
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 134
CADMIUM
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
First draft prepared by Dr. L. Friberg and Dr C.G. Elinder
(Karolinska Institute, Sweden) and Dr. T. Kjellstr÷m
(University of Auckland, New Zealand)
World Health Organization
Geneva, 1992
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Cadmium.
(Environmental health criteria ; 134)
1.Cadmium - adverse effects 2.Cadmium-toxicity
3.Environmental exposure 4.Environmental pollutants
I.Series
ISBN 92 4 157134 9 (NLM Classification: QV 290)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1992
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR CADMIUM
1. SUMMARY AND CONCLUSIONS
1.1 Identity, physical and chemical properties,
and analytical methods
1.2 Sources of human and environmental exposure
1.3 Environmental levels and human exposure
1.4 Kinetics and metabolism in laboratory animals
and humans
1.5 Effects on laboratory mammals
1.6 Effects on humans
1.7 Evaluation of human health risks
1.7.1 Conclusions
1.7.1.1 General population
1.7.1.2 Occupationally exposed population
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1 Physical and chemical properties
2.2 Analytical methods
2.2.1 Collection and preparation of samples
2.2.2 Separation and concentration
2.2.3 Methods for quantitative determination
2.2.3.1 Atomic absorption spectrometry
2.2.3.2 Electrochemical methods
2.2.3.3 Activation analysis
2.2.3.4 In vivo methods
2.3 Quality control and quality assurance
2.3.1 Principles and need for quality control
2.3.2 Comparison of methods and laboratories
2.3.3 Quality assurance
2.4 Conclusions
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence and cycling
3.2 Production
3.3 Uses
3.4 Sources of environmental exposure
3.4.1 Sources of atmospheric cadmium
3.4.2 Sources of aquatic cadmium
3.4.3 Sources of terrestrial cadmium
3.5 Conclusions
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Atmospheric deposition
4.2 Transport from water to soil
4.3 Uptake from soil by plants
4.4 Transfer to aquatic and terrestrial organisms
4.5 Conclusions
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Inhalation route of exposure
5.1.1 Ambient air
5.1.2 Air in the working environment
5.1.3 The smoking of tobacco
5.2 Ingestion routes of exposure
5.2.1 Levels in drinking-water
5.2.2 Levels in food
5.2.3 Other sources of exposure
5.2.4 Daily intake of cadmium from food
5.3 Total intake and uptake of cadmium from all
environmental pathways
5.3.1 General population, uncontaminated areas
5.3.2 General population, contaminated areas
5.3.3 Occupational exposure to cadmium
5.4 Conclusions
6. KINETICS AND METABOLISM IN LABORATORY MAMMALS AND HUMANS
6.1 Uptake
6.1.1 Absorption by inhalation
6.1.2 Absorption via the intestinal tract
6.1.3 Absorption via skin
6.1.4 Transplacental transfer
6.2 Transport
6.3 Distribution
6.3.1 In animals
6.3.1.1 Single exposure
6.3.1.2 Repeated exposure
6.3.2 In humans
6.4 Body burden and kidney burden in humans
6.5 Elimination and excretion
6.5.1 Urinary excretion
6.5.1.1 In animals
6.5.1.2 In humans
6.5.2 Gastrointestinal and other routes of
excretion
6.6 Biological half-time and metabolic models
6.6.1 In animals
6.6.2 In humans
6.7 Biological indices of cadmium exposure, body
burden, and concentrations in kidneys
6.7.1 Urine
6.7.2 Blood
6.7.3 Faeces
6.7.4 Hair
6.8 Metallothionein
6.8.1 Nature and production
6.8.2 The role of metallothionein in transport,
metabolism, and toxicity of cadmium
6.9 Conclusions
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Lethal dose and lethal effects
7.1.2 Pathological changes affecting specific
systems in the body
7.1.2.1 Acute effects on testes and ovaries
7.1.2.2 Acute effects on other organs
7.2 Repeated and/or long-term exposure
7.2.1 Effects on the kidneys
7.2.1.1 Oral route
7.2.1.2 Respiratory route
7.2.1.3 Injection route
7.2.1.4 Pathogenesis of cadmium
nephrotoxicity
7.2.1.5 General features of renal effects;
dose-effect and dose-response
relationships
7.2.2 Effects on the liver
7.2.3 Effects on the respiratory system
7.2.4 Effects on bones and calcium metabolism
7.2.5 Effects on haematopoiesis
7.2.6 Effects on blood pressure and the cardio-
vascular system
7.2.7 Effects on reproductive organs
7.2.8 Other effects
7.3 Fetal toxicity and teratogenicity
7.4 Mutagenicity
7.5 Carcinogenicity
7.6 Host and dietary factors; interactions with other
trace elements
7.7 Conclusions
8. EFFECTS ON HUMANS
8.1 Acute effects
8.1.1 Inhalation
8.1.2 Ingestion
8.2 Chronic effects
8.2.1 Renal effects and low molecular weight
proteinuria
8.2.1.1 In industry
8.2.1.2 In the general environment
8.2.1.3 Methods for detection of tubular
proteinuria
8.2.1.4 Significance of cadmium-induced
proteinuria
8.2.1.5 Glomerular effects
8.2.1.6 Relationship between renal cadmium
levels and the occurrence of effects
8.2.1.7 Reversibility of renal effects
8.2.2 Disorders of calcium metabolism and bone
effects
8.2.2.1 In industry
8.2.2.2 In the general environment
8.2.2.3 Mechanism of cadmium-induced bone
effects
8.2.3 Respiratory system effects
8.2.3.1 Upper respiratory system
8.2.3.2 Lower respiratory system
8.2.4 Hypertension and cardiovascular disease
8.2.5 Cancer
8.2.5.1 In industry
8.2.5.2 In the general environment
8.2.6 Mutagenic effects in human cells
8.2.7 Transplacental transport and fetal effects
8.2.8 Other effects
8.3 Clinical and epidemiological studies with data
on both exposure and effects
8.3.1 Studies on respiratory disorders
8.3.2 Studies on renal disorders in industry
8.3.3 Studies on renal disorders in the general
environment
8.3.3.1 Health surveys in Japan
8.3.3.2 Toyama prefecture (Fuchu area)
8.3.3.3 Hyogo prefecture (Ikuno area)
8.3.3.4 Ishikawa prefecture (Kakehashi area)
8.3.3.5 Akita prefecture (Kosaka area)
8.3.3.6 Nagasaki prefecture (Tsushima area)
8.3.3.7 Other Japanese areas
8.3.3.8 Belgium
8.3.3.9 Shipham area in the United Kingdom
8.3.3.10 USSR
8.4 Conclusions
9. EVALUATION OF HUMAN HEALTH RISKS
9.1 Exposure assessment
9.1.1 General population exposure
9.1.2 Occupational exposure
9.1.3 Amounts absorbed from air, food, and water
9.2 Dose-effect relationships
9.2.1. Renal effects
9.2.2 Bone effects
9.2.3 Pulmonary effects
9.2.4 Cardiovascular effects
9.2.5 Cancer
9.2.6 Critical organ and critical effect
9.3 Critical concentration in the kidneys
9.3.1 In animals
9.3.2 In humans
9.4 Dose-response relationships for renal effects
9.4.1 Evaluation based on data on industrial
workers
9.4.2 Evaluation based on data on the general
population
9.4.3 Evaluation based on a metabolic model and
critical concentrations
10. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
10.1 Conclusions
10.1.1 General population
10.1.2 Occupationally exposed population
10.2 Recommendations for protection of human health
11. FURTHER RESEARCH
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RESUME ET CONCLUSIONS
RESUMEN Y CONCLUSIONES
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR CADMIUM
Members
Professor K.A. Bustueva, Communal Hygiene, Central Institute
for Advanced Medical Training, Moscow, USSR
Dr S.V. Chandra, Industrial Toxicology Research Centre, Mahatma
Gandhi Marg, Lucknow, India
Dr M.G. Cherian, Department of Pathology, University of Western
Ontario, London, Ontario, Canada (Joint Rapporteur)
Dr B.A. Fowler, School of Medicine, University of Maryland,
Baltimore, Maryland, USA (Joint Rapporteur)
Dr R.A. Goyer, Department of Pathology, University of Western
Ontario, London, Ontario, Canada (Chairman)
Professor G. Kazantzis, London School of Hygiene and Tropical
Medicine, University of London, London, United Kingdom
Professor G. Nordberg, Department of Environmental
Medicine, University of Umea, Umea, Sweden
Dr J. Parizek, Czechoslovak Academy of Sciences, Institute of
Physiology, Videnska, Prague, Czechoslovakia
Dr I. Shigematsu, Radiation Effects Research Foundation, Hijiyama
Park, Minami-Ku, Hiroshima, Japan (Vice-Chairman)
Dr M.J. Thun, Division of Epidemiology and Statistics, American
Cancer Society, Atlanta, Georgia, USA
Observers
Professor K. Nogawa, Department of Hygiene, Chiba University
School of Medicine, Chiba, Japan
Dr K. Nomiyama, Department of Environmental Health, Jichi Medical
School, Minamikawachi-Machi Kawachi-Gun, Tochigi-Ken, Japan
Secretariat
Dr G.C. Becking, International Programme on Chemical Safety,
Interregional Research Unit, World Health Organization,
Research Triangle Park, North Carolina, USA (Secretary)
Dr L. Friberg, Karolinska Institute, Department of Environmental
Hygiene, Stockholm, Sweden
Dr C.G. Elinder, Section for Renal Medicine, Department of
Internal Medicine, Karolinska Hospital, Stockholm, Sweden
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the
criteria monographs as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria monographs, readers are kindly
requested to communicate any errors that may have occurred to the
Manager of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone No. 7988400 or
7985850).
ENVIRONMENTAL HEALTH CRITERIA FOR CADMIUM
A WHO Task Group on Environmental Health Criteria for Cadmium
met in Geneva from 27 November to 1 December 1989. Dr M. Mercier,
Manager, IPCS, opened the meeting on behalf of the heads of the
three IPCS cooperating organizations (UNEP/ILO/WHO). The Task Group
reviewed and revised the draft criteria document and made an
evaluation of the risks to human health from exposure to cadmium.
The first draft of this monograph, which was reviewed by a
Working Group in January 1984, was prepared by Dr L. Friberg and
Dr C.G. Elinder (Karolinska Institute, Stockholm, Sweden), and Dr T.
Kjellström (University of Auckland, New Zealand)1. Based on the
discussions of the Working Group, recent scientific data, and
comments from the IPCS Contact Points, a Task Group draft was
prepared by Dr R. Goyer (University of Western Ontario, Canada).
The Secretariat wishes to acknowledge the contributions made by
Professor K. Tsuchiya (Keio University, Tokyo, Japan), Dr M.
Piscator (Karolinska Institute), Dr G.F. Nordberg (University of
Umea, Sweden), and Professor R. Lauwerys (University of Louvain,
Brussels, Belgium) for their preparation and review of earlier draft
document on cadmium, which assisted greatly in the preparation of
this monograph.
Dr G.C. Becking (Interregional Research Unit) and Dr P.G.
Jenkins (IPCS Central Unit) were responsible for the overall
scientific content and technical editing, respectively, of this
monograph. The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
1 Present affiliation: Division of Environmental Health, World
Health Organization
ABBREVIATIONS
AAS atomic absorption spectrometry
CC critical concentration
CI confidence interval
EEC European Economic Community
ETA electrothermal atomization
GESAMP Group of Experts on the Scientific Aspects of Marine
Pollution
GFR glomerular filtration rate
GOT glutamic-oxaloacetic transaminase
GPT glutamic-pyruvic transaminase
ICD International Classification of Diseases
IDMS isotope dilution mass spectrometry
IU international units
LDH lactate dehydrogenase
LMW low molecular weight
MMAD mass median aerodynamic diameter
PCV packed-cell volume
PMR proportional mortality rate
PMSG pregnant mare serum gonadotrophin
RBP retinal binding protein
RIA radio-immuno assay
SMR standard mortality ratio
TRP tubular reabsorption of phosphate
XRF X-ray-generated atomic fluorescence
PREFACE
The definitions of terms used in this monograph were derived
from the meeting of the Scientific Committee on the Toxicology of
Metals, Permanent Commission and International Association on
Occupational Health, in Tokyo in 1974 (Task Group on Metal Toxicity,
1976). The term "critical concentration" in an organ was defined as
"the concentration of a metal in an organ at the time any of its
cells reaches a concentration at which adverse functional changes,
reversible or irreversible, occur in the cell". These first adverse
changes would be the "critical effect". The critical concentration
is thus established on an individual level and varies between
individuals. The term "critical organ" was defined as "that
particular organ which first attains the critical concentration of a
metal under specified circumstances of exposure and for a given
population".
The dose-response relationship expressing the occurrence rate
(response) of the particular effect as a function of metal
concentration in the critical organ, displays the frequency
distribution of individual critical concentrations. In risk
estimations it is thus essential to define the variability of the
critical concentration among a population or specific group of
people.
The term that was chosen to predict the variability of the
critical concentration of cadmium occurring in a particular group of
people is the predicted prevalence of the critical concentration.
For example, the critical concentration 5 (CC5) would be the
concentration at which 5% of the population had reached their
individual critical concentrations, and the CC50 would be the
critical concentration occurring in 50% of a defined group of
people. The term "critical concentration" is synonymous with the
term "population critical concentration" used in the WHO publication
on Evaluation of Certain Food Additives and Contaminants (1989).
The critical concentrations and the dose-response relationships
are very much dependent on the definition of critical effect. The
early effects of cadmium on the kidney can be measured as an
increased urinary excretion of low molecular weight (LMW) proteins.
An operational definition is needed to create a cut-off point above
which the proteinuria indicates an "adverse functional change".
Different studies of cadmium effects have used different operational
definitions, which has made it difficult to merge the data into a
dose-response relationship. Examples of these problems are given in
section 8.3.2. The relationship between dose and different types of
effect or different severities of the same effect is called the
dose-effect relationship.
In animal studies, the individual critical concentrations have
not been calculated. Both dose and effect data are based on groups
of animals, and these groups are usually rather small (section 7).
Few animal studies attempt to quantitatively measure the
dose-response relationships within the group (section 7.2.1.4). The
reports of effects occurring at a certain concentration of cadmium
in the kidney cortex may therefore best be interpreted as the
concentration at which 50% or more of the animals suffered the
effect. A 5-10% response will occur at lower cadmium concentrations.
The effects of cadmium on the environment are discussed in
Environmental Health Criteria 135: Cadmium - Environmental Aspects
(WHO, in press).
1. SUMMARY AND CONCLUSIONS
1.1 Identity, physical and chemical properties, and analytical
methods
Several methods are available for the determination of cadmium
in biological materials. Atomic absorption spectrometry is the most
widely used, but careful treatment of samples and correction for
interference is needed for the analysis of samples with low cadmium
concentrations. It is strongly recommended that analysis be
accompanied by a quality assurance programme. At present, it is
possible under ideal circumstances to determine concentrations of
about 0.1 µg/litre in urine and blood and 1-10 µg/kg in food and
tissue samples.
1.2 Sources of human and environmental exposure
Cadmium is a relatively rare element and current analytical
procedures indicate much lower concentrations of the metal in
environmental media than did previous measurements. At present, it
is not possible to determine whether human activities have caused a
historic increase in cadmium levels in the polar ice caps.
Commercial cadmium production started at the beginning of this
century. The pattern of cadmium consumption has changed in recent
years with significant decreases in electroplating and increases in
batteries and specialized electronic uses. Most of the major uses of
cadmium employ cadmium in the form of com- pounds that are present
at low concentration; these features constrain the recycling of
cadmium. Restrictions on certain uses of cadmium imposed by a few
countries may have widespread impact on these applications.
Cadmium is released to the air, land, and water by human
activities. In general, the two major sources of contamination are
the production and consumption of cadmium and other non-ferrous
metals and the disposal of wastes containing cadmium. Areas in the
vicinity of non-ferrous mines and smelters often show pronounced
cadmium contamination.
Increases in soil cadmium content result in an increase in the
uptake of cadmium by plants; the pathway of human exposure from
agricultural crops is thus susceptible to increases in soil cadmium.
The uptake by plants from soil is greater at low soil pH. Processes
that acidify soil (e.g., acid rain) may therefore increase the
average cadmium concentrations in foodstuffs. The application of
phosphate fertilizers and atmospheric deposition are significant
sources of cadmium input to arable soils in some parts of the world;
sewage sludge can also be an important source at the local level.
These sources may, in the future, cause enhanced soil and hence crop
cadmium levels, which in turn may lead to increases in dietary
cadmium exposure. In certain areas, there is evidence of increasing
cadmium content in food.
Edible free-living food organisms such as shellfish,
crustaceans, and fungi are natural accumulators of cadmium. As in
the case of humans, there are increased levels of cadmium in the
liver and kidney of horses and some feral terrestrial animals.
Regular consumption of these items can result in increased exposure.
Certain marine vertebrates contain markedly elevated renal cadmium
concentrations, which, although considered to be of natural origin,
have been linked to signs of kidney damage in the organisms
concerned.
1.3 Environmental levels and human exposure
The major route of exposure to cadmium for the non-smoking
general population is via food; the contribution from other pathways
to total uptake is small. Tobacco is an important source of cadmium
uptake in smokers. In contaminated areas, cadmium exposure via food
may be up to several hundred µg/day. In exposed workers, lung
absorption of cadmium following inhalation of workplace air is the
major route of exposure. Increased uptake can also occur as a
consequence of contamination of food and tobacco.
1.4 Kinetics and metabolism in laboratory animals and humans
Data from experimental animals and humans have shown that
pulmonary absorption is higher than gastrointestinal absorption.
Depending on chemical speciation, particle size, and solubility in
biological fluids, up to 50% of the inhaled cadmium compound may be
absorbed. The gastrointestinal absorption of cadmium is influenced
by the type of diet and nutritional status. The nutritional iron
status appears to be of particular importance. On average, 5% of the
total oral intake of cadmium is absorbed, but individual values
range from less than 1% to more than 20%. There is a maternal-fetal
gradient of cadmium. Although cadmium accumulates in the placenta,
transfer to the fetus is low. Cadmium absorbed from the lungs or the
gastrointestinal tract is mainly stored in the liver and kidneys,
where more than half of the body burden will be deposited. With
increasing exposure intensity, an increasing proportion of the
absorbed cadmium is stored in the liver. Excretion is normally slow,
and the biological half-time is very long (decades) in the muscles,
kidneys, liver, and whole body of humans. The cadmium concentrations
in most tissues increase with age. Highest concentrations are
generally found in the renal cortex, but excessive exposures may
lead to higher concentrations in the liver. In exposed people with
renal damage, urinary excretion of cadmium increases and so the
whole body half-time is shortened. The renal damage leads to losses
of cadmium from the kidney, and the renal concentrations of cadmium
will eventually be lower than in people with similar exposure but
without renal damage.
Metallothionein is an important transport and storage protein
for cadmium and other metals. Cadmium can induce metallothionein
synthesis in many organs including the liver and kidney. The binding
of intracellular cadmium to metallothionein in tissues protects
against the toxicity of cadmium. Cadmium not bound to
metallothionein may therefore play a role in the pathogenesis of
cadmium-related tissue injury. The speciation of other cadmium
complexes in tissues or biological fluids is unknown.
Urinary excretion of cadmium is related to body burden, recent
exposure, and renal damage. In people with low exposure, the urine
cadmium level is mainly related to the body burden. When
cadmium-induced renal damage has occurred, or even without renal
damage if exposure is excessive, urinary excretion increases.
Cadmium-exposed people with proteinuria generally have higher
cadmium excretion than such people without proteinuria. After high
exposure ceases, the urine cadmium level will decrease even though
renal damage persists. The interpretation of urinary cadmium is thus
dependent on a number of factors. Gastrointestinal excretion is
approximately equal to urinary excretion but cannot be easily
measured. Other excretory routes such as lactation, sweating or
placental transfer are insignificant.
The level of cadmium in faeces is a good indicator of recent
daily intake from food in the absence of inhalation exposure.
Cadmium in blood occurs mainly in the red blood cells, and the
plasma concentrations are very low. There are at least two
compartments in blood, one related to recent exposure with a
half-time of about 2-3 months, and one which is probably related to
body burden with a half-time of several years.
1.5 Effects on laboratory mammals
High inhalation exposures cause lethal pulmonary oedema. Single
high-dose injection gives rise to testicular and non-ovulating
ovarian necrosis, liver damage, and small vessel injury. Large oral
doses damage the gastric and intestinal mucosa.
Long-term inhalation exposure and intratracheal administration
give rise to chronic inflammatory changes in the lungs, fibrosis,
and appearances suggestive of emphysema. Long-term parenteral or
oral administration produces effects primarily on the kidneys, but
also on the liver and the haematopoietic, immune, skeletal, and
cardiovascular systems. Skeletal effects and hypertension have been
induced in certain species under defined conditions. The occurrence
of teratogenic effects and placental damage depends on the stage of
gestation at which exposure occurs, and may involve interactive
effects with zinc.
Of greatest relevance to human exposure are the acute
inhalation effects on the lung and the chronic effects on the
kidney. Following long-term exposure, the kidney is the critical
organ. The effects on the kidney are characterized by tubular
dysfunction and tubular cell damage, although glomerular dysfunction
may also occur. A consequence of renal tubular dysfunction is a
disturbance of calcium and vitamin D metabolism. According to some
studies, this has led to osteomalacia and/or osteoporosis, but these
effects have not been confirmed by other studies. A direct effect of
cadmium on bone mineralization cannot be excluded. The toxic effects
of cadmium in experimental animals are influenced by genetic and
nutritional factors, interactions with other metals, particularly
zinc, and pretreatment with cadmium, which may be related to the
induction of metallothionein.
In 1976 and 1987, the International Agency for Research on
Cancer accepted as sufficient the evidence that cadmium chloride,
sulfate, sulfide, and oxide can give rise to injection site sarcomas
in the rat and, for the first two compounds, induce interstitial
cell tumours of the testis in rats and mice, but found oral studies
inadequate for evaluation. Long-term inhalation studies in rats
exposed to aerosols of cadmium sulfate, cadmium oxide fumes and
cadmium sulfate dust demonstrated a high incidence of primary lung
cancer with evidence of a dose-response relationship. However, this
has not so far been demonstrated in other species. Studies on the
genotoxic effects of cadmium have given discordant results.
1.6 Effects on humans
High inhalation exposure to cadmium oxide fume results in acute
pneumonitis with pulmonary oedema, which may be lethal. High
ingestion exposure of soluble cadmium salts causes acute
gastroenteritis.
Long-term occupational exposure to cadmium has caused severe
chronic effects, predominantly in the lungs and kidneys. Chronic
renal effects have also been seen among the general population.
Following high occupational exposure, lung changes are
primarily characterized by chronic obstructive airway disease. Early
minor changes in ventilatory function tests may progress, with
continued cadmium exposure, to respiratory insufficiency. An
increased mortality rate from obstructive lung disease has been seen
in workers with high exposure, as has occurred in the past.
The accumulation of cadmium in the renal cortex leads to renal
tubular dysfunction with impaired reabsorption of, for instance,
proteins, glucose, and amino acids. A characteristic sign of tubular
dysfunction is an increased excretion of low molecular weight
proteins in urine. In some cases, the glomerular filtration rate
decreases. Increase in urine cadmium correlates with low molecular
weight proteinuria and in the absence of acute exposure to cadmium
may serve as an indicator of renal effect. In more severe cases
there is a combination of tubular and glomerular effects, with an
increase in blood creatinine in some cases. For most workers and
people in the general environment, cadmium-induced proteinuria is
irreversible.
Among other effects are disturbances in calcium metabolism,
hypercalciuria, and formation of renal stones. High exposure to
cadmium, most probably in combination with other factors such as
nutritional deficiencies, may lead to the development of
osteoporosis and/or osteomalacia.
There is evidence that long-term occupational exposure to
cadmium may contribute to the development of cancer of the lung but
observations from exposed workers have been difficult to interpret
because of confounding factors. For prostatic cancer, evidence to
date is inconclusive but does not support the suggestion from
earlier studies of a causal relationship.
At present, there is no convincing evidence for cadmium being
an etiological agent of essential hypertension. Most data speak
against a blood pressure increase due to cadmium and there is no
evidence of an increased mortality due to cardiovascular or
cerebrovascular disease.
Data from studies on groups of occupationally exposed workers
and on groups exposed in the general environment show that there is
a relationship between exposure levels, exposure durations, and the
prevalence of renal effects.
An increased prevalence of low molecular weight proteinuria in
cadmium workers after 10-20 years of exposure to cadmium levels of
about 20-50 µg/m3 has been reported.
In polluted areas of the general environment, where the
estimated cadmium intake has been 140-260 µg/day, effects in the
form of increased low molecular weight proteinuria have been seen in
some individuals following long-term exposure. More precise
dose-response estimates are given in section 8.
1.7 Evaluation of human health risks
1.7.1 Conclusions
The kidney is considered the critical target organ for the
general population as well as for occupationally exposed
populations. Chronic obstructive airway disease is associated with
long-term high-level occupational exposure by inhalation. There is
some evidence that such exposure to cadmium may contribute to the
development of cancer of the lung but observations from exposed
workers have been difficult to interpret because of confounding
factors.
1.7.1.1 General population
Food-borne cadmium is the major source of exposure for most
people. Average daily intakes from food in most areas not polluted
with cadmium are between 10-40 µg. In polluted areas it has been
found to be several hundred µg per day. In non-polluted areas,
uptake from heavy smoking may equal cadmium intake from food.
Based on a biological model, an association between cadmium
exposure and increased urinary excretion of low molecular weight
proteins has been estimated to occur in humans with a life-long
daily intake of about 140-260 µg cadmium, or a cumulative intake of
about 2000 mg or more.
1.7.1.2 Occupationally exposed population
Occupational exposure to cadmium is mainly by inhalation but
includes additional intakes through food and tobacco. The total
cadmium level in air varies according to industrial hygiene
practices and type of workplace. There is an exposure-response
relationship between airborne cadmium levels and proteinuria. An
increase in the prevalence of low molecular weight proteinuria may
occur in workers after 10-20 years of exposure to cadmium levels of
about 20-50 µg/m3. In vivo measurement of cadmium in the liver
and kidneys of people with different levels of cadmium exposure have
shown that about 10% of workers with a kidney cortex level of
200 mg/kg and about 50% of people with a kidney cortex level of
300 mg/kg would have renal tubular proteinuria.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND
ANALYTICAL METHODS
This monograph covers cadmium and its inorganic compounds
alone, since there is no evidence that organocadmium compounds
(where the metal is bound covalently to carbon) occur in nature.
Although cadmium may bind to proteins and other organic molecules
and form salts with organic acids (e.g., cadmium stearate), in these
forms it is regarded as inorganic.
The mobility of cadmium in the environment and the effects on
the ecosystem depend to a large extent on the nature of its
compounds.
Since this monograph evaluates only the health hazards for
humans (and not those for the environment), only chemical data on
cadmium compounds relevant for such an evaluation are included. Data
on cadmium compounds occurring in or toxic to lower animals and
plants are reviewed in Environmental Health Criteria 135:
Cadmium - Environmental Aspects (WHO, in press).
2.1 Physical and chemical properties
Cadmium (atomic number 48; relative atomic mass 112.40) is a
metal that belongs, together with zinc and mercury, to group IIb in
the Periodic Table. Naturally-occurring isotopes are 106 (1.22%),
108 (0.88%), 110 (12.39%), 111 (12.75%), 112 (24.07%), 113 (12.26%),
114 (28.86%), and 116 (7.50%) (Weast, 1974).
Cadmium has a relatively high vapour pressure. Its vapour is
oxidized rapidly in air to produce cadmium oxide. When reactive
gases or vapour, such as carbon dioxide, water vapour, sulfur
dioxide, sulfur trioxide or hydrogen chloride are present, cadmium
vapour reacts to produce cadmium carbonate, hydroxide, sulfite,
sulfate or chloride, respectively. These compounds may be formed in
stacks and emitted to the environment. An example of these reactions
during cadmium emissions from coal-fired power plants is described
by Kirsch et al. (1982).
Some cadmium compounds, such as cadmium sulfide, carbonate, and
oxide, are practically insoluble in water. There is, however, a lack
of data on the solubility of these compounds in biological fluids,
e.g., in the gastrointestinal tract and lung. These water-insoluble
compounds can be changed to water-soluble salts in nature under the
influence of oxygen and acids; cadmium sulfate, nitrate, and halides
are water-soluble. Most of the cadmium found in mammals, birds, and
fish is probably bound to protein molecules.
The speciation of cadmium in soil, plants, animal tissues, and
foodstuffs may be of importance for the evaluation of the health
hazards associated with areas of cadmium contamination or high
cadmium intake. For example, although soil cadmium levels in
Shipham, United Kingdom, were found to be very much higher than in
Toyama, Japan, cadmium uptake by edible plants in Shipham was only a
small fraction of that in Toyama (Tsuchiya, 1978; Sherlock et al.,
1983). Very few data on the occurrence and speciation of cadmium
compounds in nature are available.
2.2 Analytical methods
Only a few nanograms (or even less) of cadmium may be present
in collected samples of air and water, whereas hundreds of
micrograms may be present in small samples of kidney, sewage sludge,
and plastics. Different techniques are therefore required for the
collection, preparation, and analysis of the samples.
In general, the technique available for measuring cadmium in
the environment and in biological materials cannot differentiate
between the different compounds. With special separation techniques,
cadmium-containing proteins can be isolated and identified. In most
studies to date, the concentration or amount of cadmium in water,
air, soil, plants, and other environmental or biological materials
has been determined as the element.
2.2.1 Collection and preparation of samples
The degree of uncertainty in any health risk assessment of
cadmium based on the analysis of environmental or biological samples
depends on how representative the samples are. Each type of material
has specific problems in this respect, and each study should include
an evaluation of the sampling procedures utilized. For example, the
measurement of cadmium in workplace air can be made with "static"
samples or "personal" samples. The latter supposedly gives a better
estimate of true exposure levels. When both are measured, personal
samples usually give higher results, indicating that static samples
may underestimate the exposure.
For the collection of samples, standard trace element methods
can generally be used (LaFleur, 1976; Behne, 1980). The amount of
material needed for analysis varies according to the sensitivity of
the analytical methods and the cadmium concentration in the
material. During recent years, methods have improved and usually
smaller amounts (ml or g) of biological materials are now needed
than those required previously.
In the handling and storage of samples, particularly liquid
samples, special care must be taken to avoid contamination. Coloured
materials in containers, especially plastics and rubber, should be
avoided. Contamination of blood samples has been reported when blood
was collected in certain types of evacuated blood collection tubes
(Nackowski et al., 1977; Nise & Vesterberg, 1978). Disposable
coloured micropipette tips have been found to contaminate acid
solutions with cadmium (Salmela & Vuori, 1979).
Glass and transparent cadmium-free polyethylene, polypropylene
or teflon containers are usually considered as suitable for storing
samples. All containers and glassware should be pre-cleaned in
dilute nitric acid and deionized water. Water samples or standards
with low cadmium concentrations should be stored for only a short
period of time in order to avoid possible adsorption of cadmium on
the container wall. However, experiments carried out within the
UNEP/WHO programme (Vahter, 1982; Friberg & Vahter, 1983) using
haemolysed blood samples spiked with 109Cd showed that, if
properly handled, blood can be stored at room temperature for
several months without any change in the cadmium concentration. Some
solutions, such as urine, should be acidified to prevent
precipitation of salts, thus ensuring that the cadmium remains in
solution.
To prepare samples for analysis, inorganic solid samples (such
as soil or dust samples) are usually dissolved in nitric acid or
other acids. Organic samples need to be subjected to wet ashing
(digestion) or dry ashing. Wet ashing, i.e. heating under reflux
with nitric acid followed by the addition of sulfuric or perchloric
acid, is an adequate method for the digestion of most organic and
biological samples. Heating with perchloric acid is usually avoided
in modern methods because of the explosive nature of the fumes.
Biological samples may also be dissolved using tetramethylammonium
hydroxide (Kaplan et al., 1973).
Dry ashing can also be used without significant losses of
cadmium, provided that the temperature is kept at or below 450 °C
(Kjellström et al., 1974; Koirtyohann & Hopkins, 1976).
Low-temperature (about 100 °C) dry ashing at a high oxygen
concentration has also been used successfully (Gleit, 1965).
2.2.2 Separation and concentration
Some biological samples such as kidneys contain relatively high
concentrations of cadmium; this makes it possible to analyse without
significant interference from other compounds. Dry ashing, followed
by dissolving the ash in acid, is sometimes sufficient for analysis
by atomic absorption spectrometry and other modern methods. When the
cadmium concentration is low, special treatment is sometimes needed.
The procedures for separating cadmium from interfering compounds and
concentrating the samples are very important steps in obtaining
adequate results.
One technique for the solvent extraction of cadmium, which has
been widely used, is based on the APDC/MIBK system, where ammonium
pyrrolidine dithiocarbamate chelate (APDC) is extracted into methyl
isobutyl ketone (MIBK) (Mulford, 1966; Lehnert et al., 1968). Other
chelating agents that can be used to extract cadmium into an organic
solvent are dithiozone (Saltzman, 1953) and sodium diethyl
dithiocarbamate (Berman, 1967).
Ion exchange resins have also been applied for separating and
concentrating cadmium from digested food samples (Baetz & Kenner,
1974) and from urine and blood samples acidified with hydrochloric
acid (Lauwerys et al., 1974c; Vens & Lauwerys, 1982).
2.2.3 Methods for quantitative determination
A number of methods have been developed for cadmium analysis,
but none of them are known to produce absolutely "true"
concentrations of cadmium in any material. The accuracy of a method
also depends on how high the concentration is.
The nearest approximation to the "true" value when analysing
complex organic materials with low cadmium concentration is probably
attained with the isotope dilution mass spectrometry (IDMS) method
carried out in "ultraclean" facilities. However, IDMS is extremely
expensive compared with other methods, and has been used mainly for
quality control of other methods and for certified reference
materials.
The most commonly used methods, at present, are atomic
absorption spectrometry, electrochemical methods, and neutron
activation analysis. These three methods will be discussed in detail
below. Other methods are colorimetry with dithiozone, atomic
emission spectrometry, atomic fluorescence spectrometry, and
proton-induced X-ray emissions (PIXE) analysis. Analytical methods
for cadmium have been reviewed by Friberg et al. (1986).
In addition, in vivo analysis of cadmium in kidney and liver
has been carried out by certain investigators (Ellis et al. 1981a;
Roels et al. 1981b; Roels et al. 1983a, 1983b). The method uses the
principles of neutron activation and is discussed in section 8.2.1.6
of this monograph.
The validity and accuracy of any method should ideally be
ascertained by adequate quality assurance data (section 2.3). In the
absence of such data, the results should at least be accompanied by
intra-laboratory quality control data, results of analysis of
certified standard materials, or inter-laboratory comparison data
(section 2.3). Older basic chemical analysis methods may be as
accurate as newer more complex and expensive methods, at least in
the higher concentration range, and no analytical results should be
dismissed or accepted until the method used has been carefully
evaluated.
2.2.3.1 Atomic absorption spectrometry
The basic principle is to pass the sample into a
high-temperature flame (burner) or furnace and measure the
absorption from the atoms in the ground state. A lamp with a cathode
made up from the pure metal or an alloy of the desired element,
emitting the narrow line spectrum of this element, is used as an
external light source. Atomic absorption spectrometry (AAS) is the
method most commonly used at present for cadmium determination,
because the procedure is relatively simple and fast, and its
detection limit is sufficient for most environmental and biological
materials. The absorption is measured at the cadmium line
(228.8 nm).
There are two main methods for atomization of a sample, the
flame method and electrothermal atomization (ETA). The latter is
also called the heated graphite atomization, graphite furnace or
flameless method. Flame methods are generally used for liquid
samples that can be aspirated into a flame, usually an air-acetylene
flame. The detection limit for cadmium in pure water is of the order
of 1-5 mg/litre and, in biological materials, it is about 0.1 mg/kg.
At lower levels, it is usually necessary to increase the sensitivity
by some accessory or by preconcentration during sample treatment.
One important modification of the flame technique is the use of a
micro-crucible or cup made of nickel (Delves, 1970; Fernandez &
Kahn, 1971; Ediger & Coleman, 1973). The atoms are held much longer
in the light beam that passes through the tube, and this increases
the sensitivity considerably.
ETA methods have undergone rapid development in recent years.
The sample, usually in solution (1-100 ml), is first inserted into a
graphite furnace, which is surrounded by a constant flow of inert
gas, such as argon or nitrogen. The temperature is then increased in
order to dry, ash, and atomize the sample. During atomizing, the
specific absorption from cadmium is deduced from the light beams
passing through or just above the furnace. The detection limit is
extremely low (of the order of a few pg). There have been several
detailed reports describing the analysis, using ETA, of cadmium in
biological samples such as blood and urine (Lundgren, 1976; Castilho
& Herber, 1977; Stoeppler & Brandt, 1978, 1980; Vesterberg &
Wrangskogh, 1978; Gardiner et al., 1979; Delves & Woodward, 1981;
Subramanian & Meranger, 1981; Jawaid et al., 1983). The lowest
detectable concentration of cadmium in blood and urine using ETA is
of the order of 0.1-0.3 mg/litre (Delves, 1982).
Although the atomic absorption spectrometry for cadmium is
specific, the method is not free from problems when applied to
measurements in biological samples. Several important sources of
interference exist, especially light scattering from particles and
nonspecific absorption from the broad molecular absorption band
formed by, for instance, sodium chloride and phosphate ions.
Piscator (1971) showed that sodium chloride, at a concentration of
0.5 mol/litre, gave a signal corresponding to a concentration of
0.1 mg cadmium/litre when using ordinary air-acetylene flame atomic
absorption equipment without background correction. The actual
concentration was less than 0.4 mg cadmium/litre. Many problems
related to interfering salts may be compensated by the use of a
background correction system. A deuterium or hydrogen lamp is
usually used. The nonspecific absorption can thus be measured and
the signal, measured as the difference between the specific and
nonspecific absorption, is proportional to the actual cadmium
concentration (Kahn & Manning, 1972). Background correction for fine
structure nonspecific absorption can also be made by utilizing the
Zeeman effect on incoming light when it is modulated by strong
magnetic fields (Koizumi et al., 1977; Alt, 1981; Pleban et al.,
1981). Some kind of background correction is necessary when the
microcrucible or electrothermal atomization techniques are used for
cadmium analysis, since the nonspecific absorption increases as the
atoms are kept in the light for a relatively long period of time.
2.2.3.2 Electrochemical methods
Cadmium can be determined by different types of
electro-chemical methods such as classic polarographic methods or
the more recently developed anodic stripping voltammetry and
cadmium-selective electrodes. The basic principle behind the
electrochemical methods is the change in the electrochemical
potentials formed when electrons are transferred from one metal to
another. A dropping mercury electrode is placed in a solution where
the metal concentration is to be determined. By changing the charge
of the electrode, different metals will be reduced and form an
amalgam (a solid solution of metal atoms and mercury) with the
mercury electrode. Polarographic waves can thus be recorded.
Different metals can be determined simultaneously in a liquid
sample, since they form amalgams at different charges.
Anodic stripping voltammetry is based on the reverse process,
i.e. the release of metals that have already been reduced and are
bound to the mercury electrode. During oxidation and release from
the amalgam, a peak current can be recorded at a potential that is
characteristic for the particular metal. Anodic stripping
voltammetry is one of the most sensitive methods for cadmium
determination available. The most crucial aspects are complete
destruction of all organic materials and the transfer of cadmium
ions from the sample into a non-contaminated electrolyte. The method
is especially suitable for water analysis, where no sample treatment
is necessary (Piscator & Vouk, 1979), but has also been used for the
measurement of cadmium in various biological materials such as urine
(Jagner et al., 1981), foodstuffs, and tissues (Danielsson et al.,
1981). In urine, a detection limit of about 0.1 mg/litre was
obtained when using a computerized potentiometric stripping analysis
(Jagner et al., 1981).
Specific cadmium-selective electrodes are commercially
available, but their sensitivity is insufficient for cadmium
measurement in most biological materials. Furthermore, the
electrodes are not ion specific, and problems can easily arise from
various contaminants in the solution used (Hislop, 1980).
2.2.3.3 Activation analysis
Cadmium has a number of stable isotopes. Irradiation with
neutrons yields new radioactive cadmium isotopes, which can be
quantitatively measured on the basis of their specific energy and
half-life. A procedure for determining cadmium in human liver
samples by neutron activation analysis has been reported by
Halvorsen & Steinnes (1975). The irradiated sample is usually
digested before the radioactivity is measured. Sometimes, it may be
necessary to concentrate cadmium by chemical methods and to separate
the cadmium ions from other isotopes that have an energy spectrum
overlapping the one for cadmium before measurement can be carried
out. Non-radioactive cadmium can also be added after irradiation to
enable measurement of the recovery after digestion and various
concentration steps. The detection limit for neutron activation
analysis is low, of the order of 0.1-1 mg cadmium/kg or
0.1-1 mg/litre, in most biological materials. However, the method is
expensive since the samples have to be irradiated in a reactor, and
so it is not normally used for screening programmes. Neutron
activation analysis has been used as a reference method for accuracy
tests of other methods (Kjellström et al., 1975b; Kjellström, 1979;
Jawaid et al., 1983).
Neutron activation analysis is not ideal for liquid samples
such as blood and urine, where the detection limit of the method is
very close to the normal values. Furthermore, ampoules filled with
liquid samples sometimes explode as gases are formed when the sample
is irradiated in the reactor.
Irradiation with protons, proton-induced X-ray emission (PIXE),
can also be used for activation analysis of cadmium. Several
elements are measured at the same time. The main advantage of the
method is its ability to detect and quantify cadmium in very small
samples such as thin slices of tissues weighing less than 1 mg
(Hasselmann et al., 1977; Mangelson et al., 1979).
2.2.3.4 In vivo methods
A non-invasive technique for in vivo determination of liver
and kidney cadmium has been developed (Biggin et al., 1974; Harvey
et al., 1975; McLellan et al., 1975) using the principle of neutron
activation analysis and taking advantage of the very large capture
cross-section area for thermal neutrons of one of the
naturally-occurring stable isotopes of cadmium (113Cd; natural
abundance, 12.26%). A portable system using a 238Pu-Be source of
neutrons (instead of the original, which was cyclotron dependent)
has made this technique more easily available (Thomas et al., 1976).
The lowest detection limit for "field-work" techniques
currently in use for this method is about 1.5 mg/kg in liver and
15 mg/kg in whole kidney (Ellis et al., 1981a). These limits are too
high to measure accurately tissue levels in people with "normal"
environmental exposure (section 6.4). In people with occupational
exposure, cadmium levels of up to 100 mg/kg in liver and 400 mg/kg
in whole kidney have been reported (Ellis et al., 1981a; Roels et
al., 1981b). The method is still not developed to its full capacity,
and the results are greatly affected by, for instance, the
variability in the location of the kidney (Al-Haddad et al., 1981).
An alternative method for in vivo determination of cadmium
concentration in kidney cortex using X-ray-generated atomic
fluorescence (XRF method) has been reported (Ahlgren & Mattson,
1981; Christofferson & Mattson, 1983). Skerfving et al. (1987) found
the limit of detection to be 17 µg/g kidney cortex (three standard
deviations above the background). The precision is 23%.
The validity and accuracy of these in vivo neutron activation
and XRF methods have not been studied sufficiently. A comparison of
the results obtained by in situ determination of liver and kidney
cadmium in deceased people with those found by chemical analysis of
the same tissues is needed.
2.3 Quality control and quality assurance
2.3.1 Principles and need for quality control
There is a great need for strict quality control procedures in
the monitoring of trace elements in biological materials. The
purpose of these is to ensure that published data are as accurate as
possible. Quality control involves intra-laboratory or
inter-laboratory procedures that check whether the method gives
acceptable results on samples with known concentrations. Quality
assurance is usually given a broader meaning to cover the whole
system of activities that are carried out to increase the quality of
the operation. Thus, quality assurance includes not only the
chemical analysis, but also the whole pre-analytical chain, data,
handling, reporting, etc.
A review of published data (Vahter, 1982) showed that mean
blood cadmium concentrations in the general population as high as
20-50 mg/litre have been reported. Such values are definitely
unrealistic (section 6.2). Furthermore, most published reports lack
quality control or quality assurance data. Valid comparisons of
cadmium exposure based on blood cadmium levels can, therefore,
seldom be made. Results from interlaboratory comparisons amplify the
need for quality control (section 2.3.3).
2.3.2 Comparison of methods and laboratories
As indicated above, AAS (direct or combined with a separation
procedure by organic solvent extraction or ion exchange) is the
common method and can be applied to ordinary environmental or
biological samples. Each of the other methods has its particular
characteristics and can be used effectively according to the need
for sensitivity and to the type of sample. Of special concern are
methods used for the determination of cadmium in, for instance,
food, blood, and urine, where cadmium concentrations are generally
low and the matrices are complicated. Attempts to evaluate the
accuracy by comparing the proposed method with another method have
seldom been made. When testing a new method for the determination of
cadmium or a new application of a method to a different type of
sample, it is advisable to compare it with another method based on
quite different principles.
Since the principle of neutron activation analysis is quite
different from that of other methods, it is a good method for
comparison. Thus, Linnman et al. (1973) and Kjellström et al. (1974,
1975b) found good agreement between a flameless atomic absorption
method and destructive neutron activation analysis (the sample is
irradiated and then treated chemically so the original material is
"destroyed") for cadmium in wheat at concentrations down to around
20 mg/kg wheat. In the latter study (Kjellström et al., 1975b), good
agreement was also found between cadmium concentrations in urine
(above 5 µg/litre), determined by AAS after extraction into organic
solvent, and cadmium concentrations determined by neutron
activation. Because of technical problems of neutron activation
analysis of liquid samples, Kjellström et al. (1975b) could not
evaluate the accuracy at urine concentrations of around 1 µg/litre.
However, Jawaid et al. (1983) have used neutron activation to
confirm the accuracy of atomic absorption analyses of urine in the
range of 0.2-4 µg/litre.
Further comparisons of destructive neutron activation analysis
and different AAS methods conducted in different laboratories have
been carried out for faeces, rice, wheat, liver, and muscle
(Kjellström, 1979). The best agreement was found for liver, in which
the cadmium concentrations were the highest, but, there was also
reasonable agreement between most of the methods in the case of
other materials.
Another possibility for testing a method is to add radioactive
cadmium to the samples (Kjellström et al., 1974) or to inject
radioactive cadmium into animals and then compare results of
radioactive measurements with those obtained by chemical analysis.
Since there has been a need for comparing cadmium levels in
different areas of the world, studies among laboratories in
different countries have been undertaken to ensure that the
analytical methods give comparable results.
An intercomparison programme involving several European
laboratories, which used flame atomic absorption, flameless atomic
absorption, colorimetry, polarography, and anodic stripping
voltammetry, indicated great variability in results (Lauwerys et
al., 1975). Thus, reported concentrations in the same sample of
blood were from 1 to 92 µg/litre in one case, from 0 to 73 µg/litre
in another, and from 0 to 110 mg/litre in a third. A wide range of
values was also reported in the case of aqueous solutions. Only 29%
of participating laboratories measured cadmium in blood with
sufficient precision. The conclusion from this study was that
several participating laboratories had not yet adequately developed
the technique required for precisely measuring cadmium in blood,
urine, and water.
2.3.3 Quality assurance
An extensive quality assurance programme of cadmium analysis
involving laboratories in nine different countries has been carried
out (Vahter, 1982). This was a part of the UNEP/WHO Global
Environmental Monitoring Programme and involved the analysis of
cadmium in blood and kidney tissue as well as of lead in blood. A
series of quality control samples (spiked specimens), the
concentrations being known or unknown to the participating
laboratories, was used to check the accuracy of the methods before
the population samples were analysed. This procedure was repeated up
to 12 times, development work on the methods being carried out in
between, in order to improve the accuracy of the methods. After
improvement of the techniques and practice, the agreement became
excellent. An overview of various aspects of quality assurance has
been presented by Friberg (1988).
2.4 Conclusions
There are several methods available for the determination of
cadmium in biological materials. Atomic absorption spectrometry
(AAS) is the most widely used, but careful treatment of samples and
correction for interference is needed for the analysis of samples
with low cadmium concentrations. It is strongly recommended to
accompany analysis with a quality assurance programme. At present,
it is possible under ideal circumstances to determine concentrations
of about 0.1 µg/litre in urine and blood and 1-10 µg/kg in food and
tissue samples.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
The metal cadmium belongs, together with copper and zinc, to
group IIb of the Periodic Table. It is a relatively rare element and
is not found in the pure state in nature. Cadmium is mainly
associated with the sulfide ores of zinc, lead, and copper, although
purification first took place in 1817 from zinc carbonate.
Commercial production only became significant at the beginning of
this century. Cadmium is often considered as a metal of the 20th
century; indeed, over 65% of the cumulative world production has
taken place in the last two decades (Wilson, 1988).
Cadmium is commonly regarded as a pollutant of worldwide
concern. The metal has been reviewed by the International Register
of Potentially Toxic Chemicals of the United Nations Environment
Programme. As a result, it has been included on the list of chemical
substances and processes considered to be potentially dangerous at
the global level (IRPTC, 1987).
3.1 Natural occurrence and cycling
Cadmium is widely distributed in the earth's crust at an
average concentration of about 0.1 mg/kg. However, higher levels may
accumulate in sedimentary rocks, and marine phosphates often contain
about 15 mg cadmium/kg (GESAMP, 1984). Weathering also results in
the riverine transport of large quantities of cadmium to the world's
oceans and this represents a major flux of the global cadmium cycle;
an annual gross input of 15 000 tonnes has recently been estimated
(GESAMP, 1987).
Some black shale deposits in parts of the United Kingdom and
USA contain elevated cadmium levels, thus leading to high soil
concentrations in these areas (Lund et al., 1981). High soil
concentrations are more commonly found in areas containing deposits
of zinc, lead, and copper ores. Indeed, such areas are often
characterized by both soil and aquatic contamination at the local
level. The mining of these ore bodies has further increased the
extent of such contamination. In background areas away from such
deposits, surface soil concentrations of cadmium typically range
between 0.1 and 0.4 mg/kg (Page et al., 1981) while fresh water
contains < 0.01-0.06 ng/litre (Shiller & Boyle, 1987).
Volcanic activity is a major natural source of cadmium release
to the atmosphere. Emissions of cadmium take place both during
episodic eruptions and continuous low-level activity. Difficulties
exist in quantifying the global flux from this source but an
estimate of 100-500 tonnes (Nriagu, 1979) has been made. Deep sea
volcanism is also a source of environmental cadmium release, but the
role of this process in the global cadmium cycle remains to be
quantified.
Older measurements of cadmium in the atmosphere and marine
waters from background areas generally yielded much higher values
than those obtained by more recent studies. Improved sampling and
analytical techniques are considered to be responsible for these
changes. Recent measurements of atmospheric concentrations in remote
areas are typically in the range of 0.01-0.04 ng/m3 (GESAMP,
1985). Airborne cadmium concentrations around volcanoes can be
markedly elevated; for example, the plume of Mount Etna, Sicily,
contains about 90 ng/m3 (Buatmenard & Arnold, 1978).
Current measurements of dissolved cadmium in surface waters of
the open oceans give values of < 5 ng/litre. The vertical
distribution of dissolved cadmium in ocean waters is characterized
by a surface depletion and deep water enrichment, which corresponds
to the pattern of nutrient concentrations in these areas (Boyle et
al., 1976). This distribution is considered to result from the
absorption of cadmium by phytoplankton in surface waters, its
transport to the depths incorporated in biological debris, and its
subsequent release. In contrast, cadmium is enriched in the surface
waters of areas of upwelling, and this leads to elevated levels in
plankton unconnected with human activity (Martin & Broenkow, 1975;
Boyle et al., 1976). Oceanic sediments under-lying these areas of
high productivity can contain markedly elevated cadmium levels as a
result of inputs associated with biological debris (Simpson, 1981).
Ice and snow deposits from the polar regions represent a unique
historical record of pollutants in atmospheric precipitation.
However, the problems of contamination are great and no reliable
data are at present available from historic samples; this prevents
an insight into temporal changes in the cycling of cadmium.
Nevertheless, current ice samples have been analysed; those from the
Arctic contain on average 5 pg/g, while corresponding values from
the Antarctic (0.3 pg/g) are much lower (Wolff & Peel, 1985).
3.2 Production
Cadmium is a by-product of zinc production. As a result, the
level of cadmium output has closely followed the pattern of zinc
production, little being produced prior to the early 1920s. The
subsequent rapid increase corresponded to the commercial development
of cadmium electroplating. Worldwide production reached a plateau in
the 1970s but in the 1980s output appeared to be increasing again
(Wilson, 1988b). The worldwide production of cadmium in 1987 was
18 566 metric tonnes (Wilson, 1988b).
3.3 Uses
Cadmium has a limited number of applications but within this
range the metal is used in a large variety of consumer and
industrial materials. The principal applications of cadmium fall
into five categories: protective plating on steel; stabilizers for
poly-vinyl chloride (PVC); pigments in plastics and glasses;
electrode material in nickel-cadmium batteries; and as a component
of various alloys. Detailed consumption statistics are only
available for a limited number of countries but from these it is
apparent that the pattern of use can vary considerably from country
to country (Wilson, 1988b).
Examination of the reported trends in cadmium consumption over
the last 25 years reveals considerable changes in the relative
importance of the major applications. The use of cadmium for
electroplating represents the most striking decrease; in 1960 this
sector accounted for over half the cadmium consumed worldwide, but
in 1985 its share was less than 25% (Wilson, 1988b). This decline is
usually linked to the widespread introduction of progressively
stringent effluent limits from plating works and, more recently, to
the introduction of general restrictions on cadmium consumption in
certain countries. In contrast, the use of cadmium in batteries has
shown considerable growth in recent years from only 8% of the total
market in 1970 to 37% by 1985. The use of cadmium in batteries is
particularly important in Japan and represented over 75% of the
total consumption in l985 (Wilson, 1988b).
Of the remaining applications of cadmium, pigments and
stabilizers are the most important, accounting for 22% and 12%,
respectively, of the world total in 1985. The share of the market by
cadmium pigments remained relatively stable between 1970 and l985
but the use of the metal in stabilizers during this period showed a
considerable decline, largely as a result of economic factors. The
use of cadmium as a constituent of alloys is relatively small and
has also declined in importance in recent years, accounting for
about 4% of total cadmium use in l985 (Wilson, 1988b).
3.4 Sources of environmental exposure
Numerous human activities result in the release of significant
quantities of cadmium to the environment. The relative importance of
individual sources varies considerably from country to country. The
major sources of anthropogenic cadmium release can be divided into
three categories. The first is made up of those activities involved
in the mining, production, and consumption of cadmium and other
non-ferrous metals. The second category consists of inadvertent
sources where the metal is a natural constituent of the material
being processed or consumed. Sources associated with the disposal of
materials that had earlier received cadmium discharges or discarded
cadmium products make up the third category.
Table 1. Estimates of atmospheric cadmium emissions (tonnes/year) from human
activities on a national, regional and worldwide basis
Source United EECb Worldwidec
Kingdoma
Natural sources ND 20 800d
Non-ferrous metal
production
mining ND ND 0.6-3
zinc and cadmium 20 920-4600
copper 3.7 6 1700-3400
lead 7 39-195
Secondary production ND 2.3-3.6
Production of cadmium-containing
substances ND 3 ND
Iron and steel production 2.3 34 28-284
Fossil fuel combustion
coal 1.9 6 176-882
oil 0.5 41-246
Refuse incineration 5 31 56-1400
Sewage sludge incineration 0.2 2 3-36
Phosphate fertilizer manufacture ND ND 68-274
Cement manufacture 1 ND 8.9-534
Wood combustion ND ND 60-180
TOTAL EMISSIONS 14 130 3900-12800
a From: Hutton & Symon (1986); data apply to 1982-1983
b From: Hutton (1983); data apply to 1979-1980 (the EEC consisted, at
that time, of Belgium, Denmark, Federal Republic of Germany, Italy,
Luxembourg, The Netherlands, Republic of Ireland, and
the United Kingdom)
c From: Nriagu & Pacyna (1988); data apply to 1983
d From: Nriagu (1979)
ND Not determined
3.4.1 Sources of atmospheric cadmium
Estimates of cadmium emissions to the atmosphere from human and
natural sources have been carried out at the world-wide, regional,
and national levels; examples of such inventories are shown in
Table 1.
The median global total emission of the metal from human
sources in 1983 was 7570 tonnes (Nriagu & Pacyna, 1988) and
represented about half the total quantity of cadmium produced in
that year. In comparison, the worldwide emission of lead from human
activities was about 10% of the total lead produced in 1983 (Nriagu
& Pacyna 1988). In both the European Economic Community (EEC) and on
a worldwide scale (Nriagu, 1979), about 10-15% of total airborne
cadmium emissions arise from natural processes, the major source
being volcanic action.
Considerable differences exist in the relative importance of
different sources of atmospheric cadmium between the worldwide
situation and that in the United Kingdom and the EEC as a whole.
This is particularly marked for non-ferrous metal production, which
accounts for about 75% of the total anthropogenic emissions
worldwide but only 25% in the EEC. This partly reflects the
extensive emission controls operated by these industries in Europe
compared with many parts of the world. In addition, of the two basic
methods of zinc production, thermal smelting and electrolyte
refining, only the former releases significant atmospheric cadmium
emissions. In recent years, electrolytic refining has assumed the
major share of the world's production of zinc and cadmium and has
largely replaced thermal processes in Europe. The once important
vertical and horizontal retort smelters, which emit large quantities
of atmospheric cadmium, have been phased out in most developed
countries, but are still in operation in several developing
countries (ILZSG, 1988).
Other industries that employ thermal processes, e.g., iron
production, fossil fuel combustion, and cement manufacture, all
release airborne cadmium, the metal being a natural constituent of
the raw materials. The cadmium content of these materials is
generally relatively low but this is offset by the vast quantities
consumed. Furthermore, in common with other thermal processes, the
elevated temperatures employed result in the volatilization of
cadmium. It subsequently condenses in a preferential manner on the
smallest particles in the stack gases, the size range least
efficiently retained by conventional particulate control measures
(Smith, 1982). Despite mechanisms that enhance the release of
cadmium, the quantities emitted from the three processes are now
considered to be smaller than they were in the past, particularly in
the case of fossil fuel combustion (Rauhut, 1980). Municipal refuse
is a waste-related source, the cadmium being derived from discarded
nickel-cadmium batteries and plastics that contain cadmium pigments
and stabilizers. The incineration of refuse, a practice generally
restricted to developed countries, is a major source of atmospheric
cadmium release at the national, regional, and worldwide levels
(Table 1). Indeed, this activity accounts for about one third of the
total cadmium emissions in the United Kingdom and the EEC as a
whole. Cadmium release from this sector originates from a large
number of plants, while the emissions from the non-ferrous metal
industry are derived from relatively few facilities.
Sewage sludge receives cadmium from industrial sources,
particularly from the discharges of plating operations and pigment
works. One disposal option, the incineration of sewage sludge, is a
relatively minor source of airborne cadmium, reflecting the small
quantities of sludge disposed of in this manner (Table 1).
Steel production can also be considered as a waste-related
source, as large quantities of cadmium-plated steel scrap are
recycled by this industry, at least in developed countries. As a
result, steel production is responsible for considerable emissions
of atmospheric cadmium.
3.4.2 Sources of aquatic cadmium
Non-ferrous metal mines represent a major source of cadmium
release to the aquatic environment. Contamination can arise from
mine drainage water, waste water from the processing of ores,
overflow from the tailings pond, and rainwater run-off from the
general mine area. The release of these effluents to local
water-courses can lead to extensive contamination downstream of the
mining operation. The cadmium content of the ore body and mine
management policies, as well as climatic and geographical
conditions, all influence the quantities of cadmium released from
individual sites. Flood and storm conditions, for example, will
enhance the mobilization of cadmium contained in particulate
material. Aquatic inputs of cadmium are not restricted to active
mine sites, and mines disused for many years can still be
responsible for the continuing contamination of adjacent
watercourses (Johnson & Eaton, 1980).
At the global level, the smelting of non-ferrous metal ores has
been estimated to be the largest human source of cadmium release to
the aquatic environment (Nriagu & Pacyna, 1988). Discharges to fresh
and coastal waters arise from liquid effluents produced by gas
scrubbing together with the site drainage waters.
Concerning the locations where environmental health effects of
cadmium have been reported, the water and air contamination from
non-ferrous metal mining and production are the predominant sources
of cadmium. All the major areas of Japan with elevated cadmium
levels have been affected by these sources (Tsuchiya, 1978),
although contamination through the natural mobilization of cadmium
from ore bodies may also have been involved.
Cadmium is a natural constituent of rock phosphates and
deposits from some regions of the world contain markedly elevated
levels of the metal. The manufacture of phosphate fertilizer results
in a redistribution of the cadmium in the rock phosphate between the
phosphoric acid product and the gypsum waste. In many cases, the
gypsum is disposed of by dumping in coastal waters, which leads to
considerable cadmium inputs. Some countries, however, recover the
gypsum for use as a construction material and thus have negligible
cadmium discharges (Hutton, 1982).
The atmospheric fall-out of cadmium to fresh and marine waters
represents a major input of cadmium at the global level (Nriagu &
Pacyna, 1988). Indeed, a GESAMP study of the Mediterranean Sea
indicated that this source is comparable in magnitude to the total
river inputs of cadmium to the region (GESAMP, 1985). Similarly,
large cadmium inputs to the North Sea (110-430 tonnes/year) have
been estimated, based on the extrapolation from measurements of
cadmium deposition along the coast (van Aalst et al., 1983a,b).
However, another approach based on model simulation yielded a modest
annual input of 14 tonnes (Krell & Roeckner, 1988).
Acidification of soils and lakes may result in enhanced
mobilization of cadmium from soils and sediments and lead to
increased levels in surface and ground waters (WHO, 1986). The
corrosion of soldered joints or zinc galvanized plumbing by acidic
waters can dissolve cadmium and produce increased levels of the
metal in drinking-water. In one study from Sweden, cadmium levels in
tap water from areas susceptible to acidic deposition were double
those from a control area (Svensson et al., 1987).
3.4.3 Sources of terrestrial cadmium
Solid wastes from a variety of human activities are disposed of
in landfill sites, resulting in large cadmium inputs at the national
and regional levels when expressed as a total tonnage (Hutton, 1982;
Hutton & Symon, 1986). However, this simple approach exaggerates the
significance of landfilled cadmium in certain high volume wastes
with relatively low concentrations of cadmium. Examples include the
ashes from fossil fuel combustion, waste from cement manufacture,
and the disposal of municipal refuse and sewage sludge. Of greater
potential environmental significance are the solid wastes from both
non-ferrous metal production and from the manufacture of
cadmium-containing articles, as well as the ash residues from refuse
incineration. All three waste materials are characterized by
elevated cadmium levels and as such require disposal to controlled
sites to prevent the mobilization of the cadmium in ground water.
Soil cadmium contamination is a characteristic feature around
non-ferrous metal mines and smelters, particularly in the case of
those handling zinc ores. Contamination from mining is generally
local but may be widespread in areas of high mineral content
(Tsuchiya, 1978). Soil contamination from smelters is generally
greatest next to the source and decreases exponentially with
distance, although cadmium concentrations can still be above the
background level 20 km from the source (Buchauer, 1972). Shipham,
United Kingdom, is a site of extreme soil cadmium contamination.
Between 1650 and 1850 the village of Shipham was the site of a major
zinc mine. Once the mining stopped the area was flattened and
developed for agriculture and housing. Cadmium levels in
agricultural and garden soils are some of the highest ever reported
worldwide (Thornton, 1988).
The agricultural application of phosphate fertilizers
represents a direct input of cadmium to arable soils. The cadmium
content of phosphate fertilizers varies widely and depends on the
origin of the rock phosphate. It has been estimated that fertilizers
of West African origin contain 160-255 g cadmium/tonne of phosphorus
pentoxide, while those derived from the southeastern USA contain
only 35 g/tonne (Hutton, 1982).
The annual rate of cadmium input to arable land from phosphate
fertilizers had been estimated for the countries of the EEC, taking
into account differences in application rates and the cadmium
contents of the fertilizers used (Hutton, 1982). The average cadmium
input (5 g/ha) only represents about 1% of the surface soil cadmium
burden. Despite the relatively small size of this input, long-term
continuous application of phosphate fertilizers has been shown to
cause increased soil cadmium concentrations (Williams & David, 1973,
1976; Andersson & Hahlin, 1981).
The application of municipal sewage sludge to agricultural soil
as a fertilizer can also be a significant source of cadmium. In many
industrialized countries, control measures have reduced the cadmium
content of sewage sludge and at the same time national and regional
regulations have limited the input of cadmium from agricultural
sludge applications (Davis, 1984). Nevertheless, large increases in
soil cadmium concentration have resulted in the past from the
application of contaminated sludge in both North America and Europe
(Davis, 1984). Even today, the high application rates used for
sewage sludge result in relatively large cadmium inputs, a value of
80 g/ha having been estimated for the United Kingdom (Hutton &
Symon, l986). On a national or regional basis, however, these inputs
are much smaller than those from either phosphate fertilizers or
atmospheric deposition (see section 4.2).
3.5 Conclusions
Cadmium is a relatively rare element and current analytical
procedures indicate much lower concentrations of the metal in
environmental media than do older measurements. At present, it is
not possible to determine whether human activities have caused a
historic increase in cadmium levels in the polar ice caps.
Commercial cadmium production started at the beginning of this
century. The pattern of cadmium consumption has changed in recent
years with significant decreases in electroplating and increases in
batteries and specialized electronic uses. Most of the major uses of
cadmium employ it in the form of compounds that are present at low
concentration. This makes it difficult to recycle cadmium in order
to decrease the potential for environmental contamination.
Restrictions on certain uses of cadmium imposed by a few countries
may have widespread impact on the applications of cadmium.
Cadmium is released to the air, land, and water by human
activities. In general, the two major sources of contamination are
the production and consumption of cadmium and other non-ferrous
metals and the disposal of wastes containing cadmium. Areas in the
vicinity of non-ferrous mines and smelters often show pronounced
cadmium contamination.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND
TRANSFORMATION
4.1 Atmospheric deposition
Cadmium is removed from the atmosphere by dry deposition and by
precipitation. Total deposition rates have been measured at numerous
localities worldwide and values have generally been found to
increase in the order: background < rural < urban < industrial.
In rural areas of Scandinavia, annual deposition rates ranged from
0.4 to 0.9 g/ha (Laamanen, 1972; Andersson, 1977). Similarly, in a
rural region of Tennessee, USA, a deposition rate of 0.9 g/ha was
observed (Lindberg et al., 1982). Hutton (1982) concluded that
3 g/ha per year is a representative value for the atmospheric
deposition of cadmium to agricultural soils in rural areas of the
EEC. This may be compared with a corresponding input of 5 g/ha per
year for these areas from the application of phosphate fertilizers
(see 3.4).
Many industrial sources of cadmium possess tall stacks, which
bring about the wide dispersion and dilution of particulate
emissions. Indeed, it is often assumed that < 10% of such emissions
are deposited locally, the remainder being available for long-range
transport (Krell & Roeckner, 1988). Nevertheless, cadmium deposition
rates around smelter facilities are often markedly elevated nearest
the source and generally decrease rapidly with distance (Hirata,
1981). This pattern of contamination can be reflected in surface
soils and vegetation, and in the former case, contamination will
reflect the long-term history of metal inputs from the atmosphere.
As a result, soil cadmium concentrations in excess of 100 mg/kg are
commonly encountered close to long-established smelters (Buchauer,
1972). In some urban areas, the high density of non-ferrous metal
works results in a city-wide elevation of cadmium deposition (Roels
et al., 1981a).
The possibility that cadmium deposition is enhanced around
atmospheric sources of cadmium other than smelters has been
investigated on a number of occasions. One assessment of studies
conducted around coal-fired power stations concluded that this
source was unlikely to cause any marked local accumulation of
cadmium (Chadwick & Lindman, 1982). In contrast, significant cadmium
contamination was found in surface soil downwind of a phosphate
fertilizer processing plant in the USA, the levels being up to
40 mg/kg (Hutchison et al., 1979). Little attention has been paid to
the pattern of cadmium deposition around refuse incinerators; one
study of a large facility in the United Kingdom observed moderately
elevated deposition rates downwind of the plant (Hutton et al.,
1988).
Crop plants growing near to atmospheric sources of cadmium may
contain elevated cadmium levels (Carvalho et al., 1986). However, it
is not always possible to distinguish whether the cadmium is derived
directly from surface deposition or originates from root uptake,
since soil levels in such areas are generally higher than normal.
One study in Denmark has suggested that atmospheric deposition can
also be an important direct source of cadmium in crop plants even in
background areas (Hovmand et al., 1983).
4.2 Transport from water to soil
Rivers contaminated with cadmium can contaminate surrounding
land, either through irrigation for agricultural purposes, by the
dumping of dredged sediments, or through flooding (Forstner, 1980;
Sangster et al., 1984). For example, agricultural land adjacent to
the Neckar River, Germany, received dredged sediments to improve the
soil, a practice that produced soil cadmium concentrations in excess
of 70 mg/kg (Forstner, 1980).
Much of the cadmium entering fresh waters from industrial
sources is rapidly absorbed by particulate matter, where it may
settle out or remain suspended, depending on local conditions. This
can result in low concentrations of dissolved cadmium even in rivers
that receive and transport large quantities of the metal (Yamagata &
Shigematsu, 1970). Rivers can transport cadmium considerable
distances from the source of the input. In Japan, there are several
areas where soils have been contaminated with irrigation water up to
50 km from the source (Tsuchiya, 1978).
4.3 Uptake from soil by plants
It has been shown repeatedly that an increase in soil cadmium
content results in an increased plant uptake of the metal. This has
been demonstrated for soils with naturally elevated cadmium levels
(Lund et al., 1981), those contaminated by non-ferrous metal mining
(Alloway et al., 1988), and those that have received cadmium via
sewage sludge applications (Davis & Coker, 1980). It is this basic
relationship that makes the soil-crop pathway of human exposure
susceptible to increased levels of soil cadmium. Indeed, since the
major sources of cadmium exposure for the general population are
food and tobacco (see section 5), it is important to assess those
soil and plant factors that influence cadmium uptake by crop plants.
The most important soil factors influencing plant cadmium
accumulation are soil pH and cadmium concentration (Davis & Coker,
1980; Page et al., 1981). Soil cadmium is distributed between a
number of pools or fractions, of which only the cadmium in soil
solution is thought to be directly available for uptake by plants.
Soil pH is the principal factor governing the concentration of
cadmium in the soil solution. Cadmium absorption to soil particles
is greater in neutral or alkaline soils than in acidic ones and this
leads to increased cadmium levels in the soil solution. As a
consequence, plant uptake of cadmium decreases as soil pH increases.
Other soil factors that influence the distribution of cadmium
between the soil and soil solution include cation exchange capacity
and the contents of the hydrous oxides of manganese and iron,
organic matter, and calcium carbonate. Increases in these parameters
result in decreased availability of cadmium to plants owing to a
reduction of the level of cadmium in the soil solution.
A comparative study of cadmium-contaminated soils from
different sources illustrates the importance of the above soil
factors (Alloway et al., 1988). Soils from Shipham, United Kingdom,
contained the highest total cadmium levels but the soil solution
concentrations were lower than in other soils. The small proportion
of soluble cadmium in Shipham soils (0.04%) was related to the high
pH (7.7) and high calcium carbonate and hydrous oxide content of
these soils. In contrast, a paddy soil from the Junzu Valley, Japan,
contained 4% soluble cadmium and possessed a low pH (5), low calcium
carbonate content, and very low hydrous oxide concentration (Alloway
et al., 1988).
Much attention has been paid to the plant availability of
cadmium in agricultural soils to which sewage sludge has been
applied. It has been observed that the repeated application of
sludge to soils can alter the availability of cadmium, and although
soil cadmium levels may increase, crop levels do not always reflect
this increase (Page et al., l981). The long-term availability of
cadmium to plants is uncertain, availability having been reported to
remain constant, decrease, or even increase with time (Tjell et al.,
l983). In another study there were no clear changes in the plant
availability of cadmium over a period of five years after sewage
sludge was applied to the soil (Carlton-Smith, l987).
Concern over the long-term implications of present-day cadmium
inputs to European arable soils has led to modelling studies of the
future cadmium exposure for the general population (Tjell et al.,
1981; Hutton, 1982). It was estimated by Tjell et al. (1981) that
cadmium inputs from phosphate fertilizers and atmospheric deposition
will cause an annual increase of 0.6% in Danish soil cadmium levels.
The corresponding increases in crop cadmium concentrations would
lead to a predicted 70% increase in dietary cadmium intake 100 years
hence. Similar soil and dietary cadmium increases have been
predicted for the EEC as a whole, although the precise values varied
according to the soil properties and crop consumption patterns
employed (Hutton, 1982).
Indirect support for these forecasts was provided by an
investigation of the time trends in soil and crop cadmium levels
using archived samples. Jones et al. (1987) found that the cadmium
content of agricultural soils from a site in the United Kingdom had
increased by 27-55% since the 1850s. Trends in the cadmium
concentrations of wheat grain were less clear, possibly due to
confounding factors such as changes in varieties grown and altered
soil properties.
4.4 Transfer to aquatic and terrestrial organisms
In general, cadmium concentrations in terrestrial and aquatic
biota from uncontaminated localities are low, corresponding to the
geochemical abundance of this metal. However, in certain situations,
cadmium displays a propensity for marked bioaccumulation, a feature
that has implications for human dietary exposure and may be of
toxicological significance for the organisms concerned.
It appears that cadmium shows greatest mobility in certain
marine ecosystems. Phytoplankton in areas of oceanic upwelling
contain raised cadmium levels (Martin & Broenkow, 1975), and
filter-feeding molluscs can accumulate significant concentrations of
cadmium even in coastal localities that are only moderately
contaminated (Bryan et al., 1980). Oysters, in particular, are
well-known cadmium accumulators, levels of up to 8 mg/kg wet weight
having been recorded in New Zealand (Nielsen, 1975). Certain edible
crustaceans such as crab and lobster also contain relatively high
cadmium concentrations, the metal being localized in the
hepatopancreas or "brown meat" (Buchet et al., l983).
Some marine birds and mammals contain remarkably elevated
cadmium burdens in the kidney and liver (Martin et al., 1976;
Stoneburner, 1978; Nicholson & Osborn, 1983). In the case of oceanic
species, this accumulation is probably a natural process associated
with the feeding habits and longevity of the organism in question.
Even so, the high cadmium levels in pelagic sea-birds have been
linked in one study to morphological signs of kidney damage
(Nicholson & Osborn, 1983).
Terrestrial mosses and lichens display a high capacity for
retention of metals deposited from the atmosphere and these plants
have been used to map both local contamination from point sources
and regional patterns of cadmium deposition (MARC, 1986). The
fruiting bodies of some macrofungi contain remarkably high cadmium
concentrations even in areas uncontaminated with cadmium (MARC,
1986). This phenomenon has implications for human dietary exposure
as some accumulator species are edible.
In addition to humans, certain long-lived terrestrial mammals
such as the horse and moose may possess considerable cadmium burdens
in the kidney and liver (Elinder & Piscator, 1978; Frank et al.,
1981; Jeffery et al., 1989). It has been shown that cadmium
accumulates with age in horse kidney.
4.5 Conclusions
Increases in soil cadmium content result in an increase in the
uptake of cadmium by plants; the pathway of human exposure from
agricultural crops is thus susceptible to increases in soil cadmium.
The uptake by plants from soil is greater at low soil pH. Processes
that acidify soil (e.g., acid rain) may therefore increase the
average cadmium concentrations in foodstuffs. The application of
phosphate fertilizers and atmospheric deposition are significant
sources of cadmium input to arable soils in some parts of the world;
sewage sludge can also be an important source at the local level.
These sources may, in the future, cause enhanced soil and hence crop
cadmium levels, which in turn may lead to increases in dietary
cadmium exposure. In certain areas, there is evidence of increasing
cadmium content in food.
Edible free-living food organisms such as shellfish,
crustaceans, and fungi are natural accumulators of cadmium. Regular
consumption of these items can result in elevated human exposure.
Certain marine vertebrates contain markedly elevated renal cadmium
concentrations, which, although considered to be of natural origin,
have been linked to signs of kidney damage in the organisms
concerned.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Human uptake of cadmium occurs via the inhalation of air and
the ingestion of food and drinking-water. Accidental ingestion of
cadmium through the contamination of foods in contact with
cadmium-containing materials has occurred in the past. Accidental
high-level inhalation exposure during welding operations and cadmium
smelting is still a considerable hazard.
Chronic exposure to cadmium via food or workplace air is the
main concern in assessing the health risks of cadmium.
5.1 Inhalation route of exposure
5.1.1 Ambient air
Many countries carry out regular monitoring programmes for
cadmium in the air. An assessment of the available data from various
European countries showed that average values range from 1 to
5 ng/m3 in rural areas, 5 to 15 ng/m3 in urban areas, and 15 to
50 ng/m3 in industrialized areas (WHO, 1987). Examination of some
individual national data (Table 2) suggests that urban values are
likely to occupy the lower end of the range indicated above
(McInnes, 1979; RIVM, 1988).
Much higher air cadmium concentrations are found in areas close
to major atmospheric sources of the metal. However, these values can
fluctuate widely as a result of changing emission characteristics
and weather conditions (Muskett et al., 1979).
Studies of the particle size distributions of cadmium in urban
aerosols generally show that the metal is associated with
particulate matter in the respirable range (Greenberg et al., 1978).
The enrichment of cadmium on these smaller particles can be linked
to the behaviour of the metal in thermal facilities that are sources
of airborne cadmium (see section 3.4.1).
An air quality study revealed no differences between indoor and
outdoor air cadmium levels when the dwellings of non-smokers were
examined (Moschandreas, 1981). However, significantly higher indoor
air cadmium levels were observed in those houses where smoking took
place.
Table 2. Typical levels of cadmium in ambient air
Type of area Cadmium concentration Sampling Reference
range (ng/m3) periodb
Remote rural
Pacific atoll 0.0025-0.0046 NR Duce et al. (1983)
Europe 0.1-0.3 NR Heindryckx et al. (1974)
Atlantic 3 x 10-6-6.2 x 10-4 NR Duce et al. (1975)
Rural
Belgium 1a 24 h Janssens & Dams (1974)
Federal Republic
of Germany 0.1-1 < 24 h Neeb & Wahdat (1974)
Japan 1-4 24 h Japanese Environment
Agency (1974)
Urban
Belgium 50a 24 h Janssens & Dams (1974)
Federal Republic
of Germany 10-150 < 24 h Neeb & Wahdat (1974)
Japan 3-6.3 1 year Japanese Environment
Agency (1974)
Poland 2-51 1 year Just & Kelus (1971)
USA (New York) 3-23 1 year Kneip et al. (1970)
a Mean value b NR = not reported
5.1.2 Air in the working environment
Elevated air cadmium levels arise in the smelting of
non-ferrous metals and in the production and processing of
cadmium-containing articles. The thermal operations associated with
these processes are mainly responsible for producing cadmium dusts
and, if temperatures are sufficiently high, cadmium fume.
Airborne cadmium concentrations found in the occupational
setting vary considerably according to the type of industry and the
specific working conditions in each plant. Markedly elevated values,
in the mg/m3 range, were prevalent in the 1940s to 1960s (Friberg,
1950; Adams et al., 1969; Tarasenko & Vorobjeva, 1973). Considerable
improvements in occupational hygiene have taken place in developed
countries since then and these have led to progressive reductions in
ambient levels in the workplace. Table 3 illustrates the temporal
decline in air cadmium levels in a Swedish battery factory
(Adamsson, 1979). The lowest values shown in Table 3 may not be
typical for all occupational facilities; levels of 1-5 mg/m3 were
reported for one pigment plant in the mid 1970s (De Silva & Donnan,
1981).
Table 3. Average air cadmium concentrations in a Swedish cadmium
battery planta
Time period Number of Cadmium concentration
observations (µg/m3)
1946 10 5000
1947-1949 16 750
1950-1960 94 650
1965-1973 393 70
1973-1975 373 40
1975-1976 573 15
a From: Adamsson (1979)
In general, only total air cadmium concentrations are monitored
in the working environment; factors influencing respiratory
absorption, such as the speciation of cadmium and the size
distribution of the collected particles, are not taken into account.
In one study of workplaces with high total airborne cadmium levels,
Lauwerys et al. (1974b) found, in general, that less than 25% of the
total cadmium in air was in the respirable range and that this
percentage decreased as the total value increased.
Cadmium-containing dust particles that are too large to be delivered
to the pulmonary region of the lung can enter the gastrointestinal
tract by mucociliary transfer.
5.1.3 The smoking of tobacco
The tobacco plant naturally accumulates relatively high cadmium
concentrations in its leaves. As a result, this material represents
an important source of exposure for smokers. It has been reported
that one cigarette contains about 1-2 µg cadmium (Friberg et al.,
1974) and that about 10% of the cadmium content is inhaled when the
cigarette is smoked (Elinder et al., 1983). One study has suggested
that modifications in cigarette construction and the increasing
popularity of filter cigarettes have reduced cadmium exposure from
this source in recent years (Scherer & Barkemeyer, 1983). Regional
differences exist in the cadmium concentration of cigarettes, and
lower values (0.1-0.5 µg) have been found in samples from Argentina,
India, and Zambia (Nwankwo et al., 1977; Elinder et al., 1983).
Biological monitoring surveys of the general population have
shown that cigarette smoking can cause significant increases in the
concentration of cadmium in the kidney (Lewis et al., 1972; Vahter,
1982).
Occupationally exposed workers who smoke tobacco may be subject
to higher exposure levels than their non-smoking colleagues. This
may be because the original content of tobacco can be considerably
increased when handled during work (Piscator et al., 1976). In
addition, the hand-to-mouth route of exposure may be more important
in workers who are tobacco smokers (Adamsson, 1979).
5.2 Ingestion routes of exposure
5.2.1 Levels in drinking-water
Drinking-water generally contains low cadmium levels and a
value of 1 µg/litre or less is often assumed to be a representative
value in most situations (Meranger et al., 1981). Thus, cadmium
exposure from drinking-water and water-based beverages is relatively
unimportant compared with the dietary contribution.
In a study of drinking-water in Seattle, USA, Sharrett et al.
(1982) reported a median cadmium level of 0.01 µg/litre in tap water
delivered by copper pipes; the corresponding value from homes with
galvanized piping was 0.25 µg/litre. Water samples left to stand in
both types of piping showed increases in cadmium levels with median
values of 0.06 and 0.63 µg/litre in copper and galvanized supplies,
respectively. In a survey from the Netherlands, about 99% of
drinking-water samples in 1982 contained less than 0.1 µg/litre
(RIVM, 1988).
5.2.2 Levels in food
The cadmium content of agricultural crops varies according to
species, variety cultivated and season (Davis & Coker, 1980). The
results of an extensive nationwide survey of cadmium in different
classes of raw agricultural crops from uncontaminated localities
illustrate the range of values encountered within and between crop
classes (Wolnik et al., 1983, 1985). It is evident that cadmium is a
normal constituent of most foodstuffs (Tables 4 and 5).
Table 4. Cadmium concentrations in the major types of crop from
various regions of the USAa
Cadmium concentration (mg/kg wet weight)
Crop Sample size Median Minimum Maximum
Rice 166 0.0045 < 0.001 0.23
Peanuts 320 0.060 0.010 0.59
Soybeans 322 0.041 0.002 1.11
Wheat 288 0.030 < 0.0017 0.207
Potatoes 297 0.028 0.002 0.18
Carrots 207 0.017 0.002 0.13
Onions 230 0.009 0.001 0.054
Lettuce 150 0.017 0.001 0.160
Spinach 104 0.061 0.012 0.20
Tomatoes 231 0.014 0.002 0.048
a From: Wolnik et al. (1983, 1985).
Meat, fish, and fruit generally contain similar cadmium levels
and values of 5-10 µg/kg fresh weight are representative for these
food classes. Most plant-based foodstuffs contain higher cadmium
concentrations and a value of 25 µg/kg fresh weight is considered
representative for the staple items, cereals and root vegetables.
Offal from adult animals and certain shellfish contain even higher
concentrations (see section 4.4); values in excess of 50-100 µg/kg
fresh weight are considered normal. Food preparation can result in
cadmium losses from plant-based items. The milling of wheat grain
results in a reduction of about 50% in the cadmium content of the
white flour produced (Linnman et al., 1973). The washing, peeling,
and cooking of vegetables can also lead to reductions in the
concentrations of cadmium but, in general, these are relatively
small.
The use of glazed ceramic containers to store foodstuffs can
lead to significant cadmium contamination, particularly in the case
of foods that are acidic liquids (Beckman et al., 1979).
Table 5. Cadmium concentrations in different food items from various European
countries (values in µg/kg fresh weight)
Food Group United Finlandb Swedenc Denmarkd The
Kingdoma Netherlandse
Bread and cereals 20-30 20-40 31-32 30 25-35
Meat < 20-30 < 5-5 2-3 6-30 10-40
Offal
pork kidney 450 180 190 1000
pork liver 130 70 50 100
Fish < 15 < 5-20 1-20 14 15
Eggs < 30 4 1 < 10 2
Oils and dairy
products < 20-30 3-20 1-23 < 30 10-30
Sugars and preserves < 10 < 10 3 30 5
Fresh fruit < 10 < 2 1-2 11 5
Vegetables
cabbage < 10 5 4 10
cauliflower < 20 10 10
spinach 120 150 43
broccoli 10 10
legumes < 10-30 < 2-30 1-4 15
lettuce < 60 50 29 43
potatoes < 30 30 16 30 30
carrots < 50 30 41
a From: Bucke et al. (1983)
b From: Koivistoinen (1980)
c From: Jorhem et al. (1984)
d From: Andersen (1979)
e From: RIVM (1988)
Crops grown in cadmium-contaminated localities have been shown
to contain elevated levels of the metal compared with normal values.
The extent of enrichment depends on several factors (see section
4.3). The cadmium concentrations in selected vegetable crops grown
at three contaminated sites in the United Kingdom are shown in Table
6. Highest levels were generally found at Shipham, where soil
cadmium concentrations are markedly elevated, and the greatest
increase was noted in leafy vegetables. Potato, a staple food item,
showed similar values at the three locations and these were about
five times greater than background.
Large scale surveys of cadmium in rice have been carried out in
areas of Japan where environmental contamination was suspected
(Japanese Environment Agency, 1972, 1982). The results of the
earlier survey revealed that large numbers of rice samples contained
elevated cadmium levels; the corresponding data from the later study
indicated that decreases had occurred over the intervening ten
years. More detailed investigations at specific localities have also
been carried out in Japan, often as part of studies on health
effects of the general population (Table 7).
5.2.3 Other sources of exposure
Young children may ingest household dust or garden soil. This
habit may be a source of cadmium exposure, as has been identified
for lead (Duggan et al., 1985). The representative daily intake of
dust via the hands in young children is considered to be 100 mg
(Lepow et al., 1974). In an extensive survey of metals in household
dusts in the United Kingdom, an average cadmium level of 6.9 mg/kg
was obtained from over 4500 samples (Culbard et al., 1988). These
data suggest that the hand-to-mouth route is a minor source of
cadmium intake (about 0.7 µg daily).
The hand-to-mouth exposure pathway may be a significant source
of cadmium in areas around point sources of the metal. In the
vicinity of a small lead refinery in the United Kingdom, cadmium
levels in household dust were reported to be 193 mg/kg (Muskett et
al., 1979). The daily ingestion of 100 mg of this dust would result
in the intake of about 20 ßg cadmium. Buchet et al. (1983) observed
a correlation between cadmium intake from dust and the levels of
blood and urinary cadmium in children from areas of Belgium
subjected to air contamination. Despite markedly elevated soil
cadmium levels in the gardens of Shipham, United Kingdom, Thornton
(1988) found that household dust concentrations were only four times
greater (at an average of 27 mg/kg) than background.
Table 6. Mean cadmium concentrations (µg/kg fresh weight) in selected vegetable crops grown at three contaminated sites
in the United Kingdom
Location Source of cadmium Cabbage Leafy Potato Carrot Reference
contamination salad
Shipham zinc mine 250a 680 130 340 Sherlock et al. (1983)
Walsall atmospheric inputs 73 190 103 120 Tennant (1984)
from a copper
refinery
Heathrow sewage sludge 24 180 150 150 Chumbley & Unwin (1982)
applications
a Median value
5.2.4 Daily intake of cadmium from food
Three approaches are used for estimating the daily intake of
cadmium in food. The first is the total-diet collection method in
which the foods are prepared for consumption and are analysed either
individually or combined in one or more food group composites in
proportions based on available food consumption data. The total
cadmium intake is calculated as the product of the concentration and
the estimated amount of food eaten. In the second approach, a market
basket study, representative samples of individual foodstuffs are
collected from retail outlets and analysed. The cadmium
concentrations are then multiplied by the average amount of intake
of each foodstuff to give the cadmium intakes for each food item.
The sum gives the total dietary intake. The third way of estimating
cadmium intake is the collection of a duplicate sample of the meals
consumed. The combined food sample is homogenized and the cadmium
analysed. Table 8 presents some published estimates of dietary
cadmium intakes from different countries based on these three
methods.
Another method for estimating the daily intake of cadmium is to
determine the daily faecal output, because only about 5% of ingested
cadmium is absorbed on average (section 6.1.2). In Table 9 the
available data on faecal cadmium are summarized. There is general
agreement with the data presented in Table 8, but in the USA the
estimated dietary exposure based on faecal analysis is considerably
lower than direct estimates of dietary intake.
Tables 8 and 9 show that daily intakes of cadmium in Europe,
New Zealand, and the USA are usually about 10-25 ßg. These are
average values, and large individual variations do occur due to
variability in dietary habits and age-dependent changes in energy
intake. The highest daily intake of cadmium is likely to occur among
teenagers, since they have the highest caloric intake (Kjellström et
al., 1978). Individuals from the general population who are extreme
consumers of certain food items with elevated cadmium levels may
have exposure levels above the average. It has been estimated that
10% of the population consume twice the average quantity of a
particular food class and 2.5% consume three times the average
(Sherlock & Walters, 1983). Estimates of the daily cadmium intake in
areas of Japan considered normal are consistently higher than in
other parts of the world and generally range from 30 to 50 µg. In
areas of elevated exposure, average daily intakes range from 150 to
250 µg (Tables 8 and 9).
Table 7. Environmental cadmium levels in Japan: a summary of the surveys of cadmium levels in rice and
health status of local populations
Area Cadmium Daily Source of cadmium Number Health References
(prefecture) concentration cadmium contamination of effects
in rice intake peoplea reportedb
(mg/kg fresh (µg/day)c
weight)
Fuchu, Toyama 0.6-2.0 600 zinc, lead, and cadmium 7650 yes Ishizaki et al. (1969);
mine and refinery Kato & Abe (1978)
Ikuno, Hyogo 0.2-1.0 silver, copper, and zinc 13 000 yes Hyogo Prefectural Gov't (1972);
mine Tsuchiya & Nakamura (1978)
Tsushima, 0.5-0.8 213-255 lead and zinc mines 2400 yes Shigematsu et al. (1975);
Nagasaki Takabatake (1978b)
Kakehashi, 0.2-0.8 160 copper mines 2800 yes Ishizaki (1972); Kawano & Kato
Ishikawa (1978)
Kosaka, Akita 0.2-0.6 185 silver and copper mines 800 yes Kojima et al. (1975); Shigematsu &
Kawaguchi (1978)
Yoshino, Yamagata 0.6 gold, silver, copper, and 8000 NS Uruno et al. (1975); Shigematsu &
zinc mines Kawaguchi (1978)
Annaka and 0.4-0.5 281 zinc refinery 4400 NS Shigematsu et al. (1975);
Takasaki, Gunma Fukushima (1978)
Uguisuzawa, Miyagi 0.6-0.7 180 lead and zinc mines 800 NS Shigematsu et al. (1975);
Takabatake (1978a,b)
Watarase, Gunma 0.3 copper mine 4700 NS Fukushima et al. (1975);
Fukushima (1978)
Table 7 (contd).
Area Cadmium Daily Source of cadmium Number Health References
(prefecture) concentration cadmium contamination of effects
in rice intake peoplea reportedb
(mg/kg fresh (µg/day)c
weight)
Shimoda, Shizuoka 0.4-1.1 gold and copper mine 1100 NS Tsuchiya (1978)
Bandai, Fukushima 0.2-0.4 zinc refinery 1800 NS Shigematsu & Kawaguchi (1978)
Kurobe, Toyama 0.6 copper refinery 8000 NS Tsuchiya (1978)
Kiyokawa, Oita 0.2-0.5 391 tin, copper, lead, zinc, 700 NS Takabatake (1978a);
and arsenic mine Shigematsu et al. (1975)
Ohmuta, Fukuoka 0.7 zinc refinery 2540 NS Yamamoto (1972); Tsuchiya (1978)
a Indicates the approximate number of people living in exposed area. The figure usually includes only people over 30 years
old considered to consume rice with more than 0.4 mg cadmium/kg.
b NS denotes health examinations were made, but effects were not significantly different from those in control areas.
c From: Tsuchiya (1978)
Of particular interest is the village of Shipham, United
Kingdom, where markedly elevated soil cadmium levels are present.
Cadmium levels in locally grown vegetables have also been found to
be elevated and ranged from 5 to 20 times above normal values (Table
6). Three dietary and crop sampling surveys were performed to
estimate heavy metal intake from both fresh and cooked food
(Sherlock et al., 1983). A duplicate portion study and two market
basket studies were performed to coincide with periods of
significant consumption of home-grown vegetables. The total cadmium
dietary intake estimated from the market basket studies averaged
36 µg per day, of which 14 µg per day was contributed by locally
grown fruit and vegetables. The duplicate portion study gave an
average total cadmium intake of 29 µg per day, of which 17 µg per
day was attributed to locally grown fruit and vegetables. Four
individuals from the study population showed cadmium intakes greater
than 400 µg/week. Both methods indicated that cadmium intakes in
Shipham were higher than the United Kingdom average. However,
exposures were not as high as would have been expected, considering
the extent of cadmium contamination of the local vegetables,
suggesting that most inhabitants did not rely heavily on local
crops.
5.3 Total intake and uptake of cadmium from all environmental
pathways
5.3.1 General population, uncontaminated areas
Assuming an air cadmium concentration of 10 ng/m3 for both
indoor and outdoor air and a daily inhalation rate of 15 m3 for an
adult, the average intake of cadmium from the atmosphere would be
0.15 µg, of which about 25% (Friberg et al., 1974) or 0.04 µg will
be absorbed. Smoking a pack of 20 cigarettes daily can result in the
inhalation of 2-4 µg cadmium, the amount varying considerably
according to the country or origin of the tobacco. Of this amount,
25-50% may be absorbed via the lungs, resulting in an uptake of
1-2 µg, a much larger amount than from air alone. Those individuals
who smoke two or more packs of cigarettes daily will absorb
correspondingly greater quantities of cadmium.
Cadmium intake from drinking-water based on a daily consumption
of 2 litres is usually less than 1 µg. Average daily intake from
food in most countries is probably at the lower end of the range of
10-25 µg. At an absorption rate of 5%, daily uptake from water and
food would be 0.6-1.3 µg cadmium. Thus, heavy smokers from the
general population in uncontaminated areas may absorb more cadmium
from the inhalation pathway than from dietary sources.
Table 8. Estimates of average daily dietary intake of cadmium based on food
analysis in various countries
Country Method of Estimates (µg Reference
samplinga cadmium per day)
Areas of normal exposure
Belgium D 15 Buchet et al. (1983)
Finland M 13 Koivistoinen (1980)
Japan D 31 Yamagata & Iwashima (1975)
Japan D 48 Suzuki & Lu (1976)
Japan D 49 Ushio & Doguchi (1977)
Japan D 35 Iwao (1977)
Japan M 49 Ohmomo & Sumiya (1981)
Japan
(mean of 3 areas) D 59 Iwao et al. (1981a)
Japan D 43.9 (males) Watanabe et al. (1985)
37.0 (females)
New Zealand D 21 Guthrie & Robinson (1977)
Sweden D 10 Wester (1974)
Sweden M 17 Kjellström (1977)
United Kingdom M, D 10-20 Walters & Sherlock (1981)
USA M 41 Mahaffey et al. (1975)
Areas of elevated exposure
Japan M 211-245 Japan Public Health
Japan D 180-391 Association (1970)
Japan
(mean of 3 areas) D 136 Iwao et al. (1981a)
United Kingdom M 36 Sherlock et al. (1983)
United Kingdom D 29 Sherlock et al. (1983)
USA D 33 Spencer et al. (1979)
a M - Sample of foodstuffs individually analysed; market basket method
D - Duplicate portion study
Table 9. Estimates of average daily faecal cadmium elimination in various countries
Country Subjects investigated Estimates (µg Reference
cadmium/day)
Areas of normal exposure
Federal Republic
of Germany 23, sex and age of subjects 31 Essing et al. (1969)
not given
Japan 12 men, 50-59 years 81 Haga & Yamawaki (1974)
Japan 13 women, 50-59 years 56 Haga & Yamawaki (1974)
Japan 2 men, 35 and 37 years 36 Suzuki & Lu (1976)
(60 specimens)
Japan 7 men, 21-22 years 41-79 Tati et al. (1976)
(35 specimens)
Japan 64 men and women, 41 Kojima et al. (1975)
50-69 years
Japan 24-36 Iwao (1977)
Japan (rural area) 30 men, 50 years and over 49 Tsuchiya & Iwao (1978)
Sweden 4 adults (2 men, 23 years; 6-13 Wester (1974)
2 women, 28 and 31 years)
Sweden 70 men and 10 women 18 Kjellström et al. (1978)
(3-day collect)
USA 216 (men and women) 10-15 Kowal et al. (1979)
Areas of elevated exposure
Japan, Kosaka 40 men, 50-69 years 149 Haga & Yamawaki (1974)
Japan, Kosaka 47 women, 50-69 years 177 Haga & Yamawaki (1974)
Japan, Kosaka 118 men and women 146 Kojima et al. (1977)
Table 9. cont'd.
Country Subjects investigated Estimates (µg Reference
cadmium/day)
Japan, Kakehashi, 30 men, 50 years and over 149 Iwao et al. (1981b)
Kosaka, Tsushima
(rural areas)
New Zealand, 45 men and women, 50-500 McKenzie et al. (1982)
Bluff 20-70 years
5.3.2 General population, contaminated areas
Airborne cadmium in contaminated areas may reach levels of
0.5 µg/m3, which would lead to a daily inhalation of 7.5 µg and an
absorption of about 2 µg. For smokers, the contribution from tobacco
at 1-2 µg for every pack will not be changed, leading to a total
uptake of 3-4 µg from inhalation in such individuals.
The intake of cadmium from food and water varies considerably
and is related to both the extent of contamination and the reliance
on locally grown food items or local water supplies. Daily intakes
of 150-200 µg have been reported in contaminated areas where the
majority of the staple food items were of local origin. At an
absorption rate of 5%, the daily uptake from diet would be 8-10 µg.
The total daily cadmium uptake will depend on the nature of cadmium
contamination, i.e. whether food, water, and air levels are
elevated, but is unlikely to exceed 20 µg.
Cadmium intake in children via the ingestion of household dusts
is unlikely to be important except in the most contaminated
localities.
5.3.3 Occupational exposure to cadmium
Inhalation of workplace air is the dominant exposure pathway.
With air concentrations of 10-50 µg/m3 and the inhalation of
10 m3 air during a work-shift, the daily cadmium intake would be
100-500 µg. An absorption rate of 25% would thus lead to daily
uptakes of 25-125 µg. Dust particles cleared from the lungs may be
swallowed and dust-contaminated food items can also make a
significant contribution to the ingestion pathway. At an absorption
rate of 5% this could lead to an additional uptake 10-15 µg cadmium
to the total uptake.
Tobacco carried by workers can become contaminated and may
contribute up to 10 times more cadmium to the daily uptake than
under normal conditions (Piscator et al., 1976).
5.4 Conclusions
The major route of exposure to cadmium for the non-smoking
general population is via food; the contribution from other pathways
to total uptake is small. Tobacco is an important source of cadmium
uptake in smokers. In contaminated areas, cadmium exposure via food
may be up to several hundred µg/day. In exposed workers, lung
absorption of cadmium following inhalation of workplace air is the
major route of exposure. Increased uptake in workers can also occur
as a consequence of contamination of food and tobacco.
6. KINETICS AND METABOLISM IN LABORATORY MAMMALS
AND HUMANS
6.1 Uptake
6.1.1 Absorption by inhalation
Three processes in the lungs, i.e. deposition, mucociliary
clearance, and alveolar clearance, determine the absorption of
inhaled particles (Task Group on Lung Dynamics, 1966). Uptake into
epithelial cells, interstitium or the systemic circulation depends
on physical and biochemical processes in the respiratory tract after
deposition (e.g., mechanical clearance, solubilization, and
transport). The retained or accumulated dose at the local or
systemic target site resulting from the deposited dose may
eventually lead to biological effects. Length of exposure is of
major importance for chronic effects, particularly lung cancer.
Therefore, chronic effects might be expected to correlate with
retained or accumulated dose rather than deposited dose.
Extrapolation models of inhaled cadmium dosage from animal
models to humans and from high exposures (experimental) to low
(environmental) must incorporate the above variables. When
extrapolating from one species to another, specific pulmonary
retention must be taken into account. In both acute and chronic
inhalation exposures, a dose-response relationship is best described
with accumulated rather than deposited dose (Oberdorster, 1988).
The absorption of cadmium compounds may vary greatly. As
discussed in section 5.1.2, the proportion of particles in
industrial air that are respirable, i.e. up to 5 µm MMAD, may vary
widely (Materne et al., 1975). These particles will be deposited in
the alveoli (Task Group on Lung Dynamics, 1966).
There are some empirical data on the overall absorption of
cadmium. In various acute and chronic animals experiments, 5 to 20%
of inhaled cadmium has been found to be deposited in the lungs
(Friberg et al., 1986). Actual absorption may vary between 50 and
100% of the amount deposited and may continue for weeks after the
deposition of a single dose. The absorption of an aerosol of cadmium
chloride is higher than that of cadmium oxide, and alveolar
absorption is higher after intratracheal instillation than after
inhalation of an aerosol (Friberg et al., 1985).
If the particles are deposited in the alveoli, then the
majority will sooner or later be absorbed, regardless of solubility.
Cadmium chloride passes the alveolar-blood barrier with ease,
although inhaled cadmium sulfide has a greater tendency to be
retained in the lungs, indicating slower absorption. Three weeks
after exposure of Syrian hamsters to cadmium chloride aerosol, about
25-35% of the initial lung burden was present in the liver, kidneys,
and skull. The lungs still contained 50% of the initial lung burden
at this time (Henderson et al., 1979).
Data on the respiratory absorption of cadmium in humans comes
largely from comparisons of smokers and non-smokers. On the basis of
data on organ burdens of cadmium and smoking history, Elinder et al.
(1976) calculated that about 50% of the cadmium inhaled via
cigarette smoke could be absorbed.
6.1.2 Absorption via the intestinal tract
Factors affecting the absorption of ingested cadmium include
animal species, type of compound, dose, frequency of administration,
age of experimental animals, pregnancy and lactation, presence or
absence of drugs, and interactions of cadmium with various nutrients
(Nomiyama, 1978). A study in which cadmium chloride was given in
drinking-water to rats over a period of 12 months showed retention
in the kidney and liver of less than 1% of the total amount ingested
(Decker et al., 1958). There have been many reports of single
exposure studies. These may be summarized as follows: the individual
absorption of cadmium nitrate or chloride after single exposure
ranges from 0.5 to 8% (Friberg et al., 1974). Limited observations
in humans given radioactive cadmium indicate that the average
absorption is about 5% (Kitamura, 1972; Rahola et al., 1972;
Yamagata et al., 1974; Flanagan et al., 1978).
Metallothionein-bound cadmium in food does not appear to be
absorbed and/or distributed in the same way as inorganic cadmium
compounds. Mice exposed to cadmium-thionein (Cherian et al., 1978)
had lower blood and liver cadmium levels but a higher kidney level
than mice exposed to the same amount of cadmium as the chloride.
Similar results were reported by Sullivan et al. (1984) in mice fed
inorganic or oyster-incorporated radiolabelled cadmium. Cadmium in
New Zealand Bluff oysters is to a great extent bound to a
metallothionein-like protein (Nordberg et al., 1986). However, in
other species of oysters, most of the cadmium is bound to proteins
with relative molecular masses above 50 000 and lesser amounts to
small proteins (< 3000) (Casterline & Yip, 1975; Kodama et al.,
1978). Bluff oyster fishermen with an extremely high cadmium intake
(up to 500 µg per day) from oyster consumption were found to have
increased blood and urine cadmium levels (Sharma et al., 1983), but
the increase was not as great as expected from the total cadmium
ingested. This indicates that in humans, as in other animal species,
metallothionein-bound cadmium in food may be dealt with in a
different way from other cadmium compounds.
There are no data from humans studies showing a relationship
between gastrointestinal absorption of cadmium and age. Studies on
mice reported by Matsusaka et al. (1972), however, show
approximately 10% whole body retention 2 weeks after ingestion for
young mice, while the corresponding figure for adult mice was 1%.
Kello & Kostial (1977) and Engstrom & Nordberg (1979b) also
demonstrated that neonatal mice absorbed cadmium to a much greater
extent than adult mice.
Diets with low levels of calcium and protein promote increased
absorption of cadmium through the intestinal tract, up to 3 times
the absorption having been noted in several studies in experimental
animals (Friberg et al., 1974, 1975). It has also been shown that
iron-deficient animals may have a higher absorption of cadmium
(Hamilton & Valberg, 1974), and these findings have been confirmed
in humans (Flanagan et al., 1978). Women with low body iron stores,
as reflected by low serum ferritin levels, had on average, a
gastrointestinal absorption rate twice as high (about 10%) as a
control group of women. The highest individual absorption rate was
about 20%. Interrelationships between cadmium exposure and the
absorption of copper, zinc, and calcium will be discussed in section
7.5.
6.1.3 Absorption via skin
Limited skin penetration (1.8% per 5 h) of soluble cadmium
compounds can take place when they are applied as a solution to the
skin (Skog & Wahlberg, 1964). The dermal absorption rate was
estimated by Kimura & Otaki (1972) in shaved rabbits and nude mice
painted with an aqueous solution of cadmium chloride. Rabbits
painted 5 times in 3 weeks showed a combined cadmium accumulation of
0.4-0.6% of the amount applied, and mice painted 1-4 times in one
week showed an accumulation of 0.2-0.8% of the applied dose.
6.1.4 Transplacental transfer
The movement of cadmium through the placenta is limited. It has
been shown that cadmium given to pregnant mice and hamsters during
early pregnancy reaches the yolk sac and the primitive gut of the
embryo, which are connected by the vitelline duct (Dencker et al.,
1983). However, after closure of the vitelline duct during the later
stages of pregnancy, very little cadmium reaches the fetus (Ahokas &
Dilts, 1979). Sonawane et al. (1975) found that less than 0.02% of
the total dose of cadmium injected intravenously into rat dams
reached the fetus.
The cadmium concentration of the human placenta is usually
about 5-20 µg/kg wet weight (Thieme et al., 1977; Copius-Peereboom
et al., 1979). The placentas of women who smoke during pregnancy
have higher levels than those of non-smokers (Copius-Peereboom et
al., 1979).
Fetal (umbilical cord) blood cadmium levels are about 40-50%
less than those of maternal blood. However, levels of the
metabolically related essential metals zinc and copper in fetal
blood are similar to or higher than those in maternal blood, this
resulting in a fetal-maternal gradient (Lauwerys et al., 1978; Roels
et al., 1978; Kuhnert et al., 1982; Korpela et al., 1986). The
effectiveness of the gradient or its mechanism, as well as the
potential toxicity of cadmium to the fetus, is not really known.
Transplacental transport of cadmium is minimized in the normal
healthy placenta presumably by the binding of cadmium to
metallothionein. Metallo-thionein also serves as a site for
intracellular zinc and copper sequestration. These observations
suggest that there is a selective barrier to transplacental
transport of cadmium. This is not the case with lead or mercury
where fetal blood levels are similar to maternal levels (Lauwerys et
al., 1978; Korpela et al., 1986).
6.2 Transport
Human data on the transport of cadmium from the site of
absorption to the various organs are not available. This section is,
therefore, based on animal studies, although there are some
indications that similar mechanisms operate in humans. For instance,
metallothionein has been isolated from human tissues (section 6.8)
and has been measured in human plasma (Nordberg et al., 1982), where
it binds cadmium being transported between tissues.
A study on dogs showed that, immediately after parenteral
administration, most of the cadmium was present in the plasma (Walsh
& Burch, 1959). This has been verified in a large number of animal
studies (Friberg et al., 1974). Plasma concentrations decrease
rapidly during the first hours after injection, reaching a level
that is less than 1% of the initial value at 24 h, and this level
then decreases much more slowly. During the early, fast-elimination
phase, cadmium in mouse plasma is mainly bound to plasma proteins
with a molecular weight of 40 000 to 60 000 (probably albumin),
whereas in the slower phase (more than 24 h after injection), it is
partly bound to a low molecular weight (LMW) protein of the same
size as metallothionein (Nordberg, 1978). After rats were repeatedly
exposed by subcutaneous injection (up to 14 weeks), the cadmium in
plasma was partly bound to proteins with a molecular weight of
40 000 to 60 000 and partly to a LMW protein with a molecular weight
similar to that of metallothionein (Cherian & Shaikh, 1975; Shaikh &
Hirayama, 1979). The proportion of plasma cadmium bound to
metallothionein and larger proteins, respectively, is considered to
vary with the length and type of exposure. It is likely that the LMW
cadmium-binding protein is in fact metallothionein, since it was
shown by Vander Mallie & Garvey (1979) by a radioimmunological
technique that the metallothionein concentration increased in the
plasma of rats given 40 intraperitoneal injections of cadmium
chloride in saline (0.12 mg/day, five days/week).
The concentration of cadmium in blood cells increases rapidly
after a single intravenous injection (1 mg/kg body weight) and,
within a few hours, reaches a first peak concentration exceeding
that of the plasma. Although the levels of cadmium per cell may be
10 times higher in leucocytes than in red cells, the total cadmium
in the leucocyte portion of the blood is negligible compared to that
in the red cells (Garty et al., 1981).
Cadmium in erythrocytes may partly be bound to haemoglobin
(Carlson & Friberg, 1957; Nomiyama et al., 1978a). However, during
the first hour after a single subcutaneous injection, a large
proportion of the cadmium in erythrocytes is bound to proteins with
a molecular weight larger than haemoglobin (Nordberg, 1972). Between
96 and 196 h after a single injection (1 mg/kg body weight), it has
been shown in mice (Nordberg, 1972), as well as in rats (Garty et
al., 1981), that cadmium is also bound to a LMW protein. Whether
this protein is identical with metallothionein is uncertain
(Nordberg, 1984). A part of the erythrocyte cadmium in rats was also
found in erythrocyte ghosts (membranes) (Garty et al., 1981). When
mice were exposed by subcutaneous injection to cadmium chloride
(0.25 mg/kg body weight) for periods of between 6 days and 5 months
(Nordberg et al., 1971), most of the erythrocyte cadmium was bound
to a LMW protein similar to metallothionein.
Since metallothionein-bound cadmium is quickly cleared from the
plasma by the kidneys (Nordberg & Nordberg, 1975; Vostal, 1976),
this LMW fraction may be of great importance for the transport of
cadmium from liver to kidney during long-term exposure. Hepatic
metallothionein may be released into the blood in the same manner as
hepatic enzymes and transported to the kidney and urine in some
types of hepatic disorders (Tanaka, 1982).
6.3 Distribution
6.3.1 In animals
The highest cadmium levels in exposed animals are generally
found in the liver and renal cortex. However, the distribution in
the body varies according to the route of administration.
6.3.1.1 Single exposure
Studies on various species have shown that, after a single
administration of cadmium by the oral or parenteral routes, the
highest organ burden of cadmium is initially found in the liver.
However, kidney levels of cadmium increase for up to 8 months after
exposure and may then exceed the liver levels (Gunn & Gould, 1957).
The pancreas and spleen also show relatively high concentrations
(Nordberg & Nishiyama, 1972). This topic has been reviewed by
Friberg et al. (1974) and Nomiyama (1978).
6.3.1.2 Repeated exposure
The literature on the fate of cadmium in animals after repeated
exposure via various routes has been reviewed by Friberg et al.
(1985) and, with emphasis on Japanese studies, by Nomiyama (1978).
Liver cadmium levels increase rapidly, and a re-distribution of
cadmium to the kidney occurs over a period of time. The higher the
intensity of exposure, the higher the initial liver-to-kidney
concentration ratio. The route of administration has been shown to
be an important variable affecting the distribution of cadmium. When
cadmium was administered subcutaneously, 11 times more was deposited
in the liver than in the kidneys, whereas orally administered
cadmium was distributed almost equally between these two organs
(Nomiyama et al., 1976).
When rabbits were injected subcutaneously with 0.5 mg cadmium
chloride daily, concentrations of cadmium in the liver and renal
cortex reach a peak after about 10 and 15 weeks exposure,
respectively. In cases of renal damage, urinary excretion increases
(section 6.5.1.1) and the renal and liver concentrations decrease
(Bonnell et al., 1960; Nomiyama et al., 1982b).
6.3.2 In humans
Cadmium is stored to the greatest extent in the liver and
kidneys, the renal cortex showing the highest concentration in
people who have not been exposed to excess cadmium (Friberg et al.,
1974). The lowest concentrations (wet weight) are found in the
brain, bone, and fat (Sumino et al., 1975; Cherry, 1981). Cadmium
levels in the organs of second and third trimester fetuses (Chaube
et al., 1973) and in newborn babies and young children (Henke et
al., 1970) are lower by three orders of magnitude than in adult
females. The placenta contains somewhat higher concentrations than
maternal blood, brain or fat (section 6.1.4).
It has been calculated that about a third of the body burden in
a non-smoking male from the USA is in the kidney and about a quarter
in the liver and muscles. These are the tissues with the longest
biological half-time of cadmium (section 6.6.2). In spite of the low
cadmium concentration in the muscles, the contribution to the total
body burden is great due to the large weight of the muscles. Other
tissues that contribute significantly to body burden are bone, skin,
and fat (Kjellström (1979).
In cadmium workers and people in the general environment
exposed to high levels of cadmium, the liver or kidneys show the
highest concentration, depending on exposure time, exposure levels,
and the level of renal function (Friberg et al., 1974, 1985).
6.4 Body burden and kidney burden in humans
The newborn baby is practically free of cadmium (section
6.1.4), and the concentrations of cadmium in the organs increase
with age (Schroeder & Balassa, 1961; Anke & Schneider, 1974; Elinder
et al., 1976; Tsuchiya et al., 1976; Kowal et al., 1979; Chung et
al., 1986). The accumulation in human liver and muscles is shown in
Figs. 1 and 2, respectively. These data and those of Vahter (1982)
(Fig. 3) reveal important differences between people from different
countries. Great individual variation also exists, even among people
from the same area (Tsuchiya et al., 1976). For example, the
geometric mean concentration of cadmium in the renal cortex of 117
adults aged between 30 and 59 in Stockholm was 19 mg/kg (Elinder et
al., 1976). The individual concentrations followed a log-normal
distribution with a geometric standard deviation of 2.0. This means
that about 15% of the population would have values higher than
38 mg/kg, and 2.5% values higher than 76 mg/kg. Similarly shaped
distributions were found for the kidneys, liver, pancreas, and
muscle (Tsuchiya & Iwao, 1978; Kowal et al., 1979; Vuori et al.,
1979).
The critical organ in long-term exposure to low concentrations
of cadmium is the kidney (section 6.7). Initial cadmium-induced
effects occur mainly in the proximal tubules, situated in the cortex
of the kidney. Therefore, cadmium concentrations in the renal cortex
and the distribution of cadmium within the kidney are of key
importance. The weight of the renal cortex is about 2-3 times
greater than the weight of the renal medulla, and early estimates of
renal cortex cadmium concentrations (Friberg et al., 1974) were 1.5
times higher than the whole kidney concentrations. A recent study
specifically aimed at measuring this concentration ratio
(Svartengren et al., 1986) yielded an average value of 1.25 for
people aged 30-50. This figure will be used in this document when it
is necessary to recalculate whole kidney concentrations from renal
cortex concentrations for that age group. This is the best estimate
available at present, although the ratio may vary depending upon the
age groups and racial types studied.
Table 10 shows the average cadmium concentrations in renal
cortex and liver for the 20-59-year age group, and includes the
major studies that have reported age-specific data. Unfortunately,
no information on smoking habits was given in most studies. It has
been shown that smoking cigarettes may significantly increase the
body burden of cadmium (Lewis et al., 1972). Elinder et al. (1976)
showed that Swedish smokers have, on average, about twice the tissue
cadmium concentration of non-smokers. Except for the data from India
(Fig. 3), there appears to be a constant difference (10 mg/kg)
between smokers and non-smokers in the cadmium concentrations of the
renal cortex. Fig. 3 also shows that the 90th percentile is usually
about twice the geometric mean value. In most countries referred to
in Table 10, the average cortex cadmium concentration was in the
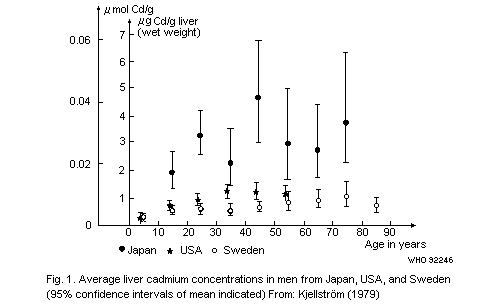
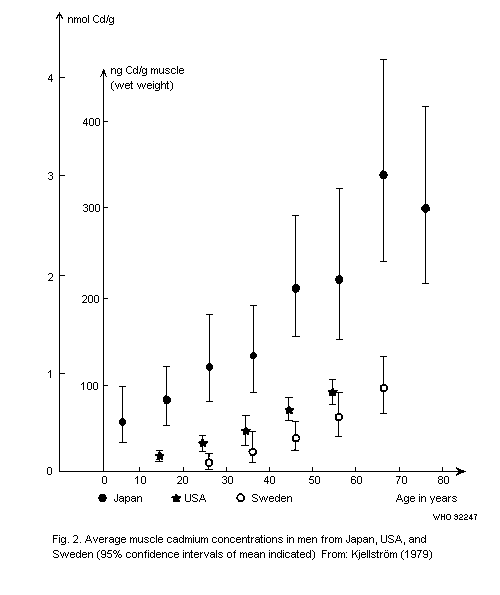
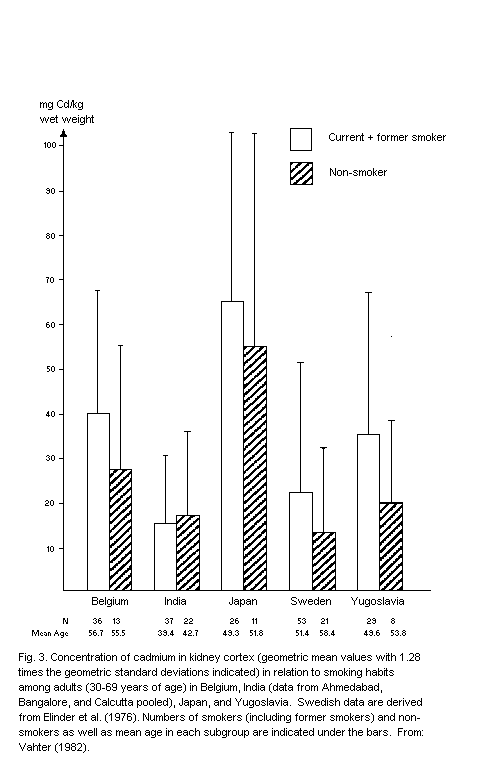 range 10-40 mg/kg, while in Japan values of between 50 and 100 mg/kg
were reported. In workers highly exposed to cadmium, but without
functional impairment of the kidney, concentrations in the renal
cortex may range from 180 to 450 mg/kg wet weight. In cases where
there is severe renal dysfunction, the cadmium concentrations are
generally lower and range between 20 and 120 mg/kg wet weight, i.e.
they are of the same magnitude as those of the general population
(Friberg et al., 1974). This seemingly paradoxical relationship is
discussed in more detail in section 6.
If exposure to cadmium throughout life remains constant and low
in amount, the concentrations in the kidneys become higher (by about
10-20 times) than those in the liver. Average liver cadmium
concentrations are about 1-2 mg/kg wet weight at age 50 in some
European countries and the USA, but in Japan average concentrations
are between 5 and 10 mg/kg (Table 10). Although renal concentrations
generally decrease after age 60 (Fig. 4), liver concentrations reach
a plateau but do not show a clear decrease in aged populations
(Fig. 1). In exposed workers, liver concentrations from 20 to about
300 mg/kg have been recorded and in Itai-itai patients they are
between 63 and 132 mg/kg (Friberg et al., 1974). In people with
severe cadmium-induced renal dysfunction, kidney cadmium levels are
low, but the liver levels may be very high (Ishizaki, 1972).
Table 10. Cadmium concentrations in the renal cortex and liver of people from various geographical areasa
Country Number Sex Age group Smoking Renal cortex Liver cadmium Reference
category cadmium level level (mg/kg
(mg/kg wet wet weight)
weight)
Belgium (Liege) 51 M, F 40-59 mixed 46b - Vahter (1982)
German Democratic 20 M 40-59 mixed 22c - Anke & Schneider (1974)
Republic
20 F 40-59 mixed 11c - Anke & Schneider (1974)
India 26 M, F 40-59 mixed 24b - Vahter (1982)
Israel (Jerusalem) 11 M, F 40-59 mixed 28b - Vahter (1982)
Japan (Kobe) 6 M, F 50-59 mixed 54 5.0 Kitamura et al. (1970)
Japan (Kanazawa) 9 M, F 40-59 mixed 95 10 Ishizaki (1972)
Japan (Tokyo) 17 M, F 40-59 mixed 99 5.7 Tsuchiya et al. (1976)
Japan (Tokyo) 23 M, F 40-59 mixed 76b - Vahter (1982)
Sweden (Stockholm) 83 M, F 40-59 mixed 20 0.76 Elinder et al. (1976)
45 M 40-59 mixed 18 0.69 Elinder et al. (1976)
38 F 40-59 mixed 23 0.88 Elinder et al. (1976)
7 M, F 40-59 non-smoker 11 0.51 Elinder et al. (1976)
28 M, F 40-59 smoker 23 0.97 Elinder et al. (1976)
USA (North Carolina) 19 M 40-59 mixed 27d 2.2 Hammer et al. (1973)
10 F 40-59 mixed 23d 2.2 Hammer et al. (1973)
10 M 40-79 non-smoker 14d 1.6 Hammer et al. (1973)
18 M 40-79 smoker 28d 3.2 Hammer et al. (1973)
Table 10 (contd).
Country Number Sex Age group Smoking Renal cortex Liver cadmium Reference
category cadmium level level (mg/kg
(mg/kg wet wet weight)
weight)
USA (Dallas) 58 M 40-59 mixed 29 1.4 Kowal et al. (1979)
47 M 20-59 non-smoker 13 1.02 Kowal et al. (1979)
115 M 20-59 smoker 24 1.31 Kowal et al. (1979)
USA (Baltimore) 10 M, F 40-59 mixed 30b - Vahter (1982)
Yugoslavia (Zagreb) 28 M, F 40-59 mixed 38b - Vahter (1982)
a The cadmium concentrations are arithmetic mean values and have been rounded off.
b Original data were geometric means. Adjusted according to the findings of Elinder et al. (1976) (x 1.18).
c Original data were for whole kidney (dry weight). Data adjusted for whole kidney (x 1.25) and dry weight (x 0.21).
d Original data were based on ash weight. Data adjusted for ash weight (x 0.011).
In vivo neutron activation analysis (see section 2.2.3.3) has
recently been used to measure kidney and liver cadmium
concentrations in exposed workers. In one study, the detection
limits were about 15 mg/kg for kidney and 1.5 mg/kg for liver (Ellis
et al., 1981a), while in another study detection limits were higher
by a factor of 2 for kidney and 5 for liver (Roels et al., 1981b).
Thus, this method is still not sufficiently sensitive to measure
in vivo tissue levels in a "normal" population. Liver levels of up
to 120 mg/kg and kidney cortex levels of up to 600 mg/kg have been
found among cadmium workers (Ellis et al., 1981a). A decreasing
trend of the kidney levels after a maximum at about 300 mg/kg in
kidney cortex was evident. At this point, the liver level was about
30 mg/kg (Fig. 5). A very similar situation was found in another
factory (Roels et al., 1981b); most workers with high liver cadmium
levels had low kidney levels, and then also showed elevated urinary
excretion of ß2-microglobulin. In exposed workers, the average
ratio of the cadmium concentration in the renal cortex to that in
the liver has been reported to be about 8 (Ellis et al., 1981a) or 7
(Roels et al., 1981b), values that are lower than for the general
population (Table 9). This corresponds to animal data (section
6.3.1.2) showing a greater proportion of accumulated cadmium in the
liver when the exposure level increases.
The total body burden of cadmium in a middle-aged person within
the general population is about twice the amount in kidneys and
liver together (Table 10), i.e. 5-7 mg in a non-smoker in Europe or
the USA and 8-13 mg in a smoker (Kjellström, 1979). In Japan, higher
body burdens have been reported (Tipton et al., 1960; Ishizaki et
al., 1971; Sumino et al., 1975; Tsuchiya et al., 1976). An extensive
review of data from several countries (Cherry, 1981) found total
body burdens to lie within the range 5-20 mg.
In conclusion, the average total body burden of a person of 50
years of age, living in an area not subject to pollution, varies
within the range 5-20 mg in different regions of the world, and the
average cadmium concentration in the renal cortex varies within the
range 11-100 mg/kg wet weight. There is a great individual
variation, and the 90th percentile in those groups studied is about
twice the median value.
Smoking increases the body burden. After long-term low-level
exposure, about half the body burden of cadmium is localized in the
kidneys and liver, a third of the total being in the kidneys. At
higher levels of exposure, a greater proportion of the body burden
is found in the liver. After the development of severe
cadmium-induced renal dysfunction, cadmium is lost from the renal
tissue.
range 10-40 mg/kg, while in Japan values of between 50 and 100 mg/kg
were reported. In workers highly exposed to cadmium, but without
functional impairment of the kidney, concentrations in the renal
cortex may range from 180 to 450 mg/kg wet weight. In cases where
there is severe renal dysfunction, the cadmium concentrations are
generally lower and range between 20 and 120 mg/kg wet weight, i.e.
they are of the same magnitude as those of the general population
(Friberg et al., 1974). This seemingly paradoxical relationship is
discussed in more detail in section 6.
If exposure to cadmium throughout life remains constant and low
in amount, the concentrations in the kidneys become higher (by about
10-20 times) than those in the liver. Average liver cadmium
concentrations are about 1-2 mg/kg wet weight at age 50 in some
European countries and the USA, but in Japan average concentrations
are between 5 and 10 mg/kg (Table 10). Although renal concentrations
generally decrease after age 60 (Fig. 4), liver concentrations reach
a plateau but do not show a clear decrease in aged populations
(Fig. 1). In exposed workers, liver concentrations from 20 to about
300 mg/kg have been recorded and in Itai-itai patients they are
between 63 and 132 mg/kg (Friberg et al., 1974). In people with
severe cadmium-induced renal dysfunction, kidney cadmium levels are
low, but the liver levels may be very high (Ishizaki, 1972).
Table 10. Cadmium concentrations in the renal cortex and liver of people from various geographical areasa
Country Number Sex Age group Smoking Renal cortex Liver cadmium Reference
category cadmium level level (mg/kg
(mg/kg wet wet weight)
weight)
Belgium (Liege) 51 M, F 40-59 mixed 46b - Vahter (1982)
German Democratic 20 M 40-59 mixed 22c - Anke & Schneider (1974)
Republic
20 F 40-59 mixed 11c - Anke & Schneider (1974)
India 26 M, F 40-59 mixed 24b - Vahter (1982)
Israel (Jerusalem) 11 M, F 40-59 mixed 28b - Vahter (1982)
Japan (Kobe) 6 M, F 50-59 mixed 54 5.0 Kitamura et al. (1970)
Japan (Kanazawa) 9 M, F 40-59 mixed 95 10 Ishizaki (1972)
Japan (Tokyo) 17 M, F 40-59 mixed 99 5.7 Tsuchiya et al. (1976)
Japan (Tokyo) 23 M, F 40-59 mixed 76b - Vahter (1982)
Sweden (Stockholm) 83 M, F 40-59 mixed 20 0.76 Elinder et al. (1976)
45 M 40-59 mixed 18 0.69 Elinder et al. (1976)
38 F 40-59 mixed 23 0.88 Elinder et al. (1976)
7 M, F 40-59 non-smoker 11 0.51 Elinder et al. (1976)
28 M, F 40-59 smoker 23 0.97 Elinder et al. (1976)
USA (North Carolina) 19 M 40-59 mixed 27d 2.2 Hammer et al. (1973)
10 F 40-59 mixed 23d 2.2 Hammer et al. (1973)
10 M 40-79 non-smoker 14d 1.6 Hammer et al. (1973)
18 M 40-79 smoker 28d 3.2 Hammer et al. (1973)
Table 10 (contd).
Country Number Sex Age group Smoking Renal cortex Liver cadmium Reference
category cadmium level level (mg/kg
(mg/kg wet wet weight)
weight)
USA (Dallas) 58 M 40-59 mixed 29 1.4 Kowal et al. (1979)
47 M 20-59 non-smoker 13 1.02 Kowal et al. (1979)
115 M 20-59 smoker 24 1.31 Kowal et al. (1979)
USA (Baltimore) 10 M, F 40-59 mixed 30b - Vahter (1982)
Yugoslavia (Zagreb) 28 M, F 40-59 mixed 38b - Vahter (1982)
a The cadmium concentrations are arithmetic mean values and have been rounded off.
b Original data were geometric means. Adjusted according to the findings of Elinder et al. (1976) (x 1.18).
c Original data were for whole kidney (dry weight). Data adjusted for whole kidney (x 1.25) and dry weight (x 0.21).
d Original data were based on ash weight. Data adjusted for ash weight (x 0.011).
In vivo neutron activation analysis (see section 2.2.3.3) has
recently been used to measure kidney and liver cadmium
concentrations in exposed workers. In one study, the detection
limits were about 15 mg/kg for kidney and 1.5 mg/kg for liver (Ellis
et al., 1981a), while in another study detection limits were higher
by a factor of 2 for kidney and 5 for liver (Roels et al., 1981b).
Thus, this method is still not sufficiently sensitive to measure
in vivo tissue levels in a "normal" population. Liver levels of up
to 120 mg/kg and kidney cortex levels of up to 600 mg/kg have been
found among cadmium workers (Ellis et al., 1981a). A decreasing
trend of the kidney levels after a maximum at about 300 mg/kg in
kidney cortex was evident. At this point, the liver level was about
30 mg/kg (Fig. 5). A very similar situation was found in another
factory (Roels et al., 1981b); most workers with high liver cadmium
levels had low kidney levels, and then also showed elevated urinary
excretion of ß2-microglobulin. In exposed workers, the average
ratio of the cadmium concentration in the renal cortex to that in
the liver has been reported to be about 8 (Ellis et al., 1981a) or 7
(Roels et al., 1981b), values that are lower than for the general
population (Table 9). This corresponds to animal data (section
6.3.1.2) showing a greater proportion of accumulated cadmium in the
liver when the exposure level increases.
The total body burden of cadmium in a middle-aged person within
the general population is about twice the amount in kidneys and
liver together (Table 10), i.e. 5-7 mg in a non-smoker in Europe or
the USA and 8-13 mg in a smoker (Kjellström, 1979). In Japan, higher
body burdens have been reported (Tipton et al., 1960; Ishizaki et
al., 1971; Sumino et al., 1975; Tsuchiya et al., 1976). An extensive
review of data from several countries (Cherry, 1981) found total
body burdens to lie within the range 5-20 mg.
In conclusion, the average total body burden of a person of 50
years of age, living in an area not subject to pollution, varies
within the range 5-20 mg in different regions of the world, and the
average cadmium concentration in the renal cortex varies within the
range 11-100 mg/kg wet weight. There is a great individual
variation, and the 90th percentile in those groups studied is about
twice the median value.
Smoking increases the body burden. After long-term low-level
exposure, about half the body burden of cadmium is localized in the
kidneys and liver, a third of the total being in the kidneys. At
higher levels of exposure, a greater proportion of the body burden
is found in the liver. After the development of severe
cadmium-induced renal dysfunction, cadmium is lost from the renal
tissue.
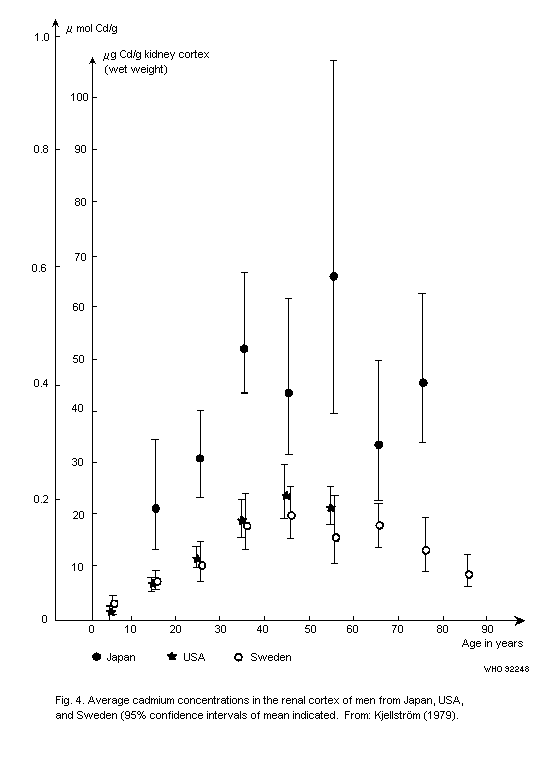
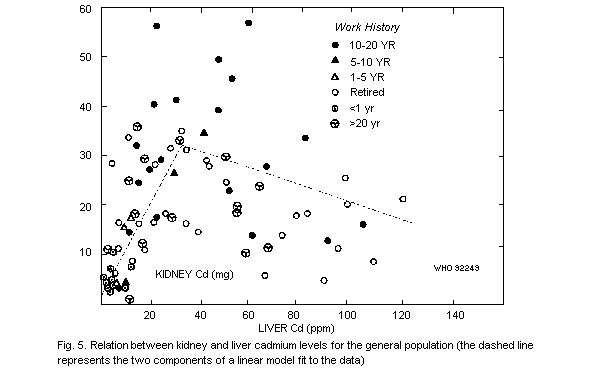 6.5 Elimination and excretion
6.5.1 Urinary excretion
6.5.1.1 In animals
Nordberg (1972) demonstrated that after subcutaneous injection
for up to 24-25 weeks, the average daily urinary cadmium excretion
(on a group basis) in mice, prior to the onset of tubular
proteinuria, represented about 0.01-0.02% of the body burden
(section 6.7.1). Elinder & Pannone (1979) showed that one month
after repeated subcutaneous exposure ceased, the excretion was only
0.001% of the body burden.
Similar low excretion rates have been found in rabbits given
subcutaneous injections (Nomiyama, 1973a; Nomiyama & Nomiyama,
1976a), and in rabbits (Nomiyama & Nomiyama, 1976a,b) and monkeys
(Nomiyama et al., 1979, 1982a) given cadmium orally. In addition, it
has been reported that, over a range of doses, an increase in
urinary excretion of cadmium is associated with an increase of
cadmium in the renal cortex (Nomiyama & Nomiyama, 1976a; Suzuki,
1980; Bernard et al., 1981).
Studies on several mammalian species, mainly involving repeated
subcutaneous injection of cadmium salts, have shown that urinary
excretion of cadmium increases slowly for a considerable time but,
as kidney dysfunction develops, a sharp increase in excretion occurs
in rabbits (Friberg, 1952; Axelsson & Piscator, 1966a; Nomiyama &
Nomiyama, 1976a), mice (Nordberg & Piscator, 1972), and rats
(Suzuki, 1980). This leads to a decrease in renal and liver cadmium
concentrations (Axelsson & Piscator, 1966a; Suzuki, 1980; Nomiyama &
Nomiyama, 1976a).
When renal tubular lesions were induced by uranyl acetate
injections in animals previously exposed to cadmium, there was no
increase in urinary cadmium excretion (Nomiyama & Nomiyama, 1976a)
or decrease in the level of cadmium in the renal cortex. This
contrasts with the increase in cadmium excretion brought about by
cadmium-induced tubular lesions.
6.5.1.2 In humans
Several studies have shown that in the general population
urinary cadmium excretion increases with age (Katagiri et al., 1971;
Tsuchiya et al., 1976; Elinder et al., 1978; Kowal et al., 1979)
(Fig. 6), this increase coinciding with the increased body burden.
Smokers have higher urinary excretion than non-smokers (Elinder et
al., 1978; Kowal et al., 1979). The mean concentration of urinary
cadmium in such groups of people not exposed to high cadmium levels
is < 0.5-2.0 µg/litre or approximately 0.01% of the total body
burden.
Increased urinary cadmium excretion occurs when tubular
proteinuria develops (Lauwerys et al., 1974a; Kojima et al., 1977).
In cadmium exposed workers, high urinary cadmium concentrations in
the absence of proteinuria can be found after only short exposures
(Lauwerys et al., 1976, 1979a,b) (section 6.7.1).
Most of the cadmium in urine is probably transported bound to
metallothionein. The urinary metallothionein concentration can now
be measured quantitatively with a sensitive radioimmunoassay (Vander
Mallie & Garvey, 1979). Using this technique, Tohyama et al. (1981b)
found good correlation between urinary metallothionein and cadmium
in 67 people exposed in the general environment, and Roels et al.
(1983b) confirmed this correlation in 94 cadmium workers.
6.5.2 Gastrointestinal and other routes of excretion
It is extremely difficult to study gastrointestinal excretion
after oral exposure, since it is not possible to distinguish net
gastroin-testinal excretion from unabsorbed cadmium in faeces.
Animal studies of gastrointestinal excretion following
injections of cadmium (summarized by Friberg et al., 1974) generally
show that a few percent of the dose is excreted in the faeces within
the first few days after injection. The faecal excretion is
initially higher than the urinary excretion after either single or
repeated exposure (Nomiyama, 1978). The mechanism for such excretion
probably involves a transfer of cadmium via the intestinal mucosa,
but biliary excretion may also be involved. The biliary excretion in
the first 24 h after intravenous injection of cadmium is dependent
on the dose (Cirkt & Tichy, 1974; Nomiyama, 1974; Klaassen &
Kotsonis, 1977). In rats given 67, 90 or 120 ßg cadmium (Cikrt &
Tichy, 1974), the cumulative 24 h excretion reached 0.83% at the
lowest dose and 5.68% at the highest dose. The highest excretion
rate was detected between 15 and 30 min after dosing. It has been
reported that after the initially rapid excretion the biliary
excretion is 0.015-0.04% of the body burden per hour over three
consecutive days (Nordberg et al., 1977; Elinder & Pannone, 1979).
Biliary cadmium has been partially characterized as a glutathione
complex (Cherian & Vostal 1977).
Both during and after parenteral exposure to cadmium, the total
gastrointestinal cadmium excretion is considerably higher than the
urinary excretion (Nordberg, 1972; Elinder & Pannone, 1979). A large
proportion of the gastrointestinal excretion is directly related to
the daily dose. After chronic exposure of rats, faecal excretion
amounted to about 0.03% of the body burden, which was considerably
more than the urinary excretion (Elinder & Pannone, 1979).
6.5 Elimination and excretion
6.5.1 Urinary excretion
6.5.1.1 In animals
Nordberg (1972) demonstrated that after subcutaneous injection
for up to 24-25 weeks, the average daily urinary cadmium excretion
(on a group basis) in mice, prior to the onset of tubular
proteinuria, represented about 0.01-0.02% of the body burden
(section 6.7.1). Elinder & Pannone (1979) showed that one month
after repeated subcutaneous exposure ceased, the excretion was only
0.001% of the body burden.
Similar low excretion rates have been found in rabbits given
subcutaneous injections (Nomiyama, 1973a; Nomiyama & Nomiyama,
1976a), and in rabbits (Nomiyama & Nomiyama, 1976a,b) and monkeys
(Nomiyama et al., 1979, 1982a) given cadmium orally. In addition, it
has been reported that, over a range of doses, an increase in
urinary excretion of cadmium is associated with an increase of
cadmium in the renal cortex (Nomiyama & Nomiyama, 1976a; Suzuki,
1980; Bernard et al., 1981).
Studies on several mammalian species, mainly involving repeated
subcutaneous injection of cadmium salts, have shown that urinary
excretion of cadmium increases slowly for a considerable time but,
as kidney dysfunction develops, a sharp increase in excretion occurs
in rabbits (Friberg, 1952; Axelsson & Piscator, 1966a; Nomiyama &
Nomiyama, 1976a), mice (Nordberg & Piscator, 1972), and rats
(Suzuki, 1980). This leads to a decrease in renal and liver cadmium
concentrations (Axelsson & Piscator, 1966a; Suzuki, 1980; Nomiyama &
Nomiyama, 1976a).
When renal tubular lesions were induced by uranyl acetate
injections in animals previously exposed to cadmium, there was no
increase in urinary cadmium excretion (Nomiyama & Nomiyama, 1976a)
or decrease in the level of cadmium in the renal cortex. This
contrasts with the increase in cadmium excretion brought about by
cadmium-induced tubular lesions.
6.5.1.2 In humans
Several studies have shown that in the general population
urinary cadmium excretion increases with age (Katagiri et al., 1971;
Tsuchiya et al., 1976; Elinder et al., 1978; Kowal et al., 1979)
(Fig. 6), this increase coinciding with the increased body burden.
Smokers have higher urinary excretion than non-smokers (Elinder et
al., 1978; Kowal et al., 1979). The mean concentration of urinary
cadmium in such groups of people not exposed to high cadmium levels
is < 0.5-2.0 µg/litre or approximately 0.01% of the total body
burden.
Increased urinary cadmium excretion occurs when tubular
proteinuria develops (Lauwerys et al., 1974a; Kojima et al., 1977).
In cadmium exposed workers, high urinary cadmium concentrations in
the absence of proteinuria can be found after only short exposures
(Lauwerys et al., 1976, 1979a,b) (section 6.7.1).
Most of the cadmium in urine is probably transported bound to
metallothionein. The urinary metallothionein concentration can now
be measured quantitatively with a sensitive radioimmunoassay (Vander
Mallie & Garvey, 1979). Using this technique, Tohyama et al. (1981b)
found good correlation between urinary metallothionein and cadmium
in 67 people exposed in the general environment, and Roels et al.
(1983b) confirmed this correlation in 94 cadmium workers.
6.5.2 Gastrointestinal and other routes of excretion
It is extremely difficult to study gastrointestinal excretion
after oral exposure, since it is not possible to distinguish net
gastroin-testinal excretion from unabsorbed cadmium in faeces.
Animal studies of gastrointestinal excretion following
injections of cadmium (summarized by Friberg et al., 1974) generally
show that a few percent of the dose is excreted in the faeces within
the first few days after injection. The faecal excretion is
initially higher than the urinary excretion after either single or
repeated exposure (Nomiyama, 1978). The mechanism for such excretion
probably involves a transfer of cadmium via the intestinal mucosa,
but biliary excretion may also be involved. The biliary excretion in
the first 24 h after intravenous injection of cadmium is dependent
on the dose (Cirkt & Tichy, 1974; Nomiyama, 1974; Klaassen &
Kotsonis, 1977). In rats given 67, 90 or 120 ßg cadmium (Cikrt &
Tichy, 1974), the cumulative 24 h excretion reached 0.83% at the
lowest dose and 5.68% at the highest dose. The highest excretion
rate was detected between 15 and 30 min after dosing. It has been
reported that after the initially rapid excretion the biliary
excretion is 0.015-0.04% of the body burden per hour over three
consecutive days (Nordberg et al., 1977; Elinder & Pannone, 1979).
Biliary cadmium has been partially characterized as a glutathione
complex (Cherian & Vostal 1977).
Both during and after parenteral exposure to cadmium, the total
gastrointestinal cadmium excretion is considerably higher than the
urinary excretion (Nordberg, 1972; Elinder & Pannone, 1979). A large
proportion of the gastrointestinal excretion is directly related to
the daily dose. After chronic exposure of rats, faecal excretion
amounted to about 0.03% of the body burden, which was considerably
more than the urinary excretion (Elinder & Pannone, 1979).
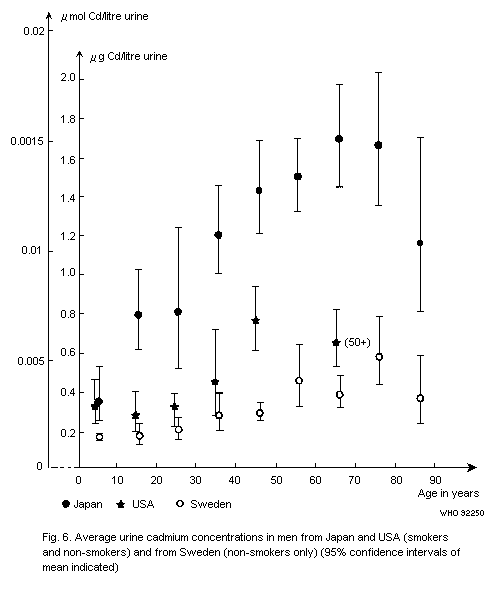 There are no available quantitative human data to indicate the
net gastrointestinal excretion.
Cadmium is also eliminated through hair (Anke et al., 1976) and
breast milk (Schroeder & Balassa, 1961), but these routes are of
limited importance for total excretion and do not significantly
alter the biological half-time.
6.6 Biological half-time and metabolic models
6.6.1 In animals
Several studies have been carried out in order to assess the
biological half-times of cadmium in experimental animals. Various
animals species, including mice, rats, rabbits, dogs, and monkeys,
have been studied, and single exposures have normally been used. The
reported half-times have varied from weeks to two years (or as long
as half the life-span of the animal). The development of metabolic
models has shown that the body contains several compartments for
cadmium accumulation, each with a different half-time. Thus, in
whole body half-time measurements, one may find several different
half-times. In order to observe the slowest half-time components, it
is necessary to study the animals for many months.
The biological half-time of cadmium in the kidney and whole
body decreases when renal tubular dysfunction occurs because of
increased urinary excretion (section 6.5.1). However, some studies
have indicated that the biological half-time may change with dose
and body burden of cadmium even before renal damage occurs. For
instance, Engstrom & Nordberg (1979a) reported that in mice
half-time increased with increasing single or repeated oral dose. In
these studies, body burden and renal burden were considerably lower
than the maximum that can be reached in long-term exposure. Nomiyama
(1978) reported that half-time decreased with increasing dose when
animals with the shortened half-time had reached the maximum renal
burden. Even shorter half-times were reported when renal tubular
dysfunction occurred after exposure to high doses (Nomiyama, 1978).
A number of studies on biological half-time and metabolic
models for animals have been reviewed by Friberg et al. (1974) and
Nomiyama (1978).
The wide difference in the results obtained by investigators
may be explained by variations in exposure level and type, the
different animal species used, and interactions between cadmium and
other exposure factors. Reported half-times range from several weeks
in mice to 22 years in monkeys (Friberg et al., 1974; Nomiyama et
al., 1979; Nomiyama et al., 1984). The variations in half-times in
specific tissues between different species or individuals may be due
to variations in the production of metallothionein, which binds
tissue cadmium and contributes to its retention.
6.6.2 In humans
Experimental and epidemiological evidence indicates strongly
that the biological half-time in the whole body is extremely long
(many years). Experimental evidence from one study (Shaikh & Smith,
1980), in which one subject was given radioactive cadmium and
examined periodically for the next 2 years, showed a biological
half-time of 26 years. In three similar studies, in which a small
number of subjects were followed up for a limited period (about 100
days), half-times of 93-202 days were reported (Rahola et al., 1972;
Flanagan et al., 1978; McLellan et al., 1978). Only one of these
studies (Rahola et al., 1972) gave confidence limits for the
estimated biological half-time (130 days to infinity).
Another approach to estimate the half-time used involves
comparing total daily excretion with total body burden, applying a
one-compartment model to the body as a whole (Friberg et al., 1974;
Task Group on Metal Toxicity, 1976). A further approach analyses the
accumulation in the kidney using a one-compartment model taking into
consideration age-related variations in daily cadmium exposure and
kidney weight (Tsuchiya & Sugita, 1971; Kjellström, 1971). More
recently, an elaborate model has been developed that includes
separate compartments for, for instance, kidney, liver, and blood,
and incorporates age-related variations in daily intake, tissue
weights, and renal function (Kjellström & Nordberg, 1978).
These models rely on many assumptions concerning the cadmium
concentration in food, calorific intake, absorption rates, and other
factors. Inevitably, the data produced by these models are only
tentative, but they are important for future research. Using data
from Japan on the accumulation of cadmium with age, Tsuchiya et al.
(1976) used a series of one-compartment mathematical models
developed by Tsuchiya & Sugita (1971) to estimate biological
half-times of cadmium in various organs. These authors estimated the
biological half-time in the kidneys to be about 17 years and that in
the liver 7 years.
Elinder et al. (1976) used autopsy data from non-smokers in
Sweden and a one-compartment model (Kjellström, 1971) to estimate
the biological half-time in the renal cortex. They assumed that the
daily intake of cadmium had doubled in 50 years (Kjellström et al.,
1975a) and estimated the half-time to be 20-50 years (30 years being
the best estimate).
Using an 8-compartment model (Kjellström & Nordberg, 1978), the
biological half-times of cadmium in the liver and kidney were
estimated to be 7.5 and 12 years, respectively (Kjellström &
Nordberg, 1978). The longest half-time was calculated for the "other
tissues" compartment. This included muscle tissue, which was found
to have the longest half-time in an autopsy study (Kjellström,
1977). However, it should be pointed out that when using this type
of model to simulate the chemobio-kinetics of cadmium, the
individual half-times of different tissues are less important than
the dynamics of the model as a whole.
After high cadmium exposure, as occurs among certain industrial
workers, the biological half-time may not be the same as that during
normal exposure in the general environment. Current models, however,
do not consider this factor. If the exposure level is very high, the
ratio of rapid components to slow components may be altered. An
example of this is the very high urinary excretion found after only
a short exposure to high air cadmium levels (Lauwerys et al., 1976,
1979b). The biological half-time is also shorter if there is renal
tubular dysfunction. Fletcher et al. (1982) carried out
in vivo neutron activation analysis of the liver of 13 cadmium
workers twice within a period of 3 to 4 years (the occupational
cadmium exposure of these workers had ceased before the first
analysis). Three workers showed proteinuria and an average cadmium
half-time in the liver of 2 years. The other 10 workers had an
average half-time of 6.4 years, nine of whom had an average
half-time of 13.5 years and no proteinuria.
Jarup et al. (1983) studied the half-time of cadmium in blood
of five smelter workers who had previously experienced high cadmium
exposure. Repeated blood analysis carried out over a 10-to 13-year
period revealed short-term (75-128 days) and a long-term (7.4-16
years) half-time components. The long-term component in two workers
with proteinuria was shorter than in the other workers.
6.7 Biological indices of cadmium exposure, body burden, and
concentrations in kidneys
There is no easy way to measure directly the whole body burden
or concentrations of cadmium in different tissues of a living
person. In vivo neutron activation methods have been used in
special circumstances (Ellis et al., 1981a; Roels et al., 1981b;
Tohyama et al., 1981a).
At present, it is necessary to study concentrations in easily
available indicator media in order to evaluate exposure and
accumulation of cadmium. The suitability of certain indicator media
for such purposes is supported by studies on both animals and
humans; urine, blood, faeces, and hair have all been used as
indicator media. Methods for the biological monitoring of cadmium
levels in blood and urine have been reviewed by Nordberg & Nordberg
(1988) and WHO (1980).
6.7.1 Urine
The human and animal studies summarized in section 6.5.1 allow
the following interpretation of the significance of cadmium in urine
(Lauwerys et al., 1980b). In the absence of episodes of high-level
exposure to cadmium and provided that cadmium-binding sites in the
organism are not saturated and cadmium-induced nephropathy has not
yet occurred, the urine cadmium level increases in proportion to the
amount of cadmium stored in the body. In such situations, which
prevail mainly in the general population and in workers moderately
exposed to cadmium, there is significant correlation between urinary
cadmium and cadmium in kidney. Episodes of high exposure to cadmium,
however, may lead to a transient increased urinary excretion.
If exposure to cadmium has been excessive, the cadmium-binding
sites in the organism become progressively saturated and, despite
continuous exposure, the cadmium concentration in the renal cortex
tends to plateau. Once this point is reached, the cadmium that is
still absorbed cannot be further retained in the kidney and is
rapidly excreted in the urine. Under these conditions, urinary
cadmium is also influenced by the recent intake. The relative
influence of the body burden and the recent exposure on urinary
cadmium depends on the exposure intensity. If exposure continues, a
certain percentage of individuals may develop renal damage. This is
associated with a progressive loss of cadmium accumulated in the
kidney, which gives rise to a further increase in urinary cadmium.
Eventually, the amount of cadmium that can be released from the
kidney decreases progressively and the urinary cadmium concentration
follows the same trend. The changes in the urinary metallothionein
level parallel those of cadmium (section 6.8.2).
In summary, several factors (duration and intensity of exposure
to cadmium, the presence of renal dysfunction and its duration) must
be taken into consideration when interpreting urinary cadmium (and
metallothionein) levels.
6.7.2 Blood
Plasma cadmium levels are considered to be related to recent
exposure but are often so low that they cannot be measured routinely
(section 6.2). Most of the cadmium in the blood is in the
erythrocytes.
Cadmium levels in whole blood mainly reflect the exposure
during recent weeks or months. In cadmium workers, the level
increases markedly within the first few months after occupational
exposure starts (Kjellström & Nordberg, 1978; Lauwerys et al.,
1979b). It is probable that a portion of the blood cadmium level
reflects body burden rather than present exposure in view of the
known transport of cadmium via blood from the liver to the kidneys
and other tissues (section 6.2) and the long-term half-time
component demonstrated by Jarup et al. (1983). Workers with
relatively long exposure durations but whose cadmium exposure has
ceased have elevated blood cadmium levels for several years (Friberg
et al., 1974; Jarup et al., 1983).
Reports of blood cadmium levels in the general population have,
in the past, often been unreliable, owing largely to the
difficulties encountered in the analysis of blood cadmium (Lauwerys
et al., 1975). However, improvements in analytical techniques have
since been achieved through biological standards for blood and
systematic quality assurance programmes (Stoeppler et al., 1979;
Vahter, 1982). Average blood cadmium values up to 10 µg/litre or
more have been reported in the past, but the analytical procedures
used mean that the accuracy of these data is in doubt (Vahter, 1982;
Friberg & Vahter, 1983).
Various aspects of blood cadmium analysis have been discussed
in a UNEP/WHO study, which also included a quality assurance
programme and data from 10 countries (Vahter, 1982; Friberg &
Vahter, 1983). It was found that even in the countries with the
highest blood cadmium levels, the average was less than 4 µg/litre.
Furthermore, smokers were found to have higher values than
non-smokers, and non-smokers, in most countries, had mean levels
below 1 µg/litre. Although only slightly above 1 µg/litre, the
levels for non-smokers in Japan were about twice as high as the
levels in the USA, which probably reflects the difference in average
daily cadmium intake via food (section 5.2.2).
Reported cadmium concentrations in the blood of exposed workers
are generally between 5 and 50 µg/litre, but levels of between 100
and 300 µg/litre have resulted from extreme exposures (Roels et al.,
1982; Hassler et al., 1983).
6.7.3 Faeces
Gastrointestinal absorption amounts to only a few percent of
the cadmium ingested daily (section 6.1.2), and the quality of
cadmium excreted gastrointestinally is small compared to the
unabsorbed portion of ingested cadmium. Thus, daily faecal cadmium
can serve as a good indicator of the daily amount of cadmium
ingested via food and water or cleared from the lungs after
occupational exposure to large dust particles. Faecal cadmium
correlates very closely with daily energy intake (section 5.2.4),
and average cadmium intake estimates for different countries agree
well with reported average faecal cadmium amounts.
A portion of the cadmium in human faeces is related to the body
burden (section 6.5.2). In workers with high body burdens but low
daily cadmium intakes via food, this portion might be greater than
the unabsorbed part of ingested cadmium, because the faecal
excretion is similar to the urinary excretion.
6.7.4 Hair
Cadmium in hair is not a reliable indicator of either recent
exposure or body burden. The main problem is external contamination
of the hair, which cannot be distinguished from the endogenous
cadmium (Nishiyama & Nordberg, 1972). However, a correlation was
found between air cadmium levels of cities in the USA and cadmium
levels in the hair of 10-year-old children living in these cities
(Hammer et al., 1971). In a study of cadmium workers (Ellis et al.,
1981b), a higher average hair cadmium level was found among exposed
workers than among controls, but there was a poor relationship
between hair cadmium and cadmium in the blood, urine, liver or
kidney. Cadmium concentrations in the hair of people without
excessive exposure are usually between 0.5 and 2 mg/kg.
6.8 Metallothionein
6.8.1 Nature and production
Metallothionein is a metal-binding protein of low molecular
weight, which has a key role in the metabolism of cadmium. It is
rich in cysteine but contains no aromatic amino acids or histidine
(Kagi & Vallee, 1960, 1961).
This protein was identified for the first time by Margoshes &
Vallee (1957) in horse kidney cortex. Its molecular weight is about
6600 (6000 for the apoprotein moiety, thionein), and it has a
non-globular shape. On gel filtration, however, it moves like a
spherical protein with a molecular weight of about 10 000 (Kagi &
Nordberg, 1979). There have been several reports dealing with the
function and biochemistry of metallothionein (Kagi & Nordberg, 1979;
Brady, 1982; Foulkes, 1982; Webb & Cain, 1982; Kagi & Kojima, 1987).
Piscator (1964) suggested that metallothionein played a role in
cadmium transport and detoxication, and it has subsequently been
identified in human kidney and liver (Pulido et al., 1966; Chung et
al., 1986) as well as in those of various experimental animals (Kagi
& Nordberg, 1979).
The structure and genetic expression of mouse and human
metallothionein have now been identified. Two major forms of
metallothionein are present in most mammalian tissues, particularly
liver and kidney, i.e. metallothionein I (Mt-I) and metallothionein
II (Mt-II). Induction of synthesis is under the control of a large
group of genes and is stimulated by glucocorticoids and the
essential metals zinc and copper, as well as by the toxic metals
cadmium and mercury (Karin et al., 1981; Karin & Richards, 1982).
In vitro binding affinities have been demonstrated for a number of
other toxic metals, including bismuth, cobalt, silver, and gold
(Cherian & Nordberg, 1983). Metallothionein binds seven metal ions
per molecule between two separate metal-cysteine clusters, and a
single molecule may contain more than one metal, e.g., cadmium and
zinc, mercury and copper.
6.8.2 The role of metallothionein in transport, metabolism, and
toxicity of cadmium
Piscator (1964) suggested that some of the cadmium-binding
metallothionein in the liver may migrate into the blood stream. As
discussed in section 6.2, part of the plasma cadmium in animals
exposed for a long time to cadmium is bound to a protein with the
same molecular weight as metallothionein. When metallothionein-bound
cadmium is present in the plasma, it is quickly cleared by
glomerular filtration and reabsorbed in the renal tubules or
excreted in the urine (Cherian & Shaikh, 1975; Nordberg et al.,
1975; Webb & Etienne, 1977; Fowler & Nordberg, 1978).
At low levels of cadmium-metallothionein in the plasma, tubular
reabsorption is almost complete, whereas the uptake in the tubular
cells from the tubular fluid is saturated in the presence of high
concentrations (Nomiyama & Foulkes, 1977; Foulkes, 1982). Thus, high
urinary excretion of cadmium-metallothionein occurs shortly after
the administration of larger doses, i.e. doses exceeding about
0.1 mg cadmium/kg body weight (Cherian & Shaikh, 1975; Nordberg &
Nordberg, 1975).
It has been demonstrated in animal and in vitro tissue
studies that metallothionein provides a protective role for cadmium
toxicity (Cherian & Nordberg, 1983). Mice pretreated with cadmium
have increased tolerance to subsequent cadmium exposure (Nordberg et
al., 1971), and exposure to cadmium may protect from subsequent
mercury toxicity (Piotrowski et al., 1974). In addition, the
inhibition of certain mixed-function oxidases by cadmium is reduced
by prior induction of metallothionein by cadmium in immature mice
(Asokan et al., 1984). Pre-exposure of cultured kidney cells to
cadmium protects from subsequent exposure (Cherian, 1980; Jin et
al., 1986).
Nordberg et al. (1975) showed that metallothionein isolated
from rabbit and mouse liver produced acute renal tubular cell
toxicity when injected subcutaneously into mice. Further study
(Cherian et al., 1976; Fowler & Nordberg, 1978; Squibb et al., 1982,
1984) suggested that parenterally administered
cadmium-metallothionein enters proximal renal tubular lining cells
in pinocytotic vesicles that fuse with lysosomes. The
metallothionein is degraded, releasing cadmium into the cytosol and
producing cellular degeneration and necrosis within 8-24 h. The
renal tubular cell toxicity produced by metallothionein with
different ratios of cadmium and zinc is proportional to the cadmium
content of the metallothionein (Suzuki, 1982). Zinc-thionein does
not have a similar effect.
The pathogenesis of renal tubular cell toxicity is thought to
be related to non-metallothionein-bound cadmium, which becomes
rapidly bound to existing metallothionein sites or induces the
synthesis of new metallothionein (section 7.2.1.4).
The prevalence of nephrotoxicity rather than hepatotoxicity in
chronic cadmium exposure may be due to several factors. Firstly, the
release of hepatic cadmium-metallothionein or its presence in the
blood can result in preferential accumulation of cadmium in the
kidneys. Secondly, it has been shown in experimental animals that
the kidney can accumulate metallothionein mRNA in response to
cadmium exposure to only about half the level of the liver
(Koropatnick & Cherian, 1988). Thus, the kidney may not be able to
synthesize metallothionein as efficiently as the liver in response
to cadmium exposure, resulting in an accumulation of
non-metallothionein cadmium in the kidney but not in the liver.
Nomiyama et al. (1982a) studied the concentrations of total
cadmium, metallothionein-cadmium, and non-metallothionein-cadmium in
the renal cortex of monkeys fed diets containing 30 mg cadmium/kg
food for 12 months. They found that the concentration of
non-metallothionein-cadmium increased with dose of cadmium to about
35 mg/kg tissue and total cadmium to about 200 mg/kg tissue when the
total dose of cadmium was 0.4 g. Similar measurements were made in
rabbits given cadmium chloride (0.5 mg cadmium/kg body weight)
subcutaneously every day for 21 weeks. There were parallel increases
of total cadmium and non-metallothionein-bound cadmium during the
initial 4 weeks of dosing; these remained unchanged until the 14th
week when striking renal dysfunction appeared. At that time, total
cadmium and non-metallothionein-bound cadmium levels fell despite
the continued administration of cadmium (Nomiyama & Nomiyama, 1982).
Increased knowledge of the intracellular binding or speciation of
non-metallothionein-bound cadmium should improve our understanding
of the relative roles of metallothionein-bound and
non-metallothionein-bound cadmium.
When Cherian et al. (1978) exposed mice by injection and
feeding to both cadmium chloride and cadmium-metallothionein, both
compounds were absorbed and distributed in the body. However, in the
short term, cadmium-metallothionein was selectively accumulated in
the kidney and cadmium chloride in the liver.
Most cadmium in human urine is bound to metallothionein
(Tohyama et al., 1981b), and good correlation has been found between
the urinary cadmium and metallothionein concentrations both in
elderly women exposed in the general environment (Tohyama et al.,
1981b) and in male cadmium workers (Nordberg et al., 1982; Roels et
al., 1983b). Measurement of urinary metallothionein thus provides a
good indication of the urinary cadmium level and offers the
advantage over cadmium analysis of avoiding the possibility of
external contamination. Women were found to have much higher urinary
metallothionein concentrations than men, even at similar cadmium
levels.
6.9 Conclusions
Data from experimental animals and humans have shown that
pulmonary cadmium absorption is greater than gastrointestinal
absorption. Depending on chemical speciation, particle size, and
solubility in biological fluids, up to 50% of the inhaled cadmium
compound may be absorbed. The gastrointestinal absorption of cadmium
is influenced by the type of diet and nutritional status, iron
status appearing to be particularly important. On average, 5% of the
total oral intake of cadmium is absorbed, but individual values
range from less than 1% to more than 20%. Cadmium may also be
transported to the fetus. However, although cadmium accumulates in
the placenta, little is transferred to the fetus.
Cadmium absorbed from the lungs or the gastrointestinal tract
is stored principally in the liver and kidneys where more than half
of the body burden is deposited. Highest cadmium concentrations are
generally found in the renal cortex, but as exposure levels
increase, a greater proportion of the absorbed cadmium is stored in
the liver. The cadmium excretion rate is normally low, and the
biological half-time is very long (decades) in the kidneys, muscles,
liver, and total body of humans. The cadmium concentrations in most
tissues increase with age. In exposed people with renal damage,
urinary excretion of cadmium increases and, thus, the whole body
half-time is shortened. The renal damage leads to losses of cadmium
from the kidney, and the renal concentrations are eventually lower
than in people with similar exposure but without renal damage.
Metallothionein is an important transport and storage protein
for cadmium and other metals. Cadmium can induce metallothionein
synthesis in many organs including the liver and kidney. The binding
of intracellular cadmium to metallothionein in tissues protects
against cadmium toxicity. Non-metallothionein-bound cadmium may,
therefore, have a role in the pathogenesis of cadmium-related tissue
injury. The speciation of other cadmium complexes in tissues or
biological fluids is unknown.
Urinary excretion of cadmium is related to body burden, recent
exposure, and renal damage. In people with low exposures, cadmium in
urine is mainly related to body burden. Cadmium-exposed people with
proteinuria generally exhibit greater cadmium excretion than such
people without proteinuria. After high exposure ceases, urinary
cadmium decreases even though renal damage persists. The
interpretation of urinary cadmium is thus dependent on a number of
factors. The magnitude of gastrointestinal excretion is similar to
that of urinary excretion, but it cannot be easily measured. Other
excretory routes such as lactation, sweating or placental transfer
are insignificant.
Cadmium in faeces is a good indicator of recent daily intake
from food in the absence of inhalation exposure. Cadmium in blood
occurs mainly in the blood cells, and the plasma concentrations are
very low. There are at least two blood compartments, one being
related to recent exposure with a half-time of about 2-3 months, and
the other probably related to body burden with a half-time of
several years.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Lethal dose and lethal effects
LD50 inhalation values are in the range of 500 to
15 000 mg/m3.min for different species (Barrett et al., 1947;
Harrison et al., 1947; Hadley et al., 1979). The cause of death is
pulmonary oedema.
The LD50 after the injection of soluble cadmium compounds is
in the range of 2.5-25 mg/kg body weight (Friberg, 1950; Eybl &
Sykora, 1966; Commission of the European Communities, 1978). Shortly
after large doses are injected, severe endothelial damage is seen in
the small vessels of the peripheral nervous system (Gabbiani, 1966)
and in the testis (Parizek, 1957). If the animal survives for some
hours, the most pronounced changes are found in the liver (Dudley et
al., 1982), and liver damage is probably the lethal effect of a
single high parenteral exposure.
For most cadmium compounds, the LD50 after oral
administration is about 10-20 times higher than after parenteral
administration, and the readily soluble compounds have a lower
LD50 values than the insoluble ones (Table 11).
Nomiyama et al. (1978b) showed that the LD50 in mice was
lower at cold temperatures (+8 °C) than at higher temperatures
(+22 or +37 °C), both after oral and peritoneal exposure.
7.1.2 Pathological changes affecting specific systems in the body
The chronic effects of long-term exposure to low doses of
cadmium constitute the main problem for non-occupationally exposed
humans. Therefore, the effects of single exposure in animals will be
dealt with only briefly, and the main emphasis will be on chronic
effects.
Specific effects from a single high dose of cadmium have been
described by several investigators and have been reviewed by Friberg
et al. (1974, 1986), Commission of the European Communities (1978),
and Kawai (1978). One of the most pronounced effects seen was in the
gonads (testis and ovary).
Table 11. LD50 values for cadmium compounds given to mice and rats by intragastric administration
Species Compound Molecular formula Relative molecular LD50 with confidence LD50 for cadmium
massa limits (mg/kg)b ion alone (mg/kg)
Mouse cadmium (element) Cd 109 890 (636-1246) 890
cadmium oxide CdO 128.4 72 (41-113) 63
cadmium sulfate CdSO4 208.5 88 (69.8-100.2) 47
cadmium chloride CdCl2 183.3 93.7 (75.5-111.9) 57
cadmium nitrate Cd(NO3)2 236.4 100 (78.7-121.8) 48
cadmium iodide CdI2 366.2 166 (139-193) 51
cadmium caprylate Cd(C7H15COO)2 394.8 300 (196-459) 85
cadmium carbonate CdCO3 169 310 (215-404) 202
cadmium stearate Cd(C17H35COO)2 679.4 590 (556-624) 98
cadmium sulfide CdS 144.5 1166 (1135-1197) 907
cadmium sulfoselenide CdSe.CdS 335.8 2425 (2393-2457) 1623
barium-cadmium stearate BaCd(C17H35COO)4 1383.7 3171 (2763-3579) 258
Rat cadmium caprylate Cd(C7H15COO)2 394.8 950 (613-1472) 270
cadmium stearate Cd(C17H35COO)2 679.4 1225 (875-1574) 203
barium-cadmium stearate BaCd(C17H35COO)4 1383.7 1980 (1736-2224) 161
a From: Weast (1974)
b From: Tarasenko et al. (1974), Vorobjeva & Sabalina (1975), and Vorobjeva & Bubnova (1981)
7.1.2.1 Acute effects on testes and ovaries
Testicular necrosis occurs in experimental animals given single
injections of salts corresponding to 2-4 mg cadmium/kg body weight
(Parizek & Zahor, 1956; Parizek, 1957). At a later stage, Leydig
cells regenerate (Parizek, 1957, 1960; Allanson & Deanesly, 1962).
Gabbiani et al. (1974) detected dilation of interendothelial clefts
in the small blood vessels of the testis as early as 15 min after an
intravenous injection of cadmium salts. Effects on the testis have
been extensively reviewed by Gunn & Gould (1970).
The marked effects on the testis after cadmium injection are
probably the result of endothelial damage. In the small vessels this
damage gives rise to increased capillary permeability. This leads to
vascular escape of fluids and blood plasma substances into the
interstitium, which results in oedema, decreased capillary blood
flow, ischaemia, and testicular cell necrosis (Aoki & Hoffer, 1978;
Francavilla et al., 1981).
A single injection of cadmium salts at a dose that induces
testicular haemorrhagic necrosis has been shown to induce
haemorrhages and necroses in the ovaries of prepubertal rats (Kar et
al., 1959), and in the ovaries of adult rats in persistent oestrus
(Parizek et al., 1968a). The effect of cadmium on the testis was not
dependent on the presence of the hypophysis (Parizek, 1960). Ovarian
effects can be prevented by the administration of PMSG hormones
(Parizek et al., 1968a). Numerous studies on the effects of cadmium
on the testes and other reproductive organs were reviewed by Barlow
& Sullivan (1982).
7.1.2.2 Acute effects on other organs
A single inhalation exposure to cadmium at concentrations of
5-20 mg/m3 for 50-120 min gives rise to pulmonary oedema in rats
and rabbits (Hayes et al., 1976; Bouley et al., 1977; Bus et al.,
1978; Dervan & Hayes, 1979; Boisset & Boudene, 1981; Fukuhara et
al., 1981). The morphological changes seen in the lung have been
described in detail by Strauss et al. (1976).
After the parenteral administration of cadmium at dose levels
similar to the LD50, pronounced effects were seen in the small
blood vessels of, for instance, the nervous system (Gabbiani et al.,
1974). Hoffman et al. (1975) noted profound morphological effects in
the liver of rats given 6 mg cadmium/kg body weight, and Dudley et
al. (1982), examining liver effects from a single injection of
cadmium (3.9 mg/kg body weight), concluded that liver was the major
target organ in rats for acute cadmium toxicity. Changes in blood
pressure shortly after the acute administration of cadmium have also
been recorded (Dalhamn & Friberg, 1954; Perry et al., 1970).
Oral administration of cadmium compounds induces epithelial
desquamation and necrosis of the gastric and intestinal mucosa,
together with dystrophic changes of the liver, heart, and kidneys
(Tarasenko et al., 1974; Vorobjeva & Sabalina, 1975).
7.2 Repeated and/or long-term exposure
7.2.1 Effects on the kidneys
Since the kidney is the critical organ in humans exposed for
long periods to relatively small amounts of cadmium (section 8.2.1),
results from relevant animal studies will be dealt with in some
detail. Even though it is difficult to extrapolate quantitative
information from the findings in animals, experiments have provided
valuable information concerning mechanisms of cadmium-induced
nephropathy and the significance of various biological indicators of
exposure and effect, and have supported the findings in humans. For
example, Friberg (1950) verified in animal experiments that exposure
to cadmium caused a type of proteinuria similar to the one he had
found in exposed workers.
Animal studies that have given data on renal effects as well as
the corresponding renal cadmium concentrations are summarized in
Table 12. An evaluation of organ dose-effect and dose-response
relationships is included in section 7.2.1.4.
7.2.1.1 Oral route
Renal lesions were first reported by Prodan (1932) and Wilson
et al. (1941) after cats and rats were given large oral doses of
cadmium for several months. Prodan (1932) reported varying degrees
of desquamation in proximal tubular epithelium (and no changes in
the glomeruli) after feeding cats 100 mg cadmium per day for one
month. Wilson et al. (1941) reported slight tubular changes in rats
after they were exposed for 3 months to a diet containing 62 mg
cadmium/kg.
Studies utilizing high exposures have also been performed by
Stowe et al. (1972). Ten rabbits received cadmium in drinking-water
(160 mg/litre) for 6 months. Kidney function was not investigated,
but histopathological examination revealed pronounced morphological
changes in the proximal tubules. The mean renal concentration of
cadmium was 170 mg/kg wet weight, which would correspond to about
210 mg/kg wet weight in the renal cortex (section 6.4). Still higher
doses (300 mg/kg diet) were given to rabbits for 54 weeks by
Nomiyama et al. (1975), who observed aminoaciduria and enzymuria
after 16 weeks. At this stage, the cadmium concentration in the
renal cortex was 200 mg/kg wet weight. Proteinuria and glycosuria
appeared at a later stage, 37 and 42 weeks, respectively, after
exposure had started. The cadmium concentration in the renal cortex
was 300 mg/kg wet weight after 40 weeks.
Table 12. Summary of animal studies with data on both renal cadmium levels and effects
Species Route of Exposure Duration Average cadmium Renal changes Reference
administration level (months) level in kidney
cortex (mg/kg
wet weight)
Mouse subcutaneous 0.25 mg/kg 6 110-170a no effects Nordberg & Piscator (1972)
body weight
Mouse subcutaneous 0.5 mg/kg 6 170a tubular protein patterns Nordberg & Piscator (1972)
body weight in urine
Rat intraperitoneal 0.75 mg/kg 3 200a no effects Bonnell et al. (1960)
body weight
Rat intraperitoneal 0.75 mg/kg 4 300a histological changes in Bonnell et al. (1960)
body weight 60% of animals
Rat subcutaneous 0.65 mg/kg 3 200 histological changes Goyer et al. (1984)
body weight
Rat water 10 mg/litre 8.5 11a no histological changes Kawai et al. (1976)
Rat water 50 mg/litre 8.5 35a slight histological changes Kawai et al. (1976)
Rat water 100 mg/litre 8.5 90a histological changes Kawai et al. (1976)
Rat water 200 mg/litre 8.5 145a histological changes Kawai et al. (1976)
Rat water 200 mg/litre 11 200 total proteinuria and low Bernard et al. (1981)
molecular weight proteinuria
Rat water 50 mg/litre 3 100a decreased insulin and PAH Kawamura et al.(1978)
clearance; histological
changes
Table 12 (contd).
Species Route of Exposure Duration Average cadmium Renal changes Reference
administration level (months) level in kidney
cortex (mg/kg
wet weight)
Rat water 50 mg/litre 2.5 235 slight histological changes Axelsson & Piscator (1966a);
in proximal tubules Axelsson et al. (1968)
Rabbit subcutaneous 0.25 mg/kg 2.5 235 slight histological changes Axelsson & Piscator (1966a);
body weight in proximal tubules Axelsson et al. (1968)
Rabbit subcutaneous 0.25 mg/kg 4 460 more severe histological Axelsson & Piscator (1966a);
body weight changes; reduction of alkaline Axelsson et al. (1968)
phosphatase activity in renal
cortex; total proteinuria
Rabbit subcutaneous 0.5 mg/kg 2.5 300 total proteinuria Nomiyama et al. (1982b)
body weight
Rabbit subcutaneous 0.5 mg/kg 0.7 200 proteinuria, glucosuria, and Nomiyama & Nomiyama
body weight aminoaciduria; decrease in (1982)
CIN and TmPAH
Rabbit subcutaneous 0.5 mg/kg 1 120 ß2-microglobulin Nomiyama et al. (1982b)
body weight
Rabbit subcutaneous 1.5 mg/kg 1 50-200 decreased tubular Nomiyama (1973a);
body weight readsorption Nomiyamaet al. (1978a)
Rabbit subcutaneous 0.5 mg/kg 2 160a slight histological changes Kawai et al. (1976)
body weight
Rabbit water 160 mg/litre 6 170a extensive fibrosis; pronounced Stowe et al. (1972)
changes
Table 12 (contd).
Species Route of Exposure Duration Average cadmium Renal changes Reference
administration level (months) level in kidney
cortex (mg/kg
wet weight)
Rabbit water 50 mg/litre 10 58 slight tubular atrophy Kawai et al. (1976)
Rabbit water 200 mg/litre 10 200 severe interstitial and Kawai et al. (1976)
tubular fibrosis
Rabbit diet 300 mg/kg 4 200 aminoaciduria, enzymuria Nomiyama et al. (1975)
Rabbit diet 300 mg/kg 10 300 proteinuria, glucosuria Nomiyama et al. (1975)
Pig diet 50-350 mg/kg < 12a equimolar increase in zinc Cousins et al. (1973)
in kidney
Pig diet 50-350 mg/kg 78a decrease in renal leucine Cousins et al. (1973)
aminopeptidase
Horse diet no cadmium lifelong 75 renal tubular interstitial Elinder et al. (1981a,b)
added (up to 240) changes and fibrosis in
25% of animals
Bird subcutaneous 0.16 mg/kg 1.5 20b histological changes Nicholson & Osborn (1983)
body weight
a These values are whole kidney concentrations; about 0.8 times kidney cortex values, on average.
b Denotes concentrations (wet weight) calculated as 0.2 times dry weight concentrations.
Morphological changes of the renal tubules were reported by
Kawai et al. (1976) in rats given 50 mg cadmium/litre drinking-water
for 8.5 months. The average renal cadmium concentration was about
38 mg/kg wet weight which corresponds to about 50 mg/kg in the renal
cortex.
Histological lesions in the proximal renal tubules were also
found in rats exposed to 200 mg cadmium/litre for 2 months (Itokawa
et al., 1978). Histochemical examination of the kidney showed that
the proximal tubular epithelium had particularly high cadmium
concentrations. The average renal concentrations were 48 mg/kg and
80 mg/kg, respectively, in rats with sufficient and deficient
calcium intakes. These levels would correspond to about 60 and
100 mg cadmium/kg in the renal cortex (section 6.4). Inulin
clearance was reduced to about a third of the control values in the
cadmium-exposed groups, indicating considerable functional damage to
the glomeruli. The only reported change in renal tubular function
was that the fractional excretion of calcium was increased about 50%
in the cadmium-exposed groups.
In a study of 50 rats exposed to 200 mg cadmium/litre in
drinking-water for up to 11 months (Bernard et al., 1981), there was
an increased prevalence of total proteinuria in the 8th month, when
the average cadmium concentration in the renal cortex was about
200 mg/kg.
Kajikawa et al. (1981) also reported morphological changes in
the kidneys of rats given drinking-water containing 200 mg cadmium
chloride/litre for 91 weeks. Histologically, they found degenerative
changes in the proximal convoluted tubules and, using electron
microscopy, proliferation of smooth endoplasmic reticulum,
vacuolization, and coagulative necrosis of the tubular cells. No
significant changes were observed in the glomeruli or interstitial
tissue.
When Cousins et al. (1973) gave large amounts of cadmium
chloride to pigs (50, 150, 450, and 1350 mg/kg diet), there was a
decrease in the activity of leucine aminopeptidase in the kidney
cortex at a renal cadmium concentration of 78 mg/kg wet weight,
corresponding to a renal cortex concentration of about 100 mg/kg wet
weight (section 6.4).
An extensive data base on the renal effects of cadmium in
monkeys has been developed in Japan. Several of these studies are
summarized in Table 13.
Study I (Nomiyama et al., 1979) was carried out using ten male
rhesus monkeys (three years of age). The monkeys were given 100 g of
solid feed containing 0, 3, 30, or 300 mg cadmium/kg daily for 37
weeks, followed by 130 g of feed for 18 weeks. Even the solid feed
given to the control group contained cadmium at a concentration of
0.13 mg/kg.
In study II, Nomiyama et al. (1987) used 36 male rhesus monkeys
(three years of age) and gave them 100 g of solid feed containing 0,
3, 10, 30, or 100 mg cadmium/kg daily for 52 weeks. During the
following 52 weeks 150 g was given, and then for the remaining 358
weeks 200 g was given. The solid feed given to the control group
contained cadmium at 0.27 mg/kg and zinc at 30 mg/kg.
Study III (Nomura et al., 1988) was performed with 40 female
rhesus monkeys given 150 g of solid feed for nine years (Table 14).
In study IV, Nomiyama & Nomiyama (1988) used nine male
crab-eating monkeys. Two of the animals were used as controls, and
three were given a diet containing cadmium concentrations of 3 mg/kg
(190 µg/day) as cadmium chloride (the pelleted food also contained
(30 mg zinc/kg)). The remaining four were fed 80 µg cadmium/day in
the form of contaminated rice.
In studies I and II, monkeys that had been given feed
containing cadmium at 300 mg/kg and 100 mg/kg showed indi-cations of
renal dysfunction, such as proteinuria, glucosuria, and
aminoaciduria, after 15-16 and 48-91 weeks, respectively. The
appearance of increased ß2-microglobulin was delayed until the
30th and 138th weeks, respectively. However, no definite disturbance
of proximal renal tubular function, such as reduced tubular
reabsorption of phosphorus, hypophosphataemia or acidosis, was noted
during the one-year follow-up. The dose-effect relationship for
renal dysfunction was similar to those which have been observed in
rabbits and rats, and thus the hypothesis that the susceptibility of
monkeys to cadmium may be exceptionally low was not corroborated.
In study II, the group of monkeys given feed containing
30 mg/kg developed urine findings (e.g., proteinuria, glucosuria,
aminoaciduria) indicative of renal dysfunction in the sixth year.
Postmortem examination revealed degeneration of the proximal renal
tubules, but there was no reduction in tubular reabsorption of
phosphorus. When the administration of cadmium was discontinued in
the fifth year, no abnormality of renal function developed during
the follow-up period of four years.
Table 13. Renal effects of cadmium in monkeysa
Study Duration Sex No. of Exposure level Average cadmium Renal effects (timing, Other effects (timing,
(weeks) monkeys (mg/kg diet) level in renal in weeks, of effects) in weeks, of effects)
cortex (mg/kg)
I 55 male 2 0 163
2 3 202 no biological effects no biological effects
3 30 596 no biological effects no biological effects
3 300 380b renal dysfunction (15-16) hepatic dysfunction (12-54)
757b ß2-microglobulinuria (30) slight anaemia (20)
II 462 male 6 0 328
8 3 700 no biological effects no biological effects
8 10 1070 no biological effects erythrocytopenia (360)
8 30 1170b renal dysfunction (300-306) erythrocytopenia (240)
ß2-microglobulinuria (311)
6 100 635b renal dysfunction (48-91) erythrocytopenia (120)
ß2-microglobulinuria (138) depressed age-related increase
in blood pressure (80)
III See Table 14.
IV 308 male 2 0 18
3 3 570 no biological effects no biological effects
4 contaminated rice 230 no biological effects no biological effects
(1.33 mg/kg)
a From: Nomiyama et al. (1979), Nomiyama et al. (1987), Nomura et al. (1988), Nomiyama & Nomiyama (1988)
b The numbers with asterisks are the critical concentrations of cadmium in the renal cortex.
Table 14. Bone and renal effects of cadmium in female monkeysa
Group No. of Exposure Low protein, Low vitamin D Average renal Renal effects Bone effectsd
monkeys level calcium and dietc cortex cadmium
(mg/kg) phosphorus dietb level (mg/kg)
1 5 0 - - 58 no biological effects no biological effects
2 4 0 + - no biological effects slightly disturbed calcification
(after 154 weeks)
3 4 0 - + no biological effects low plasma vitamin D3
4 4 0 + + no biological effects osteomalacic change (after 77
weeks) reversible by vitamin D3
5 5 30e - - 1511 no biological effects no biological effects
6 4 30e + - ß2-microglobulinuriaf disturbed calcification (after
(2000 to 12 000 µg/day, 154 weeks)
67%)
7 4 30e - + no biological effects low plasma vitamin D3
8 10 30e + + ß2-microglobulinuriag osteomalacic change (after 77
(up to 2000 µg/day) weeks) reversible by vitamin D3
a From: Nomura et al. (1988); duration of experiment was 463 weeks (9 years)
b 14% protein instead of 20%; 0.3% calcium instead of 0.9%; 0.3% phosphorus instead of 0.9%
c No vitamin D3 was added (240 IU was added to the normal diet)
d In Group 5 to 8, as depressed age-related increase in blood pressure was seen after 103 weeks of treatment.
e 3 mg/kg for the first 52 weeks
f Non-progressive lesion, reversibility uncertain; renal effect noted after 193 weeks
g Reversible by normal diet and vitamin D3 treatment; renal effect noted after 154 weeks
The monkeys in study III (Table 14) given the low nutrition
feed plus 30 mg cadmium/kg developed renal function abnormalities
after the fourth year. In addition to reduced phenolsulfonphthalein
(PSP) clearance and a variable increase in urinary
ß2-microglobulin concentration, many of the monkeys showed mild
degenerative changes of the proximal tubular epithelia, but there
was no decrease in the tubular reabsorption of phosphorus. However,
elevated urinary ß2-microglobulin did not progress further with
continued administration of cadmium. Mild degenerative changes of
the proximal tubular epithelia were also noted in the groups of
monkeys that had been given a normal diet, low vitamin D diet or low
nutrition plus low vitamin D diet, each supplemented with 30 mg
cadmium/kg. However, the elevated urinary ß2-microglobulin level
soon returned to normal in those animals fed a normal diet,
regardless of continued cadmium administration. This may indicate
that the elevated urine ß2-microglobulin in this group was not
caused solely by cadmium exposure.
In study II, some of the monkeys given feed containing 3 mg/kg
or 10 mg/kg of cadmium showed cadmium concentrations in the renal
cortex as high as 760 mg/kg and 1070 mg/kg, respectively. However,
no effect upon renal function was observed during the nine-year
period, nor was there any increase in urinary ß2-microglobulin
concentration.
The above data suggest that mild renal dysfunction
(proteinuria, glucosuria, and aminoaciduria, but no decrease in the
tubular reabsorption of phosphorus) was produced in monkeys exposed
to high concentrations of cadmium (30 mg/kg diet or more). It seems,
however, that no effects on renal function occur with low-level
exposure (10 mg/kg or less). The development of renal dysfunction is
assumed to depend upon the amount of cadmium absorbed per day rather
than the total amount absorbed in the body.
In study IV, the urinary cadmium level occasionally exceeded
10 µg/litre, but no clinical chemistry changes were reported.
Cadmium concentrations in the renal cortex increased proportionally
to the dose level and duration of exposure, reaching an average of
450 mg/kg in the group given cadmium chloride and 290 mg/kg in the
group fed contaminated rice. This suggests that the chemical form of
cadmium does not affect the severity of health effects.
7.2.1.2 Respiratory route
Princi & Geever (1950) could find no evidence of renal
morphological changes in the kidney of dogs after prolonged
inhalation exposure (up to one year) to cadmium oxide or cadmium
sulfide dust (average concentration of 4 mg/m3). Routine analysis
was performed, but neither the methods used nor the results obtained
were described. Friberg (1950) exposed rabbits for about 8 months
(3 h per day, about 20 days per month) to cadmium oxide dust with an
average concentration of about 8 mg/m3 . After 4 months of
exposure, moderate proteinuria was detected by the trichloroacetic
acid test. Histological examination of the kidneys after 8 months
revealed interstitial infiltration of leucocytes in the majority of
the exposed rabbits; this was not found in the control group.
7.2.1.3 Injection route
Friberg (1950) detected proteinuria in rabbits given
subcutaneous injections of cadmium sulfate (0.65 mg cadmium/kg body
weight) 6 days per week. Electrophoretic analysis of urine proteins
revealed that the proteinuria differed from that caused by
injections of uranium salts. More recently, many studies utilizing
parenteral administration (with doses generally in the range of
0.25-1.5 mg/kg body weight), different routes of exposure
(subcutaneous and intraperitoneal), and a duration of 1-12 months
have been performed in mice, rats, and rabbits (Table 12). These
experiments have confirmed the nephrotoxic effects of cadmium.
When rabbits were exposed for 16 weeks by subcutaneous
injection of either 0.25 mg or 0.5 mg cadmium/kg body weight 3 times
a week, there was a significant increase in urinary
ß2-microglobulin excretion indicative of renal tubular dysfunction
in the high-dose group after 7 weeks. There was only a slight
increase in the serum ß2-microglobulin/creatinine ratio. Urinary
ß2-microglobulin levels were not related to serum
ß2-microglobulin levels (Piscator et al., 1981).
Rats dosed intraperitoneally, five days/week with 0.6 mg
cadmium/kg body weight, showed no abnormal effects after 5 or 6
weeks when renal cadmium levels reached about 100 mg/kg. However, in
renal tubular lining cells an increase in lysosomes, microbodies,
and smooth endoplasmic reticulum was noted. After 8 weeks renal
cadmium levels had reached about 200 mg/kg of tissue and tissue
necrosis was observed. The early changes (with a renal cadmium
concentration of up to 100 mg/kg) were considered to be adaptive and
possibly reversible, whereas morphological changes after 8 weeks
with a renal concentration of 200 mg/kg were considered to be
irreversible (Goyer et al., 1984).
Nomiyama et al. (1982a) found that non-metallothionein-bound
cadmium increased up to about 35 mg/kg tissue in parallel with total
cadmium. At that stage, the total cadmium concentration in the renal
cortex was in the range 200-800 mg/kg, the total dose of cadmium
having been approximately 1 g.
7.2.1.4 Pathogenesis of cadmium nephrotoxicity
Various hypotheses have been proposed to explain the
pathogenesis of cadmium nephrotoxicity, particularly the role of the
metal-binding protein metallothionein (see section 6.8). This
protein is inducible by a number of essential metals (Cherian &
Goyer, 1978) and may have as its primary function the intracellular
storage of zinc and copper (Panemangalore et al., 1983; Templeton et
al., 1985). It is also induced following exposure to cadmium. It is
now thought that metallothionein protects against cadmium toxicity
and that intracellular cadmium bound to metallothionein is nontoxic
(Nordberg, 1971; Goyer et al., 1989). There is considerable support
for this hypothesis. Pre-treatment of experimental animals with
small doses of cadmium prevents the acute toxic effects of a large
dose of cadmium (Nordberg et al., 1975). Parenteral administration
of cadmium-metallothionein causes acute tubular toxic effects in the
kidney (Nordberg, 1971; Nordberg et al., 1975; Cherian & Nordberg,
1983). By treatment of animals with repeated doses of cadmium,
metallothionein synthesis in the renal cortex can be induced. This
prevents against subsequent renal toxicity by parenteral
cadmium-metallothionein at dose levels that normally give rise to
renal damage (Jin et al., 1987a). Rat renal cortical cells isolated
from animals pretreated with cadmium were resistant to normally
toxic concentrations of cadmium in vitro (Jin et al., 1987b).
Similar protective effects were observed in kidney cells pretreated
with cadmium in vitro (Jin et al., 1987b). Human cells in tissue
culture, where metallothionein has been induced by pre-treatment
with cadmium, become resistant to previously lethal exposure to
cadmium (Glennas & Rugstad, 1984).
With this evidence for the protective role of intracellular
metallothionein, several theories have been proposed to explain the
nephrotoxicity of cadmium. One hypothesis attributes the
nephro-toxicity to that fraction of intracellular cadmium not bound
to metallothionein (Nordberg et al., 1975; Nomiyama & Nomiyama,
1982; Squibb et al., 1984). Another hypothesis is that
extra-cellular cadmium bound to metallothionein is toxic (Cherian et
al., 1976). Cadmium-metallothionein derived from cadmium-induced
synthesis in reticulocytes (Tanaka et al., 1985) or released from
liver cells is filtered by the renal glomeruli and reabsorbed by the
proximal tubular lining cells where it is catabolized, releasing
cadmium ions that cause renal damage (Dudley et al., 1985). This
hypothesis is supported by the fact that parenterally administered
cadmium-metallothionein is very toxic to renal tubular cells and
that the plasma metallothionein level increases with cadmium
exposure (Goyer et al., 1984; Shaikh & Hirayama, 1979).
Still another hypothesis is that intracellular cadmium
interacts with cell membranes resulting in lipid peroxidation
(Stacey et al., 1980) and that cadmium may displace essential metals
from metallothionein (Petering et al., 1984), thereby depriving
important metalloenzymes of essential metal cofactors.
These hypotheses are not mutually exclusive and the relative
significance of each of these mechanisms may differ under particular
circumstances of exposure.
Goering et al. (1985) reported the development of calcuria in
rats injected with cadmium-metallothionein, using the model
described by Squibb et al. (1984). Jin et al. (1987a) confirmed
their observations, which suggest that this biological effect may be
an early event in the development of renal tubular damage. Data from
the above studies further validate the use of the
cadmium-metallothionein injection model for studying the mechanisms
of cadmium-induced tubular injury, since calcuria is also observed
in people with chronic elevated cadmium exposure.
7.2.1.5 General features of renal effects; dose-effect and
dose-response relationships
The available data show that long-term exposure to cadmium
leads to renal tubular lesions with proteinuria, glucosuria, and
aminoaciduria, and to histopathological changes (Table 12).
It has been reported that cadmium-induced proteinuria differs
from glomerular proteinuria (Friberg, 1950) and involves low
molecular weight proteins in particular (Axelsson & Piscator, 1966a;
Nomiyama et al., 1982b). Thus, this type of proteinuria resembles
the "tubular proteinuria" seen in humans. Microscopic examination
reveals typical tubular nephropathy, i.e. atrophy and degeneration
of tubular cells, especially proximal tubular cells, and
interstitial fibrosis (Bonnell et al., 1960; Axelsson et al., 1968;
Kawai et al., 1976).
Electron microscopic changes are characterized by interstitial
fibrosis and thickening of the basement membrane of the proximal
tubular cells (Kawai et al., 1976). The smooth endoplasmic reticulum
is dilated or undergoes proliferation, and there is apical cyst
formation (Stowe et al., 1972). An increase in the number of
lysosomes and swelling of the mitochondria have also been observed.
In addition to tubular findings, there have been reports of
pathological changes in 30% of the mesangium cells of the glomeruli
of dogs (Murase et al., 1974) and increased thickness of the
glomerular basement membrane in rats (Scott et al., 1977).
Investigations into renal function have also revealed
substantial changes, mainly in tubular function, e.g., reabsorption
of glucose (Axelsson & Piscator, 1966a), whereas the changes in
glomerular filtration are relatively small (Axelsson & Piscator,
1966a). Effects have generally been seen at average renal cortex
concentrations of 200-300 mg/kg wet weight, but some studies have
reported effects at considerably lower concentrations.
Histopathological changes in rats, rabbits, horses, and birds have
been reported at renal cortex concentrations below 100 mg/kg (Table
12). However, in chronically exposed monkeys, signs of renal tubular
changes were reported at around 400-1200 mg cadmium/kg (Table 13).
After renal cortex concentrations of cadmium have reached a
level of 200-300 mg/kg wet weight, they level off or decrease. No
further increase is seen even with continued exposure (Axelsson &
Piscator, 1966a; Nomiyama & Nomiyama, 1976a; Bernard et al., 1981).
It has also been shown (Friberg, 1952; Axelsson & Piscator, 1966a;
Nordberg & Piscator, 1972; Nomiyama & Nomiyama, 1976a) that urinary
excretion of cadmium is low during the initial exposure period but a
marked increase in the excretion of cadmium occurs subsequently,
which coincides with an increase in protein excretion (Friberg,
1952; Axelsson & Piscator, 1966a; Nordberg & Piscator, 1972)
(section 6).
Animal studies have shown that, as the cadmium concentration in
the renal cortex increases, the first effects to appear are the
histopathological changes in the renal tubular cells. The low
molecular weight proteinuria and aminoaciduria develop at somewhat
higher cadmium concentrations in the renal cortex and, at even
higher concentrations, glucosuria, total proteinuria, and other
indications of damaged renal function develop. The diagnosis of
these effects depends greatly on the sensitivity of the method for
analysing the effect. For instance, a method for analysing low
molecular weight proteinuria that can accurately measure levels
considered normal (0.1 mg/litre or less) will be able to diagnose
proteinuria at an earlier stage than a method with a detection limit
of 7 mg/litre.
Most of the studies referred to in Table 12 included no data on
the prevalence of renal effects in the animals. The results were
given in a qualitative way, stating the average cadmium level in the
renal cortex at which effects were seen.
Some dose-response data are available from animal studies.
Bernard et al. (1981) produced proteinuria in rats exposed to
cadmium in drinking-water (200 mg/litre) for up to 11 months. After
8-9 months, a significant increase in group-average proteinuria was
seen, coinciding with a 25% prevalence of increased individual
proteinuria. The renal cortex cadmium concentration at that time was
about 200 mg cadmium/kg (Bernard et al., 1981). Elinder et al.
(1981a) studied horses exposed to cadmium present in their normal
food. Histopathological changes in the renal cortex were classified
and coded in a blind manner, and the prevalence of different degrees
of change was calculated for subgroups of horses with different
renal cortex cadmium concentrations. The "background" prevalence was
25-30% and there was an increased prevalence (up to 60-75%) with
increased average renal cadmium level. At a renal cortex cadmium
concentration of about 75 mg/kg, there was a significant increase in
the prevalence of histopathological changes.
7.2.2 Effects on the liver
Friberg (1950) demonstrated fibrotic changes in the liver of
rabbits exposed to repeated subcutaneous injections of cadmium.
Periportal and interlobular collagen deposition was found in the
liver of rabbits given 160 mg cadmium/litre in drinking-water for 6
months (Stowe et al., 1972). Liver function tests, however, remained
within normal limits. The cadmium concentration in the liver was
188 mg/kg wet weight. Tarasenko et al. (1974) demonstrated by
histological techniques that dystrophic changes occur in the liver
of rats after repeated intragastric administration of cadmium
caprylate in a total dose corresponding to 47 mg cadmium/kg body
weight per day. These authors also noted an increased level of
lactic acid in the blood serum of the animals. Larionova et al.
(1974) detected decreased activity of alanine transaminase in liver
tissue and depletion of glycogen in rats given barium cadmium
laurate by gavage for 8-10 days at a dosage of 169 mg/kg body weight
per day (as the laurate). After intraperitoneal injection of cadmium
(up to 1.25 mg/kg body weight for periods of up to 6 weeks),
decreased glycogen content and increased daily activity of
gluconeogenic enzymes in rat liver were reported by Merali et al.
(1974) and Chapatwala et al. (1982).
In long-term studies, rabbits given 300 mg cadmium/kg diet for
54 weeks (Kawai et al. 1976) showed some amyloid deposition in the
liver. Studies on rats after exposure for 335 days to 1 mg
cadmium/litre in drinking-water (Sporn et al., 1970) revealed
changes in liver enzyme activities. Rhesus monkeys exposed to 300 mg
cadmium/kg in the diet for 12 weeks (Nomiyama et al., 1979)
developed increased levels of plasma enzymes (GOT, GPT, and LDH).
7.2.3 Effects on the respiratory system
Interstitial pneumonitis and emphysema were found in rabbits
exposed to cadmium iron oxide dust (approximately 8 mg/m3) for 4-8
months (Friberg, 1950) and in rats observed for 4-7 months after a
single intratracheal administration of cadmium iron oxide dust
(3.5 mg/kg body weight) (Vorobjeva, 1957). However, only very slight
pulmonary effects were detected in rabbits and rats exposed to
nickel-graphite dust at dose levels several times higher than the
concentrations of cadmium iron oxide dust.
Yoshikawa et al. (1975) exposed rats to cadmium oxide fumes
(0.1 or 1.0 mg cadmium/m3) for up to 3 months. There were 10 rats
in each group, and three of the rats in the high exposure group died
after about 7 weeks. Lung fibrosis and the first stage of emphysema
were observed at the end of the experiment in the high-dose group.
Free macrophage cells in the alveoli were more numerous in both
groups, and there was an increased surface tension of the
surfactants.
Snider et al. (1973) observed signs of emphysema in rats 10
days after 5-15 daily 1-h periods of exposure to cadmium chloride
aerosol (10 mg/m3). Also, long-term exposure to comparatively low
air concentrations of cadmium oxide (24-50 µg cadmium/m3) gave
rise to pathological changes in the lungs similar to emphysema as
well as to cell proliferation in the bronchi (Prigge, 1978). A
long-term study (14 months), in which mice and golden hamsters were
exposed to different concentrations of cadmium chloride (30 and
90 µg/m3), cadmium sulfate (30 and 90 µg/m3), cadmium sulfide
aerosols (90-100 µg/m3), cadmium oxide fume (10-90 µg/m3), and
dust (10-270 µg/m3), revealed a significantly increased incidence
of alveolar hyperplasia and interstitial fibrosis in most of the
exposed groups (Heinrich et al., 1989).
Single intratracheal administration of several cadmium
compounds (e.g., oxide, sulfide, carbonate, sulfoselenide,
caprylate, stearate, cadmium-barium laurate, and cadmium-barium
stearate) in doses from 0.5 mg (as the oxide) to 15 mg (as the
sulfide) caused the development, over 6 months, of chronic
inflammatory changes, emphysema, and atelectasis leading to
fibrosis. Exposure to cadmium oxide and caprylate gave rise to the
development of nodules of hyaline connective tissue; these resembled
silicotic nodules (Vorobjeva & Sabalina, 1975).
7.2.4 Effects on bones and calcium metabolism
Male rats fed a normal diet and exposed to cadmium sulfate by
inhalation (3 and 0.3 mg/m3, 4 h daily for 4 months) showed
decreased serum and urinary calcium concentrations compare to
controls. Female rats similarly exposed to cadmium sulfate
(2.8 mg/m3, 3 h/week) during pregnancy showed radiological
evidence of osteoporosis in addition to hypocalcaemia (Tarasenko et
al., 1975).
In a study by Kogan et al. (1972), cadmium chloride and cadmium
sulfate were administered subcutaneously to rats at a daily dose of
1 mg/kg body weight for up to 12 months. At 12 months, X-ray
analysis of the bones indicated osteoporosis and osteosclerosis.
Subsequent histopathology showed an increase in osteoclasts and a
bone structure described by the authors to be indicative of
osteomalacia.
Oral administration of cadmium chloride in drinking-water to
male rats (1 or 4 µg/kg body weight daily for six months) resulted
in changes in calcium metabolism and bone structure characteristic
of osteomalacia, which were not observed in the control group. No
effects were noted in a group of animals given a daily dose of
0.01 µg/kg body weight (Likutova & Belova, 1987).
Rats given 50 mg cadmium/litre in drinking-water for about 9
months showed reduced calcium and phosphorus absorption from the
intestine (Sugawara & Sugawara, 1974). In addition, some of the
animals showed histological changes in the duodenal mucosa, a
finding also reported in Japanese quail (Richardson & Fox, 1974).
Several mineral balance studies have been made on animals fed
cadmium. Simultaneous administration of cadmium with a low-protein,
low-calcium diet led to a decrease in the calcium and zinc content
of bone (Itokawa et al., 1973). Furthermore, Kobayashi (1974)
reported that cadmium feeding led to a negative calcium balance in
rats.
The decreased calcium absorption and negative calcium balance
in cadmium-exposed rats could result from the inhibitory effects of
cadmium on the activation of vitamin D in renal cortical cells
(Feldman & Cousins, 1973). The renal conversion of
25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol has been
found to be inhibited by high dietary cadmium exposure in rats fed a
normal calcium diet, but this effect was not seen on a low-calcium
diet (Lorentzon & Larsson, 1977). The metabolically active form of
vitamin D (1,25-dihydroxycholecalciferol) is necessary for the
normal absorption of calcium from the intestine. Ando et al. (1981)
found that the stimulation of calcium absorption by 1-alpha-hydroxy
vitamin D3 was inhibited in rats exposed to cadmium by gastric
intubation. Furthermore, the concentration of calcium-binding
protein in intestinal mucosa may be decreased by cadmium exposure
(Fullmer et al., 1980).
Administration of drinking-water containing 10 mg cadmium per
litre to rats fed a normal diet over a 9-month period gave rise to
decalcification and cortical atrophy in the skeleton (Kawai et al.,
1976). Other workers have also reported effects in the bones of rats
following several months exposure to cadmium in drinking-water
(Itokawa et al., 1974; Kawamura et al., 1978) and in the diet
(Takashima et al., 1980; Nogawa et al., 1981a), and following
subcutaneous injection (Nogawa et al., 1981a). In these studies, the
bones were reported to show more or less severe osteoporosis and
osteomalacia. On the other hand, Kajikawa et al. (1981) did not find
either osteoporosis or osteomalacia after rats were exposed for 2
years to cadmium in drinking-water (200 mg/litre).
Anderson & Danylchuk (1979) found that exposure of Beagle dogs
for six months to cadmium (25 mg/litre in drinking-water) reduced
bone turnover rate, a metabolic abnormality consistent with calcium
deficiency or osteomalacia. Kawashima et al. (1988) found that
feeding a cadmium-contaminated rice diet or a diet containing 3 mg
cadmium chloride/kg for six years to crab-eating monkeys did not
produce any change in vitamin D metabolism, and there was no
evidence of renal dysfunction. In another series of experiments
rhesus monkeys were fed diets containing 3, 10, 30 or 100 mg
cadmium/kg for 9 years. Serum vitamin D metabolites and renal
production of vitamin D remained unchanged, but in animals fed 30 or
100 mg cadmium/kg of diet there was slight but not statistically
significant depression of renal 25-hydroxy vitamin D1-hydroxylase
activity. No skeletal abnormalities were found in any of these
animals.
Bhattacharyya et al. (1988) studied the effects of 0, 0.25, 5
or 50 mg cadmium/kg diets on female mice bred for six consecutive
42-day cycles of pregnancy and lactation and on non-pregnant
controls. The multiparous mice exposed to 50 mg/kg experienced
significant decreases in body weight (3-11%) and femur calcium
content (15-27%), and the femur calcium to dry weight ratios
decreased by 5-7%. These results were thought by the authors to
provide evidence that the combination of cadmium exposure and
multiparity has a synergic effect on bone metabolism.
In the Japanese monkey study III (section 7.2.1 and Table 14),
osteomalacic changes were found in the low-nutrition plus
low-vitamin-D diet group (group 4) after 77 weeks. These effects
were not further exacerbated by feeding cadmium (group 8). These
changes were found to be reversed by the administration of vitamin
D. Renal effects were found in group 8 after 154 weeks. Therefore,
the osteomalacia found in group 8 was diagnosed as not being renal
osteomalacia.
Most of the findings discussed above indicate a direct effect
of cadmium on bone mineralization, possibly related to calcium
deficiency, and an indirect effect on calcium absorption via vitamin
D hydroxylation, perhaps leading to osteomalacia. The direct effects
develop after long-term cadmium exposure, whereas the indirect
effect on vitamin D metabolism occurs only when renal damage is seen
in the animals. Osteomalacia only occurred in monkeys fed a diet low
in protein, phosphorus, calcium, and vitamin D. Cadmium
administration did not increase these effects.
7.2.5 Effects on haematopoiesis
Anaemia is a common finding in animals after both dietary
(Wilson et al., 1941) and parenteral (Friberg, 1950) exposure to
cadmium. After dietary exposure, decreased haemoglobin concentration
(Decker et al., 1958) and decreased haematocrit (PCV) (Prigge et
al., 1977) are among the early signs of cadmium toxicity.
Fox & Fry (1970) and Fox et al. (1971) reported that the
cadmium-induced anaemia could be prevented by simultaneous feeding
with iron or ascorbic acid. Decreased gastrointestinal absorption of
iron due to cadmium may be one mechanism for this anaemia.
After parenteral exposure, iron administration has a beneficial
effect on the anaemia (Friberg, 1955). Berlin & Friberg (1960)
showed that cadmium injections caused erythrocyte destruction, but
there was no indication of an interference with haemoglobin
production. Haemolytic anaemia in rabbits was also reported by
Axelsson & Piscator (1966b).
7.2.6 Effects on blood pressure and the cardiovascular system
Chronic oral administration of cadmium compounds to rats (Perry
& Erlanger, 1974) induced statistically significant elevation of
blood pressure. However, the systolic pressure changes were much
smaller than those previously reported by Schroeder & Vinton (1962)
and by Schroeder (1964). Furthermore, a different effect was
obtained with lower, as compared with higher, doses of cadmium. Rats
given 1, 2.5, or 5 mg cadmium/litre in drinking-water for one year
had significantly higher blood pressure values than controls. Six
months after the beginning of exposure, a statistically significant
increase in blood pressure was also observed in rats given 10 or
25 mg/litre, but the increase was not statistically significant
after one year of exposure. In rats receiving 50 mg/litre, a
statistically significant increase in systolic blood pressure was
observed after 12 months of exposure. As discussed in section 8.2.4,
these results may be of basic importance in evaluating data on the
possible effects of cadmium on the human cardiovascular system.
Several mechanisms have been postulated (Perry & Erlanger,
1974) to explain the effects of chronic cadmium exposure on the
cardiovascular system. Oral adminstration of cadmium doses that
induce hypertension (and also parenteral administration of cadmium)
was shown to increase circulatory renin activity (Perry & Erlanger,
1973). Injection of cadmium into the renal artery of dogs increased
sodium reabsorption by the exposed kidney (Vander, 1962), and
repeated intramuscular (Perry et al., 1971) or chronic oral
administration of cadmium (Lener & Musil, 1971) was reported to
increase sodium retention in the body. By morpho-metric methods,
Fowler et al. (1975) demonstrated effects on the renal blood vessels
of rats exposed to various concentrations of cadmium (up to
200 mg/litre in drinking-water) for several weeks. Significantly
smaller arteriolar diameters were found in the exposed animals than
in the controls.
Perry et al. (1977) studied the influence of exposure duration
(from 3 to 24 months) and cadmium dose levels in water (from 0.1 to
10 mg/litre) on the blood pressure of Long-Evans rats. A small
increase in blood pressure occurred even at the lowest exposure
level after 3 months exposure. The greatest increase in blood
pressure (3.2 kPa; 24 mmHg) occurred after 24 months exposure to
1 mg/litre, when the average renal cortex cadmium level was 12 mg/kg
wet weight. At higher dose levels, the blood pressure increase was
less and, at the highest dosage (10 mg/litre for 24 months), the
blood pressure did in fact decrease.
Perry et al. (1976) found hypertensive effects in
Sprague-Dawley as well as Long-Evans rats. Petering et al. (1979)
reported that male rats were more susceptible to these effects than
female rats after exposure via drinking-water, but Ohanian & Iwai
(1980) found the opposite for rats exposed parenterally. In several
studies (e.g., Kotsonis & Klaassen, 1977; Whanger, 1979; Fingerle et
al., 1982), there was no increase in blood pressure after various
cadmium doses were given via drinking-water. The type of diet
appears to be crucial for the development of hypertension (Whanger,
1979); it can usually only be produced in rats fed a rye-based diet
(Perry & Erlanger, 1982). Nishiyama et al. (1986) postulated that
cadmium exposure increases sodium and water retention, which are
important factors controlling the development of hypertension.
A detailed review of factors influencing the effects of cadmium
on the cardiovascular system was reported by the Task Group on Metal
Toxicity (1976), with particular reference to those factors that
might modify the dose-effect and dose-response relationships.
Rats exposed to cadmium (5 mg/litre) in drinking-water (Kopp et
al., 1980a,b, 1983) developed electrocardiographic and biochemical
changes in the myocardium, and impairment of the functional status
of the myocardium. These effects could be related to (i) decreased
high-energy phosphate storage in the myocardium, (ii) reduced
myocardial contractility, or (iii) diminished excitability of the
cardiac conduction system. Jamall & Sprowls (1987) found that rats
fed a diet supplemented with copper (50 mg/kg), selenium
(0.5 mg/kg), and cadmium (50 mg/kg) had marked reductions in heart
cytosolic glutathione peroxidase, superoxide desmutase, and
catalase. They suggested that heart mitochondria are the site of the
cadmium-induced biochemical lesion in the myocardium.
Reviews of all aspects of the cardiovascular effects of cadmium
on experimental animals have been reported by Perry & Kopp (1983)
and Jamall & Smith (1986).
7.2.7 Effects on reproductive organs
Cadmium-induced testicular necrosis (section 7.1.2.1) generally
results in permanent infertility (Barlow & Sullivan, 1982). Ramaya &
Pomerantzeva (1977) found markedly reduced testis weights 1, 3, and
6 months after mice were administered 4 mg cadmium/kg. The animals
were sterile and microscopic examination revealed morphological
changes in the testis. Krasovskii et al. (1976) noticed decreased
spermatozoa motility and spermatogenesis index in rats continuously
exposed via food to 0.5-5.0 mg cadmium/kg body weight. In male mice
exposed repeatedly by daily subcutaneous injection of cadmium
chloride (0.5 mg/kg per day) for 6 months (Nordberg, 1975), there
was a decrease in normal testosterone-dependent proteinuria.
Morphological examination of the seminal vesicles revealed a smaller
weight and size as well as histological indications of lower
secretory activity, this being consistent with decreased
testosterone activity in these animals.
7.2.8 Other effects
Effects on the immune system have been reported after both
chronic and acute cadmium exposure. A decrease in the number of
antibody-forming cells in the spleen as well as a decrease in
antibody production was seen in mice after long-term exposure to
cadmium in drinking-water (Koller et al., 1975). An inhibition of
the cell-mediated immune response occurred in mice after repeated
intraperitoneal injections (Bozelka & Burkholder, 1982). However, no
data are available linking these effects to increased susceptibility
to infection or other secondary dysfunctions.
Gestational exposure to cadmium (4.2 and 8.4 µg/ml in
drinking-water) results in decreased birth weight, retarded growth,
delayed development of the sensory motor coordination reflexes, and
increased motor activity. Cadmium exposure during critical periods
of development might result in developmental and behavioural
deficits with long-term implications for adult behaviour (Mohd et
al., 1986).
7.3 Fetal toxicity and teratogenicity
In several species of laboratory rodents, large doses of
cadmium salts induce severe placental damage and fetal deaths when
given at a late stage of pregnancy, and teratogenic effects, such as
exencephaly, hydrocephaly, cleft lips and palate, microphthalmia,
micrognathia, clubfoot, and dysplastic tail, when given at early
stages of gestation.
A single subcutaneous injection of cadmium chloride, acetate,
or lactate (4.5 mg cadmium/kg body weight) given to Wistar rats from
the 17th to the 21st day of pregnancy (Parizek, 1964) led to the
rapid development of severe placental damage in all rats and to
fetal death. Placental damage was not dependent on the presence of
fetuses, but it was not possible to decide whether fetal lethality
resulted from the placental lesion or from a direct effect of
cadmium on the fetuses (Parizek, 1964). Similar effects were
observed at a dose level of 3.3 mg cadmium/kg body weight (Parizek
et al., 1968b). Placental damage and fetal deaths were also observed
after cadmium administration to pregnant Swiss albino mice
(Chiquoine, 1965).
Teratogenic effects can be observed when doses close to the
LD50 (Table 11) for cadmium salts are administered to pregnant
females at critical stages of embryogenesis. These effects were
demonstrated with intravenous injections of cadmium sulfate in
hamsters (Ferm & Carpenter, 1968; Mulvihill et al., 1970; Ferm,
1972) and with intraperitoneal (Barr, 1973), subcutaneous (Chernoff,
1973), or dietary (Scharpf et al., 1972) administration of cadmium
chloride in rats. Teratogenic effects induced by cadmium salts have
also been demonstrated in mice (Ishizu et al., 1973).
The character of the changes induced is dependent on the
species and on the stage of embryogenesis. As little as 123 µg/litre
in mouse embryo cultures produced exencephaly apparently by
re-opening the closed neural tube (Schmid et al., 1985). Either
facial defects or limb abnormalities were induced by cadmium when
administered to pregnant hamsters on day 8 or 9 of gestation (Ferm,
1971). Both jaw defects and cleft palate were observed in the
offspring of rats given daily subcutaneous cadmium chloride
injections (8 mg/kg body weight) on days 13-16 or 14-17 of
pregnancy, but cleft palate was not observed when this dosage was
given on days 15-18 or 16-19 of pregnancy (Chernoff, 1973).
Anophthalmia or microphthalmia and dysplastic ears were induced by
approximately 2 mg of cadmium as the chloride given
intra-peritoneally to pregnant rats on the 9th but not on the 11th
day of pregnancy (Barr, 1973). Other effects observed in these
studies included decreased lung weight in the offspring of rats
subjected to cadmium during pregnancy (Chernoff, 1973) and
deficiencies in bone formation and delays in bone ossification
(Mulvihill et al., 1970; Scharpf et al., 1972).
The dose-dependent fetal mortality and teratogenicity response
was established in studies with subcutaneous administration of
cadmium chloride to rats (Chernoff, 1973) and mice (Ishizu et al.,
1973). The no-observed-effect level with respect to malformations
was found in the latter study to be 0.33 mg/kg body weight.
All the teratogenic effects mentioned above were induced by
parenteral administration of very high doses of cadmium salts.
However, in a rat study by Scharpf et al. (1972), very high peroral
doses (20, 40, 60, or 80 mg/kg body weight given by gavage daily
from days 6 to 19 of pregnancy) of cadmium chloride were used with
the simultaneous administration of sodium chloride, and internal
teratological examinations were performed. Heart and kidney
abnormalities were the major internal defects, but their incidence
was not directly related to the dose of cadmium chloride
administered. At the lowest dose level, heart abnormalities were
detected in 19.7% of the 127 fetuses (abnormalities in the control
group were seen in 6.6% of 107 fetuses) and teratoma of the kidney
was observed in 15.7% of these fetuses.
Cvetkova (1970) exposed pregnant female rats via the
respiratory route to cadmium sulfate (2.8 mg/m3, 4 h daily) and,
on the 22nd day, killed half of them to examine the embryos. The
number of embryos in exposed rats was the same as in a control
group, but the mean weight was lower in the exposed group. In the
exposed rats, where pregnancies were allowed to proceed to full
term, the average weight of the offspring was lower than in the
controls both at birth and after 8 months. The rats born to the
exposed group also had increased mortality during the first 10 days
after birth.
When mice were exposed for several generations to cadmium in
drinking-water (10 mg/litre), fetal mortality, runting, and
malformations were observed (Schroeder & Mitchener, 1971). The
external malformations, consisting of sharp angulation of the distal
third of the tail, were observed in 16.1% of 255 offspring (F1 and
F2A generation), and 87 deaths before weaning (30.5%) were
recorded.
Ferm & Carpenter (1968) showed that zinc injected
simultaneously with cadmium could protect against the teratogenic
effects of cadmium, and a similar protective action was found for
selenium (Holmberg & Ferm, 1969). Maternal zinc deficiency can
produce congenital malformations (Hurley et al., 1971). This was
confirmed by Parzyck et al. (1978), who also found that
intraperitoneal injection of 1.5 mg cadmium/kg body weight to
pregnant rats increased the prevalence of malformations. The
increase was greater at this cadmium dose than the increase due to
zinc deficiency. Combined zinc deficiency and cadmium exposure
caused a very high incidence of fetal deaths.
Further experimental data on rats provided by Samarawickrama &
Webb (1979) indicate that maternal cadmium exposure gives rise to a
fetal zinc deficiency and that this is one cause of the teratogenic
effects observed. Intravenous cadmium injections to pregnant rats at
doses ranging from 0.25 to 1.25 mg/kg body weight on day 12 of
gestation produced a dose-related decrease in fetal uptake of a dose
of 65 mg zinc given 4 h later. Maternal cadmium exposure (1.25 mg/kg
body weight) was shown to result in decreased activity of a fetal
zinc-dependent enzyme thymidine kinase, which is responsible for the
incorporation of thymidine in DNA. Additional evidence that
cadmium-induced fetotoxicity is related to a cadmium-induced fetal
zinc deficiency was reported by Daston (1982), who found that
co-administration of zinc (12 mg/kg body weight) almost totally
eliminated severe fetal lung lesions when pregnant rats were given
cadmium (8 mg/kg body weight) on gestation days 12-15.
7.4 Mutagenicity
Studies on Drosophila (Ramel & Friberg, 1971; Vorobjeva &
Sabalina, 1975) failed to show any chromosomal abnormalities after
exposure to various cadmium compounds. Some in vitro studies of
cultured human lymphocytes and fibroblasts were also negative (Paton
& Allison, 1972; Deknudt & Deminatti, 1978; Kogan et al., 1978).
Shiraishi et al. (1972) reported a marked increase in the frequency
of chromatid breaks, translocations, and dicentric chromosomes in
leucocytes, from one person, cultured in a medium containing 62 mg
cadmium/litre (as the sulfate) for 4-8 h.
Andersen et al. (1983) found that the average chromosome length
in human lymphocytes was initially reduced when they were cultured
in a medium containing cadmium chloride (1.1 mg cadmium/litre), but
subsequently returned to normal. This effect was probably related to
the synthesis of metallothionein and complexing with cadmium.
Watanabe et al. (1979) observed aneuploidy in rat oocytes with
cadmium accumulation in the ovary after exposure to cadmium chloride
in vivo.
Rohr & Bauchinger (1976) found a reduced mitotic index in
hamster fibroblasts cultured in 100 µg cadmium/litre (as the
sulfate) and chromosome damage at concentrations above 500 µg
cadmium/litre. Deaven & Campbell (1980) showed that the effects on
cultured hamster cells depended on the type of medium used.
There appears to be an acute effect of cadmium following the
injection of 0.6-2.8 mg cadmium/kg body weight (Felten, 1979). After
6 h, there was an increased frequency of chromatid breaks in bone
marrow cells and chromosome gaps and breaks in spermatocytes, which
could be associated with the acute effects on haematopoiesis
(section 7.2.5) and on the testis (section 7.1.2.1).
A summary and graphical presentation of the available evidence
on genetic and related effects of cadmium in various in vivo and
in vitro test systems has been presented by IARC (1987a). Although
prokaryote test systems reveal no effects, variable results have
been observed in lower eukaryotes, mammalian cells in vitro, and
mammals in vivo.
7.5 Carcinogenicity
Intramuscular or subcutaneous administration of metallic
cadmium or cadmium compounds can induce sarcomata at the site of
injection. This local effect of cadmium was demonstrated with
intramuscular administration of metallic cadmium (cadmium powder in
fowl serum) to hooded rats (Heath et al., 1962), subcutaneous
administration to Chester Beatty rats of cadmium as the sulfide and
oxide (Kazantzis, 1963; Kazantzis & Hanbury, 1966) or sulfate
(Haddow et al., 1964), intramuscular injection of cadmium chloride
to Wistar rats (Gunn et al., 1967), and subcutaneous injection to
Sprague-Dawley rats (Nazari et al., 1967). Transplantability of
tumours induced in these studies (Heath & Webb, 1967) and metastases
into regional lymph nodes and into lungs (Kazantzis & Hanbury, 1966)
were reported. Intratesticular injection of cadmium chloride to
White Leghorn cockerels was reported to induce teratoma at the site
of injection (Guthrie, 1964).
After Hoffman et al. (1985) injected 1.9 mg cadmium chloride
(1.2 mg/kg body weight) directly into the ventral prostatic lobe of
100 12-month-old male rats, simple hyperplasia was found in 38 of
the rats, atypical hyperplasia in 29, atypical hyperplasia with
severe dysplasia in 11, and invasive prostatic cancer in 5 animals.
Hoffman et al. (1988) reported changes in the ultrastructure of
prostate epithelial cells in rats injected into the ventral prostate
with 2.2 or 3.3 mg cadmium/kg body weight. In animals given oral
treatment via the drinking-water (29.9 or 115 mg cadmium/kg body
weight), there were changes ranging in severity up to dysplasia but
no evidence of carcinoma.
A single parenteral administration of cadmium salts can induce
necrosis of the testis (see section 7.1.2.1). After one year, the
remnants of the necrotic testis were shown to contain masses of
cells showing the typical structure of Leydig cells (Parizek, 1960).
This regeneration of testicular Leydig cells damaged by cadmium can
result in Leydig cell neoplasia (Gunn et al., 1963, 1965; Lucis et
al., 1972). The ultrastructural features of cadmium-induced Leydig
cell tumours correspond in most respects with the fine structural
features of normal Leydig cells (Reddy et al., 1973).
Other injection studies and peroral studies did not demonstrate
increased malignancy (Schroeder et al., 1964, 1965; Loser, 1980),
but the doses were low compared to those necessary to induce renal
damage. In one peroral study (Kanisawa & Schroeder, 1969), rats were
exposed to 5 mg cadmium/litre in drinking-water for up to 2 years.
There were 7 malignant tumours among 47 cadmium-exposed male rats
and 2 tumours among 34 male control rats. This indicates a doubling
of the tumour rate, but because of the low statistical power of the
study, the increase was not statistically significant and the
authors concluded that ingestion of these cadmium doses was not
carcinogenic.
Some studies have been specially designed to investigate the
possible role of cadmium in cancer of the prostate. The prostate
gland, like the testis, is of particular interest with respect to
cadmium toxicity because these organs contain greater concentrations
of zinc than any other tissues and it has been suggested that
cadmium may affect prostate growth by competition with zinc (Gunn et
al., 1961). Levy et al. (1973) gave three groups of rats weekly
subcutaneous injections of cadmium sulfate at concentrations of
0.022, 0.044 or 0.087 mg cadmium per rat (average weight 220 g at
the start and 410 g at the end of the 2-year exposure). Weekly
injections of water were given to the 75 control rats. The liver
cadmium level was 80 mg/kg in the highest-dose group, but no
malignant changes were found in the prostate. No difference was seen
between exposed and control rats with respect to malignant changes
in other organs.
Levy & Clack (1975) and Levy et al. (1975) conducted 2-year
studies in rats and mice designed to detect carcinogenic effects in
the prostate. The animals in both experiments were given weekly
administrations of cadmium sulfate by stomach tube. The rats were
given from 0.08 to 0.35 mg/kg body weight and the mice 0.44 to
1.75 mg/kg. Extremely low levels of cadmium were found in the kidney
after 2 years (5 mg/kg wet weight in rats), but no macroscopic or
microscopic changes were seen in any tissue at these low doses.
In a long-term study on Fisher rats, Sanders & Mahaffey (1984)
administered cadmium oxide (25 µg) in single or repeated doses by
intratracheal instillations. There was no evidence for pulmonary or
prostate carcinogenicity, but increases in mammary tumours and in
tumours at multiple sites in male rats were reported.
It has been reported that inhalation of a cadmium aerosol
causes lung cancer in Wistar rats (Takenaka et al., 1983). Three
groups of 40 rats were continuously exposed to cadmium chloride
aerosols for 18 months, the air cadmium concentrations being 12.5,
25, and 50 µg/m3. A control group of 41 rats was also studied. The
study was terminated after 31 months, and no lung cancers were seen
in the control group. However, in the exposed groups, the incidence
was 15%, 53% and 71%, respectively, at increasing exposure levels.
Even at these relatively low exposure levels, there was a clear
dose-response relationship. Histologically the experimentally
induced tumours were adenocarcinomas, epidermoid carcinomas,
mucoepidermoid carcinomas, and combined epidermoid and
adenocarcinomas.
In a subsequent study, rats were exposed to inhalable aerosols
of cadmium sulfate and cadmium oxide and fume and dust at > 30 µg
cadmium/m3 and to cadmium sulfide at > 90 µg cadmium per m3 for
periods of up to 18 months. Bronchoalveolar benign and malignant
adenomas, squamous cell carcinomas, and combined forms developed at
high primary tumour rates with all four forms of cadmium tested even
after discontinuous exposure for 40 h/week for 6 months. No primary
tumour was found with cadmium oxide fume at a concentration of 10 µg
cadmium/m3 or cadmium oxide dust (at 30 µg cadmium/m3) when
combined with a zinc oxide aerosol (Oldiges et al., 1989).
In a further study, male and female Syrian golden hamsters and
female NMRT mice were exposed to cadmium chloride, sulfate, oxide,
and sulfide at concentrations of between 10 and 270 µg cadmium/m3.
The exposure was continuous (19 h/day, 5 days/week) for 50 to 70
weeks and was followed by a 50-week observation period. No increase
in the lung tumour rate was observed in either the mice or hamsters
(Heinrich et al., 1989), but in both species exposure to cadmium
caused multifocal bronchoalveolar hyperplasia, the extent of which
varied with the compound used, its concentration, and the length of
exposure. The most severe changes were found after cadmium oxide
inhalation (Aufderheide et al., 1990).
A synergistic effect has been shown in rat renal tumours
induced by dimethylnitrosamine when followed by cadmium chloride
given by intramuscular injection. In this study, cadmium appeared to
enhance the initiation of dimethylnitrosamine-induced cancer (Wade,
1987).
7.6 Host and dietary factors; interactions with other trace
elements
The toxic effects of cadmium in experimental animals have been
shown to be dependent on genetic factors, stage of ontogenic
development, functional state of the organism, and simultaneous or
previous exposure to certain environmental influences, including
exposure to certain nutrients.
Resistance to cadmium-induced testicular necrosis is determined
by a single autosomal recessive gene (cdm) in inbred mice (Taylor et
al., 1973). The teratogenic effects of cadmium are dependent on the
stage of embryogenesis (section 6.3). The stage of postnatal
development of certain organs may be of importance for the toxic
effects of cadmium, as has been shown for the testis (Parizek, 1957,
1960), ovaries (Kar et al., 1959), and central nervous system
(Gabbiani et al., 1967).
Pretreatment with small, non-toxic doses of cadmium salts has
been shown to induce resistance to testicular or lethal effects
(Terhaar et al., 1965; Ito & Sawauchi, 1966). The probable mechanism
is induction of metallothionein synthesis by the pretreatment. This
enables the subsequent dose of cadmium to be bound rapidly to
metallothionein, which renders it less acutely toxic (Nordberg,
1971). Similarly, the protective effect of zinc against cadmium
toxicity could also be, at least in part, dependent on the induction
of an increased synthesis of metallothionein-like proteins (Webb,
1972; Davies et al., 1973).
Selenium compounds are known to be highly effective in
preventing the reproductive toxic effects of cadmium (Kar et al.,
1960; Mason & Young, 1967; Parizek et al., 1968a,b), lethality to
rats (Parizek et al., 1968b) and mice (Gunn et al., 1968), and
teratogenicity (Holmberg & Ferm, 1969). Fetal lethality (Parizek et
al., 1968b) and teratogenic effects (Holmberg & Ferm, 1969) can be
prevented when selenium compounds are given at the same time as
cadmium.
Simultaneous administration of mercuric and cadmium compounds
has been shown to have an additive effect (Gale, 1973). Oral
administration of nitrilotriacetate with large oral doses of cadmium
chloride provided protection against the lethality of cadmium and
had no potentiating effect on the teratogenicity and fetal
accumulation of cadmium (Scharpf et al., 1972). This was confirmed
by Engström (1979), who also showed that simultaneous oral exposure
to cadmium and sodium tripolyphosphate decreased the mortality
expected at the cadmium level used. However, when nitrilotriacetate
or sodium tripolyphosphate was given subcutaneously with cadmium,
the mortality rates were increased (Engström & Nordberg, 1978;
Andersen et al., 1982). The chelation of cadmium in the
gastrointestinal tract decreased the uptake of cadmium, whereas
parenteral exposure to cadmium and chelating agents caused a higher
renal cadmium concentration than cadmium alone.
The interaction of cadmium with certain trace elements can
produce symptoms characteristic of trace element deficiencies. As a
result, chronic cadmium toxicity in certain animal species closely
resembles zinc and/or copper deficiency and can be prevented by
administering higher doses of the salts of these trace elements
(Petering et al., 1971, 1979; Mills & Delgarno, 1972). Cadmium-
calcium interactions are discussed in section 7.2.4.
An increased toxicity of cadmium was reported in animals on
low-protein diets (Fitzhugh & Meiller, 1941), this being due partly
to rapid intestinal absorption of cadmium (Suzuki et al., 1969).
Lack of dietary calcium seems to play a role similar to lack of
dietary protein in increasing the toxicity of cadmium (Suzuki et
al., 1969). Supplements of dietary ascorbic acid almost completely
prevented cadmium-induced anaemia and improved the growth rate (Fox
& Fry, 1970).
Ambient temperature (Nomiyama et al., 1978b) and the energy or
protein level in the diet have been reported to influence the LD50
of cadmium in mice.
Various aspects of the interactions between cadmium and other
trace elements were discussed in greater detail by the Task Group on
Metal Interactions (1978).
7.7 Conclusions
Inhalation exposure at high levels causes lethal pulmonary
oedema. Single high-dose injection gives rise to testicular and
non-ovulating ovarian necrosis, liver damage, and small vessel
injury. Large oral doses damage the gastric and intestinal mucosa.
Long-term inhalation exposure and intratracheal administration
give rise to chronic inflammatory changes in the lungs, fibrosis,
and appearances suggestive of emphysema. Long-term parenteral or
oral administration produces effects primarily on the kidneys, but
also on the liver and the haematopoietic, immune, skeletal, and
cardiovascular systems. Skeletal effects and hypertension have been
induced in certain species under defined conditions. Teratogenic
effects and placental damage occur, depending on the relation
between the exposure and the stage of gestation, and may involve
interactive effects with zinc.
Of greatest relevance to human exposure are the acute
inhalation effects on the lung and the chronic effects on the
kidney. Following long-term exposure, the kidney is regarded as the
critical organ. The effects on the kidney are characterized by
tubular dysfunction and cell damage, although glomerular dysfunction
may also occur. A consequence of renal tubular dysfunction is a
disturbance of calcium and vitamin D metabolism. According to some
studies, this has led to osteomalacia and/or osteoporosis, but these
effects have not been confirmed by other studies. A direct effect of
cadmium on bone mineralization cannot be excluded. The toxic effects
of cadmium in experimental animals are influenced by genetic and
nutritional factors, interactions with other metals, in particular
zinc, and pretreatment with cadmium, which may be related to the
induction of metallothionein.
IARC (1976, 1987b) accepted as sufficient the evidence that
cadmium chloride, sulfate, sulfide, and oxide can give rise to
injection-site sarcomata in the rat and that the chloride and
sulfate can induce interstitial cell tumours in the testis of rats
and mice, but found oral studies inadequate for evaluation. One
recent life-time study (18 months), in which rats were subjected to
continuous inhalation of a cadmium chloride aerosol at low
concentration, showed a high incidence of primary lung cancer with
evidence of a dose-response relationship. Studies on the genotoxic
effects of cadmium have given discordant results, most of the
positive results indicating chromosomal effects after short-term
high-level exposure.
8. EFFECTS ON HUMANS
Most of the available epidemiological studies or group
observations, as well as the clinical studies, have been performed
either on occupationally exposed workers or on Japanese populations
in cadmium-polluted areas. A great deal of epidemiological data has
resulted from studies in polluted areas of Japan (Cooperative
Research Committee on Itai-itai Disease, 1967; Shigematsu et al.,
1978; Japan Cadmium Research Committee, 1989) and, more recently,
from smaller studies in other countries (Drasch et al., 1985;
Philipp, 1985; Hahn et al., 1987; Roels et al., 1989; Thun et al.,
1989; Likutova, 1989). Comprehensive summaries of these studies have
also been published (Tsuchiya, 1978; Friberg et al., 1986; Nomiyama,
1986).
Many of these studies have focused on the detection of early
signs of kidney dysfunction. Others have investigated clinical signs
of disease such as renal stones and pulmonary impairment. Until the
middle of the 1970s, particular attention was given in Japan to the
detection of and screening for bone disease (e.g., Itai-itai
disease). More recently the role of cadmium in human carcinogenesis
and mortality has also been studied.
Exposure to cadmium produces a wide variety of effects
involving many organs and systems. From the point of view of
preventive medicine, the detection of early effects on the kidneys
is of particular importance in order to prevent more serious renal
effects and those on the lungs or bones. Recent studies indicating
that chronic exposure to cadmium may give rise to cancer will be
reviewed in some detail.
8.1 Acute Effects
8.1.1 Inhalation
Acute cadmium poisoning and, in some cases, death have been
reported among workers shortly after exposure to fumes when cadmium
metal or cadmium-containing materials have been heated to high
temperatures (Beton et al., 1966; Blejer, 1966; Dunphy, 1967). The
principal symptom in acute cases, both fatal and non-fatal, is
respiratory distress due to chemical pneumonitis and oedema
(MacFarland, 1979; Lucas et al., 1980). At an early stage, the
symptoms may be confused with those of "metal fume fever".
In working environments where cases of acute poisoning
occurred, cadmium concentrations were usually very high. For
instance, in one case the fatal air concentration of cadmium oxide
fume from a furnace was approximately 50 mg/m3 for a period of
about 1 h (a dose of 2900 mg/m3.min) (Barrett & Card, 1947). In
another case, the lethal dose was 2600 mg/m3.min (Beton et al.,
1966), i.e. a 5-h exposure to 8.6 mg/m3. Friberg et al. (1974)
estimated that an 8-h exposure to 5 mg cadmium/m3 may well be
lethal.
8.1.2 Ingestion
During the period 1940-50, cases of acute food poisoning
occurred mainly due to the substitution of cadmium for scarce
chromium in the plating of many cooking utensils and containers.
Food contamination arose when acid foods and drinks were prepared
and stored in contact with cadmium-plated surfaces. Rapid onset with
severe nausea, vomiting, and abdominal pain were characteristic
symptoms (US Public Health Service, 1942; Cole & Baer, l944; Lufkin
& Hodges, 1944). Effects also occurred following the consumption of
drinks with a cadmium concentration of approximately 16 mg/litre
from an automatic vending machine in which drinking-water was cooled
in a tank constructed with cadmium-containing solder (Nordberg et
al., 1973). Recovery from acute poisoning appears to be rapid and
complete. The amount of cadmium absorbed is probably very limited
due to vomiting and the consequential short presence of cadmium in
the gastrointestinal tract. However, no follow-up studies of people
who have experienced acute cadmium poisoning have been reported.
8.2 Chronic Effects
Lower cadmium concentrations with longer periods of exposure
than those described above will cause chronic cadmium poisoning.
Fully developed poisoning among industrial workers shows two main
effects: renal dysfunction and emphysema (Friberg, 1948a,b, 1950).
The kidney is most frequently the critical organ, but under certain
conditions (short-term peak exposures) it may be the lung (Bonnell,
1955). For people in the general environment, exposure is usually by
the oral route and the kidney is the critical organ.
8.2.1 Renal effects and low molecular weight proteinuria
8.2.1.1 In industry
Renal dysfunction is one of the characteristic signs of cadmium
poisoning, and many cadmium workers have developed proteinuria,
renal glucosuria, and aminoaciduria. In working environments with
high cadmium exposure levels, workers have also developed
hypercalciuria, phosphaturia, and polyuria (Friberg, 1950; Clarkson
& Kench, 1956; Kazantzis et al., 1963; Tsuchiya, 1967; Lauwerys et
al., 1974a, 1979b), and some have suffered from renal colic due to
recurrent stone formation (Friberg, 1950; Ahlmark et al., 1961;
Adams et al., 1969; Scott et al., 1976; Kanzantzis, 1979). The
polyuria is due to loss of urinary concentrating ability (Kazantzis,
1979), and, in addition, the kidneys of cadmium-poisoned workers
lose their ability to handle an acid load after a standard
NH4Cl-loading test. These are signs of distal tubular damage, and
in a few severe cases, the renal damage progresses to a reduction in
glomerular filtrations (see section 8.2.1.5).
