
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 138
2-NITROPROPANE
This report contains the collective views of an
international group of experts and does not necessarily
represent the decisions or the stated policy of the
United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr. R.B. Williams,
United States Environmental Protection Agency
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Organization
Geneva, 1992
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
2-Nitropropane.
(Environmental health criteria; 138)
1. Environmental exposure 2. Propane - analogs & derivatives
3. Propane - toxicity I. Series
ISBN 92 4 157138 1 (NLM Classification QV 633)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1992
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the impression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city, or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR 2-NITROPROPANE
1. SUMMARY
1.1. Properties and analytical methods
1.2. Uses and sources of exposure
1.2.1. Production
1.2.2. Uses and loss to the environment
1.3. Environmental transport and distribution
1.4. Environmental levels and human exposure
1.5. Kinetics and metabolism
1.6. Effects on laboratory mammals and in vitro
systems
1.7. Effects on humans
1.8. Effects on other organisms in the laboratory
and field
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND
ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factors
2.4. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels and processes
3.2.2. Uses
3.3. Release into the environment
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport in the environment
4.2. Biotic and abiotic transformation
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels and general population
exposure
5.2. Potential occupational exposure
6. KINETICS AND METABOLISM
6.1. Absorption
6.2. Distribution
6.3. Metabolic transformation
6.4. Elimination and excretion
6.5. Retention and turnover
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.2. Short-term and long-term repeated exposure
7.3. Reproduction, embryotoxicity, and teratogenicity
7.4. Mutagenicity and related end-points
7.4.1. Prokaryotes and yeast
7.4.2. Eukaryotes
7.5. Carcinogenicity
7.6. Pharmacological effects
8. EFFECTS ON HUMANS
8.1. General population exposure
8.2. Occupational exposure
8.2.1. Acute toxicity
8.2.2. Effects of long-term exposure
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON
THE ENVIRONMENT
10.1. Human health risks
10.2. Effects on the environment
11. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
12. FURTHER RESEARCH
12.1. Environment
12.2. Epidemiology
12.3. Toxicokinetics
12.4. Carcinogenesis
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RESUME
RESUMEN
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR 2-NITROPROPANE
Members
Dr D. Anderson, British Industrial Biological Research
Association, Carshalton, Surrey, United Kingdom
Dr U. Andrae, Institute for Toxicology, Research Centre for
Environment and Health, Neuherberg, Munich, Germany
(Vice-chairman)
Dr B. Baranski, Hofer Institute of Occupational Medicine,
Lodz, Poland
Dr S. Dobson, Institute of Terrestral Ecology, Monks Wood
Experimental Station, Abbots Ripton, Huntingdon, United
Kingdom
Dr E.S. Fiala, Naylor Dana Institute for Disease Prevention,
American Health Foundation, Valhalla, New York, USA
(Chairman)
Dr P. Lundberg, Department of Toxicology, National Institute
of Occupational Health, Solna, Sweden
Dr M.H. Noweir, Industrial Engineering Department, College of
Engineering, King Abdul Aziz University, Jeddah, Saudi
Arabia
Dr C.N. Ong, Department of Community, Occupational and
Family Medicine, National University of Singapore, Singapore
(Joint Rapporteur)
Dr R.B. Williams, Exploratory Research, US Environmental
Protection Agency, Washington DC, USA (Joint Rapporteur)
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P.G. Jenkins, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Mr J. Wilbourn, International Agency for Research on Cancer,
Lyon, France
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly delaying
their publication. In the interest of all users of the Environmental
Health Criteria documents, readers are kindly requested to
communicate any errors that may have occurred to the Director of the
International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland, in order that they may be
included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone No. 7988400 or
7985850).
ENVIRONMENTAL HEALTH CRITERIA FOR 2-NITROPROPANE
A WHO Task Group on Environmental Health Criteria for
2-Nitropropane met in Geneva from 4 to 8 November 1991. Dr B.H.
Chen, IPCS, welcomed the participants on behalf of the Director,
IPCS, and the three IPCS cooperating organizations (UNEP/ILO/WHO).
The Task Group reviewed and revised the draft criteria document and
made an evaluation of the risks for human health and the environment
from exposure to 2-nitro-propane.
The first draft of this monograph was prepared by Dr R.B.
Williams of the US Environmental Protection Agency. The second draft
was also prepared by Dr R.B. Williams incorporating comments
received following the circulation of the first draft to the IPCS
Contact Points for Environmental Health Criteria documents.
Dr B.H. Chen and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content
and technical editing, respectively.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
ABBREVIATIONS
DDT dichlorodiphenyltrichloroethane
DMSO dimethyl sulfoxide
SCE sister chromatid exchange
SGPT serum glutamic pyruvic transaminase
STEL short-term exposure limit
TWA time-weighted average
SUMMARY
1.1 Properties and analytical methods
2-Nitropropane (2-NP) is a colourless, oily liquid with a mild
odour. It is flammable, only moderately volatile, and stable under
ordinary conditions. It is only slightly soluble in water but
miscible with many organic liquids, and it is an excellent solvent
for many types of organic compounds. Adequate analytical methods
exist for the identification and measurement of 2-NP at
environmental concentrations. Current methods use gas chromatography
and a flame ionization or electron capture detector or,
alternatively, high-performance liquid chromatography with an
ultraviolet detector. For measurement in air, 2-NP must first be
trapped and concentrated in a solid sorbent.
1.2 Uses and sources of exposure
1.2.1 Production
Current world production figures are not available. In 1977
production in the USA was approximately 13 600 tonnes. 2-NP is
currently manufactured by two USA companies and one French company.
It is produced naturally in trace amounts in the combustion of
tobacco and other nitrate-rich organic matter, but there is no
evidence that it is produced by any biological processes.
1.2.2 Uses and loss to the environment
2-NP is used as a solvent, principally in blends, and has many
industrial applications as a solvent for printing inks, paints,
varnishes, adhesives and other coatings such as beverage container
linings. It has also been used as a solvent to separate closely
related substances such as fatty acids, as an intermediate in
chemical syntheses, and as a fuel additive. Losses to the
environment are mainly to the air and are due principally to solvent
evaporation from coated surfaces.
1.3 Environmental transport and distribution
2-NP appears to be highly mobile in the natural environment.
Since it is slightly water soluble, slightly adsorbed by sediment,
slightly bioaccumulated, and evaporates readily into the atmosphere,
it will be distributed in both air and water and not accumulated in
any individual environmental compartment. Ultraviolet
photoabsorption by 2-NP is within the range of wavelengths occurring
naturally in the environment, and it is thus likely that 2-NP
undergoes slow photolysis in the atmosphere. Slow biological
conversion of 2-NP to less toxic compounds also appears likely in
both aquatic and terrestrial environments.
1.4 Environmental levels and human exposure
General population exposure to 2-NP appears to be very low and
is derived from cigarette smoke (1.1 to 1.2 µg/cigarette), from
residues in coatings such as beverage can coatings, adhesives and
print, and from vegetable oils fractionated with 2-NP. Industrial
exposure worldwide is unknown, but in the USA appears to be limited
to 0.02-0.19% of the workforce. Significant exposure (exposure to
9.1 mg/m3 (2.5 ppm) or more) in the USA may be limited to about
4000 workers (approximately 0.005% of the workforce). Occupational
exposure limits in the air vary among different countries and range
from 3.6 mg/m3 (1 ppm) (TWA) to 146 mg/m3 (40 ppm) (STEL).
Manufacture of 2-NP is an enclosed process and usually involves
little employee exposure, but some workers in industries such as
painting, printing, and solvent extraction have in the past been
exposed to levels much greater than occupational exposure limits.
Concentrations as high as 6 g/m3 (1640 ppm) in air were recorded
in a drum-filling operation.
1.5 Kinetics and metabolism
Human uptake of 2-NP occurs mainly through the lungs. In
experimental animals, 2-NP has been shown to be rapidly absorbed not
only via the lungs but also from the peritoneal cavity and the
gastrointestinal tract. There is no satisfactory information on
absorption via the skin. Information on distribution in rats is
somewhat contradictory. 2-NP is rapidly metabolized, mainly to
acetone and nitrite. Some isopropyl alcohol may also be formed.
Following intraperitoneal injection, 2-NP and its carbon-containing
metabolites are concentrated initially in fat and subsequently in
bone marrow as well as in the adrenal glands and other internal
organs. Following inhalation, 2-NP and its carbon-containing
metabolites are concentrated in the liver and kidney, with
relatively little in fat. Several different enzyme systems may be
involved and there are species differences concerning rates and
pathways. 2-NP and its carbon-containing metabolites are rapidly
lost from the body by metabolic transformation, exhalation, and
excretion in the urine and faeces. Satisfactory information on the
distribution and excretion of nitro moiety metabolites is lacking.
1.6 Effects on laboratory mammals and in vitro systems
2-NP has moderate acute toxicity for mammals. Males are more
sensitive than females, at least among rats, and sensitivity differs
widely among the species that have been tested. The LC50
(concentration causing 50% mortality within 14 days) for rats
following a 6-h exposure was 1.5 g/m3 (400 ppm) for males and
2.6 g/m3 (720 ppm) for females. Lethality appeared to be
associated mainly with the narcotic effects, but mammals exposed to
concentrations of at least 8.4 g/m3 (2300 ppm) for one hour or
longer displayed severe pathological changes including
hepatocellular damage, pulmonary oedema, and haemorrhage.
There is clear evidence that 2-NP is carcinogenic in rats.
Long-term inhalation exposure of rats to 0.36 g/m3 (100 ppm) for
18 months (7 h/day, 5 days/week) induced destructive changes in the
liver, including hepatocellular carcinomas in some males. A
concentration of 0.75 g/m3 (207 ppm) induced more severe damage,
including a high incidence of hepatocellular carcinomas, more
quickly. Moderate-chronic oral dosage also induced excess
hepatocellular carcinomas in rats. However, long-term inhalation
exposure of rats to 91 or 98.3 mg/m3 (25 or 27 ppm) produced no
detectable injury. Exposure of mice and rabbits to concentrations of
2-NP that induced hepatocellular carcinomas in rats had little or no
effect, but these studies were too limited to completely rule out
2-NP carcinogenicity in these two species. 2-NP slightly retarded
fetal development of rats, but there is a paucity of data on
embryotoxicity, teratogenicity, and reproductive toxicity. 2-NP was
found to be strongly genotoxic in rat hepatocytes both in vitro
and in vivo, but no significant genotoxicity was observed in other
organs of the rat or in cell lines of extrahepatic origin without
exogenous metabolic activation. 2NP has been shown to be mutagenic
in bacteria both in the presence and absence of exogenous metabolic
activation.
1.7 Effects on humans
Human exposure to high concentrations of 2-NP is largely or
entirely occupationally related. High concentrations (actual values
are unknown but in one case they were estimated to be 2184 mg/m3
(600 ppm)) are acutely toxic and have produced industrial
fatalities. Initial symptoms included headache, nausea, drowsiness,
vomiting, diarrhoea, and pain. Victims often showed temporary
improvement, but in some cases death occurred 4 to 26 days after
exposure. Hepatic failure was the primary cause of death, and lung
oedema, gastrointestinal bleeding, and respiratory and kidney
failure were contributing factors. Occupational exposure to
estimated levels of 73 to 164 mg/m3 (20 to 45 ppm) induced nausea
and loss of appetite, which persisted for several hours after
leaving the workplace, whereas occupational exposure to estimated
levels of 36.4 to 109 mg/m3 (10 to 30 ppm) (< 4 h/day for
< 3 days/week) produced no noticeable ill effects.
Although available data are inadequate, there is no indication
that chronic occupational exposure to 2-NP at concentrations usually
encountered in the workplace induces hepatic or other neoplasms, or
other long-term adverse effects.
1.8 Effects on other organisms in the laboratory and field
The few studies performed on microorganisms, invertebrates, and
fish indicate low toxicity of 2-NP for non-mammalian organisms.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Chemical structure: NO2
|
H3C - C - CH3
|
H
Empirical formula: C3H7NO2
Synonyms: Dimethylnitromethane, isonitropropane,
nitroisopropane, 2-NP
Trade names: NiPar S-20 (solvent), NiPar S-30 (solvent, a
mixture of 1- and 2-nitro-propane)
CAS registry number: 79-46-9
RTECS number: TZ 5250000
Relative molecular
mass: 89.09
2.2 Physical and chemical properties
2-Nitropropane (2-NP) is an important synthetic organic
chemical. Its physical properties have been described by Angus
Chemical Co. (1985), Baker & Bollmeier, (1981), Woo et al. (1985),
and Weast (1986) and are summarized in Table 1. It is a colourless,
oily liquid with a mild odour and remains liquid over a relatively
broad temperature range, i.e. -93 to 120 °C. 2-NP is flammable, and
although only moderately volatile, its vapour forms flammable or
explosive mixtures with air. It is stable under ordinary
circumstances, but may undergo explosive decomposition under
conditions of extreme shock combined with heavy confinement and
elevated temperature. 2-NP is only slightly soluble in water (17
ml/litre at 20 °C) and water is even less soluble in 2-NP (5 ml/l at
20 °C). With increasing temperature, solubility of both 2-NP in
water and water in 2-NP increases, and an azeotrope containing 29.4%
water ultimately is formed. Its boiling point is 88.6 °C. 2-NP is,
however, miscible with many organic compounds including chloroform,
aromatic hydrocarbons, alcohols, esters, ketones, ethers, and higher
aliphatic carboxylic acids. Alkanes and cycloalkanes have more
limited solubility in 2-NP (Baker & Bollmeier, 1981). Azeotropes are
formed with some organic liquids.
Table 1. Physical properties of 2-nitropropane
Reference
Appearance colourless, oily Stokinger (1982)
liquid
Relative molecular mass 89.09 Stokinger (1982)
Weast (1986)
Windholz (1983)
Specific gravity 0.988 Baker & Bollmeier (1981)
(liquid density) Stokinger (1982)
(at 20 °/4 °C)
Vapour density (air = 1.00) 3.06 Stokinger (1982)
Vapour pressure (20 °C) 1.72 MPa Stokinger (1982)
(12.9 torr)
Boiling point 120.3 °C Baker & Bollmeier (1981)
Stokinger (1982)
Windholz (1983)
Melting point -93 °C Weast (1986)
Water solubility (20 °C) 17 ml/l Baker & Bollmeier (1981)
Stokinger (1982)
Windholz (1983)
Refractive index (20 °C) 1.3944 Baker & Bollmeier (1981)
Weast (1986)
Flash point (open cup) 38 °C Stokinger (1982)
Lower inflammability limit 2.6 volume % National Fire Protection
in air Association (1968)
Partition coefficients
water/air 128 Filser & Baumann (1988)
olive oil/air 710 Filser & Baumann (1988)
in vivo whole body 175 Filser & Baumann (1988)
(rat)/air
2-NP, like other nitroparaffins, undergoes a variety of chemical
reactions. The chemistry of nitroparaffins has been the subject of a
number of reviews and symposia and has been summarized by Baker &
Bollmeier (1981), Goldwhite (1965), Stokinger (1982), Woo et al.
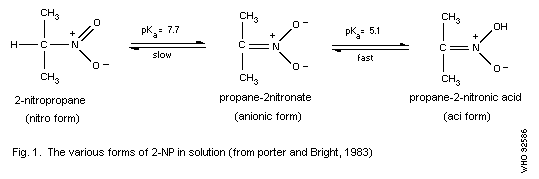
(1985), and others. 2-NP is an acidic substance. The nitro form,
which is mildly acidic, exists in equilibrium with its more strongly
acidic "aci" tautomer and with the anionic form (nitronate) of the
latter (Fig. 1). The aci tautomer is referred to as a nitronic acid
and forms metal salts. It can be dissolved and neutralized by strong
bases and gives a characteristic colour reaction with ferric
chloride. Prolonged action of bases leads to decomposition. Aqueous
acids hydrolyse 2-NP first to a hydroxamic acid and ultimately to
carboxylic acids and hydroxylammonium salts. 2-NP reacts with
nitrous acid to form a pseudonitrole which is colourless in
crystalline form but blue when melted or in solution. The carbon
atom bearing the nitro group is easily halogenated in the presence
of a base. Photochemical chlorination, however, yields reaction
products in which chlorine atoms are attached to the terminal
carbons. In the presence of a base, 2-NP condenses with carbonyl
compounds to yield a ß-nitroalcohol, which may dehydrate
spontaneously to a nitro-olefin. Nitro-olefins thus formed, and a
variety of other unsaturated compounds, undergo Michael addition
reactions with 2-NP in the presence of a catalytic amount of base.
2-NP will condense with formaldehyde and a secondary amine (the
Mannich reaction). Mild reduction of 2-NP yields
isopropylhydroxylamine, and strong reduction produces
isopropylamine. Auto-oxidation catalysed by cuprous chloride yields
2-hydroperoxy-2-nitropropane (Fieser & Fieser, 1972, 1974).
Taste and odour are subjective biological properties derived
from chemical and physical properties. The odour has been described
as "sweet-solventy, rubbery, and alcohol-like" (letter from S.E.
Ellis of Arthur D. Little, Inc. to G. Crawford of Occusafe Inc.,
1982). There also is some uncertainty concerning odour threshold.
Treon & Dutra (1952) stated that the odour of 2-NP was detectable at
1070 mg/m3 (294 ppm) but not at 302 mg/m3 (83 ppm), without
describing the methodology by which these values were determined;
their values have nevertheless been incorporated into various
guidelines (Crawford et al., 1984). Two recent studies redetermined
the odour threshold for 2-NP. In one the ED50 (minimum concentration
detected by 50% of the population) was estimated to be 18.2 mg/m3
(5.0 ppm) with 95% confidence limits from 11.3 mg/m3 (3.1 ppm) to
28.76 mg/m3 (7.9 ppm), and, in the other, all of a four-member
test panel detected 2-NP at 11.3 mg/m3 (Crawford et al., 1984).
There was no consensus as to the taste of a 6.4 g/litre (0.072
mol/litre) solution of 2-NP in water (Marcstrom, 1967). The most
frequent response was bitter, but other tasters found it (1) sweet,
(2) bitter and sour, (3) bitter, cool, and anaesthetizing, (4)
burning, (5) burning and cool, or (6) burning, sweet, bitter, and
sour. Wilks & Gilbert (1972a) reported a taste detection threshold
for 2-NP in water of 12.5 mg/litre.
 2.3 Conversion factors
1 ppm 2-NP in air = 3.64 mg/m3
1 mg/m3 = 0.27 ppm 2-NP in air
2.4 Analytical methods
Analytical methods for 2-NP appear limited to the analysis of
air, water, blood plasma, coatings, and cigarette smoke (Table 2).
Since the colorimetric methods are less sensitive or are cumbersome,
the method of choice is probably gas chromatography with either a
flame ionization or an election capture detection. Charcoal is not a
satisfactory adsorbent for 2-NP since recovery is poor (Andersson et
al., 1983) and there may be decomposition (Glaser & Woodfin, 1981).
In addition to Chromosorb 106, Amberlite XAD-7 appears satisfactory
as a solid sorbent for quantitatively collecting 2-NP from the air
(Andersson et al., 1983), although its collection efficiency is
markedly reduced in humid air (Andersson et al., 1984). Use of a
collection tube with two sections, however, can compensate for the
reduced efficiency (Andersson et al., 1984). The high-performance
liquid chromatography method developed for blood (Derks et al.,
1988) could probably be adapted to other biological materials.
Table 2. Analytical techniques for determining 2-nitropropanea
Methods Detection limit Comments Reference
Air
Trapping in ethanol; 0.1 mg/ml ethanol absorption is linear between 0.1 and Treon & Dutra (1952)
concentration determined 2.0 mg/ml; ethanol was especially
spectrophotometrically at purified and redistilled
277.5 nm
Trapping in concentrated ca. 1 µg/ml sulfuric absorption is linear between 1 and 5 µg/ml Jones & Riddick (1952);
sulfuric acid; resulting acid sulfuric acid; no interference from primary Jones (1963)
nitrous acid combined nitroparaffins, but all other secondary,
with resorcinol to form a some tertiary and some halogenated
red-blue colour; measured nitroparaffins interfere
spectro-photometrically
at 560 nm
Trapping in solid sorbent tube 3.6 mg/m3 working range is 3.6 to 36 mg/m3; 2-NP Glaser & Woodfin (1981)
(Chromosorb 106, 60/80 mesh); stable on absorbent for at least 28 days
desorption: ethyl acetate;
separation-detection: GC-FID
Methodology similar to above 3.1 mg/m3 Method is a modification of that proposed US NIOSH (1987a)
by Glaser & Woodfin (1981); range: 3.1 to
28.3 mg/m3; no interference from methyl
butyl ketone, heptane, 1-nitropropane,
toluene, and xylene
Table 2 (contd).
Methods Detection limit Comments Reference
Water
Sample (40 µl) collected ca. 0.5 mg/m3 water elutes quickly extinguishing flame Wilks & Gilbert (1972a)
with a syringe and injected in FID; flame can be reignited before 2-NP
directly into GC; emerges
detection: FID
Blood
Blood collected in chilled, 1 ng UV absorption linear from 0 to 250 ng; Derks et al. (1988)
screw-capped vial with heparin; uses 0.3 ml blood sample; samples are
centrifuged; deproteinized with unstable and must be analysed promptly
acetonitrile; Tris buffer added;
separation: HPLC; detection:
UV at 224 nm
Coating (beverage can)
Redissolve coating in solvent not given reference provides few details on Wilks & Gilbert (1972b)
suitably distinct from 2-NP; methodology
acetone suitable for vinyl
co-polymer coatings;
separation: GC
Table 2 (contd).
Methods Detection limit Comments Reference
Coating (beverage can)
Can (empty) simultaneously not given method captures about 90% of Wilks & Gilbert (1972b)
perforated and fitted with a residual 2-NP
diaphragm; hypodermic needle
inserted through diaphragm and
fitted with stopcock; can heated
(150 °C, 15 min); headspace
sampled with heated syringe;
separation: GC
Cigarette smoke
Steam distillation of smoke 0.8 µg/cigarette may be adapted for air and water Hoffmann & Rathkamp
condensate on filters, analysis (1968)
extraction re-extraction in
NaOH, in ethyl ether,
neutralization with H2SO4 and
re-extraction in ethyl ether,
concentration of extract and
injection in GC equipped with
FID or ECD detectors
a Abbreviations: ECD = electron capture detector; FID = flame ionization detector; GC = gas chromatograph;
HPLC = high-performance liquid chromatograph; UV = ultraviolet
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
There is no evidence that 2-NP and other nitroaliphatic
compounds are produced by biological processes, although a related
organic compound, ß-nitropropionic acid, has been isolated from
plants and microorganisms (Goldwhite, 1965). However, nitroaliphatic
compounds are produced in low concentrations by combustion of
organic matter and have been detected in tobacco smoke. Hoffmann &
Rathkamp (1968) reported 1.1-1.2 µg 2-NP in the smoke from single
85-mm USA blended non-filter cigarettes, and ascribed the production
of this and other nitroaliphatics to interactions in the combustion
zone between hydrocarbons and nitrogen dioxide generated by
decomposition of nitrates.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
Although 2-NP is an important industrial chemical, current world
production figures are not available. In 1977 production by the sole
USA manufacturer was estimated to be 13 600 tonnes, of which 5400
tonnes were sold in the USA and 8200 tonnes were either exported or
used internally (Finklea, 1977). 2-NP currently is produced by two
USA manufacturers, Angus Chemical Co., Sterlington, Louisiana, and
W. R. Grace Co., Deer Park, Texas (SRI International, 1988, 1990;
USITC, 1990), and by one European manufacturer, Société Chimique de
la Grande Paroisse, France (Anon., 1976, 1982; IARC, 1982). In the
USA, 2-NP, together with nitromethane, nitroethane and
1-nitropropane, is manufactured by a vapour phase reaction of nitric
acid with an excess of propane at high temperature and pressure
(370-450 °C, 0.8-1.2 MPa (8-12 atm.)) (Baker & Bollmeier, 1981). The
proportions of the four nitroaliphatic compounds in the reaction
product are a function of the reaction temperature. In Europe,
propane is reacted with nitrogen peroxide (N2O4) and an excess
of oxygen at 150-330 °C and 0.9-1.2 MPa (9-12 atm.), yielding the
same nitroaliphatic compounds in slightly different proportions as
produced by the USA process (Anon., 1976). Reaction products are
condensed, washed, and separated by fractional distillation. There
is no evidence that 2-NP is produced through human EHC 138:
2-Nitropropane activities except by combustion and deliberate
manufacture, although nitromethane has been detected in vehicle
exhaust (Seizinger & Dimitrades, 1972).
3.2.2 Uses
The importance of 2-NP as an industrial chemical stems mainly
from its desirable and occasionally unique characteristics as a
solvent (Purcell, 1967; Anon., 1976; Fishbein, 1981; Baker &
Bollmeier, 1981; IARC, 1982; ACGIH, 1986). It is an excellent
solvent or cosolvent for a variety of fats, waxes, gums, resins,
dyes and other organic compounds, including vinyl, acrylic,
polyamide and epoxy resins, chlorinated rubbers, and organic
cellulose esters. The ability of 2-NP to form an azeotrope with
water and the associated large heat of absorption permit it to
displace monomolecular layers of water molecules and secure a better
bond between pigments and the surfaces to which they are applied.
Its major use is as a solvent for inks, paints, varnishes, adhesives
and other coatings such as beverage container linings. It is used
principally in blends with other solvents to impart desirable
characteristics, such as greater solvency, better flow
characteristics and film integrity, greater pigment dispersion,
increased wetting ability, improved electrostatic spraying
properties, or reduced drying time. 2-NP is also used industrially
as a processing solvent for separating closely related substances in
natural products or reaction mixtures. These have included, for
example, separation of oleic acid from polyunsaturated fatty acids
and cetyl from oleyl alcohols.
In addition to the above, 2-NP has a number of minor uses
(Anon., 1976; Baker & Bollmeier, 1981). These include a medium for
chemical reactions, an intermediate for the manufacture of
2-nitro-2-methyl-1-propanol, 2,2-dinitropropane, 2-amino-2-
methyl-1-propanol and other propane derivatives, and a component of
explosives, propellants, and fuels for internal combustion engines.
The latter usage appears limited to model engines used by hobbyists
and to racing cars. Although the addition of 2-NP to fuel improves
diesel engine performance, it is not used commercially as a diesel
fuel additive since superior alternatives are available (Banes,
1989)a. In the USA, mixed isomers of nitropropane are used to
denature ethanol (US FDA, 1987). The addition of 2-NP to hydrocarbon
mixtures has been shown to inhibit corrosion of tin-plated steel
aerosol cans (Flanner, 1972).
3.3 Release into the environment
There is some quantitative data on releases of 2-NP into the
environment. The US Environmental Protection Agency has supported a
thorough, though largely speculative, analysis of the problem (US
EPA, 1980). Releases of 2-NP occur mainly into the atmosphere and
can result from spillage, from venting of gases and fugitive
emissions during manufacture, transfer and use, and from solvent
evaporation from coated surfaces. The US EPA document estimated that
of the 14 000 tonnes of 2-NP produced in the USA in 1979, 5714
tonnes (41%) was released into the air, and 1 tonne into water. Only
230 tonnes (1.6%) was destroyed by incineration or waste treatment.
The major contributor to this release estimate was evaporation of
2-NP used as a solvent in printing ink and surface coatings (4450
a Personal communication from the US Environmental Protection
Agency, Ann Arbor, Minneapolis
tonnes, 78% of releases). Manufacture of 2-NP is a largely enclosed
process and in 1979 it accounted for only 21 tonnes (0.3%) of the
amount released into the environment. A more recent examination of
this problem (National Library of Medicine, 1989) reported a similar
situation concerning environmental releases of 2-NP in the USA. Out
of a yearly total of 299 tonnes, 205 tonnes (69%) was released into
the air with 123 tonnes coming from point (large, easily identified)
sources and 82 tonnes from non-point (small, not easily identified)
sources. Only 2 tonnes (< 1%) was released directly into water, and
1 tonne into municipal sewage treatment plants. The remainder was
buried in closed containers (76 tonnes, 25%) or was disposed of in
unspecified ways (15 tonnes, 5%).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport in the environment
2-NP appears to be highly mobile in the natural environment.
Cupitt (1980) considered physical removal of 2-NP from the
atmosphere unlikely because it was not soluble enough to be rapidly
washed out and had a vapour pressure too great for strong adsorption
on particles. The partition of 2-NP between air and water at
equilibrium was estimated by the method of Swann et al. (1983) to be
about 0.5% in air and 99.5% in water (US EPA, 1985). These values
indicate rapid and easy exchange between air and water. The soil
sorption coefficient (ratio of soil concentration to water
concentration) and the bioconcentration factor were estimated by the
methods of Kenaga (1980) to be 20 and 2.5, respectively (US EPA,
1985). Measured values for absorption and bioaccumulation were
somewhat greater than these estimates. Freitag et al. (1982, 1985)
obtained concentration factors over water for activated sludge,
unicellular algae (Chlorella fusca), and fish (golden ide) of 70,
20, and < 10, respectively. These values indicate that 2-NP is not
strongly bioaccumulated and is readily desorbed from sediment
particles and leached from soil. Thus, in summary, since 2-NP is
slightly water soluble, slightly adsorbed by sediment, slightly
bioaccumulated, and evaporates readily into the atmosphere, it will
be distributed in both air and water and not accumulated in any
individual environmental compartment.
4.2 Biotic and abiotic transformation
Data concerning the destruction of 2-NP by biotic and abiotic
processes are limited. 2-NP has significant photoabsorption in the
environmentally relevant range of > 290 nm (Sadtler, 1961) and is
likely to undergo photolysis (Cupitt, 1980; US EPA, 1985). On the
basis of physical and chemical properties Cupitt (1980) hypothesized
that it would be rapidly removed from the atmosphere by photolysis
and estimated a reduction in concentration of 1/e (0.369) in 0.2
days. This is equivalent to a half-life of 0.14 days (3.36 h).
However, measurements of photochemical reactivity do not support
such a rapid destruction of 2-NP. Studies aimed at defining the
relationship between organic solvents and photochemical smog
production ranked 2-NP as low to moderate in terms of its
interactions with oxidants and its ability to produce formaldehyde
and other lachrymators (Levy, 1973). Laboratory measurements also
suggested slow decomposition (Freitag et al., 1985). The methodology
employed (Korte et al., 1978; Lotz et al., 1979; Freitag et al.,
1982), i.e. irradiation of the solvent adsorbed on silica gel by
light from a high pressure mercury lamp filtered through pyrex, was
too different from natural conditions to permit quantitative
extrapolation from laboratory results to rates of photolysis in the
atmosphere. The study provided comparative photodecomposition rates
for a large number of solvents. The rate of photodecomposition for
2-NP was roughly half that of dichlorodiphenyltrichloroethane (DDT),
similar to the rates for dodecane and 2,4-dichlorobenzoic acid, and
roughly twice those for kepone and dieldrin. Paszyc (1971) reported
that the major decomposition products for both gaseous and liquid
2-NP under laboratory conditions were nitrogen dioxide, acetone,
isopropyl nitrite, isopropanol, methylcyanide, water, and propane,
regardless of whether irradiation was monochromatic 253.7 nm light
or the full spectrum of light produced by a high pressure mercury
lamp. Cupitt (1980) speculated that the major products of
photodecomposition in nature would be formaldehyde and acetaldehyde.
Biological decomposition of 2-NP appears likely, but is probably
rather slow in nature. Enzymes capable of oxidizing or initiating
non-enzymatic oxidation of 2-NP have been identified in horseradish
(De Rycker & Halliwell, 1978; Porter & Bright, 1983; Indig &
Cilento, 1987), pea seedlings (Little, 1957), and a variety of
microorganisms including bacteria, yeasts, and fungi (Little, 1951;
Kido et al., 1975; Soda et al., 1977; Dhawale & Hornemann, 1979;
Patel et al., 1982). In in vitro preparations of horseradish
(Dhawale & Hornemann, 1979), pea seedlings (Little, 1957), a fungus
(Streptomyces achromogenes) (Dhawale & Hornemann, 1979), and a
yeast (Hansenula mrakii) (Kido et al., 1975), 2-NP was converted
to a less toxic compound, acetone, and a moderately toxic compound,
nitrite. In addition to nitrite, some nitrate may also be formed
(Indig & Cilento, 1987). In the yeast, nitrite was subsequently
reduced to ammonia. The importance of these processes in nature is
unknown. Kido et al. (1975), however, reported that only 4 out of 14
species of microorganisms tested would grow in a medium containing
5 g 2-NP/litre. The only study of 2-NP decomposition by a population
of microorganisms (Freitag et al., 1985) utilized activated sludge
grown at 25 °C. Solvent concentrations in these experiments were low
(50 µg/litre) to prevent adaptation to the substances tested (Korte
et al., 1978). In 5 days only 0.4% of the 2-NP was converted to
carbon dioxide. In summary, these data suggest that in both
terrestrial and aquatic communities 2-NP is biologically decomposed
and that the rate may be slow, but they offer no definite
information on the problem.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels and general population exposure
General population exposure to 2-NP appears to be very low.
There seem to be no records of its occurrence in water or in outdoor
air away from areas of manufacture and use. The only information on
intake exists in the form of a memorandum from Modderman (1983)a.
The daily intake per person in the USA was estimated to be 50 to
100 mg. The residuum from its use as a solvent for beverage can
coatings, film laminating adhesives and printing inks for flexible
food packaging may account for as much as 37 ng/day and from
vegetable oils fractionated with 2-NP, 30 ng/day. 2-NP residues of
0.077 mg/litre (77 ppb) to 0.24 mg/litre (204 ppb) have been found
in such oils. In a further report on the evaluation of
2-nitropropane as a food processing solvent, it was assumed that
residues of less than 10 µg/kg would occur in oils, giving rise to
estimated daily intakes of 10 ng/day. The use of 2-NP as a food
processing solvent was not recommended (FAO/WHO, 1990a,b). As
mentioned in section 3.1, smokers are exposed regularly to low
concentrations of 2-NP. Hoffmann & Rathkamp (1968) reported 1.1 to
1.2 µg in the smoke of a single cigarette. 2-NP was reported to
occur in the expired air of 11.1% (5 of 54 individuals) of a sample
of healthy adult urban dwellers (Krotoszynski et al., 1979). The
geometric mean was 0.406 ng/litre with one-sigma limits of
0.119 ng/litre and 1.38 ng/litre, (at 25 °C, 98 MPa (760 mmHg)). The
sample was entirely of non-smokers who had avoided medication and
prolonged exposure to perfume, paint, glue, aerosols, dust, tobacco
smoke and areas polluted with industrial wastes during, and for at
least 7 days prior to, the sampling period, and also avoided
cosmetics, spices, seasonings, and alcoholic beverages during and
immediately prior to sampling. The origin of the exhaled 2-NP is
unclear. Exposure to 2-NP may be further reduced in the future since
a number of regulations have been enacted and recommendations made
to declare it a harmful, carcinogenic substance and a toxic waste,
and to discourage its use (IARC, 1982; IRPTC, 1986; US FDA, 1987; US
NIOSH, 1988).
a Memorandum from Modderman, J.P. to Shibko, S., Associate
Director for Regulatory Evaluation, Department of Health & Human
Services, USA, 8 pp. "Exposure estimates for chemicals to be
included in the NTP annual report on carcinogens".
5.2 Potential occupational exposure
The number of workers in the USA who handle 2-NP or mixtures
containing 2-NP has been variously estimated as 15 000 (US EPA,
1977), 38 600 (Occupational Health Services, Inc., 1982), 100 000
(Finklea, 1977), and 185 000 (Beall et al., 1980). Based on
employment values of the US Bureau of the Census (1987) these
estimates of exposed workers represent from 0.02% to 0.19% of the
civilian workforce in the USA. The low estimate of 15 000, although
quoted in a US Environmental Protection Agency report, originated
with a manufacturer of 2-NP. The estimate generated by Occupational
Health Services, Inc., carried out under contract with a
manufacturer of 2-NP, was based on a detailed survey of
distributors, manufacturers, and users, and thus may represent a
reasonable approximation. This report considered 38 600 to be the
best estimate of the total number of exposed workers in the USA and
set 126 600 workers as an upper boundary. It further estimated that
significant exposure (defined as exposure to at least 10% of the US
OSHA exposure limit or 9.1 mg/m3 (2.5 ppm)) ranged from 4000 (best
estimate) to 10 600 (upper boundary) workers. Sources of worker
exposure identified in a survey conducted in the USA by the National
Institute for Occupational Safety and Health (Finklea, 1977)
included rotogravure and flexographic inks used in printing, and
coatings and adhesives used in industrial construction and
maintenance, highway marking, ship building and maintenance,
furniture manufacture, and food packaging. A US NIOSH survey
estimated that 9815 workers in the USA were exposed to 2-NP or to
trade-name products containing 2-NP (US NIOSH, 1983).
Occupational exposure limits are summarized in Table 3.
There is little data on actual occupational exposure, although
the limited information on conditions in the USA summarized in
Table 4 suggests that it is highly variable. Manufacture of 2-NP, an
enclosed process, appears to involve little employee exposure much
of the time, but spills and operations such as filling drums can
briefly expose a few workers to high concentrations. In general, low
exposures may be typical in some painting operations and in the
manufacture of tyres, but other painting and manufacturing
operations appear, at least in the past, to have exposed workers to
dangerously high concentrations. Workers were exposed to
concentrations of 2-NP up to at least 2744 mg/m3 (754 pm) in a
pigment production facility and up to at least 265 mg/m3 (73 ppm)
in a solvent extraction plant. Evidence exists that concentrations
of 2-NP at the solvent extraction plant prior to the investigation
were at times substantially greater than the values measured during
the investigation (Crawford et al., 1985). Exposure levels mentioned
in the report by Occupational Health Services, Inc. (1982) were
mainly in the vicinity of 3.6 mg/m3 (1 ppm), although one printing
Table 3. Occupational exposure limits for 2-nitropropane in aira
Exposure limit
Country mg/m3 ppm Category of limit
Australia 36 10 TWA
Belgium 36 10 TWA
Brazil 70 20 AL
Canada 90 25 CLV
Denmark 36 10 TWA
Finland 18 5 TWA
150 40 STEL
Germany 18 5 1-year TWA(TRK)
Hungary 10 3 CLV
Netherlands 3.6 1 8-h TWA
7.3 2 STEL
Poland 30 8 TWA
70 20 STEL
Romania 47 13 TWA
70 20 STEL
Sweden 18 5 TWA
36 10 CLV
Switzerland 18 5 TWA
United Kingdom 90 25 8-h TWA
USA 90 25 8-h TWA (OSHA)
36 10 8-h TWA (ACGIH)
Yugoslavia 90 25 TWA
a From: IRPTC (1986)
ACGIH = American Conference of Governmental Industrial Hygienists
AL = Acceptable or tolerable limit
CLV = Ceiling value
MAK = Maximum worksite concentration
OSHA = Occupational Safety and Health Administration
STEL = Short-term exposure limit
TLV = Threshold limit value
TWA = Time-weighted average (MAK in Switzerland)
TRK = Technical guiding concentration
plant (Table 4) reported a peak concentration of 237 mg/m3
(65 ppm) which lasted 30 min, and a time-weighted average of
36-44 mg/m3 (10-12 ppm). In addition to inhalation, it is likely
that workers using 2-NP as a solvent will have at least occasional
contact with the liquid. 2-NP also has been reported to be a minor
component of both fresh and used machine cutting fluid emulsion
(Yasuhara et al., 1986). The importance of exposure to 2-NP as a
contaminant of 1-nitropropane is unknown. In an investigation
involving 1-nitropropane-sensitized ammonium nitrate blasting agents
(Cocalis, 1982), 2-NP was below the detectable limit. Occupational
exposure may be reduced in the future since strong recommendations
have been issued on minimizing all worker contact with 2-NP and its
fumes and on substituting other less toxic solvents where possible
due to the carcinogenicity of 2-NP (IRPTC, 1986; US EPA, 1986; US
NIOSH 1988).
Table 4. Air concentration of 2-NP in the work place
Concentration
Activity mg/m3 ppm Reference
Manufacture of 2-NP 3.64 approx 1 Brown & Dobbin (1977)
Manufacture of 2-NP 0.7-364; 0.2-100; Miller & Temple (1979)
98% of 98% of
samples samples
were < 36.4 were < 10
Vulcanizing tyres 0-0.18 < 0.05 Hollett & Schloemer
(1978)
Painting (bus 0.11 < 0.03a Love & Kern (1981)
maintenance)
Painting (railway 1.46 < 0.4 Hartle (1980)
cars)
Solvent extraction 167.4 0-100 Crawford et al. (1985)
(average
46)
Laboratory (1958) 14.6 0-21 Angus Chemical Co. &
(average 4) Occusafe, Inc. (1986)
2-NP storage & 2111-6000 580-1640 Angus Chemical Co. &
transfer area (1962); Occusafe, Inc. (1986)
drum filling
operation
Pigment production 109-2745 30-754 Angus Chemical Co. &
facility (1970) Occusafe, Inc. (1986)
Painting (battery 36.4-109 10-30 Skinner (1947)
cases)
Manufacturing 72.8-164 20-45 Skinner (1947)
(coating forms)
Printing approx 40 1-65 Occupational Health
(approx 11) Services, Inc. (1982)
a below limit of detection
6. KINETICS AND METABOLISM
6.1 Absorption
2-NP is absorbed via the lungs, the peritoneal cavity, the
gastrointestinal tract, and possibly, to a lesser extent, via the
skin. Absorption via the lungs, peritoneal cavity, and
gastrointestinal tract have been used in experimental studies and
have been investigated using rats and 14C-labelled 2-NP. Pulmonary
absorption was examined by Nolan et al. (1982), Müller et al.
(1983), and Filser & Baumann (1988). Nolan et al. (1982) considered,
on the basis of respiratory rate and tidal volume of the rat and the
accumulation of 2-NP during the 6-h period of exposure, that a
minimum of 40% of the inhaled 2-NP was absorbed. This value is
minimal since it does not include 2-NP metabolized and eliminated
during exposure. The data of Müller et al. (1983) suggested that
immediately following exposure to 728 mg/m3 (200 ppm) for 3 h,
plasma contained approximately 0.4% as 2-NP and 7.2% as metabolites
of the 2-NP inhaled. The metabolites were mainly acetone but also
included a small amount of isopropanol. These percentages were
estimated from the results of Müller et al. (1983) and normative
data for the laboratory rat (Baker et al., 1979), and support rapid
pulmonary uptake of 2-NP since 2-NP and its metabolites sequestered
elsewhere in the body and loss of metabolized 2-NP during exposure
were not considered. Filser & Baumann (1988) reported that uptake of
gaseous 2-NP was rapid, the clearance rate being equal to the
ventilation rate. The value they cite for the latter, 32
litres.h-1.kg-1), seems large in comparison with normative data
for the laboratory rat (Baker et al., 1979).
The rate of 2-NP uptake by rats from intraperitoneal injection
was examined by Müller et al. (1983). Ten minutes after an injection
of 25 mg/kg, the blood plasma contained 3.3% of the dose as 2-NP and
1.9% as metabolites, acetone, and isopropanol, indicating an uptake
of greater than 5.2% since presumably some of the dose was already
lost from the body and to other tissues in the body during this
initial period. These percentages were estimated from the data of
Müller et al. (1983) and normative data for the laboratory rat
(Baker et al., 1979). A dose of 50 mg/kg yielded partially
dissimilar results. The 10-min average value was low and also had a
very large standard deviation. This may have reflected large
differences among the rats in their initial uptake rates for 2-NP or
an experimental problem such as injection into the gastrointestinal
tract rather than the peritoneal cavity. Blood plasma contained, 10
min after injection, only 1.4% of the dose as 2-NP and 1.3% as
acetone and isopropanol. These data indicate that uptake from the
peritoneal cavity is fairly rapid, and suggest that the relatively
slower uptake of the 50-mg/kg dose may have reflected saturation of
the uptake mechanisms, since initial concentrations in the plasma
did not exceed those following the 25-mg/kg dose. Intraperitoneal
injections were used by Andrae et al. (1988), Guo et
al. (1990), Hussain et al. (1990), and Conaway et al. (1991) to
demonstrate that 2-NP induced nucleic acid damage in the livers of
Wistar, F-344 and Sprague-Dawley rats.
Absorption of 2-NP via the gastrointestinal tract was
investigated with male Wistar rats by Derks et al. (1989). They
found that the systemic availability of orally administered 2-NP
from a water solution was very high (90%) and absorption was rapid,
maximum plasma values being reached within 15 min after dosage.
Absorption of 2-NP given in olive oil was much slower and
availability was only 34% during the initial 3 h following dosage.
The authors suggested that absorption from oil was incomplete at 3 h
and might ultimately be much higher, since the olive oil was
absorbed and the 2-NP redistributed between the oil and aqueous
phases.
Although workers are cautioned against dermal contact with 2-NP
(Beall et al., 1980; US EPA, 1986), there appear to be no
quantitative data on dermal absorption. The solubility of 2-NP in
both polar and non-polar solvents, together with its small molecular
size, suggests that it should be absorbed readily through the skin
(Malkinson & Gehlmann, 1977). Dermal application of 2 g 2-NP/kg to
rabbits produced no obvious symptoms (Wilbur & Parekh, 1982);
however, as noted below, the rabbit is relatively resistant to the
toxicity of 2-NP.
6.2 Distribution
The distribution of 2-NP and its carbon-containing metabolites
among the organs and tissues in Sprague-Dawley rats was examined by
Nolan et al. (1982) via inhalation, and by Müller et al. (1983) via
intraperitoneal injection. Both utilized 14C-labelled 2-NP and
thus their data do not reveal the distribution of nitrite and other
nitrogen-containing metabolites generated from the nitro portion of
the 2-NP molecule. One hour after intraperitoneal injection,
radioactivity was concentrated in fat; there were intermediate
amounts in the blood, liver, and kidney, and lower amounts in other
organs and tissues (Müller et al., 1983). By 40 h, the highest
concentrations were in bone marrow and adrenal tissue, intermediate
amounts being found in the kidney, liver, spleen, lungs, and omental
fat, and by 8 days only the concentration of 14C in adrenal tissue
was noticeably greater than elsewhere in the body. However, Nolan et
al. (1982), found, both immediately and 48 h after a 6-h period of
2-NP inhalation, that highest concentrations of carbon from 2-NP
were in the liver and kidney and relatively little in the fat.
Differences in methodology limit intercomparison of these
studies. Their major consistency is the presence of high
concentrations of 2-NP and its labelled carbon in the liver and
kidney, organs (as discussed below) actively involved in the
metabolism of 2-NP and excretion of its metabolites.
The relevance of these studies on tissue distribution of 2-NP
and its carbon-containing metabolites to the toxicity of 2-NP is
unclear, since (as discussed below) most of the dose is rapidly
metabolized initially to acetone and nitrite. The 14C label used
in these studies thus traced mainly the distribution of acetone and
its metabolites in measurements made more than a few hours after
dosing.
Dequidt et al. (1972) provided limited data on the distribution
of nitrite among body organs of the rat following inhalation and
intraperitoneal injection of 2-NP. The data suggest a fairly uniform
distribution among the heart, lungs, kidney, spleen, and,
sometimes, the liver. In the majority of experiments, however, no
nitrite was detected in the liver. No explanation is offered for the
latter observation, and data in the paper are so erratic as to
suggest the possibility of analytical problems.
6.3 Metabolic transformation
Starting with a report by Scott (1943), there have been numerous
studies on the metabolic transformation of 2-NP by mammals,
mammalian cells, microorganisms, and isolated enzymes. These studies
have shown that the major pathway for metabolic transformation of
2-NP involves oxidation to nitrite and acetone. Evidence for the
formation both of nitrite and acetone was reported from studies on
liver microsomes from rats pretreated with phenobarbital or
3-methylcholanthrene (Ullrich et al., 1978), cultured hepatocytes
from untreated rats (Haas-Jobelius et al., 1991), liver microsomes
from untreated mice (Marker & Kulkarni 1986a,b; Dayal et al., 1991),
V79 Chinese hamster cells (Haas-Jobelius et al., 1991), and a yeast
(Kido et al., 1975). Nitrite was reported to be a major metabolite
of 2-NP in rabbits (Scott, 1943), rats (Dequidt et al., 1972), and
liver microsomes from rats (Sakurai et al., 1980) and mice (Marker &
Kulkarni, 1985). Acetone was identified as a major metabolite of
2-NP in rats and chimpanzees (Muller et al., 1983).
Enzymatic oxidation of the nitronate form of 2-NP to nitrite and
acetone by horseradish peroxidase (Porter & Bright, 1983), a
dioxygenase from the yeast Hansenula mrakii (Kido et al., 1984),
and mouse liver microsomes (Dayal et al., 1991) was several times
more rapid than that of 2-NP under identical conditions. In addition
to acetone, a smaller amount of isopropanol is produced at least in
rats and chimpanzees (Muller et al., 1983). The source of the
isopropanol was not specified in this study, but since reduction of
acetone in the body is negligible (De Bruin, 1976), isopropanol may
be formed directly by oxidation of 2-NP. The formation of a
hydroxyisopropyl radical during the oxidation of 2-NP was suggested
by Kuo & Fridovich (1986).
The metabolic fates of these metabolites of 2-NP are well known.
Acetone is produced by a minor metabolic pathway in the mammalian
body (Smith et al., 1983) and has been detected in small amounts in
the blood, urine, and expired air of normal humans (Mabuchi, 1979;
Conkle et al., 1975). It may be excreted directly via expired air,
urine, and loss through the skin, or may enter into the general
metabolism either via cleavage to a 2-carbon acetyl fragment and a
1-carbon formyl fragment or via oxidation to pyruvic acid (De Bruin,
1976). The proportion excreted unchanged increases with increasing
dosage, suggesting an easily saturable metabolic pathway.
Isopropanol is oxidized to acetone (De Bruin, 1976).
Nitrite may exist as a minor constituent of the mammalian body.
It is constantly replenished by ingestion and synthesis, and
constantly removed by oxidation to nitrate. Nitrite and nitrate in
the blood stream are rapidly and homogeneously distributed
throughout the body (Parks et al., 1981). Nitrite rapidly oxidizes
divalent ferrous haemoglobin to trivalent ferric methaemoglobin
(Burrows, 1979). Little is transported to the tissues or excreted,
at least in dogs, sheep, and ponies (Schneider & Yeary, 1975).
Dequidt et al. (1972), however, reported substantial urinary
excretion of nitrite by rats following inhalation or intravenous
injection of 2-NP. Methaemoglobin is incapable of transporting
oxygen and, during enzymatic repair of this defect, nitrite is
reoxidized to nitrate. Parks et al. (1981) reported that 10 min
after intratracheal instillation of labelled nitrite into mice, 70%
of the label in plasma was in nitrate, 3% in nonionic compounds, and
only 27% remained as nitrite. Similar results were obtained with
rabbits. Nitrate is slowly excreted through the kidneys (Schneider &
Yeary, 1975) and also into saliva where it is reduced back to
nitrite by bacteria and reabsorbed into the body via the
gastrointestinal tract (Friedman et al., 1972). Small amounts of
nitrite in the stomach may react with secondary amines and other
amino substrates to form N-nitroso compounds which might be absorbed
(Sander & Schweinsberg, 1972; Fine et al., 1982).
The enzymatic system oxidizing 2-NP to acetone and nitrite was
identified through in vitro experiments using microsomes isolated
from mammalian liver. Ullrich & Schnabel (1973) determined that
cytochrome P-450, in liver microsomes from phenobarbital-pretreated
rats, bound 2-NP. Ullrich et al. (1978) subsequently reported that
liver microsomes from rats pretreated with phenobarbital or
3-methylcholanthrene rapidly catalysed the oxidation of 2-NP to
acetone and nitrite. The latter were produced in roughly equal
quantities. Surprisingly, however, the rate of this reaction was not
diminished under conditions of reduced oxygen pressure. The activity
of preparations from untreated control rats was generally very low.
Sakurai et al. (1980) demonstrated that this enzyme system in rats
was active in metabolizing other aliphatic nitro compounds. Marker &
Kulkarni (1985, 1986a, 1986b), working with mice, obtained somewhat
different results. They reported rapid denitrification of
2-NP to nitrite and acetone by liver microsomes from untreated mice,
and an acetone production at least twice the nitrite release. These
authors suggested that multiple forms of cytochrome P-450 are
involved, and claimed that nitrite is sequestered in the reaction
mixture and that denitrification of 2-NP may involve a reductive or
at least non-oxidative pathway as well as an oxidative pathway. They
also noted large differences in the rates of hepatic microsomal
enzymatic nitrite release among the five strains of mice tested.
Jonsson et al. (1977) demonstrated that hepatic microsomes from
uninduced rabbits could denitrify a compound related to 2-NP,
2-nitro-1-phenylpropane.
In addition to oxidative denitrification, a reductive pathway
has been shown to occur in cultured hepatocytes from Wistar rats and
in V79 Chinese hamster cells. Nitroreduction was indicated by the
fact that the cells formed acetone oxime, the tautomeric form of
nitrosopropane (Haas-Jobelius et al., 1991).
Evidence for the involvement of more than one pathway for the
metabolism of 2-NP in the rat was also obtained by Denk et al.
(1989). Their experiments on the pharmacokinetics of 2-NP in rats
exposed by inhalation suggested that there are two different
pathways both in male and female animals, a saturable one of low
capacity and high affinity according to Michaelis-Menten kinetics
and a non-saturable one following first-order kinetics. First-order
kinetics was similar in the two sexes, but striking differences
between sexes were observed in the kinetics of the saturable
pathway. The authors showed that in females more 2-NP was
metabolized by the non-saturable pathway at concentrations above 655
mg/m3 (180 ppm), and in males at concentrations above 218 mg/m3
(60 ppm), and linked their observations to the reported higher
susceptibility to liver damage of males as compared to females
(Griffin & Coulston, 1983). Denk et al. (1989) suggested that it is
the first-order metabolic process which results in the formation of
toxic products whereas the saturable pathway was suggested to lead
to less toxic metabolites.
These observations on the hepatic metabolism of 2-NP and related
compounds by rats, mice, and rabbits indicate differences among
species and even strains. It is probable that more than one enzyme
system is involved. In mice as well as rats hepatic cytochrome P-450
may be important in the metabolism of this xenobiotic.
Observations by Ivanetich et al. (1978) suggested an additional
detoxifying role for hepatic microsomal cytochrome P-450. They
demonstrated that under aerobic conditions in vitro 2-NP could
degrade the haem moiety of cytochrome P-450 in phenobarbital-induced
rats and speculated that this provided an additional mechanism for
trapping reactive metabolites before these could damage essential
cellular constituents.
In addition to the hepatic enzymatic systems examined in rats
and mice, Mochizuki et al. (1988), as mentioned above, described a
2-NP denitrifying system in adrenal microsomes of uninduced
guinea-pigs. They identified this cytochrome-P-450-dependent
monooxygenase as benzo[ a]pyrene hydroxylase.
6.4 Elimination and excretion
Elimination of 2-NP and its metabolites has been examined mainly
in rats and, to a much lesser extent, in chimpanzees in studies
which utilized measurements of radioactivity from 14C-labelled
2-NP as well as measurements of 2-NP and its metabolites. Dosage by
inhalation, intravenous injection, and intraperitoneal injection all
yielded fairly similar results. During a 48-h period after a 6-h
exposure of rats to 73 mg/m3 (20 ppm) and to 560.6 mg/m3
(154 ppm) of 14C-labelled 2-NP, about 50% of the radioactivity in
the absorbed dose was excreted via the lungs as carbon dioxide
(Nolan et al., 1982). The proportion of the absorbed dose excreted
via the lungs as unchanged 2-NP was 4% at the low dose level and 22%
at the high level. Still less of the labelled carbon was eliminated
via faeces and urine, i.e. 11% and 8%, respectively, at the low dose
level, and 5% and 11%, respectively, at the high level.
Disappearance of 2-NP from the blood after exposure at the high dose
level followed a first-order relationship and yielded a half-life of
48 min. Limited data in Müller et al. (1983) yielded a half-life for
rats of approximately 80 min for 2-NP in plasma following a 3-h
exposure to a concentration of 728 mg/m3 (200 ppm). Nolan et al.
(1982), however, found that disappearance of the 14C label of the
2-NP from the plasma was markedly slower and biphasic. During the
first 12 h following exposure to 560.6 mg/m3 (154 ppm), the
half-life for plasma radioactivity was 172 min and, following
exposure to 73 mg/m3 (20 ppm), 354 min. After 12 h, loss of
radioactivity from plasma was much slower, the half-life being
approximtely 35-36 h for both doses. These data on loss of 2-NP and
its 14C label indicate that 2-NP is rapidly eliminated from the
body mainly by metabolic transformation and to a lesser degree by
pulmonary excretion of the unchanged compound. The major
carbon-containing metabolites of 2-NP, acetone and isopropanol,
presumably enter into the general metabolism of the body and are
eliminated via the intermediary metabolism as part of a much larger
carbon pool.
Pulmonary excretion of 2-NP, like loss of the 14C label from
plasma, is dose dependent, biphasic, and follows first-order
kinetics (Nolan et al., 1982). Fifty times more 2-NP was exhaled
during the first hour following exposure to 560.6 mg/m3 (154 ppm)
than during the first hour following exposure to 73 mg/m3
(20 ppm). Following exposure to 73 mg/m3, 2-NP was excreted for
the first 7 h at a rate which decreased by one half every 64 min,
and subsequently decreased by one half every 16 h, whereas following
exposure to 560.6 mg/m3, the half-times of excretion were 71 min
for the first 12 h, and 16 h for the subsequent period. Changes in
the rates of pulmonary excretion of 14C-labelled carbon dioxide
were similar for 48 h following exposure to 73 and 560.6 mg/m3.
Eighty seven per cent of the total was eliminated during the first
12 h after exposure; loss was somewhat less rapid thereafter. The
dose-dependent nature of pulmonary excretion of 2-NP suggests that
greater concentrations of 2-NP in the blood markedly increase
exhalation of the unchanged compound and reduce the percentage
metabolized. Thus exposure of the tissues to 2-NP and its
metabolites may not be a linear function of the inhaled dose.
Derks et al. (1989) found that the plasma half-life of
intravenous doses of 0.01-0.05 g/kg in rats was 45 min during the
first 4 h. Loss was linear over this dose range and could be
described by an open single-compartment model. They suggested that
the measured loss from plasma may be due in part to spontaneous
conversion of 2-NP to its anionic form, 2-NP nitronate.
Elimination of 2-NP by Sprague-Dawley rats following
intraperitoneal injection of 14C-labelled 2-NP was studied by
Müller et al. (1983) and was generally similar to the elimination of
2-NP following inhalation. The concentration of 2-NP in plasma
declined exponentially with time, with half-lives of 70 and 125 min
during at least the initial 6 h following injections of 25 and
50 mg/kg, respectively. Metabolites of 2-NP, acetone and
isopropanol, reached maxima 2-4 h after injection of 25 mg/kg, and
at least 4-6 h after injection of 50 mg/kg. The concentration of
isopropanol ranged from 1/16 to 1/34 the concentration of acetone.
During the initial 40 h after injection of 50 mg/kg, 4.5% of the
dose was exhaled as 2-NP, 10.4% as acetone, and 38.1% as carbon
dioxide. Losses via urine (5.9%) and faeces (0.7%) were small in
comparison with the 53% loss via exhalation. Müller et al. (1983),
however, reported that only 12% of the dose was recovered from the
carcasses, leaving a large amount (28.4%) unaccounted for and thus
casting some doubt on their values.
With one exception, results similar to the above were obtained
following intravenous injection of 14C-labelled 2-NP (10 mg/kg)
into male chimpanzees (Müller et al., 1983). The concentration of
2-NP in plasma declined exponentially with time, the half-life being
92 min. The concentration of acetone reached a maximum 6 h after
injection and remained high for at least 48 h. The concentration of
isopropanol peaked 3 h after injection when it approached that of
acetone, but otherwise was a third to a quarter that of acetone. The
concentration of 2-NP and its carbon-containing metabolites (i.e.
the concentration of 14C) in plasma declined exponentially with a
half-life of 5.5 h for the initial 10 h, and a half-life of 48 h
thereafter. As in rats, exhalation was the major means of
elimination of 2-NP and its carbon-containing metabolites. During
the first 3 days after injection, only 5-6% of the 14C in the dose
was recovered in urine and only 0.4-0.5% in faeces. Acetone,
isopropanol, and 2-NP were mainly eliminated via renal excretion;
urine collected during 6 to 24 h after injection contained 3.1 mg
acetone/litre, 7.2 mg isopropanol/litre, and 1.8 mg 2-NP/litre.
Isopropanol may thus be maintained at low concentrations in the
plasma both by oxidation to acetone and by rapid excretion. In
addition to acetone, isopropanol, and 2-NP, 14C was excreted as an
unidentified polar metabolite which by 24 h after injection
contained 90% of the radioactivity in the urine. The one striking
difference between results with rats and with chimpanzees, i.e. the
relatively much higher plasma concentrations of isopropanol in
comparison to acetone, might indicate interspecific variation in the
excretion of 2-NP and its metabolites.
Dequidt et al. (1972) provided limited information on the
excretion of nitrite following inhalation and intraperitoneal
injection of 2-NP. Rats weighing approximately 250 g each were given
a daily injection of 0.11 g/kg. Urinary excretion of nitrite was 10
to 35 µg/animal during the first day following the initial
injection, and reached a daily rate of 11 mg/animal by the fourth
injection. The latter rate of excretion represents three quarters of
the nitrogen injected daily as 2-NP, and stands in sharp contrast to
the results of Schneider & Yeary (1975), who reported that little
intravenously injected nitrite was excreted by dogs, sheep or
ponies. Following exposure of rats to a 2-NP concentration of
2766 mg/m3 (760 ppm) for 8 h on each of two successive days, daily
elimination of nitrite was approximately 30 mg/animal. This was
equivalent to about 20% of the 2-NP inhaled daily and possibly as
much as 50% of the absorbed daily dose. The amount of 2-NP inhaled
was estimated from normative data for the rat (Baker et al., 1979),
and absorption was assumed to be 40% of the amount inhaled (Müller
et al., 1983). No nitrite was detected in urine during exposure of
rats to 291 mg/m3 (80 ppm) for 8 h per day on 5 successive days.
6.5 Retention and turnover
There is no evidence that 2-NP is retained for more than a few
hours in the body. It is rapidly lost by exhalation and metabolic
transformation. The known carbon-containing metabolites, acetone and
isopropanol, are excreted rapidly and are also transformed into
compounds which are normal to the body and enter into its general
intermediary metabolism. There is less information on nitrite, the
major metabolite of the nitro moiety. In rats much of the nitrite is
excreted as such in the urine. There is no evidence for excessive
accumulation of 2-NP or its metabolites in any organ or tissue.
Information is also lacking on possible N-nitroso and other toxic
compounds synthesized from nitrite or the nitro moiety.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Data on single exposures to experimental animals, summarized in
Table 5, indicate substantial differences in sensitivity among the
species tested. The route of administration was mainly via
inhalation, but other routes were also used. A quantitative
comparison of the results from these studies is difficult due to a
lack of information on both strain and sex of the animals used, to
differences in the route of administration, and to large variations
in dosage. The LC50 for mortality within 14 days following a 6-h
exposure was estimated to be 1456 mg/m3 (400 ppm) for male rats
and 2621 mg/m3 (720 ppm) for females (Baldwin & Williams, 1977).
Exposure of rats (of unspecified sex) via inhalation to 14 058
mg/m3 (3862 ppm) for 1 h or to 9584 mg/m3 (2633 ppm) for 2.25 h
killed some of the animals within days. Exposure of rats to a much
higher concentration of 2-NP, i.e. 53 508 mg/m3 (14 700 ppm),
killed all animals within 4 h. An inhalation exposure of 4994
mg/m3 (1372 ppm) or less for 2.25 h was not lethal to rats. Unlike
rats, LC50 values were similar, i.e. 2031 and 2038 mg/m3
(558 and 560 ppm), respectively, for male and female mice following
a 6-h exposure (Baldwin & Williams, 1977). Cats appeared more
sensitive to acute exposure to 2-NP than rats. Exposure to
8565 mg/m3 (2353 ppm) for 1 h (or lower concentrations for
proportionately longer) was lethal to some cats. Rabbits and
guinea-pigs, on the other hand, appeared far less sensitive to 2-NP
than rats. Rabbits survived a 2.25-h exposure to 9584 mg/m3
(2633 ppm) and guinea-pigs a 2.25-h exposure to 15 699 mg/m3
(4313 ppm). The sequence in acute sensitivity of animals to inhaled
2-NP (from most to least) was cat, rat and mouse, rabbit, and
guinea-pig. The cat was almost an order of magnitude more sensitive
than the guinea-pig.
There are limited data on lethality via routes of administration
other than inhalation. Large intraperitoneal doses were found to be
promptly lethal to rats; 1.7 and 1.1 g/kg killed animals within 2 h
and 4 h, respectively (Dequidt et al., 1972). The 14-day oral LD50
for mice was 0.40 g/kg. The minimal oral lethal dose for the rabbit,
0.50-0.75 g/kg, was much larger than the estimated minimum lethal
dose via inhalation, 0.24 g/kg. Dermal application of 2 g/kg to
rabbits produced no obvious local or systemic effects.
Table 5. Effects of single exposure to 2-nitropropane in mammalsa
a) Lethality
Species Sex Concentration Estimated total Effects/results Reference
doseb (g/kg)
g/m3 ppm
Oral administration
Rabbitcd lethal dose estimated as 0.50- Machle et al. (1940)
0.75 g/kg
Mouse M/F 14-day LD50: 0.40 g/kg Hite & Skeggs (1979)
Inhalation
Ratc (Wistar) 53.5 14 700 1.51 all animals died within 4 h Dequidt et al (1972)
Ratcd 5.5-14.1 1513-3865 0.10-0.17 death of some animalsc Treon & Dutra (1952)
2.6-8-57d 714-2353 0.06-0.08 no deathsd Treon & Dutra (1952)
Ratcd (Wistar) 2.77 760 0.16 death within 48 h; Dequidtet al. (1972)
Rate (CD) M 2.93 805 0.16 8/10 died within 14 days Baldwin & Williams (1977)f
F 2.19 602 0.13 no deaths within 14 dayse Baldwin & Williams (1977)f
M 2.10 574 0.14 8/8 died within 14 days Baldwin & Williams (1977)f
M 1.68 461 0.10 7/8 died within 14 days Baldwin & Williams (1977)f
Table 5 (contd)
Species Sex Concentration Estimated total Effects/results Reference
doseb (g/kg)
g/m3 ppm
Inhalation (contd)
Rate (CD) M 1.47 405 0.08 5/8 died within 14 days Baldwin & Williams (1977)f
1.34 367 0.08 no deaths within 14 days Baldwin & Williams (1977)f
Mousee (ICR) F 2.70 740 0.47 14/14 died within 14 days Baldwin & Williams (1977)f
2.33 640 0.41 11/14 died within 14 days Baldwin & Williams (1977)f
1.8 495 0.32 2/14 died within 14 days Baldwin & Williams (1977)f
Mouse (ICR) M 2.69 738 0.41 9/14 died within 14 days Baldwin & Williams (1977)f
2.08 558 0.28 7/14 died within 14 days Baldwin & Williams (1977)f
1.65 454 0.23 no deaths within 14 days Baldwin & Williams (1977)f
Catcd 2.6-8.56 714-2353 0.07-0.19 death of some animals Treon & Dutra (1952)
1.19-2.87 328-787 0.02 no deaths Treon & Dutra (1952)
Guinea-pigcd 16.8-35.0 4622-9607 0.53-0.63 death of some animals Treon & Dutra (1952)
8.67-34.7 2381-9523 0.23-0.32 no deaths Treon & Dutra (1952)
Rabbitc 8.67-34.7 2381-9523 0.24-0.27 death of some animals Treon & Dutra (1952)
5.1-14.1 1401-3865 0.10-0.16 no deaths Treon & Dutra (1952)
Table 5 (contd)
Species Sex Concentration Estimated total Effects/results Reference
doseb (g/kg)
g/m3 ppm
Intraperitoneal administration
Ratcf 1.7 death within 2 h Dequidt et al. (1972)
1.1 death within 4 h Dequidt et al. (1972)
Intraperitoneal administration (contd)
Mousec M 0.80 LD50 Friedman et al. (1976)
(Swiss ICR/HQ)
a Values recalculated as necessary to ppm and g/kg
b Estimated dose calculated by the formula: tidal volume x respiration frequency x exposure time x 2-NP conc. x alveolar
retention/animal weight. Tidal volume and respiration frequency from Kaplan et al. (1983) for mice (0.15 ml, 163/min);
from Baker et al. (1979) for rats (0.86 ml, 85.5/min); from Hoar (1976) for guinea-pigs (1.68 ml, 84/min); from Kozma
et al. 81974) for rabbits (15.8 ml, 45/min); from Reece (1984) and Breazile (1971) for cats (42 ml, 31/min); alveolar
retention in rat (0.40) from Nolan et al. (1982), used for all species; where not stated animal weights assumed to be
average values, 20 g for mice, 250 g for rats, 500 g for guinea-pigs, 2.5 kg for rabbits, and 4.0 kg for cats
c Sex not specified
d Exposure time varied from 1 to 7 h
e Exposure time was 6 h
f Baldwin & Williams (1977) also exposed female rats for 6 h to concentrations of 2-NP lower than 2.2 g/m3 (602 ppm); these
concentrations, i.e. 1.15, 1.35 and 1.69 g/m3 (316, 370 and 464 ppm), like 2.2 g/m3, produced no deaths within 14 days
Table 5. Effects of single exposure to 2-nitropropane
b) other effects
Route of Species Sex Estimated Effects/results Reference
administration total doseb
g/kg)
Intraperitoneal rat M 0.15 maximum hepatic injury achieved Filser & Daumann (1988)
(Sprague-Dawley) with this dose
Intraperitoneal rat M 0.05 lipid accumulation, centrilobular Zitting et al. (1981)
(Wistar) necrosis, mitochondrial
abnormalities, and changes in
endoplasmic reticulum and
glutathione content in liver
within 24 h, as well as changes
in enzyme activity in liver and
brain
Dermal rabbit M & F 2 no toxic effects observed Wilbur & Parekh (1982)
b See footnote b in Table 5a.
The effects of acute exposure to 2-NP, in addition to lethality,
are characterized primarily by hepatotoxicity and, at high exposure
levels, methaemoglobin formation and depression of the central
nervous system. Machle et al. (1940), in a description of symptoms
resulting from exposure of laboratory animals to simple
nitroparaffins, listed the following progression for guinea-pigs and
rabbits after a latent period of 20 to 40 min: progressive weakness,
unsteadiness, and incoordination ending in complete ataxia. The rate
of respiration at first slowed and later became increasingly rapid.
Most of these symptoms appear to reflect the narcotic effects
normally associated with inhalation of volatile hydrocarbons, but
the more rapid breathing may reflect an attempt by the body to
compensate for the formation of methaemoglobin and loss of
oxygen-carrying capacity of the red blood cells. Treon & Dutra
(1952) noted similar progression from exposure to high
concentrations of 2-NP vapour, i.e. lethargy and weakness, dyspnoea,
cyanosis, prostration, and ultimately coma and death, but did not
report more rapid breathing following a depression in rate of
respiration. They also noted lacrimation, salivation, and gastric
regurgitation in cats. In addition, the authors observed that, even
with animals which died promptly (within 9.5 h) following a single
exposure, there was a loss in body weight averaging 2.8%. Animals
exposed to 8.56 g/m3 (2353 ppm) or higher concentrations of 2-NP
displayed pathological changes including hepatocellular damage,
pulmonary oedema and haemorrhage, some disintegration of neurones in
the brain, and widespread damage to the endothelium.
Methaemoglobin and Heinz bodies (masses of denatured haemoglobin
within erythrocytes) were found in the blood of animals following
single exposures to high concentrations of 2-NP. In the case of
cats, 60 to 80% of the haemoglobin was converted to methaemoglobin
by exposure to 15.9 to 33.6 g/m3 (4360 to 9230 ppm) for 1 to 2 h,
whereas much longer exposure of rabbits to these concentrations
converted only 4 to 8% of the haemoglobin (Treon & Dutra, 1952).
Dequidt et al. (1972) reported high levels of methaemoglobin in
rats, i.e. 84% and 89%, following 1-h inhalation exposure to
53.5 g/m3 (14 700 ppm) and intraperitoneal injection of 1.7 g/kg,
respectively. Much lower methaemoglobin concentrations, 0.2 to 8.6%,
resulted from a 1-h exposure to 2.8 g/m3 (760 ppm). One day after
exposure to 15.4 g/m3 (4230 ppm) for 4.5 h or 8.5 g/m3
(2335 ppm) for 2.25 h, rabbits had Heinz bodies in 45 to 80% of
their red cells. These bodies disappeared gradually over 9 to 16
days (Treon & Dutra, 1952). Exposure of rabbits to 13.8 g/m3
(3790 ppm) for 1 h or 9.4 g/m3 (2580 ppm) for 2.25 h resulted in
the formation of Heinz bodies in only 0 to 2% of the red cells.
Formation of Heinz bodies in the cat may have reflected its high
sensitivity to 2-NP. A 1-h exposure to 13.8 g/m3 (3790 ppm) and a
20 min exposure to 16.4 g/m3 (4505 ppm) resulted in the appearance
of Heinz bodies in 27% and 16% of the erythrocytes, respectively.
Observations by Zitting et al. (1981) indicated that a single
intraperitoneal injection of 0.05 g 2-NP/kg to rats can produce
significant changes in the fine structure of the liver and in the
physiology of both liver and brain. 2-NP produced a visible
accumulation of lipid in hepatocytes, especially in periportal areas
after 4 h, and the lipid level continued to increase for the next
20 h. Within 4 h after injection there was also degranulation of the
rough endoplasmic reticulum in hepatocytes and proliferation of the
smooth endoplasmic reticulum. Within 24 h the former had almost
disappeared and the latter was vacuolated or compacted. In addition,
some hepatocytes had abnormal mitochondria and there was necrosis of
hepatocytes around the central vein. The latter was reflected in a
concurrent fourfold increase of serum alanine aminotransferase.
Other enzymatic parameters in the liver were also markedly affected
within 24 h. Cytochrome P-450 was markedly depressed,
7-ethoxycoumarin O-deethylase and 7-ethoxyresorufin O-deethylase
were diminished in activity, and microsomal epoxide hydratase,
UDP-glucuronosyltransferase and glutathione peroxidase were
increased in activity. In addition, the liver concentration of
glutathione nearly doubled.
The major observed neurochemical effect was a significant
increase in acetylcholine esterase activity in the cerebrum and in
isolated synaptosomes. There was little or no change in RNA,
2',3'-cyclic nucleotide 3'-phosphohydrolase, or acid proteinase in
the brain.
Zitting et al. (1981) noted that these histopathological and
enzymatic changes induced by 2-NP are nearly identical to the
effects of carbon tetrachloride on the rat and are thus indicative
of lipid peroxidation.
Hepatotoxicity following exposure to 2-NP has also been
observed in mice (Dayal et al., 1989). Intraperitoneal doses of
0.8 g/kg (9 mmol/kg) in male mice and 0.6 g/kg (6.7 mmol/kg) in
female mice significantly increased plasma activities of enzymes
indicative of hepatic damage (sorbitol dehydrogenase, alanine
aminotransferase, and aspartate aminotransferase) 48, 72, and 96 h
after injection. These enzyme activities were not elevated 24 h
after this dosage nor after small doses of 2-NP.
7.2 Short-term and long-term repeated exposure
Data for repeated exposure, like that for single exposure,
indicate that the cat is more sensitive to 2-NP than the other
species tested (Table 6). Rats, rabbits, guinea-pigs, and monkeys
survived 1.2 g/m3 (328 ppm) (7 h/day, 5 days/week) throughout
approximately 6 months of exposure (Treon & Dutra, 1952). However,
cats exposed to the same concentrations of 2-NP began dying after
the third day of exposure and were all dead by the end of the 17th
day (Treon & Dutra, 1952). Rats and guinea-pigs survived 5 days of
Table 6. Short-term and long-term toxicity of 2-nitropropane in mammalsa
Species Sex Dose and/or concentrationb Effects/results Reference
Oral studies
Rat (Wistar) M & F 0.25 g/kg, 5/week for 4 mortality of males (4/10); decreased growth in Wester et al. (1989)
weeks first week; increased urine, ALAT, ASAT, and
gamma-GT (males only), anaemia, thrombocyte
and leucocyte count, liver, spleen and heart
weight, and haemosiderin content of spleen;
cellular and nuclear polymorphism, single cell
necrosis, and proliferation of oval cells
and/or bile ducts in liver
Rat M 0.089 g/kg (1 mmol), some deaths by 16 week; throughout study body Fiala et al. (1987b)
(Sprague-Dawley) 3/week for 16 weeks, weights significantly lower than controls; all
maintained but not dosed rats exposed 16 weeks or longer developed
for next 61 weeks massive hepatocellular carcinomas; metastases
to the lungs in 4 animals
Rat M & F 0.05 g/kg, 5/week for 4 increased anaemia, thrombocyte conc., and Wester et al (1989)
(Wistar) weeks heart weight
Rat (Wistar) M & F 0.002 and 0.01 g/kg, 5 increased water intake by males dosed with Wester et al. (1989)
week for 4 weeks 0.002 g/kg
Inhalation studies
Ratd 2.77 g/m3 (760 ppm), 8 animals dead within 2 h after end of second Dequidt et al. (1972)
(Wistar) h/day for 2 days; inhalation session; 2-NP conc. in liver =
estimated dose over 8 h, 180 ppm; methaemoglobin = 2.4%
0.16 g/kg
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Inhalation studies (contd)
Rate 2.45 g/m3 (672 ppm), 7 no deaths Treon & Dutra (1952)
h/day for 5 days;
estimated dose over
7 h, 0.12 g/kg
1.20 g/m3 (328 ppm), 7 no deaths Treon & Dutra (1952)
h/day, 5 days/week for
130 days over 199 days;
estimated dose over 7 h,
0.06 g/kg
Rat M 0.75 g/m3 (207 ppm), 7 body weight and haematological parameters Lewis et al. (1979)
(Sprague-Dawley) h/day, 5 days/week for up unaffected; some pulmonary oedema and some
to 24 weeks; estimated pulmonary lesions within 3 months; liver
dose over 7 h, 0.04 g/kg weight elevated; hepatocellular hypertrophy,
hyperplasia and liver necrosis in all rats
within 3 months; liver neoplasms in all rats
within 6 months; these hepatocellular
carcinomas appeared to be growing rapidly and
deforming surrounding tissues
Rate 0.73 g/m3 (200 ppm), 7 growth slightly reduced and SGPT elevated in Griffin et al. (1978)
h/day, 5 days/week for 6 male rats; liver weight increased in both sexes;
months; estimated dose morphological changes in liver more pronounced
over 7 h, 0.03 g/kg in males; these included fatty metamorphosis
and hepatic nodules consisting mainly of
hyperplastic areas with distortion of lobular
architecture, necrosis and peripheral
compression
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Inhalation studies (contd)
Ratd 0.73 g/m3 (200 ppm), 7 no effect on body or organ weight to end of Griffin et al. (1986)
(Sprague-Dawley) h/day for 5 days experiment (94 week) aside from a brief
decrease in weight gain immediately following
exposure; no significant effects on mortality
or pathology
Ratc M 0.73 g/m3 (200 ppm), 7 severe liver damage with vacolar degeneration Coulston (1982)
h/day, 5 days/week for in exposed rats after 3 months up
to 7 months
Rate 0.36 g/m3 (100 ppm), 7 male rats had lower body weight, increased Griffin & Coulston (1983);
h/day, 5 days/week for up renal calcification, elevated SGPT, and Coulston et al. (1985)
to 18 months; estimated enlarged livers with necrosis, vacuolar
dose over 7 h, 0.013 g/kg degeneration and probable hepatocellular
carcinomas; female rats had increased renal
calcification and occasional hepatic masses
and nodules showing hyperplasia and vacuolar
degeneration
Rate 0.29 g/m3 (80 ppm), 8 no deaths; no trace of 2-NP in organs at end of Dequidt et al. (1972)
h/day for 5 days; estimated experiment; methaemoglobin = 0; nitrite = 0-10
dose over 8 h, 0.016 g/kg ppm in tissues, but no nitrite in urine
Rat M 0.1 g/m3 (27 ppm), 7 no gross or microscopic alteration of any Lewis et al. (1979)
(Sprague-Dawley) h/day, 5 days/week for up tissue, haematological parameter or serum
to 24 weeks; estimated biochemistry
dose over 7 h, 0.005 g/kg
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Inhalation studies (contd)
Rate 91 mg/m3 (25 ppm), 7 no changes in behaviour, appearance, rate of Griffin et al.
h/day, 5 days/week for up weight gain, final weight, serum chemistry or (1980, 1981)
to 22 months; estimated haematology; no significant increase in tumours
dose over 7 h, 0.003 g/kg and lesions associated with exposure; no
evidence of methaemoglobinaemia
Moused 0.73 g/m3 (200 ppm), 7 depression in body weight during first 3 months Griffin et al. (1984)
(ICR) h/day, 5 days/week for in females and throughout experiment in males;
48 weeks increased liver weight and elevation of liver
transaminases in females; toxic hyperplasia of
liver predominantly in females
Mousee 0.36 g/m3 (100 ppm), 7 slight depression of body weight during first 8 Coulston et al. (1986);
h/day, 5 days/week for months in males; no effects on organ weight; no Griffin et al. (1987)
18 months evidence of hepatocellular carcinoma; some
indications of liver toxicity (nodular
hyperplasia in females)
Cate 2.6 g/m3 (714 ppm), 4.5 deaths starting with first exposure, but Treon & Dutra (1952)
h/day for 4 days; some animals survived 4 exposures
estimated dose over 7 h,
0.10 g/kg
1.2 g/m3 (328 ppm), 7 deaths starting with third exposure; all Treon & Dutra (1952)
h/day, 5 days/week for 17 animals dead by end of 17th exposure
exposures; estimated dose
over 7 h, 0.07 g/kg
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Inhalation studies (contd)
Cate 1.15 g/m3 (317 ppm), 7 no deaths Treon & Dutra (1952)
h/day for 2 days;
estimated dose over 7 h,
0.07 g/kg
0.3 g/m3 (83 ppm), 7 no deaths Treon & Dutra (1952)
h/day, for 130 out of 191
days; estimated dose over
7 h, 0.02 g/kg
Rabbite 1.2 g/m3 (328 ppm), 7 no deaths Treon & Dutra (1952)
h/day, ca. 5 days/wk for
up to 130 out of 199 days;
estimated dose over 7 h,
0.06 g/kg
Rabbit M 0.75 g/m3 (207 ppm), 7 no gross or microscopic alterations to Lewis et al. (1979)
(white, NZ) h/day, 5 days/week for 24 tissues
weeks; estimated dose
over 7 h, 0.03 g/kg
Rabbite 0.3 g/m3 (83 ppm), 7 h/day no deaths Treon & Dutra (1952)
for 130 out of 191 days;
estimated dose over 7 h,
0.014 g/kg
Rabbite 0.1 mg/m3 (27 ppm), 7 no gross or microscopic alterations to Lewis et al (1979)
h/day, 5 days/week for 6 tissues
months; estimated dose
over 7 h, 0.005 g/kg
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Inhalation studies (contd)
Guinea-pige 2.45 g/m3 (672 ppm), 7 no deaths Treon & Dutra (1952)
h/day for 5 days;
estimated dose over
7 h, 0.12 g/kg
Guinea-pige 1.2 g/m3 (328 ppm), 7 no deaths Treon & Dutra (1952)
h/day, ca. 5 days/week
for 95-130 days out of
up to 199 days; estimated
dose over 7 h, 0.06 g/kg
Monkeye 1.2 g/m3 (328 ppm), 7 no deaths Treon & Dutra (1952)
h/day, ca. 5 days/week
for 100 days exposure
0.3 g/m3 (83 ppm), no deaths Treon & Dutra (1952)
7 h/day for 130 out of
191 days
Intraperitoneal studies
Ratd 0.11 g/kg, 1/day for apparently no deaths prior to sacrifice Dequidt et al. (1972)
(Wistar) 7 days 3 days after the last injection;
methaemoglobin = 4.3%; nitrite = 0.29-1.15 ppm
in organs (heart, lungs, kidneys, spleen)
Ratd 0.11 g/kg, 1/day for 15 apparently no deaths prior to sacrifice 36 h
(Wistar) days after the last injection; methaemoglobin = 0;
nitrite = 0-10.8 ppm in organs (heart, lungs,
kidneys, spleen)
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Intraperitoneal studies
Rat F 0.001 g/kg, 5/week for no significant effects on kidney function Bernard et al. (1989)
(Sprague-Dawley) 2 weeks
Dermal study
Rabbite 1 application/day on no skin irritation, illness, systemic effects Machle et al. (1940)
clipped anterior abdomen or deaths
for 5 days; dose not
stated
a values from the literature recalculated as necessary to ppm or g/kg
b estimated dose calculated by the formula: tidal volume x respiration frequency x 2-NP conc. x alveolar retention/animal weight.
Tidal volume and respiration frequency from Baker et al. (1979) for rats (0.086 ml, 85.5 /min); from Hoar (1976) for guinea-pigs
(1.68 ml, 84/min); from Kozma et al. (1974) for rabbits (15.8 ml, 45/min); from Reece (1984) and Breazile (1971) for cats
(42 ml, 31/min); alveolar retention in rat (0.40) from Nolan et al. (1982), used for all species; where not stated in reference,
animal weights assumed to be average values, 250 g for rats, 500 g for guinea-pigs, 2.5 kg for rabbits, and 4.0 kg for cats
c strain not specified
d sex not specified
e neither strain nor sex specified
exposure (7 h/day) to 2.46 g/m3 (672 ppm), but death occurred in
rats exposed for 2 days (8 h/day) to 2.77 g/m3 (760 ppm). Rats
were reported to survive 15 days of daily intra-peritoneal
injections of 0.11 g/kg (Dequidt et al., 1972). The doses used in
these repeated exposures were found to produce no more than trace
levels of methaemoglobin (maximum = 4.3%) and low concentrations
(0 to 11 mg/kg) of nitrite in the tissues.
Non-lethal chronic doses of 2-NP have been shown to produce a
number of harmful effects in rats (Table 6). Exposure of rats to
0.75 g/kg (207 ppm) for up to 24 weeks (7 h/day, 5 days/week)
initially induced pulmonary lesions and oedema, hepatocellular
hypertrophy, hyperplasia, and necrosis of the liver (Lewis et al.,
1979). By the end of 24 weeks all the rats developed rapidly growing
hepatocellular carcinomas. A similar exposure to a slightly lower
concentration of 2-NP, 0.73 g/m3 (200 ppm), induced hepatic
nodules and other destructive changes in the liver, especially in
male rats (Griffin & Coulston, 1983). These changes included fatty
degeneration, nodules consisting mainly of hyperplastic areas,
distortion of lobular architecture, necrosis and peripheral
compression. Male rats also had an elevated serum alanine
aminotransferase level (an indicator of liver damage) and slightly
reduced growth. Chronic exposure of rats to 0.36 g/m3 (100 ppm)
for up to 18 months produced similar, although slightly less severe,
damage than that resulting from exposure to 0.73 g/m3 (200 ppm)
(Griffin & Coulston, 1983; Coulston et al., 1985). Only male rats
developed hepatocellular carcinoma; female rats had increased renal
calcification and occasional hepatic masses and nodules showing
hyperplasia and vacuolar degeneration. No toxic effects were
reported after chronic exposure of rats to 90 mg/m3 (25 ppm) or
100 mg/m3 (27 ppm) (Lewis et al., 1979; Griffin et al., 1980,
1981). Daily intraperitoneal injections of 1 mg/kg (5/week for 2
weeks) had no significant effect on kidney function (Bernard et al.,
1989).
Chronic oral dosage of 2-NP by gavage for 16 weeks produced
tumours in rats (Fiala et al., 1987b). The dose was 0.089 g/kg
(1 mmol/kg), given 3 times per week, and this yielded a weekly
dosage of 0.27 g/kg, an amount similar to the highest sustained
estimated inhalation dosage. The body weights of rats treated with
2-NP were significantly lower than those of controls, and all
treated rats surviving 16 weeks or longer developed both benign
tumours and massive hepatocellular carcinomas. Metastases to the
lungs were observed in four of the 22 surviving animals (Fiala et
al., 1987b). Wester et al. (1989) treated rats with oral doses of
0.002, 0.01, 0.05, and 0.25 g/kg, 5 times per week for 4 weeks by
gavage. With a dose of 0.25 g/kg there was some mortality among male
rats, and in both sexes there was decreased growth, anaemia,
increased liver and heart weights, and severe damage to the liver.
