 and 6090 mg/litre and for
growth were 15 380 and <3330 mg/litre, respectively. The higher
toxicity of formulations was ascribed to other unknown constituents of
the formulations, including rust inhibitors, buffers, polymers, and
surfactants. Hartwell et al. (1995) conducted toxicity tests using
ethylene glycol-based deicer and determined 96-h LC50s for fathead
minnow, Daphnia magna, D. pulex, and C. dubia at 10 802, 4213,
4675, and 9845 mg glycol/litre, respectively. Seven-day exposure of
fathead minnows produced an identical LC50. A maximum acceptable
toxicant concentration (MATC) for reproduction of Ceriodaphnia was
calculated at 418 mg/litre. Gill and kidney lesions and calcium
oxalate crystals were found in exposed fish. The same species were
also cultured in stream water taken from an outflow stream from
stormwater basins at Baltimore Washington International Airport,
Maryland, USA, receiving runoff from deicing of runways. No fish
mortality was seen in either March or April water samples over 7 days.
However, oxalate crystals were seen after 7 days' exposure to the
March water. Significant reduction in survival of D. magna and D.
pulex was recorded over 96 h in the March water sample. Ceriodaphnia
dubia showed reduced survival only after 7 days, and production of
neonates was also reduced to 55% of controls. No significant adverse
effects on daphnids was seen with the April water (neonate production
was significantly increased in Ceriodaphnia).
Toxicity of formulations will vary considerably depending on the
particular constituents. For example, Union Carbide's UCAR 50/50
EG-Based Type I fluid for the 1997-98 aircraft deicing season has
lower aquatic toxicity figures than those quoted in the literature:
D. magna, 48-h EC50 88 000 mg/litre; fathead minnow, 96-h LC50
44 000 mg/litre; rainbow trout, 96-h LC50 34 200 mg/litre.1 For an
assessment of likely effects in the field, toxicity values for
particular formulations used will need to be determined.
7.1.2 Field effects
During early summer, 3 months after release of glycols into
streams draining from airport stormwater basins (Baltimore Washington
International Airport), fish were sampled from the stream. In
tesselated darters (Etheostoma olmstedi), oxalate crystals appeared
in the interstitial tissue of the kidneys and basal layers of tubules.
American eels (Aguilla rostrata) exhibited kidney lesions consistent
with oxalate damage, but no crystals were found.2 Pillard (1995)
cites his own unpublished report as showing fish kills in streams near
airports and aquatic community impairment in three streams receiving
runoff from airports.
1 Personal communication to IPCS.
2 Unpublished reports cited by Hartwell et al. (1995).
7.2 Terrestrial organisms
Incubation of yeast (Saccharomyces cerevisiae) in ethylene
glycol at a concentration of 150 g/litre produced a 1% reduction in
glucose utilization; a concentration of 172.5 g/litre produced <10%
inhibition (Gray & Sova, 1956). Concentrations of 200 g ethylene
glycol/litre prevented germination of conidia of the ascomycete fungus
Neurospora crassa; return to clean medium allowed germination.
Concentrations greater than 200 g/litre killed the spores (Bates &
Wilson, 1974). Using oxygen uptake and growth (turbidity) as
end-points, Khoury et al. (1990) reported an IC50 for heterotrophic
soil microorganisms at 114 300 mg/litre.
Bose & Bandyopadhyay (1975) soaked tomato seeds in ethylene
glycol solution at 5.5 g/litre. Only 50% of the soaked seeds
germinated, but those that did grew higher, bloomed earlier, and
carried twice the crop of untreated plants. Soaking of cluster bean
(Cyamopsis tetragonoloba) in aqueous ethylene glycol solutions at 10
or 20 g/litre for 8 h, following an initial 4-h soak in water, led to
some plants showing small leaves with shortened petioles, stunted
growth, and sterility (Bose & Naskar, 1975). Twenty-three percent of
rice seeds soaked in aqueous ethylene glycol at 10 g/litre for 24 h
germinated (compared with 48% of controls). Germinated plants showed
only marginal effects on growth, panicle length, grain weight, and
fertility, but tiller numbers were reduced by 40-50% compared with
controls (Bose & Bhattacharyya, 1975). Jute (Corchorus capsularis)
seeds soaked in ethylene glycol solution at 2 g/litre showed 84% of
control levels of germination. Plants that germinated following
treatment required 8 days longer to blossom, on average, showed a
higher degree of pollen sterility, and produced fewer and lighter
seeds than controls (Bose & Datta, 1973). Tobacco (Nicotiana xanthi)
plants sprayed with 5 ml of a solution of ethylene glycol at 34.
51.5, or 69 g/litre showed a dose-dependent 10-33% reduction in
terminal bud fresh weight, but no other overt effects were noted
(Steffens & Barer, 1984).
Toxic effects (unspecified) were noted in chickens fed a diet
containing 5% ethylene glycol for 27 days (Yoshida et al., 1969). An
LC50 for ethylene glycol in drinking-water at 75 100 mg/litre over 24
h was reported for chickens (Riddell et al., 1967). No deaths were
seen in chickens exposed through drinking-water at 27 800 mg/litre,
but renal oxalosis was observed. Chickens exposed at 14 500 mg/litre
drinking-water showed calcium oxalate crystals in renal tubules, but
no clinical signs were reported. Beasley & Buck (1980) reported lethal
doses for poultry to lie within the range of 7790-8900 mg/kg body
weight. A NOEL of 1221 mg/kg body weight and a lowest-observed-effect
level (LOEL) of 2553 mg/kg body weight were reported for orally dosed
mallard ducks (Anas platyrhynchos) (Stowe et al., 1981).
Table 1: Acute toxicity of ethylene glycol to aquatic organisms.
Organism End-point Concentration (mg/litre) Reference
Microorganisms
bacterium Pseudomonas Toxic threshold >10 000 Bringmann & Kuhn (1980a,b)
putida; protozoa (cell multiplication)
Entosiphon sulcatum,
Uronema parduczi
cyanobacterium Microcystis Toxic threshold 2000 Bringmann & Kuhn (1976)
aeruginosa (cell multiplication)
bacterium Pseudomonas EC0 (growth) 1000 Daugherty (1980)
aeruginosa EC100 (growth) 2000
bacterium Photobacterium 30-min EC50 (luminescence) 621 Kaiser & Palabrica (1991)
phosphoreum 5-min EC50 112 220 Calleja et al. (1993)
5-min EC50 166 000 Kahru et al. (1996)
bacteria from aquatic sediment EC50 (growth) 114 300 Khoury et al. (1990)
and sewage sludge
bacteria from sewage sludge EC50 (oxygen uptake) 224 600 Kilroy & Gray (1992)
anaerobic bacteria from sewage Toxic threshold 5000 Hoechst (1975)
sludge
flagellate euglenoid EC5 (growth in population) >10 000 AQUIREa
Algae
green alga Scenedesmus quadricauda Toxic threshold >10 000 Bringmann & Kuhn (1980a)
green alga Selenastrum capricornutum 96-h EC50 (growth, cell counts) 6500-7500 Dowb
96-h EC50 (growth, cell volume) 9500-13 000
168-h EC50 (growth, cell volume) 24 000
Table 1 (cont'd)
Organism End-point Concentration (mg/litre) Reference
Invertebrates
water flea Daphnia magna 48-h LC50 (immobilization) >10 000 Conway et al. (1983)
50 000 Hermens et al. (1984)
41 000-51 000 Gersich et al. (1986)
74 400 Calleja et al. (1994)
14 828c Hartwell et al. (1995)
24-h LC50 >10 000 Bringmann & Kuhn (1977)
24-h NOEC 2500
water flea Ceriodaphnia dubia 48-h LC50 25 800 Cowgill et al. (1985)
(22 600-29 900)
34 440 Pillard (1995)
crayfish Procambarus sp. 96-h LC50 91 430 Khoury et al. (1990)
common shrimp Crangon vulgaris 96-h LC50 50 000 AQUIREa
brine shrimp Artemia salina 24-h LC50 >20 000 Price et al. (1974)
180 420 Calleja et al. (1994)
brown shrimp Crangon crangon 96-h LC50 approx. 50 000 Blackman (1974)
Fish
rainbow trout Oncorhynchus mykiss 96-h LC50 >18 500 Jank et al. (1974)
17 800-45 600 Mayer & Ellersieck
(1986)
guppy Poecilia reticulata 168-h LC50 49 300 Konnemann (1981)
bluegill sunfish Lepomis macrochirus 96-h LC50 >111 300 Mayer & Ellersieck
(1986)
27 540 Khoury et al. (1990)
Table 1 (cont'd)
Organism End-point Concentration (mg/litre) Reference
fathead minnow Pimephales promelas 96-h LC50 >10 000 Conway et al. (1983)
49 000-57 000 Mayes et al. (1983)
72 860 Pillard (1995)
goldfish Carassius auratus 24-h LC50 >5000 Bridie et al. (1979)
Japanese killifish Oryzias latipes 48-h NOEC 900 Tsuji et al. (1986)
Amphibians
frog (tadpoles) Rana brevipoda 48-h LC50 17 000 Nishiushi (1984)
a AQUIRE (Aquatic Information Retrieval) Computerized database developed by the US Environmental Protection Agency.
b Dow (undated) Personal communication to IPCS.
c Value based on ethylene glycol content of a deicing product.
8. EFFECTS EVALUATION
Ethylene glycol released to the atmosphere will be degraded by
reaction with hydroxyl radicals; the half-life for this reaction has
been estimated at between 0.3 and 3.5 days.
No hydrolysis of ethylene glycol is expected in surface waters.
The compound has little or no capacity to bind to particulates
and will be mobile in soil.
The low octanol/water partition coefficient and measured
bioconcentration factors in a few organisms indicate low capacity for
bioaccumulation.
Ethylene glycol is readily biodegradable in standard tests using
sewage sludge. Many studies show biodegradation under both aerobic and
anaerobic conditions. Some studies suggest a lag phase before
degradation, but many do not. Degradation occurs in both adapted and
unadapted sludges. Rapid degradation has been reported in surface
waters (less in salt water than in fresh water), groundwater, and soil
inocula. Several strains of microorganisms capable of utilizing
ethylene glycol as a carbon source have been identified.
Limited data are available on measured concentrations of ethylene
glycol in environmental compartments. Levels measured in surface
waters have been generally low, at a few micrograms per litre.
Concentrations in wastewater from production plants, prior to
treatment, have averaged up to 1300 mg/litre. By far the highest
reported concentrations relate to runoff water from airports, with
levels up to 19 000 mg/litre.
Ethylene glycol has generally low toxicity to aquatic organisms.
Toxic thresholds for microorganisms are above 1000 mg/litre. EC50s
for growth in microalgae are 6500 mg/litre or higher. Acute toxicity
tests with aquatic invertebrates where a value could be determined
show LC50s above 20 000 mg/litre, and those with fish show LC50s
above 17 800 mg/litre. The only valid acute toxicity value for
amphibians is 17 000 mg/litre for Rana brevipoda tadpoles. A NOEC
for chronic tests on daphnids of 8590 mg/litre (for reproductive
end-points) has been reported. A NOEC following short-term exposure of
fish has been reported at 15 380 mg/litre for growth.
Tests using deicer containing ethylene glycol generally showed
greater toxicity to aquatic organisms than the pure compound,
indicating other toxic components of the formulations.