Renal function, as measured by inulin or creatinine clearance
and urine concentrating capacity, was depressed in several poisoning
cases (Friberg, 1950; Bonnell, 1955; Bernard et al., 1979). Thus, in
the more advanced cases, there is a combination of tubular and
glomerular effects. In most of the early cases, only proteinuria,
mild in comparison with the proteinuria in many other renal
disorders, has been reported as a sign of renal dysfunction, and
other signs of kidney dysfunction were not evident (Piscator,
1966a,b).
Since Friberg first observed the urinary proteins of cadmium
workers (Friberg, 1950), the proteinuria has proved to involve
proteins with a molecular weight of 10 000 to 40 000 and is the
so-called tubular proteinuria (Butler & Flynn, 1958). Table 15
contains data on proteins in urine useful for the diagnosis of
cadmium-induced proteinuria. The increased excretion of low
molecular weight proteins in urine from cadmium-exposed workers has
been found to apply to ß2-microglobulin, lysozyme (muramidase),
ribonuclease, immunoglobin chains, retinol-binding protein, and
alpha1-microglobulin (Piscator, 1966a; Peterson et al., 1969;
Peterson & Berggård, 1971; Lauwerys et al., 1974a; Bernard et al.,
1976, 1982b). In groups of exposed and unexposed workers, the
urinary ß2-microglobulin concentrations follow log-normal
distributions, and an operational definition for what is an
"increased" level should be established for each population studied
(Kjellström et al., 1977a).
Proteinuria is known to be an early sign of cadmium poisoning,
but the degree of proteinuria varies with time. In a group of 40
workers with heavy exposure to cadmium, it was found that
proteinuria was persistent and even increased several years after
cessation of exposure, as evaluated by qualitative methods (Friberg
& Nystrom, 1952; Piscator, 1966a). Tsuchiya (1976) examined five
cadmium-exposed workers who showed proteinuria. Ten years after
cessation of exposure, three of them no longer revealed proteinuria,
but two of these showed a high urinary ß2-microglobulin level, as
did the two workers with persistent total proteinuria. Four workers
in a British pigment factory still had grossly elevated
ß2-microglobulin levels despite removal from exposure many years
earlier (Stewart & Hughes, 1980).
Table 15. Excretion of urinary proteins in healthy people and in cases of glomerular and tubular disorders
Protein type Normal plasma Normal filtered Urinary excretion (mg/24 h) Reference
concentration amount in primary Healthy Glomerular Tubular
(mg/ml) urine (mg/24 h) people disorders disorders
Total protein 43-127 310-54 100 129-1570 Peterson et al. (1969)
Albumin 50 500 3.9-24 88-48 800 13.8-578 Mogensen & Solling (1977)
Retinol-binding 0.11 20-150 Peterson & Beggard (1971)
protein
0.04 3 45 Kanai et al. (1971)
ß2-microglobulin 0.002 300 0.06-0.21 0.06-4.7 1.1-105 Peterson (1971)
0.073 Evrin et al. (1971)
Lysozyme 0-2a Prockop & Davidson (1964)
0.07-1.1a 47-130a Harrison et al. (1968)
Ribonuclease 0.24-1.5a 1.9-10a Harrison et al. (1968)
a Values reported as mg/litre
According to recent observations using quantitative proteinuria
methods (Roels et al., 1982), total proteinuria in 19 workers had
not changed 4 years after exposure ceased. In those 11 workers for
whom urinary ß2-microglobulin was measured before and after
cessation of exposure, an increase was invariably seen. Eight of the
workers had abnormal ß2-microglobulin levels before exposure
ceased, whereas three had normal levels before and developed
abnormal levels after cessation. It can be concluded that
cadmium-induced tubular proteinuria is irreversible in most workers,
at least for several years.
A marked increase in urine cadmium level may reflect
cadmium-induced nephropathy if exposure has been chronic and
correlates with low molecular weight proteinuria (section 6.5.1.2).
Lauwerys et al. (1979b) proposed a biological threshold of 10 µg
cadmium/µg urinary creatinine for males occupationally exposed to
cadmium. Smith et al. (1980) found that workers with low exposure to
airborne cadmium had an average urinary cadmium level of
13.1 µg/litre, whereas workers with long histories of work in areas
with substantial airborne cadmium had an average level of
45.7 µg/litre. The high-exposure group showed a significant
reduction in urinary clearance and increased ß2-microglobulin
excretion. Buchet et al. (1980) found increased excretion of both
low and high molecular weight proteins and tubular enzymes in
workers excreting more than 10 µg cadmium/g creatinine or with a
blood cadmium level above 10 µg cadmium per litre.
Retinol binding protein (RBP) has been shown to correlate well
with ß2-microglobulin in urine with a pH value greater than 5.5.
and is equally sensitive for detection of tubular proteinuria
(Bernard et al., 1982a,b). This protein occurs in serum complexed to
prealbumin and retinol, but, after retinol is delivered to target
cells, RBP rapidly dissociates from prealbumin, is filtered through
the glomerulus, and is reabsorbed by the tubule (Peterson, 1971).
Renal tubular brush border enzymes may also be excreted in
chronic cadmium poisoning. In patients with Itai-itai disease,
urinary trehalase activity correlates inversely with tubular
resorption of phosphorus (Nakano et al., 1987) and there is a
statistical correlation between urinary trehalase and other urinary
indicators of renal tubular dysfunction, such as glucose,
ß2-microglobulin, cadmium, and alpha-amino nitrogen, in
inhabitants of chronic cadmium-polluted areas in Japan (Nogawa et
al., 1980).
Other renal effects in cadmium workers include glucosuria,
aminoaciduria, impaired concentrating abilities, and hypercalciuria
(Kazantzis et al., 1963; Scott et al., 1976), which may cause
disturbances in bone and calcium metabolism (section 8.2.2). The
hypercalciuria leads to renal stone formation in some workers
(section 8.2.2.1). An increased excretion of amino acids,
particularly of serine and threonine, has been shown in industrial
workers (Clarkson & Kench, 1956), but the amino acid excretion
pattern was not consistent. In a cadmium worker with osteomalacia
(Kazantzis, 1979), there was an increase excretion of
hydroxyproline, which could be an effect of changes in collagen
metabolism related to the bone disorder (section 7.2.4).
The renal effects of cadmium that lead to proteinuria may
progress and, in some cases, with high exposures, lead to an
increase in blood creatinine. This has contributed to a
higher-than-expected mortality rate among highly exposed workers
(Kjellström et al., l979). In a Swedish battery factory, there were
four deaths from nephritis or nephrosis among 185 workers, all of
whom had been exposed to cadmium for more than 15 years. The
expected number of deaths due to these causes in this group of
workers was 0.4 (P = 0.05) (Andersson et al., l984). In a study of
about 6995 cadmium-exposed British workers (Armstrong & Kazantzis,
1983), there were 10 deaths due to nephritis or nephrosis, whereas
15.3 deaths were expected. In the subgroup with the highest exposure
(211 workers), one death occurred (0.3 expected). A 5-year follow-up
of this study (Kazantzis et al., 1988) confirmed no excess mortality
from nephritis and nephrosis (ICD 580-584), the number of observed
deaths now having increased to 16 (18.9 expected). Armstrong &
Kazantzis (1985) conducted a case control study of this cohort in
which a more detailed assessment of the past exposure of workers was
obtained. There was a marginally increased, but not statistically
significant, risk from nephritis and nephrosis (ICD 580-584) in
workers with "ever high" or "ever medium" exposure to cadmium.
Existing studies of mortality from nephritis/nephrosis have
been based upon epidemiological studies of renal failure given as
the underlying cause of death on death certificates. With the advent
of kidney dialysis and transplantation, patients with kidney failure
frequently survive and die of other causes. If kidney failure is
indicated at all on their death certificates, it is frequently given
as a contributing rather than underlying cause of death.
No studies on the contribution of cadmium-induced renal effects
to morbidity, absence from work, etc., have been published, although
one study (Vorobjeva & Eremeeva, 1980) reported increased
cardiovascular disease and related increased work absence among
cadmium workers (section 8.2.4).
8.2.1.2 In the general environment
In Japanese cadmium-polluted areas, signs of renal dysfunction
very similar to those in cadmium-exposed industrial workers have
been found. Proteinuria and glucosuria were found to be common
(30-80%) among the exposed people in one area (Ishizaki, 1969) and
less common among people living in control areas and areas bordering
a polluted area. In the exposed groups, a positive correlation
between age and the prevalence of signs was also seen (section
8.3.2). Due to the cumulative nature of cadmium, the total dose is
directly correlated to age.
With the large number of elderly people and women included in
the groups exposed in the general environment, factors other than
cadmium that can affect renal function may make direct comparisons
with industrial workers difficult. Nevertheless, the tubular
proteinuria (Shiroishi et al., 1977), aminoaciduria, and other signs
of renal tubular damage (Saito et al., 1977; Nogawa et al. 1984)
were very similar to the findings for industrial workers. In
addition, as in the case of exposed workers, elevated urinary
excretion of metallothionein occurs as a result of environmental
cadmium exposure (Tohyama et al., 1981b).
Among cadmium-exposed people in the general environment, the
mean urine ß2-microglobulin excretion was highest (Shiroishi et
al., 1977) in patients with Itai-itai disease (section 8.2.2.2).
Many of the Itai-itai patients also have signs of decreased
glomerular filtration, as indicated by decreased urea clearance
(Nakagawa, 1960) and increased serum creatinine (Nogawa et al.,
1979).
8.2.1.3 Methods for detection of tubular proteinuria
Determination of total protein and electrophoretic analysis of
concentrated urinary protein were originally the common methods for
the detection and diagnosis of tubular proteinuria. Quantitative
immunological methods (detection limit, 0.002-0.003 mg/litre) for
the measurement of ß2-microglobulin (Evrin et al., 1971) and RBP
(Bernard et al., 1982b) in urine ("normal" level about 0.1 mg/litre)
are available, and these methods facilitate the detection of tubular
dysfunction. An electrophoretic method with reasonable sensitivity
(0.8 mg/litre) for measuring specific proteins in the urine utilizes
staining of proteins in sodium dodecyl sulfate acrylamide gel
electrophoresis (Nomiyama et al., 1982b). More recently, radio-,
latex-, and enzyme-linked immunoassays have been developed (Evrin et
al., 1971; Bernard et al., 1982a; Carlier et al., 1981). However,
the disadvantage with ß2-microglobulin as a marker for renal
tubular dysfunction is that this protein is unstable if urinary pH
is less than 5.5.
RBP has an advantage over ß2-microglobulin, particularly for
screening purposes, in that serum levels and, hence, excretion are
not affected as readily by concomitant immunological disease and are
more stable at an acidic pH (Bernard et al., 1982b). An enzyme-
linked immunosorbent assay (ELISA) for urinary RBP has also been
described (Topping et al., 1986).
Common tests for qualitative determination of proteinuria, such
as paper tests (dip sticks), the nitric acid test, and the boiling
test, should not be used for screening cadmium-induced proteinuria,
since positive readings will be obtained only at fairly high urine
protein concentrations (Piscator, 1962). Trichloroacetic acid (TCA)
or sulfosalicylic acid (SA) can be used for qualitative tests, but a
negative result does not exclude a moderate increase in low
molecular weight proteinuria.
8.2.1.4 Significance of cadmium-induced proteinuria
More than 70% of proteins with a molecular weight less than
15 000 but less than 5% of those with a molecular weight greater
than 40 000 pass through the glomerular membrane (Squire et al.,
1962). The glomerular filtrate contains relatively large amounts of
plasma proteins, which are normally almost completely reabsorbed in
the proximal tubules and only small amounts are found in the urine.
The increased excretion of tubular proteins in cadmium nephropathy
is thought to be due mainly to a decreased tubular reabsorption
capacity. This provides early evidence of renal tubular dysfunction.
The concentration of albumin in normal serum is about 25 000
times higher than the concentration of ß2-microglobulin (Table
15). In spite of the fact that very little of the albumin but about
80% of the ß2-microglobulin is filtered through the glomeruli
(Maack et al., 1979), the filtered amount of albumin is still higher
than the amount of ß2-microglobulin (Table 15).
Only a small fraction of the albumin or the other proteins is
excreted in normal urine due to the normally efficient (more than
99%) tubular reabsorption of all proteins (Table 15). The urinary
albumin excretion is about 100 times greater than the
ß2-microglo-bulin excretion (Table 15).
ß2-Microglobulin is a subunit of a major immunoglobulin
complex with a molecular weight of 12 000 and normally occurs in
serum at a concentration of approximately 2.0 mg/litre. In the case
of a decreased glomerular filtration rate, the serum concentration
of ß2-microglobulin will also increase. In certain conditions, for
example, where excessive production occurs as in some cancers and
autoimmune disorders, serum levels increase, the tubular capacity
for reabsorption may be exceeded, and the concentration in the urine
will rise. In tubular dysfunction, the capacity for absorption is
impaired and, again, this is reflected by an increased excretion in
the urine. Such renal disorders include the congenital and acquired
Fanconi syndrome, diabetic nephropathy, certain cases of reflux
nephropathy, and advanced glomerular disease (Squire et al., 1962).
A raised urinary excretion of ß2-microglobulin or other low
molecular weight protein is not, therefore, specific to renal
dysfunction induced by cadmium, and a differential diagnosis should
be considered in all cases where this occurs.
The "normal" average urinary excretion of ß2-microglobulin
measured in several populations was in the range 0.05-0.1 mg/24 h
(or mg/litre or mg/g creatine) (Kjellström & Piscator, 1977). Below
age 65, there was very little or no change with age in the urinary
ß2-microglobulin (Kjellström & Piscator, 1977), a fact confirmed
by later studies (Tsuchiya et al., 1979; Kowal & Kraemer, 1982). In
all of the studies, some high individual values were found in the
age group above 65 years, and the studies of Tsuchiya et al. (1979)
and Kowal & Kraemer (1982) reported age-regression coefficients
indicating an increase with age. However, there was wide variation
with age and the average urinary ß2-microglobulin levels in the
oldest age groups (above 65 years) were only 10-20% higher than in
the other age groups (Kowal & Kraemer, 1982). The prevalence of
increased low molecular weight proteinuria (above 0.5 mg/litre) was
less than 5% in these studies, but in a control group of people
above age 80 from a Japanese cadmium-polluted area (section 8.3.2.2)
the prevalence was about 15%.
8.2.1.5 Glomerular effects
Although renal tubular dysfunction with its accompanying low
molecular weight proteinuria is thought to be the most prominent
renal effect of cadmium, the ß2-microglobulinuria is sometimes
accompanied by the excretion of high molecular weight proteins such
as albumin (molecular weight, 69 000). This albuminuria may
occasionally occur as a result of cadmium exposure without any
concomitant increase in the urinary excretion of low molecular
weight proteins (Bernard et al., 1976, 1979); this indicates that
cadmium, in some cases, may produce a change in the glomerular
permeability to larger proteins.
Cadmium may also affect the glomerular filtration rate (GFR).
Friberg (1950) reported decreased inulin clearance in
cadmium-exposed battery workers. Elinder et al. (1985a) measured GFR
by chromium-EDTA in 17 workers previously exposed to cadmium fumes.
They found a significant negative correlation between decreasing GFR
and tubular reabsorption loss, and reported that GFR decreased with
increasing cumulative exposure to cadmium fumes. The urinary
clearance of ß2-microglobulin increased with decreasing GFR.
Several other occupational studies have reported increased
serum concentrations of creatinine and/or ß2-microglobulin,
indicating reduced GFR, in cadmium-exposed workers (Thun et al.,
1989; Roels et al., 1989).
Thun et al. (1989) found a small increase in mean serum
creatinine in a group of 45 cadmium-exposed workmen. Serum
creatinine also increased with cadmium dose, suggesting decreased
glomerular function. Cadmium dose remained the important predictor
of serum creatinine even after controlling for age, blood pressure,
body size, and other extraneous factors.
Roels et al. (1989) measured the serum creatinine and serum
ß2-microglobulin levels of 23 workers, removed from cadmium
exposure, on several occasions over a period of six years. The
average yearly decrease in GFR was estimated to be 6 ml/min per
1.73 m2, which is considerably more than the normal value
(< 1 ml/min per 1.73 m2) and significantly more than that of a
control group examined at the same time.
There is also evidence of glomerular effects in people exposed
to cadmium in the environment. Nogawa et al. (1980) suggest that a
reduction in creatinine clearance may be detected at the early stage
of cadmium poisoning in a polluted area. In addition, Nogawa et al.
(1984) reported a significant correlation between decreased tubular
reabsorption of phosphate and decreased GFR in farmers living in a
cadmium-polluted area.
The mechanism for the glomerular effects from cadmium is
uncertain. It has been suggested that cadmium-induced tubular damage
leads to a certain degree of interstitial nephritis which in turn
results in a decreased GFR (Elinder et al., 1985a). It has also been
proposed that cadmium exerts a direct toxic effect on the glomerulus
(Roels et al., 1989).
8.2.1.6 Relationship between renal cadmium levels and the
occurrence of effects
The number of reports of renal pathology in autopsy cases and
renal biopsies that contain data on kidney cortex concentrations of
cadmium is small. Thus, it is difficult to establish a dose-response
relationship between cadmium content and pathology or dysfunction.
Nomiyama (1977) summarized data from 26 cadmium-exposed workers
and 16 cadmium-exposed people from the general environment. The
criteria for choosing the subjects were that they possessed high
renal and/or high liver concentrations of cadmium, morphological
studies on the kidney had been performed, and data were available on
the occurrence of proteinuria while the person was alive. Among the
42 cases reviewed, those exhibiting slight or no proteinuria and no
morphological alterations had higher concentrations of cadmium in
the renal cortex than non-exposed people. Most cases with
morphological changes plus proteinuria had lower renal cadmium
concentrations that those without proteinuria and/or morphological
changes. In more recent studies, Ellis et al. (1985) found that in
cases of renal dysfunction the mean liver and kidney cadmium values
for retired workers were lower than those for active workers. These
findings are similar to those from animal studies (section 6.5.1.2),
where kidney concentrations levelled off or even declined in the
presence of kidney damage.
The use of in vivo neutron activation analysis has
facilitated the study of the relationship between renal cadmium
levels and occurrence of effects (Ellis et al., 1981a; Roels et al.,
1981b). However, the data must be assessed with caution as the
accuracy of this method has not yet been fully determined. For
instance, the exact location of the kidney needs to be known.
Erroneously low renal cadmium levels were reported by Roels et al.
(1981b) due to an error in adjusting for the distance between skin
and kidney (Roels et al., 1983a). Data from Ellis et al. (1981a) and
Roels et al. (1983a,b) have shown that few cases with increased
urinary ß2-microglobulin concentrations are seen when the level of
renal cortex cadmium is less than 150 mg/kg tissue and that of liver
cadmium is less than 40 mg/kg. There is a pattern of liver and
kidney cadmium levels increasing simultaneously until the average
renal cortex cadmium concentration is about 300 mg/kg and the
average liver level is about 60 mg/kg. At higher liver levels, the
renal cortex level is disproportionately low in most cases, and, in
addition, many of these workers have increased urinary
ß2-microglobulin.
Skerfving et al. (1987) measured kidney cortex cadmium levels
by X-ray fluorescence in a group of 20 workers from a factory
producing alkaline batteries and found an average value of 147 mg/kg
(range 53-317). When compared to a control group, these workers had
higher average urine levels of cadmium (5.4 vs 0.8 nmol/mmol
creatinine) and ß2-microglobulin (14.6 vs 6.6 µg/mmol creatinine).
Six workers had ß2-microglobulin levels exceeding 22 µg/mmol
creatinine. Due to selection procedures the results are, however,
not predictive for cadmium-exposed workers in general. Nevertheless,
it is clear that there are no significant correlations between
levels of cadmium or ß2-microglobulin in urine and cadmium levels
in the kidney. These results suggest that there is a relationship
between renal cadmium and occurrences of effects on a group basis
but renal cadmium levels per se are not always predictive of
pathological effects on an individual level.
8.2.1.7 Reversibility of renal effects
The potential for reversibility of renal effects has been
studied in populations of workers with occupationally induced
cadmium nephropathy as well as in residents of cadmium-polluted
areas.
a) Occupational exposures
In a group of 40 workers heavily exposed to cadmium, it was
found, using qualitative methods, that proteinuria was persistent
and sometimes even increased several years after cessation of
exposure (Friberg & Nystrom, 1952; Piscator, 1966a).
Tsuchiya (1976) studied a group of 13 workers who had been
exposed to cadmium fumes (133 µg/m3) and who had proteinuria
(determined by the trichloroacetic acid method) and abnormal
electrophoretic urine patterns (ß2-microglobulin levels above
40 000 µg/litre). A 10-year follow-up study of five of these
patients was carried out after improvements had been made in their
working environment (cadmium fumes, 20 µg/m3). The proteinuria was
reversed (measured using a single radial immuno-diffusion method) in
three of the five patients: ß2-microglobulin values were 3500,
2600 µg/litre, and not detectable (limit of detection
2000 µg/litre), and retinol-binding proteins (RBP) were not
detectable (limit of detection 500 µg/litre). In addition, there
were improvements in the remaining two patients (ß2-microglobulin,
9700 and 5500 µg/litre; RBP, 34 000 and 120 000 µg/litre). Tsuchiya
(1976) suggested that the difference in the period of exposure to
cadmium was the reason for this difference in the degree of recovery
from the effects of cadmium.
Stewart & Hughes (1981) reported on similar cases from a
British pigment factory. Despite the fact that exposure ceased many
years earlier, grossly elevated ß2-microglobulin levels were still
detected.
Using quantitative methods to detect proteinuria, Roels et al.
(1982) found that total proteinuria in 19 workers was unchanged 4
years after exposure had ceased. In those 11 workers for whom
urinary ß2-microglobulin was measured before and after cessation
of exposure, levels were invariably increased. Eight of the workers
had abnormal levels of ß2-microglobulin even before cessation of
exposure, whereas three had normal levels before cessation and
developed abnormal levels afterwards.
Roels et al. (1989) examined 23 workers once a year for 5 years
after removal from exposure to cadmium. These workers had been
exposed to cadmium for periods of 6 to 41.7 years (mean 25 years),
and their first follow-up examination took place when they had been
removed from exposure for an average of 6 years. Their mean age at
that time was 58.6 years (range 45.5-68.1 years). Cadmiumuria in
these workers had been assessed three years previously by measuring
the cadmium levels in liver and kidney using neutron activation
analysis. The cadmium concentrations (mg/kg wet weight) in the liver
and kidney cortex ranged from 24 to 158 (mean 61) and from 133 to
355 (mean 231), respectively. Although cadmium concentrations in the
blood and urine decreased significantly over the five-year period,
urine concentrations of albumin, ß2-microglobulin, and RBP did not
change significantly.
Harada (1987) conducted studies on the health status of seven
workers exposed prior to 1972 to high cadmium levels in a cadmium
sulfide dye manufacturing factory. These workers were examine for 15
years, improvements having been made to working conditions in 1974
which led to markedly decreased cadmium exposures. The cadmium
content of the blood and urine declined after the improvements in
working conditions but increased again when production rose. The
working condition improvements resulted in a marked reduction in
urine ß2-microglobulin level in five workers (e.g., from 1272 to
520 µg/g creatinine in 1 year in one worker and from 2090 to
503 µg/g creatinine in 6 months in another), but there was elevated
urinary ß2-microglobulin excretion when production increased. One
worker had fairly constant near-normal urinary levels of
ß2-microglobulins (55 to 183 µg/g creatinine) regardless of
workplace improvements or production levels. Initially, four workers
had low GFR values, but none of the seven workers showed any
decrease in glomerular filtration during the 15-year follow-up
period. TRP rates decreased in three workers but remained relatively
unchanged in the other four. These changes in GFR and TRP seemed to
be independent of cadmium exposure levels.
Elinder et al. (1985b) found that urine cadmium excretion
decreased in 14 out of 19 workers re-examined at least once five
years or more after exposure to cadmium, but renal tubular function,
as measured by urinary ß2-microglobulin excretion, had
deteriorated or not improved in nearly all of the workers. Thun et
al. (1989) concluded that "time since last exposure to cadmium" was
not an important determinant of renal outcome whether considered on
its own or together with the cadmium dose. In their study of 45
workers at a plant that recovered cadmium from industrial waste, 9
out of 15 workers with the highest ß2-microglobulin excretion had
not been exposed to cadmium for at least five years and one for 45
years. This study suggested that if cadmium nephropathy is
reversible, the recovery is so slow as to be indiscernible after
decades of non-exposure.
Ellis et al. (1985) showed that the liver cadmium levels in
workers no longer exposed to cadmium gradually declines. Persistence
of renal tubular dysfunction after cessation of exposure may reflect
the level of body burden and the transfer of cadmium from liver to
kidney.
b) Residents of cadmium-polluted areas
Reversibility of renal tubular dysfunction has been
investigated in residents of cadmium-polluted areas in Japan. Kasuya
et al. (1986) carried out a comparative study of urine
ß2-microglobulin determinations made in 1975 and 1985 for 93
people with Itai-itai disease and their family members. Urine
ß2-microglobulin levels improved in the group with
ß2-microglobulin levels of 1000 µg/g creatinine or less but
worsened in the group with ß2-microglobulin levels of 3000 µg/g
creatinine or more. Most of the people who recovered were aged 39
years or less and had been resident for 30 years or less. It was
considered that mild renal dysfunction among young individuals was
reversible.
Saito (1987) measured the urine ß2-microglobulin levels of
residents of cadmium-polluted areas for 3 years after improvements
had been made in the level of soil contamination and compared the
results with determinations obtained 7 years previously. During this
3-year period, the urine ß2-microglobulin levels tended to remain
unchanged in people with a concentration of around 1000 µg/litre.
The reversibility of ß2-microglobulinuria, glucosuria, and
aminoaciduria was examined in 74 inhabitants (32 males and 42
females) over 50 years of age who lived in a cadmium-polluted area.
Examinations were conducted just after the cessation of cadmium
exposure and 5 years later. The geometric mean concentrations of
ß2-microglobulinuria, glucosuria, and aminoaciduria indicated a
significant increase in excretion during the 5-year period. In cases
where the level of ß2-microglobulinuria exceeded 1000 µg/g
creatinine at the time cadmium exposure ceased, evidence was found
indicating significant increases in proteinuria after 5 years,
whereas in cases where the excretion of ß2-micro-globulin had been
less than 1000 µg/g creatinine no significant changes were observed
(Kido et al. 1988).
8.2.2 Disorders of calcium metabolism and bone effects
8.2.2.1 In industry
Friberg (1950) observed 7 cases of renal stones among 43
cadmium workers and drew attention to the possibility of renal
stones being associated with exposure to cadmium. Ahlmark et al.
(1961) found that 44% of a group of 39 cadmium workers exposed to
cadmium oxide dust for more than 15 years had a history of renal
stone formation. Nine stones from six workers were analysed, and in
four workers the stones were composed of basic calcium phosphate
(Axelsson, 1963). There was an increase in the mean calcium
excretion rate in the cadmium-exposed group as compared to a control
group. Kidney stones were also found in 12 out of 43 British workers
at an accumulator factory (Adams et al., 1969). It is noteworthy
that in both of these studies (Axelsson, 1963 and Adams et al.,
l969) there was a higher prevalence of renal stones in workers
without proteinuria than in those with proteinuria. The men with
proteinuria had, as a group, increased urinary excretion of calcium
and phosphate, whereas in the group without proteinuria there were a
few cases with hypercalciuria. Hypercalciuria (81%) and renal stones
(19%) were also reported among 27 coppersmiths exposed to cadmium
(Scott et al., 1976, 1978).
Elinder et al. (1985a) found an increased prevalence of renal
stones among cadmium workers with tubular proteinuria, and Mason et
al. (1988) observed decreased renal reabsorption of calcium among
cadmium alloy workers.
Seven of the 12 workers in a cadmium pigment factory
investigated by Kazantzis et al. (1963) were found to have a urinary
calcium excretion rate greater than 300 mg/day (at least in 1 of 2
specimens). There was no evidence of excessive calcium intake in
these men. Five of these seven workers had been exposed to cadmium
compounds for more than 25 years and also had tubular proteinuria.
The remaining two, who had been exposed for 2 and 12 years,
respectively, had no other abnormality except for a urinary calcium
excretion of 308 and 403 mg/day. Follow-up was possible with six of
the twelve men, including all five with hypercalciuria and
proteinuria (Kazantzis, 1979). Six of the seven who had
hypercalciuria when first examined continued to have a raised
urinary calcium excretion, and one further worker developed
hypercalciuria during the follow-up period. All those with
hypercalciuria also had tubular proteinuria, although this was
marginal in one of the workers. Blood calcium levels remained within
normal limits in all cases (Kazantzis, 1979). The occurrence of
disordered calcium metabolism in all seven men followed-up for a
number of years makes it very likely that a common environmental
factor, such as occupational exposure to cadmium compounds, was
causative.
The data of Kazantzis (1979) agree with the findings of
hypercalciuria among 27 coppersmiths with high cadmium exposure
(Scott et al., 1976, 1978). Scott et al. (1980) reported that in 15
cadmium-exposed men the amount of calcium in the whole body was
lower than that of controls and decreased with duration of an
increased exposure to cadmium. The cadmium-induced hypercalciuria
could be reduced by thiazide treatment (Scott et al., l979).
In a study by Thun et al. (1989) of workers at a plant that
recovered cadmium from industrial waste, 8 of 45 exposed workers had
experienced kidney stones, in contrast to one of 32 unexposed
workers. Increase in the urinary excretion of ß2-microglobulin and
RBP was accompanied by decreased renal tubular reabsorption of
calcium and phosphates.
In contrast to the above findings, a low urinary calcium
excretion was detected in 47 out of 81 workers with exposure to a
variety of cadmium compounds and also cadmium oxide fumes (Tarasenko
& Vorobjeva, 1973; Tarasenko et al., 1975). The 24-h excretion of
calcium in these workers was below 100 mg, compared with 115-210 mg
in a control group of 21 people. Blood calcium values were within
the normal range in all cases.
Radiological examination was performed on 32 workers exposed
for 4-20 years to cadmium compounds (concentrations ranging from 0.1
to 5.5 mg/m3). All of them complained of pains in the bones.
Pseudofractures suggestive of osteomalacia were seen in two workers
exposed for 16 and 19 years, but no histological confirmation of
osteomalacia was obtained. Radiological appearances described as
enostosis were reported in five cases and periosteal proliferation
and consolidation in a further three cases (Tarasenko & Vorobjeva,
1973; Tarasenko et al., l975).
Horstowa et al. (1966) performed radiological examination of
the skeleton in 26 alkaline battery workers with signs of chronic
cadmium intoxication out of 80 workers exposed to 0.13-1.17 mg
cadmium/m3 for 1-12 years. Seven of these workers had proteinuria
detected by sulfosalicyclic acid; pseudofractures were found in 3
workers, sclerotic foci in 13, and osteoporosis in 10. In another
alkaline battery factory, where a number of cases of severe cadmium
poisoning were diagnosed (Friberg, 1950), X-ray examinations
revealed no signs of bone disease.
One of the workers with multiple tubular defects studied by
Kazantzis et al. (1963) developed osteomalacia confirmed by
histological examination 10 years after the initial investigation
(Kazantzis, 1979). He previously displayed hypercalciuria but, at
the time of diagnosis, his urinary calcium excretion was low.
Extensive investigation failed to reveal any of the other generally
accepted causes of osteomalacia such as malabsorption or nutritional
deficiency.
In a study of 43 workers at a battery plant, one worker
developed osteomalacia without evidence of malabsorption or
nutritional deficiency but with multiple renal tubular defects
(Adams et al., 1969). Another case of osteomalacia from the same
factory was subsequently detected in a man who had been a cadmium
battery worker for 40 years (Adams, 1980). Eight years before
retirement he had a partial gastrectomy due to a duodenal ulcer.
Proteinuria was first diagnosed 6 years before retirement, but
otherwise he was in "apparent good health" and "on a balanced diet"
until 8 years after retirement. He was then frail, had pains in his
legs, and a "waddling gait". His serum alkaline phosphatase level
was increased, X-rays showed generalized osteoporosis, and a bone
biopsy showed osteomalacia. After 1 year of treatment with large
doses of vitamin D, he could walk well again.
It also seems likely that the six workers exposed to cadmium
oxide dust described by Nicaud et al. (1942), who had pains in the
back and limbs and showed multiple pseudofractures on radiological
examination, suffered from osteomalacia. More detailed data on these
workers was presented by Valetas (1946), who also pointed out that
"massive doses" of vitamin D were needed to improve the symptoms. It
took several months for improvement to occur and the vitamin D
treatment had to be maintained for several years to keep the workers
in stable health. Valetas (1946) concluded that this bone disease
was caused by occupational exposure to cadmium. Eight workers with
8-30 years of exposure to lead dust and cadmium oxide fume and dust
(Gervais & Delpech, 1963) were also found to have multiple
pseudofractures and pains in the back, thorax, and legs. Very
limited biochemical investigations were carried out, but in four
cases proteinuria was found. The authors suspected that lead
exposure led to the observed effects.
8.2.2.2 In the general environment
Bone disease and abnormalities of calcium metabolism from
exposure to cadmium in the general environment have only been noted
in people in Japan with the clinical syndrome referred to as
Itai-itai disease. The main characteristics of the disease are
osteomalacia1 and osteoporosis2 with a tendency to fractures
accompanied by severe pain and renal tubular dysfunction. The
results of epidemiological and clinical investigations indicate an
association with cadmium exposure, although the Co-operative
Research Committee on Itai-itai Disease (1967) stated that
"malnutrition (low protein, low calcium diets) and multiple
pregnancies may also be involved".
1 Osteomalacia is characterized by inadequate mineralization of
bonematrix, resulting in an increase in the relative amount
of osteoid tissue. It represents the adult counterpart of
childhoodrickets (Robbins et al. 1984).
2 Osteoporosis is defined as an excessive but proportional
reduction in the amounts of both the mineral and matrix phases of
bone unaccompanied by any abnormality in structure of the residual
bone (Robbins et al., 1984).
Itai-itai disease is an endemic bone disease prevalent in the
basin of the Jinzu river, which runs through the central part of
Toyama Prefecture in West-Central Japan (Kono et el., 1956). It is
characterized by osteomalacia in combination with renal tubular
dysfunction in most cases. Patients also have osteoporosis and one
of the most characteristic symptoms is severe bone pain. Hagino &
Yoshioka (1961) reported that high concentrations of cadmium, lead,
and zinc were present in autopsy tissues from people with Itai-itai
disease and in the everyday foods of the endemic area.
Systematic epidemiological investigations, which included
extensive mass health examinations as well as case control studies
on both patients and controls, started in 1962 (Cooperative Research
Committee on Itai-itai Disease, 1967). It was reported that
Itai-itai disease in Toyama Prefecture was restricted to a limited
area (Fuchu area) irrigated by the Jinzu river, the geographical
distribution of the patients being consistent with the levels of
cadmium concentration in the paddy fields, and that the
concentrations of cadmium in urine were higher in patients than in
controls. The total number of patients was estimated in 1955 by the
Toyama Prefecture to be 41 out of a total of 1666 residents (849
women) (Cooperative Research Committee on Itai-itai Disease, 1967).
The major source of cadmium pollution in the area was a mine 50 km
upstream from the endemic area (Japan Public Health Association,
1968).
The age and sex distribution of the patients displayed a very
distinct pattern. Clinically apparent cases were limited to women
over 40 years of age who had given birth to many children (6 on
average) and had lived in the area for more than 30 years. No
detailed data on past patients were available, but it was estimated
that the age of onset of the disease was probably between 35 and 65
years, and that almost 100 deaths had been reported up to the end of
1966. The incidence was presumably very high from 1936 to 1950 and
at its highest in 1946 and 1947, but decreased thereafter even
though the same cadmium exposure levels had been maintained. By
March 1989, 150 cases of Itai-itai disease had been officially
recognized as pollution-related disease. Whereas all cases have been
reported in the Fuchu area, there have been a few suspected cases in
2 out of 12 cadmium-contaminated areas of Japan other than the Fuchu
area (Table 7). Clinical features of five suspected cases from the
Ikuno area matched those of Itai-itai disease and urine cadmium
levels were very high (Nogawa et al., 1975). In one of these cases
an autopsy was performed; the liver cadmium level was very high
(75 mg/kg) but the renal cortex cadmium level was low (53 mg/kg)
(Nogawa et al., 1975).
Takebayashi (1980a,b, 1983a,b, 1984) and Takebayashi et al.
(1985, 1987a,b,c, 1988a,b,c,d) reported pathological findings in
kidney and bone from autopsies of eleven elderly men and women (3
males and 8 females; 72-95 years of age) from Tsushima Island. The
average levels of cadmium in the liver and kidney cortex were
92.4 mg/kg and 44.0 mg/kg, respectively. The authors considered the
histological osteomalacia and renal tubulopathy noted in eight cases
(Takebayashi, 1980, 1983a, 1984, Takebayashi et al., 1985, 1987a,b,
1988c,d) to be similar to Itai-itai disease from Toyama Prefecture.
However the Japan Cadmium Research Committee (1989), supported
by the Japanese Environment Agency, concluded, after these eight
cases had been examined by the expert group, that it was clinically
difficult to diagnose them as osteomalacia.
According to the Japan Cadmium Research Committee (1989),
diagnosed cases of Itai-itai disease were reported only in the Fuchu
area of Japan. It denied the presence of osteomalacia in five cases
in the Ikuno area, and stated that osteomalacia had not been
observed "clinically" in Tsushima Island.
A study by Kido et al. (1989) indicates that exposure to
cadmium could cause osteopenia, particularly in women. Bone density
was measured in 28 women with Itai-itai disease, 92 men and 114
women with cadmium-induced renal dysfunctions, and 44 men and 66
women living in three different non-polluted areas using a
microdensitometer. The values of indices corresponding to both
cortical width and bone mineral content were significantly lower in
Itai-itai disease patients than in cadmium-exposed women with renal
dysfunctions or in non-exposed subjects. The cadmium-exposed women
also showed a decrease in bone density compared with the non-exposed
subjects. A significant decrease in bone density was also observed
in cadmium-exposed men compared with non-exposed subjects, although
the difference was not as clear as it was in women.
Reviews (in English) of Itai-itai disease have been produced by
Tsuchiya (1969), Friberg et al. (1974), Tsuchiya (1978), and Nogawa
(1981).
8.2.2.3 Mechanism of cadmium-induced bone effects
The available data show that cadmium can affect calcium,
phosphorous, and bone metabolism in both industrial workers and
people exposed in the general environment. These effects may be
secondary to the cadmium effects on the kidneys but there have been
few studies of calcium metabolism in people with excess exposure to
cadmium. The increased prevalence of renal stones reported from
certain industries is probably one manifestation of the
cadmium-induced kidney effects. It is not known if factors other
than cadmium play a role.
Nogawa et al. (1987) reported that serum 1,25-dihydroxy-
vitamin D levels were lower in Itai-itai disease patients and
cadmium-exposed subjects with renal damage than in non-exposed
subjects. The reduction in these levels was closely related to serum
concentrations of parathyroid hormone and ß2-micro-globulin and to
the percentage tubular reabsorption of phosphate (% TRP), suggesting
that cadmium-induced bone effects were mainly due to a disturbance
in vitamin D and parathyroid hormone metabolism.
Osteomalacia has been reported in a few heavily exposed
industrial workers and people with Itai-itai disease. The industrial
cases are mainly male, whereas Itai-itai patients are almost
exclusively female. However, the clinical features and biochemical
findings are similar, except that Itai-itai patients may also suffer
from ostoporosis.
A possible mechanism for the development of osteomalacia has
been proposed (Kjellström, 1986). It is known that normal calcium
absorption in the intestines and normal bone mineralization is
dependent upon 1,25-dihydroxycholecalciferol. Vitamin D3 taken
into the body is converted to 25-hydroxy-vitamin D3 in the liver,
and then to 1,25-dihydroxy-vitamin D3 in the mitochondria of renal
proximal tubular cells, this being the biologically active species.
Cadmium accumulates in the proximal tubular cells, depressing
cellular functions, and this may result in reduced conversion of
25-hydroxy-to 1,25-dihydroxy-vitamin D3. This is likely to lead to
decreased calcium absorption and decreased mineralization of bone,
which in turn may result in osteomalacia.
8.2.3 Respiratory system effects
Cadmium workers may develop chronic injury to the respiratory
system, depending on the level and nature of exposure. The
development of such effects is often quite slow, so that they are
apparent only after several years of exposure. The rate of
development and severity appear to be roughly proportional to the
time and level of exposure.
8.2.3.1 Upper respiratory system
Chronic inflammation of the nose, pharynx, and larynx have been
reported by Vorobjeva (1958) and Horstowa et al. (1966). Anosmia is
a frequent symptom in cadmium workers after prolonged exposure. This
has been reported by, for instance, Valetas (1946), Friberg (1950),
Baader (1951), Vorobjeva (1958), Tarasenko & Vorobjeva (1973), and
Apostolov (1979), but was not observed by Tsuchiya (1967) or Suzuki
et al. (1965).
8.2.3.2 Lower respiratory system
Chronic obstructive lung disease of varying degrees of severity
is frequently seen in cadmium workers. Friberg (1950) reported
dyspnoea, impaired lung function with increased residual volume, and
reduced working capacity in a group of 43 cadmium workers. Similar
studies, which included the use of pulmonary function measurements,
by Bonnell (1955), Buxton (1956), Kazantzis et al. (1963), and Adams
et al. (1969) all showed impairment of respiratory function in
groups of workers with prolonged exposure. The symptoms and findings
were more suggestive of emphysema than bronchitis in these cases;
they were commonly diagnosed as emphysema but pathological
confirmation of this was rare (Smith et al., l960).
Tarasenko & Vorobjeva (1973) reported the presence of increased
lung markings in the chest X-rays of 17 out of 72 cadmium workers,
which were interpreted as being due to diffuse interstitial
fibrosis. Similar lung changes were observed in 21 out of 26 workers
studied by Horstowa et al. (1966).
The presence of chronic obstructive respiratory disease in
cigarette smokers exposed to an additional harmful environmental
agent presents difficulties in determining the contribution made by
the latter. Studies on the chronic respiratory effects of cadmium in
the past have not always been standardized for smoking. Lauwerys et
al. (1974a) did take smoking habits into consideration by matching
his cadmium-exposed and control groups for smoking habits. They
reported the presence of impaired lung function in a group of
cadmium workers exposed for over 20 years, but not in those with
shorter exposure. The degree of lung impairment found was small.
The effects on the lung increases the mortality of cadmium
workers with high exposures (Kjellström et al., 1979; Armstrong &
Kazantzis, 1983). In the latter study, the mortality for diseases
coded as bronchitis (ICD 490-491) was related to the intensity of
exposure, the group with the highest exposure having a highly
significant (almost 4-fold) excess risk (observed 13 expected 3.4).
A 5-year follow-up of this study (Kazantzis et al., 1988) confirmed
the earlier finding, the marked excess mortality being related to
both intensity and duration of exposure. The follow-up revealed an
excess mortality from emphysema, but this was seen only in the
low-exposure group.
8.2.4 Hypertension and cardiovascular disease
Despite the abundance of data showing that under certain
exposure conditions cadmium induces hypertension in animals, there
are very few results available from studies of cadmium-exposed
workers. Friberg (1950) examined 43 workers with a mean period of
exposure to cadmium oxide dust of 20 years (air concentration,
3-15 mg/m3) and 15 workers with a mean exposure period of 2 years.
The study included physical and roentgenological examinations of the
heart, electrocardiographic examination at rest and after exercise,
and measurement of blood pressure. No increased prevalence of
cardiac disease or pathological electro-cardiographic changes were
found. The majority of subjects had completely normal blood
pressure, but since Friberg did not examine blood pressure in the
control group, it is not possible to draw definite conclusions.
Chest examination and blood pressure measurements have also
been reported in other studies (Bonnell, 1955; Bonnell et al., 1959;
Kazantzis et al., 1963; Holden, 1969), but in no cases were there
findings of cardiac disease or hypertension due to cadmium exposure.
Hammer et al. (1972) found no relationship between exposure to
cadmium and blood pressure in superphosphate workers.
Vorobjeva & Eremeeva (1980) examined 72 female and 20 male
workers at a battery factory exposed to cadmium oxide dust at
concentrations ranging from 0.04 to 0.5 mg/m3. Blood pressure was
measured and electrocardiograms taken, but there was no control
group. The authors reported increased prevalence of hypertension and
absence from work due to hypertensive and ischaemic heart disease
among the exposed workers compared to what was considered normal.
Furthermore, several types of abnormalities in the electrocardiogram
of the exposed workers were observed: 39% showed tachycardia,
between 11 and 13% were regarded as normal, and 26% had changes in
the "R" spike (compared to the normal 7-9%). Increased QRS period
was observed in 45% of the workers compared to the normal values of
14-16%. The data presented in this report are especially interesting
in view of the evidence in rats (section 7.2.6) that suggests
myocardial effects from cadmium exposure. The results of the study
are, however, presented in a very condensed form and it is therefore
difficult to draw clear-cut conclusions.
In a retrospective study of 311 male workers in an alkaline
battery factory it was found that hypertensive workers had a longer
employment time than an age-matched control group from the same work
environment (Engvall & Perk, 1985). Again it is difficult to draw
conclusions from this study. In a study of cadmium-exposed workers
in the United Kingdom (Kazantzis et al., 1988), mortality from
hypertensive disease (ICD 400-404) over the total study period from
1943 to 1988 was elevated but not significantly (49 deaths occurred
as opposed to 41.3 expected). There was no relationship with
intensity of exposure. However, mortality from cerebrovascular
disease (ICD 430-438) was significantly lower (178 deaths occurred
as opposed to 230.3 expected). These findings do not suggest any
association between cadmium exposure and the development of
hypertension.
In contrast, Thun et al. (1989) found that mean systolic and
diastolic blood pressures were higher in 45 cadmium workers (134 and
80 mmHg, respectively) than in 32 male controls (120 and 73 mmHg
respectively). Blood pressure was measured systematically by a
single examiner on the right arm of subjects who had been seated for
at least 15 min. Systolic but not diastolic blood pressure was
significantly associated with cadmium dose in multivariate analyses.
Schroeder (1965, 1967) observed that people in the general
population dying from hypertensive and/or cardiovascular disease had
somewhat higher cadmium concentrations in liver and kidney tissues
than people dying from other causes. He suggested that cadmium could
be a causative factor for these diseases. Unfortunately, smoking
habits were not accounted for and it is likely that this was a
confounding factor. The same problem exists with a number of
subsequent studies on hypertension and cadmium in tissues, blood,
and urine.
A correlation between average air cadmium levels in cities in
the USA and mortality associated with hypertension and heart disease
has been reported (Carroll, 1966; Hickey et al., 1967). Again,
several confounding factors such as smoking habits, air pollutants
other than cadmium, and other environmental factors make it
difficult to draw conclusions concerning the effects of cadmium. In
a study by Staessen et al. (1984), the confounding variables age,
sex, body weight, and cigarette smoking were considered in a
multiple regression analysis of systolic and diastolic blood
pressure and the urinary excretion of cadmium and
ß2-micro-globulin. Negative correlations between blood pressure
and urinary cadmium or ß2-microglobulin were found in some groups.
As there was a very strong age effect on both blood pressure and
urinary cadmium, the meaning of the negative correlations is not
clear. In any case, these data do not support cadmium exposure as a
cause of hypertension.
Shigematsu et al. (1979) could find no evidence that blood
pressure was higher in polluted areas (1611 people sampled) of Japan
compared with control areas (1826 people). In a comparison of blood
pressure by prefecture (13 570 in the cadmium-polluted areas and
7196 in the control areas), the prevalence of hyper-tension was
found to be high in the polluted area of one of the eight
prefectures investigated. However, in the other seven prefectures,
the prevalence of hypertension tended to be lower in the polluted
areas (Japan Cadmium Research Committee, 1989).
In a study on cadmium-polluted areas in Japan by Nogawa et al.
(1981b), the cerebrovascular disease mortality rate among people who
had had cadmium-induced proteinuria was twice as high as that of
people in the same area without proteinuria. However, the difference
was not statistically significant. The number of men in the cohort
with proteinuria was 81 and the number without proteinuria was 1109.
Another study comparing administrative units containing polluted
areas with those without such areas (Shigematsu et al., 1981, 1982,
1983) found no difference in the cerebrovascular disease mortality
rates.
Data on a total population of 333 000 from both
cadmium-polluted and non-polluted areas were collected
retrospectively for a period of 6-30 years, based on vital
statistics or death certificates (Shigematsu et al., 1982). The
mortalities from all causes, including cardiovascular disease such
as cerebrovascular and hypertensive disease, in the general
population in the cadmium-polluted areas were no higher than, or in
some cases even lower than, those in the non-polluted areas.
A mortality study of Shipham residents and of a nearby control
village was reported by Inskip et al. (1982). The study population
consisted of 501 Shipham residents of whom 278 had died over a
40-year follow-up period. Overall mortality was low in both villages
compared generally with England and Wales. There was a small but
statistically significant excess mortality rate in Shipham from
hypertensive and cerebrovascular disease. The highest ratio of all
was for genito-urinary disease in Shipham men (but, with only eight
observed deaths, the result was only significant at the 10% level).
The Standardized Mortality Ratios (SMR) for nephritis and nephrosis
in both sexes were also slightly elevated, but there were only two
deaths for each sex from this cause. In men, the numbers of
prostatic and lung cancer deaths were approximately equal to the
expected numbers, and in neither case was the SMR in Shipham greatly
different from that in the control village.
8.2.5 Cancer
8.2.5.1 In industry
A number of epidemiological studies have been published. In
order to facilitate the interpretation of published data on the
relationship between cadmium exposure and cancer, the studies have
been grouped according to the types of industrial plants in which
they have been conducted. In some cases, more than one study has
been conducted at the same plant.
a) Nickel-cadmium battery plants
In an early study, Potts (1965) found that three out of eight
deaths in a small cohort of nickel-cadmium battery workers in the
United Kingdom with at least 10 years of exposure to cadmium oxide
dust were from carcinoma of the prostate. This study was extended by
Kipling & Waterhouse (1967) to include 248 men with at least one
year of exposure to cadmium oxide dust. Four deaths from carcinoma
of the prostate, including the three cases previously reported by
Potts (1965), were observed as opposed to an expected number of
0.58.
Sorahan & Waterhouse (1983) carried out a further investigation
of the same plant using a cohort of 3025 employees who started work
between 1923 and 1975 and had a minimum employment period of one
month. The method of regression models in life tables was used to
compare duration of exposed employment in those dying from relevant
causes with that of matched survivors in the same year of follow-up.
No new evidence of an association between occupational exposure to
cadmium and cancer of the prostate was found. However, there was an
excess mortality from cancer of the respiratory system significant
at the 5% level (89 cases, SMR = 127). As in other studies, data on
smoking habits were not available and confounding factors were
present in the form of exposure to nickel hydroxide and welding
fumes so that no firm conclusions about the pulmonary
carcinogenicity of cadmium could be drawn from this study.
Sorahan & Waterhouse (1983) reported on the incidence of
prostatic cancer in a subgroup of 458 workers employed for at least
1 year in a job involving high exposure to cadmium oxide dust. Eight
cancers were observed compared to two expected (SMR 400, P < 0.01).
However, exclusion of the four cases previously reported by Kipling
& Waterhouse leaves a non-significant excess incidence (P = 0.21),
from which the investigators concluded that if cadmium oxide is
potentially carcinogenic current risks are likely to be small.
In the most recent update of the nickel-cadmium battery plant
workers (Sorahan, 1987), the earlier findings were confirmed and
there was some evidence of an association between risk of death from
lung cancer and duration of employment in jobs with high or moderate
exposure among workers first employed in the period 1923-1946.
However, among workers first employed from 1947 to 1975 (the group
with the higher SMR for lung cancer), there was no evidence of such
an association. The authors concluded that the findings do not
suggest these nickel-cadmium battery workers had experienced an
elevated lung cancer risk as a consequence of exposure to cadmium
oxide dust.
In Sweden, Kjellström (1979) investigated the incidence of
cancer among 269 male nickel-cadmium battery workers. All workers
had been heavily exposed (on average about 1 mg cadmium/m3) for
five years or more to cadmium dust or fume, and were alive in 1959.
Fifteen workers were found to have cancer between 1959 and 1975. It
was calculated from national incidence rates that 16.4 new cases
would have occurred; only 2 were prostatic cancers while 1.2 were
expected. In a re-examination of the same cohort, there were 8
deaths from lung cancer with a non-significantly raised SMR of 133.
The SMRs increased progressively with increasing latent periods
without reaching statistical significance (Elinder et al., 1985c).
b) Copper-cadmium alloy plants
Copper-cadmium alloy workers in the United Kingdom who had
heavy past exposure to cadmium oxide fume on two sites, one urban
the other rural, were studied by Holden (1980a). There was an
increased lung cancer mortality at the urban site (8 observed versus
4.5 expected) and a significant deficit at the rural site (2
observed; 7.8 expected). Vicinity workers in the urban plant, where
the mean cadmium concentration averaged no more than 60 µg/m3),
also experienced a significantly increased lung and prostatic cancer
mortality (36 observed; 26.1 expected).
A case control study was performed (Kazantzis et al., 1989) in
the same copper-cadmium alloy plants where workers had experienced
heavy past exposure to cadmium oxide fume and dust, which had given
rise to a number of deaths coded as chronic cadmium poisoning.
Before and during the period 1939-1945, cadmium oxide fume levels
had been estimated to be up to 4 mg/m3. Cases and controls were
selected from the cohort previously studied by Holden (1980a).
Personal interviews conducted with a small number of long-term
employees revealed that arsenical copper had been additionally
produced by adding bags of arsenic trioxide to the molten copper and
stirring manually; this resulted in the evolution of dense white
clouds of arsenic fume. The case control study showed no evidence of
an increased risk of lung cancer associated with past cadmium
exposure but an approximately two-fold excess risk associated with
arsenic exposure.
Kjellström (1979) also investigated a cadmium-copper alloy
plant in Sweden where workers had been exposed to cadmium oxide
fumes and included 94 workers employed in 1940 or who started work
after that year. Four cases of prostatic cancer occurred as opposed
to 2.7 expected.
c) Cadmium recovery plant in the USA
An increase in prostatic cancer incidence was also found by
Lemen et al. (1976) in a study of 292 male smelter workers heavily
exposed to cadmium oxide dust or fumes. Air cadmium concentrations
in 1973 were up to 24 mg/m3 but generally below 1 mg/m3. There
were four deaths from cancer of the prostate (1.15 expected). There
were also 12 deaths from lung cancer (5.1 expected); the difference
was statistically significant.
Thun et al. (1985) expanded the Lemen et al. (1976) cohort to
include 602 workers who had been employed at this cadmium production
plant between 1940 and 1969 for at least 6 months. Exposure was to
cadmium in baghouse dust, a by-product of zinc smelting which was
processed to produce cadmium metal and cadmium oxide. The plant
functioned as an arsenic smelter up to the end of 1925, and small
quantities of lead, arsenic, thallium and indium were subsequently
produced at intervals. The vital status of the workers was
determined in 1978. A dose-response relationship was observed
between lung cancer mortality and cumulative exposure and was
statistically significant for workers whose exposure exceeded
2920 mg/m3.days. The SMR for this group was 280. The lung tumours
were, as far as can be determined, mostly of bronchogenic origin.
The authors accounted for smoking habits by obtaining questionnaires
from survivors or next-of-kin in 50% of the cohort members and for
arsenic exposure by measuring arsenic in certain parts of the plant.
d) Cadmium processing plants in the United Kingdom
Kazantzis & Armstrong (1982) and Armstrong & Kazantzis (1983)
investigated a large cohort of workers in England at 17 plants with
processes using cadmium. The cohort comprised 6995 cadmium-exposed
male workers born before 1940, first exposed before 1970, and not
included in any previous mortality study. Jobs were assessed for
each relevant year involving high, medium or low exposure to cadmium
on the basis of discussions with hygienists and employees with
knowledge of past working conditions, taking into account
environmental and biological monitoring data (e.g., cadmium urine
data > 20 mg/litre in the high-exposure group. The periods at risk
of the study population were classified on the basis of these
categories and recorded job histories into three groups: (i) those
workers continuously employed for more than one year in a job
assessed as entailing high exposure - "ever high"; (ii) those
workers continuously employed for more than one year in a job
assessed as entailing medium exposure, but who were never for more
than one year in a high-exposure job - "ever medium", and (iii) all
others. Actual deaths were compared with expected numbers calculated
from mortality rates for the population of England and Wales
corrected for regional variation. The 8th revision of ICD codes was
used and results were expressed as SMRs.
Only 3% of the workers (about 200) were assigned to the "ever
high" category. The mean duration of exposure was 11 years and the
mean interval from initial exposure to the end of the follow-up was
27 years. The SMR (all causes) for the entire population was 97.
There were no prostatic cancer deaths in the "ever high" and "ever
medium" exposure categories (0.4 and 2.5, respectively, expected),
and the number of deaths (23) in the "always low" group was close to
the expected value. There was a small, but not statistically
significant, excess of lung cancer in all categories, but in those
with more than 10 years exposure in the "always low" category this
excess was significant at the 5% level (100 observed, SMR 126).
Since there was no correlation between increase in lung cancer risk
and intensity of exposure, the authors concluded that it was
unlikely that the excess in the "always low" group was due to
cadmium.
A 5-year update of this study (Kazantzis et al., 1988)
confirmed no excess risk from prostatic cancer over the total study
period from 1943 to 1984 and no cases of prostatic cancer in the
medium- or high-exposure groups. The SMR was 99 as opposed to the
value of 90 in the initial study. However, there was now a
significant excess lung cancer mortality (277 observed deaths, 240.9
expected), giving a SMR of 115 (95% confidence interval, 101-129).
This excess risk was related to intensity of exposure, there being
12 deaths in the small high-exposure group as opposed to 6.2
expected (SMR, 194; 95% CI, 100-339), 41 deaths in the
medium-exposure group and 224 deaths in the low-exposure group (SMR
121 and 112, respectively; not significant). While there appeared to
be evidence of a dose-response relationship, it was not
statistically significant. The increased cancer risk mainly involved
those employed before 1940, rising with length of employment and
with length of follow-up.
Further studies have been conducted on workers at these 17
plants (Armstrong & Kazantzis, 1985; Ades & Kazantzis, 1988). A case
control study on lung cancer was carried out on workers in a large
lead-zinc-cadmium smelter. These workers formed 64% of the cohort of
6995 men, and the study included 70% of the lung cancer deaths
observed in the cohort as a whole (Ades & Kazantzis, 1988). There
was a significant excess lung cancer risk among the smelter workers,
and a significant trend with increasing duration of employment,
particularly evident among those employed for more than 20 years.
Quantitative estimates of exposure to cadmium and ordinal rankings
for lead, arsenic, zinc, sulfur dioxide and dust were used to
calculate cumulative exposures from job histories. However, matched
logistic regression analysis showed that the increasing risk of lung
cancer associated with increasing length of employment could not be
accounted for by cadmium exposure and did not appear to be
restricted to any particular process or department.
e) Summary of industrial studies
Increased mortality from lung cancer has been observed in
several occupational cohorts exposed to cadmium, and there is some
evidence of dose-response relationships in two of the examined
populations. Case control studies have not given support for such a
relationship. It is difficult to reach a firm conclusion about
causality, because in all of the occupational cohorts there has been
simultaneous exposure to other potential carcinogens (e.g., nickel,
arsenic, polyaromatic hydrocarbons) or other environmental
pollutants (e.g., sulfur dioxide). Information on tobacco smoking is
inadequate or entirely absent in all except two studies.
Investigations of the relationships between cadmium exposure and
prostatic cancer are inconclusive.
8.2.5.2 In the general environment
Elevated cadmium levels have been found in the liver and
kidneys of patients with bronchogenic carcinoma (Morgan, 1970;
Morgan et al., 1971). However, the authors stressed the possibility
that differences observed could reflect the effect of smoking
(section 5.1.3) or could represent a non specific association.
A study of the causes of death in areas of high cadmium
exposures in Japan (Shigematsu et al., 1982) revealed no difference
in age-adjusted cancer mortality rates between polluted and control
areas of the same prefecture. The mortality rate due to prostatic
cancer was elevated in two areas but only achieved statistical
significance (P < .01) in one area. It was not significant in two
areas including Toyama prefecture, which has the largest area of
pollution.
8.2.6 Mutagenic effects in human cells
An increased frequency of chromosomal aberrations in somatic
human cells is considered to be evidence of some exposure to
mutagenic agents. Shiraishi (1975) noticed an increased frequency of
chromosomal aberrations in lymphocytes obtained from 12 Itai-itai
patients compared to 9 female controls. However, this observation
was not confirmed by Bui et al. (1975) who examined cells from 4
Itai-itai patients and 4 controls.
Among cadmium workers, an increased prevalence of chromosomal
aberrations, compared to controls, was reported by Deknudt & Leonard
(1975) and by Bauchinger et al. (1976), whereas no such effect was
seen by O'Riordan et al. (1978). In none of these occupational
studies was the actual exposure to cadmium measured, and the
possible confounding effect from other industrial chemicals and
smoking was not considered.
Nogawa et al. (1986) did not find evidence for increased sister
chromatid exchange in people exposed to cadmium in the general
environment. IARC (1987a,b) reviewed the available evidence for
mutagenic and related effects and noted the differences in results
reported from different industrial environments.
In conclusion, it is not yet possible to say whether cadmium
causes mutagenic effects in humans.
8.2.7 Transplacental transport and fetal effects
There have been few studies on the fetal toxicity of cadmium
transported across the placenta. Maternal hypertension and decrease
in birth weight have been associated with elevated levels of cadmium
in the neonate (Huel et al., 1981). In addition, it is
well-established that the babies of mothers who are cigarette
smokers are smaller at birth than are those of non-smokers. The
ratio of placental zinc to cadmium is positively related to infant
birth weight in the case of pregnant smokers, and older pregnant
smokers are at higher risk for impaired fetal growth than are
younger ones (Cnattingius et al., 1985; Kuhnert et al., 1987a,
Kuhnert et al., 1987b). Multiparity is related to an increased
placental cadmium level in smokers and to a decreased placental zinc
level in both smokers and non-smokers. These results have been
interpreted as consistent with a depletion of zinc with increasing
number of births and a progressive increase in cadmium in smokers
because of the long half-life of cadmium (Kuhnert et al., 1988).
The cellular mechanisms and factors that influence
trans-placental transport of cadmium are not known. Metallothionein
has been identified in the human placenta and in fetal membranes at
term, and metallothionein synthesis is inducible in cultured
trophoblasts by treatment with cadmium (Waalkes et al., 1984). This
effect is seen with cadmium concentrations in the culture medium as
low as 52.2 µg/litre (0.5 µmol/litre) (Lehman & Poisner, 1984).
Higher levels of exposure to cadmium may have a direct toxic effect
on the placenta. In a test system involving perfusion of maternal
and fetal blood vessels in the isolated human placenta, it was shown
that perfusion of the maternal circulation with cadmium at a
concentration of 1.12 mg/litre (10 µmol/litre) resulted in the
deposition of 2.5 µg cadmium per g placenta (22 nmoles/g), but very
little of it was detectable in the fetal circulation. Perfusion of
the maternal circulation with higher concentrations of cadmium
produced placental cadmium concentrations of 11.2-16.8 µg/g
(100-150 nmoles) with stromal oedema, syncytiotrophoblastic
vesiculation and vacuolization of Hofbauer cells within 6-8 h,
followed by placental necrosis. These changes were associated with a
decrease in human chorionic gonadotropin release and decreased
movement of zinc into the fetal circulation (Miller, 1986).
8.2.8 Other effects
Many other different symptoms and signs have been reported in
humans exposed to cadmium. These include loss of appetite, loss of
weight, fatigue, and increases in the erythrocyte sedimentation rate
(ESR). Valetas (1946) reported details of the poisoning cases in a
French accumulator factory, which were first described by Nicaud et
al. (1942). In addition to the bone effects and the pains (section
8.2.2.1), Valetas mentioned that several workers experienced
paraesthesia and involuntary muscular contractions. This could be an
effect resulting from abnormal changes in the levels of serum
electrolytes, such as calcium or potassium, which may in turn be
caused by severe kidney damage.
Mild anaemia has been more frequently observed among
cadmium-exposed workers than among controls (Friberg, 1950; Bonnell,
1955; Bernard et al., 1979).
More specific effects from cadmium are the yellow discoloration
of the proximal part of the front teeth (Barthelemy & Moline, 1946;
Valetas, 1946; Princi, 1947; Friberg, 1950; Apostolov, 1979) and
anosmia (Friberg, 1950). Anosmia was found by Friberg (1950) in
about one third of a group of workers with a mean exposure time to
cadmium oxide dust of 20 years. Baader (1951) in Germany and
Apostolov (1979) in Bulgaria also noted that anosmia was common
among workers exposed to cadmium oxide dust for long periods of
time. Suzuki et al. (1965) and Tsuchiya (1967) in Japan, found no
increase in the prevalence of anosmia in workers exposed to cadmium
stearate and cadmium oxide fumes.
Nervous system symptoms were reported by Vorobjeva (1957), who
investigated 160 workers at an accumulator factory in the USSR.
Subjective symptoms included headache, vertigo, and sleep
disturbance. Physical examination revealed increases in knee-joint
reflexes, tremor, dermographia, and sweating.
Cadmium sulfide is sometimes used as a yellow tattoo pigment,
which is deposited intradermally. Local phototoxic reactions may
take place when the skin is exposed to ultraviolet light and are
probably connected with the marked photoconducting properties of
cadmium sulfide. Of 24 patients with yellow tattoos who were
examined by Bjornberg (1963), 18 experienced skin swelling when
exposed to sunlight.
8.3 Clinical and epidemiological studies with data on both exposure
and effects
There are several clinical and epidemiological studies with
data on occupational or general environment exposure levels, but the
data concerning effects are restricted to the lungs and kidneys.
8.3.1 Studies on respiratory disorders
Friberg (1950) studied 43 male workers exposed to cadmium oxide
dust, with an average period of employment of 20 years (range 9-34
years), and 15 workers who had been employed for only 1-4 years.
They were compared with a group of 200 sawmill workers. Shortness of
breath was the common symptom among the workers with long exposure,
and an impairment of lung function (increased residual capacity in
relation to total lung capacity and a decreased working capacity)
was demonstrated. The lung function of the group with short exposure
(less than 5 years) was found to be normal. The cadmium
concentration in air varied from 3 to 15 mg/m3, measurements
having been made at five places on only one occasion. In another
battery factory (where air cadmium concentrations were
0.05-5 mg/m3, Adams et al. (1969) found a slight average decrease
in forced expiratory volume in a group of 27 male workers.
Twelve out of 96 cadmium workers exposed for up to 27 years to
cadmium oxide fume in two cadmium-copper alloy factories were found
to suffer from emphysema, as evaluated from a comprehensive lung
function test (Bonnell, 1955; Buxton, 1956; Kazantzis, 1956). These
workers were compared with a similar size control group. The average
air cadmium concentrations in the two factories were 40-50 µg/m3,
and 90% of the particles were less than 0.5 µm in diameter (King,
1955).
Lauwerys et al. (1979a) studied pulmonary ventilatory function
in three groups of workers exposed to cadmium oxide dust and in
matched control groups (the matching included smoking habits). A
slight but significant reduction in forced vital capacity, in forced
expiratory volume in one second, and in peak expiratory flow rate
was found in 22 men. These were all smokers and had been exposed for
more than 20 years to a time-weighted average air concentration of
66 µg/m3 (21 µg/m3 respirable cadmium). In another group of
workers (smokers and non-smokers) exposed for 1-20 years to an
average concentration of 134 µg/m3 (the respirable cadmium level
at the most polluted work site was 88 µg/m3), the pulmonary
indices were on average lower than in the control group, but the
differences were not statistically significant. A more thorough
examination of a subgroup of the workers with long exposures carried
out by the same research group (Stanescu et al., 1977) found more
respiratory symptoms in the cadmium-exposed group than in a control
group and also impaired lung function (not statistically
significant). However, Lauwerys et al. (1979a) reported more
extensive data from the same plants and found that the workers with
less than 20 years of exposure (average 7.5 years) showed
significant effects in the lung function tests.
Reduced forced vital capacity was also found at a cadmium
production plant in the USA (where the air concentrations were
"commonly greater than 200 µg Cd/m3") among 17 workers exposed for
more than 6 years (Smith et al., 1976). De Silva & Donnan (1981)
provided evidence that insoluble cadmium compounds may induce
emphysematous changes after more than 7 years exposure to a
time-weighted average cadmium concentration of 700 µg/m3.
Edling et al. (1986) found no lung function differences between
an exposed group of workers using cadmium-containing solders and a
control group. The level of exposure, which lasted for several
years, was estimated to be 0.05-0.5 mg cadmium/m3, but the workers
had not been exposed to cadmium for several years. Of the 57 workers
examined, 42% had cadmium-induced renal tubular dysfunction.
Davison et al. (1988) examined 101 male workers, who had worked
for 1 year or more manufacturing copper-cadmium alloys, and found,
compared with a reference group, impaired lung function. They also
compared certain parameters (transfer coefficient: KCO) with the
estimated cumulative exposure index for cadmium workers with 95%
confidence limits for the regression line. Among 35 workers exposed
for more than five years and with a cumulative cadmium exposure
index up to 14 mg/m3.years, there was no evidence of a threshold.
The authors concluded that inhaled cadmium fumes caused changes in
lung function and in chest radiographs consistent with emphysema.
This could also explain the increased mortality reported. The
impaired lung function was also related to liver cadmium levels as
measured with neutron activation in vivo.
Some studies on respiratory disorders have yielded negative
results. However, some of these studies did not use lung function
tests (Hardy & Skinner, 1947; Princi, 1947; Tsuchiya, 1967; L'Epee
et al., 1968) and another did not use a control group (Teculescu &
Stanescu, 1970). Suzuki et al. (1965) examined a group of workers
exposed for a short period (average 3.3 years) to 30-690 µg
cadmium/m3 (as cadmium stearate) and found no changes in lung
function when compared to a control group.
In summary, it is clear that exposure to cadmium dust and fume
over prolonged periods can give rise to impaired lung function and
emphysema. Such effects have been seen predominantly at high air
cadmium concentrations (above 100 µg/m3), but one study showed
effects after more than 20 years of exposure to respirable cadmium
oxide dust concentrations of 21 µg/m3.
Cadmium workers sometimes suffered from symptoms such as
coughing and throat irritation, but did not show abnormal chest
X-ray findings when exposed to cadmium oxide fume at a concentration
of 100 µg/m3 for 4-8 years (Hardy & Skinner, 1947) or
40-1440 µg/m3 for 8 years (Princi, 1947), or to cadmium oxide dust
at a concentration of 64-241 µg/m3 for up to 15 years (Tsuchiya,
1967).
8.3.2 Studies on renal disorders in industry
Friberg (1950) reported that prolonged exposure gave rise to
renal damage among a large group of workers exposed to cadmium oxide
dust at concentrations of 3-15 mg/m3 in an accumulator factory. In
one group of 43 workers with a mean exposure period of 20 years
(range 9-34 years), a high prevalence of proteinuria was
demonstrated by the nitric acid and trichloroacetic acid test. In
several of the workers, the renal damage was also manifested by a
decreased inulin clearance and decreased concentrating capacity.
Another group of 15 workers with a mean exposure period of 2 years
(range 1-4 years) showed no positive reactions.
Since 1950, there have been many studies on proteinuria among
workers in various industries. This type of proteinuria is
characterized particularly by a great relative increase in the
excretion of low molecular weight (LMW) proteins (section 8.2). In
most of the early studies, qualitative tests for detecting
proteinuria were used, but, more recently, specific methods for the
quantitative determination of LMW proteins have been developed.
Table 16 contains data on the prevalence of proteinuria from
several epidemiological studies on cadmium workers. It must be
recognized that, in most studies, the dose measurements are based on
short sampling periods (hours or a few days), whereas exposure may
have been for decades. Information on sampling method (static or
personal) and the use of respirators is usually inadequate, which
makes accurate dose estimates difficult (section 2.2.1).
It is evident that the prevalence of proteinuria in cadmium
workers increases with exposure intensity duration. The study by
Kjellström et al. (1977a) presents frequency distributions of
urinary ß2-microglobulin excretion for 240 exposed workers and a
control group. There is a general shift to higher excretion levels
among the exposed workers, and a large proportion of them have
excretion levels far outside the control distribution. Any cut-off
point (operational definition) for "abnormal" proteinuria is
arbitrary. If a cut-off point of 290 µg/litre (corresponding to the
97.5 percentile of the control group) is chosen, 26% of the whole
group of exposed workers would be classified as having LMW
proteinuria. If higher cut-off points are used, the prevalence of
proteinuria will obviously be lower.
Table 16 provides evidence of a dose-response relationship. The
lowest "dose" that gave rise to a statistically significant increase
in urinary ß2-microglobulin, as defined above, was a 6-to 12-year
exposure to 50 µg cadmium/m3 (based on personal sampling)
(Kjellström et al., 1977a).
Järup et al. (1988) recently made a reassessment of
dose-response in the same battery plant (Table 16). A pattern very
similar to Kjellström et al. (1977a) was observed with a prevalence
of ß2-microglobulinuria of 4% at a cumulative dose of 0.5 mg/m3
(corresponding to 10 years of exposure to 50 µg cadmium/m3).
Lauwerys et al. (1979a,b) studied the prevalence of increased
ß2-microglobulin clearance (cut-off point: 97.5 percentile of
controls) and found a 21% prevalence after more than 20 years of
exposure to 66 µg cadmium/m3 total dust (static samples) or 21 µg
cadmium/m3 respirable dust (Table 16).
Holden (1980b) measured urine levels of ß2-microglobulin and
found dose-response relationships using cut-off points of
200 µg/litre, 1000 µg/litre, or 10 000 µg/litre. The cut-off point
of 200 µg/litre gave a 16% LMW proteinuria prevalence rate after
6-10 years of exposure (Table 16).
Table 16 also shows that an increased prevalence of total
proteinuria, as measured by sulfosalicyclic acid, trichloroacetic
acid, or quantitative determination of total proteinuria, occurs
after 5-10 years exposure to approximately 100 µg cadmium/m3. An
increased excretion of LMW protein (e.g., ß2-microglobulin) occurs
at much lower doses.
The in vivo measurement of cadmium in the liver and kidneys
of people with various levels of cadmium exposure provides a means
for relating organ dose to effects and response rates (section
6.4.2). Some questions still remain regarding the accuracy of the
analytical method (section 2.2.3.4) and the mathematical-statistical
methodology (Kjellström et al., 1984). Nevertheless, Roels et al.
(1983a) and Ellis et al. (l984) suggested that renal tubular damage
would be experienced by about 10% of people with a kidney cortex
level of 200 mg cadmium/kg, and by about 50% of people with a kidney
cortex level of 300 mg cadmium/kg.
Table 16. Prevalence of proteinuria in cadmium workers
Cadmium Estimated air Exposure No. of Prevalence of Proteinuria and Reference
compounds concentrations period examinees proteinuria (%) characteristics of
(µg/m3)a (years)b detection
methode
Cadmium oxide 40-50 control 60 2 SA and TCA Bonnell (1955); King (1955);
fume 1-9 37 24 Bonnell et al. (1959)
> 9 63 46
64-241c control 11 0 TA Tsuchiya (1967)
< 1 4 0 < 100 mg/litre
1-4 4 50
123c > 5 4 100
(time-weighted
average
Cadmium oxide 3000-15 000 1-4 15 0 nitric acid ("Hellers Friberg (1950)
dust 9-15 12 33 test"); positive in
16-22 17 41 more than half the
23-34 14 64 test
31 (1.4)c control 31 0 Abnormal Lauwerys et al. (1974a)
1-12 (4) 31 0 electrophoretic
control 27 4 pattern as defined
by the authors;
ß2-microglobulin
clearance
134 (88)c 0.6-19.7 (9) 27 15
control 22 0
66 (21) 21-40 22 68
66 (21) > 20 42 21
Table 16 (contd).
Cadmium Estimated air Exposure No. of Prevalence of Proteinuria and Reference
compounds concentrations period examinees proteinuria (%) characteristics of
(µg/m3)a (years)b detection
methode
Cadmium oxide
dust
control 87 3.4 ß2-microglobulin Kjellström et al. (1977a)
50c 0-3 50 6.0 (RIA)
50c 3-6 30 6.6 > 290 µg/litre
50c 6-12 21 19.0 (sg = 1.023)
Cadmium stearate 30-690 control 24 17 TCA Suzuki et al. (1965)
dust (3) 19 58
Cadmium sulfide 114d < 1-5 12 17 EP Harada (1987)
dust 5-21 7 100
< 1-5 12 8 TCA
5-21 12 43
control 203 1 ß2-microglobulin Stewart & Hughes (1981)
80 0-5 105 0 (RIA)
100 6-11 41 0 > 765 µg/litre
(sg = 1.016)
100-600 11-19 13 7.7
100-600 20+ 14 57.0
Mixed exposures; not reported, controls 642 0 (0.8) ß2-microglobulin Holden (1980b)f
12 factories; but mean blood < 18 months 121 0 (10) (RIA)
mainly zinc cadmium level 19 months-5 years 168 1.8 (8.3) > 1000 µg/litre
smelters after 1 year 6-10 170 1.8 (16) (or > 200 µg/litre)
exposure was 11-15 82 7.3 (22)
about 16-20 33 24 (45)
15 µg/litre
20+ 68 25 (56)
Table 16 (contd).
Cadmium Estimate of cumulative Number of Prevalence (%) Proteinuria and Reference
compounds dose (mg/m3.year) examinees of proteinuria characteristics of
detection method
Cadmium fume < 1 16 18 ß2-microglobulin Elinder et al. (1985b)
(oxide) 1-2 22 32 > 0.034 mg/mmol creatinine
2-3 9 44
3-5 8 62
> 5 5 100
< 1 ) 6 Mason et al. (1988)
1-15 ) 67
1.5-3) 75 58
3-5 ) 86
> 5 ) 100
Cadmium oxide < 0.4 264 1.1 Jarup et al. (1988)
0.4-1.7 76 9.2
1.7-4.6 43 23.3
4.6-9.6 31 32.3
9.6-15 16 32.2
> 15 10 50
a Numbers in parentheses refer to average concentrations of the respirable particulates (< 5 µm) fraction
b Numbers in parentheses are average values
c Measured in breathing zone by personal sampler
d Calculated average exposure for the worker with the most pronounced effect
e SA = sulfosalicyclic acid method (qualitative); TCA = trichloroacetic acid method (qualitative);
TA = tungstate method (quantitative); EP = electrophoresis; RIA = radioimmunoassay; sg = specific gravity
f Values for prevalence of proteinuria refer to a ß2-microglobulin level of > 1000 µg/litre.
Values in parentheses refer to a level of > 200 µg/litre.
Ellis et al. (1985) correlated time-weighted exposure indices
(TWE), based on employment records, area monitoring techniques and
personal sampling, with body burden of cadmium measured by
in vivo neutron activation analysis of the liver and left kidney
in 82 men exposed to cadmium dust in a smelter. The workers were
grouped as follows: production workers (40 active, 21 retired);
office and laboratory workers (8 active, 4 retired); and
non-production workers (3 active, 6 retired). From these
measurements the authors were able to estimate the probability of
developing kidney dysfunction based on the workers' cumulative
exposure index. When the exposure limit was 400-500 µg/m3.years,
the prevalence for renal dysfunction was about 32%; it was 22-40%
with a wider exposure index (300-600 µg/m3). Kjellström et al.
(1977a) reported a prevalence of 19% at a battery factory at a level
of 50 µg Cd/m3, which resulted in a similar exposure index.
Lauwerys et al. (1974a) observed proteinuria in 68% of workers with
long-term exposure (20 years at 66 µg/m3), whereas the logistic
model developed by Ellis et al. (l985) would have predicted 65%
under these exposure conditions.
For exposures of 250 µg/m3.years there is a 19% probability
of experiencing renal dysfunction. In the case of workers with
normal renal function, an exposure index of 400 µg/m3 predicts a
mean cadmium concentration of 28 mg/kg in liver and 288 mg/kg of
renal cortex. The model would predict that a 10-year exposure to
25 µg/m3 would result in a mean renal cortex concentration of
252 mg/kg, which is similar to the critical concentration defined by
Friberg.
Thun et al. (1989) assessed the quantitative relationship
between exposure to airborne cadmium and various markers of renal
tubular and glomerular function in 45 male workers at a plant that
recovered cadmium from industrial waste. The dose was estimated from
historical air sampling data. In this study, the "critical dose" of
cadmium necessary to induce nephropathy was based on the 5th or 95th
percentile of test results in the unexposed population. Using this
definition, renal tubular dysfunction sharply increased as
cumulative exposure to cadmium rose above 300 mg/m3.days
(corresponding to about 0.8 mg/m3.years). Very similar
dose-response curves with an increased prevalence of
ß2-microglobulinuria at cumulative exposure levels exceeding about
0.5-1 mg cadmium/m3.year have been reported from examinations of
workers exposed to cadmium fumes (Elinder et al., 1985b; Mason et
al., 1988). These findings are consistent with the recommendations
by a working group of the World Health Organization (WHO, l980) to
limit workplace exposures to 10 µg/m3 in order to prevent tubular
proteinuria after life-time occupational exposure to cadmium.
Cumulative cadmium exposure indices have been calculated for 75
cadmium alloy workers employed for periods of up to 39 years,
together with the in vivo liver and kidney cadmium burden (Mason
et al., 1988). Several indicators of both tubular and glomerular
dysfunction correlated significantly with both cumulative exposure
index and liver cadmium burden. Using these estimates of dose, a
two-phase linear regression model was applied to identify an
inflection point of the order of 1100 µg/m3.years above which
changes in renal function occurred. A number of biochemical
variables fitted this model, including total protein, albumin, and
ß2-microglobulin. Simple dose-response analysis showed a greatly
increased incidence of tubular proteinuria when the cumulative
cadmium exposure index was greater than this value. The cumulative
exposure index was equated to about 20 to 22 years of exposure to a
cadmium level of 50 µg/m3.
Evidence of tubular damage was investigated in a group of 91
cadmium workers subjected to yearly estimation of cadmium
concentration in blood and urine over a period of eight years. In
workers with blood and urine cadmium levels constantly below the
Biological Limit Value of 10 µg/litre, the prevalence of tubular
damage, as indicated by an increased excretion of ß2-microglobulin
above 260 µg/litre, was below 3%. RBP excretion confirmed this
pattern.
8.3.3 Studies on renal disorders in the general environment
8.3.3.1 Health surveys in Japan
Following the recognition of the association between Itai-itai
disease and exposure to cadmium (Japanese Ministry of Health and
Welfare, 1968), additional general surveys of cadmium pollution were
performed in Japan, and further areas were found to be involved.
Health effects were studied first among the population in the area
where Itai-itai cases had occurred and later in other areas found to
be contaminated. The original studies were designed to find cases of
Itai-itai disease, but it was possible also to estimate the
prevalence of proteinuria and glucosuria in the examined population.
A detailed description of the methods used in these cadmium
pollution surveillance programmes has been reported by Shigematsu et
al. (1979).
At the time of the first surveillance programmes (1969-71),
methods were developed for estimating the degree of contamination
with cadmium and the total daily intake. At the early stage of the
investigations, the most common index of cadmium intake measured in
all areas was the cadmium concentration in rice. From data of the
Japan Public Health Association (1970), it was estimated that, in
areas with different exposure levels, an average of almost 50%
(range 14-71) of the daily cadmium intake came from rice. In one
area, the proportion was estimated to be 85% (Kawano & Kato, 1978).
The average cadmium concentration in rice from non-polluted
areas has been reported to be 0.066 mg/kg in polished rice
(Moritsugi & Kobayashi, 1964) and 0.09 mg/kg in unpolished rice
(Japanese Ministry of Agriculture and Forestry, 1973) (section
5.2.1). The national average consumption of rice was 364 g per
person in 1961, 308 g in 1971, and 222 g in 1981 (Japanese Ministry
of Health and Welfare, 1983).
Proteinuria was generally estimated with qualitative methods
such as the sulfosalicylic acid method or the trichloracetic acid
method according to standardized techniques (Japanese Ministry of
Health and Welfare, 1971). More recently, emphasis has been placed
on the identification of the urinary protein pattern, in particular
to detect early evidence of tubular dysfunction. The methods
currently used are electrophoresis and the quantitative
determination of lysozyme, RBP, and ß2-microglobulin. Several
studies have been published, and there are extensive reviews in
English (Tsuchiya, 1969; Yamagata & Shigematsu, 1970; Friberg et
al., 1974; Tsuchiya, 1978; Shigematsu et al., 1979, l980).
From 1976 to 1984, epidemiological health surveys of residents
in areas with environmental cadmium pollution were performed by the
Japan Environment Agency using methods including immunological tests
for the detection of low molecular weight proteinuria in eight
prefectures (Akita, Fukushisuma, Gunma, Toyama, Ishikawa, Hyogo,
Nagasaki, Oita). More than 13 000 inhabitants of polluted areas and
more than 7000 inhabitants of non-polluted areas, aged 50 years or
more in both areas, were subjected to these surveys.
The following screening method was adopted for health
examinations. The urine of those people with proteinuria
(demonstrated by a semiquantitative method) and/or glucosuria (by a
paper test) was analysed for ß2-microglobulin (> 10 mg/litre),
RBP (> 4 mg/litre), lysozyme (> 2 mg/litre), total amino acid
nitrogen (> 20 mmol/litre), and cadmium (> 30 µg/litre). Those
who exceeded the above levels in more than one item were tested for
renal function by urine and blood analysis. Finally those for whom
the TRP was less than 80% were subjected to detailed health
examination, including skeletal radiography, in order to make a
clinical diagnosis (Fig. 7).
With the exception of Oita prefecture, the number of
individuals who had or were suspected of having proximal renal
tubular dysfunction (as defined by the Japanese Cadmium Research
Committee) or related findings tended to be greater in the polluted
areas than in the non-polluted areas, and this was often
significantly related to the degree of pollution (see Table 16).
This suggests that environmental cadmium pollution is associated
with the occurrence of proximal renal tubular dysfunction.
There are no available quantitative human data to indicate the
net gastrointestinal excretion.
Cadmium is also eliminated through hair (Anke et al., 1976) and
breast milk (Schroeder & Balassa, 1961), but these routes are of
limited importance for total excretion and do not significantly
alter the biological half-time.
6.6 Biological half-time and metabolic models
6.6.1 In animals
Several studies have been carried out in order to assess the
biological half-times of cadmium in experimental animals. Various
animals species, including mice, rats, rabbits, dogs, and monkeys,
have been studied, and single exposures have normally been used. The
reported half-times have varied from weeks to two years (or as long
as half the life-span of the animal). The development of metabolic
models has shown that the body contains several compartments for
cadmium accumulation, each with a different half-time. Thus, in
whole body half-time measurements, one may find several different
half-times. In order to observe the slowest half-time components, it
is necessary to study the animals for many months.
The biological half-time of cadmium in the kidney and whole
body decreases when renal tubular dysfunction occurs because of
increased urinary excretion (section 6.5.1). However, some studies
have indicated that the biological half-time may change with dose
and body burden of cadmium even before renal damage occurs. For
instance, Engstrom & Nordberg (1979a) reported that in mice
half-time increased with increasing single or repeated oral dose. In
these studies, body burden and renal burden were considerably lower
than the maximum that can be reached in long-term exposure. Nomiyama
(1978) reported that half-time decreased with increasing dose when
animals with the shortened half-time had reached the maximum renal
burden. Even shorter half-times were reported when renal tubular
dysfunction occurred after exposure to high doses (Nomiyama, 1978).
A number of studies on biological half-time and metabolic
models for animals have been reviewed by Friberg et al. (1974) and
Nomiyama (1978).
The wide difference in the results obtained by investigators
may be explained by variations in exposure level and type, the
different animal species used, and interactions between cadmium and
other exposure factors. Reported half-times range from several weeks
in mice to 22 years in monkeys (Friberg et al., 1974; Nomiyama et
al., 1979; Nomiyama et al., 1984). The variations in half-times in
specific tissues between different species or individuals may be due
to variations in the production of metallothionein, which binds
tissue cadmium and contributes to its retention.
6.6.2 In humans
Experimental and epidemiological evidence indicates strongly
that the biological half-time in the whole body is extremely long
(many years). Experimental evidence from one study (Shaikh & Smith,
1980), in which one subject was given radioactive cadmium and
examined periodically for the next 2 years, showed a biological
half-time of 26 years. In three similar studies, in which a small
number of subjects were followed up for a limited period (about 100
days), half-times of 93-202 days were reported (Rahola et al., 1972;
Flanagan et al., 1978; McLellan et al., 1978). Only one of these
studies (Rahola et al., 1972) gave confidence limits for the
estimated biological half-time (130 days to infinity).
Another approach to estimate the half-time used involves
comparing total daily excretion with total body burden, applying a
one-compartment model to the body as a whole (Friberg et al., 1974;
Task Group on Metal Toxicity, 1976). A further approach analyses the
accumulation in the kidney using a one-compartment model taking into
consideration age-related variations in daily cadmium exposure and
kidney weight (Tsuchiya & Sugita, 1971; Kjellström, 1971). More
recently, an elaborate model has been developed that includes
separate compartments for, for instance, kidney, liver, and blood,
and incorporates age-related variations in daily intake, tissue
weights, and renal function (Kjellström & Nordberg, 1978).
These models rely on many assumptions concerning the cadmium
concentration in food, calorific intake, absorption rates, and other
factors. Inevitably, the data produced by these models are only
tentative, but they are important for future research. Using data
from Japan on the accumulation of cadmium with age, Tsuchiya et al.
(1976) used a series of one-compartment mathematical models
developed by Tsuchiya & Sugita (1971) to estimate biological
half-times of cadmium in various organs. These authors estimated the
biological half-time in the kidneys to be about 17 years and that in
the liver 7 years.
Elinder et al. (1976) used autopsy data from non-smokers in
Sweden and a one-compartment model (Kjellström, 1971) to estimate
the biological half-time in the renal cortex. They assumed that the
daily intake of cadmium had doubled in 50 years (Kjellström et al.,
1975a) and estimated the half-time to be 20-50 years (30 years being
the best estimate).
Using an 8-compartment model (Kjellström & Nordberg, 1978), the
biological half-times of cadmium in the liver and kidney were
estimated to be 7.5 and 12 years, respectively (Kjellström &
Nordberg, 1978). The longest half-time was calculated for the "other
tissues" compartment. This included muscle tissue, which was found
to have the longest half-time in an autopsy study (Kjellström,
1977). However, it should be pointed out that when using this type
of model to simulate the chemobio-kinetics of cadmium, the
individual half-times of different tissues are less important than
the dynamics of the model as a whole.
After high cadmium exposure, as occurs among certain industrial
workers, the biological half-time may not be the same as that during
normal exposure in the general environment. Current models, however,
do not consider this factor. If the exposure level is very high, the
ratio of rapid components to slow components may be altered. An
example of this is the very high urinary excretion found after only
a short exposure to high air cadmium levels (Lauwerys et al., 1976,
1979b). The biological half-time is also shorter if there is renal
tubular dysfunction. Fletcher et al. (1982) carried out
in vivo neutron activation analysis of the liver of 13 cadmium
workers twice within a period of 3 to 4 years (the occupational
cadmium exposure of these workers had ceased before the first
analysis). Three workers showed proteinuria and an average cadmium
half-time in the liver of 2 years. The other 10 workers had an
average half-time of 6.4 years, nine of whom had an average
half-time of 13.5 years and no proteinuria.
Jarup et al. (1983) studied the half-time of cadmium in blood
of five smelter workers who had previously experienced high cadmium
exposure. Repeated blood analysis carried out over a 10-to 13-year
period revealed short-term (75-128 days) and a long-term (7.4-16
years) half-time components. The long-term component in two workers
with proteinuria was shorter than in the other workers.
6.7 Biological indices of cadmium exposure, body burden, and
concentrations in kidneys
There is no easy way to measure directly the whole body burden
or concentrations of cadmium in different tissues of a living
person. In vivo neutron activation methods have been used in
special circumstances (Ellis et al., 1981a; Roels et al., 1981b;
Tohyama et al., 1981a).
At present, it is necessary to study concentrations in easily
available indicator media in order to evaluate exposure and
accumulation of cadmium. The suitability of certain indicator media
for such purposes is supported by studies on both animals and
humans; urine, blood, faeces, and hair have all been used as
indicator media. Methods for the biological monitoring of cadmium
levels in blood and urine have been reviewed by Nordberg & Nordberg
(1988) and WHO (1980).
6.7.1 Urine
The human and animal studies summarized in section 6.5.1 allow
the following interpretation of the significance of cadmium in urine
(Lauwerys et al., 1980b). In the absence of episodes of high-level
exposure to cadmium and provided that cadmium-binding sites in the
organism are not saturated and cadmium-induced nephropathy has not
yet occurred, the urine cadmium level increases in proportion to the
amount of cadmium stored in the body. In such situations, which
prevail mainly in the general population and in workers moderately
exposed to cadmium, there is significant correlation between urinary
cadmium and cadmium in kidney. Episodes of high exposure to cadmium,
however, may lead to a transient increased urinary excretion.
If exposure to cadmium has been excessive, the cadmium-binding
sites in the organism become progressively saturated and, despite
continuous exposure, the cadmium concentration in the renal cortex
tends to plateau. Once this point is reached, the cadmium that is
still absorbed cannot be further retained in the kidney and is
rapidly excreted in the urine. Under these conditions, urinary
cadmium is also influenced by the recent intake. The relative
influence of the body burden and the recent exposure on urinary
cadmium depends on the exposure intensity. If exposure continues, a
certain percentage of individuals may develop renal damage. This is
associated with a progressive loss of cadmium accumulated in the
kidney, which gives rise to a further increase in urinary cadmium.
Eventually, the amount of cadmium that can be released from the
kidney decreases progressively and the urinary cadmium concentration
follows the same trend. The changes in the urinary metallothionein
level parallel those of cadmium (section 6.8.2).
In summary, several factors (duration and intensity of exposure
to cadmium, the presence of renal dysfunction and its duration) must
be taken into consideration when interpreting urinary cadmium (and
metallothionein) levels.
6.7.2 Blood
Plasma cadmium levels are considered to be related to recent
exposure but are often so low that they cannot be measured routinely
(section 6.2). Most of the cadmium in the blood is in the
erythrocytes.
Cadmium levels in whole blood mainly reflect the exposure
during recent weeks or months. In cadmium workers, the level
increases markedly within the first few months after occupational
exposure starts (Kjellström & Nordberg, 1978; Lauwerys et al.,
1979b). It is probable that a portion of the blood cadmium level
reflects body burden rather than present exposure in view of the
known transport of cadmium via blood from the liver to the kidneys
and other tissues (section 6.2) and the long-term half-time
component demonstrated by Jarup et al. (1983). Workers with
relatively long exposure durations but whose cadmium exposure has
ceased have elevated blood cadmium levels for several years (Friberg
et al., 1974; Jarup et al., 1983).
Reports of blood cadmium levels in the general population have,
in the past, often been unreliable, owing largely to the
difficulties encountered in the analysis of blood cadmium (Lauwerys
et al., 1975). However, improvements in analytical techniques have
since been achieved through biological standards for blood and
systematic quality assurance programmes (Stoeppler et al., 1979;
Vahter, 1982). Average blood cadmium values up to 10 µg/litre or
more have been reported in the past, but the analytical procedures
used mean that the accuracy of these data is in doubt (Vahter, 1982;
Friberg & Vahter, 1983).
Various aspects of blood cadmium analysis have been discussed
in a UNEP/WHO study, which also included a quality assurance
programme and data from 10 countries (Vahter, 1982; Friberg &
Vahter, 1983). It was found that even in the countries with the
highest blood cadmium levels, the average was less than 4 µg/litre.
Furthermore, smokers were found to have higher values than
non-smokers, and non-smokers, in most countries, had mean levels
below 1 µg/litre. Although only slightly above 1 µg/litre, the
levels for non-smokers in Japan were about twice as high as the
levels in the USA, which probably reflects the difference in average
daily cadmium intake via food (section 5.2.2).
Reported cadmium concentrations in the blood of exposed workers
are generally between 5 and 50 µg/litre, but levels of between 100
and 300 µg/litre have resulted from extreme exposures (Roels et al.,
1982; Hassler et al., 1983).
6.7.3 Faeces
Gastrointestinal absorption amounts to only a few percent of
the cadmium ingested daily (section 6.1.2), and the quality of
cadmium excreted gastrointestinally is small compared to the
unabsorbed portion of ingested cadmium. Thus, daily faecal cadmium
can serve as a good indicator of the daily amount of cadmium
ingested via food and water or cleared from the lungs after
occupational exposure to large dust particles. Faecal cadmium
correlates very closely with daily energy intake (section 5.2.4),
and average cadmium intake estimates for different countries agree
well with reported average faecal cadmium amounts.
A portion of the cadmium in human faeces is related to the body
burden (section 6.5.2). In workers with high body burdens but low
daily cadmium intakes via food, this portion might be greater than
the unabsorbed part of ingested cadmium, because the faecal
excretion is similar to the urinary excretion.
6.7.4 Hair
Cadmium in hair is not a reliable indicator of either recent
exposure or body burden. The main problem is external contamination
of the hair, which cannot be distinguished from the endogenous
cadmium (Nishiyama & Nordberg, 1972). However, a correlation was
found between air cadmium levels of cities in the USA and cadmium
levels in the hair of 10-year-old children living in these cities
(Hammer et al., 1971). In a study of cadmium workers (Ellis et al.,
1981b), a higher average hair cadmium level was found among exposed
workers than among controls, but there was a poor relationship
between hair cadmium and cadmium in the blood, urine, liver or
kidney. Cadmium concentrations in the hair of people without
excessive exposure are usually between 0.5 and 2 mg/kg.
6.8 Metallothionein
6.8.1 Nature and production
Metallothionein is a metal-binding protein of low molecular
weight, which has a key role in the metabolism of cadmium. It is
rich in cysteine but contains no aromatic amino acids or histidine
(Kagi & Vallee, 1960, 1961).
This protein was identified for the first time by Margoshes &
Vallee (1957) in horse kidney cortex. Its molecular weight is about
6600 (6000 for the apoprotein moiety, thionein), and it has a
non-globular shape. On gel filtration, however, it moves like a
spherical protein with a molecular weight of about 10 000 (Kagi &
Nordberg, 1979). There have been several reports dealing with the
function and biochemistry of metallothionein (Kagi & Nordberg, 1979;
Brady, 1982; Foulkes, 1982; Webb & Cain, 1982; Kagi & Kojima, 1987).
Piscator (1964) suggested that metallothionein played a role in
cadmium transport and detoxication, and it has subsequently been
identified in human kidney and liver (Pulido et al., 1966; Chung et
al., 1986) as well as in those of various experimental animals (Kagi
& Nordberg, 1979).
The structure and genetic expression of mouse and human
metallothionein have now been identified. Two major forms of
metallothionein are present in most mammalian tissues, particularly
liver and kidney, i.e. metallothionein I (Mt-I) and metallothionein
II (Mt-II). Induction of synthesis is under the control of a large
group of genes and is stimulated by glucocorticoids and the
essential metals zinc and copper, as well as by the toxic metals
cadmium and mercury (Karin et al., 1981; Karin & Richards, 1982).
In vitro binding affinities have been demonstrated for a number of
other toxic metals, including bismuth, cobalt, silver, and gold
(Cherian & Nordberg, 1983). Metallothionein binds seven metal ions
per molecule between two separate metal-cysteine clusters, and a
single molecule may contain more than one metal, e.g., cadmium and
zinc, mercury and copper.
6.8.2 The role of metallothionein in transport, metabolism, and
toxicity of cadmium
Piscator (1964) suggested that some of the cadmium-binding
metallothionein in the liver may migrate into the blood stream. As
discussed in section 6.2, part of the plasma cadmium in animals
exposed for a long time to cadmium is bound to a protein with the
same molecular weight as metallothionein. When metallothionein-bound
cadmium is present in the plasma, it is quickly cleared by
glomerular filtration and reabsorbed in the renal tubules or
excreted in the urine (Cherian & Shaikh, 1975; Nordberg et al.,
1975; Webb & Etienne, 1977; Fowler & Nordberg, 1978).
At low levels of cadmium-metallothionein in the plasma, tubular
reabsorption is almost complete, whereas the uptake in the tubular
cells from the tubular fluid is saturated in the presence of high
concentrations (Nomiyama & Foulkes, 1977; Foulkes, 1982). Thus, high
urinary excretion of cadmium-metallothionein occurs shortly after
the administration of larger doses, i.e. doses exceeding about
0.1 mg cadmium/kg body weight (Cherian & Shaikh, 1975; Nordberg &
Nordberg, 1975).
It has been demonstrated in animal and in vitro tissue
studies that metallothionein provides a protective role for cadmium
toxicity (Cherian & Nordberg, 1983). Mice pretreated with cadmium
have increased tolerance to subsequent cadmium exposure (Nordberg et
al., 1971), and exposure to cadmium may protect from subsequent
mercury toxicity (Piotrowski et al., 1974). In addition, the
inhibition of certain mixed-function oxidases by cadmium is reduced
by prior induction of metallothionein by cadmium in immature mice
(Asokan et al., 1984). Pre-exposure of cultured kidney cells to
cadmium protects from subsequent exposure (Cherian, 1980; Jin et
al., 1986).
Nordberg et al. (1975) showed that metallothionein isolated
from rabbit and mouse liver produced acute renal tubular cell
toxicity when injected subcutaneously into mice. Further study
(Cherian et al., 1976; Fowler & Nordberg, 1978; Squibb et al., 1982,
1984) suggested that parenterally administered
cadmium-metallothionein enters proximal renal tubular lining cells
in pinocytotic vesicles that fuse with lysosomes. The
metallothionein is degraded, releasing cadmium into the cytosol and
producing cellular degeneration and necrosis within 8-24 h. The
renal tubular cell toxicity produced by metallothionein with
different ratios of cadmium and zinc is proportional to the cadmium
content of the metallothionein (Suzuki, 1982). Zinc-thionein does
not have a similar effect.
The pathogenesis of renal tubular cell toxicity is thought to
be related to non-metallothionein-bound cadmium, which becomes
rapidly bound to existing metallothionein sites or induces the
synthesis of new metallothionein (section 7.2.1.4).
The prevalence of nephrotoxicity rather than hepatotoxicity in
chronic cadmium exposure may be due to several factors. Firstly, the
release of hepatic cadmium-metallothionein or its presence in the
blood can result in preferential accumulation of cadmium in the
kidneys. Secondly, it has been shown in experimental animals that
the kidney can accumulate metallothionein mRNA in response to
cadmium exposure to only about half the level of the liver
(Koropatnick & Cherian, 1988). Thus, the kidney may not be able to
synthesize metallothionein as efficiently as the liver in response
to cadmium exposure, resulting in an accumulation of
non-metallothionein cadmium in the kidney but not in the liver.
Nomiyama et al. (1982a) studied the concentrations of total
cadmium, metallothionein-cadmium, and non-metallothionein-cadmium in
the renal cortex of monkeys fed diets containing 30 mg cadmium/kg
food for 12 months. They found that the concentration of
non-metallothionein-cadmium increased with dose of cadmium to about
35 mg/kg tissue and total cadmium to about 200 mg/kg tissue when the
total dose of cadmium was 0.4 g. Similar measurements were made in
rabbits given cadmium chloride (0.5 mg cadmium/kg body weight)
subcutaneously every day for 21 weeks. There were parallel increases
of total cadmium and non-metallothionein-bound cadmium during the
initial 4 weeks of dosing; these remained unchanged until the 14th
week when striking renal dysfunction appeared. At that time, total
cadmium and non-metallothionein-bound cadmium levels fell despite
the continued administration of cadmium (Nomiyama & Nomiyama, 1982).
Increased knowledge of the intracellular binding or speciation of
non-metallothionein-bound cadmium should improve our understanding
of the relative roles of metallothionein-bound and
non-metallothionein-bound cadmium.
When Cherian et al. (1978) exposed mice by injection and
feeding to both cadmium chloride and cadmium-metallothionein, both
compounds were absorbed and distributed in the body. However, in the
short term, cadmium-metallothionein was selectively accumulated in
the kidney and cadmium chloride in the liver.
Most cadmium in human urine is bound to metallothionein
(Tohyama et al., 1981b), and good correlation has been found between
the urinary cadmium and metallothionein concentrations both in
elderly women exposed in the general environment (Tohyama et al.,
1981b) and in male cadmium workers (Nordberg et al., 1982; Roels et
al., 1983b). Measurement of urinary metallothionein thus provides a
good indication of the urinary cadmium level and offers the
advantage over cadmium analysis of avoiding the possibility of
external contamination. Women were found to have much higher urinary
metallothionein concentrations than men, even at similar cadmium
levels.
6.9 Conclusions
Data from experimental animals and humans have shown that
pulmonary cadmium absorption is greater than gastrointestinal
absorption. Depending on chemical speciation, particle size, and
solubility in biological fluids, up to 50% of the inhaled cadmium
compound may be absorbed. The gastrointestinal absorption of cadmium
is influenced by the type of diet and nutritional status, iron
status appearing to be particularly important. On average, 5% of the
total oral intake of cadmium is absorbed, but individual values
range from less than 1% to more than 20%. Cadmium may also be
transported to the fetus. However, although cadmium accumulates in
the placenta, little is transferred to the fetus.
Cadmium absorbed from the lungs or the gastrointestinal tract
is stored principally in the liver and kidneys where more than half
of the body burden is deposited. Highest cadmium concentrations are
generally found in the renal cortex, but as exposure levels
increase, a greater proportion of the absorbed cadmium is stored in
the liver. The cadmium excretion rate is normally low, and the
biological half-time is very long (decades) in the kidneys, muscles,
liver, and total body of humans. The cadmium concentrations in most
tissues increase with age. In exposed people with renal damage,
urinary excretion of cadmium increases and, thus, the whole body
half-time is shortened. The renal damage leads to losses of cadmium
from the kidney, and the renal concentrations are eventually lower
than in people with similar exposure but without renal damage.
Metallothionein is an important transport and storage protein
for cadmium and other metals. Cadmium can induce metallothionein
synthesis in many organs including the liver and kidney. The binding
of intracellular cadmium to metallothionein in tissues protects
against cadmium toxicity. Non-metallothionein-bound cadmium may,
therefore, have a role in the pathogenesis of cadmium-related tissue
injury. The speciation of other cadmium complexes in tissues or
biological fluids is unknown.
Urinary excretion of cadmium is related to body burden, recent
exposure, and renal damage. In people with low exposures, cadmium in
urine is mainly related to body burden. Cadmium-exposed people with
proteinuria generally exhibit greater cadmium excretion than such
people without proteinuria. After high exposure ceases, urinary
cadmium decreases even though renal damage persists. The
interpretation of urinary cadmium is thus dependent on a number of
factors. The magnitude of gastrointestinal excretion is similar to
that of urinary excretion, but it cannot be easily measured. Other
excretory routes such as lactation, sweating or placental transfer
are insignificant.
Cadmium in faeces is a good indicator of recent daily intake
from food in the absence of inhalation exposure. Cadmium in blood
occurs mainly in the blood cells, and the plasma concentrations are
very low. There are at least two blood compartments, one being
related to recent exposure with a half-time of about 2-3 months, and
the other probably related to body burden with a half-time of
several years.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Lethal dose and lethal effects
LD50 inhalation values are in the range of 500 to
15 000 mg/m3.min for different species (Barrett et al., 1947;
Harrison et al., 1947; Hadley et al., 1979). The cause of death is
pulmonary oedema.
The LD50 after the injection of soluble cadmium compounds is
in the range of 2.5-25 mg/kg body weight (Friberg, 1950; Eybl &
Sykora, 1966; Commission of the European Communities, 1978). Shortly
after large doses are injected, severe endothelial damage is seen in
the small vessels of the peripheral nervous system (Gabbiani, 1966)
and in the testis (Parizek, 1957). If the animal survives for some
hours, the most pronounced changes are found in the liver (Dudley et
al., 1982), and liver damage is probably the lethal effect of a
single high parenteral exposure.
For most cadmium compounds, the LD50 after oral
administration is about 10-20 times higher than after parenteral
administration, and the readily soluble compounds have a lower
LD50 values than the insoluble ones (Table 11).
Nomiyama et al. (1978b) showed that the LD50 in mice was
lower at cold temperatures (+8 °C) than at higher temperatures
(+22 or +37 °C), both after oral and peritoneal exposure.
7.1.2 Pathological changes affecting specific systems in the body
The chronic effects of long-term exposure to low doses of
cadmium constitute the main problem for non-occupationally exposed
humans. Therefore, the effects of single exposure in animals will be
dealt with only briefly, and the main emphasis will be on chronic
effects.
Specific effects from a single high dose of cadmium have been
described by several investigators and have been reviewed by Friberg
et al. (1974, 1986), Commission of the European Communities (1978),
and Kawai (1978). One of the most pronounced effects seen was in the
gonads (testis and ovary).
Table 11. LD50 values for cadmium compounds given to mice and rats by intragastric administration
Species Compound Molecular formula Relative molecular LD50 with confidence LD50 for cadmium
massa limits (mg/kg)b ion alone (mg/kg)
Mouse cadmium (element) Cd 109 890 (636-1246) 890
cadmium oxide CdO 128.4 72 (41-113) 63
cadmium sulfate CdSO4 208.5 88 (69.8-100.2) 47
cadmium chloride CdCl2 183.3 93.7 (75.5-111.9) 57
cadmium nitrate Cd(NO3)2 236.4 100 (78.7-121.8) 48
cadmium iodide CdI2 366.2 166 (139-193) 51
cadmium caprylate Cd(C7H15COO)2 394.8 300 (196-459) 85
cadmium carbonate CdCO3 169 310 (215-404) 202
cadmium stearate Cd(C17H35COO)2 679.4 590 (556-624) 98
cadmium sulfide CdS 144.5 1166 (1135-1197) 907
cadmium sulfoselenide CdSe.CdS 335.8 2425 (2393-2457) 1623
barium-cadmium stearate BaCd(C17H35COO)4 1383.7 3171 (2763-3579) 258
Rat cadmium caprylate Cd(C7H15COO)2 394.8 950 (613-1472) 270
cadmium stearate Cd(C17H35COO)2 679.4 1225 (875-1574) 203
barium-cadmium stearate BaCd(C17H35COO)4 1383.7 1980 (1736-2224) 161
a From: Weast (1974)
b From: Tarasenko et al. (1974), Vorobjeva & Sabalina (1975), and Vorobjeva & Bubnova (1981)
7.1.2.1 Acute effects on testes and ovaries
Testicular necrosis occurs in experimental animals given single
injections of salts corresponding to 2-4 mg cadmium/kg body weight
(Parizek & Zahor, 1956; Parizek, 1957). At a later stage, Leydig
cells regenerate (Parizek, 1957, 1960; Allanson & Deanesly, 1962).
Gabbiani et al. (1974) detected dilation of interendothelial clefts
in the small blood vessels of the testis as early as 15 min after an
intravenous injection of cadmium salts. Effects on the testis have
been extensively reviewed by Gunn & Gould (1970).
The marked effects on the testis after cadmium injection are
probably the result of endothelial damage. In the small vessels this
damage gives rise to increased capillary permeability. This leads to
vascular escape of fluids and blood plasma substances into the
interstitium, which results in oedema, decreased capillary blood
flow, ischaemia, and testicular cell necrosis (Aoki & Hoffer, 1978;
Francavilla et al., 1981).
A single injection of cadmium salts at a dose that induces
testicular haemorrhagic necrosis has been shown to induce
haemorrhages and necroses in the ovaries of prepubertal rats (Kar et
al., 1959), and in the ovaries of adult rats in persistent oestrus
(Parizek et al., 1968a). The effect of cadmium on the testis was not
dependent on the presence of the hypophysis (Parizek, 1960). Ovarian
effects can be prevented by the administration of PMSG hormones
(Parizek et al., 1968a). Numerous studies on the effects of cadmium
on the testes and other reproductive organs were reviewed by Barlow
& Sullivan (1982).
7.1.2.2 Acute effects on other organs
A single inhalation exposure to cadmium at concentrations of
5-20 mg/m3 for 50-120 min gives rise to pulmonary oedema in rats
and rabbits (Hayes et al., 1976; Bouley et al., 1977; Bus et al.,
1978; Dervan & Hayes, 1979; Boisset & Boudene, 1981; Fukuhara et
al., 1981). The morphological changes seen in the lung have been
described in detail by Strauss et al. (1976).
After the parenteral administration of cadmium at dose levels
similar to the LD50, pronounced effects were seen in the small
blood vessels of, for instance, the nervous system (Gabbiani et al.,
1974). Hoffman et al. (1975) noted profound morphological effects in
the liver of rats given 6 mg cadmium/kg body weight, and Dudley et
al. (1982), examining liver effects from a single injection of
cadmium (3.9 mg/kg body weight), concluded that liver was the major
target organ in rats for acute cadmium toxicity. Changes in blood
pressure shortly after the acute administration of cadmium have also
been recorded (Dalhamn & Friberg, 1954; Perry et al., 1970).
Oral administration of cadmium compounds induces epithelial
desquamation and necrosis of the gastric and intestinal mucosa,
together with dystrophic changes of the liver, heart, and kidneys
(Tarasenko et al., 1974; Vorobjeva & Sabalina, 1975).
7.2 Repeated and/or long-term exposure
7.2.1 Effects on the kidneys
Since the kidney is the critical organ in humans exposed for
long periods to relatively small amounts of cadmium (section 8.2.1),
results from relevant animal studies will be dealt with in some
detail. Even though it is difficult to extrapolate quantitative
information from the findings in animals, experiments have provided
valuable information concerning mechanisms of cadmium-induced
nephropathy and the significance of various biological indicators of
exposure and effect, and have supported the findings in humans. For
example, Friberg (1950) verified in animal experiments that exposure
to cadmium caused a type of proteinuria similar to the one he had
found in exposed workers.
Animal studies that have given data on renal effects as well as
the corresponding renal cadmium concentrations are summarized in
Table 12. An evaluation of organ dose-effect and dose-response
relationships is included in section 7.2.1.4.
7.2.1.1 Oral route
Renal lesions were first reported by Prodan (1932) and Wilson
et al. (1941) after cats and rats were given large oral doses of
cadmium for several months. Prodan (1932) reported varying degrees
of desquamation in proximal tubular epithelium (and no changes in
the glomeruli) after feeding cats 100 mg cadmium per day for one
month. Wilson et al. (1941) reported slight tubular changes in rats
after they were exposed for 3 months to a diet containing 62 mg
cadmium/kg.
Studies utilizing high exposures have also been performed by
Stowe et al. (1972). Ten rabbits received cadmium in drinking-water
(160 mg/litre) for 6 months. Kidney function was not investigated,
but histopathological examination revealed pronounced morphological
changes in the proximal tubules. The mean renal concentration of
cadmium was 170 mg/kg wet weight, which would correspond to about
210 mg/kg wet weight in the renal cortex (section 6.4). Still higher
doses (300 mg/kg diet) were given to rabbits for 54 weeks by
Nomiyama et al. (1975), who observed aminoaciduria and enzymuria
after 16 weeks. At this stage, the cadmium concentration in the
renal cortex was 200 mg/kg wet weight. Proteinuria and glycosuria
appeared at a later stage, 37 and 42 weeks, respectively, after
exposure had started. The cadmium concentration in the renal cortex
was 300 mg/kg wet weight after 40 weeks.
Table 12. Summary of animal studies with data on both renal cadmium levels and effects
Species Route of Exposure Duration Average cadmium Renal changes Reference
administration level (months) level in kidney
cortex (mg/kg
wet weight)
Mouse subcutaneous 0.25 mg/kg 6 110-170a no effects Nordberg & Piscator (1972)
body weight
Mouse subcutaneous 0.5 mg/kg 6 170a tubular protein patterns Nordberg & Piscator (1972)
body weight in urine
Rat intraperitoneal 0.75 mg/kg 3 200a no effects Bonnell et al. (1960)
body weight
Rat intraperitoneal 0.75 mg/kg 4 300a histological changes in Bonnell et al. (1960)
body weight 60% of animals
Rat subcutaneous 0.65 mg/kg 3 200 histological changes Goyer et al. (1984)
body weight
Rat water 10 mg/litre 8.5 11a no histological changes Kawai et al. (1976)
Rat water 50 mg/litre 8.5 35a slight histological changes Kawai et al. (1976)
Rat water 100 mg/litre 8.5 90a histological changes Kawai et al. (1976)
Rat water 200 mg/litre 8.5 145a histological changes Kawai et al. (1976)
Rat water 200 mg/litre 11 200 total proteinuria and low Bernard et al. (1981)
molecular weight proteinuria
Rat water 50 mg/litre 3 100a decreased insulin and PAH Kawamura et al.(1978)
clearance; histological
changes
Table 12 (contd).
Species Route of Exposure Duration Average cadmium Renal changes Reference
administration level (months) level in kidney
cortex (mg/kg
wet weight)
Rat water 50 mg/litre 2.5 235 slight histological changes Axelsson & Piscator (1966a);
in proximal tubules Axelsson et al. (1968)
Rabbit subcutaneous 0.25 mg/kg 2.5 235 slight histological changes Axelsson & Piscator (1966a);
body weight in proximal tubules Axelsson et al. (1968)
Rabbit subcutaneous 0.25 mg/kg 4 460 more severe histological Axelsson & Piscator (1966a);
body weight changes; reduction of alkaline Axelsson et al. (1968)
phosphatase activity in renal
cortex; total proteinuria
Rabbit subcutaneous 0.5 mg/kg 2.5 300 total proteinuria Nomiyama et al. (1982b)
body weight
Rabbit subcutaneous 0.5 mg/kg 0.7 200 proteinuria, glucosuria, and Nomiyama & Nomiyama
body weight aminoaciduria; decrease in (1982)
CIN and TmPAH
Rabbit subcutaneous 0.5 mg/kg 1 120 ß2-microglobulin Nomiyama et al. (1982b)
body weight
Rabbit subcutaneous 1.5 mg/kg 1 50-200 decreased tubular Nomiyama (1973a);
body weight readsorption Nomiyamaet al. (1978a)
Rabbit subcutaneous 0.5 mg/kg 2 160a slight histological changes Kawai et al. (1976)
body weight
Rabbit water 160 mg/litre 6 170a extensive fibrosis; pronounced Stowe et al. (1972)
changes
Table 12 (contd).
Species Route of Exposure Duration Average cadmium Renal changes Reference
administration level (months) level in kidney
cortex (mg/kg
wet weight)
Rabbit water 50 mg/litre 10 58 slight tubular atrophy Kawai et al. (1976)
Rabbit water 200 mg/litre 10 200 severe interstitial and Kawai et al. (1976)
tubular fibrosis
Rabbit diet 300 mg/kg 4 200 aminoaciduria, enzymuria Nomiyama et al. (1975)
Rabbit diet 300 mg/kg 10 300 proteinuria, glucosuria Nomiyama et al. (1975)
Pig diet 50-350 mg/kg < 12a equimolar increase in zinc Cousins et al. (1973)
in kidney
Pig diet 50-350 mg/kg 78a decrease in renal leucine Cousins et al. (1973)
aminopeptidase
Horse diet no cadmium lifelong 75 renal tubular interstitial Elinder et al. (1981a,b)
added (up to 240) changes and fibrosis in
25% of animals
Bird subcutaneous 0.16 mg/kg 1.5 20b histological changes Nicholson & Osborn (1983)
body weight
a These values are whole kidney concentrations; about 0.8 times kidney cortex values, on average.
b Denotes concentrations (wet weight) calculated as 0.2 times dry weight concentrations.
Morphological changes of the renal tubules were reported by
Kawai et al. (1976) in rats given 50 mg cadmium/litre drinking-water
for 8.5 months. The average renal cadmium concentration was about
38 mg/kg wet weight which corresponds to about 50 mg/kg in the renal
cortex.
Histological lesions in the proximal renal tubules were also
found in rats exposed to 200 mg cadmium/litre for 2 months (Itokawa
et al., 1978). Histochemical examination of the kidney showed that
the proximal tubular epithelium had particularly high cadmium
concentrations. The average renal concentrations were 48 mg/kg and
80 mg/kg, respectively, in rats with sufficient and deficient
calcium intakes. These levels would correspond to about 60 and
100 mg cadmium/kg in the renal cortex (section 6.4). Inulin
clearance was reduced to about a third of the control values in the
cadmium-exposed groups, indicating considerable functional damage to
the glomeruli. The only reported change in renal tubular function
was that the fractional excretion of calcium was increased about 50%
in the cadmium-exposed groups.
In a study of 50 rats exposed to 200 mg cadmium/litre in
drinking-water for up to 11 months (Bernard et al., 1981), there was
an increased prevalence of total proteinuria in the 8th month, when
the average cadmium concentration in the renal cortex was about
200 mg/kg.
Kajikawa et al. (1981) also reported morphological changes in
the kidneys of rats given drinking-water containing 200 mg cadmium
chloride/litre for 91 weeks. Histologically, they found degenerative
changes in the proximal convoluted tubules and, using electron
microscopy, proliferation of smooth endoplasmic reticulum,
vacuolization, and coagulative necrosis of the tubular cells. No
significant changes were observed in the glomeruli or interstitial
tissue.
When Cousins et al. (1973) gave large amounts of cadmium
chloride to pigs (50, 150, 450, and 1350 mg/kg diet), there was a
decrease in the activity of leucine aminopeptidase in the kidney
cortex at a renal cadmium concentration of 78 mg/kg wet weight,
corresponding to a renal cortex concentration of about 100 mg/kg wet
weight (section 6.4).
An extensive data base on the renal effects of cadmium in
monkeys has been developed in Japan. Several of these studies are
summarized in Table 13.
Study I (Nomiyama et al., 1979) was carried out using ten male
rhesus monkeys (three years of age). The monkeys were given 100 g of
solid feed containing 0, 3, 30, or 300 mg cadmium/kg daily for 37
weeks, followed by 130 g of feed for 18 weeks. Even the solid feed
given to the control group contained cadmium at a concentration of
0.13 mg/kg.
In study II, Nomiyama et al. (1987) used 36 male rhesus monkeys
(three years of age) and gave them 100 g of solid feed containing 0,
3, 10, 30, or 100 mg cadmium/kg daily for 52 weeks. During the
following 52 weeks 150 g was given, and then for the remaining 358
weeks 200 g was given. The solid feed given to the control group
contained cadmium at 0.27 mg/kg and zinc at 30 mg/kg.
Study III (Nomura et al., 1988) was performed with 40 female
rhesus monkeys given 150 g of solid feed for nine years (Table 14).
In study IV, Nomiyama & Nomiyama (1988) used nine male
crab-eating monkeys. Two of the animals were used as controls, and
three were given a diet containing cadmium concentrations of 3 mg/kg
(190 µg/day) as cadmium chloride (the pelleted food also contained
(30 mg zinc/kg)). The remaining four were fed 80 µg cadmium/day in
the form of contaminated rice.
In studies I and II, monkeys that had been given feed
containing cadmium at 300 mg/kg and 100 mg/kg showed indi-cations of
renal dysfunction, such as proteinuria, glucosuria, and
aminoaciduria, after 15-16 and 48-91 weeks, respectively. The
appearance of increased ß2-microglobulin was delayed until the
30th and 138th weeks, respectively. However, no definite disturbance
of proximal renal tubular function, such as reduced tubular
reabsorption of phosphorus, hypophosphataemia or acidosis, was noted
during the one-year follow-up. The dose-effect relationship for
renal dysfunction was similar to those which have been observed in
rabbits and rats, and thus the hypothesis that the susceptibility of
monkeys to cadmium may be exceptionally low was not corroborated.
In study II, the group of monkeys given feed containing
30 mg/kg developed urine findings (e.g., proteinuria, glucosuria,
aminoaciduria) indicative of renal dysfunction in the sixth year.
Postmortem examination revealed degeneration of the proximal renal
tubules, but there was no reduction in tubular reabsorption of
phosphorus. When the administration of cadmium was discontinued in
the fifth year, no abnormality of renal function developed during
the follow-up period of four years.
Table 13. Renal effects of cadmium in monkeysa
Study Duration Sex No. of Exposure level Average cadmium Renal effects (timing, Other effects (timing,
(weeks) monkeys (mg/kg diet) level in renal in weeks, of effects) in weeks, of effects)
cortex (mg/kg)
I 55 male 2 0 163
2 3 202 no biological effects no biological effects
3 30 596 no biological effects no biological effects
3 300 380b renal dysfunction (15-16) hepatic dysfunction (12-54)
757b ß2-microglobulinuria (30) slight anaemia (20)
II 462 male 6 0 328
8 3 700 no biological effects no biological effects
8 10 1070 no biological effects erythrocytopenia (360)
8 30 1170b renal dysfunction (300-306) erythrocytopenia (240)
ß2-microglobulinuria (311)
6 100 635b renal dysfunction (48-91) erythrocytopenia (120)
ß2-microglobulinuria (138) depressed age-related increase
in blood pressure (80)
III See Table 14.
IV 308 male 2 0 18
3 3 570 no biological effects no biological effects
4 contaminated rice 230 no biological effects no biological effects
(1.33 mg/kg)
a From: Nomiyama et al. (1979), Nomiyama et al. (1987), Nomura et al. (1988), Nomiyama & Nomiyama (1988)
b The numbers with asterisks are the critical concentrations of cadmium in the renal cortex.
Table 14. Bone and renal effects of cadmium in female monkeysa
Group No. of Exposure Low protein, Low vitamin D Average renal Renal effects Bone effectsd
monkeys level calcium and dietc cortex cadmium
(mg/kg) phosphorus dietb level (mg/kg)
1 5 0 - - 58 no biological effects no biological effects
2 4 0 + - no biological effects slightly disturbed calcification
(after 154 weeks)
3 4 0 - + no biological effects low plasma vitamin D3
4 4 0 + + no biological effects osteomalacic change (after 77
weeks) reversible by vitamin D3
5 5 30e - - 1511 no biological effects no biological effects
6 4 30e + - ß2-microglobulinuriaf disturbed calcification (after
(2000 to 12 000 µg/day, 154 weeks)
67%)
7 4 30e - + no biological effects low plasma vitamin D3
8 10 30e + + ß2-microglobulinuriag osteomalacic change (after 77
(up to 2000 µg/day) weeks) reversible by vitamin D3
a From: Nomura et al. (1988); duration of experiment was 463 weeks (9 years)
b 14% protein instead of 20%; 0.3% calcium instead of 0.9%; 0.3% phosphorus instead of 0.9%
c No vitamin D3 was added (240 IU was added to the normal diet)
d In Group 5 to 8, as depressed age-related increase in blood pressure was seen after 103 weeks of treatment.
e 3 mg/kg for the first 52 weeks
f Non-progressive lesion, reversibility uncertain; renal effect noted after 193 weeks
g Reversible by normal diet and vitamin D3 treatment; renal effect noted after 154 weeks
The monkeys in study III (Table 14) given the low nutrition
feed plus 30 mg cadmium/kg developed renal function abnormalities
after the fourth year. In addition to reduced phenolsulfonphthalein
(PSP) clearance and a variable increase in urinary
ß2-microglobulin concentration, many of the monkeys showed mild
degenerative changes of the proximal tubular epithelia, but there
was no decrease in the tubular reabsorption of phosphorus. However,
elevated urinary ß2-microglobulin did not progress further with
continued administration of cadmium. Mild degenerative changes of
the proximal tubular epithelia were also noted in the groups of
monkeys that had been given a normal diet, low vitamin D diet or low
nutrition plus low vitamin D diet, each supplemented with 30 mg
cadmium/kg. However, the elevated urinary ß2-microglobulin level
soon returned to normal in those animals fed a normal diet,
regardless of continued cadmium administration. This may indicate
that the elevated urine ß2-microglobulin in this group was not
caused solely by cadmium exposure.
In study II, some of the monkeys given feed containing 3 mg/kg
or 10 mg/kg of cadmium showed cadmium concentrations in the renal
cortex as high as 760 mg/kg and 1070 mg/kg, respectively. However,
no effect upon renal function was observed during the nine-year
period, nor was there any increase in urinary ß2-microglobulin
concentration.
The above data suggest that mild renal dysfunction
(proteinuria, glucosuria, and aminoaciduria, but no decrease in the
tubular reabsorption of phosphorus) was produced in monkeys exposed
to high concentrations of cadmium (30 mg/kg diet or more). It seems,
however, that no effects on renal function occur with low-level
exposure (10 mg/kg or less). The development of renal dysfunction is
assumed to depend upon the amount of cadmium absorbed per day rather
than the total amount absorbed in the body.
In study IV, the urinary cadmium level occasionally exceeded
10 µg/litre, but no clinical chemistry changes were reported.
Cadmium concentrations in the renal cortex increased proportionally
to the dose level and duration of exposure, reaching an average of
450 mg/kg in the group given cadmium chloride and 290 mg/kg in the
group fed contaminated rice. This suggests that the chemical form of
cadmium does not affect the severity of health effects.
7.2.1.2 Respiratory route
Princi & Geever (1950) could find no evidence of renal
morphological changes in the kidney of dogs after prolonged
inhalation exposure (up to one year) to cadmium oxide or cadmium
sulfide dust (average concentration of 4 mg/m3). Routine analysis
was performed, but neither the methods used nor the results obtained
were described. Friberg (1950) exposed rabbits for about 8 months
(3 h per day, about 20 days per month) to cadmium oxide dust with an
average concentration of about 8 mg/m3 . After 4 months of
exposure, moderate proteinuria was detected by the trichloroacetic
acid test. Histological examination of the kidneys after 8 months
revealed interstitial infiltration of leucocytes in the majority of
the exposed rabbits; this was not found in the control group.
7.2.1.3 Injection route
Friberg (1950) detected proteinuria in rabbits given
subcutaneous injections of cadmium sulfate (0.65 mg cadmium/kg body
weight) 6 days per week. Electrophoretic analysis of urine proteins
revealed that the proteinuria differed from that caused by
injections of uranium salts. More recently, many studies utilizing
parenteral administration (with doses generally in the range of
0.25-1.5 mg/kg body weight), different routes of exposure
(subcutaneous and intraperitoneal), and a duration of 1-12 months
have been performed in mice, rats, and rabbits (Table 12). These
experiments have confirmed the nephrotoxic effects of cadmium.
When rabbits were exposed for 16 weeks by subcutaneous
injection of either 0.25 mg or 0.5 mg cadmium/kg body weight 3 times
a week, there was a significant increase in urinary
ß2-microglobulin excretion indicative of renal tubular dysfunction
in the high-dose group after 7 weeks. There was only a slight
increase in the serum ß2-microglobulin/creatinine ratio. Urinary
ß2-microglobulin levels were not related to serum
ß2-microglobulin levels (Piscator et al., 1981).
Rats dosed intraperitoneally, five days/week with 0.6 mg
cadmium/kg body weight, showed no abnormal effects after 5 or 6
weeks when renal cadmium levels reached about 100 mg/kg. However, in
renal tubular lining cells an increase in lysosomes, microbodies,
and smooth endoplasmic reticulum was noted. After 8 weeks renal
cadmium levels had reached about 200 mg/kg of tissue and tissue
necrosis was observed. The early changes (with a renal cadmium
concentration of up to 100 mg/kg) were considered to be adaptive and
possibly reversible, whereas morphological changes after 8 weeks
with a renal concentration of 200 mg/kg were considered to be
irreversible (Goyer et al., 1984).
Nomiyama et al. (1982a) found that non-metallothionein-bound
cadmium increased up to about 35 mg/kg tissue in parallel with total
cadmium. At that stage, the total cadmium concentration in the renal
cortex was in the range 200-800 mg/kg, the total dose of cadmium
having been approximately 1 g.
7.2.1.4 Pathogenesis of cadmium nephrotoxicity
Various hypotheses have been proposed to explain the
pathogenesis of cadmium nephrotoxicity, particularly the role of the
metal-binding protein metallothionein (see section 6.8). This
protein is inducible by a number of essential metals (Cherian &
Goyer, 1978) and may have as its primary function the intracellular
storage of zinc and copper (Panemangalore et al., 1983; Templeton et
al., 1985). It is also induced following exposure to cadmium. It is
now thought that metallothionein protects against cadmium toxicity
and that intracellular cadmium bound to metallothionein is nontoxic
(Nordberg, 1971; Goyer et al., 1989). There is considerable support
for this hypothesis. Pre-treatment of experimental animals with
small doses of cadmium prevents the acute toxic effects of a large
dose of cadmium (Nordberg et al., 1975). Parenteral administration
of cadmium-metallothionein causes acute tubular toxic effects in the
kidney (Nordberg, 1971; Nordberg et al., 1975; Cherian & Nordberg,
1983). By treatment of animals with repeated doses of cadmium,
metallothionein synthesis in the renal cortex can be induced. This
prevents against subsequent renal toxicity by parenteral
cadmium-metallothionein at dose levels that normally give rise to
renal damage (Jin et al., 1987a). Rat renal cortical cells isolated
from animals pretreated with cadmium were resistant to normally
toxic concentrations of cadmium in vitro (Jin et al., 1987b).
Similar protective effects were observed in kidney cells pretreated
with cadmium in vitro (Jin et al., 1987b). Human cells in tissue
culture, where metallothionein has been induced by pre-treatment
with cadmium, become resistant to previously lethal exposure to
cadmium (Glennas & Rugstad, 1984).
With this evidence for the protective role of intracellular
metallothionein, several theories have been proposed to explain the
nephrotoxicity of cadmium. One hypothesis attributes the
nephro-toxicity to that fraction of intracellular cadmium not bound
to metallothionein (Nordberg et al., 1975; Nomiyama & Nomiyama,
1982; Squibb et al., 1984). Another hypothesis is that
extra-cellular cadmium bound to metallothionein is toxic (Cherian et
al., 1976). Cadmium-metallothionein derived from cadmium-induced
synthesis in reticulocytes (Tanaka et al., 1985) or released from
liver cells is filtered by the renal glomeruli and reabsorbed by the
proximal tubular lining cells where it is catabolized, releasing
cadmium ions that cause renal damage (Dudley et al., 1985). This
hypothesis is supported by the fact that parenterally administered
cadmium-metallothionein is very toxic to renal tubular cells and
that the plasma metallothionein level increases with cadmium
exposure (Goyer et al., 1984; Shaikh & Hirayama, 1979).
Still another hypothesis is that intracellular cadmium
interacts with cell membranes resulting in lipid peroxidation
(Stacey et al., 1980) and that cadmium may displace essential metals
from metallothionein (Petering et al., 1984), thereby depriving
important metalloenzymes of essential metal cofactors.
These hypotheses are not mutually exclusive and the relative
significance of each of these mechanisms may differ under particular
circumstances of exposure.
Goering et al. (1985) reported the development of calcuria in
rats injected with cadmium-metallothionein, using the model
described by Squibb et al. (1984). Jin et al. (1987a) confirmed
their observations, which suggest that this biological effect may be
an early event in the development of renal tubular damage. Data from
the above studies further validate the use of the
cadmium-metallothionein injection model for studying the mechanisms
of cadmium-induced tubular injury, since calcuria is also observed
in people with chronic elevated cadmium exposure.
7.2.1.5 General features of renal effects; dose-effect and
dose-response relationships
The available data show that long-term exposure to cadmium
leads to renal tubular lesions with proteinuria, glucosuria, and
aminoaciduria, and to histopathological changes (Table 12).
It has been reported that cadmium-induced proteinuria differs
from glomerular proteinuria (Friberg, 1950) and involves low
molecular weight proteins in particular (Axelsson & Piscator, 1966a;
Nomiyama et al., 1982b). Thus, this type of proteinuria resembles
the "tubular proteinuria" seen in humans. Microscopic examination
reveals typical tubular nephropathy, i.e. atrophy and degeneration
of tubular cells, especially proximal tubular cells, and
interstitial fibrosis (Bonnell et al., 1960; Axelsson et al., 1968;
Kawai et al., 1976).
Electron microscopic changes are characterized by interstitial
fibrosis and thickening of the basement membrane of the proximal
tubular cells (Kawai et al., 1976). The smooth endoplasmic reticulum
is dilated or undergoes proliferation, and there is apical cyst
formation (Stowe et al., 1972). An increase in the number of
lysosomes and swelling of the mitochondria have also been observed.
In addition to tubular findings, there have been reports of
pathological changes in 30% of the mesangium cells of the glomeruli
of dogs (Murase et al., 1974) and increased thickness of the
glomerular basement membrane in rats (Scott et al., 1977).
Investigations into renal function have also revealed
substantial changes, mainly in tubular function, e.g., reabsorption
of glucose (Axelsson & Piscator, 1966a), whereas the changes in
glomerular filtration are relatively small (Axelsson & Piscator,
1966a). Effects have generally been seen at average renal cortex
concentrations of 200-300 mg/kg wet weight, but some studies have
reported effects at considerably lower concentrations.
Histopathological changes in rats, rabbits, horses, and birds have
been reported at renal cortex concentrations below 100 mg/kg (Table
12). However, in chronically exposed monkeys, signs of renal tubular
changes were reported at around 400-1200 mg cadmium/kg (Table 13).
After renal cortex concentrations of cadmium have reached a
level of 200-300 mg/kg wet weight, they level off or decrease. No
further increase is seen even with continued exposure (Axelsson &
Piscator, 1966a; Nomiyama & Nomiyama, 1976a; Bernard et al., 1981).
It has also been shown (Friberg, 1952; Axelsson & Piscator, 1966a;
Nordberg & Piscator, 1972; Nomiyama & Nomiyama, 1976a) that urinary
excretion of cadmium is low during the initial exposure period but a
marked increase in the excretion of cadmium occurs subsequently,
which coincides with an increase in protein excretion (Friberg,
1952; Axelsson & Piscator, 1966a; Nordberg & Piscator, 1972)
(section 6).
Animal studies have shown that, as the cadmium concentration in
the renal cortex increases, the first effects to appear are the
histopathological changes in the renal tubular cells. The low
molecular weight proteinuria and aminoaciduria develop at somewhat
higher cadmium concentrations in the renal cortex and, at even
higher concentrations, glucosuria, total proteinuria, and other
indications of damaged renal function develop. The diagnosis of
these effects depends greatly on the sensitivity of the method for
analysing the effect. For instance, a method for analysing low
molecular weight proteinuria that can accurately measure levels
considered normal (0.1 mg/litre or less) will be able to diagnose
proteinuria at an earlier stage than a method with a detection limit
of 7 mg/litre.
Most of the studies referred to in Table 12 included no data on
the prevalence of renal effects in the animals. The results were
given in a qualitative way, stating the average cadmium level in the
renal cortex at which effects were seen.
Some dose-response data are available from animal studies.
Bernard et al. (1981) produced proteinuria in rats exposed to
cadmium in drinking-water (200 mg/litre) for up to 11 months. After
8-9 months, a significant increase in group-average proteinuria was
seen, coinciding with a 25% prevalence of increased individual
proteinuria. The renal cortex cadmium concentration at that time was
about 200 mg cadmium/kg (Bernard et al., 1981). Elinder et al.
(1981a) studied horses exposed to cadmium present in their normal
food. Histopathological changes in the renal cortex were classified
and coded in a blind manner, and the prevalence of different degrees
of change was calculated for subgroups of horses with different
renal cortex cadmium concentrations. The "background" prevalence was
25-30% and there was an increased prevalence (up to 60-75%) with
increased average renal cadmium level. At a renal cortex cadmium
concentration of about 75 mg/kg, there was a significant increase in
the prevalence of histopathological changes.
7.2.2 Effects on the liver
Friberg (1950) demonstrated fibrotic changes in the liver of
rabbits exposed to repeated subcutaneous injections of cadmium.
Periportal and interlobular collagen deposition was found in the
liver of rabbits given 160 mg cadmium/litre in drinking-water for 6
months (Stowe et al., 1972). Liver function tests, however, remained
within normal limits. The cadmium concentration in the liver was
188 mg/kg wet weight. Tarasenko et al. (1974) demonstrated by
histological techniques that dystrophic changes occur in the liver
of rats after repeated intragastric administration of cadmium
caprylate in a total dose corresponding to 47 mg cadmium/kg body
weight per day. These authors also noted an increased level of
lactic acid in the blood serum of the animals. Larionova et al.
(1974) detected decreased activity of alanine transaminase in liver
tissue and depletion of glycogen in rats given barium cadmium
laurate by gavage for 8-10 days at a dosage of 169 mg/kg body weight
per day (as the laurate). After intraperitoneal injection of cadmium
(up to 1.25 mg/kg body weight for periods of up to 6 weeks),
decreased glycogen content and increased daily activity of
gluconeogenic enzymes in rat liver were reported by Merali et al.
(1974) and Chapatwala et al. (1982).
In long-term studies, rabbits given 300 mg cadmium/kg diet for
54 weeks (Kawai et al. 1976) showed some amyloid deposition in the
liver. Studies on rats after exposure for 335 days to 1 mg
cadmium/litre in drinking-water (Sporn et al., 1970) revealed
changes in liver enzyme activities. Rhesus monkeys exposed to 300 mg
cadmium/kg in the diet for 12 weeks (Nomiyama et al., 1979)
developed increased levels of plasma enzymes (GOT, GPT, and LDH).
7.2.3 Effects on the respiratory system
Interstitial pneumonitis and emphysema were found in rabbits
exposed to cadmium iron oxide dust (approximately 8 mg/m3) for 4-8
months (Friberg, 1950) and in rats observed for 4-7 months after a
single intratracheal administration of cadmium iron oxide dust
(3.5 mg/kg body weight) (Vorobjeva, 1957). However, only very slight
pulmonary effects were detected in rabbits and rats exposed to
nickel-graphite dust at dose levels several times higher than the
concentrations of cadmium iron oxide dust.
Yoshikawa et al. (1975) exposed rats to cadmium oxide fumes
(0.1 or 1.0 mg cadmium/m3) for up to 3 months. There were 10 rats
in each group, and three of the rats in the high exposure group died
after about 7 weeks. Lung fibrosis and the first stage of emphysema
were observed at the end of the experiment in the high-dose group.
Free macrophage cells in the alveoli were more numerous in both
groups, and there was an increased surface tension of the
surfactants.
Snider et al. (1973) observed signs of emphysema in rats 10
days after 5-15 daily 1-h periods of exposure to cadmium chloride
aerosol (10 mg/m3). Also, long-term exposure to comparatively low
air concentrations of cadmium oxide (24-50 µg cadmium/m3) gave
rise to pathological changes in the lungs similar to emphysema as
well as to cell proliferation in the bronchi (Prigge, 1978). A
long-term study (14 months), in which mice and golden hamsters were
exposed to different concentrations of cadmium chloride (30 and
90 µg/m3), cadmium sulfate (30 and 90 µg/m3), cadmium sulfide
aerosols (90-100 µg/m3), cadmium oxide fume (10-90 µg/m3), and
dust (10-270 µg/m3), revealed a significantly increased incidence
of alveolar hyperplasia and interstitial fibrosis in most of the
exposed groups (Heinrich et al., 1989).
Single intratracheal administration of several cadmium
compounds (e.g., oxide, sulfide, carbonate, sulfoselenide,
caprylate, stearate, cadmium-barium laurate, and cadmium-barium
stearate) in doses from 0.5 mg (as the oxide) to 15 mg (as the
sulfide) caused the development, over 6 months, of chronic
inflammatory changes, emphysema, and atelectasis leading to
fibrosis. Exposure to cadmium oxide and caprylate gave rise to the
development of nodules of hyaline connective tissue; these resembled
silicotic nodules (Vorobjeva & Sabalina, 1975).
7.2.4 Effects on bones and calcium metabolism
Male rats fed a normal diet and exposed to cadmium sulfate by
inhalation (3 and 0.3 mg/m3, 4 h daily for 4 months) showed
decreased serum and urinary calcium concentrations compare to
controls. Female rats similarly exposed to cadmium sulfate
(2.8 mg/m3, 3 h/week) during pregnancy showed radiological
evidence of osteoporosis in addition to hypocalcaemia (Tarasenko et
al., 1975).
In a study by Kogan et al. (1972), cadmium chloride and cadmium
sulfate were administered subcutaneously to rats at a daily dose of
1 mg/kg body weight for up to 12 months. At 12 months, X-ray
analysis of the bones indicated osteoporosis and osteosclerosis.
Subsequent histopathology showed an increase in osteoclasts and a
bone structure described by the authors to be indicative of
osteomalacia.
Oral administration of cadmium chloride in drinking-water to
male rats (1 or 4 µg/kg body weight daily for six months) resulted
in changes in calcium metabolism and bone structure characteristic
of osteomalacia, which were not observed in the control group. No
effects were noted in a group of animals given a daily dose of
0.01 µg/kg body weight (Likutova & Belova, 1987).
Rats given 50 mg cadmium/litre in drinking-water for about 9
months showed reduced calcium and phosphorus absorption from the
intestine (Sugawara & Sugawara, 1974). In addition, some of the
animals showed histological changes in the duodenal mucosa, a
finding also reported in Japanese quail (Richardson & Fox, 1974).
Several mineral balance studies have been made on animals fed
cadmium. Simultaneous administration of cadmium with a low-protein,
low-calcium diet led to a decrease in the calcium and zinc content
of bone (Itokawa et al., 1973). Furthermore, Kobayashi (1974)
reported that cadmium feeding led to a negative calcium balance in
rats.
The decreased calcium absorption and negative calcium balance
in cadmium-exposed rats could result from the inhibitory effects of
cadmium on the activation of vitamin D in renal cortical cells
(Feldman & Cousins, 1973). The renal conversion of
25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol has been
found to be inhibited by high dietary cadmium exposure in rats fed a
normal calcium diet, but this effect was not seen on a low-calcium
diet (Lorentzon & Larsson, 1977). The metabolically active form of
vitamin D (1,25-dihydroxycholecalciferol) is necessary for the
normal absorption of calcium from the intestine. Ando et al. (1981)
found that the stimulation of calcium absorption by 1-alpha-hydroxy
vitamin D3 was inhibited in rats exposed to cadmium by gastric
intubation. Furthermore, the concentration of calcium-binding
protein in intestinal mucosa may be decreased by cadmium exposure
(Fullmer et al., 1980).
Administration of drinking-water containing 10 mg cadmium per
litre to rats fed a normal diet over a 9-month period gave rise to
decalcification and cortical atrophy in the skeleton (Kawai et al.,
1976). Other workers have also reported effects in the bones of rats
following several months exposure to cadmium in drinking-water
(Itokawa et al., 1974; Kawamura et al., 1978) and in the diet
(Takashima et al., 1980; Nogawa et al., 1981a), and following
subcutaneous injection (Nogawa et al., 1981a). In these studies, the
bones were reported to show more or less severe osteoporosis and
osteomalacia. On the other hand, Kajikawa et al. (1981) did not find
either osteoporosis or osteomalacia after rats were exposed for 2
years to cadmium in drinking-water (200 mg/litre).
Anderson & Danylchuk (1979) found that exposure of Beagle dogs
for six months to cadmium (25 mg/litre in drinking-water) reduced
bone turnover rate, a metabolic abnormality consistent with calcium
deficiency or osteomalacia. Kawashima et al. (1988) found that
feeding a cadmium-contaminated rice diet or a diet containing 3 mg
cadmium chloride/kg for six years to crab-eating monkeys did not
produce any change in vitamin D metabolism, and there was no
evidence of renal dysfunction. In another series of experiments
rhesus monkeys were fed diets containing 3, 10, 30 or 100 mg
cadmium/kg for 9 years. Serum vitamin D metabolites and renal
production of vitamin D remained unchanged, but in animals fed 30 or
100 mg cadmium/kg of diet there was slight but not statistically
significant depression of renal 25-hydroxy vitamin D1-hydroxylase
activity. No skeletal abnormalities were found in any of these
animals.
Bhattacharyya et al. (1988) studied the effects of 0, 0.25, 5
or 50 mg cadmium/kg diets on female mice bred for six consecutive
42-day cycles of pregnancy and lactation and on non-pregnant
controls. The multiparous mice exposed to 50 mg/kg experienced
significant decreases in body weight (3-11%) and femur calcium
content (15-27%), and the femur calcium to dry weight ratios
decreased by 5-7%. These results were thought by the authors to
provide evidence that the combination of cadmium exposure and
multiparity has a synergic effect on bone metabolism.
In the Japanese monkey study III (section 7.2.1 and Table 14),
osteomalacic changes were found in the low-nutrition plus
low-vitamin-D diet group (group 4) after 77 weeks. These effects
were not further exacerbated by feeding cadmium (group 8). These
changes were found to be reversed by the administration of vitamin
D. Renal effects were found in group 8 after 154 weeks. Therefore,
the osteomalacia found in group 8 was diagnosed as not being renal
osteomalacia.
Most of the findings discussed above indicate a direct effect
of cadmium on bone mineralization, possibly related to calcium
deficiency, and an indirect effect on calcium absorption via vitamin
D hydroxylation, perhaps leading to osteomalacia. The direct effects
develop after long-term cadmium exposure, whereas the indirect
effect on vitamin D metabolism occurs only when renal damage is seen
in the animals. Osteomalacia only occurred in monkeys fed a diet low
in protein, phosphorus, calcium, and vitamin D. Cadmium
administration did not increase these effects.
7.2.5 Effects on haematopoiesis
Anaemia is a common finding in animals after both dietary
(Wilson et al., 1941) and parenteral (Friberg, 1950) exposure to
cadmium. After dietary exposure, decreased haemoglobin concentration
(Decker et al., 1958) and decreased haematocrit (PCV) (Prigge et
al., 1977) are among the early signs of cadmium toxicity.
Fox & Fry (1970) and Fox et al. (1971) reported that the
cadmium-induced anaemia could be prevented by simultaneous feeding
with iron or ascorbic acid. Decreased gastrointestinal absorption of
iron due to cadmium may be one mechanism for this anaemia.
After parenteral exposure, iron administration has a beneficial
effect on the anaemia (Friberg, 1955). Berlin & Friberg (1960)
showed that cadmium injections caused erythrocyte destruction, but
there was no indication of an interference with haemoglobin
production. Haemolytic anaemia in rabbits was also reported by
Axelsson & Piscator (1966b).
7.2.6 Effects on blood pressure and the cardiovascular system
Chronic oral administration of cadmium compounds to rats (Perry
& Erlanger, 1974) induced statistically significant elevation of
blood pressure. However, the systolic pressure changes were much
smaller than those previously reported by Schroeder & Vinton (1962)
and by Schroeder (1964). Furthermore, a different effect was
obtained with lower, as compared with higher, doses of cadmium. Rats
given 1, 2.5, or 5 mg cadmium/litre in drinking-water for one year
had significantly higher blood pressure values than controls. Six
months after the beginning of exposure, a statistically significant
increase in blood pressure was also observed in rats given 10 or
25 mg/litre, but the increase was not statistically significant
after one year of exposure. In rats receiving 50 mg/litre, a
statistically significant increase in systolic blood pressure was
observed after 12 months of exposure. As discussed in section 8.2.4,
these results may be of basic importance in evaluating data on the
possible effects of cadmium on the human cardiovascular system.
Several mechanisms have been postulated (Perry & Erlanger,
1974) to explain the effects of chronic cadmium exposure on the
cardiovascular system. Oral adminstration of cadmium doses that
induce hypertension (and also parenteral administration of cadmium)
was shown to increase circulatory renin activity (Perry & Erlanger,
1973). Injection of cadmium into the renal artery of dogs increased
sodium reabsorption by the exposed kidney (Vander, 1962), and
repeated intramuscular (Perry et al., 1971) or chronic oral
administration of cadmium (Lener & Musil, 1971) was reported to
increase sodium retention in the body. By morpho-metric methods,
Fowler et al. (1975) demonstrated effects on the renal blood vessels
of rats exposed to various concentrations of cadmium (up to
200 mg/litre in drinking-water) for several weeks. Significantly
smaller arteriolar diameters were found in the exposed animals than
in the controls.
Perry et al. (1977) studied the influence of exposure duration
(from 3 to 24 months) and cadmium dose levels in water (from 0.1 to
10 mg/litre) on the blood pressure of Long-Evans rats. A small
increase in blood pressure occurred even at the lowest exposure
level after 3 months exposure. The greatest increase in blood
pressure (3.2 kPa; 24 mmHg) occurred after 24 months exposure to
1 mg/litre, when the average renal cortex cadmium level was 12 mg/kg
wet weight. At higher dose levels, the blood pressure increase was
less and, at the highest dosage (10 mg/litre for 24 months), the
blood pressure did in fact decrease.
Perry et al. (1976) found hypertensive effects in
Sprague-Dawley as well as Long-Evans rats. Petering et al. (1979)
reported that male rats were more susceptible to these effects than
female rats after exposure via drinking-water, but Ohanian & Iwai
(1980) found the opposite for rats exposed parenterally. In several
studies (e.g., Kotsonis & Klaassen, 1977; Whanger, 1979; Fingerle et
al., 1982), there was no increase in blood pressure after various
cadmium doses were given via drinking-water. The type of diet
appears to be crucial for the development of hypertension (Whanger,
1979); it can usually only be produced in rats fed a rye-based diet
(Perry & Erlanger, 1982). Nishiyama et al. (1986) postulated that
cadmium exposure increases sodium and water retention, which are
important factors controlling the development of hypertension.
A detailed review of factors influencing the effects of cadmium
on the cardiovascular system was reported by the Task Group on Metal
Toxicity (1976), with particular reference to those factors that
might modify the dose-effect and dose-response relationships.
Rats exposed to cadmium (5 mg/litre) in drinking-water (Kopp et
al., 1980a,b, 1983) developed electrocardiographic and biochemical
changes in the myocardium, and impairment of the functional status
of the myocardium. These effects could be related to (i) decreased
high-energy phosphate storage in the myocardium, (ii) reduced
myocardial contractility, or (iii) diminished excitability of the
cardiac conduction system. Jamall & Sprowls (1987) found that rats
fed a diet supplemented with copper (50 mg/kg), selenium
(0.5 mg/kg), and cadmium (50 mg/kg) had marked reductions in heart
cytosolic glutathione peroxidase, superoxide desmutase, and
catalase. They suggested that heart mitochondria are the site of the
cadmium-induced biochemical lesion in the myocardium.
Reviews of all aspects of the cardiovascular effects of cadmium
on experimental animals have been reported by Perry & Kopp (1983)
and Jamall & Smith (1986).
7.2.7 Effects on reproductive organs
Cadmium-induced testicular necrosis (section 7.1.2.1) generally
results in permanent infertility (Barlow & Sullivan, 1982). Ramaya &
Pomerantzeva (1977) found markedly reduced testis weights 1, 3, and
6 months after mice were administered 4 mg cadmium/kg. The animals
were sterile and microscopic examination revealed morphological
changes in the testis. Krasovskii et al. (1976) noticed decreased
spermatozoa motility and spermatogenesis index in rats continuously
exposed via food to 0.5-5.0 mg cadmium/kg body weight. In male mice
exposed repeatedly by daily subcutaneous injection of cadmium
chloride (0.5 mg/kg per day) for 6 months (Nordberg, 1975), there
was a decrease in normal testosterone-dependent proteinuria.
Morphological examination of the seminal vesicles revealed a smaller
weight and size as well as histological indications of lower
secretory activity, this being consistent with decreased
testosterone activity in these animals.
7.2.8 Other effects
Effects on the immune system have been reported after both
chronic and acute cadmium exposure. A decrease in the number of
antibody-forming cells in the spleen as well as a decrease in
antibody production was seen in mice after long-term exposure to
cadmium in drinking-water (Koller et al., 1975). An inhibition of
the cell-mediated immune response occurred in mice after repeated
intraperitoneal injections (Bozelka & Burkholder, 1982). However, no
data are available linking these effects to increased susceptibility
to infection or other secondary dysfunctions.
Gestational exposure to cadmium (4.2 and 8.4 µg/ml in
drinking-water) results in decreased birth weight, retarded growth,
delayed development of the sensory motor coordination reflexes, and
increased motor activity. Cadmium exposure during critical periods
of development might result in developmental and behavioural
deficits with long-term implications for adult behaviour (Mohd et
al., 1986).
7.3 Fetal toxicity and teratogenicity
In several species of laboratory rodents, large doses of
cadmium salts induce severe placental damage and fetal deaths when
given at a late stage of pregnancy, and teratogenic effects, such as
exencephaly, hydrocephaly, cleft lips and palate, microphthalmia,
micrognathia, clubfoot, and dysplastic tail, when given at early
stages of gestation.
A single subcutaneous injection of cadmium chloride, acetate,
or lactate (4.5 mg cadmium/kg body weight) given to Wistar rats from
the 17th to the 21st day of pregnancy (Parizek, 1964) led to the
rapid development of severe placental damage in all rats and to
fetal death. Placental damage was not dependent on the presence of
fetuses, but it was not possible to decide whether fetal lethality
resulted from the placental lesion or from a direct effect of
cadmium on the fetuses (Parizek, 1964). Similar effects were
observed at a dose level of 3.3 mg cadmium/kg body weight (Parizek
et al., 1968b). Placental damage and fetal deaths were also observed
after cadmium administration to pregnant Swiss albino mice
(Chiquoine, 1965).
Teratogenic effects can be observed when doses close to the
LD50 (Table 11) for cadmium salts are administered to pregnant
females at critical stages of embryogenesis. These effects were
demonstrated with intravenous injections of cadmium sulfate in
hamsters (Ferm & Carpenter, 1968; Mulvihill et al., 1970; Ferm,
1972) and with intraperitoneal (Barr, 1973), subcutaneous (Chernoff,
1973), or dietary (Scharpf et al., 1972) administration of cadmium
chloride in rats. Teratogenic effects induced by cadmium salts have
also been demonstrated in mice (Ishizu et al., 1973).
The character of the changes induced is dependent on the
species and on the stage of embryogenesis. As little as 123 µg/litre
in mouse embryo cultures produced exencephaly apparently by
re-opening the closed neural tube (Schmid et al., 1985). Either
facial defects or limb abnormalities were induced by cadmium when
administered to pregnant hamsters on day 8 or 9 of gestation (Ferm,
1971). Both jaw defects and cleft palate were observed in the
offspring of rats given daily subcutaneous cadmium chloride
injections (8 mg/kg body weight) on days 13-16 or 14-17 of
pregnancy, but cleft palate was not observed when this dosage was
given on days 15-18 or 16-19 of pregnancy (Chernoff, 1973).
Anophthalmia or microphthalmia and dysplastic ears were induced by
approximately 2 mg of cadmium as the chloride given
intra-peritoneally to pregnant rats on the 9th but not on the 11th
day of pregnancy (Barr, 1973). Other effects observed in these
studies included decreased lung weight in the offspring of rats
subjected to cadmium during pregnancy (Chernoff, 1973) and
deficiencies in bone formation and delays in bone ossification
(Mulvihill et al., 1970; Scharpf et al., 1972).
The dose-dependent fetal mortality and teratogenicity response
was established in studies with subcutaneous administration of
cadmium chloride to rats (Chernoff, 1973) and mice (Ishizu et al.,
1973). The no-observed-effect level with respect to malformations
was found in the latter study to be 0.33 mg/kg body weight.
All the teratogenic effects mentioned above were induced by
parenteral administration of very high doses of cadmium salts.
However, in a rat study by Scharpf et al. (1972), very high peroral
doses (20, 40, 60, or 80 mg/kg body weight given by gavage daily
from days 6 to 19 of pregnancy) of cadmium chloride were used with
the simultaneous administration of sodium chloride, and internal
teratological examinations were performed. Heart and kidney
abnormalities were the major internal defects, but their incidence
was not directly related to the dose of cadmium chloride
administered. At the lowest dose level, heart abnormalities were
detected in 19.7% of the 127 fetuses (abnormalities in the control
group were seen in 6.6% of 107 fetuses) and teratoma of the kidney
was observed in 15.7% of these fetuses.
Cvetkova (1970) exposed pregnant female rats via the
respiratory route to cadmium sulfate (2.8 mg/m3, 4 h daily) and,
on the 22nd day, killed half of them to examine the embryos. The
number of embryos in exposed rats was the same as in a control
group, but the mean weight was lower in the exposed group. In the
exposed rats, where pregnancies were allowed to proceed to full
term, the average weight of the offspring was lower than in the
controls both at birth and after 8 months. The rats born to the
exposed group also had increased mortality during the first 10 days
after birth.
When mice were exposed for several generations to cadmium in
drinking-water (10 mg/litre), fetal mortality, runting, and
malformations were observed (Schroeder & Mitchener, 1971). The
external malformations, consisting of sharp angulation of the distal
third of the tail, were observed in 16.1% of 255 offspring (F1 and
F2A generation), and 87 deaths before weaning (30.5%) were
recorded.
Ferm & Carpenter (1968) showed that zinc injected
simultaneously with cadmium could protect against the teratogenic
effects of cadmium, and a similar protective action was found for
selenium (Holmberg & Ferm, 1969). Maternal zinc deficiency can
produce congenital malformations (Hurley et al., 1971). This was
confirmed by Parzyck et al. (1978), who also found that
intraperitoneal injection of 1.5 mg cadmium/kg body weight to
pregnant rats increased the prevalence of malformations. The
increase was greater at this cadmium dose than the increase due to
zinc deficiency. Combined zinc deficiency and cadmium exposure
caused a very high incidence of fetal deaths.
Further experimental data on rats provided by Samarawickrama &
Webb (1979) indicate that maternal cadmium exposure gives rise to a
fetal zinc deficiency and that this is one cause of the teratogenic
effects observed. Intravenous cadmium injections to pregnant rats at
doses ranging from 0.25 to 1.25 mg/kg body weight on day 12 of
gestation produced a dose-related decrease in fetal uptake of a dose
of 65 mg zinc given 4 h later. Maternal cadmium exposure (1.25 mg/kg
body weight) was shown to result in decreased activity of a fetal
zinc-dependent enzyme thymidine kinase, which is responsible for the
incorporation of thymidine in DNA. Additional evidence that
cadmium-induced fetotoxicity is related to a cadmium-induced fetal
zinc deficiency was reported by Daston (1982), who found that
co-administration of zinc (12 mg/kg body weight) almost totally
eliminated severe fetal lung lesions when pregnant rats were given
cadmium (8 mg/kg body weight) on gestation days 12-15.
7.4 Mutagenicity
Studies on Drosophila (Ramel & Friberg, 1971; Vorobjeva &
Sabalina, 1975) failed to show any chromosomal abnormalities after
exposure to various cadmium compounds. Some in vitro studies of
cultured human lymphocytes and fibroblasts were also negative (Paton
& Allison, 1972; Deknudt & Deminatti, 1978; Kogan et al., 1978).
Shiraishi et al. (1972) reported a marked increase in the frequency
of chromatid breaks, translocations, and dicentric chromosomes in
leucocytes, from one person, cultured in a medium containing 62 mg
cadmium/litre (as the sulfate) for 4-8 h.
Andersen et al. (1983) found that the average chromosome length
in human lymphocytes was initially reduced when they were cultured
in a medium containing cadmium chloride (1.1 mg cadmium/litre), but
subsequently returned to normal. This effect was probably related to
the synthesis of metallothionein and complexing with cadmium.
Watanabe et al. (1979) observed aneuploidy in rat oocytes with
cadmium accumulation in the ovary after exposure to cadmium chloride
in vivo.
Rohr & Bauchinger (1976) found a reduced mitotic index in
hamster fibroblasts cultured in 100 µg cadmium/litre (as the
sulfate) and chromosome damage at concentrations above 500 µg
cadmium/litre. Deaven & Campbell (1980) showed that the effects on
cultured hamster cells depended on the type of medium used.
There appears to be an acute effect of cadmium following the
injection of 0.6-2.8 mg cadmium/kg body weight (Felten, 1979). After
6 h, there was an increased frequency of chromatid breaks in bone
marrow cells and chromosome gaps and breaks in spermatocytes, which
could be associated with the acute effects on haematopoiesis
(section 7.2.5) and on the testis (section 7.1.2.1).
A summary and graphical presentation of the available evidence
on genetic and related effects of cadmium in various in vivo and
in vitro test systems has been presented by IARC (1987a). Although
prokaryote test systems reveal no effects, variable results have
been observed in lower eukaryotes, mammalian cells in vitro, and
mammals in vivo.
7.5 Carcinogenicity
Intramuscular or subcutaneous administration of metallic
cadmium or cadmium compounds can induce sarcomata at the site of
injection. This local effect of cadmium was demonstrated with
intramuscular administration of metallic cadmium (cadmium powder in
fowl serum) to hooded rats (Heath et al., 1962), subcutaneous
administration to Chester Beatty rats of cadmium as the sulfide and
oxide (Kazantzis, 1963; Kazantzis & Hanbury, 1966) or sulfate
(Haddow et al., 1964), intramuscular injection of cadmium chloride
to Wistar rats (Gunn et al., 1967), and subcutaneous injection to
Sprague-Dawley rats (Nazari et al., 1967). Transplantability of
tumours induced in these studies (Heath & Webb, 1967) and metastases
into regional lymph nodes and into lungs (Kazantzis & Hanbury, 1966)
were reported. Intratesticular injection of cadmium chloride to
White Leghorn cockerels was reported to induce teratoma at the site
of injection (Guthrie, 1964).
After Hoffman et al. (1985) injected 1.9 mg cadmium chloride
(1.2 mg/kg body weight) directly into the ventral prostatic lobe of
100 12-month-old male rats, simple hyperplasia was found in 38 of
the rats, atypical hyperplasia in 29, atypical hyperplasia with
severe dysplasia in 11, and invasive prostatic cancer in 5 animals.
Hoffman et al. (1988) reported changes in the ultrastructure of
prostate epithelial cells in rats injected into the ventral prostate
with 2.2 or 3.3 mg cadmium/kg body weight. In animals given oral
treatment via the drinking-water (29.9 or 115 mg cadmium/kg body
weight), there were changes ranging in severity up to dysplasia but
no evidence of carcinoma.
A single parenteral administration of cadmium salts can induce
necrosis of the testis (see section 7.1.2.1). After one year, the
remnants of the necrotic testis were shown to contain masses of
cells showing the typical structure of Leydig cells (Parizek, 1960).
This regeneration of testicular Leydig cells damaged by cadmium can
result in Leydig cell neoplasia (Gunn et al., 1963, 1965; Lucis et
al., 1972). The ultrastructural features of cadmium-induced Leydig
cell tumours correspond in most respects with the fine structural
features of normal Leydig cells (Reddy et al., 1973).
Other injection studies and peroral studies did not demonstrate
increased malignancy (Schroeder et al., 1964, 1965; Loser, 1980),
but the doses were low compared to those necessary to induce renal
damage. In one peroral study (Kanisawa & Schroeder, 1969), rats were
exposed to 5 mg cadmium/litre in drinking-water for up to 2 years.
There were 7 malignant tumours among 47 cadmium-exposed male rats
and 2 tumours among 34 male control rats. This indicates a doubling
of the tumour rate, but because of the low statistical power of the
study, the increase was not statistically significant and the
authors concluded that ingestion of these cadmium doses was not
carcinogenic.
Some studies have been specially designed to investigate the
possible role of cadmium in cancer of the prostate. The prostate
gland, like the testis, is of particular interest with respect to
cadmium toxicity because these organs contain greater concentrations
of zinc than any other tissues and it has been suggested that
cadmium may affect prostate growth by competition with zinc (Gunn et
al., 1961). Levy et al. (1973) gave three groups of rats weekly
subcutaneous injections of cadmium sulfate at concentrations of
0.022, 0.044 or 0.087 mg cadmium per rat (average weight 220 g at
the start and 410 g at the end of the 2-year exposure). Weekly
injections of water were given to the 75 control rats. The liver
cadmium level was 80 mg/kg in the highest-dose group, but no
malignant changes were found in the prostate. No difference was seen
between exposed and control rats with respect to malignant changes
in other organs.
Levy & Clack (1975) and Levy et al. (1975) conducted 2-year
studies in rats and mice designed to detect carcinogenic effects in
the prostate. The animals in both experiments were given weekly
administrations of cadmium sulfate by stomach tube. The rats were
given from 0.08 to 0.35 mg/kg body weight and the mice 0.44 to
1.75 mg/kg. Extremely low levels of cadmium were found in the kidney
after 2 years (5 mg/kg wet weight in rats), but no macroscopic or
microscopic changes were seen in any tissue at these low doses.
In a long-term study on Fisher rats, Sanders & Mahaffey (1984)
administered cadmium oxide (25 µg) in single or repeated doses by
intratracheal instillations. There was no evidence for pulmonary or
prostate carcinogenicity, but increases in mammary tumours and in
tumours at multiple sites in male rats were reported.
It has been reported that inhalation of a cadmium aerosol
causes lung cancer in Wistar rats (Takenaka et al., 1983). Three
groups of 40 rats were continuously exposed to cadmium chloride
aerosols for 18 months, the air cadmium concentrations being 12.5,
25, and 50 µg/m3. A control group of 41 rats was also studied. The
study was terminated after 31 months, and no lung cancers were seen
in the control group. However, in the exposed groups, the incidence
was 15%, 53% and 71%, respectively, at increasing exposure levels.
Even at these relatively low exposure levels, there was a clear
dose-response relationship. Histologically the experimentally
induced tumours were adenocarcinomas, epidermoid carcinomas,
mucoepidermoid carcinomas, and combined epidermoid and
adenocarcinomas.
In a subsequent study, rats were exposed to inhalable aerosols
of cadmium sulfate and cadmium oxide and fume and dust at > 30 µg
cadmium/m3 and to cadmium sulfide at > 90 µg cadmium per m3 for
periods of up to 18 months. Bronchoalveolar benign and malignant
adenomas, squamous cell carcinomas, and combined forms developed at
high primary tumour rates with all four forms of cadmium tested even
after discontinuous exposure for 40 h/week for 6 months. No primary
tumour was found with cadmium oxide fume at a concentration of 10 µg
cadmium/m3 or cadmium oxide dust (at 30 µg cadmium/m3) when
combined with a zinc oxide aerosol (Oldiges et al., 1989).
In a further study, male and female Syrian golden hamsters and
female NMRT mice were exposed to cadmium chloride, sulfate, oxide,
and sulfide at concentrations of between 10 and 270 µg cadmium/m3.
The exposure was continuous (19 h/day, 5 days/week) for 50 to 70
weeks and was followed by a 50-week observation period. No increase
in the lung tumour rate was observed in either the mice or hamsters
(Heinrich et al., 1989), but in both species exposure to cadmium
caused multifocal bronchoalveolar hyperplasia, the extent of which
varied with the compound used, its concentration, and the length of
exposure. The most severe changes were found after cadmium oxide
inhalation (Aufderheide et al., 1990).
A synergistic effect has been shown in rat renal tumours
induced by dimethylnitrosamine when followed by cadmium chloride
given by intramuscular injection. In this study, cadmium appeared to
enhance the initiation of dimethylnitrosamine-induced cancer (Wade,
1987).
7.6 Host and dietary factors; interactions with other trace
elements
The toxic effects of cadmium in experimental animals have been
shown to be dependent on genetic factors, stage of ontogenic
development, functional state of the organism, and simultaneous or
previous exposure to certain environmental influences, including
exposure to certain nutrients.
Resistance to cadmium-induced testicular necrosis is determined
by a single autosomal recessive gene (cdm) in inbred mice (Taylor et
al., 1973). The teratogenic effects of cadmium are dependent on the
stage of embryogenesis (section 6.3). The stage of postnatal
development of certain organs may be of importance for the toxic
effects of cadmium, as has been shown for the testis (Parizek, 1957,
1960), ovaries (Kar et al., 1959), and central nervous system
(Gabbiani et al., 1967).
Pretreatment with small, non-toxic doses of cadmium salts has
been shown to induce resistance to testicular or lethal effects
(Terhaar et al., 1965; Ito & Sawauchi, 1966). The probable mechanism
is induction of metallothionein synthesis by the pretreatment. This
enables the subsequent dose of cadmium to be bound rapidly to
metallothionein, which renders it less acutely toxic (Nordberg,
1971). Similarly, the protective effect of zinc against cadmium
toxicity could also be, at least in part, dependent on the induction
of an increased synthesis of metallothionein-like proteins (Webb,
1972; Davies et al., 1973).
Selenium compounds are known to be highly effective in
preventing the reproductive toxic effects of cadmium (Kar et al.,
1960; Mason & Young, 1967; Parizek et al., 1968a,b), lethality to
rats (Parizek et al., 1968b) and mice (Gunn et al., 1968), and
teratogenicity (Holmberg & Ferm, 1969). Fetal lethality (Parizek et
al., 1968b) and teratogenic effects (Holmberg & Ferm, 1969) can be
prevented when selenium compounds are given at the same time as
cadmium.
Simultaneous administration of mercuric and cadmium compounds
has been shown to have an additive effect (Gale, 1973). Oral
administration of nitrilotriacetate with large oral doses of cadmium
chloride provided protection against the lethality of cadmium and
had no potentiating effect on the teratogenicity and fetal
accumulation of cadmium (Scharpf et al., 1972). This was confirmed
by Engström (1979), who also showed that simultaneous oral exposure
to cadmium and sodium tripolyphosphate decreased the mortality
expected at the cadmium level used. However, when nitrilotriacetate
or sodium tripolyphosphate was given subcutaneously with cadmium,
the mortality rates were increased (Engström & Nordberg, 1978;
Andersen et al., 1982). The chelation of cadmium in the
gastrointestinal tract decreased the uptake of cadmium, whereas
parenteral exposure to cadmium and chelating agents caused a higher
renal cadmium concentration than cadmium alone.
The interaction of cadmium with certain trace elements can
produce symptoms characteristic of trace element deficiencies. As a
result, chronic cadmium toxicity in certain animal species closely
resembles zinc and/or copper deficiency and can be prevented by
administering higher doses of the salts of these trace elements
(Petering et al., 1971, 1979; Mills & Delgarno, 1972). Cadmium-
calcium interactions are discussed in section 7.2.4.
An increased toxicity of cadmium was reported in animals on
low-protein diets (Fitzhugh & Meiller, 1941), this being due partly
to rapid intestinal absorption of cadmium (Suzuki et al., 1969).
Lack of dietary calcium seems to play a role similar to lack of
dietary protein in increasing the toxicity of cadmium (Suzuki et
al., 1969). Supplements of dietary ascorbic acid almost completely
prevented cadmium-induced anaemia and improved the growth rate (Fox
& Fry, 1970).
Ambient temperature (Nomiyama et al., 1978b) and the energy or
protein level in the diet have been reported to influence the LD50
of cadmium in mice.
Various aspects of the interactions between cadmium and other
trace elements were discussed in greater detail by the Task Group on
Metal Interactions (1978).
7.7 Conclusions
Inhalation exposure at high levels causes lethal pulmonary
oedema. Single high-dose injection gives rise to testicular and
non-ovulating ovarian necrosis, liver damage, and small vessel
injury. Large oral doses damage the gastric and intestinal mucosa.
Long-term inhalation exposure and intratracheal administration
give rise to chronic inflammatory changes in the lungs, fibrosis,
and appearances suggestive of emphysema. Long-term parenteral or
oral administration produces effects primarily on the kidneys, but
also on the liver and the haematopoietic, immune, skeletal, and
cardiovascular systems. Skeletal effects and hypertension have been
induced in certain species under defined conditions. Teratogenic
effects and placental damage occur, depending on the relation
between the exposure and the stage of gestation, and may involve
interactive effects with zinc.
Of greatest relevance to human exposure are the acute
inhalation effects on the lung and the chronic effects on the
kidney. Following long-term exposure, the kidney is regarded as the
critical organ. The effects on the kidney are characterized by
tubular dysfunction and cell damage, although glomerular dysfunction
may also occur. A consequence of renal tubular dysfunction is a
disturbance of calcium and vitamin D metabolism. According to some
studies, this has led to osteomalacia and/or osteoporosis, but these
effects have not been confirmed by other studies. A direct effect of
cadmium on bone mineralization cannot be excluded. The toxic effects
of cadmium in experimental animals are influenced by genetic and
nutritional factors, interactions with other metals, in particular
zinc, and pretreatment with cadmium, which may be related to the
induction of metallothionein.
IARC (1976, 1987b) accepted as sufficient the evidence that
cadmium chloride, sulfate, sulfide, and oxide can give rise to
injection-site sarcomata in the rat and that the chloride and
sulfate can induce interstitial cell tumours in the testis of rats
and mice, but found oral studies inadequate for evaluation. One
recent life-time study (18 months), in which rats were subjected to
continuous inhalation of a cadmium chloride aerosol at low
concentration, showed a high incidence of primary lung cancer with
evidence of a dose-response relationship. Studies on the genotoxic
effects of cadmium have given discordant results, most of the
positive results indicating chromosomal effects after short-term
high-level exposure.
8. EFFECTS ON HUMANS
Most of the available epidemiological studies or group
observations, as well as the clinical studies, have been performed
either on occupationally exposed workers or on Japanese populations
in cadmium-polluted areas. A great deal of epidemiological data has
resulted from studies in polluted areas of Japan (Cooperative
Research Committee on Itai-itai Disease, 1967; Shigematsu et al.,
1978; Japan Cadmium Research Committee, 1989) and, more recently,
from smaller studies in other countries (Drasch et al., 1985;
Philipp, 1985; Hahn et al., 1987; Roels et al., 1989; Thun et al.,
1989; Likutova, 1989). Comprehensive summaries of these studies have
also been published (Tsuchiya, 1978; Friberg et al., 1986; Nomiyama,
1986).
Many of these studies have focused on the detection of early
signs of kidney dysfunction. Others have investigated clinical signs
of disease such as renal stones and pulmonary impairment. Until the
middle of the 1970s, particular attention was given in Japan to the
detection of and screening for bone disease (e.g., Itai-itai
disease). More recently the role of cadmium in human carcinogenesis
and mortality has also been studied.
Exposure to cadmium produces a wide variety of effects
involving many organs and systems. From the point of view of
preventive medicine, the detection of early effects on the kidneys
is of particular importance in order to prevent more serious renal
effects and those on the lungs or bones. Recent studies indicating
that chronic exposure to cadmium may give rise to cancer will be
reviewed in some detail.
8.1 Acute Effects
8.1.1 Inhalation
Acute cadmium poisoning and, in some cases, death have been
reported among workers shortly after exposure to fumes when cadmium
metal or cadmium-containing materials have been heated to high
temperatures (Beton et al., 1966; Blejer, 1966; Dunphy, 1967). The
principal symptom in acute cases, both fatal and non-fatal, is
respiratory distress due to chemical pneumonitis and oedema
(MacFarland, 1979; Lucas et al., 1980). At an early stage, the
symptoms may be confused with those of "metal fume fever".
In working environments where cases of acute poisoning
occurred, cadmium concentrations were usually very high. For
instance, in one case the fatal air concentration of cadmium oxide
fume from a furnace was approximately 50 mg/m3 for a period of
about 1 h (a dose of 2900 mg/m3.min) (Barrett & Card, 1947). In
another case, the lethal dose was 2600 mg/m3.min (Beton et al.,
1966), i.e. a 5-h exposure to 8.6 mg/m3. Friberg et al. (1974)
estimated that an 8-h exposure to 5 mg cadmium/m3 may well be
lethal.
8.1.2 Ingestion
During the period 1940-50, cases of acute food poisoning
occurred mainly due to the substitution of cadmium for scarce
chromium in the plating of many cooking utensils and containers.
Food contamination arose when acid foods and drinks were prepared
and stored in contact with cadmium-plated surfaces. Rapid onset with
severe nausea, vomiting, and abdominal pain were characteristic
symptoms (US Public Health Service, 1942; Cole & Baer, l944; Lufkin
& Hodges, 1944). Effects also occurred following the consumption of
drinks with a cadmium concentration of approximately 16 mg/litre
from an automatic vending machine in which drinking-water was cooled
in a tank constructed with cadmium-containing solder (Nordberg et
al., 1973). Recovery from acute poisoning appears to be rapid and
complete. The amount of cadmium absorbed is probably very limited
due to vomiting and the consequential short presence of cadmium in
the gastrointestinal tract. However, no follow-up studies of people
who have experienced acute cadmium poisoning have been reported.
8.2 Chronic Effects
Lower cadmium concentrations with longer periods of exposure
than those described above will cause chronic cadmium poisoning.
Fully developed poisoning among industrial workers shows two main
effects: renal dysfunction and emphysema (Friberg, 1948a,b, 1950).
The kidney is most frequently the critical organ, but under certain
conditions (short-term peak exposures) it may be the lung (Bonnell,
1955). For people in the general environment, exposure is usually by
the oral route and the kidney is the critical organ.
8.2.1 Renal effects and low molecular weight proteinuria
8.2.1.1 In industry
Renal dysfunction is one of the characteristic signs of cadmium
poisoning, and many cadmium workers have developed proteinuria,
renal glucosuria, and aminoaciduria. In working environments with
high cadmium exposure levels, workers have also developed
hypercalciuria, phosphaturia, and polyuria (Friberg, 1950; Clarkson
& Kench, 1956; Kazantzis et al., 1963; Tsuchiya, 1967; Lauwerys et
al., 1974a, 1979b), and some have suffered from renal colic due to
recurrent stone formation (Friberg, 1950; Ahlmark et al., 1961;
Adams et al., 1969; Scott et al., 1976; Kanzantzis, 1979). The
polyuria is due to loss of urinary concentrating ability (Kazantzis,
1979), and, in addition, the kidneys of cadmium-poisoned workers
lose their ability to handle an acid load after a standard
NH4Cl-loading test. These are signs of distal tubular damage, and
in a few severe cases, the renal damage progresses to a reduction in
glomerular filtrations (see section 8.2.1.5).
Renal function, as measured by inulin or creatinine clearance
and urine concentrating capacity, was depressed in several poisoning
cases (Friberg, 1950; Bonnell, 1955; Bernard et al., 1979). Thus, in
the more advanced cases, there is a combination of tubular and
glomerular effects. In most of the early cases, only proteinuria,
mild in comparison with the proteinuria in many other renal
disorders, has been reported as a sign of renal dysfunction, and
other signs of kidney dysfunction were not evident (Piscator,
1966a,b).
Since Friberg first observed the urinary proteins of cadmium
workers (Friberg, 1950), the proteinuria has proved to involve
proteins with a molecular weight of 10 000 to 40 000 and is the
so-called tubular proteinuria (Butler & Flynn, 1958). Table 15
contains data on proteins in urine useful for the diagnosis of
cadmium-induced proteinuria. The increased excretion of low
molecular weight proteins in urine from cadmium-exposed workers has
been found to apply to ß2-microglobulin, lysozyme (muramidase),
ribonuclease, immunoglobin chains, retinol-binding protein, and
alpha1-microglobulin (Piscator, 1966a; Peterson et al., 1969;
Peterson & Berggård, 1971; Lauwerys et al., 1974a; Bernard et al.,
1976, 1982b). In groups of exposed and unexposed workers, the
urinary ß2-microglobulin concentrations follow log-normal
distributions, and an operational definition for what is an
"increased" level should be established for each population studied
(Kjellström et al., 1977a).
Proteinuria is known to be an early sign of cadmium poisoning,
but the degree of proteinuria varies with time. In a group of 40
workers with heavy exposure to cadmium, it was found that
proteinuria was persistent and even increased several years after
cessation of exposure, as evaluated by qualitative methods (Friberg
& Nystrom, 1952; Piscator, 1966a). Tsuchiya (1976) examined five
cadmium-exposed workers who showed proteinuria. Ten years after
cessation of exposure, three of them no longer revealed proteinuria,
but two of these showed a high urinary ß2-microglobulin level, as
did the two workers with persistent total proteinuria. Four workers
in a British pigment factory still had grossly elevated
ß2-microglobulin levels despite removal from exposure many years
earlier (Stewart & Hughes, 1980).
Table 15. Excretion of urinary proteins in healthy people and in cases of glomerular and tubular disorders
Protein type Normal plasma Normal filtered Urinary excretion (mg/24 h) Reference
concentration amount in primary Healthy Glomerular Tubular
(mg/ml) urine (mg/24 h) people disorders disorders
Total protein 43-127 310-54 100 129-1570 Peterson et al. (1969)
Albumin 50 500 3.9-24 88-48 800 13.8-578 Mogensen & Solling (1977)
Retinol-binding 0.11 20-150 Peterson & Beggard (1971)
protein
0.04 3 45 Kanai et al. (1971)
ß2-microglobulin 0.002 300 0.06-0.21 0.06-4.7 1.1-105 Peterson (1971)
0.073 Evrin et al. (1971)
Lysozyme 0-2a Prockop & Davidson (1964)
0.07-1.1a 47-130a Harrison et al. (1968)
Ribonuclease 0.24-1.5a 1.9-10a Harrison et al. (1968)
a Values reported as mg/litre
According to recent observations using quantitative proteinuria
methods (Roels et al., 1982), total proteinuria in 19 workers had
not changed 4 years after exposure ceased. In those 11 workers for
whom urinary ß2-microglobulin was measured before and after
cessation of exposure, an increase was invariably seen. Eight of the
workers had abnormal ß2-microglobulin levels before exposure
ceased, whereas three had normal levels before and developed
abnormal levels after cessation. It can be concluded that
cadmium-induced tubular proteinuria is irreversible in most workers,
at least for several years.
A marked increase in urine cadmium level may reflect
cadmium-induced nephropathy if exposure has been chronic and
correlates with low molecular weight proteinuria (section 6.5.1.2).
Lauwerys et al. (1979b) proposed a biological threshold of 10 µg
cadmium/µg urinary creatinine for males occupationally exposed to
cadmium. Smith et al. (1980) found that workers with low exposure to
airborne cadmium had an average urinary cadmium level of
13.1 µg/litre, whereas workers with long histories of work in areas
with substantial airborne cadmium had an average level of
45.7 µg/litre. The high-exposure group showed a significant
reduction in urinary clearance and increased ß2-microglobulin
excretion. Buchet et al. (1980) found increased excretion of both
low and high molecular weight proteins and tubular enzymes in
workers excreting more than 10 µg cadmium/g creatinine or with a
blood cadmium level above 10 µg cadmium per litre.
Retinol binding protein (RBP) has been shown to correlate well
with ß2-microglobulin in urine with a pH value greater than 5.5.
and is equally sensitive for detection of tubular proteinuria
(Bernard et al., 1982a,b). This protein occurs in serum complexed to
prealbumin and retinol, but, after retinol is delivered to target
cells, RBP rapidly dissociates from prealbumin, is filtered through
the glomerulus, and is reabsorbed by the tubule (Peterson, 1971).
Renal tubular brush border enzymes may also be excreted in
chronic cadmium poisoning. In patients with Itai-itai disease,
urinary trehalase activity correlates inversely with tubular
resorption of phosphorus (Nakano et al., 1987) and there is a
statistical correlation between urinary trehalase and other urinary
indicators of renal tubular dysfunction, such as glucose,
ß2-microglobulin, cadmium, and alpha-amino nitrogen, in
inhabitants of chronic cadmium-polluted areas in Japan (Nogawa et
al., 1980).
Other renal effects in cadmium workers include glucosuria,
aminoaciduria, impaired concentrating abilities, and hypercalciuria
(Kazantzis et al., 1963; Scott et al., 1976), which may cause
disturbances in bone and calcium metabolism (section 8.2.2). The
hypercalciuria leads to renal stone formation in some workers
(section 8.2.2.1). An increased excretion of amino acids,
particularly of serine and threonine, has been shown in industrial
workers (Clarkson & Kench, 1956), but the amino acid excretion
pattern was not consistent. In a cadmium worker with osteomalacia
(Kazantzis, 1979), there was an increase excretion of
hydroxyproline, which could be an effect of changes in collagen
metabolism related to the bone disorder (section 7.2.4).
The renal effects of cadmium that lead to proteinuria may
progress and, in some cases, with high exposures, lead to an
increase in blood creatinine. This has contributed to a
higher-than-expected mortality rate among highly exposed workers
(Kjellström et al., l979). In a Swedish battery factory, there were
four deaths from nephritis or nephrosis among 185 workers, all of
whom had been exposed to cadmium for more than 15 years. The
expected number of deaths due to these causes in this group of
workers was 0.4 (P = 0.05) (Andersson et al., l984). In a study of
about 6995 cadmium-exposed British workers (Armstrong & Kazantzis,
1983), there were 10 deaths due to nephritis or nephrosis, whereas
15.3 deaths were expected. In the subgroup with the highest exposure
(211 workers), one death occurred (0.3 expected). A 5-year follow-up
of this study (Kazantzis et al., 1988) confirmed no excess mortality
from nephritis and nephrosis (ICD 580-584), the number of observed
deaths now having increased to 16 (18.9 expected). Armstrong &
Kazantzis (1985) conducted a case control study of this cohort in
which a more detailed assessment of the past exposure of workers was
obtained. There was a marginally increased, but not statistically
significant, risk from nephritis and nephrosis (ICD 580-584) in
workers with "ever high" or "ever medium" exposure to cadmium.
Existing studies of mortality from nephritis/nephrosis have
been based upon epidemiological studies of renal failure given as
the underlying cause of death on death certificates. With the advent
of kidney dialysis and transplantation, patients with kidney failure
frequently survive and die of other causes. If kidney failure is
indicated at all on their death certificates, it is frequently given
as a contributing rather than underlying cause of death.
No studies on the contribution of cadmium-induced renal effects
to morbidity, absence from work, etc., have been published, although
one study (Vorobjeva & Eremeeva, 1980) reported increased
cardiovascular disease and related increased work absence among
cadmium workers (section 8.2.4).
8.2.1.2 In the general environment
In Japanese cadmium-polluted areas, signs of renal dysfunction
very similar to those in cadmium-exposed industrial workers have
been found. Proteinuria and glucosuria were found to be common
(30-80%) among the exposed people in one area (Ishizaki, 1969) and
less common among people living in control areas and areas bordering
a polluted area. In the exposed groups, a positive correlation
between age and the prevalence of signs was also seen (section
8.3.2). Due to the cumulative nature of cadmium, the total dose is
directly correlated to age.
With the large number of elderly people and women included in
the groups exposed in the general environment, factors other than
cadmium that can affect renal function may make direct comparisons
with industrial workers difficult. Nevertheless, the tubular
proteinuria (Shiroishi et al., 1977), aminoaciduria, and other signs
of renal tubular damage (Saito et al., 1977; Nogawa et al. 1984)
were very similar to the findings for industrial workers. In
addition, as in the case of exposed workers, elevated urinary
excretion of metallothionein occurs as a result of environmental
cadmium exposure (Tohyama et al., 1981b).
Among cadmium-exposed people in the general environment, the
mean urine ß2-microglobulin excretion was highest (Shiroishi et
al., 1977) in patients with Itai-itai disease (section 8.2.2.2).
Many of the Itai-itai patients also have signs of decreased
glomerular filtration, as indicated by decreased urea clearance
(Nakagawa, 1960) and increased serum creatinine (Nogawa et al.,
1979).
8.2.1.3 Methods for detection of tubular proteinuria
Determination of total protein and electrophoretic analysis of
concentrated urinary protein were originally the common methods for
the detection and diagnosis of tubular proteinuria. Quantitative
immunological methods (detection limit, 0.002-0.003 mg/litre) for
the measurement of ß2-microglobulin (Evrin et al., 1971) and RBP
(Bernard et al., 1982b) in urine ("normal" level about 0.1 mg/litre)
are available, and these methods facilitate the detection of tubular
dysfunction. An electrophoretic method with reasonable sensitivity
(0.8 mg/litre) for measuring specific proteins in the urine utilizes
staining of proteins in sodium dodecyl sulfate acrylamide gel
electrophoresis (Nomiyama et al., 1982b). More recently, radio-,
latex-, and enzyme-linked immunoassays have been developed (Evrin et
al., 1971; Bernard et al., 1982a; Carlier et al., 1981). However,
the disadvantage with ß2-microglobulin as a marker for renal
tubular dysfunction is that this protein is unstable if urinary pH
is less than 5.5.
RBP has an advantage over ß2-microglobulin, particularly for
screening purposes, in that serum levels and, hence, excretion are
not affected as readily by concomitant immunological disease and are
more stable at an acidic pH (Bernard et al., 1982b). An enzyme-
linked immunosorbent assay (ELISA) for urinary RBP has also been
described (Topping et al., 1986).
Common tests for qualitative determination of proteinuria, such
as paper tests (dip sticks), the nitric acid test, and the boiling
test, should not be used for screening cadmium-induced proteinuria,
since positive readings will be obtained only at fairly high urine
protein concentrations (Piscator, 1962). Trichloroacetic acid (TCA)
or sulfosalicylic acid (SA) can be used for qualitative tests, but a
negative result does not exclude a moderate increase in low
molecular weight proteinuria.
8.2.1.4 Significance of cadmium-induced proteinuria
More than 70% of proteins with a molecular weight less than
15 000 but less than 5% of those with a molecular weight greater
than 40 000 pass through the glomerular membrane (Squire et al.,
1962). The glomerular filtrate contains relatively large amounts of
plasma proteins, which are normally almost completely reabsorbed in
the proximal tubules and only small amounts are found in the urine.
The increased excretion of tubular proteins in cadmium nephropathy
is thought to be due mainly to a decreased tubular reabsorption
capacity. This provides early evidence of renal tubular dysfunction.
The concentration of albumin in normal serum is about 25 000
times higher than the concentration of ß2-microglobulin (Table
15). In spite of the fact that very little of the albumin but about
80% of the ß2-microglobulin is filtered through the glomeruli
(Maack et al., 1979), the filtered amount of albumin is still higher
than the amount of ß2-microglobulin (Table 15).
Only a small fraction of the albumin or the other proteins is
excreted in normal urine due to the normally efficient (more than
99%) tubular reabsorption of all proteins (Table 15). The urinary
albumin excretion is about 100 times greater than the
ß2-microglo-bulin excretion (Table 15).
ß2-Microglobulin is a subunit of a major immunoglobulin
complex with a molecular weight of 12 000 and normally occurs in
serum at a concentration of approximately 2.0 mg/litre. In the case
of a decreased glomerular filtration rate, the serum concentration
of ß2-microglobulin will also increase. In certain conditions, for
example, where excessive production occurs as in some cancers and
autoimmune disorders, serum levels increase, the tubular capacity
for reabsorption may be exceeded, and the concentration in the urine
will rise. In tubular dysfunction, the capacity for absorption is
impaired and, again, this is reflected by an increased excretion in
the urine. Such renal disorders include the congenital and acquired
Fanconi syndrome, diabetic nephropathy, certain cases of reflux
nephropathy, and advanced glomerular disease (Squire et al., 1962).
A raised urinary excretion of ß2-microglobulin or other low
molecular weight protein is not, therefore, specific to renal
dysfunction induced by cadmium, and a differential diagnosis should
be considered in all cases where this occurs.
The "normal" average urinary excretion of ß2-microglobulin
measured in several populations was in the range 0.05-0.1 mg/24 h
(or mg/litre or mg/g creatine) (Kjellström & Piscator, 1977). Below
age 65, there was very little or no change with age in the urinary
ß2-microglobulin (Kjellström & Piscator, 1977), a fact confirmed
by later studies (Tsuchiya et al., 1979; Kowal & Kraemer, 1982). In
all of the studies, some high individual values were found in the
age group above 65 years, and the studies of Tsuchiya et al. (1979)
and Kowal & Kraemer (1982) reported age-regression coefficients
indicating an increase with age. However, there was wide variation
with age and the average urinary ß2-microglobulin levels in the
oldest age groups (above 65 years) were only 10-20% higher than in
the other age groups (Kowal & Kraemer, 1982). The prevalence of
increased low molecular weight proteinuria (above 0.5 mg/litre) was
less than 5% in these studies, but in a control group of people
above age 80 from a Japanese cadmium-polluted area (section 8.3.2.2)
the prevalence was about 15%.
8.2.1.5 Glomerular effects
Although renal tubular dysfunction with its accompanying low
molecular weight proteinuria is thought to be the most prominent
renal effect of cadmium, the ß2-microglobulinuria is sometimes
accompanied by the excretion of high molecular weight proteins such
as albumin (molecular weight, 69 000). This albuminuria may
occasionally occur as a result of cadmium exposure without any
concomitant increase in the urinary excretion of low molecular
weight proteins (Bernard et al., 1976, 1979); this indicates that
cadmium, in some cases, may produce a change in the glomerular
permeability to larger proteins.
Cadmium may also affect the glomerular filtration rate (GFR).
Friberg (1950) reported decreased inulin clearance in
cadmium-exposed battery workers. Elinder et al. (1985a) measured GFR
by chromium-EDTA in 17 workers previously exposed to cadmium fumes.
They found a significant negative correlation between decreasing GFR
and tubular reabsorption loss, and reported that GFR decreased with
increasing cumulative exposure to cadmium fumes. The urinary
clearance of ß2-microglobulin increased with decreasing GFR.
Several other occupational studies have reported increased
serum concentrations of creatinine and/or ß2-microglobulin,
indicating reduced GFR, in cadmium-exposed workers (Thun et al.,
1989; Roels et al., 1989).
Thun et al. (1989) found a small increase in mean serum
creatinine in a group of 45 cadmium-exposed workmen. Serum
creatinine also increased with cadmium dose, suggesting decreased
glomerular function. Cadmium dose remained the important predictor
of serum creatinine even after controlling for age, blood pressure,
body size, and other extraneous factors.
Roels et al. (1989) measured the serum creatinine and serum
ß2-microglobulin levels of 23 workers, removed from cadmium
exposure, on several occasions over a period of six years. The
average yearly decrease in GFR was estimated to be 6 ml/min per
1.73 m2, which is considerably more than the normal value
(< 1 ml/min per 1.73 m2) and significantly more than that of a
control group examined at the same time.
There is also evidence of glomerular effects in people exposed
to cadmium in the environment. Nogawa et al. (1980) suggest that a
reduction in creatinine clearance may be detected at the early stage
of cadmium poisoning in a polluted area. In addition, Nogawa et al.
(1984) reported a significant correlation between decreased tubular
reabsorption of phosphate and decreased GFR in farmers living in a
cadmium-polluted area.
The mechanism for the glomerular effects from cadmium is
uncertain. It has been suggested that cadmium-induced tubular damage
leads to a certain degree of interstitial nephritis which in turn
results in a decreased GFR (Elinder et al., 1985a). It has also been
proposed that cadmium exerts a direct toxic effect on the glomerulus
(Roels et al., 1989).
8.2.1.6 Relationship between renal cadmium levels and the
occurrence of effects
The number of reports of renal pathology in autopsy cases and
renal biopsies that contain data on kidney cortex concentrations of
cadmium is small. Thus, it is difficult to establish a dose-response
relationship between cadmium content and pathology or dysfunction.
Nomiyama (1977) summarized data from 26 cadmium-exposed workers
and 16 cadmium-exposed people from the general environment. The
criteria for choosing the subjects were that they possessed high
renal and/or high liver concentrations of cadmium, morphological
studies on the kidney had been performed, and data were available on
the occurrence of proteinuria while the person was alive. Among the
42 cases reviewed, those exhibiting slight or no proteinuria and no
morphological alterations had higher concentrations of cadmium in
the renal cortex than non-exposed people. Most cases with
morphological changes plus proteinuria had lower renal cadmium
concentrations that those without proteinuria and/or morphological
changes. In more recent studies, Ellis et al. (1985) found that in
cases of renal dysfunction the mean liver and kidney cadmium values
for retired workers were lower than those for active workers. These
findings are similar to those from animal studies (section 6.5.1.2),
where kidney concentrations levelled off or even declined in the
presence of kidney damage.
The use of in vivo neutron activation analysis has
facilitated the study of the relationship between renal cadmium
levels and occurrence of effects (Ellis et al., 1981a; Roels et al.,
1981b). However, the data must be assessed with caution as the
accuracy of this method has not yet been fully determined. For
instance, the exact location of the kidney needs to be known.
Erroneously low renal cadmium levels were reported by Roels et al.
(1981b) due to an error in adjusting for the distance between skin
and kidney (Roels et al., 1983a). Data from Ellis et al. (1981a) and
Roels et al. (1983a,b) have shown that few cases with increased
urinary ß2-microglobulin concentrations are seen when the level of
renal cortex cadmium is less than 150 mg/kg tissue and that of liver
cadmium is less than 40 mg/kg. There is a pattern of liver and
kidney cadmium levels increasing simultaneously until the average
renal cortex cadmium concentration is about 300 mg/kg and the
average liver level is about 60 mg/kg. At higher liver levels, the
renal cortex level is disproportionately low in most cases, and, in
addition, many of these workers have increased urinary
ß2-microglobulin.
Skerfving et al. (1987) measured kidney cortex cadmium levels
by X-ray fluorescence in a group of 20 workers from a factory
producing alkaline batteries and found an average value of 147 mg/kg
(range 53-317). When compared to a control group, these workers had
higher average urine levels of cadmium (5.4 vs 0.8 nmol/mmol
creatinine) and ß2-microglobulin (14.6 vs 6.6 µg/mmol creatinine).
Six workers had ß2-microglobulin levels exceeding 22 µg/mmol
creatinine. Due to selection procedures the results are, however,
not predictive for cadmium-exposed workers in general. Nevertheless,
it is clear that there are no significant correlations between
levels of cadmium or ß2-microglobulin in urine and cadmium levels
in the kidney. These results suggest that there is a relationship
between renal cadmium and occurrences of effects on a group basis
but renal cadmium levels per se are not always predictive of
pathological effects on an individual level.
8.2.1.7 Reversibility of renal effects
The potential for reversibility of renal effects has been
studied in populations of workers with occupationally induced
cadmium nephropathy as well as in residents of cadmium-polluted
areas.
a) Occupational exposures
In a group of 40 workers heavily exposed to cadmium, it was
found, using qualitative methods, that proteinuria was persistent
and sometimes even increased several years after cessation of
exposure (Friberg & Nystrom, 1952; Piscator, 1966a).
Tsuchiya (1976) studied a group of 13 workers who had been
exposed to cadmium fumes (133 µg/m3) and who had proteinuria
(determined by the trichloroacetic acid method) and abnormal
electrophoretic urine patterns (ß2-microglobulin levels above
40 000 µg/litre). A 10-year follow-up study of five of these
patients was carried out after improvements had been made in their
working environment (cadmium fumes, 20 µg/m3). The proteinuria was
reversed (measured using a single radial immuno-diffusion method) in
three of the five patients: ß2-microglobulin values were 3500,
2600 µg/litre, and not detectable (limit of detection
2000 µg/litre), and retinol-binding proteins (RBP) were not
detectable (limit of detection 500 µg/litre). In addition, there
were improvements in the remaining two patients (ß2-microglobulin,
9700 and 5500 µg/litre; RBP, 34 000 and 120 000 µg/litre). Tsuchiya
(1976) suggested that the difference in the period of exposure to
cadmium was the reason for this difference in the degree of recovery
from the effects of cadmium.
Stewart & Hughes (1981) reported on similar cases from a
British pigment factory. Despite the fact that exposure ceased many
years earlier, grossly elevated ß2-microglobulin levels were still
detected.
Using quantitative methods to detect proteinuria, Roels et al.
(1982) found that total proteinuria in 19 workers was unchanged 4
years after exposure had ceased. In those 11 workers for whom
urinary ß2-microglobulin was measured before and after cessation
of exposure, levels were invariably increased. Eight of the workers
had abnormal levels of ß2-microglobulin even before cessation of
exposure, whereas three had normal levels before cessation and
developed abnormal levels afterwards.
Roels et al. (1989) examined 23 workers once a year for 5 years
after removal from exposure to cadmium. These workers had been
exposed to cadmium for periods of 6 to 41.7 years (mean 25 years),
and their first follow-up examination took place when they had been
removed from exposure for an average of 6 years. Their mean age at
that time was 58.6 years (range 45.5-68.1 years). Cadmiumuria in
these workers had been assessed three years previously by measuring
the cadmium levels in liver and kidney using neutron activation
analysis. The cadmium concentrations (mg/kg wet weight) in the liver
and kidney cortex ranged from 24 to 158 (mean 61) and from 133 to
355 (mean 231), respectively. Although cadmium concentrations in the
blood and urine decreased significantly over the five-year period,
urine concentrations of albumin, ß2-microglobulin, and RBP did not
change significantly.
Harada (1987) conducted studies on the health status of seven
workers exposed prior to 1972 to high cadmium levels in a cadmium
sulfide dye manufacturing factory. These workers were examine for 15
years, improvements having been made to working conditions in 1974
which led to markedly decreased cadmium exposures. The cadmium
content of the blood and urine declined after the improvements in
working conditions but increased again when production rose. The
working condition improvements resulted in a marked reduction in
urine ß2-microglobulin level in five workers (e.g., from 1272 to
520 µg/g creatinine in 1 year in one worker and from 2090 to
503 µg/g creatinine in 6 months in another), but there was elevated
urinary ß2-microglobulin excretion when production increased. One
worker had fairly constant near-normal urinary levels of
ß2-microglobulins (55 to 183 µg/g creatinine) regardless of
workplace improvements or production levels. Initially, four workers
had low GFR values, but none of the seven workers showed any
decrease in glomerular filtration during the 15-year follow-up
period. TRP rates decreased in three workers but remained relatively
unchanged in the other four. These changes in GFR and TRP seemed to
be independent of cadmium exposure levels.
Elinder et al. (1985b) found that urine cadmium excretion
decreased in 14 out of 19 workers re-examined at least once five
years or more after exposure to cadmium, but renal tubular function,
as measured by urinary ß2-microglobulin excretion, had
deteriorated or not improved in nearly all of the workers. Thun et
al. (1989) concluded that "time since last exposure to cadmium" was
not an important determinant of renal outcome whether considered on
its own or together with the cadmium dose. In their study of 45
workers at a plant that recovered cadmium from industrial waste, 9
out of 15 workers with the highest ß2-microglobulin excretion had
not been exposed to cadmium for at least five years and one for 45
years. This study suggested that if cadmium nephropathy is
reversible, the recovery is so slow as to be indiscernible after
decades of non-exposure.
Ellis et al. (1985) showed that the liver cadmium levels in
workers no longer exposed to cadmium gradually declines. Persistence
of renal tubular dysfunction after cessation of exposure may reflect
the level of body burden and the transfer of cadmium from liver to
kidney.
b) Residents of cadmium-polluted areas
Reversibility of renal tubular dysfunction has been
investigated in residents of cadmium-polluted areas in Japan. Kasuya
et al. (1986) carried out a comparative study of urine
ß2-microglobulin determinations made in 1975 and 1985 for 93
people with Itai-itai disease and their family members. Urine
ß2-microglobulin levels improved in the group with
ß2-microglobulin levels of 1000 µg/g creatinine or less but
worsened in the group with ß2-microglobulin levels of 3000 µg/g
creatinine or more. Most of the people who recovered were aged 39
years or less and had been resident for 30 years or less. It was
considered that mild renal dysfunction among young individuals was
reversible.
Saito (1987) measured the urine ß2-microglobulin levels of
residents of cadmium-polluted areas for 3 years after improvements
had been made in the level of soil contamination and compared the
results with determinations obtained 7 years previously. During this
3-year period, the urine ß2-microglobulin levels tended to remain
unchanged in people with a concentration of around 1000 µg/litre.
The reversibility of ß2-microglobulinuria, glucosuria, and
aminoaciduria was examined in 74 inhabitants (32 males and 42
females) over 50 years of age who lived in a cadmium-polluted area.
Examinations were conducted just after the cessation of cadmium
exposure and 5 years later. The geometric mean concentrations of
ß2-microglobulinuria, glucosuria, and aminoaciduria indicated a
significant increase in excretion during the 5-year period. In cases
where the level of ß2-microglobulinuria exceeded 1000 µg/g
creatinine at the time cadmium exposure ceased, evidence was found
indicating significant increases in proteinuria after 5 years,
whereas in cases where the excretion of ß2-micro-globulin had been
less than 1000 µg/g creatinine no significant changes were observed
(Kido et al. 1988).
8.2.2 Disorders of calcium metabolism and bone effects
8.2.2.1 In industry
Friberg (1950) observed 7 cases of renal stones among 43
cadmium workers and drew attention to the possibility of renal
stones being associated with exposure to cadmium. Ahlmark et al.
(1961) found that 44% of a group of 39 cadmium workers exposed to
cadmium oxide dust for more than 15 years had a history of renal
stone formation. Nine stones from six workers were analysed, and in
four workers the stones were composed of basic calcium phosphate
(Axelsson, 1963). There was an increase in the mean calcium
excretion rate in the cadmium-exposed group as compared to a control
group. Kidney stones were also found in 12 out of 43 British workers
at an accumulator factory (Adams et al., 1969). It is noteworthy
that in both of these studies (Axelsson, 1963 and Adams et al.,
l969) there was a higher prevalence of renal stones in workers
without proteinuria than in those with proteinuria. The men with
proteinuria had, as a group, increased urinary excretion of calcium
and phosphate, whereas in the group without proteinuria there were a
few cases with hypercalciuria. Hypercalciuria (81%) and renal stones
(19%) were also reported among 27 coppersmiths exposed to cadmium
(Scott et al., 1976, 1978).
Elinder et al. (1985a) found an increased prevalence of renal
stones among cadmium workers with tubular proteinuria, and Mason et
al. (1988) observed decreased renal reabsorption of calcium among
cadmium alloy workers.
Seven of the 12 workers in a cadmium pigment factory
investigated by Kazantzis et al. (1963) were found to have a urinary
calcium excretion rate greater than 300 mg/day (at least in 1 of 2
specimens). There was no evidence of excessive calcium intake in
these men. Five of these seven workers had been exposed to cadmium
compounds for more than 25 years and also had tubular proteinuria.
The remaining two, who had been exposed for 2 and 12 years,
respectively, had no other abnormality except for a urinary calcium
excretion of 308 and 403 mg/day. Follow-up was possible with six of
the twelve men, including all five with hypercalciuria and
proteinuria (Kazantzis, 1979). Six of the seven who had
hypercalciuria when first examined continued to have a raised
urinary calcium excretion, and one further worker developed
hypercalciuria during the follow-up period. All those with
hypercalciuria also had tubular proteinuria, although this was
marginal in one of the workers. Blood calcium levels remained within
normal limits in all cases (Kazantzis, 1979). The occurrence of
disordered calcium metabolism in all seven men followed-up for a
number of years makes it very likely that a common environmental
factor, such as occupational exposure to cadmium compounds, was
causative.
The data of Kazantzis (1979) agree with the findings of
hypercalciuria among 27 coppersmiths with high cadmium exposure
(Scott et al., 1976, 1978). Scott et al. (1980) reported that in 15
cadmium-exposed men the amount of calcium in the whole body was
lower than that of controls and decreased with duration of an
increased exposure to cadmium. The cadmium-induced hypercalciuria
could be reduced by thiazide treatment (Scott et al., l979).
In a study by Thun et al. (1989) of workers at a plant that
recovered cadmium from industrial waste, 8 of 45 exposed workers had
experienced kidney stones, in contrast to one of 32 unexposed
workers. Increase in the urinary excretion of ß2-microglobulin and
RBP was accompanied by decreased renal tubular reabsorption of
calcium and phosphates.
In contrast to the above findings, a low urinary calcium
excretion was detected in 47 out of 81 workers with exposure to a
variety of cadmium compounds and also cadmium oxide fumes (Tarasenko
& Vorobjeva, 1973; Tarasenko et al., 1975). The 24-h excretion of
calcium in these workers was below 100 mg, compared with 115-210 mg
in a control group of 21 people. Blood calcium values were within
the normal range in all cases.
Radiological examination was performed on 32 workers exposed
for 4-20 years to cadmium compounds (concentrations ranging from 0.1
to 5.5 mg/m3). All of them complained of pains in the bones.
Pseudofractures suggestive of osteomalacia were seen in two workers
exposed for 16 and 19 years, but no histological confirmation of
osteomalacia was obtained. Radiological appearances described as
enostosis were reported in five cases and periosteal proliferation
and consolidation in a further three cases (Tarasenko & Vorobjeva,
1973; Tarasenko et al., l975).
Horstowa et al. (1966) performed radiological examination of
the skeleton in 26 alkaline battery workers with signs of chronic
cadmium intoxication out of 80 workers exposed to 0.13-1.17 mg
cadmium/m3 for 1-12 years. Seven of these workers had proteinuria
detected by sulfosalicyclic acid; pseudofractures were found in 3
workers, sclerotic foci in 13, and osteoporosis in 10. In another
alkaline battery factory, where a number of cases of severe cadmium
poisoning were diagnosed (Friberg, 1950), X-ray examinations
revealed no signs of bone disease.
One of the workers with multiple tubular defects studied by
Kazantzis et al. (1963) developed osteomalacia confirmed by
histological examination 10 years after the initial investigation
(Kazantzis, 1979). He previously displayed hypercalciuria but, at
the time of diagnosis, his urinary calcium excretion was low.
Extensive investigation failed to reveal any of the other generally
accepted causes of osteomalacia such as malabsorption or nutritional
deficiency.
In a study of 43 workers at a battery plant, one worker
developed osteomalacia without evidence of malabsorption or
nutritional deficiency but with multiple renal tubular defects
(Adams et al., 1969). Another case of osteomalacia from the same
factory was subsequently detected in a man who had been a cadmium
battery worker for 40 years (Adams, 1980). Eight years before
retirement he had a partial gastrectomy due to a duodenal ulcer.
Proteinuria was first diagnosed 6 years before retirement, but
otherwise he was in "apparent good health" and "on a balanced diet"
until 8 years after retirement. He was then frail, had pains in his
legs, and a "waddling gait". His serum alkaline phosphatase level
was increased, X-rays showed generalized osteoporosis, and a bone
biopsy showed osteomalacia. After 1 year of treatment with large
doses of vitamin D, he could walk well again.
It also seems likely that the six workers exposed to cadmium
oxide dust described by Nicaud et al. (1942), who had pains in the
back and limbs and showed multiple pseudofractures on radiological
examination, suffered from osteomalacia. More detailed data on these
workers was presented by Valetas (1946), who also pointed out that
"massive doses" of vitamin D were needed to improve the symptoms. It
took several months for improvement to occur and the vitamin D
treatment had to be maintained for several years to keep the workers
in stable health. Valetas (1946) concluded that this bone disease
was caused by occupational exposure to cadmium. Eight workers with
8-30 years of exposure to lead dust and cadmium oxide fume and dust
(Gervais & Delpech, 1963) were also found to have multiple
pseudofractures and pains in the back, thorax, and legs. Very
limited biochemical investigations were carried out, but in four
cases proteinuria was found. The authors suspected that lead
exposure led to the observed effects.
8.2.2.2 In the general environment
Bone disease and abnormalities of calcium metabolism from
exposure to cadmium in the general environment have only been noted
in people in Japan with the clinical syndrome referred to as
Itai-itai disease. The main characteristics of the disease are
osteomalacia1 and osteoporosis2 with a tendency to fractures
accompanied by severe pain and renal tubular dysfunction. The
results of epidemiological and clinical investigations indicate an
association with cadmium exposure, although the Co-operative
Research Committee on Itai-itai Disease (1967) stated that
"malnutrition (low protein, low calcium diets) and multiple
pregnancies may also be involved".
1 Osteomalacia is characterized by inadequate mineralization of
bonematrix, resulting in an increase in the relative amount
of osteoid tissue. It represents the adult counterpart of
childhoodrickets (Robbins et al. 1984).
2 Osteoporosis is defined as an excessive but proportional
reduction in the amounts of both the mineral and matrix phases of
bone unaccompanied by any abnormality in structure of the residual
bone (Robbins et al., 1984).
Itai-itai disease is an endemic bone disease prevalent in the
basin of the Jinzu river, which runs through the central part of
Toyama Prefecture in West-Central Japan (Kono et el., 1956). It is
characterized by osteomalacia in combination with renal tubular
dysfunction in most cases. Patients also have osteoporosis and one
of the most characteristic symptoms is severe bone pain. Hagino &
Yoshioka (1961) reported that high concentrations of cadmium, lead,
and zinc were present in autopsy tissues from people with Itai-itai
disease and in the everyday foods of the endemic area.
Systematic epidemiological investigations, which included
extensive mass health examinations as well as case control studies
on both patients and controls, started in 1962 (Cooperative Research
Committee on Itai-itai Disease, 1967). It was reported that
Itai-itai disease in Toyama Prefecture was restricted to a limited
area (Fuchu area) irrigated by the Jinzu river, the geographical
distribution of the patients being consistent with the levels of
cadmium concentration in the paddy fields, and that the
concentrations of cadmium in urine were higher in patients than in
controls. The total number of patients was estimated in 1955 by the
Toyama Prefecture to be 41 out of a total of 1666 residents (849
women) (Cooperative Research Committee on Itai-itai Disease, 1967).
The major source of cadmium pollution in the area was a mine 50 km
upstream from the endemic area (Japan Public Health Association,
1968).
The age and sex distribution of the patients displayed a very
distinct pattern. Clinically apparent cases were limited to women
over 40 years of age who had given birth to many children (6 on
average) and had lived in the area for more than 30 years. No
detailed data on past patients were available, but it was estimated
that the age of onset of the disease was probably between 35 and 65
years, and that almost 100 deaths had been reported up to the end of
1966. The incidence was presumably very high from 1936 to 1950 and
at its highest in 1946 and 1947, but decreased thereafter even
though the same cadmium exposure levels had been maintained. By
March 1989, 150 cases of Itai-itai disease had been officially
recognized as pollution-related disease. Whereas all cases have been
reported in the Fuchu area, there have been a few suspected cases in
2 out of 12 cadmium-contaminated areas of Japan other than the Fuchu
area (Table 7). Clinical features of five suspected cases from the
Ikuno area matched those of Itai-itai disease and urine cadmium
levels were very high (Nogawa et al., 1975). In one of these cases
an autopsy was performed; the liver cadmium level was very high
(75 mg/kg) but the renal cortex cadmium level was low (53 mg/kg)
(Nogawa et al., 1975).
Takebayashi (1980a,b, 1983a,b, 1984) and Takebayashi et al.
(1985, 1987a,b,c, 1988a,b,c,d) reported pathological findings in
kidney and bone from autopsies of eleven elderly men and women (3
males and 8 females; 72-95 years of age) from Tsushima Island. The
average levels of cadmium in the liver and kidney cortex were
92.4 mg/kg and 44.0 mg/kg, respectively. The authors considered the
histological osteomalacia and renal tubulopathy noted in eight cases
(Takebayashi, 1980, 1983a, 1984, Takebayashi et al., 1985, 1987a,b,
1988c,d) to be similar to Itai-itai disease from Toyama Prefecture.
However the Japan Cadmium Research Committee (1989), supported
by the Japanese Environment Agency, concluded, after these eight
cases had been examined by the expert group, that it was clinically
difficult to diagnose them as osteomalacia.
According to the Japan Cadmium Research Committee (1989),
diagnosed cases of Itai-itai disease were reported only in the Fuchu
area of Japan. It denied the presence of osteomalacia in five cases
in the Ikuno area, and stated that osteomalacia had not been
observed "clinically" in Tsushima Island.
A study by Kido et al. (1989) indicates that exposure to
cadmium could cause osteopenia, particularly in women. Bone density
was measured in 28 women with Itai-itai disease, 92 men and 114
women with cadmium-induced renal dysfunctions, and 44 men and 66
women living in three different non-polluted areas using a
microdensitometer. The values of indices corresponding to both
cortical width and bone mineral content were significantly lower in
Itai-itai disease patients than in cadmium-exposed women with renal
dysfunctions or in non-exposed subjects. The cadmium-exposed women
also showed a decrease in bone density compared with the non-exposed
subjects. A significant decrease in bone density was also observed
in cadmium-exposed men compared with non-exposed subjects, although
the difference was not as clear as it was in women.
Reviews (in English) of Itai-itai disease have been produced by
Tsuchiya (1969), Friberg et al. (1974), Tsuchiya (1978), and Nogawa
(1981).
8.2.2.3 Mechanism of cadmium-induced bone effects
The available data show that cadmium can affect calcium,
phosphorous, and bone metabolism in both industrial workers and
people exposed in the general environment. These effects may be
secondary to the cadmium effects on the kidneys but there have been
few studies of calcium metabolism in people with excess exposure to
cadmium. The increased prevalence of renal stones reported from
certain industries is probably one manifestation of the
cadmium-induced kidney effects. It is not known if factors other
than cadmium play a role.
Nogawa et al. (1987) reported that serum 1,25-dihydroxy-
vitamin D levels were lower in Itai-itai disease patients and
cadmium-exposed subjects with renal damage than in non-exposed
subjects. The reduction in these levels was closely related to serum
concentrations of parathyroid hormone and ß2-micro-globulin and to
the percentage tubular reabsorption of phosphate (% TRP), suggesting
that cadmium-induced bone effects were mainly due to a disturbance
in vitamin D and parathyroid hormone metabolism.
Osteomalacia has been reported in a few heavily exposed
industrial workers and people with Itai-itai disease. The industrial
cases are mainly male, whereas Itai-itai patients are almost
exclusively female. However, the clinical features and biochemical
findings are similar, except that Itai-itai patients may also suffer
from ostoporosis.
A possible mechanism for the development of osteomalacia has
been proposed (Kjellström, 1986). It is known that normal calcium
absorption in the intestines and normal bone mineralization is
dependent upon 1,25-dihydroxycholecalciferol. Vitamin D3 taken
into the body is converted to 25-hydroxy-vitamin D3 in the liver,
and then to 1,25-dihydroxy-vitamin D3 in the mitochondria of renal
proximal tubular cells, this being the biologically active species.
Cadmium accumulates in the proximal tubular cells, depressing
cellular functions, and this may result in reduced conversion of
25-hydroxy-to 1,25-dihydroxy-vitamin D3. This is likely to lead to
decreased calcium absorption and decreased mineralization of bone,
which in turn may result in osteomalacia.
8.2.3 Respiratory system effects
Cadmium workers may develop chronic injury to the respiratory
system, depending on the level and nature of exposure. The
development of such effects is often quite slow, so that they are
apparent only after several years of exposure. The rate of
development and severity appear to be roughly proportional to the
time and level of exposure.
8.2.3.1 Upper respiratory system
Chronic inflammation of the nose, pharynx, and larynx have been
reported by Vorobjeva (1958) and Horstowa et al. (1966). Anosmia is
a frequent symptom in cadmium workers after prolonged exposure. This
has been reported by, for instance, Valetas (1946), Friberg (1950),
Baader (1951), Vorobjeva (1958), Tarasenko & Vorobjeva (1973), and
Apostolov (1979), but was not observed by Tsuchiya (1967) or Suzuki
et al. (1965).
8.2.3.2 Lower respiratory system
Chronic obstructive lung disease of varying degrees of severity
is frequently seen in cadmium workers. Friberg (1950) reported
dyspnoea, impaired lung function with increased residual volume, and
reduced working capacity in a group of 43 cadmium workers. Similar
studies, which included the use of pulmonary function measurements,
by Bonnell (1955), Buxton (1956), Kazantzis et al. (1963), and Adams
et al. (1969) all showed impairment of respiratory function in
groups of workers with prolonged exposure. The symptoms and findings
were more suggestive of emphysema than bronchitis in these cases;
they were commonly diagnosed as emphysema but pathological
confirmation of this was rare (Smith et al., l960).
Tarasenko & Vorobjeva (1973) reported the presence of increased
lung markings in the chest X-rays of 17 out of 72 cadmium workers,
which were interpreted as being due to diffuse interstitial
fibrosis. Similar lung changes were observed in 21 out of 26 workers
studied by Horstowa et al. (1966).
The presence of chronic obstructive respiratory disease in
cigarette smokers exposed to an additional harmful environmental
agent presents difficulties in determining the contribution made by
the latter. Studies on the chronic respiratory effects of cadmium in
the past have not always been standardized for smoking. Lauwerys et
al. (1974a) did take smoking habits into consideration by matching
his cadmium-exposed and control groups for smoking habits. They
reported the presence of impaired lung function in a group of
cadmium workers exposed for over 20 years, but not in those with
shorter exposure. The degree of lung impairment found was small.
The effects on the lung increases the mortality of cadmium
workers with high exposures (Kjellström et al., 1979; Armstrong &
Kazantzis, 1983). In the latter study, the mortality for diseases
coded as bronchitis (ICD 490-491) was related to the intensity of
exposure, the group with the highest exposure having a highly
significant (almost 4-fold) excess risk (observed 13 expected 3.4).
A 5-year follow-up of this study (Kazantzis et al., 1988) confirmed
the earlier finding, the marked excess mortality being related to
both intensity and duration of exposure. The follow-up revealed an
excess mortality from emphysema, but this was seen only in the
low-exposure group.
8.2.4 Hypertension and cardiovascular disease
Despite the abundance of data showing that under certain
exposure conditions cadmium induces hypertension in animals, there
are very few results available from studies of cadmium-exposed
workers. Friberg (1950) examined 43 workers with a mean period of
exposure to cadmium oxide dust of 20 years (air concentration,
3-15 mg/m3) and 15 workers with a mean exposure period of 2 years.
The study included physical and roentgenological examinations of the
heart, electrocardiographic examination at rest and after exercise,
and measurement of blood pressure. No increased prevalence of
cardiac disease or pathological electro-cardiographic changes were
found. The majority of subjects had completely normal blood
pressure, but since Friberg did not examine blood pressure in the
control group, it is not possible to draw definite conclusions.
Chest examination and blood pressure measurements have also
been reported in other studies (Bonnell, 1955; Bonnell et al., 1959;
Kazantzis et al., 1963; Holden, 1969), but in no cases were there
findings of cardiac disease or hypertension due to cadmium exposure.
Hammer et al. (1972) found no relationship between exposure to
cadmium and blood pressure in superphosphate workers.
Vorobjeva & Eremeeva (1980) examined 72 female and 20 male
workers at a battery factory exposed to cadmium oxide dust at
concentrations ranging from 0.04 to 0.5 mg/m3. Blood pressure was
measured and electrocardiograms taken, but there was no control
group. The authors reported increased prevalence of hypertension and
absence from work due to hypertensive and ischaemic heart disease
among the exposed workers compared to what was considered normal.
Furthermore, several types of abnormalities in the electrocardiogram
of the exposed workers were observed: 39% showed tachycardia,
between 11 and 13% were regarded as normal, and 26% had changes in
the "R" spike (compared to the normal 7-9%). Increased QRS period
was observed in 45% of the workers compared to the normal values of
14-16%. The data presented in this report are especially interesting
in view of the evidence in rats (section 7.2.6) that suggests
myocardial effects from cadmium exposure. The results of the study
are, however, presented in a very condensed form and it is therefore
difficult to draw clear-cut conclusions.
In a retrospective study of 311 male workers in an alkaline
battery factory it was found that hypertensive workers had a longer
employment time than an age-matched control group from the same work
environment (Engvall & Perk, 1985). Again it is difficult to draw
conclusions from this study. In a study of cadmium-exposed workers
in the United Kingdom (Kazantzis et al., 1988), mortality from
hypertensive disease (ICD 400-404) over the total study period from
1943 to 1988 was elevated but not significantly (49 deaths occurred
as opposed to 41.3 expected). There was no relationship with
intensity of exposure. However, mortality from cerebrovascular
disease (ICD 430-438) was significantly lower (178 deaths occurred
as opposed to 230.3 expected). These findings do not suggest any
association between cadmium exposure and the development of
hypertension.
In contrast, Thun et al. (1989) found that mean systolic and
diastolic blood pressures were higher in 45 cadmium workers (134 and
80 mmHg, respectively) than in 32 male controls (120 and 73 mmHg
respectively). Blood pressure was measured systematically by a
single examiner on the right arm of subjects who had been seated for
at least 15 min. Systolic but not diastolic blood pressure was
significantly associated with cadmium dose in multivariate analyses.
Schroeder (1965, 1967) observed that people in the general
population dying from hypertensive and/or cardiovascular disease had
somewhat higher cadmium concentrations in liver and kidney tissues
than people dying from other causes. He suggested that cadmium could
be a causative factor for these diseases. Unfortunately, smoking
habits were not accounted for and it is likely that this was a
confounding factor. The same problem exists with a number of
subsequent studies on hypertension and cadmium in tissues, blood,
and urine.
A correlation between average air cadmium levels in cities in
the USA and mortality associated with hypertension and heart disease
has been reported (Carroll, 1966; Hickey et al., 1967). Again,
several confounding factors such as smoking habits, air pollutants
other than cadmium, and other environmental factors make it
difficult to draw conclusions concerning the effects of cadmium. In
a study by Staessen et al. (1984), the confounding variables age,
sex, body weight, and cigarette smoking were considered in a
multiple regression analysis of systolic and diastolic blood
pressure and the urinary excretion of cadmium and
ß2-micro-globulin. Negative correlations between blood pressure
and urinary cadmium or ß2-microglobulin were found in some groups.
As there was a very strong age effect on both blood pressure and
urinary cadmium, the meaning of the negative correlations is not
clear. In any case, these data do not support cadmium exposure as a
cause of hypertension.
Shigematsu et al. (1979) could find no evidence that blood
pressure was higher in polluted areas (1611 people sampled) of Japan
compared with control areas (1826 people). In a comparison of blood
pressure by prefecture (13 570 in the cadmium-polluted areas and
7196 in the control areas), the prevalence of hyper-tension was
found to be high in the polluted area of one of the eight
prefectures investigated. However, in the other seven prefectures,
the prevalence of hypertension tended to be lower in the polluted
areas (Japan Cadmium Research Committee, 1989).
In a study on cadmium-polluted areas in Japan by Nogawa et al.
(1981b), the cerebrovascular disease mortality rate among people who
had had cadmium-induced proteinuria was twice as high as that of
people in the same area without proteinuria. However, the difference
was not statistically significant. The number of men in the cohort
with proteinuria was 81 and the number without proteinuria was 1109.
Another study comparing administrative units containing polluted
areas with those without such areas (Shigematsu et al., 1981, 1982,
1983) found no difference in the cerebrovascular disease mortality
rates.
Data on a total population of 333 000 from both
cadmium-polluted and non-polluted areas were collected
retrospectively for a period of 6-30 years, based on vital
statistics or death certificates (Shigematsu et al., 1982). The
mortalities from all causes, including cardiovascular disease such
as cerebrovascular and hypertensive disease, in the general
population in the cadmium-polluted areas were no higher than, or in
some cases even lower than, those in the non-polluted areas.
A mortality study of Shipham residents and of a nearby control
village was reported by Inskip et al. (1982). The study population
consisted of 501 Shipham residents of whom 278 had died over a
40-year follow-up period. Overall mortality was low in both villages
compared generally with England and Wales. There was a small but
statistically significant excess mortality rate in Shipham from
hypertensive and cerebrovascular disease. The highest ratio of all
was for genito-urinary disease in Shipham men (but, with only eight
observed deaths, the result was only significant at the 10% level).
The Standardized Mortality Ratios (SMR) for nephritis and nephrosis
in both sexes were also slightly elevated, but there were only two
deaths for each sex from this cause. In men, the numbers of
prostatic and lung cancer deaths were approximately equal to the
expected numbers, and in neither case was the SMR in Shipham greatly
different from that in the control village.
8.2.5 Cancer
8.2.5.1 In industry
A number of epidemiological studies have been published. In
order to facilitate the interpretation of published data on the
relationship between cadmium exposure and cancer, the studies have
been grouped according to the types of industrial plants in which
they have been conducted. In some cases, more than one study has
been conducted at the same plant.
a) Nickel-cadmium battery plants
In an early study, Potts (1965) found that three out of eight
deaths in a small cohort of nickel-cadmium battery workers in the
United Kingdom with at least 10 years of exposure to cadmium oxide
dust were from carcinoma of the prostate. This study was extended by
Kipling & Waterhouse (1967) to include 248 men with at least one
year of exposure to cadmium oxide dust. Four deaths from carcinoma
of the prostate, including the three cases previously reported by
Potts (1965), were observed as opposed to an expected number of
0.58.
Sorahan & Waterhouse (1983) carried out a further investigation
of the same plant using a cohort of 3025 employees who started work
between 1923 and 1975 and had a minimum employment period of one
month. The method of regression models in life tables was used to
compare duration of exposed employment in those dying from relevant
causes with that of matched survivors in the same year of follow-up.
No new evidence of an association between occupational exposure to
cadmium and cancer of the prostate was found. However, there was an
excess mortality from cancer of the respiratory system significant
at the 5% level (89 cases, SMR = 127). As in other studies, data on
smoking habits were not available and confounding factors were
present in the form of exposure to nickel hydroxide and welding
fumes so that no firm conclusions about the pulmonary
carcinogenicity of cadmium could be drawn from this study.
Sorahan & Waterhouse (1983) reported on the incidence of
prostatic cancer in a subgroup of 458 workers employed for at least
1 year in a job involving high exposure to cadmium oxide dust. Eight
cancers were observed compared to two expected (SMR 400, P < 0.01).
However, exclusion of the four cases previously reported by Kipling
& Waterhouse leaves a non-significant excess incidence (P = 0.21),
from which the investigators concluded that if cadmium oxide is
potentially carcinogenic current risks are likely to be small.
In the most recent update of the nickel-cadmium battery plant
workers (Sorahan, 1987), the earlier findings were confirmed and
there was some evidence of an association between risk of death from
lung cancer and duration of employment in jobs with high or moderate
exposure among workers first employed in the period 1923-1946.
However, among workers first employed from 1947 to 1975 (the group
with the higher SMR for lung cancer), there was no evidence of such
an association. The authors concluded that the findings do not
suggest these nickel-cadmium battery workers had experienced an
elevated lung cancer risk as a consequence of exposure to cadmium
oxide dust.
In Sweden, Kjellström (1979) investigated the incidence of
cancer among 269 male nickel-cadmium battery workers. All workers
had been heavily exposed (on average about 1 mg cadmium/m3) for
five years or more to cadmium dust or fume, and were alive in 1959.
Fifteen workers were found to have cancer between 1959 and 1975. It
was calculated from national incidence rates that 16.4 new cases
would have occurred; only 2 were prostatic cancers while 1.2 were
expected. In a re-examination of the same cohort, there were 8
deaths from lung cancer with a non-significantly raised SMR of 133.
The SMRs increased progressively with increasing latent periods
without reaching statistical significance (Elinder et al., 1985c).
b) Copper-cadmium alloy plants
Copper-cadmium alloy workers in the United Kingdom who had
heavy past exposure to cadmium oxide fume on two sites, one urban
the other rural, were studied by Holden (1980a). There was an
increased lung cancer mortality at the urban site (8 observed versus
4.5 expected) and a significant deficit at the rural site (2
observed; 7.8 expected). Vicinity workers in the urban plant, where
the mean cadmium concentration averaged no more than 60 µg/m3),
also experienced a significantly increased lung and prostatic cancer
mortality (36 observed; 26.1 expected).
A case control study was performed (Kazantzis et al., 1989) in
the same copper-cadmium alloy plants where workers had experienced
heavy past exposure to cadmium oxide fume and dust, which had given
rise to a number of deaths coded as chronic cadmium poisoning.
Before and during the period 1939-1945, cadmium oxide fume levels
had been estimated to be up to 4 mg/m3. Cases and controls were
selected from the cohort previously studied by Holden (1980a).
Personal interviews conducted with a small number of long-term
employees revealed that arsenical copper had been additionally
produced by adding bags of arsenic trioxide to the molten copper and
stirring manually; this resulted in the evolution of dense white
clouds of arsenic fume. The case control study showed no evidence of
an increased risk of lung cancer associated with past cadmium
exposure but an approximately two-fold excess risk associated with
arsenic exposure.
Kjellström (1979) also investigated a cadmium-copper alloy
plant in Sweden where workers had been exposed to cadmium oxide
fumes and included 94 workers employed in 1940 or who started work
after that year. Four cases of prostatic cancer occurred as opposed
to 2.7 expected.
c) Cadmium recovery plant in the USA
An increase in prostatic cancer incidence was also found by
Lemen et al. (1976) in a study of 292 male smelter workers heavily
exposed to cadmium oxide dust or fumes. Air cadmium concentrations
in 1973 were up to 24 mg/m3 but generally below 1 mg/m3. There
were four deaths from cancer of the prostate (1.15 expected). There
were also 12 deaths from lung cancer (5.1 expected); the difference
was statistically significant.
Thun et al. (1985) expanded the Lemen et al. (1976) cohort to
include 602 workers who had been employed at this cadmium production
plant between 1940 and 1969 for at least 6 months. Exposure was to
cadmium in baghouse dust, a by-product of zinc smelting which was
processed to produce cadmium metal and cadmium oxide. The plant
functioned as an arsenic smelter up to the end of 1925, and small
quantities of lead, arsenic, thallium and indium were subsequently
produced at intervals. The vital status of the workers was
determined in 1978. A dose-response relationship was observed
between lung cancer mortality and cumulative exposure and was
statistically significant for workers whose exposure exceeded
2920 mg/m3.days. The SMR for this group was 280. The lung tumours
were, as far as can be determined, mostly of bronchogenic origin.
The authors accounted for smoking habits by obtaining questionnaires
from survivors or next-of-kin in 50% of the cohort members and for
arsenic exposure by measuring arsenic in certain parts of the plant.
d) Cadmium processing plants in the United Kingdom
Kazantzis & Armstrong (1982) and Armstrong & Kazantzis (1983)
investigated a large cohort of workers in England at 17 plants with
processes using cadmium. The cohort comprised 6995 cadmium-exposed
male workers born before 1940, first exposed before 1970, and not
included in any previous mortality study. Jobs were assessed for
each relevant year involving high, medium or low exposure to cadmium
on the basis of discussions with hygienists and employees with
knowledge of past working conditions, taking into account
environmental and biological monitoring data (e.g., cadmium urine
data > 20 mg/litre in the high-exposure group. The periods at risk
of the study population were classified on the basis of these
categories and recorded job histories into three groups: (i) those
workers continuously employed for more than one year in a job
assessed as entailing high exposure - "ever high"; (ii) those
workers continuously employed for more than one year in a job
assessed as entailing medium exposure, but who were never for more
than one year in a high-exposure job - "ever medium", and (iii) all
others. Actual deaths were compared with expected numbers calculated
from mortality rates for the population of England and Wales
corrected for regional variation. The 8th revision of ICD codes was
used and results were expressed as SMRs.
Only 3% of the workers (about 200) were assigned to the "ever
high" category. The mean duration of exposure was 11 years and the
mean interval from initial exposure to the end of the follow-up was
27 years. The SMR (all causes) for the entire population was 97.
There were no prostatic cancer deaths in the "ever high" and "ever
medium" exposure categories (0.4 and 2.5, respectively, expected),
and the number of deaths (23) in the "always low" group was close to
the expected value. There was a small, but not statistically
significant, excess of lung cancer in all categories, but in those
with more than 10 years exposure in the "always low" category this
excess was significant at the 5% level (100 observed, SMR 126).
Since there was no correlation between increase in lung cancer risk
and intensity of exposure, the authors concluded that it was
unlikely that the excess in the "always low" group was due to
cadmium.
A 5-year update of this study (Kazantzis et al., 1988)
confirmed no excess risk from prostatic cancer over the total study
period from 1943 to 1984 and no cases of prostatic cancer in the
medium- or high-exposure groups. The SMR was 99 as opposed to the
value of 90 in the initial study. However, there was now a
significant excess lung cancer mortality (277 observed deaths, 240.9
expected), giving a SMR of 115 (95% confidence interval, 101-129).
This excess risk was related to intensity of exposure, there being
12 deaths in the small high-exposure group as opposed to 6.2
expected (SMR, 194; 95% CI, 100-339), 41 deaths in the
medium-exposure group and 224 deaths in the low-exposure group (SMR
121 and 112, respectively; not significant). While there appeared to
be evidence of a dose-response relationship, it was not
statistically significant. The increased cancer risk mainly involved
those employed before 1940, rising with length of employment and
with length of follow-up.
Further studies have been conducted on workers at these 17
plants (Armstrong & Kazantzis, 1985; Ades & Kazantzis, 1988). A case
control study on lung cancer was carried out on workers in a large
lead-zinc-cadmium smelter. These workers formed 64% of the cohort of
6995 men, and the study included 70% of the lung cancer deaths
observed in the cohort as a whole (Ades & Kazantzis, 1988). There
was a significant excess lung cancer risk among the smelter workers,
and a significant trend with increasing duration of employment,
particularly evident among those employed for more than 20 years.
Quantitative estimates of exposure to cadmium and ordinal rankings
for lead, arsenic, zinc, sulfur dioxide and dust were used to
calculate cumulative exposures from job histories. However, matched
logistic regression analysis showed that the increasing risk of lung
cancer associated with increasing length of employment could not be
accounted for by cadmium exposure and did not appear to be
restricted to any particular process or department.
e) Summary of industrial studies
Increased mortality from lung cancer has been observed in
several occupational cohorts exposed to cadmium, and there is some
evidence of dose-response relationships in two of the examined
populations. Case control studies have not given support for such a
relationship. It is difficult to reach a firm conclusion about
causality, because in all of the occupational cohorts there has been
simultaneous exposure to other potential carcinogens (e.g., nickel,
arsenic, polyaromatic hydrocarbons) or other environmental
pollutants (e.g., sulfur dioxide). Information on tobacco smoking is
inadequate or entirely absent in all except two studies.
