
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 197
Demeton-S-methyl
The issue of this document does not constitute formal publication.
It should not be reviewed, abstracted, or quoted without the written
permission of the Manager, International Programme on Chemical Safety,
WHO, Geneva, Switzerland.
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Environmental Health Criteria 197
First draft prepared by Dr. A. Moretto, Institute of Occupational
Medicine, University of Padua, Italy
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1997
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Demeton-S-Methyl.
(Environmental health criteria ; 197)
1.Insecticides, Organophosphate - toxicity
2.Insecticides, Organophosphate - adverse effects
3.Environmental exposure 4.Occupational exposure
I.International Programme on Chemical Safety II.Series
ISBN 92 4 157197 7 (NLM Classification: WA 240)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1997
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The designations
employed and the presentation of the material in this publication do
not imply the expression of any opinion whatsoever on the part of the
Secretariat of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities,
or concerning the delimitation of its frontiers or boundaries. The
mention of specific companies or of certain manufacturers' products
does not imply that they are endorsed or recommended by the World
Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR DEMETON-S-METHYL
PREAMBLE
ABBREVIATIONS
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1. Summary and evaluation
1.1.1. Identity, physical and chemical properties, and
analytical methods
1.1.2. Sources of human and environmental exposure
1.1.3. Environmental transport, distribution and
transformation
1.1.4. Environmental levels and human exposure
1.1.5. Kinetics and metabolism
1.1.6. Effects on laboratory animals and in vitro
test systems
1.1.6.1 Single exposure
1.1.6.2 Short-term exposure
1.1.6.3 Long-term exposure
1.1.6.4 Skin and eye irritation and
sensitization
1.1.6.5 Reproduction, embryotoxicity and
teratogenicity
1.1.6.6 Mutagenicity and related end-points
1.1.6.7 Delayed neurotoxicity
1.1.6.8 Toxicity of metabolites
1.1.7. Mechanism of toxicity - mode of action
1.1.8. Effects on humans
1.1.9. Effects on other organisms in the laboratory
and field
1.2. Conclusions
1.3. Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factors
2.4. Analytical methods
2.5. Formation of derivatives during storage
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrences
3.2. Man-made sources
3.2.1. Production
3.2.2. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Abiotic and biotic transformation
4.2.1. Hydrolytic degradation
4.2.2. Photodegradation
4.2.3. Degradation in soil
4.2.4. Biodegradation in plants
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. General population exposure
5.2. Occupational exposure during manufacture, formulation or
use
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1. Absorption, distribution and excretion
6.2. Metabolic transformation
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.1.1. Oral
7.1.2. Inhalation
7.1.3. Dermal
7.2. Short-term exposure
7.2.1. Rat
7.2.2. Dog
7.3. Long-term exposure
7.3.1. Mouse
7.3.2. Rat
7.4. Skin and eye irritation and sensitization
7.4.1. Skin and eye irritation
7.4.2. Skin sensitization
7.5. Reproduction, embryotoxicity and teratogenicity
7.5.1. Reproduction
7.5.2. Embryotoxicity and teratogenicity
7.5.2.1 Rat
7.5.2.2 Rabbit
7.6. Mutagenicity and related end-points
7.6.1. DNA damage and repair
7.6.2. Mutation
7.6.3. Chromosomal effects
7.7. Delayed neurotoxicity
7.8. Toxicity of metabolites
7.9. Mechanism of toxicity - mode of action
7.10. Potentiation
8. EFFECTS ON HUMANS
8.1. General population exposure
8.2. Occupational exposure
8.2.1. Acute poisoning
8.2.2. Effects of short- and long-term exposure
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Aquatic organisms
9.1.1. Algae
9.1.2. Invertebrates
9.1.3. Fish
9.2. Terrestrial organisms
9.2.1. Soil microorganisms
9.2.2. Invertebrates
9.2.3. Birds
9.2.4. Effects in field
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1. Evaluation of human health risks
10.2. Evaluation of effects on the environment
10.2.1. Aquatic organisms
10.2.1.1 Acute risk
10.2.1.2 Chronic risk
10.2.2. Terrestrial organisms
10.2.2.1 Birds
10.2.2.2 Mammals
10.2.2.3 Bees
10.2.2.4 Earthworms
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RÉSUMÉ ET ÉVALUATION, CONCLUSIONS ET RECOMMANDATIONS
RÉSUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (telephone no. + 41 22 -
9799111, fax no. + 41 22 - 7973460, E-mail irptc@unep.ch).
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an ever-increasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As such,
they include and review studies that are of direct relevance for the
evaluation. However, they do not describe every study carried out.
