
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 8
SULFUR OXIDES AND SUSPENDED PARTICULATE MATTER
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of either the World Health Organization or the United Nations
Environment Programme
Published under the joint sponsorship of the United Nations
Environment Programme and the World Health Organization
World Health Organization Geneva, 1979
ISBN 92 4 154068 0
(c) World Health Organization 1979
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. For rights of reproduction or
translation of WHO publications, in part or in toto, application
should be made to the Office of Publications, World Health
Organization, Geneva, Switzerland. The World Health Organization
welcomes such applications.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR SULFUR OXIDES AND SUSPENDED
PARTICULATE MATTER
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER RESEARCH AND ACTION
1.1. Summary
1.1.1. Chemistry and analytical methods
1.1.2. Sources of sulfur oxides and particulate matter
1.1.3. Dispersion and environmental transformations
1.1.4. Environmental concentrations and exposures
1.1.5. Absorption, distribution, and elimination
1.1.6. Effects on experimental animals
1.1.7. Effects on man
1.1.7.1 Controlled exposures
1.1.7.2 Industrial exposure
1.1.7.3 Community exposure
1.1.8. Evaluation of health risks
1.2. Recommendations for further research and action
2. CHEMISTRY AND ANALYTICAL METHODS
2.1. Chemical and physical properties
2.1.1. Sulfur oxides
2.1.2. Suspended particulate matter
2.2. Methods of sampling and analysis
2.2.1. Sulfur dioxide
2.2.2. Suspended sulfates and surfuric acid
2.2.3. Suspended particulate matter
2.2.4. Dustfall (deposited matter)
3. SOURCES OF SULFUR OXIDES AND PARTICULATE MATTER
3.1. Natural occurrence
3.2. Man-made sources
3.3. Characteristics of sources
4. DISPERSION AND ENVIRONMENTAL TRANSFORMATIONS
4.1. Dispersion
4.2. Transformation and degradation
5. ENVIRONMENTAL CONCENTRATIONS AND EXPOSURES
5.1. Concentrations in outdoor air
5.2. Concentrations in indoor air
5.3. Concentrations in work places
5.4. Assessment of exposures
6. ABSORPTION, DISTRIBUTION, AND ELIMINATION
6.1. Absorption and deposition in the respiratory tract
6.1.1. Sulfur dioxide
6.1.2. Airborne particles
6.2. Clearance from the respiratory tract and distribution
6.2.1. Sulfur dioxide
6.2.2. Particulate matter
7. EFFECTS ON EXPERIMENTAL ANIMALS
7.1. Short-term exposure studies
7.1.1. Exposure to sulfur dioxide singly or in combination
with other agents
7.1.2. Exposure to sulfuric acid aerosols or suspended
sulfates
7.2. Long-term exposure studies
7.2.1. Exposure to sulfur dioxide
7.2.2. Exposure to sulfuric acid aerosols
7.2.3. Exposure to a mixture of sulfur dioxide and
surfuric acid aerosols or this mixture combined
with other agents
7.2.4. Combined exposure to sulfur dioxide and particulate
matter or other gaseous pollutants
8. EFFECTS ON MAN
8.1. Controlled exposures
8.1.1. Effects on respiratory organs
8.1.1.1 Exposure to sulfur dioxide
8.1.1.2 Exposure to sulfuric acid aerosols
8.1.1.3 Exposure to mixtures of sulfur dioxide
and other compounds
8.1.2. Effects on sensory or reflex functions
8.2. Industrial exposure
8.2.1. Exposure to sulfur dioxide singly or in combination
with particulate matter
8.2.2. Exposure to surfuric acid mist:
8.3. Community exposure
8.3.1. Mortality -- effects of short-term exposures
8.3.2. Mortality -- effects of long-term exposures
8.3.3. Morbidity -- effects of short-term exposures
8.3.4. Morbidity in adults -- effects of long-term
exposures
8.3.5. Morbidity in children
8.3.6. CHESS studies
8.3.7. Lung cancer and air pollution
8.3.8. Annoyance
8.4. Exposure-effect relationships
9. EVALUATION OF HEALTH RISKS FROM EXPOSURE TO SULFUR OXIDES,
SMOKE, AND SUSPENDED PARTICULATE MATTER
9.1. Exposure levels
9.2. Experimental animal studies
9.3. Controlled studies in man
9.4. Effects of industrial exposures
9.5. Effects of community exposures
9.6. Guidelines for the protection of public health
REFERENCES
NOTE TO READERS OF THE CRITERIA DOCUMENTS
While every effort has been made to present information in the
criteria documents as accurately as possible without unduly delaying
their publication, mistakes might have occurred and are likely to
occur in the future. In the interest of all users of the environmental
health criteria documents, readers are kindly requested to communicate
any errors found to the Division of Environmental Health, World Health
Organization, Geneva, Switzerland, in order that they may be included
in corrigenda which will appear in subsequent volumes.
In addition, experts in any particular field dealt with in the
criteria documents are kindly requested to make available to the WHO
Secretariat any important published information that may have
inadvertently been omitted and which may change the evaluation of
health risks from exposure to the environmental agent under
examination, so that the information may be considered in the event of
updating and re-evaluation of the conclusions contained in the
criteria documents.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR SULFUR OXIDES
AND SUSPENDED PARTICULATE MATTER
Participants
Membersa
Professor K. Biersteker, Medical Research Division, Municipal Health
Department, Rotterdam, Netherlands (Vice-Chairman).
Professor K. A. Bustueva, Department of Community Hygiene, Central
Institute for Advanced Medical Training, Moscow, USSR
Dr P. Camner, Department of Environmental Hygiene, The Karolinska
Institute, Stockholm, Sweden
Professor L. Friberg, Department of Environmental Hygiene, The
Karolinska Institute, Stockholm, Sweden (Chairman)
Mrs M. Fugas Laboratory for Environmental Hygiene, Institute for
Medical Research and Occupational Health, Zagreb, Yugoslavia
Dr R. J. M. Horton, Health Effects Research Laboratory, US
Environmental Protection Agency, Research Triangle Park, NC, USA
Professor S. Maziarka, National Institute of Hygiene, Warsaw, Poland
Dr B. Prinz, State Institute for Protection of Air Quality and Land
Usage, Essen, Federal Republic of Germany
Dr H. P. Ribeiro, Laboratory of Pulmonary Function, Santa Casa de
Misericordia de Sao Paulo, Sao Paulo, Brazil
Dr T. Suzuki, Institute of Public Health, Tokyo, Japan
Mr G. Verduyn, Institut d'Hygiene et d'Epidemiologie, Brussels,
Belgium
Mr R. E. Waller, Medical Research Council, Air Pollution Unit, St
Bartholomew's Hospital Medical College, London, England
(Rapporteur)
Mr D. A. Williams, Surveillance Division, Air Pollution Control
Directorate, Environment Canada, Ottawa, Ontario, Canada
Professor M. H. Wahdan, High Institute of Public Health, University of
Alexandria, Alexandria, Egypt
a Unable to attend:
Representatives of other Organizations
Mr J. Janczak, Environment and Housing Division, United Nations
Economic Commission for Europe, Geneva, Switzerland
Mr D. Larré, Division of Geophysics, Global Pollution and Health,
United Nations Environmental Programme, Nairobi, Kenya
Dr D. Djordevic, Occupational Safety and Health Branch, International
Labour Organisation, Geneva, Switzerland
Mr G. W. Kronebach, Technical Supporting Services Branch, World
Meteorological Organization, Geneva, Switzerland
Dr A. Berlin, Health Protection Directorate, Commission of the
European Communities, Luxembourg
Mr J. A. Bromley, Environmental Directorate, Organization for Economic
Co-operation and Development, Paris, France
Secretariat
Professor B. G. Ferris, Jr, Department of Physiology, Harvard
University School of Public Health, Boston, MA, USA (Temporary
Adviser)
Dr Y. Hasegawa, Medical Officer, Control of Environmental Pollution
and Hazards, World Health Organization, Geneva, Switzerland
(Secretary)
Dr H. W. de Koning, Scientist, Control of Environmental Pollution and
Hazards, World Health Organization, Geneva, Switzerland
Dr B. Marschall, Medical Officer, Occupational Health, World Health
Organization, Geneva, Switzerland
Dr R. Masironi, Scientist, Cardiovascular Diseases, World Health
Organization, Geneva, Switzerland
Dr S. I. Muravieva, Institute of Industrial Hygiene and Occupational
Diseases, Academy of Medical Sciences of the USSR, Moscow, USSR
(Temporary Adviser)
Dr V. B. Vouk, Chief, Control of Environmental Pollution and Hazards,
World Health Organization, Geneva, Switzerland
ENVIRONMENTAL HEALTH CRITERIA FOR SULFUR OXIDES AND SUSPENDED
PARTICULATE MATTER
A WHO Task Group on Environmental Health Criteria for Sulfur
Oxides and Suspended Particulate Matter met in Geneva from 6 to 12
January 1976. The meeting was opened by Dr B. H. Dieterich, Director,
Division of Environmental Health, who welcomed the participants and
the representatives of other international organizations on behalf of
the Director-General. Dr Dieterich briefly outlined the history and
purpose of the WHO Environmental Health Criteria Programme and the
progress made in its implementation, thanks to the active
collaboration of WHO Member States and the support of the United
Nations Environment Programme (UNEP).
The Task Group reviewed and revised the second draft criteria
document and made an evaluation of the health risks from exposure to
these substances.
The first and second drafts were prepared by Professor B. G.
Ferris, Jr, Harvard University School of Public Health, USA. The
comments on which the second draft was based were received from the
national focal points collaborating in the WHO Environmental Health
Criteria Programme in Belgium, Bulgaria, Canada, Czechoslovakia, the
Federal Republic of Germany, Greece, Japan, New Zealand, Poland,
Sweden, USA, USSR and from the Food and Agriculture Organization of
the United Nations (FAO), the United Nations Educational Scientific
and Cultural Organization (UNESCO), the United Nations Industrial
Development Organization (UNIDO), the World Meteorological
Organization (WMO), the International Atomic Energy Agency (IAEA), and
the Commission of European Communities (CEC). Comments were also
received from Professor H. Antweiler and Dr B. Prinz (Federal Republic
of Germany), Professor K. Biersteker and Dr R. van der Lende
(Netherlands), Professor F. Sawicki (Poland), and Professor W. W.
Holland and Professor P. J. Lawther (United Kingdom).
The collaboration of these national institutions, international
organizations and individual experts is gratefully acknowledged. The
Secretariat also wishes to thank Professor B. G. Ferris, Jr and Mr R.
E. Waller for their invaluable assistance in the final stages of the
preparation of the document.
In view of the substantial amendments made to the document
(particularly within sections 2 to 5) since the meeting of the Task
Group, a revised version was circulated to all members in February
1978. At the same time, copies of a newly-produced review of the
health effects of particulate pollution (Holland et al., in press),
that had been submitted for consideration, were distributed to the
members. Comments were sought on the draft of the criteria document
itself, and on any amendments or additions considered necessary in
light of the new report. These comments, together with others received
from the International Petroleum Industry Environmental Conservation
Association, and the International Iron and Steel Institute, were then
considered by a small group consisting of the Chairman of the Task
Group meeting, the Rapporteur and some members of the Secretariat. The
alterations suggested (mainly within section 9) were circulated again
to the original members of the Task Group prior to publication.
The document has been based, primarily, on original publications
listed in the reference section. However, several recent reviews of
health aspects of sulfur oxides and suspended particulate matter have
also been used including those by Katz (1969), Committee on the
Challenges of Modern Society (1971), Organization for Economic
Cooperation and Development (1965), Rall (1974), Task Group on Lung
Dynamics (1966), Task Group on Metal Accumulation (1973), US
Department of Health, Education and Welfare (1969a), US Environmental
Protection Agency (1974), World Health Organization (1976a), and World
Meteorological Organization (1974).
The purpose of this document is to review and evaluate available
information on the biological effects of sulfur oxides and suspended
particulate matter including suspended sulfates and sulfuric acid
aerosols, and to provide a scientific basis for decisions aimed at the
protection of human health from the adverse consequences of exposure
to these substances in both occupational and general environments.
Although there are various routes of exposure, such as inhalation,
ingestion (World Health Organization, 1971, 1974) and contact with
skin, attention in this report has been concentrated upon the effects
of inhalation of these substances, since this is the most important
route of exposure. The discussion has also been limited to sulfur
dioxide, sulfur trioxide, sulfate ions, and particulate matter
primarily resulting from the combustion of fossil fuels. The sulfate
ion has been considered in the variety of forms in which it occurs in
the atmosphere, e.g., sulfuric acid and various sulfate salts.
The vast literature on these pollutants has been carefully
evaluated and selected according to its validity and relevance for
assessing human exposure, for understanding the mechanisms of the
biological action of the pollutants and for establishing environmental
health criteria, i.e., exposure-effect/response relationships in man.
Environmental considerations have been limited to elucidating the
pathways leading from the natural and man-made sources of these
substances to the sites of toxic action in the human organism. The
non-human targets (plants, animals, ecosystems) have not been
considered unless the effects of their contamination were judged to be
of direct relevance to human health. For similar reasons, much of the
published information on the effects of these pollutants on
experimental animals has not been included.
Details concerning the WHO Environmental Health Criteria Programme
including some terms frequently used in the document may be found in
the general introduction to the Environmental Health Criteria
Programme published together with the environmental health criteria
document on mercury (Environmental Health Criteria 1, Mercury, Geneva,
World Health Organization, 1976), now also available as a reprint.
The following conversion factors have been used in the present
document:a
Sulfur dioxide 1 ppm = 2856 µg/m3
Ozone 1 ppm = 2140 µg/m3
Carbon monoxide 1 ppm = 1250 µg/m3
a When converting values expressed in ppm to µg/m3, the numbers
have been rounded up to 2 or, exceptionally, 3 significant figures,
and concentrations higher than 10 000 µg/m3 have been expressed in
mg/m3.
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER RESEARCH AND ACTION
1.1 Summary
1.1.1 Chemistry and analytical methods
Procedures in common use for the sampling and determination of
sulfur dioxide, sulfates, sulfuric acid, and suspended particulate
matter have been discussed, noting their limitations and stressing the
need to specify the method of measurement when quoting results in
relation to studies on the effects of health.
Several alternative methods, already in common use, can be
recommended for the determination of sulfur dioxide using manually
operated sampling and, providing the extent of interference from other
pollutants is taken into account, results are reasonably comparable
with one other. A wide range of continuous automatic instruments is
available and, where the expense is justified, they can provide
additional information on short-term variations in concentrationsb of
sulfur dioxide.
Methods for the determination of particulate sulfate do not
present any special problems, but, at present, there does not appear
to be any wholly satisfactory way of determining sulfuric acid
separately from sulfates and other interrelated components.
Much attention was given by the Task Group to the sampling and
determination of suspended particulate matter, stressing that this was
not a well-defined entity, and that it could only be assessed in terms
of certain physical properties. The several methods in common use are
based on different characteristics, and the Task Group felt that clear
distinctions should be made between them, particularly in relation to
those based on blackness (the "smoke" measurements commonly made in
Europe) and those based on weight (the total suspended particulate
matter commonly measured in USA). The need to limit the measurements
to particles within the respiratory size range, and to consider the
wide range in chemical composition of the samples was also stressed.
b Throughout the document, the word "concentration" refers to mass
concentration unless otherwise stated.
1.1.2 Sources of sulfur oxides and particulate matter
Despite the fact that some sulfur oxides and particulate matter
occur naturally in air in large amounts, contributions from man's
activities are generally of prime importance in urban areas. In
particular, the combustion of fuels for heating and power generation
is considered responsible for most of the sulfur dioxide and
particulate pollution to which the general population is exposed. The
three broad categories of sources are: domestic sources associated
with the use of coal and some other fuels for heating and cooking;
industrial sources; and motor vehicles. Domestic and motor vehicle
sources have a disproportionate effect on concentrations in the
immediate vicinity, because the pollution is emitted close to ground
level.
1.1.3 Dispersion and environmental transformations
The temperature of the gases, the efflux velocity, and the height
of the chimney are important factors in securing effective dispersion
of emissions from combustion sources. The topography of the
surrounding area and meteorological factors determine the extent to
which these pollutants are dispersed and diluted to tolerable levels.
Temperature inversion can trap emissions over urban areas to produce
concentrations up to several hundred times the normal values.
Several processes, including photochemical reactions in the
presence of hydrocarbons, catalytic oxidation in the presence of
particulate matter containing iron or manganese compounds, and
reaction with ammonia, leading to the transformation of sulfur dioxide
to sulfates or sulfuric acid, are involved in the atmospheric
reactions of sulfur dioxide and suspended particulate matter. The
relative importance of each of these is not well established, but
together they account for the gradual removal of most of the sulfur
dioxide dispersed into the air, the remainder being deposited directly
on soil, water, vegetation, or other surfaces.
1.1.4 Environmental concentrations and exposures
Sulfur dioxide and suspended particulate matter are measured
routinely in many areas throughout the world, but care is needed to
ensure that observations from monitoring networks set up for other
purposes are suitable for assessing risks to health. The location of
samplers in relation to sources, the surrounding topography, and the
population at risk need to be considered, and also the time-resolution
of the observations. Averaging periods of 24 h are commonly used in
relation to short-term exposures, though, in some circumstances, still
shorter periods are required. For long-term exposures, annual means
based on a series of daily observations may be adequate.
Examination of concentrations of sulfur dioxide and suspended
particulate matter in the air of a number of cities throughout the
world has revealed a wide range in annual mean values and an even
wider range of peak values, reflecting the effects of climatic factors
and liability to temperature inversions. The typical, annual,
arithmetic mean concentrations of sulfur dioxide in urban areas range
from 100-200 µg/m3 (0.035-0.07 ppm) with the highest daily means from
300-900 µg/m3 (0.1.-0.3 ppm). For smoke, the corresponding values are
30-200 µg/m3 and 150-900 µg/m3 respectively, and for suspended
particulate matter, measured by the high volume sampler, the annual
arithmetic means are 60-500 µg/m3 with maximum daily means of about
150-1000 µg/m3. Relatively little information is available on
sulfates but some data have been obtained in the USA.
Indoor concentrations of these pollutants also deserve attention.
In the absence of specific sources of sulfur dioxide or particulate
matter, concentrations are generally lower indoors than outdoors.
Proposals for assessing weekly-weighted average exposures of people in
terms of the proportion of time spent in various locations were
discussed.
Industrial situations in which high concentrations of sulfur
dioxide or sulfuric acid occur should be carefully assessed in each
specific case, but it should be noted that industrial dusts are
generally very different in character from the suspended particulate
matter in urban air.
1.1.5 Absorption, distribution, and elimination
Although the major route of absorption of the relevant sulfur
compounds and particulate matter into the body is through the
intestinal tract, the respiratory tract is the most vulnerable area
for airborne materials.
Most studies on both man and animals have indicated that 40 to 90%
or more of inhaled sulfur dioxide is absorbed in the upper respiratory
tract. Taken into the blood stream, it appears to be widely
distributed throughout the body, metabolized, and excreted via the
urinary tract.
The deposition pattern of particulate matter varies with particle
size, shape, and density, and also with airflow conditions. Deposited
particles are largely phagocytized and transported to the mucociliary
escalator, into the interstitium, or to the lymphatic system. The
biological half-times range from days to years depending on their
chemical composition.
Soluble particles may dissolve in the mucous or aqueous lining of
the lungs. In the first case, they will be eliminated via the
mucociliary route. In the second, they may diffuse into the lymph or
blood.
1.1.6 Effects on experimental animals
Selected studies on animals that involve both short-term (24-h or
less) and long-term (more than 24-h) exposures have been reviewed in
this document; certain interactions between the effects of sulfur
oxides, particulate matter, and other air contaminants have also been
reported. The lowest, adverse-effect concentrations vary considerably
from study to study. The discrepancies may be due to differences in
the sensitivity of the test animals used or in exposure conditions
including the duration of exposure and the pattern of exposure
(single, continuous, repeated, or intermittent). Furthermore, exposure
may have been to a single pollutant or to a mixture of various agents,
or different effects may have been analysed.
In general, however, it has been noted that sulfuric acid aerosols
and some sulfate salts such as zinc ammonium sulfate are more
irritating to respiratory organs than sulfur dioxide, and that some
aerosols, particularly those in the submicron size range, enhance the
effect of sulfur dioxide when they are present simultaneously.
Caution must be exercised in light of the fact that differences in
metabolism and life span make extrapolation of results of animal
experiments to man difficult. However, some of these studies have
indicated possible mechanisms of biological action on the respiratory
system -- e.g., interference with mechanisms for the clearance of
bacteria and inert particles from the lung.
1.1.7 Effects on man
1.1.7.1 Controlled exposures
Inhalation studies on human volunteers have been performed under
controlled, short-term exposure conditions with sulfur dioxide or
sulfuric acid mist singly or in combination, or with mixtures of these
and other compounds. Some of these studies have proved useful for
developing exposure-effect relationships.
When exposed to sulfur dioxide alone, slight effects on
respiratory function were demonstrated at a concentration of
2.1 mg/m3 (0.75 ppm) but not at 1.1 mg/m3 (0.37 ppm), while sulfuric
acid mist affected respiratory function at levels as low as
0.35 mg/m3. Synergistic effects on pulmonary function were reported
from joint exposure to sulfur dioxide and hydrogen peroxide as well as
sulfur dioxide and ozone.
The effects of sulfuric acid mist and sulfur dioxide on sensory
receptors and cerebral cortical function have been studied extensively
in the USSR. In these reflex actions, threshold levels for sulfuric
acid were always much lower than those for sulfur dioxide. Synergistic
effects of these compounds have also been noted.
1.1.7.2 Industrial exposure
Effects of exposure to sulfur dioxide, particulate matter, or
sulfuric acid mist have been studied in workers in refrigerator
manufacturing plants, steel mills, paper and pulp mills, and in the
battery industries.
Although the exposure levels were very high (daily mean
concentrations of sulfur dioxide of up to 70 mg/m3 or 25 ppm) in some
studies, no significant differences in effects were found when
compared with the controls. In another study, effects on respiratory
function were not detected with joint exposure to sulfur dioxide and
suspended particulate matter at mean concentrations over 3 years of
1.8 to 2.1 mg/m3 (0.6-0.7 ppm) and 600 to 1800 µg/m3, respectively.
Exposure to sulfuric acid mist produced effects (nose and throat
irritation) at a concentration of 2.0 mg/m3, while exposure to a
concentration of 1.4 mg/m3 did not affect pulmonary function.
However, the effects of this pollutant are also closely dependent on
particle size.
1.1.7.3 Community exposure
The large amount of literature on the effects of community
exposure has been reviewed and detailed consideration has been given
to those studies that appeared to have adequate data and design, in
particular, due control for cigarette smoking and satisfactory
measurements of exposure levels. Certain of these studies were
selected to develop exposure-effect relationships. In the evaluation
of the studies it became apparent that it was not possible to compare
two fundamentally different methods of measuring exposure to
particulate matter -- one measuring black smoke and the other
measuring total suspended particulates, usually by the high-volume
sampling method.
Studies have been performed in terms of both short and long-term
exposures and in relation to changes in the incidences of mortality
and morbidity. In morbidity studies concerned with short-term exposure
to a combination of sulfur dioxide and total particulates, the lowest
concentrations (24-h mean) at which adverse effects were noted were
200 µg/m3 (0.07 ppm) and 150 µg/m3 (high volume sampler),
respectively. In long-term, joint exposure studies, effects were noted
at annual mean concentrations of 60-140 µg/m3 (0.02-0.05 ppm) for
sulfur dioxide and 100-200 µg/m3 for total suspended particulates
(light-scattering method). However, there were reservations about the
validity of some of these studies.
Increases in mortality were reported in relation to episodes of
high pollution with 24-h mean concentrations of the order of
500 µg/m3 (0.18 ppm) for sulfur dioxide and 500 µg/m3 for smoke.
The question as to whether carcinogenic components of suspended
particulate matter, such as benzo(a)pyrene may have some influence on
the incidence of lung cancer was not discussed by the Task Group.a
1.1.8 Evaluation of health risks
From a critical evaluation of the studies on the health effects of
community exposures, the Task Group developed two summary tables; one
for the expected effects on the health of selected populations of
short-term exposures to sulfur dioxide and smoke; the other for the
effects of long-term exposures to these substances.
As an estimate of the lowest adverse-effect levels for short-term
exposures, the Group selected the 24-h mean concentrations of
500 µg/m3 (0.18 ppm) for sulfur dioxide and 500 µg/m3 for smoke, as
levels at which excess mortality might be expected among elderly
people or patients with pulmonary diseases, and a sulfur dioxide
concentration of 250 µg/m3 (0.09 ppm) and a smoke concentration of
250 µg/m3 as levels at which the conditions of patients with
respiratory disease might become worse.
a Since the Task Group meeting, an International Symposium on Air
Pollution and Cancer has been held at the Karolinska Institute,
Stockholm, with the collaboration of the World Health Organization.
One of the conclusions quoted in the report (Task Group on Air
Pollution and Cancer, 1978) is as follows: "Combustion products of
fossil fuels in ambient air, probably acting together with cigarette
smoke, have been responsible for cases of lung cancer in large urban
areas, the numbers produced being of the order of 5-10 cases per
100 000 males per year. The actual rate will vary from place to place
and from time to time, depending on local conditions over the
previous few decades."
For long-term exposures, annual mean concentrations of 100 µg/m3
(0.035 ppm) for sulfur dioxide and 100 µg/m3 for smoke were selected
as the lowest concentrations at which adverse health effects such as
increases in respiratory symptoms, or respiratory disease incidence in
the general population might be expected.
Based on these evaluations, guidelines for the protection of the
health of the public were developed in terms of 24-h values
(100-150 µg/m3 for sulfur dioxide, and for smoke) and in terms of
annual means (40-60 µg/m3 for sulfur dioxide, and for smoke). In view
of the limited amount of data available in relation to total suspended
particulates, firm guidelines could not be recommended, but it was
suggested that interim guidelines might be of the order of
60-90 µg/m3 for annual arithmetic means and 150-230 µg/m3 for 24-h
values. Guidelines were not developed for sulfuric acid or sulfates,
also because of lack of data.
1.2 Recommendations for Further Research and Action
(a) As most of the knowledge of effects discussed in this
document relates to combinations of sulfur oxides with smoke, and as
these pollutants are not wholly representative of the current exposure
situation in a number of communities, there is a need to carry out
epidemiological studies where possible effects can be related to
particulate pollutants of other types, including sulfuric acid and
sulfates, and to other gaseous components of the mixture.
(b) As epidemiological studies are still being carried out that
do not take other variables, particularly smoking, into account when
considering effects, and in which the exposure to pollution is not
adequately assessed, it is recommended that the World Health
Organization should provide guidance and advice on the minimum
requirements for such studies.
(c) As a consequence of the conclusions reached on the expected
effects on health of sulfur oxides, smoke, and total suspended
particulates, and on the related guidelines for the protection of
public health, it is recommended that existing monitoring practices
should be reviewed and, if necessary, appropriately modified. To
assist regulatory agencies in this respect, it is recommended that the
World Health Organization should undertake consultations with
environmental scientists, inhalation toxicologists, and
epidemiologists to consider both the epidemiological and control
aspects of the problem.
(d) As there is little information from occupational exposures
that can be used for exposure-effect evaluations, it is recommended
that such studies should be carried out particularly in relation to
sulfur dioxide and related pollutants. These studies should include
measurements of pollution over complete working shifts, taking into
account variations with space and time over shorter periods, and
exposures outside the working environment. The importance of following
up people who may have left work because of effects on their health
must also be stressed.
(e) The Group did not carry out a thorough evaluation of any
possible association between lung cancer and air pollution. It is
recommended that a separate evaluation should be carried out.
(f) As deposition and clearance of particles from the
respiratory system is of fundamental importance for evaluating risks,
and for the design of measuring instruments for use in monitoring
systems, it is recommended that a thorough review of the relevance of
existing information on the mixture of pollutants present in the
ambient air be carried out, taking into account particle size
distribution and chemical composition.
(g) Some laboratory experiments on the effects of sulfur oxides,
smoke, and total suspended particulates do not need to be repeated,
but the Task Group considered that it was necessary for more
experimental work to be carried out on the mechanism of the biological
action of these pollutants and of their interactions with other
agents.
(h) The Task Group found that information concerning the nature
and effects of pollution had considerably increased since the WHO
meeting on air quality criteria and guides in 1972, and that this had
resulted in a somewhat different approach to the preparation of
criteria for sulfur oxides and suspended particulate matter and to the
recommendations for future action. It was considered, therefore, that
the criteria should be reviewed at least every five years to take into
account any new data on effects that may become available and the
implications of any further changes in the character of pollution.
(i) Since observations on sulfur oxides, smoke, and total
suspended particulates are considered mainly as indices of the complex
mixture of pollutants in the ambient air, the Task Group recommended
that, in addition to the continuation of efforts to reduce these
pollutants, efforts should be made to control other pollutants.
2. CHEMISTRY AND ANALYTICAL METHODS
2.1 Chemical and Physical Properties
2.1.1 Sulfur oxides
Sulfur dioxide is a colourless gas that can be detected by taste
by most people at concentrations in the range of 1000 to 3000 µg/m3
(0.35-1.05 ppm). At higher concentrations (above about 10 000 µg/m3;
3.5 ppm), it has a pungent, irritating odour. It dissolves readily in
water to form sulfurous acid (H2SO3), and in pure solutions this is
slowly oxidized to sulfuric acid by the oxygen from the air. In the
presence of catalysing impurities such as manganese or iron salts, it
is more rapidly converted (Freiberg, 1975, Johnstone & Coughanowr,
1958). Sulfur dioxide can also react either catalytically or
photochemically in the gas phase with other air pollutants to form
sulfur trioxide, sulfuric acid, and sulfates (see section 4).
Sulfur trioxide (SO3) is a highly reactive gas, and, in the
presence of moisture in the air, it is rapidly hydrated to sulfuric
acid. In the air, therefore, it is sulfuric acid in the form of an
aerosol that is found rather than sulfur trioxide, and, in general, it
is associated with other pollutants in droplets or solid particles
extending over a wide range of sizes (Waller, 1963). It can be emitted
into the atmosphere directly, or may result from the various reactions
mentioned earlier. Sulfuric acid may also be formed from the oxidation
of hydrogen sulfide in the air. The acid is strongly hygroscopic, and
droplets containing it readily take up further moisture from the air
until they are in equilibrium with their surroundings. If there is any
ammonia present, it will rapidly react with sulfuric acid to form
ammonium sulfate, which will continue to exist as an aerosol (in
droplet or crystalline form, depending on the relative humidity). The
sulfuric acid may react further with compounds in the air to produce
other sulfates. Some sulfate reaches the air directly, from combustion
sources or industrial emissions, and, in the proximity of oceans,
magnesium sulfate is present in the aerosol generated from ocean
spray.
A wide range of sulfur compounds is represented in the complex
mixture of urban air pollutants, but, from a practical point of view,
only the gas sulfur dioxide, and sulfuric acid and sulfates as
components of the suspended particulate matter need be considered.
2.1.2 Suspended particulate matter
The term suspended particulate matter covers a wide range of
finely divided solids or liquids that may be dispersed into the air
from combustion processes, industrial activities, or natural sources,
as discussed further in section 3. The composition of this material is
dependent upon the types of sources contributing to it, and the broad
definition is in terms of the settling velocity of the particles. For
ideal spherical particles, the velocity can be predicted from Stokes'
Law (Fuchs, 1964, see Table 1).
In the size range under about 10 µm, the settling velocity is
negligible compared with the movement produced by wind and air
turbulence, and such particles are liable to remain in suspension for
periods of the order of hours or days, until they are removed by
impaction or diffusion on to surfaces or are scavenged by rain. It is
these particles, with diameters ranging from well below 0.1 µm, up to
about 5 to 10 µm, that are referred to as suspended particulates, but
there is clearly no sharp dividing line between them and the larger
particles of deposited matter (or "dustfall") that are liable to fall
out rapidly, close to their source.
The suspended particulates are important in relation to health not
only because they persist in the atmosphere longer than larger
particles, but also because they are small enough to be inhaled and to
penetrate deeply into the respiratory tract, as discussed in section
6. They are also responsible for reduction in visibility, and take
part in reactions with other air pollutants.
Many of the particles in the air have complex shapes, as
illustrated in the electron micrograph (Fig. 1). Among the particles
shown are a number of "smoke aggregates", typical of the incomplete
combustion of hydrocarbon fuels, consisting of small spherical
particles of carbon or higher hydrocarbons having diameters of the
order of 0.05 µm, clustered Together in loose structures with overall
diameters up to several micrometres. From the point of view of their
behaviour during sampling or inhalation, such particles are classified
in terms of their equivalent aerodynamic diameters, i.e., the
diameters of unit density spheres having the same settling velocities.
Some truly spherical material may be present, mainly as aqueous
droplets containing dissolved salts, sulfuric acid, or occluded solid
particles. These cannot be examined directly under the electron
microscope, since the aqueous component evaporates completely, but
some residues can be seen in Fig. 1, and the rings of small droplets
indicate the presence of sulfuric acid (Waller et al., 1963). Many
other types of particles, including small flakes and fibres can be
Table 1. Settling velocities of spherical particles of unit density in still air
Diameter Settling
µm velocity, ms-1
^
|
Suspended particulate matter | 0.1 8 x 10-7
|
| 4 x 10-5
|
| | 10 3 x 10-3
| |
| | 100 0.25
|
Deposited matter v 1000 3.9
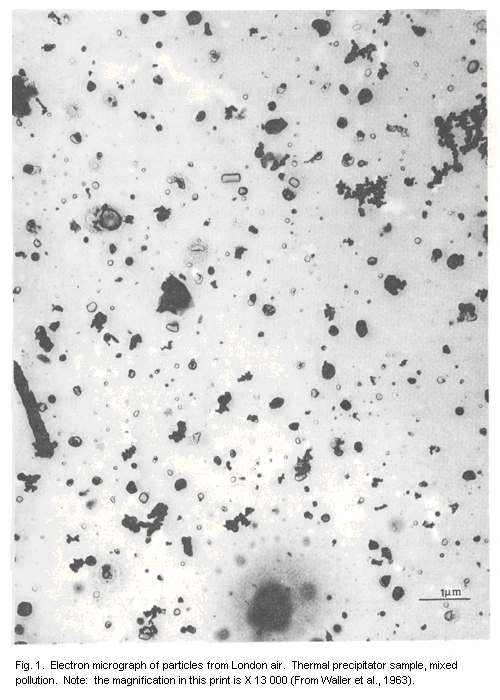 seen in Fig. 1. and a wide range of sizes, shapes, and densities is
commonly seen in all samples of suspended particulates in urban areas.
For routine monitoring purposes, it is clearly out of the question to
characterize the material completely in terms of size distribution and
composition, but it is important to recognize that the suspended
particulates generally comprise a heterogenous mixture, that can
differ greatly in its characteristics from one location to another,
and even from one occasion to another at any one site.
Estimates of size distribution can be obtained from electron
micrographs by considering the particles compressed into equivalent
spheres. The results are commonly plotted as cumulative frequency
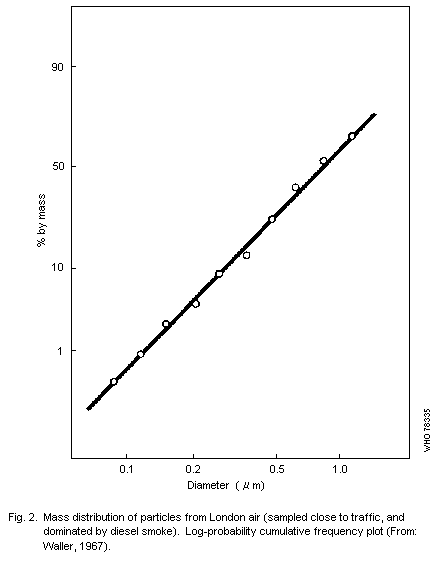
curves, and Fig. 2 shows results for a sample consisting largely of
smoke aggregates. In this the mass median diameter (MMD) is
approximately 1 µm, i.e., half the mass of material collected is
contained in particles having effective (aerodynamic) diameters under
one micrometer.a
The results shown in Fig. 2 indicate a log-normal distribution of
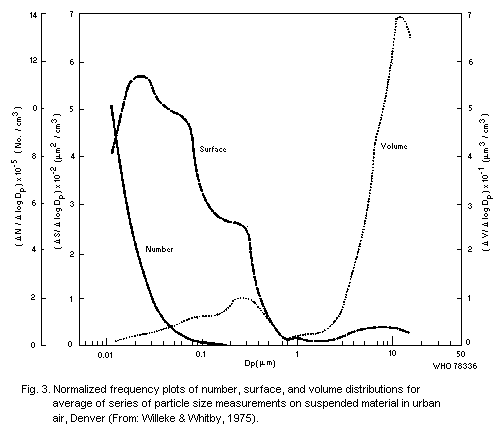
particle size in that specific sample, but Willeke & Whitby (1975)
have shown that distributions are often multimodal. These authors also
stressed the importance of examining the distribution in terms of
numbers of particles, and surface area, in addition to volume (or
mass), for each curve may reveal features not shown by the others. An
example of results obtained with their Minnesota Aerosol Analyzing
System is shown in Fig. 3. This shows a mode in the volume
distribution in the range 0.1 to 1 µm that is related to particles
formed by coagulation or condensation from smaller units, and a
further mode of the order of 10 µm that corresponds with
mechanically-produced particles, some of which are large enough to
settle rapidly, and are not, therefore, strictly part of the suspended
particulates.
The impression gained of size distributions will, however, always
depend on the characteristics of the instruments used. The most
extensive series of results that has been reported was based on a
modified Andersen cascade impactor (Lee & Goranson, 1972). This allows
samples to be collected in five, roughly size-graded fractions, with a
back-up filter as a sixth stage to collect the finest particles. Mass
median diameters have generally been found to be below 1 µm in samples
collected in urban areas of the USA, but this method cannot describe
the size distribution as completely as that of Willeke & Whitby
(1975).
a For further details concerning the definition of poly-dispersed
aerosols containing particles of irregular shape see, for example,
Fuchs (1964) or Task Group on Lung Dynamics (1966).
seen in Fig. 1. and a wide range of sizes, shapes, and densities is
commonly seen in all samples of suspended particulates in urban areas.
For routine monitoring purposes, it is clearly out of the question to
characterize the material completely in terms of size distribution and
composition, but it is important to recognize that the suspended
particulates generally comprise a heterogenous mixture, that can
differ greatly in its characteristics from one location to another,
and even from one occasion to another at any one site.
Estimates of size distribution can be obtained from electron
micrographs by considering the particles compressed into equivalent
spheres. The results are commonly plotted as cumulative frequency
curves, and Fig. 2 shows results for a sample consisting largely of
smoke aggregates. In this the mass median diameter (MMD) is
approximately 1 µm, i.e., half the mass of material collected is
contained in particles having effective (aerodynamic) diameters under
one micrometer.a
The results shown in Fig. 2 indicate a log-normal distribution of
particle size in that specific sample, but Willeke & Whitby (1975)
have shown that distributions are often multimodal. These authors also
stressed the importance of examining the distribution in terms of
numbers of particles, and surface area, in addition to volume (or
mass), for each curve may reveal features not shown by the others. An
example of results obtained with their Minnesota Aerosol Analyzing
System is shown in Fig. 3. This shows a mode in the volume
distribution in the range 0.1 to 1 µm that is related to particles
formed by coagulation or condensation from smaller units, and a
further mode of the order of 10 µm that corresponds with
mechanically-produced particles, some of which are large enough to
settle rapidly, and are not, therefore, strictly part of the suspended
particulates.
The impression gained of size distributions will, however, always
depend on the characteristics of the instruments used. The most
extensive series of results that has been reported was based on a
modified Andersen cascade impactor (Lee & Goranson, 1972). This allows
samples to be collected in five, roughly size-graded fractions, with a
back-up filter as a sixth stage to collect the finest particles. Mass
median diameters have generally been found to be below 1 µm in samples
collected in urban areas of the USA, but this method cannot describe
the size distribution as completely as that of Willeke & Whitby
(1975).
a For further details concerning the definition of poly-dispersed
aerosols containing particles of irregular shape see, for example,
Fuchs (1964) or Task Group on Lung Dynamics (1966).
 Although it is possible to investigate the composition of
individual particles to a certain extent, data on chemical composition
are usually derived from larger samples as collected for the
determination of the total mass of suspended particulates. Among the
principal components are carbon, tarry material (hydrocarbons, soluble
in organic solvents such as benzene), water soluble material (such as
ammonium sulfate), and insoluble ash (containing small amounts of
iron, lead, and a wide variety of other elements).
The proportions of these components vary widely, depending on the
types of sources in the locality. For example, the special feature of
the suspended particulate matter in the United Kingdom prior to the
implementation of the Clean Air Act was their high tar content, and
this was particularly evident in high pollution episodes (Table 2).
Table 2. Examples of analyses of suspended particulates sampled in London prior
to smoke control (high volume samples)a
Typical summer Typical winter High pollution
sample sample episode
(July 1955) (February 1955) (January 1956)
Total suspended
particulates (µg/m3) 97 485 5111
Components as %
of total:
Organic (tar) 7.5 19.1 45.7
Sulfate 11.3 9.0 5.8
Chloride 0.8 0.2 1.0
Nitrate 0.8 0.5 0.7
Iron 1.3 1.4 0.1
Lead 0.7 0.4 0.1
Zinc 0.5 0.2 0.8
a From: The Medical Research Council Air Pollution Unit, now. Clinical
Section of Medical Research Council Toxicology Unit
(unpublished data)
Results from the extensive series of analyses of high volume
samples of suspended particulate matter at sites in the USA indicate
organic contents of the order of 10% of the total particulates (US
Environmental Protection Agency, 1974a). Although little is known of
the influence of the composition of the suspended particulate matter
on effects on health, detailed analyses can be of value in identifying
sources and are relevant in the study of reactions between pollutants.
Thus iron and manganese, although only trace constituents, may be of
importance in catalysing the oxidation of sulfur dioxide to sulfuric
acid or sulfates. The lead content is usually related to pollution
from motor vehicles, and traces of vanadium that are present come
mainly from the combustion of residual oils. There may also be a
variety of substances from noncombustion sources, such as road dust,
material from the degradation of tyres, windblown soil, pollen, and
emissions from industrial processes such as cement manufacturing or
steel making.
The most important distinction to be made in relation to suspended
particulate matter at the present time, however, is neither the
precise size distribution, nor the detailed chemical composition, but
the very broad characterization that results from the different
methods of assessment that are in common use for routine monitoring
purposes. These are discussed further in section 2.2.3. In subsequent
sections, the term "smoke" has been used for observations of suspended
particulate matter based on its soiling properties, and "total
suspended particulates" for those based directly on weight. Since the
former is mainly influenced by incomplete combustion products from the
burning of fossil fuels, and is little affected by white or colourless
materials such as ammonium sulfate, it is clear that the two terms are
not interchangeable.
2.2 Methods of Sampling and Analysis
In general, the outdoor air has been sampled for sulfur oxides and
suspended particulate matter in relation to community exposures,
whereas indoor environments have been examined in connection with
occupational exposures.
The most commonly used methods have been described in detail in a
recently published manual (World Health Organization, 1976a) and their
application in monitoring networks has been discussed in a further
publication (World Health Organization, 1977). However, the bases of
these and some other methods, that have been used in reporting
concentrations of sulfur oxides and suspended particulates in the air,
are discussed below to provide a better understanding of the
measurements cited in epidemiological studies. It is important to
ensure that any measurements that are made are supervised by someone
competent in the field of air pollution monitoring, and the methods
used must be reported together with the results.
Table 3. Methods of analysis for sulfur dioxidea
Method Principle Comment
Pararosaniline Absorption of sulfur dioxide in solution of Uses simple apparatus, and suitable
methodb potassium tetrachloromercurate (TCM); for sampling periods ranging from
complex formed reacts with pararosaniline 30 min to 24 h; samples should be
and formaldehyde to produce a red-purple analysed soon after collection;
colour, determined colorimetrically (West & specific for sulfur dioxide, and
Gaeke, 1956). possible interference from oxides of
nitrogen and some metals can be
eliminated (Pate et al., 1965;
Scaringelli et al., 1967) Widely used
in USA.
Acidimetric Simple apparatus, often combined with smoke Absorption of sulfur dioxide in
methodb filter (see the Organization for Economic dilute hydrogen peroxide solution;
Cooperation and Development filter soiling the sulfuric acid formed is titrated
method in Table 5); suitable for sampling against standard alkali (Organization
periods of 24-h, or less in some circumstances for Economic Cooperation and
(e.g. high pollution episodes, or occupational Development, 1965).
environments).
Conductivity Sulfur dioxide is sampled in deionized water Simple apparatus, suitable for
measurements containing hydrogen peroxide where it is sampling periods of the order of
oxidized to sulfuric acid, as in the acidimetric 24-h, usually combined with a filter
method; increase in conductivity measured to remove particulate matter; less
with a conductivity bridge (Adams et al., 1971). reliable than acidimetric method,
and not widely used in manual
form, but the principle often used
in automatic instruments (Derrett
Table 3. (cont'd).
Method Principle Comment
Conductivity & Brown, 1978), applicable also to
measurements simple portable instruments for
cont'd. spot checks in urban or industrial
environments (Nash, 1964), and to
personal samplers for assessing
occupational exposures (Sherwood,
1969).
Detector tube Air is drawn through tubes containing silica Portable, and no power supply
measurements gel impregnated with indicator sensitive to required. Widely used for spot
sulfur dioxide; concentration assessed from the checks in occupational environments,
length of the stain (Ash & Lynch, 1972). or in other situations where
the concentrations may be high
(from about 3000 µg/m3 upwards).
Iodine Sulfur dioxide absorbed in a solution of iodine Applicable to occupational
method contained in a wash bottle with a fritted bubbler, environments, but not now widely used;
and solution titrated with thiosulfate (Elkins, the method has been modified for
1959). colorimetric assessment, providing
a basis for portable instruments for
survey use (Cummings & Redfearn,
1957).
Table 3. (cont'd).
Method Principle Comment
Automatic Based on conductivity, colorimetry, coulometry Particularly valuable for following
instruments flame photometry, or gas chromatography short-term variations in
(Hollowell et al., 1973). concentration, but difficult to
assess 24-h average concentrations,
unless linked with data processing
equipment; instruments expensive, and
must be under the control of
experienced operators.
Sulfation Sulfur compounds in the air react with an Simple and requires no power
rate exposed cylinder or plate covered with a paste supply; sampling period long (30
containing lead peroxide; sulfate formed is days); results expressed in
determined by precipitation with barium SO3/100cm2/day, indicating rate
chloride (British Standards Institution, 1969a). of reaction of sulfur compounds
with surfaces; not specific for sulfur
dioxide, does not indicate
concentrations in the air, and although
often quoted in epidemiological
studies, of little value for these.
a From: Pate et al., (1965)
b Methods that are described fully in the manual of the World Health Organization (1976a).
2.2.1 Sulfur dioxide
If sulfur dioxide were the only contaminant of the air and
providing the samples were of adequate size, each of the methods
mentioned in Table 3 would give comparable results, indicating the
true amount of sulfur dioxide. In normal urban environments, however,
other pollutants are always present and although the sampling
procedure can be arranged to minimize interference from particulate
matter by filtering the air first, errors can still arise due to the
presence of various gases and vapours. The choice of method depends on
many factors, including the averaging time required: 24-h sampling
periods are commonly used, and many of the methods are suitable for
this. For shorter periods the choice is more limited, and for detailed
information on short-term variations in concentration, instrumental
methods are required.
In occupational environments, the mixture of air pollutants may be
simple and more clearly defined than that in urban air. Sulfur dioxide
may be emitted from a specific process rather than from a variety of
combustion sources, and the air may then be relatively free from
interfering gases. Concentrations may also be much higher than those
encountered in urban air, allowing short sampling periods to be used,
and, since concentrations are liable to change rapidly, this may even
be essential. Also, concentrations may vary greatly over short
distances, depending on the proximity of the source of pollution; this
makes the assessment of exposure based on measurements at fixed sites
difficult. There may be a preference in these circumstances for
methods suitable for use with portable instruments.
2.2.2 Suspended sulfates and sulfuric acid
Most of the methods mentioned in Table 4 assess the total
water-soluble sulfates collected on filters as part of the suspended
particulates. In general, any sulfuric acid present is included with
this, and some of the material present as acid in the air may be
converted to neutral sulfate on the filter during sampling. There is
no completely satisfactory method for the determination of sulfuric
acid in the presence of other pollutants, but some procedures for
examining the acidic properties of suspended particulates have been
referred to. There is an urgent need for more research in this field.
No methods, other than those mentioned in Table 4, are, as yet,
sufficiently well established for widespread application in
epidemiological studies, but much research work is in progress.
Table 4. Methods of analysis for suspended sulfates and sulfuric acid
Method Principle Comment
Turbidimetric Sample collected on sulfate-free glass Samples normally collected
method fibre or other efficient filter: sulfate over 24-h periods by high
extracted and precipitated with barium volume sampler (see Table 5).
chloride, measuring the turbidity of the No distinction made between
suspension spectrophotometrically (US sulfates and sulfuric acid.
Environmental Protection Agency,
1974b).
Methylthymol Samples collected as in the turbidimetric This modification allows the
blue method method above and extract reacted with procedure to be automated,
barium chloride, but barium remaining comments as in the turbidimetric
in solution then reacts with methylthymol method apply.
blue; sulfate determined colorimetrically
by measurement of uncomplexed methylthymol
blue (US Environmental Protection Agency,
1974b).
The most recent trends are towards the application of more
sensitive techniques, such as X-ray fluorescence (Dzubay & Stevens,
1973), or the thermal conversion of sulfates, measuring the resulting
sulfur dioxide by flame photometry (Husar et al., 1975), or by the
pararosaniline method (Maddalone et al., 1975).
Approaches to the difficult problem of determining sulfuric acid
have been made by back-titrating a sodium tetraborate extract of
suspended particulates collected on a small filter paper (Commins,
1963), and by observing the acidic properties of individual particles
collected on indicator-treated slides in a cascade impactor (Waller,
1963). A procedure for the separate determination of sulfuric acid and
ammonium sulfate by nephelometry of a humidified sample of air has
also been described (Charlson et al., 1973) and work is in progress on
the prevention of the reaction of sulfuric acid on filter papers
(Thomas et al., 1976). However, at present, there is not enough field
experience with methods for sulfuric acid to warrant their general use
in connexion with epidemiological studies.
2.2.3 Suspended particulate matter
In general, it is not practicable to discriminate on the basis of
either particle size or chemical composition when assessing
particulate matter for routine monitoring purposes. The
characteristics of the sample are determined by the types of sources
in the vicinity, the weather conditions, and the sampling procedure
adopted. The difficulties that result and the limitations of
measurements have been discussed by Ellison (1965)and are illustrated
in the discussion of the merits and shortcomings of the various
methods described below and in Table 5.
When considering measurements of suspended particulate matter, it
is essential to specify the method used and to recognize that, even
then, results obtained in one set of circumstances will not
necessarily be applicable to others. The main difficulty has arisen in
attempts to apply findings based on smoke measurements that relate
only to the dark coloured material characteristic of the incomplete
combustion of coal or other hydrocarbon fuels, to situations involving
total suspended particulates assessed more directly in terms of
weight. Because the former have been used in much of the early
epidemiological work and the latter are now used for monitoring
purposes in many countries, some kind of conversion from one type of
measurement to the other would be desirable, but, for the reasons
already stated, there can be no generally applicable conversion
factor. Comparative evaluation of the two methods has been undertaken
at a number of sites (Ball & Hume, 1977; Commins & Waller, 1967; Lee
et al., 1972), but the results have only served to emphasize that they
measure different qualities of the particulate matter and that they
should not be compared with one another.
Table 5. Methods of analysis for suspended particulate matter
Method Principle Comment
Smoke measurement: Air is drawn through a white filter paper, Widely used in Europe and recommended
Organization for usually over periods of 24 h, and the darkness by the Organization for Economic
Economic Cooperation of the stain obtained measured by reflectometer; Cooperation and Development (1965);
and Development filter values converted to equivalent international low intake velocity ensures sample
soiling method smoke units, expressed conventionally, in restricted to respirable size range; often
µg/m3; simple apparatus, suitable for combined with sulfur dioxide measurement
continuous operation. by acidimetric method (see Table
2-3); results influenced primarily by
black material do not necessarily
represent true weights; only a limited
range of chemical analyses possible on
these small samples.
Smoke measurement: Similar to the Organization for Economic Flow rate a little greater than in the
American Society Cooperation and Development filter soiling Organization for Economic Cooperation
for Testing and method, but samples collected on a filter and Development filter soiling method
Materials filter paper tape moved on automatically to provide but sample still effectively within
soiling method a series of stains over intervals of 2-6 h respirable size range used in USA;
(American Society for Testing and Materials, interrelationships between Coh units and
1964); results usually assessed by transmittance, RUDS investigated by Saucier & Sansone
and expressed in coefficient of haze (COH) (1972); suitable for continuous
units (Hemeon et al., 1953; reflectance operation.
has sometimes been used, expressing results
in reflectance units of dirt shade (RUDS)
(Gruber & Alpaugh, 1954).
Table 5. (cont'd).
Method Principle Comment
Determination of Air drawn through a glass fibre filter sheet, Widely used in USA. Liable to collect
total suspended usually with a turbine blower, and the amount particles well beyond the respiratory size
particulates, of material collected determined by weighing range and this may bias results,
gravimetric high under controlled temperature and humidity particularly in dry, dusty locations;
volume conditions; the most widely used instrument not very suitable for continuous
is the high volume sampler (US Department operation, samples commonly collected
of Health, Education and Welfare, 1962), but over 24-h periods every sixth day;
instruments based on rotary pumps with samples large enough for a wide range
membrane rather than glass fibre filters have of chemical analyses.
been used (Verein Deutscher Ingenieure,
1974).
Indirect determination Series of samples collected on filter paper Instrument relatively expensive; used for
of mass concentration: strip over selected periods (usually 30 min), monitoring purposes in Federal Republic
ß ray sampler and mass of material determined by of Germany, but not to any large extent
attenuation of ß radiation from a built-in elsewhere; valuable for studying
source (Husar, 1974). short-term variations in total
suspended particulates.
Light scattering Direct determination of suspended particulate Used to some extent in Japan for monitoring
matter as aerosols by light scattering, either suspended particulate matter, but
counting and sizing individual particles (Liu et calibration required and results not
al., 1974) or integrating light scattered from necessarily comparable with those from
given volume of air (Horvath & Charlson, direct weighings; otherwise main
1969). application in industrial environments,
some instruments allow particles to be
counted and classified within a large
number of size ranges.
Table 5. (cont'd).
Method Principle Comment
Size selective Particles separated into several roughly size- Allows concentrations to be assessed
sampling: graded fractions by impaction, the amount of within specified size ranges; some series
modified cascade material in each being determined by direct of results available from USA but not
impactor weighing (Carson & Paulus, 1974). yet widely adopted; applicable also to
the sampling of dusts in industrial
environments.
Electrostatic Particles charged by passing through metal Not suitable for outdoor measurements,
precipitators tube with large potential gradient between but useful in occupational environments;
wall and needle along centre; deposited on advantage over direct weighing of filters
wall and determined by direct weighing is that the collector is unaffected by
(Lauterbach et al., 1954). changes in humidity.
Personal samplers Air drawn through small glass-fibre filters Applicable primarily to industrial
using battery operated pump, so that environments to assess exposures in
instrument can be worn by individuals series of working shifts; elutriator can be
(Sherwood & Greenhalgh, 1960); particulates added to exclude large particles.
assessed by weighing, or analysed for specific
constituents.
From their study in central London, Commins & Waller (1967) showed
that additional material was collected by the high volume sampler that
had little effect on smoke measurements and that for their particular
series, the total suspended particulate results were approximately
100 µg/m3 higher than the corresponding smoke figures. Other authors
have calculated regression equations for their series and, although
there are variations in these relationships with time and place, the
general picture is of a large proportional difference between total
suspended particulate and smoke figures at low values, but relatively
little difference at high values (of the order of 500 µg of smoke/m3
or more).
Thus, it is recommended that "smoke" and "total suspended
particulate" measured by the various methods described should be
regarded as separate entities; this principle has been adopted in
later sections relating to the effects on health.
In occupational environments, suspended particulate matter from
combustion sources may be of some concern, but more commonly it is the
dusts and aerosols associated with particular occupations or processes
that are of interest. In such cases, the composition of the material
may be relatively uniform and well established, and specific methods
of assessment can be devised. There has, for example, been a great
deal of research and development on methods for determining dust
concentrations in coal mines (Jacobsen, 1972). With industrial dusts,
the particle size distribution must always be considered carefully,
for it is liable to extend beyond the respirable range, and
elutriators or cyclones may be needed in conjunction with gravimetric
samplers. A valuable discussion of methods for collecting size-graded
samples has been included in a recent review (International Atomic
Energy Agency, 1978).
2.2.4 Dustfall (deposited matter)
In some of the older epidemiological studies, measurements of
dustfall were quoted as an index of particulate pollution. This is
inappropriate as the results are influenced primarily by large
particles (diameters from about 10 µm upwards: see section 2.1.2) that
do not penetrate the respiratory system and, generally, are not
relevant to health problems, apart from possible annoyance reactions.
For reference purposes, however, a brief description of one of the
more commonly used instruments is included (Table 6).
Table 6. Method of analysis for dust fall
Method Principle Comment
Deposit A receiver containing a Results expressed in terms
gauge nonfreezing solution is left in of deposit per unit area and time,
the open and the quantity of not convertible in any way to
material collected (usually concentrations of suspended matter
over 1-month periods) is determined in the air; strongly influenced by
by weighing, water-soluble and sources nearby, hence results only
insoluble components being considered relevant to immediate vicinity
separately (British Standards
Institution, 1969b)
3. SOURCES OF SULFUR OXIDES AND PARTICULATE MATTER
3.1 Natural Occurrence
Compounds of sulfur are found in small quantities in ambient air,
even in remote areas far from sources of pollution. In the gas phase,
they are present as hydrogen sulfide or sulfur dioxide, and in
particulate form they may be present as sulfate. Sulfur dioxide and
hydrogen sulfide are emitted by volcanoes and the latter is also
produced by anaerobic bacteria in soil, marshes, and tidal flats (Grey
& Jensen, 1972). Some of the particulate sulfate may also be emitted
directly by volcanoes or sea spray, but most of it is the end-product
of the oxidation of hydrogen sulfide or sulfur dioxide.
In general, suspended particulate matter can result from volcanic
activity, from dust storms, or from strong winds blowing over dry soil
and may include pollen from trees and other plants. Forest fires also
produce large amounts of particulate matter.
Some of these natural contributions to the particulate matter in
the air consist of particles too large to remain in suspension for
long periods, and their composition and properties may be quite
different from those of the emissions from man's activities.
3.2 Man-made sources
Most emissions of sulfur into the air are in the form of sulfur
dioxide resulting from the combustion of fossil fuel for heating and
energy production. Various industrial activities such as petroleum
processing, smelter operations, wood-pulping, etc., also produce
significant emissions of sulfur dioxide and other sulfur compounds.
It has been estimated (Robinson & Robbins, 1968) that on a
worldwide scale about 146 × 106 tonnes of sulfur dioxide are emitted
annually, 70% of which result from coal burning, 16% from the
combustion of petroleum products, and the remainder from petroleum
refining and nonferrous smelting. These estimates are based mainly on
1965 world figures for coal production, petroleum refining, and
smelter operations, each combined with an estimate of a sulfur dioxide
"emission factor" per unit of production. A similar basis was used for
the Committee on the Challenges of Modern Society (1971) assessment of
emissions in the northern and southern hemispheres, reproduced in
Table 7.
Table 7. Hemispheric sulfur dioxide emmissions due to man's
activities (106 tonnes per year)a
Source Total Northern Southern
Hemisphere Hemisphere
Coal 102 98 (96%) 4 (4%)
Petroleum,
combustion
and refining 28.5 27.1 (95%) 1.4 (5%)
Smelting, copper 12.9 8.6 (67%) 4.3 (33%)
lead 1.5 1.2 (80%) 0.3 (20%)
zinc 1.3 1.2 (90%) 0.1 (10%)
Total 146 136 (93%) 10 (7%)
a From: Committee on the Challenges of Modern Society (1971).
On a global scale, the emissions of sulfur compounds into the
atmosphere by man-made activities are about equal to those from
natural sources. On the other hand, the emissions from man's
activities are the main contributors to pollution in large cities and
their surrounding areas. Assuming that world energy demand increases
at its historic rate, the total emissions of sulfur dioxide will
increase unless appropriate control measures are applied, or there
is a shift from the use of fossil fuels to the use of nonpolluting
energy sources. However, with the stabilization of the population in
some countries, including the United Kingdom and USA, and increasing
concern about the use of limited fuel reserves, there are prospects
that the rate of increase in emissions may be reduced in some parts
of the world.
Combustion and industrial processes are also prime sources of
particulate emissions. As with sulfur dioxide, the burning of fuel
(especially coal) for heating and for the generation of power has been
one of the major contributors to the suspended particulate matter in
urban air. Vehicular traffic also generates dust from the road and
from the wear of tyres as well as particulate lead compounds from the
exhausts of petrol-engined vehicles, and black smoke from those of
diesel vehicles. The incineration of domestic and industrial refuse
may disperse particulate matter and other pollutants into the air
unless carefully controlled. Table 8 shows estimates of the global
emission of all particulate matter (Robinson & Robbins, 1968).
Table 8. Global emission of all particulate matter (106 tonnes
per year)a
Man-made
Particles 92
Gas-particle conversion: sulfur dioxide 147
oxides of nitrogen 30
Photochemical compounds from hydrocarbons 27
296
Natural
Soil dust 200
Gas-particle conversion: hydrogen-sulfide 204
oxides of nitrogen 432
ammonia 269
Photochemical compounds from terpenes, etc 200
Volcanic 4
Forest fires 3
Sea salt 1000
2312
a From: Robinson & Robbins (1968).
3.3 Characteristics of Sources
In urban areas, most of the sulfur dioxide and suspended
particulate matter in the air come from the combustion of fuels, but
many factors, including the type of fuel, the combustion efficiency,
and the flue velocity, influence the quantity and quality of
emissions. The incomplete combustion of soft coal in domestic fires,
for example, produces much smoke, consisting of finely divided
particles of carbon and tarry material, whereas the efficient burning
of pulverised coal in a power station leads to little or no smoke, but
the production of coarser ash particles, which must be removed at
source to avoid their being carried up the flues at a high velocity
and dispersed into the air. The relationship between types of sources
and emissions is summarized in Table 9.
Table 9. Pollutants from combustion sources
Type of source Fuel Sulfur dioxide Particulate matter
Smoke Ash etc.
Domestic heating Wood, peat etc. - + +
or cooking Soft coal ++ ++ +
Hard coal, coke ++ - +
Oil (light distillates) + - -
Gas - - -
Industrial heating Coal, coke ++ - ++
and power generation Oil (heavy residuals) ++ - -
Motor vehicles Petrol - - +
Diesel + + -
Notes: The term smoke is used for incomplete combustion products (notably carbon and tar),
and ash for inorganic components from complete combustion (including lead compounds
in the case of petrol). The signs give only a rough indication of emissions, in the
absence of direct control at source:
- = little or none, + = moderate quantities, ++ = large quantities
In cold and temperate parts of the world, the burning of coal for
domestic heating purposes has been a major contributor to both the
sulfur dioxide and suspended particulate contents of urban air. This
is particularly true of the situation in the United Kingdom prior to
the implementation of the Clean Air Act (Committee on Air Pollution,
1954). Such sources are liable to have a disproportionate effect on
concentrations in the immediate vicinity, because of the low levels of
the chimneys and the low emission velocity. Even in warmer climates,
domestic sources may be of importance, particularly if coal is used
for cooking purposes.
In densely populated areas where domestic sources dominate, the
many chimneys can be considered for some purposes as a diffuse area
source, and, within such an area, concentrations of pollutants in air
remain relatively stable over short distances and short periods of
time. In contrast, large industrial sources should be treated as point
sources, and, at any given location around them, the concentration of
air pollution is liable to vary greatly, even from minute to minute.
The emission into the air of sulfur dioxide and particulate matter
from motor vehicles is relatively small in comparison with those from
domestic and industrial chimneys but it is close to the ground and
within the breathing zone. In these circumstances, concentrations vary
greatly over short distances as well as over short time intervals,
depending on the proximity of the traffic. At points very close to
mixed traffic, smoke from diesel engines may make a substantial
contribution to the concentration of suspended particulate matter in
air (Waller et al., 1965).
Source strength may vary with time of day, day of week, and season
of the year. Accompanying meteorological conditions are also important
in determining the ultimate air concentrations of pollutants arising
from sources. Where heating is required during the winter season,
emissions of sulfur dioxide and particulate matter are usually much
higher than they are in the summer. In a number of cities, however,
where a considerable amount of fuel is used for running cooling
systems, emissions of these pollutants during the summer time are not
always lower than those in winter. Some industrial sources of
pollution may emit little at weekends, and emissions for most sources
are at a minimum during the night.
Although the control of emissions is outside the scope of the
present discussion, the general point can be made that while the
control of particulate emissions is a practicable proposition in many
circumstances, the control of sulfur dioxide at source is relatively
difficult and costly, and the more effective means of reducing
emissions is to change to fuels with a lower sulfur content.
4. DISPERSION AND ENVIRONMENTAL TRANSFORMATIONS
Sulfur compounds dispersed into the air eventually return to the
land or oceans either unchanged, or converted into sulfates. The
sulfur cycle so set up is shown diagrammatically in Fig. 4 (Kellogg et
al., 1972). Particulate matter also returns, its residence time in the
air varying widely according to its physical and chemical
characteristics. As far as direct effects on health are concerned, it
is the local concentration of pollutants in the air at a given time
that is important, as discussed in section 5, but an outline of
dispersion and transformation phenomena is given here.
4.1 Dispersion
The maintenance of a tolerable environment in modern towns depends
very much on the ability of wind and turbulence to disperse the
pollutants rapidly as they are emitted. When these processes fail, the
results can be disastrous, as they were in London in 1952 (Ministry of
Health, United Kingdom, 1954). There are some localities where natural
ventilation is so poor that the emission of pollutants must, at all
times, be carefully controlled. This is especially true of the Los
Angeles area, where emissions of sulfur dioxide and particulate matter
have been successfully curtailed, leaving, however, the major problems
associated with the emission of oxides of nitrogen, hydrocarbons, and
carbon monoxide from motor vehicles (Goldsmith, 1969).
Factors affecting the dispersion of sulfur dioxide and particulate
matter from combustion sources, include:
(a) Temperature and efflux velocity of the gases. Emissions from
small sources, such as domestic fires or incinerators have relatively
little buoyancy, since the temperature at the point of emission is not
much greater than that of the surrounding air. Such sources are liable
therefore to have their greatest impact in the immediate vicinity
(Williams, 1960). Emissions from large-scale industrial installations,
on the other hand, may be at higher temperatures, or may be assisted
by forced-draught to rise more rapidly. Thus, any major impact in the
immediate vicinity may be avoided but weaker effects may be produced
over a wider area (Bosanquet, 1957).
(b) Stack height. Dilution and dispersion over a wide area is
also aided by the use of tall stacks. Much is known of the
relationship between source strengths, stack heights, and ground-level
concentrations of pollutants, through the application of mathematical
modelling techniques, coupled with observations around selected
sources (Briggs, 1965, Pasquill, 1971, Turner, 1968). It is also
possible to devise models to predict concentrations of sulfur dioxide
in urban areas on the basis of emissions from multiple sources
(Fortak, 1970). Conversely, techniques have also been developed for
estimating the pollution inventory of an area from measurements of
sulfur dioxide concentration, fitted to a dispersion model (East,
1972). The height of emission of sulfur dioxide and particulate matter
from domestic sources is primarily a function of the height of the
building itself. Thus the effect on ground level concentrations in the
vicinity is liable to be greater in areas with closely-packed, single
or two-storey houses than in those with high-rise apartments. Tall
stacks are widely used for electricity-generating stations and other
major industrial sources, but the pollutants may then be carried great
distances, often over national boundaries, to be deposited eventually
far from their source (Royal Ministry of Foreign Affairs, Sweden,
1971; Zeeduk & Velds, 1973).
Although it is possible to investigate the composition of
individual particles to a certain extent, data on chemical composition
are usually derived from larger samples as collected for the
determination of the total mass of suspended particulates. Among the
principal components are carbon, tarry material (hydrocarbons, soluble
in organic solvents such as benzene), water soluble material (such as
ammonium sulfate), and insoluble ash (containing small amounts of
iron, lead, and a wide variety of other elements).
The proportions of these components vary widely, depending on the
types of sources in the locality. For example, the special feature of
the suspended particulate matter in the United Kingdom prior to the
implementation of the Clean Air Act was their high tar content, and
this was particularly evident in high pollution episodes (Table 2).
Table 2. Examples of analyses of suspended particulates sampled in London prior
to smoke control (high volume samples)a
Typical summer Typical winter High pollution
sample sample episode
(July 1955) (February 1955) (January 1956)
Total suspended
particulates (µg/m3) 97 485 5111
Components as %
of total:
Organic (tar) 7.5 19.1 45.7
Sulfate 11.3 9.0 5.8
Chloride 0.8 0.2 1.0
Nitrate 0.8 0.5 0.7
Iron 1.3 1.4 0.1
Lead 0.7 0.4 0.1
Zinc 0.5 0.2 0.8
a From: The Medical Research Council Air Pollution Unit, now. Clinical
Section of Medical Research Council Toxicology Unit
(unpublished data)
Results from the extensive series of analyses of high volume
samples of suspended particulate matter at sites in the USA indicate
organic contents of the order of 10% of the total particulates (US
Environmental Protection Agency, 1974a). Although little is known of
the influence of the composition of the suspended particulate matter
on effects on health, detailed analyses can be of value in identifying
sources and are relevant in the study of reactions between pollutants.
Thus iron and manganese, although only trace constituents, may be of
importance in catalysing the oxidation of sulfur dioxide to sulfuric
acid or sulfates. The lead content is usually related to pollution
from motor vehicles, and traces of vanadium that are present come
mainly from the combustion of residual oils. There may also be a
variety of substances from noncombustion sources, such as road dust,
material from the degradation of tyres, windblown soil, pollen, and
emissions from industrial processes such as cement manufacturing or
steel making.
The most important distinction to be made in relation to suspended
particulate matter at the present time, however, is neither the
precise size distribution, nor the detailed chemical composition, but
the very broad characterization that results from the different
methods of assessment that are in common use for routine monitoring
purposes. These are discussed further in section 2.2.3. In subsequent
sections, the term "smoke" has been used for observations of suspended
particulate matter based on its soiling properties, and "total
suspended particulates" for those based directly on weight. Since the
former is mainly influenced by incomplete combustion products from the
burning of fossil fuels, and is little affected by white or colourless
materials such as ammonium sulfate, it is clear that the two terms are
not interchangeable.
2.2 Methods of Sampling and Analysis
In general, the outdoor air has been sampled for sulfur oxides and
suspended particulate matter in relation to community exposures,
whereas indoor environments have been examined in connection with
occupational exposures.
The most commonly used methods have been described in detail in a
recently published manual (World Health Organization, 1976a) and their
application in monitoring networks has been discussed in a further
publication (World Health Organization, 1977). However, the bases of
these and some other methods, that have been used in reporting
concentrations of sulfur oxides and suspended particulates in the air,
are discussed below to provide a better understanding of the
measurements cited in epidemiological studies. It is important to
ensure that any measurements that are made are supervised by someone
competent in the field of air pollution monitoring, and the methods
used must be reported together with the results.
Table 3. Methods of analysis for sulfur dioxidea
Method Principle Comment
Pararosaniline Absorption of sulfur dioxide in solution of Uses simple apparatus, and suitable
methodb potassium tetrachloromercurate (TCM); for sampling periods ranging from
complex formed reacts with pararosaniline 30 min to 24 h; samples should be
and formaldehyde to produce a red-purple analysed soon after collection;
colour, determined colorimetrically (West & specific for sulfur dioxide, and
Gaeke, 1956). possible interference from oxides of
nitrogen and some metals can be
eliminated (Pate et al., 1965;
Scaringelli et al., 1967) Widely used
in USA.
Acidimetric Simple apparatus, often combined with smoke Absorption of sulfur dioxide in
methodb filter (see the Organization for Economic dilute hydrogen peroxide solution;
Cooperation and Development filter soiling the sulfuric acid formed is titrated
method in Table 5); suitable for sampling against standard alkali (Organization
periods of 24-h, or less in some circumstances for Economic Cooperation and
(e.g. high pollution episodes, or occupational Development, 1965).
environments).
Conductivity Sulfur dioxide is sampled in deionized water Simple apparatus, suitable for
measurements containing hydrogen peroxide where it is sampling periods of the order of
oxidized to sulfuric acid, as in the acidimetric 24-h, usually combined with a filter
method; increase in conductivity measured to remove particulate matter; less
with a conductivity bridge (Adams et al., 1971). reliable than acidimetric method,
and not widely used in manual
form, but the principle often used
in automatic instruments (Derrett
Table 3. (cont'd).
Method Principle Comment
Conductivity & Brown, 1978), applicable also to
measurements simple portable instruments for
cont'd. spot checks in urban or industrial
environments (Nash, 1964), and to
personal samplers for assessing
occupational exposures (Sherwood,
1969).
Detector tube Air is drawn through tubes containing silica Portable, and no power supply
measurements gel impregnated with indicator sensitive to required. Widely used for spot
sulfur dioxide; concentration assessed from the checks in occupational environments,
length of the stain (Ash & Lynch, 1972). or in other situations where
the concentrations may be high
(from about 3000 µg/m3 upwards).
Iodine Sulfur dioxide absorbed in a solution of iodine Applicable to occupational
method contained in a wash bottle with a fritted bubbler, environments, but not now widely used;
and solution titrated with thiosulfate (Elkins, the method has been modified for
1959). colorimetric assessment, providing
a basis for portable instruments for
survey use (Cummings & Redfearn,
1957).
Table 3. (cont'd).
Method Principle Comment
Automatic Based on conductivity, colorimetry, coulometry Particularly valuable for following
instruments flame photometry, or gas chromatography short-term variations in
(Hollowell et al., 1973). concentration, but difficult to
assess 24-h average concentrations,
unless linked with data processing
equipment; instruments expensive, and
must be under the control of
experienced operators.
Sulfation Sulfur compounds in the air react with an Simple and requires no power
rate exposed cylinder or plate covered with a paste supply; sampling period long (30
containing lead peroxide; sulfate formed is days); results expressed in
determined by precipitation with barium SO3/100cm2/day, indicating rate
chloride (British Standards Institution, 1969a). of reaction of sulfur compounds
with surfaces; not specific for sulfur
dioxide, does not indicate
concentrations in the air, and although
often quoted in epidemiological
studies, of little value for these.
a From: Pate et al., (1965)
b Methods that are described fully in the manual of the World Health Organization (1976a).
2.2.1 Sulfur dioxide
If sulfur dioxide were the only contaminant of the air and
providing the samples were of adequate size, each of the methods
mentioned in Table 3 would give comparable results, indicating the
true amount of sulfur dioxide. In normal urban environments, however,
other pollutants are always present and although the sampling
procedure can be arranged to minimize interference from particulate
matter by filtering the air first, errors can still arise due to the
presence of various gases and vapours. The choice of method depends on
many factors, including the averaging time required: 24-h sampling
periods are commonly used, and many of the methods are suitable for
this. For shorter periods the choice is more limited, and for detailed
information on short-term variations in concentration, instrumental
methods are required.
In occupational environments, the mixture of air pollutants may be
simple and more clearly defined than that in urban air. Sulfur dioxide
may be emitted from a specific process rather than from a variety of
combustion sources, and the air may then be relatively free from
interfering gases. Concentrations may also be much higher than those
encountered in urban air, allowing short sampling periods to be used,
and, since concentrations are liable to change rapidly, this may even
be essential. Also, concentrations may vary greatly over short
distances, depending on the proximity of the source of pollution; this
makes the assessment of exposure based on measurements at fixed sites
difficult. There may be a preference in these circumstances for
methods suitable for use with portable instruments.
2.2.2 Suspended sulfates and sulfuric acid
Most of the methods mentioned in Table 4 assess the total
water-soluble sulfates collected on filters as part of the suspended
particulates. In general, any sulfuric acid present is included with
this, and some of the material present as acid in the air may be
converted to neutral sulfate on the filter during sampling. There is
no completely satisfactory method for the determination of sulfuric
acid in the presence of other pollutants, but some procedures for
examining the acidic properties of suspended particulates have been
referred to. There is an urgent need for more research in this field.
No methods, other than those mentioned in Table 4, are, as yet,
sufficiently well established for widespread application in
epidemiological studies, but much research work is in progress.
Table 4. Methods of analysis for suspended sulfates and sulfuric acid
Method Principle Comment
Turbidimetric Sample collected on sulfate-free glass Samples normally collected
method fibre or other efficient filter: sulfate over 24-h periods by high
extracted and precipitated with barium volume sampler (see Table 5).
chloride, measuring the turbidity of the No distinction made between
suspension spectrophotometrically (US sulfates and sulfuric acid.
Environmental Protection Agency,
1974b).
Methylthymol Samples collected as in the turbidimetric This modification allows the
blue method method above and extract reacted with procedure to be automated,
barium chloride, but barium remaining comments as in the turbidimetric
in solution then reacts with methylthymol method apply.
blue; sulfate determined colorimetrically
by measurement of uncomplexed methylthymol
blue (US Environmental Protection Agency,
1974b).
The most recent trends are towards the application of more
sensitive techniques, such as X-ray fluorescence (Dzubay & Stevens,
1973), or the thermal conversion of sulfates, measuring the resulting
sulfur dioxide by flame photometry (Husar et al., 1975), or by the
pararosaniline method (Maddalone et al., 1975).
Approaches to the difficult problem of determining sulfuric acid
have been made by back-titrating a sodium tetraborate extract of
suspended particulates collected on a small filter paper (Commins,
1963), and by observing the acidic properties of individual particles
collected on indicator-treated slides in a cascade impactor (Waller,
1963). A procedure for the separate determination of sulfuric acid and
ammonium sulfate by nephelometry of a humidified sample of air has
also been described (Charlson et al., 1973) and work is in progress on
the prevention of the reaction of sulfuric acid on filter papers
(Thomas et al., 1976). However, at present, there is not enough field
experience with methods for sulfuric acid to warrant their general use
in connexion with epidemiological studies.
2.2.3 Suspended particulate matter
In general, it is not practicable to discriminate on the basis of
either particle size or chemical composition when assessing
particulate matter for routine monitoring purposes. The
characteristics of the sample are determined by the types of sources
in the vicinity, the weather conditions, and the sampling procedure
adopted. The difficulties that result and the limitations of
measurements have been discussed by Ellison (1965)and are illustrated
in the discussion of the merits and shortcomings of the various
methods described below and in Table 5.
When considering measurements of suspended particulate matter, it
is essential to specify the method used and to recognize that, even
then, results obtained in one set of circumstances will not
necessarily be applicable to others. The main difficulty has arisen in
attempts to apply findings based on smoke measurements that relate
only to the dark coloured material characteristic of the incomplete
combustion of coal or other hydrocarbon fuels, to situations involving
total suspended particulates assessed more directly in terms of
weight. Because the former have been used in much of the early
epidemiological work and the latter are now used for monitoring
purposes in many countries, some kind of conversion from one type of
measurement to the other would be desirable, but, for the reasons
already stated, there can be no generally applicable conversion
factor. Comparative evaluation of the two methods has been undertaken
at a number of sites (Ball & Hume, 1977; Commins & Waller, 1967; Lee
et al., 1972), but the results have only served to emphasize that they
measure different qualities of the particulate matter and that they
should not be compared with one another.
Table 5. Methods of analysis for suspended particulate matter
Method Principle Comment
Smoke measurement: Air is drawn through a white filter paper, Widely used in Europe and recommended
Organization for usually over periods of 24 h, and the darkness by the Organization for Economic
Economic Cooperation of the stain obtained measured by reflectometer; Cooperation and Development (1965);
and Development filter values converted to equivalent international low intake velocity ensures sample
soiling method smoke units, expressed conventionally, in restricted to respirable size range; often
µg/m3; simple apparatus, suitable for combined with sulfur dioxide measurement
continuous operation. by acidimetric method (see Table
2-3); results influenced primarily by
black material do not necessarily
represent true weights; only a limited
range of chemical analyses possible on
these small samples.
Smoke measurement: Similar to the Organization for Economic Flow rate a little greater than in the
American Society Cooperation and Development filter soiling Organization for Economic Cooperation
for Testing and method, but samples collected on a filter and Development filter soiling method
Materials filter paper tape moved on automatically to provide but sample still effectively within
soiling method a series of stains over intervals of 2-6 h respirable size range used in USA;
(American Society for Testing and Materials, interrelationships between Coh units and
1964); results usually assessed by transmittance, RUDS investigated by Saucier & Sansone
and expressed in coefficient of haze (COH) (1972); suitable for continuous
units (Hemeon et al., 1953; reflectance operation.
has sometimes been used, expressing results
in reflectance units of dirt shade (RUDS)
(Gruber & Alpaugh, 1954).
Table 5. (cont'd).
Method Principle Comment
Determination of Air drawn through a glass fibre filter sheet, Widely used in USA. Liable to collect
total suspended usually with a turbine blower, and the amount particles well beyond the respiratory size
particulates, of material collected determined by weighing range and this may bias results,
gravimetric high under controlled temperature and humidity particularly in dry, dusty locations;
volume conditions; the most widely used instrument not very suitable for continuous
is the high volume sampler (US Department operation, samples commonly collected
of Health, Education and Welfare, 1962), but over 24-h periods every sixth day;
instruments based on rotary pumps with samples large enough for a wide range
membrane rather than glass fibre filters have of chemical analyses.
been used (Verein Deutscher Ingenieure,
1974).
Indirect determination Series of samples collected on filter paper Instrument relatively expensive; used for
of mass concentration: strip over selected periods (usually 30 min), monitoring purposes in Federal Republic
ß ray sampler and mass of material determined by of Germany, but not to any large extent
attenuation of ß radiation from a built-in elsewhere; valuable for studying
source (Husar, 1974). short-term variations in total
suspended particulates.
Light scattering Direct determination of suspended particulate Used to some extent in Japan for monitoring
matter as aerosols by light scattering, either suspended particulate matter, but
counting and sizing individual particles (Liu et calibration required and results not
al., 1974) or integrating light scattered from necessarily comparable with those from
given volume of air (Horvath & Charlson, direct weighings; otherwise main
1969). application in industrial environments,
some instruments allow particles to be
counted and classified within a large
number of size ranges.
Table 5. (cont'd).
Method Principle Comment
Size selective Particles separated into several roughly size- Allows concentrations to be assessed
sampling: graded fractions by impaction, the amount of within specified size ranges; some series
modified cascade material in each being determined by direct of results available from USA but not
impactor weighing (Carson & Paulus, 1974). yet widely adopted; applicable also to
the sampling of dusts in industrial
environments.
Electrostatic Particles charged by passing through metal Not suitable for outdoor measurements,
precipitators tube with large potential gradient between but useful in occupational environments;
wall and needle along centre; deposited on advantage over direct weighing of filters
wall and determined by direct weighing is that the collector is unaffected by
(Lauterbach et al., 1954). changes in humidity.
Personal samplers Air drawn through small glass-fibre filters Applicable primarily to industrial
using battery operated pump, so that environments to assess exposures in
instrument can be worn by individuals series of working shifts; elutriator can be
(Sherwood & Greenhalgh, 1960); particulates added to exclude large particles.
assessed by weighing, or analysed for specific
constituents.
From their study in central London, Commins & Waller (1967) showed
that additional material was collected by the high volume sampler that
had little effect on smoke measurements and that for their particular
series, the total suspended particulate results were approximately
100 µg/m3 higher than the corresponding smoke figures. Other authors
have calculated regression equations for their series and, although
there are variations in these relationships with time and place, the
general picture is of a large proportional difference between total
suspended particulate and smoke figures at low values, but relatively
little difference at high values (of the order of 500 µg of smoke/m3
or more).
Thus, it is recommended that "smoke" and "total suspended
particulate" measured by the various methods described should be
regarded as separate entities; this principle has been adopted in
later sections relating to the effects on health.
In occupational environments, suspended particulate matter from
combustion sources may be of some concern, but more commonly it is the
dusts and aerosols associated with particular occupations or processes
that are of interest. In such cases, the composition of the material
may be relatively uniform and well established, and specific methods
of assessment can be devised. There has, for example, been a great
deal of research and development on methods for determining dust
concentrations in coal mines (Jacobsen, 1972). With industrial dusts,
the particle size distribution must always be considered carefully,
for it is liable to extend beyond the respirable range, and
elutriators or cyclones may be needed in conjunction with gravimetric
samplers. A valuable discussion of methods for collecting size-graded
samples has been included in a recent review (International Atomic
Energy Agency, 1978).
2.2.4 Dustfall (deposited matter)
In some of the older epidemiological studies, measurements of
dustfall were quoted as an index of particulate pollution. This is
inappropriate as the results are influenced primarily by large
particles (diameters from about 10 µm upwards: see section 2.1.2) that
do not penetrate the respiratory system and, generally, are not
relevant to health problems, apart from possible annoyance reactions.
For reference purposes, however, a brief description of one of the
more commonly used instruments is included (Table 6).
Table 6. Method of analysis for dust fall
Method Principle Comment
Deposit A receiver containing a Results expressed in terms
gauge nonfreezing solution is left in of deposit per unit area and time,
the open and the quantity of not convertible in any way to
material collected (usually concentrations of suspended matter
over 1-month periods) is determined in the air; strongly influenced by
by weighing, water-soluble and sources nearby, hence results only
insoluble components being considered relevant to immediate vicinity
separately (British Standards
Institution, 1969b)
3. SOURCES OF SULFUR OXIDES AND PARTICULATE MATTER
3.1 Natural Occurrence
Compounds of sulfur are found in small quantities in ambient air,
even in remote areas far from sources of pollution. In the gas phase,
they are present as hydrogen sulfide or sulfur dioxide, and in
particulate form they may be present as sulfate. Sulfur dioxide and
hydrogen sulfide are emitted by volcanoes and the latter is also
produced by anaerobic bacteria in soil, marshes, and tidal flats (Grey
& Jensen, 1972). Some of the particulate sulfate may also be emitted
directly by volcanoes or sea spray, but most of it is the end-product
of the oxidation of hydrogen sulfide or sulfur dioxide.
In general, suspended particulate matter can result from volcanic
activity, from dust storms, or from strong winds blowing over dry soil
and may include pollen from trees and other plants. Forest fires also
produce large amounts of particulate matter.
Some of these natural contributions to the particulate matter in
the air consist of particles too large to remain in suspension for
long periods, and their composition and properties may be quite
different from those of the emissions from man's activities.
3.2 Man-made sources
Most emissions of sulfur into the air are in the form of sulfur
dioxide resulting from the combustion of fossil fuel for heating and
energy production. Various industrial activities such as petroleum
processing, smelter operations, wood-pulping, etc., also produce
significant emissions of sulfur dioxide and other sulfur compounds.
It has been estimated (Robinson & Robbins, 1968) that on a
worldwide scale about 146 × 106 tonnes of sulfur dioxide are emitted
annually, 70% of which result from coal burning, 16% from the
combustion of petroleum products, and the remainder from petroleum
refining and nonferrous smelting. These estimates are based mainly on
1965 world figures for coal production, petroleum refining, and
smelter operations, each combined with an estimate of a sulfur dioxide
"emission factor" per unit of production. A similar basis was used for
the Committee on the Challenges of Modern Society (1971) assessment of
emissions in the northern and southern hemispheres, reproduced in
Table 7.
Table 7. Hemispheric sulfur dioxide emmissions due to man's
activities (106 tonnes per year)a
Source Total Northern Southern
Hemisphere Hemisphere
Coal 102 98 (96%) 4 (4%)
Petroleum,
combustion
and refining 28.5 27.1 (95%) 1.4 (5%)
Smelting, copper 12.9 8.6 (67%) 4.3 (33%)
lead 1.5 1.2 (80%) 0.3 (20%)
zinc 1.3 1.2 (90%) 0.1 (10%)
Total 146 136 (93%) 10 (7%)
a From: Committee on the Challenges of Modern Society (1971).
On a global scale, the emissions of sulfur compounds into the
atmosphere by man-made activities are about equal to those from
natural sources. On the other hand, the emissions from man's
activities are the main contributors to pollution in large cities and
their surrounding areas. Assuming that world energy demand increases
at its historic rate, the total emissions of sulfur dioxide will
increase unless appropriate control measures are applied, or there
is a shift from the use of fossil fuels to the use of nonpolluting
energy sources. However, with the stabilization of the population in
some countries, including the United Kingdom and USA, and increasing
concern about the use of limited fuel reserves, there are prospects
that the rate of increase in emissions may be reduced in some parts
of the world.
Combustion and industrial processes are also prime sources of
particulate emissions. As with sulfur dioxide, the burning of fuel
(especially coal) for heating and for the generation of power has been
one of the major contributors to the suspended particulate matter in
urban air. Vehicular traffic also generates dust from the road and
from the wear of tyres as well as particulate lead compounds from the
exhausts of petrol-engined vehicles, and black smoke from those of
diesel vehicles. The incineration of domestic and industrial refuse
may disperse particulate matter and other pollutants into the air
unless carefully controlled. Table 8 shows estimates of the global
emission of all particulate matter (Robinson & Robbins, 1968).
Table 8. Global emission of all particulate matter (106 tonnes
per year)a
Man-made
Particles 92
Gas-particle conversion: sulfur dioxide 147
oxides of nitrogen 30
Photochemical compounds from hydrocarbons 27
296
Natural
Soil dust 200
Gas-particle conversion: hydrogen-sulfide 204
oxides of nitrogen 432
ammonia 269
Photochemical compounds from terpenes, etc 200
Volcanic 4
Forest fires 3
Sea salt 1000
2312
a From: Robinson & Robbins (1968).
3.3 Characteristics of Sources
In urban areas, most of the sulfur dioxide and suspended
particulate matter in the air come from the combustion of fuels, but
many factors, including the type of fuel, the combustion efficiency,
and the flue velocity, influence the quantity and quality of
emissions. The incomplete combustion of soft coal in domestic fires,
for example, produces much smoke, consisting of finely divided
particles of carbon and tarry material, whereas the efficient burning
of pulverised coal in a power station leads to little or no smoke, but
the production of coarser ash particles, which must be removed at
source to avoid their being carried up the flues at a high velocity
and dispersed into the air. The relationship between types of sources
and emissions is summarized in Table 9.
Table 9. Pollutants from combustion sources
Type of source Fuel Sulfur dioxide Particulate matter
Smoke Ash etc.
Domestic heating Wood, peat etc. - + +
or cooking Soft coal ++ ++ +
Hard coal, coke ++ - +
Oil (light distillates) + - -
Gas - - -
Industrial heating Coal, coke ++ - ++
and power generation Oil (heavy residuals) ++ - -
Motor vehicles Petrol - - +
Diesel + + -
Notes: The term smoke is used for incomplete combustion products (notably carbon and tar),
and ash for inorganic components from complete combustion (including lead compounds
in the case of petrol). The signs give only a rough indication of emissions, in the
absence of direct control at source:
- = little or none, + = moderate quantities, ++ = large quantities
In cold and temperate parts of the world, the burning of coal for
domestic heating purposes has been a major contributor to both the
sulfur dioxide and suspended particulate contents of urban air. This
is particularly true of the situation in the United Kingdom prior to
the implementation of the Clean Air Act (Committee on Air Pollution,
1954). Such sources are liable to have a disproportionate effect on
concentrations in the immediate vicinity, because of the low levels of
the chimneys and the low emission velocity. Even in warmer climates,
domestic sources may be of importance, particularly if coal is used
for cooking purposes.
In densely populated areas where domestic sources dominate, the
many chimneys can be considered for some purposes as a diffuse area
source, and, within such an area, concentrations of pollutants in air
remain relatively stable over short distances and short periods of
time. In contrast, large industrial sources should be treated as point
sources, and, at any given location around them, the concentration of
air pollution is liable to vary greatly, even from minute to minute.
The emission into the air of sulfur dioxide and particulate matter
from motor vehicles is relatively small in comparison with those from
domestic and industrial chimneys but it is close to the ground and
within the breathing zone. In these circumstances, concentrations vary
greatly over short distances as well as over short time intervals,
depending on the proximity of the traffic. At points very close to
mixed traffic, smoke from diesel engines may make a substantial
contribution to the concentration of suspended particulate matter in
air (Waller et al., 1965).
Source strength may vary with time of day, day of week, and season
of the year. Accompanying meteorological conditions are also important
in determining the ultimate air concentrations of pollutants arising
from sources. Where heating is required during the winter season,
emissions of sulfur dioxide and particulate matter are usually much
higher than they are in the summer. In a number of cities, however,
where a considerable amount of fuel is used for running cooling
systems, emissions of these pollutants during the summer time are not
always lower than those in winter. Some industrial sources of
pollution may emit little at weekends, and emissions for most sources
are at a minimum during the night.
Although the control of emissions is outside the scope of the
present discussion, the general point can be made that while the
control of particulate emissions is a practicable proposition in many
circumstances, the control of sulfur dioxide at source is relatively
difficult and costly, and the more effective means of reducing
emissions is to change to fuels with a lower sulfur content.
4. DISPERSION AND ENVIRONMENTAL TRANSFORMATIONS
Sulfur compounds dispersed into the air eventually return to the
land or oceans either unchanged, or converted into sulfates. The
sulfur cycle so set up is shown diagrammatically in Fig. 4 (Kellogg et
al., 1972). Particulate matter also returns, its residence time in the
air varying widely according to its physical and chemical
characteristics. As far as direct effects on health are concerned, it
is the local concentration of pollutants in the air at a given time
that is important, as discussed in section 5, but an outline of
dispersion and transformation phenomena is given here.
4.1 Dispersion
The maintenance of a tolerable environment in modern towns depends
very much on the ability of wind and turbulence to disperse the
pollutants rapidly as they are emitted. When these processes fail, the
results can be disastrous, as they were in London in 1952 (Ministry of
Health, United Kingdom, 1954). There are some localities where natural
ventilation is so poor that the emission of pollutants must, at all
times, be carefully controlled. This is especially true of the Los
Angeles area, where emissions of sulfur dioxide and particulate matter
have been successfully curtailed, leaving, however, the major problems
associated with the emission of oxides of nitrogen, hydrocarbons, and
carbon monoxide from motor vehicles (Goldsmith, 1969).
Factors affecting the dispersion of sulfur dioxide and particulate
matter from combustion sources, include:
(a) Temperature and efflux velocity of the gases. Emissions from
small sources, such as domestic fires or incinerators have relatively
little buoyancy, since the temperature at the point of emission is not
much greater than that of the surrounding air. Such sources are liable
therefore to have their greatest impact in the immediate vicinity
(Williams, 1960). Emissions from large-scale industrial installations,
on the other hand, may be at higher temperatures, or may be assisted
by forced-draught to rise more rapidly. Thus, any major impact in the
immediate vicinity may be avoided but weaker effects may be produced
over a wider area (Bosanquet, 1957).
(b) Stack height. Dilution and dispersion over a wide area is
also aided by the use of tall stacks. Much is known of the
relationship between source strengths, stack heights, and ground-level
concentrations of pollutants, through the application of mathematical
modelling techniques, coupled with observations around selected
sources (Briggs, 1965, Pasquill, 1971, Turner, 1968). It is also
possible to devise models to predict concentrations of sulfur dioxide
in urban areas on the basis of emissions from multiple sources
(Fortak, 1970). Conversely, techniques have also been developed for
estimating the pollution inventory of an area from measurements of
sulfur dioxide concentration, fitted to a dispersion model (East,
1972). The height of emission of sulfur dioxide and particulate matter
from domestic sources is primarily a function of the height of the
building itself. Thus the effect on ground level concentrations in the
vicinity is liable to be greater in areas with closely-packed, single
or two-storey houses than in those with high-rise apartments. Tall
stacks are widely used for electricity-generating stations and other
major industrial sources, but the pollutants may then be carried great
distances, often over national boundaries, to be deposited eventually
far from their source (Royal Ministry of Foreign Affairs, Sweden,
1971; Zeeduk & Velds, 1973).
 (c) Topography and the proximity of other buildings. The
presence of hills or tall buildings, and many other features of the
landscape, have important effects on the dispersion of plumes from
individual stacks, or of the pollution from an area source as a whole.
Many industrial cities have developed in river valleys, initially to
take advantage of water transport, but, in general, the dispersion of
pollutants in such a situation is poorer than it would be from a more
exposed location.
(d) Meteorology. Meteorological factors are of fundamental
importance in determining the whole spatial and temporal distribution
of pollution, and the subject has been well reviewed in a recent
publication (Munn, 1976). Apart from the general influence of the
local climate, the great variability of the weather in any one
locality is liable to lead to considerable changes in the
concentrations of sulfur dioxide. In particular, temperature
inversions can trap these pollutants to produce concentrations up to
several hundred times the usual values (Waller & Commins, 1966).
4.2 Transformation and Degradation
In recent years, there has been a rapid escalation of interest in
the ultimate fate of sulfur dioxide and particulate matter emitted
into the air. This is concerned partly with the nature of reaction
products and their possible effects on health, and also with the
ecological effects of these products when deposited (Brosset, 1973)
and their possible role, as aerosols, in modifying the climate on a
global scale (Hobbs et al., 1974).
Some of the sulfur dioxide emitted into the air is removed
unchanged by various surfaces, including soil (Abeles et al., 1971),
water (Liss, 1971; Spedding, 1972), grass (Garland et al., 1973) and
vegetation in general (Hill, 1971). It has been estimated that, in the
United Kingdom, about 25% of the sulfur dioxide is removed by these
direct ("dry" deposition) processes (Garland et al., 1974). The
remainder is transformed into sulfuric acid or sulfates by a variety
of processes, in the presence of moisture, and is then mainly washed
out in rain. Although this self-cleansing process limits the build-up
of sulfur compounds in the air, so minimizing the effects on health,
the "acid rain" produced is considered to be a serious general
environmental problem in some areas (Likens & Bormann, 1974).
A schematic representation of a natural sulfur cycle is shown in
Fig. 5 (Kellogg et al., 1972). Additions to this cycle due to man's
activity are possible at each stage, although 95% of such
contributions are added as sulfur dioxide. Also represented here are
the possible reactions with sunlight. There have been many laboratory
investigations of this process: the reaction is slow (Allen et al.,
1972; Cox & Penkett, 1970), but it is enhanced in the presence of
hydrocarbons and other pollutants associated with motor vehicle
emissions (Cox & Penkett, 1971; Wilson & Levy, 1970). In general,
however, other processes are of even greater importance in the
transformation and removal of sulfur dioxide, including reactions in
water droplets with ammonia (McKay, 1971), and catalytic oxidation in
the presence of manganese or iron (Barrie & Georgii, 1976; Chun &
Quon, 1973). These various reactions involving sulfur dioxide,
(c) Topography and the proximity of other buildings. The
presence of hills or tall buildings, and many other features of the
landscape, have important effects on the dispersion of plumes from
individual stacks, or of the pollution from an area source as a whole.
Many industrial cities have developed in river valleys, initially to
take advantage of water transport, but, in general, the dispersion of
pollutants in such a situation is poorer than it would be from a more
exposed location.
(d) Meteorology. Meteorological factors are of fundamental
importance in determining the whole spatial and temporal distribution
of pollution, and the subject has been well reviewed in a recent
publication (Munn, 1976). Apart from the general influence of the
local climate, the great variability of the weather in any one
locality is liable to lead to considerable changes in the
concentrations of sulfur dioxide. In particular, temperature
inversions can trap these pollutants to produce concentrations up to
several hundred times the usual values (Waller & Commins, 1966).
4.2 Transformation and Degradation
In recent years, there has been a rapid escalation of interest in
the ultimate fate of sulfur dioxide and particulate matter emitted
into the air. This is concerned partly with the nature of reaction
products and their possible effects on health, and also with the
ecological effects of these products when deposited (Brosset, 1973)
and their possible role, as aerosols, in modifying the climate on a
global scale (Hobbs et al., 1974).
Some of the sulfur dioxide emitted into the air is removed
unchanged by various surfaces, including soil (Abeles et al., 1971),
water (Liss, 1971; Spedding, 1972), grass (Garland et al., 1973) and
vegetation in general (Hill, 1971). It has been estimated that, in the
United Kingdom, about 25% of the sulfur dioxide is removed by these
direct ("dry" deposition) processes (Garland et al., 1974). The
remainder is transformed into sulfuric acid or sulfates by a variety
of processes, in the presence of moisture, and is then mainly washed
out in rain. Although this self-cleansing process limits the build-up
of sulfur compounds in the air, so minimizing the effects on health,
the "acid rain" produced is considered to be a serious general
environmental problem in some areas (Likens & Bormann, 1974).
A schematic representation of a natural sulfur cycle is shown in
Fig. 5 (Kellogg et al., 1972). Additions to this cycle due to man's
activity are possible at each stage, although 95% of such
contributions are added as sulfur dioxide. Also represented here are
the possible reactions with sunlight. There have been many laboratory
investigations of this process: the reaction is slow (Allen et al.,
1972; Cox & Penkett, 1970), but it is enhanced in the presence of
hydrocarbons and other pollutants associated with motor vehicle
emissions (Cox & Penkett, 1971; Wilson & Levy, 1970). In general,
however, other processes are of even greater importance in the
transformation and removal of sulfur dioxide, including reactions in
water droplets with ammonia (McKay, 1971), and catalytic oxidation in
the presence of manganese or iron (Barrie & Georgii, 1976; Chun &
Quon, 1973). These various reactions involving sulfur dioxide,
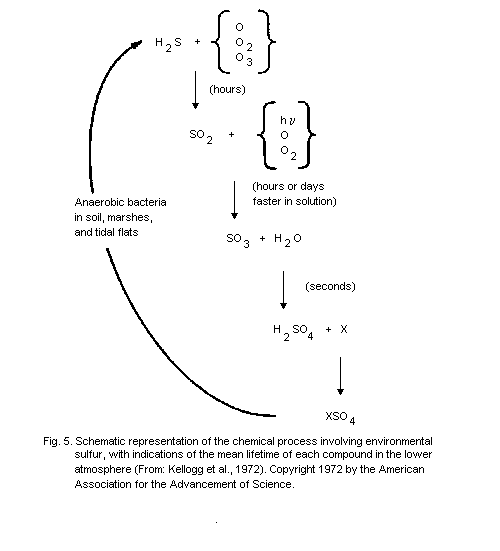 particulate matter, and other pollutants have been discussed in a
number of reviews (Bufalini, 1971; Calvert, 1973). There may be
limitations to the catalytic processes because of the restricted
availability of reactive metallic oxide, catalytic particles, and
neutralizing compounds in the air. Some of the photochemical reactions
are severely rate-limited, but in others, sulfur dioxide can be
oxidized at an appreciable rate and it has been estimated that
conversion rates as high as 18% per hour might be possible (Rall,
1974). The end-products are similar in all these reactions, i.e., the
formation of aerosols, initially in the submicron size-range,
consisting of a mixture of sulfates and sulfuric acid. There is no
uniform relationship between the proportions of sulfur dioxide,
sulfates, or sulfuric acid in the total sulfur pollution. Emissions
are primarily in the form of sulfur dioxide; thus, in cities close to
sources, the major proportion is in this form, but it has been
reported that, in the USA, the proportion present as sulfate is higher
in western than in eastern urban areas (Altshuller, 1973). At nonurban
sites, concentrations of sulfates may be similar to those of sulfur
dioxide.
The overall conversion of sulfur dioxide to sulfate is an
extremely complex process with many interrelated variables that are
poorly characterized. These include the absorption rate of sulfur
dioxide, the sizes of the particles or droplets involved, their
chemical composition, the rate of diffusion of reactants within the
aerosol, and the relative humidity. The last of these variables is a
major factor, as the catalytic reactions occur with water droplets
containing absorbed sulfur dioxide and other pollutants. Furthermore,
as the pH decreases, the rate of oxidation of sulfur dioxide also
decreases (Junge & Ryan, 1958). Thus, the formation of sulfuric acid
tends to be self-limiting unless the fall in pH is offset by
additional water vapour. On the other hand, the presence of alkaline
compounds, such as ammonia, in the droplet can enhance the reaction
rate due to its buffering capacity. Extrapolated levels for the rate
of oxidation of sulfur dioxide by catalytic processes in urban air
range upwards from 2% per hour (Rall, 1974). Overall, the half-life of
sulfur dioxide in ambient air is estimated to be three to five hours.
The physical and chemical forms of suspended particulate matter in
general may be changed in the air. Some components, such as
hydrocarbons, absorbed initially onto particulate matter, may
evaporate or be oxidized. Even some of the complex hydrocarbons in the
tarry matter from coal burning may be volatile enough to be lost
gradually, and there is evidence that they can be lost from filters
during sampling (Commins & Lawther, 1958). The sizes of the particles
may vary according to the relative humidity, particularly if sulfuric
acid, sulfates, or other salts are present, and this can lead to
precipitation even before the onset of rain (Waller, 1963). The
question of overwhelming importance is the role of particulate matter
in the conversion of sulfur dioxide to sulfuric acid and sulfates.
Traces of metallic compounds, some of which serve as catalysts in
these reactions, are present in particulate matter from the combustion
of coal, and also in the relatively small amount of particulate matter
that may come from the combustion of oil. It has long been considered
that the acute effects on health seen in episodes of high pollution
are crucially dependent on the mixture of sulfur dioxide and
particulate matter present, together with the relative humidity: the
worst effects have been seen with each of these factors in a high
range (Ministry of Health, United Kingdom, 1954).
5. ENVIRONMENTAL CONCENTRATIONS AND EXPOSURES
Sulfur dioxide and suspended particulate matter are the most
widely monitored air pollutants. National sampling networks exist in
many of the industrialized countries of the world, and summaries of
the observations are commonly published in annual reports. Results
from selected sampling sites are also collated by a number of
international organizations (Commission of the European Communities,
1976; Pan American Health Organization, 1976; World Health
Organization, 1976b).
5.1 Concentrations in Outdoor Air
Most sampling networks for sulfur dioxide, smoke, and suspended
particulate matter have been set up for control purposes, to examine
the distribution of these pollutants in various areas and to follow
the long-term trends. Such measurements are normally made on outdoor
air. Where adequate networks exist, there is obviously an advantage in
trying to use them to assess exposure, but this may be far from ideal.
The limitations of the data obtained from the usual monitoring sites
may, to some extent, account for inconsistencies in results in studies
reviewed in section 8. Requirements for monitoring networks have been
discussed further in a recent report (World Health Organization,
1977).
Concentrations of sulfur dioxide and suspended particulate matter
vary greatly from one area to another depending on the nature and
intensity of local sources, and on other factors such as topography,
general weather conditions, and liability to temperature inversions.
Even within a single city there may be large differences in
concentrations. Where sufficient monitoring stations exist, it may be
possible to construct isopleths, showing "contours" of equal
concentration. An example, for the city of Antwerp, is shown in Fig. 6
(Derouane et al., 1972). From this, it is clear that the distribution
of sulfur dioxide is not necessarily the same as that of smoke. There
is a general tendency for the concentrations of these pollutants to be
highest in the largest cities of the world, and within them for the
highest concentrations to be in the central areas. The implementation
of control measures has, however, changed the situation in recent
years, for, in some instances, these have been applied most vigorously
in the central areas of large cities (Masters, 1974).
particulate matter, and other pollutants have been discussed in a
number of reviews (Bufalini, 1971; Calvert, 1973). There may be
limitations to the catalytic processes because of the restricted
availability of reactive metallic oxide, catalytic particles, and
neutralizing compounds in the air. Some of the photochemical reactions
are severely rate-limited, but in others, sulfur dioxide can be
oxidized at an appreciable rate and it has been estimated that
conversion rates as high as 18% per hour might be possible (Rall,
1974). The end-products are similar in all these reactions, i.e., the
formation of aerosols, initially in the submicron size-range,
consisting of a mixture of sulfates and sulfuric acid. There is no
uniform relationship between the proportions of sulfur dioxide,
sulfates, or sulfuric acid in the total sulfur pollution. Emissions
are primarily in the form of sulfur dioxide; thus, in cities close to
sources, the major proportion is in this form, but it has been
reported that, in the USA, the proportion present as sulfate is higher
in western than in eastern urban areas (Altshuller, 1973). At nonurban
sites, concentrations of sulfates may be similar to those of sulfur
dioxide.
The overall conversion of sulfur dioxide to sulfate is an
extremely complex process with many interrelated variables that are
poorly characterized. These include the absorption rate of sulfur
dioxide, the sizes of the particles or droplets involved, their
chemical composition, the rate of diffusion of reactants within the
aerosol, and the relative humidity. The last of these variables is a
major factor, as the catalytic reactions occur with water droplets
containing absorbed sulfur dioxide and other pollutants. Furthermore,
as the pH decreases, the rate of oxidation of sulfur dioxide also
decreases (Junge & Ryan, 1958). Thus, the formation of sulfuric acid
tends to be self-limiting unless the fall in pH is offset by
additional water vapour. On the other hand, the presence of alkaline
compounds, such as ammonia, in the droplet can enhance the reaction
rate due to its buffering capacity. Extrapolated levels for the rate
of oxidation of sulfur dioxide by catalytic processes in urban air
range upwards from 2% per hour (Rall, 1974). Overall, the half-life of
sulfur dioxide in ambient air is estimated to be three to five hours.
The physical and chemical forms of suspended particulate matter in
general may be changed in the air. Some components, such as
hydrocarbons, absorbed initially onto particulate matter, may
evaporate or be oxidized. Even some of the complex hydrocarbons in the
tarry matter from coal burning may be volatile enough to be lost
gradually, and there is evidence that they can be lost from filters
during sampling (Commins & Lawther, 1958). The sizes of the particles
may vary according to the relative humidity, particularly if sulfuric
acid, sulfates, or other salts are present, and this can lead to
precipitation even before the onset of rain (Waller, 1963). The
question of overwhelming importance is the role of particulate matter
in the conversion of sulfur dioxide to sulfuric acid and sulfates.
Traces of metallic compounds, some of which serve as catalysts in
these reactions, are present in particulate matter from the combustion
of coal, and also in the relatively small amount of particulate matter
that may come from the combustion of oil. It has long been considered
that the acute effects on health seen in episodes of high pollution
are crucially dependent on the mixture of sulfur dioxide and
particulate matter present, together with the relative humidity: the
worst effects have been seen with each of these factors in a high
range (Ministry of Health, United Kingdom, 1954).
5. ENVIRONMENTAL CONCENTRATIONS AND EXPOSURES
Sulfur dioxide and suspended particulate matter are the most
widely monitored air pollutants. National sampling networks exist in
many of the industrialized countries of the world, and summaries of
the observations are commonly published in annual reports. Results
from selected sampling sites are also collated by a number of
international organizations (Commission of the European Communities,
1976; Pan American Health Organization, 1976; World Health
Organization, 1976b).
5.1 Concentrations in Outdoor Air
Most sampling networks for sulfur dioxide, smoke, and suspended
particulate matter have been set up for control purposes, to examine
the distribution of these pollutants in various areas and to follow
the long-term trends. Such measurements are normally made on outdoor
air. Where adequate networks exist, there is obviously an advantage in
trying to use them to assess exposure, but this may be far from ideal.
The limitations of the data obtained from the usual monitoring sites
may, to some extent, account for inconsistencies in results in studies
reviewed in section 8. Requirements for monitoring networks have been
discussed further in a recent report (World Health Organization,
1977).
Concentrations of sulfur dioxide and suspended particulate matter
vary greatly from one area to another depending on the nature and
intensity of local sources, and on other factors such as topography,
general weather conditions, and liability to temperature inversions.
Even within a single city there may be large differences in
concentrations. Where sufficient monitoring stations exist, it may be
possible to construct isopleths, showing "contours" of equal
concentration. An example, for the city of Antwerp, is shown in Fig. 6
(Derouane et al., 1972). From this, it is clear that the distribution
of sulfur dioxide is not necessarily the same as that of smoke. There
is a general tendency for the concentrations of these pollutants to be
highest in the largest cities of the world, and within them for the
highest concentrations to be in the central areas. The implementation
of control measures has, however, changed the situation in recent
years, for, in some instances, these have been applied most vigorously
in the central areas of large cities (Masters, 1974).
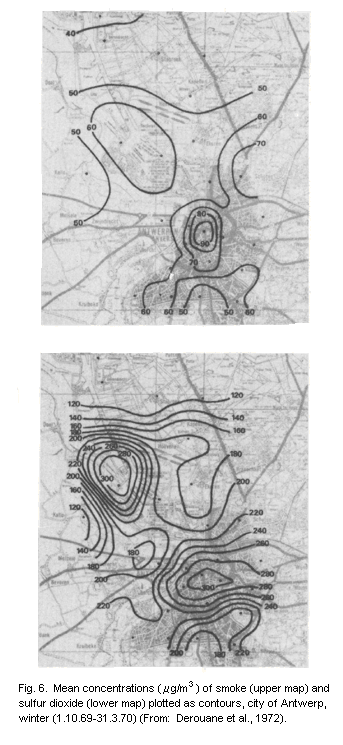 Table 10. Concentrations of sulfur dioxide, smoke, and suspended particulate
matter (1974)a
Site Concentration (µg/m 3)
annual arithmetic mean maximum daily mean
Sulfur dioxide
Brussels 107 347
Frankfurt 119 455
London 150 503
Madrid 161 763
Prague 126 482
Rome 108 600
Zagreb 173 893
Smoke, by reflectance
Brussels 37 -
London 26 149
Madrid 190 908
Rome 60 160
Suspended particulate by high volume sampler
Calcutta 519 1090
St Louis 87 189
Vancouver 64 134
Zagreb 167 806
a From: World Health Organization (1976b). Sites selected for inclusion
here are all classified as city centre commercial sites. The
selection is also limited to sites using 24-h averaging periods.
Further information is available in the WHO report of frequency
distributions, standard deviations, and monthly and annual
geometric means.
Examples of concentrations of sulfur dioxide and smoke or suspended
particulate matter, drawn from the WHO air quality monitoring
programme (World Health Organization, 1976b) are shown in Table 10.
For present purposes, results included in this table are limited to
those from one type of site (city centre commercial sites), where the
sampling methods and averaging periods are also comparable with one
another. Even so, much caution must be exercised in drawing
comparisons between cities, for the sites can only be representative
of their immediate surroundings.
The annual mean concentrations of sulfur dioxide are fairly
uniform at the particular sites quoted in Table 10, ranging from about
100 to 200 µg/m3 (0.035-0.070 ppm). For particulate matter, however,
the variation between cities appears to be much greater and it seems
likely that some of the results are unduly influenced by sources close
to the samplers, or by high background levels of dust from
noncombustion sources.
There are few reports about ambient levels of sulfates. A study
from the USA (Altshuller, 1973) reported annual, arithmetic mean
concentrations at urban sites in the range of 2.4 to 48.7 µg/m3, with
an average ratio of sulfur dioxide to sulfate of 4.7. The relationship
between these two pollutants was not, however, entirely consistent and
the ratio tended to be higher at western sites than at eastern sites.