Investigations of the relationships between cadmium exposure and
prostatic cancer are inconclusive.
8.2.5.2 In the general environment
Elevated cadmium levels have been found in the liver and
kidneys of patients with bronchogenic carcinoma (Morgan, 1970;
Morgan et al., 1971). However, the authors stressed the possibility
that differences observed could reflect the effect of smoking
(section 5.1.3) or could represent a non specific association.
A study of the causes of death in areas of high cadmium
exposures in Japan (Shigematsu et al., 1982) revealed no difference
in age-adjusted cancer mortality rates between polluted and control
areas of the same prefecture. The mortality rate due to prostatic
cancer was elevated in two areas but only achieved statistical
significance (P < .01) in one area. It was not significant in two
areas including Toyama prefecture, which has the largest area of
pollution.
8.2.6 Mutagenic effects in human cells
An increased frequency of chromosomal aberrations in somatic
human cells is considered to be evidence of some exposure to
mutagenic agents. Shiraishi (1975) noticed an increased frequency of
chromosomal aberrations in lymphocytes obtained from 12 Itai-itai
patients compared to 9 female controls. However, this observation
was not confirmed by Bui et al. (1975) who examined cells from 4
Itai-itai patients and 4 controls.
Among cadmium workers, an increased prevalence of chromosomal
aberrations, compared to controls, was reported by Deknudt & Leonard
(1975) and by Bauchinger et al. (1976), whereas no such effect was
seen by O'Riordan et al. (1978). In none of these occupational
studies was the actual exposure to cadmium measured, and the
possible confounding effect from other industrial chemicals and
smoking was not considered.
Nogawa et al. (1986) did not find evidence for increased sister
chromatid exchange in people exposed to cadmium in the general
environment. IARC (1987a,b) reviewed the available evidence for
mutagenic and related effects and noted the differences in results
reported from different industrial environments.
In conclusion, it is not yet possible to say whether cadmium
causes mutagenic effects in humans.
8.2.7 Transplacental transport and fetal effects
There have been few studies on the fetal toxicity of cadmium
transported across the placenta. Maternal hypertension and decrease
in birth weight have been associated with elevated levels of cadmium
in the neonate (Huel et al., 1981). In addition, it is
well-established that the babies of mothers who are cigarette
smokers are smaller at birth than are those of non-smokers. The
ratio of placental zinc to cadmium is positively related to infant
birth weight in the case of pregnant smokers, and older pregnant
smokers are at higher risk for impaired fetal growth than are
younger ones (Cnattingius et al., 1985; Kuhnert et al., 1987a,
Kuhnert et al., 1987b). Multiparity is related to an increased
placental cadmium level in smokers and to a decreased placental zinc
level in both smokers and non-smokers. These results have been
interpreted as consistent with a depletion of zinc with increasing
number of births and a progressive increase in cadmium in smokers
because of the long half-life of cadmium (Kuhnert et al., 1988).
The cellular mechanisms and factors that influence
trans-placental transport of cadmium are not known. Metallothionein
has been identified in the human placenta and in fetal membranes at
term, and metallothionein synthesis is inducible in cultured
trophoblasts by treatment with cadmium (Waalkes et al., 1984). This
effect is seen with cadmium concentrations in the culture medium as
low as 52.2 µg/litre (0.5 µmol/litre) (Lehman & Poisner, 1984).
Higher levels of exposure to cadmium may have a direct toxic effect
on the placenta. In a test system involving perfusion of maternal
and fetal blood vessels in the isolated human placenta, it was shown
that perfusion of the maternal circulation with cadmium at a
concentration of 1.12 mg/litre (10 µmol/litre) resulted in the
deposition of 2.5 µg cadmium per g placenta (22 nmoles/g), but very
little of it was detectable in the fetal circulation. Perfusion of
the maternal circulation with higher concentrations of cadmium
produced placental cadmium concentrations of 11.2-16.8 µg/g
(100-150 nmoles) with stromal oedema, syncytiotrophoblastic
vesiculation and vacuolization of Hofbauer cells within 6-8 h,
followed by placental necrosis. These changes were associated with a
decrease in human chorionic gonadotropin release and decreased
movement of zinc into the fetal circulation (Miller, 1986).
8.2.8 Other effects
Many other different symptoms and signs have been reported in
humans exposed to cadmium. These include loss of appetite, loss of
weight, fatigue, and increases in the erythrocyte sedimentation rate
(ESR). Valetas (1946) reported details of the poisoning cases in a
French accumulator factory, which were first described by Nicaud et
al. (1942). In addition to the bone effects and the pains (section
8.2.2.1), Valetas mentioned that several workers experienced
paraesthesia and involuntary muscular contractions. This could be an
effect resulting from abnormal changes in the levels of serum
electrolytes, such as calcium or potassium, which may in turn be
caused by severe kidney damage.
Mild anaemia has been more frequently observed among
cadmium-exposed workers than among controls (Friberg, 1950; Bonnell,
1955; Bernard et al., 1979).
More specific effects from cadmium are the yellow discoloration
of the proximal part of the front teeth (Barthelemy & Moline, 1946;
Valetas, 1946; Princi, 1947; Friberg, 1950; Apostolov, 1979) and
anosmia (Friberg, 1950). Anosmia was found by Friberg (1950) in
about one third of a group of workers with a mean exposure time to
cadmium oxide dust of 20 years. Baader (1951) in Germany and
Apostolov (1979) in Bulgaria also noted that anosmia was common
among workers exposed to cadmium oxide dust for long periods of
time. Suzuki et al. (1965) and Tsuchiya (1967) in Japan, found no
increase in the prevalence of anosmia in workers exposed to cadmium
stearate and cadmium oxide fumes.
Nervous system symptoms were reported by Vorobjeva (1957), who
investigated 160 workers at an accumulator factory in the USSR.
Subjective symptoms included headache, vertigo, and sleep
disturbance. Physical examination revealed increases in knee-joint
reflexes, tremor, dermographia, and sweating.
Cadmium sulfide is sometimes used as a yellow tattoo pigment,
which is deposited intradermally. Local phototoxic reactions may
take place when the skin is exposed to ultraviolet light and are
probably connected with the marked photoconducting properties of
cadmium sulfide. Of 24 patients with yellow tattoos who were
examined by Bjornberg (1963), 18 experienced skin swelling when
exposed to sunlight.
8.3 Clinical and epidemiological studies with data on both exposure
and effects
There are several clinical and epidemiological studies with
data on occupational or general environment exposure levels, but the
data concerning effects are restricted to the lungs and kidneys.
8.3.1 Studies on respiratory disorders
Friberg (1950) studied 43 male workers exposed to cadmium oxide
dust, with an average period of employment of 20 years (range 9-34
years), and 15 workers who had been employed for only 1-4 years.
They were compared with a group of 200 sawmill workers. Shortness of
breath was the common symptom among the workers with long exposure,
and an impairment of lung function (increased residual capacity in
relation to total lung capacity and a decreased working capacity)
was demonstrated. The lung function of the group with short exposure
(less than 5 years) was found to be normal. The cadmium
concentration in air varied from 3 to 15 mg/m3, measurements
having been made at five places on only one occasion. In another
battery factory (where air cadmium concentrations were
0.05-5 mg/m3, Adams et al. (1969) found a slight average decrease
in forced expiratory volume in a group of 27 male workers.
Twelve out of 96 cadmium workers exposed for up to 27 years to
cadmium oxide fume in two cadmium-copper alloy factories were found
to suffer from emphysema, as evaluated from a comprehensive lung
function test (Bonnell, 1955; Buxton, 1956; Kazantzis, 1956). These
workers were compared with a similar size control group. The average
air cadmium concentrations in the two factories were 40-50 µg/m3,
and 90% of the particles were less than 0.5 µm in diameter (King,
1955).
Lauwerys et al. (1979a) studied pulmonary ventilatory function
in three groups of workers exposed to cadmium oxide dust and in
matched control groups (the matching included smoking habits). A
slight but significant reduction in forced vital capacity, in forced
expiratory volume in one second, and in peak expiratory flow rate
was found in 22 men. These were all smokers and had been exposed for
more than 20 years to a time-weighted average air concentration of
66 µg/m3 (21 µg/m3 respirable cadmium). In another group of
workers (smokers and non-smokers) exposed for 1-20 years to an
average concentration of 134 µg/m3 (the respirable cadmium level
at the most polluted work site was 88 µg/m3), the pulmonary
indices were on average lower than in the control group, but the
differences were not statistically significant. A more thorough
examination of a subgroup of the workers with long exposures carried
out by the same research group (Stanescu et al., 1977) found more
respiratory symptoms in the cadmium-exposed group than in a control
group and also impaired lung function (not statistically
significant). However, Lauwerys et al. (1979a) reported more
extensive data from the same plants and found that the workers with
less than 20 years of exposure (average 7.5 years) showed
significant effects in the lung function tests.
Reduced forced vital capacity was also found at a cadmium
production plant in the USA (where the air concentrations were
"commonly greater than 200 µg Cd/m3") among 17 workers exposed for
more than 6 years (Smith et al., 1976). De Silva & Donnan (1981)
provided evidence that insoluble cadmium compounds may induce
emphysematous changes after more than 7 years exposure to a
time-weighted average cadmium concentration of 700 µg/m3.
Edling et al. (1986) found no lung function differences between
an exposed group of workers using cadmium-containing solders and a
control group. The level of exposure, which lasted for several
years, was estimated to be 0.05-0.5 mg cadmium/m3, but the workers
had not been exposed to cadmium for several years. Of the 57 workers
examined, 42% had cadmium-induced renal tubular dysfunction.
Davison et al. (1988) examined 101 male workers, who had worked
for 1 year or more manufacturing copper-cadmium alloys, and found,
compared with a reference group, impaired lung function. They also
compared certain parameters (transfer coefficient: KCO) with the
estimated cumulative exposure index for cadmium workers with 95%
confidence limits for the regression line. Among 35 workers exposed
for more than five years and with a cumulative cadmium exposure
index up to 14 mg/m3.years, there was no evidence of a threshold.
The authors concluded that inhaled cadmium fumes caused changes in
lung function and in chest radiographs consistent with emphysema.
This could also explain the increased mortality reported. The
impaired lung function was also related to liver cadmium levels as
measured with neutron activation in vivo.
Some studies on respiratory disorders have yielded negative
results. However, some of these studies did not use lung function
tests (Hardy & Skinner, 1947; Princi, 1947; Tsuchiya, 1967; L'Epee
et al., 1968) and another did not use a control group (Teculescu &
Stanescu, 1970). Suzuki et al. (1965) examined a group of workers
exposed for a short period (average 3.3 years) to 30-690 µg
cadmium/m3 (as cadmium stearate) and found no changes in lung
function when compared to a control group.
In summary, it is clear that exposure to cadmium dust and fume
over prolonged periods can give rise to impaired lung function and
emphysema. Such effects have been seen predominantly at high air
cadmium concentrations (above 100 µg/m3), but one study showed
effects after more than 20 years of exposure to respirable cadmium
oxide dust concentrations of 21 µg/m3.
Cadmium workers sometimes suffered from symptoms such as
coughing and throat irritation, but did not show abnormal chest
X-ray findings when exposed to cadmium oxide fume at a concentration
of 100 µg/m3 for 4-8 years (Hardy & Skinner, 1947) or
40-1440 µg/m3 for 8 years (Princi, 1947), or to cadmium oxide dust
at a concentration of 64-241 µg/m3 for up to 15 years (Tsuchiya,
1967).
8.3.2 Studies on renal disorders in industry
Friberg (1950) reported that prolonged exposure gave rise to
renal damage among a large group of workers exposed to cadmium oxide
dust at concentrations of 3-15 mg/m3 in an accumulator factory. In
one group of 43 workers with a mean exposure period of 20 years
(range 9-34 years), a high prevalence of proteinuria was
demonstrated by the nitric acid and trichloroacetic acid test. In
several of the workers, the renal damage was also manifested by a
decreased inulin clearance and decreased concentrating capacity.
Another group of 15 workers with a mean exposure period of 2 years
(range 1-4 years) showed no positive reactions.
Since 1950, there have been many studies on proteinuria among
workers in various industries. This type of proteinuria is
characterized particularly by a great relative increase in the
excretion of low molecular weight (LMW) proteins (section 8.2). In
most of the early studies, qualitative tests for detecting
proteinuria were used, but, more recently, specific methods for the
quantitative determination of LMW proteins have been developed.
Table 16 contains data on the prevalence of proteinuria from
several epidemiological studies on cadmium workers. It must be
recognized that, in most studies, the dose measurements are based on
short sampling periods (hours or a few days), whereas exposure may
have been for decades. Information on sampling method (static or
personal) and the use of respirators is usually inadequate, which
makes accurate dose estimates difficult (section 2.2.1).
It is evident that the prevalence of proteinuria in cadmium
workers increases with exposure intensity duration. The study by
Kjellström et al. (1977a) presents frequency distributions of
urinary ß2-microglobulin excretion for 240 exposed workers and a
control group. There is a general shift to higher excretion levels
among the exposed workers, and a large proportion of them have
excretion levels far outside the control distribution. Any cut-off
point (operational definition) for "abnormal" proteinuria is
arbitrary. If a cut-off point of 290 µg/litre (corresponding to the
97.5 percentile of the control group) is chosen, 26% of the whole
group of exposed workers would be classified as having LMW
proteinuria. If higher cut-off points are used, the prevalence of
proteinuria will obviously be lower.
Table 16 provides evidence of a dose-response relationship. The
lowest "dose" that gave rise to a statistically significant increase
in urinary ß2-microglobulin, as defined above, was a 6-to 12-year
exposure to 50 µg cadmium/m3 (based on personal sampling)
(Kjellström et al., 1977a).
Järup et al. (1988) recently made a reassessment of
dose-response in the same battery plant (Table 16). A pattern very
similar to Kjellström et al. (1977a) was observed with a prevalence
of ß2-microglobulinuria of 4% at a cumulative dose of 0.5 mg/m3
(corresponding to 10 years of exposure to 50 µg cadmium/m3).
Lauwerys et al. (1979a,b) studied the prevalence of increased
ß2-microglobulin clearance (cut-off point: 97.5 percentile of
controls) and found a 21% prevalence after more than 20 years of
exposure to 66 µg cadmium/m3 total dust (static samples) or 21 µg
cadmium/m3 respirable dust (Table 16).
Holden (1980b) measured urine levels of ß2-microglobulin and
found dose-response relationships using cut-off points of
200 µg/litre, 1000 µg/litre, or 10 000 µg/litre. The cut-off point
of 200 µg/litre gave a 16% LMW proteinuria prevalence rate after
6-10 years of exposure (Table 16).
Table 16 also shows that an increased prevalence of total
proteinuria, as measured by sulfosalicyclic acid, trichloroacetic
acid, or quantitative determination of total proteinuria, occurs
after 5-10 years exposure to approximately 100 µg cadmium/m3. An
increased excretion of LMW protein (e.g., ß2-microglobulin) occurs
at much lower doses.
The in vivo measurement of cadmium in the liver and kidneys
of people with various levels of cadmium exposure provides a means
for relating organ dose to effects and response rates (section
6.4.2). Some questions still remain regarding the accuracy of the
analytical method (section 2.2.3.4) and the mathematical-statistical
methodology (Kjellström et al., 1984). Nevertheless, Roels et al.
(1983a) and Ellis et al. (l984) suggested that renal tubular damage
would be experienced by about 10% of people with a kidney cortex
level of 200 mg cadmium/kg, and by about 50% of people with a kidney
cortex level of 300 mg cadmium/kg.
Table 16. Prevalence of proteinuria in cadmium workers
Cadmium Estimated air Exposure No. of Prevalence of Proteinuria and Reference
compounds concentrations period examinees proteinuria (%) characteristics of
(µg/m3)a (years)b detection
methode
Cadmium oxide 40-50 control 60 2 SA and TCA Bonnell (1955); King (1955);
fume 1-9 37 24 Bonnell et al. (1959)
> 9 63 46
64-241c control 11 0 TA Tsuchiya (1967)
< 1 4 0 < 100 mg/litre
1-4 4 50
123c > 5 4 100
(time-weighted
average
Cadmium oxide 3000-15 000 1-4 15 0 nitric acid ("Hellers Friberg (1950)
dust 9-15 12 33 test"); positive in
16-22 17 41 more than half the
23-34 14 64 test
31 (1.4)c control 31 0 Abnormal Lauwerys et al. (1974a)
1-12 (4) 31 0 electrophoretic
control 27 4 pattern as defined
by the authors;
ß2-microglobulin
clearance
134 (88)c 0.6-19.7 (9) 27 15
control 22 0
66 (21) 21-40 22 68
66 (21) > 20 42 21
Table 16 (contd).
Cadmium Estimated air Exposure No. of Prevalence of Proteinuria and Reference
compounds concentrations period examinees proteinuria (%) characteristics of
(µg/m3)a (years)b detection
methode
Cadmium oxide
dust
control 87 3.4 ß2-microglobulin Kjellström et al. (1977a)
50c 0-3 50 6.0 (RIA)
50c 3-6 30 6.6 > 290 µg/litre
50c 6-12 21 19.0 (sg = 1.023)
Cadmium stearate 30-690 control 24 17 TCA Suzuki et al. (1965)
dust (3) 19 58
Cadmium sulfide 114d < 1-5 12 17 EP Harada (1987)
dust 5-21 7 100
< 1-5 12 8 TCA
5-21 12 43
control 203 1 ß2-microglobulin Stewart & Hughes (1981)
80 0-5 105 0 (RIA)
100 6-11 41 0 > 765 µg/litre
(sg = 1.016)
100-600 11-19 13 7.7
100-600 20+ 14 57.0
Mixed exposures; not reported, controls 642 0 (0.8) ß2-microglobulin Holden (1980b)f
12 factories; but mean blood < 18 months 121 0 (10) (RIA)
mainly zinc cadmium level 19 months-5 years 168 1.8 (8.3) > 1000 µg/litre
smelters after 1 year 6-10 170 1.8 (16) (or > 200 µg/litre)
exposure was 11-15 82 7.3 (22)
about 16-20 33 24 (45)
15 µg/litre
20+ 68 25 (56)
Table 16 (contd).
Cadmium Estimate of cumulative Number of Prevalence (%) Proteinuria and Reference
compounds dose (mg/m3.year) examinees of proteinuria characteristics of
detection method
Cadmium fume < 1 16 18 ß2-microglobulin Elinder et al. (1985b)
(oxide) 1-2 22 32 > 0.034 mg/mmol creatinine
2-3 9 44
3-5 8 62
> 5 5 100
< 1 ) 6 Mason et al. (1988)
1-15 ) 67
1.5-3) 75 58
3-5 ) 86
> 5 ) 100
Cadmium oxide < 0.4 264 1.1 Jarup et al. (1988)
0.4-1.7 76 9.2
1.7-4.6 43 23.3
4.6-9.6 31 32.3
9.6-15 16 32.2
> 15 10 50
a Numbers in parentheses refer to average concentrations of the respirable particulates (< 5 µm) fraction
b Numbers in parentheses are average values
c Measured in breathing zone by personal sampler
d Calculated average exposure for the worker with the most pronounced effect
e SA = sulfosalicyclic acid method (qualitative); TCA = trichloroacetic acid method (qualitative);
TA = tungstate method (quantitative); EP = electrophoresis; RIA = radioimmunoassay; sg = specific gravity
f Values for prevalence of proteinuria refer to a ß2-microglobulin level of > 1000 µg/litre.
Values in parentheses refer to a level of > 200 µg/litre.
Ellis et al. (1985) correlated time-weighted exposure indices
(TWE), based on employment records, area monitoring techniques and
personal sampling, with body burden of cadmium measured by
in vivo neutron activation analysis of the liver and left kidney
in 82 men exposed to cadmium dust in a smelter. The workers were
grouped as follows: production workers (40 active, 21 retired);
office and laboratory workers (8 active, 4 retired); and
non-production workers (3 active, 6 retired). From these
measurements the authors were able to estimate the probability of
developing kidney dysfunction based on the workers' cumulative
exposure index. When the exposure limit was 400-500 µg/m3.years,
the prevalence for renal dysfunction was about 32%; it was 22-40%
with a wider exposure index (300-600 µg/m3). Kjellström et al.
(1977a) reported a prevalence of 19% at a battery factory at a level
of 50 µg Cd/m3, which resulted in a similar exposure index.
Lauwerys et al. (1974a) observed proteinuria in 68% of workers with
long-term exposure (20 years at 66 µg/m3), whereas the logistic
model developed by Ellis et al. (l985) would have predicted 65%
under these exposure conditions.
For exposures of 250 µg/m3.years there is a 19% probability
of experiencing renal dysfunction. In the case of workers with
normal renal function, an exposure index of 400 µg/m3 predicts a
mean cadmium concentration of 28 mg/kg in liver and 288 mg/kg of
renal cortex. The model would predict that a 10-year exposure to
25 µg/m3 would result in a mean renal cortex concentration of
252 mg/kg, which is similar to the critical concentration defined by
Friberg.
Thun et al. (1989) assessed the quantitative relationship
between exposure to airborne cadmium and various markers of renal
tubular and glomerular function in 45 male workers at a plant that
recovered cadmium from industrial waste. The dose was estimated from
historical air sampling data. In this study, the "critical dose" of
cadmium necessary to induce nephropathy was based on the 5th or 95th
percentile of test results in the unexposed population. Using this
definition, renal tubular dysfunction sharply increased as
cumulative exposure to cadmium rose above 300 mg/m3.days
(corresponding to about 0.8 mg/m3.years). Very similar
dose-response curves with an increased prevalence of
ß2-microglobulinuria at cumulative exposure levels exceeding about
0.5-1 mg cadmium/m3.year have been reported from examinations of
workers exposed to cadmium fumes (Elinder et al., 1985b; Mason et
al., 1988). These findings are consistent with the recommendations
by a working group of the World Health Organization (WHO, l980) to
limit workplace exposures to 10 µg/m3 in order to prevent tubular
proteinuria after life-time occupational exposure to cadmium.
Cumulative cadmium exposure indices have been calculated for 75
cadmium alloy workers employed for periods of up to 39 years,
together with the in vivo liver and kidney cadmium burden (Mason
et al., 1988). Several indicators of both tubular and glomerular
dysfunction correlated significantly with both cumulative exposure
index and liver cadmium burden. Using these estimates of dose, a
two-phase linear regression model was applied to identify an
inflection point of the order of 1100 µg/m3.years above which
changes in renal function occurred. A number of biochemical
variables fitted this model, including total protein, albumin, and
ß2-microglobulin. Simple dose-response analysis showed a greatly
increased incidence of tubular proteinuria when the cumulative
cadmium exposure index was greater than this value. The cumulative
exposure index was equated to about 20 to 22 years of exposure to a
cadmium level of 50 µg/m3.
Evidence of tubular damage was investigated in a group of 91
cadmium workers subjected to yearly estimation of cadmium
concentration in blood and urine over a period of eight years. In
workers with blood and urine cadmium levels constantly below the
Biological Limit Value of 10 µg/litre, the prevalence of tubular
damage, as indicated by an increased excretion of ß2-microglobulin
above 260 µg/litre, was below 3%. RBP excretion confirmed this
pattern.
8.3.3 Studies on renal disorders in the general environment
8.3.3.1 Health surveys in Japan
Following the recognition of the association between Itai-itai
disease and exposure to cadmium (Japanese Ministry of Health and
Welfare, 1968), additional general surveys of cadmium pollution were
performed in Japan, and further areas were found to be involved.
Health effects were studied first among the population in the area
where Itai-itai cases had occurred and later in other areas found to
be contaminated. The original studies were designed to find cases of
Itai-itai disease, but it was possible also to estimate the
prevalence of proteinuria and glucosuria in the examined population.
A detailed description of the methods used in these cadmium
pollution surveillance programmes has been reported by Shigematsu et
al. (1979).
At the time of the first surveillance programmes (1969-71),
methods were developed for estimating the degree of contamination
with cadmium and the total daily intake. At the early stage of the
investigations, the most common index of cadmium intake measured in
all areas was the cadmium concentration in rice. From data of the
Japan Public Health Association (1970), it was estimated that, in
areas with different exposure levels, an average of almost 50%
(range 14-71) of the daily cadmium intake came from rice. In one
area, the proportion was estimated to be 85% (Kawano & Kato, 1978).
The average cadmium concentration in rice from non-polluted
areas has been reported to be 0.066 mg/kg in polished rice
(Moritsugi & Kobayashi, 1964) and 0.09 mg/kg in unpolished rice
(Japanese Ministry of Agriculture and Forestry, 1973) (section
5.2.1). The national average consumption of rice was 364 g per
person in 1961, 308 g in 1971, and 222 g in 1981 (Japanese Ministry
of Health and Welfare, 1983).
Proteinuria was generally estimated with qualitative methods
such as the sulfosalicylic acid method or the trichloracetic acid
method according to standardized techniques (Japanese Ministry of
Health and Welfare, 1971). More recently, emphasis has been placed
on the identification of the urinary protein pattern, in particular
to detect early evidence of tubular dysfunction. The methods
currently used are electrophoresis and the quantitative
determination of lysozyme, RBP, and ß2-microglobulin. Several
studies have been published, and there are extensive reviews in
English (Tsuchiya, 1969; Yamagata & Shigematsu, 1970; Friberg et
al., 1974; Tsuchiya, 1978; Shigematsu et al., 1979, l980).
From 1976 to 1984, epidemiological health surveys of residents
in areas with environmental cadmium pollution were performed by the
Japan Environment Agency using methods including immunological tests
for the detection of low molecular weight proteinuria in eight
prefectures (Akita, Fukushisuma, Gunma, Toyama, Ishikawa, Hyogo,
Nagasaki, Oita). More than 13 000 inhabitants of polluted areas and
more than 7000 inhabitants of non-polluted areas, aged 50 years or
more in both areas, were subjected to these surveys.
The following screening method was adopted for health
examinations. The urine of those people with proteinuria
(demonstrated by a semiquantitative method) and/or glucosuria (by a
paper test) was analysed for ß2-microglobulin (> 10 mg/litre),
RBP (> 4 mg/litre), lysozyme (> 2 mg/litre), total amino acid
nitrogen (> 20 mmol/litre), and cadmium (> 30 µg/litre). Those
who exceeded the above levels in more than one item were tested for
renal function by urine and blood analysis. Finally those for whom
the TRP was less than 80% were subjected to detailed health
examination, including skeletal radiography, in order to make a
clinical diagnosis (Fig. 7).
With the exception of Oita prefecture, the number of
individuals who had or were suspected of having proximal renal
tubular dysfunction (as defined by the Japanese Cadmium Research
Committee) or related findings tended to be greater in the polluted
areas than in the non-polluted areas, and this was often
significantly related to the degree of pollution (see Table 16).
This suggests that environmental cadmium pollution is associated
with the occurrence of proximal renal tubular dysfunction.
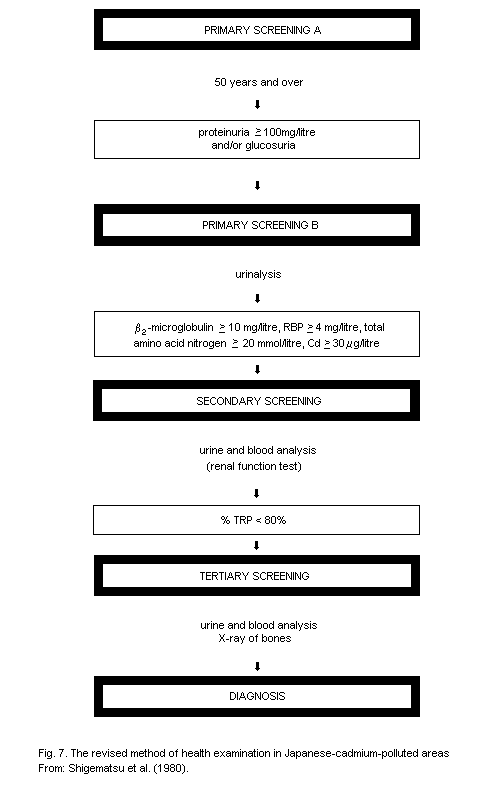 Five areas in which significantly increased
ß2-microglobulinuria was found are reviewed in detail below. In
addition there is a description of some other Japanese polluted
areas and three European polluted areas.
8.3.3.2 Toyama prefecture (Fuchu area)
This is the area where the Itai-itai disease was first
described (Kono et al., 1956). Exposure levels in polluted villages,
as measured by cadmium concentrations in rice during the 1960s,
varied greatly but in some villages the level was as high as 2 mg/kg
(Ishizaki et al., 1969). A zinc and lead mine was the major source
of pollution, and cadmium concentrations in soil were elevated
(Japan Public Health Association, 1968). Many studies have been
performed with sulfosalicyclic acid and trichloroacetic acid for the
identification of proteinuria. Both proteinuria and glucosuria were
common findings in the polluted area (Ishizaki et al., 1969;
Fukushima et al., 1974). There was a strong relationship between the
degree of proteinuria and age, and a greatly increased prevalence in
the older age groups compared with controls. The proteinuria was
similar to that seen in cadmium workers as evaluated by
electrophoresis (Piscator & Tsuchiya, 1971) or gel filtration
(Fukuyama, 1972). Quantitative estimation of the LMW urinary
proteins ß2-microglobulin (Shiroishi et al., 1975, 1977) and
retinol-binding protein (Kanai et al., 1971) confirmed that the
proteinuria was tubular.
Fukushima et al. (1973) reported on the cadmium concentration
in rice and the prevalence of renal effects in various hamlets in
the Fuchu and control areas. In control hamlets situated outside the
Jinzu river basin, the cadmium concentration in rice varied between
0.05 and 0.2 mg/kg wet weight, and the prevalence of concurrent
proteinuria and glycosuria varied between 0 and 9%. In the polluted
villages, where cadmium levels in polished rice were 0.5-1.0 mg/kg,
the prevalence was 15-20%, and, in all the 20 hamlets, the
correlation coefficient between cadmium in rice and prevalence of
renal effects was 0.68 (P < 0.05) (Fukushima et al., 1973). The
prevalence had a tendency to be somewhat higher in the hamlets where
Itai-itai disease was endemic, as compared with the hamlets where it
did not occur, even though the latter hamlets had similar cadmium
concentrations in rice.
Using a disc electrophoresis technique (Shiroishi et al., 1972)
in the age groups over 40 years, a tubular urinary protein pattern
was found in about 25% of exposed persons but not found at all in
the control group. Quantitative determination of ß2-microglobulin
in the urine of patients with Itai-itai disease and so-called
observation patients (people in polluted areas with likely
cadmium-induced renal damage) showed a marked difference between the
exposed and control groups (Shiroishi et al., 1977).
The level of urinary ß2-microglobulin in patients with
Itai-itai disease was on average 43 mg/litre, i.e. 100 times higher
than in the controls, and the level in observation patients was on
average 65 times higher than it was in the controls.
A comparison by Kjellström et al. (1977b) of 138 cadmium-
exposed women in the age-group 51-60 and 40 controls revealed
large differences. The exposed women were selected on the
basis of their consumption of polluted rice (average cadmium
concentration above 0.7 mg/kg); no health data influenced the
selection. On average, the urinary ß2-microglobulin excretion was
10 times greater among the exposed women than among the controls,
and the individual urinary levels increased as a function of the
cadmium dose. Additional data from the same area for women in the
age-group 40-45 (Kjellström, 1977) also showed an increase
prevalence of high ß2-microglobulin levels in urine.
Nogawa & Ishizaki (1979) reported a significant increase in the
prevalence of both proteinuria and concurrent proteinuria and
glucosuria at an average rice cadmium level of 0.41 mg/kg. In a
further study (Nogawa et al., 1979), the prevalence of proteinuria
was analysed as a function of urinary cadmium levels. The
correlation between the two variables was good, but urinary cadmium
may not be a suitable measure of dose as it also increases as a
consequence of renal damage (section 8.2.1).
A mathematical dose-response analysis was carried out by Hutton
(1983) based on the data of Shiroishi et al. (1977) on urinary
ß2-microglobulin excretion. For each individual the cadmium dose
index (µg/day.years) was based on the estimated daily cadmium intake
via rice and other foodstuffs and the number of years the person had
lived in the polluted area. The age-groups 51-60 and 40-45 were both
divided into three sub-groups with different dose index levels. In
the analysis of Hutton (1983), three groups from Kosaka area were
included (section 8.3.3.4) for whom the same type of data was
available (Kojima et al., 1977). Fig. 8 shows that the prevalence of
increased LMW proteinuria (response rate) increased with dose. These
prevalences were adjusted for a control group prevalence of 2.5%
(Kjellström, 1977), giving an expected "background" prevalence
without cadmium exposure of 0%. The 95% fiducial limits were quite
wide. For instance, at a cadmium intake of 55 µg/day (95% fiducial
limits 25-123), there would be a 1% increase of proteinuria in the
population. At a dose index of 5000 µg/day.years (or 50 years at
100 µg cadmium/day intake), the expected response rate was within
the range 2-12%.
Five areas in which significantly increased
ß2-microglobulinuria was found are reviewed in detail below. In
addition there is a description of some other Japanese polluted
areas and three European polluted areas.
8.3.3.2 Toyama prefecture (Fuchu area)
This is the area where the Itai-itai disease was first
described (Kono et al., 1956). Exposure levels in polluted villages,
as measured by cadmium concentrations in rice during the 1960s,
varied greatly but in some villages the level was as high as 2 mg/kg
(Ishizaki et al., 1969). A zinc and lead mine was the major source
of pollution, and cadmium concentrations in soil were elevated
(Japan Public Health Association, 1968). Many studies have been
performed with sulfosalicyclic acid and trichloroacetic acid for the
identification of proteinuria. Both proteinuria and glucosuria were
common findings in the polluted area (Ishizaki et al., 1969;
Fukushima et al., 1974). There was a strong relationship between the
degree of proteinuria and age, and a greatly increased prevalence in
the older age groups compared with controls. The proteinuria was
similar to that seen in cadmium workers as evaluated by
electrophoresis (Piscator & Tsuchiya, 1971) or gel filtration
(Fukuyama, 1972). Quantitative estimation of the LMW urinary
proteins ß2-microglobulin (Shiroishi et al., 1975, 1977) and
retinol-binding protein (Kanai et al., 1971) confirmed that the
proteinuria was tubular.
Fukushima et al. (1973) reported on the cadmium concentration
in rice and the prevalence of renal effects in various hamlets in
the Fuchu and control areas. In control hamlets situated outside the
Jinzu river basin, the cadmium concentration in rice varied between
0.05 and 0.2 mg/kg wet weight, and the prevalence of concurrent
proteinuria and glycosuria varied between 0 and 9%. In the polluted
villages, where cadmium levels in polished rice were 0.5-1.0 mg/kg,
the prevalence was 15-20%, and, in all the 20 hamlets, the
correlation coefficient between cadmium in rice and prevalence of
renal effects was 0.68 (P < 0.05) (Fukushima et al., 1973). The
prevalence had a tendency to be somewhat higher in the hamlets where
Itai-itai disease was endemic, as compared with the hamlets where it
did not occur, even though the latter hamlets had similar cadmium
concentrations in rice.
Using a disc electrophoresis technique (Shiroishi et al., 1972)
in the age groups over 40 years, a tubular urinary protein pattern
was found in about 25% of exposed persons but not found at all in
the control group. Quantitative determination of ß2-microglobulin
in the urine of patients with Itai-itai disease and so-called
observation patients (people in polluted areas with likely
cadmium-induced renal damage) showed a marked difference between the
exposed and control groups (Shiroishi et al., 1977).
The level of urinary ß2-microglobulin in patients with
Itai-itai disease was on average 43 mg/litre, i.e. 100 times higher
than in the controls, and the level in observation patients was on
average 65 times higher than it was in the controls.
A comparison by Kjellström et al. (1977b) of 138 cadmium-
exposed women in the age-group 51-60 and 40 controls revealed
large differences. The exposed women were selected on the
basis of their consumption of polluted rice (average cadmium
concentration above 0.7 mg/kg); no health data influenced the
selection. On average, the urinary ß2-microglobulin excretion was
10 times greater among the exposed women than among the controls,
and the individual urinary levels increased as a function of the
cadmium dose. Additional data from the same area for women in the
age-group 40-45 (Kjellström, 1977) also showed an increase
prevalence of high ß2-microglobulin levels in urine.
Nogawa & Ishizaki (1979) reported a significant increase in the
prevalence of both proteinuria and concurrent proteinuria and
glucosuria at an average rice cadmium level of 0.41 mg/kg. In a
further study (Nogawa et al., 1979), the prevalence of proteinuria
was analysed as a function of urinary cadmium levels. The
correlation between the two variables was good, but urinary cadmium
may not be a suitable measure of dose as it also increases as a
consequence of renal damage (section 8.2.1).
A mathematical dose-response analysis was carried out by Hutton
(1983) based on the data of Shiroishi et al. (1977) on urinary
ß2-microglobulin excretion. For each individual the cadmium dose
index (µg/day.years) was based on the estimated daily cadmium intake
via rice and other foodstuffs and the number of years the person had
lived in the polluted area. The age-groups 51-60 and 40-45 were both
divided into three sub-groups with different dose index levels. In
the analysis of Hutton (1983), three groups from Kosaka area were
included (section 8.3.3.4) for whom the same type of data was
available (Kojima et al., 1977). Fig. 8 shows that the prevalence of
increased LMW proteinuria (response rate) increased with dose. These
prevalences were adjusted for a control group prevalence of 2.5%
(Kjellström, 1977), giving an expected "background" prevalence
without cadmium exposure of 0%. The 95% fiducial limits were quite
wide. For instance, at a cadmium intake of 55 µg/day (95% fiducial
limits 25-123), there would be a 1% increase of proteinuria in the
population. At a dose index of 5000 µg/day.years (or 50 years at
100 µg cadmium/day intake), the expected response rate was within
the range 2-12%.
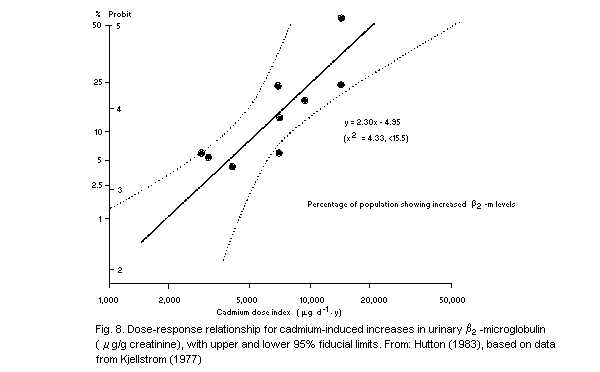 8.3.3.3 Hyogo prefecture (Ikuno area)
In the Ikuno area of Hyogo prefecture, an inactive zinc and
copper mine is the probable source of pollution of the Ichi river
basin. The average cadmium concentration in rice in the most
polluted part was found to be 0.69 mg/kg in one study and 1.10 mg/kg
in another (Hyogo Prefectural Government, 1972).
In 1972, urine from 1560 people (of both sexes, over 30 years
of age) from the polluted area and groups of 1574, 2002, and 638
people (over 30) from three control areas were examined (Tsuchiya,
1978). The prevalence of proteinuria, as measured by the
sulfosalicyclic acid methods was 58% and 33%, respectively, a
statistically significant difference. The reason for the high
prevalence in the control area is not known.
In a study by Watanabe & Murayama (1975), a search was made for
LMW proteins among 39 people in a polluted area and 56 in a control
area (all the people were above 70 years of age). Urinary
ß2-microglobulin excretion exceeding 10 000 µg/litre was found in
41% of the examined people in the polluted area and 4% of those in
the control area.
Kitamura & Koizumi (1975) used disc electrophoresis to study
tubular-type proteinuria among 224 people (above 50 years of age)
from a polluted area and compared the results with those from a
study of old bedridden people. Fig. 9 demonstrates the considerably
higher prevalence of tubular proteinuria among people from the
polluted area. There was also a definite increase in the occurrence
of tubular proteinuria with age in the exposed and bedridden control
groups.
8.3.3.4 Ishikawa prefecture (Kakehashi area)
In the Kakahaski river basin of Ishikawa prefecture, several
mines had polluted the river with cadmium and copper (Tsuchiya,
1978). Rice samples were studied in a number of villages along the
river, and village-average levels of up to about 0.7 g/kg were
found. Values were higher in paddy fields on the shores of a narrow
river valley close to the mine.
In 1974-1976, health examinations of 2805 inhabitants over the
age of 50 were carried out using test tape for proteinuria and
glucosuria examination as well as single radial immunodiffusion
analysis for RBP in urine (Tsuchiya, 1978). Based on the findings,
some people were selected for secondary and tertiary examinations,
and 39 were considered to require consultation for renal tubular
dysfunction. However, no cases with severe bone disease were found
at that time.
This area is the only one where quantitative measurement of LMW
protein in urine was carried out in the first screening (Nogawa et
al., 1978). The prevalence data in Table 17 are therefore of
particular interest. None of the other LMW proteinuria studies
mentioned in Table 16 were carried out on such a large group using a
broad epidemiological approach. A scatter in the prevalence values
among the villages is seen in Fig. 10, but there is no doubt that
the villages with the highest rice cadmium values had an increased
prevalence of high urinary RBP. This is also evident when the data
from different villages with the same rice cadmium levels are
combined (Table 17). In all of the exposed groups with different
rice cadmium levels (Table 17), the prevalence of tubular
proteinuria increases with age, but that is not seen in the control
group. It is not known whether the age effect reflects increased
cadmium dose rather than age itself.
Further analysis of these data using a mathematical
dose-response approach (Hutton, 1983) clearly showed the effect of
calculated cadmium intake on the prevalence of proteinuria
(Fig. 11). The fiducial limits are narrower than in Fig. 8 because
of the larger number of people studied.
8.3.3.3 Hyogo prefecture (Ikuno area)
In the Ikuno area of Hyogo prefecture, an inactive zinc and
copper mine is the probable source of pollution of the Ichi river
basin. The average cadmium concentration in rice in the most
polluted part was found to be 0.69 mg/kg in one study and 1.10 mg/kg
in another (Hyogo Prefectural Government, 1972).
In 1972, urine from 1560 people (of both sexes, over 30 years
of age) from the polluted area and groups of 1574, 2002, and 638
people (over 30) from three control areas were examined (Tsuchiya,
1978). The prevalence of proteinuria, as measured by the
sulfosalicyclic acid methods was 58% and 33%, respectively, a
statistically significant difference. The reason for the high
prevalence in the control area is not known.
In a study by Watanabe & Murayama (1975), a search was made for
LMW proteins among 39 people in a polluted area and 56 in a control
area (all the people were above 70 years of age). Urinary
ß2-microglobulin excretion exceeding 10 000 µg/litre was found in
41% of the examined people in the polluted area and 4% of those in
the control area.
Kitamura & Koizumi (1975) used disc electrophoresis to study
tubular-type proteinuria among 224 people (above 50 years of age)
from a polluted area and compared the results with those from a
study of old bedridden people. Fig. 9 demonstrates the considerably
higher prevalence of tubular proteinuria among people from the
polluted area. There was also a definite increase in the occurrence
of tubular proteinuria with age in the exposed and bedridden control
groups.
8.3.3.4 Ishikawa prefecture (Kakehashi area)
In the Kakahaski river basin of Ishikawa prefecture, several
mines had polluted the river with cadmium and copper (Tsuchiya,
1978). Rice samples were studied in a number of villages along the
river, and village-average levels of up to about 0.7 g/kg were
found. Values were higher in paddy fields on the shores of a narrow
river valley close to the mine.
In 1974-1976, health examinations of 2805 inhabitants over the
age of 50 were carried out using test tape for proteinuria and
glucosuria examination as well as single radial immunodiffusion
analysis for RBP in urine (Tsuchiya, 1978). Based on the findings,
some people were selected for secondary and tertiary examinations,
and 39 were considered to require consultation for renal tubular
dysfunction. However, no cases with severe bone disease were found
at that time.
This area is the only one where quantitative measurement of LMW
protein in urine was carried out in the first screening (Nogawa et
al., 1978). The prevalence data in Table 17 are therefore of
particular interest. None of the other LMW proteinuria studies
mentioned in Table 16 were carried out on such a large group using a
broad epidemiological approach. A scatter in the prevalence values
among the villages is seen in Fig. 10, but there is no doubt that
the villages with the highest rice cadmium values had an increased
prevalence of high urinary RBP. This is also evident when the data
from different villages with the same rice cadmium levels are
combined (Table 17). In all of the exposed groups with different
rice cadmium levels (Table 17), the prevalence of tubular
proteinuria increases with age, but that is not seen in the control
group. It is not known whether the age effect reflects increased
cadmium dose rather than age itself.
Further analysis of these data using a mathematical
dose-response approach (Hutton, 1983) clearly showed the effect of
calculated cadmium intake on the prevalence of proteinuria
(Fig. 11). The fiducial limits are narrower than in Fig. 8 because
of the larger number of people studied.
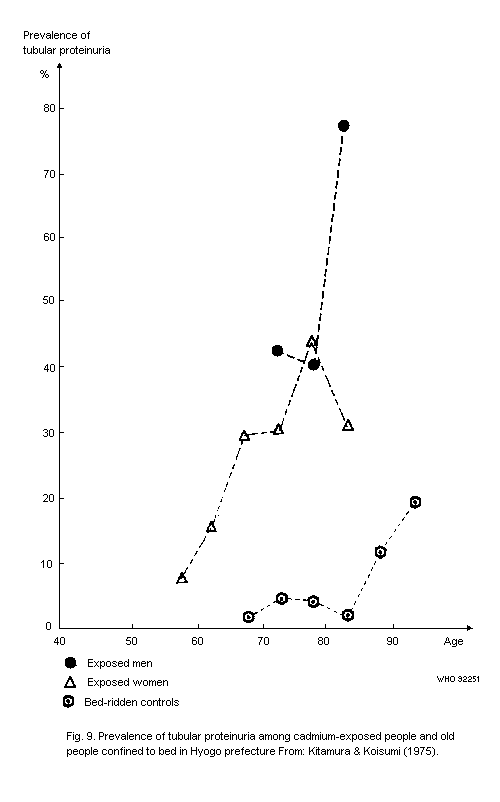 In a study by Nogawa et al. (1978), laboratory determinations
related to proximal renal tubular function, etc., were compared by
age group. The findings in the most polluted areas hardly differed
from those in the non-polluted areas in the age-group 50-59
(Table 17). At age 60 and over, however, the frequency of findings
tended to increase with age, except in the case of total
aminoaciduria. The difference in the age-adjusted rates for these
determinations between the polluted and non-polluted areas tended to
be enhanced by aging. Table 18 shows how the prevalence of tubular
proteinuria varies according to age and average rice cadmium
concentration. Of the 438 participants in the final detailed
examinations (426 in the polluted areas and 12 in the non-polluted
areas), findings of "possible proximal renal tubular dysfunction"
were noted in 334 people (333 in the polluted areas and 1 in the
non-polluted areas). Among these cases, 202 in the polluted areas
were determined to have proximal renal tubular dysfunction and 116
of them were considered to require medical supervision in view of
the severity of the dysfunction. The urinary ß2-microglobulin
level in Itai-itai disease patients was on average 43 mg/litre, 100
times higher than that in the controls, and the patients
investigated by Nogawa et al. (1978) had an average urinary level of
ß2-microglobulin 65 times the controls.
Table 17. Age-adjusted prevalence rate (%) of renal tubular dysfunction and related conditionsa
Prefecture Year of Polluted (P) or No. of examinees ß2-Microglobulinuria Decreased TRP Tubular dysfunction
studiedb investigation non-polluted male female (> 10 mg/litre) (< 80%) male female
(NP) areac male female male female
Toyama 1979-1984 P 3432 4099 6.5 10.8 4.6 5.5 1.4 3.3
(Fuchu area) NP 944 1205 0.4d 0.5d 0.6d 0.2d 0.0d 0.0d
Hyogo 1977 P 230 280 12.8 16.8 4.4 6.8 2.0 3.5
(Ikuno area) NP 212 251 2.7d 1.9d 0.9e 0.4d 0.0 0.0e
Ishikawa 1976 P 260 306 7.6 10.9 6.9 5.7 2.4 3.2
(Kakehashi area) NP 200 275 1.1d 1.5d 0.5d 0.0d 0.0 0.0d
Akita 1976 P 179 247 6.4 5.0 2.3 0.7 0.0 0.0
(Kosaka area) NP 168 234 0.0d 0.0d 0.0 0.0 0.0 0.0
Nagasaki 1976 P 143 191 3.4 10.6 4.6 9.1 1.7 6.2
(Tsushima area) NP 210 291 1.9 0.3d 1.5 0.0d 0.0 0.0d
Fukushima 1977 P 307 425 0.4 0.5 0.6 0.5 0.0 0.3
NP 246 396 0.0 0.0 0.4 0.0 0.0 0.0
Gunma 1976-1978 P 937 1160 1.6 0.6 1.6 0.5 0.0 0.0
NP 620 786 0.8 0.1 0.6 0.3 0.0 0.0
Table 17 (cont'd).
Prefecture Year of Polluted (P) or No. of examinees ß2-Microglobulinuria Decreased TRP Tubular dysfunction
studiedb investigation non-polluted male female (> 10 mg/litre) (< 80%) male female
(NP) areac male female male female
Oita 1978 P 169 194 1.7 1.5 1.1 0.5 0.0 0.0
NP 182 215 1.7 1.0 1.2 0.0 0.0 0.0
a From: Japan Cadmium Research Committee (1989). The response rates for these studies were greater than 90% of the target
population. The age composition (50-59, 60-69, 70-79, and 80+) in each prefecture was adjusted to the Japanese population
in 1980. The criteria for renal tubular dysfunction were: one out of three signs (low molecular weight proteinuria, glucosuria,
and generalized aminoaciduria), %TRP < 80% and acidosis (blood hydrogen carbonate below 23 mEq/litre).
b The total number of people living in polluted areas in each prefecture is shown in Table 7.
c Total number of people examined was 5657 males and 6902 females in polluted areas and 2782 males and 3653 females in non-polluted areas.
d Significant difference (P < 0.01)
e Significant difference (P < 0.05)
Table 18. Prevalence (%) of tubular proteinuriaa in relation to age (age-groups 50-59, 60-69, and > 69) and
village-average rice cadmium concentrationsb
Rice cadmium 50-59 60-69 > 69
concentration
(µg/g) No. A (%) B (%) No. A (%) B (%) No. A (%) B (%)
Control (< 0.1) 104 0.96 0 80 2.50 1.25 64 0 0
0.19-0.29 477 0.84 0.42 377 2.65 0.80 268 13.81c 4.48
0.30-0.39 184 3.26 2.72 138 5.07 0.72 91 17.58c 7.69d
0.40-0.49 295 1.69 0.34 204 6.86 2.94 117 14.53c 8.55c
0.50-0.59 140 0.71 0 120 10.83d 5.00 93 34.41c 25.81c
0.60-0.69 60 18.33c 5.00d 57 24.56c 12.28c 23 73.91c 43.48c
a Prevalence of increased RBP in Ishikawa prefecture only
b From: Nogawa et al. (1978); No. = number of people examined; RBP was measured by a semiquantitative method; values in column A refer
to the prevalence of RBP in urine at levels above 4 mg/litre; values in column B refer to the prevalence at values above 16 mg/litre
c Significant difference (P < 0.01) compared with control
d Significant difference (P < 0.05) compared with control
In a study by Nogawa et al. (1978), laboratory determinations
related to proximal renal tubular function, etc., were compared by
age group. The findings in the most polluted areas hardly differed
from those in the non-polluted areas in the age-group 50-59
(Table 17). At age 60 and over, however, the frequency of findings
tended to increase with age, except in the case of total
aminoaciduria. The difference in the age-adjusted rates for these
determinations between the polluted and non-polluted areas tended to
be enhanced by aging. Table 18 shows how the prevalence of tubular
proteinuria varies according to age and average rice cadmium
concentration. Of the 438 participants in the final detailed
examinations (426 in the polluted areas and 12 in the non-polluted
areas), findings of "possible proximal renal tubular dysfunction"
were noted in 334 people (333 in the polluted areas and 1 in the
non-polluted areas). Among these cases, 202 in the polluted areas
were determined to have proximal renal tubular dysfunction and 116
of them were considered to require medical supervision in view of
the severity of the dysfunction. The urinary ß2-microglobulin
level in Itai-itai disease patients was on average 43 mg/litre, 100
times higher than that in the controls, and the patients
investigated by Nogawa et al. (1978) had an average urinary level of
ß2-microglobulin 65 times the controls.
Table 17. Age-adjusted prevalence rate (%) of renal tubular dysfunction and related conditionsa
Prefecture Year of Polluted (P) or No. of examinees ß2-Microglobulinuria Decreased TRP Tubular dysfunction
studiedb investigation non-polluted male female (> 10 mg/litre) (< 80%) male female
(NP) areac male female male female
Toyama 1979-1984 P 3432 4099 6.5 10.8 4.6 5.5 1.4 3.3
(Fuchu area) NP 944 1205 0.4d 0.5d 0.6d 0.2d 0.0d 0.0d
Hyogo 1977 P 230 280 12.8 16.8 4.4 6.8 2.0 3.5
(Ikuno area) NP 212 251 2.7d 1.9d 0.9e 0.4d 0.0 0.0e
Ishikawa 1976 P 260 306 7.6 10.9 6.9 5.7 2.4 3.2
(Kakehashi area) NP 200 275 1.1d 1.5d 0.5d 0.0d 0.0 0.0d
Akita 1976 P 179 247 6.4 5.0 2.3 0.7 0.0 0.0
(Kosaka area) NP 168 234 0.0d 0.0d 0.0 0.0 0.0 0.0
Nagasaki 1976 P 143 191 3.4 10.6 4.6 9.1 1.7 6.2
(Tsushima area) NP 210 291 1.9 0.3d 1.5 0.0d 0.0 0.0d
Fukushima 1977 P 307 425 0.4 0.5 0.6 0.5 0.0 0.3
NP 246 396 0.0 0.0 0.4 0.0 0.0 0.0
Gunma 1976-1978 P 937 1160 1.6 0.6 1.6 0.5 0.0 0.0
NP 620 786 0.8 0.1 0.6 0.3 0.0 0.0
Table 17 (cont'd).
Prefecture Year of Polluted (P) or No. of examinees ß2-Microglobulinuria Decreased TRP Tubular dysfunction
studiedb investigation non-polluted male female (> 10 mg/litre) (< 80%) male female
(NP) areac male female male female
Oita 1978 P 169 194 1.7 1.5 1.1 0.5 0.0 0.0
NP 182 215 1.7 1.0 1.2 0.0 0.0 0.0
a From: Japan Cadmium Research Committee (1989). The response rates for these studies were greater than 90% of the target
population. The age composition (50-59, 60-69, 70-79, and 80+) in each prefecture was adjusted to the Japanese population
in 1980. The criteria for renal tubular dysfunction were: one out of three signs (low molecular weight proteinuria, glucosuria,
and generalized aminoaciduria), %TRP < 80% and acidosis (blood hydrogen carbonate below 23 mEq/litre).
b The total number of people living in polluted areas in each prefecture is shown in Table 7.
c Total number of people examined was 5657 males and 6902 females in polluted areas and 2782 males and 3653 females in non-polluted areas.
d Significant difference (P < 0.01)
e Significant difference (P < 0.05)
Table 18. Prevalence (%) of tubular proteinuriaa in relation to age (age-groups 50-59, 60-69, and > 69) and
village-average rice cadmium concentrationsb
Rice cadmium 50-59 60-69 > 69
concentration
(µg/g) No. A (%) B (%) No. A (%) B (%) No. A (%) B (%)
Control (< 0.1) 104 0.96 0 80 2.50 1.25 64 0 0
0.19-0.29 477 0.84 0.42 377 2.65 0.80 268 13.81c 4.48
0.30-0.39 184 3.26 2.72 138 5.07 0.72 91 17.58c 7.69d
0.40-0.49 295 1.69 0.34 204 6.86 2.94 117 14.53c 8.55c
0.50-0.59 140 0.71 0 120 10.83d 5.00 93 34.41c 25.81c
0.60-0.69 60 18.33c 5.00d 57 24.56c 12.28c 23 73.91c 43.48c
a Prevalence of increased RBP in Ishikawa prefecture only
b From: Nogawa et al. (1978); No. = number of people examined; RBP was measured by a semiquantitative method; values in column A refer
to the prevalence of RBP in urine at levels above 4 mg/litre; values in column B refer to the prevalence at values above 16 mg/litre
c Significant difference (P < 0.01) compared with control
d Significant difference (P < 0.05) compared with control
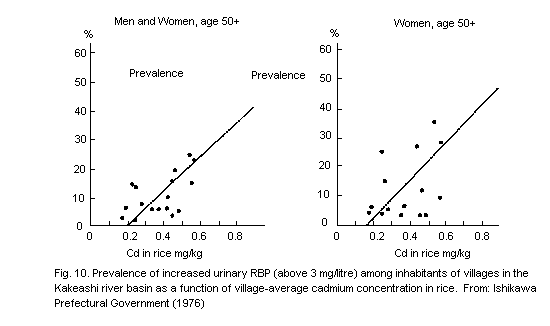
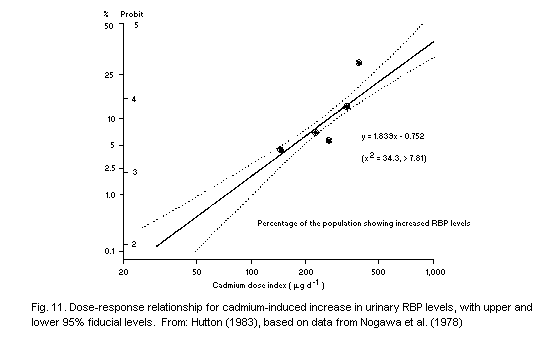 In a later epidemiological study, Nogawa et al. (1989)
investigated the dose-response relationship in 1850 cadmium-exposed
and 294 non-exposed inhabitants of the Kakehashi River basin. Using
a urine concentration of 1000 µg ß2-microglobulin/g creatinine as
an index of renal tubular dysfunction, and the average rice cadmium
concentration as an index of cadmium exposure, the authors
determined linear regression equations for men and women. These are
related to the prevalence of ß2-microglobulinuria and total
cadmium intake and are shown in Table 19. The authors concluded that
the total cadmium intake that produced an adverse effect on health
was approximately 2000 mg for both men and women. On the basis of
the linear regression equations shown in Table 19, an average daily
cadmium intake of 440 µg/day in men and 350 µg/day in women would
be expected to cause a 50% response rate (> 1000 µg
ß2-microglobulin/litre urine). Response rates of 20, 10 and 5%
would occur at daily intakes of 220, 150, and 110 µg/day for men and
200, 150, and 120 µg/day for women. The authors indicated that these
data are in general agreement with results from other studies
involving the consumption of various levels of cadmium in rice.
8.3.3.5 Akita prefecture (Kosaka area)
Around Kosaka mine and refinery areas, increased cadmium levels
in rice were first reported by the Akita Prefectural Government
(1973). Kojima et al. (1976) gave further data from the Kosaka area,
where the reported average cadmium level in rice varied between 0.26
and 0.56 mg/kg. The latter average was based on 41 samples where one
was reported to contain 4.81 mg/kg. In the exposed area, the
weighted average of cadmium levels in rice in each district
according to the number of examinees was calculated by Kojima et al.
(1976) to be 0.57 mg/kg in 1973 and 0.50 mg/kg in 1974. These
values, according to Kojima et al. (1976), represented the level of
cadmium exposure in this study and were considered more accurate
than the general average given above.
In an epidemiological investigation of the total population in
the age-group 50-69 of defined geographical areas (93 out of 98 in
the control area and 156 out of 190 in the exposed area
participated), Kojima et al. (1976, 1977) obtained data on faecal
excretion of cadmium. The cadmium level in 24-h faeces samples was
analysed only for those participants who said that they defaecated
once a day (64 in the control area and 118 in the polluted area).
Average rates were 51 µg/day and 177 µg/day for the control and
exposed groups, respectively (Kojima et al., 1976). The prevalence
of proteinuria exceeding 150 mg/litre (using the Tsuchiya biuret
method) and of combined proteinuria and glucosuria (test tape) was
significantly higher in the exposed group than in the control group
(Kojima et al., 1976).
Table 19. Linear regression equations relating total cadmium intake and prevalence of ß2-microglobulinuriaa
Sex ß2-microglobulinuria Linear regression Prevalence of Total cadmium intake
equationb ß2-microglobulinuria (mg)c
in the control group (%)
Male > 1000 µg/litre Y = 0.0076X - 10.33 5.3 2057
> 1000 µg/g creatinine Y = 0.0083X - 7.93 6.0 1678
Female > 1000 µg/litre Y = 0.011X - 19.61 3.1 2065
> 1000 µg/g creatinine Y = 0.012X - 16.16 5.0 1763
a From: Nogawa et al. (1989)
b Y = prevalence of ß2-microglobulinuria (%); X = total cadmium intake (mg)
c Total cadmium intake yielding a ß2-microglobulinuria prevalence equivalent to the control group
Quantitative analysis of urinary ß2-microglobulin with
radioimmunoassay (RIA) was performed on the same population (Kojima
et al., 1977). The frequency distributions were log-normal, as for
occupationally exposed people, and the prevalence of
ß2-microglobulin excretion was 15% in the whole exposed group and
3% in the control group. The exposed and control groups showed
average faecal cadmium excretion rates of 139 and 41 µg per day,
respectively (Kojima et al., 1977).
The ß2-microglobulin data and cadmium intake data in the
study by Kojima et al. (1977) were divided into three dose groups
(Kjellström et al., 1977b) and analysed for a dose-response
relationship by Hutton (1983).
Further studies of urinary ß2-microglobulin over the age
range 5-90 years were reported by Saito et al. (1981). The RIA
method was used, and a control area in Akita prefecture was compared
with cadmium-polluted areas in Akita prefecture (Kosakai), Ishikawa
prefecture (Kakehashi) (section 8.3.3.3), and Nagasaki prefecture
(Tsushima) (section 8.3.3.5). For people over the age of 40, there
were significant increases in average urinary ß2-microglobulin
excretion in all the polluted areas. In the older age-groups, the
increase was 10-100 times above control values.
In the first comprehensive study of proximal renal tubular
functions performed on a population living in a cadmium-polluted
area, Saito et al. (1977) conducted health examinations within Akita
prefecture. Renal tubular function tests consisted of renal
glucosuria, uric acid clearance, low molecular weight proteinuria,
tubular reabsorption of phosphate, hydrogen carbonate threshold,
acid-base balance, concentrating and acidifying ability of urine,
endogenous creatinine clearance, and renal plasma flow. Of the 147
residents (97% of target population) examined, 33 (22%) had some
indications of proximal renal tubular dysfunction, such as renal
glucosuria and low molecular weight proteinuria. In addition, 10
subjects (7%) were diagnosed as having multiple proximal renal
tubular dysfunctions. Detailed examinations revealed that none of
these 10 subjects had experienced any other environmental exposures
or diseases that could have caused the renal dysfunction. They were
therefore diagnosed as suffering from the effects of chronic cadmium
poisoning (Saito et al., 1977).
8.3.3.6 Nagasaki prefecture (Tsushima area)
This area has been a lead and zinc mining district from ancient
times (Takabatake, 1978b). Modern operations started in 1948, and
mining wastes have been scattered throughout the local area. In
1952, local farmers complained about the poor growth of crops. In
the 1960s, studies of cadmium pollution were carried out. Health
examinations for cadmium effects have been conducted since 1966
(Takabatake, 1978b).
Early studies showed an increased prevalence of proteinuria in
the most polluted village, and an average rice cadmium level of
0.75 mg/kg was reported (Takabatake, 1978b).
The study of urinary ß2-microglobulin according to age
referred to in section 8.3.3.4 (Saito et al., 1981) included an
exposed group of people from Tsushima. The age-specific average
urinary excretions were much higher than the control values and
similar to those found in the Kakehashi and Kosaka areas. For women,
the Tsushima values appear higher than those of the other two
polluted areas (Table 17), which may reflect the higher estimated
average cadmium intake in the Tsushima area.
8.3.3.7 Other Japanese areas
As shown in Table 7, health surveys have been performed in
areas other than Fuchu, Ikuno, Kakehashi, Kosaka, and Tsushima.
According to the Japanese Cadmium Research Committee (1989), it
should be emphasized, however, that no cadmium health effects,
including elevated prevalence of ß2-microglobulinuria, were
observed in some areas with higher levels of cadmium (daily intakes
of 180-309 µg in Bandai, Fukushima; 180-380 µg in Annaka, Gunma; and
222-391 µg in Okutake river basin, Oita) even than Kosaka and
Kakehashi (cadmium in rice = 0.16-0.58 mg/kg, daily intake of
cadmium = 139-177 µg in Kosaka, Akita; cadmium in rice =
0.2-0.8 mg/kg, daily intake of cadmium = 160-190 µg in the Kakehashi
river basin, Ishikawa). Also, no health effects were found in the
Uguisuzawa river basin, Miyagi (cadmium in rice = 0.6-0.7 mg/kg),
Watarase river basin, Gunma (0.32 mg/kg), Shimoda, Shizuoka
(0.4-1.1 mg/kg), and Ohmuta, Fukuoka (0.72 mg/kg), even though
residents consumed rice heavily contaminated with cadmium.
8.3.3.8 Belgium
The Liege area of Belgium is known to be polluted by cadmium,
mainly due to the activities of non-ferrous smelters since the end
of the 19th century (Kretzschmar et al., 1980; Lauwerys et al.,
1980a).
A pilot study was performed in 1979 on a group of 60 elderly
non-smoking women who had spent most of their lives in the Liege
area and had never been occupationally exposed to cadmium (Roels et
al., 1981a). Daily intakes of cadmium ranged from 2-88 µg/day with
an average of 15 µg/day. Their average blood cadmium level
(1.6 µg/litre) and their urinary excretion rates of cadmium
(0.093 µg/h), total protein (17.3 mg/h), amino acids (5.45 mg amino
acid N/h), and albumin (1.54 mg/h) were higher than those found in a
group of 70 women of the same age and socio-economic status who
lived in another industrial area (Charleroi) less polluted by
cadmium. Although the average excretion rate of ß2-microglobulin
was greater in Liege (93.6 µg/h) than in Charleroi (22 µg/h), the
difference between the geometric means was not statistically
significant. The two areas were well matched with respect to their
environmental pollution by sulfur dioxide, fume, suspended
particles, and various metals, including lead, vanadium, nickel,
chromium, and iron.
Following these observations, a mortality study and a
preliminary autopsy study were undertaken (Lauwerys & De Wals, 1981;
Lauwerys et al., 1984a). It was found that, although the overall
mortality was not markedly different, the standard mortality ratio
(SMR) and proportional mortality rate (PMR) from nephritis and
nephrosis for the years 1967-1976 were higher in Liege than in
Charleroi or in Belgium as a whole (SMR: Belgium, 100; Charleroi,
102; Liege, 196; PMR: Belgium, 3.3; Charleroi, 3.0; Liege, 6.0).
Since the increased mortality rate for renal diseases was observed
in both males and females, the influence of environmental factors
other than occupation is probable.
The results of the preliminary autopsy study indicated that, in
the age-group 40-60, the average body burden of cadmium was
approximately twice as high in people autopsied in Liege as it was
in those autopsied in a city (Brussels) less polluted by cadmium
(Lauwerys et al., 1984a). The geometric mean values of cadmium
concentration in the kidney cortex were 38.3 and 22.8 mg/kg wet
weight in Liege and Brussels, respectively.
According to the authors of these reports, the studies
performed so far in the Liege area do not refute the hypothesis that
environmental pollution by cadmium in the area has increased the
body burden of cadmium of the inhabitants and has affected their
renal function. A large-scale morbidity and autopsy study is at
present underway (Braux et al., 1987).
8.3.3.9 Shipham area in the United Kingdom
The village of Shipham is located on the slag heaps of an old
zinc mine and high levels of cadmium have been found in soil and
dust (section 3.4.3).
The exposed population has been studied using both mortality
and morbidity end-points. A census in 1979 identified 1092
residents, of whom 64% had resided in the village for more than 5
years, and 548 participated in a health study coordinated by the
United Kingdom Department of the Environment (Barltrop & Strehlow,
1982a). A similar study of 543 age-and sex-matched individuals was
performed in a nearby control village (Barltrop & Strehlow, 1982b).
The daily intake of cadmium in Shipham was an average of 35 µg/day
(section 4.2.4), which is about twice as high as the estimated
United Kingdom national average but much lower than in polluted
areas of Japan (section 5.2.4).
A health inventory was compiled by means of a questionnaire,
which included information on smoking habits, alcohol consumption,
medication, and occupation. Blood samples were analysed for
haemoglobin, haematocrit, serum protein, ß2-microglobulin,
creatinine, erythrocyte protoporphyrin, lead, and cadmium. Urine
samples were analysed for total protein, creatinine, ß2-micro-
globulin, and cadmium.
The mean 24-h urinary concentration of cadmium for Shipham
residents was 0.68 µg cadmium/g creatinine and, in the control area,
0.60 µg cadmium/g creatinine with 97.7% of values less than 3.4 µg
cadmium/litre. The difference was statistically significant
(P < 0.03) (Barltrop & Strehlow, 1982b), but the similarity of the
values for average urinary cadmium concentrations between the two
areas and the generally low levels of cadmium in Shipham suggest a
rather low daily cadmium intake.
However, there are data showing that some individuals in
Shipham had high cadmium exposures. Liver cadmium concentrations,
measured by means of in vivo neutron activation analysis, were
determine for 21 adult volunteers living in the most heavily
contaminated areas of Shipham (Harvey et al., 1979). Their age range
was 40-62 years (mean, 53 years) and, with one exception, they had
lived in Shipham for 9-50 years (mean, 23 years). On average, half
of their vegetable consumption was of local origin. The mean liver
cadmium concentration was 11.0 (+ 2.0) mg/kg, compared with 2.2
(+ 2.0) mg/kg in 20 age-matched, non-Shipham controls (P < 0.001).
The maximum concentration in the Shipham group was 28 mg/kg, which
would correspond to levels in the kidney cortex of at least
200-300 mg/kg (Friberg, 1979) (section 6.4). Health effects were not
studied in this investigation.
In the health study by the Department of the Environment
(Barltrop & Strehlow, 1982a), the comparison of ß2-microglobulin
data from the two villages showed similar distributions, and all
other laboratory data, including blood pressure levels, were
distributed within the normal range. However, the poor participation
rate in this health study (50%), makes it difficult to interpret the
findings. Another study of 31 volunteers from Shipham (Carruthers &
Smith, 1979) reported a high prevalence of hypertension and LMW
proteinuria, but the methodology of the study has been criticized
(Hughes & Stewart, 1979; Kraemer et al., 1979).
Examination of the data in relation to soil cadmium levels
showed no evidence of an increased mortality from any cause in those
living in the most polluted areas. It was concluded from this study
that, if cadmium contamination had any effect on the mortality
pattern in Shipham, this effect was only slight and did not present
a serious health hazard to residents. No case resembling Itai-itai
disease has at any time been reported in Shipham. All the authors
involved in the health studies in Shipham pointed to the possible
protective effect of high levels of zinc also present in soil, and
Kraemer et al. (1979) pointed to the need to assess dietary zinc and
the intake of nutrients other than cadmium.
8.3.3.10 USSR
Screening of populations, both environmentally and
occupationally exposed, which included measurements of urinary
ß2-microglobulin, has been carried out within the USSR (Likutova,
1989). Increased prevalence (up to 6%) of ß2-micro-globulinuria
(> 280 µg/g creatinine) was observed in females (20-50 years old)
in some of the most heavily polluted cities, i.e. Odjonikidze and
Kursk. The air cadmium concentrations in these two cities were
0.085 µg/m3 and 0.005-0.027 µg/m3, respectively. The examination
of workers (50-300 µg/m3) exposed to cadmium also revealed an
increased prevalence of ß2-microglobulinuria (up to 19% in the
most heavily exposed group). The findings are in good agreement with
the data presented in Table 15.
8.4 Conclusions
High inhalation exposure to cadmium oxide fume results in acute
pneumonitis with pulmonary oedema, which may be lethal. High
ingestion exposure of soluble cadmium salts causes acute
gastroenteritis.
Long-term occupational exposure to cadmium has caused severe
chronic effects, predominantly in the lungs and kidneys. Chronic
renal effects have also been seen among the general population.
Following high occupational exposure, lung changes are
primarily characterized by chronic obstructive airway disease. Early
minor changes in ventilatory function tests may progress, with
continued cadmium exposure, to respiratory insufficiency. An
increased mortality rate from obstructive lung disease has been seen
in workers with high exposure, as has occurred in the past.
The accumulation of cadmium in the renal cortex leads to renal
tubular dysfunction with impaired reabsorption of, for instance,
proteins, glucose, and amino acids. A characteristic sign of tubular
dysfunction is an increased excretion of low molecular weight
proteins in urine. In some cases, the glomerular filtration rate
decreases. Increase in urine cadmium correlates with low molecular
weight proteinuria and in the absence of acute exposure to cadmium
may serve as an indicator of renal effect. In more severe cases
there is a combination of tubular and glomerular effects, which may
progress in some cases to decreased glomerular filtration. For most
workers and people in the general environment, cadmium-induced
proteinuria is irreversible.
Among other effects are disturbances in calcium metabolism,
hypercalciuria, and formation of renal stones. High exposure to
cadmium, most probably in combination with other factors such as
nutritional deficiencies, may lead to the development of
osteoporosis and/or osteomalacia.
There is evidence that long-term occupational exposure to
cadmium may contribute to the development of cancer of the lung but
observations from exposed workers have been difficult to interpret
because of confounding factors. For prostatic cancer, evidence to
date is inconclusive but does not support the suggestion from
earlier studies of a causal relationship.
At present, there is no convincing evidence for cadmium being
an etiological agent of essential hypertension. Most data speak
against a blood pressure increase due to cadmium and there is no
evidence of an increased mortality due to cardiovascular or
cerebrovascular disease.
Data from studies on groups of occupationally exposed workers
and on groups exposed in the general environment show that there is
a relationship between exposure levels, exposure durations, and the
prevalence of renal effects.
An increased prevalence of low molecular weight proteinuria in
cadmium workers after 10-20 years of exposure to cadmium levels of
about 20-50 µg/m3 has been reported.
In polluted areas of the general environment, where the
estimated cadmium intake has been 140-260 µg/day, effects in the
form of increased low molecular weight proteinuria have been seen in
some individuals following long-term exposure.
9. EVALUATION OF HUMAN HEALTH RISKS
9.1 Exposure assessment
9.1.1 General population exposure
In the ambient air, cadmium concentrations based on long-term
sampling periods indicate, in most cases, a range of
0.001-0.015 µg/m3 in rural areas, 0.005-0.05 µg/m3 in urban
areas, and up to 0.6 µg/m3 near sources of pollution (section
5.1.1).
One cigarette usually contains 1-2 µg cadmium, of which about
10% may be inhaled (section 5.1.3).
Among staple foods, rice and wheat usually contain less than
0.1 mg/kg and other foods usually less than 0.05 mg/kg wet weight,
but liver and kidney may contain 1-2 mg/kg wet weight and certain
sea-foods as much as 10 mg/kg wet weight (section 5.2). Certain
animals, e.g., the horse, may accumulate considerably higher
concentrations in the liver and kidney. In polluted areas, these
levels are further increased.
The content of natural waters is usually less than 1 µg/litre,
but higher levels may be found near sources of pollution.
The total daily intake in non-polluted areas of most countries
from food, water and air is estimated to be approximately
10-40 µg/day (food, 10-40 µg/day; water, < 1 µg; and air,
< 0.5 µg/day for non-smokers).
Twenty cigarettes per day would contribute a further 2-4 µg. In
polluted areas, the daily intake may be much higher, and intakes of
several hundred µg/day have been reported (section 5.3.2).
9.1.2 Occupational exposure
Air is the main source of additional cadmium exposure for
industrial workers. In many countries such exposures have now been
reduced considerably. In the past, levels of several mg/m3 were
recorded in workplaces. Now, with proper industrial hygiene
practices, levels of 0.02-0.05 mg/m3 would be more typical
(section 5.1.2).
9.1.3 Amounts absorbed from air, food, and water
The proportion of cadmium from food and water that is absorbed
will depend on the chemical nature of the cadmium compounds, but
estimates based on the available data indicate that gastrointestinal
absorption is approximately 5%, with considerable individual
variation (section 6.1.2). Similarly, the amount absorbed from the
air will depend on the chemical nature and the particle size of the
inhaled material. The absorption varies between 25 and 50% depending
on particle size and solubility (section 6.1.1). About 10% of the
cadmium inhaled in cigarette smoke is absorbed.
Thus, the average amount absorbed from food and water in a
person from a non-polluted area would be about 0.5-1.3 µg/day. The
absorbed amount from smoking 20 cigarettes per day would be
1-2 µg/day and that from workroom air could be many times greater
(section 5.3).
9.2 Dose-effect relationships
9.2.1 Renal effects
Long-term exposure to cadmium causes renal tubular dysfunction
with proteinuria, glucosuria, and aminoaciduria, as well as
histopathological changes, in both experimental animals and humans
(sections 7.2.1.4 and 8.2.1, respectively). These are usually the
first effects to occur after ingestion or inhalation exposure. As
the renal dysfunction progresses in severity, the glomeruli may also
be affected and, in a few cases, the cadmium-induced damage may lead
to renal failure (section 8.2.1). Daily cadmium intakes in food of
140-260 µg/day for more than 50 years or workplace air exposures of
50 µg/m3 for more than 10 years have produced an increase in renal
tubular dysfunction in some exposed popu-lations (section 8.3.3.2).
9.2.2 Bone effects
Cadmium may produce bone effects in both humans and animals.
The most notable clinical entity in these cases is osteomalacia, but
many subjects also show osteoporosis. Animal experiments show that
both can be produced by long-term cadmium exposure (section 7.2.4).
In animals and humans, osteomalacia has been seen in combination
with cadmium-induced renal damage. The bone effects may be linked to
cadmium effects on calcium and vitamin D metabolism in the kidney.
The daily intakes via food and exposure levels in air at which the
bone effects occur are uncertain, but they must be higher than those
causing renal effects. Bone effects have been seen among both the
general population and industrial workers in the past when exposure
levels were very high. Host and nutritional factors influence the
development and severity of cadmium-induced bone effects.
9.2.3 Pulmonary effects
Chronic obstructive airway disease has been reported in a
number of studies of cadmium workers (section 8.2.3). This has, in
severe cases, led to an increased mortality. The dose needed to
produce these effects is uncertain, but it is higher than the dose
needed to produce renal effects, as most workers reported to have
lung effects also had renal effects. On the other hand, many workers
with renal effects, who had been exposed to cadmium oxide dust and
fume, had no lung effects.
9.2.4 Cardiovascular effects
Some animal studies have shown that, under certain exposure
conditions, increased blood pressure and effects on the myocardium
occur. Studies of cadmium-exposed workers and people in the general
environment have been carried out, but most data do not support the
animal findings.
9.2.5 Cancer
There is evidence that cadmium chloride, sulfate, sulfide and
oxide give rise to injection site sarcomata in the rat and that the
chloride and sulfate induce interstitial cell tumours of the testis
in rats and mice.
Long-term inhalation studies in rats exposed to aerosols of
cadmium chloride, sulfate, and oxide fume and dust at low
concentrations demonstrated a high incidence of primary lung cancer
with evidence of a dose-response relationship. This has not so far
been shown in other animals.
There is evidence that long-term occupational exposure to
cadmium may contribute to the development of cancer of the lung, but
observations from exposed workers have been difficult to interpret
because of inadequate exposure data and confounding factors. The
evidence to date is inconclusive, but does not support the
suggestion from earlier studies that cadmium can cause prostatic
cancer.
IARC (1987a) considered that there was sufficient evidence for
the carcinogenicity of specified cadmium compounds in experimental
animals and limited evidence for carcinogenicity in humans exposed
to cadmium. A combined evaluation of human and animal data by IARC
(1987b) classified cadmium as a probable human carcinogen (IARC
group 2A). The IPCS Task Group found no reason to deviate from this
IARC evaluation.
9.2.6 Critical organ and critical effect
The kidney is the critical organ for chronic cadmium poisoning.
Within the kidney, the cortex is the site where the first adverse
effect occurs. Therefore, in assessing dose-response relationships,
the cadmium concentration in the kidney cortex is of prime
importance.
The critical effect is renal tubular dysfunction, which is most
often manifested as low molecular weight proteinuria. Animal studies
indicate that histological changes in the renal tubules occur at a
dose level lower than that needed to produce low molecular weight
proteinuria.
9.3 Critical concentration in the kidneys
9.3.1 In animals
Several studies with data on both cadmium concentrations in the
renal cortex and the occurrence of tubular damage were discussed in
section 7.2.1. The findings were summarized in Table 12. They showed
that histological tubular lesions or proteinuria was usually seen at
cadmium renal cortex levels of 200-300 mg/kg wet weight. In some
studies on rats, monkeys, horses, and birds, certain effects were
seen at lower levels.
As no dose-response data are given in most animal studies, it
may be assumed that these renal cortex levels correspond to a 50%
response rate (CC50). Naturally, the cadmium levels at which lower
response rates occur would be lower.
In studies on monkeys conducted in Japan, kidney cadmium levels
were related to dose and duration of exposure. At the two highest
dose levels, acute liver effects occurred. If one wishes to
establish a range of values for the critical concentration in
individuals at which a small but significant part of an exposed
population will show effects, animal studies indicate that a renal
cortex level of about 100-200 mg/kg is likely to coincide with such
a range. There is some evidence that the average critical
concentration (CC50) could be as high as 300 or 400 mg/kg for the
more severe signs of renal tubular damage, but such high levels
should not be used as a starting point for calculations of
"acceptable daily exposures".
9.3.2 In humans
Section 8.2.1.5 reviewed all available data from cases in which
both renal cortex cadmium levels and renal effects were measured.
Data from autopsies or biopsies have mainly been cross-sectional,
i.e. the renal cadmium concentrations and the effects were measured
more or less simultaneously. This has made it difficult to interpret
the data from a critical concentration point-of-view, as the cases
with the most severe cadmium-induced kidney dysfunction had the
lowest renal cadmium levels. Cadmium is lost from the kidney when
the damage progresses (section 6.5.1.2).
In vivo neutron activation analysis has provided a new tool
for establishing the human critical concentration of cadmium in the
renal cortex. Longitudinal studies measuring the renal cortex
cadmium concentration several times during continued exposure can
now be carried out. The cadmium level at which the first measurable
signs of renal tubular dysfunction occurs can be estimated. However,
only two studies using in vivo neutron activation have been
published to date, and both of them are cross-sectional.
The renal cadmium concentrations are disproportionately low
when the liver cadmium concentrations are high and renal effects
have developed. Of the several methods available to estimate the
average critical kidney concentration in these groups of exposed
workers, the method of preference assumes that the peak for renal
cortex cadmium level, plotted against liver cadmium, is equivalent
to the point where renal tubular dysfunction occurs. This results in
a value of 319 mg/kg tissue (based on a ratio of renal cortex
cadmium to whole kidney cadmium of 1.5). There is considerable
variance in the individual values, the 95% tolerance (which
corresponds to a confidence interval) being in the range ± 90 mg/kg
from the mean. Other studies, using similar assumptions, have
reported a value of 332 mg/kg, 10% of the workers having a peak
cadmium level of about 216 mg/kg tissue. A re-evaluation of the
original study resulted in a calculated cadmium level of about
200 mg/kg. It was concluded that for the purposes of dose-response
calculations, using a metabolic model, this concentration could be
used as a starting point for renal effects occurring in an exposed
population.
9.4 Dose-response relationships for renal effects
Two approaches can be used to estimate dose-response
relationships. One employs epidemiological data from industry and
the general environment studying associations between exposure and
response. The other begins with a critical concentration in the
kidney cortex and employs a metabolic model to calculate, on the
basis of certain given assumptions, the exposure that is required to
reach a critical concentration.
9.4.1 Evaluation based on data on industrial workers
Table 16 contains data from various group studies on cadmium
workers. In most of these studies, the dose measurements were based
on short sampling periods (hours or a few days). However, exposure
may have lasted for decades, levels usually being higher in the
past. The use of protective devices may also confound the picture.
As discussed in section 8.3.2, there are now several reports
available that show a clear exposure-response relationship between
cadmium in workplace air and the prevalence of proteinuria.
An increased prevalence of overt proteinuria, as measured by
sulfosalicylic acid, trichloroacetic acid, or quantitative
determination of total proteinuria, can occur after only 5-10 years
of exposure to approximately 100 µg cadmium/m3. If instead, the
increased excretion of low molecular weight proteins (more than 97.5
percentile of control group) is used as the critical effect, 10-20%
of workers would have this effect after a cumulative dose
corresponding to 10-20 years of exposure to 50 µg cadmium/m3.
These evaluations are all based on levels of total cadmium in
inhaled dust or air.
9.4.2 Evaluation based on data on the general population
As indicated in chapter 8, there exists a considerable amount
of information from epidemiological studies carried out on the
general population in Japan. It was shown that in some areas of high
cadmium exposure the prevalence of low molecular weight proteinuria
was significantly higher than in control areas. This may be
considered in relation to the known cadmium concentrations in rice
and the daily cadmium intakes in the affected areas (Tables 7 and
17). Contamination of drinking-water in some areas may be a
complicating factor (section 8.3.3).
Taking all of the data in section 8.3.3 together, it seems
that, when the most sensitive method for diagnosis of low molecular
weight proteinuria is applied, there is an association between
cadmium exposure and increased excretion of low molecular weight
proteins among some people over 50 years of age at a daily intake of
about 140-260 µg cadmium or a cumulative cadmium intake of about
2000 mg or more (for both men and women).
9.4.3 Evaluation based on a metabolic model and critical
concentrations
Using the data on critical concentrations and kinetic models of
cadmium metabolism, attempts have been made to calculate the
dose-response relationship for cadmium. Assuming that cadmium in the
kidney is accumulated in accordance with a one-compartment model and
that a third or a quarter of the body burden of cadmium is in the
kidney (and making certain other assumptions indicated in Tables 20
and 21), the daily cadmium intake via food and the occupational air
concentrations needed to reach the critical concentration have been
calculated (Tables 20 and 21).
As the values calculated in Tables 20 and 21 are for an average
person, not all of those exposed to these levels would have reached
the renal cortex cadmium concentration of 200 mg/kg tissue or their
individual critical concentration. Nevertheless, these calculations
produce values that are similar to the levels at which effects have
been observed, and the model approach may be a useful way to
quantify the response rates at levels lower than those easily
measurable.
Calculations have been reported of the relationship between
intake and response rates using the observed frequency distributions
of daily intake and renal cortex cadmium concentrations, and
utilizing multi-compartment metabolic model values in the same range
as those given in Tables 20 and 21. Further development of these
modelling techniques would be of value.
Using a single-compartment model for the accumulation of
cadmium in the kidney, the average daily intake that would give rise
to an average concentration of 200 mg/kg wet weight in the kidney
cortex at age 50 would be 260-480 µg/day, assuming 5%
gastrointestinal absorption, various biological half-times, and
different proportions of the body burden in the kidneys (Table 19).
Assuming a 10% absorption rate, the intake needed would be
140-260 µg per day. These estimates will vary depending on the body
weight estimates for different populations.
Table 20. Calculated daily cadmium intake via ingestion required by a
non-smoker to reach a kidney cortex concentration of 200 mg/kg
at age 50 (using a one-compartment model)a
Gastrointestinal Proportion of body Estimated half-time in kidney cortexb
absorption rate burden in kidney
(%) 17 years 30 years
5 one-third 365 µg (286 µg) 265 µg (208 µg)
10 182 µg (143 µg) 133 µg (104 µg)
5 one-quarter 486 µg (382 µg) 353 µg (277 µg)
10 243 µg (191 µg) 177 µg (139 µg)
a The data in the table are based on the following assumptions:
gastrointestinal absorption, either 5% or 10%;
half-time in kidney cortex, either 17 years or 30 years (as reported in
section 6.6.2);
one-third or one-quarter of body burden in the kidneys;
cadmium concentration in renal cortex 25% higher than renal average;
average weight of both kidneys at age 50 of 300 g for a 70-kg person
or 235 g for a 55-kg person;
average cadmium concentration in foodstuffs constant during
the last 50 years;
variation of daily intake with age has been disregarded since such
variation would influence the values by less than 10%.
b Data have been calculated for a 70-kg person; values in parentheses
are for a 55-kg person.
Table 21. Calculated concentration of cadmium in industrial air required
for a kidney cortex concentration of 200 mg/kg to be reacheda
Proportion of body burden Estimated half-time in kidney cortexb
in kidney
17 years 30 years
one-third 16 µg/m3 (13 µg/m3) 14 µg/m3 (11 µg/m3)
one-quarter 21 µg/m3 (17 µg/m3) 19 µg/m3 (15 µg/m3)
a The data in the table are based on the following assumptions:
those assumptions given in Table 20;
exposure time of 25 years;
225 working days per year;
10 m3 of air inhaled per day;
25% pulmonary absorption
b Data have been calculated for a 70-kg worker; values in parentheses
are for an average 55-kg person
10. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF
HUMAN HEALTH
10.1 Conclusions
The kidney is considered the critical target organ for the
general population as well as for occupationally exposed
populations. Chronic obstructive airway disease is associated with
long-term high-level occupational exposure by inhalation. There is
some evidence that such exposure to cadmium may contribute to the
development of cancer of the lung but observations from exposed
workers have been difficult to interpret because of confounding
factors.
10.1.1 General population
Food-borne cadmium is the major source of exposure for most
people. Average daily intakes from food in most areas not polluted
with cadmium are 10-40 µg. In polluted areas the value has been
found to be several hundred µg per day. In non-polluted areas,
uptake from heavy smoking may equal cadmium intake from food.
An association between cadmium exposure and increased urinary
excretion of low molecular weight proteins has been noted in humans
with a life-long daily intake of about 140-260 µg cadmium, or a
cumulative intake of about 2000 mg or more.
10.1.2 Occupationally exposed population
Occupational exposure to cadmium is mainly by inhalation but
includes additional intakes through food and tobacco. The total
cadmium level in air varies according to industrial hygiene
practices and type of workplace. There is an exposure-response
relationship between airborne cadmium levels and proteinuria. An
increase in the prevalence of low molecular weight proteinuria may
occur in workers after 10-20 years of exposure to cadmium levels of
about 20-50 µg/m3. In vivo measurement of cadmium in the liver
and kidneys of people with different levels of cadmium exposure have
shown that about 10% of workers with a kidney cortex level of
200 mg/kg and about 50% of people with a kidney cortex level of
300 mg/kg would have renal tubular proteinuria.
10.2 Recommendations for protection of human health
a) Measures to increase recycling of cadmium should be
systematically examined and promising ideas encouraged.
b) Information on the importance of minimizing waste discharge
of cadmium, particularly into surface waters, should be
supplied to countries.
c) Public health measures for protection from cadmium
exposures would be improved by:
i) collection of more data from countries on cadmium
levels in foodstuffs and the environment;
ii) determination of tissue cadmium levels and
monitoring of health parameters in non-exposed
populations and in those living near mines or
smelters or exposed to elevated levels of the metal
in foodstuffs;
iii) technical assistance to developing countries for
the training of staff, particularly for cadmium
analysis;
iv) development of means of reducing cadmium exposure
by, for instance, improved working conditions and
the dissemination of information on the proper use
of fertilizers (which sometimes contain high levels
of cadmium), techniques for the disposal of
cadmium-containing wastes, etc.
11. FURTHER RESEARCH
a) There is a need for improved analytical techniques for
measuring cadmium species and biological indicators of cadmium
exposure/toxicity, such as ß2-microglobulin, in various
matrices, and for international centres for quality assurance
and training.
b) The assessment of human exposure to cadmium from all media
needs to be improved by increased monitoring of cadmium levels
in the environment. Changes in cadmium levels with time are of
particular importance.
c) Populations with ß2-microglobinuria (both those in the
workplace and in the general environment) should be
longitudinally investigated to determine the nature, severity,
and prognosis of adverse health effects associated with this
finding. Further research is needed on ß2-microglobulin as a
biological indicator of exposure and effect.
d) International collaborative efforts should be encouraged to
examine further the role of cadmium in the development of human
cancer. Both the general population and industrial workers
should be studied with special emphasis on the development of a
common format for analysing and presenting data and the
collection of additional information on exposure to cadmium,
tobacco, and other confounding factors. Multiple exposures must
be considered. It is proposed that a collaborative study
coordinated by an international body (e.g., IARC) should
include the existing cohorts in order to obtain better exposure
data. It should also collect both exposure and effects data in
a standardized manner, so that the results of different studies
may be more readily compared. A further approach would be to
perform a collaborative prospective study identifying all those
workers who have shown evidence of an effect of cadmium on the
kidney and who would therefore be considered to have had
unusually heavy exposure. In such a study, both morbidity and
mortality data would be collected. Outcome would be studied not
only for cancer but also for sequelae to renal dysfunction.
e) Existing occupational cohorts should be linked, where
possible, to regional cancer registers to determine the
incidence of prostatic cancer (morbidity) in relation to
cadmium exposure.
f) To understand the mechanism(s) of cancer induction,
experimental studies on the bioavailability of cadmium at the
target site and the interactions between zinc and cadmium would
be of value. The role of metallothionein induction in the
target cells of the respiratory tract and its relationship to
such phenomena as DNA damage and repair and oncogene protein
structure would be of interest.
g) Further information on the long-term health consequences of
cadmium exposures in the general environment is essential, with
emphasis on renal dysfunction and other end-points such as
neurotoxicity and immunotoxicity.
h) Studies of the effects of cadmium on calcium-phosphorus
metabolism and bone density should be conducted on female
workers to clarify whether these workers are at special risk in
the occupational setting. The effect of cadmium on the placenta
and subsequent effects on the fetus, especially in multiple
pregnancies, need further study.
i) The effects of various nutritional deficiencies and of exposure
to other metals on the transport, accumulation, and toxicity of
cadmium should be investigated with special reference to bone
toxicity. These studies should be conducted in humans and
experimental animals with respect to age, sex, dose-dependence,
biological half-time, and estimation of critical concentration.
j) To provide additional scientific support for the assessment of
human health risks from cadmium exposure, studies in
experimental animals addressing the following issues should be
initiated:
* mechanism of cadmium transport into the cell and factors
controlling the process;
* mechanism of cadmium-induced toxicity with particular
emphasis on kidney and bone and the role(s) of
non-metallothionein-bound cadmium in these processes;
* mechanisms of cadmium-induced calcuria and the
relationship of this phenomenon to tubular proteinuria and
osteomalacia.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenic potential of cadmium was evaluated in 1976 by
the International Agency for Research on Cancer (IARC, 1976) and
re-evaluated in 1987 (IARC, 1987a). It was concluded in the
re-evaluation that there was limited evidence that cadmium and
cadmium compounds are carcinogenic in humans. Sufficient evidence
was available to show that cadmium and specified cadmium compounds
cause cancer in experimental animals. Cadmium was classified as a
probable human carcinogen (group 2A) (IARC, 1987a,b).
To prevent adverse pulmonary and renal effects the following
health-based limits for occupational exposure to cadmium fumes and
respirable dust were proposed by WHO (WHO, 1980): 250 µg Cd/m3 for
short-term exposures provided the recommended time-weighted average
(40 h/week) of 10 µg Cd/m3 is respected. It was further
recommended that control measures be applied when cadmium levels in
urine and blood of individuals exceed 5 µg Cd/g creatinine and
5 µg Cd/litre of whole blood, respectively.
A drinking-water guideline value of 0.005 mg/litre has been set
for cadmium by the World Health Organization (WHO, 1984).
Cadmium was evaluated by a WHO Working Group developing air
quality guidelines (WHO, 1987). Based on non-carcinogenic effects,
the following recommendations were made:
a) in rural areas, levels of < 1-5 ng/m3 should not be allowed
to increase, and
b) in urban and industrialized areas without agricultural
activities, levels of 10-20 ng/m3 may be tolerable. However,
increases in the present levels of airborne cadmium should not
be permitted (WHO, 1987).
At the thirty-third meeting of the Joint FAO/WHO Expert
Committee on Food Additives and Food Contaminants, the previous
recommendation was reaffirmed, i.e. the provisional tolerable weekly
cadmium intake of 400-500 µg for an adult should not be exceeded
(WHO, 1989).
Regulatory standards established by national bodies in several
countries and the EEC are summarized in the legal file of the
International Register of Potentially Toxic Chemicals (IRPTC, 1987).
REFERENCES
ADAMS, R.G. (1980) Osteopathy associated with tubular nephropathy in
employees in an alkaline battery factory. In: Shigematsu, I. &
Nomiyama, K., ed. Cadmium-induced osteopathy, Tokyo, Japan Public
Health Association, pp. 67-73.
ADAMS, R.C., HARRISON, J.G., & SCOTT, P. (1969) The development of
cadmium-induced proteinuria, impaired renal function, and
osteomalacia in alkaline battery workers. Q. J. Med., 38: 425-443.
ADAMSSON, E. (1979) Long-term sampling of air-borne cadmium dust in
an alkaline battery factory. Scand. J. Work Environ. Health,
5: 178-187.
ADES A.E. & KAZANTZIS, G. (1988) Lung cancer in a non-ferrous
smelter: the role of cadmium. Br. J. ind. Med., 45: 435-442.
AHLGREN, L. & MATTSON, S. (1981) Cadmium in man measured
in vivo by X-ray fluorescence analysis. Phys. Med. Biol.,
26: 19-26.
AHLMARK, S., AXELSSON, B., FRIBERG, L., & PISCATOR, M. (1961)
Further investigations into kidney function and proteinuria in
chronic cadmium poisoning. Int. Congr. occup. Health, 13: 201-203.
AHOKAS, R.A. & DILTS, P.V. (1979) Cadmium uptake by the rat embryo
as a function of gestational age. Am. J. Obstet. Gynecol.,
135: 219-222.
AKITA PREFECTURAL GOVERNMENT (1973) [Surveys on environmental
pollution due to heavy metals: Annual report of the Technical Centre
for Environmental Protection], Akita, Japan, Akita Prefectural
Government (Akita Prefecture Report No. 1: 1970 and 1971) (in
Japanese).
AL-HADDAD, I.K., CHETTLE, D.R., FLETCHER, J.G., & FREMLIN, J.H.
(1981) A transportable system for measurement of kidney cadmium
in vivo. Int. J. appl. Radiat. Isot., 32: 109-112.
ALLANSON, M. & DEANESLY, R. (1962) Observations on cadmium damage
and repair in rat testes and the effects on the pituitary
gonadotrophs. J. Endocrinol., 24: 453-462.
ALLOWAY, B.J., THORNTON, I., SMART, G.A., SHERLOCK, J.C., & QUINN,
M.J. (1988) Metal availability. Sci. total Environ., 75: 41-69.
ALT, F. (1981) [Comparative determination of cadmium in blood by
four different methods.] Fresenius Z. anal. Chem., 308: 137-142 (in
German).
ANDERSEN, A. (1979) [Lead, cadmium, copper and zinc in the Danish
diet], Copenhagen, Statens Levendsmiddelinstitut, 89 pp, (Report
No. 40) (in Danish).
ANDERSEN, O., HAGERSTRAND, I., & NORDBERG, G.F. (1982) Effect of the
chelating agent sodium tripolyphosphate on cadmium toxicity in mice.
Environ. Res., 29: 54-61.
ANDERSEN, O., RONNE, M., & NORDBERG, G.F. (1983) Effects of
inorganic metal salts on chromosome length in human lymphocytes.
Hereditas, 98: 65-70.
ANDERSON, C. & DANYLCHUK, K.D. (1979) Plasma levels of
immunoreactive parathyroid hormone in dogs chronically exposed to
low levels of cadmium chloride. J. environ. Pathol. Toxicol.,
2: 1151-1159.
ANDERSSON, A. (1977) Heavy metals in Swedish soils: on their
retention, distribution, and amounts. Swed. J. agric. Res., 7: 7-20.
ANDERSSON, A. & HAHLIN, M. (1981) Cadmium effects from phosphorus
fertilization in field experiments. Swed. J. agric. Res., 11: 3-10.
ANDERSSON, K., ELINDER, C.-G., HOGSTEDT, C., KJELLSTROM, T., &
SPANG, G. (1984) Mortality among cadmium workers in a Swedish
battery factory. In: Proceedings of the 4th International Cadmium
Conference, Munich, March 1983, London, Cadmium Association,
pp. 152-154.
ANDO, M., SHIMIZU, M., SAYATA, Y., TANIMURA, A., & TOBE, M. (1981)
The inhibition of vitamin D-stimulated intestinal calcium transport
in rats after continuous oral administration of cadmium. Toxicol.
appl. Pharmacol., 61: 297-301.
ANKE, M. & SCHNEIDER, H.J. (1974) [The trace element concentration
of human kidneys as a function of age and sex.] Z. Urol.,
67: 357-362 (in German).
ANKE, M., HENNIG, A., SCHNEIDER, H.J., GROPPEL, B., GRUN, M.,
PARTSCHEFELD, M., & LUDKE, H. (1976) [The influence of the toxic
element cadmium on the metabolism and health of animals and human.]
Math. naturwiss. Unterr. R 25: 241-261 (in German).
AOKI, A. & HOFFER, A.P. (1978) Re-examination of the lesions in rat
testis caused by cadmium. Biol. Reprod., 18: 579-591.
APOSTOLOV, CH. (1979) [The effects on the upper respiratory tract
from long-term work with cadmium.] In: [Proceedings of the Cadmium
Symposium, Jena, 1-3 August, 1977], Jena, Friedrich-Schiller
Universität, pp. 322-325 (in German).
ARMSTRONG, B.G. & KAZANTZIS, G. (1983) The mortality of cadmium
workers. Lancet, 1: 1424-1427.
ARMSTRONG, B.G. & KAZANTZIS, G. (1985) Prostatic cancer and chronic
respiratory and renal disease in British cadmium workers: a case
control study. Br. J. ind. Med., 42: 540-545.
ASOKAN, P., DIXIT, R., MUKHTAR, H., & MURTI, C.R.K. (1981) Effect of
cadmium on hepatic mixed function oxidases during the early
development of rats; possible protective role of metallothionein.
Biochem. Pharmacol., 30(22): 3095-3098.
AUFDERHEIDE, M., MOHR, U., THIEDEMANN, K.-U., & HEINRICH, U. (1990)
Quantification of hyperplastic areas in hamster lungs after chronic
inhalation of different cadmium compounds. Toxicol. environ. Chem.,
27: 173-180.
AXELSSON, B. (1963) Urinary calculus in long-term exposure to
cadmium. In: Sangro, P., Akoun, G., & Beate, H.L., ed. Proceedings
of the 14th International Congress on Occupational Health, Madrid,
Amsterdam, Excerpta Medica Foundation, pp 939-942 (International
Congress Series No. 62).
AXELSSON, B. & PISCATOR, M. (1966a) Renal damage after prolonged
exposure to cadmium: an experimental study. Arch. environ. Health,
12: 360-373.
AXELSSON, B. & PISCATOR, M. (1966b) Serum proteins in
cadmium-poisoned rabbits with special reference to haemolytic
anaemia. Arch. environ. Health, 12: 374-381.
AXELSSON, B., DAHLGREN, S.E., & PISCATOR, M. (1968) Renal lesions in
the rabbit after long-term exposure to cadmium. Arch. environ.
Health, 17: 24-28.
BAADER, E.W. (1951) [Health concerns and occupational medicine:
chronic cadmium poisoning.] Dtsch. med. Wschr., 76: 484-487 (in
German).
BAETZ, R.A. & KENNER, C.T. (1974) Determination of low levels of
cadmium in foods using a chelating ion exchange resin. J. Assoc.
Off. Anal. Chem., 57: 14-17.
BARLOW, S.M. & SULLIVAN, F.M. (1982) Cadmium and its compounds. In:
Barlow, S.M., ed. Reproductive hazards of industrial chemicals. An
evaluation of animal and human data, New York, London, Academic
Press, pp. 137-173.
BARLTROP, D. & STREHLOW, C.D. (1982a) Clinical and biochemical
indices of cadmium exposure in the population of Shipham. In:
Proceedings of the 3rd International Cadmium Conference, Miami,
Florida, 3-5 February, 1981, London, Cadmium Association,
pp. 112-113.
BARLTROP, D. & STREHLOW, C.D. (1982b) Cadmium and health in Shipham.
Lancet, 2: 1394-1395.
BARR, M., Jr (1973) The teratogenicity of cadmium chloride in two
stocks of Wistar rats. Teratology, 7: 237-242.
BARRETT, H.M. & CARD, B.Y. (1947) Studies on the toxicity of inhaled
cadmium. II. The acute lethal dose of cadmium oxide for man. J. ind.
Hyg. Toxicol., 29: 286-293.
BARRETT, H.M., IRWIN, D.A., & SEMMONS, E. (1947) Studies on the
toxicity of inhaled cadmium. I. The acute toxicity of cadmium oxide
by inhalation. J. ind. Hyg. Toxicol., 29: 279-285.
BARTHELEMY, P. & MOLINE, R. (1946) Intoxication chronique par
l'hydrate de cadmium, son signe précoce: La bague jaune dentaire.
Paris Med., 1: 71-78.
BAUCHINGER, M., SCHMID, E., EINBRODT, H.J., & DRESP, J. (1976)
Chromosome aberrations in lymphocytes after occupational exposure to
lead and cadmium. Mutat. Res., 40: 57-62.
BECKMAN, I., MOVITZ, I., NYGREN, M., & SLORACH, S. (1979) [Release
of lead and cadmium from glazed and enamelled foodware.] Var Föda,
31: 193-197 (in Swedish).
BEHNE, D. (1980) Problems of sampling and sample preparation for
trace element analysis in the health sciences. In: Bratter, P. &
Schramel, P., ed. Trace element analytical chemistry in medicine and
biology. Proceedings of the First International Workshop,
Neuherberg, Federal Republic of Germany, Berlin, New York, Walter de
Gruyter & Co., pp. 769-782.
BERLIN, M. & FRIBERG, L. (1960) Bone marrow activity and erythrocyte
destruction in chronic cadmium poisoning. Arch. environ. Health,
1: 478-486.
BERMAN, E. (1967) Determination of Cd, Tl, and Hg in biological
materials by AA. At. Absorpt. Newsl., 6: 57-60.
BERNARD, A.M., ROELS, H., HUBERMONT, G., BUCHET, J.P., MASSON, P.L.,
& LAUWERYS, R. (1976) Characterization of the proteinuria in
cadmium-exposed workers. Int. Arch. occup. environ. Health,
38: 19-30.
BERNARD, A.M., BUCHET, J.P., ROELS, H., MASSON, P., & LAUWERYS, R.
(1979) Renal excretion of proteins and enzymes in workers exposed
to cadmium. Eur. J. clin. Invest., 9: 11-22.
BERNARD, A.M., LAUWERYS, R., & GENGOUX, P. (1981) Characterization
of the proteinuria induced by prolonged oral administration of
cadmium in female rats. Toxicology, 20: 345-357.
BERNARD, A.M., MOREAU, D., & LAUWERYS, R. (1982a) Latex immunoassay
of retinol binding protein. Clin. Chem., 28: 1167-1171.
BERNARD, A.M., MOREAU, D., & LAUWERYS, R. (1982b) Comparison of
retinol-binding protein and ß-2-microglobulin determination in urine
for the early detection of tubular proteinuria. Clin. Chim. Acta,
126: 1-9.
BETON, D.C., ANDREWS, G.S., DAVIES, H.J., HOWELLS, L., & SMITH, G.F.
(1966) Acute cadmium fume poisoning: five cases with one death from
renal necrosis. Br. J. ind. Med., 23: 292-301.
BHATTACHARYYA, M.H., WHELTON, B.D., PETERSON, D.P., CARNES, B.A.,
MORETTI, E.S., TOOMEY, J.M., & WILLIAMS, L.L. (1988) Skeletal
changes in multiparous mice fed a nutrient-sufficient diet
containing cadmium. Toxicology, 50: 193-204.
BIGGIN, H.C., CHEN, N.S., ETTINGER, K.V., FREMLIN, J.H., MORGAN,
W.D., NOWOTNY, R., CHAMBERLAIN, M.J., & HARVEY, T.C. (1974) Cadmium
by in vivo neutron activation analysis. J. radioanal. Chem.,
19: 207-214.
BJORNBERG, A. (1963) Reactions to light in yellow tattoos from
cadmium sulfide. Arch. Dermatol., 88: 267-271.
BLEJER, H.P. (1966) Death due to cadmium oxide fumes. Ind. Med.
Surg., 35: 363-364.
BOISSET, M. & BOUDENE, C. (1981) Effect of a single exposure to
cadmium oxide fumes on rat lung microsomal enzymes. Toxicol. appl.
Pharmacol., 57: 335-345.
BONNELL, J.A. (1955) Emphysema and proteinuria in men casting
copper-cadmium alloys. Br. J. ind. Med., 12: 181-197.
BONNELL, J.A., KAZANTZIS, G., & KING, E. (1959) A follow-up study of
men exposed to cadmium oxide fume. Br. J. ind. Med., 16: 135-147.
BONNELL, J.A., ROSS, J.H., & KING, E. (1960) Renal lesions in
experimental cadmium poisoning. Br. J. ind. Med., 17: 69-80.
BOULEY, G., DUBREUIL, A., DESPAUX, N., & BOUDENE, C. (1977) Toxic
effects of cadmium microparticles on the respiratory system. An
experimental study on rats and mice. Scand J. Work Environ. Health,
3: 116-121.
BOYLE, E.A., SCALTER, F., & EDMOND, J.M. (1976) On the marine
geochemistry of cadmium. Nature (Lond.), 263: 42-44.
BOZELKA, B.E. & BURKHOLDER, P.M. (1982) Inhibition of mixed
leukocyte culture response in cadmium-treated mice. Environ. Res.,
27: 421-432.
BRADY, F.O. (1982) The physiological function of metallothionein.
Trends biochem. Sci., 7: 143-145.
BRAUX, P., CLAEYS-THOREAU, F., DE PLAAN, P., DUCOFFRE, G., AMERY,
A., LAUWERYS, R., RONDIA, D., STAESSEN, J., ROELS, H., SARTON, F.,
BERNARD, A., & BUCHET, J.P. (1987) The Belgian Cadmibel Study:
Environmental exposure to cadmium and lead-health effects. In:
Lindberg, S.F. & Hutchinson, J.C., ed. Proceedings of an
International Conference on Heavy Metals in the Environment, New
Orleans, Edinburgh, CEP Consultants Ltd, pp. 349-351.
BRYAN, G.W., LANGSTON, W.J., & HUMMERSTONE L.G. (1980) The use of
biological indicators of heavy-metal contamination in estuaries with
special reference to an assessment of the biological availability of
metals in estuarine sediments from south-west Britain, Citadel Hill,
Devon, The Marine Biological Association of the United Kingdom,
73 pp (Occasional Publication No. 1).
BUATMENARD, P. & ARNOLD, M. (1978) Heavy-metal chemistry of
atmospheric particulate matter emitted by Mount Etna volcano. Geogr.
Res. Lett., 5: 245-248.
BUCHAUER, M.J. (1972) The effects of zinc and cadmium pollution on
forest vegetation. Bull. New Jersey Acad. Sci., 17(2): 42.
BUCHET, J.P., ROELS, H., BERNARD, A., & LAUWERYS, R. (1980)
Assessment of renal function of workers exposed to inorganic lead,
cadmium or mercury vapor. J. occup. Med., 22: 741-748.
BUCHET, J.P., ROELS, H., LAUWERYS, R., VANDEVOORDE, A., & PYCKE,
J.M. (1983) Oral daily intake of cadmium, lead, manganese, copper,
chromium, mercury, calcium, zinc, and arsenic in Belgium: a
duplicate meal study. Food chem. Toxicol., 21: 19-24.
BUCKE, D., NORTON, M.G., & ROLFE, M.S. (1983) Field assessment of
effects of dumping wastes at sea: II. Epidermal lesions and
abnormalities of fish in the outer Thames estuary, London, Ministry
of Agriculture, Fisheries and Food, 29 pp (Technical Report No. 72).
BUI, T.-H-. LINDSTEN, J., & NORDBERG, G.F. (1975) Chromosome
analysis of lymphocytes from cadmium workers and Itai-itai patients.
Environ. Res., 9: 187-195.
BUS, J.S., VINEGAR, A., & BROOKS, S.M. (1978) Biochemical and
physiologic changes in lungs of rats exposed to a cadmium chloride
aerosol. Am. Rev. respir. Dis., 118: 573-580.
BUTLER, E.A. & FLYNN, F.V. (1958) The proteinuria of renal tubular
disorders. Lancet, 2: 978-980.
BUXTON, R., St J. (1956) Respiratory function in cadmium alloy
casters. Part 2. The estimation of the total lung volume, its
subdivisions and the mixing coefficient. Br. J. ind. Med.,
13: 36-40.
CARLIER, Y., COLLE, A., TACHON, P., BOUT, D., & CAPRON, A. (1981)
Quantification of B-2-microglobulin by enzyme inhibition
immunoassay. J. immunol. Methods, 40: 231-238.
CARLSON, L.A. & FRIBERG, L. (1957) The distribution of cadmium in
blood after repeated exposure. Scand. J. clin. lab. Invest., 9: 1-4.
CARLTON-SMITH, C.H. (1987) Effects of metals in sludge-treated soils
on crops, Medmenham, United Kingdom, Water Research Centre
(Environment TR/251).
CARROLL, R.E. (1966) The relationship of cadmium in the air to
cardiovascular disease death rates. J. Am. Med. Assoc.,
198: 267-269.
CARRUTHERS, M. & SMITH, B. (1979) Evidence of cadmium toxicity in a
population living in a zinc-mining area. Lancet, 1: 845-847.
CARVALHO, F.M., TAVARES, T.M., SILVANY-NETO, A.M., LIMA, M.E.C., &
ALT, F. (1986) Cadmium concentrations in blood of children living
near a lead smelter in Bahia, Brazil. Environ. Res., 40: 437-449.
CASTERLINE, J.L. & YIP, G. (1975) The distribution and binding of
cadmium in oyster, soybean, rat liver, and kidney. Arch. environ.
Contam. Toxicol., 3: 319-329.
CASTILHO, P.D. & HERBER, R.F.M. (1977) The rapid determination of
cadmium, lead, copper, and zinc in whole blood by atomic absorption
spectrometry with electrothermal atomization. Improvements in
precision with a peak-shape monitoring device. Anal. Chim. Acta,
94: 269-270.
CHADWICK, M.J. & LINDMAN, N. (1982) Environmental implications of
expanded coal utilization, Oxford, New York, Pergamon Press, 283 pp.
CHAPATWALA, K.D., HOBSON, M., DESAIAH, D., & RAJANNA, B. (1982)
Effect of cadmium on hepatic and renal gluconeogenic enzymes in
female rats. Toxicol. Lett., 12: 27-34.
CHAUBE, S., NISHIMURA, H., & SWINYARD, C.A. (1973) Zinc and cadmium
in normal human embryos and fetuses. Arch. environ. Health,
26: 237-240.
CHERIAN, M.G. (1980) The synthesis of metallothionein and cellular
adaptation to metal toxicity in primary kidney epithelial cell
cultures. Toxicology, 17: 225-231.
CHERIAN, M.G. & GOYER, R.A. (1978) Metallothioneins and their role
in the metabolism and toxicity of metals. Life Sci., 23: 1-10.
CHERIAN, M.G. & NORDBERG, M. (1983) Cellular adaptation in metal
toxicology and metallothionein. Toxicology, 28: 1-15.
CHERIAN, M.G. & SHAIKH, Z.A. (1975) Metabolism of
intravenously-injected cadmium-binding protein. Biochem. biophys.
Res. Commun., 65: 863-869.
CHERIAN, M.G. & VOSTAL, J.J. (1977) Biliary excretion of cadmium in
rat. I. Dose-dependent biliary excretion and the form of cadmium in
the bile. J. Toxicol. environ. Health, 2: 945-954.
CHERIAN, M.G., GOYER, R.A., & RICHARDSON, L.D. (1976) Cadmium
metallothionein-induced nephropathy. Toxicol. appl. Pharmacol.,
38: 399-408.
CHERIAN, M.G., GOYER, R.A., & VALBERG, L.S. (1978) Gastrointestinal
absorption and organ distribution of oral cadmium chloride and
cadmium metallothionein in mice. J. Toxicol. environ. Health,
4: 861-868.
CHERNOFF, N. (1973) Teratogenic effects of cadmium in rats.
Teratology, 8: 29-32.
CHERRY, W.H. (1981) Distribution of cadmium in human tissues. In:
Nriagu, J.O., ed. Cadmium in the environment - II, New York,
Chichester, John Wiley & Sons, pp. 111-122.
CHIQUOINE, A.D. (1965) Effect of cadmium chloride on the pregnant
albino mouse. J. Reprod. Fertil., 10: 263-265.
CHRISTOFFERSON, J.O. & MATTSON, S. (1983) Polarized X-rays in
XRF-analysis for improved in vivo detectability of cadmium in man.
Phys. Med. Biol., 28: 1135-1144.
CHUMBLEY, C.G. & UNWIN, R.J. (1982) Cadmium and lead content of
vegetable crops grown on land with a history of sewage sludge
application. Environ. Pollut. Ser. B Chem. Phys., 4(3): 231-238.
CHUNG, J., NARTY, N.O., & CHERIAN, M.G. (1986) Metallothionein
levels in liver and kidney of Canadians - A potential indicator of
environmental exposure to cadmium. Arch. environ. Health, 41(5):
319-323.
CIRKT, M. & TICHY, M. (1974) Excretion of cadmium through bile and
intestinal wall in rats. Br. J. ind. Med., 31: 134-139.
CLARKSON, T.W. & KENCH, J.E. (1956) Urinary excretion of amino acids
by men absorbing heavy metals. Biochem. J., 62: 361-372.
CNATTINGIUS, S., AXELSSON, O., EKLUND, G., & LINDMARK, G. (1985)
Smoking, maternal age and fetal growth. Obstet. Gynecol.,
66: 449-452.
COLE, G.M. & BAER, L.S. (1944) Food poisoning from cadmium. US nav.
med. Bull., 43: 398-399.
COMMISSION OF THE EUROPEAN COMMUNITIES (1978) Criteria (dose-effect
relationships) for cadmium, Oxford, Pergamon Press, 202 pp (Report
of the Working Group of Experts prepared for the CEC,
Directorate-General for Social Affairs, Health and Safety
Directorate, Luxembourg).
COOPERATIVE RESEARCH COMMITTEE ON ITAI-ITAI DISEASE (1967) [Report
on a study on the so-called Itai-itai disease], Tokyo, Ministry of
Education and Ministry of Health and Welfare, 206 pp (in Japanese).
COPIUS-PEEREBOOM, J.W., DE VOOGT, P., VAN HATTUM, B., VAN DER VELDE,
W., & COPIUS-PEEREBOOM-STEGEMAN, J.H.J. (1979) The use of the human
placenta as a biological indicator for cadmium exposure. In:
International Conference on Management and Control of Heavy Metals
in the Environment, London, September, 1979, Edinburgh, CEP
Consultants Ltd, pp. 8-10.
COUSINS, R.J., BARBER, A.K., & TROUT, J.R. (1973) Cadmium toxicity
in growing swine. J. Nutr., 103: 964-972.
CULBARD, E.B., THORNTON, I., WATT, J., WHEATLEY, M., MOORCROFT, S.,
& THOMPSON, M. (1988) Metal contamination in British urban dusts and
soils. J. environ. Qual., 17(2): 227-234.
CVETKOVA, R.P. (1970) [Materials on the study of the influence of
cadmium compounds on the generative function.] Gig. Tr. prof.
Zabol., 14: 31-33 (in Russian with English summary).
DALHAMN, T. & FRIBERG, L. (1954) The effect of cadmium on blood
pressure and respiration and the use of dimercaprol (BAL) as
antidote. Acta pharmacol. toxicol., 10: 199-203.
DANIELSSON, L.-G., JAGNER, D., JOSEFSON, M., & WESTERLUND, S. (1981)
Computerized potentiometric stripping analysis for the determination
of cadmium, lead, copper, and zinc in biological materials. Anal.
Chim. Acta, 127: 147-156.
DASTON, G.P. (1982) Fetal zinc deficiency on a mechanism for
cadmium-induced toxicity to the developing rat lung and pulmonary
surfactant. Toxicology, 24: 55-63.
DAVIES, N.T., BREMNER, I., & MILLS, C.F. (1973) Studies on the
induction of a low molecular weight zinc-binding protein in rat
liver. Biochem. Soc. Trans., 1: 985-988.
DAVIS, R.D. (1984) Cadmium - a complex environmental problem. Part
II. Cadmium in sludges used as fertilizer. Experientia (Basel),
40(2): 117-126.
DAVIS, R.D. & COKER, E.G. (1980) Cadmium in agriculture, with
special reference to the utilisation of sewage sludge on land,
Medmenham, United Kingdom, Water Research Centre (Technical Report
TR/139).
DAVISON, A.G., NEWMAN-TAYLOR, A.J., DARBYSHIRE, J., & CHETTLE, D.R.
(1988) Cadmium fume inhalation and emphysema. Lancet, 1: 663-667.
DEAVEN, L.L. & CAMPBELL, E.W. (1980) Factors affecting the induction
of chromosomal aberrations by cadmium in Chinese hamster cells.
Cytogenet. cell Genet., 26: 251-260.
DECKER, L.E., BYERRUM, R.U., DECKER, C.F., HEPPERT, C.A., & LANGHAM,
R.F. (1958) Chronic toxicity studies. I. Cadmium administered in
drinking-water to rats. Am. Med. Assoc. Arch. ind. Health,
18: 228-231.
DEKNUDT, G. & DEMINATTI, M.L. (1978) Chromosome studies in human
lymphocytes after in vitro exposure to metal salts. Toxicology,
10: 67-75.
DEKNUDT, G. & LEONARD, A. (1975) Cytogenetic investigations on
leukocytes of workers from a cadmium plant. Environ. Physiol.
Biochem., 5: 319-327. DELVES, H.T. (1970) Micro-sampling method for
the rapid determination of lead in blood by atomic absorption
spectrophotometry. Analyst, 95: 431-438.
DELVES, H.T. (1982) Analytical techniques for measuring cadmium in
blood. In: Proceedings of the International Workshop on Biological
Indicators on Cadmium Exposure Diagnostic and Analytical
Reliability, Luxembourg, 7-9 July, 1982, Luxembourg, Commission of
the European Communities, pp. 1-15.
DELVES, H.T. & WOODWARD, J. (1981) Determination of low levels of
cadmium in blood by electrothermal atomization and atomic absorption
spectrophotometry. At. Spectrosci., 2: 65-67.
DENCKER, L., DANIELSSON, B., KHAYAT, A., & LINDGREN, A. (1983)
Disposition of metals in the embryo and fetus. In: Clarkson, T.W.,
Nordberg, G.F., & Sager, P.R., ed. Reproductive and developmental
toxicity of metals, New York, London, Plenum Press, pp. 607-632.
DERVAN, P.A. & HAYES, J.A. (1979) Peribronchiolar fibrosis following
acute experimental lung damage by cadmium aerosol. J. Pathol.,
128: 143-148.
DE SILVA, P.E. & DONNAN, M.B. (1981) Chronic cadmium poisoning in a
pigment-manufacturing plant. Br. J. ind. Med., 38: 76-86.
DUCE, R.A., HOFFMAN, G.L., & ZELLER, W.H. (1975) Atmospheric trace
metals at remote northern and southern hemisphere sites: pollution
or natural(?). Science, 187: 59-61.
DUCE, R.A., ARIMOTO, R., RAY, B.J., UNNI, C.K., & HARDER, P.J.
(1983) Atmospheric trace elements at Enewetok Atoll. I.
Concentration, sources, and temporal variability. J. geophys. Res.,
88: 5321-5342.
DUDLEY, R.E., SVOBODA, D.J., & KLAASSEN, C.O. (1982) Acute exposure
to cadmium causes severe liver injury in rats. Toxicol. appl.
Pharmacol., 65: 302-313.
DUDLEY, R.E., GAMMAL, L.M., & KLAASSEN, C.D. (1985) Cadmium-induced
hepatic and renal injury in chronically exposed rats: likely role of
hepatic cadmium-metallothionein in nephrotoxicity. Toxicol. appl.
Pharmacol., 77: 414-426.
DUGGAN, M.J., INSKIP, M.J., RUNDLE, S.A., & MOORCROFT, J.S. (1985)
Lead in playground dust and on the hands of schoolchildren. Sci.
total Environ., 44(1): 65-79.
DUNPHY, B. (1967) Acute occupational cadmium poisoning: a critical
review of the literature. J. occup. Med., 9: 22-26.
EDIGER, R.D. & COLEMAN, R.L. (1973) Determination of cadmium in
blood by a Delves cup technique. At. Absorpt. Newsl., 12: 3-6.
EDLING, C., ELINDER, C.G., & RANDMA, E. (1986) Lung function in
workers using cadmium containing solders. Br. J. ind. Med.,
43: 657-662.
ELINDER, C.-G. & PANNONE, M. (1979) Biliary excretion of cadmium.
Environ. Health Perspect., 28: 123-126.
ELINDER, C.-G. & PISCATOR, M. (1978) Cadmium and zinc in horses. In:
Kirchgessner, M., ed. Proceedings of the 3rd International
Conference on Trace Elements in Man and Animals,
Freising-Weihenstephan, Federal Republic of Germany, 25-29 July,
1977, Freising-Weihenstephan, Munich Technical University,
pp. 569-572.
ELINDER, C.-G., KJELLSTRÖM, T., LIND, B., & LINNMAN, L. (1976)
Cadmium concentration in kidney cortex, liver, and pancreas among
autopsied Swedes. Arch. environ. Health, 31: 292-302.
ELINDER, C.-G., KJELLSTRÖM, T., LINNMAN, L., & PERSHAGEN, G. (1978)
Urinary excretion of cadmium and zinc among persons from Sweden.
Environ. Res., 15: 473-484.
ELINDER, C.-G., JONSSON, L., PISCATOR, M., & RAHNSTER, B. (1981a)
Histopathological changes in relation to cadmium concentration in
horse kidneys. Environ. Res., 26: 1-21.
ELINDER, C.-G., NORDBERG, M., PALM, B., & PISCATOR, M. (1981b)
Cadmium, zinc, and copper in horse kidney and in horse liver
metallothionein: comparison with kidney cortex. Environ. Res.,
26: 22-32.
ELINDER, C.-G., KJELLSTRÖM, T., LIND, B., LINNMAN, L., PISCATOR, M.,
& SUNDSTEDT, K. (1983) Cadmium exposure from smoking cigarettes:
variations with time and country where purchased. Environ. Res.,
32: 220-227.
ELINDER, C.-G., EDLING, C., LINDBERG, E., KAGEDAL, B., & VESTERBERG,
O. (1985a) Assessment of renal function in workers previously
exposed to cadmium. Br. J. ind. Med., 42: 754-760.
ELINDER, C.-G., EDLING, C., LINDBERG, E., KAGEDAL, B., & VESTERBERG,
O. (1985b) B-2-microglobulinuria among workers previously exposed
to cadmium: follow-up and dose-response analyses. Am. J. ind. Med.,
8: 553-564.
ELINDER, C.-G., KJELLSTRÖM, T., HOGSTEDT, C., ANDERSON, K., & SPENG,
G. (1985c) Cancer mortality of cadmium workers. Br. J. ind. Med.,
42: 651-655.
ELLIS, K.J., MORGAN, W.D., ZANZI, I., YASUMURA, S., VARTSKY, D.D., &
COHN, S.H. (1981a) Critical concentrations of cadmium in human renal
cortex: dose-effect studies in cadmium smelter workers. J. Toxicol.
environ. Health, 7: 691-703.
ELLIS, K.J., YASAMURA, S., & COHN, S.H. (1981b) Hair cadmium
content: is it a biological indicator of the body burden of cadmium
for the occupationally-exposed worker? Am. J. ind. Med., 2: 323-333.
ELLIS, K.J., YUEN, K., YASAMURA, S., & COHN, S.H. (1984)
Dose-response analysis of cadmium in man: body burden vs kidney
dysfunction. Environ. Res., 33: 216-226.
ELLIS, K.J., COHN, S.H., & SMITH, T.J. (1985) Cadmium inhalation
exposure estimates: their significance with respect to kidney and
liver cadmium burden. J. Toxicol. environ. Health, 15: 173-187.
ENGSTROM, B. (1979) Factors influencing metabolism and toxicity of
cadmium. Experimental studies on mice with special reference to
chelating agents, Stockholm, Department of Environmental Hygiene,
Karolinska Institute, 80 pp (Doctoral thesis). ENGSTROM, B. &
NORDBERG, G.F. (1978) Effects of detergent formula chelating agents
on the metabolism and toxicity of cadmium in mice. Acta pharmacol.
toxicol., 43: 387-397.
ENGSTROM, B. & NORDBERG, G.F. (1979a) Dose dependence of
gastrointestinal absorption and biological half-time of cadmium in
mice. Toxicology, 13: 215-222.
ENGSTROM, B. & NORDBERG, G.F. (1979b) Factors influencing absorption
and retention of oral 109Cd in mice: age, pretreatment, and
subsequent pretreatment with non-radioactive cadmium. Acta
pharmacol. toxicol., 45: 315-324.
ENGVALL, J. & PERK, J. (1985) Prevalence of hypertension among
cadmium-exposed workers. Arch. environ. Health, 40: 185-190.
ESSING, H.-G., SCHALLER, K.-H., SZADOWSKI, D., & LEHNERT, G. (1969)
[Normal cadmium load on man from food and beverages.] Arch. Hyg.
Bakteriol., 153: 490-494 (in German).
EVRIN, P.E., PETERSON, P.A., WIDE, L., & BERGGARD, I. (1971)
Radioimmuno-assay of ß-2-microglobulin in human biological fluids.
Scand. J. clin. lab. Invest., 28: 439-443.
EYBL, V. & SYKORA, J. (1966) [The protection by chelating agents in
acute cadmium poisoning.] Acta biol. med. Germ., 16: 61-64 (in
German).
FELDMAN, S.L. & COUSINS, R.G. (1973) Influence of cadmium on the
metabolism of 25-hydroxycholecalciferol in chicks. Nutr. Rep. Int.,
8: 251-259.
FELTEN, T.L. (1979) A preliminary report of cadmium-induced
chromosomal changes in somatic and germinal tissues of C57BI/6J male
mice. Genetics, 88: 26-27.
FERM, V.H. (1971) Developmental malformations induced by cadmium.
Biol. Neonat., 19: 101-107.
FERM, V.H. (1972) The teratogenic effects of metals on mammalian
embryos. Adv. Teratol., 5: 51-75.
FERM, V.H. & CARPENTER, S.J. (1968) The relationship of cadmium and
zinc in experimental mammalian teratogenesis. Lab. Invest.,
18: 429-432.
FERNANDEZ, F.J. & KAHN, H.L. (1971) The determination of lead in
whole blood by atomic absorption spectrophotometry with the "Delves
Sampling Cup" technique. At. Absorpt. Newsl., 10: 1-5.
FINGERLE, H., FISCHER, G., & CLASSEN, H.G. (1982) Failure to produce
hypertension in rats by chronic exposure to cadmium. Food chem.
Toxicol., 20: 301-306.
FITZHUGH, O.G. & MEILLER, F.H. (1941) The chronic toxicity of
cadmium. J. Pharmacol. exp. Ther., 72: 15-20.
FLANAGAN, P.R., MCLELLAN, J.S., HAIST, J., CHERIAN, M.G.,
CHAMBERLAIN, M.J., & VALBERG, L.S. (1978) Increased dietary cadmium
absorption in mice and human subjects with iron deficiency.
Gastroenterology, 74: 841-846.
FLETCHER, J.C., CHETTLE, D.R., & AL-HADDAD, I.K. (1982) Experience
with the use of cadmium measurements of liver and kidney. J.
radioanal. Chem., 71: 547-560.
FORSTNER, U. (1980) Cadmium in the environment, Part I. In: Nriagu,
J.O., ed. Cadmium in polluted sediments, New York, Chichester, John
Wiley & Sons, pp. 305-363.
FOULKES, E.C. (1982) Role of metallothionein in transport of heavy
metals. In: Foulkes, E.C., ed. Biological roles of metallothionein,
Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp. 131-140.
FOWLER, B.A. & NORDBERG, G.F. (1978) The renal toxicity of cadmium
metallothionein: morphometric and X-ray microanalytical studies.
Toxicol. appl. Pharmacol., 46: 609-623.
FOWLER, B.A., JONES, H.S., BROWN, H.W., & HASEMAN, J.K. (1975) The
morphological effects of chronic cadmium administration on the renal
vasculature of rats given low and normal calcium diets. Toxicol.
appl. Pharmacol., 34: 233-252.
FOX, M.R.S. & FRY, B.E., Jr (1970) Cadmium toxicity decreased by
dietary ascorbic acid supplements. Science, 169: 989-991.
FOX, M.R.S., FRY, B.E., Jr, HARLAND, B.F., SCHERTAL, M.E., & WEEKS,
C.E. (1971) Effect of ascorbic acid on cadmium toxicity in the
young coturnix. J. Nutr., 101: 1295-1305.
FRANCAVILLA, S., MOSCARDELLI, S., FRANCAVILLA, F., CASASANTA, N.,
PROPERZI, G., MARTINI, M., & SANTIEMMA, V. (1981) Acute cadmium
intoxication: influence of cyproterone acetate on the testis and
epididymis of the rat. Arch. Andrology, 6: 1-11.
FRANK, A., PETERSON, L., & MORNER, T. (1981) [Lead and cadmium
levels in organs from moose, deer, and hare.] Svensk
Veterinaertidn., 33: 151-156 (in Swedish).
FRIBERG, L. (1948a) Proteinuria and kidney injury among workmen
exposed to cadmium and nickel dust. J. ind. Hyg. Toxicol.,
30: 32-36.
FRIBERG, L. (1948b) Proteinuria and emphysema among workers exposed
to cadmium and nickel dust in a storage battery plant. Proc. Int.
Congr. Ind. Med., 9: 641-644.
FRIBERG, L. (1950) Health hazards in the manufacture of alkaline
accumulators with special reference to chronic cadmium poisoning.
Acta med. Scand., 138(Suppl. 240): 1-124.
FRIBERG, L. (1952) Further investigations on chronic cadmium
poisoning. A study on rabbits with radioactive cadmium. Arch. ind.
occup. Med., 5: 30-36.
FRIBERG, L. (1955) Iron and liver administration in chronic cadmium
poisoning and studies on the distribution and excretion of cadmium.
Experimental investigations in rabbits. Acta pharmacol.,
11: 168-178.
FRIBERG, L. (1979) Cadmium in Shipham. Lancet, 1: 823-824.
FRIBERG, L. (1988) Biological monitoring of toxic metals, New York,
London, Plenum Press, pp. 103-126.
FRIBERG, L. & NYSTROM, A. (1952) [Aspects on the prognosis of
chronic cadmium poisoning.] Läkartidningen, 49: 2629-2639 (in
Swedish).
FRIBERG, L. & VAHTER, M. (1983) Assessment of exposure to lead and
cadmium through biological monitoring: results of a UNEP/WHO global
study. Environ. Res., 30: 95-128.
FRIBERG, L., PISCATOR, M., NORDBERG, G., & KJELLSTRÖM, T. (1974)
Cadmium in the environment, 2nd ed., Cleveland, Ohio, CRC Press,
248 pp.
FRIBERG, L., KJELLSTRÖM, T., NORDBERG, G., & PISCATOR, M. (1975)
Cadmium in the environment. III. A toxicological and epidemiological
appraisal, Washington, DC, US Environmental Protection Agency,
Office of Research and Development, 217 pp.
FRIBERG, L., ELINDER, C.G., KJELLSTRÖM, T., & NORDBERG, G.F. (1985)
Cadmium and health, a toxicological and epidemiological appraisal.
Vol. I. Exposure, dose and metabolism, Cleveland, Ohio, CRC Press,
209 pp.
FRIBERG, L., ELINDER, C.G., KJELLSTRÖM, T., & NORDBERG, G.F. (1986)
Cadmium and health, a toxicological and epidemiological appraisal.
Vol. II. Effects and response, Cleveland, Ohio, CRC Press, 303 pp.
FUKUHARA, M., BOULEY, G., GODIN, J., GIRARD, F., BOISSET, M., &
BOUDENE, C. (1981) Effects of short-term inhalation of cadmium
oxides on rabbit pulmonary microsomal enzymes. Biochem. Pharmacol.,
30: 715-720.
FUKUSHIMA, I. (1978) Environmental pollution and health effects -
Gunma Prefecture. In: Tsuchiya, K., ed. Cadmium studies in Japan: a
review, Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp. 170-181.
FUKUSHIMA, M., ISHIZAKI, A., SAKAMOTO, M., & KOBAYASHI, E. (1973)
[Cadmium concentrations in rice eaten by farmers in the Jinzu river
basin.] Jpn J. Hyg., 28: 406-415 (in Japanese with English summary).
FUKUSHIMA, M., ISHIZAKI, A., NOGAWA, K., SAKAMOTO, M., & KOBAYASHI,
E. (1974) [Epidemiological studies on renal failure of inhabitants
in "Itai-itai" disease endemic district. I. Some urinary findings of
inhabitants living in and around the endemic district of the Jinzu
river basin.] Jpn. J. Public Health, 21: 65-73 (in Japanese with
English summary).
FUKUSHIMA, I., NAKAJIMA, T., HIGUSHI, Y., TSUCHIYA, T., NAKAJIMA,
K., TANAKA, T., FUKAMACHI, M., ARAI, K., & SHIONO, T. (1975)
[Results of screening examination on tubular dysfunction in aged
persons confined to bed], Tokyo, Japan Public Health Association,
pp. 138-142 (Kankyo Hoken Report No. 36) (in Japanese).
FUKUYAMA, Y. (1972) [Proteinuria in Itai-itai disease and cadmium
poisoning.] Med. Biol., 84: 41-46 (in Japanese).
FULLMER, C.S., OKU, T., & WASSERMAN, R.H. (1980) Effect of cadmium
administration on intestinal calcium absorption and vitamin
D-dependent calcium-binding protein. Environ. Res., 22: 386-399.
GABBIANI, G. (1966) Action of cadmium chloride on sensory ganglia.
Experientia (Basel), 22: 261-264.
GABBIANI, G., BAIC, D., & DELZIEL, C. (1967) Toxicity of cadmium for
the central nervous system. Exp. Neurol., 18: 154-160.
GABBIANI, G., BADONNEL, M.-C., MATHEWSON, S.M., & RYAN, G.G. (1974)
Acute cadmium intoxication: early selective lesions of endothelial
clefts. Lab. Invest., 30: 686-695.
GALE, T.F. (1973) The interaction of mercury with cadmium and zinc
in mammalian embryonic development. Environ. Res., 6: 95-105.
GARDINER, P.E., OTTAWAY, J.M., & FELL, G.S. (1979) Accuracy of the
direct determination of cadmium in urine by carbon-furnace atomic
absorption spectrometry. Talanta, 26: 841-847.
GARTY, M., WONG, K.-L., & KLAASSEN, C.D. (1981) Redistribution of
cadmium to blood of rats. Toxicol. appl. Pharmacol., 59: 548-554.
GERVAIS, J. & DELPECH, P. (1963) L'intoxication cadmique. Arch. Mal.
prof. Méd. Trav. Séc. soc., 24: 803-815.
GESAMP (1984) IMO/FAO/UNESCO/WMO/IAEA/UN/UNEP Joint Group of Experts
on the Scientific Aspects of Marine Pollution: Report of the
Fourteenth Session, Vienna, 26-30 March, 1984, Vienna, International
Atomic Energy Agency (Reports and Studies No. 21).
GESAMP (1985) IMO/FAO/UNESCO/WMO/IAEA/UN/UNEP Joint Group of Experts
on the Scientific Aspects of Marine Pollution: Atmospheric transport
of contaminants into the mediterranean region, Athens, Geneva, World
Meteorological Association (Reports and Studies No. 26).
GESAMP (1987) IMO/FAO/UNESCO/WMO/WHO/IAEA/UN/UNEP Joint Group of
Experts on the Scientific Aspects of Marine Pollution: Report of the
seventeenth session, Rome, Geneva, World Health Organization
(Reports and Studies No. 31).
GLEIT, C.E. (1965) High-frequency electrodeless discharge system for
ashing organic matter. Anal. Chem., 37: 314-315.
GLENNAS, A. & RUGSTAD, H.E. (1984) Cadmium uptake and metabolism in
cultured cells. Environ. Health Perspect., 54: 45-50.
GOERING, P.L., SQUIBB, K.S., & FOWLER, B.A. (1985) Calcuria and
proteinuria during cadmium-metallothionein-induced proximal tubule
cell injury. In: Memphill, D., ed. Proceedings of the University of
Missouri's 19th Annual Conference on Trace Substances in
Environmental Health, 3-6 June 1985, Columbia, Missouri, University
of Missouri Press, pp. 22-35.
GOYER, R.A., CHERIAN, M.G., & DELAQUERRIERE-RICHARDSON, L. (1984)
Correlation of parameters of cadmium exposure with onset of
cadmium-induced nephropathy in rats. J. environ. Pathol. Toxicol.
Oncol., 5: 89-100.
GOYER, R.A., HAUST, M.D., & CHERIAN, M.G. (1989) Localization of
metallothionein in placenta. Toxicologist, 9: 130.
GREENBERG, R.R., GORDON, G.E., ZOLLER, W.H., JACKO, R.B., NEVENDORF,
D.W., & YOST, K.J. (1978) Composition of particles emitted from the
Nocosia municipal incinerator. Environ. Sci. Technol., 12(12):
1329-1332.
GUNN, S.A. & GOULD, T.C. (1957) Selective accumulation of 115Cd by
cortex of rat kidney. Proc. Soc. Exp. Biol. Med., 96: 820-823.
GUNN, S.A. & GOULD, T.C. (1970) Cadmium and other mineral elements.
In: Johnson, A.D., Gomes, W.R., & VanDeMark, N.L., ed. The testis,
New York, London, Academic Press, Vol. 3, pp. 377-481.
GUNN, S.A., GOULD, T.C., & ANDERSON, W.A.D. (1961) Zinc protection
against cadmium injury to rat testis. Arch. Pathol., 71: 274.
GUNN, S.A., GOULD, T.C., & ANDERSON, W.A.D. (1963) Cadmium-induced
inter-stitial cell tumours in rats and mice and their prevention by
zinc. J. Natl Cancer Inst., 31: 745-759.
GUNN, S.A., GOULD, T.C., & ANDERSON, W.A.D. (1965) Strain
differences in susceptibility of mice and rats to cadmium-induced
testicular damage. J. Reprod. Fertil., 10: 273-275.
GUNN, S.A., GOULD, T.C., & ANDERSON, W.A.D. (1967) Specific response
of mesenchymal tissue to cancerogenesis by cadmium. Arch. Pathol.,
83: 439-499.
GUNN, S.A., GOULD, T.C., & ANDERSON, W.A.D. (1968) Specificity in
protection against lethality and testicular toxicity from cadmium.
Proc. Soc. Exp. Biol. Med., 128: 591-595.
GUTHRIE, B.E. & ROBINSON, M.F. (1977) Daily intakes of manganese,
copper, zinc, and cadmium by New Zealand women. Br. J. Nutr.,
38: 55-63.
GUTHRIE, J. (1964) Histological effects of intratesticular
injections of cadmium chloride in domestic fowl. Br. J. Cancer,
18: 255-260.
HADDOW, A., ROE, F.J.C., DUKES, C.E., & MITCHELEY, B.C.V. (1964)
Cadmium neoplasia: sarcomata at the site for injection of cadmium
sulfate in rats and mice. Br. J. Cancer, 18: 667-673.
HADLEY, J.G., CONKLIN, A.W., & SANDERS, C.L. (1979) Systemic
toxicity of inhaled cadmium oxide. Toxicol. Lett., 4: 107-111.
HAGA, Y. & YAMAWAKI, T. (1974) [Research report on environmental
health studies of heavy metal pollution], Akita, Japan, Akita
Prefecture Institute of Public Health (Report No. 18) (in Japanese).
HAGINO, N. & YOSHIOKA, K. (1961) [A study on the etiology of
Itai-itai disease.] J. Jpn. Orthop. Assoc., 35: 812-815 (in
Japanese).
HAHN, R., EWERS, O., JERMANN, E., FREIER, E., BROCKHAUS, A., &
SCHLIPKOTER, H.W. (1987) Cadmium in kidney cortex of inhabitants of
North-West Germany: in relationship to age, sex, smoking and
environmental pollution by cadmium. Int. Arch. occup. environ.
Health, 59: 165-176.
HALVORSEN, C. & STEINNES, E. (1975) Simple and precise determination
of Zn and Cd in human liver by neutron activation analysis. Z. anal.
Chem., 274: 199-202.
HAMILTON, D.L. & VALBERG, L.S. (1974) Relationship between cadmium
and iron absorption. Am. J. Physiol., 227: 1033-1037.
HAMMER, D.I., FINKLEA, J.F., HENDRICKS, R.H., SHY, C.M., & HORTON,
R.J.M. (1971) Hair trace metal levels and environmental exposure.
Am. J. Epidemiol., 93: 84-92.
HAMMER, D.I., FINKLEA, J.F., CREASON, J.P., SANDIFER, S.H., KEIL,
J.E., PRIESTER, L.E., & STARA, J.F. (1972) Cadmium exposure and
human health effects. In: Hemphill, D.D., ed. Trace substances in
environmental health - V, Columbia, Missouri, University of Missouri
Press, pp. 269-283.
HAMMER, D.I., COLUCCI, A.V., HASSELBLAD, V., WILLIAMS, M.E., &
PINKERTON, C. (1973) Cadmium and lead in autopsy tissues. J. occup.
Med., 15: 956-963.
HARADA, A. (1987) [Results of fifteen years health examinations on
cadmium workers in a cadmium pigment factory (2nd report)], Tokyo,
Japan Public Health Association, pp. 219-233 (Kankyo Hoken Report
No. 53) (in Japanese).
HARDY, H.L. & SKINNER, J.B. (1947) The possibility of chronic
cadmium poisoning. J. ind. Hyg. Toxicol., 29: 321-325.
HARRISON, H.E., BUNTING, H., ORDWAY, N., & ALBRINK, W.S. (1947) The
effects and treatment of inhalation of cadmium chloride aerosols in
the dog. J. ind. Hyg. Toxicol., 29: 302-314.
HARRISON, J.F., LUNT, G.S., SCOTT, P., & BLAINEY, J.D. (1968)
Urinary lysozyme, ribonuclease, and low molecular weight protein in
renal disease. Lancet, 1: 371-375.
HARVEY, T.C., MCLELLAN, J.S., THOMAS, B.J., & FREMLIN, J.H. (1975)
Measurement of liver cadmium concentrations in patients and
industrial workers by neutron activation analysis. Lancet,
1: 1269-1272.
HARVEY, T.C., CHETTLE, D.R., FREMLIN, J.H., AL-HADDAD, I.K., &
DOWNES, S.P.M. (1979) Cadmium in Shipham. Lancet, 1(8115).
HASSELMANN, J., KOENIG, W., RICHTER, F.W., STEINER, U., & WATJEN, U.
(1977) Application of PIXE to trace element analysis in biological
tissues. Nucl. Instrum. Methods, 142: 163-169.
HASSLER, E., LIND, B., & PISCATOR, M. (1983) Cadmium in blood and
urine related to present and past exposure. A study of workers in an
alkaline battery factory. Br. J. ind. Med., 40: 420-425.
HAYES, J.A., SNIDER, G.L., & PALMER, K.C. (1976) The evolution of
biochemical damage in the rat lung after acute cadmium exposure. Am.
Rev. respir. Dis., 113: 121-130.
HEATH, J.C. & WEBB, M. (1967) Content and intracellular distribution
of the inducing metal in the primary rhabdomyosarcomata induced in
the rat by cobalt, nickel, and cadmium. Br. J. Cancer, 21: 768-779.
HEATH, J.C., DANIEL, M.R., DINGLE, J.T., & WEBB, M. (1962) Cadmium
as a carcinogen. Nature (Lond.), 193: 592-593.
HEINDRYCKX, R., DEMUYNCK, M., DAMS, R., JANSSENS, M., & RAHN, K.A.
(1974) Mercury and cadmium in Belgian aerosols. In: Problems of the
contamination of man and his environment by mercury and cadmium. CEC
European Colloquium, July, 1973, Luxembourg, Commission of the
European Communities, pp. 135-148.
HEINRICH, U., PETERS, L., ERNST, H., RITTINGHAUSEN, S., DASENBROCK,
C., & KÖNIG, H. (1989) Investigation on the carcinogenic effects of
various cadmium compounds after inhalation exposure in hamsters and
mice. Exp. Pathol., 37: 253-258.
HENDERSON, R.F., REBAR, A.H., PICKRELL, J.A., & NEWTON, G.J. (1979)
Early damage indicators in the lung III. Biochemical and cytological
response of the lung to inhaled metal salts. Toxicol. appl.
Pharmacol., 50: 123-136.
HENKE, G., SACHS, H.W., & BOHN, G. (1970) [Cadmium determination by
neutron activation analysis of liver and kidneys from children and
young people.] Arch. Toxikol., 26: 8-16 (in German).
HICKEY, R.J., SCHOFF, E.P., & CLELLAND, R.C. (1967) Relationship
between air pollution and certain chronic disease death rates. Arch.
environ. Health, 15: 728-738.
HIRATA, H. (1981) Annaka: land polluted mainly by fumes and dust
from a zinc smelter. In: Kitagishi, K. & Yamane, I., ed. Heavy metal
pollution in soils of Japan, Tokyo, Japan Scientific Societies
Press, pp. 149-163.
HISLOP, J.S. (1980) Choice of analytical method. In: Bratter, P. &
Schramel, P., ed. Trace element analytical chemistry in medicine
and biology, Berlin, New York, Walter de Gruyter Co., pp. 747-767.
HOFFMAN, E.O., COOK, J.A., DILUZIO, N.R., & COOVER, J.A. (1975) The
effects of acute cadmium administration in the liver and kidney of
the rat. Lab. Invest., 32: 655-661.
HOFFMAN, L., PUTZKE, H.-P., KAMPEHL, H.-J., RUSSBULT, R., GASE, P.,
SIMONN, C., ERDMANN, T., & HUCKSTORF, C. (1985) Carcinogenic effects
of cadmium on the prostate of the rat. J. Cancer Res. clin. Oncol.,
109: 193-199.
HOFFMAN, L., PUTZKE, H.-P., BENDEL, L., ERDMANN, T., & HUCKSTORF, C.
(1988) Electron microscopic results on the ventral prostate of the
rat after CdC12 administration. A contribution towards etiology of
the cancer of the prostate. J. Cancer Res. clin. Oncol.,
114: 273-278.
HOLDEN, H. (1969) Cadmium toxicology. Lancet, 2: 57.
HOLDEN, H. (1980a) A mortality study of workers exposed to cadmium
fumes. In: Proceedings of the 2nd International Cadmium Conference,
Cannes, 6-8 February, 1979, London, Cadmium Association,
pp. 211-216.
HOLDEN, H. (1980b) Health status of European cadmium workers. In:
Proceedings of the Seminar on Occupational Exposure to Cadmium,
London, 20 March, 1980, London, Cadmium Association, pp. 33-37.
HOLMBERG, R.E., Jr & FERM, V.H. (1969) Inter-relationship of
selenium, cadmium, and arsenic in mammalian teratogenesis. Arch.
environ. Health, 18: 873-877.
HORSTOWA, H., SIKORSKI, M., & TYBORSKI, H. (1966) [Chronic cadmium
poisoning in the clinical and radiological picture.] Med. Prac.
occup. Med., 17: 13-25 (in Polish).
HOVMAND, M.F., TJELL, J.C., & MOSBACK, H. (1983) Plant uptake of
airborne cadmium. Environ. Pollut., 30: 27-38.
HUEL, G., BOUDENE, C., & IBRAHIM, M.A. (1981) Cadmium and lead
content of maternal and newborn hair: relationship to parity, birth
weight and hypertension. Arch. environ. Health, 36: 221-227.
HUGHES, E.G. & STEWART, M. (1979) Cadmium in Shipham. Lancet,
1: 973-974.
HURLEY, L.S., GOWAN, J., & SWENERTON, H. (1971) Teratogenic effects
of short-term and transitory zinc deficiency in rats. Teratology,
4: 199-204.
HUTCHISON, F., WAI, C.M., JERNEGAN, M., HICKS, W., & LONG, R. (1979)
Distribution of cadmium, lead and zinc in soil around two phosphate
processing plants in Pocatello, Idaho. Bull. environ. Contam.
Toxicol., 22: 426-429.
HUTTON, M., ed. (1982) Cadmium in the European Community: a
prospective assessment of sources, human exposure, and environmental
impact, London, Monitoring and Assessment Research Centre, Chelsea
College, University of London, 100 pp (MARC Report Number 26).
HUTTON, M. (1983) Evaluation of the relationships between cadmium
exposure and indicators of kidney function, London, Monitoring and
Assessment Research Centre, Chelsea College, University of London,
46 pp (MARC Report Number 29).
HUTTON, M. & SYMON, C. (1986) The quantities of cadmium, lead,
mercury and arsenic entering the U.K. environment from human
activities. Sci. total Environ., 57: 129-150.
HUTTON, M., WADGE, A., & MILLIGAN, P.J. (1988) Environmental levels
of cadmium and lead in the vicinity of a major refuse incinerator.
Atmos. Environ., 22: 411-416.
HYOGO PREFECTURAL GOVERNMENT (1972) [Results of a comprehensive
study on cadmium pollution in the area around Ikuno Mine and an
outline of counter-measures], Kobe, Japan, Hyogo Prefectural
Government, 156 pp (in Japanese).
IARC (1976) Cadmium, nickel, some epoxides, miscellaneous
industrial chemicals, and general considerations on volatile
anaesthetics, Lyon, International Agency for Research on Cancer,
306 pp (Monographs on the Evaluation of Carcinogenic Risk of Chemicals
to Man, Vol. 11).
IARC (1987a) Genetic and related effects: an updating of selected
IARC Monographs from Volumes 1 to 42. Lyon, International Agency for
Research on Cancer (IARC Monographs on the Evaluations of
Carcinogenic Risks to Humans, Supplement 6).
IARC (1987b) Cadmium and cadmium compounds. In: Overall evaluations
of carcinogenicity: an updating of IARC Monographs, Volumes 1 to 42,
Lyon, International Agency for Research on Cancer, pp. 139-142 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals
to Humans, Supplement 7).
ILZSG (1988) International Lead and Zinc Study Group - World
directory: Lead and zinc mines and primary metallurgical works,
London, Chameleon Press, Ltd.
INSKIP, H., BERAL, V., & MCDOWALL, M. (1982) Mortality in Shipman
residents: 40-year follow-up. Lancet, 1: 896-899.
IRPTC (1987) IRPTC legal file 1986, Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment
Programme, Vol. I.
ISHIKAWA PREFECTURAL GOVERNMENT (1976) [Report on the health
examination of residents of the Kakehashi river basin], Kanazawa,
Japan, Health and Welfare Department, 104 pp (in Japanese).
ISHIZAKI, A. (1969) [On the so-called Itai-itai disease.] J. Jpn.
Med. Soc., 62: 242-249 (in Japanese).
ISHIZAKI, A. (1972) [Concentrations of cadmium and zinc in the
organs of Itai-itai disease patients and residents in the Hokuriku
region], Tokyo, Japanese Society of Public Health, pp. 154-157
(Kankyo Hoken Report No. 11) (in Japanese).
ISHIZAKI, A., FUKUSHIMA, M., SAKOMOTO, M., KURACHI, T., & HAYASHI,
E. (1969) [Cadmium content of rice eaten in the Itai-itai disease
area.] In: [Proceedings of the 27th Annual Meeting of the Japanese
Society of Public Health], Tokyo, Japan Public Health Association,
p. 111 (in Japanese).
ISHIZAKI, A., FUKUSHIMA, M., & SAKAMOTO, M. (1971) [Contents of
cadmium and zinc in organs of Itai-itai disease patients and
residents of Hakuriku district.] Jpn. J. Hyg., 26: 268-273 (in
Japanese).
ISHIZU, S., MINAMI, M., SUZUKI, A., YAMADA, M., SATO, M., &
YAMAMURA, K. (1973) An experimental study of teratogenic effect of
cadmium. Ind. Health, 11: 127-139.
ITO, K. & SAWAUCHI, K. (1966) Inhibitory effects on cadmium-induced
testicular damage by pretreatment with smaller cadmium doses.
Okajimas Folia anat. Jpn. 42: 107-117.
ITOKAWA, Y., ABE, T., & TANAKA, S. (1973) Bone changes in
experimental chronic cadmium poisoning. Arch. environ. Health,
26: 241-244.
ITOKAWA, Y., ABE, T., TABEI, R., & TANAKA, S. (1974) Renal and
skeletal lesions in experimental cadmium poisoning. Arch. environ.
Health, 28: 149-154.
ITOKAWA, Y., NISHINO, K., TAKASHIMA, M., NAKATA, T., KAITO, H.,
OKAMOTO, E., DAIJO, K., & KAWAMURA, J. (1978) Renal and skeletal
lesions in experimental cadmium poisoning of rats. Histology and
renal function. Environ. Res., 15: 206-217.
IWAO, S. (1977) Cadmium, lead, copper, and zinc in food, faeces, and
organs of humans. Keio J. Med., 26: 63-78.
IWAO, S., SUGITA, M., & TSUCHIYA, K. (1981a) Some metabolic
interrelationships among cadmium, lead, copper, and zinc: results
from a field survey in Cd-polluted areas in Japan. I. Dietary intake
of the heavy metals. Keio J. Med., 30: 17-36.
IWAO, S., SUGITA, M., & TSUCHIYA, K. (1981b) Some metabolic
interrelationships among cadmium, lead, copper, and zinc: results
from a field survey in Cd-polluted areas in Japan. II. Faecal
excretion of the heavy metals. Keio J. Med., 30: 71-82.
JAGNER, D., JOSEFSON, M., & WESTERLUND, S. (1981) Simultaneous
determination of cadmium and lead in urine by means of computerized
potentiometric stripping analysis. Anal. Chim. Acta, 128: 155-161.
JAMALL, I.S. & SMITH, J.C. (1986) The effect of dietary selenium on
cadmium cardiotoxicity. In: Born, G.V.R., Rensselaer, A.F., Herken,
H., & Welch, A.D., ed. Handbook of experimental pharmacology,
Berlin, Heidelberg, Springer Verlag, Vol. 80, pp. 351-361.
JAMALL, I.S. & SPROWLS, J.J. (1987) Effects of cadmium and dietary
selenium on cytoplasmic and mitochondrial antioxidant defense
systems in the heart of rats fed high dietary copper. Toxicol. appl.
Pharmacol., 87: 102-110.
JANSSENS, M. & DAMS, R. (1974) Determination of cadmium in air
particulates by flameless atomic absorption spectrometry with a
graphite tube. Anal. Chim. Acta, 70: 25-33.
JAPAN CADMIUM RESEARCH COMMITTEE (1989) [Summary report on studies
of health effects of cadmium], Tokyo, Japan Public Health
Association, pp. 53-67 (Kankyo Hoken Report No. 56) (in Japanese).
JAPAN PUBLIC HEALTH ASSOCIATION (1968) [A study of the cause of
Itai-itai disease], Tokyo, Japan Public Health Association, 73 pp
(in Japanese).
JAPAN PUBLIC HEALTH ASSOCIATION (1970) [Study on cadmium intake and
accumulation in areas requiring official observation], Tokyo, Japan
Public Health Association, 49 pp (in Japanese).
JAPANESE ENVIRONMENT AGENCY (1972) [Results of soil pollution survey
in 1971], Tokyo, Japanese Environment Agency, Soil Agricultural
Chemical Division, Water Quality Bureau, 129 pp (in Japanese).
JAPANESE ENVIRONMENT AGENCY (1974) [Results of measurement at the
national air sampling network stations], Tokyo, Japanese Environment
Agency, Air Pollution Control Division, Air Quality Bureau, 159 pp
(in Japanese).
JAPANESE ENVIRONMENT AGENCY (1982) [Results of soil pollution survey
in 1981], Tokyo, Japanese Government Agency, Soil Agricultural
Chemical Division, Water Quality Bureau, pp. 1-29 (in Japanese).
JAPANESE MINISTRY OF AGRICULTURE AND FORESTRY (1973) [Results of a
survey for protection measures of soil contamination in 1972.] In:
[Materials for soil protection measures], 197 pp (Report No. 44) (in
Japanese).
JAPANESE MINISTRY OF HEALTH AND WELFARE (1968) [Opinion of the
Ministry with regard to Itai-itai disease and its causes], Tokyo,
Japanese Ministry of Health and Welfare (in Japanese).
JAPANESE MINISTRY OF HEALTH AND WELFARE (1971) [Partial revision of
health examination methods in the provisional counter-measures
against environmental pollution by cadmium], Tokyo, Japanese
Ministry of Health and Welfare, Environmental Pollution Section
(Unpublished document) (in Japanese).
JAPANESE MINISTRY OF HEALTH AND WELFARE (1983) [The present status
of nutrition among the people], Tokyo, Japanese Ministry of Health
and Welfare, 56 pp (in Japanese).
JÄRUP, L., ROGENFELT, A., ELINDER, C.-G., NOGAWA, K., & KJELLSTRÖM,
T. (1983) Biological half-time of cadmium in the blood of workers
after cessation of exposure. Scand. J. Work Environ. Health,
9: 327-331.
JÄRUP, L., ELINDER, C.G., & SPANG, G. (1988) Cumulative
blood-cadmium and tubular proteinuria: a dose-response relationship.
Int. Arch. occup. environ. Health, 60: 223-229.
JAWAID, M., LIND, B., & ELINDER, C.-G (1983) Determination of
cadmium in urine by extraction and flameless atomic absorption
spectrophotometry. Comparison of urine from smokers and non-smokers
of different sex and age. Talanta, 30: 509-513.
JEFFERY, E.H., NOSEWORTHY, R., & CHERIAN, M.G. (1989) Age dependent
changes in metallothionein and accumulation of cadmium in horses.
Comp. Biochem. Physiol., 93C: 327-332.
JIN, T., NORDBERG, G.F., & NORDBERG, M. (1987a) Resistance to acute
nephrotoxicity induced by cadmium-metallothionein dependence on
pretreatment with cadmium chloride. Pharmacol. Toxicol., 61: 89-93.
JIN, T., NORDBERG, G.F., & NORDBERG, M. (1987b) Influence of
cadmium-metallothionein pretreatment on tolerance of rat kidney
cortical cells to cadmium toxicity in vitro and in vivo.
Pharmacol. Toxicol., 60: 345-349.
JIN, T., NORDBERG, G.F., & NORDBERG, M. (1986) Uptake of cadmium in
isolated kidney cells - influence of binding form and
in vivo pretreatment. J. appl. Toxicol., 6(6): 397-400.
JOHNSON, M.S. & EATON, J.W. (1980) Environmental contamination
through residual trace metal dispersal from derelict lead-zinc mine.
J. environ. Qual., 9(2): 175-179.
JONES, K.C., SYMON, C.J., & JOHNSTON, A.E. (1987) Long-term changes
in soil and cereal grain cadmium: studies at Rothamsted Experimental
Station. In: Hemphill, D.D., ed. Trace substances in environmental
health - XXI. Proceedings of the University of Missouri's 21st
Annual Conference on Trace Substances in Environmental Health, St.
Louis, Missouri, 25-28 May, 1987, Columbia, Missouri, University of
Missouri Press, pp. 450-460.
JORHEM, L., MATTSON, P., & SLORACH, S. (1984) Lead, cadmium, zinc
and certain other metals in foods on the Swedish market. Var Föda,
36: Suppl. 3.
JUST, J. & KELUS, J. (1971) [Cadmium in the air atmosphere of ten
selected cities in Poland.] Rocz. Panstw. Zakl. Hig., 22: 249-256
(in Polish).
KAGI, J.H.R. & KOJIMA, Y. (1987) Metallothionein II. Proceedings of
the Second International Meeting on Metallothionein and Other Low
Molecular Weight Metal-Binding Proteins, Zurich, 21-24 August, 1985,
Basel, Birkhauser Verlag.
KAGI, J.H.R. & NORDBERG, M., ed. (1979) Metallothionein, Basel,
Birkhauser Verlag.
KAGI, J.H.R. & VALLEE, B.L. (1960) Metallothionein: a cadmium- and
zinc-containing protein from equine renal cortex. I. J. biol. Chem.,
235: 3460-3465.
KAGI, J.H.R. & VALLEE, B.L. (1961) Metallothionein: a cadmium- and
zinc-containing protein from equine renal cortex. II. Physiochemical
properties. J. biol. Chem., 236: 2435-2442.
KAHN, H.L. & MANNING, D.C. (1972) Background correction in atomic
absorption spectroscopy. Am. Lab., August: 51-56.
KAJIKAWA, K., NAKANISHI, I., & KURODA, K. (1981) Morphological
changes of the kidney and bone of rats in chronic cadmium poisoning.
Exp. mol. Pathol., 34: 9-24.
KANAI, M., NOMOTO, S., SASAOKA, S., & NAIKI, M. (1971) Clinical
significance of urinary excretion of retinol-binding protein in
patients with Itai-itai disease. Proc. Symp. Physiol. Pathol.,
11: 194-199.
KANISAWA, M. & SCHROEDER, H.A. (1969) Life term studies on the
effect of trace elements on spontaneous tumours in mice and rats.
Cancer Res., 21: 892-895.
KAPLAN, P.D., BLACKSTONE, M., & RICHDALE, N. (1973) Direct
determination of cadmium, nickel, and zinc in rat lungs. Arch.
environ. Health, 27: 387-389.
KAR, A.B., DAS, R.P., & KARKUN, J.N. (1959) Ovarian changes in
prepubertal rats after treatment with cadmium chloride. Acta biol.
med. Germ., 3: 372-399.
KAR, A.B., DAS, R.P., & MUKERJI, B. (1960) Prevention of
cadmium-induced changes in the gonads of rat by zinc and selenium: a
study in antagonism between metals in the biological system. Proc.
Natl Inst. Sci. India Part B. Biol. Sci., 26: 40-50.
KARIN, M. & RICHARDS, R.I. (1982) Human metallothionein genes:
primary structure of the metallothionein-II gene and a related
processed gene. Nature (Lond.), 299: 797-802.
KARIN, M., SLATER, E.P., & HERSCHMAN, H.R. (1981) Regulation of
metallothionein synthesis in HeLa cells by heavy metals and
glucocorticoids. J. cell Physiol., 106: 63-74.
KASUYA, M., TERANISHI, H., KUBOTA, Y., KATO, T., AOSHIMA, K., SAIJO,
A., IWATA, K., & KANAI, M. (1986) [Biological meaning and blood and
urine low-molecular-weight protein determinations of Itai-itai
disease patient and their families, 10 years follow-up study on
urine low-molecular weight protein], Tokyo, Japan Public Health
Association, pp. 176-180 (Kankyo Hoken Report No. 52) (in Japanese).
KATAGIRI, Y., TATI, M., IWATA, H., & KANAI, M. (1971) [Concentration
of cadmium in urine by age.] Med. Biol., 82: 239-243 (in Japanese).
KATO, T. & ABE, K. (1978) Environmental pollution and health effects
- Toyama Prefecture. In: Tsuchiya, K., ed. Cadmium studies in Japan:
a review, Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp. 225-249.
KAWAI, K. (1978) Experimental studies on animals. Morphological
changes of the kidney and effects on bones. In: Tsuchiya, K., ed.
Cadmium studies in Japan: a review, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 86-97.
KAWAI, M., FUKUDA, K., & KIMURA, M. (1976) Morphological alterations
in experimental cadmium exposure with special reference to the onset
of renal lesion. In: Nordberg, G.F., ed. Effects and dose-response
relationships of toxic metals, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 343-370.
KAWAMURA, J., YOSHIDA, O., NISHINO, K., & ITOKAWA, Y. (1978)
Disturbances in kidney functions and calcium and phosphate
metabolism in cadmium-poisoned rats. Nephron, 20: 101-110.
KAWANO, S. & KATO, T. (1978) Environmental pollution and health
effects - Ishikawa Prefecture. In: Tsuchiya, K., ed. Cadmium studies
in Japan: a review, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 220-225.
KAWASHIMA, H., NOMIYAMA, H., & NOMIYAMA, K. (1988) Chronic exposure
to cadmium did not impair vitamin D metabolism in monkeys. Environ.
Res., 46: 48-58.
KAZANTZIS, G. (1956) Respiratory function in men casting cadmium
alloys. Part I. Assessment of ventilatory function. Br. J. ind.
Med., 13: 30-36.
KAZANTZIS, G. (1963) Induction of sarcoma in the rat by cadmium
sulfide pigment. Nature ( Lond.), 198: 1213-1214.
KAZANTZIS, G. (1979) Renal tubular dysfunction and abnormalities of
calcium metabolism in cadmium workers. Environ. Health Perspect.,
28: 155-160.
KAZANTZIS, G. & ARMSTRONG, B.G. (1982) A mortality study of cadmium
workers in the United Kingdom. Scand. J. Work Environ. Health,
8(1): 157-160.
KAZANTZIS, G. & HANBURY, W.J. (1966) The induction of sarcoma in the
rat by cadmium sulfide and by cadmium oxide. Br. J. Cancer,
20: 190-199.
KAZANTZIS, G., FLYNN, F.V., SPOWAGE, J.S., & TROTT, D.G. (1963)
Renal tubular malfunction and pulmonary emphysema in cadmium pigment
workers. Q. J. Med., 32: 165-192.
KAZANTZIS, G., LAM, T.-H., & SULLIVAN, K.R. (1988) Mortality of
cadmium-exposed workers. A five-year update. Scand. J. Work Environ.
Health, 14: 220-223.
KAZANTZIS, G., BLANKS, R., & TEATHER, S. (1989) The mortality of
cadmium exposed workers. In: Vernet, J.-P., ed. Proceedings of the
International Conference on Heavy Metals in the Environment, Geneva,
September 1989, Edinburgh, CEP Consultants Ltd, Vol. 1, pp. 304-307.
KELLO, D. & KOSTIAL, K. (1977) Influence of age and milk diet on
cadmium absorption from the gut. Toxicol. appl. Pharmacol.,
40: 277-282.
KIDO, T., HONDA, R., TSURITANI, I., YAMAYA, H., ISHIZAKI, M.,
YAMADA, Y., & NOGAWA, K. (1988) Progress of renal dysfunction in
inhabitants environmentally exposed to cadmium. Arch. environ.
Health, 43: 213-217.
KIDO, T., NOGAWA, K., YAMADA, Y., HONDA, R., TSURITANI, I.,
ISHIZAKI, M., & YAMAYA, H. (1989) Osteopenia in inhabitants with
renal dysfunction induced by exposure to environmental cadmium. Int.
Arch. occup. environ. Health, 61: 271-276.
KIMURA, M. & OTAKI, N. (1972) Percutaneous absorption of cadmium in
rabbit and hairless mouse. Ind. Health (Tokyo), 10: 7-10.
KING, E. (1955) An environmental study of casting copper-cadmium
alloys. Br. J. ind. Med., 12: 198-205.
KIPLING, M.D. & WATERHOUSE, J.A.H. (1967) Cadmium and prostatic
carcinoma. Lancet, 1: 730-731.
KIRSCH, H., PADBERG, W., SCHOLZ, A., & ZIMMERMEYER, G. (1982)
Cadmium emissions from coal-fired power plants. In: Proceedings of
the 3rd International Cadmium Conference, Miami, Florida, 3-5
February, 1981, London, Cadmium Association, pp. 64-68.
KITAMURA, M. (1972) [Absorption and deposition of cadmium,
especially in human subjects], Tokyo, Japan Public Health
Association, pp. 42-45 (Kankyo Hoken Report No. 11) (in Japanese).
KITAMURA, M. & KOIZUMI, N. (1975) [Results of urine tests for
bedridden old persons in Hyogo Prefecture. Report 2], Tokyo, Japan
Public Health Association, pp. 135-137 (Kankyo Hoken Report No. 36)
(in Japanese).
KITAMURA, S., SUMINO, K., & KAMATANI, N. (1970) [Cadmium
concentrations in liver, kidneys, and bones of human bodies.] Jpn.
J. public Health, 17: 177 (in Japanese).
KJELLSTRÖM, T. (1971) A mathematical model for the accumulation of
cadmium in human kidney cortex. Nord. Hyg. Tidskr., 53: 111-119.
KJELLSTRÖM, T. (1977) Accumulation and renal effects of cadmium in
men. A dose-response study, Stockholm, Karolinska Institute, 80 pp
(Doctoral thesis).
KJELLSTRÖM, T. (1979) Exposure and accumulation of cadmium in
populations from Japan, the United States, and Sweden. Environ.
Health Perspect., 28: 169-197.
KJELLSTRÖM, T. (1986) Effects on bone, on vitamin D, and calcium
metabolism. In: Friberg, L., Elinder, C.-G., Kjellström, T., &
Nordberg, G.F., ed. Cadmium and health: A toxicological and
epidemiological appraisal, Boca Raton, Florida, CRC Press, Vol. II,
pp. 111-158.
KJELLSTRÖM, T. & NORDBERG, G.F. (1978) A kinetic model of cadmium
metabolism in the human being. Environ. Res., 16: 248-269.
KJELLSTRÖM, T. & PISCATOR, M. (1977) Quantitative analysis of
ß-2-microglobulin in urine as an indicator of renal tubular damage
by cadmium. Phadedoc No. 1. Diagnostic communications, Uppsala,
Sweden, Pharmacia Diagnostics, 21 pp.
KJELLSTRÖM, T., LIND, B., LINNMAN, L., & NORDBERG, G.F. (1974) A
comparative study on methods for cadmium analysis of grain with an
application to pollution evaluation. Environ. Res., 8: 92-106.
KJELLSTRÖM, T., LIND, B., LINNMAN, L., & ELINDER, C.-G. (1975a)
Variation of cadmium concentration in Swedish wheat and barley. An
indicator of changes in daily cadmium intake during the 20th
century. Arch. environ. Health, 30: 321-328.
KJELLSTRÖM, T., TSUCHIYA, K., TOMPKINS, E., TAKABATAKE, E., LIND,
B., & LINNMAN, L. (1975b) A comparison of methods for analysis of
cadmium in food and biological material. A cooperative study between
Sweden, Japan, and the USA. In: Recent advances in the assessment of
the health effects of environmental pollution, Luxembourg,
Commission of the European Communities, pp. 2197-2213.
KJELLSTRÖM, T., EVRIN, P.E., & RAHNSTER, B. (1977a) Dose-response
analysis of cadmium-induced tubular proteinuria. A study of urinary
ß-2-microglobulin excretion among workers in a battery factory.
Environ. Res., 13: 303-317.
KJELLSTRÖM, T., SHIROISHI, K., & EVRIN, P.E. (1977b) Urinary
ß-2-microglobulin excretion among people exposed to cadmium in the
general environment. An epidemio-logical study in cooperation
between Japan and Sweden. Environ. Res., 13: 318-344.
KJELLSTRÖM, T., BORG, K., & LIND, B. (1978) Cadmium in faeces as an
estimator of daily cadmium intake in Sweden. Environ. Res.,
15: 242-251.
KJELLSTRÖM, T., FRIBERG, L., & RAHNSTER, D. (1979) Mortality and
cancer morbidity among cadmium-exposed workers. Environ. Health
Perspect., 28: 199-204.
KJELLSTRÖM, T., ELINDER, C.-G., & FRIBERG, L. (1984) Conceptual
problems in establishing the critical concentration of cadmium in
human kidney cortex. Environ. Res., 33: 284-295.
KLAASSEN, C.D. & KOTSONIS, F.N. (1977) Biliary excretion of cadmium
in the rat, rabbit, and dog. Toxicol. appl. Pharmacol., 41: 101-112.
KNEIP, T.J., EISENBUD, M., STREHLOW, C.D., & FREUDENTHAL, P.C.
(1970) Airborne particulates in New York City. J. Air Pollut.
Control Assoc., 20: 144-149.
KOBAYASHI, J. (1974) Effects of cadmium on calcium metabolism of
rats. In: Hemphill, D.D., ed. Trace substances in environmental
health - VII, Columbia, Missouri, University of Missouri Press,
pp. 295-305.
KODAMA, Y., MORIMOTO, K., & NAKAJIMA, T. (1978) [Studies on the
chemical form of heavy metal in oysters.] Eisei Kagaku, 24: 132-138
(in Japanese).
KOGAN, I.G., GROZDOVA, T.YA., & KHOLIKOVA, T.A. (1978)
[Investigation of the mutagenic effect of cadmium chloride on
Drosophila melanogaster germ cells.] Genetika, 16: 2136-2140 (in
Russian).
KOGAN, V.H., MALISHEVA, N.B., & FIDAROVA, A.M. (1972) On the role of
visceral lesions under chronic cadmium intoxications in the
development of bone changes. Trans. Kuban Med. Inst., 36: 75-78.
KOIRTYOHANN, S.R. & HOPKINS, C.A. (1976) Losses of trace metals
during the ashing of biological materials. Analyst, 101: 870-875.
KOIVISTOINEN, P. (1980) Mineral element composition of Finnish
foods: N, K, Ca, Mg, P, S, Fe, Cu, Mn, Zn, Mo, Co, Ni, Cr, F, Se,
Si, Rb, Al, B, Br, Hg, As, Cd, Pb, and Ash. Acta agric. Scand.,
22: Suppl.
KOIZUMI, H., YASUDA, K., & KATAYAMA, M. (1977) Atomic absorption
spectrophotometry based on the polarization characteristics of the
Zeeman effects. Anal. Chem., 49: 1106-1112.
KOJIMA, S., HAGA, Y., KURIHARA, T., & YAMAWAKI, T. (1975) [Report
from Akita Prefecture], Tokyo, Japan Public Health Association, pp.
114-123 (Kankyo Hoken Report No. 36) (in Japanese).
KOJIMA, S., HAGA, Y., KURIHARA, T., & YAMAWAKI, T. (1976) [Report on
cadmium pollution in Akita Prefecture], Tokyo, Japan Public Health
Association, pp. 114-120 (Kankyo Hoken Report No. 36) (in Japanese).
KOJIMA, S., HAGA, Y., KURIHARA, T., YAMAWAKI, T., & KJELLSTRÖM, T.
(1977) A comparison between faecal cadmium and urinary
ß-2-microglobulin total protein and cadmium among Japanese farmers.
An epidemiological study in cooperation between Japan and Sweden.
Environ. Res., 14: 436-451.
KOLLER, L.D., EXON, J.H., & ROAN, J.G. (1975) Antibody suppression
by cadmium. Arch. environ. Health, 30: 598-601.
KONO, M., YOSHIDA, T., SUGIHARA, H., & HAGINO, N. (1956) [Report on
so-called Itai-itai disease - First report.] J. Jap. Soc.
Orthopaedis., 30: 100-101 (in Japanese).
KOPP, S.J., GLONEK, T., ERLANGER, M., PERRY, E.F., PERRY, H.M., Jr,
& BARANY, M. (1980a) Cadmium and lead effects on myocardial function
and metabolism. J. environ. Pathol. Toxicol., 4: 205-227.
KOPP, S.J., PERRY, H.M., Jr, GLONEK, T., ERLANGER, M., PERRY, E.F.,
BARANY, M., & D'AGROSA, L.S. (1980b) Cardiac physiologic-metabolic
changes after chronic low-level heavy metal feeding. Am. J.
Physiol., 239: H22-H30.
KOPP, S.J., PERRY, H.M., Jr, PERRY, E.F., & ERLANGER, M. (1983)
Cardiac physiologic and tissue metabolic changes following chronic
low-level cadmium and cadmium plus lead ingestion in the rats.
Toxicol. appl. Pharmacol., 69: 149-160.
KOROPATNICK, J. & CHERIAN, M.G. (1988) Exposure to different forms
of cadmium in mice: differences in metallothionein and
alphafetoprotein mRNA induction in liver and kidney. J. biochem.
Toxicol., 3: 159-172.
KORPELA, H., LOUENIVA, R., YRJANHEIKKI, E., & KAUPPILA, A. (1986)
Lead and cadmium concentrations in maternal and umbilical cord
blood, amniotic fluid, placenta, and amniotic membranes. Am J.
Obstet. Gynecol., 155: 1086-1089.
KOTSONIS, F.N. & KLAASSEN, C.D. (1977) Toxicity and distribution of
cadmium administered to rats at sublethal doses. Toxicol. appl.
Pharmacol., 41: 667-680.
KOWAL, N.E. & KRAEMER, D.F. (1982) Urinary cadmium and
ß-2-microglobulin levels of persons aged 20-74 years from a
sub-sample of the national HANES II survey, 1976-1980. In:
Proceedings of the 3rd International Cadmium Conference, Miami,
Florida, 3-5 February, 1981, London, Cadmium Association,
pp. 119-122.
KOWAL, N.E., JOHNSON, D.E., KRAEMER, D.F., & PAHREN, H.R. (1979)
Normal levels of cadmium in diet, urine, blood, and tissues of
inhabitants of the United States. J. Toxicol. environ. Health,
5: 995-1014.
KRAEMER, D.F., LUCAS, J.B., PAHREN, H.R., RYAN, J.A., & KOWAL, N.E.
(1979) Cadmium toxicity. Lancet, 1: 1241-1242.
KRASOVSKII, G.N., VARSHAVSKAYA, S.P., & BORISOV, A.I. (1976) Toxic
and gonadotropic effects of cadmium and boron relative to standards
for these substances in drinking-water. Environ. Health Perspect.,
13: 69-75.
KRELL, U. & ROECKNER, E. (1988) Model simulation of the atmospheric
input of lead and cadmium into the North Sea. Atmos. Environ.,
22(2): 375-381.
KRETZSCHMAR, J.G., DELESPAUL, I., & DE RIJK, T.H. (1980) Heavy
levels in Belgium: a five-year survey. Sci. total Environ.,
14: 85-97.
KUHNERT, P.M., KUHNERT, B.R., BOTTOMS, S.F., & ERHARD, P. (1982)
Cadmium levels in maternal blood, fetal cord blood, and placental
tissues of pregnant women who smoke. Am. J. Obstet. Gynecol.,
142: 1021-1028.
KUHNERT, P.M., KUHNERT, B.R., ERHARD, P., BRASHEAR, W.T.,
GROH-WARGO, S.L., & WEBSTER, S. (1987a) The effect of smoking on
placental and fetal zinc status. Am. J. Obstet. Gynecol.,
157: 1241-1246.
KUHNERT, B.R., KUHNERT, P.M., DEBANNE, S., & WILLIAMS, T.G. (1987b)
The relationship between cadmium, zinc and birth weight in pregnant
women who smoke. Am. J. Obstet. Gynecol., 157: 1247-1251.
KUHNERT, B.R., KUHNERT, P.M., & ZARLINGO, T.J. (1988) Associations
between placental cadmium and zinc and age and parity in pregnant
women who smoke. Obstet. Gynecol., 71: 67-70.
LAAMANEN, A. (1972) Functions, progress, and prospects for an
environmental subarctic base level station. Work environ. Health,
9: 17-25.
LAFLEUR, P.D., ed. (1976) Accuracy in trace analysis: sampling,
sample handling, analysis, Washington, DC, US Department of
Commerce, National Bureau of Standards, Vol. 1 and 2, 1304 pp.
LARIONOVA, T.I., ZILLE, L.N., & VOROBJEVA, R.S. (1974) [The
influence of barium-cadmium laurate on some fermentative processes
in the liver.] Gig. Tr. prof. Zabol., 3: 50-52 (in Russian).
LAUWERYS, R. & DE WALS, PH. (1981) Environmental pollution by
cadmium and mortality from renal diseases. Lancet, 1: 383.
LAUWERYS, R., BUCHET, J.P., ROELS, H., BROUWERS, J., & STANESCU, D.
(1974a) Epidemiological survey of workers exposed to cadmium. Arch.
environ. Health, 28: 145-148.
LAUWERYS, R., BUCHET, J.P., & ROELS, H. (1974b) Effets subcliniques
de l'exposition humaine au cadmium. In: Proceedings of the
International Symposium on Problems of the Contamination of Man and
His Environment by Mercury and Cadmium, Luxembourg, Commission of
the European Communities, pp. 447-461.
LAUWERYS, R., BUCHET, J.P., & ROELS, H. (1974c) Dosage du cadmium et
du plomb dans le sang et les urines par spectrophotométrie
d'absorption atomique précedée d'une chromatographie sur résine
échangeuse d'ions. In: Proceedings of the International Symposium on
Problems of the Contamination of Man and His Environment by Mercury
and Cadmium, Luxembourg, Commission of the European Communities,
pp. 231-238.
LAUWERYS, R., BUCHET, J.P., ROELS, H., BERLIN, A., & SMEETS, J.
(1975) Intercomparison program of lead, mercury, and cadmium
analysis in blood, urine, and aqueous solutions. Clin. Chem.,
21: 551-557.
LAUWERYS, R., BUCHET, J.P., & ROELS, H. (1976) The relationship
between cadmium exposure or body burden and the concentration of
cadmium in blood and urine in man. Int. Arch. occup. environ.
Health, 36: 275-285.
LAUWERYS, R., BUCHET, J.P., ROELS, H., & HUBERMONT, G. (1978)
Placental transfer of lead, mercury, cadmium and carbon monoxide in
women. I. Comparison of the frequency distributions of the
biological indices in maternal and umbilical cord blood. Environ.
Res., 15: 278-290.
LAUWERYS, R., ROELS, H., BUCHET, J.P., BERNARD, A., & STANESCU, D.
(1979a) Investigations on the lung and kidney functions in workers
exposed to cadmium. Environ. Health Perspect., 28: 137-145.
LAUWERYS, R., ROELS, H., REGNIERS, M., BUCHET, J.P., BERNARD, A., &
GORET, A. (1979b) Significance of cadmium concentration in blood and
in urine in workers exposed to cadmium. Environ. Res., 20: 375-391.
LAUWERYS, R., ROELS, H., BERNARD, A., & BUCHET, J.P. (1980a) Renal
response to cadmium in a population living in a non-ferrous smelter
area in Belgium. Int. Arch. occup. environ. Health, 45: 271.
LAUWERYS, R., BUCHET, J.P., ROELS, H., BERNARD, A., CHETTLE, D.R.,
HARVEY, T.C., & ALHADDAD, I.K. (1980b) Biological significance of
cadmium concentration in blood and urine and their application in
monitoring workers exposed to cadmium. In: Proceedings of the 2nd
International Cadmium Conference, Cannes, 6-8 February, 1977,
London, Cadmium Association, pp. 164-167.
LAUWERYS, R., BUCHET, J.P., ROELS, H., VIAU, C., BERNARD, A.,
BRUAUX, P., CLAEYS-THOREAU, F., & RONDIA, D. (1984a) Potential risk
of cadmium for the general population: Update of Liege study. In:
Proceedings of the 4th International Cadmium Conference, Munich, 2-4
March, 1983, London, Cadmium Association, pp. 113-114.
LEHMAN, L.D. & POISNER, A.M. (1984) Induction of metallothionein
synthesis in cultured human trophoblasts by cadmium and zinc. J.
Toxicol. environ. Health, 14: 419-432.
LEHNERT, G., VON, SCHALLER, K.H., & HAAS, T. (1968) [Cadmium
analysis of serum and urine using atomic absorption spectrometry.]
Z. klin. Chem. klin. Biochem., 6: 174-176 (in German).
LEMEN, R.A., LESS, J.S., WAGONER, J.K., & BLEJER, H.P. (1976) Cancer
mortality among cadmium production workers. Ann. NY Acad Sci.,
271: 273-279.
LENER, J. & MUSIL, J. (1971) Cadmium influence on the excretion of
sodium by kidneys. Experientia (Basel), 27: 902.
L'EPEE, P., LAZARINI, H., FRANCHOME, J., N'DOKY, T., & LARRIVET, C.
(1968) Contribution à l'étude de l'intoxication cadmique. Arch.
Mal. prof. Méd. Trav. Sécur. soc., 29: 485-490.
LEPOW, M.L., BRUCKMAN, L., RUBINO, R.A., MARKOWITZ, S., GILLETTE,
M., & KAPISH, J. (1974) Role of airborne lead in increased body
burden of lead in Hartford children. Environ. Health Perspect.,
7: 99-102.
LEVY, L.S. & CLACK, J. (1975) Further studies on the effect of
cadmium on the prostate gland. I. Absence of prostatic changes in
rats given oral cadmium sulfate for two years. Ann. occup. Hyg.,
17: 205-211.
LEVY, L.S., ROE, F.J.C., MALCOLM, D., KAZANTIZIS, G., CLACK, J., &
PLATT, H.S. (1973) Absence of prostatic changes in rats exposed to
cadmium. Ann. occup. Hyg., 16: 111-118.
LEVY, L.S., CLACK, J., & ROE, F.J.C. (1975) Further studies on the
effect of cadmium on the prostate gland. II. Absence of prostatic
changes in mice given oral cadmium sulfate for eighteen months. Ann.
occup. Hyg., 17: 213-220.
LEWIS, G.P., JUSKO, W.J., COUGHLIN, L.L., & HARTZ, S. (1972) Cadmium
accumulation in man: influence of smoking, occupation, alcoholic
habit, and disease. J. chron. Dis., 25: 717-726.
LIKUTOVA, I.V. (1989) [Specific effect of inorganic cadmium
compounds on health and its hygienic assessment.] Abstract from a
dissertation for degree of Candidate of Medical Sciences, Moscow,
Central Institute for the Advanced Training of Physicians, 21 pp (in
Russian).
LIKUTOVA, I.V. & BELOVA, E.A. (1987) [Specific action of cadmium in
its peroral intake with water.] Gig. i Sanit., 1987(6): 70-72 (in
Russian).
LINDBERG, S.E., TURNER, R.R., & LOVETT, G.M. (1982) Processes of
atmospheric deposition of metals and acids to forests. Conference
Proceeding 75. Annual Meeting on the Air Pollution Control
Association, New Orleans, 20 June, 1982, Pittsburgh, Pennsylvania,
Air Pollution Control Association, 14 pp.
LINNMAN, L., ANDERSSON, A., NILSSON, K.O., LIND, B., KJELLSTRÖM, T.,
& FRIBERG, L. (1973) Cadmium uptake by wheat from sewage sludge used
as a plant nutrient source. Arch. environ. Health, 27: 45-47.
LORENTZON, R. & LARSSON, S.E. (1977) Vitamin D metabolism in adult
rats at low and normal calcium intake and the effect of cadmium
exposure. Clin. Sci. mol. Med., 53: 439-446.
LOSER, E. (1980) A 2-year oral carcinogenicity study with cadmium on
rats. Cancer Lett., 9: 191-198.
LUCAS, P.A., JARIVALLA, A.G., JONES, J.H., GOUGH, J., & VALE, P.T.
(1980) Total cadmium fume inhalation. Lancet, 2(8187): 205.
LUCIS, O.J., LUCIS, R., & ATERMAN, K. (1972) Tumorigenesis by
cadmium. Oncology, 26: 53-76.
LUFKIN, N.H. & HODGES, F.T. (1944) Cadmium poisoning. Report of
outbreak. US nav. med. Bull., 43: 1273-1276.
LUND, L.J., BETTY, E.E., PAGE, A.L., & ELLIOTT, R.A. (1981)
Occurrence of naturally high cadmium levels in soils and its
accumulation by vegetation. J. environ. Qual., 10: 551-556.
LUNDGREN, G. (1976) Direct determination of cadmium in blood with a
temperature- controlled heated graphite-tube atomizer. Talenta,
23: 309-312.
MAACK, T., JOHNSON, V., KAN, S.T., FIGUEIREDO, J., & SIGULEM, D.
(1979) Renal filtration, transport, and metabolism of low molecular
weight proteins. A review. Kidney Int., 16: 251-270.
MACFARLAND, H.N. (1979) Pulmonary effects of cadmium. In: Mennear,
J., ed. Cadmium toxicity, New York, Basel, Marcel Dekker, Inc.,
pp. 113-132.
MCINNES, G. (1979) Multi-element survey: analysis of the first two
years' results. Warren Spring Laboratory, United Kingdom Department
of Industry, 44 pp (Report No. LR305 AP).
MCKENZIE, J., KJELLSTRÖM, T., & SHARMA, R.P. (1982) Cadmium intake
metabolism and effects in people with a high intake of oysters in
New Zealand, Washington, DC, US Environmental Protection Agency,
210 pp.
MCLELLAN, J.S., THOMAS, B.J., & FREMLIN, J.H. (1975) Cadmium: its
in vivo detection in man. Phys. Med. Biol., 20: 88-95.
MCLELLAN, J.S., FLANAGAN, P.R., CHAMBERLAIN, M.J., & VALBERG, L.S.
(1978) Measurements of dietary cadmium absorption in humans. J.
Toxicol. environ. Health, 4: 131-138.
MAHAFFEY, K.R., CORNELIUSSEN, P.E., JELINEK, C.F., & FIORINO, J.A.
(1975) Heavy metal exposure from foods. Environ. Health Perspect.,
12: 63-69.
MANGELSON, N.F., HILL, M.W., NIELSON, K.K., EATOUGH, D.J.,
CHRISTENSEN, J.J., IZATT, R.M., & RICHARDS, D.O. (1979)
Proton-induced X-ray emission analysis of Pima Indian autopsy
tissues. Anal. Chem., 51: 1187-1194.
MARC (1986) Biological monitoring of environmental contaminants
(plants), London, Monitoring and Assessment Research Centre, Chelsea
College, University of London, 247 pp (MARC Report Number 32).
MARGOSHES, M. & VALLEE, B.L. (1957) A cadmium kidney protein from
equine kidney cortex. J. Am. Chem. Soc., 79: 4813-4814.
MARTIN, J.H. & BROENKOW, W.W. (1975) Cadmium in plankton: elevated
concentrations off Baja California. Science, 190: 884-885.
MARTIN, J.H., ELLIOTT, P.D., ANDERLINI, V.C., GIRVIN, D., JACOBS,
S.A., RISEBROUGH, R.W., DELONG, R.L., & GILMARTIN, W.G. (1976)
Mercury-selenium-bromine imbalance in premature parturient
California sea lions. Mar. Biol., 35: 91-104.
MASON, H.J., DAVISON, A.G., & WRIGHT, A.L. (1988) Relations between
liver cadmium, cumulative exposure, and renal function in cadmium
alloy workers. Br. J. ind. Med., 45: 793-802.
MASON, K.E. & YOUNG, J.O. (1967) Effectiveness of selenium and zinc
in protecting against cadmium-induced injury of rat testis. In:
Muth, O.H., ed. Symposium: Selenium in Biomedicine, Westport,
Connecticut, Avi, pp. 383-394.
MATERNE, D., LAUWERYS, R., BUCHET, J.P., ROELS, H., BROUWERS, J., &
STANESCU, D. (1975) Investigations sur les risques résultant de
l'exposition au cadmium dans deux entreprises de production et deux
entreprises d'utilisation du cadmium. Cah. Méd. Trav., 12: 1-76.
MATSUSAKA, N., TANAKA, M., NISHIMURA, Y., YUYAMA, A., & KOBAYASHI,
H. (1972) [Whole body retention and intestinal absorption of 115Cd
in young and adult mice.] Med. Biol., 85: 275-279 (in Japanese).
MERALI, Z., KACEW, S., & SINGHAL, R.L. (1974) Response of hepatic
carbohydrate and cyclic AMP metabolism to cadmium treatment in rats.
Can. J. Physiol. Pharmacol., 53: 174-184.
MERANGER, J.C., SUBRAMANIAN, K.S., & CHALIFOUX, C. (1981) Metals and
other elements. Survey for cadmium, cobalt, chromium, copper,
nickel, lead, zinc, calcium, and magnesium in Canadian
drinking-water supplies. J. Assoc. Off. Anal. Chem., 64: 44-53.
MILLER, R.K. (1986) Placental transfer and function. The interface
for drugs and chemicals in the conceptus. In: Fabro, S. & Scialli,
A.S., ed. Drug and chemical action in pregnancy, New York, Basel,
Marcel Dekker, Inc., pp. 123-152.
MILLS, C.F. & DELGARNO, A.C. (1972) Copper and zinc status of ewes
and lambs receiving increased dietary concentration of cadmium.
Nature ( Lond.), 239: 171-173.
MOGENSEN, C.E. & SOLLING, K. (1977) Studies on renal tubular protein
reabsorption: partial and near complete inhibition by certain amino
acids. Scand. J. clin. lab. Invest., 37: 477-486.
MOHD, M.A., MURTHY, R.C., & CHANDRA, S.V. (1986) Developmental and
long-term neurobehavioral toxicity of low-level in utero Cd
exposure in rats. Toxicol. Teratol., 8: 463.
MORGAN, J.M. (1970) Cadmium and zinc abnormalities in bronchogenic
carcinoma. Cancer, 25: 1394-1398.
MORGAN, J.M., BURCH, H.B., & WATKISS, J.B. (1971) Tissue cadmium and
zinc content in emphysema and bronchogenic carcinoma. J. chron.
Dis., 24: 107-110.
MORITSUGI, M. & KOBAYASHI, J. (1964) Study on trace elements in
biomaterials. II. Cadmium content in polished rice. Ber. Ohara Inst.
Landwirtsch. Biol. Okayama Univ., 12: 145-158.
MOSCHANDREAS, D.J. (1981) Exposure to pollutants and daily time
budgets of people. Bull. NY Acad. Med., 57: 845-859.
MULFORD, C.E. (1966) Solvent extraction techniques for atomic
absorption spectoscopy. At. Absorpt. Newsl., 5: 88-90.
MULVIHILL, J.E., GAMM, S.H., & FERM, V.H. (1970) Facial formation in
normal and cadmium-treated golden hamsters. J. Embryol. exp.
Morphol., 24: 393-403.
MURASE, H., INOUE, T., SAGA, M., IWATA, T., & KUBOTA, K. (1974)
[Microscopic pathology of dogs chronically exposed to oral cadmium],
Tokyo, Japan Public Health Association, pp. 72-73 (Kankyo Hoken
Report No. 31) (in Japanese).
MUSKETT, C.J., ROBERTS, L.H., & PAGE, B.J. (1979) Cadmium and lead
pollution from secondary metal refinery operations. Sci. total
Environ., 11: 73-87.
NACKOWSKI, S.B., PUTNAM, R.D., ROBBINS, D.A., VARNER, M.O., WHIT,
L.D., & NELSON, K.W. (1977) Trace metal contamination of evacuated
blood collection tubes. Am. Ind. Hyg. Assoc. J., 38: 503-508.
NAKAGAWA, S. (1960) [A study of osteomalacia in Toyama Prefecture
(so-called Itai-itai disease).] J. Radiol. Phys. Ther. Univ.
Kanazawa, 56: 1-51 (in Japanese with English summary).
NAKANO, M., AOSHIMA, K., KATOH, T., TERANISHI, H., & KATOH, T.
(1987) Severity of tubular brush border damage in a
cadmium-polluted area (Jinzu River basin): clinical role of urinary
trehalase. Environ. Res., 44: 161-168.
NAZARI, G., FAVINO, A., & POZZI, U. (1967) [Effects of a single
subcutaneous injection of cadmium chloride in male rats.] Riv. Anat.
Pathol. Oncol., 31: 251-271 (in Italian).
NEEB, R. & WAHDAT, F. (1974) [About inverse voltammetry analysis of
cadmium in aerosols.] Z. anal. Chem., 269: 275-279 (in German).
NICAUD, P., LAFITTE, A., & GROS, A. (1942) Les troubles de
l'intoxication chronique par le cadmium. Arch. Mal. prof.,
4: 192-202.
NICHOLSON, J.K. & OSBORN, O. (1983) Kidney lesions in pelagic
seabirds with high tissue levels of cadmium and mercury. J. Zool.
(Lond.), 200: 88-118. NIELSEN, S.A. (1975) Cadmium in New Zealand
dredge oysters, geographic distribution. Int. J. environ. anal.
Chem., 4: 1-7.
NISE, G. & VESTERBERG, O. (1978) [Sampling vessels influence on
content of heavy metals (Cd, Cr, Hg, Ni, and Pb) in blood and urine
samples.] Läkartidningen, 75: 19-20 (in Swedish).
NISHIYAMA, K. & NORDBERG, G. (1972) Adsorption and elution of
cadmium on hair. Arch. environ. Health, 25: 92-96.
NISHIYAMA, S., NAKAMURA, K., & KONISHI, Y. (1986) Blood pressure and
urinary sodium and potassium excretion in cadmium-treated male rats.
Environ. Res., 40: 357-364.
NOGAWA, K. (1981) Itai-itai disease and follow-up studies. In:
Nriagu, J.O., ed. Cad-mium in the environment. II. Health effects,
New York, John Wiley and Sons, pp. 1-38.
NOGAWA, K. & ISHIZAKI, A. (1979) A comparison between cadmium in
rice and renal effects among inhabitants of the Jinzu River Basin.
Environ. Res., 18: 410-420.
NOGAWA, K., ISHIZAKI, A., FUKUSHIMA, M., SHIBATA, I., & HAGINO, N.
(1975) Studies on the women with acquired Fanconi syndrome observed
in the Ichi River basin polluted by cadmium. Environ. Res.,
10: 280-307.
NOGAWA, K., ISHIZAKI, A., & KAWANO, S. (1978) Statistical
observations of the dose-response relationships of cadmium based on
epidemiological studies in the Kakehashi River basin. Environ. Res.,
15: 185-198.
NOGAWA, K., KOBAYASHI, E., HONDA, R., & ISHIZAKI, A. (1979)
Clinico-chemical studies on chronic cadmium poisoning. I. Results of
urinary examinations. Jpn. J. Hyg., 34: 407-414.
NOGAWA, K., KOBAYASHI, E., HONDA, R., ISHIZAKI, A., KAWANO, S., &
MATUSDA, H. (1980) Renal dysfunction of inhabitants in a
cadmium-polluted area. Environ. Res., 23: 13-23.
NOGAWA, K., KOBAYASHI, E., & KONISHI, F. (1981a) Comparison of bone
lesions in chronic cadmium intoxication and vitamin D deficiency.
Environ. Res., 24: 233-249.
NOGAWA, K., KAWANO, S., & NISHI, M. (1981b) Mortality study of
inhabitants in a cadmium-polluted area with special reference to low
molecular weight proteinuria. In: Heavy metals in the environment,
Edinburgh, CEP Consultants Ltd, pp. 538-540.
NOGAWA, K., KIDO, T., YAMADA, Y., TSURITANI, I., HONDA, R.,
ISHIZAKI, N., & TERAHATA, K. (1984) Alpha-1-microglobulin in urine
as an indicator of renal tubular damage caused by environmental
cadmium exposure. Toxicol. Lett., 22: 63-68.
NOGAWA, K., TSURITANI, I., YAMADA, Y., KIDO, T., HONDA, R.,
ISHIZAKI, M., & KURIHARA, T. (1986) Sister-chromatid exchanges in
the lymphocytes of people exposed to environmental cadmium. Toxicol.
Lett., 32: 283-288.
NOGAWA, K., TSURITANI, I., KIDO, T., HONDA, R., YAMADA, Y., &
ISHIZAKI, M. (1987) Mechanism for bone disease found in inhabitants
environmentally exposed to cadmium: decreased serum
1alpha,25-dihydroxyvitamin D level. Int. Arch. occup. environ.
Health, 59: 21-30.
NOGAWA, K., HONDA, R., KIDO, T., TSURITANI, I., YAMADA, Y.,
ISHIZAKI, M., & YAMAYA, H. (1989) A dose-response analysis of
cadmium in the general environment with special reference to total
cadmium intake limit. Environ. Res., 48: 7-16.
NOMIYAMA, K. (1973) Early signs of cadmium intoxication in rabbits.
Toxicol. appl. Pharmacol., 24: P625-P635.
NOMIYAMA, K. (1974) [Studies concerning cadmium metabolism and the
etiology of poisoning], Tokyo, Japan Public Health Association,
pp. 53-59 (Kankyo Hoken Report No. 31) (in Japanese).
NOMIYAMA, K. (1977) Does a critical concentration of cadmium in
human renal cortex exist? J. Toxicol. environ. Health, 3: 607-609.
NOMIYAMA, K. (1978) Experimental studies on animals:
in vivo experiments. In: Tsuchiya, K., ed. Cadmium studies in
Japan: a review, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 47-86.
NOMIYAMA, K. (1986) The chronic toxicity of cadmium: influence of
environmental and other variables. In: Born, G.V.R., Rensselaer,
A.F., Herken, H., & Welch, A.D., ed. Handbook of experimental
pharmacology, Berlin, Heidelberg, Springer-Verlag, Vol. 80,
pp. 101-133.
NOMIYAMA, K. & FOULKES, E.C. (1977) Reabsorption of filtered cadmium
metallothionein in the rabbit kidney. Proc. Soc. Exp. Biol. Med.,
156: 97-99.
NOMIYAMA, K. & NOMIYAMA, H. (1976a) Mechanisms of urinary excretion
of cadmium: experimental studies in rabbits. In: Nordberg, G.F., ed.
Effects and dose-response relationships of toxic metals, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 371-379.
NOMIYAMA, K. & NOMIYAMA, H. (1976b) [Long-term experiment with
300 ppm oral cadmium exposure to rabbits. Intermediate report after
12 months], Tokyo, Japan Public Health Association, pp. 161-173
(Kankyo Hoken Report No. 38) (in Japanese).
NOMIYAMA, K. & NOMIYAMA, H. (1982) Tissue metallothionein in rabbits
chronically exposed to cadmium, with special reference to the
critical concentration of cadmium in the renal cortex. In: Foulkes,
E.C., ed. Biological roles of metallothionein, Amsterdam, Oxford,
New York, Elsevier Science Publishers, pp. 47-67.
NOMIYAMA, K. & NOMIYAMA, H. (1988) Health effect of six years of
dietary cadmium (cadmium contaminated rice) in monkeys. In:
Essential and toxic trace elements in human health and disease, New
York, Alan K. Liss, Inc., pp. 589-609.
NOMIYAMA, K., SUGATA, Y., YAMAMOTO, A., & NOMIYAMA, H. (1975)
Effects of dietary cadmium on rabbits. I. Early signs of cadmium
intoxication. Toxicol. appl. Pharmacol., 31: 4-12.
NOMIYAMA, K., SUGATA, Y., NOMIYAMA, H., & YAMAMOTO, A. (1976)
Dose-response relationship for cadmium. In: Nordberg, G.F., ed.
Effects and dose-response relationships of toxic metals, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 380-385.
NOMIYAMA, K., NOMIYAMA, H., YOTORIYAMA, M., & TAGUCHI, T. (1978a)
Some recent studies on the renal effects of cadmium. In: Proceedings
of the First International Cadmium Conference, San Francisco,
California, 31 January-2 February, 1977, London, Cadmium
Association, pp. 186-194.
NOMIYAMA, K., NOMIYAMA, H., & TAGUCHI, T. (1978b) Effects of
environmental temperatures on the toxicity of cadmium in mice. In:
Proceedings of the 8th Asian Conference on Occupational Health,
Tokyo, Japan Industrial Safety Association, pp. 221-225.
NOMIYAMA, K., NOMIYAMA, H., NOMURA, M., TAGUCHI, T., MATSUI, J.,
YOTORIYAMA, M., AKAHORI, F., IWAO, S., KOIZUMI, N., MASAOKA, T.,
KITAMURA, S., TSUCHIYA, K., SUZUKI, T., & KOBAYASHI, K. (1979)
Effects of dietary cadmium on rhesus monkeys. Environ. Health
Perspect., 28: 223-243.
NOMIYAMA, K., NOMIYAMA, H., AKAHORI, F., & MASAOKA, T. (1982a)
Cadmium health effects in monkeys with special reference to the
critical concentration of cadmium in the renal cortex. In:
Proceedings of the 3rd International Cadmium Conference, Miami,
Florida, 3-5 February, 1981, London, Cadmium Association,
pp. 151-156.
NOMIYAMA, K., NOMIYAMA, H., YOTORIYAMA, M., & MATSUI, K. (1982b)
Sodium dodecyl sulfate acrylamide gel electrophoretic studies on low
molecular weight proteinuria, an early sign of cadmium health
effects in rabbits. Ind. Health, 20: 11-18.
NOMIYAMA, K., NOMIYAMA, H., YOTORIYAMA, M., AKAHORI, F., & MASAOKA,
T. (1984) Some comments and proposals on dose and effects for
estimating critical concentration of cadmium in the renal cortex.
In: Proceedings of the Fourth International Cadmium Conference,
London, Cadmium Association, pp. 167-171.
NOMIYAMA, K., AKAHORI, F., MASAOKA, T., ARAI, S., NOMURA, Y.,
YOTORIYAMA, M., KOBAYASHI, K., SUZUKI, T., KAWASHIMA, H., & ONOZAWA,
A. (1987) [Dose effect and dose response relationships of 9 years
dietary cadmium in rhesus monkeys], Tokyo, Japan Public Health
Association, pp. 1-86 (Kankyo Hoken Report No. 53) (in Japanese).
NOMURA, T., KIMURA, M., TANIOKA, Y., TANIMOTO, Y., SAEGUSA, J.,
KOTAKI, N., TAZAKI, H., HATA, R., DEGUCHI, N., SUDA, T., & YOSHIKI,
S. (1988) [Influence of nutritional factors on cadmium-administered
monkeys], Tokyo, Japan Public Health Association, pp. 42-122 (Kankyo
Hoken Report No. 54) (in Japanese).
NORDBERG, G.F. (1971) Effects of acute and chronic cadmium exposure
on the testicles of mice. With special reference to protective
effects of metallothionein. Environ. Physiol. Biochem., 1: 171-187.
NORDBERG, G.F. (1972) Cadmium metabolism and toxicity. Environ.
Physiol. Biochem., 2: 7-36.
NORDBERG, G.F. (1975) Effects of long-term cadmium exposure on the
seminal vesicles of mice. J. Reprod. Fertil., 45: 165-167.
NORDBERG, G.F. & NISHIYAMA, K. (1972) Whole-body and hair retention
of cadmium in mice. Arch. environ. Health, 24: 209-214.
NORDBERG, G.F. & NORDBERG, M. (1988) Biological monitoring of toxic
metals, New York, London, Plenum Press, pp. 151-168.
NORDBERG, G.F. & PISCATOR, M. (1972) Influence of long-term cadmium
exposure on urinary excretion of protein and cadmium in mice.
Environ. Physiol. Biochem., 2: 37-49.
NORDBERG, G.F., PISCATOR, M., & LIND, B. (1971) Distribution of
cadmium among protein fractions of mouse liver. Acta pharmacol.
toxicol., 29: 456-470.
NORDBERG, G.F., SLORACH, S., & STENSTROM, T. (1973) [Cadmium
poisoning caused by a cooled soft drink machine.] Läkartidningen,
70: 601-604 (in Swedish with English summary).
NORDBERG, G.F., GOYER, R.A., & NORDBERG, M. (1975) Comparative
toxicity of cadmium metallothionein and cadmium chloride on mouse
kidney. Arch. Pathol., 99: 192-197.
NORDBERG, G.F., ROBERT, K.-H., & PANNONE, M. (1977) Pancreatic and
biliary excretion of cadmium in the rat. Acta pharmacol. toxicol.,
41: 84-88.
NORDBERG, G.F., GARVEY, J.S., & CHANG, C.C. (1982) Metallothionein
in plasma and urine of cadmium workers. Environ. Res., 28: 179-182.
NORDBERG, M. (1978) Studies on metallothionein and cadmium. Environ.
Res., 15: 381-404.
NORDBERG, M. (1984) General aspects of cadmium transport, uptake,
and metabolism by the kidney. Environ. Health Perspect., 54: 13-20.
NORDBERG, M. & NORDBERG, G.F. (1975) Distribution of metallothionein-
bound cadmium and cadmium chloride in mice: preliminary studies.
Environ. Health Perspect., 12: 103-108.
NORDBERG, M., NUOTTANIEMI, I., CHERIAN, M.G., NORDBERG, G.F.,
KJELLSTRÖM, T., & GARVEY, J.S. (1986) Characterization studies on
the cadmium-binding proteins from two species of New Zealand
oysters. Environ. Health Perspect., 65: 57-62.
NRIAGU, J.O. (1979) Global inventory of natural and anthropogenic
emissions of trace metals to the atmosphere. Nature (Lond.),
279: 409.
NRIAGU, J.O. & PACYNA, J.M. (1988) Quantitative assessment of
worldwide contamination of air, water and soils by trace metals.
Nature (Lond.), 333: 134-139.
NWANKWO, J.N., ELINDER, C.G., PISCATOR, M., & LIND, B. (1977)
Cadmium in Zambian cigarettes: an interlaboratory comparison in
analysis. Zambia J. Sci. Technol., 2(4): 1-4.
OBERDORSTER, G. (1988) Deposition and retention modeling of inhaled
cadmium in rat and human lung: An example for extrapolation of
effects and risk estimation. In: Crapo, J.D., Miller, F.J., Smolko,
E.D., Graham, J.A., & Hayes, A.W., ed. Extrapolation of dosimetric
relationships for inhaled particles and gases, New York, London,
Academic Press, pp. 345-370.
OHANIAN, E.V. & IWAI, J. (1980) Etiological role of cadmium in
hypertension in an animal model. J. environ. Pathol. Toxicol.,
3: 229-241.
OHMOMO, Y. & SUMIYA, M. (1981) Estimation of heavy metal intake from
agricultural products. In: Kitagishi, K. & Yamane, J., ed. Heavy
metal pollution in soils of Japan, Tokyo, Japan Scientific Societies
Press, pp. 235-244.
OLDIGES, H., HOCHRAINER, D., & GLASER, U. (1989) Long-term
inhalation study with Wistar rats and four cadmium compounds.
Toxicol. environ. Chem., 19: 217-222.
O'RIORDAN, M.L., HUGHES, E.G., & EVANS, H.J. (1978) Chromosome
studies on blood lymphocytes of men occupationally exposed to
cadmium. Mutat. Res., 58: 305-311.
PAGE, A.L. (1981) Cadmium in soils and its accumulation by food
crops. In: Proceedings of an International Conference on Heavy
Metals in the Environment, Amsterdam, September 1981, Edinburgh, CEP
Consultants, Ltd, pp. 206-213.
PAGE, A.L., BINGHAM, F.T., & SHANG, A.C. (1981) Cadmium. In: Lepp,
N.W., ed. Effect of heavy metal pollution on plants, Barking,
Essex, Applied Science Publishers, Vol. 1, pp. 77-109.
PANEMANGALORE, M., BANERJEE, D., ONOSAKA, S., & CHERIAN, M.G. (1983)
Changes in the intracellular accumulation and distribution of
metallothionein in rat liver and kidney during postnatal
development. Dev. Biol., 97: 95-102.
PARIZEK, J. (1957) The destructive effect of cadmium ion on
testicular tissue and its prevention by zinc. J. Endocrinol.,
15: 56-63.
PARIZEK, J. (1960) Sterilization of the male by cadmium salts. J.
Reprod. Fertil., 1: 294-309.
PARIZEK, J. (1964) Vascular changes at sites of oestrogen
biosynthesis produced by parenteral injection of cadmium salts: the
destruction of placenta by cadmium salts. J. Reprod. Fertil.,
7: 263-265.
PARIZEK, J. & ZAHOR, Z. (1956) Effects of cadmium salts on
testicular tissue. Nature (Lond.), 177: 1036-1037.
PARIZEK, J., OSTADALOVA, I., BENES, I., & PITHA, J. (1968a) The
effect of a subcutaneous injection of cadmium salts on the ovaries
of adult rats in persistent oestrus. J. Reprod. Fertil., 17:
559-562.
PARIZEK, J., OSTADALOVA, I., BENES, I., & BABICKY, A. (1968b)
Pregnancy and trace elements: the protective effect of compounds of
an essential trace element, selenium, against the peculiar toxic
effects of cadmium during pregnancy. J. Reprod. Fertil., 16:
507-509.
PARZYCK, D.C., SHAW, S.M., KESSLER, W.V., VETTER, R.J., VAN SICKLE,
D.C., & MAYES, R.A. (1978) Fetal effects of cadmium in pregnant rats
on normal and zinc-deficient diets. Bull. environ. Contam. Toxicol.,
19: 206-214.
PATON, G.R. & ALLISON, A.C. (1972) Chromosome damage in human cell
cultures induced by metal salts. Mutat. Res., 16: 332-336.
PERRY, H.M., Jr & ERLANGER, M.W. (1973) Elevated circulating renin
activity in rats following doses of cadmium known to induce
hypertension. J. lab. clin. Med., 82: 399-405.
PERRY, H.M., Jr & ERLANGER, M.W. (1974) Metal-induced hypertension
following chronic feeding of low doses of cadmium and mercury. J.
lab. clin. Med., 83: 541-547.
PERRY, H.M., Jr & ERLANGER, M.W. (1982) Effect of diet on increases
in systolic pressure induced in rats by chronic cadmium feeding. J.
Nutr., 112: 1983-1989.
PERRY, H.M., Jr & KOPP, S.J. (1983) Does cadmium contribute to human
hypertension? Sci. total Environ., 26: 223-232.
PERRY, H.M., Jr, ERLANGER, M.W., YUNICE, A., SCHOEPFLE, E., & PERRY,
E.F. (1970) Hypertension and tissue metal levels following
intravenous cadmium, mercury, and zinc. Am. J. Physiol., 219:
755-761.
PERRY, H.M., Jr, PERRY, E.F., & PURIFOY, J.E. (1971) Antinatriuretic
effect of intramuscular cadmium in rats. Proc. Soc. Exp. Biol. Med.,
136: 1240-1244.
PERRY, H.M., Jr, ERLANGER, M.W., & PERRY, E.F. (1976) Limiting
conditions for the induction of hypertension in rats by cadmium. In:
Hemphill, D.D., ed. Trace substances in environmental health - VIII,
Columbia, Missouri, University of Missouri Press, pp. 51-57.
PERRY, H.M., Jr, ERLANGER, M., & PERRY, E.F. (1977) Hypertension
following chronic, very low-dose cadmium feeding. Proc. Soc. Exp.
Biol. Med., 156: 173-176.
PETERING, D.H., LOFTSGAARDEN, J., SCHNEIDER, J., & FOWLER, B. (1984)
Metabolism of cadmium, zinc and copper in the rat kidney: the role
of metallothionein and other binding sites. Environ. Health
Perspect., 54: 73-81.
PETERING, H.G., JOHNSON, M.A., & STEMMER, K. (1971) Studies of zinc
metabolism in the rat. I. Dose-response effects of cadmium. Arch.
environ. Health, 23: 93-96.
PETERING, H.G., CHOUDHURY, H., & STEMMER, K.L. (1979) Some effects
of oral ingestion of cadmium on zinc, copper, and iron metabolism.
Environ. Health Perspect., 28: 97-106.
PETERSON, P.A. (1971) Characteristics of a vitamin A-transporting
protein complex occurring in human serum. J. biol. Chem.,
246: 34-43.
PETERSON, P.A. & BERGGÅRD, I. (1971) Isolation and properties of a
human retinol-transporting protein. J. biol. Chem., 246: 25-33.
PETERSON, P.A., EVRIN, P.-E., & BERGGÅRD, I. (1969) Differentiation
of glomerular, tubular, and normal proteinuria: determinations of
urinary excretion of ß-2-microglobulin, albumin, and total protein.
J. clin. Invest., 48: 1189-1198.
PHILIPP, R. (1985) Cadmium-risk assessment of an exposed residential
population: a review. J. R. Soc. Med., 78: 328-333.
PIOTROWSKI, J.K., TROJANOWSKA, B., WISNIEWSKA-KNYPL, J.M., &
BOLANOWSKI, W. (1974) Mercury binding in the kidney and liver of
rats repeatedly exposed to mercuric chloride: induction of
metallothionein by mercury and cadmium. Toxicol. appl. Pharmacol.,
27: 11-19.
PISCATOR, M. (1962) Proteinuria in chronic cadmium poisoning. II.
The applicability of quantitative and qualitative methods of protein
determination for the demonstration of cadmium proteinuria. Arch.
environ. Health, 5: 325-332.
PISCATOR, M. (1964) [On cadmium in normal kidneys together with a
report on the isolation of metallothionein from livers of
cadmium-exposed rabbits.] Nord. Hyg. Tidskr., 45: 76-82 (in
Swedish).
PISCATOR, M. (1966a) Proteinuria in chronic cadmium poisoning. III.
Electrophoretic and immunoelectrophoretic studies on urinary
proteins from cadmium workers, with special reference to the
excretion of low molecular weight proteins. Arch. environ. Health,
12: 335-344.
PISCATOR, M. (1966b) Proteinuria in chronic cadmium poisoning. IV.
Gel filtration and ion exchange chromatography of urinary proteins
from cadmium workers. Arch. environ. Health, 12: 345-359.
PISCATOR, M. (1971) Data on interferences in cadmium analysis. In:
Friberg, L., Piscator, M., & Nordberg, G., ed. Cadmium in the
environment, Cleveland, Ohio, CRC Press, p. 17.
PISCATOR, M. & TSUCHIYA, K. (1971) In: Friberg, L., Piscator, M., &
Nordberg, G., ed. Cadmium in the environment, Cleveland, Ohio,
Chemical Rubber Company Press, pp. 112-114.
PISCATOR, M. & VOUK, V.B. (1979) Sampling and analytical methods.
In: Friberg, L., Nordberg, G.F., & Vouk, V.B., ed. Handbook on the
toxicology of metals, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 33-46.
PISCATOR, M., KJELLSTRÖM, T., & LIND, B. (1976) Contamination of
cigarettes and pipe tobacco by cadmium-oxide dust. Lancet, 2: 587.
PISCATOR, M., BJORK, L., & NORDBERG, M. (1981) Beta-2-microglobulin
levels in serum and urine of cadmium exposed rabbits. Acta.
pharmacol. toxicol., 49: 1-7.
PLEBAN, P.A., KERKAY, I., & PEARSON, K.H. (1981) Polarized
Zeeman-effect flameless atomic absorption spectrometry of cadmium,
copper, lead, and manganese in human kidney cortex. Clin. Chem.,
27: 68-72.
POTTS, C.L. (1965) Cadmium proteinuria - the health of battery
workers exposed to cadmium oxide dust. Ann. occup. Hyg., 8: 55-61.
PRIGGE, E. (1978) Early signs of oral and inhalative cadmium uptake
in rats. Arch. Toxicol., 40: 231-247.
PRIGGE, E., BAUMERT, H.P., & MUHLE, H. (1977) Effects of dietary and
inhalative cadmium on haemoglobin and haematocrit in rats. Bull.
environ. Contam. Toxicol., 17: 585-590.
PRINCI, F. (1947) A study of industrial exposure to cadmium. J. ind.
Hyg. Toxicol., 29: 315-320.
PRINCI, F. & GEEVER, E.F. (1950) Prolonged inhalation of cadmium.
Arch. ind. Hyg. occup. Med., 1: 651-661.
PROCKOP, D.J. & DAVIDSON, W.D. (1964) A study of urinary and serum
lysozyme in patients with renal disease. New Engl. J. Med.,
270: 269-274.
PRODAN, L. (1932) Cadmium poisoning. II. Experimental cadmium
poisoning. J. ind. Hyg. Toxicol., 14: 174-196.
PULIDO, P., KAGI, J.H., & VALLEE, B.L. (1966) Isolation and some
properties of human metallothionein. Biochemistry, 5: 1768-1777.
RAHOLA, T., AARAN, R.-K., & MIETTINEN, J.K. (1972) Half-time studies
of mercury and cadmium by whole-body counting. In: Assessment of
radioactive contamination in man, New York, International Atomic
Energy Agency Unipublishers, pp. 553-562 (IAEA Proceedings Series
No. Sm-150/13).
RAMAYA, L.K. & POMERANTZEVA, M.O. (1977) [Investigation of cadmium
chloride mutagenic effect on germ cells of male mice.] Genetika,
13: 59-63 (in Russian).
RAMEL, C. & FRIBERG, L. (1971) Carcinogenic and mutagenic effects.
In: Friberg, L., Piscator, M., & Nordberg, G.F., ed. Cadmium in the
environment, Cleveland, Ohio, CRC Press, pp. 109-110.
RAUHUT, A. (1980) Survey of industrial emission of cadmium in the
European Economic Community: Final Report, Brussels, Commission of
the European Communities, Vol. 2, pp. 1-44.
REDDY, C.S., SVOBODA, D., AZARNOFF, D., & DEWAR, R. (1973)
Cadmium-induced Leydig cell tumors of rat testis: morphologic and
histochemical study. J. Natl Cancer Inst., 51: 891-903.
RICHARDSON, M.E. & FOX, M.R.S. (1974) Dietary cadmium and
enteropathy in the Japanese quail. Lab. Invest., 31: 722-731.
RIVM (1988) In: Ros, J.P.M. & Sloof, W., ed. Integrated criteria
document cadmium, Bilthoven, The Netherlands, National Institute of
Public Health and Environmental Protection (RIVM-Report No.
758476004).
ROBBINS, S.L., COTRAN, R.S., & KUMAR, U. (1984) Pathological basis
of disease, 3rd ed., Philadelphia, Pennsylvania, W.B. Saunders &
Co., pp. 1327-1328.
ROELS, H., HUBERMONT, G., BUCHET, J.P., & LAUWERYS, R. (1978)
Placental transfer of lead, mercury, cadmium and carbon monoxide in
women. III. Factors influencing the accumulation of heavy metals in
the aplacenta and the relationship between metal concentration in
the placenta and in maternal and cord blood. Environ. Res.,
16: 236-245.
ROELS, H., LAUWERYS, R.R., BUCHET, J.P., & BERNARD, A. (1981a)
Environmental exposure to cadmium and renal function of aged women
in three areas of Belgium. Environ. Res., 24: 117-130.
ROELS, H., LAUWERYS, R.R., BUCHET, J.P., BERNARD, A., CHETTLE, D.R.,
HARVEY, T.C., & AL-HADDAD, J.K. (1981b) In vivo measurement of
liver and kidney cadmium in workers exposed to this metal: its
significance with respect to cadmium in blood and urine. Environ.
Res., 26: 217-240.
ROELS, H., DJUBGANG, J., BUCHET, J.P., BERNARD, A., & LAUWERYS, R.R.
(1982) Evolution of cadmium-induced renal dysfunction in workers
removed from exposure. Scand. J. Work Environ. Health, 8: 191-200.
ROELS, H., LAUWERYS, R.R., & DARDENNE, A.N. (1983a) The critical
level of cadmium in human renal cortex: a reevaluation. Toxicol.
Lett., 15: 357-360.
ROELS, H., LAUWERYS, R.R., BUCHET, J.P., BERNARD, A., GARVEY, J.S.,
& LINTON, H.J. (1983b) Significance of urinary metallothionein in
workers exposed to cadmium. Int. Arch. occup. environ. Health,
52: 159-166.
ROELS, H., LAUWERYS, R.R., BUCHET, J.P., BERNARD, A.M., VOS, A., &
OVERSTEYNS, M. (1989) Health significance of cadmium induced renal
dysfunction: a five year follow up. Br. J. ind. Med., 46: 755-764.
ROHR, G. & BAUCHINGER, M. (1976) Chromosome analyses in cell
cultures of the Chinese hamster after application of cadmium
sulfate. Mutat. Res., 40: 125-130.
SAITO, H. (1987) [Follow-up study on health status on residents in a
cadmium polluted area (Sasu, Izahara town of Nagasaki Prefecture)
after improvement of cadmium-polluted lands], Tokyo, Japan Public
Health Association, pp. 215-216 (Kankyo Hoken Report No. 53) (in
Japanese).
SAITO, H., SHIOJI, R., FURUKAWA, Y., NAGAI, K., ARIKAWA, T., SAITO,
T., SASAKI, Y., FURUYAMA, T., & YOSHINAGA, K. (1977) Cadmium-induced
proximal tubular dysfunction in a cadmium-polluted area. Nephrology,
6: 1-12.
SAITO, H., NAKANO, A., TOHYAMA, C., MITANE, Y., SUGIHIRA, N., &
WAKISAKA, I. (1983) [Report on a health examination of inhabitants
of areas with cadmium pollution of soil], Tokyo, Japan Public Health
Association, pp. 91-95 (Kankyo Hoken Report No. 40) (in Japanese).
SALMELA, S. & VUORI, E. (1979) Contamination with cadmium from
micropipette tips. Talanta, 26: 175-176.
SALTZMAN, B.E. (1953) Colorimetric microdetermination of cadmium
with dithizone. Anal. Chem., 25: 493-496.
SAMARAWICKRAMA, G.P. & WEBB, M. (1979) Acute effects of cadmium on
the pregnant rat and embryo-fetal development. Environ. Health
Perspect., 28: 245-249.
SANDERS, C.L. & MAHAFFEY, J.A. (1984) Carcinogenicity of single and
multiple intratracheal instillations of cadmium oxide in the rat.
Environ. Res., 33: 227-233.
SANGSTER, B., DE GROOT, G., LOEBER, J.G., DERKS, H.J.G.M., KRAJNC,
E.I., & SAVELKOUL, T.J.F. (1984) Urinary excretion of cadmium,
protein, beta-2-microglobulin and glucose in individuals living in a
cadmium-polluted area. Hum. Toxicol., 3: 7-21.
SCHARPF, L.G., Jr, HILL, I.D., WRIGHT, P.L., PLANK, J.B., KEPLINGER,
M.L., & CALANDRA, J.C. (1972) Effect of sodium nitrilotriacetate on
toxicity, teratogenicity, and tissue distribution of cadmium. Nature
(Lond.), 239: 231-234.
SCHERER, G. & BARKEMEYER, H. (1983) Cadmium concentrations in
tobacco and tobacco smoke. Ecotoxicol. environ. Saf., 7: 71-78.
SCHMID, B.P., KAO, J., & GOULDING, E. (1985) Evidence for reopening
of the cranial tube in mouse embryo treated with cadmium chloride.
Experientia (Basel), 41: 271-272.
SCHROEDER, H.A. (1964) Cadmium hypertension in rats. Am. J.
Physiol., 207: 62-66.
SCHROEDER, H.A. (1965) Cadmium as a factor in hypertension. J.
chron. Dis., 18: 647-656.
SCHROEDER, H.A. (1967) Cadmium, chromium, and cardiovascular
disease. Circulation, 35: 570.
SCHROEDER, H.A. & BALASSA, J.J. (1961) Abnormal trace metals in man:
cadmium. J. chron. Dis., 14: 236-258.
SCHROEDER, H.A. & MITCHENER, M. (1971) Toxic effects of trace
elements on the reproduction of mice and rats. Arch. environ.
Health, 23: 102-106.
SCHROEDER, H.A. & VINTON, W.H., Jr (1962) Hypertension induced in
rats by small doses of cadmium. Am. J. Physiol., 202: 515-518.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H., Jr (1964) Chromium,
lead, cadmium, nickel, and titanium in mice: effect on mortality,
tumours, and tissue levels. J. Nutr., 83: 239-250.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H., Jr (1965) Chromium,
cadmium, and lead in rats: effects on life span, tumours, and tissue
levels. J. Nutr., 86: 51-66.
SCOTT, R., MILLS, E.A., FELL, G.S., HUSAIN, F.E.R., YATES, A.J.,
PATERSON, P.J., MCKIRDY, A., OTTOWAY, J.M., FITZGERALD-FINCH, O.P.,
& LAMONT, A. (1976) Clinical and biochemical abnormalities in
coppersmiths exposed to cadmium. Lancet, 2: 396-398.
SCOTT, R., AUGHEY, E., & SINCLAIR, J. (1977) Histological and
ultrastructural changes in rat kidney following cadmium injection.
Urol. Res., 5: 15-20.
SCOTT, R., PATERSON, P.J., BURNS, R., OTTOWAY, J.M., HUSAIN, F.E.R.,
FELL, G.S., DUMBUYA, S., & IGBAL, M. (1978) Hypercalciuria related
to cadmium exposure. Urology, 11: 462-465.
SCOTT, R., PATERSON, P.J., BURNS, R., FELL, G.S., & OTTOWAY, J.M.
(1979) The effects of treatment on the hypercalciuria of chronic
cadmium poisoning. Urol. Res., 7: 285-289.
SCOTT, R., HAYWOOD, J.K., BODDY, K., WILLIAMS, E.D., HARVEY, M., &
PATERSON, P.J. (1980) Whole body calcium deficit in cadmium-exposed
workers with hypercalciuria. Urology, 15: 356-359.
SHAIKH, Z.A. & HIRAYAMA, K. (1979) Metallothionein in the
extracellular fluids as an index of cadmium toxicity. Environ.
Health Perspect., 28: 267-272.
SHAIKH, Z.A. & SMITH, J.C. (1980) Metabolism of orally ingested
cadmium in humans. In: Holmstedt, B., Lauwerys, R., Mercier, M., &
Roberfroid, M., ed. Mechanisms of toxicity and hazard evaluation,
Amsterdam, Elsevier/North Holland Biomedical Press, pp. 569-574.
SHARMA, R.P., KJELLSTRÖM, T., & MCKENZIE, J.M. (1983) Cadmium in
blood and urine among smokers and non-smokers with high cadmium
intake via food. Toxicology, 29: 163-171.
SHARRETT, A.R., CARTER, A.P., ORHEIM, R.M., & FEINLEIB, M. (1982)
Daily intake of lead, cadmium, copper, and zinc from drinking-water:
the Seattle study of trace metal exposure. Environ. Res.,
28: 456-475.
SHERLOCK, J. & WALTERS, B. (1983) Dietary intake of heavy metals and
its estimation. Chem. Ind., 13: 505-508.
SHERLOCK, J., SMART, G.A., & WALTERS, B. (1983) Dietary surveys on a
population at Shipham, Somerset, United Kingdom. Sci. total
Environ., 29: 121-142.
SHIGEMATSU, I. & KAWAGUCHI, T. (1978) Environmental pollution and
health effects, Yamagata Prefecture. In: Tsuchiya, K., ed. Cadmium
studies in Japan: a review, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 199-211.
SHIGEMATSU, I., KAWAGUCHI, T., & YANAGAWA, H. (1978) Reanalysis of
the results of health examinations conducted on the general
population in the cadmium polluted areas of Japan. In: Tsuchiya, K.
ed. Cadmium studies in Japan: a review, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 253-264.
SHIGEMATSU, I., YANAGAWA, H., & KAWAGUCHI, T. (1975) [Record filling
of health examinations for inhabitants in the Cd-polluted areas (2nd
and 3rd reports)], Tokyo, Japan Public Health Association,
pp. 72-101 (Kankyo Hoken Report No. 36) (in Japanese).
SHIGEMATSU, I., MINOWA, M., YOSHIDA, T., & MIYAMOTO, K. (1979)
Recent results of health examinations on the general population in
cadmium-polluted and control areas in Japan. Environ. Health
Perspect., 28: 205-210.
SHIGEMATSU, I., MINOWA, M., YOSHIDA, T., & MIYAMOTO, K. (1980)
Epidemiological aspects of environmental cadmium and its effects in
Japan. In: Proceedings of the 2nd International Cadmium Conference,
Cannes, 6-8 February, 1979, London, Cadmium Association,
pp. 111-117.
SHIGEMATSU, I., KITAMURA, S., TAKEUCHI, J., MINOWA, M., NAGAI, M.,
USUI, T., & FUKUSHIMA, M. (1981) A retrospective mortality study on
cadmium-exposed populations in Japan. In: Recent studies on health
effects of cadmium in Japan, Tokyo, The Japan Cadmium Research
Committee, Japan Environment Agency, pp. 303-317.
SHIGEMATSU, I., MITAMURA, S., TAKEUCHI, J., MINOWA, M., NAGAI, M., &
FUKUSHIMA, M. (1982) A retrospective mortality study on
cadmium-exposed populations in Japan. In: Proceedings of the 3rd
International Cadmium Conference, Miami, Florida, 3-5 February,
1981, London, Cadmium Association, pp. 115-118.
SHIGEMATSU, I., MINOWA, M., NAGAI, M., OHMURA, T., & TAKEUCHI, K.
(1983) A retrospective mortality study on cadmium-exposed
populations in Japan (supplement): mortalities by level of pollution
in Toyama Prefecture. In: The influence of nutritional factors on
cadmium administered to monkeys, Tokyo, Japan Environment Agency,
pp. 158-174.
SHILLER, A.M. & BOYLE, E.A. (1987) Variability of dissolved trace
metals in the Mississippi River. Geochim. Cosmochim. Acta.,
51: 3273-3277.
SHIRAISHI, Y. (1975) Cytogenetic studies in 12 patients with
Itai-itai disease. Humangenetik, 27: 31-44.
SHIRAISHI, Y., KURAHASHI, H., & YOSHIDA, T.H. (1972) Chromosomal
aberrations in cultured human leukocytes induced cadmium sulfide.
Proc. Jpn. Acad., 48(21): 133-137.
SHIROISHI, K., ANAYAMA, M., TANII, M., FUKUYAMA, Y., & KUBOTA, K.
(1972) [Urinary findings of different age groups in the inhabitants
of Itai-itai disease districts.] Med. Biol., 85: 263-267 (in
Japanese).
SHIROISHI, K., KJELLSTROM, T., ANAYAMA, M., SHIMADA, T., IWATA, T.,
NISHINO, H., MATSUNAGA, A., WATANABE, M., & KUBOTA, K. (1975)
[Studies on the urinary ß-2-microglobulin level of the inhabitants
in Cd-polluted districts.] Jpn J. Hyg., 30: 71 (in Japanese).
SHIROISHI, K., KJELLSTRÖM, T., KUBOTA, K., EVRIN, P.E., ANAYAMA, M.,
VESTERBERG, O., SHIMADA, T., PISCATOR, M., IWATA, T., & NISHINO, H.
(1977) Urine analysis for detection of cadmium-induced renal
changes, with special reference to ß-2-microglobulin. Environ. Res.,
13: 407-424.
SIMPSON, W.R. (1981) A critical review of cadmium in the marine
environment. Prog. Oceanogr., 10: 1-70.
SKERFVING, S., CHRISTOFFERSON, J.O., SHUTZ, A., WELINDER, H., SPANG,
G., AHLGREN, L., & MATTSON, S. (1987) Biological monitoring, by in
vivo XRF measurements, of occupational exposure to lead, cadmium
and mercury. Biol. Trace Elem. Res., 13: 241-251.
SKOG, E. & WAHLBERG, J.E. (1964) A comparative investigation of
percutaneous absorption of metal compounds in guinea-pig by means of
the radioactive isotopes, 51Cr, 58Co, 65Zn, 110Ag, 115Cd,
203Hg. J. invest. Dermatol., 43: 187-192.
SMITH, J.P., SMITH, J.C., & MCCALL, A.J. (1960) Chronic poisoning
from cadmium fume. J. Pathol. Bacteriol., 80: 287-296.
SMITH, T. (1982) Emission inventory case studies - policy aspects.
J. Air Pollut. Control Assoc., 32(10): 1017-1020.
SMITH, T., PETTY, T.L., READING, J.C., & LAKSHMINARAYAN, S. (1976)
Pulmonary effects of chronic exposure to airborne cadmium. Am. Rev.
respir. Dis., 114: 161-169.
SMITH, T., ANDERSON, R.J., & READING, J.C. (1980) Chronic cadmium
exposures associated with kidney function effects. Am. J. ind. Med.,
1: 319-337. SNIDER, G.L., HAYES, J.A., KORTHY, A.L., & LEWIS, G.P.
(1973) Centrilobular emphysema experimentally induced by cadmium
chloride aerosol. Am. Rev. respir. Dis., 108: 40-48.
SONAWANE, B.R., NORDBERG, M., NORDBERG, G.F., & LUCIER, G.W. (1975)
Placental transfer of cadmium in rats: influence of dose and
gestational age. Environ. Health Perspect., 12: 97-102.
SORAHAN, T. (1987) Mortality from lung cancer among a cohort of
nickel cadmium battery workers: 1946-84. Br. J. ind. Med.,
44: 803-809.
SORAHAN, T. & WATERHOUSE, J.A.H. (1983) Mortality study of
nickel-cadmium battery workers by the method of regression models in
life tables. Br. J. ind. Med., 40: 293-300.
SPENCER, H., ASMUSSEN, C.R., HOLTZMAN, R.B., & KRAMER, L. (1979)
Metabolic balances of cadmium, copper, manganese, and zinc in man.
Am. J. clin. Nutr., 32: 1867-1875.
SPORN, A., DINU, I., & STOENESCU, L. (1970) Influence of cadmium
administration on carbohydrate and cellular energetic metabolism in
the rat liver. Rev. roum. Biochim., 7: 299-305.
SQUIBB, K.S., PRITCHARD, J.B., & FOWLER, B.A. (1982) Renal
metabolism and toxicity of metallothionein. In: Foulkes, E.C., ed.