At a dose of 0.05 g/kg the major harmful effect appeared to be
anaemia. Lower concentrations (0.01 and 0.002 g/kg) did not produce
obvious harm over the period of the experiment.
Rabbits and mice appear more resistant to the sublethal effects
of 2-NP than rats (Table 6). Chronic inhalation exposure of five
rabbits to 0.75 g/m3 (207 ppm) (7 h/day, 5 days/week) for 24
weeks, a treatment which induced hepatocellular carcinomas and
severe liver damage in rats, had no detectable effect (Lewis et al.,
1979). Rabbits also were unaffected by repeated dermal application
of 2-NP (Machle et al., 1940). Liver damage was found in mice,
especially females, during chronic exposure to 0.72 g/m3 (200 ppm)
(7 h/day, 5 days/week) for 48 weeks, but hepatocellular or other
carcinomas were not detected (Griffin et al., 1984). Exposure of
mice to 0.36 g/m3 (100 ppm) (7 h/day, 5 days/week) for 18 months
produced some liver damage, especially in females, as shown by
nodular hyperplasia (Coulston et al., 1986, Griffin et al., 1987).
7.3 Reproduction, embryotoxicity, and teratogenicity
There is limited information on the embryotoxicity and
teratogenicity of 2-NP. Hardin et al. (1981) gave intraperitoneal
injections of 0.17 g/kg (1.91 mmol/kg) of 2-NP in corn oil to
pregnant rats on days 1-5 of gestation. The dosage was a previously
determined maximum tolerated dose which produced no mortality, no
marked signs of toxicity, and less than a 10% reduction in body
weight gain during dosing or within 2 weeks following 15 daily
intraperitoneal injections to non-pregnant rats. In pregnant rats,
this dose was reported to give no evidence of maternal toxicity or
teratogenicity, but produced a significant incidence of delayed
fetal development. Harris et al. (1979) reported that 2-NP at a dose
of 0.17 g/kg retarded fetal heart development by 1 to 2 days in pups
from 9 out of 10 litters produced by female rats treated with 2-NP.
In the affected litters, 30% to 86% of the pups had retarded heart
development.
There appear to be no studies that have examined specifically
the effects of 2-NP on reproductive function. No effects on
reproductive organs, however, were noted in the above two studies
nor in any of those studies on the effects of single exposures or
short-term or long-term administration of 2-NP (Tables 5 and 6).
There also was no evidence of an increase in dominant lethality or
in sperm abnormality in genetic studies relating to reproduction
(McGregor, 1981).
7.4 Mutagenicity and related end-points
7.4.1 Prokaryotes and yeast
2-NP has been found to be mutagenic in a variety of test
systems. All investigators reported mutagenicity in several strains
of Salmonella typhimurium used with the Ames test, both with and
without an exogenous activating system (S9) (Table 7). In addition,
Kawai et al. (1987) reported mutagenicity with a strain of
Escherichia coli, but Litton Bionetics, Inc. (1977) did not find
mutagenicity with a strain of Saccharomyces cerevisiae. Most
investigators found greater mutagenicity with activation by S9 than
without. With the strain that was generally most sensitive to 2-NP,
i.e. TA100, most investigators providing detailed results observed
an approximate doubling in the number of mutants at a concentration
of 3 mg 2-NP/plate. Göggelmann et al. (1988), however, observed a
10- to 12-fold increase in mutant numbers at 2.45 mg/plate, with or
without activation by S9. Fiala et al. (1987a) reported that
non-ionic 2-NP yielded only a doubling of mutant numbers at
4.9 mg/plate with S9 activation and no increase without activation,
whereas 2-NP nitronate at this concentration yielded a threefold
increase in mutant numbers with activation. Without activation 2-NP
nitronate at 2.5 mg/plate yielded a nearly 6-fold increase in mutant
numbers; nitronate at 4.9 mg/plate was toxic to this test organism.
Fiala et al. (1987a) concluded that the mutagenicity of 2-NP is
produced mainly or entirely by the nitronate anion and suggested
that the low level of mutagenicity observed with non-ionic 2-NP may
have resulted from its conversion to the nitronate form within the
microorganisms. A similar conclusion was drawn by Dayal et al.
(1989) from their comparison of 2-NP, nitromethane, nitroethane, and
their nitronates for mutagenicity in Salmonella. Some of the
variability in results obtained by others may stem from varying
proportions of non-ionic 2-NP and 2-NP nitronate in the stock 2-NP
used in their tests. Fiala et al. (1987a) further observed that
dimethyl sulfoxide (DMSO), a solvent used to solubilize 2-NP in
these tests, modified the mutagenicity of 2-NP nitronate in a
variable manner.
Several of the studies discussed above examined the question of
how 2-NP induces mutations in the test systems. In addition to 2-NP,
1-and 2-aminopropane and a number of mononitroalkanes (nitromethane,
nitroethane, 1-nitropropane, 1-and 2-nitrobutane, and 1-and
2-nitropentane) were tested for mutagenicity with bacterial systems,
mainly strains of Salmonella typhimurium (Hite & Skeggs, 1979;
Speck et al., 1982; Löfroth et al., 1986; Kawai et al., 1987;
Göggelmann et al., 1988; Dayal et al., 1989). Only 2-NP proved to be
significantly mutagenic. Some investigators observed weak
mutagenicity with nitroethane, 1-nitropropane, 2-nitrobutane, and
2-nitropentane, which, at least in the case of nitromethane and
nitroethane, may have been produced by the 2-NP present in these
solvents as a contaminant.
Table 7. Genotoxicity of 2-nitropropane
Test system Resulta Referencesb
Prokaryotes and yeastc
Salmonella typhimurium
strain TA92 + 1
strain TA92 with S9 + 1
strain TA98 + 1,2,5,7,8,9
- 6,16,19
strain TA98 with S9 + 1,2,3,5,8,9,17
strain TA98NR + 2,9
strain TA98NR with S9 + 2,9
strain TA100 + 1,2,4,5,6,7,8,9,19
- 17
strain TA100 with S9 + 1,2,3,4,5,6,8,9,17,19
strain TA100NR + 2,9
strain TA100NR with S9 + 2,9
strain TA102 + 4,8,19
strain TA102 with S9 + 4,8,19
Table 7 (contd)
Test system Resulta Referencesb
strain TA1535 ? 5
- 17
strain TA1535 with S9 + 3
? 5
- 17
strain TA1537 - 17
strain TA1537 with S9 - 3,17
strain TA1538 - 17
strain TA1538 with S9 - 17
Escherichia coli
strain WP2 uvrA/pKM101 + 7
strain WP2 uvrA/pKM101 + 7
Saccharomyces cerevisiae
strain D4 - 17
strain D4 with S9 - 17
Table 7 (contd)
Test system Resulta Referencesb
Eukaryotesf
In vivo
Drosophila
Sex-linked recessive lethal - 10,11
Mouse
erythrocytes
(micronuclei) m, f - 1,16,23
sperm (abnormality) ? 11
Rat
Liver (DNA repair synthesis) m, f + 12,13,23
(micronuclei) + 23
(DNA and RNA base + 18,20,21,22
modifications) m, f
(DNA strand breakage) m + 24
Kidney (DNA strand breakage) m - 24
(DNA and RNA base - 22
modifications) m, f
Table 7 (contd)
Test system Resulta Referencesb
Brain (DNA strand breakage) m - 24
Lung (DNA strand breakage) m - 24
Bone marrow (chromosome - 11
aberrations) m, f
(micronuclei) m - 23
Testes
(dominant lethal test) m - 11
In Vitro
Mouse
3T3-NIH fibroblasts - 13
(DNA repair synthesis)
Rat
hepatocytes + 12,13,26
(DNA repair synthesis) m, f
hepatoma cells, 2sFou + 25
(DNA repair synthesis and micronuclei)g
hepatoma cells, C2Rev7 + 25
(DNA repair synthesis and micronuclei)g
Table 7 (contd)
Test system Resulta Referencesb
hepatoma cells, H4IIEC3/G- + 25
(DNA repair synthesis, micronuclei and
gene mutations)g
208F - embryonic fibroblasts - 13
(DNA repair synthesis)
LLC WRC 256 carcinoma Walker rat - 13
(DNA repair synthesis)
Hamster
CHO cells (chromosome aberrations - 14
and sister chromatid exchanges)
CHO cells (DNA repair synthesis) - 13
V79 cells (DNA repair synthesis) - 12,13,25,26
V79 cells (mutations) + 25
V79 cells (micrnuclei) - 25,26
Human
Lymphocytes (chromosome aberrations -d 9,15
and sister chromatid exchanges)
Lymphocytes (chromosome aberrations -e 9,15
and sister chromatid exchanges) with
S9
Table 7 (contd)
Test system Resulta Referencesb
Human (contd)
Fibroblasts (DNA repair synthesis) - 11
W138 embryonic lung fibroblasts NC1- - 13
H322 adenocarcinoma lung cells A549
adenocarcinoma lung cells HEp2 epidermal
carcinoma larynx cells (DNA repair synthesis)
a + = positive response; - = negative response; ? = inconclusive result
b (1) Hite & Skeggs (1979) (2) Speck et al. (1982)
(3) Haworth et al. (1983) (4) Simmons et al. (1986)
(5) Löfroth et al. (1986) (6) Hughes et al. (1987)
(7) Kawai et al. (1987) (8) Fiala et al. (1987a)
(9) Göggelmann et al. (1988) (10) Zimmering et al. (1985)
(11) McGregor (1981) (12) Ziegler-Skylakakis et al. (1987)
(13) Andrae et al. (1988) (14) Galloway et al. (1987)
(15) Bauchinger et al. (1987) (16) Kliesch & Adler (1987)
(17) Litton Bionetics, Inc. (1977) (18) Conaway et al. (1991a)
(19) Conaway et al. (1991b) (20) Fiala et al. (1989)
(21) Hussain et al. (1990) (22) Guo et al. (1990)
(23) George et al. (1989) (24) Robbiano et al. (1991)
(25) Roscher et al. (1990) (26) Haas-Jobelius et al. (1991)
c Liver S9 fractions are prepared by treating rats, mice or hamsters with Aroclor
1254 or another microsomal enzyme inducer, and, after several days, removing
the livers, homogenizing them, and centrifuging. The supernatant, the S9
fraction, contains liver microsomal enzymes.
d Bauchiner et al. (1987) (ref. 15) reported a weak but significantly positive result
on one test, but considered results overall to be negative
e Göggelmann et al. (1988) (ref. 9) reported a very weak positive result
f m = male; f = female
g pretreated with dexamethasone
Löfroth et al. (1986) and Fiala et al. (1987a) speculated that
the relative mutagenicity of 2-NP and other nitroalkanes correlated
with the concentrations of their nitronate anions. The more highly
mutagenic compounds, such as 2-NP and 1,1-dinitroethane, had high
concentrations of nitronate at cellular pH. A thorough comparison of
2-NP, other secondary nitroalkanes, and their nitronates (Conaway et
al., 1991) showed greater mutagenicity of the nitronates and
confirmed these speculations.
These studies do not support the hypothesis that mutagenicity of
2-NP is produced largely or entirely by nitrite resulting from its
metabolic breakdown, since the response of tester strains to sodium
nitrite was quite different from their response to 2-NP (Löfroth et
al, 1986). Unlike nitroarenes and nitroheterocyclics, 2-NP probably
does not derive its mutagenicity exclusively from enzymatic
reduction to a hydroxylamine. Strains of S. typhimurium, i.e.
TA98NR and TA100NR, lacking the "classical" nitroreductase
demonstrated either a very slightly (Speck et al., 1982) or markedly
(Göggelmann et al., 1988) reduced, but still significant, level of
mutagenicity. Göggelmann et al. (1988) suggested that some of this
mutagenicity may be due to some residual nitroreductase activity
still present in these strains.
The increased mutagenicity generally observed with S9 activation
of 2-NP and the reduced mutagenicity in tester strains deficient in
nitroreductase activity suggest an involvement of metabolism in the
mutagenicity of 2-NP in S. typhimurium. Fiala et al. (1987a)
presented evidence that metabolic oxidation of the 2-NP anion can
result in the formation of reactive species such as hydroxyl
radicals, which are capable of damaging DNA bases. Incubation of
2-NP nitronate under roughly physiological conditions with thymidine
and a 1-electron oxidation system, horseradish peroxidase and
hydrogen peroxide, yielded 2-NP free radicals (which in part
condensed into 2,3-dimethyl-2,3-dinitrobutane) and oxidation
products of thymidine of the type produced by hydroxyl radical
attack. A common source of hydroxyl radicals is superoxide, which
is known to be produced during oxidation of 2-NP nitronate by
horseradish peroxidase and hydrogen peroxide (Porter & Bright,
1983). The extent to which such reactions may occur in the
Salmonella tester strains or in vivo is unknown. There is,
however, evidence from the literature for microbial oxidation of
2-NP and 2-NP nitronate (Kido et al., 1984; Fiala et al., 1987a).
The existence of this proposed mechanism for inducing mutations
through the production of DNA-damaging hydroxyl radicals is further
supported by the reduction in 2-NP mutagenicity in TA102 by DMSO, a
known scavenger of such radicals (Fiala et al., 1987a). The fact
that DMSO had little effect on the mutagenicity of 2-NP in TA100
indicated that formation of hydroxyl radicals cannot be the only
mechanism inducing mutations. Fiala et al. (1987a) hypothesized that
the 2-NP radical may act directly by forming adducts with DNA bases.
This would provide an additional mechanism for inducing mutations
that would not require hydroxyl radicals.
7.4.2 Eukaryotes
The genotoxic effects of 2-NP in eukaryotic organisms are
summarized in Table 7. Results were negative in two sex-linked
recessive lethal tests using Drosophila despite exposure to high
concentrations of 2-NP. Zimmering et al. (1985) dosed the flies by
injection and by feeding with concentrations that induced an
approximately 30% mortality, whereas McGregor (1981) exposed the
flies to 2-NP vapour at a concentration of 2.55 g/m3 (700 ppm) for
4.5 h. Sex-linked recessive lethal mutation frequency was not
increased except in mature spermatozoa in one stock of flies. Since
this increase was not reproducible, its significance is doubtful.
Results were variable but mainly negative in a variety of
in vivo mammalian test systems. In the dominant lethal test and
the bone marrow chromosomal aberration test using rats and in the
sperm abnormality test using mice, animals were exposed to 91 or
728 mg/m3 (25 or 200 ppm) for 7 h/day on 5 consecutive days.
Neither exposure level produced a positive response in any of the test
systems used (McGregor, 1981). Negative results were obtained with
the mouse micronucleus test even with an almost lethal oral dose of
0.3 g/kg on 2 consecutive days (Hite & Skeggs, 1979), and with a
single intraperitoneal dose of 0.3 g/kg (Kliesch & Adler, 1987).
One set of genotoxicity tests using rats, however, yielded
strongly positive results (Andrae et al., 1988; Ziegler-Skylakakis
et al., 1987). In both in vivo and in vitro tests, 2-NP induced
DNA repair synthesis in rat liver cells. In the in vitro
experiments, cultures of rat hepatocytes were incubated with 2-NP,
while in the in vivo experiments rats were injected
intraperitoneally with 2-NP, sacrificed 4 h later, and cultures of
their hepatocytes were examined for DNA repair synthesis. Exposure
of hepatocyte cultures for 18 to 20 h to concentrations of 2-NP as
low as 2.7 mg/litre (30 µmol/litre) induced a detectable
(approximately 2-fold above the control level) increase in repair
synthesis. The highest concentration tested, i.e. 89 mg/litre
(10 mmol/litre), induced a 12- to 15-fold increase above control
levels in hepatocytes from male rats and a 25- to 30-fold increase
in hepatocytes from female rats. The in vivo experiments also
demonstrated the existence of a sexual difference in susceptibility
to 2-NP. In males, the lowest dose (20 mg/kg) induced a doubling in
repair synthesis and the highest dose (80 mg/kg) a 3.6-fold
increase, whereas in females these doses induced a very small
increase and a doubling, respectively. In contrast to 2-NP, 1-NP
given in vivo had no effect on DNA repair synthesis and did not
increase in vitro repair synthesis above that expected from its
contamination with 2-NP. Andrae et al. (1988) considered that their
results from the in vivo experiments were in agreement with the
observed greater hepatocarcinogenicity of 2-NP in male rats than in
females. Their observation that 2-NP did not induce any increase in
repair synthesis in any of nine non-hepatic cell lines derived from
human, mouse, hamster, and rat tissues led the authors to suggest
that 2-NP is not a direct-acting genotoxic agent but rather requires
metabolic activation by liver-specific metabolism.
Several papers have confirmed and expanded these initial
observations on the genotoxicity of 2-NP to rat liver cells. George
et al. (1989) showed that oral dosage similarly induced unscheduled
DNA synthesis and also resulted in the formation of micronuclei in
rat liver. 2-NP did not, however, significantly increase the
frequency of micronuclei in mouse bone marrow. Intraperitoneal
injection of 0.1 g/kg produced in 6 h a significant increase in
8-hydroxydeoxyguanosine and 8-hydroxyguanosine, products
respectively of DNA and RNA damage caused by hydroxyl or other
oxygen-radical-forming agents (Fiala et al., 1989; Hussain et al.,
1990). This treatment produced significantly lower levels of
8-hydroxydeoxyguanosine, 8-hydroxyguanosine, and other presumed
modified nucleosides in female rats than in males, and had little
effect on the nucleic acids of the kidney, findings that are in
agreement with the known carcinogenicity of 2-NP (Guo et al., 1990).
The organ specificity of the genotoxicity of 2-NP in the rat was
confirmed by Robbiano et al. (1991) who reported that oral doses of
45-713 mg/kg produced maximum numbers of single strand breaks in the
liver 6 h after administration and did not induce DNA fragmentation
in lung, kidney, bone marrow or brain. Damage to rat liver nucleic
acids was also caused by intraperitoneal injection of other
secondary nitroalkanes and a ketoxime capable of being converted to
a secondary nitroalkane, but not with a primary or a tertiary
nitroalkane (Conaway et al., 1991). These authors suggest that the
greater genotoxicity of the secondary nitroalkanes may stem from the
greater stability of their nitronate forms at physiological pH
values.
Observations by Roscher et al. (1990) and Robbiano et al. (1991)
suggest that cytochrome P-450-dependent monooxygenases are important
in the activation of 2-NP in liver. Robbiano et al. (1991) found an
increase in damage to liver DNA in rats pretreated with
phenobarbital or ß-naphthoflavone, inducers of cytochrome
P-450-dependent monooxygenases, and a reduction in liver DNA damage
in rats pretreated with methoxsalen, an inhibitor of cytochrome
P-450. Roscher et al. (1990) examined the effect of 2-NP on rat
hepatoma cell lines that express various forms of cytochrome
P-450-dependent monooxygenases and V79 Chinese hamster cells that
lack these enzyme activities. 2-NP increased DNA repair synthesis,
micronuclei formation, and the frequency of mutants resistant to
6-thioguanine in hepatoma cells pretreated with dexamethasone, an
inducer of various liver-specific cytochrome P-450 forms.
Genotoxicity was reduced or absent in hepatoma cells not treated
with the inducer. In the V79 cells, 2-NP produced only mutations to
6-thioguanine resistance.
2-NP demonstrated only a very limited level of genotoxicity in
other cell lines. Results were negative in a DNA repair synthesis
assay with human diploid fibroblasts exposed for 3 h to 2-NP
concentrations up to 5 g/litre (56 mmol/litre) (McGregor, 1981), as
well as with various cell lines of extrahepatic origin (Andrae et
al., 1988). 2-NP did not cause chromosomal aberrations or SCEs in
Chinese hamster ovary (CHO) cells either with or without S9
(Galloway et al., 1987). In these experiments the maximum
concentrations used, i.e. 1.6 and 5.0 g/litre (18 and 56 mmol/litre)
were estimated to reduce cell growth by 50%. Weakly positive
results, however, were obtained in cytogenetic tests using human
lymphocytes (Bauchinger et al., 1987; Göggelmann et al., 1988).
Exposure of cells to high concentrations of 2-NP (commercial grade,
at least 94% 2-NP) for 1 h induced a significant increase in
chromosomal aberrations (open breaks accompanied by gaps) at a
concentration of 5.3 g/litre (60 mmol/litre) with S9 activation and
at 7.1 g/litre (80 mmol/litre) without S9 activation (Bauchinger et
al., 1987). In addition, the frequency of sister chromatid exchange
was significantly increased at all concentrations of 2-NP (0.7 to
7.1 g/litre; 7.5 to 80 mmol/litre), but only with S9 activation.
Repetition of the experiment with 2-NP of greater than 99% purity
yielded similar results (Göggelmann et al., 1988). There was no
significant increase in chromosomal changes without S9 activation,
but there was a small but significant increase at the highest
treatment levels, i.e. 7.1 and 10 g/litre (80 and 111 mmol/litre),
with S9 activation. 1-NP of 97% purity produced no significant
mutagenic or cytogenetic effects on this test system with or without
S9 activation. The authors concluded that 2-NP can exert a
clastogenic effect on human lymphocytes only with metabolic
activation, and hypothesized that it acted via a different metabolic
pathway in the lymphocytes than it did in the bacterial
S. typhimurium system, where it induced mutations without
exogenous activation.
7.5 Carcinogenicity
That 2-NP is unquestionably a potent carcinogen in rats was
demonstrated by Lewis et al. (1979). Inhalation exposure of male
Sprague-Dawley rats to 0.75 g/m3 (207 ppm) for 7 h/day, 5
days/week, over a 24-week period induced hepatocellular neoplasms in
all surviving animals. Inhalation exposure of rats to 0.36 g/m3
(100 ppm) similarly administered over 18 months was associated with
hepatocellular carcinomas in male rats and produced changes in the
livers of female rats (nodules showing hyperplasia and vacuolar
degeneration) which may have been precursors to carcinoma (Griffin &
Coulston, 1983; Coulston et al., 1985). Oral dosing of male rats
with 89 mg/kg (1 mmol/kg), three times a week for 16 weeks, induced
massive hepatocellular carcinomas in all rats, observed when they
were sacrificed 40 weeks later (Fiala et al., 1987a). Metastases to
the lungs were present in 4 out of 22 surviving animals, suggesting
a high degree of malignancy. Long-term inhalation exposure of rats
to low concentrations (100 mg/m3; 27 ppm) did not produce any
evidence of increased hepatocellular carcinoma or precursor tumour
lesions of any sort (Lewis et al., 1979; Griffin et al., 1980,
1981). Evidence that inhalation exposure to low doses of 2-NP can
cause DNA damage in rats was presented by Denk et al. (1990). Male
and female Sprague-Dawley rats, 4-6 days old at the beginning of
treatment, were exposed to 0, 91, 146, 182, 291, and 455 mg/m3 (0,
25, 40, 50, 80 and 125 ppm) for 6 h/day, 5 days/week for 3 weeks,
and this was followed by promotion with polychlorinated biphenyls
(Clophen A50) for 8 weeks. This treatment produced a dose-dependent
increase in the numbers of preneoplastic liver foci deficient in
adenosine-5'-triphosphatase. Cunningham & Matthews (1991) showed
that 2-NP can also induce cell proliferation in rat liver. Rats were
exposed to daily oral doses of 20, 40 or 80 mg/kg by gavage for 10
days, and cell proliferation during exposure was quantified by
measuring the incorporation of bromodeoxyuridine into newly
synthesized DNA. Exposure to 40 and 80 mg/kg resulted in
statistically significant increases in the frequency of S-phase
cells from 1.9% in the vehicle-treated animals to 6.3% and 11%,
respectively. Exposure to 20 mg/kg had no effect on the labelling
index. The non-carcinogenic isomer of 2-NP, 1-NP, did not affect DNA
synthesis at doses of 20, 40 and 80 mg/kg.
There is no substantial evidence that 2-NP induces cancer in
species other than the rat (Table 6). Inhalation exposure of rabbits
to 750 mg/m3 (207 ppm) for 1 h/day, 5 days/week for 24 weeks,
initially produced some evidence of liver damage. At the end of 6
months of exposure, the five rabbits examined displayed no more than
minor changes in the liver and no evidence of carcinoma (Lewis et
al., 1979). Exposure of mice to 728 mg/m3 (200 ppm), similarly
administered for 48 weeks, produced damage to the liver, especially
in females, but no carcinoma (Griffin et al., 1984). It is possible
that degenerative changes observed in the livers of exposed females
may have been precursors to subsequent tumour development. A lower
concentration of 2-NP, 360 mg/m3 (100 ppm), similarly
administered to ICR mice for 18 months, induced liver damage in the
form of nodular hyperplasia in females but no increase in
hepatocellular carcinoma (Coulston et al., 1986; Griffin et al.,
1987). The latter study is the only inhalation study in a species
other than the rat that was of sufficient duration to allow for
latency in tumour development. No studies of carcinogenicity using
oral dosing in any species except the rat have been reported. Thus,
despite the absence of clear evidence, the possibility of
carcinogenicity in species other than the rat cannot be discounted.
There appear to be no laboratory studies on species other than
rats, mice and rabbits suitable for assessing the potential
carcinogenicity of 2-NP. As discussed in section 8.2.2, there is no
evidence from limited epidemiological studies that 2-NP is
carcinogenic in humans.
Griffin & Coulston (1983) argued that 2-NP does not act as an
initiating carcinogen in the rat, but rather induces cancer as a
response to extensive liver damage (Angus Chemical Co., 1985). They
offered as evidence the results of an experiment demonstrating the
absence of long-term effects from short-term exposure to 2-NP
(Griffin et al., 1986). Exposure of rats of both sexes to 730 g/m3
(200 ppm), 7 h/day for 5 days, did not produce any effect on
longevity or on the development of hepatocellular or other
carcinomas up to the end of the experiment, which lasted for 94
weeks. This argument is markedly weakened by the observation of
Andrae et al. (1988) and others (Fiala et al., 1989; George et al.,
1989; Guo et al., 1990; Conaway et al., 1991) that 2-NP is an active
genotoxic agent in rat hepatocytes both in vitro and in vivo.
7.6 Pharmacological effects
In vivo pharmacological studies have not been performed.
However in vitro studies with guinea-pig tissues suggest that 2-NP
may have several pharmacological effects. A 0.1 µM (8.9 µg/litre)
solution inhibited oxygen consumption of polymorphonuclear
leucocytes by 50% (Estes & Gast, 1960). These authors further
reported that, with increasing concentrations of 2-NP, respiration
of heart homogenate was initially depressed and subsequently
stimulated.
2-NP also produces two opposite neural effects on smooth muscle
preparations (Bergman et al., 1962). Contraction is induced partly
by ganglionic stimulation and partly by direct liberation of a
transmitter from nerve endings, but at the same time 2-NP inhibits
the response to acetylcholine and other smooth muscle stimulants.
Bergman et al. (1962) found that the action of nitroparaffins on
smooth muscle was similar to that of nicotine in that their ability
to induce contraction was inhibited by morphine and by high, but not
low, concentrations of atropine.
8. EFFECTS ON HUMANS
8.1 General population exposure
As indicated in section 5.1, general population exposure to 2-NP
appears to be very low, and there is no information on the effects
of this exposure. 2-NP has been used as a component of paints and
other consumer products. There is no evidence that exposure to 2-NP
from the use of such products has resulted in detectable injury or
illness in the general population.
8.2 Occupational exposure
8.2.1 Acute toxicity
Significant human exposure to 2-NP is largely or entirely
occupationally related. High concentrations are acutely toxic and
seven industrial fatalities have been attributed to inhalation of
2-NP fumes (Gautier et al., 1964; Hine et al., 1978; Rondia, 1979;
Harrison et al., 1985, 1987; US NIOSH, 1987b). In all cases,
exposure was to a solvent mixture containing 2-NP, was for a total
of 5 to 16 h in the course of 1 to 3 days, and occurred while paints
or coatings were being applied in confined spaces, such as tank
interiors, underground vaults and ship's holds, with little or no
ventilation. The actual concentrations of 2-NP that caused deaths
were not measured, but were thought to be high and in one case
estimated at 2.18 g/m3 (600 ppm), as determined by GC-FID from the
2-NP content of the victim's blood (0.013 g/litre) (Harrison et al.,
1987). Initial symptoms requiring medical treatment appeared during
exposure or within a few hours following exposure and included
headache, nausea, dizziness, drowsiness, weakness, anorexia,
vomiting, diarrhoea, and neck, thoracic, and abdominal pain. Victims
were hospitalized and in general initially showed improvement, in
some cases to the point of being discharged after a day or less. But
improvement, where present, was temporary and in all seven cases was
followed within a few days by worsening condition and
rehospitalization of those previously discharged. Later symptoms
included persistent nausea, vomiting, anorexia, jaundice, reduced
urine output, diarrhoea, bloody stools, mental confusion,
restlessness, loss of reflexes, and increases in serum
aminotransferases and other indicators of hepatic lesions. Death
occurred within 4 to 26 days (average, 10 days) following exposure.
In all cases the primary cause was acute hepatic failure.
Contributing factors included lung oedema, gastrointestinal
bleeding, and respiratory and kidney failure. Postmortem microscopic
examination of the liver revealed necrosis of hepatic tissue and in
some cases fatty degeneration. None of the victims had a past
history of liver disease or drank excessively.
In addition to these fatalities, there have been four additional
serious but nonlethal cases attributed to acute exposure to 2-NP
(Gautier et al., 1964; Hine et al., 1978; Harrison et al., 1985,
1987; US NIOSH, 1987b). All but one were colleagues of the deceased
described above, but may have received lesser doses. Initial
symptoms were similar to those of the deceased, but were followed by
full recovery rather than decline and death. Serum enzyme levels,
however, remained mildly elevated for months following exposure. As
in the case of the deceased, the actual concentrations of 2-NP to
which these workers were exposed are unknown. One of the survivors
had a 2-NP serum concentration of 8.5 mg/litre when hospitalized.
Since his coworker hospitalized at the same time with a serum
concentration of 13 mg/litre subsequently died, Harrison et al.
(1987) speculated that either 2-NP has a very steep dose-response
curve or that there are substantial differences in individual
susceptibility. Six men (out of a group of approximately 300)
developed toxic hepatitis after exposure to an epoxy resin coating
containing 2-NP (Williams et al., 1974). The development of toxic
hepatitis was ascribed to methylenedianiline in the coating, and the
possibility that 2-NP may have been at least a partial cause was not
considered.
Exposure to lower concentrations of 2-NP appears to produce some
of the symptoms described above. Brief and intermittent exposure to
concentrations above 364 mg/m3 (100 ppm) may produce headache and
nausea (Angus Chemical Co. & Occusafe, Inc., 1986). Exposure to
vapours (estimated to contain 73 to 164 mg/m3 (20 to 45 ppm) using
colorimetric methods available at the time of the study) of a
solvent mixture that polluted the workplace air produced a daily
cycle in symptoms (Skinner, 1947). Workers started the day feeling
well, but by noon some exhibited nausea and lack of appetite. Later
in the working day these symptoms intensified and were accompanied
by vomiting and diarrhoea. Vomiting continued after the workers left
the workplace, and they were unable to eat supper but were able to
sleep. By morning they were feeling well again. Colleagues not
experiencing nausea and vomiting had occipital headaches, which
appeared and gradually intensified during the working day. All of
the workers were free of symptoms when away from the workplace for a
day or more, and the substitution of methyl ethyl ketone for 2-NP
brought complete relief. Skinner (1947) noted that in another plant
exposure of workers to 36-109 mg/m3 (10-30 ppm) for less than
4 h/day on not more than 3 days per week produced no noticeable ill
effects.
The flaw in all but one (Hine et al., 1978) of these case
studies is that exposure was to a solvent mixture containing 2-NP
and not to 2-NP alone (Hine et al., 1978). Thus the observed effects
cannot be assigned to 2-NP with total confidence. A number of
factors, however, point to 2-NP as the main, if not sole, causative
agent. It was the only solvent common to all the mixtures, and the
only solvent present in the mixtures known to be hepatotoxic.
Symptoms and damaging effects on the body from the solvent mixtures
were fairly similar to each other despite widely different solvent
compositions (apart from the common presence of 2-NP), and were
fairly similar to those in the single case resulting from exposure
to 2-NP alone. In addition, as described above, substitution of
another solvent for 2-NP eliminated all symptoms. The weight of
evidence thus supports the view that the symptoms and damage to the
human body described above which followed exposure to solvent
mixtures containing 2-NP, resulted mainly, if not entirely, from
2-NP.
8.2.2 Effects of long-term exposure
Despite the fact that 2-NP has been in use for over 40 years,
there are very few data on long-term effects and such information is
derived mainly from unpublished reports from manufacturers of 2-NP.
Angus Chemical Co., a major manufacturer of 2-NP, has assembled
information on more than 1800 occupationally exposed workers from a
variety of plants in the USA, Mexico, Germany, Sweden, and the
Netherlands (Table 8). Where information was available, medical
records on living employees indicated no problems with liver
functions and no obvious symptoms or conditions that could be
connected with chronic exposure to 2-NP (Angus Chemical Company and
Occusafe, Incorporated, 1986).
A major portion of the above investigation was an earlier
mortality study conducted by Miller & Temple (1979) on 1481 workers
employed in the manufacture of 2-NP during the period 1955 to 1977.
The exposures were defined as direct, indirect or zero exposure.
Formal industrial hygiene monitoring was not performed until 1977.
Individual exposures were based on job titles rather than actual
exposure data. Angus Chemical Company and Occusafe, Incorporated
(1986) concluded that analysis of these data did not suggest any
unusual cancer or other disease pattern in this group of workers.
They noted, however, that because the cohort was small and the
duration of exposure and observation was relatively short, it was
not possible to conclude from these data that 2-NP is not
carcinogenic to humans. In a follow-up report on the same cohort
(Bolender, 1983), the findings did not change the earlier
conclusion.
It is necessary to emphasize that this cohort had a limited
number of workers with long exposure (> 15 years). Since the
individual exposure data are not available, it cannot be concluded
from available data that 2-NP is non-carcinogenic in humans.
Table 8. Studies of long-term occupational exposure to 2-nitropropane
Activitya Exposure concentrationb Exposure duration No. of exposed Reference
mg/m3 (ppm) (years) employees
TWA STEL
Manufacture of 2-NP, 25-91 > 218 < 1-15 55 Angus Chem. Co. & Occusafe, Inc. (1986)
1940-1955 (7-25) (> 60) average 2.5
Manufacture of 2-NP, 3.6-36 91-5970 < 5-> 21 804 Angus Chem. Co. & Occusafe, Inc. (1986);
1946-1982, (1-10) (25-1640) average 8c Miller & Temple (1979); Bolender (1983)
Sterlington, LA, USA
Extraction of 3.6-193 109-473 < 5-ca. 25 18-19 Angus Chem. Co. & Occusafe, Inc. (1986);
triglycerides, USA (1-53; (30-130) Crawford et al. (1985); Tabershaw
average 9c) Occupational Medicine Assoc. (1980);
Life Extension Inst. (1983)
Automobile assembly unknown < 5-> 25 456 Angus Chem. Co. & Occusafe, Inc. (1986)
plant, Germany
Paint mfg., Germany unknown 5-10 6 Angus Chem. Co. & Occusafe, Inc. (1986)
Paint mfg., Germany 40-91 11-15 5 Angus Chem. Co. & Occusafe, Inc. (1986)
(11-25)
Chemical mfg., unknown 5-10 80 Angus Chem. Co. & Occusafe, Inc. (1986)
Germany
Chemical mfg., unknown < 5 4 Angus Chem. Co. & Occusafe, Inc. (1986)
Germany
Extraction plant, 3.6-18.2 73-364 5-10 15 Angus Chem. Co. & Occusafe, Inc. (1986)
Sweden (1-5) (20-100)
Table 8 (contd)
Activitya Exposure concentrationb Exposure duration No. of exposed Reference
mg/m3 (ppm) (years) employees
TWA STEL
Paint mfg., unknown 5-10 180 Angus Chem. Co. & Occusafe, Inc. (1986)
Netherlands
Chemical Co., USA 3.6-36 65.6-364 < 5-20 41 Angus Chem. Co. & Occusafe, Inc. (1986)
(1-10) (18-100)
Printing Co., USA 1.8-87 2.5-124 ? 140 Angus Chem. Co. & Occusafe, Inc. (1986)
(0.5-24) (0.7-34)
Paint mfg., Mexico 3.6 < 91 5-10 100 Angus Chem. Co. & Occusafe, Inc. (1986)
(1) (< 25)
Ink mfg., Mexico 11-18 73-80 5-10 8 Angus Chem. Co. & Occusafe, Inc. (1986)
(3-5) (20-22)
Coatings mfg., 14.6-91 237-251 < 5-20 30 Angus Chem. Co. & Occusafe, Inc. (1986)
Mexico (4-25) (65-69)
Chemical distilation, 36-55 > 91 < 5-10 2 Angus Chem. Co. & Occusafe, Inc. (1986)
Mexico (10-15) (> 25)
Paint mfg., USA unknown < 20 150 Angus Chem. Co. & Occusafe, Inc. (1986)
Automotive mfg., USA 3.6-36 142 < 18 10 Angus Chem. Co. & Occusafe, Inc. (1986)
(1-10) (39)
a mfg. = manufacturing; co. = company
b TWA = time weighted average; STEL = short-term exposure level; values in parentheses are in ppm
c This average is an approximation
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
The limited data on the effects of 2-NP on organisms other than
mammals is summarized below. Kido et al. (1975) reported that 2-NP
(5 g/litre) inhibited the growth of 10 of the 14 species of
microorganisms tested, although all 14 species contained enzymes
capable of oxidizing 2-NP to acetone and nitrite (see section 4.2).
Organisms inhibited by 2-NP included bacteria (Escherichia coli,
Pseudomonas iodinum), yeasts (Endomyces fibuliger, Hansenula
anomala, H. octospora, H. sauveolens, H. matritensis), and fungi
(Aspergillus niger, Penicillium oxalicum, Fusarium oxysporum).
Organisms capable of growing on the medium containing 2-NP similarly
included bacteria (Sarcina lutea, Brevibacterium protophormiae), a
yeast (Hansenula mrakii), and a fungus (Rhizopus batatas).
Fridman et al. (1976) reported that the minimum concentration of
2-NP inhibiting the growth of E. coli and Staphylococcus aureus
was 1000 mg/litre. Observations on aquatic macroorganisms appear
more limited than those on microorganisms. The lowest concentration
of 2-NP in sea water inducing narcosis in barnacle larvae at 18-22
°C was approximately 0.7-0.8 g/litre (Crisp et al., 1967). At 22 °C
the 96-h LC50 for fathead minnows (Pimephales promelas) was >
210 mg/litre (Curtis et al., 1980; Curtis & Ward, 1981). Aeration of
the holding tanks during the toxicity test produced flawed results
because 2-NP was lost continuously by volatilization and a nominal
highest concentration of 612.5 mg/litre was reduced to 496 mg/litre
at the start of the test and to 210 mg/litre by 96 h. There was no
significant mortality at concentrations of 2-NP below 210 mg/litre
although at lower concentrations it did produce severe (though
undescribed) sublethal effects. Observations on non-mammalian
terrestrial organisms are limited to two related species, the
oriental fruit fly (Dacus dorsalis) and the Mediterranean fruit
fly (Ceratitis capitata). Burditt et al. (1963) found that 2-NP
was not especially effective as a fumigant against eggs and larvae
of these species. The 2-h LC50 values for eggs and larvae of the
oriental fruit fly were > 103 and 75 g/m3 (> 28 300 and
20 600 ppm), respectively, and for the Mediterranean fruit fly, > 103
and 35 g/m3 (> 28 300 and 9600 ppm), respectively. These
observations, in summary, suggest a fairly low acute toxicity to
non-mammalian organisms.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Human health risks
Although there are no known biological sources of 2-NP, very low
level non-occupational exposure must be almost universal because
2-NP is known to be a minor component of tobacco smoke and probably
is present in smoke from other types of nitrate-rich organic matter.
Residues in food products containing fatty acids separated with 2-NP
and in beverage can linings and other coatings may represent
additional sources of exposure to low (µg) amounts of 2-NP. The
effects, if any, on human health of low-level exposure are unknown.
Sources of worker exposure include rotogravure and flexographic
inks used in printing, and coatings and adhesives used in industrial
construction and maintenance, highway markings, ship building and
maintenance, furniture manufacture, and food packaging. Based on a
survey conducted in the USA in 1980, occupational exposure to 2-NP
appeared to be limited to a fraction of 1% of the workforce, and
significant occupational exposure (exposure to > 9.1 mg/m3 (>
2.5 ppm)) to about 0.005% of the workforce. Thus, tens of thousands
of workers may have received significant occupational exposure
worldwide. The effects of this occupational exposure are unclear.
Very few industrial fatalities have been attributed to 2-NP. All
involved acute exposure to high concentrations of vapours from
solvent mixtures containing 2-NP during applications of coatings in
confined spaces; all deaths resulted primarily from hepatic failure.
Exposure to non-lethal concentrations of 2-NP may result in
temporary illness or discomfort, but there is no case history or
epidemiological evidence that the chemical induces cancer or other
long-term harmful effects in humans. On the other hand, the numbers
of individuals studied and the periods since first exposure to 2-NP
were inadequate to detect possible long-term harmful effects.
Animal data has demonstrated that 2-NP is a strong
hepatocarcinogen in rats. Chronic exposure to high but non-lethal
concentrations (at least 0.36 g/m3, 100 ppm) induced a high
frequency of hepatic tumours and hepatic cancer in rats but these
observations were not reproduced in limited studies on mice and
rabbits. The mechanism by which 2-NP induces cancer in rats has not
been elucidated as yet. 2-NP is strongly genotoxic to rat
hepatocytes both in vitro and in vivo. It appears likely that
the hepatocarcinogenicity of the compound in rats is a consequence
of liver-specific formation of reactive products. The DNA-damaging
species has not yet been identified. Since it can induce liver cell
proliferation, 2-NP may also have tumour-promoting activity.
Similarities or differences between metabolic activation of 2-NP in
rats and in humans are largely unknown. At present, any substance
shown to be carcinogenic in any mammalian species is considered a
potential human carcinogen. Accurate risk assessment from exposure
to 2-NP requires further studies.
10.2 Effects on the environment
2-NP does not represent a threat to the environment. It appears
highly mobile in the natural environment and is not accumulated in
any individual compartment. It is likely that 2-NP will be destroyed
by photolysis when exposed in the atmosphere to sunlight, and by
biological processes in soil and water. There are, however,
insufficient experimental and observational data on the behaviour of
2-NP in the environment to validate these assumptions or to estimate
rates of degradation in nature. Limited observations on
microorganisms, invertebrates, and fish show a low level of acute
toxicity to non-mammalian organisms.
11. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
There are no indications that 2-NP is carcinogenic in humans.
However, in view of its carcinogenicity in rats, it is recommended
that occupational exposure and its presence in consumer products
such as paints and varnishes be minimized and that it be replaced
with a less toxic solvent whenever practical. Monitoring of the
workplace should be continued to control actual worker exposure.
Although it is not feasible to eliminate non-occupational exposure,
such exposure should be minimized. 2-NP should not be used in food
processing.
12. FURTHER RESEARCH
12.1 Environment
Although it appears likely that 2-NP is highly mobile in the
environment, is not accumulated in any environmental compartment,
and is degraded in the environment to less toxic substances by
microorganisms and ultraviolet radiation, it would be desirable to
confirm experimentally these assumptions and to quantify the speed
of degradation in various environmental compartments.
12.2 Epidemiology
Cohorts of workers in production plants as well as cohorts of
workers using products containing 2-NP (e.g., inks, paints) should
be investigated for incidence of cancer and other ill effects.
12.3 Toxicokinetics
A more detailed examination of the metabolism and distribution
in the body of 2-NP and its metabolites is needed to facilitate an
understanding of the marked species and sex differences in toxic
effects. Studies on distribution and metabolism should include
metabolites of both the carbon and the nitrogen moieties.
Experimental data should be obtained on dermal uptake. Studies on
the metabolism of 2-NP in human cells are needed to facilitate the
eventual extrapolation of data obtained with laboratory animals to
humans.
12.4 Carcinogenesis
Continued efforts should be made to elucidate the biochemical
and molecular mechanisms whereby 2-NP induces genotoxic effects and
cancer.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
2-NP was evaluated by the Joint FAO/WHO Expert Committee on Food
Additives in 1979 and 1981, but no ADI was allocated. The Committee
recommended that 2-NP should not be used as a solvent in food
processing, but it was temporarily acceptable for use as a
fractionating solvent in the production of fats and oils (FAO/WHO,
1984). 2-NP was re-evaluated by JECFA in 1989; it was considered to
be a potent liver carcinogen in rats, and the temporary acceptance
for use as a fractionating solvent in the production of fats and
oils was not extended (FAO/WHO, 1990a, 1990b). The European Economic
Community found 2-NP to be flammable and to be harmful by
inhalation, ingestion, and dermal contact. It required that member
states ensure that it receives proper packaging, labelling, and
storage (IRPTC, 1986).
The International Agency for Research on Cancer evaluated 2-NP
in 1981 (IARC, 1982) and concluded that there was sufficient
evidence for its carcinogenicity in rats but that epidemiological
information was inadequate to evaluate its carcinogenicity to
humans. 2-NP is classified in Group 2B, i.e. as possibly
carcinogenic to humans (IARC, 1987).
REFERENCES
ACGIH (1986) Documentation of the threshold limit values and
biological exposure indices, 5th ed. Cincinnati, Ohio, American
Conference of Governmental Industrial Hygienists, Inc. pp 441-442.
Andersson K, Levin J-O, & Nilsson C-A (1983) Evaluation of solid
sorbents for sampling aliphatic and aromatic nitro compounds.
Chemosphere, 12: 377-384.
Andersson K, Levin J-O, Lindahl R, & Nilsson C-A (1984) Influence of
air humidity on sampling efficiency of some solid adsorbents used
for sampling organics from work-room air. Chemosphere, 13: 437-444.
Andrae U, Homfeld H, Vogl L, Lichtmannegger J, & Summer KH (1988)
2-Nitropropane induces DNA repair synthesis in rat hepatocytes
in vitro and in vivo. Carcinogenesis, 9: 811-815.
Angus Chemical Company (1985) Technical data sheet: NiPar S-20TM
nitropropane solvent, TDS 20B. Northbrook, Illinois, Angus Chemical
Company, 12 pp.
Angus Chemical Company & Occusafe, Incorporated (1986) Industrial
hygiene and occupational health information on 2-nitropropane,
December 1982, and updates for December 1983, 1984, 1985.
Northbrook, Illinois, Angus Chemical Company, 39 pp and 46
attachments (Unpublished report presented to the ACGIH TLV
Committee).
Anon (1976) Grande Paroisse looks for growth in nitroparaffins
market. Eur Chem News, 6 August: 23-24, 30.
Anon (1982) International mineral and chemical's nitroparaffins
business will be acquired by Alberto Natural Gas (Canada) and
Pacific Gas Transmission (US) for up to $ 55 million. Chem Week, 7
April: 24,251.
Baker PJ & Bollmeier AF (1981) Nitroparaffins. In: Grayson M &
Eckroth D ed. Kirk-Othmer encyclopedia of chemical technology, 3rd
ed. New York, John Wiley and Sons, vol 15, pp 969-987.
Baker HJ, Lindsey JR, & Weisbroth SH (1979) Appendix 1. Selected
normative data. In: Baker HJ, Lindsey JR, & Weisbroth SH ed. The
laboratory rat. Volume I: Biology and diseases. Orlando, Florida,
Academic Press, append 1.
Baldwin RS & Williams R (1977) 2-Nitropropane LC50. Northbrook,
Illinois, International Minerals and Chemical Corporation, 6 pp
(Unpublished report No. PLR-218/AMR-072).
Bauchinger M, Kulka U, & Schmid E (1987) Analysis of cytogenetic
effect in human lymphocytes induced by metabolically activated
2-nitropropane. Mutat Res, 190: 217-219.
Beall LR, Alexander V, Bien CT, Chen C, & Infante P (1980) Health
hazard alert - 2-Nitropropane. Cincinnati, Ohio, National Institute
for Occupational Safety and Health, 8 pp (Publication No. 80-142).
Bergman F, Chaimovitz M, & Wind E (1962) Dual action of
nitroparaffins on the guinea-pig ileum. Brit J Pharmacol,
18: 381-396.
Bernard AM, De Russis R, Normand J-C, & Lauwerys RR (1989)
Evaluation of the subacute nephrotoxicity of cyclohexane and other
industrial solvents in the female Sprague-Dawley rat. Toxicol Lett,
45: 271-280.
Bolender FL (1983) 2-NP mortality epidemiology study of the
Sterlington, LA employees: An update, 1-1-46 thru 12-31-81.
Northbrook, Illinois, International Minerals and Chemical
Corporation, 24 pp (Unpublished report).
Breazile JE (1971) Text book of veterinary physiology. Philadelphia,
Pennsylvania, Lea and Febiger, p 347.
Brown D & Dobbin R (1977) Industrial hygiene survey report. IMC,
Sterlington, Louisiana. Cincinnati, Ohio, National Institute for
Occupational Safety and Health, 14 pp.
Burditt AK, Hinman FG, & Balock FG (1963) Screening of fumigants for
toxicity to eggs and larvae of the oriental fruit fly and
Mediterranean fruit fly. J Econ Entomol, 56: 261-265.
Burrows GE (1979) Methylene blue or tolonium chloride antagonism of
sodium nitrite induced methemoglobinemia. J Vet Pharmacol Ther,
2: 81-86.
Cocalis JC (1982) Mining target environmental surveillance study.
Nitropropane sensitized, ammonium nitrate blasting agents.
Morgantown, West Virginia, National Institute for Occupational
Safety and Health, Environmental Investigations Branch, Division of
Respiratory Diseases Studies, 25 pp.
Conkle JP, Camp BJ, & Welch BE (1975) Trace composition of human
respiratory gas. Arch Environ Health, 30: 290-295.
Conaway CC, Nie G, Hussain NS, & Fiala ES (1991a) Comparison of
oxidative damage to rat liver DNA and RNA by primary nitroalkanes,
secondary nitroalkanes, cyclopentanone oxime, and related compounds.
Cancer Res, 51: 3143-3147.
Conaway CC, Nie G, Hussain NS, & Fiala ES (1991b) Evaluation of
secondary nitro-alkanes, their nitronates, primary nitroalkanes,
nitrocarbinols, and other aliphatic nitro compounds in the Ames
Salmonella assay. Mutat Res, 261: 197-207.
Coulston F (1982) Influence of glutathione or diethyl maleate in the
induction of hepatocellular carcinomas in rats exposed to 200 ppm of
2-nitropropane. Alamogordo, New Mexico, Coulston International
Corporation, 14 pp (Unpublished report).
Coulston F, Stein AA, & Griffin TB (1985) Pathology final report.
Histologic study of selected tissues from rats exposed to
2-nitropropane at 100 ppm. Alamogordo, New Mexico, Coulston
International Corporation, 222 pp.
Coulston F, Griffin T, & Eason R (1986) Chronic inhalation exposure
of mice to 2-nitropropane (100 ppm). Alamogordo, New Mexico,
Coulston International Corporation, 5 pp (Unpublished report).
Crawford GN, Garrison RP, & McFee DR (1984) Odor threshold
determination for 2-nitropropane. Am Ind Hyg Assoc J, 45: B7-B8.
Crawford GN, Garrison RP, & McFee, DR (1985) Health examination and
air monitoring evaluation for workers exposed to 2-nitropropane. Am
Ind Hyg Assoc J, 46: 45-47. Crisp DJ, Christie AO, & Ghobashy AFA
(1967) Narcotic and toxic action of organic compounds on barnacle
larvae. Comp Biochem Physiol, 22: 629-649.
Cunningham ML & Matthews HB (1991) Relationship of
hepatocarcinogenicity and hepatocellular proliferation induced by
mutagenic noncarcinogens vs carcinogens. Toxicol Appl Pharmacol,
110: 505-513.
Cupitt LT (1980) Fate of toxic and hazardous materials in the air
environment. Research Triangle Park, North Carolina, US
Environmental Protection Agency, 28 pp (EPA-600/3-80-084).
Curtis MW & Ward CH (1981) Aquatic toxicity of forty industrial
chemicals: Testing in support of hazardous substance spill
prevention regulation. J Hydrol, 51: 359-367.
Curtis MW, Curran CM, & Ward CH (1980) Aquatic toxicity testing as
fundament for a spill prevention program. In: Proceedings of the
1980 Conference on Control of Hazardous Material Spills. Nashville,
Tennessee, Vanderbilt University, pp 284-288.
Dayal R, Gescher A, Harpur ES, Pratt I, & Chipman JK (1989)
Comparison of the hepatotoxicity in mice and the mutagenicity of
three nitroalkanes. Fundam Appl Toxicol, 13: 341-348.
Dayal R, Goodwin B, Linhart I, Mynett K, & Grescher A (1991)
Oxidative denitrification of 2-nitropropane and propane-2-nitronate
by mouse liver microsomes: lack of correlation with hepatic
potential. Chem Biol Interact, 79(1): 103-114.
De Bruin A (1976) Biochemical toxicology of environmental agents.
Amsterdam, Oxford, New York, Elsevier Science Publishers, p 104.
Denk B, Bauman M, & Filser JG (1989) Pharmacokinetics and
hepatotoxicity of 2-nitropropane in rats. Arch Toxicol, 13(suppl):
330-332.
Denk B, Filser JG, Demi E, Kessler W, Shen J, & Oesterle D (1990)
Dose-dependent emergence of preneoplastic foci in rat livers after
exposure to 2-nitropropane. Arch Toxicol, 64: 329-331.
Dequidt J, Vasseur P, & Potencier J (1972) Etude toxicologique
expérimentale de quelques nitroparaffines : 1. Etude du
2-nitropropane. Bull Soc Pharm Lille, 1972: 83-89.
Derks HJGM, Van Heiningen A, Klaassen R, & Olling M (1988) High
performance liquid chromatographic method for the determination of
2-nitropropane in rat plasma. J Chromatogr, 442: 436-440.
Derks HJGM, Van Heiningen A, Klaassen R, & Olling M (1989)
Toxicokinetics of 2-nitropropane in the rat. Bilthoven, The
Netherlands, National Institute of Public Health and Environmental
Protection, 19 pp (Report No. 328605.003).
De Rycker J & Halliwell B (1978) Oxidation of 2-nitropropane by
horseradish peroxidase. Biochem J, 175: 601-606.
Dhawale MR & Hornemann U (1979) Nitroalkane oxidation by
Streptomycetes. J Bacteriol, 137: 916-924.
Estes FL & Gast JH (1960) The in vitro effects of aliphatic nitro
compounds on tissues. Arch Environ Health, 1: 59-64.
FAO/WHO (1984) Evaluation of certain food additives and
contaminants. Twenty-eighth report of the Joint FAO/WHO Expert
Committee on Food Additives. Geneva, World Health Organization,
44 pp (WHO Technical Report Series 710).
FAO/WHO (1990a) Evaluation of certain food additives and
contaminants. Thirty-fifth report of the Joint FAO/WHO Expert
Committee on Food Additives. Geneva, World Health Organization,
48 pp (WHO Technical Report Series 789).
FAO/WHO (1990b) Toxicological evaluation of certain food additives
and contaminants, prepared by the 35th meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA). Geneva, World Health
Organization, 189 pp (WHO Food Additives Series 26).
Fiala ES, Czerniak R, & Williams GM (1986) Formation of carcinogenic
and/or genotoxic aliphatic azoxy compounds from the respective
nitroalkanes by direct mild chemical reduction. Toxicologist,
6: 40.
Fiala ES, Conaway CC, Biles WT, & Johnson B (1987a) Enhanced
mutagenicity of 2-nitropropane nitronate with respect to
2-nitropropane possible involvement of free radical species. Mutat
Res, 179: 15-22.
Fiala ES, Czerniak R, Castonguay A, Conaway CC, & Rivenson A (1987b)
Assay of 1-nitropropane, 2-nitropane, 1-azoxypropane and
2-azoxypropane for cancinogenicity by gavage in Sprague-Dawley rats.
Carcinogenesis, 8: 1947-1949.
Fiala ES, Conaway CC, & Mathis JE (1989) Oxidative DNA and RNA
damage in the livers of Sprague-Dawley rats treated with the
hepatocarcinogen 2-nitropropane. Cancer Res, 49: 5518-5522.
Fieser LF & Fieser M (1972) Reagents for organic synthesis. New
York, Wiley-Interscience, vol 3, pp 213-214.
Fieser LF & Fieser M (1974) Reagents for organic synthesis. New
York, Wiley-Interscience, vol 4, p 256.
Filser JG & Baumann M (1988) Pharmokinetics of 2-nitropropane in
rats and determination of enzymes in serum specific for liver
injury. Naunyn Schmiedeberg's Arch Pharmacol, 337(suppl): R17.
Fine DH, Challis BC, Hartman P, & Van Ryzin J (1982) Endogenous
synthesis of volatile nitrosamines: model calculations and risk
assessment. In: N-nitroso compounds: Occurrence and biological
effects. Proceedings of the VIIth International Symposium on
N-Nitroso Compounds, Tokyo, 28 September-1 October 1981. Lyon,
International Agency for Research on Cancer, pp 379-396 (IARC
Scientific Publications No. 41).
Finklea JF (1977) Current NIOSH intelligence bulletin:
2-nitropropane. Am Ind Hyg Assoc J, 38(7): A-15, A-17, A-19.
Fishbein L (1981) Carcinogenicity and mutagenicity of solvents. I.
Glycidyl ethers, dioxane, nitroalkanes, dimethylformamide and allyl
derivatives. Sci Total Environ, 17: 97-110.
Flanner LT (1972) 2-Nitropropane or nitromethane for inhibiting the
corrosion of tin-plated steel aerosol cans: US Patent US 3650982
(21 March 1972). USA, Allied Chemical Corporation, 2 pp (Abstract
only).
Freitag D, Geyer H, Kraus A, Viswanathan R, Kotzias D, Attar A,
Klein W, & Korte F (1982) Ecotoxicological profile analysis. VII.
Screening chemicals for their environmental behavior by comparative
evaluation. Ecotoxicol Environ Saf, 6: 60-81.
Freitag D, Ballhorn L, Geyer H, & Korte F (1985) Environmental
hazard profile of organic chemicals. Chemosphere, 14: 1589-1616.
Friedman MA, Greene EJ, & Epstein SS (1972) Rapid gastric absorption
of sodium nitrite in mice. J Pharm Sci, 61: 1492-1494.
Friedman AL, Zalesov VS, Surkov VD, Kratynskaya LV, & Plaksina AN
(1976) [Synthesis and study of the physiological activity of
aliphatic nitro compounds. X. Relation among structure, toxicity,
and antimicrobial activity in a series of nitroalkanes and their
alpha-halo derivatives.] Khim-Farm Zh, 10: 53-56 (in Russian).
Galloway SM, Armstrong MJ, Reuben C, Colman S, Brown B, Cannon C,
Bloom AD, Nakamura F, Ahmed M, Duk S, Rimpo J, Margolin BH, Resnick
MA, Anderson B, & Zeiger E (1987) Chromosome aberrations and sister
chromatid exchanges in Chinese hamster ovary cells: Evaluations of
108 chemicals. Environ Mol Mutagen, 10(suppl 10): 1-175.
Gautier M, Fournier P-E, Gervais P, & Sicot C (1964) Intoxication
par le nitropropane. Arch Mal Prof, 25: 425-428.
George E, Burlinson B, & Gatehouse D (1989) Genotoxicity of 1- and
2-nitropropane in the rat. Carcinogenesis, 10: 2329-2334.
Glaser RA, & Woodfin WJ (1981) A method for sampling and analysis of
2-nitropropane in air. Am Ind Hyg Assoc J, 42: 18-22.
Goggelmann W, Bauchinger M, Kulka U, & Schmid E. (1988) Genotoxicity
of 2-nitropropane and 1-nitropropane in Salmonella typhimurium and
human lymphocytes. Mutagenesis, 3: 137-140.
Goldwhite H (1965) Nitrogen derivatives of the aliphatic
hydrocarbons. In: Coffey S ed. Rodd's chemistry of carbon compounds,
Part B, 2nd ed. Amsterdam, Oxford, New York, Elsevier Science
Publishers, vol. 1, pp 93-164.
Griffin TB & Coulston F (1983) Final report. Chronic inhalation
exposure of rats to vapors of 2-nitropropane at 100 ppm. Alamogordo,
New Mexico, Coulston International Corporation, 13 pp.
Griffin TB, Benitz K-F, Coulston F, & Rosenblum I (1978) Chronic
inhalation toxicity of 2-nitropropane in rats. Pharmacologist, 20:
145.
Griffin TB, Coulston F, & Stein AA (1980) Chronic inhalation
exposure of rats to vapors of 2-nitropropane at 25 ppm. Ecotoxicol
Environ Saf, 4: 267-281.
Griffin TB, Stein AA, & Coulston F (1981) Histologic study of
tissues and organs from rats exposed to vapors of 2-nitropropane at
25 ppm. Ecotoxicol Environ Saf, 5: 194-201. Griffin T, Coulston F, &
Stein AA (1984) Chronic inhalation exposure of mice to
2-nitropropane. Preliminary report: Laboratory findings and
histopathology of liver. Alamogordo, New Mexico, Coulston
International Corporation, 14 pp (Unpublished report).
Griffin T, Coulston F, & Stein AA (1986) Long-term effects of acute
exposure of rats to 2-nitropropane. Final Report, Study No. 820202.
Alamogordo, New Mexico, Coulston International Corporation, 17 pp
(Unpublished report).
Griffin T, Coulston F, & Stein AA (1987) Chronic inhalation exposure
of mice to 2-nitropropane (100 ppm): Final report. Alamogordo, New
Mexico, Coulston International Corporation, 24 pp (Unpublished
report).
Guo N, Conaway CC, Hussain NS, & Fiala ES (1990) Sex and organ
differences in oxidative DNA and RNA damage due to treatment of
Sprague-Dawley rats with acetoxime or 2-nitropropane.
Carcinogenesis, 11: 1659-1662.
Haas-Jobelius M, Ziegler-Skylakakis K, & Andrae U (1991)
Nitroreduction is not involved in the genotoxicity of 2-nitropropane
in cultured mammalian cells. Mutagenesis, 6: 87-91.
Hardin BD, Bond GP, Sikov MR, Andrew FD, Beliles RP, & Niemeier RW
(1981) Testing of selected workplace chemicals for teratogenic
potential. Scand J Work Environ Health, 7(suppl 4): 66-75.
Harris SJ, Bond GP, & Niemeier RW (1979) The effects of
2-nitropropane, naphthalene, and hexachlorobutadiene on fetal rat
development. Toxicol Appl Pharmacol, 48: A35.
Harrison RJ, Pasternak G, Blanc P, Basuk P, & Letz G (1985) Acute
hepatic failure after occupational exposure to 2-nitropropane. J Am
Med Assoc, 254(24): 3415-3416.
Harrison RJ, Letz G, Pasternak G, & Blanc P (1987) Fulminant hepatic
failure after occupation exposure to 2-nitropropane. Ann Intern Med,
107: 466-468.
Hartle RW (1980) Health hazard evaluation report No. 80-057-781 at
Long Island Railroad, Richmond Hill, New York, Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 17 pp.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, 5 (suppl 1): 3-142.
Hine CH, Pasi A, & Stephens BG (1978) Fatalities following exposure
to 2-nitropropane. J Occup Med, 20: 333-337.
Hite M & Skeggs H (1979) Mutagenic evaluation of nitroparaffins in
the Salmonella typhimurium/mammalian microsome test and the
micronucleus test. Environ Mutagen, 1: 383-389.
Hoar RM (1976) Chapter 3. Biomethodology. In: Wagner JE & Manning PJ
ed. The biology of the guinea pig. New York, London, Academic Press,
p 16.
Hoffmann D & Rathkamp C (1968) Chemical studies on tobacco smoke.
III. Primary and secondary nitroalkanes in cigarette smoke. Beitr
Tabakforsch, 4: 124-134.
Hollett BA & Schloemer J (1978) Hazard evaluation technical
assistance report No. TA 76-90: Newport Industrial Products
Firestone Tire Rubber Co., Newport, Tennessee. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 52 pp.
Hughes TJ, Simmons DS, Monteith LG, Myers LD, & Claxton LD (1987)
Mutagenicity of 31 organic compounds in a modified preincubation
Ames assay with Salmonella typhimumium strains TA100 and TA102.
Environ Mutagen, 9 (suppl 8): 49.
Hussain NS, Conaway CC, Guo N, Asaad W, & Fiala ES (1990) Oxidative
DNA and RNA damage in rat liver due to acetoxime: similarity to
effects of 2-nitropropane. Carcinogenesis, 11: 1013-1016.
Indig GL & Cilento G (1987) Peroxidase-promoted aerobic oxidation of
2-nitropropane: Mechanism of excited state formation. Biochim
Biophys Acta, 923: 347-354.
IARC (1982) 2-Nitropropane. In: Some industrial chemicals and
dyestuffs. Lyon, International Agency for Research on Cancer, pp
331-343 (IARC Monographs on the Evaluation of the Carcinogenic Risk
of Chemicals to Humans, Volume 29).
IARC (1987) Overall evaluations of carconogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer, p 67 (IARC Monographs on the Evaluation of the
Carconogenic Risk of Chemicals to Humans, Supplement 7).
IRPTC (1986) IRPTC data profile on 2-nitropropane. Geneva,
International Register of Potentially Toxic Chemicals, United
Nations Environment Programme, pp 17/1-17/3.
Ivanetich KM, Lucas S, Marsh JA, Ziman MR, Katz ID, & Bradshaw JJ
(1978) Organic compounds. Their interaction with and degradation of
hepatic microsomal drug-metabolizing enzymes in vitro. Drug Metab
Disp, 6: 218-225.
Jones LR (1963) The determination of 2-nitropropane in air. Ind Hyg
J, January-February: 11-16.
Jones LR & Riddick JA (1952) Colorimetric determination of
nitroparaffins. Anal Chem, 24: 1533-1536.
Jonsson J, Kammerer RC, & Cho AK (1977) Metabolism of
2-nitro-1-phenylpropane by rabbit liver microsomes. Res Commun chem
Pathol Pharmacol, 18: 75-82.
Kaplan HM, Brewer NR, & Blair WH (1983) Physiology. In: Foster HL,
Small JD, & Fox JG ed. The mouse in biomedical research. Volume III
Normative biology, immunology, and husbandry. New York, London,
Academic Press, p 255.
Kawai A, Goto S, Matsumoto Y, & Matsushita H (1987) [Mutagenicity of
aliphatic and aromatic nitro compounds. Industrial materials and
related compounds.] Jpn J ind Health, 29: 34-54 (in Japanese).
Kenaga EE (1980) Predicted bioconcentration factors and soil
sorption coefficients of pesticides and other chemicals. Ecotoxicol
environ Saf, 4: 26-38.
Kido T, Yamamoto T, & Soda K (1975) Microbial assimilation of alkyl
nitro compounds and formation of nitrite. Arch Microbiol, 106:
165-169.
Kido T, Tanizawa K, Inagaki K, Yoshimura T, Ishida M, Hashizume K, &
Soda K (1984) 2-Nitropropane dioxygenase from Hansenula mrakii:
Re-characterization of the enzyme and oxidation of anionic
nitroalkanes. Agric Biol Chem, 48: 2549-2554.
Kliesch U & Adler I-D (1987) Micronucleus test in bone marrow of
mice treated with 1-nitropropane, 2-nitropropane and cisplatin.
Mutat Res, 192: 181-184.
Korte F, Freitag D, Geyer H, Klein W, Kraus AG, & Lahaniatis E
(1978) Ecotoxicologic profile analysis. Chemosphere, 1: 79-102.
Kozma C, Macklin W, Cummins LM, & Mauer R (1974) Chapter 3. Anatomy,
physiology, and biochemistry of the rabbit. In: Weisbroth SH, Flatt
RE, & Kraus AL ed. The biology of the laboratory rabbit. New York,
London, Academic Press, pp 56-57.
Krotoszynski BK, Bruneau GM, & O'Neill HJ (1979) Measurement of
chemical inhalation exposure in urban population in the presence of
endogenous effluents. J Anal Toxicol, 3: 225-234.
Kuo FK & Fridovich (1986) Free-radical chain oxidation of 2-NP
initiated and propagated by superoxide. Biochem J, 237: 505-510.
Levy A (1973) The photochemical smog reactivity of organic solvents.
Adv Chem Ser, 124: 70-94.
Lewis TR, Ulrich CE, & Busey WM (1979) Subchronic inhalation
toxicity of nitromethane and 2-nitropropane. J Environ Pathol
Toxicol, 2: 233-249.
Life Extension Institute (1983) Summary report of medical findings
for selected employees at one plant. Rockville, Maryland, Life
Extension Institute, 12 pp.
Little HN (1951) Oxidation of nitroethane by extracts from
Neurospora. J Biol Chem, 193: 347-358.
Little HN (1957) The oxidation of 2-nitropropane by extracts of pea
plants. J Biol Chem, 229: 231-238.
Litton Bionetics, Inc. (1977) Mutagenicity evaluation of P-1357
66459T: Final report. Terre Haute, Indiana, IMC Chemical Group,
Inc., 10 pp (Unpublished report).
Löfroth G, Nilsson L, & Andersen JR (1986) Structure-activity
relationship of nitroalkane-induced mutagenicity in the Ames
Salmonella assay. In: Genetic toxicology of environmental
chemicals. Part B: Genetic effects and applied mutagenesis. New
York, Alan R. Liss, Inc., pp 149-155.
Lotz F, Nitz S, & Korte F (1979) [Photomineralization of adsorbed
organic chemicals on a microscale.] Chemosphere, 10: 763-768 (in
German).
Love JR & Kern M (1981) Health hazard evaluation report no.
81-065-938. Metro bus maintenance shop, Washington, DC. Cincinnati,
Ohio, National Institute for Occupational Safety and Health, 13 pp.
Mabuchi H (1979) [Urinary and serum volatile substances in
diabetics.] Nippon Naika Gakkai Zasshi, 58: 731-743 (Abstract only)
(in Japanese).
McGregor DB (1981) Tier II mutagenic screening of 13 NIOSH priority
compounds. Individual compound report: 2-Nitropropane. Musselburgh,
Scotland, Inveresk Research International Ltd, 194 pp (Report No.
31).
Machle W, Scott EW, & Treon J (1940) The physiological response of
animals to some simple mononitroparaffins and to certain derivatives
of these compounds. J Ind Hyg Toxicol, 22: 315-332.
Malkinson FD & Gehlmann L (1977) Factors affecting percutaneous
absorption. In: Drill VA & Lazar P, ed. Cutaneous toxicity. New
York, London, Academic Press, pp 63-82.
Marcstrom A (1967) Studies on the connection between physicochemical
properties and stimulating abilities of some sweet and bitter
compounds. Ark Zool, 19: 421-535.
Marker EK & Kulkarni AP (1985) Enzymatic denitrification of
2-nitropropane in uninduced mouse liver microsomes. Toxicol Lett,
26: 181-185.
Marker EK & Kulkarni AP (1986a) Cumene hydroperoxide-supported
denitrification of 2-nitropropane in uninduced mouse liver
microsomes. Int J Biochem, 18: 595-601.
Marker EK & Kulkarni AP (1986b) Cytochrome P-450-mediated
denitrification of 2-nitropropane in mouse liver microsomes. J
Biochem Toxicol, 1: 71-83.
Miller ME & Temple GW (1979) 2-NP mortality epidemiology study of
the Sterlington, LA Employees, 1-1-46 thru 6-30-77. Northbrook,
Illinois, International Minerals and Chemical Corporation, 34 pp
(Unpublished report).
Mochizuki H, Kominami S, & Takemori S (1988) Examination of
differences between benzo[ a]pyrene and steroid hydroxylases in
guinea pig adrenal microsomes. Biochem Biophys Acta, 964: 83-89.
Müller WF, Coulston F, & Korte F (1983) Comparative metabolism of
2-nitropropane in rats and chimpanzees. Chemosphere, 12: 231-237.
National Fire Protection Association (1968) Hazardous chemicals
data. J Chem Educ, 45: A313-A314, A317-A318, 320-322.
National Library of Medicine (1989) Toxic chemical release
inventory. Washington, DC, National Library of Medicine (TOXNET
system, TRI data base, 12 June 1989).
Nielsen AT (1981) Nitronic acids and esters. In: Feurer H ed. The
chemistry of the nitro and nitroso groups. Huntington, New York,
Robert Krieger Publishing Co., Part I, pp 349-386.
Nolan RJ, Unger SM, & Muller CJ (1982) Pharmacokinetics of inhaled
[14C]-2-nitropropane in male Sprague-Dawley rats. Ecotoxicol
Environ Saf, 6: 388-397.
Occupational Health Services, Inc. (1982) An evaluation of the
number of workers occupationally exposed to 2-nitropropane.
Cambridge, Massachusetts, Occupational Health Services, Inc., 22 pp
(Unpublished report prepared for IMC Corporation, Des Plaines,
Illinois).
Parks NJ, Krohn KA, Mathis CA, Chasko JH, Geiger KR, Gregor ME, &
Peek NF (1981) Nitrogen-13-labeled nitrite and nitrate: Distribution
and metabolism after intratracheal administration. Science, 212:
58-61.
Paszyc S (1971) [Photolysis of 2-nitropropane in gaseous and liquid
phases.] Poznan Tow Przyj Nauk Pr Kom Mat-Przyr, Pr Chem, 12: 79-85
(in Polish).
Patel RN, Hou CT, Laskin AI, & Felix A (1982) Epoxidation of alkenes
and hydroxylation of alkanes by soluble methane monooxygenase:
Regeneration of cofactor NADH2. J Appl Biochem, 4: 175-184.
Porter DJ & Bright HJ (1983) The mechanism of oxidation of
nitroalkanes by horseradish peroxidase. J Biol Chem, 258: 9913-9924.
Purcell RF (1967) Use of 2-nitropropane under rule 66. West Paint
Rev, 53: 22A, 24A.
Reece WO (1984) Respiration in mammals. In: Swenson MJ ed. Duke's
physiology of domestic animals, 10th ed. Cornell, New York, Comstalk
Press, p 231.
Robbiano L, Mattioli F, & Brambilla G (1991) DNA fragmentation by
2-nitropropane in rat tissues, and effects of the modulation of
biotransformation processes. Cancer Lett. 57: 61-66.
Rondia D (1979) 2-nitropropane: One more death. Vet Hum Toxicol, 21:
183-185.
Roscher E, Ziegler-Skylakakis K, & Andrae U (1990) Involvement of
different pathways in the genotoxicity of nitropropanes in cultured
mammalian cells. Mutagenesis, 5: 375-380.
Sadtler SP (1961) Sadtler standard ultraviolet spectra.
Philadelphia, Pennsylvania, Sadtler Research Laboratory, p 1.
Sakurai H, Hermann G, Ruf HH, & Ullrich V (1980) The interaction of
aliphatic nitro compounds with the liver microsomal monooxygenase
system. Biochem Pharmacol, 29: 341-345.
Sander J & Schweinsberg F (1972) [Interrelationships between
nitrate, nitrite and carcinogenic N-nitroso-compounds. 1.
Communication: nitrate, nitrite and nitrosable amino-compounds in
food and drugs, chemistry of N-nitroso compounds.] Zentrabl Bakeriol
Parasitenkd Infektionsk Hyg Abt 1: Orig Reihe B, 156: 299-340 (in
German).
Schneider NR & Yeary RA (1975) Nitrite and nitrate pharmacokinetics
in the dog, sheep, and pony. Am J Vet Res, 36: 941-947.
Scott EW (1943) The metabolism of monoparaffins. III. The
concentration of nitroethane, nitrite and nitrate in the blood of
rabbits during exposure by inhalation and oral administration. J Ind
Hyg Toxicol, 25: 20-25.
Seizinger DC & Dimitrades B (1972) Oxygenates in exhaust from simple
hydrocarbon fuels. J Air Pollut Control Assoc, 22: 47-51.
Simmons DM, Monteith LG, Hughes TJ, & Claxton LD (1986) Effect of
preincubation time on mutagenic activity in the Ames/ Salmonella
assay. Environ Mutagen, 8(suppl 6): 78.
Skinner JB (1947) The toxicity of 2-nitropropane. Ind Med, 16:
441-443.
Smith EL, Hill RL, Lehman IR, Lefkowitz RJ, Handler P, & White A
(1983) Principles of biochemistry. General aspects. New York,
McGraw-Hill, pp 539-541.
Soda K, Kido T, & Asada K (1977) 2-Nitropropane dioxygenase from
Hansenula mrakii: Generation and participation of superoxide anion
in the reaction. In: Hayashi O & Asada K ed. Biochemical and medical
aspects of active oxygen. Baltimore, Maryland, University Park
Press, pp 119-133.
Speck WT, Meyer LW, Zeiger E, & Rosenkranz HS (1982) Mutagenicity
and DNA-modifying activity of 2-nitropropane. Mutat Res, 104: 49-54.
SRI International (1988) 1988 Directory of chemical producers,
United States of America. Menlo Park, California, SRI International,
pp 29, 168, 803.
SRI International (1990) 1990 Directory of chemical producers,
United States of America. Menlo Park, California, SRI International,
p 815.
Stokinger HE (1982) Aliphatic nitro compounds, nitrates, nitrites.
In: Clayton GD & Clayton FE ed. Patty's industrial hygiene and
toxicology, 3rd revised ed. New York, John Wiley and Sons, vol 2C,
pp 4141-4208.
Swann RL, Laskowski DA, McCall PJ, Vander Kuy K, & Dishburger HJ
(1983) A rapid method for the estimation of the environmental
parameters octanol/water partition coefficient, soil sorption
constant, water to air ratio, and water solubility. Residue Rev.
85 : 17-28.
Tabershaw Occupational Medicine Association (1980) Cross-sectional
morbidity study of workers. Rockville, Maryland, Tabershaw
Occupational Medicine Association, 68 pp (Unpublished report).
Treon JF & Dutra FR (1952) Physiological response of experimental
animals to the vapor of 2-nitropropane. Arch Ind Hyg Occup Med,
5 : 52-61.
Ullrich V & Schnabel KN (1973) Formation and binding of carbanions
by cytochrome P-450 of liver microsomes. Drug Metab Disp, 1:
176-183.
Ullrich V, Hermann G, & Weber P (1978) Nitrite formation from
2-nitropropane by microsomal monooxygenases. Biochem Pharmacol,
27 : 2301-2304.
US Bureau of the Census (1987) Statistical abstract of the United
States: 1988, 108th ed. Washington, DC, US Bureau of the Census,
p 368.
US EPA (1977) TSCA chemical assessment series. Chemical hazard
information profiles (CHIPS), August 1976-August 1978. Washington,
DC, US Environmental Protection Agency, pp 212-217
(EPA-560/11-80-011).
US EPA (1980) Materials balance 2-nitropropane, Level 1. Washington,
DC, US Environmental Protection Agency, 70 pp (EPA-560/13-89-011).
US EPA (1985) Health and environmental effects profile for
2-nitropropane. Washington, DC, US Environmental Protection Agency,
pp 3-4 (EPA-600/X-85-112).
US EPA (1986) Notice of potential risk, 2-nitropropane. Washington,
DC, US Environmental Protection Agency, 5 pp.
US FDA (Food and Drug Administration) (1987) 2-Nitropropane. Code
Fed Regul, 27 (Parts 1 to 199): Docket No. 78N-0221.
USITC (1990) Synthetic organic chemicals. United States production
and sales, 1989. Washington, DC, United States International Trade
Commission, pp 15-17, 15-35-15-36 (USITC Publication No. 2338).
US NIOSH (1983) National occupational exposure survey, 1981-83:
Actual observation and trade-name exposure to 2-nitropropane.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health (Unpublished database).
US NIOSH (1987a) NIOSH manual of analytical methods. Second
supplement, 3rd ed. Cincinnati, Ohio, National Institute for
Occupational Safety and Health, pp 2528/1-2528/4 (NIOSH Publication
No. 87-117).
US NIOSH (1987b) Safe sheet #1: Summary of accidental fatality
evaluations, confined spaces, Case #3. Morgantown, West Virginia,
National Institute for Occupational Safety and Health, Environmental
Investigations Branch, Division of Respiratory Disease Studies, p 2.
US NIOSH (1988) Current intelligence bulletins: Summaries, September
1988. Cincinnati, Ohio, National Institute for Occupational Safety
and Health, p 6.
US OSHA (Occupational Safety and Health Administration) (1988)
1-Nitropropane and 2-nitropropane. Code Fed Regul, 29: 705, 708
(Parts 1900 to 1910).
Weast RC ed. (1986) CRC handbook of chemistry and physics, 67th ed.
Boca Raton, Florida, CRC Press, p C-444.
Wester PW, Van Leeuwen FXR, & De Vries T (1989) 4-Week toxicity
study with 2-nitropropane (2-NP) by gavage in rats. Bilthoven, The
Netherlands, National Institute of Public Health and Environmental
Protection, 20 pp (Report No. 658501 001).
Wilbur SZ & Parekh CK (1982) Dermal toxicity potential of
2-nitropropane (P-1357). Northbrook, Illinois, International
Minerals and Mining Corporation, 5 pp (Unpublished report No.
PLR-281/AMR-072).
Wilks RA & Gilbert SG (1972a) Sensory and instrumental evaluation in
model systems of residues migrating from can coatings. J Food Sci,
37: 72-76.
Wilks RA & Gilbert SG (1972b) Quick test for liner solvents. Modern
Packag, May: 52-55.
Williams SV, Bryan JA, Burk JR, & Wolf FS (1974) Toxic hepatitis and
methylenedianiline. New Engl J Med, 291: 1256.
Windholz M ed. (1983) The Merck index, 10th ed. Rahway, New Jersey,
Merck & Co., p 6482.
Woo Y-T, Lai DY, Arcos JC, & Argus MF (1985) Chemical induction of
cancer: structural bases and biological mechanisms, Vol. III B:
Aliphatic and polyhalogenated carcinogens. New York, London,
Academic Press, pp 329-341.
Yasuhara A, Yamanaka Y, & Ogawa T (1986) Volatile compounds in
machine cutting-fluid emulsion. Agric Biol Chem, 50: 1765-1770.
Ziegler-Skylakakis K, Homfeldt H, & Andrae U (1987) In vitro and
in vivo genotoxicity of 2-nitropropane and 1-nitropropane in
mammalian cells. Naunyn-Schmiedeberg's Arch Pharmacol, 335(suppl):
R25.
Zimmering S, Mason JM, Valencia R, & Woodruff RC (1985) Chemical
mutagenesis testing in Drosophila. II. Results of 20 coded
compounds tested for the National Toxicology Program. Environ
Mutagen, 7: 87-100.
Zitting A, Savolainen H, & Nickels J (1981) Acute effects of
2-nitropropane on rat liver and brain. Toxicol Lett, 9: 237-246.
RESUME
1. Propriétés et méthodes d'analyse
Le 2-nitropropane (2-NP) est un liquide huileux, incolore, à
l'odeur douceâtre. Il est inflammable, moyennement volatil et stable
dans les conditions ordinaires. Il n'est que légèrement soluble dans
l'eau mais miscible à de nombreux liquides organiques et c'est un
excellent solvant de nombreux composés organiques. Il existe de
bonnes méthodes d'analyse pour la recherche et le dosage du
2-nitropropane présent dans l'environnement. On a actuellement
recours à la chromatographie en phase gazeuse avec détection par
ionisation de flamme ou capture d'électrons, ou bien à la
chromatographie en phase liquide à haute performance avec détecteur
u.v. Pour le dosage dans l'air, il faut tout d'abord piéger le
2-nitropropane et le concentrer par adsorption sur phase solide.
2. Emploi et sources d'exposition
2.1 Production
Les chiffres de production actuels ne sont pas connus. En 1977
les Etats-Unis d'Amérique en ont produit environ 13 600 tonnes. Le
2-nitropropane est actuellement produit par deux firmes américaines
et une firme française. Il peut prendre naissance naturellement à
l'état de traces lors de la combustion du tabac et d'autres matières
organiques riches en nitrates mais rien n'indique qu'il puisse se
former à l'issue d'un processus biologique quelconque.
2.2 Emploi et passage dans l'environnement
Le 2-nitropropane est utilisé comme solvant, principalement en
mélange, et il a de nombreuses applications industrielles en tant
que tel pour la confection d'encres d'imprimerie, de peintures, de
vernis, d'adhésifs et autres revêtements, comme par exemple ceux que
l'on utilise dans les récipients contenant des boissons. On
l'utilise également comme solvant pour séparer des composés très
voisins comme les acides gras, comme intermédiaire en synthèse
chimique et comme additif dans les carburants. C'est principalement
par l'intermédiaire de l'air qu'il passe dans l'environnement,
surtout par évaporation à partir des surfaces enduites de produits
qui en contiennent.
3. Transport et distribution dans l'environnement
Le 2-nitropropane se révèle très mobile dans le milieu naturel.
Du fait que sa solubilité dans l'eau, son adsorption par les
sédiments et sa bioaccumulation sont faibles et qu'il s'évapore
rapidement dans l'atmosphère, il se répartit dans l'air et l'eau
sans s'accumuler dans un compartiment particulier du milieu. Le
2-nitropropane absorbe le rayonnement ultra-violet aux longueurs
d'onde que l'on rencontre dans l'environnement et il est donc
probable qu'il subisse une lente photolyse dans l'atmosphère. Il est
également probable qu'il soit lentement transformé par voie
biologique en composés moins toxiques, tant dans l'environnement
aquatique que dans l'environnement terrestre.
4. Concentrations dans l'environnement et exposition humaine
Il semble que l'exposition de la population générale au
2-nitropropane soit très faible et provienne de la fumée de
cigarettes (1,1 à 1,2 µg/cigarette), de résidus présents dans des
enduits ou revêtements tels qu'on en utilise pour les récipients
contenant des boissons, dans les adhésifs, les encres d'imprimerie,
ainsi que dans les huiles végétales que l'on fractionne au moyen de
ce solvant. On ignore quelle est l'importance de l'exposition des
travailleurs de l'industrie dans le monde, mais aux Etats-Unis
d'Amérique, il semble qu'elle soit limitée à 0,02-0,19 % du
personnel. Dans ce même pays, environ 4000 travailleurs (soit à peu
près 0,005 % de la main-d'oeuvre) subissent sans doute une
exposition notable (de l'ordre de 9,1 mg/m3(2,5 ppm) ou
davantage). Selon les pays, les limites d'exposition professionnelle
dans l'air vont de 36 mg/m3 (1 ppm) (TWA) à 146 mg/m3 (40 ppm)
(STEL). La production du 2-nitropropane s'effectue en circuit fermé
et en général, le personnel n'est guère exposé; toutefois dans
certaines industries (peinture, imprimerie ou extraction par
solvant), il est arrivé que des travailleurs soient exposés à des
concentrations dépassant largement les limites d'exposition
professionnelle. C'est ainsi que des concentrations atteignant
6 g/m3 (1640 ppm) dans l'air ont été enregistrées lors du
remplissage de fûts.
5. Cinétique et métabolisme
Le 2-nitropropane est principalement résorbé au niveau des
poumons. Chez l'animal de laboratoire, on a montré qu'il était
rapidement absorbé non seulement à ce niveau mais également au
niveau de la cavité péritonéale et des voies digestives. On est mal
renseigné sur son absorption percutanée. Quant aux données
concernant sa distribution dans l'organisme du rat, elles sont
quelque peu contradictoires. Le 2-nitropropane est rapidement
métabolisé, essentiellement sous forme d'acétone et de nitrite. Il
se forme peut-être aussi un peu d'alcool isopropylique. Après
injection par voie intrapéritonéale, le 2-nitropropane et ses
métabolites carbonés se concentrent d'abord dans les graisses, puis
dans la moelle osseuse ainsi que dans les surrénales et les autres
organes internes; après inhalation, ils se concentrent dans le foie
et les reins, mais relativement peu dans les graisses. Il est
possible que plusieurs systèmes enzymatiques interviennent dans ce
métabolisme et les vitesses, de même que les voies de
métabolisation, varient selon les espèces. Le 2-nitropropane et ses
métabolites carbonés disparaissent rapidement de l'organisme par
métabolisation, exhalation ou excrétion dans les urines et les
matières fécales. On manque de données satisfaisantes sur la
distribution et l'excrétion de la fraction nitrée de ces
métabolites.