Laboratory tests exposing aquatic organisms to stream water
receiving runoff from airports have demonstrated toxic effects and
death. Field studies in the vicinity of an airport have reported toxic
signs consistent with ethylene glycol poisoning (oxalate crystal
formation), fish kills, and reduced biodiversity. These effects cannot
definitively be ascribed to ethylene glycol.
Terrestrial organisms are much less likely to be exposed to
ethylene glycol and generally show low sensitivity to the compound.
Concentrations above 100 000 mg/litre were needed to produce toxic
effects on yeasts and fungi from soil. Very high concentrations and
soaking of seeds produced inhibition of germination in some
experiments; these are not considered of environmental significance. A
NOEL for orally dosed ducks at 1221 mg/kg body weight and reported
lethal doses for poultry at around 8000 mg/kg body weight indicate low
toxicity to birds.
8.1 Predicted environmental concentration
There are reported measurements of ethylene glycol in the influx
wastewater to treatment plants at industrial sites manufacturing the
compound. These will be used as the basis for calculating a predicted
environmental concentration (PEC) after treatment. Average
concentrations up to 1306 mg/litre have been reported.
Based on this emission concentration, and using mainly default
values from the OECD Technical Guidance Manual, the initial
concentration in river water would be as follows:
PEClocal (water) = Ceffluent/[(1 + Kp(susp) × C(susp)) × D]
where:
* PEClocal (water) is the predicted environmental concentration (g/litre)
* Ceffluent is the concentration of the chemical in the wastewater
treatment plant effluent (g/litre), calculated as Ceffluent = I
× (100 - P)/100,
where:
I = input concentration to the wastewater treatment plant
(1.3 g/litre)
P = percent removal in the wastewater treatment plant (91%,
based on the "ready biodegradability" of the compound)
* Kp(susp) is the suspended matter/water adsorption coefficient,
calculated as Kp(susp) = foc(susp) × Koc, where:
foc(susp) = the fraction of organic carbon in suspended matter
(default 0.1)
Koc = 0.411 × Kow
where:
Kow = the octanol/water partition
coefficient (log Kow = -1.36)
* C(susp) is the concentration of suspended matter in the river
water in kg/litre (default concentration 15 mg/litre)
* D is the dilution factor for river flow (a conservative default
value of 10)
Under these very conservative conditions, PEClocal (water) = 11.7
mg/litre. This is substantially higher than reported concentrations in
surface water and represents a conservative estimate of initial
maximum concentration.
8.2 Predicted no-effect concentration
There is a substantial database on the toxicity of ethylene
glycol to aquatic organisms, representing acute and chronic test
results for two trophic levels and acute and short-term results for a
third. The distribution of test results is presented in Figure 1 for
different types of organism. The shaded points represent toxic
thresholds for microorganisms or algae, and these are not considered a
suitable basis for estimating a predicted no-effect concentration
(PNEC). It would be justifiable to apply an uncertainty factor of 10
to the chronic NOEC for daphnid reproduction at 8590 mg/litre given
the wide range of available data. This gives a PNEC of 859 mg/litre.
8.3 Environmental risk factors
It is clear from Figure 1 that risk to aquatic organisms from
production of ethylene glycol is very low, even based on conservative
assumptions; a risk factor of 0.013 is generated by comparing PEClocal
(water) against PNEC. Based on the few measured values in surface waters,
risk would be negligible (risk factor at 2.3 × 10-6).
It is also clear that concentrations in airport runoff would be
expected to cause severe field effects without mitigation. It is
difficult to estimate likely dilution of runoff in generalized terms;
however, dilution factors of at least 100-fold would be needed for the
reported concentrations. Concentrations may be substantially higher in
runoff water at particular airport sites. There is indication that
formulations might be significantly more toxic to aquatic organisms
than the pure ethylene glycol. It is also unlikely that only ethylene
glycol formulations would be used. The ready biodegradability of
glycols also increases risk to organisms from oxygen depletion in
surface waters. Risk assessment and field monitoring of overt effects
should be applied on a case-by-case basis to determine pollution
control measures needed.
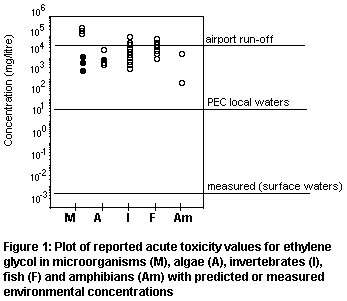 INTERNATIONAL CHEMICAL SAFETY CARD
ETHYLENE GLYCOL ICSC: 0270
March 1999
CAS # 107-21-1 1,2-Ethanediol
RTECS # KW2975000 1,2-Dihydroxyethane
EC # 803-027-00-1 HOCH2CH2OH
Molecular mass: 82.1
TYPES OF HAZARD ACUTE HAZARDS / PREVENTION FIRST AID / FIRE
/EXPOSURE SYMPTOMS FIGHTING
FIRE Combustible. NO open flames. Powder, alcohol-resistant
foam, water spray,
carbon dioxide.
EXPLOSION
EXPOSURE PREVENT GENERATION OF
MISTS!
Inhalation Cough. Dizziness. Ventilation. Fresh air, rest. Artificial
Headache. respiration if indicated. Refer
for medical attention.
Skin Dry skin. Protective gloves. Remove contaminated clothes.
Rinse skin with plenty of water
or shower.
Eyes Redness. Pain. Safety goggles. First rinse with plenty of water
for several minutes (remove contact
lenses if easily possible),
then take to a doctor.
Ingestion Abdominal pain. Dullness. Do not eat, drink, Rinse mouth. Induce vomiting (ONLY
Nausea. Unconsciousness. or smoke during work. IN CONSCIOUS PERSONS!). Refer for
Vomiting. medical attention. If no medical
personnel are available and the
patient is conscious, ingestion
of alcoholic beverage may prevent
kidney failure.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Collect leaking and spilled liquid EU Classification
in sealable containers as far as possible. Wash Symbol: Xn
away remainder with plenty of water. (Extra R: 22
personal protection: A/P2 filter respirator S: (2-)
for organic vapour and UN Classification
harmful dust).
EMERGENCY RESPONSE STORAGE
NFPA Code: H1; F1; R0; Separated from strong
oxidants, strong bases. Dry.
Ventilation along the floor.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
ODOURLESS, COLOURLESS, VISCOUS, The substance can be absorbed
HYDROSCOPIC LIQUID into the body by inhalation and
through the skin.
CHEMICAL DANGERS: INHALATION RISK:
On combustion, forms toxic gases. A harmful contamination of the air will
Reacts with strong oxidants and strong bases. be reached rather slowly on
evaporation of this substance at 20°C.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF SHORT-TERM EXPOSURE:
TLV (as STEL): ppm; 100 mg/m3 The substance Irritates the eyes and the
(ceiling values) (ACGIH 1998). respiratory tract. The substance
may cause effects on the the kidneys and
central nervous system, resulting
in renal failure and brain Injury.
Exposure could cause lowering of
consciousness.
EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
The substance may have effects on the central
nervous system, resulting
in abnormal eye movements (nystagmus).
PHYSICAL PROPERTIES
Boiling point: 198°C Auto-ignition temperature: 398°C
Melting point: -13°C Explosive limits, vol% in air. 3.2-15.3
Relative density (water = 1): 1.1 Octanol/water partition coefficient as log Pow: -1.93
Solubility in water: miscible
Vapour pressure, Pa at 20°C: 7
Relative vapour density (air = 1): 2.1
Relative density of the
vapour/air-mixture at 20°C (air = 1): 1.00
Flash point: 111°C (C.C.)
ENVIRONMENTAL DATA
NOTES
The occupational exposure limit value should not be exceeded during any part of the working exposure.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on behalf of the CEC or the IPCS is
responsible for the use which might be made of this information
(c) IPCS, CEC 1999
REFERENCES
Ahn JS, Lee KH (1986) Studies on the volatile aroma components of
edible mushroom (Tricholoma matsutake) of Korea. Journal of the
Korean Society for Food and Nutrition, 15:253-257.
ATSDR (1997) Toxicological profile for ethylene glycol and propylene
glycol. Atlanta, GA, US Department of Health and Human Services,
Public Health Service, Agency for Toxic Substances and Disease
Registry. 249 pp.
Bates WK, Wilson JF (1974) Ethylene glycol-induced alteration of
conidial germination in Neurospora crassa. Journal of bacteriology,
117:560-567.
Battersby NS, Wilson V (1989) Survey of the anaerobic biodegradation
potential of organic chemicals in digesting sludge. Applied
environmental microbiology, 55(2):433-439.
Beasley VR, Buck WB (1980) Acute ethylene glycol toxicosis: A review.
Veterinary and human toxicology, 22(4):255-263.
Blackman RAA (1974) Toxicity of oil-sinking agents. Marine pollution
bulletin, 5:116-118.
Blomstrom DC, Beyer EM (1980) Plants metabolise ethylene to ethylene
glycol. Nature, 283(5742):66-68.
Boatman RJ, Cunningham SL, Ziegler DA (1986) A method for measuring
the biodegradation of organic chemicals. Environmental toxicology and
chemistry, 5:233-243.
Bose S, Bandyopadhyay M (1975) Effect of dimethyl sulfoxide, ethylene
glycol and hydroxylamine on tomato (Lycopersicon esculentum Mill.).
Science and culture, 41:240-241.
Bose S, Bhattacharyya SK (1975) Studies on the effect of single and
combined treatments of x-rays, ethylene glycol and hydroxylamine in
rice (Oryza sativa L.). Plant science, 7:19-22.
Bose S, Datta GC (1973) Effect of treatments of colchicine,
dimethylsulphoxide, ethylene glycol, hydroxylamine and triethanolamine
in jute (Corchorus capsularis L.). Bangladesh journal of botany,
2:1-6.
Bose S, Naskar SK (1975) Effect of dimethyl sulfoxide, ethylene
glycol, hydroxylamine and triethanolamine in M1 generation in cluster
bean. Bulletin of the Botanical Society of Bengal, 29:49-52.
Bridie A, Wolff CJM, Winter M (1979) BOD and COD of some
petrochemicals. Water research, 13:627-630.
Bringmann G, Kuhn R (1976) Comparative findings on the damaging
effects of water pollutants in bacteria (Pseudomonas putida) and
blue-green algae (Microcystis aeruginosa). GasWasserfach: Wasser
Abwasser, 117(9):410-413 (in German).
Bringmann G, Kuhn R (1977) Results of the damaging effect of water
pollutants on Daphnia magna. Zeitschrift fuer Wasser und Abwasser
Forschung, 10:161-166 (in German).
Bringmann G, Kuhn R (1980a) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae and protozoa in the cell
multiplication inhibition test. Water research, 14:231-241.
Bringmann G, Kuhn R (1980b) Determination of the biological effects of
water pollutants in protozoa. II. Ciliated bacteria. Zeitschrift fuer
Wasser und Abwasser Forschung, 13(1):26-31 (in German).
BUA (1991) Ethylene glycol. GDCh-Advisory Committee on Existing
Chemicals of Environmental Relevance (BUA). Hirzel, Wissenschaftliche
Verlagsgesellschaft. 139 pp. (BUA Report 92.S).
Budavari S, ed. (1989) The Merck Index. An encyclopaedia of
chemicals, drugs and biologicals, 11th ed. Rahway, NJ, Merck
and Co. Inc.