Biological roles of metallothionein, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 181-190.
SQUIBB, K.S., PRITCHARD, J.B., & FOWLER, B.A. (1984)
Cadmium-metallothionein nephropathy: relationships between
ultrastructural/biochemical alterations and intra-cellular cadmium
binding. J. Pharmacol. exp. Ther., 229(1): 311-321.
SQUIRE, J.R., HARDWICKE, J., & SOOTHILL, J.F. (1962) Proteinuria.
In: Black, D.A.K., ed. Renal disease, Oxford, Blackwell Scientific
Publishers, pp. 218-231.
STACEY, N.H., CANTILENA, L.R., & KLAASSEN, C.D. (1980) Cadmium
toxicity and lipid peroxidation in isolated rat hepatocytes.
Toxicol. appl. Pharmacol., 53: 470-480.
STAESSEN, J., BULPITT, C.J., ROELS, H., BERNARD, A., FAGARD, R.,
JOOSSENS, J.V., LAUWERYS, R.R., LIJNEN, P., & AMERY, A. (1984)
Urinary cadmium and lead and their relationship to blood pressure in
a population with low average exposure. Br. J. ind. Med.,
41: 241-248.
STANESCU, D., VEITNER, C., FRANS, A., CONCETTE, L., ROELS, H.,
LAUWERYS, R.R., & BRASSEUR, L. (1977) Effects on lung of chronic
occupational exposure to cadmium. Scand. J. respir. Dis.,
58: 289-303.
STEWART, M. & HUGHES, E.G. (1981) Urinary beta-2-microglobin in the
biological monitoring of cadmium workers. Br. J. Ind. Med.,
38: 170-174.
STOEPPLER, M. & BRANDT, K. (1978) Determination of lead and cadmium
in whole blood by electrothermal atomic absorption spectroscopy. In:
Doss, M., ed. Proceedings of an International Symposium on Clinical
Biochemistry on Diagnosis and Therapy of Porphyrias and Lead
Intoxication, New York, Springer-Verlag, pp. 185-187.
STOEPPLER, M. & BRANDT, K. (1980) Contributions to automated trace
analysis. V. Determination of cadmium in whole blood and urine by
electrothermal atomic absorption spectrophotometry. Fresenius Z.
anal. Chem., 300: 372-380.
STOEPPLER, M., VALENTA, P., & NURNBERG, H.W. (1979) Applications of
independent methods and standard materials: an effective approach to
reliable trace and ultra trace analysis of metals and metalloids in
environmental and biological materials. Fresenius Z. anal. Chem.,
297: 22-34.
STONEBURNER, D.L. (1978) Heavy metals in tissues of stranded
short-finned pilot whales. Sci. total Environ., 9: 293-297.
STOWE, H.D., WILSON, M., & GOYER, R.A. (1972) Clinical and
morphological effects of oral cadmium toxicity in rabbits. Arch.
Pathol., 94: 389-405.
STRAUSS, R.H., PALMER, K.C., & HAYES, J.A. (1976) Acute lung injury
induced by cadmium aerosol. I. Evolution of alveolar cell damage.
Am. J. Pathol., 84: 561-568.
SUBRAMANIAN, K.S. & MERANGER, J.C. (1981) A rapid electrothermal
atomic absorption spectrophotometric method for cadmium and lead in
human whole blood. Clin. Chem., 27: 1866-1871.
SUGAWARA, C. & SUGAWARA, N. (1974) Cadmium toxicity for rat
intestine, especially on the absorption of calcium and phosphorus.
Jpn. J. Hyg., 28: 511-516.
SULLIVAN, M.F., HARDY, J.T., MILLER, B.M., BUSCHBOM, R.L., &
SIEWICKI, T.C. (1984) Absorption and distribution of cadmium in
mice fed diets containing either inorganic or oyster-incorporated
cadmium. Toxicol. appl. Pharmacol., 72: 210-217.
SUMINO, K., HAYAKAWA, K., SHIBATA, T., & KITAMURA, S. (1975) Heavy
metals in normal Japanese tissues. Arch. environ. Health,
30: 487-494.
SUZUKI, S. & LU, C.C. (1976) A balance study of cadmium: an
estimation of daily input, output, and retained amount in two
subjects. Ind. Health, 14: 53-65.
SUZUKI, S., SUZUKI, T., & ASHIZAWA, M. (1965) Proteinuria due to
inhalation of cadmium stearate dust. Ind. Health, 3: 73-85.
SUZUKI, S., TAGUCHI, T., & YOKOHASHI, G. (1969) Dietary factors
influencing upon the retention rate of orally-administered
115CdCl2 in mice, with special reference to calcium and protein
concentration in diet. Ind. Health, 7: 155-162.
SUZUKI, Y. (1980) Cadmium metabolism and toxicity in rats after
long-term subcutaneous administration. J. Toxicol. environ. Health,
6: 469-482.
SUZUKI, Y. (1982) Metal-binding properties of metallothionein in
extracellular fluids and its role in cadmium-exposed rats. In:
Foulkes, E.C., ed. Biological roles of metallothionein, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 25-67.
SVARTENGREN, M., ELINDER, C.G., FRIBERG, L., & LIND, B. (1986)
Distribution and concentration of cadmium in human kidney. Environ.
Res., 39: 1-7.
SVENSSON, B.-G., BJORNHAM, A., SCHUTZ, A., LETTEVALL, U., NILSSON,
A., & SKERFVING, S. (1987) Acidic deposition and human exposure to
toxic metals. Sci. total Environ., 67: 101-115.
TAKABATAKE, E. (1978a) Environmental pollution and health effects:
Oita Prefecture. In: Tsuchiya, K., ed. Cadmium studies in Japan: a
review, Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp. 215-218.
TAKABATAKE, E. (1978b) Nagasaki Prefecture. In: Tsuchiya, K., ed.
Cadmium studies in Japan: a review, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 181-192.
TAKASHIMA, M., MORIWAKI, S., & ITOKAWA, Y. (1980) Osteomalacic
change induced by long-term administration of cadmium to rats.
Toxicol. appl. Pharmacol., 54: 223-228.
TAKEBAYASHI, S. (1980a) First autopsy case, suspicious of cadmium
intoxication, from the cadmium-polluted area in Tsushima, Nagasaki
Prefecture. In: Shigematsu, I. & Nomiyama, K., ed. Cadmium-induced
osteopathy, Tokyo, Japan Public Health Association, pp. 124-138.
TAKEBAYASHI, S. (1980b) [Clinical and pathological findings of a
case with osteomalacia in a cadmium-polluted area (Tsushima Island)
(1st autopsied case)], Tokyo, Japan Public Health Association,
pp. 259-262 (Kankyo Hoken Report No. 46) (in Japanese).
TAKEBAYASHI, S. (1983a) [Clinical and pathological findings of a
case with osteomalacia in a cadmium-polluted area (Tsushima Island)
(2nd autopsied case)], Tokyo, Japan Public Health Association,
pp. 207-211 (Kankyo Hoken Report No. 49) (in Japanese).
TAKEBAYASHI, S. (1983b) [Clinical and pathological findings of a
case with diabetic nephropathy in a cadmium-polluted area (Tsushima
Island) (3rd autopsied case)], Tokyo, Japan Public Health
Association, pp. 212-217 (Kankyo Hoken Report No. 49) (in Japanese).
TAKEBAYASHI, S. (1984) [Clinical and pathological findings of a case
with osteomalacia in a cadmium-polluted area (Tsushima Island) (4th
autopsied case)], Tokyo, Japan Public Health Association,
pp. 238-249 (Kankyo Hoken Report No. 50) (in Japanese).
TAKEBAYASHI, S., SATO, T., HARADA, K., & YOSHIMURA, S. (1985)
[Clinical and pathological findings of a case with osteomalacia in a
cadmium-polluted area (Tsushima Island) (5th autopsied case)],
Tokyo, Japan Public Health Association, pp. 245-251 (Kankyo Hoken
Report No. 51) (in Japanese).
TAKEBAYASHI, S., SATO, T., HARADA, K., HIRAI, Y., & YOSHIMURA, S.
(1987a) [Clinical and pathological findings of a case with
osteomalacia in a cadmium-polluted area (Tsushima Island) (6th
autopsied case)], Tokyo, Japan Public Health Association,
pp. 294-305 (Kankyo Hoken Report No. 53) (in Japanese).
TAKEBAYASHI, S., SATO, T., HARADA, K., HIRAI, Y., & YOSHIMURA, S.
(1987b) [Clinical and pathological findings of a case with
osteomalacia in a cadmium-polluted area (Tsushima Island) (7th
autopsied case)], Tokyo, Japan Public Health Association,
pp. 306-319 (Kankyo Hoken Report No. 53) (in Japanese).
TAKEBAYASHI, S., HIRAI, Y., & YOSHIMURA, S. (1988a) [Clinical and
pathological findings of a case with osteomalacia in a
cadmium-polluted area (Tsushima Island) (8th autopsied case)],
Tokyo, Japan Public Health Association, pp. 187-192 (Kankyo Hoken
Report No. 54) (in Japanese).
TAKEBAYASHI, S., HIRAI, Y., & YOSHIMURA, S. (1988b) [Clinical and
pathological findings of a case with osteomalacia in a
cadmium-polluted area (Tsushima Island) (9th autopsied case)],
Tokyo, Japan Public Health Association, pp. 193-199 (Kankyo Hoken
Report No. 54) (in Japanese).
TAKEBAYASHI, S., HIRAI, Y., & YOSHIMURA, S. (1988c) [Clinical and
pathological findings of a case with osteomalacia in a
cadmium-polluted area (Tsushima Island) (10th autopsied case)],
Tokyo, Japan Public Health Association, pp. 200-206 (Kankyo Hoken
Report No. 54) (in Japanese).
TAKEBAYASHI, S., HARADA, K., & YOSHIMURA, S. (1988d) [Clinical and
pathological findings of a case with osteomalacia in a
cadmium-polluted area (Tsushima Island) (11th autopsied case)],
Tokyo, Japan Public Health Association, pp. 207-213 (Kankyo Hoken
Report No. 54) (in Japanese).
TAKENAKA, S., OLDIGES, H., KOENIG, H., HOCHRAINER, D., &
OBERDOERSTER, G. (1983) Carcinogenicity of cadmium aerosols in
Wistar rats. J. Natl Cancer Inst., 70: 367-371.
TANAKA, K. (1982) Effects of hepatic disorders on the fate of
cadmium in rats. In: Foulkes, E.C., ed. Biological roles of
metallothionein, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 237-249.
TANAKA, K., MIN, K.-S., ONOSAKA, S., FUKUHARA, C., & UEDA, M. (1985)
The origin of metallothionein in red blood cells. Toxicol. appl.
Pharmacol., 78: 63-66.
TARASENKO, N.YU. & VOROBJEVA, R.S. (1973) [Hygienic problems
connected with the use of cadmium.] Vestn. Akad. Med. Nauk. SSR,
28: 37-43 (in Russian with English abstract).
TARASENKO, N.YU., VOROBJEVA, R.S., SPIRIDINOVA, V.S., & SABALINA,
L.P. (1974) Experimental investigation of toxicity of cadmium and
zinc caprylates. J. Hyg. Epidemiol. Microbiol. Immunol.,
18: 144-153.
TARASENKO, N.YU., VOROBJEVA, R.S., SABALINA, L.P., & CVETKOVA, R.P.
(1975) [Cadmium in the environment and its effect on calcium
metabolism.] Gig. i Sanit., 9: 22-25 (in Russian).
TASK GROUP ON LUNG DYNAMICS (1966) Deposition and retention models
for internal dosimetry of the human respiratory tract. Health Phys.,
12: 173-208.
TASK GROUP ON METAL INTERACTIONS (1978) Factors influencing
metabolism and toxicity of metals: a consensus report. Environ.
Health Perspect., 25: 3-42.
TASK GROUP ON METAL TOXICITY (1976) Conceptual considerations:
critical organ, critical concentration in cells and organs, critical
effect, subcritical effect, dose-effect and dose-response
relationships. In: Nordberg, G.F., ed. Effects and dose-response
relationships of toxic metals, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 10-13.
TATI, M., KATAGIRI, Y., & KAWAI, M. (1976) Urinary and faecal
excretion of cadmium in normal Japanese: an approach to non-toxic
levels of cadmium. In: Nordberg, G.F., ed. Effects and dose-response
relationships of toxic metals, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 331-341.
TAYLOR, B.A., HEINIGER, H.J., & MEIER, H. (1973) Genetic analysis of
resistance to cadmium-induced testicular damage in mice (37380).
Proc. Soc. Exp. Biol. Med., 143: 629-633.
TECULESCU, D.B. & STANESCU, D.C. (1970) Pulmonary function in
workers with chronic exposure to cadmium oxide fumes. Int. Arch.
Arbeitsmed., 26: 335-345.
TEMPLETON, D.M., BANERJEE, D., & CHERIAN, M.G. (1985)
Metallothionein synthesis and localization in relation to metal
storage in rat liver during gestation. Can. J. Biochem. cell Biol.,
63: 16-22.
TENNANT, C.J. (1984) Cadmium in the environment and high risk
population groups, Birmingham, University of Aston (Ph.D. Thesis).
TERHAAR, C.J., VIS, E., ROUDABUSH, R.L., & FASSETT, D.W. (1965)
Protective effects of low doses of cadmium chloride against
subsequent high oral doses in the rat. Toxicol. appl. Pharmacol.,
7: 500-505.
THIEME, R., SCHRAMEL, P., & KURZ, E. (1977) [Trace element
concentration in the human placenta in a heavily-contaminated
environment.] Geburtshilfe Frauenheilkd., 37: 756-761 (in German).
THOMAS, B.J., HARVEY, T.C., CHETTLE, D.R., MCLELLAN, J.S., &
FREMLIN, J.H. (1979) A transportable system for the measurement of
liver cadmium in vivo. Phys. Med. Biol., 24: 432-437.
THORNTON, I. (1988) The Shipham report. An investigation into
cadmium contamination and its implications for human health. Metal
content of soils and dusts. Sci. total Environ., 75: 21-39.
THUN, M.T., SCHNORR, T.M., SMITH, A.B., HALPERIN, W.E., & LEMEN,
R.A. (1985) Mortality among a cohort of U.S. cadmium production
workers: An update. J. Natl Cancer Inst., 74: 325-333.
THUN, M.J., OSORIO, A.M., SCHOBER, S., HANNON, W.H., & HALPERIN, W.
(1989) Nephropathy in cadmium workers - assessment of risk from
airborne occupational cadmium exposure. Br. J. ind. Med.,
46: 689-697.
TIPTON, I.H., SCHROEDER, H.A., PERRY, H.M., & COOK, M.J. (1960)
Trace elements in human tissues. Part III. Subjects from Africa, the
Near and Far East, and Europe. Health Phys., 11: 403-451.
TJELL, J.C., HANSEN, J.A., CHRISTENSEN T.H., & HOVMAND, M.F. (1981)
Prediction of cadmium concentrations in Danish soils. In: L'Hermite,
P. & Ott, H., ed. Characterization, treatment and use of sewage
sludge, Dordrecht, Reidel Publishing Co., pp. 652-664.
TJELL, J.C., CHRISTENSEN, T.H., & BRO-RASMUSSEN, F. (1983) Cadmium
in soil and terrestrial biota, with emphasis on the Danish
situation. Ecotoxicol. environ. Saf., 7: 122-140.
TOHYAMA, C., SHAIKH, Z.A., ELLIS, K.J., & COHN, S.H. (1981a)
Metallothionein excretion in urine upon cadmium exposure: its
relationship with liver and kidney cadmium. Toxicology, 20: 181-191.
TOHYAMA, C., SHAIKH, Z.A., NOGAWA, K., KOBAYASHI, E., & HONDA, R.
(1981b) Elevated urinary excretion of metallothionein due to
environmental cadmium exposure. Toxicology, 22: 289-297.
TOPPING, M.D., FORSTER, H.W., DOLMAN, C., LUCZYNSKA, C.M., &
BERNARD, A.M. (1986) Measurement of urinary retinol-binding protein
by enzyme-linked immunosorbent assay and its application to
detection of tubular proteinuria. Clin. Chem., 32: 1863-1866.
TSUCHIYA, K. (1967) Proteinuria of workers exposed to cadmium fume.
The relationship to concentration in the working environment. Arch.
environ. Health, 14: 875-880.
TSUCHIYA, K. (1969) Causation of Ouch-ouch disease (Itai-itai byo):
an introduction review. Part 1. Nature of the disease. Keio J. Med.,
18: 181-194.
TSUCHIYA, K. (1976) Proteinuria of cadmium workers. J. occup. Med.,
18: 463-466.
TSUCHIYA, K., ed. (1978) Cadmium studies in Japan: a review,
Amsterdam, Oxford, New York, Elsevier Science Publishers, 376 pp.
TSUCHIYA, K. & IWAO, S. (1978) Interrelationships among zinc,
copper, lead, and cadmium in food, faeces, and organs of humans.
Environ. Health Perspect., 25: 119-124.
TSUCHIYA, K. & NAKAMURA, K.I. (1978) Environmental pollution and
health effects: Hyogo prefecture. In: Tsuchiya, K., ed. Cadmium
studies in Japan: a review, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 146-167.
TSUCHIYA, K. & SUGITA, M. (1971) A mathematical model for deriving
the biological half-life of a chemical. Nord. Hyg. Tidskr.,
53: 105-110.
TSUCHIYA, K., SEKI, Y., & SUGITA, M. (1976) Cadmium concentrations
in the organs and tissues of cadavers from accidental deaths. Keio
J. Med., 25: 83-90.
TSUCHIYA, K., IWAO, S., SUGITA, M., & SAKURAI, H. (1979) Increased
urinary ß-2-microglobulin in cadmium exposure: dose-effect
relationship and biological significance of ß-2-microglobulin.
Environ. Health Perspect., 28: 147-153.
URUNO, K., OTOYAMA, S., NAKANO, N., & ITAGAKI, Z. (1975) [Results
of health examination for inhabitants in Yoshinokawa area polluted
with cadmium], Tokyo, Japan Public Health Association, pp. 109-113
(Kankyo Hoken Report No. 36) (in Japanese).
USHIO, F. & DOGUCHI, M. (1977) Dietary intakes of some chlorinated
hydrocarbons and heavy metals estimated on the
experimentally-prepared diets. Bull. environ. Contam. Toxicol.,
17: 707-711.
US PUBLIC HEALTH SERVICE (1942) Cadmium poisoning. Public Health
Rep., 57: 602-612.
VAHTER, M., ed. (1982) Assessment of human exposure to lead and
cadmium through biological monitoring, Stockholm, National Swedish
Institute of Environmental Medicine and Department of Environmental
Hygiene, Karolinska Institute, 136 pp (Report prepared for the
United Nations Environment Programme and the World Health
Organization).
VALETAS, P. (1946) "Cadmiose" ou l'intoxication du cadmium, Paris,
School of Medicine, 40 pp (Medical Faculty Thesis Report).
VAN AALST, R.M., VAN ARDENNE, R.A.M., DE KRUK, J.F., & LEMS, T.
(1983a) Pollution of the North Sea from the atmosphere, Apeldoorn,
The Netherlands Organization for Applied Scientific Research (TNO)
(Report No. C182/152).
VAN AALST, R.M., DUYZER, J.H., & VELDT, C. (1983b) Atmospheric
deposition of lead and cadmium in the southern part of the North
Sea. I. Emission and preliminary model calculations, Apeldoorn, The
Netherlands Organization for Applied Scientific Research (TNO)
(Report No. R83/222).
VANDER, A.J. (1962) Cadmium enhancement of proximal tubular sodium
reabsorption. Am. J. Physiol., 203: 1005-1007.
VANDER MALLIE, R.J. & GARVEY, J.S. (1979) Radioimmunoassay of
metallothioneins. J. biol. Chem., 254: 8416-8421.
VENS, M.D. & LAUWERYS, R.R. (1982) Détermination simultanée du plomb
et du cadmium dans le sang et l'urine par le couplage des techniques
de chromatographie sur résine échangeuse d'ions et de
spectrophotométrie d'absorption atomique. Arch. Mal. prof. Méd.
Trav. Sécur. soc., 33: 97-105.
VESTERBERG, O. & WRANGSKOGH, K. (1978) Determination of cadmium in
urine by graphite furnace atomic absorption spectroscopy. Clin.
Chem., 24: 681-685.
VOROBJEVA, R.S. (1957) [Investigations of the nervous system
function in workers exposed to cadmium oxide.] Neuropatol.
Psikkiatr., 57: 385-388 (in Russian).
VOROBJEVA, R.S. (1958) [About occupational cadmium oxide
poisonings.] J. Hyg. Epidemiol. Microbiol. Immunol., 11: 244-249 (in
German).
VOROBJEVA, R.S. & BUBNOVA, N.I. (1981) [Effects of elemental cadmium
and telluric cadmium on organisms.] Gig. Trud. prof. Zabol.,
2: 42-43 (in Russian).
VOROBJEVA, R.S. & EREMEEVA, E.P. (1980) [Cardiovascular function in
workers exposed to cadmium.] Gig. i Sanit., 10: 22-25 (in Russian).
VOROBJEVA, R.S. & SABALINA, L.P. (1975) [Experimental investigations
of toxic properties of various cadmium compounds.] Gig. i Sanit.,
2: 102-104 (in Russian).
VOSTAL, J.J. (1976) Dose-response relationship of mercury and
cadmium effects on renal cadmium transport. In: Nordberg, G.F., ed.
Effects and dose-response relationships of toxic metals, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 299-308.
VUORI, E., HUUNAN-SEPPALA, A., KILPIO, J.O., & SALMELA, S.S. (1979)
Biologically-active metals in human tissues. II. The effect of age
on the concentration of cadmium in aorta, heart, kidney, liver,
lung, pancreas, and skeletal muscle. Scand. J. Work Environ. Health,
5: 16-22.
WAALKES, M.P., POISNER, A.M., WOOD, G.W., & KLAASSEN, C.D. (1984)
Metallothionein-like proteins in human placenta and fetal membranes.
Toxicol. appl. Pharmacol., 74: 179-184.
WADE, G.G. (1987) Marked synergism of dimethylnitrosamine
carcinogenesis in rats exposed to cadmium. Cancer Res.,
47: 6606-6613.
WALSH, J.J. & BURCH, G.E. (1959) The rate of disappearance from
plasma and subsequent distribution of radiocadmium ( 115 m Cd) in
normal dogs. J. clin. Med., 54: 59-65.
WALTERS, B. & SHERLOCK, J. (1981) Studies on the dietary intake of
heavy metals. In: Proceedings of the International Conference on
Heavy Metals in the Environment, Amsterdam, September 1981,
Edinburgh, CEP Consultants Ltd, pp. 506-512.
WATANABE, H. & MURAYAMA, H. (1975) A study on health effect indices
concerning population in a cadmium-polluted area. In: International
Symposium on Recent Advances in the Assessment of the Health Effects
of Environmental Pollution, Luxembourg, December 1975, Luxembourg,
Commission of the European Communities, pp. 2185-2195 (Report EURO
5360).
WATANABE, I., KOIZUMI, A., FUJITA, H., KUMAI, M., & IKEDA, M. (1985)
Dietary cadmium intakes of farmers in nonpolluted areas in Japan,
and the relation with blood cadmium levels. Environ. Res.,
37(1): 33-43.
WATANABE, T., SHIMADA, T., & ENDO, A. (1979) Mutagenic effects of
cadmium on mammalian oocyte chromosomes. Mutat. Res., 67: 349-356.
WEAST, R.C. (1974) Handbook of chemistry and physics, 55th ed.,
Cleveland, Ohio, CRC Press, 500 pp.
WEBB, M. (1972) Protection by zinc against cadmium toxicity.
Biochem. Pharmacol., 21: 2767-2771.
WEBB, M. & CAIN, K. (1982) Functions of metallothionein. Biochem.
Pharmacol., 31: 137-142.
WEBB, M. & ETIENNE, A.T. (1977) Studies on the toxicity and
metabolism of cadmium-thionein. Biochem. Pharmacol., 26: 25-30.
WESTER, P.O. (1974) Trace element balances in relation to variations
in calcium intake. Atherosclerosis, 20: 207-215.
WHANGER, P.D. (1979) Cadmium effects in rats on tissue iron,
selenium, and blood pressure: blood and hair cadmium in some Oregon
residents. Environ. Health Perspect., 28: 115-121.
WHO (1980) Recommended health-based limits in occupational exposure
to heavy metals. Report of a study group, Geneva, World Health
Organization (Technical Report Series No. 647).
WHO (1984) Guidelines for drinking-water quality: Health criteria
and other supporting information, Geneva, World Health Organization,
Vol. 2, pp. 84-90.
WHO (1986) Health impact of acidic deposition. Sci. total Environ.,
52: 157-187.
WHO (1987) Air quality guidelines for Europe, Copenhagen, World
Health Organization, Regional Office for Europe, pp. 200-209
(European Series, Vol. 23).
WHO (1989) Cadmium. In: Evaluation of certain food additives and
contaminants. Thirty-third Report of the Joint FAO/WHO Expert
Committee on Food Additives, Geneva, World Health Organization,
pp. 28-31 (Technical Report Series No. 776).
WILLIAMS, C.H. & DAVID, D.J. (1973) The effect of superphosphate on
the cadmium content of soils and plants. Aust. J. Soil Res.,
11: 43-56.
WILLIAMS, C.H. & DAVID, D.J. (1976) The accumulation in soil of
cadmium residues from phosphate for fertilizers and their effect on
the cadmium content of plants. Soil Sci., 121: 86-93.
WILSON, B. (1988a) Investigation of trace metals in the aqueous
environment: Final report (January 1986-December 1987), Houston,
Texas Southern University, p. 28 (Report No. DOE/CH/10255-T1,
prepared for the US Department of Energy, Washington).
WILSON, D.N. (1988b) Cadmium - market trends and influences. In:
Cadmium 87. Proceedings of the 6th International Cadmium
Conference, London, Cadmium Association, pp. 9-16.
WILSON, R.H., DE EDS, F., & COX, A.J., Jr (1941) Effects of
continued cadmium feeding. J. Pharmacol. exp. Ther., 71: 222-235.
WOLFF, E.W. & PEEL, D.A. (1985) The record of global pollution in
polar snow and ice. Nature (Lond.), 313: 535-540.
WOLNIK, K.A., FRICKE, F.L., CAPER, S.G., BRAUDE, G.L., MEYER, M.W.,
SATZGER, R.D., & BONNIN, E. (1983) Elements in major raw
agricultural crops in the United States. I. Cadmium and lead in
lettuce, peanuts, potatoes, soybeans, sweet corn, and wheat. J.
agric. food Chem., 31: 1240-1244.
WOLNIK, K.A., FRICKE, F.L., CAPAR, S.G., MEYER, M.W., & SATZGER,
R.D. (1985) Elements in major raw agricultural crops in the United
States. 3. Cadmium, lead, and eleven other elements in carrots,
field corn, onion, rice, spinach, and tomatoes. J. agric. food
Chem., 33: 807-811.
YAMAGATA, H. & IWASHIMA, K. (1975) Average cadmium intake of the
Japanese people. Bull. Inst. Public Health (Tokyo), 24: 18-24.
YAMAGATA, N. & SHIGEMATSU, I. (1970) Cadmium pollution in
perspective. Bull. Inst. Public Health (Tokyo), 19: 1-27.
YAMAGATA, N., IWASHIMA, K., & NAGAI, T. (1974) [Gastrointestinal
absorption of cadmium in normal humans], Tokyo, Japan Public Health
Association, pp. 84-85 (Kankyo Hoken Report No. 31) (in Japanese).
YAMAMOTO, Y. (1972) [Present status of cadmium environmental
pollution], Tokyo, Japan Public Health Association, pp. 7-12 (Kankyo
Hoken Report No. 11) (in Japanese).
YOSHIKAWA, H., KAWAI, K., SUZUKI, Y., NOZAKI, K., & OHSAWA, M.
(1975) [Studies on cadmium effects in living organisms: impairment
due to inhalation], Tokyo, Japanese Environment Agency, 40 pp (in
Japanese).
RESUME ET CONCLUSIONS
1. Identité, propriétés physiques et chimiques et méthodes
d'analyse
Il existe plusieurs méthodes pour le dosage du cadmium dans les
échantillons biologiques. La plus utilisée est la spectrométrie
d'absorption atomique, mais elle nécessite un traitement minutieux
de la prise d'essai et une correction pour tenir compte des
interférences lorsqu'elle est appliquée à des échantillons de faible
teneur en cadmium. Il est tout à fait souhaitable que l'analyse
s'accompagne d'un programme d'assurance de la qualité. A l'heure
actuelle, il est possible, dans des conditions optimales, de doser
environ 0,1 µg/litre dans l'urine et le sang et de 1 à 10 µg/litre
dans les aliments et les tissus.
2. Sources d'exposition humaine et environnementale
Le cadmium est un élément relativement rare et les méthodes
actuelles d'analyse indiquent, dans les divers compartiments de
l'environnement, des concentrations beaucoup plus faibles que les
mesures antérieures. Pour l'instant, il n'est pas possible de
déterminer si l'activité humaine est à l'origine d'un accroissement
de la teneur des calottes polaires en cadmium à l'échelle des temps
historiques.
La production commerciale de cadmium a commencé au tournant du
siècle. La consommation a changé de caractère ces dernières années
avec un recul sensible de la galvanoplastie et une utilisation
accrue dans la fabrication de batteries et de composants
électroniques spéciaux. Dans la plupart des cas, le cadmium est
utilisé sous la forme de dérivés peu concentrés, ce qui rend le
recyclage indispensable. Les restrictions à l'usage du cadmium
imposées par certains pays pourraient avoir des répercussions
importantes sur ces applications.
Les activités humaines entraînent la libération de cadmium dans
l'air, le sol et l'eau. D'une façon générale, les deux principales
sources de contamination sont la production et la consommation de
cadmium et d'autres métaux non ferreux ainsi que le rejet de déchets
contenant du cadmium. Dans les zones proches de mines ou de
fonderies de métaux non ferreux, la contamination par le cadmium est
souvent importante.
Plus le sol contient de cadmium, plus la quantité fixée par les
plantes est importante. L'exposition humaine par l'intermédiaire des
cultures sera donc sensible à toute augmentation de la teneur du sol
en cadmium. La fixation par les plantes est plus importante dans les
sols de faible pH. Les processus qui acidifient le sol (pluies
acides, par exemple) sont donc susceptibles de provoquer une
augmentation de la concentration moyenne du cadmium dans les denrées
alimentaires. Dans certaines régions du monde, l'utilisation
d'engrais phosphatés et les dépôts d'origine atmosphérique
constituent une source non négligeable de contamination des terres
arables; les boues d'égout peuvent aussi, localement, entraîner une
forte pollution. A l'avenir, ces sources risquent d'accroître la
contamination du sol et celle des cultures, ce qui débouchera sur
une exposition plus importante au cadmium par la voie alimentaire.
Dans certaines régions, on peut constater une augmentation de la
teneur des aliments en cadmium.
Les animaux et les plantes comestibles qui vivent à l'état
sauvage, comme les coquillages, les crustacés et les champignons
accumulent naturellement le cadmium. Comme chez l'homme, on constate
une augmentation de la concentration en cadmium dans le foie et les
reins des chevaux et de certains animaux terrestres vivant à l'état
sauvage. La consommation régulière de ces abats peut entraîner une
exposition accrue. Dans les reins de certains vertébrés marins on
trouve des concentrations assez fortes en cadmium, qui, même si
elles sont d'origine naturelle, n'en provoquent pas moins des
lésions au niveau de ces organes.
3. Concentrations dans l'environnement et exposition humaine
La principale voie d'exposition au cadmium des non fumeurs est
la voie alimentaire. Les autres voies sont peu importantes. Chez les
fumeurs, l'apport de cadmium par le tabac est notable. Dans les
zones contaminées, l'exposition d'origine alimentaire peut atteindre
plusieurs centaines de microgrammes par jour. Chez les travailleurs
exposés, la principale voie de pénétration est la voie pulmonaire,
après inhalation d'air contaminé sur le lieu de travail. Le
tabagisme et la consommation d'aliments contaminés ajoutent encore à
la charge de cadmium de l'organisme.
4. Cinétique et métabolisme chez les animaux de laboratoire et
chez l'homme
Les données tirées de l'expérimentation animale et humaine
montrent que l'absorption est plus importante au niveau des poumons
qu'au niveau des voies digestives. Selon l'espèce chimique en cause,
la granulométrie et la solubilité dans les liquides biologiques, le
taux d'absorption peut atteindre 50% après inhalation. L'absorption
gastro-intestinale dépend du régime alimentaire et de l'état
nutritionnel. En particulier, le bilan martial est particulièrement
important. En moyenne, le cadmium total contenu dans les aliments
est absorbé à hauteur de 5%, avec un intervalle de variation de
1%-20% selon les individus. Il existe un gradient materno-foetal de
cadmium. Le cadmium peut également parvenir jusqu'au foetus, mais en
faibles quantités malgré son accumulation dans le placenta.
Le cadmium absorbé au niveau des poumons ou des voies
digestives s'accumule principalement dans le foie et les reins où il
représente plus de la moitié de la charge totale de l'organisme.
Plus l'exposition est intense, plus l'accumulation du cadmium dans
le foie est importante. En principe, l'excrétion est lente et la
période biologique du cadmium dans les muscles, les reins, le foie
et l'organisme dans son ensemble, est très longue, de l'ordre de
plusieurs décennies. La teneur en cadmium de la plupart des tissus
augmente avec l'âge. C'est en général dans le cortex rénal que la
concentration est la plus élevée, mais en cas d'exposition
excessive, elle peut l'être encore plus dans le foie. Chez les
personnes exposées atteintes de lésions rénales, il y a augmentation
de l'excrétion urinaire du cadmium de sorte que la période
biologique pour l'ensemble de l'organisme est raccourcie. Du fait
des lésions, le rein perd son cadmium et ces malades finissent par
présenter une concentration rénale de cadmium plus faible que les
individus en bonne santé soumis à la même exposition.
La métallothionéine est une protéine qui joue un rôle important
dans le transport et le stockage du cadmium et d'autres métaux. Le
cadmium est capable d'induire la synthèse de cette protéine dans de
nombreux organes, notamment le foie et le rein. En fixant le cadmium
intracellulaire, la métallothionéine protège les tissus contre les
effets toxiques de ce métal. Il est possible, par conséquent, que le
cadmium non lié à la métallothionéine ait une responsabilité dans
les lésions tissulaires. On ignore quels peuvent être les autres
complexes du cadmium présents dans les liquides biologiques.
L'excrétion urinaire du cadmium dépend de divers facteurs:
charge totale de l'organisme, exposition récente et lésions rénales.
Chez les individus peu exposés, la concentration urinaire du cadmium
dépend principalement de la charge de l'organisme. En cas de lésions
rénales dues au cadmium, ou même sans lésions de ce genre mais en
présence d'une exposition excessive, il y a augmentation de
l'excrétion urinaire. Les individus exposés au cadmium en excrètent
davantage dans leurs urines lorsqu'ils présentent une protéinurie.
Après cessation d'une exposition intense, le taux urinaire décroît,
même s'il y a persistance des lésions rénales. Il faut donc prendre
plusieurs facteurs en considération pour interpréter le cadmium
urinaire. L'excrétion par la voie digestive est sensiblement
équivalente à l'excrétion urinaire mais elle est difficile à
mesurer. L'excrétion par d'autres voies (lactation, sueur ou passage
transplacentaire) est négligeable.
La teneur des matières fécales en cadmium est un bon indicateur
d'une ingestion récente en l'absence d'exposition par la voie
respiratoire. Le cadmium sanguin est présent principalement dans les
hématies, la concentration plasmatique étant très faible. Il existe
au moins deux compartiments dans le sang, l'un qui correspond à une
exposition récente, avec une demi-vie de 2-3 mois et l'autre, qui
est probablement lié à la charge totale de l'organisme et se
caractérise par une demi-vie de plusieurs années.
5. Effets sur les animaux de laboratoire
Une forte exposition par la voie respiratoire entraîne un
oedème mortel du poumon. Après injection d'une seule dose,
apparaissent des lésions testiculaires, une nécrose ovarienne, des
lésions hépatiques et une atteinte des petits vaisseaux. L'ingestion
de fortes doses provoque des lésions de la muqueuse gastrique et
intestinale.
Une exposition prolongée par la voie respiratoire ou une
administration intratrachéenne entraîne des altérations pulmonaires
de nature inflammatoire ainsi qu'une fibrose et donne au tissu
pulmonaire un aspect qui évoque l'emphysème. L'administration
prolongée par voie orale ou parentérale affecte principalement le
rein mais elle a aussi des effets sur le foie et les systèmes
hématopoiétique, immunitaire et cardio-vasculaire ainsi que sur le
squelette. Chez certaines espèces et dans des conditions
déterminées, on a provoqué une hypertension et constaté des effets
sur le squelette. C'est le stade de la gestation où se produit
l'exposition qui conditionne les effets tératogènes et les lésions
placentaires et il peut y avoir interaction avec le zinc.
Ce sont les effets aigus produits par l'inhalation du cadmium
ainsi que sa néphrotoxicité chronique qui sont les plus importants
du point de vue de l'exposition humaine. En cas d'exposition
prolongée, c'est le rein qui est l'organe critique. Les effets sont
caractérisés par une lésion des cellules tubulaires entraînant une
insuffisance tubulaire parfois accompagnée d'insuffisance
glomérulaire. L'insuffisance tubulaire a pour conséquence une
perturbation du métabolisme du calcium et de la vitamine D. Selon
certains travaux, ces troubles pourraient provoquer une ostéomalacie
ou une ostéoporose. Cependant ces résultats n'ont pas été confirmés
par d'autres études. On ne peut exclure un effet direct du cadmium
sur la minéralisation de l'os. Chez l'animal de laboratoire, les
effets toxiques du cadmium dépendent de certains facteurs génétiques
et nutritionnels, des interactions avec d'autres métaux, en
particulier le zinc, et d'un premier traitement éventuel par le
cadmium susceptible d'avoir stimulé la synthèse de métallothionéine.
En 1976 et 1987, le Centre international de recherche sur le
cancer a admis posséder suffisamment de preuves que l'injection de
chlorure, de sulfate, de sulfure et d'oxyde de cadmium pouvait
entraîner l'apparition d'un sarcome local chez le rat et, dans le
cas des deux premiers composés, de tumeurs testiculaires
interstitielles chez ce même animal et chez la souris. Toutefois, il
a considéré que les études basées sur l'administration par voie
orale ne permettaient pas de procéder à une évaluation. Lors
d'études au cours desquelles on a fait respirer à des rats des
aérosols de sulfate de cadmium, des vapeurs d'oxyde de cadmium et de
la poussière de sulfate de cadmium, on a observé une forte incidence
de cancers primitifs du poumon, avec probablement une relation entre
la dose et la réponse. Toutefois, ces résultats n'ont pu être
reproduits chez d'autres espèces. Les travaux relatifs aux effets
génotoxiques du cadmium ont donné des résultats contradictoires.
6. Effets sur l'homme
Une forte exposition à des vapeurs d'oxyde de cadmium par la
voie respiratoire entraîne une pneumopathie aiguë, accompagnée d'un
oedème du poumon qui peut être mortel. L'ingestion de grandes
quantités de sels solubles de cadmium provoque une gastro-entérite
aiguë.
A la suite d'une exposition professionnelle prolongée au
cadmium, on a observé de graves effets chroniques, principalement au
niveau des poumons et des reins. On a également observé une
néphrotoxicité chronique dans la population générale.
Les altérations pulmonaires consécutives à une exposition
professionnelle intense sont essentiellement caractérisées par une
obstruction des voies aériennes. Si l'exposition se poursuit, les
légers troubles ventilatoires initiaux peuvent déboucher sur une
insuffisance respiratoire. On a observé un accroissement de la
mortalité par pneumopathie obstructive chez des travailleurs
fortement exposés, comme cela se produisait auparavant.
L'accumulation de cadmium dans le cortex rénal entraîne des
troubles de la fonction tubulaire et une réabsorption insuffisante,
par exemple, des protéines, du glucose et des acides aminés.
L'accroissement de l'excrétion urinaire des protéines de faible
masse moléculaire est un signe caractéristique de l'insuffisance
tubulaire. Parfois il y a aussi baisse du taux de filtration
glomérulaire. L'augmentation du cadmium urinaire est corrélée avec
une protéinurie de faible masse moléculaire et, en l'absence
d'exposition, peut servir d'indicateur de l'atteinte rénale. Dans
les cas graves, les effets tubulaires et glomérulaires s'ajoutent
s'accompagnant parfois d'une élévation du taux sanguin de
créatinine. Chez la plupart des travailleurs et autres personnes, la
protéinurie due à une néphropathie cadmique est irréversible.
Parmi les autres effets, on peut citer les troubles du
métabolisme calcique, l'hypercalciurie et la formation de calculs
rénaux. Une exposition intense au cadmium peut, selon toute
probabilité lorsqu'elle s'accompagne d'autres facteurs comme une
carence nutritionnelle, provoquer l'apparition d'une ostéoporose
et/ou d'une ostéomalacie.
On est fondé à penser qu'une exposition professionnelle
prolongée au cadmium peut favoriser l'apparition d'un cancer du
poumon, mais la présence de facteurs de confusion ne facilite pas
l'interprétation des observations effectuées sur les travailleurs
exposés. En ce qui concerne le cancer de la prostate, les données ne
sont pas concluantes et ne confirment pas, en tout cas, l'hypothèse
antérieure d'une relation de cause à effet.
A l'heure actuelle, on ne possède pas de preuve convaincante
que le cadmium provoque une hypertension essentielle. La plupart des
données contredisent cette hypothèse et rien n'indique un
accroissement de la mortalité par maladie cardio-vasculaire ou
accident vasculaire cérébral chez les personnes exposées.
D'après les résultats d'études relatives à des groupes exposés
de par leur profession ou simplement du fait de leur environnement
général, il semble que la prévalence des effets néphrotoxiques soit
liée à la durée et à l'intensité de l'exposition.
Chez des ouvriers de l'industrie du cadmium, on a signalé,
après 10 à 20 ans d'exposition à des concentrations de l'ordre de
20-50 µg par mètre cube, une augmentation de la prévalence des cas
de protéinurie à faible masse moléculaire.
Dans des zones polluées, où l'on évalue l'apport de cadmium par
voie orale à environ 140-260 µg/jour, on a observé des effets du
genre protéinurie à faible masse moléculaire chez des sujets exposés
pendant une longue période. On trouvera à la section 8 une
estimation plus précise de la relation dose-réponse.
7. Evaluation des risques pour la santé humaine
7.1 Conclusions
On estime que le rein est l'organe cible tant dans la
population générale que chez les groupes professionnellement
exposés. Une exposition prolongée par inhalation entraîne
l'apparition d'un syndrome respiratoire obstructif chez certains
groupes professionnels. Certains détails incitent à penser que cette
exposition au cadmium pourrait favoriser l'apparition d'un cancer du
poumon, mais les observations effectuées sur des travailleurs
exposés sont difficiles à interpréter en raison de la présence de
facteurs de confusion.
7.1.1 Population générale
Pour la plupart des individus, les aliments constituent la
principale voie d'exposition au cadmium. Dans la plupart des régions
non polluées par ce métal, l'apport alimentaire journalier est de
l'ordre de 10-40 µg. Dans les zones polluées, il peut atteindre
plusieurs centaines de microgrammes par jour. Dans les zones non
polluées, l'apport dû au tabac peut être égal à l'apport alimentaire
chez les gros fumeurs.
D'après un modele biologique, on estime qu'il existe une
association entre l'exposition au cadmium et l'excrétion urinaire de
protéines de faible masse moléculaire chez les sujets qui absorbent
pendant toute leur vie une dose journalière d'environ 140-260 µg de
cadmium, ce qui correspond à une dose cumulée d'environ 2000 mg ou
davantage.
7.1.2 Groupes professionnellement exposés
Dans ce cas, l'exposition est essentiellement respiratoire,
mais il s'y ajoute l'apport alimentaire et tabagique. La teneur
totale de l'air en cadmium varie selon les pratiques en matière
d'hygiène industrielle et selon le lieu de travail. Il existe une
relation de type exposition-réponse entre la teneur de l'air en
cadmium et la protéinurie de faible masse moléculaire. La prévalence
de cette protéinurie peut augmenter après 10 à 20 ans d'exposition à
des concentrations de cadmium de l'ordre de 20-50 µg par mètre cube.
In vivo, le dosage du cadmium dans les reins et le foie de sujets
plus ou moins exposés a montré que 10 % environ des travailleurs
dont le cortex rénal contenait 200 mg/kg de cadmium et 50 % de ceux
chez qui cette concentration atteignait 300 mg/kg, feraient un jour
ou l'autre une protéinurie par insuffisance tubulaire.
RESUMEN Y CONCLUSIONES
1. Identidad, propiedades físicas y químicas, y métodos analíticos
Se dispone de varios métodos para determinar el cadmio presente
en el material biológico. El más utilizado es la espectrometría de
absorción atómica, aunque el análisis de muestras con
concentraciones bajas de cadmio exige un tratamiento cuidadoso de
las muestras y correcciones para tener en cuenta la interferencia.
Se recomienda encarecidamente acompañar el análisis con un programa
de garantía de la calidad. Actualmente, en circunstancias ideales
pueden determinarse concentraciones de alrededor de 0,1 µg/litro en
la orina y la sangre y de 1-10 µg/kg en alimentos y muestras de
tejidos.
2. Fuentes de exposición humana y ambiental
El cadmio es un elemento relativamente raro; los procedimientos
analíticos actuales indican que las concentraciones del metal en el
medio ambiente son mucho más bajas que las obtenidas en medidas
anteriores. Hoy en día no es posible determinar si la actividad
humana ha provocado un aumento histórico de los niveles de cadmio en
los casquetes polares.
La producción comercial de cadmio comenzó a principios de este
siglo. La pauta de consumo de cadmio se ha modificado en los últimos
años debido al notable descenso del uso de la galvano-plastia y al
importante aumento de la producción de baterías y de las
aplicaciones electrónicas especializadas. En las principales
aplicaciones del cadmio éste se utiliza en forma de compuestos que
se hallan presentes en bajas concentraciones; ello obstaculiza el
reciclaje del metal. Las restricciones impuestas por algunos países
a ciertas aplicaciones del cadmio pueden tener un efecto
generalizado en esas aplicaciones.
El cadmio se libera al aire, los suelos y las aguas debido a la
actividad humana. En general, las dos fuentes principales de
contaminación son la producción y el consumo de cadmio y de otros
metales no ferrosos y la evacuación de desechos que contienen
cadmio. Las zonas próximas a minas no ferrosas y fundiciones suelen
estar muy contaminadas por cadmio.
Al aumentar el contenido de cadmio del suelo, aumenta la
absorción del metal por las plantas; la exposición humana a partir
de las cosechas agrícolas está por tanto sometida a los aumentos del
contenido de cadmio del suelo. Dado que la absorción por las plantas
desde el suelo es mayor cuando el pH de éste es bajo, los procesos
que acidifican el suelo (por ejemplo, las lluvias ácidas) pueden
aumentar las concentraciones medias de cadmio en los alimentos. La
aplicación de fertilizantes a base de fosfato y la deposición
atmosférica son fuentes importantes de aportación de cadmio a las
tierras cultivables en ciertas zonas del mundo; los fangos de
alcantarillado también pueden ser una fuente de importancia a nivel
local. Estas fuentes pueden, en el futuro, aumentar los niveles de
cadmio en el suelo y con ello en las cosechas, lo que a su vez puede
acrecentar la exposición al cadmio en la dieta. En ciertas zonas, se
ha demostrado que está aumentando el contenido de cadmio en los
alimentos.
Ciertos organismos comestibles de vida libre como los mariscos,
los crustáceos y los hongos son acumuladores naturales de cadmio.
Como en el caso del ser humano, se observan niveles mayores de
cadmio en el hígado y el riñón de los caballos y de algunos animales
terrestres silvestres. El consumo habitual de estos alimentos puede
aumentar la exposición. Ciertos vertebrados marinos contienen
concentraciones notablemente elevadas de cadmio en el riñón,
fenómeno que, aunque se considera de origen natural, se ha vinculado
a signos de lesiones renales en esos organismos.
3. Niveles ambientales y exposición humana
La principal fuente de exposición al cadmio en la población
general no fumadora son los alimentos; la proporción de cadmio que
se absorbe por otras vías es pequeña. El tabaco es una importante
fuente de absorción de cadmio en los fumadores. En las zonas
contaminadas, la exposición al cadmio por los alimentos puede
alcanzar varios cientos de µg/día. En los trabajadores expuestos, la
absorción pulmonar de cadmio por inhalación en el lugar de trabajo
es la principal vía de exposición. También puede aumentar la
absorción por la contaminación de los alimentos y por el consumo de
tabaco.
4. Cinética y metabolismo en animales de experimentación y en
el ser humano
Los datos obtenidos en animales de experimentación y en el ser
humano han demostrado que la absorción pulmonar es mayor que la
gastrointestinal. Atendiendo a la especiación química, el tamaño de
las partículas y la solubilidad en fluidos biológicos, puede
absorberse hasta el 50% del compuesto de cadmio inhalado. La
absorción gastrointestinal de cadmio depende del tipo de dieta y del
estado nutricional. El estado nutricional respecto del hierro parece
revestir particular importancia. Aunque en promedio se absorbe el 5%
de la ingesta oral total de cadmio, los valores individuales varían
entre menos del 1% hasta más del 20%. Existe un gradiente
maternofetal de cadmio. Aunque se acumula en la placenta, la
transferencia al feto es baja.
El cadmio absorbido en los pulmones o el tracto
gastrointestinal se almacena principalmente en el hígado y el riñón,
donde se deposita más de la mitad de la carga corporal. Al aumentar
la intensidad de la exposición, aumenta la proporción del cadmio
absorbido que se almacena en el hígado. En el ser humano la
excreción suele ser lenta y la semivida biológica es muy larga
(decenios) en el músculo, el riñón, el hígado y el organismo entero.
Las concentraciones de cadmio en la mayoría de los tejidos aumentan
con la edad. Aunque las concentraciones más elevadas suelen
encontrarse en la corteza renal, con exposiciones excesivas pueden
producirse concentraciones mayores en el hígado. En las personas
expuestas que padecen lesiones renales, aumenta la excreción
urinaria de cadmio con lo que se reduce la semivida en el organismo
entero. Las lesiones renales producen pérdidas del cadmio contenido
en el riñón, y las concentraciones renales acaban con el tiempo
siendo inferiores a las observadas en personas con un grado de
exposición similar pero sin lesiones renales.
La metalotioneína es una importante proteína de transporte y
almacenamiento de cadmio y otros metales. El cadmio puede inducir la
síntesis de metalotioneína en muchos órganos, en particular el
hígado y el riñón. La unión del cadmio intracelular a la
metalotioneína en los tejidos protege contra la toxicidad del metal.
El cadmio libre puede por tanto tener una función en la patogenia de
las lesiones tisulares debidas a ese metal. Se desconoce la
especiación de otros complejos de cadmio en los tejidos o en los
fluidos biológicos.
La excreción urinaria de cadmio guarda relación con la carga
corporal, la exposición reciente y la lesión renal. En personas poco
expuestas, el nivel de cadmio en la orina depende principalmente de
la carga corporal. Una vez que se ha producido la lesión renal
inducida por el cadmio, o incluso en ausencia de lesión renal si la
exposición es excesiva, aumenta la excreción urinaria. En las
personas expuestas al cadmio que padecen proteinuria la excreción de
cadmio suele ser mayor que en las que no padecen proteinuria. Cuando
cesa la exposición intensa, el nivel de cadmio en la orina desciende
aunque persista la lesión renal. En la interpretación de la
presencia de cadmio en la orina hay que tener en cuenta, pues,
varios factores. La excreción gastrointestinal es aproximadamente
igual a la urinaria pero no puede medirse fácilmente. Otras vías
excretoras como la leche, el sudor o la transferencia placentaria
son insignificantes.
El nivel de cadmio en las heces es un buen indicador de la
ingesta diaria reciente a partir de los alimentos en ausencia de
exposición por inhalación. En la sangre, el cadmio aparece
principalmente en los glóbulos rojos y las concentraciones en el
plasma son muy bajas. Existen al menos dos compartimentos en la
sangre, uno referido a la exposición reciente, con una semivida de
alrededor de 2-3 meses, y otro probablemente relacionado con la
carga corporal, con una semivida de varios años.
5. Efectos en mamíferos de laboratorio
Las exposiciones elevadas por inhalación provocan edema
pulmonar letal. La inyección de una sola dosis elevada produce
necrosis en el testículo y en el ovario no ovulante, lesiones
hepáticas y lesiones en los vasos de menor tamaño. La administración
oral de dosis elevadas produce lesiones en la mucosa gástrica e
intestinal.
La exposición por inhalación prolongada y la administración
intratraqueal producen modificaciones crónicas de tipo inflamatorio
en los pulmones, fibrosis y fenómenos indicativos de enfisema. La
administración parenteral u oral prolongada afecta principalmente al
riñón aunque también al hígado y a los sistemas hematopoyético,
inmunitario, esquelético y cardiovascular. En ciertas especies y en
determinadas condiciones se han inducido efectos esqueléticos e
hipertensión. La aparición de efectos teratogénicos y lesiones
placentarias depende de la fase gestacional en que se produzca la
exposición y puede entrañar interacción con el zinc.
En cuanto a la exposición humana, lo más notable son los
efectos agudos por inhalación en el pulmón y los efectos renales
crónicos. Tras la exposición prolongada, el riñón es el órgano
crítico. Los efectos en este órgano se caracterizan por disfunción
tubular y lesiones en las células tubulares, si bien pueden
producirse también disfunciones glomerulares. Una de las
consecuencias de la disfunción tubular renal es la alteración del
metabolismo del calcio y de la vitamina D. Según algunos estudios,
ello ha producido casos de osteomalacia y/o osteoporosis, pero esos
efectos no se han confirmado en otros estudios. No debe excluirse el
efecto directo del cadmio en la mineralización ósea.
Los efectos tóxicos del cadmio en animales de experimentación
están sometidos a la influencia de factores genéticos y
nutricionales, las interacciones con otros metales, en particular el
zinc, y el pretratamiento con cadmio, que puede guardar relación con
la inducción de la metalotioneína.
En 1976 y 1987, el Centro Internacional de Investigaciones
sobre el Cáncer consideró suficientes las pruebas de que el cloruro,
el sulfato, el sulfuro y el óxido de cadmio pueden producir sarcomas
en el lugar de inyección en la rata y, en el caso de los dos
primeros compuestos, inducir tumores en las células intersticiales
del testículo en la rata y el ratón, pero consideró que los estudios
de administración oral eran insuficientes para la evaluación. En
estudios de inhalación prolongada en ratas expuestas a aerosoles de
sulfato de cadmio, vapores de óxido de cadmio y polvos de sulfato de
cadmio se observó una elevada incidencia de cáncer primario del
pulmón con pruebas de proporcionalidad entre la dosis y la
respuesta. Hasta el momento, sin embargo, esa observación no se ha
confirmado en otras especies. Los estudios de los efectos
genotóxicos del cadmio han dado resultados discordantes.
6. Efectos en el ser humano
La exposición intensa por inhalación de vapores de óxido de
cadmio produce neumonitis aguda con edema pulmonar, que puede ser
letal. La ingestión de dosis elevadas de sales solubles de cadmio
produce gastroenteritis aguda.
La exposición ocupacional prolongada al cadmio ha producido
efectos crónicos graves, principalmente en el pulmón y el riñón.
También se han observado efectos renales crónicos en la población
general.
Los cambios pulmonares observados tras una intensa exposición
ocupacional se caracterizan principalmente por la aparición de
afecciones crónicas obstructivas de las vías aéreas. Los primeros
cambios leves en las pruebas de la función ventilatoria pueden
avanzar, si prosigue la exposición al cadmio, hasta insuficiencia
respiratoria. Se ha observado una mayor tasa de mortalidad por
enfermedad pulmonar obstructiva en trabajadores sometidos a
exposiciones intensas, al igual que en otras épocas.
La acumulación de cadmio en la corteza renal produce disfunción
tubulorrenal con trastornos de la reabsorción de proteínas, glucosa
y aminoácidos, entre otros. Un signo característico de la disfunción
tubular es la mayor excreción de proteínas de bajo peso molecular en
la orina. En algunos casos, disminuye la tasa de filtración
glomerular. El aumento de la concentración de cadmio en la orina
está correlacionado con la presencia de proteínas de bajo peso
molecular en la orina y, en ausencia de exposición aguda al cadmio,
puede servir como indicador de efectos renales. En los casos más
graves se combinan los efectos tubulares y glomerulares, con aumento
del nivel de creatinina en la sangre en algunos casos. Para la
mayoría de los trabajadores y de las personas expuestas al medio
ambiente general, la proteinuria inducida por el cadmio es
irreversible.
Entre otros efectos figuran los trastornos del metabolismo del
calcio, la hipercalciuria y la formación de cálculos renales. La
exposición intensa al cadmio, con toda probabilidad combinado con
otros factores como carencias nutricionales, puede llevar a la
aparición de osteoporosis y/o osteomalacia.
Hay pruebas de que la exposición profesional prolongada al
cadmio puede contribuir a la aparición de cáncer del pulmón aunque
las observaciones obtenidas en trabajadores expuestos han sido
difíciles de interpretar a causa de factores que inducen a
confusión. En el caso del cáncer de la próstata, las pruebas
obtenidas hasta la fecha no son concluyentes pero no apoyan la
existencia de una relación causal, indicada en estudios anteriores.
Actualmente no hay pruebas convincentes de que el cadmio sea
agente etiológico de la hipertensión esencial. La mayor parte de los
datos indican que no se debe al cadmio el aumento de la tensión y no
hay pruebas de que la mortalidad por enfermedades cardio-vasculares
o cerebrovasculares sea mayor.
Los datos obtenidos en estudios de grupos de trabajadores
expuestos y de grupos expuestos en el medio ambiente general
demuestran que existe una relación entre los niveles de exposición,
la duración de ésta y la prevalencia de los efectos renales.
Se ha comunicado una mayor prevalencia de la proteinuria de
bajo peso molecular en trabajadores del cadmio tras 10-20 años de
exposición a niveles del metal de aproximadamente 20-50 µg/m3. En
zonas contaminadas del medio general, en las que la ingesta de
cadmio estimada ha sido de 140-260 µg/día, se han observado efectos
en forma de aumento de la cantidad de proteínas de bajo peso
molecular en la orina en algunos individuos tras una exposición
prolongada. En la sección 8 se dan estimaciones más precisas de la
relación dosis-respuesta.
7. Evaluación de los riesgos para la salud humana
7.1 Conclusiones
Se considera que el riñón es el órgano diana crítico en la
población general así como en las poblaciones expuestas
profesionalmente. Las enfermedades crónicas obstructivas de las vías
respiratorias están asociadas a la exposición profesional prolongada
e intensa por inhalación. Hay pruebas de que esa exposición al
cadmio puede contribuir al desarrollo de cáncer del pulmón aunque
las observaciones en trabajadores expuestos han sido difíciles de
interpretar a causa de la presencia de factores que inducen a
confusión.
7.1.1 Población general
El cadmio presente en los alimentos es la principal fuente de
exposición para la mayoría de las personas. En la mayoría de las
zonas no contaminadas con cadmio las ingestas diarias medias con los
alimentos se encuentran entre 10 y 40 µg. En zonas contaminadas se
ha observado que alcanza varios cientos de µg al día. En zonas no
contaminadas, la absorción debida al consumo de tabaco puede igualar
la ingestión de cadmio a partir de los alimentos.
Basandose en un modelo biologico, se ha estimado que con una
ingesta diaria de 140-260 µg de cadmio durante toda la vida, o una
ingesta acumulativa de unos 2000 mg o más, se produce en el ser
humano una asociación entre la exposición al cadmio y una mayor
excreción de proteínas de bajo peso molecular en la orina.
7.1.2 Población expuesta profesionalmente
La exposición ocupacional al cadmio se produce principalmente
por inhalación aunque comprende ingestas suplementarias con los
alimentos y el tabaco. El nivel total de cadmio en el aire varía
según las prácticas de higiene industrial y el tipo de lugar de
trabajo. Existe una relación exposición-respuesta entre los niveles
de cadmio en el aire y la proteinuria. Puede aumentar la prevalencia
de la proteinuria de bajo peso molecular en trabajadores a los 10-20
años de exposición a niveles de cadmio de unos 20-50 µg/m3. La
medida in vivo del cadmio en el riñón y el hígado de personas con
distintos niveles de exposición al metal ha demostrado que alrededor
del 10% de los trabajadores con un nivel en la corteza renal de
200 mg/kg y aproximadamente el 50% de las personas con un nivel en
la corteza renal de 300 mg/kg tendrían proteinuria tubulorrenal.
In a later epidemiological study, Nogawa et al. (1989)
investigated the dose-response relationship in 1850 cadmium-exposed
and 294 non-exposed inhabitants of the Kakehashi River basin. Using
a urine concentration of 1000 µg ß2-microglobulin/g creatinine as
an index of renal tubular dysfunction, and the average rice cadmium
concentration as an index of cadmium exposure, the authors
determined linear regression equations for men and women. These are
related to the prevalence of ß2-microglobulinuria and total
cadmium intake and are shown in Table 19. The authors concluded that
the total cadmium intake that produced an adverse effect on health
was approximately 2000 mg for both men and women. On the basis of
the linear regression equations shown in Table 19, an average daily
cadmium intake of 440 µg/day in men and 350 µg/day in women would
be expected to cause a 50% response rate (> 1000 µg
ß2-microglobulin/litre urine). Response rates of 20, 10 and 5%
would occur at daily intakes of 220, 150, and 110 µg/day for men and
200, 150, and 120 µg/day for women. The authors indicated that these
data are in general agreement with results from other studies
involving the consumption of various levels of cadmium in rice.
8.3.3.5 Akita prefecture (Kosaka area)
Around Kosaka mine and refinery areas, increased cadmium levels
in rice were first reported by the Akita Prefectural Government
(1973). Kojima et al. (1976) gave further data from the Kosaka area,
where the reported average cadmium level in rice varied between 0.26
and 0.56 mg/kg. The latter average was based on 41 samples where one
was reported to contain 4.81 mg/kg. In the exposed area, the
weighted average of cadmium levels in rice in each district
according to the number of examinees was calculated by Kojima et al.
(1976) to be 0.57 mg/kg in 1973 and 0.50 mg/kg in 1974. These
values, according to Kojima et al. (1976), represented the level of
cadmium exposure in this study and were considered more accurate
than the general average given above.
In an epidemiological investigation of the total population in
the age-group 50-69 of defined geographical areas (93 out of 98 in
the control area and 156 out of 190 in the exposed area
participated), Kojima et al. (1976, 1977) obtained data on faecal
excretion of cadmium. The cadmium level in 24-h faeces samples was
analysed only for those participants who said that they defaecated
once a day (64 in the control area and 118 in the polluted area).
Average rates were 51 µg/day and 177 µg/day for the control and
exposed groups, respectively (Kojima et al., 1976). The prevalence
of proteinuria exceeding 150 mg/litre (using the Tsuchiya biuret
method) and of combined proteinuria and glucosuria (test tape) was
significantly higher in the exposed group than in the control group
(Kojima et al., 1976).
Table 19. Linear regression equations relating total cadmium intake and prevalence of ß2-microglobulinuriaa
Sex ß2-microglobulinuria Linear regression Prevalence of Total cadmium intake
equationb ß2-microglobulinuria (mg)c
in the control group (%)
Male > 1000 µg/litre Y = 0.0076X - 10.33 5.3 2057
> 1000 µg/g creatinine Y = 0.0083X - 7.93 6.0 1678
Female > 1000 µg/litre Y = 0.011X - 19.61 3.1 2065
> 1000 µg/g creatinine Y = 0.012X - 16.16 5.0 1763
a From: Nogawa et al. (1989)
b Y = prevalence of ß2-microglobulinuria (%); X = total cadmium intake (mg)
c Total cadmium intake yielding a ß2-microglobulinuria prevalence equivalent to the control group
Quantitative analysis of urinary ß2-microglobulin with
radioimmunoassay (RIA) was performed on the same population (Kojima
et al., 1977). The frequency distributions were log-normal, as for
occupationally exposed people, and the prevalence of
ß2-microglobulin excretion was 15% in the whole exposed group and
3% in the control group. The exposed and control groups showed
average faecal cadmium excretion rates of 139 and 41 µg per day,
respectively (Kojima et al., 1977).
The ß2-microglobulin data and cadmium intake data in the
study by Kojima et al. (1977) were divided into three dose groups
(Kjellström et al., 1977b) and analysed for a dose-response
relationship by Hutton (1983).
Further studies of urinary ß2-microglobulin over the age
range 5-90 years were reported by Saito et al. (1981). The RIA
method was used, and a control area in Akita prefecture was compared
with cadmium-polluted areas in Akita prefecture (Kosakai), Ishikawa
prefecture (Kakehashi) (section 8.3.3.3), and Nagasaki prefecture
(Tsushima) (section 8.3.3.5). For people over the age of 40, there
were significant increases in average urinary ß2-microglobulin
excretion in all the polluted areas. In the older age-groups, the
increase was 10-100 times above control values.
In the first comprehensive study of proximal renal tubular
functions performed on a population living in a cadmium-polluted
area, Saito et al. (1977) conducted health examinations within Akita
prefecture. Renal tubular function tests consisted of renal
glucosuria, uric acid clearance, low molecular weight proteinuria,
tubular reabsorption of phosphate, hydrogen carbonate threshold,
acid-base balance, concentrating and acidifying ability of urine,
endogenous creatinine clearance, and renal plasma flow. Of the 147
residents (97% of target population) examined, 33 (22%) had some
indications of proximal renal tubular dysfunction, such as renal
glucosuria and low molecular weight proteinuria. In addition, 10
subjects (7%) were diagnosed as having multiple proximal renal
tubular dysfunctions. Detailed examinations revealed that none of
these 10 subjects had experienced any other environmental exposures
or diseases that could have caused the renal dysfunction. They were
therefore diagnosed as suffering from the effects of chronic cadmium
poisoning (Saito et al., 1977).
8.3.3.6 Nagasaki prefecture (Tsushima area)
This area has been a lead and zinc mining district from ancient
times (Takabatake, 1978b). Modern operations started in 1948, and
mining wastes have been scattered throughout the local area. In
1952, local farmers complained about the poor growth of crops. In
the 1960s, studies of cadmium pollution were carried out. Health
examinations for cadmium effects have been conducted since 1966
(Takabatake, 1978b).
Early studies showed an increased prevalence of proteinuria in
the most polluted village, and an average rice cadmium level of
0.75 mg/kg was reported (Takabatake, 1978b).
The study of urinary ß2-microglobulin according to age
referred to in section 8.3.3.4 (Saito et al., 1981) included an
exposed group of people from Tsushima. The age-specific average
urinary excretions were much higher than the control values and
similar to those found in the Kakehashi and Kosaka areas. For women,
the Tsushima values appear higher than those of the other two
polluted areas (Table 17), which may reflect the higher estimated
average cadmium intake in the Tsushima area.
8.3.3.7 Other Japanese areas
As shown in Table 7, health surveys have been performed in
areas other than Fuchu, Ikuno, Kakehashi, Kosaka, and Tsushima.
According to the Japanese Cadmium Research Committee (1989), it
should be emphasized, however, that no cadmium health effects,
including elevated prevalence of ß2-microglobulinuria, were
observed in some areas with higher levels of cadmium (daily intakes
of 180-309 µg in Bandai, Fukushima; 180-380 µg in Annaka, Gunma; and
222-391 µg in Okutake river basin, Oita) even than Kosaka and
Kakehashi (cadmium in rice = 0.16-0.58 mg/kg, daily intake of
cadmium = 139-177 µg in Kosaka, Akita; cadmium in rice =
0.2-0.8 mg/kg, daily intake of cadmium = 160-190 µg in the Kakehashi
river basin, Ishikawa). Also, no health effects were found in the
Uguisuzawa river basin, Miyagi (cadmium in rice = 0.6-0.7 mg/kg),
Watarase river basin, Gunma (0.32 mg/kg), Shimoda, Shizuoka
(0.4-1.1 mg/kg), and Ohmuta, Fukuoka (0.72 mg/kg), even though
residents consumed rice heavily contaminated with cadmium.
8.3.3.8 Belgium
The Liege area of Belgium is known to be polluted by cadmium,
mainly due to the activities of non-ferrous smelters since the end
of the 19th century (Kretzschmar et al., 1980; Lauwerys et al.,
1980a).
A pilot study was performed in 1979 on a group of 60 elderly
non-smoking women who had spent most of their lives in the Liege
area and had never been occupationally exposed to cadmium (Roels et
al., 1981a). Daily intakes of cadmium ranged from 2-88 µg/day with
an average of 15 µg/day. Their average blood cadmium level
(1.6 µg/litre) and their urinary excretion rates of cadmium
(0.093 µg/h), total protein (17.3 mg/h), amino acids (5.45 mg amino
acid N/h), and albumin (1.54 mg/h) were higher than those found in a
group of 70 women of the same age and socio-economic status who
lived in another industrial area (Charleroi) less polluted by
cadmium. Although the average excretion rate of ß2-microglobulin
was greater in Liege (93.6 µg/h) than in Charleroi (22 µg/h), the
difference between the geometric means was not statistically
significant. The two areas were well matched with respect to their
environmental pollution by sulfur dioxide, fume, suspended
particles, and various metals, including lead, vanadium, nickel,
chromium, and iron.
Following these observations, a mortality study and a
preliminary autopsy study were undertaken (Lauwerys & De Wals, 1981;
Lauwerys et al., 1984a). It was found that, although the overall
mortality was not markedly different, the standard mortality ratio
(SMR) and proportional mortality rate (PMR) from nephritis and
nephrosis for the years 1967-1976 were higher in Liege than in
Charleroi or in Belgium as a whole (SMR: Belgium, 100; Charleroi,
102; Liege, 196; PMR: Belgium, 3.3; Charleroi, 3.0; Liege, 6.0).
Since the increased mortality rate for renal diseases was observed
in both males and females, the influence of environmental factors
other than occupation is probable.
The results of the preliminary autopsy study indicated that, in
the age-group 40-60, the average body burden of cadmium was
approximately twice as high in people autopsied in Liege as it was
in those autopsied in a city (Brussels) less polluted by cadmium
(Lauwerys et al., 1984a). The geometric mean values of cadmium
concentration in the kidney cortex were 38.3 and 22.8 mg/kg wet
weight in Liege and Brussels, respectively.
According to the authors of these reports, the studies
performed so far in the Liege area do not refute the hypothesis that
environmental pollution by cadmium in the area has increased the
body burden of cadmium of the inhabitants and has affected their
renal function. A large-scale morbidity and autopsy study is at
present underway (Braux et al., 1987).
8.3.3.9 Shipham area in the United Kingdom
The village of Shipham is located on the slag heaps of an old
zinc mine and high levels of cadmium have been found in soil and
dust (section 3.4.3).
The exposed population has been studied using both mortality
and morbidity end-points. A census in 1979 identified 1092
residents, of whom 64% had resided in the village for more than 5
years, and 548 participated in a health study coordinated by the
United Kingdom Department of the Environment (Barltrop & Strehlow,
1982a). A similar study of 543 age-and sex-matched individuals was
performed in a nearby control village (Barltrop & Strehlow, 1982b).
The daily intake of cadmium in Shipham was an average of 35 µg/day
(section 4.2.4), which is about twice as high as the estimated
United Kingdom national average but much lower than in polluted
areas of Japan (section 5.2.4).
A health inventory was compiled by means of a questionnaire,
which included information on smoking habits, alcohol consumption,
medication, and occupation. Blood samples were analysed for
haemoglobin, haematocrit, serum protein, ß2-microglobulin,
creatinine, erythrocyte protoporphyrin, lead, and cadmium. Urine
samples were analysed for total protein, creatinine, ß2-micro-
globulin, and cadmium.
The mean 24-h urinary concentration of cadmium for Shipham
residents was 0.68 µg cadmium/g creatinine and, in the control area,
0.60 µg cadmium/g creatinine with 97.7% of values less than 3.4 µg
cadmium/litre. The difference was statistically significant
(P < 0.03) (Barltrop & Strehlow, 1982b), but the similarity of the
values for average urinary cadmium concentrations between the two
areas and the generally low levels of cadmium in Shipham suggest a
rather low daily cadmium intake.
However, there are data showing that some individuals in
Shipham had high cadmium exposures. Liver cadmium concentrations,
measured by means of in vivo neutron activation analysis, were
determine for 21 adult volunteers living in the most heavily
contaminated areas of Shipham (Harvey et al., 1979). Their age range
was 40-62 years (mean, 53 years) and, with one exception, they had
lived in Shipham for 9-50 years (mean, 23 years). On average, half
of their vegetable consumption was of local origin. The mean liver
cadmium concentration was 11.0 (+ 2.0) mg/kg, compared with 2.2
(+ 2.0) mg/kg in 20 age-matched, non-Shipham controls (P < 0.001).
The maximum concentration in the Shipham group was 28 mg/kg, which
would correspond to levels in the kidney cortex of at least
200-300 mg/kg (Friberg, 1979) (section 6.4). Health effects were not
studied in this investigation.
In the health study by the Department of the Environment
(Barltrop & Strehlow, 1982a), the comparison of ß2-microglobulin
data from the two villages showed similar distributions, and all
other laboratory data, including blood pressure levels, were
distributed within the normal range. However, the poor participation
rate in this health study (50%), makes it difficult to interpret the
findings. Another study of 31 volunteers from Shipham (Carruthers &
Smith, 1979) reported a high prevalence of hypertension and LMW
proteinuria, but the methodology of the study has been criticized
(Hughes & Stewart, 1979; Kraemer et al., 1979).
Examination of the data in relation to soil cadmium levels
showed no evidence of an increased mortality from any cause in those
living in the most polluted areas. It was concluded from this study
that, if cadmium contamination had any effect on the mortality
pattern in Shipham, this effect was only slight and did not present
a serious health hazard to residents. No case resembling Itai-itai
disease has at any time been reported in Shipham. All the authors
involved in the health studies in Shipham pointed to the possible
protective effect of high levels of zinc also present in soil, and
Kraemer et al. (1979) pointed to the need to assess dietary zinc and
the intake of nutrients other than cadmium.
8.3.3.10 USSR
Screening of populations, both environmentally and
occupationally exposed, which included measurements of urinary
ß2-microglobulin, has been carried out within the USSR (Likutova,
1989). Increased prevalence (up to 6%) of ß2-micro-globulinuria
(> 280 µg/g creatinine) was observed in females (20-50 years old)
in some of the most heavily polluted cities, i.e. Odjonikidze and
Kursk. The air cadmium concentrations in these two cities were
0.085 µg/m3 and 0.005-0.027 µg/m3, respectively. The examination
of workers (50-300 µg/m3) exposed to cadmium also revealed an
increased prevalence of ß2-microglobulinuria (up to 19% in the
most heavily exposed group). The findings are in good agreement with
the data presented in Table 15.
8.4 Conclusions
High inhalation exposure to cadmium oxide fume results in acute
pneumonitis with pulmonary oedema, which may be lethal. High
ingestion exposure of soluble cadmium salts causes acute
gastroenteritis.
Long-term occupational exposure to cadmium has caused severe
chronic effects, predominantly in the lungs and kidneys. Chronic
renal effects have also been seen among the general population.
Following high occupational exposure, lung changes are
primarily characterized by chronic obstructive airway disease. Early
minor changes in ventilatory function tests may progress, with
continued cadmium exposure, to respiratory insufficiency. An
increased mortality rate from obstructive lung disease has been seen
in workers with high exposure, as has occurred in the past.
The accumulation of cadmium in the renal cortex leads to renal
tubular dysfunction with impaired reabsorption of, for instance,
proteins, glucose, and amino acids. A characteristic sign of tubular
dysfunction is an increased excretion of low molecular weight
proteins in urine. In some cases, the glomerular filtration rate
decreases. Increase in urine cadmium correlates with low molecular
weight proteinuria and in the absence of acute exposure to cadmium
may serve as an indicator of renal effect. In more severe cases
there is a combination of tubular and glomerular effects, which may
progress in some cases to decreased glomerular filtration. For most
workers and people in the general environment, cadmium-induced
proteinuria is irreversible.
Among other effects are disturbances in calcium metabolism,
hypercalciuria, and formation of renal stones. High exposure to
cadmium, most probably in combination with other factors such as
nutritional deficiencies, may lead to the development of
osteoporosis and/or osteomalacia.
There is evidence that long-term occupational exposure to
cadmium may contribute to the development of cancer of the lung but
observations from exposed workers have been difficult to interpret
because of confounding factors. For prostatic cancer, evidence to
date is inconclusive but does not support the suggestion from
earlier studies of a causal relationship.
At present, there is no convincing evidence for cadmium being
an etiological agent of essential hypertension. Most data speak
against a blood pressure increase due to cadmium and there is no
evidence of an increased mortality due to cardiovascular or
cerebrovascular disease.
Data from studies on groups of occupationally exposed workers
and on groups exposed in the general environment show that there is
a relationship between exposure levels, exposure durations, and the
prevalence of renal effects.
An increased prevalence of low molecular weight proteinuria in
cadmium workers after 10-20 years of exposure to cadmium levels of
about 20-50 µg/m3 has been reported.
In polluted areas of the general environment, where the
estimated cadmium intake has been 140-260 µg/day, effects in the
form of increased low molecular weight proteinuria have been seen in
some individuals following long-term exposure.
9. EVALUATION OF HUMAN HEALTH RISKS
9.1 Exposure assessment
9.1.1 General population exposure
In the ambient air, cadmium concentrations based on long-term
sampling periods indicate, in most cases, a range of
0.001-0.015 µg/m3 in rural areas, 0.005-0.05 µg/m3 in urban
areas, and up to 0.6 µg/m3 near sources of pollution (section
5.1.1).
One cigarette usually contains 1-2 µg cadmium, of which about
10% may be inhaled (section 5.1.3).
Among staple foods, rice and wheat usually contain less than
0.1 mg/kg and other foods usually less than 0.05 mg/kg wet weight,
but liver and kidney may contain 1-2 mg/kg wet weight and certain
sea-foods as much as 10 mg/kg wet weight (section 5.2). Certain
animals, e.g., the horse, may accumulate considerably higher
concentrations in the liver and kidney. In polluted areas, these
levels are further increased.
The content of natural waters is usually less than 1 µg/litre,
but higher levels may be found near sources of pollution.
The total daily intake in non-polluted areas of most countries
from food, water and air is estimated to be approximately
10-40 µg/day (food, 10-40 µg/day; water, < 1 µg; and air,
< 0.5 µg/day for non-smokers).
Twenty cigarettes per day would contribute a further 2-4 µg. In
polluted areas, the daily intake may be much higher, and intakes of
several hundred µg/day have been reported (section 5.3.2).
9.1.2 Occupational exposure
Air is the main source of additional cadmium exposure for
industrial workers. In many countries such exposures have now been
reduced considerably. In the past, levels of several mg/m3 were
recorded in workplaces. Now, with proper industrial hygiene
practices, levels of 0.02-0.05 mg/m3 would be more typical
(section 5.1.2).
9.1.3 Amounts absorbed from air, food, and water
The proportion of cadmium from food and water that is absorbed
will depend on the chemical nature of the cadmium compounds, but
estimates based on the available data indicate that gastrointestinal
absorption is approximately 5%, with considerable individual
variation (section 6.1.2). Similarly, the amount absorbed from the
air will depend on the chemical nature and the particle size of the
inhaled material. The absorption varies between 25 and 50% depending
on particle size and solubility (section 6.1.1). About 10% of the
cadmium inhaled in cigarette smoke is absorbed.
Thus, the average amount absorbed from food and water in a
person from a non-polluted area would be about 0.5-1.3 µg/day. The
absorbed amount from smoking 20 cigarettes per day would be
1-2 µg/day and that from workroom air could be many times greater
(section 5.3).
9.2 Dose-effect relationships
9.2.1 Renal effects
Long-term exposure to cadmium causes renal tubular dysfunction
with proteinuria, glucosuria, and aminoaciduria, as well as
histopathological changes, in both experimental animals and humans
(sections 7.2.1.4 and 8.2.1, respectively). These are usually the
first effects to occur after ingestion or inhalation exposure. As
the renal dysfunction progresses in severity, the glomeruli may also
be affected and, in a few cases, the cadmium-induced damage may lead
to renal failure (section 8.2.1). Daily cadmium intakes in food of
140-260 µg/day for more than 50 years or workplace air exposures of
50 µg/m3 for more than 10 years have produced an increase in renal
tubular dysfunction in some exposed popu-lations (section 8.3.3.2).
9.2.2 Bone effects
Cadmium may produce bone effects in both humans and animals.
The most notable clinical entity in these cases is osteomalacia, but
many subjects also show osteoporosis. Animal experiments show that
both can be produced by long-term cadmium exposure (section 7.2.4).
In animals and humans, osteomalacia has been seen in combination
with cadmium-induced renal damage. The bone effects may be linked to
cadmium effects on calcium and vitamin D metabolism in the kidney.
The daily intakes via food and exposure levels in air at which the
bone effects occur are uncertain, but they must be higher than those
causing renal effects. Bone effects have been seen among both the
general population and industrial workers in the past when exposure
levels were very high. Host and nutritional factors influence the
development and severity of cadmium-induced bone effects.
9.2.3 Pulmonary effects
Chronic obstructive airway disease has been reported in a
number of studies of cadmium workers (section 8.2.3). This has, in
severe cases, led to an increased mortality. The dose needed to
produce these effects is uncertain, but it is higher than the dose
needed to produce renal effects, as most workers reported to have
lung effects also had renal effects. On the other hand, many workers
with renal effects, who had been exposed to cadmium oxide dust and
fume, had no lung effects.
9.2.4 Cardiovascular effects
Some animal studies have shown that, under certain exposure
conditions, increased blood pressure and effects on the myocardium
occur. Studies of cadmium-exposed workers and people in the general
environment have been carried out, but most data do not support the
animal findings.
9.2.5 Cancer
There is evidence that cadmium chloride, sulfate, sulfide and
oxide give rise to injection site sarcomata in the rat and that the
chloride and sulfate induce interstitial cell tumours of the testis
in rats and mice.
Long-term inhalation studies in rats exposed to aerosols of
cadmium chloride, sulfate, and oxide fume and dust at low
concentrations demonstrated a high incidence of primary lung cancer
with evidence of a dose-response relationship. This has not so far
been shown in other animals.
There is evidence that long-term occupational exposure to
cadmium may contribute to the development of cancer of the lung, but
observations from exposed workers have been difficult to interpret
because of inadequate exposure data and confounding factors. The
evidence to date is inconclusive, but does not support the
suggestion from earlier studies that cadmium can cause prostatic
cancer.
IARC (1987a) considered that there was sufficient evidence for
the carcinogenicity of specified cadmium compounds in experimental
animals and limited evidence for carcinogenicity in humans exposed
to cadmium. A combined evaluation of human and animal data by IARC
(1987b) classified cadmium as a probable human carcinogen (IARC
group 2A). The IPCS Task Group found no reason to deviate from this
IARC evaluation.
9.2.6 Critical organ and critical effect
The kidney is the critical organ for chronic cadmium poisoning.
Within the kidney, the cortex is the site where the first adverse
effect occurs. Therefore, in assessing dose-response relationships,
the cadmium concentration in the kidney cortex is of prime
importance.
The critical effect is renal tubular dysfunction, which is most
often manifested as low molecular weight proteinuria. Animal studies
indicate that histological changes in the renal tubules occur at a
dose level lower than that needed to produce low molecular weight
proteinuria.
9.3 Critical concentration in the kidneys
9.3.1 In animals
Several studies with data on both cadmium concentrations in the
renal cortex and the occurrence of tubular damage were discussed in
section 7.2.1. The findings were summarized in Table 12. They showed
that histological tubular lesions or proteinuria was usually seen at
cadmium renal cortex levels of 200-300 mg/kg wet weight. In some
studies on rats, monkeys, horses, and birds, certain effects were
seen at lower levels.
As no dose-response data are given in most animal studies, it
may be assumed that these renal cortex levels correspond to a 50%
response rate (CC50). Naturally, the cadmium levels at which lower
response rates occur would be lower.
In studies on monkeys conducted in Japan, kidney cadmium levels
were related to dose and duration of exposure. At the two highest
dose levels, acute liver effects occurred. If one wishes to
establish a range of values for the critical concentration in
individuals at which a small but significant part of an exposed
population will show effects, animal studies indicate that a renal
cortex level of about 100-200 mg/kg is likely to coincide with such
a range. There is some evidence that the average critical
concentration (CC50) could be as high as 300 or 400 mg/kg for the
more severe signs of renal tubular damage, but such high levels
should not be used as a starting point for calculations of
"acceptable daily exposures".
9.3.2 In humans
Section 8.2.1.5 reviewed all available data from cases in which
both renal cortex cadmium levels and renal effects were measured.
Data from autopsies or biopsies have mainly been cross-sectional,
i.e. the renal cadmium concentrations and the effects were measured
more or less simultaneously. This has made it difficult to interpret
the data from a critical concentration point-of-view, as the cases
with the most severe cadmium-induced kidney dysfunction had the
lowest renal cadmium levels. Cadmium is lost from the kidney when
the damage progresses (section 6.5.1.2).
In vivo neutron activation analysis has provided a new tool
for establishing the human critical concentration of cadmium in the
renal cortex. Longitudinal studies measuring the renal cortex
cadmium concentration several times during continued exposure can
now be carried out. The cadmium level at which the first measurable
signs of renal tubular dysfunction occurs can be estimated. However,
only two studies using in vivo neutron activation have been
published to date, and both of them are cross-sectional.
The renal cadmium concentrations are disproportionately low
when the liver cadmium concentrations are high and renal effects
have developed. Of the several methods available to estimate the
average critical kidney concentration in these groups of exposed
workers, the method of preference assumes that the peak for renal
cortex cadmium level, plotted against liver cadmium, is equivalent
to the point where renal tubular dysfunction occurs. This results in
a value of 319 mg/kg tissue (based on a ratio of renal cortex
cadmium to whole kidney cadmium of 1.5). There is considerable
variance in the individual values, the 95% tolerance (which
corresponds to a confidence interval) being in the range ± 90 mg/kg
from the mean. Other studies, using similar assumptions, have
reported a value of 332 mg/kg, 10% of the workers having a peak
cadmium level of about 216 mg/kg tissue. A re-evaluation of the
original study resulted in a calculated cadmium level of about
200 mg/kg. It was concluded that for the purposes of dose-response
calculations, using a metabolic model, this concentration could be
used as a starting point for renal effects occurring in an exposed
population.
9.4 Dose-response relationships for renal effects
Two approaches can be used to estimate dose-response
relationships. One employs epidemiological data from industry and
the general environment studying associations between exposure and
response. The other begins with a critical concentration in the
kidney cortex and employs a metabolic model to calculate, on the
basis of certain given assumptions, the exposure that is required to
reach a critical concentration.
9.4.1 Evaluation based on data on industrial workers
Table 16 contains data from various group studies on cadmium
workers. In most of these studies, the dose measurements were based
on short sampling periods (hours or a few days). However, exposure
may have lasted for decades, levels usually being higher in the
past. The use of protective devices may also confound the picture.
As discussed in section 8.3.2, there are now several reports
available that show a clear exposure-response relationship between
cadmium in workplace air and the prevalence of proteinuria.
An increased prevalence of overt proteinuria, as measured by
sulfosalicylic acid, trichloroacetic acid, or quantitative
determination of total proteinuria, can occur after only 5-10 years
of exposure to approximately 100 µg cadmium/m3. If instead, the
increased excretion of low molecular weight proteins (more than 97.5
percentile of control group) is used as the critical effect, 10-20%
of workers would have this effect after a cumulative dose
corresponding to 10-20 years of exposure to 50 µg cadmium/m3.
These evaluations are all based on levels of total cadmium in
inhaled dust or air.
9.4.2 Evaluation based on data on the general population
As indicated in chapter 8, there exists a considerable amount
of information from epidemiological studies carried out on the
general population in Japan. It was shown that in some areas of high
cadmium exposure the prevalence of low molecular weight proteinuria
was significantly higher than in control areas. This may be
considered in relation to the known cadmium concentrations in rice
and the daily cadmium intakes in the affected areas (Tables 7 and
17). Contamination of drinking-water in some areas may be a
complicating factor (section 8.3.3).
Taking all of the data in section 8.3.3 together, it seems
that, when the most sensitive method for diagnosis of low molecular
weight proteinuria is applied, there is an association between
cadmium exposure and increased excretion of low molecular weight
proteins among some people over 50 years of age at a daily intake of
about 140-260 µg cadmium or a cumulative cadmium intake of about
2000 mg or more (for both men and women).
9.4.3 Evaluation based on a metabolic model and critical
concentrations
Using the data on critical concentrations and kinetic models of
cadmium metabolism, attempts have been made to calculate the
dose-response relationship for cadmium. Assuming that cadmium in the
kidney is accumulated in accordance with a one-compartment model and
that a third or a quarter of the body burden of cadmium is in the
kidney (and making certain other assumptions indicated in Tables 20
and 21), the daily cadmium intake via food and the occupational air
concentrations needed to reach the critical concentration have been
calculated (Tables 20 and 21).
As the values calculated in Tables 20 and 21 are for an average
person, not all of those exposed to these levels would have reached
the renal cortex cadmium concentration of 200 mg/kg tissue or their
individual critical concentration. Nevertheless, these calculations
produce values that are similar to the levels at which effects have
been observed, and the model approach may be a useful way to
quantify the response rates at levels lower than those easily
measurable.
Calculations have been reported of the relationship between
intake and response rates using the observed frequency distributions
of daily intake and renal cortex cadmium concentrations, and
utilizing multi-compartment metabolic model values in the same range
as those given in Tables 20 and 21. Further development of these
modelling techniques would be of value.
Using a single-compartment model for the accumulation of
cadmium in the kidney, the average daily intake that would give rise
to an average concentration of 200 mg/kg wet weight in the kidney
cortex at age 50 would be 260-480 µg/day, assuming 5%
gastrointestinal absorption, various biological half-times, and
different proportions of the body burden in the kidneys (Table 19).
Assuming a 10% absorption rate, the intake needed would be
140-260 µg per day. These estimates will vary depending on the body
weight estimates for different populations.
Table 20. Calculated daily cadmium intake via ingestion required by a
non-smoker to reach a kidney cortex concentration of 200 mg/kg
at age 50 (using a one-compartment model)a
Gastrointestinal Proportion of body Estimated half-time in kidney cortexb
absorption rate burden in kidney
(%) 17 years 30 years
5 one-third 365 µg (286 µg) 265 µg (208 µg)
10 182 µg (143 µg) 133 µg (104 µg)
5 one-quarter 486 µg (382 µg) 353 µg (277 µg)
10 243 µg (191 µg) 177 µg (139 µg)
a The data in the table are based on the following assumptions:
gastrointestinal absorption, either 5% or 10%;
half-time in kidney cortex, either 17 years or 30 years (as reported in
section 6.6.2);
one-third or one-quarter of body burden in the kidneys;
cadmium concentration in renal cortex 25% higher than renal average;
average weight of both kidneys at age 50 of 300 g for a 70-kg person
or 235 g for a 55-kg person;
average cadmium concentration in foodstuffs constant during
the last 50 years;
variation of daily intake with age has been disregarded since such
variation would influence the values by less than 10%.
b Data have been calculated for a 70-kg person; values in parentheses
are for a 55-kg person.
Table 21. Calculated concentration of cadmium in industrial air required
for a kidney cortex concentration of 200 mg/kg to be reacheda
Proportion of body burden Estimated half-time in kidney cortexb
in kidney
17 years 30 years
one-third 16 µg/m3 (13 µg/m3) 14 µg/m3 (11 µg/m3)
one-quarter 21 µg/m3 (17 µg/m3) 19 µg/m3 (15 µg/m3)
a The data in the table are based on the following assumptions:
those assumptions given in Table 20;
exposure time of 25 years;
225 working days per year;
10 m3 of air inhaled per day;
25% pulmonary absorption
b Data have been calculated for a 70-kg worker; values in parentheses
are for an average 55-kg person
10. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF
HUMAN HEALTH
10.1 Conclusions
The kidney is considered the critical target organ for the
general population as well as for occupationally exposed
populations. Chronic obstructive airway disease is associated with
long-term high-level occupational exposure by inhalation. There is
some evidence that such exposure to cadmium may contribute to the
development of cancer of the lung but observations from exposed
workers have been difficult to interpret because of confounding
factors.
10.1.1 General population
Food-borne cadmium is the major source of exposure for most
people. Average daily intakes from food in most areas not polluted
with cadmium are 10-40 µg. In polluted areas the value has been
found to be several hundred µg per day. In non-polluted areas,
uptake from heavy smoking may equal cadmium intake from food.
An association between cadmium exposure and increased urinary
excretion of low molecular weight proteins has been noted in humans
with a life-long daily intake of about 140-260 µg cadmium, or a
cumulative intake of about 2000 mg or more.
10.1.2 Occupationally exposed population
Occupational exposure to cadmium is mainly by inhalation but
includes additional intakes through food and tobacco. The total
cadmium level in air varies according to industrial hygiene
practices and type of workplace. There is an exposure-response
relationship between airborne cadmium levels and proteinuria. An
increase in the prevalence of low molecular weight proteinuria may
occur in workers after 10-20 years of exposure to cadmium levels of
about 20-50 µg/m3. In vivo measurement of cadmium in the liver
and kidneys of people with different levels of cadmium exposure have
shown that about 10% of workers with a kidney cortex level of
200 mg/kg and about 50% of people with a kidney cortex level of
300 mg/kg would have renal tubular proteinuria.
10.2 Recommendations for protection of human health
a) Measures to increase recycling of cadmium should be
systematically examined and promising ideas encouraged.
b) Information on the importance of minimizing waste discharge
of cadmium, particularly into surface waters, should be
supplied to countries.
c) Public health measures for protection from cadmium
exposures would be improved by:
i) collection of more data from countries on cadmium
levels in foodstuffs and the environment;
ii) determination of tissue cadmium levels and
monitoring of health parameters in non-exposed
populations and in those living near mines or
smelters or exposed to elevated levels of the metal
in foodstuffs;
iii) technical assistance to developing countries for
the training of staff, particularly for cadmium
analysis;
iv) development of means of reducing cadmium exposure
by, for instance, improved working conditions and
the dissemination of information on the proper use
of fertilizers (which sometimes contain high levels
of cadmium), techniques for the disposal of
cadmium-containing wastes, etc.
11. FURTHER RESEARCH
a) There is a need for improved analytical techniques for
measuring cadmium species and biological indicators of cadmium
exposure/toxicity, such as ß2-microglobulin, in various
matrices, and for international centres for quality assurance
and training.
b) The assessment of human exposure to cadmium from all media
needs to be improved by increased monitoring of cadmium levels
in the environment. Changes in cadmium levels with time are of
particular importance.
c) Populations with ß2-microglobinuria (both those in the
workplace and in the general environment) should be
longitudinally investigated to determine the nature, severity,
and prognosis of adverse health effects associated with this
finding. Further research is needed on ß2-microglobulin as a
biological indicator of exposure and effect.
d) International collaborative efforts should be encouraged to
examine further the role of cadmium in the development of human
cancer. Both the general population and industrial workers
should be studied with special emphasis on the development of a
common format for analysing and presenting data and the
collection of additional information on exposure to cadmium,
tobacco, and other confounding factors. Multiple exposures must
be considered. It is proposed that a collaborative study
coordinated by an international body (e.g., IARC) should
include the existing cohorts in order to obtain better exposure
data. It should also collect both exposure and effects data in
a standardized manner, so that the results of different studies
may be more readily compared. A further approach would be to
perform a collaborative prospective study identifying all those
workers who have shown evidence of an effect of cadmium on the
kidney and who would therefore be considered to have had
unusually heavy exposure. In such a study, both morbidity and
mortality data would be collected. Outcome would be studied not
only for cancer but also for sequelae to renal dysfunction.
e) Existing occupational cohorts should be linked, where
possible, to regional cancer registers to determine the
incidence of prostatic cancer (morbidity) in relation to
cadmium exposure.
f) To understand the mechanism(s) of cancer induction,
experimental studies on the bioavailability of cadmium at the
target site and the interactions between zinc and cadmium would
be of value. The role of metallothionein induction in the
target cells of the respiratory tract and its relationship to
such phenomena as DNA damage and repair and oncogene protein
structure would be of interest.
g) Further information on the long-term health consequences of
cadmium exposures in the general environment is essential, with
emphasis on renal dysfunction and other end-points such as
neurotoxicity and immunotoxicity.
h) Studies of the effects of cadmium on calcium-phosphorus
metabolism and bone density should be conducted on female
workers to clarify whether these workers are at special risk in
the occupational setting. The effect of cadmium on the placenta
and subsequent effects on the fetus, especially in multiple
pregnancies, need further study.
i) The effects of various nutritional deficiencies and of exposure
to other metals on the transport, accumulation, and toxicity of
cadmium should be investigated with special reference to bone
toxicity. These studies should be conducted in humans and
experimental animals with respect to age, sex, dose-dependence,
biological half-time, and estimation of critical concentration.
j) To provide additional scientific support for the assessment of
human health risks from cadmium exposure, studies in
experimental animals addressing the following issues should be
initiated:
* mechanism of cadmium transport into the cell and factors
controlling the process;
* mechanism of cadmium-induced toxicity with particular
emphasis on kidney and bone and the role(s) of
non-metallothionein-bound cadmium in these processes;
* mechanisms of cadmium-induced calcuria and the
relationship of this phenomenon to tubular proteinuria and
osteomalacia.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenic potential of cadmium was evaluated in 1976 by
the International Agency for Research on Cancer (IARC, 1976) and
re-evaluated in 1987 (IARC, 1987a). It was concluded in the
re-evaluation that there was limited evidence that cadmium and
cadmium compounds are carcinogenic in humans. Sufficient evidence
was available to show that cadmium and specified cadmium compounds
cause cancer in experimental animals. Cadmium was classified as a
probable human carcinogen (group 2A) (IARC, 1987a,b).
To prevent adverse pulmonary and renal effects the following
health-based limits for occupational exposure to cadmium fumes and
respirable dust were proposed by WHO (WHO, 1980): 250 µg Cd/m3 for
short-term exposures provided the recommended time-weighted average
(40 h/week) of 10 µg Cd/m3 is respected. It was further
recommended that control measures be applied when cadmium levels in
urine and blood of individuals exceed 5 µg Cd/g creatinine and
5 µg Cd/litre of whole blood, respectively.
A drinking-water guideline value of 0.005 mg/litre has been set
for cadmium by the World Health Organization (WHO, 1984).
Cadmium was evaluated by a WHO Working Group developing air
quality guidelines (WHO, 1987). Based on non-carcinogenic effects,
the following recommendations were made:
a) in rural areas, levels of < 1-5 ng/m3 should not be allowed
to increase, and
b) in urban and industrialized areas without agricultural
activities, levels of 10-20 ng/m3 may be tolerable. However,
increases in the present levels of airborne cadmium should not
be permitted (WHO, 1987).
At the thirty-third meeting of the Joint FAO/WHO Expert
Committee on Food Additives and Food Contaminants, the previous
recommendation was reaffirmed, i.e. the provisional tolerable weekly
cadmium intake of 400-500 µg for an adult should not be exceeded
(WHO, 1989).
Regulatory standards established by national bodies in several
countries and the EEC are summarized in the legal file of the
International Register of Potentially Toxic Chemicals (IRPTC, 1987).
REFERENCES
ADAMS, R.G. (1980) Osteopathy associated with tubular nephropathy in
employees in an alkaline battery factory. In: Shigematsu, I. &
Nomiyama, K., ed. Cadmium-induced osteopathy, Tokyo, Japan Public
Health Association, pp. 67-73.
ADAMS, R.C., HARRISON, J.G., & SCOTT, P. (1969) The development of
cadmium-induced proteinuria, impaired renal function, and
osteomalacia in alkaline battery workers. Q. J. Med., 38: 425-443.
ADAMSSON, E. (1979) Long-term sampling of air-borne cadmium dust in
an alkaline battery factory. Scand. J. Work Environ. Health,
5: 178-187.
ADES A.E. & KAZANTZIS, G. (1988) Lung cancer in a non-ferrous
smelter: the role of cadmium. Br. J. ind. Med., 45: 435-442.
AHLGREN, L. & MATTSON, S. (1981) Cadmium in man measured
in vivo by X-ray fluorescence analysis. Phys. Med. Biol.,
26: 19-26.
AHLMARK, S., AXELSSON, B., FRIBERG, L., & PISCATOR, M. (1961)
Further investigations into kidney function and proteinuria in
chronic cadmium poisoning. Int. Congr. occup. Health, 13: 201-203.
AHOKAS, R.A. & DILTS, P.V. (1979) Cadmium uptake by the rat embryo
as a function of gestational age. Am. J. Obstet. Gynecol.,
135: 219-222.
AKITA PREFECTURAL GOVERNMENT (1973) [Surveys on environmental
pollution due to heavy metals: Annual report of the Technical Centre
for Environmental Protection], Akita, Japan, Akita Prefectural
Government (Akita Prefecture Report No. 1: 1970 and 1971) (in
Japanese).
AL-HADDAD, I.K., CHETTLE, D.R., FLETCHER, J.G., & FREMLIN, J.H.
(1981) A transportable system for measurement of kidney cadmium
in vivo. Int. J. appl. Radiat. Isot., 32: 109-112.
ALLANSON, M. & DEANESLY, R. (1962) Observations on cadmium damage
and repair in rat testes and the effects on the pituitary
gonadotrophs. J. Endocrinol., 24: 453-462.
ALLOWAY, B.J., THORNTON, I., SMART, G.A., SHERLOCK, J.C., & QUINN,
M.J. (1988) Metal availability. Sci. total Environ., 75: 41-69.
ALT, F. (1981) [Comparative determination of cadmium in blood by
four different methods.] Fresenius Z. anal. Chem., 308: 137-142 (in
German).
ANDERSEN, A. (1979) [Lead, cadmium, copper and zinc in the Danish
diet], Copenhagen, Statens Levendsmiddelinstitut, 89 pp, (Report
No. 40) (in Danish).
ANDERSEN, O., HAGERSTRAND, I., & NORDBERG, G.F. (1982) Effect of the
chelating agent sodium tripolyphosphate on cadmium toxicity in mice.
Environ. Res., 29: 54-61.
ANDERSEN, O., RONNE, M., & NORDBERG, G.F. (1983) Effects of
inorganic metal salts on chromosome length in human lymphocytes.
Hereditas, 98: 65-70.
ANDERSON, C. & DANYLCHUK, K.D. (1979) Plasma levels of
immunoreactive parathyroid hormone in dogs chronically exposed to
low levels of cadmium chloride. J. environ. Pathol. Toxicol.,
2: 1151-1159.
ANDERSSON, A. (1977) Heavy metals in Swedish soils: on their
retention, distribution, and amounts. Swed. J. agric. Res., 7: 7-20.
ANDERSSON, A. & HAHLIN, M. (1981) Cadmium effects from phosphorus
fertilization in field experiments. Swed. J. agric. Res., 11: 3-10.
ANDERSSON, K., ELINDER, C.-G., HOGSTEDT, C., KJELLSTROM, T., &
SPANG, G. (1984) Mortality among cadmium workers in a Swedish
battery factory. In: Proceedings of the 4th International Cadmium
Conference, Munich, March 1983, London, Cadmium Association,
pp. 152-154.
ANDO, M., SHIMIZU, M., SAYATA, Y., TANIMURA, A., & TOBE, M. (1981)
The inhibition of vitamin D-stimulated intestinal calcium transport
in rats after continuous oral administration of cadmium. Toxicol.
appl. Pharmacol., 61: 297-301.
ANKE, M. & SCHNEIDER, H.J. (1974) [The trace element concentration
of human kidneys as a function of age and sex.] Z. Urol.,
67: 357-362 (in German).
ANKE, M., HENNIG, A., SCHNEIDER, H.J., GROPPEL, B., GRUN, M.,
PARTSCHEFELD, M., & LUDKE, H. (1976) [The influence of the toxic
element cadmium on the metabolism and health of animals and human.]
Math. naturwiss. Unterr. R 25: 241-261 (in German).
AOKI, A. & HOFFER, A.P. (1978) Re-examination of the lesions in rat
testis caused by cadmium. Biol. Reprod., 18: 579-591.
APOSTOLOV, CH. (1979) [The effects on the upper respiratory tract
from long-term work with cadmium.] In: [Proceedings of the Cadmium
Symposium, Jena, 1-3 August, 1977], Jena, Friedrich-Schiller
Universität, pp. 322-325 (in German).
ARMSTRONG, B.G. & KAZANTZIS, G. (1983) The mortality of cadmium
workers. Lancet, 1: 1424-1427.
ARMSTRONG, B.G. & KAZANTZIS, G. (1985) Prostatic cancer and chronic
respiratory and renal disease in British cadmium workers: a case
control study. Br. J. ind. Med., 42: 540-545.
ASOKAN, P., DIXIT, R., MUKHTAR, H., & MURTI, C.R.K. (1981) Effect of
cadmium on hepatic mixed function oxidases during the early
development of rats; possible protective role of metallothionein.
Biochem. Pharmacol., 30(22): 3095-3098.
AUFDERHEIDE, M., MOHR, U., THIEDEMANN, K.-U., & HEINRICH, U. (1990)
Quantification of hyperplastic areas in hamster lungs after chronic
inhalation of different cadmium compounds. Toxicol. environ. Chem.,
27: 173-180.
AXELSSON, B. (1963) Urinary calculus in long-term exposure to
cadmium. In: Sangro, P., Akoun, G., & Beate, H.L., ed. Proceedings
of the 14th International Congress on Occupational Health, Madrid,
Amsterdam, Excerpta Medica Foundation, pp 939-942 (International
Congress Series No. 62).
AXELSSON, B. & PISCATOR, M. (1966a) Renal damage after prolonged
exposure to cadmium: an experimental study. Arch. environ. Health,
12: 360-373.
AXELSSON, B. & PISCATOR, M. (1966b) Serum proteins in
cadmium-poisoned rabbits with special reference to haemolytic
anaemia. Arch. environ. Health, 12: 374-381.
AXELSSON, B., DAHLGREN, S.E., & PISCATOR, M. (1968) Renal lesions in
the rabbit after long-term exposure to cadmium. Arch. environ.
Health, 17: 24-28.
BAADER, E.W. (1951) [Health concerns and occupational medicine:
chronic cadmium poisoning.] Dtsch. med. Wschr., 76: 484-487 (in
German).
BAETZ, R.A. & KENNER, C.T. (1974) Determination of low levels of
cadmium in foods using a chelating ion exchange resin. J. Assoc.
Off. Anal. Chem., 57: 14-17.
BARLOW, S.M. & SULLIVAN, F.M. (1982) Cadmium and its compounds. In:
Barlow, S.M., ed. Reproductive hazards of industrial chemicals. An
evaluation of animal and human data, New York, London, Academic
Press, pp. 137-173.
BARLTROP, D. & STREHLOW, C.D. (1982a) Clinical and biochemical
indices of cadmium exposure in the population of Shipham. In:
Proceedings of the 3rd International Cadmium Conference, Miami,
Florida, 3-5 February, 1981, London, Cadmium Association,
pp. 112-113.
BARLTROP, D. & STREHLOW, C.D. (1982b) Cadmium and health in Shipham.
Lancet, 2: 1394-1395.
BARR, M., Jr (1973) The teratogenicity of cadmium chloride in two
stocks of Wistar rats. Teratology, 7: 237-242.
BARRETT, H.M. & CARD, B.Y. (1947) Studies on the toxicity of inhaled
cadmium. II. The acute lethal dose of cadmium oxide for man. J. ind.
Hyg. Toxicol., 29: 286-293.
BARRETT, H.M., IRWIN, D.A., & SEMMONS, E. (1947) Studies on the
toxicity of inhaled cadmium. I. The acute toxicity of cadmium oxide
by inhalation. J. ind. Hyg. Toxicol., 29: 279-285.
BARTHELEMY, P. & MOLINE, R. (1946) Intoxication chronique par
l'hydrate de cadmium, son signe précoce: La bague jaune dentaire.
Paris Med., 1: 71-78.
BAUCHINGER, M., SCHMID, E., EINBRODT, H.J., & DRESP, J. (1976)
Chromosome aberrations in lymphocytes after occupational exposure to
lead and cadmium. Mutat. Res., 40: 57-62.
BECKMAN, I., MOVITZ, I., NYGREN, M., & SLORACH, S. (1979) [Release
of lead and cadmium from glazed and enamelled foodware.] Var Föda,
31: 193-197 (in Swedish).
BEHNE, D. (1980) Problems of sampling and sample preparation for
trace element analysis in the health sciences. In: Bratter, P. &
Schramel, P., ed. Trace element analytical chemistry in medicine and
biology. Proceedings of the First International Workshop,
Neuherberg, Federal Republic of Germany, Berlin, New York, Walter de
Gruyter & Co., pp. 769-782.
BERLIN, M. & FRIBERG, L. (1960) Bone marrow activity and erythrocyte
destruction in chronic cadmium poisoning. Arch. environ. Health,
1: 478-486.
BERMAN, E. (1967) Determination of Cd, Tl, and Hg in biological
materials by AA. At. Absorpt. Newsl., 6: 57-60.
BERNARD, A.M., ROELS, H., HUBERMONT, G., BUCHET, J.P., MASSON, P.L.,
& LAUWERYS, R. (1976) Characterization of the proteinuria in
cadmium-exposed workers. Int. Arch. occup. environ. Health,
38: 19-30.
BERNARD, A.M., BUCHET, J.P., ROELS, H., MASSON, P., & LAUWERYS, R.
(1979) Renal excretion of proteins and enzymes in workers exposed
to cadmium. Eur. J. clin. Invest., 9: 11-22.
BERNARD, A.M., LAUWERYS, R., & GENGOUX, P. (1981) Characterization
of the proteinuria induced by prolonged oral administration of
cadmium in female rats. Toxicology, 20: 345-357.
BERNARD, A.M., MOREAU, D., & LAUWERYS, R. (1982a) Latex immunoassay
of retinol binding protein. Clin. Chem., 28: 1167-1171.
BERNARD, A.M., MOREAU, D., & LAUWERYS, R. (1982b) Comparison of
retinol-binding protein and ß-2-microglobulin determination in urine
for the early detection of tubular proteinuria. Clin. Chim. Acta,
126: 1-9.
BETON, D.C., ANDREWS, G.S., DAVIES, H.J., HOWELLS, L., & SMITH, G.F.
(1966) Acute cadmium fume poisoning: five cases with one death from
renal necrosis. Br. J. ind. Med., 23: 292-301.
BHATTACHARYYA, M.H., WHELTON, B.D., PETERSON, D.P., CARNES, B.A.,
MORETTI, E.S., TOOMEY, J.M., & WILLIAMS, L.L. (1988) Skeletal
changes in multiparous mice fed a nutrient-sufficient diet
containing cadmium. Toxicology, 50: 193-204.
BIGGIN, H.C., CHEN, N.S., ETTINGER, K.V., FREMLIN, J.H., MORGAN,
W.D., NOWOTNY, R., CHAMBERLAIN, M.J., & HARVEY, T.C. (1974) Cadmium
by in vivo neutron activation analysis. J. radioanal. Chem.,
19: 207-214.
BJORNBERG, A. (1963) Reactions to light in yellow tattoos from
cadmium sulfide. Arch. Dermatol., 88: 267-271.
BLEJER, H.P. (1966) Death due to cadmium oxide fumes. Ind. Med.
Surg., 35: 363-364.
BOISSET, M. & BOUDENE, C. (1981) Effect of a single exposure to
cadmium oxide fumes on rat lung microsomal enzymes. Toxicol. appl.
Pharmacol., 57: 335-345.
BONNELL, J.A. (1955) Emphysema and proteinuria in men casting
copper-cadmium alloys. Br. J. ind. Med., 12: 181-197.
BONNELL, J.A., KAZANTZIS, G., & KING, E. (1959) A follow-up study of
men exposed to cadmium oxide fume. Br. J. ind. Med., 16: 135-147.
BONNELL, J.A., ROSS, J.H., & KING, E. (1960) Renal lesions in
experimental cadmium poisoning. Br. J. ind. Med., 17: 69-80.
BOULEY, G., DUBREUIL, A., DESPAUX, N., & BOUDENE, C. (1977) Toxic
effects of cadmium microparticles on the respiratory system. An
experimental study on rats and mice. Scand J. Work Environ. Health,
3: 116-121.
BOYLE, E.A., SCALTER, F., & EDMOND, J.M. (1976) On the marine
geochemistry of cadmium. Nature (Lond.), 263: 42-44.
BOZELKA, B.E. & BURKHOLDER, P.M. (1982) Inhibition of mixed
leukocyte culture response in cadmium-treated mice. Environ. Res.,
27: 421-432.
BRADY, F.O. (1982) The physiological function of metallothionein.
Trends biochem. Sci., 7: 143-145.
BRAUX, P., CLAEYS-THOREAU, F., DE PLAAN, P., DUCOFFRE, G., AMERY,
A., LAUWERYS, R., RONDIA, D., STAESSEN, J., ROELS, H., SARTON, F.,
BERNARD, A., & BUCHET, J.P. (1987) The Belgian Cadmibel Study:
Environmental exposure to cadmium and lead-health effects. In:
Lindberg, S.F. & Hutchinson, J.C., ed. Proceedings of an
International Conference on Heavy Metals in the Environment, New
Orleans, Edinburgh, CEP Consultants Ltd, pp. 349-351.
BRYAN, G.W., LANGSTON, W.J., & HUMMERSTONE L.G. (1980) The use of
biological indicators of heavy-metal contamination in estuaries with
special reference to an assessment of the biological availability of
metals in estuarine sediments from south-west Britain, Citadel Hill,
Devon, The Marine Biological Association of the United Kingdom,
73 pp (Occasional Publication No. 1).
BUATMENARD, P. & ARNOLD, M. (1978) Heavy-metal chemistry of
atmospheric particulate matter emitted by Mount Etna volcano. Geogr.
Res. Lett., 5: 245-248.
BUCHAUER, M.J. (1972) The effects of zinc and cadmium pollution on
forest vegetation. Bull. New Jersey Acad. Sci., 17(2): 42.
BUCHET, J.P., ROELS, H., BERNARD, A., & LAUWERYS, R. (1980)
Assessment of renal function of workers exposed to inorganic lead,
cadmium or mercury vapor. J. occup. Med., 22: 741-748.
BUCHET, J.P., ROELS, H., LAUWERYS, R., VANDEVOORDE, A., & PYCKE,
J.M. (1983) Oral daily intake of cadmium, lead, manganese, copper,
chromium, mercury, calcium, zinc, and arsenic in Belgium: a
duplicate meal study. Food chem. Toxicol., 21: 19-24.
BUCKE, D., NORTON, M.G., & ROLFE, M.S. (1983) Field assessment of
effects of dumping wastes at sea: II. Epidermal lesions and
abnormalities of fish in the outer Thames estuary, London, Ministry
of Agriculture, Fisheries and Food, 29 pp (Technical Report No. 72).
BUI, T.-H-. LINDSTEN, J., & NORDBERG, G.F. (1975) Chromosome
analysis of lymphocytes from cadmium workers and Itai-itai patients.
Environ. Res., 9: 187-195.
BUS, J.S., VINEGAR, A., & BROOKS, S.M. (1978) Biochemical and
physiologic changes in lungs of rats exposed to a cadmium chloride
aerosol. Am. Rev. respir. Dis., 118: 573-580.
BUTLER, E.A. & FLYNN, F.V. (1958) The proteinuria of renal tubular
disorders. Lancet, 2: 978-980.
BUXTON, R., St J. (1956) Respiratory function in cadmium alloy
casters. Part 2. The estimation of the total lung volume, its
subdivisions and the mixing coefficient. Br. J. ind. Med.,
13: 36-40.
CARLIER, Y., COLLE, A., TACHON, P., BOUT, D., & CAPRON, A. (1981)
Quantification of B-2-microglobulin by enzyme inhibition
immunoassay. J. immunol. Methods, 40: 231-238.
CARLSON, L.A. & FRIBERG, L. (1957) The distribution of cadmium in
blood after repeated exposure. Scand. J. clin. lab. Invest., 9: 1-4.
CARLTON-SMITH, C.H. (1987) Effects of metals in sludge-treated soils
on crops, Medmenham, United Kingdom, Water Research Centre
(Environment TR/251).
CARROLL, R.E. (1966) The relationship of cadmium in the air to
cardiovascular disease death rates. J. Am. Med. Assoc.,
198: 267-269.
CARRUTHERS, M. & SMITH, B. (1979) Evidence of cadmium toxicity in a
population living in a zinc-mining area. Lancet, 1: 845-847.
CARVALHO, F.M., TAVARES, T.M., SILVANY-NETO, A.M., LIMA, M.E.C., &
ALT, F. (1986) Cadmium concentrations in blood of children living
near a lead smelter in Bahia, Brazil. Environ. Res., 40: 437-449.
CASTERLINE, J.L. & YIP, G. (1975) The distribution and binding of
cadmium in oyster, soybean, rat liver, and kidney. Arch. environ.
Contam. Toxicol., 3: 319-329.
CASTILHO, P.D. & HERBER, R.F.M. (1977) The rapid determination of
cadmium, lead, copper, and zinc in whole blood by atomic absorption
spectrometry with electrothermal atomization. Improvements in
precision with a peak-shape monitoring device. Anal. Chim. Acta,
94: 269-270.
CHADWICK, M.J. & LINDMAN, N. (1982) Environmental implications of
expanded coal utilization, Oxford, New York, Pergamon Press, 283 pp.
CHAPATWALA, K.D., HOBSON, M., DESAIAH, D., & RAJANNA, B. (1982)
Effect of cadmium on hepatic and renal gluconeogenic enzymes in
female rats. Toxicol. Lett., 12: 27-34.
CHAUBE, S., NISHIMURA, H., & SWINYARD, C.A. (1973) Zinc and cadmium
in normal human embryos and fetuses. Arch. environ. Health,
26: 237-240.
CHERIAN, M.G. (1980) The synthesis of metallothionein and cellular
adaptation to metal toxicity in primary kidney epithelial cell
cultures. Toxicology, 17: 225-231.
CHERIAN, M.G. & GOYER, R.A. (1978) Metallothioneins and their role
in the metabolism and toxicity of metals. Life Sci., 23: 1-10.
CHERIAN, M.G. & NORDBERG, M. (1983) Cellular adaptation in metal
toxicology and metallothionein. Toxicology, 28: 1-15.
CHERIAN, M.G. & SHAIKH, Z.A. (1975) Metabolism of
intravenously-injected cadmium-binding protein. Biochem. biophys.
Res. Commun., 65: 863-869.
CHERIAN, M.G. & VOSTAL, J.J. (1977) Biliary excretion of cadmium in
rat. I. Dose-dependent biliary excretion and the form of cadmium in
the bile. J. Toxicol. environ. Health, 2: 945-954.
CHERIAN, M.G., GOYER, R.A., & RICHARDSON, L.D. (1976) Cadmium
metallothionein-induced nephropathy. Toxicol. appl. Pharmacol.,
38: 399-408.
CHERIAN, M.G., GOYER, R.A., & VALBERG, L.S. (1978) Gastrointestinal
absorption and organ distribution of oral cadmium chloride and
cadmium metallothionein in mice. J. Toxicol. environ. Health,
4: 861-868.
CHERNOFF, N. (1973) Teratogenic effects of cadmium in rats.
Teratology, 8: 29-32.
CHERRY, W.H. (1981) Distribution of cadmium in human tissues. In:
Nriagu, J.O., ed. Cadmium in the environment - II, New York,
Chichester, John Wiley & Sons, pp. 111-122.
CHIQUOINE, A.D. (1965) Effect of cadmium chloride on the pregnant
albino mouse. J. Reprod. Fertil., 10: 263-265.
CHRISTOFFERSON, J.O. & MATTSON, S. (1983) Polarized X-rays in
XRF-analysis for improved in vivo detectability of cadmium in man.
Phys. Med. Biol., 28: 1135-1144.
CHUMBLEY, C.G. & UNWIN, R.J. (1982) Cadmium and lead content of
vegetable crops grown on land with a history of sewage sludge
application. Environ. Pollut. Ser. B Chem. Phys., 4(3): 231-238.
CHUNG, J., NARTY, N.O., & CHERIAN, M.G. (1986) Metallothionein
levels in liver and kidney of Canadians - A potential indicator of
environmental exposure to cadmium. Arch. environ. Health, 41(5):
319-323.
CIRKT, M. & TICHY, M. (1974) Excretion of cadmium through bile and
intestinal wall in rats. Br. J. ind. Med., 31: 134-139.
CLARKSON, T.W. & KENCH, J.E. (1956) Urinary excretion of amino acids
by men absorbing heavy metals. Biochem. J., 62: 361-372.
CNATTINGIUS, S., AXELSSON, O., EKLUND, G., & LINDMARK, G. (1985)
Smoking, maternal age and fetal growth. Obstet. Gynecol.,
66: 449-452.
COLE, G.M. & BAER, L.S. (1944) Food poisoning from cadmium. US nav.
med. Bull., 43: 398-399.
COMMISSION OF THE EUROPEAN COMMUNITIES (1978) Criteria (dose-effect
relationships) for cadmium, Oxford, Pergamon Press, 202 pp (Report
of the Working Group of Experts prepared for the CEC,
Directorate-General for Social Affairs, Health and Safety
Directorate, Luxembourg).
COOPERATIVE RESEARCH COMMITTEE ON ITAI-ITAI DISEASE (1967) [Report
on a study on the so-called Itai-itai disease], Tokyo, Ministry of
Education and Ministry of Health and Welfare, 206 pp (in Japanese).
COPIUS-PEEREBOOM, J.W., DE VOOGT, P., VAN HATTUM, B., VAN DER VELDE,
W., & COPIUS-PEEREBOOM-STEGEMAN, J.H.J. (1979) The use of the human
placenta as a biological indicator for cadmium exposure. In:
International Conference on Management and Control of Heavy Metals
in the Environment, London, September, 1979, Edinburgh, CEP
Consultants Ltd, pp. 8-10.
COUSINS, R.J., BARBER, A.K., & TROUT, J.R. (1973) Cadmium toxicity
in growing swine. J. Nutr., 103: 964-972.
CULBARD, E.B., THORNTON, I., WATT, J., WHEATLEY, M., MOORCROFT, S.,
& THOMPSON, M. (1988) Metal contamination in British urban dusts and
soils. J. environ. Qual., 17(2): 227-234.
CVETKOVA, R.P. (1970) [Materials on the study of the influence of
cadmium compounds on the generative function.] Gig. Tr. prof.
Zabol., 14: 31-33 (in Russian with English summary).
DALHAMN, T. & FRIBERG, L. (1954) The effect of cadmium on blood
pressure and respiration and the use of dimercaprol (BAL) as
antidote. Acta pharmacol. toxicol., 10: 199-203.
DANIELSSON, L.-G., JAGNER, D., JOSEFSON, M., & WESTERLUND, S. (1981)
Computerized potentiometric stripping analysis for the determination
of cadmium, lead, copper, and zinc in biological materials. Anal.
Chim. Acta, 127: 147-156.
DASTON, G.P. (1982) Fetal zinc deficiency on a mechanism for
cadmium-induced toxicity to the developing rat lung and pulmonary
surfactant. Toxicology, 24: 55-63.
DAVIES, N.T., BREMNER, I., & MILLS, C.F. (1973) Studies on the
induction of a low molecular weight zinc-binding protein in rat
liver. Biochem. Soc. Trans., 1: 985-988.
DAVIS, R.D. (1984) Cadmium - a complex environmental problem. Part
II. Cadmium in sludges used as fertilizer. Experientia (Basel),
40(2): 117-126.
DAVIS, R.D. & COKER, E.G. (1980) Cadmium in agriculture, with
special reference to the utilisation of sewage sludge on land,
Medmenham, United Kingdom, Water Research Centre (Technical Report
TR/139).
DAVISON, A.G., NEWMAN-TAYLOR, A.J., DARBYSHIRE, J., & CHETTLE, D.R.
(1988) Cadmium fume inhalation and emphysema. Lancet, 1: 663-667.
DEAVEN, L.L. & CAMPBELL, E.W. (1980) Factors affecting the induction
of chromosomal aberrations by cadmium in Chinese hamster cells.
Cytogenet. cell Genet., 26: 251-260.
DECKER, L.E., BYERRUM, R.U., DECKER, C.F., HEPPERT, C.A., & LANGHAM,
R.F. (1958) Chronic toxicity studies. I. Cadmium administered in
drinking-water to rats. Am. Med. Assoc. Arch. ind. Health,
18: 228-231.
DEKNUDT, G. & DEMINATTI, M.L. (1978) Chromosome studies in human
lymphocytes after in vitro exposure to metal salts. Toxicology,
10: 67-75.
DEKNUDT, G. & LEONARD, A. (1975) Cytogenetic investigations on
leukocytes of workers from a cadmium plant. Environ. Physiol.
Biochem., 5: 319-327. DELVES, H.T. (1970) Micro-sampling method for
the rapid determination of lead in blood by atomic absorption
spectrophotometry. Analyst, 95: 431-438.
DELVES, H.T. (1982) Analytical techniques for measuring cadmium in
blood. In: Proceedings of the International Workshop on Biological
Indicators on Cadmium Exposure Diagnostic and Analytical
Reliability, Luxembourg, 7-9 July, 1982, Luxembourg, Commission of
the European Communities, pp. 1-15.
DELVES, H.T. & WOODWARD, J. (1981) Determination of low levels of
cadmium in blood by electrothermal atomization and atomic absorption
spectrophotometry. At. Spectrosci., 2: 65-67.
DENCKER, L., DANIELSSON, B., KHAYAT, A., & LINDGREN, A. (1983)
Disposition of metals in the embryo and fetus. In: Clarkson, T.W.,
Nordberg, G.F., & Sager, P.R., ed. Reproductive and developmental
toxicity of metals, New York, London, Plenum Press, pp. 607-632.
DERVAN, P.A. & HAYES, J.A. (1979) Peribronchiolar fibrosis following
acute experimental lung damage by cadmium aerosol. J. Pathol.,
128: 143-148.
DE SILVA, P.E. & DONNAN, M.B. (1981) Chronic cadmium poisoning in a
pigment-manufacturing plant. Br. J. ind. Med., 38: 76-86.
DUCE, R.A., HOFFMAN, G.L., & ZELLER, W.H. (1975) Atmospheric trace
metals at remote northern and southern hemisphere sites: pollution
or natural(?). Science, 187: 59-61.
DUCE, R.A., ARIMOTO, R., RAY, B.J., UNNI, C.K., & HARDER, P.J.
(1983) Atmospheric trace elements at Enewetok Atoll. I.
Concentration, sources, and temporal variability. J. geophys. Res.,
88: 5321-5342.
DUDLEY, R.E., SVOBODA, D.J., & KLAASSEN, C.O. (1982) Acute exposure
to cadmium causes severe liver injury in rats. Toxicol. appl.
Pharmacol., 65: 302-313.
DUDLEY, R.E., GAMMAL, L.M., & KLAASSEN, C.D. (1985) Cadmium-induced
hepatic and renal injury in chronically exposed rats: likely role of
hepatic cadmium-metallothionein in nephrotoxicity. Toxicol. appl.
Pharmacol., 77: 414-426.
DUGGAN, M.J., INSKIP, M.J., RUNDLE, S.A., & MOORCROFT, J.S. (1985)
Lead in playground dust and on the hands of schoolchildren. Sci.
total Environ., 44(1): 65-79.
DUNPHY, B. (1967) Acute occupational cadmium poisoning: a critical
review of the literature. J. occup. Med., 9: 22-26.
EDIGER, R.D. & COLEMAN, R.L. (1973) Determination of cadmium in
blood by a Delves cup technique. At. Absorpt. Newsl., 12: 3-6.
EDLING, C., ELINDER, C.G., & RANDMA, E. (1986) Lung function in
workers using cadmium containing solders. Br. J. ind. Med.,
43: 657-662.
ELINDER, C.-G. & PANNONE, M. (1979) Biliary excretion of cadmium.
Environ. Health Perspect., 28: 123-126.
ELINDER, C.-G. & PISCATOR, M. (1978) Cadmium and zinc in horses. In:
Kirchgessner, M., ed. Proceedings of the 3rd International
Conference on Trace Elements in Man and Animals,
Freising-Weihenstephan, Federal Republic of Germany, 25-29 July,
1977, Freising-Weihenstephan, Munich Technical University,
pp. 569-572.
ELINDER, C.-G., KJELLSTRÖM, T., LIND, B., & LINNMAN, L. (1976)
Cadmium concentration in kidney cortex, liver, and pancreas among
autopsied Swedes. Arch. environ. Health, 31: 292-302.
ELINDER, C.-G., KJELLSTRÖM, T., LINNMAN, L., & PERSHAGEN, G. (1978)
Urinary excretion of cadmium and zinc among persons from Sweden.
Environ. Res., 15: 473-484.
ELINDER, C.-G., JONSSON, L., PISCATOR, M., & RAHNSTER, B. (1981a)
Histopathological changes in relation to cadmium concentration in
horse kidneys. Environ. Res., 26: 1-21.
ELINDER, C.-G., NORDBERG, M., PALM, B., & PISCATOR, M. (1981b)
Cadmium, zinc, and copper in horse kidney and in horse liver
metallothionein: comparison with kidney cortex. Environ. Res.,
26: 22-32.
ELINDER, C.-G., KJELLSTRÖM, T., LIND, B., LINNMAN, L., PISCATOR, M.,
& SUNDSTEDT, K. (1983) Cadmium exposure from smoking cigarettes:
variations with time and country where purchased. Environ. Res.,
32: 220-227.
ELINDER, C.-G., EDLING, C., LINDBERG, E., KAGEDAL, B., & VESTERBERG,
O. (1985a) Assessment of renal function in workers previously
exposed to cadmium. Br. J. ind. Med., 42: 754-760.
ELINDER, C.-G., EDLING, C., LINDBERG, E., KAGEDAL, B., & VESTERBERG,
O. (1985b) B-2-microglobulinuria among workers previously exposed
to cadmium: follow-up and dose-response analyses. Am. J. ind. Med.,
8: 553-564.
ELINDER, C.-G., KJELLSTRÖM, T., HOGSTEDT, C., ANDERSON, K., & SPENG,
G. (1985c) Cancer mortality of cadmium workers. Br. J. ind. Med.,
42: 651-655.
ELLIS, K.J., MORGAN, W.D., ZANZI, I., YASUMURA, S., VARTSKY, D.D., &
COHN, S.H. (1981a) Critical concentrations of cadmium in human renal
cortex: dose-effect studies in cadmium smelter workers. J. Toxicol.
environ. Health, 7: 691-703.
ELLIS, K.J., YASAMURA, S., & COHN, S.H. (1981b) Hair cadmium
content: is it a biological indicator of the body burden of cadmium
for the occupationally-exposed worker? Am. J. ind. Med., 2: 323-333.
ELLIS, K.J., YUEN, K., YASAMURA, S., & COHN, S.H. (1984)
Dose-response analysis of cadmium in man: body burden vs kidney
dysfunction. Environ. Res., 33: 216-226.
ELLIS, K.J., COHN, S.H., & SMITH, T.J. (1985) Cadmium inhalation
exposure estimates: their significance with respect to kidney and
liver cadmium burden. J. Toxicol. environ. Health, 15: 173-187.
ENGSTROM, B. (1979) Factors influencing metabolism and toxicity of
cadmium. Experimental studies on mice with special reference to
chelating agents, Stockholm, Department of Environmental Hygiene,
Karolinska Institute, 80 pp (Doctoral thesis). ENGSTROM, B. &
NORDBERG, G.F. (1978) Effects of detergent formula chelating agents
on the metabolism and toxicity of cadmium in mice. Acta pharmacol.
toxicol., 43: 387-397.
ENGSTROM, B. & NORDBERG, G.F. (1979a) Dose dependence of
gastrointestinal absorption and biological half-time of cadmium in
mice. Toxicology, 13: 215-222.
ENGSTROM, B. & NORDBERG, G.F. (1979b) Factors influencing absorption
and retention of oral 109Cd in mice: age, pretreatment, and
subsequent pretreatment with non-radioactive cadmium. Acta
pharmacol. toxicol., 45: 315-324.
ENGVALL, J. & PERK, J. (1985) Prevalence of hypertension among
cadmium-exposed workers. Arch. environ. Health, 40: 185-190.
ESSING, H.-G., SCHALLER, K.-H., SZADOWSKI, D., & LEHNERT, G. (1969)
[Normal cadmium load on man from food and beverages.] Arch. Hyg.
Bakteriol., 153: 490-494 (in German).
EVRIN, P.E., PETERSON, P.A., WIDE, L., & BERGGARD, I. (1971)
Radioimmuno-assay of ß-2-microglobulin in human biological fluids.
Scand. J. clin. lab. Invest., 28: 439-443.
EYBL, V. & SYKORA, J. (1966) [The protection by chelating agents in
acute cadmium poisoning.] Acta biol. med. Germ., 16: 61-64 (in
German).
FELDMAN, S.L. & COUSINS, R.G. (1973) Influence of cadmium on the
metabolism of 25-hydroxycholecalciferol in chicks. Nutr. Rep. Int.,
8: 251-259.
FELTEN, T.L. (1979) A preliminary report of cadmium-induced
chromosomal changes in somatic and germinal tissues of C57BI/6J male
mice. Genetics, 88: 26-27.
FERM, V.H. (1971) Developmental malformations induced by cadmium.
Biol. Neonat., 19: 101-107.
FERM, V.H. (1972) The teratogenic effects of metals on mammalian
embryos. Adv. Teratol., 5: 51-75.
FERM, V.H. & CARPENTER, S.J. (1968) The relationship of cadmium and
zinc in experimental mammalian teratogenesis. Lab. Invest.,
18: 429-432.
FERNANDEZ, F.J. & KAHN, H.L. (1971) The determination of lead in
whole blood by atomic absorption spectrophotometry with the "Delves
Sampling Cup" technique. At. Absorpt. Newsl., 10: 1-5.
FINGERLE, H., FISCHER, G., & CLASSEN, H.G. (1982) Failure to produce
hypertension in rats by chronic exposure to cadmium. Food chem.
Toxicol., 20: 301-306.
FITZHUGH, O.G. & MEILLER, F.H. (1941) The chronic toxicity of
cadmium. J. Pharmacol. exp. Ther., 72: 15-20.
FLANAGAN, P.R., MCLELLAN, J.S., HAIST, J., CHERIAN, M.G.,
CHAMBERLAIN, M.J., & VALBERG, L.S. (1978) Increased dietary cadmium
absorption in mice and human subjects with iron deficiency.
Gastroenterology, 74: 841-846.
FLETCHER, J.C., CHETTLE, D.R., & AL-HADDAD, I.K. (1982) Experience
with the use of cadmium measurements of liver and kidney. J.
radioanal. Chem., 71: 547-560.
FORSTNER, U. (1980) Cadmium in the environment, Part I. In: Nriagu,
J.O., ed. Cadmium in polluted sediments, New York, Chichester, John
Wiley & Sons, pp. 305-363.
FOULKES, E.C. (1982) Role of metallothionein in transport of heavy
metals. In: Foulkes, E.C., ed. Biological roles of metallothionein,
Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp. 131-140.
FOWLER, B.A. & NORDBERG, G.F. (1978) The renal toxicity of cadmium
metallothionein: morphometric and X-ray microanalytical studies.
Toxicol. appl. Pharmacol., 46: 609-623.
FOWLER, B.A., JONES, H.S., BROWN, H.W., & HASEMAN, J.K. (1975) The
morphological effects of chronic cadmium administration on the renal
vasculature of rats given low and normal calcium diets. Toxicol.
appl. Pharmacol., 34: 233-252.
FOX, M.R.S. & FRY, B.E., Jr (1970) Cadmium toxicity decreased by
dietary ascorbic acid supplements. Science, 169: 989-991.
FOX, M.R.S., FRY, B.E., Jr, HARLAND, B.F., SCHERTAL, M.E., & WEEKS,
C.E. (1971) Effect of ascorbic acid on cadmium toxicity in the
young coturnix. J. Nutr., 101: 1295-1305.
FRANCAVILLA, S., MOSCARDELLI, S., FRANCAVILLA, F., CASASANTA, N.,
PROPERZI, G., MARTINI, M., & SANTIEMMA, V. (1981) Acute cadmium
intoxication: influence of cyproterone acetate on the testis and
epididymis of the rat. Arch. Andrology, 6: 1-11.
FRANK, A., PETERSON, L., & MORNER, T. (1981) [Lead and cadmium
levels in organs from moose, deer, and hare.] Svensk
Veterinaertidn., 33: 151-156 (in Swedish).
FRIBERG, L. (1948a) Proteinuria and kidney injury among workmen
exposed to cadmium and nickel dust. J. ind. Hyg. Toxicol.,
30: 32-36.
FRIBERG, L. (1948b) Proteinuria and emphysema among workers exposed
to cadmium and nickel dust in a storage battery plant. Proc. Int.
Congr. Ind. Med., 9: 641-644.
FRIBERG, L. (1950) Health hazards in the manufacture of alkaline
accumulators with special reference to chronic cadmium poisoning.
Acta med. Scand., 138(Suppl. 240): 1-124.
FRIBERG, L. (1952) Further investigations on chronic cadmium
poisoning. A study on rabbits with radioactive cadmium. Arch. ind.
occup. Med., 5: 30-36.
FRIBERG, L. (1955) Iron and liver administration in chronic cadmium
poisoning and studies on the distribution and excretion of cadmium.
Experimental investigations in rabbits. Acta pharmacol.,
11: 168-178.
FRIBERG, L. (1979) Cadmium in Shipham. Lancet, 1: 823-824.
FRIBERG, L. (1988) Biological monitoring of toxic metals, New York,
London, Plenum Press, pp. 103-126.
FRIBERG, L. & NYSTROM, A. (1952) [Aspects on the prognosis of
chronic cadmium poisoning.] Läkartidningen, 49: 2629-2639 (in
Swedish).
FRIBERG, L. & VAHTER, M. (1983) Assessment of exposure to lead and
cadmium through biological monitoring: results of a UNEP/WHO global
study. Environ. Res., 30: 95-128.
FRIBERG, L., PISCATOR, M., NORDBERG, G., & KJELLSTRÖM, T. (1974)
Cadmium in the environment, 2nd ed., Cleveland, Ohio, CRC Press,
248 pp.
FRIBERG, L., KJELLSTRÖM, T., NORDBERG, G., & PISCATOR, M. (1975)
Cadmium in the environment. III. A toxicological and epidemiological
appraisal, Washington, DC, US Environmental Protection Agency,
Office of Research and Development, 217 pp.
FRIBERG, L., ELINDER, C.G., KJELLSTRÖM, T., & NORDBERG, G.F. (1985)
Cadmium and health, a toxicological and epidemiological appraisal.
Vol. I. Exposure, dose and metabolism, Cleveland, Ohio, CRC Press,
209 pp.
FRIBERG, L., ELINDER, C.G., KJELLSTRÖM, T., & NORDBERG, G.F. (1986)
Cadmium and health, a toxicological and epidemiological appraisal.
Vol. II. Effects and response, Cleveland, Ohio, CRC Press, 303 pp.
FUKUHARA, M., BOULEY, G., GODIN, J., GIRARD, F., BOISSET, M., &
BOUDENE, C. (1981) Effects of short-term inhalation of cadmium
oxides on rabbit pulmonary microsomal enzymes. Biochem. Pharmacol.,
30: 715-720.
FUKUSHIMA, I. (1978) Environmental pollution and health effects -
Gunma Prefecture. In: Tsuchiya, K., ed. Cadmium studies in Japan: a
review, Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp. 170-181.
FUKUSHIMA, M., ISHIZAKI, A., SAKAMOTO, M., & KOBAYASHI, E. (1973)
[Cadmium concentrations in rice eaten by farmers in the Jinzu river
basin.] Jpn J. Hyg., 28: 406-415 (in Japanese with English summary).
FUKUSHIMA, M., ISHIZAKI, A., NOGAWA, K., SAKAMOTO, M., & KOBAYASHI,
E. (1974) [Epidemiological studies on renal failure of inhabitants
in "Itai-itai" disease endemic district. I. Some urinary findings of
inhabitants living in and around the endemic district of the Jinzu
river basin.] Jpn. J. Public Health, 21: 65-73 (in Japanese with
English summary).
FUKUSHIMA, I., NAKAJIMA, T., HIGUSHI, Y., TSUCHIYA, T., NAKAJIMA,
K., TANAKA, T., FUKAMACHI, M., ARAI, K., & SHIONO, T. (1975)
[Results of screening examination on tubular dysfunction in aged
persons confined to bed], Tokyo, Japan Public Health Association,
pp. 138-142 (Kankyo Hoken Report No. 36) (in Japanese).
FUKUYAMA, Y. (1972) [Proteinuria in Itai-itai disease and cadmium
poisoning.] Med. Biol., 84: 41-46 (in Japanese).
FULLMER, C.S., OKU, T., & WASSERMAN, R.H. (1980) Effect of cadmium
administration on intestinal calcium absorption and vitamin
D-dependent calcium-binding protein. Environ. Res., 22: 386-399.
GABBIANI, G. (1966) Action of cadmium chloride on sensory ganglia.
Experientia (Basel), 22: 261-264.
GABBIANI, G., BAIC, D., & DELZIEL, C. (1967) Toxicity of cadmium for
the central nervous system. Exp. Neurol., 18: 154-160.
GABBIANI, G., BADONNEL, M.-C., MATHEWSON, S.M., & RYAN, G.G. (1974)
Acute cadmium intoxication: early selective lesions of endothelial
clefts. Lab. Invest., 30: 686-695.
GALE, T.F. (1973) The interaction of mercury with cadmium and zinc
in mammalian embryonic development. Environ. Res., 6: 95-105.
GARDINER, P.E., OTTAWAY, J.M., & FELL, G.S. (1979) Accuracy of the
direct determination of cadmium in urine by carbon-furnace atomic
absorption spectrometry. Talanta, 26: 841-847.
GARTY, M., WONG, K.-L., & KLAASSEN, C.D. (1981) Redistribution of
cadmium to blood of rats. Toxicol. appl. Pharmacol., 59: 548-554.
GERVAIS, J. & DELPECH, P. (1963) L'intoxication cadmique. Arch. Mal.
prof. Méd. Trav. Séc. soc., 24: 803-815.
GESAMP (1984) IMO/FAO/UNESCO/WMO/IAEA/UN/UNEP Joint Group of Experts
on the Scientific Aspects of Marine Pollution: Report of the
Fourteenth Session, Vienna, 26-30 March, 1984, Vienna, International
Atomic Energy Agency (Reports and Studies No. 21).
GESAMP (1985) IMO/FAO/UNESCO/WMO/IAEA/UN/UNEP Joint Group of Experts
on the Scientific Aspects of Marine Pollution: Atmospheric transport
of contaminants into the mediterranean region, Athens, Geneva, World
Meteorological Association (Reports and Studies No. 26).
GESAMP (1987) IMO/FAO/UNESCO/WMO/WHO/IAEA/UN/UNEP Joint Group of
Experts on the Scientific Aspects of Marine Pollution: Report of the
seventeenth session, Rome, Geneva, World Health Organization
(Reports and Studies No. 31).
GLEIT, C.E. (1965) High-frequency electrodeless discharge system for
ashing organic matter. Anal. Chem., 37: 314-315.
GLENNAS, A. & RUGSTAD, H.E. (1984) Cadmium uptake and metabolism in
cultured cells. Environ. Health Perspect., 54: 45-50.
GOERING, P.L., SQUIBB, K.S., & FOWLER, B.A. (1985) Calcuria and
proteinuria during cadmium-metallothionein-induced proximal tubule
cell injury. In: Memphill, D., ed. Proceedings of the University of
Missouri's 19th Annual Conference on Trace Substances in
Environmental Health, 3-6 June 1985, Columbia, Missouri, University
of Missouri Press, pp. 22-35.
GOYER, R.A., CHERIAN, M.G., & DELAQUERRIERE-RICHARDSON, L. (1984)
Correlation of parameters of cadmium exposure with onset of
cadmium-induced nephropathy in rats. J. environ. Pathol. Toxicol.
Oncol., 5: 89-100.
GOYER, R.A., HAUST, M.D., & CHERIAN, M.G. (1989) Localization of
metallothionein in placenta. Toxicologist, 9: 130.
GREENBERG, R.R., GORDON, G.E., ZOLLER, W.H., JACKO, R.B., NEVENDORF,
D.W., & YOST, K.J. (1978) Composition of particles emitted from the
Nocosia municipal incinerator. Environ. Sci. Technol., 12(12):
1329-1332.
GUNN, S.A. & GOULD, T.C. (1957) Selective accumulation of 115Cd by
cortex of rat kidney. Proc. Soc. Exp. Biol. Med., 96: 820-823.
GUNN, S.A. & GOULD, T.C. (1970) Cadmium and other mineral elements.
In: Johnson, A.D., Gomes, W.R., & VanDeMark, N.L., ed. The testis,
New York, London, Academic Press, Vol. 3, pp. 377-481.
GUNN, S.A., GOULD, T.C., & ANDERSON, W.A.D. (1961) Zinc protection
against cadmium injury to rat testis. Arch. Pathol., 71: 274.
GUNN, S.A., GOULD, T.C., & ANDERSON, W.A.D. (1963) Cadmium-induced
inter-stitial cell tumours in rats and mice and their prevention by
zinc. J. Natl Cancer Inst., 31: 745-759.
GUNN, S.A., GOULD, T.C., & ANDERSON, W.A.D. (1965) Strain
differences in susceptibility of mice and rats to cadmium-induced
testicular damage. J. Reprod. Fertil., 10: 273-275.
GUNN, S.A., GOULD, T.C., & ANDERSON, W.A.D. (1967) Specific response
of mesenchymal tissue to cancerogenesis by cadmium. Arch. Pathol.,
83: 439-499.
GUNN, S.A., GOULD, T.C., & ANDERSON, W.A.D. (1968) Specificity in
protection against lethality and testicular toxicity from cadmium.
Proc. Soc. Exp. Biol. Med., 128: 591-595.
GUTHRIE, B.E. & ROBINSON, M.F. (1977) Daily intakes of manganese,
copper, zinc, and cadmium by New Zealand women. Br. J. Nutr.,
38: 55-63.
GUTHRIE, J. (1964) Histological effects of intratesticular
injections of cadmium chloride in domestic fowl. Br. J. Cancer,
18: 255-260.
HADDOW, A., ROE, F.J.C., DUKES, C.E., & MITCHELEY, B.C.V. (1964)
Cadmium neoplasia: sarcomata at the site for injection of cadmium
sulfate in rats and mice. Br. J. Cancer, 18: 667-673.
HADLEY, J.G., CONKLIN, A.W., & SANDERS, C.L. (1979) Systemic
toxicity of inhaled cadmium oxide. Toxicol. Lett., 4: 107-111.
HAGA, Y. & YAMAWAKI, T. (1974) [Research report on environmental
health studies of heavy metal pollution], Akita, Japan, Akita
Prefecture Institute of Public Health (Report No. 18) (in Japanese).
HAGINO, N. & YOSHIOKA, K. (1961) [A study on the etiology of
Itai-itai disease.] J. Jpn. Orthop. Assoc., 35: 812-815 (in
Japanese).
HAHN, R., EWERS, O., JERMANN, E., FREIER, E., BROCKHAUS, A., &
SCHLIPKOTER, H.W. (1987) Cadmium in kidney cortex of inhabitants of
North-West Germany: in relationship to age, sex, smoking and
environmental pollution by cadmium. Int. Arch. occup. environ.
Health, 59: 165-176.
HALVORSEN, C. & STEINNES, E. (1975) Simple and precise determination
of Zn and Cd in human liver by neutron activation analysis. Z. anal.
Chem., 274: 199-202.
HAMILTON, D.L. & VALBERG, L.S. (1974) Relationship between cadmium
and iron absorption. Am. J. Physiol., 227: 1033-1037.
HAMMER, D.I., FINKLEA, J.F., HENDRICKS, R.H., SHY, C.M., & HORTON,
R.J.M. (1971) Hair trace metal levels and environmental exposure.
Am. J. Epidemiol., 93: 84-92.
HAMMER, D.I., FINKLEA, J.F., CREASON, J.P., SANDIFER, S.H., KEIL,
J.E., PRIESTER, L.E., & STARA, J.F. (1972) Cadmium exposure and
human health effects. In: Hemphill, D.D., ed. Trace substances in
environmental health - V, Columbia, Missouri, University of Missouri
Press, pp. 269-283.
HAMMER, D.I., COLUCCI, A.V., HASSELBLAD, V., WILLIAMS, M.E., &
PINKERTON, C. (1973) Cadmium and lead in autopsy tissues. J. occup.
Med., 15: 956-963.
HARADA, A. (1987) [Results of fifteen years health examinations on
cadmium workers in a cadmium pigment factory (2nd report)], Tokyo,
Japan Public Health Association, pp. 219-233 (Kankyo Hoken Report
No. 53) (in Japanese).
HARDY, H.L. & SKINNER, J.B. (1947) The possibility of chronic
cadmium poisoning. J. ind. Hyg. Toxicol., 29: 321-325.
HARRISON, H.E., BUNTING, H., ORDWAY, N., & ALBRINK, W.S. (1947) The
effects and treatment of inhalation of cadmium chloride aerosols in
the dog. J. ind. Hyg. Toxicol., 29: 302-314.
HARRISON, J.F., LUNT, G.S., SCOTT, P., & BLAINEY, J.D. (1968)
Urinary lysozyme, ribonuclease, and low molecular weight protein in
renal disease. Lancet, 1: 371-375.
HARVEY, T.C., MCLELLAN, J.S., THOMAS, B.J., & FREMLIN, J.H. (1975)
Measurement of liver cadmium concentrations in patients and
industrial workers by neutron activation analysis. Lancet,
1: 1269-1272.
HARVEY, T.C., CHETTLE, D.R., FREMLIN, J.H., AL-HADDAD, I.K., &
DOWNES, S.P.M. (1979) Cadmium in Shipham. Lancet, 1(8115).
HASSELMANN, J., KOENIG, W., RICHTER, F.W., STEINER, U., & WATJEN, U.
(1977) Application of PIXE to trace element analysis in biological
tissues. Nucl. Instrum. Methods, 142: 163-169.
HASSLER, E., LIND, B., & PISCATOR, M. (1983) Cadmium in blood and
urine related to present and past exposure. A study of workers in an
alkaline battery factory. Br. J. ind. Med., 40: 420-425.
HAYES, J.A., SNIDER, G.L., & PALMER, K.C. (1976) The evolution of
biochemical damage in the rat lung after acute cadmium exposure. Am.
Rev. respir. Dis., 113: 121-130.
HEATH, J.C. & WEBB, M. (1967) Content and intracellular distribution
of the inducing metal in the primary rhabdomyosarcomata induced in
the rat by cobalt, nickel, and cadmium. Br. J. Cancer, 21: 768-779.
HEATH, J.C., DANIEL, M.R., DINGLE, J.T., & WEBB, M. (1962) Cadmium
as a carcinogen. Nature (Lond.), 193: 592-593.
HEINDRYCKX, R., DEMUYNCK, M., DAMS, R., JANSSENS, M., & RAHN, K.A.
(1974) Mercury and cadmium in Belgian aerosols. In: Problems of the
contamination of man and his environment by mercury and cadmium. CEC
European Colloquium, July, 1973, Luxembourg, Commission of the
European Communities, pp. 135-148.
HEINRICH, U., PETERS, L., ERNST, H., RITTINGHAUSEN, S., DASENBROCK,
C., & KÖNIG, H. (1989) Investigation on the carcinogenic effects of
various cadmium compounds after inhalation exposure in hamsters and
mice. Exp. Pathol., 37: 253-258.
HENDERSON, R.F., REBAR, A.H., PICKRELL, J.A., & NEWTON, G.J. (1979)
Early damage indicators in the lung III. Biochemical and cytological
response of the lung to inhaled metal salts. Toxicol. appl.
Pharmacol., 50: 123-136.
HENKE, G., SACHS, H.W., & BOHN, G. (1970) [Cadmium determination by
neutron activation analysis of liver and kidneys from children and
young people.] Arch. Toxikol., 26: 8-16 (in German).
HICKEY, R.J., SCHOFF, E.P., & CLELLAND, R.C. (1967) Relationship
between air pollution and certain chronic disease death rates. Arch.
environ. Health, 15: 728-738.
HIRATA, H. (1981) Annaka: land polluted mainly by fumes and dust
from a zinc smelter. In: Kitagishi, K. & Yamane, I., ed. Heavy metal
pollution in soils of Japan, Tokyo, Japan Scientific Societies
Press, pp. 149-163.
HISLOP, J.S. (1980) Choice of analytical method. In: Bratter, P. &
Schramel, P., ed. Trace element analytical chemistry in medicine
and biology, Berlin, New York, Walter de Gruyter Co., pp. 747-767.
HOFFMAN, E.O., COOK, J.A., DILUZIO, N.R., & COOVER, J.A. (1975) The
effects of acute cadmium administration in the liver and kidney of
the rat. Lab. Invest., 32: 655-661.
HOFFMAN, L., PUTZKE, H.-P., KAMPEHL, H.-J., RUSSBULT, R., GASE, P.,
SIMONN, C., ERDMANN, T., & HUCKSTORF, C. (1985) Carcinogenic effects
of cadmium on the prostate of the rat. J. Cancer Res. clin. Oncol.,
109: 193-199.
HOFFMAN, L., PUTZKE, H.-P., BENDEL, L., ERDMANN, T., & HUCKSTORF, C.
(1988) Electron microscopic results on the ventral prostate of the
rat after CdC12 administration. A contribution towards etiology of
the cancer of the prostate. J. Cancer Res. clin. Oncol.,
114: 273-278.
HOLDEN, H. (1969) Cadmium toxicology. Lancet, 2: 57.
HOLDEN, H. (1980a) A mortality study of workers exposed to cadmium
fumes. In: Proceedings of the 2nd International Cadmium Conference,
Cannes, 6-8 February, 1979, London, Cadmium Association,
pp. 211-216.
HOLDEN, H. (1980b) Health status of European cadmium workers. In:
Proceedings of the Seminar on Occupational Exposure to Cadmium,
London, 20 March, 1980, London, Cadmium Association, pp. 33-37.
HOLMBERG, R.E., Jr & FERM, V.H. (1969) Inter-relationship of
selenium, cadmium, and arsenic in mammalian teratogenesis. Arch.
environ. Health, 18: 873-877.
HORSTOWA, H., SIKORSKI, M., & TYBORSKI, H. (1966) [Chronic cadmium
poisoning in the clinical and radiological picture.] Med. Prac.
occup. Med., 17: 13-25 (in Polish).
HOVMAND, M.F., TJELL, J.C., & MOSBACK, H. (1983) Plant uptake of
airborne cadmium. Environ. Pollut., 30: 27-38.
HUEL, G., BOUDENE, C., & IBRAHIM, M.A. (1981) Cadmium and lead
content of maternal and newborn hair: relationship to parity, birth
weight and hypertension. Arch. environ. Health, 36: 221-227.
HUGHES, E.G. & STEWART, M. (1979) Cadmium in Shipham. Lancet,
1: 973-974.
HURLEY, L.S., GOWAN, J., & SWENERTON, H. (1971) Teratogenic effects
of short-term and transitory zinc deficiency in rats. Teratology,
4: 199-204.
HUTCHISON, F., WAI, C.M., JERNEGAN, M., HICKS, W., & LONG, R. (1979)
Distribution of cadmium, lead and zinc in soil around two phosphate
processing plants in Pocatello, Idaho. Bull. environ. Contam.
Toxicol., 22: 426-429.
HUTTON, M., ed. (1982) Cadmium in the European Community: a
prospective assessment of sources, human exposure, and environmental
impact, London, Monitoring and Assessment Research Centre, Chelsea
College, University of London, 100 pp (MARC Report Number 26).
HUTTON, M. (1983) Evaluation of the relationships between cadmium
exposure and indicators of kidney function, London, Monitoring and
Assessment Research Centre, Chelsea College, University of London,
46 pp (MARC Report Number 29).
HUTTON, M. & SYMON, C. (1986) The quantities of cadmium, lead,
mercury and arsenic entering the U.K. environment from human
activities. Sci. total Environ., 57: 129-150.
HUTTON, M., WADGE, A., & MILLIGAN, P.J. (1988) Environmental levels
of cadmium and lead in the vicinity of a major refuse incinerator.
Atmos. Environ., 22: 411-416.
HYOGO PREFECTURAL GOVERNMENT (1972) [Results of a comprehensive
study on cadmium pollution in the area around Ikuno Mine and an
outline of counter-measures], Kobe, Japan, Hyogo Prefectural
Government, 156 pp (in Japanese).
IARC (1976) Cadmium, nickel, some epoxides, miscellaneous
industrial chemicals, and general considerations on volatile
anaesthetics, Lyon, International Agency for Research on Cancer,
306 pp (Monographs on the Evaluation of Carcinogenic Risk of Chemicals
to Man, Vol. 11).
IARC (1987a) Genetic and related effects: an updating of selected
IARC Monographs from Volumes 1 to 42. Lyon, International Agency for
Research on Cancer (IARC Monographs on the Evaluations of
Carcinogenic Risks to Humans, Supplement 6).
IARC (1987b) Cadmium and cadmium compounds. In: Overall evaluations
of carcinogenicity: an updating of IARC Monographs, Volumes 1 to 42,
Lyon, International Agency for Research on Cancer, pp. 139-142 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals
to Humans, Supplement 7).
ILZSG (1988) International Lead and Zinc Study Group - World
directory: Lead and zinc mines and primary metallurgical works,
London, Chameleon Press, Ltd.
INSKIP, H., BERAL, V., & MCDOWALL, M. (1982) Mortality in Shipman
residents: 40-year follow-up. Lancet, 1: 896-899.
IRPTC (1987) IRPTC legal file 1986, Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment
Programme, Vol. I.
ISHIKAWA PREFECTURAL GOVERNMENT (1976) [Report on the health
examination of residents of the Kakehashi river basin], Kanazawa,
Japan, Health and Welfare Department, 104 pp (in Japanese).
ISHIZAKI, A. (1969) [On the so-called Itai-itai disease.] J. Jpn.
Med. Soc., 62: 242-249 (in Japanese).
ISHIZAKI, A. (1972) [Concentrations of cadmium and zinc in the
organs of Itai-itai disease patients and residents in the Hokuriku
region], Tokyo, Japanese Society of Public Health, pp. 154-157
(Kankyo Hoken Report No. 11) (in Japanese).
ISHIZAKI, A., FUKUSHIMA, M., SAKOMOTO, M., KURACHI, T., & HAYASHI,
E. (1969) [Cadmium content of rice eaten in the Itai-itai disease
area.] In: [Proceedings of the 27th Annual Meeting of the Japanese
Society of Public Health], Tokyo, Japan Public Health Association,
p. 111 (in Japanese).
ISHIZAKI, A., FUKUSHIMA, M., & SAKAMOTO, M. (1971) [Contents of
cadmium and zinc in organs of Itai-itai disease patients and
residents of Hakuriku district.] Jpn. J. Hyg., 26: 268-273 (in
Japanese).
ISHIZU, S., MINAMI, M., SUZUKI, A., YAMADA, M., SATO, M., &
YAMAMURA, K. (1973) An experimental study of teratogenic effect of
cadmium. Ind. Health, 11: 127-139.
ITO, K. & SAWAUCHI, K. (1966) Inhibitory effects on cadmium-induced
testicular damage by pretreatment with smaller cadmium doses.
Okajimas Folia anat. Jpn. 42: 107-117.
ITOKAWA, Y., ABE, T., & TANAKA, S. (1973) Bone changes in
experimental chronic cadmium poisoning. Arch. environ. Health,
26: 241-244.
ITOKAWA, Y., ABE, T., TABEI, R., & TANAKA, S. (1974) Renal and
skeletal lesions in experimental cadmium poisoning. Arch. environ.
Health, 28: 149-154.
ITOKAWA, Y., NISHINO, K., TAKASHIMA, M., NAKATA, T., KAITO, H.,
OKAMOTO, E., DAIJO, K., & KAWAMURA, J. (1978) Renal and skeletal
lesions in experimental cadmium poisoning of rats. Histology and
renal function. Environ. Res., 15: 206-217.
IWAO, S. (1977) Cadmium, lead, copper, and zinc in food, faeces, and
organs of humans. Keio J. Med., 26: 63-78.
IWAO, S., SUGITA, M., & TSUCHIYA, K. (1981a) Some metabolic
interrelationships among cadmium, lead, copper, and zinc: results
from a field survey in Cd-polluted areas in Japan. I. Dietary intake
of the heavy metals. Keio J. Med., 30: 17-36.
IWAO, S., SUGITA, M., & TSUCHIYA, K. (1981b) Some metabolic
interrelationships among cadmium, lead, copper, and zinc: results
from a field survey in Cd-polluted areas in Japan. II. Faecal
excretion of the heavy metals. Keio J. Med., 30: 71-82.
JAGNER, D., JOSEFSON, M., & WESTERLUND, S. (1981) Simultaneous
determination of cadmium and lead in urine by means of computerized
potentiometric stripping analysis. Anal. Chim. Acta, 128: 155-161.
JAMALL, I.S. & SMITH, J.C. (1986) The effect of dietary selenium on
cadmium cardiotoxicity. In: Born, G.V.R., Rensselaer, A.F., Herken,
H., & Welch, A.D., ed. Handbook of experimental pharmacology,
Berlin, Heidelberg, Springer Verlag, Vol. 80, pp. 351-361.
JAMALL, I.S. & SPROWLS, J.J. (1987) Effects of cadmium and dietary
selenium on cytoplasmic and mitochondrial antioxidant defense
systems in the heart of rats fed high dietary copper. Toxicol. appl.
Pharmacol., 87: 102-110.
JANSSENS, M. & DAMS, R. (1974) Determination of cadmium in air
particulates by flameless atomic absorption spectrometry with a
graphite tube. Anal. Chim. Acta, 70: 25-33.
JAPAN CADMIUM RESEARCH COMMITTEE (1989) [Summary report on studies
of health effects of cadmium], Tokyo, Japan Public Health
Association, pp. 53-67 (Kankyo Hoken Report No. 56) (in Japanese).
JAPAN PUBLIC HEALTH ASSOCIATION (1968) [A study of the cause of
Itai-itai disease], Tokyo, Japan Public Health Association, 73 pp
(in Japanese).
JAPAN PUBLIC HEALTH ASSOCIATION (1970) [Study on cadmium intake and
accumulation in areas requiring official observation], Tokyo, Japan
Public Health Association, 49 pp (in Japanese).
JAPANESE ENVIRONMENT AGENCY (1972) [Results of soil pollution survey
in 1971], Tokyo, Japanese Environment Agency, Soil Agricultural
Chemical Division, Water Quality Bureau, 129 pp (in Japanese).
JAPANESE ENVIRONMENT AGENCY (1974) [Results of measurement at the
national air sampling network stations], Tokyo, Japanese Environment
Agency, Air Pollution Control Division, Air Quality Bureau, 159 pp
(in Japanese).
JAPANESE ENVIRONMENT AGENCY (1982) [Results of soil pollution survey
in 1981], Tokyo, Japanese Government Agency, Soil Agricultural
Chemical Division, Water Quality Bureau, pp. 1-29 (in Japanese).
JAPANESE MINISTRY OF AGRICULTURE AND FORESTRY (1973) [Results of a
survey for protection measures of soil contamination in 1972.] In:
[Materials for soil protection measures], 197 pp (Report No. 44) (in
Japanese).
JAPANESE MINISTRY OF HEALTH AND WELFARE (1968) [Opinion of the
Ministry with regard to Itai-itai disease and its causes], Tokyo,
Japanese Ministry of Health and Welfare (in Japanese).
JAPANESE MINISTRY OF HEALTH AND WELFARE (1971) [Partial revision of
health examination methods in the provisional counter-measures
against environmental pollution by cadmium], Tokyo, Japanese
Ministry of Health and Welfare, Environmental Pollution Section
(Unpublished document) (in Japanese).
JAPANESE MINISTRY OF HEALTH AND WELFARE (1983) [The present status
of nutrition among the people], Tokyo, Japanese Ministry of Health
and Welfare, 56 pp (in Japanese).
JÄRUP, L., ROGENFELT, A., ELINDER, C.-G., NOGAWA, K., & KJELLSTRÖM,
T. (1983) Biological half-time of cadmium in the blood of workers
after cessation of exposure. Scand. J. Work Environ. Health,
9: 327-331.
JÄRUP, L., ELINDER, C.G., & SPANG, G. (1988) Cumulative
blood-cadmium and tubular proteinuria: a dose-response relationship.
Int. Arch. occup. environ. Health, 60: 223-229.
JAWAID, M., LIND, B., & ELINDER, C.-G (1983) Determination of
cadmium in urine by extraction and flameless atomic absorption
spectrophotometry. Comparison of urine from smokers and non-smokers
of different sex and age. Talanta, 30: 509-513.
JEFFERY, E.H., NOSEWORTHY, R., & CHERIAN, M.G. (1989) Age dependent
changes in metallothionein and accumulation of cadmium in horses.
Comp. Biochem. Physiol., 93C: 327-332.
JIN, T., NORDBERG, G.F., & NORDBERG, M. (1987a) Resistance to acute
nephrotoxicity induced by cadmium-metallothionein dependence on
pretreatment with cadmium chloride. Pharmacol. Toxicol., 61: 89-93.
JIN, T., NORDBERG, G.F., & NORDBERG, M. (1987b) Influence of
cadmium-metallothionein pretreatment on tolerance of rat kidney
cortical cells to cadmium toxicity in vitro and in vivo.
Pharmacol. Toxicol., 60: 345-349.
JIN, T., NORDBERG, G.F., & NORDBERG, M. (1986) Uptake of cadmium in
isolated kidney cells - influence of binding form and
in vivo pretreatment. J. appl. Toxicol., 6(6): 397-400.
JOHNSON, M.S. & EATON, J.W. (1980) Environmental contamination
through residual trace metal dispersal from derelict lead-zinc mine.
J. environ. Qual., 9(2): 175-179.
JONES, K.C., SYMON, C.J., & JOHNSTON, A.E. (1987) Long-term changes
in soil and cereal grain cadmium: studies at Rothamsted Experimental
Station. In: Hemphill, D.D., ed. Trace substances in environmental
health - XXI. Proceedings of the University of Missouri's 21st
Annual Conference on Trace Substances in Environmental Health, St.
Louis, Missouri, 25-28 May, 1987, Columbia, Missouri, University of
Missouri Press, pp. 450-460.
JORHEM, L., MATTSON, P., & SLORACH, S. (1984) Lead, cadmium, zinc
and certain other metals in foods on the Swedish market. Var Föda,
36: Suppl. 3.
JUST, J. & KELUS, J. (1971) [Cadmium in the air atmosphere of ten
selected cities in Poland.] Rocz. Panstw. Zakl. Hig., 22: 249-256
(in Polish).
KAGI, J.H.R. & KOJIMA, Y. (1987) Metallothionein II. Proceedings of
the Second International Meeting on Metallothionein and Other Low
Molecular Weight Metal-Binding Proteins, Zurich, 21-24 August, 1985,
Basel, Birkhauser Verlag.
KAGI, J.H.R. & NORDBERG, M., ed. (1979) Metallothionein, Basel,
Birkhauser Verlag.
KAGI, J.H.R. & VALLEE, B.L. (1960) Metallothionein: a cadmium- and
zinc-containing protein from equine renal cortex. I. J. biol. Chem.,
235: 3460-3465.
KAGI, J.H.R. & VALLEE, B.L. (1961) Metallothionein: a cadmium- and
zinc-containing protein from equine renal cortex. II. Physiochemical
properties. J. biol. Chem., 236: 2435-2442.
KAHN, H.L. & MANNING, D.C. (1972) Background correction in atomic
absorption spectroscopy. Am. Lab., August: 51-56.
KAJIKAWA, K., NAKANISHI, I., & KURODA, K. (1981) Morphological
changes of the kidney and bone of rats in chronic cadmium poisoning.
Exp. mol. Pathol., 34: 9-24.
KANAI, M., NOMOTO, S., SASAOKA, S., & NAIKI, M. (1971) Clinical
significance of urinary excretion of retinol-binding protein in
patients with Itai-itai disease. Proc. Symp. Physiol. Pathol.,
11: 194-199.
KANISAWA, M. & SCHROEDER, H.A. (1969) Life term studies on the
effect of trace elements on spontaneous tumours in mice and rats.
Cancer Res., 21: 892-895.
KAPLAN, P.D., BLACKSTONE, M., & RICHDALE, N. (1973) Direct
determination of cadmium, nickel, and zinc in rat lungs. Arch.
environ. Health, 27: 387-389.
KAR, A.B., DAS, R.P., & KARKUN, J.N. (1959) Ovarian changes in
prepubertal rats after treatment with cadmium chloride. Acta biol.
med. Germ., 3: 372-399.
KAR, A.B., DAS, R.P., & MUKERJI, B. (1960) Prevention of
cadmium-induced changes in the gonads of rat by zinc and selenium: a
study in antagonism between metals in the biological system. Proc.
Natl Inst. Sci. India Part B. Biol. Sci., 26: 40-50.
KARIN, M. & RICHARDS, R.I. (1982) Human metallothionein genes:
primary structure of the metallothionein-II gene and a related
processed gene. Nature (Lond.), 299: 797-802.
KARIN, M., SLATER, E.P., & HERSCHMAN, H.R. (1981) Regulation of
metallothionein synthesis in HeLa cells by heavy metals and
glucocorticoids. J. cell Physiol., 106: 63-74.
KASUYA, M., TERANISHI, H., KUBOTA, Y., KATO, T., AOSHIMA, K., SAIJO,
A., IWATA, K., & KANAI, M. (1986) [Biological meaning and blood and
urine low-molecular-weight protein determinations of Itai-itai
disease patient and their families, 10 years follow-up study on
urine low-molecular weight protein], Tokyo, Japan Public Health
Association, pp. 176-180 (Kankyo Hoken Report No. 52) (in Japanese).
KATAGIRI, Y., TATI, M., IWATA, H., & KANAI, M. (1971) [Concentration
of cadmium in urine by age.] Med. Biol., 82: 239-243 (in Japanese).
KATO, T. & ABE, K. (1978) Environmental pollution and health effects
- Toyama Prefecture. In: Tsuchiya, K., ed. Cadmium studies in Japan:
a review, Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp. 225-249.
KAWAI, K. (1978) Experimental studies on animals. Morphological
changes of the kidney and effects on bones. In: Tsuchiya, K., ed.
Cadmium studies in Japan: a review, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 86-97.
KAWAI, M., FUKUDA, K., & KIMURA, M. (1976) Morphological alterations
in experimental cadmium exposure with special reference to the onset
of renal lesion. In: Nordberg, G.F., ed. Effects and dose-response
relationships of toxic metals, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 343-370.
KAWAMURA, J., YOSHIDA, O., NISHINO, K., & ITOKAWA, Y. (1978)
Disturbances in kidney functions and calcium and phosphate
metabolism in cadmium-poisoned rats. Nephron, 20: 101-110.
KAWANO, S. & KATO, T. (1978) Environmental pollution and health
effects - Ishikawa Prefecture. In: Tsuchiya, K., ed. Cadmium studies
in Japan: a review, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 220-225.
KAWASHIMA, H., NOMIYAMA, H., & NOMIYAMA, K. (1988) Chronic exposure
to cadmium did not impair vitamin D metabolism in monkeys. Environ.
Res., 46: 48-58.
KAZANTZIS, G. (1956) Respiratory function in men casting cadmium
alloys. Part I. Assessment of ventilatory function. Br. J. ind.
Med., 13: 30-36.
KAZANTZIS, G. (1963) Induction of sarcoma in the rat by cadmium
sulfide pigment. Nature ( Lond.), 198: 1213-1214.
KAZANTZIS, G. (1979) Renal tubular dysfunction and abnormalities of
calcium metabolism in cadmium workers. Environ. Health Perspect.,
28: 155-160.
KAZANTZIS, G. & ARMSTRONG, B.G. (1982) A mortality study of cadmium
workers in the United Kingdom. Scand. J. Work Environ. Health,
8(1): 157-160.
KAZANTZIS, G. & HANBURY, W.J. (1966) The induction of sarcoma in the
rat by cadmium sulfide and by cadmium oxide. Br. J. Cancer,
20: 190-199.
KAZANTZIS, G., FLYNN, F.V., SPOWAGE, J.S., & TROTT, D.G. (1963)
Renal tubular malfunction and pulmonary emphysema in cadmium pigment
workers. Q. J. Med., 32: 165-192.
KAZANTZIS, G., LAM, T.-H., & SULLIVAN, K.R. (1988) Mortality of
cadmium-exposed workers. A five-year update. Scand. J. Work Environ.
Health, 14: 220-223.
KAZANTZIS, G., BLANKS, R., & TEATHER, S. (1989) The mortality of
cadmium exposed workers. In: Vernet, J.-P., ed. Proceedings of the
International Conference on Heavy Metals in the Environment, Geneva,
September 1989, Edinburgh, CEP Consultants Ltd, Vol. 1, pp. 304-307.
KELLO, D. & KOSTIAL, K. (1977) Influence of age and milk diet on
cadmium absorption from the gut. Toxicol. appl. Pharmacol.,
40: 277-282.
KIDO, T., HONDA, R., TSURITANI, I., YAMAYA, H., ISHIZAKI, M.,
YAMADA, Y., & NOGAWA, K. (1988) Progress of renal dysfunction in
inhabitants environmentally exposed to cadmium. Arch. environ.
Health, 43: 213-217.
KIDO, T., NOGAWA, K., YAMADA, Y., HONDA, R., TSURITANI, I.,
ISHIZAKI, M., & YAMAYA, H. (1989) Osteopenia in inhabitants with
renal dysfunction induced by exposure to environmental cadmium. Int.
Arch. occup. environ. Health, 61: 271-276.
KIMURA, M. & OTAKI, N. (1972) Percutaneous absorption of cadmium in
rabbit and hairless mouse. Ind. Health (Tokyo), 10: 7-10.
KING, E. (1955) An environmental study of casting copper-cadmium
alloys. Br. J. ind. Med., 12: 198-205.
KIPLING, M.D. & WATERHOUSE, J.A.H. (1967) Cadmium and prostatic
carcinoma. Lancet, 1: 730-731.
KIRSCH, H., PADBERG, W., SCHOLZ, A., & ZIMMERMEYER, G. (1982)
Cadmium emissions from coal-fired power plants. In: Proceedings of
the 3rd International Cadmium Conference, Miami, Florida, 3-5
February, 1981, London, Cadmium Association, pp. 64-68.
KITAMURA, M. (1972) [Absorption and deposition of cadmium,
especially in human subjects], Tokyo, Japan Public Health
Association, pp. 42-45 (Kankyo Hoken Report No. 11) (in Japanese).
KITAMURA, M. & KOIZUMI, N. (1975) [Results of urine tests for
bedridden old persons in Hyogo Prefecture. Report 2], Tokyo, Japan
Public Health Association, pp. 135-137 (Kankyo Hoken Report No. 36)
(in Japanese).
KITAMURA, S., SUMINO, K., & KAMATANI, N. (1970) [Cadmium
concentrations in liver, kidneys, and bones of human bodies.] Jpn.
J. public Health, 17: 177 (in Japanese).
KJELLSTRÖM, T. (1971) A mathematical model for the accumulation of
cadmium in human kidney cortex. Nord. Hyg. Tidskr., 53: 111-119.
KJELLSTRÖM, T. (1977) Accumulation and renal effects of cadmium in
men. A dose-response study, Stockholm, Karolinska Institute, 80 pp
(Doctoral thesis).
KJELLSTRÖM, T. (1979) Exposure and accumulation of cadmium in
populations from Japan, the United States, and Sweden. Environ.
Health Perspect., 28: 169-197.
KJELLSTRÖM, T. (1986) Effects on bone, on vitamin D, and calcium
metabolism. In: Friberg, L., Elinder, C.-G., Kjellström, T., &
Nordberg, G.F., ed. Cadmium and health: A toxicological and
epidemiological appraisal, Boca Raton, Florida, CRC Press, Vol. II,
pp. 111-158.
KJELLSTRÖM, T. & NORDBERG, G.F. (1978) A kinetic model of cadmium
metabolism in the human being. Environ. Res., 16: 248-269.
KJELLSTRÖM, T. & PISCATOR, M. (1977) Quantitative analysis of
ß-2-microglobulin in urine as an indicator of renal tubular damage
by cadmium. Phadedoc No. 1. Diagnostic communications, Uppsala,
Sweden, Pharmacia Diagnostics, 21 pp.
KJELLSTRÖM, T., LIND, B., LINNMAN, L., & NORDBERG, G.F. (1974) A
comparative study on methods for cadmium analysis of grain with an
application to pollution evaluation. Environ. Res., 8: 92-106.
KJELLSTRÖM, T., LIND, B., LINNMAN, L., & ELINDER, C.-G. (1975a)
Variation of cadmium concentration in Swedish wheat and barley. An
indicator of changes in daily cadmium intake during the 20th
century. Arch. environ. Health, 30: 321-328.
KJELLSTRÖM, T., TSUCHIYA, K., TOMPKINS, E., TAKABATAKE, E., LIND,
B., & LINNMAN, L. (1975b) A comparison of methods for analysis of
cadmium in food and biological material. A cooperative study between
Sweden, Japan, and the USA. In: Recent advances in the assessment of
the health effects of environmental pollution, Luxembourg,
Commission of the European Communities, pp. 2197-2213.
KJELLSTRÖM, T., EVRIN, P.E., & RAHNSTER, B. (1977a) Dose-response
analysis of cadmium-induced tubular proteinuria. A study of urinary
ß-2-microglobulin excretion among workers in a battery factory.
Environ. Res., 13: 303-317.
KJELLSTRÖM, T., SHIROISHI, K., & EVRIN, P.E. (1977b) Urinary
ß-2-microglobulin excretion among people exposed to cadmium in the
general environment. An epidemio-logical study in cooperation
between Japan and Sweden. Environ. Res., 13: 318-344.
KJELLSTRÖM, T., BORG, K., & LIND, B. (1978) Cadmium in faeces as an
estimator of daily cadmium intake in Sweden. Environ. Res.,
15: 242-251.
KJELLSTRÖM, T., FRIBERG, L., & RAHNSTER, D. (1979) Mortality and
cancer morbidity among cadmium-exposed workers. Environ. Health
Perspect., 28: 199-204.
KJELLSTRÖM, T., ELINDER, C.-G., & FRIBERG, L. (1984) Conceptual
problems in establishing the critical concentration of cadmium in
human kidney cortex. Environ. Res., 33: 284-295.
KLAASSEN, C.D. & KOTSONIS, F.N. (1977) Biliary excretion of cadmium
in the rat, rabbit, and dog. Toxicol. appl. Pharmacol., 41: 101-112.
KNEIP, T.J., EISENBUD, M., STREHLOW, C.D., & FREUDENTHAL, P.C.
(1970) Airborne particulates in New York City. J. Air Pollut.
Control Assoc., 20: 144-149.
KOBAYASHI, J. (1974) Effects of cadmium on calcium metabolism of
rats. In: Hemphill, D.D., ed. Trace substances in environmental
health - VII, Columbia, Missouri, University of Missouri Press,
pp. 295-305.
KODAMA, Y., MORIMOTO, K., & NAKAJIMA, T. (1978) [Studies on the
chemical form of heavy metal in oysters.] Eisei Kagaku, 24: 132-138
(in Japanese).
KOGAN, I.G., GROZDOVA, T.YA., & KHOLIKOVA, T.A. (1978)
[Investigation of the mutagenic effect of cadmium chloride on
Drosophila melanogaster germ cells.] Genetika, 16: 2136-2140 (in
Russian).
KOGAN, V.H., MALISHEVA, N.B., & FIDAROVA, A.M. (1972) On the role of
visceral lesions under chronic cadmium intoxications in the
development of bone changes. Trans. Kuban Med. Inst., 36: 75-78.
KOIRTYOHANN, S.R. & HOPKINS, C.A. (1976) Losses of trace metals
during the ashing of biological materials. Analyst, 101: 870-875.
KOIVISTOINEN, P. (1980) Mineral element composition of Finnish
foods: N, K, Ca, Mg, P, S, Fe, Cu, Mn, Zn, Mo, Co, Ni, Cr, F, Se,
Si, Rb, Al, B, Br, Hg, As, Cd, Pb, and Ash. Acta agric. Scand.,
22: Suppl.
KOIZUMI, H., YASUDA, K., & KATAYAMA, M. (1977) Atomic absorption
spectrophotometry based on the polarization characteristics of the
Zeeman effects. Anal. Chem., 49: 1106-1112.
KOJIMA, S., HAGA, Y., KURIHARA, T., & YAMAWAKI, T. (1975) [Report
from Akita Prefecture], Tokyo, Japan Public Health Association, pp.
114-123 (Kankyo Hoken Report No. 36) (in Japanese).
KOJIMA, S., HAGA, Y., KURIHARA, T., & YAMAWAKI, T. (1976) [Report on
cadmium pollution in Akita Prefecture], Tokyo, Japan Public Health
Association, pp. 114-120 (Kankyo Hoken Report No. 36) (in Japanese).
KOJIMA, S., HAGA, Y., KURIHARA, T., YAMAWAKI, T., & KJELLSTRÖM, T.
(1977) A comparison between faecal cadmium and urinary
ß-2-microglobulin total protein and cadmium among Japanese farmers.
An epidemiological study in cooperation between Japan and Sweden.
Environ. Res., 14: 436-451.
KOLLER, L.D., EXON, J.H., & ROAN, J.G. (1975) Antibody suppression
by cadmium. Arch. environ. Health, 30: 598-601.
KONO, M., YOSHIDA, T., SUGIHARA, H., & HAGINO, N. (1956) [Report on
so-called Itai-itai disease - First report.] J. Jap. Soc.
Orthopaedis., 30: 100-101 (in Japanese).
KOPP, S.J., GLONEK, T., ERLANGER, M., PERRY, E.F., PERRY, H.M., Jr,
& BARANY, M. (1980a) Cadmium and lead effects on myocardial function
and metabolism. J. environ. Pathol. Toxicol., 4: 205-227.
KOPP, S.J., PERRY, H.M., Jr, GLONEK, T., ERLANGER, M., PERRY, E.F.,
BARANY, M., & D'AGROSA, L.S. (1980b) Cardiac physiologic-metabolic
changes after chronic low-level heavy metal feeding. Am. J.
Physiol., 239: H22-H30.
KOPP, S.J., PERRY, H.M., Jr, PERRY, E.F., & ERLANGER, M. (1983)
Cardiac physiologic and tissue metabolic changes following chronic
low-level cadmium and cadmium plus lead ingestion in the rats.
Toxicol. appl. Pharmacol., 69: 149-160.
KOROPATNICK, J. & CHERIAN, M.G. (1988) Exposure to different forms
of cadmium in mice: differences in metallothionein and
alphafetoprotein mRNA induction in liver and kidney. J. biochem.
Toxicol., 3: 159-172.
KORPELA, H., LOUENIVA, R., YRJANHEIKKI, E., & KAUPPILA, A. (1986)
Lead and cadmium concentrations in maternal and umbilical cord
blood, amniotic fluid, placenta, and amniotic membranes. Am J.
Obstet. Gynecol., 155: 1086-1089.
KOTSONIS, F.N. & KLAASSEN, C.D. (1977) Toxicity and distribution of
cadmium administered to rats at sublethal doses. Toxicol. appl.
Pharmacol., 41: 667-680.
KOWAL, N.E. & KRAEMER, D.F. (1982) Urinary cadmium and
ß-2-microglobulin levels of persons aged 20-74 years from a
sub-sample of the national HANES II survey, 1976-1980. In:
Proceedings of the 3rd International Cadmium Conference, Miami,
Florida, 3-5 February, 1981, London, Cadmium Association,
pp. 119-122.
KOWAL, N.E., JOHNSON, D.E., KRAEMER, D.F., & PAHREN, H.R. (1979)
Normal levels of cadmium in diet, urine, blood, and tissues of
inhabitants of the United States. J. Toxicol. environ. Health,
5: 995-1014.
KRAEMER, D.F., LUCAS, J.B., PAHREN, H.R., RYAN, J.A., & KOWAL, N.E.
(1979) Cadmium toxicity. Lancet, 1: 1241-1242.
KRASOVSKII, G.N., VARSHAVSKAYA, S.P., & BORISOV, A.I. (1976) Toxic
and gonadotropic effects of cadmium and boron relative to standards
for these substances in drinking-water. Environ. Health Perspect.,
13: 69-75.
KRELL, U. & ROECKNER, E. (1988) Model simulation of the atmospheric
input of lead and cadmium into the North Sea. Atmos. Environ.,
22(2): 375-381.
KRETZSCHMAR, J.G., DELESPAUL, I., & DE RIJK, T.H. (1980) Heavy
levels in Belgium: a five-year survey. Sci. total Environ.,
14: 85-97.
KUHNERT, P.M., KUHNERT, B.R., BOTTOMS, S.F., & ERHARD, P. (1982)
Cadmium levels in maternal blood, fetal cord blood, and placental
tissues of pregnant women who smoke. Am. J. Obstet. Gynecol.,
142: 1021-1028.
KUHNERT, P.M., KUHNERT, B.R., ERHARD, P., BRASHEAR, W.T.,
GROH-WARGO, S.L., & WEBSTER, S. (1987a) The effect of smoking on
placental and fetal zinc status. Am. J. Obstet. Gynecol.,
157: 1241-1246.
KUHNERT, B.R., KUHNERT, P.M., DEBANNE, S., & WILLIAMS, T.G. (1987b)
The relationship between cadmium, zinc and birth weight in pregnant
women who smoke. Am. J. Obstet. Gynecol., 157: 1247-1251.
KUHNERT, B.R., KUHNERT, P.M., & ZARLINGO, T.J. (1988) Associations
between placental cadmium and zinc and age and parity in pregnant
women who smoke. Obstet. Gynecol., 71: 67-70.
LAAMANEN, A. (1972) Functions, progress, and prospects for an
environmental subarctic base level station. Work environ. Health,
9: 17-25.
LAFLEUR, P.D., ed. (1976) Accuracy in trace analysis: sampling,
sample handling, analysis, Washington, DC, US Department of
Commerce, National Bureau of Standards, Vol. 1 and 2, 1304 pp.
LARIONOVA, T.I., ZILLE, L.N., & VOROBJEVA, R.S. (1974) [The
influence of barium-cadmium laurate on some fermentative processes
in the liver.] Gig. Tr. prof. Zabol., 3: 50-52 (in Russian).
LAUWERYS, R. & DE WALS, PH. (1981) Environmental pollution by
cadmium and mortality from renal diseases. Lancet, 1: 383.
LAUWERYS, R., BUCHET, J.P., ROELS, H., BROUWERS, J., & STANESCU, D.
(1974a) Epidemiological survey of workers exposed to cadmium. Arch.
environ. Health, 28: 145-148.
LAUWERYS, R., BUCHET, J.P., & ROELS, H. (1974b) Effets subcliniques
de l'exposition humaine au cadmium. In: Proceedings of the
International Symposium on Problems of the Contamination of Man and
His Environment by Mercury and Cadmium, Luxembourg, Commission of
the European Communities, pp. 447-461.
LAUWERYS, R., BUCHET, J.P., & ROELS, H. (1974c) Dosage du cadmium et
du plomb dans le sang et les urines par spectrophotométrie
d'absorption atomique précedée d'une chromatographie sur résine
échangeuse d'ions. In: Proceedings of the International Symposium on
Problems of the Contamination of Man and His Environment by Mercury
and Cadmium, Luxembourg, Commission of the European Communities,
pp. 231-238.
LAUWERYS, R., BUCHET, J.P., ROELS, H., BERLIN, A., & SMEETS, J.
(1975) Intercomparison program of lead, mercury, and cadmium
analysis in blood, urine, and aqueous solutions. Clin. Chem.,
21: 551-557.
LAUWERYS, R., BUCHET, J.P., & ROELS, H. (1976) The relationship
between cadmium exposure or body burden and the concentration of
cadmium in blood and urine in man. Int. Arch. occup. environ.
Health, 36: 275-285.
LAUWERYS, R., BUCHET, J.P., ROELS, H., & HUBERMONT, G. (1978)
Placental transfer of lead, mercury, cadmium and carbon monoxide in
women. I. Comparison of the frequency distributions of the
biological indices in maternal and umbilical cord blood. Environ.
Res., 15: 278-290.
LAUWERYS, R., ROELS, H., BUCHET, J.P., BERNARD, A., & STANESCU, D.
(1979a) Investigations on the lung and kidney functions in workers
exposed to cadmium. Environ. Health Perspect., 28: 137-145.
LAUWERYS, R., ROELS, H., REGNIERS, M., BUCHET, J.P., BERNARD, A., &
GORET, A. (1979b) Significance of cadmium concentration in blood and
in urine in workers exposed to cadmium. Environ. Res., 20: 375-391.
LAUWERYS, R., ROELS, H., BERNARD, A., & BUCHET, J.P. (1980a) Renal
response to cadmium in a population living in a non-ferrous smelter
area in Belgium. Int. Arch. occup. environ. Health, 45: 271.
LAUWERYS, R., BUCHET, J.P., ROELS, H., BERNARD, A., CHETTLE, D.R.,
HARVEY, T.C., & ALHADDAD, I.K. (1980b) Biological significance of
cadmium concentration in blood and urine and their application in
monitoring workers exposed to cadmium. In: Proceedings of the 2nd
International Cadmium Conference, Cannes, 6-8 February, 1977,
London, Cadmium Association, pp. 164-167.
LAUWERYS, R., BUCHET, J.P., ROELS, H., VIAU, C., BERNARD, A.,
BRUAUX, P., CLAEYS-THOREAU, F., & RONDIA, D. (1984a) Potential risk
of cadmium for the general population: Update of Liege study. In:
Proceedings of the 4th International Cadmium Conference, Munich, 2-4
March, 1983, London, Cadmium Association, pp. 113-114.
LEHMAN, L.D. & POISNER, A.M. (1984) Induction of metallothionein
synthesis in cultured human trophoblasts by cadmium and zinc. J.
Toxicol. environ. Health, 14: 419-432.
LEHNERT, G., VON, SCHALLER, K.H., & HAAS, T. (1968) [Cadmium
analysis of serum and urine using atomic absorption spectrometry.]
Z. klin. Chem. klin. Biochem., 6: 174-176 (in German).
LEMEN, R.A., LESS, J.S., WAGONER, J.K., & BLEJER, H.P. (1976) Cancer
mortality among cadmium production workers. Ann. NY Acad Sci.,
271: 273-279.
LENER, J. & MUSIL, J. (1971) Cadmium influence on the excretion of
sodium by kidneys. Experientia (Basel), 27: 902.
L'EPEE, P., LAZARINI, H., FRANCHOME, J., N'DOKY, T., & LARRIVET, C.
(1968) Contribution à l'étude de l'intoxication cadmique. Arch.
Mal. prof. Méd. Trav. Sécur. soc., 29: 485-490.
LEPOW, M.L., BRUCKMAN, L., RUBINO, R.A., MARKOWITZ, S., GILLETTE,
M., & KAPISH, J. (1974) Role of airborne lead in increased body
burden of lead in Hartford children. Environ. Health Perspect.,
7: 99-102.
LEVY, L.S. & CLACK, J. (1975) Further studies on the effect of
cadmium on the prostate gland. I. Absence of prostatic changes in
rats given oral cadmium sulfate for two years. Ann. occup. Hyg.,
17: 205-211.
LEVY, L.S., ROE, F.J.C., MALCOLM, D., KAZANTIZIS, G., CLACK, J., &
PLATT, H.S. (1973) Absence of prostatic changes in rats exposed to
cadmium. Ann. occup. Hyg., 16: 111-118.
LEVY, L.S., CLACK, J., & ROE, F.J.C. (1975) Further studies on the
effect of cadmium on the prostate gland. II. Absence of prostatic
changes in mice given oral cadmium sulfate for eighteen months. Ann.
occup. Hyg., 17: 213-220.
LEWIS, G.P., JUSKO, W.J., COUGHLIN, L.L., & HARTZ, S. (1972) Cadmium
accumulation in man: influence of smoking, occupation, alcoholic
habit, and disease. J. chron. Dis., 25: 717-726.
LIKUTOVA, I.V. (1989) [Specific effect of inorganic cadmium
compounds on health and its hygienic assessment.] Abstract from a
dissertation for degree of Candidate of Medical Sciences, Moscow,
Central Institute for the Advanced Training of Physicians, 21 pp (in
Russian).
LIKUTOVA, I.V. & BELOVA, E.A. (1987) [Specific action of cadmium in
its peroral intake with water.] Gig. i Sanit., 1987(6): 70-72 (in
Russian).
LINDBERG, S.E., TURNER, R.R., & LOVETT, G.M. (1982) Processes of
atmospheric deposition of metals and acids to forests. Conference
Proceeding 75. Annual Meeting on the Air Pollution Control
Association, New Orleans, 20 June, 1982, Pittsburgh, Pennsylvania,
Air Pollution Control Association, 14 pp.
LINNMAN, L., ANDERSSON, A., NILSSON, K.O., LIND, B., KJELLSTRÖM, T.,
& FRIBERG, L. (1973) Cadmium uptake by wheat from sewage sludge used
as a plant nutrient source. Arch. environ. Health, 27: 45-47.
LORENTZON, R. & LARSSON, S.E. (1977) Vitamin D metabolism in adult
rats at low and normal calcium intake and the effect of cadmium
exposure. Clin. Sci. mol. Med., 53: 439-446.
LOSER, E. (1980) A 2-year oral carcinogenicity study with cadmium on
rats. Cancer Lett., 9: 191-198.
LUCAS, P.A., JARIVALLA, A.G., JONES, J.H., GOUGH, J., & VALE, P.T.
(1980) Total cadmium fume inhalation. Lancet, 2(8187): 205.
LUCIS, O.J., LUCIS, R., & ATERMAN, K. (1972) Tumorigenesis by
cadmium. Oncology, 26: 53-76.
LUFKIN, N.H. & HODGES, F.T. (1944) Cadmium poisoning. Report of
outbreak. US nav. med. Bull., 43: 1273-1276.
LUND, L.J., BETTY, E.E., PAGE, A.L., & ELLIOTT, R.A. (1981)
Occurrence of naturally high cadmium levels in soils and its
accumulation by vegetation. J. environ. Qual., 10: 551-556.
LUNDGREN, G. (1976) Direct determination of cadmium in blood with a
temperature- controlled heated graphite-tube atomizer. Talenta,
23: 309-312.
MAACK, T., JOHNSON, V., KAN, S.T., FIGUEIREDO, J., & SIGULEM, D.
(1979) Renal filtration, transport, and metabolism of low molecular
weight proteins. A review. Kidney Int., 16: 251-270.
MACFARLAND, H.N. (1979) Pulmonary effects of cadmium. In: Mennear,
J., ed. Cadmium toxicity, New York, Basel, Marcel Dekker, Inc.,
pp. 113-132.
MCINNES, G. (1979) Multi-element survey: analysis of the first two
years' results. Warren Spring Laboratory, United Kingdom Department
of Industry, 44 pp (Report No. LR305 AP).
MCKENZIE, J., KJELLSTRÖM, T., & SHARMA, R.P. (1982) Cadmium intake
metabolism and effects in people with a high intake of oysters in
New Zealand, Washington, DC, US Environmental Protection Agency,
210 pp.
MCLELLAN, J.S., THOMAS, B.J., & FREMLIN, J.H. (1975) Cadmium: its
in vivo detection in man. Phys. Med. Biol., 20: 88-95.
MCLELLAN, J.S., FLANAGAN, P.R., CHAMBERLAIN, M.J., & VALBERG, L.S.
(1978) Measurements of dietary cadmium absorption in humans. J.
Toxicol. environ. Health, 4: 131-138.
MAHAFFEY, K.R., CORNELIUSSEN, P.E., JELINEK, C.F., & FIORINO, J.A.
(1975) Heavy metal exposure from foods. Environ. Health Perspect.,
12: 63-69.
MANGELSON, N.F., HILL, M.W., NIELSON, K.K., EATOUGH, D.J.,
CHRISTENSEN, J.J., IZATT, R.M., & RICHARDS, D.O. (1979)
Proton-induced X-ray emission analysis of Pima Indian autopsy
tissues. Anal. Chem., 51: 1187-1194.
MARC (1986) Biological monitoring of environmental contaminants
(plants), London, Monitoring and Assessment Research Centre, Chelsea
College, University of London, 247 pp (MARC Report Number 32).
MARGOSHES, M. & VALLEE, B.L. (1957) A cadmium kidney protein from
equine kidney cortex. J. Am. Chem. Soc., 79: 4813-4814.
MARTIN, J.H. & BROENKOW, W.W. (1975) Cadmium in plankton: elevated
concentrations off Baja California. Science, 190: 884-885.
MARTIN, J.H., ELLIOTT, P.D., ANDERLINI, V.C., GIRVIN, D., JACOBS,
S.A., RISEBROUGH, R.W., DELONG, R.L., & GILMARTIN, W.G. (1976)
Mercury-selenium-bromine imbalance in premature parturient
California sea lions. Mar. Biol., 35: 91-104.
MASON, H.J., DAVISON, A.G., & WRIGHT, A.L. (1988) Relations between
liver cadmium, cumulative exposure, and renal function in cadmium
alloy workers. Br. J. ind. Med., 45: 793-802.
MASON, K.E. & YOUNG, J.O. (1967) Effectiveness of selenium and zinc
in protecting against cadmium-induced injury of rat testis. In:
Muth, O.H., ed. Symposium: Selenium in Biomedicine, Westport,
Connecticut, Avi, pp. 383-394.
MATERNE, D., LAUWERYS, R., BUCHET, J.P., ROELS, H., BROUWERS, J., &
STANESCU, D. (1975) Investigations sur les risques résultant de
l'exposition au cadmium dans deux entreprises de production et deux
entreprises d'utilisation du cadmium. Cah. Méd. Trav., 12: 1-76.
MATSUSAKA, N., TANAKA, M., NISHIMURA, Y., YUYAMA, A., & KOBAYASHI,
H. (1972) [Whole body retention and intestinal absorption of 115Cd
in young and adult mice.] Med. Biol., 85: 275-279 (in Japanese).
MERALI, Z., KACEW, S., & SINGHAL, R.L. (1974) Response of hepatic
carbohydrate and cyclic AMP metabolism to cadmium treatment in rats.
Can. J. Physiol. Pharmacol., 53: 174-184.
MERANGER, J.C., SUBRAMANIAN, K.S., & CHALIFOUX, C. (1981) Metals and
other elements. Survey for cadmium, cobalt, chromium, copper,
nickel, lead, zinc, calcium, and magnesium in Canadian
drinking-water supplies. J. Assoc. Off. Anal. Chem., 64: 44-53.
MILLER, R.K. (1986) Placental transfer and function. The interface
for drugs and chemicals in the conceptus. In: Fabro, S. & Scialli,
A.S., ed. Drug and chemical action in pregnancy, New York, Basel,
Marcel Dekker, Inc., pp. 123-152.
MILLS, C.F. & DELGARNO, A.C. (1972) Copper and zinc status of ewes
and lambs receiving increased dietary concentration of cadmium.
Nature ( Lond.), 239: 171-173.
MOGENSEN, C.E. & SOLLING, K. (1977) Studies on renal tubular protein
reabsorption: partial and near complete inhibition by certain amino
acids. Scand. J. clin. lab. Invest., 37: 477-486.
MOHD, M.A., MURTHY, R.C., & CHANDRA, S.V. (1986) Developmental and
long-term neurobehavioral toxicity of low-level in utero Cd
exposure in rats. Toxicol. Teratol., 8: 463.
MORGAN, J.M. (1970) Cadmium and zinc abnormalities in bronchogenic
carcinoma. Cancer, 25: 1394-1398.
MORGAN, J.M., BURCH, H.B., & WATKISS, J.B. (1971) Tissue cadmium and
zinc content in emphysema and bronchogenic carcinoma. J. chron.
Dis., 24: 107-110.
MORITSUGI, M. & KOBAYASHI, J. (1964) Study on trace elements in
biomaterials. II. Cadmium content in polished rice. Ber. Ohara Inst.
Landwirtsch. Biol. Okayama Univ., 12: 145-158.
MOSCHANDREAS, D.J. (1981) Exposure to pollutants and daily time
budgets of people. Bull. NY Acad. Med., 57: 845-859.
MULFORD, C.E. (1966) Solvent extraction techniques for atomic
absorption spectoscopy. At. Absorpt. Newsl., 5: 88-90.
MULVIHILL, J.E., GAMM, S.H., & FERM, V.H. (1970) Facial formation in
normal and cadmium-treated golden hamsters. J. Embryol. exp.
Morphol., 24: 393-403.
MURASE, H., INOUE, T., SAGA, M., IWATA, T., & KUBOTA, K. (1974)
[Microscopic pathology of dogs chronically exposed to oral cadmium],
Tokyo, Japan Public Health Association, pp. 72-73 (Kankyo Hoken
Report No. 31) (in Japanese).
MUSKETT, C.J., ROBERTS, L.H., & PAGE, B.J. (1979) Cadmium and lead
pollution from secondary metal refinery operations. Sci. total
Environ., 11: 73-87.
NACKOWSKI, S.B., PUTNAM, R.D., ROBBINS, D.A., VARNER, M.O., WHIT,
L.D., & NELSON, K.W. (1977) Trace metal contamination of evacuated
blood collection tubes. Am. Ind. Hyg. Assoc. J., 38: 503-508.
NAKAGAWA, S. (1960) [A study of osteomalacia in Toyama Prefecture
(so-called Itai-itai disease).] J. Radiol. Phys. Ther. Univ.
Kanazawa, 56: 1-51 (in Japanese with English summary).
NAKANO, M., AOSHIMA, K., KATOH, T., TERANISHI, H., & KATOH, T.
(1987) Severity of tubular brush border damage in a
cadmium-polluted area (Jinzu River basin): clinical role of urinary
trehalase. Environ. Res., 44: 161-168.
NAZARI, G., FAVINO, A., & POZZI, U. (1967) [Effects of a single
subcutaneous injection of cadmium chloride in male rats.] Riv. Anat.
Pathol. Oncol., 31: 251-271 (in Italian).
NEEB, R. & WAHDAT, F. (1974) [About inverse voltammetry analysis of
cadmium in aerosols.] Z. anal. Chem., 269: 275-279 (in German).
NICAUD, P., LAFITTE, A., & GROS, A. (1942) Les troubles de
l'intoxication chronique par le cadmium. Arch. Mal. prof.,
4: 192-202.
NICHOLSON, J.K. & OSBORN, O. (1983) Kidney lesions in pelagic
seabirds with high tissue levels of cadmium and mercury. J. Zool.
(Lond.), 200: 88-118. NIELSEN, S.A. (1975) Cadmium in New Zealand
dredge oysters, geographic distribution. Int. J. environ. anal.
Chem., 4: 1-7.
NISE, G. & VESTERBERG, O. (1978) [Sampling vessels influence on
content of heavy metals (Cd, Cr, Hg, Ni, and Pb) in blood and urine
samples.] Läkartidningen, 75: 19-20 (in Swedish).
NISHIYAMA, K. & NORDBERG, G. (1972) Adsorption and elution of
cadmium on hair. Arch. environ. Health, 25: 92-96.
NISHIYAMA, S., NAKAMURA, K., & KONISHI, Y. (1986) Blood pressure and
urinary sodium and potassium excretion in cadmium-treated male rats.
Environ. Res., 40: 357-364.
NOGAWA, K. (1981) Itai-itai disease and follow-up studies. In:
Nriagu, J.O., ed. Cad-mium in the environment. II. Health effects,
New York, John Wiley and Sons, pp. 1-38.
NOGAWA, K. & ISHIZAKI, A. (1979) A comparison between cadmium in
rice and renal effects among inhabitants of the Jinzu River Basin.
Environ. Res., 18: 410-420.
NOGAWA, K., ISHIZAKI, A., FUKUSHIMA, M., SHIBATA, I., & HAGINO, N.
(1975) Studies on the women with acquired Fanconi syndrome observed
in the Ichi River basin polluted by cadmium. Environ. Res.,
10: 280-307.
NOGAWA, K., ISHIZAKI, A., & KAWANO, S. (1978) Statistical
observations of the dose-response relationships of cadmium based on
epidemiological studies in the Kakehashi River basin. Environ. Res.,
15: 185-198.
NOGAWA, K., KOBAYASHI, E., HONDA, R., & ISHIZAKI, A. (1979)
Clinico-chemical studies on chronic cadmium poisoning. I. Results of
urinary examinations. Jpn. J. Hyg., 34: 407-414.
NOGAWA, K., KOBAYASHI, E., HONDA, R., ISHIZAKI, A., KAWANO, S., &
MATUSDA, H. (1980) Renal dysfunction of inhabitants in a
cadmium-polluted area. Environ. Res., 23: 13-23.
NOGAWA, K., KOBAYASHI, E., & KONISHI, F. (1981a) Comparison of bone
lesions in chronic cadmium intoxication and vitamin D deficiency.
Environ. Res., 24: 233-249.
NOGAWA, K., KAWANO, S., & NISHI, M. (1981b) Mortality study of
inhabitants in a cadmium-polluted area with special reference to low
molecular weight proteinuria. In: Heavy metals in the environment,
Edinburgh, CEP Consultants Ltd, pp. 538-540.
NOGAWA, K., KIDO, T., YAMADA, Y., TSURITANI, I., HONDA, R.,
ISHIZAKI, N., & TERAHATA, K. (1984) Alpha-1-microglobulin in urine
as an indicator of renal tubular damage caused by environmental
cadmium exposure. Toxicol. Lett., 22: 63-68.
NOGAWA, K., TSURITANI, I., YAMADA, Y., KIDO, T., HONDA, R.,
ISHIZAKI, M., & KURIHARA, T. (1986) Sister-chromatid exchanges in
the lymphocytes of people exposed to environmental cadmium. Toxicol.
Lett., 32: 283-288.
NOGAWA, K., TSURITANI, I., KIDO, T., HONDA, R., YAMADA, Y., &
ISHIZAKI, M. (1987) Mechanism for bone disease found in inhabitants
environmentally exposed to cadmium: decreased serum
1alpha,25-dihydroxyvitamin D level. Int. Arch. occup. environ.
Health, 59: 21-30.
NOGAWA, K., HONDA, R., KIDO, T., TSURITANI, I., YAMADA, Y.,
ISHIZAKI, M., & YAMAYA, H. (1989) A dose-response analysis of
cadmium in the general environment with special reference to total
cadmium intake limit. Environ. Res., 48: 7-16.
NOMIYAMA, K. (1973) Early signs of cadmium intoxication in rabbits.
Toxicol. appl. Pharmacol., 24: P625-P635.
NOMIYAMA, K. (1974) [Studies concerning cadmium metabolism and the
etiology of poisoning], Tokyo, Japan Public Health Association,
pp. 53-59 (Kankyo Hoken Report No. 31) (in Japanese).
NOMIYAMA, K. (1977) Does a critical concentration of cadmium in
human renal cortex exist? J. Toxicol. environ. Health, 3: 607-609.
NOMIYAMA, K. (1978) Experimental studies on animals:
in vivo experiments. In: Tsuchiya, K., ed. Cadmium studies in
Japan: a review, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 47-86.
NOMIYAMA, K. (1986) The chronic toxicity of cadmium: influence of
environmental and other variables. In: Born, G.V.R., Rensselaer,
A.F., Herken, H., & Welch, A.D., ed. Handbook of experimental
pharmacology, Berlin, Heidelberg, Springer-Verlag, Vol. 80,
pp. 101-133.
NOMIYAMA, K. & FOULKES, E.C. (1977) Reabsorption of filtered cadmium
metallothionein in the rabbit kidney. Proc. Soc. Exp. Biol. Med.,
156: 97-99.
NOMIYAMA, K. & NOMIYAMA, H. (1976a) Mechanisms of urinary excretion
of cadmium: experimental studies in rabbits. In: Nordberg, G.F., ed.
Effects and dose-response relationships of toxic metals, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 371-379.
NOMIYAMA, K. & NOMIYAMA, H. (1976b) [Long-term experiment with
300 ppm oral cadmium exposure to rabbits. Intermediate report after
12 months], Tokyo, Japan Public Health Association, pp. 161-173
(Kankyo Hoken Report No. 38) (in Japanese).
NOMIYAMA, K. & NOMIYAMA, H. (1982) Tissue metallothionein in rabbits
chronically exposed to cadmium, with special reference to the
critical concentration of cadmium in the renal cortex. In: Foulkes,
E.C., ed. Biological roles of metallothionein, Amsterdam, Oxford,
New York, Elsevier Science Publishers, pp. 47-67.
NOMIYAMA, K. & NOMIYAMA, H. (1988) Health effect of six years of
dietary cadmium (cadmium contaminated rice) in monkeys. In:
Essential and toxic trace elements in human health and disease, New
York, Alan K. Liss, Inc., pp. 589-609.
NOMIYAMA, K., SUGATA, Y., YAMAMOTO, A., & NOMIYAMA, H. (1975)
Effects of dietary cadmium on rabbits. I. Early signs of cadmium
intoxication. Toxicol. appl. Pharmacol., 31: 4-12.
NOMIYAMA, K., SUGATA, Y., NOMIYAMA, H., & YAMAMOTO, A. (1976)
Dose-response relationship for cadmium. In: Nordberg, G.F., ed.
Effects and dose-response relationships of toxic metals, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 380-385.
NOMIYAMA, K., NOMIYAMA, H., YOTORIYAMA, M., & TAGUCHI, T. (1978a)
Some recent studies on the renal effects of cadmium. In: Proceedings
of the First International Cadmium Conference, San Francisco,
California, 31 January-2 February, 1977, London, Cadmium
Association, pp. 186-194.
NOMIYAMA, K., NOMIYAMA, H., & TAGUCHI, T. (1978b) Effects of
environmental temperatures on the toxicity of cadmium in mice. In:
Proceedings of the 8th Asian Conference on Occupational Health,
Tokyo, Japan Industrial Safety Association, pp. 221-225.
NOMIYAMA, K., NOMIYAMA, H., NOMURA, M., TAGUCHI, T., MATSUI, J.,
YOTORIYAMA, M., AKAHORI, F., IWAO, S., KOIZUMI, N., MASAOKA, T.,
KITAMURA, S., TSUCHIYA, K., SUZUKI, T., & KOBAYASHI, K. (1979)
Effects of dietary cadmium on rhesus monkeys. Environ. Health
Perspect., 28: 223-243.
NOMIYAMA, K., NOMIYAMA, H., AKAHORI, F., & MASAOKA, T. (1982a)
Cadmium health effects in monkeys with special reference to the
critical concentration of cadmium in the renal cortex. In:
Proceedings of the 3rd International Cadmium Conference, Miami,
Florida, 3-5 February, 1981, London, Cadmium Association,
pp. 151-156.
NOMIYAMA, K., NOMIYAMA, H., YOTORIYAMA, M., & MATSUI, K. (1982b)
Sodium dodecyl sulfate acrylamide gel electrophoretic studies on low
molecular weight proteinuria, an early sign of cadmium health
effects in rabbits. Ind. Health, 20: 11-18.
NOMIYAMA, K., NOMIYAMA, H., YOTORIYAMA, M., AKAHORI, F., & MASAOKA,
T. (1984) Some comments and proposals on dose and effects for
estimating critical concentration of cadmium in the renal cortex.
In: Proceedings of the Fourth International Cadmium Conference,
London, Cadmium Association, pp. 167-171.
NOMIYAMA, K., AKAHORI, F., MASAOKA, T., ARAI, S., NOMURA, Y.,
YOTORIYAMA, M., KOBAYASHI, K., SUZUKI, T., KAWASHIMA, H., & ONOZAWA,
A. (1987) [Dose effect and dose response relationships of 9 years
dietary cadmium in rhesus monkeys], Tokyo, Japan Public Health
Association, pp. 1-86 (Kankyo Hoken Report No. 53) (in Japanese).
NOMURA, T., KIMURA, M., TANIOKA, Y., TANIMOTO, Y., SAEGUSA, J.,
KOTAKI, N., TAZAKI, H., HATA, R., DEGUCHI, N., SUDA, T., & YOSHIKI,
S. (1988) [Influence of nutritional factors on cadmium-administered
monkeys], Tokyo, Japan Public Health Association, pp. 42-122 (Kankyo
Hoken Report No. 54) (in Japanese).
NORDBERG, G.F. (1971) Effects of acute and chronic cadmium exposure
on the testicles of mice. With special reference to protective
effects of metallothionein. Environ. Physiol. Biochem., 1: 171-187.
NORDBERG, G.F. (1972) Cadmium metabolism and toxicity. Environ.
Physiol. Biochem., 2: 7-36.
NORDBERG, G.F. (1975) Effects of long-term cadmium exposure on the
seminal vesicles of mice. J. Reprod. Fertil., 45: 165-167.
NORDBERG, G.F. & NISHIYAMA, K. (1972) Whole-body and hair retention
of cadmium in mice. Arch. environ. Health, 24: 209-214.
NORDBERG, G.F. & NORDBERG, M. (1988) Biological monitoring of toxic
metals, New York, London, Plenum Press, pp. 151-168.
NORDBERG, G.F. & PISCATOR, M. (1972) Influence of long-term cadmium
exposure on urinary excretion of protein and cadmium in mice.
Environ. Physiol. Biochem., 2: 37-49.
NORDBERG, G.F., PISCATOR, M., & LIND, B. (1971) Distribution of
cadmium among protein fractions of mouse liver. Acta pharmacol.
toxicol., 29: 456-470.
NORDBERG, G.F., SLORACH, S., & STENSTROM, T. (1973) [Cadmium
poisoning caused by a cooled soft drink machine.] Läkartidningen,
70: 601-604 (in Swedish with English summary).
NORDBERG, G.F., GOYER, R.A., & NORDBERG, M. (1975) Comparative
toxicity of cadmium metallothionein and cadmium chloride on mouse
kidney. Arch. Pathol., 99: 192-197.
NORDBERG, G.F., ROBERT, K.-H., & PANNONE, M. (1977) Pancreatic and
biliary excretion of cadmium in the rat. Acta pharmacol. toxicol.,
41: 84-88.
NORDBERG, G.F., GARVEY, J.S., & CHANG, C.C. (1982) Metallothionein
in plasma and urine of cadmium workers. Environ. Res., 28: 179-182.
NORDBERG, M. (1978) Studies on metallothionein and cadmium. Environ.
Res., 15: 381-404.
NORDBERG, M. (1984) General aspects of cadmium transport, uptake,
and metabolism by the kidney. Environ. Health Perspect., 54: 13-20.
NORDBERG, M. & NORDBERG, G.F. (1975) Distribution of metallothionein-
bound cadmium and cadmium chloride in mice: preliminary studies.
Environ. Health Perspect., 12: 103-108.
NORDBERG, M., NUOTTANIEMI, I., CHERIAN, M.G., NORDBERG, G.F.,
KJELLSTRÖM, T., & GARVEY, J.S. (1986) Characterization studies on
the cadmium-binding proteins from two species of New Zealand
oysters. Environ. Health Perspect., 65: 57-62.
NRIAGU, J.O. (1979) Global inventory of natural and anthropogenic
emissions of trace metals to the atmosphere. Nature (Lond.),
279: 409.
NRIAGU, J.O. & PACYNA, J.M. (1988) Quantitative assessment of
worldwide contamination of air, water and soils by trace metals.
Nature (Lond.), 333: 134-139.
NWANKWO, J.N., ELINDER, C.G., PISCATOR, M., & LIND, B. (1977)
Cadmium in Zambian cigarettes: an interlaboratory comparison in
analysis. Zambia J. Sci. Technol., 2(4): 1-4.
OBERDORSTER, G. (1988) Deposition and retention modeling of inhaled
cadmium in rat and human lung: An example for extrapolation of
effects and risk estimation. In: Crapo, J.D., Miller, F.J., Smolko,
E.D., Graham, J.A., & Hayes, A.W., ed. Extrapolation of dosimetric
relationships for inhaled particles and gases, New York, London,
Academic Press, pp. 345-370.
OHANIAN, E.V. & IWAI, J. (1980) Etiological role of cadmium in
hypertension in an animal model. J. environ. Pathol. Toxicol.,
3: 229-241.
OHMOMO, Y. & SUMIYA, M. (1981) Estimation of heavy metal intake from
agricultural products. In: Kitagishi, K. & Yamane, J., ed. Heavy
metal pollution in soils of Japan, Tokyo, Japan Scientific Societies
Press, pp. 235-244.
OLDIGES, H., HOCHRAINER, D., & GLASER, U. (1989) Long-term
inhalation study with Wistar rats and four cadmium compounds.
Toxicol. environ. Chem., 19: 217-222.
O'RIORDAN, M.L., HUGHES, E.G., & EVANS, H.J. (1978) Chromosome
studies on blood lymphocytes of men occupationally exposed to
cadmium. Mutat. Res., 58: 305-311.
PAGE, A.L. (1981) Cadmium in soils and its accumulation by food
crops. In: Proceedings of an International Conference on Heavy
Metals in the Environment, Amsterdam, September 1981, Edinburgh, CEP
Consultants, Ltd, pp. 206-213.
PAGE, A.L., BINGHAM, F.T., & SHANG, A.C. (1981) Cadmium. In: Lepp,
N.W., ed. Effect of heavy metal pollution on plants, Barking,
Essex, Applied Science Publishers, Vol. 1, pp. 77-109.
PANEMANGALORE, M., BANERJEE, D., ONOSAKA, S., & CHERIAN, M.G. (1983)
Changes in the intracellular accumulation and distribution of
metallothionein in rat liver and kidney during postnatal
development. Dev. Biol., 97: 95-102.
PARIZEK, J. (1957) The destructive effect of cadmium ion on
testicular tissue and its prevention by zinc. J. Endocrinol.,
15: 56-63.
PARIZEK, J. (1960) Sterilization of the male by cadmium salts. J.
Reprod. Fertil., 1: 294-309.
PARIZEK, J. (1964) Vascular changes at sites of oestrogen
biosynthesis produced by parenteral injection of cadmium salts: the
destruction of placenta by cadmium salts. J. Reprod. Fertil.,
7: 263-265.
PARIZEK, J. & ZAHOR, Z. (1956) Effects of cadmium salts on
testicular tissue. Nature (Lond.), 177: 1036-1037.
PARIZEK, J., OSTADALOVA, I., BENES, I., & PITHA, J. (1968a) The
effect of a subcutaneous injection of cadmium salts on the ovaries
of adult rats in persistent oestrus. J. Reprod. Fertil., 17:
559-562.
PARIZEK, J., OSTADALOVA, I., BENES, I., & BABICKY, A. (1968b)
Pregnancy and trace elements: the protective effect of compounds of
an essential trace element, selenium, against the peculiar toxic
effects of cadmium during pregnancy. J. Reprod. Fertil., 16:
507-509.
PARZYCK, D.C., SHAW, S.M., KESSLER, W.V., VETTER, R.J., VAN SICKLE,
D.C., & MAYES, R.A. (1978) Fetal effects of cadmium in pregnant rats
on normal and zinc-deficient diets. Bull. environ. Contam. Toxicol.,
19: 206-214.
PATON, G.R. & ALLISON, A.C. (1972) Chromosome damage in human cell
cultures induced by metal salts. Mutat. Res., 16: 332-336.
PERRY, H.M., Jr & ERLANGER, M.W. (1973) Elevated circulating renin
activity in rats following doses of cadmium known to induce
hypertension. J. lab. clin. Med., 82: 399-405.
PERRY, H.M., Jr & ERLANGER, M.W. (1974) Metal-induced hypertension
following chronic feeding of low doses of cadmium and mercury. J.
lab. clin. Med., 83: 541-547.
PERRY, H.M., Jr & ERLANGER, M.W. (1982) Effect of diet on increases
in systolic pressure induced in rats by chronic cadmium feeding. J.
Nutr., 112: 1983-1989.
PERRY, H.M., Jr & KOPP, S.J. (1983) Does cadmium contribute to human
hypertension? Sci. total Environ., 26: 223-232.
PERRY, H.M., Jr, ERLANGER, M.W., YUNICE, A., SCHOEPFLE, E., & PERRY,
E.F. (1970) Hypertension and tissue metal levels following
intravenous cadmium, mercury, and zinc. Am. J. Physiol., 219:
755-761.
PERRY, H.M., Jr, PERRY, E.F., & PURIFOY, J.E. (1971) Antinatriuretic
effect of intramuscular cadmium in rats. Proc. Soc. Exp. Biol. Med.,
136: 1240-1244.
PERRY, H.M., Jr, ERLANGER, M.W., & PERRY, E.F. (1976) Limiting
conditions for the induction of hypertension in rats by cadmium. In:
Hemphill, D.D., ed. Trace substances in environmental health - VIII,
Columbia, Missouri, University of Missouri Press, pp. 51-57.
PERRY, H.M., Jr, ERLANGER, M., & PERRY, E.F. (1977) Hypertension
following chronic, very low-dose cadmium feeding. Proc. Soc. Exp.
Biol. Med., 156: 173-176.
PETERING, D.H., LOFTSGAARDEN, J., SCHNEIDER, J., & FOWLER, B. (1984)
Metabolism of cadmium, zinc and copper in the rat kidney: the role
of metallothionein and other binding sites. Environ. Health
Perspect., 54: 73-81.
PETERING, H.G., JOHNSON, M.A., & STEMMER, K. (1971) Studies of zinc
metabolism in the rat. I. Dose-response effects of cadmium. Arch.
environ. Health, 23: 93-96.
PETERING, H.G., CHOUDHURY, H., & STEMMER, K.L. (1979) Some effects
of oral ingestion of cadmium on zinc, copper, and iron metabolism.
Environ. Health Perspect., 28: 97-106.
PETERSON, P.A. (1971) Characteristics of a vitamin A-transporting
protein complex occurring in human serum. J. biol. Chem.,
246: 34-43.
PETERSON, P.A. & BERGGÅRD, I. (1971) Isolation and properties of a
human retinol-transporting protein. J. biol. Chem., 246: 25-33.
PETERSON, P.A., EVRIN, P.-E., & BERGGÅRD, I. (1969) Differentiation
of glomerular, tubular, and normal proteinuria: determinations of
urinary excretion of ß-2-microglobulin, albumin, and total protein.
J. clin. Invest., 48: 1189-1198.
PHILIPP, R. (1985) Cadmium-risk assessment of an exposed residential
population: a review. J. R. Soc. Med., 78: 328-333.
PIOTROWSKI, J.K., TROJANOWSKA, B., WISNIEWSKA-KNYPL, J.M., &
BOLANOWSKI, W. (1974) Mercury binding in the kidney and liver of
rats repeatedly exposed to mercuric chloride: induction of
metallothionein by mercury and cadmium. Toxicol. appl. Pharmacol.,
27: 11-19.
PISCATOR, M. (1962) Proteinuria in chronic cadmium poisoning. II.
The applicability of quantitative and qualitative methods of protein
determination for the demonstration of cadmium proteinuria. Arch.
environ. Health, 5: 325-332.
PISCATOR, M. (1964) [On cadmium in normal kidneys together with a
report on the isolation of metallothionein from livers of
cadmium-exposed rabbits.] Nord. Hyg. Tidskr., 45: 76-82 (in
Swedish).
PISCATOR, M. (1966a) Proteinuria in chronic cadmium poisoning. III.
Electrophoretic and immunoelectrophoretic studies on urinary
proteins from cadmium workers, with special reference to the
excretion of low molecular weight proteins. Arch. environ. Health,
12: 335-344.
PISCATOR, M. (1966b) Proteinuria in chronic cadmium poisoning. IV.
Gel filtration and ion exchange chromatography of urinary proteins
from cadmium workers. Arch. environ. Health, 12: 345-359.
PISCATOR, M. (1971) Data on interferences in cadmium analysis. In:
Friberg, L., Piscator, M., & Nordberg, G., ed. Cadmium in the
environment, Cleveland, Ohio, CRC Press, p. 17.
PISCATOR, M. & TSUCHIYA, K. (1971) In: Friberg, L., Piscator, M., &
Nordberg, G., ed. Cadmium in the environment, Cleveland, Ohio,
Chemical Rubber Company Press, pp. 112-114.
PISCATOR, M. & VOUK, V.B. (1979) Sampling and analytical methods.
In: Friberg, L., Nordberg, G.F., & Vouk, V.B., ed. Handbook on the
toxicology of metals, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 33-46.
PISCATOR, M., KJELLSTRÖM, T., & LIND, B. (1976) Contamination of
cigarettes and pipe tobacco by cadmium-oxide dust. Lancet, 2: 587.
PISCATOR, M., BJORK, L., & NORDBERG, M. (1981) Beta-2-microglobulin
levels in serum and urine of cadmium exposed rabbits. Acta.
pharmacol. toxicol., 49: 1-7.
PLEBAN, P.A., KERKAY, I., & PEARSON, K.H. (1981) Polarized
Zeeman-effect flameless atomic absorption spectrometry of cadmium,
copper, lead, and manganese in human kidney cortex. Clin. Chem.,
27: 68-72.
POTTS, C.L. (1965) Cadmium proteinuria - the health of battery
workers exposed to cadmium oxide dust. Ann. occup. Hyg., 8: 55-61.
PRIGGE, E. (1978) Early signs of oral and inhalative cadmium uptake
in rats. Arch. Toxicol., 40: 231-247.
PRIGGE, E., BAUMERT, H.P., & MUHLE, H. (1977) Effects of dietary and
inhalative cadmium on haemoglobin and haematocrit in rats. Bull.
environ. Contam. Toxicol., 17: 585-590.
PRINCI, F. (1947) A study of industrial exposure to cadmium. J. ind.
Hyg. Toxicol., 29: 315-320.
PRINCI, F. & GEEVER, E.F. (1950) Prolonged inhalation of cadmium.
Arch. ind. Hyg. occup. Med., 1: 651-661.
PROCKOP, D.J. & DAVIDSON, W.D. (1964) A study of urinary and serum
lysozyme in patients with renal disease. New Engl. J. Med.,
270: 269-274.
PRODAN, L. (1932) Cadmium poisoning. II. Experimental cadmium
poisoning. J. ind. Hyg. Toxicol., 14: 174-196.
PULIDO, P., KAGI, J.H., & VALLEE, B.L. (1966) Isolation and some
properties of human metallothionein. Biochemistry, 5: 1768-1777.
RAHOLA, T., AARAN, R.-K., & MIETTINEN, J.K. (1972) Half-time studies
of mercury and cadmium by whole-body counting. In: Assessment of
radioactive contamination in man, New York, International Atomic
Energy Agency Unipublishers, pp. 553-562 (IAEA Proceedings Series
No. Sm-150/13).
RAMAYA, L.K. & POMERANTZEVA, M.O. (1977) [Investigation of cadmium
chloride mutagenic effect on germ cells of male mice.] Genetika,
13: 59-63 (in Russian).
RAMEL, C. & FRIBERG, L. (1971) Carcinogenic and mutagenic effects.
In: Friberg, L., Piscator, M., & Nordberg, G.F., ed. Cadmium in the
environment, Cleveland, Ohio, CRC Press, pp. 109-110.
RAUHUT, A. (1980) Survey of industrial emission of cadmium in the
European Economic Community: Final Report, Brussels, Commission of
the European Communities, Vol. 2, pp. 1-44.
REDDY, C.S., SVOBODA, D., AZARNOFF, D., & DEWAR, R. (1973)
Cadmium-induced Leydig cell tumors of rat testis: morphologic and
histochemical study. J. Natl Cancer Inst., 51: 891-903.
RICHARDSON, M.E. & FOX, M.R.S. (1974) Dietary cadmium and
enteropathy in the Japanese quail. Lab. Invest., 31: 722-731.
RIVM (1988) In: Ros, J.P.M. & Sloof, W., ed. Integrated criteria
document cadmium, Bilthoven, The Netherlands, National Institute of
Public Health and Environmental Protection (RIVM-Report No.
758476004).
ROBBINS, S.L., COTRAN, R.S., & KUMAR, U. (1984) Pathological basis
of disease, 3rd ed., Philadelphia, Pennsylvania, W.B. Saunders &
Co., pp. 1327-1328.
ROELS, H., HUBERMONT, G., BUCHET, J.P., & LAUWERYS, R. (1978)
Placental transfer of lead, mercury, cadmium and carbon monoxide in
women. III. Factors influencing the accumulation of heavy metals in
the aplacenta and the relationship between metal concentration in
the placenta and in maternal and cord blood. Environ. Res.,
16: 236-245.
ROELS, H., LAUWERYS, R.R., BUCHET, J.P., & BERNARD, A. (1981a)
Environmental exposure to cadmium and renal function of aged women
in three areas of Belgium. Environ. Res., 24: 117-130.
ROELS, H., LAUWERYS, R.R., BUCHET, J.P., BERNARD, A., CHETTLE, D.R.,
HARVEY, T.C., & AL-HADDAD, J.K. (1981b) In vivo measurement of
liver and kidney cadmium in workers exposed to this metal: its
significance with respect to cadmium in blood and urine. Environ.
Res., 26: 217-240.
ROELS, H., DJUBGANG, J., BUCHET, J.P., BERNARD, A., & LAUWERYS, R.R.
(1982) Evolution of cadmium-induced renal dysfunction in workers
removed from exposure. Scand. J. Work Environ. Health, 8: 191-200.
ROELS, H., LAUWERYS, R.R., & DARDENNE, A.N. (1983a) The critical
level of cadmium in human renal cortex: a reevaluation. Toxicol.
Lett., 15: 357-360.
ROELS, H., LAUWERYS, R.R., BUCHET, J.P., BERNARD, A., GARVEY, J.S.,
& LINTON, H.J. (1983b) Significance of urinary metallothionein in
workers exposed to cadmium. Int. Arch. occup. environ. Health,
52: 159-166.
ROELS, H., LAUWERYS, R.R., BUCHET, J.P., BERNARD, A.M., VOS, A., &
OVERSTEYNS, M. (1989) Health significance of cadmium induced renal
dysfunction: a five year follow up. Br. J. ind. Med., 46: 755-764.
ROHR, G. & BAUCHINGER, M. (1976) Chromosome analyses in cell
cultures of the Chinese hamster after application of cadmium
sulfate. Mutat. Res., 40: 125-130.
SAITO, H. (1987) [Follow-up study on health status on residents in a
cadmium polluted area (Sasu, Izahara town of Nagasaki Prefecture)
after improvement of cadmium-polluted lands], Tokyo, Japan Public
Health Association, pp. 215-216 (Kankyo Hoken Report No. 53) (in
Japanese).
SAITO, H., SHIOJI, R., FURUKAWA, Y., NAGAI, K., ARIKAWA, T., SAITO,
T., SASAKI, Y., FURUYAMA, T., & YOSHINAGA, K. (1977) Cadmium-induced
proximal tubular dysfunction in a cadmium-polluted area. Nephrology,
6: 1-12.
SAITO, H., NAKANO, A., TOHYAMA, C., MITANE, Y., SUGIHIRA, N., &
WAKISAKA, I. (1983) [Report on a health examination of inhabitants
of areas with cadmium pollution of soil], Tokyo, Japan Public Health
Association, pp. 91-95 (Kankyo Hoken Report No. 40) (in Japanese).
SALMELA, S. & VUORI, E. (1979) Contamination with cadmium from
micropipette tips. Talanta, 26: 175-176.
SALTZMAN, B.E. (1953) Colorimetric microdetermination of cadmium
with dithizone. Anal. Chem., 25: 493-496.
SAMARAWICKRAMA, G.P. & WEBB, M. (1979) Acute effects of cadmium on
the pregnant rat and embryo-fetal development. Environ. Health
Perspect., 28: 245-249.
SANDERS, C.L. & MAHAFFEY, J.A. (1984) Carcinogenicity of single and
multiple intratracheal instillations of cadmium oxide in the rat.
Environ. Res., 33: 227-233.
SANGSTER, B., DE GROOT, G., LOEBER, J.G., DERKS, H.J.G.M., KRAJNC,
E.I., & SAVELKOUL, T.J.F. (1984) Urinary excretion of cadmium,
protein, beta-2-microglobulin and glucose in individuals living in a
cadmium-polluted area. Hum. Toxicol., 3: 7-21.
SCHARPF, L.G., Jr, HILL, I.D., WRIGHT, P.L., PLANK, J.B., KEPLINGER,
M.L., & CALANDRA, J.C. (1972) Effect of sodium nitrilotriacetate on
toxicity, teratogenicity, and tissue distribution of cadmium. Nature
(Lond.), 239: 231-234.
SCHERER, G. & BARKEMEYER, H. (1983) Cadmium concentrations in
tobacco and tobacco smoke. Ecotoxicol. environ. Saf., 7: 71-78.
SCHMID, B.P., KAO, J., & GOULDING, E. (1985) Evidence for reopening
of the cranial tube in mouse embryo treated with cadmium chloride.
Experientia (Basel), 41: 271-272.
SCHROEDER, H.A. (1964) Cadmium hypertension in rats. Am. J.
Physiol., 207: 62-66.
SCHROEDER, H.A. (1965) Cadmium as a factor in hypertension. J.
chron. Dis., 18: 647-656.
SCHROEDER, H.A. (1967) Cadmium, chromium, and cardiovascular
disease. Circulation, 35: 570.
SCHROEDER, H.A. & BALASSA, J.J. (1961) Abnormal trace metals in man:
cadmium. J. chron. Dis., 14: 236-258.
SCHROEDER, H.A. & MITCHENER, M. (1971) Toxic effects of trace
elements on the reproduction of mice and rats. Arch. environ.
Health, 23: 102-106.
SCHROEDER, H.A. & VINTON, W.H., Jr (1962) Hypertension induced in
rats by small doses of cadmium. Am. J. Physiol., 202: 515-518.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H., Jr (1964) Chromium,
lead, cadmium, nickel, and titanium in mice: effect on mortality,
tumours, and tissue levels. J. Nutr., 83: 239-250.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H., Jr (1965) Chromium,
cadmium, and lead in rats: effects on life span, tumours, and tissue
levels. J. Nutr., 86: 51-66.
SCOTT, R., MILLS, E.A., FELL, G.S., HUSAIN, F.E.R., YATES, A.J.,
PATERSON, P.J., MCKIRDY, A., OTTOWAY, J.M., FITZGERALD-FINCH, O.P.,
& LAMONT, A. (1976) Clinical and biochemical abnormalities in
coppersmiths exposed to cadmium. Lancet, 2: 396-398.
SCOTT, R., AUGHEY, E., & SINCLAIR, J. (1977) Histological and
ultrastructural changes in rat kidney following cadmium injection.
Urol. Res., 5: 15-20.
SCOTT, R., PATERSON, P.J., BURNS, R., OTTOWAY, J.M., HUSAIN, F.E.R.,
FELL, G.S., DUMBUYA, S., & IGBAL, M. (1978) Hypercalciuria related
to cadmium exposure. Urology, 11: 462-465.
SCOTT, R., PATERSON, P.J., BURNS, R., FELL, G.S., & OTTOWAY, J.M.
(1979) The effects of treatment on the hypercalciuria of chronic
cadmium poisoning. Urol. Res., 7: 285-289.
SCOTT, R., HAYWOOD, J.K., BODDY, K., WILLIAMS, E.D., HARVEY, M., &
PATERSON, P.J. (1980) Whole body calcium deficit in cadmium-exposed
workers with hypercalciuria. Urology, 15: 356-359.
SHAIKH, Z.A. & HIRAYAMA, K. (1979) Metallothionein in the
extracellular fluids as an index of cadmium toxicity. Environ.
Health Perspect., 28: 267-272.
SHAIKH, Z.A. & SMITH, J.C. (1980) Metabolism of orally ingested
cadmium in humans. In: Holmstedt, B., Lauwerys, R., Mercier, M., &
Roberfroid, M., ed. Mechanisms of toxicity and hazard evaluation,
Amsterdam, Elsevier/North Holland Biomedical Press, pp. 569-574.
SHARMA, R.P., KJELLSTRÖM, T., & MCKENZIE, J.M. (1983) Cadmium in
blood and urine among smokers and non-smokers with high cadmium
intake via food. Toxicology, 29: 163-171.
SHARRETT, A.R., CARTER, A.P., ORHEIM, R.M., & FEINLEIB, M. (1982)
Daily intake of lead, cadmium, copper, and zinc from drinking-water:
the Seattle study of trace metal exposure. Environ. Res.,
28: 456-475.
SHERLOCK, J. & WALTERS, B. (1983) Dietary intake of heavy metals and
its estimation. Chem. Ind., 13: 505-508.
SHERLOCK, J., SMART, G.A., & WALTERS, B. (1983) Dietary surveys on a
population at Shipham, Somerset, United Kingdom. Sci. total
Environ., 29: 121-142.
SHIGEMATSU, I. & KAWAGUCHI, T. (1978) Environmental pollution and
health effects, Yamagata Prefecture. In: Tsuchiya, K., ed. Cadmium
studies in Japan: a review, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 199-211.
SHIGEMATSU, I., KAWAGUCHI, T., & YANAGAWA, H. (1978) Reanalysis of
the results of health examinations conducted on the general
population in the cadmium polluted areas of Japan. In: Tsuchiya, K.
ed. Cadmium studies in Japan: a review, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 253-264.
SHIGEMATSU, I., YANAGAWA, H., & KAWAGUCHI, T. (1975) [Record filling
of health examinations for inhabitants in the Cd-polluted areas (2nd
and 3rd reports)], Tokyo, Japan Public Health Association,
pp. 72-101 (Kankyo Hoken Report No. 36) (in Japanese).
SHIGEMATSU, I., MINOWA, M., YOSHIDA, T., & MIYAMOTO, K. (1979)
Recent results of health examinations on the general population in
cadmium-polluted and control areas in Japan. Environ. Health
Perspect., 28: 205-210.
SHIGEMATSU, I., MINOWA, M., YOSHIDA, T., & MIYAMOTO, K. (1980)
Epidemiological aspects of environmental cadmium and its effects in
Japan. In: Proceedings of the 2nd International Cadmium Conference,
Cannes, 6-8 February, 1979, London, Cadmium Association,
pp. 111-117.
SHIGEMATSU, I., KITAMURA, S., TAKEUCHI, J., MINOWA, M., NAGAI, M.,
USUI, T., & FUKUSHIMA, M. (1981) A retrospective mortality study on
cadmium-exposed populations in Japan. In: Recent studies on health
effects of cadmium in Japan, Tokyo, The Japan Cadmium Research
Committee, Japan Environment Agency, pp. 303-317.
SHIGEMATSU, I., MITAMURA, S., TAKEUCHI, J., MINOWA, M., NAGAI, M., &
FUKUSHIMA, M. (1982) A retrospective mortality study on
cadmium-exposed populations in Japan. In: Proceedings of the 3rd
International Cadmium Conference, Miami, Florida, 3-5 February,
1981, London, Cadmium Association, pp. 115-118.
SHIGEMATSU, I., MINOWA, M., NAGAI, M., OHMURA, T., & TAKEUCHI, K.
(1983) A retrospective mortality study on cadmium-exposed
populations in Japan (supplement): mortalities by level of pollution
in Toyama Prefecture. In: The influence of nutritional factors on
cadmium administered to monkeys, Tokyo, Japan Environment Agency,
pp. 158-174.
SHILLER, A.M. & BOYLE, E.A. (1987) Variability of dissolved trace
metals in the Mississippi River. Geochim. Cosmochim. Acta.,
51: 3273-3277.
SHIRAISHI, Y. (1975) Cytogenetic studies in 12 patients with
Itai-itai disease. Humangenetik, 27: 31-44.
SHIRAISHI, Y., KURAHASHI, H., & YOSHIDA, T.H. (1972) Chromosomal
aberrations in cultured human leukocytes induced cadmium sulfide.
Proc. Jpn. Acad., 48(21): 133-137.
SHIROISHI, K., ANAYAMA, M., TANII, M., FUKUYAMA, Y., & KUBOTA, K.
(1972) [Urinary findings of different age groups in the inhabitants
of Itai-itai disease districts.] Med. Biol., 85: 263-267 (in
Japanese).
SHIROISHI, K., KJELLSTROM, T., ANAYAMA, M., SHIMADA, T., IWATA, T.,
NISHINO, H., MATSUNAGA, A., WATANABE, M., & KUBOTA, K. (1975)
[Studies on the urinary ß-2-microglobulin level of the inhabitants
in Cd-polluted districts.] Jpn J. Hyg., 30: 71 (in Japanese).
SHIROISHI, K., KJELLSTRÖM, T., KUBOTA, K., EVRIN, P.E., ANAYAMA, M.,
VESTERBERG, O., SHIMADA, T., PISCATOR, M., IWATA, T., & NISHINO, H.