6. Effets sur les mammifères de laboratoire et les systèmes
in vitro
La toxicité aiguë du 2-nitropropane pour les mammifères est
modérée. Les mâles sont plus sensibles que les femelles, tout au
moins en ce qui concerne le rat, et l'expérience montre que la
sensibilité varie largement d'une espèce à l'autre. La CL50
(concentration produisant une mortalité de 50 % en 14 jours) a été
de 1,5 g/m3 (400 ppm) pour les rats mâles et de 2,6 g/m3
(720 ppm) pour les rats femelles. Il semble que la mortalité soit
associée principalement aux effets narcotiques, mais les mammifères
exposés à des concentrations d'au moins 8,4 g/m3 (2300 ppm)
pendant une heure ou davantage ont présenté des anomalies
anatomopathologiques graves, notamment des lésions
hépatocellulaires, un oedème du poumon et des hémorragies.
Il est indiscutable que le 2-nitropropane est cancérogène pour
le rat. L'exposition de rats pendant de longues durées à du
2-nitropropane par voie respiratoire à raison de 0,36 g/m3
(100 ppm) (pendant 18 mois à raison de sept heures par jour et cinq
jours par semaine) a causé des lésions destructrices au niveau du
foie, et en particulier des carcinomes hépatocellulaires chez
certains rats mâles. A la concentration de 0,75 g/m3 (207 ppm) les
lésions étaient encore plus graves, avec une forte incidence de
carcinomes hépatocellulaires à survenue plus rapide.
L'administration chronique de doses modérées par voie orale a
également provoqué une surincidence de carcinomes hépatocellulaires.
Toutefois, l'inhalation prolongée par des rats de 2-nitropropane aux
doses respectives de 91 ou 98,3 mg/m3 (25 ou 27 ppm) n'a pas
provoqué de lésions décelables. L'exposition de souris et de lapins
à des concentrations de 2-nitropropane capables de provoquer des
carcinomes hépatocellulaires chez le rat n'a guère eu d'effets chez
ces animaux, mais il est vrai que les études en question étaient
trop limitées pour qu'on puisse exclure totalement un effet
cancérogène du 2-nitropropane chez ces deux espèces. Le
2-nitropropane a légèrement retardé le développement foetal des rats
mais les données relatives à l'embryotoxicité, à la tératogénicité
et à la toxicité du 2-NP pour la fonction de reproduction restent
très fragmentaires. Le produit s'est révélé fortement génotoxique
pour les hépatocytes de rats tant in vivo qu' in vitro; en
revanche aucune génotoxicité sensible n'a été observée au niveau des
autres organes chez le rat ou sur des lignées cellulaires d'origine
extrahépatique, en l'absence d'activation métabolique exogène. On a
également montré que le 2-nitropropane était mutagène chez les
bactéries en présence ou en l'absence d'activation métabolique
exogène.
7. Effets sur l'homme
L'exposition humaine à de fortes concentrations de
2-nitropropane est largement, voire totalement d'origine
professionnelle. A concentration élevée, le 2-nitropropane présente
une forte toxicité aiguë et il a provoqué des accidents mortels dans
l'industrie - encore qu'on ignore la valeur exacte de cette
concentration, sauf dans un cas où on a pu estimer l'exposition à
2184 mg/m3, soit 600 ppm. Les premiers symptômes consistaient en
céphalées, nausées, somnolence, vomissements, diarrhées et douleurs.
Malgré une amélioration temporaire de l'état général, la mort est
quelquefois survenue dans les 4 à 26 jours suivant l'exposition. La
cause initiale de la mort était une insuffisance hépatique à
laquelle s'ajoutaient un oedème du poumon, des hémorragies des voies
digestives et une insuffisance respiratoire et rénale. A des doses
évaluées à 73-164 mg/m3 (20 à 45 ppm) on a constaté que
l'exposition professionnelle provoquait des nausées et une perte
d'appétit pouvant persister plusieurs heures après le départ du lieu
de travail, aucun effet indésirable n'étant observé après exposition
à des doses de 36,4 à 109 mg/m3 (10 à 30 ppm) (moins de quatre
heures par jour pendant une durée inférieure ou égale à trois jours
par semaine).
Malgré l'insuffisance des données disponibles, rien n'indique
qu'une exposition professionelle de longue durée au 2-nitropropane
aux concentrations généralement présentes sur les lieux de travail
puisse provoquer des cancers du foie ou d'autres organes, ni plus
généralement des effets indésirables à longue échéance.
8. Effets sur les autres organismes au laboratoire et dans
leur milieu naturel
Les quelques études effectuées sur des microorganismes, des
invertébrés et des poissons montrent que le 2-nitropropane est peu
toxique pour les organismes non mammaliens.
RESUMEN
1. Propiedades y métodos analíticos
El 2-nitropropano (2-NP) es un líquido incoloro y oleoso de olor
ligero. Es inflamable, moderadamente volátil, y estable en
condiciones normales. Es sólo ligeramente hidrosoluble pero miscible
con numerosos líquidos orgánicos, y es un excelente disolvente para
muchos tipos de compuestos orgánicos. Existen métodos analíticos
adecuados para identificar y medir el 2-NP en concentraciones
ambientales. Los métodos de uso corriente son la cromatografía de
gases y un detector de ionización de llama o de captura electrónica;
también se usa la cromatografía líquida de alto rendimiento con
detector de ultravioleta. Para medirlo en el aire, primero es
necesario capturarlo y concentrarlo en un sorbente sólido.
2. Usos y fuentes de exposición
2.1 Producción
No se dispone de cifras recientes de producción mundial. En
1977, la producción en los Estados Unidos fue de aproximadamente
13 600 toneladas. Actualmente, el 2-NP se fabrica en dos empresas
estadounidenses y una francesa. Se origina por mecanismos naturales
como trazas en la combustión del tabaco y de otras sustancias
orgánicas ricas en nitratos, pero nada indica que se origine en
procesos biológicos.
2.2 Usos y pérdidas al medio ambiente
El 2-NP se utiliza como disolvente, principalmente en mezclas, y
tiene numerosas aplicaciones industriales como disolvente para
tintas de impresión, pinturas, barnices, adhesivos y otros
revestimientos como los de recipientes de bebidas. Se ha utilizado
asimismo como disolvente para separar sustancias estrechamente
relacionadas como ácidos grasos, como intermediario en síntesis
químicas, y como aditivo en combustibles. Las pérdidas al medio
ambiente ingresan principalmente en el aire y se deben sobre todo a
la evaporación del disolvente a partir de superficies revestidas.
3. Transporte y distribución en el medio ambiente
El 2-NP parece tener gran movilidad en el medio ambiente
natural. Dada su baja solubilidad en el agua, escasa absorción por
el sedimento, reducida bioacumulación y facil evaporación a la
atmósfera, se distribuye tanto en el aire como en el agua y no se
acumula en ningún compartimento ambiental definido. La fotoabsorción
ultravioleta del 2-NP se encuentra en la escala de frecuencias
normales en el medio ambiente, por lo que es probable que la
sustancia sea objeto de fotólisis lenta en la atmósfera. La
biotransformación lenta del 2-NP a compuestos menos tóxicos también
parece probable en los medios acuático y terrestre.
4. Niveles ambientales y exposición humana
La exposición de la población general al 2-NP parece ser muy
baja y se debe al humo de cigarrillos (1,1 a 1,2 µg/cigarrillo), a
los residuos en revestimientos, como los de las latas de bebidas,
adhesivos y material impreso, y a los aceites vegetales fraccionados
con esa sustancia. Se desconoce la exposición industrial a escala
mundial, pero en los Estados Unidos parece limitarse al 0,02-0,19%
de la población trabajadora. Los niveles de exposición de cierta
importancia (exposición a 9,1 mg/m3 (2,5 ppm) o más) en los
Estados Unidos probablemente no afectan más que a unos 4000
trabajadores (aproximadamente 0,005% de la población trabajadora).
Los límites de exposición ocupacional en el aire varían de un paíse
a otro y van desde 3,6 mg/m3 (1 ppm) (promedio ponderado en
función del tiempo) a 146 mg/m3 (40 ppm) (STEL). La fabricación
del 2-NP es un proceso cerrado y entraña por lo general una
exposición reducida de los trabajadores, pero algunos obreros de
industrias como la pintura, la impresión y la extracción de
disolventes se han visto expuestos en otras épocas a niveles muy
superiores a los límites de exposición ocupacional. Durante ciertas
operaciones industriales han llegado a registrarse concentraciones
de hasta 6 g/m3 (1640 ppm) en el aire.
5. Cinética y metabolismo
En el ser humano, la absorción de 2-NP se produce principalmente
por los pulmones. En animales de experimentación, se ha demostrado
que el 2-NP se absorbe rápidamente no sólo por vía pulmonar sino
también a partir de la cavidad peritoneal y del tracto
gastrointestinal. No se dispone de datos satisfactorios sobre la
absorción por vía cutánea. La información sobre la distribución en
la rata es ligeramente contradictoria. El 2-NP se metaboliza
rápidamente, principalmente a acetona y nitrito. También puede
formarse isopropil alcohol en pequeñas cantidades. Tras la inyección
intraperitoneal, el 2-NP y sus metabolitos carbonados se concentran
inicialmente en la grasa y después en la médula ósea, así como en
las glándulas suprarrenales y otros órganos internos. Tras la
inhalación, el 2-NP y sus metabolitos carbonados se concentran en el
hígado y el riñón; la cantidad que se acumula en la grasa es
relativamente pequeña. Varios sistemas enzimáticos diferentes pueden
participar y existen diferencias de unas especies a otras en cuanto
a la velocidad y las rutas metabólicas. El 2-NP y sus metabolitos
carbonados desaparecen rápidamente del organismo por transformación
metabólica, exhalación y excreción en orina y heces. No se dispone
de datos satisfactorios sobre la distribución y la excreción de
metabolitos que llevan el radical nitro.
6 Efectos en mamíferos de laboratorio y sistemas in vitro
El 2-NP tiene una toxicidad aguda moderada para los mamíferos.
Los machos son más sensibles que las hembras, por lo menos en la
rata, y la sensibilidad difiere ampliamente entre las especies que
se han ensayado. La CL50 (concentración que causa una mortalidad
del 50% en un plazo de 14 días) para la rata tras una exposición de
6 horas fue de 1,5 g/m3 (400 ppm) en los machos y 2,6 g/m3
(720 ppm) en las hembras. La letalidad parecía asociada
principalmente a los efectos narcóticos, si bien los mamíferos
expuestos a concentraciones de al menos 8,4 g/m3 (2300 ppm)
durante una hora o más mostraron alteraciones patológicas graves,
entre ellas lesiones hepatocelulares, edema pulmonar y hemorragia.
Existen pruebas claras de que el 2-NP es carcinogénico en la
rata. La exposición prolongada de ratas por inhalación de
0,36 g/m3 (100 ppm) durante 18 meses (7 h/día, 5 días/semana)
indujo cambios destructivos en el hígado, inclusive carcinomas
hepatocelulares en algunos machos. Una concentración de 0,75 g/m3
(207 ppm) indujo lesiones más graves, entre ellas una elevada
incidencia de carcinomas hepatocelulares, con más rapidez. La
administración crónica de dosis orales moderadas indujo también un
exceso de carcinomas hepatocelulares en ratas. En cambio, la
inhalación prolongada de 91 o 98,3 mg/m3 (25 ó 27 ppm) no produjo
lesiones detectables en las ratas. La exposición de ratones y
conejos a concentraciones de 2-NP que inducían carcinomas
hepatocelulares en la rata tuvieron escaso efecto o ninguno, pero
esos estudios eran demasiado limitados para descartar por completo
la carcinogenicidad de la sustancia en ambas especies. El 2-NP
retrasó ligeramente el desarrollo fetal en la rata, pero escasean
los datos sobre embriotoxicidad, teratogenicidad y toxicidad
reproductiva. Se observó que el 2-NP era sumamente genotóxico en
hepatocitos de rata tanto in vitro como in vivo, pero no se
observó genotoxicidad significativa en otros órganos de la rata ni
en líneas celulares de origen extrahepático sin activación
metabólica exógena. Se ha demostrado que el 2-NP es mutagénico en
bacterias tanto en presencia como en ausencia de activación
metabólica exógena.
7. Efectos en el ser humano
La exposición humana a concentraciones elevadas de 2-NP es en su
mayor parte o totalmente de origen ocupacional. Las concentraciones
elevadas (se desconocen los valores reales, si bien en un caso se
calcularon en 2184 mg/m3 (600 ppm)) producen toxicidad aguda y
accidentes mortales en la industria. Entre los síntomas iniciales
figuran dolores de cabeza, náuseas, mareos, vómitos, diarrea y
dolores. Las víctimas a menudo mostraban una mejoría temporal,
aunque en algunos casos sobrevino la muerte entre 4 y 26 días
despues de la exposición. El fallo hepático fue la principal causa
de muerte, con edema pulmonar, hemorragias gastrointestinales fallo
respiratorio y renal como factores contribuyentes. La exposición
ocupacional a niveles estimados en 73 a 164 mg/m3 (20 a 45 ppm)
indujo náuseas y pérdida de apetito, que persistieron durante varias
horas tras abandonar el lugar de trabajo, mientras que la exposición
ocupacional a niveles estimados en 36,4 a 109 mg/m3 (10 a 30 ppm)
(< de 4 h/día durante < 3 días/semana) no produjo efectos
nocivos detectables.
Aunque los datos disponibles son insuficientes, nada indica que
la exposición ocupacional crónica al 2-NP en concentraciones
normales en el lugar de trabajo induzca neoplasmas hepáticos o de
otro tipo ni otros efectos adversos a largo plazo.
8. Efectos en otros organismos en el laboratorio y sobre el terreno
Los escasos estudios realizados en microorganismos,
invertebrados y peces indican una baja toxicidad del 2-NP para
organismos no mamíferos.
2.3 Conversion factors
1 ppm 2-NP in air = 3.64 mg/m3
1 mg/m3 = 0.27 ppm 2-NP in air
2.4 Analytical methods
Analytical methods for 2-NP appear limited to the analysis of
air, water, blood plasma, coatings, and cigarette smoke (Table 2).
Since the colorimetric methods are less sensitive or are cumbersome,
the method of choice is probably gas chromatography with either a
flame ionization or an election capture detection. Charcoal is not a
satisfactory adsorbent for 2-NP since recovery is poor (Andersson et
al., 1983) and there may be decomposition (Glaser & Woodfin, 1981).
In addition to Chromosorb 106, Amberlite XAD-7 appears satisfactory
as a solid sorbent for quantitatively collecting 2-NP from the air
(Andersson et al., 1983), although its collection efficiency is
markedly reduced in humid air (Andersson et al., 1984). Use of a
collection tube with two sections, however, can compensate for the
reduced efficiency (Andersson et al., 1984). The high-performance
liquid chromatography method developed for blood (Derks et al.,
1988) could probably be adapted to other biological materials.
Table 2. Analytical techniques for determining 2-nitropropanea
Methods Detection limit Comments Reference
Air
Trapping in ethanol; 0.1 mg/ml ethanol absorption is linear between 0.1 and Treon & Dutra (1952)
concentration determined 2.0 mg/ml; ethanol was especially
spectrophotometrically at purified and redistilled
277.5 nm
Trapping in concentrated ca. 1 µg/ml sulfuric absorption is linear between 1 and 5 µg/ml Jones & Riddick (1952);
sulfuric acid; resulting acid sulfuric acid; no interference from primary Jones (1963)
nitrous acid combined nitroparaffins, but all other secondary,
with resorcinol to form a some tertiary and some halogenated
red-blue colour; measured nitroparaffins interfere
spectro-photometrically
at 560 nm
Trapping in solid sorbent tube 3.6 mg/m3 working range is 3.6 to 36 mg/m3; 2-NP Glaser & Woodfin (1981)
(Chromosorb 106, 60/80 mesh); stable on absorbent for at least 28 days
desorption: ethyl acetate;
separation-detection: GC-FID
Methodology similar to above 3.1 mg/m3 Method is a modification of that proposed US NIOSH (1987a)
by Glaser & Woodfin (1981); range: 3.1 to
28.3 mg/m3; no interference from methyl
butyl ketone, heptane, 1-nitropropane,
toluene, and xylene
Table 2 (contd).
Methods Detection limit Comments Reference
Water
Sample (40 µl) collected ca. 0.5 mg/m3 water elutes quickly extinguishing flame Wilks & Gilbert (1972a)
with a syringe and injected in FID; flame can be reignited before 2-NP
directly into GC; emerges
detection: FID
Blood
Blood collected in chilled, 1 ng UV absorption linear from 0 to 250 ng; Derks et al. (1988)
screw-capped vial with heparin; uses 0.3 ml blood sample; samples are
centrifuged; deproteinized with unstable and must be analysed promptly
acetonitrile; Tris buffer added;
separation: HPLC; detection:
UV at 224 nm
Coating (beverage can)
Redissolve coating in solvent not given reference provides few details on Wilks & Gilbert (1972b)
suitably distinct from 2-NP; methodology
acetone suitable for vinyl
co-polymer coatings;
separation: GC
Table 2 (contd).
Methods Detection limit Comments Reference
Coating (beverage can)
Can (empty) simultaneously not given method captures about 90% of Wilks & Gilbert (1972b)
perforated and fitted with a residual 2-NP
diaphragm; hypodermic needle
inserted through diaphragm and
fitted with stopcock; can heated
(150 °C, 15 min); headspace
sampled with heated syringe;
separation: GC
Cigarette smoke
Steam distillation of smoke 0.8 µg/cigarette may be adapted for air and water Hoffmann & Rathkamp
condensate on filters, analysis (1968)
extraction re-extraction in
NaOH, in ethyl ether,
neutralization with H2SO4 and
re-extraction in ethyl ether,
concentration of extract and
injection in GC equipped with
FID or ECD detectors
a Abbreviations: ECD = electron capture detector; FID = flame ionization detector; GC = gas chromatograph;
HPLC = high-performance liquid chromatograph; UV = ultraviolet
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
There is no evidence that 2-NP and other nitroaliphatic
compounds are produced by biological processes, although a related
organic compound, ß-nitropropionic acid, has been isolated from
plants and microorganisms (Goldwhite, 1965). However, nitroaliphatic
compounds are produced in low concentrations by combustion of
organic matter and have been detected in tobacco smoke. Hoffmann &
Rathkamp (1968) reported 1.1-1.2 µg 2-NP in the smoke from single
85-mm USA blended non-filter cigarettes, and ascribed the production
of this and other nitroaliphatics to interactions in the combustion
zone between hydrocarbons and nitrogen dioxide generated by
decomposition of nitrates.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
Although 2-NP is an important industrial chemical, current world
production figures are not available. In 1977 production by the sole
USA manufacturer was estimated to be 13 600 tonnes, of which 5400
tonnes were sold in the USA and 8200 tonnes were either exported or
used internally (Finklea, 1977). 2-NP currently is produced by two
USA manufacturers, Angus Chemical Co., Sterlington, Louisiana, and
W. R. Grace Co., Deer Park, Texas (SRI International, 1988, 1990;
USITC, 1990), and by one European manufacturer, Société Chimique de
la Grande Paroisse, France (Anon., 1976, 1982; IARC, 1982). In the
USA, 2-NP, together with nitromethane, nitroethane and
1-nitropropane, is manufactured by a vapour phase reaction of nitric
acid with an excess of propane at high temperature and pressure
(370-450 °C, 0.8-1.2 MPa (8-12 atm.)) (Baker & Bollmeier, 1981). The
proportions of the four nitroaliphatic compounds in the reaction
product are a function of the reaction temperature. In Europe,
propane is reacted with nitrogen peroxide (N2O4) and an excess
of oxygen at 150-330 °C and 0.9-1.2 MPa (9-12 atm.), yielding the
same nitroaliphatic compounds in slightly different proportions as
produced by the USA process (Anon., 1976). Reaction products are
condensed, washed, and separated by fractional distillation. There
is no evidence that 2-NP is produced through human EHC 138:
2-Nitropropane activities except by combustion and deliberate
manufacture, although nitromethane has been detected in vehicle
exhaust (Seizinger & Dimitrades, 1972).
3.2.2 Uses
The importance of 2-NP as an industrial chemical stems mainly
from its desirable and occasionally unique characteristics as a
solvent (Purcell, 1967; Anon., 1976; Fishbein, 1981; Baker &
Bollmeier, 1981; IARC, 1982; ACGIH, 1986). It is an excellent
solvent or cosolvent for a variety of fats, waxes, gums, resins,
dyes and other organic compounds, including vinyl, acrylic,
polyamide and epoxy resins, chlorinated rubbers, and organic
cellulose esters. The ability of 2-NP to form an azeotrope with
water and the associated large heat of absorption permit it to
displace monomolecular layers of water molecules and secure a better
bond between pigments and the surfaces to which they are applied.
Its major use is as a solvent for inks, paints, varnishes, adhesives
and other coatings such as beverage container linings. It is used
principally in blends with other solvents to impart desirable
characteristics, such as greater solvency, better flow
characteristics and film integrity, greater pigment dispersion,
increased wetting ability, improved electrostatic spraying
properties, or reduced drying time. 2-NP is also used industrially
as a processing solvent for separating closely related substances in
natural products or reaction mixtures. These have included, for
example, separation of oleic acid from polyunsaturated fatty acids
and cetyl from oleyl alcohols.
In addition to the above, 2-NP has a number of minor uses
(Anon., 1976; Baker & Bollmeier, 1981). These include a medium for
chemical reactions, an intermediate for the manufacture of
2-nitro-2-methyl-1-propanol, 2,2-dinitropropane, 2-amino-2-
methyl-1-propanol and other propane derivatives, and a component of
explosives, propellants, and fuels for internal combustion engines.
The latter usage appears limited to model engines used by hobbyists
and to racing cars. Although the addition of 2-NP to fuel improves
diesel engine performance, it is not used commercially as a diesel
fuel additive since superior alternatives are available (Banes,
1989)a. In the USA, mixed isomers of nitropropane are used to
denature ethanol (US FDA, 1987). The addition of 2-NP to hydrocarbon
mixtures has been shown to inhibit corrosion of tin-plated steel
aerosol cans (Flanner, 1972).
3.3 Release into the environment
There is some quantitative data on releases of 2-NP into the
environment. The US Environmental Protection Agency has supported a
thorough, though largely speculative, analysis of the problem (US
EPA, 1980). Releases of 2-NP occur mainly into the atmosphere and
can result from spillage, from venting of gases and fugitive
emissions during manufacture, transfer and use, and from solvent
evaporation from coated surfaces. The US EPA document estimated that
of the 14 000 tonnes of 2-NP produced in the USA in 1979, 5714
tonnes (41%) was released into the air, and 1 tonne into water. Only
230 tonnes (1.6%) was destroyed by incineration or waste treatment.
The major contributor to this release estimate was evaporation of
2-NP used as a solvent in printing ink and surface coatings (4450
a Personal communication from the US Environmental Protection
Agency, Ann Arbor, Minneapolis
tonnes, 78% of releases). Manufacture of 2-NP is a largely enclosed
process and in 1979 it accounted for only 21 tonnes (0.3%) of the
amount released into the environment. A more recent examination of
this problem (National Library of Medicine, 1989) reported a similar
situation concerning environmental releases of 2-NP in the USA. Out
of a yearly total of 299 tonnes, 205 tonnes (69%) was released into
the air with 123 tonnes coming from point (large, easily identified)
sources and 82 tonnes from non-point (small, not easily identified)
sources. Only 2 tonnes (< 1%) was released directly into water, and
1 tonne into municipal sewage treatment plants. The remainder was
buried in closed containers (76 tonnes, 25%) or was disposed of in
unspecified ways (15 tonnes, 5%).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport in the environment
2-NP appears to be highly mobile in the natural environment.
Cupitt (1980) considered physical removal of 2-NP from the
atmosphere unlikely because it was not soluble enough to be rapidly
washed out and had a vapour pressure too great for strong adsorption
on particles. The partition of 2-NP between air and water at
equilibrium was estimated by the method of Swann et al. (1983) to be
about 0.5% in air and 99.5% in water (US EPA, 1985). These values
indicate rapid and easy exchange between air and water. The soil
sorption coefficient (ratio of soil concentration to water
concentration) and the bioconcentration factor were estimated by the
methods of Kenaga (1980) to be 20 and 2.5, respectively (US EPA,
1985). Measured values for absorption and bioaccumulation were
somewhat greater than these estimates. Freitag et al. (1982, 1985)
obtained concentration factors over water for activated sludge,
unicellular algae (Chlorella fusca), and fish (golden ide) of 70,
20, and < 10, respectively. These values indicate that 2-NP is not
strongly bioaccumulated and is readily desorbed from sediment
particles and leached from soil. Thus, in summary, since 2-NP is
slightly water soluble, slightly adsorbed by sediment, slightly
bioaccumulated, and evaporates readily into the atmosphere, it will
be distributed in both air and water and not accumulated in any
individual environmental compartment.
4.2 Biotic and abiotic transformation
Data concerning the destruction of 2-NP by biotic and abiotic
processes are limited. 2-NP has significant photoabsorption in the
environmentally relevant range of > 290 nm (Sadtler, 1961) and is
likely to undergo photolysis (Cupitt, 1980; US EPA, 1985). On the
basis of physical and chemical properties Cupitt (1980) hypothesized
that it would be rapidly removed from the atmosphere by photolysis
and estimated a reduction in concentration of 1/e (0.369) in 0.2
days. This is equivalent to a half-life of 0.14 days (3.36 h).
However, measurements of photochemical reactivity do not support
such a rapid destruction of 2-NP. Studies aimed at defining the
relationship between organic solvents and photochemical smog
production ranked 2-NP as low to moderate in terms of its
interactions with oxidants and its ability to produce formaldehyde
and other lachrymators (Levy, 1973). Laboratory measurements also
suggested slow decomposition (Freitag et al., 1985). The methodology
employed (Korte et al., 1978; Lotz et al., 1979; Freitag et al.,
1982), i.e. irradiation of the solvent adsorbed on silica gel by
light from a high pressure mercury lamp filtered through pyrex, was
too different from natural conditions to permit quantitative
extrapolation from laboratory results to rates of photolysis in the
atmosphere. The study provided comparative photodecomposition rates
for a large number of solvents. The rate of photodecomposition for
2-NP was roughly half that of dichlorodiphenyltrichloroethane (DDT),
similar to the rates for dodecane and 2,4-dichlorobenzoic acid, and
roughly twice those for kepone and dieldrin. Paszyc (1971) reported
that the major decomposition products for both gaseous and liquid
2-NP under laboratory conditions were nitrogen dioxide, acetone,
isopropyl nitrite, isopropanol, methylcyanide, water, and propane,
regardless of whether irradiation was monochromatic 253.7 nm light
or the full spectrum of light produced by a high pressure mercury
lamp. Cupitt (1980) speculated that the major products of
photodecomposition in nature would be formaldehyde and acetaldehyde.
Biological decomposition of 2-NP appears likely, but is probably
rather slow in nature. Enzymes capable of oxidizing or initiating
non-enzymatic oxidation of 2-NP have been identified in horseradish
(De Rycker & Halliwell, 1978; Porter & Bright, 1983; Indig &
Cilento, 1987), pea seedlings (Little, 1957), and a variety of
microorganisms including bacteria, yeasts, and fungi (Little, 1951;
Kido et al., 1975; Soda et al., 1977; Dhawale & Hornemann, 1979;
Patel et al., 1982). In in vitro preparations of horseradish
(Dhawale & Hornemann, 1979), pea seedlings (Little, 1957), a fungus
(Streptomyces achromogenes) (Dhawale & Hornemann, 1979), and a
yeast (Hansenula mrakii) (Kido et al., 1975), 2-NP was converted
to a less toxic compound, acetone, and a moderately toxic compound,
nitrite. In addition to nitrite, some nitrate may also be formed
(Indig & Cilento, 1987). In the yeast, nitrite was subsequently
reduced to ammonia. The importance of these processes in nature is
unknown. Kido et al. (1975), however, reported that only 4 out of 14
species of microorganisms tested would grow in a medium containing
5 g 2-NP/litre. The only study of 2-NP decomposition by a population
of microorganisms (Freitag et al., 1985) utilized activated sludge
grown at 25 °C. Solvent concentrations in these experiments were low
(50 µg/litre) to prevent adaptation to the substances tested (Korte
et al., 1978). In 5 days only 0.4% of the 2-NP was converted to
carbon dioxide. In summary, these data suggest that in both
terrestrial and aquatic communities 2-NP is biologically decomposed
and that the rate may be slow, but they offer no definite
information on the problem.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels and general population exposure
General population exposure to 2-NP appears to be very low.
There seem to be no records of its occurrence in water or in outdoor
air away from areas of manufacture and use. The only information on
intake exists in the form of a memorandum from Modderman (1983)a.
The daily intake per person in the USA was estimated to be 50 to
100 mg. The residuum from its use as a solvent for beverage can
coatings, film laminating adhesives and printing inks for flexible
food packaging may account for as much as 37 ng/day and from
vegetable oils fractionated with 2-NP, 30 ng/day. 2-NP residues of
0.077 mg/litre (77 ppb) to 0.24 mg/litre (204 ppb) have been found
in such oils. In a further report on the evaluation of
2-nitropropane as a food processing solvent, it was assumed that
residues of less than 10 µg/kg would occur in oils, giving rise to
estimated daily intakes of 10 ng/day. The use of 2-NP as a food
processing solvent was not recommended (FAO/WHO, 1990a,b). As
mentioned in section 3.1, smokers are exposed regularly to low
concentrations of 2-NP. Hoffmann & Rathkamp (1968) reported 1.1 to
1.2 µg in the smoke of a single cigarette. 2-NP was reported to
occur in the expired air of 11.1% (5 of 54 individuals) of a sample
of healthy adult urban dwellers (Krotoszynski et al., 1979). The
geometric mean was 0.406 ng/litre with one-sigma limits of
0.119 ng/litre and 1.38 ng/litre, (at 25 °C, 98 MPa (760 mmHg)). The
sample was entirely of non-smokers who had avoided medication and
prolonged exposure to perfume, paint, glue, aerosols, dust, tobacco
smoke and areas polluted with industrial wastes during, and for at
least 7 days prior to, the sampling period, and also avoided
cosmetics, spices, seasonings, and alcoholic beverages during and
immediately prior to sampling. The origin of the exhaled 2-NP is
unclear. Exposure to 2-NP may be further reduced in the future since
a number of regulations have been enacted and recommendations made
to declare it a harmful, carcinogenic substance and a toxic waste,
and to discourage its use (IARC, 1982; IRPTC, 1986; US FDA, 1987; US
NIOSH, 1988).
a Memorandum from Modderman, J.P. to Shibko, S., Associate
Director for Regulatory Evaluation, Department of Health & Human
Services, USA, 8 pp. "Exposure estimates for chemicals to be
included in the NTP annual report on carcinogens".
5.2 Potential occupational exposure
The number of workers in the USA who handle 2-NP or mixtures
containing 2-NP has been variously estimated as 15 000 (US EPA,
1977), 38 600 (Occupational Health Services, Inc., 1982), 100 000
(Finklea, 1977), and 185 000 (Beall et al., 1980). Based on
employment values of the US Bureau of the Census (1987) these
estimates of exposed workers represent from 0.02% to 0.19% of the
civilian workforce in the USA. The low estimate of 15 000, although
quoted in a US Environmental Protection Agency report, originated
with a manufacturer of 2-NP. The estimate generated by Occupational
Health Services, Inc., carried out under contract with a
manufacturer of 2-NP, was based on a detailed survey of
distributors, manufacturers, and users, and thus may represent a
reasonable approximation. This report considered 38 600 to be the
best estimate of the total number of exposed workers in the USA and
set 126 600 workers as an upper boundary. It further estimated that
significant exposure (defined as exposure to at least 10% of the US
OSHA exposure limit or 9.1 mg/m3 (2.5 ppm)) ranged from 4000 (best
estimate) to 10 600 (upper boundary) workers. Sources of worker
exposure identified in a survey conducted in the USA by the National
Institute for Occupational Safety and Health (Finklea, 1977)
included rotogravure and flexographic inks used in printing, and
coatings and adhesives used in industrial construction and
maintenance, highway marking, ship building and maintenance,
furniture manufacture, and food packaging. A US NIOSH survey
estimated that 9815 workers in the USA were exposed to 2-NP or to
trade-name products containing 2-NP (US NIOSH, 1983).
Occupational exposure limits are summarized in Table 3.
There is little data on actual occupational exposure, although
the limited information on conditions in the USA summarized in
Table 4 suggests that it is highly variable. Manufacture of 2-NP, an
enclosed process, appears to involve little employee exposure much
of the time, but spills and operations such as filling drums can
briefly expose a few workers to high concentrations. In general, low
exposures may be typical in some painting operations and in the
manufacture of tyres, but other painting and manufacturing
operations appear, at least in the past, to have exposed workers to
dangerously high concentrations. Workers were exposed to
concentrations of 2-NP up to at least 2744 mg/m3 (754 pm) in a
pigment production facility and up to at least 265 mg/m3 (73 ppm)
in a solvent extraction plant. Evidence exists that concentrations
of 2-NP at the solvent extraction plant prior to the investigation
were at times substantially greater than the values measured during
the investigation (Crawford et al., 1985). Exposure levels mentioned
in the report by Occupational Health Services, Inc. (1982) were
mainly in the vicinity of 3.6 mg/m3 (1 ppm), although one printing
Table 3. Occupational exposure limits for 2-nitropropane in aira
Exposure limit
Country mg/m3 ppm Category of limit
Australia 36 10 TWA
Belgium 36 10 TWA
Brazil 70 20 AL
Canada 90 25 CLV
Denmark 36 10 TWA
Finland 18 5 TWA
150 40 STEL
Germany 18 5 1-year TWA(TRK)
Hungary 10 3 CLV
Netherlands 3.6 1 8-h TWA
7.3 2 STEL
Poland 30 8 TWA
70 20 STEL
Romania 47 13 TWA
70 20 STEL
Sweden 18 5 TWA
36 10 CLV
Switzerland 18 5 TWA
United Kingdom 90 25 8-h TWA
USA 90 25 8-h TWA (OSHA)
36 10 8-h TWA (ACGIH)
Yugoslavia 90 25 TWA
a From: IRPTC (1986)
ACGIH = American Conference of Governmental Industrial Hygienists
AL = Acceptable or tolerable limit
CLV = Ceiling value
MAK = Maximum worksite concentration
OSHA = Occupational Safety and Health Administration
STEL = Short-term exposure limit
TLV = Threshold limit value
TWA = Time-weighted average (MAK in Switzerland)
TRK = Technical guiding concentration
plant (Table 4) reported a peak concentration of 237 mg/m3
(65 ppm) which lasted 30 min, and a time-weighted average of
36-44 mg/m3 (10-12 ppm). In addition to inhalation, it is likely
that workers using 2-NP as a solvent will have at least occasional
contact with the liquid. 2-NP also has been reported to be a minor
component of both fresh and used machine cutting fluid emulsion
(Yasuhara et al., 1986). The importance of exposure to 2-NP as a
contaminant of 1-nitropropane is unknown. In an investigation
involving 1-nitropropane-sensitized ammonium nitrate blasting agents
(Cocalis, 1982), 2-NP was below the detectable limit. Occupational
exposure may be reduced in the future since strong recommendations
have been issued on minimizing all worker contact with 2-NP and its
fumes and on substituting other less toxic solvents where possible
due to the carcinogenicity of 2-NP (IRPTC, 1986; US EPA, 1986; US
NIOSH 1988).
Table 4. Air concentration of 2-NP in the work place
Concentration
Activity mg/m3 ppm Reference
Manufacture of 2-NP 3.64 approx 1 Brown & Dobbin (1977)
Manufacture of 2-NP 0.7-364; 0.2-100; Miller & Temple (1979)
98% of 98% of
samples samples
were < 36.4 were < 10
Vulcanizing tyres 0-0.18 < 0.05 Hollett & Schloemer
(1978)
Painting (bus 0.11 < 0.03a Love & Kern (1981)
maintenance)
Painting (railway 1.46 < 0.4 Hartle (1980)
cars)
Solvent extraction 167.4 0-100 Crawford et al. (1985)
(average
46)
Laboratory (1958) 14.6 0-21 Angus Chemical Co. &
(average 4) Occusafe, Inc. (1986)
2-NP storage & 2111-6000 580-1640 Angus Chemical Co. &
transfer area (1962); Occusafe, Inc. (1986)
drum filling
operation
Pigment production 109-2745 30-754 Angus Chemical Co. &
facility (1970) Occusafe, Inc. (1986)
Painting (battery 36.4-109 10-30 Skinner (1947)
cases)
Manufacturing 72.8-164 20-45 Skinner (1947)
(coating forms)
Printing approx 40 1-65 Occupational Health
(approx 11) Services, Inc. (1982)
a below limit of detection
6. KINETICS AND METABOLISM
6.1 Absorption
2-NP is absorbed via the lungs, the peritoneal cavity, the
gastrointestinal tract, and possibly, to a lesser extent, via the
skin. Absorption via the lungs, peritoneal cavity, and
gastrointestinal tract have been used in experimental studies and
have been investigated using rats and 14C-labelled 2-NP. Pulmonary
absorption was examined by Nolan et al. (1982), Müller et al.
(1983), and Filser & Baumann (1988). Nolan et al. (1982) considered,
on the basis of respiratory rate and tidal volume of the rat and the
accumulation of 2-NP during the 6-h period of exposure, that a
minimum of 40% of the inhaled 2-NP was absorbed. This value is
minimal since it does not include 2-NP metabolized and eliminated
during exposure. The data of Müller et al. (1983) suggested that
immediately following exposure to 728 mg/m3 (200 ppm) for 3 h,
plasma contained approximately 0.4% as 2-NP and 7.2% as metabolites
of the 2-NP inhaled. The metabolites were mainly acetone but also
included a small amount of isopropanol. These percentages were
estimated from the results of Müller et al. (1983) and normative
data for the laboratory rat (Baker et al., 1979), and support rapid
pulmonary uptake of 2-NP since 2-NP and its metabolites sequestered
elsewhere in the body and loss of metabolized 2-NP during exposure
were not considered. Filser & Baumann (1988) reported that uptake of
gaseous 2-NP was rapid, the clearance rate being equal to the
ventilation rate. The value they cite for the latter, 32
litres.h-1.kg-1), seems large in comparison with normative data
for the laboratory rat (Baker et al., 1979).
The rate of 2-NP uptake by rats from intraperitoneal injection
was examined by Müller et al. (1983). Ten minutes after an injection
of 25 mg/kg, the blood plasma contained 3.3% of the dose as 2-NP and
1.9% as metabolites, acetone, and isopropanol, indicating an uptake
of greater than 5.2% since presumably some of the dose was already
lost from the body and to other tissues in the body during this
initial period. These percentages were estimated from the data of
Müller et al. (1983) and normative data for the laboratory rat
(Baker et al., 1979). A dose of 50 mg/kg yielded partially
dissimilar results. The 10-min average value was low and also had a
very large standard deviation. This may have reflected large
differences among the rats in their initial uptake rates for 2-NP or
an experimental problem such as injection into the gastrointestinal
tract rather than the peritoneal cavity. Blood plasma contained, 10
min after injection, only 1.4% of the dose as 2-NP and 1.3% as
acetone and isopropanol. These data indicate that uptake from the
peritoneal cavity is fairly rapid, and suggest that the relatively
slower uptake of the 50-mg/kg dose may have reflected saturation of
the uptake mechanisms, since initial concentrations in the plasma
did not exceed those following the 25-mg/kg dose. Intraperitoneal
injections were used by Andrae et al. (1988), Guo et
al. (1990), Hussain et al. (1990), and Conaway et al. (1991) to
demonstrate that 2-NP induced nucleic acid damage in the livers of
Wistar, F-344 and Sprague-Dawley rats.
Absorption of 2-NP via the gastrointestinal tract was
investigated with male Wistar rats by Derks et al. (1989). They
found that the systemic availability of orally administered 2-NP
from a water solution was very high (90%) and absorption was rapid,
maximum plasma values being reached within 15 min after dosage.
Absorption of 2-NP given in olive oil was much slower and
availability was only 34% during the initial 3 h following dosage.
The authors suggested that absorption from oil was incomplete at 3 h
and might ultimately be much higher, since the olive oil was
absorbed and the 2-NP redistributed between the oil and aqueous
phases.
Although workers are cautioned against dermal contact with 2-NP
(Beall et al., 1980; US EPA, 1986), there appear to be no
quantitative data on dermal absorption. The solubility of 2-NP in
both polar and non-polar solvents, together with its small molecular
size, suggests that it should be absorbed readily through the skin
(Malkinson & Gehlmann, 1977). Dermal application of 2 g 2-NP/kg to
rabbits produced no obvious symptoms (Wilbur & Parekh, 1982);
however, as noted below, the rabbit is relatively resistant to the
toxicity of 2-NP.
6.2 Distribution
The distribution of 2-NP and its carbon-containing metabolites
among the organs and tissues in Sprague-Dawley rats was examined by
Nolan et al. (1982) via inhalation, and by Müller et al. (1983) via
intraperitoneal injection. Both utilized 14C-labelled 2-NP and
thus their data do not reveal the distribution of nitrite and other
nitrogen-containing metabolites generated from the nitro portion of
the 2-NP molecule. One hour after intraperitoneal injection,
radioactivity was concentrated in fat; there were intermediate
amounts in the blood, liver, and kidney, and lower amounts in other
organs and tissues (Müller et al., 1983). By 40 h, the highest
concentrations were in bone marrow and adrenal tissue, intermediate
amounts being found in the kidney, liver, spleen, lungs, and omental
fat, and by 8 days only the concentration of 14C in adrenal tissue
was noticeably greater than elsewhere in the body. However, Nolan et
al. (1982), found, both immediately and 48 h after a 6-h period of
2-NP inhalation, that highest concentrations of carbon from 2-NP
were in the liver and kidney and relatively little in the fat.
Differences in methodology limit intercomparison of these
studies. Their major consistency is the presence of high
concentrations of 2-NP and its labelled carbon in the liver and
kidney, organs (as discussed below) actively involved in the
metabolism of 2-NP and excretion of its metabolites.
The relevance of these studies on tissue distribution of 2-NP
and its carbon-containing metabolites to the toxicity of 2-NP is
unclear, since (as discussed below) most of the dose is rapidly
metabolized initially to acetone and nitrite. The 14C label used
in these studies thus traced mainly the distribution of acetone and
its metabolites in measurements made more than a few hours after
dosing.
Dequidt et al. (1972) provided limited data on the distribution
of nitrite among body organs of the rat following inhalation and
intraperitoneal injection of 2-NP. The data suggest a fairly uniform
distribution among the heart, lungs, kidney, spleen, and,
sometimes, the liver. In the majority of experiments, however, no
nitrite was detected in the liver. No explanation is offered for the
latter observation, and data in the paper are so erratic as to
suggest the possibility of analytical problems.
6.3 Metabolic transformation
Starting with a report by Scott (1943), there have been numerous
studies on the metabolic transformation of 2-NP by mammals,
mammalian cells, microorganisms, and isolated enzymes. These studies
have shown that the major pathway for metabolic transformation of
2-NP involves oxidation to nitrite and acetone. Evidence for the
formation both of nitrite and acetone was reported from studies on
liver microsomes from rats pretreated with phenobarbital or
3-methylcholanthrene (Ullrich et al., 1978), cultured hepatocytes
from untreated rats (Haas-Jobelius et al., 1991), liver microsomes
from untreated mice (Marker & Kulkarni 1986a,b; Dayal et al., 1991),
V79 Chinese hamster cells (Haas-Jobelius et al., 1991), and a yeast
(Kido et al., 1975). Nitrite was reported to be a major metabolite
of 2-NP in rabbits (Scott, 1943), rats (Dequidt et al., 1972), and
liver microsomes from rats (Sakurai et al., 1980) and mice (Marker &
Kulkarni, 1985). Acetone was identified as a major metabolite of
2-NP in rats and chimpanzees (Muller et al., 1983).
Enzymatic oxidation of the nitronate form of 2-NP to nitrite and
acetone by horseradish peroxidase (Porter & Bright, 1983), a
dioxygenase from the yeast Hansenula mrakii (Kido et al., 1984),
and mouse liver microsomes (Dayal et al., 1991) was several times
more rapid than that of 2-NP under identical conditions. In addition
to acetone, a smaller amount of isopropanol is produced at least in
rats and chimpanzees (Muller et al., 1983). The source of the
isopropanol was not specified in this study, but since reduction of
acetone in the body is negligible (De Bruin, 1976), isopropanol may
be formed directly by oxidation of 2-NP. The formation of a
hydroxyisopropyl radical during the oxidation of 2-NP was suggested
by Kuo & Fridovich (1986).
The metabolic fates of these metabolites of 2-NP are well known.
Acetone is produced by a minor metabolic pathway in the mammalian
body (Smith et al., 1983) and has been detected in small amounts in
the blood, urine, and expired air of normal humans (Mabuchi, 1979;
Conkle et al., 1975). It may be excreted directly via expired air,
urine, and loss through the skin, or may enter into the general
metabolism either via cleavage to a 2-carbon acetyl fragment and a
1-carbon formyl fragment or via oxidation to pyruvic acid (De Bruin,
1976). The proportion excreted unchanged increases with increasing
dosage, suggesting an easily saturable metabolic pathway.
Isopropanol is oxidized to acetone (De Bruin, 1976).
Nitrite may exist as a minor constituent of the mammalian body.
It is constantly replenished by ingestion and synthesis, and
constantly removed by oxidation to nitrate. Nitrite and nitrate in
the blood stream are rapidly and homogeneously distributed
throughout the body (Parks et al., 1981). Nitrite rapidly oxidizes
divalent ferrous haemoglobin to trivalent ferric methaemoglobin
(Burrows, 1979). Little is transported to the tissues or excreted,
at least in dogs, sheep, and ponies (Schneider & Yeary, 1975).
Dequidt et al. (1972), however, reported substantial urinary
excretion of nitrite by rats following inhalation or intravenous
injection of 2-NP. Methaemoglobin is incapable of transporting
oxygen and, during enzymatic repair of this defect, nitrite is
reoxidized to nitrate. Parks et al. (1981) reported that 10 min
after intratracheal instillation of labelled nitrite into mice, 70%
of the label in plasma was in nitrate, 3% in nonionic compounds, and
only 27% remained as nitrite. Similar results were obtained with
rabbits. Nitrate is slowly excreted through the kidneys (Schneider &
Yeary, 1975) and also into saliva where it is reduced back to
nitrite by bacteria and reabsorbed into the body via the
gastrointestinal tract (Friedman et al., 1972). Small amounts of
nitrite in the stomach may react with secondary amines and other
amino substrates to form N-nitroso compounds which might be absorbed
(Sander & Schweinsberg, 1972; Fine et al., 1982).
The enzymatic system oxidizing 2-NP to acetone and nitrite was
identified through in vitro experiments using microsomes isolated
from mammalian liver. Ullrich & Schnabel (1973) determined that
cytochrome P-450, in liver microsomes from phenobarbital-pretreated
rats, bound 2-NP. Ullrich et al. (1978) subsequently reported that
liver microsomes from rats pretreated with phenobarbital or
3-methylcholanthrene rapidly catalysed the oxidation of 2-NP to
acetone and nitrite. The latter were produced in roughly equal
quantities. Surprisingly, however, the rate of this reaction was not
diminished under conditions of reduced oxygen pressure. The activity
of preparations from untreated control rats was generally very low.
Sakurai et al. (1980) demonstrated that this enzyme system in rats
was active in metabolizing other aliphatic nitro compounds. Marker &
Kulkarni (1985, 1986a, 1986b), working with mice, obtained somewhat
different results. They reported rapid denitrification of
2-NP to nitrite and acetone by liver microsomes from untreated mice,
and an acetone production at least twice the nitrite release. These
authors suggested that multiple forms of cytochrome P-450 are
involved, and claimed that nitrite is sequestered in the reaction
mixture and that denitrification of 2-NP may involve a reductive or
at least non-oxidative pathway as well as an oxidative pathway. They
also noted large differences in the rates of hepatic microsomal
enzymatic nitrite release among the five strains of mice tested.
Jonsson et al. (1977) demonstrated that hepatic microsomes from
uninduced rabbits could denitrify a compound related to 2-NP,
2-nitro-1-phenylpropane.
In addition to oxidative denitrification, a reductive pathway
has been shown to occur in cultured hepatocytes from Wistar rats and
in V79 Chinese hamster cells. Nitroreduction was indicated by the
fact that the cells formed acetone oxime, the tautomeric form of
nitrosopropane (Haas-Jobelius et al., 1991).
Evidence for the involvement of more than one pathway for the
metabolism of 2-NP in the rat was also obtained by Denk et al.
(1989). Their experiments on the pharmacokinetics of 2-NP in rats
exposed by inhalation suggested that there are two different
pathways both in male and female animals, a saturable one of low
capacity and high affinity according to Michaelis-Menten kinetics
and a non-saturable one following first-order kinetics. First-order
kinetics was similar in the two sexes, but striking differences
between sexes were observed in the kinetics of the saturable
pathway. The authors showed that in females more 2-NP was
metabolized by the non-saturable pathway at concentrations above 655
mg/m3 (180 ppm), and in males at concentrations above 218 mg/m3
(60 ppm), and linked their observations to the reported higher
susceptibility to liver damage of males as compared to females
(Griffin & Coulston, 1983). Denk et al. (1989) suggested that it is
the first-order metabolic process which results in the formation of
toxic products whereas the saturable pathway was suggested to lead
to less toxic metabolites.
These observations on the hepatic metabolism of 2-NP and related
compounds by rats, mice, and rabbits indicate differences among
species and even strains. It is probable that more than one enzyme
system is involved. In mice as well as rats hepatic cytochrome P-450
may be important in the metabolism of this xenobiotic.
Observations by Ivanetich et al. (1978) suggested an additional
detoxifying role for hepatic microsomal cytochrome P-450. They
demonstrated that under aerobic conditions in vitro 2-NP could
degrade the haem moiety of cytochrome P-450 in phenobarbital-induced
rats and speculated that this provided an additional mechanism for
trapping reactive metabolites before these could damage essential
cellular constituents.
In addition to the hepatic enzymatic systems examined in rats
and mice, Mochizuki et al. (1988), as mentioned above, described a
2-NP denitrifying system in adrenal microsomes of uninduced
guinea-pigs. They identified this cytochrome-P-450-dependent
monooxygenase as benzo[ a]pyrene hydroxylase.
6.4 Elimination and excretion
Elimination of 2-NP and its metabolites has been examined mainly
in rats and, to a much lesser extent, in chimpanzees in studies
which utilized measurements of radioactivity from 14C-labelled
2-NP as well as measurements of 2-NP and its metabolites. Dosage by
inhalation, intravenous injection, and intraperitoneal injection all
yielded fairly similar results. During a 48-h period after a 6-h
exposure of rats to 73 mg/m3 (20 ppm) and to 560.6 mg/m3
(154 ppm) of 14C-labelled 2-NP, about 50% of the radioactivity in
the absorbed dose was excreted via the lungs as carbon dioxide
(Nolan et al., 1982). The proportion of the absorbed dose excreted
via the lungs as unchanged 2-NP was 4% at the low dose level and 22%
at the high level. Still less of the labelled carbon was eliminated
via faeces and urine, i.e. 11% and 8%, respectively, at the low dose
level, and 5% and 11%, respectively, at the high level.
Disappearance of 2-NP from the blood after exposure at the high dose
level followed a first-order relationship and yielded a half-life of
48 min. Limited data in Müller et al. (1983) yielded a half-life for
rats of approximately 80 min for 2-NP in plasma following a 3-h
exposure to a concentration of 728 mg/m3 (200 ppm). Nolan et al.
(1982), however, found that disappearance of the 14C label of the
2-NP from the plasma was markedly slower and biphasic. During the
first 12 h following exposure to 560.6 mg/m3 (154 ppm), the
half-life for plasma radioactivity was 172 min and, following
exposure to 73 mg/m3 (20 ppm), 354 min. After 12 h, loss of
radioactivity from plasma was much slower, the half-life being
approximtely 35-36 h for both doses. These data on loss of 2-NP and
its 14C label indicate that 2-NP is rapidly eliminated from the
body mainly by metabolic transformation and to a lesser degree by
pulmonary excretion of the unchanged compound. The major
carbon-containing metabolites of 2-NP, acetone and isopropanol,
presumably enter into the general metabolism of the body and are
eliminated via the intermediary metabolism as part of a much larger
carbon pool.
Pulmonary excretion of 2-NP, like loss of the 14C label from
plasma, is dose dependent, biphasic, and follows first-order
kinetics (Nolan et al., 1982). Fifty times more 2-NP was exhaled
during the first hour following exposure to 560.6 mg/m3 (154 ppm)
than during the first hour following exposure to 73 mg/m3
(20 ppm). Following exposure to 73 mg/m3, 2-NP was excreted for
the first 7 h at a rate which decreased by one half every 64 min,
and subsequently decreased by one half every 16 h, whereas following
exposure to 560.6 mg/m3, the half-times of excretion were 71 min
for the first 12 h, and 16 h for the subsequent period. Changes in
the rates of pulmonary excretion of 14C-labelled carbon dioxide
were similar for 48 h following exposure to 73 and 560.6 mg/m3.
Eighty seven per cent of the total was eliminated during the first
12 h after exposure; loss was somewhat less rapid thereafter. The
dose-dependent nature of pulmonary excretion of 2-NP suggests that
greater concentrations of 2-NP in the blood markedly increase
exhalation of the unchanged compound and reduce the percentage
metabolized. Thus exposure of the tissues to 2-NP and its
metabolites may not be a linear function of the inhaled dose.
Derks et al. (1989) found that the plasma half-life of
intravenous doses of 0.01-0.05 g/kg in rats was 45 min during the
first 4 h. Loss was linear over this dose range and could be
described by an open single-compartment model. They suggested that
the measured loss from plasma may be due in part to spontaneous
conversion of 2-NP to its anionic form, 2-NP nitronate.
Elimination of 2-NP by Sprague-Dawley rats following
intraperitoneal injection of 14C-labelled 2-NP was studied by
Müller et al. (1983) and was generally similar to the elimination of
2-NP following inhalation. The concentration of 2-NP in plasma
declined exponentially with time, with half-lives of 70 and 125 min
during at least the initial 6 h following injections of 25 and
50 mg/kg, respectively. Metabolites of 2-NP, acetone and
isopropanol, reached maxima 2-4 h after injection of 25 mg/kg, and
at least 4-6 h after injection of 50 mg/kg. The concentration of
isopropanol ranged from 1/16 to 1/34 the concentration of acetone.
During the initial 40 h after injection of 50 mg/kg, 4.5% of the
dose was exhaled as 2-NP, 10.4% as acetone, and 38.1% as carbon
dioxide. Losses via urine (5.9%) and faeces (0.7%) were small in
comparison with the 53% loss via exhalation. Müller et al. (1983),
however, reported that only 12% of the dose was recovered from the
carcasses, leaving a large amount (28.4%) unaccounted for and thus
casting some doubt on their values.
With one exception, results similar to the above were obtained
following intravenous injection of 14C-labelled 2-NP (10 mg/kg)
into male chimpanzees (Müller et al., 1983). The concentration of
2-NP in plasma declined exponentially with time, the half-life being
92 min. The concentration of acetone reached a maximum 6 h after
injection and remained high for at least 48 h. The concentration of
isopropanol peaked 3 h after injection when it approached that of
acetone, but otherwise was a third to a quarter that of acetone. The
concentration of 2-NP and its carbon-containing metabolites (i.e.
the concentration of 14C) in plasma declined exponentially with a
half-life of 5.5 h for the initial 10 h, and a half-life of 48 h
thereafter. As in rats, exhalation was the major means of
elimination of 2-NP and its carbon-containing metabolites. During
the first 3 days after injection, only 5-6% of the 14C in the dose
was recovered in urine and only 0.4-0.5% in faeces. Acetone,
isopropanol, and 2-NP were mainly eliminated via renal excretion;
urine collected during 6 to 24 h after injection contained 3.1 mg
acetone/litre, 7.2 mg isopropanol/litre, and 1.8 mg 2-NP/litre.
Isopropanol may thus be maintained at low concentrations in the
plasma both by oxidation to acetone and by rapid excretion. In
addition to acetone, isopropanol, and 2-NP, 14C was excreted as an
unidentified polar metabolite which by 24 h after injection
contained 90% of the radioactivity in the urine. The one striking
difference between results with rats and with chimpanzees, i.e. the
relatively much higher plasma concentrations of isopropanol in
comparison to acetone, might indicate interspecific variation in the
excretion of 2-NP and its metabolites.
Dequidt et al. (1972) provided limited information on the
excretion of nitrite following inhalation and intraperitoneal
injection of 2-NP. Rats weighing approximately 250 g each were given
a daily injection of 0.11 g/kg. Urinary excretion of nitrite was 10
to 35 µg/animal during the first day following the initial
injection, and reached a daily rate of 11 mg/animal by the fourth
injection. The latter rate of excretion represents three quarters of
the nitrogen injected daily as 2-NP, and stands in sharp contrast to
the results of Schneider & Yeary (1975), who reported that little
intravenously injected nitrite was excreted by dogs, sheep or
ponies. Following exposure of rats to a 2-NP concentration of
2766 mg/m3 (760 ppm) for 8 h on each of two successive days, daily
elimination of nitrite was approximately 30 mg/animal. This was
equivalent to about 20% of the 2-NP inhaled daily and possibly as
much as 50% of the absorbed daily dose. The amount of 2-NP inhaled
was estimated from normative data for the rat (Baker et al., 1979),
and absorption was assumed to be 40% of the amount inhaled (Müller
et al., 1983). No nitrite was detected in urine during exposure of
rats to 291 mg/m3 (80 ppm) for 8 h per day on 5 successive days.
6.5 Retention and turnover
There is no evidence that 2-NP is retained for more than a few
hours in the body. It is rapidly lost by exhalation and metabolic
transformation. The known carbon-containing metabolites, acetone and
isopropanol, are excreted rapidly and are also transformed into
compounds which are normal to the body and enter into its general
intermediary metabolism. There is less information on nitrite, the
major metabolite of the nitro moiety. In rats much of the nitrite is
excreted as such in the urine. There is no evidence for excessive
accumulation of 2-NP or its metabolites in any organ or tissue.
Information is also lacking on possible N-nitroso and other toxic
compounds synthesized from nitrite or the nitro moiety.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Data on single exposures to experimental animals, summarized in
Table 5, indicate substantial differences in sensitivity among the
species tested. The route of administration was mainly via
inhalation, but other routes were also used. A quantitative
comparison of the results from these studies is difficult due to a
lack of information on both strain and sex of the animals used, to
differences in the route of administration, and to large variations
in dosage. The LC50 for mortality within 14 days following a 6-h
exposure was estimated to be 1456 mg/m3 (400 ppm) for male rats
and 2621 mg/m3 (720 ppm) for females (Baldwin & Williams, 1977).
Exposure of rats (of unspecified sex) via inhalation to 14 058
mg/m3 (3862 ppm) for 1 h or to 9584 mg/m3 (2633 ppm) for 2.25 h
killed some of the animals within days. Exposure of rats to a much
higher concentration of 2-NP, i.e. 53 508 mg/m3 (14 700 ppm),
killed all animals within 4 h. An inhalation exposure of 4994
mg/m3 (1372 ppm) or less for 2.25 h was not lethal to rats. Unlike
rats, LC50 values were similar, i.e. 2031 and 2038 mg/m3
(558 and 560 ppm), respectively, for male and female mice following
a 6-h exposure (Baldwin & Williams, 1977). Cats appeared more
sensitive to acute exposure to 2-NP than rats. Exposure to
8565 mg/m3 (2353 ppm) for 1 h (or lower concentrations for
proportionately longer) was lethal to some cats. Rabbits and
guinea-pigs, on the other hand, appeared far less sensitive to 2-NP
than rats. Rabbits survived a 2.25-h exposure to 9584 mg/m3
(2633 ppm) and guinea-pigs a 2.25-h exposure to 15 699 mg/m3
(4313 ppm). The sequence in acute sensitivity of animals to inhaled
2-NP (from most to least) was cat, rat and mouse, rabbit, and
guinea-pig. The cat was almost an order of magnitude more sensitive
than the guinea-pig.
There are limited data on lethality via routes of administration
other than inhalation. Large intraperitoneal doses were found to be
promptly lethal to rats; 1.7 and 1.1 g/kg killed animals within 2 h
and 4 h, respectively (Dequidt et al., 1972). The 14-day oral LD50
for mice was 0.40 g/kg. The minimal oral lethal dose for the rabbit,
0.50-0.75 g/kg, was much larger than the estimated minimum lethal
dose via inhalation, 0.24 g/kg. Dermal application of 2 g/kg to
rabbits produced no obvious local or systemic effects.
Table 5. Effects of single exposure to 2-nitropropane in mammalsa
a) Lethality
Species Sex Concentration Estimated total Effects/results Reference
doseb (g/kg)
g/m3 ppm
Oral administration
Rabbitcd lethal dose estimated as 0.50- Machle et al. (1940)
0.75 g/kg
Mouse M/F 14-day LD50: 0.40 g/kg Hite & Skeggs (1979)
Inhalation
Ratc (Wistar) 53.5 14 700 1.51 all animals died within 4 h Dequidt et al (1972)
Ratcd 5.5-14.1 1513-3865 0.10-0.17 death of some animalsc Treon & Dutra (1952)
2.6-8-57d 714-2353 0.06-0.08 no deathsd Treon & Dutra (1952)
Ratcd (Wistar) 2.77 760 0.16 death within 48 h; Dequidtet al. (1972)
Rate (CD) M 2.93 805 0.16 8/10 died within 14 days Baldwin & Williams (1977)f
F 2.19 602 0.13 no deaths within 14 dayse Baldwin & Williams (1977)f
M 2.10 574 0.14 8/8 died within 14 days Baldwin & Williams (1977)f
M 1.68 461 0.10 7/8 died within 14 days Baldwin & Williams (1977)f
Table 5 (contd)
Species Sex Concentration Estimated total Effects/results Reference
doseb (g/kg)
g/m3 ppm
Inhalation (contd)
Rate (CD) M 1.47 405 0.08 5/8 died within 14 days Baldwin & Williams (1977)f
1.34 367 0.08 no deaths within 14 days Baldwin & Williams (1977)f
Mousee (ICR) F 2.70 740 0.47 14/14 died within 14 days Baldwin & Williams (1977)f
2.33 640 0.41 11/14 died within 14 days Baldwin & Williams (1977)f
1.8 495 0.32 2/14 died within 14 days Baldwin & Williams (1977)f
Mouse (ICR) M 2.69 738 0.41 9/14 died within 14 days Baldwin & Williams (1977)f
2.08 558 0.28 7/14 died within 14 days Baldwin & Williams (1977)f
1.65 454 0.23 no deaths within 14 days Baldwin & Williams (1977)f
Catcd 2.6-8.56 714-2353 0.07-0.19 death of some animals Treon & Dutra (1952)
1.19-2.87 328-787 0.02 no deaths Treon & Dutra (1952)
Guinea-pigcd 16.8-35.0 4622-9607 0.53-0.63 death of some animals Treon & Dutra (1952)
8.67-34.7 2381-9523 0.23-0.32 no deaths Treon & Dutra (1952)
Rabbitc 8.67-34.7 2381-9523 0.24-0.27 death of some animals Treon & Dutra (1952)
5.1-14.1 1401-3865 0.10-0.16 no deaths Treon & Dutra (1952)
Table 5 (contd)
Species Sex Concentration Estimated total Effects/results Reference
doseb (g/kg)
g/m3 ppm
Intraperitoneal administration
Ratcf 1.7 death within 2 h Dequidt et al. (1972)
1.1 death within 4 h Dequidt et al. (1972)
Intraperitoneal administration (contd)
Mousec M 0.80 LD50 Friedman et al. (1976)
(Swiss ICR/HQ)
a Values recalculated as necessary to ppm and g/kg
b Estimated dose calculated by the formula: tidal volume x respiration frequency x exposure time x 2-NP conc. x alveolar
retention/animal weight. Tidal volume and respiration frequency from Kaplan et al. (1983) for mice (0.15 ml, 163/min);
from Baker et al. (1979) for rats (0.86 ml, 85.5/min); from Hoar (1976) for guinea-pigs (1.68 ml, 84/min); from Kozma
et al. 81974) for rabbits (15.8 ml, 45/min); from Reece (1984) and Breazile (1971) for cats (42 ml, 31/min); alveolar
retention in rat (0.40) from Nolan et al. (1982), used for all species; where not stated animal weights assumed to be
average values, 20 g for mice, 250 g for rats, 500 g for guinea-pigs, 2.5 kg for rabbits, and 4.0 kg for cats
c Sex not specified
d Exposure time varied from 1 to 7 h
e Exposure time was 6 h
f Baldwin & Williams (1977) also exposed female rats for 6 h to concentrations of 2-NP lower than 2.2 g/m3 (602 ppm); these
concentrations, i.e. 1.15, 1.35 and 1.69 g/m3 (316, 370 and 464 ppm), like 2.2 g/m3, produced no deaths within 14 days
Table 5. Effects of single exposure to 2-nitropropane
b) other effects
Route of Species Sex Estimated Effects/results Reference
administration total doseb
g/kg)
Intraperitoneal rat M 0.15 maximum hepatic injury achieved Filser & Daumann (1988)
(Sprague-Dawley) with this dose
Intraperitoneal rat M 0.05 lipid accumulation, centrilobular Zitting et al. (1981)
(Wistar) necrosis, mitochondrial
abnormalities, and changes in
endoplasmic reticulum and
glutathione content in liver
within 24 h, as well as changes
in enzyme activity in liver and
brain
Dermal rabbit M & F 2 no toxic effects observed Wilbur & Parekh (1982)
b See footnote b in Table 5a.
The effects of acute exposure to 2-NP, in addition to lethality,
are characterized primarily by hepatotoxicity and, at high exposure
levels, methaemoglobin formation and depression of the central
nervous system. Machle et al. (1940), in a description of symptoms
resulting from exposure of laboratory animals to simple
nitroparaffins, listed the following progression for guinea-pigs and
rabbits after a latent period of 20 to 40 min: progressive weakness,
unsteadiness, and incoordination ending in complete ataxia. The rate
of respiration at first slowed and later became increasingly rapid.
Most of these symptoms appear to reflect the narcotic effects
normally associated with inhalation of volatile hydrocarbons, but
the more rapid breathing may reflect an attempt by the body to
compensate for the formation of methaemoglobin and loss of
oxygen-carrying capacity of the red blood cells. Treon & Dutra
(1952) noted similar progression from exposure to high
concentrations of 2-NP vapour, i.e. lethargy and weakness, dyspnoea,
cyanosis, prostration, and ultimately coma and death, but did not
report more rapid breathing following a depression in rate of
respiration. They also noted lacrimation, salivation, and gastric
regurgitation in cats. In addition, the authors observed that, even
with animals which died promptly (within 9.5 h) following a single
exposure, there was a loss in body weight averaging 2.8%. Animals
exposed to 8.56 g/m3 (2353 ppm) or higher concentrations of 2-NP
displayed pathological changes including hepatocellular damage,
pulmonary oedema and haemorrhage, some disintegration of neurones in
the brain, and widespread damage to the endothelium.
Methaemoglobin and Heinz bodies (masses of denatured haemoglobin
within erythrocytes) were found in the blood of animals following
single exposures to high concentrations of 2-NP. In the case of
cats, 60 to 80% of the haemoglobin was converted to methaemoglobin
by exposure to 15.9 to 33.6 g/m3 (4360 to 9230 ppm) for 1 to 2 h,
whereas much longer exposure of rabbits to these concentrations
converted only 4 to 8% of the haemoglobin (Treon & Dutra, 1952).
Dequidt et al. (1972) reported high levels of methaemoglobin in
rats, i.e. 84% and 89%, following 1-h inhalation exposure to
53.5 g/m3 (14 700 ppm) and intraperitoneal injection of 1.7 g/kg,
respectively. Much lower methaemoglobin concentrations, 0.2 to 8.6%,
resulted from a 1-h exposure to 2.8 g/m3 (760 ppm). One day after
exposure to 15.4 g/m3 (4230 ppm) for 4.5 h or 8.5 g/m3
(2335 ppm) for 2.25 h, rabbits had Heinz bodies in 45 to 80% of
their red cells. These bodies disappeared gradually over 9 to 16
days (Treon & Dutra, 1952). Exposure of rabbits to 13.8 g/m3
(3790 ppm) for 1 h or 9.4 g/m3 (2580 ppm) for 2.25 h resulted in
the formation of Heinz bodies in only 0 to 2% of the red cells.
Formation of Heinz bodies in the cat may have reflected its high
sensitivity to 2-NP. A 1-h exposure to 13.8 g/m3 (3790 ppm) and a
20 min exposure to 16.4 g/m3 (4505 ppm) resulted in the appearance
of Heinz bodies in 27% and 16% of the erythrocytes, respectively.
Observations by Zitting et al. (1981) indicated that a single
intraperitoneal injection of 0.05 g 2-NP/kg to rats can produce
significant changes in the fine structure of the liver and in the
physiology of both liver and brain. 2-NP produced a visible
accumulation of lipid in hepatocytes, especially in periportal areas
after 4 h, and the lipid level continued to increase for the next
20 h. Within 4 h after injection there was also degranulation of the
rough endoplasmic reticulum in hepatocytes and proliferation of the
smooth endoplasmic reticulum. Within 24 h the former had almost
disappeared and the latter was vacuolated or compacted. In addition,
some hepatocytes had abnormal mitochondria and there was necrosis of
hepatocytes around the central vein. The latter was reflected in a
concurrent fourfold increase of serum alanine aminotransferase.
Other enzymatic parameters in the liver were also markedly affected
within 24 h. Cytochrome P-450 was markedly depressed,
7-ethoxycoumarin O-deethylase and 7-ethoxyresorufin O-deethylase
were diminished in activity, and microsomal epoxide hydratase,
UDP-glucuronosyltransferase and glutathione peroxidase were
increased in activity. In addition, the liver concentration of
glutathione nearly doubled.
The major observed neurochemical effect was a significant
increase in acetylcholine esterase activity in the cerebrum and in
isolated synaptosomes. There was little or no change in RNA,
2',3'-cyclic nucleotide 3'-phosphohydrolase, or acid proteinase in
the brain.
Zitting et al. (1981) noted that these histopathological and
enzymatic changes induced by 2-NP are nearly identical to the
effects of carbon tetrachloride on the rat and are thus indicative
of lipid peroxidation.
Hepatotoxicity following exposure to 2-NP has also been
observed in mice (Dayal et al., 1989). Intraperitoneal doses of
0.8 g/kg (9 mmol/kg) in male mice and 0.6 g/kg (6.7 mmol/kg) in
female mice significantly increased plasma activities of enzymes
indicative of hepatic damage (sorbitol dehydrogenase, alanine
aminotransferase, and aspartate aminotransferase) 48, 72, and 96 h
after injection. These enzyme activities were not elevated 24 h
after this dosage nor after small doses of 2-NP.
7.2 Short-term and long-term repeated exposure
Data for repeated exposure, like that for single exposure,
indicate that the cat is more sensitive to 2-NP than the other
species tested (Table 6). Rats, rabbits, guinea-pigs, and monkeys
survived 1.2 g/m3 (328 ppm) (7 h/day, 5 days/week) throughout
approximately 6 months of exposure (Treon & Dutra, 1952). However,
cats exposed to the same concentrations of 2-NP began dying after
the third day of exposure and were all dead by the end of the 17th
day (Treon & Dutra, 1952). Rats and guinea-pigs survived 5 days of
Table 6. Short-term and long-term toxicity of 2-nitropropane in mammalsa
Species Sex Dose and/or concentrationb Effects/results Reference
Oral studies
Rat (Wistar) M & F 0.25 g/kg, 5/week for 4 mortality of males (4/10); decreased growth in Wester et al. (1989)
weeks first week; increased urine, ALAT, ASAT, and
gamma-GT (males only), anaemia, thrombocyte
and leucocyte count, liver, spleen and heart
weight, and haemosiderin content of spleen;
cellular and nuclear polymorphism, single cell
necrosis, and proliferation of oval cells
and/or bile ducts in liver
Rat M 0.089 g/kg (1 mmol), some deaths by 16 week; throughout study body Fiala et al. (1987b)
(Sprague-Dawley) 3/week for 16 weeks, weights significantly lower than controls; all
maintained but not dosed rats exposed 16 weeks or longer developed
for next 61 weeks massive hepatocellular carcinomas; metastases
to the lungs in 4 animals
Rat M & F 0.05 g/kg, 5/week for 4 increased anaemia, thrombocyte conc., and Wester et al (1989)
(Wistar) weeks heart weight
Rat (Wistar) M & F 0.002 and 0.01 g/kg, 5 increased water intake by males dosed with Wester et al. (1989)
week for 4 weeks 0.002 g/kg
Inhalation studies
Ratd 2.77 g/m3 (760 ppm), 8 animals dead within 2 h after end of second Dequidt et al. (1972)
(Wistar) h/day for 2 days; inhalation session; 2-NP conc. in liver =
estimated dose over 8 h, 180 ppm; methaemoglobin = 2.4%
0.16 g/kg
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Inhalation studies (contd)
Rate 2.45 g/m3 (672 ppm), 7 no deaths Treon & Dutra (1952)
h/day for 5 days;
estimated dose over
7 h, 0.12 g/kg
1.20 g/m3 (328 ppm), 7 no deaths Treon & Dutra (1952)
h/day, 5 days/week for
130 days over 199 days;
estimated dose over 7 h,
0.06 g/kg
Rat M 0.75 g/m3 (207 ppm), 7 body weight and haematological parameters Lewis et al. (1979)
(Sprague-Dawley) h/day, 5 days/week for up unaffected; some pulmonary oedema and some
to 24 weeks; estimated pulmonary lesions within 3 months; liver
dose over 7 h, 0.04 g/kg weight elevated; hepatocellular hypertrophy,
hyperplasia and liver necrosis in all rats
within 3 months; liver neoplasms in all rats
within 6 months; these hepatocellular
carcinomas appeared to be growing rapidly and
deforming surrounding tissues
Rate 0.73 g/m3 (200 ppm), 7 growth slightly reduced and SGPT elevated in Griffin et al. (1978)
h/day, 5 days/week for 6 male rats; liver weight increased in both sexes;
months; estimated dose morphological changes in liver more pronounced
over 7 h, 0.03 g/kg in males; these included fatty metamorphosis
and hepatic nodules consisting mainly of
hyperplastic areas with distortion of lobular
architecture, necrosis and peripheral
compression
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Inhalation studies (contd)
Ratd 0.73 g/m3 (200 ppm), 7 no effect on body or organ weight to end of Griffin et al. (1986)
(Sprague-Dawley) h/day for 5 days experiment (94 week) aside from a brief
decrease in weight gain immediately following
exposure; no significant effects on mortality
or pathology
Ratc M 0.73 g/m3 (200 ppm), 7 severe liver damage with vacolar degeneration Coulston (1982)
h/day, 5 days/week for in exposed rats after 3 months up
to 7 months
Rate 0.36 g/m3 (100 ppm), 7 male rats had lower body weight, increased Griffin & Coulston (1983);
h/day, 5 days/week for up renal calcification, elevated SGPT, and Coulston et al. (1985)
to 18 months; estimated enlarged livers with necrosis, vacuolar
dose over 7 h, 0.013 g/kg degeneration and probable hepatocellular
carcinomas; female rats had increased renal
calcification and occasional hepatic masses
and nodules showing hyperplasia and vacuolar
degeneration
Rate 0.29 g/m3 (80 ppm), 8 no deaths; no trace of 2-NP in organs at end of Dequidt et al. (1972)
h/day for 5 days; estimated experiment; methaemoglobin = 0; nitrite = 0-10
dose over 8 h, 0.016 g/kg ppm in tissues, but no nitrite in urine
Rat M 0.1 g/m3 (27 ppm), 7 no gross or microscopic alteration of any Lewis et al. (1979)
(Sprague-Dawley) h/day, 5 days/week for up tissue, haematological parameter or serum
to 24 weeks; estimated biochemistry
dose over 7 h, 0.005 g/kg
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Inhalation studies (contd)
Rate 91 mg/m3 (25 ppm), 7 no changes in behaviour, appearance, rate of Griffin et al.
h/day, 5 days/week for up weight gain, final weight, serum chemistry or (1980, 1981)
to 22 months; estimated haematology; no significant increase in tumours
dose over 7 h, 0.003 g/kg and lesions associated with exposure; no
evidence of methaemoglobinaemia
Moused 0.73 g/m3 (200 ppm), 7 depression in body weight during first 3 months Griffin et al. (1984)
(ICR) h/day, 5 days/week for in females and throughout experiment in males;
48 weeks increased liver weight and elevation of liver
transaminases in females; toxic hyperplasia of
liver predominantly in females
Mousee 0.36 g/m3 (100 ppm), 7 slight depression of body weight during first 8 Coulston et al. (1986);
h/day, 5 days/week for months in males; no effects on organ weight; no Griffin et al. (1987)
18 months evidence of hepatocellular carcinoma; some
indications of liver toxicity (nodular
hyperplasia in females)
Cate 2.6 g/m3 (714 ppm), 4.5 deaths starting with first exposure, but Treon & Dutra (1952)
h/day for 4 days; some animals survived 4 exposures
estimated dose over 7 h,
0.10 g/kg
1.2 g/m3 (328 ppm), 7 deaths starting with third exposure; all Treon & Dutra (1952)
h/day, 5 days/week for 17 animals dead by end of 17th exposure
exposures; estimated dose
over 7 h, 0.07 g/kg
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Inhalation studies (contd)
Cate 1.15 g/m3 (317 ppm), 7 no deaths Treon & Dutra (1952)
h/day for 2 days;
estimated dose over 7 h,
0.07 g/kg
0.3 g/m3 (83 ppm), 7 no deaths Treon & Dutra (1952)
h/day, for 130 out of 191
days; estimated dose over
7 h, 0.02 g/kg
Rabbite 1.2 g/m3 (328 ppm), 7 no deaths Treon & Dutra (1952)
h/day, ca. 5 days/wk for
up to 130 out of 199 days;
estimated dose over 7 h,
0.06 g/kg
Rabbit M 0.75 g/m3 (207 ppm), 7 no gross or microscopic alterations to Lewis et al. (1979)
(white, NZ) h/day, 5 days/week for 24 tissues
weeks; estimated dose
over 7 h, 0.03 g/kg
Rabbite 0.3 g/m3 (83 ppm), 7 h/day no deaths Treon & Dutra (1952)
for 130 out of 191 days;
estimated dose over 7 h,
0.014 g/kg
Rabbite 0.1 mg/m3 (27 ppm), 7 no gross or microscopic alterations to Lewis et al (1979)
h/day, 5 days/week for 6 tissues
months; estimated dose
over 7 h, 0.005 g/kg
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Inhalation studies (contd)
Guinea-pige 2.45 g/m3 (672 ppm), 7 no deaths Treon & Dutra (1952)
h/day for 5 days;
estimated dose over
7 h, 0.12 g/kg
Guinea-pige 1.2 g/m3 (328 ppm), 7 no deaths Treon & Dutra (1952)
h/day, ca. 5 days/week
for 95-130 days out of
up to 199 days; estimated
dose over 7 h, 0.06 g/kg
Monkeye 1.2 g/m3 (328 ppm), 7 no deaths Treon & Dutra (1952)
h/day, ca. 5 days/week
for 100 days exposure
0.3 g/m3 (83 ppm), no deaths Treon & Dutra (1952)
7 h/day for 130 out of
191 days
Intraperitoneal studies
Ratd 0.11 g/kg, 1/day for apparently no deaths prior to sacrifice Dequidt et al. (1972)
(Wistar) 7 days 3 days after the last injection;
methaemoglobin = 4.3%; nitrite = 0.29-1.15 ppm
in organs (heart, lungs, kidneys, spleen)
Ratd 0.11 g/kg, 1/day for 15 apparently no deaths prior to sacrifice 36 h
(Wistar) days after the last injection; methaemoglobin = 0;
nitrite = 0-10.8 ppm in organs (heart, lungs,
kidneys, spleen)
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Intraperitoneal studies
Rat F 0.001 g/kg, 5/week for no significant effects on kidney function Bernard et al. (1989)
(Sprague-Dawley) 2 weeks
Dermal study
Rabbite 1 application/day on no skin irritation, illness, systemic effects Machle et al. (1940)
clipped anterior abdomen or deaths
for 5 days; dose not
stated
a values from the literature recalculated as necessary to ppm or g/kg
b estimated dose calculated by the formula: tidal volume x respiration frequency x 2-NP conc. x alveolar retention/animal weight.
Tidal volume and respiration frequency from Baker et al. (1979) for rats (0.086 ml, 85.5 /min); from Hoar (1976) for guinea-pigs
(1.68 ml, 84/min); from Kozma et al. (1974) for rabbits (15.8 ml, 45/min); from Reece (1984) and Breazile (1971) for cats
(42 ml, 31/min); alveolar retention in rat (0.40) from Nolan et al. (1982), used for all species; where not stated in reference,
animal weights assumed to be average values, 250 g for rats, 500 g for guinea-pigs, 2.5 kg for rabbits, and 4.0 kg for cats
c strain not specified
d sex not specified
e neither strain nor sex specified
exposure (7 h/day) to 2.46 g/m3 (672 ppm), but death occurred in
rats exposed for 2 days (8 h/day) to 2.77 g/m3 (760 ppm). Rats
were reported to survive 15 days of daily intra-peritoneal
injections of 0.11 g/kg (Dequidt et al., 1972). The doses used in
these repeated exposures were found to produce no more than trace
levels of methaemoglobin (maximum = 4.3%) and low concentrations
(0 to 11 mg/kg) of nitrite in the tissues.
Non-lethal chronic doses of 2-NP have been shown to produce a
number of harmful effects in rats (Table 6). Exposure of rats to
0.75 g/kg (207 ppm) for up to 24 weeks (7 h/day, 5 days/week)
initially induced pulmonary lesions and oedema, hepatocellular
hypertrophy, hyperplasia, and necrosis of the liver (Lewis et al.,
1979). By the end of 24 weeks all the rats developed rapidly growing
hepatocellular carcinomas. A similar exposure to a slightly lower
concentration of 2-NP, 0.73 g/m3 (200 ppm), induced hepatic
nodules and other destructive changes in the liver, especially in
male rats (Griffin & Coulston, 1983). These changes included fatty
degeneration, nodules consisting mainly of hyperplastic areas,
distortion of lobular architecture, necrosis and peripheral
compression. Male rats also had an elevated serum alanine
aminotransferase level (an indicator of liver damage) and slightly
reduced growth. Chronic exposure of rats to 0.36 g/m3 (100 ppm)
for up to 18 months produced similar, although slightly less severe,
damage than that resulting from exposure to 0.73 g/m3 (200 ppm)
(Griffin & Coulston, 1983; Coulston et al., 1985). Only male rats
developed hepatocellular carcinoma; female rats had increased renal
calcification and occasional hepatic masses and nodules showing
hyperplasia and vacuolar degeneration. No toxic effects were
reported after chronic exposure of rats to 90 mg/m3 (25 ppm) or
100 mg/m3 (27 ppm) (Lewis et al., 1979; Griffin et al., 1980,
1981). Daily intraperitoneal injections of 1 mg/kg (5/week for 2
weeks) had no significant effect on kidney function (Bernard et al.,
1989).
Chronic oral dosage of 2-NP by gavage for 16 weeks produced
tumours in rats (Fiala et al., 1987b). The dose was 0.089 g/kg
(1 mmol/kg), given 3 times per week, and this yielded a weekly
dosage of 0.27 g/kg, an amount similar to the highest sustained
estimated inhalation dosage. The body weights of rats treated with
2-NP were significantly lower than those of controls, and all
treated rats surviving 16 weeks or longer developed both benign
tumours and massive hepatocellular carcinomas. Metastases to the
lungs were observed in four of the 22 surviving animals (Fiala et
al., 1987b). Wester et al. (1989) treated rats with oral doses of
0.002, 0.01, 0.05, and 0.25 g/kg, 5 times per week for 4 weeks by
gavage. With a dose of 0.25 g/kg there was some mortality among male
rats, and in both sexes there was decreased growth, anaemia,
increased liver and heart weights, and severe damage to the liver.
At a dose of 0.05 g/kg the major harmful effect appeared to be
anaemia. Lower concentrations (0.01 and 0.002 g/kg) did not produce
obvious harm over the period of the experiment.
Rabbits and mice appear more resistant to the sublethal effects
of 2-NP than rats (Table 6). Chronic inhalation exposure of five
rabbits to 0.75 g/m3 (207 ppm) (7 h/day, 5 days/week) for 24
weeks, a treatment which induced hepatocellular carcinomas and
severe liver damage in rats, had no detectable effect (Lewis et al.,
1979). Rabbits also were unaffected by repeated dermal application
of 2-NP (Machle et al., 1940). Liver damage was found in mice,
especially females, during chronic exposure to 0.72 g/m3 (200 ppm)
(7 h/day, 5 days/week) for 48 weeks, but hepatocellular or other
carcinomas were not detected (Griffin et al., 1984). Exposure of
mice to 0.36 g/m3 (100 ppm) (7 h/day, 5 days/week) for 18 months
produced some liver damage, especially in females, as shown by
nodular hyperplasia (Coulston et al., 1986, Griffin et al., 1987).
7.3 Reproduction, embryotoxicity, and teratogenicity
There is limited information on the embryotoxicity and
teratogenicity of 2-NP. Hardin et al. (1981) gave intraperitoneal
injections of 0.17 g/kg (1.91 mmol/kg) of 2-NP in corn oil to
pregnant rats on days 1-5 of gestation. The dosage was a previously
determined maximum tolerated dose which produced no mortality, no
marked signs of toxicity, and less than a 10% reduction in body
weight gain during dosing or within 2 weeks following 15 daily
intraperitoneal injections to non-pregnant rats. In pregnant rats,
this dose was reported to give no evidence of maternal toxicity or
teratogenicity, but produced a significant incidence of delayed
fetal development. Harris et al. (1979) reported that 2-NP at a dose
of 0.17 g/kg retarded fetal heart development by 1 to 2 days in pups
from 9 out of 10 litters produced by female rats treated with 2-NP.
In the affected litters, 30% to 86% of the pups had retarded heart
development.
There appear to be no studies that have examined specifically
the effects of 2-NP on reproductive function. No effects on
reproductive organs, however, were noted in the above two studies
nor in any of those studies on the effects of single exposures or
short-term or long-term administration of 2-NP (Tables 5 and 6).
There also was no evidence of an increase in dominant lethality or
in sperm abnormality in genetic studies relating to reproduction
(McGregor, 1981).
7.4 Mutagenicity and related end-points
7.4.1 Prokaryotes and yeast
2-NP has been found to be mutagenic in a variety of test
systems. All investigators reported mutagenicity in several strains
of Salmonella typhimurium used with the Ames test, both with and
without an exogenous activating system (S9) (Table 7). In addition,
Kawai et al. (1987) reported mutagenicity with a strain of
Escherichia coli, but Litton Bionetics, Inc. (1977) did not find
mutagenicity with a strain of Saccharomyces cerevisiae. Most
investigators found greater mutagenicity with activation by S9 than
without. With the strain that was generally most sensitive to 2-NP,
i.e. TA100, most investigators providing detailed results observed
an approximate doubling in the number of mutants at a concentration
of 3 mg 2-NP/plate. Göggelmann et al. (1988), however, observed a
10- to 12-fold increase in mutant numbers at 2.45 mg/plate, with or
without activation by S9. Fiala et al. (1987a) reported that
non-ionic 2-NP yielded only a doubling of mutant numbers at
4.9 mg/plate with S9 activation and no increase without activation,
whereas 2-NP nitronate at this concentration yielded a threefold
increase in mutant numbers with activation. Without activation 2-NP
nitronate at 2.5 mg/plate yielded a nearly 6-fold increase in mutant
numbers; nitronate at 4.9 mg/plate was toxic to this test organism.
Fiala et al. (1987a) concluded that the mutagenicity of 2-NP is
produced mainly or entirely by the nitronate anion and suggested
that the low level of mutagenicity observed with non-ionic 2-NP may
have resulted from its conversion to the nitronate form within the
microorganisms. A similar conclusion was drawn by Dayal et al.
(1989) from their comparison of 2-NP, nitromethane, nitroethane, and
their nitronates for mutagenicity in Salmonella. Some of the
variability in results obtained by others may stem from varying
proportions of non-ionic 2-NP and 2-NP nitronate in the stock 2-NP
used in their tests. Fiala et al. (1987a) further observed that
dimethyl sulfoxide (DMSO), a solvent used to solubilize 2-NP in
these tests, modified the mutagenicity of 2-NP nitronate in a
variable manner.