Calleja MC, Persoone G, Geladi P (1993) The predictive potential of a
battery of ecotoxicological tests for human acute toxicity as
evaluated with the first 50 MEIC chemicals. Alternatives to
laboratory animals, 21:330-349.
Calleja MC, Persoone G, Geladi P (1994) Comparative acute toxicity of
the first 50 multicentre evaluation of in vivo cytotoxicity
chemicals to aquatic non-vertebrates. Archives of environmental
contamination and toxicology, 26:69-78.
Chemical Daily Company (1997) Annual of chemical industry. Tokyo,
The Chemical Daily Company Ltd.
Conway RA, Waggy GT, Spiegel MH, Berglund RL (1983) Environmental fate
and effects of ethylene oxide. Environmental science and technology,
17(2):107-112.
Cowgill UM, Takahashi IT, Applegath SL (1985) A comparison of the
effect of four benchmark chemicals on Daphnia magna and
Ceriodaphnia dubia-affinis tested at two different temperatures.
Environmental toxicology and chemistry, 4:415-422.
Daugherty LC (1980) The growth of Pseudomonas aeruginosa on glycols
of industrial importance. Lubrication engineering, 36(12):718-723.
DeZwart D, Slooff W (1987) Toxicity of mixtures of heavy metals and
petrochemicals to Xenopus laevis. Bulletin of environmental
contamination and toxicology, 38:345-351.
Dwyer DF, Tiedje JM (1983) Degradation of ethylene glycol and
polyethylene glycols by methanogenic consortia. Applied environmental
microbiology, 46(1):185-190.
Eisenreich SJ, Looney BB, Thornton JD (1981) Airborne organic
contaminants in the Great Lakes ecosystem. Environmental science and
technology, 15(1):30-38.
Evans WH, David EJ (1974) Biodegradation of mono-, di-, and
triethylene glycols in river waters under controlled laboratory
conditions. Water research, 8(2):97-100.
Flathman PE, Jerger DE, Bottomley LS (1989) Remediation of
contaminated ground water using biological techniques. Ground water
monitoring review, 9:105-119.
Freitag D, Ballhorn L, Geyer H, Korte F (1985) Environmental hazard
profile of organic chemicals: An experimental method for the
assessment of the behaviour of organic chemicals in the ecosphere by
means of simple laboratory tests with 14C labeled chemicals.
Chemosphere, 14(10):1589-1616.
Gaston LW, Stadtman ER (1963) Fermentation of ethylene glycol by
Clostridium glycolicum. Journal of bacteriology, 85:356-362.
Gersich FM, Blanchard FA, Applegath SL, Park CN (1986) The precision
of daphnid (Daphnia magna Straus, 1820) static acute toxicity tests.
Archives of environmental contamination and toxicology,
15(6):741-749.
Grabinska-Loniewska A (1974) Studies on the activated sludge bacteria
participating in the biodegradation of methanol, formaldehyde and
ethylene glycol: II. Utilization of various carbon and nitrogen
compounds. Acta Microbiologica Polonica, Series B: Microbiologia
Applicata, 6(2):83-88.
Gray WD, Sova C (1956) Relation of molecule size and structure to
alcohol inhibition of glucose utilization by yeast. Journal of
bacteriology, 72:349-356.
Haines JR, Alexander M (1975) Microbial degradation of polyethylene
glycols. Applied microbiology, 29:621-625.
Hansch C, Leo AJ (1979) Substituent constants for correlation analysis
in chemistry and biology. New York, NY, John Wiley & Sons.
Hartwell SI, Jordahl DM, Evans JE, May EB (1995) Toxicity of aircraft
de-icer and anti-icer solutions to aquatic organisms. Environmental
toxicology and chemistry, 14:1375-1386.
Hermens J, Canton H, Janssen P, De Jong R (1984) Quantitative
structure activity relationships and toxicity studies of mixtures of
chemicals with anaesthetic potency: Acute lethal and sublethal
toxicity to Daphnia magna. Aquatic toxicology, 5:143-154.
Hoechst (1975) Investigation of the biodegradation of ethylene
glycol. Frankfurt/Main, Germany, Hoechst AG, Abteilung Reinhaltung
von Wasser und Luft (in German).
Hovious JC, Conway RA, Ganze CW (1973) Anaerobic lagoon pretreatment
of petrochemical wastes. Journal of the Water Pollution Control
Federation, 45:71-84.
Howard PH, Boethling RS, Jarvis WF, Meylan WM, Michalenko EM (eds.)
(1991) Handbook of environmental degradation rates. Chelsea, MI,
Lewis Publishers, Inc., pp. 392-393.
Hrotmatka O, Polesofsky W (1962) Untersuchungen uber die Essiggarung.
VII. Uber die Oxydation verschiedener primarer Alkohole und Glykole.
Enzymologia, 24:372-384.
HSDB (1998) Hazardous substances data bank. Micromedex Inc. (CD-ROM
version).
IPCS (1993) International Chemical Safety Card -- Ethylene glycol.
Geneva, World Health Organization, International Programme on
Chemical Safety (ICSC 0270).
Jank BE, Guo HM, Cairns VW (1974) Activated sludge treatment of
airport wastewater containing de-icing fluids. Water research,
8:875-880.
Japan Environment Agency (1991) Chemicals in the environment. Report
on environmental survey and wildlife monitoring of chemicals in FY
1988 and 1989. Tokyo, Japan Environment Agency, Department of
Environmental Health, Office of Health Studies.
Kahru A, Tomson K, Pall T, Kulm I (1996) Study of toxicity of
pesticides using luminescent bacteria Photobacterium phosphoreum.
Water science and technology, 33(6):147-154.
Kaiser KLE, Palabrica VS (1991) Photobacterium phosphoreum toxicity
data index. Water pollution research journal of Canada, 26:361-431.
Kameya T, Murayama T, Urano K, Kitano M (1995) Biodegradation ranks of
priority organic compounds under anaerobic conditions. Science of the
total environment, 170:43-51.
Kaushal R, Walker TK (1951) Formation of cellulose by certain species
of Acetobacter. Biochemical journal, 48:618-621.
Kent JA, ed. (1974) Riegel's handbook of industrial chemistry, 7th
ed. New York, NY, Van Nostrand Reinhold Company.
Khoury GA, Abdelghani AA, Anderson AC, Monkiedje A (1990) Acute
toxicity of ethylene glycol to crayfish, bluegill sunfish and soil
micro-organisms. Trace substances in environmental health,
23:371-378.
Khoury GA, Adbelghani AA, Anderson AC (1993) Bioaccumulation and
depuration of ethylene glycol by crayfish (Procambarus spp.).
Environmental toxicology and water quality, 8:25-31.
Kilroy AC, Gray NF (1992) Toxicity of four organic solvents commonly
used in the pharmaceutical industry to activated sludge. Water
research, 26:887-892.
Klecka GM, Carpenter CL, Landenberger BD (1993) Biodegradation of
aircraft deicing fluids in soil at low temperatures. Ecotoxicology
and environmental safety, 25:280-295.
Konnemann H (1981) Quantitative structure-activity relationships in
fish toxicity studies. I. Relationship for 50 industrial pollutants.
Toxicology, 19:209-221.
LDOTD (1990) Fate of ethylene glycol in the environment. Baton
Rouge, LA, Louisiana Department of Transportation and Development,
Louisiana Transportation Research Center.
Lee NE, Haag WR, Jolley RL (1983) Cooling water pollutants:
bioaccumulation by Corbicula. In: Jolley RL, Brungs WA, Cotruvo
JA, Cumming RB, Mattice JS, Jacobs VA, eds. Water chlorination:
Chemistry, environmental impact and health effects. Vol. 4. Ann
Arbor, MI, Ann Arbor Science Publishers, pp. 851-870.
Lipnick RL, ed. (1991) Studies of narcosis. London, Chapman and
Hall, pp. 123-124.
Lokke H (1984) Leaching of ethylene glycol and ethanol in subsoils.
Water, air, and soil pollution, 22:373-387.
Lyman WJ, Reehl WF, Rosenblatt DH (1982) Handbook of chemical
property estimation methods. New York, NY, McGraw-Hill.
Masters JA, Lewis MA, Davidson DH, Bruce RD (1991) Validation of a
4-day Ceriodaphnia toxicity test and statistical considerations in
data analysis. Environmental toxicology and chemistry, 10:47-55.
Matsui S, Murakami T, Sasaki T, Hirose Y, Iguma Y (1975) Activated
sludge degradability of organic substances in the waste water of the
Kashima petroleum and petrochemical industrial complex in Japan.
Progress in water technology, 7(3-4):645-650.
Mayer FL, Ellersieck MR (1986) Manual of acute toxicity:
interpretation and database for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service (Resource Publication No. 160).
Mayes MA, Alexander HC, Dill DC (1983) A study to assess the influence
of age on the response of fathead minnows in static acute toxicity
tests. Bulletin of environmental contamination and toxicology,
31:139-147.
McGahey C, Bouwer EJ (1992) Biodegradation of ethylene glycol in
simulated subsurface environments. Water science and technology,
26:41-49.
Means JL, Anderson SJ (1981) Comparison of five different methods for
measuring biodegradability in aqueous environments. Water, air, and
soil pollution, 16:301-315.
Nielsen IR, Malcolm HM, Dobson S (1993) Environmental hazard
assessment: Ethylene glycol. Garston, United Kingdom Department of
the Environment, Building Research Establishment, Toxic Substances
Division. 33 pp. (TSD/16).
Nishiushi Y (1984) Toxicity of agrochemicals to freshwater organisms.
III. Solvents. Suisan Zoshoku, 32:115-119.
Pillard DA (1995) Comparative toxicity of formulated glycol de-icers
and pure ethylene and propylene glycol to Ceridaphnia dubia and
Pimephales promelas. Environmental toxicology and chemistry,
14:311-315.
Pitt WW, Jolley RL, Scott CD (1975) Determination of trace organics in
municipal sewage effluents and natural waters by high resolution ion
exchange chromatography. Environmental science and technology,
9:1068-1073.
Pitter P (1976) Determination of biological degradability of organic
substances. Water research, 10:231-235.
Price KS, Waggy GT, Conway RA (1974) Brine shrimp bioassay and
seawater BOD of petrochemicals. Journal of the Water Pollution
Control Federation, 46(1):63-77.
Riddell C, Nielsen SW, Kersting EJ (1967) Ethylene glycol poisoning in
poultry. Journal of the American Veterinary Medical Association,
150:1531-1535.
Ross D, Stroo HF, Bourquin AW, Sikes DJ (1988) Bioremediation of
hazardous waste sites in the USA: case histories. In: Proceedings of
the American Pollution Control Association Annual Meeting (Paper
88-6B.2, 81, 9s).
Schramm M, Warrick AW, Fuller WH (1986) Permeability of soils to four
organic liquids and water. Hazardous waste and hazardous materials,
3:21-27.
Sills RD, Blakeslee PA (1992) The environmental impact of deicers in
airport stormwater runoff. In: Chemical deicers and the environment.
Boca Raton, FL, Lewis Publishers, pp. 323-340.
SRI (1993) Directory of chemical producers -- United States of
America. Menlo Park, CA, Stanford Research Institute International
(598; 890).
Steffens GL, Barer SJ (1984) The inhibition of axillary and terminal
bud growth on tobacco by a series of C2 to C10 diol formulations.
Beitrage zur Tabakforschung International, 12:279-284.