(1977) Urine analysis for detection of cadmium-induced renal
changes, with special reference to ß-2-microglobulin. Environ. Res.,
13: 407-424.
SIMPSON, W.R. (1981) A critical review of cadmium in the marine
environment. Prog. Oceanogr., 10: 1-70.
SKERFVING, S., CHRISTOFFERSON, J.O., SHUTZ, A., WELINDER, H., SPANG,
G., AHLGREN, L., & MATTSON, S. (1987) Biological monitoring, by in
vivo XRF measurements, of occupational exposure to lead, cadmium
and mercury. Biol. Trace Elem. Res., 13: 241-251.
SKOG, E. & WAHLBERG, J.E. (1964) A comparative investigation of
percutaneous absorption of metal compounds in guinea-pig by means of
the radioactive isotopes, 51Cr, 58Co, 65Zn, 110Ag, 115Cd,
203Hg. J. invest. Dermatol., 43: 187-192.
SMITH, J.P., SMITH, J.C., & MCCALL, A.J. (1960) Chronic poisoning
from cadmium fume. J. Pathol. Bacteriol., 80: 287-296.
SMITH, T. (1982) Emission inventory case studies - policy aspects.
J. Air Pollut. Control Assoc., 32(10): 1017-1020.
SMITH, T., PETTY, T.L., READING, J.C., & LAKSHMINARAYAN, S. (1976)
Pulmonary effects of chronic exposure to airborne cadmium. Am. Rev.
respir. Dis., 114: 161-169.
SMITH, T., ANDERSON, R.J., & READING, J.C. (1980) Chronic cadmium
exposures associated with kidney function effects. Am. J. ind. Med.,
1: 319-337. SNIDER, G.L., HAYES, J.A., KORTHY, A.L., & LEWIS, G.P.
(1973) Centrilobular emphysema experimentally induced by cadmium
chloride aerosol. Am. Rev. respir. Dis., 108: 40-48.
SONAWANE, B.R., NORDBERG, M., NORDBERG, G.F., & LUCIER, G.W. (1975)
Placental transfer of cadmium in rats: influence of dose and
gestational age. Environ. Health Perspect., 12: 97-102.
SORAHAN, T. (1987) Mortality from lung cancer among a cohort of
nickel cadmium battery workers: 1946-84. Br. J. ind. Med.,
44: 803-809.
SORAHAN, T. & WATERHOUSE, J.A.H. (1983) Mortality study of
nickel-cadmium battery workers by the method of regression models in
life tables. Br. J. ind. Med., 40: 293-300.
SPENCER, H., ASMUSSEN, C.R., HOLTZMAN, R.B., & KRAMER, L. (1979)
Metabolic balances of cadmium, copper, manganese, and zinc in man.
Am. J. clin. Nutr., 32: 1867-1875.
SPORN, A., DINU, I., & STOENESCU, L. (1970) Influence of cadmium
administration on carbohydrate and cellular energetic metabolism in
the rat liver. Rev. roum. Biochim., 7: 299-305.
SQUIBB, K.S., PRITCHARD, J.B., & FOWLER, B.A. (1982) Renal
metabolism and toxicity of metallothionein. In: Foulkes, E.C., ed.
Biological roles of metallothionein, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 181-190.
SQUIBB, K.S., PRITCHARD, J.B., & FOWLER, B.A. (1984)
Cadmium-metallothionein nephropathy: relationships between
ultrastructural/biochemical alterations and intra-cellular cadmium
binding. J. Pharmacol. exp. Ther., 229(1): 311-321.
SQUIRE, J.R., HARDWICKE, J., & SOOTHILL, J.F. (1962) Proteinuria.
In: Black, D.A.K., ed. Renal disease, Oxford, Blackwell Scientific
Publishers, pp. 218-231.
STACEY, N.H., CANTILENA, L.R., & KLAASSEN, C.D. (1980) Cadmium
toxicity and lipid peroxidation in isolated rat hepatocytes.
Toxicol. appl. Pharmacol., 53: 470-480.
STAESSEN, J., BULPITT, C.J., ROELS, H., BERNARD, A., FAGARD, R.,
JOOSSENS, J.V., LAUWERYS, R.R., LIJNEN, P., & AMERY, A. (1984)
Urinary cadmium and lead and their relationship to blood pressure in
a population with low average exposure. Br. J. ind. Med.,
41: 241-248.
STANESCU, D., VEITNER, C., FRANS, A., CONCETTE, L., ROELS, H.,
LAUWERYS, R.R., & BRASSEUR, L. (1977) Effects on lung of chronic
occupational exposure to cadmium. Scand. J. respir. Dis.,
58: 289-303.
STEWART, M. & HUGHES, E.G. (1981) Urinary beta-2-microglobin in the
biological monitoring of cadmium workers. Br. J. Ind. Med.,
38: 170-174.
STOEPPLER, M. & BRANDT, K. (1978) Determination of lead and cadmium
in whole blood by electrothermal atomic absorption spectroscopy. In:
Doss, M., ed. Proceedings of an International Symposium on Clinical
Biochemistry on Diagnosis and Therapy of Porphyrias and Lead
Intoxication, New York, Springer-Verlag, pp. 185-187.
STOEPPLER, M. & BRANDT, K. (1980) Contributions to automated trace
analysis. V. Determination of cadmium in whole blood and urine by
electrothermal atomic absorption spectrophotometry. Fresenius Z.
anal. Chem., 300: 372-380.
STOEPPLER, M., VALENTA, P., & NURNBERG, H.W. (1979) Applications of
independent methods and standard materials: an effective approach to
reliable trace and ultra trace analysis of metals and metalloids in
environmental and biological materials. Fresenius Z. anal. Chem.,
297: 22-34.
STONEBURNER, D.L. (1978) Heavy metals in tissues of stranded
short-finned pilot whales. Sci. total Environ., 9: 293-297.
STOWE, H.D., WILSON, M., & GOYER, R.A. (1972) Clinical and
morphological effects of oral cadmium toxicity in rabbits. Arch.
Pathol., 94: 389-405.
STRAUSS, R.H., PALMER, K.C., & HAYES, J.A. (1976) Acute lung injury
induced by cadmium aerosol. I. Evolution of alveolar cell damage.
Am. J. Pathol., 84: 561-568.
SUBRAMANIAN, K.S. & MERANGER, J.C. (1981) A rapid electrothermal
atomic absorption spectrophotometric method for cadmium and lead in
human whole blood. Clin. Chem., 27: 1866-1871.
SUGAWARA, C. & SUGAWARA, N. (1974) Cadmium toxicity for rat
intestine, especially on the absorption of calcium and phosphorus.
Jpn. J. Hyg., 28: 511-516.
SULLIVAN, M.F., HARDY, J.T., MILLER, B.M., BUSCHBOM, R.L., &
SIEWICKI, T.C. (1984) Absorption and distribution of cadmium in
mice fed diets containing either inorganic or oyster-incorporated
cadmium. Toxicol. appl. Pharmacol., 72: 210-217.
SUMINO, K., HAYAKAWA, K., SHIBATA, T., & KITAMURA, S. (1975) Heavy
metals in normal Japanese tissues. Arch. environ. Health,
30: 487-494.
SUZUKI, S. & LU, C.C. (1976) A balance study of cadmium: an
estimation of daily input, output, and retained amount in two
subjects. Ind. Health, 14: 53-65.
SUZUKI, S., SUZUKI, T., & ASHIZAWA, M. (1965) Proteinuria due to
inhalation of cadmium stearate dust. Ind. Health, 3: 73-85.
SUZUKI, S., TAGUCHI, T., & YOKOHASHI, G. (1969) Dietary factors
influencing upon the retention rate of orally-administered
115CdCl2 in mice, with special reference to calcium and protein
concentration in diet. Ind. Health, 7: 155-162.
SUZUKI, Y. (1980) Cadmium metabolism and toxicity in rats after
long-term subcutaneous administration. J. Toxicol. environ. Health,
6: 469-482.
SUZUKI, Y. (1982) Metal-binding properties of metallothionein in
extracellular fluids and its role in cadmium-exposed rats. In:
Foulkes, E.C., ed. Biological roles of metallothionein, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 25-67.
SVARTENGREN, M., ELINDER, C.G., FRIBERG, L., & LIND, B. (1986)
Distribution and concentration of cadmium in human kidney. Environ.
Res., 39: 1-7.
SVENSSON, B.-G., BJORNHAM, A., SCHUTZ, A., LETTEVALL, U., NILSSON,
A., & SKERFVING, S. (1987) Acidic deposition and human exposure to
toxic metals. Sci. total Environ., 67: 101-115.
TAKABATAKE, E. (1978a) Environmental pollution and health effects:
Oita Prefecture. In: Tsuchiya, K., ed. Cadmium studies in Japan: a
review, Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp. 215-218.
TAKABATAKE, E. (1978b) Nagasaki Prefecture. In: Tsuchiya, K., ed.
Cadmium studies in Japan: a review, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 181-192.
TAKASHIMA, M., MORIWAKI, S., & ITOKAWA, Y. (1980) Osteomalacic
change induced by long-term administration of cadmium to rats.
Toxicol. appl. Pharmacol., 54: 223-228.
TAKEBAYASHI, S. (1980a) First autopsy case, suspicious of cadmium
intoxication, from the cadmium-polluted area in Tsushima, Nagasaki
Prefecture. In: Shigematsu, I. & Nomiyama, K., ed. Cadmium-induced
osteopathy, Tokyo, Japan Public Health Association, pp. 124-138.
TAKEBAYASHI, S. (1980b) [Clinical and pathological findings of a
case with osteomalacia in a cadmium-polluted area (Tsushima Island)
(1st autopsied case)], Tokyo, Japan Public Health Association,
pp. 259-262 (Kankyo Hoken Report No. 46) (in Japanese).
TAKEBAYASHI, S. (1983a) [Clinical and pathological findings of a
case with osteomalacia in a cadmium-polluted area (Tsushima Island)
(2nd autopsied case)], Tokyo, Japan Public Health Association,
pp. 207-211 (Kankyo Hoken Report No. 49) (in Japanese).
TAKEBAYASHI, S. (1983b) [Clinical and pathological findings of a
case with diabetic nephropathy in a cadmium-polluted area (Tsushima
Island) (3rd autopsied case)], Tokyo, Japan Public Health
Association, pp. 212-217 (Kankyo Hoken Report No. 49) (in Japanese).
TAKEBAYASHI, S. (1984) [Clinical and pathological findings of a case
with osteomalacia in a cadmium-polluted area (Tsushima Island) (4th
autopsied case)], Tokyo, Japan Public Health Association,
pp. 238-249 (Kankyo Hoken Report No. 50) (in Japanese).
TAKEBAYASHI, S., SATO, T., HARADA, K., & YOSHIMURA, S. (1985)
[Clinical and pathological findings of a case with osteomalacia in a
cadmium-polluted area (Tsushima Island) (5th autopsied case)],
Tokyo, Japan Public Health Association, pp. 245-251 (Kankyo Hoken
Report No. 51) (in Japanese).
TAKEBAYASHI, S., SATO, T., HARADA, K., HIRAI, Y., & YOSHIMURA, S.
(1987a) [Clinical and pathological findings of a case with
osteomalacia in a cadmium-polluted area (Tsushima Island) (6th
autopsied case)], Tokyo, Japan Public Health Association,
pp. 294-305 (Kankyo Hoken Report No. 53) (in Japanese).
TAKEBAYASHI, S., SATO, T., HARADA, K., HIRAI, Y., & YOSHIMURA, S.
(1987b) [Clinical and pathological findings of a case with
osteomalacia in a cadmium-polluted area (Tsushima Island) (7th
autopsied case)], Tokyo, Japan Public Health Association,
pp. 306-319 (Kankyo Hoken Report No. 53) (in Japanese).
TAKEBAYASHI, S., HIRAI, Y., & YOSHIMURA, S. (1988a) [Clinical and
pathological findings of a case with osteomalacia in a
cadmium-polluted area (Tsushima Island) (8th autopsied case)],
Tokyo, Japan Public Health Association, pp. 187-192 (Kankyo Hoken
Report No. 54) (in Japanese).
TAKEBAYASHI, S., HIRAI, Y., & YOSHIMURA, S. (1988b) [Clinical and
pathological findings of a case with osteomalacia in a
cadmium-polluted area (Tsushima Island) (9th autopsied case)],
Tokyo, Japan Public Health Association, pp. 193-199 (Kankyo Hoken
Report No. 54) (in Japanese).
TAKEBAYASHI, S., HIRAI, Y., & YOSHIMURA, S. (1988c) [Clinical and
pathological findings of a case with osteomalacia in a
cadmium-polluted area (Tsushima Island) (10th autopsied case)],
Tokyo, Japan Public Health Association, pp. 200-206 (Kankyo Hoken
Report No. 54) (in Japanese).
TAKEBAYASHI, S., HARADA, K., & YOSHIMURA, S. (1988d) [Clinical and
pathological findings of a case with osteomalacia in a
cadmium-polluted area (Tsushima Island) (11th autopsied case)],
Tokyo, Japan Public Health Association, pp. 207-213 (Kankyo Hoken
Report No. 54) (in Japanese).
TAKENAKA, S., OLDIGES, H., KOENIG, H., HOCHRAINER, D., &
OBERDOERSTER, G. (1983) Carcinogenicity of cadmium aerosols in
Wistar rats. J. Natl Cancer Inst., 70: 367-371.
TANAKA, K. (1982) Effects of hepatic disorders on the fate of
cadmium in rats. In: Foulkes, E.C., ed. Biological roles of
metallothionein, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 237-249.
TANAKA, K., MIN, K.-S., ONOSAKA, S., FUKUHARA, C., & UEDA, M. (1985)
The origin of metallothionein in red blood cells. Toxicol. appl.
Pharmacol., 78: 63-66.
TARASENKO, N.YU. & VOROBJEVA, R.S. (1973) [Hygienic problems
connected with the use of cadmium.] Vestn. Akad. Med. Nauk. SSR,
28: 37-43 (in Russian with English abstract).
TARASENKO, N.YU., VOROBJEVA, R.S., SPIRIDINOVA, V.S., & SABALINA,
L.P. (1974) Experimental investigation of toxicity of cadmium and
zinc caprylates. J. Hyg. Epidemiol. Microbiol. Immunol.,
18: 144-153.
TARASENKO, N.YU., VOROBJEVA, R.S., SABALINA, L.P., & CVETKOVA, R.P.
(1975) [Cadmium in the environment and its effect on calcium
metabolism.] Gig. i Sanit., 9: 22-25 (in Russian).
TASK GROUP ON LUNG DYNAMICS (1966) Deposition and retention models
for internal dosimetry of the human respiratory tract. Health Phys.,
12: 173-208.
TASK GROUP ON METAL INTERACTIONS (1978) Factors influencing
metabolism and toxicity of metals: a consensus report. Environ.
Health Perspect., 25: 3-42.
TASK GROUP ON METAL TOXICITY (1976) Conceptual considerations:
critical organ, critical concentration in cells and organs, critical
effect, subcritical effect, dose-effect and dose-response
relationships. In: Nordberg, G.F., ed. Effects and dose-response
relationships of toxic metals, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 10-13.
TATI, M., KATAGIRI, Y., & KAWAI, M. (1976) Urinary and faecal
excretion of cadmium in normal Japanese: an approach to non-toxic
levels of cadmium. In: Nordberg, G.F., ed. Effects and dose-response
relationships of toxic metals, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 331-341.
TAYLOR, B.A., HEINIGER, H.J., & MEIER, H. (1973) Genetic analysis of
resistance to cadmium-induced testicular damage in mice (37380).
Proc. Soc. Exp. Biol. Med., 143: 629-633.
TECULESCU, D.B. & STANESCU, D.C. (1970) Pulmonary function in
workers with chronic exposure to cadmium oxide fumes. Int. Arch.
Arbeitsmed., 26: 335-345.
TEMPLETON, D.M., BANERJEE, D., & CHERIAN, M.G. (1985)
Metallothionein synthesis and localization in relation to metal
storage in rat liver during gestation. Can. J. Biochem. cell Biol.,
63: 16-22.
TENNANT, C.J. (1984) Cadmium in the environment and high risk
population groups, Birmingham, University of Aston (Ph.D. Thesis).
TERHAAR, C.J., VIS, E., ROUDABUSH, R.L., & FASSETT, D.W. (1965)
Protective effects of low doses of cadmium chloride against
subsequent high oral doses in the rat. Toxicol. appl. Pharmacol.,
7: 500-505.
THIEME, R., SCHRAMEL, P., & KURZ, E. (1977) [Trace element
concentration in the human placenta in a heavily-contaminated
environment.] Geburtshilfe Frauenheilkd., 37: 756-761 (in German).
THOMAS, B.J., HARVEY, T.C., CHETTLE, D.R., MCLELLAN, J.S., &
FREMLIN, J.H. (1979) A transportable system for the measurement of
liver cadmium in vivo. Phys. Med. Biol., 24: 432-437.
THORNTON, I. (1988) The Shipham report. An investigation into
cadmium contamination and its implications for human health. Metal
content of soils and dusts. Sci. total Environ., 75: 21-39.
THUN, M.T., SCHNORR, T.M., SMITH, A.B., HALPERIN, W.E., & LEMEN,
R.A. (1985) Mortality among a cohort of U.S. cadmium production
workers: An update. J. Natl Cancer Inst., 74: 325-333.
THUN, M.J., OSORIO, A.M., SCHOBER, S., HANNON, W.H., & HALPERIN, W.
(1989) Nephropathy in cadmium workers - assessment of risk from
airborne occupational cadmium exposure. Br. J. ind. Med.,
46: 689-697.
TIPTON, I.H., SCHROEDER, H.A., PERRY, H.M., & COOK, M.J. (1960)
Trace elements in human tissues. Part III. Subjects from Africa, the
Near and Far East, and Europe. Health Phys., 11: 403-451.
TJELL, J.C., HANSEN, J.A., CHRISTENSEN T.H., & HOVMAND, M.F. (1981)
Prediction of cadmium concentrations in Danish soils. In: L'Hermite,
P. & Ott, H., ed. Characterization, treatment and use of sewage
sludge, Dordrecht, Reidel Publishing Co., pp. 652-664.
TJELL, J.C., CHRISTENSEN, T.H., & BRO-RASMUSSEN, F. (1983) Cadmium
in soil and terrestrial biota, with emphasis on the Danish
situation. Ecotoxicol. environ. Saf., 7: 122-140.
TOHYAMA, C., SHAIKH, Z.A., ELLIS, K.J., & COHN, S.H. (1981a)
Metallothionein excretion in urine upon cadmium exposure: its
relationship with liver and kidney cadmium. Toxicology, 20: 181-191.
TOHYAMA, C., SHAIKH, Z.A., NOGAWA, K., KOBAYASHI, E., & HONDA, R.
(1981b) Elevated urinary excretion of metallothionein due to
environmental cadmium exposure. Toxicology, 22: 289-297.
TOPPING, M.D., FORSTER, H.W., DOLMAN, C., LUCZYNSKA, C.M., &
BERNARD, A.M. (1986) Measurement of urinary retinol-binding protein
by enzyme-linked immunosorbent assay and its application to
detection of tubular proteinuria. Clin. Chem., 32: 1863-1866.
TSUCHIYA, K. (1967) Proteinuria of workers exposed to cadmium fume.
The relationship to concentration in the working environment. Arch.
environ. Health, 14: 875-880.
TSUCHIYA, K. (1969) Causation of Ouch-ouch disease (Itai-itai byo):
an introduction review. Part 1. Nature of the disease. Keio J. Med.,
18: 181-194.
TSUCHIYA, K. (1976) Proteinuria of cadmium workers. J. occup. Med.,
18: 463-466.
TSUCHIYA, K., ed. (1978) Cadmium studies in Japan: a review,
Amsterdam, Oxford, New York, Elsevier Science Publishers, 376 pp.
TSUCHIYA, K. & IWAO, S. (1978) Interrelationships among zinc,
copper, lead, and cadmium in food, faeces, and organs of humans.
Environ. Health Perspect., 25: 119-124.
TSUCHIYA, K. & NAKAMURA, K.I. (1978) Environmental pollution and
health effects: Hyogo prefecture. In: Tsuchiya, K., ed. Cadmium
studies in Japan: a review, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 146-167.
TSUCHIYA, K. & SUGITA, M. (1971) A mathematical model for deriving
the biological half-life of a chemical. Nord. Hyg. Tidskr.,
53: 105-110.
TSUCHIYA, K., SEKI, Y., & SUGITA, M. (1976) Cadmium concentrations
in the organs and tissues of cadavers from accidental deaths. Keio
J. Med., 25: 83-90.
TSUCHIYA, K., IWAO, S., SUGITA, M., & SAKURAI, H. (1979) Increased
urinary ß-2-microglobulin in cadmium exposure: dose-effect
relationship and biological significance of ß-2-microglobulin.
Environ. Health Perspect., 28: 147-153.
URUNO, K., OTOYAMA, S., NAKANO, N., & ITAGAKI, Z. (1975) [Results
of health examination for inhabitants in Yoshinokawa area polluted
with cadmium], Tokyo, Japan Public Health Association, pp. 109-113
(Kankyo Hoken Report No. 36) (in Japanese).
USHIO, F. & DOGUCHI, M. (1977) Dietary intakes of some chlorinated
hydrocarbons and heavy metals estimated on the
experimentally-prepared diets. Bull. environ. Contam. Toxicol.,
17: 707-711.
US PUBLIC HEALTH SERVICE (1942) Cadmium poisoning. Public Health
Rep., 57: 602-612.
VAHTER, M., ed. (1982) Assessment of human exposure to lead and
cadmium through biological monitoring, Stockholm, National Swedish
Institute of Environmental Medicine and Department of Environmental
Hygiene, Karolinska Institute, 136 pp (Report prepared for the
United Nations Environment Programme and the World Health
Organization).
VALETAS, P. (1946) "Cadmiose" ou l'intoxication du cadmium, Paris,
School of Medicine, 40 pp (Medical Faculty Thesis Report).
VAN AALST, R.M., VAN ARDENNE, R.A.M., DE KRUK, J.F., & LEMS, T.
(1983a) Pollution of the North Sea from the atmosphere, Apeldoorn,
The Netherlands Organization for Applied Scientific Research (TNO)
(Report No. C182/152).
VAN AALST, R.M., DUYZER, J.H., & VELDT, C. (1983b) Atmospheric
deposition of lead and cadmium in the southern part of the North
Sea. I. Emission and preliminary model calculations, Apeldoorn, The
Netherlands Organization for Applied Scientific Research (TNO)
(Report No. R83/222).
VANDER, A.J. (1962) Cadmium enhancement of proximal tubular sodium
reabsorption. Am. J. Physiol., 203: 1005-1007.
VANDER MALLIE, R.J. & GARVEY, J.S. (1979) Radioimmunoassay of
metallothioneins. J. biol. Chem., 254: 8416-8421.
VENS, M.D. & LAUWERYS, R.R. (1982) Détermination simultanée du plomb
et du cadmium dans le sang et l'urine par le couplage des techniques
de chromatographie sur résine échangeuse d'ions et de
spectrophotométrie d'absorption atomique. Arch. Mal. prof. Méd.
Trav. Sécur. soc., 33: 97-105.
VESTERBERG, O. & WRANGSKOGH, K. (1978) Determination of cadmium in
urine by graphite furnace atomic absorption spectroscopy. Clin.
Chem., 24: 681-685.
VOROBJEVA, R.S. (1957) [Investigations of the nervous system
function in workers exposed to cadmium oxide.] Neuropatol.
Psikkiatr., 57: 385-388 (in Russian).
VOROBJEVA, R.S. (1958) [About occupational cadmium oxide
poisonings.] J. Hyg. Epidemiol. Microbiol. Immunol., 11: 244-249 (in
German).
VOROBJEVA, R.S. & BUBNOVA, N.I. (1981) [Effects of elemental cadmium
and telluric cadmium on organisms.] Gig. Trud. prof. Zabol.,
2: 42-43 (in Russian).
VOROBJEVA, R.S. & EREMEEVA, E.P. (1980) [Cardiovascular function in
workers exposed to cadmium.] Gig. i Sanit., 10: 22-25 (in Russian).
VOROBJEVA, R.S. & SABALINA, L.P. (1975) [Experimental investigations
of toxic properties of various cadmium compounds.] Gig. i Sanit.,
2: 102-104 (in Russian).
VOSTAL, J.J. (1976) Dose-response relationship of mercury and
cadmium effects on renal cadmium transport. In: Nordberg, G.F., ed.
Effects and dose-response relationships of toxic metals, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 299-308.
VUORI, E., HUUNAN-SEPPALA, A., KILPIO, J.O., & SALMELA, S.S. (1979)
Biologically-active metals in human tissues. II. The effect of age
on the concentration of cadmium in aorta, heart, kidney, liver,
lung, pancreas, and skeletal muscle. Scand. J. Work Environ. Health,
5: 16-22.
WAALKES, M.P., POISNER, A.M., WOOD, G.W., & KLAASSEN, C.D. (1984)
Metallothionein-like proteins in human placenta and fetal membranes.
Toxicol. appl. Pharmacol., 74: 179-184.
WADE, G.G. (1987) Marked synergism of dimethylnitrosamine
carcinogenesis in rats exposed to cadmium. Cancer Res.,
47: 6606-6613.
WALSH, J.J. & BURCH, G.E. (1959) The rate of disappearance from
plasma and subsequent distribution of radiocadmium ( 115 m Cd) in
normal dogs. J. clin. Med., 54: 59-65.
WALTERS, B. & SHERLOCK, J. (1981) Studies on the dietary intake of
heavy metals. In: Proceedings of the International Conference on
Heavy Metals in the Environment, Amsterdam, September 1981,
Edinburgh, CEP Consultants Ltd, pp. 506-512.
WATANABE, H. & MURAYAMA, H. (1975) A study on health effect indices
concerning population in a cadmium-polluted area. In: International
Symposium on Recent Advances in the Assessment of the Health Effects
of Environmental Pollution, Luxembourg, December 1975, Luxembourg,
Commission of the European Communities, pp. 2185-2195 (Report EURO
5360).
WATANABE, I., KOIZUMI, A., FUJITA, H., KUMAI, M., & IKEDA, M. (1985)
Dietary cadmium intakes of farmers in nonpolluted areas in Japan,
and the relation with blood cadmium levels. Environ. Res.,
37(1): 33-43.
WATANABE, T., SHIMADA, T., & ENDO, A. (1979) Mutagenic effects of
cadmium on mammalian oocyte chromosomes. Mutat. Res., 67: 349-356.
WEAST, R.C. (1974) Handbook of chemistry and physics, 55th ed.,
Cleveland, Ohio, CRC Press, 500 pp.
WEBB, M. (1972) Protection by zinc against cadmium toxicity.
Biochem. Pharmacol., 21: 2767-2771.
WEBB, M. & CAIN, K. (1982) Functions of metallothionein. Biochem.
Pharmacol., 31: 137-142.
WEBB, M. & ETIENNE, A.T. (1977) Studies on the toxicity and
metabolism of cadmium-thionein. Biochem. Pharmacol., 26: 25-30.
WESTER, P.O. (1974) Trace element balances in relation to variations
in calcium intake. Atherosclerosis, 20: 207-215.
WHANGER, P.D. (1979) Cadmium effects in rats on tissue iron,
selenium, and blood pressure: blood and hair cadmium in some Oregon
residents. Environ. Health Perspect., 28: 115-121.
WHO (1980) Recommended health-based limits in occupational exposure
to heavy metals. Report of a study group, Geneva, World Health
Organization (Technical Report Series No. 647).
WHO (1984) Guidelines for drinking-water quality: Health criteria
and other supporting information, Geneva, World Health Organization,
Vol. 2, pp. 84-90.
WHO (1986) Health impact of acidic deposition. Sci. total Environ.,
52: 157-187.
WHO (1987) Air quality guidelines for Europe, Copenhagen, World
Health Organization, Regional Office for Europe, pp. 200-209
(European Series, Vol. 23).
WHO (1989) Cadmium. In: Evaluation of certain food additives and
contaminants. Thirty-third Report of the Joint FAO/WHO Expert
Committee on Food Additives, Geneva, World Health Organization,
pp. 28-31 (Technical Report Series No. 776).
WILLIAMS, C.H. & DAVID, D.J. (1973) The effect of superphosphate on
the cadmium content of soils and plants. Aust. J. Soil Res.,
11: 43-56.
WILLIAMS, C.H. & DAVID, D.J. (1976) The accumulation in soil of
cadmium residues from phosphate for fertilizers and their effect on
the cadmium content of plants. Soil Sci., 121: 86-93.
WILSON, B. (1988a) Investigation of trace metals in the aqueous
environment: Final report (January 1986-December 1987), Houston,
Texas Southern University, p. 28 (Report No. DOE/CH/10255-T1,
prepared for the US Department of Energy, Washington).
WILSON, D.N. (1988b) Cadmium - market trends and influences. In:
Cadmium 87. Proceedings of the 6th International Cadmium
Conference, London, Cadmium Association, pp. 9-16.
WILSON, R.H., DE EDS, F., & COX, A.J., Jr (1941) Effects of
continued cadmium feeding. J. Pharmacol. exp. Ther., 71: 222-235.
WOLFF, E.W. & PEEL, D.A. (1985) The record of global pollution in
polar snow and ice. Nature (Lond.), 313: 535-540.
WOLNIK, K.A., FRICKE, F.L., CAPER, S.G., BRAUDE, G.L., MEYER, M.W.,
SATZGER, R.D., & BONNIN, E. (1983) Elements in major raw
agricultural crops in the United States. I. Cadmium and lead in
lettuce, peanuts, potatoes, soybeans, sweet corn, and wheat. J.
agric. food Chem., 31: 1240-1244.
WOLNIK, K.A., FRICKE, F.L., CAPAR, S.G., MEYER, M.W., & SATZGER,
R.D. (1985) Elements in major raw agricultural crops in the United
States. 3. Cadmium, lead, and eleven other elements in carrots,
field corn, onion, rice, spinach, and tomatoes. J. agric. food
Chem., 33: 807-811.
YAMAGATA, H. & IWASHIMA, K. (1975) Average cadmium intake of the
Japanese people. Bull. Inst. Public Health (Tokyo), 24: 18-24.
YAMAGATA, N. & SHIGEMATSU, I. (1970) Cadmium pollution in
perspective. Bull. Inst. Public Health (Tokyo), 19: 1-27.
YAMAGATA, N., IWASHIMA, K., & NAGAI, T. (1974) [Gastrointestinal
absorption of cadmium in normal humans], Tokyo, Japan Public Health
Association, pp. 84-85 (Kankyo Hoken Report No. 31) (in Japanese).
YAMAMOTO, Y. (1972) [Present status of cadmium environmental
pollution], Tokyo, Japan Public Health Association, pp. 7-12 (Kankyo
Hoken Report No. 11) (in Japanese).
YOSHIKAWA, H., KAWAI, K., SUZUKI, Y., NOZAKI, K., & OHSAWA, M.
(1975) [Studies on cadmium effects in living organisms: impairment
due to inhalation], Tokyo, Japanese Environment Agency, 40 pp (in
Japanese).
RESUME ET CONCLUSIONS
1. Identité, propriétés physiques et chimiques et méthodes
d'analyse
Il existe plusieurs méthodes pour le dosage du cadmium dans les
échantillons biologiques. La plus utilisée est la spectrométrie
d'absorption atomique, mais elle nécessite un traitement minutieux
de la prise d'essai et une correction pour tenir compte des
interférences lorsqu'elle est appliquée à des échantillons de faible
teneur en cadmium. Il est tout à fait souhaitable que l'analyse
s'accompagne d'un programme d'assurance de la qualité. A l'heure
actuelle, il est possible, dans des conditions optimales, de doser
environ 0,1 µg/litre dans l'urine et le sang et de 1 à 10 µg/litre
dans les aliments et les tissus.
2. Sources d'exposition humaine et environnementale
Le cadmium est un élément relativement rare et les méthodes
actuelles d'analyse indiquent, dans les divers compartiments de
l'environnement, des concentrations beaucoup plus faibles que les
mesures antérieures. Pour l'instant, il n'est pas possible de
déterminer si l'activité humaine est à l'origine d'un accroissement
de la teneur des calottes polaires en cadmium à l'échelle des temps
historiques.
La production commerciale de cadmium a commencé au tournant du
siècle. La consommation a changé de caractère ces dernières années
avec un recul sensible de la galvanoplastie et une utilisation
accrue dans la fabrication de batteries et de composants
électroniques spéciaux. Dans la plupart des cas, le cadmium est
utilisé sous la forme de dérivés peu concentrés, ce qui rend le
recyclage indispensable. Les restrictions à l'usage du cadmium
imposées par certains pays pourraient avoir des répercussions
importantes sur ces applications.
Les activités humaines entraînent la libération de cadmium dans
l'air, le sol et l'eau. D'une façon générale, les deux principales
sources de contamination sont la production et la consommation de
cadmium et d'autres métaux non ferreux ainsi que le rejet de déchets
contenant du cadmium. Dans les zones proches de mines ou de
fonderies de métaux non ferreux, la contamination par le cadmium est
souvent importante.
Plus le sol contient de cadmium, plus la quantité fixée par les
plantes est importante. L'exposition humaine par l'intermédiaire des
cultures sera donc sensible à toute augmentation de la teneur du sol
en cadmium. La fixation par les plantes est plus importante dans les
sols de faible pH. Les processus qui acidifient le sol (pluies
acides, par exemple) sont donc susceptibles de provoquer une
augmentation de la concentration moyenne du cadmium dans les denrées
alimentaires. Dans certaines régions du monde, l'utilisation
d'engrais phosphatés et les dépôts d'origine atmosphérique
constituent une source non négligeable de contamination des terres
arables; les boues d'égout peuvent aussi, localement, entraîner une
forte pollution. A l'avenir, ces sources risquent d'accroître la
contamination du sol et celle des cultures, ce qui débouchera sur
une exposition plus importante au cadmium par la voie alimentaire.
Dans certaines régions, on peut constater une augmentation de la
teneur des aliments en cadmium.
Les animaux et les plantes comestibles qui vivent à l'état
sauvage, comme les coquillages, les crustacés et les champignons
accumulent naturellement le cadmium. Comme chez l'homme, on constate
une augmentation de la concentration en cadmium dans le foie et les
reins des chevaux et de certains animaux terrestres vivant à l'état
sauvage. La consommation régulière de ces abats peut entraîner une
exposition accrue. Dans les reins de certains vertébrés marins on
trouve des concentrations assez fortes en cadmium, qui, même si
elles sont d'origine naturelle, n'en provoquent pas moins des
lésions au niveau de ces organes.
3. Concentrations dans l'environnement et exposition humaine
La principale voie d'exposition au cadmium des non fumeurs est
la voie alimentaire. Les autres voies sont peu importantes. Chez les
fumeurs, l'apport de cadmium par le tabac est notable. Dans les
zones contaminées, l'exposition d'origine alimentaire peut atteindre
plusieurs centaines de microgrammes par jour. Chez les travailleurs
exposés, la principale voie de pénétration est la voie pulmonaire,
après inhalation d'air contaminé sur le lieu de travail. Le
tabagisme et la consommation d'aliments contaminés ajoutent encore à
la charge de cadmium de l'organisme.
4. Cinétique et métabolisme chez les animaux de laboratoire et
chez l'homme
Les données tirées de l'expérimentation animale et humaine
montrent que l'absorption est plus importante au niveau des poumons
qu'au niveau des voies digestives. Selon l'espèce chimique en cause,
la granulométrie et la solubilité dans les liquides biologiques, le
taux d'absorption peut atteindre 50% après inhalation. L'absorption
gastro-intestinale dépend du régime alimentaire et de l'état
nutritionnel. En particulier, le bilan martial est particulièrement
important. En moyenne, le cadmium total contenu dans les aliments
est absorbé à hauteur de 5%, avec un intervalle de variation de
1%-20% selon les individus. Il existe un gradient materno-foetal de
cadmium. Le cadmium peut également parvenir jusqu'au foetus, mais en
faibles quantités malgré son accumulation dans le placenta.
Le cadmium absorbé au niveau des poumons ou des voies
digestives s'accumule principalement dans le foie et les reins où il
représente plus de la moitié de la charge totale de l'organisme.
Plus l'exposition est intense, plus l'accumulation du cadmium dans
le foie est importante. En principe, l'excrétion est lente et la
période biologique du cadmium dans les muscles, les reins, le foie
et l'organisme dans son ensemble, est très longue, de l'ordre de
plusieurs décennies. La teneur en cadmium de la plupart des tissus
augmente avec l'âge. C'est en général dans le cortex rénal que la
concentration est la plus élevée, mais en cas d'exposition
excessive, elle peut l'être encore plus dans le foie. Chez les
personnes exposées atteintes de lésions rénales, il y a augmentation
de l'excrétion urinaire du cadmium de sorte que la période
biologique pour l'ensemble de l'organisme est raccourcie. Du fait
des lésions, le rein perd son cadmium et ces malades finissent par
présenter une concentration rénale de cadmium plus faible que les
individus en bonne santé soumis à la même exposition.
La métallothionéine est une protéine qui joue un rôle important
dans le transport et le stockage du cadmium et d'autres métaux. Le
cadmium est capable d'induire la synthèse de cette protéine dans de
nombreux organes, notamment le foie et le rein. En fixant le cadmium
intracellulaire, la métallothionéine protège les tissus contre les
effets toxiques de ce métal. Il est possible, par conséquent, que le
cadmium non lié à la métallothionéine ait une responsabilité dans
les lésions tissulaires. On ignore quels peuvent être les autres
complexes du cadmium présents dans les liquides biologiques.
L'excrétion urinaire du cadmium dépend de divers facteurs:
charge totale de l'organisme, exposition récente et lésions rénales.
Chez les individus peu exposés, la concentration urinaire du cadmium
dépend principalement de la charge de l'organisme. En cas de lésions
rénales dues au cadmium, ou même sans lésions de ce genre mais en
présence d'une exposition excessive, il y a augmentation de
l'excrétion urinaire. Les individus exposés au cadmium en excrètent
davantage dans leurs urines lorsqu'ils présentent une protéinurie.
Après cessation d'une exposition intense, le taux urinaire décroît,
même s'il y a persistance des lésions rénales. Il faut donc prendre
plusieurs facteurs en considération pour interpréter le cadmium
urinaire. L'excrétion par la voie digestive est sensiblement
équivalente à l'excrétion urinaire mais elle est difficile à
mesurer. L'excrétion par d'autres voies (lactation, sueur ou passage
transplacentaire) est négligeable.
La teneur des matières fécales en cadmium est un bon indicateur
d'une ingestion récente en l'absence d'exposition par la voie
respiratoire. Le cadmium sanguin est présent principalement dans les
hématies, la concentration plasmatique étant très faible. Il existe
au moins deux compartiments dans le sang, l'un qui correspond à une
exposition récente, avec une demi-vie de 2-3 mois et l'autre, qui
est probablement lié à la charge totale de l'organisme et se
caractérise par une demi-vie de plusieurs années.
5. Effets sur les animaux de laboratoire
Une forte exposition par la voie respiratoire entraîne un
oedème mortel du poumon. Après injection d'une seule dose,
apparaissent des lésions testiculaires, une nécrose ovarienne, des
lésions hépatiques et une atteinte des petits vaisseaux. L'ingestion
de fortes doses provoque des lésions de la muqueuse gastrique et
intestinale.
Une exposition prolongée par la voie respiratoire ou une
administration intratrachéenne entraîne des altérations pulmonaires
de nature inflammatoire ainsi qu'une fibrose et donne au tissu
pulmonaire un aspect qui évoque l'emphysème. L'administration
prolongée par voie orale ou parentérale affecte principalement le
rein mais elle a aussi des effets sur le foie et les systèmes
hématopoiétique, immunitaire et cardio-vasculaire ainsi que sur le
squelette. Chez certaines espèces et dans des conditions
déterminées, on a provoqué une hypertension et constaté des effets
sur le squelette. C'est le stade de la gestation où se produit
l'exposition qui conditionne les effets tératogènes et les lésions
placentaires et il peut y avoir interaction avec le zinc.
Ce sont les effets aigus produits par l'inhalation du cadmium
ainsi que sa néphrotoxicité chronique qui sont les plus importants
du point de vue de l'exposition humaine. En cas d'exposition
prolongée, c'est le rein qui est l'organe critique. Les effets sont
caractérisés par une lésion des cellules tubulaires entraînant une
insuffisance tubulaire parfois accompagnée d'insuffisance
glomérulaire. L'insuffisance tubulaire a pour conséquence une
perturbation du métabolisme du calcium et de la vitamine D. Selon
certains travaux, ces troubles pourraient provoquer une ostéomalacie
ou une ostéoporose. Cependant ces résultats n'ont pas été confirmés
par d'autres études. On ne peut exclure un effet direct du cadmium
sur la minéralisation de l'os. Chez l'animal de laboratoire, les
effets toxiques du cadmium dépendent de certains facteurs génétiques
et nutritionnels, des interactions avec d'autres métaux, en
particulier le zinc, et d'un premier traitement éventuel par le
cadmium susceptible d'avoir stimulé la synthèse de métallothionéine.
En 1976 et 1987, le Centre international de recherche sur le
cancer a admis posséder suffisamment de preuves que l'injection de
chlorure, de sulfate, de sulfure et d'oxyde de cadmium pouvait
entraîner l'apparition d'un sarcome local chez le rat et, dans le
cas des deux premiers composés, de tumeurs testiculaires
interstitielles chez ce même animal et chez la souris. Toutefois, il
a considéré que les études basées sur l'administration par voie
orale ne permettaient pas de procéder à une évaluation. Lors
d'études au cours desquelles on a fait respirer à des rats des
aérosols de sulfate de cadmium, des vapeurs d'oxyde de cadmium et de
la poussière de sulfate de cadmium, on a observé une forte incidence
de cancers primitifs du poumon, avec probablement une relation entre
la dose et la réponse. Toutefois, ces résultats n'ont pu être
reproduits chez d'autres espèces. Les travaux relatifs aux effets
génotoxiques du cadmium ont donné des résultats contradictoires.
6. Effets sur l'homme
Une forte exposition à des vapeurs d'oxyde de cadmium par la
voie respiratoire entraîne une pneumopathie aiguë, accompagnée d'un
oedème du poumon qui peut être mortel. L'ingestion de grandes
quantités de sels solubles de cadmium provoque une gastro-entérite
aiguë.
A la suite d'une exposition professionnelle prolongée au
cadmium, on a observé de graves effets chroniques, principalement au
niveau des poumons et des reins. On a également observé une
néphrotoxicité chronique dans la population générale.
Les altérations pulmonaires consécutives à une exposition
professionnelle intense sont essentiellement caractérisées par une
obstruction des voies aériennes. Si l'exposition se poursuit, les
légers troubles ventilatoires initiaux peuvent déboucher sur une
insuffisance respiratoire. On a observé un accroissement de la
mortalité par pneumopathie obstructive chez des travailleurs
fortement exposés, comme cela se produisait auparavant.
L'accumulation de cadmium dans le cortex rénal entraîne des
troubles de la fonction tubulaire et une réabsorption insuffisante,
par exemple, des protéines, du glucose et des acides aminés.
L'accroissement de l'excrétion urinaire des protéines de faible
masse moléculaire est un signe caractéristique de l'insuffisance
tubulaire. Parfois il y a aussi baisse du taux de filtration
glomérulaire. L'augmentation du cadmium urinaire est corrélée avec
une protéinurie de faible masse moléculaire et, en l'absence
d'exposition, peut servir d'indicateur de l'atteinte rénale. Dans
les cas graves, les effets tubulaires et glomérulaires s'ajoutent
s'accompagnant parfois d'une élévation du taux sanguin de
créatinine. Chez la plupart des travailleurs et autres personnes, la
protéinurie due à une néphropathie cadmique est irréversible.
Parmi les autres effets, on peut citer les troubles du
métabolisme calcique, l'hypercalciurie et la formation de calculs
rénaux. Une exposition intense au cadmium peut, selon toute
probabilité lorsqu'elle s'accompagne d'autres facteurs comme une
carence nutritionnelle, provoquer l'apparition d'une ostéoporose
et/ou d'une ostéomalacie.
On est fondé à penser qu'une exposition professionnelle
prolongée au cadmium peut favoriser l'apparition d'un cancer du
poumon, mais la présence de facteurs de confusion ne facilite pas
l'interprétation des observations effectuées sur les travailleurs
exposés. En ce qui concerne le cancer de la prostate, les données ne
sont pas concluantes et ne confirment pas, en tout cas, l'hypothèse
antérieure d'une relation de cause à effet.
A l'heure actuelle, on ne possède pas de preuve convaincante
que le cadmium provoque une hypertension essentielle. La plupart des
données contredisent cette hypothèse et rien n'indique un
accroissement de la mortalité par maladie cardio-vasculaire ou
accident vasculaire cérébral chez les personnes exposées.
D'après les résultats d'études relatives à des groupes exposés
de par leur profession ou simplement du fait de leur environnement
général, il semble que la prévalence des effets néphrotoxiques soit
liée à la durée et à l'intensité de l'exposition.
Chez des ouvriers de l'industrie du cadmium, on a signalé,
après 10 à 20 ans d'exposition à des concentrations de l'ordre de
20-50 µg par mètre cube, une augmentation de la prévalence des cas
de protéinurie à faible masse moléculaire.
Dans des zones polluées, où l'on évalue l'apport de cadmium par
voie orale à environ 140-260 µg/jour, on a observé des effets du
genre protéinurie à faible masse moléculaire chez des sujets exposés
pendant une longue période. On trouvera à la section 8 une
estimation plus précise de la relation dose-réponse.
7. Evaluation des risques pour la santé humaine
7.1 Conclusions
On estime que le rein est l'organe cible tant dans la
population générale que chez les groupes professionnellement
exposés. Une exposition prolongée par inhalation entraîne
l'apparition d'un syndrome respiratoire obstructif chez certains
groupes professionnels. Certains détails incitent à penser que cette
exposition au cadmium pourrait favoriser l'apparition d'un cancer du
poumon, mais les observations effectuées sur des travailleurs
exposés sont difficiles à interpréter en raison de la présence de
facteurs de confusion.
7.1.1 Population générale
Pour la plupart des individus, les aliments constituent la
principale voie d'exposition au cadmium. Dans la plupart des régions
non polluées par ce métal, l'apport alimentaire journalier est de
l'ordre de 10-40 µg. Dans les zones polluées, il peut atteindre
plusieurs centaines de microgrammes par jour. Dans les zones non
polluées, l'apport dû au tabac peut être égal à l'apport alimentaire
chez les gros fumeurs.
D'après un modele biologique, on estime qu'il existe une
association entre l'exposition au cadmium et l'excrétion urinaire de
protéines de faible masse moléculaire chez les sujets qui absorbent
pendant toute leur vie une dose journalière d'environ 140-260 µg de
cadmium, ce qui correspond à une dose cumulée d'environ 2000 mg ou
davantage.
7.1.2 Groupes professionnellement exposés
Dans ce cas, l'exposition est essentiellement respiratoire,
mais il s'y ajoute l'apport alimentaire et tabagique. La teneur
totale de l'air en cadmium varie selon les pratiques en matière
d'hygiène industrielle et selon le lieu de travail. Il existe une
relation de type exposition-réponse entre la teneur de l'air en
cadmium et la protéinurie de faible masse moléculaire. La prévalence
de cette protéinurie peut augmenter après 10 à 20 ans d'exposition à
des concentrations de cadmium de l'ordre de 20-50 µg par mètre cube.
In vivo, le dosage du cadmium dans les reins et le foie de sujets
plus ou moins exposés a montré que 10 % environ des travailleurs
dont le cortex rénal contenait 200 mg/kg de cadmium et 50 % de ceux
chez qui cette concentration atteignait 300 mg/kg, feraient un jour
ou l'autre une protéinurie par insuffisance tubulaire.
RESUMEN Y CONCLUSIONES
1. Identidad, propiedades físicas y químicas, y métodos analíticos
Se dispone de varios métodos para determinar el cadmio presente
en el material biológico. El más utilizado es la espectrometría de
absorción atómica, aunque el análisis de muestras con
concentraciones bajas de cadmio exige un tratamiento cuidadoso de
las muestras y correcciones para tener en cuenta la interferencia.
Se recomienda encarecidamente acompañar el análisis con un programa
de garantía de la calidad. Actualmente, en circunstancias ideales
pueden determinarse concentraciones de alrededor de 0,1 µg/litro en
la orina y la sangre y de 1-10 µg/kg en alimentos y muestras de
tejidos.
2. Fuentes de exposición humana y ambiental
El cadmio es un elemento relativamente raro; los procedimientos
analíticos actuales indican que las concentraciones del metal en el
medio ambiente son mucho más bajas que las obtenidas en medidas
anteriores. Hoy en día no es posible determinar si la actividad
humana ha provocado un aumento histórico de los niveles de cadmio en
los casquetes polares.
La producción comercial de cadmio comenzó a principios de este
siglo. La pauta de consumo de cadmio se ha modificado en los últimos
años debido al notable descenso del uso de la galvano-plastia y al
importante aumento de la producción de baterías y de las
aplicaciones electrónicas especializadas. En las principales
aplicaciones del cadmio éste se utiliza en forma de compuestos que
se hallan presentes en bajas concentraciones; ello obstaculiza el
reciclaje del metal. Las restricciones impuestas por algunos países
a ciertas aplicaciones del cadmio pueden tener un efecto
generalizado en esas aplicaciones.
El cadmio se libera al aire, los suelos y las aguas debido a la
actividad humana. En general, las dos fuentes principales de
contaminación son la producción y el consumo de cadmio y de otros
metales no ferrosos y la evacuación de desechos que contienen
cadmio. Las zonas próximas a minas no ferrosas y fundiciones suelen
estar muy contaminadas por cadmio.
Al aumentar el contenido de cadmio del suelo, aumenta la
absorción del metal por las plantas; la exposición humana a partir
de las cosechas agrícolas está por tanto sometida a los aumentos del
contenido de cadmio del suelo. Dado que la absorción por las plantas
desde el suelo es mayor cuando el pH de éste es bajo, los procesos
que acidifican el suelo (por ejemplo, las lluvias ácidas) pueden
aumentar las concentraciones medias de cadmio en los alimentos. La
aplicación de fertilizantes a base de fosfato y la deposición
atmosférica son fuentes importantes de aportación de cadmio a las
tierras cultivables en ciertas zonas del mundo; los fangos de
alcantarillado también pueden ser una fuente de importancia a nivel
local. Estas fuentes pueden, en el futuro, aumentar los niveles de
cadmio en el suelo y con ello en las cosechas, lo que a su vez puede
acrecentar la exposición al cadmio en la dieta. En ciertas zonas, se
ha demostrado que está aumentando el contenido de cadmio en los
alimentos.
Ciertos organismos comestibles de vida libre como los mariscos,
los crustáceos y los hongos son acumuladores naturales de cadmio.
Como en el caso del ser humano, se observan niveles mayores de
cadmio en el hígado y el riñón de los caballos y de algunos animales
terrestres silvestres. El consumo habitual de estos alimentos puede
aumentar la exposición. Ciertos vertebrados marinos contienen
concentraciones notablemente elevadas de cadmio en el riñón,
fenómeno que, aunque se considera de origen natural, se ha vinculado
a signos de lesiones renales en esos organismos.
3. Niveles ambientales y exposición humana
La principal fuente de exposición al cadmio en la población
general no fumadora son los alimentos; la proporción de cadmio que
se absorbe por otras vías es pequeña. El tabaco es una importante
fuente de absorción de cadmio en los fumadores. En las zonas
contaminadas, la exposición al cadmio por los alimentos puede
alcanzar varios cientos de µg/día. En los trabajadores expuestos, la
absorción pulmonar de cadmio por inhalación en el lugar de trabajo
es la principal vía de exposición. También puede aumentar la
absorción por la contaminación de los alimentos y por el consumo de
tabaco.
4. Cinética y metabolismo en animales de experimentación y en
el ser humano
Los datos obtenidos en animales de experimentación y en el ser
humano han demostrado que la absorción pulmonar es mayor que la
gastrointestinal. Atendiendo a la especiación química, el tamaño de
las partículas y la solubilidad en fluidos biológicos, puede
absorberse hasta el 50% del compuesto de cadmio inhalado. La
absorción gastrointestinal de cadmio depende del tipo de dieta y del
estado nutricional. El estado nutricional respecto del hierro parece
revestir particular importancia. Aunque en promedio se absorbe el 5%
de la ingesta oral total de cadmio, los valores individuales varían
entre menos del 1% hasta más del 20%. Existe un gradiente
maternofetal de cadmio. Aunque se acumula en la placenta, la
transferencia al feto es baja.
El cadmio absorbido en los pulmones o el tracto
gastrointestinal se almacena principalmente en el hígado y el riñón,
donde se deposita más de la mitad de la carga corporal. Al aumentar
la intensidad de la exposición, aumenta la proporción del cadmio
absorbido que se almacena en el hígado. En el ser humano la
excreción suele ser lenta y la semivida biológica es muy larga
(decenios) en el músculo, el riñón, el hígado y el organismo entero.
Las concentraciones de cadmio en la mayoría de los tejidos aumentan
con la edad. Aunque las concentraciones más elevadas suelen
encontrarse en la corteza renal, con exposiciones excesivas pueden
producirse concentraciones mayores en el hígado. En las personas
expuestas que padecen lesiones renales, aumenta la excreción
urinaria de cadmio con lo que se reduce la semivida en el organismo
entero. Las lesiones renales producen pérdidas del cadmio contenido
en el riñón, y las concentraciones renales acaban con el tiempo
siendo inferiores a las observadas en personas con un grado de
exposición similar pero sin lesiones renales.
La metalotioneína es una importante proteína de transporte y
almacenamiento de cadmio y otros metales. El cadmio puede inducir la
síntesis de metalotioneína en muchos órganos, en particular el
hígado y el riñón. La unión del cadmio intracelular a la
metalotioneína en los tejidos protege contra la toxicidad del metal.
El cadmio libre puede por tanto tener una función en la patogenia de
las lesiones tisulares debidas a ese metal. Se desconoce la
especiación de otros complejos de cadmio en los tejidos o en los
fluidos biológicos.
La excreción urinaria de cadmio guarda relación con la carga
corporal, la exposición reciente y la lesión renal. En personas poco
expuestas, el nivel de cadmio en la orina depende principalmente de
la carga corporal. Una vez que se ha producido la lesión renal
inducida por el cadmio, o incluso en ausencia de lesión renal si la
exposición es excesiva, aumenta la excreción urinaria. En las
personas expuestas al cadmio que padecen proteinuria la excreción de
cadmio suele ser mayor que en las que no padecen proteinuria. Cuando
cesa la exposición intensa, el nivel de cadmio en la orina desciende
aunque persista la lesión renal. En la interpretación de la
presencia de cadmio en la orina hay que tener en cuenta, pues,
varios factores. La excreción gastrointestinal es aproximadamente
igual a la urinaria pero no puede medirse fácilmente. Otras vías
excretoras como la leche, el sudor o la transferencia placentaria
son insignificantes.
El nivel de cadmio en las heces es un buen indicador de la
ingesta diaria reciente a partir de los alimentos en ausencia de
exposición por inhalación. En la sangre, el cadmio aparece
principalmente en los glóbulos rojos y las concentraciones en el
plasma son muy bajas. Existen al menos dos compartimentos en la
sangre, uno referido a la exposición reciente, con una semivida de
alrededor de 2-3 meses, y otro probablemente relacionado con la
carga corporal, con una semivida de varios años.
5. Efectos en mamíferos de laboratorio
Las exposiciones elevadas por inhalación provocan edema
pulmonar letal. La inyección de una sola dosis elevada produce
necrosis en el testículo y en el ovario no ovulante, lesiones
hepáticas y lesiones en los vasos de menor tamaño. La administración
oral de dosis elevadas produce lesiones en la mucosa gástrica e
intestinal.
La exposición por inhalación prolongada y la administración
intratraqueal producen modificaciones crónicas de tipo inflamatorio
en los pulmones, fibrosis y fenómenos indicativos de enfisema. La
administración parenteral u oral prolongada afecta principalmente al
riñón aunque también al hígado y a los sistemas hematopoyético,
inmunitario, esquelético y cardiovascular. En ciertas especies y en
determinadas condiciones se han inducido efectos esqueléticos e
hipertensión. La aparición de efectos teratogénicos y lesiones
placentarias depende de la fase gestacional en que se produzca la
exposición y puede entrañar interacción con el zinc.
En cuanto a la exposición humana, lo más notable son los
efectos agudos por inhalación en el pulmón y los efectos renales
crónicos. Tras la exposición prolongada, el riñón es el órgano
crítico. Los efectos en este órgano se caracterizan por disfunción
tubular y lesiones en las células tubulares, si bien pueden
producirse también disfunciones glomerulares. Una de las
consecuencias de la disfunción tubular renal es la alteración del
metabolismo del calcio y de la vitamina D. Según algunos estudios,
ello ha producido casos de osteomalacia y/o osteoporosis, pero esos
efectos no se han confirmado en otros estudios. No debe excluirse el
efecto directo del cadmio en la mineralización ósea.
Los efectos tóxicos del cadmio en animales de experimentación
están sometidos a la influencia de factores genéticos y
nutricionales, las interacciones con otros metales, en particular el
zinc, y el pretratamiento con cadmio, que puede guardar relación con
la inducción de la metalotioneína.
En 1976 y 1987, el Centro Internacional de Investigaciones
sobre el Cáncer consideró suficientes las pruebas de que el cloruro,
el sulfato, el sulfuro y el óxido de cadmio pueden producir sarcomas
en el lugar de inyección en la rata y, en el caso de los dos
primeros compuestos, inducir tumores en las células intersticiales
del testículo en la rata y el ratón, pero consideró que los estudios
de administración oral eran insuficientes para la evaluación. En
estudios de inhalación prolongada en ratas expuestas a aerosoles de
sulfato de cadmio, vapores de óxido de cadmio y polvos de sulfato de
cadmio se observó una elevada incidencia de cáncer primario del
pulmón con pruebas de proporcionalidad entre la dosis y la
respuesta. Hasta el momento, sin embargo, esa observación no se ha
confirmado en otras especies. Los estudios de los efectos
genotóxicos del cadmio han dado resultados discordantes.
6. Efectos en el ser humano
La exposición intensa por inhalación de vapores de óxido de
cadmio produce neumonitis aguda con edema pulmonar, que puede ser
letal. La ingestión de dosis elevadas de sales solubles de cadmio
produce gastroenteritis aguda.
La exposición ocupacional prolongada al cadmio ha producido
efectos crónicos graves, principalmente en el pulmón y el riñón.
También se han observado efectos renales crónicos en la población
general.
Los cambios pulmonares observados tras una intensa exposición
ocupacional se caracterizan principalmente por la aparición de
afecciones crónicas obstructivas de las vías aéreas. Los primeros
cambios leves en las pruebas de la función ventilatoria pueden
avanzar, si prosigue la exposición al cadmio, hasta insuficiencia
respiratoria. Se ha observado una mayor tasa de mortalidad por
enfermedad pulmonar obstructiva en trabajadores sometidos a
exposiciones intensas, al igual que en otras épocas.
La acumulación de cadmio en la corteza renal produce disfunción
tubulorrenal con trastornos de la reabsorción de proteínas, glucosa
y aminoácidos, entre otros. Un signo característico de la disfunción
tubular es la mayor excreción de proteínas de bajo peso molecular en
la orina. En algunos casos, disminuye la tasa de filtración
glomerular. El aumento de la concentración de cadmio en la orina
está correlacionado con la presencia de proteínas de bajo peso
molecular en la orina y, en ausencia de exposición aguda al cadmio,
puede servir como indicador de efectos renales. En los casos más
graves se combinan los efectos tubulares y glomerulares, con aumento
del nivel de creatinina en la sangre en algunos casos. Para la
mayoría de los trabajadores y de las personas expuestas al medio
ambiente general, la proteinuria inducida por el cadmio es
irreversible.
Entre otros efectos figuran los trastornos del metabolismo del
calcio, la hipercalciuria y la formación de cálculos renales. La
exposición intensa al cadmio, con toda probabilidad combinado con
otros factores como carencias nutricionales, puede llevar a la
aparición de osteoporosis y/o osteomalacia.
Hay pruebas de que la exposición profesional prolongada al
cadmio puede contribuir a la aparición de cáncer del pulmón aunque
las observaciones obtenidas en trabajadores expuestos han sido
difíciles de interpretar a causa de factores que inducen a
confusión. En el caso del cáncer de la próstata, las pruebas
obtenidas hasta la fecha no son concluyentes pero no apoyan la
existencia de una relación causal, indicada en estudios anteriores.
Actualmente no hay pruebas convincentes de que el cadmio sea
agente etiológico de la hipertensión esencial. La mayor parte de los
datos indican que no se debe al cadmio el aumento de la tensión y no
hay pruebas de que la mortalidad por enfermedades cardio-vasculares
o cerebrovasculares sea mayor.
Los datos obtenidos en estudios de grupos de trabajadores
expuestos y de grupos expuestos en el medio ambiente general
demuestran que existe una relación entre los niveles de exposición,
la duración de ésta y la prevalencia de los efectos renales.
Se ha comunicado una mayor prevalencia de la proteinuria de
bajo peso molecular en trabajadores del cadmio tras 10-20 años de
exposición a niveles del metal de aproximadamente 20-50 µg/m3. En
zonas contaminadas del medio general, en las que la ingesta de
cadmio estimada ha sido de 140-260 µg/día, se han observado efectos
en forma de aumento de la cantidad de proteínas de bajo peso
molecular en la orina en algunos individuos tras una exposición
prolongada. En la sección 8 se dan estimaciones más precisas de la
relación dosis-respuesta.
7. Evaluación de los riesgos para la salud humana
7.1 Conclusiones
Se considera que el riñón es el órgano diana crítico en la
población general así como en las poblaciones expuestas
profesionalmente. Las enfermedades crónicas obstructivas de las vías
respiratorias están asociadas a la exposición profesional prolongada
e intensa por inhalación. Hay pruebas de que esa exposición al
cadmio puede contribuir al desarrollo de cáncer del pulmón aunque
las observaciones en trabajadores expuestos han sido difíciles de
interpretar a causa de la presencia de factores que inducen a
confusión.
7.1.1 Población general
El cadmio presente en los alimentos es la principal fuente de
exposición para la mayoría de las personas. En la mayoría de las
zonas no contaminadas con cadmio las ingestas diarias medias con los
alimentos se encuentran entre 10 y 40 µg. En zonas contaminadas se
ha observado que alcanza varios cientos de µg al día. En zonas no
contaminadas, la absorción debida al consumo de tabaco puede igualar
la ingestión de cadmio a partir de los alimentos.
Basandose en un modelo biologico, se ha estimado que con una
ingesta diaria de 140-260 µg de cadmio durante toda la vida, o una
ingesta acumulativa de unos 2000 mg o más, se produce en el ser
humano una asociación entre la exposición al cadmio y una mayor
excreción de proteínas de bajo peso molecular en la orina.
7.1.2 Población expuesta profesionalmente
La exposición ocupacional al cadmio se produce principalmente
por inhalación aunque comprende ingestas suplementarias con los
alimentos y el tabaco. El nivel total de cadmio en el aire varía
según las prácticas de higiene industrial y el tipo de lugar de
trabajo. Existe una relación exposición-respuesta entre los niveles
de cadmio en el aire y la proteinuria. Puede aumentar la prevalencia
de la proteinuria de bajo peso molecular en trabajadores a los 10-20
años de exposición a niveles de cadmio de unos 20-50 µg/m3. La
medida in vivo del cadmio en el riñón y el hígado de personas con
distintos niveles de exposición al metal ha demostrado que alrededor
del 10% de los trabajadores con un nivel en la corteza renal de
200 mg/kg y aproximadamente el 50% de las personas con un nivel en
la corteza renal de 300 mg/kg tendrían proteinuria tubulorrenal.


 range 10-40 mg/kg, while in Japan values of between 50 and 100 mg/kg
were reported. In workers highly exposed to cadmium, but without
functional impairment of the kidney, concentrations in the renal
cortex may range from 180 to 450 mg/kg wet weight. In cases where
there is severe renal dysfunction, the cadmium concentrations are
generally lower and range between 20 and 120 mg/kg wet weight, i.e.
they are of the same magnitude as those of the general population
(Friberg et al., 1974). This seemingly paradoxical relationship is
discussed in more detail in section 6.
If exposure to cadmium throughout life remains constant and low
in amount, the concentrations in the kidneys become higher (by about
10-20 times) than those in the liver. Average liver cadmium
concentrations are about 1-2 mg/kg wet weight at age 50 in some
European countries and the USA, but in Japan average concentrations
are between 5 and 10 mg/kg (Table 10). Although renal concentrations
generally decrease after age 60 (Fig. 4), liver concentrations reach
a plateau but do not show a clear decrease in aged populations
(Fig. 1). In exposed workers, liver concentrations from 20 to about
300 mg/kg have been recorded and in Itai-itai patients they are
between 63 and 132 mg/kg (Friberg et al., 1974). In people with
severe cadmium-induced renal dysfunction, kidney cadmium levels are
low, but the liver levels may be very high (Ishizaki, 1972).
Table 10. Cadmium concentrations in the renal cortex and liver of people from various geographical areasa
Country Number Sex Age group Smoking Renal cortex Liver cadmium Reference
category cadmium level level (mg/kg
(mg/kg wet wet weight)
weight)
Belgium (Liege) 51 M, F 40-59 mixed 46b - Vahter (1982)
German Democratic 20 M 40-59 mixed 22c - Anke & Schneider (1974)
Republic
20 F 40-59 mixed 11c - Anke & Schneider (1974)
India 26 M, F 40-59 mixed 24b - Vahter (1982)
Israel (Jerusalem) 11 M, F 40-59 mixed 28b - Vahter (1982)
Japan (Kobe) 6 M, F 50-59 mixed 54 5.0 Kitamura et al. (1970)
Japan (Kanazawa) 9 M, F 40-59 mixed 95 10 Ishizaki (1972)
Japan (Tokyo) 17 M, F 40-59 mixed 99 5.7 Tsuchiya et al. (1976)
Japan (Tokyo) 23 M, F 40-59 mixed 76b - Vahter (1982)
Sweden (Stockholm) 83 M, F 40-59 mixed 20 0.76 Elinder et al. (1976)
45 M 40-59 mixed 18 0.69 Elinder et al. (1976)
38 F 40-59 mixed 23 0.88 Elinder et al. (1976)
7 M, F 40-59 non-smoker 11 0.51 Elinder et al. (1976)
28 M, F 40-59 smoker 23 0.97 Elinder et al. (1976)
USA (North Carolina) 19 M 40-59 mixed 27d 2.2 Hammer et al. (1973)
10 F 40-59 mixed 23d 2.2 Hammer et al. (1973)
10 M 40-79 non-smoker 14d 1.6 Hammer et al. (1973)
18 M 40-79 smoker 28d 3.2 Hammer et al. (1973)
Table 10 (contd).
Country Number Sex Age group Smoking Renal cortex Liver cadmium Reference
category cadmium level level (mg/kg
(mg/kg wet wet weight)
weight)
USA (Dallas) 58 M 40-59 mixed 29 1.4 Kowal et al. (1979)
47 M 20-59 non-smoker 13 1.02 Kowal et al. (1979)
115 M 20-59 smoker 24 1.31 Kowal et al. (1979)
USA (Baltimore) 10 M, F 40-59 mixed 30b - Vahter (1982)
Yugoslavia (Zagreb) 28 M, F 40-59 mixed 38b - Vahter (1982)
a The cadmium concentrations are arithmetic mean values and have been rounded off.
b Original data were geometric means. Adjusted according to the findings of Elinder et al. (1976) (x 1.18).
c Original data were for whole kidney (dry weight). Data adjusted for whole kidney (x 1.25) and dry weight (x 0.21).
d Original data were based on ash weight. Data adjusted for ash weight (x 0.011).
In vivo neutron activation analysis (see section 2.2.3.3) has
recently been used to measure kidney and liver cadmium
concentrations in exposed workers. In one study, the detection
limits were about 15 mg/kg for kidney and 1.5 mg/kg for liver (Ellis
et al., 1981a), while in another study detection limits were higher
by a factor of 2 for kidney and 5 for liver (Roels et al., 1981b).
Thus, this method is still not sufficiently sensitive to measure
in vivo tissue levels in a "normal" population. Liver levels of up
to 120 mg/kg and kidney cortex levels of up to 600 mg/kg have been
found among cadmium workers (Ellis et al., 1981a). A decreasing
trend of the kidney levels after a maximum at about 300 mg/kg in
kidney cortex was evident. At this point, the liver level was about
30 mg/kg (Fig. 5). A very similar situation was found in another
factory (Roels et al., 1981b); most workers with high liver cadmium
levels had low kidney levels, and then also showed elevated urinary
excretion of ß2-microglobulin. In exposed workers, the average
ratio of the cadmium concentration in the renal cortex to that in
the liver has been reported to be about 8 (Ellis et al., 1981a) or 7
(Roels et al., 1981b), values that are lower than for the general
population (Table 9). This corresponds to animal data (section
6.3.1.2) showing a greater proportion of accumulated cadmium in the
liver when the exposure level increases.
The total body burden of cadmium in a middle-aged person within
the general population is about twice the amount in kidneys and
liver together (Table 10), i.e. 5-7 mg in a non-smoker in Europe or
the USA and 8-13 mg in a smoker (Kjellström, 1979). In Japan, higher
body burdens have been reported (Tipton et al., 1960; Ishizaki et
al., 1971; Sumino et al., 1975; Tsuchiya et al., 1976). An extensive
review of data from several countries (Cherry, 1981) found total
body burdens to lie within the range 5-20 mg.
In conclusion, the average total body burden of a person of 50
years of age, living in an area not subject to pollution, varies
within the range 5-20 mg in different regions of the world, and the
average cadmium concentration in the renal cortex varies within the
range 11-100 mg/kg wet weight. There is a great individual
variation, and the 90th percentile in those groups studied is about
twice the median value.
Smoking increases the body burden. After long-term low-level
exposure, about half the body burden of cadmium is localized in the
kidneys and liver, a third of the total being in the kidneys. At
higher levels of exposure, a greater proportion of the body burden
is found in the liver. After the development of severe
cadmium-induced renal dysfunction, cadmium is lost from the renal
tissue.
range 10-40 mg/kg, while in Japan values of between 50 and 100 mg/kg
were reported. In workers highly exposed to cadmium, but without
functional impairment of the kidney, concentrations in the renal
cortex may range from 180 to 450 mg/kg wet weight. In cases where
there is severe renal dysfunction, the cadmium concentrations are
generally lower and range between 20 and 120 mg/kg wet weight, i.e.
they are of the same magnitude as those of the general population
(Friberg et al., 1974). This seemingly paradoxical relationship is
discussed in more detail in section 6.
If exposure to cadmium throughout life remains constant and low
in amount, the concentrations in the kidneys become higher (by about
10-20 times) than those in the liver. Average liver cadmium
concentrations are about 1-2 mg/kg wet weight at age 50 in some
European countries and the USA, but in Japan average concentrations
are between 5 and 10 mg/kg (Table 10). Although renal concentrations
generally decrease after age 60 (Fig. 4), liver concentrations reach
a plateau but do not show a clear decrease in aged populations
(Fig. 1). In exposed workers, liver concentrations from 20 to about
300 mg/kg have been recorded and in Itai-itai patients they are
between 63 and 132 mg/kg (Friberg et al., 1974). In people with
severe cadmium-induced renal dysfunction, kidney cadmium levels are
low, but the liver levels may be very high (Ishizaki, 1972).
Table 10. Cadmium concentrations in the renal cortex and liver of people from various geographical areasa
Country Number Sex Age group Smoking Renal cortex Liver cadmium Reference
category cadmium level level (mg/kg
(mg/kg wet wet weight)
weight)
Belgium (Liege) 51 M, F 40-59 mixed 46b - Vahter (1982)
German Democratic 20 M 40-59 mixed 22c - Anke & Schneider (1974)
Republic
20 F 40-59 mixed 11c - Anke & Schneider (1974)
India 26 M, F 40-59 mixed 24b - Vahter (1982)
Israel (Jerusalem) 11 M, F 40-59 mixed 28b - Vahter (1982)
Japan (Kobe) 6 M, F 50-59 mixed 54 5.0 Kitamura et al. (1970)
Japan (Kanazawa) 9 M, F 40-59 mixed 95 10 Ishizaki (1972)
Japan (Tokyo) 17 M, F 40-59 mixed 99 5.7 Tsuchiya et al. (1976)
Japan (Tokyo) 23 M, F 40-59 mixed 76b - Vahter (1982)
Sweden (Stockholm) 83 M, F 40-59 mixed 20 0.76 Elinder et al. (1976)
45 M 40-59 mixed 18 0.69 Elinder et al. (1976)
38 F 40-59 mixed 23 0.88 Elinder et al. (1976)
7 M, F 40-59 non-smoker 11 0.51 Elinder et al. (1976)
28 M, F 40-59 smoker 23 0.97 Elinder et al. (1976)
USA (North Carolina) 19 M 40-59 mixed 27d 2.2 Hammer et al. (1973)
10 F 40-59 mixed 23d 2.2 Hammer et al. (1973)
10 M 40-79 non-smoker 14d 1.6 Hammer et al. (1973)
18 M 40-79 smoker 28d 3.2 Hammer et al. (1973)
Table 10 (contd).
Country Number Sex Age group Smoking Renal cortex Liver cadmium Reference
category cadmium level level (mg/kg
(mg/kg wet wet weight)
weight)
USA (Dallas) 58 M 40-59 mixed 29 1.4 Kowal et al. (1979)
47 M 20-59 non-smoker 13 1.02 Kowal et al. (1979)
115 M 20-59 smoker 24 1.31 Kowal et al. (1979)
USA (Baltimore) 10 M, F 40-59 mixed 30b - Vahter (1982)
Yugoslavia (Zagreb) 28 M, F 40-59 mixed 38b - Vahter (1982)
a The cadmium concentrations are arithmetic mean values and have been rounded off.
b Original data were geometric means. Adjusted according to the findings of Elinder et al. (1976) (x 1.18).
c Original data were for whole kidney (dry weight). Data adjusted for whole kidney (x 1.25) and dry weight (x 0.21).
d Original data were based on ash weight. Data adjusted for ash weight (x 0.011).
In vivo neutron activation analysis (see section 2.2.3.3) has
recently been used to measure kidney and liver cadmium
concentrations in exposed workers. In one study, the detection
limits were about 15 mg/kg for kidney and 1.5 mg/kg for liver (Ellis
et al., 1981a), while in another study detection limits were higher
by a factor of 2 for kidney and 5 for liver (Roels et al., 1981b).
Thus, this method is still not sufficiently sensitive to measure
in vivo tissue levels in a "normal" population. Liver levels of up
to 120 mg/kg and kidney cortex levels of up to 600 mg/kg have been
found among cadmium workers (Ellis et al., 1981a). A decreasing
trend of the kidney levels after a maximum at about 300 mg/kg in
kidney cortex was evident. At this point, the liver level was about
30 mg/kg (Fig. 5). A very similar situation was found in another
factory (Roels et al., 1981b); most workers with high liver cadmium
levels had low kidney levels, and then also showed elevated urinary
excretion of ß2-microglobulin. In exposed workers, the average
ratio of the cadmium concentration in the renal cortex to that in
the liver has been reported to be about 8 (Ellis et al., 1981a) or 7
(Roels et al., 1981b), values that are lower than for the general
population (Table 9). This corresponds to animal data (section
6.3.1.2) showing a greater proportion of accumulated cadmium in the
liver when the exposure level increases.
The total body burden of cadmium in a middle-aged person within
the general population is about twice the amount in kidneys and
liver together (Table 10), i.e. 5-7 mg in a non-smoker in Europe or
the USA and 8-13 mg in a smoker (Kjellström, 1979). In Japan, higher
body burdens have been reported (Tipton et al., 1960; Ishizaki et
al., 1971; Sumino et al., 1975; Tsuchiya et al., 1976). An extensive
review of data from several countries (Cherry, 1981) found total
body burdens to lie within the range 5-20 mg.
In conclusion, the average total body burden of a person of 50
years of age, living in an area not subject to pollution, varies
within the range 5-20 mg in different regions of the world, and the
average cadmium concentration in the renal cortex varies within the
range 11-100 mg/kg wet weight. There is a great individual
variation, and the 90th percentile in those groups studied is about
twice the median value.
Smoking increases the body burden. After long-term low-level
exposure, about half the body burden of cadmium is localized in the
kidneys and liver, a third of the total being in the kidneys. At
higher levels of exposure, a greater proportion of the body burden
is found in the liver. After the development of severe
cadmium-induced renal dysfunction, cadmium is lost from the renal
tissue.

 6.5 Elimination and excretion
6.5.1 Urinary excretion
6.5.1.1 In animals
Nordberg (1972) demonstrated that after subcutaneous injection
for up to 24-25 weeks, the average daily urinary cadmium excretion
(on a group basis) in mice, prior to the onset of tubular
proteinuria, represented about 0.01-0.02% of the body burden
(section 6.7.1). Elinder & Pannone (1979) showed that one month
after repeated subcutaneous exposure ceased, the excretion was only
0.001% of the body burden.
Similar low excretion rates have been found in rabbits given
subcutaneous injections (Nomiyama, 1973a; Nomiyama & Nomiyama,
1976a), and in rabbits (Nomiyama & Nomiyama, 1976a,b) and monkeys
(Nomiyama et al., 1979, 1982a) given cadmium orally. In addition, it
has been reported that, over a range of doses, an increase in
urinary excretion of cadmium is associated with an increase of
cadmium in the renal cortex (Nomiyama & Nomiyama, 1976a; Suzuki,
1980; Bernard et al., 1981).
Studies on several mammalian species, mainly involving repeated
subcutaneous injection of cadmium salts, have shown that urinary
excretion of cadmium increases slowly for a considerable time but,
as kidney dysfunction develops, a sharp increase in excretion occurs
in rabbits (Friberg, 1952; Axelsson & Piscator, 1966a; Nomiyama &
Nomiyama, 1976a), mice (Nordberg & Piscator, 1972), and rats
(Suzuki, 1980). This leads to a decrease in renal and liver cadmium
concentrations (Axelsson & Piscator, 1966a; Suzuki, 1980; Nomiyama &
Nomiyama, 1976a).
When renal tubular lesions were induced by uranyl acetate
injections in animals previously exposed to cadmium, there was no
increase in urinary cadmium excretion (Nomiyama & Nomiyama, 1976a)
or decrease in the level of cadmium in the renal cortex. This
contrasts with the increase in cadmium excretion brought about by
cadmium-induced tubular lesions.
6.5.1.2 In humans
Several studies have shown that in the general population
urinary cadmium excretion increases with age (Katagiri et al., 1971;
Tsuchiya et al., 1976; Elinder et al., 1978; Kowal et al., 1979)
(Fig. 6), this increase coinciding with the increased body burden.
Smokers have higher urinary excretion than non-smokers (Elinder et
al., 1978; Kowal et al., 1979). The mean concentration of urinary
cadmium in such groups of people not exposed to high cadmium levels
is < 0.5-2.0 µg/litre or approximately 0.01% of the total body
burden.
Increased urinary cadmium excretion occurs when tubular
proteinuria develops (Lauwerys et al., 1974a; Kojima et al., 1977).
In cadmium exposed workers, high urinary cadmium concentrations in
the absence of proteinuria can be found after only short exposures
(Lauwerys et al., 1976, 1979a,b) (section 6.7.1).
Most of the cadmium in urine is probably transported bound to
metallothionein. The urinary metallothionein concentration can now
be measured quantitatively with a sensitive radioimmunoassay (Vander
Mallie & Garvey, 1979). Using this technique, Tohyama et al. (1981b)
found good correlation between urinary metallothionein and cadmium
in 67 people exposed in the general environment, and Roels et al.
(1983b) confirmed this correlation in 94 cadmium workers.
6.5.2 Gastrointestinal and other routes of excretion
It is extremely difficult to study gastrointestinal excretion
after oral exposure, since it is not possible to distinguish net
gastroin-testinal excretion from unabsorbed cadmium in faeces.
Animal studies of gastrointestinal excretion following
injections of cadmium (summarized by Friberg et al., 1974) generally
show that a few percent of the dose is excreted in the faeces within
the first few days after injection. The faecal excretion is
initially higher than the urinary excretion after either single or
repeated exposure (Nomiyama, 1978). The mechanism for such excretion
probably involves a transfer of cadmium via the intestinal mucosa,
but biliary excretion may also be involved. The biliary excretion in
the first 24 h after intravenous injection of cadmium is dependent
on the dose (Cirkt & Tichy, 1974; Nomiyama, 1974; Klaassen &
Kotsonis, 1977). In rats given 67, 90 or 120 ßg cadmium (Cikrt &
Tichy, 1974), the cumulative 24 h excretion reached 0.83% at the
lowest dose and 5.68% at the highest dose. The highest excretion
rate was detected between 15 and 30 min after dosing. It has been
reported that after the initially rapid excretion the biliary
excretion is 0.015-0.04% of the body burden per hour over three
consecutive days (Nordberg et al., 1977; Elinder & Pannone, 1979).
Biliary cadmium has been partially characterized as a glutathione
complex (Cherian & Vostal 1977).
Both during and after parenteral exposure to cadmium, the total
gastrointestinal cadmium excretion is considerably higher than the
urinary excretion (Nordberg, 1972; Elinder & Pannone, 1979). A large
proportion of the gastrointestinal excretion is directly related to
the daily dose. After chronic exposure of rats, faecal excretion
amounted to about 0.03% of the body burden, which was considerably
more than the urinary excretion (Elinder & Pannone, 1979).
6.5 Elimination and excretion
6.5.1 Urinary excretion
6.5.1.1 In animals
Nordberg (1972) demonstrated that after subcutaneous injection
for up to 24-25 weeks, the average daily urinary cadmium excretion
(on a group basis) in mice, prior to the onset of tubular
proteinuria, represented about 0.01-0.02% of the body burden
(section 6.7.1). Elinder & Pannone (1979) showed that one month
after repeated subcutaneous exposure ceased, the excretion was only
0.001% of the body burden.
Similar low excretion rates have been found in rabbits given
subcutaneous injections (Nomiyama, 1973a; Nomiyama & Nomiyama,
1976a), and in rabbits (Nomiyama & Nomiyama, 1976a,b) and monkeys
(Nomiyama et al., 1979, 1982a) given cadmium orally. In addition, it
has been reported that, over a range of doses, an increase in
urinary excretion of cadmium is associated with an increase of
cadmium in the renal cortex (Nomiyama & Nomiyama, 1976a; Suzuki,
1980; Bernard et al., 1981).
Studies on several mammalian species, mainly involving repeated
subcutaneous injection of cadmium salts, have shown that urinary
excretion of cadmium increases slowly for a considerable time but,
as kidney dysfunction develops, a sharp increase in excretion occurs
in rabbits (Friberg, 1952; Axelsson & Piscator, 1966a; Nomiyama &
Nomiyama, 1976a), mice (Nordberg & Piscator, 1972), and rats
(Suzuki, 1980). This leads to a decrease in renal and liver cadmium
concentrations (Axelsson & Piscator, 1966a; Suzuki, 1980; Nomiyama &
Nomiyama, 1976a).
When renal tubular lesions were induced by uranyl acetate
injections in animals previously exposed to cadmium, there was no
increase in urinary cadmium excretion (Nomiyama & Nomiyama, 1976a)
or decrease in the level of cadmium in the renal cortex. This
contrasts with the increase in cadmium excretion brought about by
cadmium-induced tubular lesions.
6.5.1.2 In humans
Several studies have shown that in the general population
urinary cadmium excretion increases with age (Katagiri et al., 1971;
Tsuchiya et al., 1976; Elinder et al., 1978; Kowal et al., 1979)
(Fig. 6), this increase coinciding with the increased body burden.
Smokers have higher urinary excretion than non-smokers (Elinder et
al., 1978; Kowal et al., 1979). The mean concentration of urinary
cadmium in such groups of people not exposed to high cadmium levels
is < 0.5-2.0 µg/litre or approximately 0.01% of the total body
burden.
Increased urinary cadmium excretion occurs when tubular
proteinuria develops (Lauwerys et al., 1974a; Kojima et al., 1977).
In cadmium exposed workers, high urinary cadmium concentrations in
the absence of proteinuria can be found after only short exposures
(Lauwerys et al., 1976, 1979a,b) (section 6.7.1).
Most of the cadmium in urine is probably transported bound to
metallothionein. The urinary metallothionein concentration can now
be measured quantitatively with a sensitive radioimmunoassay (Vander
Mallie & Garvey, 1979). Using this technique, Tohyama et al. (1981b)
found good correlation between urinary metallothionein and cadmium
in 67 people exposed in the general environment, and Roels et al.
(1983b) confirmed this correlation in 94 cadmium workers.
6.5.2 Gastrointestinal and other routes of excretion
It is extremely difficult to study gastrointestinal excretion
after oral exposure, since it is not possible to distinguish net
gastroin-testinal excretion from unabsorbed cadmium in faeces.
Animal studies of gastrointestinal excretion following
injections of cadmium (summarized by Friberg et al., 1974) generally
show that a few percent of the dose is excreted in the faeces within
the first few days after injection. The faecal excretion is
initially higher than the urinary excretion after either single or
repeated exposure (Nomiyama, 1978). The mechanism for such excretion
probably involves a transfer of cadmium via the intestinal mucosa,
but biliary excretion may also be involved. The biliary excretion in
the first 24 h after intravenous injection of cadmium is dependent
on the dose (Cirkt & Tichy, 1974; Nomiyama, 1974; Klaassen &
Kotsonis, 1977). In rats given 67, 90 or 120 ßg cadmium (Cikrt &
Tichy, 1974), the cumulative 24 h excretion reached 0.83% at the
lowest dose and 5.68% at the highest dose. The highest excretion
rate was detected between 15 and 30 min after dosing. It has been
reported that after the initially rapid excretion the biliary
excretion is 0.015-0.04% of the body burden per hour over three
consecutive days (Nordberg et al., 1977; Elinder & Pannone, 1979).
Biliary cadmium has been partially characterized as a glutathione
complex (Cherian & Vostal 1977).
Both during and after parenteral exposure to cadmium, the total
gastrointestinal cadmium excretion is considerably higher than the
urinary excretion (Nordberg, 1972; Elinder & Pannone, 1979). A large
proportion of the gastrointestinal excretion is directly related to
the daily dose. After chronic exposure of rats, faecal excretion
amounted to about 0.03% of the body burden, which was considerably
more than the urinary excretion (Elinder & Pannone, 1979).
 There are no available quantitative human data to indicate the
net gastrointestinal excretion.
Cadmium is also eliminated through hair (Anke et al., 1976) and
breast milk (Schroeder & Balassa, 1961), but these routes are of
limited importance for total excretion and do not significantly
alter the biological half-time.
6.6 Biological half-time and metabolic models
6.6.1 In animals
Several studies have been carried out in order to assess the
biological half-times of cadmium in experimental animals. Various
animals species, including mice, rats, rabbits, dogs, and monkeys,
have been studied, and single exposures have normally been used. The
reported half-times have varied from weeks to two years (or as long
as half the life-span of the animal). The development of metabolic
models has shown that the body contains several compartments for
cadmium accumulation, each with a different half-time. Thus, in
whole body half-time measurements, one may find several different
half-times. In order to observe the slowest half-time components, it
is necessary to study the animals for many months.
The biological half-time of cadmium in the kidney and whole
body decreases when renal tubular dysfunction occurs because of
increased urinary excretion (section 6.5.1). However, some studies
have indicated that the biological half-time may change with dose
and body burden of cadmium even before renal damage occurs. For
instance, Engstrom & Nordberg (1979a) reported that in mice
half-time increased with increasing single or repeated oral dose. In
these studies, body burden and renal burden were considerably lower
than the maximum that can be reached in long-term exposure. Nomiyama
(1978) reported that half-time decreased with increasing dose when
animals with the shortened half-time had reached the maximum renal
burden. Even shorter half-times were reported when renal tubular
dysfunction occurred after exposure to high doses (Nomiyama, 1978).
A number of studies on biological half-time and metabolic
models for animals have been reviewed by Friberg et al. (1974) and
Nomiyama (1978).
The wide difference in the results obtained by investigators
may be explained by variations in exposure level and type, the
different animal species used, and interactions between cadmium and
other exposure factors. Reported half-times range from several weeks
in mice to 22 years in monkeys (Friberg et al., 1974; Nomiyama et
al., 1979; Nomiyama et al., 1984). The variations in half-times in
specific tissues between different species or individuals may be due
to variations in the production of metallothionein, which binds
tissue cadmium and contributes to its retention.
6.6.2 In humans
Experimental and epidemiological evidence indicates strongly
that the biological half-time in the whole body is extremely long
(many years). Experimental evidence from one study (Shaikh & Smith,
1980), in which one subject was given radioactive cadmium and
examined periodically for the next 2 years, showed a biological
half-time of 26 years. In three similar studies, in which a small
number of subjects were followed up for a limited period (about 100
days), half-times of 93-202 days were reported (Rahola et al., 1972;
Flanagan et al., 1978; McLellan et al., 1978). Only one of these
studies (Rahola et al., 1972) gave confidence limits for the
estimated biological half-time (130 days to infinity).
Another approach to estimate the half-time used involves
comparing total daily excretion with total body burden, applying a
one-compartment model to the body as a whole (Friberg et al., 1974;
Task Group on Metal Toxicity, 1976). A further approach analyses the
accumulation in the kidney using a one-compartment model taking into
consideration age-related variations in daily cadmium exposure and
kidney weight (Tsuchiya & Sugita, 1971; Kjellström, 1971). More
recently, an elaborate model has been developed that includes
separate compartments for, for instance, kidney, liver, and blood,
and incorporates age-related variations in daily intake, tissue
weights, and renal function (Kjellström & Nordberg, 1978).
These models rely on many assumptions concerning the cadmium
concentration in food, calorific intake, absorption rates, and other
factors. Inevitably, the data produced by these models are only
tentative, but they are important for future research. Using data
from Japan on the accumulation of cadmium with age, Tsuchiya et al.
(1976) used a series of one-compartment mathematical models
developed by Tsuchiya & Sugita (1971) to estimate biological
half-times of cadmium in various organs. These authors estimated the
biological half-time in the kidneys to be about 17 years and that in
the liver 7 years.
Elinder et al. (1976) used autopsy data from non-smokers in
Sweden and a one-compartment model (Kjellström, 1971) to estimate
the biological half-time in the renal cortex. They assumed that the
daily intake of cadmium had doubled in 50 years (Kjellström et al.,
1975a) and estimated the half-time to be 20-50 years (30 years being
the best estimate).
Using an 8-compartment model (Kjellström & Nordberg, 1978), the
biological half-times of cadmium in the liver and kidney were
estimated to be 7.5 and 12 years, respectively (Kjellström &
Nordberg, 1978). The longest half-time was calculated for the "other
tissues" compartment. This included muscle tissue, which was found
to have the longest half-time in an autopsy study (Kjellström,
1977). However, it should be pointed out that when using this type
of model to simulate the chemobio-kinetics of cadmium, the
individual half-times of different tissues are less important than
the dynamics of the model as a whole.
After high cadmium exposure, as occurs among certain industrial
workers, the biological half-time may not be the same as that during
normal exposure in the general environment. Current models, however,
do not consider this factor. If the exposure level is very high, the
ratio of rapid components to slow components may be altered. An
example of this is the very high urinary excretion found after only
a short exposure to high air cadmium levels (Lauwerys et al., 1976,
1979b). The biological half-time is also shorter if there is renal
tubular dysfunction. Fletcher et al. (1982) carried out
in vivo neutron activation analysis of the liver of 13 cadmium
workers twice within a period of 3 to 4 years (the occupational
cadmium exposure of these workers had ceased before the first
analysis). Three workers showed proteinuria and an average cadmium
half-time in the liver of 2 years. The other 10 workers had an
average half-time of 6.4 years, nine of whom had an average
half-time of 13.5 years and no proteinuria.
Jarup et al. (1983) studied the half-time of cadmium in blood
of five smelter workers who had previously experienced high cadmium
exposure. Repeated blood analysis carried out over a 10-to 13-year
period revealed short-term (75-128 days) and a long-term (7.4-16
years) half-time components. The long-term component in two workers
with proteinuria was shorter than in the other workers.
6.7 Biological indices of cadmium exposure, body burden, and
concentrations in kidneys
There is no easy way to measure directly the whole body burden
or concentrations of cadmium in different tissues of a living
person. In vivo neutron activation methods have been used in
special circumstances (Ellis et al., 1981a; Roels et al., 1981b;
Tohyama et al., 1981a).
At present, it is necessary to study concentrations in easily
available indicator media in order to evaluate exposure and
accumulation of cadmium. The suitability of certain indicator media
for such purposes is supported by studies on both animals and
humans; urine, blood, faeces, and hair have all been used as
indicator media. Methods for the biological monitoring of cadmium
levels in blood and urine have been reviewed by Nordberg & Nordberg
(1988) and WHO (1980).
6.7.1 Urine
The human and animal studies summarized in section 6.5.1 allow
the following interpretation of the significance of cadmium in urine
(Lauwerys et al., 1980b). In the absence of episodes of high-level
exposure to cadmium and provided that cadmium-binding sites in the
organism are not saturated and cadmium-induced nephropathy has not
yet occurred, the urine cadmium level increases in proportion to the
amount of cadmium stored in the body. In such situations, which
prevail mainly in the general population and in workers moderately
exposed to cadmium, there is significant correlation between urinary
cadmium and cadmium in kidney. Episodes of high exposure to cadmium,
however, may lead to a transient increased urinary excretion.
If exposure to cadmium has been excessive, the cadmium-binding
sites in the organism become progressively saturated and, despite
continuous exposure, the cadmium concentration in the renal cortex
tends to plateau. Once this point is reached, the cadmium that is
still absorbed cannot be further retained in the kidney and is
rapidly excreted in the urine. Under these conditions, urinary
cadmium is also influenced by the recent intake. The relative
influence of the body burden and the recent exposure on urinary
cadmium depends on the exposure intensity. If exposure continues, a
certain percentage of individuals may develop renal damage. This is
associated with a progressive loss of cadmium accumulated in the
kidney, which gives rise to a further increase in urinary cadmium.
Eventually, the amount of cadmium that can be released from the
kidney decreases progressively and the urinary cadmium concentration
follows the same trend. The changes in the urinary metallothionein
level parallel those of cadmium (section 6.8.2).
In summary, several factors (duration and intensity of exposure
to cadmium, the presence of renal dysfunction and its duration) must
be taken into consideration when interpreting urinary cadmium (and
metallothionein) levels.
6.7.2 Blood
Plasma cadmium levels are considered to be related to recent
exposure but are often so low that they cannot be measured routinely
(section 6.2). Most of the cadmium in the blood is in the
erythrocytes.
Cadmium levels in whole blood mainly reflect the exposure
during recent weeks or months. In cadmium workers, the level
increases markedly within the first few months after occupational
exposure starts (Kjellström & Nordberg, 1978; Lauwerys et al.,
1979b). It is probable that a portion of the blood cadmium level
reflects body burden rather than present exposure in view of the
known transport of cadmium via blood from the liver to the kidneys
and other tissues (section 6.2) and the long-term half-time
component demonstrated by Jarup et al. (1983). Workers with
relatively long exposure durations but whose cadmium exposure has
ceased have elevated blood cadmium levels for several years (Friberg
et al., 1974; Jarup et al., 1983).
Reports of blood cadmium levels in the general population have,
in the past, often been unreliable, owing largely to the
difficulties encountered in the analysis of blood cadmium (Lauwerys
et al., 1975). However, improvements in analytical techniques have
since been achieved through biological standards for blood and
systematic quality assurance programmes (Stoeppler et al., 1979;
Vahter, 1982). Average blood cadmium values up to 10 µg/litre or
more have been reported in the past, but the analytical procedures
used mean that the accuracy of these data is in doubt (Vahter, 1982;
Friberg & Vahter, 1983).
Various aspects of blood cadmium analysis have been discussed
in a UNEP/WHO study, which also included a quality assurance
programme and data from 10 countries (Vahter, 1982; Friberg &
Vahter, 1983). It was found that even in the countries with the
highest blood cadmium levels, the average was less than 4 µg/litre.
Furthermore, smokers were found to have higher values than
non-smokers, and non-smokers, in most countries, had mean levels
below 1 µg/litre. Although only slightly above 1 µg/litre, the
levels for non-smokers in Japan were about twice as high as the
levels in the USA, which probably reflects the difference in average
daily cadmium intake via food (section 5.2.2).
Reported cadmium concentrations in the blood of exposed workers
are generally between 5 and 50 µg/litre, but levels of between 100
and 300 µg/litre have resulted from extreme exposures (Roels et al.,
1982; Hassler et al., 1983).
6.7.3 Faeces
Gastrointestinal absorption amounts to only a few percent of
the cadmium ingested daily (section 6.1.2), and the quality of
cadmium excreted gastrointestinally is small compared to the
unabsorbed portion of ingested cadmium. Thus, daily faecal cadmium
can serve as a good indicator of the daily amount of cadmium
ingested via food and water or cleared from the lungs after
occupational exposure to large dust particles. Faecal cadmium
correlates very closely with daily energy intake (section 5.2.4),
and average cadmium intake estimates for different countries agree
well with reported average faecal cadmium amounts.
A portion of the cadmium in human faeces is related to the body
burden (section 6.5.2). In workers with high body burdens but low
daily cadmium intakes via food, this portion might be greater than
the unabsorbed part of ingested cadmium, because the faecal
excretion is similar to the urinary excretion.
6.7.4 Hair
Cadmium in hair is not a reliable indicator of either recent
exposure or body burden. The main problem is external contamination
of the hair, which cannot be distinguished from the endogenous
cadmium (Nishiyama & Nordberg, 1972). However, a correlation was
found between air cadmium levels of cities in the USA and cadmium
levels in the hair of 10-year-old children living in these cities
(Hammer et al., 1971). In a study of cadmium workers (Ellis et al.,
1981b), a higher average hair cadmium level was found among exposed
workers than among controls, but there was a poor relationship
between hair cadmium and cadmium in the blood, urine, liver or
kidney. Cadmium concentrations in the hair of people without
excessive exposure are usually between 0.5 and 2 mg/kg.
6.8 Metallothionein
6.8.1 Nature and production
Metallothionein is a metal-binding protein of low molecular
weight, which has a key role in the metabolism of cadmium. It is
rich in cysteine but contains no aromatic amino acids or histidine
(Kagi & Vallee, 1960, 1961).
This protein was identified for the first time by Margoshes &
Vallee (1957) in horse kidney cortex. Its molecular weight is about
6600 (6000 for the apoprotein moiety, thionein), and it has a
non-globular shape. On gel filtration, however, it moves like a
spherical protein with a molecular weight of about 10 000 (Kagi &
Nordberg, 1979). There have been several reports dealing with the
function and biochemistry of metallothionein (Kagi & Nordberg, 1979;
Brady, 1982; Foulkes, 1982; Webb & Cain, 1982; Kagi & Kojima, 1987).
Piscator (1964) suggested that metallothionein played a role in
cadmium transport and detoxication, and it has subsequently been
identified in human kidney and liver (Pulido et al., 1966; Chung et
al., 1986) as well as in those of various experimental animals (Kagi
& Nordberg, 1979).
The structure and genetic expression of mouse and human
metallothionein have now been identified. Two major forms of
metallothionein are present in most mammalian tissues, particularly
liver and kidney, i.e. metallothionein I (Mt-I) and metallothionein
II (Mt-II). Induction of synthesis is under the control of a large
group of genes and is stimulated by glucocorticoids and the
essential metals zinc and copper, as well as by the toxic metals
cadmium and mercury (Karin et al., 1981; Karin & Richards, 1982).
In vitro binding affinities have been demonstrated for a number of
other toxic metals, including bismuth, cobalt, silver, and gold
(Cherian & Nordberg, 1983). Metallothionein binds seven metal ions
per molecule between two separate metal-cysteine clusters, and a
single molecule may contain more than one metal, e.g., cadmium and
zinc, mercury and copper.
6.8.2 The role of metallothionein in transport, metabolism, and
toxicity of cadmium
Piscator (1964) suggested that some of the cadmium-binding
metallothionein in the liver may migrate into the blood stream. As
discussed in section 6.2, part of the plasma cadmium in animals
exposed for a long time to cadmium is bound to a protein with the
same molecular weight as metallothionein. When metallothionein-bound
cadmium is present in the plasma, it is quickly cleared by
glomerular filtration and reabsorbed in the renal tubules or
excreted in the urine (Cherian & Shaikh, 1975; Nordberg et al.,
1975; Webb & Etienne, 1977; Fowler & Nordberg, 1978).
At low levels of cadmium-metallothionein in the plasma, tubular
reabsorption is almost complete, whereas the uptake in the tubular
cells from the tubular fluid is saturated in the presence of high
concentrations (Nomiyama & Foulkes, 1977; Foulkes, 1982). Thus, high
urinary excretion of cadmium-metallothionein occurs shortly after
the administration of larger doses, i.e. doses exceeding about
0.1 mg cadmium/kg body weight (Cherian & Shaikh, 1975; Nordberg &
Nordberg, 1975).
It has been demonstrated in animal and in vitro tissue
studies that metallothionein provides a protective role for cadmium
toxicity (Cherian & Nordberg, 1983). Mice pretreated with cadmium
have increased tolerance to subsequent cadmium exposure (Nordberg et
al., 1971), and exposure to cadmium may protect from subsequent
mercury toxicity (Piotrowski et al., 1974). In addition, the
inhibition of certain mixed-function oxidases by cadmium is reduced
by prior induction of metallothionein by cadmium in immature mice
(Asokan et al., 1984). Pre-exposure of cultured kidney cells to
cadmium protects from subsequent exposure (Cherian, 1980; Jin et
al., 1986).
Nordberg et al. (1975) showed that metallothionein isolated
from rabbit and mouse liver produced acute renal tubular cell
toxicity when injected subcutaneously into mice. Further study
(Cherian et al., 1976; Fowler & Nordberg, 1978; Squibb et al., 1982,
1984) suggested that parenterally administered
cadmium-metallothionein enters proximal renal tubular lining cells
in pinocytotic vesicles that fuse with lysosomes. The
metallothionein is degraded, releasing cadmium into the cytosol and
producing cellular degeneration and necrosis within 8-24 h. The
renal tubular cell toxicity produced by metallothionein with
different ratios of cadmium and zinc is proportional to the cadmium
content of the metallothionein (Suzuki, 1982). Zinc-thionein does
not have a similar effect.
The pathogenesis of renal tubular cell toxicity is thought to
be related to non-metallothionein-bound cadmium, which becomes
rapidly bound to existing metallothionein sites or induces the
synthesis of new metallothionein (section 7.2.1.4).
The prevalence of nephrotoxicity rather than hepatotoxicity in
chronic cadmium exposure may be due to several factors. Firstly, the
release of hepatic cadmium-metallothionein or its presence in the
blood can result in preferential accumulation of cadmium in the
kidneys. Secondly, it has been shown in experimental animals that
the kidney can accumulate metallothionein mRNA in response to
cadmium exposure to only about half the level of the liver
(Koropatnick & Cherian, 1988). Thus, the kidney may not be able to
synthesize metallothionein as efficiently as the liver in response
to cadmium exposure, resulting in an accumulation of
non-metallothionein cadmium in the kidney but not in the liver.
Nomiyama et al. (1982a) studied the concentrations of total
cadmium, metallothionein-cadmium, and non-metallothionein-cadmium in
the renal cortex of monkeys fed diets containing 30 mg cadmium/kg
food for 12 months. They found that the concentration of
non-metallothionein-cadmium increased with dose of cadmium to about
35 mg/kg tissue and total cadmium to about 200 mg/kg tissue when the
total dose of cadmium was 0.4 g. Similar measurements were made in
rabbits given cadmium chloride (0.5 mg cadmium/kg body weight)
subcutaneously every day for 21 weeks. There were parallel increases
of total cadmium and non-metallothionein-bound cadmium during the
initial 4 weeks of dosing; these remained unchanged until the 14th
week when striking renal dysfunction appeared. At that time, total
cadmium and non-metallothionein-bound cadmium levels fell despite
the continued administration of cadmium (Nomiyama & Nomiyama, 1982).
Increased knowledge of the intracellular binding or speciation of
non-metallothionein-bound cadmium should improve our understanding
of the relative roles of metallothionein-bound and
non-metallothionein-bound cadmium.
When Cherian et al. (1978) exposed mice by injection and
feeding to both cadmium chloride and cadmium-metallothionein, both
compounds were absorbed and distributed in the body. However, in the
short term, cadmium-metallothionein was selectively accumulated in
the kidney and cadmium chloride in the liver.
Most cadmium in human urine is bound to metallothionein
(Tohyama et al., 1981b), and good correlation has been found between
the urinary cadmium and metallothionein concentrations both in
elderly women exposed in the general environment (Tohyama et al.,
1981b) and in male cadmium workers (Nordberg et al., 1982; Roels et
al., 1983b). Measurement of urinary metallothionein thus provides a
good indication of the urinary cadmium level and offers the
advantage over cadmium analysis of avoiding the possibility of
external contamination. Women were found to have much higher urinary
metallothionein concentrations than men, even at similar cadmium
levels.
6.9 Conclusions
Data from experimental animals and humans have shown that
pulmonary cadmium absorption is greater than gastrointestinal
absorption. Depending on chemical speciation, particle size, and
solubility in biological fluids, up to 50% of the inhaled cadmium
compound may be absorbed. The gastrointestinal absorption of cadmium
is influenced by the type of diet and nutritional status, iron
status appearing to be particularly important. On average, 5% of the
total oral intake of cadmium is absorbed, but individual values
range from less than 1% to more than 20%. Cadmium may also be
transported to the fetus. However, although cadmium accumulates in
the placenta, little is transferred to the fetus.
Cadmium absorbed from the lungs or the gastrointestinal tract
is stored principally in the liver and kidneys where more than half
of the body burden is deposited. Highest cadmium concentrations are
generally found in the renal cortex, but as exposure levels
increase, a greater proportion of the absorbed cadmium is stored in
the liver. The cadmium excretion rate is normally low, and the
biological half-time is very long (decades) in the kidneys, muscles,
liver, and total body of humans. The cadmium concentrations in most
tissues increase with age. In exposed people with renal damage,
urinary excretion of cadmium increases and, thus, the whole body
half-time is shortened. The renal damage leads to losses of cadmium
from the kidney, and the renal concentrations are eventually lower
than in people with similar exposure but without renal damage.
Metallothionein is an important transport and storage protein
for cadmium and other metals. Cadmium can induce metallothionein
synthesis in many organs including the liver and kidney. The binding
of intracellular cadmium to metallothionein in tissues protects
against cadmium toxicity. Non-metallothionein-bound cadmium may,
therefore, have a role in the pathogenesis of cadmium-related tissue
injury. The speciation of other cadmium complexes in tissues or
biological fluids is unknown.
Urinary excretion of cadmium is related to body burden, recent
exposure, and renal damage. In people with low exposures, cadmium in
urine is mainly related to body burden. Cadmium-exposed people with
proteinuria generally exhibit greater cadmium excretion than such
people without proteinuria. After high exposure ceases, urinary
cadmium decreases even though renal damage persists. The
interpretation of urinary cadmium is thus dependent on a number of
factors. The magnitude of gastrointestinal excretion is similar to
that of urinary excretion, but it cannot be easily measured. Other
excretory routes such as lactation, sweating or placental transfer
are insignificant.
Cadmium in faeces is a good indicator of recent daily intake
from food in the absence of inhalation exposure. Cadmium in blood
occurs mainly in the blood cells, and the plasma concentrations are
very low. There are at least two blood compartments, one being
related to recent exposure with a half-time of about 2-3 months, and
the other probably related to body burden with a half-time of
several years.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Lethal dose and lethal effects
LD50 inhalation values are in the range of 500 to
15 000 mg/m3.min for different species (Barrett et al., 1947;
Harrison et al., 1947; Hadley et al., 1979). The cause of death is
pulmonary oedema.
The LD50 after the injection of soluble cadmium compounds is
in the range of 2.5-25 mg/kg body weight (Friberg, 1950; Eybl &
Sykora, 1966; Commission of the European Communities, 1978). Shortly
after large doses are injected, severe endothelial damage is seen in
the small vessels of the peripheral nervous system (Gabbiani, 1966)
and in the testis (Parizek, 1957). If the animal survives for some
hours, the most pronounced changes are found in the liver (Dudley et
al., 1982), and liver damage is probably the lethal effect of a
single high parenteral exposure.
For most cadmium compounds, the LD50 after oral
administration is about 10-20 times higher than after parenteral
administration, and the readily soluble compounds have a lower
LD50 values than the insoluble ones (Table 11).
Nomiyama et al. (1978b) showed that the LD50 in mice was
lower at cold temperatures (+8 °C) than at higher temperatures
(+22 or +37 °C), both after oral and peritoneal exposure.
7.1.2 Pathological changes affecting specific systems in the body
The chronic effects of long-term exposure to low doses of
cadmium constitute the main problem for non-occupationally exposed
humans. Therefore, the effects of single exposure in animals will be
dealt with only briefly, and the main emphasis will be on chronic
effects.
Specific effects from a single high dose of cadmium have been
described by several investigators and have been reviewed by Friberg
et al. (1974, 1986), Commission of the European Communities (1978),
and Kawai (1978). One of the most pronounced effects seen was in the
gonads (testis and ovary).
Table 11. LD50 values for cadmium compounds given to mice and rats by intragastric administration
Species Compound Molecular formula Relative molecular LD50 with confidence LD50 for cadmium
massa limits (mg/kg)b ion alone (mg/kg)
Mouse cadmium (element) Cd 109 890 (636-1246) 890
cadmium oxide CdO 128.4 72 (41-113) 63
cadmium sulfate CdSO4 208.5 88 (69.8-100.2) 47
cadmium chloride CdCl2 183.3 93.7 (75.5-111.9) 57
cadmium nitrate Cd(NO3)2 236.4 100 (78.7-121.8) 48
cadmium iodide CdI2 366.2 166 (139-193) 51
cadmium caprylate Cd(C7H15COO)2 394.8 300 (196-459) 85
cadmium carbonate CdCO3 169 310 (215-404) 202
cadmium stearate Cd(C17H35COO)2 679.4 590 (556-624) 98
cadmium sulfide CdS 144.5 1166 (1135-1197) 907
cadmium sulfoselenide CdSe.CdS 335.8 2425 (2393-2457) 1623
barium-cadmium stearate BaCd(C17H35COO)4 1383.7 3171 (2763-3579) 258
Rat cadmium caprylate Cd(C7H15COO)2 394.8 950 (613-1472) 270
cadmium stearate Cd(C17H35COO)2 679.4 1225 (875-1574) 203
barium-cadmium stearate BaCd(C17H35COO)4 1383.7 1980 (1736-2224) 161
a From: Weast (1974)
b From: Tarasenko et al. (1974), Vorobjeva & Sabalina (1975), and Vorobjeva & Bubnova (1981)
7.1.2.1 Acute effects on testes and ovaries
Testicular necrosis occurs in experimental animals given single
injections of salts corresponding to 2-4 mg cadmium/kg body weight
(Parizek & Zahor, 1956; Parizek, 1957). At a later stage, Leydig
cells regenerate (Parizek, 1957, 1960; Allanson & Deanesly, 1962).
Gabbiani et al. (1974) detected dilation of interendothelial clefts
in the small blood vessels of the testis as early as 15 min after an
intravenous injection of cadmium salts. Effects on the testis have
been extensively reviewed by Gunn & Gould (1970).
The marked effects on the testis after cadmium injection are
probably the result of endothelial damage. In the small vessels this
damage gives rise to increased capillary permeability. This leads to
vascular escape of fluids and blood plasma substances into the
interstitium, which results in oedema, decreased capillary blood
flow, ischaemia, and testicular cell necrosis (Aoki & Hoffer, 1978;
Francavilla et al., 1981).
A single injection of cadmium salts at a dose that induces
testicular haemorrhagic necrosis has been shown to induce
haemorrhages and necroses in the ovaries of prepubertal rats (Kar et
al., 1959), and in the ovaries of adult rats in persistent oestrus
(Parizek et al., 1968a). The effect of cadmium on the testis was not
dependent on the presence of the hypophysis (Parizek, 1960). Ovarian
effects can be prevented by the administration of PMSG hormones
(Parizek et al., 1968a). Numerous studies on the effects of cadmium
on the testes and other reproductive organs were reviewed by Barlow
& Sullivan (1982).
7.1.2.2 Acute effects on other organs
A single inhalation exposure to cadmium at concentrations of
5-20 mg/m3 for 50-120 min gives rise to pulmonary oedema in rats
and rabbits (Hayes et al., 1976; Bouley et al., 1977; Bus et al.,
1978; Dervan & Hayes, 1979; Boisset & Boudene, 1981; Fukuhara et
al., 1981). The morphological changes seen in the lung have been
described in detail by Strauss et al. (1976).
After the parenteral administration of cadmium at dose levels
similar to the LD50, pronounced effects were seen in the small
blood vessels of, for instance, the nervous system (Gabbiani et al.,
1974). Hoffman et al. (1975) noted profound morphological effects in
the liver of rats given 6 mg cadmium/kg body weight, and Dudley et
al. (1982), examining liver effects from a single injection of
cadmium (3.9 mg/kg body weight), concluded that liver was the major
target organ in rats for acute cadmium toxicity. Changes in blood
pressure shortly after the acute administration of cadmium have also
been recorded (Dalhamn & Friberg, 1954; Perry et al., 1970).
Oral administration of cadmium compounds induces epithelial
desquamation and necrosis of the gastric and intestinal mucosa,
together with dystrophic changes of the liver, heart, and kidneys
(Tarasenko et al., 1974; Vorobjeva & Sabalina, 1975).
7.2 Repeated and/or long-term exposure
7.2.1 Effects on the kidneys
Since the kidney is the critical organ in humans exposed for
long periods to relatively small amounts of cadmium (section 8.2.1),
results from relevant animal studies will be dealt with in some
detail. Even though it is difficult to extrapolate quantitative
information from the findings in animals, experiments have provided
valuable information concerning mechanisms of cadmium-induced
nephropathy and the significance of various biological indicators of
exposure and effect, and have supported the findings in humans. For
example, Friberg (1950) verified in animal experiments that exposure
to cadmium caused a type of proteinuria similar to the one he had
found in exposed workers.
Animal studies that have given data on renal effects as well as
the corresponding renal cadmium concentrations are summarized in
Table 12. An evaluation of organ dose-effect and dose-response
relationships is included in section 7.2.1.4.
7.2.1.1 Oral route
Renal lesions were first reported by Prodan (1932) and Wilson
et al. (1941) after cats and rats were given large oral doses of
cadmium for several months. Prodan (1932) reported varying degrees
of desquamation in proximal tubular epithelium (and no changes in
the glomeruli) after feeding cats 100 mg cadmium per day for one
month. Wilson et al. (1941) reported slight tubular changes in rats
after they were exposed for 3 months to a diet containing 62 mg
cadmium/kg.
Studies utilizing high exposures have also been performed by
Stowe et al. (1972). Ten rabbits received cadmium in drinking-water
(160 mg/litre) for 6 months. Kidney function was not investigated,
but histopathological examination revealed pronounced morphological
changes in the proximal tubules. The mean renal concentration of
cadmium was 170 mg/kg wet weight, which would correspond to about
210 mg/kg wet weight in the renal cortex (section 6.4). Still higher
doses (300 mg/kg diet) were given to rabbits for 54 weeks by
Nomiyama et al. (1975), who observed aminoaciduria and enzymuria
after 16 weeks. At this stage, the cadmium concentration in the
renal cortex was 200 mg/kg wet weight. Proteinuria and glycosuria
appeared at a later stage, 37 and 42 weeks, respectively, after
exposure had started. The cadmium concentration in the renal cortex
was 300 mg/kg wet weight after 40 weeks.
Table 12. Summary of animal studies with data on both renal cadmium levels and effects
Species Route of Exposure Duration Average cadmium Renal changes Reference
administration level (months) level in kidney
cortex (mg/kg
wet weight)
Mouse subcutaneous 0.25 mg/kg 6 110-170a no effects Nordberg & Piscator (1972)
body weight
Mouse subcutaneous 0.5 mg/kg 6 170a tubular protein patterns Nordberg & Piscator (1972)
body weight in urine
Rat intraperitoneal 0.75 mg/kg 3 200a no effects Bonnell et al. (1960)
body weight
Rat intraperitoneal 0.75 mg/kg 4 300a histological changes in Bonnell et al. (1960)
body weight 60% of animals
Rat subcutaneous 0.65 mg/kg 3 200 histological changes Goyer et al. (1984)
body weight
Rat water 10 mg/litre 8.5 11a no histological changes Kawai et al. (1976)
Rat water 50 mg/litre 8.5 35a slight histological changes Kawai et al. (1976)
Rat water 100 mg/litre 8.5 90a histological changes Kawai et al. (1976)
Rat water 200 mg/litre 8.5 145a histological changes Kawai et al. (1976)
Rat water 200 mg/litre 11 200 total proteinuria and low Bernard et al. (1981)
molecular weight proteinuria
Rat water 50 mg/litre 3 100a decreased insulin and PAH Kawamura et al.(1978)
clearance; histological
changes
Table 12 (contd).
Species Route of Exposure Duration Average cadmium Renal changes Reference
administration level (months) level in kidney
cortex (mg/kg
wet weight)
Rat water 50 mg/litre 2.5 235 slight histological changes Axelsson & Piscator (1966a);
in proximal tubules Axelsson et al. (1968)
Rabbit subcutaneous 0.25 mg/kg 2.5 235 slight histological changes Axelsson & Piscator (1966a);
body weight in proximal tubules Axelsson et al. (1968)
Rabbit subcutaneous 0.25 mg/kg 4 460 more severe histological Axelsson & Piscator (1966a);
body weight changes; reduction of alkaline Axelsson et al. (1968)
phosphatase activity in renal
cortex; total proteinuria
Rabbit subcutaneous 0.5 mg/kg 2.5 300 total proteinuria Nomiyama et al. (1982b)
body weight
Rabbit subcutaneous 0.5 mg/kg 0.7 200 proteinuria, glucosuria, and Nomiyama & Nomiyama
body weight aminoaciduria; decrease in (1982)
CIN and TmPAH
Rabbit subcutaneous 0.5 mg/kg 1 120 ß2-microglobulin Nomiyama et al. (1982b)
body weight
Rabbit subcutaneous 1.5 mg/kg 1 50-200 decreased tubular Nomiyama (1973a);
body weight readsorption Nomiyamaet al. (1978a)
Rabbit subcutaneous 0.5 mg/kg 2 160a slight histological changes Kawai et al. (1976)
body weight
Rabbit water 160 mg/litre 6 170a extensive fibrosis; pronounced Stowe et al. (1972)
changes
Table 12 (contd).
Species Route of Exposure Duration Average cadmium Renal changes Reference
administration level (months) level in kidney
cortex (mg/kg
wet weight)
Rabbit water 50 mg/litre 10 58 slight tubular atrophy Kawai et al. (1976)
Rabbit water 200 mg/litre 10 200 severe interstitial and Kawai et al. (1976)
tubular fibrosis
Rabbit diet 300 mg/kg 4 200 aminoaciduria, enzymuria Nomiyama et al. (1975)
Rabbit diet 300 mg/kg 10 300 proteinuria, glucosuria Nomiyama et al. (1975)
Pig diet 50-350 mg/kg < 12a equimolar increase in zinc Cousins et al. (1973)
in kidney
Pig diet 50-350 mg/kg 78a decrease in renal leucine Cousins et al. (1973)
aminopeptidase
Horse diet no cadmium lifelong 75 renal tubular interstitial Elinder et al. (1981a,b)
added (up to 240) changes and fibrosis in
25% of animals
Bird subcutaneous 0.16 mg/kg 1.5 20b histological changes Nicholson & Osborn (1983)
body weight
a These values are whole kidney concentrations; about 0.8 times kidney cortex values, on average.
b Denotes concentrations (wet weight) calculated as 0.2 times dry weight concentrations.
Morphological changes of the renal tubules were reported by
Kawai et al. (1976) in rats given 50 mg cadmium/litre drinking-water
for 8.5 months. The average renal cadmium concentration was about
38 mg/kg wet weight which corresponds to about 50 mg/kg in the renal
cortex.
Histological lesions in the proximal renal tubules were also
found in rats exposed to 200 mg cadmium/litre for 2 months (Itokawa
et al., 1978). Histochemical examination of the kidney showed that
the proximal tubular epithelium had particularly high cadmium
concentrations. The average renal concentrations were 48 mg/kg and
80 mg/kg, respectively, in rats with sufficient and deficient
calcium intakes. These levels would correspond to about 60 and
100 mg cadmium/kg in the renal cortex (section 6.4). Inulin
clearance was reduced to about a third of the control values in the
cadmium-exposed groups, indicating considerable functional damage to
the glomeruli. The only reported change in renal tubular function
was that the fractional excretion of calcium was increased about 50%
in the cadmium-exposed groups.
In a study of 50 rats exposed to 200 mg cadmium/litre in
drinking-water for up to 11 months (Bernard et al., 1981), there was
an increased prevalence of total proteinuria in the 8th month, when
the average cadmium concentration in the renal cortex was about
200 mg/kg.
Kajikawa et al. (1981) also reported morphological changes in
the kidneys of rats given drinking-water containing 200 mg cadmium
chloride/litre for 91 weeks. Histologically, they found degenerative
changes in the proximal convoluted tubules and, using electron
microscopy, proliferation of smooth endoplasmic reticulum,
vacuolization, and coagulative necrosis of the tubular cells. No
significant changes were observed in the glomeruli or interstitial
tissue.
When Cousins et al. (1973) gave large amounts of cadmium
chloride to pigs (50, 150, 450, and 1350 mg/kg diet), there was a
decrease in the activity of leucine aminopeptidase in the kidney
cortex at a renal cadmium concentration of 78 mg/kg wet weight,
corresponding to a renal cortex concentration of about 100 mg/kg wet
weight (section 6.4).
An extensive data base on the renal effects of cadmium in
monkeys has been developed in Japan. Several of these studies are
summarized in Table 13.
Study I (Nomiyama et al., 1979) was carried out using ten male
rhesus monkeys (three years of age). The monkeys were given 100 g of
solid feed containing 0, 3, 30, or 300 mg cadmium/kg daily for 37
weeks, followed by 130 g of feed for 18 weeks. Even the solid feed
given to the control group contained cadmium at a concentration of
0.13 mg/kg.
In study II, Nomiyama et al. (1987) used 36 male rhesus monkeys
(three years of age) and gave them 100 g of solid feed containing 0,
3, 10, 30, or 100 mg cadmium/kg daily for 52 weeks. During the
following 52 weeks 150 g was given, and then for the remaining 358
weeks 200 g was given. The solid feed given to the control group
contained cadmium at 0.27 mg/kg and zinc at 30 mg/kg.
Study III (Nomura et al., 1988) was performed with 40 female
rhesus monkeys given 150 g of solid feed for nine years (Table 14).
In study IV, Nomiyama & Nomiyama (1988) used nine male
crab-eating monkeys. Two of the animals were used as controls, and
three were given a diet containing cadmium concentrations of 3 mg/kg
(190 µg/day) as cadmium chloride (the pelleted food also contained
(30 mg zinc/kg)). The remaining four were fed 80 µg cadmium/day in
the form of contaminated rice.
In studies I and II, monkeys that had been given feed
containing cadmium at 300 mg/kg and 100 mg/kg showed indi-cations of
renal dysfunction, such as proteinuria, glucosuria, and
aminoaciduria, after 15-16 and 48-91 weeks, respectively. The
appearance of increased ß2-microglobulin was delayed until the
30th and 138th weeks, respectively. However, no definite disturbance
of proximal renal tubular function, such as reduced tubular
reabsorption of phosphorus, hypophosphataemia or acidosis, was noted
during the one-year follow-up. The dose-effect relationship for
renal dysfunction was similar to those which have been observed in
rabbits and rats, and thus the hypothesis that the susceptibility of
monkeys to cadmium may be exceptionally low was not corroborated.
In study II, the group of monkeys given feed containing
30 mg/kg developed urine findings (e.g., proteinuria, glucosuria,
aminoaciduria) indicative of renal dysfunction in the sixth year.
Postmortem examination revealed degeneration of the proximal renal
tubules, but there was no reduction in tubular reabsorption of
phosphorus. When the administration of cadmium was discontinued in
the fifth year, no abnormality of renal function developed during
the follow-up period of four years.
Table 13. Renal effects of cadmium in monkeysa
Study Duration Sex No. of Exposure level Average cadmium Renal effects (timing, Other effects (timing,
(weeks) monkeys (mg/kg diet) level in renal in weeks, of effects) in weeks, of effects)
cortex (mg/kg)
I 55 male 2 0 163
2 3 202 no biological effects no biological effects
3 30 596 no biological effects no biological effects
3 300 380b renal dysfunction (15-16) hepatic dysfunction (12-54)
757b ß2-microglobulinuria (30) slight anaemia (20)
II 462 male 6 0 328
8 3 700 no biological effects no biological effects
8 10 1070 no biological effects erythrocytopenia (360)
8 30 1170b renal dysfunction (300-306) erythrocytopenia (240)
ß2-microglobulinuria (311)
6 100 635b renal dysfunction (48-91) erythrocytopenia (120)
ß2-microglobulinuria (138) depressed age-related increase
in blood pressure (80)
III See Table 14.
IV 308 male 2 0 18
3 3 570 no biological effects no biological effects
4 contaminated rice 230 no biological effects no biological effects
(1.33 mg/kg)
a From: Nomiyama et al. (1979), Nomiyama et al. (1987), Nomura et al. (1988), Nomiyama & Nomiyama (1988)
b The numbers with asterisks are the critical concentrations of cadmium in the renal cortex.
Table 14. Bone and renal effects of cadmium in female monkeysa
Group No. of Exposure Low protein, Low vitamin D Average renal Renal effects Bone effectsd
monkeys level calcium and dietc cortex cadmium
(mg/kg) phosphorus dietb level (mg/kg)
1 5 0 - - 58 no biological effects no biological effects
2 4 0 + - no biological effects slightly disturbed calcification
(after 154 weeks)
3 4 0 - + no biological effects low plasma vitamin D3
4 4 0 + + no biological effects osteomalacic change (after 77
weeks) reversible by vitamin D3
5 5 30e - - 1511 no biological effects no biological effects
6 4 30e + - ß2-microglobulinuriaf disturbed calcification (after
(2000 to 12 000 µg/day, 154 weeks)
67%)
7 4 30e - + no biological effects low plasma vitamin D3
8 10 30e + + ß2-microglobulinuriag osteomalacic change (after 77
(up to 2000 µg/day) weeks) reversible by vitamin D3
a From: Nomura et al. (1988); duration of experiment was 463 weeks (9 years)
b 14% protein instead of 20%; 0.3% calcium instead of 0.9%; 0.3% phosphorus instead of 0.9%
c No vitamin D3 was added (240 IU was added to the normal diet)
d In Group 5 to 8, as depressed age-related increase in blood pressure was seen after 103 weeks of treatment.
e 3 mg/kg for the first 52 weeks
f Non-progressive lesion, reversibility uncertain; renal effect noted after 193 weeks
g Reversible by normal diet and vitamin D3 treatment; renal effect noted after 154 weeks
The monkeys in study III (Table 14) given the low nutrition
feed plus 30 mg cadmium/kg developed renal function abnormalities
after the fourth year. In addition to reduced phenolsulfonphthalein
(PSP) clearance and a variable increase in urinary
ß2-microglobulin concentration, many of the monkeys showed mild
degenerative changes of the proximal tubular epithelia, but there
was no decrease in the tubular reabsorption of phosphorus. However,
elevated urinary ß2-microglobulin did not progress further with
continued administration of cadmium. Mild degenerative changes of
the proximal tubular epithelia were also noted in the groups of
monkeys that had been given a normal diet, low vitamin D diet or low
nutrition plus low vitamin D diet, each supplemented with 30 mg
cadmium/kg. However, the elevated urinary ß2-microglobulin level
soon returned to normal in those animals fed a normal diet,
regardless of continued cadmium administration. This may indicate
that the elevated urine ß2-microglobulin in this group was not
caused solely by cadmium exposure.
In study II, some of the monkeys given feed containing 3 mg/kg
or 10 mg/kg of cadmium showed cadmium concentrations in the renal
cortex as high as 760 mg/kg and 1070 mg/kg, respectively. However,
no effect upon renal function was observed during the nine-year
period, nor was there any increase in urinary ß2-microglobulin
concentration.
The above data suggest that mild renal dysfunction
(proteinuria, glucosuria, and aminoaciduria, but no decrease in the
tubular reabsorption of phosphorus) was produced in monkeys exposed
to high concentrations of cadmium (30 mg/kg diet or more). It seems,
however, that no effects on renal function occur with low-level
exposure (10 mg/kg or less). The development of renal dysfunction is
assumed to depend upon the amount of cadmium absorbed per day rather
than the total amount absorbed in the body.
In study IV, the urinary cadmium level occasionally exceeded
10 µg/litre, but no clinical chemistry changes were reported.
Cadmium concentrations in the renal cortex increased proportionally
to the dose level and duration of exposure, reaching an average of
450 mg/kg in the group given cadmium chloride and 290 mg/kg in the
group fed contaminated rice. This suggests that the chemical form of
cadmium does not affect the severity of health effects.
7.2.1.2 Respiratory route
Princi & Geever (1950) could find no evidence of renal
morphological changes in the kidney of dogs after prolonged
inhalation exposure (up to one year) to cadmium oxide or cadmium
sulfide dust (average concentration of 4 mg/m3). Routine analysis
was performed, but neither the methods used nor the results obtained
were described. Friberg (1950) exposed rabbits for about 8 months
(3 h per day, about 20 days per month) to cadmium oxide dust with an
average concentration of about 8 mg/m3 . After 4 months of
exposure, moderate proteinuria was detected by the trichloroacetic
acid test. Histological examination of the kidneys after 8 months
revealed interstitial infiltration of leucocytes in the majority of
the exposed rabbits; this was not found in the control group.
7.2.1.3 Injection route
Friberg (1950) detected proteinuria in rabbits given
subcutaneous injections of cadmium sulfate (0.65 mg cadmium/kg body
weight) 6 days per week. Electrophoretic analysis of urine proteins
revealed that the proteinuria differed from that caused by
injections of uranium salts. More recently, many studies utilizing
parenteral administration (with doses generally in the range of
0.25-1.5 mg/kg body weight), different routes of exposure
(subcutaneous and intraperitoneal), and a duration of 1-12 months
have been performed in mice, rats, and rabbits (Table 12). These
experiments have confirmed the nephrotoxic effects of cadmium.
When rabbits were exposed for 16 weeks by subcutaneous
injection of either 0.25 mg or 0.5 mg cadmium/kg body weight 3 times
a week, there was a significant increase in urinary
ß2-microglobulin excretion indicative of renal tubular dysfunction
in the high-dose group after 7 weeks. There was only a slight
increase in the serum ß2-microglobulin/creatinine ratio. Urinary
ß2-microglobulin levels were not related to serum
ß2-microglobulin levels (Piscator et al., 1981).
Rats dosed intraperitoneally, five days/week with 0.6 mg
cadmium/kg body weight, showed no abnormal effects after 5 or 6
weeks when renal cadmium levels reached about 100 mg/kg. However, in
renal tubular lining cells an increase in lysosomes, microbodies,
and smooth endoplasmic reticulum was noted. After 8 weeks renal
cadmium levels had reached about 200 mg/kg of tissue and tissue
necrosis was observed. The early changes (with a renal cadmium
concentration of up to 100 mg/kg) were considered to be adaptive and
possibly reversible, whereas morphological changes after 8 weeks
with a renal concentration of 200 mg/kg were considered to be
irreversible (Goyer et al., 1984).
Nomiyama et al. (1982a) found that non-metallothionein-bound
cadmium increased up to about 35 mg/kg tissue in parallel with total
cadmium. At that stage, the total cadmium concentration in the renal
cortex was in the range 200-800 mg/kg, the total dose of cadmium
having been approximately 1 g.
7.2.1.4 Pathogenesis of cadmium nephrotoxicity
Various hypotheses have been proposed to explain the
pathogenesis of cadmium nephrotoxicity, particularly the role of the
metal-binding protein metallothionein (see section 6.8). This
protein is inducible by a number of essential metals (Cherian &
Goyer, 1978) and may have as its primary function the intracellular
storage of zinc and copper (Panemangalore et al., 1983; Templeton et
al., 1985). It is also induced following exposure to cadmium. It is
now thought that metallothionein protects against cadmium toxicity
and that intracellular cadmium bound to metallothionein is nontoxic
(Nordberg, 1971; Goyer et al., 1989). There is considerable support
for this hypothesis. Pre-treatment of experimental animals with
small doses of cadmium prevents the acute toxic effects of a large
dose of cadmium (Nordberg et al., 1975). Parenteral administration
of cadmium-metallothionein causes acute tubular toxic effects in the
kidney (Nordberg, 1971; Nordberg et al., 1975; Cherian & Nordberg,
1983). By treatment of animals with repeated doses of cadmium,
metallothionein synthesis in the renal cortex can be induced. This
prevents against subsequent renal toxicity by parenteral
cadmium-metallothionein at dose levels that normally give rise to
renal damage (Jin et al., 1987a). Rat renal cortical cells isolated
from animals pretreated with cadmium were resistant to normally
toxic concentrations of cadmium in vitro (Jin et al., 1987b).
Similar protective effects were observed in kidney cells pretreated
with cadmium in vitro (Jin et al., 1987b). Human cells in tissue
culture, where metallothionein has been induced by pre-treatment
with cadmium, become resistant to previously lethal exposure to
cadmium (Glennas & Rugstad, 1984).
With this evidence for the protective role of intracellular
metallothionein, several theories have been proposed to explain the
nephrotoxicity of cadmium. One hypothesis attributes the
nephro-toxicity to that fraction of intracellular cadmium not bound
to metallothionein (Nordberg et al., 1975; Nomiyama & Nomiyama,
1982; Squibb et al., 1984). Another hypothesis is that
extra-cellular cadmium bound to metallothionein is toxic (Cherian et
al., 1976). Cadmium-metallothionein derived from cadmium-induced
synthesis in reticulocytes (Tanaka et al., 1985) or released from
liver cells is filtered by the renal glomeruli and reabsorbed by the
proximal tubular lining cells where it is catabolized, releasing
cadmium ions that cause renal damage (Dudley et al., 1985). This
hypothesis is supported by the fact that parenterally administered
cadmium-metallothionein is very toxic to renal tubular cells and
that the plasma metallothionein level increases with cadmium
exposure (Goyer et al., 1984; Shaikh & Hirayama, 1979).
Still another hypothesis is that intracellular cadmium
interacts with cell membranes resulting in lipid peroxidation
(Stacey et al., 1980) and that cadmium may displace essential metals
from metallothionein (Petering et al., 1984), thereby depriving
important metalloenzymes of essential metal cofactors.
These hypotheses are not mutually exclusive and the relative
significance of each of these mechanisms may differ under particular
circumstances of exposure.
Goering et al. (1985) reported the development of calcuria in
rats injected with cadmium-metallothionein, using the model
described by Squibb et al. (1984). Jin et al. (1987a) confirmed
their observations, which suggest that this biological effect may be
an early event in the development of renal tubular damage. Data from
the above studies further validate the use of the
cadmium-metallothionein injection model for studying the mechanisms
of cadmium-induced tubular injury, since calcuria is also observed
in people with chronic elevated cadmium exposure.
7.2.1.5 General features of renal effects; dose-effect and
dose-response relationships
The available data show that long-term exposure to cadmium
leads to renal tubular lesions with proteinuria, glucosuria, and
aminoaciduria, and to histopathological changes (Table 12).
It has been reported that cadmium-induced proteinuria differs
from glomerular proteinuria (Friberg, 1950) and involves low
molecular weight proteins in particular (Axelsson & Piscator, 1966a;
Nomiyama et al., 1982b). Thus, this type of proteinuria resembles
the "tubular proteinuria" seen in humans. Microscopic examination
reveals typical tubular nephropathy, i.e. atrophy and degeneration
of tubular cells, especially proximal tubular cells, and
interstitial fibrosis (Bonnell et al., 1960; Axelsson et al., 1968;
Kawai et al., 1976).
Electron microscopic changes are characterized by interstitial
fibrosis and thickening of the basement membrane of the proximal
tubular cells (Kawai et al., 1976). The smooth endoplasmic reticulum
is dilated or undergoes proliferation, and there is apical cyst
formation (Stowe et al., 1972). An increase in the number of
lysosomes and swelling of the mitochondria have also been observed.
In addition to tubular findings, there have been reports of
pathological changes in 30% of the mesangium cells of the glomeruli
of dogs (Murase et al., 1974) and increased thickness of the
glomerular basement membrane in rats (Scott et al., 1977).
Investigations into renal function have also revealed
substantial changes, mainly in tubular function, e.g., reabsorption
of glucose (Axelsson & Piscator, 1966a), whereas the changes in
glomerular filtration are relatively small (Axelsson & Piscator,
1966a). Effects have generally been seen at average renal cortex
concentrations of 200-300 mg/kg wet weight, but some studies have
reported effects at considerably lower concentrations.
Histopathological changes in rats, rabbits, horses, and birds have
been reported at renal cortex concentrations below 100 mg/kg (Table
12). However, in chronically exposed monkeys, signs of renal tubular
changes were reported at around 400-1200 mg cadmium/kg (Table 13).
After renal cortex concentrations of cadmium have reached a
level of 200-300 mg/kg wet weight, they level off or decrease. No
further increase is seen even with continued exposure (Axelsson &
Piscator, 1966a; Nomiyama & Nomiyama, 1976a; Bernard et al., 1981).
It has also been shown (Friberg, 1952; Axelsson & Piscator, 1966a;
Nordberg & Piscator, 1972; Nomiyama & Nomiyama, 1976a) that urinary
excretion of cadmium is low during the initial exposure period but a
marked increase in the excretion of cadmium occurs subsequently,
which coincides with an increase in protein excretion (Friberg,
1952; Axelsson & Piscator, 1966a; Nordberg & Piscator, 1972)
(section 6).
Animal studies have shown that, as the cadmium concentration in
the renal cortex increases, the first effects to appear are the
histopathological changes in the renal tubular cells. The low
molecular weight proteinuria and aminoaciduria develop at somewhat
higher cadmium concentrations in the renal cortex and, at even
higher concentrations, glucosuria, total proteinuria, and other
indications of damaged renal function develop. The diagnosis of
these effects depends greatly on the sensitivity of the method for
analysing the effect. For instance, a method for analysing low
molecular weight proteinuria that can accurately measure levels
considered normal (0.1 mg/litre or less) will be able to diagnose
proteinuria at an earlier stage than a method with a detection limit
of 7 mg/litre.
Most of the studies referred to in Table 12 included no data on
the prevalence of renal effects in the animals. The results were
given in a qualitative way, stating the average cadmium level in the
renal cortex at which effects were seen.
Some dose-response data are available from animal studies.
Bernard et al. (1981) produced proteinuria in rats exposed to
cadmium in drinking-water (200 mg/litre) for up to 11 months. After
8-9 months, a significant increase in group-average proteinuria was
seen, coinciding with a 25% prevalence of increased individual
proteinuria. The renal cortex cadmium concentration at that time was
about 200 mg cadmium/kg (Bernard et al., 1981). Elinder et al.
(1981a) studied horses exposed to cadmium present in their normal
food. Histopathological changes in the renal cortex were classified
and coded in a blind manner, and the prevalence of different degrees
of change was calculated for subgroups of horses with different
renal cortex cadmium concentrations. The "background" prevalence was
25-30% and there was an increased prevalence (up to 60-75%) with
increased average renal cadmium level. At a renal cortex cadmium
concentration of about 75 mg/kg, there was a significant increase in
the prevalence of histopathological changes.
7.2.2 Effects on the liver
Friberg (1950) demonstrated fibrotic changes in the liver of
rabbits exposed to repeated subcutaneous injections of cadmium.
Periportal and interlobular collagen deposition was found in the
liver of rabbits given 160 mg cadmium/litre in drinking-water for 6
months (Stowe et al., 1972). Liver function tests, however, remained
within normal limits. The cadmium concentration in the liver was
188 mg/kg wet weight. Tarasenko et al. (1974) demonstrated by
histological techniques that dystrophic changes occur in the liver
of rats after repeated intragastric administration of cadmium
caprylate in a total dose corresponding to 47 mg cadmium/kg body
weight per day. These authors also noted an increased level of
lactic acid in the blood serum of the animals. Larionova et al.
(1974) detected decreased activity of alanine transaminase in liver
tissue and depletion of glycogen in rats given barium cadmium
laurate by gavage for 8-10 days at a dosage of 169 mg/kg body weight
per day (as the laurate). After intraperitoneal injection of cadmium
(up to 1.25 mg/kg body weight for periods of up to 6 weeks),
decreased glycogen content and increased daily activity of
gluconeogenic enzymes in rat liver were reported by Merali et al.
(1974) and Chapatwala et al. (1982).
In long-term studies, rabbits given 300 mg cadmium/kg diet for
54 weeks (Kawai et al. 1976) showed some amyloid deposition in the
liver. Studies on rats after exposure for 335 days to 1 mg
cadmium/litre in drinking-water (Sporn et al., 1970) revealed
changes in liver enzyme activities. Rhesus monkeys exposed to 300 mg
cadmium/kg in the diet for 12 weeks (Nomiyama et al., 1979)
developed increased levels of plasma enzymes (GOT, GPT, and LDH).
7.2.3 Effects on the respiratory system
Interstitial pneumonitis and emphysema were found in rabbits
exposed to cadmium iron oxide dust (approximately 8 mg/m3) for 4-8
months (Friberg, 1950) and in rats observed for 4-7 months after a
single intratracheal administration of cadmium iron oxide dust
(3.5 mg/kg body weight) (Vorobjeva, 1957). However, only very slight
pulmonary effects were detected in rabbits and rats exposed to
nickel-graphite dust at dose levels several times higher than the
concentrations of cadmium iron oxide dust.
Yoshikawa et al. (1975) exposed rats to cadmium oxide fumes
(0.1 or 1.0 mg cadmium/m3) for up to 3 months. There were 10 rats
in each group, and three of the rats in the high exposure group died
after about 7 weeks. Lung fibrosis and the first stage of emphysema
were observed at the end of the experiment in the high-dose group.
Free macrophage cells in the alveoli were more numerous in both
groups, and there was an increased surface tension of the
surfactants.
Snider et al. (1973) observed signs of emphysema in rats 10
days after 5-15 daily 1-h periods of exposure to cadmium chloride
aerosol (10 mg/m3). Also, long-term exposure to comparatively low
air concentrations of cadmium oxide (24-50 µg cadmium/m3) gave
rise to pathological changes in the lungs similar to emphysema as
well as to cell proliferation in the bronchi (Prigge, 1978). A
long-term study (14 months), in which mice and golden hamsters were
exposed to different concentrations of cadmium chloride (30 and
90 µg/m3), cadmium sulfate (30 and 90 µg/m3), cadmium sulfide
aerosols (90-100 µg/m3), cadmium oxide fume (10-90 µg/m3), and
dust (10-270 µg/m3), revealed a significantly increased incidence
of alveolar hyperplasia and interstitial fibrosis in most of the
exposed groups (Heinrich et al., 1989).
Single intratracheal administration of several cadmium
compounds (e.g., oxide, sulfide, carbonate, sulfoselenide,
caprylate, stearate, cadmium-barium laurate, and cadmium-barium
stearate) in doses from 0.5 mg (as the oxide) to 15 mg (as the
sulfide) caused the development, over 6 months, of chronic
inflammatory changes, emphysema, and atelectasis leading to
fibrosis. Exposure to cadmium oxide and caprylate gave rise to the
development of nodules of hyaline connective tissue; these resembled
silicotic nodules (Vorobjeva & Sabalina, 1975).
7.2.4 Effects on bones and calcium metabolism
Male rats fed a normal diet and exposed to cadmium sulfate by
inhalation (3 and 0.3 mg/m3, 4 h daily for 4 months) showed
decreased serum and urinary calcium concentrations compare to
controls. Female rats similarly exposed to cadmium sulfate
(2.8 mg/m3, 3 h/week) during pregnancy showed radiological
evidence of osteoporosis in addition to hypocalcaemia (Tarasenko et
al., 1975).
In a study by Kogan et al. (1972), cadmium chloride and cadmium
sulfate were administered subcutaneously to rats at a daily dose of
1 mg/kg body weight for up to 12 months. At 12 months, X-ray
analysis of the bones indicated osteoporosis and osteosclerosis.
Subsequent histopathology showed an increase in osteoclasts and a
bone structure described by the authors to be indicative of
osteomalacia.
Oral administration of cadmium chloride in drinking-water to
male rats (1 or 4 µg/kg body weight daily for six months) resulted
in changes in calcium metabolism and bone structure characteristic
of osteomalacia, which were not observed in the control group. No
effects were noted in a group of animals given a daily dose of
0.01 µg/kg body weight (Likutova & Belova, 1987).
Rats given 50 mg cadmium/litre in drinking-water for about 9
months showed reduced calcium and phosphorus absorption from the
intestine (Sugawara & Sugawara, 1974). In addition, some of the
animals showed histological changes in the duodenal mucosa, a
finding also reported in Japanese quail (Richardson & Fox, 1974).
Several mineral balance studies have been made on animals fed
cadmium. Simultaneous administration of cadmium with a low-protein,
low-calcium diet led to a decrease in the calcium and zinc content
of bone (Itokawa et al., 1973). Furthermore, Kobayashi (1974)
reported that cadmium feeding led to a negative calcium balance in
rats.
The decreased calcium absorption and negative calcium balance
in cadmium-exposed rats could result from the inhibitory effects of
cadmium on the activation of vitamin D in renal cortical cells
(Feldman & Cousins, 1973). The renal conversion of
25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol has been
found to be inhibited by high dietary cadmium exposure in rats fed a
normal calcium diet, but this effect was not seen on a low-calcium
diet (Lorentzon & Larsson, 1977). The metabolically active form of
vitamin D (1,25-dihydroxycholecalciferol) is necessary for the
normal absorption of calcium from the intestine. Ando et al. (1981)
found that the stimulation of calcium absorption by 1-alpha-hydroxy
vitamin D3 was inhibited in rats exposed to cadmium by gastric
intubation. Furthermore, the concentration of calcium-binding
protein in intestinal mucosa may be decreased by cadmium exposure
(Fullmer et al., 1980).
Administration of drinking-water containing 10 mg cadmium per
litre to rats fed a normal diet over a 9-month period gave rise to
decalcification and cortical atrophy in the skeleton (Kawai et al.,
1976). Other workers have also reported effects in the bones of rats
following several months exposure to cadmium in drinking-water
(Itokawa et al., 1974; Kawamura et al., 1978) and in the diet
(Takashima et al., 1980; Nogawa et al., 1981a), and following
subcutaneous injection (Nogawa et al., 1981a). In these studies, the
bones were reported to show more or less severe osteoporosis and
osteomalacia. On the other hand, Kajikawa et al. (1981) did not find
either osteoporosis or osteomalacia after rats were exposed for 2
years to cadmium in drinking-water (200 mg/litre).
Anderson & Danylchuk (1979) found that exposure of Beagle dogs
for six months to cadmium (25 mg/litre in drinking-water) reduced
bone turnover rate, a metabolic abnormality consistent with calcium
deficiency or osteomalacia. Kawashima et al. (1988) found that
feeding a cadmium-contaminated rice diet or a diet containing 3 mg
cadmium chloride/kg for six years to crab-eating monkeys did not
produce any change in vitamin D metabolism, and there was no
evidence of renal dysfunction. In another series of experiments
rhesus monkeys were fed diets containing 3, 10, 30 or 100 mg
cadmium/kg for 9 years. Serum vitamin D metabolites and renal
production of vitamin D remained unchanged, but in animals fed 30 or
100 mg cadmium/kg of diet there was slight but not statistically
significant depression of renal 25-hydroxy vitamin D1-hydroxylase
activity. No skeletal abnormalities were found in any of these
animals.
Bhattacharyya et al. (1988) studied the effects of 0, 0.25, 5
or 50 mg cadmium/kg diets on female mice bred for six consecutive
42-day cycles of pregnancy and lactation and on non-pregnant
controls. The multiparous mice exposed to 50 mg/kg experienced
significant decreases in body weight (3-11%) and femur calcium
content (15-27%), and the femur calcium to dry weight ratios
decreased by 5-7%. These results were thought by the authors to
provide evidence that the combination of cadmium exposure and
multiparity has a synergic effect on bone metabolism.
In the Japanese monkey study III (section 7.2.1 and Table 14),
osteomalacic changes were found in the low-nutrition plus
low-vitamin-D diet group (group 4) after 77 weeks. These effects
were not further exacerbated by feeding cadmium (group 8). These
changes were found to be reversed by the administration of vitamin
D. Renal effects were found in group 8 after 154 weeks. Therefore,
the osteomalacia found in group 8 was diagnosed as not being renal
osteomalacia.
Most of the findings discussed above indicate a direct effect
of cadmium on bone mineralization, possibly related to calcium
deficiency, and an indirect effect on calcium absorption via vitamin
D hydroxylation, perhaps leading to osteomalacia. The direct effects
develop after long-term cadmium exposure, whereas the indirect
effect on vitamin D metabolism occurs only when renal damage is seen
in the animals. Osteomalacia only occurred in monkeys fed a diet low
in protein, phosphorus, calcium, and vitamin D. Cadmium
administration did not increase these effects.
7.2.5 Effects on haematopoiesis
Anaemia is a common finding in animals after both dietary
(Wilson et al., 1941) and parenteral (Friberg, 1950) exposure to
cadmium. After dietary exposure, decreased haemoglobin concentration
(Decker et al., 1958) and decreased haematocrit (PCV) (Prigge et
al., 1977) are among the early signs of cadmium toxicity.
Fox & Fry (1970) and Fox et al. (1971) reported that the
cadmium-induced anaemia could be prevented by simultaneous feeding
with iron or ascorbic acid. Decreased gastrointestinal absorption of
iron due to cadmium may be one mechanism for this anaemia.
After parenteral exposure, iron administration has a beneficial
effect on the anaemia (Friberg, 1955). Berlin & Friberg (1960)
showed that cadmium injections caused erythrocyte destruction, but
there was no indication of an interference with haemoglobin
production. Haemolytic anaemia in rabbits was also reported by
Axelsson & Piscator (1966b).
7.2.6 Effects on blood pressure and the cardiovascular system
Chronic oral administration of cadmium compounds to rats (Perry
& Erlanger, 1974) induced statistically significant elevation of
blood pressure. However, the systolic pressure changes were much
smaller than those previously reported by Schroeder & Vinton (1962)
and by Schroeder (1964). Furthermore, a different effect was
obtained with lower, as compared with higher, doses of cadmium. Rats
given 1, 2.5, or 5 mg cadmium/litre in drinking-water for one year
had significantly higher blood pressure values than controls. Six
months after the beginning of exposure, a statistically significant
increase in blood pressure was also observed in rats given 10 or
25 mg/litre, but the increase was not statistically significant
after one year of exposure. In rats receiving 50 mg/litre, a
statistically significant increase in systolic blood pressure was
observed after 12 months of exposure. As discussed in section 8.2.4,
these results may be of basic importance in evaluating data on the
possible effects of cadmium on the human cardiovascular system.
Several mechanisms have been postulated (Perry & Erlanger,
1974) to explain the effects of chronic cadmium exposure on the
cardiovascular system. Oral adminstration of cadmium doses that
induce hypertension (and also parenteral administration of cadmium)
was shown to increase circulatory renin activity (Perry & Erlanger,
1973). Injection of cadmium into the renal artery of dogs increased
sodium reabsorption by the exposed kidney (Vander, 1962), and
repeated intramuscular (Perry et al., 1971) or chronic oral
administration of cadmium (Lener & Musil, 1971) was reported to
increase sodium retention in the body. By morpho-metric methods,
Fowler et al. (1975) demonstrated effects on the renal blood vessels
of rats exposed to various concentrations of cadmium (up to
200 mg/litre in drinking-water) for several weeks. Significantly
smaller arteriolar diameters were found in the exposed animals than
in the controls.
Perry et al. (1977) studied the influence of exposure duration
(from 3 to 24 months) and cadmium dose levels in water (from 0.1 to
10 mg/litre) on the blood pressure of Long-Evans rats. A small
increase in blood pressure occurred even at the lowest exposure
level after 3 months exposure. The greatest increase in blood
pressure (3.2 kPa; 24 mmHg) occurred after 24 months exposure to
1 mg/litre, when the average renal cortex cadmium level was 12 mg/kg
wet weight. At higher dose levels, the blood pressure increase was
less and, at the highest dosage (10 mg/litre for 24 months), the
blood pressure did in fact decrease.
Perry et al. (1976) found hypertensive effects in
Sprague-Dawley as well as Long-Evans rats. Petering et al. (1979)
reported that male rats were more susceptible to these effects than
female rats after exposure via drinking-water, but Ohanian & Iwai
(1980) found the opposite for rats exposed parenterally. In several
studies (e.g., Kotsonis & Klaassen, 1977; Whanger, 1979; Fingerle et
al., 1982), there was no increase in blood pressure after various
cadmium doses were given via drinking-water. The type of diet
appears to be crucial for the development of hypertension (Whanger,
1979); it can usually only be produced in rats fed a rye-based diet
(Perry & Erlanger, 1982). Nishiyama et al. (1986) postulated that
cadmium exposure increases sodium and water retention, which are
important factors controlling the development of hypertension.
A detailed review of factors influencing the effects of cadmium
on the cardiovascular system was reported by the Task Group on Metal
Toxicity (1976), with particular reference to those factors that
might modify the dose-effect and dose-response relationships.
Rats exposed to cadmium (5 mg/litre) in drinking-water (Kopp et
al., 1980a,b, 1983) developed electrocardiographic and biochemical
changes in the myocardium, and impairment of the functional status
of the myocardium. These effects could be related to (i) decreased
high-energy phosphate storage in the myocardium, (ii) reduced
myocardial contractility, or (iii) diminished excitability of the
cardiac conduction system. Jamall & Sprowls (1987) found that rats
fed a diet supplemented with copper (50 mg/kg), selenium
(0.5 mg/kg), and cadmium (50 mg/kg) had marked reductions in heart
cytosolic glutathione peroxidase, superoxide desmutase, and
catalase. They suggested that heart mitochondria are the site of the
cadmium-induced biochemical lesion in the myocardium.
Reviews of all aspects of the cardiovascular effects of cadmium
on experimental animals have been reported by Perry & Kopp (1983)
and Jamall & Smith (1986).
7.2.7 Effects on reproductive organs
Cadmium-induced testicular necrosis (section 7.1.2.1) generally
results in permanent infertility (Barlow & Sullivan, 1982). Ramaya &
Pomerantzeva (1977) found markedly reduced testis weights 1, 3, and
6 months after mice were administered 4 mg cadmium/kg. The animals
were sterile and microscopic examination revealed morphological
changes in the testis. Krasovskii et al. (1976) noticed decreased
spermatozoa motility and spermatogenesis index in rats continuously
exposed via food to 0.5-5.0 mg cadmium/kg body weight. In male mice
exposed repeatedly by daily subcutaneous injection of cadmium
chloride (0.5 mg/kg per day) for 6 months (Nordberg, 1975), there
was a decrease in normal testosterone-dependent proteinuria.
Morphological examination of the seminal vesicles revealed a smaller
weight and size as well as histological indications of lower
secretory activity, this being consistent with decreased
testosterone activity in these animals.
7.2.8 Other effects
Effects on the immune system have been reported after both
chronic and acute cadmium exposure. A decrease in the number of
antibody-forming cells in the spleen as well as a decrease in
antibody production was seen in mice after long-term exposure to
cadmium in drinking-water (Koller et al., 1975). An inhibition of
the cell-mediated immune response occurred in mice after repeated
intraperitoneal injections (Bozelka & Burkholder, 1982). However, no
data are available linking these effects to increased susceptibility
to infection or other secondary dysfunctions.
Gestational exposure to cadmium (4.2 and 8.4 µg/ml in
drinking-water) results in decreased birth weight, retarded growth,
delayed development of the sensory motor coordination reflexes, and
increased motor activity. Cadmium exposure during critical periods
of development might result in developmental and behavioural
deficits with long-term implications for adult behaviour (Mohd et
al., 1986).
7.3 Fetal toxicity and teratogenicity
In several species of laboratory rodents, large doses of
cadmium salts induce severe placental damage and fetal deaths when
given at a late stage of pregnancy, and teratogenic effects, such as
exencephaly, hydrocephaly, cleft lips and palate, microphthalmia,
micrognathia, clubfoot, and dysplastic tail, when given at early
stages of gestation.
A single subcutaneous injection of cadmium chloride, acetate,
or lactate (4.5 mg cadmium/kg body weight) given to Wistar rats from
the 17th to the 21st day of pregnancy (Parizek, 1964) led to the
rapid development of severe placental damage in all rats and to
fetal death. Placental damage was not dependent on the presence of
fetuses, but it was not possible to decide whether fetal lethality
resulted from the placental lesion or from a direct effect of
cadmium on the fetuses (Parizek, 1964). Similar effects were
observed at a dose level of 3.3 mg cadmium/kg body weight (Parizek
et al., 1968b). Placental damage and fetal deaths were also observed
after cadmium administration to pregnant Swiss albino mice
(Chiquoine, 1965).
Teratogenic effects can be observed when doses close to the
LD50 (Table 11) for cadmium salts are administered to pregnant
females at critical stages of embryogenesis. These effects were
demonstrated with intravenous injections of cadmium sulfate in
hamsters (Ferm & Carpenter, 1968; Mulvihill et al., 1970; Ferm,
1972) and with intraperitoneal (Barr, 1973), subcutaneous (Chernoff,
1973), or dietary (Scharpf et al., 1972) administration of cadmium
chloride in rats. Teratogenic effects induced by cadmium salts have
also been demonstrated in mice (Ishizu et al., 1973).
The character of the changes induced is dependent on the
species and on the stage of embryogenesis. As little as 123 µg/litre
in mouse embryo cultures produced exencephaly apparently by
re-opening the closed neural tube (Schmid et al., 1985). Either
facial defects or limb abnormalities were induced by cadmium when
administered to pregnant hamsters on day 8 or 9 of gestation (Ferm,
1971). Both jaw defects and cleft palate were observed in the
offspring of rats given daily subcutaneous cadmium chloride
injections (8 mg/kg body weight) on days 13-16 or 14-17 of
pregnancy, but cleft palate was not observed when this dosage was
given on days 15-18 or 16-19 of pregnancy (Chernoff, 1973).
Anophthalmia or microphthalmia and dysplastic ears were induced by
approximately 2 mg of cadmium as the chloride given
intra-peritoneally to pregnant rats on the 9th but not on the 11th
day of pregnancy (Barr, 1973). Other effects observed in these
studies included decreased lung weight in the offspring of rats
subjected to cadmium during pregnancy (Chernoff, 1973) and
deficiencies in bone formation and delays in bone ossification
(Mulvihill et al., 1970; Scharpf et al., 1972).
The dose-dependent fetal mortality and teratogenicity response
was established in studies with subcutaneous administration of
cadmium chloride to rats (Chernoff, 1973) and mice (Ishizu et al.,
1973). The no-observed-effect level with respect to malformations
was found in the latter study to be 0.33 mg/kg body weight.
All the teratogenic effects mentioned above were induced by
parenteral administration of very high doses of cadmium salts.
However, in a rat study by Scharpf et al. (1972), very high peroral
doses (20, 40, 60, or 80 mg/kg body weight given by gavage daily
from days 6 to 19 of pregnancy) of cadmium chloride were used with
the simultaneous administration of sodium chloride, and internal
teratological examinations were performed. Heart and kidney
abnormalities were the major internal defects, but their incidence
was not directly related to the dose of cadmium chloride
administered. At the lowest dose level, heart abnormalities were
detected in 19.7% of the 127 fetuses (abnormalities in the control
group were seen in 6.6% of 107 fetuses) and teratoma of the kidney
was observed in 15.7% of these fetuses.
Cvetkova (1970) exposed pregnant female rats via the
respiratory route to cadmium sulfate (2.8 mg/m3, 4 h daily) and,
on the 22nd day, killed half of them to examine the embryos. The
number of embryos in exposed rats was the same as in a control
group, but the mean weight was lower in the exposed group. In the
exposed rats, where pregnancies were allowed to proceed to full
term, the average weight of the offspring was lower than in the
controls both at birth and after 8 months. The rats born to the
exposed group also had increased mortality during the first 10 days
after birth.
When mice were exposed for several generations to cadmium in
drinking-water (10 mg/litre), fetal mortality, runting, and
malformations were observed (Schroeder & Mitchener, 1971). The
external malformations, consisting of sharp angulation of the distal
third of the tail, were observed in 16.1% of 255 offspring (F1 and
F2A generation), and 87 deaths before weaning (30.5%) were
recorded.
Ferm & Carpenter (1968) showed that zinc injected
simultaneously with cadmium could protect against the teratogenic
effects of cadmium, and a similar protective action was found for
selenium (Holmberg & Ferm, 1969). Maternal zinc deficiency can
produce congenital malformations (Hurley et al., 1971). This was
confirmed by Parzyck et al. (1978), who also found that
intraperitoneal injection of 1.5 mg cadmium/kg body weight to
pregnant rats increased the prevalence of malformations. The
increase was greater at this cadmium dose than the increase due to
zinc deficiency. Combined zinc deficiency and cadmium exposure
caused a very high incidence of fetal deaths.
Further experimental data on rats provided by Samarawickrama &
Webb (1979) indicate that maternal cadmium exposure gives rise to a
fetal zinc deficiency and that this is one cause of the teratogenic
effects observed. Intravenous cadmium injections to pregnant rats at
doses ranging from 0.25 to 1.25 mg/kg body weight on day 12 of
gestation produced a dose-related decrease in fetal uptake of a dose
of 65 mg zinc given 4 h later. Maternal cadmium exposure (1.25 mg/kg
body weight) was shown to result in decreased activity of a fetal
zinc-dependent enzyme thymidine kinase, which is responsible for the
incorporation of thymidine in DNA. Additional evidence that
cadmium-induced fetotoxicity is related to a cadmium-induced fetal
zinc deficiency was reported by Daston (1982), who found that
co-administration of zinc (12 mg/kg body weight) almost totally
eliminated severe fetal lung lesions when pregnant rats were given
cadmium (8 mg/kg body weight) on gestation days 12-15.
7.4 Mutagenicity
Studies on Drosophila (Ramel & Friberg, 1971; Vorobjeva &
Sabalina, 1975) failed to show any chromosomal abnormalities after
exposure to various cadmium compounds. Some in vitro studies of
cultured human lymphocytes and fibroblasts were also negative (Paton
& Allison, 1972; Deknudt & Deminatti, 1978; Kogan et al., 1978).
Shiraishi et al. (1972) reported a marked increase in the frequency
of chromatid breaks, translocations, and dicentric chromosomes in
leucocytes, from one person, cultured in a medium containing 62 mg
cadmium/litre (as the sulfate) for 4-8 h.
Andersen et al. (1983) found that the average chromosome length
in human lymphocytes was initially reduced when they were cultured
in a medium containing cadmium chloride (1.1 mg cadmium/litre), but
subsequently returned to normal. This effect was probably related to
the synthesis of metallothionein and complexing with cadmium.
Watanabe et al. (1979) observed aneuploidy in rat oocytes with
cadmium accumulation in the ovary after exposure to cadmium chloride
in vivo.
Rohr & Bauchinger (1976) found a reduced mitotic index in
hamster fibroblasts cultured in 100 µg cadmium/litre (as the
sulfate) and chromosome damage at concentrations above 500 µg
cadmium/litre. Deaven & Campbell (1980) showed that the effects on
cultured hamster cells depended on the type of medium used.
There appears to be an acute effect of cadmium following the
injection of 0.6-2.8 mg cadmium/kg body weight (Felten, 1979). After
6 h, there was an increased frequency of chromatid breaks in bone
marrow cells and chromosome gaps and breaks in spermatocytes, which
could be associated with the acute effects on haematopoiesis
(section 7.2.5) and on the testis (section 7.1.2.1).
A summary and graphical presentation of the available evidence
on genetic and related effects of cadmium in various in vivo and
in vitro test systems has been presented by IARC (1987a). Although
prokaryote test systems reveal no effects, variable results have
been observed in lower eukaryotes, mammalian cells in vitro, and
mammals in vivo.
7.5 Carcinogenicity
Intramuscular or subcutaneous administration of metallic
cadmium or cadmium compounds can induce sarcomata at the site of
injection. This local effect of cadmium was demonstrated with
intramuscular administration of metallic cadmium (cadmium powder in
fowl serum) to hooded rats (Heath et al., 1962), subcutaneous
administration to Chester Beatty rats of cadmium as the sulfide and
oxide (Kazantzis, 1963; Kazantzis & Hanbury, 1966) or sulfate
(Haddow et al., 1964), intramuscular injection of cadmium chloride
to Wistar rats (Gunn et al., 1967), and subcutaneous injection to
Sprague-Dawley rats (Nazari et al., 1967). Transplantability of
tumours induced in these studies (Heath & Webb, 1967) and metastases
into regional lymph nodes and into lungs (Kazantzis & Hanbury, 1966)
were reported. Intratesticular injection of cadmium chloride to
White Leghorn cockerels was reported to induce teratoma at the site
of injection (Guthrie, 1964).
After Hoffman et al. (1985) injected 1.9 mg cadmium chloride
(1.2 mg/kg body weight) directly into the ventral prostatic lobe of
100 12-month-old male rats, simple hyperplasia was found in 38 of
the rats, atypical hyperplasia in 29, atypical hyperplasia with
severe dysplasia in 11, and invasive prostatic cancer in 5 animals.
Hoffman et al. (1988) reported changes in the ultrastructure of
prostate epithelial cells in rats injected into the ventral prostate
with 2.2 or 3.3 mg cadmium/kg body weight. In animals given oral
treatment via the drinking-water (29.9 or 115 mg cadmium/kg body
weight), there were changes ranging in severity up to dysplasia but
no evidence of carcinoma.
A single parenteral administration of cadmium salts can induce
necrosis of the testis (see section 7.1.2.1). After one year, the
remnants of the necrotic testis were shown to contain masses of
cells showing the typical structure of Leydig cells (Parizek, 1960).
This regeneration of testicular Leydig cells damaged by cadmium can
result in Leydig cell neoplasia (Gunn et al., 1963, 1965; Lucis et
al., 1972). The ultrastructural features of cadmium-induced Leydig
cell tumours correspond in most respects with the fine structural
features of normal Leydig cells (Reddy et al., 1973).
Other injection studies and peroral studies did not demonstrate
increased malignancy (Schroeder et al., 1964, 1965; Loser, 1980),
but the doses were low compared to those necessary to induce renal
damage. In one peroral study (Kanisawa & Schroeder, 1969), rats were
exposed to 5 mg cadmium/litre in drinking-water for up to 2 years.
There were 7 malignant tumours among 47 cadmium-exposed male rats
and 2 tumours among 34 male control rats. This indicates a doubling
of the tumour rate, but because of the low statistical power of the
study, the increase was not statistically significant and the
authors concluded that ingestion of these cadmium doses was not
carcinogenic.
Some studies have been specially designed to investigate the
possible role of cadmium in cancer of the prostate. The prostate
gland, like the testis, is of particular interest with respect to
cadmium toxicity because these organs contain greater concentrations
of zinc than any other tissues and it has been suggested that
cadmium may affect prostate growth by competition with zinc (Gunn et
al., 1961). Levy et al. (1973) gave three groups of rats weekly
subcutaneous injections of cadmium sulfate at concentrations of
0.022, 0.044 or 0.087 mg cadmium per rat (average weight 220 g at
the start and 410 g at the end of the 2-year exposure). Weekly
injections of water were given to the 75 control rats. The liver
cadmium level was 80 mg/kg in the highest-dose group, but no
malignant changes were found in the prostate. No difference was seen
between exposed and control rats with respect to malignant changes
in other organs.
Levy & Clack (1975) and Levy et al. (1975) conducted 2-year
studies in rats and mice designed to detect carcinogenic effects in
the prostate. The animals in both experiments were given weekly
administrations of cadmium sulfate by stomach tube. The rats were
given from 0.08 to 0.35 mg/kg body weight and the mice 0.44 to
1.75 mg/kg. Extremely low levels of cadmium were found in the kidney
after 2 years (5 mg/kg wet weight in rats), but no macroscopic or
microscopic changes were seen in any tissue at these low doses.
In a long-term study on Fisher rats, Sanders & Mahaffey (1984)
administered cadmium oxide (25 µg) in single or repeated doses by
intratracheal instillations. There was no evidence for pulmonary or
prostate carcinogenicity, but increases in mammary tumours and in
tumours at multiple sites in male rats were reported.
It has been reported that inhalation of a cadmium aerosol
causes lung cancer in Wistar rats (Takenaka et al., 1983). Three
groups of 40 rats were continuously exposed to cadmium chloride
aerosols for 18 months, the air cadmium concentrations being 12.5,
25, and 50 µg/m3. A control group of 41 rats was also studied. The
study was terminated after 31 months, and no lung cancers were seen
in the control group. However, in the exposed groups, the incidence
was 15%, 53% and 71%, respectively, at increasing exposure levels.
Even at these relatively low exposure levels, there was a clear
dose-response relationship. Histologically the experimentally
induced tumours were adenocarcinomas, epidermoid carcinomas,
mucoepidermoid carcinomas, and combined epidermoid and
adenocarcinomas.
In a subsequent study, rats were exposed to inhalable aerosols
of cadmium sulfate and cadmium oxide and fume and dust at > 30 µg
cadmium/m3 and to cadmium sulfide at > 90 µg cadmium per m3 for
periods of up to 18 months. Bronchoalveolar benign and malignant
adenomas, squamous cell carcinomas, and combined forms developed at
high primary tumour rates with all four forms of cadmium tested even
after discontinuous exposure for 40 h/week for 6 months. No primary
tumour was found with cadmium oxide fume at a concentration of 10 µg
cadmium/m3 or cadmium oxide dust (at 30 µg cadmium/m3) when
combined with a zinc oxide aerosol (Oldiges et al., 1989).
In a further study, male and female Syrian golden hamsters and
female NMRT mice were exposed to cadmium chloride, sulfate, oxide,
and sulfide at concentrations of between 10 and 270 µg cadmium/m3.
The exposure was continuous (19 h/day, 5 days/week) for 50 to 70
weeks and was followed by a 50-week observation period. No increase
in the lung tumour rate was observed in either the mice or hamsters
(Heinrich et al., 1989), but in both species exposure to cadmium
caused multifocal bronchoalveolar hyperplasia, the extent of which
varied with the compound used, its concentration, and the length of
exposure. The most severe changes were found after cadmium oxide
inhalation (Aufderheide et al., 1990).
A synergistic effect has been shown in rat renal tumours
induced by dimethylnitrosamine when followed by cadmium chloride
given by intramuscular injection. In this study, cadmium appeared to
enhance the initiation of dimethylnitrosamine-induced cancer (Wade,
1987).
7.6 Host and dietary factors; interactions with other trace
elements
The toxic effects of cadmium in experimental animals have been
shown to be dependent on genetic factors, stage of ontogenic
development, functional state of the organism, and simultaneous or
previous exposure to certain environmental influences, including
exposure to certain nutrients.
Resistance to cadmium-induced testicular necrosis is determined
by a single autosomal recessive gene (cdm) in inbred mice (Taylor et
al., 1973). The teratogenic effects of cadmium are dependent on the
stage of embryogenesis (section 6.3). The stage of postnatal
development of certain organs may be of importance for the toxic
effects of cadmium, as has been shown for the testis (Parizek, 1957,
1960), ovaries (Kar et al., 1959), and central nervous system
(Gabbiani et al., 1967).
Pretreatment with small, non-toxic doses of cadmium salts has
been shown to induce resistance to testicular or lethal effects
(Terhaar et al., 1965; Ito & Sawauchi, 1966). The probable mechanism
is induction of metallothionein synthesis by the pretreatment. This
enables the subsequent dose of cadmium to be bound rapidly to
metallothionein, which renders it less acutely toxic (Nordberg,
1971). Similarly, the protective effect of zinc against cadmium
toxicity could also be, at least in part, dependent on the induction
of an increased synthesis of metallothionein-like proteins (Webb,
1972; Davies et al., 1973).
Selenium compounds are known to be highly effective in
preventing the reproductive toxic effects of cadmium (Kar et al.,
1960; Mason & Young, 1967; Parizek et al., 1968a,b), lethality to
rats (Parizek et al., 1968b) and mice (Gunn et al., 1968), and
teratogenicity (Holmberg & Ferm, 1969). Fetal lethality (Parizek et
al., 1968b) and teratogenic effects (Holmberg & Ferm, 1969) can be
prevented when selenium compounds are given at the same time as
cadmium.
Simultaneous administration of mercuric and cadmium compounds
has been shown to have an additive effect (Gale, 1973). Oral
administration of nitrilotriacetate with large oral doses of cadmium
chloride provided protection against the lethality of cadmium and
had no potentiating effect on the teratogenicity and fetal
accumulation of cadmium (Scharpf et al., 1972). This was confirmed
by Engström (1979), who also showed that simultaneous oral exposure
to cadmium and sodium tripolyphosphate decreased the mortality
expected at the cadmium level used. However, when nitrilotriacetate
or sodium tripolyphosphate was given subcutaneously with cadmium,
the mortality rates were increased (Engström & Nordberg, 1978;
Andersen et al., 1982). The chelation of cadmium in the
gastrointestinal tract decreased the uptake of cadmium, whereas
parenteral exposure to cadmium and chelating agents caused a higher
renal cadmium concentration than cadmium alone.
The interaction of cadmium with certain trace elements can
produce symptoms characteristic of trace element deficiencies. As a
result, chronic cadmium toxicity in certain animal species closely
resembles zinc and/or copper deficiency and can be prevented by
administering higher doses of the salts of these trace elements
(Petering et al., 1971, 1979; Mills & Delgarno, 1972). Cadmium-
calcium interactions are discussed in section 7.2.4.
An increased toxicity of cadmium was reported in animals on
low-protein diets (Fitzhugh & Meiller, 1941), this being due partly
to rapid intestinal absorption of cadmium (Suzuki et al., 1969).
Lack of dietary calcium seems to play a role similar to lack of
dietary protein in increasing the toxicity of cadmium (Suzuki et
al., 1969). Supplements of dietary ascorbic acid almost completely
prevented cadmium-induced anaemia and improved the growth rate (Fox
& Fry, 1970).
Ambient temperature (Nomiyama et al., 1978b) and the energy or
protein level in the diet have been reported to influence the LD50
of cadmium in mice.
Various aspects of the interactions between cadmium and other
trace elements were discussed in greater detail by the Task Group on
Metal Interactions (1978).
7.7 Conclusions
Inhalation exposure at high levels causes lethal pulmonary
oedema. Single high-dose injection gives rise to testicular and
non-ovulating ovarian necrosis, liver damage, and small vessel
injury. Large oral doses damage the gastric and intestinal mucosa.
Long-term inhalation exposure and intratracheal administration
give rise to chronic inflammatory changes in the lungs, fibrosis,
and appearances suggestive of emphysema. Long-term parenteral or
oral administration produces effects primarily on the kidneys, but
also on the liver and the haematopoietic, immune, skeletal, and
cardiovascular systems. Skeletal effects and hypertension have been
induced in certain species under defined conditions. Teratogenic
effects and placental damage occur, depending on the relation
between the exposure and the stage of gestation, and may involve
interactive effects with zinc.
Of greatest relevance to human exposure are the acute
inhalation effects on the lung and the chronic effects on the
kidney. Following long-term exposure, the kidney is regarded as the
critical organ. The effects on the kidney are characterized by
tubular dysfunction and cell damage, although glomerular dysfunction
may also occur. A consequence of renal tubular dysfunction is a
disturbance of calcium and vitamin D metabolism. According to some
studies, this has led to osteomalacia and/or osteoporosis, but these
effects have not been confirmed by other studies. A direct effect of
cadmium on bone mineralization cannot be excluded. The toxic effects
of cadmium in experimental animals are influenced by genetic and
nutritional factors, interactions with other metals, in particular
zinc, and pretreatment with cadmium, which may be related to the
induction of metallothionein.
IARC (1976, 1987b) accepted as sufficient the evidence that
cadmium chloride, sulfate, sulfide, and oxide can give rise to
injection-site sarcomata in the rat and that the chloride and
sulfate can induce interstitial cell tumours in the testis of rats
and mice, but found oral studies inadequate for evaluation. One
recent life-time study (18 months), in which rats were subjected to
continuous inhalation of a cadmium chloride aerosol at low
concentration, showed a high incidence of primary lung cancer with
evidence of a dose-response relationship. Studies on the genotoxic
effects of cadmium have given discordant results, most of the
positive results indicating chromosomal effects after short-term
high-level exposure.
8. EFFECTS ON HUMANS
Most of the available epidemiological studies or group
observations, as well as the clinical studies, have been performed
either on occupationally exposed workers or on Japanese populations
in cadmium-polluted areas. A great deal of epidemiological data has
resulted from studies in polluted areas of Japan (Cooperative
Research Committee on Itai-itai Disease, 1967; Shigematsu et al.,
1978; Japan Cadmium Research Committee, 1989) and, more recently,
from smaller studies in other countries (Drasch et al., 1985;
Philipp, 1985; Hahn et al., 1987; Roels et al., 1989; Thun et al.,
1989; Likutova, 1989). Comprehensive summaries of these studies have
also been published (Tsuchiya, 1978; Friberg et al., 1986; Nomiyama,
1986).
Many of these studies have focused on the detection of early
signs of kidney dysfunction. Others have investigated clinical signs
of disease such as renal stones and pulmonary impairment. Until the
middle of the 1970s, particular attention was given in Japan to the
detection of and screening for bone disease (e.g., Itai-itai
disease). More recently the role of cadmium in human carcinogenesis
and mortality has also been studied.
Exposure to cadmium produces a wide variety of effects
involving many organs and systems. From the point of view of
preventive medicine, the detection of early effects on the kidneys
is of particular importance in order to prevent more serious renal
effects and those on the lungs or bones. Recent studies indicating
that chronic exposure to cadmium may give rise to cancer will be
reviewed in some detail.
8.1 Acute Effects
8.1.1 Inhalation
Acute cadmium poisoning and, in some cases, death have been
reported among workers shortly after exposure to fumes when cadmium
metal or cadmium-containing materials have been heated to high
temperatures (Beton et al., 1966; Blejer, 1966; Dunphy, 1967). The
principal symptom in acute cases, both fatal and non-fatal, is
respiratory distress due to chemical pneumonitis and oedema
(MacFarland, 1979; Lucas et al., 1980). At an early stage, the
symptoms may be confused with those of "metal fume fever".
In working environments where cases of acute poisoning
occurred, cadmium concentrations were usually very high. For
instance, in one case the fatal air concentration of cadmium oxide
fume from a furnace was approximately 50 mg/m3 for a period of
about 1 h (a dose of 2900 mg/m3.min) (Barrett & Card, 1947). In
another case, the lethal dose was 2600 mg/m3.min (Beton et al.,
1966), i.e. a 5-h exposure to 8.6 mg/m3. Friberg et al. (1974)
estimated that an 8-h exposure to 5 mg cadmium/m3 may well be
lethal.
8.1.2 Ingestion
During the period 1940-50, cases of acute food poisoning
occurred mainly due to the substitution of cadmium for scarce
chromium in the plating of many cooking utensils and containers.
Food contamination arose when acid foods and drinks were prepared
and stored in contact with cadmium-plated surfaces. Rapid onset with
severe nausea, vomiting, and abdominal pain were characteristic
symptoms (US Public Health Service, 1942; Cole & Baer, l944; Lufkin
& Hodges, 1944). Effects also occurred following the consumption of
drinks with a cadmium concentration of approximately 16 mg/litre
from an automatic vending machine in which drinking-water was cooled
in a tank constructed with cadmium-containing solder (Nordberg et
al., 1973). Recovery from acute poisoning appears to be rapid and
complete. The amount of cadmium absorbed is probably very limited
due to vomiting and the consequential short presence of cadmium in
the gastrointestinal tract. However, no follow-up studies of people
who have experienced acute cadmium poisoning have been reported.
8.2 Chronic Effects
Lower cadmium concentrations with longer periods of exposure
than those described above will cause chronic cadmium poisoning.
Fully developed poisoning among industrial workers shows two main
effects: renal dysfunction and emphysema (Friberg, 1948a,b, 1950).
The kidney is most frequently the critical organ, but under certain
conditions (short-term peak exposures) it may be the lung (Bonnell,
1955). For people in the general environment, exposure is usually by
the oral route and the kidney is the critical organ.
8.2.1 Renal effects and low molecular weight proteinuria
8.2.1.1 In industry
Renal dysfunction is one of the characteristic signs of cadmium
poisoning, and many cadmium workers have developed proteinuria,
renal glucosuria, and aminoaciduria. In working environments with
high cadmium exposure levels, workers have also developed
hypercalciuria, phosphaturia, and polyuria (Friberg, 1950; Clarkson
& Kench, 1956; Kazantzis et al., 1963; Tsuchiya, 1967; Lauwerys et
al., 1974a, 1979b), and some have suffered from renal colic due to
recurrent stone formation (Friberg, 1950; Ahlmark et al., 1961;
Adams et al., 1969; Scott et al., 1976; Kanzantzis, 1979). The
polyuria is due to loss of urinary concentrating ability (Kazantzis,
1979), and, in addition, the kidneys of cadmium-poisoned workers
lose their ability to handle an acid load after a standard
NH4Cl-loading test. These are signs of distal tubular damage, and
in a few severe cases, the renal damage progresses to a reduction in
glomerular filtrations (see section 8.2.1.5).
Renal function, as measured by inulin or creatinine clearance
and urine concentrating capacity, was depressed in several poisoning
cases (Friberg, 1950; Bonnell, 1955; Bernard et al., 1979). Thus, in
the more advanced cases, there is a combination of tubular and
glomerular effects. In most of the early cases, only proteinuria,
mild in comparison with the proteinuria in many other renal
disorders, has been reported as a sign of renal dysfunction, and
other signs of kidney dysfunction were not evident (Piscator,
1966a,b).
Since Friberg first observed the urinary proteins of cadmium
workers (Friberg, 1950), the proteinuria has proved to involve
proteins with a molecular weight of 10 000 to 40 000 and is the
so-called tubular proteinuria (Butler & Flynn, 1958). Table 15
contains data on proteins in urine useful for the diagnosis of
cadmium-induced proteinuria. The increased excretion of low
molecular weight proteins in urine from cadmium-exposed workers has
been found to apply to ß2-microglobulin, lysozyme (muramidase),
ribonuclease, immunoglobin chains, retinol-binding protein, and
alpha1-microglobulin (Piscator, 1966a; Peterson et al., 1969;
Peterson & Berggård, 1971; Lauwerys et al., 1974a; Bernard et al.,
1976, 1982b). In groups of exposed and unexposed workers, the
urinary ß2-microglobulin concentrations follow log-normal
distributions, and an operational definition for what is an
"increased" level should be established for each population studied
(Kjellström et al., 1977a).
Proteinuria is known to be an early sign of cadmium poisoning,
but the degree of proteinuria varies with time. In a group of 40
workers with heavy exposure to cadmium, it was found that
proteinuria was persistent and even increased several years after
cessation of exposure, as evaluated by qualitative methods (Friberg
& Nystrom, 1952; Piscator, 1966a). Tsuchiya (1976) examined five
cadmium-exposed workers who showed proteinuria. Ten years after
cessation of exposure, three of them no longer revealed proteinuria,
but two of these showed a high urinary ß2-microglobulin level, as
did the two workers with persistent total proteinuria. Four workers
in a British pigment factory still had grossly elevated
ß2-microglobulin levels despite removal from exposure many years
earlier (Stewart & Hughes, 1980).
Table 15. Excretion of urinary proteins in healthy people and in cases of glomerular and tubular disorders
Protein type Normal plasma Normal filtered Urinary excretion (mg/24 h) Reference
concentration amount in primary Healthy Glomerular Tubular
(mg/ml) urine (mg/24 h) people disorders disorders
Total protein 43-127 310-54 100 129-1570 Peterson et al. (1969)
Albumin 50 500 3.9-24 88-48 800 13.8-578 Mogensen & Solling (1977)
Retinol-binding 0.11 20-150 Peterson & Beggard (1971)
protein
0.04 3 45 Kanai et al. (1971)
ß2-microglobulin 0.002 300 0.06-0.21 0.06-4.7 1.1-105 Peterson (1971)
0.073 Evrin et al. (1971)
Lysozyme 0-2a Prockop & Davidson (1964)
0.07-1.1a 47-130a Harrison et al. (1968)
Ribonuclease 0.24-1.5a 1.9-10a Harrison et al. (1968)
a Values reported as mg/litre
According to recent observations using quantitative proteinuria
methods (Roels et al., 1982), total proteinuria in 19 workers had
not changed 4 years after exposure ceased. In those 11 workers for
whom urinary ß2-microglobulin was measured before and after
cessation of exposure, an increase was invariably seen. Eight of the
workers had abnormal ß2-microglobulin levels before exposure
ceased, whereas three had normal levels before and developed
abnormal levels after cessation. It can be concluded that
cadmium-induced tubular proteinuria is irreversible in most workers,
at least for several years.
A marked increase in urine cadmium level may reflect
cadmium-induced nephropathy if exposure has been chronic and
correlates with low molecular weight proteinuria (section 6.5.1.2).
Lauwerys et al. (1979b) proposed a biological threshold of 10 µg
cadmium/µg urinary creatinine for males occupationally exposed to
cadmium. Smith et al. (1980) found that workers with low exposure to
airborne cadmium had an average urinary cadmium level of
13.1 µg/litre, whereas workers with long histories of work in areas
with substantial airborne cadmium had an average level of
45.7 µg/litre. The high-exposure group showed a significant
reduction in urinary clearance and increased ß2-microglobulin
excretion. Buchet et al. (1980) found increased excretion of both
low and high molecular weight proteins and tubular enzymes in
workers excreting more than 10 µg cadmium/g creatinine or with a
blood cadmium level above 10 µg cadmium per litre.
Retinol binding protein (RBP) has been shown to correlate well
with ß2-microglobulin in urine with a pH value greater than 5.5.
and is equally sensitive for detection of tubular proteinuria
(Bernard et al., 1982a,b). This protein occurs in serum complexed to
prealbumin and retinol, but, after retinol is delivered to target
cells, RBP rapidly dissociates from prealbumin, is filtered through
the glomerulus, and is reabsorbed by the tubule (Peterson, 1971).
Renal tubular brush border enzymes may also be excreted in
chronic cadmium poisoning. In patients with Itai-itai disease,
urinary trehalase activity correlates inversely with tubular
resorption of phosphorus (Nakano et al., 1987) and there is a
statistical correlation between urinary trehalase and other urinary
indicators of renal tubular dysfunction, such as glucose,
ß2-microglobulin, cadmium, and alpha-amino nitrogen, in
inhabitants of chronic cadmium-polluted areas in Japan (Nogawa et
al., 1980).
Other renal effects in cadmium workers include glucosuria,
aminoaciduria, impaired concentrating abilities, and hypercalciuria
(Kazantzis et al., 1963; Scott et al., 1976), which may cause
disturbances in bone and calcium metabolism (section 8.2.2). The
hypercalciuria leads to renal stone formation in some workers
(section 8.2.2.1). An increased excretion of amino acids,
particularly of serine and threonine, has been shown in industrial
workers (Clarkson & Kench, 1956), but the amino acid excretion
pattern was not consistent. In a cadmium worker with osteomalacia
(Kazantzis, 1979), there was an increase excretion of
hydroxyproline, which could be an effect of changes in collagen
metabolism related to the bone disorder (section 7.2.4).
The renal effects of cadmium that lead to proteinuria may
progress and, in some cases, with high exposures, lead to an
increase in blood creatinine. This has contributed to a
higher-than-expected mortality rate among highly exposed workers
(Kjellström et al., l979). In a Swedish battery factory, there were
four deaths from nephritis or nephrosis among 185 workers, all of
whom had been exposed to cadmium for more than 15 years. The
expected number of deaths due to these causes in this group of
workers was 0.4 (P = 0.05) (Andersson et al., l984). In a study of
about 6995 cadmium-exposed British workers (Armstrong & Kazantzis,
1983), there were 10 deaths due to nephritis or nephrosis, whereas
15.3 deaths were expected. In the subgroup with the highest exposure
(211 workers), one death occurred (0.3 expected). A 5-year follow-up
of this study (Kazantzis et al., 1988) confirmed no excess mortality
from nephritis and nephrosis (ICD 580-584), the number of observed
deaths now having increased to 16 (18.9 expected). Armstrong &
Kazantzis (1985) conducted a case control study of this cohort in
which a more detailed assessment of the past exposure of workers was
obtained. There was a marginally increased, but not statistically
significant, risk from nephritis and nephrosis (ICD 580-584) in
workers with "ever high" or "ever medium" exposure to cadmium.
Existing studies of mortality from nephritis/nephrosis have
been based upon epidemiological studies of renal failure given as
the underlying cause of death on death certificates. With the advent
of kidney dialysis and transplantation, patients with kidney failure
frequently survive and die of other causes. If kidney failure is
indicated at all on their death certificates, it is frequently given
as a contributing rather than underlying cause of death.
No studies on the contribution of cadmium-induced renal effects
to morbidity, absence from work, etc., have been published, although
one study (Vorobjeva & Eremeeva, 1980) reported increased
cardiovascular disease and related increased work absence among
cadmium workers (section 8.2.4).
8.2.1.2 In the general environment
In Japanese cadmium-polluted areas, signs of renal dysfunction
very similar to those in cadmium-exposed industrial workers have
been found. Proteinuria and glucosuria were found to be common
(30-80%) among the exposed people in one area (Ishizaki, 1969) and
less common among people living in control areas and areas bordering
a polluted area. In the exposed groups, a positive correlation
between age and the prevalence of signs was also seen (section
8.3.2). Due to the cumulative nature of cadmium, the total dose is
directly correlated to age.
With the large number of elderly people and women included in
the groups exposed in the general environment, factors other than
cadmium that can affect renal function may make direct comparisons
with industrial workers difficult. Nevertheless, the tubular
proteinuria (Shiroishi et al., 1977), aminoaciduria, and other signs
of renal tubular damage (Saito et al., 1977; Nogawa et al. 1984)
were very similar to the findings for industrial workers. In
addition, as in the case of exposed workers, elevated urinary
excretion of metallothionein occurs as a result of environmental
cadmium exposure (Tohyama et al., 1981b).
Among cadmium-exposed people in the general environment, the
mean urine ß2-microglobulin excretion was highest (Shiroishi et
al., 1977) in patients with Itai-itai disease (section 8.2.2.2).
Many of the Itai-itai patients also have signs of decreased
glomerular filtration, as indicated by decreased urea clearance
(Nakagawa, 1960) and increased serum creatinine (Nogawa et al.,
1979).
8.2.1.3 Methods for detection of tubular proteinuria
Determination of total protein and electrophoretic analysis of
concentrated urinary protein were originally the common methods for
the detection and diagnosis of tubular proteinuria. Quantitative
immunological methods (detection limit, 0.002-0.003 mg/litre) for
the measurement of ß2-microglobulin (Evrin et al., 1971) and RBP
(Bernard et al., 1982b) in urine ("normal" level about 0.1 mg/litre)
are available, and these methods facilitate the detection of tubular
dysfunction. An electrophoretic method with reasonable sensitivity
(0.8 mg/litre) for measuring specific proteins in the urine utilizes
staining of proteins in sodium dodecyl sulfate acrylamide gel
electrophoresis (Nomiyama et al., 1982b). More recently, radio-,
latex-, and enzyme-linked immunoassays have been developed (Evrin et
al., 1971; Bernard et al., 1982a; Carlier et al., 1981). However,
the disadvantage with ß2-microglobulin as a marker for renal
tubular dysfunction is that this protein is unstable if urinary pH
is less than 5.5.
RBP has an advantage over ß2-microglobulin, particularly for
screening purposes, in that serum levels and, hence, excretion are
not affected as readily by concomitant immunological disease and are
more stable at an acidic pH (Bernard et al., 1982b). An enzyme-
linked immunosorbent assay (ELISA) for urinary RBP has also been
described (Topping et al., 1986).
Common tests for qualitative determination of proteinuria, such
as paper tests (dip sticks), the nitric acid test, and the boiling
test, should not be used for screening cadmium-induced proteinuria,
since positive readings will be obtained only at fairly high urine
protein concentrations (Piscator, 1962). Trichloroacetic acid (TCA)
or sulfosalicylic acid (SA) can be used for qualitative tests, but a
negative result does not exclude a moderate increase in low
molecular weight proteinuria.
8.2.1.4 Significance of cadmium-induced proteinuria
More than 70% of proteins with a molecular weight less than
15 000 but less than 5% of those with a molecular weight greater
than 40 000 pass through the glomerular membrane (Squire et al.,
1962). The glomerular filtrate contains relatively large amounts of
plasma proteins, which are normally almost completely reabsorbed in
the proximal tubules and only small amounts are found in the urine.
The increased excretion of tubular proteins in cadmium nephropathy
is thought to be due mainly to a decreased tubular reabsorption
capacity. This provides early evidence of renal tubular dysfunction.
The concentration of albumin in normal serum is about 25 000
times higher than the concentration of ß2-microglobulin (Table
15). In spite of the fact that very little of the albumin but about
80% of the ß2-microglobulin is filtered through the glomeruli
(Maack et al., 1979), the filtered amount of albumin is still higher
than the amount of ß2-microglobulin (Table 15).
Only a small fraction of the albumin or the other proteins is
excreted in normal urine due to the normally efficient (more than
99%) tubular reabsorption of all proteins (Table 15). The urinary
albumin excretion is about 100 times greater than the
ß2-microglo-bulin excretion (Table 15).
ß2-Microglobulin is a subunit of a major immunoglobulin
complex with a molecular weight of 12 000 and normally occurs in
serum at a concentration of approximately 2.0 mg/litre. In the case
of a decreased glomerular filtration rate, the serum concentration
of ß2-microglobulin will also increase. In certain conditions, for
example, where excessive production occurs as in some cancers and
autoimmune disorders, serum levels increase, the tubular capacity
for reabsorption may be exceeded, and the concentration in the urine
will rise. In tubular dysfunction, the capacity for absorption is
impaired and, again, this is reflected by an increased excretion in
the urine. Such renal disorders include the congenital and acquired
Fanconi syndrome, diabetic nephropathy, certain cases of reflux
nephropathy, and advanced glomerular disease (Squire et al., 1962).
A raised urinary excretion of ß2-microglobulin or other low
molecular weight protein is not, therefore, specific to renal
dysfunction induced by cadmium, and a differential diagnosis should
be considered in all cases where this occurs.
The "normal" average urinary excretion of ß2-microglobulin
measured in several populations was in the range 0.05-0.1 mg/24 h
(or mg/litre or mg/g creatine) (Kjellström & Piscator, 1977). Below
age 65, there was very little or no change with age in the urinary
ß2-microglobulin (Kjellström & Piscator, 1977), a fact confirmed
by later studies (Tsuchiya et al., 1979; Kowal & Kraemer, 1982). In
all of the studies, some high individual values were found in the
age group above 65 years, and the studies of Tsuchiya et al. (1979)
and Kowal & Kraemer (1982) reported age-regression coefficients
indicating an increase with age. However, there was wide variation
with age and the average urinary ß2-microglobulin levels in the
oldest age groups (above 65 years) were only 10-20% higher than in
the other age groups (Kowal & Kraemer, 1982). The prevalence of
increased low molecular weight proteinuria (above 0.5 mg/litre) was
less than 5% in these studies, but in a control group of people
above age 80 from a Japanese cadmium-polluted area (section 8.3.2.2)
the prevalence was about 15%.
8.2.1.5 Glomerular effects
Although renal tubular dysfunction with its accompanying low
molecular weight proteinuria is thought to be the most prominent
renal effect of cadmium, the ß2-microglobulinuria is sometimes
accompanied by the excretion of high molecular weight proteins such
as albumin (molecular weight, 69 000). This albuminuria may
occasionally occur as a result of cadmium exposure without any
concomitant increase in the urinary excretion of low molecular
weight proteins (Bernard et al., 1976, 1979); this indicates that
cadmium, in some cases, may produce a change in the glomerular
permeability to larger proteins.
Cadmium may also affect the glomerular filtration rate (GFR).
Friberg (1950) reported decreased inulin clearance in
cadmium-exposed battery workers. Elinder et al. (1985a) measured GFR
by chromium-EDTA in 17 workers previously exposed to cadmium fumes.
They found a significant negative correlation between decreasing GFR
and tubular reabsorption loss, and reported that GFR decreased with
increasing cumulative exposure to cadmium fumes. The urinary
clearance of ß2-microglobulin increased with decreasing GFR.
Several other occupational studies have reported increased
serum concentrations of creatinine and/or ß2-microglobulin,
indicating reduced GFR, in cadmium-exposed workers (Thun et al.,
1989; Roels et al., 1989).
Thun et al. (1989) found a small increase in mean serum
creatinine in a group of 45 cadmium-exposed workmen. Serum
creatinine also increased with cadmium dose, suggesting decreased
glomerular function. Cadmium dose remained the important predictor
of serum creatinine even after controlling for age, blood pressure,
body size, and other extraneous factors.
Roels et al. (1989) measured the serum creatinine and serum
ß2-microglobulin levels of 23 workers, removed from cadmium
exposure, on several occasions over a period of six years. The
average yearly decrease in GFR was estimated to be 6 ml/min per
1.73 m2, which is considerably more than the normal value
(< 1 ml/min per 1.73 m2) and significantly more than that of a
control group examined at the same time.
There is also evidence of glomerular effects in people exposed
to cadmium in the environment. Nogawa et al. (1980) suggest that a
reduction in creatinine clearance may be detected at the early stage
of cadmium poisoning in a polluted area. In addition, Nogawa et al.
(1984) reported a significant correlation between decreased tubular
reabsorption of phosphate and decreased GFR in farmers living in a
cadmium-polluted area.
The mechanism for the glomerular effects from cadmium is
uncertain. It has been suggested that cadmium-induced tubular damage
leads to a certain degree of interstitial nephritis which in turn
results in a decreased GFR (Elinder et al., 1985a). It has also been
proposed that cadmium exerts a direct toxic effect on the glomerulus
(Roels et al., 1989).
8.2.1.6 Relationship between renal cadmium levels and the
occurrence of effects
The number of reports of renal pathology in autopsy cases and
renal biopsies that contain data on kidney cortex concentrations of
cadmium is small. Thus, it is difficult to establish a dose-response
relationship between cadmium content and pathology or dysfunction.
Nomiyama (1977) summarized data from 26 cadmium-exposed workers
and 16 cadmium-exposed people from the general environment. The
criteria for choosing the subjects were that they possessed high
renal and/or high liver concentrations of cadmium, morphological
studies on the kidney had been performed, and data were available on
the occurrence of proteinuria while the person was alive. Among the
42 cases reviewed, those exhibiting slight or no proteinuria and no
morphological alterations had higher concentrations of cadmium in
the renal cortex than non-exposed people. Most cases with
morphological changes plus proteinuria had lower renal cadmium
concentrations that those without proteinuria and/or morphological
changes. In more recent studies, Ellis et al. (1985) found that in
cases of renal dysfunction the mean liver and kidney cadmium values
for retired workers were lower than those for active workers. These
findings are similar to those from animal studies (section 6.5.1.2),
where kidney concentrations levelled off or even declined in the
presence of kidney damage.
The use of in vivo neutron activation analysis has
facilitated the study of the relationship between renal cadmium
levels and occurrence of effects (Ellis et al., 1981a; Roels et al.,
1981b). However, the data must be assessed with caution as the
accuracy of this method has not yet been fully determined. For
instance, the exact location of the kidney needs to be known.
Erroneously low renal cadmium levels were reported by Roels et al.
(1981b) due to an error in adjusting for the distance between skin
and kidney (Roels et al., 1983a). Data from Ellis et al. (1981a) and
Roels et al. (1983a,b) have shown that few cases with increased
urinary ß2-microglobulin concentrations are seen when the level of
renal cortex cadmium is less than 150 mg/kg tissue and that of liver
cadmium is less than 40 mg/kg. There is a pattern of liver and
kidney cadmium levels increasing simultaneously until the average
renal cortex cadmium concentration is about 300 mg/kg and the
average liver level is about 60 mg/kg. At higher liver levels, the
renal cortex level is disproportionately low in most cases, and, in
addition, many of these workers have increased urinary
ß2-microglobulin.
Skerfving et al. (1987) measured kidney cortex cadmium levels
by X-ray fluorescence in a group of 20 workers from a factory
producing alkaline batteries and found an average value of 147 mg/kg
(range 53-317). When compared to a control group, these workers had
higher average urine levels of cadmium (5.4 vs 0.8 nmol/mmol
creatinine) and ß2-microglobulin (14.6 vs 6.6 µg/mmol creatinine).
Six workers had ß2-microglobulin levels exceeding 22 µg/mmol
creatinine. Due to selection procedures the results are, however,
not predictive for cadmium-exposed workers in general. Nevertheless,
it is clear that there are no significant correlations between
levels of cadmium or ß2-microglobulin in urine and cadmium levels
in the kidney. These results suggest that there is a relationship
between renal cadmium and occurrences of effects on a group basis
but renal cadmium levels per se are not always predictive of
pathological effects on an individual level.
8.2.1.7 Reversibility of renal effects
The potential for reversibility of renal effects has been
studied in populations of workers with occupationally induced
cadmium nephropathy as well as in residents of cadmium-polluted
areas.
a) Occupational exposures
In a group of 40 workers heavily exposed to cadmium, it was
found, using qualitative methods, that proteinuria was persistent
and sometimes even increased several years after cessation of
exposure (Friberg & Nystrom, 1952; Piscator, 1966a).
Tsuchiya (1976) studied a group of 13 workers who had been
exposed to cadmium fumes (133 µg/m3) and who had proteinuria
(determined by the trichloroacetic acid method) and abnormal
electrophoretic urine patterns (ß2-microglobulin levels above
40 000 µg/litre). A 10-year follow-up study of five of these
patients was carried out after improvements had been made in their
working environment (cadmium fumes, 20 µg/m3). The proteinuria was
reversed (measured using a single radial immuno-diffusion method) in
three of the five patients: ß2-microglobulin values were 3500,
2600 µg/litre, and not detectable (limit of detection
2000 µg/litre), and retinol-binding proteins (RBP) were not
detectable (limit of detection 500 µg/litre). In addition, there
were improvements in the remaining two patients (ß2-microglobulin,
9700 and 5500 µg/litre; RBP, 34 000 and 120 000 µg/litre). Tsuchiya
(1976) suggested that the difference in the period of exposure to
cadmium was the reason for this difference in the degree of recovery
from the effects of cadmium.
Stewart & Hughes (1981) reported on similar cases from a
British pigment factory. Despite the fact that exposure ceased many
years earlier, grossly elevated ß2-microglobulin levels were still
detected.
Using quantitative methods to detect proteinuria, Roels et al.
(1982) found that total proteinuria in 19 workers was unchanged 4
years after exposure had ceased. In those 11 workers for whom
urinary ß2-microglobulin was measured before and after cessation
of exposure, levels were invariably increased. Eight of the workers
had abnormal levels of ß2-microglobulin even before cessation of
exposure, whereas three had normal levels before cessation and
developed abnormal levels afterwards.
Roels et al. (1989) examined 23 workers once a year for 5 years
after removal from exposure to cadmium. These workers had been
exposed to cadmium for periods of 6 to 41.7 years (mean 25 years),
and their first follow-up examination took place when they had been
removed from exposure for an average of 6 years. Their mean age at
that time was 58.6 years (range 45.5-68.1 years). Cadmiumuria in
these workers had been assessed three years previously by measuring
the cadmium levels in liver and kidney using neutron activation
analysis. The cadmium concentrations (mg/kg wet weight) in the liver
and kidney cortex ranged from 24 to 158 (mean 61) and from 133 to
355 (mean 231), respectively. Although cadmium concentrations in the
blood and urine decreased significantly over the five-year period,
urine concentrations of albumin, ß2-microglobulin, and RBP did not
change significantly.
Harada (1987) conducted studies on the health status of seven
workers exposed prior to 1972 to high cadmium levels in a cadmium
sulfide dye manufacturing factory. These workers were examine for 15
years, improvements having been made to working conditions in 1974
which led to markedly decreased cadmium exposures. The cadmium
content of the blood and urine declined after the improvements in
working conditions but increased again when production rose. The
working condition improvements resulted in a marked reduction in
urine ß2-microglobulin level in five workers (e.g., from 1272 to
520 µg/g creatinine in 1 year in one worker and from 2090 to
503 µg/g creatinine in 6 months in another), but there was elevated
urinary ß2-microglobulin excretion when production increased. One
worker had fairly constant near-normal urinary levels of
ß2-microglobulins (55 to 183 µg/g creatinine) regardless of
workplace improvements or production levels. Initially, four workers
had low GFR values, but none of the seven workers showed any
decrease in glomerular filtration during the 15-year follow-up
period. TRP rates decreased in three workers but remained relatively
unchanged in the other four. These changes in GFR and TRP seemed to
be independent of cadmium exposure levels.
Elinder et al. (1985b) found that urine cadmium excretion
decreased in 14 out of 19 workers re-examined at least once five
years or more after exposure to cadmium, but renal tubular function,
as measured by urinary ß2-microglobulin excretion, had
deteriorated or not improved in nearly all of the workers. Thun et
al. (1989) concluded that "time since last exposure to cadmium" was
not an important determinant of renal outcome whether considered on
its own or together with the cadmium dose. In their study of 45
workers at a plant that recovered cadmium from industrial waste, 9
out of 15 workers with the highest ß2-microglobulin excretion had
not been exposed to cadmium for at least five years and one for 45
years. This study suggested that if cadmium nephropathy is
reversible, the recovery is so slow as to be indiscernible after
decades of non-exposure.
Ellis et al. (1985) showed that the liver cadmium levels in
workers no longer exposed to cadmium gradually declines. Persistence
of renal tubular dysfunction after cessation of exposure may reflect
the level of body burden and the transfer of cadmium from liver to
kidney.
b) Residents of cadmium-polluted areas
Reversibility of renal tubular dysfunction has been
investigated in residents of cadmium-polluted areas in Japan. Kasuya
et al. (1986) carried out a comparative study of urine
ß2-microglobulin determinations made in 1975 and 1985 for 93
people with Itai-itai disease and their family members. Urine
ß2-microglobulin levels improved in the group with
ß2-microglobulin levels of 1000 µg/g creatinine or less but
worsened in the group with ß2-microglobulin levels of 3000 µg/g
creatinine or more. Most of the people who recovered were aged 39
years or less and had been resident for 30 years or less. It was
considered that mild renal dysfunction among young individuals was
reversible.
Saito (1987) measured the urine ß2-microglobulin levels of
residents of cadmium-polluted areas for 3 years after improvements
had been made in the level of soil contamination and compared the
results with determinations obtained 7 years previously. During this
3-year period, the urine ß2-microglobulin levels tended to remain
unchanged in people with a concentration of around 1000 µg/litre.
The reversibility of ß2-microglobulinuria, glucosuria, and
aminoaciduria was examined in 74 inhabitants (32 males and 42
females) over 50 years of age who lived in a cadmium-polluted area.
Examinations were conducted just after the cessation of cadmium
exposure and 5 years later. The geometric mean concentrations of
ß2-microglobulinuria, glucosuria, and aminoaciduria indicated a
significant increase in excretion during the 5-year period. In cases
where the level of ß2-microglobulinuria exceeded 1000 µg/g
creatinine at the time cadmium exposure ceased, evidence was found
indicating significant increases in proteinuria after 5 years,
whereas in cases where the excretion of ß2-micro-globulin had been
less than 1000 µg/g creatinine no significant changes were observed
(Kido et al. 1988).
8.2.2 Disorders of calcium metabolism and bone effects
8.2.2.1 In industry
Friberg (1950) observed 7 cases of renal stones among 43
cadmium workers and drew attention to the possibility of renal
stones being associated with exposure to cadmium. Ahlmark et al.
(1961) found that 44% of a group of 39 cadmium workers exposed to
cadmium oxide dust for more than 15 years had a history of renal
stone formation. Nine stones from six workers were analysed, and in
four workers the stones were composed of basic calcium phosphate
(Axelsson, 1963). There was an increase in the mean calcium
excretion rate in the cadmium-exposed group as compared to a control
group. Kidney stones were also found in 12 out of 43 British workers
at an accumulator factory (Adams et al., 1969). It is noteworthy
that in both of these studies (Axelsson, 1963 and Adams et al.,
l969) there was a higher prevalence of renal stones in workers
without proteinuria than in those with proteinuria. The men with
proteinuria had, as a group, increased urinary excretion of calcium
and phosphate, whereas in the group without proteinuria there were a
few cases with hypercalciuria. Hypercalciuria (81%) and renal stones
(19%) were also reported among 27 coppersmiths exposed to cadmium
(Scott et al., 1976, 1978).
Elinder et al. (1985a) found an increased prevalence of renal
stones among cadmium workers with tubular proteinuria, and Mason et
al. (1988) observed decreased renal reabsorption of calcium among
cadmium alloy workers.
Seven of the 12 workers in a cadmium pigment factory
investigated by Kazantzis et al. (1963) were found to have a urinary
calcium excretion rate greater than 300 mg/day (at least in 1 of 2
specimens). There was no evidence of excessive calcium intake in
these men. Five of these seven workers had been exposed to cadmium
compounds for more than 25 years and also had tubular proteinuria.
The remaining two, who had been exposed for 2 and 12 years,
respectively, had no other abnormality except for a urinary calcium
excretion of 308 and 403 mg/day. Follow-up was possible with six of
the twelve men, including all five with hypercalciuria and
proteinuria (Kazantzis, 1979). Six of the seven who had
hypercalciuria when first examined continued to have a raised
urinary calcium excretion, and one further worker developed
hypercalciuria during the follow-up period. All those with
hypercalciuria also had tubular proteinuria, although this was
marginal in one of the workers. Blood calcium levels remained within
normal limits in all cases (Kazantzis, 1979). The occurrence of
disordered calcium metabolism in all seven men followed-up for a
number of years makes it very likely that a common environmental
factor, such as occupational exposure to cadmium compounds, was
causative.
The data of Kazantzis (1979) agree with the findings of
hypercalciuria among 27 coppersmiths with high cadmium exposure
(Scott et al., 1976, 1978). Scott et al. (1980) reported that in 15
cadmium-exposed men the amount of calcium in the whole body was
lower than that of controls and decreased with duration of an
increased exposure to cadmium. The cadmium-induced hypercalciuria
could be reduced by thiazide treatment (Scott et al., l979).
In a study by Thun et al. (1989) of workers at a plant that
recovered cadmium from industrial waste, 8 of 45 exposed workers had
experienced kidney stones, in contrast to one of 32 unexposed
workers. Increase in the urinary excretion of ß2-microglobulin and
RBP was accompanied by decreased renal tubular reabsorption of
calcium and phosphates.
In contrast to the above findings, a low urinary calcium
excretion was detected in 47 out of 81 workers with exposure to a
variety of cadmium compounds and also cadmium oxide fumes (Tarasenko
& Vorobjeva, 1973; Tarasenko et al., 1975). The 24-h excretion of
calcium in these workers was below 100 mg, compared with 115-210 mg
in a control group of 21 people. Blood calcium values were within
the normal range in all cases.
Radiological examination was performed on 32 workers exposed
for 4-20 years to cadmium compounds (concentrations ranging from 0.1
to 5.5 mg/m3). All of them complained of pains in the bones.
Pseudofractures suggestive of osteomalacia were seen in two workers
exposed for 16 and 19 years, but no histological confirmation of
osteomalacia was obtained. Radiological appearances described as
enostosis were reported in five cases and periosteal proliferation
and consolidation in a further three cases (Tarasenko & Vorobjeva,
1973; Tarasenko et al., l975).
Horstowa et al. (1966) performed radiological examination of
the skeleton in 26 alkaline battery workers with signs of chronic
cadmium intoxication out of 80 workers exposed to 0.13-1.17 mg
cadmium/m3 for 1-12 years. Seven of these workers had proteinuria
detected by sulfosalicyclic acid; pseudofractures were found in 3
workers, sclerotic foci in 13, and osteoporosis in 10. In another
alkaline battery factory, where a number of cases of severe cadmium
poisoning were diagnosed (Friberg, 1950), X-ray examinations
revealed no signs of bone disease.
One of the workers with multiple tubular defects studied by
Kazantzis et al. (1963) developed osteomalacia confirmed by
histological examination 10 years after the initial investigation
(Kazantzis, 1979). He previously displayed hypercalciuria but, at
the time of diagnosis, his urinary calcium excretion was low.
Extensive investigation failed to reveal any of the other generally
accepted causes of osteomalacia such as malabsorption or nutritional
deficiency.
In a study of 43 workers at a battery plant, one worker
developed osteomalacia without evidence of malabsorption or
nutritional deficiency but with multiple renal tubular defects
(Adams et al., 1969). Another case of osteomalacia from the same
factory was subsequently detected in a man who had been a cadmium
battery worker for 40 years (Adams, 1980). Eight years before
retirement he had a partial gastrectomy due to a duodenal ulcer.
Proteinuria was first diagnosed 6 years before retirement, but
otherwise he was in "apparent good health" and "on a balanced diet"
until 8 years after retirement. He was then frail, had pains in his
legs, and a "waddling gait". His serum alkaline phosphatase level
was increased, X-rays showed generalized osteoporosis, and a bone
biopsy showed osteomalacia. After 1 year of treatment with large
doses of vitamin D, he could walk well again.
It also seems likely that the six workers exposed to cadmium
oxide dust described by Nicaud et al. (1942), who had pains in the
back and limbs and showed multiple pseudofractures on radiological
examination, suffered from osteomalacia. More detailed data on these
workers was presented by Valetas (1946), who also pointed out that
"massive doses" of vitamin D were needed to improve the symptoms. It
took several months for improvement to occur and the vitamin D
treatment had to be maintained for several years to keep the workers
in stable health. Valetas (1946) concluded that this bone disease
was caused by occupational exposure to cadmium. Eight workers with
8-30 years of exposure to lead dust and cadmium oxide fume and dust
(Gervais & Delpech, 1963) were also found to have multiple
pseudofractures and pains in the back, thorax, and legs. Very
limited biochemical investigations were carried out, but in four
cases proteinuria was found. The authors suspected that lead
exposure led to the observed effects.
8.2.2.2 In the general environment
Bone disease and abnormalities of calcium metabolism from
exposure to cadmium in the general environment have only been noted
in people in Japan with the clinical syndrome referred to as
Itai-itai disease. The main characteristics of the disease are
osteomalacia1 and osteoporosis2 with a tendency to fractures
accompanied by severe pain and renal tubular dysfunction. The
results of epidemiological and clinical investigations indicate an
association with cadmium exposure, although the Co-operative
Research Committee on Itai-itai Disease (1967) stated that
"malnutrition (low protein, low calcium diets) and multiple
pregnancies may also be involved".
1 Osteomalacia is characterized by inadequate mineralization of
bonematrix, resulting in an increase in the relative amount
of osteoid tissue. It represents the adult counterpart of
childhoodrickets (Robbins et al. 1984).
2 Osteoporosis is defined as an excessive but proportional
reduction in the amounts of both the mineral and matrix phases of
bone unaccompanied by any abnormality in structure of the residual
bone (Robbins et al., 1984).
Itai-itai disease is an endemic bone disease prevalent in the
basin of the Jinzu river, which runs through the central part of
Toyama Prefecture in West-Central Japan (Kono et el., 1956). It is
characterized by osteomalacia in combination with renal tubular
dysfunction in most cases. Patients also have osteoporosis and one
of the most characteristic symptoms is severe bone pain. Hagino &
Yoshioka (1961) reported that high concentrations of cadmium, lead,
and zinc were present in autopsy tissues from people with Itai-itai
disease and in the everyday foods of the endemic area.
Systematic epidemiological investigations, which included
extensive mass health examinations as well as case control studies
on both patients and controls, started in 1962 (Cooperative Research
Committee on Itai-itai Disease, 1967). It was reported that
Itai-itai disease in Toyama Prefecture was restricted to a limited
area (Fuchu area) irrigated by the Jinzu river, the geographical
distribution of the patients being consistent with the levels of
cadmium concentration in the paddy fields, and that the
concentrations of cadmium in urine were higher in patients than in
controls. The total number of patients was estimated in 1955 by the
Toyama Prefecture to be 41 out of a total of 1666 residents (849
women) (Cooperative Research Committee on Itai-itai Disease, 1967).
The major source of cadmium pollution in the area was a mine 50 km
upstream from the endemic area (Japan Public Health Association,
1968).
The age and sex distribution of the patients displayed a very
distinct pattern. Clinically apparent cases were limited to women
over 40 years of age who had given birth to many children (6 on
average) and had lived in the area for more than 30 years. No
detailed data on past patients were available, but it was estimated
that the age of onset of the disease was probably between 35 and 65
years, and that almost 100 deaths had been reported up to the end of
1966. The incidence was presumably very high from 1936 to 1950 and
at its highest in 1946 and 1947, but decreased thereafter even
though the same cadmium exposure levels had been maintained. By
March 1989, 150 cases of Itai-itai disease had been officially
recognized as pollution-related disease. Whereas all cases have been
reported in the Fuchu area, there have been a few suspected cases in
2 out of 12 cadmium-contaminated areas of Japan other than the Fuchu
area (Table 7). Clinical features of five suspected cases from the
Ikuno area matched those of Itai-itai disease and urine cadmium
levels were very high (Nogawa et al., 1975). In one of these cases
an autopsy was performed; the liver cadmium level was very high
(75 mg/kg) but the renal cortex cadmium level was low (53 mg/kg)
(Nogawa et al., 1975).
Takebayashi (1980a,b, 1983a,b, 1984) and Takebayashi et al.
(1985, 1987a,b,c, 1988a,b,c,d) reported pathological findings in
kidney and bone from autopsies of eleven elderly men and women (3
males and 8 females; 72-95 years of age) from Tsushima Island. The
average levels of cadmium in the liver and kidney cortex were
92.4 mg/kg and 44.0 mg/kg, respectively. The authors considered the
histological osteomalacia and renal tubulopathy noted in eight cases
(Takebayashi, 1980, 1983a, 1984, Takebayashi et al., 1985, 1987a,b,
1988c,d) to be similar to Itai-itai disease from Toyama Prefecture.
However the Japan Cadmium Research Committee (1989), supported
by the Japanese Environment Agency, concluded, after these eight
cases had been examined by the expert group, that it was clinically
difficult to diagnose them as osteomalacia.
According to the Japan Cadmium Research Committee (1989),
diagnosed cases of Itai-itai disease were reported only in the Fuchu
area of Japan. It denied the presence of osteomalacia in five cases
in the Ikuno area, and stated that osteomalacia had not been
observed "clinically" in Tsushima Island.
A study by Kido et al. (1989) indicates that exposure to
cadmium could cause osteopenia, particularly in women. Bone density
was measured in 28 women with Itai-itai disease, 92 men and 114
women with cadmium-induced renal dysfunctions, and 44 men and 66
women living in three different non-polluted areas using a
microdensitometer. The values of indices corresponding to both
cortical width and bone mineral content were significantly lower in
Itai-itai disease patients than in cadmium-exposed women with renal
dysfunctions or in non-exposed subjects. The cadmium-exposed women
also showed a decrease in bone density compared with the non-exposed
subjects. A significant decrease in bone density was also observed
in cadmium-exposed men compared with non-exposed subjects, although
the difference was not as clear as it was in women.
Reviews (in English) of Itai-itai disease have been produced by
Tsuchiya (1969), Friberg et al. (1974), Tsuchiya (1978), and Nogawa
(1981).
8.2.2.3 Mechanism of cadmium-induced bone effects
The available data show that cadmium can affect calcium,
phosphorous, and bone metabolism in both industrial workers and
people exposed in the general environment. These effects may be
secondary to the cadmium effects on the kidneys but there have been
few studies of calcium metabolism in people with excess exposure to
cadmium. The increased prevalence of renal stones reported from
certain industries is probably one manifestation of the
cadmium-induced kidney effects. It is not known if factors other
than cadmium play a role.
Nogawa et al. (1987) reported that serum 1,25-dihydroxy-
vitamin D levels were lower in Itai-itai disease patients and
cadmium-exposed subjects with renal damage than in non-exposed
subjects. The reduction in these levels was closely related to serum
concentrations of parathyroid hormone and ß2-micro-globulin and to
the percentage tubular reabsorption of phosphate (% TRP), suggesting
that cadmium-induced bone effects were mainly due to a disturbance
in vitamin D and parathyroid hormone metabolism.
Osteomalacia has been reported in a few heavily exposed
industrial workers and people with Itai-itai disease. The industrial
cases are mainly male, whereas Itai-itai patients are almost
exclusively female. However, the clinical features and biochemical
findings are similar, except that Itai-itai patients may also suffer
from ostoporosis.
A possible mechanism for the development of osteomalacia has
been proposed (Kjellström, 1986). It is known that normal calcium
absorption in the intestines and normal bone mineralization is
dependent upon 1,25-dihydroxycholecalciferol. Vitamin D3 taken
into the body is converted to 25-hydroxy-vitamin D3 in the liver,
and then to 1,25-dihydroxy-vitamin D3 in the mitochondria of renal
proximal tubular cells, this being the biologically active species.
Cadmium accumulates in the proximal tubular cells, depressing
cellular functions, and this may result in reduced conversion of
25-hydroxy-to 1,25-dihydroxy-vitamin D3. This is likely to lead to
decreased calcium absorption and decreased mineralization of bone,
which in turn may result in osteomalacia.
8.2.3 Respiratory system effects
Cadmium workers may develop chronic injury to the respiratory
system, depending on the level and nature of exposure. The
development of such effects is often quite slow, so that they are
apparent only after several years of exposure. The rate of
development and severity appear to be roughly proportional to the
time and level of exposure.
8.2.3.1 Upper respiratory system
Chronic inflammation of the nose, pharynx, and larynx have been
reported by Vorobjeva (1958) and Horstowa et al. (1966). Anosmia is
a frequent symptom in cadmium workers after prolonged exposure. This
has been reported by, for instance, Valetas (1946), Friberg (1950),
Baader (1951), Vorobjeva (1958), Tarasenko & Vorobjeva (1973), and
Apostolov (1979), but was not observed by Tsuchiya (1967) or Suzuki
et al. (1965).
8.2.3.2 Lower respiratory system
Chronic obstructive lung disease of varying degrees of severity
is frequently seen in cadmium workers. Friberg (1950) reported
dyspnoea, impaired lung function with increased residual volume, and
reduced working capacity in a group of 43 cadmium workers. Similar
studies, which included the use of pulmonary function measurements,
by Bonnell (1955), Buxton (1956), Kazantzis et al. (1963), and Adams
et al. (1969) all showed impairment of respiratory function in
groups of workers with prolonged exposure. The symptoms and findings
were more suggestive of emphysema than bronchitis in these cases;
they were commonly diagnosed as emphysema but pathological
confirmation of this was rare (Smith et al., l960).
Tarasenko & Vorobjeva (1973) reported the presence of increased
lung markings in the chest X-rays of 17 out of 72 cadmium workers,
which were interpreted as being due to diffuse interstitial
fibrosis. Similar lung changes were observed in 21 out of 26 workers
studied by Horstowa et al. (1966).
The presence of chronic obstructive respiratory disease in
cigarette smokers exposed to an additional harmful environmental
agent presents difficulties in determining the contribution made by
the latter. Studies on the chronic respiratory effects of cadmium in
the past have not always been standardized for smoking. Lauwerys et
al. (1974a) did take smoking habits into consideration by matching
his cadmium-exposed and control groups for smoking habits. They
reported the presence of impaired lung function in a group of
cadmium workers exposed for over 20 years, but not in those with
shorter exposure. The degree of lung impairment found was small.
The effects on the lung increases the mortality of cadmium
workers with high exposures (Kjellström et al., 1979; Armstrong &
Kazantzis, 1983). In the latter study, the mortality for diseases
coded as bronchitis (ICD 490-491) was related to the intensity of
exposure, the group with the highest exposure having a highly
significant (almost 4-fold) excess risk (observed 13 expected 3.4).
A 5-year follow-up of this study (Kazantzis et al., 1988) confirmed
the earlier finding, the marked excess mortality being related to
both intensity and duration of exposure. The follow-up revealed an
excess mortality from emphysema, but this was seen only in the
low-exposure group.
8.2.4 Hypertension and cardiovascular disease
Despite the abundance of data showing that under certain
exposure conditions cadmium induces hypertension in animals, there
are very few results available from studies of cadmium-exposed
workers. Friberg (1950) examined 43 workers with a mean period of
exposure to cadmium oxide dust of 20 years (air concentration,
3-15 mg/m3) and 15 workers with a mean exposure period of 2 years.
The study included physical and roentgenological examinations of the
heart, electrocardiographic examination at rest and after exercise,
and measurement of blood pressure. No increased prevalence of
cardiac disease or pathological electro-cardiographic changes were
found. The majority of subjects had completely normal blood
pressure, but since Friberg did not examine blood pressure in the
control group, it is not possible to draw definite conclusions.
Chest examination and blood pressure measurements have also
been reported in other studies (Bonnell, 1955; Bonnell et al., 1959;
Kazantzis et al., 1963; Holden, 1969), but in no cases were there
findings of cardiac disease or hypertension due to cadmium exposure.
Hammer et al. (1972) found no relationship between exposure to
cadmium and blood pressure in superphosphate workers.
Vorobjeva & Eremeeva (1980) examined 72 female and 20 male
workers at a battery factory exposed to cadmium oxide dust at
concentrations ranging from 0.04 to 0.5 mg/m3. Blood pressure was
measured and electrocardiograms taken, but there was no control
group. The authors reported increased prevalence of hypertension and
absence from work due to hypertensive and ischaemic heart disease
among the exposed workers compared to what was considered normal.
Furthermore, several types of abnormalities in the electrocardiogram
of the exposed workers were observed: 39% showed tachycardia,
between 11 and 13% were regarded as normal, and 26% had changes in
the "R" spike (compared to the normal 7-9%). Increased QRS period
was observed in 45% of the workers compared to the normal values of
14-16%. The data presented in this report are especially interesting
in view of the evidence in rats (section 7.2.6) that suggests
myocardial effects from cadmium exposure. The results of the study
are, however, presented in a very condensed form and it is therefore
difficult to draw clear-cut conclusions.
In a retrospective study of 311 male workers in an alkaline
battery factory it was found that hypertensive workers had a longer
employment time than an age-matched control group from the same work
environment (Engvall & Perk, 1985). Again it is difficult to draw
conclusions from this study. In a study of cadmium-exposed workers
in the United Kingdom (Kazantzis et al., 1988), mortality from
hypertensive disease (ICD 400-404) over the total study period from
1943 to 1988 was elevated but not significantly (49 deaths occurred
as opposed to 41.3 expected). There was no relationship with
intensity of exposure. However, mortality from cerebrovascular
disease (ICD 430-438) was significantly lower (178 deaths occurred
as opposed to 230.3 expected). These findings do not suggest any
association between cadmium exposure and the development of
hypertension.
In contrast, Thun et al. (1989) found that mean systolic and
diastolic blood pressures were higher in 45 cadmium workers (134 and
80 mmHg, respectively) than in 32 male controls (120 and 73 mmHg
respectively). Blood pressure was measured systematically by a
single examiner on the right arm of subjects who had been seated for
at least 15 min. Systolic but not diastolic blood pressure was
significantly associated with cadmium dose in multivariate analyses.
Schroeder (1965, 1967) observed that people in the general
population dying from hypertensive and/or cardiovascular disease had
somewhat higher cadmium concentrations in liver and kidney tissues
than people dying from other causes. He suggested that cadmium could
be a causative factor for these diseases. Unfortunately, smoking
habits were not accounted for and it is likely that this was a
confounding factor. The same problem exists with a number of
subsequent studies on hypertension and cadmium in tissues, blood,
and urine.
A correlation between average air cadmium levels in cities in
the USA and mortality associated with hypertension and heart disease
has been reported (Carroll, 1966; Hickey et al., 1967). Again,
several confounding factors such as smoking habits, air pollutants
other than cadmium, and other environmental factors make it
difficult to draw conclusions concerning the effects of cadmium. In
a study by Staessen et al. (1984), the confounding variables age,
sex, body weight, and cigarette smoking were considered in a
multiple regression analysis of systolic and diastolic blood
pressure and the urinary excretion of cadmium and
ß2-micro-globulin. Negative correlations between blood pressure
and urinary cadmium or ß2-microglobulin were found in some groups.
As there was a very strong age effect on both blood pressure and
urinary cadmium, the meaning of the negative correlations is not
clear. In any case, these data do not support cadmium exposure as a
cause of hypertension.
Shigematsu et al. (1979) could find no evidence that blood
pressure was higher in polluted areas (1611 people sampled) of Japan
compared with control areas (1826 people). In a comparison of blood
pressure by prefecture (13 570 in the cadmium-polluted areas and
7196 in the control areas), the prevalence of hyper-tension was
found to be high in the polluted area of one of the eight
prefectures investigated. However, in the other seven prefectures,
the prevalence of hypertension tended to be lower in the polluted
areas (Japan Cadmium Research Committee, 1989).
In a study on cadmium-polluted areas in Japan by Nogawa et al.
(1981b), the cerebrovascular disease mortality rate among people who
had had cadmium-induced proteinuria was twice as high as that of
people in the same area without proteinuria. However, the difference
was not statistically significant. The number of men in the cohort
with proteinuria was 81 and the number without proteinuria was 1109.
Another study comparing administrative units containing polluted
areas with those without such areas (Shigematsu et al., 1981, 1982,
1983) found no difference in the cerebrovascular disease mortality
rates.
Data on a total population of 333 000 from both
cadmium-polluted and non-polluted areas were collected
retrospectively for a period of 6-30 years, based on vital
statistics or death certificates (Shigematsu et al., 1982). The
mortalities from all causes, including cardiovascular disease such
as cerebrovascular and hypertensive disease, in the general
population in the cadmium-polluted areas were no higher than, or in
some cases even lower than, those in the non-polluted areas.
A mortality study of Shipham residents and of a nearby control
village was reported by Inskip et al. (1982). The study population
consisted of 501 Shipham residents of whom 278 had died over a
40-year follow-up period. Overall mortality was low in both villages
compared generally with England and Wales. There was a small but
statistically significant excess mortality rate in Shipham from
hypertensive and cerebrovascular disease. The highest ratio of all
was for genito-urinary disease in Shipham men (but, with only eight
observed deaths, the result was only significant at the 10% level).
The Standardized Mortality Ratios (SMR) for nephritis and nephrosis
in both sexes were also slightly elevated, but there were only two
deaths for each sex from this cause. In men, the numbers of
prostatic and lung cancer deaths were approximately equal to the
expected numbers, and in neither case was the SMR in Shipham greatly
different from that in the control village.
8.2.5 Cancer
8.2.5.1 In industry
A number of epidemiological studies have been published. In
order to facilitate the interpretation of published data on the
relationship between cadmium exposure and cancer, the studies have
been grouped according to the types of industrial plants in which
they have been conducted. In some cases, more than one study has
been conducted at the same plant.
a) Nickel-cadmium battery plants
In an early study, Potts (1965) found that three out of eight
deaths in a small cohort of nickel-cadmium battery workers in the
United Kingdom with at least 10 years of exposure to cadmium oxide
dust were from carcinoma of the prostate. This study was extended by
Kipling & Waterhouse (1967) to include 248 men with at least one
year of exposure to cadmium oxide dust. Four deaths from carcinoma
of the prostate, including the three cases previously reported by
Potts (1965), were observed as opposed to an expected number of
0.58.
Sorahan & Waterhouse (1983) carried out a further investigation
of the same plant using a cohort of 3025 employees who started work
between 1923 and 1975 and had a minimum employment period of one
month. The method of regression models in life tables was used to
compare duration of exposed employment in those dying from relevant
causes with that of matched survivors in the same year of follow-up.
No new evidence of an association between occupational exposure to
cadmium and cancer of the prostate was found. However, there was an
excess mortality from cancer of the respiratory system significant
at the 5% level (89 cases, SMR = 127). As in other studies, data on
smoking habits were not available and confounding factors were
present in the form of exposure to nickel hydroxide and welding
fumes so that no firm conclusions about the pulmonary
carcinogenicity of cadmium could be drawn from this study.
Sorahan & Waterhouse (1983) reported on the incidence of
prostatic cancer in a subgroup of 458 workers employed for at least
1 year in a job involving high exposure to cadmium oxide dust. Eight
cancers were observed compared to two expected (SMR 400, P < 0.01).
However, exclusion of the four cases previously reported by Kipling
& Waterhouse leaves a non-significant excess incidence (P = 0.21),
from which the investigators concluded that if cadmium oxide is
potentially carcinogenic current risks are likely to be small.
In the most recent update of the nickel-cadmium battery plant
workers (Sorahan, 1987), the earlier findings were confirmed and
there was some evidence of an association between risk of death from
lung cancer and duration of employment in jobs with high or moderate
exposure among workers first employed in the period 1923-1946.
However, among workers first employed from 1947 to 1975 (the group
with the higher SMR for lung cancer), there was no evidence of such
an association. The authors concluded that the findings do not
suggest these nickel-cadmium battery workers had experienced an
elevated lung cancer risk as a consequence of exposure to cadmium
oxide dust.
In Sweden, Kjellström (1979) investigated the incidence of
cancer among 269 male nickel-cadmium battery workers. All workers
had been heavily exposed (on average about 1 mg cadmium/m3) for
five years or more to cadmium dust or fume, and were alive in 1959.
Fifteen workers were found to have cancer between 1959 and 1975. It
was calculated from national incidence rates that 16.4 new cases
would have occurred; only 2 were prostatic cancers while 1.2 were
expected. In a re-examination of the same cohort, there were 8
deaths from lung cancer with a non-significantly raised SMR of 133.
The SMRs increased progressively with increasing latent periods
without reaching statistical significance (Elinder et al., 1985c).
b) Copper-cadmium alloy plants
Copper-cadmium alloy workers in the United Kingdom who had
heavy past exposure to cadmium oxide fume on two sites, one urban
the other rural, were studied by Holden (1980a). There was an
increased lung cancer mortality at the urban site (8 observed versus
4.5 expected) and a significant deficit at the rural site (2
observed; 7.8 expected). Vicinity workers in the urban plant, where
the mean cadmium concentration averaged no more than 60 µg/m3),
also experienced a significantly increased lung and prostatic cancer
mortality (36 observed; 26.1 expected).
A case control study was performed (Kazantzis et al., 1989) in
the same copper-cadmium alloy plants where workers had experienced
heavy past exposure to cadmium oxide fume and dust, which had given
rise to a number of deaths coded as chronic cadmium poisoning.
Before and during the period 1939-1945, cadmium oxide fume levels
had been estimated to be up to 4 mg/m3. Cases and controls were
selected from the cohort previously studied by Holden (1980a).
Personal interviews conducted with a small number of long-term
employees revealed that arsenical copper had been additionally
produced by adding bags of arsenic trioxide to the molten copper and
stirring manually; this resulted in the evolution of dense white
clouds of arsenic fume. The case control study showed no evidence of
an increased risk of lung cancer associated with past cadmium
exposure but an approximately two-fold excess risk associated with
arsenic exposure.
Kjellström (1979) also investigated a cadmium-copper alloy
plant in Sweden where workers had been exposed to cadmium oxide
fumes and included 94 workers employed in 1940 or who started work
after that year. Four cases of prostatic cancer occurred as opposed
to 2.7 expected.
c) Cadmium recovery plant in the USA
An increase in prostatic cancer incidence was also found by
Lemen et al. (1976) in a study of 292 male smelter workers heavily
exposed to cadmium oxide dust or fumes. Air cadmium concentrations
in 1973 were up to 24 mg/m3 but generally below 1 mg/m3. There
were four deaths from cancer of the prostate (1.15 expected). There
were also 12 deaths from lung cancer (5.1 expected); the difference
was statistically significant.
Thun et al. (1985) expanded the Lemen et al. (1976) cohort to
include 602 workers who had been employed at this cadmium production
plant between 1940 and 1969 for at least 6 months. Exposure was to
cadmium in baghouse dust, a by-product of zinc smelting which was
processed to produce cadmium metal and cadmium oxide. The plant
functioned as an arsenic smelter up to the end of 1925, and small
quantities of lead, arsenic, thallium and indium were subsequently
produced at intervals. The vital status of the workers was
determined in 1978. A dose-response relationship was observed
between lung cancer mortality and cumulative exposure and was
statistically significant for workers whose exposure exceeded
2920 mg/m3.days. The SMR for this group was 280. The lung tumours
were, as far as can be determined, mostly of bronchogenic origin.
The authors accounted for smoking habits by obtaining questionnaires
from survivors or next-of-kin in 50% of the cohort members and for
arsenic exposure by measuring arsenic in certain parts of the plant.
d) Cadmium processing plants in the United Kingdom
Kazantzis & Armstrong (1982) and Armstrong & Kazantzis (1983)
investigated a large cohort of workers in England at 17 plants with
processes using cadmium. The cohort comprised 6995 cadmium-exposed
male workers born before 1940, first exposed before 1970, and not
included in any previous mortality study. Jobs were assessed for
each relevant year involving high, medium or low exposure to cadmium
on the basis of discussions with hygienists and employees with
knowledge of past working conditions, taking into account
environmental and biological monitoring data (e.g., cadmium urine
data > 20 mg/litre in the high-exposure group. The periods at risk
of the study population were classified on the basis of these
categories and recorded job histories into three groups: (i) those
workers continuously employed for more than one year in a job
assessed as entailing high exposure - "ever high"; (ii) those
workers continuously employed for more than one year in a job
assessed as entailing medium exposure, but who were never for more
than one year in a high-exposure job - "ever medium", and (iii) all
others. Actual deaths were compared with expected numbers calculated
from mortality rates for the population of England and Wales
corrected for regional variation. The 8th revision of ICD codes was
used and results were expressed as SMRs.
Only 3% of the workers (about 200) were assigned to the "ever
high" category. The mean duration of exposure was 11 years and the
mean interval from initial exposure to the end of the follow-up was
27 years. The SMR (all causes) for the entire population was 97.
There were no prostatic cancer deaths in the "ever high" and "ever
medium" exposure categories (0.4 and 2.5, respectively, expected),
and the number of deaths (23) in the "always low" group was close to
the expected value. There was a small, but not statistically
significant, excess of lung cancer in all categories, but in those
with more than 10 years exposure in the "always low" category this
excess was significant at the 5% level (100 observed, SMR 126).
Since there was no correlation between increase in lung cancer risk
and intensity of exposure, the authors concluded that it was
unlikely that the excess in the "always low" group was due to
cadmium.
A 5-year update of this study (Kazantzis et al., 1988)
confirmed no excess risk from prostatic cancer over the total study
period from 1943 to 1984 and no cases of prostatic cancer in the
medium- or high-exposure groups. The SMR was 99 as opposed to the
value of 90 in the initial study. However, there was now a
significant excess lung cancer mortality (277 observed deaths, 240.9
expected), giving a SMR of 115 (95% confidence interval, 101-129).
This excess risk was related to intensity of exposure, there being
12 deaths in the small high-exposure group as opposed to 6.2
expected (SMR, 194; 95% CI, 100-339), 41 deaths in the
medium-exposure group and 224 deaths in the low-exposure group (SMR
121 and 112, respectively; not significant). While there appeared to
be evidence of a dose-response relationship, it was not
statistically significant. The increased cancer risk mainly involved
those employed before 1940, rising with length of employment and
with length of follow-up.
Further studies have been conducted on workers at these 17
plants (Armstrong & Kazantzis, 1985; Ades & Kazantzis, 1988). A case
control study on lung cancer was carried out on workers in a large
lead-zinc-cadmium smelter. These workers formed 64% of the cohort of
6995 men, and the study included 70% of the lung cancer deaths
observed in the cohort as a whole (Ades & Kazantzis, 1988). There
was a significant excess lung cancer risk among the smelter workers,
and a significant trend with increasing duration of employment,
particularly evident among those employed for more than 20 years.
Quantitative estimates of exposure to cadmium and ordinal rankings
for lead, arsenic, zinc, sulfur dioxide and dust were used to
calculate cumulative exposures from job histories. However, matched
logistic regression analysis showed that the increasing risk of lung
cancer associated with increasing length of employment could not be
accounted for by cadmium exposure and did not appear to be
restricted to any particular process or department.
e) Summary of industrial studies
Increased mortality from lung cancer has been observed in
several occupational cohorts exposed to cadmium, and there is some
evidence of dose-response relationships in two of the examined
populations. Case control studies have not given support for such a
relationship. It is difficult to reach a firm conclusion about
causality, because in all of the occupational cohorts there has been
simultaneous exposure to other potential carcinogens (e.g., nickel,
arsenic, polyaromatic hydrocarbons) or other environmental
pollutants (e.g., sulfur dioxide). Information on tobacco smoking is
inadequate or entirely absent in all except two studies.