Several of the studies discussed above examined the question of
how 2-NP induces mutations in the test systems. In addition to 2-NP,
1-and 2-aminopropane and a number of mononitroalkanes (nitromethane,
nitroethane, 1-nitropropane, 1-and 2-nitrobutane, and 1-and
2-nitropentane) were tested for mutagenicity with bacterial systems,
mainly strains of Salmonella typhimurium (Hite & Skeggs, 1979;
Speck et al., 1982; Löfroth et al., 1986; Kawai et al., 1987;
Göggelmann et al., 1988; Dayal et al., 1989). Only 2-NP proved to be
significantly mutagenic. Some investigators observed weak
mutagenicity with nitroethane, 1-nitropropane, 2-nitrobutane, and
2-nitropentane, which, at least in the case of nitromethane and
nitroethane, may have been produced by the 2-NP present in these
solvents as a contaminant.
Table 7. Genotoxicity of 2-nitropropane
Test system Resulta Referencesb
Prokaryotes and yeastc
Salmonella typhimurium
strain TA92 + 1
strain TA92 with S9 + 1
strain TA98 + 1,2,5,7,8,9
- 6,16,19
strain TA98 with S9 + 1,2,3,5,8,9,17
strain TA98NR + 2,9
strain TA98NR with S9 + 2,9
strain TA100 + 1,2,4,5,6,7,8,9,19
- 17
strain TA100 with S9 + 1,2,3,4,5,6,8,9,17,19
strain TA100NR + 2,9
strain TA100NR with S9 + 2,9
strain TA102 + 4,8,19
strain TA102 with S9 + 4,8,19
Table 7 (contd)
Test system Resulta Referencesb
strain TA1535 ? 5
- 17
strain TA1535 with S9 + 3
? 5
- 17
strain TA1537 - 17
strain TA1537 with S9 - 3,17
strain TA1538 - 17
strain TA1538 with S9 - 17
Escherichia coli
strain WP2 uvrA/pKM101 + 7
strain WP2 uvrA/pKM101 + 7
Saccharomyces cerevisiae
strain D4 - 17
strain D4 with S9 - 17
Table 7 (contd)
Test system Resulta Referencesb
Eukaryotesf
In vivo
Drosophila
Sex-linked recessive lethal - 10,11
Mouse
erythrocytes
(micronuclei) m, f - 1,16,23
sperm (abnormality) ? 11
Rat
Liver (DNA repair synthesis) m, f + 12,13,23
(micronuclei) + 23
(DNA and RNA base + 18,20,21,22
modifications) m, f
(DNA strand breakage) m + 24
Kidney (DNA strand breakage) m - 24
(DNA and RNA base - 22
modifications) m, f
Table 7 (contd)
Test system Resulta Referencesb
Brain (DNA strand breakage) m - 24
Lung (DNA strand breakage) m - 24
Bone marrow (chromosome - 11
aberrations) m, f
(micronuclei) m - 23
Testes
(dominant lethal test) m - 11
In Vitro
Mouse
3T3-NIH fibroblasts - 13
(DNA repair synthesis)
Rat
hepatocytes + 12,13,26
(DNA repair synthesis) m, f
hepatoma cells, 2sFou + 25
(DNA repair synthesis and micronuclei)g
hepatoma cells, C2Rev7 + 25
(DNA repair synthesis and micronuclei)g
Table 7 (contd)
Test system Resulta Referencesb
hepatoma cells, H4IIEC3/G- + 25
(DNA repair synthesis, micronuclei and
gene mutations)g
208F - embryonic fibroblasts - 13
(DNA repair synthesis)
LLC WRC 256 carcinoma Walker rat - 13
(DNA repair synthesis)
Hamster
CHO cells (chromosome aberrations - 14
and sister chromatid exchanges)
CHO cells (DNA repair synthesis) - 13
V79 cells (DNA repair synthesis) - 12,13,25,26
V79 cells (mutations) + 25
V79 cells (micrnuclei) - 25,26
Human
Lymphocytes (chromosome aberrations -d 9,15
and sister chromatid exchanges)
Lymphocytes (chromosome aberrations -e 9,15
and sister chromatid exchanges) with
S9
Table 7 (contd)
Test system Resulta Referencesb
Human (contd)
Fibroblasts (DNA repair synthesis) - 11
W138 embryonic lung fibroblasts NC1- - 13
H322 adenocarcinoma lung cells A549
adenocarcinoma lung cells HEp2 epidermal
carcinoma larynx cells (DNA repair synthesis)
a + = positive response; - = negative response; ? = inconclusive result
b (1) Hite & Skeggs (1979) (2) Speck et al. (1982)
(3) Haworth et al. (1983) (4) Simmons et al. (1986)
(5) Löfroth et al. (1986) (6) Hughes et al. (1987)
(7) Kawai et al. (1987) (8) Fiala et al. (1987a)
(9) Göggelmann et al. (1988) (10) Zimmering et al. (1985)
(11) McGregor (1981) (12) Ziegler-Skylakakis et al. (1987)
(13) Andrae et al. (1988) (14) Galloway et al. (1987)
(15) Bauchinger et al. (1987) (16) Kliesch & Adler (1987)
(17) Litton Bionetics, Inc. (1977) (18) Conaway et al. (1991a)
(19) Conaway et al. (1991b) (20) Fiala et al. (1989)
(21) Hussain et al. (1990) (22) Guo et al. (1990)
(23) George et al. (1989) (24) Robbiano et al. (1991)
(25) Roscher et al. (1990) (26) Haas-Jobelius et al. (1991)
c Liver S9 fractions are prepared by treating rats, mice or hamsters with Aroclor
1254 or another microsomal enzyme inducer, and, after several days, removing
the livers, homogenizing them, and centrifuging. The supernatant, the S9
fraction, contains liver microsomal enzymes.
d Bauchiner et al. (1987) (ref. 15) reported a weak but significantly positive result
on one test, but considered results overall to be negative
e Göggelmann et al. (1988) (ref. 9) reported a very weak positive result
f m = male; f = female
g pretreated with dexamethasone
Löfroth et al. (1986) and Fiala et al. (1987a) speculated that
the relative mutagenicity of 2-NP and other nitroalkanes correlated
with the concentrations of their nitronate anions. The more highly
mutagenic compounds, such as 2-NP and 1,1-dinitroethane, had high
concentrations of nitronate at cellular pH. A thorough comparison of
2-NP, other secondary nitroalkanes, and their nitronates (Conaway et
al., 1991) showed greater mutagenicity of the nitronates and
confirmed these speculations.
These studies do not support the hypothesis that mutagenicity of
2-NP is produced largely or entirely by nitrite resulting from its
metabolic breakdown, since the response of tester strains to sodium
nitrite was quite different from their response to 2-NP (Löfroth et
al, 1986). Unlike nitroarenes and nitroheterocyclics, 2-NP probably
does not derive its mutagenicity exclusively from enzymatic
reduction to a hydroxylamine. Strains of S. typhimurium, i.e.
TA98NR and TA100NR, lacking the "classical" nitroreductase
demonstrated either a very slightly (Speck et al., 1982) or markedly
(Göggelmann et al., 1988) reduced, but still significant, level of
mutagenicity. Göggelmann et al. (1988) suggested that some of this
mutagenicity may be due to some residual nitroreductase activity
still present in these strains.
The increased mutagenicity generally observed with S9 activation
of 2-NP and the reduced mutagenicity in tester strains deficient in
nitroreductase activity suggest an involvement of metabolism in the
mutagenicity of 2-NP in S. typhimurium. Fiala et al. (1987a)
presented evidence that metabolic oxidation of the 2-NP anion can
result in the formation of reactive species such as hydroxyl
radicals, which are capable of damaging DNA bases. Incubation of
2-NP nitronate under roughly physiological conditions with thymidine
and a 1-electron oxidation system, horseradish peroxidase and
hydrogen peroxide, yielded 2-NP free radicals (which in part
condensed into 2,3-dimethyl-2,3-dinitrobutane) and oxidation
products of thymidine of the type produced by hydroxyl radical
attack. A common source of hydroxyl radicals is superoxide, which
is known to be produced during oxidation of 2-NP nitronate by
horseradish peroxidase and hydrogen peroxide (Porter & Bright,
1983). The extent to which such reactions may occur in the
Salmonella tester strains or in vivo is unknown. There is,
however, evidence from the literature for microbial oxidation of
2-NP and 2-NP nitronate (Kido et al., 1984; Fiala et al., 1987a).
The existence of this proposed mechanism for inducing mutations
through the production of DNA-damaging hydroxyl radicals is further
supported by the reduction in 2-NP mutagenicity in TA102 by DMSO, a
known scavenger of such radicals (Fiala et al., 1987a). The fact
that DMSO had little effect on the mutagenicity of 2-NP in TA100
indicated that formation of hydroxyl radicals cannot be the only
mechanism inducing mutations. Fiala et al. (1987a) hypothesized that
the 2-NP radical may act directly by forming adducts with DNA bases.
This would provide an additional mechanism for inducing mutations
that would not require hydroxyl radicals.
7.4.2 Eukaryotes
The genotoxic effects of 2-NP in eukaryotic organisms are
summarized in Table 7. Results were negative in two sex-linked
recessive lethal tests using Drosophila despite exposure to high
concentrations of 2-NP. Zimmering et al. (1985) dosed the flies by
injection and by feeding with concentrations that induced an
approximately 30% mortality, whereas McGregor (1981) exposed the
flies to 2-NP vapour at a concentration of 2.55 g/m3 (700 ppm) for
4.5 h. Sex-linked recessive lethal mutation frequency was not
increased except in mature spermatozoa in one stock of flies. Since
this increase was not reproducible, its significance is doubtful.
Results were variable but mainly negative in a variety of
in vivo mammalian test systems. In the dominant lethal test and
the bone marrow chromosomal aberration test using rats and in the
sperm abnormality test using mice, animals were exposed to 91 or
728 mg/m3 (25 or 200 ppm) for 7 h/day on 5 consecutive days.
Neither exposure level produced a positive response in any of the test
systems used (McGregor, 1981). Negative results were obtained with
the mouse micronucleus test even with an almost lethal oral dose of
0.3 g/kg on 2 consecutive days (Hite & Skeggs, 1979), and with a
single intraperitoneal dose of 0.3 g/kg (Kliesch & Adler, 1987).
One set of genotoxicity tests using rats, however, yielded
strongly positive results (Andrae et al., 1988; Ziegler-Skylakakis
et al., 1987). In both in vivo and in vitro tests, 2-NP induced
DNA repair synthesis in rat liver cells. In the in vitro
experiments, cultures of rat hepatocytes were incubated with 2-NP,
while in the in vivo experiments rats were injected
intraperitoneally with 2-NP, sacrificed 4 h later, and cultures of
their hepatocytes were examined for DNA repair synthesis. Exposure
of hepatocyte cultures for 18 to 20 h to concentrations of 2-NP as
low as 2.7 mg/litre (30 µmol/litre) induced a detectable
(approximately 2-fold above the control level) increase in repair
synthesis. The highest concentration tested, i.e. 89 mg/litre
(10 mmol/litre), induced a 12- to 15-fold increase above control
levels in hepatocytes from male rats and a 25- to 30-fold increase
in hepatocytes from female rats. The in vivo experiments also
demonstrated the existence of a sexual difference in susceptibility
to 2-NP. In males, the lowest dose (20 mg/kg) induced a doubling in
repair synthesis and the highest dose (80 mg/kg) a 3.6-fold
increase, whereas in females these doses induced a very small
increase and a doubling, respectively. In contrast to 2-NP, 1-NP
given in vivo had no effect on DNA repair synthesis and did not
increase in vitro repair synthesis above that expected from its
contamination with 2-NP. Andrae et al. (1988) considered that their
results from the in vivo experiments were in agreement with the
observed greater hepatocarcinogenicity of 2-NP in male rats than in
females. Their observation that 2-NP did not induce any increase in
repair synthesis in any of nine non-hepatic cell lines derived from
human, mouse, hamster, and rat tissues led the authors to suggest
that 2-NP is not a direct-acting genotoxic agent but rather requires
metabolic activation by liver-specific metabolism.
Several papers have confirmed and expanded these initial
observations on the genotoxicity of 2-NP to rat liver cells. George
et al. (1989) showed that oral dosage similarly induced unscheduled
DNA synthesis and also resulted in the formation of micronuclei in
rat liver. 2-NP did not, however, significantly increase the
frequency of micronuclei in mouse bone marrow. Intraperitoneal
injection of 0.1 g/kg produced in 6 h a significant increase in
8-hydroxydeoxyguanosine and 8-hydroxyguanosine, products
respectively of DNA and RNA damage caused by hydroxyl or other
oxygen-radical-forming agents (Fiala et al., 1989; Hussain et al.,
1990). This treatment produced significantly lower levels of
8-hydroxydeoxyguanosine, 8-hydroxyguanosine, and other presumed
modified nucleosides in female rats than in males, and had little
effect on the nucleic acids of the kidney, findings that are in
agreement with the known carcinogenicity of 2-NP (Guo et al., 1990).
The organ specificity of the genotoxicity of 2-NP in the rat was
confirmed by Robbiano et al. (1991) who reported that oral doses of
45-713 mg/kg produced maximum numbers of single strand breaks in the
liver 6 h after administration and did not induce DNA fragmentation
in lung, kidney, bone marrow or brain. Damage to rat liver nucleic
acids was also caused by intraperitoneal injection of other
secondary nitroalkanes and a ketoxime capable of being converted to
a secondary nitroalkane, but not with a primary or a tertiary
nitroalkane (Conaway et al., 1991). These authors suggest that the
greater genotoxicity of the secondary nitroalkanes may stem from the
greater stability of their nitronate forms at physiological pH
values.
Observations by Roscher et al. (1990) and Robbiano et al. (1991)
suggest that cytochrome P-450-dependent monooxygenases are important
in the activation of 2-NP in liver. Robbiano et al. (1991) found an
increase in damage to liver DNA in rats pretreated with
phenobarbital or ß-naphthoflavone, inducers of cytochrome
P-450-dependent monooxygenases, and a reduction in liver DNA damage
in rats pretreated with methoxsalen, an inhibitor of cytochrome
P-450. Roscher et al. (1990) examined the effect of 2-NP on rat
hepatoma cell lines that express various forms of cytochrome
P-450-dependent monooxygenases and V79 Chinese hamster cells that
lack these enzyme activities. 2-NP increased DNA repair synthesis,
micronuclei formation, and the frequency of mutants resistant to
6-thioguanine in hepatoma cells pretreated with dexamethasone, an
inducer of various liver-specific cytochrome P-450 forms.
Genotoxicity was reduced or absent in hepatoma cells not treated
with the inducer. In the V79 cells, 2-NP produced only mutations to
6-thioguanine resistance.
2-NP demonstrated only a very limited level of genotoxicity in
other cell lines. Results were negative in a DNA repair synthesis
assay with human diploid fibroblasts exposed for 3 h to 2-NP
concentrations up to 5 g/litre (56 mmol/litre) (McGregor, 1981), as
well as with various cell lines of extrahepatic origin (Andrae et
al., 1988). 2-NP did not cause chromosomal aberrations or SCEs in
Chinese hamster ovary (CHO) cells either with or without S9
(Galloway et al., 1987). In these experiments the maximum
concentrations used, i.e. 1.6 and 5.0 g/litre (18 and 56 mmol/litre)
were estimated to reduce cell growth by 50%. Weakly positive
results, however, were obtained in cytogenetic tests using human
lymphocytes (Bauchinger et al., 1987; Göggelmann et al., 1988).
Exposure of cells to high concentrations of 2-NP (commercial grade,
at least 94% 2-NP) for 1 h induced a significant increase in
chromosomal aberrations (open breaks accompanied by gaps) at a
concentration of 5.3 g/litre (60 mmol/litre) with S9 activation and
at 7.1 g/litre (80 mmol/litre) without S9 activation (Bauchinger et
al., 1987). In addition, the frequency of sister chromatid exchange
was significantly increased at all concentrations of 2-NP (0.7 to
7.1 g/litre; 7.5 to 80 mmol/litre), but only with S9 activation.
Repetition of the experiment with 2-NP of greater than 99% purity
yielded similar results (Göggelmann et al., 1988). There was no
significant increase in chromosomal changes without S9 activation,
but there was a small but significant increase at the highest
treatment levels, i.e. 7.1 and 10 g/litre (80 and 111 mmol/litre),
with S9 activation. 1-NP of 97% purity produced no significant
mutagenic or cytogenetic effects on this test system with or without
S9 activation. The authors concluded that 2-NP can exert a
clastogenic effect on human lymphocytes only with metabolic
activation, and hypothesized that it acted via a different metabolic
pathway in the lymphocytes than it did in the bacterial
S. typhimurium system, where it induced mutations without
exogenous activation.
7.5 Carcinogenicity
That 2-NP is unquestionably a potent carcinogen in rats was
demonstrated by Lewis et al. (1979). Inhalation exposure of male
Sprague-Dawley rats to 0.75 g/m3 (207 ppm) for 7 h/day, 5
days/week, over a 24-week period induced hepatocellular neoplasms in
all surviving animals. Inhalation exposure of rats to 0.36 g/m3
(100 ppm) similarly administered over 18 months was associated with
hepatocellular carcinomas in male rats and produced changes in the
livers of female rats (nodules showing hyperplasia and vacuolar
degeneration) which may have been precursors to carcinoma (Griffin &
Coulston, 1983; Coulston et al., 1985). Oral dosing of male rats
with 89 mg/kg (1 mmol/kg), three times a week for 16 weeks, induced
massive hepatocellular carcinomas in all rats, observed when they
were sacrificed 40 weeks later (Fiala et al., 1987a). Metastases to
the lungs were present in 4 out of 22 surviving animals, suggesting
a high degree of malignancy. Long-term inhalation exposure of rats
to low concentrations (100 mg/m3; 27 ppm) did not produce any
evidence of increased hepatocellular carcinoma or precursor tumour
lesions of any sort (Lewis et al., 1979; Griffin et al., 1980,
1981). Evidence that inhalation exposure to low doses of 2-NP can
cause DNA damage in rats was presented by Denk et al. (1990). Male
and female Sprague-Dawley rats, 4-6 days old at the beginning of
treatment, were exposed to 0, 91, 146, 182, 291, and 455 mg/m3 (0,
25, 40, 50, 80 and 125 ppm) for 6 h/day, 5 days/week for 3 weeks,
and this was followed by promotion with polychlorinated biphenyls
(Clophen A50) for 8 weeks. This treatment produced a dose-dependent
increase in the numbers of preneoplastic liver foci deficient in
adenosine-5'-triphosphatase. Cunningham & Matthews (1991) showed
that 2-NP can also induce cell proliferation in rat liver. Rats were
exposed to daily oral doses of 20, 40 or 80 mg/kg by gavage for 10
days, and cell proliferation during exposure was quantified by
measuring the incorporation of bromodeoxyuridine into newly
synthesized DNA. Exposure to 40 and 80 mg/kg resulted in
statistically significant increases in the frequency of S-phase
cells from 1.9% in the vehicle-treated animals to 6.3% and 11%,
respectively. Exposure to 20 mg/kg had no effect on the labelling
index. The non-carcinogenic isomer of 2-NP, 1-NP, did not affect DNA
synthesis at doses of 20, 40 and 80 mg/kg.
There is no substantial evidence that 2-NP induces cancer in
species other than the rat (Table 6). Inhalation exposure of rabbits
to 750 mg/m3 (207 ppm) for 1 h/day, 5 days/week for 24 weeks,
initially produced some evidence of liver damage. At the end of 6
months of exposure, the five rabbits examined displayed no more than
minor changes in the liver and no evidence of carcinoma (Lewis et
al., 1979). Exposure of mice to 728 mg/m3 (200 ppm), similarly
administered for 48 weeks, produced damage to the liver, especially
in females, but no carcinoma (Griffin et al., 1984). It is possible
that degenerative changes observed in the livers of exposed females
may have been precursors to subsequent tumour development. A lower
concentration of 2-NP, 360 mg/m3 (100 ppm), similarly
administered to ICR mice for 18 months, induced liver damage in the
form of nodular hyperplasia in females but no increase in
hepatocellular carcinoma (Coulston et al., 1986; Griffin et al.,
1987). The latter study is the only inhalation study in a species
other than the rat that was of sufficient duration to allow for
latency in tumour development. No studies of carcinogenicity using
oral dosing in any species except the rat have been reported. Thus,
despite the absence of clear evidence, the possibility of
carcinogenicity in species other than the rat cannot be discounted.
There appear to be no laboratory studies on species other than
rats, mice and rabbits suitable for assessing the potential
carcinogenicity of 2-NP. As discussed in section 8.2.2, there is no
evidence from limited epidemiological studies that 2-NP is
carcinogenic in humans.
Griffin & Coulston (1983) argued that 2-NP does not act as an
initiating carcinogen in the rat, but rather induces cancer as a
response to extensive liver damage (Angus Chemical Co., 1985). They
offered as evidence the results of an experiment demonstrating the
absence of long-term effects from short-term exposure to 2-NP
(Griffin et al., 1986). Exposure of rats of both sexes to 730 g/m3
(200 ppm), 7 h/day for 5 days, did not produce any effect on
longevity or on the development of hepatocellular or other
carcinomas up to the end of the experiment, which lasted for 94
weeks. This argument is markedly weakened by the observation of
Andrae et al. (1988) and others (Fiala et al., 1989; George et al.,
1989; Guo et al., 1990; Conaway et al., 1991) that 2-NP is an active
genotoxic agent in rat hepatocytes both in vitro and in vivo.
7.6 Pharmacological effects
In vivo pharmacological studies have not been performed.
However in vitro studies with guinea-pig tissues suggest that 2-NP
may have several pharmacological effects. A 0.1 µM (8.9 µg/litre)
solution inhibited oxygen consumption of polymorphonuclear
leucocytes by 50% (Estes & Gast, 1960). These authors further
reported that, with increasing concentrations of 2-NP, respiration
of heart homogenate was initially depressed and subsequently
stimulated.
2-NP also produces two opposite neural effects on smooth muscle
preparations (Bergman et al., 1962). Contraction is induced partly
by ganglionic stimulation and partly by direct liberation of a
transmitter from nerve endings, but at the same time 2-NP inhibits
the response to acetylcholine and other smooth muscle stimulants.
Bergman et al. (1962) found that the action of nitroparaffins on
smooth muscle was similar to that of nicotine in that their ability
to induce contraction was inhibited by morphine and by high, but not
low, concentrations of atropine.
8. EFFECTS ON HUMANS
8.1 General population exposure
As indicated in section 5.1, general population exposure to 2-NP
appears to be very low, and there is no information on the effects
of this exposure. 2-NP has been used as a component of paints and
other consumer products. There is no evidence that exposure to 2-NP
from the use of such products has resulted in detectable injury or
illness in the general population.
8.2 Occupational exposure
8.2.1 Acute toxicity
Significant human exposure to 2-NP is largely or entirely
occupationally related. High concentrations are acutely toxic and
seven industrial fatalities have been attributed to inhalation of
2-NP fumes (Gautier et al., 1964; Hine et al., 1978; Rondia, 1979;
Harrison et al., 1985, 1987; US NIOSH, 1987b). In all cases,
exposure was to a solvent mixture containing 2-NP, was for a total
of 5 to 16 h in the course of 1 to 3 days, and occurred while paints
or coatings were being applied in confined spaces, such as tank
interiors, underground vaults and ship's holds, with little or no
ventilation. The actual concentrations of 2-NP that caused deaths
were not measured, but were thought to be high and in one case
estimated at 2.18 g/m3 (600 ppm), as determined by GC-FID from the
2-NP content of the victim's blood (0.013 g/litre) (Harrison et al.,
1987). Initial symptoms requiring medical treatment appeared during
exposure or within a few hours following exposure and included
headache, nausea, dizziness, drowsiness, weakness, anorexia,
vomiting, diarrhoea, and neck, thoracic, and abdominal pain. Victims
were hospitalized and in general initially showed improvement, in
some cases to the point of being discharged after a day or less. But
improvement, where present, was temporary and in all seven cases was
followed within a few days by worsening condition and
rehospitalization of those previously discharged. Later symptoms
included persistent nausea, vomiting, anorexia, jaundice, reduced
urine output, diarrhoea, bloody stools, mental confusion,
restlessness, loss of reflexes, and increases in serum
aminotransferases and other indicators of hepatic lesions. Death
occurred within 4 to 26 days (average, 10 days) following exposure.
In all cases the primary cause was acute hepatic failure.
Contributing factors included lung oedema, gastrointestinal
bleeding, and respiratory and kidney failure. Postmortem microscopic
examination of the liver revealed necrosis of hepatic tissue and in
some cases fatty degeneration. None of the victims had a past
history of liver disease or drank excessively.
In addition to these fatalities, there have been four additional
serious but nonlethal cases attributed to acute exposure to 2-NP
(Gautier et al., 1964; Hine et al., 1978; Harrison et al., 1985,
1987; US NIOSH, 1987b). All but one were colleagues of the deceased
described above, but may have received lesser doses. Initial
symptoms were similar to those of the deceased, but were followed by
full recovery rather than decline and death. Serum enzyme levels,
however, remained mildly elevated for months following exposure. As
in the case of the deceased, the actual concentrations of 2-NP to
which these workers were exposed are unknown. One of the survivors
had a 2-NP serum concentration of 8.5 mg/litre when hospitalized.
Since his coworker hospitalized at the same time with a serum
concentration of 13 mg/litre subsequently died, Harrison et al.
(1987) speculated that either 2-NP has a very steep dose-response
curve or that there are substantial differences in individual
susceptibility. Six men (out of a group of approximately 300)
developed toxic hepatitis after exposure to an epoxy resin coating
containing 2-NP (Williams et al., 1974). The development of toxic
hepatitis was ascribed to methylenedianiline in the coating, and the
possibility that 2-NP may have been at least a partial cause was not
considered.
Exposure to lower concentrations of 2-NP appears to produce some
of the symptoms described above. Brief and intermittent exposure to
concentrations above 364 mg/m3 (100 ppm) may produce headache and
nausea (Angus Chemical Co. & Occusafe, Inc., 1986). Exposure to
vapours (estimated to contain 73 to 164 mg/m3 (20 to 45 ppm) using
colorimetric methods available at the time of the study) of a
solvent mixture that polluted the workplace air produced a daily
cycle in symptoms (Skinner, 1947). Workers started the day feeling
well, but by noon some exhibited nausea and lack of appetite. Later
in the working day these symptoms intensified and were accompanied
by vomiting and diarrhoea. Vomiting continued after the workers left
the workplace, and they were unable to eat supper but were able to
sleep. By morning they were feeling well again. Colleagues not
experiencing nausea and vomiting had occipital headaches, which
appeared and gradually intensified during the working day. All of
the workers were free of symptoms when away from the workplace for a
day or more, and the substitution of methyl ethyl ketone for 2-NP
brought complete relief. Skinner (1947) noted that in another plant
exposure of workers to 36-109 mg/m3 (10-30 ppm) for less than
4 h/day on not more than 3 days per week produced no noticeable ill
effects.
The flaw in all but one (Hine et al., 1978) of these case
studies is that exposure was to a solvent mixture containing 2-NP
and not to 2-NP alone (Hine et al., 1978). Thus the observed effects
cannot be assigned to 2-NP with total confidence. A number of
factors, however, point to 2-NP as the main, if not sole, causative
agent. It was the only solvent common to all the mixtures, and the
only solvent present in the mixtures known to be hepatotoxic.
Symptoms and damaging effects on the body from the solvent mixtures
were fairly similar to each other despite widely different solvent
compositions (apart from the common presence of 2-NP), and were
fairly similar to those in the single case resulting from exposure
to 2-NP alone. In addition, as described above, substitution of
another solvent for 2-NP eliminated all symptoms. The weight of
evidence thus supports the view that the symptoms and damage to the
human body described above which followed exposure to solvent
mixtures containing 2-NP, resulted mainly, if not entirely, from
2-NP.
8.2.2 Effects of long-term exposure
Despite the fact that 2-NP has been in use for over 40 years,
there are very few data on long-term effects and such information is
derived mainly from unpublished reports from manufacturers of 2-NP.
Angus Chemical Co., a major manufacturer of 2-NP, has assembled
information on more than 1800 occupationally exposed workers from a
variety of plants in the USA, Mexico, Germany, Sweden, and the
Netherlands (Table 8). Where information was available, medical
records on living employees indicated no problems with liver
functions and no obvious symptoms or conditions that could be
connected with chronic exposure to 2-NP (Angus Chemical Company and
Occusafe, Incorporated, 1986).
A major portion of the above investigation was an earlier
mortality study conducted by Miller & Temple (1979) on 1481 workers
employed in the manufacture of 2-NP during the period 1955 to 1977.
The exposures were defined as direct, indirect or zero exposure.
Formal industrial hygiene monitoring was not performed until 1977.
Individual exposures were based on job titles rather than actual
exposure data. Angus Chemical Company and Occusafe, Incorporated
(1986) concluded that analysis of these data did not suggest any
unusual cancer or other disease pattern in this group of workers.
They noted, however, that because the cohort was small and the
duration of exposure and observation was relatively short, it was
not possible to conclude from these data that 2-NP is not
carcinogenic to humans. In a follow-up report on the same cohort
(Bolender, 1983), the findings did not change the earlier
conclusion.
It is necessary to emphasize that this cohort had a limited
number of workers with long exposure (> 15 years). Since the
individual exposure data are not available, it cannot be concluded
from available data that 2-NP is non-carcinogenic in humans.
Table 8. Studies of long-term occupational exposure to 2-nitropropane
Activitya Exposure concentrationb Exposure duration No. of exposed Reference
mg/m3 (ppm) (years) employees
TWA STEL
Manufacture of 2-NP, 25-91 > 218 < 1-15 55 Angus Chem. Co. & Occusafe, Inc. (1986)
1940-1955 (7-25) (> 60) average 2.5
Manufacture of 2-NP, 3.6-36 91-5970 < 5-> 21 804 Angus Chem. Co. & Occusafe, Inc. (1986);
1946-1982, (1-10) (25-1640) average 8c Miller & Temple (1979); Bolender (1983)
Sterlington, LA, USA
Extraction of 3.6-193 109-473 < 5-ca. 25 18-19 Angus Chem. Co. & Occusafe, Inc. (1986);
triglycerides, USA (1-53; (30-130) Crawford et al. (1985); Tabershaw
average 9c) Occupational Medicine Assoc. (1980);
Life Extension Inst. (1983)
Automobile assembly unknown < 5-> 25 456 Angus Chem. Co. & Occusafe, Inc. (1986)
plant, Germany
Paint mfg., Germany unknown 5-10 6 Angus Chem. Co. & Occusafe, Inc. (1986)
Paint mfg., Germany 40-91 11-15 5 Angus Chem. Co. & Occusafe, Inc. (1986)
(11-25)
Chemical mfg., unknown 5-10 80 Angus Chem. Co. & Occusafe, Inc. (1986)
Germany
Chemical mfg., unknown < 5 4 Angus Chem. Co. & Occusafe, Inc. (1986)
Germany
Extraction plant, 3.6-18.2 73-364 5-10 15 Angus Chem. Co. & Occusafe, Inc. (1986)
Sweden (1-5) (20-100)
Table 8 (contd)
Activitya Exposure concentrationb Exposure duration No. of exposed Reference
mg/m3 (ppm) (years) employees
TWA STEL
Paint mfg., unknown 5-10 180 Angus Chem. Co. & Occusafe, Inc. (1986)
Netherlands
Chemical Co., USA 3.6-36 65.6-364 < 5-20 41 Angus Chem. Co. & Occusafe, Inc. (1986)
(1-10) (18-100)
Printing Co., USA 1.8-87 2.5-124 ? 140 Angus Chem. Co. & Occusafe, Inc. (1986)
(0.5-24) (0.7-34)
Paint mfg., Mexico 3.6 < 91 5-10 100 Angus Chem. Co. & Occusafe, Inc. (1986)
(1) (< 25)
Ink mfg., Mexico 11-18 73-80 5-10 8 Angus Chem. Co. & Occusafe, Inc. (1986)
(3-5) (20-22)
Coatings mfg., 14.6-91 237-251 < 5-20 30 Angus Chem. Co. & Occusafe, Inc. (1986)
Mexico (4-25) (65-69)
Chemical distilation, 36-55 > 91 < 5-10 2 Angus Chem. Co. & Occusafe, Inc. (1986)
Mexico (10-15) (> 25)
Paint mfg., USA unknown < 20 150 Angus Chem. Co. & Occusafe, Inc. (1986)
Automotive mfg., USA 3.6-36 142 < 18 10 Angus Chem. Co. & Occusafe, Inc. (1986)
(1-10) (39)
a mfg. = manufacturing; co. = company
b TWA = time weighted average; STEL = short-term exposure level; values in parentheses are in ppm
c This average is an approximation
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
The limited data on the effects of 2-NP on organisms other than
mammals is summarized below. Kido et al. (1975) reported that 2-NP
(5 g/litre) inhibited the growth of 10 of the 14 species of
microorganisms tested, although all 14 species contained enzymes
capable of oxidizing 2-NP to acetone and nitrite (see section 4.2).
Organisms inhibited by 2-NP included bacteria (Escherichia coli,
Pseudomonas iodinum), yeasts (Endomyces fibuliger, Hansenula
anomala, H. octospora, H. sauveolens, H. matritensis), and fungi
(Aspergillus niger, Penicillium oxalicum, Fusarium oxysporum).
Organisms capable of growing on the medium containing 2-NP similarly
included bacteria (Sarcina lutea, Brevibacterium protophormiae), a
yeast (Hansenula mrakii), and a fungus (Rhizopus batatas).
Fridman et al. (1976) reported that the minimum concentration of
2-NP inhibiting the growth of E. coli and Staphylococcus aureus
was 1000 mg/litre. Observations on aquatic macroorganisms appear
more limited than those on microorganisms. The lowest concentration
of 2-NP in sea water inducing narcosis in barnacle larvae at 18-22
°C was approximately 0.7-0.8 g/litre (Crisp et al., 1967). At 22 °C
the 96-h LC50 for fathead minnows (Pimephales promelas) was >
210 mg/litre (Curtis et al., 1980; Curtis & Ward, 1981). Aeration of
the holding tanks during the toxicity test produced flawed results
because 2-NP was lost continuously by volatilization and a nominal
highest concentration of 612.5 mg/litre was reduced to 496 mg/litre
at the start of the test and to 210 mg/litre by 96 h. There was no
significant mortality at concentrations of 2-NP below 210 mg/litre
although at lower concentrations it did produce severe (though
undescribed) sublethal effects. Observations on non-mammalian
terrestrial organisms are limited to two related species, the
oriental fruit fly (Dacus dorsalis) and the Mediterranean fruit
fly (Ceratitis capitata). Burditt et al. (1963) found that 2-NP
was not especially effective as a fumigant against eggs and larvae
of these species. The 2-h LC50 values for eggs and larvae of the
oriental fruit fly were > 103 and 75 g/m3 (> 28 300 and
20 600 ppm), respectively, and for the Mediterranean fruit fly, > 103
and 35 g/m3 (> 28 300 and 9600 ppm), respectively. These
observations, in summary, suggest a fairly low acute toxicity to
non-mammalian organisms.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Human health risks
Although there are no known biological sources of 2-NP, very low
level non-occupational exposure must be almost universal because
2-NP is known to be a minor component of tobacco smoke and probably
is present in smoke from other types of nitrate-rich organic matter.
Residues in food products containing fatty acids separated with 2-NP
and in beverage can linings and other coatings may represent
additional sources of exposure to low (µg) amounts of 2-NP. The
effects, if any, on human health of low-level exposure are unknown.
Sources of worker exposure include rotogravure and flexographic
inks used in printing, and coatings and adhesives used in industrial
construction and maintenance, highway markings, ship building and
maintenance, furniture manufacture, and food packaging. Based on a
survey conducted in the USA in 1980, occupational exposure to 2-NP
appeared to be limited to a fraction of 1% of the workforce, and
significant occupational exposure (exposure to > 9.1 mg/m3 (>
2.5 ppm)) to about 0.005% of the workforce. Thus, tens of thousands
of workers may have received significant occupational exposure
worldwide. The effects of this occupational exposure are unclear.
Very few industrial fatalities have been attributed to 2-NP. All
involved acute exposure to high concentrations of vapours from
solvent mixtures containing 2-NP during applications of coatings in
confined spaces; all deaths resulted primarily from hepatic failure.
Exposure to non-lethal concentrations of 2-NP may result in
temporary illness or discomfort, but there is no case history or
epidemiological evidence that the chemical induces cancer or other
long-term harmful effects in humans. On the other hand, the numbers
of individuals studied and the periods since first exposure to 2-NP
were inadequate to detect possible long-term harmful effects.
Animal data has demonstrated that 2-NP is a strong
hepatocarcinogen in rats. Chronic exposure to high but non-lethal
concentrations (at least 0.36 g/m3, 100 ppm) induced a high
frequency of hepatic tumours and hepatic cancer in rats but these
observations were not reproduced in limited studies on mice and
rabbits. The mechanism by which 2-NP induces cancer in rats has not
been elucidated as yet. 2-NP is strongly genotoxic to rat
hepatocytes both in vitro and in vivo. It appears likely that
the hepatocarcinogenicity of the compound in rats is a consequence
of liver-specific formation of reactive products. The DNA-damaging
species has not yet been identified. Since it can induce liver cell
proliferation, 2-NP may also have tumour-promoting activity.
Similarities or differences between metabolic activation of 2-NP in
rats and in humans are largely unknown. At present, any substance
shown to be carcinogenic in any mammalian species is considered a
potential human carcinogen. Accurate risk assessment from exposure
to 2-NP requires further studies.
10.2 Effects on the environment
2-NP does not represent a threat to the environment. It appears
highly mobile in the natural environment and is not accumulated in
any individual compartment. It is likely that 2-NP will be destroyed
by photolysis when exposed in the atmosphere to sunlight, and by
biological processes in soil and water. There are, however,
insufficient experimental and observational data on the behaviour of
2-NP in the environment to validate these assumptions or to estimate
rates of degradation in nature. Limited observations on
microorganisms, invertebrates, and fish show a low level of acute
toxicity to non-mammalian organisms.
11. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
There are no indications that 2-NP is carcinogenic in humans.
However, in view of its carcinogenicity in rats, it is recommended
that occupational exposure and its presence in consumer products
such as paints and varnishes be minimized and that it be replaced
with a less toxic solvent whenever practical. Monitoring of the
workplace should be continued to control actual worker exposure.
Although it is not feasible to eliminate non-occupational exposure,
such exposure should be minimized. 2-NP should not be used in food
processing.
12. FURTHER RESEARCH
12.1 Environment
Although it appears likely that 2-NP is highly mobile in the
environment, is not accumulated in any environmental compartment,
and is degraded in the environment to less toxic substances by
microorganisms and ultraviolet radiation, it would be desirable to
confirm experimentally these assumptions and to quantify the speed
of degradation in various environmental compartments.
12.2 Epidemiology
Cohorts of workers in production plants as well as cohorts of
workers using products containing 2-NP (e.g., inks, paints) should
be investigated for incidence of cancer and other ill effects.
12.3 Toxicokinetics
A more detailed examination of the metabolism and distribution
in the body of 2-NP and its metabolites is needed to facilitate an
understanding of the marked species and sex differences in toxic
effects. Studies on distribution and metabolism should include
metabolites of both the carbon and the nitrogen moieties.
Experimental data should be obtained on dermal uptake. Studies on
the metabolism of 2-NP in human cells are needed to facilitate the
eventual extrapolation of data obtained with laboratory animals to
humans.
12.4 Carcinogenesis
Continued efforts should be made to elucidate the biochemical
and molecular mechanisms whereby 2-NP induces genotoxic effects and
cancer.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
2-NP was evaluated by the Joint FAO/WHO Expert Committee on Food
Additives in 1979 and 1981, but no ADI was allocated. The Committee
recommended that 2-NP should not be used as a solvent in food
processing, but it was temporarily acceptable for use as a
fractionating solvent in the production of fats and oils (FAO/WHO,
1984). 2-NP was re-evaluated by JECFA in 1989; it was considered to
be a potent liver carcinogen in rats, and the temporary acceptance
for use as a fractionating solvent in the production of fats and
oils was not extended (FAO/WHO, 1990a, 1990b). The European Economic
Community found 2-NP to be flammable and to be harmful by
inhalation, ingestion, and dermal contact. It required that member
states ensure that it receives proper packaging, labelling, and
storage (IRPTC, 1986).
The International Agency for Research on Cancer evaluated 2-NP
in 1981 (IARC, 1982) and concluded that there was sufficient
evidence for its carcinogenicity in rats but that epidemiological
information was inadequate to evaluate its carcinogenicity to
humans. 2-NP is classified in Group 2B, i.e. as possibly
carcinogenic to humans (IARC, 1987).
REFERENCES
ACGIH (1986) Documentation of the threshold limit values and
biological exposure indices, 5th ed. Cincinnati, Ohio, American
Conference of Governmental Industrial Hygienists, Inc. pp 441-442.
Andersson K, Levin J-O, & Nilsson C-A (1983) Evaluation of solid
sorbents for sampling aliphatic and aromatic nitro compounds.
Chemosphere, 12: 377-384.
Andersson K, Levin J-O, Lindahl R, & Nilsson C-A (1984) Influence of
air humidity on sampling efficiency of some solid adsorbents used
for sampling organics from work-room air. Chemosphere, 13: 437-444.
Andrae U, Homfeld H, Vogl L, Lichtmannegger J, & Summer KH (1988)
2-Nitropropane induces DNA repair synthesis in rat hepatocytes
in vitro and in vivo. Carcinogenesis, 9: 811-815.
Angus Chemical Company (1985) Technical data sheet: NiPar S-20TM
nitropropane solvent, TDS 20B. Northbrook, Illinois, Angus Chemical
Company, 12 pp.
Angus Chemical Company & Occusafe, Incorporated (1986) Industrial
hygiene and occupational health information on 2-nitropropane,
December 1982, and updates for December 1983, 1984, 1985.
Northbrook, Illinois, Angus Chemical Company, 39 pp and 46
attachments (Unpublished report presented to the ACGIH TLV
Committee).
Anon (1976) Grande Paroisse looks for growth in nitroparaffins
market. Eur Chem News, 6 August: 23-24, 30.
Anon (1982) International mineral and chemical's nitroparaffins
business will be acquired by Alberto Natural Gas (Canada) and
Pacific Gas Transmission (US) for up to $ 55 million. Chem Week, 7
April: 24,251.
Baker PJ & Bollmeier AF (1981) Nitroparaffins. In: Grayson M &
Eckroth D ed. Kirk-Othmer encyclopedia of chemical technology, 3rd
ed. New York, John Wiley and Sons, vol 15, pp 969-987.
Baker HJ, Lindsey JR, & Weisbroth SH (1979) Appendix 1. Selected
normative data. In: Baker HJ, Lindsey JR, & Weisbroth SH ed. The
laboratory rat. Volume I: Biology and diseases. Orlando, Florida,
Academic Press, append 1.
Baldwin RS & Williams R (1977) 2-Nitropropane LC50. Northbrook,
Illinois, International Minerals and Chemical Corporation, 6 pp
(Unpublished report No. PLR-218/AMR-072).
Bauchinger M, Kulka U, & Schmid E (1987) Analysis of cytogenetic
effect in human lymphocytes induced by metabolically activated
2-nitropropane. Mutat Res, 190: 217-219.
Beall LR, Alexander V, Bien CT, Chen C, & Infante P (1980) Health
hazard alert - 2-Nitropropane. Cincinnati, Ohio, National Institute
for Occupational Safety and Health, 8 pp (Publication No. 80-142).
Bergman F, Chaimovitz M, & Wind E (1962) Dual action of
nitroparaffins on the guinea-pig ileum. Brit J Pharmacol,
18: 381-396.
Bernard AM, De Russis R, Normand J-C, & Lauwerys RR (1989)
Evaluation of the subacute nephrotoxicity of cyclohexane and other
industrial solvents in the female Sprague-Dawley rat. Toxicol Lett,
45: 271-280.
Bolender FL (1983) 2-NP mortality epidemiology study of the
Sterlington, LA employees: An update, 1-1-46 thru 12-31-81.
Northbrook, Illinois, International Minerals and Chemical
Corporation, 24 pp (Unpublished report).
Breazile JE (1971) Text book of veterinary physiology. Philadelphia,
Pennsylvania, Lea and Febiger, p 347.
Brown D & Dobbin R (1977) Industrial hygiene survey report. IMC,
Sterlington, Louisiana. Cincinnati, Ohio, National Institute for
Occupational Safety and Health, 14 pp.
Burditt AK, Hinman FG, & Balock FG (1963) Screening of fumigants for
toxicity to eggs and larvae of the oriental fruit fly and
Mediterranean fruit fly. J Econ Entomol, 56: 261-265.
Burrows GE (1979) Methylene blue or tolonium chloride antagonism of
sodium nitrite induced methemoglobinemia. J Vet Pharmacol Ther,
2: 81-86.
Cocalis JC (1982) Mining target environmental surveillance study.
Nitropropane sensitized, ammonium nitrate blasting agents.
Morgantown, West Virginia, National Institute for Occupational
Safety and Health, Environmental Investigations Branch, Division of
Respiratory Diseases Studies, 25 pp.
Conkle JP, Camp BJ, & Welch BE (1975) Trace composition of human
respiratory gas. Arch Environ Health, 30: 290-295.
Conaway CC, Nie G, Hussain NS, & Fiala ES (1991a) Comparison of
oxidative damage to rat liver DNA and RNA by primary nitroalkanes,
secondary nitroalkanes, cyclopentanone oxime, and related compounds.
Cancer Res, 51: 3143-3147.
Conaway CC, Nie G, Hussain NS, & Fiala ES (1991b) Evaluation of
secondary nitro-alkanes, their nitronates, primary nitroalkanes,
nitrocarbinols, and other aliphatic nitro compounds in the Ames
Salmonella assay. Mutat Res, 261: 197-207.
Coulston F (1982) Influence of glutathione or diethyl maleate in the
induction of hepatocellular carcinomas in rats exposed to 200 ppm of
2-nitropropane. Alamogordo, New Mexico, Coulston International
Corporation, 14 pp (Unpublished report).
Coulston F, Stein AA, & Griffin TB (1985) Pathology final report.
Histologic study of selected tissues from rats exposed to
2-nitropropane at 100 ppm. Alamogordo, New Mexico, Coulston
International Corporation, 222 pp.
Coulston F, Griffin T, & Eason R (1986) Chronic inhalation exposure
of mice to 2-nitropropane (100 ppm). Alamogordo, New Mexico,
Coulston International Corporation, 5 pp (Unpublished report).
Crawford GN, Garrison RP, & McFee DR (1984) Odor threshold
determination for 2-nitropropane. Am Ind Hyg Assoc J, 45: B7-B8.
Crawford GN, Garrison RP, & McFee, DR (1985) Health examination and
air monitoring evaluation for workers exposed to 2-nitropropane. Am
Ind Hyg Assoc J, 46: 45-47. Crisp DJ, Christie AO, & Ghobashy AFA
(1967) Narcotic and toxic action of organic compounds on barnacle
larvae. Comp Biochem Physiol, 22: 629-649.
Cunningham ML & Matthews HB (1991) Relationship of
hepatocarcinogenicity and hepatocellular proliferation induced by
mutagenic noncarcinogens vs carcinogens. Toxicol Appl Pharmacol,
110: 505-513.
Cupitt LT (1980) Fate of toxic and hazardous materials in the air
environment. Research Triangle Park, North Carolina, US
Environmental Protection Agency, 28 pp (EPA-600/3-80-084).
Curtis MW & Ward CH (1981) Aquatic toxicity of forty industrial
chemicals: Testing in support of hazardous substance spill
prevention regulation. J Hydrol, 51: 359-367.
Curtis MW, Curran CM, & Ward CH (1980) Aquatic toxicity testing as
fundament for a spill prevention program. In: Proceedings of the
1980 Conference on Control of Hazardous Material Spills. Nashville,
Tennessee, Vanderbilt University, pp 284-288.
Dayal R, Gescher A, Harpur ES, Pratt I, & Chipman JK (1989)
Comparison of the hepatotoxicity in mice and the mutagenicity of
three nitroalkanes. Fundam Appl Toxicol, 13: 341-348.
Dayal R, Goodwin B, Linhart I, Mynett K, & Grescher A (1991)
Oxidative denitrification of 2-nitropropane and propane-2-nitronate
by mouse liver microsomes: lack of correlation with hepatic
potential. Chem Biol Interact, 79(1): 103-114.
De Bruin A (1976) Biochemical toxicology of environmental agents.
Amsterdam, Oxford, New York, Elsevier Science Publishers, p 104.
Denk B, Bauman M, & Filser JG (1989) Pharmacokinetics and
hepatotoxicity of 2-nitropropane in rats. Arch Toxicol, 13(suppl):
330-332.
Denk B, Filser JG, Demi E, Kessler W, Shen J, & Oesterle D (1990)
Dose-dependent emergence of preneoplastic foci in rat livers after
exposure to 2-nitropropane. Arch Toxicol, 64: 329-331.
Dequidt J, Vasseur P, & Potencier J (1972) Etude toxicologique
expérimentale de quelques nitroparaffines : 1. Etude du
2-nitropropane. Bull Soc Pharm Lille, 1972: 83-89.
Derks HJGM, Van Heiningen A, Klaassen R, & Olling M (1988) High
performance liquid chromatographic method for the determination of
2-nitropropane in rat plasma. J Chromatogr, 442: 436-440.
Derks HJGM, Van Heiningen A, Klaassen R, & Olling M (1989)
Toxicokinetics of 2-nitropropane in the rat. Bilthoven, The
Netherlands, National Institute of Public Health and Environmental
Protection, 19 pp (Report No. 328605.003).
De Rycker J & Halliwell B (1978) Oxidation of 2-nitropropane by
horseradish peroxidase. Biochem J, 175: 601-606.
Dhawale MR & Hornemann U (1979) Nitroalkane oxidation by
Streptomycetes. J Bacteriol, 137: 916-924.
Estes FL & Gast JH (1960) The in vitro effects of aliphatic nitro
compounds on tissues. Arch Environ Health, 1: 59-64.
FAO/WHO (1984) Evaluation of certain food additives and
contaminants. Twenty-eighth report of the Joint FAO/WHO Expert
Committee on Food Additives. Geneva, World Health Organization,
44 pp (WHO Technical Report Series 710).
FAO/WHO (1990a) Evaluation of certain food additives and
contaminants. Thirty-fifth report of the Joint FAO/WHO Expert
Committee on Food Additives. Geneva, World Health Organization,
48 pp (WHO Technical Report Series 789).
FAO/WHO (1990b) Toxicological evaluation of certain food additives
and contaminants, prepared by the 35th meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA). Geneva, World Health
Organization, 189 pp (WHO Food Additives Series 26).
Fiala ES, Czerniak R, & Williams GM (1986) Formation of carcinogenic
and/or genotoxic aliphatic azoxy compounds from the respective
nitroalkanes by direct mild chemical reduction. Toxicologist,
6: 40.
Fiala ES, Conaway CC, Biles WT, & Johnson B (1987a) Enhanced
mutagenicity of 2-nitropropane nitronate with respect to
2-nitropropane possible involvement of free radical species. Mutat
Res, 179: 15-22.
Fiala ES, Czerniak R, Castonguay A, Conaway CC, & Rivenson A (1987b)
Assay of 1-nitropropane, 2-nitropane, 1-azoxypropane and
2-azoxypropane for cancinogenicity by gavage in Sprague-Dawley rats.
Carcinogenesis, 8: 1947-1949.
Fiala ES, Conaway CC, & Mathis JE (1989) Oxidative DNA and RNA
damage in the livers of Sprague-Dawley rats treated with the
hepatocarcinogen 2-nitropropane. Cancer Res, 49: 5518-5522.
Fieser LF & Fieser M (1972) Reagents for organic synthesis. New
York, Wiley-Interscience, vol 3, pp 213-214.
Fieser LF & Fieser M (1974) Reagents for organic synthesis. New
York, Wiley-Interscience, vol 4, p 256.
Filser JG & Baumann M (1988) Pharmokinetics of 2-nitropropane in
rats and determination of enzymes in serum specific for liver
injury. Naunyn Schmiedeberg's Arch Pharmacol, 337(suppl): R17.
Fine DH, Challis BC, Hartman P, & Van Ryzin J (1982) Endogenous
synthesis of volatile nitrosamines: model calculations and risk
assessment. In: N-nitroso compounds: Occurrence and biological
effects. Proceedings of the VIIth International Symposium on
N-Nitroso Compounds, Tokyo, 28 September-1 October 1981. Lyon,
International Agency for Research on Cancer, pp 379-396 (IARC
Scientific Publications No. 41).
Finklea JF (1977) Current NIOSH intelligence bulletin:
2-nitropropane. Am Ind Hyg Assoc J, 38(7): A-15, A-17, A-19.
Fishbein L (1981) Carcinogenicity and mutagenicity of solvents. I.
Glycidyl ethers, dioxane, nitroalkanes, dimethylformamide and allyl
derivatives. Sci Total Environ, 17: 97-110.
Flanner LT (1972) 2-Nitropropane or nitromethane for inhibiting the
corrosion of tin-plated steel aerosol cans: US Patent US 3650982
(21 March 1972). USA, Allied Chemical Corporation, 2 pp (Abstract
only).
Freitag D, Geyer H, Kraus A, Viswanathan R, Kotzias D, Attar A,
Klein W, & Korte F (1982) Ecotoxicological profile analysis. VII.
Screening chemicals for their environmental behavior by comparative
evaluation. Ecotoxicol Environ Saf, 6: 60-81.
Freitag D, Ballhorn L, Geyer H, & Korte F (1985) Environmental
hazard profile of organic chemicals. Chemosphere, 14: 1589-1616.
Friedman MA, Greene EJ, & Epstein SS (1972) Rapid gastric absorption
of sodium nitrite in mice. J Pharm Sci, 61: 1492-1494.
Friedman AL, Zalesov VS, Surkov VD, Kratynskaya LV, & Plaksina AN
(1976) [Synthesis and study of the physiological activity of
aliphatic nitro compounds. X. Relation among structure, toxicity,
and antimicrobial activity in a series of nitroalkanes and their
alpha-halo derivatives.] Khim-Farm Zh, 10: 53-56 (in Russian).
Galloway SM, Armstrong MJ, Reuben C, Colman S, Brown B, Cannon C,
Bloom AD, Nakamura F, Ahmed M, Duk S, Rimpo J, Margolin BH, Resnick
MA, Anderson B, & Zeiger E (1987) Chromosome aberrations and sister
chromatid exchanges in Chinese hamster ovary cells: Evaluations of
108 chemicals. Environ Mol Mutagen, 10(suppl 10): 1-175.
Gautier M, Fournier P-E, Gervais P, & Sicot C (1964) Intoxication
par le nitropropane. Arch Mal Prof, 25: 425-428.
George E, Burlinson B, & Gatehouse D (1989) Genotoxicity of 1- and
2-nitropropane in the rat. Carcinogenesis, 10: 2329-2334.
Glaser RA, & Woodfin WJ (1981) A method for sampling and analysis of
2-nitropropane in air. Am Ind Hyg Assoc J, 42: 18-22.
Goggelmann W, Bauchinger M, Kulka U, & Schmid E. (1988) Genotoxicity
of 2-nitropropane and 1-nitropropane in Salmonella typhimurium and
human lymphocytes. Mutagenesis, 3: 137-140.
Goldwhite H (1965) Nitrogen derivatives of the aliphatic
hydrocarbons. In: Coffey S ed. Rodd's chemistry of carbon compounds,
Part B, 2nd ed. Amsterdam, Oxford, New York, Elsevier Science
Publishers, vol. 1, pp 93-164.
Griffin TB & Coulston F (1983) Final report. Chronic inhalation
exposure of rats to vapors of 2-nitropropane at 100 ppm. Alamogordo,
New Mexico, Coulston International Corporation, 13 pp.
Griffin TB, Benitz K-F, Coulston F, & Rosenblum I (1978) Chronic
inhalation toxicity of 2-nitropropane in rats. Pharmacologist, 20:
145.
Griffin TB, Coulston F, & Stein AA (1980) Chronic inhalation
exposure of rats to vapors of 2-nitropropane at 25 ppm. Ecotoxicol
Environ Saf, 4: 267-281.
Griffin TB, Stein AA, & Coulston F (1981) Histologic study of
tissues and organs from rats exposed to vapors of 2-nitropropane at
25 ppm. Ecotoxicol Environ Saf, 5: 194-201. Griffin T, Coulston F, &
Stein AA (1984) Chronic inhalation exposure of mice to
2-nitropropane. Preliminary report: Laboratory findings and
histopathology of liver. Alamogordo, New Mexico, Coulston
International Corporation, 14 pp (Unpublished report).
Griffin T, Coulston F, & Stein AA (1986) Long-term effects of acute
exposure of rats to 2-nitropropane. Final Report, Study No. 820202.
Alamogordo, New Mexico, Coulston International Corporation, 17 pp
(Unpublished report).
Griffin T, Coulston F, & Stein AA (1987) Chronic inhalation exposure
of mice to 2-nitropropane (100 ppm): Final report. Alamogordo, New
Mexico, Coulston International Corporation, 24 pp (Unpublished
report).
Guo N, Conaway CC, Hussain NS, & Fiala ES (1990) Sex and organ
differences in oxidative DNA and RNA damage due to treatment of
Sprague-Dawley rats with acetoxime or 2-nitropropane.
Carcinogenesis, 11: 1659-1662.
Haas-Jobelius M, Ziegler-Skylakakis K, & Andrae U (1991)
Nitroreduction is not involved in the genotoxicity of 2-nitropropane
in cultured mammalian cells. Mutagenesis, 6: 87-91.
Hardin BD, Bond GP, Sikov MR, Andrew FD, Beliles RP, & Niemeier RW
(1981) Testing of selected workplace chemicals for teratogenic
potential. Scand J Work Environ Health, 7(suppl 4): 66-75.
Harris SJ, Bond GP, & Niemeier RW (1979) The effects of
2-nitropropane, naphthalene, and hexachlorobutadiene on fetal rat
development. Toxicol Appl Pharmacol, 48: A35.
Harrison RJ, Pasternak G, Blanc P, Basuk P, & Letz G (1985) Acute
hepatic failure after occupational exposure to 2-nitropropane. J Am
Med Assoc, 254(24): 3415-3416.
Harrison RJ, Letz G, Pasternak G, & Blanc P (1987) Fulminant hepatic
failure after occupation exposure to 2-nitropropane. Ann Intern Med,
107: 466-468.
Hartle RW (1980) Health hazard evaluation report No. 80-057-781 at
Long Island Railroad, Richmond Hill, New York, Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 17 pp.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, 5 (suppl 1): 3-142.
Hine CH, Pasi A, & Stephens BG (1978) Fatalities following exposure
to 2-nitropropane. J Occup Med, 20: 333-337.
Hite M & Skeggs H (1979) Mutagenic evaluation of nitroparaffins in
the Salmonella typhimurium/mammalian microsome test and the
micronucleus test. Environ Mutagen, 1: 383-389.
Hoar RM (1976) Chapter 3. Biomethodology. In: Wagner JE & Manning PJ
ed. The biology of the guinea pig. New York, London, Academic Press,
p 16.
Hoffmann D & Rathkamp C (1968) Chemical studies on tobacco smoke.
III. Primary and secondary nitroalkanes in cigarette smoke. Beitr
Tabakforsch, 4: 124-134.
Hollett BA & Schloemer J (1978) Hazard evaluation technical
assistance report No. TA 76-90: Newport Industrial Products
Firestone Tire Rubber Co., Newport, Tennessee. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 52 pp.
Hughes TJ, Simmons DS, Monteith LG, Myers LD, & Claxton LD (1987)
Mutagenicity of 31 organic compounds in a modified preincubation
Ames assay with Salmonella typhimumium strains TA100 and TA102.
Environ Mutagen, 9 (suppl 8): 49.
Hussain NS, Conaway CC, Guo N, Asaad W, & Fiala ES (1990) Oxidative
DNA and RNA damage in rat liver due to acetoxime: similarity to
effects of 2-nitropropane. Carcinogenesis, 11: 1013-1016.
Indig GL & Cilento G (1987) Peroxidase-promoted aerobic oxidation of
2-nitropropane: Mechanism of excited state formation. Biochim
Biophys Acta, 923: 347-354.
IARC (1982) 2-Nitropropane. In: Some industrial chemicals and
dyestuffs. Lyon, International Agency for Research on Cancer, pp
331-343 (IARC Monographs on the Evaluation of the Carcinogenic Risk
of Chemicals to Humans, Volume 29).
IARC (1987) Overall evaluations of carconogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer, p 67 (IARC Monographs on the Evaluation of the
Carconogenic Risk of Chemicals to Humans, Supplement 7).
IRPTC (1986) IRPTC data profile on 2-nitropropane. Geneva,
International Register of Potentially Toxic Chemicals, United
Nations Environment Programme, pp 17/1-17/3.
Ivanetich KM, Lucas S, Marsh JA, Ziman MR, Katz ID, & Bradshaw JJ
(1978) Organic compounds. Their interaction with and degradation of
hepatic microsomal drug-metabolizing enzymes in vitro. Drug Metab
Disp, 6: 218-225.
Jones LR (1963) The determination of 2-nitropropane in air. Ind Hyg
J, January-February: 11-16.
Jones LR & Riddick JA (1952) Colorimetric determination of
nitroparaffins. Anal Chem, 24: 1533-1536.
Jonsson J, Kammerer RC, & Cho AK (1977) Metabolism of
2-nitro-1-phenylpropane by rabbit liver microsomes. Res Commun chem
Pathol Pharmacol, 18: 75-82.
Kaplan HM, Brewer NR, & Blair WH (1983) Physiology. In: Foster HL,
Small JD, & Fox JG ed. The mouse in biomedical research. Volume III
Normative biology, immunology, and husbandry. New York, London,
Academic Press, p 255.
Kawai A, Goto S, Matsumoto Y, & Matsushita H (1987) [Mutagenicity of
aliphatic and aromatic nitro compounds. Industrial materials and
related compounds.] Jpn J ind Health, 29: 34-54 (in Japanese).
Kenaga EE (1980) Predicted bioconcentration factors and soil
sorption coefficients of pesticides and other chemicals. Ecotoxicol
environ Saf, 4: 26-38.
Kido T, Yamamoto T, & Soda K (1975) Microbial assimilation of alkyl
nitro compounds and formation of nitrite. Arch Microbiol, 106:
165-169.
Kido T, Tanizawa K, Inagaki K, Yoshimura T, Ishida M, Hashizume K, &
Soda K (1984) 2-Nitropropane dioxygenase from Hansenula mrakii:
Re-characterization of the enzyme and oxidation of anionic
nitroalkanes. Agric Biol Chem, 48: 2549-2554.
Kliesch U & Adler I-D (1987) Micronucleus test in bone marrow of
mice treated with 1-nitropropane, 2-nitropropane and cisplatin.
Mutat Res, 192: 181-184.
Korte F, Freitag D, Geyer H, Klein W, Kraus AG, & Lahaniatis E
(1978) Ecotoxicologic profile analysis. Chemosphere, 1: 79-102.
Kozma C, Macklin W, Cummins LM, & Mauer R (1974) Chapter 3. Anatomy,
physiology, and biochemistry of the rabbit. In: Weisbroth SH, Flatt
RE, & Kraus AL ed. The biology of the laboratory rabbit. New York,
London, Academic Press, pp 56-57.
Krotoszynski BK, Bruneau GM, & O'Neill HJ (1979) Measurement of
chemical inhalation exposure in urban population in the presence of
endogenous effluents. J Anal Toxicol, 3: 225-234.
Kuo FK & Fridovich (1986) Free-radical chain oxidation of 2-NP
initiated and propagated by superoxide. Biochem J, 237: 505-510.
Levy A (1973) The photochemical smog reactivity of organic solvents.
Adv Chem Ser, 124: 70-94.
Lewis TR, Ulrich CE, & Busey WM (1979) Subchronic inhalation
toxicity of nitromethane and 2-nitropropane. J Environ Pathol
Toxicol, 2: 233-249.
Life Extension Institute (1983) Summary report of medical findings
for selected employees at one plant. Rockville, Maryland, Life
Extension Institute, 12 pp.
Little HN (1951) Oxidation of nitroethane by extracts from
Neurospora. J Biol Chem, 193: 347-358.
Little HN (1957) The oxidation of 2-nitropropane by extracts of pea
plants. J Biol Chem, 229: 231-238.
Litton Bionetics, Inc. (1977) Mutagenicity evaluation of P-1357
66459T: Final report. Terre Haute, Indiana, IMC Chemical Group,
Inc., 10 pp (Unpublished report).
Löfroth G, Nilsson L, & Andersen JR (1986) Structure-activity
relationship of nitroalkane-induced mutagenicity in the Ames
Salmonella assay. In: Genetic toxicology of environmental
chemicals. Part B: Genetic effects and applied mutagenesis. New
York, Alan R. Liss, Inc., pp 149-155.
Lotz F, Nitz S, & Korte F (1979) [Photomineralization of adsorbed
organic chemicals on a microscale.] Chemosphere, 10: 763-768 (in
German).
Love JR & Kern M (1981) Health hazard evaluation report no.
81-065-938. Metro bus maintenance shop, Washington, DC. Cincinnati,
Ohio, National Institute for Occupational Safety and Health, 13 pp.
Mabuchi H (1979) [Urinary and serum volatile substances in
diabetics.] Nippon Naika Gakkai Zasshi, 58: 731-743 (Abstract only)
(in Japanese).
McGregor DB (1981) Tier II mutagenic screening of 13 NIOSH priority
compounds. Individual compound report: 2-Nitropropane. Musselburgh,
Scotland, Inveresk Research International Ltd, 194 pp (Report No.
31).
Machle W, Scott EW, & Treon J (1940) The physiological response of
animals to some simple mononitroparaffins and to certain derivatives
of these compounds. J Ind Hyg Toxicol, 22: 315-332.
Malkinson FD & Gehlmann L (1977) Factors affecting percutaneous
absorption. In: Drill VA & Lazar P, ed. Cutaneous toxicity. New
York, London, Academic Press, pp 63-82.
Marcstrom A (1967) Studies on the connection between physicochemical
properties and stimulating abilities of some sweet and bitter
compounds. Ark Zool, 19: 421-535.
Marker EK & Kulkarni AP (1985) Enzymatic denitrification of
2-nitropropane in uninduced mouse liver microsomes. Toxicol Lett,
26: 181-185.
Marker EK & Kulkarni AP (1986a) Cumene hydroperoxide-supported
denitrification of 2-nitropropane in uninduced mouse liver
microsomes. Int J Biochem, 18: 595-601.
Marker EK & Kulkarni AP (1986b) Cytochrome P-450-mediated
denitrification of 2-nitropropane in mouse liver microsomes. J
Biochem Toxicol, 1: 71-83.
Miller ME & Temple GW (1979) 2-NP mortality epidemiology study of
the Sterlington, LA Employees, 1-1-46 thru 6-30-77. Northbrook,
Illinois, International Minerals and Chemical Corporation, 34 pp
(Unpublished report).
Mochizuki H, Kominami S, & Takemori S (1988) Examination of
differences between benzo[ a]pyrene and steroid hydroxylases in
guinea pig adrenal microsomes. Biochem Biophys Acta, 964: 83-89.
Müller WF, Coulston F, & Korte F (1983) Comparative metabolism of
2-nitropropane in rats and chimpanzees. Chemosphere, 12: 231-237.
National Fire Protection Association (1968) Hazardous chemicals
data. J Chem Educ, 45: A313-A314, A317-A318, 320-322.
National Library of Medicine (1989) Toxic chemical release
inventory. Washington, DC, National Library of Medicine (TOXNET
system, TRI data base, 12 June 1989).
Nielsen AT (1981) Nitronic acids and esters. In: Feurer H ed. The
chemistry of the nitro and nitroso groups. Huntington, New York,
Robert Krieger Publishing Co., Part I, pp 349-386.
Nolan RJ, Unger SM, & Muller CJ (1982) Pharmacokinetics of inhaled
[14C]-2-nitropropane in male Sprague-Dawley rats. Ecotoxicol
Environ Saf, 6: 388-397.
Occupational Health Services, Inc. (1982) An evaluation of the
number of workers occupationally exposed to 2-nitropropane.
Cambridge, Massachusetts, Occupational Health Services, Inc., 22 pp
(Unpublished report prepared for IMC Corporation, Des Plaines,
Illinois).
Parks NJ, Krohn KA, Mathis CA, Chasko JH, Geiger KR, Gregor ME, &
Peek NF (1981) Nitrogen-13-labeled nitrite and nitrate: Distribution
and metabolism after intratracheal administration. Science, 212:
58-61.
Paszyc S (1971) [Photolysis of 2-nitropropane in gaseous and liquid
phases.] Poznan Tow Przyj Nauk Pr Kom Mat-Przyr, Pr Chem, 12: 79-85
(in Polish).
Patel RN, Hou CT, Laskin AI, & Felix A (1982) Epoxidation of alkenes
and hydroxylation of alkanes by soluble methane monooxygenase:
Regeneration of cofactor NADH2. J Appl Biochem, 4: 175-184.
Porter DJ & Bright HJ (1983) The mechanism of oxidation of
nitroalkanes by horseradish peroxidase. J Biol Chem, 258: 9913-9924.
Purcell RF (1967) Use of 2-nitropropane under rule 66. West Paint
Rev, 53: 22A, 24A.
Reece WO (1984) Respiration in mammals. In: Swenson MJ ed. Duke's
physiology of domestic animals, 10th ed. Cornell, New York, Comstalk
Press, p 231.
Robbiano L, Mattioli F, & Brambilla G (1991) DNA fragmentation by
2-nitropropane in rat tissues, and effects of the modulation of
biotransformation processes. Cancer Lett. 57: 61-66.
Rondia D (1979) 2-nitropropane: One more death. Vet Hum Toxicol, 21:
183-185.
Roscher E, Ziegler-Skylakakis K, & Andrae U (1990) Involvement of
different pathways in the genotoxicity of nitropropanes in cultured
mammalian cells. Mutagenesis, 5: 375-380.
Sadtler SP (1961) Sadtler standard ultraviolet spectra.
Philadelphia, Pennsylvania, Sadtler Research Laboratory, p 1.
Sakurai H, Hermann G, Ruf HH, & Ullrich V (1980) The interaction of
aliphatic nitro compounds with the liver microsomal monooxygenase
system. Biochem Pharmacol, 29: 341-345.
Sander J & Schweinsberg F (1972) [Interrelationships between
nitrate, nitrite and carcinogenic N-nitroso-compounds. 1.
Communication: nitrate, nitrite and nitrosable amino-compounds in
food and drugs, chemistry of N-nitroso compounds.] Zentrabl Bakeriol
Parasitenkd Infektionsk Hyg Abt 1: Orig Reihe B, 156: 299-340 (in
German).
Schneider NR & Yeary RA (1975) Nitrite and nitrate pharmacokinetics
in the dog, sheep, and pony. Am J Vet Res, 36: 941-947.
Scott EW (1943) The metabolism of monoparaffins. III. The
concentration of nitroethane, nitrite and nitrate in the blood of
rabbits during exposure by inhalation and oral administration. J Ind
Hyg Toxicol, 25: 20-25.
Seizinger DC & Dimitrades B (1972) Oxygenates in exhaust from simple
hydrocarbon fuels. J Air Pollut Control Assoc, 22: 47-51.
Simmons DM, Monteith LG, Hughes TJ, & Claxton LD (1986) Effect of
preincubation time on mutagenic activity in the Ames/ Salmonella
assay. Environ Mutagen, 8(suppl 6): 78.
Skinner JB (1947) The toxicity of 2-nitropropane. Ind Med, 16:
441-443.
Smith EL, Hill RL, Lehman IR, Lefkowitz RJ, Handler P, & White A
(1983) Principles of biochemistry. General aspects. New York,
McGraw-Hill, pp 539-541.
Soda K, Kido T, & Asada K (1977) 2-Nitropropane dioxygenase from
Hansenula mrakii: Generation and participation of superoxide anion
in the reaction. In: Hayashi O & Asada K ed. Biochemical and medical
aspects of active oxygen. Baltimore, Maryland, University Park
Press, pp 119-133.
Speck WT, Meyer LW, Zeiger E, & Rosenkranz HS (1982) Mutagenicity
and DNA-modifying activity of 2-nitropropane. Mutat Res, 104: 49-54.
SRI International (1988) 1988 Directory of chemical producers,
United States of America. Menlo Park, California, SRI International,
pp 29, 168, 803.
SRI International (1990) 1990 Directory of chemical producers,
United States of America. Menlo Park, California, SRI International,
p 815.
Stokinger HE (1982) Aliphatic nitro compounds, nitrates, nitrites.
In: Clayton GD & Clayton FE ed. Patty's industrial hygiene and
toxicology, 3rd revised ed. New York, John Wiley and Sons, vol 2C,
pp 4141-4208.
Swann RL, Laskowski DA, McCall PJ, Vander Kuy K, & Dishburger HJ
(1983) A rapid method for the estimation of the environmental
parameters octanol/water partition coefficient, soil sorption
constant, water to air ratio, and water solubility. Residue Rev.
85 : 17-28.
Tabershaw Occupational Medicine Association (1980) Cross-sectional
morbidity study of workers. Rockville, Maryland, Tabershaw
Occupational Medicine Association, 68 pp (Unpublished report).
Treon JF & Dutra FR (1952) Physiological response of experimental
animals to the vapor of 2-nitropropane. Arch Ind Hyg Occup Med,
5 : 52-61.
Ullrich V & Schnabel KN (1973) Formation and binding of carbanions
by cytochrome P-450 of liver microsomes. Drug Metab Disp, 1:
176-183.
Ullrich V, Hermann G, & Weber P (1978) Nitrite formation from
2-nitropropane by microsomal monooxygenases. Biochem Pharmacol,
27 : 2301-2304.
US Bureau of the Census (1987) Statistical abstract of the United
States: 1988, 108th ed. Washington, DC, US Bureau of the Census,
p 368.
US EPA (1977) TSCA chemical assessment series. Chemical hazard
information profiles (CHIPS), August 1976-August 1978. Washington,
DC, US Environmental Protection Agency, pp 212-217
(EPA-560/11-80-011).
US EPA (1980) Materials balance 2-nitropropane, Level 1. Washington,
DC, US Environmental Protection Agency, 70 pp (EPA-560/13-89-011).
US EPA (1985) Health and environmental effects profile for
2-nitropropane. Washington, DC, US Environmental Protection Agency,
pp 3-4 (EPA-600/X-85-112).
US EPA (1986) Notice of potential risk, 2-nitropropane. Washington,
DC, US Environmental Protection Agency, 5 pp.
US FDA (Food and Drug Administration) (1987) 2-Nitropropane. Code
Fed Regul, 27 (Parts 1 to 199): Docket No. 78N-0221.
USITC (1990) Synthetic organic chemicals. United States production
and sales, 1989. Washington, DC, United States International Trade
Commission, pp 15-17, 15-35-15-36 (USITC Publication No. 2338).
US NIOSH (1983) National occupational exposure survey, 1981-83:
Actual observation and trade-name exposure to 2-nitropropane.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health (Unpublished database).
US NIOSH (1987a) NIOSH manual of analytical methods. Second
supplement, 3rd ed. Cincinnati, Ohio, National Institute for
Occupational Safety and Health, pp 2528/1-2528/4 (NIOSH Publication
No. 87-117).
US NIOSH (1987b) Safe sheet #1: Summary of accidental fatality
evaluations, confined spaces, Case #3. Morgantown, West Virginia,
National Institute for Occupational Safety and Health, Environmental
Investigations Branch, Division of Respiratory Disease Studies, p 2.
US NIOSH (1988) Current intelligence bulletins: Summaries, September
1988. Cincinnati, Ohio, National Institute for Occupational Safety
and Health, p 6.
US OSHA (Occupational Safety and Health Administration) (1988)
1-Nitropropane and 2-nitropropane. Code Fed Regul, 29: 705, 708
(Parts 1900 to 1910).
Weast RC ed. (1986) CRC handbook of chemistry and physics, 67th ed.
Boca Raton, Florida, CRC Press, p C-444.
Wester PW, Van Leeuwen FXR, & De Vries T (1989) 4-Week toxicity
study with 2-nitropropane (2-NP) by gavage in rats. Bilthoven, The
Netherlands, National Institute of Public Health and Environmental
Protection, 20 pp (Report No. 658501 001).
Wilbur SZ & Parekh CK (1982) Dermal toxicity potential of
2-nitropropane (P-1357). Northbrook, Illinois, International
Minerals and Mining Corporation, 5 pp (Unpublished report No.
PLR-281/AMR-072).
Wilks RA & Gilbert SG (1972a) Sensory and instrumental evaluation in
model systems of residues migrating from can coatings. J Food Sci,
37: 72-76.
Wilks RA & Gilbert SG (1972b) Quick test for liner solvents. Modern
Packag, May: 52-55.
Williams SV, Bryan JA, Burk JR, & Wolf FS (1974) Toxic hepatitis and
methylenedianiline. New Engl J Med, 291: 1256.
Windholz M ed. (1983) The Merck index, 10th ed. Rahway, New Jersey,
Merck & Co., p 6482.
Woo Y-T, Lai DY, Arcos JC, & Argus MF (1985) Chemical induction of
cancer: structural bases and biological mechanisms, Vol. III B:
Aliphatic and polyhalogenated carcinogens. New York, London,
Academic Press, pp 329-341.
Yasuhara A, Yamanaka Y, & Ogawa T (1986) Volatile compounds in
machine cutting-fluid emulsion. Agric Biol Chem, 50: 1765-1770.
Ziegler-Skylakakis K, Homfeldt H, & Andrae U (1987) In vitro and
in vivo genotoxicity of 2-nitropropane and 1-nitropropane in
mammalian cells. Naunyn-Schmiedeberg's Arch Pharmacol, 335(suppl):
R25.
Zimmering S, Mason JM, Valencia R, & Woodruff RC (1985) Chemical
mutagenesis testing in Drosophila. II. Results of 20 coded
compounds tested for the National Toxicology Program. Environ
Mutagen, 7: 87-100.
Zitting A, Savolainen H, & Nickels J (1981) Acute effects of
2-nitropropane on rat liver and brain. Toxicol Lett, 9: 237-246.
RESUME
1. Propriétés et méthodes d'analyse
Le 2-nitropropane (2-NP) est un liquide huileux, incolore, à
l'odeur douceâtre. Il est inflammable, moyennement volatil et stable
dans les conditions ordinaires. Il n'est que légèrement soluble dans
l'eau mais miscible à de nombreux liquides organiques et c'est un
excellent solvant de nombreux composés organiques. Il existe de
bonnes méthodes d'analyse pour la recherche et le dosage du
2-nitropropane présent dans l'environnement. On a actuellement
recours à la chromatographie en phase gazeuse avec détection par
ionisation de flamme ou capture d'électrons, ou bien à la
chromatographie en phase liquide à haute performance avec détecteur
u.v. Pour le dosage dans l'air, il faut tout d'abord piéger le
2-nitropropane et le concentrer par adsorption sur phase solide.
2. Emploi et sources d'exposition
2.1 Production
Les chiffres de production actuels ne sont pas connus. En 1977
les Etats-Unis d'Amérique en ont produit environ 13 600 tonnes. Le
2-nitropropane est actuellement produit par deux firmes américaines
et une firme française. Il peut prendre naissance naturellement à
l'état de traces lors de la combustion du tabac et d'autres matières
organiques riches en nitrates mais rien n'indique qu'il puisse se
former à l'issue d'un processus biologique quelconque.
2.2 Emploi et passage dans l'environnement
Le 2-nitropropane est utilisé comme solvant, principalement en
mélange, et il a de nombreuses applications industrielles en tant
que tel pour la confection d'encres d'imprimerie, de peintures, de
vernis, d'adhésifs et autres revêtements, comme par exemple ceux que
l'on utilise dans les récipients contenant des boissons. On
l'utilise également comme solvant pour séparer des composés très
voisins comme les acides gras, comme intermédiaire en synthèse
chimique et comme additif dans les carburants. C'est principalement
par l'intermédiaire de l'air qu'il passe dans l'environnement,
surtout par évaporation à partir des surfaces enduites de produits
qui en contiennent.
3. Transport et distribution dans l'environnement
Le 2-nitropropane se révèle très mobile dans le milieu naturel.
Du fait que sa solubilité dans l'eau, son adsorption par les
sédiments et sa bioaccumulation sont faibles et qu'il s'évapore
rapidement dans l'atmosphère, il se répartit dans l'air et l'eau
sans s'accumuler dans un compartiment particulier du milieu. Le
2-nitropropane absorbe le rayonnement ultra-violet aux longueurs
d'onde que l'on rencontre dans l'environnement et il est donc
probable qu'il subisse une lente photolyse dans l'atmosphère. Il est
également probable qu'il soit lentement transformé par voie
biologique en composés moins toxiques, tant dans l'environnement
aquatique que dans l'environnement terrestre.
4. Concentrations dans l'environnement et exposition humaine
Il semble que l'exposition de la population générale au
2-nitropropane soit très faible et provienne de la fumée de
cigarettes (1,1 à 1,2 µg/cigarette), de résidus présents dans des
enduits ou revêtements tels qu'on en utilise pour les récipients
contenant des boissons, dans les adhésifs, les encres d'imprimerie,
ainsi que dans les huiles végétales que l'on fractionne au moyen de
ce solvant. On ignore quelle est l'importance de l'exposition des
travailleurs de l'industrie dans le monde, mais aux Etats-Unis
d'Amérique, il semble qu'elle soit limitée à 0,02-0,19 % du
personnel. Dans ce même pays, environ 4000 travailleurs (soit à peu
près 0,005 % de la main-d'oeuvre) subissent sans doute une
exposition notable (de l'ordre de 9,1 mg/m3(2,5 ppm) ou
davantage). Selon les pays, les limites d'exposition professionnelle
dans l'air vont de 36 mg/m3 (1 ppm) (TWA) à 146 mg/m3 (40 ppm)
(STEL). La production du 2-nitropropane s'effectue en circuit fermé
et en général, le personnel n'est guère exposé; toutefois dans
certaines industries (peinture, imprimerie ou extraction par
solvant), il est arrivé que des travailleurs soient exposés à des
concentrations dépassant largement les limites d'exposition
professionnelle. C'est ainsi que des concentrations atteignant
6 g/m3 (1640 ppm) dans l'air ont été enregistrées lors du
remplissage de fûts.
5. Cinétique et métabolisme
Le 2-nitropropane est principalement résorbé au niveau des
poumons. Chez l'animal de laboratoire, on a montré qu'il était
rapidement absorbé non seulement à ce niveau mais également au
niveau de la cavité péritonéale et des voies digestives. On est mal
renseigné sur son absorption percutanée. Quant aux données
concernant sa distribution dans l'organisme du rat, elles sont
quelque peu contradictoires. Le 2-nitropropane est rapidement
métabolisé, essentiellement sous forme d'acétone et de nitrite. Il
se forme peut-être aussi un peu d'alcool isopropylique. Après
injection par voie intrapéritonéale, le 2-nitropropane et ses
métabolites carbonés se concentrent d'abord dans les graisses, puis
dans la moelle osseuse ainsi que dans les surrénales et les autres
organes internes; après inhalation, ils se concentrent dans le foie
et les reins, mais relativement peu dans les graisses. Il est
possible que plusieurs systèmes enzymatiques interviennent dans ce
métabolisme et les vitesses, de même que les voies de
métabolisation, varient selon les espèces. Le 2-nitropropane et ses
métabolites carbonés disparaissent rapidement de l'organisme par
métabolisation, exhalation ou excrétion dans les urines et les
matières fécales. On manque de données satisfaisantes sur la
distribution et l'excrétion de la fraction nitrée de ces
métabolites.
6. Effets sur les mammifères de laboratoire et les systèmes
in vitro
La toxicité aiguë du 2-nitropropane pour les mammifères est
modérée. Les mâles sont plus sensibles que les femelles, tout au
moins en ce qui concerne le rat, et l'expérience montre que la
sensibilité varie largement d'une espèce à l'autre. La CL50
(concentration produisant une mortalité de 50 % en 14 jours) a été
de 1,5 g/m3 (400 ppm) pour les rats mâles et de 2,6 g/m3
(720 ppm) pour les rats femelles. Il semble que la mortalité soit
associée principalement aux effets narcotiques, mais les mammifères
exposés à des concentrations d'au moins 8,4 g/m3 (2300 ppm)
pendant une heure ou davantage ont présenté des anomalies
anatomopathologiques graves, notamment des lésions
hépatocellulaires, un oedème du poumon et des hémorragies.
Il est indiscutable que le 2-nitropropane est cancérogène pour
le rat. L'exposition de rats pendant de longues durées à du
2-nitropropane par voie respiratoire à raison de 0,36 g/m3
(100 ppm) (pendant 18 mois à raison de sept heures par jour et cinq
jours par semaine) a causé des lésions destructrices au niveau du
foie, et en particulier des carcinomes hépatocellulaires chez
certains rats mâles. A la concentration de 0,75 g/m3 (207 ppm) les
lésions étaient encore plus graves, avec une forte incidence de
carcinomes hépatocellulaires à survenue plus rapide.
L'administration chronique de doses modérées par voie orale a
également provoqué une surincidence de carcinomes hépatocellulaires.
Toutefois, l'inhalation prolongée par des rats de 2-nitropropane aux
doses respectives de 91 ou 98,3 mg/m3 (25 ou 27 ppm) n'a pas
provoqué de lésions décelables. L'exposition de souris et de lapins
à des concentrations de 2-nitropropane capables de provoquer des
carcinomes hépatocellulaires chez le rat n'a guère eu d'effets chez
ces animaux, mais il est vrai que les études en question étaient
trop limitées pour qu'on puisse exclure totalement un effet
cancérogène du 2-nitropropane chez ces deux espèces. Le
2-nitropropane a légèrement retardé le développement foetal des rats
mais les données relatives à l'embryotoxicité, à la tératogénicité
et à la toxicité du 2-NP pour la fonction de reproduction restent
très fragmentaires. Le produit s'est révélé fortement génotoxique
pour les hépatocytes de rats tant in vivo qu' in vitro; en
revanche aucune génotoxicité sensible n'a été observée au niveau des
autres organes chez le rat ou sur des lignées cellulaires d'origine
extrahépatique, en l'absence d'activation métabolique exogène. On a
également montré que le 2-nitropropane était mutagène chez les
bactéries en présence ou en l'absence d'activation métabolique
exogène.
7. Effets sur l'homme
L'exposition humaine à de fortes concentrations de
2-nitropropane est largement, voire totalement d'origine
professionnelle. A concentration élevée, le 2-nitropropane présente
une forte toxicité aiguë et il a provoqué des accidents mortels dans
l'industrie - encore qu'on ignore la valeur exacte de cette
concentration, sauf dans un cas où on a pu estimer l'exposition à
2184 mg/m3, soit 600 ppm. Les premiers symptômes consistaient en
céphalées, nausées, somnolence, vomissements, diarrhées et douleurs.
Malgré une amélioration temporaire de l'état général, la mort est
quelquefois survenue dans les 4 à 26 jours suivant l'exposition. La
cause initiale de la mort était une insuffisance hépatique à
laquelle s'ajoutaient un oedème du poumon, des hémorragies des voies
digestives et une insuffisance respiratoire et rénale. A des doses
évaluées à 73-164 mg/m3 (20 à 45 ppm) on a constaté que
l'exposition professionnelle provoquait des nausées et une perte
d'appétit pouvant persister plusieurs heures après le départ du lieu
de travail, aucun effet indésirable n'étant observé après exposition
à des doses de 36,4 à 109 mg/m3 (10 à 30 ppm) (moins de quatre
heures par jour pendant une durée inférieure ou égale à trois jours
par semaine).
Malgré l'insuffisance des données disponibles, rien n'indique
qu'une exposition professionelle de longue durée au 2-nitropropane
aux concentrations généralement présentes sur les lieux de travail
puisse provoquer des cancers du foie ou d'autres organes, ni plus
généralement des effets indésirables à longue échéance.
8. Effets sur les autres organismes au laboratoire et dans
leur milieu naturel
Les quelques études effectuées sur des microorganismes, des
invertébrés et des poissons montrent que le 2-nitropropane est peu
toxique pour les organismes non mammaliens.