Worldwide data are used and are quoted from original studies, not from
abstracts or reviews. Both published and unpublished reports are
considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant published
data are absent or when they are pivotal to the risk assessment. A
detailed policy statement is available that describes the procedures
used for unpublished proprietary data so that this information can be
used in the evaluation without compromising its confidential nature
(WHO (1990) Revised Guidelines for the Preparation of Environmental
Health Criteria Monographs. PCS/90.69, Geneva, World Health
Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and
in vitro studies provide support and are used mainly to supply
evidence missing from human studies. It is mandatory that research on
human subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined
below.
* Summary - a review of the salient facts and the risk evaluation
of the chemical
* Identity - physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for layout
and language. The first draft, prepared by consultants or, more
usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
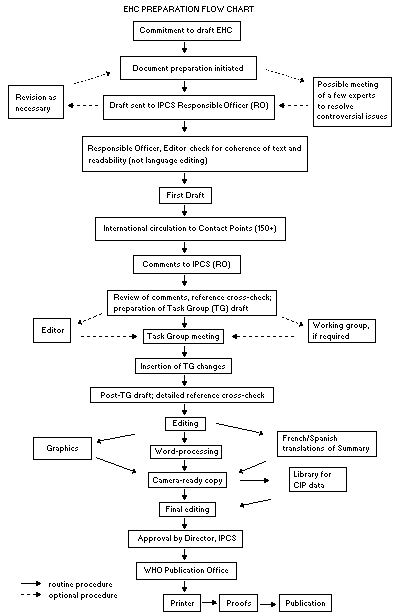 150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize
the important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DEMETON-S-METHYL
Members
Dr P.J. Abbott, Australia and New Zealand Food Authority
(ANZFA), Canberra, Australia
Dr K. Barabas, Department of Public Health, Albert Szent-Gyorgyi,
University Medical School, Szeged, Hungary
Dr A.L. Black, Woden, ACT, Australia
Professor J.F. Borzelleca, Pharmacology and Toxicology,
Richmond, Virginia, USA
Dr P.J. Campbell, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries and Food, Kings Pool, York,
United Kingdom
Professor L.G. Costa, Department of Environmental Health,
University of Washington, Seattle, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr I. Dewhurst, Mammalian Toxicology Branch, Pesticides Safety
Directorate, Ministry of Agriculture, Fisheries and Food,
Kings Pool, York, United Kingdom
Dr V. Drevenkar, Institute for Medical Research and Occupational
Health, Zagreb, Croatia
Dr W. Erickson, Environmental Fate and Effects Division,
US Environmental Protection Agency, Washington, D.C., USA
Dr A. Finizio, Group of Ecotoxicology, Institute of Agricultural
Entomology, University of Milan, Milan, Italy
Mr K. Garvey, Office of Pesticide Programs (7501C),
US Environmental Protection Agency, Washington, D.C., USA
Dr A.B. Kocialski, Health Effects Division, Office of Pesticide
Programs, US Environmental Protection Agency,
Washington, D.C., USA
Dr A. Moretto, Institute of Occupational Medicine, University
of Padua, Padua, Italy
Professor O. Pelkonen, Department of Pharmacology and
Toxicology, University of Oulu, Oulu, Finland
Dr D. Ray, Medical Research Council Toxicology Unit, University
of Leicester, Leicester, United Kingdom
Dr J.H.M. Temmink, Department of Toxicology, Wageningen
Agricultural University, Wageningen, The Netherlands
Observers
Dr J.W. Adcock, AgrEvo UK Limited, Chesterford Park, Saffron,
Waldon, Essex, United Kingdom
Mr D. Arnold, Environmental Sciences, AgrEvo UK Ltd.,
Chesterford Park, Saffron Waldon, Essex, United Kingdom
Dr E. Bellet, CCII, Overland Park, Kansas, USA
Mr Jan Chart, AMVAC Chemical Corporation, Newport Beach,
California, USA
Dr H. Egli, Novartis Crop Protection AG, Basel, Switzerland
Dr P. Harvey, AgrEvo UK Ltd., Chesterford Park, Saffron Walden,
Essex, United Kingdom
Dr G. Krinke, Novartis Crop Protection AG, Basel, Switzerland
Dr A. McReath, DowElanco Limited, Letcombe Regis, Wantage,
Oxford, United Kingdom
Dr H. Scheffler, Novartis Crop Protection AG, Basel, Switzerland
Dr A.E. Smith, Novartis Crop Protection AG, Basel, Switzerland
Secretariat
Dr L. Harrison, Health and Safety Executive, Bootle, Merseyside,
United Kingdom
Dr J.L. Herrman, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P.G. Jenkins, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr D. McGregor, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
Dr R. Plestina, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr E. Smith, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P. Toft, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DEMETON-S-METHYL
The Core Assessment Group (CAG) of the Joint Meeting on
Pesticides (JMP) met at the Institute for Environment and Health,
Leicester, United Kingdom, from 3 to 8 March 1997. Dr L.L. Smith
welcomed the participants on behalf of the Institute, and
Dr R. Plestina on behalf of the three IPCS cooperating organizations
(UNEP/ILO/WHO). The CAG reviewed and revised the draft monograph and
made an evaluation of the risks for human health and the environment
from exposure to demeton-S-methyl.