Investigations of the relationships between cadmium exposure and
prostatic cancer are inconclusive.
8.2.5.2 In the general environment
Elevated cadmium levels have been found in the liver and
kidneys of patients with bronchogenic carcinoma (Morgan, 1970;
Morgan et al., 1971). However, the authors stressed the possibility
that differences observed could reflect the effect of smoking
(section 5.1.3) or could represent a non specific association.
A study of the causes of death in areas of high cadmium
exposures in Japan (Shigematsu et al., 1982) revealed no difference
in age-adjusted cancer mortality rates between polluted and control
areas of the same prefecture. The mortality rate due to prostatic
cancer was elevated in two areas but only achieved statistical
significance (P < .01) in one area. It was not significant in two
areas including Toyama prefecture, which has the largest area of
pollution.
8.2.6 Mutagenic effects in human cells
An increased frequency of chromosomal aberrations in somatic
human cells is considered to be evidence of some exposure to
mutagenic agents. Shiraishi (1975) noticed an increased frequency of
chromosomal aberrations in lymphocytes obtained from 12 Itai-itai
patients compared to 9 female controls. However, this observation
was not confirmed by Bui et al. (1975) who examined cells from 4
Itai-itai patients and 4 controls.
Among cadmium workers, an increased prevalence of chromosomal
aberrations, compared to controls, was reported by Deknudt & Leonard
(1975) and by Bauchinger et al. (1976), whereas no such effect was
seen by O'Riordan et al. (1978). In none of these occupational
studies was the actual exposure to cadmium measured, and the
possible confounding effect from other industrial chemicals and
smoking was not considered.
Nogawa et al. (1986) did not find evidence for increased sister
chromatid exchange in people exposed to cadmium in the general
environment. IARC (1987a,b) reviewed the available evidence for
mutagenic and related effects and noted the differences in results
reported from different industrial environments.
In conclusion, it is not yet possible to say whether cadmium
causes mutagenic effects in humans.
8.2.7 Transplacental transport and fetal effects
There have been few studies on the fetal toxicity of cadmium
transported across the placenta. Maternal hypertension and decrease
in birth weight have been associated with elevated levels of cadmium
in the neonate (Huel et al., 1981). In addition, it is
well-established that the babies of mothers who are cigarette
smokers are smaller at birth than are those of non-smokers. The
ratio of placental zinc to cadmium is positively related to infant
birth weight in the case of pregnant smokers, and older pregnant
smokers are at higher risk for impaired fetal growth than are
younger ones (Cnattingius et al., 1985; Kuhnert et al., 1987a,
Kuhnert et al., 1987b). Multiparity is related to an increased
placental cadmium level in smokers and to a decreased placental zinc
level in both smokers and non-smokers. These results have been
interpreted as consistent with a depletion of zinc with increasing
number of births and a progressive increase in cadmium in smokers
because of the long half-life of cadmium (Kuhnert et al., 1988).
The cellular mechanisms and factors that influence
trans-placental transport of cadmium are not known. Metallothionein
has been identified in the human placenta and in fetal membranes at
term, and metallothionein synthesis is inducible in cultured
trophoblasts by treatment with cadmium (Waalkes et al., 1984). This
effect is seen with cadmium concentrations in the culture medium as
low as 52.2 µg/litre (0.5 µmol/litre) (Lehman & Poisner, 1984).
Higher levels of exposure to cadmium may have a direct toxic effect
on the placenta. In a test system involving perfusion of maternal
and fetal blood vessels in the isolated human placenta, it was shown
that perfusion of the maternal circulation with cadmium at a
concentration of 1.12 mg/litre (10 µmol/litre) resulted in the
deposition of 2.5 µg cadmium per g placenta (22 nmoles/g), but very
little of it was detectable in the fetal circulation. Perfusion of
the maternal circulation with higher concentrations of cadmium
produced placental cadmium concentrations of 11.2-16.8 µg/g
(100-150 nmoles) with stromal oedema, syncytiotrophoblastic
vesiculation and vacuolization of Hofbauer cells within 6-8 h,
followed by placental necrosis. These changes were associated with a
decrease in human chorionic gonadotropin release and decreased
movement of zinc into the fetal circulation (Miller, 1986).
8.2.8 Other effects
Many other different symptoms and signs have been reported in
humans exposed to cadmium. These include loss of appetite, loss of
weight, fatigue, and increases in the erythrocyte sedimentation rate
(ESR). Valetas (1946) reported details of the poisoning cases in a
French accumulator factory, which were first described by Nicaud et
al. (1942). In addition to the bone effects and the pains (section
8.2.2.1), Valetas mentioned that several workers experienced
paraesthesia and involuntary muscular contractions. This could be an
effect resulting from abnormal changes in the levels of serum
electrolytes, such as calcium or potassium, which may in turn be
caused by severe kidney damage.
Mild anaemia has been more frequently observed among
cadmium-exposed workers than among controls (Friberg, 1950; Bonnell,
1955; Bernard et al., 1979).
More specific effects from cadmium are the yellow discoloration
of the proximal part of the front teeth (Barthelemy & Moline, 1946;
Valetas, 1946; Princi, 1947; Friberg, 1950; Apostolov, 1979) and
anosmia (Friberg, 1950). Anosmia was found by Friberg (1950) in
about one third of a group of workers with a mean exposure time to
cadmium oxide dust of 20 years. Baader (1951) in Germany and
Apostolov (1979) in Bulgaria also noted that anosmia was common
among workers exposed to cadmium oxide dust for long periods of
time. Suzuki et al. (1965) and Tsuchiya (1967) in Japan, found no
increase in the prevalence of anosmia in workers exposed to cadmium
stearate and cadmium oxide fumes.
Nervous system symptoms were reported by Vorobjeva (1957), who
investigated 160 workers at an accumulator factory in the USSR.
Subjective symptoms included headache, vertigo, and sleep
disturbance. Physical examination revealed increases in knee-joint
reflexes, tremor, dermographia, and sweating.
Cadmium sulfide is sometimes used as a yellow tattoo pigment,
which is deposited intradermally. Local phototoxic reactions may
take place when the skin is exposed to ultraviolet light and are
probably connected with the marked photoconducting properties of
cadmium sulfide. Of 24 patients with yellow tattoos who were
examined by Bjornberg (1963), 18 experienced skin swelling when
exposed to sunlight.
8.3 Clinical and epidemiological studies with data on both exposure
and effects
There are several clinical and epidemiological studies with
data on occupational or general environment exposure levels, but the
data concerning effects are restricted to the lungs and kidneys.
8.3.1 Studies on respiratory disorders
Friberg (1950) studied 43 male workers exposed to cadmium oxide
dust, with an average period of employment of 20 years (range 9-34
years), and 15 workers who had been employed for only 1-4 years.
They were compared with a group of 200 sawmill workers. Shortness of
breath was the common symptom among the workers with long exposure,
and an impairment of lung function (increased residual capacity in
relation to total lung capacity and a decreased working capacity)
was demonstrated. The lung function of the group with short exposure
(less than 5 years) was found to be normal. The cadmium
concentration in air varied from 3 to 15 mg/m3, measurements
having been made at five places on only one occasion. In another
battery factory (where air cadmium concentrations were
0.05-5 mg/m3, Adams et al. (1969) found a slight average decrease
in forced expiratory volume in a group of 27 male workers.
Twelve out of 96 cadmium workers exposed for up to 27 years to
cadmium oxide fume in two cadmium-copper alloy factories were found
to suffer from emphysema, as evaluated from a comprehensive lung
function test (Bonnell, 1955; Buxton, 1956; Kazantzis, 1956). These
workers were compared with a similar size control group. The average
air cadmium concentrations in the two factories were 40-50 µg/m3,
and 90% of the particles were less than 0.5 µm in diameter (King,
1955).
Lauwerys et al. (1979a) studied pulmonary ventilatory function
in three groups of workers exposed to cadmium oxide dust and in
matched control groups (the matching included smoking habits). A
slight but significant reduction in forced vital capacity, in forced
expiratory volume in one second, and in peak expiratory flow rate
was found in 22 men. These were all smokers and had been exposed for
more than 20 years to a time-weighted average air concentration of
66 µg/m3 (21 µg/m3 respirable cadmium). In another group of
workers (smokers and non-smokers) exposed for 1-20 years to an
average concentration of 134 µg/m3 (the respirable cadmium level
at the most polluted work site was 88 µg/m3), the pulmonary
indices were on average lower than in the control group, but the
differences were not statistically significant. A more thorough
examination of a subgroup of the workers with long exposures carried
out by the same research group (Stanescu et al., 1977) found more
respiratory symptoms in the cadmium-exposed group than in a control
group and also impaired lung function (not statistically
significant). However, Lauwerys et al. (1979a) reported more
extensive data from the same plants and found that the workers with
less than 20 years of exposure (average 7.5 years) showed
significant effects in the lung function tests.
Reduced forced vital capacity was also found at a cadmium
production plant in the USA (where the air concentrations were
"commonly greater than 200 µg Cd/m3") among 17 workers exposed for
more than 6 years (Smith et al., 1976). De Silva & Donnan (1981)
provided evidence that insoluble cadmium compounds may induce
emphysematous changes after more than 7 years exposure to a
time-weighted average cadmium concentration of 700 µg/m3.
Edling et al. (1986) found no lung function differences between
an exposed group of workers using cadmium-containing solders and a
control group. The level of exposure, which lasted for several
years, was estimated to be 0.05-0.5 mg cadmium/m3, but the workers
had not been exposed to cadmium for several years. Of the 57 workers
examined, 42% had cadmium-induced renal tubular dysfunction.
Davison et al. (1988) examined 101 male workers, who had worked
for 1 year or more manufacturing copper-cadmium alloys, and found,
compared with a reference group, impaired lung function. They also
compared certain parameters (transfer coefficient: KCO) with the
estimated cumulative exposure index for cadmium workers with 95%
confidence limits for the regression line. Among 35 workers exposed
for more than five years and with a cumulative cadmium exposure
index up to 14 mg/m3.years, there was no evidence of a threshold.
The authors concluded that inhaled cadmium fumes caused changes in
lung function and in chest radiographs consistent with emphysema.
This could also explain the increased mortality reported. The
impaired lung function was also related to liver cadmium levels as
measured with neutron activation in vivo.
Some studies on respiratory disorders have yielded negative
results. However, some of these studies did not use lung function
tests (Hardy & Skinner, 1947; Princi, 1947; Tsuchiya, 1967; L'Epee
et al., 1968) and another did not use a control group (Teculescu &
Stanescu, 1970). Suzuki et al. (1965) examined a group of workers
exposed for a short period (average 3.3 years) to 30-690 µg
cadmium/m3 (as cadmium stearate) and found no changes in lung
function when compared to a control group.
In summary, it is clear that exposure to cadmium dust and fume
over prolonged periods can give rise to impaired lung function and
emphysema. Such effects have been seen predominantly at high air
cadmium concentrations (above 100 µg/m3), but one study showed
effects after more than 20 years of exposure to respirable cadmium
oxide dust concentrations of 21 µg/m3.
Cadmium workers sometimes suffered from symptoms such as
coughing and throat irritation, but did not show abnormal chest
X-ray findings when exposed to cadmium oxide fume at a concentration
of 100 µg/m3 for 4-8 years (Hardy & Skinner, 1947) or
40-1440 µg/m3 for 8 years (Princi, 1947), or to cadmium oxide dust
at a concentration of 64-241 µg/m3 for up to 15 years (Tsuchiya,
1967).
8.3.2 Studies on renal disorders in industry
Friberg (1950) reported that prolonged exposure gave rise to
renal damage among a large group of workers exposed to cadmium oxide
dust at concentrations of 3-15 mg/m3 in an accumulator factory. In
one group of 43 workers with a mean exposure period of 20 years
(range 9-34 years), a high prevalence of proteinuria was
demonstrated by the nitric acid and trichloroacetic acid test. In
several of the workers, the renal damage was also manifested by a
decreased inulin clearance and decreased concentrating capacity.
Another group of 15 workers with a mean exposure period of 2 years
(range 1-4 years) showed no positive reactions.
Since 1950, there have been many studies on proteinuria among
workers in various industries. This type of proteinuria is
characterized particularly by a great relative increase in the
excretion of low molecular weight (LMW) proteins (section 8.2). In
most of the early studies, qualitative tests for detecting
proteinuria were used, but, more recently, specific methods for the
quantitative determination of LMW proteins have been developed.
Table 16 contains data on the prevalence of proteinuria from
several epidemiological studies on cadmium workers. It must be
recognized that, in most studies, the dose measurements are based on
short sampling periods (hours or a few days), whereas exposure may
have been for decades. Information on sampling method (static or
personal) and the use of respirators is usually inadequate, which
makes accurate dose estimates difficult (section 2.2.1).
It is evident that the prevalence of proteinuria in cadmium
workers increases with exposure intensity duration. The study by
Kjellström et al. (1977a) presents frequency distributions of
urinary ß2-microglobulin excretion for 240 exposed workers and a
control group. There is a general shift to higher excretion levels
among the exposed workers, and a large proportion of them have
excretion levels far outside the control distribution. Any cut-off
point (operational definition) for "abnormal" proteinuria is
arbitrary. If a cut-off point of 290 µg/litre (corresponding to the
97.5 percentile of the control group) is chosen, 26% of the whole
group of exposed workers would be classified as having LMW
proteinuria. If higher cut-off points are used, the prevalence of
proteinuria will obviously be lower.
Table 16 provides evidence of a dose-response relationship. The
lowest "dose" that gave rise to a statistically significant increase
in urinary ß2-microglobulin, as defined above, was a 6-to 12-year
exposure to 50 µg cadmium/m3 (based on personal sampling)
(Kjellström et al., 1977a).
Järup et al. (1988) recently made a reassessment of
dose-response in the same battery plant (Table 16). A pattern very
similar to Kjellström et al. (1977a) was observed with a prevalence
of ß2-microglobulinuria of 4% at a cumulative dose of 0.5 mg/m3
(corresponding to 10 years of exposure to 50 µg cadmium/m3).
Lauwerys et al. (1979a,b) studied the prevalence of increased
ß2-microglobulin clearance (cut-off point: 97.5 percentile of
controls) and found a 21% prevalence after more than 20 years of
exposure to 66 µg cadmium/m3 total dust (static samples) or 21 µg
cadmium/m3 respirable dust (Table 16).
Holden (1980b) measured urine levels of ß2-microglobulin and
found dose-response relationships using cut-off points of
200 µg/litre, 1000 µg/litre, or 10 000 µg/litre. The cut-off point
of 200 µg/litre gave a 16% LMW proteinuria prevalence rate after
6-10 years of exposure (Table 16).
Table 16 also shows that an increased prevalence of total
proteinuria, as measured by sulfosalicyclic acid, trichloroacetic
acid, or quantitative determination of total proteinuria, occurs
after 5-10 years exposure to approximately 100 µg cadmium/m3. An
increased excretion of LMW protein (e.g., ß2-microglobulin) occurs
at much lower doses.
The in vivo measurement of cadmium in the liver and kidneys
of people with various levels of cadmium exposure provides a means
for relating organ dose to effects and response rates (section
6.4.2). Some questions still remain regarding the accuracy of the
analytical method (section 2.2.3.4) and the mathematical-statistical
methodology (Kjellström et al., 1984). Nevertheless, Roels et al.
(1983a) and Ellis et al. (l984) suggested that renal tubular damage
would be experienced by about 10% of people with a kidney cortex
level of 200 mg cadmium/kg, and by about 50% of people with a kidney
cortex level of 300 mg cadmium/kg.
Table 16. Prevalence of proteinuria in cadmium workers
Cadmium Estimated air Exposure No. of Prevalence of Proteinuria and Reference
compounds concentrations period examinees proteinuria (%) characteristics of
(µg/m3)a (years)b detection
methode
Cadmium oxide 40-50 control 60 2 SA and TCA Bonnell (1955); King (1955);
fume 1-9 37 24 Bonnell et al. (1959)
> 9 63 46
64-241c control 11 0 TA Tsuchiya (1967)
< 1 4 0 < 100 mg/litre
1-4 4 50
123c > 5 4 100
(time-weighted
average
Cadmium oxide 3000-15 000 1-4 15 0 nitric acid ("Hellers Friberg (1950)
dust 9-15 12 33 test"); positive in
16-22 17 41 more than half the
23-34 14 64 test
31 (1.4)c control 31 0 Abnormal Lauwerys et al. (1974a)
1-12 (4) 31 0 electrophoretic
control 27 4 pattern as defined
by the authors;
ß2-microglobulin
clearance
134 (88)c 0.6-19.7 (9) 27 15
control 22 0
66 (21) 21-40 22 68
66 (21) > 20 42 21
Table 16 (contd).
Cadmium Estimated air Exposure No. of Prevalence of Proteinuria and Reference
compounds concentrations period examinees proteinuria (%) characteristics of
(µg/m3)a (years)b detection
methode
Cadmium oxide
dust
control 87 3.4 ß2-microglobulin Kjellström et al. (1977a)
50c 0-3 50 6.0 (RIA)
50c 3-6 30 6.6 > 290 µg/litre
50c 6-12 21 19.0 (sg = 1.023)
Cadmium stearate 30-690 control 24 17 TCA Suzuki et al. (1965)
dust (3) 19 58
Cadmium sulfide 114d < 1-5 12 17 EP Harada (1987)
dust 5-21 7 100
< 1-5 12 8 TCA
5-21 12 43
control 203 1 ß2-microglobulin Stewart & Hughes (1981)
80 0-5 105 0 (RIA)
100 6-11 41 0 > 765 µg/litre
(sg = 1.016)
100-600 11-19 13 7.7
100-600 20+ 14 57.0
Mixed exposures; not reported, controls 642 0 (0.8) ß2-microglobulin Holden (1980b)f
12 factories; but mean blood < 18 months 121 0 (10) (RIA)
mainly zinc cadmium level 19 months-5 years 168 1.8 (8.3) > 1000 µg/litre
smelters after 1 year 6-10 170 1.8 (16) (or > 200 µg/litre)
exposure was 11-15 82 7.3 (22)
about 16-20 33 24 (45)
15 µg/litre
20+ 68 25 (56)
Table 16 (contd).
Cadmium Estimate of cumulative Number of Prevalence (%) Proteinuria and Reference
compounds dose (mg/m3.year) examinees of proteinuria characteristics of
detection method
Cadmium fume < 1 16 18 ß2-microglobulin Elinder et al. (1985b)
(oxide) 1-2 22 32 > 0.034 mg/mmol creatinine
2-3 9 44
3-5 8 62
> 5 5 100
< 1 ) 6 Mason et al. (1988)
1-15 ) 67
1.5-3) 75 58
3-5 ) 86
> 5 ) 100
Cadmium oxide < 0.4 264 1.1 Jarup et al. (1988)
0.4-1.7 76 9.2
1.7-4.6 43 23.3
4.6-9.6 31 32.3
9.6-15 16 32.2
> 15 10 50
a Numbers in parentheses refer to average concentrations of the respirable particulates (< 5 µm) fraction
b Numbers in parentheses are average values
c Measured in breathing zone by personal sampler
d Calculated average exposure for the worker with the most pronounced effect
e SA = sulfosalicyclic acid method (qualitative); TCA = trichloroacetic acid method (qualitative);
TA = tungstate method (quantitative); EP = electrophoresis; RIA = radioimmunoassay; sg = specific gravity
f Values for prevalence of proteinuria refer to a ß2-microglobulin level of > 1000 µg/litre.
Values in parentheses refer to a level of > 200 µg/litre.
Ellis et al. (1985) correlated time-weighted exposure indices
(TWE), based on employment records, area monitoring techniques and
personal sampling, with body burden of cadmium measured by
in vivo neutron activation analysis of the liver and left kidney
in 82 men exposed to cadmium dust in a smelter. The workers were
grouped as follows: production workers (40 active, 21 retired);
office and laboratory workers (8 active, 4 retired); and
non-production workers (3 active, 6 retired). From these
measurements the authors were able to estimate the probability of
developing kidney dysfunction based on the workers' cumulative
exposure index. When the exposure limit was 400-500 µg/m3.years,
the prevalence for renal dysfunction was about 32%; it was 22-40%
with a wider exposure index (300-600 µg/m3). Kjellström et al.
(1977a) reported a prevalence of 19% at a battery factory at a level
of 50 µg Cd/m3, which resulted in a similar exposure index.
Lauwerys et al. (1974a) observed proteinuria in 68% of workers with
long-term exposure (20 years at 66 µg/m3), whereas the logistic
model developed by Ellis et al. (l985) would have predicted 65%
under these exposure conditions.
For exposures of 250 µg/m3.years there is a 19% probability
of experiencing renal dysfunction. In the case of workers with
normal renal function, an exposure index of 400 µg/m3 predicts a
mean cadmium concentration of 28 mg/kg in liver and 288 mg/kg of
renal cortex. The model would predict that a 10-year exposure to
25 µg/m3 would result in a mean renal cortex concentration of
252 mg/kg, which is similar to the critical concentration defined by
Friberg.
Thun et al. (1989) assessed the quantitative relationship
between exposure to airborne cadmium and various markers of renal
tubular and glomerular function in 45 male workers at a plant that
recovered cadmium from industrial waste. The dose was estimated from
historical air sampling data. In this study, the "critical dose" of
cadmium necessary to induce nephropathy was based on the 5th or 95th
percentile of test results in the unexposed population. Using this
definition, renal tubular dysfunction sharply increased as
cumulative exposure to cadmium rose above 300 mg/m3.days
(corresponding to about 0.8 mg/m3.years). Very similar
dose-response curves with an increased prevalence of
ß2-microglobulinuria at cumulative exposure levels exceeding about
0.5-1 mg cadmium/m3.year have been reported from examinations of
workers exposed to cadmium fumes (Elinder et al., 1985b; Mason et
al., 1988). These findings are consistent with the recommendations
by a working group of the World Health Organization (WHO, l980) to
limit workplace exposures to 10 µg/m3 in order to prevent tubular
proteinuria after life-time occupational exposure to cadmium.
Cumulative cadmium exposure indices have been calculated for 75
cadmium alloy workers employed for periods of up to 39 years,
together with the in vivo liver and kidney cadmium burden (Mason
et al., 1988). Several indicators of both tubular and glomerular
dysfunction correlated significantly with both cumulative exposure
index and liver cadmium burden. Using these estimates of dose, a
two-phase linear regression model was applied to identify an
inflection point of the order of 1100 µg/m3.years above which
changes in renal function occurred. A number of biochemical
variables fitted this model, including total protein, albumin, and
ß2-microglobulin. Simple dose-response analysis showed a greatly
increased incidence of tubular proteinuria when the cumulative
cadmium exposure index was greater than this value. The cumulative
exposure index was equated to about 20 to 22 years of exposure to a
cadmium level of 50 µg/m3.
Evidence of tubular damage was investigated in a group of 91
cadmium workers subjected to yearly estimation of cadmium
concentration in blood and urine over a period of eight years. In
workers with blood and urine cadmium levels constantly below the
Biological Limit Value of 10 µg/litre, the prevalence of tubular
damage, as indicated by an increased excretion of ß2-microglobulin
above 260 µg/litre, was below 3%. RBP excretion confirmed this
pattern.
8.3.3 Studies on renal disorders in the general environment
8.3.3.1 Health surveys in Japan
Following the recognition of the association between Itai-itai
disease and exposure to cadmium (Japanese Ministry of Health and
Welfare, 1968), additional general surveys of cadmium pollution were
performed in Japan, and further areas were found to be involved.
Health effects were studied first among the population in the area
where Itai-itai cases had occurred and later in other areas found to
be contaminated. The original studies were designed to find cases of
Itai-itai disease, but it was possible also to estimate the
prevalence of proteinuria and glucosuria in the examined population.
A detailed description of the methods used in these cadmium
pollution surveillance programmes has been reported by Shigematsu et
al. (1979).
At the time of the first surveillance programmes (1969-71),
methods were developed for estimating the degree of contamination
with cadmium and the total daily intake. At the early stage of the
investigations, the most common index of cadmium intake measured in
all areas was the cadmium concentration in rice. From data of the
Japan Public Health Association (1970), it was estimated that, in
areas with different exposure levels, an average of almost 50%
(range 14-71) of the daily cadmium intake came from rice. In one
area, the proportion was estimated to be 85% (Kawano & Kato, 1978).
The average cadmium concentration in rice from non-polluted
areas has been reported to be 0.066 mg/kg in polished rice
(Moritsugi & Kobayashi, 1964) and 0.09 mg/kg in unpolished rice
(Japanese Ministry of Agriculture and Forestry, 1973) (section
5.2.1). The national average consumption of rice was 364 g per
person in 1961, 308 g in 1971, and 222 g in 1981 (Japanese Ministry
of Health and Welfare, 1983).
Proteinuria was generally estimated with qualitative methods
such as the sulfosalicylic acid method or the trichloracetic acid
method according to standardized techniques (Japanese Ministry of
Health and Welfare, 1971). More recently, emphasis has been placed
on the identification of the urinary protein pattern, in particular
to detect early evidence of tubular dysfunction. The methods
currently used are electrophoresis and the quantitative
determination of lysozyme, RBP, and ß2-microglobulin. Several
studies have been published, and there are extensive reviews in
English (Tsuchiya, 1969; Yamagata & Shigematsu, 1970; Friberg et
al., 1974; Tsuchiya, 1978; Shigematsu et al., 1979, l980).
From 1976 to 1984, epidemiological health surveys of residents
in areas with environmental cadmium pollution were performed by the
Japan Environment Agency using methods including immunological tests
for the detection of low molecular weight proteinuria in eight
prefectures (Akita, Fukushisuma, Gunma, Toyama, Ishikawa, Hyogo,
Nagasaki, Oita). More than 13 000 inhabitants of polluted areas and
more than 7000 inhabitants of non-polluted areas, aged 50 years or
more in both areas, were subjected to these surveys.
The following screening method was adopted for health
examinations. The urine of those people with proteinuria
(demonstrated by a semiquantitative method) and/or glucosuria (by a
paper test) was analysed for ß2-microglobulin (> 10 mg/litre),
RBP (> 4 mg/litre), lysozyme (> 2 mg/litre), total amino acid
nitrogen (> 20 mmol/litre), and cadmium (> 30 µg/litre). Those
who exceeded the above levels in more than one item were tested for
renal function by urine and blood analysis. Finally those for whom
the TRP was less than 80% were subjected to detailed health
examination, including skeletal radiography, in order to make a
clinical diagnosis (Fig. 7).
With the exception of Oita prefecture, the number of
individuals who had or were suspected of having proximal renal
tubular dysfunction (as defined by the Japanese Cadmium Research
Committee) or related findings tended to be greater in the polluted
areas than in the non-polluted areas, and this was often
significantly related to the degree of pollution (see Table 16).
This suggests that environmental cadmium pollution is associated
with the occurrence of proximal renal tubular dysfunction.
There are no available quantitative human data to indicate the
net gastrointestinal excretion.
Cadmium is also eliminated through hair (Anke et al., 1976) and
breast milk (Schroeder & Balassa, 1961), but these routes are of
limited importance for total excretion and do not significantly
alter the biological half-time.
6.6 Biological half-time and metabolic models
6.6.1 In animals
Several studies have been carried out in order to assess the
biological half-times of cadmium in experimental animals. Various
animals species, including mice, rats, rabbits, dogs, and monkeys,
have been studied, and single exposures have normally been used. The
reported half-times have varied from weeks to two years (or as long
as half the life-span of the animal). The development of metabolic
models has shown that the body contains several compartments for
cadmium accumulation, each with a different half-time. Thus, in
whole body half-time measurements, one may find several different
half-times. In order to observe the slowest half-time components, it
is necessary to study the animals for many months.
The biological half-time of cadmium in the kidney and whole
body decreases when renal tubular dysfunction occurs because of
increased urinary excretion (section 6.5.1). However, some studies
have indicated that the biological half-time may change with dose
and body burden of cadmium even before renal damage occurs. For
instance, Engstrom & Nordberg (1979a) reported that in mice
half-time increased with increasing single or repeated oral dose. In
these studies, body burden and renal burden were considerably lower
than the maximum that can be reached in long-term exposure. Nomiyama
(1978) reported that half-time decreased with increasing dose when
animals with the shortened half-time had reached the maximum renal
burden. Even shorter half-times were reported when renal tubular
dysfunction occurred after exposure to high doses (Nomiyama, 1978).
A number of studies on biological half-time and metabolic
models for animals have been reviewed by Friberg et al. (1974) and
Nomiyama (1978).
The wide difference in the results obtained by investigators
may be explained by variations in exposure level and type, the
different animal species used, and interactions between cadmium and
other exposure factors. Reported half-times range from several weeks
in mice to 22 years in monkeys (Friberg et al., 1974; Nomiyama et
al., 1979; Nomiyama et al., 1984). The variations in half-times in
specific tissues between different species or individuals may be due
to variations in the production of metallothionein, which binds
tissue cadmium and contributes to its retention.
6.6.2 In humans
Experimental and epidemiological evidence indicates strongly
that the biological half-time in the whole body is extremely long
(many years). Experimental evidence from one study (Shaikh & Smith,
1980), in which one subject was given radioactive cadmium and
examined periodically for the next 2 years, showed a biological
half-time of 26 years. In three similar studies, in which a small
number of subjects were followed up for a limited period (about 100
days), half-times of 93-202 days were reported (Rahola et al., 1972;
Flanagan et al., 1978; McLellan et al., 1978). Only one of these
studies (Rahola et al., 1972) gave confidence limits for the
estimated biological half-time (130 days to infinity).
Another approach to estimate the half-time used involves
comparing total daily excretion with total body burden, applying a
one-compartment model to the body as a whole (Friberg et al., 1974;
Task Group on Metal Toxicity, 1976). A further approach analyses the
accumulation in the kidney using a one-compartment model taking into
consideration age-related variations in daily cadmium exposure and
kidney weight (Tsuchiya & Sugita, 1971; Kjellström, 1971). More
recently, an elaborate model has been developed that includes
separate compartments for, for instance, kidney, liver, and blood,
and incorporates age-related variations in daily intake, tissue
weights, and renal function (Kjellström & Nordberg, 1978).
These models rely on many assumptions concerning the cadmium
concentration in food, calorific intake, absorption rates, and other
factors. Inevitably, the data produced by these models are only
tentative, but they are important for future research. Using data
from Japan on the accumulation of cadmium with age, Tsuchiya et al.
(1976) used a series of one-compartment mathematical models
developed by Tsuchiya & Sugita (1971) to estimate biological
half-times of cadmium in various organs. These authors estimated the
biological half-time in the kidneys to be about 17 years and that in
the liver 7 years.
Elinder et al. (1976) used autopsy data from non-smokers in
Sweden and a one-compartment model (Kjellström, 1971) to estimate
the biological half-time in the renal cortex. They assumed that the
daily intake of cadmium had doubled in 50 years (Kjellström et al.,
1975a) and estimated the half-time to be 20-50 years (30 years being
the best estimate).
Using an 8-compartment model (Kjellström & Nordberg, 1978), the
biological half-times of cadmium in the liver and kidney were
estimated to be 7.5 and 12 years, respectively (Kjellström &
Nordberg, 1978). The longest half-time was calculated for the "other
tissues" compartment. This included muscle tissue, which was found
to have the longest half-time in an autopsy study (Kjellström,
1977). However, it should be pointed out that when using this type
of model to simulate the chemobio-kinetics of cadmium, the
individual half-times of different tissues are less important than
the dynamics of the model as a whole.
After high cadmium exposure, as occurs among certain industrial
workers, the biological half-time may not be the same as that during
normal exposure in the general environment. Current models, however,
do not consider this factor. If the exposure level is very high, the
ratio of rapid components to slow components may be altered. An
example of this is the very high urinary excretion found after only
a short exposure to high air cadmium levels (Lauwerys et al., 1976,
1979b). The biological half-time is also shorter if there is renal
tubular dysfunction. Fletcher et al. (1982) carried out
in vivo neutron activation analysis of the liver of 13 cadmium
workers twice within a period of 3 to 4 years (the occupational
cadmium exposure of these workers had ceased before the first
analysis). Three workers showed proteinuria and an average cadmium
half-time in the liver of 2 years. The other 10 workers had an
average half-time of 6.4 years, nine of whom had an average
half-time of 13.5 years and no proteinuria.
Jarup et al. (1983) studied the half-time of cadmium in blood
of five smelter workers who had previously experienced high cadmium
exposure. Repeated blood analysis carried out over a 10-to 13-year
period revealed short-term (75-128 days) and a long-term (7.4-16
years) half-time components. The long-term component in two workers
with proteinuria was shorter than in the other workers.
6.7 Biological indices of cadmium exposure, body burden, and
concentrations in kidneys
There is no easy way to measure directly the whole body burden
or concentrations of cadmium in different tissues of a living
person. In vivo neutron activation methods have been used in
special circumstances (Ellis et al., 1981a; Roels et al., 1981b;
Tohyama et al., 1981a).
At present, it is necessary to study concentrations in easily
available indicator media in order to evaluate exposure and
accumulation of cadmium. The suitability of certain indicator media
for such purposes is supported by studies on both animals and
humans; urine, blood, faeces, and hair have all been used as
indicator media. Methods for the biological monitoring of cadmium
levels in blood and urine have been reviewed by Nordberg & Nordberg
(1988) and WHO (1980).
6.7.1 Urine
The human and animal studies summarized in section 6.5.1 allow
the following interpretation of the significance of cadmium in urine
(Lauwerys et al., 1980b). In the absence of episodes of high-level
exposure to cadmium and provided that cadmium-binding sites in the
organism are not saturated and cadmium-induced nephropathy has not
yet occurred, the urine cadmium level increases in proportion to the
amount of cadmium stored in the body. In such situations, which
prevail mainly in the general population and in workers moderately
exposed to cadmium, there is significant correlation between urinary
cadmium and cadmium in kidney. Episodes of high exposure to cadmium,
however, may lead to a transient increased urinary excretion.
If exposure to cadmium has been excessive, the cadmium-binding
sites in the organism become progressively saturated and, despite
continuous exposure, the cadmium concentration in the renal cortex
tends to plateau. Once this point is reached, the cadmium that is
still absorbed cannot be further retained in the kidney and is
rapidly excreted in the urine. Under these conditions, urinary
cadmium is also influenced by the recent intake. The relative
influence of the body burden and the recent exposure on urinary
cadmium depends on the exposure intensity. If exposure continues, a
certain percentage of individuals may develop renal damage. This is
associated with a progressive loss of cadmium accumulated in the
kidney, which gives rise to a further increase in urinary cadmium.
Eventually, the amount of cadmium that can be released from the
kidney decreases progressively and the urinary cadmium concentration
follows the same trend. The changes in the urinary metallothionein
level parallel those of cadmium (section 6.8.2).
In summary, several factors (duration and intensity of exposure
to cadmium, the presence of renal dysfunction and its duration) must
be taken into consideration when interpreting urinary cadmium (and
metallothionein) levels.
6.7.2 Blood
Plasma cadmium levels are considered to be related to recent
exposure but are often so low that they cannot be measured routinely
(section 6.2). Most of the cadmium in the blood is in the
erythrocytes.
Cadmium levels in whole blood mainly reflect the exposure
during recent weeks or months. In cadmium workers, the level
increases markedly within the first few months after occupational
exposure starts (Kjellström & Nordberg, 1978; Lauwerys et al.,
1979b). It is probable that a portion of the blood cadmium level
reflects body burden rather than present exposure in view of the
known transport of cadmium via blood from the liver to the kidneys
and other tissues (section 6.2) and the long-term half-time
component demonstrated by Jarup et al. (1983). Workers with
relatively long exposure durations but whose cadmium exposure has
ceased have elevated blood cadmium levels for several years (Friberg
et al., 1974; Jarup et al., 1983).
Reports of blood cadmium levels in the general population have,
in the past, often been unreliable, owing largely to the
difficulties encountered in the analysis of blood cadmium (Lauwerys
et al., 1975). However, improvements in analytical techniques have
since been achieved through biological standards for blood and
systematic quality assurance programmes (Stoeppler et al., 1979;
Vahter, 1982). Average blood cadmium values up to 10 µg/litre or
more have been reported in the past, but the analytical procedures
used mean that the accuracy of these data is in doubt (Vahter, 1982;
Friberg & Vahter, 1983).
Various aspects of blood cadmium analysis have been discussed
in a UNEP/WHO study, which also included a quality assurance
programme and data from 10 countries (Vahter, 1982; Friberg &
Vahter, 1983). It was found that even in the countries with the
highest blood cadmium levels, the average was less than 4 µg/litre.
Furthermore, smokers were found to have higher values than
non-smokers, and non-smokers, in most countries, had mean levels
below 1 µg/litre. Although only slightly above 1 µg/litre, the
levels for non-smokers in Japan were about twice as high as the
levels in the USA, which probably reflects the difference in average
daily cadmium intake via food (section 5.2.2).
Reported cadmium concentrations in the blood of exposed workers
are generally between 5 and 50 µg/litre, but levels of between 100
and 300 µg/litre have resulted from extreme exposures (Roels et al.,
1982; Hassler et al., 1983).
6.7.3 Faeces
Gastrointestinal absorption amounts to only a few percent of
the cadmium ingested daily (section 6.1.2), and the quality of
cadmium excreted gastrointestinally is small compared to the
unabsorbed portion of ingested cadmium. Thus, daily faecal cadmium
can serve as a good indicator of the daily amount of cadmium
ingested via food and water or cleared from the lungs after
occupational exposure to large dust particles. Faecal cadmium
correlates very closely with daily energy intake (section 5.2.4),
and average cadmium intake estimates for different countries agree
well with reported average faecal cadmium amounts.
A portion of the cadmium in human faeces is related to the body
burden (section 6.5.2). In workers with high body burdens but low
daily cadmium intakes via food, this portion might be greater than
the unabsorbed part of ingested cadmium, because the faecal
excretion is similar to the urinary excretion.
6.7.4 Hair
Cadmium in hair is not a reliable indicator of either recent
exposure or body burden. The main problem is external contamination
of the hair, which cannot be distinguished from the endogenous
cadmium (Nishiyama & Nordberg, 1972). However, a correlation was
found between air cadmium levels of cities in the USA and cadmium
levels in the hair of 10-year-old children living in these cities
(Hammer et al., 1971). In a study of cadmium workers (Ellis et al.,
1981b), a higher average hair cadmium level was found among exposed
workers than among controls, but there was a poor relationship
between hair cadmium and cadmium in the blood, urine, liver or
kidney. Cadmium concentrations in the hair of people without
excessive exposure are usually between 0.5 and 2 mg/kg.
6.8 Metallothionein
6.8.1 Nature and production
Metallothionein is a metal-binding protein of low molecular
weight, which has a key role in the metabolism of cadmium. It is
rich in cysteine but contains no aromatic amino acids or histidine
(Kagi & Vallee, 1960, 1961).
This protein was identified for the first time by Margoshes &
Vallee (1957) in horse kidney cortex. Its molecular weight is about
6600 (6000 for the apoprotein moiety, thionein), and it has a
non-globular shape. On gel filtration, however, it moves like a
spherical protein with a molecular weight of about 10 000 (Kagi &
Nordberg, 1979). There have been several reports dealing with the
function and biochemistry of metallothionein (Kagi & Nordberg, 1979;
Brady, 1982; Foulkes, 1982; Webb & Cain, 1982; Kagi & Kojima, 1987).
Piscator (1964) suggested that metallothionein played a role in
cadmium transport and detoxication, and it has subsequently been
identified in human kidney and liver (Pulido et al., 1966; Chung et
al., 1986) as well as in those of various experimental animals (Kagi
& Nordberg, 1979).
The structure and genetic expression of mouse and human
metallothionein have now been identified. Two major forms of
metallothionein are present in most mammalian tissues, particularly
liver and kidney, i.e. metallothionein I (Mt-I) and metallothionein
II (Mt-II). Induction of synthesis is under the control of a large
group of genes and is stimulated by glucocorticoids and the
essential metals zinc and copper, as well as by the toxic metals
cadmium and mercury (Karin et al., 1981; Karin & Richards, 1982).
In vitro binding affinities have been demonstrated for a number of
other toxic metals, including bismuth, cobalt, silver, and gold
(Cherian & Nordberg, 1983). Metallothionein binds seven metal ions
per molecule between two separate metal-cysteine clusters, and a
single molecule may contain more than one metal, e.g., cadmium and
zinc, mercury and copper.
6.8.2 The role of metallothionein in transport, metabolism, and
toxicity of cadmium
Piscator (1964) suggested that some of the cadmium-binding
metallothionein in the liver may migrate into the blood stream. As
discussed in section 6.2, part of the plasma cadmium in animals
exposed for a long time to cadmium is bound to a protein with the
same molecular weight as metallothionein. When metallothionein-bound
cadmium is present in the plasma, it is quickly cleared by
glomerular filtration and reabsorbed in the renal tubules or
excreted in the urine (Cherian & Shaikh, 1975; Nordberg et al.,
1975; Webb & Etienne, 1977; Fowler & Nordberg, 1978).
At low levels of cadmium-metallothionein in the plasma, tubular
reabsorption is almost complete, whereas the uptake in the tubular
cells from the tubular fluid is saturated in the presence of high
concentrations (Nomiyama & Foulkes, 1977; Foulkes, 1982). Thus, high
urinary excretion of cadmium-metallothionein occurs shortly after
the administration of larger doses, i.e. doses exceeding about
0.1 mg cadmium/kg body weight (Cherian & Shaikh, 1975; Nordberg &
Nordberg, 1975).
It has been demonstrated in animal and in vitro tissue
studies that metallothionein provides a protective role for cadmium
toxicity (Cherian & Nordberg, 1983). Mice pretreated with cadmium
have increased tolerance to subsequent cadmium exposure (Nordberg et
al., 1971), and exposure to cadmium may protect from subsequent
mercury toxicity (Piotrowski et al., 1974). In addition, the
inhibition of certain mixed-function oxidases by cadmium is reduced
by prior induction of metallothionein by cadmium in immature mice
(Asokan et al., 1984). Pre-exposure of cultured kidney cells to
cadmium protects from subsequent exposure (Cherian, 1980; Jin et
al., 1986).
Nordberg et al. (1975) showed that metallothionein isolated
from rabbit and mouse liver produced acute renal tubular cell
toxicity when injected subcutaneously into mice. Further study
(Cherian et al., 1976; Fowler & Nordberg, 1978; Squibb et al., 1982,
1984) suggested that parenterally administered
cadmium-metallothionein enters proximal renal tubular lining cells
in pinocytotic vesicles that fuse with lysosomes. The
metallothionein is degraded, releasing cadmium into the cytosol and
producing cellular degeneration and necrosis within 8-24 h. The
renal tubular cell toxicity produced by metallothionein with
different ratios of cadmium and zinc is proportional to the cadmium
content of the metallothionein (Suzuki, 1982). Zinc-thionein does
not have a similar effect.
The pathogenesis of renal tubular cell toxicity is thought to
be related to non-metallothionein-bound cadmium, which becomes
rapidly bound to existing metallothionein sites or induces the
synthesis of new metallothionein (section 7.2.1.4).
The prevalence of nephrotoxicity rather than hepatotoxicity in
chronic cadmium exposure may be due to several factors. Firstly, the
release of hepatic cadmium-metallothionein or its presence in the
blood can result in preferential accumulation of cadmium in the
kidneys. Secondly, it has been shown in experimental animals that
the kidney can accumulate metallothionein mRNA in response to
cadmium exposure to only about half the level of the liver
(Koropatnick & Cherian, 1988). Thus, the kidney may not be able to
synthesize metallothionein as efficiently as the liver in response
to cadmium exposure, resulting in an accumulation of
non-metallothionein cadmium in the kidney but not in the liver.
Nomiyama et al. (1982a) studied the concentrations of total
cadmium, metallothionein-cadmium, and non-metallothionein-cadmium in
the renal cortex of monkeys fed diets containing 30 mg cadmium/kg
food for 12 months. They found that the concentration of
non-metallothionein-cadmium increased with dose of cadmium to about
35 mg/kg tissue and total cadmium to about 200 mg/kg tissue when the
total dose of cadmium was 0.4 g. Similar measurements were made in
rabbits given cadmium chloride (0.5 mg cadmium/kg body weight)
subcutaneously every day for 21 weeks. There were parallel increases
of total cadmium and non-metallothionein-bound cadmium during the
initial 4 weeks of dosing; these remained unchanged until the 14th
week when striking renal dysfunction appeared. At that time, total
cadmium and non-metallothionein-bound cadmium levels fell despite
the continued administration of cadmium (Nomiyama & Nomiyama, 1982).
Increased knowledge of the intracellular binding or speciation of
non-metallothionein-bound cadmium should improve our understanding
of the relative roles of metallothionein-bound and
non-metallothionein-bound cadmium.
When Cherian et al. (1978) exposed mice by injection and
feeding to both cadmium chloride and cadmium-metallothionein, both
compounds were absorbed and distributed in the body. However, in the
short term, cadmium-metallothionein was selectively accumulated in
the kidney and cadmium chloride in the liver.
Most cadmium in human urine is bound to metallothionein
(Tohyama et al., 1981b), and good correlation has been found between
the urinary cadmium and metallothionein concentrations both in
elderly women exposed in the general environment (Tohyama et al.,
1981b) and in male cadmium workers (Nordberg et al., 1982; Roels et
al., 1983b). Measurement of urinary metallothionein thus provides a
good indication of the urinary cadmium level and offers the
advantage over cadmium analysis of avoiding the possibility of
external contamination. Women were found to have much higher urinary
metallothionein concentrations than men, even at similar cadmium
levels.
6.9 Conclusions
Data from experimental animals and humans have shown that
pulmonary cadmium absorption is greater than gastrointestinal
absorption. Depending on chemical speciation, particle size, and
solubility in biological fluids, up to 50% of the inhaled cadmium
compound may be absorbed. The gastrointestinal absorption of cadmium
is influenced by the type of diet and nutritional status, iron
status appearing to be particularly important. On average, 5% of the
total oral intake of cadmium is absorbed, but individual values
range from less than 1% to more than 20%. Cadmium may also be
transported to the fetus. However, although cadmium accumulates in
the placenta, little is transferred to the fetus.
Cadmium absorbed from the lungs or the gastrointestinal tract
is stored principally in the liver and kidneys where more than half
of the body burden is deposited. Highest cadmium concentrations are
generally found in the renal cortex, but as exposure levels
increase, a greater proportion of the absorbed cadmium is stored in
the liver. The cadmium excretion rate is normally low, and the
biological half-time is very long (decades) in the kidneys, muscles,
liver, and total body of humans. The cadmium concentrations in most
tissues increase with age. In exposed people with renal damage,
urinary excretion of cadmium increases and, thus, the whole body
half-time is shortened. The renal damage leads to losses of cadmium
from the kidney, and the renal concentrations are eventually lower
than in people with similar exposure but without renal damage.
Metallothionein is an important transport and storage protein
for cadmium and other metals. Cadmium can induce metallothionein
synthesis in many organs including the liver and kidney. The binding
of intracellular cadmium to metallothionein in tissues protects
against cadmium toxicity. Non-metallothionein-bound cadmium may,
therefore, have a role in the pathogenesis of cadmium-related tissue
injury. The speciation of other cadmium complexes in tissues or
biological fluids is unknown.
Urinary excretion of cadmium is related to body burden, recent
exposure, and renal damage. In people with low exposures, cadmium in
urine is mainly related to body burden. Cadmium-exposed people with
proteinuria generally exhibit greater cadmium excretion than such
people without proteinuria. After high exposure ceases, urinary
cadmium decreases even though renal damage persists. The
interpretation of urinary cadmium is thus dependent on a number of
factors. The magnitude of gastrointestinal excretion is similar to
that of urinary excretion, but it cannot be easily measured. Other
excretory routes such as lactation, sweating or placental transfer
are insignificant.
Cadmium in faeces is a good indicator of recent daily intake
from food in the absence of inhalation exposure. Cadmium in blood
occurs mainly in the blood cells, and the plasma concentrations are
very low. There are at least two blood compartments, one being
related to recent exposure with a half-time of about 2-3 months, and
the other probably related to body burden with a half-time of
several years.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Lethal dose and lethal effects
LD50 inhalation values are in the range of 500 to
15 000 mg/m3.min for different species (Barrett et al., 1947;
Harrison et al., 1947; Hadley et al., 1979). The cause of death is
pulmonary oedema.
The LD50 after the injection of soluble cadmium compounds is
in the range of 2.5-25 mg/kg body weight (Friberg, 1950; Eybl &
Sykora, 1966; Commission of the European Communities, 1978). Shortly
after large doses are injected, severe endothelial damage is seen in
the small vessels of the peripheral nervous system (Gabbiani, 1966)
and in the testis (Parizek, 1957). If the animal survives for some
hours, the most pronounced changes are found in the liver (Dudley et
al., 1982), and liver damage is probably the lethal effect of a
single high parenteral exposure.
For most cadmium compounds, the LD50 after oral
administration is about 10-20 times higher than after parenteral
administration, and the readily soluble compounds have a lower
LD50 values than the insoluble ones (Table 11).
Nomiyama et al. (1978b) showed that the LD50 in mice was
lower at cold temperatures (+8 °C) than at higher temperatures
(+22 or +37 °C), both after oral and peritoneal exposure.
7.1.2 Pathological changes affecting specific systems in the body
The chronic effects of long-term exposure to low doses of
cadmium constitute the main problem for non-occupationally exposed
humans. Therefore, the effects of single exposure in animals will be
dealt with only briefly, and the main emphasis will be on chronic
effects.
Specific effects from a single high dose of cadmium have been
described by several investigators and have been reviewed by Friberg
et al. (1974, 1986), Commission of the European Communities (1978),
and Kawai (1978). One of the most pronounced effects seen was in the
gonads (testis and ovary).
Table 11. LD50 values for cadmium compounds given to mice and rats by intragastric administration
Species Compound Molecular formula Relative molecular LD50 with confidence LD50 for cadmium
massa limits (mg/kg)b ion alone (mg/kg)
Mouse cadmium (element) Cd 109 890 (636-1246) 890
cadmium oxide CdO 128.4 72 (41-113) 63
cadmium sulfate CdSO4 208.5 88 (69.8-100.2) 47
cadmium chloride CdCl2 183.3 93.7 (75.5-111.9) 57
cadmium nitrate Cd(NO3)2 236.4 100 (78.7-121.8) 48
cadmium iodide CdI2 366.2 166 (139-193) 51
cadmium caprylate Cd(C7H15COO)2 394.8 300 (196-459) 85
cadmium carbonate CdCO3 169 310 (215-404) 202
cadmium stearate Cd(C17H35COO)2 679.4 590 (556-624) 98
cadmium sulfide CdS 144.5 1166 (1135-1197) 907
cadmium sulfoselenide CdSe.CdS 335.8 2425 (2393-2457) 1623
barium-cadmium stearate BaCd(C17H35COO)4 1383.7 3171 (2763-3579) 258
Rat cadmium caprylate Cd(C7H15COO)2 394.8 950 (613-1472) 270
cadmium stearate Cd(C17H35COO)2 679.4 1225 (875-1574) 203
barium-cadmium stearate BaCd(C17H35COO)4 1383.7 1980 (1736-2224) 161
a From: Weast (1974)
b From: Tarasenko et al. (1974), Vorobjeva & Sabalina (1975), and Vorobjeva & Bubnova (1981)
7.1.2.1 Acute effects on testes and ovaries
Testicular necrosis occurs in experimental animals given single
injections of salts corresponding to 2-4 mg cadmium/kg body weight
(Parizek & Zahor, 1956; Parizek, 1957). At a later stage, Leydig
cells regenerate (Parizek, 1957, 1960; Allanson & Deanesly, 1962).
Gabbiani et al. (1974) detected dilation of interendothelial clefts
in the small blood vessels of the testis as early as 15 min after an
intravenous injection of cadmium salts. Effects on the testis have
been extensively reviewed by Gunn & Gould (1970).
The marked effects on the testis after cadmium injection are
probably the result of endothelial damage. In the small vessels this
damage gives rise to increased capillary permeability. This leads to
vascular escape of fluids and blood plasma substances into the
interstitium, which results in oedema, decreased capillary blood
flow, ischaemia, and testicular cell necrosis (Aoki & Hoffer, 1978;
Francavilla et al., 1981).
A single injection of cadmium salts at a dose that induces
testicular haemorrhagic necrosis has been shown to induce
haemorrhages and necroses in the ovaries of prepubertal rats (Kar et
al., 1959), and in the ovaries of adult rats in persistent oestrus
(Parizek et al., 1968a). The effect of cadmium on the testis was not
dependent on the presence of the hypophysis (Parizek, 1960). Ovarian
effects can be prevented by the administration of PMSG hormones
(Parizek et al., 1968a). Numerous studies on the effects of cadmium
on the testes and other reproductive organs were reviewed by Barlow
& Sullivan (1982).
7.1.2.2 Acute effects on other organs
A single inhalation exposure to cadmium at concentrations of
5-20 mg/m3 for 50-120 min gives rise to pulmonary oedema in rats
and rabbits (Hayes et al., 1976; Bouley et al., 1977; Bus et al.,
1978; Dervan & Hayes, 1979; Boisset & Boudene, 1981; Fukuhara et
al., 1981). The morphological changes seen in the lung have been
described in detail by Strauss et al. (1976).
After the parenteral administration of cadmium at dose levels
similar to the LD50, pronounced effects were seen in the small
blood vessels of, for instance, the nervous system (Gabbiani et al.,
1974). Hoffman et al. (1975) noted profound morphological effects in
the liver of rats given 6 mg cadmium/kg body weight, and Dudley et
al. (1982), examining liver effects from a single injection of
cadmium (3.9 mg/kg body weight), concluded that liver was the major
target organ in rats for acute cadmium toxicity. Changes in blood
pressure shortly after the acute administration of cadmium have also
been recorded (Dalhamn & Friberg, 1954; Perry et al., 1970).
Oral administration of cadmium compounds induces epithelial
desquamation and necrosis of the gastric and intestinal mucosa,
together with dystrophic changes of the liver, heart, and kidneys
(Tarasenko et al., 1974; Vorobjeva & Sabalina, 1975).
7.2 Repeated and/or long-term exposure
7.2.1 Effects on the kidneys
Since the kidney is the critical organ in humans exposed for
long periods to relatively small amounts of cadmium (section 8.2.1),
results from relevant animal studies will be dealt with in some
detail. Even though it is difficult to extrapolate quantitative
information from the findings in animals, experiments have provided
valuable information concerning mechanisms of cadmium-induced
nephropathy and the significance of various biological indicators of
exposure and effect, and have supported the findings in humans. For
example, Friberg (1950) verified in animal experiments that exposure
to cadmium caused a type of proteinuria similar to the one he had
found in exposed workers.
Animal studies that have given data on renal effects as well as
the corresponding renal cadmium concentrations are summarized in
Table 12. An evaluation of organ dose-effect and dose-response
relationships is included in section 7.2.1.4.
7.2.1.1 Oral route
Renal lesions were first reported by Prodan (1932) and Wilson
et al. (1941) after cats and rats were given large oral doses of
cadmium for several months. Prodan (1932) reported varying degrees
of desquamation in proximal tubular epithelium (and no changes in
the glomeruli) after feeding cats 100 mg cadmium per day for one
month. Wilson et al. (1941) reported slight tubular changes in rats
after they were exposed for 3 months to a diet containing 62 mg
cadmium/kg.
Studies utilizing high exposures have also been performed by
Stowe et al. (1972). Ten rabbits received cadmium in drinking-water
(160 mg/litre) for 6 months. Kidney function was not investigated,
but histopathological examination revealed pronounced morphological
changes in the proximal tubules. The mean renal concentration of
cadmium was 170 mg/kg wet weight, which would correspond to about
210 mg/kg wet weight in the renal cortex (section 6.4). Still higher
doses (300 mg/kg diet) were given to rabbits for 54 weeks by
Nomiyama et al. (1975), who observed aminoaciduria and enzymuria
after 16 weeks. At this stage, the cadmium concentration in the
renal cortex was 200 mg/kg wet weight. Proteinuria and glycosuria
appeared at a later stage, 37 and 42 weeks, respectively, after
exposure had started. The cadmium concentration in the renal cortex
was 300 mg/kg wet weight after 40 weeks.
Table 12. Summary of animal studies with data on both renal cadmium levels and effects
Species Route of Exposure Duration Average cadmium Renal changes Reference
administration level (months) level in kidney
cortex (mg/kg
wet weight)
Mouse subcutaneous 0.25 mg/kg 6 110-170a no effects Nordberg & Piscator (1972)
body weight
Mouse subcutaneous 0.5 mg/kg 6 170a tubular protein patterns Nordberg & Piscator (1972)
body weight in urine
Rat intraperitoneal 0.75 mg/kg 3 200a no effects Bonnell et al. (1960)
body weight
Rat intraperitoneal 0.75 mg/kg 4 300a histological changes in Bonnell et al. (1960)
body weight 60% of animals
Rat subcutaneous 0.65 mg/kg 3 200 histological changes Goyer et al. (1984)
body weight
Rat water 10 mg/litre 8.5 11a no histological changes Kawai et al. (1976)
Rat water 50 mg/litre 8.5 35a slight histological changes Kawai et al. (1976)
Rat water 100 mg/litre 8.5 90a histological changes Kawai et al. (1976)
Rat water 200 mg/litre 8.5 145a histological changes Kawai et al. (1976)
Rat water 200 mg/litre 11 200 total proteinuria and low Bernard et al. (1981)
molecular weight proteinuria
Rat water 50 mg/litre 3 100a decreased insulin and PAH Kawamura et al.(1978)
clearance; histological
changes
Table 12 (contd).
Species Route of Exposure Duration Average cadmium Renal changes Reference
administration level (months) level in kidney
cortex (mg/kg
wet weight)
Rat water 50 mg/litre 2.5 235 slight histological changes Axelsson & Piscator (1966a);
in proximal tubules Axelsson et al. (1968)
Rabbit subcutaneous 0.25 mg/kg 2.5 235 slight histological changes Axelsson & Piscator (1966a);
body weight in proximal tubules Axelsson et al. (1968)
Rabbit subcutaneous 0.25 mg/kg 4 460 more severe histological Axelsson & Piscator (1966a);
body weight changes; reduction of alkaline Axelsson et al. (1968)
phosphatase activity in renal
cortex; total proteinuria
Rabbit subcutaneous 0.5 mg/kg 2.5 300 total proteinuria Nomiyama et al. (1982b)
body weight
Rabbit subcutaneous 0.5 mg/kg 0.7 200 proteinuria, glucosuria, and Nomiyama & Nomiyama
body weight aminoaciduria; decrease in (1982)
CIN and TmPAH
Rabbit subcutaneous 0.5 mg/kg 1 120 ß2-microglobulin Nomiyama et al. (1982b)
body weight
Rabbit subcutaneous 1.5 mg/kg 1 50-200 decreased tubular Nomiyama (1973a);
body weight readsorption Nomiyamaet al. (1978a)
Rabbit subcutaneous 0.5 mg/kg 2 160a slight histological changes Kawai et al. (1976)
body weight
Rabbit water 160 mg/litre 6 170a extensive fibrosis; pronounced Stowe et al. (1972)
changes
Table 12 (contd).
Species Route of Exposure Duration Average cadmium Renal changes Reference
administration level (months) level in kidney
cortex (mg/kg
wet weight)
Rabbit water 50 mg/litre 10 58 slight tubular atrophy Kawai et al. (1976)
Rabbit water 200 mg/litre 10 200 severe interstitial and Kawai et al. (1976)
tubular fibrosis
Rabbit diet 300 mg/kg 4 200 aminoaciduria, enzymuria Nomiyama et al. (1975)
Rabbit diet 300 mg/kg 10 300 proteinuria, glucosuria Nomiyama et al. (1975)
Pig diet 50-350 mg/kg < 12a equimolar increase in zinc Cousins et al. (1973)
in kidney
Pig diet 50-350 mg/kg 78a decrease in renal leucine Cousins et al. (1973)
aminopeptidase
Horse diet no cadmium lifelong 75 renal tubular interstitial Elinder et al. (1981a,b)
added (up to 240) changes and fibrosis in
25% of animals
Bird subcutaneous 0.16 mg/kg 1.5 20b histological changes Nicholson & Osborn (1983)
body weight
a These values are whole kidney concentrations; about 0.8 times kidney cortex values, on average.
b Denotes concentrations (wet weight) calculated as 0.2 times dry weight concentrations.
Morphological changes of the renal tubules were reported by
Kawai et al. (1976) in rats given 50 mg cadmium/litre drinking-water
for 8.5 months. The average renal cadmium concentration was about
38 mg/kg wet weight which corresponds to about 50 mg/kg in the renal
cortex.
Histological lesions in the proximal renal tubules were also
found in rats exposed to 200 mg cadmium/litre for 2 months (Itokawa
et al., 1978). Histochemical examination of the kidney showed that
the proximal tubular epithelium had particularly high cadmium
concentrations. The average renal concentrations were 48 mg/kg and
80 mg/kg, respectively, in rats with sufficient and deficient
calcium intakes. These levels would correspond to about 60 and
100 mg cadmium/kg in the renal cortex (section 6.4). Inulin
clearance was reduced to about a third of the control values in the
cadmium-exposed groups, indicating considerable functional damage to
the glomeruli. The only reported change in renal tubular function
was that the fractional excretion of calcium was increased about 50%
in the cadmium-exposed groups.
In a study of 50 rats exposed to 200 mg cadmium/litre in
drinking-water for up to 11 months (Bernard et al., 1981), there was
an increased prevalence of total proteinuria in the 8th month, when
the average cadmium concentration in the renal cortex was about
200 mg/kg.
Kajikawa et al. (1981) also reported morphological changes in
the kidneys of rats given drinking-water containing 200 mg cadmium
chloride/litre for 91 weeks. Histologically, they found degenerative
changes in the proximal convoluted tubules and, using electron
microscopy, proliferation of smooth endoplasmic reticulum,
vacuolization, and coagulative necrosis of the tubular cells. No
significant changes were observed in the glomeruli or interstitial
tissue.
When Cousins et al. (1973) gave large amounts of cadmium
chloride to pigs (50, 150, 450, and 1350 mg/kg diet), there was a
decrease in the activity of leucine aminopeptidase in the kidney
cortex at a renal cadmium concentration of 78 mg/kg wet weight,
corresponding to a renal cortex concentration of about 100 mg/kg wet
weight (section 6.4).
An extensive data base on the renal effects of cadmium in
monkeys has been developed in Japan. Several of these studies are
summarized in Table 13.
Study I (Nomiyama et al., 1979) was carried out using ten male
rhesus monkeys (three years of age). The monkeys were given 100 g of
solid feed containing 0, 3, 30, or 300 mg cadmium/kg daily for 37
weeks, followed by 130 g of feed for 18 weeks. Even the solid feed
given to the control group contained cadmium at a concentration of
0.13 mg/kg.
In study II, Nomiyama et al. (1987) used 36 male rhesus monkeys
(three years of age) and gave them 100 g of solid feed containing 0,
3, 10, 30, or 100 mg cadmium/kg daily for 52 weeks. During the
following 52 weeks 150 g was given, and then for the remaining 358
weeks 200 g was given. The solid feed given to the control group
contained cadmium at 0.27 mg/kg and zinc at 30 mg/kg.
Study III (Nomura et al., 1988) was performed with 40 female
rhesus monkeys given 150 g of solid feed for nine years (Table 14).
In study IV, Nomiyama & Nomiyama (1988) used nine male
crab-eating monkeys. Two of the animals were used as controls, and
three were given a diet containing cadmium concentrations of 3 mg/kg
(190 µg/day) as cadmium chloride (the pelleted food also contained
(30 mg zinc/kg)). The remaining four were fed 80 µg cadmium/day in
the form of contaminated rice.
In studies I and II, monkeys that had been given feed
containing cadmium at 300 mg/kg and 100 mg/kg showed indi-cations of
renal dysfunction, such as proteinuria, glucosuria, and
aminoaciduria, after 15-16 and 48-91 weeks, respectively. The
appearance of increased ß2-microglobulin was delayed until the
30th and 138th weeks, respectively. However, no definite disturbance
of proximal renal tubular function, such as reduced tubular
reabsorption of phosphorus, hypophosphataemia or acidosis, was noted
during the one-year follow-up. The dose-effect relationship for
renal dysfunction was similar to those which have been observed in
rabbits and rats, and thus the hypothesis that the susceptibility of
monkeys to cadmium may be exceptionally low was not corroborated.
In study II, the group of monkeys given feed containing
30 mg/kg developed urine findings (e.g., proteinuria, glucosuria,
aminoaciduria) indicative of renal dysfunction in the sixth year.
Postmortem examination revealed degeneration of the proximal renal
tubules, but there was no reduction in tubular reabsorption of
phosphorus. When the administration of cadmium was discontinued in
the fifth year, no abnormality of renal function developed during
the follow-up period of four years.
Table 13. Renal effects of cadmium in monkeysa
Study Duration Sex No. of Exposure level Average cadmium Renal effects (timing, Other effects (timing,
(weeks) monkeys (mg/kg diet) level in renal in weeks, of effects) in weeks, of effects)
cortex (mg/kg)
I 55 male 2 0 163
2 3 202 no biological effects no biological effects
3 30 596 no biological effects no biological effects
3 300 380b renal dysfunction (15-16) hepatic dysfunction (12-54)
757b ß2-microglobulinuria (30) slight anaemia (20)
II 462 male 6 0 328
8 3 700 no biological effects no biological effects
8 10 1070 no biological effects erythrocytopenia (360)
8 30 1170b renal dysfunction (300-306) erythrocytopenia (240)
ß2-microglobulinuria (311)
6 100 635b renal dysfunction (48-91) erythrocytopenia (120)
ß2-microglobulinuria (138) depressed age-related increase
in blood pressure (80)
III See Table 14.
IV 308 male 2 0 18
3 3 570 no biological effects no biological effects
4 contaminated rice 230 no biological effects no biological effects
(1.33 mg/kg)
a From: Nomiyama et al. (1979), Nomiyama et al. (1987), Nomura et al. (1988), Nomiyama & Nomiyama (1988)
b The numbers with asterisks are the critical concentrations of cadmium in the renal cortex.
Table 14. Bone and renal effects of cadmium in female monkeysa
Group No. of Exposure Low protein, Low vitamin D Average renal Renal effects Bone effectsd
monkeys level calcium and dietc cortex cadmium
(mg/kg) phosphorus dietb level (mg/kg)
1 5 0 - - 58 no biological effects no biological effects
2 4 0 + - no biological effects slightly disturbed calcification
(after 154 weeks)
3 4 0 - + no biological effects low plasma vitamin D3
4 4 0 + + no biological effects osteomalacic change (after 77
weeks) reversible by vitamin D3
5 5 30e - - 1511 no biological effects no biological effects
6 4 30e + - ß2-microglobulinuriaf disturbed calcification (after
(2000 to 12 000 µg/day, 154 weeks)
67%)
7 4 30e - + no biological effects low plasma vitamin D3
8 10 30e + + ß2-microglobulinuriag osteomalacic change (after 77
(up to 2000 µg/day) weeks) reversible by vitamin D3
a From: Nomura et al. (1988); duration of experiment was 463 weeks (9 years)
b 14% protein instead of 20%; 0.3% calcium instead of 0.9%; 0.3% phosphorus instead of 0.9%
c No vitamin D3 was added (240 IU was added to the normal diet)
d In Group 5 to 8, as depressed age-related increase in blood pressure was seen after 103 weeks of treatment.
e 3 mg/kg for the first 52 weeks
f Non-progressive lesion, reversibility uncertain; renal effect noted after 193 weeks
g Reversible by normal diet and vitamin D3 treatment; renal effect noted after 154 weeks
The monkeys in study III (Table 14) given the low nutrition
feed plus 30 mg cadmium/kg developed renal function abnormalities
after the fourth year. In addition to reduced phenolsulfonphthalein
(PSP) clearance and a variable increase in urinary
ß2-microglobulin concentration, many of the monkeys showed mild
degenerative changes of the proximal tubular epithelia, but there
was no decrease in the tubular reabsorption of phosphorus. However,
elevated urinary ß2-microglobulin did not progress further with
continued administration of cadmium. Mild degenerative changes of
the proximal tubular epithelia were also noted in the groups of
monkeys that had been given a normal diet, low vitamin D diet or low
nutrition plus low vitamin D diet, each supplemented with 30 mg
cadmium/kg. However, the elevated urinary ß2-microglobulin level
soon returned to normal in those animals fed a normal diet,
regardless of continued cadmium administration. This may indicate
that the elevated urine ß2-microglobulin in this group was not
caused solely by cadmium exposure.
In study II, some of the monkeys given feed containing 3 mg/kg
or 10 mg/kg of cadmium showed cadmium concentrations in the renal
cortex as high as 760 mg/kg and 1070 mg/kg, respectively. However,
no effect upon renal function was observed during the nine-year
period, nor was there any increase in urinary ß2-microglobulin
concentration.
The above data suggest that mild renal dysfunction
(proteinuria, glucosuria, and aminoaciduria, but no decrease in the
tubular reabsorption of phosphorus) was produced in monkeys exposed
to high concentrations of cadmium (30 mg/kg diet or more). It seems,
however, that no effects on renal function occur with low-level
exposure (10 mg/kg or less). The development of renal dysfunction is
assumed to depend upon the amount of cadmium absorbed per day rather
than the total amount absorbed in the body.
In study IV, the urinary cadmium level occasionally exceeded
10 µg/litre, but no clinical chemistry changes were reported.
Cadmium concentrations in the renal cortex increased proportionally
to the dose level and duration of exposure, reaching an average of
450 mg/kg in the group given cadmium chloride and 290 mg/kg in the
group fed contaminated rice. This suggests that the chemical form of
cadmium does not affect the severity of health effects.
7.2.1.2 Respiratory route
Princi & Geever (1950) could find no evidence of renal
morphological changes in the kidney of dogs after prolonged
inhalation exposure (up to one year) to cadmium oxide or cadmium
sulfide dust (average concentration of 4 mg/m3). Routine analysis
was performed, but neither the methods used nor the results obtained
were described. Friberg (1950) exposed rabbits for about 8 months
(3 h per day, about 20 days per month) to cadmium oxide dust with an
average concentration of about 8 mg/m3 . After 4 months of
exposure, moderate proteinuria was detected by the trichloroacetic
acid test. Histological examination of the kidneys after 8 months
revealed interstitial infiltration of leucocytes in the majority of
the exposed rabbits; this was not found in the control group.
7.2.1.3 Injection route
Friberg (1950) detected proteinuria in rabbits given
subcutaneous injections of cadmium sulfate (0.65 mg cadmium/kg body
weight) 6 days per week. Electrophoretic analysis of urine proteins
revealed that the proteinuria differed from that caused by
injections of uranium salts. More recently, many studies utilizing
parenteral administration (with doses generally in the range of
0.25-1.5 mg/kg body weight), different routes of exposure
(subcutaneous and intraperitoneal), and a duration of 1-12 months
have been performed in mice, rats, and rabbits (Table 12). These
experiments have confirmed the nephrotoxic effects of cadmium.
When rabbits were exposed for 16 weeks by subcutaneous
injection of either 0.25 mg or 0.5 mg cadmium/kg body weight 3 times
a week, there was a significant increase in urinary
ß2-microglobulin excretion indicative of renal tubular dysfunction
in the high-dose group after 7 weeks. There was only a slight
increase in the serum ß2-microglobulin/creatinine ratio. Urinary
ß2-microglobulin levels were not related to serum
ß2-microglobulin levels (Piscator et al., 1981).
Rats dosed intraperitoneally, five days/week with 0.6 mg
cadmium/kg body weight, showed no abnormal effects after 5 or 6
weeks when renal cadmium levels reached about 100 mg/kg. However, in
renal tubular lining cells an increase in lysosomes, microbodies,
and smooth endoplasmic reticulum was noted. After 8 weeks renal
cadmium levels had reached about 200 mg/kg of tissue and tissue
necrosis was observed. The early changes (with a renal cadmium
concentration of up to 100 mg/kg) were considered to be adaptive and
possibly reversible, whereas morphological changes after 8 weeks
with a renal concentration of 200 mg/kg were considered to be
irreversible (Goyer et al., 1984).
Nomiyama et al. (1982a) found that non-metallothionein-bound
cadmium increased up to about 35 mg/kg tissue in parallel with total
cadmium. At that stage, the total cadmium concentration in the renal
cortex was in the range 200-800 mg/kg, the total dose of cadmium
having been approximately 1 g.
7.2.1.4 Pathogenesis of cadmium nephrotoxicity
Various hypotheses have been proposed to explain the
pathogenesis of cadmium nephrotoxicity, particularly the role of the
metal-binding protein metallothionein (see section 6.8). This
protein is inducible by a number of essential metals (Cherian &
Goyer, 1978) and may have as its primary function the intracellular
storage of zinc and copper (Panemangalore et al., 1983; Templeton et
al., 1985). It is also induced following exposure to cadmium. It is
now thought that metallothionein protects against cadmium toxicity
and that intracellular cadmium bound to metallothionein is nontoxic
(Nordberg, 1971; Goyer et al., 1989). There is considerable support
for this hypothesis. Pre-treatment of experimental animals with
small doses of cadmium prevents the acute toxic effects of a large
dose of cadmium (Nordberg et al., 1975). Parenteral administration
of cadmium-metallothionein causes acute tubular toxic effects in the
kidney (Nordberg, 1971; Nordberg et al., 1975; Cherian & Nordberg,
1983). By treatment of animals with repeated doses of cadmium,
metallothionein synthesis in the renal cortex can be induced. This
prevents against subsequent renal toxicity by parenteral
cadmium-metallothionein at dose levels that normally give rise to
renal damage (Jin et al., 1987a). Rat renal cortical cells isolated
from animals pretreated with cadmium were resistant to normally
toxic concentrations of cadmium in vitro (Jin et al., 1987b).
Similar protective effects were observed in kidney cells pretreated
with cadmium in vitro (Jin et al., 1987b). Human cells in tissue
culture, where metallothionein has been induced by pre-treatment
with cadmium, become resistant to previously lethal exposure to
cadmium (Glennas & Rugstad, 1984).
With this evidence for the protective role of intracellular
metallothionein, several theories have been proposed to explain the
nephrotoxicity of cadmium. One hypothesis attributes the
nephro-toxicity to that fraction of intracellular cadmium not bound
to metallothionein (Nordberg et al., 1975; Nomiyama & Nomiyama,
1982; Squibb et al., 1984). Another hypothesis is that
extra-cellular cadmium bound to metallothionein is toxic (Cherian et
al., 1976). Cadmium-metallothionein derived from cadmium-induced
synthesis in reticulocytes (Tanaka et al., 1985) or released from
liver cells is filtered by the renal glomeruli and reabsorbed by the
proximal tubular lining cells where it is catabolized, releasing
cadmium ions that cause renal damage (Dudley et al., 1985). This
hypothesis is supported by the fact that parenterally administered
cadmium-metallothionein is very toxic to renal tubular cells and
that the plasma metallothionein level increases with cadmium
exposure (Goyer et al., 1984; Shaikh & Hirayama, 1979).
Still another hypothesis is that intracellular cadmium
interacts with cell membranes resulting in lipid peroxidation
(Stacey et al., 1980) and that cadmium may displace essential metals
from metallothionein (Petering et al., 1984), thereby depriving
important metalloenzymes of essential metal cofactors.
These hypotheses are not mutually exclusive and the relative
significance of each of these mechanisms may differ under particular
circumstances of exposure.
Goering et al. (1985) reported the development of calcuria in
rats injected with cadmium-metallothionein, using the model
described by Squibb et al. (1984). Jin et al. (1987a) confirmed
their observations, which suggest that this biological effect may be
an early event in the development of renal tubular damage. Data from
the above studies further validate the use of the
cadmium-metallothionein injection model for studying the mechanisms
of cadmium-induced tubular injury, since calcuria is also observed
in people with chronic elevated cadmium exposure.
7.2.1.5 General features of renal effects; dose-effect and
dose-response relationships
The available data show that long-term exposure to cadmium
leads to renal tubular lesions with proteinuria, glucosuria, and
aminoaciduria, and to histopathological changes (Table 12).
It has been reported that cadmium-induced proteinuria differs
from glomerular proteinuria (Friberg, 1950) and involves low
molecular weight proteins in particular (Axelsson & Piscator, 1966a;
Nomiyama et al., 1982b). Thus, this type of proteinuria resembles
the "tubular proteinuria" seen in humans. Microscopic examination
reveals typical tubular nephropathy, i.e. atrophy and degeneration
of tubular cells, especially proximal tubular cells, and
interstitial fibrosis (Bonnell et al., 1960; Axelsson et al., 1968;
Kawai et al., 1976).
Electron microscopic changes are characterized by interstitial
fibrosis and thickening of the basement membrane of the proximal
tubular cells (Kawai et al., 1976). The smooth endoplasmic reticulum
is dilated or undergoes proliferation, and there is apical cyst
formation (Stowe et al., 1972). An increase in the number of
lysosomes and swelling of the mitochondria have also been observed.
In addition to tubular findings, there have been reports of
pathological changes in 30% of the mesangium cells of the glomeruli
of dogs (Murase et al., 1974) and increased thickness of the
glomerular basement membrane in rats (Scott et al., 1977).
Investigations into renal function have also revealed
substantial changes, mainly in tubular function, e.g., reabsorption
of glucose (Axelsson & Piscator, 1966a), whereas the changes in
glomerular filtration are relatively small (Axelsson & Piscator,
1966a). Effects have generally been seen at average renal cortex
concentrations of 200-300 mg/kg wet weight, but some studies have
reported effects at considerably lower concentrations.
Histopathological changes in rats, rabbits, horses, and birds have
been reported at renal cortex concentrations below 100 mg/kg (Table
12). However, in chronically exposed monkeys, signs of renal tubular
changes were reported at around 400-1200 mg cadmium/kg (Table 13).
After renal cortex concentrations of cadmium have reached a
level of 200-300 mg/kg wet weight, they level off or decrease. No
further increase is seen even with continued exposure (Axelsson &
Piscator, 1966a; Nomiyama & Nomiyama, 1976a; Bernard et al., 1981).
It has also been shown (Friberg, 1952; Axelsson & Piscator, 1966a;
Nordberg & Piscator, 1972; Nomiyama & Nomiyama, 1976a) that urinary
excretion of cadmium is low during the initial exposure period but a
marked increase in the excretion of cadmium occurs subsequently,
which coincides with an increase in protein excretion (Friberg,
1952; Axelsson & Piscator, 1966a; Nordberg & Piscator, 1972)
(section 6).
Animal studies have shown that, as the cadmium concentration in
the renal cortex increases, the first effects to appear are the
histopathological changes in the renal tubular cells. The low
molecular weight proteinuria and aminoaciduria develop at somewhat
higher cadmium concentrations in the renal cortex and, at even
higher concentrations, glucosuria, total proteinuria, and other
indications of damaged renal function develop. The diagnosis of
these effects depends greatly on the sensitivity of the method for
analysing the effect. For instance, a method for analysing low
molecular weight proteinuria that can accurately measure levels
considered normal (0.1 mg/litre or less) will be able to diagnose
proteinuria at an earlier stage than a method with a detection limit
of 7 mg/litre.
Most of the studies referred to in Table 12 included no data on
the prevalence of renal effects in the animals. The results were
given in a qualitative way, stating the average cadmium level in the
renal cortex at which effects were seen.
Some dose-response data are available from animal studies.
Bernard et al. (1981) produced proteinuria in rats exposed to
cadmium in drinking-water (200 mg/litre) for up to 11 months. After
8-9 months, a significant increase in group-average proteinuria was
seen, coinciding with a 25% prevalence of increased individual
proteinuria. The renal cortex cadmium concentration at that time was
about 200 mg cadmium/kg (Bernard et al., 1981). Elinder et al.
(1981a) studied horses exposed to cadmium present in their normal
food. Histopathological changes in the renal cortex were classified
and coded in a blind manner, and the prevalence of different degrees
of change was calculated for subgroups of horses with different
renal cortex cadmium concentrations. The "background" prevalence was
25-30% and there was an increased prevalence (up to 60-75%) with
increased average renal cadmium level. At a renal cortex cadmium
concentration of about 75 mg/kg, there was a significant increase in
the prevalence of histopathological changes.
7.2.2 Effects on the liver
Friberg (1950) demonstrated fibrotic changes in the liver of
rabbits exposed to repeated subcutaneous injections of cadmium.
Periportal and interlobular collagen deposition was found in the
liver of rabbits given 160 mg cadmium/litre in drinking-water for 6
months (Stowe et al., 1972). Liver function tests, however, remained
within normal limits. The cadmium concentration in the liver was
188 mg/kg wet weight. Tarasenko et al. (1974) demonstrated by
histological techniques that dystrophic changes occur in the liver
of rats after repeated intragastric administration of cadmium
caprylate in a total dose corresponding to 47 mg cadmium/kg body
weight per day. These authors also noted an increased level of
lactic acid in the blood serum of the animals. Larionova et al.
(1974) detected decreased activity of alanine transaminase in liver
tissue and depletion of glycogen in rats given barium cadmium
laurate by gavage for 8-10 days at a dosage of 169 mg/kg body weight
per day (as the laurate). After intraperitoneal injection of cadmium
(up to 1.25 mg/kg body weight for periods of up to 6 weeks),
decreased glycogen content and increased daily activity of
gluconeogenic enzymes in rat liver were reported by Merali et al.
(1974) and Chapatwala et al. (1982).
In long-term studies, rabbits given 300 mg cadmium/kg diet for
54 weeks (Kawai et al. 1976) showed some amyloid deposition in the
liver. Studies on rats after exposure for 335 days to 1 mg
cadmium/litre in drinking-water (Sporn et al., 1970) revealed
changes in liver enzyme activities. Rhesus monkeys exposed to 300 mg
cadmium/kg in the diet for 12 weeks (Nomiyama et al., 1979)
developed increased levels of plasma enzymes (GOT, GPT, and LDH).
7.2.3 Effects on the respiratory system
Interstitial pneumonitis and emphysema were found in rabbits
exposed to cadmium iron oxide dust (approximately 8 mg/m3) for 4-8
months (Friberg, 1950) and in rats observed for 4-7 months after a
single intratracheal administration of cadmium iron oxide dust
(3.5 mg/kg body weight) (Vorobjeva, 1957). However, only very slight
pulmonary effects were detected in rabbits and rats exposed to
nickel-graphite dust at dose levels several times higher than the
concentrations of cadmium iron oxide dust.
Yoshikawa et al. (1975) exposed rats to cadmium oxide fumes
(0.1 or 1.0 mg cadmium/m3) for up to 3 months. There were 10 rats
in each group, and three of the rats in the high exposure group died
after about 7 weeks. Lung fibrosis and the first stage of emphysema
were observed at the end of the experiment in the high-dose group.
Free macrophage cells in the alveoli were more numerous in both
groups, and there was an increased surface tension of the
surfactants.
Snider et al. (1973) observed signs of emphysema in rats 10
days after 5-15 daily 1-h periods of exposure to cadmium chloride
aerosol (10 mg/m3). Also, long-term exposure to comparatively low
air concentrations of cadmium oxide (24-50 µg cadmium/m3) gave
rise to pathological changes in the lungs similar to emphysema as
well as to cell proliferation in the bronchi (Prigge, 1978). A
long-term study (14 months), in which mice and golden hamsters were
exposed to different concentrations of cadmium chloride (30 and
90 µg/m3), cadmium sulfate (30 and 90 µg/m3), cadmium sulfide
aerosols (90-100 µg/m3), cadmium oxide fume (10-90 µg/m3), and
dust (10-270 µg/m3), revealed a significantly increased incidence
of alveolar hyperplasia and interstitial fibrosis in most of the
exposed groups (Heinrich et al., 1989).
Single intratracheal administration of several cadmium
compounds (e.g., oxide, sulfide, carbonate, sulfoselenide,
caprylate, stearate, cadmium-barium laurate, and cadmium-barium
stearate) in doses from 0.5 mg (as the oxide) to 15 mg (as the
sulfide) caused the development, over 6 months, of chronic
inflammatory changes, emphysema, and atelectasis leading to
fibrosis. Exposure to cadmium oxide and caprylate gave rise to the
development of nodules of hyaline connective tissue; these resembled
silicotic nodules (Vorobjeva & Sabalina, 1975).
7.2.4 Effects on bones and calcium metabolism
Male rats fed a normal diet and exposed to cadmium sulfate by
inhalation (3 and 0.3 mg/m3, 4 h daily for 4 months) showed
decreased serum and urinary calcium concentrations compare to
controls. Female rats similarly exposed to cadmium sulfate
(2.8 mg/m3, 3 h/week) during pregnancy showed radiological
evidence of osteoporosis in addition to hypocalcaemia (Tarasenko et
al., 1975).
In a study by Kogan et al. (1972), cadmium chloride and cadmium
sulfate were administered subcutaneously to rats at a daily dose of
1 mg/kg body weight for up to 12 months. At 12 months, X-ray
analysis of the bones indicated osteoporosis and osteosclerosis.
Subsequent histopathology showed an increase in osteoclasts and a
bone structure described by the authors to be indicative of
osteomalacia.
Oral administration of cadmium chloride in drinking-water to
male rats (1 or 4 µg/kg body weight daily for six months) resulted
in changes in calcium metabolism and bone structure characteristic
of osteomalacia, which were not observed in the control group. No
effects were noted in a group of animals given a daily dose of
0.01 µg/kg body weight (Likutova & Belova, 1987).
Rats given 50 mg cadmium/litre in drinking-water for about 9
months showed reduced calcium and phosphorus absorption from the
intestine (Sugawara & Sugawara, 1974). In addition, some of the
animals showed histological changes in the duodenal mucosa, a
finding also reported in Japanese quail (Richardson & Fox, 1974).
Several mineral balance studies have been made on animals fed
cadmium. Simultaneous administration of cadmium with a low-protein,
low-calcium diet led to a decrease in the calcium and zinc content
of bone (Itokawa et al., 1973). Furthermore, Kobayashi (1974)
reported that cadmium feeding led to a negative calcium balance in
rats.
The decreased calcium absorption and negative calcium balance
in cadmium-exposed rats could result from the inhibitory effects of
cadmium on the activation of vitamin D in renal cortical cells
(Feldman & Cousins, 1973). The renal conversion of
25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol has been
found to be inhibited by high dietary cadmium exposure in rats fed a
normal calcium diet, but this effect was not seen on a low-calcium
diet (Lorentzon & Larsson, 1977). The metabolically active form of
vitamin D (1,25-dihydroxycholecalciferol) is necessary for the
normal absorption of calcium from the intestine. Ando et al. (1981)
found that the stimulation of calcium absorption by 1-alpha-hydroxy
vitamin D3 was inhibited in rats exposed to cadmium by gastric
intubation. Furthermore, the concentration of calcium-binding
protein in intestinal mucosa may be decreased by cadmium exposure
(Fullmer et al., 1980).
Administration of drinking-water containing 10 mg cadmium per
litre to rats fed a normal diet over a 9-month period gave rise to
decalcification and cortical atrophy in the skeleton (Kawai et al.,
1976). Other workers have also reported effects in the bones of rats
following several months exposure to cadmium in drinking-water
(Itokawa et al., 1974; Kawamura et al., 1978) and in the diet
(Takashima et al., 1980; Nogawa et al., 1981a), and following
subcutaneous injection (Nogawa et al., 1981a). In these studies, the
bones were reported to show more or less severe osteoporosis and
osteomalacia. On the other hand, Kajikawa et al. (1981) did not find
either osteoporosis or osteomalacia after rats were exposed for 2
years to cadmium in drinking-water (200 mg/litre).
Anderson & Danylchuk (1979) found that exposure of Beagle dogs
for six months to cadmium (25 mg/litre in drinking-water) reduced
bone turnover rate, a metabolic abnormality consistent with calcium
deficiency or osteomalacia. Kawashima et al. (1988) found that
feeding a cadmium-contaminated rice diet or a diet containing 3 mg
cadmium chloride/kg for six years to crab-eating monkeys did not
produce any change in vitamin D metabolism, and there was no
evidence of renal dysfunction. In another series of experiments
rhesus monkeys were fed diets containing 3, 10, 30 or 100 mg
cadmium/kg for 9 years. Serum vitamin D metabolites and renal
production of vitamin D remained unchanged, but in animals fed 30 or
100 mg cadmium/kg of diet there was slight but not statistically
significant depression of renal 25-hydroxy vitamin D1-hydroxylase
activity. No skeletal abnormalities were found in any of these
animals.
Bhattacharyya et al. (1988) studied the effects of 0, 0.25, 5
or 50 mg cadmium/kg diets on female mice bred for six consecutive
42-day cycles of pregnancy and lactation and on non-pregnant
controls. The multiparous mice exposed to 50 mg/kg experienced
significant decreases in body weight (3-11%) and femur calcium
content (15-27%), and the femur calcium to dry weight ratios
decreased by 5-7%. These results were thought by the authors to
provide evidence that the combination of cadmium exposure and
multiparity has a synergic effect on bone metabolism.
In the Japanese monkey study III (section 7.2.1 and Table 14),
osteomalacic changes were found in the low-nutrition plus
low-vitamin-D diet group (group 4) after 77 weeks. These effects
were not further exacerbated by feeding cadmium (group 8). These
changes were found to be reversed by the administration of vitamin
D. Renal effects were found in group 8 after 154 weeks. Therefore,
the osteomalacia found in group 8 was diagnosed as not being renal
osteomalacia.
Most of the findings discussed above indicate a direct effect
of cadmium on bone mineralization, possibly related to calcium
deficiency, and an indirect effect on calcium absorption via vitamin
D hydroxylation, perhaps leading to osteomalacia. The direct effects
develop after long-term cadmium exposure, whereas the indirect
effect on vitamin D metabolism occurs only when renal damage is seen
in the animals. Osteomalacia only occurred in monkeys fed a diet low
in protein, phosphorus, calcium, and vitamin D. Cadmium
administration did not increase these effects.
7.2.5 Effects on haematopoiesis
Anaemia is a common finding in animals after both dietary
(Wilson et al., 1941) and parenteral (Friberg, 1950) exposure to
cadmium. After dietary exposure, decreased haemoglobin concentration
(Decker et al., 1958) and decreased haematocrit (PCV) (Prigge et
al., 1977) are among the early signs of cadmium toxicity.
Fox & Fry (1970) and Fox et al. (1971) reported that the
cadmium-induced anaemia could be prevented by simultaneous feeding
with iron or ascorbic acid. Decreased gastrointestinal absorption of
iron due to cadmium may be one mechanism for this anaemia.
After parenteral exposure, iron administration has a beneficial
effect on the anaemia (Friberg, 1955). Berlin & Friberg (1960)
showed that cadmium injections caused erythrocyte destruction, but
there was no indication of an interference with haemoglobin
production. Haemolytic anaemia in rabbits was also reported by
Axelsson & Piscator (1966b).
7.2.6 Effects on blood pressure and the cardiovascular system
Chronic oral administration of cadmium compounds to rats (Perry
& Erlanger, 1974) induced statistically significant elevation of
blood pressure. However, the systolic pressure changes were much
smaller than those previously reported by Schroeder & Vinton (1962)
and by Schroeder (1964). Furthermore, a different effect was
obtained with lower, as compared with higher, doses of cadmium. Rats
given 1, 2.5, or 5 mg cadmium/litre in drinking-water for one year
had significantly higher blood pressure values than controls. Six
months after the beginning of exposure, a statistically significant
increase in blood pressure was also observed in rats given 10 or
25 mg/litre, but the increase was not statistically significant
after one year of exposure. In rats receiving 50 mg/litre, a
statistically significant increase in systolic blood pressure was
observed after 12 months of exposure. As discussed in section 8.2.4,
these results may be of basic importance in evaluating data on the
possible effects of cadmium on the human cardiovascular system.
Several mechanisms have been postulated (Perry & Erlanger,
1974) to explain the effects of chronic cadmium exposure on the
cardiovascular system. Oral adminstration of cadmium doses that
induce hypertension (and also parenteral administration of cadmium)
was shown to increase circulatory renin activity (Perry & Erlanger,
1973). Injection of cadmium into the renal artery of dogs increased
sodium reabsorption by the exposed kidney (Vander, 1962), and
repeated intramuscular (Perry et al., 1971) or chronic oral
administration of cadmium (Lener & Musil, 1971) was reported to
increase sodium retention in the body. By morpho-metric methods,
Fowler et al. (1975) demonstrated effects on the renal blood vessels
of rats exposed to various concentrations of cadmium (up to
200 mg/litre in drinking-water) for several weeks. Significantly
smaller arteriolar diameters were found in the exposed animals than
in the controls.
Perry et al. (1977) studied the influence of exposure duration
(from 3 to 24 months) and cadmium dose levels in water (from 0.1 to
10 mg/litre) on the blood pressure of Long-Evans rats. A small
increase in blood pressure occurred even at the lowest exposure
level after 3 months exposure. The greatest increase in blood
pressure (3.2 kPa; 24 mmHg) occurred after 24 months exposure to
1 mg/litre, when the average renal cortex cadmium level was 12 mg/kg
wet weight. At higher dose levels, the blood pressure increase was
less and, at the highest dosage (10 mg/litre for 24 months), the
blood pressure did in fact decrease.
Perry et al. (1976) found hypertensive effects in
Sprague-Dawley as well as Long-Evans rats. Petering et al. (1979)
reported that male rats were more susceptible to these effects than
female rats after exposure via drinking-water, but Ohanian & Iwai
(1980) found the opposite for rats exposed parenterally. In several
studies (e.g., Kotsonis & Klaassen, 1977; Whanger, 1979; Fingerle et
al., 1982), there was no increase in blood pressure after various
cadmium doses were given via drinking-water. The type of diet
appears to be crucial for the development of hypertension (Whanger,
1979); it can usually only be produced in rats fed a rye-based diet
(Perry & Erlanger, 1982). Nishiyama et al. (1986) postulated that
cadmium exposure increases sodium and water retention, which are
important factors controlling the development of hypertension.
A detailed review of factors influencing the effects of cadmium
on the cardiovascular system was reported by the Task Group on Metal
Toxicity (1976), with particular reference to those factors that
might modify the dose-effect and dose-response relationships.
Rats exposed to cadmium (5 mg/litre) in drinking-water (Kopp et
al., 1980a,b, 1983) developed electrocardiographic and biochemical
changes in the myocardium, and impairment of the functional status
of the myocardium. These effects could be related to (i) decreased
high-energy phosphate storage in the myocardium, (ii) reduced
myocardial contractility, or (iii) diminished excitability of the
cardiac conduction system. Jamall & Sprowls (1987) found that rats
fed a diet supplemented with copper (50 mg/kg), selenium
(0.5 mg/kg), and cadmium (50 mg/kg) had marked reductions in heart
cytosolic glutathione peroxidase, superoxide desmutase, and
catalase. They suggested that heart mitochondria are the site of the
cadmium-induced biochemical lesion in the myocardium.
Reviews of all aspects of the cardiovascular effects of cadmium
on experimental animals have been reported by Perry & Kopp (1983)
and Jamall & Smith (1986).
7.2.7 Effects on reproductive organs
Cadmium-induced testicular necrosis (section 7.1.2.1) generally
results in permanent infertility (Barlow & Sullivan, 1982). Ramaya &
Pomerantzeva (1977) found markedly reduced testis weights 1, 3, and
6 months after mice were administered 4 mg cadmium/kg. The animals
were sterile and microscopic examination revealed morphological
changes in the testis. Krasovskii et al. (1976) noticed decreased
spermatozoa motility and spermatogenesis index in rats continuously
exposed via food to 0.5-5.0 mg cadmium/kg body weight. In male mice
exposed repeatedly by daily subcutaneous injection of cadmium
chloride (0.5 mg/kg per day) for 6 months (Nordberg, 1975), there
was a decrease in normal testosterone-dependent proteinuria.
Morphological examination of the seminal vesicles revealed a smaller
weight and size as well as histological indications of lower
secretory activity, this being consistent with decreased
testosterone activity in these animals.
7.2.8 Other effects
Effects on the immune system have been reported after both
chronic and acute cadmium exposure. A decrease in the number of
antibody-forming cells in the spleen as well as a decrease in
antibody production was seen in mice after long-term exposure to
cadmium in drinking-water (Koller et al., 1975). An inhibition of
the cell-mediated immune response occurred in mice after repeated
intraperitoneal injections (Bozelka & Burkholder, 1982). However, no
data are available linking these effects to increased susceptibility
to infection or other secondary dysfunctions.
Gestational exposure to cadmium (4.2 and 8.4 µg/ml in
drinking-water) results in decreased birth weight, retarded growth,
delayed development of the sensory motor coordination reflexes, and
increased motor activity. Cadmium exposure during critical periods
of development might result in developmental and behavioural
deficits with long-term implications for adult behaviour (Mohd et
al., 1986).
7.3 Fetal toxicity and teratogenicity
In several species of laboratory rodents, large doses of
cadmium salts induce severe placental damage and fetal deaths when
given at a late stage of pregnancy, and teratogenic effects, such as
exencephaly, hydrocephaly, cleft lips and palate, microphthalmia,
micrognathia, clubfoot, and dysplastic tail, when given at early
stages of gestation.
A single subcutaneous injection of cadmium chloride, acetate,
or lactate (4.5 mg cadmium/kg body weight) given to Wistar rats from
the 17th to the 21st day of pregnancy (Parizek, 1964) led to the
rapid development of severe placental damage in all rats and to
fetal death. Placental damage was not dependent on the presence of
fetuses, but it was not possible to decide whether fetal lethality
resulted from the placental lesion or from a direct effect of
cadmium on the fetuses (Parizek, 1964). Similar effects were
observed at a dose level of 3.3 mg cadmium/kg body weight (Parizek
et al., 1968b). Placental damage and fetal deaths were also observed
after cadmium administration to pregnant Swiss albino mice
(Chiquoine, 1965).
Teratogenic effects can be observed when doses close to the
LD50 (Table 11) for cadmium salts are administered to pregnant
females at critical stages of embryogenesis. These effects were
demonstrated with intravenous injections of cadmium sulfate in
hamsters (Ferm & Carpenter, 1968; Mulvihill et al., 1970; Ferm,
1972) and with intraperitoneal (Barr, 1973), subcutaneous (Chernoff,
1973), or dietary (Scharpf et al., 1972) administration of cadmium
chloride in rats. Teratogenic effects induced by cadmium salts have
also been demonstrated in mice (Ishizu et al., 1973).
The character of the changes induced is dependent on the
species and on the stage of embryogenesis. As little as 123 µg/litre
in mouse embryo cultures produced exencephaly apparently by
re-opening the closed neural tube (Schmid et al., 1985). Either
facial defects or limb abnormalities were induced by cadmium when
administered to pregnant hamsters on day 8 or 9 of gestation (Ferm,
1971). Both jaw defects and cleft palate were observed in the
offspring of rats given daily subcutaneous cadmium chloride
injections (8 mg/kg body weight) on days 13-16 or 14-17 of
pregnancy, but cleft palate was not observed when this dosage was
given on days 15-18 or 16-19 of pregnancy (Chernoff, 1973).
Anophthalmia or microphthalmia and dysplastic ears were induced by
approximately 2 mg of cadmium as the chloride given
intra-peritoneally to pregnant rats on the 9th but not on the 11th
day of pregnancy (Barr, 1973). Other effects observed in these
studies included decreased lung weight in the offspring of rats
subjected to cadmium during pregnancy (Chernoff, 1973) and
deficiencies in bone formation and delays in bone ossification
(Mulvihill et al., 1970; Scharpf et al., 1972).
The dose-dependent fetal mortality and teratogenicity response
was established in studies with subcutaneous administration of
cadmium chloride to rats (Chernoff, 1973) and mice (Ishizu et al.,
1973). The no-observed-effect level with respect to malformations
was found in the latter study to be 0.33 mg/kg body weight.
All the teratogenic effects mentioned above were induced by
parenteral administration of very high doses of cadmium salts.
However, in a rat study by Scharpf et al. (1972), very high peroral
doses (20, 40, 60, or 80 mg/kg body weight given by gavage daily
from days 6 to 19 of pregnancy) of cadmium chloride were used with
the simultaneous administration of sodium chloride, and internal
teratological examinations were performed. Heart and kidney
abnormalities were the major internal defects, but their incidence
was not directly related to the dose of cadmium chloride
administered. At the lowest dose level, heart abnormalities were
detected in 19.7% of the 127 fetuses (abnormalities in the control
group were seen in 6.6% of 107 fetuses) and teratoma of the kidney
was observed in 15.7% of these fetuses.
Cvetkova (1970) exposed pregnant female rats via the
respiratory route to cadmium sulfate (2.8 mg/m3, 4 h daily) and,
on the 22nd day, killed half of them to examine the embryos. The
number of embryos in exposed rats was the same as in a control
group, but the mean weight was lower in the exposed group. In the
exposed rats, where pregnancies were allowed to proceed to full
term, the average weight of the offspring was lower than in the
controls both at birth and after 8 months. The rats born to the
exposed group also had increased mortality during the first 10 days
after birth.
When mice were exposed for several generations to cadmium in
drinking-water (10 mg/litre), fetal mortality, runting, and
malformations were observed (Schroeder & Mitchener, 1971). The
external malformations, consisting of sharp angulation of the distal
third of the tail, were observed in 16.1% of 255 offspring (F1 and
F2A generation), and 87 deaths before weaning (30.5%) were
recorded.
Ferm & Carpenter (1968) showed that zinc injected
simultaneously with cadmium could protect against the teratogenic
effects of cadmium, and a similar protective action was found for
selenium (Holmberg & Ferm, 1969). Maternal zinc deficiency can
produce congenital malformations (Hurley et al., 1971). This was
confirmed by Parzyck et al. (1978), who also found that
intraperitoneal injection of 1.5 mg cadmium/kg body weight to
pregnant rats increased the prevalence of malformations. The
increase was greater at this cadmium dose than the increase due to
zinc deficiency. Combined zinc deficiency and cadmium exposure
caused a very high incidence of fetal deaths.
Further experimental data on rats provided by Samarawickrama &
Webb (1979) indicate that maternal cadmium exposure gives rise to a
fetal zinc deficiency and that this is one cause of the teratogenic
effects observed. Intravenous cadmium injections to pregnant rats at
doses ranging from 0.25 to 1.25 mg/kg body weight on day 12 of
gestation produced a dose-related decrease in fetal uptake of a dose
of 65 mg zinc given 4 h later. Maternal cadmium exposure (1.25 mg/kg
body weight) was shown to result in decreased activity of a fetal
zinc-dependent enzyme thymidine kinase, which is responsible for the
incorporation of thymidine in DNA. Additional evidence that
cadmium-induced fetotoxicity is related to a cadmium-induced fetal
zinc deficiency was reported by Daston (1982), who found that
co-administration of zinc (12 mg/kg body weight) almost totally
eliminated severe fetal lung lesions when pregnant rats were given
cadmium (8 mg/kg body weight) on gestation days 12-15.
7.4 Mutagenicity
Studies on Drosophila (Ramel & Friberg, 1971; Vorobjeva &
Sabalina, 1975) failed to show any chromosomal abnormalities after
exposure to various cadmium compounds. Some in vitro studies of
cultured human lymphocytes and fibroblasts were also negative (Paton
& Allison, 1972; Deknudt & Deminatti, 1978; Kogan et al., 1978).
Shiraishi et al. (1972) reported a marked increase in the frequency
of chromatid breaks, translocations, and dicentric chromosomes in
leucocytes, from one person, cultured in a medium containing 62 mg
cadmium/litre (as the sulfate) for 4-8 h.
Andersen et al. (1983) found that the average chromosome length
in human lymphocytes was initially reduced when they were cultured
in a medium containing cadmium chloride (1.1 mg cadmium/litre), but
subsequently returned to normal. This effect was probably related to
the synthesis of metallothionein and complexing with cadmium.
Watanabe et al. (1979) observed aneuploidy in rat oocytes with
cadmium accumulation in the ovary after exposure to cadmium chloride
in vivo.
Rohr & Bauchinger (1976) found a reduced mitotic index in
hamster fibroblasts cultured in 100 µg cadmium/litre (as the
sulfate) and chromosome damage at concentrations above 500 µg
cadmium/litre. Deaven & Campbell (1980) showed that the effects on
cultured hamster cells depended on the type of medium used.
There appears to be an acute effect of cadmium following the
injection of 0.6-2.8 mg cadmium/kg body weight (Felten, 1979). After
6 h, there was an increased frequency of chromatid breaks in bone
marrow cells and chromosome gaps and breaks in spermatocytes, which
could be associated with the acute effects on haematopoiesis
(section 7.2.5) and on the testis (section 7.1.2.1).
A summary and graphical presentation of the available evidence
on genetic and related effects of cadmium in various in vivo and
in vitro test systems has been presented by IARC (1987a). Although
prokaryote test systems reveal no effects, variable results have
been observed in lower eukaryotes, mammalian cells in vitro, and
mammals in vivo.
7.5 Carcinogenicity
Intramuscular or subcutaneous administration of metallic
cadmium or cadmium compounds can induce sarcomata at the site of
injection. This local effect of cadmium was demonstrated with
intramuscular administration of metallic cadmium (cadmium powder in
fowl serum) to hooded rats (Heath et al., 1962), subcutaneous
administration to Chester Beatty rats of cadmium as the sulfide and
oxide (Kazantzis, 1963; Kazantzis & Hanbury, 1966) or sulfate
(Haddow et al., 1964), intramuscular injection of cadmium chloride
to Wistar rats (Gunn et al., 1967), and subcutaneous injection to
Sprague-Dawley rats (Nazari et al., 1967). Transplantability of
tumours induced in these studies (Heath & Webb, 1967) and metastases
into regional lymph nodes and into lungs (Kazantzis & Hanbury, 1966)
were reported. Intratesticular injection of cadmium chloride to
White Leghorn cockerels was reported to induce teratoma at the site
of injection (Guthrie, 1964).
After Hoffman et al. (1985) injected 1.9 mg cadmium chloride
(1.2 mg/kg body weight) directly into the ventral prostatic lobe of
100 12-month-old male rats, simple hyperplasia was found in 38 of
the rats, atypical hyperplasia in 29, atypical hyperplasia with
severe dysplasia in 11, and invasive prostatic cancer in 5 animals.
Hoffman et al. (1988) reported changes in the ultrastructure of
prostate epithelial cells in rats injected into the ventral prostate
with 2.2 or 3.3 mg cadmium/kg body weight. In animals given oral
treatment via the drinking-water (29.9 or 115 mg cadmium/kg body
weight), there were changes ranging in severity up to dysplasia but
no evidence of carcinoma.
A single parenteral administration of cadmium salts can induce
necrosis of the testis (see section 7.1.2.1). After one year, the
remnants of the necrotic testis were shown to contain masses of
cells showing the typical structure of Leydig cells (Parizek, 1960).
This regeneration of testicular Leydig cells damaged by cadmium can
result in Leydig cell neoplasia (Gunn et al., 1963, 1965; Lucis et
al., 1972). The ultrastructural features of cadmium-induced Leydig
cell tumours correspond in most respects with the fine structural
features of normal Leydig cells (Reddy et al., 1973).
Other injection studies and peroral studies did not demonstrate
increased malignancy (Schroeder et al., 1964, 1965; Loser, 1980),
but the doses were low compared to those necessary to induce renal
damage. In one peroral study (Kanisawa & Schroeder, 1969), rats were
exposed to 5 mg cadmium/litre in drinking-water for up to 2 years.
There were 7 malignant tumours among 47 cadmium-exposed male rats
and 2 tumours among 34 male control rats. This indicates a doubling
of the tumour rate, but because of the low statistical power of the
study, the increase was not statistically significant and the
authors concluded that ingestion of these cadmium doses was not
carcinogenic.
Some studies have been specially designed to investigate the
possible role of cadmium in cancer of the prostate. The prostate
gland, like the testis, is of particular interest with respect to
cadmium toxicity because these organs contain greater concentrations
of zinc than any other tissues and it has been suggested that
cadmium may affect prostate growth by competition with zinc (Gunn et
al., 1961). Levy et al. (1973) gave three groups of rats weekly
subcutaneous injections of cadmium sulfate at concentrations of
0.022, 0.044 or 0.087 mg cadmium per rat (average weight 220 g at
the start and 410 g at the end of the 2-year exposure). Weekly
injections of water were given to the 75 control rats. The liver
cadmium level was 80 mg/kg in the highest-dose group, but no
malignant changes were found in the prostate. No difference was seen
between exposed and control rats with respect to malignant changes
in other organs.
Levy & Clack (1975) and Levy et al. (1975) conducted 2-year
studies in rats and mice designed to detect carcinogenic effects in
the prostate. The animals in both experiments were given weekly
administrations of cadmium sulfate by stomach tube. The rats were
given from 0.08 to 0.35 mg/kg body weight and the mice 0.44 to
1.75 mg/kg. Extremely low levels of cadmium were found in the kidney
after 2 years (5 mg/kg wet weight in rats), but no macroscopic or
microscopic changes were seen in any tissue at these low doses.
In a long-term study on Fisher rats, Sanders & Mahaffey (1984)
administered cadmium oxide (25 µg) in single or repeated doses by
intratracheal instillations. There was no evidence for pulmonary or
prostate carcinogenicity, but increases in mammary tumours and in
tumours at multiple sites in male rats were reported.
It has been reported that inhalation of a cadmium aerosol
causes lung cancer in Wistar rats (Takenaka et al., 1983). Three
groups of 40 rats were continuously exposed to cadmium chloride
aerosols for 18 months, the air cadmium concentrations being 12.5,
25, and 50 µg/m3. A control group of 41 rats was also studied. The
study was terminated after 31 months, and no lung cancers were seen
in the control group. However, in the exposed groups, the incidence
was 15%, 53% and 71%, respectively, at increasing exposure levels.
Even at these relatively low exposure levels, there was a clear
dose-response relationship. Histologically the experimentally
induced tumours were adenocarcinomas, epidermoid carcinomas,
mucoepidermoid carcinomas, and combined epidermoid and
adenocarcinomas.
In a subsequent study, rats were exposed to inhalable aerosols
of cadmium sulfate and cadmium oxide and fume and dust at > 30 µg
cadmium/m3 and to cadmium sulfide at > 90 µg cadmium per m3 for
periods of up to 18 months. Bronchoalveolar benign and malignant
adenomas, squamous cell carcinomas, and combined forms developed at
high primary tumour rates with all four forms of cadmium tested even
after discontinuous exposure for 40 h/week for 6 months. No primary
tumour was found with cadmium oxide fume at a concentration of 10 µg
cadmium/m3 or cadmium oxide dust (at 30 µg cadmium/m3) when
combined with a zinc oxide aerosol (Oldiges et al., 1989).
In a further study, male and female Syrian golden hamsters and
female NMRT mice were exposed to cadmium chloride, sulfate, oxide,
and sulfide at concentrations of between 10 and 270 µg cadmium/m3.
The exposure was continuous (19 h/day, 5 days/week) for 50 to 70
weeks and was followed by a 50-week observation period. No increase
in the lung tumour rate was observed in either the mice or hamsters
(Heinrich et al., 1989), but in both species exposure to cadmium
caused multifocal bronchoalveolar hyperplasia, the extent of which
varied with the compound used, its concentration, and the length of
exposure. The most severe changes were found after cadmium oxide
inhalation (Aufderheide et al., 1990).
A synergistic effect has been shown in rat renal tumours
induced by dimethylnitrosamine when followed by cadmium chloride
given by intramuscular injection. In this study, cadmium appeared to
enhance the initiation of dimethylnitrosamine-induced cancer (Wade,
1987).
7.6 Host and dietary factors; interactions with other trace
elements
The toxic effects of cadmium in experimental animals have been
shown to be dependent on genetic factors, stage of ontogenic
development, functional state of the organism, and simultaneous or
previous exposure to certain environmental influences, including
exposure to certain nutrients.
Resistance to cadmium-induced testicular necrosis is determined
by a single autosomal recessive gene (cdm) in inbred mice (Taylor et
al., 1973). The teratogenic effects of cadmium are dependent on the
stage of embryogenesis (section 6.3). The stage of postnatal
development of certain organs may be of importance for the toxic
effects of cadmium, as has been shown for the testis (Parizek, 1957,
1960), ovaries (Kar et al., 1959), and central nervous system
(Gabbiani et al., 1967).
Pretreatment with small, non-toxic doses of cadmium salts has
been shown to induce resistance to testicular or lethal effects
(Terhaar et al., 1965; Ito & Sawauchi, 1966). The probable mechanism
is induction of metallothionein synthesis by the pretreatment. This
enables the subsequent dose of cadmium to be bound rapidly to
metallothionein, which renders it less acutely toxic (Nordberg,
1971). Similarly, the protective effect of zinc against cadmium
toxicity could also be, at least in part, dependent on the induction
of an increased synthesis of metallothionein-like proteins (Webb,
1972; Davies et al., 1973).
Selenium compounds are known to be highly effective in
preventing the reproductive toxic effects of cadmium (Kar et al.,
1960; Mason & Young, 1967; Parizek et al., 1968a,b), lethality to
rats (Parizek et al., 1968b) and mice (Gunn et al., 1968), and
teratogenicity (Holmberg & Ferm, 1969). Fetal lethality (Parizek et
al., 1968b) and teratogenic effects (Holmberg & Ferm, 1969) can be
prevented when selenium compounds are given at the same time as
cadmium.
Simultaneous administration of mercuric and cadmium compounds
has been shown to have an additive effect (Gale, 1973). Oral
administration of nitrilotriacetate with large oral doses of cadmium
chloride provided protection against the lethality of cadmium and
had no potentiating effect on the teratogenicity and fetal
accumulation of cadmium (Scharpf et al., 1972). This was confirmed
by Engström (1979), who also showed that simultaneous oral exposure
to cadmium and sodium tripolyphosphate decreased the mortality
expected at the cadmium level used. However, when nitrilotriacetate
or sodium tripolyphosphate was given subcutaneously with cadmium,
the mortality rates were increased (Engström & Nordberg, 1978;
Andersen et al., 1982). The chelation of cadmium in the
gastrointestinal tract decreased the uptake of cadmium, whereas
parenteral exposure to cadmium and chelating agents caused a higher
renal cadmium concentration than cadmium alone.
The interaction of cadmium with certain trace elements can
produce symptoms characteristic of trace element deficiencies. As a
result, chronic cadmium toxicity in certain animal species closely
resembles zinc and/or copper deficiency and can be prevented by
administering higher doses of the salts of these trace elements
(Petering et al., 1971, 1979; Mills & Delgarno, 1972). Cadmium-
calcium interactions are discussed in section 7.2.4.
An increased toxicity of cadmium was reported in animals on
low-protein diets (Fitzhugh & Meiller, 1941), this being due partly
to rapid intestinal absorption of cadmium (Suzuki et al., 1969).
Lack of dietary calcium seems to play a role similar to lack of
dietary protein in increasing the toxicity of cadmium (Suzuki et
al., 1969). Supplements of dietary ascorbic acid almost completely
prevented cadmium-induced anaemia and improved the growth rate (Fox
& Fry, 1970).
Ambient temperature (Nomiyama et al., 1978b) and the energy or
protein level in the diet have been reported to influence the LD50
of cadmium in mice.
Various aspects of the interactions between cadmium and other
trace elements were discussed in greater detail by the Task Group on
Metal Interactions (1978).
7.7 Conclusions
Inhalation exposure at high levels causes lethal pulmonary
oedema. Single high-dose injection gives rise to testicular and
non-ovulating ovarian necrosis, liver damage, and small vessel
injury. Large oral doses damage the gastric and intestinal mucosa.
Long-term inhalation exposure and intratracheal administration
give rise to chronic inflammatory changes in the lungs, fibrosis,
and appearances suggestive of emphysema. Long-term parenteral or
oral administration produces effects primarily on the kidneys, but
also on the liver and the haematopoietic, immune, skeletal, and
cardiovascular systems. Skeletal effects and hypertension have been
induced in certain species under defined conditions. Teratogenic
effects and placental damage occur, depending on the relation
between the exposure and the stage of gestation, and may involve
interactive effects with zinc.
Of greatest relevance to human exposure are the acute
inhalation effects on the lung and the chronic effects on the
kidney. Following long-term exposure, the kidney is regarded as the
critical organ. The effects on the kidney are characterized by
tubular dysfunction and cell damage, although glomerular dysfunction
may also occur. A consequence of renal tubular dysfunction is a
disturbance of calcium and vitamin D metabolism. According to some
studies, this has led to osteomalacia and/or osteoporosis, but these
effects have not been confirmed by other studies. A direct effect of
cadmium on bone mineralization cannot be excluded. The toxic effects
of cadmium in experimental animals are influenced by genetic and
nutritional factors, interactions with other metals, in particular
zinc, and pretreatment with cadmium, which may be related to the
induction of metallothionein.
IARC (1976, 1987b) accepted as sufficient the evidence that
cadmium chloride, sulfate, sulfide, and oxide can give rise to
injection-site sarcomata in the rat and that the chloride and
sulfate can induce interstitial cell tumours in the testis of rats
and mice, but found oral studies inadequate for evaluation. One
recent life-time study (18 months), in which rats were subjected to
continuous inhalation of a cadmium chloride aerosol at low
concentration, showed a high incidence of primary lung cancer with
evidence of a dose-response relationship. Studies on the genotoxic
effects of cadmium have given discordant results, most of the
positive results indicating chromosomal effects after short-term
high-level exposure.
8. EFFECTS ON HUMANS
Most of the available epidemiological studies or group
observations, as well as the clinical studies, have been performed
either on occupationally exposed workers or on Japanese populations
in cadmium-polluted areas. A great deal of epidemiological data has
resulted from studies in polluted areas of Japan (Cooperative
Research Committee on Itai-itai Disease, 1967; Shigematsu et al.,
1978; Japan Cadmium Research Committee, 1989) and, more recently,
from smaller studies in other countries (Drasch et al., 1985;
Philipp, 1985; Hahn et al., 1987; Roels et al., 1989; Thun et al.,
1989; Likutova, 1989). Comprehensive summaries of these studies have
also been published (Tsuchiya, 1978; Friberg et al., 1986; Nomiyama,
1986).
Many of these studies have focused on the detection of early
signs of kidney dysfunction. Others have investigated clinical signs
of disease such as renal stones and pulmonary impairment. Until the
middle of the 1970s, particular attention was given in Japan to the
detection of and screening for bone disease (e.g., Itai-itai
disease). More recently the role of cadmium in human carcinogenesis
and mortality has also been studied.
Exposure to cadmium produces a wide variety of effects
involving many organs and systems. From the point of view of
preventive medicine, the detection of early effects on the kidneys
is of particular importance in order to prevent more serious renal
effects and those on the lungs or bones. Recent studies indicating
that chronic exposure to cadmium may give rise to cancer will be
reviewed in some detail.
8.1 Acute Effects
8.1.1 Inhalation
Acute cadmium poisoning and, in some cases, death have been
reported among workers shortly after exposure to fumes when cadmium
metal or cadmium-containing materials have been heated to high
temperatures (Beton et al., 1966; Blejer, 1966; Dunphy, 1967). The
principal symptom in acute cases, both fatal and non-fatal, is
respiratory distress due to chemical pneumonitis and oedema
(MacFarland, 1979; Lucas et al., 1980). At an early stage, the
symptoms may be confused with those of "metal fume fever".
In working environments where cases of acute poisoning
occurred, cadmium concentrations were usually very high. For
instance, in one case the fatal air concentration of cadmium oxide
fume from a furnace was approximately 50 mg/m3 for a period of
about 1 h (a dose of 2900 mg/m3.min) (Barrett & Card, 1947). In
another case, the lethal dose was 2600 mg/m3.min (Beton et al.,
1966), i.e. a 5-h exposure to 8.6 mg/m3. Friberg et al. (1974)
estimated that an 8-h exposure to 5 mg cadmium/m3 may well be
lethal.
8.1.2 Ingestion
During the period 1940-50, cases of acute food poisoning
occurred mainly due to the substitution of cadmium for scarce
chromium in the plating of many cooking utensils and containers.
Food contamination arose when acid foods and drinks were prepared
and stored in contact with cadmium-plated surfaces. Rapid onset with
severe nausea, vomiting, and abdominal pain were characteristic
symptoms (US Public Health Service, 1942; Cole & Baer, l944; Lufkin
& Hodges, 1944). Effects also occurred following the consumption of
drinks with a cadmium concentration of approximately 16 mg/litre
from an automatic vending machine in which drinking-water was cooled
in a tank constructed with cadmium-containing solder (Nordberg et
al., 1973). Recovery from acute poisoning appears to be rapid and
complete. The amount of cadmium absorbed is probably very limited
due to vomiting and the consequential short presence of cadmium in
the gastrointestinal tract. However, no follow-up studies of people
who have experienced acute cadmium poisoning have been reported.
8.2 Chronic Effects
Lower cadmium concentrations with longer periods of exposure
than those described above will cause chronic cadmium poisoning.
Fully developed poisoning among industrial workers shows two main
effects: renal dysfunction and emphysema (Friberg, 1948a,b, 1950).
The kidney is most frequently the critical organ, but under certain
conditions (short-term peak exposures) it may be the lung (Bonnell,
1955). For people in the general environment, exposure is usually by
the oral route and the kidney is the critical organ.
8.2.1 Renal effects and low molecular weight proteinuria
8.2.1.1 In industry
Renal dysfunction is one of the characteristic signs of cadmium
poisoning, and many cadmium workers have developed proteinuria,
renal glucosuria, and aminoaciduria. In working environments with
high cadmium exposure levels, workers have also developed
hypercalciuria, phosphaturia, and polyuria (Friberg, 1950; Clarkson
& Kench, 1956; Kazantzis et al., 1963; Tsuchiya, 1967; Lauwerys et
al., 1974a, 1979b), and some have suffered from renal colic due to
recurrent stone formation (Friberg, 1950; Ahlmark et al., 1961;
Adams et al., 1969; Scott et al., 1976; Kanzantzis, 1979). The
polyuria is due to loss of urinary concentrating ability (Kazantzis,
1979), and, in addition, the kidneys of cadmium-poisoned workers
lose their ability to handle an acid load after a standard
NH4Cl-loading test. These are signs of distal tubular damage, and
in a few severe cases, the renal damage progresses to a reduction in
glomerular filtrations (see section 8.2.1.5).
Renal function, as measured by inulin or creatinine clearance
and urine concentrating capacity, was depressed in several poisoning
cases (Friberg, 1950; Bonnell, 1955; Bernard et al., 1979). Thus, in
the more advanced cases, there is a combination of tubular and
glomerular effects. In most of the early cases, only proteinuria,
mild in comparison with the proteinuria in many other renal
disorders, has been reported as a sign of renal dysfunction, and
other signs of kidney dysfunction were not evident (Piscator,
1966a,b).
Since Friberg first observed the urinary proteins of cadmium
workers (Friberg, 1950), the proteinuria has proved to involve
proteins with a molecular weight of 10 000 to 40 000 and is the
so-called tubular proteinuria (Butler & Flynn, 1958). Table 15
contains data on proteins in urine useful for the diagnosis of
cadmium-induced proteinuria. The increased excretion of low
molecular weight proteins in urine from cadmium-exposed workers has
been found to apply to ß2-microglobulin, lysozyme (muramidase),
ribonuclease, immunoglobin chains, retinol-binding protein, and
alpha1-microglobulin (Piscator, 1966a; Peterson et al., 1969;
Peterson & Berggård, 1971; Lauwerys et al., 1974a; Bernard et al.,
1976, 1982b). In groups of exposed and unexposed workers, the
urinary ß2-microglobulin concentrations follow log-normal
distributions, and an operational definition for what is an
"increased" level should be established for each population studied
(Kjellström et al., 1977a).
Proteinuria is known to be an early sign of cadmium poisoning,
but the degree of proteinuria varies with time. In a group of 40
workers with heavy exposure to cadmium, it was found that
proteinuria was persistent and even increased several years after
cessation of exposure, as evaluated by qualitative methods (Friberg
& Nystrom, 1952; Piscator, 1966a). Tsuchiya (1976) examined five
cadmium-exposed workers who showed proteinuria. Ten years after
cessation of exposure, three of them no longer revealed proteinuria,
but two of these showed a high urinary ß2-microglobulin level, as
did the two workers with persistent total proteinuria. Four workers
in a British pigment factory still had grossly elevated
ß2-microglobulin levels despite removal from exposure many years
earlier (Stewart & Hughes, 1980).
Table 15. Excretion of urinary proteins in healthy people and in cases of glomerular and tubular disorders
Protein type Normal plasma Normal filtered Urinary excretion (mg/24 h) Reference
concentration amount in primary Healthy Glomerular Tubular
(mg/ml) urine (mg/24 h) people disorders disorders
Total protein 43-127 310-54 100 129-1570 Peterson et al. (1969)
Albumin 50 500 3.9-24 88-48 800 13.8-578 Mogensen & Solling (1977)
Retinol-binding 0.11 20-150 Peterson & Beggard (1971)
protein
0.04 3 45 Kanai et al. (1971)
ß2-microglobulin 0.002 300 0.06-0.21 0.06-4.7 1.1-105 Peterson (1971)
0.073 Evrin et al. (1971)
Lysozyme 0-2a Prockop & Davidson (1964)
0.07-1.1a 47-130a Harrison et al. (1968)
Ribonuclease 0.24-1.5a 1.9-10a Harrison et al. (1968)
a Values reported as mg/litre
According to recent observations using quantitative proteinuria
methods (Roels et al., 1982), total proteinuria in 19 workers had
not changed 4 years after exposure ceased. In those 11 workers for
whom urinary ß2-microglobulin was measured before and after
cessation of exposure, an increase was invariably seen. Eight of the
workers had abnormal ß2-microglobulin levels before exposure
ceased, whereas three had normal levels before and developed
abnormal levels after cessation. It can be concluded that
cadmium-induced tubular proteinuria is irreversible in most workers,
at least for several years.
A marked increase in urine cadmium level may reflect
cadmium-induced nephropathy if exposure has been chronic and
correlates with low molecular weight proteinuria (section 6.5.1.2).
Lauwerys et al. (1979b) proposed a biological threshold of 10 µg
cadmium/µg urinary creatinine for males occupationally exposed to
cadmium. Smith et al. (1980) found that workers with low exposure to
airborne cadmium had an average urinary cadmium level of
13.1 µg/litre, whereas workers with long histories of work in areas
with substantial airborne cadmium had an average level of
45.7 µg/litre. The high-exposure group showed a significant
reduction in urinary clearance and increased ß2-microglobulin
excretion. Buchet et al. (1980) found increased excretion of both
low and high molecular weight proteins and tubular enzymes in
workers excreting more than 10 µg cadmium/g creatinine or with a
blood cadmium level above 10 µg cadmium per litre.
Retinol binding protein (RBP) has been shown to correlate well
with ß2-microglobulin in urine with a pH value greater than 5.5.
and is equally sensitive for detection of tubular proteinuria
(Bernard et al., 1982a,b). This protein occurs in serum complexed to
prealbumin and retinol, but, after retinol is delivered to target
cells, RBP rapidly dissociates from prealbumin, is filtered through
the glomerulus, and is reabsorbed by the tubule (Peterson, 1971).
Renal tubular brush border enzymes may also be excreted in
chronic cadmium poisoning. In patients with Itai-itai disease,
urinary trehalase activity correlates inversely with tubular
resorption of phosphorus (Nakano et al., 1987) and there is a
statistical correlation between urinary trehalase and other urinary
indicators of renal tubular dysfunction, such as glucose,
ß2-microglobulin, cadmium, and alpha-amino nitrogen, in
inhabitants of chronic cadmium-polluted areas in Japan (Nogawa et
al., 1980).
Other renal effects in cadmium workers include glucosuria,
aminoaciduria, impaired concentrating abilities, and hypercalciuria
(Kazantzis et al., 1963; Scott et al., 1976), which may cause
disturbances in bone and calcium metabolism (section 8.2.2). The
hypercalciuria leads to renal stone formation in some workers
(section 8.2.2.1). An increased excretion of amino acids,
particularly of serine and threonine, has been shown in industrial
workers (Clarkson & Kench, 1956), but the amino acid excretion
pattern was not consistent. In a cadmium worker with osteomalacia
(Kazantzis, 1979), there was an increase excretion of
hydroxyproline, which could be an effect of changes in collagen
metabolism related to the bone disorder (section 7.2.4).
The renal effects of cadmium that lead to proteinuria may
progress and, in some cases, with high exposures, lead to an
increase in blood creatinine. This has contributed to a
higher-than-expected mortality rate among highly exposed workers
(Kjellström et al., l979). In a Swedish battery factory, there were
four deaths from nephritis or nephrosis among 185 workers, all of
whom had been exposed to cadmium for more than 15 years. The
expected number of deaths due to these causes in this group of
workers was 0.4 (P = 0.05) (Andersson et al., l984). In a study of
about 6995 cadmium-exposed British workers (Armstrong & Kazantzis,
1983), there were 10 deaths due to nephritis or nephrosis, whereas
15.3 deaths were expected. In the subgroup with the highest exposure
(211 workers), one death occurred (0.3 expected). A 5-year follow-up
of this study (Kazantzis et al., 1988) confirmed no excess mortality
from nephritis and nephrosis (ICD 580-584), the number of observed
deaths now having increased to 16 (18.9 expected). Armstrong &
Kazantzis (1985) conducted a case control study of this cohort in
which a more detailed assessment of the past exposure of workers was
obtained. There was a marginally increased, but not statistically
significant, risk from nephritis and nephrosis (ICD 580-584) in
workers with "ever high" or "ever medium" exposure to cadmium.
Existing studies of mortality from nephritis/nephrosis have
been based upon epidemiological studies of renal failure given as
the underlying cause of death on death certificates. With the advent
of kidney dialysis and transplantation, patients with kidney failure
frequently survive and die of other causes. If kidney failure is
indicated at all on their death certificates, it is frequently given
as a contributing rather than underlying cause of death.
No studies on the contribution of cadmium-induced renal effects
to morbidity, absence from work, etc., have been published, although
one study (Vorobjeva & Eremeeva, 1980) reported increased
cardiovascular disease and related increased work absence among
cadmium workers (section 8.2.4).
8.2.1.2 In the general environment
In Japanese cadmium-polluted areas, signs of renal dysfunction
very similar to those in cadmium-exposed industrial workers have
been found. Proteinuria and glucosuria were found to be common
(30-80%) among the exposed people in one area (Ishizaki, 1969) and
less common among people living in control areas and areas bordering
a polluted area. In the exposed groups, a positive correlation
between age and the prevalence of signs was also seen (section
8.3.2). Due to the cumulative nature of cadmium, the total dose is
directly correlated to age.
With the large number of elderly people and women included in
the groups exposed in the general environment, factors other than
cadmium that can affect renal function may make direct comparisons
with industrial workers difficult. Nevertheless, the tubular
proteinuria (Shiroishi et al., 1977), aminoaciduria, and other signs
of renal tubular damage (Saito et al., 1977; Nogawa et al. 1984)
were very similar to the findings for industrial workers. In
addition, as in the case of exposed workers, elevated urinary
excretion of metallothionein occurs as a result of environmental
cadmium exposure (Tohyama et al., 1981b).
Among cadmium-exposed people in the general environment, the
mean urine ß2-microglobulin excretion was highest (Shiroishi et
al., 1977) in patients with Itai-itai disease (section 8.2.2.2).
Many of the Itai-itai patients also have signs of decreased
glomerular filtration, as indicated by decreased urea clearance
(Nakagawa, 1960) and increased serum creatinine (Nogawa et al.,
1979).
8.2.1.3 Methods for detection of tubular proteinuria
Determination of total protein and electrophoretic analysis of
concentrated urinary protein were originally the common methods for
the detection and diagnosis of tubular proteinuria. Quantitative
immunological methods (detection limit, 0.002-0.003 mg/litre) for
the measurement of ß2-microglobulin (Evrin et al., 1971) and RBP
(Bernard et al., 1982b) in urine ("normal" level about 0.1 mg/litre)
are available, and these methods facilitate the detection of tubular
dysfunction. An electrophoretic method with reasonable sensitivity
(0.8 mg/litre) for measuring specific proteins in the urine utilizes
staining of proteins in sodium dodecyl sulfate acrylamide gel
electrophoresis (Nomiyama et al., 1982b). More recently, radio-,
latex-, and enzyme-linked immunoassays have been developed (Evrin et
al., 1971; Bernard et al., 1982a; Carlier et al., 1981). However,
the disadvantage with ß2-microglobulin as a marker for renal
tubular dysfunction is that this protein is unstable if urinary pH
is less than 5.5.
RBP has an advantage over ß2-microglobulin, particularly for
screening purposes, in that serum levels and, hence, excretion are
not affected as readily by concomitant immunological disease and are
more stable at an acidic pH (Bernard et al., 1982b). An enzyme-
linked immunosorbent assay (ELISA) for urinary RBP has also been
described (Topping et al., 1986).
Common tests for qualitative determination of proteinuria, such
as paper tests (dip sticks), the nitric acid test, and the boiling
test, should not be used for screening cadmium-induced proteinuria,
since positive readings will be obtained only at fairly high urine
protein concentrations (Piscator, 1962). Trichloroacetic acid (TCA)
or sulfosalicylic acid (SA) can be used for qualitative tests, but a
negative result does not exclude a moderate increase in low
molecular weight proteinuria.
8.2.1.4 Significance of cadmium-induced proteinuria
More than 70% of proteins with a molecular weight less than
15 000 but less than 5% of those with a molecular weight greater
than 40 000 pass through the glomerular membrane (Squire et al.,
1962). The glomerular filtrate contains relatively large amounts of
plasma proteins, which are normally almost completely reabsorbed in
the proximal tubules and only small amounts are found in the urine.
The increased excretion of tubular proteins in cadmium nephropathy
is thought to be due mainly to a decreased tubular reabsorption
capacity. This provides early evidence of renal tubular dysfunction.
The concentration of albumin in normal serum is about 25 000
times higher than the concentration of ß2-microglobulin (Table
15). In spite of the fact that very little of the albumin but about
80% of the ß2-microglobulin is filtered through the glomeruli
(Maack et al., 1979), the filtered amount of albumin is still higher
than the amount of ß2-microglobulin (Table 15).
Only a small fraction of the albumin or the other proteins is
excreted in normal urine due to the normally efficient (more than
99%) tubular reabsorption of all proteins (Table 15). The urinary
albumin excretion is about 100 times greater than the
ß2-microglo-bulin excretion (Table 15).
ß2-Microglobulin is a subunit of a major immunoglobulin
complex with a molecular weight of 12 000 and normally occurs in
serum at a concentration of approximately 2.0 mg/litre. In the case
of a decreased glomerular filtration rate, the serum concentration
of ß2-microglobulin will also increase. In certain conditions, for
example, where excessive production occurs as in some cancers and
autoimmune disorders, serum levels increase, the tubular capacity
for reabsorption may be exceeded, and the concentration in the urine
will rise. In tubular dysfunction, the capacity for absorption is
impaired and, again, this is reflected by an increased excretion in
the urine. Such renal disorders include the congenital and acquired
Fanconi syndrome, diabetic nephropathy, certain cases of reflux
nephropathy, and advanced glomerular disease (Squire et al., 1962).
A raised urinary excretion of ß2-microglobulin or other low
molecular weight protein is not, therefore, specific to renal
dysfunction induced by cadmium, and a differential diagnosis should
be considered in all cases where this occurs.
The "normal" average urinary excretion of ß2-microglobulin
measured in several populations was in the range 0.05-0.1 mg/24 h
(or mg/litre or mg/g creatine) (Kjellström & Piscator, 1977). Below
age 65, there was very little or no change with age in the urinary
ß2-microglobulin (Kjellström & Piscator, 1977), a fact confirmed
by later studies (Tsuchiya et al., 1979; Kowal & Kraemer, 1982). In
all of the studies, some high individual values were found in the
age group above 65 years, and the studies of Tsuchiya et al. (1979)
and Kowal & Kraemer (1982) reported age-regression coefficients
indicating an increase with age. However, there was wide variation
with age and the average urinary ß2-microglobulin levels in the
oldest age groups (above 65 years) were only 10-20% higher than in
the other age groups (Kowal & Kraemer, 1982). The prevalence of
increased low molecular weight proteinuria (above 0.5 mg/litre) was
less than 5% in these studies, but in a control group of people
above age 80 from a Japanese cadmium-polluted area (section 8.3.2.2)
the prevalence was about 15%.
8.2.1.5 Glomerular effects
Although renal tubular dysfunction with its accompanying low
molecular weight proteinuria is thought to be the most prominent
renal effect of cadmium, the ß2-microglobulinuria is sometimes
accompanied by the excretion of high molecular weight proteins such
as albumin (molecular weight, 69 000). This albuminuria may
occasionally occur as a result of cadmium exposure without any
concomitant increase in the urinary excretion of low molecular
weight proteins (Bernard et al., 1976, 1979); this indicates that
cadmium, in some cases, may produce a change in the glomerular
permeability to larger proteins.
Cadmium may also affect the glomerular filtration rate (GFR).
Friberg (1950) reported decreased inulin clearance in
cadmium-exposed battery workers. Elinder et al. (1985a) measured GFR
by chromium-EDTA in 17 workers previously exposed to cadmium fumes.
They found a significant negative correlation between decreasing GFR
and tubular reabsorption loss, and reported that GFR decreased with
increasing cumulative exposure to cadmium fumes. The urinary
clearance of ß2-microglobulin increased with decreasing GFR.
Several other occupational studies have reported increased
serum concentrations of creatinine and/or ß2-microglobulin,
indicating reduced GFR, in cadmium-exposed workers (Thun et al.,
1989; Roels et al., 1989).
Thun et al. (1989) found a small increase in mean serum
creatinine in a group of 45 cadmium-exposed workmen. Serum
creatinine also increased with cadmium dose, suggesting decreased
glomerular function. Cadmium dose remained the important predictor
of serum creatinine even after controlling for age, blood pressure,
body size, and other extraneous factors.
Roels et al. (1989) measured the serum creatinine and serum
ß2-microglobulin levels of 23 workers, removed from cadmium
exposure, on several occasions over a period of six years. The
average yearly decrease in GFR was estimated to be 6 ml/min per
1.73 m2, which is considerably more than the normal value
(< 1 ml/min per 1.73 m2) and significantly more than that of a
control group examined at the same time.
There is also evidence of glomerular effects in people exposed
to cadmium in the environment. Nogawa et al. (1980) suggest that a
reduction in creatinine clearance may be detected at the early stage
of cadmium poisoning in a polluted area. In addition, Nogawa et al.
(1984) reported a significant correlation between decreased tubular
reabsorption of phosphate and decreased GFR in farmers living in a
cadmium-polluted area.
The mechanism for the glomerular effects from cadmium is
uncertain. It has been suggested that cadmium-induced tubular damage
leads to a certain degree of interstitial nephritis which in turn
results in a decreased GFR (Elinder et al., 1985a). It has also been
proposed that cadmium exerts a direct toxic effect on the glomerulus
(Roels et al., 1989).
8.2.1.6 Relationship between renal cadmium levels and the
occurrence of effects
The number of reports of renal pathology in autopsy cases and
renal biopsies that contain data on kidney cortex concentrations of
cadmium is small. Thus, it is difficult to establish a dose-response
relationship between cadmium content and pathology or dysfunction.
Nomiyama (1977) summarized data from 26 cadmium-exposed workers
and 16 cadmium-exposed people from the general environment. The
criteria for choosing the subjects were that they possessed high
renal and/or high liver concentrations of cadmium, morphological
studies on the kidney had been performed, and data were available on
the occurrence of proteinuria while the person was alive. Among the
42 cases reviewed, those exhibiting slight or no proteinuria and no
morphological alterations had higher concentrations of cadmium in
the renal cortex than non-exposed people. Most cases with
morphological changes plus proteinuria had lower renal cadmium
concentrations that those without proteinuria and/or morphological
changes. In more recent studies, Ellis et al. (1985) found that in
cases of renal dysfunction the mean liver and kidney cadmium values
for retired workers were lower than those for active workers. These
findings are similar to those from animal studies (section 6.5.1.2),
where kidney concentrations levelled off or even declined in the
presence of kidney damage.
The use of in vivo neutron activation analysis has
facilitated the study of the relationship between renal cadmium
levels and occurrence of effects (Ellis et al., 1981a; Roels et al.,
1981b). However, the data must be assessed with caution as the
accuracy of this method has not yet been fully determined. For
instance, the exact location of the kidney needs to be known.
Erroneously low renal cadmium levels were reported by Roels et al.
(1981b) due to an error in adjusting for the distance between skin
and kidney (Roels et al., 1983a). Data from Ellis et al. (1981a) and
Roels et al. (1983a,b) have shown that few cases with increased
urinary ß2-microglobulin concentrations are seen when the level of
renal cortex cadmium is less than 150 mg/kg tissue and that of liver
cadmium is less than 40 mg/kg. There is a pattern of liver and
kidney cadmium levels increasing simultaneously until the average
renal cortex cadmium concentration is about 300 mg/kg and the
average liver level is about 60 mg/kg. At higher liver levels, the
renal cortex level is disproportionately low in most cases, and, in
addition, many of these workers have increased urinary
ß2-microglobulin.
Skerfving et al. (1987) measured kidney cortex cadmium levels
by X-ray fluorescence in a group of 20 workers from a factory
producing alkaline batteries and found an average value of 147 mg/kg
(range 53-317). When compared to a control group, these workers had
higher average urine levels of cadmium (5.4 vs 0.8 nmol/mmol
creatinine) and ß2-microglobulin (14.6 vs 6.6 µg/mmol creatinine).
Six workers had ß2-microglobulin levels exceeding 22 µg/mmol
creatinine. Due to selection procedures the results are, however,
not predictive for cadmium-exposed workers in general. Nevertheless,
it is clear that there are no significant correlations between
levels of cadmium or ß2-microglobulin in urine and cadmium levels
in the kidney. These results suggest that there is a relationship
between renal cadmium and occurrences of effects on a group basis
but renal cadmium levels per se are not always predictive of
pathological effects on an individual level.
8.2.1.7 Reversibility of renal effects
The potential for reversibility of renal effects has been
studied in populations of workers with occupationally induced
cadmium nephropathy as well as in residents of cadmium-polluted
areas.
a) Occupational exposures
In a group of 40 workers heavily exposed to cadmium, it was
found, using qualitative methods, that proteinuria was persistent
and sometimes even increased several years after cessation of
exposure (Friberg & Nystrom, 1952; Piscator, 1966a).
Tsuchiya (1976) studied a group of 13 workers who had been
exposed to cadmium fumes (133 µg/m3) and who had proteinuria
(determined by the trichloroacetic acid method) and abnormal
electrophoretic urine patterns (ß2-microglobulin levels above
40 000 µg/litre). A 10-year follow-up study of five of these
patients was carried out after improvements had been made in their
working environment (cadmium fumes, 20 µg/m3). The proteinuria was
reversed (measured using a single radial immuno-diffusion method) in
three of the five patients: ß2-microglobulin values were 3500,
2600 µg/litre, and not detectable (limit of detection
2000 µg/litre), and retinol-binding proteins (RBP) were not
detectable (limit of detection 500 µg/litre). In addition, there
were improvements in the remaining two patients (ß2-microglobulin,
9700 and 5500 µg/litre; RBP, 34 000 and 120 000 µg/litre). Tsuchiya
(1976) suggested that the difference in the period of exposure to
cadmium was the reason for this difference in the degree of recovery
from the effects of cadmium.
Stewart & Hughes (1981) reported on similar cases from a
British pigment factory. Despite the fact that exposure ceased many
years earlier, grossly elevated ß2-microglobulin levels were still
detected.
Using quantitative methods to detect proteinuria, Roels et al.
(1982) found that total proteinuria in 19 workers was unchanged 4
years after exposure had ceased. In those 11 workers for whom
urinary ß2-microglobulin was measured before and after cessation
of exposure, levels were invariably increased. Eight of the workers
had abnormal levels of ß2-microglobulin even before cessation of
exposure, whereas three had normal levels before cessation and
developed abnormal levels afterwards.
Roels et al. (1989) examined 23 workers once a year for 5 years
after removal from exposure to cadmium. These workers had been
exposed to cadmium for periods of 6 to 41.7 years (mean 25 years),
and their first follow-up examination took place when they had been
removed from exposure for an average of 6 years. Their mean age at
that time was 58.6 years (range 45.5-68.1 years). Cadmiumuria in
these workers had been assessed three years previously by measuring
the cadmium levels in liver and kidney using neutron activation
analysis. The cadmium concentrations (mg/kg wet weight) in the liver
and kidney cortex ranged from 24 to 158 (mean 61) and from 133 to
355 (mean 231), respectively. Although cadmium concentrations in the
blood and urine decreased significantly over the five-year period,
urine concentrations of albumin, ß2-microglobulin, and RBP did not
change significantly.
Harada (1987) conducted studies on the health status of seven
workers exposed prior to 1972 to high cadmium levels in a cadmium
sulfide dye manufacturing factory. These workers were examine for 15
years, improvements having been made to working conditions in 1974
which led to markedly decreased cadmium exposures. The cadmium
content of the blood and urine declined after the improvements in
working conditions but increased again when production rose. The
working condition improvements resulted in a marked reduction in
urine ß2-microglobulin level in five workers (e.g., from 1272 to
520 µg/g creatinine in 1 year in one worker and from 2090 to
503 µg/g creatinine in 6 months in another), but there was elevated
urinary ß2-microglobulin excretion when production increased. One
worker had fairly constant near-normal urinary levels of
ß2-microglobulins (55 to 183 µg/g creatinine) regardless of
workplace improvements or production levels. Initially, four workers
had low GFR values, but none of the seven workers showed any
decrease in glomerular filtration during the 15-year follow-up
period. TRP rates decreased in three workers but remained relatively
unchanged in the other four. These changes in GFR and TRP seemed to
be independent of cadmium exposure levels.
Elinder et al. (1985b) found that urine cadmium excretion
decreased in 14 out of 19 workers re-examined at least once five
years or more after exposure to cadmium, but renal tubular function,
as measured by urinary ß2-microglobulin excretion, had
deteriorated or not improved in nearly all of the workers. Thun et
al. (1989) concluded that "time since last exposure to cadmium" was
not an important determinant of renal outcome whether considered on
its own or together with the cadmium dose. In their study of 45
workers at a plant that recovered cadmium from industrial waste, 9
out of 15 workers with the highest ß2-microglobulin excretion had
not been exposed to cadmium for at least five years and one for 45
years. This study suggested that if cadmium nephropathy is
reversible, the recovery is so slow as to be indiscernible after
decades of non-exposure.
Ellis et al. (1985) showed that the liver cadmium levels in
workers no longer exposed to cadmium gradually declines. Persistence
of renal tubular dysfunction after cessation of exposure may reflect
the level of body burden and the transfer of cadmium from liver to
kidney.
b) Residents of cadmium-polluted areas
Reversibility of renal tubular dysfunction has been
investigated in residents of cadmium-polluted areas in Japan. Kasuya
et al. (1986) carried out a comparative study of urine
ß2-microglobulin determinations made in 1975 and 1985 for 93
people with Itai-itai disease and their family members. Urine
ß2-microglobulin levels improved in the group with
ß2-microglobulin levels of 1000 µg/g creatinine or less but
worsened in the group with ß2-microglobulin levels of 3000 µg/g
creatinine or more. Most of the people who recovered were aged 39
years or less and had been resident for 30 years or less. It was
considered that mild renal dysfunction among young individuals was
reversible.
Saito (1987) measured the urine ß2-microglobulin levels of
residents of cadmium-polluted areas for 3 years after improvements
had been made in the level of soil contamination and compared the
results with determinations obtained 7 years previously. During this
3-year period, the urine ß2-microglobulin levels tended to remain
unchanged in people with a concentration of around 1000 µg/litre.
The reversibility of ß2-microglobulinuria, glucosuria, and
aminoaciduria was examined in 74 inhabitants (32 males and 42
females) over 50 years of age who lived in a cadmium-polluted area.
Examinations were conducted just after the cessation of cadmium
exposure and 5 years later. The geometric mean concentrations of
ß2-microglobulinuria, glucosuria, and aminoaciduria indicated a
significant increase in excretion during the 5-year period. In cases
where the level of ß2-microglobulinuria exceeded 1000 µg/g
creatinine at the time cadmium exposure ceased, evidence was found
indicating significant increases in proteinuria after 5 years,
whereas in cases where the excretion of ß2-micro-globulin had been
less than 1000 µg/g creatinine no significant changes were observed
(Kido et al. 1988).
8.2.2 Disorders of calcium metabolism and bone effects
8.2.2.1 In industry
Friberg (1950) observed 7 cases of renal stones among 43
cadmium workers and drew attention to the possibility of renal
stones being associated with exposure to cadmium. Ahlmark et al.
(1961) found that 44% of a group of 39 cadmium workers exposed to
cadmium oxide dust for more than 15 years had a history of renal
stone formation. Nine stones from six workers were analysed, and in
four workers the stones were composed of basic calcium phosphate
(Axelsson, 1963). There was an increase in the mean calcium
excretion rate in the cadmium-exposed group as compared to a control
group. Kidney stones were also found in 12 out of 43 British workers
at an accumulator factory (Adams et al., 1969). It is noteworthy
that in both of these studies (Axelsson, 1963 and Adams et al.,
l969) there was a higher prevalence of renal stones in workers
without proteinuria than in those with proteinuria. The men with
proteinuria had, as a group, increased urinary excretion of calcium
and phosphate, whereas in the group without proteinuria there were a
few cases with hypercalciuria. Hypercalciuria (81%) and renal stones
(19%) were also reported among 27 coppersmiths exposed to cadmium
(Scott et al., 1976, 1978).
Elinder et al. (1985a) found an increased prevalence of renal
stones among cadmium workers with tubular proteinuria, and Mason et
al. (1988) observed decreased renal reabsorption of calcium among
cadmium alloy workers.
Seven of the 12 workers in a cadmium pigment factory
investigated by Kazantzis et al. (1963) were found to have a urinary
calcium excretion rate greater than 300 mg/day (at least in 1 of 2
specimens). There was no evidence of excessive calcium intake in
these men. Five of these seven workers had been exposed to cadmium
compounds for more than 25 years and also had tubular proteinuria.
The remaining two, who had been exposed for 2 and 12 years,
respectively, had no other abnormality except for a urinary calcium
excretion of 308 and 403 mg/day. Follow-up was possible with six of
the twelve men, including all five with hypercalciuria and
proteinuria (Kazantzis, 1979). Six of the seven who had
hypercalciuria when first examined continued to have a raised
urinary calcium excretion, and one further worker developed
hypercalciuria during the follow-up period. All those with
hypercalciuria also had tubular proteinuria, although this was
marginal in one of the workers. Blood calcium levels remained within
normal limits in all cases (Kazantzis, 1979). The occurrence of
disordered calcium metabolism in all seven men followed-up for a
number of years makes it very likely that a common environmental
factor, such as occupational exposure to cadmium compounds, was
causative.
The data of Kazantzis (1979) agree with the findings of
hypercalciuria among 27 coppersmiths with high cadmium exposure
(Scott et al., 1976, 1978). Scott et al. (1980) reported that in 15
cadmium-exposed men the amount of calcium in the whole body was
lower than that of controls and decreased with duration of an
increased exposure to cadmium. The cadmium-induced hypercalciuria
could be reduced by thiazide treatment (Scott et al., l979).
In a study by Thun et al. (1989) of workers at a plant that
recovered cadmium from industrial waste, 8 of 45 exposed workers had
experienced kidney stones, in contrast to one of 32 unexposed
workers. Increase in the urinary excretion of ß2-microglobulin and
RBP was accompanied by decreased renal tubular reabsorption of
calcium and phosphates.
In contrast to the above findings, a low urinary calcium
excretion was detected in 47 out of 81 workers with exposure to a
variety of cadmium compounds and also cadmium oxide fumes (Tarasenko
& Vorobjeva, 1973; Tarasenko et al., 1975). The 24-h excretion of
calcium in these workers was below 100 mg, compared with 115-210 mg
in a control group of 21 people. Blood calcium values were within
the normal range in all cases.
Radiological examination was performed on 32 workers exposed
for 4-20 years to cadmium compounds (concentrations ranging from 0.1
to 5.5 mg/m3). All of them complained of pains in the bones.
Pseudofractures suggestive of osteomalacia were seen in two workers
exposed for 16 and 19 years, but no histological confirmation of
osteomalacia was obtained. Radiological appearances described as
enostosis were reported in five cases and periosteal proliferation
and consolidation in a further three cases (Tarasenko & Vorobjeva,
1973; Tarasenko et al., l975).
Horstowa et al. (1966) performed radiological examination of
the skeleton in 26 alkaline battery workers with signs of chronic
cadmium intoxication out of 80 workers exposed to 0.13-1.17 mg
cadmium/m3 for 1-12 years. Seven of these workers had proteinuria
detected by sulfosalicyclic acid; pseudofractures were found in 3
workers, sclerotic foci in 13, and osteoporosis in 10. In another
alkaline battery factory, where a number of cases of severe cadmium
poisoning were diagnosed (Friberg, 1950), X-ray examinations
revealed no signs of bone disease.
One of the workers with multiple tubular defects studied by
Kazantzis et al. (1963) developed osteomalacia confirmed by
histological examination 10 years after the initial investigation
(Kazantzis, 1979). He previously displayed hypercalciuria but, at
the time of diagnosis, his urinary calcium excretion was low.
Extensive investigation failed to reveal any of the other generally
accepted causes of osteomalacia such as malabsorption or nutritional
deficiency.
In a study of 43 workers at a battery plant, one worker
developed osteomalacia without evidence of malabsorption or
nutritional deficiency but with multiple renal tubular defects
(Adams et al., 1969). Another case of osteomalacia from the same
factory was subsequently detected in a man who had been a cadmium
battery worker for 40 years (Adams, 1980). Eight years before
retirement he had a partial gastrectomy due to a duodenal ulcer.
Proteinuria was first diagnosed 6 years before retirement, but
otherwise he was in "apparent good health" and "on a balanced diet"
until 8 years after retirement. He was then frail, had pains in his
legs, and a "waddling gait". His serum alkaline phosphatase level
was increased, X-rays showed generalized osteoporosis, and a bone
biopsy showed osteomalacia. After 1 year of treatment with large
doses of vitamin D, he could walk well again.
It also seems likely that the six workers exposed to cadmium
oxide dust described by Nicaud et al. (1942), who had pains in the
back and limbs and showed multiple pseudofractures on radiological
examination, suffered from osteomalacia. More detailed data on these
workers was presented by Valetas (1946), who also pointed out that
"massive doses" of vitamin D were needed to improve the symptoms. It
took several months for improvement to occur and the vitamin D
treatment had to be maintained for several years to keep the workers
in stable health. Valetas (1946) concluded that this bone disease
was caused by occupational exposure to cadmium. Eight workers with
8-30 years of exposure to lead dust and cadmium oxide fume and dust
(Gervais & Delpech, 1963) were also found to have multiple
pseudofractures and pains in the back, thorax, and legs. Very
limited biochemical investigations were carried out, but in four
cases proteinuria was found. The authors suspected that lead
exposure led to the observed effects.
8.2.2.2 In the general environment
Bone disease and abnormalities of calcium metabolism from
exposure to cadmium in the general environment have only been noted
in people in Japan with the clinical syndrome referred to as
Itai-itai disease. The main characteristics of the disease are
osteomalacia1 and osteoporosis2 with a tendency to fractures
accompanied by severe pain and renal tubular dysfunction. The
results of epidemiological and clinical investigations indicate an
association with cadmium exposure, although the Co-operative
Research Committee on Itai-itai Disease (1967) stated that
"malnutrition (low protein, low calcium diets) and multiple
pregnancies may also be involved".
1 Osteomalacia is characterized by inadequate mineralization of
bonematrix, resulting in an increase in the relative amount
of osteoid tissue. It represents the adult counterpart of
childhoodrickets (Robbins et al. 1984).
2 Osteoporosis is defined as an excessive but proportional
reduction in the amounts of both the mineral and matrix phases of
bone unaccompanied by any abnormality in structure of the residual
bone (Robbins et al., 1984).
Itai-itai disease is an endemic bone disease prevalent in the
basin of the Jinzu river, which runs through the central part of
Toyama Prefecture in West-Central Japan (Kono et el., 1956). It is
characterized by osteomalacia in combination with renal tubular
dysfunction in most cases. Patients also have osteoporosis and one
of the most characteristic symptoms is severe bone pain. Hagino &
Yoshioka (1961) reported that high concentrations of cadmium, lead,
and zinc were present in autopsy tissues from people with Itai-itai
disease and in the everyday foods of the endemic area.
Systematic epidemiological investigations, which included
extensive mass health examinations as well as case control studies
on both patients and controls, started in 1962 (Cooperative Research
Committee on Itai-itai Disease, 1967). It was reported that
Itai-itai disease in Toyama Prefecture was restricted to a limited
area (Fuchu area) irrigated by the Jinzu river, the geographical
distribution of the patients being consistent with the levels of
cadmium concentration in the paddy fields, and that the
concentrations of cadmium in urine were higher in patients than in
controls. The total number of patients was estimated in 1955 by the
Toyama Prefecture to be 41 out of a total of 1666 residents (849
women) (Cooperative Research Committee on Itai-itai Disease, 1967).
The major source of cadmium pollution in the area was a mine 50 km
upstream from the endemic area (Japan Public Health Association,
1968).
The age and sex distribution of the patients displayed a very
distinct pattern. Clinically apparent cases were limited to women
over 40 years of age who had given birth to many children (6 on
average) and had lived in the area for more than 30 years. No
detailed data on past patients were available, but it was estimated
that the age of onset of the disease was probably between 35 and 65
years, and that almost 100 deaths had been reported up to the end of
1966. The incidence was presumably very high from 1936 to 1950 and
at its highest in 1946 and 1947, but decreased thereafter even
though the same cadmium exposure levels had been maintained. By
March 1989, 150 cases of Itai-itai disease had been officially
recognized as pollution-related disease. Whereas all cases have been
reported in the Fuchu area, there have been a few suspected cases in
2 out of 12 cadmium-contaminated areas of Japan other than the Fuchu
area (Table 7). Clinical features of five suspected cases from the
Ikuno area matched those of Itai-itai disease and urine cadmium
levels were very high (Nogawa et al., 1975). In one of these cases
an autopsy was performed; the liver cadmium level was very high
(75 mg/kg) but the renal cortex cadmium level was low (53 mg/kg)
(Nogawa et al., 1975).
Takebayashi (1980a,b, 1983a,b, 1984) and Takebayashi et al.
(1985, 1987a,b,c, 1988a,b,c,d) reported pathological findings in
kidney and bone from autopsies of eleven elderly men and women (3
males and 8 females; 72-95 years of age) from Tsushima Island. The
average levels of cadmium in the liver and kidney cortex were
92.4 mg/kg and 44.0 mg/kg, respectively. The authors considered the
histological osteomalacia and renal tubulopathy noted in eight cases
(Takebayashi, 1980, 1983a, 1984, Takebayashi et al., 1985, 1987a,b,
1988c,d) to be similar to Itai-itai disease from Toyama Prefecture.
However the Japan Cadmium Research Committee (1989), supported
by the Japanese Environment Agency, concluded, after these eight
cases had been examined by the expert group, that it was clinically
difficult to diagnose them as osteomalacia.
According to the Japan Cadmium Research Committee (1989),
diagnosed cases of Itai-itai disease were reported only in the Fuchu
area of Japan. It denied the presence of osteomalacia in five cases
in the Ikuno area, and stated that osteomalacia had not been
observed "clinically" in Tsushima Island.
A study by Kido et al. (1989) indicates that exposure to
cadmium could cause osteopenia, particularly in women. Bone density
was measured in 28 women with Itai-itai disease, 92 men and 114
women with cadmium-induced renal dysfunctions, and 44 men and 66
women living in three different non-polluted areas using a
microdensitometer. The values of indices corresponding to both
cortical width and bone mineral content were significantly lower in
Itai-itai disease patients than in cadmium-exposed women with renal
dysfunctions or in non-exposed subjects. The cadmium-exposed women
also showed a decrease in bone density compared with the non-exposed
subjects. A significant decrease in bone density was also observed
in cadmium-exposed men compared with non-exposed subjects, although
the difference was not as clear as it was in women.
Reviews (in English) of Itai-itai disease have been produced by
Tsuchiya (1969), Friberg et al. (1974), Tsuchiya (1978), and Nogawa
(1981).
8.2.2.3 Mechanism of cadmium-induced bone effects
The available data show that cadmium can affect calcium,
phosphorous, and bone metabolism in both industrial workers and
people exposed in the general environment. These effects may be
secondary to the cadmium effects on the kidneys but there have been
few studies of calcium metabolism in people with excess exposure to
cadmium. The increased prevalence of renal stones reported from
certain industries is probably one manifestation of the
cadmium-induced kidney effects. It is not known if factors other
than cadmium play a role.
Nogawa et al. (1987) reported that serum 1,25-dihydroxy-
vitamin D levels were lower in Itai-itai disease patients and
cadmium-exposed subjects with renal damage than in non-exposed
subjects. The reduction in these levels was closely related to serum
concentrations of parathyroid hormone and ß2-micro-globulin and to
the percentage tubular reabsorption of phosphate (% TRP), suggesting
that cadmium-induced bone effects were mainly due to a disturbance
in vitamin D and parathyroid hormone metabolism.
Osteomalacia has been reported in a few heavily exposed
industrial workers and people with Itai-itai disease. The industrial
cases are mainly male, whereas Itai-itai patients are almost
exclusively female. However, the clinical features and biochemical
findings are similar, except that Itai-itai patients may also suffer
from ostoporosis.
A possible mechanism for the development of osteomalacia has
been proposed (Kjellström, 1986). It is known that normal calcium
absorption in the intestines and normal bone mineralization is
dependent upon 1,25-dihydroxycholecalciferol. Vitamin D3 taken
into the body is converted to 25-hydroxy-vitamin D3 in the liver,
and then to 1,25-dihydroxy-vitamin D3 in the mitochondria of renal
proximal tubular cells, this being the biologically active species.
Cadmium accumulates in the proximal tubular cells, depressing
cellular functions, and this may result in reduced conversion of
25-hydroxy-to 1,25-dihydroxy-vitamin D3. This is likely to lead to
decreased calcium absorption and decreased mineralization of bone,
which in turn may result in osteomalacia.
8.2.3 Respiratory system effects
Cadmium workers may develop chronic injury to the respiratory
system, depending on the level and nature of exposure. The
development of such effects is often quite slow, so that they are
apparent only after several years of exposure. The rate of
development and severity appear to be roughly proportional to the
time and level of exposure.
8.2.3.1 Upper respiratory system
Chronic inflammation of the nose, pharynx, and larynx have been
reported by Vorobjeva (1958) and Horstowa et al. (1966). Anosmia is
a frequent symptom in cadmium workers after prolonged exposure. This
has been reported by, for instance, Valetas (1946), Friberg (1950),
Baader (1951), Vorobjeva (1958), Tarasenko & Vorobjeva (1973), and
Apostolov (1979), but was not observed by Tsuchiya (1967) or Suzuki
et al. (1965).
8.2.3.2 Lower respiratory system
Chronic obstructive lung disease of varying degrees of severity
is frequently seen in cadmium workers. Friberg (1950) reported
dyspnoea, impaired lung function with increased residual volume, and
reduced working capacity in a group of 43 cadmium workers. Similar
studies, which included the use of pulmonary function measurements,
by Bonnell (1955), Buxton (1956), Kazantzis et al. (1963), and Adams
et al. (1969) all showed impairment of respiratory function in
groups of workers with prolonged exposure. The symptoms and findings
were more suggestive of emphysema than bronchitis in these cases;
they were commonly diagnosed as emphysema but pathological
confirmation of this was rare (Smith et al., l960).
Tarasenko & Vorobjeva (1973) reported the presence of increased
lung markings in the chest X-rays of 17 out of 72 cadmium workers,
which were interpreted as being due to diffuse interstitial
fibrosis. Similar lung changes were observed in 21 out of 26 workers
studied by Horstowa et al. (1966).
The presence of chronic obstructive respiratory disease in
cigarette smokers exposed to an additional harmful environmental
agent presents difficulties in determining the contribution made by
the latter. Studies on the chronic respiratory effects of cadmium in
the past have not always been standardized for smoking. Lauwerys et
al. (1974a) did take smoking habits into consideration by matching
his cadmium-exposed and control groups for smoking habits. They
reported the presence of impaired lung function in a group of
cadmium workers exposed for over 20 years, but not in those with
shorter exposure. The degree of lung impairment found was small.
The effects on the lung increases the mortality of cadmium
workers with high exposures (Kjellström et al., 1979; Armstrong &
Kazantzis, 1983). In the latter study, the mortality for diseases
coded as bronchitis (ICD 490-491) was related to the intensity of
exposure, the group with the highest exposure having a highly
significant (almost 4-fold) excess risk (observed 13 expected 3.4).
A 5-year follow-up of this study (Kazantzis et al., 1988) confirmed
the earlier finding, the marked excess mortality being related to
both intensity and duration of exposure. The follow-up revealed an
excess mortality from emphysema, but this was seen only in the
low-exposure group.
8.2.4 Hypertension and cardiovascular disease
Despite the abundance of data showing that under certain
exposure conditions cadmium induces hypertension in animals, there
are very few results available from studies of cadmium-exposed
workers. Friberg (1950) examined 43 workers with a mean period of
exposure to cadmium oxide dust of 20 years (air concentration,
3-15 mg/m3) and 15 workers with a mean exposure period of 2 years.
The study included physical and roentgenological examinations of the
heart, electrocardiographic examination at rest and after exercise,
and measurement of blood pressure. No increased prevalence of
cardiac disease or pathological electro-cardiographic changes were
found. The majority of subjects had completely normal blood
pressure, but since Friberg did not examine blood pressure in the
control group, it is not possible to draw definite conclusions.
Chest examination and blood pressure measurements have also
been reported in other studies (Bonnell, 1955; Bonnell et al., 1959;
Kazantzis et al., 1963; Holden, 1969), but in no cases were there
findings of cardiac disease or hypertension due to cadmium exposure.
Hammer et al. (1972) found no relationship between exposure to
cadmium and blood pressure in superphosphate workers.
Vorobjeva & Eremeeva (1980) examined 72 female and 20 male
workers at a battery factory exposed to cadmium oxide dust at
concentrations ranging from 0.04 to 0.5 mg/m3. Blood pressure was
measured and electrocardiograms taken, but there was no control
group. The authors reported increased prevalence of hypertension and
absence from work due to hypertensive and ischaemic heart disease
among the exposed workers compared to what was considered normal.
Furthermore, several types of abnormalities in the electrocardiogram
of the exposed workers were observed: 39% showed tachycardia,
between 11 and 13% were regarded as normal, and 26% had changes in
the "R" spike (compared to the normal 7-9%). Increased QRS period
was observed in 45% of the workers compared to the normal values of
14-16%. The data presented in this report are especially interesting
in view of the evidence in rats (section 7.2.6) that suggests
myocardial effects from cadmium exposure. The results of the study
are, however, presented in a very condensed form and it is therefore
difficult to draw clear-cut conclusions.
In a retrospective study of 311 male workers in an alkaline
battery factory it was found that hypertensive workers had a longer
employment time than an age-matched control group from the same work
environment (Engvall & Perk, 1985). Again it is difficult to draw
conclusions from this study. In a study of cadmium-exposed workers
in the United Kingdom (Kazantzis et al., 1988), mortality from
hypertensive disease (ICD 400-404) over the total study period from
1943 to 1988 was elevated but not significantly (49 deaths occurred
as opposed to 41.3 expected). There was no relationship with
intensity of exposure. However, mortality from cerebrovascular
disease (ICD 430-438) was significantly lower (178 deaths occurred
as opposed to 230.3 expected). These findings do not suggest any
association between cadmium exposure and the development of
hypertension.
In contrast, Thun et al. (1989) found that mean systolic and
diastolic blood pressures were higher in 45 cadmium workers (134 and
80 mmHg, respectively) than in 32 male controls (120 and 73 mmHg
respectively). Blood pressure was measured systematically by a
single examiner on the right arm of subjects who had been seated for
at least 15 min. Systolic but not diastolic blood pressure was
significantly associated with cadmium dose in multivariate analyses.
Schroeder (1965, 1967) observed that people in the general
population dying from hypertensive and/or cardiovascular disease had
somewhat higher cadmium concentrations in liver and kidney tissues
than people dying from other causes. He suggested that cadmium could
be a causative factor for these diseases. Unfortunately, smoking
habits were not accounted for and it is likely that this was a
confounding factor. The same problem exists with a number of
subsequent studies on hypertension and cadmium in tissues, blood,
and urine.
A correlation between average air cadmium levels in cities in
the USA and mortality associated with hypertension and heart disease
has been reported (Carroll, 1966; Hickey et al., 1967). Again,
several confounding factors such as smoking habits, air pollutants
other than cadmium, and other environmental factors make it
difficult to draw conclusions concerning the effects of cadmium. In
a study by Staessen et al. (1984), the confounding variables age,
sex, body weight, and cigarette smoking were considered in a
multiple regression analysis of systolic and diastolic blood
pressure and the urinary excretion of cadmium and
ß2-micro-globulin. Negative correlations between blood pressure
and urinary cadmium or ß2-microglobulin were found in some groups.
As there was a very strong age effect on both blood pressure and
urinary cadmium, the meaning of the negative correlations is not
clear. In any case, these data do not support cadmium exposure as a
cause of hypertension.
Shigematsu et al. (1979) could find no evidence that blood
pressure was higher in polluted areas (1611 people sampled) of Japan
compared with control areas (1826 people). In a comparison of blood
pressure by prefecture (13 570 in the cadmium-polluted areas and
7196 in the control areas), the prevalence of hyper-tension was
found to be high in the polluted area of one of the eight
prefectures investigated. However, in the other seven prefectures,
the prevalence of hypertension tended to be lower in the polluted
areas (Japan Cadmium Research Committee, 1989).
In a study on cadmium-polluted areas in Japan by Nogawa et al.
(1981b), the cerebrovascular disease mortality rate among people who
had had cadmium-induced proteinuria was twice as high as that of
people in the same area without proteinuria. However, the difference
was not statistically significant. The number of men in the cohort
with proteinuria was 81 and the number without proteinuria was 1109.
Another study comparing administrative units containing polluted
areas with those without such areas (Shigematsu et al., 1981, 1982,
1983) found no difference in the cerebrovascular disease mortality
rates.
Data on a total population of 333 000 from both
cadmium-polluted and non-polluted areas were collected
retrospectively for a period of 6-30 years, based on vital
statistics or death certificates (Shigematsu et al., 1982). The
mortalities from all causes, including cardiovascular disease such
as cerebrovascular and hypertensive disease, in the general
population in the cadmium-polluted areas were no higher than, or in
some cases even lower than, those in the non-polluted areas.
A mortality study of Shipham residents and of a nearby control
village was reported by Inskip et al. (1982). The study population
consisted of 501 Shipham residents of whom 278 had died over a
40-year follow-up period. Overall mortality was low in both villages
compared generally with England and Wales. There was a small but
statistically significant excess mortality rate in Shipham from
hypertensive and cerebrovascular disease. The highest ratio of all
was for genito-urinary disease in Shipham men (but, with only eight
observed deaths, the result was only significant at the 10% level).
The Standardized Mortality Ratios (SMR) for nephritis and nephrosis
in both sexes were also slightly elevated, but there were only two
deaths for each sex from this cause. In men, the numbers of
prostatic and lung cancer deaths were approximately equal to the
expected numbers, and in neither case was the SMR in Shipham greatly
different from that in the control village.
8.2.5 Cancer
8.2.5.1 In industry
A number of epidemiological studies have been published. In
order to facilitate the interpretation of published data on the
relationship between cadmium exposure and cancer, the studies have
been grouped according to the types of industrial plants in which
they have been conducted. In some cases, more than one study has
been conducted at the same plant.
a) Nickel-cadmium battery plants
In an early study, Potts (1965) found that three out of eight
deaths in a small cohort of nickel-cadmium battery workers in the
United Kingdom with at least 10 years of exposure to cadmium oxide
dust were from carcinoma of the prostate. This study was extended by
Kipling & Waterhouse (1967) to include 248 men with at least one
year of exposure to cadmium oxide dust. Four deaths from carcinoma
of the prostate, including the three cases previously reported by
Potts (1965), were observed as opposed to an expected number of
0.58.
Sorahan & Waterhouse (1983) carried out a further investigation
of the same plant using a cohort of 3025 employees who started work
between 1923 and 1975 and had a minimum employment period of one
month. The method of regression models in life tables was used to
compare duration of exposed employment in those dying from relevant
causes with that of matched survivors in the same year of follow-up.
No new evidence of an association between occupational exposure to
cadmium and cancer of the prostate was found. However, there was an
excess mortality from cancer of the respiratory system significant
at the 5% level (89 cases, SMR = 127). As in other studies, data on
smoking habits were not available and confounding factors were
present in the form of exposure to nickel hydroxide and welding
fumes so that no firm conclusions about the pulmonary
carcinogenicity of cadmium could be drawn from this study.
Sorahan & Waterhouse (1983) reported on the incidence of
prostatic cancer in a subgroup of 458 workers employed for at least
1 year in a job involving high exposure to cadmium oxide dust. Eight
cancers were observed compared to two expected (SMR 400, P < 0.01).
However, exclusion of the four cases previously reported by Kipling
& Waterhouse leaves a non-significant excess incidence (P = 0.21),
from which the investigators concluded that if cadmium oxide is
potentially carcinogenic current risks are likely to be small.
In the most recent update of the nickel-cadmium battery plant
workers (Sorahan, 1987), the earlier findings were confirmed and
there was some evidence of an association between risk of death from
lung cancer and duration of employment in jobs with high or moderate
exposure among workers first employed in the period 1923-1946.
However, among workers first employed from 1947 to 1975 (the group
with the higher SMR for lung cancer), there was no evidence of such
an association. The authors concluded that the findings do not
suggest these nickel-cadmium battery workers had experienced an
elevated lung cancer risk as a consequence of exposure to cadmium
oxide dust.
In Sweden, Kjellström (1979) investigated the incidence of
cancer among 269 male nickel-cadmium battery workers. All workers
had been heavily exposed (on average about 1 mg cadmium/m3) for
five years or more to cadmium dust or fume, and were alive in 1959.
Fifteen workers were found to have cancer between 1959 and 1975. It
was calculated from national incidence rates that 16.4 new cases
would have occurred; only 2 were prostatic cancers while 1.2 were
expected. In a re-examination of the same cohort, there were 8
deaths from lung cancer with a non-significantly raised SMR of 133.
The SMRs increased progressively with increasing latent periods
without reaching statistical significance (Elinder et al., 1985c).
b) Copper-cadmium alloy plants
Copper-cadmium alloy workers in the United Kingdom who had
heavy past exposure to cadmium oxide fume on two sites, one urban
the other rural, were studied by Holden (1980a). There was an
increased lung cancer mortality at the urban site (8 observed versus
4.5 expected) and a significant deficit at the rural site (2
observed; 7.8 expected). Vicinity workers in the urban plant, where
the mean cadmium concentration averaged no more than 60 µg/m3),
also experienced a significantly increased lung and prostatic cancer
mortality (36 observed; 26.1 expected).
A case control study was performed (Kazantzis et al., 1989) in
the same copper-cadmium alloy plants where workers had experienced
heavy past exposure to cadmium oxide fume and dust, which had given
rise to a number of deaths coded as chronic cadmium poisoning.
Before and during the period 1939-1945, cadmium oxide fume levels
had been estimated to be up to 4 mg/m3. Cases and controls were
selected from the cohort previously studied by Holden (1980a).
Personal interviews conducted with a small number of long-term
employees revealed that arsenical copper had been additionally
produced by adding bags of arsenic trioxide to the molten copper and
stirring manually; this resulted in the evolution of dense white
clouds of arsenic fume. The case control study showed no evidence of
an increased risk of lung cancer associated with past cadmium
exposure but an approximately two-fold excess risk associated with
arsenic exposure.
Kjellström (1979) also investigated a cadmium-copper alloy
plant in Sweden where workers had been exposed to cadmium oxide
fumes and included 94 workers employed in 1940 or who started work
after that year. Four cases of prostatic cancer occurred as opposed
to 2.7 expected.
c) Cadmium recovery plant in the USA
An increase in prostatic cancer incidence was also found by
Lemen et al. (1976) in a study of 292 male smelter workers heavily
exposed to cadmium oxide dust or fumes. Air cadmium concentrations
in 1973 were up to 24 mg/m3 but generally below 1 mg/m3. There
were four deaths from cancer of the prostate (1.15 expected). There
were also 12 deaths from lung cancer (5.1 expected); the difference
was statistically significant.
Thun et al. (1985) expanded the Lemen et al. (1976) cohort to
include 602 workers who had been employed at this cadmium production
plant between 1940 and 1969 for at least 6 months. Exposure was to
cadmium in baghouse dust, a by-product of zinc smelting which was
processed to produce cadmium metal and cadmium oxide. The plant
functioned as an arsenic smelter up to the end of 1925, and small
quantities of lead, arsenic, thallium and indium were subsequently
produced at intervals. The vital status of the workers was
determined in 1978. A dose-response relationship was observed
between lung cancer mortality and cumulative exposure and was
statistically significant for workers whose exposure exceeded
2920 mg/m3.days. The SMR for this group was 280. The lung tumours
were, as far as can be determined, mostly of bronchogenic origin.
The authors accounted for smoking habits by obtaining questionnaires
from survivors or next-of-kin in 50% of the cohort members and for
arsenic exposure by measuring arsenic in certain parts of the plant.
d) Cadmium processing plants in the United Kingdom
Kazantzis & Armstrong (1982) and Armstrong & Kazantzis (1983)
investigated a large cohort of workers in England at 17 plants with
processes using cadmium. The cohort comprised 6995 cadmium-exposed
male workers born before 1940, first exposed before 1970, and not
included in any previous mortality study. Jobs were assessed for
each relevant year involving high, medium or low exposure to cadmium
on the basis of discussions with hygienists and employees with
knowledge of past working conditions, taking into account
environmental and biological monitoring data (e.g., cadmium urine
data > 20 mg/litre in the high-exposure group. The periods at risk
of the study population were classified on the basis of these
categories and recorded job histories into three groups: (i) those
workers continuously employed for more than one year in a job
assessed as entailing high exposure - "ever high"; (ii) those
workers continuously employed for more than one year in a job
assessed as entailing medium exposure, but who were never for more
than one year in a high-exposure job - "ever medium", and (iii) all
others. Actual deaths were compared with expected numbers calculated
from mortality rates for the population of England and Wales
corrected for regional variation. The 8th revision of ICD codes was
used and results were expressed as SMRs.
Only 3% of the workers (about 200) were assigned to the "ever
high" category. The mean duration of exposure was 11 years and the
mean interval from initial exposure to the end of the follow-up was
27 years. The SMR (all causes) for the entire population was 97.
There were no prostatic cancer deaths in the "ever high" and "ever
medium" exposure categories (0.4 and 2.5, respectively, expected),
and the number of deaths (23) in the "always low" group was close to
the expected value. There was a small, but not statistically
significant, excess of lung cancer in all categories, but in those
with more than 10 years exposure in the "always low" category this
excess was significant at the 5% level (100 observed, SMR 126).
Since there was no correlation between increase in lung cancer risk
and intensity of exposure, the authors concluded that it was
unlikely that the excess in the "always low" group was due to
cadmium.
A 5-year update of this study (Kazantzis et al., 1988)
confirmed no excess risk from prostatic cancer over the total study
period from 1943 to 1984 and no cases of prostatic cancer in the
medium- or high-exposure groups. The SMR was 99 as opposed to the
value of 90 in the initial study. However, there was now a
significant excess lung cancer mortality (277 observed deaths, 240.9
expected), giving a SMR of 115 (95% confidence interval, 101-129).
This excess risk was related to intensity of exposure, there being
12 deaths in the small high-exposure group as opposed to 6.2
expected (SMR, 194; 95% CI, 100-339), 41 deaths in the
medium-exposure group and 224 deaths in the low-exposure group (SMR
121 and 112, respectively; not significant). While there appeared to
be evidence of a dose-response relationship, it was not
statistically significant. The increased cancer risk mainly involved
those employed before 1940, rising with length of employment and
with length of follow-up.
Further studies have been conducted on workers at these 17
plants (Armstrong & Kazantzis, 1985; Ades & Kazantzis, 1988). A case
control study on lung cancer was carried out on workers in a large
lead-zinc-cadmium smelter. These workers formed 64% of the cohort of
6995 men, and the study included 70% of the lung cancer deaths
observed in the cohort as a whole (Ades & Kazantzis, 1988). There
was a significant excess lung cancer risk among the smelter workers,
and a significant trend with increasing duration of employment,
particularly evident among those employed for more than 20 years.
Quantitative estimates of exposure to cadmium and ordinal rankings
for lead, arsenic, zinc, sulfur dioxide and dust were used to
calculate cumulative exposures from job histories. However, matched
logistic regression analysis showed that the increasing risk of lung
cancer associated with increasing length of employment could not be
accounted for by cadmium exposure and did not appear to be
restricted to any particular process or department.
e) Summary of industrial studies
Increased mortality from lung cancer has been observed in
several occupational cohorts exposed to cadmium, and there is some
evidence of dose-response relationships in two of the examined
populations. Case control studies have not given support for such a
relationship. It is difficult to reach a firm conclusion about
causality, because in all of the occupational cohorts there has been
simultaneous exposure to other potential carcinogens (e.g., nickel,
arsenic, polyaromatic hydrocarbons) or other environmental
pollutants (e.g., sulfur dioxide). Information on tobacco smoking is
inadequate or entirely absent in all except two studies.
Investigations of the relationships between cadmium exposure and
prostatic cancer are inconclusive.
8.2.5.2 In the general environment
Elevated cadmium levels have been found in the liver and
kidneys of patients with bronchogenic carcinoma (Morgan, 1970;
Morgan et al., 1971). However, the authors stressed the possibility
that differences observed could reflect the effect of smoking
(section 5.1.3) or could represent a non specific association.
A study of the causes of death in areas of high cadmium
exposures in Japan (Shigematsu et al., 1982) revealed no difference
in age-adjusted cancer mortality rates between polluted and control
areas of the same prefecture. The mortality rate due to prostatic
cancer was elevated in two areas but only achieved statistical
significance (P < .01) in one area. It was not significant in two
areas including Toyama prefecture, which has the largest area of
pollution.
8.2.6 Mutagenic effects in human cells
An increased frequency of chromosomal aberrations in somatic
human cells is considered to be evidence of some exposure to
mutagenic agents. Shiraishi (1975) noticed an increased frequency of
chromosomal aberrations in lymphocytes obtained from 12 Itai-itai
patients compared to 9 female controls. However, this observation
was not confirmed by Bui et al. (1975) who examined cells from 4
Itai-itai patients and 4 controls.
Among cadmium workers, an increased prevalence of chromosomal
aberrations, compared to controls, was reported by Deknudt & Leonard
(1975) and by Bauchinger et al. (1976), whereas no such effect was
seen by O'Riordan et al. (1978). In none of these occupational
studies was the actual exposure to cadmium measured, and the
possible confounding effect from other industrial chemicals and
smoking was not considered.
Nogawa et al. (1986) did not find evidence for increased sister
chromatid exchange in people exposed to cadmium in the general
environment. IARC (1987a,b) reviewed the available evidence for
mutagenic and related effects and noted the differences in results
reported from different industrial environments.
In conclusion, it is not yet possible to say whether cadmium
causes mutagenic effects in humans.
8.2.7 Transplacental transport and fetal effects
There have been few studies on the fetal toxicity of cadmium
transported across the placenta. Maternal hypertension and decrease
in birth weight have been associated with elevated levels of cadmium
in the neonate (Huel et al., 1981). In addition, it is
well-established that the babies of mothers who are cigarette
smokers are smaller at birth than are those of non-smokers. The
ratio of placental zinc to cadmium is positively related to infant
birth weight in the case of pregnant smokers, and older pregnant
smokers are at higher risk for impaired fetal growth than are
younger ones (Cnattingius et al., 1985; Kuhnert et al., 1987a,
Kuhnert et al., 1987b). Multiparity is related to an increased
placental cadmium level in smokers and to a decreased placental zinc
level in both smokers and non-smokers. These results have been
interpreted as consistent with a depletion of zinc with increasing
number of births and a progressive increase in cadmium in smokers
because of the long half-life of cadmium (Kuhnert et al., 1988).
The cellular mechanisms and factors that influence
trans-placental transport of cadmium are not known. Metallothionein
has been identified in the human placenta and in fetal membranes at
term, and metallothionein synthesis is inducible in cultured
trophoblasts by treatment with cadmium (Waalkes et al., 1984). This
effect is seen with cadmium concentrations in the culture medium as
low as 52.2 µg/litre (0.5 µmol/litre) (Lehman & Poisner, 1984).
Higher levels of exposure to cadmium may have a direct toxic effect
on the placenta. In a test system involving perfusion of maternal
and fetal blood vessels in the isolated human placenta, it was shown
that perfusion of the maternal circulation with cadmium at a
concentration of 1.12 mg/litre (10 µmol/litre) resulted in the
deposition of 2.5 µg cadmium per g placenta (22 nmoles/g), but very
little of it was detectable in the fetal circulation. Perfusion of
the maternal circulation with higher concentrations of cadmium
produced placental cadmium concentrations of 11.2-16.8 µg/g
(100-150 nmoles) with stromal oedema, syncytiotrophoblastic
vesiculation and vacuolization of Hofbauer cells within 6-8 h,
followed by placental necrosis. These changes were associated with a
decrease in human chorionic gonadotropin release and decreased
movement of zinc into the fetal circulation (Miller, 1986).
8.2.8 Other effects
Many other different symptoms and signs have been reported in
humans exposed to cadmium. These include loss of appetite, loss of
weight, fatigue, and increases in the erythrocyte sedimentation rate
(ESR). Valetas (1946) reported details of the poisoning cases in a
French accumulator factory, which were first described by Nicaud et
al. (1942). In addition to the bone effects and the pains (section
8.2.2.1), Valetas mentioned that several workers experienced
paraesthesia and involuntary muscular contractions. This could be an
effect resulting from abnormal changes in the levels of serum
electrolytes, such as calcium or potassium, which may in turn be
caused by severe kidney damage.
Mild anaemia has been more frequently observed among
cadmium-exposed workers than among controls (Friberg, 1950; Bonnell,
1955; Bernard et al., 1979).
More specific effects from cadmium are the yellow discoloration
of the proximal part of the front teeth (Barthelemy & Moline, 1946;
Valetas, 1946; Princi, 1947; Friberg, 1950; Apostolov, 1979) and
anosmia (Friberg, 1950). Anosmia was found by Friberg (1950) in
about one third of a group of workers with a mean exposure time to
cadmium oxide dust of 20 years. Baader (1951) in Germany and
Apostolov (1979) in Bulgaria also noted that anosmia was common
among workers exposed to cadmium oxide dust for long periods of
time. Suzuki et al. (1965) and Tsuchiya (1967) in Japan, found no
increase in the prevalence of anosmia in workers exposed to cadmium
stearate and cadmium oxide fumes.
Nervous system symptoms were reported by Vorobjeva (1957), who
investigated 160 workers at an accumulator factory in the USSR.
Subjective symptoms included headache, vertigo, and sleep
disturbance. Physical examination revealed increases in knee-joint
reflexes, tremor, dermographia, and sweating.
Cadmium sulfide is sometimes used as a yellow tattoo pigment,
which is deposited intradermally. Local phototoxic reactions may
take place when the skin is exposed to ultraviolet light and are
probably connected with the marked photoconducting properties of
cadmium sulfide. Of 24 patients with yellow tattoos who were
examined by Bjornberg (1963), 18 experienced skin swelling when
exposed to sunlight.
8.3 Clinical and epidemiological studies with data on both exposure
and effects
There are several clinical and epidemiological studies with
data on occupational or general environment exposure levels, but the
data concerning effects are restricted to the lungs and kidneys.
8.3.1 Studies on respiratory disorders
Friberg (1950) studied 43 male workers exposed to cadmium oxide
dust, with an average period of employment of 20 years (range 9-34
years), and 15 workers who had been employed for only 1-4 years.
They were compared with a group of 200 sawmill workers. Shortness of
breath was the common symptom among the workers with long exposure,
and an impairment of lung function (increased residual capacity in
relation to total lung capacity and a decreased working capacity)
was demonstrated. The lung function of the group with short exposure
(less than 5 years) was found to be normal. The cadmium
concentration in air varied from 3 to 15 mg/m3, measurements
having been made at five places on only one occasion. In another
battery factory (where air cadmium concentrations were
0.05-5 mg/m3, Adams et al. (1969) found a slight average decrease
in forced expiratory volume in a group of 27 male workers.
Twelve out of 96 cadmium workers exposed for up to 27 years to
cadmium oxide fume in two cadmium-copper alloy factories were found
to suffer from emphysema, as evaluated from a comprehensive lung
function test (Bonnell, 1955; Buxton, 1956; Kazantzis, 1956). These
workers were compared with a similar size control group. The average
air cadmium concentrations in the two factories were 40-50 µg/m3,
and 90% of the particles were less than 0.5 µm in diameter (King,
1955).
Lauwerys et al. (1979a) studied pulmonary ventilatory function
in three groups of workers exposed to cadmium oxide dust and in
matched control groups (the matching included smoking habits). A
slight but significant reduction in forced vital capacity, in forced
expiratory volume in one second, and in peak expiratory flow rate
was found in 22 men. These were all smokers and had been exposed for
more than 20 years to a time-weighted average air concentration of
66 µg/m3 (21 µg/m3 respirable cadmium). In another group of
workers (smokers and non-smokers) exposed for 1-20 years to an
average concentration of 134 µg/m3 (the respirable cadmium level
at the most polluted work site was 88 µg/m3), the pulmonary
indices were on average lower than in the control group, but the
differences were not statistically significant. A more thorough
examination of a subgroup of the workers with long exposures carried
out by the same research group (Stanescu et al., 1977) found more
respiratory symptoms in the cadmium-exposed group than in a control
group and also impaired lung function (not statistically
significant). However, Lauwerys et al. (1979a) reported more
extensive data from the same plants and found that the workers with
less than 20 years of exposure (average 7.5 years) showed
significant effects in the lung function tests.
Reduced forced vital capacity was also found at a cadmium
production plant in the USA (where the air concentrations were
"commonly greater than 200 µg Cd/m3") among 17 workers exposed for
more than 6 years (Smith et al., 1976). De Silva & Donnan (1981)
provided evidence that insoluble cadmium compounds may induce
emphysematous changes after more than 7 years exposure to a
time-weighted average cadmium concentration of 700 µg/m3.
Edling et al. (1986) found no lung function differences between
an exposed group of workers using cadmium-containing solders and a
control group. The level of exposure, which lasted for several
years, was estimated to be 0.05-0.5 mg cadmium/m3, but the workers
had not been exposed to cadmium for several years. Of the 57 workers
examined, 42% had cadmium-induced renal tubular dysfunction.
Davison et al. (1988) examined 101 male workers, who had worked
for 1 year or more manufacturing copper-cadmium alloys, and found,
compared with a reference group, impaired lung function. They also
compared certain parameters (transfer coefficient: KCO) with the
estimated cumulative exposure index for cadmium workers with 95%
confidence limits for the regression line. Among 35 workers exposed
for more than five years and with a cumulative cadmium exposure
index up to 14 mg/m3.years, there was no evidence of a threshold.
The authors concluded that inhaled cadmium fumes caused changes in
lung function and in chest radiographs consistent with emphysema.
This could also explain the increased mortality reported. The
impaired lung function was also related to liver cadmium levels as
measured with neutron activation in vivo.
Some studies on respiratory disorders have yielded negative
results. However, some of these studies did not use lung function
tests (Hardy & Skinner, 1947; Princi, 1947; Tsuchiya, 1967; L'Epee
et al., 1968) and another did not use a control group (Teculescu &
Stanescu, 1970). Suzuki et al. (1965) examined a group of workers
exposed for a short period (average 3.3 years) to 30-690 µg
cadmium/m3 (as cadmium stearate) and found no changes in lung
function when compared to a control group.
In summary, it is clear that exposure to cadmium dust and fume
over prolonged periods can give rise to impaired lung function and
emphysema. Such effects have been seen predominantly at high air
cadmium concentrations (above 100 µg/m3), but one study showed
effects after more than 20 years of exposure to respirable cadmium
oxide dust concentrations of 21 µg/m3.
Cadmium workers sometimes suffered from symptoms such as
coughing and throat irritation, but did not show abnormal chest
X-ray findings when exposed to cadmium oxide fume at a concentration
of 100 µg/m3 for 4-8 years (Hardy & Skinner, 1947) or
40-1440 µg/m3 for 8 years (Princi, 1947), or to cadmium oxide dust
at a concentration of 64-241 µg/m3 for up to 15 years (Tsuchiya,
1967).
8.3.2 Studies on renal disorders in industry
Friberg (1950) reported that prolonged exposure gave rise to
renal damage among a large group of workers exposed to cadmium oxide
dust at concentrations of 3-15 mg/m3 in an accumulator factory. In
one group of 43 workers with a mean exposure period of 20 years
(range 9-34 years), a high prevalence of proteinuria was
demonstrated by the nitric acid and trichloroacetic acid test. In
several of the workers, the renal damage was also manifested by a
decreased inulin clearance and decreased concentrating capacity.
Another group of 15 workers with a mean exposure period of 2 years
(range 1-4 years) showed no positive reactions.
Since 1950, there have been many studies on proteinuria among
workers in various industries. This type of proteinuria is
characterized particularly by a great relative increase in the
excretion of low molecular weight (LMW) proteins (section 8.2). In
most of the early studies, qualitative tests for detecting
proteinuria were used, but, more recently, specific methods for the
quantitative determination of LMW proteins have been developed.
Table 16 contains data on the prevalence of proteinuria from
several epidemiological studies on cadmium workers. It must be
recognized that, in most studies, the dose measurements are based on
short sampling periods (hours or a few days), whereas exposure may
have been for decades. Information on sampling method (static or
personal) and the use of respirators is usually inadequate, which
makes accurate dose estimates difficult (section 2.2.1).
It is evident that the prevalence of proteinuria in cadmium
workers increases with exposure intensity duration. The study by
Kjellström et al. (1977a) presents frequency distributions of
urinary ß2-microglobulin excretion for 240 exposed workers and a
control group. There is a general shift to higher excretion levels
among the exposed workers, and a large proportion of them have
excretion levels far outside the control distribution. Any cut-off
point (operational definition) for "abnormal" proteinuria is
arbitrary. If a cut-off point of 290 µg/litre (corresponding to the
97.5 percentile of the control group) is chosen, 26% of the whole
group of exposed workers would be classified as having LMW
proteinuria. If higher cut-off points are used, the prevalence of
proteinuria will obviously be lower.
Table 16 provides evidence of a dose-response relationship. The
lowest "dose" that gave rise to a statistically significant increase
in urinary ß2-microglobulin, as defined above, was a 6-to 12-year
exposure to 50 µg cadmium/m3 (based on personal sampling)
(Kjellström et al., 1977a).
Järup et al. (1988) recently made a reassessment of
dose-response in the same battery plant (Table 16). A pattern very
similar to Kjellström et al. (1977a) was observed with a prevalence
of ß2-microglobulinuria of 4% at a cumulative dose of 0.5 mg/m3
(corresponding to 10 years of exposure to 50 µg cadmium/m3).
Lauwerys et al. (1979a,b) studied the prevalence of increased
ß2-microglobulin clearance (cut-off point: 97.5 percentile of
controls) and found a 21% prevalence after more than 20 years of
exposure to 66 µg cadmium/m3 total dust (static samples) or 21 µg
cadmium/m3 respirable dust (Table 16).
Holden (1980b) measured urine levels of ß2-microglobulin and
found dose-response relationships using cut-off points of
200 µg/litre, 1000 µg/litre, or 10 000 µg/litre. The cut-off point
of 200 µg/litre gave a 16% LMW proteinuria prevalence rate after
6-10 years of exposure (Table 16).
Table 16 also shows that an increased prevalence of total
proteinuria, as measured by sulfosalicyclic acid, trichloroacetic
acid, or quantitative determination of total proteinuria, occurs
after 5-10 years exposure to approximately 100 µg cadmium/m3. An
increased excretion of LMW protein (e.g., ß2-microglobulin) occurs
at much lower doses.
The in vivo measurement of cadmium in the liver and kidneys
of people with various levels of cadmium exposure provides a means
for relating organ dose to effects and response rates (section
6.4.2). Some questions still remain regarding the accuracy of the
analytical method (section 2.2.3.4) and the mathematical-statistical
methodology (Kjellström et al., 1984). Nevertheless, Roels et al.
(1983a) and Ellis et al. (l984) suggested that renal tubular damage
would be experienced by about 10% of people with a kidney cortex
level of 200 mg cadmium/kg, and by about 50% of people with a kidney
cortex level of 300 mg cadmium/kg.
Table 16. Prevalence of proteinuria in cadmium workers
Cadmium Estimated air Exposure No. of Prevalence of Proteinuria and Reference
compounds concentrations period examinees proteinuria (%) characteristics of
(µg/m3)a (years)b detection
methode
Cadmium oxide 40-50 control 60 2 SA and TCA Bonnell (1955); King (1955);
fume 1-9 37 24 Bonnell et al. (1959)
> 9 63 46
64-241c control 11 0 TA Tsuchiya (1967)
< 1 4 0 < 100 mg/litre
1-4 4 50
123c > 5 4 100
(time-weighted
average
Cadmium oxide 3000-15 000 1-4 15 0 nitric acid ("Hellers Friberg (1950)
dust 9-15 12 33 test"); positive in
16-22 17 41 more than half the
23-34 14 64 test
31 (1.4)c control 31 0 Abnormal Lauwerys et al. (1974a)
1-12 (4) 31 0 electrophoretic
control 27 4 pattern as defined
by the authors;
ß2-microglobulin
clearance
134 (88)c 0.6-19.7 (9) 27 15
control 22 0
66 (21) 21-40 22 68
66 (21) > 20 42 21
Table 16 (contd).
Cadmium Estimated air Exposure No. of Prevalence of Proteinuria and Reference
compounds concentrations period examinees proteinuria (%) characteristics of
(µg/m3)a (years)b detection
methode
Cadmium oxide
dust
control 87 3.4 ß2-microglobulin Kjellström et al. (1977a)
50c 0-3 50 6.0 (RIA)
50c 3-6 30 6.6 > 290 µg/litre
50c 6-12 21 19.0 (sg = 1.023)
Cadmium stearate 30-690 control 24 17 TCA Suzuki et al. (1965)
dust (3) 19 58
Cadmium sulfide 114d < 1-5 12 17 EP Harada (1987)
dust 5-21 7 100
< 1-5 12 8 TCA
5-21 12 43
control 203 1 ß2-microglobulin Stewart & Hughes (1981)
80 0-5 105 0 (RIA)
100 6-11 41 0 > 765 µg/litre
(sg = 1.016)
100-600 11-19 13 7.7
100-600 20+ 14 57.0
Mixed exposures; not reported, controls 642 0 (0.8) ß2-microglobulin Holden (1980b)f
12 factories; but mean blood < 18 months 121 0 (10) (RIA)
mainly zinc cadmium level 19 months-5 years 168 1.8 (8.3) > 1000 µg/litre
smelters after 1 year 6-10 170 1.8 (16) (or > 200 µg/litre)
exposure was 11-15 82 7.3 (22)
about 16-20 33 24 (45)
15 µg/litre
20+ 68 25 (56)
Table 16 (contd).
Cadmium Estimate of cumulative Number of Prevalence (%) Proteinuria and Reference
compounds dose (mg/m3.year) examinees of proteinuria characteristics of
detection method
Cadmium fume < 1 16 18 ß2-microglobulin Elinder et al. (1985b)
(oxide) 1-2 22 32 > 0.034 mg/mmol creatinine
2-3 9 44
3-5 8 62
> 5 5 100
< 1 ) 6 Mason et al. (1988)
1-15 ) 67
1.5-3) 75 58
3-5 ) 86
> 5 ) 100
Cadmium oxide < 0.4 264 1.1 Jarup et al. (1988)
0.4-1.7 76 9.2
1.7-4.6 43 23.3
4.6-9.6 31 32.3
9.6-15 16 32.2
> 15 10 50
a Numbers in parentheses refer to average concentrations of the respirable particulates (< 5 µm) fraction
b Numbers in parentheses are average values
c Measured in breathing zone by personal sampler
d Calculated average exposure for the worker with the most pronounced effect
e SA = sulfosalicyclic acid method (qualitative); TCA = trichloroacetic acid method (qualitative);
TA = tungstate method (quantitative); EP = electrophoresis; RIA = radioimmunoassay; sg = specific gravity
f Values for prevalence of proteinuria refer to a ß2-microglobulin level of > 1000 µg/litre.
Values in parentheses refer to a level of > 200 µg/litre.
Ellis et al. (1985) correlated time-weighted exposure indices
(TWE), based on employment records, area monitoring techniques and
personal sampling, with body burden of cadmium measured by
in vivo neutron activation analysis of the liver and left kidney
in 82 men exposed to cadmium dust in a smelter. The workers were
grouped as follows: production workers (40 active, 21 retired);
office and laboratory workers (8 active, 4 retired); and
non-production workers (3 active, 6 retired). From these
measurements the authors were able to estimate the probability of
developing kidney dysfunction based on the workers' cumulative
exposure index. When the exposure limit was 400-500 µg/m3.years,
the prevalence for renal dysfunction was about 32%; it was 22-40%
with a wider exposure index (300-600 µg/m3). Kjellström et al.
(1977a) reported a prevalence of 19% at a battery factory at a level
of 50 µg Cd/m3, which resulted in a similar exposure index.
Lauwerys et al. (1974a) observed proteinuria in 68% of workers with
long-term exposure (20 years at 66 µg/m3), whereas the logistic
model developed by Ellis et al. (l985) would have predicted 65%
under these exposure conditions.
For exposures of 250 µg/m3.years there is a 19% probability
of experiencing renal dysfunction. In the case of workers with
normal renal function, an exposure index of 400 µg/m3 predicts a
mean cadmium concentration of 28 mg/kg in liver and 288 mg/kg of
renal cortex. The model would predict that a 10-year exposure to
25 µg/m3 would result in a mean renal cortex concentration of
252 mg/kg, which is similar to the critical concentration defined by
Friberg.
Thun et al. (1989) assessed the quantitative relationship
between exposure to airborne cadmium and various markers of renal
tubular and glomerular function in 45 male workers at a plant that
recovered cadmium from industrial waste. The dose was estimated from
historical air sampling data. In this study, the "critical dose" of
cadmium necessary to induce nephropathy was based on the 5th or 95th
percentile of test results in the unexposed population. Using this
definition, renal tubular dysfunction sharply increased as
cumulative exposure to cadmium rose above 300 mg/m3.days
(corresponding to about 0.8 mg/m3.years). Very similar
dose-response curves with an increased prevalence of
ß2-microglobulinuria at cumulative exposure levels exceeding about
0.5-1 mg cadmium/m3.year have been reported from examinations of
workers exposed to cadmium fumes (Elinder et al., 1985b; Mason et
al., 1988). These findings are consistent with the recommendations
by a working group of the World Health Organization (WHO, l980) to
limit workplace exposures to 10 µg/m3 in order to prevent tubular
proteinuria after life-time occupational exposure to cadmium.
Cumulative cadmium exposure indices have been calculated for 75
cadmium alloy workers employed for periods of up to 39 years,
together with the in vivo liver and kidney cadmium burden (Mason
et al., 1988). Several indicators of both tubular and glomerular
dysfunction correlated significantly with both cumulative exposure
index and liver cadmium burden. Using these estimates of dose, a
two-phase linear regression model was applied to identify an
inflection point of the order of 1100 µg/m3.years above which
changes in renal function occurred. A number of biochemical
variables fitted this model, including total protein, albumin, and
ß2-microglobulin. Simple dose-response analysis showed a greatly
increased incidence of tubular proteinuria when the cumulative
cadmium exposure index was greater than this value. The cumulative
exposure index was equated to about 20 to 22 years of exposure to a
cadmium level of 50 µg/m3.
Evidence of tubular damage was investigated in a group of 91
cadmium workers subjected to yearly estimation of cadmium
concentration in blood and urine over a period of eight years. In
workers with blood and urine cadmium levels constantly below the
Biological Limit Value of 10 µg/litre, the prevalence of tubular
damage, as indicated by an increased excretion of ß2-microglobulin
above 260 µg/litre, was below 3%. RBP excretion confirmed this
pattern.
8.3.3 Studies on renal disorders in the general environment
8.3.3.1 Health surveys in Japan
Following the recognition of the association between Itai-itai
disease and exposure to cadmium (Japanese Ministry of Health and
Welfare, 1968), additional general surveys of cadmium pollution were
performed in Japan, and further areas were found to be involved.
Health effects were studied first among the population in the area
where Itai-itai cases had occurred and later in other areas found to
be contaminated. The original studies were designed to find cases of
Itai-itai disease, but it was possible also to estimate the
prevalence of proteinuria and glucosuria in the examined population.
A detailed description of the methods used in these cadmium
pollution surveillance programmes has been reported by Shigematsu et
al. (1979).
At the time of the first surveillance programmes (1969-71),
methods were developed for estimating the degree of contamination
with cadmium and the total daily intake. At the early stage of the
investigations, the most common index of cadmium intake measured in
all areas was the cadmium concentration in rice. From data of the
Japan Public Health Association (1970), it was estimated that, in
areas with different exposure levels, an average of almost 50%
(range 14-71) of the daily cadmium intake came from rice. In one
area, the proportion was estimated to be 85% (Kawano & Kato, 1978).
The average cadmium concentration in rice from non-polluted
areas has been reported to be 0.066 mg/kg in polished rice
(Moritsugi & Kobayashi, 1964) and 0.09 mg/kg in unpolished rice
(Japanese Ministry of Agriculture and Forestry, 1973) (section
5.2.1). The national average consumption of rice was 364 g per
person in 1961, 308 g in 1971, and 222 g in 1981 (Japanese Ministry
of Health and Welfare, 1983).
Proteinuria was generally estimated with qualitative methods
such as the sulfosalicylic acid method or the trichloracetic acid
method according to standardized techniques (Japanese Ministry of
Health and Welfare, 1971). More recently, emphasis has been placed
on the identification of the urinary protein pattern, in particular
to detect early evidence of tubular dysfunction. The methods
currently used are electrophoresis and the quantitative
determination of lysozyme, RBP, and ß2-microglobulin. Several
studies have been published, and there are extensive reviews in
English (Tsuchiya, 1969; Yamagata & Shigematsu, 1970; Friberg et
al., 1974; Tsuchiya, 1978; Shigematsu et al., 1979, l980).
From 1976 to 1984, epidemiological health surveys of residents
in areas with environmental cadmium pollution were performed by the
Japan Environment Agency using methods including immunological tests
for the detection of low molecular weight proteinuria in eight
prefectures (Akita, Fukushisuma, Gunma, Toyama, Ishikawa, Hyogo,
Nagasaki, Oita). More than 13 000 inhabitants of polluted areas and
more than 7000 inhabitants of non-polluted areas, aged 50 years or
more in both areas, were subjected to these surveys.
The following screening method was adopted for health
examinations. The urine of those people with proteinuria
(demonstrated by a semiquantitative method) and/or glucosuria (by a
paper test) was analysed for ß2-microglobulin (> 10 mg/litre),
RBP (> 4 mg/litre), lysozyme (> 2 mg/litre), total amino acid
nitrogen (> 20 mmol/litre), and cadmium (> 30 µg/litre). Those
who exceeded the above levels in more than one item were tested for
renal function by urine and blood analysis. Finally those for whom
the TRP was less than 80% were subjected to detailed health
examination, including skeletal radiography, in order to make a
clinical diagnosis (Fig. 7).
With the exception of Oita prefecture, the number of
individuals who had or were suspected of having proximal renal
tubular dysfunction (as defined by the Japanese Cadmium Research
Committee) or related findings tended to be greater in the polluted
areas than in the non-polluted areas, and this was often
significantly related to the degree of pollution (see Table 16).
This suggests that environmental cadmium pollution is associated
with the occurrence of proximal renal tubular dysfunction.
 Five areas in which significantly increased
ß2-microglobulinuria was found are reviewed in detail below. In
addition there is a description of some other Japanese polluted
areas and three European polluted areas.
8.3.3.2 Toyama prefecture (Fuchu area)
This is the area where the Itai-itai disease was first
described (Kono et al., 1956). Exposure levels in polluted villages,
as measured by cadmium concentrations in rice during the 1960s,
varied greatly but in some villages the level was as high as 2 mg/kg
(Ishizaki et al., 1969). A zinc and lead mine was the major source
of pollution, and cadmium concentrations in soil were elevated
(Japan Public Health Association, 1968). Many studies have been
performed with sulfosalicyclic acid and trichloroacetic acid for the
identification of proteinuria. Both proteinuria and glucosuria were
common findings in the polluted area (Ishizaki et al., 1969;
Fukushima et al., 1974). There was a strong relationship between the
degree of proteinuria and age, and a greatly increased prevalence in
the older age groups compared with controls. The proteinuria was
similar to that seen in cadmium workers as evaluated by
electrophoresis (Piscator & Tsuchiya, 1971) or gel filtration
(Fukuyama, 1972). Quantitative estimation of the LMW urinary
proteins ß2-microglobulin (Shiroishi et al., 1975, 1977) and
retinol-binding protein (Kanai et al., 1971) confirmed that the
proteinuria was tubular.
Fukushima et al. (1973) reported on the cadmium concentration
in rice and the prevalence of renal effects in various hamlets in
the Fuchu and control areas. In control hamlets situated outside the
Jinzu river basin, the cadmium concentration in rice varied between
0.05 and 0.2 mg/kg wet weight, and the prevalence of concurrent
proteinuria and glycosuria varied between 0 and 9%. In the polluted
villages, where cadmium levels in polished rice were 0.5-1.0 mg/kg,
the prevalence was 15-20%, and, in all the 20 hamlets, the
correlation coefficient between cadmium in rice and prevalence of
renal effects was 0.68 (P < 0.05) (Fukushima et al., 1973). The
prevalence had a tendency to be somewhat higher in the hamlets where
Itai-itai disease was endemic, as compared with the hamlets where it
did not occur, even though the latter hamlets had similar cadmium
concentrations in rice.
Using a disc electrophoresis technique (Shiroishi et al., 1972)
in the age groups over 40 years, a tubular urinary protein pattern
was found in about 25% of exposed persons but not found at all in
the control group. Quantitative determination of ß2-microglobulin
in the urine of patients with Itai-itai disease and so-called
observation patients (people in polluted areas with likely
cadmium-induced renal damage) showed a marked difference between the
exposed and control groups (Shiroishi et al., 1977).
The level of urinary ß2-microglobulin in patients with
Itai-itai disease was on average 43 mg/litre, i.e. 100 times higher
than in the controls, and the level in observation patients was on
average 65 times higher than it was in the controls.
A comparison by Kjellström et al. (1977b) of 138 cadmium-
exposed women in the age-group 51-60 and 40 controls revealed
large differences. The exposed women were selected on the
basis of their consumption of polluted rice (average cadmium
concentration above 0.7 mg/kg); no health data influenced the
selection. On average, the urinary ß2-microglobulin excretion was
10 times greater among the exposed women than among the controls,
and the individual urinary levels increased as a function of the
cadmium dose. Additional data from the same area for women in the
age-group 40-45 (Kjellström, 1977) also showed an increase
prevalence of high ß2-microglobulin levels in urine.
Nogawa & Ishizaki (1979) reported a significant increase in the
prevalence of both proteinuria and concurrent proteinuria and
glucosuria at an average rice cadmium level of 0.41 mg/kg. In a
further study (Nogawa et al., 1979), the prevalence of proteinuria
was analysed as a function of urinary cadmium levels. The
correlation between the two variables was good, but urinary cadmium
may not be a suitable measure of dose as it also increases as a
consequence of renal damage (section 8.2.1).
A mathematical dose-response analysis was carried out by Hutton
(1983) based on the data of Shiroishi et al. (1977) on urinary
ß2-microglobulin excretion. For each individual the cadmium dose
index (µg/day.years) was based on the estimated daily cadmium intake
via rice and other foodstuffs and the number of years the person had
lived in the polluted area. The age-groups 51-60 and 40-45 were both
divided into three sub-groups with different dose index levels. In
the analysis of Hutton (1983), three groups from Kosaka area were
included (section 8.3.3.4) for whom the same type of data was
available (Kojima et al., 1977). Fig. 8 shows that the prevalence of
increased LMW proteinuria (response rate) increased with dose. These
prevalences were adjusted for a control group prevalence of 2.5%
(Kjellström, 1977), giving an expected "background" prevalence
without cadmium exposure of 0%. The 95% fiducial limits were quite
wide. For instance, at a cadmium intake of 55 µg/day (95% fiducial
limits 25-123), there would be a 1% increase of proteinuria in the
population. At a dose index of 5000 µg/day.years (or 50 years at
100 µg cadmium/day intake), the expected response rate was within
the range 2-12%.
Five areas in which significantly increased
ß2-microglobulinuria was found are reviewed in detail below. In
addition there is a description of some other Japanese polluted
areas and three European polluted areas.
8.3.3.2 Toyama prefecture (Fuchu area)
This is the area where the Itai-itai disease was first
described (Kono et al., 1956). Exposure levels in polluted villages,
as measured by cadmium concentrations in rice during the 1960s,
varied greatly but in some villages the level was as high as 2 mg/kg
(Ishizaki et al., 1969). A zinc and lead mine was the major source
of pollution, and cadmium concentrations in soil were elevated
(Japan Public Health Association, 1968). Many studies have been
performed with sulfosalicyclic acid and trichloroacetic acid for the
identification of proteinuria. Both proteinuria and glucosuria were
common findings in the polluted area (Ishizaki et al., 1969;
Fukushima et al., 1974). There was a strong relationship between the
degree of proteinuria and age, and a greatly increased prevalence in
the older age groups compared with controls. The proteinuria was
similar to that seen in cadmium workers as evaluated by
electrophoresis (Piscator & Tsuchiya, 1971) or gel filtration
(Fukuyama, 1972). Quantitative estimation of the LMW urinary
proteins ß2-microglobulin (Shiroishi et al., 1975, 1977) and
retinol-binding protein (Kanai et al., 1971) confirmed that the
proteinuria was tubular.
Fukushima et al. (1973) reported on the cadmium concentration
in rice and the prevalence of renal effects in various hamlets in
the Fuchu and control areas. In control hamlets situated outside the
Jinzu river basin, the cadmium concentration in rice varied between
0.05 and 0.2 mg/kg wet weight, and the prevalence of concurrent
proteinuria and glycosuria varied between 0 and 9%. In the polluted
villages, where cadmium levels in polished rice were 0.5-1.0 mg/kg,
the prevalence was 15-20%, and, in all the 20 hamlets, the
correlation coefficient between cadmium in rice and prevalence of
renal effects was 0.68 (P < 0.05) (Fukushima et al., 1973). The
prevalence had a tendency to be somewhat higher in the hamlets where
Itai-itai disease was endemic, as compared with the hamlets where it
did not occur, even though the latter hamlets had similar cadmium
concentrations in rice.
Using a disc electrophoresis technique (Shiroishi et al., 1972)
in the age groups over 40 years, a tubular urinary protein pattern
was found in about 25% of exposed persons but not found at all in
the control group. Quantitative determination of ß2-microglobulin
in the urine of patients with Itai-itai disease and so-called
observation patients (people in polluted areas with likely
cadmium-induced renal damage) showed a marked difference between the
exposed and control groups (Shiroishi et al., 1977).
The level of urinary ß2-microglobulin in patients with
Itai-itai disease was on average 43 mg/litre, i.e. 100 times higher
than in the controls, and the level in observation patients was on
average 65 times higher than it was in the controls.
A comparison by Kjellström et al. (1977b) of 138 cadmium-
exposed women in the age-group 51-60 and 40 controls revealed
large differences. The exposed women were selected on the
basis of their consumption of polluted rice (average cadmium
concentration above 0.7 mg/kg); no health data influenced the
selection. On average, the urinary ß2-microglobulin excretion was
10 times greater among the exposed women than among the controls,
and the individual urinary levels increased as a function of the
cadmium dose. Additional data from the same area for women in the
age-group 40-45 (Kjellström, 1977) also showed an increase
prevalence of high ß2-microglobulin levels in urine.
Nogawa & Ishizaki (1979) reported a significant increase in the
prevalence of both proteinuria and concurrent proteinuria and
glucosuria at an average rice cadmium level of 0.41 mg/kg. In a
further study (Nogawa et al., 1979), the prevalence of proteinuria
was analysed as a function of urinary cadmium levels. The
correlation between the two variables was good, but urinary cadmium
may not be a suitable measure of dose as it also increases as a
consequence of renal damage (section 8.2.1).
A mathematical dose-response analysis was carried out by Hutton
(1983) based on the data of Shiroishi et al. (1977) on urinary
ß2-microglobulin excretion. For each individual the cadmium dose
index (µg/day.years) was based on the estimated daily cadmium intake
via rice and other foodstuffs and the number of years the person had
lived in the polluted area. The age-groups 51-60 and 40-45 were both
divided into three sub-groups with different dose index levels. In
the analysis of Hutton (1983), three groups from Kosaka area were
included (section 8.3.3.4) for whom the same type of data was
available (Kojima et al., 1977). Fig. 8 shows that the prevalence of
increased LMW proteinuria (response rate) increased with dose. These
prevalences were adjusted for a control group prevalence of 2.5%
(Kjellström, 1977), giving an expected "background" prevalence
without cadmium exposure of 0%. The 95% fiducial limits were quite
wide. For instance, at a cadmium intake of 55 µg/day (95% fiducial
limits 25-123), there would be a 1% increase of proteinuria in the
population. At a dose index of 5000 µg/day.years (or 50 years at
100 µg cadmium/day intake), the expected response rate was within
the range 2-12%.
 8.3.3.3 Hyogo prefecture (Ikuno area)
In the Ikuno area of Hyogo prefecture, an inactive zinc and
copper mine is the probable source of pollution of the Ichi river
basin. The average cadmium concentration in rice in the most
polluted part was found to be 0.69 mg/kg in one study and 1.10 mg/kg
in another (Hyogo Prefectural Government, 1972).
In 1972, urine from 1560 people (of both sexes, over 30 years
of age) from the polluted area and groups of 1574, 2002, and 638
people (over 30) from three control areas were examined (Tsuchiya,
1978). The prevalence of proteinuria, as measured by the
sulfosalicyclic acid methods was 58% and 33%, respectively, a
statistically significant difference. The reason for the high
prevalence in the control area is not known.
In a study by Watanabe & Murayama (1975), a search was made for
LMW proteins among 39 people in a polluted area and 56 in a control
area (all the people were above 70 years of age). Urinary
ß2-microglobulin excretion exceeding 10 000 µg/litre was found in
41% of the examined people in the polluted area and 4% of those in
the control area.
Kitamura & Koizumi (1975) used disc electrophoresis to study
tubular-type proteinuria among 224 people (above 50 years of age)
from a polluted area and compared the results with those from a
study of old bedridden people. Fig. 9 demonstrates the considerably
higher prevalence of tubular proteinuria among people from the
polluted area. There was also a definite increase in the occurrence
of tubular proteinuria with age in the exposed and bedridden control
groups.
8.3.3.4 Ishikawa prefecture (Kakehashi area)
In the Kakahaski river basin of Ishikawa prefecture, several
mines had polluted the river with cadmium and copper (Tsuchiya,
1978). Rice samples were studied in a number of villages along the
river, and village-average levels of up to about 0.7 g/kg were
found. Values were higher in paddy fields on the shores of a narrow
river valley close to the mine.
In 1974-1976, health examinations of 2805 inhabitants over the
age of 50 were carried out using test tape for proteinuria and
glucosuria examination as well as single radial immunodiffusion
analysis for RBP in urine (Tsuchiya, 1978). Based on the findings,
some people were selected for secondary and tertiary examinations,
and 39 were considered to require consultation for renal tubular
dysfunction. However, no cases with severe bone disease were found
at that time.
This area is the only one where quantitative measurement of LMW
protein in urine was carried out in the first screening (Nogawa et
al., 1978). The prevalence data in Table 17 are therefore of
particular interest. None of the other LMW proteinuria studies
mentioned in Table 16 were carried out on such a large group using a
broad epidemiological approach. A scatter in the prevalence values
among the villages is seen in Fig. 10, but there is no doubt that
the villages with the highest rice cadmium values had an increased
prevalence of high urinary RBP. This is also evident when the data
from different villages with the same rice cadmium levels are
combined (Table 17). In all of the exposed groups with different
rice cadmium levels (Table 17), the prevalence of tubular
proteinuria increases with age, but that is not seen in the control
group. It is not known whether the age effect reflects increased
cadmium dose rather than age itself.
Further analysis of these data using a mathematical
dose-response approach (Hutton, 1983) clearly showed the effect of
calculated cadmium intake on the prevalence of proteinuria
(Fig. 11). The fiducial limits are narrower than in Fig. 8 because
of the larger number of people studied.
8.3.3.3 Hyogo prefecture (Ikuno area)
In the Ikuno area of Hyogo prefecture, an inactive zinc and
copper mine is the probable source of pollution of the Ichi river
basin. The average cadmium concentration in rice in the most
polluted part was found to be 0.69 mg/kg in one study and 1.10 mg/kg
in another (Hyogo Prefectural Government, 1972).
In 1972, urine from 1560 people (of both sexes, over 30 years
of age) from the polluted area and groups of 1574, 2002, and 638
people (over 30) from three control areas were examined (Tsuchiya,
1978). The prevalence of proteinuria, as measured by the
sulfosalicyclic acid methods was 58% and 33%, respectively, a
statistically significant difference. The reason for the high
prevalence in the control area is not known.
In a study by Watanabe & Murayama (1975), a search was made for
LMW proteins among 39 people in a polluted area and 56 in a control
area (all the people were above 70 years of age). Urinary
ß2-microglobulin excretion exceeding 10 000 µg/litre was found in
41% of the examined people in the polluted area and 4% of those in
the control area.
Kitamura & Koizumi (1975) used disc electrophoresis to study
tubular-type proteinuria among 224 people (above 50 years of age)
from a polluted area and compared the results with those from a
study of old bedridden people. Fig. 9 demonstrates the considerably
higher prevalence of tubular proteinuria among people from the
polluted area. There was also a definite increase in the occurrence
of tubular proteinuria with age in the exposed and bedridden control
groups.
8.3.3.4 Ishikawa prefecture (Kakehashi area)
In the Kakahaski river basin of Ishikawa prefecture, several
mines had polluted the river with cadmium and copper (Tsuchiya,
1978). Rice samples were studied in a number of villages along the
river, and village-average levels of up to about 0.7 g/kg were
found. Values were higher in paddy fields on the shores of a narrow
river valley close to the mine.
In 1974-1976, health examinations of 2805 inhabitants over the
age of 50 were carried out using test tape for proteinuria and
glucosuria examination as well as single radial immunodiffusion
analysis for RBP in urine (Tsuchiya, 1978). Based on the findings,
some people were selected for secondary and tertiary examinations,
and 39 were considered to require consultation for renal tubular
dysfunction. However, no cases with severe bone disease were found
at that time.
This area is the only one where quantitative measurement of LMW
protein in urine was carried out in the first screening (Nogawa et
al., 1978). The prevalence data in Table 17 are therefore of
particular interest. None of the other LMW proteinuria studies
mentioned in Table 16 were carried out on such a large group using a
broad epidemiological approach. A scatter in the prevalence values
among the villages is seen in Fig. 10, but there is no doubt that
the villages with the highest rice cadmium values had an increased
prevalence of high urinary RBP. This is also evident when the data
from different villages with the same rice cadmium levels are
combined (Table 17). In all of the exposed groups with different
rice cadmium levels (Table 17), the prevalence of tubular
proteinuria increases with age, but that is not seen in the control
group. It is not known whether the age effect reflects increased
cadmium dose rather than age itself.
Further analysis of these data using a mathematical
dose-response approach (Hutton, 1983) clearly showed the effect of
calculated cadmium intake on the prevalence of proteinuria
(Fig. 11). The fiducial limits are narrower than in Fig. 8 because
of the larger number of people studied.
 In a study by Nogawa et al. (1978), laboratory determinations
related to proximal renal tubular function, etc., were compared by
age group. The findings in the most polluted areas hardly differed
from those in the non-polluted areas in the age-group 50-59
(Table 17). At age 60 and over, however, the frequency of findings
tended to increase with age, except in the case of total
aminoaciduria. The difference in the age-adjusted rates for these
determinations between the polluted and non-polluted areas tended to
be enhanced by aging. Table 18 shows how the prevalence of tubular
proteinuria varies according to age and average rice cadmium
concentration. Of the 438 participants in the final detailed
examinations (426 in the polluted areas and 12 in the non-polluted
areas), findings of "possible proximal renal tubular dysfunction"
were noted in 334 people (333 in the polluted areas and 1 in the
non-polluted areas). Among these cases, 202 in the polluted areas
were determined to have proximal renal tubular dysfunction and 116
of them were considered to require medical supervision in view of
the severity of the dysfunction. The urinary ß2-microglobulin
level in Itai-itai disease patients was on average 43 mg/litre, 100
times higher than that in the controls, and the patients
investigated by Nogawa et al. (1978) had an average urinary level of
ß2-microglobulin 65 times the controls.
Table 17. Age-adjusted prevalence rate (%) of renal tubular dysfunction and related conditionsa
Prefecture Year of Polluted (P) or No. of examinees ß2-Microglobulinuria Decreased TRP Tubular dysfunction
studiedb investigation non-polluted male female (> 10 mg/litre) (< 80%) male female
(NP) areac male female male female
Toyama 1979-1984 P 3432 4099 6.5 10.8 4.6 5.5 1.4 3.3
(Fuchu area) NP 944 1205 0.4d 0.5d 0.6d 0.2d 0.0d 0.0d
Hyogo 1977 P 230 280 12.8 16.8 4.4 6.8 2.0 3.5
(Ikuno area) NP 212 251 2.7d 1.9d 0.9e 0.4d 0.0 0.0e
Ishikawa 1976 P 260 306 7.6 10.9 6.9 5.7 2.4 3.2
(Kakehashi area) NP 200 275 1.1d 1.5d 0.5d 0.0d 0.0 0.0d
Akita 1976 P 179 247 6.4 5.0 2.3 0.7 0.0 0.0
(Kosaka area) NP 168 234 0.0d 0.0d 0.0 0.0 0.0 0.0
Nagasaki 1976 P 143 191 3.4 10.6 4.6 9.1 1.7 6.2
(Tsushima area) NP 210 291 1.9 0.3d 1.5 0.0d 0.0 0.0d
Fukushima 1977 P 307 425 0.4 0.5 0.6 0.5 0.0 0.3
NP 246 396 0.0 0.0 0.4 0.0 0.0 0.0
Gunma 1976-1978 P 937 1160 1.6 0.6 1.6 0.5 0.0 0.0
NP 620 786 0.8 0.1 0.6 0.3 0.0 0.0
Table 17 (cont'd).
Prefecture Year of Polluted (P) or No. of examinees ß2-Microglobulinuria Decreased TRP Tubular dysfunction
studiedb investigation non-polluted male female (> 10 mg/litre) (< 80%) male female
(NP) areac male female male female
Oita 1978 P 169 194 1.7 1.5 1.1 0.5 0.0 0.0
NP 182 215 1.7 1.0 1.2 0.0 0.0 0.0
a From: Japan Cadmium Research Committee (1989). The response rates for these studies were greater than 90% of the target
population. The age composition (50-59, 60-69, 70-79, and 80+) in each prefecture was adjusted to the Japanese population
in 1980. The criteria for renal tubular dysfunction were: one out of three signs (low molecular weight proteinuria, glucosuria,
and generalized aminoaciduria), %TRP < 80% and acidosis (blood hydrogen carbonate below 23 mEq/litre).
b The total number of people living in polluted areas in each prefecture is shown in Table 7.
c Total number of people examined was 5657 males and 6902 females in polluted areas and 2782 males and 3653 females in non-polluted areas.
d Significant difference (P < 0.01)
e Significant difference (P < 0.05)
Table 18. Prevalence (%) of tubular proteinuriaa in relation to age (age-groups 50-59, 60-69, and > 69) and
village-average rice cadmium concentrationsb
Rice cadmium 50-59 60-69 > 69
concentration
(µg/g) No. A (%) B (%) No. A (%) B (%) No. A (%) B (%)
Control (< 0.1) 104 0.96 0 80 2.50 1.25 64 0 0
0.19-0.29 477 0.84 0.42 377 2.65 0.80 268 13.81c 4.48
0.30-0.39 184 3.26 2.72 138 5.07 0.72 91 17.58c 7.69d
0.40-0.49 295 1.69 0.34 204 6.86 2.94 117 14.53c 8.55c
0.50-0.59 140 0.71 0 120 10.83d 5.00 93 34.41c 25.81c
0.60-0.69 60 18.33c 5.00d 57 24.56c 12.28c 23 73.91c 43.48c
a Prevalence of increased RBP in Ishikawa prefecture only
b From: Nogawa et al. (1978); No. = number of people examined; RBP was measured by a semiquantitative method; values in column A refer
to the prevalence of RBP in urine at levels above 4 mg/litre; values in column B refer to the prevalence at values above 16 mg/litre
c Significant difference (P < 0.01) compared with control
d Significant difference (P < 0.05) compared with control
In a study by Nogawa et al. (1978), laboratory determinations
related to proximal renal tubular function, etc., were compared by
age group. The findings in the most polluted areas hardly differed
from those in the non-polluted areas in the age-group 50-59
(Table 17). At age 60 and over, however, the frequency of findings
tended to increase with age, except in the case of total
aminoaciduria. The difference in the age-adjusted rates for these
determinations between the polluted and non-polluted areas tended to
be enhanced by aging. Table 18 shows how the prevalence of tubular
proteinuria varies according to age and average rice cadmium
concentration. Of the 438 participants in the final detailed
examinations (426 in the polluted areas and 12 in the non-polluted
areas), findings of "possible proximal renal tubular dysfunction"
were noted in 334 people (333 in the polluted areas and 1 in the
non-polluted areas). Among these cases, 202 in the polluted areas
were determined to have proximal renal tubular dysfunction and 116
of them were considered to require medical supervision in view of
the severity of the dysfunction. The urinary ß2-microglobulin
level in Itai-itai disease patients was on average 43 mg/litre, 100
times higher than that in the controls, and the patients
investigated by Nogawa et al. (1978) had an average urinary level of
ß2-microglobulin 65 times the controls.
Table 17. Age-adjusted prevalence rate (%) of renal tubular dysfunction and related conditionsa
Prefecture Year of Polluted (P) or No. of examinees ß2-Microglobulinuria Decreased TRP Tubular dysfunction
studiedb investigation non-polluted male female (> 10 mg/litre) (< 80%) male female
(NP) areac male female male female
Toyama 1979-1984 P 3432 4099 6.5 10.8 4.6 5.5 1.4 3.3
(Fuchu area) NP 944 1205 0.4d 0.5d 0.6d 0.2d 0.0d 0.0d
Hyogo 1977 P 230 280 12.8 16.8 4.4 6.8 2.0 3.5
(Ikuno area) NP 212 251 2.7d 1.9d 0.9e 0.4d 0.0 0.0e
Ishikawa 1976 P 260 306 7.6 10.9 6.9 5.7 2.4 3.2
(Kakehashi area) NP 200 275 1.1d 1.5d 0.5d 0.0d 0.0 0.0d
Akita 1976 P 179 247 6.4 5.0 2.3 0.7 0.0 0.0
(Kosaka area) NP 168 234 0.0d 0.0d 0.0 0.0 0.0 0.0
Nagasaki 1976 P 143 191 3.4 10.6 4.6 9.1 1.7 6.2
(Tsushima area) NP 210 291 1.9 0.3d 1.5 0.0d 0.0 0.0d
Fukushima 1977 P 307 425 0.4 0.5 0.6 0.5 0.0 0.3
NP 246 396 0.0 0.0 0.4 0.0 0.0 0.0
Gunma 1976-1978 P 937 1160 1.6 0.6 1.6 0.5 0.0 0.0
NP 620 786 0.8 0.1 0.6 0.3 0.0 0.0
Table 17 (cont'd).
Prefecture Year of Polluted (P) or No. of examinees ß2-Microglobulinuria Decreased TRP Tubular dysfunction
studiedb investigation non-polluted male female (> 10 mg/litre) (< 80%) male female
(NP) areac male female male female
Oita 1978 P 169 194 1.7 1.5 1.1 0.5 0.0 0.0
NP 182 215 1.7 1.0 1.2 0.0 0.0 0.0
a From: Japan Cadmium Research Committee (1989). The response rates for these studies were greater than 90% of the target
population. The age composition (50-59, 60-69, 70-79, and 80+) in each prefecture was adjusted to the Japanese population
in 1980. The criteria for renal tubular dysfunction were: one out of three signs (low molecular weight proteinuria, glucosuria,
and generalized aminoaciduria), %TRP < 80% and acidosis (blood hydrogen carbonate below 23 mEq/litre).
b The total number of people living in polluted areas in each prefecture is shown in Table 7.
c Total number of people examined was 5657 males and 6902 females in polluted areas and 2782 males and 3653 females in non-polluted areas.
d Significant difference (P < 0.01)
e Significant difference (P < 0.05)
Table 18. Prevalence (%) of tubular proteinuriaa in relation to age (age-groups 50-59, 60-69, and > 69) and
village-average rice cadmium concentrationsb
Rice cadmium 50-59 60-69 > 69
concentration
(µg/g) No. A (%) B (%) No. A (%) B (%) No. A (%) B (%)
Control (< 0.1) 104 0.96 0 80 2.50 1.25 64 0 0
0.19-0.29 477 0.84 0.42 377 2.65 0.80 268 13.81c 4.48
0.30-0.39 184 3.26 2.72 138 5.07 0.72 91 17.58c 7.69d
0.40-0.49 295 1.69 0.34 204 6.86 2.94 117 14.53c 8.55c
0.50-0.59 140 0.71 0 120 10.83d 5.00 93 34.41c 25.81c
0.60-0.69 60 18.33c 5.00d 57 24.56c 12.28c 23 73.91c 43.48c
a Prevalence of increased RBP in Ishikawa prefecture only
b From: Nogawa et al. (1978); No. = number of people examined; RBP was measured by a semiquantitative method; values in column A refer
to the prevalence of RBP in urine at levels above 4 mg/litre; values in column B refer to the prevalence at values above 16 mg/litre
c Significant difference (P < 0.01) compared with control
d Significant difference (P < 0.05) compared with control

 In a later epidemiological study, Nogawa et al. (1989)
investigated the dose-response relationship in 1850 cadmium-exposed
and 294 non-exposed inhabitants of the Kakehashi River basin. Using
a urine concentration of 1000 µg ß2-microglobulin/g creatinine as
an index of renal tubular dysfunction, and the average rice cadmium
concentration as an index of cadmium exposure, the authors
determined linear regression equations for men and women. These are
related to the prevalence of ß2-microglobulinuria and total
cadmium intake and are shown in Table 19. The authors concluded that
the total cadmium intake that produced an adverse effect on health
was approximately 2000 mg for both men and women. On the basis of
the linear regression equations shown in Table 19, an average daily
cadmium intake of 440 µg/day in men and 350 µg/day in women would
be expected to cause a 50% response rate (> 1000 µg
ß2-microglobulin/litre urine). Response rates of 20, 10 and 5%
would occur at daily intakes of 220, 150, and 110 µg/day for men and
200, 150, and 120 µg/day for women. The authors indicated that these
data are in general agreement with results from other studies
involving the consumption of various levels of cadmium in rice.
8.3.3.5 Akita prefecture (Kosaka area)
Around Kosaka mine and refinery areas, increased cadmium levels
in rice were first reported by the Akita Prefectural Government
(1973). Kojima et al. (1976) gave further data from the Kosaka area,
where the reported average cadmium level in rice varied between 0.26
and 0.56 mg/kg. The latter average was based on 41 samples where one
was reported to contain 4.81 mg/kg. In the exposed area, the
weighted average of cadmium levels in rice in each district
according to the number of examinees was calculated by Kojima et al.
(1976) to be 0.57 mg/kg in 1973 and 0.50 mg/kg in 1974. These
values, according to Kojima et al. (1976), represented the level of
cadmium exposure in this study and were considered more accurate
than the general average given above.
In an epidemiological investigation of the total population in
the age-group 50-69 of defined geographical areas (93 out of 98 in
the control area and 156 out of 190 in the exposed area
participated), Kojima et al. (1976, 1977) obtained data on faecal
excretion of cadmium. The cadmium level in 24-h faeces samples was
analysed only for those participants who said that they defaecated
once a day (64 in the control area and 118 in the polluted area).
Average rates were 51 µg/day and 177 µg/day for the control and
exposed groups, respectively (Kojima et al., 1976). The prevalence
of proteinuria exceeding 150 mg/litre (using the Tsuchiya biuret
method) and of combined proteinuria and glucosuria (test tape) was
significantly higher in the exposed group than in the control group
(Kojima et al., 1976).
Table 19. Linear regression equations relating total cadmium intake and prevalence of ß2-microglobulinuriaa
Sex ß2-microglobulinuria Linear regression Prevalence of Total cadmium intake
equationb ß2-microglobulinuria (mg)c
in the control group (%)
Male > 1000 µg/litre Y = 0.0076X - 10.33 5.3 2057
> 1000 µg/g creatinine Y = 0.0083X - 7.93 6.0 1678
Female > 1000 µg/litre Y = 0.011X - 19.61 3.1 2065
> 1000 µg/g creatinine Y = 0.012X - 16.16 5.0 1763
a From: Nogawa et al. (1989)
b Y = prevalence of ß2-microglobulinuria (%); X = total cadmium intake (mg)
c Total cadmium intake yielding a ß2-microglobulinuria prevalence equivalent to the control group
Quantitative analysis of urinary ß2-microglobulin with
radioimmunoassay (RIA) was performed on the same population (Kojima
et al., 1977). The frequency distributions were log-normal, as for
occupationally exposed people, and the prevalence of
ß2-microglobulin excretion was 15% in the whole exposed group and
3% in the control group. The exposed and control groups showed
average faecal cadmium excretion rates of 139 and 41 µg per day,
respectively (Kojima et al., 1977).
The ß2-microglobulin data and cadmium intake data in the
study by Kojima et al. (1977) were divided into three dose groups
(Kjellström et al., 1977b) and analysed for a dose-response
relationship by Hutton (1983).
Further studies of urinary ß2-microglobulin over the age
range 5-90 years were reported by Saito et al. (1981). The RIA
method was used, and a control area in Akita prefecture was compared
with cadmium-polluted areas in Akita prefecture (Kosakai), Ishikawa
prefecture (Kakehashi) (section 8.3.3.3), and Nagasaki prefecture
(Tsushima) (section 8.3.3.5). For people over the age of 40, there
were significant increases in average urinary ß2-microglobulin
excretion in all the polluted areas. In the older age-groups, the
increase was 10-100 times above control values.
In the first comprehensive study of proximal renal tubular
functions performed on a population living in a cadmium-polluted
area, Saito et al. (1977) conducted health examinations within Akita
prefecture. Renal tubular function tests consisted of renal
glucosuria, uric acid clearance, low molecular weight proteinuria,
tubular reabsorption of phosphate, hydrogen carbonate threshold,
acid-base balance, concentrating and acidifying ability of urine,
endogenous creatinine clearance, and renal plasma flow. Of the 147
residents (97% of target population) examined, 33 (22%) had some
indications of proximal renal tubular dysfunction, such as renal
glucosuria and low molecular weight proteinuria. In addition, 10
subjects (7%) were diagnosed as having multiple proximal renal
tubular dysfunctions. Detailed examinations revealed that none of
these 10 subjects had experienced any other environmental exposures
or diseases that could have caused the renal dysfunction. They were
therefore diagnosed as suffering from the effects of chronic cadmium
poisoning (Saito et al., 1977).
8.3.3.6 Nagasaki prefecture (Tsushima area)
This area has been a lead and zinc mining district from ancient
times (Takabatake, 1978b). Modern operations started in 1948, and
mining wastes have been scattered throughout the local area. In
1952, local farmers complained about the poor growth of crops. In
the 1960s, studies of cadmium pollution were carried out. Health
examinations for cadmium effects have been conducted since 1966
(Takabatake, 1978b).
Early studies showed an increased prevalence of proteinuria in
the most polluted village, and an average rice cadmium level of
0.75 mg/kg was reported (Takabatake, 1978b).