In the east, there was a general background level of sulfate of about
5 µg/m3, even at nonurban sites, and this was attributed to the long
distance transport of sulfur dioxide, with conversion to sulfate
during transport. There also appeared to be a "saturation" level of
sulfate at about 17 µg/m3 at eastern urban sites, within the sulfur
dioxide range of 100-200 µg/m3 (0.035-0.070 ppm).
There is even less published information concerning concentrations
in air of sulfuric acid, and such observations must always be related
to the method of measurement. One series of measurements of net
particulate acid, as determined by titration of samples collected on
filter papers, has indicated a mean concentration in London of
approximately 4 µg/m3 representing a few percent of the
corresponding concentration of sulfur dioxide (Commins & Waller,
unpublished data). However, the concentration of this pollutant is
liable to increase rapidly during temperature inversions, particularly
if the relative humidity is high (Commins, 1967).
For each of the pollutants considered, there are generally large
variations in concentration with time at any one place. The extent to
which this can be followed depends on the time resolution of the
sampling instruments. Usually, integrated samples are collected over
24-h periods to yield daily mean values, and from these monthly,
seasonal, and annual means are calculated. Shorter sampling periods
may however be used, and where continuous automatic instruments are
used, virtually instantaneous values can be obtained. Relationships
between values averaged over different periods have been extensively
studied in the USA (Larsen, 1971). An indication of the day-to-day
variation in smoke and sulfur dioxide concentrations in a large city
(London) is given in Fig. 7.
Table 10. Concentrations of sulfur dioxide, smoke, and suspended particulate
matter (1974)a
Site Concentration (µg/m 3)
annual arithmetic mean maximum daily mean
Sulfur dioxide
Brussels 107 347
Frankfurt 119 455
London 150 503
Madrid 161 763
Prague 126 482
Rome 108 600
Zagreb 173 893
Smoke, by reflectance
Brussels 37 -
London 26 149
Madrid 190 908
Rome 60 160
Suspended particulate by high volume sampler
Calcutta 519 1090
St Louis 87 189
Vancouver 64 134
Zagreb 167 806
a From: World Health Organization (1976b). Sites selected for inclusion
here are all classified as city centre commercial sites. The
selection is also limited to sites using 24-h averaging periods.
Further information is available in the WHO report of frequency
distributions, standard deviations, and monthly and annual
geometric means.
Examples of concentrations of sulfur dioxide and smoke or suspended
particulate matter, drawn from the WHO air quality monitoring
programme (World Health Organization, 1976b) are shown in Table 10.
For present purposes, results included in this table are limited to
those from one type of site (city centre commercial sites), where the
sampling methods and averaging periods are also comparable with one
another. Even so, much caution must be exercised in drawing
comparisons between cities, for the sites can only be representative
of their immediate surroundings.
The annual mean concentrations of sulfur dioxide are fairly
uniform at the particular sites quoted in Table 10, ranging from about
100 to 200 µg/m3 (0.035-0.070 ppm). For particulate matter, however,
the variation between cities appears to be much greater and it seems
likely that some of the results are unduly influenced by sources close
to the samplers, or by high background levels of dust from
noncombustion sources.
There are few reports about ambient levels of sulfates. A study
from the USA (Altshuller, 1973) reported annual, arithmetic mean
concentrations at urban sites in the range of 2.4 to 48.7 µg/m3, with
an average ratio of sulfur dioxide to sulfate of 4.7. The relationship
between these two pollutants was not, however, entirely consistent and
the ratio tended to be higher at western sites than at eastern sites.
In the east, there was a general background level of sulfate of about
5 µg/m3, even at nonurban sites, and this was attributed to the long
distance transport of sulfur dioxide, with conversion to sulfate
during transport. There also appeared to be a "saturation" level of
sulfate at about 17 µg/m3 at eastern urban sites, within the sulfur
dioxide range of 100-200 µg/m3 (0.035-0.070 ppm).
There is even less published information concerning concentrations
in air of sulfuric acid, and such observations must always be related
to the method of measurement. One series of measurements of net
particulate acid, as determined by titration of samples collected on
filter papers, has indicated a mean concentration in London of
approximately 4 µg/m3 representing a few percent of the
corresponding concentration of sulfur dioxide (Commins & Waller,
unpublished data). However, the concentration of this pollutant is
liable to increase rapidly during temperature inversions, particularly
if the relative humidity is high (Commins, 1967).
For each of the pollutants considered, there are generally large
variations in concentration with time at any one place. The extent to
which this can be followed depends on the time resolution of the
sampling instruments. Usually, integrated samples are collected over
24-h periods to yield daily mean values, and from these monthly,
seasonal, and annual means are calculated. Shorter sampling periods
may however be used, and where continuous automatic instruments are
used, virtually instantaneous values can be obtained. Relationships
between values averaged over different periods have been extensively
studied in the USA (Larsen, 1971). An indication of the day-to-day
variation in smoke and sulfur dioxide concentrations in a large city
(London) is given in Fig. 7.
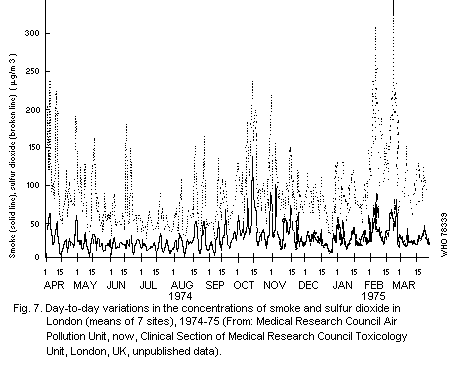 Although annual means, coupled with daily maxima, give a general
impression of pollution levels in any given locality, long series of
results are often summarized as frequency distributions. These have
been shown to be log-normal for a wide range of averaging times,
pollutants, and localities (Pollack, 1975). This suggests that the
geometric mean, which, in such a case, is equivalent to the median, is
perhaps the most appropriate central value to use. Historically,
however, the arithmetic mean has been more widely used. For the usual
log-normal distribution, the geometric mean is a little lower than the
arithmetic mean. Percentiles of the frequency distribution are
tabulated in some monitoring networks (US Environmental Protection
Agency, 1974a), and the complete distributions can conveniently be
plotted on log-probability paper, as in the example in Fig. 8 drawn
from data for a recent 5-year period in London (Commins & Waller,
unpublished data).
Relationships between peak and mean values for sulfur dioxide have
also been considered on an empirical basis. For a number of cities in
Europe, the highest daily mean concentrations during the year have
been found to be of the order of four times the annual means
(Commission of the European Communities, 1976). Transient peaks in
continuous records have been examined in the USA in relation to
averaging times: the ratio of peak to mean values has been found to be
2.3 for hourly averaging periods, increasing for successively longer
periods (Montgomery & Coleman, 1975). Some highly sophisticated
networks exist for the measurement of sulfur dioxide on a continuous
basis at many points. The collection and interpretation of such data
then presents a formidable task, and in some of these networks on-line
computers are used for data acquisition (Lauer & Benson, 1975).
The examination of trends in the concentrations of sulfur dioxide
and particulate matter is important when the effects of long-term
exposures are investigated. In urban areas of most developed
countries, there has been a tendency for levels to decline in recent
years as a result of control efforts, although elsewhere, and
particularly where concentrations of these pollutants had previously
been low, increases have occurred as emissions from industrial and
other sources have increased. Since variations in weather patterns
from year-to-year can affect even the annual mean concentrations, long
series are required to examine trends adequately. The declining trend
in sulfur dioxide concentrations seen in a number of large cities in
Europe during the 1960s (Commission of the European Communities, 1976)
may, to a large extent, reflect declining emissions or improved
dispersion from chimneys, but some authors have considered that
changing weather conditions have been a contributory factor (Van Dop &
Kruizinga, 1976). In the United Kingdom, there has been an overall
decline in sulfur dioxide concentrations without a corresponding
decline in total emissions (Fig. 9). This is attributable to the
Although annual means, coupled with daily maxima, give a general
impression of pollution levels in any given locality, long series of
results are often summarized as frequency distributions. These have
been shown to be log-normal for a wide range of averaging times,
pollutants, and localities (Pollack, 1975). This suggests that the
geometric mean, which, in such a case, is equivalent to the median, is
perhaps the most appropriate central value to use. Historically,
however, the arithmetic mean has been more widely used. For the usual
log-normal distribution, the geometric mean is a little lower than the
arithmetic mean. Percentiles of the frequency distribution are
tabulated in some monitoring networks (US Environmental Protection
Agency, 1974a), and the complete distributions can conveniently be
plotted on log-probability paper, as in the example in Fig. 8 drawn
from data for a recent 5-year period in London (Commins & Waller,
unpublished data).
Relationships between peak and mean values for sulfur dioxide have
also been considered on an empirical basis. For a number of cities in
Europe, the highest daily mean concentrations during the year have
been found to be of the order of four times the annual means
(Commission of the European Communities, 1976). Transient peaks in
continuous records have been examined in the USA in relation to
averaging times: the ratio of peak to mean values has been found to be
2.3 for hourly averaging periods, increasing for successively longer
periods (Montgomery & Coleman, 1975). Some highly sophisticated
networks exist for the measurement of sulfur dioxide on a continuous
basis at many points. The collection and interpretation of such data
then presents a formidable task, and in some of these networks on-line
computers are used for data acquisition (Lauer & Benson, 1975).
The examination of trends in the concentrations of sulfur dioxide
and particulate matter is important when the effects of long-term
exposures are investigated. In urban areas of most developed
countries, there has been a tendency for levels to decline in recent
years as a result of control efforts, although elsewhere, and
particularly where concentrations of these pollutants had previously
been low, increases have occurred as emissions from industrial and
other sources have increased. Since variations in weather patterns
from year-to-year can affect even the annual mean concentrations, long
series are required to examine trends adequately. The declining trend
in sulfur dioxide concentrations seen in a number of large cities in
Europe during the 1960s (Commission of the European Communities, 1976)
may, to a large extent, reflect declining emissions or improved
dispersion from chimneys, but some authors have considered that
changing weather conditions have been a contributory factor (Van Dop &
Kruizinga, 1976). In the United Kingdom, there has been an overall
decline in sulfur dioxide concentrations without a corresponding
decline in total emissions (Fig. 9). This is attributable to the
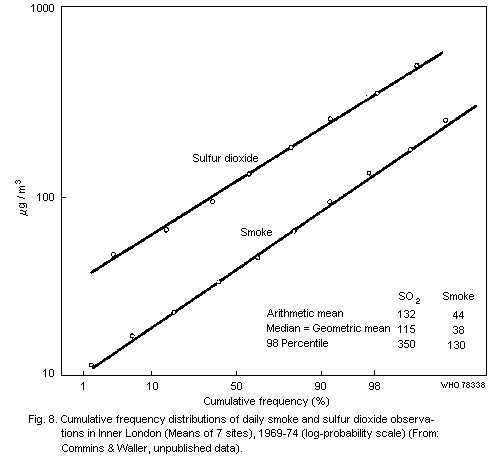
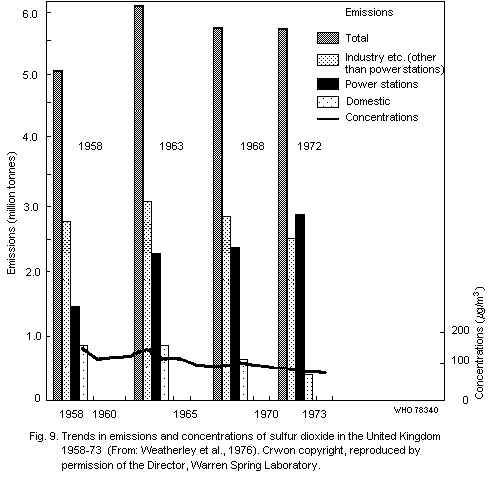 gradual elimination of sources, such as domestic fires, that had a
substantial effect on local concentrations, and their replacement by a
smaller number of large sources dispersing the sulfur dioxide more
widely. In the case of smoke, concentrations in the United Kingdom
have declined in parallel with the emissions (Fig. 10). Domestic fires
always had a dominant effect, and these have been subject to control
in an increasing number of urban areas.
5.2 Concentrations in Indoor Air
As yet, there is relatively little information available
concerning the concentrations of sulfur dioxide and particulate matter
in indoor environments (excluding those specifically related to
occupational exposures). It is quite possible to make such
measurements indoors by most of the methods mentioned in section 2,
subject to additional care about interfering substances, and
limitations of noise for equipment such as the high volume sampler.
The results are, however, of limited value for general monitoring
purposes, because of the additional variability introduced by the
circumstances within each building. As far as human exposure is
concerned, much time is spent indoors, particularly by the oldest and
youngest members of the community, and information on indoor
concentrations is required for epidemiological studies.
Whether there are substantial differences in indoor and outdoor
concentrations of sulfur dioxide and particulate matter will depend on
the degree of ventilation, the capacity of surfaces within to absorb
or otherwise collect these pollutants, and the presence of sources
either of the pollutants themselves, or of others that may interact
with them.
In warm climates not subject to frequent rain or other adverse
weather conditions, buildings may be left open enough to ensure that
indoor concentrations of pollutants are virtually the same as those
outdoors. Even so, there can be local problems associated with the use
of fuels in equipment with poor flues or without flues. Extremely high
concentrations of pollutants, including smoke, have been reported
inside primitive dwellings in tropical regions, where cooking is
carried out over open fires (Sofoluwe, 1968). The open coal fires that
were so widely used in the United Kingdom prior to the Clean Air Act
of 1956 had two possible, and opposing, effects on indoor
concentrations. The very large ventilation rate that they induced
helped to maintain indoor concentrations of smoke and sulfur dioxide
close to those outdoors, but, in unfavourable wind conditions,
downdraughts could force these pollutants from the fire itself into
the room, producing concentrations far in excess of those outdoors.
Examples of indoor concentrations of suspended particulate matter or
(more rarely) of sulfur dioxide exceeding those out of doors have also
been found in studies in the Netherlands (Biersteker et al., 1956) and
in the USA (Yocom et al., 1971).
gradual elimination of sources, such as domestic fires, that had a
substantial effect on local concentrations, and their replacement by a
smaller number of large sources dispersing the sulfur dioxide more
widely. In the case of smoke, concentrations in the United Kingdom
have declined in parallel with the emissions (Fig. 10). Domestic fires
always had a dominant effect, and these have been subject to control
in an increasing number of urban areas.
5.2 Concentrations in Indoor Air
As yet, there is relatively little information available
concerning the concentrations of sulfur dioxide and particulate matter
in indoor environments (excluding those specifically related to
occupational exposures). It is quite possible to make such
measurements indoors by most of the methods mentioned in section 2,
subject to additional care about interfering substances, and
limitations of noise for equipment such as the high volume sampler.
The results are, however, of limited value for general monitoring
purposes, because of the additional variability introduced by the
circumstances within each building. As far as human exposure is
concerned, much time is spent indoors, particularly by the oldest and
youngest members of the community, and information on indoor
concentrations is required for epidemiological studies.
Whether there are substantial differences in indoor and outdoor
concentrations of sulfur dioxide and particulate matter will depend on
the degree of ventilation, the capacity of surfaces within to absorb
or otherwise collect these pollutants, and the presence of sources
either of the pollutants themselves, or of others that may interact
with them.
In warm climates not subject to frequent rain or other adverse
weather conditions, buildings may be left open enough to ensure that
indoor concentrations of pollutants are virtually the same as those
outdoors. Even so, there can be local problems associated with the use
of fuels in equipment with poor flues or without flues. Extremely high
concentrations of pollutants, including smoke, have been reported
inside primitive dwellings in tropical regions, where cooking is
carried out over open fires (Sofoluwe, 1968). The open coal fires that
were so widely used in the United Kingdom prior to the Clean Air Act
of 1956 had two possible, and opposing, effects on indoor
concentrations. The very large ventilation rate that they induced
helped to maintain indoor concentrations of smoke and sulfur dioxide
close to those outdoors, but, in unfavourable wind conditions,
downdraughts could force these pollutants from the fire itself into
the room, producing concentrations far in excess of those outdoors.
Examples of indoor concentrations of suspended particulate matter or
(more rarely) of sulfur dioxide exceeding those out of doors have also
been found in studies in the Netherlands (Biersteker et al., 1956) and
in the USA (Yocom et al., 1971).
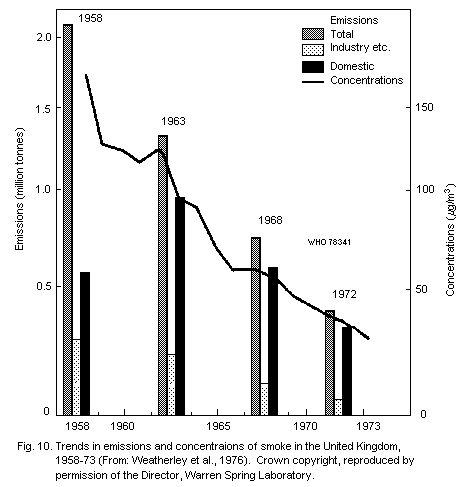 In general, however, in the absence of specific sources of sulfur
dioxide or fine particulate matter indoors, concentrations are
generally less than those outdoors. Sulfur dioxide as a gas, can
diffuse readily onto walls and other surfaces. There is evidence that
it reacts with ammonia from the indoor air on painted surfaces,
particularly in the presence of moisture (Holbrow, 1958), but it is
most effectively absorbed on clothing, curtains, carpets, and other
soft furnishings, so that in domestic surroundings where these abound
concentrations of sulfur dioxide are only of the order of 20% of those
outdoors (Weatherley, 1966), while in offices and other buildings
containing less absorbing material, concentrations may be 40-50% of
those outdoors (Andersen, 1972; Derouane, 1972). The presence of
ammonia in occupied rooms is important in relation to measurements of
sulfur dioxide. Concentrations of this "natural" pollutant may be much
higher than outdoors, particularly where there are young babies or old
people with incontinence problems. The ammonia will only react
effectively with sulfur dioxide in the presence of moisture, but it
will, in any case, interfere with the determination of the sulfur
dioxide by acidimetric or conductometric methods.
Smoke, that is to say the finely divided black material from
incomplete combustion, can penetrate fairly readily into buildings,
and since the mobility of the particles is less than that of sulfur
dioxide molecules, they are less rapidly removed onto surfaces.
Concentrations of smoke indoors, assessed by soiling methods, have
been found to be in the range of 50-90% of those outdoors (Derouane,
1972; Yocom et al., 1971), again assuming there is no specific source
of this material inside. Cigarette smoke can make a very substantial
contribution to the finely divided particulate matter indoors (Elliott
& Rowe, 1976; Hoegg, 1972). It is liable to affect direct gravimetric
concentrations, but it has relatively little effect on concentrations
measured by blackness. The composition of cigarette smoke is quite
different from that of the general urban particulate matter, and it
has been stressed by De Graaf & Biersteker (1972) that there may be
many differences in the type and composition of suspended particulate
matter indoors and outdoors. This point is particularly important in
relation to samples obtained with the high volume sampler. The larger
particles (greater than 10 µm diameter) that are liable to be
collected by this method would penetrate into buildings less readily
than the fine particles, and, with reduced air movement inside, they
may fall out fairly rapidly under gravity. This may account for the
smaller proportion of total suspended particles (as compared with
smoke) found indoors in some studies (Yocom et al., 1971). However, on
the other hand, there is a risk that textile and other dusts dispersed
in the home may be sampled and assessed as part of the general
suspended particulate matter.
Sulfuric acid is unlikely to remain for any appreciable time in
occupied rooms, as it is readily neutralized by ammonia. However,
finely divided sulfate particles are likely to behave in the same way
as smoke, with a modest reduction indoors compared with outdoors.
Relative humidity is normally lower indoors than outdoors and this
will help to keep sulfate particles in suspension, but where the
humidity is especially high, as it may be in kitchens, particles
containing sulfates or other salts will grow rapidly and be deposited.
Studies on indoor concentrations of sulfur dioxide and particulate
matter are limited primarily by the need to consider the circumstances
of each location individually; nevertheless, there is a growing body
of literature on the subject that has been assembled and reviewed in
recent reports from the USA (Benson et al., 1972; Henderson et al,
1973).
5.3 Concentrations in Work Places
Concentrations of sulfur dioxide, much higher than those commonly
found in urban air, may be present in some industrial environments,
arising from processes in which the gas is handled or evolved, as well
as from combustion sources. Paper mills, sulfuric acid plants, steel
works, nonferrous metal foundries, and oil refineries are among the
places where such concentrations may be found. However, emissions are
usually highly localized and intermittent, presenting major problems
in assessing concentrations to which workers may be exposed. Results
are not normally published, but in a number of countries there is a
requirement to ensure that a specified limit is not exceeded. For
example, in the USSR, the maximum permissible concentration is
10 mg/m3 (3.8 ppm) (ILO/WHO Committee on Occupational Health, 1970)
whereas in the USA (American Conference of Government Industrial
Hygienists, 1977), this value, averaged over 8-h shifts, is 13 mg/m3
(5 ppm). This figure is of the order of 100 times the average values
in urban air and it is higher than the maximum values reported, even
in episodes of high pollution (Waller & Commins, 1966). Mean values
over 3 years of 1800-2100 µg/m3 (0.6-0.7 ppm) have been reported
close to blast furnaces in steelworks, with occasional 24-h mean
values up to 17 000 µg/m3 (5.95 ppm) (Lowe et al., 1970).
In steelworks, substantial quantities of suspended particulate
matter may be present as well as sulfur dioxide and other pollutants.
A 3-year mean concentration of about 1000 µg/m3 (respirable dust, as
measured with a Hexhlet sampler) has been reported in the study cited
above (Lowe et al., 1970). Even so, the situation does not necessarily
parallel that in urban atmospheres, for the particulate matter is
liable to include dust which differs in composition and size
distribution from that produced in the usual range of combustion
processes.
Dusts encountered in industry must, in general, be considered
separately from the suspended particulate matter in urban air. Some,
such as the coal-dust in mines, may be present at high concentrations,
and have substantial effects on health, but these are specific to that
dust, and are outside the scope of the present discussion. However,
some of the chemical compounds in dust from industrial activities are
covered by other documents of the WHO environmental health criteria
programme.
In some industries, such as wood-pulping and paper-making, sulfur
dioxide may be evolved in the process, producing high concentrations
locally without an accompanying particulate matter problem.
Concentrations in the range 6 to 100 mg/m3 (2 to 36 ppm) have been
reported at a plant in Norway (Skalpe, 1964) and similar
concentrations were found in a study in the USA (Ferris et al., 1967)
but these were gradually reduced over a 5-year period. High
concentrations, averaging about 71 mg/m3 (25 ppm), also existed at
one time in charging rooms for refrigerators when sulfur dioxide was
used as a refrigerant (Kehoe et al., 1932) and similar concentrations
have been reported in certain areas of oil refineries (Anderson 1950).
There is a specific problem of sulfuric acid mist in the forming
departments of works making lead-acid accumulators, where
concentrations up to 16.6 mg/m3 have been observed (Malcolm & Paul,
1961; Williams, 1970). This is very much greater than the
concentrations ever found in urban air (Commins & Waller, 1978) and
the physical form of the aerosol is different: mass median diameters
of the droplets have been reported to be over 10 µm (Williams, 1970)
whereas those found in urban air are of the order of 0.5 µm (Waller,
1963).
5.4 Assessment of Exposures
In the present context, the term exposure relates to the
concentrations of sulfur dioxide or suspended particulate matter in
the air breathed by individuals or populations, averaged over
specified periods. Broadly speaking, two types of exposure are
considered: short-term exposure, in which the relevant concentrations
are averaged over periods of the order of a day, and long-term
exposure, for which averages over periods of the order of a year are
commonly used. These periods are to a certain extent arbitrary,
imposed by the time-resolution of the pollution measurements and by
the nature of the health indices examined, and there is no clear
guidance on the most relevant averaging periods to use in relation
either to short-or to long-term exposure.
Exposures are usually estimated from measurements of concentration
at fixed sampling sites. The number of sampling sites required to give
an adequate representation of the exposure of people living in a given
area will depend on the topography, the distribution of sources, and
other factors. In urban areas, there is usually a close correlation
between values for neighbouring stations (Prinz, 1970). Care must be
taken, however, to ensure that these stations are not used to
represent environmental levels in areas much larger than their
coverage. Furthermore, the instruments for measurement may be sited on
the roof of a building for protection and convenience, and the levels
measured may not necessarily represent those in the ordinary breathing
zone.
The usual practice in epidemiological studies is to assume that
measurements made on outdoor air in the areas where people live or
work provide an index of exposure that allows comparison to be made
between different groups. It has been demonstrated, however, that
estimated weekly exposures calculated as averages of concentrations
measured indoors and at various outdoor sites and weighted according
to the length of time likely to be spent in each location, may differ
substantially from those indicated by measurements at just one site
close to the place of residence or work (Fugas, 1976). To validate
this approach, further measurements using personal samplers would be
required. These have been used in studies on specific components of
suspended particulate matter such as lead (Azar et al., 1972) and they
have wide application in assessing the exposures of individuals to
pollutants, including sulfur dioxide, in industry, but they are not
generally practicable for large-scale epidemiological studies on the
general population.
A further problem in assessing exposures is the great variation in
concentrations with time for, as far as sulfur dioxide is concerned, a
brief exposure to a very high concentration, with no appreciable
exposure during the rest of the day, would create a different
situation from a steady exposure to a low level throughout the day. At
present, there is no satisfactory way of measuring such situations, at
least for people moving about during the course of the day. Providing
studies are confined to groups of people living or working in
reasonably uniform circumstances, measurements made over extended
periods at a few fixed sites may give results that are valid for
comparative purposes. Difficulties are liable to arise however if
dissimilar groups are compared. In particular, people such as the very
old and the very young may be partially protected from sulfur dioxide
in the outside air if they spend long periods indoors, whereas an
active worker may be exposed not only to a higher mean concentration
in the course of the day, but also to a greater range. A further point
is that increased activity will also lead to a greater ventilation
rate, increasing the overall intake, and, in the case of sulfur
dioxide, the deep inspirations associated with exercise are liable to
increase the penetration of the gas and to enhance its effects
(Lawther et al., 1975). The situation is even more complex for
sulfuric acid aerosols. Because of the hygroscopic properties of the
droplets, they take up moisture rapidly on inspiration, becoming
larger and more dilute, and it has been suggested, on the basis of
experimental work, that those from urban air could be diluted to such
an extent that their irritant properties would be lost (Carabine &
Maddock, 1976).
When assessing long-term exposures, annual mean concentrations at
fixed sampling sites are commonly used as the basis. Short-term
variations are then of little consequence, but it may still be
necessary to consider the extent of seasonal swings in concentration,
or the frequency of occurrence of days of exceptionally high
pollution. Also, it is often necessary to examine trends in
concentrations for many years back, for the relevant exposure may have
been one that occurred earlier in life, rather than the current level.
Since there are very few long series of measurements of sulfur dioxide
or suspended particulate matter made by uniform methods, it is very
difficult to assess the true exposure of people to these pollutants.
6. ABSORPTION, DISTRIBUTION, AND ELIMINATION
Most of the sulfur compounds discussed in this report are absorbed
via the digestive system. Relatively little is absorbed from the
respiratory tract, even in areas with highly polluted ambient air.
Even so, it is reasonable to believe that the respiratory tract is the
most vulnerable organ for the local effects of sulfur oxides and
particulate matter in the ambient air. Thus, this section will deal
mainly with the absorption, deposition, and clearance of sulfur
dioxide and particulate matter in the respiratory tract.
6.1 Absorption and Deposition in the Respiratory Tract
6.1.1 Sulfur dioxide
Sulfur dioxide is highly soluble in aqueous media. Absorption
after inhalation has been studied in rabbits (Dalhamn & Strandberg,
1961; Strandberg, 1964), dogs (Balchum et al., 1959, 1960a, 1960b;
Frank et al., 1969) and man (Speizer & Frank, 1966a, 1966b).
In rabbits, about 40% of the inhaled sulfur dioxide is absorbed in
the nose and pharynx when concentrations of about 290 µg/m3 (0.1 ppm)
are inhaled. At higher concentrations (29-290 mg/m3; 10-100 ppm), the
fraction absorbed is much higher (about 95%),The reasons for these
different rates of absorption are not clear. In dogs, more than 99% of
the inhaled sulfur dioxide is absorbed by the nose at exposure levels
of 2.9-140 mg/m3 (1-50 ppm). These observations in dogs have been
confirmed in man by studies on human volunteers, with levels of
exposure to sulfur dioxide ranging from 2.9 to 420 mg/m3 (1 to
140 ppm) and exposure times of a few minutes at the higher levels and
30-40 minutes at the lower levels. Absorption can occur during
mouth-breathing, but is less efficient than during nose-breathing,
especially with increased ventilation.
6.1.2 Airborne particles
Particles smaller than about 10 µm are deposited at different
levels in the respiratory tract. The exact deposition pattern is
determined by the interaction of size, shape, and density (expressed
as aerodynamic diameter of unit density spheres) of the particles and
by airflow conditions. Some particles in the air, such as sulfuric
acid particles are hygroscopic. Such particles will take up water,
expand in the respiratory tract, and be deposited in a manner other
than would be expected from their diameters in the ambient air (Hatch
& Gross, 1964; Stuart, 1973; Task Group on Lung Dynamics, 1966;
Vigdorcik, 1948). Sophisticated theoretical models for the deposition
of particles in the lung such as the ICRP model have been constructed
(Task Group on Lung Dynamics, 1966). Deposition is caused by
impaction, sedimentation, and diffusion. Impaction is an important
mechanism for the deposition of the larger or heavier particles
(5-30 µm aerodynamic diameter) and where the air velocity is
relatively high. Impaction occurs therefore at sites where the air
stream is turbulent and is of most importance in the nose, mouth,
pharynx, and the upper part of the tracheobronchial tree.
Sedimentation or settling out of particles is also a function of
particle size and density, as well as of residence time in the
airways. It is important for the deposition of larger particles
(1-5 µm) providing they have not been deposited by impaction and is
active in the trachea, bronchi, and bronchioles. Diffusion is of
importance for particles smaller than a few tenths of 1 µm (its effect
increasing with decreasing particle size), with respect to the smaller
bronchioles and especially the alveoli. In the size range of 2-5 µm,
experimental data have fitted quite well with the ICRP model, but
deposition has varied widely (with an order of magnitude of 2 to 3)
among human subjects (Lippmann et al., 1971). In experiments on
animals, large reproducible differences in deposition have been seen
among individuals within the same species (Albert et al., 1968;
Tomenius, 1973). In the submicron range, theoretical models have not
yet been satisfactorily verified by experiments.
6.2 Clearance from the Respiratory Tract and Distribution
6.2.1 Sulfur dioxide
Sulfur dioxide is absorbed from the respiratory tract into the
blood stream. It can then be widely distributed throughout the body
(Balchum et al., 1960a, 1960b; Bystrova, 1957; Frank et al., 1967;
Yokoyama et al., 1971), where it appears to be metabolized and
excreted via the urinary tract.
6.2.2 Particulate matter
Knowledge concerning the elimination of insoluble particles
deposited in the alveoli is incomplete (Hatch & Gross, 1964; Morrow,
1973). It is clear that these particles are, to a large extent,
phagocytized by alveolar macrophages within hours, but it is not known
to what extent they are actively carried to the ciliated part of the
lung or to the lymphatic system. The particles can be transported by
the mucociliary escalator into the interstitium (where they can remain
for a long time) or the lymphatic system. Solubility in vivo is
important for the clearance of "insoluble" particles deposited in the
alveoli (Mercer, 1967; Morrow, 1973). The biological half-lives range
from days to years depending on the chemical composition of the
particles (Brain & Valberg, 1974; Task Group on Lung Dynamics, 1966;
Task Group on Metal Accumulation, 1973).
Mucociliary transport, and therefore clearance of particles, can
be affected by various factors and can be impaired by long-term
cigarette smoking and acute infection in the respiratory tract (Camner
& Philipson, 1972; Camner et al., 1973a; Jarstrand et al, 1974).
Subjects suffering from chronic obstructive lung disease may also have
an impaired mucociliary transport (Camner et al., 1973b; Toigo et al.,
1963). Studies in animals have shown that the inhalation of sulfur
dioxide can interfere with the clearance of bacteria (Rylander et al.,
1971) and inert particles (Ferin & Leach, 1973) from the lungs, but
how much is due to the action of the sulfur dioxide on the mucociliary
mechanism and how much to its action on the macrophages is not clear.
The clearance of soluble particles may follow the above pathways
depending upon their solubility. They may dissolve in the mucus in
which case they will probably be eliminated via the mucociliary route
and be swallowed and removed or absorbed via the intestinal tract. In
the alveoli, the particles may diffuse into the lymph or blood and
thus be removed from the lung.
7. EFFECTS ON EXPERIMENTAL ANIMALS
It has been difficult to separate the relative effects of sulfur
dioxide, sulfuric acid mists, sulfate salts, and particulate matter in
the ambient air by epidemiological techniques. The effects of these
substances individually and in various combinations have been studied
in the laboratory. These studies have been useful in explaining some
of the mechanisms of action but their application has been rather
limited for the establishment of safe ambient levels, particularly for
complex mixtures such as exist in the ambient air. In the past, the
levels used in laboratory studies on animals have usually been far in
excess of those seen in the ambient air and therefore have had little
relevance for ambient air quality standards. Later studies have been
at more relevant levels.
The following discussion separates work on animals into short-term
and long-term exposure studies. Short-term exposure studies are those
that involve exposures of 24-h or less and usually last from minutes
to at the most a few hours. Long-term exposure studies refer to
exposures that last longer than 24-h and are usually extended over
months. In the short-term exposure studies, only immediate or acute
effects have been studied. In long-term exposure studies, animals may
be studied during the period of exposure to determine whether there
are progressive changes or not and at the end of the exposure period
to quantify any chronic effect.
7.1 Short-term Exposure Studies
7.1.1 Exposure to sulfur dioxide singly or in combination with
other agents
Sulfur dioxide is a respiratory irritant that is very soluble in
the aqueous surfaces of the respiratory airways. Because of this high
solubility, most of the sulfur dioxide is absorbed in the nose and
upper airways (section 6.1) and very little reaches the lungs
directly. At extremely high concentrations, the absorptive capacity of
the upper airways can be overwhelmed and death or pathological changes
including laryngotracheal and pulmonary oedema can be induced in the
respiratory tract of experimental animals.
Exposure-effect curves have been developed for guineapigs (Amdur,
1966) and dogs (Frank & Speizer, 1965). In Amdur's study, a linear
relationship was obtained between exposure for 1 h to sulfur dioxide
concentrations ranging from 0.46 to 2380 mg/m3 (0.16-835 ppm) and
corresponding increases in pulmonary flow resistance. The second study
showed that nasal flow resistance increased roughly in proportion to
exposure to sulfur dioxide concentrations ranging from 20 to
660 mg/m3 (7-230 ppm) for a 15-20 minute period.
Studies with guineapigs at lower concentrations have shown that a
sodium chloride aerosol, administered concomitantly enhances the
effects of sulfur dioxide on the lungs in the form of
bronchoconstriction and increased airway resistance (Amdur, 1957). It
was postulated that the sodium chloride could act as a carrier to
deliver the absorbed sulfur dioxide deep into the lungs, or that the
increased humidity in the respiratory tract could react with the
sulfur dioxide to form sulfurous acid, especially if catalysts were
present (Amdur & Underhill, 1968). McJilton et al. (1973) have
reaffirmed the importance of humidity in this reaction, exposing
guineapigs for 1 h to sulfur dioxide at 3.1 mg/m3 (1.1 ppm) and a
sodium chloride aerosol of about 1 mg/m3. They allowed the mixture to
"age" in a reaction chamber for 8-10 minutes at various relative
humidities. The chamber temperature was 22°C and the average aerosol
particle size was 0.1 µm with a maximum size of less than 2 µm. At
relative humidities above 80%, they noted a marked increase in
pulmonary resistance, whereas administration of the individual
components produced little or no effect. The droplets formed at high
humidity and in the presence of the aerosol had a pH of 3.2 ± 0.5.
Analysis of these particles by mass spectrometer revealed sulfur
dioxide and bisulfite ions (HSO-3)in the solution but no sulfuric
acid. In the high humidity, the sodium chloride aerosol apparently
became hydrated and could then absorb sulfur dioxide. The authors
indicated that the mixture had to "age" to ensure absorption of the
sulfur dioxide; otherwise there would be too much competition with the
moist surfaces in the nasopharynx which could sweep out the sulfur
dioxide. Thus, this is another mechanism whereby the effect of sulfur
dioxide can be enhanced. If such mixtures were allowed to "age"
longer, it seems likely that the sulfur dioxide could be changed to
sulfuric acid. Studies using other animal species such as the cat have
not shown this enhancement of the effect of sulfur dioxide by sodium
chloride (Corn et al., 1972).
Matsumura (1970a) exposed guineapigs to ozone at 2.1, 11, or
21 mg/m3 (1, 5, or 10 ppm), nitrogen dioxide at 40, 80, or 140 µg/m3
(20, 40, or 70 ppm), or sulfur dioxide at 60, 170, 510, or 940 mg/m3
(20, 60, 180, or 330 ppm) for 30-50 minutes. Each group with unexposed
controls was then exposed to an aerosolized antigen (2 µm) of egg
albumin and bovine albumin for 45 minutes, 5-7 times at intervals of a
day or more. Two weeks after the last exposure, the animals were
killed and blood was drawn for assay of the immunological response.
Enhanced sensitization was noted only at the highest levels of
exposure. During these exposures a number of animals died of
anaphylactic reactions at the fifth or sixth inhalation. In another
study, sulfur dioxide at a concentration of 1100 mg/m3 (400 ppm) did
not have any effect on dyspnoeic attacks (Matsumura, 1970b).
7.1.2 Exposure to sulfuric acid aerosols or suspended sulfates
Sulfuric acid mist and some of the sulfate salts are more powerful
respiratory irritants than sulfur dioxide, and this effect is also
related to particle size (smaller particles tending to be more
irritating) (Amdur, 1958). Treon et al. (1950) exposed rabbits, rats,
mice, and guineapigs to sulfuric acid mist, 93-99% of which was less
than 2 µm in diameter, many droplets being about 1 µm in diameter.
Concentrations ranged from 87 to 1610 mg/m3. Despite the relatively
small number of animals used, a clear-cut species difference was shown
at these high concentrations. The order of increasing sensitivity was
rabbits, rats, mice, guineapigs.
It was reported by Amdur et al. (1952a) that the 8-h LC50 of
sulfuric acid mist with a mass median diameter (MMD) of about 1 µm was
18 mg/m3 for 1-2 month old guineapigs. Pattle et al. (1956) showed
that sulfuric acid mist of MMD 2.7 µm was more toxic to guineapigs
than a mist of 0.8 µm, and that the toxicity of the smaller particles
increased when the exposure occurred at 0°C. However, this might be a
response of the guineapig to low temperature rather than to the
sulfuric acid mist. Concomitant exposures with ammonium carbonate had
a protective action, apparently because it neutralized the sulfuric
acid.
Table 11. Sulfuric acid and some sulfates in descending order of
their irritative capacity for animals. Presented for
equivalent amounts of sulfur and at comparable particle
size i.e., sub-micron; short-term exposuresa
Sulfuric acid H2SO4
Zinc ammonium sulfate ZnSO4(NH4)2 SO4
Iron (III) sulfate Fe2 (SO4)3
Zinc sulfate ZnSO4
Ammonium sulfate (NH4)2 SO4
Nonreactive
Iron (II) sulfate FeSO4
Manganese (II) sulfate MnSO4
a From: Amdur (1969, 1970, 1971).
Studies on guineapigs have shown that, for equivalent amounts of
sulfur and in comparable particle size, sulfuric acid is more
irritative than any of the sulfate compounds, some of which appear to
be nonreactive in animals (Table 11). Whether these compounds would
behave in the same manner in complex mixtures such as those found in
polluted air is not known. Some of the nonreactive compounds such as
manganese salts or oxides can catalyze the reaction of sulfur dioxide
to sulfuric acid.
In short-term exposure studies by Amdur (1958), concentration
seemed to be more important than duration and death was related to
laryngospasm and bronchospasm. Sulfuric acid mist also caused
parenchymal lung damage that seemed to be related to the total dose
(Amdur, 1958; Pattle et al., 1956).
7.2 Long-term Exposure Studies
7.2.1 Exposure to sulfur dioxide
Rats were exposed for 96 days to sulfur dioxide at concentrations
of 0.1, 0.5, and 1.5 mg/m3 (0.04, 0.18 & 0.53 ppm). Histological
examination showed interstitial pneumonia, bronchitis, tracheitis, and
peribronchitis after exposures to the two higher levels (Elfimova &
Gusev, 1969).
Misiakiewicz (1970) exposed rats continuously during 5 months to
sulfur dioxide at concentrations of 0.3, 0.5, 1.0, 2.0, and
20.0 mg/m3 (0.11, 0.18, 0.35, 0.7 and 7.0 ppm). Exposures to
2.0 mg/m3 (0.7 ppm) and 20.0 mg/m3 (7.0 ppm) increased the activity
of serum cholinesterase (EC 3.1.1.8) and aspartate aminotransferase
(EC 2.6.1.1) and caused morphological changes in the upper respiratory
tract.
After a 120-h exposure to a sulfur dioxide concentration of
3 mg/m3 (1.1 ppm), guineapigs showed proliferative interstitial
pneumonia, bronchitis, and tracheitis and an increased histamine
content in the lungs, while exposure to 167 µg/m3 (0.06 ppm) of
sulfur dioxide for one month led to interstitial changes in the
respiratory tract (Bustueva, 1961a). Bustueva (1966) also exposed rats
for 65 days (24 h per day) to sulfur dioxide at 4.86 mg/m3 (1.7 ppm).
Tracheitis, desquamation of epithelial cells, an increased amount of
purulent mucus, and interstitial pneumonia were found. It was not
clear, however, whether this was due to the sulfur dioxide or to the
pulmonary infection that can develop in rats.
Beagle dogs exposed to a sulfur dioxide concentration of
13.4 mg/m3 (4.7 ppm) for 21 h per day, for 620 days, did not develop
any specific histopathological changes (Lewis et al., 1973).
Guineapigs have been exposed to sulfur dioxide levels up to
16.3 mg/m3 (5.7 ppm) for 12 months without definite effects except
for slight cytoplasmic vacuolation in the liver (Alarie et al., 1970).
Cynomolgus monkeys exposed continuously for 78 weeks to sulfur dioxide
levels up to 3.7 mg/m3 (1.3 ppm) did not show any significant
pathological changes (Alarie et al., 1972). However, rats exposed to
sulfur dioxide at 2.9 mg/m3 (1 ppm) for 170 h showed a significant
reduction in clearance of inert particles from the lung (Ferin &
Leach, 1973). Syrian hamsters, made emphysematous by previous exposure
to aerosolized papain, tolerated concentrations of sulfur dioxide up
to 1900 mg/m3 (650 ppm). Exposures were for 4 h per day, 5 days per
week, for a total of 19-74 exposures. Only slight changes in the
mechanical properties of the lung were noted as well as slight
bronchitis (Goldring et al., 1970). As Syrian hamsters are
exceptionally resistant to the effects of sulfur dioxide, these data
must be extrapolated with caution to other species.
7.2.2 Exposure to sulfuric acid aerosols
Alarie et al. (1973) exposed cynomolgus monkeys for 78 weeks to
sulfuric acid mist at concentrations of 0.38 to 4.79 mg/m3 and
particle sizes of 0.54 to 3.60 µm. At concentrations of 2.43 and
4.79 mg/m3 and particle sizes of 3.60 and 0.73 µm, definite damage to
the pulmonary structure was evident and there was deterioration in
pulmonary function. At the lower concentrations, changes were slight
or absent. The authors also exposed guineapigs for 52 weeks to
concentrations of 0.08-0.1 mg/m3 and particle sizes of 0.84 and
2.78 µm; no detectable effects were seen.
When beagle dogs were exposed to sulfuric acid at a concentration
of about 0.9 mg/m3 for 21 h per day, for 620 days, there was a
significant reduction in pulmonary function; 90%, of the sulfuric acid
mist was less than 0.5 µm in diameter (Lewis et al., 1973).
Histopathological changes were produced in the alveolar part of the
lung, especially in the elastic tissue, of rats exposed for 65 days
(24 h per day) to a sulfuric acid concentration of 1 mg/m3. Ninety
per cent of the particles were less than 2 µm in diameter in this
study (Bustueva, 1966).
Guineapigs were exposed to sulfuric acid aerosol for 120 h at 3
different concentrations (1.98 ± 0.03; 4.20 ± 0.06; and 8.27 ±
0.15 mg/m3). Exposure to the highest concentration led to oedema of
the lungs, changes in the interalveolar walls, sharp, diffused,
interstitial changes, and increased histamine content in the lung
tissue. Three weeks after exposure, sclerosis appeared (Bustueva,
1957). After a 1-month exposure to 0.1 mg/m3, no changes were found
(Bustueva, 1961a).
7.2.3 Exposure to a mixture of sulfur dioxide and sulfuric acid
aerosols or this mixture combined with other agents
Rats were exposed for 65 days (24 h per day) to a combination of
sulfur dioxide at 4.86 mg/m3 (1.7 ppm) and a sulfuric acid aerosol at
1 mg/m3 (90% of the aerosol particles were less than 2 µm in
diameter). There was a summation of the effects (histopathological
changes in alveolar tissue) produced by exposure to each of the
pollutants alone (Bustueva, 1966).
Lewis et al. (1973) exposed beagle dogs to a combination of sulfur
dioxide at 13.4 mg/m3 (4.7 ppm) and sulfuric acid at about
0.9 mg/m3. Ninety percent of the sulfuric acid mist was less than
0.5 µm in diameter. The duration of exposure was 21 h per day, for 620
days. Some of the dogs had been pre-exposed to nitrogen dioxide. This
group tended to show less response in the form of changes in pulmonary
function than dogs not pre-exposed to nitrogen dioxide.
Alarie et al. (1975) exposed cynomolgus monkeys and guineapigs to
mixtures of sulfur dioxide, fly ash, and sulfuric acid mist, for 18
months after an 8-week baseline period. Exposure concentrations varied
from 0.29 to 143 mg/m3 (0.1 to 5.0 ppm) for sulfur dioxide and from
0.1 to 1 mg/m3 for sulfuric acid mist; the concentration of fly ash
was approximately 0.5 mg/m3. Particle size (MMD) varied from 0.53 to
3.11 µm in the acid mist and from 4.1 to 5.8 µm in the fly ash.
Pulmonary function tests and serum biochemical and haematological
analyses were conducted prior to, and periodically during, the
exposure. Lungs were examined microscopically at the end of the
experiment. Sulfuric acid mist appeared to be responsible for the
effects observed. These were largely histopathological changes in the
lungs. No synergistic action was noted between the pollutants.
7.2.4 Combined exposure to sulfur dioxide and particulate matter
or other gaseous pollutants
In studies by Frazer et al. (1968), white albino rats were exposed
to sulfur dioxide at 2.9 and 8.6 mg/m3 (1 and 3 ppm) and graphite
dust at 1 mg/m3. The mean particle size of the dust was less than
1 µm and the MMD was 3 µm. The animals were exposed for 12 hours per
day, 7 days per week, for 4 months. Both ciliary activity, and the
number of dust-containing cells per 100 lung cells appeared to be
unaffected after 56-109 days of exposure.
Various aerosols that may react with sulfur dioxide have been
studied either singly or in combination with sulfur dioxide in
experimental animals, particularly in guineapigs. Amdur (1969, 1971)
emphasized that the particle size of the aerosol as well as the
concentration was extremely important in the determination of toxicity
and that the most important size range was the submicron level. The
changes induced in pulmonary mechanics were slow to return to
pre-exposure levels. This indicates either that deposited aerosols may
not be cleared promptly but remain in the lungs exerting their effect
for a period of time, or that the repair mechanisms are slow, or that
both conditions are present. There was also a variation in the
toxicity of different sulfates for the same particle size and sulfur
content (Amdur, 1969) (Table 11).
Studies by Battigelli et al. (1969) in which rats were exposed to
sulfur dioxide at 2.9 mg/m3 (1 ppm) and graphite dust at 1 mg/m3
(particle size not stated) did not show any effect other than the
accumulation of dust in the lung after exposure for 4 months, for 12
hours per day. Amdur & Underhill (1968) pointed out that not all
aerosols enhance the effect of sulfur dioxide and that only those
aerosols composed of droplets in which the sulfur dioxide could
dissolve were active.
Mice were exposed by Zarkower (1972) to carbon particles (1.8 to
2.2 µm MMD) at a concentration of 558 ± 154 µg/m3 and sulfur dioxide
at 5.7 mg/m3 (2 ppm). The animals were exposed to carbon alone,
carbon with sulfur dioxide, and sulfur dioxide alone; controls were
unexposed. Killed Escherichia coli was used as an antigen and
antibody production was measured. Exposures for 192 days produced
significant immunosuppression. Shorter periods of exposure resulted in
variable effects including stimulation of antibody production.
A variety of metallic aerosols can catalyze the oxidation of
sulfur dioxide including the soluble salts of ferrous iron, manganese,
and vanadium.
Rylander & Bergström (1973) exposed animals with latent upper
respiratory disease and controls for 4 weeks to various combinations
of sulfur dioxide at 57 mg/m3 (20 ppm), manganese dioxide at
12-20 mg/m3 (particle size between 5.0 and 0.5 µm), and carbon
monoxide at 187-250 mg/m3 (150-200 ppm). Animals with latent upper
respiratory disease showed more extensive histological changes and an
increase in the number of free lung cells. The combination of sulfur
dioxide and manganese dioxide produced the greatest changes.
An addition of effects in rats was reported by Salamberidze (1969)
from joint exposure to sulfur dioxide at 0.15 mg/m3 (0.05 ppm) and
nitrogen dioxide at 0.1 mg/m3 (0.05 ppm) over a 3-month period.
The precise mechanism by which the oxides of sulfur and
particulate matter can affect the lungs is not known. Sulfur dioxide
(and presumably sulfuric acid as well) can interfere with the
clearance of bacteria (Rylander et al., 1971) and inert particles
(Ferin & Leach, 1973) from the lungs (see also section 6). Chronic
exposure to sulfur dioxide increased the number and area of goblet
cells in guineapigs and lambs (Mawdesley-Thomas et al., 1971).
The considerable variations in the results of these experiments on
animals reflect differences in sensitivity of individual species,
exposure levels, and methods used to assess the effects.
It should be emphasized that extrapolation of these results from
animals to human beings is not easy. These findings, however, do give
some insight into possible mechanisms of action and reactions that can
occur.
8. EFFECTS ON MAN
8.1 Controlled Exposures
A number of studies have been performed on volunteers under
controlled conditions of exposure to sulfur dioxide or sulfuric acid
aerosols, singly or in combination, or to mixtures of these with other
compounds such as ozone and hydrogen peroxide. These studies, all
conducted under short-term exposure (up to 24 h), include those on
changes in respiratory function and effects on sensory and reflex
organs.
8.1.1 Effects on respiratory organs
8.1.1.1 Exposure to sulfur dioxide
Amdur et al. (1953) exposed 14 healthy volunteers (inhaling
through the mouth) to sulfur dioxide at concentrations of
2.9-23 mg/m3 (1-8 ppm) for 10 min. They noted an increased pulse
frequency, decreased tidal volume, and an increased respiratory
frequency which returned to normal levels after exposure. The sequence
of exposures was randomized. Effects increased with increasing levels
of sulfur dioxide and were detectable at the lowest concentration
tested (2.9 mg/m3; 1 ppm). Sulfur dioxide levels were monitored by
the conductimetric method. However, Lawther (1955) was unable to
reproduce these effects in any consistent manner, either in urban or
in rural dwellings. Eleven healthy volunteers were exposed to sulfur
dioxide at levels of 2.9, 14, and 37 mg/m3 (1, 5, and 13 ppm) in
studies by Frank et al. (1962). Respiratory mechanics were measured by
means of a body plethysmograph and an oesophageal balloon. Exposures
lasted 10-30 min. Only one of the 11 subjects exposed showed an
increased pulmonary resistance at 2.9 mg/m3 (1 ppm). Increased
pulmonary resistance was noted in all subjects and was greater at the
higher concentration. The change occurred within 1 min of exposure and
increased up to 10 min, after which no further increase was noted.
In studies by Snell & Luchsinger (1969), exposure to a sulfur
dioxide concentration of 2.9 mg/m3 (1 ppm) for 15 min produced a
slight effect on total respiratory resistance in 9 healthy volunteers.
Andersen et al. (1974) exposed healthy male subjects to sulfur
dioxide at levels of 2.9, 14, and 71 mg/m3 (1, 5, and 25 ppm) for up
to 6 h. With exposures of 1-3 h at 2.9 mg/m3 (1 ppm), there was a
decrease in the flow of nasal mucus and a decrease in the cross
section of the nasal passages. Thus it appears that exposure to
concentrations of 2.9 mg/m3 (1 ppm) or more may result in impairment
of mucociliary transport in the nose.
Four healthy volunteers were exposed to sulfur dioxide and the
forced vital capacity (FVC), one second forced expiratory volume
(FEV1.0), mid-maximal flow rates (MMFR), maximal expiratory flow rate
at 50% (MEFR50) and closing volume and capacity were measured.
Although no changes were found in these tests at 1.1 mg/m3
(0.37 ppm), slight changes in FVC, FEV1.0, MMFR, and MEFR50 were
noticed at 2.1 mg/m3 (0.75 ppm) after exposure for 30 min; no effect
on closing volume was detected (Bates & Hazucha, 1973).
The results of these studies on human volunteers are summarized in
Table 12.
8.1.1.2 Exposure to sulfuric acid aerosols
Amdur et al. (1952b) exposed 15 healthy men to sulfuric acid mist,
through mouth breathing, at concentrations of 0.35-5 mg/m3 (particle
size of approximately 1 µm) for periods of 5-15 min. At concentrations
below 1 mg/m3, the mist did not produce any subjective sensations
although 5 of the 15 subjects showed a slightly increased respiratory
rate and a decreased tidal volume at 0.35 mg/m3. All subjects noted
irritation at a concentration of 3 mg/m3. Respiration was monitored
by means of a pneumotachograph. The respiratory changes as well as the
subjective sensations increased with increasing concentrations of
sulfuric acid. Healthy male volunteers were also exposed to sulfuric
acid mist by Sim & Pattle (1957), either by mask or in a chamber for
periods ranging from 10 to 60 min. Twelve men were exposed to the
mist. The temperature of the air was 18.4°C with a relative humidity
of 62%. The MMD of the aerosol was 0.99 µm and the concentration was
39.4 mg/m3. The men noted minor irritation, and lung resistance (as
measured by the interrupter technique) rose by 35-100%. With
re-exposure for 30 min to the mist at a temperature of 24.5°C, a
relative humidity of 91%, a concentration of 20.8 mg/m3, and a MMD of
1.54 µm, severe coughing and irritation of the throat occurred. The
men found it almost intolerable. Lung resistance had risen by 43-150%
when measured after 10 minutes of exposure and when coughing had
ceased. No changes in respiration, blood pressure, or pulse rate were
noted. Two of the men exposed to these conditions had persistent
symptoms for some days after exposure ended.
It is difficult to evaluate these two studies. Amdur did not
report the temperature, though it was probably in the neighbourhood of
24°C, or the relative humidity. The studies of Sim & Pattle were at
relatively high levels but they demonstrated the importance of the
relative humidity or perhaps even more important, the absolute
humidity. The results of these studies are summarized in Table 13.
None of these studies used sulfates.
Table 12. Selected laboratory studies on the effects of short-term exposures to sulfur dioxide on respiratory
function in volunteers
Concentration Length of Effects Subjects Reference
exposure
(mg/m3)a (ppm) (min)
2.9-23 1-8 10 Increased pulse rate, decreased 14 healthy males Amdur et al. (1953)
tidal volume, and increased
respiratory rate
2.9 1 10-30 Increased pulmonary/resistance 11 healthy males Frank et al. (1962)
2.9 1 15 Increased respiratory resistance 9 healthy subjects Snell & Luchsinger (1969)
(5 males &
4 females)
2.9 1 60-180 Decreased nasal mucus flow 15 healthy males Andersen et al. (1974)
and decreased cross section of
nasal passages
2.1 0.75 120 Slight effect in 30 min on 4 healthy subjects Bates & Hazucha (1973)
FVC, FEV1-0, MMFR, and
MEFR50; no effect on
closing volume
1.1 0.37 120 No effect on above tests of 4 healthy subjects Bates & Hazucha (1973)
pulmonary function throughout
exposure period
a Original levels reported as ppm have been converted to mg/m3 and rounded off.
Table 13. Selected laboratory studies on the effects of short-term exposures to sulfuric acid mist on respiratory
function in volunteers
Concentration Particle Size Length of Relative Temperature Effects Subjects Reference
(mg/m3) (µm) exposure Humidity (°C)
(min) (%)
0.35 1 5-15 ? Room (?24) 5 of 15 subjects 15 healthy Amdur et al.
increased subjects (1952b)
respiratory rate
and decreased
tidal volume
39.4 0.99 60 62 18.4 Increased 12 healthy Sim & Pattle
pulmonary males (1957)
resistance and
minor irritation
20.8 1.54 30 91 24.5 Marked increase 12 healthy Sim & Pattle
of pulmonary males (1957)
resistance and
severe irritation
8.1.1.3 Exposure to mixtures of sulfur dioxide and other compounds
Frank et al. (1964) repeated the above exposures in combination
with a sodium chloride aerosol. Concentrations of sulfur dioxide were:
2.9-5.7 mg/m3 (1-2 ppm), 11-17 mg/m3 (4-6 ppm), and 40-49 mg/m3
(14-17 ppm). The sodium chloride aerosol had a geometric mean diameter
of 0.15 µm with a geometric standard deviation of 2.3 µm and an
average concentration of 18 mg/m3. As in the earlier studies, little
change in pulmonary flow resistance was noted at the lower levels of
sulfur dioxide alone (2.9-5.7 mg/m3; 1-2 ppm) but a progressive
increase was noted at higher levels. The authors did not find any
systematic differences between the responses to sulfur dioxide alone
or to the gas plus the aerosol. As mentioned in section 8.1.1.1, Snell
& Luchsinger (1969) noted a slight effect of sulfur dioxide at
2.9 mg/m3 (1 ppm) on total respiratory resistance but could not
demonstrate an enhancing effect of either a sodium chloride aerosol or
a distilled water aerosol. Burton et al. (1969) exposed volunteers to
a concentration of sulfur dioxide of 6 mg/m3 (2.1 ppm) with or
without a sodium chloride aerosol (MMD of less than 0.4 µm) at a
concentration of 2.2 mg/m3. The inhaled air was warmed and moistened.
However, they too failed to demonstrate any effect of the sodium
chloride aerosol on the response to sulfur dioxide.
Thus, three groups have not been able to demonstrate any enhancing
effect of sodium chloride aerosol on respiratory resistance in man as
had been demonstrated in guineapigs. This does not exclude the
possibility that other particulate matter might enhance the effect of
sulfur dioxide on the human respiratory system. These studies should
be repeated at levels of humidity above 80% and the mixture should be
allowed to "age".
The possible interaction of sulfur dioxide and hydrogen peroxide
has been examined by Toyama & Nakamura (1964) and that of sulfur
dioxide and ozone by Bates & Hazucha (1973). Toyama & Nakamura (1964)
studied 24 healthy male volunteers by means of a pneumotachograph and
the interrupter technique to measure alveolar pressure; airway
resistance was determined from these measurements. The particle sizes
of the hydrogen peroxide were reported to be 1.8 and 4.6 µm.
Concentrations of hydrogen peroxide were 0.8-1.4 mg/m3 for the larger
particles and 0.01-0.1 mg/m3 for the smaller ones. Levels of sulfur
dioxide ranged from 2.9 to 170 mg/m3 (1-60 ppm). The subjects were
not aware whether they were breathing hydrogen peroxide, sulfur
dioxide, or combinations. It was not stated whether the order of
administration was randomized or not. The investigators noted a marked
increase in airway resistance with the mixture compared with the
individual components. They also noted that the larger particles had
more effect than the smaller particles. This was probably due to the
greater dose delivered, although they did not comment on this. It was
the authors' opinion that the enhancement of the response to the
combination was due to the conversion of sulfur dioxide to sulfuric
acid, although no measurement for sulfuric acid was made.
In similar studies by Bates & Hazucha (1973) healthy male
volunteers were exposed in a chamber to sulfur dioxide, or ozone, or a
combination of the two. Changes in FVC, FEV1.0, MMFR, MEFR at 50%
vital capacity and peak expiratory flow rates were studied and some
effects were noted during exposure to ozone at 540-1610 µg/m3
(0.25-0.75 ppm). Sulfur dioxide by itself had little effect over a
similar range. Joint exposure to sulfur dioxide and ozone at
1060 µg/m3 (0.37 ppm) and 790 µg/m3 (0.37 ppm), respectively,
produced a greater effect than ozone alone. Changes were noted after
exposure for 30 min and were marked after 2 h of exposure. Exercise
during exposure enhanced the effect.
8.1.2 Effects on sensory or reflex functions
Studies in the USSR have concentrated on the effects of sulfuric
acid aerosols and sulfur dioxide on sensory receptors, cerebral
cortical function, and their interrelationships. When sulfuric acid
mist produced subjective sensory stimulation such as odour or
irritation of mucous membranes, or both, then, invariably, objective
evidence of central nervous system depression could be demonstrated
(Rjazanov, 1962).
Bustueva (1961b) did not note any change in optical chronaxy in
volunteers exposed to concentrations of sulfur dioxide of 0.5 mg/m3
(0.18 ppm) and sulfuric acid of 0.3 mg/m3. However, when levels of
1.5 mg/m3 for sulfur dioxide, 0.73 mg/m3 for sulfuric acid, and
combined levels of 1.2 mg/m3 and 0.6 mg/m3, respectively, were
exceeded, optical chronaxy increased (Table 14). Similar effects were
seen in dark adaptation responses (Rjazanov, 1962).
Studies have also been carried out in which the cerebral cortex
was monitored by electroencephalography. Alpha rhythm suppression was
used as an index of response. Threshold levels at which a response was
noted are given in Table 14. (Bustueva et al. 1960).
Table 14. Threshold levels of sulfur dioxide and sulfuric acid required for effects on sensory or
reflex functions in volunteers during short-term exposuresa
Threshold levels (mg/m3)
Effects Sulfuric acid Sulfur dioxide Sulfuric acid + Sulfur dioxide
Perception of odour and 0.6 to 0.85 1.6 to 2.8 0.3 + 0.5
irritation of mucosa
Suppression of dark 0.63 to 0.73 0.92 0.3 + 0.5
adaptation
Elevation of optical 0.73 1.5 0.6 + 1.2
chronaxy
Disruption of alpha 0.63 0.9 0.3 + 0.5
rhythm
Conditioning of 0.4 0.6 0.15 + 0.5
electrocortical reflex or 0.3 + 0.25
a Summarized from studies in the USSR (Bustueva, 1961b; Bustueva et al., 1960; Rjazanov, 1962).
The electrocortical conditioned reflex is a central nervous system
phenomenon elicited only after a succession of repeated, conditioned
reflex trials. After exposure to a combination of irritants (sulfur
dioxide or sulfuric acid) and light has been repeated several times,
desynchronization begins to appear before the light is switched on.
This can be produced at levels generally not sensorially perceived.
Thus, unperceived odour or stimulus appears to become the conditioning
stimulus and generates the conditioned electrocortical reflex
(Rjazanov, 1962).
Elfimova & Hacaturjan (1968) exposed volunteers to sulfur dioxide,
phenol, and carbon monoxide and noted a summation of effects as
measured by reflex action. No interaction was noted between sulfur
dioxide at 0.5 mg/m3 (0.18 ppm) and carbon monoxide at 3 mg/m3
(2.4 ppm) as measured in volunteers by the sensory reflex technique
(Mamacasvili, 1968).
8.2 Industrial Exposure
Workers are exposed to sulfur dioxide or sulfuric acid mist in a
number of industries; sometimes exposure is not solely to sulfur
dioxide or sulfuric acid. Exposed populations have been studied with
respect to the effects of exposure on their health status or on their
respiratory system. However, in many of these studies, only the
currently employed workers were examined and a serious effort was not
made to locate subjects who had left the industry and who may have
suffered more from the disease or could have been more sensitive to
the materials. It should also be emphasized that, in general, in the
following reports, the exposure levels studied were from spot samples
or for very short time intervals.
8.2.1 Exposure to sulfur dioxide singly or in combination with
particulate matter
Kehoe et al. (1932) studied men working in a refrigerator company
in the USA where sulfur dioxide was the refrigerant. Exposures
averaged 60-90 mg/m3 (20-32 ppm) with peaks as high as 200 mg/m3
(70 ppm). These peaks had probably been higher in the past ranging up
to 290 mg/m3 (100 ppm) or more. The exposed group had significantly
more respiratory symptoms and colds. They also complained more of
fatigue and shortness of breath on exertion. Chest X-rays of the
exposed and unexposed groups showed the same distribution of
abnormalities. The authors concluded on their inadequate evidence that
there was no injury to the tracheobronchial tree or alveoli.
In studies on men working in smelters in Sweden, Sjörstrand (1947)
found that those who had worked at the roasting and reverberatory
furnaces and in the converter hall for 8 years or more had poorer
respiratory function than men working in other parts of the smelter.
Levels of exposure and the smoking histories of the subjects were not
reported. Men working in smelters are exposed to a variety of dusts as
well as to sulfur dioxide and it is difficult to separate the effects
of such exposures from those due to sulfur dioxide.
Men exposed to sulfur dioxide at daily mean concentrations up to
70 mg/m3 (25 ppm) with occasional peaks of 290 mg/m3 (100 ppm) in
certain areas of a petroleum refining plant in Abadan (Iran) were
compared by Anderson (1950) with an unexposed group. No differences
were found between the two groups. This study also did not refer to
the smoking histories of the subjects.
In a study in Norway, pulp mill workers were compared with paper
mill workers using a standard questionnaire on respiration and simple
tests of pulmonary function. The smoking histories of the subjects
were also studied. Levels of sulfur dioxide ranged from 6-100 mg/m3
(2-36 ppm) with peaks of 290 mg/m3 (100 ppm) when the digester was
blown. The exposed group had more cough, sputum, and dyspnoea than the
unexposed group but the vital capacities were similar in both groups.
The expiratory peak flows, however, of the exposed men under 50 years
of age were lower than those in a comparable unexposed group (Skalpe,
1964).
A similar study was carried out in the USA by Ferris et al. (1967)
who reported that there was no difference between men in a pulp mill
and men in a paper mill. Both groups had less respiratory disease than
was reported from a survey of the general population. The authors
noted that some of the men working in the paper mill had worked in the
pulp mill but had left because they could not tolerate the conditions.
Occupational levels of exposure to sulfur dioxide ranged from a trace
to 95 mg/m3 (33 ppm). Average levels ranged from 6 to 35 mg/m3
(2-13 ppm). Smoking habits were considered.
Huhti et al. (1970) studied pulp and paper mill workers in Finland
and noted that the effects of smoking were much more significant than
the exposures at work or the effects of climate. Levels of exposure
were not reported in this study.
Men working in two integrated steel mills in Wales were studied by
Lowe et al. (1968, 1970) and Warner et al. (1969). Mean concentrations
of sulfur dioxide over 3 years ranged from 1.8 to 2.1 mg/m3 (0.6 to
0.7 ppm) and those of suspended particulate matter in the respirable
range, ranged from 600 to 1800 µg/m3 (by elutriation technique).
Analysis of the particulate matter showed that it was mainly composed
of iron oxides and calcium sulfate. No effects on respiratory symptoms
or on simple tests of pulmonary function were found after
standardization for cigarette smoking and for age. These observations
may reflect the limitation of studying occupational groups because of
the effect of the selection processes, or this may be an example in
which the suspended particulate matter present was not interacting
with the sulfur dioxide to produce an effect.
8.2.2 Exposure to sulfuric acid mist
Dorsch (1913) studied men exposed to sulfuric acid mist in a plant
manufacturing storage batteries in Germany. He reported that at a
concentration of 0.5 mg/m3, the mist was barely noticeable; at
2.0 mg/m3, there was nose and throat irritation, at 3-4 mg/m3, there
was distinct discomfort, and at 6-8 mg/m3, there was marked
discomfort. These responses are comparable to those reported in
laboratory studies on human beings in section 8.1. No particle size
was given but, as noted in another survey reported below, they were
probably relatively large.
Men in the battery industry were also examined by Malcolm & Paul
(1961) in the United Kingdom who reported that there was significant
erosion of the teeth of the battery room workers. This was confirmed
by ten Bruggen Cate (1968). Apparently this was due to the direct
impingement of relatively large droplets of sulfuric acid on the
teeth.
Williams (1970) studied men from the same works as Malcolm & Paul
(1961) and reported that there was no difference in the forced vital
capacity and the one-second forced expiratory volume between the men
in the forming and control departments. Levels of sulfuric acid mist
averaged 1.4 mg/m3 during working hours over two days and ranged from
a trace to 6.1 mg/m3 in 1968. An earlier survey reported higher
values. Particle size reported from a survey in another firming
department doing comparable work was 14 µm MMD; 4% of the particles
being less than 4 µm MMD. The level of sulfuric acid in this
department was 2.7 mg/m3.
8.3 Community Exposure
Much of the information that has been gained concerning the
effects on health of exposure to realistic concentrations of sulfur
oxides and particulate matter has come from epidemiological studies,
carried out on segments of population chosen by virtue of place of
residence, age, existing state of health, or other characteristics, in
order to present contrasts in exposure or sensitivity to these
pollutants. Some studies have been based on the complete populations
of urban areas, observing the total number of deaths, or the incidence
or prevalence of illness within them in relation to differences in
pollution between areas, or with time in any one area.
Many epidemiological studies concerning the health effects of
exposure to sulfur oxides and particulate matter have been reported in
the literature. In the discussion that follows, attention has been
directed to papers that yield information relevant to the development
of exposure-effect and exposure-response relationships for sulfur
oxides, smoke, and suspended particulate matter, and to some others
that are of interest from the point of view of the method of approach.
8.3.1 Mortality -- effects of short-term exposures
The most clearly defined effects on mortality arising from
exposure to sulfur oxides and particulate matter have been the sudden
increases in the number of deaths occurring, on a day-to-day basis, in
episodes of high pollution. The most notable of these occurred in the
Meuse Valley in 1930 (Firket, 1931), in Donora in 1948 (Schrenk et
al., 1949), and in London in 1952 (Ministry of Health, UK, 1954). The
people primarily affected were those with pre-existing heart or lung
disease or both, and the elderly. The London episode lasted for 5 days
and it was estimated that the number of deaths during and immediately
after this period was about 4000 more than expected under normal
circumstances. On one day, the number of deaths was about three times
the number expected at that time of the year. Concentrations of sulfur
dioxide as high as 3.7 mg/m3 (1.3 ppm) were recorded in the centre of
the urban area (48-h average). Concentrations of particulate matter
were too great to be measured properly (British daily smoke/sulfur
dioxide method), and the 48-h average of about 4.5 mg/m3 at a central
site must be regarded as a conservative estimate. These were rough
estimates for the exposures and, probably, there was considerable
variation in individual exposures.
Following these major episodes, attention was turned to studies on
more moderate day-to-day variations in mortality within large cities,
in relation to pollution. Gore & Shaddick (1958) correlated mortality
in the County of London (the inner part of the Greater London Area)
with pollution by smoke and sulfur dioxide for 4 foggy periods in
1954-56, using 7-day moving averages to smooth out the data. The
authors considered that, in two of the episodes, there was a marked
increase in mortality from bronchitis and other lung diseases,
particularly in the elderly. They concluded that when the 24-h mean
concentration of smoke exceeded 2.0 mg/m3 at the same time as the
24-h mean concentration of sulfur dioxide exceeded 1.1 mg/m3
(0.4 ppm) (British daily smoke/sulfur dioxide method), there would be
increased mortality. Care was taken in this study to ensure that the
measurements were reasonably representative of the exposure of people
anywhere within the study area: the figures quoted were the mean
values from a group of 7 sites, all situated close to ground level in
mainly residential areas. However, there remained the problem that the
people at risk in a study of this type were the elderly sick, who were
likely to remain indoors, and that outdoor measurements might not have
provided an adequate assessment of exposure (Biersteker et al., 1965).
The relationship between daily mortality in the more extensive
area of Greater London and day-to-day variations in pollution (smoke
and sulfur dioxide) and visibility was examined by Martin & Bradley
(1960) in the winter of 1958-59. They noted that on days when the
smoke concentration increased by more than 100 µg/m3 compared with
the previous day, or when the sulfur dioxide concentration increased
by 70 µg/m3 (0.025 ppm), there was likely to be increased mortality
(British daily smoke/sulfur dioxide method). The increases in daily
mortality were up to about 1.25 times expected values assessed from
15-day moving averages. Thick fog (visibility less than 200 metres)
was also associated with increases in mortality. The relative
importance of the 3 factors could not be determined but, on the basis
of other work, the authors considered that the smoke was probably the
most important. It is not clear whether the results are best
interpreted in terms of change in pollution from one day to the next,
rather than in terms of absolute values, but there is support for the
former approach from studies carried out elsewhere. When results were
considered on an absolute basis (Lawther, 1963), it was concluded that
increases in mortality became evident when the 24-h mean
concentrations of smoke and sulfur dioxide exceeded 750 µg/m3 and
710 µg/m3 (0.25 ppm), respectively. The measurement sites were the
same as those used by Gore & Shaddick (1958). They could still be
considered reasonably representative of outdoor concentrations in the
areas where people lived, although the inclusion of outer,
less-densely populated areas meant that the average exposures would
tend to have been underestimated.
Studies on day-to-day variations in mortality in London were
continued in successive winters, and coupled with the records of
emergency hospital admissions. In a later paper, Martin (1964) showed
correlations between both the daily mortality and hospital admission
data and concentrations of smoke or sulfur dioxide. There was no
clearly defined level above which effects were seen, but there were
fairly consistent increases in both mortality and hospital admissions
when the concentrations of smoke and sulfur dioxide each exceeded a
24- mean of about 500 µg/m3 (0.18 ppm of sulfur dioxide, British
daily smoke/sulfur dioxide method). In 1962, there was a major episode
of high pollution in London, similar in terms of duration and of
sulfur dioxide concentrations to the one in 1952, but with lower smoke
concentrations. Again, there was a sudden increase in deaths, but the
number of deaths was not as great as before (about 700, compared with
4000). Whether the change in medical care could have influenced these
results is not clear. The greater use of antibiotics in 1962 compared
with 1952 might have reduced the number of deaths, and a greater
awareness of the risk together with clear advice to the elderly and
infirm to remain indoors could have had an effect. The dramatic
reduction in smoke concentrations in London brought about by the
implementation of the Clean Air Act, and the more gradual reduction in
sulfur dioxide that has followed it, have meant that in more recent
years there have been few occasions when levels of 500 µg/m3 have
been exceeded simultaneously for smoke and sulfur dioxide (Waller et
al., 1969).
Biersteker (1966) published a study of an episode of high
pollution in Rotterdam in December 1962, when concentrations of smoke
and sulfur dioxide of approximately 500 µg/m3 and 1000 µg/m3
(0.35 ppm), respectively, were recorded (24-h means, OECD smoke/sulfur
dioxide method). There were increases in admissions to local hospitals
of people over 50 years of age with cardiovascular diseases, and there
was also some indication of an increase in mortality. This was
observed only once in Rotterdam and could have been due to other
causes. Further observations during a similar episode would be needed
to provide a convincing statistical relationship between hospital
admissions and these levels of pollution.
A relationship between day-to-day changes in mortality and
pollution has also been reported from Osaka (Watanabe 1966). There
appeared to be increases in mortality (about 20%) on days when the
concentration of suspended particulate matter (light scattering
method) exceeded 1 mg/m3 (4-day average) and was associated with a
level of sulfur dioxide of 200 µg/m3 (0.07 ppm). Low temperatures may
have been partly responsible for the effects.
Variations in daily mortality in New York in relation to sulfur
dioxide concentrations were studied by Buechley et al. (1973). They
examined correlations and developed regressions between a number of
daily climatic factors and indices of pollution (sulfur dioxide,
conductimetric method and coefficient of haze (Cohs)), and the
mortality residuals for a given day. They noted that the day of the
week had a special correlation with mortality (mortality rates were
considerably higher on Mondays). Regression analysis indicated that
heat waves and seasonal cycle were major predictors of mortality.
Other factors were much weaker (about one third as strong) but were
all of equal strength. Partial residual mortality values were computed
and showed a significant correlation with the levels of air pollution
(sulfur dioxide r = 0.14). Mortality could be predicted equally as
well from Cohs as from sulfur dioxide levels. Mortality was 1.5% less
than expected on 232 days when sulfur dioxide levels were below
30 µg/m3 (0.01 ppm) and 2% greater than expected on 260 days when the
sulfur dioxide levels were above 500 µg/m3 (0.18 ppm) after
correcting for the other factors. The crossover point (i.e., that
point below which deaths were less than expected and above which
deaths were greater than expected) was in the vicinity of a
concentration of sulfur dioxide of 260 µg/m3 (0.09 ppm). On the other
hand, the data from these studies could be interpreted to show a
continuum of an effect across the levels of sulfur dioxide, which, in
turn, should not be considered the causative agent but rather an index
of pollution.
Schimmel & Murawski (1975) have reported on their regression
analysis of daily deaths and levels of pollution (smoke shade and
sulfur dioxide) in New York City for 1963-1972. This was an extension
of an earlier study (Schimmel & Greenberg, 1972). The authors
controlled for season, day of week, and temperature. During this time
there was a marked reduction in the average level of sulfur dioxide
from 510 µg/m3 (0.18 ppm) to 170 µg/m3 (0.06 ppm) but virtually no
change in smoke shade. Their observations indicated that, despite this
reduction in sulfur dioxide, there had been no reduction in adverse
health effects. Analysis indicated that the adverse health effects
were associated principally (80%) with the particulate matter and only
to a small extent (20%) with the sulfur dioxide. However, the authors
also pointed out that, when they regressed mortality on temperature
and sulfur dioxide alone, the effects attributable to the sulfur
dioxide increased three-fold.
These findings are provocative but they must be interpreted
cautiously because of a number of limitations in the data. Air
pollution levels were from data obtained at a single monitoring
station and were probably not truly representative of exposures for
the community. The standard errors reported with their data are large.
The report should be considered to be an indicator for further studies
in which age-specific death rates and causes of death should be
included in the analysis as well as more relevant air pollution
measurements. The results should not be used for developing an
exposure-effect relationship.
A study involving comparisons between daily mortality data in New
York and Tokyo was carried out by Lebowitz et al. (1973). They applied
a "stimulus-response" technique to identify associations between days
of high pollution and days with increased mortality, and showed strong
relationships in both cities. However, their findings do not provide
information of direct value in the assessment of exposure-effect
relationships.
8.3.2 Mortality -- effects of long-term exposures
In countries having reliable systems for the collection and
analysis of data on deaths, based on cause and area of residence,
death rates for respiratory diseases have commonly been found to be
higher in towns than in rural areas. Many factors, such as differences
in smoking habits, occupation, or social conditions may be involved in
these contrasts, but, in a number of countries, a general association
between death rates from respiratory diseases and air pollution has
been apparent for many decades.
Analyses of these data have been of great value as a lead for
epidemiological studies, but the absence of information concerning
other relevant variables, such as smoking, and the relatively crude
nature of the indices of pollution used in many of these studies make
them unsuitable for the assessment of exposure-effect relationships.
The studies of Daly (1954, 1959), Pemberton & Goldberg (1954), and
Stocks (1959) were all based on mortality data from towns in England
and Wales, and each showed a positive correlation between bronchitis
or pneumonia death rates and some index of pollution by sulfur oxides
or particulate matter, as assessed for periods close to those for
which death rates were calculated. The most detailed investigation of
this type, taking into account social factors as well as pollution,
but still not smoking, was that conducted by Gardner et al. (1969).
One interesting feature of their findings was a slight improvement in
correlation when the index of pollution used was related to a period
some 10 years earlier than that for which the death rates were
calculated (deaths 1958-64, pollution index 1952). This illustrates
another of the problems that has been widely recognized when trying to
use mortality records to assess the effects of pollution i.e., that it
may not be recent exposures that are most relevant, but those earlier
in life; where concentrations have changed markedly over the years,
current measurements may not provide an adequate index.
Lave & Seskin (1970) reanalyzed some of the mortality data from
England and Wales, and developed multiple regression equations in
terms of pollution and socioeconomic indices. Again their findings of
positive correlations with pollution are of general interest but
cannot contribute to the development of dose-response relationships.
These authors also examined analogous data for Standard Metropolitan
Statistical Areas (SMSAs) in USA and in a later paper (Lave & Seskin,
1972) they attempted to assess the relative effects of air pollution,
climate, and home heating on mortality rates. Although equations were
obtained relating death rates to measurements of suspended particulate
matter and total sulfates (both by high volume sampler), it is
doubtful whether these can be regarded as valid in the absence of
adequate information on smoking.
8.3.3 Morbidity -- effects of short-term exposures
Prospective studies on specific occupational groups, not
professionally exposed, can be useful in assessing the effects of air
pollution in different communities or in areas where a change in air
pollution is expected. In such studies, where respiratory diseases are
followed, it is necessary to control for age distribution and
household composition, and to employ adequate statistical methods. In
studies on the Philadelphia area, USA, Dohan & Taylor (1960) used
absences of seven days or more because of respiratory disease as the
index and related this to the levels of sulfate. A later report by
Dohan (1961) noted that this relationship was stronger during an
epidemic of influenza. Ipsen et al., (1969) repeated this type of
study in the same area on a slightly different occupational group
using more detailed statistical analyses. They were not able to
confirm the earlier observations that the sulfate levels were related
to absences due to respiratory diseases.
Results have been reported (Lawther et al., 1970) of a series of
studies extending from 1954 to 1968 that were carried out mainly in
London, but also in some other large cities in England, using a diary
technique for the self-assessment of day-to-day changes in conditions
among bronchitic patients. A daily illness score was calculated from
the data contained in the diaries and this was related to the
concentrations of smoke and sulfur dioxide (British daily smoke/sulfur
dioxide method) and to weather variables. The pollution figures used
for most of the London studies were the mean values from the group of
sites associated with the mortality/morbidity studies of Martin (1964)
and Gore & Shaddick (1958). Many of the subjects in the series were
active enough to be out and about and at work, and the measurements
were considered to give a reasonable assessment of the average
exposures in the areas where they lived or worked. The method used in
these studies has not been validated, for the subjects recorded only
their own assessment of their condition, and this was not checked
against regular clinical examinations or ventilatory function
measurements, but the changes appeared to have some real meaning. In
the earlier years of the series, when the general level of pollution
was high, well defined peaks in the illness score were seen when
concentrations of either smoke or sulfur dioxide exceeded 1000 µg/m3.
With the reductions in pollution that followed the gradual
implementation of the Clean Air Act, these changes in condition became
less frequent and of smaller magnitude, and the conclusion from the
series as a whole, up to 1968, was that the minimum pollution
associated with significant changes in the condition of the patients
was a smoke level of about 250 µg/m3 together with a sulfur dioxide
concentration of about 500 µg/m3 (0.18 ppm) (24-h means, British
daily smoke/sulfur dioxide method). At these levels, there was still
some evidence that the peaks were associated specifically with
pollution rather than with adverse weather conditions. A later study
that has been reported by Waller (1971), showed that, with much
reduced average levels of pollution, there was an almost complete
disappearance of days with smoke levels exceeding 250 µg/m3 and
sulfur dioxide levels exceeding 500 µg/m3 (0.18 ppm). As in earlier
studies, some correlation remained between changes in the condition of
the patients and daily concentrations of smoke and sulfur dioxide but
the changes were small at these levels. At this low range of
pollution, discrimination between the effects of pollution and those
of adverse weather was poor.
Cohen et al. (1974) studied symptoms of irritation during a
publicized and an unpublicized period of air pollution as well as
during a control period in 3 communities in the New York metropolitan
area during the summer of 1970. They used a telephone survey technique
to inquire about specific symptoms such as eye irritation, throat
irritation, chest discomfort, shortness of breath, restricted
activity, and medical visits. No difference was noted between the 2
episodes of pollution. Both episodes showed significantly increased
symptoms compared with the control period. The results indicated that
irritative symptoms increased significantly when sulfur dioxide levels
exceeded 310 µg/m3 (0.11 ppm, West-Gaeke method) and total suspended
particulates exceeded 145 µg/m3 (high volume sampler) as a 3 day
average. Sulfate levels ranged from 6.6 to 7.6 µg/m3 in one area and
5.8 to 12.3 µg/m3 in another area. In the second area, sulfate levels
during the 2 periods of air pollution were 8.5 and 12.3 µg/m3,
respectively. Sulfate levels were not reported from the third area but
were probably low. The authors drew attention to some of the problems
associated with their study. The persons interviewed were generally
wives and the symptomatology in the male population could have been
underestimated. Also, there was an internal inconsistency possibly due
to intercurrent infectious disease or socioeconomic differences.
During the publicized episode, particulate pollution was considerably
higher in the Bronx than in Queens whereas irritation symptoms were
somewhat higher in Queens. The authors concluded that there could have
been confounding effects of other air pollutants, intercurrent
infection, or sociocultural factors.
Spirometric measurements were made at approximately weekly
intervals on 18 patients with chronic obstructive lung disease for
various periods during 1969-71 (Emerson, 1973). The spirometric values
(FEV1.0 and MEFR) were correlated with the levels of pollution (sulfur
dioxide and smoke, British daily smoke/sulfur dioxide method) and
climatic factors (temperature and humidity). Changes in FEV1.0 in
these patients were more strongly correlated with temperature and
humidity than with concentrations of sulfur dioxide or smoke. Levels
of air pollution in London were: sulfur dioxide, mean 190 µg/m3
(0.07 ppm), maximum, 720 µg/m3 (0.25 ppm) and smoke mean, 44 µg/m3,
maximum, 240 µg/m3. One limitation of the study was that the
pollution figures were averaged for 5-day periods, whilst the
spirometric measurements were made on specific days.
Studies of patients with chronic bronchitis in Chicago, USA
(Burrows et al., 1968; Carnow et al., 1969) showed conflicting
results. The reasons for these differences are not clear but may be
that different criteria for the selection of patients as well as
different methods for the determination of the exposure of the
individuals and their responses were used.
An unexpected finding of a possible effect of short-term exposure
to pollution arose from a study primarily concerned with long-term
exposures. In a resurvey of adults in Vlaardingen (Netherlands) who
had been interviewed and had lung function measurements made in 1969,
Van der Lende et al. (1975) found that the average lung function
values were higher in 1972 than in 1969, even though the subjects were
3 years older. When the authors examined the concentrations of
pollution on the 2 occasions (each survey having been done within a
5-day period), they found that levels were relatively high in 1969
with daily values ranging from 15 to 140 µg/m3 for smoke and from 120
to 300 µg/m3 (0.04-0.11 ppm) for sulfur dioxide compared with values
ranging from 15 to 40 µg/m3 and 45-100 µg/m3 (0.02-0.04 ppm),
respectively, in 1972. The increase in lung function was most
pronounced on the days with the greatest difference in the levels of
pollution. A control population in a rural area showed no comparable
changes over the same period, and temperature differences did not
explain the effect.
Asthmatic subjects have also been studied. These patients
represent a heterogeneous group and this may account for the variable
responses that have been reported. Cohen et al. (1972) studied 20
asthmatic subjects living in a small town (Cumberland, WV, USA) in the
vicinity of a coal-fired power plant. They found that when the soiling
index exceeded 1.0 Coh unit, or sulfur dioxide concentrations
(West-Gaeke method) exceeded 200 µg/m3 (0.07 ppm), or total suspended
particulates exceeded 150 µg/m3 (high volume sampling method), or the
temperature was lower than 0°C, there was a significant increase in
the frequency of asthmatic attacks. Levels of sulfates exceeding
20 µg/m3 and nitrate levels exceeding 2 µg/m3 did not result in such
an effect. In general, the effect of temperature was stronger than
that of the air pollutants although each of the 5 air pollutants
measured including sulfates and nitrates showed a correlation. When
temperature and any one of the pollutants were controlled for in the
analysis, the effect of any of the other 4 pollutants was eliminated.
Temperatures below 0°C overwhelmed any effect of the pollutants and
higher levels of the pollutants reduced the effect of temperature. One
further feature of this study should be stressed. Pollution in
Cumberland was dominated by emissions from a large single source, and
this might have led to high transient exposures to the pollutants,
which included oxides of nitrogen as well as sulfur oxides and
suspended particulate matter. In these circumstances it is doubtful
whether the 24-h values provided an adequate index of exposure of the
population.
Several studies on asthmatic subjects in Yokkaichi, Japan, have
been reported by Yoshida et al. (1966) including one on a group of 13
patients in which the number of attacks increased from 1 to 4 per week
when the concentration of sulfur dioxide was in the range of
140-230 µg/m3 (0.05-0.08 ppm), rising to about 12 per week when the
sulfur dioxide level reached 740 µg/m3 (0.26 ppm), all expressed as
weekly means. Suspended particulate or smoke levels were not reported.
8.3.4 Morbidity in adults -- effects of long-term exposures
Random samples of populations can be used for international
comparisons where there are gradations of air pollution. The major
difficulty here has been to ensure comparability with respect to
occupational exposures and ethnic groups. Reid et al. (1964) reported
such a comparison based on a study in the United Kingdom (College of
General Practitioners' Study, 1961) and a survey in Berlin, NH, USA
(Ferris & Anderson, 1962). The same questionnaire was used in both
studies. In the United Kingdom, it was completed by a large number of
practitioners who made up the survey group. In the USA, it was
completed by 2 physicians who tried to maintain the criteria developed
in the British survey. Results were standardized for cigarette
smoking. The effects of air pollution were then examined by age group
and sex. When simple bronchitis was present, i.e., phlegm production
for 3 months out of the year for 2-3 years, standardizing for
cigarette smoking removed any effect of air pollution for both males
and females. A more severe form of chronic bronchitis characterized by
phlegm production, exacerbations of colds that went to the chest, and
shortness of breath when walking on the level at one's own pace did
show an association with air pollution for both males and females,
even after standardizing for cigarette smoking. Levels of air
pollution were measured in Berlin, NH, by the lead peroxide candle,
dustfall, and high-volume samplers. Data for the United Kingdom were
estimated from similar measurements obtained in comparable towns and
cities where the general practitioners collected the data. In Berlin,
NH, the sulfation rate (lead candle) was 730 µg SO3/100 cm2 per day;
in the United Kingdom, in the large towns it was 950 and in the large
conurbations 1650 µg SO3/100 cm2 per day. The results of this study
seem to be consistent with those of other studies but it is not known
whether differences in socioeconomic or ethnic status could have been
relevant factors. The population of Berlin, NH, was resurveyed in
1967, and the results were compared with those obtained in 1961
(Ferris et al., 1973). There had been some decrease in pollution
levels in the interval, the sulfation rate being 470 µg SO3/100 cm2
per day in 1967, compared with the earlier figure of 730, while the
concentration of total suspended particulates had fallen from 180 to
132 µg/m3 (high-volume samples). Small reductions in the prevalence
of respiratory disease were noted after standardizing for age and
cigarette smoking. Slight improvements in forced vital capacity (FVC)
and peak expiratory flow rate (PEFR) were also noted, but there was
little change in FEV1.0. There is some doubt about the relevance of
the 180 µg/m3 figure quoted for total suspended particulates in the
1961 study, since it referred only to a 2-month period at a single
site. A random population sample from Chilliwack, BC, Canada, an
unpolluted community, was studied by Ferris & Anderson (1964) and the
results were compared with the results of the 1961 study in Berlin,
NH. Respiratory symptoms, after standardization for age and cigarette
smoking, tended to be higher in Berlin than in Chilliwack. Pulmonary
function (FEV1.0 and PEFR) was lower in Berlin than in Chilliwack,
after standardization for age, height, sex, and smoking category.
Pollution levels in Chilliwack (based on lead-candle measurements)
were about one-tenth to one-sixth of those in Berlin, NH.
A third survey in this series was carried out in 1973 (Ferris et
al., 1976). By this time, there had been a further decline in
pollution by particulate matter, the annual mean concentration of
total suspended particulates (high volume sampler) being quoted as
80 µg/m3. Only limited data on sulfur dioxide were available; the
mean of a series of 8-h samples for selected weeks was quoted as
0.01 ppm (30 µg/m3). On this occasion, the authors did not find any
appreciable differences in the prevalence of respiratory symptoms or
in measures of lung function, as compared with the 1973 report. The
interpretation of these findings is difficult, for the studies were
concerned largely with consecutive investigations of survivors of the
original group, over a 12-year period. Although the authors took into
account, as far as possible, the effect of selective losses of some of
the population, and changes in smoking habits among those who
remained, there is still some doubt as to whether the three sets of
results are truly comparable. The authors themselves concluded that
either the changes in air pollution levels from 1967 to 1973 (which
included a decline in total suspended particulates from about 130 to
80 µg/m3 (annual mean) with possibly a slight increase rather than
decrease in sulfur dioxide) were not associated with a beneficial
effect on health, or that their methods were not sufficiently
sensitive at the levels involved.
One of the difficulties in interpreting these data is that
exposure to odorous air pollutants (Berlin, NH) also occurred,
indicating an unconventional type of air pollution. It does seem
reasonable, however, to interpret the results of these surveys as
showing that slight changes in respiratory symptoms and pulmonary
function were related to levels of pollution. Sulfur oxides and
particulate matter may have been of importance. Only sulfation data
are available for sulfur oxides in the 1961 study.
Extensive studies have been made of post office and telephone
workers in the United Kingdom and USA (Holland & Reid, 1965; Holland &
Stone, 1965; Holland et al., 1965). The authors carefully considered
most of the relevant epidemiological variables and showed a gradation
of symptoms across the levels of pollution, particularly in the 50 to
59-year-old category. However, since pollution was measured in
different ways in each part of the studies, it is difficult to deduce
any quantitative relationships with sulfur dioxide and particulate
matter. The differences in the prevalence of symptoms persisted when
the authors examined the various smoking categories. They converted
the small amount of pipe and cigar smoking to cigarette equivalents,
which is not advisable, since other studies have indicated that pipe
and cigar smoking are less markedly associated with respiratory
symptoms. Lower levels of FEV1.0 and PEFR were observed in the areas
of higher pollution. The authors indicated that a difference in height
of 2-4 cm between the two populations studied could not account for
the differences seen in pulmonary function. Presumably, these
pulmonary function values had been corrected for standard temperature
and saturated vapour pressure. If not, some of the differences between
values in the USA and United Kingdom could be explained by the fact
that lower temperatures in England could have resulted in lower
measured air volumes. Similar spirometers were used in both studies.
In another study in the United Kingdom, Lambert & Reid (1970)
questioned about 10 000 adults by post. They estimated that their
sample represented about 74% of those able to reply. The positive
responses from the questionnaire (which had been recommended by the
British Medical Research Council) were correlated with levels of
pollution estimated from data used earlier by Douglas & Waller (1966)
and from some data from the National Air Pollution Survey. The
responses were controlled for social class and cigarette smoking. The
authors reported that whereas nonsmokers showed little response to the
levels of air pollution, cigarette smokers did respond and appeared to
be more sensitive. They also noted a considerable rural/urban
gradient, more pronounced in men than in women, that could not be
explained by differences in smoking habits.
Extensive studies were carried out in the Ruhr area of the Federal
Republic of Germany (Reichel et al., 1970; Ulmer et al., 1970) based
on a short questionnaire on respiratory symptoms, physical
examinations, and measurements of airways resistance using a body
plethysmograph. There were no clear differences in the results between
areas with different levels of pollution, but because of selection
factors and a low response rate, no definite conclusions can be drawn
from these studies regarding relationships with sulfur dioxide and
particulate matter.
A study in Vlaardingen in the Netherlands by Van der Lende (1969)
indicated that increased cough and phlegm production were associated
with air pollution but decreased lung function was not. However, the
author considered that the study might have been affected by the
presence of allergens, such as spores and bacteria in the agricultural
regions. Comparisons were made between an industrial area, polluted by
sulfur dioxide and particulate matter, and an agricultural area with
little pollution. In a later report, Van der Lende et al. (1973)
indicated that the effect of pollution could have been masked by
"self-selection" of the populations concerned.
The relationship between chronic bronchitis and air pollution was
studied in Osaka Prefecture, Japan, by Tsunetoshi et al. (1971).
Surveys were made of all persons over 40 years of age in selected
areas using self-administered questionnaires similar to that
recommended by the British Medical Research Council. The authors'
definition of chronic bronchitis was cough with sputum for 3 or more
months, for at least 2 successive years. Pulmonary function was
measured by the Vitalor, using the maximum value. An equation was
developed relating prevalence of bronchitis to age, smoking, and
sulfation rate (lead candle). The prevalence of bronchitis in the
study areas ranged from about 4% (in males aged 40-59 years) in areas
where the sulfation rate was close to 1 mg/100 cm2 per day to about
10% in those around 3 mg/100 cm2 per day. No data were given
concerning the levels of particulate matter.
Many other studies on the prevalence of respiratory symptoms in
relation to air pollution have been carried out in Japan such as that
by Toyama et al. (1966). They reported prevalences ranging from 2.8 to
3.7% (males aged 40-59 years, adjusted for age and smoking to accord
with other Japanese studies) in areas of Kashima (a nonindustrialized
rural town, at that time) with sulfur dioxide concentrations of less
than 30 µg/m3 (0.01 ppm, automatic conductimetric method) and
suspended particulate concentrations (high-volume sample) of
106-341 µg/m3 (mean 197). An extensive survey in Tokyo, (Suzuki &
Hitosugi, 1970, unpublished data)a showed a higher prevalence of
chronic bronchitis in areas that were more polluted, ranging from 5.5%
(males aged 40-59 years) and 1.1% (females 40-59 years) where the
sulfur dioxide concentration was below 60 µg/m3 (0.02 ppm, automatic
conductimetric method), and the suspended particulate level was below
100 µg/m3 (light-scattering method), to 6.7% (males) and 3.9%
(females), where sulfur dioxide was over 140 µg/m3 (0.05 ppm) and
suspended particulates more than 200 µg/m3. There was also some
increase in prevalence in an intermediate area with sulfur dioxide
concentrations of 60-140 µg/m3 (0.02-0.05 ppm) and a suspended
particulate concentration of 100-200 µg/m3. Smoking habits were
standardized in this study. In a recent study by Tani (1975) in the
vicinity of a pulp mill at Fuji City, and in control areas, there
appeared to be a consistent relationship between the prevalence of
bronchitis and sulfation rates, as measured by lead candles, with a
prevalence of about 3% (males and females combined, aged 40-59 years)
in areas where the sulfation rate was around 0.6 mg/100 cm2 per day
to about 8% where it was 1.2 mg/100 cm2 per day.
In studies on a random sample of adults in Cracow, Polandm the
levels of pollution measured made it possible to classify the subjects
into high and low exposure groups (Sawicki, 1972). The levels in the
high pollution area were an annual mean smoke level of 170 µg/m3 and
an annual mean sulfur dioxide level of 125 µg/m3 (0.04 ppm). In the
low pollution area, the standard smoke annual mean was 90 µg/m3 and
the sulfur dioxide annual mean was 45 µg/m3 (0.02 ppm). Persons
a Suzuki, T. & Hitosugi, M. Prevalence study of pulmonary symptoms
in Tokyo Prefecture employees. A paper presented at a meeting of a
Working Group on Air Pollution and Health, USA-Japan Cooperative
Science Programme, East-West Center, Honolulu, 21-23 April, 1970. The
pollution levels quoted in this study were obtained from reports of
the Tokyo Metropolitan Government concerning air pollution levels for
the years 1966-69 (Department of Environmental Pollution Control
Tokyo, 1976).
residing in the more polluted area had more respiratory symptoms and
poorer pulmonary function than those residing in the area with lower
pollution (chronic bronchitis 19% in more polluted area and 11% in
less polluted area and asthmatic disease 11% in more polluted area and
5% in less polluted area). Sawicki considered that there was a
synergistic interaction with cigarette smoking. He also pointed out
that many of the inhabitants worked outside the area in which they
lived and, therefore, the exposure levels at their places of residence
might not represent their true exposures.
8.3.5 Morbidity in children
Studies of the health of children have also provided useful
information.
Douglas & Waller (1966) performed a cohort study based on a
national sample of children born in the United Kingdom in the first
week of March 1946. The children were followed medically for 15 years
by health visitors and school doctors. The study was restricted to the
3131 children who remained at the same address for the first 11 years
of the enquiry, or who moved to an area that was in a similar
pollution group. Levels of air pollution were estimated from the
amount of coal consumed in a given area where each child lived, and it
was shown at the end of the study that the 4 pollution categories that
had been defined provided a satisfactory gradation in terms of the
measurements of smoke and sulfur dioxide that were then available. In
each of the several periods of life considered, from birth onwards,
the authors noted a consistent relationship between the frequency of
lower respiratory tract infections and pollution category. There was
no sharp change at any particular pollution level, but if the rates
within the lowest category (mainly rural areas) were taken as a
baseline, then increased frequencies were seen in the next category
up, with smoke and sulfur dioxide each of the order of 140 µg/m3
(0.05 ppm sulfur dioxide) or more. Annual means were estimates based
on the British daily smoke/sulfur dioxide method for a period after
the end of the study and probably underestimated earlier level.
Socioeconomic status was important in the study, but a relationship
between air pollution and frequency of lower respiratory tract
infections still existed within separate social classes. In a later
follow-up of these subjects, Colley et al. (1973) showed that, at the
age of 20 years, respiratory symptoms were related to smoking habits
rather than to the pollution of the areas in which the subjects were
then living. However, there was some relationship between the
prevalence of symptoms and earlier histories of lower respiratory
tract infections, which, in turn, were related to estimated pollution
exposures during childhood.
Lunn et al. (1967) studied 819 children in their first year at
school in the industrial city of Sheffield, in the United Kingdom.
Medical examinations were carried out on the children, the parents
were questioned concerning the previous health of the children, and
FEV0.75 and FVC were measured. Various socioeconomic factors were also
carefully considered. The families were stable, and there had been
little or no migration into or out of the specific communities.
Measurements of pollution were made at the schools attended by the
children (British daily smoke/sulfur dioxide method). The authors
found increased frequencies of both upper and lower respiratory tract
infections in the more polluted areas. When the "cleanest" area was
taken as the baseline, most of the illness indices considered showed
an increase in the next area up in order of pollution levels, i.e.,
where the annual mean concentrations of smoke and sulfur dioxide were
each about 200 µg/m3 (0.07 ppm sulfur dioxide) taking figures for the
middle year of the survey. A follow-up study was carried out on some
of these children when they were 4 years older (i.e., aged 9) (Lunn et
al., 1970). By then, the implementation of the Clean Air Act had
reduced the contrast in pollution between the areas, so that the mean
concentration of smoke in the 3 "dirty" areas combined was about
140 µg/m3 and the mean sulfur dioxide concentration was 200 µg/m3
(0.07 ppm), compared with about 50 and 100 µg/m3 (0.04 ppm sulfur
dioxide), respectively, in the "clean" area. At this time, there was
no significant difference in the illnesses reported by the 9-year-olds
in the different areas and the authors concluded that this was in line
with the reduced pollution levels.
Data were collected (Holland et al., 1969) concerning about 11 000
children attending school in 4 areas of Kent, England, 2 of which were
predominantly urban, and 2, rural. As part of the children's regular
medical examinations, peak expiratory flow rates, height, weight, and
the results of examinations of ears and tonsils were recorded. Smoking
habits and school absences were also recorded. Questionnaires
concerning such factors as previous respiratory diseases, father's
occupation, and size of family were completed by the parents. Smoke
and sulfur dioxide were measured in 3 of the areas (British daily
smoke/sulfur dioxide method) and information on population density and
housing was collected. Four factors emerged as important in relation
to decreased peak expiratory flow rates. Place of residence was most
important followed by previous history of respiratory disease; family
size and social class were of least importance. These 4 factors seemed
to be additive but they only accounted for 10-15% of the total
variation. Levels of air pollution for smoke were reported to range
from 34 to 69 µg/m3 in the 3 communities. Values for sulfur dioxide
were not reported but were stated to parallel those for smoke. There
were clearly other factors affecting area difference, and there is
some doubt as to whether the small contrasts in pollution could have
had much effect.
Colley & Reid (1970) surveyed about 11 000 children from 6 to 10
years of age, living in urban and rural areas of England and Wales.
The prevalence of respiratory disease was assessed in the autumn.
Pollution was assessed in terms of sulfur dioxide concentration, from
some direct measurements (British daily smoke/sulfur dioxide method)
coupled with estimates based on lead candle measurements of sulfation.
Within the English areas studied (winter mean concentrations of sulfur
dioxide ranging from about 30 to 150 µg/m3 or 0.01-0.05 ppm) there
was a gradient in the prevalence of symptoms, but the rates were much
higher in Wales for comparable levels of pollution. The reasons for
this difference were not clear, although it has been suggested that it
could be related to the fact that solid-fuel consumption was high in
Wales.
Respiratory function measurements were made on children aged 10-11
years from 2 different areas of the German Democratic Republic (Berlin
and Bitterfeld) with different levels of pollution (Grosser et al.,
1971). The groups were matched for social class, age, and height. In
the low pollution area, the concentration of respirable dust (method
not mentioned) was 110 µg/m3 and that of sulfur dioxide (method not
mentioned) was 50 µg/m3 (0.02 ppm); in the other area, the figures
were 290 and 360 µg/m3 (0.13 ppm), respectively (based on 24-h
measurements, averaged for July and December). Two studies were made,
6 months apart, in each of the 2 areas. Each of the spirometric values
studied (FVC, FEV1.0, FEV0.7) was higher in the area with lower
pollution.
A variety of other techniques has been used to investigate
possible effects of exposure to pollution. These include the work of
Yoshii et al. (1969) who noted an association between chronic
pharyngitis accompanied by histopathological changes at biopsy and
level of sulfation, expressed as the annual mean, in persons attending
their clinic in Yokkaichi, Japan, and in sixth-grade children. In the
heavily polluted districts sulfation rate was more than 1.0 mg/100
cm2 per day, in moderately polluted districts it ranged from 0.25 to
1.0 mg/100 cm2 per day, and in the control area it was less than this
level (lead candle measurements).
Conjunctivitis, both acute and chronic, has been reported in
Zabrze, Poland, in relation to industrial air pollution (Maziarka &
Moroz, 1968). In Czechoslovakia, Schmidt et al. (1966) reported
differences in the blood cells, tonsils, and cervical lymph nodes of
children living in a highly polluted atmosphere compared with those
living in a relatively clean atmosphere and Symon et al. (1969)
reported retardation in growth and ossification and a reduced colour
index in red blood cells in children living in areas with high air
levels of sulfur dioxide (up to 2-3 mg/m3) and fly ash. None of these
studies can be used for the exposure-effect relationship.
8.3.6 CHESS studies
The US Environmental Protection Agency has recognized the need for
studies at relatively low levels of air pollution in order to
determine whether a no-effect level can be identified to develop a
dose-response relationship and to monitor areas where there might be a
change in the levels of exposure. The CHESS (Community Health and
Environmental Surveillance System) studies were developed with these
purposes in mind. In some aspects these studies have been well
designed and executed but in other aspects there have been
difficulties. In many ways these are preliminary studies that need to
be continued. The studies have included adults, children, asthmatic
subjects, and patients with cardiovascular and pulmonary diseases.
Both acute and chronic effects have been studied. Methods have
involved mailed questionnaires on respiratory symptoms and illnesses
(adults and children), mailed questionnaires on family composition,
housing, and socioeconomic status, telephone interviews, tests of
pulmonary function (children), and diary techniques with patients.
Previous exposures to air pollutants were estimated from
historical data on emissions or known production figures from the
local industries and the application of mathematical models. More
recent exposures were based on actual measurements. These were
generally conducted at monitoring stations (each of which covered a
radius of up to 2 km), and involved high volume sampling for total
suspended particulates and suspended sulfates, and the West-Gaeke
method for sulfur dioxide. Mathematical models were used to estimate
the variation in exposure of the different groups. Various problems
discussed in a summary of the results (US Environmental Protection
Agency, 1974) include poor response rates (i.e., low participation
rates) in some of the studies on children, high dropout rates in
studies on patients, and, in at least one of the studies on children,
the fact that their spirometry was probably in error.
There was a general tendency for the authors to over-interpret the
CHESS data. In some of the studies on children, the results were
grouped according to high and low levels of pollution and a difference
was found between the two groups. However, the relationship of the
prevalence of disease to levels of pollution was not clear -- namely,
when the individual groups were examined, some exposed to the high
levels of pollution had a lower disease prevalence than some of the
groups exposed to low levels of pollution.
The design for the study of the impact on respiratory disease was
excellent and took into account family composition, housing, and
socioeconomic status. It is unfortunate that these studies were not
continued for more than one year of observation before reporting. A
number of years of observations are needed to rule out natural
fluctuations in respiratory disease.
These studies have been reviewed by an expert committee in the USA
under the chairmanship of Dr D. Rall (Director, National Institute of
Environmental Health Sciences, US Department of Health, Education and
Welfare) (Rall, 1974). The Committee's evaluation of the studies was
similar to that of the Task Group and the Committee concluded that the
results of the studies did not warrant any change in the present US
Federal Air Quality standards.
It was the opinion of the WHO Task Group that these data could not
be used for the estimation of an exposure-effect relationship.
8.3.7 Lung cancer and air pollution
The possibility that air pollution is a causal factor in cancer of
the lung has given rise to considerable concern. The evidence in
favour of a causal relationship is briefly: (a) the excess
occurrence of the disease in urban areas; (b) the presence in the
suspended matter in urban air of substances such as benzo(a)pyrene
that can cause cancer under experimental conditions; and (c) the
general rise in lung cancer that appeared, at one time, to follow
certain assumed trends in pollution.
Early studies in the United Kingdom (Stocks, 1959, 1966) indicated
that variations in lung cancer mortality in urban areas were
associated with variations in amounts of pollution and, following a
recommendation by a WHO Study Group in 1959 (World Health
Organization, 1960), a pilot international study was undertaken in
several cities where there were contrasts in lung cancer death rates.
The results did not show any clear-cut relationship with measurements
of particulate matter or its benzo(a)pyrene content (Waller & Commins,
1967) and it was clear that apart from the difficulties of making
proper allowances for differences in smoking habits, it seemed likely
that present-day measurements of polycyclic hydrocarbons gave an
inadequate assessment of past exposures to these compounds.
The Royal College of Physicians of London (1970) reviewed the
issue, and concluded that the evidence against community air pollution
being a causal factor in lung cancer was stronger than the evidence
for it. The urban/rural differential is greatest in countries with
relatively low urban air pollution (Sweden, Norway, Denmark). The
upward trend in mortality as well as other experimental and
epidemiological evidence are best explained by the causal role of
cigarette smoking.
Nevertheless, in a review by Cleary (1967), evidence is presented
to show that, in Australia, New Zealand, South Africa, and the USA,
immigrants from the United Kingdom have a higher lung cancer death
rate than those born in these countries: immigrants from Norway have a
lower rate than native-born citizens of the USA, and the lung cancer
mortality rates for all these migrants are intermediate between those
of their countries of origin and destination, strongly suggesting an
environmental factor in early life.
While the existence of an urban excess of lung cancer has been
proved, it is uncertain that air pollution is the "urban factor"
responsible. In contrast, recent work incriminates cigarette smoking
more strongly than ever; there is also a contribution from some
specific occupational exposures (see footnote in section 1.1.7).
The consideration of criteria for environmental carcinogenesis
specifically in relation to any possible effects on lung cancer, is
outside the scope of the present discussion, but it may be mentioned
that any action to reduce smoke and especially that from the
inefficient combustion of coal in domestic fires, is likely to make
substantial reductions in the benzo(a)pyrene content of the air. Such
a change has already been noted in London, where the concentration of
this compound is now only about one-tenth of what it was 25 years ago
(Lawther & Waller, 1976). A reduction of sulfur dioxide may also be
important for any possible interactions relating to the production of
lung cancer. Some experimental studies (Kuschner & Laskin, 1971;
Skvorcova et al., 1973) showed an increased carcinogenic response when
laboratory animals (rats and hamsters) were exposed to sulfur dioxide
in addition to benzo(a)pyrene.
8.3.8 Annoyance
Annoyance may be defined as "a feeling of displeasure associated
with any agent or condition believed to affect adversely an individual
or a group". This definition was adopted by an international symposium
on annoyance in Stockholm in 1971 (Lindvall & Radford, 1973). Very few
studies have been performed that would make quantitative evaluations
of this effect possible.
The social awareness of pollution caused by particulate matter has
been studied in a few areas. The results from different studies have
been presented in a document on particulate matter (US Department of
Health, Education and Welfare, 1969a); they include those from a study
carried out in St. Louis (Schusky, 1966), where values for suspended
particulates of around 100 µg/m3 produced annoyance reactions from a
considerable number of people.
A similar study was carried out in Birmingham, Alabama, USA
(Stalker & Robinson, 1967), in which levels of air pollution were
correlated with annoyance. They found that, as dustfall reached or
exceeded 14.1 g/m2 per month, about one-half of the population
considered it to be a nuisance; at 10.5 g/m2 per month, about
one-third of the population considered air pollution a nuisance.
Stepwise multiple regression analysis showed that the variation due to
dust fall alone explained 49% during the spring season and 68% during
the winter season of the total association between the air pollutants
measured and public annoyance. The association between levels of
suspended particulate matter and public opinion, which was weaker than
that of dustfall, was strongest during the summer season (r = 0.59).
About one-half of the persons interviewed thought that air pollution
was a general nuisance, when mean annual or mean summer concentrations
of particulate matter reached 230 µg/m3 and one-third, when they
reached 150 µg/m3.
No such relationship was shown with sulfur dioxide concentrations.
However, these levels were low. Further studies are indicated, since
it may be that effects such as annoyance reactions will, in the
future, be the critical effects on which criteria, as regards the
protection of public health, will be based. Since annoyance reactions
have a large sociocultural component, these levels may vary from place
to place and should be determined for each locality. Surveys on
annoyance are fraught with many problems. When proper survey
techniques are expertly applied, however, it will be possible to
assess reactions in a quantitative manner.
8.4 Exposure-Effect Relationships
Some of the studies reported in this section can be used to
develop exposure-effect relationships. It has been necessary to
develop 2 tables, one for the effects of short-term exposures
(Table 15, p. 107) and another for the effects of long-term exposures
(Table 16). The results from the studies have been based on different
methods of measuring sulfur oxides and particulate matter. The values
for the sulfur oxides are treated as if they were comparable. For the
particulate matter, two categories are listed: smoke as measured by
the Organization for Economic Cooperation and Development or British
daily smoke/sulfur oxide methods and total suspended particulates as
measured by the high volume sampler or light scattering. These factors
should be considered in interpreting these tables.
Table 15. Exposure-effect relationships of sulfur dioxide, smoke, and total suspended
particulates: effects of short-term exposures
concentration
24-h mean
values (µg/m3)
Total
suspended
Sulfur dioxide Smoke particulates Effects
> 1000 > 1000 -- London, 1952. Very large increase in mortality to about 3
times normal, during 5-day fog. Pollution figures represent
means for whole area: maximum (central site) sulfur dioxide
3700 µg/m3, smoke 4500 µg/m3 (Ministry of Health, UK, (1954).