The first draft of the monograph was prepared by Dr A. Moretto,
Institute of Occupational Medicine, University of Padua, Italy.
Extensive scientific comments were received following circulation of
the first draft to the IPCS contact points for Environmental Health
Criteria monographs and these comments were incorporated into the
second draft by the Secretariat.
Dr R. Plestina and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively. The efforts of all who helped in the
preparation and finalization of the monograph are gratefully
acknowledged.
ABBREVIATIONS
ACGIH American Conference of Governmental Industrial Hygienists
AChE acetylcholinesterase
ADI acceptable daily intake
a.i. active ingredient
BuChE butyrylcholinesterase
b.w. body weight
ChE cholinesterase
EC50 median effective concentration
GLC gas-liquid chromatography
HID highest ineffective dose
I50 concentration inhibiting 50% of the enzyme activity
i.p. intraperitoneal administration
i.v. intravenous administration
JMPR Joint Meeting on Pesticide Residues
Kd sorption coefficient
LC50 median lethal concentration
LD50 median lethal dose
LED lowest effective dose
LOEC lowest-observed-effect concentration
MRL maximum residue level
NT not tested
NTE neuropathy target esterase
NOAEL no-observed-adverse-effect level
NOEC no-observed-effect concentration
PEC predicted environmental concentration
RBC red blood cell
s.c. subcutaneous
SCE sister chromatid exchange
STS standard type of soil
TER toxicity-exposure ratio
TLC thin layer chromatography
TLV threshold limit value
TWA time-weighted average
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Identity, physical and chemical properties, and analytical
methods
Demeton-S-methyl, a pale yellow oily liquid with a penetrating
odour, is a systemic and contact organophosphate insecticide and
acaricide used to control Acarina, Thysanoptera, Hymenoptera and
Homoptera in fruits, cereals, ornamentals and vegetables. It has a
vapour pressure of 63.8 mPa at 20°C, is readily soluble in most
organic solvents, has a high water solubility of 3.3 g/litre at room
temperature and an octanol-water partition coefficient (log Pow) of
1.32. Demeton-S-methyl is stable in non-aqueous solvents.
Residual and environmental analyses are performed by extraction
with an organic solvent, followed by oxidation to the corresponding
sulfone. Measurement is then performed by gas chromatography, using a
phosphorus-specific detector.
1.1.2 Sources of human and environmental exposure
Prior to 1957, methyl-demeton was marketed as a mixture of
demeton-S-methyl and demeton-O-methyl isomers.
Demeton-S-methyl has been in use since 1957. It is formulated as
an emulsifiable concentrate and used as a spray on cereals, fruits,
ornamentals and vegetables. It is being replaced by oxydemeton-methyl,
which is a plant, soil and mammalian metabolite of demeton-S-methyl.
1.1.3 Environmental transport, distribution and transformation
Hydrolytic degradation of demeton-S-methyl depends on the pH of
the solution; at 22°C the half-life is 63 days at pH 4, 56 days at pH
7 and 8 days at pH 9. In soil, biodegradation is the primary route of
degradation. The half-life of demeton-S-methyl in soil is about 4 h.
However, after 24 h, oxydemeton-methyl still represents 20-30% of the
applied dose of demeton-S-methyl. The sorption coefficient (Kd) of
demeton-S-methyl in soil is 0.68 to 2.66, depending on the soil
composition.
Photolysis is not one of the major mechanisms of degradation of
demeton-S-methyl in the environment.
Metabolism in spring wheat is rapid and similar to that in soil
and mammals.
1.1.4 Environmental levels and human exposure
Primary exposure for the general human population is from
residues of demeton-S-methyl on food crops. The Joint FAO/WHO Meeting
on Pesticide Residues (JMPR) recommended acceptable daily intake (ADI)
is 0.0003 mg/kg body weight. This is a group ADI for demeton-S-methyl,
oxydemeton-methyl and demeton-S-methyl-sulfone, since the routine
analytical methods do not discriminate between these three compounds.