RESUMEN
1. Propiedades y métodos analíticos
El 2-nitropropano (2-NP) es un líquido incoloro y oleoso de olor
ligero. Es inflamable, moderadamente volátil, y estable en
condiciones normales. Es sólo ligeramente hidrosoluble pero miscible
con numerosos líquidos orgánicos, y es un excelente disolvente para
muchos tipos de compuestos orgánicos. Existen métodos analíticos
adecuados para identificar y medir el 2-NP en concentraciones
ambientales. Los métodos de uso corriente son la cromatografía de
gases y un detector de ionización de llama o de captura electrónica;
también se usa la cromatografía líquida de alto rendimiento con
detector de ultravioleta. Para medirlo en el aire, primero es
necesario capturarlo y concentrarlo en un sorbente sólido.
2. Usos y fuentes de exposición
2.1 Producción
No se dispone de cifras recientes de producción mundial. En
1977, la producción en los Estados Unidos fue de aproximadamente
13 600 toneladas. Actualmente, el 2-NP se fabrica en dos empresas
estadounidenses y una francesa. Se origina por mecanismos naturales
como trazas en la combustión del tabaco y de otras sustancias
orgánicas ricas en nitratos, pero nada indica que se origine en
procesos biológicos.
2.2 Usos y pérdidas al medio ambiente
El 2-NP se utiliza como disolvente, principalmente en mezclas, y
tiene numerosas aplicaciones industriales como disolvente para
tintas de impresión, pinturas, barnices, adhesivos y otros
revestimientos como los de recipientes de bebidas. Se ha utilizado
asimismo como disolvente para separar sustancias estrechamente
relacionadas como ácidos grasos, como intermediario en síntesis
químicas, y como aditivo en combustibles. Las pérdidas al medio
ambiente ingresan principalmente en el aire y se deben sobre todo a
la evaporación del disolvente a partir de superficies revestidas.
3. Transporte y distribución en el medio ambiente
El 2-NP parece tener gran movilidad en el medio ambiente
natural. Dada su baja solubilidad en el agua, escasa absorción por
el sedimento, reducida bioacumulación y facil evaporación a la
atmósfera, se distribuye tanto en el aire como en el agua y no se
acumula en ningún compartimento ambiental definido. La fotoabsorción
ultravioleta del 2-NP se encuentra en la escala de frecuencias
normales en el medio ambiente, por lo que es probable que la
sustancia sea objeto de fotólisis lenta en la atmósfera. La
biotransformación lenta del 2-NP a compuestos menos tóxicos también
parece probable en los medios acuático y terrestre.
4. Niveles ambientales y exposición humana
La exposición de la población general al 2-NP parece ser muy
baja y se debe al humo de cigarrillos (1,1 a 1,2 µg/cigarrillo), a
los residuos en revestimientos, como los de las latas de bebidas,
adhesivos y material impreso, y a los aceites vegetales fraccionados
con esa sustancia. Se desconoce la exposición industrial a escala
mundial, pero en los Estados Unidos parece limitarse al 0,02-0,19%
de la población trabajadora. Los niveles de exposición de cierta
importancia (exposición a 9,1 mg/m3 (2,5 ppm) o más) en los
Estados Unidos probablemente no afectan más que a unos 4000
trabajadores (aproximadamente 0,005% de la población trabajadora).
Los límites de exposición ocupacional en el aire varían de un paíse
a otro y van desde 3,6 mg/m3 (1 ppm) (promedio ponderado en
función del tiempo) a 146 mg/m3 (40 ppm) (STEL). La fabricación
del 2-NP es un proceso cerrado y entraña por lo general una
exposición reducida de los trabajadores, pero algunos obreros de
industrias como la pintura, la impresión y la extracción de
disolventes se han visto expuestos en otras épocas a niveles muy
superiores a los límites de exposición ocupacional. Durante ciertas
operaciones industriales han llegado a registrarse concentraciones
de hasta 6 g/m3 (1640 ppm) en el aire.
5. Cinética y metabolismo
En el ser humano, la absorción de 2-NP se produce principalmente
por los pulmones. En animales de experimentación, se ha demostrado
que el 2-NP se absorbe rápidamente no sólo por vía pulmonar sino
también a partir de la cavidad peritoneal y del tracto
gastrointestinal. No se dispone de datos satisfactorios sobre la
absorción por vía cutánea. La información sobre la distribución en
la rata es ligeramente contradictoria. El 2-NP se metaboliza
rápidamente, principalmente a acetona y nitrito. También puede
formarse isopropil alcohol en pequeñas cantidades. Tras la inyección
intraperitoneal, el 2-NP y sus metabolitos carbonados se concentran
inicialmente en la grasa y después en la médula ósea, así como en
las glándulas suprarrenales y otros órganos internos. Tras la
inhalación, el 2-NP y sus metabolitos carbonados se concentran en el
hígado y el riñón; la cantidad que se acumula en la grasa es
relativamente pequeña. Varios sistemas enzimáticos diferentes pueden
participar y existen diferencias de unas especies a otras en cuanto
a la velocidad y las rutas metabólicas. El 2-NP y sus metabolitos
carbonados desaparecen rápidamente del organismo por transformación
metabólica, exhalación y excreción en orina y heces. No se dispone
de datos satisfactorios sobre la distribución y la excreción de
metabolitos que llevan el radical nitro.
6 Efectos en mamíferos de laboratorio y sistemas in vitro
El 2-NP tiene una toxicidad aguda moderada para los mamíferos.
Los machos son más sensibles que las hembras, por lo menos en la
rata, y la sensibilidad difiere ampliamente entre las especies que
se han ensayado. La CL50 (concentración que causa una mortalidad
del 50% en un plazo de 14 días) para la rata tras una exposición de
6 horas fue de 1,5 g/m3 (400 ppm) en los machos y 2,6 g/m3
(720 ppm) en las hembras. La letalidad parecía asociada
principalmente a los efectos narcóticos, si bien los mamíferos
expuestos a concentraciones de al menos 8,4 g/m3 (2300 ppm)
durante una hora o más mostraron alteraciones patológicas graves,
entre ellas lesiones hepatocelulares, edema pulmonar y hemorragia.
Existen pruebas claras de que el 2-NP es carcinogénico en la
rata. La exposición prolongada de ratas por inhalación de
0,36 g/m3 (100 ppm) durante 18 meses (7 h/día, 5 días/semana)
indujo cambios destructivos en el hígado, inclusive carcinomas
hepatocelulares en algunos machos. Una concentración de 0,75 g/m3
(207 ppm) indujo lesiones más graves, entre ellas una elevada
incidencia de carcinomas hepatocelulares, con más rapidez. La
administración crónica de dosis orales moderadas indujo también un
exceso de carcinomas hepatocelulares en ratas. En cambio, la
inhalación prolongada de 91 o 98,3 mg/m3 (25 ó 27 ppm) no produjo
lesiones detectables en las ratas. La exposición de ratones y
conejos a concentraciones de 2-NP que inducían carcinomas
hepatocelulares en la rata tuvieron escaso efecto o ninguno, pero
esos estudios eran demasiado limitados para descartar por completo
la carcinogenicidad de la sustancia en ambas especies. El 2-NP
retrasó ligeramente el desarrollo fetal en la rata, pero escasean
los datos sobre embriotoxicidad, teratogenicidad y toxicidad
reproductiva. Se observó que el 2-NP era sumamente genotóxico en
hepatocitos de rata tanto in vitro como in vivo, pero no se
observó genotoxicidad significativa en otros órganos de la rata ni
en líneas celulares de origen extrahepático sin activación
metabólica exógena. Se ha demostrado que el 2-NP es mutagénico en
bacterias tanto en presencia como en ausencia de activación
metabólica exógena.
7. Efectos en el ser humano
La exposición humana a concentraciones elevadas de 2-NP es en su
mayor parte o totalmente de origen ocupacional. Las concentraciones
elevadas (se desconocen los valores reales, si bien en un caso se
calcularon en 2184 mg/m3 (600 ppm)) producen toxicidad aguda y
accidentes mortales en la industria. Entre los síntomas iniciales
figuran dolores de cabeza, náuseas, mareos, vómitos, diarrea y
dolores. Las víctimas a menudo mostraban una mejoría temporal,
aunque en algunos casos sobrevino la muerte entre 4 y 26 días
despues de la exposición. El fallo hepático fue la principal causa
de muerte, con edema pulmonar, hemorragias gastrointestinales fallo
respiratorio y renal como factores contribuyentes. La exposición
ocupacional a niveles estimados en 73 a 164 mg/m3 (20 a 45 ppm)
indujo náuseas y pérdida de apetito, que persistieron durante varias
horas tras abandonar el lugar de trabajo, mientras que la exposición
ocupacional a niveles estimados en 36,4 a 109 mg/m3 (10 a 30 ppm)
(< de 4 h/día durante < 3 días/semana) no produjo efectos
nocivos detectables.
Aunque los datos disponibles son insuficientes, nada indica que
la exposición ocupacional crónica al 2-NP en concentraciones
normales en el lugar de trabajo induzca neoplasmas hepáticos o de
otro tipo ni otros efectos adversos a largo plazo.
8. Efectos en otros organismos en el laboratorio y sobre el terreno
Los escasos estudios realizados en microorganismos,
invertebrados y peces indican una baja toxicidad del 2-NP para
organismos no mamíferos.
 2.3 Conversion factors
1 ppm 2-NP in air = 3.64 mg/m3
1 mg/m3 = 0.27 ppm 2-NP in air
2.4 Analytical methods
Analytical methods for 2-NP appear limited to the analysis of
air, water, blood plasma, coatings, and cigarette smoke (Table 2).
Since the colorimetric methods are less sensitive or are cumbersome,
the method of choice is probably gas chromatography with either a
flame ionization or an election capture detection. Charcoal is not a
satisfactory adsorbent for 2-NP since recovery is poor (Andersson et
al., 1983) and there may be decomposition (Glaser & Woodfin, 1981).
In addition to Chromosorb 106, Amberlite XAD-7 appears satisfactory
as a solid sorbent for quantitatively collecting 2-NP from the air
(Andersson et al., 1983), although its collection efficiency is
markedly reduced in humid air (Andersson et al., 1984). Use of a
collection tube with two sections, however, can compensate for the
reduced efficiency (Andersson et al., 1984). The high-performance
liquid chromatography method developed for blood (Derks et al.,
1988) could probably be adapted to other biological materials.
Table 2. Analytical techniques for determining 2-nitropropanea
Methods Detection limit Comments Reference
Air
Trapping in ethanol; 0.1 mg/ml ethanol absorption is linear between 0.1 and Treon & Dutra (1952)
concentration determined 2.0 mg/ml; ethanol was especially
spectrophotometrically at purified and redistilled
277.5 nm
Trapping in concentrated ca. 1 µg/ml sulfuric absorption is linear between 1 and 5 µg/ml Jones & Riddick (1952);
sulfuric acid; resulting acid sulfuric acid; no interference from primary Jones (1963)
nitrous acid combined nitroparaffins, but all other secondary,
with resorcinol to form a some tertiary and some halogenated
red-blue colour; measured nitroparaffins interfere
spectro-photometrically
at 560 nm
Trapping in solid sorbent tube 3.6 mg/m3 working range is 3.6 to 36 mg/m3; 2-NP Glaser & Woodfin (1981)
(Chromosorb 106, 60/80 mesh); stable on absorbent for at least 28 days
desorption: ethyl acetate;
separation-detection: GC-FID
Methodology similar to above 3.1 mg/m3 Method is a modification of that proposed US NIOSH (1987a)
by Glaser & Woodfin (1981); range: 3.1 to
28.3 mg/m3; no interference from methyl
butyl ketone, heptane, 1-nitropropane,
toluene, and xylene
Table 2 (contd).
Methods Detection limit Comments Reference
Water
Sample (40 µl) collected ca. 0.5 mg/m3 water elutes quickly extinguishing flame Wilks & Gilbert (1972a)
with a syringe and injected in FID; flame can be reignited before 2-NP
directly into GC; emerges
detection: FID
Blood
Blood collected in chilled, 1 ng UV absorption linear from 0 to 250 ng; Derks et al. (1988)
screw-capped vial with heparin; uses 0.3 ml blood sample; samples are
centrifuged; deproteinized with unstable and must be analysed promptly
acetonitrile; Tris buffer added;
separation: HPLC; detection:
UV at 224 nm
Coating (beverage can)
Redissolve coating in solvent not given reference provides few details on Wilks & Gilbert (1972b)
suitably distinct from 2-NP; methodology
acetone suitable for vinyl
co-polymer coatings;
separation: GC
Table 2 (contd).
Methods Detection limit Comments Reference
Coating (beverage can)
Can (empty) simultaneously not given method captures about 90% of Wilks & Gilbert (1972b)
perforated and fitted with a residual 2-NP
diaphragm; hypodermic needle
inserted through diaphragm and
fitted with stopcock; can heated
(150 °C, 15 min); headspace
sampled with heated syringe;
separation: GC
Cigarette smoke
Steam distillation of smoke 0.8 µg/cigarette may be adapted for air and water Hoffmann & Rathkamp
condensate on filters, analysis (1968)
extraction re-extraction in
NaOH, in ethyl ether,
neutralization with H2SO4 and
re-extraction in ethyl ether,
concentration of extract and
injection in GC equipped with
FID or ECD detectors
a Abbreviations: ECD = electron capture detector; FID = flame ionization detector; GC = gas chromatograph;
HPLC = high-performance liquid chromatograph; UV = ultraviolet
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
There is no evidence that 2-NP and other nitroaliphatic
compounds are produced by biological processes, although a related
organic compound, ß-nitropropionic acid, has been isolated from
plants and microorganisms (Goldwhite, 1965). However, nitroaliphatic
compounds are produced in low concentrations by combustion of
organic matter and have been detected in tobacco smoke. Hoffmann &
Rathkamp (1968) reported 1.1-1.2 µg 2-NP in the smoke from single
85-mm USA blended non-filter cigarettes, and ascribed the production
of this and other nitroaliphatics to interactions in the combustion
zone between hydrocarbons and nitrogen dioxide generated by
decomposition of nitrates.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
Although 2-NP is an important industrial chemical, current world
production figures are not available. In 1977 production by the sole
USA manufacturer was estimated to be 13 600 tonnes, of which 5400
tonnes were sold in the USA and 8200 tonnes were either exported or
used internally (Finklea, 1977). 2-NP currently is produced by two
USA manufacturers, Angus Chemical Co., Sterlington, Louisiana, and
W. R. Grace Co., Deer Park, Texas (SRI International, 1988, 1990;
USITC, 1990), and by one European manufacturer, Société Chimique de
la Grande Paroisse, France (Anon., 1976, 1982; IARC, 1982). In the
USA, 2-NP, together with nitromethane, nitroethane and
1-nitropropane, is manufactured by a vapour phase reaction of nitric
acid with an excess of propane at high temperature and pressure
(370-450 °C, 0.8-1.2 MPa (8-12 atm.)) (Baker & Bollmeier, 1981). The
proportions of the four nitroaliphatic compounds in the reaction
product are a function of the reaction temperature. In Europe,
propane is reacted with nitrogen peroxide (N2O4) and an excess
of oxygen at 150-330 °C and 0.9-1.2 MPa (9-12 atm.), yielding the
same nitroaliphatic compounds in slightly different proportions as
produced by the USA process (Anon., 1976). Reaction products are
condensed, washed, and separated by fractional distillation. There
is no evidence that 2-NP is produced through human EHC 138:
2-Nitropropane activities except by combustion and deliberate
manufacture, although nitromethane has been detected in vehicle
exhaust (Seizinger & Dimitrades, 1972).
3.2.2 Uses
The importance of 2-NP as an industrial chemical stems mainly
from its desirable and occasionally unique characteristics as a
solvent (Purcell, 1967; Anon., 1976; Fishbein, 1981; Baker &
Bollmeier, 1981; IARC, 1982; ACGIH, 1986). It is an excellent
solvent or cosolvent for a variety of fats, waxes, gums, resins,
dyes and other organic compounds, including vinyl, acrylic,
polyamide and epoxy resins, chlorinated rubbers, and organic
cellulose esters. The ability of 2-NP to form an azeotrope with
water and the associated large heat of absorption permit it to
displace monomolecular layers of water molecules and secure a better
bond between pigments and the surfaces to which they are applied.
Its major use is as a solvent for inks, paints, varnishes, adhesives
and other coatings such as beverage container linings. It is used
principally in blends with other solvents to impart desirable
characteristics, such as greater solvency, better flow
characteristics and film integrity, greater pigment dispersion,
increased wetting ability, improved electrostatic spraying
properties, or reduced drying time. 2-NP is also used industrially
as a processing solvent for separating closely related substances in
natural products or reaction mixtures. These have included, for
example, separation of oleic acid from polyunsaturated fatty acids
and cetyl from oleyl alcohols.
In addition to the above, 2-NP has a number of minor uses
(Anon., 1976; Baker & Bollmeier, 1981). These include a medium for
chemical reactions, an intermediate for the manufacture of
2-nitro-2-methyl-1-propanol, 2,2-dinitropropane, 2-amino-2-
methyl-1-propanol and other propane derivatives, and a component of
explosives, propellants, and fuels for internal combustion engines.
The latter usage appears limited to model engines used by hobbyists
and to racing cars. Although the addition of 2-NP to fuel improves
diesel engine performance, it is not used commercially as a diesel
fuel additive since superior alternatives are available (Banes,
1989)a. In the USA, mixed isomers of nitropropane are used to
denature ethanol (US FDA, 1987). The addition of 2-NP to hydrocarbon
mixtures has been shown to inhibit corrosion of tin-plated steel
aerosol cans (Flanner, 1972).
3.3 Release into the environment
There is some quantitative data on releases of 2-NP into the
environment. The US Environmental Protection Agency has supported a
thorough, though largely speculative, analysis of the problem (US
EPA, 1980). Releases of 2-NP occur mainly into the atmosphere and
can result from spillage, from venting of gases and fugitive
emissions during manufacture, transfer and use, and from solvent
evaporation from coated surfaces. The US EPA document estimated that
of the 14 000 tonnes of 2-NP produced in the USA in 1979, 5714
tonnes (41%) was released into the air, and 1 tonne into water. Only
230 tonnes (1.6%) was destroyed by incineration or waste treatment.
The major contributor to this release estimate was evaporation of
2-NP used as a solvent in printing ink and surface coatings (4450
a Personal communication from the US Environmental Protection
Agency, Ann Arbor, Minneapolis
tonnes, 78% of releases). Manufacture of 2-NP is a largely enclosed
process and in 1979 it accounted for only 21 tonnes (0.3%) of the
amount released into the environment. A more recent examination of
this problem (National Library of Medicine, 1989) reported a similar
situation concerning environmental releases of 2-NP in the USA. Out
of a yearly total of 299 tonnes, 205 tonnes (69%) was released into
the air with 123 tonnes coming from point (large, easily identified)
sources and 82 tonnes from non-point (small, not easily identified)
sources. Only 2 tonnes (< 1%) was released directly into water, and
1 tonne into municipal sewage treatment plants. The remainder was
buried in closed containers (76 tonnes, 25%) or was disposed of in
unspecified ways (15 tonnes, 5%).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport in the environment
2-NP appears to be highly mobile in the natural environment.
Cupitt (1980) considered physical removal of 2-NP from the
atmosphere unlikely because it was not soluble enough to be rapidly
washed out and had a vapour pressure too great for strong adsorption
on particles. The partition of 2-NP between air and water at
equilibrium was estimated by the method of Swann et al. (1983) to be
about 0.5% in air and 99.5% in water (US EPA, 1985). These values
indicate rapid and easy exchange between air and water. The soil
sorption coefficient (ratio of soil concentration to water
concentration) and the bioconcentration factor were estimated by the
methods of Kenaga (1980) to be 20 and 2.5, respectively (US EPA,
1985). Measured values for absorption and bioaccumulation were
somewhat greater than these estimates. Freitag et al. (1982, 1985)
obtained concentration factors over water for activated sludge,
unicellular algae (Chlorella fusca), and fish (golden ide) of 70,
20, and < 10, respectively. These values indicate that 2-NP is not
strongly bioaccumulated and is readily desorbed from sediment
particles and leached from soil. Thus, in summary, since 2-NP is
slightly water soluble, slightly adsorbed by sediment, slightly
bioaccumulated, and evaporates readily into the atmosphere, it will
be distributed in both air and water and not accumulated in any
individual environmental compartment.
4.2 Biotic and abiotic transformation
Data concerning the destruction of 2-NP by biotic and abiotic
processes are limited. 2-NP has significant photoabsorption in the
environmentally relevant range of > 290 nm (Sadtler, 1961) and is
likely to undergo photolysis (Cupitt, 1980; US EPA, 1985). On the
basis of physical and chemical properties Cupitt (1980) hypothesized
that it would be rapidly removed from the atmosphere by photolysis
and estimated a reduction in concentration of 1/e (0.369) in 0.2
days. This is equivalent to a half-life of 0.14 days (3.36 h).
However, measurements of photochemical reactivity do not support
such a rapid destruction of 2-NP. Studies aimed at defining the
relationship between organic solvents and photochemical smog
production ranked 2-NP as low to moderate in terms of its
interactions with oxidants and its ability to produce formaldehyde
and other lachrymators (Levy, 1973). Laboratory measurements also
suggested slow decomposition (Freitag et al., 1985). The methodology
employed (Korte et al., 1978; Lotz et al., 1979; Freitag et al.,
1982), i.e. irradiation of the solvent adsorbed on silica gel by
light from a high pressure mercury lamp filtered through pyrex, was
too different from natural conditions to permit quantitative
extrapolation from laboratory results to rates of photolysis in the
atmosphere. The study provided comparative photodecomposition rates
for a large number of solvents. The rate of photodecomposition for
2-NP was roughly half that of dichlorodiphenyltrichloroethane (DDT),
similar to the rates for dodecane and 2,4-dichlorobenzoic acid, and
roughly twice those for kepone and dieldrin. Paszyc (1971) reported
that the major decomposition products for both gaseous and liquid
2-NP under laboratory conditions were nitrogen dioxide, acetone,
isopropyl nitrite, isopropanol, methylcyanide, water, and propane,
regardless of whether irradiation was monochromatic 253.7 nm light
or the full spectrum of light produced by a high pressure mercury
lamp. Cupitt (1980) speculated that the major products of
photodecomposition in nature would be formaldehyde and acetaldehyde.
Biological decomposition of 2-NP appears likely, but is probably
rather slow in nature. Enzymes capable of oxidizing or initiating
non-enzymatic oxidation of 2-NP have been identified in horseradish
(De Rycker & Halliwell, 1978; Porter & Bright, 1983; Indig &
Cilento, 1987), pea seedlings (Little, 1957), and a variety of
microorganisms including bacteria, yeasts, and fungi (Little, 1951;
Kido et al., 1975; Soda et al., 1977; Dhawale & Hornemann, 1979;
Patel et al., 1982). In in vitro preparations of horseradish
(Dhawale & Hornemann, 1979), pea seedlings (Little, 1957), a fungus
(Streptomyces achromogenes) (Dhawale & Hornemann, 1979), and a
yeast (Hansenula mrakii) (Kido et al., 1975), 2-NP was converted
to a less toxic compound, acetone, and a moderately toxic compound,
nitrite. In addition to nitrite, some nitrate may also be formed
(Indig & Cilento, 1987). In the yeast, nitrite was subsequently
reduced to ammonia. The importance of these processes in nature is
unknown. Kido et al. (1975), however, reported that only 4 out of 14
species of microorganisms tested would grow in a medium containing
5 g 2-NP/litre. The only study of 2-NP decomposition by a population
of microorganisms (Freitag et al., 1985) utilized activated sludge
grown at 25 °C. Solvent concentrations in these experiments were low
(50 µg/litre) to prevent adaptation to the substances tested (Korte
et al., 1978). In 5 days only 0.4% of the 2-NP was converted to
carbon dioxide. In summary, these data suggest that in both
terrestrial and aquatic communities 2-NP is biologically decomposed
and that the rate may be slow, but they offer no definite
information on the problem.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels and general population exposure
General population exposure to 2-NP appears to be very low.
There seem to be no records of its occurrence in water or in outdoor
air away from areas of manufacture and use. The only information on
intake exists in the form of a memorandum from Modderman (1983)a.
The daily intake per person in the USA was estimated to be 50 to
100 mg. The residuum from its use as a solvent for beverage can
coatings, film laminating adhesives and printing inks for flexible
food packaging may account for as much as 37 ng/day and from
vegetable oils fractionated with 2-NP, 30 ng/day. 2-NP residues of
0.077 mg/litre (77 ppb) to 0.24 mg/litre (204 ppb) have been found
in such oils. In a further report on the evaluation of
2-nitropropane as a food processing solvent, it was assumed that
residues of less than 10 µg/kg would occur in oils, giving rise to
estimated daily intakes of 10 ng/day. The use of 2-NP as a food
processing solvent was not recommended (FAO/WHO, 1990a,b). As
mentioned in section 3.1, smokers are exposed regularly to low
concentrations of 2-NP. Hoffmann & Rathkamp (1968) reported 1.1 to
1.2 µg in the smoke of a single cigarette. 2-NP was reported to
occur in the expired air of 11.1% (5 of 54 individuals) of a sample
of healthy adult urban dwellers (Krotoszynski et al., 1979). The
geometric mean was 0.406 ng/litre with one-sigma limits of
0.119 ng/litre and 1.38 ng/litre, (at 25 °C, 98 MPa (760 mmHg)). The
sample was entirely of non-smokers who had avoided medication and
prolonged exposure to perfume, paint, glue, aerosols, dust, tobacco
smoke and areas polluted with industrial wastes during, and for at
least 7 days prior to, the sampling period, and also avoided
cosmetics, spices, seasonings, and alcoholic beverages during and
immediately prior to sampling. The origin of the exhaled 2-NP is
unclear. Exposure to 2-NP may be further reduced in the future since
a number of regulations have been enacted and recommendations made
to declare it a harmful, carcinogenic substance and a toxic waste,
and to discourage its use (IARC, 1982; IRPTC, 1986; US FDA, 1987; US
NIOSH, 1988).
a Memorandum from Modderman, J.P. to Shibko, S., Associate
Director for Regulatory Evaluation, Department of Health & Human
Services, USA, 8 pp. "Exposure estimates for chemicals to be
included in the NTP annual report on carcinogens".
5.2 Potential occupational exposure
The number of workers in the USA who handle 2-NP or mixtures
containing 2-NP has been variously estimated as 15 000 (US EPA,
1977), 38 600 (Occupational Health Services, Inc., 1982), 100 000
(Finklea, 1977), and 185 000 (Beall et al., 1980). Based on
employment values of the US Bureau of the Census (1987) these
estimates of exposed workers represent from 0.02% to 0.19% of the
civilian workforce in the USA. The low estimate of 15 000, although
quoted in a US Environmental Protection Agency report, originated
with a manufacturer of 2-NP. The estimate generated by Occupational
Health Services, Inc., carried out under contract with a
manufacturer of 2-NP, was based on a detailed survey of
distributors, manufacturers, and users, and thus may represent a
reasonable approximation. This report considered 38 600 to be the
best estimate of the total number of exposed workers in the USA and
set 126 600 workers as an upper boundary. It further estimated that
significant exposure (defined as exposure to at least 10% of the US
OSHA exposure limit or 9.1 mg/m3 (2.5 ppm)) ranged from 4000 (best
estimate) to 10 600 (upper boundary) workers. Sources of worker
exposure identified in a survey conducted in the USA by the National
Institute for Occupational Safety and Health (Finklea, 1977)
included rotogravure and flexographic inks used in printing, and
coatings and adhesives used in industrial construction and
maintenance, highway marking, ship building and maintenance,
furniture manufacture, and food packaging. A US NIOSH survey
estimated that 9815 workers in the USA were exposed to 2-NP or to
trade-name products containing 2-NP (US NIOSH, 1983).
Occupational exposure limits are summarized in Table 3.
There is little data on actual occupational exposure, although
the limited information on conditions in the USA summarized in
Table 4 suggests that it is highly variable. Manufacture of 2-NP, an
enclosed process, appears to involve little employee exposure much
of the time, but spills and operations such as filling drums can
briefly expose a few workers to high concentrations. In general, low
exposures may be typical in some painting operations and in the
manufacture of tyres, but other painting and manufacturing
operations appear, at least in the past, to have exposed workers to
dangerously high concentrations. Workers were exposed to
concentrations of 2-NP up to at least 2744 mg/m3 (754 pm) in a
pigment production facility and up to at least 265 mg/m3 (73 ppm)
in a solvent extraction plant. Evidence exists that concentrations
of 2-NP at the solvent extraction plant prior to the investigation
were at times substantially greater than the values measured during
the investigation (Crawford et al., 1985). Exposure levels mentioned
in the report by Occupational Health Services, Inc. (1982) were
mainly in the vicinity of 3.6 mg/m3 (1 ppm), although one printing
Table 3. Occupational exposure limits for 2-nitropropane in aira
Exposure limit
Country mg/m3 ppm Category of limit
Australia 36 10 TWA
Belgium 36 10 TWA
Brazil 70 20 AL
Canada 90 25 CLV
Denmark 36 10 TWA
Finland 18 5 TWA
150 40 STEL
Germany 18 5 1-year TWA(TRK)
Hungary 10 3 CLV
Netherlands 3.6 1 8-h TWA
7.3 2 STEL
Poland 30 8 TWA
70 20 STEL
Romania 47 13 TWA
70 20 STEL
Sweden 18 5 TWA
36 10 CLV
Switzerland 18 5 TWA
United Kingdom 90 25 8-h TWA
USA 90 25 8-h TWA (OSHA)
36 10 8-h TWA (ACGIH)
Yugoslavia 90 25 TWA
a From: IRPTC (1986)
ACGIH = American Conference of Governmental Industrial Hygienists
AL = Acceptable or tolerable limit
CLV = Ceiling value
MAK = Maximum worksite concentration
OSHA = Occupational Safety and Health Administration
STEL = Short-term exposure limit
TLV = Threshold limit value
TWA = Time-weighted average (MAK in Switzerland)
TRK = Technical guiding concentration
plant (Table 4) reported a peak concentration of 237 mg/m3
(65 ppm) which lasted 30 min, and a time-weighted average of
36-44 mg/m3 (10-12 ppm). In addition to inhalation, it is likely
that workers using 2-NP as a solvent will have at least occasional
contact with the liquid. 2-NP also has been reported to be a minor
component of both fresh and used machine cutting fluid emulsion
(Yasuhara et al., 1986). The importance of exposure to 2-NP as a
contaminant of 1-nitropropane is unknown. In an investigation
involving 1-nitropropane-sensitized ammonium nitrate blasting agents
(Cocalis, 1982), 2-NP was below the detectable limit. Occupational
exposure may be reduced in the future since strong recommendations
have been issued on minimizing all worker contact with 2-NP and its
fumes and on substituting other less toxic solvents where possible
due to the carcinogenicity of 2-NP (IRPTC, 1986; US EPA, 1986; US
NIOSH 1988).
Table 4. Air concentration of 2-NP in the work place
Concentration
Activity mg/m3 ppm Reference
Manufacture of 2-NP 3.64 approx 1 Brown & Dobbin (1977)
Manufacture of 2-NP 0.7-364; 0.2-100; Miller & Temple (1979)
98% of 98% of
samples samples
were < 36.4 were < 10
Vulcanizing tyres 0-0.18 < 0.05 Hollett & Schloemer
(1978)
Painting (bus 0.11 < 0.03a Love & Kern (1981)
maintenance)
Painting (railway 1.46 < 0.4 Hartle (1980)
cars)
Solvent extraction 167.4 0-100 Crawford et al. (1985)
(average
46)
Laboratory (1958) 14.6 0-21 Angus Chemical Co. &
(average 4) Occusafe, Inc. (1986)
2-NP storage & 2111-6000 580-1640 Angus Chemical Co. &
transfer area (1962); Occusafe, Inc. (1986)
drum filling
operation
Pigment production 109-2745 30-754 Angus Chemical Co. &
facility (1970) Occusafe, Inc. (1986)
Painting (battery 36.4-109 10-30 Skinner (1947)
cases)
Manufacturing 72.8-164 20-45 Skinner (1947)
(coating forms)
Printing approx 40 1-65 Occupational Health
(approx 11) Services, Inc. (1982)
a below limit of detection
6. KINETICS AND METABOLISM
6.1 Absorption
2-NP is absorbed via the lungs, the peritoneal cavity, the
gastrointestinal tract, and possibly, to a lesser extent, via the
skin. Absorption via the lungs, peritoneal cavity, and
gastrointestinal tract have been used in experimental studies and
have been investigated using rats and 14C-labelled 2-NP. Pulmonary
absorption was examined by Nolan et al. (1982), Müller et al.
(1983), and Filser & Baumann (1988). Nolan et al. (1982) considered,
on the basis of respiratory rate and tidal volume of the rat and the
accumulation of 2-NP during the 6-h period of exposure, that a
minimum of 40% of the inhaled 2-NP was absorbed. This value is
minimal since it does not include 2-NP metabolized and eliminated
during exposure. The data of Müller et al. (1983) suggested that
immediately following exposure to 728 mg/m3 (200 ppm) for 3 h,
plasma contained approximately 0.4% as 2-NP and 7.2% as metabolites
of the 2-NP inhaled. The metabolites were mainly acetone but also
included a small amount of isopropanol. These percentages were
estimated from the results of Müller et al. (1983) and normative
data for the laboratory rat (Baker et al., 1979), and support rapid
pulmonary uptake of 2-NP since 2-NP and its metabolites sequestered
elsewhere in the body and loss of metabolized 2-NP during exposure
were not considered. Filser & Baumann (1988) reported that uptake of
gaseous 2-NP was rapid, the clearance rate being equal to the
ventilation rate. The value they cite for the latter, 32
litres.h-1.kg-1), seems large in comparison with normative data
for the laboratory rat (Baker et al., 1979).
The rate of 2-NP uptake by rats from intraperitoneal injection
was examined by Müller et al. (1983). Ten minutes after an injection
of 25 mg/kg, the blood plasma contained 3.3% of the dose as 2-NP and
1.9% as metabolites, acetone, and isopropanol, indicating an uptake
of greater than 5.2% since presumably some of the dose was already
lost from the body and to other tissues in the body during this
initial period. These percentages were estimated from the data of
Müller et al. (1983) and normative data for the laboratory rat
(Baker et al., 1979). A dose of 50 mg/kg yielded partially
dissimilar results. The 10-min average value was low and also had a
very large standard deviation. This may have reflected large
differences among the rats in their initial uptake rates for 2-NP or
an experimental problem such as injection into the gastrointestinal
tract rather than the peritoneal cavity. Blood plasma contained, 10
min after injection, only 1.4% of the dose as 2-NP and 1.3% as
acetone and isopropanol. These data indicate that uptake from the
peritoneal cavity is fairly rapid, and suggest that the relatively
slower uptake of the 50-mg/kg dose may have reflected saturation of
the uptake mechanisms, since initial concentrations in the plasma
did not exceed those following the 25-mg/kg dose. Intraperitoneal
injections were used by Andrae et al. (1988), Guo et
al. (1990), Hussain et al. (1990), and Conaway et al. (1991) to
demonstrate that 2-NP induced nucleic acid damage in the livers of
Wistar, F-344 and Sprague-Dawley rats.
Absorption of 2-NP via the gastrointestinal tract was
investigated with male Wistar rats by Derks et al. (1989). They
found that the systemic availability of orally administered 2-NP
from a water solution was very high (90%) and absorption was rapid,
maximum plasma values being reached within 15 min after dosage.
Absorption of 2-NP given in olive oil was much slower and
availability was only 34% during the initial 3 h following dosage.
The authors suggested that absorption from oil was incomplete at 3 h
and might ultimately be much higher, since the olive oil was
absorbed and the 2-NP redistributed between the oil and aqueous
phases.
Although workers are cautioned against dermal contact with 2-NP
(Beall et al., 1980; US EPA, 1986), there appear to be no
quantitative data on dermal absorption. The solubility of 2-NP in
both polar and non-polar solvents, together with its small molecular
size, suggests that it should be absorbed readily through the skin
(Malkinson & Gehlmann, 1977). Dermal application of 2 g 2-NP/kg to
rabbits produced no obvious symptoms (Wilbur & Parekh, 1982);
however, as noted below, the rabbit is relatively resistant to the
toxicity of 2-NP.
6.2 Distribution
The distribution of 2-NP and its carbon-containing metabolites
among the organs and tissues in Sprague-Dawley rats was examined by
Nolan et al. (1982) via inhalation, and by Müller et al. (1983) via
intraperitoneal injection. Both utilized 14C-labelled 2-NP and
thus their data do not reveal the distribution of nitrite and other
nitrogen-containing metabolites generated from the nitro portion of
the 2-NP molecule. One hour after intraperitoneal injection,
radioactivity was concentrated in fat; there were intermediate
amounts in the blood, liver, and kidney, and lower amounts in other
organs and tissues (Müller et al., 1983). By 40 h, the highest
concentrations were in bone marrow and adrenal tissue, intermediate
amounts being found in the kidney, liver, spleen, lungs, and omental
fat, and by 8 days only the concentration of 14C in adrenal tissue
was noticeably greater than elsewhere in the body. However, Nolan et
al. (1982), found, both immediately and 48 h after a 6-h period of
2-NP inhalation, that highest concentrations of carbon from 2-NP
were in the liver and kidney and relatively little in the fat.
Differences in methodology limit intercomparison of these
studies. Their major consistency is the presence of high
concentrations of 2-NP and its labelled carbon in the liver and
kidney, organs (as discussed below) actively involved in the
metabolism of 2-NP and excretion of its metabolites.
The relevance of these studies on tissue distribution of 2-NP
and its carbon-containing metabolites to the toxicity of 2-NP is
unclear, since (as discussed below) most of the dose is rapidly
metabolized initially to acetone and nitrite. The 14C label used
in these studies thus traced mainly the distribution of acetone and
its metabolites in measurements made more than a few hours after
dosing.
Dequidt et al. (1972) provided limited data on the distribution
of nitrite among body organs of the rat following inhalation and
intraperitoneal injection of 2-NP. The data suggest a fairly uniform
distribution among the heart, lungs, kidney, spleen, and,
sometimes, the liver. In the majority of experiments, however, no
nitrite was detected in the liver. No explanation is offered for the
latter observation, and data in the paper are so erratic as to
suggest the possibility of analytical problems.
6.3 Metabolic transformation
Starting with a report by Scott (1943), there have been numerous
studies on the metabolic transformation of 2-NP by mammals,
mammalian cells, microorganisms, and isolated enzymes. These studies
have shown that the major pathway for metabolic transformation of
2-NP involves oxidation to nitrite and acetone. Evidence for the
formation both of nitrite and acetone was reported from studies on
liver microsomes from rats pretreated with phenobarbital or
3-methylcholanthrene (Ullrich et al., 1978), cultured hepatocytes
from untreated rats (Haas-Jobelius et al., 1991), liver microsomes
from untreated mice (Marker & Kulkarni 1986a,b; Dayal et al., 1991),
V79 Chinese hamster cells (Haas-Jobelius et al., 1991), and a yeast
(Kido et al., 1975). Nitrite was reported to be a major metabolite
of 2-NP in rabbits (Scott, 1943), rats (Dequidt et al., 1972), and
liver microsomes from rats (Sakurai et al., 1980) and mice (Marker &
Kulkarni, 1985). Acetone was identified as a major metabolite of
2-NP in rats and chimpanzees (Muller et al., 1983).
Enzymatic oxidation of the nitronate form of 2-NP to nitrite and
acetone by horseradish peroxidase (Porter & Bright, 1983), a
dioxygenase from the yeast Hansenula mrakii (Kido et al., 1984),
and mouse liver microsomes (Dayal et al., 1991) was several times
more rapid than that of 2-NP under identical conditions. In addition
to acetone, a smaller amount of isopropanol is produced at least in
rats and chimpanzees (Muller et al., 1983). The source of the
isopropanol was not specified in this study, but since reduction of
acetone in the body is negligible (De Bruin, 1976), isopropanol may
be formed directly by oxidation of 2-NP. The formation of a
hydroxyisopropyl radical during the oxidation of 2-NP was suggested
by Kuo & Fridovich (1986).
The metabolic fates of these metabolites of 2-NP are well known.
Acetone is produced by a minor metabolic pathway in the mammalian
body (Smith et al., 1983) and has been detected in small amounts in
the blood, urine, and expired air of normal humans (Mabuchi, 1979;
Conkle et al., 1975). It may be excreted directly via expired air,
urine, and loss through the skin, or may enter into the general
metabolism either via cleavage to a 2-carbon acetyl fragment and a
1-carbon formyl fragment or via oxidation to pyruvic acid (De Bruin,
1976). The proportion excreted unchanged increases with increasing
dosage, suggesting an easily saturable metabolic pathway.
Isopropanol is oxidized to acetone (De Bruin, 1976).
Nitrite may exist as a minor constituent of the mammalian body.
It is constantly replenished by ingestion and synthesis, and
constantly removed by oxidation to nitrate. Nitrite and nitrate in
the blood stream are rapidly and homogeneously distributed
throughout the body (Parks et al., 1981). Nitrite rapidly oxidizes
divalent ferrous haemoglobin to trivalent ferric methaemoglobin
(Burrows, 1979). Little is transported to the tissues or excreted,
at least in dogs, sheep, and ponies (Schneider & Yeary, 1975).
Dequidt et al. (1972), however, reported substantial urinary
excretion of nitrite by rats following inhalation or intravenous
injection of 2-NP. Methaemoglobin is incapable of transporting
oxygen and, during enzymatic repair of this defect, nitrite is
reoxidized to nitrate. Parks et al. (1981) reported that 10 min
after intratracheal instillation of labelled nitrite into mice, 70%
of the label in plasma was in nitrate, 3% in nonionic compounds, and
only 27% remained as nitrite. Similar results were obtained with
rabbits. Nitrate is slowly excreted through the kidneys (Schneider &
Yeary, 1975) and also into saliva where it is reduced back to
nitrite by bacteria and reabsorbed into the body via the
gastrointestinal tract (Friedman et al., 1972). Small amounts of
nitrite in the stomach may react with secondary amines and other
amino substrates to form N-nitroso compounds which might be absorbed
(Sander & Schweinsberg, 1972; Fine et al., 1982).
The enzymatic system oxidizing 2-NP to acetone and nitrite was
identified through in vitro experiments using microsomes isolated
from mammalian liver. Ullrich & Schnabel (1973) determined that
cytochrome P-450, in liver microsomes from phenobarbital-pretreated
rats, bound 2-NP. Ullrich et al. (1978) subsequently reported that
liver microsomes from rats pretreated with phenobarbital or
3-methylcholanthrene rapidly catalysed the oxidation of 2-NP to
acetone and nitrite. The latter were produced in roughly equal
quantities. Surprisingly, however, the rate of this reaction was not
diminished under conditions of reduced oxygen pressure. The activity
of preparations from untreated control rats was generally very low.
Sakurai et al. (1980) demonstrated that this enzyme system in rats
was active in metabolizing other aliphatic nitro compounds. Marker &
Kulkarni (1985, 1986a, 1986b), working with mice, obtained somewhat
different results. They reported rapid denitrification of
2-NP to nitrite and acetone by liver microsomes from untreated mice,
and an acetone production at least twice the nitrite release. These
authors suggested that multiple forms of cytochrome P-450 are
involved, and claimed that nitrite is sequestered in the reaction
mixture and that denitrification of 2-NP may involve a reductive or
at least non-oxidative pathway as well as an oxidative pathway. They
also noted large differences in the rates of hepatic microsomal
enzymatic nitrite release among the five strains of mice tested.
Jonsson et al. (1977) demonstrated that hepatic microsomes from
uninduced rabbits could denitrify a compound related to 2-NP,
2-nitro-1-phenylpropane.
In addition to oxidative denitrification, a reductive pathway
has been shown to occur in cultured hepatocytes from Wistar rats and
in V79 Chinese hamster cells. Nitroreduction was indicated by the
fact that the cells formed acetone oxime, the tautomeric form of
nitrosopropane (Haas-Jobelius et al., 1991).
Evidence for the involvement of more than one pathway for the
metabolism of 2-NP in the rat was also obtained by Denk et al.
(1989). Their experiments on the pharmacokinetics of 2-NP in rats
exposed by inhalation suggested that there are two different
pathways both in male and female animals, a saturable one of low
capacity and high affinity according to Michaelis-Menten kinetics
and a non-saturable one following first-order kinetics. First-order
kinetics was similar in the two sexes, but striking differences
between sexes were observed in the kinetics of the saturable
pathway. The authors showed that in females more 2-NP was
metabolized by the non-saturable pathway at concentrations above 655
mg/m3 (180 ppm), and in males at concentrations above 218 mg/m3
(60 ppm), and linked their observations to the reported higher
susceptibility to liver damage of males as compared to females
(Griffin & Coulston, 1983). Denk et al. (1989) suggested that it is
the first-order metabolic process which results in the formation of
toxic products whereas the saturable pathway was suggested to lead
to less toxic metabolites.
These observations on the hepatic metabolism of 2-NP and related
compounds by rats, mice, and rabbits indicate differences among
species and even strains. It is probable that more than one enzyme
system is involved. In mice as well as rats hepatic cytochrome P-450
may be important in the metabolism of this xenobiotic.
Observations by Ivanetich et al. (1978) suggested an additional
detoxifying role for hepatic microsomal cytochrome P-450. They
demonstrated that under aerobic conditions in vitro 2-NP could
degrade the haem moiety of cytochrome P-450 in phenobarbital-induced
rats and speculated that this provided an additional mechanism for
trapping reactive metabolites before these could damage essential
cellular constituents.
In addition to the hepatic enzymatic systems examined in rats
and mice, Mochizuki et al. (1988), as mentioned above, described a
2-NP denitrifying system in adrenal microsomes of uninduced
guinea-pigs. They identified this cytochrome-P-450-dependent
monooxygenase as benzo[ a]pyrene hydroxylase.
6.4 Elimination and excretion
Elimination of 2-NP and its metabolites has been examined mainly
in rats and, to a much lesser extent, in chimpanzees in studies
which utilized measurements of radioactivity from 14C-labelled
2-NP as well as measurements of 2-NP and its metabolites. Dosage by
inhalation, intravenous injection, and intraperitoneal injection all
yielded fairly similar results. During a 48-h period after a 6-h
exposure of rats to 73 mg/m3 (20 ppm) and to 560.6 mg/m3
(154 ppm) of 14C-labelled 2-NP, about 50% of the radioactivity in
the absorbed dose was excreted via the lungs as carbon dioxide
(Nolan et al., 1982). The proportion of the absorbed dose excreted
via the lungs as unchanged 2-NP was 4% at the low dose level and 22%
at the high level. Still less of the labelled carbon was eliminated
via faeces and urine, i.e. 11% and 8%, respectively, at the low dose
level, and 5% and 11%, respectively, at the high level.
Disappearance of 2-NP from the blood after exposure at the high dose
level followed a first-order relationship and yielded a half-life of
48 min. Limited data in Müller et al. (1983) yielded a half-life for
rats of approximately 80 min for 2-NP in plasma following a 3-h
exposure to a concentration of 728 mg/m3 (200 ppm). Nolan et al.
(1982), however, found that disappearance of the 14C label of the
2-NP from the plasma was markedly slower and biphasic. During the
first 12 h following exposure to 560.6 mg/m3 (154 ppm), the
half-life for plasma radioactivity was 172 min and, following
exposure to 73 mg/m3 (20 ppm), 354 min. After 12 h, loss of
radioactivity from plasma was much slower, the half-life being
approximtely 35-36 h for both doses. These data on loss of 2-NP and
its 14C label indicate that 2-NP is rapidly eliminated from the
body mainly by metabolic transformation and to a lesser degree by
pulmonary excretion of the unchanged compound. The major
carbon-containing metabolites of 2-NP, acetone and isopropanol,
presumably enter into the general metabolism of the body and are
eliminated via the intermediary metabolism as part of a much larger
carbon pool.
Pulmonary excretion of 2-NP, like loss of the 14C label from
plasma, is dose dependent, biphasic, and follows first-order
kinetics (Nolan et al., 1982). Fifty times more 2-NP was exhaled
during the first hour following exposure to 560.6 mg/m3 (154 ppm)
than during the first hour following exposure to 73 mg/m3
(20 ppm). Following exposure to 73 mg/m3, 2-NP was excreted for
the first 7 h at a rate which decreased by one half every 64 min,
and subsequently decreased by one half every 16 h, whereas following
exposure to 560.6 mg/m3, the half-times of excretion were 71 min
for the first 12 h, and 16 h for the subsequent period. Changes in
the rates of pulmonary excretion of 14C-labelled carbon dioxide
were similar for 48 h following exposure to 73 and 560.6 mg/m3.
Eighty seven per cent of the total was eliminated during the first
12 h after exposure; loss was somewhat less rapid thereafter. The
dose-dependent nature of pulmonary excretion of 2-NP suggests that
greater concentrations of 2-NP in the blood markedly increase
exhalation of the unchanged compound and reduce the percentage
metabolized. Thus exposure of the tissues to 2-NP and its
metabolites may not be a linear function of the inhaled dose.
Derks et al. (1989) found that the plasma half-life of
intravenous doses of 0.01-0.05 g/kg in rats was 45 min during the
first 4 h. Loss was linear over this dose range and could be
described by an open single-compartment model. They suggested that
the measured loss from plasma may be due in part to spontaneous
conversion of 2-NP to its anionic form, 2-NP nitronate.
Elimination of 2-NP by Sprague-Dawley rats following
intraperitoneal injection of 14C-labelled 2-NP was studied by
Müller et al. (1983) and was generally similar to the elimination of
2-NP following inhalation. The concentration of 2-NP in plasma
declined exponentially with time, with half-lives of 70 and 125 min
during at least the initial 6 h following injections of 25 and
50 mg/kg, respectively. Metabolites of 2-NP, acetone and
isopropanol, reached maxima 2-4 h after injection of 25 mg/kg, and
at least 4-6 h after injection of 50 mg/kg. The concentration of
isopropanol ranged from 1/16 to 1/34 the concentration of acetone.
During the initial 40 h after injection of 50 mg/kg, 4.5% of the
dose was exhaled as 2-NP, 10.4% as acetone, and 38.1% as carbon
dioxide. Losses via urine (5.9%) and faeces (0.7%) were small in
comparison with the 53% loss via exhalation. Müller et al. (1983),
however, reported that only 12% of the dose was recovered from the
carcasses, leaving a large amount (28.4%) unaccounted for and thus
casting some doubt on their values.
With one exception, results similar to the above were obtained
following intravenous injection of 14C-labelled 2-NP (10 mg/kg)
into male chimpanzees (Müller et al., 1983). The concentration of
2-NP in plasma declined exponentially with time, the half-life being
92 min. The concentration of acetone reached a maximum 6 h after
injection and remained high for at least 48 h. The concentration of
isopropanol peaked 3 h after injection when it approached that of
acetone, but otherwise was a third to a quarter that of acetone. The
concentration of 2-NP and its carbon-containing metabolites (i.e.
the concentration of 14C) in plasma declined exponentially with a
half-life of 5.5 h for the initial 10 h, and a half-life of 48 h
thereafter. As in rats, exhalation was the major means of
elimination of 2-NP and its carbon-containing metabolites. During
the first 3 days after injection, only 5-6% of the 14C in the dose
was recovered in urine and only 0.4-0.5% in faeces. Acetone,
isopropanol, and 2-NP were mainly eliminated via renal excretion;
urine collected during 6 to 24 h after injection contained 3.1 mg
acetone/litre, 7.2 mg isopropanol/litre, and 1.8 mg 2-NP/litre.
Isopropanol may thus be maintained at low concentrations in the
plasma both by oxidation to acetone and by rapid excretion. In
addition to acetone, isopropanol, and 2-NP, 14C was excreted as an
unidentified polar metabolite which by 24 h after injection
contained 90% of the radioactivity in the urine. The one striking
difference between results with rats and with chimpanzees, i.e. the
relatively much higher plasma concentrations of isopropanol in
comparison to acetone, might indicate interspecific variation in the
excretion of 2-NP and its metabolites.
Dequidt et al. (1972) provided limited information on the
excretion of nitrite following inhalation and intraperitoneal
injection of 2-NP. Rats weighing approximately 250 g each were given
a daily injection of 0.11 g/kg. Urinary excretion of nitrite was 10
to 35 µg/animal during the first day following the initial
injection, and reached a daily rate of 11 mg/animal by the fourth
injection. The latter rate of excretion represents three quarters of
the nitrogen injected daily as 2-NP, and stands in sharp contrast to
the results of Schneider & Yeary (1975), who reported that little
intravenously injected nitrite was excreted by dogs, sheep or
ponies. Following exposure of rats to a 2-NP concentration of
2766 mg/m3 (760 ppm) for 8 h on each of two successive days, daily
elimination of nitrite was approximately 30 mg/animal. This was
equivalent to about 20% of the 2-NP inhaled daily and possibly as
much as 50% of the absorbed daily dose. The amount of 2-NP inhaled
was estimated from normative data for the rat (Baker et al., 1979),
and absorption was assumed to be 40% of the amount inhaled (Müller
et al., 1983). No nitrite was detected in urine during exposure of
rats to 291 mg/m3 (80 ppm) for 8 h per day on 5 successive days.
6.5 Retention and turnover
There is no evidence that 2-NP is retained for more than a few
hours in the body. It is rapidly lost by exhalation and metabolic
transformation. The known carbon-containing metabolites, acetone and
isopropanol, are excreted rapidly and are also transformed into
compounds which are normal to the body and enter into its general
intermediary metabolism. There is less information on nitrite, the
major metabolite of the nitro moiety. In rats much of the nitrite is
excreted as such in the urine. There is no evidence for excessive
accumulation of 2-NP or its metabolites in any organ or tissue.
Information is also lacking on possible N-nitroso and other toxic
compounds synthesized from nitrite or the nitro moiety.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Data on single exposures to experimental animals, summarized in
Table 5, indicate substantial differences in sensitivity among the
species tested. The route of administration was mainly via
inhalation, but other routes were also used. A quantitative
comparison of the results from these studies is difficult due to a
lack of information on both strain and sex of the animals used, to
differences in the route of administration, and to large variations
in dosage. The LC50 for mortality within 14 days following a 6-h
exposure was estimated to be 1456 mg/m3 (400 ppm) for male rats
and 2621 mg/m3 (720 ppm) for females (Baldwin & Williams, 1977).
Exposure of rats (of unspecified sex) via inhalation to 14 058
mg/m3 (3862 ppm) for 1 h or to 9584 mg/m3 (2633 ppm) for 2.25 h
killed some of the animals within days. Exposure of rats to a much
higher concentration of 2-NP, i.e. 53 508 mg/m3 (14 700 ppm),
killed all animals within 4 h. An inhalation exposure of 4994
mg/m3 (1372 ppm) or less for 2.25 h was not lethal to rats. Unlike
rats, LC50 values were similar, i.e. 2031 and 2038 mg/m3
(558 and 560 ppm), respectively, for male and female mice following
a 6-h exposure (Baldwin & Williams, 1977). Cats appeared more
sensitive to acute exposure to 2-NP than rats. Exposure to
8565 mg/m3 (2353 ppm) for 1 h (or lower concentrations for
proportionately longer) was lethal to some cats. Rabbits and
guinea-pigs, on the other hand, appeared far less sensitive to 2-NP
than rats. Rabbits survived a 2.25-h exposure to 9584 mg/m3
(2633 ppm) and guinea-pigs a 2.25-h exposure to 15 699 mg/m3
(4313 ppm). The sequence in acute sensitivity of animals to inhaled
2-NP (from most to least) was cat, rat and mouse, rabbit, and
guinea-pig. The cat was almost an order of magnitude more sensitive
than the guinea-pig.
There are limited data on lethality via routes of administration
other than inhalation. Large intraperitoneal doses were found to be
promptly lethal to rats; 1.7 and 1.1 g/kg killed animals within 2 h
and 4 h, respectively (Dequidt et al., 1972). The 14-day oral LD50
for mice was 0.40 g/kg. The minimal oral lethal dose for the rabbit,
0.50-0.75 g/kg, was much larger than the estimated minimum lethal
dose via inhalation, 0.24 g/kg. Dermal application of 2 g/kg to
rabbits produced no obvious local or systemic effects.
Table 5. Effects of single exposure to 2-nitropropane in mammalsa
a) Lethality
Species Sex Concentration Estimated total Effects/results Reference
doseb (g/kg)
g/m3 ppm
Oral administration
Rabbitcd lethal dose estimated as 0.50- Machle et al. (1940)
0.75 g/kg
Mouse M/F 14-day LD50: 0.40 g/kg Hite & Skeggs (1979)
Inhalation
Ratc (Wistar) 53.5 14 700 1.51 all animals died within 4 h Dequidt et al (1972)
Ratcd 5.5-14.1 1513-3865 0.10-0.17 death of some animalsc Treon & Dutra (1952)
2.6-8-57d 714-2353 0.06-0.08 no deathsd Treon & Dutra (1952)
Ratcd (Wistar) 2.77 760 0.16 death within 48 h; Dequidtet al. (1972)
Rate (CD) M 2.93 805 0.16 8/10 died within 14 days Baldwin & Williams (1977)f
F 2.19 602 0.13 no deaths within 14 dayse Baldwin & Williams (1977)f
M 2.10 574 0.14 8/8 died within 14 days Baldwin & Williams (1977)f
M 1.68 461 0.10 7/8 died within 14 days Baldwin & Williams (1977)f
Table 5 (contd)
Species Sex Concentration Estimated total Effects/results Reference
doseb (g/kg)
g/m3 ppm
Inhalation (contd)
Rate (CD) M 1.47 405 0.08 5/8 died within 14 days Baldwin & Williams (1977)f
1.34 367 0.08 no deaths within 14 days Baldwin & Williams (1977)f
Mousee (ICR) F 2.70 740 0.47 14/14 died within 14 days Baldwin & Williams (1977)f
2.33 640 0.41 11/14 died within 14 days Baldwin & Williams (1977)f
1.8 495 0.32 2/14 died within 14 days Baldwin & Williams (1977)f
Mouse (ICR) M 2.69 738 0.41 9/14 died within 14 days Baldwin & Williams (1977)f
2.08 558 0.28 7/14 died within 14 days Baldwin & Williams (1977)f
1.65 454 0.23 no deaths within 14 days Baldwin & Williams (1977)f
Catcd 2.6-8.56 714-2353 0.07-0.19 death of some animals Treon & Dutra (1952)
1.19-2.87 328-787 0.02 no deaths Treon & Dutra (1952)
Guinea-pigcd 16.8-35.0 4622-9607 0.53-0.63 death of some animals Treon & Dutra (1952)
8.67-34.7 2381-9523 0.23-0.32 no deaths Treon & Dutra (1952)
Rabbitc 8.67-34.7 2381-9523 0.24-0.27 death of some animals Treon & Dutra (1952)
5.1-14.1 1401-3865 0.10-0.16 no deaths Treon & Dutra (1952)
Table 5 (contd)
Species Sex Concentration Estimated total Effects/results Reference
doseb (g/kg)
g/m3 ppm
Intraperitoneal administration
Ratcf 1.7 death within 2 h Dequidt et al. (1972)
1.1 death within 4 h Dequidt et al. (1972)
Intraperitoneal administration (contd)
Mousec M 0.80 LD50 Friedman et al. (1976)
(Swiss ICR/HQ)
a Values recalculated as necessary to ppm and g/kg
b Estimated dose calculated by the formula: tidal volume x respiration frequency x exposure time x 2-NP conc. x alveolar
retention/animal weight. Tidal volume and respiration frequency from Kaplan et al. (1983) for mice (0.15 ml, 163/min);
from Baker et al. (1979) for rats (0.86 ml, 85.5/min); from Hoar (1976) for guinea-pigs (1.68 ml, 84/min); from Kozma
et al. 81974) for rabbits (15.8 ml, 45/min); from Reece (1984) and Breazile (1971) for cats (42 ml, 31/min); alveolar
retention in rat (0.40) from Nolan et al. (1982), used for all species; where not stated animal weights assumed to be
average values, 20 g for mice, 250 g for rats, 500 g for guinea-pigs, 2.5 kg for rabbits, and 4.0 kg for cats
c Sex not specified
d Exposure time varied from 1 to 7 h
e Exposure time was 6 h
f Baldwin & Williams (1977) also exposed female rats for 6 h to concentrations of 2-NP lower than 2.2 g/m3 (602 ppm); these
concentrations, i.e. 1.15, 1.35 and 1.69 g/m3 (316, 370 and 464 ppm), like 2.2 g/m3, produced no deaths within 14 days
Table 5. Effects of single exposure to 2-nitropropane
b) other effects
Route of Species Sex Estimated Effects/results Reference
administration total doseb
g/kg)
Intraperitoneal rat M 0.15 maximum hepatic injury achieved Filser & Daumann (1988)
(Sprague-Dawley) with this dose
Intraperitoneal rat M 0.05 lipid accumulation, centrilobular Zitting et al. (1981)
(Wistar) necrosis, mitochondrial
abnormalities, and changes in
endoplasmic reticulum and
glutathione content in liver
within 24 h, as well as changes
in enzyme activity in liver and
brain
Dermal rabbit M & F 2 no toxic effects observed Wilbur & Parekh (1982)
b See footnote b in Table 5a.
The effects of acute exposure to 2-NP, in addition to lethality,
are characterized primarily by hepatotoxicity and, at high exposure
levels, methaemoglobin formation and depression of the central
nervous system. Machle et al. (1940), in a description of symptoms
resulting from exposure of laboratory animals to simple
nitroparaffins, listed the following progression for guinea-pigs and
rabbits after a latent period of 20 to 40 min: progressive weakness,
unsteadiness, and incoordination ending in complete ataxia. The rate
of respiration at first slowed and later became increasingly rapid.
Most of these symptoms appear to reflect the narcotic effects
normally associated with inhalation of volatile hydrocarbons, but
the more rapid breathing may reflect an attempt by the body to
compensate for the formation of methaemoglobin and loss of
oxygen-carrying capacity of the red blood cells. Treon & Dutra
(1952) noted similar progression from exposure to high
concentrations of 2-NP vapour, i.e. lethargy and weakness, dyspnoea,
cyanosis, prostration, and ultimately coma and death, but did not
report more rapid breathing following a depression in rate of
respiration. They also noted lacrimation, salivation, and gastric
regurgitation in cats. In addition, the authors observed that, even
with animals which died promptly (within 9.5 h) following a single
exposure, there was a loss in body weight averaging 2.8%. Animals
exposed to 8.56 g/m3 (2353 ppm) or higher concentrations of 2-NP
displayed pathological changes including hepatocellular damage,
pulmonary oedema and haemorrhage, some disintegration of neurones in
the brain, and widespread damage to the endothelium.
Methaemoglobin and Heinz bodies (masses of denatured haemoglobin
within erythrocytes) were found in the blood of animals following
single exposures to high concentrations of 2-NP. In the case of
cats, 60 to 80% of the haemoglobin was converted to methaemoglobin
by exposure to 15.9 to 33.6 g/m3 (4360 to 9230 ppm) for 1 to 2 h,
whereas much longer exposure of rabbits to these concentrations
converted only 4 to 8% of the haemoglobin (Treon & Dutra, 1952).
Dequidt et al. (1972) reported high levels of methaemoglobin in
rats, i.e. 84% and 89%, following 1-h inhalation exposure to
53.5 g/m3 (14 700 ppm) and intraperitoneal injection of 1.7 g/kg,
respectively. Much lower methaemoglobin concentrations, 0.2 to 8.6%,
resulted from a 1-h exposure to 2.8 g/m3 (760 ppm). One day after
exposure to 15.4 g/m3 (4230 ppm) for 4.5 h or 8.5 g/m3
(2335 ppm) for 2.25 h, rabbits had Heinz bodies in 45 to 80% of
their red cells. These bodies disappeared gradually over 9 to 16
days (Treon & Dutra, 1952). Exposure of rabbits to 13.8 g/m3
(3790 ppm) for 1 h or 9.4 g/m3 (2580 ppm) for 2.25 h resulted in
the formation of Heinz bodies in only 0 to 2% of the red cells.
Formation of Heinz bodies in the cat may have reflected its high
sensitivity to 2-NP. A 1-h exposure to 13.8 g/m3 (3790 ppm) and a
20 min exposure to 16.4 g/m3 (4505 ppm) resulted in the appearance
of Heinz bodies in 27% and 16% of the erythrocytes, respectively.
Observations by Zitting et al. (1981) indicated that a single
intraperitoneal injection of 0.05 g 2-NP/kg to rats can produce
significant changes in the fine structure of the liver and in the
physiology of both liver and brain. 2-NP produced a visible
accumulation of lipid in hepatocytes, especially in periportal areas
after 4 h, and the lipid level continued to increase for the next
20 h. Within 4 h after injection there was also degranulation of the
rough endoplasmic reticulum in hepatocytes and proliferation of the
smooth endoplasmic reticulum. Within 24 h the former had almost
disappeared and the latter was vacuolated or compacted. In addition,
some hepatocytes had abnormal mitochondria and there was necrosis of
hepatocytes around the central vein. The latter was reflected in a
concurrent fourfold increase of serum alanine aminotransferase.
Other enzymatic parameters in the liver were also markedly affected
within 24 h. Cytochrome P-450 was markedly depressed,
7-ethoxycoumarin O-deethylase and 7-ethoxyresorufin O-deethylase
were diminished in activity, and microsomal epoxide hydratase,
UDP-glucuronosyltransferase and glutathione peroxidase were
increased in activity. In addition, the liver concentration of
glutathione nearly doubled.
The major observed neurochemical effect was a significant
increase in acetylcholine esterase activity in the cerebrum and in
isolated synaptosomes. There was little or no change in RNA,
2',3'-cyclic nucleotide 3'-phosphohydrolase, or acid proteinase in
the brain.
Zitting et al. (1981) noted that these histopathological and
enzymatic changes induced by 2-NP are nearly identical to the
effects of carbon tetrachloride on the rat and are thus indicative
of lipid peroxidation.
Hepatotoxicity following exposure to 2-NP has also been
observed in mice (Dayal et al., 1989). Intraperitoneal doses of
0.8 g/kg (9 mmol/kg) in male mice and 0.6 g/kg (6.7 mmol/kg) in
female mice significantly increased plasma activities of enzymes
indicative of hepatic damage (sorbitol dehydrogenase, alanine
aminotransferase, and aspartate aminotransferase) 48, 72, and 96 h
after injection. These enzyme activities were not elevated 24 h
after this dosage nor after small doses of 2-NP.
7.2 Short-term and long-term repeated exposure
Data for repeated exposure, like that for single exposure,
indicate that the cat is more sensitive to 2-NP than the other
species tested (Table 6). Rats, rabbits, guinea-pigs, and monkeys
survived 1.2 g/m3 (328 ppm) (7 h/day, 5 days/week) throughout
approximately 6 months of exposure (Treon & Dutra, 1952). However,
cats exposed to the same concentrations of 2-NP began dying after
the third day of exposure and were all dead by the end of the 17th
day (Treon & Dutra, 1952). Rats and guinea-pigs survived 5 days of
Table 6. Short-term and long-term toxicity of 2-nitropropane in mammalsa
Species Sex Dose and/or concentrationb Effects/results Reference
Oral studies
Rat (Wistar) M & F 0.25 g/kg, 5/week for 4 mortality of males (4/10); decreased growth in Wester et al. (1989)
weeks first week; increased urine, ALAT, ASAT, and
gamma-GT (males only), anaemia, thrombocyte
and leucocyte count, liver, spleen and heart
weight, and haemosiderin content of spleen;
cellular and nuclear polymorphism, single cell
necrosis, and proliferation of oval cells
and/or bile ducts in liver
Rat M 0.089 g/kg (1 mmol), some deaths by 16 week; throughout study body Fiala et al. (1987b)
(Sprague-Dawley) 3/week for 16 weeks, weights significantly lower than controls; all
maintained but not dosed rats exposed 16 weeks or longer developed
for next 61 weeks massive hepatocellular carcinomas; metastases
to the lungs in 4 animals
Rat M & F 0.05 g/kg, 5/week for 4 increased anaemia, thrombocyte conc., and Wester et al (1989)
(Wistar) weeks heart weight
Rat (Wistar) M & F 0.002 and 0.01 g/kg, 5 increased water intake by males dosed with Wester et al. (1989)
week for 4 weeks 0.002 g/kg
Inhalation studies
Ratd 2.77 g/m3 (760 ppm), 8 animals dead within 2 h after end of second Dequidt et al. (1972)
(Wistar) h/day for 2 days; inhalation session; 2-NP conc. in liver =
estimated dose over 8 h, 180 ppm; methaemoglobin = 2.4%
0.16 g/kg
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Inhalation studies (contd)
Rate 2.45 g/m3 (672 ppm), 7 no deaths Treon & Dutra (1952)
h/day for 5 days;
estimated dose over
7 h, 0.12 g/kg
1.20 g/m3 (328 ppm), 7 no deaths Treon & Dutra (1952)
h/day, 5 days/week for
130 days over 199 days;
estimated dose over 7 h,
0.06 g/kg
Rat M 0.75 g/m3 (207 ppm), 7 body weight and haematological parameters Lewis et al. (1979)
(Sprague-Dawley) h/day, 5 days/week for up unaffected; some pulmonary oedema and some
to 24 weeks; estimated pulmonary lesions within 3 months; liver
dose over 7 h, 0.04 g/kg weight elevated; hepatocellular hypertrophy,
hyperplasia and liver necrosis in all rats
within 3 months; liver neoplasms in all rats
within 6 months; these hepatocellular
carcinomas appeared to be growing rapidly and
deforming surrounding tissues
Rate 0.73 g/m3 (200 ppm), 7 growth slightly reduced and SGPT elevated in Griffin et al. (1978)
h/day, 5 days/week for 6 male rats; liver weight increased in both sexes;
months; estimated dose morphological changes in liver more pronounced
over 7 h, 0.03 g/kg in males; these included fatty metamorphosis
and hepatic nodules consisting mainly of
hyperplastic areas with distortion of lobular
architecture, necrosis and peripheral
compression
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Inhalation studies (contd)
Ratd 0.73 g/m3 (200 ppm), 7 no effect on body or organ weight to end of Griffin et al. (1986)
(Sprague-Dawley) h/day for 5 days experiment (94 week) aside from a brief
decrease in weight gain immediately following
exposure; no significant effects on mortality
or pathology
Ratc M 0.73 g/m3 (200 ppm), 7 severe liver damage with vacolar degeneration Coulston (1982)
h/day, 5 days/week for in exposed rats after 3 months up
to 7 months
Rate 0.36 g/m3 (100 ppm), 7 male rats had lower body weight, increased Griffin & Coulston (1983);
h/day, 5 days/week for up renal calcification, elevated SGPT, and Coulston et al. (1985)
to 18 months; estimated enlarged livers with necrosis, vacuolar
dose over 7 h, 0.013 g/kg degeneration and probable hepatocellular
carcinomas; female rats had increased renal
calcification and occasional hepatic masses
and nodules showing hyperplasia and vacuolar
degeneration
Rate 0.29 g/m3 (80 ppm), 8 no deaths; no trace of 2-NP in organs at end of Dequidt et al. (1972)
h/day for 5 days; estimated experiment; methaemoglobin = 0; nitrite = 0-10
dose over 8 h, 0.016 g/kg ppm in tissues, but no nitrite in urine
Rat M 0.1 g/m3 (27 ppm), 7 no gross or microscopic alteration of any Lewis et al. (1979)
(Sprague-Dawley) h/day, 5 days/week for up tissue, haematological parameter or serum
to 24 weeks; estimated biochemistry
dose over 7 h, 0.005 g/kg
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Inhalation studies (contd)
Rate 91 mg/m3 (25 ppm), 7 no changes in behaviour, appearance, rate of Griffin et al.
h/day, 5 days/week for up weight gain, final weight, serum chemistry or (1980, 1981)
to 22 months; estimated haematology; no significant increase in tumours
dose over 7 h, 0.003 g/kg and lesions associated with exposure; no
evidence of methaemoglobinaemia
Moused 0.73 g/m3 (200 ppm), 7 depression in body weight during first 3 months Griffin et al. (1984)
(ICR) h/day, 5 days/week for in females and throughout experiment in males;
48 weeks increased liver weight and elevation of liver
transaminases in females; toxic hyperplasia of
liver predominantly in females
Mousee 0.36 g/m3 (100 ppm), 7 slight depression of body weight during first 8 Coulston et al. (1986);
h/day, 5 days/week for months in males; no effects on organ weight; no Griffin et al. (1987)
18 months evidence of hepatocellular carcinoma; some
indications of liver toxicity (nodular
hyperplasia in females)
Cate 2.6 g/m3 (714 ppm), 4.5 deaths starting with first exposure, but Treon & Dutra (1952)
h/day for 4 days; some animals survived 4 exposures
estimated dose over 7 h,
0.10 g/kg
1.2 g/m3 (328 ppm), 7 deaths starting with third exposure; all Treon & Dutra (1952)
h/day, 5 days/week for 17 animals dead by end of 17th exposure
exposures; estimated dose
over 7 h, 0.07 g/kg
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Inhalation studies (contd)
Cate 1.15 g/m3 (317 ppm), 7 no deaths Treon & Dutra (1952)
h/day for 2 days;
estimated dose over 7 h,
0.07 g/kg
0.3 g/m3 (83 ppm), 7 no deaths Treon & Dutra (1952)
h/day, for 130 out of 191
days; estimated dose over
7 h, 0.02 g/kg
Rabbite 1.2 g/m3 (328 ppm), 7 no deaths Treon & Dutra (1952)
h/day, ca. 5 days/wk for
up to 130 out of 199 days;
estimated dose over 7 h,
0.06 g/kg
Rabbit M 0.75 g/m3 (207 ppm), 7 no gross or microscopic alterations to Lewis et al. (1979)
(white, NZ) h/day, 5 days/week for 24 tissues
weeks; estimated dose
over 7 h, 0.03 g/kg
Rabbite 0.3 g/m3 (83 ppm), 7 h/day no deaths Treon & Dutra (1952)
for 130 out of 191 days;
estimated dose over 7 h,
0.014 g/kg
Rabbite 0.1 mg/m3 (27 ppm), 7 no gross or microscopic alterations to Lewis et al (1979)
h/day, 5 days/week for 6 tissues
months; estimated dose
over 7 h, 0.005 g/kg
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Inhalation studies (contd)
Guinea-pige 2.45 g/m3 (672 ppm), 7 no deaths Treon & Dutra (1952)
h/day for 5 days;
estimated dose over
7 h, 0.12 g/kg
Guinea-pige 1.2 g/m3 (328 ppm), 7 no deaths Treon & Dutra (1952)
h/day, ca. 5 days/week
for 95-130 days out of
up to 199 days; estimated
dose over 7 h, 0.06 g/kg
Monkeye 1.2 g/m3 (328 ppm), 7 no deaths Treon & Dutra (1952)
h/day, ca. 5 days/week
for 100 days exposure
0.3 g/m3 (83 ppm), no deaths Treon & Dutra (1952)
7 h/day for 130 out of
191 days
Intraperitoneal studies
Ratd 0.11 g/kg, 1/day for apparently no deaths prior to sacrifice Dequidt et al. (1972)
(Wistar) 7 days 3 days after the last injection;
methaemoglobin = 4.3%; nitrite = 0.29-1.15 ppm
in organs (heart, lungs, kidneys, spleen)
Ratd 0.11 g/kg, 1/day for 15 apparently no deaths prior to sacrifice 36 h
(Wistar) days after the last injection; methaemoglobin = 0;
nitrite = 0-10.8 ppm in organs (heart, lungs,
kidneys, spleen)
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Intraperitoneal studies
Rat F 0.001 g/kg, 5/week for no significant effects on kidney function Bernard et al. (1989)
(Sprague-Dawley) 2 weeks
Dermal study
Rabbite 1 application/day on no skin irritation, illness, systemic effects Machle et al. (1940)
clipped anterior abdomen or deaths
for 5 days; dose not
stated
a values from the literature recalculated as necessary to ppm or g/kg
b estimated dose calculated by the formula: tidal volume x respiration frequency x 2-NP conc. x alveolar retention/animal weight.
Tidal volume and respiration frequency from Baker et al. (1979) for rats (0.086 ml, 85.5 /min); from Hoar (1976) for guinea-pigs
(1.68 ml, 84/min); from Kozma et al. (1974) for rabbits (15.8 ml, 45/min); from Reece (1984) and Breazile (1971) for cats
(42 ml, 31/min); alveolar retention in rat (0.40) from Nolan et al. (1982), used for all species; where not stated in reference,
animal weights assumed to be average values, 250 g for rats, 500 g for guinea-pigs, 2.5 kg for rabbits, and 4.0 kg for cats
c strain not specified
d sex not specified
e neither strain nor sex specified
exposure (7 h/day) to 2.46 g/m3 (672 ppm), but death occurred in
rats exposed for 2 days (8 h/day) to 2.77 g/m3 (760 ppm). Rats
were reported to survive 15 days of daily intra-peritoneal
injections of 0.11 g/kg (Dequidt et al., 1972). The doses used in
these repeated exposures were found to produce no more than trace
levels of methaemoglobin (maximum = 4.3%) and low concentrations
(0 to 11 mg/kg) of nitrite in the tissues.
Non-lethal chronic doses of 2-NP have been shown to produce a
number of harmful effects in rats (Table 6). Exposure of rats to
0.75 g/kg (207 ppm) for up to 24 weeks (7 h/day, 5 days/week)
initially induced pulmonary lesions and oedema, hepatocellular
hypertrophy, hyperplasia, and necrosis of the liver (Lewis et al.,
1979). By the end of 24 weeks all the rats developed rapidly growing
hepatocellular carcinomas. A similar exposure to a slightly lower
concentration of 2-NP, 0.73 g/m3 (200 ppm), induced hepatic
nodules and other destructive changes in the liver, especially in
male rats (Griffin & Coulston, 1983). These changes included fatty
degeneration, nodules consisting mainly of hyperplastic areas,
distortion of lobular architecture, necrosis and peripheral
compression. Male rats also had an elevated serum alanine
aminotransferase level (an indicator of liver damage) and slightly
reduced growth. Chronic exposure of rats to 0.36 g/m3 (100 ppm)
for up to 18 months produced similar, although slightly less severe,
damage than that resulting from exposure to 0.73 g/m3 (200 ppm)
(Griffin & Coulston, 1983; Coulston et al., 1985). Only male rats
developed hepatocellular carcinoma; female rats had increased renal
calcification and occasional hepatic masses and nodules showing
hyperplasia and vacuolar degeneration. No toxic effects were
reported after chronic exposure of rats to 90 mg/m3 (25 ppm) or
100 mg/m3 (27 ppm) (Lewis et al., 1979; Griffin et al., 1980,
1981). Daily intraperitoneal injections of 1 mg/kg (5/week for 2
weeks) had no significant effect on kidney function (Bernard et al.,
1989).
Chronic oral dosage of 2-NP by gavage for 16 weeks produced
tumours in rats (Fiala et al., 1987b). The dose was 0.089 g/kg
(1 mmol/kg), given 3 times per week, and this yielded a weekly
dosage of 0.27 g/kg, an amount similar to the highest sustained
estimated inhalation dosage. The body weights of rats treated with
2-NP were significantly lower than those of controls, and all
treated rats surviving 16 weeks or longer developed both benign
tumours and massive hepatocellular carcinomas. Metastases to the
lungs were observed in four of the 22 surviving animals (Fiala et
al., 1987b). Wester et al. (1989) treated rats with oral doses of
0.002, 0.01, 0.05, and 0.25 g/kg, 5 times per week for 4 weeks by
gavage. With a dose of 0.25 g/kg there was some mortality among male
rats, and in both sexes there was decreased growth, anaemia,
increased liver and heart weights, and severe damage to the liver.
At a dose of 0.05 g/kg the major harmful effect appeared to be
anaemia. Lower concentrations (0.01 and 0.002 g/kg) did not produce
obvious harm over the period of the experiment.
Rabbits and mice appear more resistant to the sublethal effects
of 2-NP than rats (Table 6). Chronic inhalation exposure of five
rabbits to 0.75 g/m3 (207 ppm) (7 h/day, 5 days/week) for 24
weeks, a treatment which induced hepatocellular carcinomas and
severe liver damage in rats, had no detectable effect (Lewis et al.,
1979). Rabbits also were unaffected by repeated dermal application
of 2-NP (Machle et al., 1940). Liver damage was found in mice,
especially females, during chronic exposure to 0.72 g/m3 (200 ppm)
(7 h/day, 5 days/week) for 48 weeks, but hepatocellular or other
carcinomas were not detected (Griffin et al., 1984). Exposure of
mice to 0.36 g/m3 (100 ppm) (7 h/day, 5 days/week) for 18 months
produced some liver damage, especially in females, as shown by
nodular hyperplasia (Coulston et al., 1986, Griffin et al., 1987).
7.3 Reproduction, embryotoxicity, and teratogenicity
There is limited information on the embryotoxicity and
teratogenicity of 2-NP. Hardin et al. (1981) gave intraperitoneal
injections of 0.17 g/kg (1.91 mmol/kg) of 2-NP in corn oil to
pregnant rats on days 1-5 of gestation. The dosage was a previously
determined maximum tolerated dose which produced no mortality, no
marked signs of toxicity, and less than a 10% reduction in body
weight gain during dosing or within 2 weeks following 15 daily
intraperitoneal injections to non-pregnant rats. In pregnant rats,
this dose was reported to give no evidence of maternal toxicity or
teratogenicity, but produced a significant incidence of delayed
fetal development. Harris et al. (1979) reported that 2-NP at a dose
of 0.17 g/kg retarded fetal heart development by 1 to 2 days in pups
from 9 out of 10 litters produced by female rats treated with 2-NP.
In the affected litters, 30% to 86% of the pups had retarded heart
development.
There appear to be no studies that have examined specifically
the effects of 2-NP on reproductive function. No effects on
reproductive organs, however, were noted in the above two studies
nor in any of those studies on the effects of single exposures or
short-term or long-term administration of 2-NP (Tables 5 and 6).
There also was no evidence of an increase in dominant lethality or
in sperm abnormality in genetic studies relating to reproduction
(McGregor, 1981).
7.4 Mutagenicity and related end-points
7.4.1 Prokaryotes and yeast
2-NP has been found to be mutagenic in a variety of test
systems. All investigators reported mutagenicity in several strains
of Salmonella typhimurium used with the Ames test, both with and
without an exogenous activating system (S9) (Table 7). In addition,
Kawai et al. (1987) reported mutagenicity with a strain of
Escherichia coli, but Litton Bionetics, Inc. (1977) did not find
mutagenicity with a strain of Saccharomyces cerevisiae. Most
investigators found greater mutagenicity with activation by S9 than
without. With the strain that was generally most sensitive to 2-NP,
i.e. TA100, most investigators providing detailed results observed
an approximate doubling in the number of mutants at a concentration
of 3 mg 2-NP/plate. Göggelmann et al. (1988), however, observed a
10- to 12-fold increase in mutant numbers at 2.45 mg/plate, with or
without activation by S9. Fiala et al. (1987a) reported that
non-ionic 2-NP yielded only a doubling of mutant numbers at
4.9 mg/plate with S9 activation and no increase without activation,
whereas 2-NP nitronate at this concentration yielded a threefold
increase in mutant numbers with activation. Without activation 2-NP
nitronate at 2.5 mg/plate yielded a nearly 6-fold increase in mutant
numbers; nitronate at 4.9 mg/plate was toxic to this test organism.
Fiala et al. (1987a) concluded that the mutagenicity of 2-NP is
produced mainly or entirely by the nitronate anion and suggested
that the low level of mutagenicity observed with non-ionic 2-NP may
have resulted from its conversion to the nitronate form within the
microorganisms. A similar conclusion was drawn by Dayal et al.
(1989) from their comparison of 2-NP, nitromethane, nitroethane, and
their nitronates for mutagenicity in Salmonella. Some of the
variability in results obtained by others may stem from varying
proportions of non-ionic 2-NP and 2-NP nitronate in the stock 2-NP
used in their tests. Fiala et al. (1987a) further observed that
dimethyl sulfoxide (DMSO), a solvent used to solubilize 2-NP in
these tests, modified the mutagenicity of 2-NP nitronate in a
variable manner.
Several of the studies discussed above examined the question of
how 2-NP induces mutations in the test systems. In addition to 2-NP,
1-and 2-aminopropane and a number of mononitroalkanes (nitromethane,
nitroethane, 1-nitropropane, 1-and 2-nitrobutane, and 1-and
2-nitropentane) were tested for mutagenicity with bacterial systems,
mainly strains of Salmonella typhimurium (Hite & Skeggs, 1979;
Speck et al., 1982; Löfroth et al., 1986; Kawai et al., 1987;
Göggelmann et al., 1988; Dayal et al., 1989). Only 2-NP proved to be
significantly mutagenic. Some investigators observed weak
mutagenicity with nitroethane, 1-nitropropane, 2-nitrobutane, and
2-nitropentane, which, at least in the case of nitromethane and
nitroethane, may have been produced by the 2-NP present in these
solvents as a contaminant.
Table 7. Genotoxicity of 2-nitropropane
Test system Resulta Referencesb
Prokaryotes and yeastc
Salmonella typhimurium
strain TA92 + 1
strain TA92 with S9 + 1
strain TA98 + 1,2,5,7,8,9
- 6,16,19
strain TA98 with S9 + 1,2,3,5,8,9,17
strain TA98NR + 2,9
strain TA98NR with S9 + 2,9
strain TA100 + 1,2,4,5,6,7,8,9,19
- 17
strain TA100 with S9 + 1,2,3,4,5,6,8,9,17,19
strain TA100NR + 2,9
strain TA100NR with S9 + 2,9
strain TA102 + 4,8,19
strain TA102 with S9 + 4,8,19
Table 7 (contd)
Test system Resulta Referencesb
strain TA1535 ? 5
- 17
strain TA1535 with S9 + 3
? 5
- 17
strain TA1537 - 17
strain TA1537 with S9 - 3,17
strain TA1538 - 17
strain TA1538 with S9 - 17
Escherichia coli
strain WP2 uvrA/pKM101 + 7
strain WP2 uvrA/pKM101 + 7
Saccharomyces cerevisiae
strain D4 - 17
strain D4 with S9 - 17
Table 7 (contd)
Test system Resulta Referencesb
Eukaryotesf
In vivo
Drosophila
Sex-linked recessive lethal - 10,11
Mouse
erythrocytes
(micronuclei) m, f - 1,16,23
sperm (abnormality) ? 11
Rat
Liver (DNA repair synthesis) m, f + 12,13,23
(micronuclei) + 23
(DNA and RNA base + 18,20,21,22
modifications) m, f
(DNA strand breakage) m + 24
Kidney (DNA strand breakage) m - 24
(DNA and RNA base - 22
modifications) m, f
Table 7 (contd)
Test system Resulta Referencesb
Brain (DNA strand breakage) m - 24
Lung (DNA strand breakage) m - 24
Bone marrow (chromosome - 11
aberrations) m, f
(micronuclei) m - 23
Testes
(dominant lethal test) m - 11
In Vitro
Mouse
3T3-NIH fibroblasts - 13
(DNA repair synthesis)
Rat
hepatocytes + 12,13,26
(DNA repair synthesis) m, f
hepatoma cells, 2sFou + 25
(DNA repair synthesis and micronuclei)g
hepatoma cells, C2Rev7 + 25
(DNA repair synthesis and micronuclei)g
Table 7 (contd)
Test system Resulta Referencesb
hepatoma cells, H4IIEC3/G- + 25
(DNA repair synthesis, micronuclei and
gene mutations)g
208F - embryonic fibroblasts - 13
(DNA repair synthesis)
LLC WRC 256 carcinoma Walker rat - 13
(DNA repair synthesis)
Hamster
CHO cells (chromosome aberrations - 14
and sister chromatid exchanges)
CHO cells (DNA repair synthesis) - 13
V79 cells (DNA repair synthesis) - 12,13,25,26
V79 cells (mutations) + 25
V79 cells (micrnuclei) - 25,26
Human
Lymphocytes (chromosome aberrations -d 9,15
and sister chromatid exchanges)
Lymphocytes (chromosome aberrations -e 9,15
and sister chromatid exchanges) with
S9
Table 7 (contd)
Test system Resulta Referencesb
Human (contd)
Fibroblasts (DNA repair synthesis) - 11
W138 embryonic lung fibroblasts NC1- - 13
H322 adenocarcinoma lung cells A549
adenocarcinoma lung cells HEp2 epidermal
carcinoma larynx cells (DNA repair synthesis)
a + = positive response; - = negative response; ? = inconclusive result
b (1) Hite & Skeggs (1979) (2) Speck et al. (1982)
(3) Haworth et al. (1983) (4) Simmons et al. (1986)
(5) Löfroth et al. (1986) (6) Hughes et al. (1987)
(7) Kawai et al. (1987) (8) Fiala et al. (1987a)
(9) Göggelmann et al. (1988) (10) Zimmering et al. (1985)
(11) McGregor (1981) (12) Ziegler-Skylakakis et al. (1987)
(13) Andrae et al. (1988) (14) Galloway et al. (1987)
(15) Bauchinger et al. (1987) (16) Kliesch & Adler (1987)
(17) Litton Bionetics, Inc. (1977) (18) Conaway et al. (1991a)
(19) Conaway et al. (1991b) (20) Fiala et al. (1989)
(21) Hussain et al. (1990) (22) Guo et al. (1990)
(23) George et al. (1989) (24) Robbiano et al. (1991)
(25) Roscher et al. (1990) (26) Haas-Jobelius et al. (1991)
c Liver S9 fractions are prepared by treating rats, mice or hamsters with Aroclor
1254 or another microsomal enzyme inducer, and, after several days, removing
the livers, homogenizing them, and centrifuging. The supernatant, the S9
fraction, contains liver microsomal enzymes.
d Bauchiner et al. (1987) (ref. 15) reported a weak but significantly positive result
on one test, but considered results overall to be negative
e Göggelmann et al. (1988) (ref. 9) reported a very weak positive result
f m = male; f = female
g pretreated with dexamethasone
Löfroth et al. (1986) and Fiala et al. (1987a) speculated that
the relative mutagenicity of 2-NP and other nitroalkanes correlated
with the concentrations of their nitronate anions. The more highly
mutagenic compounds, such as 2-NP and 1,1-dinitroethane, had high
concentrations of nitronate at cellular pH. A thorough comparison of
2-NP, other secondary nitroalkanes, and their nitronates (Conaway et
al., 1991) showed greater mutagenicity of the nitronates and
confirmed these speculations.