710 750 -- London, 1958-59. Increases in daily mortality up to about 1.25
times expected value (Lawther, 1963; Martin & Bradley, 1960).
500 500 -- London, 1968-60. Increases in daily mortality (as above) and
increases in hospital admissions, becoming evident when
pollution levels shown were exceeded (magnitude increasing
steadily with pollution) (Martin, 1964).
500 -- -- New York 1962-66. Mortality correlated with pollution: 2%
excess at level shown (Buechley, 1973).
500 250 -- London, 1954-68. Increases in illness score by diary technique
among bronchitic patients seen above pollution levels shown
(means for whole area) (Lawther et al., 1970).
Table 15 (cont'd).
concentration
24-h mean
values (µg/m3)
Total
suspended
Sulfur dioxide Smoke particulates Effects
300 140 -- Vlaardingen, Netherlands, 1969-72. Temporary decrease in
ventilatory function (Van der Lende et al., 1975).
200a -- 150b Cumberland, WV, USA. Increased asthma attack rate among
small group of patients, when pollution levels shown were
exceeded (Cohen et al., 1972).
a West-Gaeke method
b High volume sampling method
Other measurements by Organization for Economic Cooperation and Development or British daily smoke/sulfur
dioxide methods (Ministry of Technology, UK, 1966; Organization for Economic Cooperation and Development, 1965).
Table 16. Exposure-effect relationships of sulfur dioxide, smoke, and
total suspended particulates: effects of long-term exposures
Concentration
Annual means of 24-h
mean values (µg/m3)
Total
suspended
Sulfur dioxide Smoke particulates Effects
200 200 -- Sheffield, England. Increased
respiratory illnesses in children
(Lunn et al., 1967, 1970)
-- -- 180b Berlin, NH, USA. Increased respiratory
symptoms, decreased respiratory
function in adults (Ferris et al., 1973)
150 -- -- England & Wales. Increased respiratory
symptoms in children (Colley & Reid, 1970)
125 170 -- Cracow, Poland. Increased respiratory
symptoms in adults (Sawicki, 1972)
140d 140d -- Great Britain. Increased lower
respiratory tract illnesses in
children (Douglas & Waller, 1966)
60-140a -- 100-200c Tokyo, Increased respiratory symptoms
in adults (Suzuki & Hitosugi, unpublished
data, 1970)
a Automatic conductimetric method
b High volume sampler (2-month mean, possible underestimation of annual mean).
c Light-scattering method, results not directly comparable with others.
d Estimates based on observations after end of study; probable underestimation
of exposures in early years of study.
Other measurements by Organization for Economic Cooperation and Development or
British daily smoke/sulfur dioxide methods (Ministry of Technology, UK, 1966;
Organization for Economic Cooperation and Development, 1965).
9. EVALUATION OF HEALTH RISKS FROM EXPOSURE TO SULFUR OXIDES, SMOKE,
AND SUSPENDED PARTICULATE MATTER
It is well established that respiratory diseases are important
causes of disability and death, and that, for some of them, there is
evidence of association with environmental factors in the ambient air.
There is evidence that exposure to mixtures of urban air pollutants
containing sulfur oxides and particulate matter is related to a
variety of adverse effects on health, even when other factors are
controlled (section 8 -- Tables 15 and 16). Although in most of the
studies considered the levels of air pollution have been expressed in
terms of sulfur dioxide, smoke, or suspended particulate matter, this
does not necessarily imply that these are the causative agents. They
provide only indices of pollution, and certain components, such as
sulfuric acid or sulfates, may be of particular importance.
Measurements of some of these components have now been made in a
number of areas and used in some of the more recent epidemiological
studies. The Task Group concluded, however, that, at present, there
are not enough data on any of these other indices of pollution to
allow exposure-response relationships to be established. Thus, the
present discussion is confined to the effects of pollution expressed
in terms of sulfur dioxide, smoke, and total suspended particulate
matter.
9.1 Exposure Levels
Concentrations of sulfur dioxide, smoke, and suspended particulate
matter vary greatly from place to place and from time to time. Many
factors including sources, topography, and weather conditions may be
involved in these variations.
An annual arithmetic mean of sulfur dioxide concentrations in
urban areas typically ranges from 100-200 µg/m3 (0.035-0.070 ppm),
whereas a maximum daily mean ranges from 300-900 µg/m3
(0.11-0.32 ppm). For smoke, an annual arithmetic mean ranges from
30-200 µg/m3 with a maximum daily mean of 150-900 µg/m3; for total
suspended particulates, these levels range from 60-500 µg/m3 and
150-1000 µg/m3, respectively.
Comparatively little information is available concerning indoor
concentrations of sulfur dioxide and particulate matter, though, in
general, these levels are known to be lower than those outdoors,
except at work places.
Levels in working environments are considerably higher than
general community levels and are thought to have been much higher in
the past.
In evaluating exposure levels that have been used in the past in
connexion with epidemiological studies, a serious question arises as
to how far these measurements, often intended primarily for control or
monitoring purposes, can be considered as providing adequate measures
of exposure. As discussed in section 5, it must be recognized that all
the figures quoted in subsequent sections are based on measurements
that have been made outdoors, usually at only a limited and not wholly
representative set of fixed sites, though most people spend much time
indoors, where concentrations may differ substantially from those
outside. However, this does not necessarily invalidate these data
which are serving primarily as indices.
So far, there has been little information on the particle size
distribution and the chemical composition of selected particle size
ranges that would make the nature of exposure more understandable.
Comparable assessment is often difficult because of differences in
the methods of measurement of particulate matter that have been used
in the health effect studies. Smoke, assessed in terms of blackness,
is a measure of pollution associated with the incomplete combustion of
fuel, and total suspended particulates, determined by weight, is a
wider concept that includes all material which, by virtue of its
particle size, remains in suspension for long periods.
9.2 Experimental Animal Studies
Laboratory studies have been performed using a variety of test
animals, of periods of exposure, of experimental designs in exposure,
and of combinations of pollutants and other agents.
Some of these studies have provided useful information on the
mechanisms of the biological action of sulfur dioxide, sulfuric acid
mist, or particulate matter. However, the results are of limited value
in developing guidelines for the protection of human health from
effects of these pollutants as they exist in urban areas, and for this
reason it is necessary to turn to the available epidemiological
evidence.
9.3 Controlled Studies in Man
Effects studied in volunteers under controlled conditions include
those on the functions of the respiratory system, sensory organs, and
cerebral cortex. Durations of exposure have been very short, usually
less than a few hours.
Slight effects on pulmonary function were observed with exposure
to sulfur dioxide at a concentration of 2100 µg/m3 (0.75 ppm), but
not with exposure to a concentration of 1100 µg/m3 (0.37 ppm).
With exposure to sulfuric acid, a decrease in tidal volume was
found at a concentration as low as 350 µg/m3.
Combined exposures to sulfur dioxide and ozone or hydrogen
peroxide produced a greater effect on respiratory function than
exposure to each compound alone.
Studies on sensory and cerebral cortical functions showed that
sulfuric acid was more toxic than sulfur dioxide and that a
combination of these two substances produced an approximately additive
effect.
There is a limit to the use of these studies for the development
of criteria for community exposures, particularly when long-term
exposure to complex mixtures of air pollutants is involved.
9.4 Effects of Industrial Exposures
Effects on the health of workers have been studied in relation to
exposure to sulfur dioxide, particulate matter, or sulfuric acid mist
arising from various manufacturing processes. In many of these
studies, exposure levels were relatively high and, in some studies,
adverse effects were detected only at a daily mean sulfur dioxide
concentration as high as 70 000 µg/m3 (25 ppm).
It has also been reported that exposure to sulfuric acid at a mean
concentration of 1400 µg/m3 during working hours for 2 days did not
produce any effect on lung function.
The fact that adverse effects have not been reported at
comparatively high levels of sulfur dioxide or surfuric acid aerosols
may well be explained by the influence of biasing factors, such as
that the workers remaining in jobs with exposures to pollutants
consist of people especially resistant to their effects. Therefore, it
should not be considered an indication that such concentrations are
without effects for the average working population and particularly
not for workers with pre-existing pulmonary diseases.
In evaluating the effects from industrial exposures, due attention
must also be given to the variation in chemical composition and size
distribution of particulate matter.
9.5 Effects of Community Exposures
Many of the epidemiological studies in relation to community
exposures that were considered by a WHO Expert Committee in 1972
(World Health Organization, 1972), and which must still be relied on
to a large extent today, were based on the measurement of smoke rather
than of total suspended particulates. However, some new data on the
effects of total suspended particulates have become available and have
been included in Tables 15 and 16 (section 8).
Tables 17 and 18 show the levels above which some effects on
health might be expected among specified populations for short-term
and long-term exposures, respectively. These are based on the critical
evaluation of the results of studies reviewed in section 8.
In developing Table 17, greater emphasis had to be placed on some
earlier results related to major effects seen when concentrations of
pollution were much higher than those commonly experienced today. With
the effective control of at least some components in areas where
pollution was, at one time, very high, most of the more recent studies
have failed to isolate the effects of the pollutants in question from
effects similar in nature but arising from other factors.
The figures for sulfur dioxide and smoke are essentially the same
as those proposed by the Expert Committee in 1972 (World Health
Organization, 1972) the only change being the adoption of 250 µg/m3
(0.09 ppm) instead of 250-500 µg/m3 (0.09-0.18 ppm) as the level at
which the worsening of the condition of patients from short-term
exposures to sulfur dioxide might be expected. It was recognized that
the magnitude of responses at this level appeared to be small and
difficult to separate from effects due to other factors such as
weather or infections. It is possible that a lower figure for smoke
could now be adopted, but, in view of uncertainties concerning the
differences in composition of this component of pollution from one
area to another, and the changes with time even within one locality,
it was felt that any further modification of the figure determined on
the basis of the older studies from London would need new evidence.
Table 18 represents an overall assessment of the effects of
long-term exposure to sulfur dioxide and smoke in terms of increases
in the prevalence of respiratory symptoms among both adults and
children, and in terms of the increased frequency of acute respiratory
illnesses, that has been demonstrated particularly among children.
Table 17. Expected effects of air pollutants on health in selected segments of
the population: effects of short-term exposuresa
24-h mean concentration (µg/m3)
Expected effects Sulfur dioxide Smoke
Excess mortality among the elderly
or the chronically sick 500 500
Worsening of the condition of patients
with existing respiratory disease 250 250
a Concentrations of sulfur dioxide and smoke as measured by OECD or British daily
smoke/sulfur dioxide method (Ministry of Technology, UK, 1966; Organization for
Economic Cooperation and Development, 1965). These values may have to be adjusted
in terms of measurements made by other procedures.
Table 18. Expected effects of air pollutants on health in selected segments of
the population: effects of long-term exposuresa
Annual mean concentration (µg/m3)
Expected effects Sulfur dioxide Smoke
Increased respiratory symptoms among
samples of the general population 100 100
(adults and children) and increased
frequencies of respiratory illnesses
among children
a Concentrations of sulfur dioxide and smoke as measured by OECD or British daily
smoke/sulfur dioxide method (Ministry of Technology, UK, 1966; Organization for
Economic Cooperation and Developments, 1965). These values may have to be adjusted
in terms of measurements made by other procedures.
For each pollutant there remains much uncertainty about the
minimum levels associated with demonstrable effects. Where populations
exposed to different levels of pollution have been compared, it cannot
necessarily be assumed that even those who were exposed to a level
lower than the known lowest effect level are entirely unaffected by
pollution. There must also be doubts as to whether the effects
observed in some studies were due in part to other pollutants, or to
socioeconomic or other factors that had not been adjusted for
completely.
No firm conclusion was reached on the effects of total suspended
particulates as a component of air pollution, together with sulfur
dioxide, because of the limited amount of information available. For
the effects of long-term exposure, a tentative figure of 150 µg/m3
(annual arithmetic mean) was suggested, based on the 2 entries in
Table 16, recognising that one of these was based on light-scattering
observations, and the other on high volume sampler measurements, but
for a 2-month period only. It was noted that the one study on the
effects of short-term exposure to total suspended particulates had
been included in Table 15, but it was felt that this could not provide
satisfactory information for an assessment of these effects.
The figures in Table 18 have been expressed in terms of annual
arithmetic mean concentrations, although it is not known whether the
effects are related to extended exposures to these average levels, or
more particularly to days of high pollution within each series. In
view of the limited quantitative information on effects of pollution
in terms of annoyance, this aspect has been omitted from Tables 17 and
18.
9.6 Guidelines for the Protection of Public Health
Tables 17 and 18 present two different sets of criteria, one
relating to effects of short-term exposure, in terms of 24-h average
concentrations, and the other to effects of long-term exposure in
terms of annual means. These effects may be interrelated; the gradual
development of respiratory symptoms may, for example, be a reaction to
repeated short-term exposure to peak 24-h values, or even to transient
peaks lasting for still shorter periods, but in the absence of any
substantial evidence on this point, the two criteria must, for the
time being, be considered separately.
With the present state of knowledge, it was considered that a
safety factor of two below the figures given in Tables 17 and 18 would
be reasonable to ensure the protection of public health, and,
accordingly, Table 19 was developed, still considering the effects of
short-term and long-term exposures separately. As an indication of the
uncertainty surrounding these estimates, the figures have further been
expressed with a range of ± 20%.
The values proposed in Table 19 are in general agreement with
those suggested as long-term goals in the earlier report (World Health
Organization, 1972). In the case of sulfur dioxide, there has been
some reduction in the 24-h figure (if this is regarded as a level not
to be exceeded on more than 7 days a year), and this is in line with
the revision of the "effect" level from a range of 250 to 500 µg/m3
in the 1972 report, to the present figure of 250 µg/m3 (Table 17).
Table 19 requires careful interpretation, for none of the figures
can be considered as absolute limits. In the first place, day-to-day
variations in the concentration of smoke and sulfur dioxide are
determined largely by weather conditions, and occasional peaks far
beyond the usual daily values may well occur, even with careful
control of emissions.
Table 19. Guidelines for exposure limits consistent with
the protection of public healtha
Concentration (µg/m3)
Sulfur dioxide Smoke
24-h mean 100-150 100-150
Annual arithmetic mean 40-60 40-60
a Values for sulfur dioxide and smoke as measured by OECD or
British daily smoke/sulfur dioxide method (Ministry of
Technology, UK, 1966; Organization for Economic Cooperation
and Development, 1965). Adjustments may be necessary where
measurements are made by other methods.
Although there are two sets of conditions specified in Table 19,
determined independently from evidence on the effects of short- and
long-term exposures, they can generally be considered to be consistent
with one another. If the proportion of days with 24-h values above
those in Table 19 is small (e.g., of the order of 7 days per year),
then the annual means may well fall within or below the ranges
specified in the second row of the table. Annual means are specified
here in terms of arithmetic means: the corresponding geometric means
would generally be a little lower (see section 5).
Much consideration was given to the possibility of extending
Table 19 to include guidelines for total suspended particulates as
measured by the high volume sampler but it was concluded that the
available evidence on the effects associated with exposure to
suspended particulate matter was highly unsatisfactory.
The Task Group felt, however, that some recommendations for a
guideline should be made. A tentative annual mean value of 150 µg/m3
has been suggested in section 9.5 as a level beyond which effects of
long-term exposure to total suspended particulates might be observed.
Applying a safety factor of two and introducing a ± 20% range, as in
the case of smoke and sulfur dioxide, would then provide a range of
levels from 60 to 90 µg/m3 as a possible guideline.
For short-term exposures, no satisfactory, direct evidence
relating concentrations of total suspended particulates to effects is
available. Because of this, a guideline for short-term exposure levels
can only be inferred. Assuming that the same ratio of 24-h mean
concentrations to the annual mean (each derived independently, see
beginning of section) given in the guidelines for smoke, is applicable
to suspended particulate matter, then a very approximate 24-h
guideline for suspended particulate matter, as measured by the high
volume sampler, would be in the order of 150 to 230 µg/m3.
While the Group felt that it was reasonable and prudent to
consider the above figures as interim guidelines consistent with the
protection of public health, it stressed the very urgent need for
additional information on the effects of exposure to suspended
particulate matter (measured by the high-volume sampler). Furthermore,
it recognized the fact that the toxicological significance of total
suspended particulates might vary depending on their chemical
composition and particle size, and that under certain circumstances,
the suggested guidelines might need to be reconsidered. It should be
noted that the discussion above does not imply any preference for
smoke measurements over those of total suspended particulates; indeed
it is highly desirable to develop more appropriate methods for the
measurement of suspended particulates, especially those limited to the
measurement of respirable particles.
It was recognized that in many urban and industrial areas existing
levels of pollution by sulfur dioxide and suspended particulate matter
were substantially above these guidelines. Furthermore, there was the
problem that the long-distance transport of these pollutants from
major sources could, in some circumstances, result in comparatively
high background concentrations in rural areas, and high levels in the
incoming air in towns striving to meet their own air quality
standards. It was considered, however, that every effort should be
made to develop control procedures that would allow these guidelines
to be met.
These guidelines are based on observations among populations in
the community exposed to a mixture of sulfur dioxide and smoke or
total suspended particulates and they may not apply to situations
where only one of the components is present. On grounds of prudence,
however, it is recommended that the levels of each pollutant should be
below the values stated. It should be stressed again, however, that
the data on which the guidelines are based are uncertain and each of
the guidelines is tentative and subject to review when further
information becomes available.
It was the opinion of the Group that there is not yet sufficient
information available on the effects of community exposures to
sulfuric acid aerosols or suspended sulphates to develop guidelines
for these air pollutants.
REFERENCES
ABELES, F. B., CRAKER, K. E., FORRENCE, L. E., & LEATHER, G. R. (1971)
Fate of air pollutants: removal of ethylene, sulfur dioxide and
nitrogen dioxide by soil. Science, 173: 914-916.
ADAMS, D. F., FALGOUT, D., FROHLINGER, J. O., PATE, J. B., PLUMLEY, A.
L., SCARINGELLI, F. P., & URONE, P. (1971) Tentative method of
analysis for sulfur dioxide content of the atmosphere (manual
conductimetric method). Health Sci. Lab., 8: 42-47.
ALARIE Y., ULRICH, C. E., BUSEY, W. M., SWANN, H. E., & MACFARLAND, H.
N. (1970) Long-term continuous exposure of guinea pigs to sulfur
dioxide. Arch. environ. Health, 21: 769-777.
ALARIE, Y., ULRICH, C. E., BUSEY, W. M., KRUMM, A. A., & MACFARLAND,
H. N. (1972) Long-term continuous exposure to sulfur dioxide in
cynomolgus monkeys. Arch. environ. Health, 24: 115-128.
ALARIE, Y., BUSEY, W. M., KRUMM, A. A., & ULRICH, C. E. (1973)
Long-term continuous exposure to sulfuric acid mist in cynomolgus
monkeys and guinea pigs. Arch. environ. Health, 27: 16-24.
ALARIE, Y., KRUMM, A. A., BUSEY, W. M., ULRICH, C. E., & KANTZ, R. J.
II. (1975) Long-term exposure to sulfur dioxide, sulfuric acid
mist, fly ash and their mixtures. Results of studies in monkeys
and guinea pigs. Arch. environ. Health, 30: 254-262.
ALBERT, R. E., SPIEGELMAN, J., LIPPMANN, M., & BENNET, R. (1968) The
characteristics of bronchial clearance in the miniature donkey.
Arch. environ. Health, 17: 50-58.
ALLEN, E. R., MCQUIGG, R. D., & CADLE, R. D. (1972) The
photo-oxidation of gaseous sulfur dioxide in the air.
Chemosphere, 1: 23-32.
ALTSHULLER, A. P. (1973) Atmospheric sulfur dioxide and sulfate:
distribution of concentrations at urban and non-urban sites in the
United States. Environ. Sci. Technol., 7: 709-712.
AMDUR, M. O. (1957) The influence of aerosols upon the respiratory
response of guinea pigs to sulfur dioxide. Am. Ind. Hyg. Assoc.
Q., 18: 149-155.
AMDUR, M. O. (1958) The respiratory response of guinea pigs to
sulfuric acid mist. Am. Med. Assoc. Arch. ind. Health,
18: 407-414.
AMDUR, M. O. (1961) Report on tentative ambient air standards for
sulfur dioxide and sulfuric acid. Ann. occup. Hyg., 3: 71-83.
AMDUR, M. O. (1966) Respiratory absorption data and SO2 dose-response
curves. Arch. environ. Health, 12: 729-732.
AMDUR, M. O. (1969) Toxicological appraisal of particulate matter,
oxides of sulfur and sulfuric acid. J. Air Pollut. Control
Assoc., 19: 638-644.
AMDUR, M. O. (1970) The impact of air pollutants on physiological
responses of the respiratory tract. Proc. Am. Philos. Soc.,
114: 3-8.
AMDUR, M. O. (1971) Aerosols formed by oxidation of sulfur dioxide.
Review of their toxicology. Arch. environ. Health, 23: 459-468.
AMDUR, M. O. & UNDERHILL, D. (1968) The effect of various aerosols on
the response of guinea pigs to sulfur dioxide. Arch. environ.
Health, 16: 460-468.
AMDUR, M. O., SCHULZ, R. Z., & DRINKER, P. (1952a) Toxicity of
sulfuric acid mist to guinea pigs. Am. Med. Assoc. ind. Hyg.
occup. Med., 5: 318-329.
AMDUR, M. O., SILVERMAN, L., & DRINKER, P. (1952b) Inhalation of
sulphuric acid mist by human subjects. Am. Med. Assoc. Arch. ind.
Hyg. occup. Med., 6: 305-313.
AMDUR, M. O., MELVIN, W. W., Jr, & DRINKER, P. (1953) Effects of
inhalation of sulfur dioxide by man. Lancet, 2: 758-759.
AMERICAN CONFERENCE OF GOVERNMENT INDUSTRIAL HYGIENISTS (1977)
TLVs threshold limit values for chemical substances and physical
agents in the workroom environment with intended changes for
1977, Cincinnati, OH, ACGIH, p. 28.
AMERICAN SOCIETY FOR TESTING AND MATERIALS (1964) Standard test
method for particulate matter in the atmosphere. Optical density
of filtered deposit. ASTM standards on methods of atmospheric
sampling and analysis, Philadelphia, USA, ASTM, pp. 661-668.
ANDERSEN, I. (1972) Relationships between outdoor and indoor air
pollution. Atmos. Environ. 6: 275-278.
ANDERSEN, I., LUNDQVIST, G. R., JENSEN, P. L., & PROCTOR, D. F. (1974)
Human response to controlled levels of sulfur dioxide. Arch.
environ. Health, 28: 31-39.
ANDERSON, A. (1950) Possible long-term effects of exposure to sulfur
dioxide. Br. J. ind. Med., 7: 82-86.
ASH, R. & LYNCH, J. (1972) The evaluation of gas detector tube
systems: sulfur dioxide. Am. Ind. Hyg. Assoc. J., 32: 490-491.
AZAR, A., SNEE, R. D., & HABIBI, K. (1972) Relationship of community
levels of air lead and indices of lead absorption. In:
Proceedings of the International Symposium on Environmental
Aspects of Lead, Luxembourg, Commission of the European
Communities, pp. 581-594.
BALCHUM, O. J., DYBICKI, J., & MENECLY, G. R. (1959) Absorption and
distribution of 35SO2 inhaled through the nose and mouth by
dogs. Am. J. Physiol., 197: 1317-1321.
BALCHUM, O. J., DYBICKI, J., & MENECLY, G. R (1960a) The dynamics of
sulfur dioxide inhalation, absorption, distribution and retention.
Arch. ind. Health, 21: 564-569.
BALCHUM, O. J., DYBICKI, J., & MENECLY, G. R. (1960b) Pulmonary
resistance and compliance with concurrent radioactive sulfur
distribution in dogs breathing 35SO2. J. appl. Physiol.,
15: 62-66.
BALL, D. J. & HUME, R. (1977) The relative importance of vehicular and
domestic emissions of dark smoke in Greater London in the mid
1970s, the significance of smoke shade measurements, and an
explanation of the relationship of smoke shade to gravimetric
measurements of particulate. Atmos. Environ., 2: 1065-1073.
BARRIE, L. A. & GEORGII, H. W. (1976) An experimental investigation of
the absorption of sulfur dioxide by water drops containing heavy
metal ions. Atmos. Environ., 10: 743-749.
BATES, D. V. & HAZUCHA, M. (1973) The short-term effects of ozone on
the human lung. Proceedings of the Conference on Health Effects of
Air Pollution, NAS-NRC, Washington, DC, Oct. 3-5. In: Committee
Print, Committee on Public Works, United States Senate, US Govt.
Printing Office, pp. 507-540.
BATTIGELLI, M. C., COLE, H. M., FRASER, D. A., & MAH, R. A. (1969)
Long-term effect of sulfur dioxide and graphite dust on rats.
Arch. environ. Health, 18: 602-608.
BENSON, E. B., HENDERSON, J. J., & CALDWELL, D. E. (1972)
Indoor-outdoor air pollution relationships: a literature review,
Research Triangle Park, NC, US Environmental Protection Agency,
83 pp. (Publ. No. AP 112).
BIERSTEKER, K. (1966) [Polluted air causes, epidemiological
significance and prevention of atmospheric pollution.] Assen,
Netherlands, Van Gorcum & Co., pp. 21-23 (in Dutch).
BIERSTEKER, K., DE GRAAF, H., & NASS, C. A. G. (1965) Indoor air
pollution in Rotterdam homes. Int. J. Air Water Pollut.,
9: 343-350.
BOSANQUET, C. H. (1957) The rise of a hot waste gas plume. J. Inst.
Fuel, 30: 322-328.
BRAIN, J. D. & VALBERG, P. A. (1974) Models of lung retention based
ion ICRP Task Group Report. Arch. environ. Health, 28: 1-11.
BRIGGS, G. A. (1965) A plume rise model compared with observations.
J. Air Pollut. Control Assoc., 15: 433-438.
BRITISH STANDARDS INSTITUTION (1969a) Methods for the measurement of
air pollution. BS1747 Part 4. The lead dioxide method.
London, BSI, 16 pp.
BRITISH STANDARDS INSTITUTION (1969b) Methods for the measurement of
air pollution. BS1747 Part 1. Deposit gauges. London, BSI,
15 pp.
BROSSET, C. (1973) Air-borne acid. Ambio, 2: 2-9.
BUECHLEY, R. W., RIGGAN, W. B., HASSELBLAD, V., & VAN BRUGGEN, J. B.
(1973) SO2 levels and perturbations in mortality -- a study in
the New York/New Jersey metropolis. Arch. environ. Health,
27: 134-137.
BUFALINI, M. (1971) Oxidation of SO2 in polluted atmospheres -- a
review. Environ. Sci. Technol., 5: 685-703.
BURROWS, B., KELLOG, A. L., & BUSKEY, J. (1968) Relationships of
symptoms of chronic bronchitis and emphysema to weather and air
pollution. Arch. environ. Health, 16: 406-413.
BURTON, G. G., CORN, M., GEE, J. B. L., VASALLO, C., & THOMAS, A. P.
(1969) Response of healthy men to inhaled low concentration of
gas-aerosol mixtures. Arch. environ. Health, 18: 681-692.
BUSTUEVA, K. A. (1957) [On the toxicology of sulfuric acid aerosol.]
Gig. i Sanit., No. 2, pp. 17-22 (in Russian).
BUSTUEVA, K. A. (1961a) Experimental data on the effect of small
concentration of sulfur oxides, maximum permissible concentration
of air pollutants, Moscow, Medgiz, pp. 126-141.
BUSTUEVA, K. A. (1961b) Threshold reflex effect of SO2 and H2SO4
aerosols simultaneously present in the air. In: Ryazanov, V. A.,
ed. Limits of allowable concentrations of atmospheric pollutants,
Book 4, Washington DC, US Department of Commerce, Office of
Technical Services, pp. 92-101.
BUSTUEVA, K. A. (1964) [Resorptive action of sulfur oxides.] Gig. i
Sanit., No. 10, pp. 8-12 (in Russian).
BUSTUEVA, K. A. (1966) The toxicity of continuous exposure to sulfur
oxides. Biological action and hygienic significance of
atmospheric pollution, Moscow, Medgiz, pp. 142-172.
BUSTUEVA, K. A., POLEZAEV, E.G., & SEMENENKO, A.D. (1960)
[Electroencephalographic determination of threshold reflex effect
of atmospheric pollutants.] Gig. i Sanit., 25: 57-61
(in Russian).
BYSTROVA, T. A. (1957) [Some effects of sulfurous gas determined by
the method of tracer atoms.] Gig. i Sanit., 5: 30-37
(in Russian).
CALVERT, J. G. (1973) Interaction of air pollutants. In: Proceedings
of the Conference on the Health Effects of Air Pollution,
Washington, DC, US Government Printing Office, pp. 19-101
(Serial No. 93-15).
CAMNER, P. & PHILIPSON, K. (1972) Tracheobronchial clearance in
smoking discordant twins. Arch. environ. Health, 25: 60-63.
CAMNER, P., JARSTRAND, C., & PHILIPSON, K. (1973a) Tracheobronchial
clearance in patients with influenza. Am. Rev. respir. Dis.,
108: 131-135.
CAMNER, P., MOSSBERG, B., & PHILIPSON, K. (1973b) Tracheobronchial
clearance and chronic obstructive lung disease. Scand. J. respir.
Dis., 54: 272-281.
CARABINE, M.D. & MADDOCK, J. E. L. (1976) The growth of sulfuric acid
aerosol particles when contacted with water vapour. Atmos.
Environ., 10: 735-742.
CARNOW, B. W., LEPPER, M. H., SHEKELLE, R. B., & STAMLER, J. (1969)
Chicago air pollution study: SO2 levels and acute illness in
patients with chronic bronchopulmonary disease. Arch. environ.
Health,. 18: 768-776.
CARSON, G. A.. & PAULUS, H. J. (1974) A high volume cascade sieve
impactor. Am. Ind. Hyg. Assoc. J., 35: 262-268.
CHARLSON, R. J., VANDERPOL, A. H., COVERT, D. S., WAGGONER, A. P., &
AHLQVIST, N. C. (1973) Sulfuric acid-ammonium sulfate aerosol:
Optical detection in the St Louis region. Science, 184: 156-158.
CHUN, K. C. & QUON, J. E. (1973) Capacity of ferric oxide particles to
oxidize sulfur dioxide in air. Environ. Sci. Technol.,
7: 532-538.
CLEARY, G. J. (1967) Some arguments suggesting a causative
relationship between air pollutants and lung cancer. Clean Air,
September: 15-18.
COHEN, A. A., BROMBERG, S., BUECHLEY, R. W., HEIDERSCHEIT, L. T., &
SHY, C. M. (1972) Asthma and air pollution from a coal-fuelled
power plant. Am. J. public Health, 62: 1181-1188.
COHEN, A. A., NELSON, C. J., BROMBERG, S. M., PRAVDA, M., FERRAND, E.
F., & LEONE, G. (1974) Symptom reporting during recent publicized
and unpublicized air pollution episodes. Am. J. public Health,
64: 442-449.
COLLEGE OF GENERAL PRACTITIONERS (1961) Chronic bronchitis in Great
Britain: A national survey carried out by the respiratory diseases
study group of the College of General Practitioners. Br. med. J.,
2: 973-979.
COLLEY, J. R. T. & REID, D. D. (1970) Urban and social origins of
childhood bronchitis in England and Wales. Br. med. J.,
2: 213-217.
COLLEY, J. R. T., DOUGLAS, J. W. B., & REID, D. D. (1973) Respiratory
disease in young adults: influence of early childhood lower
respiratory tract illness, social class, air pollution and
smoking. Br. med. J., 3: 195-198.
COMMINS, B. T. (1963) Determination of particulate acid in town air.
Analyst, 88: 364-367.
COMMINS, B. T. (1967) Some studies of the particulate acid sulfate
from the products of combustion of fuels and measurement of the
acid in polluted atmospheres. In: Proceedings of the Symposium on
the Transformation of Sulfur Compounds, Mainz, Germany,
7-9 June, Paris, OECD, pp. 39-46.
COMMINS, B. T. & LAWTHER, P. J. (1958) Volatility of 3,4-benzpyrene in
relation to the collection of smoke samples. Br. J. Cancer,
12: 351-354.
COMMINS, B. T. & WALLER, R. E. (1967) Observations from a 10-year
study of pollution at a site in the city of London. Atmos.
Environ., 1: 49-68.
COMMINS, B. T. & WALLER, R. E. (unpublished) Long-term trends in
pollution in central London. Atmos. Environ.
COMMISSION OF THE EUROPEAN COMMUNITIES (1976) Air sulfur dioxide
concentrations in the European community, Luxembourg, CEC,
pp. 34-51 (Report EUR 5417e).
COMMITTEE ON AIR POLLUTION (1954) Report, London, Her Majesty's
Stationery Office, pp. 7-25 (Report Cmd 9322).
COMMITTEE ON THE CHALLENGES OF MODERN SOCIETY (1971) Air pollution.
Air quality criteria for sulfur oxides. NATO/CCMS, No. 7,
pp. 1-1 to 8-85.
CORN, M., KOTSKO, N., STANTON, D., BELL, W., & THOMAS, A. P. (1972)
Response of cats to inhaled mixtures of SO2 and SO2-NaCl aerosol
in air. Arch. environ. Health, 24: 248-256.
COX, R. A. & PENKETT, S. A. (1970) The photo-oxidation of sulfur
dioxide in sunlight. Atmos. Environ., 4: 425-433.
COX, R. A. & PENKETT, S. A. (1971) Oxidation of atmospheric SO2 by
products of the ozone-olefin reaction. Nature (Lond.),
230: 321-322.
CUMMINGS, W. G. & REDFEARN, M. W. (1957)Instruments for measuring
small quantities of sulfur dioxide in the atmosphere. J. Inst.
Fuel, 30: 628-635.
DALHAMN, T. & STRANDBERG, L. (1961) Acute effect of sulfur dioxide on
the rate of ciliary beat in the trachea of rabbit, in vivo and
in vitro, with studies on the absorptional capacity of the nasal
cavity. Int. J. Air Water Pollut., 4: 154-167.
DALY, C. (1954) Air pollution and bronchitis. Br. med. J.,
2: 687-688.
DALY, C. (1959) Air pollution and causes of death. Br. J. prev. soc.
Med., 13: 14-27.
DE GRAAF, H. & BIERSTEKER, K. (1972) Comparison of smoke concentration
outside and inside two selected sites. Atmos. Environ., 6: 697.
DEPARTMENT OF ENVIRONMENTAL POLLUTION CONTROL, Tokyo (1976) Report of
air pollution measurements. Tokyo, Continuous Measurement
Stations, Tokyo Metropolitan Government, 119 pp.
DEROUANE, A. (1972) Comparaison des concentrations en fumées à
l'extérieur et à l'interiéur des lieux d'habitation. Atmos.
Environ., 6: 209-220.
DEROUANE, A., GRANDJEAN, L., LEGRAND, M., & VERDUYN, G. (1972)
Atmospheric pollution by oxides of sulfur and smoke in Belgium,
1969-70. Inst. R. Met. Belg., Publ. A. No. 78: 1-54.
DERRETT, C. J. & BROWN, C. (in press) A continuous running
direct-reading SO2 recorder. J. Phys. E. Sci. Instrum., 11:
DOHAN, F. C. (1961) Air pollutants and incidence of respiratory
disease. Arch. environ. Health, 3: 387-395.
DOHAN, F. C. & TAYLOR, E. W. (1960) Air pollution and respiratory
disease: a preliminary report. Am. J. med. Sci., 240: 337-339.
DORSCH, R. (1913) [The pollution of the air in the storage battery
room and its surroundings by sulfuric acid.] Unpublished
dissertation, Wurzberg, Germany (in German). Quoted in: Lewis, T.
R., ed. Toxicology of atmospheric sulfur dioxide decay products,
Research Triangle Park, NC, US Environmental Protection Agency,
p. 19 (Report No. AP111).
DOUGLAS, J. W. B. & WALLER, R. E. (1966) Air pollution and respiratory
infection in children. Br. J. prev. soc. Med., 20 (1): 1-8.
DZUBAY, T. G. & STEVENS, R. K. (1973) Ambient air analysis with
dictomous sampler and X-ray fluorescence spectrometer. Environ.
Sci. Technol., 9: 663-668.
EAST, C. (1972) Sulfur dioxide emissions estimated from airborne
measurements. Atmos. Environ., 6: 399-408.
ELFIMOVA, E. V. & HACATURJAN, M. K. (1968) [Features specific to the
reflex action of sub-threshold concentrations of sulfurous
anhydride in combustion with phenol and carbon monoxide in the
atmosphere.] Gig. i Sanit., 33 (11): 3-6 (in Russian).
ELFIMOVA, E. V. & GUSEV, M. I. (1969) [Health effects of low sulfur
dioxide concentrations in air.] Gig. i Sanit., 34 (2): 3-7
(in Russian).
ELKINS, H. (1959) The chemistry of industrial toxicology. New York,
John Wiley, pp. 396-397
ELLIOTT, L. P. & ROWE, D. R. (1976) Air quality during public
gatherings. J. Air Pollut. Control Assoc'., 25: 635-636.
ELLISON, J. M. (1965) The nature of air pollution and the methods
available for measuring it. Bull. World Health Organ.,
32: 399-409.
EMERSON, P. A. (1973) Air pollution, atmospheric conditions and
chronic airways obstruction. J. occup. Med., 15: 635-638
FERIN, J. & LEACH, L. J. (1973) The effect of SO2 on lung clearance
of TiO2 particles in rats. Am. Ind. Hyg. Assoc. J.,
34: 260-263.
FERRIS, B. G., Jr & ANDERSON, D. O. (1962) The prevalence of chronic
respiratory disease in a New Hampshire town. Am. Rev. respir.
Dis., 86: 165-185.
FERRIS, B. G., Jr & ANDERSON, D. O. (1964) Epidemiological studies
related to air pollution: A comparison of Berlin, NH and
Chilliwack, British Columbia. Proc. R. Soc. Med.,
57 (Suppl.): 979-983
FERRIS, B. G., Jr, BURGESS, W. A., & WORCESTER, J. (1967) Prevalence
of chronic respiratory disease in a pulp mill and a paper mill in
the United States. Br. J. ind. Med., 24: 26-37.
FERRIS, B. G., Jr, HIGGINS, I. T. T., HIGGINS, M. W., & PETERS, J. M.
(1973) Chronic nonspecific respiratory disease in Berlin, New
Hampshire 1961-1967. A follow-up study. Am. Rev. respir. Dis.,
107: 110-122.
FERRIS, B. G., Jr, CHEN, H., PULEO, S., & MURPHY, L. H., Jr (1976)
Chronic nonspecific respiratory disease in Berlin, New Hampshire,
1967 to 1973. A further follow-up study. Amer. Rev. resp. Dis.,
113: 475-485.
FIRKET, H. (1931) Sur les causes des accidents survenue dans la vallée
de la Meuse, lors des brouillards de Décembre 1930. Bull. R.
Acad. Med. Belg., 11: 683-739.
FORTAK, H. G. (1970) Numerical simulation of the temporal and spatial
distributions of urban air pollution concentrations. In:
Proceedings of a Symposium on Multiple Source Urban Diffusion
Models. Research Triangle Park, NC, US Environmental Protection
Agency.
FRANK, N. R. & SPEIZER, F. E. (1965) SO2 effects on the respiratory
system in dogs. Arch. environ. Health, 11: 624-634.
FRANK, N. R., AMDUR, M. O., WORCESTER, J., & WHITTENBERGER, J. L.
(1962) Effects of acute controlled exposure to SO2 on respiratory
mechanics in healthy male adults J. appl. Physiol., 17: 252-258.
FRANK, N. R., AMDUR, M, O., & WHITTENBERGER, J. L. (1964) A comparison
of the acute effects of SO2 administered alone or in combination
with NaCl particles on the respiratory mechanics of healthy
adults. Int. J. Air Water Pollut., 8: 125-133.
FRANK, N. R., YODER, R. E., YOKOYAMA, E., & SPEIZER, F. E. (1967) The
diffusion of 35SO2 from tissue fluids into the lungs following
exposure of dogs to 35SO2. Health Phys., 13: 31-38.
FRANK, N. R., YODER, R. E., BRAIN, J. D., & YOKOYAMA, E. (1969) SO2
(35S labelled) absorption by the nose and mouth under conditions
of varying concentration and flow. Arch. environ. Health,
18: 315-322.
FRASER, D. A., BATTIGELLI, M. C., & COLE, H. M. (1968) Ciliary
activity and pulmonary retention of inhaled dust in rats exposed
to sulfur dioxide. J. Air Pollut. Control Assoc., 18: 821-823.
FREIBERG, J. (1975) The mechanism of iron catalyzed oxidation of SO2
in oxygenated solutions. Atmos. Environ., 9: 661-672.
FUCHS, N. A. (1964) The mechanics of aerosols, Oxford, Pergamon
Press, p. 28.
FUGAS, M. (1976) Assessment of total exposure to an air pollutant. In:
International Conference on Environmental Sensing and Assessment,
Las Vegas, September 14-19, 1975, New York, IEEE, Vol. 2,
paper 38-5. pp. 1-3.
GARDNER, M. J., CRAWFORD, M. D., & MORRIS, J. N. (1969) Patterns of
mortality in middle and early old age in the county boroughs of
England and Wales. Br. J. prev. soc. Med., 23: 133-140.
GARLAND, J. A., CLOUGH, W. S., & FOWLER, D. (1973) Deposition of
sulfur dioxide on grass. Nature (Lond.). 242: 256-257.
GARLAND, J. A., ATKINS, D. H. F., READINGS, C. J., & CAUGHEY, S. J.
(1974) Deposition of gaseous sulfur dioxide to the ground. Atmos.
Environ., 8: 75-79.
GOLDRING, I. P., GREENBURG, L., PARK, S.S., & RATNER, I. M. (1970)
Pulmonary effects of sulfur dioxide exposure in the Syrian
hamster. Arch. environ. Health, 21: 32-37.
GOLDSMITH, J. R. (1969) Los Angeles smog. Sci. J., 5: 44-49.
GORE, A. T. & SHADDICK, C. W. (1958) Atmospheric pollution and
mortality in the County of London. Br. J. prev. soc. Med.,
12: 104-113.
GREY, D.C. & JENSEN, M. L. (1972) Bacteriogenic sulfur in air
pollution. Science, 177: 1099-1100.
GROSSER, P. J., STARK, C., JECH, J., & MEHLHORN, H. (1971) [Vital
capacity and respiration tests of school children of the 4th and
5th grade in areas with different air quality situations.]
Zeitschr. Erkr. Atmungsorgane, 134: 255-265 (in German).
GRUBER, C. W. & ALPAUGH, E. L. (1954) The automatic filter paper
sampler in an air pollution measurement programme. J. Air Pollut.
Control Assoc., 4: 143-147.
HATCH, T. & GROSS, P. (1964) Pulmonary deposition and retention of
inhaled aerosols, New York, Academic Press.
HEMEON, W. C. L., HAINES, G. F., Jr, & IDE, H. M. (1953) Determination
of haze and smoke concentrations by filter paper samplers. J. Air
Pollut. Control Assoc., 3: 22-28.
HENDERSON, J. J., BENSON, F. B., & CALDWELL, D. E. (1973)
Indoor-outdoor air pollution relationships. Vol. II. An annotated
bibliography. Washington, DC, US EPA (Publ. No. AP 112b).
HILL, A. C. (1971) Vegetation: a sink for atmospheric pollutants.
J. Air Pollut. Control Assoc., 21: 341-346.
HOBBS, P. V., HARRISON, H., & ROBINSON, E. (1974) Atmospheric effects
of pollutants. Science, 183: 909-915.
HOEGG, U. R. (1972) Cigarette smoke in closed places. Environ. Health
Perspect., October, No. 2: 117-128.
HOLBROW, G. L. (1958) Crystalline bloom, Teddington, UK, Paint
Research Station, p. 4 (Tech. Paper No. 208).
HOLLAND, W. W. & REID, D. D. (1965) The urban factor on chronic
bronchitis. Lancet, 1: 445-448.
HOLLAND, W. W. & STONE, R. W. (1965) Respiratory disorders in United
States East Coast telephone men. Am. J. Epidemiol., 82: 92-101.
HOLLAND, W. W., REID, D. D., SELTSER, R., & STONE, R. W. (1965)
Respiratory disease in England and the United States: Studies of
comparative prevalence. Arch. environ. Health, 10: 338-343.
HOLLAND, W. W., HALIL, T., BENNETT, A. E., & ELLIOTT, A. (1969)
Factors influencing the onset of chronic respiratory disease.
Br. med. J., 2: 205-208.
HOLLAND, W. W., BENNETT, A. E., CAMERON, I. R., FLOREY, C DU V.,
LEEDER, S. R., SCHILLING, R. S. F., SWAN, A. V, & WALLER, R. E.
(in press) Health effects of particulate pollution. Reappraising
the evidence. Am. J. Epidemiol.
HOLLOWELL, C. D., GEE, G. Y., & McLAUGHLIN, R. D. (1973) Current
instrumentation for continuous monitoring for SO2. Anal. Chem.,
45: 63A-72A.
HORVATH, H. & CHARLSON, R. J. (1969) The direct optical measurement of
atmospheric air pollution. Am. Ind. Hyg. Assoc. J., 30: 500-509.
HUHTI, E., RYHANEN, P., VUOPALA, U., & TAKKANEN, J. (1970) Chronic
respiratory disease among pulp mill workers in an arctic area in
Northern Finland. Acta Med. Scand., 187: 433-444.
HUSAR, J. D., HUSAR, R. B., & STUBITS, P. K. (1975) Determination of
submicrogram amounts of atmospheric particulate sulfur. Anal.
Chem., 47: 2062-2065.
HUSAR, R. B. (1974) Atmospheric particulate mass monitoring with a
radiation detector. Atmos. Environ., 8: 183-188.
INTERNATIONAL ATOMIC ENERGY AGENCY (1978) Particle size analysis in
estimating the significance of airborne contamination, Vienna,
International Atomic Energy Agency, pp. 71-170 (Technical Report
Series, No. 179).
ILO/WHO COMMITTEE ON OCCUPATIONAL HEALTH (1970) Permissible levels of
toxic substances in the working environment. Geneva, International
Labour Office, p. 337 (Occupational Safety and Health Series).
IPSEN, J., DEANE, M., & INGENITO, F. E. (1969) Relationships of acute
respiratory disease to atmospheric pollution and meteorological
conditions. Arch. environ. Health, 18: 462-472.
JACOBSEN, M. (1972) Sampling and evaluating respirable coal mine dust.
Ann. NY Acad. Sci., 200: 661-665.
JARSTRAND, C., CAMNER, P., & PHILIPSON, K. (1974) Mycoplasma
pneumoniae and tracheobronchial clearance. Am. Rev. respir. Dis.,
110: 415-419.
JOHNSTONE, H. F. & COUGHANOWR, D. R. (1958) Absorption of SO2 from
air. Oxidation in drops containing dissolved catalyst. Ind. eng.
Chem., 50: 1169-1172.
JUNGE, C. E. & RYAN, T. G. (1958) Study of the SO2 oxidation in
solution and its role in atmospheric chemistry. Q. J. R. Med.
Soc., 84: 46-55.
KATZ, M. (1969) Measurement of air pollutants, guide to the selection
of methods, Geneva, WHO, 123 pp.
KEHOE, R. A., MACHLE, W. H., KITZMILLER, K., & LEBLANC, T. J. (1932)
On the effects of prolonged exposure to sulfur dioxide. J. ind.
Hyg., 14: 159-173.
KELLOGG, W. W., CADLE, R. D., ALLEN, E. R., LAZRUS, A. Z., & MARTELL,
E. A. (1972) The sulfur cycle. Science, 175: 587-596.
KRUSHNER, M. & LASKIN, S. (1971) Experimental models in environmental
carcinogenesis. Am. J. Pathol., 64: 183-196.
LAMBERT P. M. & REID, D. D. (1970) Smoking, air pollution and
bronchitis in Britain. Lancet, 25 April 1970: 853-857.
LARSEN, R. I. (1971) A mathematical model for relating air quality
measurements to air quality standards, Research Triangle Park,
NC, US Environmental Protection Agency, 61 pp. (Rpt No. AP-89).
LAUER, G. & BENSON, F. B. (1975) The CHAMP air quality monitoring
programme. In: Proceedings of the International Symposium on
Recent Advances in the Assessment of the Health Effects of
Environmental Pollution, Paris, 24-28 June 1974, Luxembourg,
Commission of the European Communities, Vol. 1, pp. 423-430.
LAUTERBACH, K. E., MERCER, T. T., HAYES, A.D., & MARROW, P. E. (1954)
Efficiency studies of the electrostatic precipitator. Arch. ind.
Hyg., 9: 69-75.
LAVE, L. B. & SESKIN, E. P. (1970) Air pollution and human health.
Science, 169: 723-733.
LAVE, L. B. & SESKIN, E. P. (1972) Air pollution, climate, and home
heating: their effects on US mortality rates. Am. J. public
Health, 62: 909-916.
LAWTHER, P. J. (1963) Compliance with the clean air act: medical
aspects. J. Inst. Fuel, 36: 341-344.
LAWTHER, P. J. (1955) Effects of inhalation of sulfur dioxide on
respiration and pulse-rate in normal subjects. Lancet,
2: 745-748.
LAWTHER, P. J. & WALLER, R. E. (1976) Coal fires, industrial emissions
and motor vehicles as sources of environmental carcinogens. In:
Proceedings of Symposium on Environmental Pollution and
Carcinogenic risks, Lyons, 3-5 November 1975, Paris, INSERM,
Vol. 52, pp. 27-40.
LAWTHER, P. J., WALLER, R. E., & HENDERSON, M. (1970) Air pollution
and exacerbations of bronchitis. Thorax, 25: 525-539.
LAWTHER, P. J., MACFARLANE, A. J., WALLER, R. E., & BROOKS, A. G. F.
(1975) Pulmonary function and sulfur dioxide: some preliminary
findings. Environ. Res., 10: 355-367.
LEBOWITZ, M.D., TOYAMA, T., & MCCARROLL, J. (1973) The relationship
between air pollution and weather as stimuli and daily mortality
as responses in Tokyo, Japan, with comparisons with other cities.
Environ. Res., 6: 327-333.
LEE, R. E., Jr & GORANSON, S. (1972) Cascade impactor network,
Research Triangle Park, NC, US Environmental Protection Agency,
132 pp. (Rep. No. AP108).
LEE, R. E., CALDWELL, J. S., & MORGAN, G. B. (1972) The evaluation of
methods for measuring suspended particulates in air. Atmos.
Environ., 6: 593-622.
LEWIS, T. R., MOORMAN, W. J., LUDMAN, W. F., & CAMPBELL, K. I. (1973)
Toxicity of long-term exposure to oxides of sulfur. Arch.
environ. Health, 26: 16-21.
LIKENS, G. E. & BORMANN, F. H. (1974) Acid rain: a serious regional
environmental problem. Science, 184: 1176-1179.
LINDVALL, T. & RADFORD, E. P. (1973) Measurement of annoyance due to
exposure to environmental factors. Environ. Res., 6: 1-36.
LIPPMANN, M., ALBERT, R. E., & PETERSON, H. T., Jr (1971) The regional
deposition of inhaled aerosols in man. In: Walton, W. H., ed.
Inhaled Particles III, London, Unwin, Vol. 1, pp. 105-120.
LISS, P.S. (1971) Exchange of SO2 between the atmosphere and natural
waters. Nature (Lond.), 233: 327-329.
LIU, B. Y. H., BERGLUND, R. N., & AGARWAL, J. M. (1974) Experimental
studies of optical particle counters. Atmos. Environ.,
8: 717-732.
LOWE, C. R., PELMEAR, P. L., CAMPBELL, H, HITCHENS, R. A. N., KHOSLA,
T., & KING, T. C. (1968) Bronchitis in two integrated steel works.
I. Ventilatory capacity, age, and physique of non-bronchitic men.
Br. J. prev. soc. Med., 22: 1-11.
LOWE, C. R., CAMPBELL, H., & KHOSLA, T. (1970) Bronchitis in two
integrated steel works. III. Respiratory symptoms and ventilatory
capacity related to atmospheric pollution. Br. J. Ind. Med.,
27: 121-120.
LUNN, J. E., KNOWELDEN, J., & HANDYSIDE, A. J. (1967) Patterns of
respiratory illness in Sheffield infant school-children. Br. J.
prev. soc. Med., 21: 7-16.
LUNN, J. E., KNOWELDEN, J., & ROE, J. W. (1970) Patterns of
respiratory illness in Sheffield junior school-children -- A
follow-up study. Br. J. prev. soc. Med., 24: 223-228.
MADDALONE, R. F., MCCLURE, G. L., & WEST, P. W. (1975) Determination
of sulfate by thermal reduction of perimidylammonium sulfate.
Anal. Chem., 47: 316-322.
MALCOLM, D. & PAUL, E. (1961) Erosion of the teeth due to sulfuric
acid in the battery industry. Br. J. ind. Med., 18: 63-69.
MAMACASVILI, M. L (1968) Determination of the reflex action of SO2
and CO by adaptometer method and coloured vision. In: Biological
action and hygienic significance of atmospheric pollutants,
Moscow, Medgiz, pp. 179-188.
MARTIN, A. E. (1961) Epidemiological studies of atmospheric pollution:
A review of British methodology. Mon. Bull. Minist. Health Public
Health Lab. Serv., 20: 42-49.
MARTIN, A. E. (1964) Mortality and morbidity statistics and air
pollution. Proc. R. Soc. Med., 57: 969-975.
MARTIN, A. E. & BRADLEY, W. H. (1960) Mortality, fog and atmospheric
pollution. An investigation during the winter of 1958-59. Mon.
Bull. Minist. Health Public Health Lab. Serv., 19: 56-72.
MASTERS, B. R. (1974) The city of London and clean air. Clean Air,
4: 22-26.
MATSUMURA, Y. (1970a) The effects of ozone, nitrogen dioxide and
sulfur dioxide on the experimentally induced allergic respiratory
disorder in guinea pigs. I. The effect on sensitization with
albumin through the airway. Am. Rev. respir. Dis., 102: 430-437.
MATSUMURA, Y. (1970b) The effects of ozone, nitrogen dioxide and
sulfur dioxide on the experimentally induced allergic respiratory
disorder in guinea pigs. III. The effect on the occurrence of
dyspneic attacks. Am. Rev. respir. Dis., 102: 444-447.
MAWDESLEY-THOMAS, L. E., HEALEY, P., & BARRY, D. H. (1971)
Experimental bronchitis in animals due to sulfur dioxide and
cigarette smoke. In: WALTON, W. H., ed. Inhaled Particles III,
London, Unwin, Vol. 1, pp. 504-525.
MAZIARKA, S. & MROZ, E. (1968) [The influence of air pollution on the
protective apparatus of the eye in school children.] Rocz. PZH,
19: (1) 31-36 (in Polish).
MCJILTON, C., FRANK, R., & CHARLSON, R. (1973) Role of relative
humidity in the synergistic effect of a sulfur dioxide-aerosol
mixture on the lung. Science, 182: 503-504.
MCKAY, H. A. C. (1971) The atmospheric oxidation of SO2 in water
droplets in presence of NH3. Atmos. Environ., 5: 7-14.
MERCER, T. T. (1967) On the role of particle size in the dissolution
of lung burdens. Health Phys., 13: 1211-1222.
MINISTRY OF HEALTH, UNITED KINGDOM (1954) Mortality and morbidity
during the London fog of December 1952, London, HMSO (Report on
Public Health and Medical Subjects No. 95).
MINISTRY OF TECHNOLOGY, UK (1966) National survey of smoke and sulfur
dioxide: Instruction manual, Stevenage, Warren Spring
Laboratory, 142 pp.
MISIAKIEWICZ, Z. (1970) The effects on rat organism of prolonged
exposure to low concentrations of sulfur dioxide in the air.
J. Natl Inst. Hyg. (Warsaw), 21: 469-487.
MONTGOMERY, T. L. & COLEMAN, J. H. (1975) Empirical relationships
between time-averaged SO2 concentrations. Environ. Sci.
Technol., 9: 953-957.
MORROW, P. E. (1973) Alveolar clearance of aerosols. Arch. intern.
Med., 131: 101-108.
MUNN, R. E. (1976) Air pollution meteorology. Ch. 7 in Manual on
urban air quality measurement. Copenhagen, WHO (WHO Regional
publ. European Series, No. 1).
NASH, T. (1964) A "personal" measuring instrument for atmospheric
sulfur dioxide. Int. J. Air Water Pollut., 8: 121-124.
ORGANISATION FOR ECONOMIC CO-OPERATION AND DEVELOPMENT (1965) Methods
of measurement of air pollution, Paris, OECD, 94 pp.
PAN AMERICAN HEALTH ORGANIZATION (1976) Pan American air pollution
monitoring network (Redpanaire), Lima, Pan American Center for
Sanitary Engineering and Environmental Sciences, 50 pp.
(Pub. No. 30).
PASQUILL, F. (1971) Atmospheric dispersion of pollution. Q. J. R.
Med. Soc., 97: 369-395.
PATE, J. B., AMMONS, B. E., SWANSON, G. A., & LODGE, J.P. (1965)
Nitrite interference in spectrophotometric determination of
atmospheric sulfur dioxide. Anal. Chem. 37: 942-945.
PATTLE, R. E., BURGESS, F., & CULLUMBINE, H. (1956) The effects of a
cold environment and of ammonia on the toxicity of sulfuric acid
mist to guinea pigs. J. Pathol. Bacteriol., 72: 219-232.
PEMBERTON, J. & GOLDBERG, C. (1954) Air pollution and bronchitis.
Br. med. J., 2: 567-570.
POLLACK, R. I. (1975) Studies of pollutant concentration frequency
distributions, Washington DC, US Environmental Protection
Agency, 93 pp. (EPA-650/4-75-004).
PRINZ, B. (1970) Use of statistical methods in random sampling for the
preparation of plans for measurement of emissions. Staub,
30: 204-210.
RALL, D. P. (1974) Review of the health effects of sulfur oxides.
Environ. Health Perspect., 8: 97-121.
REICHEL, G., ULMER, W. T., GARY, K., LEUSCHNER, A., & ROSKE, G. (1970)
[Influence of the locally different grade of pollution in the
township of Duisburg on the incidence of non-specific respiratory
diseases. V. Communication.] Int. Arch. Arbeitsmed., 27: 110-129
(in German).
REID, D. D., ANDERSON, D. O., FERRIS, B. G., & FLETCHER, C. M. (1970)
An Anglo-American comparison of the prevalence of bronchitis.
Br. med. J., 2: 1487-1491.
ROBINSON, E. & ROBBINS, R. A. (1968) Sources, abundance and fate of
gaseous atmospheric pollutants. Menlo Park, CA, Stanford
Research Institute (Final Report, SRI project SCC-8501).
ROBINSON, E. & ROBBINS, R. A. (1972) Emissions, concentrations and
fate of gaseous atmospheric pollutants. In: Strauss, W., ed.
Air pollution control, II, New York, Wiley-Interscience,
pp. 1-93.
ROYAL COLLEGE OF PHYSICIANS, London (1970) Air pollution and health,
London, Pitman, pp. 48-57.
ROYAL MINISTRY OF FOREIGN AFFAIRS, SWEDEN (1971) Air pollution across
national boundaries. The impact on the environment of sulfur in
air and precipitation. Stockholm. Kungl. Bokytrckerict P.A.
Norsted & Söner 710396, 96 pp.
RJAZANOV, V. (1962) Sensory physiology as basis for air quality
standards. Arch. environ. Health, 5: 480-494.
RYLANDER, R. & BERGSTROM, R. (1973) Particles and SO2 -- synergistic
effects for pulmonary damage. In: Proceedings of the Third
International Clean Air Congress, Düsseldorf, 1973, Düsseldorf,
VDI Verlag, pp. A23-A25.
RYLANDER, R., OHRSTROM, M., HELLSTROM, P. A., & BERGSTROM, R. (1971)
SO2 and particles -- synergistic effects on guinea pig lungs. In:
Walton, W. H., ed. Inhaled Particles III, London, Unwin, Vol. 1,
pp. 535-540.
SALAMBERIDZE, O. P. (1969) [The joint action of small concentrations
of sulfur dioxide and nitrogen dioxide gases under conditions of a
chronic test.] Gig. i Sanit., 34(4): 10-14 (in Russian).
SAUCIER, J. Y. & SANSONE, E. B. (1972) The relationship between
transmittance and reflectance measurements of soiling index.
Atmos. Environ., 6: 37-43.
SAWICKI, F. (1972) Chronic non-specific respiratory diseases in
Cracow. Epidemiol. Rev., 26: 229-250.
SCARINGELLI, F. B., SALTZMAN, B. E., & FREG, S. A. (1967)
Spectrophotometric determination of atmospheric sulfur dioxide.
Anal. Chem., 39: 1709-1719.
SCHIMMEL, H. & GREENBERG, L. (1972) A study of the relation of
pollution to mortality, New York City, 1963-1968, J. Air Pollut.
Control Assoc., 22: 607-616.
SCHIMMEL, H. & MURAWSKI, T. J. (1975) SO2 -- Harmful pollutant or air
quality indicator? J. Air Pollut. Control Assoc., 25: 739-740.
SCHMIDT, P., PETR, B., & PICKO, V. (1966) [The indices of white blood
sequence, the tonsils and neck lymphatic nodules state as the
diagnostic criteria of subtle changes within the child's
organism.] Cesk. Hyg., 11: 473-478 (in Czech).
SCHRENK, H. H., HEIMANN, H., CLAYTON, G. O., GAFAER, W. M., & WEXLER,
H. (1949) Air pollution, Donora, Pennsylvania -- Epidemiology of
the unusual smog episode of October 1948. Public Health Bull.
(Fed. Sec. Agency, Washington DC). 306: 1-173.
SCHUSKY, J. (1966) Public awareness and concern with air pollution in
the St. Louis, metropolitan area. J. Air Pollut. Control Assoc.,
16: 72-76.
SHERWOOD, R. J. (1969) Miniature air samplers for sulfur dioxide.
Am. Ind. Hyg. Assoc. J., 30: 614-619.
SHERWOOD, R. J. & GREENHALGH, D. M. S. (1960) A personal air sampler.
Ann. occup. Hyg., 2: 127-132.
SIM, V. M. & PATTLE, R. E. (1957) Effect of possible smog irritants on
human subjects. J. Am. Med. Assoc., 165: 1918-1913.
SJORSTRAND, T. (1947) Changes in respiratory organs of workmen at an
ore smelting works. Acta Med. Scand., 196 (Suppl.): 687-699.
SKALPE, I. O. (1964) Long term effects of sulfur dioxide exposure in
pulp mills. Br. J. ind. Med., 21: 69-73.
SKVORCOVA, N. N., OSINCEVA, V. P., PUSKINA, N. N., & VYSOCINA, I. V.
(1973) [Role of some air pollutants in the genesis of lung tumour
provoked by chemical carcinogens. In: Prevention of environmental
pollution due to carcinogenic substances.], Moscow, Medicina,
pp. 61-64 (in Russian).
SNELL, R. E. & LUCHSINGER, P. C. (1969) Effects of sulfur dioxide on
expiratory flow rates and total respiratory resistance in normal
human subjects. Arch. environ. Health, 18: 693-698.
SOFOLUWE, G. O. (1968) Smoke pollution in dwellings of infants with
bronchopneumomia. Arch. environ. Health, 16: 670-672.
SPEDDING, D. J. (1972) Sulfur dioxide absorption by sea water. Atmos.
Environ., 6: 583-586.
SPEIZER, F. E. & FRANK, N. R. (1966a) The uptake and release of SO2
by the human nose. Arch. environ. Health, 12: 725-728.
SPEIZER, F. E. & FRANK, N. R. (1966b) A comparison of changes in
pulmonary flow resistance in healthy volunteers acutely exposed to
SO2 by mouth and nose. Br. J. ind. Med., 23: 75-79.
STALKER, W. W. & ROBINSON, C. B. (1967) A method for using air
pollution measurements and public opinion to establish ambient air
quality standards. J. Air Pollut. Control Assoc., 17: 142-144.
STOCKS, P. (1959) Cancer and bronchitis mortality in relation to
atmospheric deposit and smoke. Br. med. J., 1: 74-79.
STOCKS, P. (1966) Recent epidemiological studies of lung cancer
mortality, cigarette smoking and air pollution, with discussion of
a new hypothesis of causation. Br. J. Cancer, 20: 495-623.
STOCKS, P. (1967) Lung cancer and bronchitis in relation to cigarette
smoking and fuel consumption in twenty countries. Br. J. prev.
soc. Med., 21: 181-185.
STRANDBERG, L. G. (1964) SO2 absorption in the respiratory tract.
Arch. environ. Health, 9: 160-166.
STUART, B. O. (1973) Deposition of inhaled aerosols. Arch. intern.
Med., 131: 60-73.
SYMON, K., PETR, B., & KAPALIN, V. (1966) [The influence of pollution
caused by gaseous pollutants on the health of children.] Prakt.
Lek., 46: 19-22 (in Czech).
TANI, S. (1975) [Epidemiological study on chronic bronchitis.] Jpn.
J. public Health, 22: 431-438 (in Japanese).
TASK GROUP ON AIR POLLUTION AND CANCER (unpublished) Air pollution and
cancer. Risk assessment methodology and epidemiological evidence.
Environ. Health Perspect. (March, 1978.)
TASK GROUP ON LUNG DYNAMICS (1966) Deposition and retention models for
internal dosimetry of the human respiratory tract. Health Phys.,
12: 173-207.
TASK GROUP ON METAL ACCUMULATION (1973) Accumulation of toxic metals
with special reference to their absorption, excretion and
biological half-times. Environ. Physiol. Biochem., 3: 65-107.
TEN BRUGGEN CATE, H. J. (1968) Dental erosion in industry. Br. J.
ind. Med., 25: 249-266.
THOMAS, R. L., DHARMARAJAN, V., LUNDQUIST, G. L., & WEST, P. W. (1976)
Measurement of sulfuric acid aerosol, sulfur trioxide and the
total sulfate content of the ambient air. Anal. Chem.,
48: 639-642.
TOIGO, A., IMARISIO, J. J., MURMALL, H., & LEPPER, M. N. (1963)
Clearance of large carbon particles from the human
tracheobronchial tree. Am. Rev. respir. Dis., 87: 487-492.
TOMENIUS, L. (1973) A study on the role of deposition for
tracheobronchial clearance. Physiol. Biochem., 3: 111-116.
TOYAMA, T. & NAKAMURA, K. (1964) Synergistic response of hydrogen
peroxide aerosols and sulfur dioxide to pulmonary airway
resistance. Ind. Health, 2: 34-45.
TOYAMA T., KAHYO, H., NAKAMURA, K., KAGAWA, J., YAKURA, S., ADACHI,
S., YAMAMOTO, N., IRIYAMA, F., KUMAGAYA, F., OSAWA, S., &
NAKAMURA, T. (1966) [Study on the prevalence of respiratory
symptoms in a rural area (Kashima, Ibaragi Pref.) in Japan.]
J. Jpn Soc. Air Pollut., 1: 24-35 (in Japanese).
TREON, J. F., DUTRA, F. R., CAPPEL, J., SIGMON, H., & YOUNKER, W.
(1950) Toxicity of sulfuric acid mist. Arch. ind. Hyg. occup.
Med., 2: 716-734.
TSUNETOSHI, Y., SHIMIZU, T., TAKAHASHI, H., ICHINOSAWA, A., UEDA, M.,
NAKAYAMA, N., YAMAGATA, Y., & OHSHINO, A. (1971) Epidemiological
study of chronic bronchitis with special reference to effect of
air pollution. Int. Arch. Arbeitsmed., 29: 1-27.
TURNER, D. B. (1968) Workbook of atmospheric dispersion estimates,
Washington, DC, US Department of Health, Education and Welfare,
84 pp.
ULMER, W. T., REICHEL, G., CZEIKE, A., & LEUSCHNER, A. (1970)
[Regional incidence of non-specific respiratory diseases. IV.
Communication.] Int. Arch. Arbeitsmed., 27: 73-109 (in German).
UNITED STATES DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1962)
Air pollution measurements of the National Air Sampling Network
1957-1961, Cincinnati, OH, Division of Air Pollution, pp. 3-4
(Publ. No. 978).
UNITED STATES DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1969)
Air quality criteria for particulate matter, Washington, DC,
DHEW, National Air Pollution Control Administration, pp. 1-211
(AP. 49).
US ENVIRONMENTAL PROTECTION AGENCY (1974a) Air quality data -- 1972
annual statistics, Research Triangle Park, NC, USEPA, 145 pp.
(EPA450/2-74-001).
US ENVIRONMENTAL PROTECTION AGENCY (1974b) Health consequences of
sulfur oxides. A report from CHESS, 1970-71, Research Triangle
Park, NC, US Government Printing Office, 265 pp.
(EPA650/1-74-604).
VAN DER LENDE, R. (1969) Epidemiology of chronic non-specific lung
disease (chronic bronchitis), Thesis, University of Groningen,
2 vols. Assen, Van Gorcum.
VAN DER LENDE, R., VISSER, B. F., WEVER-HESS, J., TAMMELIFG, G. J., DE
VRIES, K., ORIE, N. G. M. (1973) Epidemiological investigations in
the Netherlands into the influence of smoking and atmospheric
pollution on respiratory symptoms and lung function disturbances.
Pneumologie, 149: 119-126.
VAN DER LENDE, R., HUYGEN, C., JANSEN-KOSTER, E. J., KNIFPSTRA, S.,
PESEL, R., VISSER, B. F., WOLFS, E. H. E., & ORIE, N. G. M. (1975)
A temporary decrease in the ventilatory function of an urban
population during an acute increase in air pollution. Bull.
Physiopathol. Respir., 11: 31-43.
VAN DOP, H. & KRUIZINGA, S. (1976) The decrease of sulfur dioxide
concentration near Rotterdam and their relation to some
meteorological parameters during thirteen consecutive winters
(1961-74). Atmos. Environ., 10: 1-4.
VEREIN DEUTSCHER INGENIEURE (1974) [Measurements of the wet
concentrations of particles in the outdoor air (LIB-filter method)
VDI2463 Sheet 4.] (in German).
VIGDORCIK, E. A. (1948) Retention of inhaled aerosols (resume),
Leningrad, Leningrad Research Institute of Labour Hygiene and
Occupational Health Publication, Vol. XI, Part 2, pp. 227-230.
WALLER, R. E. (1963) Acid droplets in town air. Int, J. Air Water
Pollut., 7: 773-778.
WALLER, R. E. (1967) Studies on the nature of urban air pollution. In:
Conference on Museum Climatology, London, International
Institute of Works of Art, pp. 65-69.
WALLER, R. E. (1971) Air pollution and community health. J. R. Coll.
Physicians, Lond., 5: 362-368
WALLER, R. E. & COMMINS, B. T. (1966) High pollution episodes in
London, 1952-1966. In: Proceedings of the International Clean Air
Conference, London, Natural Society of Clean Air, pp. 228-231.
WALLER, R. E. & COMMINS, B. T. (1967) Studies of the smoke and
polycyclic aromatic hydrocarbon content of the air in large urban
areas. Environ. Res., 1: 295-306.
WALLER, R. E., BROOKS, A. G. F., & CARTWRIGHT, J. (1963) An electron
microscope study of particles in town air. Int. J. Air Water
Pollut. Control., 7: 779-786.
WALLER, R. E., COMMINS, B. T., & LAWTHER, P. J. (1965) Air pollution
in a city street. Br. J. ind. Med., 22: 128-138.
WALLER, R. E., LAWTHER, P. J., & MARTIN, A. E. (1969) Clean air and
health in London. In: Proceedings of the International Clean Air
Conference, London, Natural Society of Clean Air, pp. 71-79.
WARNER, C. G., DAVIES, G. M., JONES, J. G., & LOWE, C. R. (1969)
Bronchitis in two integrated steel works, II. Sulfur dioxide and
particulate atmospheric pollution in and around the two works.
Ann. occup. Hyg., 12: 151-170.
WATANABE, H. (1966) [Health effects of air pollution in Osaka City.]
J. Osaka Life Hyg. Assoc., 10: 147-157 (in Japanese).
WEATHERLEY, M. L. (1966) Air pollution inside the home. Int. J. Air
Water Pollut. Control, 10: 404-409.
WEATHERLEY, M. L., GOORIAH, B. D., & CHARNOCK, J. (1976) Fuel
consumption, smoke and sulfur dioxide emissions and
concentrations, and grit and dust deposition in the U.K. up to
1973-74. Stevenage, UK, Warren Spring Laboratory
(WSL Report LR214AP).
WEST, P.M. & GAEKE, G. (1956) Fixation of sulfur dioxide as
sulfitomercurate III and subsequent calorimetric determination.
Anal. Chem., 28: 1816-1819.
WILLEKE, K. & WHITBY, K. T. (1975) Atmospheric aerosols: size
distribution interpretation. J. Air Pollut. Control Assoc.,
25: 529-534.
WILLIAMS, F. P. (1960) Pollution levels in cities. In: Proceedings of
the Harrogate Conference, London Natural Society of Clean Air,
pp. 83-88.
WILLIAMS, M. K. (1970) Sickness absence and ventilatory capacity of
workers exposed to sulfuric acid mist. Br. J. ind. Med.,
27: 61-66.
WILSON, W. E., Jr & LEVY, A. (1970) A study of sulfur dioxide in
photochemical smog. I. Effect of SO2 and water vapour
concentration in the 1-butene/NOx/SO2 system. J. Air Pollut.
Control Assoc., 20: 385-390.
WORLD HEALTH ORGANIZATION (1960) WHO Technical Report Series No. 192
(Epidemiology of cancer of the lung: Report of a study group).
Geneva, WHO, 13 pp.
WORLD HEALTH ORGANIZATION (1971) International standards for
drinking-water. Geneva, WHO, 70 pp.
WORLD HEALTH ORGANIZATION (1972) WHO Technical Report Series No. 506
(Air quality criteria and guides for urban air pollutants),
Geneva, WHO, 35 pp.
WORLD HEALTH ORGANIZATION (1974) WHO Technical Report Series No. 539
(Toxicological evaluation of certain food additives with a review
of general principles and of specifications), Geneva, WHO, 40 pp.
WORLD HEALTH ORGANIZATION (1976a) Selected methods of measuring air
pollutants, Geneva, WHO, 110 pp. (WHO Offset Publ. No. 24).
WORLD HEALTH ORGANIZATION (1976b) Air quality in selected urban
areas, 1973-1974, Geneva, WHO, 65 pp. (WHO Offset Publ.,
No. 30).
WORLD HEALTH ORGANIZATION (1977) Air monitoring programme design for
urban and industrial areas, Geneva, WHO, 46 pp. (WHO Offset
Publ., No. 33).
WORLD METEOROLOGICAL ORGANIZATION (1974) WMO operations manual for
sampling and analysis techniques for chemical constituents in air
and precipitation, Geneva, WMO, 56 pp. (No. 299).
YOCOM, J. E., CLINK, W. L., & COTE, W. A. (1971) Indoor/outdoor air
quality relationships. J. Air Pollut. Control Assoc.,
21: 251-259.
YOKOYAMA, E., YODER, R. E., & FRANK, N. R. (1971) Distribution of 35S
in the blood and its excretion in urine of dogs exposed to
35SO2. Arch. environ. Health, 22: 389-395.
YOSHIDA, K., OSHIMA, H., & IMAI, M. (1966) Air pollution and asthma in
Yokkaichi. Arch. environ. Health, 13: 763-768.
YOSHII, M., NONOYAMA, J., OSHIMA, H., YAMAGIWA, H., & TAKEDA, S.
(1969) Chronic pharyngitis in air-polluted districts of Yokkaichi
in Japan. Mie med. J., 19: 17-27.
ZARKOWER, A. (1972) Alterations in antibody response induced by
chronic inhalation of SO2 and carbon. Arch. environ. Health,
25: 45-50.
ZEEDUK, H. & VELDS, C. A. (1973) The transport of sulfur dioxide over
a long distance. Atmos. Environ., 7: 849-862.
In general, however, in the absence of specific sources of sulfur
dioxide or fine particulate matter indoors, concentrations are
generally less than those outdoors. Sulfur dioxide as a gas, can
diffuse readily onto walls and other surfaces. There is evidence that
it reacts with ammonia from the indoor air on painted surfaces,
particularly in the presence of moisture (Holbrow, 1958), but it is
most effectively absorbed on clothing, curtains, carpets, and other
soft furnishings, so that in domestic surroundings where these abound
concentrations of sulfur dioxide are only of the order of 20% of those
outdoors (Weatherley, 1966), while in offices and other buildings
containing less absorbing material, concentrations may be 40-50% of
those outdoors (Andersen, 1972; Derouane, 1972). The presence of
ammonia in occupied rooms is important in relation to measurements of
sulfur dioxide. Concentrations of this "natural" pollutant may be much
higher than outdoors, particularly where there are young babies or old
people with incontinence problems. The ammonia will only react
effectively with sulfur dioxide in the presence of moisture, but it
will, in any case, interfere with the determination of the sulfur
dioxide by acidimetric or conductometric methods.
Smoke, that is to say the finely divided black material from
incomplete combustion, can penetrate fairly readily into buildings,
and since the mobility of the particles is less than that of sulfur
dioxide molecules, they are less rapidly removed onto surfaces.
Concentrations of smoke indoors, assessed by soiling methods, have
been found to be in the range of 50-90% of those outdoors (Derouane,
1972; Yocom et al., 1971), again assuming there is no specific source
of this material inside. Cigarette smoke can make a very substantial
contribution to the finely divided particulate matter indoors (Elliott
& Rowe, 1976; Hoegg, 1972). It is liable to affect direct gravimetric
concentrations, but it has relatively little effect on concentrations
measured by blackness. The composition of cigarette smoke is quite
different from that of the general urban particulate matter, and it
has been stressed by De Graaf & Biersteker (1972) that there may be
many differences in the type and composition of suspended particulate
matter indoors and outdoors. This point is particularly important in
relation to samples obtained with the high volume sampler. The larger
particles (greater than 10 µm diameter) that are liable to be
collected by this method would penetrate into buildings less readily
than the fine particles, and, with reduced air movement inside, they
may fall out fairly rapidly under gravity. This may account for the
smaller proportion of total suspended particles (as compared with
smoke) found indoors in some studies (Yocom et al., 1971). However, on
the other hand, there is a risk that textile and other dusts dispersed
in the home may be sampled and assessed as part of the general
suspended particulate matter.
Sulfuric acid is unlikely to remain for any appreciable time in
occupied rooms, as it is readily neutralized by ammonia. However,
finely divided sulfate particles are likely to behave in the same way
as smoke, with a modest reduction indoors compared with outdoors.
Relative humidity is normally lower indoors than outdoors and this
will help to keep sulfate particles in suspension, but where the
humidity is especially high, as it may be in kitchens, particles
containing sulfates or other salts will grow rapidly and be deposited.
Studies on indoor concentrations of sulfur dioxide and particulate
matter are limited primarily by the need to consider the circumstances
of each location individually; nevertheless, there is a growing body
of literature on the subject that has been assembled and reviewed in
recent reports from the USA (Benson et al., 1972; Henderson et al,
1973).
5.3 Concentrations in Work Places
Concentrations of sulfur dioxide, much higher than those commonly
found in urban air, may be present in some industrial environments,
arising from processes in which the gas is handled or evolved, as well
as from combustion sources. Paper mills, sulfuric acid plants, steel
works, nonferrous metal foundries, and oil refineries are among the
places where such concentrations may be found. However, emissions are
usually highly localized and intermittent, presenting major problems
in assessing concentrations to which workers may be exposed. Results
are not normally published, but in a number of countries there is a
requirement to ensure that a specified limit is not exceeded. For
example, in the USSR, the maximum permissible concentration is
10 mg/m3 (3.8 ppm) (ILO/WHO Committee on Occupational Health, 1970)
whereas in the USA (American Conference of Government Industrial
Hygienists, 1977), this value, averaged over 8-h shifts, is 13 mg/m3
(5 ppm). This figure is of the order of 100 times the average values
in urban air and it is higher than the maximum values reported, even
in episodes of high pollution (Waller & Commins, 1966). Mean values
over 3 years of 1800-2100 µg/m3 (0.6-0.7 ppm) have been reported
close to blast furnaces in steelworks, with occasional 24-h mean
values up to 17 000 µg/m3 (5.95 ppm) (Lowe et al., 1970).
In steelworks, substantial quantities of suspended particulate
matter may be present as well as sulfur dioxide and other pollutants.
A 3-year mean concentration of about 1000 µg/m3 (respirable dust, as
measured with a Hexhlet sampler) has been reported in the study cited
above (Lowe et al., 1970). Even so, the situation does not necessarily
parallel that in urban atmospheres, for the particulate matter is
liable to include dust which differs in composition and size
distribution from that produced in the usual range of combustion
processes.
Dusts encountered in industry must, in general, be considered
separately from the suspended particulate matter in urban air. Some,
such as the coal-dust in mines, may be present at high concentrations,
and have substantial effects on health, but these are specific to that
dust, and are outside the scope of the present discussion. However,
some of the chemical compounds in dust from industrial activities are
covered by other documents of the WHO environmental health criteria
programme.
In some industries, such as wood-pulping and paper-making, sulfur
dioxide may be evolved in the process, producing high concentrations
locally without an accompanying particulate matter problem.
Concentrations in the range 6 to 100 mg/m3 (2 to 36 ppm) have been
reported at a plant in Norway (Skalpe, 1964) and similar
concentrations were found in a study in the USA (Ferris et al., 1967)
but these were gradually reduced over a 5-year period. High
concentrations, averaging about 71 mg/m3 (25 ppm), also existed at
one time in charging rooms for refrigerators when sulfur dioxide was
used as a refrigerant (Kehoe et al., 1932) and similar concentrations
have been reported in certain areas of oil refineries (Anderson 1950).
There is a specific problem of sulfuric acid mist in the forming
departments of works making lead-acid accumulators, where
concentrations up to 16.6 mg/m3 have been observed (Malcolm & Paul,
1961; Williams, 1970). This is very much greater than the
concentrations ever found in urban air (Commins & Waller, 1978) and
the physical form of the aerosol is different: mass median diameters
of the droplets have been reported to be over 10 µm (Williams, 1970)
whereas those found in urban air are of the order of 0.5 µm (Waller,
1963).
5.4 Assessment of Exposures
In the present context, the term exposure relates to the
concentrations of sulfur dioxide or suspended particulate matter in
the air breathed by individuals or populations, averaged over
specified periods. Broadly speaking, two types of exposure are
considered: short-term exposure, in which the relevant concentrations
are averaged over periods of the order of a day, and long-term
exposure, for which averages over periods of the order of a year are
commonly used. These periods are to a certain extent arbitrary,
imposed by the time-resolution of the pollution measurements and by
the nature of the health indices examined, and there is no clear
guidance on the most relevant averaging periods to use in relation
either to short-or to long-term exposure.
Exposures are usually estimated from measurements of concentration
at fixed sampling sites. The number of sampling sites required to give
an adequate representation of the exposure of people living in a given
area will depend on the topography, the distribution of sources, and
other factors. In urban areas, there is usually a close correlation
between values for neighbouring stations (Prinz, 1970). Care must be
taken, however, to ensure that these stations are not used to
represent environmental levels in areas much larger than their
coverage. Furthermore, the instruments for measurement may be sited on
the roof of a building for protection and convenience, and the levels
measured may not necessarily represent those in the ordinary breathing
zone.
The usual practice in epidemiological studies is to assume that
measurements made on outdoor air in the areas where people live or
work provide an index of exposure that allows comparison to be made
between different groups. It has been demonstrated, however, that
estimated weekly exposures calculated as averages of concentrations
measured indoors and at various outdoor sites and weighted according
to the length of time likely to be spent in each location, may differ
substantially from those indicated by measurements at just one site
close to the place of residence or work (Fugas, 1976). To validate
this approach, further measurements using personal samplers would be
required. These have been used in studies on specific components of
suspended particulate matter such as lead (Azar et al., 1972) and they
have wide application in assessing the exposures of individuals to
pollutants, including sulfur dioxide, in industry, but they are not
generally practicable for large-scale epidemiological studies on the
general population.
A further problem in assessing exposures is the great variation in
concentrations with time for, as far as sulfur dioxide is concerned, a
brief exposure to a very high concentration, with no appreciable
exposure during the rest of the day, would create a different
situation from a steady exposure to a low level throughout the day. At
present, there is no satisfactory way of measuring such situations, at
least for people moving about during the course of the day. Providing
studies are confined to groups of people living or working in
reasonably uniform circumstances, measurements made over extended
periods at a few fixed sites may give results that are valid for
comparative purposes. Difficulties are liable to arise however if
dissimilar groups are compared. In particular, people such as the very
old and the very young may be partially protected from sulfur dioxide
in the outside air if they spend long periods indoors, whereas an
active worker may be exposed not only to a higher mean concentration
in the course of the day, but also to a greater range. A further point
is that increased activity will also lead to a greater ventilation
rate, increasing the overall intake, and, in the case of sulfur
dioxide, the deep inspirations associated with exercise are liable to
increase the penetration of the gas and to enhance its effects
(Lawther et al., 1975). The situation is even more complex for
sulfuric acid aerosols. Because of the hygroscopic properties of the
droplets, they take up moisture rapidly on inspiration, becoming
larger and more dilute, and it has been suggested, on the basis of
experimental work, that those from urban air could be diluted to such
an extent that their irritant properties would be lost (Carabine &
Maddock, 1976).
When assessing long-term exposures, annual mean concentrations at
fixed sampling sites are commonly used as the basis. Short-term
variations are then of little consequence, but it may still be
necessary to consider the extent of seasonal swings in concentration,
or the frequency of occurrence of days of exceptionally high
pollution. Also, it is often necessary to examine trends in
concentrations for many years back, for the relevant exposure may have
been one that occurred earlier in life, rather than the current level.
Since there are very few long series of measurements of sulfur dioxide
or suspended particulate matter made by uniform methods, it is very
difficult to assess the true exposure of people to these pollutants.
6. ABSORPTION, DISTRIBUTION, AND ELIMINATION
Most of the sulfur compounds discussed in this report are absorbed
via the digestive system. Relatively little is absorbed from the
respiratory tract, even in areas with highly polluted ambient air.
Even so, it is reasonable to believe that the respiratory tract is the
most vulnerable organ for the local effects of sulfur oxides and
particulate matter in the ambient air. Thus, this section will deal
mainly with the absorption, deposition, and clearance of sulfur
dioxide and particulate matter in the respiratory tract.
6.1 Absorption and Deposition in the Respiratory Tract
6.1.1 Sulfur dioxide
Sulfur dioxide is highly soluble in aqueous media. Absorption
after inhalation has been studied in rabbits (Dalhamn & Strandberg,
1961; Strandberg, 1964), dogs (Balchum et al., 1959, 1960a, 1960b;
Frank et al., 1969) and man (Speizer & Frank, 1966a, 1966b).
In rabbits, about 40% of the inhaled sulfur dioxide is absorbed in
the nose and pharynx when concentrations of about 290 µg/m3 (0.1 ppm)
are inhaled. At higher concentrations (29-290 mg/m3; 10-100 ppm), the
fraction absorbed is much higher (about 95%),The reasons for these
different rates of absorption are not clear. In dogs, more than 99% of
the inhaled sulfur dioxide is absorbed by the nose at exposure levels
of 2.9-140 mg/m3 (1-50 ppm). These observations in dogs have been
confirmed in man by studies on human volunteers, with levels of
exposure to sulfur dioxide ranging from 2.9 to 420 mg/m3 (1 to
140 ppm) and exposure times of a few minutes at the higher levels and
30-40 minutes at the lower levels. Absorption can occur during
mouth-breathing, but is less efficient than during nose-breathing,
especially with increased ventilation.
6.1.2 Airborne particles
Particles smaller than about 10 µm are deposited at different
levels in the respiratory tract. The exact deposition pattern is
determined by the interaction of size, shape, and density (expressed
as aerodynamic diameter of unit density spheres) of the particles and
by airflow conditions. Some particles in the air, such as sulfuric
acid particles are hygroscopic. Such particles will take up water,
expand in the respiratory tract, and be deposited in a manner other
than would be expected from their diameters in the ambient air (Hatch
& Gross, 1964; Stuart, 1973; Task Group on Lung Dynamics, 1966;
Vigdorcik, 1948). Sophisticated theoretical models for the deposition
of particles in the lung such as the ICRP model have been constructed
(Task Group on Lung Dynamics, 1966). Deposition is caused by
impaction, sedimentation, and diffusion. Impaction is an important
mechanism for the deposition of the larger or heavier particles
(5-30 µm aerodynamic diameter) and where the air velocity is
relatively high. Impaction occurs therefore at sites where the air
stream is turbulent and is of most importance in the nose, mouth,
pharynx, and the upper part of the tracheobronchial tree.
Sedimentation or settling out of particles is also a function of
particle size and density, as well as of residence time in the
airways. It is important for the deposition of larger particles
(1-5 µm) providing they have not been deposited by impaction and is
active in the trachea, bronchi, and bronchioles. Diffusion is of
importance for particles smaller than a few tenths of 1 µm (its effect
increasing with decreasing particle size), with respect to the smaller
bronchioles and especially the alveoli. In the size range of 2-5 µm,
experimental data have fitted quite well with the ICRP model, but
deposition has varied widely (with an order of magnitude of 2 to 3)
among human subjects (Lippmann et al., 1971). In experiments on
animals, large reproducible differences in deposition have been seen
among individuals within the same species (Albert et al., 1968;
Tomenius, 1973). In the submicron range, theoretical models have not
yet been satisfactorily verified by experiments.
6.2 Clearance from the Respiratory Tract and Distribution
6.2.1 Sulfur dioxide
Sulfur dioxide is absorbed from the respiratory tract into the
blood stream. It can then be widely distributed throughout the body
(Balchum et al., 1960a, 1960b; Bystrova, 1957; Frank et al., 1967;
Yokoyama et al., 1971), where it appears to be metabolized and
excreted via the urinary tract.
6.2.2 Particulate matter
Knowledge concerning the elimination of insoluble particles
deposited in the alveoli is incomplete (Hatch & Gross, 1964; Morrow,
1973). It is clear that these particles are, to a large extent,
phagocytized by alveolar macrophages within hours, but it is not known
to what extent they are actively carried to the ciliated part of the
lung or to the lymphatic system. The particles can be transported by
the mucociliary escalator into the interstitium (where they can remain
for a long time) or the lymphatic system. Solubility in vivo is
important for the clearance of "insoluble" particles deposited in the
alveoli (Mercer, 1967; Morrow, 1973). The biological half-lives range
from days to years depending on the chemical composition of the
particles (Brain & Valberg, 1974; Task Group on Lung Dynamics, 1966;
Task Group on Metal Accumulation, 1973).
Mucociliary transport, and therefore clearance of particles, can
be affected by various factors and can be impaired by long-term
cigarette smoking and acute infection in the respiratory tract (Camner
& Philipson, 1972; Camner et al., 1973a; Jarstrand et al, 1974).
Subjects suffering from chronic obstructive lung disease may also have
an impaired mucociliary transport (Camner et al., 1973b; Toigo et al.,
1963). Studies in animals have shown that the inhalation of sulfur
dioxide can interfere with the clearance of bacteria (Rylander et al.,
1971) and inert particles (Ferin & Leach, 1973) from the lungs, but
how much is due to the action of the sulfur dioxide on the mucociliary
mechanism and how much to its action on the macrophages is not clear.
The clearance of soluble particles may follow the above pathways
depending upon their solubility. They may dissolve in the mucus in
which case they will probably be eliminated via the mucociliary route
and be swallowed and removed or absorbed via the intestinal tract. In
the alveoli, the particles may diffuse into the lymph or blood and
thus be removed from the lung.
7. EFFECTS ON EXPERIMENTAL ANIMALS
It has been difficult to separate the relative effects of sulfur
dioxide, sulfuric acid mists, sulfate salts, and particulate matter in
the ambient air by epidemiological techniques. The effects of these
substances individually and in various combinations have been studied
in the laboratory. These studies have been useful in explaining some
of the mechanisms of action but their application has been rather
limited for the establishment of safe ambient levels, particularly for
complex mixtures such as exist in the ambient air. In the past, the
levels used in laboratory studies on animals have usually been far in
excess of those seen in the ambient air and therefore have had little
relevance for ambient air quality standards. Later studies have been
at more relevant levels.
The following discussion separates work on animals into short-term
and long-term exposure studies. Short-term exposure studies are those
that involve exposures of 24-h or less and usually last from minutes
to at the most a few hours. Long-term exposure studies refer to
exposures that last longer than 24-h and are usually extended over
months. In the short-term exposure studies, only immediate or acute
effects have been studied. In long-term exposure studies, animals may
be studied during the period of exposure to determine whether there
are progressive changes or not and at the end of the exposure period
to quantify any chronic effect.
7.1 Short-term Exposure Studies
7.1.1 Exposure to sulfur dioxide singly or in combination with
other agents
Sulfur dioxide is a respiratory irritant that is very soluble in
the aqueous surfaces of the respiratory airways. Because of this high
solubility, most of the sulfur dioxide is absorbed in the nose and
upper airways (section 6.1) and very little reaches the lungs
directly. At extremely high concentrations, the absorptive capacity of
the upper airways can be overwhelmed and death or pathological changes
including laryngotracheal and pulmonary oedema can be induced in the
respiratory tract of experimental animals.
Exposure-effect curves have been developed for guineapigs (Amdur,
1966) and dogs (Frank & Speizer, 1965). In Amdur's study, a linear
relationship was obtained between exposure for 1 h to sulfur dioxide
concentrations ranging from 0.46 to 2380 mg/m3 (0.16-835 ppm) and
corresponding increases in pulmonary flow resistance. The second study
showed that nasal flow resistance increased roughly in proportion to
exposure to sulfur dioxide concentrations ranging from 20 to
660 mg/m3 (7-230 ppm) for a 15-20 minute period.
Studies with guineapigs at lower concentrations have shown that a
sodium chloride aerosol, administered concomitantly enhances the
effects of sulfur dioxide on the lungs in the form of
bronchoconstriction and increased airway resistance (Amdur, 1957). It
was postulated that the sodium chloride could act as a carrier to
deliver the absorbed sulfur dioxide deep into the lungs, or that the
increased humidity in the respiratory tract could react with the
sulfur dioxide to form sulfurous acid, especially if catalysts were
present (Amdur & Underhill, 1968). McJilton et al. (1973) have
reaffirmed the importance of humidity in this reaction, exposing
guineapigs for 1 h to sulfur dioxide at 3.1 mg/m3 (1.1 ppm) and a
sodium chloride aerosol of about 1 mg/m3. They allowed the mixture to
"age" in a reaction chamber for 8-10 minutes at various relative
humidities. The chamber temperature was 22°C and the average aerosol
particle size was 0.1 µm with a maximum size of less than 2 µm. At
relative humidities above 80%, they noted a marked increase in
pulmonary resistance, whereas administration of the individual
components produced little or no effect. The droplets formed at high
humidity and in the presence of the aerosol had a pH of 3.2 ± 0.5.
Analysis of these particles by mass spectrometer revealed sulfur
dioxide and bisulfite ions (HSO-3)in the solution but no sulfuric
acid. In the high humidity, the sodium chloride aerosol apparently
became hydrated and could then absorb sulfur dioxide. The authors
indicated that the mixture had to "age" to ensure absorption of the
sulfur dioxide; otherwise there would be too much competition with the
moist surfaces in the nasopharynx which could sweep out the sulfur
dioxide. Thus, this is another mechanism whereby the effect of sulfur
dioxide can be enhanced. If such mixtures were allowed to "age"
longer, it seems likely that the sulfur dioxide could be changed to
sulfuric acid. Studies using other animal species such as the cat have
not shown this enhancement of the effect of sulfur dioxide by sodium
chloride (Corn et al., 1972).
Matsumura (1970a) exposed guineapigs to ozone at 2.1, 11, or
21 mg/m3 (1, 5, or 10 ppm), nitrogen dioxide at 40, 80, or 140 µg/m3
(20, 40, or 70 ppm), or sulfur dioxide at 60, 170, 510, or 940 mg/m3
(20, 60, 180, or 330 ppm) for 30-50 minutes. Each group with unexposed
controls was then exposed to an aerosolized antigen (2 µm) of egg
albumin and bovine albumin for 45 minutes, 5-7 times at intervals of a
day or more. Two weeks after the last exposure, the animals were
killed and blood was drawn for assay of the immunological response.
Enhanced sensitization was noted only at the highest levels of
exposure. During these exposures a number of animals died of
anaphylactic reactions at the fifth or sixth inhalation. In another
study, sulfur dioxide at a concentration of 1100 mg/m3 (400 ppm) did
not have any effect on dyspnoeic attacks (Matsumura, 1970b).
7.1.2 Exposure to sulfuric acid aerosols or suspended sulfates
Sulfuric acid mist and some of the sulfate salts are more powerful
respiratory irritants than sulfur dioxide, and this effect is also
related to particle size (smaller particles tending to be more
irritating) (Amdur, 1958). Treon et al. (1950) exposed rabbits, rats,
mice, and guineapigs to sulfuric acid mist, 93-99% of which was less
than 2 µm in diameter, many droplets being about 1 µm in diameter.
Concentrations ranged from 87 to 1610 mg/m3. Despite the relatively
small number of animals used, a clear-cut species difference was shown
at these high concentrations. The order of increasing sensitivity was
rabbits, rats, mice, guineapigs.
It was reported by Amdur et al. (1952a) that the 8-h LC50 of
sulfuric acid mist with a mass median diameter (MMD) of about 1 µm was
18 mg/m3 for 1-2 month old guineapigs. Pattle et al. (1956) showed
that sulfuric acid mist of MMD 2.7 µm was more toxic to guineapigs
than a mist of 0.8 µm, and that the toxicity of the smaller particles
increased when the exposure occurred at 0°C. However, this might be a
response of the guineapig to low temperature rather than to the
sulfuric acid mist. Concomitant exposures with ammonium carbonate had
a protective action, apparently because it neutralized the sulfuric
acid.
Table 11. Sulfuric acid and some sulfates in descending order of
their irritative capacity for animals. Presented for
equivalent amounts of sulfur and at comparable particle
size i.e., sub-micron; short-term exposuresa
Sulfuric acid H2SO4
Zinc ammonium sulfate ZnSO4(NH4)2 SO4
Iron (III) sulfate Fe2 (SO4)3
Zinc sulfate ZnSO4
Ammonium sulfate (NH4)2 SO4
Nonreactive
Iron (II) sulfate FeSO4
Manganese (II) sulfate MnSO4
a From: Amdur (1969, 1970, 1971).
Studies on guineapigs have shown that, for equivalent amounts of
sulfur and in comparable particle size, sulfuric acid is more
irritative than any of the sulfate compounds, some of which appear to
be nonreactive in animals (Table 11). Whether these compounds would
behave in the same manner in complex mixtures such as those found in
polluted air is not known. Some of the nonreactive compounds such as
manganese salts or oxides can catalyze the reaction of sulfur dioxide
to sulfuric acid.
In short-term exposure studies by Amdur (1958), concentration
seemed to be more important than duration and death was related to
laryngospasm and bronchospasm. Sulfuric acid mist also caused
parenchymal lung damage that seemed to be related to the total dose
(Amdur, 1958; Pattle et al., 1956).
7.2 Long-term Exposure Studies
7.2.1 Exposure to sulfur dioxide
Rats were exposed for 96 days to sulfur dioxide at concentrations
of 0.1, 0.5, and 1.5 mg/m3 (0.04, 0.18 & 0.53 ppm). Histological
examination showed interstitial pneumonia, bronchitis, tracheitis, and
peribronchitis after exposures to the two higher levels (Elfimova &
Gusev, 1969).
Misiakiewicz (1970) exposed rats continuously during 5 months to
sulfur dioxide at concentrations of 0.3, 0.5, 1.0, 2.0, and
20.0 mg/m3 (0.11, 0.18, 0.35, 0.7 and 7.0 ppm). Exposures to
2.0 mg/m3 (0.7 ppm) and 20.0 mg/m3 (7.0 ppm) increased the activity
of serum cholinesterase (EC 3.1.1.8) and aspartate aminotransferase
(EC 2.6.1.1) and caused morphological changes in the upper respiratory
tract.
After a 120-h exposure to a sulfur dioxide concentration of
3 mg/m3 (1.1 ppm), guineapigs showed proliferative interstitial
pneumonia, bronchitis, and tracheitis and an increased histamine
content in the lungs, while exposure to 167 µg/m3 (0.06 ppm) of
sulfur dioxide for one month led to interstitial changes in the
respiratory tract (Bustueva, 1961a). Bustueva (1966) also exposed rats
for 65 days (24 h per day) to sulfur dioxide at 4.86 mg/m3 (1.7 ppm).
Tracheitis, desquamation of epithelial cells, an increased amount of
purulent mucus, and interstitial pneumonia were found. It was not
clear, however, whether this was due to the sulfur dioxide or to the
pulmonary infection that can develop in rats.
Beagle dogs exposed to a sulfur dioxide concentration of
13.4 mg/m3 (4.7 ppm) for 21 h per day, for 620 days, did not develop
any specific histopathological changes (Lewis et al., 1973).
Guineapigs have been exposed to sulfur dioxide levels up to
16.3 mg/m3 (5.7 ppm) for 12 months without definite effects except
for slight cytoplasmic vacuolation in the liver (Alarie et al., 1970).
Cynomolgus monkeys exposed continuously for 78 weeks to sulfur dioxide
levels up to 3.7 mg/m3 (1.3 ppm) did not show any significant
pathological changes (Alarie et al., 1972). However, rats exposed to
sulfur dioxide at 2.9 mg/m3 (1 ppm) for 170 h showed a significant
reduction in clearance of inert particles from the lung (Ferin &
Leach, 1973). Syrian hamsters, made emphysematous by previous exposure
to aerosolized papain, tolerated concentrations of sulfur dioxide up
to 1900 mg/m3 (650 ppm). Exposures were for 4 h per day, 5 days per
week, for a total of 19-74 exposures. Only slight changes in the
mechanical properties of the lung were noted as well as slight
bronchitis (Goldring et al., 1970). As Syrian hamsters are
exceptionally resistant to the effects of sulfur dioxide, these data
must be extrapolated with caution to other species.
7.2.2 Exposure to sulfuric acid aerosols
Alarie et al. (1973) exposed cynomolgus monkeys for 78 weeks to
sulfuric acid mist at concentrations of 0.38 to 4.79 mg/m3 and
particle sizes of 0.54 to 3.60 µm. At concentrations of 2.43 and
4.79 mg/m3 and particle sizes of 3.60 and 0.73 µm, definite damage to
the pulmonary structure was evident and there was deterioration in
pulmonary function. At the lower concentrations, changes were slight
or absent. The authors also exposed guineapigs for 52 weeks to
concentrations of 0.08-0.1 mg/m3 and particle sizes of 0.84 and
2.78 µm; no detectable effects were seen.
When beagle dogs were exposed to sulfuric acid at a concentration
of about 0.9 mg/m3 for 21 h per day, for 620 days, there was a
significant reduction in pulmonary function; 90%, of the sulfuric acid
mist was less than 0.5 µm in diameter (Lewis et al., 1973).
Histopathological changes were produced in the alveolar part of the
lung, especially in the elastic tissue, of rats exposed for 65 days
(24 h per day) to a sulfuric acid concentration of 1 mg/m3. Ninety
per cent of the particles were less than 2 µm in diameter in this
study (Bustueva, 1966).
Guineapigs were exposed to sulfuric acid aerosol for 120 h at 3
different concentrations (1.98 ± 0.03; 4.20 ± 0.06; and 8.27 ±
0.15 mg/m3). Exposure to the highest concentration led to oedema of
the lungs, changes in the interalveolar walls, sharp, diffused,
interstitial changes, and increased histamine content in the lung
tissue. Three weeks after exposure, sclerosis appeared (Bustueva,
1957). After a 1-month exposure to 0.1 mg/m3, no changes were found
(Bustueva, 1961a).
7.2.3 Exposure to a mixture of sulfur dioxide and sulfuric acid
aerosols or this mixture combined with other agents
Rats were exposed for 65 days (24 h per day) to a combination of
sulfur dioxide at 4.86 mg/m3 (1.7 ppm) and a sulfuric acid aerosol at
1 mg/m3 (90% of the aerosol particles were less than 2 µm in
diameter). There was a summation of the effects (histopathological
changes in alveolar tissue) produced by exposure to each of the
pollutants alone (Bustueva, 1966).
Lewis et al. (1973) exposed beagle dogs to a combination of sulfur
dioxide at 13.4 mg/m3 (4.7 ppm) and sulfuric acid at about
0.9 mg/m3. Ninety percent of the sulfuric acid mist was less than
0.5 µm in diameter. The duration of exposure was 21 h per day, for 620
days. Some of the dogs had been pre-exposed to nitrogen dioxide. This
group tended to show less response in the form of changes in pulmonary
function than dogs not pre-exposed to nitrogen dioxide.
Alarie et al. (1975) exposed cynomolgus monkeys and guineapigs to
mixtures of sulfur dioxide, fly ash, and sulfuric acid mist, for 18
months after an 8-week baseline period. Exposure concentrations varied
from 0.29 to 143 mg/m3 (0.1 to 5.0 ppm) for sulfur dioxide and from
0.1 to 1 mg/m3 for sulfuric acid mist; the concentration of fly ash
was approximately 0.5 mg/m3. Particle size (MMD) varied from 0.53 to
3.11 µm in the acid mist and from 4.1 to 5.8 µm in the fly ash.
Pulmonary function tests and serum biochemical and haematological
analyses were conducted prior to, and periodically during, the
exposure. Lungs were examined microscopically at the end of the
experiment. Sulfuric acid mist appeared to be responsible for the
effects observed. These were largely histopathological changes in the
lungs. No synergistic action was noted between the pollutants.
7.2.4 Combined exposure to sulfur dioxide and particulate matter
or other gaseous pollutants
In studies by Frazer et al. (1968), white albino rats were exposed
to sulfur dioxide at 2.9 and 8.6 mg/m3 (1 and 3 ppm) and graphite
dust at 1 mg/m3. The mean particle size of the dust was less than
1 µm and the MMD was 3 µm. The animals were exposed for 12 hours per
day, 7 days per week, for 4 months. Both ciliary activity, and the
number of dust-containing cells per 100 lung cells appeared to be
unaffected after 56-109 days of exposure.
Various aerosols that may react with sulfur dioxide have been
studied either singly or in combination with sulfur dioxide in
experimental animals, particularly in guineapigs. Amdur (1969, 1971)
emphasized that the particle size of the aerosol as well as the
concentration was extremely important in the determination of toxicity
and that the most important size range was the submicron level. The
changes induced in pulmonary mechanics were slow to return to
pre-exposure levels. This indicates either that deposited aerosols may
not be cleared promptly but remain in the lungs exerting their effect
for a period of time, or that the repair mechanisms are slow, or that
both conditions are present. There was also a variation in the
toxicity of different sulfates for the same particle size and sulfur
content (Amdur, 1969) (Table 11).
Studies by Battigelli et al. (1969) in which rats were exposed to
sulfur dioxide at 2.9 mg/m3 (1 ppm) and graphite dust at 1 mg/m3
(particle size not stated) did not show any effect other than the
accumulation of dust in the lung after exposure for 4 months, for 12
hours per day. Amdur & Underhill (1968) pointed out that not all
aerosols enhance the effect of sulfur dioxide and that only those
aerosols composed of droplets in which the sulfur dioxide could
dissolve were active.
Mice were exposed by Zarkower (1972) to carbon particles (1.8 to
2.2 µm MMD) at a concentration of 558 ± 154 µg/m3 and sulfur dioxide
at 5.7 mg/m3 (2 ppm). The animals were exposed to carbon alone,
carbon with sulfur dioxide, and sulfur dioxide alone; controls were
unexposed. Killed Escherichia coli was used as an antigen and
antibody production was measured. Exposures for 192 days produced
significant immunosuppression. Shorter periods of exposure resulted in
variable effects including stimulation of antibody production.
A variety of metallic aerosols can catalyze the oxidation of
sulfur dioxide including the soluble salts of ferrous iron, manganese,
and vanadium.
Rylander & Bergström (1973) exposed animals with latent upper
respiratory disease and controls for 4 weeks to various combinations
of sulfur dioxide at 57 mg/m3 (20 ppm), manganese dioxide at
12-20 mg/m3 (particle size between 5.0 and 0.5 µm), and carbon
monoxide at 187-250 mg/m3 (150-200 ppm). Animals with latent upper
respiratory disease showed more extensive histological changes and an
increase in the number of free lung cells. The combination of sulfur
dioxide and manganese dioxide produced the greatest changes.
An addition of effects in rats was reported by Salamberidze (1969)
from joint exposure to sulfur dioxide at 0.15 mg/m3 (0.05 ppm) and
nitrogen dioxide at 0.1 mg/m3 (0.05 ppm) over a 3-month period.
The precise mechanism by which the oxides of sulfur and
particulate matter can affect the lungs is not known. Sulfur dioxide
(and presumably sulfuric acid as well) can interfere with the
clearance of bacteria (Rylander et al., 1971) and inert particles
(Ferin & Leach, 1973) from the lungs (see also section 6). Chronic
exposure to sulfur dioxide increased the number and area of goblet
cells in guineapigs and lambs (Mawdesley-Thomas et al., 1971).
The considerable variations in the results of these experiments on
animals reflect differences in sensitivity of individual species,
exposure levels, and methods used to assess the effects.
It should be emphasized that extrapolation of these results from
animals to human beings is not easy. These findings, however, do give
some insight into possible mechanisms of action and reactions that can
occur.
8. EFFECTS ON MAN
8.1 Controlled Exposures
A number of studies have been performed on volunteers under
controlled conditions of exposure to sulfur dioxide or sulfuric acid
aerosols, singly or in combination, or to mixtures of these with other
compounds such as ozone and hydrogen peroxide. These studies, all
conducted under short-term exposure (up to 24 h), include those on
changes in respiratory function and effects on sensory and reflex
organs.
8.1.1 Effects on respiratory organs
8.1.1.1 Exposure to sulfur dioxide
Amdur et al. (1953) exposed 14 healthy volunteers (inhaling
through the mouth) to sulfur dioxide at concentrations of
2.9-23 mg/m3 (1-8 ppm) for 10 min. They noted an increased pulse
frequency, decreased tidal volume, and an increased respiratory
frequency which returned to normal levels after exposure. The sequence
of exposures was randomized. Effects increased with increasing levels
of sulfur dioxide and were detectable at the lowest concentration
tested (2.9 mg/m3; 1 ppm). Sulfur dioxide levels were monitored by
the conductimetric method. However, Lawther (1955) was unable to
reproduce these effects in any consistent manner, either in urban or
in rural dwellings. Eleven healthy volunteers were exposed to sulfur
dioxide at levels of 2.9, 14, and 37 mg/m3 (1, 5, and 13 ppm) in
studies by Frank et al. (1962). Respiratory mechanics were measured by
means of a body plethysmograph and an oesophageal balloon. Exposures
lasted 10-30 min. Only one of the 11 subjects exposed showed an
increased pulmonary resistance at 2.9 mg/m3 (1 ppm). Increased
pulmonary resistance was noted in all subjects and was greater at the
higher concentration. The change occurred within 1 min of exposure and
increased up to 10 min, after which no further increase was noted.
In studies by Snell & Luchsinger (1969), exposure to a sulfur
dioxide concentration of 2.9 mg/m3 (1 ppm) for 15 min produced a
slight effect on total respiratory resistance in 9 healthy volunteers.
Andersen et al. (1974) exposed healthy male subjects to sulfur
dioxide at levels of 2.9, 14, and 71 mg/m3 (1, 5, and 25 ppm) for up
to 6 h. With exposures of 1-3 h at 2.9 mg/m3 (1 ppm), there was a
decrease in the flow of nasal mucus and a decrease in the cross
section of the nasal passages. Thus it appears that exposure to
concentrations of 2.9 mg/m3 (1 ppm) or more may result in impairment
of mucociliary transport in the nose.
Four healthy volunteers were exposed to sulfur dioxide and the
forced vital capacity (FVC), one second forced expiratory volume
(FEV1.0), mid-maximal flow rates (MMFR), maximal expiratory flow rate
at 50% (MEFR50) and closing volume and capacity were measured.
Although no changes were found in these tests at 1.1 mg/m3
(0.37 ppm), slight changes in FVC, FEV1.0, MMFR, and MEFR50 were
noticed at 2.1 mg/m3 (0.75 ppm) after exposure for 30 min; no effect
on closing volume was detected (Bates & Hazucha, 1973).
The results of these studies on human volunteers are summarized in
Table 12.
8.1.1.2 Exposure to sulfuric acid aerosols
Amdur et al. (1952b) exposed 15 healthy men to sulfuric acid mist,
through mouth breathing, at concentrations of 0.35-5 mg/m3 (particle
size of approximately 1 µm) for periods of 5-15 min. At concentrations
below 1 mg/m3, the mist did not produce any subjective sensations
although 5 of the 15 subjects showed a slightly increased respiratory
rate and a decreased tidal volume at 0.35 mg/m3. All subjects noted
irritation at a concentration of 3 mg/m3. Respiration was monitored
by means of a pneumotachograph. The respiratory changes as well as the
subjective sensations increased with increasing concentrations of
sulfuric acid. Healthy male volunteers were also exposed to sulfuric
acid mist by Sim & Pattle (1957), either by mask or in a chamber for
periods ranging from 10 to 60 min. Twelve men were exposed to the
mist. The temperature of the air was 18.4°C with a relative humidity
of 62%. The MMD of the aerosol was 0.99 µm and the concentration was
39.4 mg/m3. The men noted minor irritation, and lung resistance (as
measured by the interrupter technique) rose by 35-100%. With
re-exposure for 30 min to the mist at a temperature of 24.5°C, a
relative humidity of 91%, a concentration of 20.8 mg/m3, and a MMD of
1.54 µm, severe coughing and irritation of the throat occurred. The
men found it almost intolerable. Lung resistance had risen by 43-150%
when measured after 10 minutes of exposure and when coughing had
ceased. No changes in respiration, blood pressure, or pulse rate were
noted. Two of the men exposed to these conditions had persistent
symptoms for some days after exposure ended.
It is difficult to evaluate these two studies. Amdur did not
report the temperature, though it was probably in the neighbourhood of
24°C, or the relative humidity. The studies of Sim & Pattle were at
relatively high levels but they demonstrated the importance of the
relative humidity or perhaps even more important, the absolute
humidity. The results of these studies are summarized in Table 13.
None of these studies used sulfates.