Stowe CM, Barnes DM, Arendt TD (1981) Ethylene glycol intoxication in
ducks. Avian diseases, 25:538-541.
Tsuji S, Tonogai Y, Ito Y, Kanoh S (1986) The influence of rearing
temperature on the toxicity of various environmental pollutants for
killifish (Oryzias latipes). Eisei Kagaku, 32:46-53.
US EPA (1980) Organic chemical manufacturing. Vol. 9: Selected
processes. Prepared by R.J. Lovell et al., US Environmental
Protection Agency (Report No. EPA-450/3-80-028d).
Watson GK, Jones N (1977) The biodegradation of polyethylene glycols
by sewage bacteria. Water research, 11:95-100.
Willetts A (1981) Bacterial metabolism of ethylene glycol.
Biochimica Biophysica Acta, 677(2):194-199.
Yoshida M, Hoshii H, Morimoto H (1969) Nutritive values of glycols for
poultry feeds . Japanese poultry science, 6:73-81.
Zahn R, Wellens H (1980) Prufung der biologischen Abbaubarkeit im
Standversuch - weitere Erfahrungen und neue Einsatzmoglichkeiten.
Zeitschrift fuer Wasser und Abwasser Forschung, 13:1-7.
Zeithoun MA, McIllhenny WF (1971) Treatment of wastewater from the
production of polyhydric organics. Produced for the US Environmental
Protection Agency (PB-213841).
APPENDIX 1 -- SOURCE DOCUMENTS
Nielsen IR, Malcolm HM, Dobson S (1993) Environmental hazard
assessment: Ethylene glycol. Garston, United Kingdom Department of
the Environment, Building Research Establishment, Toxic Substances
Division (TSD/16)
The first draft of the Environmental Hazard Assessment (EHA)
documents are extensively circulated both within the United Kingdom
and internationally for peer review. Comments received are dealt with
in the final published version. For this EHA document on ethylene
glycol, comments were received from the United Kingdom Department of
the Environment (Wastes Technical Division and Global Atmosphere
Division), the Health and Safety Executive (United Kingdom), the
Ministry of Agriculture, Fisheries and Food (United Kingdom), the
Water Research Centre (United Kingdom), The Edinburgh Centre for
Toxicology, Heriot-Watt University, the US Environmental Protection
Agency, the Swedish National Chemicals Inspectorate, the
Umweltbundesamt, Germany, and ICI Chemicals and Polymers Ltd.
BUA (1991) Ethylene glycol. GDCh-Advisory Committee on Existing
Chemicals of Environmental Relevance (BUA). Hirzel, Wissenschaftliche
Verlagsgesellschaft (BUA Report 92.S)
For the BUA review process, the company that is in charge of
writing the report (usually the largest producer in Germany) prepares
a draft report using literature from an extensive literature search as
well as internal company studies. This draft is subject to a peer
review during several readings of a working group consisting of
representatives from government agencies, the scientific community,
and industry.
The English translation of this report was published in 1994.
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on ethylene glycol was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS national Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Chemical Manufacturers' Association, Arlington, USA
Chinese Academy of Preventive Medicine, Beijing, People's
Republic of China
European Chemical Industry Council (CEFIC), Brussels, Belgium
Health and Safety Executive, Bootle, United Kingdom
Health Department of Western Australia, Perth, Australia
National Institute of Health Sciences, Tokyo, Japan
National Institute of Public Health, Prague, Czech Republic
Senatskommission der Deutschen Forschungsgemeinschaft, Bonn,
Germany
United States Department of Health and Human Services (National
Institute of Environmental Health Sciences, Research Triangle
Park), USA
United States Environmental Protection Agency (Region VIII;
National Center for Environmental Assessment, Washington, DC),
USA
World Health Organization/International Programme on Chemical
Safety, Montreal, Canada
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Washington, DC, USA, 8-11 December 1998
Members
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
(Vice-Chairperson)
Mr R. Cary, Toxicology Unit, Health Directorate, Health and Safety
Executive, Bootle, Merseyside, United Kingdom (Rapporteur)
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr O. Faroon, Agency for Toxic Substances and Disease Registry,
Centers for Disease Control and Prevention, Atlanta, GA, USA
Dr G. Foureman, National Center for Environmental Assessment, US
Environmental Protection Agency, Research Triangle Park, NC, USA
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA (Chairperson)
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr E.V. Ohanian, Office of Water/Office of Science and Technology,
Health and Ecological Criteria Division, US Environmental Protection
Agency, Washington, DC, USA
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Ministry of Health, Beijing, People's Republic
of China
Observers
Dr K. Austin, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr I. Daly (ICCA representative), Regulatory and Technical Associates,
Lebanon, NJ, USA
Ms K.L. Lang (CEFIC, European Chemical Industry Council,
representative), Shell International, London, United Kingdom
Ms K. Roberts (ICCA representative), Chemical Self-funded Technical
Advocacy and Research (CHEMSTAR), Chemical Manufacturers Association,
Arlington, VA, USA
Dr W. Snellings (ICCA representative), Union Carbide Corporation,
Danbury, CN, USA
Dr M. Sweeney, Document Development Branch, National Institute for
Occupational Safety and Health, Cincinnati, OH, USA
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Dr M. Baril, Institut de Recherches en Santé et Sécurité du Travail du
Québec (IRSST), Montreal, Quebec, Canada
Dr H. Galal-Gorchev, Chevy Chase, MD, USA
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr R.G. Liteplo, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Ms L. Regis, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif aux problèmes d'ordre écologique posés par
l'éthylène-glycol a été préparé par l'Institut d'Ecologie terrestre
(Royaume-Uni) sur la base d'un rapport intitulé Environmental hazard
assessment: Ethylene glycol (Nielsen et al., 1993). Le rapport sur
l'éthylène-glycol rédigé par le Comité consultatif de la Société
allemande de Chimie pour les produits chimiques qui posent des
problèmes écologiques (BUA, 1991) a également été utilisé comme source
de données. Parallèlement, il a été procédé à un dépouillement de la
littérature récente (jusqu'en 1998).On trouvera à l'appendice 1 des
indications sur la méthode utilisée par les pairs pour examiner les
principales sources documentaires. Les renseignements concernant
l'examen du CICAD par les pairs font l'objet de l'appendice 2. Ce
CICAD a été approuvé en tant qu'évaluation internationale lors de la
réunion du Comité d'évaluation finale qui s'est tenue à Washington du
8 au 11 décembre 1998. La liste des participants à cette réunion
figure à l'appendice 3. La fiche d'information internationale sur la
sécurité chimique (ICSC No 0270) relative à l'éthylène-glycol, établie
par le Programme international sur la sécurité chimique (IPCS, 1993)
est également reproduite dans ce document.
L'éthylène-glycol (No CAS 107-21-1) se présente sous la forme
d'un liquide limpide, incolore et sirupeux, de saveur sucrée mais
dépourvu d'odeur. Il est peu volatil. Il est miscible à l'eau et à
certains autres solvants, légèrement soluble dans l'éther mais
pratiquement insoluble dans le benzène, les hydrocarbures chlorés,
l'éther de pétrole et les huiles. Son coefficient de partage entre
l'octanol et l'eau (log Kow) est compris entre -1,93 et -1,36.
On estime que la capacité de production mondiale était de 9,4
millions de tonnes en 1993. La libération d'éthylène-glycol dans
l'environnement se produit principalement au niveau de l'hydrosphère.
Localement, c'est par suite de l'utilisation du composé dans les
aéroports pour dégivrer les pistes et les avions que les décharges
dans l'environnement devraient être les plus importantes. Dans
l'ensemble du monde, environ les deux tiers de la production
d'éthylène-glycol sont utilisés comme intermédiaire dans la
préparation d'autres composés et à peu près un quart comme antigel
pour moteurs.
L'éthylène-glycol libéré dans l'atmosphère subit une
décomposition par suite de sa réaction sur les radicaux hydroxyle;
dans ces conditions, sa demi-vie se situe entre 0,3 et 3,5 jours.
Il ne devrait pas subir d'hydrolyse dans les eaux de surface.
Il a peu, voire pas de propension à se fixer aux particules et
présente une certaine mobilité pédologique.
La faible valeur de son coefficient de partage entre l'octanol et
l'eau et de son facteur de bioconcentration dans un certain nombre
d'organismes fait présager une tendance peu marquée à la
bioaccumulation.
Les tests habituels sur boues d'égouts révèlent une bonne
biodégradabilité. De nombreuses études montrent qu'il y a
biodégradation en aérobiose comme en anaérobiose. Selon certains
travaux, la biodégradation est retardée, mais selon d'autres elle ne
l'est pas. La décomposition se produit dans les boues adaptées comme
dans celles qui ne le sont pas. On a fait état d'une décomposition
rapide dans les eaux de surface (moindre dans l'eau salée que dans
l'eau douce), les eaux souterraines et les inoculums de sol. Certaines
souches de microorganismes sont capables d'utiliser l'éthylène-glycol
comme source de carbone.
On n'a guère de données sur les concentrations mesurées dans les
divers compartiments de l'environnement. Dans les eaux superficielles,
la concentration d'éthylène-glycol est généralement faible, de l'ordre
de quelques microgrammes par litre. Dans des effluents industriels
provenant d'unités de production, on a mesuré des concentrations avant
traitement allant jusqu'à 1 300 mg/litre en moyenne. Les
concentrations de loin les plus élevées sont celles que l'on trouve
dans les eaux de ruissellement des aéroports, avec des valeurs qui
peuvent atteindre 19 000 mg/litre.
L'éthylène-glycol est généralement peu toxique pour les
organismes aquatiques. Pour les microorganismes, le seuil de toxicité
est supérieur à 1 000 mg/litre. Dans le cas des algues microscopiques,
la CE50 relative à la croissance est supérieure ou égale à 6 500
mg/litre. Les tests de toxicité aiguë sur invertébrés aquatiques dont
on a pu tirer une valeur, montrent que la CL50 se situe au-delà de 20
000 mg/litre; ceux qui ont été pratiqués sur des poissons donnent des
valeurs supérieures à 17 800 mg/litre. Un test sur amphibien a montré
que la CL50 pour les têtards était égale à 17 000 mg/litre. Les
études de toxicité chronique portant sur la reproduction des daphnies
ont permis de fixer à 8 590 mg/litre la concentration sans effet
observable (NOEC). En prenant la croissance pour critère, on a obtenu
une NOEC de 15 380 mg/litre pour des poissons brièvement exposés.
Les tests effectués avec des dégivrants à base d'éthylène-glycol
montrent que ces produits sont plus toxiques pour les organismes
aquatiques que l'éthylène-glycol pur, ce qui indique que ces
dégivrants contiennent d'autres substances toxiques.
Lors de tests de laboratoire comportant l'exposition d'organismes
aquatiques à l'eau d'une rivière recevant les eaux de ruissellement
d'un aéroport, on a constaté des effets toxiques pouvant aller jusqu'à
la mort. Des études effectuées sur le terrain à proximité d'un
aéroport ont révélé que les organismes aquatiques présentaient des
signes d'intoxication qui pourraient être dus à l'éthylène-glycol,
avec en outre présence de poissons morts et réduction de la
biodiversité. Il n'est toutefois pas absolument certain que ces effets
puissent être attribués à l'éthylène-glycol.