These studies do not support the hypothesis that mutagenicity of
2-NP is produced largely or entirely by nitrite resulting from its
metabolic breakdown, since the response of tester strains to sodium
nitrite was quite different from their response to 2-NP (Löfroth et
al, 1986). Unlike nitroarenes and nitroheterocyclics, 2-NP probably
does not derive its mutagenicity exclusively from enzymatic
reduction to a hydroxylamine. Strains of S. typhimurium, i.e.
TA98NR and TA100NR, lacking the "classical" nitroreductase
demonstrated either a very slightly (Speck et al., 1982) or markedly
(Göggelmann et al., 1988) reduced, but still significant, level of
mutagenicity. Göggelmann et al. (1988) suggested that some of this
mutagenicity may be due to some residual nitroreductase activity
still present in these strains.
The increased mutagenicity generally observed with S9 activation
of 2-NP and the reduced mutagenicity in tester strains deficient in
nitroreductase activity suggest an involvement of metabolism in the
mutagenicity of 2-NP in S. typhimurium. Fiala et al. (1987a)
presented evidence that metabolic oxidation of the 2-NP anion can
result in the formation of reactive species such as hydroxyl
radicals, which are capable of damaging DNA bases. Incubation of
2-NP nitronate under roughly physiological conditions with thymidine
and a 1-electron oxidation system, horseradish peroxidase and
hydrogen peroxide, yielded 2-NP free radicals (which in part
condensed into 2,3-dimethyl-2,3-dinitrobutane) and oxidation
products of thymidine of the type produced by hydroxyl radical
attack. A common source of hydroxyl radicals is superoxide, which
is known to be produced during oxidation of 2-NP nitronate by
horseradish peroxidase and hydrogen peroxide (Porter & Bright,
1983). The extent to which such reactions may occur in the
Salmonella tester strains or in vivo is unknown. There is,
however, evidence from the literature for microbial oxidation of
2-NP and 2-NP nitronate (Kido et al., 1984; Fiala et al., 1987a).
The existence of this proposed mechanism for inducing mutations
through the production of DNA-damaging hydroxyl radicals is further
supported by the reduction in 2-NP mutagenicity in TA102 by DMSO, a
known scavenger of such radicals (Fiala et al., 1987a). The fact
that DMSO had little effect on the mutagenicity of 2-NP in TA100
indicated that formation of hydroxyl radicals cannot be the only
mechanism inducing mutations. Fiala et al. (1987a) hypothesized that
the 2-NP radical may act directly by forming adducts with DNA bases.
This would provide an additional mechanism for inducing mutations
that would not require hydroxyl radicals.
7.4.2 Eukaryotes
The genotoxic effects of 2-NP in eukaryotic organisms are
summarized in Table 7. Results were negative in two sex-linked
recessive lethal tests using Drosophila despite exposure to high
concentrations of 2-NP. Zimmering et al. (1985) dosed the flies by
injection and by feeding with concentrations that induced an
approximately 30% mortality, whereas McGregor (1981) exposed the
flies to 2-NP vapour at a concentration of 2.55 g/m3 (700 ppm) for
4.5 h. Sex-linked recessive lethal mutation frequency was not
increased except in mature spermatozoa in one stock of flies. Since
this increase was not reproducible, its significance is doubtful.
Results were variable but mainly negative in a variety of
in vivo mammalian test systems. In the dominant lethal test and
the bone marrow chromosomal aberration test using rats and in the
sperm abnormality test using mice, animals were exposed to 91 or
728 mg/m3 (25 or 200 ppm) for 7 h/day on 5 consecutive days.
Neither exposure level produced a positive response in any of the test
systems used (McGregor, 1981). Negative results were obtained with
the mouse micronucleus test even with an almost lethal oral dose of
0.3 g/kg on 2 consecutive days (Hite & Skeggs, 1979), and with a
single intraperitoneal dose of 0.3 g/kg (Kliesch & Adler, 1987).
One set of genotoxicity tests using rats, however, yielded
strongly positive results (Andrae et al., 1988; Ziegler-Skylakakis
et al., 1987). In both in vivo and in vitro tests, 2-NP induced
DNA repair synthesis in rat liver cells. In the in vitro
experiments, cultures of rat hepatocytes were incubated with 2-NP,
while in the in vivo experiments rats were injected
intraperitoneally with 2-NP, sacrificed 4 h later, and cultures of
their hepatocytes were examined for DNA repair synthesis. Exposure
of hepatocyte cultures for 18 to 20 h to concentrations of 2-NP as
low as 2.7 mg/litre (30 µmol/litre) induced a detectable
(approximately 2-fold above the control level) increase in repair
synthesis. The highest concentration tested, i.e. 89 mg/litre
(10 mmol/litre), induced a 12- to 15-fold increase above control
levels in hepatocytes from male rats and a 25- to 30-fold increase
in hepatocytes from female rats. The in vivo experiments also
demonstrated the existence of a sexual difference in susceptibility
to 2-NP. In males, the lowest dose (20 mg/kg) induced a doubling in
repair synthesis and the highest dose (80 mg/kg) a 3.6-fold
increase, whereas in females these doses induced a very small
increase and a doubling, respectively. In contrast to 2-NP, 1-NP
given in vivo had no effect on DNA repair synthesis and did not
increase in vitro repair synthesis above that expected from its
contamination with 2-NP. Andrae et al. (1988) considered that their
results from the in vivo experiments were in agreement with the
observed greater hepatocarcinogenicity of 2-NP in male rats than in
females. Their observation that 2-NP did not induce any increase in
repair synthesis in any of nine non-hepatic cell lines derived from
human, mouse, hamster, and rat tissues led the authors to suggest
that 2-NP is not a direct-acting genotoxic agent but rather requires
metabolic activation by liver-specific metabolism.
Several papers have confirmed and expanded these initial
observations on the genotoxicity of 2-NP to rat liver cells. George
et al. (1989) showed that oral dosage similarly induced unscheduled
DNA synthesis and also resulted in the formation of micronuclei in
rat liver. 2-NP did not, however, significantly increase the
frequency of micronuclei in mouse bone marrow. Intraperitoneal
injection of 0.1 g/kg produced in 6 h a significant increase in
8-hydroxydeoxyguanosine and 8-hydroxyguanosine, products
respectively of DNA and RNA damage caused by hydroxyl or other
oxygen-radical-forming agents (Fiala et al., 1989; Hussain et al.,
1990). This treatment produced significantly lower levels of
8-hydroxydeoxyguanosine, 8-hydroxyguanosine, and other presumed
modified nucleosides in female rats than in males, and had little
effect on the nucleic acids of the kidney, findings that are in
agreement with the known carcinogenicity of 2-NP (Guo et al., 1990).
The organ specificity of the genotoxicity of 2-NP in the rat was
confirmed by Robbiano et al. (1991) who reported that oral doses of
45-713 mg/kg produced maximum numbers of single strand breaks in the
liver 6 h after administration and did not induce DNA fragmentation
in lung, kidney, bone marrow or brain. Damage to rat liver nucleic
acids was also caused by intraperitoneal injection of other
secondary nitroalkanes and a ketoxime capable of being converted to
a secondary nitroalkane, but not with a primary or a tertiary
nitroalkane (Conaway et al., 1991). These authors suggest that the
greater genotoxicity of the secondary nitroalkanes may stem from the
greater stability of their nitronate forms at physiological pH
values.
Observations by Roscher et al. (1990) and Robbiano et al. (1991)
suggest that cytochrome P-450-dependent monooxygenases are important
in the activation of 2-NP in liver. Robbiano et al. (1991) found an
increase in damage to liver DNA in rats pretreated with
phenobarbital or ß-naphthoflavone, inducers of cytochrome
P-450-dependent monooxygenases, and a reduction in liver DNA damage
in rats pretreated with methoxsalen, an inhibitor of cytochrome
P-450. Roscher et al. (1990) examined the effect of 2-NP on rat
hepatoma cell lines that express various forms of cytochrome
P-450-dependent monooxygenases and V79 Chinese hamster cells that
lack these enzyme activities. 2-NP increased DNA repair synthesis,
micronuclei formation, and the frequency of mutants resistant to
6-thioguanine in hepatoma cells pretreated with dexamethasone, an
inducer of various liver-specific cytochrome P-450 forms.
Genotoxicity was reduced or absent in hepatoma cells not treated
with the inducer. In the V79 cells, 2-NP produced only mutations to
6-thioguanine resistance.
2-NP demonstrated only a very limited level of genotoxicity in
other cell lines. Results were negative in a DNA repair synthesis
assay with human diploid fibroblasts exposed for 3 h to 2-NP
concentrations up to 5 g/litre (56 mmol/litre) (McGregor, 1981), as
well as with various cell lines of extrahepatic origin (Andrae et
al., 1988). 2-NP did not cause chromosomal aberrations or SCEs in
Chinese hamster ovary (CHO) cells either with or without S9
(Galloway et al., 1987). In these experiments the maximum
concentrations used, i.e. 1.6 and 5.0 g/litre (18 and 56 mmol/litre)
were estimated to reduce cell growth by 50%. Weakly positive
results, however, were obtained in cytogenetic tests using human
lymphocytes (Bauchinger et al., 1987; Göggelmann et al., 1988).
Exposure of cells to high concentrations of 2-NP (commercial grade,
at least 94% 2-NP) for 1 h induced a significant increase in
chromosomal aberrations (open breaks accompanied by gaps) at a
concentration of 5.3 g/litre (60 mmol/litre) with S9 activation and
at 7.1 g/litre (80 mmol/litre) without S9 activation (Bauchinger et
al., 1987). In addition, the frequency of sister chromatid exchange
was significantly increased at all concentrations of 2-NP (0.7 to
7.1 g/litre; 7.5 to 80 mmol/litre), but only with S9 activation.
Repetition of the experiment with 2-NP of greater than 99% purity
yielded similar results (Göggelmann et al., 1988). There was no
significant increase in chromosomal changes without S9 activation,
but there was a small but significant increase at the highest
treatment levels, i.e. 7.1 and 10 g/litre (80 and 111 mmol/litre),
with S9 activation. 1-NP of 97% purity produced no significant
mutagenic or cytogenetic effects on this test system with or without
S9 activation. The authors concluded that 2-NP can exert a
clastogenic effect on human lymphocytes only with metabolic
activation, and hypothesized that it acted via a different metabolic
pathway in the lymphocytes than it did in the bacterial
S. typhimurium system, where it induced mutations without
exogenous activation.
7.5 Carcinogenicity
That 2-NP is unquestionably a potent carcinogen in rats was
demonstrated by Lewis et al. (1979). Inhalation exposure of male
Sprague-Dawley rats to 0.75 g/m3 (207 ppm) for 7 h/day, 5
days/week, over a 24-week period induced hepatocellular neoplasms in
all surviving animals. Inhalation exposure of rats to 0.36 g/m3
(100 ppm) similarly administered over 18 months was associated with
hepatocellular carcinomas in male rats and produced changes in the
livers of female rats (nodules showing hyperplasia and vacuolar
degeneration) which may have been precursors to carcinoma (Griffin &
Coulston, 1983; Coulston et al., 1985). Oral dosing of male rats
with 89 mg/kg (1 mmol/kg), three times a week for 16 weeks, induced
massive hepatocellular carcinomas in all rats, observed when they
were sacrificed 40 weeks later (Fiala et al., 1987a). Metastases to
the lungs were present in 4 out of 22 surviving animals, suggesting
a high degree of malignancy. Long-term inhalation exposure of rats
to low concentrations (100 mg/m3; 27 ppm) did not produce any
evidence of increased hepatocellular carcinoma or precursor tumour
lesions of any sort (Lewis et al., 1979; Griffin et al., 1980,
1981). Evidence that inhalation exposure to low doses of 2-NP can
cause DNA damage in rats was presented by Denk et al. (1990). Male
and female Sprague-Dawley rats, 4-6 days old at the beginning of
treatment, were exposed to 0, 91, 146, 182, 291, and 455 mg/m3 (0,
25, 40, 50, 80 and 125 ppm) for 6 h/day, 5 days/week for 3 weeks,
and this was followed by promotion with polychlorinated biphenyls
(Clophen A50) for 8 weeks. This treatment produced a dose-dependent
increase in the numbers of preneoplastic liver foci deficient in
adenosine-5'-triphosphatase. Cunningham & Matthews (1991) showed
that 2-NP can also induce cell proliferation in rat liver. Rats were
exposed to daily oral doses of 20, 40 or 80 mg/kg by gavage for 10
days, and cell proliferation during exposure was quantified by
measuring the incorporation of bromodeoxyuridine into newly
synthesized DNA. Exposure to 40 and 80 mg/kg resulted in
statistically significant increases in the frequency of S-phase
cells from 1.9% in the vehicle-treated animals to 6.3% and 11%,
respectively. Exposure to 20 mg/kg had no effect on the labelling
index. The non-carcinogenic isomer of 2-NP, 1-NP, did not affect DNA
synthesis at doses of 20, 40 and 80 mg/kg.
There is no substantial evidence that 2-NP induces cancer in
species other than the rat (Table 6). Inhalation exposure of rabbits
to 750 mg/m3 (207 ppm) for 1 h/day, 5 days/week for 24 weeks,
initially produced some evidence of liver damage. At the end of 6
months of exposure, the five rabbits examined displayed no more than
minor changes in the liver and no evidence of carcinoma (Lewis et
al., 1979). Exposure of mice to 728 mg/m3 (200 ppm), similarly
administered for 48 weeks, produced damage to the liver, especially
in females, but no carcinoma (Griffin et al., 1984). It is possible
that degenerative changes observed in the livers of exposed females
may have been precursors to subsequent tumour development. A lower
concentration of 2-NP, 360 mg/m3 (100 ppm), similarly
administered to ICR mice for 18 months, induced liver damage in the
form of nodular hyperplasia in females but no increase in
hepatocellular carcinoma (Coulston et al., 1986; Griffin et al.,
1987). The latter study is the only inhalation study in a species
other than the rat that was of sufficient duration to allow for
latency in tumour development. No studies of carcinogenicity using
oral dosing in any species except the rat have been reported. Thus,
despite the absence of clear evidence, the possibility of
carcinogenicity in species other than the rat cannot be discounted.
There appear to be no laboratory studies on species other than
rats, mice and rabbits suitable for assessing the potential
carcinogenicity of 2-NP. As discussed in section 8.2.2, there is no
evidence from limited epidemiological studies that 2-NP is
carcinogenic in humans.
Griffin & Coulston (1983) argued that 2-NP does not act as an
initiating carcinogen in the rat, but rather induces cancer as a
response to extensive liver damage (Angus Chemical Co., 1985). They
offered as evidence the results of an experiment demonstrating the
absence of long-term effects from short-term exposure to 2-NP
(Griffin et al., 1986). Exposure of rats of both sexes to 730 g/m3
(200 ppm), 7 h/day for 5 days, did not produce any effect on
longevity or on the development of hepatocellular or other
carcinomas up to the end of the experiment, which lasted for 94
weeks. This argument is markedly weakened by the observation of
Andrae et al. (1988) and others (Fiala et al., 1989; George et al.,
1989; Guo et al., 1990; Conaway et al., 1991) that 2-NP is an active
genotoxic agent in rat hepatocytes both in vitro and in vivo.
7.6 Pharmacological effects
In vivo pharmacological studies have not been performed.
However in vitro studies with guinea-pig tissues suggest that 2-NP
may have several pharmacological effects. A 0.1 µM (8.9 µg/litre)
solution inhibited oxygen consumption of polymorphonuclear
leucocytes by 50% (Estes & Gast, 1960). These authors further
reported that, with increasing concentrations of 2-NP, respiration
of heart homogenate was initially depressed and subsequently
stimulated.
2-NP also produces two opposite neural effects on smooth muscle
preparations (Bergman et al., 1962). Contraction is induced partly
by ganglionic stimulation and partly by direct liberation of a
transmitter from nerve endings, but at the same time 2-NP inhibits
the response to acetylcholine and other smooth muscle stimulants.
Bergman et al. (1962) found that the action of nitroparaffins on
smooth muscle was similar to that of nicotine in that their ability
to induce contraction was inhibited by morphine and by high, but not
low, concentrations of atropine.
8. EFFECTS ON HUMANS
8.1 General population exposure
As indicated in section 5.1, general population exposure to 2-NP
appears to be very low, and there is no information on the effects
of this exposure. 2-NP has been used as a component of paints and
other consumer products. There is no evidence that exposure to 2-NP
from the use of such products has resulted in detectable injury or
illness in the general population.
8.2 Occupational exposure
8.2.1 Acute toxicity
Significant human exposure to 2-NP is largely or entirely
occupationally related. High concentrations are acutely toxic and
seven industrial fatalities have been attributed to inhalation of
2-NP fumes (Gautier et al., 1964; Hine et al., 1978; Rondia, 1979;
Harrison et al., 1985, 1987; US NIOSH, 1987b). In all cases,
exposure was to a solvent mixture containing 2-NP, was for a total
of 5 to 16 h in the course of 1 to 3 days, and occurred while paints
or coatings were being applied in confined spaces, such as tank
interiors, underground vaults and ship's holds, with little or no
ventilation. The actual concentrations of 2-NP that caused deaths
were not measured, but were thought to be high and in one case
estimated at 2.18 g/m3 (600 ppm), as determined by GC-FID from the
2-NP content of the victim's blood (0.013 g/litre) (Harrison et al.,
1987). Initial symptoms requiring medical treatment appeared during
exposure or within a few hours following exposure and included
headache, nausea, dizziness, drowsiness, weakness, anorexia,
vomiting, diarrhoea, and neck, thoracic, and abdominal pain. Victims
were hospitalized and in general initially showed improvement, in
some cases to the point of being discharged after a day or less. But
improvement, where present, was temporary and in all seven cases was
followed within a few days by worsening condition and
rehospitalization of those previously discharged. Later symptoms
included persistent nausea, vomiting, anorexia, jaundice, reduced
urine output, diarrhoea, bloody stools, mental confusion,
restlessness, loss of reflexes, and increases in serum
aminotransferases and other indicators of hepatic lesions. Death
occurred within 4 to 26 days (average, 10 days) following exposure.
In all cases the primary cause was acute hepatic failure.
Contributing factors included lung oedema, gastrointestinal
bleeding, and respiratory and kidney failure. Postmortem microscopic
examination of the liver revealed necrosis of hepatic tissue and in
some cases fatty degeneration. None of the victims had a past
history of liver disease or drank excessively.
In addition to these fatalities, there have been four additional
serious but nonlethal cases attributed to acute exposure to 2-NP
(Gautier et al., 1964; Hine et al., 1978; Harrison et al., 1985,
1987; US NIOSH, 1987b). All but one were colleagues of the deceased
described above, but may have received lesser doses. Initial
symptoms were similar to those of the deceased, but were followed by
full recovery rather than decline and death. Serum enzyme levels,
however, remained mildly elevated for months following exposure. As
in the case of the deceased, the actual concentrations of 2-NP to
which these workers were exposed are unknown. One of the survivors
had a 2-NP serum concentration of 8.5 mg/litre when hospitalized.
Since his coworker hospitalized at the same time with a serum
concentration of 13 mg/litre subsequently died, Harrison et al.
(1987) speculated that either 2-NP has a very steep dose-response
curve or that there are substantial differences in individual
susceptibility. Six men (out of a group of approximately 300)
developed toxic hepatitis after exposure to an epoxy resin coating
containing 2-NP (Williams et al., 1974). The development of toxic
hepatitis was ascribed to methylenedianiline in the coating, and the
possibility that 2-NP may have been at least a partial cause was not
considered.
Exposure to lower concentrations of 2-NP appears to produce some
of the symptoms described above. Brief and intermittent exposure to
concentrations above 364 mg/m3 (100 ppm) may produce headache and
nausea (Angus Chemical Co. & Occusafe, Inc., 1986). Exposure to
vapours (estimated to contain 73 to 164 mg/m3 (20 to 45 ppm) using
colorimetric methods available at the time of the study) of a
solvent mixture that polluted the workplace air produced a daily
cycle in symptoms (Skinner, 1947). Workers started the day feeling
well, but by noon some exhibited nausea and lack of appetite. Later
in the working day these symptoms intensified and were accompanied
by vomiting and diarrhoea. Vomiting continued after the workers left
the workplace, and they were unable to eat supper but were able to
sleep. By morning they were feeling well again. Colleagues not
experiencing nausea and vomiting had occipital headaches, which
appeared and gradually intensified during the working day. All of
the workers were free of symptoms when away from the workplace for a
day or more, and the substitution of methyl ethyl ketone for 2-NP
brought complete relief. Skinner (1947) noted that in another plant
exposure of workers to 36-109 mg/m3 (10-30 ppm) for less than
4 h/day on not more than 3 days per week produced no noticeable ill
effects.
The flaw in all but one (Hine et al., 1978) of these case
studies is that exposure was to a solvent mixture containing 2-NP
and not to 2-NP alone (Hine et al., 1978). Thus the observed effects
cannot be assigned to 2-NP with total confidence. A number of
factors, however, point to 2-NP as the main, if not sole, causative
agent. It was the only solvent common to all the mixtures, and the
only solvent present in the mixtures known to be hepatotoxic.
Symptoms and damaging effects on the body from the solvent mixtures
were fairly similar to each other despite widely different solvent
compositions (apart from the common presence of 2-NP), and were
fairly similar to those in the single case resulting from exposure
to 2-NP alone. In addition, as described above, substitution of
another solvent for 2-NP eliminated all symptoms. The weight of
evidence thus supports the view that the symptoms and damage to the
human body described above which followed exposure to solvent
mixtures containing 2-NP, resulted mainly, if not entirely, from
2-NP.
8.2.2 Effects of long-term exposure
Despite the fact that 2-NP has been in use for over 40 years,
there are very few data on long-term effects and such information is
derived mainly from unpublished reports from manufacturers of 2-NP.
Angus Chemical Co., a major manufacturer of 2-NP, has assembled
information on more than 1800 occupationally exposed workers from a
variety of plants in the USA, Mexico, Germany, Sweden, and the
Netherlands (Table 8). Where information was available, medical
records on living employees indicated no problems with liver
functions and no obvious symptoms or conditions that could be
connected with chronic exposure to 2-NP (Angus Chemical Company and
Occusafe, Incorporated, 1986).
A major portion of the above investigation was an earlier
mortality study conducted by Miller & Temple (1979) on 1481 workers
employed in the manufacture of 2-NP during the period 1955 to 1977.
The exposures were defined as direct, indirect or zero exposure.
Formal industrial hygiene monitoring was not performed until 1977.
Individual exposures were based on job titles rather than actual
exposure data. Angus Chemical Company and Occusafe, Incorporated
(1986) concluded that analysis of these data did not suggest any
unusual cancer or other disease pattern in this group of workers.
They noted, however, that because the cohort was small and the
duration of exposure and observation was relatively short, it was
not possible to conclude from these data that 2-NP is not
carcinogenic to humans. In a follow-up report on the same cohort
(Bolender, 1983), the findings did not change the earlier
conclusion.
It is necessary to emphasize that this cohort had a limited
number of workers with long exposure (> 15 years). Since the
individual exposure data are not available, it cannot be concluded
from available data that 2-NP is non-carcinogenic in humans.
Table 8. Studies of long-term occupational exposure to 2-nitropropane
Activitya Exposure concentrationb Exposure duration No. of exposed Reference
mg/m3 (ppm) (years) employees
TWA STEL
Manufacture of 2-NP, 25-91 > 218 < 1-15 55 Angus Chem. Co. & Occusafe, Inc. (1986)
1940-1955 (7-25) (> 60) average 2.5
Manufacture of 2-NP, 3.6-36 91-5970 < 5-> 21 804 Angus Chem. Co. & Occusafe, Inc. (1986);
1946-1982, (1-10) (25-1640) average 8c Miller & Temple (1979); Bolender (1983)
Sterlington, LA, USA
Extraction of 3.6-193 109-473 < 5-ca. 25 18-19 Angus Chem. Co. & Occusafe, Inc. (1986);
triglycerides, USA (1-53; (30-130) Crawford et al. (1985); Tabershaw
average 9c) Occupational Medicine Assoc. (1980);
Life Extension Inst. (1983)
Automobile assembly unknown < 5-> 25 456 Angus Chem. Co. & Occusafe, Inc. (1986)
plant, Germany
Paint mfg., Germany unknown 5-10 6 Angus Chem. Co. & Occusafe, Inc. (1986)
Paint mfg., Germany 40-91 11-15 5 Angus Chem. Co. & Occusafe, Inc. (1986)
(11-25)
Chemical mfg., unknown 5-10 80 Angus Chem. Co. & Occusafe, Inc. (1986)
Germany
Chemical mfg., unknown < 5 4 Angus Chem. Co. & Occusafe, Inc. (1986)
Germany
Extraction plant, 3.6-18.2 73-364 5-10 15 Angus Chem. Co. & Occusafe, Inc. (1986)
Sweden (1-5) (20-100)
Table 8 (contd)
Activitya Exposure concentrationb Exposure duration No. of exposed Reference
mg/m3 (ppm) (years) employees
TWA STEL
Paint mfg., unknown 5-10 180 Angus Chem. Co. & Occusafe, Inc. (1986)
Netherlands
Chemical Co., USA 3.6-36 65.6-364 < 5-20 41 Angus Chem. Co. & Occusafe, Inc. (1986)
(1-10) (18-100)
Printing Co., USA 1.8-87 2.5-124 ? 140 Angus Chem. Co. & Occusafe, Inc. (1986)
(0.5-24) (0.7-34)
Paint mfg., Mexico 3.6 < 91 5-10 100 Angus Chem. Co. & Occusafe, Inc. (1986)
(1) (< 25)
Ink mfg., Mexico 11-18 73-80 5-10 8 Angus Chem. Co. & Occusafe, Inc. (1986)
(3-5) (20-22)
Coatings mfg., 14.6-91 237-251 < 5-20 30 Angus Chem. Co. & Occusafe, Inc. (1986)
Mexico (4-25) (65-69)
Chemical distilation, 36-55 > 91 < 5-10 2 Angus Chem. Co. & Occusafe, Inc. (1986)
Mexico (10-15) (> 25)
Paint mfg., USA unknown < 20 150 Angus Chem. Co. & Occusafe, Inc. (1986)
Automotive mfg., USA 3.6-36 142 < 18 10 Angus Chem. Co. & Occusafe, Inc. (1986)
(1-10) (39)
a mfg. = manufacturing; co. = company
b TWA = time weighted average; STEL = short-term exposure level; values in parentheses are in ppm
c This average is an approximation
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
The limited data on the effects of 2-NP on organisms other than
mammals is summarized below. Kido et al. (1975) reported that 2-NP
(5 g/litre) inhibited the growth of 10 of the 14 species of
microorganisms tested, although all 14 species contained enzymes
capable of oxidizing 2-NP to acetone and nitrite (see section 4.2).
Organisms inhibited by 2-NP included bacteria (Escherichia coli,
Pseudomonas iodinum), yeasts (Endomyces fibuliger, Hansenula
anomala, H. octospora, H. sauveolens, H. matritensis), and fungi
(Aspergillus niger, Penicillium oxalicum, Fusarium oxysporum).
Organisms capable of growing on the medium containing 2-NP similarly
included bacteria (Sarcina lutea, Brevibacterium protophormiae), a
yeast (Hansenula mrakii), and a fungus (Rhizopus batatas).
Fridman et al. (1976) reported that the minimum concentration of
2-NP inhibiting the growth of E. coli and Staphylococcus aureus
was 1000 mg/litre. Observations on aquatic macroorganisms appear
more limited than those on microorganisms. The lowest concentration
of 2-NP in sea water inducing narcosis in barnacle larvae at 18-22
°C was approximately 0.7-0.8 g/litre (Crisp et al., 1967). At 22 °C
the 96-h LC50 for fathead minnows (Pimephales promelas) was >
210 mg/litre (Curtis et al., 1980; Curtis & Ward, 1981). Aeration of
the holding tanks during the toxicity test produced flawed results
because 2-NP was lost continuously by volatilization and a nominal
highest concentration of 612.5 mg/litre was reduced to 496 mg/litre
at the start of the test and to 210 mg/litre by 96 h. There was no
significant mortality at concentrations of 2-NP below 210 mg/litre
although at lower concentrations it did produce severe (though
undescribed) sublethal effects. Observations on non-mammalian
terrestrial organisms are limited to two related species, the
oriental fruit fly (Dacus dorsalis) and the Mediterranean fruit
fly (Ceratitis capitata). Burditt et al. (1963) found that 2-NP
was not especially effective as a fumigant against eggs and larvae
of these species. The 2-h LC50 values for eggs and larvae of the
oriental fruit fly were > 103 and 75 g/m3 (> 28 300 and
20 600 ppm), respectively, and for the Mediterranean fruit fly, > 103
and 35 g/m3 (> 28 300 and 9600 ppm), respectively. These
observations, in summary, suggest a fairly low acute toxicity to
non-mammalian organisms.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Human health risks
Although there are no known biological sources of 2-NP, very low
level non-occupational exposure must be almost universal because
2-NP is known to be a minor component of tobacco smoke and probably
is present in smoke from other types of nitrate-rich organic matter.
Residues in food products containing fatty acids separated with 2-NP
and in beverage can linings and other coatings may represent
additional sources of exposure to low (µg) amounts of 2-NP. The
effects, if any, on human health of low-level exposure are unknown.
Sources of worker exposure include rotogravure and flexographic
inks used in printing, and coatings and adhesives used in industrial
construction and maintenance, highway markings, ship building and
maintenance, furniture manufacture, and food packaging. Based on a
survey conducted in the USA in 1980, occupational exposure to 2-NP
appeared to be limited to a fraction of 1% of the workforce, and
significant occupational exposure (exposure to > 9.1 mg/m3 (>
2.5 ppm)) to about 0.005% of the workforce. Thus, tens of thousands
of workers may have received significant occupational exposure
worldwide. The effects of this occupational exposure are unclear.
Very few industrial fatalities have been attributed to 2-NP. All
involved acute exposure to high concentrations of vapours from
solvent mixtures containing 2-NP during applications of coatings in
confined spaces; all deaths resulted primarily from hepatic failure.
Exposure to non-lethal concentrations of 2-NP may result in
temporary illness or discomfort, but there is no case history or
epidemiological evidence that the chemical induces cancer or other
long-term harmful effects in humans. On the other hand, the numbers
of individuals studied and the periods since first exposure to 2-NP
were inadequate to detect possible long-term harmful effects.
Animal data has demonstrated that 2-NP is a strong
hepatocarcinogen in rats. Chronic exposure to high but non-lethal
concentrations (at least 0.36 g/m3, 100 ppm) induced a high
frequency of hepatic tumours and hepatic cancer in rats but these
observations were not reproduced in limited studies on mice and
rabbits. The mechanism by which 2-NP induces cancer in rats has not
been elucidated as yet. 2-NP is strongly genotoxic to rat
hepatocytes both in vitro and in vivo. It appears likely that
the hepatocarcinogenicity of the compound in rats is a consequence
of liver-specific formation of reactive products. The DNA-damaging
species has not yet been identified. Since it can induce liver cell
proliferation, 2-NP may also have tumour-promoting activity.
Similarities or differences between metabolic activation of 2-NP in
rats and in humans are largely unknown. At present, any substance
shown to be carcinogenic in any mammalian species is considered a
potential human carcinogen. Accurate risk assessment from exposure
to 2-NP requires further studies.
10.2 Effects on the environment
2-NP does not represent a threat to the environment. It appears
highly mobile in the natural environment and is not accumulated in
any individual compartment. It is likely that 2-NP will be destroyed
by photolysis when exposed in the atmosphere to sunlight, and by
biological processes in soil and water. There are, however,
insufficient experimental and observational data on the behaviour of
2-NP in the environment to validate these assumptions or to estimate
rates of degradation in nature. Limited observations on
microorganisms, invertebrates, and fish show a low level of acute
toxicity to non-mammalian organisms.
11. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
There are no indications that 2-NP is carcinogenic in humans.
However, in view of its carcinogenicity in rats, it is recommended
that occupational exposure and its presence in consumer products
such as paints and varnishes be minimized and that it be replaced
with a less toxic solvent whenever practical. Monitoring of the
workplace should be continued to control actual worker exposure.
Although it is not feasible to eliminate non-occupational exposure,
such exposure should be minimized. 2-NP should not be used in food
processing.
12. FURTHER RESEARCH
12.1 Environment
Although it appears likely that 2-NP is highly mobile in the
environment, is not accumulated in any environmental compartment,
and is degraded in the environment to less toxic substances by
microorganisms and ultraviolet radiation, it would be desirable to
confirm experimentally these assumptions and to quantify the speed
of degradation in various environmental compartments.
12.2 Epidemiology
Cohorts of workers in production plants as well as cohorts of
workers using products containing 2-NP (e.g., inks, paints) should
be investigated for incidence of cancer and other ill effects.
12.3 Toxicokinetics
A more detailed examination of the metabolism and distribution
in the body of 2-NP and its metabolites is needed to facilitate an
understanding of the marked species and sex differences in toxic
effects. Studies on distribution and metabolism should include
metabolites of both the carbon and the nitrogen moieties.
Experimental data should be obtained on dermal uptake. Studies on
the metabolism of 2-NP in human cells are needed to facilitate the
eventual extrapolation of data obtained with laboratory animals to
humans.
12.4 Carcinogenesis
Continued efforts should be made to elucidate the biochemical
and molecular mechanisms whereby 2-NP induces genotoxic effects and
cancer.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
2-NP was evaluated by the Joint FAO/WHO Expert Committee on Food
Additives in 1979 and 1981, but no ADI was allocated. The Committee
recommended that 2-NP should not be used as a solvent in food
processing, but it was temporarily acceptable for use as a
fractionating solvent in the production of fats and oils (FAO/WHO,
1984). 2-NP was re-evaluated by JECFA in 1989; it was considered to
be a potent liver carcinogen in rats, and the temporary acceptance
for use as a fractionating solvent in the production of fats and
oils was not extended (FAO/WHO, 1990a, 1990b). The European Economic
Community found 2-NP to be flammable and to be harmful by
inhalation, ingestion, and dermal contact. It required that member
states ensure that it receives proper packaging, labelling, and
storage (IRPTC, 1986).
The International Agency for Research on Cancer evaluated 2-NP
in 1981 (IARC, 1982) and concluded that there was sufficient
evidence for its carcinogenicity in rats but that epidemiological
information was inadequate to evaluate its carcinogenicity to
humans. 2-NP is classified in Group 2B, i.e. as possibly
carcinogenic to humans (IARC, 1987).
REFERENCES
ACGIH (1986) Documentation of the threshold limit values and
biological exposure indices, 5th ed. Cincinnati, Ohio, American
Conference of Governmental Industrial Hygienists, Inc. pp 441-442.
Andersson K, Levin J-O, & Nilsson C-A (1983) Evaluation of solid
sorbents for sampling aliphatic and aromatic nitro compounds.
Chemosphere, 12: 377-384.
Andersson K, Levin J-O, Lindahl R, & Nilsson C-A (1984) Influence of
air humidity on sampling efficiency of some solid adsorbents used
for sampling organics from work-room air. Chemosphere, 13: 437-444.
Andrae U, Homfeld H, Vogl L, Lichtmannegger J, & Summer KH (1988)
2-Nitropropane induces DNA repair synthesis in rat hepatocytes
in vitro and in vivo. Carcinogenesis, 9: 811-815.
Angus Chemical Company (1985) Technical data sheet: NiPar S-20TM
nitropropane solvent, TDS 20B. Northbrook, Illinois, Angus Chemical
Company, 12 pp.
Angus Chemical Company & Occusafe, Incorporated (1986) Industrial
hygiene and occupational health information on 2-nitropropane,
December 1982, and updates for December 1983, 1984, 1985.
Northbrook, Illinois, Angus Chemical Company, 39 pp and 46
attachments (Unpublished report presented to the ACGIH TLV
Committee).
Anon (1976) Grande Paroisse looks for growth in nitroparaffins
market. Eur Chem News, 6 August: 23-24, 30.
Anon (1982) International mineral and chemical's nitroparaffins
business will be acquired by Alberto Natural Gas (Canada) and
Pacific Gas Transmission (US) for up to $ 55 million. Chem Week, 7
April: 24,251.
Baker PJ & Bollmeier AF (1981) Nitroparaffins. In: Grayson M &
Eckroth D ed. Kirk-Othmer encyclopedia of chemical technology, 3rd
ed. New York, John Wiley and Sons, vol 15, pp 969-987.
Baker HJ, Lindsey JR, & Weisbroth SH (1979) Appendix 1. Selected
normative data. In: Baker HJ, Lindsey JR, & Weisbroth SH ed. The
laboratory rat. Volume I: Biology and diseases. Orlando, Florida,
Academic Press, append 1.
Baldwin RS & Williams R (1977) 2-Nitropropane LC50. Northbrook,
Illinois, International Minerals and Chemical Corporation, 6 pp
(Unpublished report No. PLR-218/AMR-072).
Bauchinger M, Kulka U, & Schmid E (1987) Analysis of cytogenetic
effect in human lymphocytes induced by metabolically activated
2-nitropropane. Mutat Res, 190: 217-219.
Beall LR, Alexander V, Bien CT, Chen C, & Infante P (1980) Health
hazard alert - 2-Nitropropane. Cincinnati, Ohio, National Institute
for Occupational Safety and Health, 8 pp (Publication No. 80-142).
Bergman F, Chaimovitz M, & Wind E (1962) Dual action of
nitroparaffins on the guinea-pig ileum. Brit J Pharmacol,
18: 381-396.
Bernard AM, De Russis R, Normand J-C, & Lauwerys RR (1989)
Evaluation of the subacute nephrotoxicity of cyclohexane and other
industrial solvents in the female Sprague-Dawley rat. Toxicol Lett,
45: 271-280.
Bolender FL (1983) 2-NP mortality epidemiology study of the
Sterlington, LA employees: An update, 1-1-46 thru 12-31-81.
Northbrook, Illinois, International Minerals and Chemical
Corporation, 24 pp (Unpublished report).
Breazile JE (1971) Text book of veterinary physiology. Philadelphia,
Pennsylvania, Lea and Febiger, p 347.
Brown D & Dobbin R (1977) Industrial hygiene survey report. IMC,
Sterlington, Louisiana. Cincinnati, Ohio, National Institute for
Occupational Safety and Health, 14 pp.
Burditt AK, Hinman FG, & Balock FG (1963) Screening of fumigants for
toxicity to eggs and larvae of the oriental fruit fly and
Mediterranean fruit fly. J Econ Entomol, 56: 261-265.
Burrows GE (1979) Methylene blue or tolonium chloride antagonism of
sodium nitrite induced methemoglobinemia. J Vet Pharmacol Ther,
2: 81-86.
Cocalis JC (1982) Mining target environmental surveillance study.
Nitropropane sensitized, ammonium nitrate blasting agents.
Morgantown, West Virginia, National Institute for Occupational
Safety and Health, Environmental Investigations Branch, Division of
Respiratory Diseases Studies, 25 pp.
Conkle JP, Camp BJ, & Welch BE (1975) Trace composition of human
respiratory gas. Arch Environ Health, 30: 290-295.
Conaway CC, Nie G, Hussain NS, & Fiala ES (1991a) Comparison of
oxidative damage to rat liver DNA and RNA by primary nitroalkanes,
secondary nitroalkanes, cyclopentanone oxime, and related compounds.
Cancer Res, 51: 3143-3147.
Conaway CC, Nie G, Hussain NS, & Fiala ES (1991b) Evaluation of
secondary nitro-alkanes, their nitronates, primary nitroalkanes,
nitrocarbinols, and other aliphatic nitro compounds in the Ames
Salmonella assay. Mutat Res, 261: 197-207.
Coulston F (1982) Influence of glutathione or diethyl maleate in the
induction of hepatocellular carcinomas in rats exposed to 200 ppm of
2-nitropropane. Alamogordo, New Mexico, Coulston International
Corporation, 14 pp (Unpublished report).
Coulston F, Stein AA, & Griffin TB (1985) Pathology final report.
Histologic study of selected tissues from rats exposed to
2-nitropropane at 100 ppm. Alamogordo, New Mexico, Coulston
International Corporation, 222 pp.
Coulston F, Griffin T, & Eason R (1986) Chronic inhalation exposure
of mice to 2-nitropropane (100 ppm). Alamogordo, New Mexico,
Coulston International Corporation, 5 pp (Unpublished report).
Crawford GN, Garrison RP, & McFee DR (1984) Odor threshold
determination for 2-nitropropane. Am Ind Hyg Assoc J, 45: B7-B8.
Crawford GN, Garrison RP, & McFee, DR (1985) Health examination and
air monitoring evaluation for workers exposed to 2-nitropropane. Am
Ind Hyg Assoc J, 46: 45-47. Crisp DJ, Christie AO, & Ghobashy AFA
(1967) Narcotic and toxic action of organic compounds on barnacle
larvae. Comp Biochem Physiol, 22: 629-649.
Cunningham ML & Matthews HB (1991) Relationship of
hepatocarcinogenicity and hepatocellular proliferation induced by
mutagenic noncarcinogens vs carcinogens. Toxicol Appl Pharmacol,
110: 505-513.
Cupitt LT (1980) Fate of toxic and hazardous materials in the air
environment. Research Triangle Park, North Carolina, US
Environmental Protection Agency, 28 pp (EPA-600/3-80-084).
Curtis MW & Ward CH (1981) Aquatic toxicity of forty industrial
chemicals: Testing in support of hazardous substance spill
prevention regulation. J Hydrol, 51: 359-367.
Curtis MW, Curran CM, & Ward CH (1980) Aquatic toxicity testing as
fundament for a spill prevention program. In: Proceedings of the
1980 Conference on Control of Hazardous Material Spills. Nashville,
Tennessee, Vanderbilt University, pp 284-288.
Dayal R, Gescher A, Harpur ES, Pratt I, & Chipman JK (1989)
Comparison of the hepatotoxicity in mice and the mutagenicity of
three nitroalkanes. Fundam Appl Toxicol, 13: 341-348.
Dayal R, Goodwin B, Linhart I, Mynett K, & Grescher A (1991)
Oxidative denitrification of 2-nitropropane and propane-2-nitronate
by mouse liver microsomes: lack of correlation with hepatic
potential. Chem Biol Interact, 79(1): 103-114.
De Bruin A (1976) Biochemical toxicology of environmental agents.
Amsterdam, Oxford, New York, Elsevier Science Publishers, p 104.
Denk B, Bauman M, & Filser JG (1989) Pharmacokinetics and
hepatotoxicity of 2-nitropropane in rats. Arch Toxicol, 13(suppl):
330-332.
Denk B, Filser JG, Demi E, Kessler W, Shen J, & Oesterle D (1990)
Dose-dependent emergence of preneoplastic foci in rat livers after
exposure to 2-nitropropane. Arch Toxicol, 64: 329-331.
Dequidt J, Vasseur P, & Potencier J (1972) Etude toxicologique
expérimentale de quelques nitroparaffines : 1. Etude du
2-nitropropane. Bull Soc Pharm Lille, 1972: 83-89.
Derks HJGM, Van Heiningen A, Klaassen R, & Olling M (1988) High
performance liquid chromatographic method for the determination of
2-nitropropane in rat plasma. J Chromatogr, 442: 436-440.
Derks HJGM, Van Heiningen A, Klaassen R, & Olling M (1989)
Toxicokinetics of 2-nitropropane in the rat. Bilthoven, The
Netherlands, National Institute of Public Health and Environmental
Protection, 19 pp (Report No. 328605.003).
De Rycker J & Halliwell B (1978) Oxidation of 2-nitropropane by
horseradish peroxidase. Biochem J, 175: 601-606.
Dhawale MR & Hornemann U (1979) Nitroalkane oxidation by
Streptomycetes. J Bacteriol, 137: 916-924.
Estes FL & Gast JH (1960) The in vitro effects of aliphatic nitro
compounds on tissues. Arch Environ Health, 1: 59-64.
FAO/WHO (1984) Evaluation of certain food additives and
contaminants. Twenty-eighth report of the Joint FAO/WHO Expert
Committee on Food Additives. Geneva, World Health Organization,
44 pp (WHO Technical Report Series 710).
FAO/WHO (1990a) Evaluation of certain food additives and
contaminants. Thirty-fifth report of the Joint FAO/WHO Expert
Committee on Food Additives. Geneva, World Health Organization,
48 pp (WHO Technical Report Series 789).
FAO/WHO (1990b) Toxicological evaluation of certain food additives
and contaminants, prepared by the 35th meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA). Geneva, World Health
Organization, 189 pp (WHO Food Additives Series 26).
Fiala ES, Czerniak R, & Williams GM (1986) Formation of carcinogenic
and/or genotoxic aliphatic azoxy compounds from the respective
nitroalkanes by direct mild chemical reduction. Toxicologist,
6: 40.
Fiala ES, Conaway CC, Biles WT, & Johnson B (1987a) Enhanced
mutagenicity of 2-nitropropane nitronate with respect to
2-nitropropane possible involvement of free radical species. Mutat
Res, 179: 15-22.
Fiala ES, Czerniak R, Castonguay A, Conaway CC, & Rivenson A (1987b)
Assay of 1-nitropropane, 2-nitropane, 1-azoxypropane and
2-azoxypropane for cancinogenicity by gavage in Sprague-Dawley rats.
Carcinogenesis, 8: 1947-1949.
Fiala ES, Conaway CC, & Mathis JE (1989) Oxidative DNA and RNA
damage in the livers of Sprague-Dawley rats treated with the
hepatocarcinogen 2-nitropropane. Cancer Res, 49: 5518-5522.
Fieser LF & Fieser M (1972) Reagents for organic synthesis. New
York, Wiley-Interscience, vol 3, pp 213-214.
Fieser LF & Fieser M (1974) Reagents for organic synthesis. New
York, Wiley-Interscience, vol 4, p 256.
Filser JG & Baumann M (1988) Pharmokinetics of 2-nitropropane in
rats and determination of enzymes in serum specific for liver
injury. Naunyn Schmiedeberg's Arch Pharmacol, 337(suppl): R17.
Fine DH, Challis BC, Hartman P, & Van Ryzin J (1982) Endogenous
synthesis of volatile nitrosamines: model calculations and risk
assessment. In: N-nitroso compounds: Occurrence and biological
effects. Proceedings of the VIIth International Symposium on
N-Nitroso Compounds, Tokyo, 28 September-1 October 1981. Lyon,
International Agency for Research on Cancer, pp 379-396 (IARC
Scientific Publications No. 41).
Finklea JF (1977) Current NIOSH intelligence bulletin:
2-nitropropane. Am Ind Hyg Assoc J, 38(7): A-15, A-17, A-19.
Fishbein L (1981) Carcinogenicity and mutagenicity of solvents. I.
Glycidyl ethers, dioxane, nitroalkanes, dimethylformamide and allyl
derivatives. Sci Total Environ, 17: 97-110.
Flanner LT (1972) 2-Nitropropane or nitromethane for inhibiting the
corrosion of tin-plated steel aerosol cans: US Patent US 3650982
(21 March 1972). USA, Allied Chemical Corporation, 2 pp (Abstract
only).
Freitag D, Geyer H, Kraus A, Viswanathan R, Kotzias D, Attar A,
Klein W, & Korte F (1982) Ecotoxicological profile analysis. VII.
Screening chemicals for their environmental behavior by comparative
evaluation. Ecotoxicol Environ Saf, 6: 60-81.
Freitag D, Ballhorn L, Geyer H, & Korte F (1985) Environmental
hazard profile of organic chemicals. Chemosphere, 14: 1589-1616.
Friedman MA, Greene EJ, & Epstein SS (1972) Rapid gastric absorption
of sodium nitrite in mice. J Pharm Sci, 61: 1492-1494.
Friedman AL, Zalesov VS, Surkov VD, Kratynskaya LV, & Plaksina AN
(1976) [Synthesis and study of the physiological activity of
aliphatic nitro compounds. X. Relation among structure, toxicity,
and antimicrobial activity in a series of nitroalkanes and their
alpha-halo derivatives.] Khim-Farm Zh, 10: 53-56 (in Russian).
Galloway SM, Armstrong MJ, Reuben C, Colman S, Brown B, Cannon C,
Bloom AD, Nakamura F, Ahmed M, Duk S, Rimpo J, Margolin BH, Resnick
MA, Anderson B, & Zeiger E (1987) Chromosome aberrations and sister
chromatid exchanges in Chinese hamster ovary cells: Evaluations of
108 chemicals. Environ Mol Mutagen, 10(suppl 10): 1-175.
Gautier M, Fournier P-E, Gervais P, & Sicot C (1964) Intoxication
par le nitropropane. Arch Mal Prof, 25: 425-428.
George E, Burlinson B, & Gatehouse D (1989) Genotoxicity of 1- and
2-nitropropane in the rat. Carcinogenesis, 10: 2329-2334.
Glaser RA, & Woodfin WJ (1981) A method for sampling and analysis of
2-nitropropane in air. Am Ind Hyg Assoc J, 42: 18-22.
Goggelmann W, Bauchinger M, Kulka U, & Schmid E. (1988) Genotoxicity
of 2-nitropropane and 1-nitropropane in Salmonella typhimurium and
human lymphocytes. Mutagenesis, 3: 137-140.
Goldwhite H (1965) Nitrogen derivatives of the aliphatic
hydrocarbons. In: Coffey S ed. Rodd's chemistry of carbon compounds,
Part B, 2nd ed. Amsterdam, Oxford, New York, Elsevier Science
Publishers, vol. 1, pp 93-164.
Griffin TB & Coulston F (1983) Final report. Chronic inhalation
exposure of rats to vapors of 2-nitropropane at 100 ppm. Alamogordo,
New Mexico, Coulston International Corporation, 13 pp.
Griffin TB, Benitz K-F, Coulston F, & Rosenblum I (1978) Chronic
inhalation toxicity of 2-nitropropane in rats. Pharmacologist, 20:
145.
Griffin TB, Coulston F, & Stein AA (1980) Chronic inhalation
exposure of rats to vapors of 2-nitropropane at 25 ppm. Ecotoxicol
Environ Saf, 4: 267-281.
Griffin TB, Stein AA, & Coulston F (1981) Histologic study of
tissues and organs from rats exposed to vapors of 2-nitropropane at
25 ppm. Ecotoxicol Environ Saf, 5: 194-201. Griffin T, Coulston F, &
Stein AA (1984) Chronic inhalation exposure of mice to
2-nitropropane. Preliminary report: Laboratory findings and
histopathology of liver. Alamogordo, New Mexico, Coulston
International Corporation, 14 pp (Unpublished report).
Griffin T, Coulston F, & Stein AA (1986) Long-term effects of acute
exposure of rats to 2-nitropropane. Final Report, Study No. 820202.
Alamogordo, New Mexico, Coulston International Corporation, 17 pp
(Unpublished report).
Griffin T, Coulston F, & Stein AA (1987) Chronic inhalation exposure
of mice to 2-nitropropane (100 ppm): Final report. Alamogordo, New
Mexico, Coulston International Corporation, 24 pp (Unpublished
report).
Guo N, Conaway CC, Hussain NS, & Fiala ES (1990) Sex and organ
differences in oxidative DNA and RNA damage due to treatment of
Sprague-Dawley rats with acetoxime or 2-nitropropane.
Carcinogenesis, 11: 1659-1662.
Haas-Jobelius M, Ziegler-Skylakakis K, & Andrae U (1991)
Nitroreduction is not involved in the genotoxicity of 2-nitropropane
in cultured mammalian cells. Mutagenesis, 6: 87-91.
Hardin BD, Bond GP, Sikov MR, Andrew FD, Beliles RP, & Niemeier RW
(1981) Testing of selected workplace chemicals for teratogenic
potential. Scand J Work Environ Health, 7(suppl 4): 66-75.
Harris SJ, Bond GP, & Niemeier RW (1979) The effects of
2-nitropropane, naphthalene, and hexachlorobutadiene on fetal rat
development. Toxicol Appl Pharmacol, 48: A35.
Harrison RJ, Pasternak G, Blanc P, Basuk P, & Letz G (1985) Acute
hepatic failure after occupational exposure to 2-nitropropane. J Am
Med Assoc, 254(24): 3415-3416.
Harrison RJ, Letz G, Pasternak G, & Blanc P (1987) Fulminant hepatic
failure after occupation exposure to 2-nitropropane. Ann Intern Med,
107: 466-468.
Hartle RW (1980) Health hazard evaluation report No. 80-057-781 at
Long Island Railroad, Richmond Hill, New York, Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 17 pp.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, 5 (suppl 1): 3-142.
Hine CH, Pasi A, & Stephens BG (1978) Fatalities following exposure
to 2-nitropropane. J Occup Med, 20: 333-337.
Hite M & Skeggs H (1979) Mutagenic evaluation of nitroparaffins in
the Salmonella typhimurium/mammalian microsome test and the
micronucleus test. Environ Mutagen, 1: 383-389.
Hoar RM (1976) Chapter 3. Biomethodology. In: Wagner JE & Manning PJ
ed. The biology of the guinea pig. New York, London, Academic Press,
p 16.
Hoffmann D & Rathkamp C (1968) Chemical studies on tobacco smoke.
III. Primary and secondary nitroalkanes in cigarette smoke. Beitr
Tabakforsch, 4: 124-134.
Hollett BA & Schloemer J (1978) Hazard evaluation technical
assistance report No. TA 76-90: Newport Industrial Products
Firestone Tire Rubber Co., Newport, Tennessee. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 52 pp.
Hughes TJ, Simmons DS, Monteith LG, Myers LD, & Claxton LD (1987)
Mutagenicity of 31 organic compounds in a modified preincubation
Ames assay with Salmonella typhimumium strains TA100 and TA102.
Environ Mutagen, 9 (suppl 8): 49.
Hussain NS, Conaway CC, Guo N, Asaad W, & Fiala ES (1990) Oxidative
DNA and RNA damage in rat liver due to acetoxime: similarity to
effects of 2-nitropropane. Carcinogenesis, 11: 1013-1016.
Indig GL & Cilento G (1987) Peroxidase-promoted aerobic oxidation of
2-nitropropane: Mechanism of excited state formation. Biochim
Biophys Acta, 923: 347-354.
IARC (1982) 2-Nitropropane. In: Some industrial chemicals and
dyestuffs. Lyon, International Agency for Research on Cancer, pp
331-343 (IARC Monographs on the Evaluation of the Carcinogenic Risk
of Chemicals to Humans, Volume 29).
IARC (1987) Overall evaluations of carconogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer, p 67 (IARC Monographs on the Evaluation of the
Carconogenic Risk of Chemicals to Humans, Supplement 7).
IRPTC (1986) IRPTC data profile on 2-nitropropane. Geneva,
International Register of Potentially Toxic Chemicals, United
Nations Environment Programme, pp 17/1-17/3.
Ivanetich KM, Lucas S, Marsh JA, Ziman MR, Katz ID, & Bradshaw JJ
(1978) Organic compounds. Their interaction with and degradation of
hepatic microsomal drug-metabolizing enzymes in vitro. Drug Metab
Disp, 6: 218-225.
Jones LR (1963) The determination of 2-nitropropane in air. Ind Hyg
J, January-February: 11-16.
Jones LR & Riddick JA (1952) Colorimetric determination of
nitroparaffins. Anal Chem, 24: 1533-1536.
Jonsson J, Kammerer RC, & Cho AK (1977) Metabolism of
2-nitro-1-phenylpropane by rabbit liver microsomes. Res Commun chem
Pathol Pharmacol, 18: 75-82.
Kaplan HM, Brewer NR, & Blair WH (1983) Physiology. In: Foster HL,
Small JD, & Fox JG ed. The mouse in biomedical research. Volume III
Normative biology, immunology, and husbandry. New York, London,
Academic Press, p 255.
Kawai A, Goto S, Matsumoto Y, & Matsushita H (1987) [Mutagenicity of
aliphatic and aromatic nitro compounds. Industrial materials and
related compounds.] Jpn J ind Health, 29: 34-54 (in Japanese).
Kenaga EE (1980) Predicted bioconcentration factors and soil
sorption coefficients of pesticides and other chemicals. Ecotoxicol
environ Saf, 4: 26-38.
Kido T, Yamamoto T, & Soda K (1975) Microbial assimilation of alkyl
nitro compounds and formation of nitrite. Arch Microbiol, 106:
165-169.
Kido T, Tanizawa K, Inagaki K, Yoshimura T, Ishida M, Hashizume K, &
Soda K (1984) 2-Nitropropane dioxygenase from Hansenula mrakii:
Re-characterization of the enzyme and oxidation of anionic
nitroalkanes. Agric Biol Chem, 48: 2549-2554.
Kliesch U & Adler I-D (1987) Micronucleus test in bone marrow of
mice treated with 1-nitropropane, 2-nitropropane and cisplatin.
Mutat Res, 192: 181-184.
Korte F, Freitag D, Geyer H, Klein W, Kraus AG, & Lahaniatis E
(1978) Ecotoxicologic profile analysis. Chemosphere, 1: 79-102.
Kozma C, Macklin W, Cummins LM, & Mauer R (1974) Chapter 3. Anatomy,
physiology, and biochemistry of the rabbit. In: Weisbroth SH, Flatt
RE, & Kraus AL ed. The biology of the laboratory rabbit. New York,
London, Academic Press, pp 56-57.
Krotoszynski BK, Bruneau GM, & O'Neill HJ (1979) Measurement of
chemical inhalation exposure in urban population in the presence of
endogenous effluents. J Anal Toxicol, 3: 225-234.
Kuo FK & Fridovich (1986) Free-radical chain oxidation of 2-NP
initiated and propagated by superoxide. Biochem J, 237: 505-510.
Levy A (1973) The photochemical smog reactivity of organic solvents.
Adv Chem Ser, 124: 70-94.
Lewis TR, Ulrich CE, & Busey WM (1979) Subchronic inhalation
toxicity of nitromethane and 2-nitropropane. J Environ Pathol
Toxicol, 2: 233-249.
Life Extension Institute (1983) Summary report of medical findings
for selected employees at one plant. Rockville, Maryland, Life
Extension Institute, 12 pp.
Little HN (1951) Oxidation of nitroethane by extracts from
Neurospora. J Biol Chem, 193: 347-358.
Little HN (1957) The oxidation of 2-nitropropane by extracts of pea
plants. J Biol Chem, 229: 231-238.
Litton Bionetics, Inc. (1977) Mutagenicity evaluation of P-1357
66459T: Final report. Terre Haute, Indiana, IMC Chemical Group,
Inc., 10 pp (Unpublished report).
Löfroth G, Nilsson L, & Andersen JR (1986) Structure-activity
relationship of nitroalkane-induced mutagenicity in the Ames
Salmonella assay. In: Genetic toxicology of environmental
chemicals. Part B: Genetic effects and applied mutagenesis. New
York, Alan R. Liss, Inc., pp 149-155.
Lotz F, Nitz S, & Korte F (1979) [Photomineralization of adsorbed
organic chemicals on a microscale.] Chemosphere, 10: 763-768 (in
German).
Love JR & Kern M (1981) Health hazard evaluation report no.
81-065-938. Metro bus maintenance shop, Washington, DC. Cincinnati,
Ohio, National Institute for Occupational Safety and Health, 13 pp.
Mabuchi H (1979) [Urinary and serum volatile substances in
diabetics.] Nippon Naika Gakkai Zasshi, 58: 731-743 (Abstract only)
(in Japanese).
McGregor DB (1981) Tier II mutagenic screening of 13 NIOSH priority
compounds. Individual compound report: 2-Nitropropane. Musselburgh,
Scotland, Inveresk Research International Ltd, 194 pp (Report No.
31).
Machle W, Scott EW, & Treon J (1940) The physiological response of
animals to some simple mononitroparaffins and to certain derivatives
of these compounds. J Ind Hyg Toxicol, 22: 315-332.
Malkinson FD & Gehlmann L (1977) Factors affecting percutaneous
absorption. In: Drill VA & Lazar P, ed. Cutaneous toxicity. New
York, London, Academic Press, pp 63-82.
Marcstrom A (1967) Studies on the connection between physicochemical
properties and stimulating abilities of some sweet and bitter
compounds. Ark Zool, 19: 421-535.
Marker EK & Kulkarni AP (1985) Enzymatic denitrification of
2-nitropropane in uninduced mouse liver microsomes. Toxicol Lett,
26: 181-185.
Marker EK & Kulkarni AP (1986a) Cumene hydroperoxide-supported
denitrification of 2-nitropropane in uninduced mouse liver
microsomes. Int J Biochem, 18: 595-601.
Marker EK & Kulkarni AP (1986b) Cytochrome P-450-mediated
denitrification of 2-nitropropane in mouse liver microsomes. J
Biochem Toxicol, 1: 71-83.
Miller ME & Temple GW (1979) 2-NP mortality epidemiology study of
the Sterlington, LA Employees, 1-1-46 thru 6-30-77. Northbrook,
Illinois, International Minerals and Chemical Corporation, 34 pp
(Unpublished report).
Mochizuki H, Kominami S, & Takemori S (1988) Examination of
differences between benzo[ a]pyrene and steroid hydroxylases in
guinea pig adrenal microsomes. Biochem Biophys Acta, 964: 83-89.
Müller WF, Coulston F, & Korte F (1983) Comparative metabolism of
2-nitropropane in rats and chimpanzees. Chemosphere, 12: 231-237.
National Fire Protection Association (1968) Hazardous chemicals
data. J Chem Educ, 45: A313-A314, A317-A318, 320-322.
National Library of Medicine (1989) Toxic chemical release
inventory. Washington, DC, National Library of Medicine (TOXNET
system, TRI data base, 12 June 1989).
Nielsen AT (1981) Nitronic acids and esters. In: Feurer H ed. The
chemistry of the nitro and nitroso groups. Huntington, New York,
Robert Krieger Publishing Co., Part I, pp 349-386.
Nolan RJ, Unger SM, & Muller CJ (1982) Pharmacokinetics of inhaled
[14C]-2-nitropropane in male Sprague-Dawley rats. Ecotoxicol
Environ Saf, 6: 388-397.
Occupational Health Services, Inc. (1982) An evaluation of the
number of workers occupationally exposed to 2-nitropropane.
Cambridge, Massachusetts, Occupational Health Services, Inc., 22 pp
(Unpublished report prepared for IMC Corporation, Des Plaines,
Illinois).
Parks NJ, Krohn KA, Mathis CA, Chasko JH, Geiger KR, Gregor ME, &
Peek NF (1981) Nitrogen-13-labeled nitrite and nitrate: Distribution
and metabolism after intratracheal administration. Science, 212:
58-61.
Paszyc S (1971) [Photolysis of 2-nitropropane in gaseous and liquid
phases.] Poznan Tow Przyj Nauk Pr Kom Mat-Przyr, Pr Chem, 12: 79-85
(in Polish).
Patel RN, Hou CT, Laskin AI, & Felix A (1982) Epoxidation of alkenes
and hydroxylation of alkanes by soluble methane monooxygenase:
Regeneration of cofactor NADH2. J Appl Biochem, 4: 175-184.
Porter DJ & Bright HJ (1983) The mechanism of oxidation of
nitroalkanes by horseradish peroxidase. J Biol Chem, 258: 9913-9924.
Purcell RF (1967) Use of 2-nitropropane under rule 66. West Paint
Rev, 53: 22A, 24A.
Reece WO (1984) Respiration in mammals. In: Swenson MJ ed. Duke's
physiology of domestic animals, 10th ed. Cornell, New York, Comstalk
Press, p 231.
Robbiano L, Mattioli F, & Brambilla G (1991) DNA fragmentation by
2-nitropropane in rat tissues, and effects of the modulation of
biotransformation processes. Cancer Lett. 57: 61-66.
Rondia D (1979) 2-nitropropane: One more death. Vet Hum Toxicol, 21:
183-185.
Roscher E, Ziegler-Skylakakis K, & Andrae U (1990) Involvement of
different pathways in the genotoxicity of nitropropanes in cultured
mammalian cells. Mutagenesis, 5: 375-380.
Sadtler SP (1961) Sadtler standard ultraviolet spectra.
Philadelphia, Pennsylvania, Sadtler Research Laboratory, p 1.
Sakurai H, Hermann G, Ruf HH, & Ullrich V (1980) The interaction of
aliphatic nitro compounds with the liver microsomal monooxygenase
system. Biochem Pharmacol, 29: 341-345.
Sander J & Schweinsberg F (1972) [Interrelationships between
nitrate, nitrite and carcinogenic N-nitroso-compounds. 1.
Communication: nitrate, nitrite and nitrosable amino-compounds in
food and drugs, chemistry of N-nitroso compounds.] Zentrabl Bakeriol
Parasitenkd Infektionsk Hyg Abt 1: Orig Reihe B, 156: 299-340 (in
German).
Schneider NR & Yeary RA (1975) Nitrite and nitrate pharmacokinetics
in the dog, sheep, and pony. Am J Vet Res, 36: 941-947.
Scott EW (1943) The metabolism of monoparaffins. III. The
concentration of nitroethane, nitrite and nitrate in the blood of
rabbits during exposure by inhalation and oral administration. J Ind
Hyg Toxicol, 25: 20-25.
Seizinger DC & Dimitrades B (1972) Oxygenates in exhaust from simple
hydrocarbon fuels. J Air Pollut Control Assoc, 22: 47-51.
Simmons DM, Monteith LG, Hughes TJ, & Claxton LD (1986) Effect of
preincubation time on mutagenic activity in the Ames/ Salmonella
assay. Environ Mutagen, 8(suppl 6): 78.
Skinner JB (1947) The toxicity of 2-nitropropane. Ind Med, 16:
441-443.
Smith EL, Hill RL, Lehman IR, Lefkowitz RJ, Handler P, & White A
(1983) Principles of biochemistry. General aspects. New York,
McGraw-Hill, pp 539-541.
Soda K, Kido T, & Asada K (1977) 2-Nitropropane dioxygenase from
Hansenula mrakii: Generation and participation of superoxide anion
in the reaction. In: Hayashi O & Asada K ed. Biochemical and medical
aspects of active oxygen. Baltimore, Maryland, University Park
Press, pp 119-133.
Speck WT, Meyer LW, Zeiger E, & Rosenkranz HS (1982) Mutagenicity
and DNA-modifying activity of 2-nitropropane. Mutat Res, 104: 49-54.
SRI International (1988) 1988 Directory of chemical producers,
United States of America. Menlo Park, California, SRI International,
pp 29, 168, 803.
SRI International (1990) 1990 Directory of chemical producers,
United States of America. Menlo Park, California, SRI International,
p 815.
Stokinger HE (1982) Aliphatic nitro compounds, nitrates, nitrites.
In: Clayton GD & Clayton FE ed. Patty's industrial hygiene and
toxicology, 3rd revised ed. New York, John Wiley and Sons, vol 2C,
pp 4141-4208.
Swann RL, Laskowski DA, McCall PJ, Vander Kuy K, & Dishburger HJ
(1983) A rapid method for the estimation of the environmental
parameters octanol/water partition coefficient, soil sorption
constant, water to air ratio, and water solubility. Residue Rev.
85 : 17-28.
Tabershaw Occupational Medicine Association (1980) Cross-sectional
morbidity study of workers. Rockville, Maryland, Tabershaw
Occupational Medicine Association, 68 pp (Unpublished report).
Treon JF & Dutra FR (1952) Physiological response of experimental
animals to the vapor of 2-nitropropane. Arch Ind Hyg Occup Med,
5 : 52-61.
Ullrich V & Schnabel KN (1973) Formation and binding of carbanions
by cytochrome P-450 of liver microsomes. Drug Metab Disp, 1:
176-183.
Ullrich V, Hermann G, & Weber P (1978) Nitrite formation from
2-nitropropane by microsomal monooxygenases. Biochem Pharmacol,
27 : 2301-2304.
US Bureau of the Census (1987) Statistical abstract of the United
States: 1988, 108th ed. Washington, DC, US Bureau of the Census,
p 368.
US EPA (1977) TSCA chemical assessment series. Chemical hazard
information profiles (CHIPS), August 1976-August 1978. Washington,
DC, US Environmental Protection Agency, pp 212-217
(EPA-560/11-80-011).
US EPA (1980) Materials balance 2-nitropropane, Level 1. Washington,
DC, US Environmental Protection Agency, 70 pp (EPA-560/13-89-011).
US EPA (1985) Health and environmental effects profile for
2-nitropropane. Washington, DC, US Environmental Protection Agency,
pp 3-4 (EPA-600/X-85-112).
US EPA (1986) Notice of potential risk, 2-nitropropane. Washington,
DC, US Environmental Protection Agency, 5 pp.
US FDA (Food and Drug Administration) (1987) 2-Nitropropane. Code
Fed Regul, 27 (Parts 1 to 199): Docket No. 78N-0221.
USITC (1990) Synthetic organic chemicals. United States production
and sales, 1989. Washington, DC, United States International Trade
Commission, pp 15-17, 15-35-15-36 (USITC Publication No. 2338).
US NIOSH (1983) National occupational exposure survey, 1981-83:
Actual observation and trade-name exposure to 2-nitropropane.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health (Unpublished database).
US NIOSH (1987a) NIOSH manual of analytical methods. Second
supplement, 3rd ed. Cincinnati, Ohio, National Institute for
Occupational Safety and Health, pp 2528/1-2528/4 (NIOSH Publication
No. 87-117).
US NIOSH (1987b) Safe sheet #1: Summary of accidental fatality
evaluations, confined spaces, Case #3. Morgantown, West Virginia,
National Institute for Occupational Safety and Health, Environmental
Investigations Branch, Division of Respiratory Disease Studies, p 2.
US NIOSH (1988) Current intelligence bulletins: Summaries, September
1988. Cincinnati, Ohio, National Institute for Occupational Safety
and Health, p 6.
US OSHA (Occupational Safety and Health Administration) (1988)
1-Nitropropane and 2-nitropropane. Code Fed Regul, 29: 705, 708
(Parts 1900 to 1910).
Weast RC ed. (1986) CRC handbook of chemistry and physics, 67th ed.
Boca Raton, Florida, CRC Press, p C-444.
Wester PW, Van Leeuwen FXR, & De Vries T (1989) 4-Week toxicity
study with 2-nitropropane (2-NP) by gavage in rats. Bilthoven, The
Netherlands, National Institute of Public Health and Environmental
Protection, 20 pp (Report No. 658501 001).
Wilbur SZ & Parekh CK (1982) Dermal toxicity potential of
2-nitropropane (P-1357). Northbrook, Illinois, International
Minerals and Mining Corporation, 5 pp (Unpublished report No.
PLR-281/AMR-072).
Wilks RA & Gilbert SG (1972a) Sensory and instrumental evaluation in
model systems of residues migrating from can coatings. J Food Sci,
37: 72-76.
Wilks RA & Gilbert SG (1972b) Quick test for liner solvents. Modern
Packag, May: 52-55.
Williams SV, Bryan JA, Burk JR, & Wolf FS (1974) Toxic hepatitis and
methylenedianiline. New Engl J Med, 291: 1256.
Windholz M ed. (1983) The Merck index, 10th ed. Rahway, New Jersey,
Merck & Co., p 6482.
Woo Y-T, Lai DY, Arcos JC, & Argus MF (1985) Chemical induction of
cancer: structural bases and biological mechanisms, Vol. III B:
Aliphatic and polyhalogenated carcinogens. New York, London,
Academic Press, pp 329-341.
Yasuhara A, Yamanaka Y, & Ogawa T (1986) Volatile compounds in
machine cutting-fluid emulsion. Agric Biol Chem, 50: 1765-1770.
Ziegler-Skylakakis K, Homfeldt H, & Andrae U (1987) In vitro and
in vivo genotoxicity of 2-nitropropane and 1-nitropropane in
mammalian cells. Naunyn-Schmiedeberg's Arch Pharmacol, 335(suppl):
R25.
Zimmering S, Mason JM, Valencia R, & Woodruff RC (1985) Chemical
mutagenesis testing in Drosophila. II. Results of 20 coded
compounds tested for the National Toxicology Program. Environ
Mutagen, 7: 87-100.
Zitting A, Savolainen H, & Nickels J (1981) Acute effects of
2-nitropropane on rat liver and brain. Toxicol Lett, 9: 237-246.
RESUME
1. Propriétés et méthodes d'analyse
Le 2-nitropropane (2-NP) est un liquide huileux, incolore, à
l'odeur douceâtre. Il est inflammable, moyennement volatil et stable
dans les conditions ordinaires. Il n'est que légèrement soluble dans
l'eau mais miscible à de nombreux liquides organiques et c'est un
excellent solvant de nombreux composés organiques. Il existe de
bonnes méthodes d'analyse pour la recherche et le dosage du
2-nitropropane présent dans l'environnement. On a actuellement
recours à la chromatographie en phase gazeuse avec détection par
ionisation de flamme ou capture d'électrons, ou bien à la
chromatographie en phase liquide à haute performance avec détecteur
u.v. Pour le dosage dans l'air, il faut tout d'abord piéger le
2-nitropropane et le concentrer par adsorption sur phase solide.
2. Emploi et sources d'exposition
2.1 Production
Les chiffres de production actuels ne sont pas connus. En 1977
les Etats-Unis d'Amérique en ont produit environ 13 600 tonnes. Le
2-nitropropane est actuellement produit par deux firmes américaines
et une firme française. Il peut prendre naissance naturellement à
l'état de traces lors de la combustion du tabac et d'autres matières
organiques riches en nitrates mais rien n'indique qu'il puisse se
former à l'issue d'un processus biologique quelconque.
2.2 Emploi et passage dans l'environnement
Le 2-nitropropane est utilisé comme solvant, principalement en
mélange, et il a de nombreuses applications industrielles en tant
que tel pour la confection d'encres d'imprimerie, de peintures, de
vernis, d'adhésifs et autres revêtements, comme par exemple ceux que
l'on utilise dans les récipients contenant des boissons. On
l'utilise également comme solvant pour séparer des composés très
voisins comme les acides gras, comme intermédiaire en synthèse
chimique et comme additif dans les carburants. C'est principalement
par l'intermédiaire de l'air qu'il passe dans l'environnement,
surtout par évaporation à partir des surfaces enduites de produits
qui en contiennent.
3. Transport et distribution dans l'environnement
Le 2-nitropropane se révèle très mobile dans le milieu naturel.
Du fait que sa solubilité dans l'eau, son adsorption par les
sédiments et sa bioaccumulation sont faibles et qu'il s'évapore
rapidement dans l'atmosphère, il se répartit dans l'air et l'eau
sans s'accumuler dans un compartiment particulier du milieu. Le
2-nitropropane absorbe le rayonnement ultra-violet aux longueurs
d'onde que l'on rencontre dans l'environnement et il est donc
probable qu'il subisse une lente photolyse dans l'atmosphère. Il est
également probable qu'il soit lentement transformé par voie
biologique en composés moins toxiques, tant dans l'environnement
aquatique que dans l'environnement terrestre.
4. Concentrations dans l'environnement et exposition humaine
Il semble que l'exposition de la population générale au
2-nitropropane soit très faible et provienne de la fumée de
cigarettes (1,1 à 1,2 µg/cigarette), de résidus présents dans des
enduits ou revêtements tels qu'on en utilise pour les récipients
contenant des boissons, dans les adhésifs, les encres d'imprimerie,
ainsi que dans les huiles végétales que l'on fractionne au moyen de
ce solvant. On ignore quelle est l'importance de l'exposition des
travailleurs de l'industrie dans le monde, mais aux Etats-Unis
d'Amérique, il semble qu'elle soit limitée à 0,02-0,19 % du
personnel. Dans ce même pays, environ 4000 travailleurs (soit à peu
près 0,005 % de la main-d'oeuvre) subissent sans doute une
exposition notable (de l'ordre de 9,1 mg/m3(2,5 ppm) ou
davantage). Selon les pays, les limites d'exposition professionnelle
dans l'air vont de 36 mg/m3 (1 ppm) (TWA) à 146 mg/m3 (40 ppm)
(STEL). La production du 2-nitropropane s'effectue en circuit fermé
et en général, le personnel n'est guère exposé; toutefois dans
certaines industries (peinture, imprimerie ou extraction par
solvant), il est arrivé que des travailleurs soient exposés à des
concentrations dépassant largement les limites d'exposition
professionnelle. C'est ainsi que des concentrations atteignant
6 g/m3 (1640 ppm) dans l'air ont été enregistrées lors du
remplissage de fûts.
5. Cinétique et métabolisme
Le 2-nitropropane est principalement résorbé au niveau des
poumons. Chez l'animal de laboratoire, on a montré qu'il était
rapidement absorbé non seulement à ce niveau mais également au
niveau de la cavité péritonéale et des voies digestives. On est mal
renseigné sur son absorption percutanée. Quant aux données
concernant sa distribution dans l'organisme du rat, elles sont
quelque peu contradictoires. Le 2-nitropropane est rapidement
métabolisé, essentiellement sous forme d'acétone et de nitrite. Il
se forme peut-être aussi un peu d'alcool isopropylique. Après
injection par voie intrapéritonéale, le 2-nitropropane et ses
métabolites carbonés se concentrent d'abord dans les graisses, puis
dans la moelle osseuse ainsi que dans les surrénales et les autres
organes internes; après inhalation, ils se concentrent dans le foie
et les reins, mais relativement peu dans les graisses. Il est
possible que plusieurs systèmes enzymatiques interviennent dans ce
métabolisme et les vitesses, de même que les voies de
métabolisation, varient selon les espèces. Le 2-nitropropane et ses
métabolites carbonés disparaissent rapidement de l'organisme par
métabolisation, exhalation ou excrétion dans les urines et les
matières fécales. On manque de données satisfaisantes sur la
distribution et l'excrétion de la fraction nitrée de ces
métabolites.
6. Effets sur les mammifères de laboratoire et les systèmes
in vitro
La toxicité aiguë du 2-nitropropane pour les mammifères est
modérée. Les mâles sont plus sensibles que les femelles, tout au
moins en ce qui concerne le rat, et l'expérience montre que la
sensibilité varie largement d'une espèce à l'autre. La CL50
(concentration produisant une mortalité de 50 % en 14 jours) a été
de 1,5 g/m3 (400 ppm) pour les rats mâles et de 2,6 g/m3
(720 ppm) pour les rats femelles. Il semble que la mortalité soit
associée principalement aux effets narcotiques, mais les mammifères
exposés à des concentrations d'au moins 8,4 g/m3 (2300 ppm)
pendant une heure ou davantage ont présenté des anomalies
anatomopathologiques graves, notamment des lésions
hépatocellulaires, un oedème du poumon et des hémorragies.
Il est indiscutable que le 2-nitropropane est cancérogène pour
le rat. L'exposition de rats pendant de longues durées à du
2-nitropropane par voie respiratoire à raison de 0,36 g/m3
(100 ppm) (pendant 18 mois à raison de sept heures par jour et cinq
jours par semaine) a causé des lésions destructrices au niveau du
foie, et en particulier des carcinomes hépatocellulaires chez
certains rats mâles. A la concentration de 0,75 g/m3 (207 ppm) les
lésions étaient encore plus graves, avec une forte incidence de
carcinomes hépatocellulaires à survenue plus rapide.
L'administration chronique de doses modérées par voie orale a
également provoqué une surincidence de carcinomes hépatocellulaires.
Toutefois, l'inhalation prolongée par des rats de 2-nitropropane aux
doses respectives de 91 ou 98,3 mg/m3 (25 ou 27 ppm) n'a pas
provoqué de lésions décelables. L'exposition de souris et de lapins
à des concentrations de 2-nitropropane capables de provoquer des
carcinomes hépatocellulaires chez le rat n'a guère eu d'effets chez
ces animaux, mais il est vrai que les études en question étaient
trop limitées pour qu'on puisse exclure totalement un effet
cancérogène du 2-nitropropane chez ces deux espèces. Le
2-nitropropane a légèrement retardé le développement foetal des rats
mais les données relatives à l'embryotoxicité, à la tératogénicité
et à la toxicité du 2-NP pour la fonction de reproduction restent
très fragmentaires. Le produit s'est révélé fortement génotoxique
pour les hépatocytes de rats tant in vivo qu' in vitro; en
revanche aucune génotoxicité sensible n'a été observée au niveau des
autres organes chez le rat ou sur des lignées cellulaires d'origine
extrahépatique, en l'absence d'activation métabolique exogène. On a
également montré que le 2-nitropropane était mutagène chez les
bactéries en présence ou en l'absence d'activation métabolique
exogène.
7. Effets sur l'homme
L'exposition humaine à de fortes concentrations de
2-nitropropane est largement, voire totalement d'origine
professionnelle. A concentration élevée, le 2-nitropropane présente
une forte toxicité aiguë et il a provoqué des accidents mortels dans
l'industrie - encore qu'on ignore la valeur exacte de cette
concentration, sauf dans un cas où on a pu estimer l'exposition à
2184 mg/m3, soit 600 ppm. Les premiers symptômes consistaient en
céphalées, nausées, somnolence, vomissements, diarrhées et douleurs.
Malgré une amélioration temporaire de l'état général, la mort est
quelquefois survenue dans les 4 à 26 jours suivant l'exposition. La
cause initiale de la mort était une insuffisance hépatique à
laquelle s'ajoutaient un oedème du poumon, des hémorragies des voies
digestives et une insuffisance respiratoire et rénale. A des doses
évaluées à 73-164 mg/m3 (20 à 45 ppm) on a constaté que
l'exposition professionnelle provoquait des nausées et une perte
d'appétit pouvant persister plusieurs heures après le départ du lieu
de travail, aucun effet indésirable n'étant observé après exposition
à des doses de 36,4 à 109 mg/m3 (10 à 30 ppm) (moins de quatre
heures par jour pendant une durée inférieure ou égale à trois jours
par semaine).
Malgré l'insuffisance des données disponibles, rien n'indique
qu'une exposition professionelle de longue durée au 2-nitropropane
aux concentrations généralement présentes sur les lieux de travail
puisse provoquer des cancers du foie ou d'autres organes, ni plus
généralement des effets indésirables à longue échéance.
8. Effets sur les autres organismes au laboratoire et dans
leur milieu naturel
Les quelques études effectuées sur des microorganismes, des
invertébrés et des poissons montrent que le 2-nitropropane est peu
toxique pour les organismes non mammaliens.
RESUMEN
1. Propiedades y métodos analíticos
El 2-nitropropano (2-NP) es un líquido incoloro y oleoso de olor
ligero. Es inflamable, moderadamente volátil, y estable en
condiciones normales. Es sólo ligeramente hidrosoluble pero miscible
con numerosos líquidos orgánicos, y es un excelente disolvente para
muchos tipos de compuestos orgánicos. Existen métodos analíticos
adecuados para identificar y medir el 2-NP en concentraciones
ambientales. Los métodos de uso corriente son la cromatografía de
gases y un detector de ionización de llama o de captura electrónica;
también se usa la cromatografía líquida de alto rendimiento con
detector de ultravioleta. Para medirlo en el aire, primero es
necesario capturarlo y concentrarlo en un sorbente sólido.
2. Usos y fuentes de exposición
2.1 Producción
No se dispone de cifras recientes de producción mundial. En
1977, la producción en los Estados Unidos fue de aproximadamente
13 600 toneladas. Actualmente, el 2-NP se fabrica en dos empresas
estadounidenses y una francesa. Se origina por mecanismos naturales
como trazas en la combustión del tabaco y de otras sustancias
orgánicas ricas en nitratos, pero nada indica que se origine en
procesos biológicos.
2.2 Usos y pérdidas al medio ambiente
El 2-NP se utiliza como disolvente, principalmente en mezclas, y
tiene numerosas aplicaciones industriales como disolvente para
tintas de impresión, pinturas, barnices, adhesivos y otros
revestimientos como los de recipientes de bebidas. Se ha utilizado
asimismo como disolvente para separar sustancias estrechamente
relacionadas como ácidos grasos, como intermediario en síntesis
químicas, y como aditivo en combustibles. Las pérdidas al medio
ambiente ingresan principalmente en el aire y se deben sobre todo a
la evaporación del disolvente a partir de superficies revestidas.
3. Transporte y distribución en el medio ambiente
El 2-NP parece tener gran movilidad en el medio ambiente
natural. Dada su baja solubilidad en el agua, escasa absorción por
el sedimento, reducida bioacumulación y facil evaporación a la
atmósfera, se distribuye tanto en el aire como en el agua y no se
acumula en ningún compartimento ambiental definido. La fotoabsorción
ultravioleta del 2-NP se encuentra en la escala de frecuencias
normales en el medio ambiente, por lo que es probable que la
sustancia sea objeto de fotólisis lenta en la atmósfera. La
biotransformación lenta del 2-NP a compuestos menos tóxicos también
parece probable en los medios acuático y terrestre.
4. Niveles ambientales y exposición humana
La exposición de la población general al 2-NP parece ser muy
baja y se debe al humo de cigarrillos (1,1 a 1,2 µg/cigarrillo), a
los residuos en revestimientos, como los de las latas de bebidas,
adhesivos y material impreso, y a los aceites vegetales fraccionados
con esa sustancia. Se desconoce la exposición industrial a escala
mundial, pero en los Estados Unidos parece limitarse al 0,02-0,19%
de la población trabajadora. Los niveles de exposición de cierta
importancia (exposición a 9,1 mg/m3 (2,5 ppm) o más) en los
Estados Unidos probablemente no afectan más que a unos 4000
trabajadores (aproximadamente 0,005% de la población trabajadora).
Los límites de exposición ocupacional en el aire varían de un paíse
a otro y van desde 3,6 mg/m3 (1 ppm) (promedio ponderado en
función del tiempo) a 146 mg/m3 (40 ppm) (STEL). La fabricación
del 2-NP es un proceso cerrado y entraña por lo general una
exposición reducida de los trabajadores, pero algunos obreros de
industrias como la pintura, la impresión y la extracción de
disolventes se han visto expuestos en otras épocas a niveles muy
superiores a los límites de exposición ocupacional. Durante ciertas
operaciones industriales han llegado a registrarse concentraciones
de hasta 6 g/m3 (1640 ppm) en el aire.
5. Cinética y metabolismo
En el ser humano, la absorción de 2-NP se produce principalmente
por los pulmones. En animales de experimentación, se ha demostrado
que el 2-NP se absorbe rápidamente no sólo por vía pulmonar sino
también a partir de la cavidad peritoneal y del tracto
gastrointestinal. No se dispone de datos satisfactorios sobre la
absorción por vía cutánea. La información sobre la distribución en
la rata es ligeramente contradictoria. El 2-NP se metaboliza
rápidamente, principalmente a acetona y nitrito. También puede
formarse isopropil alcohol en pequeñas cantidades. Tras la inyección
intraperitoneal, el 2-NP y sus metabolitos carbonados se concentran
inicialmente en la grasa y después en la médula ósea, así como en
las glándulas suprarrenales y otros órganos internos. Tras la
inhalación, el 2-NP y sus metabolitos carbonados se concentran en el
hígado y el riñón; la cantidad que se acumula en la grasa es
relativamente pequeña. Varios sistemas enzimáticos diferentes pueden
participar y existen diferencias de unas especies a otras en cuanto
a la velocidad y las rutas metabólicas. El 2-NP y sus metabolitos
carbonados desaparecen rápidamente del organismo por transformación
metabólica, exhalación y excreción en orina y heces. No se dispone
de datos satisfactorios sobre la distribución y la excreción de
metabolitos que llevan el radical nitro.