Table 12. Selected laboratory studies on the effects of short-term exposures to sulfur dioxide on respiratory
function in volunteers
Concentration Length of Effects Subjects Reference
exposure
(mg/m3)a (ppm) (min)
2.9-23 1-8 10 Increased pulse rate, decreased 14 healthy males Amdur et al. (1953)
tidal volume, and increased
respiratory rate
2.9 1 10-30 Increased pulmonary/resistance 11 healthy males Frank et al. (1962)
2.9 1 15 Increased respiratory resistance 9 healthy subjects Snell & Luchsinger (1969)
(5 males &
4 females)
2.9 1 60-180 Decreased nasal mucus flow 15 healthy males Andersen et al. (1974)
and decreased cross section of
nasal passages
2.1 0.75 120 Slight effect in 30 min on 4 healthy subjects Bates & Hazucha (1973)
FVC, FEV1-0, MMFR, and
MEFR50; no effect on
closing volume
1.1 0.37 120 No effect on above tests of 4 healthy subjects Bates & Hazucha (1973)
pulmonary function throughout
exposure period
a Original levels reported as ppm have been converted to mg/m3 and rounded off.
Table 13. Selected laboratory studies on the effects of short-term exposures to sulfuric acid mist on respiratory
function in volunteers
Concentration Particle Size Length of Relative Temperature Effects Subjects Reference
(mg/m3) (µm) exposure Humidity (°C)
(min) (%)
0.35 1 5-15 ? Room (?24) 5 of 15 subjects 15 healthy Amdur et al.
increased subjects (1952b)
respiratory rate
and decreased
tidal volume
39.4 0.99 60 62 18.4 Increased 12 healthy Sim & Pattle
pulmonary males (1957)
resistance and
minor irritation
20.8 1.54 30 91 24.5 Marked increase 12 healthy Sim & Pattle
of pulmonary males (1957)
resistance and
severe irritation
8.1.1.3 Exposure to mixtures of sulfur dioxide and other compounds
Frank et al. (1964) repeated the above exposures in combination
with a sodium chloride aerosol. Concentrations of sulfur dioxide were:
2.9-5.7 mg/m3 (1-2 ppm), 11-17 mg/m3 (4-6 ppm), and 40-49 mg/m3
(14-17 ppm). The sodium chloride aerosol had a geometric mean diameter
of 0.15 µm with a geometric standard deviation of 2.3 µm and an
average concentration of 18 mg/m3. As in the earlier studies, little
change in pulmonary flow resistance was noted at the lower levels of
sulfur dioxide alone (2.9-5.7 mg/m3; 1-2 ppm) but a progressive
increase was noted at higher levels. The authors did not find any
systematic differences between the responses to sulfur dioxide alone
or to the gas plus the aerosol. As mentioned in section 8.1.1.1, Snell
& Luchsinger (1969) noted a slight effect of sulfur dioxide at
2.9 mg/m3 (1 ppm) on total respiratory resistance but could not
demonstrate an enhancing effect of either a sodium chloride aerosol or
a distilled water aerosol. Burton et al. (1969) exposed volunteers to
a concentration of sulfur dioxide of 6 mg/m3 (2.1 ppm) with or
without a sodium chloride aerosol (MMD of less than 0.4 µm) at a
concentration of 2.2 mg/m3. The inhaled air was warmed and moistened.
However, they too failed to demonstrate any effect of the sodium
chloride aerosol on the response to sulfur dioxide.
Thus, three groups have not been able to demonstrate any enhancing
effect of sodium chloride aerosol on respiratory resistance in man as
had been demonstrated in guineapigs. This does not exclude the
possibility that other particulate matter might enhance the effect of
sulfur dioxide on the human respiratory system. These studies should
be repeated at levels of humidity above 80% and the mixture should be
allowed to "age".
The possible interaction of sulfur dioxide and hydrogen peroxide
has been examined by Toyama & Nakamura (1964) and that of sulfur
dioxide and ozone by Bates & Hazucha (1973). Toyama & Nakamura (1964)
studied 24 healthy male volunteers by means of a pneumotachograph and
the interrupter technique to measure alveolar pressure; airway
resistance was determined from these measurements. The particle sizes
of the hydrogen peroxide were reported to be 1.8 and 4.6 µm.
Concentrations of hydrogen peroxide were 0.8-1.4 mg/m3 for the larger
particles and 0.01-0.1 mg/m3 for the smaller ones. Levels of sulfur
dioxide ranged from 2.9 to 170 mg/m3 (1-60 ppm). The subjects were
not aware whether they were breathing hydrogen peroxide, sulfur
dioxide, or combinations. It was not stated whether the order of
administration was randomized or not. The investigators noted a marked
increase in airway resistance with the mixture compared with the
individual components. They also noted that the larger particles had
more effect than the smaller particles. This was probably due to the
greater dose delivered, although they did not comment on this. It was
the authors' opinion that the enhancement of the response to the
combination was due to the conversion of sulfur dioxide to sulfuric
acid, although no measurement for sulfuric acid was made.
In similar studies by Bates & Hazucha (1973) healthy male
volunteers were exposed in a chamber to sulfur dioxide, or ozone, or a
combination of the two. Changes in FVC, FEV1.0, MMFR, MEFR at 50%
vital capacity and peak expiratory flow rates were studied and some
effects were noted during exposure to ozone at 540-1610 µg/m3
(0.25-0.75 ppm). Sulfur dioxide by itself had little effect over a
similar range. Joint exposure to sulfur dioxide and ozone at
1060 µg/m3 (0.37 ppm) and 790 µg/m3 (0.37 ppm), respectively,
produced a greater effect than ozone alone. Changes were noted after
exposure for 30 min and were marked after 2 h of exposure. Exercise
during exposure enhanced the effect.
8.1.2 Effects on sensory or reflex functions
Studies in the USSR have concentrated on the effects of sulfuric
acid aerosols and sulfur dioxide on sensory receptors, cerebral
cortical function, and their interrelationships. When sulfuric acid
mist produced subjective sensory stimulation such as odour or
irritation of mucous membranes, or both, then, invariably, objective
evidence of central nervous system depression could be demonstrated
(Rjazanov, 1962).
Bustueva (1961b) did not note any change in optical chronaxy in
volunteers exposed to concentrations of sulfur dioxide of 0.5 mg/m3
(0.18 ppm) and sulfuric acid of 0.3 mg/m3. However, when levels of
1.5 mg/m3 for sulfur dioxide, 0.73 mg/m3 for sulfuric acid, and
combined levels of 1.2 mg/m3 and 0.6 mg/m3, respectively, were
exceeded, optical chronaxy increased (Table 14). Similar effects were
seen in dark adaptation responses (Rjazanov, 1962).
Studies have also been carried out in which the cerebral cortex
was monitored by electroencephalography. Alpha rhythm suppression was
used as an index of response. Threshold levels at which a response was
noted are given in Table 14. (Bustueva et al. 1960).
Table 14. Threshold levels of sulfur dioxide and sulfuric acid required for effects on sensory or
reflex functions in volunteers during short-term exposuresa
Threshold levels (mg/m3)
Effects Sulfuric acid Sulfur dioxide Sulfuric acid + Sulfur dioxide
Perception of odour and 0.6 to 0.85 1.6 to 2.8 0.3 + 0.5
irritation of mucosa
Suppression of dark 0.63 to 0.73 0.92 0.3 + 0.5
adaptation
Elevation of optical 0.73 1.5 0.6 + 1.2
chronaxy
Disruption of alpha 0.63 0.9 0.3 + 0.5
rhythm
Conditioning of 0.4 0.6 0.15 + 0.5
electrocortical reflex or 0.3 + 0.25
a Summarized from studies in the USSR (Bustueva, 1961b; Bustueva et al., 1960; Rjazanov, 1962).
The electrocortical conditioned reflex is a central nervous system
phenomenon elicited only after a succession of repeated, conditioned
reflex trials. After exposure to a combination of irritants (sulfur
dioxide or sulfuric acid) and light has been repeated several times,
desynchronization begins to appear before the light is switched on.
This can be produced at levels generally not sensorially perceived.
Thus, unperceived odour or stimulus appears to become the conditioning
stimulus and generates the conditioned electrocortical reflex
(Rjazanov, 1962).
Elfimova & Hacaturjan (1968) exposed volunteers to sulfur dioxide,
phenol, and carbon monoxide and noted a summation of effects as
measured by reflex action. No interaction was noted between sulfur
dioxide at 0.5 mg/m3 (0.18 ppm) and carbon monoxide at 3 mg/m3
(2.4 ppm) as measured in volunteers by the sensory reflex technique
(Mamacasvili, 1968).
8.2 Industrial Exposure
Workers are exposed to sulfur dioxide or sulfuric acid mist in a
number of industries; sometimes exposure is not solely to sulfur
dioxide or sulfuric acid. Exposed populations have been studied with
respect to the effects of exposure on their health status or on their
respiratory system. However, in many of these studies, only the
currently employed workers were examined and a serious effort was not
made to locate subjects who had left the industry and who may have
suffered more from the disease or could have been more sensitive to
the materials. It should also be emphasized that, in general, in the
following reports, the exposure levels studied were from spot samples
or for very short time intervals.
8.2.1 Exposure to sulfur dioxide singly or in combination with
particulate matter
Kehoe et al. (1932) studied men working in a refrigerator company
in the USA where sulfur dioxide was the refrigerant. Exposures
averaged 60-90 mg/m3 (20-32 ppm) with peaks as high as 200 mg/m3
(70 ppm). These peaks had probably been higher in the past ranging up
to 290 mg/m3 (100 ppm) or more. The exposed group had significantly
more respiratory symptoms and colds. They also complained more of
fatigue and shortness of breath on exertion. Chest X-rays of the
exposed and unexposed groups showed the same distribution of
abnormalities. The authors concluded on their inadequate evidence that
there was no injury to the tracheobronchial tree or alveoli.
In studies on men working in smelters in Sweden, Sjörstrand (1947)
found that those who had worked at the roasting and reverberatory
furnaces and in the converter hall for 8 years or more had poorer
respiratory function than men working in other parts of the smelter.
Levels of exposure and the smoking histories of the subjects were not
reported. Men working in smelters are exposed to a variety of dusts as
well as to sulfur dioxide and it is difficult to separate the effects
of such exposures from those due to sulfur dioxide.
Men exposed to sulfur dioxide at daily mean concentrations up to
70 mg/m3 (25 ppm) with occasional peaks of 290 mg/m3 (100 ppm) in
certain areas of a petroleum refining plant in Abadan (Iran) were
compared by Anderson (1950) with an unexposed group. No differences
were found between the two groups. This study also did not refer to
the smoking histories of the subjects.
In a study in Norway, pulp mill workers were compared with paper
mill workers using a standard questionnaire on respiration and simple
tests of pulmonary function. The smoking histories of the subjects
were also studied. Levels of sulfur dioxide ranged from 6-100 mg/m3
(2-36 ppm) with peaks of 290 mg/m3 (100 ppm) when the digester was
blown. The exposed group had more cough, sputum, and dyspnoea than the
unexposed group but the vital capacities were similar in both groups.
The expiratory peak flows, however, of the exposed men under 50 years
of age were lower than those in a comparable unexposed group (Skalpe,
1964).
A similar study was carried out in the USA by Ferris et al. (1967)
who reported that there was no difference between men in a pulp mill
and men in a paper mill. Both groups had less respiratory disease than
was reported from a survey of the general population. The authors
noted that some of the men working in the paper mill had worked in the
pulp mill but had left because they could not tolerate the conditions.
Occupational levels of exposure to sulfur dioxide ranged from a trace
to 95 mg/m3 (33 ppm). Average levels ranged from 6 to 35 mg/m3
(2-13 ppm). Smoking habits were considered.
Huhti et al. (1970) studied pulp and paper mill workers in Finland
and noted that the effects of smoking were much more significant than
the exposures at work or the effects of climate. Levels of exposure
were not reported in this study.
Men working in two integrated steel mills in Wales were studied by
Lowe et al. (1968, 1970) and Warner et al. (1969). Mean concentrations
of sulfur dioxide over 3 years ranged from 1.8 to 2.1 mg/m3 (0.6 to
0.7 ppm) and those of suspended particulate matter in the respirable
range, ranged from 600 to 1800 µg/m3 (by elutriation technique).
Analysis of the particulate matter showed that it was mainly composed
of iron oxides and calcium sulfate. No effects on respiratory symptoms
or on simple tests of pulmonary function were found after
standardization for cigarette smoking and for age. These observations
may reflect the limitation of studying occupational groups because of
the effect of the selection processes, or this may be an example in
which the suspended particulate matter present was not interacting
with the sulfur dioxide to produce an effect.
8.2.2 Exposure to sulfuric acid mist
Dorsch (1913) studied men exposed to sulfuric acid mist in a plant
manufacturing storage batteries in Germany. He reported that at a
concentration of 0.5 mg/m3, the mist was barely noticeable; at
2.0 mg/m3, there was nose and throat irritation, at 3-4 mg/m3, there
was distinct discomfort, and at 6-8 mg/m3, there was marked
discomfort. These responses are comparable to those reported in
laboratory studies on human beings in section 8.1. No particle size
was given but, as noted in another survey reported below, they were
probably relatively large.
Men in the battery industry were also examined by Malcolm & Paul
(1961) in the United Kingdom who reported that there was significant
erosion of the teeth of the battery room workers. This was confirmed
by ten Bruggen Cate (1968). Apparently this was due to the direct
impingement of relatively large droplets of sulfuric acid on the
teeth.
Williams (1970) studied men from the same works as Malcolm & Paul
(1961) and reported that there was no difference in the forced vital
capacity and the one-second forced expiratory volume between the men
in the forming and control departments. Levels of sulfuric acid mist
averaged 1.4 mg/m3 during working hours over two days and ranged from
a trace to 6.1 mg/m3 in 1968. An earlier survey reported higher
values. Particle size reported from a survey in another firming
department doing comparable work was 14 µm MMD; 4% of the particles
being less than 4 µm MMD. The level of sulfuric acid in this
department was 2.7 mg/m3.
8.3 Community Exposure
Much of the information that has been gained concerning the
effects on health of exposure to realistic concentrations of sulfur
oxides and particulate matter has come from epidemiological studies,
carried out on segments of population chosen by virtue of place of
residence, age, existing state of health, or other characteristics, in
order to present contrasts in exposure or sensitivity to these
pollutants. Some studies have been based on the complete populations
of urban areas, observing the total number of deaths, or the incidence
or prevalence of illness within them in relation to differences in
pollution between areas, or with time in any one area.
Many epidemiological studies concerning the health effects of
exposure to sulfur oxides and particulate matter have been reported in
the literature. In the discussion that follows, attention has been
directed to papers that yield information relevant to the development
of exposure-effect and exposure-response relationships for sulfur
oxides, smoke, and suspended particulate matter, and to some others
that are of interest from the point of view of the method of approach.
8.3.1 Mortality -- effects of short-term exposures
The most clearly defined effects on mortality arising from
exposure to sulfur oxides and particulate matter have been the sudden
increases in the number of deaths occurring, on a day-to-day basis, in
episodes of high pollution. The most notable of these occurred in the
Meuse Valley in 1930 (Firket, 1931), in Donora in 1948 (Schrenk et
al., 1949), and in London in 1952 (Ministry of Health, UK, 1954). The
people primarily affected were those with pre-existing heart or lung
disease or both, and the elderly. The London episode lasted for 5 days
and it was estimated that the number of deaths during and immediately
after this period was about 4000 more than expected under normal
circumstances. On one day, the number of deaths was about three times
the number expected at that time of the year. Concentrations of sulfur
dioxide as high as 3.7 mg/m3 (1.3 ppm) were recorded in the centre of
the urban area (48-h average). Concentrations of particulate matter
were too great to be measured properly (British daily smoke/sulfur
dioxide method), and the 48-h average of about 4.5 mg/m3 at a central
site must be regarded as a conservative estimate. These were rough
estimates for the exposures and, probably, there was considerable
variation in individual exposures.
Following these major episodes, attention was turned to studies on
more moderate day-to-day variations in mortality within large cities,
in relation to pollution. Gore & Shaddick (1958) correlated mortality
in the County of London (the inner part of the Greater London Area)
with pollution by smoke and sulfur dioxide for 4 foggy periods in
1954-56, using 7-day moving averages to smooth out the data. The
authors considered that, in two of the episodes, there was a marked
increase in mortality from bronchitis and other lung diseases,
particularly in the elderly. They concluded that when the 24-h mean
concentration of smoke exceeded 2.0 mg/m3 at the same time as the
24-h mean concentration of sulfur dioxide exceeded 1.1 mg/m3
(0.4 ppm) (British daily smoke/sulfur dioxide method), there would be
increased mortality. Care was taken in this study to ensure that the
measurements were reasonably representative of the exposure of people
anywhere within the study area: the figures quoted were the mean
values from a group of 7 sites, all situated close to ground level in
mainly residential areas. However, there remained the problem that the
people at risk in a study of this type were the elderly sick, who were
likely to remain indoors, and that outdoor measurements might not have
provided an adequate assessment of exposure (Biersteker et al., 1965).
The relationship between daily mortality in the more extensive
area of Greater London and day-to-day variations in pollution (smoke
and sulfur dioxide) and visibility was examined by Martin & Bradley
(1960) in the winter of 1958-59. They noted that on days when the
smoke concentration increased by more than 100 µg/m3 compared with
the previous day, or when the sulfur dioxide concentration increased
by 70 µg/m3 (0.025 ppm), there was likely to be increased mortality
(British daily smoke/sulfur dioxide method). The increases in daily
mortality were up to about 1.25 times expected values assessed from
15-day moving averages. Thick fog (visibility less than 200 metres)
was also associated with increases in mortality. The relative
importance of the 3 factors could not be determined but, on the basis
of other work, the authors considered that the smoke was probably the
most important. It is not clear whether the results are best
interpreted in terms of change in pollution from one day to the next,
rather than in terms of absolute values, but there is support for the
former approach from studies carried out elsewhere. When results were
considered on an absolute basis (Lawther, 1963), it was concluded that
increases in mortality became evident when the 24-h mean
concentrations of smoke and sulfur dioxide exceeded 750 µg/m3 and
710 µg/m3 (0.25 ppm), respectively. The measurement sites were the
same as those used by Gore & Shaddick (1958). They could still be
considered reasonably representative of outdoor concentrations in the
areas where people lived, although the inclusion of outer,
less-densely populated areas meant that the average exposures would
tend to have been underestimated.
Studies on day-to-day variations in mortality in London were
continued in successive winters, and coupled with the records of
emergency hospital admissions. In a later paper, Martin (1964) showed
correlations between both the daily mortality and hospital admission
data and concentrations of smoke or sulfur dioxide. There was no
clearly defined level above which effects were seen, but there were
fairly consistent increases in both mortality and hospital admissions
when the concentrations of smoke and sulfur dioxide each exceeded a
24- mean of about 500 µg/m3 (0.18 ppm of sulfur dioxide, British
daily smoke/sulfur dioxide method). In 1962, there was a major episode
of high pollution in London, similar in terms of duration and of
sulfur dioxide concentrations to the one in 1952, but with lower smoke
concentrations. Again, there was a sudden increase in deaths, but the
number of deaths was not as great as before (about 700, compared with
4000). Whether the change in medical care could have influenced these
results is not clear. The greater use of antibiotics in 1962 compared
with 1952 might have reduced the number of deaths, and a greater
awareness of the risk together with clear advice to the elderly and
infirm to remain indoors could have had an effect. The dramatic
reduction in smoke concentrations in London brought about by the
implementation of the Clean Air Act, and the more gradual reduction in
sulfur dioxide that has followed it, have meant that in more recent
years there have been few occasions when levels of 500 µg/m3 have
been exceeded simultaneously for smoke and sulfur dioxide (Waller et
al., 1969).
Biersteker (1966) published a study of an episode of high
pollution in Rotterdam in December 1962, when concentrations of smoke
and sulfur dioxide of approximately 500 µg/m3 and 1000 µg/m3
(0.35 ppm), respectively, were recorded (24-h means, OECD smoke/sulfur
dioxide method). There were increases in admissions to local hospitals
of people over 50 years of age with cardiovascular diseases, and there
was also some indication of an increase in mortality. This was
observed only once in Rotterdam and could have been due to other
causes. Further observations during a similar episode would be needed
to provide a convincing statistical relationship between hospital
admissions and these levels of pollution.
A relationship between day-to-day changes in mortality and
pollution has also been reported from Osaka (Watanabe 1966). There
appeared to be increases in mortality (about 20%) on days when the
concentration of suspended particulate matter (light scattering
method) exceeded 1 mg/m3 (4-day average) and was associated with a
level of sulfur dioxide of 200 µg/m3 (0.07 ppm). Low temperatures may
have been partly responsible for the effects.
Variations in daily mortality in New York in relation to sulfur
dioxide concentrations were studied by Buechley et al. (1973). They
examined correlations and developed regressions between a number of
daily climatic factors and indices of pollution (sulfur dioxide,
conductimetric method and coefficient of haze (Cohs)), and the
mortality residuals for a given day. They noted that the day of the
week had a special correlation with mortality (mortality rates were
considerably higher on Mondays). Regression analysis indicated that
heat waves and seasonal cycle were major predictors of mortality.
Other factors were much weaker (about one third as strong) but were
all of equal strength. Partial residual mortality values were computed
and showed a significant correlation with the levels of air pollution
(sulfur dioxide r = 0.14). Mortality could be predicted equally as
well from Cohs as from sulfur dioxide levels. Mortality was 1.5% less
than expected on 232 days when sulfur dioxide levels were below
30 µg/m3 (0.01 ppm) and 2% greater than expected on 260 days when the
sulfur dioxide levels were above 500 µg/m3 (0.18 ppm) after
correcting for the other factors. The crossover point (i.e., that
point below which deaths were less than expected and above which
deaths were greater than expected) was in the vicinity of a
concentration of sulfur dioxide of 260 µg/m3 (0.09 ppm). On the other
hand, the data from these studies could be interpreted to show a
continuum of an effect across the levels of sulfur dioxide, which, in
turn, should not be considered the causative agent but rather an index
of pollution.
Schimmel & Murawski (1975) have reported on their regression
analysis of daily deaths and levels of pollution (smoke shade and
sulfur dioxide) in New York City for 1963-1972. This was an extension
of an earlier study (Schimmel & Greenberg, 1972). The authors
controlled for season, day of week, and temperature. During this time
there was a marked reduction in the average level of sulfur dioxide
from 510 µg/m3 (0.18 ppm) to 170 µg/m3 (0.06 ppm) but virtually no
change in smoke shade. Their observations indicated that, despite this
reduction in sulfur dioxide, there had been no reduction in adverse
health effects. Analysis indicated that the adverse health effects
were associated principally (80%) with the particulate matter and only
to a small extent (20%) with the sulfur dioxide. However, the authors
also pointed out that, when they regressed mortality on temperature
and sulfur dioxide alone, the effects attributable to the sulfur
dioxide increased three-fold.
These findings are provocative but they must be interpreted
cautiously because of a number of limitations in the data. Air
pollution levels were from data obtained at a single monitoring
station and were probably not truly representative of exposures for
the community. The standard errors reported with their data are large.
The report should be considered to be an indicator for further studies
in which age-specific death rates and causes of death should be
included in the analysis as well as more relevant air pollution
measurements. The results should not be used for developing an
exposure-effect relationship.
A study involving comparisons between daily mortality data in New
York and Tokyo was carried out by Lebowitz et al. (1973). They applied
a "stimulus-response" technique to identify associations between days
of high pollution and days with increased mortality, and showed strong
relationships in both cities. However, their findings do not provide
information of direct value in the assessment of exposure-effect
relationships.
8.3.2 Mortality -- effects of long-term exposures
In countries having reliable systems for the collection and
analysis of data on deaths, based on cause and area of residence,
death rates for respiratory diseases have commonly been found to be
higher in towns than in rural areas. Many factors, such as differences
in smoking habits, occupation, or social conditions may be involved in
these contrasts, but, in a number of countries, a general association
between death rates from respiratory diseases and air pollution has
been apparent for many decades.
Analyses of these data have been of great value as a lead for
epidemiological studies, but the absence of information concerning
other relevant variables, such as smoking, and the relatively crude
nature of the indices of pollution used in many of these studies make
them unsuitable for the assessment of exposure-effect relationships.
The studies of Daly (1954, 1959), Pemberton & Goldberg (1954), and
Stocks (1959) were all based on mortality data from towns in England
and Wales, and each showed a positive correlation between bronchitis
or pneumonia death rates and some index of pollution by sulfur oxides
or particulate matter, as assessed for periods close to those for
which death rates were calculated. The most detailed investigation of
this type, taking into account social factors as well as pollution,
but still not smoking, was that conducted by Gardner et al. (1969).
One interesting feature of their findings was a slight improvement in
correlation when the index of pollution used was related to a period
some 10 years earlier than that for which the death rates were
calculated (deaths 1958-64, pollution index 1952). This illustrates
another of the problems that has been widely recognized when trying to
use mortality records to assess the effects of pollution i.e., that it
may not be recent exposures that are most relevant, but those earlier
in life; where concentrations have changed markedly over the years,
current measurements may not provide an adequate index.
Lave & Seskin (1970) reanalyzed some of the mortality data from
England and Wales, and developed multiple regression equations in
terms of pollution and socioeconomic indices. Again their findings of
positive correlations with pollution are of general interest but
cannot contribute to the development of dose-response relationships.
These authors also examined analogous data for Standard Metropolitan
Statistical Areas (SMSAs) in USA and in a later paper (Lave & Seskin,
1972) they attempted to assess the relative effects of air pollution,
climate, and home heating on mortality rates. Although equations were
obtained relating death rates to measurements of suspended particulate
matter and total sulfates (both by high volume sampler), it is
doubtful whether these can be regarded as valid in the absence of
adequate information on smoking.
8.3.3 Morbidity -- effects of short-term exposures
Prospective studies on specific occupational groups, not
professionally exposed, can be useful in assessing the effects of air
pollution in different communities or in areas where a change in air
pollution is expected. In such studies, where respiratory diseases are
followed, it is necessary to control for age distribution and
household composition, and to employ adequate statistical methods. In
studies on the Philadelphia area, USA, Dohan & Taylor (1960) used
absences of seven days or more because of respiratory disease as the
index and related this to the levels of sulfate. A later report by
Dohan (1961) noted that this relationship was stronger during an
epidemic of influenza. Ipsen et al., (1969) repeated this type of
study in the same area on a slightly different occupational group
using more detailed statistical analyses. They were not able to
confirm the earlier observations that the sulfate levels were related
to absences due to respiratory diseases.
Results have been reported (Lawther et al., 1970) of a series of
studies extending from 1954 to 1968 that were carried out mainly in
London, but also in some other large cities in England, using a diary
technique for the self-assessment of day-to-day changes in conditions
among bronchitic patients. A daily illness score was calculated from
the data contained in the diaries and this was related to the
concentrations of smoke and sulfur dioxide (British daily smoke/sulfur
dioxide method) and to weather variables. The pollution figures used
for most of the London studies were the mean values from the group of
sites associated with the mortality/morbidity studies of Martin (1964)
and Gore & Shaddick (1958). Many of the subjects in the series were
active enough to be out and about and at work, and the measurements
were considered to give a reasonable assessment of the average
exposures in the areas where they lived or worked. The method used in
these studies has not been validated, for the subjects recorded only
their own assessment of their condition, and this was not checked
against regular clinical examinations or ventilatory function
measurements, but the changes appeared to have some real meaning. In
the earlier years of the series, when the general level of pollution
was high, well defined peaks in the illness score were seen when
concentrations of either smoke or sulfur dioxide exceeded 1000 µg/m3.
With the reductions in pollution that followed the gradual
implementation of the Clean Air Act, these changes in condition became
less frequent and of smaller magnitude, and the conclusion from the
series as a whole, up to 1968, was that the minimum pollution
associated with significant changes in the condition of the patients
was a smoke level of about 250 µg/m3 together with a sulfur dioxide
concentration of about 500 µg/m3 (0.18 ppm) (24-h means, British
daily smoke/sulfur dioxide method). At these levels, there was still
some evidence that the peaks were associated specifically with
pollution rather than with adverse weather conditions. A later study
that has been reported by Waller (1971), showed that, with much
reduced average levels of pollution, there was an almost complete
disappearance of days with smoke levels exceeding 250 µg/m3 and
sulfur dioxide levels exceeding 500 µg/m3 (0.18 ppm). As in earlier
studies, some correlation remained between changes in the condition of
the patients and daily concentrations of smoke and sulfur dioxide but
the changes were small at these levels. At this low range of
pollution, discrimination between the effects of pollution and those
of adverse weather was poor.
Cohen et al. (1974) studied symptoms of irritation during a
publicized and an unpublicized period of air pollution as well as
during a control period in 3 communities in the New York metropolitan
area during the summer of 1970. They used a telephone survey technique
to inquire about specific symptoms such as eye irritation, throat
irritation, chest discomfort, shortness of breath, restricted
activity, and medical visits. No difference was noted between the 2
episodes of pollution. Both episodes showed significantly increased
symptoms compared with the control period. The results indicated that
irritative symptoms increased significantly when sulfur dioxide levels
exceeded 310 µg/m3 (0.11 ppm, West-Gaeke method) and total suspended
particulates exceeded 145 µg/m3 (high volume sampler) as a 3 day
average. Sulfate levels ranged from 6.6 to 7.6 µg/m3 in one area and
5.8 to 12.3 µg/m3 in another area. In the second area, sulfate levels
during the 2 periods of air pollution were 8.5 and 12.3 µg/m3,
respectively. Sulfate levels were not reported from the third area but
were probably low. The authors drew attention to some of the problems
associated with their study. The persons interviewed were generally
wives and the symptomatology in the male population could have been
underestimated. Also, there was an internal inconsistency possibly due
to intercurrent infectious disease or socioeconomic differences.
During the publicized episode, particulate pollution was considerably
higher in the Bronx than in Queens whereas irritation symptoms were
somewhat higher in Queens. The authors concluded that there could have
been confounding effects of other air pollutants, intercurrent
infection, or sociocultural factors.
Spirometric measurements were made at approximately weekly
intervals on 18 patients with chronic obstructive lung disease for
various periods during 1969-71 (Emerson, 1973). The spirometric values
(FEV1.0 and MEFR) were correlated with the levels of pollution (sulfur
dioxide and smoke, British daily smoke/sulfur dioxide method) and
climatic factors (temperature and humidity). Changes in FEV1.0 in
these patients were more strongly correlated with temperature and
humidity than with concentrations of sulfur dioxide or smoke. Levels
of air pollution in London were: sulfur dioxide, mean 190 µg/m3
(0.07 ppm), maximum, 720 µg/m3 (0.25 ppm) and smoke mean, 44 µg/m3,
maximum, 240 µg/m3. One limitation of the study was that the
pollution figures were averaged for 5-day periods, whilst the
spirometric measurements were made on specific days.
Studies of patients with chronic bronchitis in Chicago, USA
(Burrows et al., 1968; Carnow et al., 1969) showed conflicting
results. The reasons for these differences are not clear but may be
that different criteria for the selection of patients as well as
different methods for the determination of the exposure of the
individuals and their responses were used.
An unexpected finding of a possible effect of short-term exposure
to pollution arose from a study primarily concerned with long-term
exposures. In a resurvey of adults in Vlaardingen (Netherlands) who
had been interviewed and had lung function measurements made in 1969,
Van der Lende et al. (1975) found that the average lung function
values were higher in 1972 than in 1969, even though the subjects were
3 years older. When the authors examined the concentrations of
pollution on the 2 occasions (each survey having been done within a
5-day period), they found that levels were relatively high in 1969
with daily values ranging from 15 to 140 µg/m3 for smoke and from 120
to 300 µg/m3 (0.04-0.11 ppm) for sulfur dioxide compared with values
ranging from 15 to 40 µg/m3 and 45-100 µg/m3 (0.02-0.04 ppm),
respectively, in 1972. The increase in lung function was most
pronounced on the days with the greatest difference in the levels of
pollution. A control population in a rural area showed no comparable
changes over the same period, and temperature differences did not
explain the effect.
Asthmatic subjects have also been studied. These patients
represent a heterogeneous group and this may account for the variable
responses that have been reported. Cohen et al. (1972) studied 20
asthmatic subjects living in a small town (Cumberland, WV, USA) in the
vicinity of a coal-fired power plant. They found that when the soiling
index exceeded 1.0 Coh unit, or sulfur dioxide concentrations
(West-Gaeke method) exceeded 200 µg/m3 (0.07 ppm), or total suspended
particulates exceeded 150 µg/m3 (high volume sampling method), or the
temperature was lower than 0°C, there was a significant increase in
the frequency of asthmatic attacks. Levels of sulfates exceeding
20 µg/m3 and nitrate levels exceeding 2 µg/m3 did not result in such
an effect. In general, the effect of temperature was stronger than
that of the air pollutants although each of the 5 air pollutants
measured including sulfates and nitrates showed a correlation. When
temperature and any one of the pollutants were controlled for in the
analysis, the effect of any of the other 4 pollutants was eliminated.
Temperatures below 0°C overwhelmed any effect of the pollutants and
higher levels of the pollutants reduced the effect of temperature. One
further feature of this study should be stressed. Pollution in
Cumberland was dominated by emissions from a large single source, and
this might have led to high transient exposures to the pollutants,
which included oxides of nitrogen as well as sulfur oxides and
suspended particulate matter. In these circumstances it is doubtful
whether the 24-h values provided an adequate index of exposure of the
population.
Several studies on asthmatic subjects in Yokkaichi, Japan, have
been reported by Yoshida et al. (1966) including one on a group of 13
patients in which the number of attacks increased from 1 to 4 per week
when the concentration of sulfur dioxide was in the range of
140-230 µg/m3 (0.05-0.08 ppm), rising to about 12 per week when the
sulfur dioxide level reached 740 µg/m3 (0.26 ppm), all expressed as
weekly means. Suspended particulate or smoke levels were not reported.
8.3.4 Morbidity in adults -- effects of long-term exposures
Random samples of populations can be used for international
comparisons where there are gradations of air pollution. The major
difficulty here has been to ensure comparability with respect to
occupational exposures and ethnic groups. Reid et al. (1964) reported
such a comparison based on a study in the United Kingdom (College of
General Practitioners' Study, 1961) and a survey in Berlin, NH, USA
(Ferris & Anderson, 1962). The same questionnaire was used in both
studies. In the United Kingdom, it was completed by a large number of
practitioners who made up the survey group. In the USA, it was
completed by 2 physicians who tried to maintain the criteria developed
in the British survey. Results were standardized for cigarette
smoking. The effects of air pollution were then examined by age group
and sex. When simple bronchitis was present, i.e., phlegm production
for 3 months out of the year for 2-3 years, standardizing for
cigarette smoking removed any effect of air pollution for both males
and females. A more severe form of chronic bronchitis characterized by
phlegm production, exacerbations of colds that went to the chest, and
shortness of breath when walking on the level at one's own pace did
show an association with air pollution for both males and females,
even after standardizing for cigarette smoking. Levels of air
pollution were measured in Berlin, NH, by the lead peroxide candle,
dustfall, and high-volume samplers. Data for the United Kingdom were
estimated from similar measurements obtained in comparable towns and
cities where the general practitioners collected the data. In Berlin,
NH, the sulfation rate (lead candle) was 730 µg SO3/100 cm2 per day;
in the United Kingdom, in the large towns it was 950 and in the large
conurbations 1650 µg SO3/100 cm2 per day. The results of this study
seem to be consistent with those of other studies but it is not known
whether differences in socioeconomic or ethnic status could have been
relevant factors. The population of Berlin, NH, was resurveyed in
1967, and the results were compared with those obtained in 1961
(Ferris et al., 1973). There had been some decrease in pollution
levels in the interval, the sulfation rate being 470 µg SO3/100 cm2
per day in 1967, compared with the earlier figure of 730, while the
concentration of total suspended particulates had fallen from 180 to
132 µg/m3 (high-volume samples). Small reductions in the prevalence
of respiratory disease were noted after standardizing for age and
cigarette smoking. Slight improvements in forced vital capacity (FVC)
and peak expiratory flow rate (PEFR) were also noted, but there was
little change in FEV1.0. There is some doubt about the relevance of
the 180 µg/m3 figure quoted for total suspended particulates in the
1961 study, since it referred only to a 2-month period at a single
site. A random population sample from Chilliwack, BC, Canada, an
unpolluted community, was studied by Ferris & Anderson (1964) and the
results were compared with the results of the 1961 study in Berlin,
NH. Respiratory symptoms, after standardization for age and cigarette
smoking, tended to be higher in Berlin than in Chilliwack. Pulmonary
function (FEV1.0 and PEFR) was lower in Berlin than in Chilliwack,
after standardization for age, height, sex, and smoking category.
Pollution levels in Chilliwack (based on lead-candle measurements)
were about one-tenth to one-sixth of those in Berlin, NH.
A third survey in this series was carried out in 1973 (Ferris et
al., 1976). By this time, there had been a further decline in
pollution by particulate matter, the annual mean concentration of
total suspended particulates (high volume sampler) being quoted as
80 µg/m3. Only limited data on sulfur dioxide were available; the
mean of a series of 8-h samples for selected weeks was quoted as
0.01 ppm (30 µg/m3). On this occasion, the authors did not find any
appreciable differences in the prevalence of respiratory symptoms or
in measures of lung function, as compared with the 1973 report. The
interpretation of these findings is difficult, for the studies were
concerned largely with consecutive investigations of survivors of the
original group, over a 12-year period. Although the authors took into
account, as far as possible, the effect of selective losses of some of
the population, and changes in smoking habits among those who
remained, there is still some doubt as to whether the three sets of
results are truly comparable. The authors themselves concluded that
either the changes in air pollution levels from 1967 to 1973 (which
included a decline in total suspended particulates from about 130 to
80 µg/m3 (annual mean) with possibly a slight increase rather than
decrease in sulfur dioxide) were not associated with a beneficial
effect on health, or that their methods were not sufficiently
sensitive at the levels involved.
One of the difficulties in interpreting these data is that
exposure to odorous air pollutants (Berlin, NH) also occurred,
indicating an unconventional type of air pollution. It does seem
reasonable, however, to interpret the results of these surveys as
showing that slight changes in respiratory symptoms and pulmonary
function were related to levels of pollution. Sulfur oxides and
particulate matter may have been of importance. Only sulfation data
are available for sulfur oxides in the 1961 study.
Extensive studies have been made of post office and telephone
workers in the United Kingdom and USA (Holland & Reid, 1965; Holland &
Stone, 1965; Holland et al., 1965). The authors carefully considered
most of the relevant epidemiological variables and showed a gradation
of symptoms across the levels of pollution, particularly in the 50 to
59-year-old category. However, since pollution was measured in
different ways in each part of the studies, it is difficult to deduce
any quantitative relationships with sulfur dioxide and particulate
matter. The differences in the prevalence of symptoms persisted when
the authors examined the various smoking categories. They converted
the small amount of pipe and cigar smoking to cigarette equivalents,
which is not advisable, since other studies have indicated that pipe
and cigar smoking are less markedly associated with respiratory
symptoms. Lower levels of FEV1.0 and PEFR were observed in the areas
of higher pollution. The authors indicated that a difference in height
of 2-4 cm between the two populations studied could not account for
the differences seen in pulmonary function. Presumably, these
pulmonary function values had been corrected for standard temperature
and saturated vapour pressure. If not, some of the differences between
values in the USA and United Kingdom could be explained by the fact
that lower temperatures in England could have resulted in lower
measured air volumes. Similar spirometers were used in both studies.
In another study in the United Kingdom, Lambert & Reid (1970)
questioned about 10 000 adults by post. They estimated that their
sample represented about 74% of those able to reply. The positive
responses from the questionnaire (which had been recommended by the
British Medical Research Council) were correlated with levels of
pollution estimated from data used earlier by Douglas & Waller (1966)
and from some data from the National Air Pollution Survey. The
responses were controlled for social class and cigarette smoking. The
authors reported that whereas nonsmokers showed little response to the
levels of air pollution, cigarette smokers did respond and appeared to
be more sensitive. They also noted a considerable rural/urban
gradient, more pronounced in men than in women, that could not be
explained by differences in smoking habits.
Extensive studies were carried out in the Ruhr area of the Federal
Republic of Germany (Reichel et al., 1970; Ulmer et al., 1970) based
on a short questionnaire on respiratory symptoms, physical
examinations, and measurements of airways resistance using a body
plethysmograph. There were no clear differences in the results between
areas with different levels of pollution, but because of selection
factors and a low response rate, no definite conclusions can be drawn
from these studies regarding relationships with sulfur dioxide and
particulate matter.
A study in Vlaardingen in the Netherlands by Van der Lende (1969)
indicated that increased cough and phlegm production were associated
with air pollution but decreased lung function was not. However, the
author considered that the study might have been affected by the
presence of allergens, such as spores and bacteria in the agricultural
regions. Comparisons were made between an industrial area, polluted by
sulfur dioxide and particulate matter, and an agricultural area with
little pollution. In a later report, Van der Lende et al. (1973)
indicated that the effect of pollution could have been masked by
"self-selection" of the populations concerned.
The relationship between chronic bronchitis and air pollution was
studied in Osaka Prefecture, Japan, by Tsunetoshi et al. (1971).
Surveys were made of all persons over 40 years of age in selected
areas using self-administered questionnaires similar to that
recommended by the British Medical Research Council. The authors'
definition of chronic bronchitis was cough with sputum for 3 or more
months, for at least 2 successive years. Pulmonary function was
measured by the Vitalor, using the maximum value. An equation was
developed relating prevalence of bronchitis to age, smoking, and
sulfation rate (lead candle). The prevalence of bronchitis in the
study areas ranged from about 4% (in males aged 40-59 years) in areas
where the sulfation rate was close to 1 mg/100 cm2 per day to about
10% in those around 3 mg/100 cm2 per day. No data were given
concerning the levels of particulate matter.
Many other studies on the prevalence of respiratory symptoms in
relation to air pollution have been carried out in Japan such as that
by Toyama et al. (1966). They reported prevalences ranging from 2.8 to
3.7% (males aged 40-59 years, adjusted for age and smoking to accord
with other Japanese studies) in areas of Kashima (a nonindustrialized
rural town, at that time) with sulfur dioxide concentrations of less
than 30 µg/m3 (0.01 ppm, automatic conductimetric method) and
suspended particulate concentrations (high-volume sample) of
106-341 µg/m3 (mean 197). An extensive survey in Tokyo, (Suzuki &
Hitosugi, 1970, unpublished data)a showed a higher prevalence of
chronic bronchitis in areas that were more polluted, ranging from 5.5%
(males aged 40-59 years) and 1.1% (females 40-59 years) where the
sulfur dioxide concentration was below 60 µg/m3 (0.02 ppm, automatic
conductimetric method), and the suspended particulate level was below
100 µg/m3 (light-scattering method), to 6.7% (males) and 3.9%
(females), where sulfur dioxide was over 140 µg/m3 (0.05 ppm) and
suspended particulates more than 200 µg/m3. There was also some
increase in prevalence in an intermediate area with sulfur dioxide
concentrations of 60-140 µg/m3 (0.02-0.05 ppm) and a suspended
particulate concentration of 100-200 µg/m3. Smoking habits were
standardized in this study. In a recent study by Tani (1975) in the
vicinity of a pulp mill at Fuji City, and in control areas, there
appeared to be a consistent relationship between the prevalence of
bronchitis and sulfation rates, as measured by lead candles, with a
prevalence of about 3% (males and females combined, aged 40-59 years)
in areas where the sulfation rate was around 0.6 mg/100 cm2 per day
to about 8% where it was 1.2 mg/100 cm2 per day.
In studies on a random sample of adults in Cracow, Polandm the
levels of pollution measured made it possible to classify the subjects
into high and low exposure groups (Sawicki, 1972). The levels in the
high pollution area were an annual mean smoke level of 170 µg/m3 and
an annual mean sulfur dioxide level of 125 µg/m3 (0.04 ppm). In the
low pollution area, the standard smoke annual mean was 90 µg/m3 and
the sulfur dioxide annual mean was 45 µg/m3 (0.02 ppm). Persons
a Suzuki, T. & Hitosugi, M. Prevalence study of pulmonary symptoms
in Tokyo Prefecture employees. A paper presented at a meeting of a
Working Group on Air Pollution and Health, USA-Japan Cooperative
Science Programme, East-West Center, Honolulu, 21-23 April, 1970. The
pollution levels quoted in this study were obtained from reports of
the Tokyo Metropolitan Government concerning air pollution levels for
the years 1966-69 (Department of Environmental Pollution Control
Tokyo, 1976).
residing in the more polluted area had more respiratory symptoms and
poorer pulmonary function than those residing in the area with lower
pollution (chronic bronchitis 19% in more polluted area and 11% in
less polluted area and asthmatic disease 11% in more polluted area and
5% in less polluted area). Sawicki considered that there was a
synergistic interaction with cigarette smoking. He also pointed out
that many of the inhabitants worked outside the area in which they
lived and, therefore, the exposure levels at their places of residence
might not represent their true exposures.
8.3.5 Morbidity in children
Studies of the health of children have also provided useful
information.
Douglas & Waller (1966) performed a cohort study based on a
national sample of children born in the United Kingdom in the first
week of March 1946. The children were followed medically for 15 years
by health visitors and school doctors. The study was restricted to the
3131 children who remained at the same address for the first 11 years
of the enquiry, or who moved to an area that was in a similar
pollution group. Levels of air pollution were estimated from the
amount of coal consumed in a given area where each child lived, and it
was shown at the end of the study that the 4 pollution categories that
had been defined provided a satisfactory gradation in terms of the
measurements of smoke and sulfur dioxide that were then available. In
each of the several periods of life considered, from birth onwards,
the authors noted a consistent relationship between the frequency of
lower respiratory tract infections and pollution category. There was
no sharp change at any particular pollution level, but if the rates
within the lowest category (mainly rural areas) were taken as a
baseline, then increased frequencies were seen in the next category
up, with smoke and sulfur dioxide each of the order of 140 µg/m3
(0.05 ppm sulfur dioxide) or more. Annual means were estimates based
on the British daily smoke/sulfur dioxide method for a period after
the end of the study and probably underestimated earlier level.
Socioeconomic status was important in the study, but a relationship
between air pollution and frequency of lower respiratory tract
infections still existed within separate social classes. In a later
follow-up of these subjects, Colley et al. (1973) showed that, at the
age of 20 years, respiratory symptoms were related to smoking habits
rather than to the pollution of the areas in which the subjects were
then living. However, there was some relationship between the
prevalence of symptoms and earlier histories of lower respiratory
tract infections, which, in turn, were related to estimated pollution
exposures during childhood.
Lunn et al. (1967) studied 819 children in their first year at
school in the industrial city of Sheffield, in the United Kingdom.
Medical examinations were carried out on the children, the parents
were questioned concerning the previous health of the children, and
FEV0.75 and FVC were measured. Various socioeconomic factors were also
carefully considered. The families were stable, and there had been
little or no migration into or out of the specific communities.
Measurements of pollution were made at the schools attended by the
children (British daily smoke/sulfur dioxide method). The authors
found increased frequencies of both upper and lower respiratory tract
infections in the more polluted areas. When the "cleanest" area was
taken as the baseline, most of the illness indices considered showed
an increase in the next area up in order of pollution levels, i.e.,
where the annual mean concentrations of smoke and sulfur dioxide were
each about 200 µg/m3 (0.07 ppm sulfur dioxide) taking figures for the
middle year of the survey. A follow-up study was carried out on some
of these children when they were 4 years older (i.e., aged 9) (Lunn et
al., 1970). By then, the implementation of the Clean Air Act had
reduced the contrast in pollution between the areas, so that the mean
concentration of smoke in the 3 "dirty" areas combined was about
140 µg/m3 and the mean sulfur dioxide concentration was 200 µg/m3
(0.07 ppm), compared with about 50 and 100 µg/m3 (0.04 ppm sulfur
dioxide), respectively, in the "clean" area. At this time, there was
no significant difference in the illnesses reported by the 9-year-olds
in the different areas and the authors concluded that this was in line
with the reduced pollution levels.
Data were collected (Holland et al., 1969) concerning about 11 000
children attending school in 4 areas of Kent, England, 2 of which were
predominantly urban, and 2, rural. As part of the children's regular
medical examinations, peak expiratory flow rates, height, weight, and
the results of examinations of ears and tonsils were recorded. Smoking
habits and school absences were also recorded. Questionnaires
concerning such factors as previous respiratory diseases, father's
occupation, and size of family were completed by the parents. Smoke
and sulfur dioxide were measured in 3 of the areas (British daily
smoke/sulfur dioxide method) and information on population density and
housing was collected. Four factors emerged as important in relation
to decreased peak expiratory flow rates. Place of residence was most
important followed by previous history of respiratory disease; family
size and social class were of least importance. These 4 factors seemed
to be additive but they only accounted for 10-15% of the total
variation. Levels of air pollution for smoke were reported to range
from 34 to 69 µg/m3 in the 3 communities. Values for sulfur dioxide
were not reported but were stated to parallel those for smoke. There
were clearly other factors affecting area difference, and there is
some doubt as to whether the small contrasts in pollution could have
had much effect.
Colley & Reid (1970) surveyed about 11 000 children from 6 to 10
years of age, living in urban and rural areas of England and Wales.
The prevalence of respiratory disease was assessed in the autumn.
Pollution was assessed in terms of sulfur dioxide concentration, from
some direct measurements (British daily smoke/sulfur dioxide method)
coupled with estimates based on lead candle measurements of sulfation.
Within the English areas studied (winter mean concentrations of sulfur
dioxide ranging from about 30 to 150 µg/m3 or 0.01-0.05 ppm) there
was a gradient in the prevalence of symptoms, but the rates were much
higher in Wales for comparable levels of pollution. The reasons for
this difference were not clear, although it has been suggested that it
could be related to the fact that solid-fuel consumption was high in
Wales.
Respiratory function measurements were made on children aged 10-11
years from 2 different areas of the German Democratic Republic (Berlin
and Bitterfeld) with different levels of pollution (Grosser et al.,
1971). The groups were matched for social class, age, and height. In
the low pollution area, the concentration of respirable dust (method
not mentioned) was 110 µg/m3 and that of sulfur dioxide (method not
mentioned) was 50 µg/m3 (0.02 ppm); in the other area, the figures
were 290 and 360 µg/m3 (0.13 ppm), respectively (based on 24-h
measurements, averaged for July and December). Two studies were made,
6 months apart, in each of the 2 areas. Each of the spirometric values
studied (FVC, FEV1.0, FEV0.7) was higher in the area with lower
pollution.
A variety of other techniques has been used to investigate
possible effects of exposure to pollution. These include the work of
Yoshii et al. (1969) who noted an association between chronic
pharyngitis accompanied by histopathological changes at biopsy and
level of sulfation, expressed as the annual mean, in persons attending
their clinic in Yokkaichi, Japan, and in sixth-grade children. In the
heavily polluted districts sulfation rate was more than 1.0 mg/100
cm2 per day, in moderately polluted districts it ranged from 0.25 to
1.0 mg/100 cm2 per day, and in the control area it was less than this
level (lead candle measurements).
Conjunctivitis, both acute and chronic, has been reported in
Zabrze, Poland, in relation to industrial air pollution (Maziarka &
Moroz, 1968). In Czechoslovakia, Schmidt et al. (1966) reported
differences in the blood cells, tonsils, and cervical lymph nodes of
children living in a highly polluted atmosphere compared with those
living in a relatively clean atmosphere and Symon et al. (1969)
reported retardation in growth and ossification and a reduced colour
index in red blood cells in children living in areas with high air
levels of sulfur dioxide (up to 2-3 mg/m3) and fly ash. None of these
studies can be used for the exposure-effect relationship.
8.3.6 CHESS studies
The US Environmental Protection Agency has recognized the need for
studies at relatively low levels of air pollution in order to
determine whether a no-effect level can be identified to develop a
dose-response relationship and to monitor areas where there might be a
change in the levels of exposure. The CHESS (Community Health and
Environmental Surveillance System) studies were developed with these
purposes in mind. In some aspects these studies have been well
designed and executed but in other aspects there have been
difficulties. In many ways these are preliminary studies that need to
be continued. The studies have included adults, children, asthmatic
subjects, and patients with cardiovascular and pulmonary diseases.
Both acute and chronic effects have been studied. Methods have
involved mailed questionnaires on respiratory symptoms and illnesses
(adults and children), mailed questionnaires on family composition,
housing, and socioeconomic status, telephone interviews, tests of
pulmonary function (children), and diary techniques with patients.
Previous exposures to air pollutants were estimated from
historical data on emissions or known production figures from the
local industries and the application of mathematical models. More
recent exposures were based on actual measurements. These were
generally conducted at monitoring stations (each of which covered a
radius of up to 2 km), and involved high volume sampling for total
suspended particulates and suspended sulfates, and the West-Gaeke
method for sulfur dioxide. Mathematical models were used to estimate
the variation in exposure of the different groups. Various problems
discussed in a summary of the results (US Environmental Protection
Agency, 1974) include poor response rates (i.e., low participation
rates) in some of the studies on children, high dropout rates in
studies on patients, and, in at least one of the studies on children,
the fact that their spirometry was probably in error.
There was a general tendency for the authors to over-interpret the
CHESS data. In some of the studies on children, the results were
grouped according to high and low levels of pollution and a difference
was found between the two groups. However, the relationship of the
prevalence of disease to levels of pollution was not clear -- namely,
when the individual groups were examined, some exposed to the high
levels of pollution had a lower disease prevalence than some of the
groups exposed to low levels of pollution.
The design for the study of the impact on respiratory disease was
excellent and took into account family composition, housing, and
socioeconomic status. It is unfortunate that these studies were not
continued for more than one year of observation before reporting. A
number of years of observations are needed to rule out natural
fluctuations in respiratory disease.
These studies have been reviewed by an expert committee in the USA
under the chairmanship of Dr D. Rall (Director, National Institute of
Environmental Health Sciences, US Department of Health, Education and
Welfare) (Rall, 1974). The Committee's evaluation of the studies was
similar to that of the Task Group and the Committee concluded that the
results of the studies did not warrant any change in the present US
Federal Air Quality standards.
It was the opinion of the WHO Task Group that these data could not
be used for the estimation of an exposure-effect relationship.
8.3.7 Lung cancer and air pollution
The possibility that air pollution is a causal factor in cancer of
the lung has given rise to considerable concern. The evidence in
favour of a causal relationship is briefly: (a) the excess
occurrence of the disease in urban areas; (b) the presence in the
suspended matter in urban air of substances such as benzo(a)pyrene
that can cause cancer under experimental conditions; and (c) the
general rise in lung cancer that appeared, at one time, to follow
certain assumed trends in pollution.
Early studies in the United Kingdom (Stocks, 1959, 1966) indicated
that variations in lung cancer mortality in urban areas were
associated with variations in amounts of pollution and, following a
recommendation by a WHO Study Group in 1959 (World Health
Organization, 1960), a pilot international study was undertaken in
several cities where there were contrasts in lung cancer death rates.
The results did not show any clear-cut relationship with measurements
of particulate matter or its benzo(a)pyrene content (Waller & Commins,
1967) and it was clear that apart from the difficulties of making
proper allowances for differences in smoking habits, it seemed likely
that present-day measurements of polycyclic hydrocarbons gave an
inadequate assessment of past exposures to these compounds.
The Royal College of Physicians of London (1970) reviewed the
issue, and concluded that the evidence against community air pollution
being a causal factor in lung cancer was stronger than the evidence
for it. The urban/rural differential is greatest in countries with
relatively low urban air pollution (Sweden, Norway, Denmark). The
upward trend in mortality as well as other experimental and
epidemiological evidence are best explained by the causal role of
cigarette smoking.
Nevertheless, in a review by Cleary (1967), evidence is presented
to show that, in Australia, New Zealand, South Africa, and the USA,
immigrants from the United Kingdom have a higher lung cancer death
rate than those born in these countries: immigrants from Norway have a
lower rate than native-born citizens of the USA, and the lung cancer
mortality rates for all these migrants are intermediate between those
of their countries of origin and destination, strongly suggesting an
environmental factor in early life.
While the existence of an urban excess of lung cancer has been
proved, it is uncertain that air pollution is the "urban factor"
responsible. In contrast, recent work incriminates cigarette smoking
more strongly than ever; there is also a contribution from some
specific occupational exposures (see footnote in section 1.1.7).
The consideration of criteria for environmental carcinogenesis
specifically in relation to any possible effects on lung cancer, is
outside the scope of the present discussion, but it may be mentioned
that any action to reduce smoke and especially that from the
inefficient combustion of coal in domestic fires, is likely to make
substantial reductions in the benzo(a)pyrene content of the air. Such
a change has already been noted in London, where the concentration of
this compound is now only about one-tenth of what it was 25 years ago
(Lawther & Waller, 1976). A reduction of sulfur dioxide may also be
important for any possible interactions relating to the production of
lung cancer. Some experimental studies (Kuschner & Laskin, 1971;
Skvorcova et al., 1973) showed an increased carcinogenic response when
laboratory animals (rats and hamsters) were exposed to sulfur dioxide
in addition to benzo(a)pyrene.
8.3.8 Annoyance
Annoyance may be defined as "a feeling of displeasure associated
with any agent or condition believed to affect adversely an individual
or a group". This definition was adopted by an international symposium
on annoyance in Stockholm in 1971 (Lindvall & Radford, 1973). Very few
studies have been performed that would make quantitative evaluations
of this effect possible.
The social awareness of pollution caused by particulate matter has
been studied in a few areas. The results from different studies have
been presented in a document on particulate matter (US Department of
Health, Education and Welfare, 1969a); they include those from a study
carried out in St. Louis (Schusky, 1966), where values for suspended
particulates of around 100 µg/m3 produced annoyance reactions from a
considerable number of people.
A similar study was carried out in Birmingham, Alabama, USA
(Stalker & Robinson, 1967), in which levels of air pollution were
correlated with annoyance. They found that, as dustfall reached or
exceeded 14.1 g/m2 per month, about one-half of the population
considered it to be a nuisance; at 10.5 g/m2 per month, about
one-third of the population considered air pollution a nuisance.
Stepwise multiple regression analysis showed that the variation due to
dust fall alone explained 49% during the spring season and 68% during
the winter season of the total association between the air pollutants
measured and public annoyance. The association between levels of
suspended particulate matter and public opinion, which was weaker than
that of dustfall, was strongest during the summer season (r = 0.59).
About one-half of the persons interviewed thought that air pollution
was a general nuisance, when mean annual or mean summer concentrations
of particulate matter reached 230 µg/m3 and one-third, when they
reached 150 µg/m3.
No such relationship was shown with sulfur dioxide concentrations.
However, these levels were low. Further studies are indicated, since
it may be that effects such as annoyance reactions will, in the
future, be the critical effects on which criteria, as regards the
protection of public health, will be based. Since annoyance reactions
have a large sociocultural component, these levels may vary from place
to place and should be determined for each locality. Surveys on
annoyance are fraught with many problems. When proper survey
techniques are expertly applied, however, it will be possible to
assess reactions in a quantitative manner.
8.4 Exposure-Effect Relationships
Some of the studies reported in this section can be used to
develop exposure-effect relationships. It has been necessary to
develop 2 tables, one for the effects of short-term exposures
(Table 15, p. 107) and another for the effects of long-term exposures
(Table 16). The results from the studies have been based on different
methods of measuring sulfur oxides and particulate matter. The values
for the sulfur oxides are treated as if they were comparable. For the
particulate matter, two categories are listed: smoke as measured by
the Organization for Economic Cooperation and Development or British
daily smoke/sulfur oxide methods and total suspended particulates as
measured by the high volume sampler or light scattering. These factors
should be considered in interpreting these tables.
Table 15. Exposure-effect relationships of sulfur dioxide, smoke, and total suspended
particulates: effects of short-term exposures
concentration
24-h mean
values (µg/m3)
Total
suspended
Sulfur dioxide Smoke particulates Effects
> 1000 > 1000 -- London, 1952. Very large increase in mortality to about 3
times normal, during 5-day fog. Pollution figures represent
means for whole area: maximum (central site) sulfur dioxide
3700 µg/m3, smoke 4500 µg/m3 (Ministry of Health, UK, (1954).
710 750 -- London, 1958-59. Increases in daily mortality up to about 1.25
times expected value (Lawther, 1963; Martin & Bradley, 1960).
500 500 -- London, 1968-60. Increases in daily mortality (as above) and
increases in hospital admissions, becoming evident when
pollution levels shown were exceeded (magnitude increasing
steadily with pollution) (Martin, 1964).
500 -- -- New York 1962-66. Mortality correlated with pollution: 2%
excess at level shown (Buechley, 1973).
500 250 -- London, 1954-68. Increases in illness score by diary technique
among bronchitic patients seen above pollution levels shown
(means for whole area) (Lawther et al., 1970).
Table 15 (cont'd).
concentration
24-h mean
values (µg/m3)
Total
suspended
Sulfur dioxide Smoke particulates Effects
300 140 -- Vlaardingen, Netherlands, 1969-72. Temporary decrease in
ventilatory function (Van der Lende et al., 1975).
200a -- 150b Cumberland, WV, USA. Increased asthma attack rate among
small group of patients, when pollution levels shown were
exceeded (Cohen et al., 1972).
a West-Gaeke method
b High volume sampling method
Other measurements by Organization for Economic Cooperation and Development or British daily smoke/sulfur
dioxide methods (Ministry of Technology, UK, 1966; Organization for Economic Cooperation and Development, 1965).
Table 16. Exposure-effect relationships of sulfur dioxide, smoke, and
total suspended particulates: effects of long-term exposures
Concentration
Annual means of 24-h
mean values (µg/m3)
Total
suspended
Sulfur dioxide Smoke particulates Effects
200 200 -- Sheffield, England. Increased
respiratory illnesses in children
(Lunn et al., 1967, 1970)
-- -- 180b Berlin, NH, USA. Increased respiratory
symptoms, decreased respiratory
function in adults (Ferris et al., 1973)
150 -- -- England & Wales. Increased respiratory
symptoms in children (Colley & Reid, 1970)
125 170 -- Cracow, Poland. Increased respiratory
symptoms in adults (Sawicki, 1972)
140d 140d -- Great Britain. Increased lower
respiratory tract illnesses in
children (Douglas & Waller, 1966)
60-140a -- 100-200c Tokyo, Increased respiratory symptoms
in adults (Suzuki & Hitosugi, unpublished
data, 1970)
a Automatic conductimetric method
b High volume sampler (2-month mean, possible underestimation of annual mean).
c Light-scattering method, results not directly comparable with others.
d Estimates based on observations after end of study; probable underestimation
of exposures in early years of study.
Other measurements by Organization for Economic Cooperation and Development or
British daily smoke/sulfur dioxide methods (Ministry of Technology, UK, 1966;
Organization for Economic Cooperation and Development, 1965).
9. EVALUATION OF HEALTH RISKS FROM EXPOSURE TO SULFUR OXIDES, SMOKE,
AND SUSPENDED PARTICULATE MATTER
It is well established that respiratory diseases are important
causes of disability and death, and that, for some of them, there is
evidence of association with environmental factors in the ambient air.
There is evidence that exposure to mixtures of urban air pollutants
containing sulfur oxides and particulate matter is related to a
variety of adverse effects on health, even when other factors are
controlled (section 8 -- Tables 15 and 16). Although in most of the
studies considered the levels of air pollution have been expressed in
terms of sulfur dioxide, smoke, or suspended particulate matter, this
does not necessarily imply that these are the causative agents. They
provide only indices of pollution, and certain components, such as
sulfuric acid or sulfates, may be of particular importance.
Measurements of some of these components have now been made in a
number of areas and used in some of the more recent epidemiological
studies. The Task Group concluded, however, that, at present, there
are not enough data on any of these other indices of pollution to
allow exposure-response relationships to be established. Thus, the
present discussion is confined to the effects of pollution expressed
in terms of sulfur dioxide, smoke, and total suspended particulate
matter.
9.1 Exposure Levels
Concentrations of sulfur dioxide, smoke, and suspended particulate
matter vary greatly from place to place and from time to time. Many
factors including sources, topography, and weather conditions may be
involved in these variations.
An annual arithmetic mean of sulfur dioxide concentrations in
urban areas typically ranges from 100-200 µg/m3 (0.035-0.070 ppm),
whereas a maximum daily mean ranges from 300-900 µg/m3
(0.11-0.32 ppm). For smoke, an annual arithmetic mean ranges from
30-200 µg/m3 with a maximum daily mean of 150-900 µg/m3; for total
suspended particulates, these levels range from 60-500 µg/m3 and
150-1000 µg/m3, respectively.
Comparatively little information is available concerning indoor
concentrations of sulfur dioxide and particulate matter, though, in
general, these levels are known to be lower than those outdoors,
except at work places.
Levels in working environments are considerably higher than
general community levels and are thought to have been much higher in
the past.
In evaluating exposure levels that have been used in the past in
connexion with epidemiological studies, a serious question arises as
to how far these measurements, often intended primarily for control or
monitoring purposes, can be considered as providing adequate measures
of exposure. As discussed in section 5, it must be recognized that all
the figures quoted in subsequent sections are based on measurements
that have been made outdoors, usually at only a limited and not wholly
representative set of fixed sites, though most people spend much time
indoors, where concentrations may differ substantially from those
outside. However, this does not necessarily invalidate these data
which are serving primarily as indices.
So far, there has been little information on the particle size
distribution and the chemical composition of selected particle size
ranges that would make the nature of exposure more understandable.
Comparable assessment is often difficult because of differences in
the methods of measurement of particulate matter that have been used
in the health effect studies. Smoke, assessed in terms of blackness,
is a measure of pollution associated with the incomplete combustion of
fuel, and total suspended particulates, determined by weight, is a
wider concept that includes all material which, by virtue of its
particle size, remains in suspension for long periods.
9.2 Experimental Animal Studies
Laboratory studies have been performed using a variety of test
animals, of periods of exposure, of experimental designs in exposure,
and of combinations of pollutants and other agents.
Some of these studies have provided useful information on the
mechanisms of the biological action of sulfur dioxide, sulfuric acid
mist, or particulate matter. However, the results are of limited value
in developing guidelines for the protection of human health from
effects of these pollutants as they exist in urban areas, and for this
reason it is necessary to turn to the available epidemiological
evidence.
9.3 Controlled Studies in Man
Effects studied in volunteers under controlled conditions include
those on the functions of the respiratory system, sensory organs, and
cerebral cortex. Durations of exposure have been very short, usually
less than a few hours.
Slight effects on pulmonary function were observed with exposure
to sulfur dioxide at a concentration of 2100 µg/m3 (0.75 ppm), but
not with exposure to a concentration of 1100 µg/m3 (0.37 ppm).
With exposure to sulfuric acid, a decrease in tidal volume was
found at a concentration as low as 350 µg/m3.
Combined exposures to sulfur dioxide and ozone or hydrogen
peroxide produced a greater effect on respiratory function than
exposure to each compound alone.
Studies on sensory and cerebral cortical functions showed that
sulfuric acid was more toxic than sulfur dioxide and that a
combination of these two substances produced an approximately additive
effect.
There is a limit to the use of these studies for the development
of criteria for community exposures, particularly when long-term
exposure to complex mixtures of air pollutants is involved.
9.4 Effects of Industrial Exposures
Effects on the health of workers have been studied in relation to
exposure to sulfur dioxide, particulate matter, or sulfuric acid mist
arising from various manufacturing processes. In many of these
studies, exposure levels were relatively high and, in some studies,
adverse effects were detected only at a daily mean sulfur dioxide
concentration as high as 70 000 µg/m3 (25 ppm).
It has also been reported that exposure to sulfuric acid at a mean
concentration of 1400 µg/m3 during working hours for 2 days did not
produce any effect on lung function.
The fact that adverse effects have not been reported at
comparatively high levels of sulfur dioxide or surfuric acid aerosols
may well be explained by the influence of biasing factors, such as
that the workers remaining in jobs with exposures to pollutants
consist of people especially resistant to their effects. Therefore, it
should not be considered an indication that such concentrations are
without effects for the average working population and particularly
not for workers with pre-existing pulmonary diseases.
In evaluating the effects from industrial exposures, due attention
must also be given to the variation in chemical composition and size
distribution of particulate matter.
9.5 Effects of Community Exposures
Many of the epidemiological studies in relation to community
exposures that were considered by a WHO Expert Committee in 1972
(World Health Organization, 1972), and which must still be relied on
to a large extent today, were based on the measurement of smoke rather
than of total suspended particulates. However, some new data on the
effects of total suspended particulates have become available and have
been included in Tables 15 and 16 (section 8).
Tables 17 and 18 show the levels above which some effects on
health might be expected among specified populations for short-term
and long-term exposures, respectively. These are based on the critical
evaluation of the results of studies reviewed in section 8.
In developing Table 17, greater emphasis had to be placed on some
earlier results related to major effects seen when concentrations of
pollution were much higher than those commonly experienced today. With
the effective control of at least some components in areas where
pollution was, at one time, very high, most of the more recent studies
have failed to isolate the effects of the pollutants in question from
effects similar in nature but arising from other factors.
The figures for sulfur dioxide and smoke are essentially the same
as those proposed by the Expert Committee in 1972 (World Health
Organization, 1972) the only change being the adoption of 250 µg/m3
(0.09 ppm) instead of 250-500 µg/m3 (0.09-0.18 ppm) as the level at
which the worsening of the condition of patients from short-term
exposures to sulfur dioxide might be expected. It was recognized that
the magnitude of responses at this level appeared to be small and
difficult to separate from effects due to other factors such as
weather or infections. It is possible that a lower figure for smoke
could now be adopted, but, in view of uncertainties concerning the
differences in composition of this component of pollution from one
area to another, and the changes with time even within one locality,
it was felt that any further modification of the figure determined on
the basis of the older studies from London would need new evidence.
Table 18 represents an overall assessment of the effects of
long-term exposure to sulfur dioxide and smoke in terms of increases
in the prevalence of respiratory symptoms among both adults and
children, and in terms of the increased frequency of acute respiratory
illnesses, that has been demonstrated particularly among children.
Table 17. Expected effects of air pollutants on health in selected segments of
the population: effects of short-term exposuresa
24-h mean concentration (µg/m3)
Expected effects Sulfur dioxide Smoke
Excess mortality among the elderly
or the chronically sick 500 500
Worsening of the condition of patients
with existing respiratory disease 250 250
a Concentrations of sulfur dioxide and smoke as measured by OECD or British daily
smoke/sulfur dioxide method (Ministry of Technology, UK, 1966; Organization for
Economic Cooperation and Development, 1965). These values may have to be adjusted
in terms of measurements made by other procedures.
Table 18. Expected effects of air pollutants on health in selected segments of
the population: effects of long-term exposuresa
Annual mean concentration (µg/m3)
Expected effects Sulfur dioxide Smoke
Increased respiratory symptoms among
samples of the general population 100 100
(adults and children) and increased
frequencies of respiratory illnesses
among children
a Concentrations of sulfur dioxide and smoke as measured by OECD or British daily
smoke/sulfur dioxide method (Ministry of Technology, UK, 1966; Organization for
Economic Cooperation and Developments, 1965). These values may have to be adjusted
in terms of measurements made by other procedures.
For each pollutant there remains much uncertainty about the
minimum levels associated with demonstrable effects. Where populations
exposed to different levels of pollution have been compared, it cannot
necessarily be assumed that even those who were exposed to a level
lower than the known lowest effect level are entirely unaffected by
pollution. There must also be doubts as to whether the effects
observed in some studies were due in part to other pollutants, or to
socioeconomic or other factors that had not been adjusted for
completely.
No firm conclusion was reached on the effects of total suspended
particulates as a component of air pollution, together with sulfur
dioxide, because of the limited amount of information available. For
the effects of long-term exposure, a tentative figure of 150 µg/m3
(annual arithmetic mean) was suggested, based on the 2 entries in
Table 16, recognising that one of these was based on light-scattering
observations, and the other on high volume sampler measurements, but
for a 2-month period only. It was noted that the one study on the
effects of short-term exposure to total suspended particulates had
been included in Table 15, but it was felt that this could not provide
satisfactory information for an assessment of these effects.
The figures in Table 18 have been expressed in terms of annual
arithmetic mean concentrations, although it is not known whether the
effects are related to extended exposures to these average levels, or
more particularly to days of high pollution within each series. In
view of the limited quantitative information on effects of pollution
in terms of annoyance, this aspect has been omitted from Tables 17 and
18.
9.6 Guidelines for the Protection of Public Health
Tables 17 and 18 present two different sets of criteria, one
relating to effects of short-term exposure, in terms of 24-h average
concentrations, and the other to effects of long-term exposure in
terms of annual means. These effects may be interrelated; the gradual
development of respiratory symptoms may, for example, be a reaction to
repeated short-term exposure to peak 24-h values, or even to transient
peaks lasting for still shorter periods, but in the absence of any
substantial evidence on this point, the two criteria must, for the
time being, be considered separately.
With the present state of knowledge, it was considered that a
safety factor of two below the figures given in Tables 17 and 18 would
be reasonable to ensure the protection of public health, and,
accordingly, Table 19 was developed, still considering the effects of
short-term and long-term exposures separately. As an indication of the
uncertainty surrounding these estimates, the figures have further been
expressed with a range of ± 20%.
The values proposed in Table 19 are in general agreement with
those suggested as long-term goals in the earlier report (World Health
Organization, 1972). In the case of sulfur dioxide, there has been
some reduction in the 24-h figure (if this is regarded as a level not
to be exceeded on more than 7 days a year), and this is in line with
the revision of the "effect" level from a range of 250 to 500 µg/m3
in the 1972 report, to the present figure of 250 µg/m3 (Table 17).
Table 19 requires careful interpretation, for none of the figures
can be considered as absolute limits. In the first place, day-to-day
variations in the concentration of smoke and sulfur dioxide are
determined largely by weather conditions, and occasional peaks far
beyond the usual daily values may well occur, even with careful
control of emissions.
Table 19. Guidelines for exposure limits consistent with
the protection of public healtha
Concentration (µg/m3)
Sulfur dioxide Smoke
24-h mean 100-150 100-150
Annual arithmetic mean 40-60 40-60
a Values for sulfur dioxide and smoke as measured by OECD or
British daily smoke/sulfur dioxide method (Ministry of
Technology, UK, 1966; Organization for Economic Cooperation
and Development, 1965). Adjustments may be necessary where
measurements are made by other methods.
Although there are two sets of conditions specified in Table 19,
determined independently from evidence on the effects of short- and
long-term exposures, they can generally be considered to be consistent
with one another. If the proportion of days with 24-h values above
those in Table 19 is small (e.g., of the order of 7 days per year),
then the annual means may well fall within or below the ranges
specified in the second row of the table. Annual means are specified
here in terms of arithmetic means: the corresponding geometric means
would generally be a little lower (see section 5).
Much consideration was given to the possibility of extending
Table 19 to include guidelines for total suspended particulates as
measured by the high volume sampler but it was concluded that the
available evidence on the effects associated with exposure to
suspended particulate matter was highly unsatisfactory.
The Task Group felt, however, that some recommendations for a
guideline should be made. A tentative annual mean value of 150 µg/m3
has been suggested in section 9.5 as a level beyond which effects of
long-term exposure to total suspended particulates might be observed.
Applying a safety factor of two and introducing a ± 20% range, as in
the case of smoke and sulfur dioxide, would then provide a range of
levels from 60 to 90 µg/m3 as a possible guideline.
For short-term exposures, no satisfactory, direct evidence
relating concentrations of total suspended particulates to effects is
available. Because of this, a guideline for short-term exposure levels
can only be inferred. Assuming that the same ratio of 24-h mean
concentrations to the annual mean (each derived independently, see
beginning of section) given in the guidelines for smoke, is applicable
to suspended particulate matter, then a very approximate 24-h
guideline for suspended particulate matter, as measured by the high
volume sampler, would be in the order of 150 to 230 µg/m3.
While the Group felt that it was reasonable and prudent to
consider the above figures as interim guidelines consistent with the
protection of public health, it stressed the very urgent need for
additional information on the effects of exposure to suspended
particulate matter (measured by the high-volume sampler). Furthermore,
it recognized the fact that the toxicological significance of total
suspended particulates might vary depending on their chemical
composition and particle size, and that under certain circumstances,
the suggested guidelines might need to be reconsidered. It should be
noted that the discussion above does not imply any preference for
smoke measurements over those of total suspended particulates; indeed
it is highly desirable to develop more appropriate methods for the
measurement of suspended particulates, especially those limited to the
measurement of respirable particles.
It was recognized that in many urban and industrial areas existing
levels of pollution by sulfur dioxide and suspended particulate matter
were substantially above these guidelines. Furthermore, there was the
problem that the long-distance transport of these pollutants from
major sources could, in some circumstances, result in comparatively
high background concentrations in rural areas, and high levels in the
incoming air in towns striving to meet their own air quality
standards. It was considered, however, that every effort should be
made to develop control procedures that would allow these guidelines
to be met.
These guidelines are based on observations among populations in
the community exposed to a mixture of sulfur dioxide and smoke or
total suspended particulates and they may not apply to situations
where only one of the components is present. On grounds of prudence,
however, it is recommended that the levels of each pollutant should be
below the values stated. It should be stressed again, however, that
the data on which the guidelines are based are uncertain and each of
the guidelines is tentative and subject to review when further
information becomes available.
It was the opinion of the Group that there is not yet sufficient
information available on the effects of community exposures to
sulfuric acid aerosols or suspended sulphates to develop guidelines
for these air pollutants.
REFERENCES
ABELES, F. B., CRAKER, K. E., FORRENCE, L. E., & LEATHER, G. R. (1971)
Fate of air pollutants: removal of ethylene, sulfur dioxide and
nitrogen dioxide by soil. Science, 173: 914-916.
ADAMS, D. F., FALGOUT, D., FROHLINGER, J. O., PATE, J. B., PLUMLEY, A.
L., SCARINGELLI, F. P., & URONE, P. (1971) Tentative method of
analysis for sulfur dioxide content of the atmosphere (manual
conductimetric method). Health Sci. Lab., 8: 42-47.
ALARIE Y., ULRICH, C. E., BUSEY, W. M., SWANN, H. E., & MACFARLAND, H.
N. (1970) Long-term continuous exposure of guinea pigs to sulfur
dioxide. Arch. environ. Health, 21: 769-777.
ALARIE, Y., ULRICH, C. E., BUSEY, W. M., KRUMM, A. A., & MACFARLAND,
H. N. (1972) Long-term continuous exposure to sulfur dioxide in
cynomolgus monkeys. Arch. environ. Health, 24: 115-128.
ALARIE, Y., BUSEY, W. M., KRUMM, A. A., & ULRICH, C. E. (1973)
Long-term continuous exposure to sulfuric acid mist in cynomolgus
monkeys and guinea pigs. Arch. environ. Health, 27: 16-24.
ALARIE, Y., KRUMM, A. A., BUSEY, W. M., ULRICH, C. E., & KANTZ, R. J.
II. (1975) Long-term exposure to sulfur dioxide, sulfuric acid
mist, fly ash and their mixtures. Results of studies in monkeys
and guinea pigs. Arch. environ. Health, 30: 254-262.
ALBERT, R. E., SPIEGELMAN, J., LIPPMANN, M., & BENNET, R. (1968) The
characteristics of bronchial clearance in the miniature donkey.
Arch. environ. Health, 17: 50-58.
ALLEN, E. R., MCQUIGG, R. D., & CADLE, R. D. (1972) The
photo-oxidation of gaseous sulfur dioxide in the air.
Chemosphere, 1: 23-32.
ALTSHULLER, A. P. (1973) Atmospheric sulfur dioxide and sulfate:
distribution of concentrations at urban and non-urban sites in the
United States. Environ. Sci. Technol., 7: 709-712.
AMDUR, M. O. (1957) The influence of aerosols upon the respiratory
response of guinea pigs to sulfur dioxide. Am. Ind. Hyg. Assoc.
Q., 18: 149-155.
AMDUR, M. O. (1958) The respiratory response of guinea pigs to
sulfuric acid mist. Am. Med. Assoc. Arch. ind. Health,
18: 407-414.
AMDUR, M. O. (1961) Report on tentative ambient air standards for
sulfur dioxide and sulfuric acid. Ann. occup. Hyg., 3: 71-83.
AMDUR, M. O. (1966) Respiratory absorption data and SO2 dose-response
curves. Arch. environ. Health, 12: 729-732.
AMDUR, M. O. (1969) Toxicological appraisal of particulate matter,
oxides of sulfur and sulfuric acid. J. Air Pollut. Control
Assoc., 19: 638-644.
AMDUR, M. O. (1970) The impact of air pollutants on physiological
responses of the respiratory tract. Proc. Am. Philos. Soc.,
114: 3-8.
AMDUR, M. O. (1971) Aerosols formed by oxidation of sulfur dioxide.
Review of their toxicology. Arch. environ. Health, 23: 459-468.
AMDUR, M. O. & UNDERHILL, D. (1968) The effect of various aerosols on
the response of guinea pigs to sulfur dioxide. Arch. environ.
Health, 16: 460-468.
AMDUR, M. O., SCHULZ, R. Z., & DRINKER, P. (1952a) Toxicity of
sulfuric acid mist to guinea pigs. Am. Med. Assoc. ind. Hyg.
occup. Med., 5: 318-329.
AMDUR, M. O., SILVERMAN, L., & DRINKER, P. (1952b) Inhalation of
sulphuric acid mist by human subjects. Am. Med. Assoc. Arch. ind.
Hyg. occup. Med., 6: 305-313.
AMDUR, M. O., MELVIN, W. W., Jr, & DRINKER, P. (1953) Effects of
inhalation of sulfur dioxide by man. Lancet, 2: 758-759.
AMERICAN CONFERENCE OF GOVERNMENT INDUSTRIAL HYGIENISTS (1977)
TLVs threshold limit values for chemical substances and physical
agents in the workroom environment with intended changes for
1977, Cincinnati, OH, ACGIH, p. 28.
AMERICAN SOCIETY FOR TESTING AND MATERIALS (1964) Standard test
method for particulate matter in the atmosphere. Optical density
of filtered deposit. ASTM standards on methods of atmospheric
sampling and analysis, Philadelphia, USA, ASTM, pp. 661-668.
ANDERSEN, I. (1972) Relationships between outdoor and indoor air
pollution. Atmos. Environ. 6: 275-278.
ANDERSEN, I., LUNDQVIST, G. R., JENSEN, P. L., & PROCTOR, D. F. (1974)
Human response to controlled levels of sulfur dioxide. Arch.
environ. Health, 28: 31-39.
ANDERSON, A. (1950) Possible long-term effects of exposure to sulfur
dioxide. Br. J. ind. Med., 7: 82-86.
ASH, R. & LYNCH, J. (1972) The evaluation of gas detector tube
systems: sulfur dioxide. Am. Ind. Hyg. Assoc. J., 32: 490-491.
AZAR, A., SNEE, R. D., & HABIBI, K. (1972) Relationship of community
levels of air lead and indices of lead absorption. In:
Proceedings of the International Symposium on Environmental
Aspects of Lead, Luxembourg, Commission of the European
Communities, pp. 581-594.
BALCHUM, O. J., DYBICKI, J., & MENECLY, G. R. (1959) Absorption and
distribution of 35SO2 inhaled through the nose and mouth by
dogs. Am. J. Physiol., 197: 1317-1321.
BALCHUM, O. J., DYBICKI, J., & MENECLY, G. R (1960a) The dynamics of
sulfur dioxide inhalation, absorption, distribution and retention.
Arch. ind. Health, 21: 564-569.
BALCHUM, O. J., DYBICKI, J., & MENECLY, G. R. (1960b) Pulmonary
resistance and compliance with concurrent radioactive sulfur
distribution in dogs breathing 35SO2. J. appl. Physiol.,
15: 62-66.
BALL, D. J. & HUME, R. (1977) The relative importance of vehicular and
domestic emissions of dark smoke in Greater London in the mid
1970s, the significance of smoke shade measurements, and an
explanation of the relationship of smoke shade to gravimetric
measurements of particulate. Atmos. Environ., 2: 1065-1073.
BARRIE, L. A. & GEORGII, H. W. (1976) An experimental investigation of
the absorption of sulfur dioxide by water drops containing heavy
metal ions. Atmos. Environ., 10: 743-749.
BATES, D. V. & HAZUCHA, M. (1973) The short-term effects of ozone on
the human lung. Proceedings of the Conference on Health Effects of
Air Pollution, NAS-NRC, Washington, DC, Oct. 3-5. In: Committee
Print, Committee on Public Works, United States Senate, US Govt.
Printing Office, pp. 507-540.
BATTIGELLI, M. C., COLE, H. M., FRASER, D. A., & MAH, R. A. (1969)
Long-term effect of sulfur dioxide and graphite dust on rats.
Arch. environ. Health, 18: 602-608.
BENSON, E. B., HENDERSON, J. J., & CALDWELL, D. E. (1972)
Indoor-outdoor air pollution relationships: a literature review,
Research Triangle Park, NC, US Environmental Protection Agency,
83 pp. (Publ. No. AP 112).
BIERSTEKER, K. (1966) [Polluted air causes, epidemiological
significance and prevention of atmospheric pollution.] Assen,
Netherlands, Van Gorcum & Co., pp. 21-23 (in Dutch).
BIERSTEKER, K., DE GRAAF, H., & NASS, C. A. G. (1965) Indoor air
pollution in Rotterdam homes. Int. J. Air Water Pollut.,
9: 343-350.
BOSANQUET, C. H. (1957) The rise of a hot waste gas plume. J. Inst.
Fuel, 30: 322-328.
BRAIN, J. D. & VALBERG, P. A. (1974) Models of lung retention based
ion ICRP Task Group Report. Arch. environ. Health, 28: 1-11.
BRIGGS, G. A. (1965) A plume rise model compared with observations.
J. Air Pollut. Control Assoc., 15: 433-438.
BRITISH STANDARDS INSTITUTION (1969a) Methods for the measurement of
air pollution. BS1747 Part 4. The lead dioxide method.
London, BSI, 16 pp.
BRITISH STANDARDS INSTITUTION (1969b) Methods for the measurement of
air pollution. BS1747 Part 1. Deposit gauges. London, BSI,
15 pp.
BROSSET, C. (1973) Air-borne acid. Ambio, 2: 2-9.
BUECHLEY, R. W., RIGGAN, W. B., HASSELBLAD, V., & VAN BRUGGEN, J. B.
(1973) SO2 levels and perturbations in mortality -- a study in
the New York/New Jersey metropolis. Arch. environ. Health,
27: 134-137.
BUFALINI, M. (1971) Oxidation of SO2 in polluted atmospheres -- a
review. Environ. Sci. Technol., 5: 685-703.
BURROWS, B., KELLOG, A. L., & BUSKEY, J. (1968) Relationships of
symptoms of chronic bronchitis and emphysema to weather and air
pollution. Arch. environ. Health, 16: 406-413.
BURTON, G. G., CORN, M., GEE, J. B. L., VASALLO, C., & THOMAS, A. P.
(1969) Response of healthy men to inhaled low concentration of
gas-aerosol mixtures. Arch. environ. Health, 18: 681-692.
BUSTUEVA, K. A. (1957) [On the toxicology of sulfuric acid aerosol.]
Gig. i Sanit., No. 2, pp. 17-22 (in Russian).
BUSTUEVA, K. A. (1961a) Experimental data on the effect of small
concentration of sulfur oxides, maximum permissible concentration
of air pollutants, Moscow, Medgiz, pp. 126-141.
BUSTUEVA, K. A. (1961b) Threshold reflex effect of SO2 and H2SO4
aerosols simultaneously present in the air. In: Ryazanov, V. A.,
ed. Limits of allowable concentrations of atmospheric pollutants,
Book 4, Washington DC, US Department of Commerce, Office of
Technical Services, pp. 92-101.
BUSTUEVA, K. A. (1964) [Resorptive action of sulfur oxides.] Gig. i
Sanit., No. 10, pp. 8-12 (in Russian).
BUSTUEVA, K. A. (1966) The toxicity of continuous exposure to sulfur
oxides. Biological action and hygienic significance of
atmospheric pollution, Moscow, Medgiz, pp. 142-172.
BUSTUEVA, K. A., POLEZAEV, E.G., & SEMENENKO, A.D. (1960)
[Electroencephalographic determination of threshold reflex effect
of atmospheric pollutants.] Gig. i Sanit., 25: 57-61
(in Russian).
BYSTROVA, T. A. (1957) [Some effects of sulfurous gas determined by
the method of tracer atoms.] Gig. i Sanit., 5: 30-37
(in Russian).
CALVERT, J. G. (1973) Interaction of air pollutants. In: Proceedings
of the Conference on the Health Effects of Air Pollution,
Washington, DC, US Government Printing Office, pp. 19-101
(Serial No. 93-15).
CAMNER, P. & PHILIPSON, K. (1972) Tracheobronchial clearance in
smoking discordant twins. Arch. environ. Health, 25: 60-63.
CAMNER, P., JARSTRAND, C., & PHILIPSON, K. (1973a) Tracheobronchial
clearance in patients with influenza. Am. Rev. respir. Dis.,
108: 131-135.
CAMNER, P., MOSSBERG, B., & PHILIPSON, K. (1973b) Tracheobronchial
clearance and chronic obstructive lung disease. Scand. J. respir.
Dis., 54: 272-281.
CARABINE, M.D. & MADDOCK, J. E. L. (1976) The growth of sulfuric acid
aerosol particles when contacted with water vapour. Atmos.
Environ., 10: 735-742.
CARNOW, B. W., LEPPER, M. H., SHEKELLE, R. B., & STAMLER, J. (1969)
Chicago air pollution study: SO2 levels and acute illness in
patients with chronic bronchopulmonary disease. Arch. environ.
Health,. 18: 768-776.
CARSON, G. A.. & PAULUS, H. J. (1974) A high volume cascade sieve
impactor. Am. Ind. Hyg. Assoc. J., 35: 262-268.
CHARLSON, R. J., VANDERPOL, A. H., COVERT, D. S., WAGGONER, A. P., &
AHLQVIST, N. C. (1973) Sulfuric acid-ammonium sulfate aerosol:
Optical detection in the St Louis region. Science, 184: 156-158.
CHUN, K. C. & QUON, J. E. (1973) Capacity of ferric oxide particles to
oxidize sulfur dioxide in air. Environ. Sci. Technol.,
7: 532-538.
CLEARY, G. J. (1967) Some arguments suggesting a causative
relationship between air pollutants and lung cancer. Clean Air,
September: 15-18.
COHEN, A. A., BROMBERG, S., BUECHLEY, R. W., HEIDERSCHEIT, L. T., &
SHY, C. M. (1972) Asthma and air pollution from a coal-fuelled
power plant. Am. J. public Health, 62: 1181-1188.
COHEN, A. A., NELSON, C. J., BROMBERG, S. M., PRAVDA, M., FERRAND, E.
F., & LEONE, G. (1974) Symptom reporting during recent publicized
and unpublicized air pollution episodes. Am. J. public Health,
64: 442-449.
COLLEGE OF GENERAL PRACTITIONERS (1961) Chronic bronchitis in Great
Britain: A national survey carried out by the respiratory diseases
study group of the College of General Practitioners. Br. med. J.,
2: 973-979.
COLLEY, J. R. T. & REID, D. D. (1970) Urban and social origins of
childhood bronchitis in England and Wales. Br. med. J.,
2: 213-217.
COLLEY, J. R. T., DOUGLAS, J. W. B., & REID, D. D. (1973) Respiratory
disease in young adults: influence of early childhood lower
respiratory tract illness, social class, air pollution and
smoking. Br. med. J., 3: 195-198.
COMMINS, B. T. (1963) Determination of particulate acid in town air.
Analyst, 88: 364-367.
COMMINS, B. T. (1967) Some studies of the particulate acid sulfate
from the products of combustion of fuels and measurement of the
acid in polluted atmospheres. In: Proceedings of the Symposium on
the Transformation of Sulfur Compounds, Mainz, Germany,
7-9 June, Paris, OECD, pp. 39-46.
COMMINS, B. T. & LAWTHER, P. J. (1958) Volatility of 3,4-benzpyrene in
relation to the collection of smoke samples. Br. J. Cancer,
12: 351-354.
COMMINS, B. T. & WALLER, R. E. (1967) Observations from a 10-year
study of pollution at a site in the city of London. Atmos.
Environ., 1: 49-68.
COMMINS, B. T. & WALLER, R. E. (unpublished) Long-term trends in
pollution in central London. Atmos. Environ.
COMMISSION OF THE EUROPEAN COMMUNITIES (1976) Air sulfur dioxide
concentrations in the European community, Luxembourg, CEC,
pp. 34-51 (Report EUR 5417e).
COMMITTEE ON AIR POLLUTION (1954) Report, London, Her Majesty's
Stationery Office, pp. 7-25 (Report Cmd 9322).
COMMITTEE ON THE CHALLENGES OF MODERN SOCIETY (1971) Air pollution.
Air quality criteria for sulfur oxides. NATO/CCMS, No. 7,
pp. 1-1 to 8-85.
CORN, M., KOTSKO, N., STANTON, D., BELL, W., & THOMAS, A. P. (1972)
Response of cats to inhaled mixtures of SO2 and SO2-NaCl aerosol
in air. Arch. environ. Health, 24: 248-256.
COX, R. A. & PENKETT, S. A. (1970) The photo-oxidation of sulfur
dioxide in sunlight. Atmos. Environ., 4: 425-433.
COX, R. A. & PENKETT, S. A. (1971) Oxidation of atmospheric SO2 by
products of the ozone-olefin reaction. Nature (Lond.),
230: 321-322.
CUMMINGS, W. G. & REDFEARN, M. W. (1957)Instruments for measuring
small quantities of sulfur dioxide in the atmosphere. J. Inst.
Fuel, 30: 628-635.
DALHAMN, T. & STRANDBERG, L. (1961) Acute effect of sulfur dioxide on
the rate of ciliary beat in the trachea of rabbit, in vivo and
in vitro, with studies on the absorptional capacity of the nasal
cavity. Int. J. Air Water Pollut., 4: 154-167.
DALY, C. (1954) Air pollution and bronchitis. Br. med. J.,
2: 687-688.
DALY, C. (1959) Air pollution and causes of death. Br. J. prev. soc.
Med., 13: 14-27.
DE GRAAF, H. & BIERSTEKER, K. (1972) Comparison of smoke concentration
outside and inside two selected sites. Atmos. Environ., 6: 697.
DEPARTMENT OF ENVIRONMENTAL POLLUTION CONTROL, Tokyo (1976) Report of
air pollution measurements. Tokyo, Continuous Measurement
Stations, Tokyo Metropolitan Government, 119 pp.
DEROUANE, A. (1972) Comparaison des concentrations en fumées à
l'extérieur et à l'interiéur des lieux d'habitation. Atmos.
Environ., 6: 209-220.
DEROUANE, A., GRANDJEAN, L., LEGRAND, M., & VERDUYN, G. (1972)
Atmospheric pollution by oxides of sulfur and smoke in Belgium,
1969-70. Inst. R. Met. Belg., Publ. A. No. 78: 1-54.
DERRETT, C. J. & BROWN, C. (in press) A continuous running
direct-reading SO2 recorder. J. Phys. E. Sci. Instrum., 11:
DOHAN, F. C. (1961) Air pollutants and incidence of respiratory
disease. Arch. environ. Health, 3: 387-395.
DOHAN, F. C. & TAYLOR, E. W. (1960) Air pollution and respiratory
disease: a preliminary report. Am. J. med. Sci., 240: 337-339.
DORSCH, R. (1913) [The pollution of the air in the storage battery
room and its surroundings by sulfuric acid.] Unpublished
dissertation, Wurzberg, Germany (in German). Quoted in: Lewis, T.
R., ed. Toxicology of atmospheric sulfur dioxide decay products,
Research Triangle Park, NC, US Environmental Protection Agency,
p. 19 (Report No. AP111).
DOUGLAS, J. W. B. & WALLER, R. E. (1966) Air pollution and respiratory
infection in children. Br. J. prev. soc. Med., 20 (1): 1-8.
DZUBAY, T. G. & STEVENS, R. K. (1973) Ambient air analysis with
dictomous sampler and X-ray fluorescence spectrometer. Environ.
Sci. Technol., 9: 663-668.
EAST, C. (1972) Sulfur dioxide emissions estimated from airborne
measurements. Atmos. Environ., 6: 399-408.
ELFIMOVA, E. V. & HACATURJAN, M. K. (1968) [Features specific to the
reflex action of sub-threshold concentrations of sulfurous
anhydride in combustion with phenol and carbon monoxide in the
atmosphere.] Gig. i Sanit., 33 (11): 3-6 (in Russian).
ELFIMOVA, E. V. & GUSEV, M. I. (1969) [Health effects of low sulfur
dioxide concentrations in air.] Gig. i Sanit., 34 (2): 3-7
(in Russian).
ELKINS, H. (1959) The chemistry of industrial toxicology. New York,
John Wiley, pp. 396-397
ELLIOTT, L. P. & ROWE, D. R. (1976) Air quality during public
gatherings. J. Air Pollut. Control Assoc'., 25: 635-636.
ELLISON, J. M. (1965) The nature of air pollution and the methods
available for measuring it. Bull. World Health Organ.,
32: 399-409.
EMERSON, P. A. (1973) Air pollution, atmospheric conditions and
chronic airways obstruction. J. occup. Med., 15: 635-638
FERIN, J. & LEACH, L. J. (1973) The effect of SO2 on lung clearance
of TiO2 particles in rats. Am. Ind. Hyg. Assoc. J.,
34: 260-263.
FERRIS, B. G., Jr & ANDERSON, D. O. (1962) The prevalence of chronic
respiratory disease in a New Hampshire town. Am. Rev. respir.
Dis., 86: 165-185.
FERRIS, B. G., Jr & ANDERSON, D. O. (1964) Epidemiological studies
related to air pollution: A comparison of Berlin, NH and
Chilliwack, British Columbia. Proc. R. Soc. Med.,
57 (Suppl.): 979-983
FERRIS, B. G., Jr, BURGESS, W. A., & WORCESTER, J. (1967) Prevalence
of chronic respiratory disease in a pulp mill and a paper mill in
the United States. Br. J. ind. Med., 24: 26-37.
FERRIS, B. G., Jr, HIGGINS, I. T. T., HIGGINS, M. W., & PETERS, J. M.
(1973) Chronic nonspecific respiratory disease in Berlin, New
Hampshire 1961-1967. A follow-up study. Am. Rev. respir. Dis.,
107: 110-122.
FERRIS, B. G., Jr, CHEN, H., PULEO, S., & MURPHY, L. H., Jr (1976)
Chronic nonspecific respiratory disease in Berlin, New Hampshire,
1967 to 1973. A further follow-up study. Amer. Rev. resp. Dis.,
113: 475-485.
FIRKET, H. (1931) Sur les causes des accidents survenue dans la vallée
de la Meuse, lors des brouillards de Décembre 1930. Bull. R.
Acad. Med. Belg., 11: 683-739.
FORTAK, H. G. (1970) Numerical simulation of the temporal and spatial
distributions of urban air pollution concentrations. In:
Proceedings of a Symposium on Multiple Source Urban Diffusion
Models. Research Triangle Park, NC, US Environmental Protection
Agency.
FRANK, N. R. & SPEIZER, F. E. (1965) SO2 effects on the respiratory
system in dogs. Arch. environ. Health, 11: 624-634.
FRANK, N. R., AMDUR, M. O., WORCESTER, J., & WHITTENBERGER, J. L.
(1962) Effects of acute controlled exposure to SO2 on respiratory
mechanics in healthy male adults J. appl. Physiol., 17: 252-258.
FRANK, N. R., AMDUR, M, O., & WHITTENBERGER, J. L. (1964) A comparison
of the acute effects of SO2 administered alone or in combination
with NaCl particles on the respiratory mechanics of healthy
adults. Int. J. Air Water Pollut., 8: 125-133.
FRANK, N. R., YODER, R. E., YOKOYAMA, E., & SPEIZER, F. E. (1967) The
diffusion of 35SO2 from tissue fluids into the lungs following
exposure of dogs to 35SO2. Health Phys., 13: 31-38.
FRANK, N. R., YODER, R. E., BRAIN, J. D., & YOKOYAMA, E. (1969) SO2
(35S labelled) absorption by the nose and mouth under conditions
of varying concentration and flow. Arch. environ. Health,
18: 315-322.
FRASER, D. A., BATTIGELLI, M. C., & COLE, H. M. (1968) Ciliary
activity and pulmonary retention of inhaled dust in rats exposed
to sulfur dioxide. J. Air Pollut. Control Assoc., 18: 821-823.
FREIBERG, J. (1975) The mechanism of iron catalyzed oxidation of SO2
in oxygenated solutions. Atmos. Environ., 9: 661-672.
FUCHS, N. A. (1964) The mechanics of aerosols, Oxford, Pergamon
Press, p. 28.
FUGAS, M. (1976) Assessment of total exposure to an air pollutant. In:
International Conference on Environmental Sensing and Assessment,
Las Vegas, September 14-19, 1975, New York, IEEE, Vol. 2,
paper 38-5. pp. 1-3.
GARDNER, M. J., CRAWFORD, M. D., & MORRIS, J. N. (1969) Patterns of
mortality in middle and early old age in the county boroughs of
England and Wales. Br. J. prev. soc. Med., 23: 133-140.
GARLAND, J. A., CLOUGH, W. S., & FOWLER, D. (1973) Deposition of
sulfur dioxide on grass. Nature (Lond.). 242: 256-257.
GARLAND, J. A., ATKINS, D. H. F., READINGS, C. J., & CAUGHEY, S. J.
(1974) Deposition of gaseous sulfur dioxide to the ground. Atmos.
Environ., 8: 75-79.
GOLDRING, I. P., GREENBURG, L., PARK, S.S., & RATNER, I. M. (1970)
Pulmonary effects of sulfur dioxide exposure in the Syrian
hamster. Arch. environ. Health, 21: 32-37.
GOLDSMITH, J. R. (1969) Los Angeles smog. Sci. J., 5: 44-49.
GORE, A. T. & SHADDICK, C. W. (1958) Atmospheric pollution and
mortality in the County of London. Br. J. prev. soc. Med.,
12: 104-113.
GREY, D.C. & JENSEN, M. L. (1972) Bacteriogenic sulfur in air
pollution. Science, 177: 1099-1100.
GROSSER, P. J., STARK, C., JECH, J., & MEHLHORN, H. (1971) [Vital
capacity and respiration tests of school children of the 4th and
5th grade in areas with different air quality situations.]
Zeitschr. Erkr. Atmungsorgane, 134: 255-265 (in German).
GRUBER, C. W. & ALPAUGH, E. L. (1954) The automatic filter paper
sampler in an air pollution measurement programme. J. Air Pollut.
Control Assoc., 4: 143-147.
HATCH, T. & GROSS, P. (1964) Pulmonary deposition and retention of
inhaled aerosols, New York, Academic Press.
HEMEON, W. C. L., HAINES, G. F., Jr, & IDE, H. M. (1953) Determination
of haze and smoke concentrations by filter paper samplers. J. Air
Pollut. Control Assoc., 3: 22-28.
HENDERSON, J. J., BENSON, F. B., & CALDWELL, D. E. (1973)
Indoor-outdoor air pollution relationships. Vol. II. An annotated
bibliography. Washington, DC, US EPA (Publ. No. AP 112b).
HILL, A. C. (1971) Vegetation: a sink for atmospheric pollutants.
J. Air Pollut. Control Assoc., 21: 341-346.
HOBBS, P. V., HARRISON, H., & ROBINSON, E. (1974) Atmospheric effects
of pollutants. Science, 183: 909-915.
HOEGG, U. R. (1972) Cigarette smoke in closed places. Environ. Health
Perspect., October, No. 2: 117-128.
HOLBROW, G. L. (1958) Crystalline bloom, Teddington, UK, Paint
Research Station, p. 4 (Tech. Paper No. 208).
HOLLAND, W. W. & REID, D. D. (1965) The urban factor on chronic
bronchitis. Lancet, 1: 445-448.
HOLLAND, W. W. & STONE, R. W. (1965) Respiratory disorders in United
States East Coast telephone men. Am. J. Epidemiol., 82: 92-101.
HOLLAND, W. W., REID, D. D., SELTSER, R., & STONE, R. W. (1965)
Respiratory disease in England and the United States: Studies of
comparative prevalence. Arch. environ. Health, 10: 338-343.
HOLLAND, W. W., HALIL, T., BENNETT, A. E., & ELLIOTT, A. (1969)
Factors influencing the onset of chronic respiratory disease.
Br. med. J., 2: 205-208.
HOLLAND, W. W., BENNETT, A. E., CAMERON, I. R., FLOREY, C DU V.,
LEEDER, S. R., SCHILLING, R. S. F., SWAN, A. V, & WALLER, R. E.
(in press) Health effects of particulate pollution. Reappraising
the evidence. Am. J. Epidemiol.
HOLLOWELL, C. D., GEE, G. Y., & McLAUGHLIN, R. D. (1973) Current
instrumentation for continuous monitoring for SO2. Anal. Chem.,
45: 63A-72A.
HORVATH, H. & CHARLSON, R. J. (1969) The direct optical measurement of
atmospheric air pollution. Am. Ind. Hyg. Assoc. J., 30: 500-509.
HUHTI, E., RYHANEN, P., VUOPALA, U., & TAKKANEN, J. (1970) Chronic
respiratory disease among pulp mill workers in an arctic area in
Northern Finland. Acta Med. Scand., 187: 433-444.
HUSAR, J. D., HUSAR, R. B., & STUBITS, P. K. (1975) Determination of
submicrogram amounts of atmospheric particulate sulfur. Anal.
Chem., 47: 2062-2065.
HUSAR, R. B. (1974) Atmospheric particulate mass monitoring with a
radiation detector. Atmos. Environ., 8: 183-188.
INTERNATIONAL ATOMIC ENERGY AGENCY (1978) Particle size analysis in
estimating the significance of airborne contamination, Vienna,
International Atomic Energy Agency, pp. 71-170 (Technical Report
Series, No. 179).
ILO/WHO COMMITTEE ON OCCUPATIONAL HEALTH (1970) Permissible levels of
toxic substances in the working environment. Geneva, International
Labour Office, p. 337 (Occupational Safety and Health Series).
IPSEN, J., DEANE, M., & INGENITO, F. E. (1969) Relationships of acute
respiratory disease to atmospheric pollution and meteorological
conditions. Arch. environ. Health, 18: 462-472.
JACOBSEN, M. (1972) Sampling and evaluating respirable coal mine dust.
Ann. NY Acad. Sci., 200: 661-665.
JARSTRAND, C., CAMNER, P., & PHILIPSON, K. (1974) Mycoplasma
pneumoniae and tracheobronchial clearance. Am. Rev. respir. Dis.,
110: 415-419.
JOHNSTONE, H. F. & COUGHANOWR, D. R. (1958) Absorption of SO2 from
air. Oxidation in drops containing dissolved catalyst. Ind. eng.
Chem., 50: 1169-1172.
JUNGE, C. E. & RYAN, T. G. (1958) Study of the SO2 oxidation in
solution and its role in atmospheric chemistry. Q. J. R. Med.
Soc., 84: 46-55.
KATZ, M. (1969) Measurement of air pollutants, guide to the selection
of methods, Geneva, WHO, 123 pp.
KEHOE, R. A., MACHLE, W. H., KITZMILLER, K., & LEBLANC, T. J. (1932)
On the effects of prolonged exposure to sulfur dioxide. J. ind.
Hyg., 14: 159-173.
KELLOGG, W. W., CADLE, R. D., ALLEN, E. R., LAZRUS, A. Z., & MARTELL,
E. A. (1972) The sulfur cycle. Science, 175: 587-596.
KRUSHNER, M. & LASKIN, S. (1971) Experimental models in environmental
carcinogenesis. Am. J. Pathol., 64: 183-196.
LAMBERT P. M. & REID, D. D. (1970) Smoking, air pollution and
bronchitis in Britain. Lancet, 25 April 1970: 853-857.
LARSEN, R. I. (1971) A mathematical model for relating air quality
measurements to air quality standards, Research Triangle Park,
NC, US Environmental Protection Agency, 61 pp. (Rpt No. AP-89).
LAUER, G. & BENSON, F. B. (1975) The CHAMP air quality monitoring
programme. In: Proceedings of the International Symposium on
Recent Advances in the Assessment of the Health Effects of
Environmental Pollution, Paris, 24-28 June 1974, Luxembourg,
Commission of the European Communities, Vol. 1, pp. 423-430.
LAUTERBACH, K. E., MERCER, T. T., HAYES, A.D., & MARROW, P. E. (1954)
Efficiency studies of the electrostatic precipitator. Arch. ind.
Hyg., 9: 69-75.
LAVE, L. B. & SESKIN, E. P. (1970) Air pollution and human health.
Science, 169: 723-733.
LAVE, L. B. & SESKIN, E. P. (1972) Air pollution, climate, and home
heating: their effects on US mortality rates. Am. J. public
Health, 62: 909-916.
LAWTHER, P. J. (1963) Compliance with the clean air act: medical
aspects. J. Inst. Fuel, 36: 341-344.
LAWTHER, P. J. (1955) Effects of inhalation of sulfur dioxide on
respiration and pulse-rate in normal subjects. Lancet,
2: 745-748.
LAWTHER, P. J. & WALLER, R. E. (1976) Coal fires, industrial emissions
and motor vehicles as sources of environmental carcinogens. In:
Proceedings of Symposium on Environmental Pollution and
Carcinogenic risks, Lyons, 3-5 November 1975, Paris, INSERM,
Vol. 52, pp. 27-40.
LAWTHER, P. J., WALLER, R. E., & HENDERSON, M. (1970) Air pollution
and exacerbations of bronchitis. Thorax, 25: 525-539.
LAWTHER, P. J., MACFARLANE, A. J., WALLER, R. E., & BROOKS, A. G. F.
(1975) Pulmonary function and sulfur dioxide: some preliminary
findings. Environ. Res., 10: 355-367.
LEBOWITZ, M.D., TOYAMA, T., & MCCARROLL, J. (1973) The relationship
between air pollution and weather as stimuli and daily mortality
as responses in Tokyo, Japan, with comparisons with other cities.
Environ. Res., 6: 327-333.
LEE, R. E., Jr & GORANSON, S. (1972) Cascade impactor network,
Research Triangle Park, NC, US Environmental Protection Agency,
132 pp. (Rep. No. AP108).
LEE, R. E., CALDWELL, J. S., & MORGAN, G. B. (1972) The evaluation of
methods for measuring suspended particulates in air. Atmos.
Environ., 6: 593-622.
LEWIS, T. R., MOORMAN, W. J., LUDMAN, W. F., & CAMPBELL, K. I. (1973)
Toxicity of long-term exposure to oxides of sulfur. Arch.
environ. Health, 26: 16-21.
LIKENS, G. E. & BORMANN, F. H. (1974) Acid rain: a serious regional
environmental problem. Science, 184: 1176-1179.
LINDVALL, T. & RADFORD, E. P. (1973) Measurement of annoyance due to
exposure to environmental factors. Environ. Res., 6: 1-36.
LIPPMANN, M., ALBERT, R. E., & PETERSON, H. T., Jr (1971) The regional
deposition of inhaled aerosols in man. In: Walton, W. H., ed.
Inhaled Particles III, London, Unwin, Vol. 1, pp. 105-120.
LISS, P.S. (1971) Exchange of SO2 between the atmosphere and natural
waters. Nature (Lond.), 233: 327-329.
LIU, B. Y. H., BERGLUND, R. N., & AGARWAL, J. M. (1974) Experimental
studies of optical particle counters. Atmos. Environ.,
8: 717-732.
LOWE, C. R., PELMEAR, P. L., CAMPBELL, H, HITCHENS, R. A. N., KHOSLA,
T., & KING, T. C. (1968) Bronchitis in two integrated steel works.
I. Ventilatory capacity, age, and physique of non-bronchitic men.
Br. J. prev. soc. Med., 22: 1-11.
LOWE, C. R., CAMPBELL, H., & KHOSLA, T. (1970) Bronchitis in two
integrated steel works. III. Respiratory symptoms and ventilatory
capacity related to atmospheric pollution. Br. J. Ind. Med.,
27: 121-120.
LUNN, J. E., KNOWELDEN, J., & HANDYSIDE, A. J. (1967) Patterns of
respiratory illness in Sheffield infant school-children. Br. J.
prev. soc. Med., 21: 7-16.
LUNN, J. E., KNOWELDEN, J., & ROE, J. W. (1970) Patterns of
respiratory illness in Sheffield junior school-children -- A
follow-up study. Br. J. prev. soc. Med., 24: 223-228.
MADDALONE, R. F., MCCLURE, G. L., & WEST, P. W. (1975) Determination
of sulfate by thermal reduction of perimidylammonium sulfate.
Anal. Chem., 47: 316-322.
MALCOLM, D. & PAUL, E. (1961) Erosion of the teeth due to sulfuric
acid in the battery industry. Br. J. ind. Med., 18: 63-69.
MAMACASVILI, M. L (1968) Determination of the reflex action of SO2
and CO by adaptometer method and coloured vision. In: Biological
action and hygienic significance of atmospheric pollutants,
Moscow, Medgiz, pp. 179-188.
MARTIN, A. E. (1961) Epidemiological studies of atmospheric pollution:
A review of British methodology. Mon. Bull. Minist. Health Public
Health Lab. Serv., 20: 42-49.
MARTIN, A. E. (1964) Mortality and morbidity statistics and air
pollution. Proc. R. Soc. Med., 57: 969-975.
MARTIN, A. E. & BRADLEY, W. H. (1960) Mortality, fog and atmospheric
pollution. An investigation during the winter of 1958-59. Mon.
Bull. Minist. Health Public Health Lab. Serv., 19: 56-72.
MASTERS, B. R. (1974) The city of London and clean air. Clean Air,
4: 22-26.
MATSUMURA, Y. (1970a) The effects of ozone, nitrogen dioxide and
sulfur dioxide on the experimentally induced allergic respiratory
disorder in guinea pigs. I. The effect on sensitization with
albumin through the airway. Am. Rev. respir. Dis., 102: 430-437.
MATSUMURA, Y. (1970b) The effects of ozone, nitrogen dioxide and
sulfur dioxide on the experimentally induced allergic respiratory
disorder in guinea pigs. III. The effect on the occurrence of
dyspneic attacks. Am. Rev. respir. Dis., 102: 444-447.
MAWDESLEY-THOMAS, L. E., HEALEY, P., & BARRY, D. H. (1971)
Experimental bronchitis in animals due to sulfur dioxide and
cigarette smoke. In: WALTON, W. H., ed. Inhaled Particles III,
London, Unwin, Vol. 1, pp. 504-525.
MAZIARKA, S. & MROZ, E. (1968) [The influence of air pollution on the
protective apparatus of the eye in school children.] Rocz. PZH,
19: (1) 31-36 (in Polish).
MCJILTON, C., FRANK, R., & CHARLSON, R. (1973) Role of relative
humidity in the synergistic effect of a sulfur dioxide-aerosol
mixture on the lung. Science, 182: 503-504.
MCKAY, H. A. C. (1971) The atmospheric oxidation of SO2 in water
droplets in presence of NH3. Atmos. Environ., 5: 7-14.
MERCER, T. T. (1967) On the role of particle size in the dissolution
of lung burdens. Health Phys., 13: 1211-1222.
MINISTRY OF HEALTH, UNITED KINGDOM (1954) Mortality and morbidity
during the London fog of December 1952, London, HMSO (Report on
Public Health and Medical Subjects No. 95).
MINISTRY OF TECHNOLOGY, UK (1966) National survey of smoke and sulfur
dioxide: Instruction manual, Stevenage, Warren Spring
Laboratory, 142 pp.
MISIAKIEWICZ, Z. (1970) The effects on rat organism of prolonged
exposure to low concentrations of sulfur dioxide in the air.