Les organismes terrestres ont beaucoup moins de chances d'être
exposés à de l'éthylène-glycol et ils sont généralement peu sensibles
à ce composé. Il a fallu des concentrations supérieures à 100 000
mg/litre pour produire des effets toxiques sur des champignons et des
levures prélevés dans le sol. On a pu provoquer une inhibition de la
germination en plongeant des semences dans des bains contenant une
très forte concentration d'éthylène-glycol, mais ces résultats n'ont
aucune signification sur le plan écologique. Chez des canards ayant
reçu de l'éthylène-glycol par voie digestive, la dose sans effet
observable (NOEL) se situait à 1221 mg/kg de poids corporel; pour les
poulets, la dose létale serait d'environ 8 000 mg/kg p.c. Ces valeurs
montrent que le composé est peu toxique pour les volatiles.
RESUMEN DE ORIENTACION
El presente CICAD sobre los aspectos ambientales del
etilenglicol, preparado por el Instituto de Ecología Terrestre del
Reino Unido se basa en el informe de Evaluación de los peligros para
el medio ambiente: Etilenglicol (Nielsen et al., 1993). Se utilizó
también como documento original el informe sobre el etilenglicol que
había preparado el Comité Consultivo sobre Sustancias Químicas
Importantes para el Medio Ambiente de la Sociedad Alemana de Química
(BUA, 1991). Además de usar estos documentos, se realizó una búsqueda
de la bibliografía reciente hasta 1998. La información acerca del
carácter del proceso de examen colegiado para los principales
documentos originales figura en el apéndice 1. La información relativa
al examen colegiado de este CICAD se presenta en el apéndice 2. Su
aprobación como evaluación internacional se realizó en una reunión de
la Junta de Evaluación Final, celebrada en Washington, DC, Estados
Unidos, los días 8-11 de diciembre de 1998. La lista de participantes
en esta reunión figura en el apéndice 3. La Ficha internacional de
seguridad química (ICSC 0270), preparada por el Programa Internacional
de Seguridad de las Sustancias Químicas (IPCS, 1993), también se
reproduce en el presente documento.
El etilenglicol (CAS No 107-21-1) es un líquido denso, claro,
incoloro, de sabor dulce, pero inodoro. Tiene una volatilidad baja. Es
miscible con el agua y algunos otros disolventes, ligeramente soluble
en éter, pero prácticamente insoluble en benceno, hidrocarburos
clorados, éteres de petróleo y aceites. El log del coeficiente de
reparto octanol/agua oscila entre -1,93 y -1,36.
La capacidad de producción mundial estimada en 1993 fue de 9,4
millones de toneladas. La liberación en el medio ambiente se produce
fundamentalmente en la hidrosfera. La liberación local más importante
a las aguas superficiales es consecuencia de la utilización de
etilenglicol como descongelante en las pistas de los aeropuerto y en
los aviones. Unos dos tercios de la producción mundial de etilenglicol
se utilizan como intermediario químico, con otra cuarta parte como
anticongelante en los refrigerantes de los motores.
El etilenglicol que se libera en la atmósfera se degrada por
reacción con radicales hidroxilo; la semivida del compuesto en esta
reacción se ha calculado entre 0,3 y 3,5 días.
No se prevé que haya hidrólisis del etilenglicol en aguas
superficiales.
El compuesto tiene poca o ninguna capacidad de unión a partículas
y es móvil en el suelo.
El bajo coeficiente de reparto octanol/agua y los factores de
bioacumulación medidos en un pequeño número de organismos indican una
capacidad escasa de bioacumulación.
El etilenglicol es fácilmente biodegradable en pruebas
normalizadas utilizando lodos cloacales. En numerosos estudios se ha
puesto de manifiesto su biodegradación en condiciones tanto aerobias
como anaerobias. Algunos estudios parecen indicar una fase
estacionaria antes de la degradación, pero otros muchos no. Se produce
degradación tanto en lodos adaptados como no adaptados. Se ha
notificado una degradación rápida en las agua superficiales (inferior
en la salada que en la dulce), el agua freática y los inóculos del
suelo. Se han identificado varias cepas de microorganismos capaces
utilizar el etilenglicol como fuente de carbono.
Se dispone de datos limitados sobre las concentraciones de
etilenglicol medidas en los compartimentos del medio ambiente. Los
niveles medidos en las aguas superficiales generalmente han sido
bajos, de algunos microgramos por litro. Las concentraciones en las
aguas residuales de instalaciones de producción antes del tratamiento
han alcanzado un promedio de hasta 1 300 mg/litro. Las concentraciones
con diferencia más altas de las notificadas corresponden al agua de
escorrentía de los aeropuertos, con concentraciones de hasta 19 000
mg/litro.
El etilenglicol suele tener una toxicidad baja para los
organismos acuáticos. El umbral tóxico para los microorganismos es
superior a 1 000 mg/litro. Las CE50 para el crecimiento en las
microalgas son de 6 500 mg/litro o superiores. En las pruebas de
toxicidad aguda con invertebrados acuáticos en las que se pudo
determinar un valor se obtuvieron CL50 superiores a 20 000 mg/litro,
y con peces por encima de 17 800 mg/litro. En una prueba realizada con
anfibios se observó una CL50 para los renacuajos de 17 000 mg/litro.
Se ha notificado una concentración sin efectos observados (NOEC) para
pruebas crónicas en dáfnidos de 8 590 mg/litro (para los efectos
finales reproductivos). Tras una exposición breve de peces se notificó
una NOEC para el crecimiento de 15 380 mg/litro.
En pruebas en las que se utilizó descongelante que contenía
etilenglicol se puso de manifiesto una toxicidad para los organismos
acuáticos superior a la observada con el compuesto puro, lo que indica
la presencia de otros componentes tóxicos en las formulaciones.
En pruebas de laboratorio de exposición de organismos acuáticos a
una corriente de agua receptora de la escorrentía de los aeropuertos
aparecieron efectos tóxicos y letales. En estudios sobre el terreno
realizados en las cercanías de un aeropuerto se han notificado signos
tóxicos compatibles con la intoxicación por etilenglicol, muerte de
peces y reducción de la biodiversidad. Estos efectos no se pueden
atribuir de manera definitiva al etilenglicol.
Es mucho menos probable que los organismos terrestres estén
expuestos al etilenglicol y en general muestran una sensibilidad baja
al compuesto. Se necesitaron concentraciones superiores a 100 000
mg/litro para producir efectos tóxicos en levaduras y hongos del
suelo. En algunos experimentos se observó que las concentraciones muy
altas y la impregnación de las semillas inhibían la germinación; estos
efectos no se consideran importantes para el medio ambiente. La
concentración sin efectos observados (NOEL) para patos a los que se
administró por vía oral 1 221 mg/kg de peso corporal y las dosis
letales notificadas para aves de corral de alrededor de 8 000 mg/kg de
peso corporal indican una toxicidad baja para las aves.
INTERNATIONAL CHEMICAL SAFETY CARD
ETHYLENE GLYCOL ICSC: 0270
March 1999
CAS # 107-21-1 1,2-Ethanediol
RTECS # KW2975000 1,2-Dihydroxyethane
EC # 803-027-00-1 HOCH2CH2OH
Molecular mass: 82.1
TYPES OF HAZARD ACUTE HAZARDS / PREVENTION FIRST AID / FIRE
/EXPOSURE SYMPTOMS FIGHTING
FIRE Combustible. NO open flames. Powder, alcohol-resistant
foam, water spray,
carbon dioxide.
EXPLOSION
EXPOSURE PREVENT GENERATION OF
MISTS!
Inhalation Cough. Dizziness. Ventilation. Fresh air, rest. Artificial
Headache. respiration if indicated. Refer
for medical attention.
Skin Dry skin. Protective gloves. Remove contaminated clothes.
Rinse skin with plenty of water
or shower.
Eyes Redness. Pain. Safety goggles. First rinse with plenty of water
for several minutes (remove contact
lenses if easily possible),
then take to a doctor.
Ingestion Abdominal pain. Dullness. Do not eat, drink, Rinse mouth. Induce vomiting (ONLY
Nausea. Unconsciousness. or smoke during work. IN CONSCIOUS PERSONS!). Refer for
Vomiting. medical attention. If no medical
personnel are available and the
patient is conscious, ingestion
of alcoholic beverage may prevent
kidney failure.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Collect leaking and spilled liquid EU Classification
in sealable containers as far as possible. Wash Symbol: Xn
away remainder with plenty of water. (Extra R: 22
personal protection: A/P2 filter respirator S: (2-)
for organic vapour and UN Classification
harmful dust).
EMERGENCY RESPONSE STORAGE
NFPA Code: H1; F1; R0; Separated from strong
oxidants, strong bases. Dry.
Ventilation along the floor.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
ODOURLESS, COLOURLESS, VISCOUS, The substance can be absorbed
HYDROSCOPIC LIQUID into the body by inhalation and
through the skin.
CHEMICAL DANGERS: INHALATION RISK:
On combustion, forms toxic gases. A harmful contamination of the air will
Reacts with strong oxidants and strong bases. be reached rather slowly on
evaporation of this substance at 20°C.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF SHORT-TERM EXPOSURE:
TLV (as STEL): ppm; 100 mg/m3 The substance Irritates the eyes and the
(ceiling values) (ACGIH 1998). respiratory tract. The substance
may cause effects on the the kidneys and
central nervous system, resulting
in renal failure and brain Injury.
Exposure could cause lowering of
consciousness.
EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
The substance may have effects on the central
nervous system, resulting
in abnormal eye movements (nystagmus).
PHYSICAL PROPERTIES
Boiling point: 198°C Auto-ignition temperature: 398°C
Melting point: -13°C Explosive limits, vol% in air. 3.2-15.3
Relative density (water = 1): 1.1 Octanol/water partition coefficient as log Pow: -1.93
Solubility in water: miscible
Vapour pressure, Pa at 20°C: 7
Relative vapour density (air = 1): 2.1
Relative density of the
vapour/air-mixture at 20°C (air = 1): 1.00
Flash point: 111°C (C.C.)
ENVIRONMENTAL DATA
NOTES
The occupational exposure limit value should not be exceeded during any part of the working exposure.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on behalf of the CEC or the IPCS is
responsible for the use which might be made of this information
(c) IPCS, CEC 1999
REFERENCES
Ahn JS, Lee KH (1986) Studies on the volatile aroma components of
edible mushroom (Tricholoma matsutake) of Korea. Journal of the
Korean Society for Food and Nutrition, 15:253-257.
ATSDR (1997) Toxicological profile for ethylene glycol and propylene
glycol. Atlanta, GA, US Department of Health and Human Services,
Public Health Service, Agency for Toxic Substances and Disease
Registry. 249 pp.
Bates WK, Wilson JF (1974) Ethylene glycol-induced alteration of
conidial germination in Neurospora crassa. Journal of bacteriology,
117:560-567.
Battersby NS, Wilson V (1989) Survey of the anaerobic biodegradation
potential of organic chemicals in digesting sludge. Applied
environmental microbiology, 55(2):433-439.
Beasley VR, Buck WB (1980) Acute ethylene glycol toxicosis: A review.
Veterinary and human toxicology, 22(4):255-263.
Blackman RAA (1974) Toxicity of oil-sinking agents. Marine pollution
bulletin, 5:116-118.
Blomstrom DC, Beyer EM (1980) Plants metabolise ethylene to ethylene
glycol. Nature, 283(5742):66-68.