The study of urinary ß2-microglobulin according to age
referred to in section 8.3.3.4 (Saito et al., 1981) included an
exposed group of people from Tsushima. The age-specific average
urinary excretions were much higher than the control values and
similar to those found in the Kakehashi and Kosaka areas. For women,
the Tsushima values appear higher than those of the other two
polluted areas (Table 17), which may reflect the higher estimated
average cadmium intake in the Tsushima area.
8.3.3.7 Other Japanese areas
As shown in Table 7, health surveys have been performed in
areas other than Fuchu, Ikuno, Kakehashi, Kosaka, and Tsushima.
According to the Japanese Cadmium Research Committee (1989), it
should be emphasized, however, that no cadmium health effects,
including elevated prevalence of ß2-microglobulinuria, were
observed in some areas with higher levels of cadmium (daily intakes
of 180-309 µg in Bandai, Fukushima; 180-380 µg in Annaka, Gunma; and
222-391 µg in Okutake river basin, Oita) even than Kosaka and
Kakehashi (cadmium in rice = 0.16-0.58 mg/kg, daily intake of
cadmium = 139-177 µg in Kosaka, Akita; cadmium in rice =
0.2-0.8 mg/kg, daily intake of cadmium = 160-190 µg in the Kakehashi
river basin, Ishikawa). Also, no health effects were found in the
Uguisuzawa river basin, Miyagi (cadmium in rice = 0.6-0.7 mg/kg),
Watarase river basin, Gunma (0.32 mg/kg), Shimoda, Shizuoka
(0.4-1.1 mg/kg), and Ohmuta, Fukuoka (0.72 mg/kg), even though
residents consumed rice heavily contaminated with cadmium.
8.3.3.8 Belgium
The Liege area of Belgium is known to be polluted by cadmium,
mainly due to the activities of non-ferrous smelters since the end
of the 19th century (Kretzschmar et al., 1980; Lauwerys et al.,
1980a).
A pilot study was performed in 1979 on a group of 60 elderly
non-smoking women who had spent most of their lives in the Liege
area and had never been occupationally exposed to cadmium (Roels et
al., 1981a). Daily intakes of cadmium ranged from 2-88 µg/day with
an average of 15 µg/day. Their average blood cadmium level
(1.6 µg/litre) and their urinary excretion rates of cadmium
(0.093 µg/h), total protein (17.3 mg/h), amino acids (5.45 mg amino
acid N/h), and albumin (1.54 mg/h) were higher than those found in a
group of 70 women of the same age and socio-economic status who
lived in another industrial area (Charleroi) less polluted by
cadmium. Although the average excretion rate of ß2-microglobulin
was greater in Liege (93.6 µg/h) than in Charleroi (22 µg/h), the
difference between the geometric means was not statistically
significant. The two areas were well matched with respect to their
environmental pollution by sulfur dioxide, fume, suspended
particles, and various metals, including lead, vanadium, nickel,
chromium, and iron.
Following these observations, a mortality study and a
preliminary autopsy study were undertaken (Lauwerys & De Wals, 1981;
Lauwerys et al., 1984a). It was found that, although the overall
mortality was not markedly different, the standard mortality ratio
(SMR) and proportional mortality rate (PMR) from nephritis and
nephrosis for the years 1967-1976 were higher in Liege than in
Charleroi or in Belgium as a whole (SMR: Belgium, 100; Charleroi,
102; Liege, 196; PMR: Belgium, 3.3; Charleroi, 3.0; Liege, 6.0).
Since the increased mortality rate for renal diseases was observed
in both males and females, the influence of environmental factors
other than occupation is probable.
The results of the preliminary autopsy study indicated that, in
the age-group 40-60, the average body burden of cadmium was
approximately twice as high in people autopsied in Liege as it was
in those autopsied in a city (Brussels) less polluted by cadmium
(Lauwerys et al., 1984a). The geometric mean values of cadmium
concentration in the kidney cortex were 38.3 and 22.8 mg/kg wet
weight in Liege and Brussels, respectively.
According to the authors of these reports, the studies
performed so far in the Liege area do not refute the hypothesis that
environmental pollution by cadmium in the area has increased the
body burden of cadmium of the inhabitants and has affected their
renal function. A large-scale morbidity and autopsy study is at
present underway (Braux et al., 1987).
8.3.3.9 Shipham area in the United Kingdom
The village of Shipham is located on the slag heaps of an old
zinc mine and high levels of cadmium have been found in soil and
dust (section 3.4.3).
The exposed population has been studied using both mortality
and morbidity end-points. A census in 1979 identified 1092
residents, of whom 64% had resided in the village for more than 5
years, and 548 participated in a health study coordinated by the
United Kingdom Department of the Environment (Barltrop & Strehlow,
1982a). A similar study of 543 age-and sex-matched individuals was
performed in a nearby control village (Barltrop & Strehlow, 1982b).
The daily intake of cadmium in Shipham was an average of 35 µg/day
(section 4.2.4), which is about twice as high as the estimated
United Kingdom national average but much lower than in polluted
areas of Japan (section 5.2.4).
A health inventory was compiled by means of a questionnaire,
which included information on smoking habits, alcohol consumption,
medication, and occupation. Blood samples were analysed for
haemoglobin, haematocrit, serum protein, ß2-microglobulin,
creatinine, erythrocyte protoporphyrin, lead, and cadmium. Urine
samples were analysed for total protein, creatinine, ß2-micro-
globulin, and cadmium.
The mean 24-h urinary concentration of cadmium for Shipham
residents was 0.68 µg cadmium/g creatinine and, in the control area,
0.60 µg cadmium/g creatinine with 97.7% of values less than 3.4 µg
cadmium/litre. The difference was statistically significant
(P < 0.03) (Barltrop & Strehlow, 1982b), but the similarity of the
values for average urinary cadmium concentrations between the two
areas and the generally low levels of cadmium in Shipham suggest a
rather low daily cadmium intake.
However, there are data showing that some individuals in
Shipham had high cadmium exposures. Liver cadmium concentrations,
measured by means of in vivo neutron activation analysis, were
determine for 21 adult volunteers living in the most heavily
contaminated areas of Shipham (Harvey et al., 1979). Their age range
was 40-62 years (mean, 53 years) and, with one exception, they had
lived in Shipham for 9-50 years (mean, 23 years). On average, half
of their vegetable consumption was of local origin. The mean liver
cadmium concentration was 11.0 (+ 2.0) mg/kg, compared with 2.2
(+ 2.0) mg/kg in 20 age-matched, non-Shipham controls (P < 0.001).
The maximum concentration in the Shipham group was 28 mg/kg, which
would correspond to levels in the kidney cortex of at least
200-300 mg/kg (Friberg, 1979) (section 6.4). Health effects were not
studied in this investigation.
In the health study by the Department of the Environment
(Barltrop & Strehlow, 1982a), the comparison of ß2-microglobulin
data from the two villages showed similar distributions, and all
other laboratory data, including blood pressure levels, were
distributed within the normal range. However, the poor participation
rate in this health study (50%), makes it difficult to interpret the
findings. Another study of 31 volunteers from Shipham (Carruthers &
Smith, 1979) reported a high prevalence of hypertension and LMW
proteinuria, but the methodology of the study has been criticized
(Hughes & Stewart, 1979; Kraemer et al., 1979).
Examination of the data in relation to soil cadmium levels
showed no evidence of an increased mortality from any cause in those
living in the most polluted areas. It was concluded from this study
that, if cadmium contamination had any effect on the mortality
pattern in Shipham, this effect was only slight and did not present
a serious health hazard to residents. No case resembling Itai-itai
disease has at any time been reported in Shipham. All the authors
involved in the health studies in Shipham pointed to the possible
protective effect of high levels of zinc also present in soil, and
Kraemer et al. (1979) pointed to the need to assess dietary zinc and
the intake of nutrients other than cadmium.
8.3.3.10 USSR
Screening of populations, both environmentally and
occupationally exposed, which included measurements of urinary
ß2-microglobulin, has been carried out within the USSR (Likutova,
1989). Increased prevalence (up to 6%) of ß2-micro-globulinuria
(> 280 µg/g creatinine) was observed in females (20-50 years old)
in some of the most heavily polluted cities, i.e. Odjonikidze and
Kursk. The air cadmium concentrations in these two cities were
0.085 µg/m3 and 0.005-0.027 µg/m3, respectively. The examination
of workers (50-300 µg/m3) exposed to cadmium also revealed an
increased prevalence of ß2-microglobulinuria (up to 19% in the
most heavily exposed group). The findings are in good agreement with
the data presented in Table 15.
8.4 Conclusions
High inhalation exposure to cadmium oxide fume results in acute
pneumonitis with pulmonary oedema, which may be lethal. High
ingestion exposure of soluble cadmium salts causes acute
gastroenteritis.
Long-term occupational exposure to cadmium has caused severe
chronic effects, predominantly in the lungs and kidneys. Chronic
renal effects have also been seen among the general population.
Following high occupational exposure, lung changes are
primarily characterized by chronic obstructive airway disease. Early
minor changes in ventilatory function tests may progress, with
continued cadmium exposure, to respiratory insufficiency. An
increased mortality rate from obstructive lung disease has been seen
in workers with high exposure, as has occurred in the past.
The accumulation of cadmium in the renal cortex leads to renal
tubular dysfunction with impaired reabsorption of, for instance,
proteins, glucose, and amino acids. A characteristic sign of tubular
dysfunction is an increased excretion of low molecular weight
proteins in urine. In some cases, the glomerular filtration rate
decreases. Increase in urine cadmium correlates with low molecular
weight proteinuria and in the absence of acute exposure to cadmium
may serve as an indicator of renal effect. In more severe cases
there is a combination of tubular and glomerular effects, which may
progress in some cases to decreased glomerular filtration. For most
workers and people in the general environment, cadmium-induced
proteinuria is irreversible.
Among other effects are disturbances in calcium metabolism,
hypercalciuria, and formation of renal stones. High exposure to
cadmium, most probably in combination with other factors such as
nutritional deficiencies, may lead to the development of
osteoporosis and/or osteomalacia.
There is evidence that long-term occupational exposure to
cadmium may contribute to the development of cancer of the lung but
observations from exposed workers have been difficult to interpret
because of confounding factors. For prostatic cancer, evidence to
date is inconclusive but does not support the suggestion from
earlier studies of a causal relationship.
At present, there is no convincing evidence for cadmium being
an etiological agent of essential hypertension. Most data speak
against a blood pressure increase due to cadmium and there is no
evidence of an increased mortality due to cardiovascular or
cerebrovascular disease.
Data from studies on groups of occupationally exposed workers
and on groups exposed in the general environment show that there is
a relationship between exposure levels, exposure durations, and the
prevalence of renal effects.
An increased prevalence of low molecular weight proteinuria in
cadmium workers after 10-20 years of exposure to cadmium levels of
about 20-50 µg/m3 has been reported.
In polluted areas of the general environment, where the
estimated cadmium intake has been 140-260 µg/day, effects in the
form of increased low molecular weight proteinuria have been seen in
some individuals following long-term exposure.
9. EVALUATION OF HUMAN HEALTH RISKS
9.1 Exposure assessment
9.1.1 General population exposure
In the ambient air, cadmium concentrations based on long-term
sampling periods indicate, in most cases, a range of
0.001-0.015 µg/m3 in rural areas, 0.005-0.05 µg/m3 in urban
areas, and up to 0.6 µg/m3 near sources of pollution (section
5.1.1).
One cigarette usually contains 1-2 µg cadmium, of which about
10% may be inhaled (section 5.1.3).
Among staple foods, rice and wheat usually contain less than
0.1 mg/kg and other foods usually less than 0.05 mg/kg wet weight,
but liver and kidney may contain 1-2 mg/kg wet weight and certain
sea-foods as much as 10 mg/kg wet weight (section 5.2). Certain
animals, e.g., the horse, may accumulate considerably higher
concentrations in the liver and kidney. In polluted areas, these
levels are further increased.
The content of natural waters is usually less than 1 µg/litre,
but higher levels may be found near sources of pollution.
The total daily intake in non-polluted areas of most countries
from food, water and air is estimated to be approximately
10-40 µg/day (food, 10-40 µg/day; water, < 1 µg; and air,
< 0.5 µg/day for non-smokers).
Twenty cigarettes per day would contribute a further 2-4 µg. In
polluted areas, the daily intake may be much higher, and intakes of
several hundred µg/day have been reported (section 5.3.2).
9.1.2 Occupational exposure
Air is the main source of additional cadmium exposure for
industrial workers. In many countries such exposures have now been
reduced considerably. In the past, levels of several mg/m3 were
recorded in workplaces. Now, with proper industrial hygiene
practices, levels of 0.02-0.05 mg/m3 would be more typical
(section 5.1.2).
9.1.3 Amounts absorbed from air, food, and water
The proportion of cadmium from food and water that is absorbed
will depend on the chemical nature of the cadmium compounds, but
estimates based on the available data indicate that gastrointestinal
absorption is approximately 5%, with considerable individual
variation (section 6.1.2). Similarly, the amount absorbed from the
air will depend on the chemical nature and the particle size of the
inhaled material. The absorption varies between 25 and 50% depending
on particle size and solubility (section 6.1.1). About 10% of the
cadmium inhaled in cigarette smoke is absorbed.
Thus, the average amount absorbed from food and water in a
person from a non-polluted area would be about 0.5-1.3 µg/day. The
absorbed amount from smoking 20 cigarettes per day would be
1-2 µg/day and that from workroom air could be many times greater
(section 5.3).
9.2 Dose-effect relationships
9.2.1 Renal effects
Long-term exposure to cadmium causes renal tubular dysfunction
with proteinuria, glucosuria, and aminoaciduria, as well as
histopathological changes, in both experimental animals and humans
(sections 7.2.1.4 and 8.2.1, respectively). These are usually the
first effects to occur after ingestion or inhalation exposure. As
the renal dysfunction progresses in severity, the glomeruli may also
be affected and, in a few cases, the cadmium-induced damage may lead
to renal failure (section 8.2.1). Daily cadmium intakes in food of
140-260 µg/day for more than 50 years or workplace air exposures of
50 µg/m3 for more than 10 years have produced an increase in renal
tubular dysfunction in some exposed popu-lations (section 8.3.3.2).
9.2.2 Bone effects
Cadmium may produce bone effects in both humans and animals.
The most notable clinical entity in these cases is osteomalacia, but
many subjects also show osteoporosis. Animal experiments show that
both can be produced by long-term cadmium exposure (section 7.2.4).
In animals and humans, osteomalacia has been seen in combination
with cadmium-induced renal damage. The bone effects may be linked to
cadmium effects on calcium and vitamin D metabolism in the kidney.
The daily intakes via food and exposure levels in air at which the
bone effects occur are uncertain, but they must be higher than those
causing renal effects. Bone effects have been seen among both the
general population and industrial workers in the past when exposure
levels were very high. Host and nutritional factors influence the
development and severity of cadmium-induced bone effects.
9.2.3 Pulmonary effects
Chronic obstructive airway disease has been reported in a
number of studies of cadmium workers (section 8.2.3). This has, in
severe cases, led to an increased mortality. The dose needed to
produce these effects is uncertain, but it is higher than the dose
needed to produce renal effects, as most workers reported to have
lung effects also had renal effects. On the other hand, many workers
with renal effects, who had been exposed to cadmium oxide dust and
fume, had no lung effects.
9.2.4 Cardiovascular effects
Some animal studies have shown that, under certain exposure
conditions, increased blood pressure and effects on the myocardium
occur. Studies of cadmium-exposed workers and people in the general
environment have been carried out, but most data do not support the
animal findings.
9.2.5 Cancer
There is evidence that cadmium chloride, sulfate, sulfide and
oxide give rise to injection site sarcomata in the rat and that the
chloride and sulfate induce interstitial cell tumours of the testis
in rats and mice.
Long-term inhalation studies in rats exposed to aerosols of
cadmium chloride, sulfate, and oxide fume and dust at low
concentrations demonstrated a high incidence of primary lung cancer
with evidence of a dose-response relationship. This has not so far
been shown in other animals.
There is evidence that long-term occupational exposure to
cadmium may contribute to the development of cancer of the lung, but
observations from exposed workers have been difficult to interpret
because of inadequate exposure data and confounding factors. The
evidence to date is inconclusive, but does not support the
suggestion from earlier studies that cadmium can cause prostatic
cancer.
IARC (1987a) considered that there was sufficient evidence for
the carcinogenicity of specified cadmium compounds in experimental
animals and limited evidence for carcinogenicity in humans exposed
to cadmium. A combined evaluation of human and animal data by IARC
(1987b) classified cadmium as a probable human carcinogen (IARC
group 2A). The IPCS Task Group found no reason to deviate from this
IARC evaluation.
9.2.6 Critical organ and critical effect
The kidney is the critical organ for chronic cadmium poisoning.
Within the kidney, the cortex is the site where the first adverse
effect occurs. Therefore, in assessing dose-response relationships,
the cadmium concentration in the kidney cortex is of prime
importance.
The critical effect is renal tubular dysfunction, which is most
often manifested as low molecular weight proteinuria. Animal studies
indicate that histological changes in the renal tubules occur at a
dose level lower than that needed to produce low molecular weight
proteinuria.
9.3 Critical concentration in the kidneys
9.3.1 In animals
Several studies with data on both cadmium concentrations in the
renal cortex and the occurrence of tubular damage were discussed in
section 7.2.1. The findings were summarized in Table 12. They showed
that histological tubular lesions or proteinuria was usually seen at
cadmium renal cortex levels of 200-300 mg/kg wet weight. In some
studies on rats, monkeys, horses, and birds, certain effects were
seen at lower levels.
As no dose-response data are given in most animal studies, it
may be assumed that these renal cortex levels correspond to a 50%
response rate (CC50). Naturally, the cadmium levels at which lower
response rates occur would be lower.
In studies on monkeys conducted in Japan, kidney cadmium levels
were related to dose and duration of exposure. At the two highest
dose levels, acute liver effects occurred. If one wishes to
establish a range of values for the critical concentration in
individuals at which a small but significant part of an exposed
population will show effects, animal studies indicate that a renal
cortex level of about 100-200 mg/kg is likely to coincide with such
a range. There is some evidence that the average critical
concentration (CC50) could be as high as 300 or 400 mg/kg for the
more severe signs of renal tubular damage, but such high levels
should not be used as a starting point for calculations of
"acceptable daily exposures".
9.3.2 In humans
Section 8.2.1.5 reviewed all available data from cases in which
both renal cortex cadmium levels and renal effects were measured.
Data from autopsies or biopsies have mainly been cross-sectional,
i.e. the renal cadmium concentrations and the effects were measured
more or less simultaneously. This has made it difficult to interpret
the data from a critical concentration point-of-view, as the cases
with the most severe cadmium-induced kidney dysfunction had the
lowest renal cadmium levels. Cadmium is lost from the kidney when
the damage progresses (section 6.5.1.2).
In vivo neutron activation analysis has provided a new tool
for establishing the human critical concentration of cadmium in the
renal cortex. Longitudinal studies measuring the renal cortex
cadmium concentration several times during continued exposure can
now be carried out. The cadmium level at which the first measurable
signs of renal tubular dysfunction occurs can be estimated. However,
only two studies using in vivo neutron activation have been
published to date, and both of them are cross-sectional.
The renal cadmium concentrations are disproportionately low
when the liver cadmium concentrations are high and renal effects
have developed. Of the several methods available to estimate the
average critical kidney concentration in these groups of exposed
workers, the method of preference assumes that the peak for renal
cortex cadmium level, plotted against liver cadmium, is equivalent
to the point where renal tubular dysfunction occurs. This results in
a value of 319 mg/kg tissue (based on a ratio of renal cortex
cadmium to whole kidney cadmium of 1.5). There is considerable
variance in the individual values, the 95% tolerance (which
corresponds to a confidence interval) being in the range ± 90 mg/kg
from the mean. Other studies, using similar assumptions, have
reported a value of 332 mg/kg, 10% of the workers having a peak
cadmium level of about 216 mg/kg tissue. A re-evaluation of the
original study resulted in a calculated cadmium level of about
200 mg/kg. It was concluded that for the purposes of dose-response
calculations, using a metabolic model, this concentration could be
used as a starting point for renal effects occurring in an exposed
population.
9.4 Dose-response relationships for renal effects
Two approaches can be used to estimate dose-response
relationships. One employs epidemiological data from industry and
the general environment studying associations between exposure and
response. The other begins with a critical concentration in the
kidney cortex and employs a metabolic model to calculate, on the
basis of certain given assumptions, the exposure that is required to
reach a critical concentration.
9.4.1 Evaluation based on data on industrial workers
Table 16 contains data from various group studies on cadmium
workers. In most of these studies, the dose measurements were based
on short sampling periods (hours or a few days). However, exposure
may have lasted for decades, levels usually being higher in the
past. The use of protective devices may also confound the picture.
As discussed in section 8.3.2, there are now several reports
available that show a clear exposure-response relationship between
cadmium in workplace air and the prevalence of proteinuria.
An increased prevalence of overt proteinuria, as measured by
sulfosalicylic acid, trichloroacetic acid, or quantitative
determination of total proteinuria, can occur after only 5-10 years
of exposure to approximately 100 µg cadmium/m3. If instead, the
increased excretion of low molecular weight proteins (more than 97.5
percentile of control group) is used as the critical effect, 10-20%
of workers would have this effect after a cumulative dose
corresponding to 10-20 years of exposure to 50 µg cadmium/m3.
These evaluations are all based on levels of total cadmium in
inhaled dust or air.
9.4.2 Evaluation based on data on the general population
As indicated in chapter 8, there exists a considerable amount
of information from epidemiological studies carried out on the
general population in Japan. It was shown that in some areas of high
cadmium exposure the prevalence of low molecular weight proteinuria
was significantly higher than in control areas. This may be
considered in relation to the known cadmium concentrations in rice
and the daily cadmium intakes in the affected areas (Tables 7 and
17). Contamination of drinking-water in some areas may be a
complicating factor (section 8.3.3).
Taking all of the data in section 8.3.3 together, it seems
that, when the most sensitive method for diagnosis of low molecular
weight proteinuria is applied, there is an association between
cadmium exposure and increased excretion of low molecular weight
proteins among some people over 50 years of age at a daily intake of
about 140-260 µg cadmium or a cumulative cadmium intake of about
2000 mg or more (for both men and women).
9.4.3 Evaluation based on a metabolic model and critical
concentrations
Using the data on critical concentrations and kinetic models of
cadmium metabolism, attempts have been made to calculate the
dose-response relationship for cadmium. Assuming that cadmium in the
kidney is accumulated in accordance with a one-compartment model and
that a third or a quarter of the body burden of cadmium is in the
kidney (and making certain other assumptions indicated in Tables 20
and 21), the daily cadmium intake via food and the occupational air
concentrations needed to reach the critical concentration have been
calculated (Tables 20 and 21).
As the values calculated in Tables 20 and 21 are for an average
person, not all of those exposed to these levels would have reached
the renal cortex cadmium concentration of 200 mg/kg tissue or their
individual critical concentration. Nevertheless, these calculations
produce values that are similar to the levels at which effects have
been observed, and the model approach may be a useful way to
quantify the response rates at levels lower than those easily
measurable.
Calculations have been reported of the relationship between
intake and response rates using the observed frequency distributions
of daily intake and renal cortex cadmium concentrations, and
utilizing multi-compartment metabolic model values in the same range
as those given in Tables 20 and 21. Further development of these
modelling techniques would be of value.
Using a single-compartment model for the accumulation of
cadmium in the kidney, the average daily intake that would give rise
to an average concentration of 200 mg/kg wet weight in the kidney
cortex at age 50 would be 260-480 µg/day, assuming 5%
gastrointestinal absorption, various biological half-times, and
different proportions of the body burden in the kidneys (Table 19).
Assuming a 10% absorption rate, the intake needed would be
140-260 µg per day. These estimates will vary depending on the body
weight estimates for different populations.
Table 20. Calculated daily cadmium intake via ingestion required by a
non-smoker to reach a kidney cortex concentration of 200 mg/kg
at age 50 (using a one-compartment model)a
Gastrointestinal Proportion of body Estimated half-time in kidney cortexb
absorption rate burden in kidney
(%) 17 years 30 years
5 one-third 365 µg (286 µg) 265 µg (208 µg)
10 182 µg (143 µg) 133 µg (104 µg)
5 one-quarter 486 µg (382 µg) 353 µg (277 µg)
10 243 µg (191 µg) 177 µg (139 µg)
a The data in the table are based on the following assumptions:
gastrointestinal absorption, either 5% or 10%;
half-time in kidney cortex, either 17 years or 30 years (as reported in
section 6.6.2);
one-third or one-quarter of body burden in the kidneys;
cadmium concentration in renal cortex 25% higher than renal average;
average weight of both kidneys at age 50 of 300 g for a 70-kg person
or 235 g for a 55-kg person;
average cadmium concentration in foodstuffs constant during
the last 50 years;
variation of daily intake with age has been disregarded since such
variation would influence the values by less than 10%.
b Data have been calculated for a 70-kg person; values in parentheses
are for a 55-kg person.
Table 21. Calculated concentration of cadmium in industrial air required
for a kidney cortex concentration of 200 mg/kg to be reacheda
Proportion of body burden Estimated half-time in kidney cortexb
in kidney
17 years 30 years
one-third 16 µg/m3 (13 µg/m3) 14 µg/m3 (11 µg/m3)
one-quarter 21 µg/m3 (17 µg/m3) 19 µg/m3 (15 µg/m3)
a The data in the table are based on the following assumptions:
those assumptions given in Table 20;
exposure time of 25 years;
225 working days per year;
10 m3 of air inhaled per day;
25% pulmonary absorption
b Data have been calculated for a 70-kg worker; values in parentheses
are for an average 55-kg person
10. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF
HUMAN HEALTH
10.1 Conclusions
The kidney is considered the critical target organ for the
general population as well as for occupationally exposed
populations. Chronic obstructive airway disease is associated with
long-term high-level occupational exposure by inhalation. There is
some evidence that such exposure to cadmium may contribute to the
development of cancer of the lung but observations from exposed
workers have been difficult to interpret because of confounding
factors.
10.1.1 General population
Food-borne cadmium is the major source of exposure for most
people. Average daily intakes from food in most areas not polluted
with cadmium are 10-40 µg. In polluted areas the value has been
found to be several hundred µg per day. In non-polluted areas,
uptake from heavy smoking may equal cadmium intake from food.
An association between cadmium exposure and increased urinary
excretion of low molecular weight proteins has been noted in humans
with a life-long daily intake of about 140-260 µg cadmium, or a
cumulative intake of about 2000 mg or more.
10.1.2 Occupationally exposed population
Occupational exposure to cadmium is mainly by inhalation but
includes additional intakes through food and tobacco. The total
cadmium level in air varies according to industrial hygiene
practices and type of workplace. There is an exposure-response
relationship between airborne cadmium levels and proteinuria. An
increase in the prevalence of low molecular weight proteinuria may
occur in workers after 10-20 years of exposure to cadmium levels of
about 20-50 µg/m3. In vivo measurement of cadmium in the liver
and kidneys of people with different levels of cadmium exposure have
shown that about 10% of workers with a kidney cortex level of
200 mg/kg and about 50% of people with a kidney cortex level of
300 mg/kg would have renal tubular proteinuria.
10.2 Recommendations for protection of human health
a) Measures to increase recycling of cadmium should be
systematically examined and promising ideas encouraged.
b) Information on the importance of minimizing waste discharge
of cadmium, particularly into surface waters, should be
supplied to countries.
c) Public health measures for protection from cadmium
exposures would be improved by:
i) collection of more data from countries on cadmium
levels in foodstuffs and the environment;
ii) determination of tissue cadmium levels and
monitoring of health parameters in non-exposed
populations and in those living near mines or
smelters or exposed to elevated levels of the metal
in foodstuffs;
iii) technical assistance to developing countries for
the training of staff, particularly for cadmium
analysis;
iv) development of means of reducing cadmium exposure
by, for instance, improved working conditions and
the dissemination of information on the proper use
of fertilizers (which sometimes contain high levels
of cadmium), techniques for the disposal of
cadmium-containing wastes, etc.
11. FURTHER RESEARCH
a) There is a need for improved analytical techniques for
measuring cadmium species and biological indicators of cadmium
exposure/toxicity, such as ß2-microglobulin, in various
matrices, and for international centres for quality assurance
and training.
b) The assessment of human exposure to cadmium from all media
needs to be improved by increased monitoring of cadmium levels
in the environment. Changes in cadmium levels with time are of
particular importance.
c) Populations with ß2-microglobinuria (both those in the
workplace and in the general environment) should be
longitudinally investigated to determine the nature, severity,
and prognosis of adverse health effects associated with this
finding. Further research is needed on ß2-microglobulin as a
biological indicator of exposure and effect.
d) International collaborative efforts should be encouraged to
examine further the role of cadmium in the development of human
cancer. Both the general population and industrial workers
should be studied with special emphasis on the development of a
common format for analysing and presenting data and the
collection of additional information on exposure to cadmium,
tobacco, and other confounding factors. Multiple exposures must
be considered. It is proposed that a collaborative study
coordinated by an international body (e.g., IARC) should
include the existing cohorts in order to obtain better exposure
data. It should also collect both exposure and effects data in
a standardized manner, so that the results of different studies
may be more readily compared. A further approach would be to
perform a collaborative prospective study identifying all those
workers who have shown evidence of an effect of cadmium on the
kidney and who would therefore be considered to have had
unusually heavy exposure. In such a study, both morbidity and
mortality data would be collected. Outcome would be studied not
only for cancer but also for sequelae to renal dysfunction.
e) Existing occupational cohorts should be linked, where
possible, to regional cancer registers to determine the
incidence of prostatic cancer (morbidity) in relation to
cadmium exposure.
f) To understand the mechanism(s) of cancer induction,
experimental studies on the bioavailability of cadmium at the
target site and the interactions between zinc and cadmium would
be of value. The role of metallothionein induction in the
target cells of the respiratory tract and its relationship to
such phenomena as DNA damage and repair and oncogene protein
structure would be of interest.
g) Further information on the long-term health consequences of
cadmium exposures in the general environment is essential, with
emphasis on renal dysfunction and other end-points such as
neurotoxicity and immunotoxicity.
h) Studies of the effects of cadmium on calcium-phosphorus
metabolism and bone density should be conducted on female
workers to clarify whether these workers are at special risk in
the occupational setting. The effect of cadmium on the placenta
and subsequent effects on the fetus, especially in multiple
pregnancies, need further study.
i) The effects of various nutritional deficiencies and of exposure
to other metals on the transport, accumulation, and toxicity of
cadmium should be investigated with special reference to bone
toxicity. These studies should be conducted in humans and
experimental animals with respect to age, sex, dose-dependence,
biological half-time, and estimation of critical concentration.
j) To provide additional scientific support for the assessment of
human health risks from cadmium exposure, studies in
experimental animals addressing the following issues should be
initiated:
* mechanism of cadmium transport into the cell and factors
controlling the process;
* mechanism of cadmium-induced toxicity with particular
emphasis on kidney and bone and the role(s) of
non-metallothionein-bound cadmium in these processes;
* mechanisms of cadmium-induced calcuria and the
relationship of this phenomenon to tubular proteinuria and
osteomalacia.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenic potential of cadmium was evaluated in 1976 by
the International Agency for Research on Cancer (IARC, 1976) and
re-evaluated in 1987 (IARC, 1987a). It was concluded in the
re-evaluation that there was limited evidence that cadmium and
cadmium compounds are carcinogenic in humans. Sufficient evidence
was available to show that cadmium and specified cadmium compounds
cause cancer in experimental animals. Cadmium was classified as a
probable human carcinogen (group 2A) (IARC, 1987a,b).
To prevent adverse pulmonary and renal effects the following
health-based limits for occupational exposure to cadmium fumes and
respirable dust were proposed by WHO (WHO, 1980): 250 µg Cd/m3 for
short-term exposures provided the recommended time-weighted average
(40 h/week) of 10 µg Cd/m3 is respected. It was further
recommended that control measures be applied when cadmium levels in
urine and blood of individuals exceed 5 µg Cd/g creatinine and
5 µg Cd/litre of whole blood, respectively.
A drinking-water guideline value of 0.005 mg/litre has been set
for cadmium by the World Health Organization (WHO, 1984).
Cadmium was evaluated by a WHO Working Group developing air
quality guidelines (WHO, 1987). Based on non-carcinogenic effects,
the following recommendations were made:
a) in rural areas, levels of < 1-5 ng/m3 should not be allowed
to increase, and
b) in urban and industrialized areas without agricultural
activities, levels of 10-20 ng/m3 may be tolerable. However,
increases in the present levels of airborne cadmium should not
be permitted (WHO, 1987).
At the thirty-third meeting of the Joint FAO/WHO Expert
Committee on Food Additives and Food Contaminants, the previous
recommendation was reaffirmed, i.e. the provisional tolerable weekly
cadmium intake of 400-500 µg for an adult should not be exceeded
(WHO, 1989).
Regulatory standards established by national bodies in several
countries and the EEC are summarized in the legal file of the
International Register of Potentially Toxic Chemicals (IRPTC, 1987).
REFERENCES
ADAMS, R.G. (1980) Osteopathy associated with tubular nephropathy in
employees in an alkaline battery factory. In: Shigematsu, I. &
Nomiyama, K., ed. Cadmium-induced osteopathy, Tokyo, Japan Public
Health Association, pp. 67-73.
ADAMS, R.C., HARRISON, J.G., & SCOTT, P. (1969) The development of
cadmium-induced proteinuria, impaired renal function, and
osteomalacia in alkaline battery workers. Q. J. Med., 38: 425-443.
ADAMSSON, E. (1979) Long-term sampling of air-borne cadmium dust in
an alkaline battery factory. Scand. J. Work Environ. Health,
5: 178-187.
ADES A.E. & KAZANTZIS, G. (1988) Lung cancer in a non-ferrous
smelter: the role of cadmium. Br. J. ind. Med., 45: 435-442.
AHLGREN, L. & MATTSON, S. (1981) Cadmium in man measured
in vivo by X-ray fluorescence analysis. Phys. Med. Biol.,
26: 19-26.
AHLMARK, S., AXELSSON, B., FRIBERG, L., & PISCATOR, M. (1961)
Further investigations into kidney function and proteinuria in
chronic cadmium poisoning. Int. Congr. occup. Health, 13: 201-203.
AHOKAS, R.A. & DILTS, P.V. (1979) Cadmium uptake by the rat embryo
as a function of gestational age. Am. J. Obstet. Gynecol.,
135: 219-222.
AKITA PREFECTURAL GOVERNMENT (1973) [Surveys on environmental
pollution due to heavy metals: Annual report of the Technical Centre
for Environmental Protection], Akita, Japan, Akita Prefectural
Government (Akita Prefecture Report No. 1: 1970 and 1971) (in
Japanese).
AL-HADDAD, I.K., CHETTLE, D.R., FLETCHER, J.G., & FREMLIN, J.H.
(1981) A transportable system for measurement of kidney cadmium
in vivo. Int. J. appl. Radiat. Isot., 32: 109-112.
ALLANSON, M. & DEANESLY, R. (1962) Observations on cadmium damage
and repair in rat testes and the effects on the pituitary
gonadotrophs. J. Endocrinol., 24: 453-462.
ALLOWAY, B.J., THORNTON, I., SMART, G.A., SHERLOCK, J.C., & QUINN,
M.J. (1988) Metal availability. Sci. total Environ., 75: 41-69.
ALT, F. (1981) [Comparative determination of cadmium in blood by
four different methods.] Fresenius Z. anal. Chem., 308: 137-142 (in
German).
ANDERSEN, A. (1979) [Lead, cadmium, copper and zinc in the Danish
diet], Copenhagen, Statens Levendsmiddelinstitut, 89 pp, (Report
No. 40) (in Danish).
ANDERSEN, O., HAGERSTRAND, I., & NORDBERG, G.F. (1982) Effect of the
chelating agent sodium tripolyphosphate on cadmium toxicity in mice.
Environ. Res., 29: 54-61.
ANDERSEN, O., RONNE, M., & NORDBERG, G.F. (1983) Effects of
inorganic metal salts on chromosome length in human lymphocytes.
Hereditas, 98: 65-70.
ANDERSON, C. & DANYLCHUK, K.D. (1979) Plasma levels of
immunoreactive parathyroid hormone in dogs chronically exposed to
low levels of cadmium chloride. J. environ. Pathol. Toxicol.,
2: 1151-1159.
ANDERSSON, A. (1977) Heavy metals in Swedish soils: on their
retention, distribution, and amounts. Swed. J. agric. Res., 7: 7-20.
ANDERSSON, A. & HAHLIN, M. (1981) Cadmium effects from phosphorus
fertilization in field experiments. Swed. J. agric. Res., 11: 3-10.
ANDERSSON, K., ELINDER, C.-G., HOGSTEDT, C., KJELLSTROM, T., &
SPANG, G. (1984) Mortality among cadmium workers in a Swedish
battery factory. In: Proceedings of the 4th International Cadmium
Conference, Munich, March 1983, London, Cadmium Association,
pp. 152-154.
ANDO, M., SHIMIZU, M., SAYATA, Y., TANIMURA, A., & TOBE, M. (1981)
The inhibition of vitamin D-stimulated intestinal calcium transport
in rats after continuous oral administration of cadmium. Toxicol.
appl. Pharmacol., 61: 297-301.
ANKE, M. & SCHNEIDER, H.J. (1974) [The trace element concentration
of human kidneys as a function of age and sex.] Z. Urol.,
67: 357-362 (in German).
ANKE, M., HENNIG, A., SCHNEIDER, H.J., GROPPEL, B., GRUN, M.,
PARTSCHEFELD, M., & LUDKE, H. (1976) [The influence of the toxic
element cadmium on the metabolism and health of animals and human.]
Math. naturwiss. Unterr. R 25: 241-261 (in German).
AOKI, A. & HOFFER, A.P. (1978) Re-examination of the lesions in rat
testis caused by cadmium. Biol. Reprod., 18: 579-591.
APOSTOLOV, CH. (1979) [The effects on the upper respiratory tract
from long-term work with cadmium.] In: [Proceedings of the Cadmium
Symposium, Jena, 1-3 August, 1977], Jena, Friedrich-Schiller
Universität, pp. 322-325 (in German).
ARMSTRONG, B.G. & KAZANTZIS, G. (1983) The mortality of cadmium
workers. Lancet, 1: 1424-1427.
ARMSTRONG, B.G. & KAZANTZIS, G. (1985) Prostatic cancer and chronic
respiratory and renal disease in British cadmium workers: a case
control study. Br. J. ind. Med., 42: 540-545.
ASOKAN, P., DIXIT, R., MUKHTAR, H., & MURTI, C.R.K. (1981) Effect of
cadmium on hepatic mixed function oxidases during the early
development of rats; possible protective role of metallothionein.
Biochem. Pharmacol., 30(22): 3095-3098.
AUFDERHEIDE, M., MOHR, U., THIEDEMANN, K.-U., & HEINRICH, U. (1990)
Quantification of hyperplastic areas in hamster lungs after chronic
inhalation of different cadmium compounds. Toxicol. environ. Chem.,
27: 173-180.
AXELSSON, B. (1963) Urinary calculus in long-term exposure to
cadmium. In: Sangro, P., Akoun, G., & Beate, H.L., ed. Proceedings
of the 14th International Congress on Occupational Health, Madrid,
Amsterdam, Excerpta Medica Foundation, pp 939-942 (International
Congress Series No. 62).
AXELSSON, B. & PISCATOR, M. (1966a) Renal damage after prolonged
exposure to cadmium: an experimental study. Arch. environ. Health,
12: 360-373.
AXELSSON, B. & PISCATOR, M. (1966b) Serum proteins in
cadmium-poisoned rabbits with special reference to haemolytic
anaemia. Arch. environ. Health, 12: 374-381.
AXELSSON, B., DAHLGREN, S.E., & PISCATOR, M. (1968) Renal lesions in
the rabbit after long-term exposure to cadmium. Arch. environ.
Health, 17: 24-28.
BAADER, E.W. (1951) [Health concerns and occupational medicine:
chronic cadmium poisoning.] Dtsch. med. Wschr., 76: 484-487 (in
German).
BAETZ, R.A. & KENNER, C.T. (1974) Determination of low levels of
cadmium in foods using a chelating ion exchange resin. J. Assoc.
Off. Anal. Chem., 57: 14-17.
BARLOW, S.M. & SULLIVAN, F.M. (1982) Cadmium and its compounds. In:
Barlow, S.M., ed. Reproductive hazards of industrial chemicals. An
evaluation of animal and human data, New York, London, Academic
Press, pp. 137-173.
BARLTROP, D. & STREHLOW, C.D. (1982a) Clinical and biochemical
indices of cadmium exposure in the population of Shipham. In:
Proceedings of the 3rd International Cadmium Conference, Miami,
Florida, 3-5 February, 1981, London, Cadmium Association,
pp. 112-113.
BARLTROP, D. & STREHLOW, C.D. (1982b) Cadmium and health in Shipham.
Lancet, 2: 1394-1395.
BARR, M., Jr (1973) The teratogenicity of cadmium chloride in two
stocks of Wistar rats. Teratology, 7: 237-242.
BARRETT, H.M. & CARD, B.Y. (1947) Studies on the toxicity of inhaled
cadmium. II. The acute lethal dose of cadmium oxide for man. J. ind.
Hyg. Toxicol., 29: 286-293.
BARRETT, H.M., IRWIN, D.A., & SEMMONS, E. (1947) Studies on the
toxicity of inhaled cadmium. I. The acute toxicity of cadmium oxide
by inhalation. J. ind. Hyg. Toxicol., 29: 279-285.
BARTHELEMY, P. & MOLINE, R. (1946) Intoxication chronique par
l'hydrate de cadmium, son signe précoce: La bague jaune dentaire.
Paris Med., 1: 71-78.
BAUCHINGER, M., SCHMID, E., EINBRODT, H.J., & DRESP, J. (1976)
Chromosome aberrations in lymphocytes after occupational exposure to
lead and cadmium. Mutat. Res., 40: 57-62.
BECKMAN, I., MOVITZ, I., NYGREN, M., & SLORACH, S. (1979) [Release
of lead and cadmium from glazed and enamelled foodware.] Var Föda,
31: 193-197 (in Swedish).
BEHNE, D. (1980) Problems of sampling and sample preparation for
trace element analysis in the health sciences. In: Bratter, P. &
Schramel, P., ed. Trace element analytical chemistry in medicine and
biology. Proceedings of the First International Workshop,
Neuherberg, Federal Republic of Germany, Berlin, New York, Walter de
Gruyter & Co., pp. 769-782.
BERLIN, M. & FRIBERG, L. (1960) Bone marrow activity and erythrocyte
destruction in chronic cadmium poisoning. Arch. environ. Health,
1: 478-486.
BERMAN, E. (1967) Determination of Cd, Tl, and Hg in biological
materials by AA. At. Absorpt. Newsl., 6: 57-60.
BERNARD, A.M., ROELS, H., HUBERMONT, G., BUCHET, J.P., MASSON, P.L.,
& LAUWERYS, R. (1976) Characterization of the proteinuria in
cadmium-exposed workers. Int. Arch. occup. environ. Health,
38: 19-30.
BERNARD, A.M., BUCHET, J.P., ROELS, H., MASSON, P., & LAUWERYS, R.
(1979) Renal excretion of proteins and enzymes in workers exposed
to cadmium. Eur. J. clin. Invest., 9: 11-22.
BERNARD, A.M., LAUWERYS, R., & GENGOUX, P. (1981) Characterization
of the proteinuria induced by prolonged oral administration of
cadmium in female rats. Toxicology, 20: 345-357.
BERNARD, A.M., MOREAU, D., & LAUWERYS, R. (1982a) Latex immunoassay
of retinol binding protein. Clin. Chem., 28: 1167-1171.
BERNARD, A.M., MOREAU, D., & LAUWERYS, R. (1982b) Comparison of
retinol-binding protein and ß-2-microglobulin determination in urine
for the early detection of tubular proteinuria. Clin. Chim. Acta,
126: 1-9.
BETON, D.C., ANDREWS, G.S., DAVIES, H.J., HOWELLS, L., & SMITH, G.F.
(1966) Acute cadmium fume poisoning: five cases with one death from
renal necrosis. Br. J. ind. Med., 23: 292-301.
BHATTACHARYYA, M.H., WHELTON, B.D., PETERSON, D.P., CARNES, B.A.,
MORETTI, E.S., TOOMEY, J.M., & WILLIAMS, L.L. (1988) Skeletal
changes in multiparous mice fed a nutrient-sufficient diet
containing cadmium. Toxicology, 50: 193-204.
BIGGIN, H.C., CHEN, N.S., ETTINGER, K.V., FREMLIN, J.H., MORGAN,
W.D., NOWOTNY, R., CHAMBERLAIN, M.J., & HARVEY, T.C. (1974) Cadmium
by in vivo neutron activation analysis. J. radioanal. Chem.,
19: 207-214.
BJORNBERG, A. (1963) Reactions to light in yellow tattoos from
cadmium sulfide. Arch. Dermatol., 88: 267-271.
BLEJER, H.P. (1966) Death due to cadmium oxide fumes. Ind. Med.
Surg., 35: 363-364.
BOISSET, M. & BOUDENE, C. (1981) Effect of a single exposure to
cadmium oxide fumes on rat lung microsomal enzymes. Toxicol. appl.
Pharmacol., 57: 335-345.
BONNELL, J.A. (1955) Emphysema and proteinuria in men casting
copper-cadmium alloys. Br. J. ind. Med., 12: 181-197.
BONNELL, J.A., KAZANTZIS, G., & KING, E. (1959) A follow-up study of
men exposed to cadmium oxide fume. Br. J. ind. Med., 16: 135-147.
BONNELL, J.A., ROSS, J.H., & KING, E. (1960) Renal lesions in
experimental cadmium poisoning. Br. J. ind. Med., 17: 69-80.
BOULEY, G., DUBREUIL, A., DESPAUX, N., & BOUDENE, C. (1977) Toxic
effects of cadmium microparticles on the respiratory system. An
experimental study on rats and mice. Scand J. Work Environ. Health,
3: 116-121.
BOYLE, E.A., SCALTER, F., & EDMOND, J.M. (1976) On the marine
geochemistry of cadmium. Nature (Lond.), 263: 42-44.
BOZELKA, B.E. & BURKHOLDER, P.M. (1982) Inhibition of mixed
leukocyte culture response in cadmium-treated mice. Environ. Res.,
27: 421-432.
BRADY, F.O. (1982) The physiological function of metallothionein.
Trends biochem. Sci., 7: 143-145.
BRAUX, P., CLAEYS-THOREAU, F., DE PLAAN, P., DUCOFFRE, G., AMERY,
A., LAUWERYS, R., RONDIA, D., STAESSEN, J., ROELS, H., SARTON, F.,
BERNARD, A., & BUCHET, J.P. (1987) The Belgian Cadmibel Study:
Environmental exposure to cadmium and lead-health effects. In:
Lindberg, S.F. & Hutchinson, J.C., ed. Proceedings of an
International Conference on Heavy Metals in the Environment, New
Orleans, Edinburgh, CEP Consultants Ltd, pp. 349-351.
BRYAN, G.W., LANGSTON, W.J., & HUMMERSTONE L.G. (1980) The use of
biological indicators of heavy-metal contamination in estuaries with
special reference to an assessment of the biological availability of
metals in estuarine sediments from south-west Britain, Citadel Hill,
Devon, The Marine Biological Association of the United Kingdom,
73 pp (Occasional Publication No. 1).
BUATMENARD, P. & ARNOLD, M. (1978) Heavy-metal chemistry of
atmospheric particulate matter emitted by Mount Etna volcano. Geogr.
Res. Lett., 5: 245-248.
BUCHAUER, M.J. (1972) The effects of zinc and cadmium pollution on
forest vegetation. Bull. New Jersey Acad. Sci., 17(2): 42.
BUCHET, J.P., ROELS, H., BERNARD, A., & LAUWERYS, R. (1980)
Assessment of renal function of workers exposed to inorganic lead,
cadmium or mercury vapor. J. occup. Med., 22: 741-748.
BUCHET, J.P., ROELS, H., LAUWERYS, R., VANDEVOORDE, A., & PYCKE,
J.M. (1983) Oral daily intake of cadmium, lead, manganese, copper,
chromium, mercury, calcium, zinc, and arsenic in Belgium: a
duplicate meal study. Food chem. Toxicol., 21: 19-24.
BUCKE, D., NORTON, M.G., & ROLFE, M.S. (1983) Field assessment of
effects of dumping wastes at sea: II. Epidermal lesions and
abnormalities of fish in the outer Thames estuary, London, Ministry
of Agriculture, Fisheries and Food, 29 pp (Technical Report No. 72).
BUI, T.-H-. LINDSTEN, J., & NORDBERG, G.F. (1975) Chromosome
analysis of lymphocytes from cadmium workers and Itai-itai patients.
Environ. Res., 9: 187-195.
BUS, J.S., VINEGAR, A., & BROOKS, S.M. (1978) Biochemical and
physiologic changes in lungs of rats exposed to a cadmium chloride
aerosol. Am. Rev. respir. Dis., 118: 573-580.
BUTLER, E.A. & FLYNN, F.V. (1958) The proteinuria of renal tubular
disorders. Lancet, 2: 978-980.
BUXTON, R., St J. (1956) Respiratory function in cadmium alloy
casters. Part 2. The estimation of the total lung volume, its
subdivisions and the mixing coefficient. Br. J. ind. Med.,
13: 36-40.
CARLIER, Y., COLLE, A., TACHON, P., BOUT, D., & CAPRON, A. (1981)
Quantification of B-2-microglobulin by enzyme inhibition
immunoassay. J. immunol. Methods, 40: 231-238.
CARLSON, L.A. & FRIBERG, L. (1957) The distribution of cadmium in
blood after repeated exposure. Scand. J. clin. lab. Invest., 9: 1-4.
CARLTON-SMITH, C.H. (1987) Effects of metals in sludge-treated soils
on crops, Medmenham, United Kingdom, Water Research Centre
(Environment TR/251).
CARROLL, R.E. (1966) The relationship of cadmium in the air to
cardiovascular disease death rates. J. Am. Med. Assoc.,
198: 267-269.
CARRUTHERS, M. & SMITH, B. (1979) Evidence of cadmium toxicity in a
population living in a zinc-mining area. Lancet, 1: 845-847.
CARVALHO, F.M., TAVARES, T.M., SILVANY-NETO, A.M., LIMA, M.E.C., &
ALT, F. (1986) Cadmium concentrations in blood of children living
near a lead smelter in Bahia, Brazil. Environ. Res., 40: 437-449.
CASTERLINE, J.L. & YIP, G. (1975) The distribution and binding of
cadmium in oyster, soybean, rat liver, and kidney. Arch. environ.
Contam. Toxicol., 3: 319-329.
CASTILHO, P.D. & HERBER, R.F.M. (1977) The rapid determination of
cadmium, lead, copper, and zinc in whole blood by atomic absorption
spectrometry with electrothermal atomization. Improvements in
precision with a peak-shape monitoring device. Anal. Chim. Acta,
94: 269-270.
CHADWICK, M.J. & LINDMAN, N. (1982) Environmental implications of
expanded coal utilization, Oxford, New York, Pergamon Press, 283 pp.
CHAPATWALA, K.D., HOBSON, M., DESAIAH, D., & RAJANNA, B. (1982)
Effect of cadmium on hepatic and renal gluconeogenic enzymes in
female rats. Toxicol. Lett., 12: 27-34.
CHAUBE, S., NISHIMURA, H., & SWINYARD, C.A. (1973) Zinc and cadmium
in normal human embryos and fetuses. Arch. environ. Health,
26: 237-240.
CHERIAN, M.G. (1980) The synthesis of metallothionein and cellular
adaptation to metal toxicity in primary kidney epithelial cell
cultures. Toxicology, 17: 225-231.
CHERIAN, M.G. & GOYER, R.A. (1978) Metallothioneins and their role
in the metabolism and toxicity of metals. Life Sci., 23: 1-10.
CHERIAN, M.G. & NORDBERG, M. (1983) Cellular adaptation in metal
toxicology and metallothionein. Toxicology, 28: 1-15.
CHERIAN, M.G. & SHAIKH, Z.A. (1975) Metabolism of
intravenously-injected cadmium-binding protein. Biochem. biophys.
Res. Commun., 65: 863-869.
CHERIAN, M.G. & VOSTAL, J.J. (1977) Biliary excretion of cadmium in
rat. I. Dose-dependent biliary excretion and the form of cadmium in
the bile. J. Toxicol. environ. Health, 2: 945-954.
CHERIAN, M.G., GOYER, R.A., & RICHARDSON, L.D. (1976) Cadmium
metallothionein-induced nephropathy. Toxicol. appl. Pharmacol.,
38: 399-408.
CHERIAN, M.G., GOYER, R.A., & VALBERG, L.S. (1978) Gastrointestinal
absorption and organ distribution of oral cadmium chloride and
cadmium metallothionein in mice. J. Toxicol. environ. Health,
4: 861-868.
CHERNOFF, N. (1973) Teratogenic effects of cadmium in rats.
Teratology, 8: 29-32.
CHERRY, W.H. (1981) Distribution of cadmium in human tissues. In:
Nriagu, J.O., ed. Cadmium in the environment - II, New York,
Chichester, John Wiley & Sons, pp. 111-122.
CHIQUOINE, A.D. (1965) Effect of cadmium chloride on the pregnant
albino mouse. J. Reprod. Fertil., 10: 263-265.
CHRISTOFFERSON, J.O. & MATTSON, S. (1983) Polarized X-rays in
XRF-analysis for improved in vivo detectability of cadmium in man.
Phys. Med. Biol., 28: 1135-1144.
CHUMBLEY, C.G. & UNWIN, R.J. (1982) Cadmium and lead content of
vegetable crops grown on land with a history of sewage sludge
application. Environ. Pollut. Ser. B Chem. Phys., 4(3): 231-238.
CHUNG, J., NARTY, N.O., & CHERIAN, M.G. (1986) Metallothionein
levels in liver and kidney of Canadians - A potential indicator of
environmental exposure to cadmium. Arch. environ. Health, 41(5):
319-323.
CIRKT, M. & TICHY, M. (1974) Excretion of cadmium through bile and
intestinal wall in rats. Br. J. ind. Med., 31: 134-139.
CLARKSON, T.W. & KENCH, J.E. (1956) Urinary excretion of amino acids
by men absorbing heavy metals. Biochem. J., 62: 361-372.
CNATTINGIUS, S., AXELSSON, O., EKLUND, G., & LINDMARK, G. (1985)
Smoking, maternal age and fetal growth. Obstet. Gynecol.,
66: 449-452.
COLE, G.M. & BAER, L.S. (1944) Food poisoning from cadmium. US nav.
med. Bull., 43: 398-399.
COMMISSION OF THE EUROPEAN COMMUNITIES (1978) Criteria (dose-effect
relationships) for cadmium, Oxford, Pergamon Press, 202 pp (Report
of the Working Group of Experts prepared for the CEC,
Directorate-General for Social Affairs, Health and Safety
Directorate, Luxembourg).
COOPERATIVE RESEARCH COMMITTEE ON ITAI-ITAI DISEASE (1967) [Report
on a study on the so-called Itai-itai disease], Tokyo, Ministry of
Education and Ministry of Health and Welfare, 206 pp (in Japanese).
COPIUS-PEEREBOOM, J.W., DE VOOGT, P., VAN HATTUM, B., VAN DER VELDE,
W., & COPIUS-PEEREBOOM-STEGEMAN, J.H.J. (1979) The use of the human
placenta as a biological indicator for cadmium exposure. In:
International Conference on Management and Control of Heavy Metals
in the Environment, London, September, 1979, Edinburgh, CEP
Consultants Ltd, pp. 8-10.
COUSINS, R.J., BARBER, A.K., & TROUT, J.R. (1973) Cadmium toxicity
in growing swine. J. Nutr., 103: 964-972.
CULBARD, E.B., THORNTON, I., WATT, J., WHEATLEY, M., MOORCROFT, S.,
& THOMPSON, M. (1988) Metal contamination in British urban dusts and
soils. J. environ. Qual., 17(2): 227-234.
CVETKOVA, R.P. (1970) [Materials on the study of the influence of
cadmium compounds on the generative function.] Gig. Tr. prof.
Zabol., 14: 31-33 (in Russian with English summary).
DALHAMN, T. & FRIBERG, L. (1954) The effect of cadmium on blood
pressure and respiration and the use of dimercaprol (BAL) as
antidote. Acta pharmacol. toxicol., 10: 199-203.
DANIELSSON, L.-G., JAGNER, D., JOSEFSON, M., & WESTERLUND, S. (1981)
Computerized potentiometric stripping analysis for the determination
of cadmium, lead, copper, and zinc in biological materials. Anal.
Chim. Acta, 127: 147-156.
DASTON, G.P. (1982) Fetal zinc deficiency on a mechanism for
cadmium-induced toxicity to the developing rat lung and pulmonary
surfactant. Toxicology, 24: 55-63.
DAVIES, N.T., BREMNER, I., & MILLS, C.F. (1973) Studies on the
induction of a low molecular weight zinc-binding protein in rat
liver. Biochem. Soc. Trans., 1: 985-988.
DAVIS, R.D. (1984) Cadmium - a complex environmental problem. Part
II. Cadmium in sludges used as fertilizer. Experientia (Basel),
40(2): 117-126.
DAVIS, R.D. & COKER, E.G. (1980) Cadmium in agriculture, with
special reference to the utilisation of sewage sludge on land,
Medmenham, United Kingdom, Water Research Centre (Technical Report
TR/139).
DAVISON, A.G., NEWMAN-TAYLOR, A.J., DARBYSHIRE, J., & CHETTLE, D.R.
(1988) Cadmium fume inhalation and emphysema. Lancet, 1: 663-667.
DEAVEN, L.L. & CAMPBELL, E.W. (1980) Factors affecting the induction
of chromosomal aberrations by cadmium in Chinese hamster cells.
Cytogenet. cell Genet., 26: 251-260.
DECKER, L.E., BYERRUM, R.U., DECKER, C.F., HEPPERT, C.A., & LANGHAM,
R.F. (1958) Chronic toxicity studies. I. Cadmium administered in
drinking-water to rats. Am. Med. Assoc. Arch. ind. Health,
18: 228-231.
DEKNUDT, G. & DEMINATTI, M.L. (1978) Chromosome studies in human
lymphocytes after in vitro exposure to metal salts. Toxicology,
10: 67-75.
DEKNUDT, G. & LEONARD, A. (1975) Cytogenetic investigations on
leukocytes of workers from a cadmium plant. Environ. Physiol.
Biochem., 5: 319-327. DELVES, H.T. (1970) Micro-sampling method for
the rapid determination of lead in blood by atomic absorption
spectrophotometry. Analyst, 95: 431-438.
DELVES, H.T. (1982) Analytical techniques for measuring cadmium in
blood. In: Proceedings of the International Workshop on Biological
Indicators on Cadmium Exposure Diagnostic and Analytical
Reliability, Luxembourg, 7-9 July, 1982, Luxembourg, Commission of
the European Communities, pp. 1-15.
DELVES, H.T. & WOODWARD, J. (1981) Determination of low levels of
cadmium in blood by electrothermal atomization and atomic absorption
spectrophotometry. At. Spectrosci., 2: 65-67.
DENCKER, L., DANIELSSON, B., KHAYAT, A., & LINDGREN, A. (1983)
Disposition of metals in the embryo and fetus. In: Clarkson, T.W.,
Nordberg, G.F., & Sager, P.R., ed. Reproductive and developmental
toxicity of metals, New York, London, Plenum Press, pp. 607-632.
DERVAN, P.A. & HAYES, J.A. (1979) Peribronchiolar fibrosis following
acute experimental lung damage by cadmium aerosol. J. Pathol.,
128: 143-148.
DE SILVA, P.E. & DONNAN, M.B. (1981) Chronic cadmium poisoning in a
pigment-manufacturing plant. Br. J. ind. Med., 38: 76-86.
DUCE, R.A., HOFFMAN, G.L., & ZELLER, W.H. (1975) Atmospheric trace
metals at remote northern and southern hemisphere sites: pollution
or natural(?). Science, 187: 59-61.
DUCE, R.A., ARIMOTO, R., RAY, B.J., UNNI, C.K., & HARDER, P.J.
(1983) Atmospheric trace elements at Enewetok Atoll. I.
Concentration, sources, and temporal variability. J. geophys. Res.,
88: 5321-5342.
DUDLEY, R.E., SVOBODA, D.J., & KLAASSEN, C.O. (1982) Acute exposure
to cadmium causes severe liver injury in rats. Toxicol. appl.
Pharmacol., 65: 302-313.
DUDLEY, R.E., GAMMAL, L.M., & KLAASSEN, C.D. (1985) Cadmium-induced
hepatic and renal injury in chronically exposed rats: likely role of
hepatic cadmium-metallothionein in nephrotoxicity. Toxicol. appl.
Pharmacol., 77: 414-426.
DUGGAN, M.J., INSKIP, M.J., RUNDLE, S.A., & MOORCROFT, J.S. (1985)
Lead in playground dust and on the hands of schoolchildren. Sci.
total Environ., 44(1): 65-79.
DUNPHY, B. (1967) Acute occupational cadmium poisoning: a critical
review of the literature. J. occup. Med., 9: 22-26.
EDIGER, R.D. & COLEMAN, R.L. (1973) Determination of cadmium in
blood by a Delves cup technique. At. Absorpt. Newsl., 12: 3-6.
EDLING, C., ELINDER, C.G., & RANDMA, E. (1986) Lung function in
workers using cadmium containing solders. Br. J. ind. Med.,
43: 657-662.
ELINDER, C.-G. & PANNONE, M. (1979) Biliary excretion of cadmium.
Environ. Health Perspect., 28: 123-126.
ELINDER, C.-G. & PISCATOR, M. (1978) Cadmium and zinc in horses. In:
Kirchgessner, M., ed. Proceedings of the 3rd International
Conference on Trace Elements in Man and Animals,
Freising-Weihenstephan, Federal Republic of Germany, 25-29 July,
1977, Freising-Weihenstephan, Munich Technical University,
pp. 569-572.
ELINDER, C.-G., KJELLSTRÖM, T., LIND, B., & LINNMAN, L. (1976)
Cadmium concentration in kidney cortex, liver, and pancreas among
autopsied Swedes. Arch. environ. Health, 31: 292-302.
ELINDER, C.-G., KJELLSTRÖM, T., LINNMAN, L., & PERSHAGEN, G. (1978)
Urinary excretion of cadmium and zinc among persons from Sweden.
Environ. Res., 15: 473-484.
ELINDER, C.-G., JONSSON, L., PISCATOR, M., & RAHNSTER, B. (1981a)
Histopathological changes in relation to cadmium concentration in
horse kidneys. Environ. Res., 26: 1-21.
ELINDER, C.-G., NORDBERG, M., PALM, B., & PISCATOR, M. (1981b)
Cadmium, zinc, and copper in horse kidney and in horse liver
metallothionein: comparison with kidney cortex. Environ. Res.,
26: 22-32.
ELINDER, C.-G., KJELLSTRÖM, T., LIND, B., LINNMAN, L., PISCATOR, M.,
& SUNDSTEDT, K. (1983) Cadmium exposure from smoking cigarettes:
variations with time and country where purchased. Environ. Res.,
32: 220-227.
ELINDER, C.-G., EDLING, C., LINDBERG, E., KAGEDAL, B., & VESTERBERG,
O. (1985a) Assessment of renal function in workers previously
exposed to cadmium. Br. J. ind. Med., 42: 754-760.
ELINDER, C.-G., EDLING, C., LINDBERG, E., KAGEDAL, B., & VESTERBERG,
O. (1985b) B-2-microglobulinuria among workers previously exposed
to cadmium: follow-up and dose-response analyses. Am. J. ind. Med.,
8: 553-564.
ELINDER, C.-G., KJELLSTRÖM, T., HOGSTEDT, C., ANDERSON, K., & SPENG,
G. (1985c) Cancer mortality of cadmium workers. Br. J. ind. Med.,
42: 651-655.
ELLIS, K.J., MORGAN, W.D., ZANZI, I., YASUMURA, S., VARTSKY, D.D., &
COHN, S.H. (1981a) Critical concentrations of cadmium in human renal
cortex: dose-effect studies in cadmium smelter workers. J. Toxicol.
environ. Health, 7: 691-703.
ELLIS, K.J., YASAMURA, S., & COHN, S.H. (1981b) Hair cadmium
content: is it a biological indicator of the body burden of cadmium
for the occupationally-exposed worker? Am. J. ind. Med., 2: 323-333.
ELLIS, K.J., YUEN, K., YASAMURA, S., & COHN, S.H. (1984)
Dose-response analysis of cadmium in man: body burden vs kidney
dysfunction. Environ. Res., 33: 216-226.
ELLIS, K.J., COHN, S.H., & SMITH, T.J. (1985) Cadmium inhalation
exposure estimates: their significance with respect to kidney and
liver cadmium burden. J. Toxicol. environ. Health, 15: 173-187.
ENGSTROM, B. (1979) Factors influencing metabolism and toxicity of
cadmium. Experimental studies on mice with special reference to
chelating agents, Stockholm, Department of Environmental Hygiene,
Karolinska Institute, 80 pp (Doctoral thesis). ENGSTROM, B. &
NORDBERG, G.F. (1978) Effects of detergent formula chelating agents
on the metabolism and toxicity of cadmium in mice. Acta pharmacol.
toxicol., 43: 387-397.
ENGSTROM, B. & NORDBERG, G.F. (1979a) Dose dependence of
gastrointestinal absorption and biological half-time of cadmium in
mice. Toxicology, 13: 215-222.
ENGSTROM, B. & NORDBERG, G.F. (1979b) Factors influencing absorption
and retention of oral 109Cd in mice: age, pretreatment, and
subsequent pretreatment with non-radioactive cadmium. Acta
pharmacol. toxicol., 45: 315-324.
ENGVALL, J. & PERK, J. (1985) Prevalence of hypertension among
cadmium-exposed workers. Arch. environ. Health, 40: 185-190.
ESSING, H.-G., SCHALLER, K.-H., SZADOWSKI, D., & LEHNERT, G. (1969)
[Normal cadmium load on man from food and beverages.] Arch. Hyg.
Bakteriol., 153: 490-494 (in German).
EVRIN, P.E., PETERSON, P.A., WIDE, L., & BERGGARD, I. (1971)
Radioimmuno-assay of ß-2-microglobulin in human biological fluids.
Scand. J. clin. lab. Invest., 28: 439-443.
EYBL, V. & SYKORA, J. (1966) [The protection by chelating agents in
acute cadmium poisoning.] Acta biol. med. Germ., 16: 61-64 (in
German).
FELDMAN, S.L. & COUSINS, R.G. (1973) Influence of cadmium on the
metabolism of 25-hydroxycholecalciferol in chicks. Nutr. Rep. Int.,
8: 251-259.
FELTEN, T.L. (1979) A preliminary report of cadmium-induced
chromosomal changes in somatic and germinal tissues of C57BI/6J male
mice. Genetics, 88: 26-27.
FERM, V.H. (1971) Developmental malformations induced by cadmium.
Biol. Neonat., 19: 101-107.
FERM, V.H. (1972) The teratogenic effects of metals on mammalian
embryos. Adv. Teratol., 5: 51-75.
FERM, V.H. & CARPENTER, S.J. (1968) The relationship of cadmium and
zinc in experimental mammalian teratogenesis. Lab. Invest.,
18: 429-432.
FERNANDEZ, F.J. & KAHN, H.L. (1971) The determination of lead in
whole blood by atomic absorption spectrophotometry with the "Delves
Sampling Cup" technique. At. Absorpt. Newsl., 10: 1-5.
FINGERLE, H., FISCHER, G., & CLASSEN, H.G. (1982) Failure to produce
hypertension in rats by chronic exposure to cadmium. Food chem.
Toxicol., 20: 301-306.
FITZHUGH, O.G. & MEILLER, F.H. (1941) The chronic toxicity of
cadmium. J. Pharmacol. exp. Ther., 72: 15-20.
FLANAGAN, P.R., MCLELLAN, J.S., HAIST, J., CHERIAN, M.G.,
CHAMBERLAIN, M.J., & VALBERG, L.S. (1978) Increased dietary cadmium
absorption in mice and human subjects with iron deficiency.
Gastroenterology, 74: 841-846.
FLETCHER, J.C., CHETTLE, D.R., & AL-HADDAD, I.K. (1982) Experience
with the use of cadmium measurements of liver and kidney. J.
radioanal. Chem., 71: 547-560.
FORSTNER, U. (1980) Cadmium in the environment, Part I. In: Nriagu,
J.O., ed. Cadmium in polluted sediments, New York, Chichester, John
Wiley & Sons, pp. 305-363.
FOULKES, E.C. (1982) Role of metallothionein in transport of heavy
metals. In: Foulkes, E.C., ed. Biological roles of metallothionein,
Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp. 131-140.
FOWLER, B.A. & NORDBERG, G.F. (1978) The renal toxicity of cadmium
metallothionein: morphometric and X-ray microanalytical studies.
Toxicol. appl. Pharmacol., 46: 609-623.
FOWLER, B.A., JONES, H.S., BROWN, H.W., & HASEMAN, J.K. (1975) The
morphological effects of chronic cadmium administration on the renal
vasculature of rats given low and normal calcium diets. Toxicol.
appl. Pharmacol., 34: 233-252.
FOX, M.R.S. & FRY, B.E., Jr (1970) Cadmium toxicity decreased by
dietary ascorbic acid supplements. Science, 169: 989-991.
FOX, M.R.S., FRY, B.E., Jr, HARLAND, B.F., SCHERTAL, M.E., & WEEKS,
C.E. (1971) Effect of ascorbic acid on cadmium toxicity in the
young coturnix. J. Nutr., 101: 1295-1305.
FRANCAVILLA, S., MOSCARDELLI, S., FRANCAVILLA, F., CASASANTA, N.,
PROPERZI, G., MARTINI, M., & SANTIEMMA, V. (1981) Acute cadmium
intoxication: influence of cyproterone acetate on the testis and
epididymis of the rat. Arch. Andrology, 6: 1-11.
FRANK, A., PETERSON, L., & MORNER, T. (1981) [Lead and cadmium
levels in organs from moose, deer, and hare.] Svensk
Veterinaertidn., 33: 151-156 (in Swedish).
FRIBERG, L. (1948a) Proteinuria and kidney injury among workmen
exposed to cadmium and nickel dust. J. ind. Hyg. Toxicol.,
30: 32-36.
FRIBERG, L. (1948b) Proteinuria and emphysema among workers exposed
to cadmium and nickel dust in a storage battery plant. Proc. Int.
Congr. Ind. Med., 9: 641-644.
FRIBERG, L. (1950) Health hazards in the manufacture of alkaline
accumulators with special reference to chronic cadmium poisoning.
Acta med. Scand., 138(Suppl. 240): 1-124.
FRIBERG, L. (1952) Further investigations on chronic cadmium
poisoning. A study on rabbits with radioactive cadmium. Arch. ind.
occup. Med., 5: 30-36.
FRIBERG, L. (1955) Iron and liver administration in chronic cadmium
poisoning and studies on the distribution and excretion of cadmium.
Experimental investigations in rabbits. Acta pharmacol.,
11: 168-178.
FRIBERG, L. (1979) Cadmium in Shipham. Lancet, 1: 823-824.
FRIBERG, L. (1988) Biological monitoring of toxic metals, New York,
London, Plenum Press, pp. 103-126.
FRIBERG, L. & NYSTROM, A. (1952) [Aspects on the prognosis of
chronic cadmium poisoning.] Läkartidningen, 49: 2629-2639 (in
Swedish).
FRIBERG, L. & VAHTER, M. (1983) Assessment of exposure to lead and
cadmium through biological monitoring: results of a UNEP/WHO global
study. Environ. Res., 30: 95-128.
FRIBERG, L., PISCATOR, M., NORDBERG, G., & KJELLSTRÖM, T. (1974)
Cadmium in the environment, 2nd ed., Cleveland, Ohio, CRC Press,
248 pp.
FRIBERG, L., KJELLSTRÖM, T., NORDBERG, G., & PISCATOR, M. (1975)
Cadmium in the environment. III. A toxicological and epidemiological
appraisal, Washington, DC, US Environmental Protection Agency,
Office of Research and Development, 217 pp.
FRIBERG, L., ELINDER, C.G., KJELLSTRÖM, T., & NORDBERG, G.F. (1985)
Cadmium and health, a toxicological and epidemiological appraisal.
Vol. I. Exposure, dose and metabolism, Cleveland, Ohio, CRC Press,
209 pp.
FRIBERG, L., ELINDER, C.G., KJELLSTRÖM, T., & NORDBERG, G.F. (1986)
Cadmium and health, a toxicological and epidemiological appraisal.
Vol. II. Effects and response, Cleveland, Ohio, CRC Press, 303 pp.
FUKUHARA, M., BOULEY, G., GODIN, J., GIRARD, F., BOISSET, M., &
BOUDENE, C. (1981) Effects of short-term inhalation of cadmium
oxides on rabbit pulmonary microsomal enzymes. Biochem. Pharmacol.,
30: 715-720.
FUKUSHIMA, I. (1978) Environmental pollution and health effects -
Gunma Prefecture. In: Tsuchiya, K., ed. Cadmium studies in Japan: a
review, Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp. 170-181.
FUKUSHIMA, M., ISHIZAKI, A., SAKAMOTO, M., & KOBAYASHI, E. (1973)
[Cadmium concentrations in rice eaten by farmers in the Jinzu river
basin.] Jpn J. Hyg., 28: 406-415 (in Japanese with English summary).
FUKUSHIMA, M., ISHIZAKI, A., NOGAWA, K., SAKAMOTO, M., & KOBAYASHI,
E. (1974) [Epidemiological studies on renal failure of inhabitants
in "Itai-itai" disease endemic district. I. Some urinary findings of
inhabitants living in and around the endemic district of the Jinzu
river basin.] Jpn. J. Public Health, 21: 65-73 (in Japanese with
English summary).
FUKUSHIMA, I., NAKAJIMA, T., HIGUSHI, Y., TSUCHIYA, T., NAKAJIMA,
K., TANAKA, T., FUKAMACHI, M., ARAI, K., & SHIONO, T. (1975)
[Results of screening examination on tubular dysfunction in aged
persons confined to bed], Tokyo, Japan Public Health Association,
pp. 138-142 (Kankyo Hoken Report No. 36) (in Japanese).
FUKUYAMA, Y. (1972) [Proteinuria in Itai-itai disease and cadmium
poisoning.] Med. Biol., 84: 41-46 (in Japanese).
FULLMER, C.S., OKU, T., & WASSERMAN, R.H. (1980) Effect of cadmium
administration on intestinal calcium absorption and vitamin
D-dependent calcium-binding protein. Environ. Res., 22: 386-399.
GABBIANI, G. (1966) Action of cadmium chloride on sensory ganglia.
Experientia (Basel), 22: 261-264.
GABBIANI, G., BAIC, D., & DELZIEL, C. (1967) Toxicity of cadmium for
the central nervous system. Exp. Neurol., 18: 154-160.
GABBIANI, G., BADONNEL, M.-C., MATHEWSON, S.M., & RYAN, G.G. (1974)
Acute cadmium intoxication: early selective lesions of endothelial
clefts. Lab. Invest., 30: 686-695.
GALE, T.F. (1973) The interaction of mercury with cadmium and zinc
in mammalian embryonic development. Environ. Res., 6: 95-105.
GARDINER, P.E., OTTAWAY, J.M., & FELL, G.S. (1979) Accuracy of the
direct determination of cadmium in urine by carbon-furnace atomic
absorption spectrometry. Talanta, 26: 841-847.
GARTY, M., WONG, K.-L., & KLAASSEN, C.D. (1981) Redistribution of
cadmium to blood of rats. Toxicol. appl. Pharmacol., 59: 548-554.
GERVAIS, J. & DELPECH, P. (1963) L'intoxication cadmique. Arch. Mal.
prof. Méd. Trav. Séc. soc., 24: 803-815.
GESAMP (1984) IMO/FAO/UNESCO/WMO/IAEA/UN/UNEP Joint Group of Experts
on the Scientific Aspects of Marine Pollution: Report of the
Fourteenth Session, Vienna, 26-30 March, 1984, Vienna, International
Atomic Energy Agency (Reports and Studies No. 21).
GESAMP (1985) IMO/FAO/UNESCO/WMO/IAEA/UN/UNEP Joint Group of Experts
on the Scientific Aspects of Marine Pollution: Atmospheric transport
of contaminants into the mediterranean region, Athens, Geneva, World
Meteorological Association (Reports and Studies No. 26).
GESAMP (1987) IMO/FAO/UNESCO/WMO/WHO/IAEA/UN/UNEP Joint Group of
Experts on the Scientific Aspects of Marine Pollution: Report of the
seventeenth session, Rome, Geneva, World Health Organization
(Reports and Studies No. 31).
GLEIT, C.E. (1965) High-frequency electrodeless discharge system for
ashing organic matter. Anal. Chem., 37: 314-315.
GLENNAS, A. & RUGSTAD, H.E. (1984) Cadmium uptake and metabolism in
cultured cells. Environ. Health Perspect., 54: 45-50.
GOERING, P.L., SQUIBB, K.S., & FOWLER, B.A. (1985) Calcuria and
proteinuria during cadmium-metallothionein-induced proximal tubule
cell injury. In: Memphill, D., ed. Proceedings of the University of
Missouri's 19th Annual Conference on Trace Substances in
Environmental Health, 3-6 June 1985, Columbia, Missouri, University
of Missouri Press, pp. 22-35.
GOYER, R.A., CHERIAN, M.G., & DELAQUERRIERE-RICHARDSON, L. (1984)
Correlation of parameters of cadmium exposure with onset of
cadmium-induced nephropathy in rats. J. environ. Pathol. Toxicol.
Oncol., 5: 89-100.
GOYER, R.A., HAUST, M.D., & CHERIAN, M.G. (1989) Localization of
metallothionein in placenta. Toxicologist, 9: 130.
GREENBERG, R.R., GORDON, G.E., ZOLLER, W.H., JACKO, R.B., NEVENDORF,
D.W., & YOST, K.J. (1978) Composition of particles emitted from the
Nocosia municipal incinerator. Environ. Sci. Technol., 12(12):
1329-1332.
GUNN, S.A. & GOULD, T.C. (1957) Selective accumulation of 115Cd by
cortex of rat kidney. Proc. Soc. Exp. Biol. Med., 96: 820-823.
GUNN, S.A. & GOULD, T.C. (1970) Cadmium and other mineral elements.
In: Johnson, A.D., Gomes, W.R., & VanDeMark, N.L., ed. The testis,
New York, London, Academic Press, Vol. 3, pp. 377-481.
GUNN, S.A., GOULD, T.C., & ANDERSON, W.A.D. (1961) Zinc protection
against cadmium injury to rat testis. Arch. Pathol., 71: 274.
GUNN, S.A., GOULD, T.C., & ANDERSON, W.A.D. (1963) Cadmium-induced
inter-stitial cell tumours in rats and mice and their prevention by
zinc. J. Natl Cancer Inst., 31: 745-759.
GUNN, S.A., GOULD, T.C., & ANDERSON, W.A.D. (1965) Strain
differences in susceptibility of mice and rats to cadmium-induced
testicular damage. J. Reprod. Fertil., 10: 273-275.
GUNN, S.A., GOULD, T.C., & ANDERSON, W.A.D. (1967) Specific response
of mesenchymal tissue to cancerogenesis by cadmium. Arch. Pathol.,
83: 439-499.
GUNN, S.A., GOULD, T.C., & ANDERSON, W.A.D. (1968) Specificity in
protection against lethality and testicular toxicity from cadmium.
Proc. Soc. Exp. Biol. Med., 128: 591-595.
GUTHRIE, B.E. & ROBINSON, M.F. (1977) Daily intakes of manganese,
copper, zinc, and cadmium by New Zealand women. Br. J. Nutr.,
38: 55-63.
GUTHRIE, J. (1964) Histological effects of intratesticular
injections of cadmium chloride in domestic fowl. Br. J. Cancer,
18: 255-260.
HADDOW, A., ROE, F.J.C., DUKES, C.E., & MITCHELEY, B.C.V. (1964)
Cadmium neoplasia: sarcomata at the site for injection of cadmium
sulfate in rats and mice. Br. J. Cancer, 18: 667-673.
HADLEY, J.G., CONKLIN, A.W., & SANDERS, C.L. (1979) Systemic
toxicity of inhaled cadmium oxide. Toxicol. Lett., 4: 107-111.
HAGA, Y. & YAMAWAKI, T. (1974) [Research report on environmental
health studies of heavy metal pollution], Akita, Japan, Akita
Prefecture Institute of Public Health (Report No. 18) (in Japanese).
HAGINO, N. & YOSHIOKA, K. (1961) [A study on the etiology of
Itai-itai disease.] J. Jpn. Orthop. Assoc., 35: 812-815 (in
Japanese).
HAHN, R., EWERS, O., JERMANN, E., FREIER, E., BROCKHAUS, A., &
SCHLIPKOTER, H.W. (1987) Cadmium in kidney cortex of inhabitants of
North-West Germany: in relationship to age, sex, smoking and
environmental pollution by cadmium. Int. Arch. occup. environ.
Health, 59: 165-176.
HALVORSEN, C. & STEINNES, E. (1975) Simple and precise determination
of Zn and Cd in human liver by neutron activation analysis. Z. anal.
Chem., 274: 199-202.
HAMILTON, D.L. & VALBERG, L.S. (1974) Relationship between cadmium
and iron absorption. Am. J. Physiol., 227: 1033-1037.
HAMMER, D.I., FINKLEA, J.F., HENDRICKS, R.H., SHY, C.M., & HORTON,
R.J.M. (1971) Hair trace metal levels and environmental exposure.
Am. J. Epidemiol., 93: 84-92.
HAMMER, D.I., FINKLEA, J.F., CREASON, J.P., SANDIFER, S.H., KEIL,
J.E., PRIESTER, L.E., & STARA, J.F. (1972) Cadmium exposure and
human health effects. In: Hemphill, D.D., ed. Trace substances in
environmental health - V, Columbia, Missouri, University of Missouri
Press, pp. 269-283.
HAMMER, D.I., COLUCCI, A.V., HASSELBLAD, V., WILLIAMS, M.E., &
PINKERTON, C. (1973) Cadmium and lead in autopsy tissues. J. occup.
Med., 15: 956-963.
HARADA, A. (1987) [Results of fifteen years health examinations on
cadmium workers in a cadmium pigment factory (2nd report)], Tokyo,
Japan Public Health Association, pp. 219-233 (Kankyo Hoken Report
No. 53) (in Japanese).
HARDY, H.L. & SKINNER, J.B. (1947) The possibility of chronic
cadmium poisoning. J. ind. Hyg. Toxicol., 29: 321-325.
HARRISON, H.E., BUNTING, H., ORDWAY, N., & ALBRINK, W.S. (1947) The
effects and treatment of inhalation of cadmium chloride aerosols in
the dog. J. ind. Hyg. Toxicol., 29: 302-314.
HARRISON, J.F., LUNT, G.S., SCOTT, P., & BLAINEY, J.D. (1968)
Urinary lysozyme, ribonuclease, and low molecular weight protein in
renal disease. Lancet, 1: 371-375.
HARVEY, T.C., MCLELLAN, J.S., THOMAS, B.J., & FREMLIN, J.H. (1975)
Measurement of liver cadmium concentrations in patients and
industrial workers by neutron activation analysis. Lancet,
1: 1269-1272.
HARVEY, T.C., CHETTLE, D.R., FREMLIN, J.H., AL-HADDAD, I.K., &
DOWNES, S.P.M. (1979) Cadmium in Shipham. Lancet, 1(8115).
HASSELMANN, J., KOENIG, W., RICHTER, F.W., STEINER, U., & WATJEN, U.
(1977) Application of PIXE to trace element analysis in biological
tissues. Nucl. Instrum. Methods, 142: 163-169.
HASSLER, E., LIND, B., & PISCATOR, M. (1983) Cadmium in blood and
urine related to present and past exposure. A study of workers in an
alkaline battery factory. Br. J. ind. Med., 40: 420-425.
HAYES, J.A., SNIDER, G.L., & PALMER, K.C. (1976) The evolution of
biochemical damage in the rat lung after acute cadmium exposure. Am.
Rev. respir. Dis., 113: 121-130.
HEATH, J.C. & WEBB, M. (1967) Content and intracellular distribution
of the inducing metal in the primary rhabdomyosarcomata induced in
the rat by cobalt, nickel, and cadmium. Br. J. Cancer, 21: 768-779.
HEATH, J.C., DANIEL, M.R., DINGLE, J.T., & WEBB, M. (1962) Cadmium
as a carcinogen. Nature (Lond.), 193: 592-593.
HEINDRYCKX, R., DEMUYNCK, M., DAMS, R., JANSSENS, M., & RAHN, K.A.
(1974) Mercury and cadmium in Belgian aerosols. In: Problems of the
contamination of man and his environment by mercury and cadmium. CEC
European Colloquium, July, 1973, Luxembourg, Commission of the
European Communities, pp. 135-148.
HEINRICH, U., PETERS, L., ERNST, H., RITTINGHAUSEN, S., DASENBROCK,
C., & KÖNIG, H. (1989) Investigation on the carcinogenic effects of
various cadmium compounds after inhalation exposure in hamsters and
mice. Exp. Pathol., 37: 253-258.
HENDERSON, R.F., REBAR, A.H., PICKRELL, J.A., & NEWTON, G.J. (1979)
Early damage indicators in the lung III. Biochemical and cytological
response of the lung to inhaled metal salts. Toxicol. appl.
Pharmacol., 50: 123-136.
HENKE, G., SACHS, H.W., & BOHN, G. (1970) [Cadmium determination by
neutron activation analysis of liver and kidneys from children and
young people.] Arch. Toxikol., 26: 8-16 (in German).
HICKEY, R.J., SCHOFF, E.P., & CLELLAND, R.C. (1967) Relationship
between air pollution and certain chronic disease death rates. Arch.
environ. Health, 15: 728-738.
HIRATA, H. (1981) Annaka: land polluted mainly by fumes and dust
from a zinc smelter. In: Kitagishi, K. & Yamane, I., ed. Heavy metal
pollution in soils of Japan, Tokyo, Japan Scientific Societies
Press, pp. 149-163.
HISLOP, J.S. (1980) Choice of analytical method. In: Bratter, P. &
Schramel, P., ed. Trace element analytical chemistry in medicine
and biology, Berlin, New York, Walter de Gruyter Co., pp. 747-767.
HOFFMAN, E.O., COOK, J.A., DILUZIO, N.R., & COOVER, J.A. (1975) The
effects of acute cadmium administration in the liver and kidney of
the rat. Lab. Invest., 32: 655-661.
HOFFMAN, L., PUTZKE, H.-P., KAMPEHL, H.-J., RUSSBULT, R., GASE, P.,
SIMONN, C., ERDMANN, T., & HUCKSTORF, C. (1985) Carcinogenic effects
of cadmium on the prostate of the rat. J. Cancer Res. clin. Oncol.,
109: 193-199.
HOFFMAN, L., PUTZKE, H.-P., BENDEL, L., ERDMANN, T., & HUCKSTORF, C.
(1988) Electron microscopic results on the ventral prostate of the
rat after CdC12 administration. A contribution towards etiology of
the cancer of the prostate. J. Cancer Res. clin. Oncol.,
114: 273-278.
HOLDEN, H. (1969) Cadmium toxicology. Lancet, 2: 57.
HOLDEN, H. (1980a) A mortality study of workers exposed to cadmium
fumes. In: Proceedings of the 2nd International Cadmium Conference,
Cannes, 6-8 February, 1979, London, Cadmium Association,
pp. 211-216.
HOLDEN, H. (1980b) Health status of European cadmium workers. In:
Proceedings of the Seminar on Occupational Exposure to Cadmium,
London, 20 March, 1980, London, Cadmium Association, pp. 33-37.
HOLMBERG, R.E., Jr & FERM, V.H. (1969) Inter-relationship of
selenium, cadmium, and arsenic in mammalian teratogenesis. Arch.
environ. Health, 18: 873-877.
HORSTOWA, H., SIKORSKI, M., & TYBORSKI, H. (1966) [Chronic cadmium
poisoning in the clinical and radiological picture.] Med. Prac.
occup. Med., 17: 13-25 (in Polish).
HOVMAND, M.F., TJELL, J.C., & MOSBACK, H. (1983) Plant uptake of
airborne cadmium. Environ. Pollut., 30: 27-38.
HUEL, G., BOUDENE, C., & IBRAHIM, M.A. (1981) Cadmium and lead
content of maternal and newborn hair: relationship to parity, birth
weight and hypertension. Arch. environ. Health, 36: 221-227.
HUGHES, E.G. & STEWART, M. (1979) Cadmium in Shipham. Lancet,
1: 973-974.
HURLEY, L.S., GOWAN, J., & SWENERTON, H. (1971) Teratogenic effects
of short-term and transitory zinc deficiency in rats. Teratology,
4: 199-204.
HUTCHISON, F., WAI, C.M., JERNEGAN, M., HICKS, W., & LONG, R. (1979)
Distribution of cadmium, lead and zinc in soil around two phosphate
processing plants in Pocatello, Idaho. Bull. environ. Contam.
Toxicol., 22: 426-429.
HUTTON, M., ed. (1982) Cadmium in the European Community: a
prospective assessment of sources, human exposure, and environmental
impact, London, Monitoring and Assessment Research Centre, Chelsea
College, University of London, 100 pp (MARC Report Number 26).
HUTTON, M. (1983) Evaluation of the relationships between cadmium
exposure and indicators of kidney function, London, Monitoring and
Assessment Research Centre, Chelsea College, University of London,
46 pp (MARC Report Number 29).
HUTTON, M. & SYMON, C. (1986) The quantities of cadmium, lead,
mercury and arsenic entering the U.K. environment from human
activities. Sci. total Environ., 57: 129-150.
HUTTON, M., WADGE, A., & MILLIGAN, P.J. (1988) Environmental levels
of cadmium and lead in the vicinity of a major refuse incinerator.
Atmos. Environ., 22: 411-416.
HYOGO PREFECTURAL GOVERNMENT (1972) [Results of a comprehensive
study on cadmium pollution in the area around Ikuno Mine and an
outline of counter-measures], Kobe, Japan, Hyogo Prefectural
Government, 156 pp (in Japanese).
IARC (1976) Cadmium, nickel, some epoxides, miscellaneous
industrial chemicals, and general considerations on volatile
anaesthetics, Lyon, International Agency for Research on Cancer,
306 pp (Monographs on the Evaluation of Carcinogenic Risk of Chemicals
to Man, Vol. 11).
IARC (1987a) Genetic and related effects: an updating of selected
IARC Monographs from Volumes 1 to 42. Lyon, International Agency for
Research on Cancer (IARC Monographs on the Evaluations of
Carcinogenic Risks to Humans, Supplement 6).
IARC (1987b) Cadmium and cadmium compounds. In: Overall evaluations
of carcinogenicity: an updating of IARC Monographs, Volumes 1 to 42,
Lyon, International Agency for Research on Cancer, pp. 139-142 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals
to Humans, Supplement 7).
ILZSG (1988) International Lead and Zinc Study Group - World
directory: Lead and zinc mines and primary metallurgical works,
London, Chameleon Press, Ltd.
INSKIP, H., BERAL, V., & MCDOWALL, M. (1982) Mortality in Shipman
residents: 40-year follow-up. Lancet, 1: 896-899.
IRPTC (1987) IRPTC legal file 1986, Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment
Programme, Vol. I.
ISHIKAWA PREFECTURAL GOVERNMENT (1976) [Report on the health
examination of residents of the Kakehashi river basin], Kanazawa,
Japan, Health and Welfare Department, 104 pp (in Japanese).
ISHIZAKI, A. (1969) [On the so-called Itai-itai disease.] J. Jpn.
Med. Soc., 62: 242-249 (in Japanese).
ISHIZAKI, A. (1972) [Concentrations of cadmium and zinc in the
organs of Itai-itai disease patients and residents in the Hokuriku
region], Tokyo, Japanese Society of Public Health, pp. 154-157
(Kankyo Hoken Report No. 11) (in Japanese).
ISHIZAKI, A., FUKUSHIMA, M., SAKOMOTO, M., KURACHI, T., & HAYASHI,
E. (1969) [Cadmium content of rice eaten in the Itai-itai disease
area.] In: [Proceedings of the 27th Annual Meeting of the Japanese
Society of Public Health], Tokyo, Japan Public Health Association,
p. 111 (in Japanese).
ISHIZAKI, A., FUKUSHIMA, M., & SAKAMOTO, M. (1971) [Contents of
cadmium and zinc in organs of Itai-itai disease patients and
residents of Hakuriku district.] Jpn. J. Hyg., 26: 268-273 (in
Japanese).
ISHIZU, S., MINAMI, M., SUZUKI, A., YAMADA, M., SATO, M., &
YAMAMURA, K. (1973) An experimental study of teratogenic effect of
cadmium. Ind. Health, 11: 127-139.
ITO, K. & SAWAUCHI, K. (1966) Inhibitory effects on cadmium-induced
testicular damage by pretreatment with smaller cadmium doses.
Okajimas Folia anat. Jpn. 42: 107-117.
ITOKAWA, Y., ABE, T., & TANAKA, S. (1973) Bone changes in
experimental chronic cadmium poisoning. Arch. environ. Health,
26: 241-244.
ITOKAWA, Y., ABE, T., TABEI, R., & TANAKA, S. (1974) Renal and
skeletal lesions in experimental cadmium poisoning. Arch. environ.
Health, 28: 149-154.
ITOKAWA, Y., NISHINO, K., TAKASHIMA, M., NAKATA, T., KAITO, H.,
OKAMOTO, E., DAIJO, K., & KAWAMURA, J. (1978) Renal and skeletal
lesions in experimental cadmium poisoning of rats. Histology and
renal function. Environ. Res., 15: 206-217.
IWAO, S. (1977) Cadmium, lead, copper, and zinc in food, faeces, and
organs of humans. Keio J. Med., 26: 63-78.
IWAO, S., SUGITA, M., & TSUCHIYA, K. (1981a) Some metabolic
interrelationships among cadmium, lead, copper, and zinc: results
from a field survey in Cd-polluted areas in Japan. I. Dietary intake
of the heavy metals. Keio J. Med., 30: 17-36.
IWAO, S., SUGITA, M., & TSUCHIYA, K. (1981b) Some metabolic
interrelationships among cadmium, lead, copper, and zinc: results
from a field survey in Cd-polluted areas in Japan. II. Faecal
excretion of the heavy metals. Keio J. Med., 30: 71-82.
JAGNER, D., JOSEFSON, M., & WESTERLUND, S. (1981) Simultaneous
determination of cadmium and lead in urine by means of computerized
potentiometric stripping analysis. Anal. Chim. Acta, 128: 155-161.
JAMALL, I.S. & SMITH, J.C. (1986) The effect of dietary selenium on
cadmium cardiotoxicity. In: Born, G.V.R., Rensselaer, A.F., Herken,
H., & Welch, A.D., ed. Handbook of experimental pharmacology,
Berlin, Heidelberg, Springer Verlag, Vol. 80, pp. 351-361.
JAMALL, I.S. & SPROWLS, J.J. (1987) Effects of cadmium and dietary
selenium on cytoplasmic and mitochondrial antioxidant defense
systems in the heart of rats fed high dietary copper. Toxicol. appl.
Pharmacol., 87: 102-110.
JANSSENS, M. & DAMS, R. (1974) Determination of cadmium in air
particulates by flameless atomic absorption spectrometry with a
graphite tube. Anal. Chim. Acta, 70: 25-33.
JAPAN CADMIUM RESEARCH COMMITTEE (1989) [Summary report on studies
of health effects of cadmium], Tokyo, Japan Public Health
Association, pp. 53-67 (Kankyo Hoken Report No. 56) (in Japanese).
JAPAN PUBLIC HEALTH ASSOCIATION (1968) [A study of the cause of
Itai-itai disease], Tokyo, Japan Public Health Association, 73 pp
(in Japanese).
JAPAN PUBLIC HEALTH ASSOCIATION (1970) [Study on cadmium intake and
accumulation in areas requiring official observation], Tokyo, Japan
Public Health Association, 49 pp (in Japanese).
JAPANESE ENVIRONMENT AGENCY (1972) [Results of soil pollution survey
in 1971], Tokyo, Japanese Environment Agency, Soil Agricultural
Chemical Division, Water Quality Bureau, 129 pp (in Japanese).
JAPANESE ENVIRONMENT AGENCY (1974) [Results of measurement at the
national air sampling network stations], Tokyo, Japanese Environment
Agency, Air Pollution Control Division, Air Quality Bureau, 159 pp
(in Japanese).
JAPANESE ENVIRONMENT AGENCY (1982) [Results of soil pollution survey
in 1981], Tokyo, Japanese Government Agency, Soil Agricultural
Chemical Division, Water Quality Bureau, pp. 1-29 (in Japanese).
JAPANESE MINISTRY OF AGRICULTURE AND FORESTRY (1973) [Results of a
survey for protection measures of soil contamination in 1972.] In:
[Materials for soil protection measures], 197 pp (Report No. 44) (in
Japanese).
JAPANESE MINISTRY OF HEALTH AND WELFARE (1968) [Opinion of the
Ministry with regard to Itai-itai disease and its causes], Tokyo,
Japanese Ministry of Health and Welfare (in Japanese).
JAPANESE MINISTRY OF HEALTH AND WELFARE (1971) [Partial revision of
health examination methods in the provisional counter-measures
against environmental pollution by cadmium], Tokyo, Japanese
Ministry of Health and Welfare, Environmental Pollution Section
(Unpublished document) (in Japanese).
JAPANESE MINISTRY OF HEALTH AND WELFARE (1983) [The present status
of nutrition among the people], Tokyo, Japanese Ministry of Health
and Welfare, 56 pp (in Japanese).
JÄRUP, L., ROGENFELT, A., ELINDER, C.-G., NOGAWA, K., & KJELLSTRÖM,
T. (1983) Biological half-time of cadmium in the blood of workers
after cessation of exposure. Scand. J. Work Environ. Health,
9: 327-331.
JÄRUP, L., ELINDER, C.G., & SPANG, G. (1988) Cumulative
blood-cadmium and tubular proteinuria: a dose-response relationship.
Int. Arch. occup. environ. Health, 60: 223-229.
JAWAID, M., LIND, B., & ELINDER, C.-G (1983) Determination of
cadmium in urine by extraction and flameless atomic absorption
spectrophotometry. Comparison of urine from smokers and non-smokers
of different sex and age. Talanta, 30: 509-513.
JEFFERY, E.H., NOSEWORTHY, R., & CHERIAN, M.G. (1989) Age dependent
changes in metallothionein and accumulation of cadmium in horses.
Comp. Biochem. Physiol., 93C: 327-332.
JIN, T., NORDBERG, G.F., & NORDBERG, M. (1987a) Resistance to acute
nephrotoxicity induced by cadmium-metallothionein dependence on
pretreatment with cadmium chloride. Pharmacol. Toxicol., 61: 89-93.
JIN, T., NORDBERG, G.F., & NORDBERG, M. (1987b) Influence of
cadmium-metallothionein pretreatment on tolerance of rat kidney
cortical cells to cadmium toxicity in vitro and in vivo.
Pharmacol. Toxicol., 60: 345-349.
JIN, T., NORDBERG, G.F., & NORDBERG, M. (1986) Uptake of cadmium in
isolated kidney cells - influence of binding form and
in vivo pretreatment. J. appl. Toxicol., 6(6): 397-400.
JOHNSON, M.S. & EATON, J.W. (1980) Environmental contamination
through residual trace metal dispersal from derelict lead-zinc mine.
J. environ. Qual., 9(2): 175-179.
JONES, K.C., SYMON, C.J., & JOHNSTON, A.E. (1987) Long-term changes
in soil and cereal grain cadmium: studies at Rothamsted Experimental
Station. In: Hemphill, D.D., ed. Trace substances in environmental
health - XXI. Proceedings of the University of Missouri's 21st
Annual Conference on Trace Substances in Environmental Health, St.
Louis, Missouri, 25-28 May, 1987, Columbia, Missouri, University of
Missouri Press, pp. 450-460.
JORHEM, L., MATTSON, P., & SLORACH, S. (1984) Lead, cadmium, zinc
and certain other metals in foods on the Swedish market. Var Föda,
36: Suppl. 3.
JUST, J. & KELUS, J. (1971) [Cadmium in the air atmosphere of ten
selected cities in Poland.] Rocz. Panstw. Zakl. Hig., 22: 249-256
(in Polish).
KAGI, J.H.R. & KOJIMA, Y. (1987) Metallothionein II. Proceedings of
the Second International Meeting on Metallothionein and Other Low
Molecular Weight Metal-Binding Proteins, Zurich, 21-24 August, 1985,
Basel, Birkhauser Verlag.
KAGI, J.H.R. & NORDBERG, M., ed. (1979) Metallothionein, Basel,
Birkhauser Verlag.
KAGI, J.H.R. & VALLEE, B.L. (1960) Metallothionein: a cadmium- and
zinc-containing protein from equine renal cortex. I. J. biol. Chem.,
235: 3460-3465.
KAGI, J.H.R. & VALLEE, B.L. (1961) Metallothionein: a cadmium- and
zinc-containing protein from equine renal cortex. II. Physiochemical
properties. J. biol. Chem., 236: 2435-2442.
KAHN, H.L. & MANNING, D.C. (1972) Background correction in atomic
absorption spectroscopy. Am. Lab., August: 51-56.
KAJIKAWA, K., NAKANISHI, I., & KURODA, K. (1981) Morphological
changes of the kidney and bone of rats in chronic cadmium poisoning.
Exp. mol. Pathol., 34: 9-24.
KANAI, M., NOMOTO, S., SASAOKA, S., & NAIKI, M. (1971) Clinical
significance of urinary excretion of retinol-binding protein in
patients with Itai-itai disease. Proc. Symp. Physiol. Pathol.,
11: 194-199.
KANISAWA, M. & SCHROEDER, H.A. (1969) Life term studies on the
effect of trace elements on spontaneous tumours in mice and rats.
Cancer Res., 21: 892-895.
KAPLAN, P.D., BLACKSTONE, M., & RICHDALE, N. (1973) Direct
determination of cadmium, nickel, and zinc in rat lungs. Arch.
environ. Health, 27: 387-389.
KAR, A.B., DAS, R.P., & KARKUN, J.N. (1959) Ovarian changes in
prepubertal rats after treatment with cadmium chloride. Acta biol.
med. Germ., 3: 372-399.
KAR, A.B., DAS, R.P., & MUKERJI, B. (1960) Prevention of
cadmium-induced changes in the gonads of rat by zinc and selenium: a
study in antagonism between metals in the biological system. Proc.
Natl Inst. Sci. India Part B. Biol. Sci., 26: 40-50.
KARIN, M. & RICHARDS, R.I. (1982) Human metallothionein genes:
primary structure of the metallothionein-II gene and a related
processed gene. Nature (Lond.), 299: 797-802.
KARIN, M., SLATER, E.P., & HERSCHMAN, H.R. (1981) Regulation of
metallothionein synthesis in HeLa cells by heavy metals and
glucocorticoids. J. cell Physiol., 106: 63-74.
KASUYA, M., TERANISHI, H., KUBOTA, Y., KATO, T., AOSHIMA, K., SAIJO,
A., IWATA, K., & KANAI, M. (1986) [Biological meaning and blood and
urine low-molecular-weight protein determinations of Itai-itai
disease patient and their families, 10 years follow-up study on
urine low-molecular weight protein], Tokyo, Japan Public Health
Association, pp. 176-180 (Kankyo Hoken Report No. 52) (in Japanese).
KATAGIRI, Y., TATI, M., IWATA, H., & KANAI, M. (1971) [Concentration
of cadmium in urine by age.] Med. Biol., 82: 239-243 (in Japanese).
KATO, T. & ABE, K. (1978) Environmental pollution and health effects
- Toyama Prefecture. In: Tsuchiya, K., ed. Cadmium studies in Japan:
a review, Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp. 225-249.
KAWAI, K. (1978) Experimental studies on animals. Morphological
changes of the kidney and effects on bones. In: Tsuchiya, K., ed.
Cadmium studies in Japan: a review, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 86-97.
KAWAI, M., FUKUDA, K., & KIMURA, M. (1976) Morphological alterations
in experimental cadmium exposure with special reference to the onset
of renal lesion. In: Nordberg, G.F., ed. Effects and dose-response
relationships of toxic metals, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 343-370.
KAWAMURA, J., YOSHIDA, O., NISHINO, K., & ITOKAWA, Y. (1978)
Disturbances in kidney functions and calcium and phosphate
metabolism in cadmium-poisoned rats. Nephron, 20: 101-110.
KAWANO, S. & KATO, T. (1978) Environmental pollution and health
effects - Ishikawa Prefecture. In: Tsuchiya, K., ed. Cadmium studies
in Japan: a review, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 220-225.
KAWASHIMA, H., NOMIYAMA, H., & NOMIYAMA, K. (1988) Chronic exposure
to cadmium did not impair vitamin D metabolism in monkeys. Environ.
Res., 46: 48-58.
KAZANTZIS, G. (1956) Respiratory function in men casting cadmium
alloys. Part I. Assessment of ventilatory function. Br. J. ind.
Med., 13: 30-36.
KAZANTZIS, G. (1963) Induction of sarcoma in the rat by cadmium
sulfide pigment. Nature ( Lond.), 198: 1213-1214.
KAZANTZIS, G. (1979) Renal tubular dysfunction and abnormalities of
calcium metabolism in cadmium workers. Environ. Health Perspect.,
28: 155-160.
KAZANTZIS, G. & ARMSTRONG, B.G. (1982) A mortality study of cadmium
workers in the United Kingdom. Scand. J. Work Environ. Health,
8(1): 157-160.
KAZANTZIS, G. & HANBURY, W.J. (1966) The induction of sarcoma in the
rat by cadmium sulfide and by cadmium oxide. Br. J. Cancer,
20: 190-199.
KAZANTZIS, G., FLYNN, F.V., SPOWAGE, J.S., & TROTT, D.G. (1963)
Renal tubular malfunction and pulmonary emphysema in cadmium pigment
workers. Q. J. Med., 32: 165-192.
KAZANTZIS, G., LAM, T.-H., & SULLIVAN, K.R. (1988) Mortality of
cadmium-exposed workers. A five-year update. Scand. J. Work Environ.
Health, 14: 220-223.
KAZANTZIS, G., BLANKS, R., & TEATHER, S. (1989) The mortality of
cadmium exposed workers. In: Vernet, J.-P., ed. Proceedings of the
International Conference on Heavy Metals in the Environment, Geneva,
September 1989, Edinburgh, CEP Consultants Ltd, Vol. 1, pp. 304-307.
KELLO, D. & KOSTIAL, K. (1977) Influence of age and milk diet on
cadmium absorption from the gut. Toxicol. appl. Pharmacol.,
40: 277-282.
KIDO, T., HONDA, R., TSURITANI, I., YAMAYA, H., ISHIZAKI, M.,
YAMADA, Y., & NOGAWA, K. (1988) Progress of renal dysfunction in
inhabitants environmentally exposed to cadmium. Arch. environ.
Health, 43: 213-217.
KIDO, T., NOGAWA, K., YAMADA, Y., HONDA, R., TSURITANI, I.,
ISHIZAKI, M., & YAMAYA, H. (1989) Osteopenia in inhabitants with
renal dysfunction induced by exposure to environmental cadmium. Int.
Arch. occup. environ. Health, 61: 271-276.
KIMURA, M. & OTAKI, N. (1972) Percutaneous absorption of cadmium in
rabbit and hairless mouse. Ind. Health (Tokyo), 10: 7-10.
KING, E. (1955) An environmental study of casting copper-cadmium
alloys. Br. J. ind. Med., 12: 198-205.
KIPLING, M.D. & WATERHOUSE, J.A.H. (1967) Cadmium and prostatic
carcinoma. Lancet, 1: 730-731.
KIRSCH, H., PADBERG, W., SCHOLZ, A., & ZIMMERMEYER, G. (1982)
Cadmium emissions from coal-fired power plants. In: Proceedings of
the 3rd International Cadmium Conference, Miami, Florida, 3-5
February, 1981, London, Cadmium Association, pp. 64-68.
KITAMURA, M. (1972) [Absorption and deposition of cadmium,
especially in human subjects], Tokyo, Japan Public Health
Association, pp. 42-45 (Kankyo Hoken Report No. 11) (in Japanese).
KITAMURA, M. & KOIZUMI, N. (1975) [Results of urine tests for
bedridden old persons in Hyogo Prefecture. Report 2], Tokyo, Japan
Public Health Association, pp. 135-137 (Kankyo Hoken Report No. 36)
(in Japanese).
KITAMURA, S., SUMINO, K., & KAMATANI, N. (1970) [Cadmium
concentrations in liver, kidneys, and bones of human bodies.] Jpn.
J. public Health, 17: 177 (in Japanese).
KJELLSTRÖM, T. (1971) A mathematical model for the accumulation of
cadmium in human kidney cortex. Nord. Hyg. Tidskr., 53: 111-119.
KJELLSTRÖM, T. (1977) Accumulation and renal effects of cadmium in
men. A dose-response study, Stockholm, Karolinska Institute, 80 pp
(Doctoral thesis).
KJELLSTRÖM, T. (1979) Exposure and accumulation of cadmium in
populations from Japan, the United States, and Sweden. Environ.
Health Perspect., 28: 169-197.
KJELLSTRÖM, T. (1986) Effects on bone, on vitamin D, and calcium
metabolism. In: Friberg, L., Elinder, C.-G., Kjellström, T., &
Nordberg, G.F., ed. Cadmium and health: A toxicological and
epidemiological appraisal, Boca Raton, Florida, CRC Press, Vol. II,
pp. 111-158.
KJELLSTRÖM, T. & NORDBERG, G.F. (1978) A kinetic model of cadmium
metabolism in the human being. Environ. Res., 16: 248-269.
KJELLSTRÖM, T. & PISCATOR, M. (1977) Quantitative analysis of
ß-2-microglobulin in urine as an indicator of renal tubular damage
by cadmium. Phadedoc No. 1. Diagnostic communications, Uppsala,
Sweden, Pharmacia Diagnostics, 21 pp.
KJELLSTRÖM, T., LIND, B., LINNMAN, L., & NORDBERG, G.F. (1974) A
comparative study on methods for cadmium analysis of grain with an
application to pollution evaluation. Environ. Res., 8: 92-106.
KJELLSTRÖM, T., LIND, B., LINNMAN, L., & ELINDER, C.-G. (1975a)
Variation of cadmium concentration in Swedish wheat and barley. An
indicator of changes in daily cadmium intake during the 20th
century. Arch. environ. Health, 30: 321-328.
KJELLSTRÖM, T., TSUCHIYA, K., TOMPKINS, E., TAKABATAKE, E., LIND,
B., & LINNMAN, L. (1975b) A comparison of methods for analysis of
cadmium in food and biological material. A cooperative study between
Sweden, Japan, and the USA. In: Recent advances in the assessment of
the health effects of environmental pollution, Luxembourg,
Commission of the European Communities, pp. 2197-2213.
KJELLSTRÖM, T., EVRIN, P.E., & RAHNSTER, B. (1977a) Dose-response
analysis of cadmium-induced tubular proteinuria. A study of urinary
ß-2-microglobulin excretion among workers in a battery factory.
Environ. Res., 13: 303-317.
KJELLSTRÖM, T., SHIROISHI, K., & EVRIN, P.E. (1977b) Urinary
ß-2-microglobulin excretion among people exposed to cadmium in the
general environment. An epidemio-logical study in cooperation
between Japan and Sweden. Environ. Res., 13: 318-344.
KJELLSTRÖM, T., BORG, K., & LIND, B. (1978) Cadmium in faeces as an
estimator of daily cadmium intake in Sweden. Environ. Res.,
15: 242-251.
KJELLSTRÖM, T., FRIBERG, L., & RAHNSTER, D. (1979) Mortality and
cancer morbidity among cadmium-exposed workers. Environ. Health
Perspect., 28: 199-204.
KJELLSTRÖM, T., ELINDER, C.-G., & FRIBERG, L. (1984) Conceptual
problems in establishing the critical concentration of cadmium in
human kidney cortex. Environ. Res., 33: 284-295.
KLAASSEN, C.D. & KOTSONIS, F.N. (1977) Biliary excretion of cadmium
in the rat, rabbit, and dog. Toxicol. appl. Pharmacol., 41: 101-112.
KNEIP, T.J., EISENBUD, M., STREHLOW, C.D., & FREUDENTHAL, P.C.
(1970) Airborne particulates in New York City. J. Air Pollut.
Control Assoc., 20: 144-149.
KOBAYASHI, J. (1974) Effects of cadmium on calcium metabolism of
rats. In: Hemphill, D.D., ed. Trace substances in environmental
health - VII, Columbia, Missouri, University of Missouri Press,
pp. 295-305.
KODAMA, Y., MORIMOTO, K., & NAKAJIMA, T. (1978) [Studies on the
chemical form of heavy metal in oysters.] Eisei Kagaku, 24: 132-138
(in Japanese).
KOGAN, I.G., GROZDOVA, T.YA., & KHOLIKOVA, T.A. (1978)
[Investigation of the mutagenic effect of cadmium chloride on
Drosophila melanogaster germ cells.] Genetika, 16: 2136-2140 (in
Russian).
KOGAN, V.H., MALISHEVA, N.B., & FIDAROVA, A.M. (1972) On the role of
visceral lesions under chronic cadmium intoxications in the
development of bone changes. Trans. Kuban Med. Inst., 36: 75-78.
KOIRTYOHANN, S.R. & HOPKINS, C.A. (1976) Losses of trace metals
during the ashing of biological materials. Analyst, 101: 870-875.
KOIVISTOINEN, P. (1980) Mineral element composition of Finnish
foods: N, K, Ca, Mg, P, S, Fe, Cu, Mn, Zn, Mo, Co, Ni, Cr, F, Se,
Si, Rb, Al, B, Br, Hg, As, Cd, Pb, and Ash. Acta agric. Scand.,
22: Suppl.
KOIZUMI, H., YASUDA, K., & KATAYAMA, M. (1977) Atomic absorption
spectrophotometry based on the polarization characteristics of the
Zeeman effects. Anal. Chem., 49: 1106-1112.
KOJIMA, S., HAGA, Y., KURIHARA, T., & YAMAWAKI, T. (1975) [Report
from Akita Prefecture], Tokyo, Japan Public Health Association, pp.
114-123 (Kankyo Hoken Report No. 36) (in Japanese).
KOJIMA, S., HAGA, Y., KURIHARA, T., & YAMAWAKI, T. (1976) [Report on
cadmium pollution in Akita Prefecture], Tokyo, Japan Public Health
Association, pp. 114-120 (Kankyo Hoken Report No. 36) (in Japanese).
KOJIMA, S., HAGA, Y., KURIHARA, T., YAMAWAKI, T., & KJELLSTRÖM, T.
(1977) A comparison between faecal cadmium and urinary
ß-2-microglobulin total protein and cadmium among Japanese farmers.
An epidemiological study in cooperation between Japan and Sweden.
Environ. Res., 14: 436-451.
KOLLER, L.D., EXON, J.H., & ROAN, J.G. (1975) Antibody suppression
by cadmium. Arch. environ. Health, 30: 598-601.
KONO, M., YOSHIDA, T., SUGIHARA, H., & HAGINO, N. (1956) [Report on
so-called Itai-itai disease - First report.] J. Jap. Soc.
Orthopaedis., 30: 100-101 (in Japanese).
KOPP, S.J., GLONEK, T., ERLANGER, M., PERRY, E.F., PERRY, H.M., Jr,
& BARANY, M. (1980a) Cadmium and lead effects on myocardial function
and metabolism. J. environ. Pathol. Toxicol., 4: 205-227.
KOPP, S.J., PERRY, H.M., Jr, GLONEK, T., ERLANGER, M., PERRY, E.F.,
BARANY, M., & D'AGROSA, L.S. (1980b) Cardiac physiologic-metabolic
changes after chronic low-level heavy metal feeding. Am. J.
Physiol., 239: H22-H30.
KOPP, S.J., PERRY, H.M., Jr, PERRY, E.F., & ERLANGER, M. (1983)
Cardiac physiologic and tissue metabolic changes following chronic
low-level cadmium and cadmium plus lead ingestion in the rats.
Toxicol. appl. Pharmacol., 69: 149-160.
KOROPATNICK, J. & CHERIAN, M.G. (1988) Exposure to different forms
of cadmium in mice: differences in metallothionein and
alphafetoprotein mRNA induction in liver and kidney. J. biochem.
Toxicol., 3: 159-172.
KORPELA, H., LOUENIVA, R., YRJANHEIKKI, E., & KAUPPILA, A. (1986)
Lead and cadmium concentrations in maternal and umbilical cord
blood, amniotic fluid, placenta, and amniotic membranes. Am J.
Obstet. Gynecol., 155: 1086-1089.
KOTSONIS, F.N. & KLAASSEN, C.D. (1977) Toxicity and distribution of
cadmium administered to rats at sublethal doses. Toxicol. appl.
Pharmacol., 41: 667-680.
KOWAL, N.E. & KRAEMER, D.F. (1982) Urinary cadmium and
ß-2-microglobulin levels of persons aged 20-74 years from a
sub-sample of the national HANES II survey, 1976-1980. In:
Proceedings of the 3rd International Cadmium Conference, Miami,
Florida, 3-5 February, 1981, London, Cadmium Association,
pp. 119-122.
KOWAL, N.E., JOHNSON, D.E., KRAEMER, D.F., & PAHREN, H.R. (1979)
Normal levels of cadmium in diet, urine, blood, and tissues of
inhabitants of the United States. J. Toxicol. environ. Health,
5: 995-1014.
KRAEMER, D.F., LUCAS, J.B., PAHREN, H.R., RYAN, J.A., & KOWAL, N.E.
(1979) Cadmium toxicity. Lancet, 1: 1241-1242.
KRASOVSKII, G.N., VARSHAVSKAYA, S.P., & BORISOV, A.I. (1976) Toxic
and gonadotropic effects of cadmium and boron relative to standards
for these substances in drinking-water. Environ. Health Perspect.,
13: 69-75.
KRELL, U. & ROECKNER, E. (1988) Model simulation of the atmospheric
input of lead and cadmium into the North Sea. Atmos. Environ.,
22(2): 375-381.
KRETZSCHMAR, J.G., DELESPAUL, I., & DE RIJK, T.H. (1980) Heavy
levels in Belgium: a five-year survey. Sci. total Environ.,
14: 85-97.
KUHNERT, P.M., KUHNERT, B.R., BOTTOMS, S.F., & ERHARD, P. (1982)
Cadmium levels in maternal blood, fetal cord blood, and placental
tissues of pregnant women who smoke. Am. J. Obstet. Gynecol.,
142: 1021-1028.
KUHNERT, P.M., KUHNERT, B.R., ERHARD, P., BRASHEAR, W.T.,
GROH-WARGO, S.L., & WEBSTER, S. (1987a) The effect of smoking on
placental and fetal zinc status. Am. J. Obstet. Gynecol.,
157: 1241-1246.
KUHNERT, B.R., KUHNERT, P.M., DEBANNE, S., & WILLIAMS, T.G. (1987b)
The relationship between cadmium, zinc and birth weight in pregnant
women who smoke. Am. J. Obstet. Gynecol., 157: 1247-1251.
KUHNERT, B.R., KUHNERT, P.M., & ZARLINGO, T.J. (1988) Associations
between placental cadmium and zinc and age and parity in pregnant
women who smoke. Obstet. Gynecol., 71: 67-70.
LAAMANEN, A. (1972) Functions, progress, and prospects for an
environmental subarctic base level station. Work environ. Health,
9: 17-25.
LAFLEUR, P.D., ed. (1976) Accuracy in trace analysis: sampling,
sample handling, analysis, Washington, DC, US Department of
Commerce, National Bureau of Standards, Vol. 1 and 2, 1304 pp.
LARIONOVA, T.I., ZILLE, L.N., & VOROBJEVA, R.S. (1974) [The
influence of barium-cadmium laurate on some fermentative processes
in the liver.] Gig. Tr. prof. Zabol., 3: 50-52 (in Russian).
LAUWERYS, R. & DE WALS, PH. (1981) Environmental pollution by
cadmium and mortality from renal diseases. Lancet, 1: 383.
LAUWERYS, R., BUCHET, J.P., ROELS, H., BROUWERS, J., & STANESCU, D.
(1974a) Epidemiological survey of workers exposed to cadmium. Arch.
environ. Health, 28: 145-148.
LAUWERYS, R., BUCHET, J.P., & ROELS, H. (1974b) Effets subcliniques
de l'exposition humaine au cadmium. In: Proceedings of the
International Symposium on Problems of the Contamination of Man and
His Environment by Mercury and Cadmium, Luxembourg, Commission of
the European Communities, pp. 447-461.
LAUWERYS, R., BUCHET, J.P., & ROELS, H. (1974c) Dosage du cadmium et
du plomb dans le sang et les urines par spectrophotométrie
d'absorption atomique précedée d'une chromatographie sur résine
échangeuse d'ions. In: Proceedings of the International Symposium on
Problems of the Contamination of Man and His Environment by Mercury
and Cadmium, Luxembourg, Commission of the European Communities,
pp. 231-238.
LAUWERYS, R., BUCHET, J.P., ROELS, H., BERLIN, A., & SMEETS, J.
(1975) Intercomparison program of lead, mercury, and cadmium
analysis in blood, urine, and aqueous solutions. Clin. Chem.,
21: 551-557.
LAUWERYS, R., BUCHET, J.P., & ROELS, H. (1976) The relationship
between cadmium exposure or body burden and the concentration of
cadmium in blood and urine in man. Int. Arch. occup. environ.
Health, 36: 275-285.
LAUWERYS, R., BUCHET, J.P., ROELS, H., & HUBERMONT, G. (1978)
Placental transfer of lead, mercury, cadmium and carbon monoxide in
women. I. Comparison of the frequency distributions of the
biological indices in maternal and umbilical cord blood. Environ.
Res., 15: 278-290.
LAUWERYS, R., ROELS, H., BUCHET, J.P., BERNARD, A., & STANESCU, D.
(1979a) Investigations on the lung and kidney functions in workers
exposed to cadmium. Environ. Health Perspect., 28: 137-145.
LAUWERYS, R., ROELS, H., REGNIERS, M., BUCHET, J.P., BERNARD, A., &
GORET, A. (1979b) Significance of cadmium concentration in blood and
in urine in workers exposed to cadmium. Environ. Res., 20: 375-391.
LAUWERYS, R., ROELS, H., BERNARD, A., & BUCHET, J.P. (1980a) Renal
response to cadmium in a population living in a non-ferrous smelter
area in Belgium. Int. Arch. occup. environ. Health, 45: 271.
LAUWERYS, R., BUCHET, J.P., ROELS, H., BERNARD, A., CHETTLE, D.R.,
HARVEY, T.C., & ALHADDAD, I.K. (1980b) Biological significance of
cadmium concentration in blood and urine and their application in
monitoring workers exposed to cadmium. In: Proceedings of the 2nd
International Cadmium Conference, Cannes, 6-8 February, 1977,
London, Cadmium Association, pp. 164-167.
LAUWERYS, R., BUCHET, J.P., ROELS, H., VIAU, C., BERNARD, A.,
BRUAUX, P., CLAEYS-THOREAU, F., & RONDIA, D. (1984a) Potential risk
of cadmium for the general population: Update of Liege study. In:
Proceedings of the 4th International Cadmium Conference, Munich, 2-4
March, 1983, London, Cadmium Association, pp. 113-114.
LEHMAN, L.D. & POISNER, A.M. (1984) Induction of metallothionein
synthesis in cultured human trophoblasts by cadmium and zinc. J.
Toxicol. environ. Health, 14: 419-432.
LEHNERT, G., VON, SCHALLER, K.H., & HAAS, T. (1968) [Cadmium
analysis of serum and urine using atomic absorption spectrometry.]
Z. klin. Chem. klin. Biochem., 6: 174-176 (in German).
LEMEN, R.A., LESS, J.S., WAGONER, J.K., & BLEJER, H.P. (1976) Cancer
mortality among cadmium production workers. Ann. NY Acad Sci.,
271: 273-279.
LENER, J. & MUSIL, J. (1971) Cadmium influence on the excretion of
sodium by kidneys. Experientia (Basel), 27: 902.
L'EPEE, P., LAZARINI, H., FRANCHOME, J., N'DOKY, T., & LARRIVET, C.
(1968) Contribution à l'étude de l'intoxication cadmique. Arch.
Mal. prof. Méd. Trav. Sécur. soc., 29: 485-490.
LEPOW, M.L., BRUCKMAN, L., RUBINO, R.A., MARKOWITZ, S., GILLETTE,
M., & KAPISH, J. (1974) Role of airborne lead in increased body
burden of lead in Hartford children. Environ. Health Perspect.,
7: 99-102.
LEVY, L.S. & CLACK, J. (1975) Further studies on the effect of
cadmium on the prostate gland. I. Absence of prostatic changes in
rats given oral cadmium sulfate for two years. Ann. occup. Hyg.,
17: 205-211.
LEVY, L.S., ROE, F.J.C., MALCOLM, D., KAZANTIZIS, G., CLACK, J., &
PLATT, H.S. (1973) Absence of prostatic changes in rats exposed to
cadmium. Ann. occup. Hyg., 16: 111-118.
LEVY, L.S., CLACK, J., & ROE, F.J.C. (1975) Further studies on the
effect of cadmium on the prostate gland. II. Absence of prostatic
changes in mice given oral cadmium sulfate for eighteen months. Ann.
occup. Hyg., 17: 213-220.
LEWIS, G.P., JUSKO, W.J., COUGHLIN, L.L., & HARTZ, S. (1972) Cadmium
accumulation in man: influence of smoking, occupation, alcoholic
habit, and disease. J. chron. Dis., 25: 717-726.
LIKUTOVA, I.V. (1989) [Specific effect of inorganic cadmium
compounds on health and its hygienic assessment.] Abstract from a
dissertation for degree of Candidate of Medical Sciences, Moscow,
Central Institute for the Advanced Training of Physicians, 21 pp (in
Russian).
LIKUTOVA, I.V. & BELOVA, E.A. (1987) [Specific action of cadmium in
its peroral intake with water.] Gig. i Sanit., 1987(6): 70-72 (in
Russian).
LINDBERG, S.E., TURNER, R.R., & LOVETT, G.M. (1982) Processes of
atmospheric deposition of metals and acids to forests. Conference
Proceeding 75. Annual Meeting on the Air Pollution Control
Association, New Orleans, 20 June, 1982, Pittsburgh, Pennsylvania,
Air Pollution Control Association, 14 pp.
LINNMAN, L., ANDERSSON, A., NILSSON, K.O., LIND, B., KJELLSTRÖM, T.,
& FRIBERG, L. (1973) Cadmium uptake by wheat from sewage sludge used
as a plant nutrient source. Arch. environ. Health, 27: 45-47.
LORENTZON, R. & LARSSON, S.E. (1977) Vitamin D metabolism in adult
rats at low and normal calcium intake and the effect of cadmium
exposure. Clin. Sci. mol. Med., 53: 439-446.
LOSER, E. (1980) A 2-year oral carcinogenicity study with cadmium on
rats. Cancer Lett., 9: 191-198.
LUCAS, P.A., JARIVALLA, A.G., JONES, J.H., GOUGH, J., & VALE, P.T.
(1980) Total cadmium fume inhalation. Lancet, 2(8187): 205.
LUCIS, O.J., LUCIS, R., & ATERMAN, K. (1972) Tumorigenesis by
cadmium. Oncology, 26: 53-76.
LUFKIN, N.H. & HODGES, F.T. (1944) Cadmium poisoning. Report of
outbreak. US nav. med. Bull., 43: 1273-1276.
LUND, L.J., BETTY, E.E., PAGE, A.L., & ELLIOTT, R.A. (1981)
Occurrence of naturally high cadmium levels in soils and its
accumulation by vegetation. J. environ. Qual., 10: 551-556.
LUNDGREN, G. (1976) Direct determination of cadmium in blood with a
temperature- controlled heated graphite-tube atomizer. Talenta,
23: 309-312.
MAACK, T., JOHNSON, V., KAN, S.T., FIGUEIREDO, J., & SIGULEM, D.
(1979) Renal filtration, transport, and metabolism of low molecular
weight proteins. A review. Kidney Int., 16: 251-270.
MACFARLAND, H.N. (1979) Pulmonary effects of cadmium. In: Mennear,
J., ed. Cadmium toxicity, New York, Basel, Marcel Dekker, Inc.,
pp. 113-132.
MCINNES, G. (1979) Multi-element survey: analysis of the first two
years' results. Warren Spring Laboratory, United Kingdom Department
of Industry, 44 pp (Report No. LR305 AP).
MCKENZIE, J., KJELLSTRÖM, T., & SHARMA, R.P. (1982) Cadmium intake
metabolism and effects in people with a high intake of oysters in
New Zealand, Washington, DC, US Environmental Protection Agency,
210 pp.
MCLELLAN, J.S., THOMAS, B.J., & FREMLIN, J.H. (1975) Cadmium: its
in vivo detection in man. Phys. Med. Biol., 20: 88-95.
MCLELLAN, J.S., FLANAGAN, P.R., CHAMBERLAIN, M.J., & VALBERG, L.S.
(1978) Measurements of dietary cadmium absorption in humans. J.
Toxicol. environ. Health, 4: 131-138.
MAHAFFEY, K.R., CORNELIUSSEN, P.E., JELINEK, C.F., & FIORINO, J.A.
(1975) Heavy metal exposure from foods. Environ. Health Perspect.,
12: 63-69.
MANGELSON, N.F., HILL, M.W., NIELSON, K.K., EATOUGH, D.J.,
CHRISTENSEN, J.J., IZATT, R.M., & RICHARDS, D.O. (1979)
Proton-induced X-ray emission analysis of Pima Indian autopsy
tissues. Anal. Chem., 51: 1187-1194.
MARC (1986) Biological monitoring of environmental contaminants
(plants), London, Monitoring and Assessment Research Centre, Chelsea
College, University of London, 247 pp (MARC Report Number 32).
MARGOSHES, M. & VALLEE, B.L. (1957) A cadmium kidney protein from
equine kidney cortex. J. Am. Chem. Soc., 79: 4813-4814.
MARTIN, J.H. & BROENKOW, W.W. (1975) Cadmium in plankton: elevated
concentrations off Baja California. Science, 190: 884-885.
MARTIN, J.H., ELLIOTT, P.D., ANDERLINI, V.C., GIRVIN, D., JACOBS,
S.A., RISEBROUGH, R.W., DELONG, R.L., & GILMARTIN, W.G. (1976)
Mercury-selenium-bromine imbalance in premature parturient
California sea lions. Mar. Biol., 35: 91-104.
MASON, H.J., DAVISON, A.G., & WRIGHT, A.L. (1988) Relations between
liver cadmium, cumulative exposure, and renal function in cadmium
alloy workers. Br. J. ind. Med., 45: 793-802.
MASON, K.E. & YOUNG, J.O. (1967) Effectiveness of selenium and zinc
in protecting against cadmium-induced injury of rat testis. In:
Muth, O.H., ed. Symposium: Selenium in Biomedicine, Westport,
Connecticut, Avi, pp. 383-394.
MATERNE, D., LAUWERYS, R., BUCHET, J.P., ROELS, H., BROUWERS, J., &
STANESCU, D. (1975) Investigations sur les risques résultant de
l'exposition au cadmium dans deux entreprises de production et deux
entreprises d'utilisation du cadmium. Cah. Méd. Trav., 12: 1-76.
MATSUSAKA, N., TANAKA, M., NISHIMURA, Y., YUYAMA, A., & KOBAYASHI,
H. (1972) [Whole body retention and intestinal absorption of 115Cd
in young and adult mice.] Med. Biol., 85: 275-279 (in Japanese).
MERALI, Z., KACEW, S., & SINGHAL, R.L. (1974) Response of hepatic
carbohydrate and cyclic AMP metabolism to cadmium treatment in rats.
Can. J. Physiol. Pharmacol., 53: 174-184.
MERANGER, J.C., SUBRAMANIAN, K.S., & CHALIFOUX, C. (1981) Metals and
other elements. Survey for cadmium, cobalt, chromium, copper,
nickel, lead, zinc, calcium, and magnesium in Canadian
drinking-water supplies. J. Assoc. Off. Anal. Chem., 64: 44-53.
MILLER, R.K. (1986) Placental transfer and function. The interface
for drugs and chemicals in the conceptus. In: Fabro, S. & Scialli,
A.S., ed. Drug and chemical action in pregnancy, New York, Basel,
Marcel Dekker, Inc., pp. 123-152.
MILLS, C.F. & DELGARNO, A.C. (1972) Copper and zinc status of ewes
and lambs receiving increased dietary concentration of cadmium.
Nature ( Lond.), 239: 171-173.
MOGENSEN, C.E. & SOLLING, K. (1977) Studies on renal tubular protein
reabsorption: partial and near complete inhibition by certain amino
acids. Scand. J. clin. lab. Invest., 37: 477-486.
MOHD, M.A., MURTHY, R.C., & CHANDRA, S.V. (1986) Developmental and
long-term neurobehavioral toxicity of low-level in utero Cd
exposure in rats. Toxicol. Teratol., 8: 463.
MORGAN, J.M. (1970) Cadmium and zinc abnormalities in bronchogenic
carcinoma. Cancer, 25: 1394-1398.
MORGAN, J.M., BURCH, H.B., & WATKISS, J.B. (1971) Tissue cadmium and
zinc content in emphysema and bronchogenic carcinoma. J. chron.
Dis., 24: 107-110.
MORITSUGI, M. & KOBAYASHI, J. (1964) Study on trace elements in
biomaterials. II. Cadmium content in polished rice. Ber. Ohara Inst.
Landwirtsch. Biol. Okayama Univ., 12: 145-158.
MOSCHANDREAS, D.J. (1981) Exposure to pollutants and daily time
budgets of people. Bull. NY Acad. Med., 57: 845-859.
MULFORD, C.E. (1966) Solvent extraction techniques for atomic
absorption spectoscopy. At. Absorpt. Newsl., 5: 88-90.
MULVIHILL, J.E., GAMM, S.H., & FERM, V.H. (1970) Facial formation in
normal and cadmium-treated golden hamsters. J. Embryol. exp.
Morphol., 24: 393-403.
MURASE, H., INOUE, T., SAGA, M., IWATA, T., & KUBOTA, K. (1974)
[Microscopic pathology of dogs chronically exposed to oral cadmium],
Tokyo, Japan Public Health Association, pp. 72-73 (Kankyo Hoken
Report No. 31) (in Japanese).
MUSKETT, C.J., ROBERTS, L.H., & PAGE, B.J. (1979) Cadmium and lead
pollution from secondary metal refinery operations. Sci. total
Environ., 11: 73-87.
NACKOWSKI, S.B., PUTNAM, R.D., ROBBINS, D.A., VARNER, M.O., WHIT,
L.D., & NELSON, K.W. (1977) Trace metal contamination of evacuated
blood collection tubes. Am. Ind. Hyg. Assoc. J., 38: 503-508.
NAKAGAWA, S. (1960) [A study of osteomalacia in Toyama Prefecture
(so-called Itai-itai disease).] J. Radiol. Phys. Ther. Univ.
Kanazawa, 56: 1-51 (in Japanese with English summary).
NAKANO, M., AOSHIMA, K., KATOH, T., TERANISHI, H., & KATOH, T.
(1987) Severity of tubular brush border damage in a
cadmium-polluted area (Jinzu River basin): clinical role of urinary
trehalase. Environ. Res., 44: 161-168.
NAZARI, G., FAVINO, A., & POZZI, U. (1967) [Effects of a single
subcutaneous injection of cadmium chloride in male rats.] Riv. Anat.
Pathol. Oncol., 31: 251-271 (in Italian).
NEEB, R. & WAHDAT, F. (1974) [About inverse voltammetry analysis of
cadmium in aerosols.] Z. anal. Chem., 269: 275-279 (in German).
NICAUD, P., LAFITTE, A., & GROS, A. (1942) Les troubles de
l'intoxication chronique par le cadmium. Arch. Mal. prof.,
4: 192-202.
NICHOLSON, J.K. & OSBORN, O. (1983) Kidney lesions in pelagic
seabirds with high tissue levels of cadmium and mercury. J. Zool.
(Lond.), 200: 88-118. NIELSEN, S.A. (1975) Cadmium in New Zealand
dredge oysters, geographic distribution. Int. J. environ. anal.
Chem., 4: 1-7.
NISE, G. & VESTERBERG, O. (1978) [Sampling vessels influence on
content of heavy metals (Cd, Cr, Hg, Ni, and Pb) in blood and urine
samples.] Läkartidningen, 75: 19-20 (in Swedish).
NISHIYAMA, K. & NORDBERG, G. (1972) Adsorption and elution of
cadmium on hair. Arch. environ. Health, 25: 92-96.
NISHIYAMA, S., NAKAMURA, K., & KONISHI, Y. (1986) Blood pressure and
urinary sodium and potassium excretion in cadmium-treated male rats.
Environ. Res., 40: 357-364.
NOGAWA, K. (1981) Itai-itai disease and follow-up studies. In:
Nriagu, J.O., ed. Cad-mium in the environment. II. Health effects,
New York, John Wiley and Sons, pp. 1-38.
NOGAWA, K. & ISHIZAKI, A. (1979) A comparison between cadmium in
rice and renal effects among inhabitants of the Jinzu River Basin.
Environ. Res., 18: 410-420.
NOGAWA, K., ISHIZAKI, A., FUKUSHIMA, M., SHIBATA, I., & HAGINO, N.
(1975) Studies on the women with acquired Fanconi syndrome observed
in the Ichi River basin polluted by cadmium. Environ. Res.,
10: 280-307.
NOGAWA, K., ISHIZAKI, A., & KAWANO, S. (1978) Statistical
observations of the dose-response relationships of cadmium based on
epidemiological studies in the Kakehashi River basin. Environ. Res.,
15: 185-198.
NOGAWA, K., KOBAYASHI, E., HONDA, R., & ISHIZAKI, A. (1979)
Clinico-chemical studies on chronic cadmium poisoning. I. Results of
urinary examinations. Jpn. J. Hyg., 34: 407-414.
NOGAWA, K., KOBAYASHI, E., HONDA, R., ISHIZAKI, A., KAWANO, S., &
MATUSDA, H. (1980) Renal dysfunction of inhabitants in a
cadmium-polluted area. Environ. Res., 23: 13-23.
NOGAWA, K., KOBAYASHI, E., & KONISHI, F. (1981a) Comparison of bone
lesions in chronic cadmium intoxication and vitamin D deficiency.
Environ. Res., 24: 233-249.
NOGAWA, K., KAWANO, S., & NISHI, M. (1981b) Mortality study of
inhabitants in a cadmium-polluted area with special reference to low
molecular weight proteinuria. In: Heavy metals in the environment,
Edinburgh, CEP Consultants Ltd, pp. 538-540.
NOGAWA, K., KIDO, T., YAMADA, Y., TSURITANI, I., HONDA, R.,
ISHIZAKI, N., & TERAHATA, K. (1984) Alpha-1-microglobulin in urine
as an indicator of renal tubular damage caused by environmental
cadmium exposure. Toxicol. Lett., 22: 63-68.
NOGAWA, K., TSURITANI, I., YAMADA, Y., KIDO, T., HONDA, R.,
ISHIZAKI, M., & KURIHARA, T. (1986) Sister-chromatid exchanges in
the lymphocytes of people exposed to environmental cadmium. Toxicol.
Lett., 32: 283-288.
NOGAWA, K., TSURITANI, I., KIDO, T., HONDA, R., YAMADA, Y., &
ISHIZAKI, M. (1987) Mechanism for bone disease found in inhabitants
environmentally exposed to cadmium: decreased serum
1alpha,25-dihydroxyvitamin D level. Int. Arch. occup. environ.
Health, 59: 21-30.
NOGAWA, K., HONDA, R., KIDO, T., TSURITANI, I., YAMADA, Y.,
ISHIZAKI, M., & YAMAYA, H. (1989) A dose-response analysis of
cadmium in the general environment with special reference to total
cadmium intake limit. Environ. Res., 48: 7-16.
NOMIYAMA, K. (1973) Early signs of cadmium intoxication in rabbits.
Toxicol. appl. Pharmacol., 24: P625-P635.
NOMIYAMA, K. (1974) [Studies concerning cadmium metabolism and the
etiology of poisoning], Tokyo, Japan Public Health Association,
pp. 53-59 (Kankyo Hoken Report No. 31) (in Japanese).
NOMIYAMA, K. (1977) Does a critical concentration of cadmium in
human renal cortex exist? J. Toxicol. environ. Health, 3: 607-609.
NOMIYAMA, K. (1978) Experimental studies on animals:
in vivo experiments. In: Tsuchiya, K., ed. Cadmium studies in
Japan: a review, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 47-86.
NOMIYAMA, K. (1986) The chronic toxicity of cadmium: influence of
environmental and other variables. In: Born, G.V.R., Rensselaer,
A.F., Herken, H., & Welch, A.D., ed. Handbook of experimental
pharmacology, Berlin, Heidelberg, Springer-Verlag, Vol. 80,
pp. 101-133.
NOMIYAMA, K. & FOULKES, E.C. (1977) Reabsorption of filtered cadmium
metallothionein in the rabbit kidney. Proc. Soc. Exp. Biol. Med.,
156: 97-99.
NOMIYAMA, K. & NOMIYAMA, H. (1976a) Mechanisms of urinary excretion
of cadmium: experimental studies in rabbits. In: Nordberg, G.F., ed.
Effects and dose-response relationships of toxic metals, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 371-379.
NOMIYAMA, K. & NOMIYAMA, H. (1976b) [Long-term experiment with
300 ppm oral cadmium exposure to rabbits. Intermediate report after
12 months], Tokyo, Japan Public Health Association, pp. 161-173
(Kankyo Hoken Report No. 38) (in Japanese).
NOMIYAMA, K. & NOMIYAMA, H. (1982) Tissue metallothionein in rabbits
chronically exposed to cadmium, with special reference to the
critical concentration of cadmium in the renal cortex. In: Foulkes,
E.C., ed. Biological roles of metallothionein, Amsterdam, Oxford,
New York, Elsevier Science Publishers, pp. 47-67.
NOMIYAMA, K. & NOMIYAMA, H. (1988) Health effect of six years of
dietary cadmium (cadmium contaminated rice) in monkeys. In:
Essential and toxic trace elements in human health and disease, New
York, Alan K. Liss, Inc., pp. 589-609.
NOMIYAMA, K., SUGATA, Y., YAMAMOTO, A., & NOMIYAMA, H. (1975)
Effects of dietary cadmium on rabbits. I. Early signs of cadmium
intoxication. Toxicol. appl. Pharmacol., 31: 4-12.
NOMIYAMA, K., SUGATA, Y., NOMIYAMA, H., & YAMAMOTO, A. (1976)
Dose-response relationship for cadmium. In: Nordberg, G.F., ed.
Effects and dose-response relationships of toxic metals, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 380-385.
NOMIYAMA, K., NOMIYAMA, H., YOTORIYAMA, M., & TAGUCHI, T. (1978a)
Some recent studies on the renal effects of cadmium. In: Proceedings
of the First International Cadmium Conference, San Francisco,
California, 31 January-2 February, 1977, London, Cadmium
Association, pp. 186-194.
NOMIYAMA, K., NOMIYAMA, H., & TAGUCHI, T. (1978b) Effects of
environmental temperatures on the toxicity of cadmium in mice. In:
Proceedings of the 8th Asian Conference on Occupational Health,
Tokyo, Japan Industrial Safety Association, pp. 221-225.
NOMIYAMA, K., NOMIYAMA, H., NOMURA, M., TAGUCHI, T., MATSUI, J.,
YOTORIYAMA, M., AKAHORI, F., IWAO, S., KOIZUMI, N., MASAOKA, T.,
KITAMURA, S., TSUCHIYA, K., SUZUKI, T., & KOBAYASHI, K. (1979)
Effects of dietary cadmium on rhesus monkeys. Environ. Health
Perspect., 28: 223-243.
NOMIYAMA, K., NOMIYAMA, H., AKAHORI, F., & MASAOKA, T. (1982a)
Cadmium health effects in monkeys with special reference to the
critical concentration of cadmium in the renal cortex. In:
Proceedings of the 3rd International Cadmium Conference, Miami,
Florida, 3-5 February, 1981, London, Cadmium Association,
pp. 151-156.
NOMIYAMA, K., NOMIYAMA, H., YOTORIYAMA, M., & MATSUI, K. (1982b)
Sodium dodecyl sulfate acrylamide gel electrophoretic studies on low
molecular weight proteinuria, an early sign of cadmium health
effects in rabbits. Ind. Health, 20: 11-18.
NOMIYAMA, K., NOMIYAMA, H., YOTORIYAMA, M., AKAHORI, F., & MASAOKA,
T. (1984) Some comments and proposals on dose and effects for
estimating critical concentration of cadmium in the renal cortex.
In: Proceedings of the Fourth International Cadmium Conference,
London, Cadmium Association, pp. 167-171.
NOMIYAMA, K., AKAHORI, F., MASAOKA, T., ARAI, S., NOMURA, Y.,
YOTORIYAMA, M., KOBAYASHI, K., SUZUKI, T., KAWASHIMA, H., & ONOZAWA,
A. (1987) [Dose effect and dose response relationships of 9 years
dietary cadmium in rhesus monkeys], Tokyo, Japan Public Health
Association, pp. 1-86 (Kankyo Hoken Report No. 53) (in Japanese).
NOMURA, T., KIMURA, M., TANIOKA, Y., TANIMOTO, Y., SAEGUSA, J.,
KOTAKI, N., TAZAKI, H., HATA, R., DEGUCHI, N., SUDA, T., & YOSHIKI,
S. (1988) [Influence of nutritional factors on cadmium-administered
monkeys], Tokyo, Japan Public Health Association, pp. 42-122 (Kankyo
Hoken Report No. 54) (in Japanese).
NORDBERG, G.F. (1971) Effects of acute and chronic cadmium exposure
on the testicles of mice. With special reference to protective
effects of metallothionein. Environ. Physiol. Biochem., 1: 171-187.
NORDBERG, G.F. (1972) Cadmium metabolism and toxicity. Environ.
Physiol. Biochem., 2: 7-36.
NORDBERG, G.F. (1975) Effects of long-term cadmium exposure on the
seminal vesicles of mice. J. Reprod. Fertil., 45: 165-167.
NORDBERG, G.F. & NISHIYAMA, K. (1972) Whole-body and hair retention
of cadmium in mice. Arch. environ. Health, 24: 209-214.
NORDBERG, G.F. & NORDBERG, M. (1988) Biological monitoring of toxic
metals, New York, London, Plenum Press, pp. 151-168.
NORDBERG, G.F. & PISCATOR, M. (1972) Influence of long-term cadmium
exposure on urinary excretion of protein and cadmium in mice.
Environ. Physiol. Biochem., 2: 37-49.
NORDBERG, G.F., PISCATOR, M., & LIND, B. (1971) Distribution of
cadmium among protein fractions of mouse liver. Acta pharmacol.
toxicol., 29: 456-470.
NORDBERG, G.F., SLORACH, S., & STENSTROM, T. (1973) [Cadmium
poisoning caused by a cooled soft drink machine.] Läkartidningen,
70: 601-604 (in Swedish with English summary).
NORDBERG, G.F., GOYER, R.A., & NORDBERG, M. (1975) Comparative
toxicity of cadmium metallothionein and cadmium chloride on mouse
kidney. Arch. Pathol., 99: 192-197.
NORDBERG, G.F., ROBERT, K.-H., & PANNONE, M. (1977) Pancreatic and
biliary excretion of cadmium in the rat. Acta pharmacol. toxicol.,
41: 84-88.
NORDBERG, G.F., GARVEY, J.S., & CHANG, C.C. (1982) Metallothionein
in plasma and urine of cadmium workers. Environ. Res., 28: 179-182.
NORDBERG, M. (1978) Studies on metallothionein and cadmium. Environ.
Res., 15: 381-404.
NORDBERG, M. (1984) General aspects of cadmium transport, uptake,
and metabolism by the kidney. Environ. Health Perspect., 54: 13-20.
NORDBERG, M. & NORDBERG, G.F. (1975) Distribution of metallothionein-
bound cadmium and cadmium chloride in mice: preliminary studies.
Environ. Health Perspect., 12: 103-108.
NORDBERG, M., NUOTTANIEMI, I., CHERIAN, M.G., NORDBERG, G.F.,
KJELLSTRÖM, T., & GARVEY, J.S. (1986) Characterization studies on
the cadmium-binding proteins from two species of New Zealand
oysters. Environ. Health Perspect., 65: 57-62.
NRIAGU, J.O. (1979) Global inventory of natural and anthropogenic
emissions of trace metals to the atmosphere. Nature (Lond.),
279: 409.
NRIAGU, J.O. & PACYNA, J.M. (1988) Quantitative assessment of
worldwide contamination of air, water and soils by trace metals.
Nature (Lond.), 333: 134-139.
NWANKWO, J.N., ELINDER, C.G., PISCATOR, M., & LIND, B. (1977)
Cadmium in Zambian cigarettes: an interlaboratory comparison in
analysis. Zambia J. Sci. Technol., 2(4): 1-4.
OBERDORSTER, G. (1988) Deposition and retention modeling of inhaled
cadmium in rat and human lung: An example for extrapolation of
effects and risk estimation. In: Crapo, J.D., Miller, F.J., Smolko,
E.D., Graham, J.A., & Hayes, A.W., ed. Extrapolation of dosimetric
relationships for inhaled particles and gases, New York, London,
Academic Press, pp. 345-370.
OHANIAN, E.V. & IWAI, J. (1980) Etiological role of cadmium in
hypertension in an animal model. J. environ. Pathol. Toxicol.,
3: 229-241.
OHMOMO, Y. & SUMIYA, M. (1981) Estimation of heavy metal intake from
agricultural products. In: Kitagishi, K. & Yamane, J., ed. Heavy
metal pollution in soils of Japan, Tokyo, Japan Scientific Societies
Press, pp. 235-244.
OLDIGES, H., HOCHRAINER, D., & GLASER, U. (1989) Long-term
inhalation study with Wistar rats and four cadmium compounds.
Toxicol. environ. Chem., 19: 217-222.
O'RIORDAN, M.L., HUGHES, E.G., & EVANS, H.J. (1978) Chromosome
studies on blood lymphocytes of men occupationally exposed to
cadmium. Mutat. Res., 58: 305-311.
PAGE, A.L. (1981) Cadmium in soils and its accumulation by food
crops. In: Proceedings of an International Conference on Heavy
Metals in the Environment, Amsterdam, September 1981, Edinburgh, CEP
Consultants, Ltd, pp. 206-213.
PAGE, A.L., BINGHAM, F.T., & SHANG, A.C. (1981) Cadmium. In: Lepp,
N.W., ed. Effect of heavy metal pollution on plants, Barking,
Essex, Applied Science Publishers, Vol. 1, pp. 77-109.
PANEMANGALORE, M., BANERJEE, D., ONOSAKA, S., & CHERIAN, M.G. (1983)
Changes in the intracellular accumulation and distribution of
metallothionein in rat liver and kidney during postnatal
development. Dev. Biol., 97: 95-102.
PARIZEK, J. (1957) The destructive effect of cadmium ion on
testicular tissue and its prevention by zinc. J. Endocrinol.,
15: 56-63.
PARIZEK, J. (1960) Sterilization of the male by cadmium salts. J.
Reprod. Fertil., 1: 294-309.
PARIZEK, J. (1964) Vascular changes at sites of oestrogen
biosynthesis produced by parenteral injection of cadmium salts: the
destruction of placenta by cadmium salts. J. Reprod. Fertil.,
7: 263-265.
PARIZEK, J. & ZAHOR, Z. (1956) Effects of cadmium salts on
testicular tissue. Nature (Lond.), 177: 1036-1037.
PARIZEK, J., OSTADALOVA, I., BENES, I., & PITHA, J. (1968a) The
effect of a subcutaneous injection of cadmium salts on the ovaries
of adult rats in persistent oestrus. J. Reprod. Fertil., 17:
559-562.
PARIZEK, J., OSTADALOVA, I., BENES, I., & BABICKY, A. (1968b)
Pregnancy and trace elements: the protective effect of compounds of
an essential trace element, selenium, against the peculiar toxic
effects of cadmium during pregnancy. J. Reprod. Fertil., 16:
507-509.
PARZYCK, D.C., SHAW, S.M., KESSLER, W.V., VETTER, R.J., VAN SICKLE,
D.C., & MAYES, R.A. (1978) Fetal effects of cadmium in pregnant rats
on normal and zinc-deficient diets. Bull. environ. Contam. Toxicol.,
19: 206-214.
PATON, G.R. & ALLISON, A.C. (1972) Chromosome damage in human cell
cultures induced by metal salts. Mutat. Res., 16: 332-336.
PERRY, H.M., Jr & ERLANGER, M.W. (1973) Elevated circulating renin
activity in rats following doses of cadmium known to induce
hypertension. J. lab. clin. Med., 82: 399-405.
PERRY, H.M., Jr & ERLANGER, M.W. (1974) Metal-induced hypertension
following chronic feeding of low doses of cadmium and mercury. J.
lab. clin. Med., 83: 541-547.
PERRY, H.M., Jr & ERLANGER, M.W. (1982) Effect of diet on increases
in systolic pressure induced in rats by chronic cadmium feeding. J.
Nutr., 112: 1983-1989.
PERRY, H.M., Jr & KOPP, S.J. (1983) Does cadmium contribute to human
hypertension? Sci. total Environ., 26: 223-232.
PERRY, H.M., Jr, ERLANGER, M.W., YUNICE, A., SCHOEPFLE, E., & PERRY,
E.F. (1970) Hypertension and tissue metal levels following
intravenous cadmium, mercury, and zinc. Am. J. Physiol., 219:
755-761.
PERRY, H.M., Jr, PERRY, E.F., & PURIFOY, J.E. (1971) Antinatriuretic
effect of intramuscular cadmium in rats. Proc. Soc. Exp. Biol. Med.,
136: 1240-1244.
PERRY, H.M., Jr, ERLANGER, M.W., & PERRY, E.F. (1976) Limiting
conditions for the induction of hypertension in rats by cadmium. In:
Hemphill, D.D., ed. Trace substances in environmental health - VIII,
Columbia, Missouri, University of Missouri Press, pp. 51-57.
PERRY, H.M., Jr, ERLANGER, M., & PERRY, E.F. (1977) Hypertension
following chronic, very low-dose cadmium feeding. Proc. Soc. Exp.
Biol. Med., 156: 173-176.
PETERING, D.H., LOFTSGAARDEN, J., SCHNEIDER, J., & FOWLER, B. (1984)
Metabolism of cadmium, zinc and copper in the rat kidney: the role
of metallothionein and other binding sites. Environ. Health
Perspect., 54: 73-81.
PETERING, H.G., JOHNSON, M.A., & STEMMER, K. (1971) Studies of zinc
metabolism in the rat. I. Dose-response effects of cadmium. Arch.
environ. Health, 23: 93-96.
PETERING, H.G., CHOUDHURY, H., & STEMMER, K.L. (1979) Some effects
of oral ingestion of cadmium on zinc, copper, and iron metabolism.
Environ. Health Perspect., 28: 97-106.
PETERSON, P.A. (1971) Characteristics of a vitamin A-transporting
protein complex occurring in human serum. J. biol. Chem.,
246: 34-43.
PETERSON, P.A. & BERGGÅRD, I. (1971) Isolation and properties of a
human retinol-transporting protein. J. biol. Chem., 246: 25-33.
PETERSON, P.A., EVRIN, P.-E., & BERGGÅRD, I. (1969) Differentiation
of glomerular, tubular, and normal proteinuria: determinations of
urinary excretion of ß-2-microglobulin, albumin, and total protein.
J. clin. Invest., 48: 1189-1198.
PHILIPP, R. (1985) Cadmium-risk assessment of an exposed residential
population: a review. J. R. Soc. Med., 78: 328-333.
PIOTROWSKI, J.K., TROJANOWSKA, B., WISNIEWSKA-KNYPL, J.M., &
BOLANOWSKI, W. (1974) Mercury binding in the kidney and liver of
rats repeatedly exposed to mercuric chloride: induction of
metallothionein by mercury and cadmium. Toxicol. appl. Pharmacol.,
27: 11-19.
PISCATOR, M. (1962) Proteinuria in chronic cadmium poisoning. II.
The applicability of quantitative and qualitative methods of protein
determination for the demonstration of cadmium proteinuria. Arch.
environ. Health, 5: 325-332.
PISCATOR, M. (1964) [On cadmium in normal kidneys together with a
report on the isolation of metallothionein from livers of
cadmium-exposed rabbits.] Nord. Hyg. Tidskr., 45: 76-82 (in
Swedish).
PISCATOR, M. (1966a) Proteinuria in chronic cadmium poisoning. III.
Electrophoretic and immunoelectrophoretic studies on urinary
proteins from cadmium workers, with special reference to the
excretion of low molecular weight proteins. Arch. environ. Health,
12: 335-344.
PISCATOR, M. (1966b) Proteinuria in chronic cadmium poisoning. IV.
Gel filtration and ion exchange chromatography of urinary proteins
from cadmium workers. Arch. environ. Health, 12: 345-359.
PISCATOR, M. (1971) Data on interferences in cadmium analysis. In:
Friberg, L., Piscator, M., & Nordberg, G., ed. Cadmium in the
environment, Cleveland, Ohio, CRC Press, p. 17.
PISCATOR, M. & TSUCHIYA, K. (1971) In: Friberg, L., Piscator, M., &
Nordberg, G., ed. Cadmium in the environment, Cleveland, Ohio,
Chemical Rubber Company Press, pp. 112-114.
PISCATOR, M. & VOUK, V.B. (1979) Sampling and analytical methods.
In: Friberg, L., Nordberg, G.F., & Vouk, V.B., ed. Handbook on the
toxicology of metals, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 33-46.
PISCATOR, M., KJELLSTRÖM, T., & LIND, B. (1976) Contamination of
cigarettes and pipe tobacco by cadmium-oxide dust. Lancet, 2: 587.
PISCATOR, M., BJORK, L., & NORDBERG, M. (1981) Beta-2-microglobulin
levels in serum and urine of cadmium exposed rabbits. Acta.
pharmacol. toxicol., 49: 1-7.
PLEBAN, P.A., KERKAY, I., & PEARSON, K.H. (1981) Polarized
Zeeman-effect flameless atomic absorption spectrometry of cadmium,
copper, lead, and manganese in human kidney cortex. Clin. Chem.,
27: 68-72.
POTTS, C.L. (1965) Cadmium proteinuria - the health of battery
workers exposed to cadmium oxide dust. Ann. occup. Hyg., 8: 55-61.
PRIGGE, E. (1978) Early signs of oral and inhalative cadmium uptake
in rats. Arch. Toxicol., 40: 231-247.
PRIGGE, E., BAUMERT, H.P., & MUHLE, H. (1977) Effects of dietary and
inhalative cadmium on haemoglobin and haematocrit in rats. Bull.
environ. Contam. Toxicol., 17: 585-590.
PRINCI, F. (1947) A study of industrial exposure to cadmium. J. ind.
Hyg. Toxicol., 29: 315-320.
PRINCI, F. & GEEVER, E.F. (1950) Prolonged inhalation of cadmium.
Arch. ind. Hyg. occup. Med., 1: 651-661.
PROCKOP, D.J. & DAVIDSON, W.D. (1964) A study of urinary and serum
lysozyme in patients with renal disease. New Engl. J. Med.,
270: 269-274.
PRODAN, L. (1932) Cadmium poisoning. II. Experimental cadmium
poisoning. J. ind. Hyg. Toxicol., 14: 174-196.
PULIDO, P., KAGI, J.H., & VALLEE, B.L. (1966) Isolation and some
properties of human metallothionein. Biochemistry, 5: 1768-1777.
RAHOLA, T., AARAN, R.-K., & MIETTINEN, J.K. (1972) Half-time studies
of mercury and cadmium by whole-body counting. In: Assessment of
radioactive contamination in man, New York, International Atomic
Energy Agency Unipublishers, pp. 553-562 (IAEA Proceedings Series
No. Sm-150/13).
RAMAYA, L.K. & POMERANTZEVA, M.O. (1977) [Investigation of cadmium
chloride mutagenic effect on germ cells of male mice.] Genetika,
13: 59-63 (in Russian).
RAMEL, C. & FRIBERG, L. (1971) Carcinogenic and mutagenic effects.
In: Friberg, L., Piscator, M., & Nordberg, G.F., ed. Cadmium in the
environment, Cleveland, Ohio, CRC Press, pp. 109-110.
RAUHUT, A. (1980) Survey of industrial emission of cadmium in the
European Economic Community: Final Report, Brussels, Commission of
the European Communities, Vol. 2, pp. 1-44.
REDDY, C.S., SVOBODA, D., AZARNOFF, D., & DEWAR, R. (1973)
Cadmium-induced Leydig cell tumors of rat testis: morphologic and
histochemical study. J. Natl Cancer Inst., 51: 891-903.
RICHARDSON, M.E. & FOX, M.R.S. (1974) Dietary cadmium and
enteropathy in the Japanese quail. Lab. Invest., 31: 722-731.
RIVM (1988) In: Ros, J.P.M. & Sloof, W., ed. Integrated criteria
document cadmium, Bilthoven, The Netherlands, National Institute of
Public Health and Environmental Protection (RIVM-Report No.
758476004).
ROBBINS, S.L., COTRAN, R.S., & KUMAR, U. (1984) Pathological basis
of disease, 3rd ed., Philadelphia, Pennsylvania, W.B. Saunders &
Co., pp. 1327-1328.
ROELS, H., HUBERMONT, G., BUCHET, J.P., & LAUWERYS, R. (1978)
Placental transfer of lead, mercury, cadmium and carbon monoxide in
women. III. Factors influencing the accumulation of heavy metals in
the aplacenta and the relationship between metal concentration in
the placenta and in maternal and cord blood. Environ. Res.,
16: 236-245.
ROELS, H., LAUWERYS, R.R., BUCHET, J.P., & BERNARD, A. (1981a)
Environmental exposure to cadmium and renal function of aged women
in three areas of Belgium. Environ. Res., 24: 117-130.
ROELS, H., LAUWERYS, R.R., BUCHET, J.P., BERNARD, A., CHETTLE, D.R.,
HARVEY, T.C., & AL-HADDAD, J.K. (1981b) In vivo measurement of
liver and kidney cadmium in workers exposed to this metal: its
significance with respect to cadmium in blood and urine. Environ.
Res., 26: 217-240.
ROELS, H., DJUBGANG, J., BUCHET, J.P., BERNARD, A., & LAUWERYS, R.R.
(1982) Evolution of cadmium-induced renal dysfunction in workers
removed from exposure. Scand. J. Work Environ. Health, 8: 191-200.
ROELS, H., LAUWERYS, R.R., & DARDENNE, A.N. (1983a) The critical
level of cadmium in human renal cortex: a reevaluation. Toxicol.
Lett., 15: 357-360.
ROELS, H., LAUWERYS, R.R., BUCHET, J.P., BERNARD, A., GARVEY, J.S.,
& LINTON, H.J. (1983b) Significance of urinary metallothionein in
workers exposed to cadmium. Int. Arch. occup. environ. Health,
52: 159-166.
ROELS, H., LAUWERYS, R.R., BUCHET, J.P., BERNARD, A.M., VOS, A., &
OVERSTEYNS, M. (1989) Health significance of cadmium induced renal
dysfunction: a five year follow up. Br. J. ind. Med., 46: 755-764.
ROHR, G. & BAUCHINGER, M. (1976) Chromosome analyses in cell
cultures of the Chinese hamster after application of cadmium
sulfate. Mutat. Res., 40: 125-130.
SAITO, H. (1987) [Follow-up study on health status on residents in a
cadmium polluted area (Sasu, Izahara town of Nagasaki Prefecture)
after improvement of cadmium-polluted lands], Tokyo, Japan Public
Health Association, pp. 215-216 (Kankyo Hoken Report No. 53) (in
Japanese).
SAITO, H., SHIOJI, R., FURUKAWA, Y., NAGAI, K., ARIKAWA, T., SAITO,
T., SASAKI, Y., FURUYAMA, T., & YOSHINAGA, K. (1977) Cadmium-induced
proximal tubular dysfunction in a cadmium-polluted area. Nephrology,
6: 1-12.
SAITO, H., NAKANO, A., TOHYAMA, C., MITANE, Y., SUGIHIRA, N., &
WAKISAKA, I. (1983) [Report on a health examination of inhabitants
of areas with cadmium pollution of soil], Tokyo, Japan Public Health
Association, pp. 91-95 (Kankyo Hoken Report No. 40) (in Japanese).
SALMELA, S. & VUORI, E. (1979) Contamination with cadmium from
micropipette tips. Talanta, 26: 175-176.
SALTZMAN, B.E. (1953) Colorimetric microdetermination of cadmium
with dithizone. Anal. Chem., 25: 493-496.
SAMARAWICKRAMA, G.P. & WEBB, M. (1979) Acute effects of cadmium on
the pregnant rat and embryo-fetal development. Environ. Health
Perspect., 28: 245-249.
SANDERS, C.L. & MAHAFFEY, J.A. (1984) Carcinogenicity of single and
multiple intratracheal instillations of cadmium oxide in the rat.
Environ. Res., 33: 227-233.
SANGSTER, B., DE GROOT, G., LOEBER, J.G., DERKS, H.J.G.M., KRAJNC,
E.I., & SAVELKOUL, T.J.F. (1984) Urinary excretion of cadmium,
protein, beta-2-microglobulin and glucose in individuals living in a
cadmium-polluted area. Hum. Toxicol., 3: 7-21.
SCHARPF, L.G., Jr, HILL, I.D., WRIGHT, P.L., PLANK, J.B., KEPLINGER,
M.L., & CALANDRA, J.C. (1972) Effect of sodium nitrilotriacetate on
toxicity, teratogenicity, and tissue distribution of cadmium. Nature
(Lond.), 239: 231-234.
SCHERER, G. & BARKEMEYER, H. (1983) Cadmium concentrations in
tobacco and tobacco smoke. Ecotoxicol. environ. Saf., 7: 71-78.
SCHMID, B.P., KAO, J., & GOULDING, E. (1985) Evidence for reopening
of the cranial tube in mouse embryo treated with cadmium chloride.
Experientia (Basel), 41: 271-272.
SCHROEDER, H.A. (1964) Cadmium hypertension in rats. Am. J.
Physiol., 207: 62-66.
SCHROEDER, H.A. (1965) Cadmium as a factor in hypertension. J.
chron. Dis., 18: 647-656.
SCHROEDER, H.A. (1967) Cadmium, chromium, and cardiovascular
disease. Circulation, 35: 570.
SCHROEDER, H.A. & BALASSA, J.J. (1961) Abnormal trace metals in man:
cadmium. J. chron. Dis., 14: 236-258.
SCHROEDER, H.A. & MITCHENER, M. (1971) Toxic effects of trace
elements on the reproduction of mice and rats. Arch. environ.
Health, 23: 102-106.
SCHROEDER, H.A. & VINTON, W.H., Jr (1962) Hypertension induced in
rats by small doses of cadmium. Am. J. Physiol., 202: 515-518.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H., Jr (1964) Chromium,
lead, cadmium, nickel, and titanium in mice: effect on mortality,
tumours, and tissue levels. J. Nutr., 83: 239-250.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H., Jr (1965) Chromium,
cadmium, and lead in rats: effects on life span, tumours, and tissue
levels. J. Nutr., 86: 51-66.
SCOTT, R., MILLS, E.A., FELL, G.S., HUSAIN, F.E.R., YATES, A.J.,
PATERSON, P.J., MCKIRDY, A., OTTOWAY, J.M., FITZGERALD-FINCH, O.P.,
& LAMONT, A. (1976) Clinical and biochemical abnormalities in
coppersmiths exposed to cadmium. Lancet, 2: 396-398.
SCOTT, R., AUGHEY, E., & SINCLAIR, J. (1977) Histological and
ultrastructural changes in rat kidney following cadmium injection.
Urol. Res., 5: 15-20.
SCOTT, R., PATERSON, P.J., BURNS, R., OTTOWAY, J.M., HUSAIN, F.E.R.,
FELL, G.S., DUMBUYA, S., & IGBAL, M. (1978) Hypercalciuria related
to cadmium exposure. Urology, 11: 462-465.
SCOTT, R., PATERSON, P.J., BURNS, R., FELL, G.S., & OTTOWAY, J.M.
(1979) The effects of treatment on the hypercalciuria of chronic
cadmium poisoning. Urol. Res., 7: 285-289.
SCOTT, R., HAYWOOD, J.K., BODDY, K., WILLIAMS, E.D., HARVEY, M., &
PATERSON, P.J. (1980) Whole body calcium deficit in cadmium-exposed
workers with hypercalciuria. Urology, 15: 356-359.
SHAIKH, Z.A. & HIRAYAMA, K. (1979) Metallothionein in the
extracellular fluids as an index of cadmium toxicity. Environ.
Health Perspect., 28: 267-272.
SHAIKH, Z.A. & SMITH, J.C. (1980) Metabolism of orally ingested
cadmium in humans. In: Holmstedt, B., Lauwerys, R., Mercier, M., &
Roberfroid, M., ed. Mechanisms of toxicity and hazard evaluation,
Amsterdam, Elsevier/North Holland Biomedical Press, pp. 569-574.
SHARMA, R.P., KJELLSTRÖM, T., & MCKENZIE, J.M. (1983) Cadmium in
blood and urine among smokers and non-smokers with high cadmium
intake via food. Toxicology, 29: 163-171.
SHARRETT, A.R., CARTER, A.P., ORHEIM, R.M., & FEINLEIB, M. (1982)
Daily intake of lead, cadmium, copper, and zinc from drinking-water:
the Seattle study of trace metal exposure. Environ. Res.,
28: 456-475.
SHERLOCK, J. & WALTERS, B. (1983) Dietary intake of heavy metals and
its estimation. Chem. Ind., 13: 505-508.
SHERLOCK, J., SMART, G.A., & WALTERS, B. (1983) Dietary surveys on a
population at Shipham, Somerset, United Kingdom. Sci. total
Environ., 29: 121-142.
SHIGEMATSU, I. & KAWAGUCHI, T. (1978) Environmental pollution and
health effects, Yamagata Prefecture. In: Tsuchiya, K., ed. Cadmium
studies in Japan: a review, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 199-211.
SHIGEMATSU, I., KAWAGUCHI, T., & YANAGAWA, H. (1978) Reanalysis of
the results of health examinations conducted on the general
population in the cadmium polluted areas of Japan. In: Tsuchiya, K.
ed. Cadmium studies in Japan: a review, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 253-264.
SHIGEMATSU, I., YANAGAWA, H., & KAWAGUCHI, T. (1975) [Record filling
of health examinations for inhabitants in the Cd-polluted areas (2nd
and 3rd reports)], Tokyo, Japan Public Health Association,
pp. 72-101 (Kankyo Hoken Report No. 36) (in Japanese).
SHIGEMATSU, I., MINOWA, M., YOSHIDA, T., & MIYAMOTO, K. (1979)
Recent results of health examinations on the general population in
cadmium-polluted and control areas in Japan. Environ. Health
Perspect., 28: 205-210.
SHIGEMATSU, I., MINOWA, M., YOSHIDA, T., & MIYAMOTO, K. (1980)
Epidemiological aspects of environmental cadmium and its effects in
Japan. In: Proceedings of the 2nd International Cadmium Conference,
Cannes, 6-8 February, 1979, London, Cadmium Association,
pp. 111-117.
SHIGEMATSU, I., KITAMURA, S., TAKEUCHI, J., MINOWA, M., NAGAI, M.,
USUI, T., & FUKUSHIMA, M. (1981) A retrospective mortality study on
cadmium-exposed populations in Japan. In: Recent studies on health
effects of cadmium in Japan, Tokyo, The Japan Cadmium Research
Committee, Japan Environment Agency, pp. 303-317.
SHIGEMATSU, I., MITAMURA, S., TAKEUCHI, J., MINOWA, M., NAGAI, M., &
FUKUSHIMA, M. (1982) A retrospective mortality study on
cadmium-exposed populations in Japan. In: Proceedings of the 3rd
International Cadmium Conference, Miami, Florida, 3-5 February,
1981, London, Cadmium Association, pp. 115-118.
SHIGEMATSU, I., MINOWA, M., NAGAI, M., OHMURA, T., & TAKEUCHI, K.
(1983) A retrospective mortality study on cadmium-exposed
populations in Japan (supplement): mortalities by level of pollution
in Toyama Prefecture. In: The influence of nutritional factors on
cadmium administered to monkeys, Tokyo, Japan Environment Agency,
pp. 158-174.
SHILLER, A.M. & BOYLE, E.A. (1987) Variability of dissolved trace
metals in the Mississippi River. Geochim. Cosmochim. Acta.,
51: 3273-3277.
SHIRAISHI, Y. (1975) Cytogenetic studies in 12 patients with
Itai-itai disease. Humangenetik, 27: 31-44.
SHIRAISHI, Y., KURAHASHI, H., & YOSHIDA, T.H. (1972) Chromosomal
aberrations in cultured human leukocytes induced cadmium sulfide.
Proc. Jpn. Acad., 48(21): 133-137.
SHIROISHI, K., ANAYAMA, M., TANII, M., FUKUYAMA, Y., & KUBOTA, K.
(1972) [Urinary findings of different age groups in the inhabitants
of Itai-itai disease districts.] Med. Biol., 85: 263-267 (in
Japanese).
SHIROISHI, K., KJELLSTROM, T., ANAYAMA, M., SHIMADA, T., IWATA, T.,
NISHINO, H., MATSUNAGA, A., WATANABE, M., & KUBOTA, K. (1975)
[Studies on the urinary ß-2-microglobulin level of the inhabitants
in Cd-polluted districts.] Jpn J. Hyg., 30: 71 (in Japanese).
SHIROISHI, K., KJELLSTRÖM, T., KUBOTA, K., EVRIN, P.E., ANAYAMA, M.,
VESTERBERG, O., SHIMADA, T., PISCATOR, M., IWATA, T., & NISHINO, H.
(1977) Urine analysis for detection of cadmium-induced renal
changes, with special reference to ß-2-microglobulin. Environ. Res.,
13: 407-424.
SIMPSON, W.R. (1981) A critical review of cadmium in the marine
environment. Prog. Oceanogr., 10: 1-70.
SKERFVING, S., CHRISTOFFERSON, J.O., SHUTZ, A., WELINDER, H., SPANG,
G., AHLGREN, L., & MATTSON, S. (1987) Biological monitoring, by in
vivo XRF measurements, of occupational exposure to lead, cadmium
and mercury. Biol. Trace Elem. Res., 13: 241-251.
SKOG, E. & WAHLBERG, J.E. (1964) A comparative investigation of
percutaneous absorption of metal compounds in guinea-pig by means of
the radioactive isotopes, 51Cr, 58Co, 65Zn, 110Ag, 115Cd,
203Hg. J. invest. Dermatol., 43: 187-192.
SMITH, J.P., SMITH, J.C., & MCCALL, A.J. (1960) Chronic poisoning
from cadmium fume. J. Pathol. Bacteriol., 80: 287-296.
SMITH, T. (1982) Emission inventory case studies - policy aspects.
J. Air Pollut. Control Assoc., 32(10): 1017-1020.
SMITH, T., PETTY, T.L., READING, J.C., & LAKSHMINARAYAN, S. (1976)
Pulmonary effects of chronic exposure to airborne cadmium. Am. Rev.
respir. Dis., 114: 161-169.
SMITH, T., ANDERSON, R.J., & READING, J.C. (1980) Chronic cadmium
exposures associated with kidney function effects. Am. J. ind. Med.,
1: 319-337. SNIDER, G.L., HAYES, J.A., KORTHY, A.L., & LEWIS, G.P.
(1973) Centrilobular emphysema experimentally induced by cadmium
chloride aerosol. Am. Rev. respir. Dis., 108: 40-48.
SONAWANE, B.R., NORDBERG, M., NORDBERG, G.F., & LUCIER, G.W. (1975)
Placental transfer of cadmium in rats: influence of dose and
gestational age. Environ. Health Perspect., 12: 97-102.
SORAHAN, T. (1987) Mortality from lung cancer among a cohort of
nickel cadmium battery workers: 1946-84. Br. J. ind. Med.,
44: 803-809.
SORAHAN, T. & WATERHOUSE, J.A.H. (1983) Mortality study of
nickel-cadmium battery workers by the method of regression models in
life tables. Br. J. ind. Med., 40: 293-300.
SPENCER, H., ASMUSSEN, C.R., HOLTZMAN, R.B., & KRAMER, L. (1979)
Metabolic balances of cadmium, copper, manganese, and zinc in man.
Am. J. clin. Nutr., 32: 1867-1875.
SPORN, A., DINU, I., & STOENESCU, L. (1970) Influence of cadmium
administration on carbohydrate and cellular energetic metabolism in
the rat liver. Rev. roum. Biochim., 7: 299-305.
SQUIBB, K.S., PRITCHARD, J.B., & FOWLER, B.A. (1982) Renal
metabolism and toxicity of metallothionein. In: Foulkes, E.C., ed.
Biological roles of metallothionein, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 181-190.
SQUIBB, K.S., PRITCHARD, J.B., & FOWLER, B.A. (1984)
Cadmium-metallothionein nephropathy: relationships between
ultrastructural/biochemical alterations and intra-cellular cadmium
binding. J. Pharmacol. exp. Ther., 229(1): 311-321.
SQUIRE, J.R., HARDWICKE, J., & SOOTHILL, J.F. (1962) Proteinuria.
In: Black, D.A.K., ed. Renal disease, Oxford, Blackwell Scientific
Publishers, pp. 218-231.
STACEY, N.H., CANTILENA, L.R., & KLAASSEN, C.D. (1980) Cadmium
toxicity and lipid peroxidation in isolated rat hepatocytes.
Toxicol. appl. Pharmacol., 53: 470-480.
STAESSEN, J., BULPITT, C.J., ROELS, H., BERNARD, A., FAGARD, R.,
JOOSSENS, J.V., LAUWERYS, R.R., LIJNEN, P., & AMERY, A. (1984)
Urinary cadmium and lead and their relationship to blood pressure in
a population with low average exposure. Br. J. ind. Med.,
41: 241-248.
STANESCU, D., VEITNER, C., FRANS, A., CONCETTE, L., ROELS, H.,
LAUWERYS, R.R., & BRASSEUR, L. (1977) Effects on lung of chronic
occupational exposure to cadmium. Scand. J. respir. Dis.,
58: 289-303.
STEWART, M. & HUGHES, E.G. (1981) Urinary beta-2-microglobin in the
biological monitoring of cadmium workers. Br. J. Ind. Med.,
38: 170-174.
STOEPPLER, M. & BRANDT, K. (1978) Determination of lead and cadmium
in whole blood by electrothermal atomic absorption spectroscopy. In:
Doss, M., ed. Proceedings of an International Symposium on Clinical
Biochemistry on Diagnosis and Therapy of Porphyrias and Lead
Intoxication, New York, Springer-Verlag, pp. 185-187.
STOEPPLER, M. & BRANDT, K. (1980) Contributions to automated trace
analysis. V. Determination of cadmium in whole blood and urine by
electrothermal atomic absorption spectrophotometry. Fresenius Z.
anal. Chem., 300: 372-380.
STOEPPLER, M., VALENTA, P., & NURNBERG, H.W. (1979) Applications of
independent methods and standard materials: an effective approach to
reliable trace and ultra trace analysis of metals and metalloids in
environmental and biological materials. Fresenius Z. anal. Chem.,
297: 22-34.
STONEBURNER, D.L. (1978) Heavy metals in tissues of stranded
short-finned pilot whales. Sci. total Environ., 9: 293-297.
STOWE, H.D., WILSON, M., & GOYER, R.A. (1972) Clinical and
morphological effects of oral cadmium toxicity in rabbits. Arch.
Pathol., 94: 389-405.
STRAUSS, R.H., PALMER, K.C., & HAYES, J.A. (1976) Acute lung injury
induced by cadmium aerosol. I. Evolution of alveolar cell damage.
Am. J. Pathol., 84: 561-568.
SUBRAMANIAN, K.S. & MERANGER, J.C. (1981) A rapid electrothermal
atomic absorption spectrophotometric method for cadmium and lead in
human whole blood. Clin. Chem., 27: 1866-1871.
SUGAWARA, C. & SUGAWARA, N. (1974) Cadmium toxicity for rat
intestine, especially on the absorption of calcium and phosphorus.
Jpn. J. Hyg., 28: 511-516.
SULLIVAN, M.F., HARDY, J.T., MILLER, B.M., BUSCHBOM, R.L., &
SIEWICKI, T.C. (1984) Absorption and distribution of cadmium in
mice fed diets containing either inorganic or oyster-incorporated
cadmium. Toxicol. appl. Pharmacol., 72: 210-217.
SUMINO, K., HAYAKAWA, K., SHIBATA, T., & KITAMURA, S. (1975) Heavy
metals in normal Japanese tissues. Arch. environ. Health,
30: 487-494.
SUZUKI, S. & LU, C.C. (1976) A balance study of cadmium: an
estimation of daily input, output, and retained amount in two
subjects. Ind. Health, 14: 53-65.
SUZUKI, S., SUZUKI, T., & ASHIZAWA, M. (1965) Proteinuria due to
inhalation of cadmium stearate dust. Ind. Health, 3: 73-85.
SUZUKI, S., TAGUCHI, T., & YOKOHASHI, G. (1969) Dietary factors
influencing upon the retention rate of orally-administered
115CdCl2 in mice, with special reference to calcium and protein
concentration in diet. Ind. Health, 7: 155-162.
SUZUKI, Y. (1980) Cadmium metabolism and toxicity in rats after
long-term subcutaneous administration. J. Toxicol. environ. Health,
6: 469-482.
SUZUKI, Y. (1982) Metal-binding properties of metallothionein in
extracellular fluids and its role in cadmium-exposed rats. In:
Foulkes, E.C., ed. Biological roles of metallothionein, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 25-67.
SVARTENGREN, M., ELINDER, C.G., FRIBERG, L., & LIND, B. (1986)
Distribution and concentration of cadmium in human kidney. Environ.
Res., 39: 1-7.
SVENSSON, B.-G., BJORNHAM, A., SCHUTZ, A., LETTEVALL, U., NILSSON,
A., & SKERFVING, S. (1987) Acidic deposition and human exposure to
toxic metals. Sci. total Environ., 67: 101-115.
TAKABATAKE, E. (1978a) Environmental pollution and health effects:
Oita Prefecture. In: Tsuchiya, K., ed. Cadmium studies in Japan: a
review, Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp. 215-218.
TAKABATAKE, E. (1978b) Nagasaki Prefecture. In: Tsuchiya, K., ed.
Cadmium studies in Japan: a review, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 181-192.
TAKASHIMA, M., MORIWAKI, S., & ITOKAWA, Y. (1980) Osteomalacic
change induced by long-term administration of cadmium to rats.
Toxicol. appl. Pharmacol., 54: 223-228.
TAKEBAYASHI, S. (1980a) First autopsy case, suspicious of cadmium
intoxication, from the cadmium-polluted area in Tsushima, Nagasaki
Prefecture. In: Shigematsu, I. & Nomiyama, K., ed. Cadmium-induced
osteopathy, Tokyo, Japan Public Health Association, pp. 124-138.
TAKEBAYASHI, S. (1980b) [Clinical and pathological findings of a
case with osteomalacia in a cadmium-polluted area (Tsushima Island)
(1st autopsied case)], Tokyo, Japan Public Health Association,
pp. 259-262 (Kankyo Hoken Report No. 46) (in Japanese).
TAKEBAYASHI, S. (1983a) [Clinical and pathological findings of a
case with osteomalacia in a cadmium-polluted area (Tsushima Island)
(2nd autopsied case)], Tokyo, Japan Public Health Association,
pp. 207-211 (Kankyo Hoken Report No. 49) (in Japanese).
TAKEBAYASHI, S. (1983b) [Clinical and pathological findings of a
case with diabetic nephropathy in a cadmium-polluted area (Tsushima
Island) (3rd autopsied case)], Tokyo, Japan Public Health
Association, pp. 212-217 (Kankyo Hoken Report No. 49) (in Japanese).
TAKEBAYASHI, S. (1984) [Clinical and pathological findings of a case
with osteomalacia in a cadmium-polluted area (Tsushima Island) (4th
autopsied case)], Tokyo, Japan Public Health Association,
pp. 238-249 (Kankyo Hoken Report No. 50) (in Japanese).
TAKEBAYASHI, S., SATO, T., HARADA, K., & YOSHIMURA, S. (1985)
[Clinical and pathological findings of a case with osteomalacia in a
cadmium-polluted area (Tsushima Island) (5th autopsied case)],
Tokyo, Japan Public Health Association, pp. 245-251 (Kankyo Hoken
Report No. 51) (in Japanese).
TAKEBAYASHI, S., SATO, T., HARADA, K., HIRAI, Y., & YOSHIMURA, S.
(1987a) [Clinical and pathological findings of a case with
osteomalacia in a cadmium-polluted area (Tsushima Island) (6th
autopsied case)], Tokyo, Japan Public Health Association,
pp. 294-305 (Kankyo Hoken Report No. 53) (in Japanese).
TAKEBAYASHI, S., SATO, T., HARADA, K., HIRAI, Y., & YOSHIMURA, S.
(1987b) [Clinical and pathological findings of a case with
osteomalacia in a cadmium-polluted area (Tsushima Island) (7th
autopsied case)], Tokyo, Japan Public Health Association,
pp. 306-319 (Kankyo Hoken Report No. 53) (in Japanese).
TAKEBAYASHI, S., HIRAI, Y., & YOSHIMURA, S. (1988a) [Clinical and
pathological findings of a case with osteomalacia in a
cadmium-polluted area (Tsushima Island) (8th autopsied case)],
Tokyo, Japan Public Health Association, pp. 187-192 (Kankyo Hoken
Report No. 54) (in Japanese).
TAKEBAYASHI, S., HIRAI, Y., & YOSHIMURA, S. (1988b) [Clinical and
pathological findings of a case with osteomalacia in a
cadmium-polluted area (Tsushima Island) (9th autopsied case)],
Tokyo, Japan Public Health Association, pp. 193-199 (Kankyo Hoken
Report No. 54) (in Japanese).
TAKEBAYASHI, S., HIRAI, Y., & YOSHIMURA, S. (1988c) [Clinical and
pathological findings of a case with osteomalacia in a
cadmium-polluted area (Tsushima Island) (10th autopsied case)],
Tokyo, Japan Public Health Association, pp. 200-206 (Kankyo Hoken
Report No. 54) (in Japanese).
TAKEBAYASHI, S., HARADA, K., & YOSHIMURA, S. (1988d) [Clinical and
pathological findings of a case with osteomalacia in a
cadmium-polluted area (Tsushima Island) (11th autopsied case)],
Tokyo, Japan Public Health Association, pp. 207-213 (Kankyo Hoken
Report No. 54) (in Japanese).
TAKENAKA, S., OLDIGES, H., KOENIG, H., HOCHRAINER, D., &
OBERDOERSTER, G. (1983) Carcinogenicity of cadmium aerosols in
Wistar rats. J. Natl Cancer Inst., 70: 367-371.
TANAKA, K. (1982) Effects of hepatic disorders on the fate of
cadmium in rats. In: Foulkes, E.C., ed. Biological roles of
metallothionein, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 237-249.
TANAKA, K., MIN, K.-S., ONOSAKA, S., FUKUHARA, C., & UEDA, M. (1985)
The origin of metallothionein in red blood cells. Toxicol. appl.
Pharmacol., 78: 63-66.
TARASENKO, N.YU. & VOROBJEVA, R.S. (1973) [Hygienic problems
connected with the use of cadmium.] Vestn. Akad. Med. Nauk. SSR,
28: 37-43 (in Russian with English abstract).
TARASENKO, N.YU., VOROBJEVA, R.S., SPIRIDINOVA, V.S., & SABALINA,
L.P. (1974) Experimental investigation of toxicity of cadmium and
zinc caprylates. J. Hyg. Epidemiol. Microbiol. Immunol.,
18: 144-153.
TARASENKO, N.YU., VOROBJEVA, R.S., SABALINA, L.P., & CVETKOVA, R.P.
(1975) [Cadmium in the environment and its effect on calcium
metabolism.] Gig. i Sanit., 9: 22-25 (in Russian).
TASK GROUP ON LUNG DYNAMICS (1966) Deposition and retention models
for internal dosimetry of the human respiratory tract. Health Phys.,
12: 173-208.
TASK GROUP ON METAL INTERACTIONS (1978) Factors influencing
metabolism and toxicity of metals: a consensus report. Environ.
Health Perspect., 25: 3-42.
TASK GROUP ON METAL TOXICITY (1976) Conceptual considerations:
critical organ, critical concentration in cells and organs, critical
effect, subcritical effect, dose-effect and dose-response
relationships. In: Nordberg, G.F., ed. Effects and dose-response
relationships of toxic metals, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 10-13.
TATI, M., KATAGIRI, Y., & KAWAI, M. (1976) Urinary and faecal
excretion of cadmium in normal Japanese: an approach to non-toxic
levels of cadmium. In: Nordberg, G.F., ed. Effects and dose-response
relationships of toxic metals, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 331-341.
TAYLOR, B.A., HEINIGER, H.J., & MEIER, H. (1973) Genetic analysis of
resistance to cadmium-induced testicular damage in mice (37380).
Proc. Soc. Exp. Biol. Med., 143: 629-633.
TECULESCU, D.B. & STANESCU, D.C. (1970) Pulmonary function in
workers with chronic exposure to cadmium oxide fumes. Int. Arch.
Arbeitsmed., 26: 335-345.
TEMPLETON, D.M., BANERJEE, D., & CHERIAN, M.G. (1985)
Metallothionein synthesis and localization in relation to metal
storage in rat liver during gestation. Can. J. Biochem. cell Biol.,
63: 16-22.
TENNANT, C.J. (1984) Cadmium in the environment and high risk
population groups, Birmingham, University of Aston (Ph.D. Thesis).
TERHAAR, C.J., VIS, E., ROUDABUSH, R.L., & FASSETT, D.W. (1965)
Protective effects of low doses of cadmium chloride against
subsequent high oral doses in the rat. Toxicol. appl. Pharmacol.,
7: 500-505.
THIEME, R., SCHRAMEL, P., & KURZ, E. (1977) [Trace element
concentration in the human placenta in a heavily-contaminated
environment.] Geburtshilfe Frauenheilkd., 37: 756-761 (in German).
THOMAS, B.J., HARVEY, T.C., CHETTLE, D.R., MCLELLAN, J.S., &
FREMLIN, J.H. (1979) A transportable system for the measurement of
liver cadmium in vivo. Phys. Med. Biol., 24: 432-437.
THORNTON, I. (1988) The Shipham report. An investigation into
cadmium contamination and its implications for human health. Metal
content of soils and dusts. Sci. total Environ., 75: 21-39.
THUN, M.T., SCHNORR, T.M., SMITH, A.B., HALPERIN, W.E., & LEMEN,
R.A. (1985) Mortality among a cohort of U.S. cadmium production
workers: An update. J. Natl Cancer Inst., 74: 325-333.
THUN, M.J., OSORIO, A.M., SCHOBER, S., HANNON, W.H., & HALPERIN, W.
(1989) Nephropathy in cadmium workers - assessment of risk from
airborne occupational cadmium exposure. Br. J. ind. Med.,
46: 689-697.
TIPTON, I.H., SCHROEDER, H.A., PERRY, H.M., & COOK, M.J. (1960)
Trace elements in human tissues. Part III. Subjects from Africa, the
Near and Far East, and Europe. Health Phys., 11: 403-451.
TJELL, J.C., HANSEN, J.A., CHRISTENSEN T.H., & HOVMAND, M.F. (1981)
Prediction of cadmium concentrations in Danish soils. In: L'Hermite,
P. & Ott, H., ed. Characterization, treatment and use of sewage
sludge, Dordrecht, Reidel Publishing Co., pp. 652-664.
TJELL, J.C., CHRISTENSEN, T.H., & BRO-RASMUSSEN, F. (1983) Cadmium
in soil and terrestrial biota, with emphasis on the Danish
situation. Ecotoxicol. environ. Saf., 7: 122-140.
TOHYAMA, C., SHAIKH, Z.A., ELLIS, K.J., & COHN, S.H. (1981a)
Metallothionein excretion in urine upon cadmium exposure: its
relationship with liver and kidney cadmium. Toxicology, 20: 181-191.
TOHYAMA, C., SHAIKH, Z.A., NOGAWA, K., KOBAYASHI, E., & HONDA, R.
(1981b) Elevated urinary excretion of metallothionein due to
environmental cadmium exposure. Toxicology, 22: 289-297.
TOPPING, M.D., FORSTER, H.W., DOLMAN, C., LUCZYNSKA, C.M., &
BERNARD, A.M. (1986) Measurement of urinary retinol-binding protein
by enzyme-linked immunosorbent assay and its application to
detection of tubular proteinuria. Clin. Chem., 32: 1863-1866.
TSUCHIYA, K. (1967) Proteinuria of workers exposed to cadmium fume.
The relationship to concentration in the working environment. Arch.
environ. Health, 14: 875-880.
TSUCHIYA, K. (1969) Causation of Ouch-ouch disease (Itai-itai byo):
an introduction review. Part 1. Nature of the disease. Keio J. Med.,
18: 181-194.
TSUCHIYA, K. (1976) Proteinuria of cadmium workers. J. occup. Med.,
18: 463-466.
TSUCHIYA, K., ed. (1978) Cadmium studies in Japan: a review,
Amsterdam, Oxford, New York, Elsevier Science Publishers, 376 pp.
TSUCHIYA, K. & IWAO, S. (1978) Interrelationships among zinc,
copper, lead, and cadmium in food, faeces, and organs of humans.
Environ. Health Perspect., 25: 119-124.
TSUCHIYA, K. & NAKAMURA, K.I. (1978) Environmental pollution and
health effects: Hyogo prefecture. In: Tsuchiya, K., ed. Cadmium
studies in Japan: a review, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 146-167.
TSUCHIYA, K. & SUGITA, M. (1971) A mathematical model for deriving
the biological half-life of a chemical. Nord. Hyg. Tidskr.,
53: 105-110.
TSUCHIYA, K., SEKI, Y., & SUGITA, M. (1976) Cadmium concentrations
in the organs and tissues of cadavers from accidental deaths. Keio
J. Med., 25: 83-90.
TSUCHIYA, K., IWAO, S., SUGITA, M., & SAKURAI, H. (1979) Increased
urinary ß-2-microglobulin in cadmium exposure: dose-effect
relationship and biological significance of ß-2-microglobulin.
Environ. Health Perspect., 28: 147-153.
URUNO, K., OTOYAMA, S., NAKANO, N., & ITAGAKI, Z. (1975) [Results
of health examination for inhabitants in Yoshinokawa area polluted
with cadmium], Tokyo, Japan Public Health Association, pp. 109-113
(Kankyo Hoken Report No. 36) (in Japanese).
USHIO, F. & DOGUCHI, M. (1977) Dietary intakes of some chlorinated
hydrocarbons and heavy metals estimated on the
experimentally-prepared diets. Bull. environ. Contam. Toxicol.,
17: 707-711.
US PUBLIC HEALTH SERVICE (1942) Cadmium poisoning. Public Health
Rep., 57: 602-612.
VAHTER, M., ed. (1982) Assessment of human exposure to lead and
cadmium through biological monitoring, Stockholm, National Swedish
Institute of Environmental Medicine and Department of Environmental
Hygiene, Karolinska Institute, 136 pp (Report prepared for the
United Nations Environment Programme and the World Health
Organization).
VALETAS, P. (1946) "Cadmiose" ou l'intoxication du cadmium, Paris,
School of Medicine, 40 pp (Medical Faculty Thesis Report).
VAN AALST, R.M., VAN ARDENNE, R.A.M., DE KRUK, J.F., & LEMS, T.
(1983a) Pollution of the North Sea from the atmosphere, Apeldoorn,
The Netherlands Organization for Applied Scientific Research (TNO)
(Report No. C182/152).
VAN AALST, R.M., DUYZER, J.H., & VELDT, C. (1983b) Atmospheric
deposition of lead and cadmium in the southern part of the North
Sea. I. Emission and preliminary model calculations, Apeldoorn, The
Netherlands Organization for Applied Scientific Research (TNO)
(Report No. R83/222).
VANDER, A.J. (1962) Cadmium enhancement of proximal tubular sodium
reabsorption. Am. J. Physiol., 203: 1005-1007.
VANDER MALLIE, R.J. & GARVEY, J.S. (1979) Radioimmunoassay of
metallothioneins. J. biol. Chem., 254: 8416-8421.
VENS, M.D. & LAUWERYS, R.R. (1982) Détermination simultanée du plomb
et du cadmium dans le sang et l'urine par le couplage des techniques
de chromatographie sur résine échangeuse d'ions et de
spectrophotométrie d'absorption atomique. Arch. Mal. prof. Méd.
Trav. Sécur. soc., 33: 97-105.
VESTERBERG, O. & WRANGSKOGH, K. (1978) Determination of cadmium in
urine by graphite furnace atomic absorption spectroscopy. Clin.
Chem., 24: 681-685.
VOROBJEVA, R.S. (1957) [Investigations of the nervous system
function in workers exposed to cadmium oxide.] Neuropatol.
Psikkiatr., 57: 385-388 (in Russian).
VOROBJEVA, R.S. (1958) [About occupational cadmium oxide
poisonings.] J. Hyg. Epidemiol. Microbiol. Immunol., 11: 244-249 (in
German).
VOROBJEVA, R.S. & BUBNOVA, N.I. (1981) [Effects of elemental cadmium
and telluric cadmium on organisms.] Gig. Trud. prof. Zabol.,
2: 42-43 (in Russian).
VOROBJEVA, R.S. & EREMEEVA, E.P. (1980) [Cardiovascular function in
workers exposed to cadmium.] Gig. i Sanit., 10: 22-25 (in Russian).
VOROBJEVA, R.S. & SABALINA, L.P. (1975) [Experimental investigations
of toxic properties of various cadmium compounds.] Gig. i Sanit.,
2: 102-104 (in Russian).
VOSTAL, J.J. (1976) Dose-response relationship of mercury and
cadmium effects on renal cadmium transport. In: Nordberg, G.F., ed.
Effects and dose-response relationships of toxic metals, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 299-308.
VUORI, E., HUUNAN-SEPPALA, A., KILPIO, J.O., & SALMELA, S.S. (1979)
Biologically-active metals in human tissues. II. The effect of age
on the concentration of cadmium in aorta, heart, kidney, liver,
lung, pancreas, and skeletal muscle. Scand. J. Work Environ. Health,
5: 16-22.
WAALKES, M.P., POISNER, A.M., WOOD, G.W., & KLAASSEN, C.D. (1984)
Metallothionein-like proteins in human placenta and fetal membranes.
Toxicol. appl. Pharmacol., 74: 179-184.
WADE, G.G. (1987) Marked synergism of dimethylnitrosamine
carcinogenesis in rats exposed to cadmium. Cancer Res.,
47: 6606-6613.
WALSH, J.J. & BURCH, G.E. (1959) The rate of disappearance from
plasma and subsequent distribution of radiocadmium ( 115 m Cd) in
normal dogs. J. clin. Med., 54: 59-65.
WALTERS, B. & SHERLOCK, J. (1981) Studies on the dietary intake of
heavy metals. In: Proceedings of the International Conference on
Heavy Metals in the Environment, Amsterdam, September 1981,
Edinburgh, CEP Consultants Ltd, pp. 506-512.
WATANABE, H. & MURAYAMA, H. (1975) A study on health effect indices
concerning population in a cadmium-polluted area. In: International
Symposium on Recent Advances in the Assessment of the Health Effects
of Environmental Pollution, Luxembourg, December 1975, Luxembourg,
Commission of the European Communities, pp. 2185-2195 (Report EURO
5360).
WATANABE, I., KOIZUMI, A., FUJITA, H., KUMAI, M., & IKEDA, M. (1985)
Dietary cadmium intakes of farmers in nonpolluted areas in Japan,
and the relation with blood cadmium levels. Environ. Res.,
37(1): 33-43.
WATANABE, T., SHIMADA, T., & ENDO, A. (1979) Mutagenic effects of
cadmium on mammalian oocyte chromosomes. Mutat. Res., 67: 349-356.
WEAST, R.C. (1974) Handbook of chemistry and physics, 55th ed.,
Cleveland, Ohio, CRC Press, 500 pp.
WEBB, M. (1972) Protection by zinc against cadmium toxicity.
Biochem. Pharmacol., 21: 2767-2771.
WEBB, M. & CAIN, K. (1982) Functions of metallothionein. Biochem.
Pharmacol., 31: 137-142.
WEBB, M. & ETIENNE, A.T. (1977) Studies on the toxicity and
metabolism of cadmium-thionein. Biochem. Pharmacol., 26: 25-30.
WESTER, P.O. (1974) Trace element balances in relation to variations
in calcium intake. Atherosclerosis, 20: 207-215.
WHANGER, P.D. (1979) Cadmium effects in rats on tissue iron,
selenium, and blood pressure: blood and hair cadmium in some Oregon
residents. Environ. Health Perspect., 28: 115-121.
WHO (1980) Recommended health-based limits in occupational exposure
to heavy metals. Report of a study group, Geneva, World Health
Organization (Technical Report Series No. 647).
WHO (1984) Guidelines for drinking-water quality: Health criteria
and other supporting information, Geneva, World Health Organization,
Vol. 2, pp. 84-90.
WHO (1986) Health impact of acidic deposition. Sci. total Environ.,
52: 157-187.
WHO (1987) Air quality guidelines for Europe, Copenhagen, World
Health Organization, Regional Office for Europe, pp. 200-209
(European Series, Vol. 23).
WHO (1989) Cadmium. In: Evaluation of certain food additives and
contaminants. Thirty-third Report of the Joint FAO/WHO Expert
Committee on Food Additives, Geneva, World Health Organization,
pp. 28-31 (Technical Report Series No. 776).
WILLIAMS, C.H. & DAVID, D.J. (1973) The effect of superphosphate on
the cadmium content of soils and plants. Aust. J. Soil Res.,
11: 43-56.
WILLIAMS, C.H. & DAVID, D.J. (1976) The accumulation in soil of
cadmium residues from phosphate for fertilizers and their effect on
the cadmium content of plants. Soil Sci., 121: 86-93.
WILSON, B. (1988a) Investigation of trace metals in the aqueous
environment: Final report (January 1986-December 1987), Houston,
Texas Southern University, p. 28 (Report No. DOE/CH/10255-T1,
prepared for the US Department of Energy, Washington).
WILSON, D.N. (1988b) Cadmium - market trends and influences. In:
Cadmium 87. Proceedings of the 6th International Cadmium
Conference, London, Cadmium Association, pp. 9-16.
WILSON, R.H., DE EDS, F., & COX, A.J., Jr (1941) Effects of
continued cadmium feeding. J. Pharmacol. exp. Ther., 71: 222-235.
WOLFF, E.W. & PEEL, D.A. (1985) The record of global pollution in
polar snow and ice. Nature (Lond.), 313: 535-540.
WOLNIK, K.A., FRICKE, F.L., CAPER, S.G., BRAUDE, G.L., MEYER, M.W.,
SATZGER, R.D., & BONNIN, E. (1983) Elements in major raw
agricultural crops in the United States. I. Cadmium and lead in
lettuce, peanuts, potatoes, soybeans, sweet corn, and wheat. J.
agric. food Chem., 31: 1240-1244.
WOLNIK, K.A., FRICKE, F.L., CAPAR, S.G., MEYER, M.W., & SATZGER,
R.D. (1985) Elements in major raw agricultural crops in the United
States. 3. Cadmium, lead, and eleven other elements in carrots,
field corn, onion, rice, spinach, and tomatoes. J. agric. food
Chem., 33: 807-811.
YAMAGATA, H. & IWASHIMA, K. (1975) Average cadmium intake of the
Japanese people. Bull. Inst. Public Health (Tokyo), 24: 18-24.
YAMAGATA, N. & SHIGEMATSU, I. (1970) Cadmium pollution in
perspective. Bull. Inst. Public Health (Tokyo), 19: 1-27.
YAMAGATA, N., IWASHIMA, K., & NAGAI, T. (1974) [Gastrointestinal
absorption of cadmium in normal humans], Tokyo, Japan Public Health
Association, pp. 84-85 (Kankyo Hoken Report No. 31) (in Japanese).
YAMAMOTO, Y. (1972) [Present status of cadmium environmental
pollution], Tokyo, Japan Public Health Association, pp. 7-12 (Kankyo
Hoken Report No. 11) (in Japanese).
YOSHIKAWA, H., KAWAI, K., SUZUKI, Y., NOZAKI, K., & OHSAWA, M.
(1975) [Studies on cadmium effects in living organisms: impairment
due to inhalation], Tokyo, Japanese Environment Agency, 40 pp (in
Japanese).
RESUME ET CONCLUSIONS
1. Identité, propriétés physiques et chimiques et méthodes
d'analyse
Il existe plusieurs méthodes pour le dosage du cadmium dans les
échantillons biologiques. La plus utilisée est la spectrométrie
d'absorption atomique, mais elle nécessite un traitement minutieux
de la prise d'essai et une correction pour tenir compte des
interférences lorsqu'elle est appliquée à des échantillons de faible
teneur en cadmium. Il est tout à fait souhaitable que l'analyse
s'accompagne d'un programme d'assurance de la qualité. A l'heure
actuelle, il est possible, dans des conditions optimales, de doser
environ 0,1 µg/litre dans l'urine et le sang et de 1 à 10 µg/litre
dans les aliments et les tissus.
2. Sources d'exposition humaine et environnementale
Le cadmium est un élément relativement rare et les méthodes
actuelles d'analyse indiquent, dans les divers compartiments de
l'environnement, des concentrations beaucoup plus faibles que les
mesures antérieures. Pour l'instant, il n'est pas possible de
déterminer si l'activité humaine est à l'origine d'un accroissement
de la teneur des calottes polaires en cadmium à l'échelle des temps
historiques.
La production commerciale de cadmium a commencé au tournant du
siècle. La consommation a changé de caractère ces dernières années
avec un recul sensible de la galvanoplastie et une utilisation
accrue dans la fabrication de batteries et de composants
électroniques spéciaux. Dans la plupart des cas, le cadmium est
utilisé sous la forme de dérivés peu concentrés, ce qui rend le
recyclage indispensable. Les restrictions à l'usage du cadmium
imposées par certains pays pourraient avoir des répercussions
importantes sur ces applications.
Les activités humaines entraînent la libération de cadmium dans
l'air, le sol et l'eau. D'une façon générale, les deux principales
sources de contamination sont la production et la consommation de
cadmium et d'autres métaux non ferreux ainsi que le rejet de déchets
contenant du cadmium. Dans les zones proches de mines ou de
fonderies de métaux non ferreux, la contamination par le cadmium est
souvent importante.
Plus le sol contient de cadmium, plus la quantité fixée par les
plantes est importante. L'exposition humaine par l'intermédiaire des
cultures sera donc sensible à toute augmentation de la teneur du sol
en cadmium. La fixation par les plantes est plus importante dans les
sols de faible pH. Les processus qui acidifient le sol (pluies
acides, par exemple) sont donc susceptibles de provoquer une
augmentation de la concentration moyenne du cadmium dans les denrées
alimentaires. Dans certaines régions du monde, l'utilisation
d'engrais phosphatés et les dépôts d'origine atmosphérique
constituent une source non négligeable de contamination des terres
arables; les boues d'égout peuvent aussi, localement, entraîner une
forte pollution. A l'avenir, ces sources risquent d'accroître la
contamination du sol et celle des cultures, ce qui débouchera sur
une exposition plus importante au cadmium par la voie alimentaire.
Dans certaines régions, on peut constater une augmentation de la
teneur des aliments en cadmium.
Les animaux et les plantes comestibles qui vivent à l'état
sauvage, comme les coquillages, les crustacés et les champignons
accumulent naturellement le cadmium. Comme chez l'homme, on constate
une augmentation de la concentration en cadmium dans le foie et les
reins des chevaux et de certains animaux terrestres vivant à l'état
sauvage. La consommation régulière de ces abats peut entraîner une
exposition accrue. Dans les reins de certains vertébrés marins on
trouve des concentrations assez fortes en cadmium, qui, même si
elles sont d'origine naturelle, n'en provoquent pas moins des
lésions au niveau de ces organes.
3. Concentrations dans l'environnement et exposition humaine
La principale voie d'exposition au cadmium des non fumeurs est
la voie alimentaire. Les autres voies sont peu importantes. Chez les
fumeurs, l'apport de cadmium par le tabac est notable. Dans les
zones contaminées, l'exposition d'origine alimentaire peut atteindre
plusieurs centaines de microgrammes par jour. Chez les travailleurs
exposés, la principale voie de pénétration est la voie pulmonaire,
après inhalation d'air contaminé sur le lieu de travail. Le
tabagisme et la consommation d'aliments contaminés ajoutent encore à
la charge de cadmium de l'organisme.
4. Cinétique et métabolisme chez les animaux de laboratoire et
chez l'homme
Les données tirées de l'expérimentation animale et humaine
montrent que l'absorption est plus importante au niveau des poumons
qu'au niveau des voies digestives. Selon l'espèce chimique en cause,
la granulométrie et la solubilité dans les liquides biologiques, le
taux d'absorption peut atteindre 50% après inhalation. L'absorption
gastro-intestinale dépend du régime alimentaire et de l'état
nutritionnel. En particulier, le bilan martial est particulièrement
important. En moyenne, le cadmium total contenu dans les aliments
est absorbé à hauteur de 5%, avec un intervalle de variation de
1%-20% selon les individus. Il existe un gradient materno-foetal de
cadmium. Le cadmium peut également parvenir jusqu'au foetus, mais en
faibles quantités malgré son accumulation dans le placenta.
Le cadmium absorbé au niveau des poumons ou des voies
digestives s'accumule principalement dans le foie et les reins où il
représente plus de la moitié de la charge totale de l'organisme.
Plus l'exposition est intense, plus l'accumulation du cadmium dans
le foie est importante. En principe, l'excrétion est lente et la
période biologique du cadmium dans les muscles, les reins, le foie
et l'organisme dans son ensemble, est très longue, de l'ordre de
plusieurs décennies. La teneur en cadmium de la plupart des tissus
augmente avec l'âge. C'est en général dans le cortex rénal que la
concentration est la plus élevée, mais en cas d'exposition
excessive, elle peut l'être encore plus dans le foie. Chez les
personnes exposées atteintes de lésions rénales, il y a augmentation
de l'excrétion urinaire du cadmium de sorte que la période
biologique pour l'ensemble de l'organisme est raccourcie. Du fait
des lésions, le rein perd son cadmium et ces malades finissent par
présenter une concentration rénale de cadmium plus faible que les
individus en bonne santé soumis à la même exposition.
La métallothionéine est une protéine qui joue un rôle important
dans le transport et le stockage du cadmium et d'autres métaux. Le
cadmium est capable d'induire la synthèse de cette protéine dans de
nombreux organes, notamment le foie et le rein. En fixant le cadmium
intracellulaire, la métallothionéine protège les tissus contre les
effets toxiques de ce métal. Il est possible, par conséquent, que le
cadmium non lié à la métallothionéine ait une responsabilité dans
les lésions tissulaires. On ignore quels peuvent être les autres
complexes du cadmium présents dans les liquides biologiques.
L'excrétion urinaire du cadmium dépend de divers facteurs:
charge totale de l'organisme, exposition récente et lésions rénales.
Chez les individus peu exposés, la concentration urinaire du cadmium
dépend principalement de la charge de l'organisme. En cas de lésions
rénales dues au cadmium, ou même sans lésions de ce genre mais en
présence d'une exposition excessive, il y a augmentation de
l'excrétion urinaire. Les individus exposés au cadmium en excrètent
davantage dans leurs urines lorsqu'ils présentent une protéinurie.
Après cessation d'une exposition intense, le taux urinaire décroît,
même s'il y a persistance des lésions rénales. Il faut donc prendre
plusieurs facteurs en considération pour interpréter le cadmium
urinaire. L'excrétion par la voie digestive est sensiblement
équivalente à l'excrétion urinaire mais elle est difficile à
mesurer. L'excrétion par d'autres voies (lactation, sueur ou passage
transplacentaire) est négligeable.
La teneur des matières fécales en cadmium est un bon indicateur
d'une ingestion récente en l'absence d'exposition par la voie
respiratoire. Le cadmium sanguin est présent principalement dans les
hématies, la concentration plasmatique étant très faible. Il existe
au moins deux compartiments dans le sang, l'un qui correspond à une
exposition récente, avec une demi-vie de 2-3 mois et l'autre, qui
est probablement lié à la charge totale de l'organisme et se
caractérise par une demi-vie de plusieurs années.
5. Effets sur les animaux de laboratoire
Une forte exposition par la voie respiratoire entraîne un
oedème mortel du poumon. Après injection d'une seule dose,
apparaissent des lésions testiculaires, une nécrose ovarienne, des
lésions hépatiques et une atteinte des petits vaisseaux. L'ingestion
de fortes doses provoque des lésions de la muqueuse gastrique et
intestinale.
Une exposition prolongée par la voie respiratoire ou une
administration intratrachéenne entraîne des altérations pulmonaires
de nature inflammatoire ainsi qu'une fibrose et donne au tissu
pulmonaire un aspect qui évoque l'emphysème. L'administration
prolongée par voie orale ou parentérale affecte principalement le
rein mais elle a aussi des effets sur le foie et les systèmes
hématopoiétique, immunitaire et cardio-vasculaire ainsi que sur le
squelette. Chez certaines espèces et dans des conditions
déterminées, on a provoqué une hypertension et constaté des effets
sur le squelette. C'est le stade de la gestation où se produit
l'exposition qui conditionne les effets tératogènes et les lésions
placentaires et il peut y avoir interaction avec le zinc.
Ce sont les effets aigus produits par l'inhalation du cadmium
ainsi que sa néphrotoxicité chronique qui sont les plus importants
du point de vue de l'exposition humaine. En cas d'exposition
prolongée, c'est le rein qui est l'organe critique. Les effets sont
caractérisés par une lésion des cellules tubulaires entraînant une
insuffisance tubulaire parfois accompagnée d'insuffisance
glomérulaire. L'insuffisance tubulaire a pour conséquence une
perturbation du métabolisme du calcium et de la vitamine D. Selon
certains travaux, ces troubles pourraient provoquer une ostéomalacie
ou une ostéoporose. Cependant ces résultats n'ont pas été confirmés
par d'autres études. On ne peut exclure un effet direct du cadmium
sur la minéralisation de l'os. Chez l'animal de laboratoire, les
effets toxiques du cadmium dépendent de certains facteurs génétiques
et nutritionnels, des interactions avec d'autres métaux, en
particulier le zinc, et d'un premier traitement éventuel par le
cadmium susceptible d'avoir stimulé la synthèse de métallothionéine.
En 1976 et 1987, le Centre international de recherche sur le
cancer a admis posséder suffisamment de preuves que l'injection de
chlorure, de sulfate, de sulfure et d'oxyde de cadmium pouvait
entraîner l'apparition d'un sarcome local chez le rat et, dans le
cas des deux premiers composés, de tumeurs testiculaires
interstitielles chez ce même animal et chez la souris. Toutefois, il
a considéré que les études basées sur l'administration par voie
orale ne permettaient pas de procéder à une évaluation. Lors
d'études au cours desquelles on a fait respirer à des rats des
aérosols de sulfate de cadmium, des vapeurs d'oxyde de cadmium et de
la poussière de sulfate de cadmium, on a observé une forte incidence
de cancers primitifs du poumon, avec probablement une relation entre
la dose et la réponse. Toutefois, ces résultats n'ont pu être
reproduits chez d'autres espèces. Les travaux relatifs aux effets
génotoxiques du cadmium ont donné des résultats contradictoires.
6. Effets sur l'homme
Une forte exposition à des vapeurs d'oxyde de cadmium par la
voie respiratoire entraîne une pneumopathie aiguë, accompagnée d'un
oedème du poumon qui peut être mortel. L'ingestion de grandes
quantités de sels solubles de cadmium provoque une gastro-entérite
aiguë.
A la suite d'une exposition professionnelle prolongée au
cadmium, on a observé de graves effets chroniques, principalement au
niveau des poumons et des reins. On a également observé une
néphrotoxicité chronique dans la population générale.
Les altérations pulmonaires consécutives à une exposition
professionnelle intense sont essentiellement caractérisées par une
obstruction des voies aériennes. Si l'exposition se poursuit, les
légers troubles ventilatoires initiaux peuvent déboucher sur une
insuffisance respiratoire. On a observé un accroissement de la
mortalité par pneumopathie obstructive chez des travailleurs
fortement exposés, comme cela se produisait auparavant.
L'accumulation de cadmium dans le cortex rénal entraîne des
troubles de la fonction tubulaire et une réabsorption insuffisante,
par exemple, des protéines, du glucose et des acides aminés.
L'accroissement de l'excrétion urinaire des protéines de faible
masse moléculaire est un signe caractéristique de l'insuffisance
tubulaire. Parfois il y a aussi baisse du taux de filtration
glomérulaire. L'augmentation du cadmium urinaire est corrélée avec
une protéinurie de faible masse moléculaire et, en l'absence
d'exposition, peut servir d'indicateur de l'atteinte rénale. Dans
les cas graves, les effets tubulaires et glomérulaires s'ajoutent
s'accompagnant parfois d'une élévation du taux sanguin de
créatinine. Chez la plupart des travailleurs et autres personnes, la
protéinurie due à une néphropathie cadmique est irréversible.
Parmi les autres effets, on peut citer les troubles du
métabolisme calcique, l'hypercalciurie et la formation de calculs
rénaux. Une exposition intense au cadmium peut, selon toute
probabilité lorsqu'elle s'accompagne d'autres facteurs comme une
carence nutritionnelle, provoquer l'apparition d'une ostéoporose
et/ou d'une ostéomalacie.
On est fondé à penser qu'une exposition professionnelle
prolongée au cadmium peut favoriser l'apparition d'un cancer du
poumon, mais la présence de facteurs de confusion ne facilite pas
l'interprétation des observations effectuées sur les travailleurs
exposés. En ce qui concerne le cancer de la prostate, les données ne
sont pas concluantes et ne confirment pas, en tout cas, l'hypothèse
antérieure d'une relation de cause à effet.
A l'heure actuelle, on ne possède pas de preuve convaincante
que le cadmium provoque une hypertension essentielle. La plupart des
données contredisent cette hypothèse et rien n'indique un
accroissement de la mortalité par maladie cardio-vasculaire ou
accident vasculaire cérébral chez les personnes exposées.
D'après les résultats d'études relatives à des groupes exposés
de par leur profession ou simplement du fait de leur environnement
général, il semble que la prévalence des effets néphrotoxiques soit
liée à la durée et à l'intensité de l'exposition.
Chez des ouvriers de l'industrie du cadmium, on a signalé,
après 10 à 20 ans d'exposition à des concentrations de l'ordre de
20-50 µg par mètre cube, une augmentation de la prévalence des cas
de protéinurie à faible masse moléculaire.
Dans des zones polluées, où l'on évalue l'apport de cadmium par
voie orale à environ 140-260 µg/jour, on a observé des effets du
genre protéinurie à faible masse moléculaire chez des sujets exposés
pendant une longue période. On trouvera à la section 8 une
estimation plus précise de la relation dose-réponse.
7. Evaluation des risques pour la santé humaine
7.1 Conclusions
On estime que le rein est l'organe cible tant dans la
population générale que chez les groupes professionnellement
exposés. Une exposition prolongée par inhalation entraîne
l'apparition d'un syndrome respiratoire obstructif chez certains
groupes professionnels. Certains détails incitent à penser que cette
exposition au cadmium pourrait favoriser l'apparition d'un cancer du
poumon, mais les observations effectuées sur des travailleurs
exposés sont difficiles à interpréter en raison de la présence de
facteurs de confusion.
7.1.1 Population générale
Pour la plupart des individus, les aliments constituent la
principale voie d'exposition au cadmium. Dans la plupart des régions
non polluées par ce métal, l'apport alimentaire journalier est de
l'ordre de 10-40 µg. Dans les zones polluées, il peut atteindre
plusieurs centaines de microgrammes par jour. Dans les zones non
polluées, l'apport dû au tabac peut être égal à l'apport alimentaire
chez les gros fumeurs.
D'après un modele biologique, on estime qu'il existe une
association entre l'exposition au cadmium et l'excrétion urinaire de
protéines de faible masse moléculaire chez les sujets qui absorbent
pendant toute leur vie une dose journalière d'environ 140-260 µg de
cadmium, ce qui correspond à une dose cumulée d'environ 2000 mg ou
davantage.
7.1.2 Groupes professionnellement exposés
Dans ce cas, l'exposition est essentiellement respiratoire,
mais il s'y ajoute l'apport alimentaire et tabagique. La teneur
totale de l'air en cadmium varie selon les pratiques en matière
d'hygiène industrielle et selon le lieu de travail. Il existe une
relation de type exposition-réponse entre la teneur de l'air en
cadmium et la protéinurie de faible masse moléculaire. La prévalence
de cette protéinurie peut augmenter après 10 à 20 ans d'exposition à
des concentrations de cadmium de l'ordre de 20-50 µg par mètre cube.
In vivo, le dosage du cadmium dans les reins et le foie de sujets
plus ou moins exposés a montré que 10 % environ des travailleurs
dont le cortex rénal contenait 200 mg/kg de cadmium et 50 % de ceux
chez qui cette concentration atteignait 300 mg/kg, feraient un jour
ou l'autre une protéinurie par insuffisance tubulaire.
RESUMEN Y CONCLUSIONES
1. Identidad, propiedades físicas y químicas, y métodos analíticos
Se dispone de varios métodos para determinar el cadmio presente
en el material biológico. El más utilizado es la espectrometría de
absorción atómica, aunque el análisis de muestras con
concentraciones bajas de cadmio exige un tratamiento cuidadoso de
las muestras y correcciones para tener en cuenta la interferencia.
Se recomienda encarecidamente acompañar el análisis con un programa
de garantía de la calidad. Actualmente, en circunstancias ideales
pueden determinarse concentraciones de alrededor de 0,1 µg/litro en
la orina y la sangre y de 1-10 µg/kg en alimentos y muestras de
tejidos.
2. Fuentes de exposición humana y ambiental
El cadmio es un elemento relativamente raro; los procedimientos
analíticos actuales indican que las concentraciones del metal en el
medio ambiente son mucho más bajas que las obtenidas en medidas
anteriores. Hoy en día no es posible determinar si la actividad
humana ha provocado un aumento histórico de los niveles de cadmio en
los casquetes polares.
La producción comercial de cadmio comenzó a principios de este
siglo. La pauta de consumo de cadmio se ha modificado en los últimos
años debido al notable descenso del uso de la galvano-plastia y al
importante aumento de la producción de baterías y de las
aplicaciones electrónicas especializadas. En las principales
aplicaciones del cadmio éste se utiliza en forma de compuestos que
se hallan presentes en bajas concentraciones; ello obstaculiza el
reciclaje del metal. Las restricciones impuestas por algunos países
a ciertas aplicaciones del cadmio pueden tener un efecto
generalizado en esas aplicaciones.
El cadmio se libera al aire, los suelos y las aguas debido a la
actividad humana. En general, las dos fuentes principales de
contaminación son la producción y el consumo de cadmio y de otros
metales no ferrosos y la evacuación de desechos que contienen
cadmio. Las zonas próximas a minas no ferrosas y fundiciones suelen
estar muy contaminadas por cadmio.
Al aumentar el contenido de cadmio del suelo, aumenta la
absorción del metal por las plantas; la exposición humana a partir
de las cosechas agrícolas está por tanto sometida a los aumentos del
contenido de cadmio del suelo. Dado que la absorción por las plantas
desde el suelo es mayor cuando el pH de éste es bajo, los procesos
que acidifican el suelo (por ejemplo, las lluvias ácidas) pueden
aumentar las concentraciones medias de cadmio en los alimentos. La
aplicación de fertilizantes a base de fosfato y la deposición
atmosférica son fuentes importantes de aportación de cadmio a las
tierras cultivables en ciertas zonas del mundo; los fangos de
alcantarillado también pueden ser una fuente de importancia a nivel
local. Estas fuentes pueden, en el futuro, aumentar los niveles de
cadmio en el suelo y con ello en las cosechas, lo que a su vez puede
acrecentar la exposición al cadmio en la dieta. En ciertas zonas, se
ha demostrado que está aumentando el contenido de cadmio en los
alimentos.
Ciertos organismos comestibles de vida libre como los mariscos,
los crustáceos y los hongos son acumuladores naturales de cadmio.
Como en el caso del ser humano, se observan niveles mayores de
cadmio en el hígado y el riñón de los caballos y de algunos animales
terrestres silvestres. El consumo habitual de estos alimentos puede
aumentar la exposición. Ciertos vertebrados marinos contienen
concentraciones notablemente elevadas de cadmio en el riñón,
fenómeno que, aunque se considera de origen natural, se ha vinculado
a signos de lesiones renales en esos organismos.
3. Niveles ambientales y exposición humana
La principal fuente de exposición al cadmio en la población
general no fumadora son los alimentos; la proporción de cadmio que
se absorbe por otras vías es pequeña. El tabaco es una importante
fuente de absorción de cadmio en los fumadores. En las zonas
contaminadas, la exposición al cadmio por los alimentos puede
alcanzar varios cientos de µg/día. En los trabajadores expuestos, la
absorción pulmonar de cadmio por inhalación en el lugar de trabajo
es la principal vía de exposición. También puede aumentar la
absorción por la contaminación de los alimentos y por el consumo de
tabaco.
4. Cinética y metabolismo en animales de experimentación y en
el ser humano
Los datos obtenidos en animales de experimentación y en el ser
humano han demostrado que la absorción pulmonar es mayor que la
gastrointestinal. Atendiendo a la especiación química, el tamaño de
las partículas y la solubilidad en fluidos biológicos, puede
absorberse hasta el 50% del compuesto de cadmio inhalado. La
absorción gastrointestinal de cadmio depende del tipo de dieta y del
estado nutricional. El estado nutricional respecto del hierro parece
revestir particular importancia. Aunque en promedio se absorbe el 5%
de la ingesta oral total de cadmio, los valores individuales varían
entre menos del 1% hasta más del 20%. Existe un gradiente
maternofetal de cadmio. Aunque se acumula en la placenta, la
transferencia al feto es baja.
El cadmio absorbido en los pulmones o el tracto
gastrointestinal se almacena principalmente en el hígado y el riñón,
donde se deposita más de la mitad de la carga corporal. Al aumentar
la intensidad de la exposición, aumenta la proporción del cadmio
absorbido que se almacena en el hígado. En el ser humano la
excreción suele ser lenta y la semivida biológica es muy larga
(decenios) en el músculo, el riñón, el hígado y el organismo entero.
Las concentraciones de cadmio en la mayoría de los tejidos aumentan
con la edad. Aunque las concentraciones más elevadas suelen
encontrarse en la corteza renal, con exposiciones excesivas pueden
producirse concentraciones mayores en el hígado. En las personas
expuestas que padecen lesiones renales, aumenta la excreción
urinaria de cadmio con lo que se reduce la semivida en el organismo
entero. Las lesiones renales producen pérdidas del cadmio contenido
en el riñón, y las concentraciones renales acaban con el tiempo
siendo inferiores a las observadas en personas con un grado de
exposición similar pero sin lesiones renales.
La metalotioneína es una importante proteína de transporte y
almacenamiento de cadmio y otros metales. El cadmio puede inducir la
síntesis de metalotioneína en muchos órganos, en particular el
hígado y el riñón. La unión del cadmio intracelular a la
metalotioneína en los tejidos protege contra la toxicidad del metal.
El cadmio libre puede por tanto tener una función en la patogenia de
las lesiones tisulares debidas a ese metal. Se desconoce la
especiación de otros complejos de cadmio en los tejidos o en los
fluidos biológicos.
La excreción urinaria de cadmio guarda relación con la carga
corporal, la exposición reciente y la lesión renal. En personas poco
expuestas, el nivel de cadmio en la orina depende principalmente de
la carga corporal. Una vez que se ha producido la lesión renal
inducida por el cadmio, o incluso en ausencia de lesión renal si la
exposición es excesiva, aumenta la excreción urinaria. En las
personas expuestas al cadmio que padecen proteinuria la excreción de
cadmio suele ser mayor que en las que no padecen proteinuria. Cuando
cesa la exposición intensa, el nivel de cadmio en la orina desciende
aunque persista la lesión renal. En la interpretación de la
presencia de cadmio en la orina hay que tener en cuenta, pues,
varios factores. La excreción gastrointestinal es aproximadamente
igual a la urinaria pero no puede medirse fácilmente. Otras vías
excretoras como la leche, el sudor o la transferencia placentaria
son insignificantes.
El nivel de cadmio en las heces es un buen indicador de la
ingesta diaria reciente a partir de los alimentos en ausencia de
exposición por inhalación. En la sangre, el cadmio aparece
principalmente en los glóbulos rojos y las concentraciones en el
plasma son muy bajas. Existen al menos dos compartimentos en la
sangre, uno referido a la exposición reciente, con una semivida de
alrededor de 2-3 meses, y otro probablemente relacionado con la
carga corporal, con una semivida de varios años.
5. Efectos en mamíferos de laboratorio
Las exposiciones elevadas por inhalación provocan edema
pulmonar letal. La inyección de una sola dosis elevada produce
necrosis en el testículo y en el ovario no ovulante, lesiones
hepáticas y lesiones en los vasos de menor tamaño. La administración
oral de dosis elevadas produce lesiones en la mucosa gástrica e
intestinal.
La exposición por inhalación prolongada y la administración
intratraqueal producen modificaciones crónicas de tipo inflamatorio
en los pulmones, fibrosis y fenómenos indicativos de enfisema. La
administración parenteral u oral prolongada afecta principalmente al
riñón aunque también al hígado y a los sistemas hematopoyético,
inmunitario, esquelético y cardiovascular. En ciertas especies y en
determinadas condiciones se han inducido efectos esqueléticos e
hipertensión. La aparición de efectos teratogénicos y lesiones
placentarias depende de la fase gestacional en que se produzca la
exposición y puede entrañar interacción con el zinc.
En cuanto a la exposición humana, lo más notable son los
efectos agudos por inhalación en el pulmón y los efectos renales
crónicos. Tras la exposición prolongada, el riñón es el órgano
crítico. Los efectos en este órgano se caracterizan por disfunción
tubular y lesiones en las células tubulares, si bien pueden
producirse también disfunciones glomerulares. Una de las
consecuencias de la disfunción tubular renal es la alteración del
metabolismo del calcio y de la vitamina D. Según algunos estudios,
ello ha producido casos de osteomalacia y/o osteoporosis, pero esos
efectos no se han confirmado en otros estudios. No debe excluirse el
efecto directo del cadmio en la mineralización ósea.
Los efectos tóxicos del cadmio en animales de experimentación
están sometidos a la influencia de factores genéticos y
nutricionales, las interacciones con otros metales, en particular el
zinc, y el pretratamiento con cadmio, que puede guardar relación con
la inducción de la metalotioneína.
En 1976 y 1987, el Centro Internacional de Investigaciones
sobre el Cáncer consideró suficientes las pruebas de que el cloruro,
el sulfato, el sulfuro y el óxido de cadmio pueden producir sarcomas
en el lugar de inyección en la rata y, en el caso de los dos
primeros compuestos, inducir tumores en las células intersticiales
del testículo en la rata y el ratón, pero consideró que los estudios
de administración oral eran insuficientes para la evaluación. En
estudios de inhalación prolongada en ratas expuestas a aerosoles de
sulfato de cadmio, vapores de óxido de cadmio y polvos de sulfato de
cadmio se observó una elevada incidencia de cáncer primario del
pulmón con pruebas de proporcionalidad entre la dosis y la
respuesta. Hasta el momento, sin embargo, esa observación no se ha
confirmado en otras especies. Los estudios de los efectos
genotóxicos del cadmio han dado resultados discordantes.
6. Efectos en el ser humano
La exposición intensa por inhalación de vapores de óxido de
cadmio produce neumonitis aguda con edema pulmonar, que puede ser
letal. La ingestión de dosis elevadas de sales solubles de cadmio
produce gastroenteritis aguda.
La exposición ocupacional prolongada al cadmio ha producido
efectos crónicos graves, principalmente en el pulmón y el riñón.
También se han observado efectos renales crónicos en la población
general.
Los cambios pulmonares observados tras una intensa exposición
ocupacional se caracterizan principalmente por la aparición de
afecciones crónicas obstructivas de las vías aéreas. Los primeros
cambios leves en las pruebas de la función ventilatoria pueden
avanzar, si prosigue la exposición al cadmio, hasta insuficiencia
respiratoria. Se ha observado una mayor tasa de mortalidad por
enfermedad pulmonar obstructiva en trabajadores sometidos a
exposiciones intensas, al igual que en otras épocas.
La acumulación de cadmio en la corteza renal produce disfunción
tubulorrenal con trastornos de la reabsorción de proteínas, glucosa
y aminoácidos, entre otros. Un signo característico de la disfunción
tubular es la mayor excreción de proteínas de bajo peso molecular en
la orina. En algunos casos, disminuye la tasa de filtración
glomerular. El aumento de la concentración de cadmio en la orina
está correlacionado con la presencia de proteínas de bajo peso
molecular en la orina y, en ausencia de exposición aguda al cadmio,
puede servir como indicador de efectos renales. En los casos más
graves se combinan los efectos tubulares y glomerulares, con aumento
del nivel de creatinina en la sangre en algunos casos. Para la
mayoría de los trabajadores y de las personas expuestas al medio
ambiente general, la proteinuria inducida por el cadmio es
irreversible.
Entre otros efectos figuran los trastornos del metabolismo del
calcio, la hipercalciuria y la formación de cálculos renales. La
exposición intensa al cadmio, con toda probabilidad combinado con
otros factores como carencias nutricionales, puede llevar a la
aparición de osteoporosis y/o osteomalacia.
Hay pruebas de que la exposición profesional prolongada al
cadmio puede contribuir a la aparición de cáncer del pulmón aunque
las observaciones obtenidas en trabajadores expuestos han sido
difíciles de interpretar a causa de factores que inducen a
confusión. En el caso del cáncer de la próstata, las pruebas
obtenidas hasta la fecha no son concluyentes pero no apoyan la
existencia de una relación causal, indicada en estudios anteriores.
Actualmente no hay pruebas convincentes de que el cadmio sea
agente etiológico de la hipertensión esencial. La mayor parte de los
datos indican que no se debe al cadmio el aumento de la tensión y no
hay pruebas de que la mortalidad por enfermedades cardio-vasculares
o cerebrovasculares sea mayor.
Los datos obtenidos en estudios de grupos de trabajadores
expuestos y de grupos expuestos en el medio ambiente general
demuestran que existe una relación entre los niveles de exposición,
la duración de ésta y la prevalencia de los efectos renales.
Se ha comunicado una mayor prevalencia de la proteinuria de
bajo peso molecular en trabajadores del cadmio tras 10-20 años de
exposición a niveles del metal de aproximadamente 20-50 µg/m3. En
zonas contaminadas del medio general, en las que la ingesta de
cadmio estimada ha sido de 140-260 µg/día, se han observado efectos
en forma de aumento de la cantidad de proteínas de bajo peso
molecular en la orina en algunos individuos tras una exposición
prolongada. En la sección 8 se dan estimaciones más precisas de la
relación dosis-respuesta.
7. Evaluación de los riesgos para la salud humana
7.1 Conclusiones
Se considera que el riñón es el órgano diana crítico en la
población general así como en las poblaciones expuestas
profesionalmente. Las enfermedades crónicas obstructivas de las vías
respiratorias están asociadas a la exposición profesional prolongada
e intensa por inhalación. Hay pruebas de que esa exposición al
cadmio puede contribuir al desarrollo de cáncer del pulmón aunque
las observaciones en trabajadores expuestos han sido difíciles de
interpretar a causa de la presencia de factores que inducen a
confusión.
7.1.1 Población general
El cadmio presente en los alimentos es la principal fuente de
exposición para la mayoría de las personas. En la mayoría de las
zonas no contaminadas con cadmio las ingestas diarias medias con los
alimentos se encuentran entre 10 y 40 µg. En zonas contaminadas se
ha observado que alcanza varios cientos de µg al día. En zonas no
contaminadas, la absorción debida al consumo de tabaco puede igualar
la ingestión de cadmio a partir de los alimentos.
Basandose en un modelo biologico, se ha estimado que con una
ingesta diaria de 140-260 µg de cadmio durante toda la vida, o una
ingesta acumulativa de unos 2000 mg o más, se produce en el ser
humano una asociación entre la exposición al cadmio y una mayor
excreción de proteínas de bajo peso molecular en la orina.
7.1.2 Población expuesta profesionalmente
La exposición ocupacional al cadmio se produce principalmente
por inhalación aunque comprende ingestas suplementarias con los
alimentos y el tabaco. El nivel total de cadmio en el aire varía
según las prácticas de higiene industrial y el tipo de lugar de
trabajo. Existe una relación exposición-respuesta entre los niveles
de cadmio en el aire y la proteinuria. Puede aumentar la prevalencia
de la proteinuria de bajo peso molecular en trabajadores a los 10-20
años de exposición a niveles de cadmio de unos 20-50 µg/m3. La
medida in vivo del cadmio en el riñón y el hígado de personas con
distintos niveles de exposición al metal ha demostrado que alrededor
del 10% de los trabajadores con un nivel en la corteza renal de
200 mg/kg y aproximadamente el 50% de las personas con un nivel en
la corteza renal de 300 mg/kg tendrían proteinuria tubulorrenal.
In a later epidemiological study, Nogawa et al. (1989)
investigated the dose-response relationship in 1850 cadmium-exposed
and 294 non-exposed inhabitants of the Kakehashi River basin. Using
a urine concentration of 1000 µg ß2-microglobulin/g creatinine as
an index of renal tubular dysfunction, and the average rice cadmium
concentration as an index of cadmium exposure, the authors
determined linear regression equations for men and women. These are
related to the prevalence of ß2-microglobulinuria and total
cadmium intake and are shown in Table 19. The authors concluded that
the total cadmium intake that produced an adverse effect on health
was approximately 2000 mg for both men and women. On the basis of
the linear regression equations shown in Table 19, an average daily
cadmium intake of 440 µg/day in men and 350 µg/day in women would
be expected to cause a 50% response rate (> 1000 µg
ß2-microglobulin/litre urine). Response rates of 20, 10 and 5%
would occur at daily intakes of 220, 150, and 110 µg/day for men and
200, 150, and 120 µg/day for women. The authors indicated that these
data are in general agreement with results from other studies
involving the consumption of various levels of cadmium in rice.
8.3.3.5 Akita prefecture (Kosaka area)
Around Kosaka mine and refinery areas, increased cadmium levels
in rice were first reported by the Akita Prefectural Government
(1973). Kojima et al. (1976) gave further data from the Kosaka area,
where the reported average cadmium level in rice varied between 0.26
and 0.56 mg/kg. The latter average was based on 41 samples where one
was reported to contain 4.81 mg/kg. In the exposed area, the
weighted average of cadmium levels in rice in each district
according to the number of examinees was calculated by Kojima et al.
(1976) to be 0.57 mg/kg in 1973 and 0.50 mg/kg in 1974. These
values, according to Kojima et al. (1976), represented the level of
cadmium exposure in this study and were considered more accurate
than the general average given above.
In an epidemiological investigation of the total population in
the age-group 50-69 of defined geographical areas (93 out of 98 in
the control area and 156 out of 190 in the exposed area
participated), Kojima et al. (1976, 1977) obtained data on faecal
excretion of cadmium. The cadmium level in 24-h faeces samples was
analysed only for those participants who said that they defaecated
once a day (64 in the control area and 118 in the polluted area).
Average rates were 51 µg/day and 177 µg/day for the control and
exposed groups, respectively (Kojima et al., 1976). The prevalence
of proteinuria exceeding 150 mg/litre (using the Tsuchiya biuret
method) and of combined proteinuria and glucosuria (test tape) was
significantly higher in the exposed group than in the control group
(Kojima et al., 1976).