J. Natl Inst. Hyg. (Warsaw), 21: 469-487.
MONTGOMERY, T. L. & COLEMAN, J. H. (1975) Empirical relationships
between time-averaged SO2 concentrations. Environ. Sci.
Technol., 9: 953-957.
MORROW, P. E. (1973) Alveolar clearance of aerosols. Arch. intern.
Med., 131: 101-108.
MUNN, R. E. (1976) Air pollution meteorology. Ch. 7 in Manual on
urban air quality measurement. Copenhagen, WHO (WHO Regional
publ. European Series, No. 1).
NASH, T. (1964) A "personal" measuring instrument for atmospheric
sulfur dioxide. Int. J. Air Water Pollut., 8: 121-124.
ORGANISATION FOR ECONOMIC CO-OPERATION AND DEVELOPMENT (1965) Methods
of measurement of air pollution, Paris, OECD, 94 pp.
PAN AMERICAN HEALTH ORGANIZATION (1976) Pan American air pollution
monitoring network (Redpanaire), Lima, Pan American Center for
Sanitary Engineering and Environmental Sciences, 50 pp.
(Pub. No. 30).
PASQUILL, F. (1971) Atmospheric dispersion of pollution. Q. J. R.
Med. Soc., 97: 369-395.
PATE, J. B., AMMONS, B. E., SWANSON, G. A., & LODGE, J.P. (1965)
Nitrite interference in spectrophotometric determination of
atmospheric sulfur dioxide. Anal. Chem. 37: 942-945.
PATTLE, R. E., BURGESS, F., & CULLUMBINE, H. (1956) The effects of a
cold environment and of ammonia on the toxicity of sulfuric acid
mist to guinea pigs. J. Pathol. Bacteriol., 72: 219-232.
PEMBERTON, J. & GOLDBERG, C. (1954) Air pollution and bronchitis.
Br. med. J., 2: 567-570.
POLLACK, R. I. (1975) Studies of pollutant concentration frequency
distributions, Washington DC, US Environmental Protection
Agency, 93 pp. (EPA-650/4-75-004).
PRINZ, B. (1970) Use of statistical methods in random sampling for the
preparation of plans for measurement of emissions. Staub,
30: 204-210.
RALL, D. P. (1974) Review of the health effects of sulfur oxides.
Environ. Health Perspect., 8: 97-121.
REICHEL, G., ULMER, W. T., GARY, K., LEUSCHNER, A., & ROSKE, G. (1970)
[Influence of the locally different grade of pollution in the
township of Duisburg on the incidence of non-specific respiratory
diseases. V. Communication.] Int. Arch. Arbeitsmed., 27: 110-129
(in German).
REID, D. D., ANDERSON, D. O., FERRIS, B. G., & FLETCHER, C. M. (1970)
An Anglo-American comparison of the prevalence of bronchitis.
Br. med. J., 2: 1487-1491.
ROBINSON, E. & ROBBINS, R. A. (1968) Sources, abundance and fate of
gaseous atmospheric pollutants. Menlo Park, CA, Stanford
Research Institute (Final Report, SRI project SCC-8501).
ROBINSON, E. & ROBBINS, R. A. (1972) Emissions, concentrations and
fate of gaseous atmospheric pollutants. In: Strauss, W., ed.
Air pollution control, II, New York, Wiley-Interscience,
pp. 1-93.
ROYAL COLLEGE OF PHYSICIANS, London (1970) Air pollution and health,
London, Pitman, pp. 48-57.
ROYAL MINISTRY OF FOREIGN AFFAIRS, SWEDEN (1971) Air pollution across
national boundaries. The impact on the environment of sulfur in
air and precipitation. Stockholm. Kungl. Bokytrckerict P.A.
Norsted & Söner 710396, 96 pp.
RJAZANOV, V. (1962) Sensory physiology as basis for air quality
standards. Arch. environ. Health, 5: 480-494.
RYLANDER, R. & BERGSTROM, R. (1973) Particles and SO2 -- synergistic
effects for pulmonary damage. In: Proceedings of the Third
International Clean Air Congress, Düsseldorf, 1973, Düsseldorf,
VDI Verlag, pp. A23-A25.
RYLANDER, R., OHRSTROM, M., HELLSTROM, P. A., & BERGSTROM, R. (1971)
SO2 and particles -- synergistic effects on guinea pig lungs. In:
Walton, W. H., ed. Inhaled Particles III, London, Unwin, Vol. 1,
pp. 535-540.
SALAMBERIDZE, O. P. (1969) [The joint action of small concentrations
of sulfur dioxide and nitrogen dioxide gases under conditions of a
chronic test.] Gig. i Sanit., 34(4): 10-14 (in Russian).
SAUCIER, J. Y. & SANSONE, E. B. (1972) The relationship between
transmittance and reflectance measurements of soiling index.
Atmos. Environ., 6: 37-43.
SAWICKI, F. (1972) Chronic non-specific respiratory diseases in
Cracow. Epidemiol. Rev., 26: 229-250.
SCARINGELLI, F. B., SALTZMAN, B. E., & FREG, S. A. (1967)
Spectrophotometric determination of atmospheric sulfur dioxide.
Anal. Chem., 39: 1709-1719.
SCHIMMEL, H. & GREENBERG, L. (1972) A study of the relation of
pollution to mortality, New York City, 1963-1968, J. Air Pollut.
Control Assoc., 22: 607-616.
SCHIMMEL, H. & MURAWSKI, T. J. (1975) SO2 -- Harmful pollutant or air
quality indicator? J. Air Pollut. Control Assoc., 25: 739-740.
SCHMIDT, P., PETR, B., & PICKO, V. (1966) [The indices of white blood
sequence, the tonsils and neck lymphatic nodules state as the
diagnostic criteria of subtle changes within the child's
organism.] Cesk. Hyg., 11: 473-478 (in Czech).
SCHRENK, H. H., HEIMANN, H., CLAYTON, G. O., GAFAER, W. M., & WEXLER,
H. (1949) Air pollution, Donora, Pennsylvania -- Epidemiology of
the unusual smog episode of October 1948. Public Health Bull.
(Fed. Sec. Agency, Washington DC). 306: 1-173.
SCHUSKY, J. (1966) Public awareness and concern with air pollution in
the St. Louis, metropolitan area. J. Air Pollut. Control Assoc.,
16: 72-76.
SHERWOOD, R. J. (1969) Miniature air samplers for sulfur dioxide.
Am. Ind. Hyg. Assoc. J., 30: 614-619.
SHERWOOD, R. J. & GREENHALGH, D. M. S. (1960) A personal air sampler.
Ann. occup. Hyg., 2: 127-132.
SIM, V. M. & PATTLE, R. E. (1957) Effect of possible smog irritants on
human subjects. J. Am. Med. Assoc., 165: 1918-1913.
SJORSTRAND, T. (1947) Changes in respiratory organs of workmen at an
ore smelting works. Acta Med. Scand., 196 (Suppl.): 687-699.
SKALPE, I. O. (1964) Long term effects of sulfur dioxide exposure in
pulp mills. Br. J. ind. Med., 21: 69-73.
SKVORCOVA, N. N., OSINCEVA, V. P., PUSKINA, N. N., & VYSOCINA, I. V.
(1973) [Role of some air pollutants in the genesis of lung tumour
provoked by chemical carcinogens. In: Prevention of environmental
pollution due to carcinogenic substances.], Moscow, Medicina,
pp. 61-64 (in Russian).
SNELL, R. E. & LUCHSINGER, P. C. (1969) Effects of sulfur dioxide on
expiratory flow rates and total respiratory resistance in normal
human subjects. Arch. environ. Health, 18: 693-698.
SOFOLUWE, G. O. (1968) Smoke pollution in dwellings of infants with
bronchopneumomia. Arch. environ. Health, 16: 670-672.
SPEDDING, D. J. (1972) Sulfur dioxide absorption by sea water. Atmos.
Environ., 6: 583-586.
SPEIZER, F. E. & FRANK, N. R. (1966a) The uptake and release of SO2
by the human nose. Arch. environ. Health, 12: 725-728.
SPEIZER, F. E. & FRANK, N. R. (1966b) A comparison of changes in
pulmonary flow resistance in healthy volunteers acutely exposed to
SO2 by mouth and nose. Br. J. ind. Med., 23: 75-79.
STALKER, W. W. & ROBINSON, C. B. (1967) A method for using air
pollution measurements and public opinion to establish ambient air
quality standards. J. Air Pollut. Control Assoc., 17: 142-144.
STOCKS, P. (1959) Cancer and bronchitis mortality in relation to
atmospheric deposit and smoke. Br. med. J., 1: 74-79.
STOCKS, P. (1966) Recent epidemiological studies of lung cancer
mortality, cigarette smoking and air pollution, with discussion of
a new hypothesis of causation. Br. J. Cancer, 20: 495-623.
STOCKS, P. (1967) Lung cancer and bronchitis in relation to cigarette
smoking and fuel consumption in twenty countries. Br. J. prev.
soc. Med., 21: 181-185.
STRANDBERG, L. G. (1964) SO2 absorption in the respiratory tract.
Arch. environ. Health, 9: 160-166.
STUART, B. O. (1973) Deposition of inhaled aerosols. Arch. intern.
Med., 131: 60-73.
SYMON, K., PETR, B., & KAPALIN, V. (1966) [The influence of pollution
caused by gaseous pollutants on the health of children.] Prakt.
Lek., 46: 19-22 (in Czech).
TANI, S. (1975) [Epidemiological study on chronic bronchitis.] Jpn.
J. public Health, 22: 431-438 (in Japanese).
TASK GROUP ON AIR POLLUTION AND CANCER (unpublished) Air pollution and
cancer. Risk assessment methodology and epidemiological evidence.
Environ. Health Perspect. (March, 1978.)
TASK GROUP ON LUNG DYNAMICS (1966) Deposition and retention models for
internal dosimetry of the human respiratory tract. Health Phys.,
12: 173-207.
TASK GROUP ON METAL ACCUMULATION (1973) Accumulation of toxic metals
with special reference to their absorption, excretion and
biological half-times. Environ. Physiol. Biochem., 3: 65-107.
TEN BRUGGEN CATE, H. J. (1968) Dental erosion in industry. Br. J.
ind. Med., 25: 249-266.
THOMAS, R. L., DHARMARAJAN, V., LUNDQUIST, G. L., & WEST, P. W. (1976)
Measurement of sulfuric acid aerosol, sulfur trioxide and the
total sulfate content of the ambient air. Anal. Chem.,
48: 639-642.
TOIGO, A., IMARISIO, J. J., MURMALL, H., & LEPPER, M. N. (1963)
Clearance of large carbon particles from the human
tracheobronchial tree. Am. Rev. respir. Dis., 87: 487-492.
TOMENIUS, L. (1973) A study on the role of deposition for
tracheobronchial clearance. Physiol. Biochem., 3: 111-116.
TOYAMA, T. & NAKAMURA, K. (1964) Synergistic response of hydrogen
peroxide aerosols and sulfur dioxide to pulmonary airway
resistance. Ind. Health, 2: 34-45.
TOYAMA T., KAHYO, H., NAKAMURA, K., KAGAWA, J., YAKURA, S., ADACHI,
S., YAMAMOTO, N., IRIYAMA, F., KUMAGAYA, F., OSAWA, S., &
NAKAMURA, T. (1966) [Study on the prevalence of respiratory
symptoms in a rural area (Kashima, Ibaragi Pref.) in Japan.]
J. Jpn Soc. Air Pollut., 1: 24-35 (in Japanese).
TREON, J. F., DUTRA, F. R., CAPPEL, J., SIGMON, H., & YOUNKER, W.
(1950) Toxicity of sulfuric acid mist. Arch. ind. Hyg. occup.
Med., 2: 716-734.
TSUNETOSHI, Y., SHIMIZU, T., TAKAHASHI, H., ICHINOSAWA, A., UEDA, M.,
NAKAYAMA, N., YAMAGATA, Y., & OHSHINO, A. (1971) Epidemiological
study of chronic bronchitis with special reference to effect of
air pollution. Int. Arch. Arbeitsmed., 29: 1-27.
TURNER, D. B. (1968) Workbook of atmospheric dispersion estimates,
Washington, DC, US Department of Health, Education and Welfare,
84 pp.
ULMER, W. T., REICHEL, G., CZEIKE, A., & LEUSCHNER, A. (1970)
[Regional incidence of non-specific respiratory diseases. IV.
Communication.] Int. Arch. Arbeitsmed., 27: 73-109 (in German).
UNITED STATES DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1962)
Air pollution measurements of the National Air Sampling Network
1957-1961, Cincinnati, OH, Division of Air Pollution, pp. 3-4
(Publ. No. 978).
UNITED STATES DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1969)
Air quality criteria for particulate matter, Washington, DC,
DHEW, National Air Pollution Control Administration, pp. 1-211
(AP. 49).
US ENVIRONMENTAL PROTECTION AGENCY (1974a) Air quality data -- 1972
annual statistics, Research Triangle Park, NC, USEPA, 145 pp.
(EPA450/2-74-001).
US ENVIRONMENTAL PROTECTION AGENCY (1974b) Health consequences of
sulfur oxides. A report from CHESS, 1970-71, Research Triangle
Park, NC, US Government Printing Office, 265 pp.
(EPA650/1-74-604).
VAN DER LENDE, R. (1969) Epidemiology of chronic non-specific lung
disease (chronic bronchitis), Thesis, University of Groningen,
2 vols. Assen, Van Gorcum.
VAN DER LENDE, R., VISSER, B. F., WEVER-HESS, J., TAMMELIFG, G. J., DE
VRIES, K., ORIE, N. G. M. (1973) Epidemiological investigations in
the Netherlands into the influence of smoking and atmospheric
pollution on respiratory symptoms and lung function disturbances.
Pneumologie, 149: 119-126.
VAN DER LENDE, R., HUYGEN, C., JANSEN-KOSTER, E. J., KNIFPSTRA, S.,
PESEL, R., VISSER, B. F., WOLFS, E. H. E., & ORIE, N. G. M. (1975)
A temporary decrease in the ventilatory function of an urban
population during an acute increase in air pollution. Bull.
Physiopathol. Respir., 11: 31-43.
VAN DOP, H. & KRUIZINGA, S. (1976) The decrease of sulfur dioxide
concentration near Rotterdam and their relation to some
meteorological parameters during thirteen consecutive winters
(1961-74). Atmos. Environ., 10: 1-4.
VEREIN DEUTSCHER INGENIEURE (1974) [Measurements of the wet
concentrations of particles in the outdoor air (LIB-filter method)
VDI2463 Sheet 4.] (in German).
VIGDORCIK, E. A. (1948) Retention of inhaled aerosols (resume),
Leningrad, Leningrad Research Institute of Labour Hygiene and
Occupational Health Publication, Vol. XI, Part 2, pp. 227-230.
WALLER, R. E. (1963) Acid droplets in town air. Int, J. Air Water
Pollut., 7: 773-778.
WALLER, R. E. (1967) Studies on the nature of urban air pollution. In:
Conference on Museum Climatology, London, International
Institute of Works of Art, pp. 65-69.
WALLER, R. E. (1971) Air pollution and community health. J. R. Coll.
Physicians, Lond., 5: 362-368
WALLER, R. E. & COMMINS, B. T. (1966) High pollution episodes in
London, 1952-1966. In: Proceedings of the International Clean Air
Conference, London, Natural Society of Clean Air, pp. 228-231.
WALLER, R. E. & COMMINS, B. T. (1967) Studies of the smoke and
polycyclic aromatic hydrocarbon content of the air in large urban
areas. Environ. Res., 1: 295-306.
WALLER, R. E., BROOKS, A. G. F., & CARTWRIGHT, J. (1963) An electron
microscope study of particles in town air. Int. J. Air Water
Pollut. Control., 7: 779-786.
WALLER, R. E., COMMINS, B. T., & LAWTHER, P. J. (1965) Air pollution
in a city street. Br. J. ind. Med., 22: 128-138.
WALLER, R. E., LAWTHER, P. J., & MARTIN, A. E. (1969) Clean air and
health in London. In: Proceedings of the International Clean Air
Conference, London, Natural Society of Clean Air, pp. 71-79.
WARNER, C. G., DAVIES, G. M., JONES, J. G., & LOWE, C. R. (1969)
Bronchitis in two integrated steel works, II. Sulfur dioxide and
particulate atmospheric pollution in and around the two works.
Ann. occup. Hyg., 12: 151-170.
WATANABE, H. (1966) [Health effects of air pollution in Osaka City.]
J. Osaka Life Hyg. Assoc., 10: 147-157 (in Japanese).
WEATHERLEY, M. L. (1966) Air pollution inside the home. Int. J. Air
Water Pollut. Control, 10: 404-409.
WEATHERLEY, M. L., GOORIAH, B. D., & CHARNOCK, J. (1976) Fuel
consumption, smoke and sulfur dioxide emissions and
concentrations, and grit and dust deposition in the U.K. up to
1973-74. Stevenage, UK, Warren Spring Laboratory
(WSL Report LR214AP).
WEST, P.M. & GAEKE, G. (1956) Fixation of sulfur dioxide as
sulfitomercurate III and subsequent calorimetric determination.
Anal. Chem., 28: 1816-1819.
WILLEKE, K. & WHITBY, K. T. (1975) Atmospheric aerosols: size
distribution interpretation. J. Air Pollut. Control Assoc.,
25: 529-534.
WILLIAMS, F. P. (1960) Pollution levels in cities. In: Proceedings of
the Harrogate Conference, London Natural Society of Clean Air,
pp. 83-88.
WILLIAMS, M. K. (1970) Sickness absence and ventilatory capacity of
workers exposed to sulfuric acid mist. Br. J. ind. Med.,
27: 61-66.
WILSON, W. E., Jr & LEVY, A. (1970) A study of sulfur dioxide in
photochemical smog. I. Effect of SO2 and water vapour
concentration in the 1-butene/NOx/SO2 system. J. Air Pollut.
Control Assoc., 20: 385-390.
WORLD HEALTH ORGANIZATION (1960) WHO Technical Report Series No. 192
(Epidemiology of cancer of the lung: Report of a study group).
Geneva, WHO, 13 pp.
WORLD HEALTH ORGANIZATION (1971) International standards for
drinking-water. Geneva, WHO, 70 pp.
WORLD HEALTH ORGANIZATION (1972) WHO Technical Report Series No. 506
(Air quality criteria and guides for urban air pollutants),
Geneva, WHO, 35 pp.
WORLD HEALTH ORGANIZATION (1974) WHO Technical Report Series No. 539
(Toxicological evaluation of certain food additives with a review
of general principles and of specifications), Geneva, WHO, 40 pp.
WORLD HEALTH ORGANIZATION (1976a) Selected methods of measuring air
pollutants, Geneva, WHO, 110 pp. (WHO Offset Publ. No. 24).
WORLD HEALTH ORGANIZATION (1976b) Air quality in selected urban
areas, 1973-1974, Geneva, WHO, 65 pp. (WHO Offset Publ.,
No. 30).
WORLD HEALTH ORGANIZATION (1977) Air monitoring programme design for
urban and industrial areas, Geneva, WHO, 46 pp. (WHO Offset
Publ., No. 33).
WORLD METEOROLOGICAL ORGANIZATION (1974) WMO operations manual for
sampling and analysis techniques for chemical constituents in air
and precipitation, Geneva, WMO, 56 pp. (No. 299).
YOCOM, J. E., CLINK, W. L., & COTE, W. A. (1971) Indoor/outdoor air
quality relationships. J. Air Pollut. Control Assoc.,
21: 251-259.
YOKOYAMA, E., YODER, R. E., & FRANK, N. R. (1971) Distribution of 35S
in the blood and its excretion in urine of dogs exposed to
35SO2. Arch. environ. Health, 22: 389-395.
YOSHIDA, K., OSHIMA, H., & IMAI, M. (1966) Air pollution and asthma in
Yokkaichi. Arch. environ. Health, 13: 763-768.
YOSHII, M., NONOYAMA, J., OSHIMA, H., YAMAGIWA, H., & TAKEDA, S.
(1969) Chronic pharyngitis in air-polluted districts of Yokkaichi
in Japan. Mie med. J., 19: 17-27.
ZARKOWER, A. (1972) Alterations in antibody response induced by
chronic inhalation of SO2 and carbon. Arch. environ. Health,
25: 45-50.
ZEEDUK, H. & VELDS, C. A. (1973) The transport of sulfur dioxide over
a long distance. Atmos. Environ., 7: 849-862.
 seen in Fig. 1. and a wide range of sizes, shapes, and densities is
commonly seen in all samples of suspended particulates in urban areas.
For routine monitoring purposes, it is clearly out of the question to
characterize the material completely in terms of size distribution and
composition, but it is important to recognize that the suspended
particulates generally comprise a heterogenous mixture, that can
differ greatly in its characteristics from one location to another,
and even from one occasion to another at any one site.
Estimates of size distribution can be obtained from electron
micrographs by considering the particles compressed into equivalent
spheres. The results are commonly plotted as cumulative frequency
curves, and Fig. 2 shows results for a sample consisting largely of
smoke aggregates. In this the mass median diameter (MMD) is
approximately 1 µm, i.e., half the mass of material collected is
contained in particles having effective (aerodynamic) diameters under
one micrometer.a
The results shown in Fig. 2 indicate a log-normal distribution of
particle size in that specific sample, but Willeke & Whitby (1975)
have shown that distributions are often multimodal. These authors also
stressed the importance of examining the distribution in terms of
numbers of particles, and surface area, in addition to volume (or
mass), for each curve may reveal features not shown by the others. An
example of results obtained with their Minnesota Aerosol Analyzing
System is shown in Fig. 3. This shows a mode in the volume
distribution in the range 0.1 to 1 µm that is related to particles
formed by coagulation or condensation from smaller units, and a
further mode of the order of 10 µm that corresponds with
mechanically-produced particles, some of which are large enough to
settle rapidly, and are not, therefore, strictly part of the suspended
particulates.
The impression gained of size distributions will, however, always
depend on the characteristics of the instruments used. The most
extensive series of results that has been reported was based on a
modified Andersen cascade impactor (Lee & Goranson, 1972). This allows
samples to be collected in five, roughly size-graded fractions, with a
back-up filter as a sixth stage to collect the finest particles. Mass
median diameters have generally been found to be below 1 µm in samples
collected in urban areas of the USA, but this method cannot describe
the size distribution as completely as that of Willeke & Whitby
(1975).
a For further details concerning the definition of poly-dispersed
aerosols containing particles of irregular shape see, for example,
Fuchs (1964) or Task Group on Lung Dynamics (1966).
seen in Fig. 1. and a wide range of sizes, shapes, and densities is
commonly seen in all samples of suspended particulates in urban areas.
For routine monitoring purposes, it is clearly out of the question to
characterize the material completely in terms of size distribution and
composition, but it is important to recognize that the suspended
particulates generally comprise a heterogenous mixture, that can
differ greatly in its characteristics from one location to another,
and even from one occasion to another at any one site.
Estimates of size distribution can be obtained from electron
micrographs by considering the particles compressed into equivalent
spheres. The results are commonly plotted as cumulative frequency
curves, and Fig. 2 shows results for a sample consisting largely of
smoke aggregates. In this the mass median diameter (MMD) is
approximately 1 µm, i.e., half the mass of material collected is
contained in particles having effective (aerodynamic) diameters under
one micrometer.a
The results shown in Fig. 2 indicate a log-normal distribution of
particle size in that specific sample, but Willeke & Whitby (1975)
have shown that distributions are often multimodal. These authors also
stressed the importance of examining the distribution in terms of
numbers of particles, and surface area, in addition to volume (or
mass), for each curve may reveal features not shown by the others. An
example of results obtained with their Minnesota Aerosol Analyzing
System is shown in Fig. 3. This shows a mode in the volume
distribution in the range 0.1 to 1 µm that is related to particles
formed by coagulation or condensation from smaller units, and a
further mode of the order of 10 µm that corresponds with
mechanically-produced particles, some of which are large enough to
settle rapidly, and are not, therefore, strictly part of the suspended
particulates.
The impression gained of size distributions will, however, always
depend on the characteristics of the instruments used. The most
extensive series of results that has been reported was based on a
modified Andersen cascade impactor (Lee & Goranson, 1972). This allows
samples to be collected in five, roughly size-graded fractions, with a
back-up filter as a sixth stage to collect the finest particles. Mass
median diameters have generally been found to be below 1 µm in samples
collected in urban areas of the USA, but this method cannot describe
the size distribution as completely as that of Willeke & Whitby
(1975).
a For further details concerning the definition of poly-dispersed
aerosols containing particles of irregular shape see, for example,
Fuchs (1964) or Task Group on Lung Dynamics (1966).

 Although it is possible to investigate the composition of
individual particles to a certain extent, data on chemical composition
are usually derived from larger samples as collected for the
determination of the total mass of suspended particulates. Among the
principal components are carbon, tarry material (hydrocarbons, soluble
in organic solvents such as benzene), water soluble material (such as
ammonium sulfate), and insoluble ash (containing small amounts of
iron, lead, and a wide variety of other elements).
The proportions of these components vary widely, depending on the
types of sources in the locality. For example, the special feature of
the suspended particulate matter in the United Kingdom prior to the
implementation of the Clean Air Act was their high tar content, and
this was particularly evident in high pollution episodes (Table 2).
Table 2. Examples of analyses of suspended particulates sampled in London prior
to smoke control (high volume samples)a
Typical summer Typical winter High pollution
sample sample episode
(July 1955) (February 1955) (January 1956)
Total suspended
particulates (µg/m3) 97 485 5111
Components as %
of total:
Organic (tar) 7.5 19.1 45.7
Sulfate 11.3 9.0 5.8
Chloride 0.8 0.2 1.0
Nitrate 0.8 0.5 0.7
Iron 1.3 1.4 0.1
Lead 0.7 0.4 0.1
Zinc 0.5 0.2 0.8
a From: The Medical Research Council Air Pollution Unit, now. Clinical
Section of Medical Research Council Toxicology Unit
(unpublished data)
Results from the extensive series of analyses of high volume
samples of suspended particulate matter at sites in the USA indicate
organic contents of the order of 10% of the total particulates (US
Environmental Protection Agency, 1974a). Although little is known of
the influence of the composition of the suspended particulate matter
on effects on health, detailed analyses can be of value in identifying
sources and are relevant in the study of reactions between pollutants.
Thus iron and manganese, although only trace constituents, may be of
importance in catalysing the oxidation of sulfur dioxide to sulfuric
acid or sulfates. The lead content is usually related to pollution
from motor vehicles, and traces of vanadium that are present come
mainly from the combustion of residual oils. There may also be a
variety of substances from noncombustion sources, such as road dust,
material from the degradation of tyres, windblown soil, pollen, and
emissions from industrial processes such as cement manufacturing or
steel making.
The most important distinction to be made in relation to suspended
particulate matter at the present time, however, is neither the
precise size distribution, nor the detailed chemical composition, but
the very broad characterization that results from the different
methods of assessment that are in common use for routine monitoring
purposes. These are discussed further in section 2.2.3. In subsequent
sections, the term "smoke" has been used for observations of suspended
particulate matter based on its soiling properties, and "total
suspended particulates" for those based directly on weight. Since the
former is mainly influenced by incomplete combustion products from the
burning of fossil fuels, and is little affected by white or colourless
materials such as ammonium sulfate, it is clear that the two terms are
not interchangeable.
2.2 Methods of Sampling and Analysis
In general, the outdoor air has been sampled for sulfur oxides and
suspended particulate matter in relation to community exposures,
whereas indoor environments have been examined in connection with
occupational exposures.
The most commonly used methods have been described in detail in a
recently published manual (World Health Organization, 1976a) and their
application in monitoring networks has been discussed in a further
publication (World Health Organization, 1977). However, the bases of
these and some other methods, that have been used in reporting
concentrations of sulfur oxides and suspended particulates in the air,
are discussed below to provide a better understanding of the
measurements cited in epidemiological studies. It is important to
ensure that any measurements that are made are supervised by someone
competent in the field of air pollution monitoring, and the methods
used must be reported together with the results.
Table 3. Methods of analysis for sulfur dioxidea
Method Principle Comment
Pararosaniline Absorption of sulfur dioxide in solution of Uses simple apparatus, and suitable
methodb potassium tetrachloromercurate (TCM); for sampling periods ranging from
complex formed reacts with pararosaniline 30 min to 24 h; samples should be
and formaldehyde to produce a red-purple analysed soon after collection;
colour, determined colorimetrically (West & specific for sulfur dioxide, and
Gaeke, 1956). possible interference from oxides of
nitrogen and some metals can be
eliminated (Pate et al., 1965;
Scaringelli et al., 1967) Widely used
in USA.
Acidimetric Simple apparatus, often combined with smoke Absorption of sulfur dioxide in
methodb filter (see the Organization for Economic dilute hydrogen peroxide solution;
Cooperation and Development filter soiling the sulfuric acid formed is titrated
method in Table 5); suitable for sampling against standard alkali (Organization
periods of 24-h, or less in some circumstances for Economic Cooperation and
(e.g. high pollution episodes, or occupational Development, 1965).
environments).
Conductivity Sulfur dioxide is sampled in deionized water Simple apparatus, suitable for
measurements containing hydrogen peroxide where it is sampling periods of the order of
oxidized to sulfuric acid, as in the acidimetric 24-h, usually combined with a filter
method; increase in conductivity measured to remove particulate matter; less
with a conductivity bridge (Adams et al., 1971). reliable than acidimetric method,
and not widely used in manual
form, but the principle often used
in automatic instruments (Derrett
Table 3. (cont'd).
Method Principle Comment
Conductivity & Brown, 1978), applicable also to
measurements simple portable instruments for
cont'd. spot checks in urban or industrial
environments (Nash, 1964), and to
personal samplers for assessing
occupational exposures (Sherwood,
1969).
Detector tube Air is drawn through tubes containing silica Portable, and no power supply
measurements gel impregnated with indicator sensitive to required. Widely used for spot
sulfur dioxide; concentration assessed from the checks in occupational environments,
length of the stain (Ash & Lynch, 1972). or in other situations where
the concentrations may be high
(from about 3000 µg/m3 upwards).
Iodine Sulfur dioxide absorbed in a solution of iodine Applicable to occupational
method contained in a wash bottle with a fritted bubbler, environments, but not now widely used;
and solution titrated with thiosulfate (Elkins, the method has been modified for
1959). colorimetric assessment, providing
a basis for portable instruments for
survey use (Cummings & Redfearn,
1957).
Table 3. (cont'd).
Method Principle Comment
Automatic Based on conductivity, colorimetry, coulometry Particularly valuable for following
instruments flame photometry, or gas chromatography short-term variations in
(Hollowell et al., 1973). concentration, but difficult to
assess 24-h average concentrations,
unless linked with data processing
equipment; instruments expensive, and
must be under the control of
experienced operators.
Sulfation Sulfur compounds in the air react with an Simple and requires no power
rate exposed cylinder or plate covered with a paste supply; sampling period long (30
containing lead peroxide; sulfate formed is days); results expressed in
determined by precipitation with barium SO3/100cm2/day, indicating rate
chloride (British Standards Institution, 1969a). of reaction of sulfur compounds
with surfaces; not specific for sulfur
dioxide, does not indicate
concentrations in the air, and although
often quoted in epidemiological
studies, of little value for these.
a From: Pate et al., (1965)
b Methods that are described fully in the manual of the World Health Organization (1976a).
2.2.1 Sulfur dioxide
If sulfur dioxide were the only contaminant of the air and
providing the samples were of adequate size, each of the methods
mentioned in Table 3 would give comparable results, indicating the
true amount of sulfur dioxide. In normal urban environments, however,
other pollutants are always present and although the sampling
procedure can be arranged to minimize interference from particulate
matter by filtering the air first, errors can still arise due to the
presence of various gases and vapours. The choice of method depends on
many factors, including the averaging time required: 24-h sampling
periods are commonly used, and many of the methods are suitable for
this. For shorter periods the choice is more limited, and for detailed
information on short-term variations in concentration, instrumental
methods are required.
In occupational environments, the mixture of air pollutants may be
simple and more clearly defined than that in urban air. Sulfur dioxide
may be emitted from a specific process rather than from a variety of
combustion sources, and the air may then be relatively free from
interfering gases. Concentrations may also be much higher than those
encountered in urban air, allowing short sampling periods to be used,
and, since concentrations are liable to change rapidly, this may even
be essential. Also, concentrations may vary greatly over short
distances, depending on the proximity of the source of pollution; this
makes the assessment of exposure based on measurements at fixed sites
difficult. There may be a preference in these circumstances for
methods suitable for use with portable instruments.
2.2.2 Suspended sulfates and sulfuric acid
Most of the methods mentioned in Table 4 assess the total
water-soluble sulfates collected on filters as part of the suspended
particulates. In general, any sulfuric acid present is included with
this, and some of the material present as acid in the air may be
converted to neutral sulfate on the filter during sampling. There is
no completely satisfactory method for the determination of sulfuric
acid in the presence of other pollutants, but some procedures for
examining the acidic properties of suspended particulates have been
referred to. There is an urgent need for more research in this field.
No methods, other than those mentioned in Table 4, are, as yet,
sufficiently well established for widespread application in
epidemiological studies, but much research work is in progress.
Table 4. Methods of analysis for suspended sulfates and sulfuric acid
Method Principle Comment
Turbidimetric Sample collected on sulfate-free glass Samples normally collected
method fibre or other efficient filter: sulfate over 24-h periods by high
extracted and precipitated with barium volume sampler (see Table 5).
chloride, measuring the turbidity of the No distinction made between
suspension spectrophotometrically (US sulfates and sulfuric acid.
Environmental Protection Agency,
1974b).
Methylthymol Samples collected as in the turbidimetric This modification allows the
blue method method above and extract reacted with procedure to be automated,
barium chloride, but barium remaining comments as in the turbidimetric
in solution then reacts with methylthymol method apply.
blue; sulfate determined colorimetrically
by measurement of uncomplexed methylthymol
blue (US Environmental Protection Agency,
1974b).
The most recent trends are towards the application of more
sensitive techniques, such as X-ray fluorescence (Dzubay & Stevens,
1973), or the thermal conversion of sulfates, measuring the resulting
sulfur dioxide by flame photometry (Husar et al., 1975), or by the
pararosaniline method (Maddalone et al., 1975).
Approaches to the difficult problem of determining sulfuric acid
have been made by back-titrating a sodium tetraborate extract of
suspended particulates collected on a small filter paper (Commins,
1963), and by observing the acidic properties of individual particles
collected on indicator-treated slides in a cascade impactor (Waller,
1963). A procedure for the separate determination of sulfuric acid and
ammonium sulfate by nephelometry of a humidified sample of air has
also been described (Charlson et al., 1973) and work is in progress on
the prevention of the reaction of sulfuric acid on filter papers
(Thomas et al., 1976). However, at present, there is not enough field
experience with methods for sulfuric acid to warrant their general use
in connexion with epidemiological studies.
2.2.3 Suspended particulate matter
In general, it is not practicable to discriminate on the basis of
either particle size or chemical composition when assessing
particulate matter for routine monitoring purposes. The
characteristics of the sample are determined by the types of sources
in the vicinity, the weather conditions, and the sampling procedure
adopted. The difficulties that result and the limitations of
measurements have been discussed by Ellison (1965)and are illustrated
in the discussion of the merits and shortcomings of the various
methods described below and in Table 5.
When considering measurements of suspended particulate matter, it
is essential to specify the method used and to recognize that, even
then, results obtained in one set of circumstances will not
necessarily be applicable to others. The main difficulty has arisen in
attempts to apply findings based on smoke measurements that relate
only to the dark coloured material characteristic of the incomplete
combustion of coal or other hydrocarbon fuels, to situations involving
total suspended particulates assessed more directly in terms of
weight. Because the former have been used in much of the early
epidemiological work and the latter are now used for monitoring
purposes in many countries, some kind of conversion from one type of
measurement to the other would be desirable, but, for the reasons
already stated, there can be no generally applicable conversion
factor. Comparative evaluation of the two methods has been undertaken
at a number of sites (Ball & Hume, 1977; Commins & Waller, 1967; Lee
et al., 1972), but the results have only served to emphasize that they
measure different qualities of the particulate matter and that they
should not be compared with one another.
Table 5. Methods of analysis for suspended particulate matter
Method Principle Comment
Smoke measurement: Air is drawn through a white filter paper, Widely used in Europe and recommended
Organization for usually over periods of 24 h, and the darkness by the Organization for Economic
Economic Cooperation of the stain obtained measured by reflectometer; Cooperation and Development (1965);
and Development filter values converted to equivalent international low intake velocity ensures sample
soiling method smoke units, expressed conventionally, in restricted to respirable size range; often
µg/m3; simple apparatus, suitable for combined with sulfur dioxide measurement
continuous operation. by acidimetric method (see Table
2-3); results influenced primarily by
black material do not necessarily
represent true weights; only a limited
range of chemical analyses possible on
these small samples.
Smoke measurement: Similar to the Organization for Economic Flow rate a little greater than in the
American Society Cooperation and Development filter soiling Organization for Economic Cooperation
for Testing and method, but samples collected on a filter and Development filter soiling method
Materials filter paper tape moved on automatically to provide but sample still effectively within
soiling method a series of stains over intervals of 2-6 h respirable size range used in USA;
(American Society for Testing and Materials, interrelationships between Coh units and
1964); results usually assessed by transmittance, RUDS investigated by Saucier & Sansone
and expressed in coefficient of haze (COH) (1972); suitable for continuous
units (Hemeon et al., 1953; reflectance operation.
has sometimes been used, expressing results
in reflectance units of dirt shade (RUDS)
(Gruber & Alpaugh, 1954).
Table 5. (cont'd).
Method Principle Comment
Determination of Air drawn through a glass fibre filter sheet, Widely used in USA. Liable to collect
total suspended usually with a turbine blower, and the amount particles well beyond the respiratory size
particulates, of material collected determined by weighing range and this may bias results,
gravimetric high under controlled temperature and humidity particularly in dry, dusty locations;
volume conditions; the most widely used instrument not very suitable for continuous
is the high volume sampler (US Department operation, samples commonly collected
of Health, Education and Welfare, 1962), but over 24-h periods every sixth day;
instruments based on rotary pumps with samples large enough for a wide range
membrane rather than glass fibre filters have of chemical analyses.
been used (Verein Deutscher Ingenieure,
1974).
Indirect determination Series of samples collected on filter paper Instrument relatively expensive; used for
of mass concentration: strip over selected periods (usually 30 min), monitoring purposes in Federal Republic
ß ray sampler and mass of material determined by of Germany, but not to any large extent
attenuation of ß radiation from a built-in elsewhere; valuable for studying
source (Husar, 1974). short-term variations in total
suspended particulates.
Light scattering Direct determination of suspended particulate Used to some extent in Japan for monitoring
matter as aerosols by light scattering, either suspended particulate matter, but
counting and sizing individual particles (Liu et calibration required and results not
al., 1974) or integrating light scattered from necessarily comparable with those from
given volume of air (Horvath & Charlson, direct weighings; otherwise main
1969). application in industrial environments,
some instruments allow particles to be
counted and classified within a large
number of size ranges.
Table 5. (cont'd).
Method Principle Comment
Size selective Particles separated into several roughly size- Allows concentrations to be assessed
sampling: graded fractions by impaction, the amount of within specified size ranges; some series
modified cascade material in each being determined by direct of results available from USA but not
impactor weighing (Carson & Paulus, 1974). yet widely adopted; applicable also to
the sampling of dusts in industrial
environments.
Electrostatic Particles charged by passing through metal Not suitable for outdoor measurements,
precipitators tube with large potential gradient between but useful in occupational environments;
wall and needle along centre; deposited on advantage over direct weighing of filters
wall and determined by direct weighing is that the collector is unaffected by
(Lauterbach et al., 1954). changes in humidity.
Personal samplers Air drawn through small glass-fibre filters Applicable primarily to industrial
using battery operated pump, so that environments to assess exposures in
instrument can be worn by individuals series of working shifts; elutriator can be
(Sherwood & Greenhalgh, 1960); particulates added to exclude large particles.
assessed by weighing, or analysed for specific
constituents.
From their study in central London, Commins & Waller (1967) showed
that additional material was collected by the high volume sampler that
had little effect on smoke measurements and that for their particular
series, the total suspended particulate results were approximately
100 µg/m3 higher than the corresponding smoke figures. Other authors
have calculated regression equations for their series and, although
there are variations in these relationships with time and place, the
general picture is of a large proportional difference between total
suspended particulate and smoke figures at low values, but relatively
little difference at high values (of the order of 500 µg of smoke/m3
or more).
Thus, it is recommended that "smoke" and "total suspended
particulate" measured by the various methods described should be
regarded as separate entities; this principle has been adopted in
later sections relating to the effects on health.
In occupational environments, suspended particulate matter from
combustion sources may be of some concern, but more commonly it is the
dusts and aerosols associated with particular occupations or processes
that are of interest. In such cases, the composition of the material
may be relatively uniform and well established, and specific methods
of assessment can be devised. There has, for example, been a great
deal of research and development on methods for determining dust
concentrations in coal mines (Jacobsen, 1972). With industrial dusts,
the particle size distribution must always be considered carefully,
for it is liable to extend beyond the respirable range, and
elutriators or cyclones may be needed in conjunction with gravimetric
samplers. A valuable discussion of methods for collecting size-graded
samples has been included in a recent review (International Atomic
Energy Agency, 1978).
2.2.4 Dustfall (deposited matter)
In some of the older epidemiological studies, measurements of
dustfall were quoted as an index of particulate pollution. This is
inappropriate as the results are influenced primarily by large
particles (diameters from about 10 µm upwards: see section 2.1.2) that
do not penetrate the respiratory system and, generally, are not
relevant to health problems, apart from possible annoyance reactions.
For reference purposes, however, a brief description of one of the
more commonly used instruments is included (Table 6).
Table 6. Method of analysis for dust fall
Method Principle Comment
Deposit A receiver containing a Results expressed in terms
gauge nonfreezing solution is left in of deposit per unit area and time,
the open and the quantity of not convertible in any way to
material collected (usually concentrations of suspended matter
over 1-month periods) is determined in the air; strongly influenced by
by weighing, water-soluble and sources nearby, hence results only
insoluble components being considered relevant to immediate vicinity
separately (British Standards
Institution, 1969b)
3. SOURCES OF SULFUR OXIDES AND PARTICULATE MATTER
3.1 Natural Occurrence
Compounds of sulfur are found in small quantities in ambient air,
even in remote areas far from sources of pollution. In the gas phase,
they are present as hydrogen sulfide or sulfur dioxide, and in
particulate form they may be present as sulfate. Sulfur dioxide and
hydrogen sulfide are emitted by volcanoes and the latter is also
produced by anaerobic bacteria in soil, marshes, and tidal flats (Grey
& Jensen, 1972). Some of the particulate sulfate may also be emitted
directly by volcanoes or sea spray, but most of it is the end-product
of the oxidation of hydrogen sulfide or sulfur dioxide.
In general, suspended particulate matter can result from volcanic
activity, from dust storms, or from strong winds blowing over dry soil
and may include pollen from trees and other plants. Forest fires also
produce large amounts of particulate matter.
Some of these natural contributions to the particulate matter in
the air consist of particles too large to remain in suspension for
long periods, and their composition and properties may be quite
different from those of the emissions from man's activities.
3.2 Man-made sources
Most emissions of sulfur into the air are in the form of sulfur
dioxide resulting from the combustion of fossil fuel for heating and
energy production. Various industrial activities such as petroleum
processing, smelter operations, wood-pulping, etc., also produce
significant emissions of sulfur dioxide and other sulfur compounds.
It has been estimated (Robinson & Robbins, 1968) that on a
worldwide scale about 146 × 106 tonnes of sulfur dioxide are emitted
annually, 70% of which result from coal burning, 16% from the
combustion of petroleum products, and the remainder from petroleum
refining and nonferrous smelting. These estimates are based mainly on
1965 world figures for coal production, petroleum refining, and
smelter operations, each combined with an estimate of a sulfur dioxide
"emission factor" per unit of production. A similar basis was used for
the Committee on the Challenges of Modern Society (1971) assessment of
emissions in the northern and southern hemispheres, reproduced in
Table 7.
Table 7. Hemispheric sulfur dioxide emmissions due to man's
activities (106 tonnes per year)a
Source Total Northern Southern
Hemisphere Hemisphere
Coal 102 98 (96%) 4 (4%)
Petroleum,
combustion
and refining 28.5 27.1 (95%) 1.4 (5%)
Smelting, copper 12.9 8.6 (67%) 4.3 (33%)
lead 1.5 1.2 (80%) 0.3 (20%)
zinc 1.3 1.2 (90%) 0.1 (10%)
Total 146 136 (93%) 10 (7%)
a From: Committee on the Challenges of Modern Society (1971).
On a global scale, the emissions of sulfur compounds into the
atmosphere by man-made activities are about equal to those from
natural sources. On the other hand, the emissions from man's
activities are the main contributors to pollution in large cities and
their surrounding areas. Assuming that world energy demand increases
at its historic rate, the total emissions of sulfur dioxide will
increase unless appropriate control measures are applied, or there
is a shift from the use of fossil fuels to the use of nonpolluting
energy sources. However, with the stabilization of the population in
some countries, including the United Kingdom and USA, and increasing
concern about the use of limited fuel reserves, there are prospects
that the rate of increase in emissions may be reduced in some parts
of the world.
Combustion and industrial processes are also prime sources of
particulate emissions. As with sulfur dioxide, the burning of fuel
(especially coal) for heating and for the generation of power has been
one of the major contributors to the suspended particulate matter in
urban air. Vehicular traffic also generates dust from the road and
from the wear of tyres as well as particulate lead compounds from the
exhausts of petrol-engined vehicles, and black smoke from those of
diesel vehicles. The incineration of domestic and industrial refuse
may disperse particulate matter and other pollutants into the air
unless carefully controlled. Table 8 shows estimates of the global
emission of all particulate matter (Robinson & Robbins, 1968).
Table 8. Global emission of all particulate matter (106 tonnes
per year)a
Man-made
Particles 92
Gas-particle conversion: sulfur dioxide 147
oxides of nitrogen 30
Photochemical compounds from hydrocarbons 27
296
Natural
Soil dust 200
Gas-particle conversion: hydrogen-sulfide 204
oxides of nitrogen 432
ammonia 269
Photochemical compounds from terpenes, etc 200
Volcanic 4
Forest fires 3
Sea salt 1000
2312
a From: Robinson & Robbins (1968).
3.3 Characteristics of Sources
In urban areas, most of the sulfur dioxide and suspended
particulate matter in the air come from the combustion of fuels, but
many factors, including the type of fuel, the combustion efficiency,
and the flue velocity, influence the quantity and quality of
emissions. The incomplete combustion of soft coal in domestic fires,
for example, produces much smoke, consisting of finely divided
particles of carbon and tarry material, whereas the efficient burning
of pulverised coal in a power station leads to little or no smoke, but
the production of coarser ash particles, which must be removed at
source to avoid their being carried up the flues at a high velocity
and dispersed into the air. The relationship between types of sources
and emissions is summarized in Table 9.
Table 9. Pollutants from combustion sources
Type of source Fuel Sulfur dioxide Particulate matter
Smoke Ash etc.
Domestic heating Wood, peat etc. - + +
or cooking Soft coal ++ ++ +
Hard coal, coke ++ - +
Oil (light distillates) + - -
Gas - - -
Industrial heating Coal, coke ++ - ++
and power generation Oil (heavy residuals) ++ - -
Motor vehicles Petrol - - +
Diesel + + -
Notes: The term smoke is used for incomplete combustion products (notably carbon and tar),
and ash for inorganic components from complete combustion (including lead compounds
in the case of petrol). The signs give only a rough indication of emissions, in the
absence of direct control at source:
- = little or none, + = moderate quantities, ++ = large quantities
In cold and temperate parts of the world, the burning of coal for
domestic heating purposes has been a major contributor to both the
sulfur dioxide and suspended particulate contents of urban air. This
is particularly true of the situation in the United Kingdom prior to
the implementation of the Clean Air Act (Committee on Air Pollution,
1954). Such sources are liable to have a disproportionate effect on
concentrations in the immediate vicinity, because of the low levels of
the chimneys and the low emission velocity. Even in warmer climates,
domestic sources may be of importance, particularly if coal is used
for cooking purposes.
In densely populated areas where domestic sources dominate, the
many chimneys can be considered for some purposes as a diffuse area
source, and, within such an area, concentrations of pollutants in air
remain relatively stable over short distances and short periods of
time. In contrast, large industrial sources should be treated as point
sources, and, at any given location around them, the concentration of
air pollution is liable to vary greatly, even from minute to minute.
The emission into the air of sulfur dioxide and particulate matter
from motor vehicles is relatively small in comparison with those from
domestic and industrial chimneys but it is close to the ground and
within the breathing zone. In these circumstances, concentrations vary
greatly over short distances as well as over short time intervals,
depending on the proximity of the traffic. At points very close to
mixed traffic, smoke from diesel engines may make a substantial
contribution to the concentration of suspended particulate matter in
air (Waller et al., 1965).
Source strength may vary with time of day, day of week, and season
of the year. Accompanying meteorological conditions are also important
in determining the ultimate air concentrations of pollutants arising
from sources. Where heating is required during the winter season,
emissions of sulfur dioxide and particulate matter are usually much
higher than they are in the summer. In a number of cities, however,
where a considerable amount of fuel is used for running cooling
systems, emissions of these pollutants during the summer time are not
always lower than those in winter. Some industrial sources of
pollution may emit little at weekends, and emissions for most sources
are at a minimum during the night.
Although the control of emissions is outside the scope of the
present discussion, the general point can be made that while the
control of particulate emissions is a practicable proposition in many
circumstances, the control of sulfur dioxide at source is relatively
difficult and costly, and the more effective means of reducing
emissions is to change to fuels with a lower sulfur content.
4. DISPERSION AND ENVIRONMENTAL TRANSFORMATIONS
Sulfur compounds dispersed into the air eventually return to the
land or oceans either unchanged, or converted into sulfates. The
sulfur cycle so set up is shown diagrammatically in Fig. 4 (Kellogg et
al., 1972). Particulate matter also returns, its residence time in the
air varying widely according to its physical and chemical
characteristics. As far as direct effects on health are concerned, it
is the local concentration of pollutants in the air at a given time
that is important, as discussed in section 5, but an outline of
dispersion and transformation phenomena is given here.
4.1 Dispersion
The maintenance of a tolerable environment in modern towns depends
very much on the ability of wind and turbulence to disperse the
pollutants rapidly as they are emitted. When these processes fail, the
results can be disastrous, as they were in London in 1952 (Ministry of
Health, United Kingdom, 1954). There are some localities where natural
ventilation is so poor that the emission of pollutants must, at all
times, be carefully controlled. This is especially true of the Los
Angeles area, where emissions of sulfur dioxide and particulate matter
have been successfully curtailed, leaving, however, the major problems
associated with the emission of oxides of nitrogen, hydrocarbons, and
carbon monoxide from motor vehicles (Goldsmith, 1969).
Factors affecting the dispersion of sulfur dioxide and particulate
matter from combustion sources, include:
(a) Temperature and efflux velocity of the gases. Emissions from
small sources, such as domestic fires or incinerators have relatively
little buoyancy, since the temperature at the point of emission is not
much greater than that of the surrounding air. Such sources are liable
therefore to have their greatest impact in the immediate vicinity
(Williams, 1960). Emissions from large-scale industrial installations,
on the other hand, may be at higher temperatures, or may be assisted
by forced-draught to rise more rapidly. Thus, any major impact in the
immediate vicinity may be avoided but weaker effects may be produced
over a wider area (Bosanquet, 1957).
(b) Stack height. Dilution and dispersion over a wide area is
also aided by the use of tall stacks. Much is known of the
relationship between source strengths, stack heights, and ground-level
concentrations of pollutants, through the application of mathematical
modelling techniques, coupled with observations around selected
sources (Briggs, 1965, Pasquill, 1971, Turner, 1968). It is also
possible to devise models to predict concentrations of sulfur dioxide
in urban areas on the basis of emissions from multiple sources
(Fortak, 1970). Conversely, techniques have also been developed for
estimating the pollution inventory of an area from measurements of
sulfur dioxide concentration, fitted to a dispersion model (East,
1972). The height of emission of sulfur dioxide and particulate matter
from domestic sources is primarily a function of the height of the
building itself. Thus the effect on ground level concentrations in the
vicinity is liable to be greater in areas with closely-packed, single
or two-storey houses than in those with high-rise apartments. Tall
stacks are widely used for electricity-generating stations and other
major industrial sources, but the pollutants may then be carried great
distances, often over national boundaries, to be deposited eventually
far from their source (Royal Ministry of Foreign Affairs, Sweden,
1971; Zeeduk & Velds, 1973).
Although it is possible to investigate the composition of
individual particles to a certain extent, data on chemical composition
are usually derived from larger samples as collected for the
determination of the total mass of suspended particulates. Among the
principal components are carbon, tarry material (hydrocarbons, soluble
in organic solvents such as benzene), water soluble material (such as
ammonium sulfate), and insoluble ash (containing small amounts of
iron, lead, and a wide variety of other elements).
The proportions of these components vary widely, depending on the
types of sources in the locality. For example, the special feature of
the suspended particulate matter in the United Kingdom prior to the
implementation of the Clean Air Act was their high tar content, and
this was particularly evident in high pollution episodes (Table 2).
Table 2. Examples of analyses of suspended particulates sampled in London prior
to smoke control (high volume samples)a
Typical summer Typical winter High pollution
sample sample episode
(July 1955) (February 1955) (January 1956)
Total suspended
particulates (µg/m3) 97 485 5111
Components as %
of total:
Organic (tar) 7.5 19.1 45.7
Sulfate 11.3 9.0 5.8
Chloride 0.8 0.2 1.0
Nitrate 0.8 0.5 0.7
Iron 1.3 1.4 0.1
Lead 0.7 0.4 0.1
Zinc 0.5 0.2 0.8
a From: The Medical Research Council Air Pollution Unit, now. Clinical
Section of Medical Research Council Toxicology Unit
(unpublished data)
Results from the extensive series of analyses of high volume
samples of suspended particulate matter at sites in the USA indicate
organic contents of the order of 10% of the total particulates (US
Environmental Protection Agency, 1974a). Although little is known of
the influence of the composition of the suspended particulate matter
on effects on health, detailed analyses can be of value in identifying
sources and are relevant in the study of reactions between pollutants.
Thus iron and manganese, although only trace constituents, may be of
importance in catalysing the oxidation of sulfur dioxide to sulfuric
acid or sulfates. The lead content is usually related to pollution
from motor vehicles, and traces of vanadium that are present come
mainly from the combustion of residual oils. There may also be a
variety of substances from noncombustion sources, such as road dust,
material from the degradation of tyres, windblown soil, pollen, and
emissions from industrial processes such as cement manufacturing or
steel making.
The most important distinction to be made in relation to suspended
particulate matter at the present time, however, is neither the
precise size distribution, nor the detailed chemical composition, but
the very broad characterization that results from the different
methods of assessment that are in common use for routine monitoring
purposes. These are discussed further in section 2.2.3. In subsequent
sections, the term "smoke" has been used for observations of suspended
particulate matter based on its soiling properties, and "total
suspended particulates" for those based directly on weight. Since the
former is mainly influenced by incomplete combustion products from the
burning of fossil fuels, and is little affected by white or colourless
materials such as ammonium sulfate, it is clear that the two terms are
not interchangeable.
2.2 Methods of Sampling and Analysis
In general, the outdoor air has been sampled for sulfur oxides and
suspended particulate matter in relation to community exposures,
whereas indoor environments have been examined in connection with
occupational exposures.
The most commonly used methods have been described in detail in a
recently published manual (World Health Organization, 1976a) and their
application in monitoring networks has been discussed in a further
publication (World Health Organization, 1977). However, the bases of
these and some other methods, that have been used in reporting
concentrations of sulfur oxides and suspended particulates in the air,
are discussed below to provide a better understanding of the
measurements cited in epidemiological studies. It is important to
ensure that any measurements that are made are supervised by someone
competent in the field of air pollution monitoring, and the methods
used must be reported together with the results.
Table 3. Methods of analysis for sulfur dioxidea
Method Principle Comment
Pararosaniline Absorption of sulfur dioxide in solution of Uses simple apparatus, and suitable
methodb potassium tetrachloromercurate (TCM); for sampling periods ranging from
complex formed reacts with pararosaniline 30 min to 24 h; samples should be
and formaldehyde to produce a red-purple analysed soon after collection;
colour, determined colorimetrically (West & specific for sulfur dioxide, and
Gaeke, 1956). possible interference from oxides of
nitrogen and some metals can be
eliminated (Pate et al., 1965;
Scaringelli et al., 1967) Widely used
in USA.
Acidimetric Simple apparatus, often combined with smoke Absorption of sulfur dioxide in
methodb filter (see the Organization for Economic dilute hydrogen peroxide solution;
Cooperation and Development filter soiling the sulfuric acid formed is titrated
method in Table 5); suitable for sampling against standard alkali (Organization
periods of 24-h, or less in some circumstances for Economic Cooperation and
(e.g. high pollution episodes, or occupational Development, 1965).
environments).
Conductivity Sulfur dioxide is sampled in deionized water Simple apparatus, suitable for
measurements containing hydrogen peroxide where it is sampling periods of the order of
oxidized to sulfuric acid, as in the acidimetric 24-h, usually combined with a filter
method; increase in conductivity measured to remove particulate matter; less
with a conductivity bridge (Adams et al., 1971). reliable than acidimetric method,
and not widely used in manual
form, but the principle often used
in automatic instruments (Derrett
Table 3. (cont'd).
Method Principle Comment
Conductivity & Brown, 1978), applicable also to
measurements simple portable instruments for
cont'd. spot checks in urban or industrial
environments (Nash, 1964), and to
personal samplers for assessing
occupational exposures (Sherwood,
1969).
Detector tube Air is drawn through tubes containing silica Portable, and no power supply
measurements gel impregnated with indicator sensitive to required. Widely used for spot
sulfur dioxide; concentration assessed from the checks in occupational environments,
length of the stain (Ash & Lynch, 1972). or in other situations where
the concentrations may be high
(from about 3000 µg/m3 upwards).
Iodine Sulfur dioxide absorbed in a solution of iodine Applicable to occupational
method contained in a wash bottle with a fritted bubbler, environments, but not now widely used;
and solution titrated with thiosulfate (Elkins, the method has been modified for
1959). colorimetric assessment, providing
a basis for portable instruments for
survey use (Cummings & Redfearn,
1957).
Table 3. (cont'd).
Method Principle Comment
Automatic Based on conductivity, colorimetry, coulometry Particularly valuable for following
instruments flame photometry, or gas chromatography short-term variations in
(Hollowell et al., 1973). concentration, but difficult to
assess 24-h average concentrations,
unless linked with data processing
equipment; instruments expensive, and
must be under the control of
experienced operators.
Sulfation Sulfur compounds in the air react with an Simple and requires no power
rate exposed cylinder or plate covered with a paste supply; sampling period long (30
containing lead peroxide; sulfate formed is days); results expressed in
determined by precipitation with barium SO3/100cm2/day, indicating rate
chloride (British Standards Institution, 1969a). of reaction of sulfur compounds
with surfaces; not specific for sulfur
dioxide, does not indicate
concentrations in the air, and although
often quoted in epidemiological
studies, of little value for these.
a From: Pate et al., (1965)
b Methods that are described fully in the manual of the World Health Organization (1976a).
2.2.1 Sulfur dioxide
If sulfur dioxide were the only contaminant of the air and
providing the samples were of adequate size, each of the methods
mentioned in Table 3 would give comparable results, indicating the
true amount of sulfur dioxide. In normal urban environments, however,
other pollutants are always present and although the sampling
procedure can be arranged to minimize interference from particulate
matter by filtering the air first, errors can still arise due to the
presence of various gases and vapours. The choice of method depends on
many factors, including the averaging time required: 24-h sampling
periods are commonly used, and many of the methods are suitable for
this. For shorter periods the choice is more limited, and for detailed
information on short-term variations in concentration, instrumental
methods are required.
In occupational environments, the mixture of air pollutants may be
simple and more clearly defined than that in urban air. Sulfur dioxide
may be emitted from a specific process rather than from a variety of
combustion sources, and the air may then be relatively free from
interfering gases. Concentrations may also be much higher than those
encountered in urban air, allowing short sampling periods to be used,
and, since concentrations are liable to change rapidly, this may even
be essential. Also, concentrations may vary greatly over short
distances, depending on the proximity of the source of pollution; this
makes the assessment of exposure based on measurements at fixed sites
difficult. There may be a preference in these circumstances for
methods suitable for use with portable instruments.
2.2.2 Suspended sulfates and sulfuric acid
Most of the methods mentioned in Table 4 assess the total
water-soluble sulfates collected on filters as part of the suspended
particulates. In general, any sulfuric acid present is included with
this, and some of the material present as acid in the air may be
converted to neutral sulfate on the filter during sampling. There is
no completely satisfactory method for the determination of sulfuric
acid in the presence of other pollutants, but some procedures for
examining the acidic properties of suspended particulates have been
referred to. There is an urgent need for more research in this field.
No methods, other than those mentioned in Table 4, are, as yet,
sufficiently well established for widespread application in
epidemiological studies, but much research work is in progress.
Table 4. Methods of analysis for suspended sulfates and sulfuric acid
Method Principle Comment
Turbidimetric Sample collected on sulfate-free glass Samples normally collected
method fibre or other efficient filter: sulfate over 24-h periods by high
extracted and precipitated with barium volume sampler (see Table 5).
chloride, measuring the turbidity of the No distinction made between
suspension spectrophotometrically (US sulfates and sulfuric acid.
Environmental Protection Agency,
1974b).
Methylthymol Samples collected as in the turbidimetric This modification allows the
blue method method above and extract reacted with procedure to be automated,
barium chloride, but barium remaining comments as in the turbidimetric
in solution then reacts with methylthymol method apply.
blue; sulfate determined colorimetrically
by measurement of uncomplexed methylthymol
blue (US Environmental Protection Agency,
1974b).
The most recent trends are towards the application of more
sensitive techniques, such as X-ray fluorescence (Dzubay & Stevens,
1973), or the thermal conversion of sulfates, measuring the resulting
sulfur dioxide by flame photometry (Husar et al., 1975), or by the
pararosaniline method (Maddalone et al., 1975).
Approaches to the difficult problem of determining sulfuric acid
have been made by back-titrating a sodium tetraborate extract of
suspended particulates collected on a small filter paper (Commins,
1963), and by observing the acidic properties of individual particles
collected on indicator-treated slides in a cascade impactor (Waller,
1963). A procedure for the separate determination of sulfuric acid and
ammonium sulfate by nephelometry of a humidified sample of air has
also been described (Charlson et al., 1973) and work is in progress on
the prevention of the reaction of sulfuric acid on filter papers
(Thomas et al., 1976). However, at present, there is not enough field
experience with methods for sulfuric acid to warrant their general use
in connexion with epidemiological studies.
2.2.3 Suspended particulate matter
In general, it is not practicable to discriminate on the basis of
either particle size or chemical composition when assessing
particulate matter for routine monitoring purposes. The
characteristics of the sample are determined by the types of sources
in the vicinity, the weather conditions, and the sampling procedure
adopted. The difficulties that result and the limitations of
measurements have been discussed by Ellison (1965)and are illustrated
in the discussion of the merits and shortcomings of the various
methods described below and in Table 5.
When considering measurements of suspended particulate matter, it
is essential to specify the method used and to recognize that, even
then, results obtained in one set of circumstances will not
necessarily be applicable to others. The main difficulty has arisen in
attempts to apply findings based on smoke measurements that relate
only to the dark coloured material characteristic of the incomplete
combustion of coal or other hydrocarbon fuels, to situations involving
total suspended particulates assessed more directly in terms of
weight. Because the former have been used in much of the early
epidemiological work and the latter are now used for monitoring
purposes in many countries, some kind of conversion from one type of
measurement to the other would be desirable, but, for the reasons
already stated, there can be no generally applicable conversion
factor. Comparative evaluation of the two methods has been undertaken
at a number of sites (Ball & Hume, 1977; Commins & Waller, 1967; Lee
et al., 1972), but the results have only served to emphasize that they
measure different qualities of the particulate matter and that they
should not be compared with one another.
Table 5. Methods of analysis for suspended particulate matter
Method Principle Comment
Smoke measurement: Air is drawn through a white filter paper, Widely used in Europe and recommended
Organization for usually over periods of 24 h, and the darkness by the Organization for Economic
Economic Cooperation of the stain obtained measured by reflectometer; Cooperation and Development (1965);
and Development filter values converted to equivalent international low intake velocity ensures sample
soiling method smoke units, expressed conventionally, in restricted to respirable size range; often
µg/m3; simple apparatus, suitable for combined with sulfur dioxide measurement
continuous operation. by acidimetric method (see Table
2-3); results influenced primarily by
black material do not necessarily
represent true weights; only a limited
range of chemical analyses possible on
these small samples.
Smoke measurement: Similar to the Organization for Economic Flow rate a little greater than in the
American Society Cooperation and Development filter soiling Organization for Economic Cooperation
for Testing and method, but samples collected on a filter and Development filter soiling method
Materials filter paper tape moved on automatically to provide but sample still effectively within
soiling method a series of stains over intervals of 2-6 h respirable size range used in USA;
(American Society for Testing and Materials, interrelationships between Coh units and
1964); results usually assessed by transmittance, RUDS investigated by Saucier & Sansone
and expressed in coefficient of haze (COH) (1972); suitable for continuous
units (Hemeon et al., 1953; reflectance operation.
has sometimes been used, expressing results
in reflectance units of dirt shade (RUDS)
(Gruber & Alpaugh, 1954).
Table 5. (cont'd).
Method Principle Comment
Determination of Air drawn through a glass fibre filter sheet, Widely used in USA. Liable to collect
total suspended usually with a turbine blower, and the amount particles well beyond the respiratory size
particulates, of material collected determined by weighing range and this may bias results,
gravimetric high under controlled temperature and humidity particularly in dry, dusty locations;
volume conditions; the most widely used instrument not very suitable for continuous
is the high volume sampler (US Department operation, samples commonly collected
of Health, Education and Welfare, 1962), but over 24-h periods every sixth day;
instruments based on rotary pumps with samples large enough for a wide range
membrane rather than glass fibre filters have of chemical analyses.
been used (Verein Deutscher Ingenieure,
1974).
Indirect determination Series of samples collected on filter paper Instrument relatively expensive; used for
of mass concentration: strip over selected periods (usually 30 min), monitoring purposes in Federal Republic
ß ray sampler and mass of material determined by of Germany, but not to any large extent
attenuation of ß radiation from a built-in elsewhere; valuable for studying
source (Husar, 1974). short-term variations in total
suspended particulates.
Light scattering Direct determination of suspended particulate Used to some extent in Japan for monitoring
matter as aerosols by light scattering, either suspended particulate matter, but
counting and sizing individual particles (Liu et calibration required and results not
al., 1974) or integrating light scattered from necessarily comparable with those from
given volume of air (Horvath & Charlson, direct weighings; otherwise main
1969). application in industrial environments,
some instruments allow particles to be
counted and classified within a large
number of size ranges.
Table 5. (cont'd).
Method Principle Comment
Size selective Particles separated into several roughly size- Allows concentrations to be assessed
sampling: graded fractions by impaction, the amount of within specified size ranges; some series
modified cascade material in each being determined by direct of results available from USA but not
impactor weighing (Carson & Paulus, 1974). yet widely adopted; applicable also to
the sampling of dusts in industrial
environments.
Electrostatic Particles charged by passing through metal Not suitable for outdoor measurements,
precipitators tube with large potential gradient between but useful in occupational environments;
wall and needle along centre; deposited on advantage over direct weighing of filters
wall and determined by direct weighing is that the collector is unaffected by
(Lauterbach et al., 1954). changes in humidity.
Personal samplers Air drawn through small glass-fibre filters Applicable primarily to industrial
using battery operated pump, so that environments to assess exposures in
instrument can be worn by individuals series of working shifts; elutriator can be
(Sherwood & Greenhalgh, 1960); particulates added to exclude large particles.
assessed by weighing, or analysed for specific
constituents.
From their study in central London, Commins & Waller (1967) showed
that additional material was collected by the high volume sampler that
had little effect on smoke measurements and that for their particular
series, the total suspended particulate results were approximately
100 µg/m3 higher than the corresponding smoke figures. Other authors
have calculated regression equations for their series and, although
there are variations in these relationships with time and place, the
general picture is of a large proportional difference between total
suspended particulate and smoke figures at low values, but relatively
little difference at high values (of the order of 500 µg of smoke/m3
or more).
Thus, it is recommended that "smoke" and "total suspended
particulate" measured by the various methods described should be
regarded as separate entities; this principle has been adopted in
later sections relating to the effects on health.
In occupational environments, suspended particulate matter from
combustion sources may be of some concern, but more commonly it is the
dusts and aerosols associated with particular occupations or processes
that are of interest. In such cases, the composition of the material
may be relatively uniform and well established, and specific methods
of assessment can be devised. There has, for example, been a great
deal of research and development on methods for determining dust
concentrations in coal mines (Jacobsen, 1972). With industrial dusts,
the particle size distribution must always be considered carefully,
for it is liable to extend beyond the respirable range, and
elutriators or cyclones may be needed in conjunction with gravimetric
samplers. A valuable discussion of methods for collecting size-graded
samples has been included in a recent review (International Atomic
Energy Agency, 1978).
2.2.4 Dustfall (deposited matter)
In some of the older epidemiological studies, measurements of
dustfall were quoted as an index of particulate pollution. This is
inappropriate as the results are influenced primarily by large
particles (diameters from about 10 µm upwards: see section 2.1.2) that
do not penetrate the respiratory system and, generally, are not
relevant to health problems, apart from possible annoyance reactions.
For reference purposes, however, a brief description of one of the
more commonly used instruments is included (Table 6).
Table 6. Method of analysis for dust fall
Method Principle Comment
Deposit A receiver containing a Results expressed in terms
gauge nonfreezing solution is left in of deposit per unit area and time,
the open and the quantity of not convertible in any way to
material collected (usually concentrations of suspended matter
over 1-month periods) is determined in the air; strongly influenced by
by weighing, water-soluble and sources nearby, hence results only
insoluble components being considered relevant to immediate vicinity
separately (British Standards
Institution, 1969b)
3. SOURCES OF SULFUR OXIDES AND PARTICULATE MATTER
3.1 Natural Occurrence
Compounds of sulfur are found in small quantities in ambient air,
even in remote areas far from sources of pollution. In the gas phase,
they are present as hydrogen sulfide or sulfur dioxide, and in
particulate form they may be present as sulfate. Sulfur dioxide and
hydrogen sulfide are emitted by volcanoes and the latter is also
produced by anaerobic bacteria in soil, marshes, and tidal flats (Grey
& Jensen, 1972). Some of the particulate sulfate may also be emitted
directly by volcanoes or sea spray, but most of it is the end-product
of the oxidation of hydrogen sulfide or sulfur dioxide.
In general, suspended particulate matter can result from volcanic
activity, from dust storms, or from strong winds blowing over dry soil
and may include pollen from trees and other plants. Forest fires also
produce large amounts of particulate matter.
Some of these natural contributions to the particulate matter in
the air consist of particles too large to remain in suspension for
long periods, and their composition and properties may be quite
different from those of the emissions from man's activities.
3.2 Man-made sources
Most emissions of sulfur into the air are in the form of sulfur
dioxide resulting from the combustion of fossil fuel for heating and
energy production. Various industrial activities such as petroleum
processing, smelter operations, wood-pulping, etc., also produce
significant emissions of sulfur dioxide and other sulfur compounds.
It has been estimated (Robinson & Robbins, 1968) that on a
worldwide scale about 146 × 106 tonnes of sulfur dioxide are emitted
annually, 70% of which result from coal burning, 16% from the
combustion of petroleum products, and the remainder from petroleum
refining and nonferrous smelting. These estimates are based mainly on
1965 world figures for coal production, petroleum refining, and
smelter operations, each combined with an estimate of a sulfur dioxide
"emission factor" per unit of production. A similar basis was used for
the Committee on the Challenges of Modern Society (1971) assessment of
emissions in the northern and southern hemispheres, reproduced in
Table 7.
Table 7. Hemispheric sulfur dioxide emmissions due to man's
activities (106 tonnes per year)a
Source Total Northern Southern
Hemisphere Hemisphere
Coal 102 98 (96%) 4 (4%)
Petroleum,
combustion
and refining 28.5 27.1 (95%) 1.4 (5%)
Smelting, copper 12.9 8.6 (67%) 4.3 (33%)
lead 1.5 1.2 (80%) 0.3 (20%)
zinc 1.3 1.2 (90%) 0.1 (10%)
Total 146 136 (93%) 10 (7%)
a From: Committee on the Challenges of Modern Society (1971).
On a global scale, the emissions of sulfur compounds into the
atmosphere by man-made activities are about equal to those from
natural sources. On the other hand, the emissions from man's
activities are the main contributors to pollution in large cities and
their surrounding areas. Assuming that world energy demand increases
at its historic rate, the total emissions of sulfur dioxide will
increase unless appropriate control measures are applied, or there
is a shift from the use of fossil fuels to the use of nonpolluting
energy sources. However, with the stabilization of the population in
some countries, including the United Kingdom and USA, and increasing
concern about the use of limited fuel reserves, there are prospects
that the rate of increase in emissions may be reduced in some parts
of the world.
Combustion and industrial processes are also prime sources of
particulate emissions. As with sulfur dioxide, the burning of fuel
(especially coal) for heating and for the generation of power has been
one of the major contributors to the suspended particulate matter in
urban air. Vehicular traffic also generates dust from the road and
from the wear of tyres as well as particulate lead compounds from the
exhausts of petrol-engined vehicles, and black smoke from those of
diesel vehicles. The incineration of domestic and industrial refuse
may disperse particulate matter and other pollutants into the air
unless carefully controlled. Table 8 shows estimates of the global
emission of all particulate matter (Robinson & Robbins, 1968).
Table 8. Global emission of all particulate matter (106 tonnes
per year)a
Man-made
Particles 92
Gas-particle conversion: sulfur dioxide 147
oxides of nitrogen 30
Photochemical compounds from hydrocarbons 27
296
Natural
Soil dust 200
Gas-particle conversion: hydrogen-sulfide 204
oxides of nitrogen 432
ammonia 269
Photochemical compounds from terpenes, etc 200
Volcanic 4
Forest fires 3
Sea salt 1000
2312
a From: Robinson & Robbins (1968).
3.3 Characteristics of Sources
In urban areas, most of the sulfur dioxide and suspended
particulate matter in the air come from the combustion of fuels, but
many factors, including the type of fuel, the combustion efficiency,
and the flue velocity, influence the quantity and quality of
emissions. The incomplete combustion of soft coal in domestic fires,
for example, produces much smoke, consisting of finely divided
particles of carbon and tarry material, whereas the efficient burning
of pulverised coal in a power station leads to little or no smoke, but
the production of coarser ash particles, which must be removed at
source to avoid their being carried up the flues at a high velocity
and dispersed into the air. The relationship between types of sources
and emissions is summarized in Table 9.
Table 9. Pollutants from combustion sources
Type of source Fuel Sulfur dioxide Particulate matter
Smoke Ash etc.
Domestic heating Wood, peat etc. - + +
or cooking Soft coal ++ ++ +
Hard coal, coke ++ - +
Oil (light distillates) + - -
Gas - - -
Industrial heating Coal, coke ++ - ++
and power generation Oil (heavy residuals) ++ - -
Motor vehicles Petrol - - +
Diesel + + -
Notes: The term smoke is used for incomplete combustion products (notably carbon and tar),
and ash for inorganic components from complete combustion (including lead compounds
in the case of petrol). The signs give only a rough indication of emissions, in the
absence of direct control at source:
- = little or none, + = moderate quantities, ++ = large quantities
In cold and temperate parts of the world, the burning of coal for
domestic heating purposes has been a major contributor to both the
sulfur dioxide and suspended particulate contents of urban air. This
is particularly true of the situation in the United Kingdom prior to
the implementation of the Clean Air Act (Committee on Air Pollution,
1954). Such sources are liable to have a disproportionate effect on
concentrations in the immediate vicinity, because of the low levels of
the chimneys and the low emission velocity. Even in warmer climates,
domestic sources may be of importance, particularly if coal is used
for cooking purposes.
In densely populated areas where domestic sources dominate, the
many chimneys can be considered for some purposes as a diffuse area
source, and, within such an area, concentrations of pollutants in air
remain relatively stable over short distances and short periods of
time. In contrast, large industrial sources should be treated as point
sources, and, at any given location around them, the concentration of
air pollution is liable to vary greatly, even from minute to minute.
The emission into the air of sulfur dioxide and particulate matter
from motor vehicles is relatively small in comparison with those from
domestic and industrial chimneys but it is close to the ground and
within the breathing zone. In these circumstances, concentrations vary
greatly over short distances as well as over short time intervals,
depending on the proximity of the traffic. At points very close to
mixed traffic, smoke from diesel engines may make a substantial
contribution to the concentration of suspended particulate matter in
air (Waller et al., 1965).
Source strength may vary with time of day, day of week, and season
of the year. Accompanying meteorological conditions are also important
in determining the ultimate air concentrations of pollutants arising
from sources. Where heating is required during the winter season,
emissions of sulfur dioxide and particulate matter are usually much
higher than they are in the summer. In a number of cities, however,
where a considerable amount of fuel is used for running cooling
systems, emissions of these pollutants during the summer time are not
always lower than those in winter. Some industrial sources of
pollution may emit little at weekends, and emissions for most sources
are at a minimum during the night.
Although the control of emissions is outside the scope of the
present discussion, the general point can be made that while the
control of particulate emissions is a practicable proposition in many
circumstances, the control of sulfur dioxide at source is relatively
difficult and costly, and the more effective means of reducing
emissions is to change to fuels with a lower sulfur content.
4. DISPERSION AND ENVIRONMENTAL TRANSFORMATIONS
Sulfur compounds dispersed into the air eventually return to the
land or oceans either unchanged, or converted into sulfates. The
sulfur cycle so set up is shown diagrammatically in Fig. 4 (Kellogg et
al., 1972). Particulate matter also returns, its residence time in the
air varying widely according to its physical and chemical
characteristics. As far as direct effects on health are concerned, it
is the local concentration of pollutants in the air at a given time
that is important, as discussed in section 5, but an outline of
dispersion and transformation phenomena is given here.
4.1 Dispersion
The maintenance of a tolerable environment in modern towns depends
very much on the ability of wind and turbulence to disperse the
pollutants rapidly as they are emitted. When these processes fail, the
results can be disastrous, as they were in London in 1952 (Ministry of
Health, United Kingdom, 1954). There are some localities where natural
ventilation is so poor that the emission of pollutants must, at all
times, be carefully controlled. This is especially true of the Los
Angeles area, where emissions of sulfur dioxide and particulate matter
have been successfully curtailed, leaving, however, the major problems
associated with the emission of oxides of nitrogen, hydrocarbons, and
carbon monoxide from motor vehicles (Goldsmith, 1969).
Factors affecting the dispersion of sulfur dioxide and particulate
matter from combustion sources, include:
(a) Temperature and efflux velocity of the gases. Emissions from
small sources, such as domestic fires or incinerators have relatively
little buoyancy, since the temperature at the point of emission is not
much greater than that of the surrounding air. Such sources are liable
therefore to have their greatest impact in the immediate vicinity
(Williams, 1960). Emissions from large-scale industrial installations,
on the other hand, may be at higher temperatures, or may be assisted
by forced-draught to rise more rapidly. Thus, any major impact in the
immediate vicinity may be avoided but weaker effects may be produced
over a wider area (Bosanquet, 1957).
(b) Stack height. Dilution and dispersion over a wide area is
also aided by the use of tall stacks. Much is known of the
relationship between source strengths, stack heights, and ground-level
concentrations of pollutants, through the application of mathematical
modelling techniques, coupled with observations around selected
sources (Briggs, 1965, Pasquill, 1971, Turner, 1968). It is also
possible to devise models to predict concentrations of sulfur dioxide
in urban areas on the basis of emissions from multiple sources
(Fortak, 1970). Conversely, techniques have also been developed for
estimating the pollution inventory of an area from measurements of
sulfur dioxide concentration, fitted to a dispersion model (East,
1972). The height of emission of sulfur dioxide and particulate matter
from domestic sources is primarily a function of the height of the
building itself. Thus the effect on ground level concentrations in the
vicinity is liable to be greater in areas with closely-packed, single
or two-storey houses than in those with high-rise apartments. Tall
stacks are widely used for electricity-generating stations and other
major industrial sources, but the pollutants may then be carried great
distances, often over national boundaries, to be deposited eventually
far from their source (Royal Ministry of Foreign Affairs, Sweden,
1971; Zeeduk & Velds, 1973).
 (c) Topography and the proximity of other buildings. The
presence of hills or tall buildings, and many other features of the
landscape, have important effects on the dispersion of plumes from
individual stacks, or of the pollution from an area source as a whole.
Many industrial cities have developed in river valleys, initially to
take advantage of water transport, but, in general, the dispersion of
pollutants in such a situation is poorer than it would be from a more
exposed location.
(d) Meteorology. Meteorological factors are of fundamental
importance in determining the whole spatial and temporal distribution
of pollution, and the subject has been well reviewed in a recent
publication (Munn, 1976). Apart from the general influence of the
local climate, the great variability of the weather in any one
locality is liable to lead to considerable changes in the
concentrations of sulfur dioxide. In particular, temperature
inversions can trap these pollutants to produce concentrations up to
several hundred times the usual values (Waller & Commins, 1966).
4.2 Transformation and Degradation
In recent years, there has been a rapid escalation of interest in
the ultimate fate of sulfur dioxide and particulate matter emitted
into the air. This is concerned partly with the nature of reaction
products and their possible effects on health, and also with the
ecological effects of these products when deposited (Brosset, 1973)
and their possible role, as aerosols, in modifying the climate on a
global scale (Hobbs et al., 1974).
Some of the sulfur dioxide emitted into the air is removed
unchanged by various surfaces, including soil (Abeles et al., 1971),
water (Liss, 1971; Spedding, 1972), grass (Garland et al., 1973) and
vegetation in general (Hill, 1971). It has been estimated that, in the
United Kingdom, about 25% of the sulfur dioxide is removed by these
direct ("dry" deposition) processes (Garland et al., 1974). The
remainder is transformed into sulfuric acid or sulfates by a variety
of processes, in the presence of moisture, and is then mainly washed
out in rain. Although this self-cleansing process limits the build-up
of sulfur compounds in the air, so minimizing the effects on health,
the "acid rain" produced is considered to be a serious general
environmental problem in some areas (Likens & Bormann, 1974).
A schematic representation of a natural sulfur cycle is shown in
Fig. 5 (Kellogg et al., 1972). Additions to this cycle due to man's
activity are possible at each stage, although 95% of such
contributions are added as sulfur dioxide. Also represented here are
the possible reactions with sunlight. There have been many laboratory
investigations of this process: the reaction is slow (Allen et al.,
1972; Cox & Penkett, 1970), but it is enhanced in the presence of
hydrocarbons and other pollutants associated with motor vehicle
emissions (Cox & Penkett, 1971; Wilson & Levy, 1970). In general,
however, other processes are of even greater importance in the
transformation and removal of sulfur dioxide, including reactions in
water droplets with ammonia (McKay, 1971), and catalytic oxidation in
the presence of manganese or iron (Barrie & Georgii, 1976; Chun &
Quon, 1973). These various reactions involving sulfur dioxide,
(c) Topography and the proximity of other buildings. The
presence of hills or tall buildings, and many other features of the
landscape, have important effects on the dispersion of plumes from
individual stacks, or of the pollution from an area source as a whole.
Many industrial cities have developed in river valleys, initially to
take advantage of water transport, but, in general, the dispersion of
pollutants in such a situation is poorer than it would be from a more
exposed location.
(d) Meteorology. Meteorological factors are of fundamental
importance in determining the whole spatial and temporal distribution
of pollution, and the subject has been well reviewed in a recent
publication (Munn, 1976). Apart from the general influence of the
local climate, the great variability of the weather in any one
locality is liable to lead to considerable changes in the
concentrations of sulfur dioxide. In particular, temperature
inversions can trap these pollutants to produce concentrations up to
several hundred times the usual values (Waller & Commins, 1966).
4.2 Transformation and Degradation
In recent years, there has been a rapid escalation of interest in
the ultimate fate of sulfur dioxide and particulate matter emitted
into the air. This is concerned partly with the nature of reaction
products and their possible effects on health, and also with the
ecological effects of these products when deposited (Brosset, 1973)
and their possible role, as aerosols, in modifying the climate on a
global scale (Hobbs et al., 1974).
Some of the sulfur dioxide emitted into the air is removed
unchanged by various surfaces, including soil (Abeles et al., 1971),
water (Liss, 1971; Spedding, 1972), grass (Garland et al., 1973) and
vegetation in general (Hill, 1971). It has been estimated that, in the
United Kingdom, about 25% of the sulfur dioxide is removed by these
direct ("dry" deposition) processes (Garland et al., 1974). The
remainder is transformed into sulfuric acid or sulfates by a variety
of processes, in the presence of moisture, and is then mainly washed
out in rain. Although this self-cleansing process limits the build-up
of sulfur compounds in the air, so minimizing the effects on health,
the "acid rain" produced is considered to be a serious general
environmental problem in some areas (Likens & Bormann, 1974).
A schematic representation of a natural sulfur cycle is shown in
Fig. 5 (Kellogg et al., 1972). Additions to this cycle due to man's
activity are possible at each stage, although 95% of such
contributions are added as sulfur dioxide. Also represented here are
the possible reactions with sunlight. There have been many laboratory
investigations of this process: the reaction is slow (Allen et al.,
1972; Cox & Penkett, 1970), but it is enhanced in the presence of
hydrocarbons and other pollutants associated with motor vehicle
emissions (Cox & Penkett, 1971; Wilson & Levy, 1970). In general,
however, other processes are of even greater importance in the
transformation and removal of sulfur dioxide, including reactions in
water droplets with ammonia (McKay, 1971), and catalytic oxidation in
the presence of manganese or iron (Barrie & Georgii, 1976; Chun &
Quon, 1973). These various reactions involving sulfur dioxide,
 particulate matter, and other pollutants have been discussed in a
number of reviews (Bufalini, 1971; Calvert, 1973). There may be
limitations to the catalytic processes because of the restricted
availability of reactive metallic oxide, catalytic particles, and
neutralizing compounds in the air. Some of the photochemical reactions
are severely rate-limited, but in others, sulfur dioxide can be
oxidized at an appreciable rate and it has been estimated that
conversion rates as high as 18% per hour might be possible (Rall,
1974). The end-products are similar in all these reactions, i.e., the
formation of aerosols, initially in the submicron size-range,
consisting of a mixture of sulfates and sulfuric acid. There is no
uniform relationship between the proportions of sulfur dioxide,
sulfates, or sulfuric acid in the total sulfur pollution. Emissions
are primarily in the form of sulfur dioxide; thus, in cities close to
sources, the major proportion is in this form, but it has been
reported that, in the USA, the proportion present as sulfate is higher
in western than in eastern urban areas (Altshuller, 1973). At nonurban
sites, concentrations of sulfates may be similar to those of sulfur
dioxide.
The overall conversion of sulfur dioxide to sulfate is an
extremely complex process with many interrelated variables that are
poorly characterized. These include the absorption rate of sulfur
dioxide, the sizes of the particles or droplets involved, their
chemical composition, the rate of diffusion of reactants within the
aerosol, and the relative humidity. The last of these variables is a
major factor, as the catalytic reactions occur with water droplets
containing absorbed sulfur dioxide and other pollutants. Furthermore,
as the pH decreases, the rate of oxidation of sulfur dioxide also
decreases (Junge & Ryan, 1958). Thus, the formation of sulfuric acid
tends to be self-limiting unless the fall in pH is offset by
additional water vapour. On the other hand, the presence of alkaline
compounds, such as ammonia, in the droplet can enhance the reaction
rate due to its buffering capacity. Extrapolated levels for the rate
of oxidation of sulfur dioxide by catalytic processes in urban air
range upwards from 2% per hour (Rall, 1974). Overall, the half-life of
sulfur dioxide in ambient air is estimated to be three to five hours.
The physical and chemical forms of suspended particulate matter in
general may be changed in the air. Some components, such as
hydrocarbons, absorbed initially onto particulate matter, may
evaporate or be oxidized. Even some of the complex hydrocarbons in the
tarry matter from coal burning may be volatile enough to be lost
gradually, and there is evidence that they can be lost from filters
during sampling (Commins & Lawther, 1958). The sizes of the particles
may vary according to the relative humidity, particularly if sulfuric
acid, sulfates, or other salts are present, and this can lead to
precipitation even before the onset of rain (Waller, 1963). The
question of overwhelming importance is the role of particulate matter
in the conversion of sulfur dioxide to sulfuric acid and sulfates.
Traces of metallic compounds, some of which serve as catalysts in
these reactions, are present in particulate matter from the combustion
of coal, and also in the relatively small amount of particulate matter
that may come from the combustion of oil. It has long been considered
that the acute effects on health seen in episodes of high pollution
are crucially dependent on the mixture of sulfur dioxide and
particulate matter present, together with the relative humidity: the
worst effects have been seen with each of these factors in a high
range (Ministry of Health, United Kingdom, 1954).
5. ENVIRONMENTAL CONCENTRATIONS AND EXPOSURES
Sulfur dioxide and suspended particulate matter are the most
widely monitored air pollutants. National sampling networks exist in
many of the industrialized countries of the world, and summaries of
the observations are commonly published in annual reports. Results
from selected sampling sites are also collated by a number of
international organizations (Commission of the European Communities,
1976; Pan American Health Organization, 1976; World Health
Organization, 1976b).
5.1 Concentrations in Outdoor Air
Most sampling networks for sulfur dioxide, smoke, and suspended
particulate matter have been set up for control purposes, to examine
the distribution of these pollutants in various areas and to follow
the long-term trends. Such measurements are normally made on outdoor
air. Where adequate networks exist, there is obviously an advantage in
trying to use them to assess exposure, but this may be far from ideal.
The limitations of the data obtained from the usual monitoring sites
may, to some extent, account for inconsistencies in results in studies
reviewed in section 8. Requirements for monitoring networks have been
discussed further in a recent report (World Health Organization,
1977).
Concentrations of sulfur dioxide and suspended particulate matter
vary greatly from one area to another depending on the nature and
intensity of local sources, and on other factors such as topography,
general weather conditions, and liability to temperature inversions.
Even within a single city there may be large differences in
concentrations. Where sufficient monitoring stations exist, it may be
possible to construct isopleths, showing "contours" of equal
concentration. An example, for the city of Antwerp, is shown in Fig. 6
(Derouane et al., 1972). From this, it is clear that the distribution
of sulfur dioxide is not necessarily the same as that of smoke. There
is a general tendency for the concentrations of these pollutants to be
highest in the largest cities of the world, and within them for the
highest concentrations to be in the central areas. The implementation
of control measures has, however, changed the situation in recent
years, for, in some instances, these have been applied most vigorously
in the central areas of large cities (Masters, 1974).
particulate matter, and other pollutants have been discussed in a
number of reviews (Bufalini, 1971; Calvert, 1973). There may be
limitations to the catalytic processes because of the restricted
availability of reactive metallic oxide, catalytic particles, and
neutralizing compounds in the air. Some of the photochemical reactions
are severely rate-limited, but in others, sulfur dioxide can be
oxidized at an appreciable rate and it has been estimated that
conversion rates as high as 18% per hour might be possible (Rall,
1974). The end-products are similar in all these reactions, i.e., the
formation of aerosols, initially in the submicron size-range,
consisting of a mixture of sulfates and sulfuric acid. There is no
uniform relationship between the proportions of sulfur dioxide,
sulfates, or sulfuric acid in the total sulfur pollution. Emissions
are primarily in the form of sulfur dioxide; thus, in cities close to
sources, the major proportion is in this form, but it has been
reported that, in the USA, the proportion present as sulfate is higher
in western than in eastern urban areas (Altshuller, 1973). At nonurban
sites, concentrations of sulfates may be similar to those of sulfur
dioxide.
The overall conversion of sulfur dioxide to sulfate is an
extremely complex process with many interrelated variables that are
poorly characterized. These include the absorption rate of sulfur
dioxide, the sizes of the particles or droplets involved, their
chemical composition, the rate of diffusion of reactants within the
aerosol, and the relative humidity. The last of these variables is a
major factor, as the catalytic reactions occur with water droplets
containing absorbed sulfur dioxide and other pollutants. Furthermore,
as the pH decreases, the rate of oxidation of sulfur dioxide also
decreases (Junge & Ryan, 1958). Thus, the formation of sulfuric acid
tends to be self-limiting unless the fall in pH is offset by
additional water vapour. On the other hand, the presence of alkaline
compounds, such as ammonia, in the droplet can enhance the reaction
rate due to its buffering capacity. Extrapolated levels for the rate
of oxidation of sulfur dioxide by catalytic processes in urban air
range upwards from 2% per hour (Rall, 1974). Overall, the half-life of
sulfur dioxide in ambient air is estimated to be three to five hours.
The physical and chemical forms of suspended particulate matter in
general may be changed in the air. Some components, such as
hydrocarbons, absorbed initially onto particulate matter, may
evaporate or be oxidized. Even some of the complex hydrocarbons in the
tarry matter from coal burning may be volatile enough to be lost
gradually, and there is evidence that they can be lost from filters
during sampling (Commins & Lawther, 1958). The sizes of the particles
may vary according to the relative humidity, particularly if sulfuric
acid, sulfates, or other salts are present, and this can lead to
precipitation even before the onset of rain (Waller, 1963). The
question of overwhelming importance is the role of particulate matter
in the conversion of sulfur dioxide to sulfuric acid and sulfates.
Traces of metallic compounds, some of which serve as catalysts in
these reactions, are present in particulate matter from the combustion
of coal, and also in the relatively small amount of particulate matter
that may come from the combustion of oil. It has long been considered
that the acute effects on health seen in episodes of high pollution
are crucially dependent on the mixture of sulfur dioxide and
particulate matter present, together with the relative humidity: the
worst effects have been seen with each of these factors in a high
range (Ministry of Health, United Kingdom, 1954).
5. ENVIRONMENTAL CONCENTRATIONS AND EXPOSURES
Sulfur dioxide and suspended particulate matter are the most
widely monitored air pollutants. National sampling networks exist in
many of the industrialized countries of the world, and summaries of
the observations are commonly published in annual reports. Results
from selected sampling sites are also collated by a number of
international organizations (Commission of the European Communities,
1976; Pan American Health Organization, 1976; World Health
Organization, 1976b).
5.1 Concentrations in Outdoor Air
Most sampling networks for sulfur dioxide, smoke, and suspended
particulate matter have been set up for control purposes, to examine
the distribution of these pollutants in various areas and to follow
the long-term trends. Such measurements are normally made on outdoor
air. Where adequate networks exist, there is obviously an advantage in
trying to use them to assess exposure, but this may be far from ideal.
The limitations of the data obtained from the usual monitoring sites
may, to some extent, account for inconsistencies in results in studies
reviewed in section 8. Requirements for monitoring networks have been
discussed further in a recent report (World Health Organization,
1977).
Concentrations of sulfur dioxide and suspended particulate matter
vary greatly from one area to another depending on the nature and
intensity of local sources, and on other factors such as topography,
general weather conditions, and liability to temperature inversions.
Even within a single city there may be large differences in
concentrations. Where sufficient monitoring stations exist, it may be
possible to construct isopleths, showing "contours" of equal
concentration. An example, for the city of Antwerp, is shown in Fig. 6
(Derouane et al., 1972). From this, it is clear that the distribution
of sulfur dioxide is not necessarily the same as that of smoke. There
is a general tendency for the concentrations of these pollutants to be
highest in the largest cities of the world, and within them for the
highest concentrations to be in the central areas. The implementation
of control measures has, however, changed the situation in recent
years, for, in some instances, these have been applied most vigorously
in the central areas of large cities (Masters, 1974).
 Table 10. Concentrations of sulfur dioxide, smoke, and suspended particulate
matter (1974)a
Site Concentration (µg/m 3)
annual arithmetic mean maximum daily mean
Sulfur dioxide
Brussels 107 347
Frankfurt 119 455
London 150 503
Madrid 161 763
Prague 126 482
Rome 108 600
Zagreb 173 893
Smoke, by reflectance
Brussels 37 -
London 26 149
Madrid 190 908
Rome 60 160
Suspended particulate by high volume sampler
Calcutta 519 1090
St Louis 87 189
Vancouver 64 134
Zagreb 167 806
a From: World Health Organization (1976b). Sites selected for inclusion
here are all classified as city centre commercial sites. The
selection is also limited to sites using 24-h averaging periods.
Further information is available in the WHO report of frequency
distributions, standard deviations, and monthly and annual
geometric means.
Examples of concentrations of sulfur dioxide and smoke or suspended
particulate matter, drawn from the WHO air quality monitoring
programme (World Health Organization, 1976b) are shown in Table 10.
For present purposes, results included in this table are limited to
those from one type of site (city centre commercial sites), where the
sampling methods and averaging periods are also comparable with one
another. Even so, much caution must be exercised in drawing
comparisons between cities, for the sites can only be representative
of their immediate surroundings.
The annual mean concentrations of sulfur dioxide are fairly
uniform at the particular sites quoted in Table 10, ranging from about
100 to 200 µg/m3 (0.035-0.070 ppm). For particulate matter, however,
the variation between cities appears to be much greater and it seems
likely that some of the results are unduly influenced by sources close
to the samplers, or by high background levels of dust from
noncombustion sources.
There are few reports about ambient levels of sulfates. A study
from the USA (Altshuller, 1973) reported annual, arithmetic mean
concentrations at urban sites in the range of 2.4 to 48.7 µg/m3, with
an average ratio of sulfur dioxide to sulfate of 4.7. The relationship
between these two pollutants was not, however, entirely consistent and
the ratio tended to be higher at western sites than at eastern sites.
In the east, there was a general background level of sulfate of about
5 µg/m3, even at nonurban sites, and this was attributed to the long
distance transport of sulfur dioxide, with conversion to sulfate
during transport. There also appeared to be a "saturation" level of
sulfate at about 17 µg/m3 at eastern urban sites, within the sulfur
dioxide range of 100-200 µg/m3 (0.035-0.070 ppm).
There is even less published information concerning concentrations
in air of sulfuric acid, and such observations must always be related
to the method of measurement. One series of measurements of net
particulate acid, as determined by titration of samples collected on
filter papers, has indicated a mean concentration in London of
approximately 4 µg/m3 representing a few percent of the
corresponding concentration of sulfur dioxide (Commins & Waller,
unpublished data). However, the concentration of this pollutant is
liable to increase rapidly during temperature inversions, particularly
if the relative humidity is high (Commins, 1967).
For each of the pollutants considered, there are generally large
variations in concentration with time at any one place. The extent to
which this can be followed depends on the time resolution of the
sampling instruments. Usually, integrated samples are collected over
24-h periods to yield daily mean values, and from these monthly,
seasonal, and annual means are calculated. Shorter sampling periods
may however be used, and where continuous automatic instruments are
used, virtually instantaneous values can be obtained. Relationships
between values averaged over different periods have been extensively
studied in the USA (Larsen, 1971). An indication of the day-to-day
variation in smoke and sulfur dioxide concentrations in a large city
(London) is given in Fig. 7.
Table 10. Concentrations of sulfur dioxide, smoke, and suspended particulate
matter (1974)a
Site Concentration (µg/m 3)
annual arithmetic mean maximum daily mean
Sulfur dioxide
Brussels 107 347
Frankfurt 119 455
London 150 503
Madrid 161 763
Prague 126 482
Rome 108 600
Zagreb 173 893
Smoke, by reflectance
Brussels 37 -
London 26 149
Madrid 190 908
Rome 60 160
Suspended particulate by high volume sampler
Calcutta 519 1090
St Louis 87 189
Vancouver 64 134
Zagreb 167 806
a From: World Health Organization (1976b). Sites selected for inclusion
here are all classified as city centre commercial sites. The
selection is also limited to sites using 24-h averaging periods.
Further information is available in the WHO report of frequency
distributions, standard deviations, and monthly and annual
geometric means.
Examples of concentrations of sulfur dioxide and smoke or suspended
particulate matter, drawn from the WHO air quality monitoring
programme (World Health Organization, 1976b) are shown in Table 10.
For present purposes, results included in this table are limited to
those from one type of site (city centre commercial sites), where the
sampling methods and averaging periods are also comparable with one
another. Even so, much caution must be exercised in drawing
comparisons between cities, for the sites can only be representative
of their immediate surroundings.
The annual mean concentrations of sulfur dioxide are fairly
uniform at the particular sites quoted in Table 10, ranging from about
100 to 200 µg/m3 (0.035-0.070 ppm). For particulate matter, however,
the variation between cities appears to be much greater and it seems
likely that some of the results are unduly influenced by sources close
to the samplers, or by high background levels of dust from
noncombustion sources.
There are few reports about ambient levels of sulfates. A study
from the USA (Altshuller, 1973) reported annual, arithmetic mean
concentrations at urban sites in the range of 2.4 to 48.7 µg/m3, with
an average ratio of sulfur dioxide to sulfate of 4.7. The relationship
between these two pollutants was not, however, entirely consistent and
the ratio tended to be higher at western sites than at eastern sites.
In the east, there was a general background level of sulfate of about
5 µg/m3, even at nonurban sites, and this was attributed to the long
distance transport of sulfur dioxide, with conversion to sulfate
during transport. There also appeared to be a "saturation" level of
sulfate at about 17 µg/m3 at eastern urban sites, within the sulfur
dioxide range of 100-200 µg/m3 (0.035-0.070 ppm).
There is even less published information concerning concentrations
in air of sulfuric acid, and such observations must always be related
to the method of measurement. One series of measurements of net
particulate acid, as determined by titration of samples collected on
filter papers, has indicated a mean concentration in London of
approximately 4 µg/m3 representing a few percent of the
corresponding concentration of sulfur dioxide (Commins & Waller,
unpublished data). However, the concentration of this pollutant is
liable to increase rapidly during temperature inversions, particularly
if the relative humidity is high (Commins, 1967).
For each of the pollutants considered, there are generally large
variations in concentration with time at any one place. The extent to
which this can be followed depends on the time resolution of the
sampling instruments. Usually, integrated samples are collected over
24-h periods to yield daily mean values, and from these monthly,
seasonal, and annual means are calculated. Shorter sampling periods
may however be used, and where continuous automatic instruments are
used, virtually instantaneous values can be obtained. Relationships
between values averaged over different periods have been extensively
studied in the USA (Larsen, 1971). An indication of the day-to-day
variation in smoke and sulfur dioxide concentrations in a large city
(London) is given in Fig. 7.
 Although annual means, coupled with daily maxima, give a general
impression of pollution levels in any given locality, long series of
results are often summarized as frequency distributions. These have
been shown to be log-normal for a wide range of averaging times,
pollutants, and localities (Pollack, 1975). This suggests that the
geometric mean, which, in such a case, is equivalent to the median, is
perhaps the most appropriate central value to use. Historically,
however, the arithmetic mean has been more widely used. For the usual
log-normal distribution, the geometric mean is a little lower than the
arithmetic mean. Percentiles of the frequency distribution are
tabulated in some monitoring networks (US Environmental Protection
Agency, 1974a), and the complete distributions can conveniently be
plotted on log-probability paper, as in the example in Fig. 8 drawn
from data for a recent 5-year period in London (Commins & Waller,
unpublished data).
Relationships between peak and mean values for sulfur dioxide have
also been considered on an empirical basis. For a number of cities in
Europe, the highest daily mean concentrations during the year have
been found to be of the order of four times the annual means
(Commission of the European Communities, 1976). Transient peaks in
continuous records have been examined in the USA in relation to
averaging times: the ratio of peak to mean values has been found to be
2.3 for hourly averaging periods, increasing for successively longer
periods (Montgomery & Coleman, 1975). Some highly sophisticated
networks exist for the measurement of sulfur dioxide on a continuous
basis at many points. The collection and interpretation of such data
then presents a formidable task, and in some of these networks on-line
computers are used for data acquisition (Lauer & Benson, 1975).
The examination of trends in the concentrations of sulfur dioxide
and particulate matter is important when the effects of long-term
exposures are investigated. In urban areas of most developed
countries, there has been a tendency for levels to decline in recent
years as a result of control efforts, although elsewhere, and
particularly where concentrations of these pollutants had previously
been low, increases have occurred as emissions from industrial and
other sources have increased. Since variations in weather patterns
from year-to-year can affect even the annual mean concentrations, long
series are required to examine trends adequately. The declining trend
in sulfur dioxide concentrations seen in a number of large cities in
Europe during the 1960s (Commission of the European Communities, 1976)
may, to a large extent, reflect declining emissions or improved
dispersion from chimneys, but some authors have considered that
changing weather conditions have been a contributory factor (Van Dop &
Kruizinga, 1976). In the United Kingdom, there has been an overall
decline in sulfur dioxide concentrations without a corresponding
decline in total emissions (Fig. 9). This is attributable to the
Although annual means, coupled with daily maxima, give a general
impression of pollution levels in any given locality, long series of
results are often summarized as frequency distributions. These have
been shown to be log-normal for a wide range of averaging times,
pollutants, and localities (Pollack, 1975). This suggests that the
geometric mean, which, in such a case, is equivalent to the median, is
perhaps the most appropriate central value to use. Historically,
however, the arithmetic mean has been more widely used. For the usual
log-normal distribution, the geometric mean is a little lower than the
arithmetic mean. Percentiles of the frequency distribution are
tabulated in some monitoring networks (US Environmental Protection
Agency, 1974a), and the complete distributions can conveniently be
plotted on log-probability paper, as in the example in Fig. 8 drawn
from data for a recent 5-year period in London (Commins & Waller,
unpublished data).
Relationships between peak and mean values for sulfur dioxide have
also been considered on an empirical basis. For a number of cities in
Europe, the highest daily mean concentrations during the year have
been found to be of the order of four times the annual means
(Commission of the European Communities, 1976). Transient peaks in
continuous records have been examined in the USA in relation to
averaging times: the ratio of peak to mean values has been found to be
2.3 for hourly averaging periods, increasing for successively longer
periods (Montgomery & Coleman, 1975). Some highly sophisticated
networks exist for the measurement of sulfur dioxide on a continuous
basis at many points. The collection and interpretation of such data
then presents a formidable task, and in some of these networks on-line
computers are used for data acquisition (Lauer & Benson, 1975).
The examination of trends in the concentrations of sulfur dioxide
and particulate matter is important when the effects of long-term
exposures are investigated. In urban areas of most developed
countries, there has been a tendency for levels to decline in recent
years as a result of control efforts, although elsewhere, and
particularly where concentrations of these pollutants had previously
been low, increases have occurred as emissions from industrial and
other sources have increased. Since variations in weather patterns
from year-to-year can affect even the annual mean concentrations, long
series are required to examine trends adequately. The declining trend
in sulfur dioxide concentrations seen in a number of large cities in
Europe during the 1960s (Commission of the European Communities, 1976)
may, to a large extent, reflect declining emissions or improved
dispersion from chimneys, but some authors have considered that
changing weather conditions have been a contributory factor (Van Dop &
Kruizinga, 1976). In the United Kingdom, there has been an overall
decline in sulfur dioxide concentrations without a corresponding
decline in total emissions (Fig. 9). This is attributable to the

 gradual elimination of sources, such as domestic fires, that had a
substantial effect on local concentrations, and their replacement by a
smaller number of large sources dispersing the sulfur dioxide more
widely. In the case of smoke, concentrations in the United Kingdom
have declined in parallel with the emissions (Fig. 10). Domestic fires
always had a dominant effect, and these have been subject to control
in an increasing number of urban areas.
5.2 Concentrations in Indoor Air
As yet, there is relatively little information available
concerning the concentrations of sulfur dioxide and particulate matter
in indoor environments (excluding those specifically related to
occupational exposures). It is quite possible to make such
measurements indoors by most of the methods mentioned in section 2,
subject to additional care about interfering substances, and
limitations of noise for equipment such as the high volume sampler.
The results are, however, of limited value for general monitoring
purposes, because of the additional variability introduced by the
circumstances within each building. As far as human exposure is
concerned, much time is spent indoors, particularly by the oldest and
youngest members of the community, and information on indoor
concentrations is required for epidemiological studies.
Whether there are substantial differences in indoor and outdoor
concentrations of sulfur dioxide and particulate matter will depend on
the degree of ventilation, the capacity of surfaces within to absorb
or otherwise collect these pollutants, and the presence of sources
either of the pollutants themselves, or of others that may interact
with them.
In warm climates not subject to frequent rain or other adverse
weather conditions, buildings may be left open enough to ensure that
indoor concentrations of pollutants are virtually the same as those
outdoors. Even so, there can be local problems associated with the use
of fuels in equipment with poor flues or without flues. Extremely high
concentrations of pollutants, including smoke, have been reported
inside primitive dwellings in tropical regions, where cooking is
carried out over open fires (Sofoluwe, 1968). The open coal fires that
were so widely used in the United Kingdom prior to the Clean Air Act
of 1956 had two possible, and opposing, effects on indoor
concentrations. The very large ventilation rate that they induced
helped to maintain indoor concentrations of smoke and sulfur dioxide
close to those outdoors, but, in unfavourable wind conditions,
downdraughts could force these pollutants from the fire itself into
the room, producing concentrations far in excess of those outdoors.
Examples of indoor concentrations of suspended particulate matter or
(more rarely) of sulfur dioxide exceeding those out of doors have also
been found in studies in the Netherlands (Biersteker et al., 1956) and
in the USA (Yocom et al., 1971).
gradual elimination of sources, such as domestic fires, that had a
substantial effect on local concentrations, and their replacement by a
smaller number of large sources dispersing the sulfur dioxide more
widely. In the case of smoke, concentrations in the United Kingdom
have declined in parallel with the emissions (Fig. 10). Domestic fires
always had a dominant effect, and these have been subject to control
in an increasing number of urban areas.
5.2 Concentrations in Indoor Air
As yet, there is relatively little information available
concerning the concentrations of sulfur dioxide and particulate matter
in indoor environments (excluding those specifically related to
occupational exposures). It is quite possible to make such
measurements indoors by most of the methods mentioned in section 2,
subject to additional care about interfering substances, and
limitations of noise for equipment such as the high volume sampler.
The results are, however, of limited value for general monitoring
purposes, because of the additional variability introduced by the
circumstances within each building. As far as human exposure is
concerned, much time is spent indoors, particularly by the oldest and
youngest members of the community, and information on indoor
concentrations is required for epidemiological studies.
Whether there are substantial differences in indoor and outdoor
concentrations of sulfur dioxide and particulate matter will depend on
the degree of ventilation, the capacity of surfaces within to absorb
or otherwise collect these pollutants, and the presence of sources
either of the pollutants themselves, or of others that may interact
with them.
In warm climates not subject to frequent rain or other adverse
weather conditions, buildings may be left open enough to ensure that
indoor concentrations of pollutants are virtually the same as those
outdoors. Even so, there can be local problems associated with the use
of fuels in equipment with poor flues or without flues. Extremely high
concentrations of pollutants, including smoke, have been reported
inside primitive dwellings in tropical regions, where cooking is
carried out over open fires (Sofoluwe, 1968). The open coal fires that
were so widely used in the United Kingdom prior to the Clean Air Act
of 1956 had two possible, and opposing, effects on indoor
concentrations. The very large ventilation rate that they induced
helped to maintain indoor concentrations of smoke and sulfur dioxide
close to those outdoors, but, in unfavourable wind conditions,
downdraughts could force these pollutants from the fire itself into
the room, producing concentrations far in excess of those outdoors.
Examples of indoor concentrations of suspended particulate matter or
(more rarely) of sulfur dioxide exceeding those out of doors have also
been found in studies in the Netherlands (Biersteker et al., 1956) and
in the USA (Yocom et al., 1971).
 In general, however, in the absence of specific sources of sulfur
dioxide or fine particulate matter indoors, concentrations are
generally less than those outdoors. Sulfur dioxide as a gas, can
diffuse readily onto walls and other surfaces. There is evidence that
it reacts with ammonia from the indoor air on painted surfaces,
particularly in the presence of moisture (Holbrow, 1958), but it is
most effectively absorbed on clothing, curtains, carpets, and other
soft furnishings, so that in domestic surroundings where these abound
concentrations of sulfur dioxide are only of the order of 20% of those
outdoors (Weatherley, 1966), while in offices and other buildings
containing less absorbing material, concentrations may be 40-50% of
those outdoors (Andersen, 1972; Derouane, 1972). The presence of
ammonia in occupied rooms is important in relation to measurements of
sulfur dioxide. Concentrations of this "natural" pollutant may be much
higher than outdoors, particularly where there are young babies or old
people with incontinence problems. The ammonia will only react
effectively with sulfur dioxide in the presence of moisture, but it
will, in any case, interfere with the determination of the sulfur
dioxide by acidimetric or conductometric methods.
Smoke, that is to say the finely divided black material from
incomplete combustion, can penetrate fairly readily into buildings,
and since the mobility of the particles is less than that of sulfur
dioxide molecules, they are less rapidly removed onto surfaces.
Concentrations of smoke indoors, assessed by soiling methods, have
been found to be in the range of 50-90% of those outdoors (Derouane,
1972; Yocom et al., 1971), again assuming there is no specific source
of this material inside. Cigarette smoke can make a very substantial
contribution to the finely divided particulate matter indoors (Elliott
& Rowe, 1976; Hoegg, 1972). It is liable to affect direct gravimetric
concentrations, but it has relatively little effect on concentrations
measured by blackness. The composition of cigarette smoke is quite
different from that of the general urban particulate matter, and it
has been stressed by De Graaf & Biersteker (1972) that there may be
many differences in the type and composition of suspended particulate
matter indoors and outdoors. This point is particularly important in
relation to samples obtained with the high volume sampler. The larger
particles (greater than 10 µm diameter) that are liable to be
collected by this method would penetrate into buildings less readily
than the fine particles, and, with reduced air movement inside, they
may fall out fairly rapidly under gravity. This may account for the
smaller proportion of total suspended particles (as compared with
smoke) found indoors in some studies (Yocom et al., 1971). However, on
the other hand, there is a risk that textile and other dusts dispersed
in the home may be sampled and assessed as part of the general
suspended particulate matter.
Sulfuric acid is unlikely to remain for any appreciable time in
occupied rooms, as it is readily neutralized by ammonia. However,
finely divided sulfate particles are likely to behave in the same way
as smoke, with a modest reduction indoors compared with outdoors.
Relative humidity is normally lower indoors than outdoors and this
will help to keep sulfate particles in suspension, but where the
humidity is especially high, as it may be in kitchens, particles
containing sulfates or other salts will grow rapidly and be deposited.
Studies on indoor concentrations of sulfur dioxide and particulate
matter are limited primarily by the need to consider the circumstances
of each location individually; nevertheless, there is a growing body
of literature on the subject that has been assembled and reviewed in
recent reports from the USA (Benson et al., 1972; Henderson et al,
1973).