Boatman RJ, Cunningham SL, Ziegler DA (1986) A method for measuring
the biodegradation of organic chemicals. Environmental toxicology and
chemistry, 5:233-243.
Bose S, Bandyopadhyay M (1975) Effect of dimethyl sulfoxide, ethylene
glycol and hydroxylamine on tomato (Lycopersicon esculentum Mill.).
Science and culture, 41:240-241.
Bose S, Bhattacharyya SK (1975) Studies on the effect of single and
combined treatments of x-rays, ethylene glycol and hydroxylamine in
rice (Oryza sativa L.). Plant science, 7:19-22.
Bose S, Datta GC (1973) Effect of treatments of colchicine,
dimethylsulphoxide, ethylene glycol, hydroxylamine and triethanolamine
in jute (Corchorus capsularis L.). Bangladesh journal of botany,
2:1-6.
Bose S, Naskar SK (1975) Effect of dimethyl sulfoxide, ethylene
glycol, hydroxylamine and triethanolamine in M1 generation in cluster
bean. Bulletin of the Botanical Society of Bengal, 29:49-52.
Bridie A, Wolff CJM, Winter M (1979) BOD and COD of some
petrochemicals. Water research, 13:627-630.
Bringmann G, Kuhn R (1976) Comparative findings on the damaging
effects of water pollutants in bacteria (Pseudomonas putida) and
blue-green algae (Microcystis aeruginosa). GasWasserfach: Wasser
Abwasser, 117(9):410-413 (in German).
Bringmann G, Kuhn R (1977) Results of the damaging effect of water
pollutants on Daphnia magna. Zeitschrift fuer Wasser und Abwasser
Forschung, 10:161-166 (in German).
Bringmann G, Kuhn R (1980a) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae and protozoa in the cell
multiplication inhibition test. Water research, 14:231-241.
Bringmann G, Kuhn R (1980b) Determination of the biological effects of
water pollutants in protozoa. II. Ciliated bacteria. Zeitschrift fuer
Wasser und Abwasser Forschung, 13(1):26-31 (in German).
BUA (1991) Ethylene glycol. GDCh-Advisory Committee on Existing
Chemicals of Environmental Relevance (BUA). Hirzel, Wissenschaftliche
Verlagsgesellschaft. 139 pp. (BUA Report 92.S).
Budavari S, ed. (1989) The Merck Index. An encyclopaedia of
chemicals, drugs and biologicals, 11th ed. Rahway, NJ, Merck
and Co. Inc.
Calleja MC, Persoone G, Geladi P (1993) The predictive potential of a
battery of ecotoxicological tests for human acute toxicity as
evaluated with the first 50 MEIC chemicals. Alternatives to
laboratory animals, 21:330-349.
Calleja MC, Persoone G, Geladi P (1994) Comparative acute toxicity of
the first 50 multicentre evaluation of in vivo cytotoxicity
chemicals to aquatic non-vertebrates. Archives of environmental
contamination and toxicology, 26:69-78.
Chemical Daily Company (1997) Annual of chemical industry. Tokyo,
The Chemical Daily Company Ltd.
Conway RA, Waggy GT, Spiegel MH, Berglund RL (1983) Environmental fate
and effects of ethylene oxide. Environmental science and technology,
17(2):107-112.
Cowgill UM, Takahashi IT, Applegath SL (1985) A comparison of the
effect of four benchmark chemicals on Daphnia magna and
Ceriodaphnia dubia-affinis tested at two different temperatures.
Environmental toxicology and chemistry, 4:415-422.
Daugherty LC (1980) The growth of Pseudomonas aeruginosa on glycols
of industrial importance. Lubrication engineering, 36(12):718-723.
DeZwart D, Slooff W (1987) Toxicity of mixtures of heavy metals and
petrochemicals to Xenopus laevis. Bulletin of environmental
contamination and toxicology, 38:345-351.
Dwyer DF, Tiedje JM (1983) Degradation of ethylene glycol and
polyethylene glycols by methanogenic consortia. Applied environmental
microbiology, 46(1):185-190.
Eisenreich SJ, Looney BB, Thornton JD (1981) Airborne organic
contaminants in the Great Lakes ecosystem. Environmental science and
technology, 15(1):30-38.
Evans WH, David EJ (1974) Biodegradation of mono-, di-, and
triethylene glycols in river waters under controlled laboratory
conditions. Water research, 8(2):97-100.
Flathman PE, Jerger DE, Bottomley LS (1989) Remediation of
contaminated ground water using biological techniques. Ground water
monitoring review, 9:105-119.
Freitag D, Ballhorn L, Geyer H, Korte F (1985) Environmental hazard
profile of organic chemicals: An experimental method for the
assessment of the behaviour of organic chemicals in the ecosphere by
means of simple laboratory tests with 14C labeled chemicals.
Chemosphere, 14(10):1589-1616.
Gaston LW, Stadtman ER (1963) Fermentation of ethylene glycol by
Clostridium glycolicum. Journal of bacteriology, 85:356-362.
Gersich FM, Blanchard FA, Applegath SL, Park CN (1986) The precision
of daphnid (Daphnia magna Straus, 1820) static acute toxicity tests.
Archives of environmental contamination and toxicology,
15(6):741-749.
Grabinska-Loniewska A (1974) Studies on the activated sludge bacteria
participating in the biodegradation of methanol, formaldehyde and
ethylene glycol: II. Utilization of various carbon and nitrogen
compounds. Acta Microbiologica Polonica, Series B: Microbiologia
Applicata, 6(2):83-88.
Gray WD, Sova C (1956) Relation of molecule size and structure to
alcohol inhibition of glucose utilization by yeast. Journal of
bacteriology, 72:349-356.
Haines JR, Alexander M (1975) Microbial degradation of polyethylene
glycols. Applied microbiology, 29:621-625.
Hansch C, Leo AJ (1979) Substituent constants for correlation analysis
in chemistry and biology. New York, NY, John Wiley & Sons.
Hartwell SI, Jordahl DM, Evans JE, May EB (1995) Toxicity of aircraft
de-icer and anti-icer solutions to aquatic organisms. Environmental
toxicology and chemistry, 14:1375-1386.
Hermens J, Canton H, Janssen P, De Jong R (1984) Quantitative
structure activity relationships and toxicity studies of mixtures of
chemicals with anaesthetic potency: Acute lethal and sublethal
toxicity to Daphnia magna. Aquatic toxicology, 5:143-154.
Hoechst (1975) Investigation of the biodegradation of ethylene
glycol. Frankfurt/Main, Germany, Hoechst AG, Abteilung Reinhaltung
von Wasser und Luft (in German).
Hovious JC, Conway RA, Ganze CW (1973) Anaerobic lagoon pretreatment
of petrochemical wastes. Journal of the Water Pollution Control
Federation, 45:71-84.
Howard PH, Boethling RS, Jarvis WF, Meylan WM, Michalenko EM (eds.)
(1991) Handbook of environmental degradation rates. Chelsea, MI,
Lewis Publishers, Inc., pp. 392-393.
Hrotmatka O, Polesofsky W (1962) Untersuchungen uber die Essiggarung.
VII. Uber die Oxydation verschiedener primarer Alkohole und Glykole.
Enzymologia, 24:372-384.
HSDB (1998) Hazardous substances data bank. Micromedex Inc. (CD-ROM
version).
IPCS (1993) International Chemical Safety Card -- Ethylene glycol.
Geneva, World Health Organization, International Programme on
Chemical Safety (ICSC 0270).
Jank BE, Guo HM, Cairns VW (1974) Activated sludge treatment of
airport wastewater containing de-icing fluids. Water research,
8:875-880.
Japan Environment Agency (1991) Chemicals in the environment. Report
on environmental survey and wildlife monitoring of chemicals in FY
1988 and 1989. Tokyo, Japan Environment Agency, Department of
Environmental Health, Office of Health Studies.
Kahru A, Tomson K, Pall T, Kulm I (1996) Study of toxicity of
pesticides using luminescent bacteria Photobacterium phosphoreum.
Water science and technology, 33(6):147-154.
Kaiser KLE, Palabrica VS (1991) Photobacterium phosphoreum toxicity
data index. Water pollution research journal of Canada, 26:361-431.
Kameya T, Murayama T, Urano K, Kitano M (1995) Biodegradation ranks of
priority organic compounds under anaerobic conditions. Science of the
total environment, 170:43-51.
Kaushal R, Walker TK (1951) Formation of cellulose by certain species
of Acetobacter. Biochemical journal, 48:618-621.
Kent JA, ed. (1974) Riegel's handbook of industrial chemistry, 7th
ed. New York, NY, Van Nostrand Reinhold Company.
Khoury GA, Abdelghani AA, Anderson AC, Monkiedje A (1990) Acute
toxicity of ethylene glycol to crayfish, bluegill sunfish and soil
micro-organisms. Trace substances in environmental health,
23:371-378.
Khoury GA, Adbelghani AA, Anderson AC (1993) Bioaccumulation and
depuration of ethylene glycol by crayfish (Procambarus spp.).
Environmental toxicology and water quality, 8:25-31.
Kilroy AC, Gray NF (1992) Toxicity of four organic solvents commonly
used in the pharmaceutical industry to activated sludge. Water
research, 26:887-892.
Klecka GM, Carpenter CL, Landenberger BD (1993) Biodegradation of
aircraft deicing fluids in soil at low temperatures. Ecotoxicology
and environmental safety, 25:280-295.
Konnemann H (1981) Quantitative structure-activity relationships in
fish toxicity studies. I. Relationship for 50 industrial pollutants.
Toxicology, 19:209-221.
LDOTD (1990) Fate of ethylene glycol in the environment. Baton
Rouge, LA, Louisiana Department of Transportation and Development,
Louisiana Transportation Research Center.
Lee NE, Haag WR, Jolley RL (1983) Cooling water pollutants:
bioaccumulation by Corbicula. In: Jolley RL, Brungs WA, Cotruvo
JA, Cumming RB, Mattice JS, Jacobs VA, eds. Water chlorination:
Chemistry, environmental impact and health effects. Vol. 4. Ann
Arbor, MI, Ann Arbor Science Publishers, pp. 851-870.
Lipnick RL, ed. (1991) Studies of narcosis. London, Chapman and
Hall, pp. 123-124.
Lokke H (1984) Leaching of ethylene glycol and ethanol in subsoils.
Water, air, and soil pollution, 22:373-387.
Lyman WJ, Reehl WF, Rosenblatt DH (1982) Handbook of chemical
property estimation methods. New York, NY, McGraw-Hill.
Masters JA, Lewis MA, Davidson DH, Bruce RD (1991) Validation of a
4-day Ceriodaphnia toxicity test and statistical considerations in
data analysis. Environmental toxicology and chemistry, 10:47-55.
Matsui S, Murakami T, Sasaki T, Hirose Y, Iguma Y (1975) Activated
sludge degradability of organic substances in the waste water of the
Kashima petroleum and petrochemical industrial complex in Japan.
Progress in water technology, 7(3-4):645-650.
Mayer FL, Ellersieck MR (1986) Manual of acute toxicity:
interpretation and database for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service (Resource Publication No. 160).
Mayes MA, Alexander HC, Dill DC (1983) A study to assess the influence
of age on the response of fathead minnows in static acute toxicity
tests. Bulletin of environmental contamination and toxicology,
31:139-147.
McGahey C, Bouwer EJ (1992) Biodegradation of ethylene glycol in
simulated subsurface environments. Water science and technology,
26:41-49.