6 Efectos en mamíferos de laboratorio y sistemas in vitro
El 2-NP tiene una toxicidad aguda moderada para los mamíferos.
Los machos son más sensibles que las hembras, por lo menos en la
rata, y la sensibilidad difiere ampliamente entre las especies que
se han ensayado. La CL50 (concentración que causa una mortalidad
del 50% en un plazo de 14 días) para la rata tras una exposición de
6 horas fue de 1,5 g/m3 (400 ppm) en los machos y 2,6 g/m3
(720 ppm) en las hembras. La letalidad parecía asociada
principalmente a los efectos narcóticos, si bien los mamíferos
expuestos a concentraciones de al menos 8,4 g/m3 (2300 ppm)
durante una hora o más mostraron alteraciones patológicas graves,
entre ellas lesiones hepatocelulares, edema pulmonar y hemorragia.
Existen pruebas claras de que el 2-NP es carcinogénico en la
rata. La exposición prolongada de ratas por inhalación de
0,36 g/m3 (100 ppm) durante 18 meses (7 h/día, 5 días/semana)
indujo cambios destructivos en el hígado, inclusive carcinomas
hepatocelulares en algunos machos. Una concentración de 0,75 g/m3
(207 ppm) indujo lesiones más graves, entre ellas una elevada
incidencia de carcinomas hepatocelulares, con más rapidez. La
administración crónica de dosis orales moderadas indujo también un
exceso de carcinomas hepatocelulares en ratas. En cambio, la
inhalación prolongada de 91 o 98,3 mg/m3 (25 ó 27 ppm) no produjo
lesiones detectables en las ratas. La exposición de ratones y
conejos a concentraciones de 2-NP que inducían carcinomas
hepatocelulares en la rata tuvieron escaso efecto o ninguno, pero
esos estudios eran demasiado limitados para descartar por completo
la carcinogenicidad de la sustancia en ambas especies. El 2-NP
retrasó ligeramente el desarrollo fetal en la rata, pero escasean
los datos sobre embriotoxicidad, teratogenicidad y toxicidad
reproductiva. Se observó que el 2-NP era sumamente genotóxico en
hepatocitos de rata tanto in vitro como in vivo, pero no se
observó genotoxicidad significativa en otros órganos de la rata ni
en líneas celulares de origen extrahepático sin activación
metabólica exógena. Se ha demostrado que el 2-NP es mutagénico en
bacterias tanto en presencia como en ausencia de activación
metabólica exógena.
7. Efectos en el ser humano
La exposición humana a concentraciones elevadas de 2-NP es en su
mayor parte o totalmente de origen ocupacional. Las concentraciones
elevadas (se desconocen los valores reales, si bien en un caso se
calcularon en 2184 mg/m3 (600 ppm)) producen toxicidad aguda y
accidentes mortales en la industria. Entre los síntomas iniciales
figuran dolores de cabeza, náuseas, mareos, vómitos, diarrea y
dolores. Las víctimas a menudo mostraban una mejoría temporal,
aunque en algunos casos sobrevino la muerte entre 4 y 26 días
despues de la exposición. El fallo hepático fue la principal causa
de muerte, con edema pulmonar, hemorragias gastrointestinales fallo
respiratorio y renal como factores contribuyentes. La exposición
ocupacional a niveles estimados en 73 a 164 mg/m3 (20 a 45 ppm)
indujo náuseas y pérdida de apetito, que persistieron durante varias
horas tras abandonar el lugar de trabajo, mientras que la exposición
ocupacional a niveles estimados en 36,4 a 109 mg/m3 (10 a 30 ppm)
(< de 4 h/día durante < 3 días/semana) no produjo efectos
nocivos detectables.
Aunque los datos disponibles son insuficientes, nada indica que
la exposición ocupacional crónica al 2-NP en concentraciones
normales en el lugar de trabajo induzca neoplasmas hepáticos o de
otro tipo ni otros efectos adversos a largo plazo.
8. Efectos en otros organismos en el laboratorio y sobre el terreno
Los escasos estudios realizados en microorganismos,
invertebrados y peces indican una baja toxicidad del 2-NP para
organismos no mamíferos.
2.3 Conversion factors
1 ppm 2-NP in air = 3.64 mg/m3
1 mg/m3 = 0.27 ppm 2-NP in air
2.4 Analytical methods
Analytical methods for 2-NP appear limited to the analysis of
air, water, blood plasma, coatings, and cigarette smoke (Table 2).
Since the colorimetric methods are less sensitive or are cumbersome,
the method of choice is probably gas chromatography with either a
flame ionization or an election capture detection. Charcoal is not a
satisfactory adsorbent for 2-NP since recovery is poor (Andersson et
al., 1983) and there may be decomposition (Glaser & Woodfin, 1981).
In addition to Chromosorb 106, Amberlite XAD-7 appears satisfactory
as a solid sorbent for quantitatively collecting 2-NP from the air
(Andersson et al., 1983), although its collection efficiency is
markedly reduced in humid air (Andersson et al., 1984). Use of a
collection tube with two sections, however, can compensate for the
reduced efficiency (Andersson et al., 1984). The high-performance
liquid chromatography method developed for blood (Derks et al.,
1988) could probably be adapted to other biological materials.
Table 2. Analytical techniques for determining 2-nitropropanea
Methods Detection limit Comments Reference
Air
Trapping in ethanol; 0.1 mg/ml ethanol absorption is linear between 0.1 and Treon & Dutra (1952)
concentration determined 2.0 mg/ml; ethanol was especially
spectrophotometrically at purified and redistilled
277.5 nm
Trapping in concentrated ca. 1 µg/ml sulfuric absorption is linear between 1 and 5 µg/ml Jones & Riddick (1952);
sulfuric acid; resulting acid sulfuric acid; no interference from primary Jones (1963)
nitrous acid combined nitroparaffins, but all other secondary,
with resorcinol to form a some tertiary and some halogenated
red-blue colour; measured nitroparaffins interfere
spectro-photometrically
at 560 nm
Trapping in solid sorbent tube 3.6 mg/m3 working range is 3.6 to 36 mg/m3; 2-NP Glaser & Woodfin (1981)
(Chromosorb 106, 60/80 mesh); stable on absorbent for at least 28 days
desorption: ethyl acetate;
separation-detection: GC-FID
Methodology similar to above 3.1 mg/m3 Method is a modification of that proposed US NIOSH (1987a)
by Glaser & Woodfin (1981); range: 3.1 to
28.3 mg/m3; no interference from methyl
butyl ketone, heptane, 1-nitropropane,
toluene, and xylene
Table 2 (contd).
Methods Detection limit Comments Reference
Water
Sample (40 µl) collected ca. 0.5 mg/m3 water elutes quickly extinguishing flame Wilks & Gilbert (1972a)
with a syringe and injected in FID; flame can be reignited before 2-NP
directly into GC; emerges
detection: FID
Blood
Blood collected in chilled, 1 ng UV absorption linear from 0 to 250 ng; Derks et al. (1988)
screw-capped vial with heparin; uses 0.3 ml blood sample; samples are
centrifuged; deproteinized with unstable and must be analysed promptly
acetonitrile; Tris buffer added;
separation: HPLC; detection:
UV at 224 nm
Coating (beverage can)
Redissolve coating in solvent not given reference provides few details on Wilks & Gilbert (1972b)
suitably distinct from 2-NP; methodology
acetone suitable for vinyl
co-polymer coatings;
separation: GC
Table 2 (contd).
Methods Detection limit Comments Reference
Coating (beverage can)
Can (empty) simultaneously not given method captures about 90% of Wilks & Gilbert (1972b)
perforated and fitted with a residual 2-NP
diaphragm; hypodermic needle
inserted through diaphragm and
fitted with stopcock; can heated
(150 °C, 15 min); headspace
sampled with heated syringe;
separation: GC
Cigarette smoke
Steam distillation of smoke 0.8 µg/cigarette may be adapted for air and water Hoffmann & Rathkamp
condensate on filters, analysis (1968)
extraction re-extraction in
NaOH, in ethyl ether,
neutralization with H2SO4 and
re-extraction in ethyl ether,
concentration of extract and
injection in GC equipped with
FID or ECD detectors
a Abbreviations: ECD = electron capture detector; FID = flame ionization detector; GC = gas chromatograph;
HPLC = high-performance liquid chromatograph; UV = ultraviolet
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
There is no evidence that 2-NP and other nitroaliphatic
compounds are produced by biological processes, although a related
organic compound, ß-nitropropionic acid, has been isolated from
plants and microorganisms (Goldwhite, 1965). However, nitroaliphatic
compounds are produced in low concentrations by combustion of
organic matter and have been detected in tobacco smoke. Hoffmann &
Rathkamp (1968) reported 1.1-1.2 µg 2-NP in the smoke from single
85-mm USA blended non-filter cigarettes, and ascribed the production
of this and other nitroaliphatics to interactions in the combustion
zone between hydrocarbons and nitrogen dioxide generated by
decomposition of nitrates.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
Although 2-NP is an important industrial chemical, current world
production figures are not available. In 1977 production by the sole
USA manufacturer was estimated to be 13 600 tonnes, of which 5400
tonnes were sold in the USA and 8200 tonnes were either exported or
used internally (Finklea, 1977). 2-NP currently is produced by two
USA manufacturers, Angus Chemical Co., Sterlington, Louisiana, and
W. R. Grace Co., Deer Park, Texas (SRI International, 1988, 1990;
USITC, 1990), and by one European manufacturer, Société Chimique de
la Grande Paroisse, France (Anon., 1976, 1982; IARC, 1982). In the
USA, 2-NP, together with nitromethane, nitroethane and
1-nitropropane, is manufactured by a vapour phase reaction of nitric
acid with an excess of propane at high temperature and pressure
(370-450 °C, 0.8-1.2 MPa (8-12 atm.)) (Baker & Bollmeier, 1981). The
proportions of the four nitroaliphatic compounds in the reaction
product are a function of the reaction temperature. In Europe,
propane is reacted with nitrogen peroxide (N2O4) and an excess
of oxygen at 150-330 °C and 0.9-1.2 MPa (9-12 atm.), yielding the
same nitroaliphatic compounds in slightly different proportions as
produced by the USA process (Anon., 1976). Reaction products are
condensed, washed, and separated by fractional distillation. There
is no evidence that 2-NP is produced through human EHC 138:
2-Nitropropane activities except by combustion and deliberate
manufacture, although nitromethane has been detected in vehicle
exhaust (Seizinger & Dimitrades, 1972).
3.2.2 Uses
The importance of 2-NP as an industrial chemical stems mainly
from its desirable and occasionally unique characteristics as a
solvent (Purcell, 1967; Anon., 1976; Fishbein, 1981; Baker &
Bollmeier, 1981; IARC, 1982; ACGIH, 1986). It is an excellent
solvent or cosolvent for a variety of fats, waxes, gums, resins,
dyes and other organic compounds, including vinyl, acrylic,
polyamide and epoxy resins, chlorinated rubbers, and organic
cellulose esters. The ability of 2-NP to form an azeotrope with
water and the associated large heat of absorption permit it to
displace monomolecular layers of water molecules and secure a better
bond between pigments and the surfaces to which they are applied.
Its major use is as a solvent for inks, paints, varnishes, adhesives
and other coatings such as beverage container linings. It is used
principally in blends with other solvents to impart desirable
characteristics, such as greater solvency, better flow
characteristics and film integrity, greater pigment dispersion,
increased wetting ability, improved electrostatic spraying
properties, or reduced drying time. 2-NP is also used industrially
as a processing solvent for separating closely related substances in
natural products or reaction mixtures. These have included, for
example, separation of oleic acid from polyunsaturated fatty acids
and cetyl from oleyl alcohols.
In addition to the above, 2-NP has a number of minor uses
(Anon., 1976; Baker & Bollmeier, 1981). These include a medium for
chemical reactions, an intermediate for the manufacture of
2-nitro-2-methyl-1-propanol, 2,2-dinitropropane, 2-amino-2-
methyl-1-propanol and other propane derivatives, and a component of
explosives, propellants, and fuels for internal combustion engines.
The latter usage appears limited to model engines used by hobbyists
and to racing cars. Although the addition of 2-NP to fuel improves
diesel engine performance, it is not used commercially as a diesel
fuel additive since superior alternatives are available (Banes,
1989)a. In the USA, mixed isomers of nitropropane are used to
denature ethanol (US FDA, 1987). The addition of 2-NP to hydrocarbon
mixtures has been shown to inhibit corrosion of tin-plated steel
aerosol cans (Flanner, 1972).
3.3 Release into the environment
There is some quantitative data on releases of 2-NP into the
environment. The US Environmental Protection Agency has supported a
thorough, though largely speculative, analysis of the problem (US
EPA, 1980). Releases of 2-NP occur mainly into the atmosphere and
can result from spillage, from venting of gases and fugitive
emissions during manufacture, transfer and use, and from solvent
evaporation from coated surfaces. The US EPA document estimated that
of the 14 000 tonnes of 2-NP produced in the USA in 1979, 5714
tonnes (41%) was released into the air, and 1 tonne into water. Only
230 tonnes (1.6%) was destroyed by incineration or waste treatment.
The major contributor to this release estimate was evaporation of
2-NP used as a solvent in printing ink and surface coatings (4450
a Personal communication from the US Environmental Protection
Agency, Ann Arbor, Minneapolis
tonnes, 78% of releases). Manufacture of 2-NP is a largely enclosed
process and in 1979 it accounted for only 21 tonnes (0.3%) of the
amount released into the environment. A more recent examination of
this problem (National Library of Medicine, 1989) reported a similar
situation concerning environmental releases of 2-NP in the USA. Out
of a yearly total of 299 tonnes, 205 tonnes (69%) was released into
the air with 123 tonnes coming from point (large, easily identified)
sources and 82 tonnes from non-point (small, not easily identified)
sources. Only 2 tonnes (< 1%) was released directly into water, and
1 tonne into municipal sewage treatment plants. The remainder was
buried in closed containers (76 tonnes, 25%) or was disposed of in
unspecified ways (15 tonnes, 5%).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport in the environment
2-NP appears to be highly mobile in the natural environment.
Cupitt (1980) considered physical removal of 2-NP from the
atmosphere unlikely because it was not soluble enough to be rapidly
washed out and had a vapour pressure too great for strong adsorption
on particles. The partition of 2-NP between air and water at
equilibrium was estimated by the method of Swann et al. (1983) to be
about 0.5% in air and 99.5% in water (US EPA, 1985). These values
indicate rapid and easy exchange between air and water. The soil
sorption coefficient (ratio of soil concentration to water
concentration) and the bioconcentration factor were estimated by the
methods of Kenaga (1980) to be 20 and 2.5, respectively (US EPA,
1985). Measured values for absorption and bioaccumulation were
somewhat greater than these estimates. Freitag et al. (1982, 1985)
obtained concentration factors over water for activated sludge,
unicellular algae (Chlorella fusca), and fish (golden ide) of 70,
20, and < 10, respectively. These values indicate that 2-NP is not
strongly bioaccumulated and is readily desorbed from sediment
particles and leached from soil. Thus, in summary, since 2-NP is
slightly water soluble, slightly adsorbed by sediment, slightly
bioaccumulated, and evaporates readily into the atmosphere, it will
be distributed in both air and water and not accumulated in any
individual environmental compartment.
4.2 Biotic and abiotic transformation
Data concerning the destruction of 2-NP by biotic and abiotic
processes are limited. 2-NP has significant photoabsorption in the
environmentally relevant range of > 290 nm (Sadtler, 1961) and is
likely to undergo photolysis (Cupitt, 1980; US EPA, 1985). On the
basis of physical and chemical properties Cupitt (1980) hypothesized
that it would be rapidly removed from the atmosphere by photolysis
and estimated a reduction in concentration of 1/e (0.369) in 0.2
days. This is equivalent to a half-life of 0.14 days (3.36 h).
However, measurements of photochemical reactivity do not support
such a rapid destruction of 2-NP. Studies aimed at defining the
relationship between organic solvents and photochemical smog
production ranked 2-NP as low to moderate in terms of its
interactions with oxidants and its ability to produce formaldehyde
and other lachrymators (Levy, 1973). Laboratory measurements also
suggested slow decomposition (Freitag et al., 1985). The methodology
employed (Korte et al., 1978; Lotz et al., 1979; Freitag et al.,
1982), i.e. irradiation of the solvent adsorbed on silica gel by
light from a high pressure mercury lamp filtered through pyrex, was
too different from natural conditions to permit quantitative
extrapolation from laboratory results to rates of photolysis in the
atmosphere. The study provided comparative photodecomposition rates
for a large number of solvents. The rate of photodecomposition for
2-NP was roughly half that of dichlorodiphenyltrichloroethane (DDT),
similar to the rates for dodecane and 2,4-dichlorobenzoic acid, and
roughly twice those for kepone and dieldrin. Paszyc (1971) reported
that the major decomposition products for both gaseous and liquid
2-NP under laboratory conditions were nitrogen dioxide, acetone,
isopropyl nitrite, isopropanol, methylcyanide, water, and propane,
regardless of whether irradiation was monochromatic 253.7 nm light
or the full spectrum of light produced by a high pressure mercury
lamp. Cupitt (1980) speculated that the major products of
photodecomposition in nature would be formaldehyde and acetaldehyde.
Biological decomposition of 2-NP appears likely, but is probably
rather slow in nature. Enzymes capable of oxidizing or initiating
non-enzymatic oxidation of 2-NP have been identified in horseradish
(De Rycker & Halliwell, 1978; Porter & Bright, 1983; Indig &
Cilento, 1987), pea seedlings (Little, 1957), and a variety of
microorganisms including bacteria, yeasts, and fungi (Little, 1951;
Kido et al., 1975; Soda et al., 1977; Dhawale & Hornemann, 1979;
Patel et al., 1982). In in vitro preparations of horseradish
(Dhawale & Hornemann, 1979), pea seedlings (Little, 1957), a fungus
(Streptomyces achromogenes) (Dhawale & Hornemann, 1979), and a
yeast (Hansenula mrakii) (Kido et al., 1975), 2-NP was converted
to a less toxic compound, acetone, and a moderately toxic compound,
nitrite. In addition to nitrite, some nitrate may also be formed
(Indig & Cilento, 1987). In the yeast, nitrite was subsequently
reduced to ammonia. The importance of these processes in nature is
unknown. Kido et al. (1975), however, reported that only 4 out of 14
species of microorganisms tested would grow in a medium containing
5 g 2-NP/litre. The only study of 2-NP decomposition by a population
of microorganisms (Freitag et al., 1985) utilized activated sludge
grown at 25 °C. Solvent concentrations in these experiments were low
(50 µg/litre) to prevent adaptation to the substances tested (Korte
et al., 1978). In 5 days only 0.4% of the 2-NP was converted to
carbon dioxide. In summary, these data suggest that in both
terrestrial and aquatic communities 2-NP is biologically decomposed
and that the rate may be slow, but they offer no definite
information on the problem.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels and general population exposure
General population exposure to 2-NP appears to be very low.
There seem to be no records of its occurrence in water or in outdoor
air away from areas of manufacture and use. The only information on
intake exists in the form of a memorandum from Modderman (1983)a.
The daily intake per person in the USA was estimated to be 50 to
100 mg. The residuum from its use as a solvent for beverage can
coatings, film laminating adhesives and printing inks for flexible
food packaging may account for as much as 37 ng/day and from
vegetable oils fractionated with 2-NP, 30 ng/day. 2-NP residues of
0.077 mg/litre (77 ppb) to 0.24 mg/litre (204 ppb) have been found
in such oils. In a further report on the evaluation of
2-nitropropane as a food processing solvent, it was assumed that
residues of less than 10 µg/kg would occur in oils, giving rise to
estimated daily intakes of 10 ng/day. The use of 2-NP as a food
processing solvent was not recommended (FAO/WHO, 1990a,b). As
mentioned in section 3.1, smokers are exposed regularly to low
concentrations of 2-NP. Hoffmann & Rathkamp (1968) reported 1.1 to
1.2 µg in the smoke of a single cigarette. 2-NP was reported to
occur in the expired air of 11.1% (5 of 54 individuals) of a sample
of healthy adult urban dwellers (Krotoszynski et al., 1979). The
geometric mean was 0.406 ng/litre with one-sigma limits of
0.119 ng/litre and 1.38 ng/litre, (at 25 °C, 98 MPa (760 mmHg)). The
sample was entirely of non-smokers who had avoided medication and
prolonged exposure to perfume, paint, glue, aerosols, dust, tobacco
smoke and areas polluted with industrial wastes during, and for at
least 7 days prior to, the sampling period, and also avoided
cosmetics, spices, seasonings, and alcoholic beverages during and
immediately prior to sampling. The origin of the exhaled 2-NP is
unclear. Exposure to 2-NP may be further reduced in the future since
a number of regulations have been enacted and recommendations made
to declare it a harmful, carcinogenic substance and a toxic waste,
and to discourage its use (IARC, 1982; IRPTC, 1986; US FDA, 1987; US
NIOSH, 1988).
a Memorandum from Modderman, J.P. to Shibko, S., Associate
Director for Regulatory Evaluation, Department of Health & Human
Services, USA, 8 pp. "Exposure estimates for chemicals to be
included in the NTP annual report on carcinogens".
5.2 Potential occupational exposure
The number of workers in the USA who handle 2-NP or mixtures
containing 2-NP has been variously estimated as 15 000 (US EPA,
1977), 38 600 (Occupational Health Services, Inc., 1982), 100 000
(Finklea, 1977), and 185 000 (Beall et al., 1980). Based on
employment values of the US Bureau of the Census (1987) these
estimates of exposed workers represent from 0.02% to 0.19% of the
civilian workforce in the USA. The low estimate of 15 000, although
quoted in a US Environmental Protection Agency report, originated
with a manufacturer of 2-NP. The estimate generated by Occupational
Health Services, Inc., carried out under contract with a
manufacturer of 2-NP, was based on a detailed survey of
distributors, manufacturers, and users, and thus may represent a
reasonable approximation. This report considered 38 600 to be the
best estimate of the total number of exposed workers in the USA and
set 126 600 workers as an upper boundary. It further estimated that
significant exposure (defined as exposure to at least 10% of the US
OSHA exposure limit or 9.1 mg/m3 (2.5 ppm)) ranged from 4000 (best
estimate) to 10 600 (upper boundary) workers. Sources of worker
exposure identified in a survey conducted in the USA by the National
Institute for Occupational Safety and Health (Finklea, 1977)
included rotogravure and flexographic inks used in printing, and
coatings and adhesives used in industrial construction and
maintenance, highway marking, ship building and maintenance,
furniture manufacture, and food packaging. A US NIOSH survey
estimated that 9815 workers in the USA were exposed to 2-NP or to
trade-name products containing 2-NP (US NIOSH, 1983).
Occupational exposure limits are summarized in Table 3.
There is little data on actual occupational exposure, although
the limited information on conditions in the USA summarized in
Table 4 suggests that it is highly variable. Manufacture of 2-NP, an
enclosed process, appears to involve little employee exposure much
of the time, but spills and operations such as filling drums can
briefly expose a few workers to high concentrations. In general, low
exposures may be typical in some painting operations and in the
manufacture of tyres, but other painting and manufacturing
operations appear, at least in the past, to have exposed workers to
dangerously high concentrations. Workers were exposed to
concentrations of 2-NP up to at least 2744 mg/m3 (754 pm) in a
pigment production facility and up to at least 265 mg/m3 (73 ppm)
in a solvent extraction plant. Evidence exists that concentrations
of 2-NP at the solvent extraction plant prior to the investigation
were at times substantially greater than the values measured during
the investigation (Crawford et al., 1985). Exposure levels mentioned
in the report by Occupational Health Services, Inc. (1982) were
mainly in the vicinity of 3.6 mg/m3 (1 ppm), although one printing
Table 3. Occupational exposure limits for 2-nitropropane in aira
Exposure limit
Country mg/m3 ppm Category of limit
Australia 36 10 TWA
Belgium 36 10 TWA
Brazil 70 20 AL
Canada 90 25 CLV
Denmark 36 10 TWA
Finland 18 5 TWA
150 40 STEL
Germany 18 5 1-year TWA(TRK)
Hungary 10 3 CLV
Netherlands 3.6 1 8-h TWA
7.3 2 STEL
Poland 30 8 TWA
70 20 STEL
Romania 47 13 TWA
70 20 STEL
Sweden 18 5 TWA
36 10 CLV
Switzerland 18 5 TWA
United Kingdom 90 25 8-h TWA
USA 90 25 8-h TWA (OSHA)
36 10 8-h TWA (ACGIH)
Yugoslavia 90 25 TWA
a From: IRPTC (1986)
ACGIH = American Conference of Governmental Industrial Hygienists
AL = Acceptable or tolerable limit
CLV = Ceiling value
MAK = Maximum worksite concentration
OSHA = Occupational Safety and Health Administration
STEL = Short-term exposure limit
TLV = Threshold limit value
TWA = Time-weighted average (MAK in Switzerland)
TRK = Technical guiding concentration
plant (Table 4) reported a peak concentration of 237 mg/m3
(65 ppm) which lasted 30 min, and a time-weighted average of
36-44 mg/m3 (10-12 ppm). In addition to inhalation, it is likely
that workers using 2-NP as a solvent will have at least occasional
contact with the liquid. 2-NP also has been reported to be a minor
component of both fresh and used machine cutting fluid emulsion
(Yasuhara et al., 1986). The importance of exposure to 2-NP as a
contaminant of 1-nitropropane is unknown. In an investigation
involving 1-nitropropane-sensitized ammonium nitrate blasting agents
(Cocalis, 1982), 2-NP was below the detectable limit. Occupational
exposure may be reduced in the future since strong recommendations
have been issued on minimizing all worker contact with 2-NP and its
fumes and on substituting other less toxic solvents where possible
due to the carcinogenicity of 2-NP (IRPTC, 1986; US EPA, 1986; US
NIOSH 1988).
Table 4. Air concentration of 2-NP in the work place
Concentration
Activity mg/m3 ppm Reference
Manufacture of 2-NP 3.64 approx 1 Brown & Dobbin (1977)
Manufacture of 2-NP 0.7-364; 0.2-100; Miller & Temple (1979)
98% of 98% of
samples samples
were < 36.4 were < 10
Vulcanizing tyres 0-0.18 < 0.05 Hollett & Schloemer
(1978)
Painting (bus 0.11 < 0.03a Love & Kern (1981)
maintenance)
Painting (railway 1.46 < 0.4 Hartle (1980)
cars)
Solvent extraction 167.4 0-100 Crawford et al. (1985)
(average
46)
Laboratory (1958) 14.6 0-21 Angus Chemical Co. &
(average 4) Occusafe, Inc. (1986)
2-NP storage & 2111-6000 580-1640 Angus Chemical Co. &
transfer area (1962); Occusafe, Inc. (1986)
drum filling
operation
Pigment production 109-2745 30-754 Angus Chemical Co. &
facility (1970) Occusafe, Inc. (1986)
Painting (battery 36.4-109 10-30 Skinner (1947)
cases)
Manufacturing 72.8-164 20-45 Skinner (1947)
(coating forms)
Printing approx 40 1-65 Occupational Health
(approx 11) Services, Inc. (1982)
a below limit of detection
6. KINETICS AND METABOLISM
6.1 Absorption
2-NP is absorbed via the lungs, the peritoneal cavity, the
gastrointestinal tract, and possibly, to a lesser extent, via the
skin. Absorption via the lungs, peritoneal cavity, and
gastrointestinal tract have been used in experimental studies and
have been investigated using rats and 14C-labelled 2-NP. Pulmonary
absorption was examined by Nolan et al. (1982), Müller et al.
(1983), and Filser & Baumann (1988). Nolan et al. (1982) considered,
on the basis of respiratory rate and tidal volume of the rat and the
accumulation of 2-NP during the 6-h period of exposure, that a
minimum of 40% of the inhaled 2-NP was absorbed. This value is
minimal since it does not include 2-NP metabolized and eliminated
during exposure. The data of Müller et al. (1983) suggested that
immediately following exposure to 728 mg/m3 (200 ppm) for 3 h,
plasma contained approximately 0.4% as 2-NP and 7.2% as metabolites
of the 2-NP inhaled. The metabolites were mainly acetone but also
included a small amount of isopropanol. These percentages were
estimated from the results of Müller et al. (1983) and normative
data for the laboratory rat (Baker et al., 1979), and support rapid
pulmonary uptake of 2-NP since 2-NP and its metabolites sequestered
elsewhere in the body and loss of metabolized 2-NP during exposure
were not considered. Filser & Baumann (1988) reported that uptake of
gaseous 2-NP was rapid, the clearance rate being equal to the
ventilation rate. The value they cite for the latter, 32
litres.h-1.kg-1), seems large in comparison with normative data
for the laboratory rat (Baker et al., 1979).
The rate of 2-NP uptake by rats from intraperitoneal injection
was examined by Müller et al. (1983). Ten minutes after an injection
of 25 mg/kg, the blood plasma contained 3.3% of the dose as 2-NP and
1.9% as metabolites, acetone, and isopropanol, indicating an uptake
of greater than 5.2% since presumably some of the dose was already
lost from the body and to other tissues in the body during this
initial period. These percentages were estimated from the data of
Müller et al. (1983) and normative data for the laboratory rat
(Baker et al., 1979). A dose of 50 mg/kg yielded partially
dissimilar results. The 10-min average value was low and also had a
very large standard deviation. This may have reflected large
differences among the rats in their initial uptake rates for 2-NP or
an experimental problem such as injection into the gastrointestinal
tract rather than the peritoneal cavity. Blood plasma contained, 10
min after injection, only 1.4% of the dose as 2-NP and 1.3% as
acetone and isopropanol. These data indicate that uptake from the
peritoneal cavity is fairly rapid, and suggest that the relatively
slower uptake of the 50-mg/kg dose may have reflected saturation of
the uptake mechanisms, since initial concentrations in the plasma
did not exceed those following the 25-mg/kg dose. Intraperitoneal
injections were used by Andrae et al. (1988), Guo et
al. (1990), Hussain et al. (1990), and Conaway et al. (1991) to
demonstrate that 2-NP induced nucleic acid damage in the livers of
Wistar, F-344 and Sprague-Dawley rats.
Absorption of 2-NP via the gastrointestinal tract was
investigated with male Wistar rats by Derks et al. (1989). They
found that the systemic availability of orally administered 2-NP
from a water solution was very high (90%) and absorption was rapid,
maximum plasma values being reached within 15 min after dosage.
Absorption of 2-NP given in olive oil was much slower and
availability was only 34% during the initial 3 h following dosage.
The authors suggested that absorption from oil was incomplete at 3 h
and might ultimately be much higher, since the olive oil was
absorbed and the 2-NP redistributed between the oil and aqueous
phases.
Although workers are cautioned against dermal contact with 2-NP
(Beall et al., 1980; US EPA, 1986), there appear to be no
quantitative data on dermal absorption. The solubility of 2-NP in
both polar and non-polar solvents, together with its small molecular
size, suggests that it should be absorbed readily through the skin
(Malkinson & Gehlmann, 1977). Dermal application of 2 g 2-NP/kg to
rabbits produced no obvious symptoms (Wilbur & Parekh, 1982);
however, as noted below, the rabbit is relatively resistant to the
toxicity of 2-NP.
6.2 Distribution
The distribution of 2-NP and its carbon-containing metabolites
among the organs and tissues in Sprague-Dawley rats was examined by
Nolan et al. (1982) via inhalation, and by Müller et al. (1983) via
intraperitoneal injection. Both utilized 14C-labelled 2-NP and
thus their data do not reveal the distribution of nitrite and other
nitrogen-containing metabolites generated from the nitro portion of
the 2-NP molecule. One hour after intraperitoneal injection,
radioactivity was concentrated in fat; there were intermediate
amounts in the blood, liver, and kidney, and lower amounts in other
organs and tissues (Müller et al., 1983). By 40 h, the highest
concentrations were in bone marrow and adrenal tissue, intermediate
amounts being found in the kidney, liver, spleen, lungs, and omental
fat, and by 8 days only the concentration of 14C in adrenal tissue
was noticeably greater than elsewhere in the body. However, Nolan et
al. (1982), found, both immediately and 48 h after a 6-h period of
2-NP inhalation, that highest concentrations of carbon from 2-NP
were in the liver and kidney and relatively little in the fat.
Differences in methodology limit intercomparison of these
studies. Their major consistency is the presence of high
concentrations of 2-NP and its labelled carbon in the liver and
kidney, organs (as discussed below) actively involved in the
metabolism of 2-NP and excretion of its metabolites.
The relevance of these studies on tissue distribution of 2-NP
and its carbon-containing metabolites to the toxicity of 2-NP is
unclear, since (as discussed below) most of the dose is rapidly
metabolized initially to acetone and nitrite. The 14C label used
in these studies thus traced mainly the distribution of acetone and
its metabolites in measurements made more than a few hours after
dosing.
Dequidt et al. (1972) provided limited data on the distribution
of nitrite among body organs of the rat following inhalation and
intraperitoneal injection of 2-NP. The data suggest a fairly uniform
distribution among the heart, lungs, kidney, spleen, and,
sometimes, the liver. In the majority of experiments, however, no
nitrite was detected in the liver. No explanation is offered for the
latter observation, and data in the paper are so erratic as to
suggest the possibility of analytical problems.
6.3 Metabolic transformation
Starting with a report by Scott (1943), there have been numerous
studies on the metabolic transformation of 2-NP by mammals,
mammalian cells, microorganisms, and isolated enzymes. These studies
have shown that the major pathway for metabolic transformation of
2-NP involves oxidation to nitrite and acetone. Evidence for the
formation both of nitrite and acetone was reported from studies on
liver microsomes from rats pretreated with phenobarbital or
3-methylcholanthrene (Ullrich et al., 1978), cultured hepatocytes
from untreated rats (Haas-Jobelius et al., 1991), liver microsomes
from untreated mice (Marker & Kulkarni 1986a,b; Dayal et al., 1991),
V79 Chinese hamster cells (Haas-Jobelius et al., 1991), and a yeast
(Kido et al., 1975). Nitrite was reported to be a major metabolite
of 2-NP in rabbits (Scott, 1943), rats (Dequidt et al., 1972), and
liver microsomes from rats (Sakurai et al., 1980) and mice (Marker &
Kulkarni, 1985). Acetone was identified as a major metabolite of
2-NP in rats and chimpanzees (Muller et al., 1983).
Enzymatic oxidation of the nitronate form of 2-NP to nitrite and
acetone by horseradish peroxidase (Porter & Bright, 1983), a
dioxygenase from the yeast Hansenula mrakii (Kido et al., 1984),
and mouse liver microsomes (Dayal et al., 1991) was several times
more rapid than that of 2-NP under identical conditions. In addition
to acetone, a smaller amount of isopropanol is produced at least in
rats and chimpanzees (Muller et al., 1983). The source of the
isopropanol was not specified in this study, but since reduction of
acetone in the body is negligible (De Bruin, 1976), isopropanol may
be formed directly by oxidation of 2-NP. The formation of a
hydroxyisopropyl radical during the oxidation of 2-NP was suggested
by Kuo & Fridovich (1986).
The metabolic fates of these metabolites of 2-NP are well known.
Acetone is produced by a minor metabolic pathway in the mammalian
body (Smith et al., 1983) and has been detected in small amounts in
the blood, urine, and expired air of normal humans (Mabuchi, 1979;
Conkle et al., 1975). It may be excreted directly via expired air,
urine, and loss through the skin, or may enter into the general
metabolism either via cleavage to a 2-carbon acetyl fragment and a
1-carbon formyl fragment or via oxidation to pyruvic acid (De Bruin,
1976). The proportion excreted unchanged increases with increasing
dosage, suggesting an easily saturable metabolic pathway.
Isopropanol is oxidized to acetone (De Bruin, 1976).
Nitrite may exist as a minor constituent of the mammalian body.
It is constantly replenished by ingestion and synthesis, and
constantly removed by oxidation to nitrate. Nitrite and nitrate in
the blood stream are rapidly and homogeneously distributed
throughout the body (Parks et al., 1981). Nitrite rapidly oxidizes
divalent ferrous haemoglobin to trivalent ferric methaemoglobin
(Burrows, 1979). Little is transported to the tissues or excreted,
at least in dogs, sheep, and ponies (Schneider & Yeary, 1975).
Dequidt et al. (1972), however, reported substantial urinary
excretion of nitrite by rats following inhalation or intravenous
injection of 2-NP. Methaemoglobin is incapable of transporting
oxygen and, during enzymatic repair of this defect, nitrite is
reoxidized to nitrate. Parks et al. (1981) reported that 10 min
after intratracheal instillation of labelled nitrite into mice, 70%
of the label in plasma was in nitrate, 3% in nonionic compounds, and
only 27% remained as nitrite. Similar results were obtained with
rabbits. Nitrate is slowly excreted through the kidneys (Schneider &
Yeary, 1975) and also into saliva where it is reduced back to
nitrite by bacteria and reabsorbed into the body via the
gastrointestinal tract (Friedman et al., 1972). Small amounts of
nitrite in the stomach may react with secondary amines and other
amino substrates to form N-nitroso compounds which might be absorbed
(Sander & Schweinsberg, 1972; Fine et al., 1982).
The enzymatic system oxidizing 2-NP to acetone and nitrite was
identified through in vitro experiments using microsomes isolated
from mammalian liver. Ullrich & Schnabel (1973) determined that
cytochrome P-450, in liver microsomes from phenobarbital-pretreated
rats, bound 2-NP. Ullrich et al. (1978) subsequently reported that
liver microsomes from rats pretreated with phenobarbital or
3-methylcholanthrene rapidly catalysed the oxidation of 2-NP to
acetone and nitrite. The latter were produced in roughly equal
quantities. Surprisingly, however, the rate of this reaction was not
diminished under conditions of reduced oxygen pressure. The activity
of preparations from untreated control rats was generally very low.
Sakurai et al. (1980) demonstrated that this enzyme system in rats
was active in metabolizing other aliphatic nitro compounds. Marker &
Kulkarni (1985, 1986a, 1986b), working with mice, obtained somewhat
different results. They reported rapid denitrification of
2-NP to nitrite and acetone by liver microsomes from untreated mice,
and an acetone production at least twice the nitrite release. These
authors suggested that multiple forms of cytochrome P-450 are
involved, and claimed that nitrite is sequestered in the reaction
mixture and that denitrification of 2-NP may involve a reductive or
at least non-oxidative pathway as well as an oxidative pathway. They
also noted large differences in the rates of hepatic microsomal
enzymatic nitrite release among the five strains of mice tested.
Jonsson et al. (1977) demonstrated that hepatic microsomes from
uninduced rabbits could denitrify a compound related to 2-NP,
2-nitro-1-phenylpropane.
In addition to oxidative denitrification, a reductive pathway
has been shown to occur in cultured hepatocytes from Wistar rats and
in V79 Chinese hamster cells. Nitroreduction was indicated by the
fact that the cells formed acetone oxime, the tautomeric form of
nitrosopropane (Haas-Jobelius et al., 1991).
Evidence for the involvement of more than one pathway for the
metabolism of 2-NP in the rat was also obtained by Denk et al.
(1989). Their experiments on the pharmacokinetics of 2-NP in rats
exposed by inhalation suggested that there are two different
pathways both in male and female animals, a saturable one of low
capacity and high affinity according to Michaelis-Menten kinetics
and a non-saturable one following first-order kinetics. First-order
kinetics was similar in the two sexes, but striking differences
between sexes were observed in the kinetics of the saturable
pathway. The authors showed that in females more 2-NP was
metabolized by the non-saturable pathway at concentrations above 655
mg/m3 (180 ppm), and in males at concentrations above 218 mg/m3
(60 ppm), and linked their observations to the reported higher
susceptibility to liver damage of males as compared to females
(Griffin & Coulston, 1983). Denk et al. (1989) suggested that it is
the first-order metabolic process which results in the formation of
toxic products whereas the saturable pathway was suggested to lead
to less toxic metabolites.
These observations on the hepatic metabolism of 2-NP and related
compounds by rats, mice, and rabbits indicate differences among
species and even strains. It is probable that more than one enzyme
system is involved. In mice as well as rats hepatic cytochrome P-450
may be important in the metabolism of this xenobiotic.
Observations by Ivanetich et al. (1978) suggested an additional
detoxifying role for hepatic microsomal cytochrome P-450. They
demonstrated that under aerobic conditions in vitro 2-NP could
degrade the haem moiety of cytochrome P-450 in phenobarbital-induced
rats and speculated that this provided an additional mechanism for
trapping reactive metabolites before these could damage essential
cellular constituents.
In addition to the hepatic enzymatic systems examined in rats
and mice, Mochizuki et al. (1988), as mentioned above, described a
2-NP denitrifying system in adrenal microsomes of uninduced
guinea-pigs. They identified this cytochrome-P-450-dependent
monooxygenase as benzo[ a]pyrene hydroxylase.
6.4 Elimination and excretion
Elimination of 2-NP and its metabolites has been examined mainly
in rats and, to a much lesser extent, in chimpanzees in studies
which utilized measurements of radioactivity from 14C-labelled
2-NP as well as measurements of 2-NP and its metabolites. Dosage by
inhalation, intravenous injection, and intraperitoneal injection all
yielded fairly similar results. During a 48-h period after a 6-h
exposure of rats to 73 mg/m3 (20 ppm) and to 560.6 mg/m3
(154 ppm) of 14C-labelled 2-NP, about 50% of the radioactivity in
the absorbed dose was excreted via the lungs as carbon dioxide
(Nolan et al., 1982). The proportion of the absorbed dose excreted
via the lungs as unchanged 2-NP was 4% at the low dose level and 22%
at the high level. Still less of the labelled carbon was eliminated
via faeces and urine, i.e. 11% and 8%, respectively, at the low dose
level, and 5% and 11%, respectively, at the high level.
Disappearance of 2-NP from the blood after exposure at the high dose
level followed a first-order relationship and yielded a half-life of
48 min. Limited data in Müller et al. (1983) yielded a half-life for
rats of approximately 80 min for 2-NP in plasma following a 3-h
exposure to a concentration of 728 mg/m3 (200 ppm). Nolan et al.
(1982), however, found that disappearance of the 14C label of the
2-NP from the plasma was markedly slower and biphasic. During the
first 12 h following exposure to 560.6 mg/m3 (154 ppm), the
half-life for plasma radioactivity was 172 min and, following
exposure to 73 mg/m3 (20 ppm), 354 min. After 12 h, loss of
radioactivity from plasma was much slower, the half-life being
approximtely 35-36 h for both doses. These data on loss of 2-NP and
its 14C label indicate that 2-NP is rapidly eliminated from the
body mainly by metabolic transformation and to a lesser degree by
pulmonary excretion of the unchanged compound. The major
carbon-containing metabolites of 2-NP, acetone and isopropanol,
presumably enter into the general metabolism of the body and are
eliminated via the intermediary metabolism as part of a much larger
carbon pool.
Pulmonary excretion of 2-NP, like loss of the 14C label from
plasma, is dose dependent, biphasic, and follows first-order
kinetics (Nolan et al., 1982). Fifty times more 2-NP was exhaled
during the first hour following exposure to 560.6 mg/m3 (154 ppm)
than during the first hour following exposure to 73 mg/m3
(20 ppm). Following exposure to 73 mg/m3, 2-NP was excreted for
the first 7 h at a rate which decreased by one half every 64 min,
and subsequently decreased by one half every 16 h, whereas following
exposure to 560.6 mg/m3, the half-times of excretion were 71 min
for the first 12 h, and 16 h for the subsequent period. Changes in
the rates of pulmonary excretion of 14C-labelled carbon dioxide
were similar for 48 h following exposure to 73 and 560.6 mg/m3.
Eighty seven per cent of the total was eliminated during the first
12 h after exposure; loss was somewhat less rapid thereafter. The
dose-dependent nature of pulmonary excretion of 2-NP suggests that
greater concentrations of 2-NP in the blood markedly increase
exhalation of the unchanged compound and reduce the percentage
metabolized. Thus exposure of the tissues to 2-NP and its
metabolites may not be a linear function of the inhaled dose.
Derks et al. (1989) found that the plasma half-life of
intravenous doses of 0.01-0.05 g/kg in rats was 45 min during the
first 4 h. Loss was linear over this dose range and could be
described by an open single-compartment model. They suggested that
the measured loss from plasma may be due in part to spontaneous
conversion of 2-NP to its anionic form, 2-NP nitronate.
Elimination of 2-NP by Sprague-Dawley rats following
intraperitoneal injection of 14C-labelled 2-NP was studied by
Müller et al. (1983) and was generally similar to the elimination of
2-NP following inhalation. The concentration of 2-NP in plasma
declined exponentially with time, with half-lives of 70 and 125 min
during at least the initial 6 h following injections of 25 and
50 mg/kg, respectively. Metabolites of 2-NP, acetone and
isopropanol, reached maxima 2-4 h after injection of 25 mg/kg, and
at least 4-6 h after injection of 50 mg/kg. The concentration of
isopropanol ranged from 1/16 to 1/34 the concentration of acetone.
During the initial 40 h after injection of 50 mg/kg, 4.5% of the
dose was exhaled as 2-NP, 10.4% as acetone, and 38.1% as carbon
dioxide. Losses via urine (5.9%) and faeces (0.7%) were small in
comparison with the 53% loss via exhalation. Müller et al. (1983),
however, reported that only 12% of the dose was recovered from the
carcasses, leaving a large amount (28.4%) unaccounted for and thus
casting some doubt on their values.
With one exception, results similar to the above were obtained
following intravenous injection of 14C-labelled 2-NP (10 mg/kg)
into male chimpanzees (Müller et al., 1983). The concentration of
2-NP in plasma declined exponentially with time, the half-life being
92 min. The concentration of acetone reached a maximum 6 h after
injection and remained high for at least 48 h. The concentration of
isopropanol peaked 3 h after injection when it approached that of
acetone, but otherwise was a third to a quarter that of acetone. The
concentration of 2-NP and its carbon-containing metabolites (i.e.
the concentration of 14C) in plasma declined exponentially with a
half-life of 5.5 h for the initial 10 h, and a half-life of 48 h
thereafter. As in rats, exhalation was the major means of
elimination of 2-NP and its carbon-containing metabolites. During
the first 3 days after injection, only 5-6% of the 14C in the dose
was recovered in urine and only 0.4-0.5% in faeces. Acetone,
isopropanol, and 2-NP were mainly eliminated via renal excretion;
urine collected during 6 to 24 h after injection contained 3.1 mg
acetone/litre, 7.2 mg isopropanol/litre, and 1.8 mg 2-NP/litre.
Isopropanol may thus be maintained at low concentrations in the
plasma both by oxidation to acetone and by rapid excretion. In
addition to acetone, isopropanol, and 2-NP, 14C was excreted as an
unidentified polar metabolite which by 24 h after injection
contained 90% of the radioactivity in the urine. The one striking
difference between results with rats and with chimpanzees, i.e. the
relatively much higher plasma concentrations of isopropanol in
comparison to acetone, might indicate interspecific variation in the
excretion of 2-NP and its metabolites.
Dequidt et al. (1972) provided limited information on the
excretion of nitrite following inhalation and intraperitoneal
injection of 2-NP. Rats weighing approximately 250 g each were given
a daily injection of 0.11 g/kg. Urinary excretion of nitrite was 10
to 35 µg/animal during the first day following the initial
injection, and reached a daily rate of 11 mg/animal by the fourth
injection. The latter rate of excretion represents three quarters of
the nitrogen injected daily as 2-NP, and stands in sharp contrast to
the results of Schneider & Yeary (1975), who reported that little
intravenously injected nitrite was excreted by dogs, sheep or
ponies. Following exposure of rats to a 2-NP concentration of
2766 mg/m3 (760 ppm) for 8 h on each of two successive days, daily
elimination of nitrite was approximately 30 mg/animal. This was
equivalent to about 20% of the 2-NP inhaled daily and possibly as
much as 50% of the absorbed daily dose. The amount of 2-NP inhaled
was estimated from normative data for the rat (Baker et al., 1979),
and absorption was assumed to be 40% of the amount inhaled (Müller
et al., 1983). No nitrite was detected in urine during exposure of
rats to 291 mg/m3 (80 ppm) for 8 h per day on 5 successive days.
6.5 Retention and turnover
There is no evidence that 2-NP is retained for more than a few
hours in the body. It is rapidly lost by exhalation and metabolic
transformation. The known carbon-containing metabolites, acetone and
isopropanol, are excreted rapidly and are also transformed into
compounds which are normal to the body and enter into its general
intermediary metabolism. There is less information on nitrite, the
major metabolite of the nitro moiety. In rats much of the nitrite is
excreted as such in the urine. There is no evidence for excessive
accumulation of 2-NP or its metabolites in any organ or tissue.
Information is also lacking on possible N-nitroso and other toxic
compounds synthesized from nitrite or the nitro moiety.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Data on single exposures to experimental animals, summarized in
Table 5, indicate substantial differences in sensitivity among the
species tested. The route of administration was mainly via
inhalation, but other routes were also used. A quantitative
comparison of the results from these studies is difficult due to a
lack of information on both strain and sex of the animals used, to
differences in the route of administration, and to large variations
in dosage. The LC50 for mortality within 14 days following a 6-h
exposure was estimated to be 1456 mg/m3 (400 ppm) for male rats
and 2621 mg/m3 (720 ppm) for females (Baldwin & Williams, 1977).
Exposure of rats (of unspecified sex) via inhalation to 14 058
mg/m3 (3862 ppm) for 1 h or to 9584 mg/m3 (2633 ppm) for 2.25 h
killed some of the animals within days. Exposure of rats to a much
higher concentration of 2-NP, i.e. 53 508 mg/m3 (14 700 ppm),
killed all animals within 4 h. An inhalation exposure of 4994
mg/m3 (1372 ppm) or less for 2.25 h was not lethal to rats. Unlike
rats, LC50 values were similar, i.e. 2031 and 2038 mg/m3
(558 and 560 ppm), respectively, for male and female mice following
a 6-h exposure (Baldwin & Williams, 1977). Cats appeared more
sensitive to acute exposure to 2-NP than rats. Exposure to
8565 mg/m3 (2353 ppm) for 1 h (or lower concentrations for
proportionately longer) was lethal to some cats. Rabbits and
guinea-pigs, on the other hand, appeared far less sensitive to 2-NP
than rats. Rabbits survived a 2.25-h exposure to 9584 mg/m3
(2633 ppm) and guinea-pigs a 2.25-h exposure to 15 699 mg/m3
(4313 ppm). The sequence in acute sensitivity of animals to inhaled
2-NP (from most to least) was cat, rat and mouse, rabbit, and
guinea-pig. The cat was almost an order of magnitude more sensitive
than the guinea-pig.
There are limited data on lethality via routes of administration
other than inhalation. Large intraperitoneal doses were found to be
promptly lethal to rats; 1.7 and 1.1 g/kg killed animals within 2 h
and 4 h, respectively (Dequidt et al., 1972). The 14-day oral LD50
for mice was 0.40 g/kg. The minimal oral lethal dose for the rabbit,
0.50-0.75 g/kg, was much larger than the estimated minimum lethal
dose via inhalation, 0.24 g/kg. Dermal application of 2 g/kg to
rabbits produced no obvious local or systemic effects.
Table 5. Effects of single exposure to 2-nitropropane in mammalsa
a) Lethality
Species Sex Concentration Estimated total Effects/results Reference
doseb (g/kg)
g/m3 ppm
Oral administration
Rabbitcd lethal dose estimated as 0.50- Machle et al. (1940)
0.75 g/kg
Mouse M/F 14-day LD50: 0.40 g/kg Hite & Skeggs (1979)
Inhalation
Ratc (Wistar) 53.5 14 700 1.51 all animals died within 4 h Dequidt et al (1972)
Ratcd 5.5-14.1 1513-3865 0.10-0.17 death of some animalsc Treon & Dutra (1952)
2.6-8-57d 714-2353 0.06-0.08 no deathsd Treon & Dutra (1952)
Ratcd (Wistar) 2.77 760 0.16 death within 48 h; Dequidtet al. (1972)
Rate (CD) M 2.93 805 0.16 8/10 died within 14 days Baldwin & Williams (1977)f
F 2.19 602 0.13 no deaths within 14 dayse Baldwin & Williams (1977)f
M 2.10 574 0.14 8/8 died within 14 days Baldwin & Williams (1977)f
M 1.68 461 0.10 7/8 died within 14 days Baldwin & Williams (1977)f
Table 5 (contd)
Species Sex Concentration Estimated total Effects/results Reference
doseb (g/kg)
g/m3 ppm
Inhalation (contd)
Rate (CD) M 1.47 405 0.08 5/8 died within 14 days Baldwin & Williams (1977)f
1.34 367 0.08 no deaths within 14 days Baldwin & Williams (1977)f
Mousee (ICR) F 2.70 740 0.47 14/14 died within 14 days Baldwin & Williams (1977)f
2.33 640 0.41 11/14 died within 14 days Baldwin & Williams (1977)f
1.8 495 0.32 2/14 died within 14 days Baldwin & Williams (1977)f
Mouse (ICR) M 2.69 738 0.41 9/14 died within 14 days Baldwin & Williams (1977)f
2.08 558 0.28 7/14 died within 14 days Baldwin & Williams (1977)f
1.65 454 0.23 no deaths within 14 days Baldwin & Williams (1977)f
Catcd 2.6-8.56 714-2353 0.07-0.19 death of some animals Treon & Dutra (1952)
1.19-2.87 328-787 0.02 no deaths Treon & Dutra (1952)
Guinea-pigcd 16.8-35.0 4622-9607 0.53-0.63 death of some animals Treon & Dutra (1952)
8.67-34.7 2381-9523 0.23-0.32 no deaths Treon & Dutra (1952)
Rabbitc 8.67-34.7 2381-9523 0.24-0.27 death of some animals Treon & Dutra (1952)
5.1-14.1 1401-3865 0.10-0.16 no deaths Treon & Dutra (1952)
Table 5 (contd)
Species Sex Concentration Estimated total Effects/results Reference
doseb (g/kg)
g/m3 ppm
Intraperitoneal administration
Ratcf 1.7 death within 2 h Dequidt et al. (1972)
1.1 death within 4 h Dequidt et al. (1972)
Intraperitoneal administration (contd)
Mousec M 0.80 LD50 Friedman et al. (1976)
(Swiss ICR/HQ)
a Values recalculated as necessary to ppm and g/kg
b Estimated dose calculated by the formula: tidal volume x respiration frequency x exposure time x 2-NP conc. x alveolar
retention/animal weight. Tidal volume and respiration frequency from Kaplan et al. (1983) for mice (0.15 ml, 163/min);
from Baker et al. (1979) for rats (0.86 ml, 85.5/min); from Hoar (1976) for guinea-pigs (1.68 ml, 84/min); from Kozma
et al. 81974) for rabbits (15.8 ml, 45/min); from Reece (1984) and Breazile (1971) for cats (42 ml, 31/min); alveolar
retention in rat (0.40) from Nolan et al. (1982), used for all species; where not stated animal weights assumed to be
average values, 20 g for mice, 250 g for rats, 500 g for guinea-pigs, 2.5 kg for rabbits, and 4.0 kg for cats
c Sex not specified
d Exposure time varied from 1 to 7 h
e Exposure time was 6 h
f Baldwin & Williams (1977) also exposed female rats for 6 h to concentrations of 2-NP lower than 2.2 g/m3 (602 ppm); these
concentrations, i.e. 1.15, 1.35 and 1.69 g/m3 (316, 370 and 464 ppm), like 2.2 g/m3, produced no deaths within 14 days
Table 5. Effects of single exposure to 2-nitropropane
b) other effects
Route of Species Sex Estimated Effects/results Reference
administration total doseb
g/kg)
Intraperitoneal rat M 0.15 maximum hepatic injury achieved Filser & Daumann (1988)
(Sprague-Dawley) with this dose
Intraperitoneal rat M 0.05 lipid accumulation, centrilobular Zitting et al. (1981)
(Wistar) necrosis, mitochondrial
abnormalities, and changes in
endoplasmic reticulum and
glutathione content in liver
within 24 h, as well as changes
in enzyme activity in liver and
brain
Dermal rabbit M & F 2 no toxic effects observed Wilbur & Parekh (1982)
b See footnote b in Table 5a.
The effects of acute exposure to 2-NP, in addition to lethality,
are characterized primarily by hepatotoxicity and, at high exposure
levels, methaemoglobin formation and depression of the central
nervous system. Machle et al. (1940), in a description of symptoms
resulting from exposure of laboratory animals to simple
nitroparaffins, listed the following progression for guinea-pigs and
rabbits after a latent period of 20 to 40 min: progressive weakness,
unsteadiness, and incoordination ending in complete ataxia. The rate
of respiration at first slowed and later became increasingly rapid.
Most of these symptoms appear to reflect the narcotic effects
normally associated with inhalation of volatile hydrocarbons, but
the more rapid breathing may reflect an attempt by the body to
compensate for the formation of methaemoglobin and loss of
oxygen-carrying capacity of the red blood cells. Treon & Dutra
(1952) noted similar progression from exposure to high
concentrations of 2-NP vapour, i.e. lethargy and weakness, dyspnoea,
cyanosis, prostration, and ultimately coma and death, but did not
report more rapid breathing following a depression in rate of
respiration. They also noted lacrimation, salivation, and gastric
regurgitation in cats. In addition, the authors observed that, even
with animals which died promptly (within 9.5 h) following a single
exposure, there was a loss in body weight averaging 2.8%. Animals
exposed to 8.56 g/m3 (2353 ppm) or higher concentrations of 2-NP
displayed pathological changes including hepatocellular damage,
pulmonary oedema and haemorrhage, some disintegration of neurones in
the brain, and widespread damage to the endothelium.
Methaemoglobin and Heinz bodies (masses of denatured haemoglobin
within erythrocytes) were found in the blood of animals following
single exposures to high concentrations of 2-NP. In the case of
cats, 60 to 80% of the haemoglobin was converted to methaemoglobin
by exposure to 15.9 to 33.6 g/m3 (4360 to 9230 ppm) for 1 to 2 h,
whereas much longer exposure of rabbits to these concentrations
converted only 4 to 8% of the haemoglobin (Treon & Dutra, 1952).
Dequidt et al. (1972) reported high levels of methaemoglobin in
rats, i.e. 84% and 89%, following 1-h inhalation exposure to
53.5 g/m3 (14 700 ppm) and intraperitoneal injection of 1.7 g/kg,
respectively. Much lower methaemoglobin concentrations, 0.2 to 8.6%,
resulted from a 1-h exposure to 2.8 g/m3 (760 ppm). One day after
exposure to 15.4 g/m3 (4230 ppm) for 4.5 h or 8.5 g/m3
(2335 ppm) for 2.25 h, rabbits had Heinz bodies in 45 to 80% of
their red cells. These bodies disappeared gradually over 9 to 16
days (Treon & Dutra, 1952). Exposure of rabbits to 13.8 g/m3
(3790 ppm) for 1 h or 9.4 g/m3 (2580 ppm) for 2.25 h resulted in
the formation of Heinz bodies in only 0 to 2% of the red cells.
Formation of Heinz bodies in the cat may have reflected its high
sensitivity to 2-NP. A 1-h exposure to 13.8 g/m3 (3790 ppm) and a
20 min exposure to 16.4 g/m3 (4505 ppm) resulted in the appearance
of Heinz bodies in 27% and 16% of the erythrocytes, respectively.
Observations by Zitting et al. (1981) indicated that a single
intraperitoneal injection of 0.05 g 2-NP/kg to rats can produce
significant changes in the fine structure of the liver and in the
physiology of both liver and brain. 2-NP produced a visible
accumulation of lipid in hepatocytes, especially in periportal areas
after 4 h, and the lipid level continued to increase for the next
20 h. Within 4 h after injection there was also degranulation of the
rough endoplasmic reticulum in hepatocytes and proliferation of the
smooth endoplasmic reticulum. Within 24 h the former had almost
disappeared and the latter was vacuolated or compacted. In addition,
some hepatocytes had abnormal mitochondria and there was necrosis of
hepatocytes around the central vein. The latter was reflected in a
concurrent fourfold increase of serum alanine aminotransferase.
Other enzymatic parameters in the liver were also markedly affected
within 24 h. Cytochrome P-450 was markedly depressed,
7-ethoxycoumarin O-deethylase and 7-ethoxyresorufin O-deethylase
were diminished in activity, and microsomal epoxide hydratase,
UDP-glucuronosyltransferase and glutathione peroxidase were
increased in activity. In addition, the liver concentration of
glutathione nearly doubled.
The major observed neurochemical effect was a significant
increase in acetylcholine esterase activity in the cerebrum and in
isolated synaptosomes. There was little or no change in RNA,
2',3'-cyclic nucleotide 3'-phosphohydrolase, or acid proteinase in
the brain.
Zitting et al. (1981) noted that these histopathological and
enzymatic changes induced by 2-NP are nearly identical to the
effects of carbon tetrachloride on the rat and are thus indicative
of lipid peroxidation.
Hepatotoxicity following exposure to 2-NP has also been
observed in mice (Dayal et al., 1989). Intraperitoneal doses of
0.8 g/kg (9 mmol/kg) in male mice and 0.6 g/kg (6.7 mmol/kg) in
female mice significantly increased plasma activities of enzymes
indicative of hepatic damage (sorbitol dehydrogenase, alanine
aminotransferase, and aspartate aminotransferase) 48, 72, and 96 h
after injection. These enzyme activities were not elevated 24 h
after this dosage nor after small doses of 2-NP.
7.2 Short-term and long-term repeated exposure
Data for repeated exposure, like that for single exposure,
indicate that the cat is more sensitive to 2-NP than the other
species tested (Table 6). Rats, rabbits, guinea-pigs, and monkeys
survived 1.2 g/m3 (328 ppm) (7 h/day, 5 days/week) throughout
approximately 6 months of exposure (Treon & Dutra, 1952). However,
cats exposed to the same concentrations of 2-NP began dying after
the third day of exposure and were all dead by the end of the 17th
day (Treon & Dutra, 1952). Rats and guinea-pigs survived 5 days of
Table 6. Short-term and long-term toxicity of 2-nitropropane in mammalsa
Species Sex Dose and/or concentrationb Effects/results Reference
Oral studies
Rat (Wistar) M & F 0.25 g/kg, 5/week for 4 mortality of males (4/10); decreased growth in Wester et al. (1989)
weeks first week; increased urine, ALAT, ASAT, and
gamma-GT (males only), anaemia, thrombocyte
and leucocyte count, liver, spleen and heart
weight, and haemosiderin content of spleen;
cellular and nuclear polymorphism, single cell
necrosis, and proliferation of oval cells
and/or bile ducts in liver
Rat M 0.089 g/kg (1 mmol), some deaths by 16 week; throughout study body Fiala et al. (1987b)
(Sprague-Dawley) 3/week for 16 weeks, weights significantly lower than controls; all
maintained but not dosed rats exposed 16 weeks or longer developed
for next 61 weeks massive hepatocellular carcinomas; metastases
to the lungs in 4 animals
Rat M & F 0.05 g/kg, 5/week for 4 increased anaemia, thrombocyte conc., and Wester et al (1989)
(Wistar) weeks heart weight
Rat (Wistar) M & F 0.002 and 0.01 g/kg, 5 increased water intake by males dosed with Wester et al. (1989)
week for 4 weeks 0.002 g/kg
Inhalation studies
Ratd 2.77 g/m3 (760 ppm), 8 animals dead within 2 h after end of second Dequidt et al. (1972)
(Wistar) h/day for 2 days; inhalation session; 2-NP conc. in liver =
estimated dose over 8 h, 180 ppm; methaemoglobin = 2.4%
0.16 g/kg
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Inhalation studies (contd)
Rate 2.45 g/m3 (672 ppm), 7 no deaths Treon & Dutra (1952)
h/day for 5 days;
estimated dose over
7 h, 0.12 g/kg
1.20 g/m3 (328 ppm), 7 no deaths Treon & Dutra (1952)
h/day, 5 days/week for
130 days over 199 days;
estimated dose over 7 h,
0.06 g/kg
Rat M 0.75 g/m3 (207 ppm), 7 body weight and haematological parameters Lewis et al. (1979)
(Sprague-Dawley) h/day, 5 days/week for up unaffected; some pulmonary oedema and some
to 24 weeks; estimated pulmonary lesions within 3 months; liver
dose over 7 h, 0.04 g/kg weight elevated; hepatocellular hypertrophy,
hyperplasia and liver necrosis in all rats
within 3 months; liver neoplasms in all rats
within 6 months; these hepatocellular
carcinomas appeared to be growing rapidly and
deforming surrounding tissues
Rate 0.73 g/m3 (200 ppm), 7 growth slightly reduced and SGPT elevated in Griffin et al. (1978)
h/day, 5 days/week for 6 male rats; liver weight increased in both sexes;
months; estimated dose morphological changes in liver more pronounced
over 7 h, 0.03 g/kg in males; these included fatty metamorphosis
and hepatic nodules consisting mainly of
hyperplastic areas with distortion of lobular
architecture, necrosis and peripheral
compression
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Inhalation studies (contd)
Ratd 0.73 g/m3 (200 ppm), 7 no effect on body or organ weight to end of Griffin et al. (1986)
(Sprague-Dawley) h/day for 5 days experiment (94 week) aside from a brief
decrease in weight gain immediately following
exposure; no significant effects on mortality
or pathology
Ratc M 0.73 g/m3 (200 ppm), 7 severe liver damage with vacolar degeneration Coulston (1982)
h/day, 5 days/week for in exposed rats after 3 months up
to 7 months
Rate 0.36 g/m3 (100 ppm), 7 male rats had lower body weight, increased Griffin & Coulston (1983);
h/day, 5 days/week for up renal calcification, elevated SGPT, and Coulston et al. (1985)
to 18 months; estimated enlarged livers with necrosis, vacuolar
dose over 7 h, 0.013 g/kg degeneration and probable hepatocellular
carcinomas; female rats had increased renal
calcification and occasional hepatic masses
and nodules showing hyperplasia and vacuolar
degeneration
Rate 0.29 g/m3 (80 ppm), 8 no deaths; no trace of 2-NP in organs at end of Dequidt et al. (1972)
h/day for 5 days; estimated experiment; methaemoglobin = 0; nitrite = 0-10
dose over 8 h, 0.016 g/kg ppm in tissues, but no nitrite in urine
Rat M 0.1 g/m3 (27 ppm), 7 no gross or microscopic alteration of any Lewis et al. (1979)
(Sprague-Dawley) h/day, 5 days/week for up tissue, haematological parameter or serum
to 24 weeks; estimated biochemistry
dose over 7 h, 0.005 g/kg
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Inhalation studies (contd)
Rate 91 mg/m3 (25 ppm), 7 no changes in behaviour, appearance, rate of Griffin et al.
h/day, 5 days/week for up weight gain, final weight, serum chemistry or (1980, 1981)
to 22 months; estimated haematology; no significant increase in tumours
dose over 7 h, 0.003 g/kg and lesions associated with exposure; no
evidence of methaemoglobinaemia
Moused 0.73 g/m3 (200 ppm), 7 depression in body weight during first 3 months Griffin et al. (1984)
(ICR) h/day, 5 days/week for in females and throughout experiment in males;
48 weeks increased liver weight and elevation of liver
transaminases in females; toxic hyperplasia of
liver predominantly in females
Mousee 0.36 g/m3 (100 ppm), 7 slight depression of body weight during first 8 Coulston et al. (1986);
h/day, 5 days/week for months in males; no effects on organ weight; no Griffin et al. (1987)
18 months evidence of hepatocellular carcinoma; some
indications of liver toxicity (nodular
hyperplasia in females)
Cate 2.6 g/m3 (714 ppm), 4.5 deaths starting with first exposure, but Treon & Dutra (1952)
h/day for 4 days; some animals survived 4 exposures
estimated dose over 7 h,
0.10 g/kg
1.2 g/m3 (328 ppm), 7 deaths starting with third exposure; all Treon & Dutra (1952)
h/day, 5 days/week for 17 animals dead by end of 17th exposure
exposures; estimated dose
over 7 h, 0.07 g/kg
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Inhalation studies (contd)
Cate 1.15 g/m3 (317 ppm), 7 no deaths Treon & Dutra (1952)
h/day for 2 days;
estimated dose over 7 h,
0.07 g/kg
0.3 g/m3 (83 ppm), 7 no deaths Treon & Dutra (1952)
h/day, for 130 out of 191
days; estimated dose over
7 h, 0.02 g/kg
Rabbite 1.2 g/m3 (328 ppm), 7 no deaths Treon & Dutra (1952)
h/day, ca. 5 days/wk for
up to 130 out of 199 days;
estimated dose over 7 h,
0.06 g/kg
Rabbit M 0.75 g/m3 (207 ppm), 7 no gross or microscopic alterations to Lewis et al. (1979)
(white, NZ) h/day, 5 days/week for 24 tissues
weeks; estimated dose
over 7 h, 0.03 g/kg
Rabbite 0.3 g/m3 (83 ppm), 7 h/day no deaths Treon & Dutra (1952)
for 130 out of 191 days;
estimated dose over 7 h,
0.014 g/kg
Rabbite 0.1 mg/m3 (27 ppm), 7 no gross or microscopic alterations to Lewis et al (1979)
h/day, 5 days/week for 6 tissues
months; estimated dose
over 7 h, 0.005 g/kg
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Inhalation studies (contd)
Guinea-pige 2.45 g/m3 (672 ppm), 7 no deaths Treon & Dutra (1952)
h/day for 5 days;
estimated dose over
7 h, 0.12 g/kg
Guinea-pige 1.2 g/m3 (328 ppm), 7 no deaths Treon & Dutra (1952)
h/day, ca. 5 days/week
for 95-130 days out of
up to 199 days; estimated
dose over 7 h, 0.06 g/kg
Monkeye 1.2 g/m3 (328 ppm), 7 no deaths Treon & Dutra (1952)
h/day, ca. 5 days/week
for 100 days exposure
0.3 g/m3 (83 ppm), no deaths Treon & Dutra (1952)
7 h/day for 130 out of
191 days
Intraperitoneal studies
Ratd 0.11 g/kg, 1/day for apparently no deaths prior to sacrifice Dequidt et al. (1972)
(Wistar) 7 days 3 days after the last injection;
methaemoglobin = 4.3%; nitrite = 0.29-1.15 ppm
in organs (heart, lungs, kidneys, spleen)
Ratd 0.11 g/kg, 1/day for 15 apparently no deaths prior to sacrifice 36 h
(Wistar) days after the last injection; methaemoglobin = 0;
nitrite = 0-10.8 ppm in organs (heart, lungs,
kidneys, spleen)
Table 6 (contd)
Species Sex Dose and/or concentrationb Effects/results Reference
Intraperitoneal studies
Rat F 0.001 g/kg, 5/week for no significant effects on kidney function Bernard et al. (1989)
(Sprague-Dawley) 2 weeks
Dermal study
Rabbite 1 application/day on no skin irritation, illness, systemic effects Machle et al. (1940)
clipped anterior abdomen or deaths
for 5 days; dose not
stated
a values from the literature recalculated as necessary to ppm or g/kg
b estimated dose calculated by the formula: tidal volume x respiration frequency x 2-NP conc. x alveolar retention/animal weight.
Tidal volume and respiration frequency from Baker et al. (1979) for rats (0.086 ml, 85.5 /min); from Hoar (1976) for guinea-pigs
(1.68 ml, 84/min); from Kozma et al. (1974) for rabbits (15.8 ml, 45/min); from Reece (1984) and Breazile (1971) for cats
(42 ml, 31/min); alveolar retention in rat (0.40) from Nolan et al. (1982), used for all species; where not stated in reference,
animal weights assumed to be average values, 250 g for rats, 500 g for guinea-pigs, 2.5 kg for rabbits, and 4.0 kg for cats
c strain not specified
d sex not specified
e neither strain nor sex specified
exposure (7 h/day) to 2.46 g/m3 (672 ppm), but death occurred in
rats exposed for 2 days (8 h/day) to 2.77 g/m3 (760 ppm). Rats
were reported to survive 15 days of daily intra-peritoneal
injections of 0.11 g/kg (Dequidt et al., 1972). The doses used in
these repeated exposures were found to produce no more than trace
levels of methaemoglobin (maximum = 4.3%) and low concentrations
(0 to 11 mg/kg) of nitrite in the tissues.
Non-lethal chronic doses of 2-NP have been shown to produce a
number of harmful effects in rats (Table 6). Exposure of rats to
0.75 g/kg (207 ppm) for up to 24 weeks (7 h/day, 5 days/week)
initially induced pulmonary lesions and oedema, hepatocellular
hypertrophy, hyperplasia, and necrosis of the liver (Lewis et al.,
1979). By the end of 24 weeks all the rats developed rapidly growing
hepatocellular carcinomas. A similar exposure to a slightly lower
concentration of 2-NP, 0.73 g/m3 (200 ppm), induced hepatic
nodules and other destructive changes in the liver, especially in
male rats (Griffin & Coulston, 1983). These changes included fatty
degeneration, nodules consisting mainly of hyperplastic areas,
distortion of lobular architecture, necrosis and peripheral
compression. Male rats also had an elevated serum alanine
aminotransferase level (an indicator of liver damage) and slightly
reduced growth. Chronic exposure of rats to 0.36 g/m3 (100 ppm)
for up to 18 months produced similar, although slightly less severe,
damage than that resulting from exposure to 0.73 g/m3 (200 ppm)
(Griffin & Coulston, 1983; Coulston et al., 1985). Only male rats
developed hepatocellular carcinoma; female rats had increased renal
calcification and occasional hepatic masses and nodules showing
hyperplasia and vacuolar degeneration. No toxic effects were
reported after chronic exposure of rats to 90 mg/m3 (25 ppm) or
100 mg/m3 (27 ppm) (Lewis et al., 1979; Griffin et al., 1980,
1981). Daily intraperitoneal injections of 1 mg/kg (5/week for 2
weeks) had no significant effect on kidney function (Bernard et al.,
1989).
Chronic oral dosage of 2-NP by gavage for 16 weeks produced
tumours in rats (Fiala et al., 1987b). The dose was 0.089 g/kg
(1 mmol/kg), given 3 times per week, and this yielded a weekly
dosage of 0.27 g/kg, an amount similar to the highest sustained
estimated inhalation dosage. The body weights of rats treated with
2-NP were significantly lower than those of controls, and all
treated rats surviving 16 weeks or longer developed both benign
tumours and massive hepatocellular carcinomas. Metastases to the
lungs were observed in four of the 22 surviving animals (Fiala et
al., 1987b). Wester et al. (1989) treated rats with oral doses of
0.002, 0.01, 0.05, and 0.25 g/kg, 5 times per week for 4 weeks by
gavage. With a dose of 0.25 g/kg there was some mortality among male
rats, and in both sexes there was decreased growth, anaemia,
increased liver and heart weights, and severe damage to the liver.
At a dose of 0.05 g/kg the major harmful effect appeared to be
anaemia. Lower concentrations (0.01 and 0.002 g/kg) did not produce
obvious harm over the period of the experiment.
Rabbits and mice appear more resistant to the sublethal effects
of 2-NP than rats (Table 6). Chronic inhalation exposure of five
rabbits to 0.75 g/m3 (207 ppm) (7 h/day, 5 days/week) for 24
weeks, a treatment which induced hepatocellular carcinomas and
severe liver damage in rats, had no detectable effect (Lewis et al.,
1979). Rabbits also were unaffected by repeated dermal application
of 2-NP (Machle et al., 1940). Liver damage was found in mice,
especially females, during chronic exposure to 0.72 g/m3 (200 ppm)
(7 h/day, 5 days/week) for 48 weeks, but hepatocellular or other
carcinomas were not detected (Griffin et al., 1984). Exposure of
mice to 0.36 g/m3 (100 ppm) (7 h/day, 5 days/week) for 18 months
produced some liver damage, especially in females, as shown by
nodular hyperplasia (Coulston et al., 1986, Griffin et al., 1987).
7.3 Reproduction, embryotoxicity, and teratogenicity
There is limited information on the embryotoxicity and
teratogenicity of 2-NP. Hardin et al. (1981) gave intraperitoneal
injections of 0.17 g/kg (1.91 mmol/kg) of 2-NP in corn oil to
pregnant rats on days 1-5 of gestation. The dosage was a previously
determined maximum tolerated dose which produced no mortality, no
marked signs of toxicity, and less than a 10% reduction in body
weight gain during dosing or within 2 weeks following 15 daily
intraperitoneal injections to non-pregnant rats. In pregnant rats,
this dose was reported to give no evidence of maternal toxicity or
teratogenicity, but produced a significant incidence of delayed
fetal development. Harris et al. (1979) reported that 2-NP at a dose
of 0.17 g/kg retarded fetal heart development by 1 to 2 days in pups
from 9 out of 10 litters produced by female rats treated with 2-NP.
In the affected litters, 30% to 86% of the pups had retarded heart
development.
There appear to be no studies that have examined specifically
the effects of 2-NP on reproductive function. No effects on
reproductive organs, however, were noted in the above two studies
nor in any of those studies on the effects of single exposures or
short-term or long-term administration of 2-NP (Tables 5 and 6).
There also was no evidence of an increase in dominant lethality or
in sperm abnormality in genetic studies relating to reproduction
(McGregor, 1981).
7.4 Mutagenicity and related end-points
7.4.1 Prokaryotes and yeast
2-NP has been found to be mutagenic in a variety of test
systems. All investigators reported mutagenicity in several strains
of Salmonella typhimurium used with the Ames test, both with and
without an exogenous activating system (S9) (Table 7). In addition,
Kawai et al. (1987) reported mutagenicity with a strain of
Escherichia coli, but Litton Bionetics, Inc. (1977) did not find
mutagenicity with a strain of Saccharomyces cerevisiae. Most
investigators found greater mutagenicity with activation by S9 than
without. With the strain that was generally most sensitive to 2-NP,
i.e. TA100, most investigators providing detailed results observed
an approximate doubling in the number of mutants at a concentration
of 3 mg 2-NP/plate. Göggelmann et al. (1988), however, observed a
10- to 12-fold increase in mutant numbers at 2.45 mg/plate, with or
without activation by S9. Fiala et al. (1987a) reported that
non-ionic 2-NP yielded only a doubling of mutant numbers at
4.9 mg/plate with S9 activation and no increase without activation,
whereas 2-NP nitronate at this concentration yielded a threefold
increase in mutant numbers with activation. Without activation 2-NP
nitronate at 2.5 mg/plate yielded a nearly 6-fold increase in mutant
numbers; nitronate at 4.9 mg/plate was toxic to this test organism.
Fiala et al. (1987a) concluded that the mutagenicity of 2-NP is
produced mainly or entirely by the nitronate anion and suggested
that the low level of mutagenicity observed with non-ionic 2-NP may
have resulted from its conversion to the nitronate form within the
microorganisms. A similar conclusion was drawn by Dayal et al.
(1989) from their comparison of 2-NP, nitromethane, nitroethane, and
their nitronates for mutagenicity in Salmonella. Some of the
variability in results obtained by others may stem from varying
proportions of non-ionic 2-NP and 2-NP nitronate in the stock 2-NP
used in their tests. Fiala et al. (1987a) further observed that
dimethyl sulfoxide (DMSO), a solvent used to solubilize 2-NP in
these tests, modified the mutagenicity of 2-NP nitronate in a
variable manner.
Several of the studies discussed above examined the question of
how 2-NP induces mutations in the test systems. In addition to 2-NP,
1-and 2-aminopropane and a number of mononitroalkanes (nitromethane,
nitroethane, 1-nitropropane, 1-and 2-nitrobutane, and 1-and
2-nitropentane) were tested for mutagenicity with bacterial systems,
mainly strains of Salmonella typhimurium (Hite & Skeggs, 1979;
Speck et al., 1982; Löfroth et al., 1986; Kawai et al., 1987;
Göggelmann et al., 1988; Dayal et al., 1989). Only 2-NP proved to be
significantly mutagenic. Some investigators observed weak
mutagenicity with nitroethane, 1-nitropropane, 2-nitrobutane, and
2-nitropentane, which, at least in the case of nitromethane and
nitroethane, may have been produced by the 2-NP present in these
solvents as a contaminant.
Table 7. Genotoxicity of 2-nitropropane
Test system Resulta Referencesb
Prokaryotes and yeastc
Salmonella typhimurium
strain TA92 + 1
strain TA92 with S9 + 1
strain TA98 + 1,2,5,7,8,9
- 6,16,19
strain TA98 with S9 + 1,2,3,5,8,9,17
strain TA98NR + 2,9
strain TA98NR with S9 + 2,9
strain TA100 + 1,2,4,5,6,7,8,9,19
- 17
strain TA100 with S9 + 1,2,3,4,5,6,8,9,17,19
strain TA100NR + 2,9
strain TA100NR with S9 + 2,9
strain TA102 + 4,8,19
strain TA102 with S9 + 4,8,19
Table 7 (contd)
Test system Resulta Referencesb
strain TA1535 ? 5
- 17
strain TA1535 with S9 + 3
? 5
- 17
strain TA1537 - 17
strain TA1537 with S9 - 3,17
strain TA1538 - 17
strain TA1538 with S9 - 17
Escherichia coli
strain WP2 uvrA/pKM101 + 7
strain WP2 uvrA/pKM101 + 7
Saccharomyces cerevisiae
strain D4 - 17
strain D4 with S9 - 17
Table 7 (contd)
Test system Resulta Referencesb
Eukaryotesf
In vivo
Drosophila
Sex-linked recessive lethal - 10,11
Mouse
erythrocytes
(micronuclei) m, f - 1,16,23
sperm (abnormality) ? 11
Rat
Liver (DNA repair synthesis) m, f + 12,13,23
(micronuclei) + 23
(DNA and RNA base + 18,20,21,22
modifications) m, f
(DNA strand breakage) m + 24
Kidney (DNA strand breakage) m - 24
(DNA and RNA base - 22
modifications) m, f
Table 7 (contd)
Test system Resulta Referencesb
Brain (DNA strand breakage) m - 24
Lung (DNA strand breakage) m - 24
Bone marrow (chromosome - 11
aberrations) m, f
(micronuclei) m - 23
Testes
(dominant lethal test) m - 11
In Vitro
Mouse
3T3-NIH fibroblasts - 13
(DNA repair synthesis)
Rat
hepatocytes + 12,13,26
(DNA repair synthesis) m, f
hepatoma cells, 2sFou + 25
(DNA repair synthesis and micronuclei)g
hepatoma cells, C2Rev7 + 25
(DNA repair synthesis and micronuclei)g
Table 7 (contd)
Test system Resulta Referencesb
hepatoma cells, H4IIEC3/G- + 25
(DNA repair synthesis, micronuclei and
gene mutations)g
208F - embryonic fibroblasts - 13
(DNA repair synthesis)
LLC WRC 256 carcinoma Walker rat - 13
(DNA repair synthesis)
Hamster
CHO cells (chromosome aberrations - 14
and sister chromatid exchanges)
CHO cells (DNA repair synthesis) - 13
V79 cells (DNA repair synthesis) - 12,13,25,26
V79 cells (mutations) + 25
V79 cells (micrnuclei) - 25,26
Human
Lymphocytes (chromosome aberrations -d 9,15
and sister chromatid exchanges)
Lymphocytes (chromosome aberrations -e 9,15
and sister chromatid exchanges) with
S9
Table 7 (contd)
Test system Resulta Referencesb
Human (contd)
Fibroblasts (DNA repair synthesis) - 11
W138 embryonic lung fibroblasts NC1- - 13
H322 adenocarcinoma lung cells A549
adenocarcinoma lung cells HEp2 epidermal
carcinoma larynx cells (DNA repair synthesis)
a + = positive response; - = negative response; ? = inconclusive result
b (1) Hite & Skeggs (1979) (2) Speck et al. (1982)
(3) Haworth et al. (1983) (4) Simmons et al. (1986)
(5) Löfroth et al. (1986) (6) Hughes et al. (1987)
(7) Kawai et al. (1987) (8) Fiala et al. (1987a)
(9) Göggelmann et al. (1988) (10) Zimmering et al. (1985)
(11) McGregor (1981) (12) Ziegler-Skylakakis et al. (1987)
(13) Andrae et al. (1988) (14) Galloway et al. (1987)
(15) Bauchinger et al. (1987) (16) Kliesch & Adler (1987)
(17) Litton Bionetics, Inc. (1977) (18) Conaway et al. (1991a)
(19) Conaway et al. (1991b) (20) Fiala et al. (1989)
(21) Hussain et al. (1990) (22) Guo et al. (1990)
(23) George et al. (1989) (24) Robbiano et al. (1991)
(25) Roscher et al. (1990) (26) Haas-Jobelius et al. (1991)
c Liver S9 fractions are prepared by treating rats, mice or hamsters with Aroclor
1254 or another microsomal enzyme inducer, and, after several days, removing
the livers, homogenizing them, and centrifuging. The supernatant, the S9
fraction, contains liver microsomal enzymes.
d Bauchiner et al. (1987) (ref. 15) reported a weak but significantly positive result
on one test, but considered results overall to be negative
e Göggelmann et al. (1988) (ref. 9) reported a very weak positive result
f m = male; f = female
g pretreated with dexamethasone
Löfroth et al. (1986) and Fiala et al. (1987a) speculated that
the relative mutagenicity of 2-NP and other nitroalkanes correlated
with the concentrations of their nitronate anions. The more highly
mutagenic compounds, such as 2-NP and 1,1-dinitroethane, had high
concentrations of nitronate at cellular pH. A thorough comparison of
2-NP, other secondary nitroalkanes, and their nitronates (Conaway et
al., 1991) showed greater mutagenicity of the nitronates and
confirmed these speculations.
These studies do not support the hypothesis that mutagenicity of
2-NP is produced largely or entirely by nitrite resulting from its
metabolic breakdown, since the response of tester strains to sodium
nitrite was quite different from their response to 2-NP (Löfroth et
al, 1986). Unlike nitroarenes and nitroheterocyclics, 2-NP probably
does not derive its mutagenicity exclusively from enzymatic
reduction to a hydroxylamine. Strains of S. typhimurium, i.e.
TA98NR and TA100NR, lacking the "classical" nitroreductase
demonstrated either a very slightly (Speck et al., 1982) or markedly
(Göggelmann et al., 1988) reduced, but still significant, level of
mutagenicity. Göggelmann et al. (1988) suggested that some of this
mutagenicity may be due to some residual nitroreductase activity
still present in these strains.
The increased mutagenicity generally observed with S9 activation
of 2-NP and the reduced mutagenicity in tester strains deficient in
nitroreductase activity suggest an involvement of metabolism in the
mutagenicity of 2-NP in S. typhimurium. Fiala et al. (1987a)
presented evidence that metabolic oxidation of the 2-NP anion can
result in the formation of reactive species such as hydroxyl
radicals, which are capable of damaging DNA bases. Incubation of
2-NP nitronate under roughly physiological conditions with thymidine
and a 1-electron oxidation system, horseradish peroxidase and
hydrogen peroxide, yielded 2-NP free radicals (which in part
condensed into 2,3-dimethyl-2,3-dinitrobutane) and oxidation
products of thymidine of the type produced by hydroxyl radical
attack. A common source of hydroxyl radicals is superoxide, which
is known to be produced during oxidation of 2-NP nitronate by
horseradish peroxidase and hydrogen peroxide (Porter & Bright,
1983). The extent to which such reactions may occur in the
Salmonella tester strains or in vivo is unknown. There is,
however, evidence from the literature for microbial oxidation of
2-NP and 2-NP nitronate (Kido et al., 1984; Fiala et al., 1987a).
The existence of this proposed mechanism for inducing mutations
through the production of DNA-damaging hydroxyl radicals is further
supported by the reduction in 2-NP mutagenicity in TA102 by DMSO, a
known scavenger of such radicals (Fiala et al., 1987a). The fact
that DMSO had little effect on the mutagenicity of 2-NP in TA100
indicated that formation of hydroxyl radicals cannot be the only
mechanism inducing mutations. Fiala et al. (1987a) hypothesized that
the 2-NP radical may act directly by forming adducts with DNA bases.
This would provide an additional mechanism for inducing mutations
that would not require hydroxyl radicals.