5.3 Concentrations in Work Places
Concentrations of sulfur dioxide, much higher than those commonly
found in urban air, may be present in some industrial environments,
arising from processes in which the gas is handled or evolved, as well
as from combustion sources. Paper mills, sulfuric acid plants, steel
works, nonferrous metal foundries, and oil refineries are among the
places where such concentrations may be found. However, emissions are
usually highly localized and intermittent, presenting major problems
in assessing concentrations to which workers may be exposed. Results
are not normally published, but in a number of countries there is a
requirement to ensure that a specified limit is not exceeded. For
example, in the USSR, the maximum permissible concentration is
10 mg/m3 (3.8 ppm) (ILO/WHO Committee on Occupational Health, 1970)
whereas in the USA (American Conference of Government Industrial
Hygienists, 1977), this value, averaged over 8-h shifts, is 13 mg/m3
(5 ppm). This figure is of the order of 100 times the average values
in urban air and it is higher than the maximum values reported, even
in episodes of high pollution (Waller & Commins, 1966). Mean values
over 3 years of 1800-2100 µg/m3 (0.6-0.7 ppm) have been reported
close to blast furnaces in steelworks, with occasional 24-h mean
values up to 17 000 µg/m3 (5.95 ppm) (Lowe et al., 1970).
In steelworks, substantial quantities of suspended particulate
matter may be present as well as sulfur dioxide and other pollutants.
A 3-year mean concentration of about 1000 µg/m3 (respirable dust, as
measured with a Hexhlet sampler) has been reported in the study cited
above (Lowe et al., 1970). Even so, the situation does not necessarily
parallel that in urban atmospheres, for the particulate matter is
liable to include dust which differs in composition and size
distribution from that produced in the usual range of combustion
processes.
Dusts encountered in industry must, in general, be considered
separately from the suspended particulate matter in urban air. Some,
such as the coal-dust in mines, may be present at high concentrations,
and have substantial effects on health, but these are specific to that
dust, and are outside the scope of the present discussion. However,
some of the chemical compounds in dust from industrial activities are
covered by other documents of the WHO environmental health criteria
programme.
In some industries, such as wood-pulping and paper-making, sulfur
dioxide may be evolved in the process, producing high concentrations
locally without an accompanying particulate matter problem.
Concentrations in the range 6 to 100 mg/m3 (2 to 36 ppm) have been
reported at a plant in Norway (Skalpe, 1964) and similar
concentrations were found in a study in the USA (Ferris et al., 1967)
but these were gradually reduced over a 5-year period. High
concentrations, averaging about 71 mg/m3 (25 ppm), also existed at
one time in charging rooms for refrigerators when sulfur dioxide was
used as a refrigerant (Kehoe et al., 1932) and similar concentrations
have been reported in certain areas of oil refineries (Anderson 1950).
There is a specific problem of sulfuric acid mist in the forming
departments of works making lead-acid accumulators, where
concentrations up to 16.6 mg/m3 have been observed (Malcolm & Paul,
1961; Williams, 1970). This is very much greater than the
concentrations ever found in urban air (Commins & Waller, 1978) and
the physical form of the aerosol is different: mass median diameters
of the droplets have been reported to be over 10 µm (Williams, 1970)
whereas those found in urban air are of the order of 0.5 µm (Waller,
1963).
5.4 Assessment of Exposures
In the present context, the term exposure relates to the
concentrations of sulfur dioxide or suspended particulate matter in
the air breathed by individuals or populations, averaged over
specified periods. Broadly speaking, two types of exposure are
considered: short-term exposure, in which the relevant concentrations
are averaged over periods of the order of a day, and long-term
exposure, for which averages over periods of the order of a year are
commonly used. These periods are to a certain extent arbitrary,
imposed by the time-resolution of the pollution measurements and by
the nature of the health indices examined, and there is no clear
guidance on the most relevant averaging periods to use in relation
either to short-or to long-term exposure.
Exposures are usually estimated from measurements of concentration
at fixed sampling sites. The number of sampling sites required to give
an adequate representation of the exposure of people living in a given
area will depend on the topography, the distribution of sources, and
other factors. In urban areas, there is usually a close correlation
between values for neighbouring stations (Prinz, 1970). Care must be
taken, however, to ensure that these stations are not used to
represent environmental levels in areas much larger than their
coverage. Furthermore, the instruments for measurement may be sited on
the roof of a building for protection and convenience, and the levels
measured may not necessarily represent those in the ordinary breathing
zone.
The usual practice in epidemiological studies is to assume that
measurements made on outdoor air in the areas where people live or
work provide an index of exposure that allows comparison to be made
between different groups. It has been demonstrated, however, that
estimated weekly exposures calculated as averages of concentrations
measured indoors and at various outdoor sites and weighted according
to the length of time likely to be spent in each location, may differ
substantially from those indicated by measurements at just one site
close to the place of residence or work (Fugas, 1976). To validate
this approach, further measurements using personal samplers would be
required. These have been used in studies on specific components of
suspended particulate matter such as lead (Azar et al., 1972) and they
have wide application in assessing the exposures of individuals to
pollutants, including sulfur dioxide, in industry, but they are not
generally practicable for large-scale epidemiological studies on the
general population.
A further problem in assessing exposures is the great variation in
concentrations with time for, as far as sulfur dioxide is concerned, a
brief exposure to a very high concentration, with no appreciable
exposure during the rest of the day, would create a different
situation from a steady exposure to a low level throughout the day. At
present, there is no satisfactory way of measuring such situations, at
least for people moving about during the course of the day. Providing
studies are confined to groups of people living or working in
reasonably uniform circumstances, measurements made over extended
periods at a few fixed sites may give results that are valid for
comparative purposes. Difficulties are liable to arise however if
dissimilar groups are compared. In particular, people such as the very
old and the very young may be partially protected from sulfur dioxide
in the outside air if they spend long periods indoors, whereas an
active worker may be exposed not only to a higher mean concentration
in the course of the day, but also to a greater range. A further point
is that increased activity will also lead to a greater ventilation
rate, increasing the overall intake, and, in the case of sulfur
dioxide, the deep inspirations associated with exercise are liable to
increase the penetration of the gas and to enhance its effects
(Lawther et al., 1975). The situation is even more complex for
sulfuric acid aerosols. Because of the hygroscopic properties of the
droplets, they take up moisture rapidly on inspiration, becoming
larger and more dilute, and it has been suggested, on the basis of
experimental work, that those from urban air could be diluted to such
an extent that their irritant properties would be lost (Carabine &
Maddock, 1976).
When assessing long-term exposures, annual mean concentrations at
fixed sampling sites are commonly used as the basis. Short-term
variations are then of little consequence, but it may still be
necessary to consider the extent of seasonal swings in concentration,
or the frequency of occurrence of days of exceptionally high
pollution. Also, it is often necessary to examine trends in
concentrations for many years back, for the relevant exposure may have
been one that occurred earlier in life, rather than the current level.
Since there are very few long series of measurements of sulfur dioxide
or suspended particulate matter made by uniform methods, it is very
difficult to assess the true exposure of people to these pollutants.
6. ABSORPTION, DISTRIBUTION, AND ELIMINATION
Most of the sulfur compounds discussed in this report are absorbed
via the digestive system. Relatively little is absorbed from the
respiratory tract, even in areas with highly polluted ambient air.
Even so, it is reasonable to believe that the respiratory tract is the
most vulnerable organ for the local effects of sulfur oxides and
particulate matter in the ambient air. Thus, this section will deal
mainly with the absorption, deposition, and clearance of sulfur
dioxide and particulate matter in the respiratory tract.
6.1 Absorption and Deposition in the Respiratory Tract
6.1.1 Sulfur dioxide
Sulfur dioxide is highly soluble in aqueous media. Absorption
after inhalation has been studied in rabbits (Dalhamn & Strandberg,
1961; Strandberg, 1964), dogs (Balchum et al., 1959, 1960a, 1960b;
Frank et al., 1969) and man (Speizer & Frank, 1966a, 1966b).
In rabbits, about 40% of the inhaled sulfur dioxide is absorbed in
the nose and pharynx when concentrations of about 290 µg/m3 (0.1 ppm)
are inhaled. At higher concentrations (29-290 mg/m3; 10-100 ppm), the
fraction absorbed is much higher (about 95%),The reasons for these
different rates of absorption are not clear. In dogs, more than 99% of
the inhaled sulfur dioxide is absorbed by the nose at exposure levels
of 2.9-140 mg/m3 (1-50 ppm). These observations in dogs have been
confirmed in man by studies on human volunteers, with levels of
exposure to sulfur dioxide ranging from 2.9 to 420 mg/m3 (1 to
140 ppm) and exposure times of a few minutes at the higher levels and
30-40 minutes at the lower levels. Absorption can occur during
mouth-breathing, but is less efficient than during nose-breathing,
especially with increased ventilation.
6.1.2 Airborne particles
Particles smaller than about 10 µm are deposited at different
levels in the respiratory tract. The exact deposition pattern is
determined by the interaction of size, shape, and density (expressed
as aerodynamic diameter of unit density spheres) of the particles and
by airflow conditions. Some particles in the air, such as sulfuric
acid particles are hygroscopic. Such particles will take up water,
expand in the respiratory tract, and be deposited in a manner other
than would be expected from their diameters in the ambient air (Hatch
& Gross, 1964; Stuart, 1973; Task Group on Lung Dynamics, 1966;
Vigdorcik, 1948). Sophisticated theoretical models for the deposition
of particles in the lung such as the ICRP model have been constructed
(Task Group on Lung Dynamics, 1966). Deposition is caused by
impaction, sedimentation, and diffusion. Impaction is an important
mechanism for the deposition of the larger or heavier particles
(5-30 µm aerodynamic diameter) and where the air velocity is
relatively high. Impaction occurs therefore at sites where the air
stream is turbulent and is of most importance in the nose, mouth,
pharynx, and the upper part of the tracheobronchial tree.
Sedimentation or settling out of particles is also a function of
particle size and density, as well as of residence time in the
airways. It is important for the deposition of larger particles
(1-5 µm) providing they have not been deposited by impaction and is
active in the trachea, bronchi, and bronchioles. Diffusion is of
importance for particles smaller than a few tenths of 1 µm (its effect
increasing with decreasing particle size), with respect to the smaller
bronchioles and especially the alveoli. In the size range of 2-5 µm,
experimental data have fitted quite well with the ICRP model, but
deposition has varied widely (with an order of magnitude of 2 to 3)
among human subjects (Lippmann et al., 1971). In experiments on
animals, large reproducible differences in deposition have been seen
among individuals within the same species (Albert et al., 1968;
Tomenius, 1973). In the submicron range, theoretical models have not
yet been satisfactorily verified by experiments.
6.2 Clearance from the Respiratory Tract and Distribution
6.2.1 Sulfur dioxide
Sulfur dioxide is absorbed from the respiratory tract into the
blood stream. It can then be widely distributed throughout the body
(Balchum et al., 1960a, 1960b; Bystrova, 1957; Frank et al., 1967;
Yokoyama et al., 1971), where it appears to be metabolized and
excreted via the urinary tract.
6.2.2 Particulate matter
Knowledge concerning the elimination of insoluble particles
deposited in the alveoli is incomplete (Hatch & Gross, 1964; Morrow,
1973). It is clear that these particles are, to a large extent,
phagocytized by alveolar macrophages within hours, but it is not known
to what extent they are actively carried to the ciliated part of the
lung or to the lymphatic system. The particles can be transported by
the mucociliary escalator into the interstitium (where they can remain
for a long time) or the lymphatic system. Solubility in vivo is
important for the clearance of "insoluble" particles deposited in the
alveoli (Mercer, 1967; Morrow, 1973). The biological half-lives range
from days to years depending on the chemical composition of the
particles (Brain & Valberg, 1974; Task Group on Lung Dynamics, 1966;
Task Group on Metal Accumulation, 1973).
Mucociliary transport, and therefore clearance of particles, can
be affected by various factors and can be impaired by long-term
cigarette smoking and acute infection in the respiratory tract (Camner
& Philipson, 1972; Camner et al., 1973a; Jarstrand et al, 1974).
Subjects suffering from chronic obstructive lung disease may also have
an impaired mucociliary transport (Camner et al., 1973b; Toigo et al.,
1963). Studies in animals have shown that the inhalation of sulfur
dioxide can interfere with the clearance of bacteria (Rylander et al.,
1971) and inert particles (Ferin & Leach, 1973) from the lungs, but
how much is due to the action of the sulfur dioxide on the mucociliary
mechanism and how much to its action on the macrophages is not clear.
The clearance of soluble particles may follow the above pathways
depending upon their solubility. They may dissolve in the mucus in
which case they will probably be eliminated via the mucociliary route
and be swallowed and removed or absorbed via the intestinal tract. In
the alveoli, the particles may diffuse into the lymph or blood and
thus be removed from the lung.
7. EFFECTS ON EXPERIMENTAL ANIMALS
It has been difficult to separate the relative effects of sulfur
dioxide, sulfuric acid mists, sulfate salts, and particulate matter in
the ambient air by epidemiological techniques. The effects of these
substances individually and in various combinations have been studied
in the laboratory. These studies have been useful in explaining some
of the mechanisms of action but their application has been rather
limited for the establishment of safe ambient levels, particularly for
complex mixtures such as exist in the ambient air. In the past, the
levels used in laboratory studies on animals have usually been far in
excess of those seen in the ambient air and therefore have had little
relevance for ambient air quality standards. Later studies have been
at more relevant levels.
The following discussion separates work on animals into short-term
and long-term exposure studies. Short-term exposure studies are those
that involve exposures of 24-h or less and usually last from minutes
to at the most a few hours. Long-term exposure studies refer to
exposures that last longer than 24-h and are usually extended over
months. In the short-term exposure studies, only immediate or acute
effects have been studied. In long-term exposure studies, animals may
be studied during the period of exposure to determine whether there
are progressive changes or not and at the end of the exposure period
to quantify any chronic effect.
7.1 Short-term Exposure Studies
7.1.1 Exposure to sulfur dioxide singly or in combination with
other agents
Sulfur dioxide is a respiratory irritant that is very soluble in
the aqueous surfaces of the respiratory airways. Because of this high
solubility, most of the sulfur dioxide is absorbed in the nose and
upper airways (section 6.1) and very little reaches the lungs
directly. At extremely high concentrations, the absorptive capacity of
the upper airways can be overwhelmed and death or pathological changes
including laryngotracheal and pulmonary oedema can be induced in the
respiratory tract of experimental animals.
Exposure-effect curves have been developed for guineapigs (Amdur,
1966) and dogs (Frank & Speizer, 1965). In Amdur's study, a linear
relationship was obtained between exposure for 1 h to sulfur dioxide
concentrations ranging from 0.46 to 2380 mg/m3 (0.16-835 ppm) and
corresponding increases in pulmonary flow resistance. The second study
showed that nasal flow resistance increased roughly in proportion to
exposure to sulfur dioxide concentrations ranging from 20 to
660 mg/m3 (7-230 ppm) for a 15-20 minute period.
Studies with guineapigs at lower concentrations have shown that a
sodium chloride aerosol, administered concomitantly enhances the
effects of sulfur dioxide on the lungs in the form of
bronchoconstriction and increased airway resistance (Amdur, 1957). It
was postulated that the sodium chloride could act as a carrier to
deliver the absorbed sulfur dioxide deep into the lungs, or that the
increased humidity in the respiratory tract could react with the
sulfur dioxide to form sulfurous acid, especially if catalysts were
present (Amdur & Underhill, 1968). McJilton et al. (1973) have
reaffirmed the importance of humidity in this reaction, exposing
guineapigs for 1 h to sulfur dioxide at 3.1 mg/m3 (1.1 ppm) and a
sodium chloride aerosol of about 1 mg/m3. They allowed the mixture to
"age" in a reaction chamber for 8-10 minutes at various relative
humidities. The chamber temperature was 22°C and the average aerosol
particle size was 0.1 µm with a maximum size of less than 2 µm. At
relative humidities above 80%, they noted a marked increase in
pulmonary resistance, whereas administration of the individual
components produced little or no effect. The droplets formed at high
humidity and in the presence of the aerosol had a pH of 3.2 ± 0.5.
Analysis of these particles by mass spectrometer revealed sulfur
dioxide and bisulfite ions (HSO-3)in the solution but no sulfuric
acid. In the high humidity, the sodium chloride aerosol apparently
became hydrated and could then absorb sulfur dioxide. The authors
indicated that the mixture had to "age" to ensure absorption of the
sulfur dioxide; otherwise there would be too much competition with the
moist surfaces in the nasopharynx which could sweep out the sulfur
dioxide. Thus, this is another mechanism whereby the effect of sulfur
dioxide can be enhanced. If such mixtures were allowed to "age"
longer, it seems likely that the sulfur dioxide could be changed to
sulfuric acid. Studies using other animal species such as the cat have
not shown this enhancement of the effect of sulfur dioxide by sodium
chloride (Corn et al., 1972).
Matsumura (1970a) exposed guineapigs to ozone at 2.1, 11, or
21 mg/m3 (1, 5, or 10 ppm), nitrogen dioxide at 40, 80, or 140 µg/m3
(20, 40, or 70 ppm), or sulfur dioxide at 60, 170, 510, or 940 mg/m3
(20, 60, 180, or 330 ppm) for 30-50 minutes. Each group with unexposed
controls was then exposed to an aerosolized antigen (2 µm) of egg
albumin and bovine albumin for 45 minutes, 5-7 times at intervals of a
day or more. Two weeks after the last exposure, the animals were
killed and blood was drawn for assay of the immunological response.
Enhanced sensitization was noted only at the highest levels of
exposure. During these exposures a number of animals died of
anaphylactic reactions at the fifth or sixth inhalation. In another
study, sulfur dioxide at a concentration of 1100 mg/m3 (400 ppm) did
not have any effect on dyspnoeic attacks (Matsumura, 1970b).
7.1.2 Exposure to sulfuric acid aerosols or suspended sulfates
Sulfuric acid mist and some of the sulfate salts are more powerful
respiratory irritants than sulfur dioxide, and this effect is also
related to particle size (smaller particles tending to be more
irritating) (Amdur, 1958). Treon et al. (1950) exposed rabbits, rats,
mice, and guineapigs to sulfuric acid mist, 93-99% of which was less
than 2 µm in diameter, many droplets being about 1 µm in diameter.
Concentrations ranged from 87 to 1610 mg/m3. Despite the relatively
small number of animals used, a clear-cut species difference was shown
at these high concentrations. The order of increasing sensitivity was
rabbits, rats, mice, guineapigs.
It was reported by Amdur et al. (1952a) that the 8-h LC50 of
sulfuric acid mist with a mass median diameter (MMD) of about 1 µm was
18 mg/m3 for 1-2 month old guineapigs. Pattle et al. (1956) showed
that sulfuric acid mist of MMD 2.7 µm was more toxic to guineapigs
than a mist of 0.8 µm, and that the toxicity of the smaller particles
increased when the exposure occurred at 0°C. However, this might be a
response of the guineapig to low temperature rather than to the
sulfuric acid mist. Concomitant exposures with ammonium carbonate had
a protective action, apparently because it neutralized the sulfuric
acid.
Table 11. Sulfuric acid and some sulfates in descending order of
their irritative capacity for animals. Presented for
equivalent amounts of sulfur and at comparable particle
size i.e., sub-micron; short-term exposuresa
Sulfuric acid H2SO4
Zinc ammonium sulfate ZnSO4(NH4)2 SO4
Iron (III) sulfate Fe2 (SO4)3
Zinc sulfate ZnSO4
Ammonium sulfate (NH4)2 SO4
Nonreactive
Iron (II) sulfate FeSO4
Manganese (II) sulfate MnSO4
a From: Amdur (1969, 1970, 1971).
Studies on guineapigs have shown that, for equivalent amounts of
sulfur and in comparable particle size, sulfuric acid is more
irritative than any of the sulfate compounds, some of which appear to
be nonreactive in animals (Table 11). Whether these compounds would
behave in the same manner in complex mixtures such as those found in
polluted air is not known. Some of the nonreactive compounds such as
manganese salts or oxides can catalyze the reaction of sulfur dioxide
to sulfuric acid.
In short-term exposure studies by Amdur (1958), concentration
seemed to be more important than duration and death was related to
laryngospasm and bronchospasm. Sulfuric acid mist also caused
parenchymal lung damage that seemed to be related to the total dose
(Amdur, 1958; Pattle et al., 1956).
7.2 Long-term Exposure Studies
7.2.1 Exposure to sulfur dioxide
Rats were exposed for 96 days to sulfur dioxide at concentrations
of 0.1, 0.5, and 1.5 mg/m3 (0.04, 0.18 & 0.53 ppm). Histological
examination showed interstitial pneumonia, bronchitis, tracheitis, and
peribronchitis after exposures to the two higher levels (Elfimova &
Gusev, 1969).
Misiakiewicz (1970) exposed rats continuously during 5 months to
sulfur dioxide at concentrations of 0.3, 0.5, 1.0, 2.0, and
20.0 mg/m3 (0.11, 0.18, 0.35, 0.7 and 7.0 ppm). Exposures to
2.0 mg/m3 (0.7 ppm) and 20.0 mg/m3 (7.0 ppm) increased the activity
of serum cholinesterase (EC 3.1.1.8) and aspartate aminotransferase
(EC 2.6.1.1) and caused morphological changes in the upper respiratory
tract.
After a 120-h exposure to a sulfur dioxide concentration of
3 mg/m3 (1.1 ppm), guineapigs showed proliferative interstitial
pneumonia, bronchitis, and tracheitis and an increased histamine
content in the lungs, while exposure to 167 µg/m3 (0.06 ppm) of
sulfur dioxide for one month led to interstitial changes in the
respiratory tract (Bustueva, 1961a). Bustueva (1966) also exposed rats
for 65 days (24 h per day) to sulfur dioxide at 4.86 mg/m3 (1.7 ppm).
Tracheitis, desquamation of epithelial cells, an increased amount of
purulent mucus, and interstitial pneumonia were found. It was not
clear, however, whether this was due to the sulfur dioxide or to the
pulmonary infection that can develop in rats.
Beagle dogs exposed to a sulfur dioxide concentration of
13.4 mg/m3 (4.7 ppm) for 21 h per day, for 620 days, did not develop
any specific histopathological changes (Lewis et al., 1973).
Guineapigs have been exposed to sulfur dioxide levels up to
16.3 mg/m3 (5.7 ppm) for 12 months without definite effects except
for slight cytoplasmic vacuolation in the liver (Alarie et al., 1970).
Cynomolgus monkeys exposed continuously for 78 weeks to sulfur dioxide
levels up to 3.7 mg/m3 (1.3 ppm) did not show any significant
pathological changes (Alarie et al., 1972). However, rats exposed to
sulfur dioxide at 2.9 mg/m3 (1 ppm) for 170 h showed a significant
reduction in clearance of inert particles from the lung (Ferin &
Leach, 1973). Syrian hamsters, made emphysematous by previous exposure
to aerosolized papain, tolerated concentrations of sulfur dioxide up
to 1900 mg/m3 (650 ppm). Exposures were for 4 h per day, 5 days per
week, for a total of 19-74 exposures. Only slight changes in the
mechanical properties of the lung were noted as well as slight
bronchitis (Goldring et al., 1970). As Syrian hamsters are
exceptionally resistant to the effects of sulfur dioxide, these data
must be extrapolated with caution to other species.
7.2.2 Exposure to sulfuric acid aerosols
Alarie et al. (1973) exposed cynomolgus monkeys for 78 weeks to
sulfuric acid mist at concentrations of 0.38 to 4.79 mg/m3 and
particle sizes of 0.54 to 3.60 µm. At concentrations of 2.43 and
4.79 mg/m3 and particle sizes of 3.60 and 0.73 µm, definite damage to
the pulmonary structure was evident and there was deterioration in
pulmonary function. At the lower concentrations, changes were slight
or absent. The authors also exposed guineapigs for 52 weeks to
concentrations of 0.08-0.1 mg/m3 and particle sizes of 0.84 and
2.78 µm; no detectable effects were seen.
When beagle dogs were exposed to sulfuric acid at a concentration
of about 0.9 mg/m3 for 21 h per day, for 620 days, there was a
significant reduction in pulmonary function; 90%, of the sulfuric acid
mist was less than 0.5 µm in diameter (Lewis et al., 1973).
Histopathological changes were produced in the alveolar part of the
lung, especially in the elastic tissue, of rats exposed for 65 days
(24 h per day) to a sulfuric acid concentration of 1 mg/m3. Ninety
per cent of the particles were less than 2 µm in diameter in this
study (Bustueva, 1966).
Guineapigs were exposed to sulfuric acid aerosol for 120 h at 3
different concentrations (1.98 ± 0.03; 4.20 ± 0.06; and 8.27 ±
0.15 mg/m3). Exposure to the highest concentration led to oedema of
the lungs, changes in the interalveolar walls, sharp, diffused,
interstitial changes, and increased histamine content in the lung
tissue. Three weeks after exposure, sclerosis appeared (Bustueva,
1957). After a 1-month exposure to 0.1 mg/m3, no changes were found
(Bustueva, 1961a).
7.2.3 Exposure to a mixture of sulfur dioxide and sulfuric acid
aerosols or this mixture combined with other agents
Rats were exposed for 65 days (24 h per day) to a combination of
sulfur dioxide at 4.86 mg/m3 (1.7 ppm) and a sulfuric acid aerosol at
1 mg/m3 (90% of the aerosol particles were less than 2 µm in
diameter). There was a summation of the effects (histopathological
changes in alveolar tissue) produced by exposure to each of the
pollutants alone (Bustueva, 1966).
Lewis et al. (1973) exposed beagle dogs to a combination of sulfur
dioxide at 13.4 mg/m3 (4.7 ppm) and sulfuric acid at about
0.9 mg/m3. Ninety percent of the sulfuric acid mist was less than
0.5 µm in diameter. The duration of exposure was 21 h per day, for 620
days. Some of the dogs had been pre-exposed to nitrogen dioxide. This
group tended to show less response in the form of changes in pulmonary
function than dogs not pre-exposed to nitrogen dioxide.
Alarie et al. (1975) exposed cynomolgus monkeys and guineapigs to
mixtures of sulfur dioxide, fly ash, and sulfuric acid mist, for 18
months after an 8-week baseline period. Exposure concentrations varied
from 0.29 to 143 mg/m3 (0.1 to 5.0 ppm) for sulfur dioxide and from
0.1 to 1 mg/m3 for sulfuric acid mist; the concentration of fly ash
was approximately 0.5 mg/m3. Particle size (MMD) varied from 0.53 to
3.11 µm in the acid mist and from 4.1 to 5.8 µm in the fly ash.
Pulmonary function tests and serum biochemical and haematological
analyses were conducted prior to, and periodically during, the
exposure. Lungs were examined microscopically at the end of the
experiment. Sulfuric acid mist appeared to be responsible for the
effects observed. These were largely histopathological changes in the
lungs. No synergistic action was noted between the pollutants.
7.2.4 Combined exposure to sulfur dioxide and particulate matter
or other gaseous pollutants
In studies by Frazer et al. (1968), white albino rats were exposed
to sulfur dioxide at 2.9 and 8.6 mg/m3 (1 and 3 ppm) and graphite
dust at 1 mg/m3. The mean particle size of the dust was less than
1 µm and the MMD was 3 µm. The animals were exposed for 12 hours per
day, 7 days per week, for 4 months. Both ciliary activity, and the
number of dust-containing cells per 100 lung cells appeared to be
unaffected after 56-109 days of exposure.
Various aerosols that may react with sulfur dioxide have been
studied either singly or in combination with sulfur dioxide in
experimental animals, particularly in guineapigs. Amdur (1969, 1971)
emphasized that the particle size of the aerosol as well as the
concentration was extremely important in the determination of toxicity
and that the most important size range was the submicron level. The
changes induced in pulmonary mechanics were slow to return to
pre-exposure levels. This indicates either that deposited aerosols may
not be cleared promptly but remain in the lungs exerting their effect
for a period of time, or that the repair mechanisms are slow, or that
both conditions are present. There was also a variation in the
toxicity of different sulfates for the same particle size and sulfur
content (Amdur, 1969) (Table 11).
Studies by Battigelli et al. (1969) in which rats were exposed to
sulfur dioxide at 2.9 mg/m3 (1 ppm) and graphite dust at 1 mg/m3
(particle size not stated) did not show any effect other than the
accumulation of dust in the lung after exposure for 4 months, for 12
hours per day. Amdur & Underhill (1968) pointed out that not all
aerosols enhance the effect of sulfur dioxide and that only those
aerosols composed of droplets in which the sulfur dioxide could
dissolve were active.
Mice were exposed by Zarkower (1972) to carbon particles (1.8 to
2.2 µm MMD) at a concentration of 558 ± 154 µg/m3 and sulfur dioxide
at 5.7 mg/m3 (2 ppm). The animals were exposed to carbon alone,
carbon with sulfur dioxide, and sulfur dioxide alone; controls were
unexposed. Killed Escherichia coli was used as an antigen and
antibody production was measured. Exposures for 192 days produced
significant immunosuppression. Shorter periods of exposure resulted in
variable effects including stimulation of antibody production.
A variety of metallic aerosols can catalyze the oxidation of
sulfur dioxide including the soluble salts of ferrous iron, manganese,
and vanadium.
Rylander & Bergström (1973) exposed animals with latent upper
respiratory disease and controls for 4 weeks to various combinations
of sulfur dioxide at 57 mg/m3 (20 ppm), manganese dioxide at
12-20 mg/m3 (particle size between 5.0 and 0.5 µm), and carbon
monoxide at 187-250 mg/m3 (150-200 ppm). Animals with latent upper
respiratory disease showed more extensive histological changes and an
increase in the number of free lung cells. The combination of sulfur
dioxide and manganese dioxide produced the greatest changes.
An addition of effects in rats was reported by Salamberidze (1969)
from joint exposure to sulfur dioxide at 0.15 mg/m3 (0.05 ppm) and
nitrogen dioxide at 0.1 mg/m3 (0.05 ppm) over a 3-month period.
The precise mechanism by which the oxides of sulfur and
particulate matter can affect the lungs is not known. Sulfur dioxide
(and presumably sulfuric acid as well) can interfere with the
clearance of bacteria (Rylander et al., 1971) and inert particles
(Ferin & Leach, 1973) from the lungs (see also section 6). Chronic
exposure to sulfur dioxide increased the number and area of goblet
cells in guineapigs and lambs (Mawdesley-Thomas et al., 1971).
The considerable variations in the results of these experiments on
animals reflect differences in sensitivity of individual species,
exposure levels, and methods used to assess the effects.
It should be emphasized that extrapolation of these results from
animals to human beings is not easy. These findings, however, do give
some insight into possible mechanisms of action and reactions that can
occur.
8. EFFECTS ON MAN
8.1 Controlled Exposures
A number of studies have been performed on volunteers under
controlled conditions of exposure to sulfur dioxide or sulfuric acid
aerosols, singly or in combination, or to mixtures of these with other
compounds such as ozone and hydrogen peroxide. These studies, all
conducted under short-term exposure (up to 24 h), include those on
changes in respiratory function and effects on sensory and reflex
organs.
8.1.1 Effects on respiratory organs
8.1.1.1 Exposure to sulfur dioxide
Amdur et al. (1953) exposed 14 healthy volunteers (inhaling
through the mouth) to sulfur dioxide at concentrations of
2.9-23 mg/m3 (1-8 ppm) for 10 min. They noted an increased pulse
frequency, decreased tidal volume, and an increased respiratory
frequency which returned to normal levels after exposure. The sequence
of exposures was randomized. Effects increased with increasing levels
of sulfur dioxide and were detectable at the lowest concentration
tested (2.9 mg/m3; 1 ppm). Sulfur dioxide levels were monitored by
the conductimetric method. However, Lawther (1955) was unable to
reproduce these effects in any consistent manner, either in urban or
in rural dwellings. Eleven healthy volunteers were exposed to sulfur
dioxide at levels of 2.9, 14, and 37 mg/m3 (1, 5, and 13 ppm) in
studies by Frank et al. (1962). Respiratory mechanics were measured by
means of a body plethysmograph and an oesophageal balloon. Exposures
lasted 10-30 min. Only one of the 11 subjects exposed showed an
increased pulmonary resistance at 2.9 mg/m3 (1 ppm). Increased
pulmonary resistance was noted in all subjects and was greater at the
higher concentration. The change occurred within 1 min of exposure and
increased up to 10 min, after which no further increase was noted.
In studies by Snell & Luchsinger (1969), exposure to a sulfur
dioxide concentration of 2.9 mg/m3 (1 ppm) for 15 min produced a
slight effect on total respiratory resistance in 9 healthy volunteers.
Andersen et al. (1974) exposed healthy male subjects to sulfur
dioxide at levels of 2.9, 14, and 71 mg/m3 (1, 5, and 25 ppm) for up
to 6 h. With exposures of 1-3 h at 2.9 mg/m3 (1 ppm), there was a
decrease in the flow of nasal mucus and a decrease in the cross
section of the nasal passages. Thus it appears that exposure to
concentrations of 2.9 mg/m3 (1 ppm) or more may result in impairment
of mucociliary transport in the nose.
Four healthy volunteers were exposed to sulfur dioxide and the
forced vital capacity (FVC), one second forced expiratory volume
(FEV1.0), mid-maximal flow rates (MMFR), maximal expiratory flow rate
at 50% (MEFR50) and closing volume and capacity were measured.
Although no changes were found in these tests at 1.1 mg/m3
(0.37 ppm), slight changes in FVC, FEV1.0, MMFR, and MEFR50 were
noticed at 2.1 mg/m3 (0.75 ppm) after exposure for 30 min; no effect
on closing volume was detected (Bates & Hazucha, 1973).
The results of these studies on human volunteers are summarized in
Table 12.
8.1.1.2 Exposure to sulfuric acid aerosols
Amdur et al. (1952b) exposed 15 healthy men to sulfuric acid mist,
through mouth breathing, at concentrations of 0.35-5 mg/m3 (particle
size of approximately 1 µm) for periods of 5-15 min. At concentrations
below 1 mg/m3, the mist did not produce any subjective sensations
although 5 of the 15 subjects showed a slightly increased respiratory
rate and a decreased tidal volume at 0.35 mg/m3. All subjects noted
irritation at a concentration of 3 mg/m3. Respiration was monitored
by means of a pneumotachograph. The respiratory changes as well as the
subjective sensations increased with increasing concentrations of
sulfuric acid. Healthy male volunteers were also exposed to sulfuric
acid mist by Sim & Pattle (1957), either by mask or in a chamber for
periods ranging from 10 to 60 min. Twelve men were exposed to the
mist. The temperature of the air was 18.4°C with a relative humidity
of 62%. The MMD of the aerosol was 0.99 µm and the concentration was
39.4 mg/m3. The men noted minor irritation, and lung resistance (as
measured by the interrupter technique) rose by 35-100%. With
re-exposure for 30 min to the mist at a temperature of 24.5°C, a
relative humidity of 91%, a concentration of 20.8 mg/m3, and a MMD of
1.54 µm, severe coughing and irritation of the throat occurred. The
men found it almost intolerable. Lung resistance had risen by 43-150%
when measured after 10 minutes of exposure and when coughing had
ceased. No changes in respiration, blood pressure, or pulse rate were
noted. Two of the men exposed to these conditions had persistent
symptoms for some days after exposure ended.
It is difficult to evaluate these two studies. Amdur did not
report the temperature, though it was probably in the neighbourhood of
24°C, or the relative humidity. The studies of Sim & Pattle were at
relatively high levels but they demonstrated the importance of the
relative humidity or perhaps even more important, the absolute
humidity. The results of these studies are summarized in Table 13.
None of these studies used sulfates.
Table 12. Selected laboratory studies on the effects of short-term exposures to sulfur dioxide on respiratory
function in volunteers
Concentration Length of Effects Subjects Reference
exposure
(mg/m3)a (ppm) (min)
2.9-23 1-8 10 Increased pulse rate, decreased 14 healthy males Amdur et al. (1953)
tidal volume, and increased
respiratory rate
2.9 1 10-30 Increased pulmonary/resistance 11 healthy males Frank et al. (1962)
2.9 1 15 Increased respiratory resistance 9 healthy subjects Snell & Luchsinger (1969)
(5 males &
4 females)
2.9 1 60-180 Decreased nasal mucus flow 15 healthy males Andersen et al. (1974)
and decreased cross section of
nasal passages
2.1 0.75 120 Slight effect in 30 min on 4 healthy subjects Bates & Hazucha (1973)
FVC, FEV1-0, MMFR, and
MEFR50; no effect on
closing volume
1.1 0.37 120 No effect on above tests of 4 healthy subjects Bates & Hazucha (1973)
pulmonary function throughout
exposure period
a Original levels reported as ppm have been converted to mg/m3 and rounded off.
Table 13. Selected laboratory studies on the effects of short-term exposures to sulfuric acid mist on respiratory
function in volunteers
Concentration Particle Size Length of Relative Temperature Effects Subjects Reference
(mg/m3) (µm) exposure Humidity (°C)
(min) (%)
0.35 1 5-15 ? Room (?24) 5 of 15 subjects 15 healthy Amdur et al.
increased subjects (1952b)
respiratory rate
and decreased
tidal volume
39.4 0.99 60 62 18.4 Increased 12 healthy Sim & Pattle
pulmonary males (1957)
resistance and
minor irritation
20.8 1.54 30 91 24.5 Marked increase 12 healthy Sim & Pattle
of pulmonary males (1957)
resistance and
severe irritation
8.1.1.3 Exposure to mixtures of sulfur dioxide and other compounds
Frank et al. (1964) repeated the above exposures in combination
with a sodium chloride aerosol. Concentrations of sulfur dioxide were:
2.9-5.7 mg/m3 (1-2 ppm), 11-17 mg/m3 (4-6 ppm), and 40-49 mg/m3
(14-17 ppm). The sodium chloride aerosol had a geometric mean diameter
of 0.15 µm with a geometric standard deviation of 2.3 µm and an
average concentration of 18 mg/m3. As in the earlier studies, little
change in pulmonary flow resistance was noted at the lower levels of
sulfur dioxide alone (2.9-5.7 mg/m3; 1-2 ppm) but a progressive
increase was noted at higher levels. The authors did not find any
systematic differences between the responses to sulfur dioxide alone
or to the gas plus the aerosol. As mentioned in section 8.1.1.1, Snell
& Luchsinger (1969) noted a slight effect of sulfur dioxide at
2.9 mg/m3 (1 ppm) on total respiratory resistance but could not
demonstrate an enhancing effect of either a sodium chloride aerosol or
a distilled water aerosol. Burton et al. (1969) exposed volunteers to
a concentration of sulfur dioxide of 6 mg/m3 (2.1 ppm) with or
without a sodium chloride aerosol (MMD of less than 0.4 µm) at a
concentration of 2.2 mg/m3. The inhaled air was warmed and moistened.
However, they too failed to demonstrate any effect of the sodium
chloride aerosol on the response to sulfur dioxide.
Thus, three groups have not been able to demonstrate any enhancing
effect of sodium chloride aerosol on respiratory resistance in man as
had been demonstrated in guineapigs. This does not exclude the
possibility that other particulate matter might enhance the effect of
sulfur dioxide on the human respiratory system. These studies should
be repeated at levels of humidity above 80% and the mixture should be
allowed to "age".
The possible interaction of sulfur dioxide and hydrogen peroxide
has been examined by Toyama & Nakamura (1964) and that of sulfur
dioxide and ozone by Bates & Hazucha (1973). Toyama & Nakamura (1964)
studied 24 healthy male volunteers by means of a pneumotachograph and
the interrupter technique to measure alveolar pressure; airway
resistance was determined from these measurements. The particle sizes
of the hydrogen peroxide were reported to be 1.8 and 4.6 µm.
Concentrations of hydrogen peroxide were 0.8-1.4 mg/m3 for the larger
particles and 0.01-0.1 mg/m3 for the smaller ones. Levels of sulfur
dioxide ranged from 2.9 to 170 mg/m3 (1-60 ppm). The subjects were
not aware whether they were breathing hydrogen peroxide, sulfur
dioxide, or combinations. It was not stated whether the order of
administration was randomized or not. The investigators noted a marked
increase in airway resistance with the mixture compared with the
individual components. They also noted that the larger particles had
more effect than the smaller particles. This was probably due to the
greater dose delivered, although they did not comment on this. It was
the authors' opinion that the enhancement of the response to the
combination was due to the conversion of sulfur dioxide to sulfuric
acid, although no measurement for sulfuric acid was made.
In similar studies by Bates & Hazucha (1973) healthy male
volunteers were exposed in a chamber to sulfur dioxide, or ozone, or a
combination of the two. Changes in FVC, FEV1.0, MMFR, MEFR at 50%
vital capacity and peak expiratory flow rates were studied and some
effects were noted during exposure to ozone at 540-1610 µg/m3
(0.25-0.75 ppm). Sulfur dioxide by itself had little effect over a
similar range. Joint exposure to sulfur dioxide and ozone at
1060 µg/m3 (0.37 ppm) and 790 µg/m3 (0.37 ppm), respectively,
produced a greater effect than ozone alone. Changes were noted after
exposure for 30 min and were marked after 2 h of exposure. Exercise
during exposure enhanced the effect.
8.1.2 Effects on sensory or reflex functions
Studies in the USSR have concentrated on the effects of sulfuric
acid aerosols and sulfur dioxide on sensory receptors, cerebral
cortical function, and their interrelationships. When sulfuric acid
mist produced subjective sensory stimulation such as odour or
irritation of mucous membranes, or both, then, invariably, objective
evidence of central nervous system depression could be demonstrated
(Rjazanov, 1962).
Bustueva (1961b) did not note any change in optical chronaxy in
volunteers exposed to concentrations of sulfur dioxide of 0.5 mg/m3
(0.18 ppm) and sulfuric acid of 0.3 mg/m3. However, when levels of
1.5 mg/m3 for sulfur dioxide, 0.73 mg/m3 for sulfuric acid, and
combined levels of 1.2 mg/m3 and 0.6 mg/m3, respectively, were
exceeded, optical chronaxy increased (Table 14). Similar effects were
seen in dark adaptation responses (Rjazanov, 1962).
Studies have also been carried out in which the cerebral cortex
was monitored by electroencephalography. Alpha rhythm suppression was
used as an index of response. Threshold levels at which a response was
noted are given in Table 14. (Bustueva et al. 1960).
Table 14. Threshold levels of sulfur dioxide and sulfuric acid required for effects on sensory or
reflex functions in volunteers during short-term exposuresa
Threshold levels (mg/m3)
Effects Sulfuric acid Sulfur dioxide Sulfuric acid + Sulfur dioxide
Perception of odour and 0.6 to 0.85 1.6 to 2.8 0.3 + 0.5
irritation of mucosa
Suppression of dark 0.63 to 0.73 0.92 0.3 + 0.5
adaptation
Elevation of optical 0.73 1.5 0.6 + 1.2
chronaxy
Disruption of alpha 0.63 0.9 0.3 + 0.5
rhythm
Conditioning of 0.4 0.6 0.15 + 0.5
electrocortical reflex or 0.3 + 0.25
a Summarized from studies in the USSR (Bustueva, 1961b; Bustueva et al., 1960; Rjazanov, 1962).
The electrocortical conditioned reflex is a central nervous system
phenomenon elicited only after a succession of repeated, conditioned
reflex trials. After exposure to a combination of irritants (sulfur
dioxide or sulfuric acid) and light has been repeated several times,
desynchronization begins to appear before the light is switched on.
This can be produced at levels generally not sensorially perceived.
Thus, unperceived odour or stimulus appears to become the conditioning
stimulus and generates the conditioned electrocortical reflex
(Rjazanov, 1962).
Elfimova & Hacaturjan (1968) exposed volunteers to sulfur dioxide,
phenol, and carbon monoxide and noted a summation of effects as
measured by reflex action. No interaction was noted between sulfur
dioxide at 0.5 mg/m3 (0.18 ppm) and carbon monoxide at 3 mg/m3
(2.4 ppm) as measured in volunteers by the sensory reflex technique
(Mamacasvili, 1968).
8.2 Industrial Exposure
Workers are exposed to sulfur dioxide or sulfuric acid mist in a
number of industries; sometimes exposure is not solely to sulfur
dioxide or sulfuric acid. Exposed populations have been studied with
respect to the effects of exposure on their health status or on their
respiratory system. However, in many of these studies, only the
currently employed workers were examined and a serious effort was not
made to locate subjects who had left the industry and who may have
suffered more from the disease or could have been more sensitive to
the materials. It should also be emphasized that, in general, in the
following reports, the exposure levels studied were from spot samples
or for very short time intervals.
8.2.1 Exposure to sulfur dioxide singly or in combination with
particulate matter
Kehoe et al. (1932) studied men working in a refrigerator company
in the USA where sulfur dioxide was the refrigerant. Exposures
averaged 60-90 mg/m3 (20-32 ppm) with peaks as high as 200 mg/m3
(70 ppm). These peaks had probably been higher in the past ranging up
to 290 mg/m3 (100 ppm) or more. The exposed group had significantly
more respiratory symptoms and colds. They also complained more of
fatigue and shortness of breath on exertion. Chest X-rays of the
exposed and unexposed groups showed the same distribution of
abnormalities. The authors concluded on their inadequate evidence that
there was no injury to the tracheobronchial tree or alveoli.
In studies on men working in smelters in Sweden, Sjörstrand (1947)
found that those who had worked at the roasting and reverberatory
furnaces and in the converter hall for 8 years or more had poorer
respiratory function than men working in other parts of the smelter.
Levels of exposure and the smoking histories of the subjects were not
reported. Men working in smelters are exposed to a variety of dusts as
well as to sulfur dioxide and it is difficult to separate the effects
of such exposures from those due to sulfur dioxide.
Men exposed to sulfur dioxide at daily mean concentrations up to
70 mg/m3 (25 ppm) with occasional peaks of 290 mg/m3 (100 ppm) in
certain areas of a petroleum refining plant in Abadan (Iran) were
compared by Anderson (1950) with an unexposed group. No differences
were found between the two groups. This study also did not refer to
the smoking histories of the subjects.
In a study in Norway, pulp mill workers were compared with paper
mill workers using a standard questionnaire on respiration and simple
tests of pulmonary function. The smoking histories of the subjects
were also studied. Levels of sulfur dioxide ranged from 6-100 mg/m3
(2-36 ppm) with peaks of 290 mg/m3 (100 ppm) when the digester was
blown. The exposed group had more cough, sputum, and dyspnoea than the
unexposed group but the vital capacities were similar in both groups.
The expiratory peak flows, however, of the exposed men under 50 years
of age were lower than those in a comparable unexposed group (Skalpe,
1964).
A similar study was carried out in the USA by Ferris et al. (1967)
who reported that there was no difference between men in a pulp mill
and men in a paper mill. Both groups had less respiratory disease than
was reported from a survey of the general population. The authors
noted that some of the men working in the paper mill had worked in the
pulp mill but had left because they could not tolerate the conditions.
Occupational levels of exposure to sulfur dioxide ranged from a trace
to 95 mg/m3 (33 ppm). Average levels ranged from 6 to 35 mg/m3
(2-13 ppm). Smoking habits were considered.
Huhti et al. (1970) studied pulp and paper mill workers in Finland
and noted that the effects of smoking were much more significant than
the exposures at work or the effects of climate. Levels of exposure
were not reported in this study.
Men working in two integrated steel mills in Wales were studied by
Lowe et al. (1968, 1970) and Warner et al. (1969). Mean concentrations
of sulfur dioxide over 3 years ranged from 1.8 to 2.1 mg/m3 (0.6 to
0.7 ppm) and those of suspended particulate matter in the respirable
range, ranged from 600 to 1800 µg/m3 (by elutriation technique).
Analysis of the particulate matter showed that it was mainly composed
of iron oxides and calcium sulfate. No effects on respiratory symptoms
or on simple tests of pulmonary function were found after
standardization for cigarette smoking and for age. These observations
may reflect the limitation of studying occupational groups because of
the effect of the selection processes, or this may be an example in
which the suspended particulate matter present was not interacting
with the sulfur dioxide to produce an effect.
8.2.2 Exposure to sulfuric acid mist
Dorsch (1913) studied men exposed to sulfuric acid mist in a plant
manufacturing storage batteries in Germany. He reported that at a
concentration of 0.5 mg/m3, the mist was barely noticeable; at
2.0 mg/m3, there was nose and throat irritation, at 3-4 mg/m3, there
was distinct discomfort, and at 6-8 mg/m3, there was marked
discomfort. These responses are comparable to those reported in
laboratory studies on human beings in section 8.1. No particle size
was given but, as noted in another survey reported below, they were
probably relatively large.
Men in the battery industry were also examined by Malcolm & Paul
(1961) in the United Kingdom who reported that there was significant
erosion of the teeth of the battery room workers. This was confirmed
by ten Bruggen Cate (1968). Apparently this was due to the direct
impingement of relatively large droplets of sulfuric acid on the
teeth.
Williams (1970) studied men from the same works as Malcolm & Paul
(1961) and reported that there was no difference in the forced vital
capacity and the one-second forced expiratory volume between the men
in the forming and control departments. Levels of sulfuric acid mist
averaged 1.4 mg/m3 during working hours over two days and ranged from
a trace to 6.1 mg/m3 in 1968. An earlier survey reported higher
values. Particle size reported from a survey in another firming
department doing comparable work was 14 µm MMD; 4% of the particles
being less than 4 µm MMD. The level of sulfuric acid in this
department was 2.7 mg/m3.
8.3 Community Exposure
Much of the information that has been gained concerning the
effects on health of exposure to realistic concentrations of sulfur
oxides and particulate matter has come from epidemiological studies,
carried out on segments of population chosen by virtue of place of
residence, age, existing state of health, or other characteristics, in
order to present contrasts in exposure or sensitivity to these
pollutants. Some studies have been based on the complete populations
of urban areas, observing the total number of deaths, or the incidence
or prevalence of illness within them in relation to differences in
pollution between areas, or with time in any one area.
Many epidemiological studies concerning the health effects of
exposure to sulfur oxides and particulate matter have been reported in
the literature. In the discussion that follows, attention has been
directed to papers that yield information relevant to the development
of exposure-effect and exposure-response relationships for sulfur
oxides, smoke, and suspended particulate matter, and to some others
that are of interest from the point of view of the method of approach.
8.3.1 Mortality -- effects of short-term exposures
The most clearly defined effects on mortality arising from
exposure to sulfur oxides and particulate matter have been the sudden
increases in the number of deaths occurring, on a day-to-day basis, in
episodes of high pollution. The most notable of these occurred in the
Meuse Valley in 1930 (Firket, 1931), in Donora in 1948 (Schrenk et
al., 1949), and in London in 1952 (Ministry of Health, UK, 1954). The
people primarily affected were those with pre-existing heart or lung
disease or both, and the elderly. The London episode lasted for 5 days
and it was estimated that the number of deaths during and immediately
after this period was about 4000 more than expected under normal
circumstances. On one day, the number of deaths was about three times
the number expected at that time of the year. Concentrations of sulfur
dioxide as high as 3.7 mg/m3 (1.3 ppm) were recorded in the centre of
the urban area (48-h average). Concentrations of particulate matter
were too great to be measured properly (British daily smoke/sulfur
dioxide method), and the 48-h average of about 4.5 mg/m3 at a central
site must be regarded as a conservative estimate. These were rough
estimates for the exposures and, probably, there was considerable
variation in individual exposures.
Following these major episodes, attention was turned to studies on
more moderate day-to-day variations in mortality within large cities,
in relation to pollution. Gore & Shaddick (1958) correlated mortality
in the County of London (the inner part of the Greater London Area)
with pollution by smoke and sulfur dioxide for 4 foggy periods in
1954-56, using 7-day moving averages to smooth out the data. The
authors considered that, in two of the episodes, there was a marked
increase in mortality from bronchitis and other lung diseases,
particularly in the elderly. They concluded that when the 24-h mean
concentration of smoke exceeded 2.0 mg/m3 at the same time as the
24-h mean concentration of sulfur dioxide exceeded 1.1 mg/m3
(0.4 ppm) (British daily smoke/sulfur dioxide method), there would be
increased mortality. Care was taken in this study to ensure that the
measurements were reasonably representative of the exposure of people
anywhere within the study area: the figures quoted were the mean
values from a group of 7 sites, all situated close to ground level in
mainly residential areas. However, there remained the problem that the
people at risk in a study of this type were the elderly sick, who were
likely to remain indoors, and that outdoor measurements might not have
provided an adequate assessment of exposure (Biersteker et al., 1965).
The relationship between daily mortality in the more extensive
area of Greater London and day-to-day variations in pollution (smoke
and sulfur dioxide) and visibility was examined by Martin & Bradley
(1960) in the winter of 1958-59. They noted that on days when the
smoke concentration increased by more than 100 µg/m3 compared with
the previous day, or when the sulfur dioxide concentration increased
by 70 µg/m3 (0.025 ppm), there was likely to be increased mortality
(British daily smoke/sulfur dioxide method). The increases in daily
mortality were up to about 1.25 times expected values assessed from
15-day moving averages. Thick fog (visibility less than 200 metres)
was also associated with increases in mortality. The relative
importance of the 3 factors could not be determined but, on the basis
of other work, the authors considered that the smoke was probably the
most important. It is not clear whether the results are best
interpreted in terms of change in pollution from one day to the next,
rather than in terms of absolute values, but there is support for the
former approach from studies carried out elsewhere. When results were
considered on an absolute basis (Lawther, 1963), it was concluded that
increases in mortality became evident when the 24-h mean
concentrations of smoke and sulfur dioxide exceeded 750 µg/m3 and
710 µg/m3 (0.25 ppm), respectively. The measurement sites were the
same as those used by Gore & Shaddick (1958). They could still be
considered reasonably representative of outdoor concentrations in the
areas where people lived, although the inclusion of outer,
less-densely populated areas meant that the average exposures would
tend to have been underestimated.
Studies on day-to-day variations in mortality in London were
continued in successive winters, and coupled with the records of
emergency hospital admissions. In a later paper, Martin (1964) showed
correlations between both the daily mortality and hospital admission
data and concentrations of smoke or sulfur dioxide. There was no
clearly defined level above which effects were seen, but there were
fairly consistent increases in both mortality and hospital admissions
when the concentrations of smoke and sulfur dioxide each exceeded a
24- mean of about 500 µg/m3 (0.18 ppm of sulfur dioxide, British
daily smoke/sulfur dioxide method). In 1962, there was a major episode
of high pollution in London, similar in terms of duration and of
sulfur dioxide concentrations to the one in 1952, but with lower smoke
concentrations. Again, there was a sudden increase in deaths, but the
number of deaths was not as great as before (about 700, compared with
4000). Whether the change in medical care could have influenced these
results is not clear. The greater use of antibiotics in 1962 compared
with 1952 might have reduced the number of deaths, and a greater
awareness of the risk together with clear advice to the elderly and
infirm to remain indoors could have had an effect. The dramatic
reduction in smoke concentrations in London brought about by the
implementation of the Clean Air Act, and the more gradual reduction in
sulfur dioxide that has followed it, have meant that in more recent
years there have been few occasions when levels of 500 µg/m3 have
been exceeded simultaneously for smoke and sulfur dioxide (Waller et
al., 1969).
Biersteker (1966) published a study of an episode of high
pollution in Rotterdam in December 1962, when concentrations of smoke
and sulfur dioxide of approximately 500 µg/m3 and 1000 µg/m3
(0.35 ppm), respectively, were recorded (24-h means, OECD smoke/sulfur
dioxide method). There were increases in admissions to local hospitals
of people over 50 years of age with cardiovascular diseases, and there
was also some indication of an increase in mortality. This was
observed only once in Rotterdam and could have been due to other
causes. Further observations during a similar episode would be needed
to provide a convincing statistical relationship between hospital
admissions and these levels of pollution.
A relationship between day-to-day changes in mortality and
pollution has also been reported from Osaka (Watanabe 1966). There
appeared to be increases in mortality (about 20%) on days when the
concentration of suspended particulate matter (light scattering
method) exceeded 1 mg/m3 (4-day average) and was associated with a
level of sulfur dioxide of 200 µg/m3 (0.07 ppm). Low temperatures may
have been partly responsible for the effects.
Variations in daily mortality in New York in relation to sulfur
dioxide concentrations were studied by Buechley et al. (1973). They
examined correlations and developed regressions between a number of
daily climatic factors and indices of pollution (sulfur dioxide,
conductimetric method and coefficient of haze (Cohs)), and the
mortality residuals for a given day. They noted that the day of the
week had a special correlation with mortality (mortality rates were
considerably higher on Mondays). Regression analysis indicated that
heat waves and seasonal cycle were major predictors of mortality.
Other factors were much weaker (about one third as strong) but were
all of equal strength. Partial residual mortality values were computed
and showed a significant correlation with the levels of air pollution
(sulfur dioxide r = 0.14). Mortality could be predicted equally as
well from Cohs as from sulfur dioxide levels. Mortality was 1.5% less
than expected on 232 days when sulfur dioxide levels were below
30 µg/m3 (0.01 ppm) and 2% greater than expected on 260 days when the
sulfur dioxide levels were above 500 µg/m3 (0.18 ppm) after
correcting for the other factors. The crossover point (i.e., that
point below which deaths were less than expected and above which
deaths were greater than expected) was in the vicinity of a
concentration of sulfur dioxide of 260 µg/m3 (0.09 ppm). On the other
hand, the data from these studies could be interpreted to show a
continuum of an effect across the levels of sulfur dioxide, which, in
turn, should not be considered the causative agent but rather an index
of pollution.
Schimmel & Murawski (1975) have reported on their regression
analysis of daily deaths and levels of pollution (smoke shade and
sulfur dioxide) in New York City for 1963-1972. This was an extension
of an earlier study (Schimmel & Greenberg, 1972). The authors
controlled for season, day of week, and temperature. During this time
there was a marked reduction in the average level of sulfur dioxide
from 510 µg/m3 (0.18 ppm) to 170 µg/m3 (0.06 ppm) but virtually no
change in smoke shade. Their observations indicated that, despite this
reduction in sulfur dioxide, there had been no reduction in adverse
health effects. Analysis indicated that the adverse health effects
were associated principally (80%) with the particulate matter and only
to a small extent (20%) with the sulfur dioxide. However, the authors
also pointed out that, when they regressed mortality on temperature
and sulfur dioxide alone, the effects attributable to the sulfur
dioxide increased three-fold.
These findings are provocative but they must be interpreted
cautiously because of a number of limitations in the data. Air
pollution levels were from data obtained at a single monitoring
station and were probably not truly representative of exposures for
the community. The standard errors reported with their data are large.
The report should be considered to be an indicator for further studies
in which age-specific death rates and causes of death should be
included in the analysis as well as more relevant air pollution
measurements. The results should not be used for developing an
exposure-effect relationship.
A study involving comparisons between daily mortality data in New
York and Tokyo was carried out by Lebowitz et al. (1973). They applied
a "stimulus-response" technique to identify associations between days
of high pollution and days with increased mortality, and showed strong
relationships in both cities. However, their findings do not provide
information of direct value in the assessment of exposure-effect
relationships.
8.3.2 Mortality -- effects of long-term exposures
In countries having reliable systems for the collection and
analysis of data on deaths, based on cause and area of residence,
death rates for respiratory diseases have commonly been found to be
higher in towns than in rural areas. Many factors, such as differences
in smoking habits, occupation, or social conditions may be involved in
these contrasts, but, in a number of countries, a general association
between death rates from respiratory diseases and air pollution has
been apparent for many decades.
Analyses of these data have been of great value as a lead for
epidemiological studies, but the absence of information concerning
other relevant variables, such as smoking, and the relatively crude
nature of the indices of pollution used in many of these studies make
them unsuitable for the assessment of exposure-effect relationships.
The studies of Daly (1954, 1959), Pemberton & Goldberg (1954), and
Stocks (1959) were all based on mortality data from towns in England
and Wales, and each showed a positive correlation between bronchitis
or pneumonia death rates and some index of pollution by sulfur oxides
or particulate matter, as assessed for periods close to those for
which death rates were calculated. The most detailed investigation of
this type, taking into account social factors as well as pollution,
but still not smoking, was that conducted by Gardner et al. (1969).
One interesting feature of their findings was a slight improvement in
correlation when the index of pollution used was related to a period
some 10 years earlier than that for which the death rates were
calculated (deaths 1958-64, pollution index 1952). This illustrates
another of the problems that has been widely recognized when trying to
use mortality records to assess the effects of pollution i.e., that it
may not be recent exposures that are most relevant, but those earlier
in life; where concentrations have changed markedly over the years,
current measurements may not provide an adequate index.
Lave & Seskin (1970) reanalyzed some of the mortality data from
England and Wales, and developed multiple regression equations in
terms of pollution and socioeconomic indices. Again their findings of
positive correlations with pollution are of general interest but
cannot contribute to the development of dose-response relationships.
These authors also examined analogous data for Standard Metropolitan
Statistical Areas (SMSAs) in USA and in a later paper (Lave & Seskin,
1972) they attempted to assess the relative effects of air pollution,
climate, and home heating on mortality rates. Although equations were
obtained relating death rates to measurements of suspended particulate
matter and total sulfates (both by high volume sampler), it is
doubtful whether these can be regarded as valid in the absence of
adequate information on smoking.
8.3.3 Morbidity -- effects of short-term exposures
Prospective studies on specific occupational groups, not
professionally exposed, can be useful in assessing the effects of air
pollution in different communities or in areas where a change in air
pollution is expected. In such studies, where respiratory diseases are
followed, it is necessary to control for age distribution and
household composition, and to employ adequate statistical methods. In
studies on the Philadelphia area, USA, Dohan & Taylor (1960) used
absences of seven days or more because of respiratory disease as the
index and related this to the levels of sulfate. A later report by
Dohan (1961) noted that this relationship was stronger during an
epidemic of influenza. Ipsen et al., (1969) repeated this type of
study in the same area on a slightly different occupational group
using more detailed statistical analyses. They were not able to
confirm the earlier observations that the sulfate levels were related
to absences due to respiratory diseases.
Results have been reported (Lawther et al., 1970) of a series of
studies extending from 1954 to 1968 that were carried out mainly in
London, but also in some other large cities in England, using a diary
technique for the self-assessment of day-to-day changes in conditions
among bronchitic patients. A daily illness score was calculated from
the data contained in the diaries and this was related to the
concentrations of smoke and sulfur dioxide (British daily smoke/sulfur
dioxide method) and to weather variables. The pollution figures used
for most of the London studies were the mean values from the group of
sites associated with the mortality/morbidity studies of Martin (1964)
and Gore & Shaddick (1958). Many of the subjects in the series were
active enough to be out and about and at work, and the measurements
were considered to give a reasonable assessment of the average
exposures in the areas where they lived or worked. The method used in
these studies has not been validated, for the subjects recorded only
their own assessment of their condition, and this was not checked
against regular clinical examinations or ventilatory function
measurements, but the changes appeared to have some real meaning. In
the earlier years of the series, when the general level of pollution
was high, well defined peaks in the illness score were seen when
concentrations of either smoke or sulfur dioxide exceeded 1000 µg/m3.
With the reductions in pollution that followed the gradual
implementation of the Clean Air Act, these changes in condition became
less frequent and of smaller magnitude, and the conclusion from the
series as a whole, up to 1968, was that the minimum pollution
associated with significant changes in the condition of the patients
was a smoke level of about 250 µg/m3 together with a sulfur dioxide
concentration of about 500 µg/m3 (0.18 ppm) (24-h means, British
daily smoke/sulfur dioxide method). At these levels, there was still
some evidence that the peaks were associated specifically with
pollution rather than with adverse weather conditions. A later study
that has been reported by Waller (1971), showed that, with much
reduced average levels of pollution, there was an almost complete
disappearance of days with smoke levels exceeding 250 µg/m3 and
sulfur dioxide levels exceeding 500 µg/m3 (0.18 ppm). As in earlier
studies, some correlation remained between changes in the condition of
the patients and daily concentrations of smoke and sulfur dioxide but
the changes were small at these levels. At this low range of
pollution, discrimination between the effects of pollution and those
of adverse weather was poor.
Cohen et al. (1974) studied symptoms of irritation during a
publicized and an unpublicized period of air pollution as well as
during a control period in 3 communities in the New York metropolitan
area during the summer of 1970. They used a telephone survey technique
to inquire about specific symptoms such as eye irritation, throat
irritation, chest discomfort, shortness of breath, restricted
activity, and medical visits. No difference was noted between the 2
episodes of pollution. Both episodes showed significantly increased
symptoms compared with the control period. The results indicated that
irritative symptoms increased significantly when sulfur dioxide levels
exceeded 310 µg/m3 (0.11 ppm, West-Gaeke method) and total suspended
particulates exceeded 145 µg/m3 (high volume sampler) as a 3 day
average. Sulfate levels ranged from 6.6 to 7.6 µg/m3 in one area and
5.8 to 12.3 µg/m3 in another area. In the second area, sulfate levels
during the 2 periods of air pollution were 8.5 and 12.3 µg/m3,
respectively. Sulfate levels were not reported from the third area but
were probably low. The authors drew attention to some of the problems
associated with their study. The persons interviewed were generally
wives and the symptomatology in the male population could have been
underestimated. Also, there was an internal inconsistency possibly due
to intercurrent infectious disease or socioeconomic differences.
During the publicized episode, particulate pollution was considerably
higher in the Bronx than in Queens whereas irritation symptoms were
somewhat higher in Queens. The authors concluded that there could have
been confounding effects of other air pollutants, intercurrent
infection, or sociocultural factors.
Spirometric measurements were made at approximately weekly
intervals on 18 patients with chronic obstructive lung disease for
various periods during 1969-71 (Emerson, 1973). The spirometric values
(FEV1.0 and MEFR) were correlated with the levels of pollution (sulfur
dioxide and smoke, British daily smoke/sulfur dioxide method) and
climatic factors (temperature and humidity). Changes in FEV1.0 in
these patients were more strongly correlated with temperature and
humidity than with concentrations of sulfur dioxide or smoke. Levels
of air pollution in London were: sulfur dioxide, mean 190 µg/m3
(0.07 ppm), maximum, 720 µg/m3 (0.25 ppm) and smoke mean, 44 µg/m3,
maximum, 240 µg/m3. One limitation of the study was that the
pollution figures were averaged for 5-day periods, whilst the
spirometric measurements were made on specific days.
Studies of patients with chronic bronchitis in Chicago, USA
(Burrows et al., 1968; Carnow et al., 1969) showed conflicting
results. The reasons for these differences are not clear but may be
that different criteria for the selection of patients as well as
different methods for the determination of the exposure of the
individuals and their responses were used.
An unexpected finding of a possible effect of short-term exposure
to pollution arose from a study primarily concerned with long-term
exposures. In a resurvey of adults in Vlaardingen (Netherlands) who
had been interviewed and had lung function measurements made in 1969,
Van der Lende et al. (1975) found that the average lung function
values were higher in 1972 than in 1969, even though the subjects were
3 years older. When the authors examined the concentrations of
pollution on the 2 occasions (each survey having been done within a
5-day period), they found that levels were relatively high in 1969
with daily values ranging from 15 to 140 µg/m3 for smoke and from 120
to 300 µg/m3 (0.04-0.11 ppm) for sulfur dioxide compared with values
ranging from 15 to 40 µg/m3 and 45-100 µg/m3 (0.02-0.04 ppm),
respectively, in 1972. The increase in lung function was most
pronounced on the days with the greatest difference in the levels of
pollution. A control population in a rural area showed no comparable
changes over the same period, and temperature differences did not
explain the effect.
Asthmatic subjects have also been studied. These patients
represent a heterogeneous group and this may account for the variable
responses that have been reported. Cohen et al. (1972) studied 20
asthmatic subjects living in a small town (Cumberland, WV, USA) in the
vicinity of a coal-fired power plant. They found that when the soiling
index exceeded 1.0 Coh unit, or sulfur dioxide concentrations
(West-Gaeke method) exceeded 200 µg/m3 (0.07 ppm), or total suspended
particulates exceeded 150 µg/m3 (high volume sampling method), or the
temperature was lower than 0°C, there was a significant increase in
the frequency of asthmatic attacks. Levels of sulfates exceeding
20 µg/m3 and nitrate levels exceeding 2 µg/m3 did not result in such
an effect. In general, the effect of temperature was stronger than
that of the air pollutants although each of the 5 air pollutants
measured including sulfates and nitrates showed a correlation. When
temperature and any one of the pollutants were controlled for in the
analysis, the effect of any of the other 4 pollutants was eliminated.
Temperatures below 0°C overwhelmed any effect of the pollutants and
higher levels of the pollutants reduced the effect of temperature. One
further feature of this study should be stressed. Pollution in
Cumberland was dominated by emissions from a large single source, and
this might have led to high transient exposures to the pollutants,
which included oxides of nitrogen as well as sulfur oxides and
suspended particulate matter. In these circumstances it is doubtful
whether the 24-h values provided an adequate index of exposure of the
population.
Several studies on asthmatic subjects in Yokkaichi, Japan, have
been reported by Yoshida et al. (1966) including one on a group of 13
patients in which the number of attacks increased from 1 to 4 per week
when the concentration of sulfur dioxide was in the range of
140-230 µg/m3 (0.05-0.08 ppm), rising to about 12 per week when the
sulfur dioxide level reached 740 µg/m3 (0.26 ppm), all expressed as
weekly means. Suspended particulate or smoke levels were not reported.
8.3.4 Morbidity in adults -- effects of long-term exposures
Random samples of populations can be used for international
comparisons where there are gradations of air pollution. The major
difficulty here has been to ensure comparability with respect to
occupational exposures and ethnic groups. Reid et al. (1964) reported
such a comparison based on a study in the United Kingdom (College of
General Practitioners' Study, 1961) and a survey in Berlin, NH, USA
(Ferris & Anderson, 1962). The same questionnaire was used in both
studies. In the United Kingdom, it was completed by a large number of
practitioners who made up the survey group. In the USA, it was
completed by 2 physicians who tried to maintain the criteria developed
in the British survey. Results were standardized for cigarette
smoking. The effects of air pollution were then examined by age group
and sex. When simple bronchitis was present, i.e., phlegm production
for 3 months out of the year for 2-3 years, standardizing for
cigarette smoking removed any effect of air pollution for both males
and females. A more severe form of chronic bronchitis characterized by
phlegm production, exacerbations of colds that went to the chest, and
shortness of breath when walking on the level at one's own pace did
show an association with air pollution for both males and females,
even after standardizing for cigarette smoking. Levels of air
pollution were measured in Berlin, NH, by the lead peroxide candle,
dustfall, and high-volume samplers. Data for the United Kingdom were
estimated from similar measurements obtained in comparable towns and
cities where the general practitioners collected the data. In Berlin,
NH, the sulfation rate (lead candle) was 730 µg SO3/100 cm2 per day;
in the United Kingdom, in the large towns it was 950 and in the large
conurbations 1650 µg SO3/100 cm2 per day. The results of this study
seem to be consistent with those of other studies but it is not known
whether differences in socioeconomic or ethnic status could have been
relevant factors. The population of Berlin, NH, was resurveyed in
1967, and the results were compared with those obtained in 1961
(Ferris et al., 1973). There had been some decrease in pollution
levels in the interval, the sulfation rate being 470 µg SO3/100 cm2
per day in 1967, compared with the earlier figure of 730, while the
concentration of total suspended particulates had fallen from 180 to
132 µg/m3 (high-volume samples). Small reductions in the prevalence
of respiratory disease were noted after standardizing for age and
cigarette smoking. Slight improvements in forced vital capacity (FVC)
and peak expiratory flow rate (PEFR) were also noted, but there was
little change in FEV1.0. There is some doubt about the relevance of
the 180 µg/m3 figure quoted for total suspended particulates in the
1961 study, since it referred only to a 2-month period at a single
site. A random population sample from Chilliwack, BC, Canada, an
unpolluted community, was studied by Ferris & Anderson (1964) and the
results were compared with the results of the 1961 study in Berlin,
NH. Respiratory symptoms, after standardization for age and cigarette
smoking, tended to be higher in Berlin than in Chilliwack. Pulmonary
function (FEV1.0 and PEFR) was lower in Berlin than in Chilliwack,
after standardization for age, height, sex, and smoking category.
Pollution levels in Chilliwack (based on lead-candle measurements)
were about one-tenth to one-sixth of those in Berlin, NH.
A third survey in this series was carried out in 1973 (Ferris et
al., 1976). By this time, there had been a further decline in
pollution by particulate matter, the annual mean concentration of
total suspended particulates (high volume sampler) being quoted as
80 µg/m3. Only limited data on sulfur dioxide were available; the
mean of a series of 8-h samples for selected weeks was quoted as
0.01 ppm (30 µg/m3). On this occasion, the authors did not find any
appreciable differences in the prevalence of respiratory symptoms or
in measures of lung function, as compared with the 1973 report. The
interpretation of these findings is difficult, for the studies were
concerned largely with consecutive investigations of survivors of the
original group, over a 12-year period. Although the authors took into
account, as far as possible, the effect of selective losses of some of
the population, and changes in smoking habits among those who
remained, there is still some doubt as to whether the three sets of
results are truly comparable. The authors themselves concluded that
either the changes in air pollution levels from 1967 to 1973 (which
included a decline in total suspended particulates from about 130 to
80 µg/m3 (annual mean) with possibly a slight increase rather than
decrease in sulfur dioxide) were not associated with a beneficial
effect on health, or that their methods were not sufficiently
sensitive at the levels involved.
One of the difficulties in interpreting these data is that
exposure to odorous air pollutants (Berlin, NH) also occurred,
indicating an unconventional type of air pollution. It does seem
reasonable, however, to interpret the results of these surveys as
showing that slight changes in respiratory symptoms and pulmonary
function were related to levels of pollution. Sulfur oxides and
particulate matter may have been of importance. Only sulfation data
are available for sulfur oxides in the 1961 study.
Extensive studies have been made of post office and telephone
workers in the United Kingdom and USA (Holland & Reid, 1965; Holland &
Stone, 1965; Holland et al., 1965). The authors carefully considered
most of the relevant epidemiological variables and showed a gradation
of symptoms across the levels of pollution, particularly in the 50 to
59-year-old category. However, since pollution was measured in
different ways in each part of the studies, it is difficult to deduce
any quantitative relationships with sulfur dioxide and particulate
matter. The differences in the prevalence of symptoms persisted when
the authors examined the various smoking categories. They converted
the small amount of pipe and cigar smoking to cigarette equivalents,
which is not advisable, since other studies have indicated that pipe
and cigar smoking are less markedly associated with respiratory
symptoms. Lower levels of FEV1.0 and PEFR were observed in the areas
of higher pollution. The authors indicated that a difference in height
of 2-4 cm between the two populations studied could not account for
the differences seen in pulmonary function. Presumably, these
pulmonary function values had been corrected for standard temperature
and saturated vapour pressure. If not, some of the differences between
values in the USA and United Kingdom could be explained by the fact
that lower temperatures in England could have resulted in lower
measured air volumes. Similar spirometers were used in both studies.
In another study in the United Kingdom, Lambert & Reid (1970)
questioned about 10 000 adults by post. They estimated that their
sample represented about 74% of those able to reply. The positive
responses from the questionnaire (which had been recommended by the
British Medical Research Council) were correlated with levels of
pollution estimated from data used earlier by Douglas & Waller (1966)
and from some data from the National Air Pollution Survey. The
responses were controlled for social class and cigarette smoking. The
authors reported that whereas nonsmokers showed little response to the
levels of air pollution, cigarette smokers did respond and appeared to
be more sensitive. They also noted a considerable rural/urban
gradient, more pronounced in men than in women, that could not be
explained by differences in smoking habits.
Extensive studies were carried out in the Ruhr area of the Federal
Republic of Germany (Reichel et al., 1970; Ulmer et al., 1970) based
on a short questionnaire on respiratory symptoms, physical
examinations, and measurements of airways resistance using a body
plethysmograph. There were no clear differences in the results between
areas with different levels of pollution, but because of selection
factors and a low response rate, no definite conclusions can be drawn
from these studies regarding relationships with sulfur dioxide and
particulate matter.
A study in Vlaardingen in the Netherlands by Van der Lende (1969)
indicated that increased cough and phlegm production were associated
with air pollution but decreased lung function was not. However, the
author considered that the study might have been affected by the
presence of allergens, such as spores and bacteria in the agricultural
regions. Comparisons were made between an industrial area, polluted by
sulfur dioxide and particulate matter, and an agricultural area with
little pollution. In a later report, Van der Lende et al. (1973)
indicated that the effect of pollution could have been masked by
"self-selection" of the populations concerned.
The relationship between chronic bronchitis and air pollution was
studied in Osaka Prefecture, Japan, by Tsunetoshi et al. (1971).
Surveys were made of all persons over 40 years of age in selected
areas using self-administered questionnaires similar to that
recommended by the British Medical Research Council. The authors'
definition of chronic bronchitis was cough with sputum for 3 or more
months, for at least 2 successive years. Pulmonary function was
measured by the Vitalor, using the maximum value. An equation was
developed relating prevalence of bronchitis to age, smoking, and
sulfation rate (lead candle). The prevalence of bronchitis in the
study areas ranged from about 4% (in males aged 40-59 years) in areas
where the sulfation rate was close to 1 mg/100 cm2 per day to about
10% in those around 3 mg/100 cm2 per day. No data were given
concerning the levels of particulate matter.
Many other studies on the prevalence of respiratory symptoms in
relation to air pollution have been carried out in Japan such as that
by Toyama et al. (1966). They reported prevalences ranging from 2.8 to
3.7% (males aged 40-59 years, adjusted for age and smoking to accord
with other Japanese studies) in areas of Kashima (a nonindustrialized
rural town, at that time) with sulfur dioxide concentrations of less
than 30 µg/m3 (0.01 ppm, automatic conductimetric method) and
suspended particulate concentrations (high-volume sample) of
106-341 µg/m3 (mean 197). An extensive survey in Tokyo, (Suzuki &
Hitosugi, 1970, unpublished data)a showed a higher prevalence of
chronic bronchitis in areas that were more polluted, ranging from 5.5%
(males aged 40-59 years) and 1.1% (females 40-59 years) where the
sulfur dioxide concentration was below 60 µg/m3 (0.02 ppm, automatic
conductimetric method), and the suspended particulate level was below
100 µg/m3 (light-scattering method), to 6.7% (males) and 3.9%
(females), where sulfur dioxide was over 140 µg/m3 (0.05 ppm) and
suspended particulates more than 200 µg/m3. There was also some
increase in prevalence in an intermediate area with sulfur dioxide
concentrations of 60-140 µg/m3 (0.02-0.05 ppm) and a suspended
particulate concentration of 100-200 µg/m3. Smoking habits were
standardized in this study. In a recent study by Tani (1975) in the
vicinity of a pulp mill at Fuji City, and in control areas, there
appeared to be a consistent relationship between the prevalence of
bronchitis and sulfation rates, as measured by lead candles, with a
prevalence of about 3% (males and females combined, aged 40-59 years)
in areas where the sulfation rate was around 0.6 mg/100 cm2 per day
to about 8% where it was 1.2 mg/100 cm2 per day.
In studies on a random sample of adults in Cracow, Polandm the
levels of pollution measured made it possible to classify the subjects
into high and low exposure groups (Sawicki, 1972). The levels in the
high pollution area were an annual mean smoke level of 170 µg/m3 and
an annual mean sulfur dioxide level of 125 µg/m3 (0.04 ppm). In the
low pollution area, the standard smoke annual mean was 90 µg/m3 and
the sulfur dioxide annual mean was 45 µg/m3 (0.02 ppm). Persons
a Suzuki, T. & Hitosugi, M. Prevalence study of pulmonary symptoms
in Tokyo Prefecture employees. A paper presented at a meeting of a
Working Group on Air Pollution and Health, USA-Japan Cooperative
Science Programme, East-West Center, Honolulu, 21-23 April, 1970. The
pollution levels quoted in this study were obtained from reports of
the Tokyo Metropolitan Government concerning air pollution levels for
the years 1966-69 (Department of Environmental Pollution Control
Tokyo, 1976).
residing in the more polluted area had more respiratory symptoms and
poorer pulmonary function than those residing in the area with lower
pollution (chronic bronchitis 19% in more polluted area and 11% in
less polluted area and asthmatic disease 11% in more polluted area and
5% in less polluted area). Sawicki considered that there was a
synergistic interaction with cigarette smoking. He also pointed out
that many of the inhabitants worked outside the area in which they
lived and, therefore, the exposure levels at their places of residence
might not represent their true exposures.
8.3.5 Morbidity in children
Studies of the health of children have also provided useful
information.
Douglas & Waller (1966) performed a cohort study based on a
national sample of children born in the United Kingdom in the first
week of March 1946. The children were followed medically for 15 years
by health visitors and school doctors. The study was restricted to the
3131 children who remained at the same address for the first 11 years
of the enquiry, or who moved to an area that was in a similar
pollution group. Levels of air pollution were estimated from the
amount of coal consumed in a given area where each child lived, and it
was shown at the end of the study that the 4 pollution categories that
had been defined provided a satisfactory gradation in terms of the
measurements of smoke and sulfur dioxide that were then available. In
each of the several periods of life considered, from birth onwards,
the authors noted a consistent relationship between the frequency of
lower respiratory tract infections and pollution category. There was
no sharp change at any particular pollution level, but if the rates
within the lowest category (mainly rural areas) were taken as a
baseline, then increased frequencies were seen in the next category
up, with smoke and sulfur dioxide each of the order of 140 µg/m3
(0.05 ppm sulfur dioxide) or more. Annual means were estimates based
on the British daily smoke/sulfur dioxide method for a period after
the end of the study and probably underestimated earlier level.
Socioeconomic status was important in the study, but a relationship
between air pollution and frequency of lower respiratory tract
infections still existed within separate social classes. In a later
follow-up of these subjects, Colley et al. (1973) showed that, at the
age of 20 years, respiratory symptoms were related to smoking habits
rather than to the pollution of the areas in which the subjects were
then living. However, there was some relationship between the
prevalence of symptoms and earlier histories of lower respiratory
tract infections, which, in turn, were related to estimated pollution
exposures during childhood.
Lunn et al. (1967) studied 819 children in their first year at
school in the industrial city of Sheffield, in the United Kingdom.
Medical examinations were carried out on the children, the parents
were questioned concerning the previous health of the children, and
FEV0.75 and FVC were measured. Various socioeconomic factors were also
carefully considered. The families were stable, and there had been
little or no migration into or out of the specific communities.
Measurements of pollution were made at the schools attended by the
children (British daily smoke/sulfur dioxide method). The authors
found increased frequencies of both upper and lower respiratory tract
infections in the more polluted areas. When the "cleanest" area was
taken as the baseline, most of the illness indices considered showed
an increase in the next area up in order of pollution levels, i.e.,
where the annual mean concentrations of smoke and sulfur dioxide were
each about 200 µg/m3 (0.07 ppm sulfur dioxide) taking figures for the
middle year of the survey. A follow-up study was carried out on some
of these children when they were 4 years older (i.e., aged 9) (Lunn et
al., 1970). By then, the implementation of the Clean Air Act had
reduced the contrast in pollution between the areas, so that the mean
concentration of smoke in the 3 "dirty" areas combined was about
140 µg/m3 and the mean sulfur dioxide concentration was 200 µg/m3
(0.07 ppm), compared with about 50 and 100 µg/m3 (0.04 ppm sulfur
dioxide), respectively, in the "clean" area. At this time, there was
no significant difference in the illnesses reported by the 9-year-olds
in the different areas and the authors concluded that this was in line
with the reduced pollution levels.
Data were collected (Holland et al., 1969) concerning about 11 000
children attending school in 4 areas of Kent, England, 2 of which were
predominantly urban, and 2, rural. As part of the children's regular
medical examinations, peak expiratory flow rates, height, weight, and
the results of examinations of ears and tonsils were recorded. Smoking
habits and school absences were also recorded. Questionnaires
concerning such factors as previous respiratory diseases, father's
occupation, and size of family were completed by the parents. Smoke
and sulfur dioxide were measured in 3 of the areas (British daily
smoke/sulfur dioxide method) and information on population density and
housing was collected. Four factors emerged as important in relation
to decreased peak expiratory flow rates. Place of residence was most
important followed by previous history of respiratory disease; family
size and social class were of least importance. These 4 factors seemed
to be additive but they only accounted for 10-15% of the total
variation. Levels of air pollution for smoke were reported to range
from 34 to 69 µg/m3 in the 3 communities. Values for sulfur dioxide
were not reported but were stated to parallel those for smoke. There
were clearly other factors affecting area difference, and there is
some doubt as to whether the small contrasts in pollution could have
had much effect.
Colley & Reid (1970) surveyed about 11 000 children from 6 to 10
years of age, living in urban and rural areas of England and Wales.
The prevalence of respiratory disease was assessed in the autumn.
Pollution was assessed in terms of sulfur dioxide concentration, from
some direct measurements (British daily smoke/sulfur dioxide method)
coupled with estimates based on lead candle measurements of sulfation.
Within the English areas studied (winter mean concentrations of sulfur
dioxide ranging from about 30 to 150 µg/m3 or 0.01-0.05 ppm) there
was a gradient in the prevalence of symptoms, but the rates were much
higher in Wales for comparable levels of pollution. The reasons for
this difference were not clear, although it has been suggested that it
could be related to the fact that solid-fuel consumption was high in
Wales.
Respiratory function measurements were made on children aged 10-11
years from 2 different areas of the German Democratic Republic (Berlin
and Bitterfeld) with different levels of pollution (Grosser et al.,
1971). The groups were matched for social class, age, and height. In
the low pollution area, the concentration of respirable dust (method
not mentioned) was 110 µg/m3 and that of sulfur dioxide (method not
mentioned) was 50 µg/m3 (0.02 ppm); in the other area, the figures
were 290 and 360 µg/m3 (0.13 ppm), respectively (based on 24-h
measurements, averaged for July and December). Two studies were made,
6 months apart, in each of the 2 areas. Each of the spirometric values
studied (FVC, FEV1.0, FEV0.7) was higher in the area with lower
pollution.
A variety of other techniques has been used to investigate
possible effects of exposure to pollution. These include the work of
Yoshii et al. (1969) who noted an association between chronic
pharyngitis accompanied by histopathological changes at biopsy and
level of sulfation, expressed as the annual mean, in persons attending
their clinic in Yokkaichi, Japan, and in sixth-grade children. In the
heavily polluted districts sulfation rate was more than 1.0 mg/100
cm2 per day, in moderately polluted districts it ranged from 0.25 to
1.0 mg/100 cm2 per day, and in the control area it was less than this
level (lead candle measurements).
Conjunctivitis, both acute and chronic, has been reported in
Zabrze, Poland, in relation to industrial air pollution (Maziarka &
Moroz, 1968). In Czechoslovakia, Schmidt et al. (1966) reported
differences in the blood cells, tonsils, and cervical lymph nodes of
children living in a highly polluted atmosphere compared with those
living in a relatively clean atmosphere and Symon et al. (1969)
reported retardation in growth and ossification and a reduced colour
index in red blood cells in children living in areas with high air
levels of sulfur dioxide (up to 2-3 mg/m3) and fly ash. None of these
studies can be used for the exposure-effect relationship.
8.3.6 CHESS studies
The US Environmental Protection Agency has recognized the need for
studies at relatively low levels of air pollution in order to
determine whether a no-effect level can be identified to develop a
dose-response relationship and to monitor areas where there might be a
change in the levels of exposure. The CHESS (Community Health and
Environmental Surveillance System) studies were developed with these
purposes in mind. In some aspects these studies have been well
designed and executed but in other aspects there have been
difficulties. In many ways these are preliminary studies that need to
be continued. The studies have included adults, children, asthmatic
subjects, and patients with cardiovascular and pulmonary diseases.
Both acute and chronic effects have been studied. Methods have
involved mailed questionnaires on respiratory symptoms and illnesses
(adults and children), mailed questionnaires on family composition,
housing, and socioeconomic status, telephone interviews, tests of
pulmonary function (children), and diary techniques with patients.
Previous exposures to air pollutants were estimated from
historical data on emissions or known production figures from the
local industries and the application of mathematical models. More
recent exposures were based on actual measurements. These were
generally conducted at monitoring stations (each of which covered a
radius of up to 2 km), and involved high volume sampling for total
suspended particulates and suspended sulfates, and the West-Gaeke
method for sulfur dioxide. Mathematical models were used to estimate
the variation in exposure of the different groups. Various problems
discussed in a summary of the results (US Environmental Protection
Agency, 1974) include poor response rates (i.e., low participation
rates) in some of the studies on children, high dropout rates in
studies on patients, and, in at least one of the studies on children,
the fact that their spirometry was probably in error.
There was a general tendency for the authors to over-interpret the
CHESS data. In some of the studies on children, the results were
grouped according to high and low levels of pollution and a difference
was found between the two groups. However, the relationship of the
prevalence of disease to levels of pollution was not clear -- namely,
when the individual groups were examined, some exposed to the high
levels of pollution had a lower disease prevalence than some of the
groups exposed to low levels of pollution.
The design for the study of the impact on respiratory disease was
excellent and took into account family composition, housing, and
socioeconomic status. It is unfortunate that these studies were not
continued for more than one year of observation before reporting. A
number of years of observations are needed to rule out natural
fluctuations in respiratory disease.
These studies have been reviewed by an expert committee in the USA
under the chairmanship of Dr D. Rall (Director, National Institute of
Environmental Health Sciences, US Department of Health, Education and
Welfare) (Rall, 1974). The Committee's evaluation of the studies was
similar to that of the Task Group and the Committee concluded that the
results of the studies did not warrant any change in the present US
Federal Air Quality standards.
It was the opinion of the WHO Task Group that these data could not
be used for the estimation of an exposure-effect relationship.
8.3.7 Lung cancer and air pollution
The possibility that air pollution is a causal factor in cancer of
the lung has given rise to considerable concern. The evidence in
favour of a causal relationship is briefly: (a) the excess
occurrence of the disease in urban areas; (b) the presence in the
suspended matter in urban air of substances such as benzo(a)pyrene
that can cause cancer under experimental conditions; and (c) the
general rise in lung cancer that appeared, at one time, to follow
certain assumed trends in pollution.
Early studies in the United Kingdom (Stocks, 1959, 1966) indicated
that variations in lung cancer mortality in urban areas were
associated with variations in amounts of pollution and, following a
recommendation by a WHO Study Group in 1959 (World Health
Organization, 1960), a pilot international study was undertaken in
several cities where there were contrasts in lung cancer death rates.
The results did not show any clear-cut relationship with measurements
of particulate matter or its benzo(a)pyrene content (Waller & Commins,
1967) and it was clear that apart from the difficulties of making
proper allowances for differences in smoking habits, it seemed likely
that present-day measurements of polycyclic hydrocarbons gave an
inadequate assessment of past exposures to these compounds.
The Royal College of Physicians of London (1970) reviewed the
issue, and concluded that the evidence against community air pollution
being a causal factor in lung cancer was stronger than the evidence
for it. The urban/rural differential is greatest in countries with
relatively low urban air pollution (Sweden, Norway, Denmark). The
upward trend in mortality as well as other experimental and
epidemiological evidence are best explained by the causal role of
cigarette smoking.
Nevertheless, in a review by Cleary (1967), evidence is presented
to show that, in Australia, New Zealand, South Africa, and the USA,
immigrants from the United Kingdom have a higher lung cancer death
rate than those born in these countries: immigrants from Norway have a
lower rate than native-born citizens of the USA, and the lung cancer
mortality rates for all these migrants are intermediate between those
of their countries of origin and destination, strongly suggesting an
environmental factor in early life.
While the existence of an urban excess of lung cancer has been
proved, it is uncertain that air pollution is the "urban factor"
responsible. In contrast, recent work incriminates cigarette smoking
more strongly than ever; there is also a contribution from some
specific occupational exposures (see footnote in section 1.1.7).
The consideration of criteria for environmental carcinogenesis
specifically in relation to any possible effects on lung cancer, is
outside the scope of the present discussion, but it may be mentioned
that any action to reduce smoke and especially that from the
inefficient combustion of coal in domestic fires, is likely to make
substantial reductions in the benzo(a)pyrene content of the air. Such
a change has already been noted in London, where the concentration of
this compound is now only about one-tenth of what it was 25 years ago
(Lawther & Waller, 1976). A reduction of sulfur dioxide may also be
important for any possible interactions relating to the production of
lung cancer. Some experimental studies (Kuschner & Laskin, 1971;
Skvorcova et al., 1973) showed an increased carcinogenic response when
laboratory animals (rats and hamsters) were exposed to sulfur dioxide
in addition to benzo(a)pyrene.
8.3.8 Annoyance
Annoyance may be defined as "a feeling of displeasure associated
with any agent or condition believed to affect adversely an individual
or a group". This definition was adopted by an international symposium
on annoyance in Stockholm in 1971 (Lindvall & Radford, 1973). Very few
studies have been performed that would make quantitative evaluations
of this effect possible.
The social awareness of pollution caused by particulate matter has
been studied in a few areas. The results from different studies have
been presented in a document on particulate matter (US Department of
Health, Education and Welfare, 1969a); they include those from a study
carried out in St. Louis (Schusky, 1966), where values for suspended
particulates of around 100 µg/m3 produced annoyance reactions from a
considerable number of people.
A similar study was carried out in Birmingham, Alabama, USA
(Stalker & Robinson, 1967), in which levels of air pollution were
correlated with annoyance. They found that, as dustfall reached or
exceeded 14.1 g/m2 per month, about one-half of the population
considered it to be a nuisance; at 10.5 g/m2 per month, about
one-third of the population considered air pollution a nuisance.
Stepwise multiple regression analysis showed that the variation due to
dust fall alone explained 49% during the spring season and 68% during
the winter season of the total association between the air pollutants
measured and public annoyance. The association between levels of
suspended particulate matter and public opinion, which was weaker than
that of dustfall, was strongest during the summer season (r = 0.59).
About one-half of the persons interviewed thought that air pollution
was a general nuisance, when mean annual or mean summer concentrations
of particulate matter reached 230 µg/m3 and one-third, when they
reached 150 µg/m3.
No such relationship was shown with sulfur dioxide concentrations.
However, these levels were low. Further studies are indicated, since
it may be that effects such as annoyance reactions will, in the
future, be the critical effects on which criteria, as regards the
protection of public health, will be based. Since annoyance reactions
have a large sociocultural component, these levels may vary from place
to place and should be determined for each locality. Surveys on
annoyance are fraught with many problems. When proper survey
techniques are expertly applied, however, it will be possible to
assess reactions in a quantitative manner.
8.4 Exposure-Effect Relationships
Some of the studies reported in this section can be used to
develop exposure-effect relationships. It has been necessary to
develop 2 tables, one for the effects of short-term exposures
(Table 15, p. 107) and another for the effects of long-term exposures
(Table 16). The results from the studies have been based on different
methods of measuring sulfur oxides and particulate matter. The values
for the sulfur oxides are treated as if they were comparable. For the
particulate matter, two categories are listed: smoke as measured by
the Organization for Economic Cooperation and Development or British
daily smoke/sulfur oxide methods and total suspended particulates as
measured by the high volume sampler or light scattering. These factors
should be considered in interpreting these tables.
Table 15. Exposure-effect relationships of sulfur dioxide, smoke, and total suspended
particulates: effects of short-term exposures
concentration
24-h mean
values (µg/m3)
Total
suspended
Sulfur dioxide Smoke particulates Effects
> 1000 > 1000 -- London, 1952. Very large increase in mortality to about 3
times normal, during 5-day fog. Pollution figures represent
means for whole area: maximum (central site) sulfur dioxide
3700 µg/m3, smoke 4500 µg/m3 (Ministry of Health, UK, (1954).
710 750 -- London, 1958-59. Increases in daily mortality up to about 1.25
times expected value (Lawther, 1963; Martin & Bradley, 1960).
500 500 -- London, 1968-60. Increases in daily mortality (as above) and
increases in hospital admissions, becoming evident when
pollution levels shown were exceeded (magnitude increasing
steadily with pollution) (Martin, 1964).
500 -- -- New York 1962-66. Mortality correlated with pollution: 2%
excess at level shown (Buechley, 1973).
500 250 -- London, 1954-68. Increases in illness score by diary technique
among bronchitic patients seen above pollution levels shown
(means for whole area) (Lawther et al., 1970).
Table 15 (cont'd).
concentration
24-h mean
values (µg/m3)
Total
suspended
Sulfur dioxide Smoke particulates Effects
300 140 -- Vlaardingen, Netherlands, 1969-72. Temporary decrease in
ventilatory function (Van der Lende et al., 1975).
200a -- 150b Cumberland, WV, USA. Increased asthma attack rate among
small group of patients, when pollution levels shown were
exceeded (Cohen et al., 1972).
a West-Gaeke method
b High volume sampling method
Other measurements by Organization for Economic Cooperation and Development or British daily smoke/sulfur
dioxide methods (Ministry of Technology, UK, 1966; Organization for Economic Cooperation and Development, 1965).
Table 16. Exposure-effect relationships of sulfur dioxide, smoke, and
total suspended particulates: effects of long-term exposures
Concentration
Annual means of 24-h
mean values (µg/m3)
Total
suspended
Sulfur dioxide Smoke particulates Effects
200 200 -- Sheffield, England. Increased
respiratory illnesses in children
(Lunn et al., 1967, 1970)
-- -- 180b Berlin, NH, USA. Increased respiratory
symptoms, decreased respiratory
function in adults (Ferris et al., 1973)
150 -- -- England & Wales. Increased respiratory
symptoms in children (Colley & Reid, 1970)
125 170 -- Cracow, Poland. Increased respiratory
symptoms in adults (Sawicki, 1972)
140d 140d -- Great Britain. Increased lower
respiratory tract illnesses in
children (Douglas & Waller, 1966)
60-140a -- 100-200c Tokyo, Increased respiratory symptoms
in adults (Suzuki & Hitosugi, unpublished
data, 1970)
a Automatic conductimetric method
b High volume sampler (2-month mean, possible underestimation of annual mean).
c Light-scattering method, results not directly comparable with others.
d Estimates based on observations after end of study; probable underestimation
of exposures in early years of study.
Other measurements by Organization for Economic Cooperation and Development or
British daily smoke/sulfur dioxide methods (Ministry of Technology, UK, 1966;
Organization for Economic Cooperation and Development, 1965).
9. EVALUATION OF HEALTH RISKS FROM EXPOSURE TO SULFUR OXIDES, SMOKE,
AND SUSPENDED PARTICULATE MATTER
It is well established that respiratory diseases are important
causes of disability and death, and that, for some of them, there is
evidence of association with environmental factors in the ambient air.
There is evidence that exposure to mixtures of urban air pollutants
containing sulfur oxides and particulate matter is related to a
variety of adverse effects on health, even when other factors are
controlled (section 8 -- Tables 15 and 16). Although in most of the
studies considered the levels of air pollution have been expressed in
terms of sulfur dioxide, smoke, or suspended particulate matter, this
does not necessarily imply that these are the causative agents. They
provide only indices of pollution, and certain components, such as
sulfuric acid or sulfates, may be of particular importance.
Measurements of some of these components have now been made in a
number of areas and used in some of the more recent epidemiological
studies. The Task Group concluded, however, that, at present, there
are not enough data on any of these other indices of pollution to
allow exposure-response relationships to be established. Thus, the
present discussion is confined to the effects of pollution expressed
in terms of sulfur dioxide, smoke, and total suspended particulate
matter.
9.1 Exposure Levels
Concentrations of sulfur dioxide, smoke, and suspended particulate
matter vary greatly from place to place and from time to time. Many
factors including sources, topography, and weather conditions may be
involved in these variations.
An annual arithmetic mean of sulfur dioxide concentrations in
urban areas typically ranges from 100-200 µg/m3 (0.035-0.070 ppm),
whereas a maximum daily mean ranges from 300-900 µg/m3
(0.11-0.32 ppm). For smoke, an annual arithmetic mean ranges from
30-200 µg/m3 with a maximum daily mean of 150-900 µg/m3; for total
suspended particulates, these levels range from 60-500 µg/m3 and
150-1000 µg/m3, respectively.
Comparatively little information is available concerning indoor
concentrations of sulfur dioxide and particulate matter, though, in
general, these levels are known to be lower than those outdoors,
except at work places.
Levels in working environments are considerably higher than
general community levels and are thought to have been much higher in
the past.
In evaluating exposure levels that have been used in the past in
connexion with epidemiological studies, a serious question arises as
to how far these measurements, often intended primarily for control or
monitoring purposes, can be considered as providing adequate measures
of exposure. As discussed in section 5, it must be recognized that all
the figures quoted in subsequent sections are based on measurements
that have been made outdoors, usually at only a limited and not wholly
representative set of fixed sites, though most people spend much time
indoors, where concentrations may differ substantially from those
outside. However, this does not necessarily invalidate these data
which are serving primarily as indices.
So far, there has been little information on the particle size
distribution and the chemical composition of selected particle size
ranges that would make the nature of exposure more understandable.
Comparable assessment is often difficult because of differences in
the methods of measurement of particulate matter that have been used
in the health effect studies. Smoke, assessed in terms of blackness,
is a measure of pollution associated with the incomplete combustion of
fuel, and total suspended particulates, determined by weight, is a
wider concept that includes all material which, by virtue of its
particle size, remains in suspension for long periods.
9.2 Experimental Animal Studies
Laboratory studies have been performed using a variety of test
animals, of periods of exposure, of experimental designs in exposure,
and of combinations of pollutants and other agents.
Some of these studies have provided useful information on the
mechanisms of the biological action of sulfur dioxide, sulfuric acid
mist, or particulate matter. However, the results are of limited value
in developing guidelines for the protection of human health from
effects of these pollutants as they exist in urban areas, and for this
reason it is necessary to turn to the available epidemiological
evidence.
9.3 Controlled Studies in Man
Effects studied in volunteers under controlled conditions include
those on the functions of the respiratory system, sensory organs, and
cerebral cortex. Durations of exposure have been very short, usually
less than a few hours.
Slight effects on pulmonary function were observed with exposure
to sulfur dioxide at a concentration of 2100 µg/m3 (0.75 ppm), but
not with exposure to a concentration of 1100 µg/m3 (0.37 ppm).
With exposure to sulfuric acid, a decrease in tidal volume was
found at a concentration as low as 350 µg/m3.
Combined exposures to sulfur dioxide and ozone or hydrogen
peroxide produced a greater effect on respiratory function than
exposure to each compound alone.
Studies on sensory and cerebral cortical functions showed that
sulfuric acid was more toxic than sulfur dioxide and that a
combination of these two substances produced an approximately additive
effect.
There is a limit to the use of these studies for the development
of criteria for community exposures, particularly when long-term
exposure to complex mixtures of air pollutants is involved.
9.4 Effects of Industrial Exposures
Effects on the health of workers have been studied in relation to
exposure to sulfur dioxide, particulate matter, or sulfuric acid mist
arising from various manufacturing processes. In many of these
studies, exposure levels were relatively high and, in some studies,
adverse effects were detected only at a daily mean sulfur dioxide
concentration as high as 70 000 µg/m3 (25 ppm).
It has also been reported that exposure to sulfuric acid at a mean
concentration of 1400 µg/m3 during working hours for 2 days did not
produce any effect on lung function.
The fact that adverse effects have not been reported at
comparatively high levels of sulfur dioxide or surfuric acid aerosols
may well be explained by the influence of biasing factors, such as
that the workers remaining in jobs with exposures to pollutants
consist of people especially resistant to their effects. Therefore, it
should not be considered an indication that such concentrations are
without effects for the average working population and particularly
not for workers with pre-existing pulmonary diseases.
In evaluating the effects from industrial exposures, due attention
must also be given to the variation in chemical composition and size
distribution of particulate matter.
9.5 Effects of Community Exposures
Many of the epidemiological studies in relation to community
exposures that were considered by a WHO Expert Committee in 1972
(World Health Organization, 1972), and which must still be relied on
to a large extent today, were based on the measurement of smoke rather
than of total suspended particulates. However, some new data on the
effects of total suspended particulates have become available and have
been included in Tables 15 and 16 (section 8).
Tables 17 and 18 show the levels above which some effects on
health might be expected among specified populations for short-term
and long-term exposures, respectively. These are based on the critical
evaluation of the results of studies reviewed in section 8.
In developing Table 17, greater emphasis had to be placed on some
earlier results related to major effects seen when concentrations of
pollution were much higher than those commonly experienced today. With
the effective control of at least some components in areas where
pollution was, at one time, very high, most of the more recent studies
have failed to isolate the effects of the pollutants in question from
effects similar in nature but arising from other factors.
The figures for sulfur dioxide and smoke are essentially the same
as those proposed by the Expert Committee in 1972 (World Health
Organization, 1972) the only change being the adoption of 250 µg/m3
(0.09 ppm) instead of 250-500 µg/m3 (0.09-0.18 ppm) as the level at
which the worsening of the condition of patients from short-term
exposures to sulfur dioxide might be expected. It was recognized that
the magnitude of responses at this level appeared to be small and
difficult to separate from effects due to other factors such as
weather or infections. It is possible that a lower figure for smoke
could now be adopted, but, in view of uncertainties concerning the
differences in composition of this component of pollution from one
area to another, and the changes with time even within one locality,
it was felt that any further modification of the figure determined on
the basis of the older studies from London would need new evidence.
Table 18 represents an overall assessment of the effects of
long-term exposure to sulfur dioxide and smoke in terms of increases
in the prevalence of respiratory symptoms among both adults and
children, and in terms of the increased frequency of acute respiratory
illnesses, that has been demonstrated particularly among children.
Table 17. Expected effects of air pollutants on health in selected segments of
the population: effects of short-term exposuresa
24-h mean concentration (µg/m3)
Expected effects Sulfur dioxide Smoke
Excess mortality among the elderly
or the chronically sick 500 500
Worsening of the condition of patients
with existing respiratory disease 250 250
a Concentrations of sulfur dioxide and smoke as measured by OECD or British daily
smoke/sulfur dioxide method (Ministry of Technology, UK, 1966; Organization for
Economic Cooperation and Development, 1965). These values may have to be adjusted
in terms of measurements made by other procedures.
Table 18. Expected effects of air pollutants on health in selected segments of
the population: effects of long-term exposuresa
Annual mean concentration (µg/m3)
Expected effects Sulfur dioxide Smoke
Increased respiratory symptoms among
samples of the general population 100 100
(adults and children) and increased
frequencies of respiratory illnesses
among children
a Concentrations of sulfur dioxide and smoke as measured by OECD or British daily
smoke/sulfur dioxide method (Ministry of Technology, UK, 1966; Organization for
Economic Cooperation and Developments, 1965). These values may have to be adjusted
in terms of measurements made by other procedures.
For each pollutant there remains much uncertainty about the
minimum levels associated with demonstrable effects. Where populations
exposed to different levels of pollution have been compared, it cannot
necessarily be assumed that even those who were exposed to a level
lower than the known lowest effect level are entirely unaffected by
pollution. There must also be doubts as to whether the effects
observed in some studies were due in part to other pollutants, or to
socioeconomic or other factors that had not been adjusted for
completely.
No firm conclusion was reached on the effects of total suspended
particulates as a component of air pollution, together with sulfur
dioxide, because of the limited amount of information available. For
the effects of long-term exposure, a tentative figure of 150 µg/m3
(annual arithmetic mean) was suggested, based on the 2 entries in
Table 16, recognising that one of these was based on light-scattering
observations, and the other on high volume sampler measurements, but
for a 2-month period only. It was noted that the one study on the
effects of short-term exposure to total suspended particulates had
been included in Table 15, but it was felt that this could not provide
satisfactory information for an assessment of these effects.
The figures in Table 18 have been expressed in terms of annual
arithmetic mean concentrations, although it is not known whether the
effects are related to extended exposures to these average levels, or
more particularly to days of high pollution within each series. In
view of the limited quantitative information on effects of pollution
in terms of annoyance, this aspect has been omitted from Tables 17 and
18.
9.6 Guidelines for the Protection of Public Health
Tables 17 and 18 present two different sets of criteria, one
relating to effects of short-term exposure, in terms of 24-h average
concentrations, and the other to effects of long-term exposure in
terms of annual means. These effects may be interrelated; the gradual
development of respiratory symptoms may, for example, be a reaction to
repeated short-term exposure to peak 24-h values, or even to transient
peaks lasting for still shorter periods, but in the absence of any
substantial evidence on this point, the two criteria must, for the
time being, be considered separately.
With the present state of knowledge, it was considered that a
safety factor of two below the figures given in Tables 17 and 18 would
be reasonable to ensure the protection of public health, and,
accordingly, Table 19 was developed, still considering the effects of
short-term and long-term exposures separately. As an indication of the
uncertainty surrounding these estimates, the figures have further been
expressed with a range of ± 20%.
The values proposed in Table 19 are in general agreement with
those suggested as long-term goals in the earlier report (World Health
Organization, 1972). In the case of sulfur dioxide, there has been
some reduction in the 24-h figure (if this is regarded as a level not
to be exceeded on more than 7 days a year), and this is in line with
the revision of the "effect" level from a range of 250 to 500 µg/m3
in the 1972 report, to the present figure of 250 µg/m3 (Table 17).
Table 19 requires careful interpretation, for none of the figures
can be considered as absolute limits. In the first place, day-to-day
variations in the concentration of smoke and sulfur dioxide are
determined largely by weather conditions, and occasional peaks far
beyond the usual daily values may well occur, even with careful
control of emissions.
Table 19. Guidelines for exposure limits consistent with
the protection of public healtha
Concentration (µg/m3)
Sulfur dioxide Smoke
24-h mean 100-150 100-150
Annual arithmetic mean 40-60 40-60
a Values for sulfur dioxide and smoke as measured by OECD or
British daily smoke/sulfur dioxide method (Ministry of
Technology, UK, 1966; Organization for Economic Cooperation
and Development, 1965). Adjustments may be necessary where
measurements are made by other methods.
Although there are two sets of conditions specified in Table 19,
determined independently from evidence on the effects of short- and
long-term exposures, they can generally be considered to be consistent
with one another. If the proportion of days with 24-h values above
those in Table 19 is small (e.g., of the order of 7 days per year),
then the annual means may well fall within or below the ranges
specified in the second row of the table. Annual means are specified
here in terms of arithmetic means: the corresponding geometric means
would generally be a little lower (see section 5).
Much consideration was given to the possibility of extending
Table 19 to include guidelines for total suspended particulates as
measured by the high volume sampler but it was concluded that the
available evidence on the effects associated with exposure to
suspended particulate matter was highly unsatisfactory.
The Task Group felt, however, that some recommendations for a
guideline should be made. A tentative annual mean value of 150 µg/m3
has been suggested in section 9.5 as a level beyond which effects of
long-term exposure to total suspended particulates might be observed.
Applying a safety factor of two and introducing a ± 20% range, as in
the case of smoke and sulfur dioxide, would then provide a range of
levels from 60 to 90 µg/m3 as a possible guideline.
For short-term exposures, no satisfactory, direct evidence
relating concentrations of total suspended particulates to effects is
available. Because of this, a guideline for short-term exposure levels
can only be inferred. Assuming that the same ratio of 24-h mean
concentrations to the annual mean (each derived independently, see
beginning of section) given in the guidelines for smoke, is applicable
to suspended particulate matter, then a very approximate 24-h
guideline for suspended particulate matter, as measured by the high
volume sampler, would be in the order of 150 to 230 µg/m3.
While the Group felt that it was reasonable and prudent to
consider the above figures as interim guidelines consistent with the
protection of public health, it stressed the very urgent need for
additional information on the effects of exposure to suspended
particulate matter (measured by the high-volume sampler). Furthermore,
it recognized the fact that the toxicological significance of total
suspended particulates might vary depending on their chemical
composition and particle size, and that under certain circumstances,
the suggested guidelines might need to be reconsidered. It should be
noted that the discussion above does not imply any preference for
smoke measurements over those of total suspended particulates; indeed
it is highly desirable to develop more appropriate methods for the
measurement of suspended particulates, especially those limited to the
measurement of respirable particles.
It was recognized that in many urban and industrial areas existing
levels of pollution by sulfur dioxide and suspended particulate matter
were substantially above these guidelines. Furthermore, there was the
problem that the long-distance transport of these pollutants from
major sources could, in some circumstances, result in comparatively
high background concentrations in rural areas, and high levels in the
incoming air in towns striving to meet their own air quality
standards. It was considered, however, that every effort should be
made to develop control procedures that would allow these guidelines
to be met.
These guidelines are based on observations among populations in
the community exposed to a mixture of sulfur dioxide and smoke or
total suspended particulates and they may not apply to situations
where only one of the components is present. On grounds of prudence,
however, it is recommended that the levels of each pollutant should be
below the values stated. It should be stressed again, however, that
the data on which the guidelines are based are uncertain and each of
the guidelines is tentative and subject to review when further
information becomes available.
It was the opinion of the Group that there is not yet sufficient
information available on the effects of community exposures to
sulfuric acid aerosols or suspended sulphates to develop guidelines
for these air pollutants.
REFERENCES
ABELES, F. B., CRAKER, K. E., FORRENCE, L. E., & LEATHER, G. R. (1971)
Fate of air pollutants: removal of ethylene, sulfur dioxide and
nitrogen dioxide by soil. Science, 173: 914-916.
ADAMS, D. F., FALGOUT, D., FROHLINGER, J. O., PATE, J. B., PLUMLEY, A.
L., SCARINGELLI, F. P., & URONE, P. (1971) Tentative method of
analysis for sulfur dioxide content of the atmosphere (manual
conductimetric method). Health Sci. Lab., 8: 42-47.
ALARIE Y., ULRICH, C. E., BUSEY, W. M., SWANN, H. E., & MACFARLAND, H.
N. (1970) Long-term continuous exposure of guinea pigs to sulfur
dioxide. Arch. environ. Health, 21: 769-777.
ALARIE, Y., ULRICH, C. E., BUSEY, W. M., KRUMM, A. A., & MACFARLAND,
H. N. (1972) Long-term continuous exposure to sulfur dioxide in
cynomolgus monkeys. Arch. environ. Health, 24: 115-128.
ALARIE, Y., BUSEY, W. M., KRUMM, A. A., & ULRICH, C. E. (1973)
Long-term continuous exposure to sulfuric acid mist in cynomolgus
monkeys and guinea pigs. Arch. environ. Health, 27: 16-24.
ALARIE, Y., KRUMM, A. A., BUSEY, W. M., ULRICH, C. E., & KANTZ, R. J.
II. (1975) Long-term exposure to sulfur dioxide, sulfuric acid
mist, fly ash and their mixtures. Results of studies in monkeys
and guinea pigs. Arch. environ. Health, 30: 254-262.
ALBERT, R. E., SPIEGELMAN, J., LIPPMANN, M., & BENNET, R. (1968) The
characteristics of bronchial clearance in the miniature donkey.
Arch. environ. Health, 17: 50-58.
ALLEN, E. R., MCQUIGG, R. D., & CADLE, R. D. (1972) The
photo-oxidation of gaseous sulfur dioxide in the air.
Chemosphere, 1: 23-32.
ALTSHULLER, A. P. (1973) Atmospheric sulfur dioxide and sulfate:
distribution of concentrations at urban and non-urban sites in the
United States. Environ. Sci. Technol., 7: 709-712.
AMDUR, M. O. (1957) The influence of aerosols upon the respiratory
response of guinea pigs to sulfur dioxide. Am. Ind. Hyg. Assoc.
Q., 18: 149-155.
AMDUR, M. O. (1958) The respiratory response of guinea pigs to
sulfuric acid mist. Am. Med. Assoc. Arch. ind. Health,
18: 407-414.
AMDUR, M. O. (1961) Report on tentative ambient air standards for
sulfur dioxide and sulfuric acid. Ann. occup. Hyg., 3: 71-83.