Table 19. Linear regression equations relating total cadmium intake and prevalence of ß2-microglobulinuriaa
Sex ß2-microglobulinuria Linear regression Prevalence of Total cadmium intake
equationb ß2-microglobulinuria (mg)c
in the control group (%)
Male > 1000 µg/litre Y = 0.0076X - 10.33 5.3 2057
> 1000 µg/g creatinine Y = 0.0083X - 7.93 6.0 1678
Female > 1000 µg/litre Y = 0.011X - 19.61 3.1 2065
> 1000 µg/g creatinine Y = 0.012X - 16.16 5.0 1763
a From: Nogawa et al. (1989)
b Y = prevalence of ß2-microglobulinuria (%); X = total cadmium intake (mg)
c Total cadmium intake yielding a ß2-microglobulinuria prevalence equivalent to the control group
Quantitative analysis of urinary ß2-microglobulin with
radioimmunoassay (RIA) was performed on the same population (Kojima
et al., 1977). The frequency distributions were log-normal, as for
occupationally exposed people, and the prevalence of
ß2-microglobulin excretion was 15% in the whole exposed group and
3% in the control group. The exposed and control groups showed
average faecal cadmium excretion rates of 139 and 41 µg per day,
respectively (Kojima et al., 1977).
The ß2-microglobulin data and cadmium intake data in the
study by Kojima et al. (1977) were divided into three dose groups
(Kjellström et al., 1977b) and analysed for a dose-response
relationship by Hutton (1983).
Further studies of urinary ß2-microglobulin over the age
range 5-90 years were reported by Saito et al. (1981). The RIA
method was used, and a control area in Akita prefecture was compared
with cadmium-polluted areas in Akita prefecture (Kosakai), Ishikawa
prefecture (Kakehashi) (section 8.3.3.3), and Nagasaki prefecture
(Tsushima) (section 8.3.3.5). For people over the age of 40, there
were significant increases in average urinary ß2-microglobulin
excretion in all the polluted areas. In the older age-groups, the
increase was 10-100 times above control values.
In the first comprehensive study of proximal renal tubular
functions performed on a population living in a cadmium-polluted
area, Saito et al. (1977) conducted health examinations within Akita
prefecture. Renal tubular function tests consisted of renal
glucosuria, uric acid clearance, low molecular weight proteinuria,
tubular reabsorption of phosphate, hydrogen carbonate threshold,
acid-base balance, concentrating and acidifying ability of urine,
endogenous creatinine clearance, and renal plasma flow. Of the 147
residents (97% of target population) examined, 33 (22%) had some
indications of proximal renal tubular dysfunction, such as renal
glucosuria and low molecular weight proteinuria. In addition, 10
subjects (7%) were diagnosed as having multiple proximal renal
tubular dysfunctions. Detailed examinations revealed that none of
these 10 subjects had experienced any other environmental exposures
or diseases that could have caused the renal dysfunction. They were
therefore diagnosed as suffering from the effects of chronic cadmium
poisoning (Saito et al., 1977).
8.3.3.6 Nagasaki prefecture (Tsushima area)
This area has been a lead and zinc mining district from ancient
times (Takabatake, 1978b). Modern operations started in 1948, and
mining wastes have been scattered throughout the local area. In
1952, local farmers complained about the poor growth of crops. In
the 1960s, studies of cadmium pollution were carried out. Health
examinations for cadmium effects have been conducted since 1966
(Takabatake, 1978b).
Early studies showed an increased prevalence of proteinuria in
the most polluted village, and an average rice cadmium level of
0.75 mg/kg was reported (Takabatake, 1978b).
The study of urinary ß2-microglobulin according to age
referred to in section 8.3.3.4 (Saito et al., 1981) included an
exposed group of people from Tsushima. The age-specific average
urinary excretions were much higher than the control values and
similar to those found in the Kakehashi and Kosaka areas. For women,
the Tsushima values appear higher than those of the other two
polluted areas (Table 17), which may reflect the higher estimated
average cadmium intake in the Tsushima area.
8.3.3.7 Other Japanese areas
As shown in Table 7, health surveys have been performed in
areas other than Fuchu, Ikuno, Kakehashi, Kosaka, and Tsushima.
According to the Japanese Cadmium Research Committee (1989), it
should be emphasized, however, that no cadmium health effects,
including elevated prevalence of ß2-microglobulinuria, were
observed in some areas with higher levels of cadmium (daily intakes
of 180-309 µg in Bandai, Fukushima; 180-380 µg in Annaka, Gunma; and
222-391 µg in Okutake river basin, Oita) even than Kosaka and
Kakehashi (cadmium in rice = 0.16-0.58 mg/kg, daily intake of
cadmium = 139-177 µg in Kosaka, Akita; cadmium in rice =
0.2-0.8 mg/kg, daily intake of cadmium = 160-190 µg in the Kakehashi
river basin, Ishikawa). Also, no health effects were found in the
Uguisuzawa river basin, Miyagi (cadmium in rice = 0.6-0.7 mg/kg),
Watarase river basin, Gunma (0.32 mg/kg), Shimoda, Shizuoka
(0.4-1.1 mg/kg), and Ohmuta, Fukuoka (0.72 mg/kg), even though
residents consumed rice heavily contaminated with cadmium.
8.3.3.8 Belgium
The Liege area of Belgium is known to be polluted by cadmium,
mainly due to the activities of non-ferrous smelters since the end
of the 19th century (Kretzschmar et al., 1980; Lauwerys et al.,
1980a).
A pilot study was performed in 1979 on a group of 60 elderly
non-smoking women who had spent most of their lives in the Liege
area and had never been occupationally exposed to cadmium (Roels et
al., 1981a). Daily intakes of cadmium ranged from 2-88 µg/day with
an average of 15 µg/day. Their average blood cadmium level
(1.6 µg/litre) and their urinary excretion rates of cadmium
(0.093 µg/h), total protein (17.3 mg/h), amino acids (5.45 mg amino
acid N/h), and albumin (1.54 mg/h) were higher than those found in a
group of 70 women of the same age and socio-economic status who
lived in another industrial area (Charleroi) less polluted by
cadmium. Although the average excretion rate of ß2-microglobulin
was greater in Liege (93.6 µg/h) than in Charleroi (22 µg/h), the
difference between the geometric means was not statistically
significant. The two areas were well matched with respect to their
environmental pollution by sulfur dioxide, fume, suspended
particles, and various metals, including lead, vanadium, nickel,
chromium, and iron.
Following these observations, a mortality study and a
preliminary autopsy study were undertaken (Lauwerys & De Wals, 1981;
Lauwerys et al., 1984a). It was found that, although the overall
mortality was not markedly different, the standard mortality ratio
(SMR) and proportional mortality rate (PMR) from nephritis and
nephrosis for the years 1967-1976 were higher in Liege than in
Charleroi or in Belgium as a whole (SMR: Belgium, 100; Charleroi,
102; Liege, 196; PMR: Belgium, 3.3; Charleroi, 3.0; Liege, 6.0).
Since the increased mortality rate for renal diseases was observed
in both males and females, the influence of environmental factors
other than occupation is probable.
The results of the preliminary autopsy study indicated that, in
the age-group 40-60, the average body burden of cadmium was
approximately twice as high in people autopsied in Liege as it was
in those autopsied in a city (Brussels) less polluted by cadmium
(Lauwerys et al., 1984a). The geometric mean values of cadmium
concentration in the kidney cortex were 38.3 and 22.8 mg/kg wet
weight in Liege and Brussels, respectively.
According to the authors of these reports, the studies
performed so far in the Liege area do not refute the hypothesis that
environmental pollution by cadmium in the area has increased the
body burden of cadmium of the inhabitants and has affected their
renal function. A large-scale morbidity and autopsy study is at
present underway (Braux et al., 1987).
8.3.3.9 Shipham area in the United Kingdom
The village of Shipham is located on the slag heaps of an old
zinc mine and high levels of cadmium have been found in soil and
dust (section 3.4.3).
The exposed population has been studied using both mortality
and morbidity end-points. A census in 1979 identified 1092
residents, of whom 64% had resided in the village for more than 5
years, and 548 participated in a health study coordinated by the
United Kingdom Department of the Environment (Barltrop & Strehlow,
1982a). A similar study of 543 age-and sex-matched individuals was
performed in a nearby control village (Barltrop & Strehlow, 1982b).
The daily intake of cadmium in Shipham was an average of 35 µg/day
(section 4.2.4), which is about twice as high as the estimated
United Kingdom national average but much lower than in polluted
areas of Japan (section 5.2.4).
A health inventory was compiled by means of a questionnaire,
which included information on smoking habits, alcohol consumption,
medication, and occupation. Blood samples were analysed for
haemoglobin, haematocrit, serum protein, ß2-microglobulin,
creatinine, erythrocyte protoporphyrin, lead, and cadmium. Urine
samples were analysed for total protein, creatinine, ß2-micro-
globulin, and cadmium.
The mean 24-h urinary concentration of cadmium for Shipham
residents was 0.68 µg cadmium/g creatinine and, in the control area,
0.60 µg cadmium/g creatinine with 97.7% of values less than 3.4 µg
cadmium/litre. The difference was statistically significant
(P < 0.03) (Barltrop & Strehlow, 1982b), but the similarity of the
values for average urinary cadmium concentrations between the two
areas and the generally low levels of cadmium in Shipham suggest a
rather low daily cadmium intake.
However, there are data showing that some individuals in
Shipham had high cadmium exposures. Liver cadmium concentrations,
measured by means of in vivo neutron activation analysis, were
determine for 21 adult volunteers living in the most heavily
contaminated areas of Shipham (Harvey et al., 1979). Their age range
was 40-62 years (mean, 53 years) and, with one exception, they had
lived in Shipham for 9-50 years (mean, 23 years). On average, half
of their vegetable consumption was of local origin. The mean liver
cadmium concentration was 11.0 (+ 2.0) mg/kg, compared with 2.2
(+ 2.0) mg/kg in 20 age-matched, non-Shipham controls (P < 0.001).
The maximum concentration in the Shipham group was 28 mg/kg, which
would correspond to levels in the kidney cortex of at least
200-300 mg/kg (Friberg, 1979) (section 6.4). Health effects were not
studied in this investigation.
In the health study by the Department of the Environment
(Barltrop & Strehlow, 1982a), the comparison of ß2-microglobulin
data from the two villages showed similar distributions, and all
other laboratory data, including blood pressure levels, were
distributed within the normal range. However, the poor participation
rate in this health study (50%), makes it difficult to interpret the
findings. Another study of 31 volunteers from Shipham (Carruthers &
Smith, 1979) reported a high prevalence of hypertension and LMW
proteinuria, but the methodology of the study has been criticized
(Hughes & Stewart, 1979; Kraemer et al., 1979).
Examination of the data in relation to soil cadmium levels
showed no evidence of an increased mortality from any cause in those
living in the most polluted areas. It was concluded from this study
that, if cadmium contamination had any effect on the mortality
pattern in Shipham, this effect was only slight and did not present
a serious health hazard to residents. No case resembling Itai-itai
disease has at any time been reported in Shipham. All the authors
involved in the health studies in Shipham pointed to the possible
protective effect of high levels of zinc also present in soil, and
Kraemer et al. (1979) pointed to the need to assess dietary zinc and
the intake of nutrients other than cadmium.
8.3.3.10 USSR
Screening of populations, both environmentally and
occupationally exposed, which included measurements of urinary
ß2-microglobulin, has been carried out within the USSR (Likutova,
1989). Increased prevalence (up to 6%) of ß2-micro-globulinuria
(> 280 µg/g creatinine) was observed in females (20-50 years old)
in some of the most heavily polluted cities, i.e. Odjonikidze and
Kursk. The air cadmium concentrations in these two cities were
0.085 µg/m3 and 0.005-0.027 µg/m3, respectively. The examination
of workers (50-300 µg/m3) exposed to cadmium also revealed an
increased prevalence of ß2-microglobulinuria (up to 19% in the
most heavily exposed group). The findings are in good agreement with
the data presented in Table 15.
8.4 Conclusions
High inhalation exposure to cadmium oxide fume results in acute
pneumonitis with pulmonary oedema, which may be lethal. High
ingestion exposure of soluble cadmium salts causes acute
gastroenteritis.
Long-term occupational exposure to cadmium has caused severe
chronic effects, predominantly in the lungs and kidneys. Chronic
renal effects have also been seen among the general population.
Following high occupational exposure, lung changes are
primarily characterized by chronic obstructive airway disease. Early
minor changes in ventilatory function tests may progress, with
continued cadmium exposure, to respiratory insufficiency. An
increased mortality rate from obstructive lung disease has been seen
in workers with high exposure, as has occurred in the past.
The accumulation of cadmium in the renal cortex leads to renal
tubular dysfunction with impaired reabsorption of, for instance,
proteins, glucose, and amino acids. A characteristic sign of tubular
dysfunction is an increased excretion of low molecular weight
proteins in urine. In some cases, the glomerular filtration rate
decreases. Increase in urine cadmium correlates with low molecular
weight proteinuria and in the absence of acute exposure to cadmium
may serve as an indicator of renal effect. In more severe cases
there is a combination of tubular and glomerular effects, which may
progress in some cases to decreased glomerular filtration. For most
workers and people in the general environment, cadmium-induced
proteinuria is irreversible.
Among other effects are disturbances in calcium metabolism,
hypercalciuria, and formation of renal stones. High exposure to
cadmium, most probably in combination with other factors such as
nutritional deficiencies, may lead to the development of
osteoporosis and/or osteomalacia.
There is evidence that long-term occupational exposure to
cadmium may contribute to the development of cancer of the lung but
observations from exposed workers have been difficult to interpret
because of confounding factors. For prostatic cancer, evidence to
date is inconclusive but does not support the suggestion from
earlier studies of a causal relationship.
At present, there is no convincing evidence for cadmium being
an etiological agent of essential hypertension. Most data speak
against a blood pressure increase due to cadmium and there is no
evidence of an increased mortality due to cardiovascular or
cerebrovascular disease.
Data from studies on groups of occupationally exposed workers
and on groups exposed in the general environment show that there is
a relationship between exposure levels, exposure durations, and the
prevalence of renal effects.
An increased prevalence of low molecular weight proteinuria in
cadmium workers after 10-20 years of exposure to cadmium levels of
about 20-50 µg/m3 has been reported.
In polluted areas of the general environment, where the
estimated cadmium intake has been 140-260 µg/day, effects in the
form of increased low molecular weight proteinuria have been seen in
some individuals following long-term exposure.
9. EVALUATION OF HUMAN HEALTH RISKS
9.1 Exposure assessment
9.1.1 General population exposure
In the ambient air, cadmium concentrations based on long-term
sampling periods indicate, in most cases, a range of
0.001-0.015 µg/m3 in rural areas, 0.005-0.05 µg/m3 in urban
areas, and up to 0.6 µg/m3 near sources of pollution (section
5.1.1).
One cigarette usually contains 1-2 µg cadmium, of which about
10% may be inhaled (section 5.1.3).
Among staple foods, rice and wheat usually contain less than
0.1 mg/kg and other foods usually less than 0.05 mg/kg wet weight,
but liver and kidney may contain 1-2 mg/kg wet weight and certain
sea-foods as much as 10 mg/kg wet weight (section 5.2). Certain
animals, e.g., the horse, may accumulate considerably higher
concentrations in the liver and kidney. In polluted areas, these
levels are further increased.
The content of natural waters is usually less than 1 µg/litre,
but higher levels may be found near sources of pollution.
The total daily intake in non-polluted areas of most countries
from food, water and air is estimated to be approximately
10-40 µg/day (food, 10-40 µg/day; water, < 1 µg; and air,
< 0.5 µg/day for non-smokers).
Twenty cigarettes per day would contribute a further 2-4 µg. In
polluted areas, the daily intake may be much higher, and intakes of
several hundred µg/day have been reported (section 5.3.2).
9.1.2 Occupational exposure
Air is the main source of additional cadmium exposure for
industrial workers. In many countries such exposures have now been
reduced considerably. In the past, levels of several mg/m3 were
recorded in workplaces. Now, with proper industrial hygiene
practices, levels of 0.02-0.05 mg/m3 would be more typical
(section 5.1.2).
9.1.3 Amounts absorbed from air, food, and water
The proportion of cadmium from food and water that is absorbed
will depend on the chemical nature of the cadmium compounds, but
estimates based on the available data indicate that gastrointestinal
absorption is approximately 5%, with considerable individual
variation (section 6.1.2). Similarly, the amount absorbed from the
air will depend on the chemical nature and the particle size of the
inhaled material. The absorption varies between 25 and 50% depending
on particle size and solubility (section 6.1.1). About 10% of the
cadmium inhaled in cigarette smoke is absorbed.
Thus, the average amount absorbed from food and water in a
person from a non-polluted area would be about 0.5-1.3 µg/day. The
absorbed amount from smoking 20 cigarettes per day would be
1-2 µg/day and that from workroom air could be many times greater
(section 5.3).
9.2 Dose-effect relationships
9.2.1 Renal effects
Long-term exposure to cadmium causes renal tubular dysfunction
with proteinuria, glucosuria, and aminoaciduria, as well as
histopathological changes, in both experimental animals and humans
(sections 7.2.1.4 and 8.2.1, respectively). These are usually the
first effects to occur after ingestion or inhalation exposure. As
the renal dysfunction progresses in severity, the glomeruli may also
be affected and, in a few cases, the cadmium-induced damage may lead
to renal failure (section 8.2.1). Daily cadmium intakes in food of
140-260 µg/day for more than 50 years or workplace air exposures of
50 µg/m3 for more than 10 years have produced an increase in renal
tubular dysfunction in some exposed popu-lations (section 8.3.3.2).
9.2.2 Bone effects
Cadmium may produce bone effects in both humans and animals.
The most notable clinical entity in these cases is osteomalacia, but
many subjects also show osteoporosis. Animal experiments show that
both can be produced by long-term cadmium exposure (section 7.2.4).
In animals and humans, osteomalacia has been seen in combination
with cadmium-induced renal damage. The bone effects may be linked to
cadmium effects on calcium and vitamin D metabolism in the kidney.
The daily intakes via food and exposure levels in air at which the
bone effects occur are uncertain, but they must be higher than those
causing renal effects. Bone effects have been seen among both the
general population and industrial workers in the past when exposure
levels were very high. Host and nutritional factors influence the
development and severity of cadmium-induced bone effects.
9.2.3 Pulmonary effects
Chronic obstructive airway disease has been reported in a
number of studies of cadmium workers (section 8.2.3). This has, in
severe cases, led to an increased mortality. The dose needed to
produce these effects is uncertain, but it is higher than the dose
needed to produce renal effects, as most workers reported to have
lung effects also had renal effects. On the other hand, many workers
with renal effects, who had been exposed to cadmium oxide dust and
fume, had no lung effects.
9.2.4 Cardiovascular effects
Some animal studies have shown that, under certain exposure
conditions, increased blood pressure and effects on the myocardium
occur. Studies of cadmium-exposed workers and people in the general
environment have been carried out, but most data do not support the
animal findings.
9.2.5 Cancer
There is evidence that cadmium chloride, sulfate, sulfide and
oxide give rise to injection site sarcomata in the rat and that the
chloride and sulfate induce interstitial cell tumours of the testis
in rats and mice.
Long-term inhalation studies in rats exposed to aerosols of
cadmium chloride, sulfate, and oxide fume and dust at low
concentrations demonstrated a high incidence of primary lung cancer
with evidence of a dose-response relationship. This has not so far
been shown in other animals.
There is evidence that long-term occupational exposure to
cadmium may contribute to the development of cancer of the lung, but
observations from exposed workers have been difficult to interpret
because of inadequate exposure data and confounding factors. The
evidence to date is inconclusive, but does not support the
suggestion from earlier studies that cadmium can cause prostatic
cancer.
IARC (1987a) considered that there was sufficient evidence for
the carcinogenicity of specified cadmium compounds in experimental
animals and limited evidence for carcinogenicity in humans exposed
to cadmium. A combined evaluation of human and animal data by IARC
(1987b) classified cadmium as a probable human carcinogen (IARC
group 2A). The IPCS Task Group found no reason to deviate from this
IARC evaluation.
9.2.6 Critical organ and critical effect
The kidney is the critical organ for chronic cadmium poisoning.
Within the kidney, the cortex is the site where the first adverse
effect occurs. Therefore, in assessing dose-response relationships,
the cadmium concentration in the kidney cortex is of prime
importance.
The critical effect is renal tubular dysfunction, which is most
often manifested as low molecular weight proteinuria. Animal studies
indicate that histological changes in the renal tubules occur at a
dose level lower than that needed to produce low molecular weight
proteinuria.
9.3 Critical concentration in the kidneys
9.3.1 In animals
Several studies with data on both cadmium concentrations in the
renal cortex and the occurrence of tubular damage were discussed in
section 7.2.1. The findings were summarized in Table 12. They showed
that histological tubular lesions or proteinuria was usually seen at
cadmium renal cortex levels of 200-300 mg/kg wet weight. In some
studies on rats, monkeys, horses, and birds, certain effects were
seen at lower levels.
As no dose-response data are given in most animal studies, it
may be assumed that these renal cortex levels correspond to a 50%
response rate (CC50). Naturally, the cadmium levels at which lower
response rates occur would be lower.
In studies on monkeys conducted in Japan, kidney cadmium levels
were related to dose and duration of exposure. At the two highest
dose levels, acute liver effects occurred. If one wishes to
establish a range of values for the critical concentration in
individuals at which a small but significant part of an exposed
population will show effects, animal studies indicate that a renal
cortex level of about 100-200 mg/kg is likely to coincide with such
a range. There is some evidence that the average critical
concentration (CC50) could be as high as 300 or 400 mg/kg for the
more severe signs of renal tubular damage, but such high levels
should not be used as a starting point for calculations of
"acceptable daily exposures".
9.3.2 In humans
Section 8.2.1.5 reviewed all available data from cases in which
both renal cortex cadmium levels and renal effects were measured.
Data from autopsies or biopsies have mainly been cross-sectional,
i.e. the renal cadmium concentrations and the effects were measured
more or less simultaneously. This has made it difficult to interpret
the data from a critical concentration point-of-view, as the cases
with the most severe cadmium-induced kidney dysfunction had the
lowest renal cadmium levels. Cadmium is lost from the kidney when
the damage progresses (section 6.5.1.2).
In vivo neutron activation analysis has provided a new tool
for establishing the human critical concentration of cadmium in the
renal cortex. Longitudinal studies measuring the renal cortex
cadmium concentration several times during continued exposure can
now be carried out. The cadmium level at which the first measurable
signs of renal tubular dysfunction occurs can be estimated. However,
only two studies using in vivo neutron activation have been
published to date, and both of them are cross-sectional.
The renal cadmium concentrations are disproportionately low
when the liver cadmium concentrations are high and renal effects
have developed. Of the several methods available to estimate the
average critical kidney concentration in these groups of exposed
workers, the method of preference assumes that the peak for renal
cortex cadmium level, plotted against liver cadmium, is equivalent
to the point where renal tubular dysfunction occurs. This results in
a value of 319 mg/kg tissue (based on a ratio of renal cortex
cadmium to whole kidney cadmium of 1.5). There is considerable
variance in the individual values, the 95% tolerance (which
corresponds to a confidence interval) being in the range ± 90 mg/kg
from the mean. Other studies, using similar assumptions, have
reported a value of 332 mg/kg, 10% of the workers having a peak
cadmium level of about 216 mg/kg tissue. A re-evaluation of the
original study resulted in a calculated cadmium level of about
200 mg/kg. It was concluded that for the purposes of dose-response
calculations, using a metabolic model, this concentration could be
used as a starting point for renal effects occurring in an exposed
population.
9.4 Dose-response relationships for renal effects
Two approaches can be used to estimate dose-response
relationships. One employs epidemiological data from industry and
the general environment studying associations between exposure and
response. The other begins with a critical concentration in the
kidney cortex and employs a metabolic model to calculate, on the
basis of certain given assumptions, the exposure that is required to
reach a critical concentration.
9.4.1 Evaluation based on data on industrial workers
Table 16 contains data from various group studies on cadmium
workers. In most of these studies, the dose measurements were based
on short sampling periods (hours or a few days). However, exposure
may have lasted for decades, levels usually being higher in the
past. The use of protective devices may also confound the picture.
As discussed in section 8.3.2, there are now several reports
available that show a clear exposure-response relationship between
cadmium in workplace air and the prevalence of proteinuria.
An increased prevalence of overt proteinuria, as measured by
sulfosalicylic acid, trichloroacetic acid, or quantitative
determination of total proteinuria, can occur after only 5-10 years
of exposure to approximately 100 µg cadmium/m3. If instead, the
increased excretion of low molecular weight proteins (more than 97.5
percentile of control group) is used as the critical effect, 10-20%
of workers would have this effect after a cumulative dose
corresponding to 10-20 years of exposure to 50 µg cadmium/m3.
These evaluations are all based on levels of total cadmium in
inhaled dust or air.
9.4.2 Evaluation based on data on the general population
As indicated in chapter 8, there exists a considerable amount
of information from epidemiological studies carried out on the
general population in Japan. It was shown that in some areas of high
cadmium exposure the prevalence of low molecular weight proteinuria
was significantly higher than in control areas. This may be
considered in relation to the known cadmium concentrations in rice
and the daily cadmium intakes in the affected areas (Tables 7 and
17). Contamination of drinking-water in some areas may be a
complicating factor (section 8.3.3).
Taking all of the data in section 8.3.3 together, it seems
that, when the most sensitive method for diagnosis of low molecular
weight proteinuria is applied, there is an association between
cadmium exposure and increased excretion of low molecular weight
proteins among some people over 50 years of age at a daily intake of
about 140-260 µg cadmium or a cumulative cadmium intake of about
2000 mg or more (for both men and women).
9.4.3 Evaluation based on a metabolic model and critical
concentrations
Using the data on critical concentrations and kinetic models of
cadmium metabolism, attempts have been made to calculate the
dose-response relationship for cadmium. Assuming that cadmium in the
kidney is accumulated in accordance with a one-compartment model and
that a third or a quarter of the body burden of cadmium is in the
kidney (and making certain other assumptions indicated in Tables 20
and 21), the daily cadmium intake via food and the occupational air
concentrations needed to reach the critical concentration have been
calculated (Tables 20 and 21).
As the values calculated in Tables 20 and 21 are for an average
person, not all of those exposed to these levels would have reached
the renal cortex cadmium concentration of 200 mg/kg tissue or their
individual critical concentration. Nevertheless, these calculations
produce values that are similar to the levels at which effects have
been observed, and the model approach may be a useful way to
quantify the response rates at levels lower than those easily
measurable.
Calculations have been reported of the relationship between
intake and response rates using the observed frequency distributions
of daily intake and renal cortex cadmium concentrations, and
utilizing multi-compartment metabolic model values in the same range
as those given in Tables 20 and 21. Further development of these
modelling techniques would be of value.
Using a single-compartment model for the accumulation of
cadmium in the kidney, the average daily intake that would give rise
to an average concentration of 200 mg/kg wet weight in the kidney
cortex at age 50 would be 260-480 µg/day, assuming 5%
gastrointestinal absorption, various biological half-times, and
different proportions of the body burden in the kidneys (Table 19).
Assuming a 10% absorption rate, the intake needed would be
140-260 µg per day. These estimates will vary depending on the body
weight estimates for different populations.
Table 20. Calculated daily cadmium intake via ingestion required by a
non-smoker to reach a kidney cortex concentration of 200 mg/kg
at age 50 (using a one-compartment model)a
Gastrointestinal Proportion of body Estimated half-time in kidney cortexb
absorption rate burden in kidney
(%) 17 years 30 years
5 one-third 365 µg (286 µg) 265 µg (208 µg)
10 182 µg (143 µg) 133 µg (104 µg)
5 one-quarter 486 µg (382 µg) 353 µg (277 µg)
10 243 µg (191 µg) 177 µg (139 µg)
a The data in the table are based on the following assumptions:
gastrointestinal absorption, either 5% or 10%;
half-time in kidney cortex, either 17 years or 30 years (as reported in
section 6.6.2);
one-third or one-quarter of body burden in the kidneys;
cadmium concentration in renal cortex 25% higher than renal average;
average weight of both kidneys at age 50 of 300 g for a 70-kg person
or 235 g for a 55-kg person;
average cadmium concentration in foodstuffs constant during
the last 50 years;
variation of daily intake with age has been disregarded since such
variation would influence the values by less than 10%.
b Data have been calculated for a 70-kg person; values in parentheses
are for a 55-kg person.
Table 21. Calculated concentration of cadmium in industrial air required
for a kidney cortex concentration of 200 mg/kg to be reacheda
Proportion of body burden Estimated half-time in kidney cortexb
in kidney
17 years 30 years
one-third 16 µg/m3 (13 µg/m3) 14 µg/m3 (11 µg/m3)
one-quarter 21 µg/m3 (17 µg/m3) 19 µg/m3 (15 µg/m3)
a The data in the table are based on the following assumptions:
those assumptions given in Table 20;
exposure time of 25 years;
225 working days per year;
10 m3 of air inhaled per day;
25% pulmonary absorption
b Data have been calculated for a 70-kg worker; values in parentheses
are for an average 55-kg person
10. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF
HUMAN HEALTH
10.1 Conclusions
The kidney is considered the critical target organ for the
general population as well as for occupationally exposed
populations. Chronic obstructive airway disease is associated with
long-term high-level occupational exposure by inhalation. There is
some evidence that such exposure to cadmium may contribute to the
development of cancer of the lung but observations from exposed
workers have been difficult to interpret because of confounding
factors.
10.1.1 General population
Food-borne cadmium is the major source of exposure for most
people. Average daily intakes from food in most areas not polluted
with cadmium are 10-40 µg. In polluted areas the value has been
found to be several hundred µg per day. In non-polluted areas,
uptake from heavy smoking may equal cadmium intake from food.
An association between cadmium exposure and increased urinary
excretion of low molecular weight proteins has been noted in humans
with a life-long daily intake of about 140-260 µg cadmium, or a
cumulative intake of about 2000 mg or more.
10.1.2 Occupationally exposed population
Occupational exposure to cadmium is mainly by inhalation but
includes additional intakes through food and tobacco. The total
cadmium level in air varies according to industrial hygiene
practices and type of workplace. There is an exposure-response
relationship between airborne cadmium levels and proteinuria. An
increase in the prevalence of low molecular weight proteinuria may
occur in workers after 10-20 years of exposure to cadmium levels of
about 20-50 µg/m3. In vivo measurement of cadmium in the liver
and kidneys of people with different levels of cadmium exposure have
shown that about 10% of workers with a kidney cortex level of
200 mg/kg and about 50% of people with a kidney cortex level of
300 mg/kg would have renal tubular proteinuria.
10.2 Recommendations for protection of human health
a) Measures to increase recycling of cadmium should be
systematically examined and promising ideas encouraged.
b) Information on the importance of minimizing waste discharge
of cadmium, particularly into surface waters, should be
supplied to countries.
c) Public health measures for protection from cadmium
exposures would be improved by:
i) collection of more data from countries on cadmium
levels in foodstuffs and the environment;
ii) determination of tissue cadmium levels and
monitoring of health parameters in non-exposed
populations and in those living near mines or
smelters or exposed to elevated levels of the metal
in foodstuffs;
iii) technical assistance to developing countries for
the training of staff, particularly for cadmium
analysis;
iv) development of means of reducing cadmium exposure
by, for instance, improved working conditions and
the dissemination of information on the proper use
of fertilizers (which sometimes contain high levels
of cadmium), techniques for the disposal of
cadmium-containing wastes, etc.
11. FURTHER RESEARCH
a) There is a need for improved analytical techniques for
measuring cadmium species and biological indicators of cadmium
exposure/toxicity, such as ß2-microglobulin, in various
matrices, and for international centres for quality assurance
and training.
b) The assessment of human exposure to cadmium from all media
needs to be improved by increased monitoring of cadmium levels
in the environment. Changes in cadmium levels with time are of
particular importance.
c) Populations with ß2-microglobinuria (both those in the
workplace and in the general environment) should be
longitudinally investigated to determine the nature, severity,
and prognosis of adverse health effects associated with this
finding. Further research is needed on ß2-microglobulin as a
biological indicator of exposure and effect.
d) International collaborative efforts should be encouraged to
examine further the role of cadmium in the development of human
cancer. Both the general population and industrial workers
should be studied with special emphasis on the development of a
common format for analysing and presenting data and the
collection of additional information on exposure to cadmium,
tobacco, and other confounding factors. Multiple exposures must
be considered. It is proposed that a collaborative study
coordinated by an international body (e.g., IARC) should
include the existing cohorts in order to obtain better exposure
data. It should also collect both exposure and effects data in
a standardized manner, so that the results of different studies
may be more readily compared. A further approach would be to
perform a collaborative prospective study identifying all those
workers who have shown evidence of an effect of cadmium on the
kidney and who would therefore be considered to have had
unusually heavy exposure. In such a study, both morbidity and
mortality data would be collected. Outcome would be studied not
only for cancer but also for sequelae to renal dysfunction.
e) Existing occupational cohorts should be linked, where
possible, to regional cancer registers to determine the
incidence of prostatic cancer (morbidity) in relation to
cadmium exposure.
f) To understand the mechanism(s) of cancer induction,
experimental studies on the bioavailability of cadmium at the
target site and the interactions between zinc and cadmium would
be of value. The role of metallothionein induction in the
target cells of the respiratory tract and its relationship to
such phenomena as DNA damage and repair and oncogene protein
structure would be of interest.
g) Further information on the long-term health consequences of
cadmium exposures in the general environment is essential, with
emphasis on renal dysfunction and other end-points such as
neurotoxicity and immunotoxicity.
h) Studies of the effects of cadmium on calcium-phosphorus
metabolism and bone density should be conducted on female
workers to clarify whether these workers are at special risk in
the occupational setting. The effect of cadmium on the placenta
and subsequent effects on the fetus, especially in multiple
pregnancies, need further study.
i) The effects of various nutritional deficiencies and of exposure
to other metals on the transport, accumulation, and toxicity of
cadmium should be investigated with special reference to bone
toxicity. These studies should be conducted in humans and
experimental animals with respect to age, sex, dose-dependence,
biological half-time, and estimation of critical concentration.
j) To provide additional scientific support for the assessment of
human health risks from cadmium exposure, studies in
experimental animals addressing the following issues should be
initiated:
* mechanism of cadmium transport into the cell and factors
controlling the process;
* mechanism of cadmium-induced toxicity with particular
emphasis on kidney and bone and the role(s) of
non-metallothionein-bound cadmium in these processes;
* mechanisms of cadmium-induced calcuria and the
relationship of this phenomenon to tubular proteinuria and
osteomalacia.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenic potential of cadmium was evaluated in 1976 by
the International Agency for Research on Cancer (IARC, 1976) and
re-evaluated in 1987 (IARC, 1987a). It was concluded in the
re-evaluation that there was limited evidence that cadmium and
cadmium compounds are carcinogenic in humans. Sufficient evidence
was available to show that cadmium and specified cadmium compounds
cause cancer in experimental animals. Cadmium was classified as a
probable human carcinogen (group 2A) (IARC, 1987a,b).
To prevent adverse pulmonary and renal effects the following
health-based limits for occupational exposure to cadmium fumes and
respirable dust were proposed by WHO (WHO, 1980): 250 µg Cd/m3 for
short-term exposures provided the recommended time-weighted average
(40 h/week) of 10 µg Cd/m3 is respected. It was further
recommended that control measures be applied when cadmium levels in
urine and blood of individuals exceed 5 µg Cd/g creatinine and
5 µg Cd/litre of whole blood, respectively.
A drinking-water guideline value of 0.005 mg/litre has been set
for cadmium by the World Health Organization (WHO, 1984).
Cadmium was evaluated by a WHO Working Group developing air
quality guidelines (WHO, 1987). Based on non-carcinogenic effects,
the following recommendations were made:
a) in rural areas, levels of < 1-5 ng/m3 should not be allowed
to increase, and
b) in urban and industrialized areas without agricultural
activities, levels of 10-20 ng/m3 may be tolerable. However,
increases in the present levels of airborne cadmium should not
be permitted (WHO, 1987).
At the thirty-third meeting of the Joint FAO/WHO Expert
Committee on Food Additives and Food Contaminants, the previous
recommendation was reaffirmed, i.e. the provisional tolerable weekly
cadmium intake of 400-500 µg for an adult should not be exceeded
(WHO, 1989).
Regulatory standards established by national bodies in several
countries and the EEC are summarized in the legal file of the
International Register of Potentially Toxic Chemicals (IRPTC, 1987).
REFERENCES
ADAMS, R.G. (1980) Osteopathy associated with tubular nephropathy in
employees in an alkaline battery factory. In: Shigematsu, I. &
Nomiyama, K., ed. Cadmium-induced osteopathy, Tokyo, Japan Public
Health Association, pp. 67-73.
ADAMS, R.C., HARRISON, J.G., & SCOTT, P. (1969) The development of
cadmium-induced proteinuria, impaired renal function, and
osteomalacia in alkaline battery workers. Q. J. Med., 38: 425-443.
ADAMSSON, E. (1979) Long-term sampling of air-borne cadmium dust in
an alkaline battery factory. Scand. J. Work Environ. Health,
5: 178-187.
ADES A.E. & KAZANTZIS, G. (1988) Lung cancer in a non-ferrous
smelter: the role of cadmium. Br. J. ind. Med., 45: 435-442.
AHLGREN, L. & MATTSON, S. (1981) Cadmium in man measured
in vivo by X-ray fluorescence analysis. Phys. Med. Biol.,
26: 19-26.
AHLMARK, S., AXELSSON, B., FRIBERG, L., & PISCATOR, M. (1961)
Further investigations into kidney function and proteinuria in
chronic cadmium poisoning. Int. Congr. occup. Health, 13: 201-203.
AHOKAS, R.A. & DILTS, P.V. (1979) Cadmium uptake by the rat embryo
as a function of gestational age. Am. J. Obstet. Gynecol.,
135: 219-222.
AKITA PREFECTURAL GOVERNMENT (1973) [Surveys on environmental
pollution due to heavy metals: Annual report of the Technical Centre
for Environmental Protection], Akita, Japan, Akita Prefectural
Government (Akita Prefecture Report No. 1: 1970 and 1971) (in
Japanese).
AL-HADDAD, I.K., CHETTLE, D.R., FLETCHER, J.G., & FREMLIN, J.H.
(1981) A transportable system for measurement of kidney cadmium
in vivo. Int. J. appl. Radiat. Isot., 32: 109-112.
ALLANSON, M. & DEANESLY, R. (1962) Observations on cadmium damage
and repair in rat testes and the effects on the pituitary
gonadotrophs. J. Endocrinol., 24: 453-462.
ALLOWAY, B.J., THORNTON, I., SMART, G.A., SHERLOCK, J.C., & QUINN,
M.J. (1988) Metal availability. Sci. total Environ., 75: 41-69.
ALT, F. (1981) [Comparative determination of cadmium in blood by
four different methods.] Fresenius Z. anal. Chem., 308: 137-142 (in
German).
ANDERSEN, A. (1979) [Lead, cadmium, copper and zinc in the Danish
diet], Copenhagen, Statens Levendsmiddelinstitut, 89 pp, (Report
No. 40) (in Danish).
ANDERSEN, O., HAGERSTRAND, I., & NORDBERG, G.F. (1982) Effect of the
chelating agent sodium tripolyphosphate on cadmium toxicity in mice.
Environ. Res., 29: 54-61.
ANDERSEN, O., RONNE, M., & NORDBERG, G.F. (1983) Effects of
inorganic metal salts on chromosome length in human lymphocytes.
Hereditas, 98: 65-70.
ANDERSON, C. & DANYLCHUK, K.D. (1979) Plasma levels of
immunoreactive parathyroid hormone in dogs chronically exposed to
low levels of cadmium chloride. J. environ. Pathol. Toxicol.,
2: 1151-1159.
ANDERSSON, A. (1977) Heavy metals in Swedish soils: on their
retention, distribution, and amounts. Swed. J. agric. Res., 7: 7-20.
ANDERSSON, A. & HAHLIN, M. (1981) Cadmium effects from phosphorus
fertilization in field experiments. Swed. J. agric. Res., 11: 3-10.
ANDERSSON, K., ELINDER, C.-G., HOGSTEDT, C., KJELLSTROM, T., &
SPANG, G. (1984) Mortality among cadmium workers in a Swedish
battery factory. In: Proceedings of the 4th International Cadmium
Conference, Munich, March 1983, London, Cadmium Association,
pp. 152-154.
ANDO, M., SHIMIZU, M., SAYATA, Y., TANIMURA, A., & TOBE, M. (1981)
The inhibition of vitamin D-stimulated intestinal calcium transport
in rats after continuous oral administration of cadmium. Toxicol.
appl. Pharmacol., 61: 297-301.
ANKE, M. & SCHNEIDER, H.J. (1974) [The trace element concentration
of human kidneys as a function of age and sex.] Z. Urol.,
67: 357-362 (in German).
ANKE, M., HENNIG, A., SCHNEIDER, H.J., GROPPEL, B., GRUN, M.,
PARTSCHEFELD, M., & LUDKE, H. (1976) [The influence of the toxic
element cadmium on the metabolism and health of animals and human.]
Math. naturwiss. Unterr. R 25: 241-261 (in German).
AOKI, A. & HOFFER, A.P. (1978) Re-examination of the lesions in rat
testis caused by cadmium. Biol. Reprod., 18: 579-591.
APOSTOLOV, CH. (1979) [The effects on the upper respiratory tract
from long-term work with cadmium.] In: [Proceedings of the Cadmium
Symposium, Jena, 1-3 August, 1977], Jena, Friedrich-Schiller
Universität, pp. 322-325 (in German).
ARMSTRONG, B.G. & KAZANTZIS, G. (1983) The mortality of cadmium
workers. Lancet, 1: 1424-1427.
ARMSTRONG, B.G. & KAZANTZIS, G. (1985) Prostatic cancer and chronic
respiratory and renal disease in British cadmium workers: a case
control study. Br. J. ind. Med., 42: 540-545.
ASOKAN, P., DIXIT, R., MUKHTAR, H., & MURTI, C.R.K. (1981) Effect of
cadmium on hepatic mixed function oxidases during the early
development of rats; possible protective role of metallothionein.
Biochem. Pharmacol., 30(22): 3095-3098.
AUFDERHEIDE, M., MOHR, U., THIEDEMANN, K.-U., & HEINRICH, U. (1990)
Quantification of hyperplastic areas in hamster lungs after chronic
inhalation of different cadmium compounds. Toxicol. environ. Chem.,
27: 173-180.
AXELSSON, B. (1963) Urinary calculus in long-term exposure to
cadmium. In: Sangro, P., Akoun, G., & Beate, H.L., ed. Proceedings
of the 14th International Congress on Occupational Health, Madrid,
Amsterdam, Excerpta Medica Foundation, pp 939-942 (International
Congress Series No. 62).
AXELSSON, B. & PISCATOR, M. (1966a) Renal damage after prolonged
exposure to cadmium: an experimental study. Arch. environ. Health,
12: 360-373.
AXELSSON, B. & PISCATOR, M. (1966b) Serum proteins in
cadmium-poisoned rabbits with special reference to haemolytic
anaemia. Arch. environ. Health, 12: 374-381.
AXELSSON, B., DAHLGREN, S.E., & PISCATOR, M. (1968) Renal lesions in
the rabbit after long-term exposure to cadmium. Arch. environ.
Health, 17: 24-28.
BAADER, E.W. (1951) [Health concerns and occupational medicine:
chronic cadmium poisoning.] Dtsch. med. Wschr., 76: 484-487 (in
German).
BAETZ, R.A. & KENNER, C.T. (1974) Determination of low levels of
cadmium in foods using a chelating ion exchange resin. J. Assoc.
Off. Anal. Chem., 57: 14-17.
BARLOW, S.M. & SULLIVAN, F.M. (1982) Cadmium and its compounds. In:
Barlow, S.M., ed. Reproductive hazards of industrial chemicals. An
evaluation of animal and human data, New York, London, Academic
Press, pp. 137-173.
BARLTROP, D. & STREHLOW, C.D. (1982a) Clinical and biochemical
indices of cadmium exposure in the population of Shipham. In:
Proceedings of the 3rd International Cadmium Conference, Miami,
Florida, 3-5 February, 1981, London, Cadmium Association,
pp. 112-113.
BARLTROP, D. & STREHLOW, C.D. (1982b) Cadmium and health in Shipham.
Lancet, 2: 1394-1395.
BARR, M., Jr (1973) The teratogenicity of cadmium chloride in two
stocks of Wistar rats. Teratology, 7: 237-242.
BARRETT, H.M. & CARD, B.Y. (1947) Studies on the toxicity of inhaled
cadmium. II. The acute lethal dose of cadmium oxide for man. J. ind.
Hyg. Toxicol., 29: 286-293.
BARRETT, H.M., IRWIN, D.A., & SEMMONS, E. (1947) Studies on the
toxicity of inhaled cadmium. I. The acute toxicity of cadmium oxide
by inhalation. J. ind. Hyg. Toxicol., 29: 279-285.
BARTHELEMY, P. & MOLINE, R. (1946) Intoxication chronique par
l'hydrate de cadmium, son signe précoce: La bague jaune dentaire.
Paris Med., 1: 71-78.
BAUCHINGER, M., SCHMID, E., EINBRODT, H.J., & DRESP, J. (1976)
Chromosome aberrations in lymphocytes after occupational exposure to
lead and cadmium. Mutat. Res., 40: 57-62.
BECKMAN, I., MOVITZ, I., NYGREN, M., & SLORACH, S. (1979) [Release
of lead and cadmium from glazed and enamelled foodware.] Var Föda,
31: 193-197 (in Swedish).
BEHNE, D. (1980) Problems of sampling and sample preparation for
trace element analysis in the health sciences. In: Bratter, P. &
Schramel, P., ed. Trace element analytical chemistry in medicine and
biology. Proceedings of the First International Workshop,
Neuherberg, Federal Republic of Germany, Berlin, New York, Walter de
Gruyter & Co., pp. 769-782.
BERLIN, M. & FRIBERG, L. (1960) Bone marrow activity and erythrocyte
destruction in chronic cadmium poisoning. Arch. environ. Health,
1: 478-486.
BERMAN, E. (1967) Determination of Cd, Tl, and Hg in biological
materials by AA. At. Absorpt. Newsl., 6: 57-60.
BERNARD, A.M., ROELS, H., HUBERMONT, G., BUCHET, J.P., MASSON, P.L.,
& LAUWERYS, R. (1976) Characterization of the proteinuria in
cadmium-exposed workers. Int. Arch. occup. environ. Health,
38: 19-30.
BERNARD, A.M., BUCHET, J.P., ROELS, H., MASSON, P., & LAUWERYS, R.
(1979) Renal excretion of proteins and enzymes in workers exposed
to cadmium. Eur. J. clin. Invest., 9: 11-22.
BERNARD, A.M., LAUWERYS, R., & GENGOUX, P. (1981) Characterization
of the proteinuria induced by prolonged oral administration of
cadmium in female rats. Toxicology, 20: 345-357.
BERNARD, A.M., MOREAU, D., & LAUWERYS, R. (1982a) Latex immunoassay
of retinol binding protein. Clin. Chem., 28: 1167-1171.
BERNARD, A.M., MOREAU, D., & LAUWERYS, R. (1982b) Comparison of
retinol-binding protein and ß-2-microglobulin determination in urine
for the early detection of tubular proteinuria. Clin. Chim. Acta,
126: 1-9.
BETON, D.C., ANDREWS, G.S., DAVIES, H.J., HOWELLS, L., & SMITH, G.F.
(1966) Acute cadmium fume poisoning: five cases with one death from
renal necrosis. Br. J. ind. Med., 23: 292-301.
BHATTACHARYYA, M.H., WHELTON, B.D., PETERSON, D.P., CARNES, B.A.,
MORETTI, E.S., TOOMEY, J.M., & WILLIAMS, L.L. (1988) Skeletal
changes in multiparous mice fed a nutrient-sufficient diet
containing cadmium. Toxicology, 50: 193-204.
BIGGIN, H.C., CHEN, N.S., ETTINGER, K.V., FREMLIN, J.H., MORGAN,
W.D., NOWOTNY, R., CHAMBERLAIN, M.J., & HARVEY, T.C. (1974) Cadmium
by in vivo neutron activation analysis. J. radioanal. Chem.,
19: 207-214.
BJORNBERG, A. (1963) Reactions to light in yellow tattoos from
cadmium sulfide. Arch. Dermatol., 88: 267-271.
BLEJER, H.P. (1966) Death due to cadmium oxide fumes. Ind. Med.
Surg., 35: 363-364.
BOISSET, M. & BOUDENE, C. (1981) Effect of a single exposure to
cadmium oxide fumes on rat lung microsomal enzymes. Toxicol. appl.
Pharmacol., 57: 335-345.
BONNELL, J.A. (1955) Emphysema and proteinuria in men casting
copper-cadmium alloys. Br. J. ind. Med., 12: 181-197.
BONNELL, J.A., KAZANTZIS, G., & KING, E. (1959) A follow-up study of
men exposed to cadmium oxide fume. Br. J. ind. Med., 16: 135-147.
BONNELL, J.A., ROSS, J.H., & KING, E. (1960) Renal lesions in
experimental cadmium poisoning. Br. J. ind. Med., 17: 69-80.
BOULEY, G., DUBREUIL, A., DESPAUX, N., & BOUDENE, C. (1977) Toxic
effects of cadmium microparticles on the respiratory system. An
experimental study on rats and mice. Scand J. Work Environ. Health,
3: 116-121.
BOYLE, E.A., SCALTER, F., & EDMOND, J.M. (1976) On the marine
geochemistry of cadmium. Nature (Lond.), 263: 42-44.
BOZELKA, B.E. & BURKHOLDER, P.M. (1982) Inhibition of mixed
leukocyte culture response in cadmium-treated mice. Environ. Res.,
27: 421-432.
BRADY, F.O. (1982) The physiological function of metallothionein.
Trends biochem. Sci., 7: 143-145.
BRAUX, P., CLAEYS-THOREAU, F., DE PLAAN, P., DUCOFFRE, G., AMERY,
A., LAUWERYS, R., RONDIA, D., STAESSEN, J., ROELS, H., SARTON, F.,
BERNARD, A., & BUCHET, J.P. (1987) The Belgian Cadmibel Study:
Environmental exposure to cadmium and lead-health effects. In:
Lindberg, S.F. & Hutchinson, J.C., ed. Proceedings of an
International Conference on Heavy Metals in the Environment, New
Orleans, Edinburgh, CEP Consultants Ltd, pp. 349-351.
BRYAN, G.W., LANGSTON, W.J., & HUMMERSTONE L.G. (1980) The use of
biological indicators of heavy-metal contamination in estuaries with
special reference to an assessment of the biological availability of
metals in estuarine sediments from south-west Britain, Citadel Hill,
Devon, The Marine Biological Association of the United Kingdom,
73 pp (Occasional Publication No. 1).
BUATMENARD, P. & ARNOLD, M. (1978) Heavy-metal chemistry of
atmospheric particulate matter emitted by Mount Etna volcano. Geogr.
Res. Lett., 5: 245-248.
BUCHAUER, M.J. (1972) The effects of zinc and cadmium pollution on
forest vegetation. Bull. New Jersey Acad. Sci., 17(2): 42.
BUCHET, J.P., ROELS, H., BERNARD, A., & LAUWERYS, R. (1980)
Assessment of renal function of workers exposed to inorganic lead,
cadmium or mercury vapor. J. occup. Med., 22: 741-748.
BUCHET, J.P., ROELS, H., LAUWERYS, R., VANDEVOORDE, A., & PYCKE,
J.M. (1983) Oral daily intake of cadmium, lead, manganese, copper,
chromium, mercury, calcium, zinc, and arsenic in Belgium: a
duplicate meal study. Food chem. Toxicol., 21: 19-24.
BUCKE, D., NORTON, M.G., & ROLFE, M.S. (1983) Field assessment of
effects of dumping wastes at sea: II. Epidermal lesions and
abnormalities of fish in the outer Thames estuary, London, Ministry
of Agriculture, Fisheries and Food, 29 pp (Technical Report No. 72).
BUI, T.-H-. LINDSTEN, J., & NORDBERG, G.F. (1975) Chromosome
analysis of lymphocytes from cadmium workers and Itai-itai patients.
Environ. Res., 9: 187-195.
BUS, J.S., VINEGAR, A., & BROOKS, S.M. (1978) Biochemical and
physiologic changes in lungs of rats exposed to a cadmium chloride
aerosol. Am. Rev. respir. Dis., 118: 573-580.
BUTLER, E.A. & FLYNN, F.V. (1958) The proteinuria of renal tubular
disorders. Lancet, 2: 978-980.
BUXTON, R., St J. (1956) Respiratory function in cadmium alloy
casters. Part 2. The estimation of the total lung volume, its
subdivisions and the mixing coefficient. Br. J. ind. Med.,
13: 36-40.
CARLIER, Y., COLLE, A., TACHON, P., BOUT, D., & CAPRON, A. (1981)
Quantification of B-2-microglobulin by enzyme inhibition
immunoassay. J. immunol. Methods, 40: 231-238.
CARLSON, L.A. & FRIBERG, L. (1957) The distribution of cadmium in
blood after repeated exposure. Scand. J. clin. lab. Invest., 9: 1-4.
CARLTON-SMITH, C.H. (1987) Effects of metals in sludge-treated soils
on crops, Medmenham, United Kingdom, Water Research Centre
(Environment TR/251).
CARROLL, R.E. (1966) The relationship of cadmium in the air to
cardiovascular disease death rates. J. Am. Med. Assoc.,
198: 267-269.
CARRUTHERS, M. & SMITH, B. (1979) Evidence of cadmium toxicity in a
population living in a zinc-mining area. Lancet, 1: 845-847.
CARVALHO, F.M., TAVARES, T.M., SILVANY-NETO, A.M., LIMA, M.E.C., &
ALT, F. (1986) Cadmium concentrations in blood of children living
near a lead smelter in Bahia, Brazil. Environ. Res., 40: 437-449.
CASTERLINE, J.L. & YIP, G. (1975) The distribution and binding of
cadmium in oyster, soybean, rat liver, and kidney. Arch. environ.
Contam. Toxicol., 3: 319-329.
CASTILHO, P.D. & HERBER, R.F.M. (1977) The rapid determination of
cadmium, lead, copper, and zinc in whole blood by atomic absorption
spectrometry with electrothermal atomization. Improvements in
precision with a peak-shape monitoring device. Anal. Chim. Acta,
94: 269-270.
CHADWICK, M.J. & LINDMAN, N. (1982) Environmental implications of
expanded coal utilization, Oxford, New York, Pergamon Press, 283 pp.
CHAPATWALA, K.D., HOBSON, M., DESAIAH, D., & RAJANNA, B. (1982)
Effect of cadmium on hepatic and renal gluconeogenic enzymes in
female rats. Toxicol. Lett., 12: 27-34.
CHAUBE, S., NISHIMURA, H., & SWINYARD, C.A. (1973) Zinc and cadmium
in normal human embryos and fetuses. Arch. environ. Health,
26: 237-240.
CHERIAN, M.G. (1980) The synthesis of metallothionein and cellular
adaptation to metal toxicity in primary kidney epithelial cell
cultures. Toxicology, 17: 225-231.
CHERIAN, M.G. & GOYER, R.A. (1978) Metallothioneins and their role
in the metabolism and toxicity of metals. Life Sci., 23: 1-10.
CHERIAN, M.G. & NORDBERG, M. (1983) Cellular adaptation in metal
toxicology and metallothionein. Toxicology, 28: 1-15.
CHERIAN, M.G. & SHAIKH, Z.A. (1975) Metabolism of
intravenously-injected cadmium-binding protein. Biochem. biophys.
Res. Commun., 65: 863-869.
CHERIAN, M.G. & VOSTAL, J.J. (1977) Biliary excretion of cadmium in
rat. I. Dose-dependent biliary excretion and the form of cadmium in
the bile. J. Toxicol. environ. Health, 2: 945-954.
CHERIAN, M.G., GOYER, R.A., & RICHARDSON, L.D. (1976) Cadmium
metallothionein-induced nephropathy. Toxicol. appl. Pharmacol.,
38: 399-408.
CHERIAN, M.G., GOYER, R.A., & VALBERG, L.S. (1978) Gastrointestinal
absorption and organ distribution of oral cadmium chloride and
cadmium metallothionein in mice. J. Toxicol. environ. Health,
4: 861-868.
CHERNOFF, N. (1973) Teratogenic effects of cadmium in rats.
Teratology, 8: 29-32.
CHERRY, W.H. (1981) Distribution of cadmium in human tissues. In:
Nriagu, J.O., ed. Cadmium in the environment - II, New York,
Chichester, John Wiley & Sons, pp. 111-122.
CHIQUOINE, A.D. (1965) Effect of cadmium chloride on the pregnant
albino mouse. J. Reprod. Fertil., 10: 263-265.
CHRISTOFFERSON, J.O. & MATTSON, S. (1983) Polarized X-rays in
XRF-analysis for improved in vivo detectability of cadmium in man.
Phys. Med. Biol., 28: 1135-1144.
CHUMBLEY, C.G. & UNWIN, R.J. (1982) Cadmium and lead content of
vegetable crops grown on land with a history of sewage sludge
application. Environ. Pollut. Ser. B Chem. Phys., 4(3): 231-238.
CHUNG, J., NARTY, N.O., & CHERIAN, M.G. (1986) Metallothionein
levels in liver and kidney of Canadians - A potential indicator of
environmental exposure to cadmium. Arch. environ. Health, 41(5):
319-323.
CIRKT, M. & TICHY, M. (1974) Excretion of cadmium through bile and
intestinal wall in rats. Br. J. ind. Med., 31: 134-139.
CLARKSON, T.W. & KENCH, J.E. (1956) Urinary excretion of amino acids
by men absorbing heavy metals. Biochem. J., 62: 361-372.
CNATTINGIUS, S., AXELSSON, O., EKLUND, G., & LINDMARK, G. (1985)
Smoking, maternal age and fetal growth. Obstet. Gynecol.,
66: 449-452.
COLE, G.M. & BAER, L.S. (1944) Food poisoning from cadmium. US nav.
med. Bull., 43: 398-399.
COMMISSION OF THE EUROPEAN COMMUNITIES (1978) Criteria (dose-effect
relationships) for cadmium, Oxford, Pergamon Press, 202 pp (Report
of the Working Group of Experts prepared for the CEC,
Directorate-General for Social Affairs, Health and Safety
Directorate, Luxembourg).
COOPERATIVE RESEARCH COMMITTEE ON ITAI-ITAI DISEASE (1967) [Report
on a study on the so-called Itai-itai disease], Tokyo, Ministry of
Education and Ministry of Health and Welfare, 206 pp (in Japanese).
COPIUS-PEEREBOOM, J.W., DE VOOGT, P., VAN HATTUM, B., VAN DER VELDE,
W., & COPIUS-PEEREBOOM-STEGEMAN, J.H.J. (1979) The use of the human
placenta as a biological indicator for cadmium exposure. In:
International Conference on Management and Control of Heavy Metals
in the Environment, London, September, 1979, Edinburgh, CEP
Consultants Ltd, pp. 8-10.
COUSINS, R.J., BARBER, A.K., & TROUT, J.R. (1973) Cadmium toxicity
in growing swine. J. Nutr., 103: 964-972.
CULBARD, E.B., THORNTON, I., WATT, J., WHEATLEY, M., MOORCROFT, S.,
& THOMPSON, M. (1988) Metal contamination in British urban dusts and
soils. J. environ. Qual., 17(2): 227-234.
CVETKOVA, R.P. (1970) [Materials on the study of the influence of
cadmium compounds on the generative function.] Gig. Tr. prof.
Zabol., 14: 31-33 (in Russian with English summary).
DALHAMN, T. & FRIBERG, L. (1954) The effect of cadmium on blood
pressure and respiration and the use of dimercaprol (BAL) as
antidote. Acta pharmacol. toxicol., 10: 199-203.
DANIELSSON, L.-G., JAGNER, D., JOSEFSON, M., & WESTERLUND, S. (1981)
Computerized potentiometric stripping analysis for the determination
of cadmium, lead, copper, and zinc in biological materials. Anal.
Chim. Acta, 127: 147-156.
DASTON, G.P. (1982) Fetal zinc deficiency on a mechanism for
cadmium-induced toxicity to the developing rat lung and pulmonary
surfactant. Toxicology, 24: 55-63.
DAVIES, N.T., BREMNER, I., & MILLS, C.F. (1973) Studies on the
induction of a low molecular weight zinc-binding protein in rat
liver. Biochem. Soc. Trans., 1: 985-988.
DAVIS, R.D. (1984) Cadmium - a complex environmental problem. Part
II. Cadmium in sludges used as fertilizer. Experientia (Basel),
40(2): 117-126.
DAVIS, R.D. & COKER, E.G. (1980) Cadmium in agriculture, with
special reference to the utilisation of sewage sludge on land,
Medmenham, United Kingdom, Water Research Centre (Technical Report
TR/139).
DAVISON, A.G., NEWMAN-TAYLOR, A.J., DARBYSHIRE, J., & CHETTLE, D.R.
(1988) Cadmium fume inhalation and emphysema. Lancet, 1: 663-667.
DEAVEN, L.L. & CAMPBELL, E.W. (1980) Factors affecting the induction
of chromosomal aberrations by cadmium in Chinese hamster cells.
Cytogenet. cell Genet., 26: 251-260.
DECKER, L.E., BYERRUM, R.U., DECKER, C.F., HEPPERT, C.A., & LANGHAM,
R.F. (1958) Chronic toxicity studies. I. Cadmium administered in
drinking-water to rats. Am. Med. Assoc. Arch. ind. Health,
18: 228-231.
DEKNUDT, G. & DEMINATTI, M.L. (1978) Chromosome studies in human
lymphocytes after in vitro exposure to metal salts. Toxicology,
10: 67-75.
DEKNUDT, G. & LEONARD, A. (1975) Cytogenetic investigations on
leukocytes of workers from a cadmium plant. Environ. Physiol.
Biochem., 5: 319-327. DELVES, H.T. (1970) Micro-sampling method for
the rapid determination of lead in blood by atomic absorption
spectrophotometry. Analyst, 95: 431-438.
DELVES, H.T. (1982) Analytical techniques for measuring cadmium in
blood. In: Proceedings of the International Workshop on Biological
Indicators on Cadmium Exposure Diagnostic and Analytical
Reliability, Luxembourg, 7-9 July, 1982, Luxembourg, Commission of
the European Communities, pp. 1-15.
DELVES, H.T. & WOODWARD, J. (1981) Determination of low levels of
cadmium in blood by electrothermal atomization and atomic absorption
spectrophotometry. At. Spectrosci., 2: 65-67.
DENCKER, L., DANIELSSON, B., KHAYAT, A., & LINDGREN, A. (1983)
Disposition of metals in the embryo and fetus. In: Clarkson, T.W.,
Nordberg, G.F., & Sager, P.R., ed. Reproductive and developmental
toxicity of metals, New York, London, Plenum Press, pp. 607-632.
DERVAN, P.A. & HAYES, J.A. (1979) Peribronchiolar fibrosis following
acute experimental lung damage by cadmium aerosol. J. Pathol.,
128: 143-148.
DE SILVA, P.E. & DONNAN, M.B. (1981) Chronic cadmium poisoning in a
pigment-manufacturing plant. Br. J. ind. Med., 38: 76-86.
DUCE, R.A., HOFFMAN, G.L., & ZELLER, W.H. (1975) Atmospheric trace
metals at remote northern and southern hemisphere sites: pollution
or natural(?). Science, 187: 59-61.
DUCE, R.A., ARIMOTO, R., RAY, B.J., UNNI, C.K., & HARDER, P.J.
(1983) Atmospheric trace elements at Enewetok Atoll. I.
Concentration, sources, and temporal variability. J. geophys. Res.,
88: 5321-5342.
DUDLEY, R.E., SVOBODA, D.J., & KLAASSEN, C.O. (1982) Acute exposure
to cadmium causes severe liver injury in rats. Toxicol. appl.
Pharmacol., 65: 302-313.
DUDLEY, R.E., GAMMAL, L.M., & KLAASSEN, C.D. (1985) Cadmium-induced
hepatic and renal injury in chronically exposed rats: likely role of
hepatic cadmium-metallothionein in nephrotoxicity. Toxicol. appl.
Pharmacol., 77: 414-426.
DUGGAN, M.J., INSKIP, M.J., RUNDLE, S.A., & MOORCROFT, J.S. (1985)
Lead in playground dust and on the hands of schoolchildren. Sci.
total Environ., 44(1): 65-79.
DUNPHY, B. (1967) Acute occupational cadmium poisoning: a critical
review of the literature. J. occup. Med., 9: 22-26.
EDIGER, R.D. & COLEMAN, R.L. (1973) Determination of cadmium in
blood by a Delves cup technique. At. Absorpt. Newsl., 12: 3-6.
EDLING, C., ELINDER, C.G., & RANDMA, E. (1986) Lung function in
workers using cadmium containing solders. Br. J. ind. Med.,
43: 657-662.
ELINDER, C.-G. & PANNONE, M. (1979) Biliary excretion of cadmium.
Environ. Health Perspect., 28: 123-126.
ELINDER, C.-G. & PISCATOR, M. (1978) Cadmium and zinc in horses. In:
Kirchgessner, M., ed. Proceedings of the 3rd International
Conference on Trace Elements in Man and Animals,
Freising-Weihenstephan, Federal Republic of Germany, 25-29 July,
1977, Freising-Weihenstephan, Munich Technical University,
pp. 569-572.
ELINDER, C.-G., KJELLSTRÖM, T., LIND, B., & LINNMAN, L. (1976)
Cadmium concentration in kidney cortex, liver, and pancreas among
autopsied Swedes. Arch. environ. Health, 31: 292-302.
ELINDER, C.-G., KJELLSTRÖM, T., LINNMAN, L., & PERSHAGEN, G. (1978)
Urinary excretion of cadmium and zinc among persons from Sweden.
Environ. Res., 15: 473-484.
ELINDER, C.-G., JONSSON, L., PISCATOR, M., & RAHNSTER, B. (1981a)
Histopathological changes in relation to cadmium concentration in
horse kidneys. Environ. Res., 26: 1-21.
ELINDER, C.-G., NORDBERG, M., PALM, B., & PISCATOR, M. (1981b)
Cadmium, zinc, and copper in horse kidney and in horse liver
metallothionein: comparison with kidney cortex. Environ. Res.,
26: 22-32.
ELINDER, C.-G., KJELLSTRÖM, T., LIND, B., LINNMAN, L., PISCATOR, M.,
& SUNDSTEDT, K. (1983) Cadmium exposure from smoking cigarettes:
variations with time and country where purchased. Environ. Res.,
32: 220-227.
ELINDER, C.-G., EDLING, C., LINDBERG, E., KAGEDAL, B., & VESTERBERG,
O. (1985a) Assessment of renal function in workers previously
exposed to cadmium. Br. J. ind. Med., 42: 754-760.
ELINDER, C.-G., EDLING, C., LINDBERG, E., KAGEDAL, B., & VESTERBERG,
O. (1985b) B-2-microglobulinuria among workers previously exposed
to cadmium: follow-up and dose-response analyses. Am. J. ind. Med.,
8: 553-564.
ELINDER, C.-G., KJELLSTRÖM, T., HOGSTEDT, C., ANDERSON, K., & SPENG,
G. (1985c) Cancer mortality of cadmium workers. Br. J. ind. Med.,
42: 651-655.
ELLIS, K.J., MORGAN, W.D., ZANZI, I., YASUMURA, S., VARTSKY, D.D., &
COHN, S.H. (1981a) Critical concentrations of cadmium in human renal
cortex: dose-effect studies in cadmium smelter workers. J. Toxicol.
environ. Health, 7: 691-703.
ELLIS, K.J., YASAMURA, S., & COHN, S.H. (1981b) Hair cadmium
content: is it a biological indicator of the body burden of cadmium
for the occupationally-exposed worker? Am. J. ind. Med., 2: 323-333.
ELLIS, K.J., YUEN, K., YASAMURA, S., & COHN, S.H. (1984)
Dose-response analysis of cadmium in man: body burden vs kidney
dysfunction. Environ. Res., 33: 216-226.
ELLIS, K.J., COHN, S.H., & SMITH, T.J. (1985) Cadmium inhalation
exposure estimates: their significance with respect to kidney and
liver cadmium burden. J. Toxicol. environ. Health, 15: 173-187.
ENGSTROM, B. (1979) Factors influencing metabolism and toxicity of
cadmium. Experimental studies on mice with special reference to
chelating agents, Stockholm, Department of Environmental Hygiene,
Karolinska Institute, 80 pp (Doctoral thesis). ENGSTROM, B. &
NORDBERG, G.F. (1978) Effects of detergent formula chelating agents
on the metabolism and toxicity of cadmium in mice. Acta pharmacol.
toxicol., 43: 387-397.
ENGSTROM, B. & NORDBERG, G.F. (1979a) Dose dependence of
gastrointestinal absorption and biological half-time of cadmium in
mice. Toxicology, 13: 215-222.
ENGSTROM, B. & NORDBERG, G.F. (1979b) Factors influencing absorption
and retention of oral 109Cd in mice: age, pretreatment, and
subsequent pretreatment with non-radioactive cadmium. Acta
pharmacol. toxicol., 45: 315-324.
ENGVALL, J. & PERK, J. (1985) Prevalence of hypertension among
cadmium-exposed workers. Arch. environ. Health, 40: 185-190.
ESSING, H.-G., SCHALLER, K.-H., SZADOWSKI, D., & LEHNERT, G. (1969)
[Normal cadmium load on man from food and beverages.] Arch. Hyg.
Bakteriol., 153: 490-494 (in German).
EVRIN, P.E., PETERSON, P.A., WIDE, L., & BERGGARD, I. (1971)
Radioimmuno-assay of ß-2-microglobulin in human biological fluids.
Scand. J. clin. lab. Invest., 28: 439-443.
EYBL, V. & SYKORA, J. (1966) [The protection by chelating agents in
acute cadmium poisoning.] Acta biol. med. Germ., 16: 61-64 (in
German).
FELDMAN, S.L. & COUSINS, R.G. (1973) Influence of cadmium on the
metabolism of 25-hydroxycholecalciferol in chicks. Nutr. Rep. Int.,
8: 251-259.
FELTEN, T.L. (1979) A preliminary report of cadmium-induced
chromosomal changes in somatic and germinal tissues of C57BI/6J male
mice. Genetics, 88: 26-27.
FERM, V.H. (1971) Developmental malformations induced by cadmium.
Biol. Neonat., 19: 101-107.
FERM, V.H. (1972) The teratogenic effects of metals on mammalian
embryos. Adv. Teratol., 5: 51-75.
FERM, V.H. & CARPENTER, S.J. (1968) The relationship of cadmium and
zinc in experimental mammalian teratogenesis. Lab. Invest.,
18: 429-432.
FERNANDEZ, F.J. & KAHN, H.L. (1971) The determination of lead in
whole blood by atomic absorption spectrophotometry with the "Delves
Sampling Cup" technique. At. Absorpt. Newsl., 10: 1-5.
FINGERLE, H., FISCHER, G., & CLASSEN, H.G. (1982) Failure to produce
hypertension in rats by chronic exposure to cadmium. Food chem.
Toxicol., 20: 301-306.
FITZHUGH, O.G. & MEILLER, F.H. (1941) The chronic toxicity of
cadmium. J. Pharmacol. exp. Ther., 72: 15-20.
FLANAGAN, P.R., MCLELLAN, J.S., HAIST, J., CHERIAN, M.G.,
CHAMBERLAIN, M.J., & VALBERG, L.S. (1978) Increased dietary cadmium
absorption in mice and human subjects with iron deficiency.
Gastroenterology, 74: 841-846.
FLETCHER, J.C., CHETTLE, D.R., & AL-HADDAD, I.K. (1982) Experience
with the use of cadmium measurements of liver and kidney. J.
radioanal. Chem., 71: 547-560.
FORSTNER, U. (1980) Cadmium in the environment, Part I. In: Nriagu,
J.O., ed. Cadmium in polluted sediments, New York, Chichester, John
Wiley & Sons, pp. 305-363.
FOULKES, E.C. (1982) Role of metallothionein in transport of heavy
metals. In: Foulkes, E.C., ed. Biological roles of metallothionein,
Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp. 131-140.
FOWLER, B.A. & NORDBERG, G.F. (1978) The renal toxicity of cadmium
metallothionein: morphometric and X-ray microanalytical studies.
Toxicol. appl. Pharmacol., 46: 609-623.
FOWLER, B.A., JONES, H.S., BROWN, H.W., & HASEMAN, J.K. (1975) The
morphological effects of chronic cadmium administration on the renal
vasculature of rats given low and normal calcium diets. Toxicol.
appl. Pharmacol., 34: 233-252.
FOX, M.R.S. & FRY, B.E., Jr (1970) Cadmium toxicity decreased by
dietary ascorbic acid supplements. Science, 169: 989-991.
FOX, M.R.S., FRY, B.E., Jr, HARLAND, B.F., SCHERTAL, M.E., & WEEKS,
C.E. (1971) Effect of ascorbic acid on cadmium toxicity in the
young coturnix. J. Nutr., 101: 1295-1305.
FRANCAVILLA, S., MOSCARDELLI, S., FRANCAVILLA, F., CASASANTA, N.,
PROPERZI, G., MARTINI, M., & SANTIEMMA, V. (1981) Acute cadmium
intoxication: influence of cyproterone acetate on the testis and
epididymis of the rat. Arch. Andrology, 6: 1-11.
FRANK, A., PETERSON, L., & MORNER, T. (1981) [Lead and cadmium
levels in organs from moose, deer, and hare.] Svensk
Veterinaertidn., 33: 151-156 (in Swedish).
FRIBERG, L. (1948a) Proteinuria and kidney injury among workmen
exposed to cadmium and nickel dust. J. ind. Hyg. Toxicol.,
30: 32-36.
FRIBERG, L. (1948b) Proteinuria and emphysema among workers exposed
to cadmium and nickel dust in a storage battery plant. Proc. Int.
Congr. Ind. Med., 9: 641-644.
FRIBERG, L. (1950) Health hazards in the manufacture of alkaline
accumulators with special reference to chronic cadmium poisoning.
Acta med. Scand., 138(Suppl. 240): 1-124.
FRIBERG, L. (1952) Further investigations on chronic cadmium
poisoning. A study on rabbits with radioactive cadmium. Arch. ind.
occup. Med., 5: 30-36.
FRIBERG, L. (1955) Iron and liver administration in chronic cadmium
poisoning and studies on the distribution and excretion of cadmium.
Experimental investigations in rabbits. Acta pharmacol.,
11: 168-178.
FRIBERG, L. (1979) Cadmium in Shipham. Lancet, 1: 823-824.
FRIBERG, L. (1988) Biological monitoring of toxic metals, New York,
London, Plenum Press, pp. 103-126.
FRIBERG, L. & NYSTROM, A. (1952) [Aspects on the prognosis of
chronic cadmium poisoning.] Läkartidningen, 49: 2629-2639 (in
Swedish).
FRIBERG, L. & VAHTER, M. (1983) Assessment of exposure to lead and
cadmium through biological monitoring: results of a UNEP/WHO global
study. Environ. Res., 30: 95-128.
FRIBERG, L., PISCATOR, M., NORDBERG, G., & KJELLSTRÖM, T. (1974)
Cadmium in the environment, 2nd ed., Cleveland, Ohio, CRC Press,
248 pp.
FRIBERG, L., KJELLSTRÖM, T., NORDBERG, G., & PISCATOR, M. (1975)
Cadmium in the environment. III. A toxicological and epidemiological
appraisal, Washington, DC, US Environmental Protection Agency,
Office of Research and Development, 217 pp.
FRIBERG, L., ELINDER, C.G., KJELLSTRÖM, T., & NORDBERG, G.F. (1985)
Cadmium and health, a toxicological and epidemiological appraisal.
Vol. I. Exposure, dose and metabolism, Cleveland, Ohio, CRC Press,
209 pp.
FRIBERG, L., ELINDER, C.G., KJELLSTRÖM, T., & NORDBERG, G.F. (1986)
Cadmium and health, a toxicological and epidemiological appraisal.
Vol. II. Effects and response, Cleveland, Ohio, CRC Press, 303 pp.
FUKUHARA, M., BOULEY, G., GODIN, J., GIRARD, F., BOISSET, M., &
BOUDENE, C. (1981) Effects of short-term inhalation of cadmium
oxides on rabbit pulmonary microsomal enzymes. Biochem. Pharmacol.,
30: 715-720.
FUKUSHIMA, I. (1978) Environmental pollution and health effects -
Gunma Prefecture. In: Tsuchiya, K., ed. Cadmium studies in Japan: a
review, Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp. 170-181.
FUKUSHIMA, M., ISHIZAKI, A., SAKAMOTO, M., & KOBAYASHI, E. (1973)
[Cadmium concentrations in rice eaten by farmers in the Jinzu river
basin.] Jpn J. Hyg., 28: 406-415 (in Japanese with English summary).
FUKUSHIMA, M., ISHIZAKI, A., NOGAWA, K., SAKAMOTO, M., & KOBAYASHI,
E. (1974) [Epidemiological studies on renal failure of inhabitants
in "Itai-itai" disease endemic district. I. Some urinary findings of
inhabitants living in and around the endemic district of the Jinzu
river basin.] Jpn. J. Public Health, 21: 65-73 (in Japanese with
English summary).
FUKUSHIMA, I., NAKAJIMA, T., HIGUSHI, Y., TSUCHIYA, T., NAKAJIMA,
K., TANAKA, T., FUKAMACHI, M., ARAI, K., & SHIONO, T. (1975)
[Results of screening examination on tubular dysfunction in aged
persons confined to bed], Tokyo, Japan Public Health Association,
pp. 138-142 (Kankyo Hoken Report No. 36) (in Japanese).
FUKUYAMA, Y. (1972) [Proteinuria in Itai-itai disease and cadmium
poisoning.] Med. Biol., 84: 41-46 (in Japanese).
FULLMER, C.S., OKU, T., & WASSERMAN, R.H. (1980) Effect of cadmium
administration on intestinal calcium absorption and vitamin
D-dependent calcium-binding protein. Environ. Res., 22: 386-399.
GABBIANI, G. (1966) Action of cadmium chloride on sensory ganglia.
Experientia (Basel), 22: 261-264.
GABBIANI, G., BAIC, D., & DELZIEL, C. (1967) Toxicity of cadmium for
the central nervous system. Exp. Neurol., 18: 154-160.
GABBIANI, G., BADONNEL, M.-C., MATHEWSON, S.M., & RYAN, G.G. (1974)
Acute cadmium intoxication: early selective lesions of endothelial
clefts. Lab. Invest., 30: 686-695.
GALE, T.F. (1973) The interaction of mercury with cadmium and zinc
in mammalian embryonic development. Environ. Res., 6: 95-105.
GARDINER, P.E., OTTAWAY, J.M., & FELL, G.S. (1979) Accuracy of the
direct determination of cadmium in urine by carbon-furnace atomic
absorption spectrometry. Talanta, 26: 841-847.
GARTY, M., WONG, K.-L., & KLAASSEN, C.D. (1981) Redistribution of
cadmium to blood of rats. Toxicol. appl. Pharmacol., 59: 548-554.
GERVAIS, J. & DELPECH, P. (1963) L'intoxication cadmique. Arch. Mal.
prof. Méd. Trav. Séc. soc., 24: 803-815.
GESAMP (1984) IMO/FAO/UNESCO/WMO/IAEA/UN/UNEP Joint Group of Experts
on the Scientific Aspects of Marine Pollution: Report of the
Fourteenth Session, Vienna, 26-30 March, 1984, Vienna, International
Atomic Energy Agency (Reports and Studies No. 21).
GESAMP (1985) IMO/FAO/UNESCO/WMO/IAEA/UN/UNEP Joint Group of Experts
on the Scientific Aspects of Marine Pollution: Atmospheric transport
of contaminants into the mediterranean region, Athens, Geneva, World
Meteorological Association (Reports and Studies No. 26).
GESAMP (1987) IMO/FAO/UNESCO/WMO/WHO/IAEA/UN/UNEP Joint Group of
Experts on the Scientific Aspects of Marine Pollution: Report of the
seventeenth session, Rome, Geneva, World Health Organization
(Reports and Studies No. 31).
GLEIT, C.E. (1965) High-frequency electrodeless discharge system for
ashing organic matter. Anal. Chem., 37: 314-315.
GLENNAS, A. & RUGSTAD, H.E. (1984) Cadmium uptake and metabolism in
cultured cells. Environ. Health Perspect., 54: 45-50.
GOERING, P.L., SQUIBB, K.S., & FOWLER, B.A. (1985) Calcuria and
proteinuria during cadmium-metallothionein-induced proximal tubule
cell injury. In: Memphill, D., ed. Proceedings of the University of
Missouri's 19th Annual Conference on Trace Substances in
Environmental Health, 3-6 June 1985, Columbia, Missouri, University
of Missouri Press, pp. 22-35.
GOYER, R.A., CHERIAN, M.G., & DELAQUERRIERE-RICHARDSON, L. (1984)
Correlation of parameters of cadmium exposure with onset of
cadmium-induced nephropathy in rats. J. environ. Pathol. Toxicol.
Oncol., 5: 89-100.
GOYER, R.A., HAUST, M.D., & CHERIAN, M.G. (1989) Localization of
metallothionein in placenta. Toxicologist, 9: 130.
GREENBERG, R.R., GORDON, G.E., ZOLLER, W.H., JACKO, R.B., NEVENDORF,
D.W., & YOST, K.J. (1978) Composition of particles emitted from the
Nocosia municipal incinerator. Environ. Sci. Technol., 12(12):
1329-1332.
GUNN, S.A. & GOULD, T.C. (1957) Selective accumulation of 115Cd by
cortex of rat kidney. Proc. Soc. Exp. Biol. Med., 96: 820-823.
GUNN, S.A. & GOULD, T.C. (1970) Cadmium and other mineral elements.
In: Johnson, A.D., Gomes, W.R., & VanDeMark, N.L., ed. The testis,
New York, London, Academic Press, Vol. 3, pp. 377-481.
GUNN, S.A., GOULD, T.C., & ANDERSON, W.A.D. (1961) Zinc protection
against cadmium injury to rat testis. Arch. Pathol., 71: 274.
GUNN, S.A., GOULD, T.C., & ANDERSON, W.A.D. (1963) Cadmium-induced
inter-stitial cell tumours in rats and mice and their prevention by
zinc. J. Natl Cancer Inst., 31: 745-759.
GUNN, S.A., GOULD, T.C., & ANDERSON, W.A.D. (1965) Strain
differences in susceptibility of mice and rats to cadmium-induced
testicular damage. J. Reprod. Fertil., 10: 273-275.
GUNN, S.A., GOULD, T.C., & ANDERSON, W.A.D. (1967) Specific response
of mesenchymal tissue to cancerogenesis by cadmium. Arch. Pathol.,
83: 439-499.
GUNN, S.A., GOULD, T.C., & ANDERSON, W.A.D. (1968) Specificity in
protection against lethality and testicular toxicity from cadmium.
Proc. Soc. Exp. Biol. Med., 128: 591-595.
GUTHRIE, B.E. & ROBINSON, M.F. (1977) Daily intakes of manganese,
copper, zinc, and cadmium by New Zealand women. Br. J. Nutr.,
38: 55-63.
GUTHRIE, J. (1964) Histological effects of intratesticular
injections of cadmium chloride in domestic fowl. Br. J. Cancer,
18: 255-260.
HADDOW, A., ROE, F.J.C., DUKES, C.E., & MITCHELEY, B.C.V. (1964)
Cadmium neoplasia: sarcomata at the site for injection of cadmium
sulfate in rats and mice. Br. J. Cancer, 18: 667-673.
HADLEY, J.G., CONKLIN, A.W., & SANDERS, C.L. (1979) Systemic
toxicity of inhaled cadmium oxide. Toxicol. Lett., 4: 107-111.
HAGA, Y. & YAMAWAKI, T. (1974) [Research report on environmental
health studies of heavy metal pollution], Akita, Japan, Akita
Prefecture Institute of Public Health (Report No. 18) (in Japanese).
HAGINO, N. & YOSHIOKA, K. (1961) [A study on the etiology of
Itai-itai disease.] J. Jpn. Orthop. Assoc., 35: 812-815 (in
Japanese).
HAHN, R., EWERS, O., JERMANN, E., FREIER, E., BROCKHAUS, A., &
SCHLIPKOTER, H.W. (1987) Cadmium in kidney cortex of inhabitants of
North-West Germany: in relationship to age, sex, smoking and
environmental pollution by cadmium. Int. Arch. occup. environ.
Health, 59: 165-176.
HALVORSEN, C. & STEINNES, E. (1975) Simple and precise determination
of Zn and Cd in human liver by neutron activation analysis. Z. anal.
Chem., 274: 199-202.
HAMILTON, D.L. & VALBERG, L.S. (1974) Relationship between cadmium
and iron absorption. Am. J. Physiol., 227: 1033-1037.
HAMMER, D.I., FINKLEA, J.F., HENDRICKS, R.H., SHY, C.M., & HORTON,
R.J.M. (1971) Hair trace metal levels and environmental exposure.
Am. J. Epidemiol., 93: 84-92.
HAMMER, D.I., FINKLEA, J.F., CREASON, J.P., SANDIFER, S.H., KEIL,
J.E., PRIESTER, L.E., & STARA, J.F. (1972) Cadmium exposure and
human health effects. In: Hemphill, D.D., ed. Trace substances in
environmental health - V, Columbia, Missouri, University of Missouri
Press, pp. 269-283.
HAMMER, D.I., COLUCCI, A.V., HASSELBLAD, V., WILLIAMS, M.E., &
PINKERTON, C. (1973) Cadmium and lead in autopsy tissues. J. occup.
Med., 15: 956-963.
HARADA, A. (1987) [Results of fifteen years health examinations on
cadmium workers in a cadmium pigment factory (2nd report)], Tokyo,
Japan Public Health Association, pp. 219-233 (Kankyo Hoken Report
No. 53) (in Japanese).
HARDY, H.L. & SKINNER, J.B. (1947) The possibility of chronic
cadmium poisoning. J. ind. Hyg. Toxicol., 29: 321-325.
HARRISON, H.E., BUNTING, H., ORDWAY, N., & ALBRINK, W.S. (1947) The
effects and treatment of inhalation of cadmium chloride aerosols in
the dog. J. ind. Hyg. Toxicol., 29: 302-314.
HARRISON, J.F., LUNT, G.S., SCOTT, P., & BLAINEY, J.D. (1968)
Urinary lysozyme, ribonuclease, and low molecular weight protein in
renal disease. Lancet, 1: 371-375.
HARVEY, T.C., MCLELLAN, J.S., THOMAS, B.J., & FREMLIN, J.H. (1975)
Measurement of liver cadmium concentrations in patients and
industrial workers by neutron activation analysis. Lancet,
1: 1269-1272.
HARVEY, T.C., CHETTLE, D.R., FREMLIN, J.H., AL-HADDAD, I.K., &
DOWNES, S.P.M. (1979) Cadmium in Shipham. Lancet, 1(8115).
HASSELMANN, J., KOENIG, W., RICHTER, F.W., STEINER, U., & WATJEN, U.
(1977) Application of PIXE to trace element analysis in biological
tissues. Nucl. Instrum. Methods, 142: 163-169.
HASSLER, E., LIND, B., & PISCATOR, M. (1983) Cadmium in blood and
urine related to present and past exposure. A study of workers in an
alkaline battery factory. Br. J. ind. Med., 40: 420-425.
HAYES, J.A., SNIDER, G.L., & PALMER, K.C. (1976) The evolution of
biochemical damage in the rat lung after acute cadmium exposure. Am.
Rev. respir. Dis., 113: 121-130.
HEATH, J.C. & WEBB, M. (1967) Content and intracellular distribution
of the inducing metal in the primary rhabdomyosarcomata induced in
the rat by cobalt, nickel, and cadmium. Br. J. Cancer, 21: 768-779.
HEATH, J.C., DANIEL, M.R., DINGLE, J.T., & WEBB, M. (1962) Cadmium
as a carcinogen. Nature (Lond.), 193: 592-593.
HEINDRYCKX, R., DEMUYNCK, M., DAMS, R., JANSSENS, M., & RAHN, K.A.
(1974) Mercury and cadmium in Belgian aerosols. In: Problems of the
contamination of man and his environment by mercury and cadmium. CEC
European Colloquium, July, 1973, Luxembourg, Commission of the
European Communities, pp. 135-148.
HEINRICH, U., PETERS, L., ERNST, H., RITTINGHAUSEN, S., DASENBROCK,
C., & KÖNIG, H. (1989) Investigation on the carcinogenic effects of
various cadmium compounds after inhalation exposure in hamsters and
mice. Exp. Pathol., 37: 253-258.
HENDERSON, R.F., REBAR, A.H., PICKRELL, J.A., & NEWTON, G.J. (1979)
Early damage indicators in the lung III. Biochemical and cytological
response of the lung to inhaled metal salts. Toxicol. appl.
Pharmacol., 50: 123-136.
HENKE, G., SACHS, H.W., & BOHN, G. (1970) [Cadmium determination by
neutron activation analysis of liver and kidneys from children and
young people.] Arch. Toxikol., 26: 8-16 (in German).
HICKEY, R.J., SCHOFF, E.P., & CLELLAND, R.C. (1967) Relationship
between air pollution and certain chronic disease death rates. Arch.
environ. Health, 15: 728-738.
HIRATA, H. (1981) Annaka: land polluted mainly by fumes and dust
from a zinc smelter. In: Kitagishi, K. & Yamane, I., ed. Heavy metal
pollution in soils of Japan, Tokyo, Japan Scientific Societies
Press, pp. 149-163.
HISLOP, J.S. (1980) Choice of analytical method. In: Bratter, P. &
Schramel, P., ed. Trace element analytical chemistry in medicine
and biology, Berlin, New York, Walter de Gruyter Co., pp. 747-767.
HOFFMAN, E.O., COOK, J.A., DILUZIO, N.R., & COOVER, J.A. (1975) The
effects of acute cadmium administration in the liver and kidney of
the rat. Lab. Invest., 32: 655-661.
HOFFMAN, L., PUTZKE, H.-P., KAMPEHL, H.-J., RUSSBULT, R., GASE, P.,
SIMONN, C., ERDMANN, T., & HUCKSTORF, C. (1985) Carcinogenic effects
of cadmium on the prostate of the rat. J. Cancer Res. clin. Oncol.,
109: 193-199.
HOFFMAN, L., PUTZKE, H.-P., BENDEL, L., ERDMANN, T., & HUCKSTORF, C.
(1988) Electron microscopic results on the ventral prostate of the
rat after CdC12 administration. A contribution towards etiology of
the cancer of the prostate. J. Cancer Res. clin. Oncol.,
114: 273-278.
HOLDEN, H. (1969) Cadmium toxicology. Lancet, 2: 57.
HOLDEN, H. (1980a) A mortality study of workers exposed to cadmium
fumes. In: Proceedings of the 2nd International Cadmium Conference,
Cannes, 6-8 February, 1979, London, Cadmium Association,
pp. 211-216.
HOLDEN, H. (1980b) Health status of European cadmium workers. In:
Proceedings of the Seminar on Occupational Exposure to Cadmium,
London, 20 March, 1980, London, Cadmium Association, pp. 33-37.
HOLMBERG, R.E., Jr & FERM, V.H. (1969) Inter-relationship of
selenium, cadmium, and arsenic in mammalian teratogenesis. Arch.
environ. Health, 18: 873-877.
HORSTOWA, H., SIKORSKI, M., & TYBORSKI, H. (1966) [Chronic cadmium
poisoning in the clinical and radiological picture.] Med. Prac.
occup. Med., 17: 13-25 (in Polish).
HOVMAND, M.F., TJELL, J.C., & MOSBACK, H. (1983) Plant uptake of
airborne cadmium. Environ. Pollut., 30: 27-38.
HUEL, G., BOUDENE, C., & IBRAHIM, M.A. (1981) Cadmium and lead
content of maternal and newborn hair: relationship to parity, birth
weight and hypertension. Arch. environ. Health, 36: 221-227.
HUGHES, E.G. & STEWART, M. (1979) Cadmium in Shipham. Lancet,
1: 973-974.
HURLEY, L.S., GOWAN, J., & SWENERTON, H. (1971) Teratogenic effects
of short-term and transitory zinc deficiency in rats. Teratology,
4: 199-204.
HUTCHISON, F., WAI, C.M., JERNEGAN, M., HICKS, W., & LONG, R. (1979)
Distribution of cadmium, lead and zinc in soil around two phosphate
processing plants in Pocatello, Idaho. Bull. environ. Contam.
Toxicol., 22: 426-429.
HUTTON, M., ed. (1982) Cadmium in the European Community: a
prospective assessment of sources, human exposure, and environmental
impact, London, Monitoring and Assessment Research Centre, Chelsea
College, University of London, 100 pp (MARC Report Number 26).
HUTTON, M. (1983) Evaluation of the relationships between cadmium
exposure and indicators of kidney function, London, Monitoring and
Assessment Research Centre, Chelsea College, University of London,
46 pp (MARC Report Number 29).
HUTTON, M. & SYMON, C. (1986) The quantities of cadmium, lead,
mercury and arsenic entering the U.K. environment from human
activities. Sci. total Environ., 57: 129-150.
HUTTON, M., WADGE, A., & MILLIGAN, P.J. (1988) Environmental levels
of cadmium and lead in the vicinity of a major refuse incinerator.
Atmos. Environ., 22: 411-416.
HYOGO PREFECTURAL GOVERNMENT (1972) [Results of a comprehensive
study on cadmium pollution in the area around Ikuno Mine and an
outline of counter-measures], Kobe, Japan, Hyogo Prefectural
Government, 156 pp (in Japanese).
IARC (1976) Cadmium, nickel, some epoxides, miscellaneous
industrial chemicals, and general considerations on volatile
anaesthetics, Lyon, International Agency for Research on Cancer,
306 pp (Monographs on the Evaluation of Carcinogenic Risk of Chemicals
to Man, Vol. 11).
IARC (1987a) Genetic and related effects: an updating of selected
IARC Monographs from Volumes 1 to 42. Lyon, International Agency for
Research on Cancer (IARC Monographs on the Evaluations of
Carcinogenic Risks to Humans, Supplement 6).
IARC (1987b) Cadmium and cadmium compounds. In: Overall evaluations
of carcinogenicity: an updating of IARC Monographs, Volumes 1 to 42,
Lyon, International Agency for Research on Cancer, pp. 139-142 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals
to Humans, Supplement 7).
ILZSG (1988) International Lead and Zinc Study Group - World
directory: Lead and zinc mines and primary metallurgical works,
London, Chameleon Press, Ltd.
INSKIP, H., BERAL, V., & MCDOWALL, M. (1982) Mortality in Shipman
residents: 40-year follow-up. Lancet, 1: 896-899.
IRPTC (1987) IRPTC legal file 1986, Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment
Programme, Vol. I.
ISHIKAWA PREFECTURAL GOVERNMENT (1976) [Report on the health
examination of residents of the Kakehashi river basin], Kanazawa,
Japan, Health and Welfare Department, 104 pp (in Japanese).
ISHIZAKI, A. (1969) [On the so-called Itai-itai disease.] J. Jpn.
Med. Soc., 62: 242-249 (in Japanese).
ISHIZAKI, A. (1972) [Concentrations of cadmium and zinc in the
organs of Itai-itai disease patients and residents in the Hokuriku
region], Tokyo, Japanese Society of Public Health, pp. 154-157
(Kankyo Hoken Report No. 11) (in Japanese).
ISHIZAKI, A., FUKUSHIMA, M., SAKOMOTO, M., KURACHI, T., & HAYASHI,
E. (1969) [Cadmium content of rice eaten in the Itai-itai disease
area.] In: [Proceedings of the 27th Annual Meeting of the Japanese
Society of Public Health], Tokyo, Japan Public Health Association,
p. 111 (in Japanese).
ISHIZAKI, A., FUKUSHIMA, M., & SAKAMOTO, M. (1971) [Contents of
cadmium and zinc in organs of Itai-itai disease patients and
residents of Hakuriku district.] Jpn. J. Hyg., 26: 268-273 (in
Japanese).
ISHIZU, S., MINAMI, M., SUZUKI, A., YAMADA, M., SATO, M., &
YAMAMURA, K. (1973) An experimental study of teratogenic effect of
cadmium. Ind. Health, 11: 127-139.
ITO, K. & SAWAUCHI, K. (1966) Inhibitory effects on cadmium-induced
testicular damage by pretreatment with smaller cadmium doses.
Okajimas Folia anat. Jpn. 42: 107-117.
ITOKAWA, Y., ABE, T., & TANAKA, S. (1973) Bone changes in
experimental chronic cadmium poisoning. Arch. environ. Health,
26: 241-244.
ITOKAWA, Y., ABE, T., TABEI, R., & TANAKA, S. (1974) Renal and
skeletal lesions in experimental cadmium poisoning. Arch. environ.
Health, 28: 149-154.
ITOKAWA, Y., NISHINO, K., TAKASHIMA, M., NAKATA, T., KAITO, H.,
OKAMOTO, E., DAIJO, K., & KAWAMURA, J. (1978) Renal and skeletal
lesions in experimental cadmium poisoning of rats. Histology and
renal function. Environ. Res., 15: 206-217.
IWAO, S. (1977) Cadmium, lead, copper, and zinc in food, faeces, and
organs of humans. Keio J. Med., 26: 63-78.
IWAO, S., SUGITA, M., & TSUCHIYA, K. (1981a) Some metabolic
interrelationships among cadmium, lead, copper, and zinc: results
from a field survey in Cd-polluted areas in Japan. I. Dietary intake
of the heavy metals. Keio J. Med., 30: 17-36.
IWAO, S., SUGITA, M., & TSUCHIYA, K. (1981b) Some metabolic
interrelationships among cadmium, lead, copper, and zinc: results
from a field survey in Cd-polluted areas in Japan. II. Faecal
excretion of the heavy metals. Keio J. Med., 30: 71-82.
JAGNER, D., JOSEFSON, M., & WESTERLUND, S. (1981) Simultaneous
determination of cadmium and lead in urine by means of computerized
potentiometric stripping analysis. Anal. Chim. Acta, 128: 155-161.
JAMALL, I.S. & SMITH, J.C. (1986) The effect of dietary selenium on
cadmium cardiotoxicity. In: Born, G.V.R., Rensselaer, A.F., Herken,
H., & Welch, A.D., ed. Handbook of experimental pharmacology,
Berlin, Heidelberg, Springer Verlag, Vol. 80, pp. 351-361.
JAMALL, I.S. & SPROWLS, J.J. (1987) Effects of cadmium and dietary
selenium on cytoplasmic and mitochondrial antioxidant defense
systems in the heart of rats fed high dietary copper. Toxicol. appl.
Pharmacol., 87: 102-110.
JANSSENS, M. & DAMS, R. (1974) Determination of cadmium in air
particulates by flameless atomic absorption spectrometry with a
graphite tube. Anal. Chim. Acta, 70: 25-33.
JAPAN CADMIUM RESEARCH COMMITTEE (1989) [Summary report on studies
of health effects of cadmium], Tokyo, Japan Public Health
Association, pp. 53-67 (Kankyo Hoken Report No. 56) (in Japanese).
JAPAN PUBLIC HEALTH ASSOCIATION (1968) [A study of the cause of
Itai-itai disease], Tokyo, Japan Public Health Association, 73 pp
(in Japanese).
JAPAN PUBLIC HEALTH ASSOCIATION (1970) [Study on cadmium intake and
accumulation in areas requiring official observation], Tokyo, Japan
Public Health Association, 49 pp (in Japanese).
JAPANESE ENVIRONMENT AGENCY (1972) [Results of soil pollution survey
in 1971], Tokyo, Japanese Environment Agency, Soil Agricultural
Chemical Division, Water Quality Bureau, 129 pp (in Japanese).
JAPANESE ENVIRONMENT AGENCY (1974) [Results of measurement at the
national air sampling network stations], Tokyo, Japanese Environment
Agency, Air Pollution Control Division, Air Quality Bureau, 159 pp
(in Japanese).
JAPANESE ENVIRONMENT AGENCY (1982) [Results of soil pollution survey
in 1981], Tokyo, Japanese Government Agency, Soil Agricultural
Chemical Division, Water Quality Bureau, pp. 1-29 (in Japanese).
JAPANESE MINISTRY OF AGRICULTURE AND FORESTRY (1973) [Results of a
survey for protection measures of soil contamination in 1972.] In:
[Materials for soil protection measures], 197 pp (Report No. 44) (in
Japanese).
JAPANESE MINISTRY OF HEALTH AND WELFARE (1968) [Opinion of the
Ministry with regard to Itai-itai disease and its causes], Tokyo,
Japanese Ministry of Health and Welfare (in Japanese).
JAPANESE MINISTRY OF HEALTH AND WELFARE (1971) [Partial revision of
health examination methods in the provisional counter-measures
against environmental pollution by cadmium], Tokyo, Japanese
Ministry of Health and Welfare, Environmental Pollution Section
(Unpublished document) (in Japanese).
JAPANESE MINISTRY OF HEALTH AND WELFARE (1983) [The present status
of nutrition among the people], Tokyo, Japanese Ministry of Health
and Welfare, 56 pp (in Japanese).
JÄRUP, L., ROGENFELT, A., ELINDER, C.-G., NOGAWA, K., & KJELLSTRÖM,
T. (1983) Biological half-time of cadmium in the blood of workers
after cessation of exposure. Scand. J. Work Environ. Health,
9: 327-331.
JÄRUP, L., ELINDER, C.G., & SPANG, G. (1988) Cumulative
blood-cadmium and tubular proteinuria: a dose-response relationship.
Int. Arch. occup. environ. Health, 60: 223-229.
JAWAID, M., LIND, B., & ELINDER, C.-G (1983) Determination of
cadmium in urine by extraction and flameless atomic absorption
spectrophotometry. Comparison of urine from smokers and non-smokers
of different sex and age. Talanta, 30: 509-513.
JEFFERY, E.H., NOSEWORTHY, R., & CHERIAN, M.G. (1989) Age dependent
changes in metallothionein and accumulation of cadmium in horses.
Comp. Biochem. Physiol., 93C: 327-332.
JIN, T., NORDBERG, G.F., & NORDBERG, M. (1987a) Resistance to acute
nephrotoxicity induced by cadmium-metallothionein dependence on
pretreatment with cadmium chloride. Pharmacol. Toxicol., 61: 89-93.
JIN, T., NORDBERG, G.F., & NORDBERG, M. (1987b) Influence of
cadmium-metallothionein pretreatment on tolerance of rat kidney
cortical cells to cadmium toxicity in vitro and in vivo.
Pharmacol. Toxicol., 60: 345-349.
JIN, T., NORDBERG, G.F., & NORDBERG, M. (1986) Uptake of cadmium in
isolated kidney cells - influence of binding form and
in vivo pretreatment. J. appl. Toxicol., 6(6): 397-400.
JOHNSON, M.S. & EATON, J.W. (1980) Environmental contamination
through residual trace metal dispersal from derelict lead-zinc mine.
J. environ. Qual., 9(2): 175-179.
JONES, K.C., SYMON, C.J., & JOHNSTON, A.E. (1987) Long-term changes
in soil and cereal grain cadmium: studies at Rothamsted Experimental
Station. In: Hemphill, D.D., ed. Trace substances in environmental
health - XXI. Proceedings of the University of Missouri's 21st
Annual Conference on Trace Substances in Environmental Health, St.
Louis, Missouri, 25-28 May, 1987, Columbia, Missouri, University of
Missouri Press, pp. 450-460.
JORHEM, L., MATTSON, P., & SLORACH, S. (1984) Lead, cadmium, zinc
and certain other metals in foods on the Swedish market. Var Föda,
36: Suppl. 3.
JUST, J. & KELUS, J. (1971) [Cadmium in the air atmosphere of ten
selected cities in Poland.] Rocz. Panstw. Zakl. Hig., 22: 249-256
(in Polish).
KAGI, J.H.R. & KOJIMA, Y. (1987) Metallothionein II. Proceedings of
the Second International Meeting on Metallothionein and Other Low
Molecular Weight Metal-Binding Proteins, Zurich, 21-24 August, 1985,
Basel, Birkhauser Verlag.
KAGI, J.H.R. & NORDBERG, M., ed. (1979) Metallothionein, Basel,
Birkhauser Verlag.
KAGI, J.H.R. & VALLEE, B.L. (1960) Metallothionein: a cadmium- and
zinc-containing protein from equine renal cortex. I. J. biol. Chem.,
235: 3460-3465.
KAGI, J.H.R. & VALLEE, B.L. (1961) Metallothionein: a cadmium- and
zinc-containing protein from equine renal cortex. II. Physiochemical
properties. J. biol. Chem., 236: 2435-2442.
KAHN, H.L. & MANNING, D.C. (1972) Background correction in atomic
absorption spectroscopy. Am. Lab., August: 51-56.
KAJIKAWA, K., NAKANISHI, I., & KURODA, K. (1981) Morphological
changes of the kidney and bone of rats in chronic cadmium poisoning.
Exp. mol. Pathol., 34: 9-24.
KANAI, M., NOMOTO, S., SASAOKA, S., & NAIKI, M. (1971) Clinical
significance of urinary excretion of retinol-binding protein in
patients with Itai-itai disease. Proc. Symp. Physiol. Pathol.,
11: 194-199.
KANISAWA, M. & SCHROEDER, H.A. (1969) Life term studies on the
effect of trace elements on spontaneous tumours in mice and rats.
Cancer Res., 21: 892-895.
KAPLAN, P.D., BLACKSTONE, M., & RICHDALE, N. (1973) Direct
determination of cadmium, nickel, and zinc in rat lungs. Arch.
environ. Health, 27: 387-389.
KAR, A.B., DAS, R.P., & KARKUN, J.N. (1959) Ovarian changes in
prepubertal rats after treatment with cadmium chloride. Acta biol.
med. Germ., 3: 372-399.
KAR, A.B., DAS, R.P., & MUKERJI, B. (1960) Prevention of
cadmium-induced changes in the gonads of rat by zinc and selenium: a
study in antagonism between metals in the biological system. Proc.
Natl Inst. Sci. India Part B. Biol. Sci., 26: 40-50.
KARIN, M. & RICHARDS, R.I. (1982) Human metallothionein genes:
primary structure of the metallothionein-II gene and a related
processed gene. Nature (Lond.), 299: 797-802.
KARIN, M., SLATER, E.P., & HERSCHMAN, H.R. (1981) Regulation of
metallothionein synthesis in HeLa cells by heavy metals and
glucocorticoids. J. cell Physiol., 106: 63-74.
KASUYA, M., TERANISHI, H., KUBOTA, Y., KATO, T., AOSHIMA, K., SAIJO,
A., IWATA, K., & KANAI, M. (1986) [Biological meaning and blood and
urine low-molecular-weight protein determinations of Itai-itai
disease patient and their families, 10 years follow-up study on
urine low-molecular weight protein], Tokyo, Japan Public Health
Association, pp. 176-180 (Kankyo Hoken Report No. 52) (in Japanese).
KATAGIRI, Y., TATI, M., IWATA, H., & KANAI, M. (1971) [Concentration
of cadmium in urine by age.] Med. Biol., 82: 239-243 (in Japanese).
KATO, T. & ABE, K. (1978) Environmental pollution and health effects
- Toyama Prefecture. In: Tsuchiya, K., ed. Cadmium studies in Japan:
a review, Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp. 225-249.
KAWAI, K. (1978) Experimental studies on animals. Morphological
changes of the kidney and effects on bones. In: Tsuchiya, K., ed.
Cadmium studies in Japan: a review, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 86-97.
KAWAI, M., FUKUDA, K., & KIMURA, M. (1976) Morphological alterations
in experimental cadmium exposure with special reference to the onset
of renal lesion. In: Nordberg, G.F., ed. Effects and dose-response
relationships of toxic metals, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 343-370.
KAWAMURA, J., YOSHIDA, O., NISHINO, K., & ITOKAWA, Y. (1978)
Disturbances in kidney functions and calcium and phosphate
metabolism in cadmium-poisoned rats. Nephron, 20: 101-110.
KAWANO, S. & KATO, T. (1978) Environmental pollution and health
effects - Ishikawa Prefecture. In: Tsuchiya, K., ed. Cadmium studies
in Japan: a review, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 220-225.
KAWASHIMA, H., NOMIYAMA, H., & NOMIYAMA, K. (1988) Chronic exposure
to cadmium did not impair vitamin D metabolism in monkeys. Environ.
Res., 46: 48-58.
KAZANTZIS, G. (1956) Respiratory function in men casting cadmium
alloys. Part I. Assessment of ventilatory function. Br. J. ind.
Med., 13: 30-36.
KAZANTZIS, G. (1963) Induction of sarcoma in the rat by cadmium
sulfide pigment. Nature ( Lond.), 198: 1213-1214.
KAZANTZIS, G. (1979) Renal tubular dysfunction and abnormalities of
calcium metabolism in cadmium workers. Environ. Health Perspect.,
28: 155-160.
KAZANTZIS, G. & ARMSTRONG, B.G. (1982) A mortality study of cadmium
workers in the United Kingdom. Scand. J. Work Environ. Health,
8(1): 157-160.
KAZANTZIS, G. & HANBURY, W.J. (1966) The induction of sarcoma in the
rat by cadmium sulfide and by cadmium oxide. Br. J. Cancer,
20: 190-199.
KAZANTZIS, G., FLYNN, F.V., SPOWAGE, J.S., & TROTT, D.G. (1963)
Renal tubular malfunction and pulmonary emphysema in cadmium pigment
workers. Q. J. Med., 32: 165-192.
KAZANTZIS, G., LAM, T.-H., & SULLIVAN, K.R. (1988) Mortality of
cadmium-exposed workers. A five-year update. Scand. J. Work Environ.
Health, 14: 220-223.
KAZANTZIS, G., BLANKS, R., & TEATHER, S. (1989) The mortality of
cadmium exposed workers. In: Vernet, J.-P., ed. Proceedings of the
International Conference on Heavy Metals in the Environment, Geneva,
September 1989, Edinburgh, CEP Consultants Ltd, Vol. 1, pp. 304-307.
KELLO, D. & KOSTIAL, K. (1977) Influence of age and milk diet on
cadmium absorption from the gut. Toxicol. appl. Pharmacol.,
40: 277-282.
KIDO, T., HONDA, R., TSURITANI, I., YAMAYA, H., ISHIZAKI, M.,
YAMADA, Y., & NOGAWA, K. (1988) Progress of renal dysfunction in
inhabitants environmentally exposed to cadmium. Arch. environ.
Health, 43: 213-217.
KIDO, T., NOGAWA, K., YAMADA, Y., HONDA, R., TSURITANI, I.,
ISHIZAKI, M., & YAMAYA, H. (1989) Osteopenia in inhabitants with
renal dysfunction induced by exposure to environmental cadmium. Int.
Arch. occup. environ. Health, 61: 271-276.
KIMURA, M. & OTAKI, N. (1972) Percutaneous absorption of cadmium in
rabbit and hairless mouse. Ind. Health (Tokyo), 10: 7-10.
KING, E. (1955) An environmental study of casting copper-cadmium
alloys. Br. J. ind. Med., 12: 198-205.
KIPLING, M.D. & WATERHOUSE, J.A.H. (1967) Cadmium and prostatic
carcinoma. Lancet, 1: 730-731.
KIRSCH, H., PADBERG, W., SCHOLZ, A., & ZIMMERMEYER, G. (1982)
Cadmium emissions from coal-fired power plants. In: Proceedings of
the 3rd International Cadmium Conference, Miami, Florida, 3-5
February, 1981, London, Cadmium Association, pp. 64-68.
KITAMURA, M. (1972) [Absorption and deposition of cadmium,
especially in human subjects], Tokyo, Japan Public Health
Association, pp. 42-45 (Kankyo Hoken Report No. 11) (in Japanese).
KITAMURA, M. & KOIZUMI, N. (1975) [Results of urine tests for
bedridden old persons in Hyogo Prefecture. Report 2], Tokyo, Japan
Public Health Association, pp. 135-137 (Kankyo Hoken Report No. 36)
(in Japanese).
KITAMURA, S., SUMINO, K., & KAMATANI, N. (1970) [Cadmium
concentrations in liver, kidneys, and bones of human bodies.] Jpn.
J. public Health, 17: 177 (in Japanese).
KJELLSTRÖM, T. (1971) A mathematical model for the accumulation of
cadmium in human kidney cortex. Nord. Hyg. Tidskr., 53: 111-119.
KJELLSTRÖM, T. (1977) Accumulation and renal effects of cadmium in
men. A dose-response study, Stockholm, Karolinska Institute, 80 pp
(Doctoral thesis).
KJELLSTRÖM, T. (1979) Exposure and accumulation of cadmium in
populations from Japan, the United States, and Sweden. Environ.
Health Perspect., 28: 169-197.
KJELLSTRÖM, T. (1986) Effects on bone, on vitamin D, and calcium
metabolism. In: Friberg, L., Elinder, C.-G., Kjellström, T., &
Nordberg, G.F., ed. Cadmium and health: A toxicological and
epidemiological appraisal, Boca Raton, Florida, CRC Press, Vol. II,
pp. 111-158.
KJELLSTRÖM, T. & NORDBERG, G.F. (1978) A kinetic model of cadmium
metabolism in the human being. Environ. Res., 16: 248-269.
KJELLSTRÖM, T. & PISCATOR, M. (1977) Quantitative analysis of
ß-2-microglobulin in urine as an indicator of renal tubular damage
by cadmium. Phadedoc No. 1. Diagnostic communications, Uppsala,
Sweden, Pharmacia Diagnostics, 21 pp.
KJELLSTRÖM, T., LIND, B., LINNMAN, L., & NORDBERG, G.F. (1974) A
comparative study on methods for cadmium analysis of grain with an
application to pollution evaluation. Environ. Res., 8: 92-106.
KJELLSTRÖM, T., LIND, B., LINNMAN, L., & ELINDER, C.-G. (1975a)
Variation of cadmium concentration in Swedish wheat and barley. An
indicator of changes in daily cadmium intake during the 20th
century. Arch. environ. Health, 30: 321-328.
KJELLSTRÖM, T., TSUCHIYA, K., TOMPKINS, E., TAKABATAKE, E., LIND,
B., & LINNMAN, L. (1975b) A comparison of methods for analysis of
cadmium in food and biological material. A cooperative study between
Sweden, Japan, and the USA. In: Recent advances in the assessment of
the health effects of environmental pollution, Luxembourg,
Commission of the European Communities, pp. 2197-2213.
KJELLSTRÖM, T., EVRIN, P.E., & RAHNSTER, B. (1977a) Dose-response
analysis of cadmium-induced tubular proteinuria. A study of urinary
ß-2-microglobulin excretion among workers in a battery factory.
Environ. Res., 13: 303-317.
KJELLSTRÖM, T., SHIROISHI, K., & EVRIN, P.E. (1977b) Urinary
ß-2-microglobulin excretion among people exposed to cadmium in the
general environment. An epidemio-logical study in cooperation
between Japan and Sweden. Environ. Res., 13: 318-344.
KJELLSTRÖM, T., BORG, K., & LIND, B. (1978) Cadmium in faeces as an
estimator of daily cadmium intake in Sweden. Environ. Res.,
15: 242-251.
KJELLSTRÖM, T., FRIBERG, L., & RAHNSTER, D. (1979) Mortality and
cancer morbidity among cadmium-exposed workers. Environ. Health
Perspect., 28: 199-204.
KJELLSTRÖM, T., ELINDER, C.-G., & FRIBERG, L. (1984) Conceptual
problems in establishing the critical concentration of cadmium in
human kidney cortex. Environ. Res., 33: 284-295.
KLAASSEN, C.D. & KOTSONIS, F.N. (1977) Biliary excretion of cadmium
in the rat, rabbit, and dog. Toxicol. appl. Pharmacol., 41: 101-112.
KNEIP, T.J., EISENBUD, M., STREHLOW, C.D., & FREUDENTHAL, P.C.
(1970) Airborne particulates in New York City. J. Air Pollut.
Control Assoc., 20: 144-149.
KOBAYASHI, J. (1974) Effects of cadmium on calcium metabolism of
rats. In: Hemphill, D.D., ed. Trace substances in environmental
health - VII, Columbia, Missouri, University of Missouri Press,
pp. 295-305.
KODAMA, Y., MORIMOTO, K., & NAKAJIMA, T. (1978) [Studies on the
chemical form of heavy metal in oysters.] Eisei Kagaku, 24: 132-138
(in Japanese).
KOGAN, I.G., GROZDOVA, T.YA., & KHOLIKOVA, T.A. (1978)
[Investigation of the mutagenic effect of cadmium chloride on
Drosophila melanogaster germ cells.] Genetika, 16: 2136-2140 (in
Russian).
KOGAN, V.H., MALISHEVA, N.B., & FIDAROVA, A.M. (1972) On the role of
visceral lesions under chronic cadmium intoxications in the
development of bone changes. Trans. Kuban Med. Inst., 36: 75-78.
KOIRTYOHANN, S.R. & HOPKINS, C.A. (1976) Losses of trace metals
during the ashing of biological materials. Analyst, 101: 870-875.
KOIVISTOINEN, P. (1980) Mineral element composition of Finnish
foods: N, K, Ca, Mg, P, S, Fe, Cu, Mn, Zn, Mo, Co, Ni, Cr, F, Se,
Si, Rb, Al, B, Br, Hg, As, Cd, Pb, and Ash. Acta agric. Scand.,
22: Suppl.
KOIZUMI, H., YASUDA, K., & KATAYAMA, M. (1977) Atomic absorption
spectrophotometry based on the polarization characteristics of the
Zeeman effects. Anal. Chem., 49: 1106-1112.
KOJIMA, S., HAGA, Y., KURIHARA, T., & YAMAWAKI, T. (1975) [Report
from Akita Prefecture], Tokyo, Japan Public Health Association, pp.
114-123 (Kankyo Hoken Report No. 36) (in Japanese).
KOJIMA, S., HAGA, Y., KURIHARA, T., & YAMAWAKI, T. (1976) [Report on
cadmium pollution in Akita Prefecture], Tokyo, Japan Public Health
Association, pp. 114-120 (Kankyo Hoken Report No. 36) (in Japanese).
KOJIMA, S., HAGA, Y., KURIHARA, T., YAMAWAKI, T., & KJELLSTRÖM, T.
(1977) A comparison between faecal cadmium and urinary
ß-2-microglobulin total protein and cadmium among Japanese farmers.
An epidemiological study in cooperation between Japan and Sweden.
Environ. Res., 14: 436-451.
KOLLER, L.D., EXON, J.H., & ROAN, J.G. (1975) Antibody suppression
by cadmium. Arch. environ. Health, 30: 598-601.
KONO, M., YOSHIDA, T., SUGIHARA, H., & HAGINO, N. (1956) [Report on
so-called Itai-itai disease - First report.] J. Jap. Soc.
Orthopaedis., 30: 100-101 (in Japanese).
KOPP, S.J., GLONEK, T., ERLANGER, M., PERRY, E.F., PERRY, H.M., Jr,
& BARANY, M. (1980a) Cadmium and lead effects on myocardial function
and metabolism. J. environ. Pathol. Toxicol., 4: 205-227.
KOPP, S.J., PERRY, H.M., Jr, GLONEK, T., ERLANGER, M., PERRY, E.F.,
BARANY, M., & D'AGROSA, L.S. (1980b) Cardiac physiologic-metabolic
changes after chronic low-level heavy metal feeding. Am. J.
Physiol., 239: H22-H30.
KOPP, S.J., PERRY, H.M., Jr, PERRY, E.F., & ERLANGER, M. (1983)
Cardiac physiologic and tissue metabolic changes following chronic
low-level cadmium and cadmium plus lead ingestion in the rats.
Toxicol. appl. Pharmacol., 69: 149-160.
KOROPATNICK, J. & CHERIAN, M.G. (1988) Exposure to different forms
of cadmium in mice: differences in metallothionein and
alphafetoprotein mRNA induction in liver and kidney. J. biochem.
Toxicol., 3: 159-172.
KORPELA, H., LOUENIVA, R., YRJANHEIKKI, E., & KAUPPILA, A. (1986)
Lead and cadmium concentrations in maternal and umbilical cord
blood, amniotic fluid, placenta, and amniotic membranes. Am J.
Obstet. Gynecol., 155: 1086-1089.
KOTSONIS, F.N. & KLAASSEN, C.D. (1977) Toxicity and distribution of
cadmium administered to rats at sublethal doses. Toxicol. appl.
Pharmacol., 41: 667-680.
KOWAL, N.E. & KRAEMER, D.F. (1982) Urinary cadmium and
ß-2-microglobulin levels of persons aged 20-74 years from a
sub-sample of the national HANES II survey, 1976-1980. In:
Proceedings of the 3rd International Cadmium Conference, Miami,
Florida, 3-5 February, 1981, London, Cadmium Association,
pp. 119-122.
KOWAL, N.E., JOHNSON, D.E., KRAEMER, D.F., & PAHREN, H.R. (1979)
Normal levels of cadmium in diet, urine, blood, and tissues of
inhabitants of the United States. J. Toxicol. environ. Health,
5: 995-1014.
KRAEMER, D.F., LUCAS, J.B., PAHREN, H.R., RYAN, J.A., & KOWAL, N.E.
(1979) Cadmium toxicity. Lancet, 1: 1241-1242.
KRASOVSKII, G.N., VARSHAVSKAYA, S.P., & BORISOV, A.I. (1976) Toxic
and gonadotropic effects of cadmium and boron relative to standards
for these substances in drinking-water. Environ. Health Perspect.,
13: 69-75.
KRELL, U. & ROECKNER, E. (1988) Model simulation of the atmospheric
input of lead and cadmium into the North Sea. Atmos. Environ.,
22(2): 375-381.
KRETZSCHMAR, J.G., DELESPAUL, I., & DE RIJK, T.H. (1980) Heavy
levels in Belgium: a five-year survey. Sci. total Environ.,
14: 85-97.
KUHNERT, P.M., KUHNERT, B.R., BOTTOMS, S.F., & ERHARD, P. (1982)
Cadmium levels in maternal blood, fetal cord blood, and placental
tissues of pregnant women who smoke. Am. J. Obstet. Gynecol.,
142: 1021-1028.
KUHNERT, P.M., KUHNERT, B.R., ERHARD, P., BRASHEAR, W.T.,
GROH-WARGO, S.L., & WEBSTER, S. (1987a) The effect of smoking on
placental and fetal zinc status. Am. J. Obstet. Gynecol.,
157: 1241-1246.
KUHNERT, B.R., KUHNERT, P.M., DEBANNE, S., & WILLIAMS, T.G. (1987b)
The relationship between cadmium, zinc and birth weight in pregnant
women who smoke. Am. J. Obstet. Gynecol., 157: 1247-1251.
KUHNERT, B.R., KUHNERT, P.M., & ZARLINGO, T.J. (1988) Associations
between placental cadmium and zinc and age and parity in pregnant
women who smoke. Obstet. Gynecol., 71: 67-70.
LAAMANEN, A. (1972) Functions, progress, and prospects for an
environmental subarctic base level station. Work environ. Health,
9: 17-25.
LAFLEUR, P.D., ed. (1976) Accuracy in trace analysis: sampling,
sample handling, analysis, Washington, DC, US Department of
Commerce, National Bureau of Standards, Vol. 1 and 2, 1304 pp.
LARIONOVA, T.I., ZILLE, L.N., & VOROBJEVA, R.S. (1974) [The
influence of barium-cadmium laurate on some fermentative processes
in the liver.] Gig. Tr. prof. Zabol., 3: 50-52 (in Russian).
LAUWERYS, R. & DE WALS, PH. (1981) Environmental pollution by
cadmium and mortality from renal diseases. Lancet, 1: 383.
LAUWERYS, R., BUCHET, J.P., ROELS, H., BROUWERS, J., & STANESCU, D.
(1974a) Epidemiological survey of workers exposed to cadmium. Arch.
environ. Health, 28: 145-148.
LAUWERYS, R., BUCHET, J.P., & ROELS, H. (1974b) Effets subcliniques
de l'exposition humaine au cadmium. In: Proceedings of the
International Symposium on Problems of the Contamination of Man and
His Environment by Mercury and Cadmium, Luxembourg, Commission of
the European Communities, pp. 447-461.
LAUWERYS, R., BUCHET, J.P., & ROELS, H. (1974c) Dosage du cadmium et
du plomb dans le sang et les urines par spectrophotométrie
d'absorption atomique précedée d'une chromatographie sur résine
échangeuse d'ions. In: Proceedings of the International Symposium on
Problems of the Contamination of Man and His Environment by Mercury
and Cadmium, Luxembourg, Commission of the European Communities,
pp. 231-238.
LAUWERYS, R., BUCHET, J.P., ROELS, H., BERLIN, A., & SMEETS, J.
(1975) Intercomparison program of lead, mercury, and cadmium
analysis in blood, urine, and aqueous solutions. Clin. Chem.,
21: 551-557.
LAUWERYS, R., BUCHET, J.P., & ROELS, H. (1976) The relationship
between cadmium exposure or body burden and the concentration of
cadmium in blood and urine in man. Int. Arch. occup. environ.
Health, 36: 275-285.
LAUWERYS, R., BUCHET, J.P., ROELS, H., & HUBERMONT, G. (1978)
Placental transfer of lead, mercury, cadmium and carbon monoxide in
women. I. Comparison of the frequency distributions of the
biological indices in maternal and umbilical cord blood. Environ.
Res., 15: 278-290.
LAUWERYS, R., ROELS, H., BUCHET, J.P., BERNARD, A., & STANESCU, D.
(1979a) Investigations on the lung and kidney functions in workers
exposed to cadmium. Environ. Health Perspect., 28: 137-145.
LAUWERYS, R., ROELS, H., REGNIERS, M., BUCHET, J.P., BERNARD, A., &
GORET, A. (1979b) Significance of cadmium concentration in blood and
in urine in workers exposed to cadmium. Environ. Res., 20: 375-391.
LAUWERYS, R., ROELS, H., BERNARD, A., & BUCHET, J.P. (1980a) Renal
response to cadmium in a population living in a non-ferrous smelter
area in Belgium. Int. Arch. occup. environ. Health, 45: 271.
LAUWERYS, R., BUCHET, J.P., ROELS, H., BERNARD, A., CHETTLE, D.R.,
HARVEY, T.C., & ALHADDAD, I.K. (1980b) Biological significance of
cadmium concentration in blood and urine and their application in
monitoring workers exposed to cadmium. In: Proceedings of the 2nd
International Cadmium Conference, Cannes, 6-8 February, 1977,
London, Cadmium Association, pp. 164-167.
LAUWERYS, R., BUCHET, J.P., ROELS, H., VIAU, C., BERNARD, A.,
BRUAUX, P., CLAEYS-THOREAU, F., & RONDIA, D. (1984a) Potential risk
of cadmium for the general population: Update of Liege study. In:
Proceedings of the 4th International Cadmium Conference, Munich, 2-4
March, 1983, London, Cadmium Association, pp. 113-114.
LEHMAN, L.D. & POISNER, A.M. (1984) Induction of metallothionein
synthesis in cultured human trophoblasts by cadmium and zinc. J.
Toxicol. environ. Health, 14: 419-432.
LEHNERT, G., VON, SCHALLER, K.H., & HAAS, T. (1968) [Cadmium
analysis of serum and urine using atomic absorption spectrometry.]
Z. klin. Chem. klin. Biochem., 6: 174-176 (in German).
LEMEN, R.A., LESS, J.S., WAGONER, J.K., & BLEJER, H.P. (1976) Cancer
mortality among cadmium production workers. Ann. NY Acad Sci.,
271: 273-279.
LENER, J. & MUSIL, J. (1971) Cadmium influence on the excretion of
sodium by kidneys. Experientia (Basel), 27: 902.
L'EPEE, P., LAZARINI, H., FRANCHOME, J., N'DOKY, T., & LARRIVET, C.
(1968) Contribution à l'étude de l'intoxication cadmique. Arch.
Mal. prof. Méd. Trav. Sécur. soc., 29: 485-490.
LEPOW, M.L., BRUCKMAN, L., RUBINO, R.A., MARKOWITZ, S., GILLETTE,
M., & KAPISH, J. (1974) Role of airborne lead in increased body
burden of lead in Hartford children. Environ. Health Perspect.,
7: 99-102.
LEVY, L.S. & CLACK, J. (1975) Further studies on the effect of
cadmium on the prostate gland. I. Absence of prostatic changes in
rats given oral cadmium sulfate for two years. Ann. occup. Hyg.,
17: 205-211.
LEVY, L.S., ROE, F.J.C., MALCOLM, D., KAZANTIZIS, G., CLACK, J., &
PLATT, H.S. (1973) Absence of prostatic changes in rats exposed to
cadmium. Ann. occup. Hyg., 16: 111-118.
LEVY, L.S., CLACK, J., & ROE, F.J.C. (1975) Further studies on the
effect of cadmium on the prostate gland. II. Absence of prostatic
changes in mice given oral cadmium sulfate for eighteen months. Ann.
occup. Hyg., 17: 213-220.
LEWIS, G.P., JUSKO, W.J., COUGHLIN, L.L., & HARTZ, S. (1972) Cadmium
accumulation in man: influence of smoking, occupation, alcoholic
habit, and disease. J. chron. Dis., 25: 717-726.
LIKUTOVA, I.V. (1989) [Specific effect of inorganic cadmium
compounds on health and its hygienic assessment.] Abstract from a
dissertation for degree of Candidate of Medical Sciences, Moscow,
Central Institute for the Advanced Training of Physicians, 21 pp (in
Russian).
LIKUTOVA, I.V. & BELOVA, E.A. (1987) [Specific action of cadmium in
its peroral intake with water.] Gig. i Sanit., 1987(6): 70-72 (in
Russian).
LINDBERG, S.E., TURNER, R.R., & LOVETT, G.M. (1982) Processes of
atmospheric deposition of metals and acids to forests. Conference
Proceeding 75. Annual Meeting on the Air Pollution Control
Association, New Orleans, 20 June, 1982, Pittsburgh, Pennsylvania,
Air Pollution Control Association, 14 pp.
LINNMAN, L., ANDERSSON, A., NILSSON, K.O., LIND, B., KJELLSTRÖM, T.,
& FRIBERG, L. (1973) Cadmium uptake by wheat from sewage sludge used
as a plant nutrient source. Arch. environ. Health, 27: 45-47.
LORENTZON, R. & LARSSON, S.E. (1977) Vitamin D metabolism in adult
rats at low and normal calcium intake and the effect of cadmium
exposure. Clin. Sci. mol. Med., 53: 439-446.
LOSER, E. (1980) A 2-year oral carcinogenicity study with cadmium on
rats. Cancer Lett., 9: 191-198.
LUCAS, P.A., JARIVALLA, A.G., JONES, J.H., GOUGH, J., & VALE, P.T.
(1980) Total cadmium fume inhalation. Lancet, 2(8187): 205.
LUCIS, O.J., LUCIS, R., & ATERMAN, K. (1972) Tumorigenesis by
cadmium. Oncology, 26: 53-76.
LUFKIN, N.H. & HODGES, F.T. (1944) Cadmium poisoning. Report of
outbreak. US nav. med. Bull., 43: 1273-1276.
LUND, L.J., BETTY, E.E., PAGE, A.L., & ELLIOTT, R.A. (1981)
Occurrence of naturally high cadmium levels in soils and its
accumulation by vegetation. J. environ. Qual., 10: 551-556.
LUNDGREN, G. (1976) Direct determination of cadmium in blood with a
temperature- controlled heated graphite-tube atomizer. Talenta,
23: 309-312.
MAACK, T., JOHNSON, V., KAN, S.T., FIGUEIREDO, J., & SIGULEM, D.
(1979) Renal filtration, transport, and metabolism of low molecular
weight proteins. A review. Kidney Int., 16: 251-270.
MACFARLAND, H.N. (1979) Pulmonary effects of cadmium. In: Mennear,
J., ed. Cadmium toxicity, New York, Basel, Marcel Dekker, Inc.,
pp. 113-132.
MCINNES, G. (1979) Multi-element survey: analysis of the first two
years' results. Warren Spring Laboratory, United Kingdom Department
of Industry, 44 pp (Report No. LR305 AP).
MCKENZIE, J., KJELLSTRÖM, T., & SHARMA, R.P. (1982) Cadmium intake
metabolism and effects in people with a high intake of oysters in
New Zealand, Washington, DC, US Environmental Protection Agency,
210 pp.
MCLELLAN, J.S., THOMAS, B.J., & FREMLIN, J.H. (1975) Cadmium: its
in vivo detection in man. Phys. Med. Biol., 20: 88-95.
MCLELLAN, J.S., FLANAGAN, P.R., CHAMBERLAIN, M.J., & VALBERG, L.S.
(1978) Measurements of dietary cadmium absorption in humans. J.
Toxicol. environ. Health, 4: 131-138.
MAHAFFEY, K.R., CORNELIUSSEN, P.E., JELINEK, C.F., & FIORINO, J.A.
(1975) Heavy metal exposure from foods. Environ. Health Perspect.,
12: 63-69.
MANGELSON, N.F., HILL, M.W., NIELSON, K.K., EATOUGH, D.J.,
CHRISTENSEN, J.J., IZATT, R.M., & RICHARDS, D.O. (1979)
Proton-induced X-ray emission analysis of Pima Indian autopsy
tissues. Anal. Chem., 51: 1187-1194.
MARC (1986) Biological monitoring of environmental contaminants
(plants), London, Monitoring and Assessment Research Centre, Chelsea
College, University of London, 247 pp (MARC Report Number 32).
MARGOSHES, M. & VALLEE, B.L. (1957) A cadmium kidney protein from
equine kidney cortex. J. Am. Chem. Soc., 79: 4813-4814.
MARTIN, J.H. & BROENKOW, W.W. (1975) Cadmium in plankton: elevated
concentrations off Baja California. Science, 190: 884-885.
MARTIN, J.H., ELLIOTT, P.D., ANDERLINI, V.C., GIRVIN, D., JACOBS,
S.A., RISEBROUGH, R.W., DELONG, R.L., & GILMARTIN, W.G. (1976)
Mercury-selenium-bromine imbalance in premature parturient
California sea lions. Mar. Biol., 35: 91-104.
MASON, H.J., DAVISON, A.G., & WRIGHT, A.L. (1988) Relations between
liver cadmium, cumulative exposure, and renal function in cadmium
alloy workers. Br. J. ind. Med., 45: 793-802.
MASON, K.E. & YOUNG, J.O. (1967) Effectiveness of selenium and zinc
in protecting against cadmium-induced injury of rat testis. In:
Muth, O.H., ed. Symposium: Selenium in Biomedicine, Westport,
Connecticut, Avi, pp. 383-394.
MATERNE, D., LAUWERYS, R., BUCHET, J.P., ROELS, H., BROUWERS, J., &
STANESCU, D. (1975) Investigations sur les risques résultant de
l'exposition au cadmium dans deux entreprises de production et deux
entreprises d'utilisation du cadmium. Cah. Méd. Trav., 12: 1-76.
MATSUSAKA, N., TANAKA, M., NISHIMURA, Y., YUYAMA, A., & KOBAYASHI,
H. (1972) [Whole body retention and intestinal absorption of 115Cd
in young and adult mice.] Med. Biol., 85: 275-279 (in Japanese).
MERALI, Z., KACEW, S., & SINGHAL, R.L. (1974) Response of hepatic
carbohydrate and cyclic AMP metabolism to cadmium treatment in rats.
Can. J. Physiol. Pharmacol., 53: 174-184.
MERANGER, J.C., SUBRAMANIAN, K.S., & CHALIFOUX, C. (1981) Metals and
other elements. Survey for cadmium, cobalt, chromium, copper,
nickel, lead, zinc, calcium, and magnesium in Canadian
drinking-water supplies. J. Assoc. Off. Anal. Chem., 64: 44-53.
MILLER, R.K. (1986) Placental transfer and function. The interface
for drugs and chemicals in the conceptus. In: Fabro, S. & Scialli,
A.S., ed. Drug and chemical action in pregnancy, New York, Basel,
Marcel Dekker, Inc., pp. 123-152.
MILLS, C.F. & DELGARNO, A.C. (1972) Copper and zinc status of ewes
and lambs receiving increased dietary concentration of cadmium.
Nature ( Lond.), 239: 171-173.
MOGENSEN, C.E. & SOLLING, K. (1977) Studies on renal tubular protein
reabsorption: partial and near complete inhibition by certain amino
acids. Scand. J. clin. lab. Invest., 37: 477-486.
MOHD, M.A., MURTHY, R.C., & CHANDRA, S.V. (1986) Developmental and
long-term neurobehavioral toxicity of low-level in utero Cd
exposure in rats. Toxicol. Teratol., 8: 463.
MORGAN, J.M. (1970) Cadmium and zinc abnormalities in bronchogenic
carcinoma. Cancer, 25: 1394-1398.
MORGAN, J.M., BURCH, H.B., & WATKISS, J.B. (1971) Tissue cadmium and
zinc content in emphysema and bronchogenic carcinoma. J. chron.
Dis., 24: 107-110.
MORITSUGI, M. & KOBAYASHI, J. (1964) Study on trace elements in
biomaterials. II. Cadmium content in polished rice. Ber. Ohara Inst.
Landwirtsch. Biol. Okayama Univ., 12: 145-158.
MOSCHANDREAS, D.J. (1981) Exposure to pollutants and daily time
budgets of people. Bull. NY Acad. Med., 57: 845-859.
MULFORD, C.E. (1966) Solvent extraction techniques for atomic
absorption spectoscopy. At. Absorpt. Newsl., 5: 88-90.
MULVIHILL, J.E., GAMM, S.H., & FERM, V.H. (1970) Facial formation in
normal and cadmium-treated golden hamsters. J. Embryol. exp.
Morphol., 24: 393-403.
MURASE, H., INOUE, T., SAGA, M., IWATA, T., & KUBOTA, K. (1974)
[Microscopic pathology of dogs chronically exposed to oral cadmium],
Tokyo, Japan Public Health Association, pp. 72-73 (Kankyo Hoken
Report No. 31) (in Japanese).
MUSKETT, C.J., ROBERTS, L.H., & PAGE, B.J. (1979) Cadmium and lead
pollution from secondary metal refinery operations. Sci. total
Environ., 11: 73-87.
NACKOWSKI, S.B., PUTNAM, R.D., ROBBINS, D.A., VARNER, M.O., WHIT,
L.D., & NELSON, K.W. (1977) Trace metal contamination of evacuated
blood collection tubes. Am. Ind. Hyg. Assoc. J., 38: 503-508.
NAKAGAWA, S. (1960) [A study of osteomalacia in Toyama Prefecture
(so-called Itai-itai disease).] J. Radiol. Phys. Ther. Univ.
Kanazawa, 56: 1-51 (in Japanese with English summary).
NAKANO, M., AOSHIMA, K., KATOH, T., TERANISHI, H., & KATOH, T.
(1987) Severity of tubular brush border damage in a
cadmium-polluted area (Jinzu River basin): clinical role of urinary
trehalase. Environ. Res., 44: 161-168.
NAZARI, G., FAVINO, A., & POZZI, U. (1967) [Effects of a single
subcutaneous injection of cadmium chloride in male rats.] Riv. Anat.
Pathol. Oncol., 31: 251-271 (in Italian).
NEEB, R. & WAHDAT, F. (1974) [About inverse voltammetry analysis of
cadmium in aerosols.] Z. anal. Chem., 269: 275-279 (in German).
NICAUD, P., LAFITTE, A., & GROS, A. (1942) Les troubles de
l'intoxication chronique par le cadmium. Arch. Mal. prof.,
4: 192-202.
NICHOLSON, J.K. & OSBORN, O. (1983) Kidney lesions in pelagic
seabirds with high tissue levels of cadmium and mercury. J. Zool.
(Lond.), 200: 88-118. NIELSEN, S.A. (1975) Cadmium in New Zealand
dredge oysters, geographic distribution. Int. J. environ. anal.
Chem., 4: 1-7.
NISE, G. & VESTERBERG, O. (1978) [Sampling vessels influence on
content of heavy metals (Cd, Cr, Hg, Ni, and Pb) in blood and urine
samples.] Läkartidningen, 75: 19-20 (in Swedish).
NISHIYAMA, K. & NORDBERG, G. (1972) Adsorption and elution of
cadmium on hair. Arch. environ. Health, 25: 92-96.
NISHIYAMA, S., NAKAMURA, K., & KONISHI, Y. (1986) Blood pressure and
urinary sodium and potassium excretion in cadmium-treated male rats.
Environ. Res., 40: 357-364.
NOGAWA, K. (1981) Itai-itai disease and follow-up studies. In:
Nriagu, J.O., ed. Cad-mium in the environment. II. Health effects,
New York, John Wiley and Sons, pp. 1-38.
NOGAWA, K. & ISHIZAKI, A. (1979) A comparison between cadmium in
rice and renal effects among inhabitants of the Jinzu River Basin.
Environ. Res., 18: 410-420.
NOGAWA, K., ISHIZAKI, A., FUKUSHIMA, M., SHIBATA, I., & HAGINO, N.
(1975) Studies on the women with acquired Fanconi syndrome observed
in the Ichi River basin polluted by cadmium. Environ. Res.,
10: 280-307.
NOGAWA, K., ISHIZAKI, A., & KAWANO, S. (1978) Statistical
observations of the dose-response relationships of cadmium based on
epidemiological studies in the Kakehashi River basin. Environ. Res.,
15: 185-198.
NOGAWA, K., KOBAYASHI, E., HONDA, R., & ISHIZAKI, A. (1979)
Clinico-chemical studies on chronic cadmium poisoning. I. Results of
urinary examinations. Jpn. J. Hyg., 34: 407-414.
NOGAWA, K., KOBAYASHI, E., HONDA, R., ISHIZAKI, A., KAWANO, S., &
MATUSDA, H. (1980) Renal dysfunction of inhabitants in a
cadmium-polluted area. Environ. Res., 23: 13-23.
NOGAWA, K., KOBAYASHI, E., & KONISHI, F. (1981a) Comparison of bone
lesions in chronic cadmium intoxication and vitamin D deficiency.
Environ. Res., 24: 233-249.
NOGAWA, K., KAWANO, S., & NISHI, M. (1981b) Mortality study of
inhabitants in a cadmium-polluted area with special reference to low
molecular weight proteinuria. In: Heavy metals in the environment,
Edinburgh, CEP Consultants Ltd, pp. 538-540.
NOGAWA, K., KIDO, T., YAMADA, Y., TSURITANI, I., HONDA, R.,
ISHIZAKI, N., & TERAHATA, K. (1984) Alpha-1-microglobulin in urine
as an indicator of renal tubular damage caused by environmental
cadmium exposure. Toxicol. Lett., 22: 63-68.
NOGAWA, K., TSURITANI, I., YAMADA, Y., KIDO, T., HONDA, R.,
ISHIZAKI, M., & KURIHARA, T. (1986) Sister-chromatid exchanges in
the lymphocytes of people exposed to environmental cadmium. Toxicol.
Lett., 32: 283-288.
NOGAWA, K., TSURITANI, I., KIDO, T., HONDA, R., YAMADA, Y., &
ISHIZAKI, M. (1987) Mechanism for bone disease found in inhabitants
environmentally exposed to cadmium: decreased serum
1alpha,25-dihydroxyvitamin D level. Int. Arch. occup. environ.
Health, 59: 21-30.
NOGAWA, K., HONDA, R., KIDO, T., TSURITANI, I., YAMADA, Y.,
ISHIZAKI, M., & YAMAYA, H. (1989) A dose-response analysis of
cadmium in the general environment with special reference to total
cadmium intake limit. Environ. Res., 48: 7-16.
NOMIYAMA, K. (1973) Early signs of cadmium intoxication in rabbits.
Toxicol. appl. Pharmacol., 24: P625-P635.
NOMIYAMA, K. (1974) [Studies concerning cadmium metabolism and the
etiology of poisoning], Tokyo, Japan Public Health Association,
pp. 53-59 (Kankyo Hoken Report No. 31) (in Japanese).
NOMIYAMA, K. (1977) Does a critical concentration of cadmium in
human renal cortex exist? J. Toxicol. environ. Health, 3: 607-609.
NOMIYAMA, K. (1978) Experimental studies on animals:
in vivo experiments. In: Tsuchiya, K., ed. Cadmium studies in
Japan: a review, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 47-86.
NOMIYAMA, K. (1986) The chronic toxicity of cadmium: influence of
environmental and other variables. In: Born, G.V.R., Rensselaer,
A.F., Herken, H., & Welch, A.D., ed. Handbook of experimental
pharmacology, Berlin, Heidelberg, Springer-Verlag, Vol. 80,
pp. 101-133.
NOMIYAMA, K. & FOULKES, E.C. (1977) Reabsorption of filtered cadmium
metallothionein in the rabbit kidney. Proc. Soc. Exp. Biol. Med.,
156: 97-99.
NOMIYAMA, K. & NOMIYAMA, H. (1976a) Mechanisms of urinary excretion
of cadmium: experimental studies in rabbits. In: Nordberg, G.F., ed.
Effects and dose-response relationships of toxic metals, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 371-379.
NOMIYAMA, K. & NOMIYAMA, H. (1976b) [Long-term experiment with
300 ppm oral cadmium exposure to rabbits. Intermediate report after
12 months], Tokyo, Japan Public Health Association, pp. 161-173
(Kankyo Hoken Report No. 38) (in Japanese).
NOMIYAMA, K. & NOMIYAMA, H. (1982) Tissue metallothionein in rabbits
chronically exposed to cadmium, with special reference to the
critical concentration of cadmium in the renal cortex. In: Foulkes,
E.C., ed. Biological roles of metallothionein, Amsterdam, Oxford,
New York, Elsevier Science Publishers, pp. 47-67.
NOMIYAMA, K. & NOMIYAMA, H. (1988) Health effect of six years of
dietary cadmium (cadmium contaminated rice) in monkeys. In:
Essential and toxic trace elements in human health and disease, New
York, Alan K. Liss, Inc., pp. 589-609.
NOMIYAMA, K., SUGATA, Y., YAMAMOTO, A., & NOMIYAMA, H. (1975)
Effects of dietary cadmium on rabbits. I. Early signs of cadmium
intoxication. Toxicol. appl. Pharmacol., 31: 4-12.
NOMIYAMA, K., SUGATA, Y., NOMIYAMA, H., & YAMAMOTO, A. (1976)
Dose-response relationship for cadmium. In: Nordberg, G.F., ed.
Effects and dose-response relationships of toxic metals, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 380-385.
NOMIYAMA, K., NOMIYAMA, H., YOTORIYAMA, M., & TAGUCHI, T. (1978a)
Some recent studies on the renal effects of cadmium. In: Proceedings
of the First International Cadmium Conference, San Francisco,
California, 31 January-2 February, 1977, London, Cadmium
Association, pp. 186-194.
NOMIYAMA, K., NOMIYAMA, H., & TAGUCHI, T. (1978b) Effects of
environmental temperatures on the toxicity of cadmium in mice. In:
Proceedings of the 8th Asian Conference on Occupational Health,
Tokyo, Japan Industrial Safety Association, pp. 221-225.
NOMIYAMA, K., NOMIYAMA, H., NOMURA, M., TAGUCHI, T., MATSUI, J.,
YOTORIYAMA, M., AKAHORI, F., IWAO, S., KOIZUMI, N., MASAOKA, T.,
KITAMURA, S., TSUCHIYA, K., SUZUKI, T., & KOBAYASHI, K. (1979)
Effects of dietary cadmium on rhesus monkeys. Environ. Health
Perspect., 28: 223-243.
NOMIYAMA, K., NOMIYAMA, H., AKAHORI, F., & MASAOKA, T. (1982a)
Cadmium health effects in monkeys with special reference to the
critical concentration of cadmium in the renal cortex. In:
Proceedings of the 3rd International Cadmium Conference, Miami,
Florida, 3-5 February, 1981, London, Cadmium Association,
pp. 151-156.
NOMIYAMA, K., NOMIYAMA, H., YOTORIYAMA, M., & MATSUI, K. (1982b)
Sodium dodecyl sulfate acrylamide gel electrophoretic studies on low
molecular weight proteinuria, an early sign of cadmium health
effects in rabbits. Ind. Health, 20: 11-18.
NOMIYAMA, K., NOMIYAMA, H., YOTORIYAMA, M., AKAHORI, F., & MASAOKA,
T. (1984) Some comments and proposals on dose and effects for
estimating critical concentration of cadmium in the renal cortex.
In: Proceedings of the Fourth International Cadmium Conference,
London, Cadmium Association, pp. 167-171.
NOMIYAMA, K., AKAHORI, F., MASAOKA, T., ARAI, S., NOMURA, Y.,
YOTORIYAMA, M., KOBAYASHI, K., SUZUKI, T., KAWASHIMA, H., & ONOZAWA,
A. (1987) [Dose effect and dose response relationships of 9 years
dietary cadmium in rhesus monkeys], Tokyo, Japan Public Health
Association, pp. 1-86 (Kankyo Hoken Report No. 53) (in Japanese).
NOMURA, T., KIMURA, M., TANIOKA, Y., TANIMOTO, Y., SAEGUSA, J.,
KOTAKI, N., TAZAKI, H., HATA, R., DEGUCHI, N., SUDA, T., & YOSHIKI,
S. (1988) [Influence of nutritional factors on cadmium-administered
monkeys], Tokyo, Japan Public Health Association, pp. 42-122 (Kankyo
Hoken Report No. 54) (in Japanese).
NORDBERG, G.F. (1971) Effects of acute and chronic cadmium exposure
on the testicles of mice. With special reference to protective
effects of metallothionein. Environ. Physiol. Biochem., 1: 171-187.
NORDBERG, G.F. (1972) Cadmium metabolism and toxicity. Environ.
Physiol. Biochem., 2: 7-36.
NORDBERG, G.F. (1975) Effects of long-term cadmium exposure on the
seminal vesicles of mice. J. Reprod. Fertil., 45: 165-167.
NORDBERG, G.F. & NISHIYAMA, K. (1972) Whole-body and hair retention
of cadmium in mice. Arch. environ. Health, 24: 209-214.
NORDBERG, G.F. & NORDBERG, M. (1988) Biological monitoring of toxic
metals, New York, London, Plenum Press, pp. 151-168.
NORDBERG, G.F. & PISCATOR, M. (1972) Influence of long-term cadmium
exposure on urinary excretion of protein and cadmium in mice.
Environ. Physiol. Biochem., 2: 37-49.
NORDBERG, G.F., PISCATOR, M., & LIND, B. (1971) Distribution of
cadmium among protein fractions of mouse liver. Acta pharmacol.
toxicol., 29: 456-470.
NORDBERG, G.F., SLORACH, S., & STENSTROM, T. (1973) [Cadmium
poisoning caused by a cooled soft drink machine.] Läkartidningen,
70: 601-604 (in Swedish with English summary).
NORDBERG, G.F., GOYER, R.A., & NORDBERG, M. (1975) Comparative
toxicity of cadmium metallothionein and cadmium chloride on mouse
kidney. Arch. Pathol., 99: 192-197.
NORDBERG, G.F., ROBERT, K.-H., & PANNONE, M. (1977) Pancreatic and
biliary excretion of cadmium in the rat. Acta pharmacol. toxicol.,
41: 84-88.
NORDBERG, G.F., GARVEY, J.S., & CHANG, C.C. (1982) Metallothionein
in plasma and urine of cadmium workers. Environ. Res., 28: 179-182.
NORDBERG, M. (1978) Studies on metallothionein and cadmium. Environ.
Res., 15: 381-404.
NORDBERG, M. (1984) General aspects of cadmium transport, uptake,
and metabolism by the kidney. Environ. Health Perspect., 54: 13-20.
NORDBERG, M. & NORDBERG, G.F. (1975) Distribution of metallothionein-
bound cadmium and cadmium chloride in mice: preliminary studies.
Environ. Health Perspect., 12: 103-108.
NORDBERG, M., NUOTTANIEMI, I., CHERIAN, M.G., NORDBERG, G.F.,
KJELLSTRÖM, T., & GARVEY, J.S. (1986) Characterization studies on
the cadmium-binding proteins from two species of New Zealand
oysters. Environ. Health Perspect., 65: 57-62.
NRIAGU, J.O. (1979) Global inventory of natural and anthropogenic
emissions of trace metals to the atmosphere. Nature (Lond.),
279: 409.
NRIAGU, J.O. & PACYNA, J.M. (1988) Quantitative assessment of
worldwide contamination of air, water and soils by trace metals.
Nature (Lond.), 333: 134-139.
NWANKWO, J.N., ELINDER, C.G., PISCATOR, M., & LIND, B. (1977)
Cadmium in Zambian cigarettes: an interlaboratory comparison in
analysis. Zambia J. Sci. Technol., 2(4): 1-4.
OBERDORSTER, G. (1988) Deposition and retention modeling of inhaled
cadmium in rat and human lung: An example for extrapolation of
effects and risk estimation. In: Crapo, J.D., Miller, F.J., Smolko,
E.D., Graham, J.A., & Hayes, A.W., ed. Extrapolation of dosimetric
relationships for inhaled particles and gases, New York, London,
Academic Press, pp. 345-370.
OHANIAN, E.V. & IWAI, J. (1980) Etiological role of cadmium in
hypertension in an animal model. J. environ. Pathol. Toxicol.,
3: 229-241.
OHMOMO, Y. & SUMIYA, M. (1981) Estimation of heavy metal intake from
agricultural products. In: Kitagishi, K. & Yamane, J., ed. Heavy
metal pollution in soils of Japan, Tokyo, Japan Scientific Societies
Press, pp. 235-244.
OLDIGES, H., HOCHRAINER, D., & GLASER, U. (1989) Long-term
inhalation study with Wistar rats and four cadmium compounds.
Toxicol. environ. Chem., 19: 217-222.
O'RIORDAN, M.L., HUGHES, E.G., & EVANS, H.J. (1978) Chromosome
studies on blood lymphocytes of men occupationally exposed to
cadmium. Mutat. Res., 58: 305-311.
PAGE, A.L. (1981) Cadmium in soils and its accumulation by food
crops. In: Proceedings of an International Conference on Heavy
Metals in the Environment, Amsterdam, September 1981, Edinburgh, CEP
Consultants, Ltd, pp. 206-213.
PAGE, A.L., BINGHAM, F.T., & SHANG, A.C. (1981) Cadmium. In: Lepp,
N.W., ed. Effect of heavy metal pollution on plants, Barking,
Essex, Applied Science Publishers, Vol. 1, pp. 77-109.
PANEMANGALORE, M., BANERJEE, D., ONOSAKA, S., & CHERIAN, M.G. (1983)
Changes in the intracellular accumulation and distribution of
metallothionein in rat liver and kidney during postnatal
development. Dev. Biol., 97: 95-102.
PARIZEK, J. (1957) The destructive effect of cadmium ion on
testicular tissue and its prevention by zinc. J. Endocrinol.,
15: 56-63.
PARIZEK, J. (1960) Sterilization of the male by cadmium salts. J.
Reprod. Fertil., 1: 294-309.
PARIZEK, J. (1964) Vascular changes at sites of oestrogen
biosynthesis produced by parenteral injection of cadmium salts: the
destruction of placenta by cadmium salts. J. Reprod. Fertil.,
7: 263-265.
PARIZEK, J. & ZAHOR, Z. (1956) Effects of cadmium salts on
testicular tissue. Nature (Lond.), 177: 1036-1037.
PARIZEK, J., OSTADALOVA, I., BENES, I., & PITHA, J. (1968a) The
effect of a subcutaneous injection of cadmium salts on the ovaries
of adult rats in persistent oestrus. J. Reprod. Fertil., 17:
559-562.
PARIZEK, J., OSTADALOVA, I., BENES, I., & BABICKY, A. (1968b)
Pregnancy and trace elements: the protective effect of compounds of
an essential trace element, selenium, against the peculiar toxic
effects of cadmium during pregnancy. J. Reprod. Fertil., 16:
507-509.
PARZYCK, D.C., SHAW, S.M., KESSLER, W.V., VETTER, R.J., VAN SICKLE,
D.C., & MAYES, R.A. (1978) Fetal effects of cadmium in pregnant rats
on normal and zinc-deficient diets. Bull. environ. Contam. Toxicol.,
19: 206-214.
PATON, G.R. & ALLISON, A.C. (1972) Chromosome damage in human cell
cultures induced by metal salts. Mutat. Res., 16: 332-336.
PERRY, H.M., Jr & ERLANGER, M.W. (1973) Elevated circulating renin
activity in rats following doses of cadmium known to induce
hypertension. J. lab. clin. Med., 82: 399-405.
PERRY, H.M., Jr & ERLANGER, M.W. (1974) Metal-induced hypertension
following chronic feeding of low doses of cadmium and mercury. J.
lab. clin. Med., 83: 541-547.
PERRY, H.M., Jr & ERLANGER, M.W. (1982) Effect of diet on increases
in systolic pressure induced in rats by chronic cadmium feeding. J.
Nutr., 112: 1983-1989.
PERRY, H.M., Jr & KOPP, S.J. (1983) Does cadmium contribute to human
hypertension? Sci. total Environ., 26: 223-232.
PERRY, H.M., Jr, ERLANGER, M.W., YUNICE, A., SCHOEPFLE, E., & PERRY,
E.F. (1970) Hypertension and tissue metal levels following
intravenous cadmium, mercury, and zinc. Am. J. Physiol., 219:
755-761.
PERRY, H.M., Jr, PERRY, E.F., & PURIFOY, J.E. (1971) Antinatriuretic
effect of intramuscular cadmium in rats. Proc. Soc. Exp. Biol. Med.,
136: 1240-1244.
PERRY, H.M., Jr, ERLANGER, M.W., & PERRY, E.F. (1976) Limiting
conditions for the induction of hypertension in rats by cadmium. In:
Hemphill, D.D., ed. Trace substances in environmental health - VIII,
Columbia, Missouri, University of Missouri Press, pp. 51-57.
PERRY, H.M., Jr, ERLANGER, M., & PERRY, E.F. (1977) Hypertension
following chronic, very low-dose cadmium feeding. Proc. Soc. Exp.
Biol. Med., 156: 173-176.
PETERING, D.H., LOFTSGAARDEN, J., SCHNEIDER, J., & FOWLER, B. (1984)
Metabolism of cadmium, zinc and copper in the rat kidney: the role
of metallothionein and other binding sites. Environ. Health
Perspect., 54: 73-81.
PETERING, H.G., JOHNSON, M.A., & STEMMER, K. (1971) Studies of zinc
metabolism in the rat. I. Dose-response effects of cadmium. Arch.
environ. Health, 23: 93-96.
PETERING, H.G., CHOUDHURY, H., & STEMMER, K.L. (1979) Some effects
of oral ingestion of cadmium on zinc, copper, and iron metabolism.
Environ. Health Perspect., 28: 97-106.
PETERSON, P.A. (1971) Characteristics of a vitamin A-transporting
protein complex occurring in human serum. J. biol. Chem.,
246: 34-43.
PETERSON, P.A. & BERGGÅRD, I. (1971) Isolation and properties of a
human retinol-transporting protein. J. biol. Chem., 246: 25-33.
PETERSON, P.A., EVRIN, P.-E., & BERGGÅRD, I. (1969) Differentiation
of glomerular, tubular, and normal proteinuria: determinations of
urinary excretion of ß-2-microglobulin, albumin, and total protein.
J. clin. Invest., 48: 1189-1198.
PHILIPP, R. (1985) Cadmium-risk assessment of an exposed residential
population: a review. J. R. Soc. Med., 78: 328-333.
PIOTROWSKI, J.K., TROJANOWSKA, B., WISNIEWSKA-KNYPL, J.M., &
BOLANOWSKI, W. (1974) Mercury binding in the kidney and liver of
rats repeatedly exposed to mercuric chloride: induction of
metallothionein by mercury and cadmium. Toxicol. appl. Pharmacol.,
27: 11-19.
PISCATOR, M. (1962) Proteinuria in chronic cadmium poisoning. II.
The applicability of quantitative and qualitative methods of protein
determination for the demonstration of cadmium proteinuria. Arch.
environ. Health, 5: 325-332.
PISCATOR, M. (1964) [On cadmium in normal kidneys together with a
report on the isolation of metallothionein from livers of
cadmium-exposed rabbits.] Nord. Hyg. Tidskr., 45: 76-82 (in
Swedish).
PISCATOR, M. (1966a) Proteinuria in chronic cadmium poisoning. III.
Electrophoretic and immunoelectrophoretic studies on urinary
proteins from cadmium workers, with special reference to the
excretion of low molecular weight proteins. Arch. environ. Health,
12: 335-344.
PISCATOR, M. (1966b) Proteinuria in chronic cadmium poisoning. IV.
Gel filtration and ion exchange chromatography of urinary proteins
from cadmium workers. Arch. environ. Health, 12: 345-359.
PISCATOR, M. (1971) Data on interferences in cadmium analysis. In:
Friberg, L., Piscator, M., & Nordberg, G., ed. Cadmium in the
environment, Cleveland, Ohio, CRC Press, p. 17.
PISCATOR, M. & TSUCHIYA, K. (1971) In: Friberg, L., Piscator, M., &
Nordberg, G., ed. Cadmium in the environment, Cleveland, Ohio,
Chemical Rubber Company Press, pp. 112-114.
PISCATOR, M. & VOUK, V.B. (1979) Sampling and analytical methods.
In: Friberg, L., Nordberg, G.F., & Vouk, V.B., ed. Handbook on the
toxicology of metals, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 33-46.
PISCATOR, M., KJELLSTRÖM, T., & LIND, B. (1976) Contamination of
cigarettes and pipe tobacco by cadmium-oxide dust. Lancet, 2: 587.
PISCATOR, M., BJORK, L., & NORDBERG, M. (1981) Beta-2-microglobulin
levels in serum and urine of cadmium exposed rabbits. Acta.
pharmacol. toxicol., 49: 1-7.
PLEBAN, P.A., KERKAY, I., & PEARSON, K.H. (1981) Polarized
Zeeman-effect flameless atomic absorption spectrometry of cadmium,
copper, lead, and manganese in human kidney cortex. Clin. Chem.,
27: 68-72.
POTTS, C.L. (1965) Cadmium proteinuria - the health of battery
workers exposed to cadmium oxide dust. Ann. occup. Hyg., 8: 55-61.
PRIGGE, E. (1978) Early signs of oral and inhalative cadmium uptake
in rats. Arch. Toxicol., 40: 231-247.
PRIGGE, E., BAUMERT, H.P., & MUHLE, H. (1977) Effects of dietary and
inhalative cadmium on haemoglobin and haematocrit in rats. Bull.
environ. Contam. Toxicol., 17: 585-590.
PRINCI, F. (1947) A study of industrial exposure to cadmium. J. ind.
Hyg. Toxicol., 29: 315-320.
PRINCI, F. & GEEVER, E.F. (1950) Prolonged inhalation of cadmium.
Arch. ind. Hyg. occup. Med., 1: 651-661.
PROCKOP, D.J. & DAVIDSON, W.D. (1964) A study of urinary and serum
lysozyme in patients with renal disease. New Engl. J. Med.,
270: 269-274.
PRODAN, L. (1932) Cadmium poisoning. II. Experimental cadmium
poisoning. J. ind. Hyg. Toxicol., 14: 174-196.
PULIDO, P., KAGI, J.H., & VALLEE, B.L. (1966) Isolation and some
properties of human metallothionein. Biochemistry, 5: 1768-1777.
RAHOLA, T., AARAN, R.-K., & MIETTINEN, J.K. (1972) Half-time studies
of mercury and cadmium by whole-body counting. In: Assessment of
radioactive contamination in man, New York, International Atomic
Energy Agency Unipublishers, pp. 553-562 (IAEA Proceedings Series
No. Sm-150/13).
RAMAYA, L.K. & POMERANTZEVA, M.O. (1977) [Investigation of cadmium
chloride mutagenic effect on germ cells of male mice.] Genetika,
13: 59-63 (in Russian).
RAMEL, C. & FRIBERG, L. (1971) Carcinogenic and mutagenic effects.
In: Friberg, L., Piscator, M., & Nordberg, G.F., ed. Cadmium in the
environment, Cleveland, Ohio, CRC Press, pp. 109-110.
RAUHUT, A. (1980) Survey of industrial emission of cadmium in the
European Economic Community: Final Report, Brussels, Commission of
the European Communities, Vol. 2, pp. 1-44.
REDDY, C.S., SVOBODA, D., AZARNOFF, D., & DEWAR, R. (1973)
Cadmium-induced Leydig cell tumors of rat testis: morphologic and
histochemical study. J. Natl Cancer Inst., 51: 891-903.
RICHARDSON, M.E. & FOX, M.R.S. (1974) Dietary cadmium and
enteropathy in the Japanese quail. Lab. Invest., 31: 722-731.
RIVM (1988) In: Ros, J.P.M. & Sloof, W., ed. Integrated criteria
document cadmium, Bilthoven, The Netherlands, National Institute of
Public Health and Environmental Protection (RIVM-Report No.
758476004).
ROBBINS, S.L., COTRAN, R.S., & KUMAR, U. (1984) Pathological basis
of disease, 3rd ed., Philadelphia, Pennsylvania, W.B. Saunders &
Co., pp. 1327-1328.
ROELS, H., HUBERMONT, G., BUCHET, J.P., & LAUWERYS, R. (1978)
Placental transfer of lead, mercury, cadmium and carbon monoxide in
women. III. Factors influencing the accumulation of heavy metals in
the aplacenta and the relationship between metal concentration in
the placenta and in maternal and cord blood. Environ. Res.,
16: 236-245.
ROELS, H., LAUWERYS, R.R., BUCHET, J.P., & BERNARD, A. (1981a)
Environmental exposure to cadmium and renal function of aged women
in three areas of Belgium. Environ. Res., 24: 117-130.
ROELS, H., LAUWERYS, R.R., BUCHET, J.P., BERNARD, A., CHETTLE, D.R.,
HARVEY, T.C., & AL-HADDAD, J.K. (1981b) In vivo measurement of
liver and kidney cadmium in workers exposed to this metal: its
significance with respect to cadmium in blood and urine. Environ.
Res., 26: 217-240.
ROELS, H., DJUBGANG, J., BUCHET, J.P., BERNARD, A., & LAUWERYS, R.R.
(1982) Evolution of cadmium-induced renal dysfunction in workers
removed from exposure. Scand. J. Work Environ. Health, 8: 191-200.
ROELS, H., LAUWERYS, R.R., & DARDENNE, A.N. (1983a) The critical
level of cadmium in human renal cortex: a reevaluation. Toxicol.
Lett., 15: 357-360.
ROELS, H., LAUWERYS, R.R., BUCHET, J.P., BERNARD, A., GARVEY, J.S.,
& LINTON, H.J. (1983b) Significance of urinary metallothionein in
workers exposed to cadmium. Int. Arch. occup. environ. Health,
52: 159-166.
ROELS, H., LAUWERYS, R.R., BUCHET, J.P., BERNARD, A.M., VOS, A., &
OVERSTEYNS, M. (1989) Health significance of cadmium induced renal
dysfunction: a five year follow up. Br. J. ind. Med., 46: 755-764.
ROHR, G. & BAUCHINGER, M. (1976) Chromosome analyses in cell
cultures of the Chinese hamster after application of cadmium
sulfate. Mutat. Res., 40: 125-130.
SAITO, H. (1987) [Follow-up study on health status on residents in a
cadmium polluted area (Sasu, Izahara town of Nagasaki Prefecture)
after improvement of cadmium-polluted lands], Tokyo, Japan Public
Health Association, pp. 215-216 (Kankyo Hoken Report No. 53) (in
Japanese).
SAITO, H., SHIOJI, R., FURUKAWA, Y., NAGAI, K., ARIKAWA, T., SAITO,
T., SASAKI, Y., FURUYAMA, T., & YOSHINAGA, K. (1977) Cadmium-induced
proximal tubular dysfunction in a cadmium-polluted area. Nephrology,
6: 1-12.
SAITO, H., NAKANO, A., TOHYAMA, C., MITANE, Y., SUGIHIRA, N., &
WAKISAKA, I. (1983) [Report on a health examination of inhabitants
of areas with cadmium pollution of soil], Tokyo, Japan Public Health
Association, pp. 91-95 (Kankyo Hoken Report No. 40) (in Japanese).
SALMELA, S. & VUORI, E. (1979) Contamination with cadmium from
micropipette tips. Talanta, 26: 175-176.
SALTZMAN, B.E. (1953) Colorimetric microdetermination of cadmium
with dithizone. Anal. Chem., 25: 493-496.
SAMARAWICKRAMA, G.P. & WEBB, M. (1979) Acute effects of cadmium on
the pregnant rat and embryo-fetal development. Environ. Health
Perspect., 28: 245-249.
SANDERS, C.L. & MAHAFFEY, J.A. (1984) Carcinogenicity of single and
multiple intratracheal instillations of cadmium oxide in the rat.
Environ. Res., 33: 227-233.
SANGSTER, B., DE GROOT, G., LOEBER, J.G., DERKS, H.J.G.M., KRAJNC,
E.I., & SAVELKOUL, T.J.F. (1984) Urinary excretion of cadmium,
protein, beta-2-microglobulin and glucose in individuals living in a
cadmium-polluted area. Hum. Toxicol., 3: 7-21.
SCHARPF, L.G., Jr, HILL, I.D., WRIGHT, P.L., PLANK, J.B., KEPLINGER,
M.L., & CALANDRA, J.C. (1972) Effect of sodium nitrilotriacetate on
toxicity, teratogenicity, and tissue distribution of cadmium. Nature
(Lond.), 239: 231-234.
SCHERER, G. & BARKEMEYER, H. (1983) Cadmium concentrations in
tobacco and tobacco smoke. Ecotoxicol. environ. Saf., 7: 71-78.
SCHMID, B.P., KAO, J., & GOULDING, E. (1985) Evidence for reopening
of the cranial tube in mouse embryo treated with cadmium chloride.
Experientia (Basel), 41: 271-272.
SCHROEDER, H.A. (1964) Cadmium hypertension in rats. Am. J.
Physiol., 207: 62-66.
SCHROEDER, H.A. (1965) Cadmium as a factor in hypertension. J.
chron. Dis., 18: 647-656.
SCHROEDER, H.A. (1967) Cadmium, chromium, and cardiovascular
disease. Circulation, 35: 570.
SCHROEDER, H.A. & BALASSA, J.J. (1961) Abnormal trace metals in man:
cadmium. J. chron. Dis., 14: 236-258.
SCHROEDER, H.A. & MITCHENER, M. (1971) Toxic effects of trace
elements on the reproduction of mice and rats. Arch. environ.
Health, 23: 102-106.
SCHROEDER, H.A. & VINTON, W.H., Jr (1962) Hypertension induced in
rats by small doses of cadmium. Am. J. Physiol., 202: 515-518.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H., Jr (1964) Chromium,
lead, cadmium, nickel, and titanium in mice: effect on mortality,
tumours, and tissue levels. J. Nutr., 83: 239-250.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H., Jr (1965) Chromium,
cadmium, and lead in rats: effects on life span, tumours, and tissue
levels. J. Nutr., 86: 51-66.
SCOTT, R., MILLS, E.A., FELL, G.S., HUSAIN, F.E.R., YATES, A.J.,
PATERSON, P.J., MCKIRDY, A., OTTOWAY, J.M., FITZGERALD-FINCH, O.P.,
& LAMONT, A. (1976) Clinical and biochemical abnormalities in
coppersmiths exposed to cadmium. Lancet, 2: 396-398.
SCOTT, R., AUGHEY, E., & SINCLAIR, J. (1977) Histological and
ultrastructural changes in rat kidney following cadmium injection.
Urol. Res., 5: 15-20.
SCOTT, R., PATERSON, P.J., BURNS, R., OTTOWAY, J.M., HUSAIN, F.E.R.,
FELL, G.S., DUMBUYA, S., & IGBAL, M. (1978) Hypercalciuria related
to cadmium exposure. Urology, 11: 462-465.
SCOTT, R., PATERSON, P.J., BURNS, R., FELL, G.S., & OTTOWAY, J.M.
(1979) The effects of treatment on the hypercalciuria of chronic
cadmium poisoning. Urol. Res., 7: 285-289.
SCOTT, R., HAYWOOD, J.K., BODDY, K., WILLIAMS, E.D., HARVEY, M., &
PATERSON, P.J. (1980) Whole body calcium deficit in cadmium-exposed
workers with hypercalciuria. Urology, 15: 356-359.
SHAIKH, Z.A. & HIRAYAMA, K. (1979) Metallothionein in the
extracellular fluids as an index of cadmium toxicity. Environ.
Health Perspect., 28: 267-272.
SHAIKH, Z.A. & SMITH, J.C. (1980) Metabolism of orally ingested
cadmium in humans. In: Holmstedt, B., Lauwerys, R., Mercier, M., &
Roberfroid, M., ed. Mechanisms of toxicity and hazard evaluation,
Amsterdam, Elsevier/North Holland Biomedical Press, pp. 569-574.
SHARMA, R.P., KJELLSTRÖM, T., & MCKENZIE, J.M. (1983) Cadmium in
blood and urine among smokers and non-smokers with high cadmium
intake via food. Toxicology, 29: 163-171.
SHARRETT, A.R., CARTER, A.P., ORHEIM, R.M., & FEINLEIB, M. (1982)
Daily intake of lead, cadmium, copper, and zinc from drinking-water:
the Seattle study of trace metal exposure. Environ. Res.,
28: 456-475.
SHERLOCK, J. & WALTERS, B. (1983) Dietary intake of heavy metals and
its estimation. Chem. Ind., 13: 505-508.
SHERLOCK, J., SMART, G.A., & WALTERS, B. (1983) Dietary surveys on a
population at Shipham, Somerset, United Kingdom. Sci. total
Environ., 29: 121-142.
SHIGEMATSU, I. & KAWAGUCHI, T. (1978) Environmental pollution and
health effects, Yamagata Prefecture. In: Tsuchiya, K., ed. Cadmium
studies in Japan: a review, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 199-211.
SHIGEMATSU, I., KAWAGUCHI, T., & YANAGAWA, H. (1978) Reanalysis of
the results of health examinations conducted on the general
population in the cadmium polluted areas of Japan. In: Tsuchiya, K.
ed. Cadmium studies in Japan: a review, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 253-264.
SHIGEMATSU, I., YANAGAWA, H., & KAWAGUCHI, T. (1975) [Record filling
of health examinations for inhabitants in the Cd-polluted areas (2nd
and 3rd reports)], Tokyo, Japan Public Health Association,
pp. 72-101 (Kankyo Hoken Report No. 36) (in Japanese).
SHIGEMATSU, I., MINOWA, M., YOSHIDA, T., & MIYAMOTO, K. (1979)
Recent results of health examinations on the general population in
cadmium-polluted and control areas in Japan. Environ. Health
Perspect., 28: 205-210.
SHIGEMATSU, I., MINOWA, M., YOSHIDA, T., & MIYAMOTO, K. (1980)
Epidemiological aspects of environmental cadmium and its effects in
Japan. In: Proceedings of the 2nd International Cadmium Conference,
Cannes, 6-8 February, 1979, London, Cadmium Association,
pp. 111-117.
SHIGEMATSU, I., KITAMURA, S., TAKEUCHI, J., MINOWA, M., NAGAI, M.,
USUI, T., & FUKUSHIMA, M. (1981) A retrospective mortality study on
cadmium-exposed populations in Japan. In: Recent studies on health
effects of cadmium in Japan, Tokyo, The Japan Cadmium Research
Committee, Japan Environment Agency, pp. 303-317.
SHIGEMATSU, I., MITAMURA, S., TAKEUCHI, J., MINOWA, M., NAGAI, M., &
FUKUSHIMA, M. (1982) A retrospective mortality study on
cadmium-exposed populations in Japan. In: Proceedings of the 3rd
International Cadmium Conference, Miami, Florida, 3-5 February,
1981, London, Cadmium Association, pp. 115-118.
SHIGEMATSU, I., MINOWA, M., NAGAI, M., OHMURA, T., & TAKEUCHI, K.
(1983) A retrospective mortality study on cadmium-exposed
populations in Japan (supplement): mortalities by level of pollution
in Toyama Prefecture. In: The influence of nutritional factors on
cadmium administered to monkeys, Tokyo, Japan Environment Agency,
pp. 158-174.
SHILLER, A.M. & BOYLE, E.A. (1987) Variability of dissolved trace
metals in the Mississippi River. Geochim. Cosmochim. Acta.,
51: 3273-3277.
SHIRAISHI, Y. (1975) Cytogenetic studies in 12 patients with
Itai-itai disease. Humangenetik, 27: 31-44.
SHIRAISHI, Y., KURAHASHI, H., & YOSHIDA, T.H. (1972) Chromosomal
aberrations in cultured human leukocytes induced cadmium sulfide.
Proc. Jpn. Acad., 48(21): 133-137.
SHIROISHI, K., ANAYAMA, M., TANII, M., FUKUYAMA, Y., & KUBOTA, K.
(1972) [Urinary findings of different age groups in the inhabitants
of Itai-itai disease districts.] Med. Biol., 85: 263-267 (in
Japanese).
SHIROISHI, K., KJELLSTROM, T., ANAYAMA, M., SHIMADA, T., IWATA, T.,
NISHINO, H., MATSUNAGA, A., WATANABE, M., & KUBOTA, K. (1975)
[Studies on the urinary ß-2-microglobulin level of the inhabitants
in Cd-polluted districts.] Jpn J. Hyg., 30: 71 (in Japanese).
SHIROISHI, K., KJELLSTRÖM, T., KUBOTA, K., EVRIN, P.E., ANAYAMA, M.,
VESTERBERG, O., SHIMADA, T., PISCATOR, M., IWATA, T., & NISHINO, H.
(1977) Urine analysis for detection of cadmium-induced renal
changes, with special reference to ß-2-microglobulin. Environ. Res.,
13: 407-424.
SIMPSON, W.R. (1981) A critical review of cadmium in the marine
environment. Prog. Oceanogr., 10: 1-70.
SKERFVING, S., CHRISTOFFERSON, J.O., SHUTZ, A., WELINDER, H., SPANG,
G., AHLGREN, L., & MATTSON, S. (1987) Biological monitoring, by in
vivo XRF measurements, of occupational exposure to lead, cadmium
and mercury. Biol. Trace Elem. Res., 13: 241-251.
SKOG, E. & WAHLBERG, J.E. (1964) A comparative investigation of
percutaneous absorption of metal compounds in guinea-pig by means of
the radioactive isotopes, 51Cr, 58Co, 65Zn, 110Ag, 115Cd,
203Hg. J. invest. Dermatol., 43: 187-192.
SMITH, J.P., SMITH, J.C., & MCCALL, A.J. (1960) Chronic poisoning
from cadmium fume. J. Pathol. Bacteriol., 80: 287-296.
SMITH, T. (1982) Emission inventory case studies - policy aspects.
J. Air Pollut. Control Assoc., 32(10): 1017-1020.
SMITH, T., PETTY, T.L., READING, J.C., & LAKSHMINARAYAN, S. (1976)
Pulmonary effects of chronic exposure to airborne cadmium. Am. Rev.
respir. Dis., 114: 161-169.
SMITH, T., ANDERSON, R.J., & READING, J.C. (1980) Chronic cadmium
exposures associated with kidney function effects. Am. J. ind. Med.,
1: 319-337. SNIDER, G.L., HAYES, J.A., KORTHY, A.L., & LEWIS, G.P.
(1973) Centrilobular emphysema experimentally induced by cadmium
chloride aerosol. Am. Rev. respir. Dis., 108: 40-48.
SONAWANE, B.R., NORDBERG, M., NORDBERG, G.F., & LUCIER, G.W. (1975)
Placental transfer of cadmium in rats: influence of dose and
gestational age. Environ. Health Perspect., 12: 97-102.
SORAHAN, T. (1987) Mortality from lung cancer among a cohort of
nickel cadmium battery workers: 1946-84. Br. J. ind. Med.,
44: 803-809.
SORAHAN, T. & WATERHOUSE, J.A.H. (1983) Mortality study of
nickel-cadmium battery workers by the method of regression models in
life tables. Br. J. ind. Med., 40: 293-300.
SPENCER, H., ASMUSSEN, C.R., HOLTZMAN, R.B., & KRAMER, L. (1979)
Metabolic balances of cadmium, copper, manganese, and zinc in man.
Am. J. clin. Nutr., 32: 1867-1875.
SPORN, A., DINU, I., & STOENESCU, L. (1970) Influence of cadmium
administration on carbohydrate and cellular energetic metabolism in
the rat liver. Rev. roum. Biochim., 7: 299-305.
SQUIBB, K.S., PRITCHARD, J.B., & FOWLER, B.A. (1982) Renal
metabolism and toxicity of metallothionein. In: Foulkes, E.C., ed.
Biological roles of metallothionein, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 181-190.
SQUIBB, K.S., PRITCHARD, J.B., & FOWLER, B.A. (1984)
Cadmium-metallothionein nephropathy: relationships between
ultrastructural/biochemical alterations and intra-cellular cadmium
binding. J. Pharmacol. exp. Ther., 229(1): 311-321.
SQUIRE, J.R., HARDWICKE, J., & SOOTHILL, J.F. (1962) Proteinuria.
In: Black, D.A.K., ed. Renal disease, Oxford, Blackwell Scientific
Publishers, pp. 218-231.
STACEY, N.H., CANTILENA, L.R., & KLAASSEN, C.D. (1980) Cadmium
toxicity and lipid peroxidation in isolated rat hepatocytes.
Toxicol. appl. Pharmacol., 53: 470-480.
STAESSEN, J., BULPITT, C.J., ROELS, H., BERNARD, A., FAGARD, R.,
JOOSSENS, J.V., LAUWERYS, R.R., LIJNEN, P., & AMERY, A. (1984)
Urinary cadmium and lead and their relationship to blood pressure in
a population with low average exposure. Br. J. ind. Med.,
41: 241-248.
STANESCU, D., VEITNER, C., FRANS, A., CONCETTE, L., ROELS, H.,
LAUWERYS, R.R., & BRASSEUR, L. (1977) Effects on lung of chronic
occupational exposure to cadmium. Scand. J. respir. Dis.,
58: 289-303.
STEWART, M. & HUGHES, E.G. (1981) Urinary beta-2-microglobin in the
biological monitoring of cadmium workers. Br. J. Ind. Med.,
38: 170-174.
STOEPPLER, M. & BRANDT, K. (1978) Determination of lead and cadmium
in whole blood by electrothermal atomic absorption spectroscopy. In:
Doss, M., ed. Proceedings of an International Symposium on Clinical
Biochemistry on Diagnosis and Therapy of Porphyrias and Lead
Intoxication, New York, Springer-Verlag, pp. 185-187.
STOEPPLER, M. & BRANDT, K. (1980) Contributions to automated trace
analysis. V. Determination of cadmium in whole blood and urine by
electrothermal atomic absorption spectrophotometry. Fresenius Z.
anal. Chem., 300: 372-380.
STOEPPLER, M., VALENTA, P., & NURNBERG, H.W. (1979) Applications of
independent methods and standard materials: an effective approach to
reliable trace and ultra trace analysis of metals and metalloids in
environmental and biological materials. Fresenius Z. anal. Chem.,
297: 22-34.
STONEBURNER, D.L. (1978) Heavy metals in tissues of stranded
short-finned pilot whales. Sci. total Environ., 9: 293-297.
STOWE, H.D., WILSON, M., & GOYER, R.A. (1972) Clinical and
morphological effects of oral cadmium toxicity in rabbits. Arch.
Pathol., 94: 389-405.
STRAUSS, R.H., PALMER, K.C., & HAYES, J.A. (1976) Acute lung injury
induced by cadmium aerosol. I. Evolution of alveolar cell damage.
Am. J. Pathol., 84: 561-568.
SUBRAMANIAN, K.S. & MERANGER, J.C. (1981) A rapid electrothermal
atomic absorption spectrophotometric method for cadmium and lead in
human whole blood. Clin. Chem., 27: 1866-1871.
SUGAWARA, C. & SUGAWARA, N. (1974) Cadmium toxicity for rat
intestine, especially on the absorption of calcium and phosphorus.
Jpn. J. Hyg., 28: 511-516.
SULLIVAN, M.F., HARDY, J.T., MILLER, B.M., BUSCHBOM, R.L., &
SIEWICKI, T.C. (1984) Absorption and distribution of cadmium in
mice fed diets containing either inorganic or oyster-incorporated
cadmium. Toxicol. appl. Pharmacol., 72: 210-217.
SUMINO, K., HAYAKAWA, K., SHIBATA, T., & KITAMURA, S. (1975) Heavy
metals in normal Japanese tissues. Arch. environ. Health,
30: 487-494.
SUZUKI, S. & LU, C.C. (1976) A balance study of cadmium: an
estimation of daily input, output, and retained amount in two
subjects. Ind. Health, 14: 53-65.
SUZUKI, S., SUZUKI, T., & ASHIZAWA, M. (1965) Proteinuria due to
inhalation of cadmium stearate dust. Ind. Health, 3: 73-85.
SUZUKI, S., TAGUCHI, T., & YOKOHASHI, G. (1969) Dietary factors
influencing upon the retention rate of orally-administered
115CdCl2 in mice, with special reference to calcium and protein
concentration in diet. Ind. Health, 7: 155-162.
SUZUKI, Y. (1980) Cadmium metabolism and toxicity in rats after
long-term subcutaneous administration. J. Toxicol. environ. Health,
6: 469-482.
SUZUKI, Y. (1982) Metal-binding properties of metallothionein in
extracellular fluids and its role in cadmium-exposed rats. In:
Foulkes, E.C., ed. Biological roles of metallothionein, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 25-67.
SVARTENGREN, M., ELINDER, C.G., FRIBERG, L., & LIND, B. (1986)
Distribution and concentration of cadmium in human kidney. Environ.
Res., 39: 1-7.
SVENSSON, B.-G., BJORNHAM, A., SCHUTZ, A., LETTEVALL, U., NILSSON,
A., & SKERFVING, S. (1987) Acidic deposition and human exposure to
toxic metals. Sci. total Environ., 67: 101-115.
TAKABATAKE, E. (1978a) Environmental pollution and health effects:
Oita Prefecture. In: Tsuchiya, K., ed. Cadmium studies in Japan: a
review, Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp. 215-218.
TAKABATAKE, E. (1978b) Nagasaki Prefecture. In: Tsuchiya, K., ed.
Cadmium studies in Japan: a review, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 181-192.
TAKASHIMA, M., MORIWAKI, S., & ITOKAWA, Y. (1980) Osteomalacic
change induced by long-term administration of cadmium to rats.
Toxicol. appl. Pharmacol., 54: 223-228.
TAKEBAYASHI, S. (1980a) First autopsy case, suspicious of cadmium
intoxication, from the cadmium-polluted area in Tsushima, Nagasaki
Prefecture. In: Shigematsu, I. & Nomiyama, K., ed. Cadmium-induced
osteopathy, Tokyo, Japan Public Health Association, pp. 124-138.
TAKEBAYASHI, S. (1980b) [Clinical and pathological findings of a
case with osteomalacia in a cadmium-polluted area (Tsushima Island)
(1st autopsied case)], Tokyo, Japan Public Health Association,
pp. 259-262 (Kankyo Hoken Report No. 46) (in Japanese).
TAKEBAYASHI, S. (1983a) [Clinical and pathological findings of a
case with osteomalacia in a cadmium-polluted area (Tsushima Island)
(2nd autopsied case)], Tokyo, Japan Public Health Association,
pp. 207-211 (Kankyo Hoken Report No. 49) (in Japanese).
TAKEBAYASHI, S. (1983b) [Clinical and pathological findings of a
case with diabetic nephropathy in a cadmium-polluted area (Tsushima
Island) (3rd autopsied case)], Tokyo, Japan Public Health
Association, pp. 212-217 (Kankyo Hoken Report No. 49) (in Japanese).
TAKEBAYASHI, S. (1984) [Clinical and pathological findings of a case
with osteomalacia in a cadmium-polluted area (Tsushima Island) (4th
autopsied case)], Tokyo, Japan Public Health Association,
pp. 238-249 (Kankyo Hoken Report No. 50) (in Japanese).
TAKEBAYASHI, S., SATO, T., HARADA, K., & YOSHIMURA, S. (1985)
[Clinical and pathological findings of a case with osteomalacia in a
cadmium-polluted area (Tsushima Island) (5th autopsied case)],
Tokyo, Japan Public Health Association, pp. 245-251 (Kankyo Hoken
Report No. 51) (in Japanese).
TAKEBAYASHI, S., SATO, T., HARADA, K., HIRAI, Y., & YOSHIMURA, S.
(1987a) [Clinical and pathological findings of a case with
osteomalacia in a cadmium-polluted area (Tsushima Island) (6th
autopsied case)], Tokyo, Japan Public Health Association,
pp. 294-305 (Kankyo Hoken Report No. 53) (in Japanese).
TAKEBAYASHI, S., SATO, T., HARADA, K., HIRAI, Y., & YOSHIMURA, S.
(1987b) [Clinical and pathological findings of a case with
osteomalacia in a cadmium-polluted area (Tsushima Island) (7th
autopsied case)], Tokyo, Japan Public Health Association,
pp. 306-319 (Kankyo Hoken Report No. 53) (in Japanese).
TAKEBAYASHI, S., HIRAI, Y., & YOSHIMURA, S. (1988a) [Clinical and
pathological findings of a case with osteomalacia in a
cadmium-polluted area (Tsushima Island) (8th autopsied case)],
Tokyo, Japan Public Health Association, pp. 187-192 (Kankyo Hoken
Report No. 54) (in Japanese).
TAKEBAYASHI, S., HIRAI, Y., & YOSHIMURA, S. (1988b) [Clinical and
pathological findings of a case with osteomalacia in a
cadmium-polluted area (Tsushima Island) (9th autopsied case)],
Tokyo, Japan Public Health Association, pp. 193-199 (Kankyo Hoken
Report No. 54) (in Japanese).
TAKEBAYASHI, S., HIRAI, Y., & YOSHIMURA, S. (1988c) [Clinical and
pathological findings of a case with osteomalacia in a
cadmium-polluted area (Tsushima Island) (10th autopsied case)],
Tokyo, Japan Public Health Association, pp. 200-206 (Kankyo Hoken
Report No. 54) (in Japanese).
TAKEBAYASHI, S., HARADA, K., & YOSHIMURA, S. (1988d) [Clinical and
pathological findings of a case with osteomalacia in a
cadmium-polluted area (Tsushima Island) (11th autopsied case)],
Tokyo, Japan Public Health Association, pp. 207-213 (Kankyo Hoken
Report No. 54) (in Japanese).
TAKENAKA, S., OLDIGES, H., KOENIG, H., HOCHRAINER, D., &
OBERDOERSTER, G. (1983) Carcinogenicity of cadmium aerosols in
Wistar rats. J. Natl Cancer Inst., 70: 367-371.
TANAKA, K. (1982) Effects of hepatic disorders on the fate of
cadmium in rats. In: Foulkes, E.C., ed. Biological roles of
metallothionein, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 237-249.
TANAKA, K., MIN, K.-S., ONOSAKA, S., FUKUHARA, C., & UEDA, M. (1985)
The origin of metallothionein in red blood cells. Toxicol. appl.
Pharmacol., 78: 63-66.
TARASENKO, N.YU. & VOROBJEVA, R.S. (1973) [Hygienic problems
connected with the use of cadmium.] Vestn. Akad. Med. Nauk. SSR,
28: 37-43 (in Russian with English abstract).
TARASENKO, N.YU., VOROBJEVA, R.S., SPIRIDINOVA, V.S., & SABALINA,
L.P. (1974) Experimental investigation of toxicity of cadmium and
zinc caprylates. J. Hyg. Epidemiol. Microbiol. Immunol.,
18: 144-153.
TARASENKO, N.YU., VOROBJEVA, R.S., SABALINA, L.P., & CVETKOVA, R.P.
(1975) [Cadmium in the environment and its effect on calcium
metabolism.] Gig. i Sanit., 9: 22-25 (in Russian).
TASK GROUP ON LUNG DYNAMICS (1966) Deposition and retention models
for internal dosimetry of the human respiratory tract. Health Phys.,
12: 173-208.
TASK GROUP ON METAL INTERACTIONS (1978) Factors influencing
metabolism and toxicity of metals: a consensus report. Environ.
Health Perspect., 25: 3-42.
TASK GROUP ON METAL TOXICITY (1976) Conceptual considerations:
critical organ, critical concentration in cells and organs, critical
effect, subcritical effect, dose-effect and dose-response
relationships. In: Nordberg, G.F., ed. Effects and dose-response
relationships of toxic metals, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 10-13.
TATI, M., KATAGIRI, Y., & KAWAI, M. (1976) Urinary and faecal
excretion of cadmium in normal Japanese: an approach to non-toxic
levels of cadmium. In: Nordberg, G.F., ed. Effects and dose-response
relationships of toxic metals, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 331-341.
TAYLOR, B.A., HEINIGER, H.J., & MEIER, H. (1973) Genetic analysis of
resistance to cadmium-induced testicular damage in mice (37380).
Proc. Soc. Exp. Biol. Med., 143: 629-633.
TECULESCU, D.B. & STANESCU, D.C. (1970) Pulmonary function in
workers with chronic exposure to cadmium oxide fumes. Int. Arch.
Arbeitsmed., 26: 335-345.
TEMPLETON, D.M., BANERJEE, D., & CHERIAN, M.G. (1985)
Metallothionein synthesis and localization in relation to metal
storage in rat liver during gestation. Can. J. Biochem. cell Biol.,
63: 16-22.
TENNANT, C.J. (1984) Cadmium in the environment and high risk
population groups, Birmingham, University of Aston (Ph.D. Thesis).
TERHAAR, C.J., VIS, E., ROUDABUSH, R.L., & FASSETT, D.W. (1965)
Protective effects of low doses of cadmium chloride against
subsequent high oral doses in the rat. Toxicol. appl. Pharmacol.,
7: 500-505.
THIEME, R., SCHRAMEL, P., & KURZ, E. (1977) [Trace element
concentration in the human placenta in a heavily-contaminated
environment.] Geburtshilfe Frauenheilkd., 37: 756-761 (in German).
THOMAS, B.J., HARVEY, T.C., CHETTLE, D.R., MCLELLAN, J.S., &
FREMLIN, J.H. (1979) A transportable system for the measurement of
liver cadmium in vivo. Phys. Med. Biol., 24: 432-437.
THORNTON, I. (1988) The Shipham report. An investigation into
cadmium contamination and its implications for human health. Metal
content of soils and dusts. Sci. total Environ., 75: 21-39.
THUN, M.T., SCHNORR, T.M., SMITH, A.B., HALPERIN, W.E., & LEMEN,
R.A. (1985) Mortality among a cohort of U.S. cadmium production
workers: An update. J. Natl Cancer Inst., 74: 325-333.
THUN, M.J., OSORIO, A.M., SCHOBER, S., HANNON, W.H., & HALPERIN, W.
(1989) Nephropathy in cadmium workers - assessment of risk from
airborne occupational cadmium exposure. Br. J. ind. Med.,
46: 689-697.
TIPTON, I.H., SCHROEDER, H.A., PERRY, H.M., & COOK, M.J. (1960)
Trace elements in human tissues. Part III. Subjects from Africa, the
Near and Far East, and Europe. Health Phys., 11: 403-451.
TJELL, J.C., HANSEN, J.A., CHRISTENSEN T.H., & HOVMAND, M.F. (1981)
Prediction of cadmium concentrations in Danish soils. In: L'Hermite,
P. & Ott, H., ed. Characterization, treatment and use of sewage
sludge, Dordrecht, Reidel Publishing Co., pp. 652-664.
TJELL, J.C., CHRISTENSEN, T.H., & BRO-RASMUSSEN, F. (1983) Cadmium
in soil and terrestrial biota, with emphasis on the Danish
situation. Ecotoxicol. environ. Saf., 7: 122-140.
TOHYAMA, C., SHAIKH, Z.A., ELLIS, K.J., & COHN, S.H. (1981a)
Metallothionein excretion in urine upon cadmium exposure: its
relationship with liver and kidney cadmium. Toxicology, 20: 181-191.
TOHYAMA, C., SHAIKH, Z.A., NOGAWA, K., KOBAYASHI, E., & HONDA, R.
(1981b) Elevated urinary excretion of metallothionein due to
environmental cadmium exposure. Toxicology, 22: 289-297.
TOPPING, M.D., FORSTER, H.W., DOLMAN, C., LUCZYNSKA, C.M., &
BERNARD, A.M. (1986) Measurement of urinary retinol-binding protein
by enzyme-linked immunosorbent assay and its application to
detection of tubular proteinuria. Clin. Chem., 32: 1863-1866.
TSUCHIYA, K. (1967) Proteinuria of workers exposed to cadmium fume.
The relationship to concentration in the working environment. Arch.
environ. Health, 14: 875-880.
TSUCHIYA, K. (1969) Causation of Ouch-ouch disease (Itai-itai byo):
an introduction review. Part 1. Nature of the disease. Keio J. Med.,
18: 181-194.
TSUCHIYA, K. (1976) Proteinuria of cadmium workers. J. occup. Med.,
18: 463-466.
TSUCHIYA, K., ed. (1978) Cadmium studies in Japan: a review,
Amsterdam, Oxford, New York, Elsevier Science Publishers, 376 pp.
TSUCHIYA, K. & IWAO, S. (1978) Interrelationships among zinc,
copper, lead, and cadmium in food, faeces, and organs of humans.
Environ. Health Perspect., 25: 119-124.
TSUCHIYA, K. & NAKAMURA, K.I. (1978) Environmental pollution and
health effects: Hyogo prefecture. In: Tsuchiya, K., ed. Cadmium
studies in Japan: a review, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 146-167.
TSUCHIYA, K. & SUGITA, M. (1971) A mathematical model for deriving
the biological half-life of a chemical. Nord. Hyg. Tidskr.,
53: 105-110.
TSUCHIYA, K., SEKI, Y., & SUGITA, M. (1976) Cadmium concentrations
in the organs and tissues of cadavers from accidental deaths. Keio
J. Med., 25: 83-90.
TSUCHIYA, K., IWAO, S., SUGITA, M., & SAKURAI, H. (1979) Increased
urinary ß-2-microglobulin in cadmium exposure: dose-effect
relationship and biological significance of ß-2-microglobulin.
Environ. Health Perspect., 28: 147-153.
URUNO, K., OTOYAMA, S., NAKANO, N., & ITAGAKI, Z. (1975) [Results
of health examination for inhabitants in Yoshinokawa area polluted
with cadmium], Tokyo, Japan Public Health Association, pp. 109-113
(Kankyo Hoken Report No. 36) (in Japanese).
USHIO, F. & DOGUCHI, M. (1977) Dietary intakes of some chlorinated
hydrocarbons and heavy metals estimated on the
experimentally-prepared diets. Bull. environ. Contam. Toxicol.,
17: 707-711.
US PUBLIC HEALTH SERVICE (1942) Cadmium poisoning. Public Health
Rep., 57: 602-612.
VAHTER, M., ed. (1982) Assessment of human exposure to lead and
cadmium through biological monitoring, Stockholm, National Swedish
Institute of Environmental Medicine and Department of Environmental
Hygiene, Karolinska Institute, 136 pp (Report prepared for the
United Nations Environment Programme and the World Health
Organization).
VALETAS, P. (1946) "Cadmiose" ou l'intoxication du cadmium, Paris,
School of Medicine, 40 pp (Medical Faculty Thesis Report).
VAN AALST, R.M., VAN ARDENNE, R.A.M., DE KRUK, J.F., & LEMS, T.
(1983a) Pollution of the North Sea from the atmosphere, Apeldoorn,
The Netherlands Organization for Applied Scientific Research (TNO)
(Report No. C182/152).
VAN AALST, R.M., DUYZER, J.H., & VELDT, C. (1983b) Atmospheric
deposition of lead and cadmium in the southern part of the North
Sea. I. Emission and preliminary model calculations, Apeldoorn, The
Netherlands Organization for Applied Scientific Research (TNO)
(Report No. R83/222).
VANDER, A.J. (1962) Cadmium enhancement of proximal tubular sodium
reabsorption. Am. J. Physiol., 203: 1005-1007.
VANDER MALLIE, R.J. & GARVEY, J.S. (1979) Radioimmunoassay of
metallothioneins. J. biol. Chem., 254: 8416-8421.
VENS, M.D. & LAUWERYS, R.R. (1982) Détermination simultanée du plomb
et du cadmium dans le sang et l'urine par le couplage des techniques
de chromatographie sur résine échangeuse d'ions et de
spectrophotométrie d'absorption atomique. Arch. Mal. prof. Méd.
Trav. Sécur. soc., 33: 97-105.
VESTERBERG, O. & WRANGSKOGH, K. (1978) Determination of cadmium in
urine by graphite furnace atomic absorption spectroscopy. Clin.
Chem., 24: 681-685.
VOROBJEVA, R.S. (1957) [Investigations of the nervous system
function in workers exposed to cadmium oxide.] Neuropatol.
Psikkiatr., 57: 385-388 (in Russian).
VOROBJEVA, R.S. (1958) [About occupational cadmium oxide
poisonings.] J. Hyg. Epidemiol. Microbiol. Immunol., 11: 244-249 (in
German).
VOROBJEVA, R.S. & BUBNOVA, N.I. (1981) [Effects of elemental cadmium
and telluric cadmium on organisms.] Gig. Trud. prof. Zabol.,
2: 42-43 (in Russian).
VOROBJEVA, R.S. & EREMEEVA, E.P. (1980) [Cardiovascular function in
workers exposed to cadmium.] Gig. i Sanit., 10: 22-25 (in Russian).
VOROBJEVA, R.S. & SABALINA, L.P. (1975) [Experimental investigations
of toxic properties of various cadmium compounds.] Gig. i Sanit.,
2: 102-104 (in Russian).
VOSTAL, J.J. (1976) Dose-response relationship of mercury and
cadmium effects on renal cadmium transport. In: Nordberg, G.F., ed.
Effects and dose-response relationships of toxic metals, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 299-308.
VUORI, E., HUUNAN-SEPPALA, A., KILPIO, J.O., & SALMELA, S.S. (1979)
Biologically-active metals in human tissues. II. The effect of age
on the concentration of cadmium in aorta, heart, kidney, liver,
lung, pancreas, and skeletal muscle. Scand. J. Work Environ. Health,
5: 16-22.
WAALKES, M.P., POISNER, A.M., WOOD, G.W., & KLAASSEN, C.D. (1984)
Metallothionein-like proteins in human placenta and fetal membranes.
Toxicol. appl. Pharmacol., 74: 179-184.
WADE, G.G. (1987) Marked synergism of dimethylnitrosamine
carcinogenesis in rats exposed to cadmium. Cancer Res.,
47: 6606-6613.
WALSH, J.J. & BURCH, G.E. (1959) The rate of disappearance from
plasma and subsequent distribution of radiocadmium ( 115 m Cd) in
normal dogs. J. clin. Med., 54: 59-65.
WALTERS, B. & SHERLOCK, J. (1981) Studies on the dietary intake of
heavy metals. In: Proceedings of the International Conference on
Heavy Metals in the Environment, Amsterdam, September 1981,
Edinburgh, CEP Consultants Ltd, pp. 506-512.
WATANABE, H. & MURAYAMA, H. (1975) A study on health effect indices
concerning population in a cadmium-polluted area. In: International
Symposium on Recent Advances in the Assessment of the Health Effects
of Environmental Pollution, Luxembourg, December 1975, Luxembourg,
Commission of the European Communities, pp. 2185-2195 (Report EURO
5360).
WATANABE, I., KOIZUMI, A., FUJITA, H., KUMAI, M., & IKEDA, M. (1985)
Dietary cadmium intakes of farmers in nonpolluted areas in Japan,
and the relation with blood cadmium levels. Environ. Res.,
37(1): 33-43.
WATANABE, T., SHIMADA, T., & ENDO, A. (1979) Mutagenic effects of
cadmium on mammalian oocyte chromosomes. Mutat. Res., 67: 349-356.
WEAST, R.C. (1974) Handbook of chemistry and physics, 55th ed.,
Cleveland, Ohio, CRC Press, 500 pp.
WEBB, M. (1972) Protection by zinc against cadmium toxicity.
Biochem. Pharmacol., 21: 2767-2771.
WEBB, M. & CAIN, K. (1982) Functions of metallothionein. Biochem.
Pharmacol., 31: 137-142.
WEBB, M. & ETIENNE, A.T. (1977) Studies on the toxicity and
metabolism of cadmium-thionein. Biochem. Pharmacol., 26: 25-30.
WESTER, P.O. (1974) Trace element balances in relation to variations
in calcium intake. Atherosclerosis, 20: 207-215.
WHANGER, P.D. (1979) Cadmium effects in rats on tissue iron,
selenium, and blood pressure: blood and hair cadmium in some Oregon
residents. Environ. Health Perspect., 28: 115-121.
WHO (1980) Recommended health-based limits in occupational exposure
to heavy metals. Report of a study group, Geneva, World Health
Organization (Technical Report Series No. 647).
WHO (1984) Guidelines for drinking-water quality: Health criteria
and other supporting information, Geneva, World Health Organization,
Vol. 2, pp. 84-90.
WHO (1986) Health impact of acidic deposition. Sci. total Environ.,
52: 157-187.
WHO (1987) Air quality guidelines for Europe, Copenhagen, World
Health Organization, Regional Office for Europe, pp. 200-209
(European Series, Vol. 23).
WHO (1989) Cadmium. In: Evaluation of certain food additives and
contaminants. Thirty-third Report of the Joint FAO/WHO Expert
Committee on Food Additives, Geneva, World Health Organization,
pp. 28-31 (Technical Report Series No. 776).
WILLIAMS, C.H. & DAVID, D.J. (1973) The effect of superphosphate on
the cadmium content of soils and plants. Aust. J. Soil Res.,
11: 43-56.
WILLIAMS, C.H. & DAVID, D.J. (1976) The accumulation in soil of
cadmium residues from phosphate for fertilizers and their effect on
the cadmium content of plants. Soil Sci., 121: 86-93.
WILSON, B. (1988a) Investigation of trace metals in the aqueous
environment: Final report (January 1986-December 1987), Houston,
Texas Southern University, p. 28 (Report No. DOE/CH/10255-T1,
prepared for the US Department of Energy, Washington).
WILSON, D.N. (1988b) Cadmium - market trends and influences. In:
Cadmium 87. Proceedings of the 6th International Cadmium
Conference, London, Cadmium Association, pp. 9-16.
WILSON, R.H., DE EDS, F., & COX, A.J., Jr (1941) Effects of
continued cadmium feeding. J. Pharmacol. exp. Ther., 71: 222-235.
WOLFF, E.W. & PEEL, D.A. (1985) The record of global pollution in
polar snow and ice. Nature (Lond.), 313: 535-540.
WOLNIK, K.A., FRICKE, F.L., CAPER, S.G., BRAUDE, G.L., MEYER, M.W.,
SATZGER, R.D., & BONNIN, E. (1983) Elements in major raw
agricultural crops in the United States. I. Cadmium and lead in
lettuce, peanuts, potatoes, soybeans, sweet corn, and wheat. J.
agric. food Chem., 31: 1240-1244.
WOLNIK, K.A., FRICKE, F.L., CAPAR, S.G., MEYER, M.W., & SATZGER,
R.D. (1985) Elements in major raw agricultural crops in the United
States. 3. Cadmium, lead, and eleven other elements in carrots,
field corn, onion, rice, spinach, and tomatoes. J. agric. food
Chem., 33: 807-811.
YAMAGATA, H. & IWASHIMA, K. (1975) Average cadmium intake of the
Japanese people. Bull. Inst. Public Health (Tokyo), 24: 18-24.
YAMAGATA, N. & SHIGEMATSU, I. (1970) Cadmium pollution in
perspective. Bull. Inst. Public Health (Tokyo), 19: 1-27.
YAMAGATA, N., IWASHIMA, K., & NAGAI, T. (1974) [Gastrointestinal
absorption of cadmium in normal humans], Tokyo, Japan Public Health
Association, pp. 84-85 (Kankyo Hoken Report No. 31) (in Japanese).
YAMAMOTO, Y. (1972) [Present status of cadmium environmental
pollution], Tokyo, Japan Public Health Association, pp. 7-12 (Kankyo
Hoken Report No. 11) (in Japanese).
YOSHIKAWA, H., KAWAI, K., SUZUKI, Y., NOZAKI, K., & OHSAWA, M.
(1975) [Studies on cadmium effects in living organisms: impairment
due to inhalation], Tokyo, Japanese Environment Agency, 40 pp (in
Japanese).
RESUME ET CONCLUSIONS
1. Identité, propriétés physiques et chimiques et méthodes
d'analyse
Il existe plusieurs méthodes pour le dosage du cadmium dans les
échantillons biologiques. La plus utilisée est la spectrométrie
d'absorption atomique, mais elle nécessite un traitement minutieux
de la prise d'essai et une correction pour tenir compte des
interférences lorsqu'elle est appliquée à des échantillons de faible
teneur en cadmium. Il est tout à fait souhaitable que l'analyse
s'accompagne d'un programme d'assurance de la qualité. A l'heure
actuelle, il est possible, dans des conditions optimales, de doser
environ 0,1 µg/litre dans l'urine et le sang et de 1 à 10 µg/litre
dans les aliments et les tissus.
2. Sources d'exposition humaine et environnementale
Le cadmium est un élément relativement rare et les méthodes
actuelles d'analyse indiquent, dans les divers compartiments de
l'environnement, des concentrations beaucoup plus faibles que les
mesures antérieures. Pour l'instant, il n'est pas possible de
déterminer si l'activité humaine est à l'origine d'un accroissement
de la teneur des calottes polaires en cadmium à l'échelle des temps
historiques.
La production commerciale de cadmium a commencé au tournant du
siècle. La consommation a changé de caractère ces dernières années
avec un recul sensible de la galvanoplastie et une utilisation
accrue dans la fabrication de batteries et de composants
électroniques spéciaux. Dans la plupart des cas, le cadmium est
utilisé sous la forme de dérivés peu concentrés, ce qui rend le
recyclage indispensable. Les restrictions à l'usage du cadmium
imposées par certains pays pourraient avoir des répercussions
importantes sur ces applications.
Les activités humaines entraînent la libération de cadmium dans
l'air, le sol et l'eau. D'une façon générale, les deux principales
sources de contamination sont la production et la consommation de
cadmium et d'autres métaux non ferreux ainsi que le rejet de déchets
contenant du cadmium. Dans les zones proches de mines ou de
fonderies de métaux non ferreux, la contamination par le cadmium est
souvent importante.
Plus le sol contient de cadmium, plus la quantité fixée par les
plantes est importante. L'exposition humaine par l'intermédiaire des
cultures sera donc sensible à toute augmentation de la teneur du sol
en cadmium. La fixation par les plantes est plus importante dans les
sols de faible pH. Les processus qui acidifient le sol (pluies
acides, par exemple) sont donc susceptibles de provoquer une
augmentation de la concentration moyenne du cadmium dans les denrées
alimentaires. Dans certaines régions du monde, l'utilisation
d'engrais phosphatés et les dépôts d'origine atmosphérique
constituent une source non négligeable de contamination des terres
arables; les boues d'égout peuvent aussi, localement, entraîner une
forte pollution. A l'avenir, ces sources risquent d'accroître la
contamination du sol et celle des cultures, ce qui débouchera sur
une exposition plus importante au cadmium par la voie alimentaire.
Dans certaines régions, on peut constater une augmentation de la
teneur des aliments en cadmium.
Les animaux et les plantes comestibles qui vivent à l'état
sauvage, comme les coquillages, les crustacés et les champignons
accumulent naturellement le cadmium. Comme chez l'homme, on constate
une augmentation de la concentration en cadmium dans le foie et les
reins des chevaux et de certains animaux terrestres vivant à l'état
sauvage. La consommation régulière de ces abats peut entraîner une
exposition accrue. Dans les reins de certains vertébrés marins on
trouve des concentrations assez fortes en cadmium, qui, même si
elles sont d'origine naturelle, n'en provoquent pas moins des
lésions au niveau de ces organes.
3. Concentrations dans l'environnement et exposition humaine
La principale voie d'exposition au cadmium des non fumeurs est
la voie alimentaire. Les autres voies sont peu importantes. Chez les
fumeurs, l'apport de cadmium par le tabac est notable. Dans les
zones contaminées, l'exposition d'origine alimentaire peut atteindre
plusieurs centaines de microgrammes par jour. Chez les travailleurs
exposés, la principale voie de pénétration est la voie pulmonaire,
après inhalation d'air contaminé sur le lieu de travail. Le
tabagisme et la consommation d'aliments contaminés ajoutent encore à
la charge de cadmium de l'organisme.
4. Cinétique et métabolisme chez les animaux de laboratoire et
chez l'homme
Les données tirées de l'expérimentation animale et humaine
montrent que l'absorption est plus importante au niveau des poumons
qu'au niveau des voies digestives. Selon l'espèce chimique en cause,
la granulométrie et la solubilité dans les liquides biologiques, le
taux d'absorption peut atteindre 50% après inhalation. L'absorption
gastro-intestinale dépend du régime alimentaire et de l'état
nutritionnel. En particulier, le bilan martial est particulièrement
important. En moyenne, le cadmium total contenu dans les aliments
est absorbé à hauteur de 5%, avec un intervalle de variation de
1%-20% selon les individus. Il existe un gradient materno-foetal de
cadmium. Le cadmium peut également parvenir jusqu'au foetus, mais en
faibles quantités malgré son accumulation dans le placenta.
Le cadmium absorbé au niveau des poumons ou des voies
digestives s'accumule principalement dans le foie et les reins où il
représente plus de la moitié de la charge totale de l'organisme.
Plus l'exposition est intense, plus l'accumulation du cadmium dans
le foie est importante. En principe, l'excrétion est lente et la
période biologique du cadmium dans les muscles, les reins, le foie
et l'organisme dans son ensemble, est très longue, de l'ordre de
plusieurs décennies. La teneur en cadmium de la plupart des tissus
augmente avec l'âge. C'est en général dans le cortex rénal que la
concentration est la plus élevée, mais en cas d'exposition
excessive, elle peut l'être encore plus dans le foie. Chez les
personnes exposées atteintes de lésions rénales, il y a augmentation
de l'excrétion urinaire du cadmium de sorte que la période
biologique pour l'ensemble de l'organisme est raccourcie. Du fait
des lésions, le rein perd son cadmium et ces malades finissent par
présenter une concentration rénale de cadmium plus faible que les
individus en bonne santé soumis à la même exposition.
La métallothionéine est une protéine qui joue un rôle important
dans le transport et le stockage du cadmium et d'autres métaux. Le
cadmium est capable d'induire la synthèse de cette protéine dans de
nombreux organes, notamment le foie et le rein. En fixant le cadmium
intracellulaire, la métallothionéine protège les tissus contre les
effets toxiques de ce métal. Il est possible, par conséquent, que le
cadmium non lié à la métallothionéine ait une responsabilité dans
les lésions tissulaires. On ignore quels peuvent être les autres
complexes du cadmium présents dans les liquides biologiques.
L'excrétion urinaire du cadmium dépend de divers facteurs:
charge totale de l'organisme, exposition récente et lésions rénales.
Chez les individus peu exposés, la concentration urinaire du cadmium
dépend principalement de la charge de l'organisme. En cas de lésions
rénales dues au cadmium, ou même sans lésions de ce genre mais en
présence d'une exposition excessive, il y a augmentation de
l'excrétion urinaire. Les individus exposés au cadmium en excrètent
davantage dans leurs urines lorsqu'ils présentent une protéinurie.
Après cessation d'une exposition intense, le taux urinaire décroît,
même s'il y a persistance des lésions rénales. Il faut donc prendre
plusieurs facteurs en considération pour interpréter le cadmium
urinaire. L'excrétion par la voie digestive est sensiblement
équivalente à l'excrétion urinaire mais elle est difficile à
mesurer. L'excrétion par d'autres voies (lactation, sueur ou passage
transplacentaire) est négligeable.
La teneur des matières fécales en cadmium est un bon indicateur
d'une ingestion récente en l'absence d'exposition par la voie
respiratoire. Le cadmium sanguin est présent principalement dans les
hématies, la concentration plasmatique étant très faible. Il existe
au moins deux compartiments dans le sang, l'un qui correspond à une
exposition récente, avec une demi-vie de 2-3 mois et l'autre, qui
est probablement lié à la charge totale de l'organisme et se
caractérise par une demi-vie de plusieurs années.
5. Effets sur les animaux de laboratoire
Une forte exposition par la voie respiratoire entraîne un
oedème mortel du poumon. Après injection d'une seule dose,
apparaissent des lésions testiculaires, une nécrose ovarienne, des
lésions hépatiques et une atteinte des petits vaisseaux. L'ingestion
de fortes doses provoque des lésions de la muqueuse gastrique et
intestinale.
Une exposition prolongée par la voie respiratoire ou une
administration intratrachéenne entraîne des altérations pulmonaires
de nature inflammatoire ainsi qu'une fibrose et donne au tissu
pulmonaire un aspect qui évoque l'emphysème. L'administration
prolongée par voie orale ou parentérale affecte principalement le
rein mais elle a aussi des effets sur le foie et les systèmes
hématopoiétique, immunitaire et cardio-vasculaire ainsi que sur le
squelette. Chez certaines espèces et dans des conditions
déterminées, on a provoqué une hypertension et constaté des effets
sur le squelette. C'est le stade de la gestation où se produit
l'exposition qui conditionne les effets tératogènes et les lésions
placentaires et il peut y avoir interaction avec le zinc.
Ce sont les effets aigus produits par l'inhalation du cadmium
ainsi que sa néphrotoxicité chronique qui sont les plus importants
du point de vue de l'exposition humaine. En cas d'exposition
prolongée, c'est le rein qui est l'organe critique. Les effets sont
caractérisés par une lésion des cellules tubulaires entraînant une
insuffisance tubulaire parfois accompagnée d'insuffisance
glomérulaire. L'insuffisance tubulaire a pour conséquence une
perturbation du métabolisme du calcium et de la vitamine D. Selon
certains travaux, ces troubles pourraient provoquer une ostéomalacie
ou une ostéoporose. Cependant ces résultats n'ont pas été confirmés
par d'autres études. On ne peut exclure un effet direct du cadmium
sur la minéralisation de l'os. Chez l'animal de laboratoire, les
effets toxiques du cadmium dépendent de certains facteurs génétiques
et nutritionnels, des interactions avec d'autres métaux, en
particulier le zinc, et d'un premier traitement éventuel par le
cadmium susceptible d'avoir stimulé la synthèse de métallothionéine.
En 1976 et 1987, le Centre international de recherche sur le
cancer a admis posséder suffisamment de preuves que l'injection de
chlorure, de sulfate, de sulfure et d'oxyde de cadmium pouvait
entraîner l'apparition d'un sarcome local chez le rat et, dans le
cas des deux premiers composés, de tumeurs testiculaires
interstitielles chez ce même animal et chez la souris. Toutefois, il
a considéré que les études basées sur l'administration par voie
orale ne permettaient pas de procéder à une évaluation. Lors
d'études au cours desquelles on a fait respirer à des rats des
aérosols de sulfate de cadmium, des vapeurs d'oxyde de cadmium et de
la poussière de sulfate de cadmium, on a observé une forte incidence
de cancers primitifs du poumon, avec probablement une relation entre
la dose et la réponse. Toutefois, ces résultats n'ont pu être
reproduits chez d'autres espèces. Les travaux relatifs aux effets
génotoxiques du cadmium ont donné des résultats contradictoires.
6. Effets sur l'homme
Une forte exposition à des vapeurs d'oxyde de cadmium par la
voie respiratoire entraîne une pneumopathie aiguë, accompagnée d'un
oedème du poumon qui peut être mortel. L'ingestion de grandes
quantités de sels solubles de cadmium provoque une gastro-entérite
aiguë.
A la suite d'une exposition professionnelle prolongée au
cadmium, on a observé de graves effets chroniques, principalement au
niveau des poumons et des reins. On a également observé une
néphrotoxicité chronique dans la population générale.
Les altérations pulmonaires consécutives à une exposition
professionnelle intense sont essentiellement caractérisées par une
obstruction des voies aériennes. Si l'exposition se poursuit, les
légers troubles ventilatoires initiaux peuvent déboucher sur une
insuffisance respiratoire. On a observé un accroissement de la
mortalité par pneumopathie obstructive chez des travailleurs
fortement exposés, comme cela se produisait auparavant.
L'accumulation de cadmium dans le cortex rénal entraîne des
troubles de la fonction tubulaire et une réabsorption insuffisante,
par exemple, des protéines, du glucose et des acides aminés.
L'accroissement de l'excrétion urinaire des protéines de faible
masse moléculaire est un signe caractéristique de l'insuffisance
tubulaire. Parfois il y a aussi baisse du taux de filtration
glomérulaire. L'augmentation du cadmium urinaire est corrélée avec
une protéinurie de faible masse moléculaire et, en l'absence
d'exposition, peut servir d'indicateur de l'atteinte rénale. Dans
les cas graves, les effets tubulaires et glomérulaires s'ajoutent
s'accompagnant parfois d'une élévation du taux sanguin de
créatinine. Chez la plupart des travailleurs et autres personnes, la
protéinurie due à une néphropathie cadmique est irréversible.
Parmi les autres effets, on peut citer les troubles du
métabolisme calcique, l'hypercalciurie et la formation de calculs
rénaux. Une exposition intense au cadmium peut, selon toute
probabilité lorsqu'elle s'accompagne d'autres facteurs comme une
carence nutritionnelle, provoquer l'apparition d'une ostéoporose
et/ou d'une ostéomalacie.
On est fondé à penser qu'une exposition professionnelle
prolongée au cadmium peut favoriser l'apparition d'un cancer du
poumon, mais la présence de facteurs de confusion ne facilite pas
l'interprétation des observations effectuées sur les travailleurs
exposés. En ce qui concerne le cancer de la prostate, les données ne
sont pas concluantes et ne confirment pas, en tout cas, l'hypothèse
antérieure d'une relation de cause à effet.
A l'heure actuelle, on ne possède pas de preuve convaincante
que le cadmium provoque une hypertension essentielle. La plupart des
données contredisent cette hypothèse et rien n'indique un
accroissement de la mortalité par maladie cardio-vasculaire ou
accident vasculaire cérébral chez les personnes exposées.
D'après les résultats d'études relatives à des groupes exposés
de par leur profession ou simplement du fait de leur environnement
général, il semble que la prévalence des effets néphrotoxiques soit
liée à la durée et à l'intensité de l'exposition.
Chez des ouvriers de l'industrie du cadmium, on a signalé,
après 10 à 20 ans d'exposition à des concentrations de l'ordre de
20-50 µg par mètre cube, une augmentation de la prévalence des cas
de protéinurie à faible masse moléculaire.
Dans des zones polluées, où l'on évalue l'apport de cadmium par
voie orale à environ 140-260 µg/jour, on a observé des effets du
genre protéinurie à faible masse moléculaire chez des sujets exposés
pendant une longue période. On trouvera à la section 8 une
estimation plus précise de la relation dose-réponse.
7. Evaluation des risques pour la santé humaine
7.1 Conclusions
On estime que le rein est l'organe cible tant dans la
population générale que chez les groupes professionnellement
exposés. Une exposition prolongée par inhalation entraîne
l'apparition d'un syndrome respiratoire obstructif chez certains
groupes professionnels. Certains détails incitent à penser que cette
exposition au cadmium pourrait favoriser l'apparition d'un cancer du
poumon, mais les observations effectuées sur des travailleurs
exposés sont difficiles à interpréter en raison de la présence de
facteurs de confusion.
7.1.1 Population générale
Pour la plupart des individus, les aliments constituent la
principale voie d'exposition au cadmium. Dans la plupart des régions
non polluées par ce métal, l'apport alimentaire journalier est de
l'ordre de 10-40 µg. Dans les zones polluées, il peut atteindre
plusieurs centaines de microgrammes par jour. Dans les zones non
polluées, l'apport dû au tabac peut être égal à l'apport alimentaire
chez les gros fumeurs.
D'après un modele biologique, on estime qu'il existe une
association entre l'exposition au cadmium et l'excrétion urinaire de
protéines de faible masse moléculaire chez les sujets qui absorbent
pendant toute leur vie une dose journalière d'environ 140-260 µg de
cadmium, ce qui correspond à une dose cumulée d'environ 2000 mg ou
davantage.
7.1.2 Groupes professionnellement exposés
Dans ce cas, l'exposition est essentiellement respiratoire,
mais il s'y ajoute l'apport alimentaire et tabagique. La teneur
totale de l'air en cadmium varie selon les pratiques en matière
d'hygiène industrielle et selon le lieu de travail. Il existe une
relation de type exposition-réponse entre la teneur de l'air en
cadmium et la protéinurie de faible masse moléculaire. La prévalence
de cette protéinurie peut augmenter après 10 à 20 ans d'exposition à
des concentrations de cadmium de l'ordre de 20-50 µg par mètre cube.
In vivo, le dosage du cadmium dans les reins et le foie de sujets
plus ou moins exposés a montré que 10 % environ des travailleurs
dont le cortex rénal contenait 200 mg/kg de cadmium et 50 % de ceux
chez qui cette concentration atteignait 300 mg/kg, feraient un jour
ou l'autre une protéinurie par insuffisance tubulaire.
RESUMEN Y CONCLUSIONES
1. Identidad, propiedades físicas y químicas, y métodos analíticos
Se dispone de varios métodos para determinar el cadmio presente
en el material biológico. El más utilizado es la espectrometría de
absorción atómica, aunque el análisis de muestras con
concentraciones bajas de cadmio exige un tratamiento cuidadoso de
las muestras y correcciones para tener en cuenta la interferencia.
Se recomienda encarecidamente acompañar el análisis con un programa
de garantía de la calidad. Actualmente, en circunstancias ideales
pueden determinarse concentraciones de alrededor de 0,1 µg/litro en
la orina y la sangre y de 1-10 µg/kg en alimentos y muestras de
tejidos.
2. Fuentes de exposición humana y ambiental
El cadmio es un elemento relativamente raro; los procedimientos
analíticos actuales indican que las concentraciones del metal en el
medio ambiente son mucho más bajas que las obtenidas en medidas
anteriores. Hoy en día no es posible determinar si la actividad
humana ha provocado un aumento histórico de los niveles de cadmio en
los casquetes polares.
La producción comercial de cadmio comenzó a principios de este
siglo. La pauta de consumo de cadmio se ha modificado en los últimos
años debido al notable descenso del uso de la galvano-plastia y al
importante aumento de la producción de baterías y de las
aplicaciones electrónicas especializadas. En las principales
aplicaciones del cadmio éste se utiliza en forma de compuestos que
se hallan presentes en bajas concentraciones; ello obstaculiza el
reciclaje del metal. Las restricciones impuestas por algunos países
a ciertas aplicaciones del cadmio pueden tener un efecto
generalizado en esas aplicaciones.
El cadmio se libera al aire, los suelos y las aguas debido a la
actividad humana. En general, las dos fuentes principales de
contaminación son la producción y el consumo de cadmio y de otros
metales no ferrosos y la evacuación de desechos que contienen
cadmio. Las zonas próximas a minas no ferrosas y fundiciones suelen
estar muy contaminadas por cadmio.
Al aumentar el contenido de cadmio del suelo, aumenta la
absorción del metal por las plantas; la exposición humana a partir
de las cosechas agrícolas está por tanto sometida a los aumentos del
contenido de cadmio del suelo. Dado que la absorción por las plantas
desde el suelo es mayor cuando el pH de éste es bajo, los procesos
que acidifican el suelo (por ejemplo, las lluvias ácidas) pueden
aumentar las concentraciones medias de cadmio en los alimentos. La
aplicación de fertilizantes a base de fosfato y la deposición
atmosférica son fuentes importantes de aportación de cadmio a las
tierras cultivables en ciertas zonas del mundo; los fangos de
alcantarillado también pueden ser una fuente de importancia a nivel
local. Estas fuentes pueden, en el futuro, aumentar los niveles de
cadmio en el suelo y con ello en las cosechas, lo que a su vez puede
acrecentar la exposición al cadmio en la dieta. En ciertas zonas, se
ha demostrado que está aumentando el contenido de cadmio en los
alimentos.
Ciertos organismos comestibles de vida libre como los mariscos,
los crustáceos y los hongos son acumuladores naturales de cadmio.
Como en el caso del ser humano, se observan niveles mayores de
cadmio en el hígado y el riñón de los caballos y de algunos animales
terrestres silvestres. El consumo habitual de estos alimentos puede
aumentar la exposición. Ciertos vertebrados marinos contienen
concentraciones notablemente elevadas de cadmio en el riñón,
fenómeno que, aunque se considera de origen natural, se ha vinculado
a signos de lesiones renales en esos organismos.
3. Niveles ambientales y exposición humana
La principal fuente de exposición al cadmio en la población
general no fumadora son los alimentos; la proporción de cadmio que
se absorbe por otras vías es pequeña. El tabaco es una importante
fuente de absorción de cadmio en los fumadores. En las zonas
contaminadas, la exposición al cadmio por los alimentos puede
alcanzar varios cientos de µg/día. En los trabajadores expuestos, la
absorción pulmonar de cadmio por inhalación en el lugar de trabajo
es la principal vía de exposición. También puede aumentar la
absorción por la contaminación de los alimentos y por el consumo de
tabaco.
4. Cinética y metabolismo en animales de experimentación y en
el ser humano
Los datos obtenidos en animales de experimentación y en el ser
humano han demostrado que la absorción pulmonar es mayor que la
gastrointestinal. Atendiendo a la especiación química, el tamaño de
las partículas y la solubilidad en fluidos biológicos, puede
absorberse hasta el 50% del compuesto de cadmio inhalado. La
absorción gastrointestinal de cadmio depende del tipo de dieta y del
estado nutricional. El estado nutricional respecto del hierro parece
revestir particular importancia. Aunque en promedio se absorbe el 5%
de la ingesta oral total de cadmio, los valores individuales varían
entre menos del 1% hasta más del 20%. Existe un gradiente
maternofetal de cadmio. Aunque se acumula en la placenta, la
transferencia al feto es baja.
El cadmio absorbido en los pulmones o el tracto
gastrointestinal se almacena principalmente en el hígado y el riñón,
donde se deposita más de la mitad de la carga corporal. Al aumentar
la intensidad de la exposición, aumenta la proporción del cadmio
absorbido que se almacena en el hígado. En el ser humano la
excreción suele ser lenta y la semivida biológica es muy larga
(decenios) en el músculo, el riñón, el hígado y el organismo entero.
Las concentraciones de cadmio en la mayoría de los tejidos aumentan
con la edad. Aunque las concentraciones más elevadas suelen
encontrarse en la corteza renal, con exposiciones excesivas pueden
producirse concentraciones mayores en el hígado. En las personas
expuestas que padecen lesiones renales, aumenta la excreción
urinaria de cadmio con lo que se reduce la semivida en el organismo
entero. Las lesiones renales producen pérdidas del cadmio contenido
en el riñón, y las concentraciones renales acaban con el tiempo
siendo inferiores a las observadas en personas con un grado de
exposición similar pero sin lesiones renales.
La metalotioneína es una importante proteína de transporte y
almacenamiento de cadmio y otros metales. El cadmio puede inducir la
síntesis de metalotioneína en muchos órganos, en particular el
hígado y el riñón. La unión del cadmio intracelular a la
metalotioneína en los tejidos protege contra la toxicidad del metal.
El cadmio libre puede por tanto tener una función en la patogenia de
las lesiones tisulares debidas a ese metal. Se desconoce la
especiación de otros complejos de cadmio en los tejidos o en los
fluidos biológicos.
La excreción urinaria de cadmio guarda relación con la carga
corporal, la exposición reciente y la lesión renal. En personas poco
expuestas, el nivel de cadmio en la orina depende principalmente de
la carga corporal. Una vez que se ha producido la lesión renal
inducida por el cadmio, o incluso en ausencia de lesión renal si la
exposición es excesiva, aumenta la excreción urinaria. En las
personas expuestas al cadmio que padecen proteinuria la excreción de
cadmio suele ser mayor que en las que no padecen proteinuria. Cuando
cesa la exposición intensa, el nivel de cadmio en la orina desciende
aunque persista la lesión renal. En la interpretación de la
presencia de cadmio en la orina hay que tener en cuenta, pues,
varios factores. La excreción gastrointestinal es aproximadamente
igual a la urinaria pero no puede medirse fácilmente. Otras vías
excretoras como la leche, el sudor o la transferencia placentaria
son insignificantes.
El nivel de cadmio en las heces es un buen indicador de la
ingesta diaria reciente a partir de los alimentos en ausencia de
exposición por inhalación. En la sangre, el cadmio aparece
principalmente en los glóbulos rojos y las concentraciones en el
plasma son muy bajas. Existen al menos dos compartimentos en la
sangre, uno referido a la exposición reciente, con una semivida de
alrededor de 2-3 meses, y otro probablemente relacionado con la
carga corporal, con una semivida de varios años.
5. Efectos en mamíferos de laboratorio
Las exposiciones elevadas por inhalación provocan edema
pulmonar letal. La inyección de una sola dosis elevada produce
necrosis en el testículo y en el ovario no ovulante, lesiones
hepáticas y lesiones en los vasos de menor tamaño. La administración
oral de dosis elevadas produce lesiones en la mucosa gástrica e
intestinal.
La exposición por inhalación prolongada y la administración
intratraqueal producen modificaciones crónicas de tipo inflamatorio
en los pulmones, fibrosis y fenómenos indicativos de enfisema. La
administración parenteral u oral prolongada afecta principalmente al
riñón aunque también al hígado y a los sistemas hematopoyético,
inmunitario, esquelético y cardiovascular. En ciertas especies y en
determinadas condiciones se han inducido efectos esqueléticos e
hipertensión. La aparición de efectos teratogénicos y lesiones
placentarias depende de la fase gestacional en que se produzca la
exposición y puede entrañar interacción con el zinc.
En cuanto a la exposición humana, lo más notable son los
efectos agudos por inhalación en el pulmón y los efectos renales
crónicos. Tras la exposición prolongada, el riñón es el órgano
crítico. Los efectos en este órgano se caracterizan por disfunción
tubular y lesiones en las células tubulares, si bien pueden
producirse también disfunciones glomerulares. Una de las
consecuencias de la disfunción tubular renal es la alteración del
metabolismo del calcio y de la vitamina D. Según algunos estudios,
ello ha producido casos de osteomalacia y/o osteoporosis, pero esos
efectos no se han confirmado en otros estudios. No debe excluirse el
efecto directo del cadmio en la mineralización ósea.
Los efectos tóxicos del cadmio en animales de experimentación
están sometidos a la influencia de factores genéticos y
nutricionales, las interacciones con otros metales, en particular el
zinc, y el pretratamiento con cadmio, que puede guardar relación con
la inducción de la metalotioneína.
En 1976 y 1987, el Centro Internacional de Investigaciones
sobre el Cáncer consideró suficientes las pruebas de que el cloruro,
el sulfato, el sulfuro y el óxido de cadmio pueden producir sarcomas
en el lugar de inyección en la rata y, en el caso de los dos
primeros compuestos, inducir tumores en las células intersticiales
del testículo en la rata y el ratón, pero consideró que los estudios
de administración oral eran insuficientes para la evaluación. En
estudios de inhalación prolongada en ratas expuestas a aerosoles de
sulfato de cadmio, vapores de óxido de cadmio y polvos de sulfato de
cadmio se observó una elevada incidencia de cáncer primario del
pulmón con pruebas de proporcionalidad entre la dosis y la
respuesta. Hasta el momento, sin embargo, esa observación no se ha
confirmado en otras especies. Los estudios de los efectos
genotóxicos del cadmio han dado resultados discordantes.
6. Efectos en el ser humano
La exposición intensa por inhalación de vapores de óxido de
cadmio produce neumonitis aguda con edema pulmonar, que puede ser
letal. La ingestión de dosis elevadas de sales solubles de cadmio
produce gastroenteritis aguda.
La exposición ocupacional prolongada al cadmio ha producido
efectos crónicos graves, principalmente en el pulmón y el riñón.
También se han observado efectos renales crónicos en la población
general.
Los cambios pulmonares observados tras una intensa exposición
ocupacional se caracterizan principalmente por la aparición de
afecciones crónicas obstructivas de las vías aéreas. Los primeros
cambios leves en las pruebas de la función ventilatoria pueden
avanzar, si prosigue la exposición al cadmio, hasta insuficiencia
respiratoria. Se ha observado una mayor tasa de mortalidad por
enfermedad pulmonar obstructiva en trabajadores sometidos a
exposiciones intensas, al igual que en otras épocas.
La acumulación de cadmio en la corteza renal produce disfunción
tubulorrenal con trastornos de la reabsorción de proteínas, glucosa
y aminoácidos, entre otros. Un signo característico de la disfunción
tubular es la mayor excreción de proteínas de bajo peso molecular en
la orina. En algunos casos, disminuye la tasa de filtración
glomerular. El aumento de la concentración de cadmio en la orina
está correlacionado con la presencia de proteínas de bajo peso
molecular en la orina y, en ausencia de exposición aguda al cadmio,
puede servir como indicador de efectos renales. En los casos más
graves se combinan los efectos tubulares y glomerulares, con aumento
del nivel de creatinina en la sangre en algunos casos. Para la
mayoría de los trabajadores y de las personas expuestas al medio
ambiente general, la proteinuria inducida por el cadmio es
irreversible.
Entre otros efectos figuran los trastornos del metabolismo del
calcio, la hipercalciuria y la formación de cálculos renales. La
exposición intensa al cadmio, con toda probabilidad combinado con
otros factores como carencias nutricionales, puede llevar a la
aparición de osteoporosis y/o osteomalacia.
Hay pruebas de que la exposición profesional prolongada al
cadmio puede contribuir a la aparición de cáncer del pulmón aunque
las observaciones obtenidas en trabajadores expuestos han sido
difíciles de interpretar a causa de factores que inducen a
confusión. En el caso del cáncer de la próstata, las pruebas
obtenidas hasta la fecha no son concluyentes pero no apoyan la
existencia de una relación causal, indicada en estudios anteriores.
Actualmente no hay pruebas convincentes de que el cadmio sea
agente etiológico de la hipertensión esencial. La mayor parte de los
datos indican que no se debe al cadmio el aumento de la tensión y no
hay pruebas de que la mortalidad por enfermedades cardio-vasculares
o cerebrovasculares sea mayor.
Los datos obtenidos en estudios de grupos de trabajadores
expuestos y de grupos expuestos en el medio ambiente general
demuestran que existe una relación entre los niveles de exposición,
la duración de ésta y la prevalencia de los efectos renales.
Se ha comunicado una mayor prevalencia de la proteinuria de
bajo peso molecular en trabajadores del cadmio tras 10-20 años de
exposición a niveles del metal de aproximadamente 20-50 µg/m3. En
zonas contaminadas del medio general, en las que la ingesta de
cadmio estimada ha sido de 140-260 µg/día, se han observado efectos
en forma de aumento de la cantidad de proteínas de bajo peso
molecular en la orina en algunos individuos tras una exposición
prolongada. En la sección 8 se dan estimaciones más precisas de la
relación dosis-respuesta.
7. Evaluación de los riesgos para la salud humana
7.1 Conclusiones
Se considera que el riñón es el órgano diana crítico en la
población general así como en las poblaciones expuestas
profesionalmente. Las enfermedades crónicas obstructivas de las vías
respiratorias están asociadas a la exposición profesional prolongada
e intensa por inhalación. Hay pruebas de que esa exposición al
cadmio puede contribuir al desarrollo de cáncer del pulmón aunque
las observaciones en trabajadores expuestos han sido difíciles de
interpretar a causa de la presencia de factores que inducen a
confusión.
7.1.1 Población general
El cadmio presente en los alimentos es la principal fuente de
exposición para la mayoría de las personas. En la mayoría de las
zonas no contaminadas con cadmio las ingestas diarias medias con los
alimentos se encuentran entre 10 y 40 µg. En zonas contaminadas se
ha observado que alcanza varios cientos de µg al día. En zonas no
contaminadas, la absorción debida al consumo de tabaco puede igualar
la ingestión de cadmio a partir de los alimentos.
Basandose en un modelo biologico, se ha estimado que con una
ingesta diaria de 140-260 µg de cadmio durante toda la vida, o una
ingesta acumulativa de unos 2000 mg o más, se produce en el ser
humano una asociación entre la exposición al cadmio y una mayor
excreción de proteínas de bajo peso molecular en la orina.
7.1.2 Población expuesta profesionalmente
La exposición ocupacional al cadmio se produce principalmente
por inhalación aunque comprende ingestas suplementarias con los
alimentos y el tabaco. El nivel total de cadmio en el aire varía
según las prácticas de higiene industrial y el tipo de lugar de
trabajo. Existe una relación exposición-respuesta entre los niveles
de cadmio en el aire y la proteinuria. Puede aumentar la prevalencia
de la proteinuria de bajo peso molecular en trabajadores a los 10-20
años de exposición a niveles de cadmio de unos 20-50 µg/m3. La
medida in vivo del cadmio en el riñón y el hígado de personas con
distintos niveles de exposición al metal ha demostrado que alrededor
del 10% de los trabajadores con un nivel en la corteza renal de
200 mg/kg y aproximadamente el 50% de las personas con un nivel en
la corteza renal de 300 mg/kg tendrían proteinuria tubulorrenal.