Excessive dermal exposure and absorption of demeton-S-methyl has
caused cholinergic toxicity in workers inadequately protected during
packaging of the concentrate formulation.
When volunteers engaged in a simulated spray activity with a
mixture of demeton-S-methyl and demeton-O-methyl (30 and 70%,
respectively) were exposed to 8.8-27 mg/m3 of the two active
ingredients combined, they experienced no adverse effects on plasma or
erythrocyte cholinesterase activities.
1.1.5 Kinetics and metabolism
Demeton-S-methyl is rapidly and almost completely absorbed from
the intestinal tract of rats and is uniformly (except for high
concentration in erythrocytes) distributed to body tissues. It is
rapidly metabolized and excreted via the urine. Blood concentration
decreases with an initial half-life of about 2 h. About 1% of the oral
dose is present in the body 24 h after treatment. The main metabolic
pathway of demeton-S-methyl in rats is the oxidation of the side
chain leading to the formation of the corresponding sulfoxide
(oxydemeton-methyl) and, to a lesser extent, after further oxidation,
to the sulfone. Another important metabolic route is O-demethylation.
1.1.6 Effects on laboratory animals and in vitro test systems
1.1.6.1 Single exposure
Demeton-S-methyl causes cholinergic toxicity. The LD50 values
for mammals range from 7 to 100 mg/kg body weight, depending on the
route of administration and species.
1.1.6.2 Short-term exposure
An early dietary study showed that rats fed demeton-S-methyl at
50 mg/kg diet had substantially reduced brain and erythrocyte
cholinesterase activity after 26 weeks of exposure. Cholinergic signs
were observed in rats fed 200 mg/kg diet during the first 5 weeks of
exposure.
In a one-year dietary study on dogs, a no-observed-adverse-effect
level (NOAEL) of 1 mg/kg diet (equal to 0.036 mg/kg body weight per
day) was established, based on effects on brain cholinesterase.
1.1.6.3 Long-term exposure
Mice were fed diets containing 0, 1, 15 or 75 mg/kg demeton-S-
methyl for 21 months. The NOAEL was found to be 1 mg/kg diet (equal to
0.24 mg/kg body weight per day) based on inhibition of brain
cholinesterase.
In rats fed diets containing 0, 1, 7 or 50 mg/kg demeton-S-
methyl, the NOAEL, based on inhibition of brain cholinesterase, was
1 mg/kg diet (equal to 0.05 mg/kg body weight per day).
No increased tumour incidence was found in either species.
1.1.6.4 Skin and eye irritation and sensitization
Demeton-S-methyl is a mild skin and eye irritant. Positive
results were obtained with the Magnusson and Klingman maximization
test in guinea-pigs. However, the Buehler epidermal patch test gave no
indication of skin sensitization, suggesting that sensitization should
not be a problem in the practical use of demeton-S-methyl.
1.1.6.5 Reproduction, embryotoxicity and teratogenicity
In a two-generation dietary rat study, demeton-S-methyl caused
reduced viability and body weight of pups (F1b generation only)
at a dose level of 5 mg/kg diet. The NOAEL was 1 mg/kg diet, equal to
0.07 mg/kg body weight per day.
Demeton-S-methyl was neither embryotoxic nor teratogenic in rats
and rabbits.
1.1.6.6 Mutagenicity and related end-points
Demeton-S-methyl induces point mutations in vitro. Chromosomal
effects have been demonstrated in vivo with commercial formulations
only. The available information is insufficient to permit an adequate
assessment of the genotoxic potential of demeton-S-methyl.
1.1.6.7 Delayed neurotoxicity
Demeton-S-methyl caused neither delayed polyneuropathy nor
inhibition of neuropathy target esterase (NTE) when tested in hens at
a level equal to the oral LD50.
1.1.6.8 Toxicity of metabolites
Two plant and mammalian metabolites of demeton-S-methyl
(i.e. oxydemeton-methyl and demeton-S-methylsulfone) are also
commercial pesticides and have been extensively studied. It has been
reported that the toxicological profile of these two compounds does
not significantly differ, either quantitatively or qualitatively, from
that of demeton-S-methyl.
1.1.7 Mechanism of toxicity - mode of action
Demeton-S-methyl is a direct cholinesterase inhibitor, and the
toxicity it causes is related to inhibition of acetylcholinesterase
(AChE) at nerve terminals. AChE inhibited by demeton-S-methyl
reactivates spontaneously with an in vitro half-life of about 1.3 h,
as expected for dimethyl phosphorylated AChE.