AMDUR, M. O. (1966) Respiratory absorption data and SO2 dose-response
curves. Arch. environ. Health, 12: 729-732.
AMDUR, M. O. (1969) Toxicological appraisal of particulate matter,
oxides of sulfur and sulfuric acid. J. Air Pollut. Control
Assoc., 19: 638-644.
AMDUR, M. O. (1970) The impact of air pollutants on physiological
responses of the respiratory tract. Proc. Am. Philos. Soc.,
114: 3-8.
AMDUR, M. O. (1971) Aerosols formed by oxidation of sulfur dioxide.
Review of their toxicology. Arch. environ. Health, 23: 459-468.
AMDUR, M. O. & UNDERHILL, D. (1968) The effect of various aerosols on
the response of guinea pigs to sulfur dioxide. Arch. environ.
Health, 16: 460-468.
AMDUR, M. O., SCHULZ, R. Z., & DRINKER, P. (1952a) Toxicity of
sulfuric acid mist to guinea pigs. Am. Med. Assoc. ind. Hyg.
occup. Med., 5: 318-329.
AMDUR, M. O., SILVERMAN, L., & DRINKER, P. (1952b) Inhalation of
sulphuric acid mist by human subjects. Am. Med. Assoc. Arch. ind.
Hyg. occup. Med., 6: 305-313.
AMDUR, M. O., MELVIN, W. W., Jr, & DRINKER, P. (1953) Effects of
inhalation of sulfur dioxide by man. Lancet, 2: 758-759.
AMERICAN CONFERENCE OF GOVERNMENT INDUSTRIAL HYGIENISTS (1977)
TLVs threshold limit values for chemical substances and physical
agents in the workroom environment with intended changes for
1977, Cincinnati, OH, ACGIH, p. 28.
AMERICAN SOCIETY FOR TESTING AND MATERIALS (1964) Standard test
method for particulate matter in the atmosphere. Optical density
of filtered deposit. ASTM standards on methods of atmospheric
sampling and analysis, Philadelphia, USA, ASTM, pp. 661-668.
ANDERSEN, I. (1972) Relationships between outdoor and indoor air
pollution. Atmos. Environ. 6: 275-278.
ANDERSEN, I., LUNDQVIST, G. R., JENSEN, P. L., & PROCTOR, D. F. (1974)
Human response to controlled levels of sulfur dioxide. Arch.
environ. Health, 28: 31-39.
ANDERSON, A. (1950) Possible long-term effects of exposure to sulfur
dioxide. Br. J. ind. Med., 7: 82-86.
ASH, R. & LYNCH, J. (1972) The evaluation of gas detector tube
systems: sulfur dioxide. Am. Ind. Hyg. Assoc. J., 32: 490-491.
AZAR, A., SNEE, R. D., & HABIBI, K. (1972) Relationship of community
levels of air lead and indices of lead absorption. In:
Proceedings of the International Symposium on Environmental
Aspects of Lead, Luxembourg, Commission of the European
Communities, pp. 581-594.
BALCHUM, O. J., DYBICKI, J., & MENECLY, G. R. (1959) Absorption and
distribution of 35SO2 inhaled through the nose and mouth by
dogs. Am. J. Physiol., 197: 1317-1321.
BALCHUM, O. J., DYBICKI, J., & MENECLY, G. R (1960a) The dynamics of
sulfur dioxide inhalation, absorption, distribution and retention.
Arch. ind. Health, 21: 564-569.
BALCHUM, O. J., DYBICKI, J., & MENECLY, G. R. (1960b) Pulmonary
resistance and compliance with concurrent radioactive sulfur
distribution in dogs breathing 35SO2. J. appl. Physiol.,
15: 62-66.
BALL, D. J. & HUME, R. (1977) The relative importance of vehicular and
domestic emissions of dark smoke in Greater London in the mid
1970s, the significance of smoke shade measurements, and an
explanation of the relationship of smoke shade to gravimetric
measurements of particulate. Atmos. Environ., 2: 1065-1073.
BARRIE, L. A. & GEORGII, H. W. (1976) An experimental investigation of
the absorption of sulfur dioxide by water drops containing heavy
metal ions. Atmos. Environ., 10: 743-749.
BATES, D. V. & HAZUCHA, M. (1973) The short-term effects of ozone on
the human lung. Proceedings of the Conference on Health Effects of
Air Pollution, NAS-NRC, Washington, DC, Oct. 3-5. In: Committee
Print, Committee on Public Works, United States Senate, US Govt.
Printing Office, pp. 507-540.
BATTIGELLI, M. C., COLE, H. M., FRASER, D. A., & MAH, R. A. (1969)
Long-term effect of sulfur dioxide and graphite dust on rats.
Arch. environ. Health, 18: 602-608.
BENSON, E. B., HENDERSON, J. J., & CALDWELL, D. E. (1972)
Indoor-outdoor air pollution relationships: a literature review,
Research Triangle Park, NC, US Environmental Protection Agency,
83 pp. (Publ. No. AP 112).
BIERSTEKER, K. (1966) [Polluted air causes, epidemiological
significance and prevention of atmospheric pollution.] Assen,
Netherlands, Van Gorcum & Co., pp. 21-23 (in Dutch).
BIERSTEKER, K., DE GRAAF, H., & NASS, C. A. G. (1965) Indoor air
pollution in Rotterdam homes. Int. J. Air Water Pollut.,
9: 343-350.
BOSANQUET, C. H. (1957) The rise of a hot waste gas plume. J. Inst.
Fuel, 30: 322-328.
BRAIN, J. D. & VALBERG, P. A. (1974) Models of lung retention based
ion ICRP Task Group Report. Arch. environ. Health, 28: 1-11.
BRIGGS, G. A. (1965) A plume rise model compared with observations.
J. Air Pollut. Control Assoc., 15: 433-438.
BRITISH STANDARDS INSTITUTION (1969a) Methods for the measurement of
air pollution. BS1747 Part 4. The lead dioxide method.
London, BSI, 16 pp.
BRITISH STANDARDS INSTITUTION (1969b) Methods for the measurement of
air pollution. BS1747 Part 1. Deposit gauges. London, BSI,
15 pp.
BROSSET, C. (1973) Air-borne acid. Ambio, 2: 2-9.
BUECHLEY, R. W., RIGGAN, W. B., HASSELBLAD, V., & VAN BRUGGEN, J. B.
(1973) SO2 levels and perturbations in mortality -- a study in
the New York/New Jersey metropolis. Arch. environ. Health,
27: 134-137.
BUFALINI, M. (1971) Oxidation of SO2 in polluted atmospheres -- a
review. Environ. Sci. Technol., 5: 685-703.
BURROWS, B., KELLOG, A. L., & BUSKEY, J. (1968) Relationships of
symptoms of chronic bronchitis and emphysema to weather and air
pollution. Arch. environ. Health, 16: 406-413.
BURTON, G. G., CORN, M., GEE, J. B. L., VASALLO, C., & THOMAS, A. P.
(1969) Response of healthy men to inhaled low concentration of
gas-aerosol mixtures. Arch. environ. Health, 18: 681-692.
BUSTUEVA, K. A. (1957) [On the toxicology of sulfuric acid aerosol.]
Gig. i Sanit., No. 2, pp. 17-22 (in Russian).
BUSTUEVA, K. A. (1961a) Experimental data on the effect of small
concentration of sulfur oxides, maximum permissible concentration
of air pollutants, Moscow, Medgiz, pp. 126-141.
BUSTUEVA, K. A. (1961b) Threshold reflex effect of SO2 and H2SO4
aerosols simultaneously present in the air. In: Ryazanov, V. A.,
ed. Limits of allowable concentrations of atmospheric pollutants,
Book 4, Washington DC, US Department of Commerce, Office of
Technical Services, pp. 92-101.
BUSTUEVA, K. A. (1964) [Resorptive action of sulfur oxides.] Gig. i
Sanit., No. 10, pp. 8-12 (in Russian).
BUSTUEVA, K. A. (1966) The toxicity of continuous exposure to sulfur
oxides. Biological action and hygienic significance of
atmospheric pollution, Moscow, Medgiz, pp. 142-172.
BUSTUEVA, K. A., POLEZAEV, E.G., & SEMENENKO, A.D. (1960)
[Electroencephalographic determination of threshold reflex effect
of atmospheric pollutants.] Gig. i Sanit., 25: 57-61
(in Russian).
BYSTROVA, T. A. (1957) [Some effects of sulfurous gas determined by
the method of tracer atoms.] Gig. i Sanit., 5: 30-37
(in Russian).
CALVERT, J. G. (1973) Interaction of air pollutants. In: Proceedings
of the Conference on the Health Effects of Air Pollution,
Washington, DC, US Government Printing Office, pp. 19-101
(Serial No. 93-15).
CAMNER, P. & PHILIPSON, K. (1972) Tracheobronchial clearance in
smoking discordant twins. Arch. environ. Health, 25: 60-63.
CAMNER, P., JARSTRAND, C., & PHILIPSON, K. (1973a) Tracheobronchial
clearance in patients with influenza. Am. Rev. respir. Dis.,
108: 131-135.
CAMNER, P., MOSSBERG, B., & PHILIPSON, K. (1973b) Tracheobronchial
clearance and chronic obstructive lung disease. Scand. J. respir.
Dis., 54: 272-281.
CARABINE, M.D. & MADDOCK, J. E. L. (1976) The growth of sulfuric acid
aerosol particles when contacted with water vapour. Atmos.
Environ., 10: 735-742.
CARNOW, B. W., LEPPER, M. H., SHEKELLE, R. B., & STAMLER, J. (1969)
Chicago air pollution study: SO2 levels and acute illness in
patients with chronic bronchopulmonary disease. Arch. environ.
Health,. 18: 768-776.
CARSON, G. A.. & PAULUS, H. J. (1974) A high volume cascade sieve
impactor. Am. Ind. Hyg. Assoc. J., 35: 262-268.
CHARLSON, R. J., VANDERPOL, A. H., COVERT, D. S., WAGGONER, A. P., &
AHLQVIST, N. C. (1973) Sulfuric acid-ammonium sulfate aerosol:
Optical detection in the St Louis region. Science, 184: 156-158.
CHUN, K. C. & QUON, J. E. (1973) Capacity of ferric oxide particles to
oxidize sulfur dioxide in air. Environ. Sci. Technol.,
7: 532-538.
CLEARY, G. J. (1967) Some arguments suggesting a causative
relationship between air pollutants and lung cancer. Clean Air,
September: 15-18.
COHEN, A. A., BROMBERG, S., BUECHLEY, R. W., HEIDERSCHEIT, L. T., &
SHY, C. M. (1972) Asthma and air pollution from a coal-fuelled
power plant. Am. J. public Health, 62: 1181-1188.
COHEN, A. A., NELSON, C. J., BROMBERG, S. M., PRAVDA, M., FERRAND, E.
F., & LEONE, G. (1974) Symptom reporting during recent publicized
and unpublicized air pollution episodes. Am. J. public Health,
64: 442-449.
COLLEGE OF GENERAL PRACTITIONERS (1961) Chronic bronchitis in Great
Britain: A national survey carried out by the respiratory diseases
study group of the College of General Practitioners. Br. med. J.,
2: 973-979.
COLLEY, J. R. T. & REID, D. D. (1970) Urban and social origins of
childhood bronchitis in England and Wales. Br. med. J.,
2: 213-217.
COLLEY, J. R. T., DOUGLAS, J. W. B., & REID, D. D. (1973) Respiratory
disease in young adults: influence of early childhood lower
respiratory tract illness, social class, air pollution and
smoking. Br. med. J., 3: 195-198.
COMMINS, B. T. (1963) Determination of particulate acid in town air.
Analyst, 88: 364-367.
COMMINS, B. T. (1967) Some studies of the particulate acid sulfate
from the products of combustion of fuels and measurement of the
acid in polluted atmospheres. In: Proceedings of the Symposium on
the Transformation of Sulfur Compounds, Mainz, Germany,
7-9 June, Paris, OECD, pp. 39-46.
COMMINS, B. T. & LAWTHER, P. J. (1958) Volatility of 3,4-benzpyrene in
relation to the collection of smoke samples. Br. J. Cancer,
12: 351-354.
COMMINS, B. T. & WALLER, R. E. (1967) Observations from a 10-year
study of pollution at a site in the city of London. Atmos.
Environ., 1: 49-68.
COMMINS, B. T. & WALLER, R. E. (unpublished) Long-term trends in
pollution in central London. Atmos. Environ.
COMMISSION OF THE EUROPEAN COMMUNITIES (1976) Air sulfur dioxide
concentrations in the European community, Luxembourg, CEC,
pp. 34-51 (Report EUR 5417e).
COMMITTEE ON AIR POLLUTION (1954) Report, London, Her Majesty's
Stationery Office, pp. 7-25 (Report Cmd 9322).
COMMITTEE ON THE CHALLENGES OF MODERN SOCIETY (1971) Air pollution.
Air quality criteria for sulfur oxides. NATO/CCMS, No. 7,
pp. 1-1 to 8-85.
CORN, M., KOTSKO, N., STANTON, D., BELL, W., & THOMAS, A. P. (1972)
Response of cats to inhaled mixtures of SO2 and SO2-NaCl aerosol
in air. Arch. environ. Health, 24: 248-256.
COX, R. A. & PENKETT, S. A. (1970) The photo-oxidation of sulfur
dioxide in sunlight. Atmos. Environ., 4: 425-433.
COX, R. A. & PENKETT, S. A. (1971) Oxidation of atmospheric SO2 by
products of the ozone-olefin reaction. Nature (Lond.),
230: 321-322.
CUMMINGS, W. G. & REDFEARN, M. W. (1957)Instruments for measuring
small quantities of sulfur dioxide in the atmosphere. J. Inst.
Fuel, 30: 628-635.
DALHAMN, T. & STRANDBERG, L. (1961) Acute effect of sulfur dioxide on
the rate of ciliary beat in the trachea of rabbit, in vivo and
in vitro, with studies on the absorptional capacity of the nasal
cavity. Int. J. Air Water Pollut., 4: 154-167.
DALY, C. (1954) Air pollution and bronchitis. Br. med. J.,
2: 687-688.
DALY, C. (1959) Air pollution and causes of death. Br. J. prev. soc.
Med., 13: 14-27.
DE GRAAF, H. & BIERSTEKER, K. (1972) Comparison of smoke concentration
outside and inside two selected sites. Atmos. Environ., 6: 697.
DEPARTMENT OF ENVIRONMENTAL POLLUTION CONTROL, Tokyo (1976) Report of
air pollution measurements. Tokyo, Continuous Measurement
Stations, Tokyo Metropolitan Government, 119 pp.
DEROUANE, A. (1972) Comparaison des concentrations en fumées à
l'extérieur et à l'interiéur des lieux d'habitation. Atmos.
Environ., 6: 209-220.
DEROUANE, A., GRANDJEAN, L., LEGRAND, M., & VERDUYN, G. (1972)
Atmospheric pollution by oxides of sulfur and smoke in Belgium,
1969-70. Inst. R. Met. Belg., Publ. A. No. 78: 1-54.
DERRETT, C. J. & BROWN, C. (in press) A continuous running
direct-reading SO2 recorder. J. Phys. E. Sci. Instrum., 11:
DOHAN, F. C. (1961) Air pollutants and incidence of respiratory
disease. Arch. environ. Health, 3: 387-395.
DOHAN, F. C. & TAYLOR, E. W. (1960) Air pollution and respiratory
disease: a preliminary report. Am. J. med. Sci., 240: 337-339.
DORSCH, R. (1913) [The pollution of the air in the storage battery
room and its surroundings by sulfuric acid.] Unpublished
dissertation, Wurzberg, Germany (in German). Quoted in: Lewis, T.
R., ed. Toxicology of atmospheric sulfur dioxide decay products,
Research Triangle Park, NC, US Environmental Protection Agency,
p. 19 (Report No. AP111).
DOUGLAS, J. W. B. & WALLER, R. E. (1966) Air pollution and respiratory
infection in children. Br. J. prev. soc. Med., 20 (1): 1-8.
DZUBAY, T. G. & STEVENS, R. K. (1973) Ambient air analysis with
dictomous sampler and X-ray fluorescence spectrometer. Environ.
Sci. Technol., 9: 663-668.
EAST, C. (1972) Sulfur dioxide emissions estimated from airborne
measurements. Atmos. Environ., 6: 399-408.
ELFIMOVA, E. V. & HACATURJAN, M. K. (1968) [Features specific to the
reflex action of sub-threshold concentrations of sulfurous
anhydride in combustion with phenol and carbon monoxide in the
atmosphere.] Gig. i Sanit., 33 (11): 3-6 (in Russian).
ELFIMOVA, E. V. & GUSEV, M. I. (1969) [Health effects of low sulfur
dioxide concentrations in air.] Gig. i Sanit., 34 (2): 3-7
(in Russian).
ELKINS, H. (1959) The chemistry of industrial toxicology. New York,
John Wiley, pp. 396-397
ELLIOTT, L. P. & ROWE, D. R. (1976) Air quality during public
gatherings. J. Air Pollut. Control Assoc'., 25: 635-636.
ELLISON, J. M. (1965) The nature of air pollution and the methods
available for measuring it. Bull. World Health Organ.,
32: 399-409.
EMERSON, P. A. (1973) Air pollution, atmospheric conditions and
chronic airways obstruction. J. occup. Med., 15: 635-638
FERIN, J. & LEACH, L. J. (1973) The effect of SO2 on lung clearance
of TiO2 particles in rats. Am. Ind. Hyg. Assoc. J.,
34: 260-263.
FERRIS, B. G., Jr & ANDERSON, D. O. (1962) The prevalence of chronic
respiratory disease in a New Hampshire town. Am. Rev. respir.
Dis., 86: 165-185.
FERRIS, B. G., Jr & ANDERSON, D. O. (1964) Epidemiological studies
related to air pollution: A comparison of Berlin, NH and
Chilliwack, British Columbia. Proc. R. Soc. Med.,
57 (Suppl.): 979-983
FERRIS, B. G., Jr, BURGESS, W. A., & WORCESTER, J. (1967) Prevalence
of chronic respiratory disease in a pulp mill and a paper mill in
the United States. Br. J. ind. Med., 24: 26-37.
FERRIS, B. G., Jr, HIGGINS, I. T. T., HIGGINS, M. W., & PETERS, J. M.
(1973) Chronic nonspecific respiratory disease in Berlin, New
Hampshire 1961-1967. A follow-up study. Am. Rev. respir. Dis.,
107: 110-122.
FERRIS, B. G., Jr, CHEN, H., PULEO, S., & MURPHY, L. H., Jr (1976)
Chronic nonspecific respiratory disease in Berlin, New Hampshire,
1967 to 1973. A further follow-up study. Amer. Rev. resp. Dis.,
113: 475-485.
FIRKET, H. (1931) Sur les causes des accidents survenue dans la vallée
de la Meuse, lors des brouillards de Décembre 1930. Bull. R.
Acad. Med. Belg., 11: 683-739.
FORTAK, H. G. (1970) Numerical simulation of the temporal and spatial
distributions of urban air pollution concentrations. In:
Proceedings of a Symposium on Multiple Source Urban Diffusion
Models. Research Triangle Park, NC, US Environmental Protection
Agency.
FRANK, N. R. & SPEIZER, F. E. (1965) SO2 effects on the respiratory
system in dogs. Arch. environ. Health, 11: 624-634.
FRANK, N. R., AMDUR, M. O., WORCESTER, J., & WHITTENBERGER, J. L.
(1962) Effects of acute controlled exposure to SO2 on respiratory
mechanics in healthy male adults J. appl. Physiol., 17: 252-258.
FRANK, N. R., AMDUR, M, O., & WHITTENBERGER, J. L. (1964) A comparison
of the acute effects of SO2 administered alone or in combination
with NaCl particles on the respiratory mechanics of healthy
adults. Int. J. Air Water Pollut., 8: 125-133.
FRANK, N. R., YODER, R. E., YOKOYAMA, E., & SPEIZER, F. E. (1967) The
diffusion of 35SO2 from tissue fluids into the lungs following
exposure of dogs to 35SO2. Health Phys., 13: 31-38.
FRANK, N. R., YODER, R. E., BRAIN, J. D., & YOKOYAMA, E. (1969) SO2
(35S labelled) absorption by the nose and mouth under conditions
of varying concentration and flow. Arch. environ. Health,
18: 315-322.
FRASER, D. A., BATTIGELLI, M. C., & COLE, H. M. (1968) Ciliary
activity and pulmonary retention of inhaled dust in rats exposed
to sulfur dioxide. J. Air Pollut. Control Assoc., 18: 821-823.
FREIBERG, J. (1975) The mechanism of iron catalyzed oxidation of SO2
in oxygenated solutions. Atmos. Environ., 9: 661-672.
FUCHS, N. A. (1964) The mechanics of aerosols, Oxford, Pergamon
Press, p. 28.
FUGAS, M. (1976) Assessment of total exposure to an air pollutant. In:
International Conference on Environmental Sensing and Assessment,
Las Vegas, September 14-19, 1975, New York, IEEE, Vol. 2,
paper 38-5. pp. 1-3.
GARDNER, M. J., CRAWFORD, M. D., & MORRIS, J. N. (1969) Patterns of
mortality in middle and early old age in the county boroughs of
England and Wales. Br. J. prev. soc. Med., 23: 133-140.
GARLAND, J. A., CLOUGH, W. S., & FOWLER, D. (1973) Deposition of
sulfur dioxide on grass. Nature (Lond.). 242: 256-257.
GARLAND, J. A., ATKINS, D. H. F., READINGS, C. J., & CAUGHEY, S. J.
(1974) Deposition of gaseous sulfur dioxide to the ground. Atmos.
Environ., 8: 75-79.
GOLDRING, I. P., GREENBURG, L., PARK, S.S., & RATNER, I. M. (1970)
Pulmonary effects of sulfur dioxide exposure in the Syrian
hamster. Arch. environ. Health, 21: 32-37.
GOLDSMITH, J. R. (1969) Los Angeles smog. Sci. J., 5: 44-49.
GORE, A. T. & SHADDICK, C. W. (1958) Atmospheric pollution and
mortality in the County of London. Br. J. prev. soc. Med.,
12: 104-113.
GREY, D.C. & JENSEN, M. L. (1972) Bacteriogenic sulfur in air
pollution. Science, 177: 1099-1100.
GROSSER, P. J., STARK, C., JECH, J., & MEHLHORN, H. (1971) [Vital
capacity and respiration tests of school children of the 4th and
5th grade in areas with different air quality situations.]
Zeitschr. Erkr. Atmungsorgane, 134: 255-265 (in German).
GRUBER, C. W. & ALPAUGH, E. L. (1954) The automatic filter paper
sampler in an air pollution measurement programme. J. Air Pollut.
Control Assoc., 4: 143-147.
HATCH, T. & GROSS, P. (1964) Pulmonary deposition and retention of
inhaled aerosols, New York, Academic Press.
HEMEON, W. C. L., HAINES, G. F., Jr, & IDE, H. M. (1953) Determination
of haze and smoke concentrations by filter paper samplers. J. Air
Pollut. Control Assoc., 3: 22-28.
HENDERSON, J. J., BENSON, F. B., & CALDWELL, D. E. (1973)
Indoor-outdoor air pollution relationships. Vol. II. An annotated
bibliography. Washington, DC, US EPA (Publ. No. AP 112b).
HILL, A. C. (1971) Vegetation: a sink for atmospheric pollutants.
J. Air Pollut. Control Assoc., 21: 341-346.
HOBBS, P. V., HARRISON, H., & ROBINSON, E. (1974) Atmospheric effects
of pollutants. Science, 183: 909-915.
HOEGG, U. R. (1972) Cigarette smoke in closed places. Environ. Health
Perspect., October, No. 2: 117-128.
HOLBROW, G. L. (1958) Crystalline bloom, Teddington, UK, Paint
Research Station, p. 4 (Tech. Paper No. 208).
HOLLAND, W. W. & REID, D. D. (1965) The urban factor on chronic
bronchitis. Lancet, 1: 445-448.
HOLLAND, W. W. & STONE, R. W. (1965) Respiratory disorders in United
States East Coast telephone men. Am. J. Epidemiol., 82: 92-101.
HOLLAND, W. W., REID, D. D., SELTSER, R., & STONE, R. W. (1965)
Respiratory disease in England and the United States: Studies of
comparative prevalence. Arch. environ. Health, 10: 338-343.
HOLLAND, W. W., HALIL, T., BENNETT, A. E., & ELLIOTT, A. (1969)
Factors influencing the onset of chronic respiratory disease.
Br. med. J., 2: 205-208.
HOLLAND, W. W., BENNETT, A. E., CAMERON, I. R., FLOREY, C DU V.,
LEEDER, S. R., SCHILLING, R. S. F., SWAN, A. V, & WALLER, R. E.
(in press) Health effects of particulate pollution. Reappraising
the evidence. Am. J. Epidemiol.
HOLLOWELL, C. D., GEE, G. Y., & McLAUGHLIN, R. D. (1973) Current
instrumentation for continuous monitoring for SO2. Anal. Chem.,
45: 63A-72A.
HORVATH, H. & CHARLSON, R. J. (1969) The direct optical measurement of
atmospheric air pollution. Am. Ind. Hyg. Assoc. J., 30: 500-509.
HUHTI, E., RYHANEN, P., VUOPALA, U., & TAKKANEN, J. (1970) Chronic
respiratory disease among pulp mill workers in an arctic area in
Northern Finland. Acta Med. Scand., 187: 433-444.
HUSAR, J. D., HUSAR, R. B., & STUBITS, P. K. (1975) Determination of
submicrogram amounts of atmospheric particulate sulfur. Anal.
Chem., 47: 2062-2065.
HUSAR, R. B. (1974) Atmospheric particulate mass monitoring with a
radiation detector. Atmos. Environ., 8: 183-188.
INTERNATIONAL ATOMIC ENERGY AGENCY (1978) Particle size analysis in
estimating the significance of airborne contamination, Vienna,
International Atomic Energy Agency, pp. 71-170 (Technical Report
Series, No. 179).
ILO/WHO COMMITTEE ON OCCUPATIONAL HEALTH (1970) Permissible levels of
toxic substances in the working environment. Geneva, International
Labour Office, p. 337 (Occupational Safety and Health Series).
IPSEN, J., DEANE, M., & INGENITO, F. E. (1969) Relationships of acute
respiratory disease to atmospheric pollution and meteorological
conditions. Arch. environ. Health, 18: 462-472.
JACOBSEN, M. (1972) Sampling and evaluating respirable coal mine dust.
Ann. NY Acad. Sci., 200: 661-665.
JARSTRAND, C., CAMNER, P., & PHILIPSON, K. (1974) Mycoplasma
pneumoniae and tracheobronchial clearance. Am. Rev. respir. Dis.,
110: 415-419.
JOHNSTONE, H. F. & COUGHANOWR, D. R. (1958) Absorption of SO2 from
air. Oxidation in drops containing dissolved catalyst. Ind. eng.
Chem., 50: 1169-1172.
JUNGE, C. E. & RYAN, T. G. (1958) Study of the SO2 oxidation in
solution and its role in atmospheric chemistry. Q. J. R. Med.
Soc., 84: 46-55.
KATZ, M. (1969) Measurement of air pollutants, guide to the selection
of methods, Geneva, WHO, 123 pp.
KEHOE, R. A., MACHLE, W. H., KITZMILLER, K., & LEBLANC, T. J. (1932)
On the effects of prolonged exposure to sulfur dioxide. J. ind.
Hyg., 14: 159-173.
KELLOGG, W. W., CADLE, R. D., ALLEN, E. R., LAZRUS, A. Z., & MARTELL,
E. A. (1972) The sulfur cycle. Science, 175: 587-596.
KRUSHNER, M. & LASKIN, S. (1971) Experimental models in environmental
carcinogenesis. Am. J. Pathol., 64: 183-196.
LAMBERT P. M. & REID, D. D. (1970) Smoking, air pollution and
bronchitis in Britain. Lancet, 25 April 1970: 853-857.
LARSEN, R. I. (1971) A mathematical model for relating air quality
measurements to air quality standards, Research Triangle Park,
NC, US Environmental Protection Agency, 61 pp. (Rpt No. AP-89).
LAUER, G. & BENSON, F. B. (1975) The CHAMP air quality monitoring
programme. In: Proceedings of the International Symposium on
Recent Advances in the Assessment of the Health Effects of
Environmental Pollution, Paris, 24-28 June 1974, Luxembourg,
Commission of the European Communities, Vol. 1, pp. 423-430.
LAUTERBACH, K. E., MERCER, T. T., HAYES, A.D., & MARROW, P. E. (1954)
Efficiency studies of the electrostatic precipitator. Arch. ind.
Hyg., 9: 69-75.
LAVE, L. B. & SESKIN, E. P. (1970) Air pollution and human health.
Science, 169: 723-733.
LAVE, L. B. & SESKIN, E. P. (1972) Air pollution, climate, and home
heating: their effects on US mortality rates. Am. J. public
Health, 62: 909-916.
LAWTHER, P. J. (1963) Compliance with the clean air act: medical
aspects. J. Inst. Fuel, 36: 341-344.
LAWTHER, P. J. (1955) Effects of inhalation of sulfur dioxide on
respiration and pulse-rate in normal subjects. Lancet,
2: 745-748.
LAWTHER, P. J. & WALLER, R. E. (1976) Coal fires, industrial emissions
and motor vehicles as sources of environmental carcinogens. In:
Proceedings of Symposium on Environmental Pollution and
Carcinogenic risks, Lyons, 3-5 November 1975, Paris, INSERM,
Vol. 52, pp. 27-40.
LAWTHER, P. J., WALLER, R. E., & HENDERSON, M. (1970) Air pollution
and exacerbations of bronchitis. Thorax, 25: 525-539.
LAWTHER, P. J., MACFARLANE, A. J., WALLER, R. E., & BROOKS, A. G. F.
(1975) Pulmonary function and sulfur dioxide: some preliminary
findings. Environ. Res., 10: 355-367.
LEBOWITZ, M.D., TOYAMA, T., & MCCARROLL, J. (1973) The relationship
between air pollution and weather as stimuli and daily mortality
as responses in Tokyo, Japan, with comparisons with other cities.
Environ. Res., 6: 327-333.
LEE, R. E., Jr & GORANSON, S. (1972) Cascade impactor network,
Research Triangle Park, NC, US Environmental Protection Agency,
132 pp. (Rep. No. AP108).
LEE, R. E., CALDWELL, J. S., & MORGAN, G. B. (1972) The evaluation of
methods for measuring suspended particulates in air. Atmos.
Environ., 6: 593-622.
LEWIS, T. R., MOORMAN, W. J., LUDMAN, W. F., & CAMPBELL, K. I. (1973)
Toxicity of long-term exposure to oxides of sulfur. Arch.
environ. Health, 26: 16-21.
LIKENS, G. E. & BORMANN, F. H. (1974) Acid rain: a serious regional
environmental problem. Science, 184: 1176-1179.
LINDVALL, T. & RADFORD, E. P. (1973) Measurement of annoyance due to
exposure to environmental factors. Environ. Res., 6: 1-36.
LIPPMANN, M., ALBERT, R. E., & PETERSON, H. T., Jr (1971) The regional
deposition of inhaled aerosols in man. In: Walton, W. H., ed.
Inhaled Particles III, London, Unwin, Vol. 1, pp. 105-120.
LISS, P.S. (1971) Exchange of SO2 between the atmosphere and natural
waters. Nature (Lond.), 233: 327-329.
LIU, B. Y. H., BERGLUND, R. N., & AGARWAL, J. M. (1974) Experimental
studies of optical particle counters. Atmos. Environ.,
8: 717-732.
LOWE, C. R., PELMEAR, P. L., CAMPBELL, H, HITCHENS, R. A. N., KHOSLA,
T., & KING, T. C. (1968) Bronchitis in two integrated steel works.
I. Ventilatory capacity, age, and physique of non-bronchitic men.
Br. J. prev. soc. Med., 22: 1-11.
LOWE, C. R., CAMPBELL, H., & KHOSLA, T. (1970) Bronchitis in two
integrated steel works. III. Respiratory symptoms and ventilatory
capacity related to atmospheric pollution. Br. J. Ind. Med.,
27: 121-120.
LUNN, J. E., KNOWELDEN, J., & HANDYSIDE, A. J. (1967) Patterns of
respiratory illness in Sheffield infant school-children. Br. J.
prev. soc. Med., 21: 7-16.
LUNN, J. E., KNOWELDEN, J., & ROE, J. W. (1970) Patterns of
respiratory illness in Sheffield junior school-children -- A
follow-up study. Br. J. prev. soc. Med., 24: 223-228.
MADDALONE, R. F., MCCLURE, G. L., & WEST, P. W. (1975) Determination
of sulfate by thermal reduction of perimidylammonium sulfate.
Anal. Chem., 47: 316-322.
MALCOLM, D. & PAUL, E. (1961) Erosion of the teeth due to sulfuric
acid in the battery industry. Br. J. ind. Med., 18: 63-69.
MAMACASVILI, M. L (1968) Determination of the reflex action of SO2
and CO by adaptometer method and coloured vision. In: Biological
action and hygienic significance of atmospheric pollutants,
Moscow, Medgiz, pp. 179-188.
MARTIN, A. E. (1961) Epidemiological studies of atmospheric pollution:
A review of British methodology. Mon. Bull. Minist. Health Public
Health Lab. Serv., 20: 42-49.
MARTIN, A. E. (1964) Mortality and morbidity statistics and air
pollution. Proc. R. Soc. Med., 57: 969-975.
MARTIN, A. E. & BRADLEY, W. H. (1960) Mortality, fog and atmospheric
pollution. An investigation during the winter of 1958-59. Mon.
Bull. Minist. Health Public Health Lab. Serv., 19: 56-72.
MASTERS, B. R. (1974) The city of London and clean air. Clean Air,
4: 22-26.
MATSUMURA, Y. (1970a) The effects of ozone, nitrogen dioxide and
sulfur dioxide on the experimentally induced allergic respiratory
disorder in guinea pigs. I. The effect on sensitization with
albumin through the airway. Am. Rev. respir. Dis., 102: 430-437.
MATSUMURA, Y. (1970b) The effects of ozone, nitrogen dioxide and
sulfur dioxide on the experimentally induced allergic respiratory
disorder in guinea pigs. III. The effect on the occurrence of
dyspneic attacks. Am. Rev. respir. Dis., 102: 444-447.
MAWDESLEY-THOMAS, L. E., HEALEY, P., & BARRY, D. H. (1971)
Experimental bronchitis in animals due to sulfur dioxide and
cigarette smoke. In: WALTON, W. H., ed. Inhaled Particles III,
London, Unwin, Vol. 1, pp. 504-525.
MAZIARKA, S. & MROZ, E. (1968) [The influence of air pollution on the
protective apparatus of the eye in school children.] Rocz. PZH,
19: (1) 31-36 (in Polish).
MCJILTON, C., FRANK, R., & CHARLSON, R. (1973) Role of relative
humidity in the synergistic effect of a sulfur dioxide-aerosol
mixture on the lung. Science, 182: 503-504.
MCKAY, H. A. C. (1971) The atmospheric oxidation of SO2 in water
droplets in presence of NH3. Atmos. Environ., 5: 7-14.
MERCER, T. T. (1967) On the role of particle size in the dissolution
of lung burdens. Health Phys., 13: 1211-1222.
MINISTRY OF HEALTH, UNITED KINGDOM (1954) Mortality and morbidity
during the London fog of December 1952, London, HMSO (Report on
Public Health and Medical Subjects No. 95).
MINISTRY OF TECHNOLOGY, UK (1966) National survey of smoke and sulfur
dioxide: Instruction manual, Stevenage, Warren Spring
Laboratory, 142 pp.
MISIAKIEWICZ, Z. (1970) The effects on rat organism of prolonged
exposure to low concentrations of sulfur dioxide in the air.
J. Natl Inst. Hyg. (Warsaw), 21: 469-487.
MONTGOMERY, T. L. & COLEMAN, J. H. (1975) Empirical relationships
between time-averaged SO2 concentrations. Environ. Sci.
Technol., 9: 953-957.
MORROW, P. E. (1973) Alveolar clearance of aerosols. Arch. intern.
Med., 131: 101-108.
MUNN, R. E. (1976) Air pollution meteorology. Ch. 7 in Manual on
urban air quality measurement. Copenhagen, WHO (WHO Regional
publ. European Series, No. 1).
NASH, T. (1964) A "personal" measuring instrument for atmospheric
sulfur dioxide. Int. J. Air Water Pollut., 8: 121-124.
ORGANISATION FOR ECONOMIC CO-OPERATION AND DEVELOPMENT (1965) Methods
of measurement of air pollution, Paris, OECD, 94 pp.
PAN AMERICAN HEALTH ORGANIZATION (1976) Pan American air pollution
monitoring network (Redpanaire), Lima, Pan American Center for
Sanitary Engineering and Environmental Sciences, 50 pp.
(Pub. No. 30).
PASQUILL, F. (1971) Atmospheric dispersion of pollution. Q. J. R.
Med. Soc., 97: 369-395.
PATE, J. B., AMMONS, B. E., SWANSON, G. A., & LODGE, J.P. (1965)
Nitrite interference in spectrophotometric determination of
atmospheric sulfur dioxide. Anal. Chem. 37: 942-945.
PATTLE, R. E., BURGESS, F., & CULLUMBINE, H. (1956) The effects of a
cold environment and of ammonia on the toxicity of sulfuric acid
mist to guinea pigs. J. Pathol. Bacteriol., 72: 219-232.
PEMBERTON, J. & GOLDBERG, C. (1954) Air pollution and bronchitis.
Br. med. J., 2: 567-570.
POLLACK, R. I. (1975) Studies of pollutant concentration frequency
distributions, Washington DC, US Environmental Protection
Agency, 93 pp. (EPA-650/4-75-004).
PRINZ, B. (1970) Use of statistical methods in random sampling for the
preparation of plans for measurement of emissions. Staub,
30: 204-210.
RALL, D. P. (1974) Review of the health effects of sulfur oxides.
Environ. Health Perspect., 8: 97-121.
REICHEL, G., ULMER, W. T., GARY, K., LEUSCHNER, A., & ROSKE, G. (1970)
[Influence of the locally different grade of pollution in the
township of Duisburg on the incidence of non-specific respiratory
diseases. V. Communication.] Int. Arch. Arbeitsmed., 27: 110-129
(in German).
REID, D. D., ANDERSON, D. O., FERRIS, B. G., & FLETCHER, C. M. (1970)
An Anglo-American comparison of the prevalence of bronchitis.
Br. med. J., 2: 1487-1491.
ROBINSON, E. & ROBBINS, R. A. (1968) Sources, abundance and fate of
gaseous atmospheric pollutants. Menlo Park, CA, Stanford
Research Institute (Final Report, SRI project SCC-8501).
ROBINSON, E. & ROBBINS, R. A. (1972) Emissions, concentrations and
fate of gaseous atmospheric pollutants. In: Strauss, W., ed.
Air pollution control, II, New York, Wiley-Interscience,
pp. 1-93.
ROYAL COLLEGE OF PHYSICIANS, London (1970) Air pollution and health,
London, Pitman, pp. 48-57.
ROYAL MINISTRY OF FOREIGN AFFAIRS, SWEDEN (1971) Air pollution across
national boundaries. The impact on the environment of sulfur in
air and precipitation. Stockholm. Kungl. Bokytrckerict P.A.
Norsted & Söner 710396, 96 pp.
RJAZANOV, V. (1962) Sensory physiology as basis for air quality
standards. Arch. environ. Health, 5: 480-494.
RYLANDER, R. & BERGSTROM, R. (1973) Particles and SO2 -- synergistic
effects for pulmonary damage. In: Proceedings of the Third
International Clean Air Congress, Düsseldorf, 1973, Düsseldorf,
VDI Verlag, pp. A23-A25.
RYLANDER, R., OHRSTROM, M., HELLSTROM, P. A., & BERGSTROM, R. (1971)
SO2 and particles -- synergistic effects on guinea pig lungs. In:
Walton, W. H., ed. Inhaled Particles III, London, Unwin, Vol. 1,
pp. 535-540.
SALAMBERIDZE, O. P. (1969) [The joint action of small concentrations
of sulfur dioxide and nitrogen dioxide gases under conditions of a
chronic test.] Gig. i Sanit., 34(4): 10-14 (in Russian).
SAUCIER, J. Y. & SANSONE, E. B. (1972) The relationship between
transmittance and reflectance measurements of soiling index.
Atmos. Environ., 6: 37-43.
SAWICKI, F. (1972) Chronic non-specific respiratory diseases in
Cracow. Epidemiol. Rev., 26: 229-250.
SCARINGELLI, F. B., SALTZMAN, B. E., & FREG, S. A. (1967)
Spectrophotometric determination of atmospheric sulfur dioxide.
Anal. Chem., 39: 1709-1719.
SCHIMMEL, H. & GREENBERG, L. (1972) A study of the relation of
pollution to mortality, New York City, 1963-1968, J. Air Pollut.
Control Assoc., 22: 607-616.
SCHIMMEL, H. & MURAWSKI, T. J. (1975) SO2 -- Harmful pollutant or air
quality indicator? J. Air Pollut. Control Assoc., 25: 739-740.
SCHMIDT, P., PETR, B., & PICKO, V. (1966) [The indices of white blood
sequence, the tonsils and neck lymphatic nodules state as the
diagnostic criteria of subtle changes within the child's
organism.] Cesk. Hyg., 11: 473-478 (in Czech).
SCHRENK, H. H., HEIMANN, H., CLAYTON, G. O., GAFAER, W. M., & WEXLER,
H. (1949) Air pollution, Donora, Pennsylvania -- Epidemiology of
the unusual smog episode of October 1948. Public Health Bull.
(Fed. Sec. Agency, Washington DC). 306: 1-173.
SCHUSKY, J. (1966) Public awareness and concern with air pollution in
the St. Louis, metropolitan area. J. Air Pollut. Control Assoc.,
16: 72-76.
SHERWOOD, R. J. (1969) Miniature air samplers for sulfur dioxide.
Am. Ind. Hyg. Assoc. J., 30: 614-619.
SHERWOOD, R. J. & GREENHALGH, D. M. S. (1960) A personal air sampler.
Ann. occup. Hyg., 2: 127-132.
SIM, V. M. & PATTLE, R. E. (1957) Effect of possible smog irritants on
human subjects. J. Am. Med. Assoc., 165: 1918-1913.
SJORSTRAND, T. (1947) Changes in respiratory organs of workmen at an
ore smelting works. Acta Med. Scand., 196 (Suppl.): 687-699.
SKALPE, I. O. (1964) Long term effects of sulfur dioxide exposure in
pulp mills. Br. J. ind. Med., 21: 69-73.
SKVORCOVA, N. N., OSINCEVA, V. P., PUSKINA, N. N., & VYSOCINA, I. V.
(1973) [Role of some air pollutants in the genesis of lung tumour
provoked by chemical carcinogens. In: Prevention of environmental
pollution due to carcinogenic substances.], Moscow, Medicina,
pp. 61-64 (in Russian).
SNELL, R. E. & LUCHSINGER, P. C. (1969) Effects of sulfur dioxide on
expiratory flow rates and total respiratory resistance in normal
human subjects. Arch. environ. Health, 18: 693-698.
SOFOLUWE, G. O. (1968) Smoke pollution in dwellings of infants with
bronchopneumomia. Arch. environ. Health, 16: 670-672.
SPEDDING, D. J. (1972) Sulfur dioxide absorption by sea water. Atmos.
Environ., 6: 583-586.
SPEIZER, F. E. & FRANK, N. R. (1966a) The uptake and release of SO2
by the human nose. Arch. environ. Health, 12: 725-728.
SPEIZER, F. E. & FRANK, N. R. (1966b) A comparison of changes in
pulmonary flow resistance in healthy volunteers acutely exposed to
SO2 by mouth and nose. Br. J. ind. Med., 23: 75-79.
STALKER, W. W. & ROBINSON, C. B. (1967) A method for using air
pollution measurements and public opinion to establish ambient air
quality standards. J. Air Pollut. Control Assoc., 17: 142-144.
STOCKS, P. (1959) Cancer and bronchitis mortality in relation to
atmospheric deposit and smoke. Br. med. J., 1: 74-79.
STOCKS, P. (1966) Recent epidemiological studies of lung cancer
mortality, cigarette smoking and air pollution, with discussion of
a new hypothesis of causation. Br. J. Cancer, 20: 495-623.
STOCKS, P. (1967) Lung cancer and bronchitis in relation to cigarette
smoking and fuel consumption in twenty countries. Br. J. prev.
soc. Med., 21: 181-185.
STRANDBERG, L. G. (1964) SO2 absorption in the respiratory tract.
Arch. environ. Health, 9: 160-166.
STUART, B. O. (1973) Deposition of inhaled aerosols. Arch. intern.
Med., 131: 60-73.
SYMON, K., PETR, B., & KAPALIN, V. (1966) [The influence of pollution
caused by gaseous pollutants on the health of children.] Prakt.
Lek., 46: 19-22 (in Czech).
TANI, S. (1975) [Epidemiological study on chronic bronchitis.] Jpn.
J. public Health, 22: 431-438 (in Japanese).
TASK GROUP ON AIR POLLUTION AND CANCER (unpublished) Air pollution and
cancer. Risk assessment methodology and epidemiological evidence.
Environ. Health Perspect. (March, 1978.)
TASK GROUP ON LUNG DYNAMICS (1966) Deposition and retention models for
internal dosimetry of the human respiratory tract. Health Phys.,
12: 173-207.
TASK GROUP ON METAL ACCUMULATION (1973) Accumulation of toxic metals
with special reference to their absorption, excretion and
biological half-times. Environ. Physiol. Biochem., 3: 65-107.
TEN BRUGGEN CATE, H. J. (1968) Dental erosion in industry. Br. J.
ind. Med., 25: 249-266.
THOMAS, R. L., DHARMARAJAN, V., LUNDQUIST, G. L., & WEST, P. W. (1976)
Measurement of sulfuric acid aerosol, sulfur trioxide and the
total sulfate content of the ambient air. Anal. Chem.,
48: 639-642.
TOIGO, A., IMARISIO, J. J., MURMALL, H., & LEPPER, M. N. (1963)
Clearance of large carbon particles from the human
tracheobronchial tree. Am. Rev. respir. Dis., 87: 487-492.
TOMENIUS, L. (1973) A study on the role of deposition for
tracheobronchial clearance. Physiol. Biochem., 3: 111-116.
TOYAMA, T. & NAKAMURA, K. (1964) Synergistic response of hydrogen
peroxide aerosols and sulfur dioxide to pulmonary airway
resistance. Ind. Health, 2: 34-45.
TOYAMA T., KAHYO, H., NAKAMURA, K., KAGAWA, J., YAKURA, S., ADACHI,
S., YAMAMOTO, N., IRIYAMA, F., KUMAGAYA, F., OSAWA, S., &
NAKAMURA, T. (1966) [Study on the prevalence of respiratory
symptoms in a rural area (Kashima, Ibaragi Pref.) in Japan.]
J. Jpn Soc. Air Pollut., 1: 24-35 (in Japanese).
TREON, J. F., DUTRA, F. R., CAPPEL, J., SIGMON, H., & YOUNKER, W.
(1950) Toxicity of sulfuric acid mist. Arch. ind. Hyg. occup.
Med., 2: 716-734.
TSUNETOSHI, Y., SHIMIZU, T., TAKAHASHI, H., ICHINOSAWA, A., UEDA, M.,
NAKAYAMA, N., YAMAGATA, Y., & OHSHINO, A. (1971) Epidemiological
study of chronic bronchitis with special reference to effect of
air pollution. Int. Arch. Arbeitsmed., 29: 1-27.
TURNER, D. B. (1968) Workbook of atmospheric dispersion estimates,
Washington, DC, US Department of Health, Education and Welfare,
84 pp.
ULMER, W. T., REICHEL, G., CZEIKE, A., & LEUSCHNER, A. (1970)
[Regional incidence of non-specific respiratory diseases. IV.
Communication.] Int. Arch. Arbeitsmed., 27: 73-109 (in German).
UNITED STATES DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1962)
Air pollution measurements of the National Air Sampling Network
1957-1961, Cincinnati, OH, Division of Air Pollution, pp. 3-4
(Publ. No. 978).
UNITED STATES DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1969)
Air quality criteria for particulate matter, Washington, DC,
DHEW, National Air Pollution Control Administration, pp. 1-211
(AP. 49).
US ENVIRONMENTAL PROTECTION AGENCY (1974a) Air quality data -- 1972
annual statistics, Research Triangle Park, NC, USEPA, 145 pp.
(EPA450/2-74-001).
US ENVIRONMENTAL PROTECTION AGENCY (1974b) Health consequences of
sulfur oxides. A report from CHESS, 1970-71, Research Triangle
Park, NC, US Government Printing Office, 265 pp.
(EPA650/1-74-604).
VAN DER LENDE, R. (1969) Epidemiology of chronic non-specific lung
disease (chronic bronchitis), Thesis, University of Groningen,
2 vols. Assen, Van Gorcum.
VAN DER LENDE, R., VISSER, B. F., WEVER-HESS, J., TAMMELIFG, G. J., DE
VRIES, K., ORIE, N. G. M. (1973) Epidemiological investigations in
the Netherlands into the influence of smoking and atmospheric
pollution on respiratory symptoms and lung function disturbances.
Pneumologie, 149: 119-126.
VAN DER LENDE, R., HUYGEN, C., JANSEN-KOSTER, E. J., KNIFPSTRA, S.,
PESEL, R., VISSER, B. F., WOLFS, E. H. E., & ORIE, N. G. M. (1975)
A temporary decrease in the ventilatory function of an urban
population during an acute increase in air pollution. Bull.
Physiopathol. Respir., 11: 31-43.
VAN DOP, H. & KRUIZINGA, S. (1976) The decrease of sulfur dioxide
concentration near Rotterdam and their relation to some
meteorological parameters during thirteen consecutive winters
(1961-74). Atmos. Environ., 10: 1-4.
VEREIN DEUTSCHER INGENIEURE (1974) [Measurements of the wet
concentrations of particles in the outdoor air (LIB-filter method)
VDI2463 Sheet 4.] (in German).
VIGDORCIK, E. A. (1948) Retention of inhaled aerosols (resume),
Leningrad, Leningrad Research Institute of Labour Hygiene and
Occupational Health Publication, Vol. XI, Part 2, pp. 227-230.
WALLER, R. E. (1963) Acid droplets in town air. Int, J. Air Water
Pollut., 7: 773-778.
WALLER, R. E. (1967) Studies on the nature of urban air pollution. In:
Conference on Museum Climatology, London, International
Institute of Works of Art, pp. 65-69.
WALLER, R. E. (1971) Air pollution and community health. J. R. Coll.
Physicians, Lond., 5: 362-368
WALLER, R. E. & COMMINS, B. T. (1966) High pollution episodes in
London, 1952-1966. In: Proceedings of the International Clean Air
Conference, London, Natural Society of Clean Air, pp. 228-231.
WALLER, R. E. & COMMINS, B. T. (1967) Studies of the smoke and
polycyclic aromatic hydrocarbon content of the air in large urban
areas. Environ. Res., 1: 295-306.
WALLER, R. E., BROOKS, A. G. F., & CARTWRIGHT, J. (1963) An electron
microscope study of particles in town air. Int. J. Air Water
Pollut. Control., 7: 779-786.
WALLER, R. E., COMMINS, B. T., & LAWTHER, P. J. (1965) Air pollution
in a city street. Br. J. ind. Med., 22: 128-138.
WALLER, R. E., LAWTHER, P. J., & MARTIN, A. E. (1969) Clean air and
health in London. In: Proceedings of the International Clean Air
Conference, London, Natural Society of Clean Air, pp. 71-79.
WARNER, C. G., DAVIES, G. M., JONES, J. G., & LOWE, C. R. (1969)
Bronchitis in two integrated steel works, II. Sulfur dioxide and
particulate atmospheric pollution in and around the two works.
Ann. occup. Hyg., 12: 151-170.
WATANABE, H. (1966) [Health effects of air pollution in Osaka City.]
J. Osaka Life Hyg. Assoc., 10: 147-157 (in Japanese).
WEATHERLEY, M. L. (1966) Air pollution inside the home. Int. J. Air
Water Pollut. Control, 10: 404-409.
WEATHERLEY, M. L., GOORIAH, B. D., & CHARNOCK, J. (1976) Fuel
consumption, smoke and sulfur dioxide emissions and
concentrations, and grit and dust deposition in the U.K. up to
1973-74. Stevenage, UK, Warren Spring Laboratory
(WSL Report LR214AP).
WEST, P.M. & GAEKE, G. (1956) Fixation of sulfur dioxide as
sulfitomercurate III and subsequent calorimetric determination.
Anal. Chem., 28: 1816-1819.
WILLEKE, K. & WHITBY, K. T. (1975) Atmospheric aerosols: size
distribution interpretation. J. Air Pollut. Control Assoc.,
25: 529-534.
WILLIAMS, F. P. (1960) Pollution levels in cities. In: Proceedings of
the Harrogate Conference, London Natural Society of Clean Air,
pp. 83-88.
WILLIAMS, M. K. (1970) Sickness absence and ventilatory capacity of
workers exposed to sulfuric acid mist. Br. J. ind. Med.,
27: 61-66.
WILSON, W. E., Jr & LEVY, A. (1970) A study of sulfur dioxide in
photochemical smog. I. Effect of SO2 and water vapour
concentration in the 1-butene/NOx/SO2 system. J. Air Pollut.
Control Assoc., 20: 385-390.
WORLD HEALTH ORGANIZATION (1960) WHO Technical Report Series No. 192
(Epidemiology of cancer of the lung: Report of a study group).
Geneva, WHO, 13 pp.
WORLD HEALTH ORGANIZATION (1971) International standards for
drinking-water. Geneva, WHO, 70 pp.
WORLD HEALTH ORGANIZATION (1972) WHO Technical Report Series No. 506
(Air quality criteria and guides for urban air pollutants),
Geneva, WHO, 35 pp.
WORLD HEALTH ORGANIZATION (1974) WHO Technical Report Series No. 539
(Toxicological evaluation of certain food additives with a review
of general principles and of specifications), Geneva, WHO, 40 pp.
WORLD HEALTH ORGANIZATION (1976a) Selected methods of measuring air
pollutants, Geneva, WHO, 110 pp. (WHO Offset Publ. No. 24).
WORLD HEALTH ORGANIZATION (1976b) Air quality in selected urban
areas, 1973-1974, Geneva, WHO, 65 pp. (WHO Offset Publ.,
No. 30).
WORLD HEALTH ORGANIZATION (1977) Air monitoring programme design for
urban and industrial areas, Geneva, WHO, 46 pp. (WHO Offset
Publ., No. 33).
WORLD METEOROLOGICAL ORGANIZATION (1974) WMO operations manual for
sampling and analysis techniques for chemical constituents in air
and precipitation, Geneva, WMO, 56 pp. (No. 299).
YOCOM, J. E., CLINK, W. L., & COTE, W. A. (1971) Indoor/outdoor air
quality relationships. J. Air Pollut. Control Assoc.,
21: 251-259.
YOKOYAMA, E., YODER, R. E., & FRANK, N. R. (1971) Distribution of 35S
in the blood and its excretion in urine of dogs exposed to
35SO2. Arch. environ. Health, 22: 389-395.
YOSHIDA, K., OSHIMA, H., & IMAI, M. (1966) Air pollution and asthma in
Yokkaichi. Arch. environ. Health, 13: 763-768.
YOSHII, M., NONOYAMA, J., OSHIMA, H., YAMAGIWA, H., & TAKEDA, S.
(1969) Chronic pharyngitis in air-polluted districts of Yokkaichi
in Japan. Mie med. J., 19: 17-27.
ZARKOWER, A. (1972) Alterations in antibody response induced by
chronic inhalation of SO2 and carbon. Arch. environ. Health,
25: 45-50.
ZEEDUK, H. & VELDS, C. A. (1973) The transport of sulfur dioxide over
a long distance. Atmos. Environ., 7: 849-862.
In general, however, in the absence of specific sources of sulfur
dioxide or fine particulate matter indoors, concentrations are
generally less than those outdoors. Sulfur dioxide as a gas, can
diffuse readily onto walls and other surfaces. There is evidence that
it reacts with ammonia from the indoor air on painted surfaces,
particularly in the presence of moisture (Holbrow, 1958), but it is
most effectively absorbed on clothing, curtains, carpets, and other
soft furnishings, so that in domestic surroundings where these abound
concentrations of sulfur dioxide are only of the order of 20% of those
outdoors (Weatherley, 1966), while in offices and other buildings
containing less absorbing material, concentrations may be 40-50% of
those outdoors (Andersen, 1972; Derouane, 1972). The presence of
ammonia in occupied rooms is important in relation to measurements of
sulfur dioxide. Concentrations of this "natural" pollutant may be much
higher than outdoors, particularly where there are young babies or old
people with incontinence problems. The ammonia will only react
effectively with sulfur dioxide in the presence of moisture, but it
will, in any case, interfere with the determination of the sulfur
dioxide by acidimetric or conductometric methods.
Smoke, that is to say the finely divided black material from
incomplete combustion, can penetrate fairly readily into buildings,
and since the mobility of the particles is less than that of sulfur
dioxide molecules, they are less rapidly removed onto surfaces.
Concentrations of smoke indoors, assessed by soiling methods, have
been found to be in the range of 50-90% of those outdoors (Derouane,
1972; Yocom et al., 1971), again assuming there is no specific source
of this material inside. Cigarette smoke can make a very substantial
contribution to the finely divided particulate matter indoors (Elliott
& Rowe, 1976; Hoegg, 1972). It is liable to affect direct gravimetric
concentrations, but it has relatively little effect on concentrations
measured by blackness. The composition of cigarette smoke is quite
different from that of the general urban particulate matter, and it
has been stressed by De Graaf & Biersteker (1972) that there may be
many differences in the type and composition of suspended particulate
matter indoors and outdoors. This point is particularly important in
relation to samples obtained with the high volume sampler. The larger
particles (greater than 10 µm diameter) that are liable to be
collected by this method would penetrate into buildings less readily
than the fine particles, and, with reduced air movement inside, they
may fall out fairly rapidly under gravity. This may account for the
smaller proportion of total suspended particles (as compared with
smoke) found indoors in some studies (Yocom et al., 1971). However, on
the other hand, there is a risk that textile and other dusts dispersed
in the home may be sampled and assessed as part of the general
suspended particulate matter.
Sulfuric acid is unlikely to remain for any appreciable time in
occupied rooms, as it is readily neutralized by ammonia. However,
finely divided sulfate particles are likely to behave in the same way
as smoke, with a modest reduction indoors compared with outdoors.
Relative humidity is normally lower indoors than outdoors and this
will help to keep sulfate particles in suspension, but where the
humidity is especially high, as it may be in kitchens, particles
containing sulfates or other salts will grow rapidly and be deposited.
Studies on indoor concentrations of sulfur dioxide and particulate
matter are limited primarily by the need to consider the circumstances
of each location individually; nevertheless, there is a growing body
of literature on the subject that has been assembled and reviewed in
recent reports from the USA (Benson et al., 1972; Henderson et al,
1973).
5.3 Concentrations in Work Places
Concentrations of sulfur dioxide, much higher than those commonly
found in urban air, may be present in some industrial environments,
arising from processes in which the gas is handled or evolved, as well
as from combustion sources. Paper mills, sulfuric acid plants, steel
works, nonferrous metal foundries, and oil refineries are among the
places where such concentrations may be found. However, emissions are
usually highly localized and intermittent, presenting major problems
in assessing concentrations to which workers may be exposed. Results
are not normally published, but in a number of countries there is a
requirement to ensure that a specified limit is not exceeded. For
example, in the USSR, the maximum permissible concentration is
10 mg/m3 (3.8 ppm) (ILO/WHO Committee on Occupational Health, 1970)
whereas in the USA (American Conference of Government Industrial
Hygienists, 1977), this value, averaged over 8-h shifts, is 13 mg/m3
(5 ppm). This figure is of the order of 100 times the average values
in urban air and it is higher than the maximum values reported, even
in episodes of high pollution (Waller & Commins, 1966). Mean values
over 3 years of 1800-2100 µg/m3 (0.6-0.7 ppm) have been reported
close to blast furnaces in steelworks, with occasional 24-h mean
values up to 17 000 µg/m3 (5.95 ppm) (Lowe et al., 1970).
In steelworks, substantial quantities of suspended particulate
matter may be present as well as sulfur dioxide and other pollutants.
A 3-year mean concentration of about 1000 µg/m3 (respirable dust, as
measured with a Hexhlet sampler) has been reported in the study cited
above (Lowe et al., 1970). Even so, the situation does not necessarily
parallel that in urban atmospheres, for the particulate matter is
liable to include dust which differs in composition and size
distribution from that produced in the usual range of combustion
processes.
Dusts encountered in industry must, in general, be considered
separately from the suspended particulate matter in urban air. Some,
such as the coal-dust in mines, may be present at high concentrations,
and have substantial effects on health, but these are specific to that
dust, and are outside the scope of the present discussion. However,
some of the chemical compounds in dust from industrial activities are
covered by other documents of the WHO environmental health criteria
programme.
In some industries, such as wood-pulping and paper-making, sulfur
dioxide may be evolved in the process, producing high concentrations
locally without an accompanying particulate matter problem.
Concentrations in the range 6 to 100 mg/m3 (2 to 36 ppm) have been
reported at a plant in Norway (Skalpe, 1964) and similar
concentrations were found in a study in the USA (Ferris et al., 1967)
but these were gradually reduced over a 5-year period. High
concentrations, averaging about 71 mg/m3 (25 ppm), also existed at
one time in charging rooms for refrigerators when sulfur dioxide was
used as a refrigerant (Kehoe et al., 1932) and similar concentrations
have been reported in certain areas of oil refineries (Anderson 1950).
There is a specific problem of sulfuric acid mist in the forming
departments of works making lead-acid accumulators, where
concentrations up to 16.6 mg/m3 have been observed (Malcolm & Paul,
1961; Williams, 1970). This is very much greater than the
concentrations ever found in urban air (Commins & Waller, 1978) and
the physical form of the aerosol is different: mass median diameters
of the droplets have been reported to be over 10 µm (Williams, 1970)
whereas those found in urban air are of the order of 0.5 µm (Waller,
1963).
5.4 Assessment of Exposures
In the present context, the term exposure relates to the
concentrations of sulfur dioxide or suspended particulate matter in
the air breathed by individuals or populations, averaged over
specified periods. Broadly speaking, two types of exposure are
considered: short-term exposure, in which the relevant concentrations
are averaged over periods of the order of a day, and long-term
exposure, for which averages over periods of the order of a year are
commonly used. These periods are to a certain extent arbitrary,
imposed by the time-resolution of the pollution measurements and by
the nature of the health indices examined, and there is no clear
guidance on the most relevant averaging periods to use in relation
either to short-or to long-term exposure.
Exposures are usually estimated from measurements of concentration
at fixed sampling sites. The number of sampling sites required to give
an adequate representation of the exposure of people living in a given
area will depend on the topography, the distribution of sources, and
other factors. In urban areas, there is usually a close correlation
between values for neighbouring stations (Prinz, 1970). Care must be
taken, however, to ensure that these stations are not used to
represent environmental levels in areas much larger than their
coverage. Furthermore, the instruments for measurement may be sited on
the roof of a building for protection and convenience, and the levels
measured may not necessarily represent those in the ordinary breathing
zone.
The usual practice in epidemiological studies is to assume that
measurements made on outdoor air in the areas where people live or
work provide an index of exposure that allows comparison to be made
between different groups. It has been demonstrated, however, that
estimated weekly exposures calculated as averages of concentrations
measured indoors and at various outdoor sites and weighted according
to the length of time likely to be spent in each location, may differ
substantially from those indicated by measurements at just one site
close to the place of residence or work (Fugas, 1976). To validate
this approach, further measurements using personal samplers would be
required. These have been used in studies on specific components of
suspended particulate matter such as lead (Azar et al., 1972) and they
have wide application in assessing the exposures of individuals to
pollutants, including sulfur dioxide, in industry, but they are not
generally practicable for large-scale epidemiological studies on the
general population.
A further problem in assessing exposures is the great variation in
concentrations with time for, as far as sulfur dioxide is concerned, a
brief exposure to a very high concentration, with no appreciable
exposure during the rest of the day, would create a different
situation from a steady exposure to a low level throughout the day. At
present, there is no satisfactory way of measuring such situations, at
least for people moving about during the course of the day. Providing
studies are confined to groups of people living or working in
reasonably uniform circumstances, measurements made over extended
periods at a few fixed sites may give results that are valid for
comparative purposes. Difficulties are liable to arise however if
dissimilar groups are compared. In particular, people such as the very
old and the very young may be partially protected from sulfur dioxide
in the outside air if they spend long periods indoors, whereas an
active worker may be exposed not only to a higher mean concentration
in the course of the day, but also to a greater range. A further point
is that increased activity will also lead to a greater ventilation
rate, increasing the overall intake, and, in the case of sulfur
dioxide, the deep inspirations associated with exercise are liable to
increase the penetration of the gas and to enhance its effects
(Lawther et al., 1975). The situation is even more complex for
sulfuric acid aerosols. Because of the hygroscopic properties of the
droplets, they take up moisture rapidly on inspiration, becoming
larger and more dilute, and it has been suggested, on the basis of
experimental work, that those from urban air could be diluted to such
an extent that their irritant properties would be lost (Carabine &
Maddock, 1976).
When assessing long-term exposures, annual mean concentrations at
fixed sampling sites are commonly used as the basis. Short-term
variations are then of little consequence, but it may still be
necessary to consider the extent of seasonal swings in concentration,
or the frequency of occurrence of days of exceptionally high
pollution. Also, it is often necessary to examine trends in
concentrations for many years back, for the relevant exposure may have
been one that occurred earlier in life, rather than the current level.
Since there are very few long series of measurements of sulfur dioxide
or suspended particulate matter made by uniform methods, it is very
difficult to assess the true exposure of people to these pollutants.
6. ABSORPTION, DISTRIBUTION, AND ELIMINATION
Most of the sulfur compounds discussed in this report are absorbed
via the digestive system. Relatively little is absorbed from the
respiratory tract, even in areas with highly polluted ambient air.
Even so, it is reasonable to believe that the respiratory tract is the
most vulnerable organ for the local effects of sulfur oxides and
particulate matter in the ambient air. Thus, this section will deal
mainly with the absorption, deposition, and clearance of sulfur
dioxide and particulate matter in the respiratory tract.
6.1 Absorption and Deposition in the Respiratory Tract
6.1.1 Sulfur dioxide
Sulfur dioxide is highly soluble in aqueous media. Absorption
after inhalation has been studied in rabbits (Dalhamn & Strandberg,
1961; Strandberg, 1964), dogs (Balchum et al., 1959, 1960a, 1960b;
Frank et al., 1969) and man (Speizer & Frank, 1966a, 1966b).
In rabbits, about 40% of the inhaled sulfur dioxide is absorbed in
the nose and pharynx when concentrations of about 290 µg/m3 (0.1 ppm)
are inhaled. At higher concentrations (29-290 mg/m3; 10-100 ppm), the
fraction absorbed is much higher (about 95%),The reasons for these
different rates of absorption are not clear. In dogs, more than 99% of
the inhaled sulfur dioxide is absorbed by the nose at exposure levels
of 2.9-140 mg/m3 (1-50 ppm). These observations in dogs have been
confirmed in man by studies on human volunteers, with levels of
exposure to sulfur dioxide ranging from 2.9 to 420 mg/m3 (1 to
140 ppm) and exposure times of a few minutes at the higher levels and
30-40 minutes at the lower levels. Absorption can occur during
mouth-breathing, but is less efficient than during nose-breathing,
especially with increased ventilation.
6.1.2 Airborne particles
Particles smaller than about 10 µm are deposited at different
levels in the respiratory tract. The exact deposition pattern is
determined by the interaction of size, shape, and density (expressed
as aerodynamic diameter of unit density spheres) of the particles and
by airflow conditions. Some particles in the air, such as sulfuric
acid particles are hygroscopic. Such particles will take up water,
expand in the respiratory tract, and be deposited in a manner other
than would be expected from their diameters in the ambient air (Hatch
& Gross, 1964; Stuart, 1973; Task Group on Lung Dynamics, 1966;
Vigdorcik, 1948). Sophisticated theoretical models for the deposition
of particles in the lung such as the ICRP model have been constructed
(Task Group on Lung Dynamics, 1966). Deposition is caused by
impaction, sedimentation, and diffusion. Impaction is an important
mechanism for the deposition of the larger or heavier particles
(5-30 µm aerodynamic diameter) and where the air velocity is
relatively high. Impaction occurs therefore at sites where the air
stream is turbulent and is of most importance in the nose, mouth,
pharynx, and the upper part of the tracheobronchial tree.
Sedimentation or settling out of particles is also a function of
particle size and density, as well as of residence time in the
airways. It is important for the deposition of larger particles
(1-5 µm) providing they have not been deposited by impaction and is
active in the trachea, bronchi, and bronchioles. Diffusion is of
importance for particles smaller than a few tenths of 1 µm (its effect
increasing with decreasing particle size), with respect to the smaller
bronchioles and especially the alveoli. In the size range of 2-5 µm,
experimental data have fitted quite well with the ICRP model, but
deposition has varied widely (with an order of magnitude of 2 to 3)
among human subjects (Lippmann et al., 1971). In experiments on
animals, large reproducible differences in deposition have been seen
among individuals within the same species (Albert et al., 1968;
Tomenius, 1973). In the submicron range, theoretical models have not
yet been satisfactorily verified by experiments.
6.2 Clearance from the Respiratory Tract and Distribution
6.2.1 Sulfur dioxide
Sulfur dioxide is absorbed from the respiratory tract into the
blood stream. It can then be widely distributed throughout the body
(Balchum et al., 1960a, 1960b; Bystrova, 1957; Frank et al., 1967;
Yokoyama et al., 1971), where it appears to be metabolized and
excreted via the urinary tract.
6.2.2 Particulate matter
Knowledge concerning the elimination of insoluble particles
deposited in the alveoli is incomplete (Hatch & Gross, 1964; Morrow,
1973). It is clear that these particles are, to a large extent,
phagocytized by alveolar macrophages within hours, but it is not known
to what extent they are actively carried to the ciliated part of the
lung or to the lymphatic system. The particles can be transported by
the mucociliary escalator into the interstitium (where they can remain
for a long time) or the lymphatic system. Solubility in vivo is
important for the clearance of "insoluble" particles deposited in the
alveoli (Mercer, 1967; Morrow, 1973). The biological half-lives range
from days to years depending on the chemical composition of the
particles (Brain & Valberg, 1974; Task Group on Lung Dynamics, 1966;
Task Group on Metal Accumulation, 1973).
Mucociliary transport, and therefore clearance of particles, can
be affected by various factors and can be impaired by long-term
cigarette smoking and acute infection in the respiratory tract (Camner
& Philipson, 1972; Camner et al., 1973a; Jarstrand et al, 1974).
Subjects suffering from chronic obstructive lung disease may also have
an impaired mucociliary transport (Camner et al., 1973b; Toigo et al.,
1963). Studies in animals have shown that the inhalation of sulfur
dioxide can interfere with the clearance of bacteria (Rylander et al.,
1971) and inert particles (Ferin & Leach, 1973) from the lungs, but
how much is due to the action of the sulfur dioxide on the mucociliary
mechanism and how much to its action on the macrophages is not clear.
The clearance of soluble particles may follow the above pathways
depending upon their solubility. They may dissolve in the mucus in
which case they will probably be eliminated via the mucociliary route
and be swallowed and removed or absorbed via the intestinal tract. In
the alveoli, the particles may diffuse into the lymph or blood and
thus be removed from the lung.
7. EFFECTS ON EXPERIMENTAL ANIMALS
It has been difficult to separate the relative effects of sulfur
dioxide, sulfuric acid mists, sulfate salts, and particulate matter in
the ambient air by epidemiological techniques. The effects of these
substances individually and in various combinations have been studied
in the laboratory. These studies have been useful in explaining some
of the mechanisms of action but their application has been rather
limited for the establishment of safe ambient levels, particularly for
complex mixtures such as exist in the ambient air. In the past, the
levels used in laboratory studies on animals have usually been far in
excess of those seen in the ambient air and therefore have had little
relevance for ambient air quality standards. Later studies have been
at more relevant levels.
The following discussion separates work on animals into short-term
and long-term exposure studies. Short-term exposure studies are those
that involve exposures of 24-h or less and usually last from minutes
to at the most a few hours. Long-term exposure studies refer to
exposures that last longer than 24-h and are usually extended over
months. In the short-term exposure studies, only immediate or acute
effects have been studied. In long-term exposure studies, animals may
be studied during the period of exposure to determine whether there
are progressive changes or not and at the end of the exposure period
to quantify any chronic effect.
7.1 Short-term Exposure Studies
7.1.1 Exposure to sulfur dioxide singly or in combination with
other agents
Sulfur dioxide is a respiratory irritant that is very soluble in
the aqueous surfaces of the respiratory airways. Because of this high
solubility, most of the sulfur dioxide is absorbed in the nose and
upper airways (section 6.1) and very little reaches the lungs
directly. At extremely high concentrations, the absorptive capacity of
the upper airways can be overwhelmed and death or pathological changes
including laryngotracheal and pulmonary oedema can be induced in the
respiratory tract of experimental animals.
Exposure-effect curves have been developed for guineapigs (Amdur,
1966) and dogs (Frank & Speizer, 1965). In Amdur's study, a linear
relationship was obtained between exposure for 1 h to sulfur dioxide
concentrations ranging from 0.46 to 2380 mg/m3 (0.16-835 ppm) and
corresponding increases in pulmonary flow resistance. The second study
showed that nasal flow resistance increased roughly in proportion to
exposure to sulfur dioxide concentrations ranging from 20 to
660 mg/m3 (7-230 ppm) for a 15-20 minute period.
Studies with guineapigs at lower concentrations have shown that a
sodium chloride aerosol, administered concomitantly enhances the
effects of sulfur dioxide on the lungs in the form of
bronchoconstriction and increased airway resistance (Amdur, 1957). It
was postulated that the sodium chloride could act as a carrier to
deliver the absorbed sulfur dioxide deep into the lungs, or that the
increased humidity in the respiratory tract could react with the
sulfur dioxide to form sulfurous acid, especially if catalysts were
present (Amdur & Underhill, 1968). McJilton et al. (1973) have
reaffirmed the importance of humidity in this reaction, exposing
guineapigs for 1 h to sulfur dioxide at 3.1 mg/m3 (1.1 ppm) and a
sodium chloride aerosol of about 1 mg/m3. They allowed the mixture to
"age" in a reaction chamber for 8-10 minutes at various relative
humidities. The chamber temperature was 22°C and the average aerosol
particle size was 0.1 µm with a maximum size of less than 2 µm. At
relative humidities above 80%, they noted a marked increase in
pulmonary resistance, whereas administration of the individual
components produced little or no effect. The droplets formed at high
humidity and in the presence of the aerosol had a pH of 3.2 ± 0.5.
Analysis of these particles by mass spectrometer revealed sulfur
dioxide and bisulfite ions (HSO-3)in the solution but no sulfuric
acid. In the high humidity, the sodium chloride aerosol apparently
became hydrated and could then absorb sulfur dioxide. The authors
indicated that the mixture had to "age" to ensure absorption of the
sulfur dioxide; otherwise there would be too much competition with the
moist surfaces in the nasopharynx which could sweep out the sulfur
dioxide. Thus, this is another mechanism whereby the effect of sulfur
dioxide can be enhanced. If such mixtures were allowed to "age"
longer, it seems likely that the sulfur dioxide could be changed to
sulfuric acid. Studies using other animal species such as the cat have
not shown this enhancement of the effect of sulfur dioxide by sodium
chloride (Corn et al., 1972).
Matsumura (1970a) exposed guineapigs to ozone at 2.1, 11, or
21 mg/m3 (1, 5, or 10 ppm), nitrogen dioxide at 40, 80, or 140 µg/m3
(20, 40, or 70 ppm), or sulfur dioxide at 60, 170, 510, or 940 mg/m3
(20, 60, 180, or 330 ppm) for 30-50 minutes. Each group with unexposed
controls was then exposed to an aerosolized antigen (2 µm) of egg
albumin and bovine albumin for 45 minutes, 5-7 times at intervals of a
day or more. Two weeks after the last exposure, the animals were
killed and blood was drawn for assay of the immunological response.
Enhanced sensitization was noted only at the highest levels of
exposure. During these exposures a number of animals died of
anaphylactic reactions at the fifth or sixth inhalation. In another
study, sulfur dioxide at a concentration of 1100 mg/m3 (400 ppm) did
not have any effect on dyspnoeic attacks (Matsumura, 1970b).
7.1.2 Exposure to sulfuric acid aerosols or suspended sulfates
Sulfuric acid mist and some of the sulfate salts are more powerful
respiratory irritants than sulfur dioxide, and this effect is also
related to particle size (smaller particles tending to be more
irritating) (Amdur, 1958). Treon et al. (1950) exposed rabbits, rats,
mice, and guineapigs to sulfuric acid mist, 93-99% of which was less
than 2 µm in diameter, many droplets being about 1 µm in diameter.
Concentrations ranged from 87 to 1610 mg/m3. Despite the relatively
small number of animals used, a clear-cut species difference was shown
at these high concentrations. The order of increasing sensitivity was
rabbits, rats, mice, guineapigs.
It was reported by Amdur et al. (1952a) that the 8-h LC50 of
sulfuric acid mist with a mass median diameter (MMD) of about 1 µm was
18 mg/m3 for 1-2 month old guineapigs. Pattle et al. (1956) showed
that sulfuric acid mist of MMD 2.7 µm was more toxic to guineapigs
than a mist of 0.8 µm, and that the toxicity of the smaller particles
increased when the exposure occurred at 0°C. However, this might be a
response of the guineapig to low temperature rather than to the
sulfuric acid mist. Concomitant exposures with ammonium carbonate had
a protective action, apparently because it neutralized the sulfuric
acid.
Table 11. Sulfuric acid and some sulfates in descending order of
their irritative capacity for animals. Presented for
equivalent amounts of sulfur and at comparable particle
size i.e., sub-micron; short-term exposuresa
Sulfuric acid H2SO4
Zinc ammonium sulfate ZnSO4(NH4)2 SO4
Iron (III) sulfate Fe2 (SO4)3
Zinc sulfate ZnSO4
Ammonium sulfate (NH4)2 SO4
Nonreactive
Iron (II) sulfate FeSO4
Manganese (II) sulfate MnSO4
a From: Amdur (1969, 1970, 1971).
Studies on guineapigs have shown that, for equivalent amounts of
sulfur and in comparable particle size, sulfuric acid is more
irritative than any of the sulfate compounds, some of which appear to
be nonreactive in animals (Table 11). Whether these compounds would
behave in the same manner in complex mixtures such as those found in
polluted air is not known. Some of the nonreactive compounds such as
manganese salts or oxides can catalyze the reaction of sulfur dioxide
to sulfuric acid.
In short-term exposure studies by Amdur (1958), concentration
seemed to be more important than duration and death was related to
laryngospasm and bronchospasm. Sulfuric acid mist also caused
parenchymal lung damage that seemed to be related to the total dose
(Amdur, 1958; Pattle et al., 1956).
7.2 Long-term Exposure Studies
7.2.1 Exposure to sulfur dioxide
Rats were exposed for 96 days to sulfur dioxide at concentrations
of 0.1, 0.5, and 1.5 mg/m3 (0.04, 0.18 & 0.53 ppm). Histological
examination showed interstitial pneumonia, bronchitis, tracheitis, and
peribronchitis after exposures to the two higher levels (Elfimova &
Gusev, 1969).
Misiakiewicz (1970) exposed rats continuously during 5 months to
sulfur dioxide at concentrations of 0.3, 0.5, 1.0, 2.0, and
20.0 mg/m3 (0.11, 0.18, 0.35, 0.7 and 7.0 ppm). Exposures to
2.0 mg/m3 (0.7 ppm) and 20.0 mg/m3 (7.0 ppm) increased the activity
of serum cholinesterase (EC 3.1.1.8) and aspartate aminotransferase
(EC 2.6.1.1) and caused morphological changes in the upper respiratory
tract.
After a 120-h exposure to a sulfur dioxide concentration of
3 mg/m3 (1.1 ppm), guineapigs showed proliferative interstitial
pneumonia, bronchitis, and tracheitis and an increased histamine
content in the lungs, while exposure to 167 µg/m3 (0.06 ppm) of
sulfur dioxide for one month led to interstitial changes in the
respiratory tract (Bustueva, 1961a). Bustueva (1966) also exposed rats
for 65 days (24 h per day) to sulfur dioxide at 4.86 mg/m3 (1.7 ppm).
Tracheitis, desquamation of epithelial cells, an increased amount of
purulent mucus, and interstitial pneumonia were found. It was not
clear, however, whether this was due to the sulfur dioxide or to the
pulmonary infection that can develop in rats.
Beagle dogs exposed to a sulfur dioxide concentration of
13.4 mg/m3 (4.7 ppm) for 21 h per day, for 620 days, did not develop
any specific histopathological changes (Lewis et al., 1973).
Guineapigs have been exposed to sulfur dioxide levels up to
16.3 mg/m3 (5.7 ppm) for 12 months without definite effects except
for slight cytoplasmic vacuolation in the liver (Alarie et al., 1970).
Cynomolgus monkeys exposed continuously for 78 weeks to sulfur dioxide
levels up to 3.7 mg/m3 (1.3 ppm) did not show any significant
pathological changes (Alarie et al., 1972). However, rats exposed to
sulfur dioxide at 2.9 mg/m3 (1 ppm) for 170 h showed a significant
reduction in clearance of inert particles from the lung (Ferin &
Leach, 1973). Syrian hamsters, made emphysematous by previous exposure
to aerosolized papain, tolerated concentrations of sulfur dioxide up
to 1900 mg/m3 (650 ppm). Exposures were for 4 h per day, 5 days per
week, for a total of 19-74 exposures. Only slight changes in the
mechanical properties of the lung were noted as well as slight
bronchitis (Goldring et al., 1970). As Syrian hamsters are
exceptionally resistant to the effects of sulfur dioxide, these data
must be extrapolated with caution to other species.
7.2.2 Exposure to sulfuric acid aerosols
Alarie et al. (1973) exposed cynomolgus monkeys for 78 weeks to
sulfuric acid mist at concentrations of 0.38 to 4.79 mg/m3 and
particle sizes of 0.54 to 3.60 µm. At concentrations of 2.43 and
4.79 mg/m3 and particle sizes of 3.60 and 0.73 µm, definite damage to
the pulmonary structure was evident and there was deterioration in
pulmonary function. At the lower concentrations, changes were slight
or absent. The authors also exposed guineapigs for 52 weeks to
concentrations of 0.08-0.1 mg/m3 and particle sizes of 0.84 and
2.78 µm; no detectable effects were seen.
When beagle dogs were exposed to sulfuric acid at a concentration
of about 0.9 mg/m3 for 21 h per day, for 620 days, there was a
significant reduction in pulmonary function; 90%, of the sulfuric acid
mist was less than 0.5 µm in diameter (Lewis et al., 1973).
Histopathological changes were produced in the alveolar part of the
lung, especially in the elastic tissue, of rats exposed for 65 days
(24 h per day) to a sulfuric acid concentration of 1 mg/m3. Ninety
per cent of the particles were less than 2 µm in diameter in this
study (Bustueva, 1966).
Guineapigs were exposed to sulfuric acid aerosol for 120 h at 3
different concentrations (1.98 ± 0.03; 4.20 ± 0.06; and 8.27 ±
0.15 mg/m3). Exposure to the highest concentration led to oedema of
the lungs, changes in the interalveolar walls, sharp, diffused,
interstitial changes, and increased histamine content in the lung
tissue. Three weeks after exposure, sclerosis appeared (Bustueva,
1957). After a 1-month exposure to 0.1 mg/m3, no changes were found
(Bustueva, 1961a).
7.2.3 Exposure to a mixture of sulfur dioxide and sulfuric acid
aerosols or this mixture combined with other agents
Rats were exposed for 65 days (24 h per day) to a combination of
sulfur dioxide at 4.86 mg/m3 (1.7 ppm) and a sulfuric acid aerosol at
1 mg/m3 (90% of the aerosol particles were less than 2 µm in
diameter). There was a summation of the effects (histopathological
changes in alveolar tissue) produced by exposure to each of the
pollutants alone (Bustueva, 1966).
Lewis et al. (1973) exposed beagle dogs to a combination of sulfur
dioxide at 13.4 mg/m3 (4.7 ppm) and sulfuric acid at about
0.9 mg/m3. Ninety percent of the sulfuric acid mist was less than
0.5 µm in diameter. The duration of exposure was 21 h per day, for 620
days. Some of the dogs had been pre-exposed to nitrogen dioxide. This
group tended to show less response in the form of changes in pulmonary
function than dogs not pre-exposed to nitrogen dioxide.
Alarie et al. (1975) exposed cynomolgus monkeys and guineapigs to
mixtures of sulfur dioxide, fly ash, and sulfuric acid mist, for 18
months after an 8-week baseline period. Exposure concentrations varied
from 0.29 to 143 mg/m3 (0.1 to 5.0 ppm) for sulfur dioxide and from
0.1 to 1 mg/m3 for sulfuric acid mist; the concentration of fly ash
was approximately 0.5 mg/m3. Particle size (MMD) varied from 0.53 to
3.11 µm in the acid mist and from 4.1 to 5.8 µm in the fly ash.
Pulmonary function tests and serum biochemical and haematological
analyses were conducted prior to, and periodically during, the
exposure. Lungs were examined microscopically at the end of the
experiment. Sulfuric acid mist appeared to be responsible for the
effects observed. These were largely histopathological changes in the
lungs. No synergistic action was noted between the pollutants.
7.2.4 Combined exposure to sulfur dioxide and particulate matter
or other gaseous pollutants
In studies by Frazer et al. (1968), white albino rats were exposed
to sulfur dioxide at 2.9 and 8.6 mg/m3 (1 and 3 ppm) and graphite
dust at 1 mg/m3. The mean particle size of the dust was less than
1 µm and the MMD was 3 µm. The animals were exposed for 12 hours per
day, 7 days per week, for 4 months. Both ciliary activity, and the
number of dust-containing cells per 100 lung cells appeared to be
unaffected after 56-109 days of exposure.
Various aerosols that may react with sulfur dioxide have been
studied either singly or in combination with sulfur dioxide in
experimental animals, particularly in guineapigs. Amdur (1969, 1971)
emphasized that the particle size of the aerosol as well as the
concentration was extremely important in the determination of toxicity
and that the most important size range was the submicron level. The
changes induced in pulmonary mechanics were slow to return to
pre-exposure levels. This indicates either that deposited aerosols may
not be cleared promptly but remain in the lungs exerting their effect
for a period of time, or that the repair mechanisms are slow, or that
both conditions are present. There was also a variation in the
toxicity of different sulfates for the same particle size and sulfur
content (Amdur, 1969) (Table 11).
Studies by Battigelli et al. (1969) in which rats were exposed to
sulfur dioxide at 2.9 mg/m3 (1 ppm) and graphite dust at 1 mg/m3
(particle size not stated) did not show any effect other than the
accumulation of dust in the lung after exposure for 4 months, for 12
hours per day. Amdur & Underhill (1968) pointed out that not all
aerosols enhance the effect of sulfur dioxide and that only those
aerosols composed of droplets in which the sulfur dioxide could
dissolve were active.
Mice were exposed by Zarkower (1972) to carbon particles (1.8 to
2.2 µm MMD) at a concentration of 558 ± 154 µg/m3 and sulfur dioxide
at 5.7 mg/m3 (2 ppm). The animals were exposed to carbon alone,
carbon with sulfur dioxide, and sulfur dioxide alone; controls were
unexposed. Killed Escherichia coli was used as an antigen and
antibody production was measured. Exposures for 192 days produced
significant immunosuppression. Shorter periods of exposure resulted in
variable effects including stimulation of antibody production.
A variety of metallic aerosols can catalyze the oxidation of
sulfur dioxide including the soluble salts of ferrous iron, manganese,
and vanadium.
Rylander & Bergström (1973) exposed animals with latent upper
respiratory disease and controls for 4 weeks to various combinations
of sulfur dioxide at 57 mg/m3 (20 ppm), manganese dioxide at
12-20 mg/m3 (particle size between 5.0 and 0.5 µm), and carbon
monoxide at 187-250 mg/m3 (150-200 ppm). Animals with latent upper
respiratory disease showed more extensive histological changes and an
increase in the number of free lung cells. The combination of sulfur
dioxide and manganese dioxide produced the greatest changes.
An addition of effects in rats was reported by Salamberidze (1969)
from joint exposure to sulfur dioxide at 0.15 mg/m3 (0.05 ppm) and
nitrogen dioxide at 0.1 mg/m3 (0.05 ppm) over a 3-month period.
The precise mechanism by which the oxides of sulfur and
particulate matter can affect the lungs is not known. Sulfur dioxide
(and presumably sulfuric acid as well) can interfere with the
clearance of bacteria (Rylander et al., 1971) and inert particles
(Ferin & Leach, 1973) from the lungs (see also section 6). Chronic
exposure to sulfur dioxide increased the number and area of goblet
cells in guineapigs and lambs (Mawdesley-Thomas et al., 1971).
The considerable variations in the results of these experiments on
animals reflect differences in sensitivity of individual species,
exposure levels, and methods used to assess the effects.
It should be emphasized that extrapolation of these results from
animals to human beings is not easy. These findings, however, do give
some insight into possible mechanisms of action and reactions that can
occur.
8. EFFECTS ON MAN
8.1 Controlled Exposures
A number of studies have been performed on volunteers under
controlled conditions of exposure to sulfur dioxide or sulfuric acid
aerosols, singly or in combination, or to mixtures of these with other
compounds such as ozone and hydrogen peroxide. These studies, all
conducted under short-term exposure (up to 24 h), include those on
changes in respiratory function and effects on sensory and reflex
organs.
8.1.1 Effects on respiratory organs
8.1.1.1 Exposure to sulfur dioxide
Amdur et al. (1953) exposed 14 healthy volunteers (inhaling
through the mouth) to sulfur dioxide at concentrations of
2.9-23 mg/m3 (1-8 ppm) for 10 min. They noted an increased pulse
frequency, decreased tidal volume, and an increased respiratory
frequency which returned to normal levels after exposure. The sequence
of exposures was randomized. Effects increased with increasing levels
of sulfur dioxide and were detectable at the lowest concentration
tested (2.9 mg/m3; 1 ppm). Sulfur dioxide levels were monitored by
the conductimetric method. However, Lawther (1955) was unable to
reproduce these effects in any consistent manner, either in urban or
in rural dwellings. Eleven healthy volunteers were exposed to sulfur
dioxide at levels of 2.9, 14, and 37 mg/m3 (1, 5, and 13 ppm) in
studies by Frank et al. (1962). Respiratory mechanics were measured by
means of a body plethysmograph and an oesophageal balloon. Exposures
lasted 10-30 min. Only one of the 11 subjects exposed showed an
increased pulmonary resistance at 2.9 mg/m3 (1 ppm). Increased
pulmonary resistance was noted in all subjects and was greater at the
higher concentration. The change occurred within 1 min of exposure and
increased up to 10 min, after which no further increase was noted.
In studies by Snell & Luchsinger (1969), exposure to a sulfur
dioxide concentration of 2.9 mg/m3 (1 ppm) for 15 min produced a
slight effect on total respiratory resistance in 9 healthy volunteers.
Andersen et al. (1974) exposed healthy male subjects to sulfur
dioxide at levels of 2.9, 14, and 71 mg/m3 (1, 5, and 25 ppm) for up
to 6 h. With exposures of 1-3 h at 2.9 mg/m3 (1 ppm), there was a
decrease in the flow of nasal mucus and a decrease in the cross
section of the nasal passages. Thus it appears that exposure to
concentrations of 2.9 mg/m3 (1 ppm) or more may result in impairment
of mucociliary transport in the nose.
Four healthy volunteers were exposed to sulfur dioxide and the
forced vital capacity (FVC), one second forced expiratory volume
(FEV1.0), mid-maximal flow rates (MMFR), maximal expiratory flow rate
at 50% (MEFR50) and closing volume and capacity were measured.
Although no changes were found in these tests at 1.1 mg/m3
(0.37 ppm), slight changes in FVC, FEV1.0, MMFR, and MEFR50 were
noticed at 2.1 mg/m3 (0.75 ppm) after exposure for 30 min; no effect
on closing volume was detected (Bates & Hazucha, 1973).
The results of these studies on human volunteers are summarized in
Table 12.
8.1.1.2 Exposure to sulfuric acid aerosols
Amdur et al. (1952b) exposed 15 healthy men to sulfuric acid mist,
through mouth breathing, at concentrations of 0.35-5 mg/m3 (particle
size of approximately 1 µm) for periods of 5-15 min. At concentrations
below 1 mg/m3, the mist did not produce any subjective sensations
although 5 of the 15 subjects showed a slightly increased respiratory
rate and a decreased tidal volume at 0.35 mg/m3. All subjects noted
irritation at a concentration of 3 mg/m3. Respiration was monitored
by means of a pneumotachograph. The respiratory changes as well as the
subjective sensations increased with increasing concentrations of
sulfuric acid. Healthy male volunteers were also exposed to sulfuric
acid mist by Sim & Pattle (1957), either by mask or in a chamber for
periods ranging from 10 to 60 min. Twelve men were exposed to the
mist. The temperature of the air was 18.4°C with a relative humidity
of 62%. The MMD of the aerosol was 0.99 µm and the concentration was
39.4 mg/m3. The men noted minor irritation, and lung resistance (as
measured by the interrupter technique) rose by 35-100%. With
re-exposure for 30 min to the mist at a temperature of 24.5°C, a
relative humidity of 91%, a concentration of 20.8 mg/m3, and a MMD of
1.54 µm, severe coughing and irritation of the throat occurred. The
men found it almost intolerable. Lung resistance had risen by 43-150%
when measured after 10 minutes of exposure and when coughing had
ceased. No changes in respiration, blood pressure, or pulse rate were
noted. Two of the men exposed to these conditions had persistent
symptoms for some days after exposure ended.
It is difficult to evaluate these two studies. Amdur did not
report the temperature, though it was probably in the neighbourhood of
24°C, or the relative humidity. The studies of Sim & Pattle were at
relatively high levels but they demonstrated the importance of the
relative humidity or perhaps even more important, the absolute
humidity. The results of these studies are summarized in Table 13.
None of these studies used sulfates.
Table 12. Selected laboratory studies on the effects of short-term exposures to sulfur dioxide on respiratory
function in volunteers
Concentration Length of Effects Subjects Reference
exposure
(mg/m3)a (ppm) (min)
2.9-23 1-8 10 Increased pulse rate, decreased 14 healthy males Amdur et al. (1953)
tidal volume, and increased
respiratory rate
2.9 1 10-30 Increased pulmonary/resistance 11 healthy males Frank et al. (1962)
2.9 1 15 Increased respiratory resistance 9 healthy subjects Snell & Luchsinger (1969)
(5 males &
4 females)
2.9 1 60-180 Decreased nasal mucus flow 15 healthy males Andersen et al. (1974)
and decreased cross section of
nasal passages
2.1 0.75 120 Slight effect in 30 min on 4 healthy subjects Bates & Hazucha (1973)
FVC, FEV1-0, MMFR, and
MEFR50; no effect on
closing volume
1.1 0.37 120 No effect on above tests of 4 healthy subjects Bates & Hazucha (1973)
pulmonary function throughout
exposure period
a Original levels reported as ppm have been converted to mg/m3 and rounded off.
Table 13. Selected laboratory studies on the effects of short-term exposures to sulfuric acid mist on respiratory
function in volunteers
Concentration Particle Size Length of Relative Temperature Effects Subjects Reference
(mg/m3) (µm) exposure Humidity (°C)
(min) (%)
0.35 1 5-15 ? Room (?24) 5 of 15 subjects 15 healthy Amdur et al.
increased subjects (1952b)
respiratory rate
and decreased
tidal volume
39.4 0.99 60 62 18.4 Increased 12 healthy Sim & Pattle
pulmonary males (1957)
resistance and
minor irritation
20.8 1.54 30 91 24.5 Marked increase 12 healthy Sim & Pattle
of pulmonary males (1957)
resistance and
severe irritation
8.1.1.3 Exposure to mixtures of sulfur dioxide and other compounds
Frank et al. (1964) repeated the above exposures in combination
with a sodium chloride aerosol. Concentrations of sulfur dioxide were:
2.9-5.7 mg/m3 (1-2 ppm), 11-17 mg/m3 (4-6 ppm), and 40-49 mg/m3
(14-17 ppm). The sodium chloride aerosol had a geometric mean diameter
of 0.15 µm with a geometric standard deviation of 2.3 µm and an
average concentration of 18 mg/m3. As in the earlier studies, little
change in pulmonary flow resistance was noted at the lower levels of
sulfur dioxide alone (2.9-5.7 mg/m3; 1-2 ppm) but a progressive
increase was noted at higher levels. The authors did not find any
systematic differences between the responses to sulfur dioxide alone
or to the gas plus the aerosol. As mentioned in section 8.1.1.1, Snell
& Luchsinger (1969) noted a slight effect of sulfur dioxide at
2.9 mg/m3 (1 ppm) on total respiratory resistance but could not
demonstrate an enhancing effect of either a sodium chloride aerosol or
a distilled water aerosol. Burton et al. (1969) exposed volunteers to
a concentration of sulfur dioxide of 6 mg/m3 (2.1 ppm) with or
without a sodium chloride aerosol (MMD of less than 0.4 µm) at a
concentration of 2.2 mg/m3. The inhaled air was warmed and moistened.
However, they too failed to demonstrate any effect of the sodium
chloride aerosol on the response to sulfur dioxide.
Thus, three groups have not been able to demonstrate any enhancing
effect of sodium chloride aerosol on respiratory resistance in man as
had been demonstrated in guineapigs. This does not exclude the
possibility that other particulate matter might enhance the effect of
sulfur dioxide on the human respiratory system. These studies should
be repeated at levels of humidity above 80% and the mixture should be
allowed to "age".
The possible interaction of sulfur dioxide and hydrogen peroxide
has been examined by Toyama & Nakamura (1964) and that of sulfur
dioxide and ozone by Bates & Hazucha (1973). Toyama & Nakamura (1964)
studied 24 healthy male volunteers by means of a pneumotachograph and
the interrupter technique to measure alveolar pressure; airway
resistance was determined from these measurements. The particle sizes
of the hydrogen peroxide were reported to be 1.8 and 4.6 µm.
Concentrations of hydrogen peroxide were 0.8-1.4 mg/m3 for the larger
particles and 0.01-0.1 mg/m3 for the smaller ones. Levels of sulfur
dioxide ranged from 2.9 to 170 mg/m3 (1-60 ppm). The subjects were
not aware whether they were breathing hydrogen peroxide, sulfur
dioxide, or combinations. It was not stated whether the order of
administration was randomized or not. The investigators noted a marked
increase in airway resistance with the mixture compared with the
individual components. They also noted that the larger particles had
more effect than the smaller particles. This was probably due to the
greater dose delivered, although they did not comment on this. It was
the authors' opinion that the enhancement of the response to the
combination was due to the conversion of sulfur dioxide to sulfuric
acid, although no measurement for sulfuric acid was made.
In similar studies by Bates & Hazucha (1973) healthy male
volunteers were exposed in a chamber to sulfur dioxide, or ozone, or a
combination of the two. Changes in FVC, FEV1.0, MMFR, MEFR at 50%
vital capacity and peak expiratory flow rates were studied and some
effects were noted during exposure to ozone at 540-1610 µg/m3
(0.25-0.75 ppm). Sulfur dioxide by itself had little effect over a
similar range. Joint exposure to sulfur dioxide and ozone at
1060 µg/m3 (0.37 ppm) and 790 µg/m3 (0.37 ppm), respectively,
produced a greater effect than ozone alone. Changes were noted after
exposure for 30 min and were marked after 2 h of exposure. Exercise
during exposure enhanced the effect.
8.1.2 Effects on sensory or reflex functions
Studies in the USSR have concentrated on the effects of sulfuric
acid aerosols and sulfur dioxide on sensory receptors, cerebral
cortical function, and their interrelationships. When sulfuric acid
mist produced subjective sensory stimulation such as odour or
irritation of mucous membranes, or both, then, invariably, objective
evidence of central nervous system depression could be demonstrated
(Rjazanov, 1962).
Bustueva (1961b) did not note any change in optical chronaxy in
volunteers exposed to concentrations of sulfur dioxide of 0.5 mg/m3
(0.18 ppm) and sulfuric acid of 0.3 mg/m3. However, when levels of
1.5 mg/m3 for sulfur dioxide, 0.73 mg/m3 for sulfuric acid, and
combined levels of 1.2 mg/m3 and 0.6 mg/m3, respectively, were
exceeded, optical chronaxy increased (Table 14). Similar effects were
seen in dark adaptation responses (Rjazanov, 1962).
Studies have also been carried out in which the cerebral cortex
was monitored by electroencephalography. Alpha rhythm suppression was
used as an index of response. Threshold levels at which a response was
noted are given in Table 14. (Bustueva et al. 1960).
Table 14. Threshold levels of sulfur dioxide and sulfuric acid required for effects on sensory or
reflex functions in volunteers during short-term exposuresa
Threshold levels (mg/m3)
Effects Sulfuric acid Sulfur dioxide Sulfuric acid + Sulfur dioxide
Perception of odour and 0.6 to 0.85 1.6 to 2.8 0.3 + 0.5
irritation of mucosa
Suppression of dark 0.63 to 0.73 0.92 0.3 + 0.5
adaptation
Elevation of optical 0.73 1.5 0.6 + 1.2
chronaxy
Disruption of alpha 0.63 0.9 0.3 + 0.5
rhythm
Conditioning of 0.4 0.6 0.15 + 0.5
electrocortical reflex or 0.3 + 0.25
a Summarized from studies in the USSR (Bustueva, 1961b; Bustueva et al., 1960; Rjazanov, 1962).
The electrocortical conditioned reflex is a central nervous system
phenomenon elicited only after a succession of repeated, conditioned
reflex trials. After exposure to a combination of irritants (sulfur
dioxide or sulfuric acid) and light has been repeated several times,
desynchronization begins to appear before the light is switched on.
This can be produced at levels generally not sensorially perceived.
Thus, unperceived odour or stimulus appears to become the conditioning
stimulus and generates the conditioned electrocortical reflex
(Rjazanov, 1962).
Elfimova & Hacaturjan (1968) exposed volunteers to sulfur dioxide,
phenol, and carbon monoxide and noted a summation of effects as
measured by reflex action. No interaction was noted between sulfur
dioxide at 0.5 mg/m3 (0.18 ppm) and carbon monoxide at 3 mg/m3
(2.4 ppm) as measured in volunteers by the sensory reflex technique
(Mamacasvili, 1968).
8.2 Industrial Exposure
Workers are exposed to sulfur dioxide or sulfuric acid mist in a
number of industries; sometimes exposure is not solely to sulfur
dioxide or sulfuric acid. Exposed populations have been studied with
respect to the effects of exposure on their health status or on their
respiratory system. However, in many of these studies, only the
currently employed workers were examined and a serious effort was not
made to locate subjects who had left the industry and who may have
suffered more from the disease or could have been more sensitive to
the materials. It should also be emphasized that, in general, in the
following reports, the exposure levels studied were from spot samples
or for very short time intervals.
8.2.1 Exposure to sulfur dioxide singly or in combination with
particulate matter
Kehoe et al. (1932) studied men working in a refrigerator company
in the USA where sulfur dioxide was the refrigerant. Exposures
averaged 60-90 mg/m3 (20-32 ppm) with peaks as high as 200 mg/m3
(70 ppm). These peaks had probably been higher in the past ranging up
to 290 mg/m3 (100 ppm) or more. The exposed group had significantly
more respiratory symptoms and colds. They also complained more of
fatigue and shortness of breath on exertion. Chest X-rays of the
exposed and unexposed groups showed the same distribution of
abnormalities. The authors concluded on their inadequate evidence that
there was no injury to the tracheobronchial tree or alveoli.
In studies on men working in smelters in Sweden, Sjörstrand (1947)
found that those who had worked at the roasting and reverberatory
furnaces and in the converter hall for 8 years or more had poorer
respiratory function than men working in other parts of the smelter.
Levels of exposure and the smoking histories of the subjects were not
reported. Men working in smelters are exposed to a variety of dusts as
well as to sulfur dioxide and it is difficult to separate the effects
of such exposures from those due to sulfur dioxide.
Men exposed to sulfur dioxide at daily mean concentrations up to
70 mg/m3 (25 ppm) with occasional peaks of 290 mg/m3 (100 ppm) in
certain areas of a petroleum refining plant in Abadan (Iran) were
compared by Anderson (1950) with an unexposed group. No differences
were found between the two groups. This study also did not refer to
the smoking histories of the subjects.
In a study in Norway, pulp mill workers were compared with paper
mill workers using a standard questionnaire on respiration and simple
tests of pulmonary function. The smoking histories of the subjects
were also studied. Levels of sulfur dioxide ranged from 6-100 mg/m3
(2-36 ppm) with peaks of 290 mg/m3 (100 ppm) when the digester was
blown. The exposed group had more cough, sputum, and dyspnoea than the
unexposed group but the vital capacities were similar in both groups.
The expiratory peak flows, however, of the exposed men under 50 years
of age were lower than those in a comparable unexposed group (Skalpe,
1964).
A similar study was carried out in the USA by Ferris et al. (1967)
who reported that there was no difference between men in a pulp mill
and men in a paper mill. Both groups had less respiratory disease than
was reported from a survey of the general population. The authors
noted that some of the men working in the paper mill had worked in the
pulp mill but had left because they could not tolerate the conditions.
Occupational levels of exposure to sulfur dioxide ranged from a trace
to 95 mg/m3 (33 ppm). Average levels ranged from 6 to 35 mg/m3
(2-13 ppm). Smoking habits were considered.
Huhti et al. (1970) studied pulp and paper mill workers in Finland
and noted that the effects of smoking were much more significant than
the exposures at work or the effects of climate. Levels of exposure
were not reported in this study.
Men working in two integrated steel mills in Wales were studied by
Lowe et al. (1968, 1970) and Warner et al. (1969). Mean concentrations
of sulfur dioxide over 3 years ranged from 1.8 to 2.1 mg/m3 (0.6 to
0.7 ppm) and those of suspended particulate matter in the respirable
range, ranged from 600 to 1800 µg/m3 (by elutriation technique).
Analysis of the particulate matter showed that it was mainly composed
of iron oxides and calcium sulfate. No effects on respiratory symptoms
or on simple tests of pulmonary function were found after
standardization for cigarette smoking and for age. These observations
may reflect the limitation of studying occupational groups because of
the effect of the selection processes, or this may be an example in
which the suspended particulate matter present was not interacting
with the sulfur dioxide to produce an effect.
8.2.2 Exposure to sulfuric acid mist
Dorsch (1913) studied men exposed to sulfuric acid mist in a plant
manufacturing storage batteries in Germany. He reported that at a
concentration of 0.5 mg/m3, the mist was barely noticeable; at
2.0 mg/m3, there was nose and throat irritation, at 3-4 mg/m3, there
was distinct discomfort, and at 6-8 mg/m3, there was marked
discomfort. These responses are comparable to those reported in
laboratory studies on human beings in section 8.1. No particle size
was given but, as noted in another survey reported below, they were
probably relatively large.
Men in the battery industry were also examined by Malcolm & Paul
(1961) in the United Kingdom who reported that there was significant
erosion of the teeth of the battery room workers. This was confirmed
by ten Bruggen Cate (1968). Apparently this was due to the direct
impingement of relatively large droplets of sulfuric acid on the
teeth.
Williams (1970) studied men from the same works as Malcolm & Paul
(1961) and reported that there was no difference in the forced vital
capacity and the one-second forced expiratory volume between the men
in the forming and control departments. Levels of sulfuric acid mist
averaged 1.4 mg/m3 during working hours over two days and ranged from
a trace to 6.1 mg/m3 in 1968. An earlier survey reported higher
values. Particle size reported from a survey in another firming
department doing comparable work was 14 µm MMD; 4% of the particles
being less than 4 µm MMD. The level of sulfuric acid in this
department was 2.7 mg/m3.
8.3 Community Exposure
Much of the information that has been gained concerning the
effects on health of exposure to realistic concentrations of sulfur
oxides and particulate matter has come from epidemiological studies,
carried out on segments of population chosen by virtue of place of
residence, age, existing state of health, or other characteristics, in
order to present contrasts in exposure or sensitivity to these
pollutants. Some studies have been based on the complete populations
of urban areas, observing the total number of deaths, or the incidence
or prevalence of illness within them in relation to differences in
pollution between areas, or with time in any one area.
Many epidemiological studies concerning the health effects of
exposure to sulfur oxides and particulate matter have been reported in
the literature. In the discussion that follows, attention has been
directed to papers that yield information relevant to the development
of exposure-effect and exposure-response relationships for sulfur
oxides, smoke, and suspended particulate matter, and to some others
that are of interest from the point of view of the method of approach.
8.3.1 Mortality -- effects of short-term exposures
The most clearly defined effects on mortality arising from
exposure to sulfur oxides and particulate matter have been the sudden
increases in the number of deaths occurring, on a day-to-day basis, in
episodes of high pollution. The most notable of these occurred in the
Meuse Valley in 1930 (Firket, 1931), in Donora in 1948 (Schrenk et
al., 1949), and in London in 1952 (Ministry of Health, UK, 1954). The
people primarily affected were those with pre-existing heart or lung
disease or both, and the elderly. The London episode lasted for 5 days
and it was estimated that the number of deaths during and immediately
after this period was about 4000 more than expected under normal
circumstances. On one day, the number of deaths was about three times
the number expected at that time of the year. Concentrations of sulfur
dioxide as high as 3.7 mg/m3 (1.3 ppm) were recorded in the centre of
the urban area (48-h average). Concentrations of particulate matter
were too great to be measured properly (British daily smoke/sulfur
dioxide method), and the 48-h average of about 4.5 mg/m3 at a central
site must be regarded as a conservative estimate. These were rough
estimates for the exposures and, probably, there was considerable
variation in individual exposures.
Following these major episodes, attention was turned to studies on
more moderate day-to-day variations in mortality within large cities,
in relation to pollution. Gore & Shaddick (1958) correlated mortality
in the County of London (the inner part of the Greater London Area)
with pollution by smoke and sulfur dioxide for 4 foggy periods in
1954-56, using 7-day moving averages to smooth out the data. The
authors considered that, in two of the episodes, there was a marked
increase in mortality from bronchitis and other lung diseases,
particularly in the elderly. They concluded that when the 24-h mean
concentration of smoke exceeded 2.0 mg/m3 at the same time as the
24-h mean concentration of sulfur dioxide exceeded 1.1 mg/m3
(0.4 ppm) (British daily smoke/sulfur dioxide method), there would be
increased mortality. Care was taken in this study to ensure that the
measurements were reasonably representative of the exposure of people
anywhere within the study area: the figures quoted were the mean
values from a group of 7 sites, all situated close to ground level in
mainly residential areas. However, there remained the problem that the
people at risk in a study of this type were the elderly sick, who were
likely to remain indoors, and that outdoor measurements might not have
provided an adequate assessment of exposure (Biersteker et al., 1965).
The relationship between daily mortality in the more extensive
area of Greater London and day-to-day variations in pollution (smoke
and sulfur dioxide) and visibility was examined by Martin & Bradley
(1960) in the winter of 1958-59. They noted that on days when the
smoke concentration increased by more than 100 µg/m3 compared with
the previous day, or when the sulfur dioxide concentration increased
by 70 µg/m3 (0.025 ppm), there was likely to be increased mortality
(British daily smoke/sulfur dioxide method). The increases in daily
mortality were up to about 1.25 times expected values assessed from
15-day moving averages. Thick fog (visibility less than 200 metres)
was also associated with increases in mortality. The relative
importance of the 3 factors could not be determined but, on the basis
of other work, the authors considered that the smoke was probably the
most important. It is not clear whether the results are best
interpreted in terms of change in pollution from one day to the next,
rather than in terms of absolute values, but there is support for the
former approach from studies carried out elsewhere. When results were
considered on an absolute basis (Lawther, 1963), it was concluded that
increases in mortality became evident when the 24-h mean
concentrations of smoke and sulfur dioxide exceeded 750 µg/m3 and
710 µg/m3 (0.25 ppm), respectively. The measurement sites were the
same as those used by Gore & Shaddick (1958). They could still be
considered reasonably representative of outdoor concentrations in the
areas where people lived, although the inclusion of outer,
less-densely populated areas meant that the average exposures would
tend to have been underestimated.
Studies on day-to-day variations in mortality in London were
continued in successive winters, and coupled with the records of
emergency hospital admissions. In a later paper, Martin (1964) showed
correlations between both the daily mortality and hospital admission
data and concentrations of smoke or sulfur dioxide. There was no
clearly defined level above which effects were seen, but there were
fairly consistent increases in both mortality and hospital admissions
when the concentrations of smoke and sulfur dioxide each exceeded a
24- mean of about 500 µg/m3 (0.18 ppm of sulfur dioxide, British
daily smoke/sulfur dioxide method). In 1962, there was a major episode
of high pollution in London, similar in terms of duration and of
sulfur dioxide concentrations to the one in 1952, but with lower smoke
concentrations. Again, there was a sudden increase in deaths, but the
number of deaths was not as great as before (about 700, compared with
4000). Whether the change in medical care could have influenced these
results is not clear. The greater use of antibiotics in 1962 compared
with 1952 might have reduced the number of deaths, and a greater
awareness of the risk together with clear advice to the elderly and
infirm to remain indoors could have had an effect. The dramatic
reduction in smoke concentrations in London brought about by the
implementation of the Clean Air Act, and the more gradual reduction in
sulfur dioxide that has followed it, have meant that in more recent
years there have been few occasions when levels of 500 µg/m3 have
been exceeded simultaneously for smoke and sulfur dioxide (Waller et
al., 1969).
Biersteker (1966) published a study of an episode of high
pollution in Rotterdam in December 1962, when concentrations of smoke
and sulfur dioxide of approximately 500 µg/m3 and 1000 µg/m3
(0.35 ppm), respectively, were recorded (24-h means, OECD smoke/sulfur
dioxide method). There were increases in admissions to local hospitals
of people over 50 years of age with cardiovascular diseases, and there
was also some indication of an increase in mortality. This was
observed only once in Rotterdam and could have been due to other
causes. Further observations during a similar episode would be needed
to provide a convincing statistical relationship between hospital
admissions and these levels of pollution.
A relationship between day-to-day changes in mortality and
pollution has also been reported from Osaka (Watanabe 1966). There
appeared to be increases in mortality (about 20%) on days when the
concentration of suspended particulate matter (light scattering
method) exceeded 1 mg/m3 (4-day average) and was associated with a
level of sulfur dioxide of 200 µg/m3 (0.07 ppm). Low temperatures may
have been partly responsible for the effects.