Means JL, Anderson SJ (1981) Comparison of five different methods for
measuring biodegradability in aqueous environments. Water, air, and
soil pollution, 16:301-315.
Nielsen IR, Malcolm HM, Dobson S (1993) Environmental hazard
assessment: Ethylene glycol. Garston, United Kingdom Department of
the Environment, Building Research Establishment, Toxic Substances
Division. 33 pp. (TSD/16).
Nishiushi Y (1984) Toxicity of agrochemicals to freshwater organisms.
III. Solvents. Suisan Zoshoku, 32:115-119.
Pillard DA (1995) Comparative toxicity of formulated glycol de-icers
and pure ethylene and propylene glycol to Ceridaphnia dubia and
Pimephales promelas. Environmental toxicology and chemistry,
14:311-315.
Pitt WW, Jolley RL, Scott CD (1975) Determination of trace organics in
municipal sewage effluents and natural waters by high resolution ion
exchange chromatography. Environmental science and technology,
9:1068-1073.
Pitter P (1976) Determination of biological degradability of organic
substances. Water research, 10:231-235.
Price KS, Waggy GT, Conway RA (1974) Brine shrimp bioassay and
seawater BOD of petrochemicals. Journal of the Water Pollution
Control Federation, 46(1):63-77.
Riddell C, Nielsen SW, Kersting EJ (1967) Ethylene glycol poisoning in
poultry. Journal of the American Veterinary Medical Association,
150:1531-1535.
Ross D, Stroo HF, Bourquin AW, Sikes DJ (1988) Bioremediation of
hazardous waste sites in the USA: case histories. In: Proceedings of
the American Pollution Control Association Annual Meeting (Paper
88-6B.2, 81, 9s).
Schramm M, Warrick AW, Fuller WH (1986) Permeability of soils to four
organic liquids and water. Hazardous waste and hazardous materials,
3:21-27.
Sills RD, Blakeslee PA (1992) The environmental impact of deicers in
airport stormwater runoff. In: Chemical deicers and the environment.
Boca Raton, FL, Lewis Publishers, pp. 323-340.
SRI (1993) Directory of chemical producers -- United States of
America. Menlo Park, CA, Stanford Research Institute International
(598; 890).
Steffens GL, Barer SJ (1984) The inhibition of axillary and terminal
bud growth on tobacco by a series of C2 to C10 diol formulations.
Beitrage zur Tabakforschung International, 12:279-284.
Stowe CM, Barnes DM, Arendt TD (1981) Ethylene glycol intoxication in
ducks. Avian diseases, 25:538-541.
Tsuji S, Tonogai Y, Ito Y, Kanoh S (1986) The influence of rearing
temperature on the toxicity of various environmental pollutants for
killifish (Oryzias latipes). Eisei Kagaku, 32:46-53.
US EPA (1980) Organic chemical manufacturing. Vol. 9: Selected
processes. Prepared by R.J. Lovell et al., US Environmental
Protection Agency (Report No. EPA-450/3-80-028d).
Watson GK, Jones N (1977) The biodegradation of polyethylene glycols
by sewage bacteria. Water research, 11:95-100.
Willetts A (1981) Bacterial metabolism of ethylene glycol.
Biochimica Biophysica Acta, 677(2):194-199.
Yoshida M, Hoshii H, Morimoto H (1969) Nutritive values of glycols for
poultry feeds . Japanese poultry science, 6:73-81.
Zahn R, Wellens H (1980) Prufung der biologischen Abbaubarkeit im
Standversuch - weitere Erfahrungen und neue Einsatzmoglichkeiten.
Zeitschrift fuer Wasser und Abwasser Forschung, 13:1-7.
Zeithoun MA, McIllhenny WF (1971) Treatment of wastewater from the
production of polyhydric organics. Produced for the US Environmental
Protection Agency (PB-213841).
APPENDIX 1 -- SOURCE DOCUMENTS
Nielsen IR, Malcolm HM, Dobson S (1993) Environmental hazard
assessment: Ethylene glycol. Garston, United Kingdom Department of
the Environment, Building Research Establishment, Toxic Substances
Division (TSD/16)
The first draft of the Environmental Hazard Assessment (EHA)
documents are extensively circulated both within the United Kingdom
and internationally for peer review. Comments received are dealt with
in the final published version. For this EHA document on ethylene
glycol, comments were received from the United Kingdom Department of
the Environment (Wastes Technical Division and Global Atmosphere
Division), the Health and Safety Executive (United Kingdom), the
Ministry of Agriculture, Fisheries and Food (United Kingdom), the
Water Research Centre (United Kingdom), The Edinburgh Centre for
Toxicology, Heriot-Watt University, the US Environmental Protection
Agency, the Swedish National Chemicals Inspectorate, the
Umweltbundesamt, Germany, and ICI Chemicals and Polymers Ltd.
BUA (1991) Ethylene glycol. GDCh-Advisory Committee on Existing
Chemicals of Environmental Relevance (BUA). Hirzel, Wissenschaftliche
Verlagsgesellschaft (BUA Report 92.S)
For the BUA review process, the company that is in charge of
writing the report (usually the largest producer in Germany) prepares
a draft report using literature from an extensive literature search as
well as internal company studies. This draft is subject to a peer
review during several readings of a working group consisting of
representatives from government agencies, the scientific community,
and industry.
The English translation of this report was published in 1994.
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on ethylene glycol was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS national Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Chemical Manufacturers' Association, Arlington, USA
Chinese Academy of Preventive Medicine, Beijing, People's
Republic of China
European Chemical Industry Council (CEFIC), Brussels, Belgium
Health and Safety Executive, Bootle, United Kingdom
Health Department of Western Australia, Perth, Australia
National Institute of Health Sciences, Tokyo, Japan
National Institute of Public Health, Prague, Czech Republic
Senatskommission der Deutschen Forschungsgemeinschaft, Bonn,
Germany
United States Department of Health and Human Services (National
Institute of Environmental Health Sciences, Research Triangle
Park), USA
United States Environmental Protection Agency (Region VIII;
National Center for Environmental Assessment, Washington, DC),
USA
World Health Organization/International Programme on Chemical
Safety, Montreal, Canada
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Washington, DC, USA, 8-11 December 1998
Members
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
(Vice-Chairperson)
Mr R. Cary, Toxicology Unit, Health Directorate, Health and Safety
Executive, Bootle, Merseyside, United Kingdom (Rapporteur)
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr O. Faroon, Agency for Toxic Substances and Disease Registry,
Centers for Disease Control and Prevention, Atlanta, GA, USA
Dr G. Foureman, National Center for Environmental Assessment, US
Environmental Protection Agency, Research Triangle Park, NC, USA
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA (Chairperson)
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr E.V. Ohanian, Office of Water/Office of Science and Technology,
Health and Ecological Criteria Division, US Environmental Protection
Agency, Washington, DC, USA
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Ministry of Health, Beijing, People's Republic
of China
Observers
Dr K. Austin, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr I. Daly (ICCA representative), Regulatory and Technical Associates,
Lebanon, NJ, USA
Ms K.L. Lang (CEFIC, European Chemical Industry Council,
representative), Shell International, London, United Kingdom
Ms K. Roberts (ICCA representative), Chemical Self-funded Technical
Advocacy and Research (CHEMSTAR), Chemical Manufacturers Association,
Arlington, VA, USA
Dr W. Snellings (ICCA representative), Union Carbide Corporation,
Danbury, CN, USA
Dr M. Sweeney, Document Development Branch, National Institute for
Occupational Safety and Health, Cincinnati, OH, USA
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Dr M. Baril, Institut de Recherches en Santé et Sécurité du Travail du
Québec (IRSST), Montreal, Quebec, Canada
Dr H. Galal-Gorchev, Chevy Chase, MD, USA
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr R.G. Liteplo, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Ms L. Regis, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif aux problèmes d'ordre écologique posés par
l'éthylène-glycol a été préparé par l'Institut d'Ecologie terrestre
(Royaume-Uni) sur la base d'un rapport intitulé Environmental hazard
assessment: Ethylene glycol (Nielsen et al., 1993). Le rapport sur
l'éthylène-glycol rédigé par le Comité consultatif de la Société
allemande de Chimie pour les produits chimiques qui posent des
problèmes écologiques (BUA, 1991) a également été utilisé comme source
de données. Parallèlement, il a été procédé à un dépouillement de la
littérature récente (jusqu'en 1998).On trouvera à l'appendice 1 des
indications sur la méthode utilisée par les pairs pour examiner les
principales sources documentaires. Les renseignements concernant
l'examen du CICAD par les pairs font l'objet de l'appendice 2. Ce
CICAD a été approuvé en tant qu'évaluation internationale lors de la
réunion du Comité d'évaluation finale qui s'est tenue à Washington du
8 au 11 décembre 1998. La liste des participants à cette réunion
figure à l'appendice 3. La fiche d'information internationale sur la
sécurité chimique (ICSC No 0270) relative à l'éthylène-glycol, établie
par le Programme international sur la sécurité chimique (IPCS, 1993)
est également reproduite dans ce document.
L'éthylène-glycol (No CAS 107-21-1) se présente sous la forme
d'un liquide limpide, incolore et sirupeux, de saveur sucrée mais
dépourvu d'odeur. Il est peu volatil. Il est miscible à l'eau et à
certains autres solvants, légèrement soluble dans l'éther mais
pratiquement insoluble dans le benzène, les hydrocarbures chlorés,
l'éther de pétrole et les huiles. Son coefficient de partage entre
l'octanol et l'eau (log Kow) est compris entre -1,93 et -1,36.
On estime que la capacité de production mondiale était de 9,4
millions de tonnes en 1993. La libération d'éthylène-glycol dans
l'environnement se produit principalement au niveau de l'hydrosphère.
Localement, c'est par suite de l'utilisation du composé dans les
aéroports pour dégivrer les pistes et les avions que les décharges
dans l'environnement devraient être les plus importantes. Dans
l'ensemble du monde, environ les deux tiers de la production
d'éthylène-glycol sont utilisés comme intermédiaire dans la
préparation d'autres composés et à peu près un quart comme antigel
pour moteurs.
L'éthylène-glycol libéré dans l'atmosphère subit une
décomposition par suite de sa réaction sur les radicaux hydroxyle;
dans ces conditions, sa demi-vie se situe entre 0,3 et 3,5 jours.
Il ne devrait pas subir d'hydrolyse dans les eaux de surface.
Il a peu, voire pas de propension à se fixer aux particules et
présente une certaine mobilité pédologique.
La faible valeur de son coefficient de partage entre l'octanol et
l'eau et de son facteur de bioconcentration dans un certain nombre
d'organismes fait présager une tendance peu marquée à la
bioaccumulation.
Les tests habituels sur boues d'égouts révèlent une bonne
biodégradabilité. De nombreuses études montrent qu'il y a
biodégradation en aérobiose comme en anaérobiose. Selon certains
travaux, la biodégradation est retardée, mais selon d'autres elle ne
l'est pas. La décomposition se produit dans les boues adaptées comme
dans celles qui ne le sont pas. On a fait état d'une décomposition
rapide dans les eaux de surface (moindre dans l'eau salée que dans
l'eau douce), les eaux souterraines et les inoculums de sol. Certaines
souches de microorganismes sont capables d'utiliser l'éthylène-glycol
comme source de carbone.