7.4.2 Eukaryotes
The genotoxic effects of 2-NP in eukaryotic organisms are
summarized in Table 7. Results were negative in two sex-linked
recessive lethal tests using Drosophila despite exposure to high
concentrations of 2-NP. Zimmering et al. (1985) dosed the flies by
injection and by feeding with concentrations that induced an
approximately 30% mortality, whereas McGregor (1981) exposed the
flies to 2-NP vapour at a concentration of 2.55 g/m3 (700 ppm) for
4.5 h. Sex-linked recessive lethal mutation frequency was not
increased except in mature spermatozoa in one stock of flies. Since
this increase was not reproducible, its significance is doubtful.
Results were variable but mainly negative in a variety of
in vivo mammalian test systems. In the dominant lethal test and
the bone marrow chromosomal aberration test using rats and in the
sperm abnormality test using mice, animals were exposed to 91 or
728 mg/m3 (25 or 200 ppm) for 7 h/day on 5 consecutive days.
Neither exposure level produced a positive response in any of the test
systems used (McGregor, 1981). Negative results were obtained with
the mouse micronucleus test even with an almost lethal oral dose of
0.3 g/kg on 2 consecutive days (Hite & Skeggs, 1979), and with a
single intraperitoneal dose of 0.3 g/kg (Kliesch & Adler, 1987).
One set of genotoxicity tests using rats, however, yielded
strongly positive results (Andrae et al., 1988; Ziegler-Skylakakis
et al., 1987). In both in vivo and in vitro tests, 2-NP induced
DNA repair synthesis in rat liver cells. In the in vitro
experiments, cultures of rat hepatocytes were incubated with 2-NP,
while in the in vivo experiments rats were injected
intraperitoneally with 2-NP, sacrificed 4 h later, and cultures of
their hepatocytes were examined for DNA repair synthesis. Exposure
of hepatocyte cultures for 18 to 20 h to concentrations of 2-NP as
low as 2.7 mg/litre (30 µmol/litre) induced a detectable
(approximately 2-fold above the control level) increase in repair
synthesis. The highest concentration tested, i.e. 89 mg/litre
(10 mmol/litre), induced a 12- to 15-fold increase above control
levels in hepatocytes from male rats and a 25- to 30-fold increase
in hepatocytes from female rats. The in vivo experiments also
demonstrated the existence of a sexual difference in susceptibility
to 2-NP. In males, the lowest dose (20 mg/kg) induced a doubling in
repair synthesis and the highest dose (80 mg/kg) a 3.6-fold
increase, whereas in females these doses induced a very small
increase and a doubling, respectively. In contrast to 2-NP, 1-NP
given in vivo had no effect on DNA repair synthesis and did not
increase in vitro repair synthesis above that expected from its
contamination with 2-NP. Andrae et al. (1988) considered that their
results from the in vivo experiments were in agreement with the
observed greater hepatocarcinogenicity of 2-NP in male rats than in
females. Their observation that 2-NP did not induce any increase in
repair synthesis in any of nine non-hepatic cell lines derived from
human, mouse, hamster, and rat tissues led the authors to suggest
that 2-NP is not a direct-acting genotoxic agent but rather requires
metabolic activation by liver-specific metabolism.
Several papers have confirmed and expanded these initial
observations on the genotoxicity of 2-NP to rat liver cells. George
et al. (1989) showed that oral dosage similarly induced unscheduled
DNA synthesis and also resulted in the formation of micronuclei in
rat liver. 2-NP did not, however, significantly increase the
frequency of micronuclei in mouse bone marrow. Intraperitoneal
injection of 0.1 g/kg produced in 6 h a significant increase in
8-hydroxydeoxyguanosine and 8-hydroxyguanosine, products
respectively of DNA and RNA damage caused by hydroxyl or other
oxygen-radical-forming agents (Fiala et al., 1989; Hussain et al.,
1990). This treatment produced significantly lower levels of
8-hydroxydeoxyguanosine, 8-hydroxyguanosine, and other presumed
modified nucleosides in female rats than in males, and had little
effect on the nucleic acids of the kidney, findings that are in
agreement with the known carcinogenicity of 2-NP (Guo et al., 1990).
The organ specificity of the genotoxicity of 2-NP in the rat was
confirmed by Robbiano et al. (1991) who reported that oral doses of
45-713 mg/kg produced maximum numbers of single strand breaks in the
liver 6 h after administration and did not induce DNA fragmentation
in lung, kidney, bone marrow or brain. Damage to rat liver nucleic
acids was also caused by intraperitoneal injection of other
secondary nitroalkanes and a ketoxime capable of being converted to
a secondary nitroalkane, but not with a primary or a tertiary
nitroalkane (Conaway et al., 1991). These authors suggest that the
greater genotoxicity of the secondary nitroalkanes may stem from the
greater stability of their nitronate forms at physiological pH
values.
Observations by Roscher et al. (1990) and Robbiano et al. (1991)
suggest that cytochrome P-450-dependent monooxygenases are important
in the activation of 2-NP in liver. Robbiano et al. (1991) found an
increase in damage to liver DNA in rats pretreated with
phenobarbital or ß-naphthoflavone, inducers of cytochrome
P-450-dependent monooxygenases, and a reduction in liver DNA damage
in rats pretreated with methoxsalen, an inhibitor of cytochrome
P-450. Roscher et al. (1990) examined the effect of 2-NP on rat
hepatoma cell lines that express various forms of cytochrome
P-450-dependent monooxygenases and V79 Chinese hamster cells that
lack these enzyme activities. 2-NP increased DNA repair synthesis,
micronuclei formation, and the frequency of mutants resistant to
6-thioguanine in hepatoma cells pretreated with dexamethasone, an
inducer of various liver-specific cytochrome P-450 forms.
Genotoxicity was reduced or absent in hepatoma cells not treated
with the inducer. In the V79 cells, 2-NP produced only mutations to
6-thioguanine resistance.
2-NP demonstrated only a very limited level of genotoxicity in
other cell lines. Results were negative in a DNA repair synthesis
assay with human diploid fibroblasts exposed for 3 h to 2-NP
concentrations up to 5 g/litre (56 mmol/litre) (McGregor, 1981), as
well as with various cell lines of extrahepatic origin (Andrae et
al., 1988). 2-NP did not cause chromosomal aberrations or SCEs in
Chinese hamster ovary (CHO) cells either with or without S9
(Galloway et al., 1987). In these experiments the maximum
concentrations used, i.e. 1.6 and 5.0 g/litre (18 and 56 mmol/litre)
were estimated to reduce cell growth by 50%. Weakly positive
results, however, were obtained in cytogenetic tests using human
lymphocytes (Bauchinger et al., 1987; Göggelmann et al., 1988).
Exposure of cells to high concentrations of 2-NP (commercial grade,
at least 94% 2-NP) for 1 h induced a significant increase in
chromosomal aberrations (open breaks accompanied by gaps) at a
concentration of 5.3 g/litre (60 mmol/litre) with S9 activation and
at 7.1 g/litre (80 mmol/litre) without S9 activation (Bauchinger et
al., 1987). In addition, the frequency of sister chromatid exchange
was significantly increased at all concentrations of 2-NP (0.7 to
7.1 g/litre; 7.5 to 80 mmol/litre), but only with S9 activation.
Repetition of the experiment with 2-NP of greater than 99% purity
yielded similar results (Göggelmann et al., 1988). There was no
significant increase in chromosomal changes without S9 activation,
but there was a small but significant increase at the highest
treatment levels, i.e. 7.1 and 10 g/litre (80 and 111 mmol/litre),
with S9 activation. 1-NP of 97% purity produced no significant
mutagenic or cytogenetic effects on this test system with or without
S9 activation. The authors concluded that 2-NP can exert a
clastogenic effect on human lymphocytes only with metabolic
activation, and hypothesized that it acted via a different metabolic
pathway in the lymphocytes than it did in the bacterial
S. typhimurium system, where it induced mutations without
exogenous activation.
7.5 Carcinogenicity
That 2-NP is unquestionably a potent carcinogen in rats was
demonstrated by Lewis et al. (1979). Inhalation exposure of male
Sprague-Dawley rats to 0.75 g/m3 (207 ppm) for 7 h/day, 5
days/week, over a 24-week period induced hepatocellular neoplasms in
all surviving animals. Inhalation exposure of rats to 0.36 g/m3
(100 ppm) similarly administered over 18 months was associated with
hepatocellular carcinomas in male rats and produced changes in the
livers of female rats (nodules showing hyperplasia and vacuolar
degeneration) which may have been precursors to carcinoma (Griffin &
Coulston, 1983; Coulston et al., 1985). Oral dosing of male rats
with 89 mg/kg (1 mmol/kg), three times a week for 16 weeks, induced
massive hepatocellular carcinomas in all rats, observed when they
were sacrificed 40 weeks later (Fiala et al., 1987a). Metastases to
the lungs were present in 4 out of 22 surviving animals, suggesting
a high degree of malignancy. Long-term inhalation exposure of rats
to low concentrations (100 mg/m3; 27 ppm) did not produce any
evidence of increased hepatocellular carcinoma or precursor tumour
lesions of any sort (Lewis et al., 1979; Griffin et al., 1980,
1981). Evidence that inhalation exposure to low doses of 2-NP can
cause DNA damage in rats was presented by Denk et al. (1990). Male
and female Sprague-Dawley rats, 4-6 days old at the beginning of
treatment, were exposed to 0, 91, 146, 182, 291, and 455 mg/m3 (0,
25, 40, 50, 80 and 125 ppm) for 6 h/day, 5 days/week for 3 weeks,
and this was followed by promotion with polychlorinated biphenyls
(Clophen A50) for 8 weeks. This treatment produced a dose-dependent
increase in the numbers of preneoplastic liver foci deficient in
adenosine-5'-triphosphatase. Cunningham & Matthews (1991) showed
that 2-NP can also induce cell proliferation in rat liver. Rats were
exposed to daily oral doses of 20, 40 or 80 mg/kg by gavage for 10
days, and cell proliferation during exposure was quantified by
measuring the incorporation of bromodeoxyuridine into newly
synthesized DNA. Exposure to 40 and 80 mg/kg resulted in
statistically significant increases in the frequency of S-phase
cells from 1.9% in the vehicle-treated animals to 6.3% and 11%,
respectively. Exposure to 20 mg/kg had no effect on the labelling
index. The non-carcinogenic isomer of 2-NP, 1-NP, did not affect DNA
synthesis at doses of 20, 40 and 80 mg/kg.
There is no substantial evidence that 2-NP induces cancer in
species other than the rat (Table 6). Inhalation exposure of rabbits
to 750 mg/m3 (207 ppm) for 1 h/day, 5 days/week for 24 weeks,
initially produced some evidence of liver damage. At the end of 6
months of exposure, the five rabbits examined displayed no more than
minor changes in the liver and no evidence of carcinoma (Lewis et
al., 1979). Exposure of mice to 728 mg/m3 (200 ppm), similarly
administered for 48 weeks, produced damage to the liver, especially
in females, but no carcinoma (Griffin et al., 1984). It is possible
that degenerative changes observed in the livers of exposed females
may have been precursors to subsequent tumour development. A lower
concentration of 2-NP, 360 mg/m3 (100 ppm), similarly
administered to ICR mice for 18 months, induced liver damage in the
form of nodular hyperplasia in females but no increase in
hepatocellular carcinoma (Coulston et al., 1986; Griffin et al.,
1987). The latter study is the only inhalation study in a species
other than the rat that was of sufficient duration to allow for
latency in tumour development. No studies of carcinogenicity using
oral dosing in any species except the rat have been reported. Thus,
despite the absence of clear evidence, the possibility of
carcinogenicity in species other than the rat cannot be discounted.
There appear to be no laboratory studies on species other than
rats, mice and rabbits suitable for assessing the potential
carcinogenicity of 2-NP. As discussed in section 8.2.2, there is no
evidence from limited epidemiological studies that 2-NP is
carcinogenic in humans.
Griffin & Coulston (1983) argued that 2-NP does not act as an
initiating carcinogen in the rat, but rather induces cancer as a
response to extensive liver damage (Angus Chemical Co., 1985). They
offered as evidence the results of an experiment demonstrating the
absence of long-term effects from short-term exposure to 2-NP
(Griffin et al., 1986). Exposure of rats of both sexes to 730 g/m3
(200 ppm), 7 h/day for 5 days, did not produce any effect on
longevity or on the development of hepatocellular or other
carcinomas up to the end of the experiment, which lasted for 94
weeks. This argument is markedly weakened by the observation of
Andrae et al. (1988) and others (Fiala et al., 1989; George et al.,
1989; Guo et al., 1990; Conaway et al., 1991) that 2-NP is an active
genotoxic agent in rat hepatocytes both in vitro and in vivo.
7.6 Pharmacological effects
In vivo pharmacological studies have not been performed.
However in vitro studies with guinea-pig tissues suggest that 2-NP
may have several pharmacological effects. A 0.1 µM (8.9 µg/litre)
solution inhibited oxygen consumption of polymorphonuclear
leucocytes by 50% (Estes & Gast, 1960). These authors further
reported that, with increasing concentrations of 2-NP, respiration
of heart homogenate was initially depressed and subsequently
stimulated.
2-NP also produces two opposite neural effects on smooth muscle
preparations (Bergman et al., 1962). Contraction is induced partly
by ganglionic stimulation and partly by direct liberation of a
transmitter from nerve endings, but at the same time 2-NP inhibits
the response to acetylcholine and other smooth muscle stimulants.
Bergman et al. (1962) found that the action of nitroparaffins on
smooth muscle was similar to that of nicotine in that their ability
to induce contraction was inhibited by morphine and by high, but not
low, concentrations of atropine.
8. EFFECTS ON HUMANS
8.1 General population exposure
As indicated in section 5.1, general population exposure to 2-NP
appears to be very low, and there is no information on the effects
of this exposure. 2-NP has been used as a component of paints and
other consumer products. There is no evidence that exposure to 2-NP
from the use of such products has resulted in detectable injury or
illness in the general population.
8.2 Occupational exposure
8.2.1 Acute toxicity
Significant human exposure to 2-NP is largely or entirely
occupationally related. High concentrations are acutely toxic and
seven industrial fatalities have been attributed to inhalation of
2-NP fumes (Gautier et al., 1964; Hine et al., 1978; Rondia, 1979;
Harrison et al., 1985, 1987; US NIOSH, 1987b). In all cases,
exposure was to a solvent mixture containing 2-NP, was for a total
of 5 to 16 h in the course of 1 to 3 days, and occurred while paints
or coatings were being applied in confined spaces, such as tank
interiors, underground vaults and ship's holds, with little or no
ventilation. The actual concentrations of 2-NP that caused deaths
were not measured, but were thought to be high and in one case
estimated at 2.18 g/m3 (600 ppm), as determined by GC-FID from the
2-NP content of the victim's blood (0.013 g/litre) (Harrison et al.,
1987). Initial symptoms requiring medical treatment appeared during
exposure or within a few hours following exposure and included
headache, nausea, dizziness, drowsiness, weakness, anorexia,
vomiting, diarrhoea, and neck, thoracic, and abdominal pain. Victims
were hospitalized and in general initially showed improvement, in
some cases to the point of being discharged after a day or less. But
improvement, where present, was temporary and in all seven cases was
followed within a few days by worsening condition and
rehospitalization of those previously discharged. Later symptoms
included persistent nausea, vomiting, anorexia, jaundice, reduced
urine output, diarrhoea, bloody stools, mental confusion,
restlessness, loss of reflexes, and increases in serum
aminotransferases and other indicators of hepatic lesions. Death
occurred within 4 to 26 days (average, 10 days) following exposure.
In all cases the primary cause was acute hepatic failure.
Contributing factors included lung oedema, gastrointestinal
bleeding, and respiratory and kidney failure. Postmortem microscopic
examination of the liver revealed necrosis of hepatic tissue and in
some cases fatty degeneration. None of the victims had a past
history of liver disease or drank excessively.
In addition to these fatalities, there have been four additional
serious but nonlethal cases attributed to acute exposure to 2-NP
(Gautier et al., 1964; Hine et al., 1978; Harrison et al., 1985,
1987; US NIOSH, 1987b). All but one were colleagues of the deceased
described above, but may have received lesser doses. Initial
symptoms were similar to those of the deceased, but were followed by
full recovery rather than decline and death. Serum enzyme levels,
however, remained mildly elevated for months following exposure. As
in the case of the deceased, the actual concentrations of 2-NP to
which these workers were exposed are unknown. One of the survivors
had a 2-NP serum concentration of 8.5 mg/litre when hospitalized.
Since his coworker hospitalized at the same time with a serum
concentration of 13 mg/litre subsequently died, Harrison et al.
(1987) speculated that either 2-NP has a very steep dose-response
curve or that there are substantial differences in individual
susceptibility. Six men (out of a group of approximately 300)
developed toxic hepatitis after exposure to an epoxy resin coating
containing 2-NP (Williams et al., 1974). The development of toxic
hepatitis was ascribed to methylenedianiline in the coating, and the
possibility that 2-NP may have been at least a partial cause was not
considered.
Exposure to lower concentrations of 2-NP appears to produce some
of the symptoms described above. Brief and intermittent exposure to
concentrations above 364 mg/m3 (100 ppm) may produce headache and
nausea (Angus Chemical Co. & Occusafe, Inc., 1986). Exposure to
vapours (estimated to contain 73 to 164 mg/m3 (20 to 45 ppm) using
colorimetric methods available at the time of the study) of a
solvent mixture that polluted the workplace air produced a daily
cycle in symptoms (Skinner, 1947). Workers started the day feeling
well, but by noon some exhibited nausea and lack of appetite. Later
in the working day these symptoms intensified and were accompanied
by vomiting and diarrhoea. Vomiting continued after the workers left
the workplace, and they were unable to eat supper but were able to
sleep. By morning they were feeling well again. Colleagues not
experiencing nausea and vomiting had occipital headaches, which
appeared and gradually intensified during the working day. All of
the workers were free of symptoms when away from the workplace for a
day or more, and the substitution of methyl ethyl ketone for 2-NP
brought complete relief. Skinner (1947) noted that in another plant
exposure of workers to 36-109 mg/m3 (10-30 ppm) for less than
4 h/day on not more than 3 days per week produced no noticeable ill
effects.
The flaw in all but one (Hine et al., 1978) of these case
studies is that exposure was to a solvent mixture containing 2-NP
and not to 2-NP alone (Hine et al., 1978). Thus the observed effects
cannot be assigned to 2-NP with total confidence. A number of
factors, however, point to 2-NP as the main, if not sole, causative
agent. It was the only solvent common to all the mixtures, and the
only solvent present in the mixtures known to be hepatotoxic.
Symptoms and damaging effects on the body from the solvent mixtures
were fairly similar to each other despite widely different solvent
compositions (apart from the common presence of 2-NP), and were
fairly similar to those in the single case resulting from exposure
to 2-NP alone. In addition, as described above, substitution of
another solvent for 2-NP eliminated all symptoms. The weight of
evidence thus supports the view that the symptoms and damage to the
human body described above which followed exposure to solvent
mixtures containing 2-NP, resulted mainly, if not entirely, from
2-NP.
8.2.2 Effects of long-term exposure
Despite the fact that 2-NP has been in use for over 40 years,
there are very few data on long-term effects and such information is
derived mainly from unpublished reports from manufacturers of 2-NP.
Angus Chemical Co., a major manufacturer of 2-NP, has assembled
information on more than 1800 occupationally exposed workers from a
variety of plants in the USA, Mexico, Germany, Sweden, and the
Netherlands (Table 8). Where information was available, medical
records on living employees indicated no problems with liver
functions and no obvious symptoms or conditions that could be
connected with chronic exposure to 2-NP (Angus Chemical Company and
Occusafe, Incorporated, 1986).
A major portion of the above investigation was an earlier
mortality study conducted by Miller & Temple (1979) on 1481 workers
employed in the manufacture of 2-NP during the period 1955 to 1977.
The exposures were defined as direct, indirect or zero exposure.
Formal industrial hygiene monitoring was not performed until 1977.
Individual exposures were based on job titles rather than actual
exposure data. Angus Chemical Company and Occusafe, Incorporated
(1986) concluded that analysis of these data did not suggest any
unusual cancer or other disease pattern in this group of workers.
They noted, however, that because the cohort was small and the
duration of exposure and observation was relatively short, it was
not possible to conclude from these data that 2-NP is not
carcinogenic to humans. In a follow-up report on the same cohort
(Bolender, 1983), the findings did not change the earlier
conclusion.
It is necessary to emphasize that this cohort had a limited
number of workers with long exposure (> 15 years). Since the
individual exposure data are not available, it cannot be concluded
from available data that 2-NP is non-carcinogenic in humans.
Table 8. Studies of long-term occupational exposure to 2-nitropropane
Activitya Exposure concentrationb Exposure duration No. of exposed Reference
mg/m3 (ppm) (years) employees
TWA STEL
Manufacture of 2-NP, 25-91 > 218 < 1-15 55 Angus Chem. Co. & Occusafe, Inc. (1986)
1940-1955 (7-25) (> 60) average 2.5
Manufacture of 2-NP, 3.6-36 91-5970 < 5-> 21 804 Angus Chem. Co. & Occusafe, Inc. (1986);
1946-1982, (1-10) (25-1640) average 8c Miller & Temple (1979); Bolender (1983)
Sterlington, LA, USA
Extraction of 3.6-193 109-473 < 5-ca. 25 18-19 Angus Chem. Co. & Occusafe, Inc. (1986);
triglycerides, USA (1-53; (30-130) Crawford et al. (1985); Tabershaw
average 9c) Occupational Medicine Assoc. (1980);
Life Extension Inst. (1983)
Automobile assembly unknown < 5-> 25 456 Angus Chem. Co. & Occusafe, Inc. (1986)
plant, Germany
Paint mfg., Germany unknown 5-10 6 Angus Chem. Co. & Occusafe, Inc. (1986)
Paint mfg., Germany 40-91 11-15 5 Angus Chem. Co. & Occusafe, Inc. (1986)
(11-25)
Chemical mfg., unknown 5-10 80 Angus Chem. Co. & Occusafe, Inc. (1986)
Germany
Chemical mfg., unknown < 5 4 Angus Chem. Co. & Occusafe, Inc. (1986)
Germany
Extraction plant, 3.6-18.2 73-364 5-10 15 Angus Chem. Co. & Occusafe, Inc. (1986)
Sweden (1-5) (20-100)
Table 8 (contd)
Activitya Exposure concentrationb Exposure duration No. of exposed Reference
mg/m3 (ppm) (years) employees
TWA STEL
Paint mfg., unknown 5-10 180 Angus Chem. Co. & Occusafe, Inc. (1986)
Netherlands
Chemical Co., USA 3.6-36 65.6-364 < 5-20 41 Angus Chem. Co. & Occusafe, Inc. (1986)
(1-10) (18-100)
Printing Co., USA 1.8-87 2.5-124 ? 140 Angus Chem. Co. & Occusafe, Inc. (1986)
(0.5-24) (0.7-34)
Paint mfg., Mexico 3.6 < 91 5-10 100 Angus Chem. Co. & Occusafe, Inc. (1986)
(1) (< 25)
Ink mfg., Mexico 11-18 73-80 5-10 8 Angus Chem. Co. & Occusafe, Inc. (1986)
(3-5) (20-22)
Coatings mfg., 14.6-91 237-251 < 5-20 30 Angus Chem. Co. & Occusafe, Inc. (1986)
Mexico (4-25) (65-69)
Chemical distilation, 36-55 > 91 < 5-10 2 Angus Chem. Co. & Occusafe, Inc. (1986)
Mexico (10-15) (> 25)
Paint mfg., USA unknown < 20 150 Angus Chem. Co. & Occusafe, Inc. (1986)
Automotive mfg., USA 3.6-36 142 < 18 10 Angus Chem. Co. & Occusafe, Inc. (1986)
(1-10) (39)
a mfg. = manufacturing; co. = company
b TWA = time weighted average; STEL = short-term exposure level; values in parentheses are in ppm
c This average is an approximation
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
The limited data on the effects of 2-NP on organisms other than
mammals is summarized below. Kido et al. (1975) reported that 2-NP
(5 g/litre) inhibited the growth of 10 of the 14 species of
microorganisms tested, although all 14 species contained enzymes
capable of oxidizing 2-NP to acetone and nitrite (see section 4.2).
Organisms inhibited by 2-NP included bacteria (Escherichia coli,
Pseudomonas iodinum), yeasts (Endomyces fibuliger, Hansenula
anomala, H. octospora, H. sauveolens, H. matritensis), and fungi
(Aspergillus niger, Penicillium oxalicum, Fusarium oxysporum).
Organisms capable of growing on the medium containing 2-NP similarly
included bacteria (Sarcina lutea, Brevibacterium protophormiae), a
yeast (Hansenula mrakii), and a fungus (Rhizopus batatas).
Fridman et al. (1976) reported that the minimum concentration of
2-NP inhibiting the growth of E. coli and Staphylococcus aureus
was 1000 mg/litre. Observations on aquatic macroorganisms appear
more limited than those on microorganisms. The lowest concentration
of 2-NP in sea water inducing narcosis in barnacle larvae at 18-22
°C was approximately 0.7-0.8 g/litre (Crisp et al., 1967). At 22 °C
the 96-h LC50 for fathead minnows (Pimephales promelas) was >
210 mg/litre (Curtis et al., 1980; Curtis & Ward, 1981). Aeration of
the holding tanks during the toxicity test produced flawed results
because 2-NP was lost continuously by volatilization and a nominal
highest concentration of 612.5 mg/litre was reduced to 496 mg/litre
at the start of the test and to 210 mg/litre by 96 h. There was no
significant mortality at concentrations of 2-NP below 210 mg/litre
although at lower concentrations it did produce severe (though
undescribed) sublethal effects. Observations on non-mammalian
terrestrial organisms are limited to two related species, the
oriental fruit fly (Dacus dorsalis) and the Mediterranean fruit
fly (Ceratitis capitata). Burditt et al. (1963) found that 2-NP
was not especially effective as a fumigant against eggs and larvae
of these species. The 2-h LC50 values for eggs and larvae of the
oriental fruit fly were > 103 and 75 g/m3 (> 28 300 and
20 600 ppm), respectively, and for the Mediterranean fruit fly, > 103
and 35 g/m3 (> 28 300 and 9600 ppm), respectively. These
observations, in summary, suggest a fairly low acute toxicity to
non-mammalian organisms.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Human health risks
Although there are no known biological sources of 2-NP, very low
level non-occupational exposure must be almost universal because
2-NP is known to be a minor component of tobacco smoke and probably
is present in smoke from other types of nitrate-rich organic matter.
Residues in food products containing fatty acids separated with 2-NP
and in beverage can linings and other coatings may represent
additional sources of exposure to low (µg) amounts of 2-NP. The
effects, if any, on human health of low-level exposure are unknown.
Sources of worker exposure include rotogravure and flexographic
inks used in printing, and coatings and adhesives used in industrial
construction and maintenance, highway markings, ship building and
maintenance, furniture manufacture, and food packaging. Based on a
survey conducted in the USA in 1980, occupational exposure to 2-NP
appeared to be limited to a fraction of 1% of the workforce, and
significant occupational exposure (exposure to > 9.1 mg/m3 (>
2.5 ppm)) to about 0.005% of the workforce. Thus, tens of thousands
of workers may have received significant occupational exposure
worldwide. The effects of this occupational exposure are unclear.
Very few industrial fatalities have been attributed to 2-NP. All
involved acute exposure to high concentrations of vapours from
solvent mixtures containing 2-NP during applications of coatings in
confined spaces; all deaths resulted primarily from hepatic failure.
Exposure to non-lethal concentrations of 2-NP may result in
temporary illness or discomfort, but there is no case history or
epidemiological evidence that the chemical induces cancer or other
long-term harmful effects in humans. On the other hand, the numbers
of individuals studied and the periods since first exposure to 2-NP
were inadequate to detect possible long-term harmful effects.
Animal data has demonstrated that 2-NP is a strong
hepatocarcinogen in rats. Chronic exposure to high but non-lethal
concentrations (at least 0.36 g/m3, 100 ppm) induced a high
frequency of hepatic tumours and hepatic cancer in rats but these
observations were not reproduced in limited studies on mice and
rabbits. The mechanism by which 2-NP induces cancer in rats has not
been elucidated as yet. 2-NP is strongly genotoxic to rat
hepatocytes both in vitro and in vivo. It appears likely that
the hepatocarcinogenicity of the compound in rats is a consequence
of liver-specific formation of reactive products. The DNA-damaging
species has not yet been identified. Since it can induce liver cell
proliferation, 2-NP may also have tumour-promoting activity.
Similarities or differences between metabolic activation of 2-NP in
rats and in humans are largely unknown. At present, any substance
shown to be carcinogenic in any mammalian species is considered a
potential human carcinogen. Accurate risk assessment from exposure
to 2-NP requires further studies.
10.2 Effects on the environment
2-NP does not represent a threat to the environment. It appears
highly mobile in the natural environment and is not accumulated in
any individual compartment. It is likely that 2-NP will be destroyed
by photolysis when exposed in the atmosphere to sunlight, and by
biological processes in soil and water. There are, however,
insufficient experimental and observational data on the behaviour of
2-NP in the environment to validate these assumptions or to estimate
rates of degradation in nature. Limited observations on
microorganisms, invertebrates, and fish show a low level of acute
toxicity to non-mammalian organisms.
11. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
There are no indications that 2-NP is carcinogenic in humans.
However, in view of its carcinogenicity in rats, it is recommended
that occupational exposure and its presence in consumer products
such as paints and varnishes be minimized and that it be replaced
with a less toxic solvent whenever practical. Monitoring of the
workplace should be continued to control actual worker exposure.
Although it is not feasible to eliminate non-occupational exposure,
such exposure should be minimized. 2-NP should not be used in food
processing.
12. FURTHER RESEARCH
12.1 Environment
Although it appears likely that 2-NP is highly mobile in the
environment, is not accumulated in any environmental compartment,
and is degraded in the environment to less toxic substances by
microorganisms and ultraviolet radiation, it would be desirable to
confirm experimentally these assumptions and to quantify the speed
of degradation in various environmental compartments.
12.2 Epidemiology
Cohorts of workers in production plants as well as cohorts of
workers using products containing 2-NP (e.g., inks, paints) should
be investigated for incidence of cancer and other ill effects.
12.3 Toxicokinetics
A more detailed examination of the metabolism and distribution
in the body of 2-NP and its metabolites is needed to facilitate an
understanding of the marked species and sex differences in toxic
effects. Studies on distribution and metabolism should include
metabolites of both the carbon and the nitrogen moieties.
Experimental data should be obtained on dermal uptake. Studies on
the metabolism of 2-NP in human cells are needed to facilitate the
eventual extrapolation of data obtained with laboratory animals to
humans.
12.4 Carcinogenesis
Continued efforts should be made to elucidate the biochemical
and molecular mechanisms whereby 2-NP induces genotoxic effects and
cancer.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
2-NP was evaluated by the Joint FAO/WHO Expert Committee on Food
Additives in 1979 and 1981, but no ADI was allocated. The Committee
recommended that 2-NP should not be used as a solvent in food
processing, but it was temporarily acceptable for use as a
fractionating solvent in the production of fats and oils (FAO/WHO,
1984). 2-NP was re-evaluated by JECFA in 1989; it was considered to
be a potent liver carcinogen in rats, and the temporary acceptance
for use as a fractionating solvent in the production of fats and
oils was not extended (FAO/WHO, 1990a, 1990b). The European Economic
Community found 2-NP to be flammable and to be harmful by
inhalation, ingestion, and dermal contact. It required that member
states ensure that it receives proper packaging, labelling, and
storage (IRPTC, 1986).
The International Agency for Research on Cancer evaluated 2-NP
in 1981 (IARC, 1982) and concluded that there was sufficient
evidence for its carcinogenicity in rats but that epidemiological
information was inadequate to evaluate its carcinogenicity to
humans. 2-NP is classified in Group 2B, i.e. as possibly
carcinogenic to humans (IARC, 1987).
REFERENCES
ACGIH (1986) Documentation of the threshold limit values and
biological exposure indices, 5th ed. Cincinnati, Ohio, American
Conference of Governmental Industrial Hygienists, Inc. pp 441-442.
Andersson K, Levin J-O, & Nilsson C-A (1983) Evaluation of solid
sorbents for sampling aliphatic and aromatic nitro compounds.
Chemosphere, 12: 377-384.
Andersson K, Levin J-O, Lindahl R, & Nilsson C-A (1984) Influence of
air humidity on sampling efficiency of some solid adsorbents used
for sampling organics from work-room air. Chemosphere, 13: 437-444.
Andrae U, Homfeld H, Vogl L, Lichtmannegger J, & Summer KH (1988)
2-Nitropropane induces DNA repair synthesis in rat hepatocytes
in vitro and in vivo. Carcinogenesis, 9: 811-815.
Angus Chemical Company (1985) Technical data sheet: NiPar S-20TM
nitropropane solvent, TDS 20B. Northbrook, Illinois, Angus Chemical
Company, 12 pp.
Angus Chemical Company & Occusafe, Incorporated (1986) Industrial
hygiene and occupational health information on 2-nitropropane,
December 1982, and updates for December 1983, 1984, 1985.
Northbrook, Illinois, Angus Chemical Company, 39 pp and 46
attachments (Unpublished report presented to the ACGIH TLV
Committee).
Anon (1976) Grande Paroisse looks for growth in nitroparaffins
market. Eur Chem News, 6 August: 23-24, 30.
Anon (1982) International mineral and chemical's nitroparaffins
business will be acquired by Alberto Natural Gas (Canada) and
Pacific Gas Transmission (US) for up to $ 55 million. Chem Week, 7
April: 24,251.
Baker PJ & Bollmeier AF (1981) Nitroparaffins. In: Grayson M &
Eckroth D ed. Kirk-Othmer encyclopedia of chemical technology, 3rd
ed. New York, John Wiley and Sons, vol 15, pp 969-987.
Baker HJ, Lindsey JR, & Weisbroth SH (1979) Appendix 1. Selected
normative data. In: Baker HJ, Lindsey JR, & Weisbroth SH ed. The
laboratory rat. Volume I: Biology and diseases. Orlando, Florida,
Academic Press, append 1.
Baldwin RS & Williams R (1977) 2-Nitropropane LC50. Northbrook,
Illinois, International Minerals and Chemical Corporation, 6 pp
(Unpublished report No. PLR-218/AMR-072).
Bauchinger M, Kulka U, & Schmid E (1987) Analysis of cytogenetic
effect in human lymphocytes induced by metabolically activated
2-nitropropane. Mutat Res, 190: 217-219.
Beall LR, Alexander V, Bien CT, Chen C, & Infante P (1980) Health
hazard alert - 2-Nitropropane. Cincinnati, Ohio, National Institute
for Occupational Safety and Health, 8 pp (Publication No. 80-142).
Bergman F, Chaimovitz M, & Wind E (1962) Dual action of
nitroparaffins on the guinea-pig ileum. Brit J Pharmacol,
18: 381-396.
Bernard AM, De Russis R, Normand J-C, & Lauwerys RR (1989)
Evaluation of the subacute nephrotoxicity of cyclohexane and other
industrial solvents in the female Sprague-Dawley rat. Toxicol Lett,
45: 271-280.
Bolender FL (1983) 2-NP mortality epidemiology study of the
Sterlington, LA employees: An update, 1-1-46 thru 12-31-81.
Northbrook, Illinois, International Minerals and Chemical
Corporation, 24 pp (Unpublished report).
Breazile JE (1971) Text book of veterinary physiology. Philadelphia,
Pennsylvania, Lea and Febiger, p 347.
Brown D & Dobbin R (1977) Industrial hygiene survey report. IMC,
Sterlington, Louisiana. Cincinnati, Ohio, National Institute for
Occupational Safety and Health, 14 pp.
Burditt AK, Hinman FG, & Balock FG (1963) Screening of fumigants for
toxicity to eggs and larvae of the oriental fruit fly and
Mediterranean fruit fly. J Econ Entomol, 56: 261-265.
Burrows GE (1979) Methylene blue or tolonium chloride antagonism of
sodium nitrite induced methemoglobinemia. J Vet Pharmacol Ther,
2: 81-86.
Cocalis JC (1982) Mining target environmental surveillance study.
Nitropropane sensitized, ammonium nitrate blasting agents.
Morgantown, West Virginia, National Institute for Occupational
Safety and Health, Environmental Investigations Branch, Division of
Respiratory Diseases Studies, 25 pp.
Conkle JP, Camp BJ, & Welch BE (1975) Trace composition of human
respiratory gas. Arch Environ Health, 30: 290-295.
Conaway CC, Nie G, Hussain NS, & Fiala ES (1991a) Comparison of
oxidative damage to rat liver DNA and RNA by primary nitroalkanes,
secondary nitroalkanes, cyclopentanone oxime, and related compounds.
Cancer Res, 51: 3143-3147.
Conaway CC, Nie G, Hussain NS, & Fiala ES (1991b) Evaluation of
secondary nitro-alkanes, their nitronates, primary nitroalkanes,
nitrocarbinols, and other aliphatic nitro compounds in the Ames
Salmonella assay. Mutat Res, 261: 197-207.
Coulston F (1982) Influence of glutathione or diethyl maleate in the
induction of hepatocellular carcinomas in rats exposed to 200 ppm of
2-nitropropane. Alamogordo, New Mexico, Coulston International
Corporation, 14 pp (Unpublished report).
Coulston F, Stein AA, & Griffin TB (1985) Pathology final report.
Histologic study of selected tissues from rats exposed to
2-nitropropane at 100 ppm. Alamogordo, New Mexico, Coulston
International Corporation, 222 pp.
Coulston F, Griffin T, & Eason R (1986) Chronic inhalation exposure
of mice to 2-nitropropane (100 ppm). Alamogordo, New Mexico,
Coulston International Corporation, 5 pp (Unpublished report).
Crawford GN, Garrison RP, & McFee DR (1984) Odor threshold
determination for 2-nitropropane. Am Ind Hyg Assoc J, 45: B7-B8.
Crawford GN, Garrison RP, & McFee, DR (1985) Health examination and
air monitoring evaluation for workers exposed to 2-nitropropane. Am
Ind Hyg Assoc J, 46: 45-47. Crisp DJ, Christie AO, & Ghobashy AFA
(1967) Narcotic and toxic action of organic compounds on barnacle
larvae. Comp Biochem Physiol, 22: 629-649.
Cunningham ML & Matthews HB (1991) Relationship of
hepatocarcinogenicity and hepatocellular proliferation induced by
mutagenic noncarcinogens vs carcinogens. Toxicol Appl Pharmacol,
110: 505-513.
Cupitt LT (1980) Fate of toxic and hazardous materials in the air
environment. Research Triangle Park, North Carolina, US
Environmental Protection Agency, 28 pp (EPA-600/3-80-084).
Curtis MW & Ward CH (1981) Aquatic toxicity of forty industrial
chemicals: Testing in support of hazardous substance spill
prevention regulation. J Hydrol, 51: 359-367.
Curtis MW, Curran CM, & Ward CH (1980) Aquatic toxicity testing as
fundament for a spill prevention program. In: Proceedings of the
1980 Conference on Control of Hazardous Material Spills. Nashville,
Tennessee, Vanderbilt University, pp 284-288.
Dayal R, Gescher A, Harpur ES, Pratt I, & Chipman JK (1989)
Comparison of the hepatotoxicity in mice and the mutagenicity of
three nitroalkanes. Fundam Appl Toxicol, 13: 341-348.
Dayal R, Goodwin B, Linhart I, Mynett K, & Grescher A (1991)
Oxidative denitrification of 2-nitropropane and propane-2-nitronate
by mouse liver microsomes: lack of correlation with hepatic
potential. Chem Biol Interact, 79(1): 103-114.
De Bruin A (1976) Biochemical toxicology of environmental agents.
Amsterdam, Oxford, New York, Elsevier Science Publishers, p 104.
Denk B, Bauman M, & Filser JG (1989) Pharmacokinetics and
hepatotoxicity of 2-nitropropane in rats. Arch Toxicol, 13(suppl):
330-332.
Denk B, Filser JG, Demi E, Kessler W, Shen J, & Oesterle D (1990)
Dose-dependent emergence of preneoplastic foci in rat livers after
exposure to 2-nitropropane. Arch Toxicol, 64: 329-331.
Dequidt J, Vasseur P, & Potencier J (1972) Etude toxicologique
expérimentale de quelques nitroparaffines : 1. Etude du
2-nitropropane. Bull Soc Pharm Lille, 1972: 83-89.
Derks HJGM, Van Heiningen A, Klaassen R, & Olling M (1988) High
performance liquid chromatographic method for the determination of
2-nitropropane in rat plasma. J Chromatogr, 442: 436-440.
Derks HJGM, Van Heiningen A, Klaassen R, & Olling M (1989)
Toxicokinetics of 2-nitropropane in the rat. Bilthoven, The
Netherlands, National Institute of Public Health and Environmental
Protection, 19 pp (Report No. 328605.003).
De Rycker J & Halliwell B (1978) Oxidation of 2-nitropropane by
horseradish peroxidase. Biochem J, 175: 601-606.
Dhawale MR & Hornemann U (1979) Nitroalkane oxidation by
Streptomycetes. J Bacteriol, 137: 916-924.
Estes FL & Gast JH (1960) The in vitro effects of aliphatic nitro
compounds on tissues. Arch Environ Health, 1: 59-64.
FAO/WHO (1984) Evaluation of certain food additives and
contaminants. Twenty-eighth report of the Joint FAO/WHO Expert
Committee on Food Additives. Geneva, World Health Organization,
44 pp (WHO Technical Report Series 710).
FAO/WHO (1990a) Evaluation of certain food additives and
contaminants. Thirty-fifth report of the Joint FAO/WHO Expert
Committee on Food Additives. Geneva, World Health Organization,
48 pp (WHO Technical Report Series 789).
FAO/WHO (1990b) Toxicological evaluation of certain food additives
and contaminants, prepared by the 35th meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA). Geneva, World Health
Organization, 189 pp (WHO Food Additives Series 26).
Fiala ES, Czerniak R, & Williams GM (1986) Formation of carcinogenic
and/or genotoxic aliphatic azoxy compounds from the respective
nitroalkanes by direct mild chemical reduction. Toxicologist,
6: 40.
Fiala ES, Conaway CC, Biles WT, & Johnson B (1987a) Enhanced
mutagenicity of 2-nitropropane nitronate with respect to
2-nitropropane possible involvement of free radical species. Mutat
Res, 179: 15-22.
Fiala ES, Czerniak R, Castonguay A, Conaway CC, & Rivenson A (1987b)
Assay of 1-nitropropane, 2-nitropane, 1-azoxypropane and
2-azoxypropane for cancinogenicity by gavage in Sprague-Dawley rats.
Carcinogenesis, 8: 1947-1949.
Fiala ES, Conaway CC, & Mathis JE (1989) Oxidative DNA and RNA
damage in the livers of Sprague-Dawley rats treated with the
hepatocarcinogen 2-nitropropane. Cancer Res, 49: 5518-5522.
Fieser LF & Fieser M (1972) Reagents for organic synthesis. New
York, Wiley-Interscience, vol 3, pp 213-214.
Fieser LF & Fieser M (1974) Reagents for organic synthesis. New
York, Wiley-Interscience, vol 4, p 256.
Filser JG & Baumann M (1988) Pharmokinetics of 2-nitropropane in
rats and determination of enzymes in serum specific for liver
injury. Naunyn Schmiedeberg's Arch Pharmacol, 337(suppl): R17.
Fine DH, Challis BC, Hartman P, & Van Ryzin J (1982) Endogenous
synthesis of volatile nitrosamines: model calculations and risk
assessment. In: N-nitroso compounds: Occurrence and biological
effects. Proceedings of the VIIth International Symposium on
N-Nitroso Compounds, Tokyo, 28 September-1 October 1981. Lyon,
International Agency for Research on Cancer, pp 379-396 (IARC
Scientific Publications No. 41).
Finklea JF (1977) Current NIOSH intelligence bulletin:
2-nitropropane. Am Ind Hyg Assoc J, 38(7): A-15, A-17, A-19.
Fishbein L (1981) Carcinogenicity and mutagenicity of solvents. I.
Glycidyl ethers, dioxane, nitroalkanes, dimethylformamide and allyl
derivatives. Sci Total Environ, 17: 97-110.
Flanner LT (1972) 2-Nitropropane or nitromethane for inhibiting the
corrosion of tin-plated steel aerosol cans: US Patent US 3650982
(21 March 1972). USA, Allied Chemical Corporation, 2 pp (Abstract
only).
Freitag D, Geyer H, Kraus A, Viswanathan R, Kotzias D, Attar A,
Klein W, & Korte F (1982) Ecotoxicological profile analysis. VII.
Screening chemicals for their environmental behavior by comparative
evaluation. Ecotoxicol Environ Saf, 6: 60-81.
Freitag D, Ballhorn L, Geyer H, & Korte F (1985) Environmental
hazard profile of organic chemicals. Chemosphere, 14: 1589-1616.
Friedman MA, Greene EJ, & Epstein SS (1972) Rapid gastric absorption
of sodium nitrite in mice. J Pharm Sci, 61: 1492-1494.
Friedman AL, Zalesov VS, Surkov VD, Kratynskaya LV, & Plaksina AN
(1976) [Synthesis and study of the physiological activity of
aliphatic nitro compounds. X. Relation among structure, toxicity,
and antimicrobial activity in a series of nitroalkanes and their
alpha-halo derivatives.] Khim-Farm Zh, 10: 53-56 (in Russian).
Galloway SM, Armstrong MJ, Reuben C, Colman S, Brown B, Cannon C,
Bloom AD, Nakamura F, Ahmed M, Duk S, Rimpo J, Margolin BH, Resnick
MA, Anderson B, & Zeiger E (1987) Chromosome aberrations and sister
chromatid exchanges in Chinese hamster ovary cells: Evaluations of
108 chemicals. Environ Mol Mutagen, 10(suppl 10): 1-175.
Gautier M, Fournier P-E, Gervais P, & Sicot C (1964) Intoxication
par le nitropropane. Arch Mal Prof, 25: 425-428.
George E, Burlinson B, & Gatehouse D (1989) Genotoxicity of 1- and
2-nitropropane in the rat. Carcinogenesis, 10: 2329-2334.
Glaser RA, & Woodfin WJ (1981) A method for sampling and analysis of
2-nitropropane in air. Am Ind Hyg Assoc J, 42: 18-22.
Goggelmann W, Bauchinger M, Kulka U, & Schmid E. (1988) Genotoxicity
of 2-nitropropane and 1-nitropropane in Salmonella typhimurium and
human lymphocytes. Mutagenesis, 3: 137-140.
Goldwhite H (1965) Nitrogen derivatives of the aliphatic
hydrocarbons. In: Coffey S ed. Rodd's chemistry of carbon compounds,
Part B, 2nd ed. Amsterdam, Oxford, New York, Elsevier Science
Publishers, vol. 1, pp 93-164.
Griffin TB & Coulston F (1983) Final report. Chronic inhalation
exposure of rats to vapors of 2-nitropropane at 100 ppm. Alamogordo,
New Mexico, Coulston International Corporation, 13 pp.
Griffin TB, Benitz K-F, Coulston F, & Rosenblum I (1978) Chronic
inhalation toxicity of 2-nitropropane in rats. Pharmacologist, 20:
145.
Griffin TB, Coulston F, & Stein AA (1980) Chronic inhalation
exposure of rats to vapors of 2-nitropropane at 25 ppm. Ecotoxicol
Environ Saf, 4: 267-281.
Griffin TB, Stein AA, & Coulston F (1981) Histologic study of
tissues and organs from rats exposed to vapors of 2-nitropropane at
25 ppm. Ecotoxicol Environ Saf, 5: 194-201. Griffin T, Coulston F, &
Stein AA (1984) Chronic inhalation exposure of mice to
2-nitropropane. Preliminary report: Laboratory findings and
histopathology of liver. Alamogordo, New Mexico, Coulston
International Corporation, 14 pp (Unpublished report).
Griffin T, Coulston F, & Stein AA (1986) Long-term effects of acute
exposure of rats to 2-nitropropane. Final Report, Study No. 820202.
Alamogordo, New Mexico, Coulston International Corporation, 17 pp
(Unpublished report).
Griffin T, Coulston F, & Stein AA (1987) Chronic inhalation exposure
of mice to 2-nitropropane (100 ppm): Final report. Alamogordo, New
Mexico, Coulston International Corporation, 24 pp (Unpublished
report).
Guo N, Conaway CC, Hussain NS, & Fiala ES (1990) Sex and organ
differences in oxidative DNA and RNA damage due to treatment of
Sprague-Dawley rats with acetoxime or 2-nitropropane.
Carcinogenesis, 11: 1659-1662.
Haas-Jobelius M, Ziegler-Skylakakis K, & Andrae U (1991)
Nitroreduction is not involved in the genotoxicity of 2-nitropropane
in cultured mammalian cells. Mutagenesis, 6: 87-91.
Hardin BD, Bond GP, Sikov MR, Andrew FD, Beliles RP, & Niemeier RW
(1981) Testing of selected workplace chemicals for teratogenic
potential. Scand J Work Environ Health, 7(suppl 4): 66-75.
Harris SJ, Bond GP, & Niemeier RW (1979) The effects of
2-nitropropane, naphthalene, and hexachlorobutadiene on fetal rat
development. Toxicol Appl Pharmacol, 48: A35.
Harrison RJ, Pasternak G, Blanc P, Basuk P, & Letz G (1985) Acute
hepatic failure after occupational exposure to 2-nitropropane. J Am
Med Assoc, 254(24): 3415-3416.
Harrison RJ, Letz G, Pasternak G, & Blanc P (1987) Fulminant hepatic
failure after occupation exposure to 2-nitropropane. Ann Intern Med,
107: 466-468.
Hartle RW (1980) Health hazard evaluation report No. 80-057-781 at
Long Island Railroad, Richmond Hill, New York, Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 17 pp.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, 5 (suppl 1): 3-142.
Hine CH, Pasi A, & Stephens BG (1978) Fatalities following exposure
to 2-nitropropane. J Occup Med, 20: 333-337.
Hite M & Skeggs H (1979) Mutagenic evaluation of nitroparaffins in
the Salmonella typhimurium/mammalian microsome test and the
micronucleus test. Environ Mutagen, 1: 383-389.
Hoar RM (1976) Chapter 3. Biomethodology. In: Wagner JE & Manning PJ
ed. The biology of the guinea pig. New York, London, Academic Press,
p 16.
Hoffmann D & Rathkamp C (1968) Chemical studies on tobacco smoke.
III. Primary and secondary nitroalkanes in cigarette smoke. Beitr
Tabakforsch, 4: 124-134.
Hollett BA & Schloemer J (1978) Hazard evaluation technical
assistance report No. TA 76-90: Newport Industrial Products
Firestone Tire Rubber Co., Newport, Tennessee. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 52 pp.
Hughes TJ, Simmons DS, Monteith LG, Myers LD, & Claxton LD (1987)
Mutagenicity of 31 organic compounds in a modified preincubation
Ames assay with Salmonella typhimumium strains TA100 and TA102.
Environ Mutagen, 9 (suppl 8): 49.
Hussain NS, Conaway CC, Guo N, Asaad W, & Fiala ES (1990) Oxidative
DNA and RNA damage in rat liver due to acetoxime: similarity to
effects of 2-nitropropane. Carcinogenesis, 11: 1013-1016.
Indig GL & Cilento G (1987) Peroxidase-promoted aerobic oxidation of
2-nitropropane: Mechanism of excited state formation. Biochim
Biophys Acta, 923: 347-354.
IARC (1982) 2-Nitropropane. In: Some industrial chemicals and
dyestuffs. Lyon, International Agency for Research on Cancer, pp
331-343 (IARC Monographs on the Evaluation of the Carcinogenic Risk
of Chemicals to Humans, Volume 29).
IARC (1987) Overall evaluations of carconogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer, p 67 (IARC Monographs on the Evaluation of the
Carconogenic Risk of Chemicals to Humans, Supplement 7).
IRPTC (1986) IRPTC data profile on 2-nitropropane. Geneva,
International Register of Potentially Toxic Chemicals, United
Nations Environment Programme, pp 17/1-17/3.
Ivanetich KM, Lucas S, Marsh JA, Ziman MR, Katz ID, & Bradshaw JJ
(1978) Organic compounds. Their interaction with and degradation of
hepatic microsomal drug-metabolizing enzymes in vitro. Drug Metab
Disp, 6: 218-225.
Jones LR (1963) The determination of 2-nitropropane in air. Ind Hyg
J, January-February: 11-16.
Jones LR & Riddick JA (1952) Colorimetric determination of
nitroparaffins. Anal Chem, 24: 1533-1536.
Jonsson J, Kammerer RC, & Cho AK (1977) Metabolism of
2-nitro-1-phenylpropane by rabbit liver microsomes. Res Commun chem
Pathol Pharmacol, 18: 75-82.
Kaplan HM, Brewer NR, & Blair WH (1983) Physiology. In: Foster HL,
Small JD, & Fox JG ed. The mouse in biomedical research. Volume III
Normative biology, immunology, and husbandry. New York, London,
Academic Press, p 255.
Kawai A, Goto S, Matsumoto Y, & Matsushita H (1987) [Mutagenicity of
aliphatic and aromatic nitro compounds. Industrial materials and
related compounds.] Jpn J ind Health, 29: 34-54 (in Japanese).
Kenaga EE (1980) Predicted bioconcentration factors and soil
sorption coefficients of pesticides and other chemicals. Ecotoxicol
environ Saf, 4: 26-38.
Kido T, Yamamoto T, & Soda K (1975) Microbial assimilation of alkyl
nitro compounds and formation of nitrite. Arch Microbiol, 106:
165-169.
Kido T, Tanizawa K, Inagaki K, Yoshimura T, Ishida M, Hashizume K, &
Soda K (1984) 2-Nitropropane dioxygenase from Hansenula mrakii:
Re-characterization of the enzyme and oxidation of anionic
nitroalkanes. Agric Biol Chem, 48: 2549-2554.
Kliesch U & Adler I-D (1987) Micronucleus test in bone marrow of
mice treated with 1-nitropropane, 2-nitropropane and cisplatin.
Mutat Res, 192: 181-184.
Korte F, Freitag D, Geyer H, Klein W, Kraus AG, & Lahaniatis E
(1978) Ecotoxicologic profile analysis. Chemosphere, 1: 79-102.
Kozma C, Macklin W, Cummins LM, & Mauer R (1974) Chapter 3. Anatomy,
physiology, and biochemistry of the rabbit. In: Weisbroth SH, Flatt
RE, & Kraus AL ed. The biology of the laboratory rabbit. New York,
London, Academic Press, pp 56-57.
Krotoszynski BK, Bruneau GM, & O'Neill HJ (1979) Measurement of
chemical inhalation exposure in urban population in the presence of
endogenous effluents. J Anal Toxicol, 3: 225-234.
Kuo FK & Fridovich (1986) Free-radical chain oxidation of 2-NP
initiated and propagated by superoxide. Biochem J, 237: 505-510.
Levy A (1973) The photochemical smog reactivity of organic solvents.
Adv Chem Ser, 124: 70-94.
Lewis TR, Ulrich CE, & Busey WM (1979) Subchronic inhalation
toxicity of nitromethane and 2-nitropropane. J Environ Pathol
Toxicol, 2: 233-249.
Life Extension Institute (1983) Summary report of medical findings
for selected employees at one plant. Rockville, Maryland, Life
Extension Institute, 12 pp.
Little HN (1951) Oxidation of nitroethane by extracts from
Neurospora. J Biol Chem, 193: 347-358.
Little HN (1957) The oxidation of 2-nitropropane by extracts of pea
plants. J Biol Chem, 229: 231-238.
Litton Bionetics, Inc. (1977) Mutagenicity evaluation of P-1357
66459T: Final report. Terre Haute, Indiana, IMC Chemical Group,
Inc., 10 pp (Unpublished report).
Löfroth G, Nilsson L, & Andersen JR (1986) Structure-activity
relationship of nitroalkane-induced mutagenicity in the Ames
Salmonella assay. In: Genetic toxicology of environmental
chemicals. Part B: Genetic effects and applied mutagenesis. New
York, Alan R. Liss, Inc., pp 149-155.
Lotz F, Nitz S, & Korte F (1979) [Photomineralization of adsorbed
organic chemicals on a microscale.] Chemosphere, 10: 763-768 (in
German).
Love JR & Kern M (1981) Health hazard evaluation report no.
81-065-938. Metro bus maintenance shop, Washington, DC. Cincinnati,
Ohio, National Institute for Occupational Safety and Health, 13 pp.
Mabuchi H (1979) [Urinary and serum volatile substances in
diabetics.] Nippon Naika Gakkai Zasshi, 58: 731-743 (Abstract only)
(in Japanese).
McGregor DB (1981) Tier II mutagenic screening of 13 NIOSH priority
compounds. Individual compound report: 2-Nitropropane. Musselburgh,
Scotland, Inveresk Research International Ltd, 194 pp (Report No.
31).
Machle W, Scott EW, & Treon J (1940) The physiological response of
animals to some simple mononitroparaffins and to certain derivatives
of these compounds. J Ind Hyg Toxicol, 22: 315-332.
Malkinson FD & Gehlmann L (1977) Factors affecting percutaneous
absorption. In: Drill VA & Lazar P, ed. Cutaneous toxicity. New
York, London, Academic Press, pp 63-82.
Marcstrom A (1967) Studies on the connection between physicochemical
properties and stimulating abilities of some sweet and bitter
compounds. Ark Zool, 19: 421-535.
Marker EK & Kulkarni AP (1985) Enzymatic denitrification of
2-nitropropane in uninduced mouse liver microsomes. Toxicol Lett,
26: 181-185.
Marker EK & Kulkarni AP (1986a) Cumene hydroperoxide-supported
denitrification of 2-nitropropane in uninduced mouse liver
microsomes. Int J Biochem, 18: 595-601.
Marker EK & Kulkarni AP (1986b) Cytochrome P-450-mediated
denitrification of 2-nitropropane in mouse liver microsomes. J
Biochem Toxicol, 1: 71-83.
Miller ME & Temple GW (1979) 2-NP mortality epidemiology study of
the Sterlington, LA Employees, 1-1-46 thru 6-30-77. Northbrook,
Illinois, International Minerals and Chemical Corporation, 34 pp
(Unpublished report).
Mochizuki H, Kominami S, & Takemori S (1988) Examination of
differences between benzo[ a]pyrene and steroid hydroxylases in
guinea pig adrenal microsomes. Biochem Biophys Acta, 964: 83-89.
Müller WF, Coulston F, & Korte F (1983) Comparative metabolism of
2-nitropropane in rats and chimpanzees. Chemosphere, 12: 231-237.
National Fire Protection Association (1968) Hazardous chemicals
data. J Chem Educ, 45: A313-A314, A317-A318, 320-322.
National Library of Medicine (1989) Toxic chemical release
inventory. Washington, DC, National Library of Medicine (TOXNET
system, TRI data base, 12 June 1989).
Nielsen AT (1981) Nitronic acids and esters. In: Feurer H ed. The
chemistry of the nitro and nitroso groups. Huntington, New York,
Robert Krieger Publishing Co., Part I, pp 349-386.
Nolan RJ, Unger SM, & Muller CJ (1982) Pharmacokinetics of inhaled
[14C]-2-nitropropane in male Sprague-Dawley rats. Ecotoxicol
Environ Saf, 6: 388-397.
Occupational Health Services, Inc. (1982) An evaluation of the
number of workers occupationally exposed to 2-nitropropane.
Cambridge, Massachusetts, Occupational Health Services, Inc., 22 pp
(Unpublished report prepared for IMC Corporation, Des Plaines,
Illinois).
Parks NJ, Krohn KA, Mathis CA, Chasko JH, Geiger KR, Gregor ME, &
Peek NF (1981) Nitrogen-13-labeled nitrite and nitrate: Distribution
and metabolism after intratracheal administration. Science, 212:
58-61.
Paszyc S (1971) [Photolysis of 2-nitropropane in gaseous and liquid
phases.] Poznan Tow Przyj Nauk Pr Kom Mat-Przyr, Pr Chem, 12: 79-85
(in Polish).
Patel RN, Hou CT, Laskin AI, & Felix A (1982) Epoxidation of alkenes
and hydroxylation of alkanes by soluble methane monooxygenase:
Regeneration of cofactor NADH2. J Appl Biochem, 4: 175-184.
Porter DJ & Bright HJ (1983) The mechanism of oxidation of
nitroalkanes by horseradish peroxidase. J Biol Chem, 258: 9913-9924.
Purcell RF (1967) Use of 2-nitropropane under rule 66. West Paint
Rev, 53: 22A, 24A.
Reece WO (1984) Respiration in mammals. In: Swenson MJ ed. Duke's
physiology of domestic animals, 10th ed. Cornell, New York, Comstalk
Press, p 231.
Robbiano L, Mattioli F, & Brambilla G (1991) DNA fragmentation by
2-nitropropane in rat tissues, and effects of the modulation of
biotransformation processes. Cancer Lett. 57: 61-66.
Rondia D (1979) 2-nitropropane: One more death. Vet Hum Toxicol, 21:
183-185.
Roscher E, Ziegler-Skylakakis K, & Andrae U (1990) Involvement of
different pathways in the genotoxicity of nitropropanes in cultured
mammalian cells. Mutagenesis, 5: 375-380.
Sadtler SP (1961) Sadtler standard ultraviolet spectra.
Philadelphia, Pennsylvania, Sadtler Research Laboratory, p 1.
Sakurai H, Hermann G, Ruf HH, & Ullrich V (1980) The interaction of
aliphatic nitro compounds with the liver microsomal monooxygenase
system. Biochem Pharmacol, 29: 341-345.
Sander J & Schweinsberg F (1972) [Interrelationships between
nitrate, nitrite and carcinogenic N-nitroso-compounds. 1.
Communication: nitrate, nitrite and nitrosable amino-compounds in
food and drugs, chemistry of N-nitroso compounds.] Zentrabl Bakeriol
Parasitenkd Infektionsk Hyg Abt 1: Orig Reihe B, 156: 299-340 (in
German).
Schneider NR & Yeary RA (1975) Nitrite and nitrate pharmacokinetics
in the dog, sheep, and pony. Am J Vet Res, 36: 941-947.
Scott EW (1943) The metabolism of monoparaffins. III. The
concentration of nitroethane, nitrite and nitrate in the blood of
rabbits during exposure by inhalation and oral administration. J Ind
Hyg Toxicol, 25: 20-25.
Seizinger DC & Dimitrades B (1972) Oxygenates in exhaust from simple
hydrocarbon fuels. J Air Pollut Control Assoc, 22: 47-51.
Simmons DM, Monteith LG, Hughes TJ, & Claxton LD (1986) Effect of
preincubation time on mutagenic activity in the Ames/ Salmonella
assay. Environ Mutagen, 8(suppl 6): 78.
Skinner JB (1947) The toxicity of 2-nitropropane. Ind Med, 16:
441-443.
Smith EL, Hill RL, Lehman IR, Lefkowitz RJ, Handler P, & White A
(1983) Principles of biochemistry. General aspects. New York,
McGraw-Hill, pp 539-541.
Soda K, Kido T, & Asada K (1977) 2-Nitropropane dioxygenase from
Hansenula mrakii: Generation and participation of superoxide anion
in the reaction. In: Hayashi O & Asada K ed. Biochemical and medical
aspects of active oxygen. Baltimore, Maryland, University Park
Press, pp 119-133.
Speck WT, Meyer LW, Zeiger E, & Rosenkranz HS (1982) Mutagenicity
and DNA-modifying activity of 2-nitropropane. Mutat Res, 104: 49-54.
SRI International (1988) 1988 Directory of chemical producers,
United States of America. Menlo Park, California, SRI International,
pp 29, 168, 803.
SRI International (1990) 1990 Directory of chemical producers,
United States of America. Menlo Park, California, SRI International,
p 815.
Stokinger HE (1982) Aliphatic nitro compounds, nitrates, nitrites.
In: Clayton GD & Clayton FE ed. Patty's industrial hygiene and
toxicology, 3rd revised ed. New York, John Wiley and Sons, vol 2C,
pp 4141-4208.
Swann RL, Laskowski DA, McCall PJ, Vander Kuy K, & Dishburger HJ
(1983) A rapid method for the estimation of the environmental
parameters octanol/water partition coefficient, soil sorption
constant, water to air ratio, and water solubility. Residue Rev.
85 : 17-28.
Tabershaw Occupational Medicine Association (1980) Cross-sectional
morbidity study of workers. Rockville, Maryland, Tabershaw
Occupational Medicine Association, 68 pp (Unpublished report).
Treon JF & Dutra FR (1952) Physiological response of experimental
animals to the vapor of 2-nitropropane. Arch Ind Hyg Occup Med,
5 : 52-61.
Ullrich V & Schnabel KN (1973) Formation and binding of carbanions
by cytochrome P-450 of liver microsomes. Drug Metab Disp, 1:
176-183.
Ullrich V, Hermann G, & Weber P (1978) Nitrite formation from
2-nitropropane by microsomal monooxygenases. Biochem Pharmacol,
27 : 2301-2304.
US Bureau of the Census (1987) Statistical abstract of the United
States: 1988, 108th ed. Washington, DC, US Bureau of the Census,
p 368.
US EPA (1977) TSCA chemical assessment series. Chemical hazard
information profiles (CHIPS), August 1976-August 1978. Washington,
DC, US Environmental Protection Agency, pp 212-217
(EPA-560/11-80-011).
US EPA (1980) Materials balance 2-nitropropane, Level 1. Washington,
DC, US Environmental Protection Agency, 70 pp (EPA-560/13-89-011).
US EPA (1985) Health and environmental effects profile for
2-nitropropane. Washington, DC, US Environmental Protection Agency,
pp 3-4 (EPA-600/X-85-112).
US EPA (1986) Notice of potential risk, 2-nitropropane. Washington,
DC, US Environmental Protection Agency, 5 pp.
US FDA (Food and Drug Administration) (1987) 2-Nitropropane. Code
Fed Regul, 27 (Parts 1 to 199): Docket No. 78N-0221.
USITC (1990) Synthetic organic chemicals. United States production
and sales, 1989. Washington, DC, United States International Trade
Commission, pp 15-17, 15-35-15-36 (USITC Publication No. 2338).
US NIOSH (1983) National occupational exposure survey, 1981-83:
Actual observation and trade-name exposure to 2-nitropropane.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health (Unpublished database).
US NIOSH (1987a) NIOSH manual of analytical methods. Second
supplement, 3rd ed. Cincinnati, Ohio, National Institute for
Occupational Safety and Health, pp 2528/1-2528/4 (NIOSH Publication
No. 87-117).
US NIOSH (1987b) Safe sheet #1: Summary of accidental fatality
evaluations, confined spaces, Case #3. Morgantown, West Virginia,
National Institute for Occupational Safety and Health, Environmental
Investigations Branch, Division of Respiratory Disease Studies, p 2.
US NIOSH (1988) Current intelligence bulletins: Summaries, September
1988. Cincinnati, Ohio, National Institute for Occupational Safety
and Health, p 6.
US OSHA (Occupational Safety and Health Administration) (1988)
1-Nitropropane and 2-nitropropane. Code Fed Regul, 29: 705, 708
(Parts 1900 to 1910).
Weast RC ed. (1986) CRC handbook of chemistry and physics, 67th ed.
Boca Raton, Florida, CRC Press, p C-444.
Wester PW, Van Leeuwen FXR, & De Vries T (1989) 4-Week toxicity
study with 2-nitropropane (2-NP) by gavage in rats. Bilthoven, The
Netherlands, National Institute of Public Health and Environmental
Protection, 20 pp (Report No. 658501 001).
Wilbur SZ & Parekh CK (1982) Dermal toxicity potential of
2-nitropropane (P-1357). Northbrook, Illinois, International
Minerals and Mining Corporation, 5 pp (Unpublished report No.
PLR-281/AMR-072).
Wilks RA & Gilbert SG (1972a) Sensory and instrumental evaluation in
model systems of residues migrating from can coatings. J Food Sci,
37: 72-76.
Wilks RA & Gilbert SG (1972b) Quick test for liner solvents. Modern
Packag, May: 52-55.
Williams SV, Bryan JA, Burk JR, & Wolf FS (1974) Toxic hepatitis and
methylenedianiline. New Engl J Med, 291: 1256.
Windholz M ed. (1983) The Merck index, 10th ed. Rahway, New Jersey,
Merck & Co., p 6482.
Woo Y-T, Lai DY, Arcos JC, & Argus MF (1985) Chemical induction of
cancer: structural bases and biological mechanisms, Vol. III B:
Aliphatic and polyhalogenated carcinogens. New York, London,
Academic Press, pp 329-341.
Yasuhara A, Yamanaka Y, & Ogawa T (1986) Volatile compounds in
machine cutting-fluid emulsion. Agric Biol Chem, 50: 1765-1770.
Ziegler-Skylakakis K, Homfeldt H, & Andrae U (1987) In vitro and
in vivo genotoxicity of 2-nitropropane and 1-nitropropane in
mammalian cells. Naunyn-Schmiedeberg's Arch Pharmacol, 335(suppl):
R25.
Zimmering S, Mason JM, Valencia R, & Woodruff RC (1985) Chemical
mutagenesis testing in Drosophila. II. Results of 20 coded
compounds tested for the National Toxicology Program. Environ
Mutagen, 7: 87-100.
Zitting A, Savolainen H, & Nickels J (1981) Acute effects of
2-nitropropane on rat liver and brain. Toxicol Lett, 9: 237-246.
RESUME
1. Propriétés et méthodes d'analyse
Le 2-nitropropane (2-NP) est un liquide huileux, incolore, à
l'odeur douceâtre. Il est inflammable, moyennement volatil et stable
dans les conditions ordinaires. Il n'est que légèrement soluble dans
l'eau mais miscible à de nombreux liquides organiques et c'est un
excellent solvant de nombreux composés organiques. Il existe de
bonnes méthodes d'analyse pour la recherche et le dosage du
2-nitropropane présent dans l'environnement. On a actuellement
recours à la chromatographie en phase gazeuse avec détection par
ionisation de flamme ou capture d'électrons, ou bien à la
chromatographie en phase liquide à haute performance avec détecteur
u.v. Pour le dosage dans l'air, il faut tout d'abord piéger le
2-nitropropane et le concentrer par adsorption sur phase solide.
2. Emploi et sources d'exposition
2.1 Production
Les chiffres de production actuels ne sont pas connus. En 1977
les Etats-Unis d'Amérique en ont produit environ 13 600 tonnes. Le
2-nitropropane est actuellement produit par deux firmes américaines
et une firme française. Il peut prendre naissance naturellement à
l'état de traces lors de la combustion du tabac et d'autres matières
organiques riches en nitrates mais rien n'indique qu'il puisse se
former à l'issue d'un processus biologique quelconque.
2.2 Emploi et passage dans l'environnement
Le 2-nitropropane est utilisé comme solvant, principalement en
mélange, et il a de nombreuses applications industrielles en tant
que tel pour la confection d'encres d'imprimerie, de peintures, de
vernis, d'adhésifs et autres revêtements, comme par exemple ceux que
l'on utilise dans les récipients contenant des boissons. On
l'utilise également comme solvant pour séparer des composés très
voisins comme les acides gras, comme intermédiaire en synthèse
chimique et comme additif dans les carburants. C'est principalement
par l'intermédiaire de l'air qu'il passe dans l'environnement,
surtout par évaporation à partir des surfaces enduites de produits
qui en contiennent.
3. Transport et distribution dans l'environnement
Le 2-nitropropane se révèle très mobile dans le milieu naturel.
Du fait que sa solubilité dans l'eau, son adsorption par les
sédiments et sa bioaccumulation sont faibles et qu'il s'évapore
rapidement dans l'atmosphère, il se répartit dans l'air et l'eau
sans s'accumuler dans un compartiment particulier du milieu. Le
2-nitropropane absorbe le rayonnement ultra-violet aux longueurs
d'onde que l'on rencontre dans l'environnement et il est donc
probable qu'il subisse une lente photolyse dans l'atmosphère. Il est
également probable qu'il soit lentement transformé par voie
biologique en composés moins toxiques, tant dans l'environnement
aquatique que dans l'environnement terrestre.
4. Concentrations dans l'environnement et exposition humaine
Il semble que l'exposition de la population générale au
2-nitropropane soit très faible et provienne de la fumée de
cigarettes (1,1 à 1,2 µg/cigarette), de résidus présents dans des
enduits ou revêtements tels qu'on en utilise pour les récipients
contenant des boissons, dans les adhésifs, les encres d'imprimerie,
ainsi que dans les huiles végétales que l'on fractionne au moyen de
ce solvant. On ignore quelle est l'importance de l'exposition des
travailleurs de l'industrie dans le monde, mais aux Etats-Unis
d'Amérique, il semble qu'elle soit limitée à 0,02-0,19 % du
personnel. Dans ce même pays, environ 4000 travailleurs (soit à peu
près 0,005 % de la main-d'oeuvre) subissent sans doute une
exposition notable (de l'ordre de 9,1 mg/m3(2,5 ppm) ou
davantage). Selon les pays, les limites d'exposition professionnelle
dans l'air vont de 36 mg/m3 (1 ppm) (TWA) à 146 mg/m3 (40 ppm)
(STEL). La production du 2-nitropropane s'effectue en circuit fermé
et en général, le personnel n'est guère exposé; toutefois dans
certaines industries (peinture, imprimerie ou extraction par
solvant), il est arrivé que des travailleurs soient exposés à des
concentrations dépassant largement les limites d'exposition
professionnelle. C'est ainsi que des concentrations atteignant
6 g/m3 (1640 ppm) dans l'air ont été enregistrées lors du
remplissage de fûts.
5. Cinétique et métabolisme
Le 2-nitropropane est principalement résorbé au niveau des
poumons. Chez l'animal de laboratoire, on a montré qu'il était
rapidement absorbé non seulement à ce niveau mais également au
niveau de la cavité péritonéale et des voies digestives. On est mal
renseigné sur son absorption percutanée. Quant aux données
concernant sa distribution dans l'organisme du rat, elles sont
quelque peu contradictoires. Le 2-nitropropane est rapidement
métabolisé, essentiellement sous forme d'acétone et de nitrite. Il
se forme peut-être aussi un peu d'alcool isopropylique. Après
injection par voie intrapéritonéale, le 2-nitropropane et ses
métabolites carbonés se concentrent d'abord dans les graisses, puis
dans la moelle osseuse ainsi que dans les surrénales et les autres
organes internes; après inhalation, ils se concentrent dans le foie
et les reins, mais relativement peu dans les graisses. Il est
possible que plusieurs systèmes enzymatiques interviennent dans ce
métabolisme et les vitesses, de même que les voies de
métabolisation, varient selon les espèces. Le 2-nitropropane et ses
métabolites carbonés disparaissent rapidement de l'organisme par
métabolisation, exhalation ou excrétion dans les urines et les
matières fécales. On manque de données satisfaisantes sur la
distribution et l'excrétion de la fraction nitrée de ces
métabolites.
6. Effets sur les mammifères de laboratoire et les systèmes
in vitro
La toxicité aiguë du 2-nitropropane pour les mammifères est
modérée. Les mâles sont plus sensibles que les femelles, tout au
moins en ce qui concerne le rat, et l'expérience montre que la
sensibilité varie largement d'une espèce à l'autre. La CL50
(concentration produisant une mortalité de 50 % en 14 jours) a été
de 1,5 g/m3 (400 ppm) pour les rats mâles et de 2,6 g/m3
(720 ppm) pour les rats femelles. Il semble que la mortalité soit
associée principalement aux effets narcotiques, mais les mammifères
exposés à des concentrations d'au moins 8,4 g/m3 (2300 ppm)
pendant une heure ou davantage ont présenté des anomalies
anatomopathologiques graves, notamment des lésions
hépatocellulaires, un oedème du poumon et des hémorragies.
Il est indiscutable que le 2-nitropropane est cancérogène pour
le rat. L'exposition de rats pendant de longues durées à du
2-nitropropane par voie respiratoire à raison de 0,36 g/m3
(100 ppm) (pendant 18 mois à raison de sept heures par jour et cinq
jours par semaine) a causé des lésions destructrices au niveau du
foie, et en particulier des carcinomes hépatocellulaires chez
certains rats mâles. A la concentration de 0,75 g/m3 (207 ppm) les
lésions étaient encore plus graves, avec une forte incidence de
carcinomes hépatocellulaires à survenue plus rapide.
L'administration chronique de doses modérées par voie orale a
également provoqué une surincidence de carcinomes hépatocellulaires.
Toutefois, l'inhalation prolongée par des rats de 2-nitropropane aux
doses respectives de 91 ou 98,3 mg/m3 (25 ou 27 ppm) n'a pas
provoqué de lésions décelables. L'exposition de souris et de lapins
à des concentrations de 2-nitropropane capables de provoquer des
carcinomes hépatocellulaires chez le rat n'a guère eu d'effets chez
ces animaux, mais il est vrai que les études en question étaient
trop limitées pour qu'on puisse exclure totalement un effet
cancérogène du 2-nitropropane chez ces deux espèces. Le
2-nitropropane a légèrement retardé le développement foetal des rats
mais les données relatives à l'embryotoxicité, à la tératogénicité
et à la toxicité du 2-NP pour la fonction de reproduction restent
très fragmentaires. Le produit s'est révélé fortement génotoxique
pour les hépatocytes de rats tant in vivo qu' in vitro; en
revanche aucune génotoxicité sensible n'a été observée au niveau des
autres organes chez le rat ou sur des lignées cellulaires d'origine
extrahépatique, en l'absence d'activation métabolique exogène. On a
également montré que le 2-nitropropane était mutagène chez les
bactéries en présence ou en l'absence d'activation métabolique
exogène.
7. Effets sur l'homme
L'exposition humaine à de fortes concentrations de
2-nitropropane est largement, voire totalement d'origine
professionnelle. A concentration élevée, le 2-nitropropane présente
une forte toxicité aiguë et il a provoqué des accidents mortels dans
l'industrie - encore qu'on ignore la valeur exacte de cette
concentration, sauf dans un cas où on a pu estimer l'exposition à
2184 mg/m3, soit 600 ppm. Les premiers symptômes consistaient en
céphalées, nausées, somnolence, vomissements, diarrhées et douleurs.
Malgré une amélioration temporaire de l'état général, la mort est
quelquefois survenue dans les 4 à 26 jours suivant l'exposition. La
cause initiale de la mort était une insuffisance hépatique à
laquelle s'ajoutaient un oedème du poumon, des hémorragies des voies
digestives et une insuffisance respiratoire et rénale. A des doses
évaluées à 73-164 mg/m3 (20 à 45 ppm) on a constaté que
l'exposition professionnelle provoquait des nausées et une perte
d'appétit pouvant persister plusieurs heures après le départ du lieu
de travail, aucun effet indésirable n'étant observé après exposition
à des doses de 36,4 à 109 mg/m3 (10 à 30 ppm) (moins de quatre
heures par jour pendant une durée inférieure ou égale à trois jours
par semaine).
Malgré l'insuffisance des données disponibles, rien n'indique
qu'une exposition professionelle de longue durée au 2-nitropropane
aux concentrations généralement présentes sur les lieux de travail
puisse provoquer des cancers du foie ou d'autres organes, ni plus
généralement des effets indésirables à longue échéance.
8. Effets sur les autres organismes au laboratoire et dans
leur milieu naturel
Les quelques études effectuées sur des microorganismes, des
invertébrés et des poissons montrent que le 2-nitropropane est peu
toxique pour les organismes non mammaliens.
RESUMEN
1. Propiedades y métodos analíticos
El 2-nitropropano (2-NP) es un líquido incoloro y oleoso de olor
ligero. Es inflamable, moderadamente volátil, y estable en
condiciones normales. Es sólo ligeramente hidrosoluble pero miscible
con numerosos líquidos orgánicos, y es un excelente disolvente para
muchos tipos de compuestos orgánicos. Existen métodos analíticos
adecuados para identificar y medir el 2-NP en concentraciones
ambientales. Los métodos de uso corriente son la cromatografía de
gases y un detector de ionización de llama o de captura electrónica;
también se usa la cromatografía líquida de alto rendimiento con
detector de ultravioleta. Para medirlo en el aire, primero es
necesario capturarlo y concentrarlo en un sorbente sólido.
2. Usos y fuentes de exposición
2.1 Producción
No se dispone de cifras recientes de producción mundial. En
1977, la producción en los Estados Unidos fue de aproximadamente
13 600 toneladas. Actualmente, el 2-NP se fabrica en dos empresas
estadounidenses y una francesa. Se origina por mecanismos naturales
como trazas en la combustión del tabaco y de otras sustancias
orgánicas ricas en nitratos, pero nada indica que se origine en
procesos biológicos.
2.2 Usos y pérdidas al medio ambiente
El 2-NP se utiliza como disolvente, principalmente en mezclas, y
tiene numerosas aplicaciones industriales como disolvente para
tintas de impresión, pinturas, barnices, adhesivos y otros
revestimientos como los de recipientes de bebidas. Se ha utilizado
asimismo como disolvente para separar sustancias estrechamente
relacionadas como ácidos grasos, como intermediario en síntesis
químicas, y como aditivo en combustibles. Las pérdidas al medio
ambiente ingresan principalmente en el aire y se deben sobre todo a
la evaporación del disolvente a partir de superficies revestidas.
3. Transporte y distribución en el medio ambiente
El 2-NP parece tener gran movilidad en el medio ambiente
natural. Dada su baja solubilidad en el agua, escasa absorción por
el sedimento, reducida bioacumulación y facil evaporación a la
atmósfera, se distribuye tanto en el aire como en el agua y no se
acumula en ningún compartimento ambiental definido. La fotoabsorción
ultravioleta del 2-NP se encuentra en la escala de frecuencias
normales en el medio ambiente, por lo que es probable que la
sustancia sea objeto de fotólisis lenta en la atmósfera. La
biotransformación lenta del 2-NP a compuestos menos tóxicos también
parece probable en los medios acuático y terrestre.
4. Niveles ambientales y exposición humana
La exposición de la población general al 2-NP parece ser muy
baja y se debe al humo de cigarrillos (1,1 a 1,2 µg/cigarrillo), a
los residuos en revestimientos, como los de las latas de bebidas,
adhesivos y material impreso, y a los aceites vegetales fraccionados
con esa sustancia. Se desconoce la exposición industrial a escala
mundial, pero en los Estados Unidos parece limitarse al 0,02-0,19%
de la población trabajadora. Los niveles de exposición de cierta
importancia (exposición a 9,1 mg/m3 (2,5 ppm) o más) en los
Estados Unidos probablemente no afectan más que a unos 4000
trabajadores (aproximadamente 0,005% de la población trabajadora).
Los límites de exposición ocupacional en el aire varían de un paíse
a otro y van desde 3,6 mg/m3 (1 ppm) (promedio ponderado en
función del tiempo) a 146 mg/m3 (40 ppm) (STEL). La fabricación
del 2-NP es un proceso cerrado y entraña por lo general una
exposición reducida de los trabajadores, pero algunos obreros de
industrias como la pintura, la impresión y la extracción de
disolventes se han visto expuestos en otras épocas a niveles muy
superiores a los límites de exposición ocupacional. Durante ciertas
operaciones industriales han llegado a registrarse concentraciones
de hasta 6 g/m3 (1640 ppm) en el aire.
5. Cinética y metabolismo
En el ser humano, la absorción de 2-NP se produce principalmente
por los pulmones. En animales de experimentación, se ha demostrado
que el 2-NP se absorbe rápidamente no sólo por vía pulmonar sino
también a partir de la cavidad peritoneal y del tracto
gastrointestinal. No se dispone de datos satisfactorios sobre la
absorción por vía cutánea. La información sobre la distribución en
la rata es ligeramente contradictoria. El 2-NP se metaboliza
rápidamente, principalmente a acetona y nitrito. También puede
formarse isopropil alcohol en pequeñas cantidades. Tras la inyección
intraperitoneal, el 2-NP y sus metabolitos carbonados se concentran
inicialmente en la grasa y después en la médula ósea, así como en
las glándulas suprarrenales y otros órganos internos. Tras la
inhalación, el 2-NP y sus metabolitos carbonados se concentran en el
hígado y el riñón; la cantidad que se acumula en la grasa es
relativamente pequeña. Varios sistemas enzimáticos diferentes pueden
participar y existen diferencias de unas especies a otras en cuanto
a la velocidad y las rutas metabólicas. El 2-NP y sus metabolitos
carbonados desaparecen rápidamente del organismo por transformación
metabólica, exhalación y excreción en orina y heces. No se dispone
de datos satisfactorios sobre la distribución y la excreción de
metabolitos que llevan el radical nitro.
6 Efectos en mamíferos de laboratorio y sistemas in vitro
El 2-NP tiene una toxicidad aguda moderada para los mamíferos.
Los machos son más sensibles que las hembras, por lo menos en la
rata, y la sensibilidad difiere ampliamente entre las especies que
se han ensayado. La CL50 (concentración que causa una mortalidad
del 50% en un plazo de 14 días) para la rata tras una exposición de
6 horas fue de 1,5 g/m3 (400 ppm) en los machos y 2,6 g/m3
(720 ppm) en las hembras. La letalidad parecía asociada
principalmente a los efectos narcóticos, si bien los mamíferos
expuestos a concentraciones de al menos 8,4 g/m3 (2300 ppm)
durante una hora o más mostraron alteraciones patológicas graves,
entre ellas lesiones hepatocelulares, edema pulmonar y hemorragia.
Existen pruebas claras de que el 2-NP es carcinogénico en la
rata. La exposición prolongada de ratas por inhalación de
0,36 g/m3 (100 ppm) durante 18 meses (7 h/día, 5 días/semana)
indujo cambios destructivos en el hígado, inclusive carcinomas
hepatocelulares en algunos machos. Una concentración de 0,75 g/m3
(207 ppm) indujo lesiones más graves, entre ellas una elevada
incidencia de carcinomas hepatocelulares, con más rapidez. La
administración crónica de dosis orales moderadas indujo también un
exceso de carcinomas hepatocelulares en ratas. En cambio, la
inhalación prolongada de 91 o 98,3 mg/m3 (25 ó 27 ppm) no produjo
lesiones detectables en las ratas. La exposición de ratones y
conejos a concentraciones de 2-NP que inducían carcinomas
hepatocelulares en la rata tuvieron escaso efecto o ninguno, pero
esos estudios eran demasiado limitados para descartar por completo
la carcinogenicidad de la sustancia en ambas especies. El 2-NP
retrasó ligeramente el desarrollo fetal en la rata, pero escasean
los datos sobre embriotoxicidad, teratogenicidad y toxicidad
reproductiva. Se observó que el 2-NP era sumamente genotóxico en
hepatocitos de rata tanto in vitro como in vivo, pero no se
observó genotoxicidad significativa en otros órganos de la rata ni
en líneas celulares de origen extrahepático sin activación
metabólica exógena. Se ha demostrado que el 2-NP es mutagénico en
bacterias tanto en presencia como en ausencia de activación
metabólica exógena.
7. Efectos en el ser humano
La exposición humana a concentraciones elevadas de 2-NP es en su
mayor parte o totalmente de origen ocupacional. Las concentraciones
elevadas (se desconocen los valores reales, si bien en un caso se
calcularon en 2184 mg/m3 (600 ppm)) producen toxicidad aguda y
accidentes mortales en la industria. Entre los síntomas iniciales
figuran dolores de cabeza, náuseas, mareos, vómitos, diarrea y
dolores. Las víctimas a menudo mostraban una mejoría temporal,
aunque en algunos casos sobrevino la muerte entre 4 y 26 días
despues de la exposición. El fallo hepático fue la principal causa
de muerte, con edema pulmonar, hemorragias gastrointestinales fallo
respiratorio y renal como factores contribuyentes. La exposición
ocupacional a niveles estimados en 73 a 164 mg/m3 (20 a 45 ppm)
indujo náuseas y pérdida de apetito, que persistieron durante varias
horas tras abandonar el lugar de trabajo, mientras que la exposición
ocupacional a niveles estimados en 36,4 a 109 mg/m3 (10 a 30 ppm)
(< de 4 h/día durante < 3 días/semana) no produjo efectos
nocivos detectables.
Aunque los datos disponibles son insuficientes, nada indica que
la exposición ocupacional crónica al 2-NP en concentraciones
normales en el lugar de trabajo induzca neoplasmas hepáticos o de
otro tipo ni otros efectos adversos a largo plazo.
8. Efectos en otros organismos en el laboratorio y sobre el terreno
Los escasos estudios realizados en microorganismos,
invertebrados y peces indican una baja toxicidad del 2-NP para
organismos no mamíferos.