Variations in daily mortality in New York in relation to sulfur
dioxide concentrations were studied by Buechley et al. (1973). They
examined correlations and developed regressions between a number of
daily climatic factors and indices of pollution (sulfur dioxide,
conductimetric method and coefficient of haze (Cohs)), and the
mortality residuals for a given day. They noted that the day of the
week had a special correlation with mortality (mortality rates were
considerably higher on Mondays). Regression analysis indicated that
heat waves and seasonal cycle were major predictors of mortality.
Other factors were much weaker (about one third as strong) but were
all of equal strength. Partial residual mortality values were computed
and showed a significant correlation with the levels of air pollution
(sulfur dioxide r = 0.14). Mortality could be predicted equally as
well from Cohs as from sulfur dioxide levels. Mortality was 1.5% less
than expected on 232 days when sulfur dioxide levels were below
30 µg/m3 (0.01 ppm) and 2% greater than expected on 260 days when the
sulfur dioxide levels were above 500 µg/m3 (0.18 ppm) after
correcting for the other factors. The crossover point (i.e., that
point below which deaths were less than expected and above which
deaths were greater than expected) was in the vicinity of a
concentration of sulfur dioxide of 260 µg/m3 (0.09 ppm). On the other
hand, the data from these studies could be interpreted to show a
continuum of an effect across the levels of sulfur dioxide, which, in
turn, should not be considered the causative agent but rather an index
of pollution.
Schimmel & Murawski (1975) have reported on their regression
analysis of daily deaths and levels of pollution (smoke shade and
sulfur dioxide) in New York City for 1963-1972. This was an extension
of an earlier study (Schimmel & Greenberg, 1972). The authors
controlled for season, day of week, and temperature. During this time
there was a marked reduction in the average level of sulfur dioxide
from 510 µg/m3 (0.18 ppm) to 170 µg/m3 (0.06 ppm) but virtually no
change in smoke shade. Their observations indicated that, despite this
reduction in sulfur dioxide, there had been no reduction in adverse
health effects. Analysis indicated that the adverse health effects
were associated principally (80%) with the particulate matter and only
to a small extent (20%) with the sulfur dioxide. However, the authors
also pointed out that, when they regressed mortality on temperature
and sulfur dioxide alone, the effects attributable to the sulfur
dioxide increased three-fold.
These findings are provocative but they must be interpreted
cautiously because of a number of limitations in the data. Air
pollution levels were from data obtained at a single monitoring
station and were probably not truly representative of exposures for
the community. The standard errors reported with their data are large.
The report should be considered to be an indicator for further studies
in which age-specific death rates and causes of death should be
included in the analysis as well as more relevant air pollution
measurements. The results should not be used for developing an
exposure-effect relationship.
A study involving comparisons between daily mortality data in New
York and Tokyo was carried out by Lebowitz et al. (1973). They applied
a "stimulus-response" technique to identify associations between days
of high pollution and days with increased mortality, and showed strong
relationships in both cities. However, their findings do not provide
information of direct value in the assessment of exposure-effect
relationships.
8.3.2 Mortality -- effects of long-term exposures
In countries having reliable systems for the collection and
analysis of data on deaths, based on cause and area of residence,
death rates for respiratory diseases have commonly been found to be
higher in towns than in rural areas. Many factors, such as differences
in smoking habits, occupation, or social conditions may be involved in
these contrasts, but, in a number of countries, a general association
between death rates from respiratory diseases and air pollution has
been apparent for many decades.
Analyses of these data have been of great value as a lead for
epidemiological studies, but the absence of information concerning
other relevant variables, such as smoking, and the relatively crude
nature of the indices of pollution used in many of these studies make
them unsuitable for the assessment of exposure-effect relationships.
The studies of Daly (1954, 1959), Pemberton & Goldberg (1954), and
Stocks (1959) were all based on mortality data from towns in England
and Wales, and each showed a positive correlation between bronchitis
or pneumonia death rates and some index of pollution by sulfur oxides
or particulate matter, as assessed for periods close to those for
which death rates were calculated. The most detailed investigation of
this type, taking into account social factors as well as pollution,
but still not smoking, was that conducted by Gardner et al. (1969).
One interesting feature of their findings was a slight improvement in
correlation when the index of pollution used was related to a period
some 10 years earlier than that for which the death rates were
calculated (deaths 1958-64, pollution index 1952). This illustrates
another of the problems that has been widely recognized when trying to
use mortality records to assess the effects of pollution i.e., that it
may not be recent exposures that are most relevant, but those earlier
in life; where concentrations have changed markedly over the years,
current measurements may not provide an adequate index.
Lave & Seskin (1970) reanalyzed some of the mortality data from
England and Wales, and developed multiple regression equations in
terms of pollution and socioeconomic indices. Again their findings of
positive correlations with pollution are of general interest but
cannot contribute to the development of dose-response relationships.
These authors also examined analogous data for Standard Metropolitan
Statistical Areas (SMSAs) in USA and in a later paper (Lave & Seskin,
1972) they attempted to assess the relative effects of air pollution,
climate, and home heating on mortality rates. Although equations were
obtained relating death rates to measurements of suspended particulate
matter and total sulfates (both by high volume sampler), it is
doubtful whether these can be regarded as valid in the absence of
adequate information on smoking.
8.3.3 Morbidity -- effects of short-term exposures
Prospective studies on specific occupational groups, not
professionally exposed, can be useful in assessing the effects of air
pollution in different communities or in areas where a change in air
pollution is expected. In such studies, where respiratory diseases are
followed, it is necessary to control for age distribution and
household composition, and to employ adequate statistical methods. In
studies on the Philadelphia area, USA, Dohan & Taylor (1960) used
absences of seven days or more because of respiratory disease as the
index and related this to the levels of sulfate. A later report by
Dohan (1961) noted that this relationship was stronger during an
epidemic of influenza. Ipsen et al., (1969) repeated this type of
study in the same area on a slightly different occupational group
using more detailed statistical analyses. They were not able to
confirm the earlier observations that the sulfate levels were related
to absences due to respiratory diseases.
Results have been reported (Lawther et al., 1970) of a series of
studies extending from 1954 to 1968 that were carried out mainly in
London, but also in some other large cities in England, using a diary
technique for the self-assessment of day-to-day changes in conditions
among bronchitic patients. A daily illness score was calculated from
the data contained in the diaries and this was related to the
concentrations of smoke and sulfur dioxide (British daily smoke/sulfur
dioxide method) and to weather variables. The pollution figures used
for most of the London studies were the mean values from the group of
sites associated with the mortality/morbidity studies of Martin (1964)
and Gore & Shaddick (1958). Many of the subjects in the series were
active enough to be out and about and at work, and the measurements
were considered to give a reasonable assessment of the average
exposures in the areas where they lived or worked. The method used in
these studies has not been validated, for the subjects recorded only
their own assessment of their condition, and this was not checked
against regular clinical examinations or ventilatory function
measurements, but the changes appeared to have some real meaning. In
the earlier years of the series, when the general level of pollution
was high, well defined peaks in the illness score were seen when
concentrations of either smoke or sulfur dioxide exceeded 1000 µg/m3.
With the reductions in pollution that followed the gradual
implementation of the Clean Air Act, these changes in condition became
less frequent and of smaller magnitude, and the conclusion from the
series as a whole, up to 1968, was that the minimum pollution
associated with significant changes in the condition of the patients
was a smoke level of about 250 µg/m3 together with a sulfur dioxide
concentration of about 500 µg/m3 (0.18 ppm) (24-h means, British
daily smoke/sulfur dioxide method). At these levels, there was still
some evidence that the peaks were associated specifically with
pollution rather than with adverse weather conditions. A later study
that has been reported by Waller (1971), showed that, with much
reduced average levels of pollution, there was an almost complete
disappearance of days with smoke levels exceeding 250 µg/m3 and
sulfur dioxide levels exceeding 500 µg/m3 (0.18 ppm). As in earlier
studies, some correlation remained between changes in the condition of
the patients and daily concentrations of smoke and sulfur dioxide but
the changes were small at these levels. At this low range of
pollution, discrimination between the effects of pollution and those
of adverse weather was poor.
Cohen et al. (1974) studied symptoms of irritation during a
publicized and an unpublicized period of air pollution as well as
during a control period in 3 communities in the New York metropolitan
area during the summer of 1970. They used a telephone survey technique
to inquire about specific symptoms such as eye irritation, throat
irritation, chest discomfort, shortness of breath, restricted
activity, and medical visits. No difference was noted between the 2
episodes of pollution. Both episodes showed significantly increased
symptoms compared with the control period. The results indicated that
irritative symptoms increased significantly when sulfur dioxide levels
exceeded 310 µg/m3 (0.11 ppm, West-Gaeke method) and total suspended
particulates exceeded 145 µg/m3 (high volume sampler) as a 3 day
average. Sulfate levels ranged from 6.6 to 7.6 µg/m3 in one area and
5.8 to 12.3 µg/m3 in another area. In the second area, sulfate levels
during the 2 periods of air pollution were 8.5 and 12.3 µg/m3,
respectively. Sulfate levels were not reported from the third area but
were probably low. The authors drew attention to some of the problems
associated with their study. The persons interviewed were generally
wives and the symptomatology in the male population could have been
underestimated. Also, there was an internal inconsistency possibly due
to intercurrent infectious disease or socioeconomic differences.
During the publicized episode, particulate pollution was considerably
higher in the Bronx than in Queens whereas irritation symptoms were
somewhat higher in Queens. The authors concluded that there could have
been confounding effects of other air pollutants, intercurrent
infection, or sociocultural factors.
Spirometric measurements were made at approximately weekly
intervals on 18 patients with chronic obstructive lung disease for
various periods during 1969-71 (Emerson, 1973). The spirometric values
(FEV1.0 and MEFR) were correlated with the levels of pollution (sulfur
dioxide and smoke, British daily smoke/sulfur dioxide method) and
climatic factors (temperature and humidity). Changes in FEV1.0 in
these patients were more strongly correlated with temperature and
humidity than with concentrations of sulfur dioxide or smoke. Levels
of air pollution in London were: sulfur dioxide, mean 190 µg/m3
(0.07 ppm), maximum, 720 µg/m3 (0.25 ppm) and smoke mean, 44 µg/m3,
maximum, 240 µg/m3. One limitation of the study was that the
pollution figures were averaged for 5-day periods, whilst the
spirometric measurements were made on specific days.
Studies of patients with chronic bronchitis in Chicago, USA
(Burrows et al., 1968; Carnow et al., 1969) showed conflicting
results. The reasons for these differences are not clear but may be
that different criteria for the selection of patients as well as
different methods for the determination of the exposure of the
individuals and their responses were used.
An unexpected finding of a possible effect of short-term exposure
to pollution arose from a study primarily concerned with long-term
exposures. In a resurvey of adults in Vlaardingen (Netherlands) who
had been interviewed and had lung function measurements made in 1969,
Van der Lende et al. (1975) found that the average lung function
values were higher in 1972 than in 1969, even though the subjects were
3 years older. When the authors examined the concentrations of
pollution on the 2 occasions (each survey having been done within a
5-day period), they found that levels were relatively high in 1969
with daily values ranging from 15 to 140 µg/m3 for smoke and from 120
to 300 µg/m3 (0.04-0.11 ppm) for sulfur dioxide compared with values
ranging from 15 to 40 µg/m3 and 45-100 µg/m3 (0.02-0.04 ppm),
respectively, in 1972. The increase in lung function was most
pronounced on the days with the greatest difference in the levels of
pollution. A control population in a rural area showed no comparable
changes over the same period, and temperature differences did not
explain the effect.
Asthmatic subjects have also been studied. These patients
represent a heterogeneous group and this may account for the variable
responses that have been reported. Cohen et al. (1972) studied 20
asthmatic subjects living in a small town (Cumberland, WV, USA) in the
vicinity of a coal-fired power plant. They found that when the soiling
index exceeded 1.0 Coh unit, or sulfur dioxide concentrations
(West-Gaeke method) exceeded 200 µg/m3 (0.07 ppm), or total suspended
particulates exceeded 150 µg/m3 (high volume sampling method), or the
temperature was lower than 0°C, there was a significant increase in
the frequency of asthmatic attacks. Levels of sulfates exceeding
20 µg/m3 and nitrate levels exceeding 2 µg/m3 did not result in such
an effect. In general, the effect of temperature was stronger than
that of the air pollutants although each of the 5 air pollutants
measured including sulfates and nitrates showed a correlation. When
temperature and any one of the pollutants were controlled for in the
analysis, the effect of any of the other 4 pollutants was eliminated.
Temperatures below 0°C overwhelmed any effect of the pollutants and
higher levels of the pollutants reduced the effect of temperature. One
further feature of this study should be stressed. Pollution in
Cumberland was dominated by emissions from a large single source, and
this might have led to high transient exposures to the pollutants,
which included oxides of nitrogen as well as sulfur oxides and
suspended particulate matter. In these circumstances it is doubtful
whether the 24-h values provided an adequate index of exposure of the
population.
Several studies on asthmatic subjects in Yokkaichi, Japan, have
been reported by Yoshida et al. (1966) including one on a group of 13
patients in which the number of attacks increased from 1 to 4 per week
when the concentration of sulfur dioxide was in the range of
140-230 µg/m3 (0.05-0.08 ppm), rising to about 12 per week when the
sulfur dioxide level reached 740 µg/m3 (0.26 ppm), all expressed as
weekly means. Suspended particulate or smoke levels were not reported.
8.3.4 Morbidity in adults -- effects of long-term exposures
Random samples of populations can be used for international
comparisons where there are gradations of air pollution. The major
difficulty here has been to ensure comparability with respect to
occupational exposures and ethnic groups. Reid et al. (1964) reported
such a comparison based on a study in the United Kingdom (College of
General Practitioners' Study, 1961) and a survey in Berlin, NH, USA
(Ferris & Anderson, 1962). The same questionnaire was used in both
studies. In the United Kingdom, it was completed by a large number of
practitioners who made up the survey group. In the USA, it was
completed by 2 physicians who tried to maintain the criteria developed
in the British survey. Results were standardized for cigarette
smoking. The effects of air pollution were then examined by age group
and sex. When simple bronchitis was present, i.e., phlegm production
for 3 months out of the year for 2-3 years, standardizing for
cigarette smoking removed any effect of air pollution for both males
and females. A more severe form of chronic bronchitis characterized by
phlegm production, exacerbations of colds that went to the chest, and
shortness of breath when walking on the level at one's own pace did
show an association with air pollution for both males and females,
even after standardizing for cigarette smoking. Levels of air
pollution were measured in Berlin, NH, by the lead peroxide candle,
dustfall, and high-volume samplers. Data for the United Kingdom were
estimated from similar measurements obtained in comparable towns and
cities where the general practitioners collected the data. In Berlin,
NH, the sulfation rate (lead candle) was 730 µg SO3/100 cm2 per day;
in the United Kingdom, in the large towns it was 950 and in the large
conurbations 1650 µg SO3/100 cm2 per day. The results of this study
seem to be consistent with those of other studies but it is not known
whether differences in socioeconomic or ethnic status could have been
relevant factors. The population of Berlin, NH, was resurveyed in
1967, and the results were compared with those obtained in 1961
(Ferris et al., 1973). There had been some decrease in pollution
levels in the interval, the sulfation rate being 470 µg SO3/100 cm2
per day in 1967, compared with the earlier figure of 730, while the
concentration of total suspended particulates had fallen from 180 to
132 µg/m3 (high-volume samples). Small reductions in the prevalence
of respiratory disease were noted after standardizing for age and
cigarette smoking. Slight improvements in forced vital capacity (FVC)
and peak expiratory flow rate (PEFR) were also noted, but there was
little change in FEV1.0. There is some doubt about the relevance of
the 180 µg/m3 figure quoted for total suspended particulates in the
1961 study, since it referred only to a 2-month period at a single
site. A random population sample from Chilliwack, BC, Canada, an
unpolluted community, was studied by Ferris & Anderson (1964) and the
results were compared with the results of the 1961 study in Berlin,
NH. Respiratory symptoms, after standardization for age and cigarette
smoking, tended to be higher in Berlin than in Chilliwack. Pulmonary
function (FEV1.0 and PEFR) was lower in Berlin than in Chilliwack,
after standardization for age, height, sex, and smoking category.
Pollution levels in Chilliwack (based on lead-candle measurements)
were about one-tenth to one-sixth of those in Berlin, NH.
A third survey in this series was carried out in 1973 (Ferris et
al., 1976). By this time, there had been a further decline in
pollution by particulate matter, the annual mean concentration of
total suspended particulates (high volume sampler) being quoted as
80 µg/m3. Only limited data on sulfur dioxide were available; the
mean of a series of 8-h samples for selected weeks was quoted as
0.01 ppm (30 µg/m3). On this occasion, the authors did not find any
appreciable differences in the prevalence of respiratory symptoms or
in measures of lung function, as compared with the 1973 report. The
interpretation of these findings is difficult, for the studies were
concerned largely with consecutive investigations of survivors of the
original group, over a 12-year period. Although the authors took into
account, as far as possible, the effect of selective losses of some of
the population, and changes in smoking habits among those who
remained, there is still some doubt as to whether the three sets of
results are truly comparable. The authors themselves concluded that
either the changes in air pollution levels from 1967 to 1973 (which
included a decline in total suspended particulates from about 130 to
80 µg/m3 (annual mean) with possibly a slight increase rather than
decrease in sulfur dioxide) were not associated with a beneficial
effect on health, or that their methods were not sufficiently
sensitive at the levels involved.
One of the difficulties in interpreting these data is that
exposure to odorous air pollutants (Berlin, NH) also occurred,
indicating an unconventional type of air pollution. It does seem
reasonable, however, to interpret the results of these surveys as
showing that slight changes in respiratory symptoms and pulmonary
function were related to levels of pollution. Sulfur oxides and
particulate matter may have been of importance. Only sulfation data
are available for sulfur oxides in the 1961 study.
Extensive studies have been made of post office and telephone
workers in the United Kingdom and USA (Holland & Reid, 1965; Holland &
Stone, 1965; Holland et al., 1965). The authors carefully considered
most of the relevant epidemiological variables and showed a gradation
of symptoms across the levels of pollution, particularly in the 50 to
59-year-old category. However, since pollution was measured in
different ways in each part of the studies, it is difficult to deduce
any quantitative relationships with sulfur dioxide and particulate
matter. The differences in the prevalence of symptoms persisted when
the authors examined the various smoking categories. They converted
the small amount of pipe and cigar smoking to cigarette equivalents,
which is not advisable, since other studies have indicated that pipe
and cigar smoking are less markedly associated with respiratory
symptoms. Lower levels of FEV1.0 and PEFR were observed in the areas
of higher pollution. The authors indicated that a difference in height
of 2-4 cm between the two populations studied could not account for
the differences seen in pulmonary function. Presumably, these
pulmonary function values had been corrected for standard temperature
and saturated vapour pressure. If not, some of the differences between
values in the USA and United Kingdom could be explained by the fact
that lower temperatures in England could have resulted in lower
measured air volumes. Similar spirometers were used in both studies.
In another study in the United Kingdom, Lambert & Reid (1970)
questioned about 10 000 adults by post. They estimated that their
sample represented about 74% of those able to reply. The positive
responses from the questionnaire (which had been recommended by the
British Medical Research Council) were correlated with levels of
pollution estimated from data used earlier by Douglas & Waller (1966)
and from some data from the National Air Pollution Survey. The
responses were controlled for social class and cigarette smoking. The
authors reported that whereas nonsmokers showed little response to the
levels of air pollution, cigarette smokers did respond and appeared to
be more sensitive. They also noted a considerable rural/urban
gradient, more pronounced in men than in women, that could not be
explained by differences in smoking habits.
Extensive studies were carried out in the Ruhr area of the Federal
Republic of Germany (Reichel et al., 1970; Ulmer et al., 1970) based
on a short questionnaire on respiratory symptoms, physical
examinations, and measurements of airways resistance using a body
plethysmograph. There were no clear differences in the results between
areas with different levels of pollution, but because of selection
factors and a low response rate, no definite conclusions can be drawn
from these studies regarding relationships with sulfur dioxide and
particulate matter.
A study in Vlaardingen in the Netherlands by Van der Lende (1969)
indicated that increased cough and phlegm production were associated
with air pollution but decreased lung function was not. However, the
author considered that the study might have been affected by the
presence of allergens, such as spores and bacteria in the agricultural
regions. Comparisons were made between an industrial area, polluted by
sulfur dioxide and particulate matter, and an agricultural area with
little pollution. In a later report, Van der Lende et al. (1973)
indicated that the effect of pollution could have been masked by
"self-selection" of the populations concerned.
The relationship between chronic bronchitis and air pollution was
studied in Osaka Prefecture, Japan, by Tsunetoshi et al. (1971).
Surveys were made of all persons over 40 years of age in selected
areas using self-administered questionnaires similar to that
recommended by the British Medical Research Council. The authors'
definition of chronic bronchitis was cough with sputum for 3 or more
months, for at least 2 successive years. Pulmonary function was
measured by the Vitalor, using the maximum value. An equation was
developed relating prevalence of bronchitis to age, smoking, and
sulfation rate (lead candle). The prevalence of bronchitis in the
study areas ranged from about 4% (in males aged 40-59 years) in areas
where the sulfation rate was close to 1 mg/100 cm2 per day to about
10% in those around 3 mg/100 cm2 per day. No data were given
concerning the levels of particulate matter.
Many other studies on the prevalence of respiratory symptoms in
relation to air pollution have been carried out in Japan such as that
by Toyama et al. (1966). They reported prevalences ranging from 2.8 to
3.7% (males aged 40-59 years, adjusted for age and smoking to accord
with other Japanese studies) in areas of Kashima (a nonindustrialized
rural town, at that time) with sulfur dioxide concentrations of less
than 30 µg/m3 (0.01 ppm, automatic conductimetric method) and
suspended particulate concentrations (high-volume sample) of
106-341 µg/m3 (mean 197). An extensive survey in Tokyo, (Suzuki &
Hitosugi, 1970, unpublished data)a showed a higher prevalence of
chronic bronchitis in areas that were more polluted, ranging from 5.5%
(males aged 40-59 years) and 1.1% (females 40-59 years) where the
sulfur dioxide concentration was below 60 µg/m3 (0.02 ppm, automatic
conductimetric method), and the suspended particulate level was below
100 µg/m3 (light-scattering method), to 6.7% (males) and 3.9%
(females), where sulfur dioxide was over 140 µg/m3 (0.05 ppm) and
suspended particulates more than 200 µg/m3. There was also some
increase in prevalence in an intermediate area with sulfur dioxide
concentrations of 60-140 µg/m3 (0.02-0.05 ppm) and a suspended
particulate concentration of 100-200 µg/m3. Smoking habits were
standardized in this study. In a recent study by Tani (1975) in the
vicinity of a pulp mill at Fuji City, and in control areas, there
appeared to be a consistent relationship between the prevalence of
bronchitis and sulfation rates, as measured by lead candles, with a
prevalence of about 3% (males and females combined, aged 40-59 years)
in areas where the sulfation rate was around 0.6 mg/100 cm2 per day
to about 8% where it was 1.2 mg/100 cm2 per day.
In studies on a random sample of adults in Cracow, Polandm the
levels of pollution measured made it possible to classify the subjects
into high and low exposure groups (Sawicki, 1972). The levels in the
high pollution area were an annual mean smoke level of 170 µg/m3 and
an annual mean sulfur dioxide level of 125 µg/m3 (0.04 ppm). In the
low pollution area, the standard smoke annual mean was 90 µg/m3 and
the sulfur dioxide annual mean was 45 µg/m3 (0.02 ppm). Persons
a Suzuki, T. & Hitosugi, M. Prevalence study of pulmonary symptoms
in Tokyo Prefecture employees. A paper presented at a meeting of a
Working Group on Air Pollution and Health, USA-Japan Cooperative
Science Programme, East-West Center, Honolulu, 21-23 April, 1970. The
pollution levels quoted in this study were obtained from reports of
the Tokyo Metropolitan Government concerning air pollution levels for
the years 1966-69 (Department of Environmental Pollution Control
Tokyo, 1976).
residing in the more polluted area had more respiratory symptoms and
poorer pulmonary function than those residing in the area with lower
pollution (chronic bronchitis 19% in more polluted area and 11% in
less polluted area and asthmatic disease 11% in more polluted area and
5% in less polluted area). Sawicki considered that there was a
synergistic interaction with cigarette smoking. He also pointed out
that many of the inhabitants worked outside the area in which they
lived and, therefore, the exposure levels at their places of residence
might not represent their true exposures.
8.3.5 Morbidity in children
Studies of the health of children have also provided useful
information.
Douglas & Waller (1966) performed a cohort study based on a
national sample of children born in the United Kingdom in the first
week of March 1946. The children were followed medically for 15 years
by health visitors and school doctors. The study was restricted to the
3131 children who remained at the same address for the first 11 years
of the enquiry, or who moved to an area that was in a similar
pollution group. Levels of air pollution were estimated from the
amount of coal consumed in a given area where each child lived, and it
was shown at the end of the study that the 4 pollution categories that
had been defined provided a satisfactory gradation in terms of the
measurements of smoke and sulfur dioxide that were then available. In
each of the several periods of life considered, from birth onwards,
the authors noted a consistent relationship between the frequency of
lower respiratory tract infections and pollution category. There was
no sharp change at any particular pollution level, but if the rates
within the lowest category (mainly rural areas) were taken as a
baseline, then increased frequencies were seen in the next category
up, with smoke and sulfur dioxide each of the order of 140 µg/m3
(0.05 ppm sulfur dioxide) or more. Annual means were estimates based
on the British daily smoke/sulfur dioxide method for a period after
the end of the study and probably underestimated earlier level.
Socioeconomic status was important in the study, but a relationship
between air pollution and frequency of lower respiratory tract
infections still existed within separate social classes. In a later
follow-up of these subjects, Colley et al. (1973) showed that, at the
age of 20 years, respiratory symptoms were related to smoking habits
rather than to the pollution of the areas in which the subjects were
then living. However, there was some relationship between the
prevalence of symptoms and earlier histories of lower respiratory
tract infections, which, in turn, were related to estimated pollution
exposures during childhood.
Lunn et al. (1967) studied 819 children in their first year at
school in the industrial city of Sheffield, in the United Kingdom.
Medical examinations were carried out on the children, the parents
were questioned concerning the previous health of the children, and
FEV0.75 and FVC were measured. Various socioeconomic factors were also
carefully considered. The families were stable, and there had been
little or no migration into or out of the specific communities.
Measurements of pollution were made at the schools attended by the
children (British daily smoke/sulfur dioxide method). The authors
found increased frequencies of both upper and lower respiratory tract
infections in the more polluted areas. When the "cleanest" area was
taken as the baseline, most of the illness indices considered showed
an increase in the next area up in order of pollution levels, i.e.,
where the annual mean concentrations of smoke and sulfur dioxide were
each about 200 µg/m3 (0.07 ppm sulfur dioxide) taking figures for the
middle year of the survey. A follow-up study was carried out on some
of these children when they were 4 years older (i.e., aged 9) (Lunn et
al., 1970). By then, the implementation of the Clean Air Act had
reduced the contrast in pollution between the areas, so that the mean
concentration of smoke in the 3 "dirty" areas combined was about
140 µg/m3 and the mean sulfur dioxide concentration was 200 µg/m3
(0.07 ppm), compared with about 50 and 100 µg/m3 (0.04 ppm sulfur
dioxide), respectively, in the "clean" area. At this time, there was
no significant difference in the illnesses reported by the 9-year-olds
in the different areas and the authors concluded that this was in line
with the reduced pollution levels.
Data were collected (Holland et al., 1969) concerning about 11 000
children attending school in 4 areas of Kent, England, 2 of which were
predominantly urban, and 2, rural. As part of the children's regular
medical examinations, peak expiratory flow rates, height, weight, and
the results of examinations of ears and tonsils were recorded. Smoking
habits and school absences were also recorded. Questionnaires
concerning such factors as previous respiratory diseases, father's
occupation, and size of family were completed by the parents. Smoke
and sulfur dioxide were measured in 3 of the areas (British daily
smoke/sulfur dioxide method) and information on population density and
housing was collected. Four factors emerged as important in relation
to decreased peak expiratory flow rates. Place of residence was most
important followed by previous history of respiratory disease; family
size and social class were of least importance. These 4 factors seemed
to be additive but they only accounted for 10-15% of the total
variation. Levels of air pollution for smoke were reported to range
from 34 to 69 µg/m3 in the 3 communities. Values for sulfur dioxide
were not reported but were stated to parallel those for smoke. There
were clearly other factors affecting area difference, and there is
some doubt as to whether the small contrasts in pollution could have
had much effect.
Colley & Reid (1970) surveyed about 11 000 children from 6 to 10
years of age, living in urban and rural areas of England and Wales.
The prevalence of respiratory disease was assessed in the autumn.
Pollution was assessed in terms of sulfur dioxide concentration, from
some direct measurements (British daily smoke/sulfur dioxide method)
coupled with estimates based on lead candle measurements of sulfation.
Within the English areas studied (winter mean concentrations of sulfur
dioxide ranging from about 30 to 150 µg/m3 or 0.01-0.05 ppm) there
was a gradient in the prevalence of symptoms, but the rates were much
higher in Wales for comparable levels of pollution. The reasons for
this difference were not clear, although it has been suggested that it
could be related to the fact that solid-fuel consumption was high in
Wales.
Respiratory function measurements were made on children aged 10-11
years from 2 different areas of the German Democratic Republic (Berlin
and Bitterfeld) with different levels of pollution (Grosser et al.,
1971). The groups were matched for social class, age, and height. In
the low pollution area, the concentration of respirable dust (method
not mentioned) was 110 µg/m3 and that of sulfur dioxide (method not
mentioned) was 50 µg/m3 (0.02 ppm); in the other area, the figures
were 290 and 360 µg/m3 (0.13 ppm), respectively (based on 24-h
measurements, averaged for July and December). Two studies were made,
6 months apart, in each of the 2 areas. Each of the spirometric values
studied (FVC, FEV1.0, FEV0.7) was higher in the area with lower
pollution.
A variety of other techniques has been used to investigate
possible effects of exposure to pollution. These include the work of
Yoshii et al. (1969) who noted an association between chronic
pharyngitis accompanied by histopathological changes at biopsy and
level of sulfation, expressed as the annual mean, in persons attending
their clinic in Yokkaichi, Japan, and in sixth-grade children. In the
heavily polluted districts sulfation rate was more than 1.0 mg/100
cm2 per day, in moderately polluted districts it ranged from 0.25 to
1.0 mg/100 cm2 per day, and in the control area it was less than this
level (lead candle measurements).
Conjunctivitis, both acute and chronic, has been reported in
Zabrze, Poland, in relation to industrial air pollution (Maziarka &
Moroz, 1968). In Czechoslovakia, Schmidt et al. (1966) reported
differences in the blood cells, tonsils, and cervical lymph nodes of
children living in a highly polluted atmosphere compared with those
living in a relatively clean atmosphere and Symon et al. (1969)
reported retardation in growth and ossification and a reduced colour
index in red blood cells in children living in areas with high air
levels of sulfur dioxide (up to 2-3 mg/m3) and fly ash. None of these
studies can be used for the exposure-effect relationship.
8.3.6 CHESS studies
The US Environmental Protection Agency has recognized the need for
studies at relatively low levels of air pollution in order to
determine whether a no-effect level can be identified to develop a
dose-response relationship and to monitor areas where there might be a
change in the levels of exposure. The CHESS (Community Health and
Environmental Surveillance System) studies were developed with these
purposes in mind. In some aspects these studies have been well
designed and executed but in other aspects there have been
difficulties. In many ways these are preliminary studies that need to
be continued. The studies have included adults, children, asthmatic
subjects, and patients with cardiovascular and pulmonary diseases.
Both acute and chronic effects have been studied. Methods have
involved mailed questionnaires on respiratory symptoms and illnesses
(adults and children), mailed questionnaires on family composition,
housing, and socioeconomic status, telephone interviews, tests of
pulmonary function (children), and diary techniques with patients.
Previous exposures to air pollutants were estimated from
historical data on emissions or known production figures from the
local industries and the application of mathematical models. More
recent exposures were based on actual measurements. These were
generally conducted at monitoring stations (each of which covered a
radius of up to 2 km), and involved high volume sampling for total
suspended particulates and suspended sulfates, and the West-Gaeke
method for sulfur dioxide. Mathematical models were used to estimate
the variation in exposure of the different groups. Various problems
discussed in a summary of the results (US Environmental Protection
Agency, 1974) include poor response rates (i.e., low participation
rates) in some of the studies on children, high dropout rates in
studies on patients, and, in at least one of the studies on children,
the fact that their spirometry was probably in error.
There was a general tendency for the authors to over-interpret the
CHESS data. In some of the studies on children, the results were
grouped according to high and low levels of pollution and a difference
was found between the two groups. However, the relationship of the
prevalence of disease to levels of pollution was not clear -- namely,
when the individual groups were examined, some exposed to the high
levels of pollution had a lower disease prevalence than some of the
groups exposed to low levels of pollution.
The design for the study of the impact on respiratory disease was
excellent and took into account family composition, housing, and
socioeconomic status. It is unfortunate that these studies were not
continued for more than one year of observation before reporting. A
number of years of observations are needed to rule out natural
fluctuations in respiratory disease.
These studies have been reviewed by an expert committee in the USA
under the chairmanship of Dr D. Rall (Director, National Institute of
Environmental Health Sciences, US Department of Health, Education and
Welfare) (Rall, 1974). The Committee's evaluation of the studies was
similar to that of the Task Group and the Committee concluded that the
results of the studies did not warrant any change in the present US
Federal Air Quality standards.
It was the opinion of the WHO Task Group that these data could not
be used for the estimation of an exposure-effect relationship.
8.3.7 Lung cancer and air pollution
The possibility that air pollution is a causal factor in cancer of
the lung has given rise to considerable concern. The evidence in
favour of a causal relationship is briefly: (a) the excess
occurrence of the disease in urban areas; (b) the presence in the
suspended matter in urban air of substances such as benzo(a)pyrene
that can cause cancer under experimental conditions; and (c) the
general rise in lung cancer that appeared, at one time, to follow
certain assumed trends in pollution.
Early studies in the United Kingdom (Stocks, 1959, 1966) indicated
that variations in lung cancer mortality in urban areas were
associated with variations in amounts of pollution and, following a
recommendation by a WHO Study Group in 1959 (World Health
Organization, 1960), a pilot international study was undertaken in
several cities where there were contrasts in lung cancer death rates.
The results did not show any clear-cut relationship with measurements
of particulate matter or its benzo(a)pyrene content (Waller & Commins,
1967) and it was clear that apart from the difficulties of making
proper allowances for differences in smoking habits, it seemed likely
that present-day measurements of polycyclic hydrocarbons gave an
inadequate assessment of past exposures to these compounds.
The Royal College of Physicians of London (1970) reviewed the
issue, and concluded that the evidence against community air pollution
being a causal factor in lung cancer was stronger than the evidence
for it. The urban/rural differential is greatest in countries with
relatively low urban air pollution (Sweden, Norway, Denmark). The
upward trend in mortality as well as other experimental and
epidemiological evidence are best explained by the causal role of
cigarette smoking.
Nevertheless, in a review by Cleary (1967), evidence is presented
to show that, in Australia, New Zealand, South Africa, and the USA,
immigrants from the United Kingdom have a higher lung cancer death
rate than those born in these countries: immigrants from Norway have a
lower rate than native-born citizens of the USA, and the lung cancer
mortality rates for all these migrants are intermediate between those
of their countries of origin and destination, strongly suggesting an
environmental factor in early life.
While the existence of an urban excess of lung cancer has been
proved, it is uncertain that air pollution is the "urban factor"
responsible. In contrast, recent work incriminates cigarette smoking
more strongly than ever; there is also a contribution from some
specific occupational exposures (see footnote in section 1.1.7).
The consideration of criteria for environmental carcinogenesis
specifically in relation to any possible effects on lung cancer, is
outside the scope of the present discussion, but it may be mentioned
that any action to reduce smoke and especially that from the
inefficient combustion of coal in domestic fires, is likely to make
substantial reductions in the benzo(a)pyrene content of the air. Such
a change has already been noted in London, where the concentration of
this compound is now only about one-tenth of what it was 25 years ago
(Lawther & Waller, 1976). A reduction of sulfur dioxide may also be
important for any possible interactions relating to the production of
lung cancer. Some experimental studies (Kuschner & Laskin, 1971;
Skvorcova et al., 1973) showed an increased carcinogenic response when
laboratory animals (rats and hamsters) were exposed to sulfur dioxide
in addition to benzo(a)pyrene.
8.3.8 Annoyance
Annoyance may be defined as "a feeling of displeasure associated
with any agent or condition believed to affect adversely an individual
or a group". This definition was adopted by an international symposium
on annoyance in Stockholm in 1971 (Lindvall & Radford, 1973). Very few
studies have been performed that would make quantitative evaluations
of this effect possible.
The social awareness of pollution caused by particulate matter has
been studied in a few areas. The results from different studies have
been presented in a document on particulate matter (US Department of
Health, Education and Welfare, 1969a); they include those from a study
carried out in St. Louis (Schusky, 1966), where values for suspended
particulates of around 100 µg/m3 produced annoyance reactions from a
considerable number of people.
A similar study was carried out in Birmingham, Alabama, USA
(Stalker & Robinson, 1967), in which levels of air pollution were
correlated with annoyance. They found that, as dustfall reached or
exceeded 14.1 g/m2 per month, about one-half of the population
considered it to be a nuisance; at 10.5 g/m2 per month, about
one-third of the population considered air pollution a nuisance.
Stepwise multiple regression analysis showed that the variation due to
dust fall alone explained 49% during the spring season and 68% during
the winter season of the total association between the air pollutants
measured and public annoyance. The association between levels of
suspended particulate matter and public opinion, which was weaker than
that of dustfall, was strongest during the summer season (r = 0.59).
About one-half of the persons interviewed thought that air pollution
was a general nuisance, when mean annual or mean summer concentrations
of particulate matter reached 230 µg/m3 and one-third, when they
reached 150 µg/m3.
No such relationship was shown with sulfur dioxide concentrations.
However, these levels were low. Further studies are indicated, since
it may be that effects such as annoyance reactions will, in the
future, be the critical effects on which criteria, as regards the
protection of public health, will be based. Since annoyance reactions
have a large sociocultural component, these levels may vary from place
to place and should be determined for each locality. Surveys on
annoyance are fraught with many problems. When proper survey
techniques are expertly applied, however, it will be possible to
assess reactions in a quantitative manner.
8.4 Exposure-Effect Relationships
Some of the studies reported in this section can be used to
develop exposure-effect relationships. It has been necessary to
develop 2 tables, one for the effects of short-term exposures
(Table 15, p. 107) and another for the effects of long-term exposures
(Table 16). The results from the studies have been based on different
methods of measuring sulfur oxides and particulate matter. The values
for the sulfur oxides are treated as if they were comparable. For the
particulate matter, two categories are listed: smoke as measured by
the Organization for Economic Cooperation and Development or British
daily smoke/sulfur oxide methods and total suspended particulates as
measured by the high volume sampler or light scattering. These factors
should be considered in interpreting these tables.
Table 15. Exposure-effect relationships of sulfur dioxide, smoke, and total suspended
particulates: effects of short-term exposures
concentration
24-h mean
values (µg/m3)
Total
suspended
Sulfur dioxide Smoke particulates Effects
> 1000 > 1000 -- London, 1952. Very large increase in mortality to about 3
times normal, during 5-day fog. Pollution figures represent
means for whole area: maximum (central site) sulfur dioxide
3700 µg/m3, smoke 4500 µg/m3 (Ministry of Health, UK, (1954).
710 750 -- London, 1958-59. Increases in daily mortality up to about 1.25
times expected value (Lawther, 1963; Martin & Bradley, 1960).
500 500 -- London, 1968-60. Increases in daily mortality (as above) and
increases in hospital admissions, becoming evident when
pollution levels shown were exceeded (magnitude increasing
steadily with pollution) (Martin, 1964).
500 -- -- New York 1962-66. Mortality correlated with pollution: 2%
excess at level shown (Buechley, 1973).
500 250 -- London, 1954-68. Increases in illness score by diary technique
among bronchitic patients seen above pollution levels shown
(means for whole area) (Lawther et al., 1970).
Table 15 (cont'd).
concentration
24-h mean
values (µg/m3)
Total
suspended
Sulfur dioxide Smoke particulates Effects
300 140 -- Vlaardingen, Netherlands, 1969-72. Temporary decrease in
ventilatory function (Van der Lende et al., 1975).
200a -- 150b Cumberland, WV, USA. Increased asthma attack rate among
small group of patients, when pollution levels shown were
exceeded (Cohen et al., 1972).
a West-Gaeke method
b High volume sampling method
Other measurements by Organization for Economic Cooperation and Development or British daily smoke/sulfur
dioxide methods (Ministry of Technology, UK, 1966; Organization for Economic Cooperation and Development, 1965).
Table 16. Exposure-effect relationships of sulfur dioxide, smoke, and
total suspended particulates: effects of long-term exposures
Concentration
Annual means of 24-h
mean values (µg/m3)
Total
suspended
Sulfur dioxide Smoke particulates Effects
200 200 -- Sheffield, England. Increased
respiratory illnesses in children
(Lunn et al., 1967, 1970)
-- -- 180b Berlin, NH, USA. Increased respiratory
symptoms, decreased respiratory
function in adults (Ferris et al., 1973)
150 -- -- England & Wales. Increased respiratory
symptoms in children (Colley & Reid, 1970)
125 170 -- Cracow, Poland. Increased respiratory
symptoms in adults (Sawicki, 1972)
140d 140d -- Great Britain. Increased lower
respiratory tract illnesses in
children (Douglas & Waller, 1966)
60-140a -- 100-200c Tokyo, Increased respiratory symptoms
in adults (Suzuki & Hitosugi, unpublished
data, 1970)
a Automatic conductimetric method
b High volume sampler (2-month mean, possible underestimation of annual mean).
c Light-scattering method, results not directly comparable with others.
d Estimates based on observations after end of study; probable underestimation
of exposures in early years of study.
Other measurements by Organization for Economic Cooperation and Development or
British daily smoke/sulfur dioxide methods (Ministry of Technology, UK, 1966;
Organization for Economic Cooperation and Development, 1965).
9. EVALUATION OF HEALTH RISKS FROM EXPOSURE TO SULFUR OXIDES, SMOKE,
AND SUSPENDED PARTICULATE MATTER
It is well established that respiratory diseases are important
causes of disability and death, and that, for some of them, there is
evidence of association with environmental factors in the ambient air.
There is evidence that exposure to mixtures of urban air pollutants
containing sulfur oxides and particulate matter is related to a
variety of adverse effects on health, even when other factors are
controlled (section 8 -- Tables 15 and 16). Although in most of the
studies considered the levels of air pollution have been expressed in
terms of sulfur dioxide, smoke, or suspended particulate matter, this
does not necessarily imply that these are the causative agents. They
provide only indices of pollution, and certain components, such as
sulfuric acid or sulfates, may be of particular importance.
Measurements of some of these components have now been made in a
number of areas and used in some of the more recent epidemiological
studies. The Task Group concluded, however, that, at present, there
are not enough data on any of these other indices of pollution to
allow exposure-response relationships to be established. Thus, the
present discussion is confined to the effects of pollution expressed
in terms of sulfur dioxide, smoke, and total suspended particulate
matter.
9.1 Exposure Levels
Concentrations of sulfur dioxide, smoke, and suspended particulate
matter vary greatly from place to place and from time to time. Many
factors including sources, topography, and weather conditions may be
involved in these variations.
An annual arithmetic mean of sulfur dioxide concentrations in
urban areas typically ranges from 100-200 µg/m3 (0.035-0.070 ppm),
whereas a maximum daily mean ranges from 300-900 µg/m3
(0.11-0.32 ppm). For smoke, an annual arithmetic mean ranges from
30-200 µg/m3 with a maximum daily mean of 150-900 µg/m3; for total
suspended particulates, these levels range from 60-500 µg/m3 and
150-1000 µg/m3, respectively.
Comparatively little information is available concerning indoor
concentrations of sulfur dioxide and particulate matter, though, in
general, these levels are known to be lower than those outdoors,
except at work places.
Levels in working environments are considerably higher than
general community levels and are thought to have been much higher in
the past.
In evaluating exposure levels that have been used in the past in
connexion with epidemiological studies, a serious question arises as
to how far these measurements, often intended primarily for control or
monitoring purposes, can be considered as providing adequate measures
of exposure. As discussed in section 5, it must be recognized that all
the figures quoted in subsequent sections are based on measurements
that have been made outdoors, usually at only a limited and not wholly
representative set of fixed sites, though most people spend much time
indoors, where concentrations may differ substantially from those
outside. However, this does not necessarily invalidate these data
which are serving primarily as indices.
So far, there has been little information on the particle size
distribution and the chemical composition of selected particle size
ranges that would make the nature of exposure more understandable.
Comparable assessment is often difficult because of differences in
the methods of measurement of particulate matter that have been used
in the health effect studies. Smoke, assessed in terms of blackness,
is a measure of pollution associated with the incomplete combustion of
fuel, and total suspended particulates, determined by weight, is a
wider concept that includes all material which, by virtue of its
particle size, remains in suspension for long periods.
9.2 Experimental Animal Studies
Laboratory studies have been performed using a variety of test
animals, of periods of exposure, of experimental designs in exposure,
and of combinations of pollutants and other agents.
Some of these studies have provided useful information on the
mechanisms of the biological action of sulfur dioxide, sulfuric acid
mist, or particulate matter. However, the results are of limited value
in developing guidelines for the protection of human health from
effects of these pollutants as they exist in urban areas, and for this
reason it is necessary to turn to the available epidemiological
evidence.
9.3 Controlled Studies in Man
Effects studied in volunteers under controlled conditions include
those on the functions of the respiratory system, sensory organs, and
cerebral cortex. Durations of exposure have been very short, usually
less than a few hours.
Slight effects on pulmonary function were observed with exposure
to sulfur dioxide at a concentration of 2100 µg/m3 (0.75 ppm), but
not with exposure to a concentration of 1100 µg/m3 (0.37 ppm).
With exposure to sulfuric acid, a decrease in tidal volume was
found at a concentration as low as 350 µg/m3.
Combined exposures to sulfur dioxide and ozone or hydrogen
peroxide produced a greater effect on respiratory function than
exposure to each compound alone.
Studies on sensory and cerebral cortical functions showed that
sulfuric acid was more toxic than sulfur dioxide and that a
combination of these two substances produced an approximately additive
effect.
There is a limit to the use of these studies for the development
of criteria for community exposures, particularly when long-term
exposure to complex mixtures of air pollutants is involved.
9.4 Effects of Industrial Exposures
Effects on the health of workers have been studied in relation to
exposure to sulfur dioxide, particulate matter, or sulfuric acid mist
arising from various manufacturing processes. In many of these
studies, exposure levels were relatively high and, in some studies,
adverse effects were detected only at a daily mean sulfur dioxide
concentration as high as 70 000 µg/m3 (25 ppm).
It has also been reported that exposure to sulfuric acid at a mean
concentration of 1400 µg/m3 during working hours for 2 days did not
produce any effect on lung function.
The fact that adverse effects have not been reported at
comparatively high levels of sulfur dioxide or surfuric acid aerosols
may well be explained by the influence of biasing factors, such as
that the workers remaining in jobs with exposures to pollutants
consist of people especially resistant to their effects. Therefore, it
should not be considered an indication that such concentrations are
without effects for the average working population and particularly
not for workers with pre-existing pulmonary diseases.
In evaluating the effects from industrial exposures, due attention
must also be given to the variation in chemical composition and size
distribution of particulate matter.
9.5 Effects of Community Exposures
Many of the epidemiological studies in relation to community
exposures that were considered by a WHO Expert Committee in 1972
(World Health Organization, 1972), and which must still be relied on
to a large extent today, were based on the measurement of smoke rather
than of total suspended particulates. However, some new data on the
effects of total suspended particulates have become available and have
been included in Tables 15 and 16 (section 8).
Tables 17 and 18 show the levels above which some effects on
health might be expected among specified populations for short-term
and long-term exposures, respectively. These are based on the critical
evaluation of the results of studies reviewed in section 8.
In developing Table 17, greater emphasis had to be placed on some
earlier results related to major effects seen when concentrations of
pollution were much higher than those commonly experienced today. With
the effective control of at least some components in areas where
pollution was, at one time, very high, most of the more recent studies
have failed to isolate the effects of the pollutants in question from
effects similar in nature but arising from other factors.
The figures for sulfur dioxide and smoke are essentially the same
as those proposed by the Expert Committee in 1972 (World Health
Organization, 1972) the only change being the adoption of 250 µg/m3
(0.09 ppm) instead of 250-500 µg/m3 (0.09-0.18 ppm) as the level at
which the worsening of the condition of patients from short-term
exposures to sulfur dioxide might be expected. It was recognized that
the magnitude of responses at this level appeared to be small and
difficult to separate from effects due to other factors such as
weather or infections. It is possible that a lower figure for smoke
could now be adopted, but, in view of uncertainties concerning the
differences in composition of this component of pollution from one
area to another, and the changes with time even within one locality,
it was felt that any further modification of the figure determined on
the basis of the older studies from London would need new evidence.
Table 18 represents an overall assessment of the effects of
long-term exposure to sulfur dioxide and smoke in terms of increases
in the prevalence of respiratory symptoms among both adults and
children, and in terms of the increased frequency of acute respiratory
illnesses, that has been demonstrated particularly among children.
Table 17. Expected effects of air pollutants on health in selected segments of
the population: effects of short-term exposuresa
24-h mean concentration (µg/m3)
Expected effects Sulfur dioxide Smoke
Excess mortality among the elderly
or the chronically sick 500 500
Worsening of the condition of patients
with existing respiratory disease 250 250
a Concentrations of sulfur dioxide and smoke as measured by OECD or British daily
smoke/sulfur dioxide method (Ministry of Technology, UK, 1966; Organization for
Economic Cooperation and Development, 1965). These values may have to be adjusted
in terms of measurements made by other procedures.
Table 18. Expected effects of air pollutants on health in selected segments of
the population: effects of long-term exposuresa
Annual mean concentration (µg/m3)
Expected effects Sulfur dioxide Smoke
Increased respiratory symptoms among
samples of the general population 100 100
(adults and children) and increased
frequencies of respiratory illnesses
among children
a Concentrations of sulfur dioxide and smoke as measured by OECD or British daily
smoke/sulfur dioxide method (Ministry of Technology, UK, 1966; Organization for
Economic Cooperation and Developments, 1965). These values may have to be adjusted
in terms of measurements made by other procedures.
For each pollutant there remains much uncertainty about the
minimum levels associated with demonstrable effects. Where populations
exposed to different levels of pollution have been compared, it cannot
necessarily be assumed that even those who were exposed to a level
lower than the known lowest effect level are entirely unaffected by
pollution. There must also be doubts as to whether the effects
observed in some studies were due in part to other pollutants, or to
socioeconomic or other factors that had not been adjusted for
completely.
No firm conclusion was reached on the effects of total suspended
particulates as a component of air pollution, together with sulfur
dioxide, because of the limited amount of information available. For
the effects of long-term exposure, a tentative figure of 150 µg/m3
(annual arithmetic mean) was suggested, based on the 2 entries in
Table 16, recognising that one of these was based on light-scattering
observations, and the other on high volume sampler measurements, but
for a 2-month period only. It was noted that the one study on the
effects of short-term exposure to total suspended particulates had
been included in Table 15, but it was felt that this could not provide
satisfactory information for an assessment of these effects.
The figures in Table 18 have been expressed in terms of annual
arithmetic mean concentrations, although it is not known whether the
effects are related to extended exposures to these average levels, or
more particularly to days of high pollution within each series. In
view of the limited quantitative information on effects of pollution
in terms of annoyance, this aspect has been omitted from Tables 17 and
18.
9.6 Guidelines for the Protection of Public Health
Tables 17 and 18 present two different sets of criteria, one
relating to effects of short-term exposure, in terms of 24-h average
concentrations, and the other to effects of long-term exposure in
terms of annual means. These effects may be interrelated; the gradual
development of respiratory symptoms may, for example, be a reaction to
repeated short-term exposure to peak 24-h values, or even to transient
peaks lasting for still shorter periods, but in the absence of any
substantial evidence on this point, the two criteria must, for the
time being, be considered separately.
With the present state of knowledge, it was considered that a
safety factor of two below the figures given in Tables 17 and 18 would
be reasonable to ensure the protection of public health, and,
accordingly, Table 19 was developed, still considering the effects of
short-term and long-term exposures separately. As an indication of the
uncertainty surrounding these estimates, the figures have further been
expressed with a range of ± 20%.
The values proposed in Table 19 are in general agreement with
those suggested as long-term goals in the earlier report (World Health
Organization, 1972). In the case of sulfur dioxide, there has been
some reduction in the 24-h figure (if this is regarded as a level not
to be exceeded on more than 7 days a year), and this is in line with
the revision of the "effect" level from a range of 250 to 500 µg/m3
in the 1972 report, to the present figure of 250 µg/m3 (Table 17).
Table 19 requires careful interpretation, for none of the figures
can be considered as absolute limits. In the first place, day-to-day
variations in the concentration of smoke and sulfur dioxide are
determined largely by weather conditions, and occasional peaks far
beyond the usual daily values may well occur, even with careful
control of emissions.
Table 19. Guidelines for exposure limits consistent with
the protection of public healtha
Concentration (µg/m3)
Sulfur dioxide Smoke
24-h mean 100-150 100-150
Annual arithmetic mean 40-60 40-60
a Values for sulfur dioxide and smoke as measured by OECD or
British daily smoke/sulfur dioxide method (Ministry of
Technology, UK, 1966; Organization for Economic Cooperation
and Development, 1965). Adjustments may be necessary where
measurements are made by other methods.
Although there are two sets of conditions specified in Table 19,
determined independently from evidence on the effects of short- and
long-term exposures, they can generally be considered to be consistent
with one another. If the proportion of days with 24-h values above
those in Table 19 is small (e.g., of the order of 7 days per year),
then the annual means may well fall within or below the ranges
specified in the second row of the table. Annual means are specified
here in terms of arithmetic means: the corresponding geometric means
would generally be a little lower (see section 5).
Much consideration was given to the possibility of extending
Table 19 to include guidelines for total suspended particulates as
measured by the high volume sampler but it was concluded that the
available evidence on the effects associated with exposure to
suspended particulate matter was highly unsatisfactory.
The Task Group felt, however, that some recommendations for a
guideline should be made. A tentative annual mean value of 150 µg/m3
has been suggested in section 9.5 as a level beyond which effects of
long-term exposure to total suspended particulates might be observed.
Applying a safety factor of two and introducing a ± 20% range, as in
the case of smoke and sulfur dioxide, would then provide a range of
levels from 60 to 90 µg/m3 as a possible guideline.
For short-term exposures, no satisfactory, direct evidence
relating concentrations of total suspended particulates to effects is
available. Because of this, a guideline for short-term exposure levels
can only be inferred. Assuming that the same ratio of 24-h mean
concentrations to the annual mean (each derived independently, see
beginning of section) given in the guidelines for smoke, is applicable
to suspended particulate matter, then a very approximate 24-h
guideline for suspended particulate matter, as measured by the high
volume sampler, would be in the order of 150 to 230 µg/m3.
While the Group felt that it was reasonable and prudent to
consider the above figures as interim guidelines consistent with the
protection of public health, it stressed the very urgent need for
additional information on the effects of exposure to suspended
particulate matter (measured by the high-volume sampler). Furthermore,
it recognized the fact that the toxicological significance of total
suspended particulates might vary depending on their chemical
composition and particle size, and that under certain circumstances,
the suggested guidelines might need to be reconsidered. It should be
noted that the discussion above does not imply any preference for
smoke measurements over those of total suspended particulates; indeed
it is highly desirable to develop more appropriate methods for the
measurement of suspended particulates, especially those limited to the
measurement of respirable particles.
It was recognized that in many urban and industrial areas existing
levels of pollution by sulfur dioxide and suspended particulate matter
were substantially above these guidelines. Furthermore, there was the
problem that the long-distance transport of these pollutants from
major sources could, in some circumstances, result in comparatively
high background concentrations in rural areas, and high levels in the
incoming air in towns striving to meet their own air quality
standards. It was considered, however, that every effort should be
made to develop control procedures that would allow these guidelines
to be met.
These guidelines are based on observations among populations in
the community exposed to a mixture of sulfur dioxide and smoke or
total suspended particulates and they may not apply to situations
where only one of the components is present. On grounds of prudence,
however, it is recommended that the levels of each pollutant should be
below the values stated. It should be stressed again, however, that
the data on which the guidelines are based are uncertain and each of
the guidelines is tentative and subject to review when further
information becomes available.
It was the opinion of the Group that there is not yet sufficient
information available on the effects of community exposures to
sulfuric acid aerosols or suspended sulphates to develop guidelines
for these air pollutants.
REFERENCES
ABELES, F. B., CRAKER, K. E., FORRENCE, L. E., & LEATHER, G. R. (1971)
Fate of air pollutants: removal of ethylene, sulfur dioxide and
nitrogen dioxide by soil. Science, 173: 914-916.
ADAMS, D. F., FALGOUT, D., FROHLINGER, J. O., PATE, J. B., PLUMLEY, A.
L., SCARINGELLI, F. P., & URONE, P. (1971) Tentative method of
analysis for sulfur dioxide content of the atmosphere (manual
conductimetric method). Health Sci. Lab., 8: 42-47.
ALARIE Y., ULRICH, C. E., BUSEY, W. M., SWANN, H. E., & MACFARLAND, H.
N. (1970) Long-term continuous exposure of guinea pigs to sulfur
dioxide. Arch. environ. Health, 21: 769-777.
ALARIE, Y., ULRICH, C. E., BUSEY, W. M., KRUMM, A. A., & MACFARLAND,
H. N. (1972) Long-term continuous exposure to sulfur dioxide in
cynomolgus monkeys. Arch. environ. Health, 24: 115-128.
ALARIE, Y., BUSEY, W. M., KRUMM, A. A., & ULRICH, C. E. (1973)
Long-term continuous exposure to sulfuric acid mist in cynomolgus
monkeys and guinea pigs. Arch. environ. Health, 27: 16-24.
ALARIE, Y., KRUMM, A. A., BUSEY, W. M., ULRICH, C. E., & KANTZ, R. J.
II. (1975) Long-term exposure to sulfur dioxide, sulfuric acid
mist, fly ash and their mixtures. Results of studies in monkeys
and guinea pigs. Arch. environ. Health, 30: 254-262.
ALBERT, R. E., SPIEGELMAN, J., LIPPMANN, M., & BENNET, R. (1968) The
characteristics of bronchial clearance in the miniature donkey.
Arch. environ. Health, 17: 50-58.
ALLEN, E. R., MCQUIGG, R. D., & CADLE, R. D. (1972) The
photo-oxidation of gaseous sulfur dioxide in the air.
Chemosphere, 1: 23-32.
ALTSHULLER, A. P. (1973) Atmospheric sulfur dioxide and sulfate:
distribution of concentrations at urban and non-urban sites in the
United States. Environ. Sci. Technol., 7: 709-712.
AMDUR, M. O. (1957) The influence of aerosols upon the respiratory
response of guinea pigs to sulfur dioxide. Am. Ind. Hyg. Assoc.
Q., 18: 149-155.
AMDUR, M. O. (1958) The respiratory response of guinea pigs to
sulfuric acid mist. Am. Med. Assoc. Arch. ind. Health,
18: 407-414.
AMDUR, M. O. (1961) Report on tentative ambient air standards for
sulfur dioxide and sulfuric acid. Ann. occup. Hyg., 3: 71-83.
AMDUR, M. O. (1966) Respiratory absorption data and SO2 dose-response
curves. Arch. environ. Health, 12: 729-732.
AMDUR, M. O. (1969) Toxicological appraisal of particulate matter,
oxides of sulfur and sulfuric acid. J. Air Pollut. Control
Assoc., 19: 638-644.
AMDUR, M. O. (1970) The impact of air pollutants on physiological
responses of the respiratory tract. Proc. Am. Philos. Soc.,
114: 3-8.
AMDUR, M. O. (1971) Aerosols formed by oxidation of sulfur dioxide.
Review of their toxicology. Arch. environ. Health, 23: 459-468.
AMDUR, M. O. & UNDERHILL, D. (1968) The effect of various aerosols on
the response of guinea pigs to sulfur dioxide. Arch. environ.
Health, 16: 460-468.
AMDUR, M. O., SCHULZ, R. Z., & DRINKER, P. (1952a) Toxicity of
sulfuric acid mist to guinea pigs. Am. Med. Assoc. ind. Hyg.
occup. Med., 5: 318-329.
AMDUR, M. O., SILVERMAN, L., & DRINKER, P. (1952b) Inhalation of
sulphuric acid mist by human subjects. Am. Med. Assoc. Arch. ind.
Hyg. occup. Med., 6: 305-313.
AMDUR, M. O., MELVIN, W. W., Jr, & DRINKER, P. (1953) Effects of
inhalation of sulfur dioxide by man. Lancet, 2: 758-759.
AMERICAN CONFERENCE OF GOVERNMENT INDUSTRIAL HYGIENISTS (1977)
TLVs threshold limit values for chemical substances and physical
agents in the workroom environment with intended changes for
1977, Cincinnati, OH, ACGIH, p. 28.
AMERICAN SOCIETY FOR TESTING AND MATERIALS (1964) Standard test
method for particulate matter in the atmosphere. Optical density
of filtered deposit. ASTM standards on methods of atmospheric
sampling and analysis, Philadelphia, USA, ASTM, pp. 661-668.
ANDERSEN, I. (1972) Relationships between outdoor and indoor air
pollution. Atmos. Environ. 6: 275-278.
ANDERSEN, I., LUNDQVIST, G. R., JENSEN, P. L., & PROCTOR, D. F. (1974)
Human response to controlled levels of sulfur dioxide. Arch.
environ. Health, 28: 31-39.
ANDERSON, A. (1950) Possible long-term effects of exposure to sulfur
dioxide. Br. J. ind. Med., 7: 82-86.
ASH, R. & LYNCH, J. (1972) The evaluation of gas detector tube
systems: sulfur dioxide. Am. Ind. Hyg. Assoc. J., 32: 490-491.
AZAR, A., SNEE, R. D., & HABIBI, K. (1972) Relationship of community
levels of air lead and indices of lead absorption. In:
Proceedings of the International Symposium on Environmental
Aspects of Lead, Luxembourg, Commission of the European
Communities, pp. 581-594.
BALCHUM, O. J., DYBICKI, J., & MENECLY, G. R. (1959) Absorption and
distribution of 35SO2 inhaled through the nose and mouth by
dogs. Am. J. Physiol., 197: 1317-1321.
BALCHUM, O. J., DYBICKI, J., & MENECLY, G. R (1960a) The dynamics of
sulfur dioxide inhalation, absorption, distribution and retention.
Arch. ind. Health, 21: 564-569.
BALCHUM, O. J., DYBICKI, J., & MENECLY, G. R. (1960b) Pulmonary
resistance and compliance with concurrent radioactive sulfur
distribution in dogs breathing 35SO2. J. appl. Physiol.,
15: 62-66.
BALL, D. J. & HUME, R. (1977) The relative importance of vehicular and
domestic emissions of dark smoke in Greater London in the mid
1970s, the significance of smoke shade measurements, and an
explanation of the relationship of smoke shade to gravimetric
measurements of particulate. Atmos. Environ., 2: 1065-1073.
BARRIE, L. A. & GEORGII, H. W. (1976) An experimental investigation of
the absorption of sulfur dioxide by water drops containing heavy
metal ions. Atmos. Environ., 10: 743-749.
BATES, D. V. & HAZUCHA, M. (1973) The short-term effects of ozone on
the human lung. Proceedings of the Conference on Health Effects of
Air Pollution, NAS-NRC, Washington, DC, Oct. 3-5. In: Committee
Print, Committee on Public Works, United States Senate, US Govt.
Printing Office, pp. 507-540.
BATTIGELLI, M. C., COLE, H. M., FRASER, D. A., & MAH, R. A. (1969)
Long-term effect of sulfur dioxide and graphite dust on rats.
Arch. environ. Health, 18: 602-608.
BENSON, E. B., HENDERSON, J. J., & CALDWELL, D. E. (1972)
Indoor-outdoor air pollution relationships: a literature review,
Research Triangle Park, NC, US Environmental Protection Agency,
83 pp. (Publ. No. AP 112).
BIERSTEKER, K. (1966) [Polluted air causes, epidemiological
significance and prevention of atmospheric pollution.] Assen,
Netherlands, Van Gorcum & Co., pp. 21-23 (in Dutch).
BIERSTEKER, K., DE GRAAF, H., & NASS, C. A. G. (1965) Indoor air
pollution in Rotterdam homes. Int. J. Air Water Pollut.,
9: 343-350.
BOSANQUET, C. H. (1957) The rise of a hot waste gas plume. J. Inst.
Fuel, 30: 322-328.
BRAIN, J. D. & VALBERG, P. A. (1974) Models of lung retention based
ion ICRP Task Group Report. Arch. environ. Health, 28: 1-11.
BRIGGS, G. A. (1965) A plume rise model compared with observations.
J. Air Pollut. Control Assoc., 15: 433-438.
BRITISH STANDARDS INSTITUTION (1969a) Methods for the measurement of
air pollution. BS1747 Part 4. The lead dioxide method.
London, BSI, 16 pp.
BRITISH STANDARDS INSTITUTION (1969b) Methods for the measurement of
air pollution. BS1747 Part 1. Deposit gauges. London, BSI,
15 pp.
BROSSET, C. (1973) Air-borne acid. Ambio, 2: 2-9.
BUECHLEY, R. W., RIGGAN, W. B., HASSELBLAD, V., & VAN BRUGGEN, J. B.
(1973) SO2 levels and perturbations in mortality -- a study in
the New York/New Jersey metropolis. Arch. environ. Health,
27: 134-137.
BUFALINI, M. (1971) Oxidation of SO2 in polluted atmospheres -- a
review. Environ. Sci. Technol., 5: 685-703.
BURROWS, B., KELLOG, A. L., & BUSKEY, J. (1968) Relationships of
symptoms of chronic bronchitis and emphysema to weather and air
pollution. Arch. environ. Health, 16: 406-413.
BURTON, G. G., CORN, M., GEE, J. B. L., VASALLO, C., & THOMAS, A. P.
(1969) Response of healthy men to inhaled low concentration of
gas-aerosol mixtures. Arch. environ. Health, 18: 681-692.
BUSTUEVA, K. A. (1957) [On the toxicology of sulfuric acid aerosol.]
Gig. i Sanit., No. 2, pp. 17-22 (in Russian).
BUSTUEVA, K. A. (1961a) Experimental data on the effect of small
concentration of sulfur oxides, maximum permissible concentration
of air pollutants, Moscow, Medgiz, pp. 126-141.
BUSTUEVA, K. A. (1961b) Threshold reflex effect of SO2 and H2SO4
aerosols simultaneously present in the air. In: Ryazanov, V. A.,
ed. Limits of allowable concentrations of atmospheric pollutants,
Book 4, Washington DC, US Department of Commerce, Office of
Technical Services, pp. 92-101.
BUSTUEVA, K. A. (1964) [Resorptive action of sulfur oxides.] Gig. i
Sanit., No. 10, pp. 8-12 (in Russian).
BUSTUEVA, K. A. (1966) The toxicity of continuous exposure to sulfur
oxides. Biological action and hygienic significance of
atmospheric pollution, Moscow, Medgiz, pp. 142-172.
BUSTUEVA, K. A., POLEZAEV, E.G., & SEMENENKO, A.D. (1960)
[Electroencephalographic determination of threshold reflex effect
of atmospheric pollutants.] Gig. i Sanit., 25: 57-61
(in Russian).
BYSTROVA, T. A. (1957) [Some effects of sulfurous gas determined by
the method of tracer atoms.] Gig. i Sanit., 5: 30-37
(in Russian).
CALVERT, J. G. (1973) Interaction of air pollutants. In: Proceedings
of the Conference on the Health Effects of Air Pollution,
Washington, DC, US Government Printing Office, pp. 19-101
(Serial No. 93-15).
CAMNER, P. & PHILIPSON, K. (1972) Tracheobronchial clearance in
smoking discordant twins. Arch. environ. Health, 25: 60-63.
CAMNER, P., JARSTRAND, C., & PHILIPSON, K. (1973a) Tracheobronchial
clearance in patients with influenza. Am. Rev. respir. Dis.,
108: 131-135.
CAMNER, P., MOSSBERG, B., & PHILIPSON, K. (1973b) Tracheobronchial
clearance and chronic obstructive lung disease. Scand. J. respir.
Dis., 54: 272-281.
CARABINE, M.D. & MADDOCK, J. E. L. (1976) The growth of sulfuric acid
aerosol particles when contacted with water vapour. Atmos.
Environ., 10: 735-742.
CARNOW, B. W., LEPPER, M. H., SHEKELLE, R. B., & STAMLER, J. (1969)
Chicago air pollution study: SO2 levels and acute illness in
patients with chronic bronchopulmonary disease. Arch. environ.
Health,. 18: 768-776.
CARSON, G. A.. & PAULUS, H. J. (1974) A high volume cascade sieve
impactor. Am. Ind. Hyg. Assoc. J., 35: 262-268.
CHARLSON, R. J., VANDERPOL, A. H., COVERT, D. S., WAGGONER, A. P., &
AHLQVIST, N. C. (1973) Sulfuric acid-ammonium sulfate aerosol:
Optical detection in the St Louis region. Science, 184: 156-158.
CHUN, K. C. & QUON, J. E. (1973) Capacity of ferric oxide particles to
oxidize sulfur dioxide in air. Environ. Sci. Technol.,
7: 532-538.
CLEARY, G. J. (1967) Some arguments suggesting a causative
relationship between air pollutants and lung cancer. Clean Air,
September: 15-18.
COHEN, A. A., BROMBERG, S., BUECHLEY, R. W., HEIDERSCHEIT, L. T., &
SHY, C. M. (1972) Asthma and air pollution from a coal-fuelled
power plant. Am. J. public Health, 62: 1181-1188.
COHEN, A. A., NELSON, C. J., BROMBERG, S. M., PRAVDA, M., FERRAND, E.
F., & LEONE, G. (1974) Symptom reporting during recent publicized
and unpublicized air pollution episodes. Am. J. public Health,
64: 442-449.
COLLEGE OF GENERAL PRACTITIONERS (1961) Chronic bronchitis in Great
Britain: A national survey carried out by the respiratory diseases
study group of the College of General Practitioners. Br. med. J.,
2: 973-979.
COLLEY, J. R. T. & REID, D. D. (1970) Urban and social origins of
childhood bronchitis in England and Wales. Br. med. J.,
2: 213-217.
COLLEY, J. R. T., DOUGLAS, J. W. B., & REID, D. D. (1973) Respiratory
disease in young adults: influence of early childhood lower
respiratory tract illness, social class, air pollution and
smoking. Br. med. J., 3: 195-198.
COMMINS, B. T. (1963) Determination of particulate acid in town air.
Analyst, 88: 364-367.
COMMINS, B. T. (1967) Some studies of the particulate acid sulfate
from the products of combustion of fuels and measurement of the
acid in polluted atmospheres. In: Proceedings of the Symposium on
the Transformation of Sulfur Compounds, Mainz, Germany,
7-9 June, Paris, OECD, pp. 39-46.
COMMINS, B. T. & LAWTHER, P. J. (1958) Volatility of 3,4-benzpyrene in
relation to the collection of smoke samples. Br. J. Cancer,
12: 351-354.
COMMINS, B. T. & WALLER, R. E. (1967) Observations from a 10-year
study of pollution at a site in the city of London. Atmos.
Environ., 1: 49-68.
COMMINS, B. T. & WALLER, R. E. (unpublished) Long-term trends in
pollution in central London. Atmos. Environ.
COMMISSION OF THE EUROPEAN COMMUNITIES (1976) Air sulfur dioxide
concentrations in the European community, Luxembourg, CEC,
pp. 34-51 (Report EUR 5417e).
COMMITTEE ON AIR POLLUTION (1954) Report, London, Her Majesty's
Stationery Office, pp. 7-25 (Report Cmd 9322).
COMMITTEE ON THE CHALLENGES OF MODERN SOCIETY (1971) Air pollution.
Air quality criteria for sulfur oxides. NATO/CCMS, No. 7,
pp. 1-1 to 8-85.
CORN, M., KOTSKO, N., STANTON, D., BELL, W., & THOMAS, A. P. (1972)
Response of cats to inhaled mixtures of SO2 and SO2-NaCl aerosol
in air. Arch. environ. Health, 24: 248-256.
COX, R. A. & PENKETT, S. A. (1970) The photo-oxidation of sulfur
dioxide in sunlight. Atmos. Environ., 4: 425-433.
COX, R. A. & PENKETT, S. A. (1971) Oxidation of atmospheric SO2 by
products of the ozone-olefin reaction. Nature (Lond.),
230: 321-322.
CUMMINGS, W. G. & REDFEARN, M. W. (1957)Instruments for measuring
small quantities of sulfur dioxide in the atmosphere. J. Inst.
Fuel, 30: 628-635.
DALHAMN, T. & STRANDBERG, L. (1961) Acute effect of sulfur dioxide on
the rate of ciliary beat in the trachea of rabbit, in vivo and
in vitro, with studies on the absorptional capacity of the nasal
cavity. Int. J. Air Water Pollut., 4: 154-167.
DALY, C. (1954) Air pollution and bronchitis. Br. med. J.,
2: 687-688.
DALY, C. (1959) Air pollution and causes of death. Br. J. prev. soc.
Med., 13: 14-27.
DE GRAAF, H. & BIERSTEKER, K. (1972) Comparison of smoke concentration
outside and inside two selected sites. Atmos. Environ., 6: 697.
DEPARTMENT OF ENVIRONMENTAL POLLUTION CONTROL, Tokyo (1976) Report of
air pollution measurements. Tokyo, Continuous Measurement
Stations, Tokyo Metropolitan Government, 119 pp.
DEROUANE, A. (1972) Comparaison des concentrations en fumées à
l'extérieur et à l'interiéur des lieux d'habitation. Atmos.
Environ., 6: 209-220.
DEROUANE, A., GRANDJEAN, L., LEGRAND, M., & VERDUYN, G. (1972)
Atmospheric pollution by oxides of sulfur and smoke in Belgium,
1969-70. Inst. R. Met. Belg., Publ. A. No. 78: 1-54.
DERRETT, C. J. & BROWN, C. (in press) A continuous running
direct-reading SO2 recorder. J. Phys. E. Sci. Instrum., 11:
DOHAN, F. C. (1961) Air pollutants and incidence of respiratory
disease. Arch. environ. Health, 3: 387-395.
DOHAN, F. C. & TAYLOR, E. W. (1960) Air pollution and respiratory
disease: a preliminary report. Am. J. med. Sci., 240: 337-339.
DORSCH, R. (1913) [The pollution of the air in the storage battery
room and its surroundings by sulfuric acid.] Unpublished
dissertation, Wurzberg, Germany (in German). Quoted in: Lewis, T.
R., ed. Toxicology of atmospheric sulfur dioxide decay products,
Research Triangle Park, NC, US Environmental Protection Agency,
p. 19 (Report No. AP111).
DOUGLAS, J. W. B. & WALLER, R. E. (1966) Air pollution and respiratory
infection in children. Br. J. prev. soc. Med., 20 (1): 1-8.
DZUBAY, T. G. & STEVENS, R. K. (1973) Ambient air analysis with
dictomous sampler and X-ray fluorescence spectrometer. Environ.
Sci. Technol., 9: 663-668.
EAST, C. (1972) Sulfur dioxide emissions estimated from airborne
measurements. Atmos. Environ., 6: 399-408.
ELFIMOVA, E. V. & HACATURJAN, M. K. (1968) [Features specific to the
reflex action of sub-threshold concentrations of sulfurous
anhydride in combustion with phenol and carbon monoxide in the
atmosphere.] Gig. i Sanit., 33 (11): 3-6 (in Russian).
ELFIMOVA, E. V. & GUSEV, M. I. (1969) [Health effects of low sulfur
dioxide concentrations in air.] Gig. i Sanit., 34 (2): 3-7
(in Russian).
ELKINS, H. (1959) The chemistry of industrial toxicology. New York,
John Wiley, pp. 396-397
ELLIOTT, L. P. & ROWE, D. R. (1976) Air quality during public
gatherings. J. Air Pollut. Control Assoc'., 25: 635-636.
ELLISON, J. M. (1965) The nature of air pollution and the methods
available for measuring it. Bull. World Health Organ.,
32: 399-409.
EMERSON, P. A. (1973) Air pollution, atmospheric conditions and
chronic airways obstruction. J. occup. Med., 15: 635-638
FERIN, J. & LEACH, L. J. (1973) The effect of SO2 on lung clearance
of TiO2 particles in rats. Am. Ind. Hyg. Assoc. J.,
34: 260-263.
FERRIS, B. G., Jr & ANDERSON, D. O. (1962) The prevalence of chronic
respiratory disease in a New Hampshire town. Am. Rev. respir.
Dis., 86: 165-185.
FERRIS, B. G., Jr & ANDERSON, D. O. (1964) Epidemiological studies
related to air pollution: A comparison of Berlin, NH and
Chilliwack, British Columbia. Proc. R. Soc. Med.,
57 (Suppl.): 979-983
FERRIS, B. G., Jr, BURGESS, W. A., & WORCESTER, J. (1967) Prevalence
of chronic respiratory disease in a pulp mill and a paper mill in
the United States. Br. J. ind. Med., 24: 26-37.
FERRIS, B. G., Jr, HIGGINS, I. T. T., HIGGINS, M. W., & PETERS, J. M.
(1973) Chronic nonspecific respiratory disease in Berlin, New
Hampshire 1961-1967. A follow-up study. Am. Rev. respir. Dis.,
107: 110-122.
FERRIS, B. G., Jr, CHEN, H., PULEO, S., & MURPHY, L. H., Jr (1976)
Chronic nonspecific respiratory disease in Berlin, New Hampshire,
1967 to 1973. A further follow-up study. Amer. Rev. resp. Dis.,
113: 475-485.
FIRKET, H. (1931) Sur les causes des accidents survenue dans la vallée
de la Meuse, lors des brouillards de Décembre 1930. Bull. R.
Acad. Med. Belg., 11: 683-739.
FORTAK, H. G. (1970) Numerical simulation of the temporal and spatial
distributions of urban air pollution concentrations. In:
Proceedings of a Symposium on Multiple Source Urban Diffusion
Models. Research Triangle Park, NC, US Environmental Protection
Agency.
FRANK, N. R. & SPEIZER, F. E. (1965) SO2 effects on the respiratory
system in dogs. Arch. environ. Health, 11: 624-634.
FRANK, N. R., AMDUR, M. O., WORCESTER, J., & WHITTENBERGER, J. L.
(1962) Effects of acute controlled exposure to SO2 on respiratory
mechanics in healthy male adults J. appl. Physiol., 17: 252-258.
FRANK, N. R., AMDUR, M, O., & WHITTENBERGER, J. L. (1964) A comparison
of the acute effects of SO2 administered alone or in combination
with NaCl particles on the respiratory mechanics of healthy
adults. Int. J. Air Water Pollut., 8: 125-133.
FRANK, N. R., YODER, R. E., YOKOYAMA, E., & SPEIZER, F. E. (1967) The
diffusion of 35SO2 from tissue fluids into the lungs following
exposure of dogs to 35SO2. Health Phys., 13: 31-38.
FRANK, N. R., YODER, R. E., BRAIN, J. D., & YOKOYAMA, E. (1969) SO2
(35S labelled) absorption by the nose and mouth under conditions
of varying concentration and flow. Arch. environ. Health,
18: 315-322.
FRASER, D. A., BATTIGELLI, M. C., & COLE, H. M. (1968) Ciliary
activity and pulmonary retention of inhaled dust in rats exposed
to sulfur dioxide. J. Air Pollut. Control Assoc., 18: 821-823.
FREIBERG, J. (1975) The mechanism of iron catalyzed oxidation of SO2
in oxygenated solutions. Atmos. Environ., 9: 661-672.
FUCHS, N. A. (1964) The mechanics of aerosols, Oxford, Pergamon
Press, p. 28.
FUGAS, M. (1976) Assessment of total exposure to an air pollutant. In:
International Conference on Environmental Sensing and Assessment,
Las Vegas, September 14-19, 1975, New York, IEEE, Vol. 2,
paper 38-5. pp. 1-3.
GARDNER, M. J., CRAWFORD, M. D., & MORRIS, J. N. (1969) Patterns of
mortality in middle and early old age in the county boroughs of
England and Wales. Br. J. prev. soc. Med., 23: 133-140.
GARLAND, J. A., CLOUGH, W. S., & FOWLER, D. (1973) Deposition of
sulfur dioxide on grass. Nature (Lond.). 242: 256-257.
GARLAND, J. A., ATKINS, D. H. F., READINGS, C. J., & CAUGHEY, S. J.
(1974) Deposition of gaseous sulfur dioxide to the ground. Atmos.
Environ., 8: 75-79.
GOLDRING, I. P., GREENBURG, L., PARK, S.S., & RATNER, I. M. (1970)
Pulmonary effects of sulfur dioxide exposure in the Syrian
hamster. Arch. environ. Health, 21: 32-37.
GOLDSMITH, J. R. (1969) Los Angeles smog. Sci. J., 5: 44-49.
GORE, A. T. & SHADDICK, C. W. (1958) Atmospheric pollution and
mortality in the County of London. Br. J. prev. soc. Med.,
12: 104-113.
GREY, D.C. & JENSEN, M. L. (1972) Bacteriogenic sulfur in air
pollution. Science, 177: 1099-1100.
GROSSER, P. J., STARK, C., JECH, J., & MEHLHORN, H. (1971) [Vital
capacity and respiration tests of school children of the 4th and
5th grade in areas with different air quality situations.]
Zeitschr. Erkr. Atmungsorgane, 134: 255-265 (in German).
GRUBER, C. W. & ALPAUGH, E. L. (1954) The automatic filter paper
sampler in an air pollution measurement programme. J. Air Pollut.
Control Assoc., 4: 143-147.
HATCH, T. & GROSS, P. (1964) Pulmonary deposition and retention of
inhaled aerosols, New York, Academic Press.
HEMEON, W. C. L., HAINES, G. F., Jr, & IDE, H. M. (1953) Determination
of haze and smoke concentrations by filter paper samplers. J. Air
Pollut. Control Assoc., 3: 22-28.
HENDERSON, J. J., BENSON, F. B., & CALDWELL, D. E. (1973)
Indoor-outdoor air pollution relationships. Vol. II. An annotated
bibliography. Washington, DC, US EPA (Publ. No. AP 112b).
HILL, A. C. (1971) Vegetation: a sink for atmospheric pollutants.
J. Air Pollut. Control Assoc., 21: 341-346.
HOBBS, P. V., HARRISON, H., & ROBINSON, E. (1974) Atmospheric effects
of pollutants. Science, 183: 909-915.
HOEGG, U. R. (1972) Cigarette smoke in closed places. Environ. Health
Perspect., October, No. 2: 117-128.
HOLBROW, G. L. (1958) Crystalline bloom, Teddington, UK, Paint
Research Station, p. 4 (Tech. Paper No. 208).
HOLLAND, W. W. & REID, D. D. (1965) The urban factor on chronic
bronchitis. Lancet, 1: 445-448.
HOLLAND, W. W. & STONE, R. W. (1965) Respiratory disorders in United
States East Coast telephone men. Am. J. Epidemiol., 82: 92-101.
HOLLAND, W. W., REID, D. D., SELTSER, R., & STONE, R. W. (1965)
Respiratory disease in England and the United States: Studies of
comparative prevalence. Arch. environ. Health, 10: 338-343.
HOLLAND, W. W., HALIL, T., BENNETT, A. E., & ELLIOTT, A. (1969)
Factors influencing the onset of chronic respiratory disease.
Br. med. J., 2: 205-208.
HOLLAND, W. W., BENNETT, A. E., CAMERON, I. R., FLOREY, C DU V.,
LEEDER, S. R., SCHILLING, R. S. F., SWAN, A. V, & WALLER, R. E.
(in press) Health effects of particulate pollution. Reappraising
the evidence. Am. J. Epidemiol.
HOLLOWELL, C. D., GEE, G. Y., & McLAUGHLIN, R. D. (1973) Current
instrumentation for continuous monitoring for SO2. Anal. Chem.,
45: 63A-72A.
HORVATH, H. & CHARLSON, R. J. (1969) The direct optical measurement of
atmospheric air pollution. Am. Ind. Hyg. Assoc. J., 30: 500-509.
HUHTI, E., RYHANEN, P., VUOPALA, U., & TAKKANEN, J. (1970) Chronic
respiratory disease among pulp mill workers in an arctic area in
Northern Finland. Acta Med. Scand., 187: 433-444.
HUSAR, J. D., HUSAR, R. B., & STUBITS, P. K. (1975) Determination of
submicrogram amounts of atmospheric particulate sulfur. Anal.
Chem., 47: 2062-2065.
HUSAR, R. B. (1974) Atmospheric particulate mass monitoring with a
radiation detector. Atmos. Environ., 8: 183-188.
INTERNATIONAL ATOMIC ENERGY AGENCY (1978) Particle size analysis in
estimating the significance of airborne contamination, Vienna,
International Atomic Energy Agency, pp. 71-170 (Technical Report
Series, No. 179).
ILO/WHO COMMITTEE ON OCCUPATIONAL HEALTH (1970) Permissible levels of
toxic substances in the working environment. Geneva, International
Labour Office, p. 337 (Occupational Safety and Health Series).
IPSEN, J., DEANE, M., & INGENITO, F. E. (1969) Relationships of acute
respiratory disease to atmospheric pollution and meteorological
conditions. Arch. environ. Health, 18: 462-472.
JACOBSEN, M. (1972) Sampling and evaluating respirable coal mine dust.
Ann. NY Acad. Sci., 200: 661-665.
JARSTRAND, C., CAMNER, P., & PHILIPSON, K. (1974) Mycoplasma
pneumoniae and tracheobronchial clearance. Am. Rev. respir. Dis.,
110: 415-419.
JOHNSTONE, H. F. & COUGHANOWR, D. R. (1958) Absorption of SO2 from
air. Oxidation in drops containing dissolved catalyst. Ind. eng.
Chem., 50: 1169-1172.
JUNGE, C. E. & RYAN, T. G. (1958) Study of the SO2 oxidation in
solution and its role in atmospheric chemistry. Q. J. R. Med.
Soc., 84: 46-55.
KATZ, M. (1969) Measurement of air pollutants, guide to the selection
of methods, Geneva, WHO, 123 pp.
KEHOE, R. A., MACHLE, W. H., KITZMILLER, K., & LEBLANC, T. J. (1932)
On the effects of prolonged exposure to sulfur dioxide. J. ind.
Hyg., 14: 159-173.
KELLOGG, W. W., CADLE, R. D., ALLEN, E. R., LAZRUS, A. Z., & MARTELL,
E. A. (1972) The sulfur cycle. Science, 175: 587-596.
KRUSHNER, M. & LASKIN, S. (1971) Experimental models in environmental
carcinogenesis. Am. J. Pathol., 64: 183-196.
LAMBERT P. M. & REID, D. D. (1970) Smoking, air pollution and
bronchitis in Britain. Lancet, 25 April 1970: 853-857.
LARSEN, R. I. (1971) A mathematical model for relating air quality
measurements to air quality standards, Research Triangle Park,
NC, US Environmental Protection Agency, 61 pp. (Rpt No. AP-89).
LAUER, G. & BENSON, F. B. (1975) The CHAMP air quality monitoring
programme. In: Proceedings of the International Symposium on
Recent Advances in the Assessment of the Health Effects of
Environmental Pollution, Paris, 24-28 June 1974, Luxembourg,
Commission of the European Communities, Vol. 1, pp. 423-430.
LAUTERBACH, K. E., MERCER, T. T., HAYES, A.D., & MARROW, P. E. (1954)
Efficiency studies of the electrostatic precipitator. Arch. ind.
Hyg., 9: 69-75.
LAVE, L. B. & SESKIN, E. P. (1970) Air pollution and human health.
Science, 169: 723-733.
LAVE, L. B. & SESKIN, E. P. (1972) Air pollution, climate, and home
heating: their effects on US mortality rates. Am. J. public
Health, 62: 909-916.
LAWTHER, P. J. (1963) Compliance with the clean air act: medical
aspects. J. Inst. Fuel, 36: 341-344.
LAWTHER, P. J. (1955) Effects of inhalation of sulfur dioxide on
respiration and pulse-rate in normal subjects. Lancet,
2: 745-748.
LAWTHER, P. J. & WALLER, R. E. (1976) Coal fires, industrial emissions
and motor vehicles as sources of environmental carcinogens. In:
Proceedings of Symposium on Environmental Pollution and
Carcinogenic risks, Lyons, 3-5 November 1975, Paris, INSERM,
Vol. 52, pp. 27-40.
LAWTHER, P. J., WALLER, R. E., & HENDERSON, M. (1970) Air pollution
and exacerbations of bronchitis. Thorax, 25: 525-539.
LAWTHER, P. J., MACFARLANE, A. J., WALLER, R. E., & BROOKS, A. G. F.
(1975) Pulmonary function and sulfur dioxide: some preliminary
findings. Environ. Res., 10: 355-367.
LEBOWITZ, M.D., TOYAMA, T., & MCCARROLL, J. (1973) The relationship
between air pollution and weather as stimuli and daily mortality
as responses in Tokyo, Japan, with comparisons with other cities.
Environ. Res., 6: 327-333.
LEE, R. E., Jr & GORANSON, S. (1972) Cascade impactor network,
Research Triangle Park, NC, US Environmental Protection Agency,
132 pp. (Rep. No. AP108).
LEE, R. E., CALDWELL, J. S., & MORGAN, G. B. (1972) The evaluation of
methods for measuring suspended particulates in air. Atmos.
Environ., 6: 593-622.
LEWIS, T. R., MOORMAN, W. J., LUDMAN, W. F., & CAMPBELL, K. I. (1973)
Toxicity of long-term exposure to oxides of sulfur. Arch.
environ. Health, 26: 16-21.
LIKENS, G. E. & BORMANN, F. H. (1974) Acid rain: a serious regional
environmental problem. Science, 184: 1176-1179.
LINDVALL, T. & RADFORD, E. P. (1973) Measurement of annoyance due to
exposure to environmental factors. Environ. Res., 6: 1-36.
LIPPMANN, M., ALBERT, R. E., & PETERSON, H. T., Jr (1971) The regional
deposition of inhaled aerosols in man. In: Walton, W. H., ed.
Inhaled Particles III, London, Unwin, Vol. 1, pp. 105-120.
LISS, P.S. (1971) Exchange of SO2 between the atmosphere and natural
waters. Nature (Lond.), 233: 327-329.
LIU, B. Y. H., BERGLUND, R. N., & AGARWAL, J. M. (1974) Experimental
studies of optical particle counters. Atmos. Environ.,
8: 717-732.
LOWE, C. R., PELMEAR, P. L., CAMPBELL, H, HITCHENS, R. A. N., KHOSLA,
T., & KING, T. C. (1968) Bronchitis in two integrated steel works.
I. Ventilatory capacity, age, and physique of non-bronchitic men.
Br. J. prev. soc. Med., 22: 1-11.
LOWE, C. R., CAMPBELL, H., & KHOSLA, T. (1970) Bronchitis in two
integrated steel works. III. Respiratory symptoms and ventilatory
capacity related to atmospheric pollution. Br. J. Ind. Med.,
27: 121-120.
LUNN, J. E., KNOWELDEN, J., & HANDYSIDE, A. J. (1967) Patterns of
respiratory illness in Sheffield infant school-children. Br. J.
prev. soc. Med., 21: 7-16.
LUNN, J. E., KNOWELDEN, J., & ROE, J. W. (1970) Patterns of
respiratory illness in Sheffield junior school-children -- A
follow-up study. Br. J. prev. soc. Med., 24: 223-228.
MADDALONE, R. F., MCCLURE, G. L., & WEST, P. W. (1975) Determination
of sulfate by thermal reduction of perimidylammonium sulfate.
Anal. Chem., 47: 316-322.
MALCOLM, D. & PAUL, E. (1961) Erosion of the teeth due to sulfuric
acid in the battery industry. Br. J. ind. Med., 18: 63-69.
MAMACASVILI, M. L (1968) Determination of the reflex action of SO2
and CO by adaptometer method and coloured vision. In: Biological
action and hygienic significance of atmospheric pollutants,
Moscow, Medgiz, pp. 179-188.
MARTIN, A. E. (1961) Epidemiological studies of atmospheric pollution:
A review of British methodology. Mon. Bull. Minist. Health Public
Health Lab. Serv., 20: 42-49.
MARTIN, A. E. (1964) Mortality and morbidity statistics and air
pollution. Proc. R. Soc. Med., 57: 969-975.
MARTIN, A. E. & BRADLEY, W. H. (1960) Mortality, fog and atmospheric
pollution. An investigation during the winter of 1958-59. Mon.
Bull. Minist. Health Public Health Lab. Serv., 19: 56-72.
MASTERS, B. R. (1974) The city of London and clean air. Clean Air,
4: 22-26.
MATSUMURA, Y. (1970a) The effects of ozone, nitrogen dioxide and
sulfur dioxide on the experimentally induced allergic respiratory
disorder in guinea pigs. I. The effect on sensitization with
albumin through the airway. Am. Rev. respir. Dis., 102: 430-437.
MATSUMURA, Y. (1970b) The effects of ozone, nitrogen dioxide and
sulfur dioxide on the experimentally induced allergic respiratory
disorder in guinea pigs. III. The effect on the occurrence of
dyspneic attacks. Am. Rev. respir. Dis., 102: 444-447.
MAWDESLEY-THOMAS, L. E., HEALEY, P., & BARRY, D. H. (1971)
Experimental bronchitis in animals due to sulfur dioxide and
cigarette smoke. In: WALTON, W. H., ed. Inhaled Particles III,
London, Unwin, Vol. 1, pp. 504-525.
MAZIARKA, S. & MROZ, E. (1968) [The influence of air pollution on the
protective apparatus of the eye in school children.] Rocz. PZH,
19: (1) 31-36 (in Polish).
MCJILTON, C., FRANK, R., & CHARLSON, R. (1973) Role of relative
humidity in the synergistic effect of a sulfur dioxide-aerosol
mixture on the lung. Science, 182: 503-504.
MCKAY, H. A. C. (1971) The atmospheric oxidation of SO2 in water
droplets in presence of NH3. Atmos. Environ., 5: 7-14.
MERCER, T. T. (1967) On the role of particle size in the dissolution
of lung burdens. Health Phys., 13: 1211-1222.
MINISTRY OF HEALTH, UNITED KINGDOM (1954) Mortality and morbidity
during the London fog of December 1952, London, HMSO (Report on
Public Health and Medical Subjects No. 95).
MINISTRY OF TECHNOLOGY, UK (1966) National survey of smoke and sulfur
dioxide: Instruction manual, Stevenage, Warren Spring
Laboratory, 142 pp.
MISIAKIEWICZ, Z. (1970) The effects on rat organism of prolonged
exposure to low concentrations of sulfur dioxide in the air.
J. Natl Inst. Hyg. (Warsaw), 21: 469-487.
MONTGOMERY, T. L. & COLEMAN, J. H. (1975) Empirical relationships
between time-averaged SO2 concentrations. Environ. Sci.
Technol., 9: 953-957.
MORROW, P. E. (1973) Alveolar clearance of aerosols. Arch. intern.
Med., 131: 101-108.
MUNN, R. E. (1976) Air pollution meteorology. Ch. 7 in Manual on
urban air quality measurement. Copenhagen, WHO (WHO Regional
publ. European Series, No. 1).
NASH, T. (1964) A "personal" measuring instrument for atmospheric
sulfur dioxide. Int. J. Air Water Pollut., 8: 121-124.
ORGANISATION FOR ECONOMIC CO-OPERATION AND DEVELOPMENT (1965) Methods
of measurement of air pollution, Paris, OECD, 94 pp.
PAN AMERICAN HEALTH ORGANIZATION (1976) Pan American air pollution
monitoring network (Redpanaire), Lima, Pan American Center for
Sanitary Engineering and Environmental Sciences, 50 pp.
(Pub. No. 30).
PASQUILL, F. (1971) Atmospheric dispersion of pollution. Q. J. R.
Med. Soc., 97: 369-395.
PATE, J. B., AMMONS, B. E., SWANSON, G. A., & LODGE, J.P. (1965)
Nitrite interference in spectrophotometric determination of
atmospheric sulfur dioxide. Anal. Chem. 37: 942-945.
PATTLE, R. E., BURGESS, F., & CULLUMBINE, H. (1956) The effects of a
cold environment and of ammonia on the toxicity of sulfuric acid
mist to guinea pigs. J. Pathol. Bacteriol., 72: 219-232.
PEMBERTON, J. & GOLDBERG, C. (1954) Air pollution and bronchitis.
Br. med. J., 2: 567-570.
POLLACK, R. I. (1975) Studies of pollutant concentration frequency
distributions, Washington DC, US Environmental Protection
Agency, 93 pp. (EPA-650/4-75-004).
PRINZ, B. (1970) Use of statistical methods in random sampling for the
preparation of plans for measurement of emissions. Staub,
30: 204-210.
RALL, D. P. (1974) Review of the health effects of sulfur oxides.
Environ. Health Perspect., 8: 97-121.
REICHEL, G., ULMER, W. T., GARY, K., LEUSCHNER, A., & ROSKE, G. (1970)
[Influence of the locally different grade of pollution in the
township of Duisburg on the incidence of non-specific respiratory
diseases. V. Communication.] Int. Arch. Arbeitsmed., 27: 110-129
(in German).
REID, D. D., ANDERSON, D. O., FERRIS, B. G., & FLETCHER, C. M. (1970)
An Anglo-American comparison of the prevalence of bronchitis.
Br. med. J., 2: 1487-1491.
ROBINSON, E. & ROBBINS, R. A. (1968) Sources, abundance and fate of
gaseous atmospheric pollutants. Menlo Park, CA, Stanford
Research Institute (Final Report, SRI project SCC-8501).
ROBINSON, E. & ROBBINS, R. A. (1972) Emissions, concentrations and
fate of gaseous atmospheric pollutants. In: Strauss, W., ed.
Air pollution control, II, New York, Wiley-Interscience,
pp. 1-93.
ROYAL COLLEGE OF PHYSICIANS, London (1970) Air pollution and health,
London, Pitman, pp. 48-57.
ROYAL MINISTRY OF FOREIGN AFFAIRS, SWEDEN (1971) Air pollution across
national boundaries. The impact on the environment of sulfur in
air and precipitation. Stockholm. Kungl. Bokytrckerict P.A.
Norsted & Söner 710396, 96 pp.
RJAZANOV, V. (1962) Sensory physiology as basis for air quality
standards. Arch. environ. Health, 5: 480-494.
RYLANDER, R. & BERGSTROM, R. (1973) Particles and SO2 -- synergistic
effects for pulmonary damage. In: Proceedings of the Third
International Clean Air Congress, Düsseldorf, 1973, Düsseldorf,
VDI Verlag, pp. A23-A25.
RYLANDER, R., OHRSTROM, M., HELLSTROM, P. A., & BERGSTROM, R. (1971)
SO2 and particles -- synergistic effects on guinea pig lungs. In:
Walton, W. H., ed. Inhaled Particles III, London, Unwin, Vol. 1,
pp. 535-540.
SALAMBERIDZE, O. P. (1969) [The joint action of small concentrations
of sulfur dioxide and nitrogen dioxide gases under conditions of a
chronic test.] Gig. i Sanit., 34(4): 10-14 (in Russian).
SAUCIER, J. Y. & SANSONE, E. B. (1972) The relationship between
transmittance and reflectance measurements of soiling index.
Atmos. Environ., 6: 37-43.
SAWICKI, F. (1972) Chronic non-specific respiratory diseases in
Cracow. Epidemiol. Rev., 26: 229-250.
SCARINGELLI, F. B., SALTZMAN, B. E., & FREG, S. A. (1967)
Spectrophotometric determination of atmospheric sulfur dioxide.
Anal. Chem., 39: 1709-1719.
SCHIMMEL, H. & GREENBERG, L. (1972) A study of the relation of
pollution to mortality, New York City, 1963-1968, J. Air Pollut.
Control Assoc., 22: 607-616.
SCHIMMEL, H. & MURAWSKI, T. J. (1975) SO2 -- Harmful pollutant or air
quality indicator? J. Air Pollut. Control Assoc., 25: 739-740.
SCHMIDT, P., PETR, B., & PICKO, V. (1966) [The indices of white blood
sequence, the tonsils and neck lymphatic nodules state as the
diagnostic criteria of subtle changes within the child's
organism.] Cesk. Hyg., 11: 473-478 (in Czech).
SCHRENK, H. H., HEIMANN, H., CLAYTON, G. O., GAFAER, W. M., & WEXLER,
H. (1949) Air pollution, Donora, Pennsylvania -- Epidemiology of
the unusual smog episode of October 1948. Public Health Bull.
(Fed. Sec. Agency, Washington DC). 306: 1-173.
SCHUSKY, J. (1966) Public awareness and concern with air pollution in
the St. Louis, metropolitan area. J. Air Pollut. Control Assoc.,
16: 72-76.
SHERWOOD, R. J. (1969) Miniature air samplers for sulfur dioxide.
Am. Ind. Hyg. Assoc. J., 30: 614-619.
SHERWOOD, R. J. & GREENHALGH, D. M. S. (1960) A personal air sampler.
Ann. occup. Hyg., 2: 127-132.
SIM, V. M. & PATTLE, R. E. (1957) Effect of possible smog irritants on
human subjects. J. Am. Med. Assoc., 165: 1918-1913.
SJORSTRAND, T. (1947) Changes in respiratory organs of workmen at an
ore smelting works. Acta Med. Scand., 196 (Suppl.): 687-699.
SKALPE, I. O. (1964) Long term effects of sulfur dioxide exposure in
pulp mills. Br. J. ind. Med., 21: 69-73.
SKVORCOVA, N. N., OSINCEVA, V. P., PUSKINA, N. N., & VYSOCINA, I. V.
(1973) [Role of some air pollutants in the genesis of lung tumour
provoked by chemical carcinogens. In: Prevention of environmental
pollution due to carcinogenic substances.], Moscow, Medicina,
pp. 61-64 (in Russian).
SNELL, R. E. & LUCHSINGER, P. C. (1969) Effects of sulfur dioxide on
expiratory flow rates and total respiratory resistance in normal
human subjects. Arch. environ. Health, 18: 693-698.
SOFOLUWE, G. O. (1968) Smoke pollution in dwellings of infants with
bronchopneumomia. Arch. environ. Health, 16: 670-672.
SPEDDING, D. J. (1972) Sulfur dioxide absorption by sea water. Atmos.
Environ., 6: 583-586.
SPEIZER, F. E. & FRANK, N. R. (1966a) The uptake and release of SO2
by the human nose. Arch. environ. Health, 12: 725-728.
SPEIZER, F. E. & FRANK, N. R. (1966b) A comparison of changes in
pulmonary flow resistance in healthy volunteers acutely exposed to
SO2 by mouth and nose. Br. J. ind. Med., 23: 75-79.
STALKER, W. W. & ROBINSON, C. B. (1967) A method for using air
pollution measurements and public opinion to establish ambient air
quality standards. J. Air Pollut. Control Assoc., 17: 142-144.
STOCKS, P. (1959) Cancer and bronchitis mortality in relation to
atmospheric deposit and smoke. Br. med. J., 1: 74-79.
STOCKS, P. (1966) Recent epidemiological studies of lung cancer
mortality, cigarette smoking and air pollution, with discussion of
a new hypothesis of causation. Br. J. Cancer, 20: 495-623.
STOCKS, P. (1967) Lung cancer and bronchitis in relation to cigarette
smoking and fuel consumption in twenty countries. Br. J. prev.
soc. Med., 21: 181-185.
STRANDBERG, L. G. (1964) SO2 absorption in the respiratory tract.
Arch. environ. Health, 9: 160-166.
STUART, B. O. (1973) Deposition of inhaled aerosols. Arch. intern.
Med., 131: 60-73.
SYMON, K., PETR, B., & KAPALIN, V. (1966) [The influence of pollution
caused by gaseous pollutants on the health of children.] Prakt.
Lek., 46: 19-22 (in Czech).
TANI, S. (1975) [Epidemiological study on chronic bronchitis.] Jpn.
J. public Health, 22: 431-438 (in Japanese).
TASK GROUP ON AIR POLLUTION AND CANCER (unpublished) Air pollution and
cancer. Risk assessment methodology and epidemiological evidence.
Environ. Health Perspect. (March, 1978.)
TASK GROUP ON LUNG DYNAMICS (1966) Deposition and retention models for
internal dosimetry of the human respiratory tract. Health Phys.,
12: 173-207.
TASK GROUP ON METAL ACCUMULATION (1973) Accumulation of toxic metals
with special reference to their absorption, excretion and
biological half-times. Environ. Physiol. Biochem., 3: 65-107.
TEN BRUGGEN CATE, H. J. (1968) Dental erosion in industry. Br. J.
ind. Med., 25: 249-266.
THOMAS, R. L., DHARMARAJAN, V., LUNDQUIST, G. L., & WEST, P. W. (1976)
Measurement of sulfuric acid aerosol, sulfur trioxide and the
total sulfate content of the ambient air. Anal. Chem.,
48: 639-642.
TOIGO, A., IMARISIO, J. J., MURMALL, H., & LEPPER, M. N. (1963)
Clearance of large carbon particles from the human
tracheobronchial tree. Am. Rev. respir. Dis., 87: 487-492.
TOMENIUS, L. (1973) A study on the role of deposition for
tracheobronchial clearance. Physiol. Biochem., 3: 111-116.
TOYAMA, T. & NAKAMURA, K. (1964) Synergistic response of hydrogen
peroxide aerosols and sulfur dioxide to pulmonary airway
resistance. Ind. Health, 2: 34-45.
TOYAMA T., KAHYO, H., NAKAMURA, K., KAGAWA, J., YAKURA, S., ADACHI,
S., YAMAMOTO, N., IRIYAMA, F., KUMAGAYA, F., OSAWA, S., &
NAKAMURA, T. (1966) [Study on the prevalence of respiratory
symptoms in a rural area (Kashima, Ibaragi Pref.) in Japan.]
J. Jpn Soc. Air Pollut., 1: 24-35 (in Japanese).
TREON, J. F., DUTRA, F. R., CAPPEL, J., SIGMON, H., & YOUNKER, W.
(1950) Toxicity of sulfuric acid mist. Arch. ind. Hyg. occup.
Med., 2: 716-734.
TSUNETOSHI, Y., SHIMIZU, T., TAKAHASHI, H., ICHINOSAWA, A., UEDA, M.,
NAKAYAMA, N., YAMAGATA, Y., & OHSHINO, A. (1971) Epidemiological
study of chronic bronchitis with special reference to effect of
air pollution. Int. Arch. Arbeitsmed., 29: 1-27.
TURNER, D. B. (1968) Workbook of atmospheric dispersion estimates,
Washington, DC, US Department of Health, Education and Welfare,
84 pp.
ULMER, W. T., REICHEL, G., CZEIKE, A., & LEUSCHNER, A. (1970)
[Regional incidence of non-specific respiratory diseases. IV.
Communication.] Int. Arch. Arbeitsmed., 27: 73-109 (in German).
UNITED STATES DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1962)
Air pollution measurements of the National Air Sampling Network
1957-1961, Cincinnati, OH, Division of Air Pollution, pp. 3-4
(Publ. No. 978).
UNITED STATES DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1969)
Air quality criteria for particulate matter, Washington, DC,
DHEW, National Air Pollution Control Administration, pp. 1-211
(AP. 49).
US ENVIRONMENTAL PROTECTION AGENCY (1974a) Air quality data -- 1972
annual statistics, Research Triangle Park, NC, USEPA, 145 pp.
(EPA450/2-74-001).
US ENVIRONMENTAL PROTECTION AGENCY (1974b) Health consequences of
sulfur oxides. A report from CHESS, 1970-71, Research Triangle
Park, NC, US Government Printing Office, 265 pp.
(EPA650/1-74-604).
VAN DER LENDE, R. (1969) Epidemiology of chronic non-specific lung
disease (chronic bronchitis), Thesis, University of Groningen,
2 vols. Assen, Van Gorcum.
VAN DER LENDE, R., VISSER, B. F., WEVER-HESS, J., TAMMELIFG, G. J., DE
VRIES, K., ORIE, N. G. M. (1973) Epidemiological investigations in
the Netherlands into the influence of smoking and atmospheric
pollution on respiratory symptoms and lung function disturbances.
Pneumologie, 149: 119-126.
VAN DER LENDE, R., HUYGEN, C., JANSEN-KOSTER, E. J., KNIFPSTRA, S.,
PESEL, R., VISSER, B. F., WOLFS, E. H. E., & ORIE, N. G. M. (1975)
A temporary decrease in the ventilatory function of an urban
population during an acute increase in air pollution. Bull.
Physiopathol. Respir., 11: 31-43.
VAN DOP, H. & KRUIZINGA, S. (1976) The decrease of sulfur dioxide
concentration near Rotterdam and their relation to some
meteorological parameters during thirteen consecutive winters
(1961-74). Atmos. Environ., 10: 1-4.
VEREIN DEUTSCHER INGENIEURE (1974) [Measurements of the wet
concentrations of particles in the outdoor air (LIB-filter method)
VDI2463 Sheet 4.] (in German).
VIGDORCIK, E. A. (1948) Retention of inhaled aerosols (resume),
Leningrad, Leningrad Research Institute of Labour Hygiene and
Occupational Health Publication, Vol. XI, Part 2, pp. 227-230.
WALLER, R. E. (1963) Acid droplets in town air. Int, J. Air Water
Pollut., 7: 773-778.
WALLER, R. E. (1967) Studies on the nature of urban air pollution. In:
Conference on Museum Climatology, London, International
Institute of Works of Art, pp. 65-69.
WALLER, R. E. (1971) Air pollution and community health. J. R. Coll.
Physicians, Lond., 5: 362-368
WALLER, R. E. & COMMINS, B. T. (1966) High pollution episodes in
London, 1952-1966. In: Proceedings of the International Clean Air
Conference, London, Natural Society of Clean Air, pp. 228-231.
WALLER, R. E. & COMMINS, B. T. (1967) Studies of the smoke and
polycyclic aromatic hydrocarbon content of the air in large urban
areas. Environ. Res., 1: 295-306.
WALLER, R. E., BROOKS, A. G. F., & CARTWRIGHT, J. (1963) An electron
microscope study of particles in town air. Int. J. Air Water
Pollut. Control., 7: 779-786.
WALLER, R. E., COMMINS, B. T., & LAWTHER, P. J. (1965) Air pollution
in a city street. Br. J. ind. Med., 22: 128-138.
WALLER, R. E., LAWTHER, P. J., & MARTIN, A. E. (1969) Clean air and
health in London. In: Proceedings of the International Clean Air
Conference, London, Natural Society of Clean Air, pp. 71-79.
WARNER, C. G., DAVIES, G. M., JONES, J. G., & LOWE, C. R. (1969)
Bronchitis in two integrated steel works, II. Sulfur dioxide and
particulate atmospheric pollution in and around the two works.
Ann. occup. Hyg., 12: 151-170.
WATANABE, H. (1966) [Health effects of air pollution in Osaka City.]
J. Osaka Life Hyg. Assoc., 10: 147-157 (in Japanese).
WEATHERLEY, M. L. (1966) Air pollution inside the home. Int. J. Air
Water Pollut. Control, 10: 404-409.
WEATHERLEY, M. L., GOORIAH, B. D., & CHARNOCK, J. (1976) Fuel
consumption, smoke and sulfur dioxide emissions and
concentrations, and grit and dust deposition in the U.K. up to
1973-74. Stevenage, UK, Warren Spring Laboratory
(WSL Report LR214AP).
WEST, P.M. & GAEKE, G. (1956) Fixation of sulfur dioxide as
sulfitomercurate III and subsequent calorimetric determination.
Anal. Chem., 28: 1816-1819.
WILLEKE, K. & WHITBY, K. T. (1975) Atmospheric aerosols: size
distribution interpretation. J. Air Pollut. Control Assoc.,
25: 529-534.
WILLIAMS, F. P. (1960) Pollution levels in cities. In: Proceedings of
the Harrogate Conference, London Natural Society of Clean Air,
pp. 83-88.
WILLIAMS, M. K. (1970) Sickness absence and ventilatory capacity of
workers exposed to sulfuric acid mist. Br. J. ind. Med.,
27: 61-66.
WILSON, W. E., Jr & LEVY, A. (1970) A study of sulfur dioxide in
photochemical smog. I. Effect of SO2 and water vapour
concentration in the 1-butene/NOx/SO2 system. J. Air Pollut.
Control Assoc., 20: 385-390.
WORLD HEALTH ORGANIZATION (1960) WHO Technical Report Series No. 192
(Epidemiology of cancer of the lung: Report of a study group).
Geneva, WHO, 13 pp.
WORLD HEALTH ORGANIZATION (1971) International standards for
drinking-water. Geneva, WHO, 70 pp.
WORLD HEALTH ORGANIZATION (1972) WHO Technical Report Series No. 506
(Air quality criteria and guides for urban air pollutants),
Geneva, WHO, 35 pp.
WORLD HEALTH ORGANIZATION (1974) WHO Technical Report Series No. 539
(Toxicological evaluation of certain food additives with a review
of general principles and of specifications), Geneva, WHO, 40 pp.
WORLD HEALTH ORGANIZATION (1976a) Selected methods of measuring air
pollutants, Geneva, WHO, 110 pp. (WHO Offset Publ. No. 24).
WORLD HEALTH ORGANIZATION (1976b) Air quality in selected urban
areas, 1973-1974, Geneva, WHO, 65 pp. (WHO Offset Publ.,
No. 30).
WORLD HEALTH ORGANIZATION (1977) Air monitoring programme design for
urban and industrial areas, Geneva, WHO, 46 pp. (WHO Offset
Publ., No. 33).
WORLD METEOROLOGICAL ORGANIZATION (1974) WMO operations manual for
sampling and analysis techniques for chemical constituents in air
and precipitation, Geneva, WMO, 56 pp. (No. 299).
YOCOM, J. E., CLINK, W. L., & COTE, W. A. (1971) Indoor/outdoor air
quality relationships. J. Air Pollut. Control Assoc.,
21: 251-259.
YOKOYAMA, E., YODER, R. E., & FRANK, N. R. (1971) Distribution of 35S
in the blood and its excretion in urine of dogs exposed to
35SO2. Arch. environ. Health, 22: 389-395.
YOSHIDA, K., OSHIMA, H., & IMAI, M. (1966) Air pollution and asthma in
Yokkaichi. Arch. environ. Health, 13: 763-768.
YOSHII, M., NONOYAMA, J., OSHIMA, H., YAMAGIWA, H., & TAKEDA, S.
(1969) Chronic pharyngitis in air-polluted districts of Yokkaichi
in Japan. Mie med. J., 19: 17-27.
ZARKOWER, A. (1972) Alterations in antibody response induced by
chronic inhalation of SO2 and carbon. Arch. environ. Health,
25: 45-50.
ZEEDUK, H. & VELDS, C. A. (1973) The transport of sulfur dioxide over
a long distance. Atmos. Environ., 7: 849-862.