On n'a guère de données sur les concentrations mesurées dans les
divers compartiments de l'environnement. Dans les eaux superficielles,
la concentration d'éthylène-glycol est généralement faible, de l'ordre
de quelques microgrammes par litre. Dans des effluents industriels
provenant d'unités de production, on a mesuré des concentrations avant
traitement allant jusqu'à 1 300 mg/litre en moyenne. Les
concentrations de loin les plus élevées sont celles que l'on trouve
dans les eaux de ruissellement des aéroports, avec des valeurs qui
peuvent atteindre 19 000 mg/litre.
L'éthylène-glycol est généralement peu toxique pour les
organismes aquatiques. Pour les microorganismes, le seuil de toxicité
est supérieur à 1 000 mg/litre. Dans le cas des algues microscopiques,
la CE50 relative à la croissance est supérieure ou égale à 6 500
mg/litre. Les tests de toxicité aiguë sur invertébrés aquatiques dont
on a pu tirer une valeur, montrent que la CL50 se situe au-delà de 20
000 mg/litre; ceux qui ont été pratiqués sur des poissons donnent des
valeurs supérieures à 17 800 mg/litre. Un test sur amphibien a montré
que la CL50 pour les têtards était égale à 17 000 mg/litre. Les
études de toxicité chronique portant sur la reproduction des daphnies
ont permis de fixer à 8 590 mg/litre la concentration sans effet
observable (NOEC). En prenant la croissance pour critère, on a obtenu
une NOEC de 15 380 mg/litre pour des poissons brièvement exposés.
Les tests effectués avec des dégivrants à base d'éthylène-glycol
montrent que ces produits sont plus toxiques pour les organismes
aquatiques que l'éthylène-glycol pur, ce qui indique que ces
dégivrants contiennent d'autres substances toxiques.
Lors de tests de laboratoire comportant l'exposition d'organismes
aquatiques à l'eau d'une rivière recevant les eaux de ruissellement
d'un aéroport, on a constaté des effets toxiques pouvant aller jusqu'à
la mort. Des études effectuées sur le terrain à proximité d'un
aéroport ont révélé que les organismes aquatiques présentaient des
signes d'intoxication qui pourraient être dus à l'éthylène-glycol,
avec en outre présence de poissons morts et réduction de la
biodiversité. Il n'est toutefois pas absolument certain que ces effets
puissent être attribués à l'éthylène-glycol.
Les organismes terrestres ont beaucoup moins de chances d'être
exposés à de l'éthylène-glycol et ils sont généralement peu sensibles
à ce composé. Il a fallu des concentrations supérieures à 100 000
mg/litre pour produire des effets toxiques sur des champignons et des
levures prélevés dans le sol. On a pu provoquer une inhibition de la
germination en plongeant des semences dans des bains contenant une
très forte concentration d'éthylène-glycol, mais ces résultats n'ont
aucune signification sur le plan écologique. Chez des canards ayant
reçu de l'éthylène-glycol par voie digestive, la dose sans effet
observable (NOEL) se situait à 1221 mg/kg de poids corporel; pour les
poulets, la dose létale serait d'environ 8 000 mg/kg p.c. Ces valeurs
montrent que le composé est peu toxique pour les volatiles.
RESUMEN DE ORIENTACION
El presente CICAD sobre los aspectos ambientales del
etilenglicol, preparado por el Instituto de Ecología Terrestre del
Reino Unido se basa en el informe de Evaluación de los peligros para
el medio ambiente: Etilenglicol (Nielsen et al., 1993). Se utilizó
también como documento original el informe sobre el etilenglicol que
había preparado el Comité Consultivo sobre Sustancias Químicas
Importantes para el Medio Ambiente de la Sociedad Alemana de Química
(BUA, 1991). Además de usar estos documentos, se realizó una búsqueda
de la bibliografía reciente hasta 1998. La información acerca del
carácter del proceso de examen colegiado para los principales
documentos originales figura en el apéndice 1. La información relativa
al examen colegiado de este CICAD se presenta en el apéndice 2. Su
aprobación como evaluación internacional se realizó en una reunión de
la Junta de Evaluación Final, celebrada en Washington, DC, Estados
Unidos, los días 8-11 de diciembre de 1998. La lista de participantes
en esta reunión figura en el apéndice 3. La Ficha internacional de
seguridad química (ICSC 0270), preparada por el Programa Internacional
de Seguridad de las Sustancias Químicas (IPCS, 1993), también se
reproduce en el presente documento.
El etilenglicol (CAS No 107-21-1) es un líquido denso, claro,
incoloro, de sabor dulce, pero inodoro. Tiene una volatilidad baja. Es
miscible con el agua y algunos otros disolventes, ligeramente soluble
en éter, pero prácticamente insoluble en benceno, hidrocarburos
clorados, éteres de petróleo y aceites. El log del coeficiente de
reparto octanol/agua oscila entre -1,93 y -1,36.
La capacidad de producción mundial estimada en 1993 fue de 9,4
millones de toneladas. La liberación en el medio ambiente se produce
fundamentalmente en la hidrosfera. La liberación local más importante
a las aguas superficiales es consecuencia de la utilización de
etilenglicol como descongelante en las pistas de los aeropuerto y en
los aviones. Unos dos tercios de la producción mundial de etilenglicol
se utilizan como intermediario químico, con otra cuarta parte como
anticongelante en los refrigerantes de los motores.
El etilenglicol que se libera en la atmósfera se degrada por
reacción con radicales hidroxilo; la semivida del compuesto en esta
reacción se ha calculado entre 0,3 y 3,5 días.
No se prevé que haya hidrólisis del etilenglicol en aguas
superficiales.
El compuesto tiene poca o ninguna capacidad de unión a partículas
y es móvil en el suelo.
El bajo coeficiente de reparto octanol/agua y los factores de
bioacumulación medidos en un pequeño número de organismos indican una
capacidad escasa de bioacumulación.
El etilenglicol es fácilmente biodegradable en pruebas
normalizadas utilizando lodos cloacales. En numerosos estudios se ha
puesto de manifiesto su biodegradación en condiciones tanto aerobias
como anaerobias. Algunos estudios parecen indicar una fase
estacionaria antes de la degradación, pero otros muchos no. Se produce
degradación tanto en lodos adaptados como no adaptados. Se ha
notificado una degradación rápida en las agua superficiales (inferior
en la salada que en la dulce), el agua freática y los inóculos del
suelo. Se han identificado varias cepas de microorganismos capaces
utilizar el etilenglicol como fuente de carbono.
Se dispone de datos limitados sobre las concentraciones de
etilenglicol medidas en los compartimentos del medio ambiente. Los
niveles medidos en las aguas superficiales generalmente han sido
bajos, de algunos microgramos por litro. Las concentraciones en las
aguas residuales de instalaciones de producción antes del tratamiento
han alcanzado un promedio de hasta 1 300 mg/litro. Las concentraciones
con diferencia más altas de las notificadas corresponden al agua de
escorrentía de los aeropuertos, con concentraciones de hasta 19 000
mg/litro.
El etilenglicol suele tener una toxicidad baja para los
organismos acuáticos. El umbral tóxico para los microorganismos es
superior a 1 000 mg/litro. Las CE50 para el crecimiento en las
microalgas son de 6 500 mg/litro o superiores. En las pruebas de
toxicidad aguda con invertebrados acuáticos en las que se pudo
determinar un valor se obtuvieron CL50 superiores a 20 000 mg/litro,
y con peces por encima de 17 800 mg/litro. En una prueba realizada con
anfibios se observó una CL50 para los renacuajos de 17 000 mg/litro.
Se ha notificado una concentración sin efectos observados (NOEC) para
pruebas crónicas en dáfnidos de 8 590 mg/litro (para los efectos
finales reproductivos). Tras una exposición breve de peces se notificó
una NOEC para el crecimiento de 15 380 mg/litro.
En pruebas en las que se utilizó descongelante que contenía
etilenglicol se puso de manifiesto una toxicidad para los organismos
acuáticos superior a la observada con el compuesto puro, lo que indica
la presencia de otros componentes tóxicos en las formulaciones.
En pruebas de laboratorio de exposición de organismos acuáticos a
una corriente de agua receptora de la escorrentía de los aeropuertos
aparecieron efectos tóxicos y letales. En estudios sobre el terreno
realizados en las cercanías de un aeropuerto se han notificado signos
tóxicos compatibles con la intoxicación por etilenglicol, muerte de
peces y reducción de la biodiversidad. Estos efectos no se pueden
atribuir de manera definitiva al etilenglicol.
Es mucho menos probable que los organismos terrestres estén
expuestos al etilenglicol y en general muestran una sensibilidad baja
al compuesto. Se necesitaron concentraciones superiores a 100 000
mg/litro para producir efectos tóxicos en levaduras y hongos del
suelo. En algunos experimentos se observó que las concentraciones muy
altas y la impregnación de las semillas inhibían la germinación; estos
efectos no se consideran importantes para el medio ambiente. La
concentración sin efectos observados (NOEL) para patos a los que se
administró por vía oral 1 221 mg/kg de peso corporal y las dosis
letales notificadas para aves de corral de alrededor de 8 000 mg/kg de
peso corporal indican una toxicidad baja para las aves.
 1. EXECUTIVE SUMMARY
This CICAD on the environmental aspects of ethylene glycol was
prepared by the Institute of Terrestrial Ecology, United Kingdom,
based on the report Environmental hazard assessment: Ethylene glycol
(Nielsen et al., 1993). The report on ethylene glycol prepared by the
German Chemical Society Advisory Committee on Existing Chemicals of
Environmental Relevance (BUA, 1991) was also used as a source
document. In addition to these documents, a search of recent
literature was conducted up to 1998. Information on the nature of the
peer review process for the main source documents is presented in
Appendix 1. Information on the peer review of this CICAD is presented
in Appendix 2. This CICAD was approved as an international assessment
at a meeting of the Final Review Board, held in Washington, DC, USA,
on 8-11 December 1998. Participants at the Final Review Board meeting
are listed in Appendix 3. The International Chemical Safety Card (ICSC
0270) produced by the International Programme on Chemical Safety
(IPCS, 1993) has also been reproduced in this document.
Ethylene glycol (CAS No.
1. EXECUTIVE SUMMARY
This CICAD on the environmental aspects of ethylene glycol was
prepared by the Institute of Terrestrial Ecology, United Kingdom,
based on the report Environmental hazard assessment: Ethylene glycol
(Nielsen et al., 1993). The report on ethylene glycol prepared by the
German Chemical Society Advisory Committee on Existing Chemicals of
Environmental Relevance (BUA, 1991) was also used as a source
document. In addition to these documents, a search of recent
literature was conducted up to 1998. Information on the nature of the
peer review process for the main source documents is presented in
Appendix 1. Information on the peer review of this CICAD is presented
in Appendix 2. This CICAD was approved as an international assessment
at a meeting of the Final Review Board, held in Washington, DC, USA,
on 8-11 December 1998. Participants at the Final Review Board meeting
are listed in Appendix 3. The International Chemical Safety Card (ICSC
0270) produced by the International Programme on Chemical Safety
(IPCS, 1993) has also been reproduced in this document.
Ethylene glycol (CAS No.  INTERNATIONAL CHEMICAL SAFETY CARD
ETHYLENE GLYCOL ICSC: 0270
March 1999
CAS #
INTERNATIONAL CHEMICAL SAFETY CARD
ETHYLENE GLYCOL ICSC: 0270
March 1999
CAS # 