1.1.8 Effects on humans
A few cases of acute intoxication with cholinergic syndrome,
following suicide attempts, have been reported. Surviving patients,
including a pregnant woman, did not show delayed effects.
Following careless occupational exposure during packaging of
the commercial formulation, some workers developed cholinergic
toxicity which required pharmacological treatment. Absorption of
demeton-S-methyl was probably through the skin. Similarly, improper
working conditions may have caused excessive dermal absorption during
application of demeton-S-methyl in cotton fields.
1.1.9 Effects on other organisms in the laboratory and field
The 96-h EC50s for green algae range from 8 to 37 mg/litre. The
LC50s for a range of aquatic invertebrates range from 0.004 to
1.3 mg/litre. The toxicity for fish varies, with 96-h LC50 ranging
from 0.59 mg/litre for the rainbow trout to about 40 mg/litre for the
golden orfe, the goldfish and the carp.
The acute oral LD50 for the Japanese quail and the canary is
10-50 mg/kg body weight. In starlings, a single oral dose of 2 mg/kg
body weight caused 20% inhibition of brain AChE 3 h after treatment.
The LC50 of demeton-S-methyl in soil for earthworms is 66 mg/kg
for 14 days. The acute oral and contact LD50 for demeton-S-methyl are
0.21 and 0.6 µg/bee respectively. When used on winter wheat at the
suggested rate, demeton-S-methyl significantly reduced the number of
crop foliage invertebrates (mainly Empididae flies) but not the
number of soil surface entomophagous invertebrates.
1.2 Conclusions
Demeton-S-methyl is a highly toxic (class Ib of the WHO
classification) (WHO, 1996) organophosphorus ester insecticide. The
mechanism of toxicity is that of AChE inhibition at nerve terminals.
Exposure of the general population results mainly from residues
present in crop commodities.
With good work practices, hygienic measures and safety
precautions, the use of demeton-S-methyl during manufacture or
application should not cause adverse effects. Effects due to chronic
exposure are unlikely to occur.
Demeton-S-methyl does not persist in the environment and is not
accumulated by organisms. It has high acute toxicity to aquatic
invertebrates and is toxic to fish and birds, leading to high or
moderate risk factors for these organisms. However, significant field
kills of organisms have not been reported for the compound.
Precautions should be taken to minimize exposure of non-target
organisms (e.g., do not spray over water bodies, minimize exposure by
spray drift).
1.3 Recommendations
For the health and welfare of workers and the general population,
the handling and application of demeton-S-methyl should only be
entrusted to supervised and well-trained operators who follow the
required safety measures and good application practices.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical formula: C6H15O3PS2
Chemical structure:
O
"
CH3CH2SCH2CH2SP(OCH3)2
Relative molecular mass: 230.3
Common name: demeton-S-methyl
CAS chemical name: S-[2-(ethylthio)ethyl] O,O-dimethyl
phosphorothioate
IUPAC name: S-2-ethylthioethyl O,O-dimethyl
phosphorothioate
CAS registry number: 919-86-8
RTECS number: TG1750000
Common synonyms and AI3-24963; BAY 18436; Bayer 18 436;
trade names: Bayer 25/154; Demetox; DEP 836 349;
Duratox; ethanethiol,
2-(ethylthio)-S-ester with O,O-dimethyl
phosphorothioate; HSDB 6410;
Isometasystox; Isomethylsystox;
Metaisoseptox; Metaisosystox; Metasystox
(I); metasystox forte; Metasystox I;
Metasystox J; Metasystox 55; methyl
demeton thioester; methyl isosystox;
methyl-mercaptofos teolery;
methyl-mercaptofos teolovy (USSR);
methylmercaptofostiol (USSR); Mifatox;
O,O-dimethyl S-(2-(ethylthio)
ethylphosphorothioate; O,O-dimethyl
S-ethylmercaptoethyl thiophosphate;
O,O-dimethyl 2-ethylmercaptoethyl
thiophosphate, thiolo isomer;
phosphorothioic acid, O,O-dimethyl
S-(2-(ethylthio)ethyl) ester;
phosphorothioic acid,
S-(2-(ethylthio)ethyl) O,O-dimethyl
ester; S-(2-(ethylthio)ethyl);
dimethyl phosphorothiolate;
S-(2-(Ethylthio)ethyl) O,O-dimethyl
phosphorothioate (8CI)(9CI);
S-(2-(ethylthio)ethyl) O,O-dimethyl
phosphorothioate; S-(2-ethylthio)ethyl)
O,O-dimethyl phosphorothioate;
S-(2-ethylthioetyl)0,0-dimethyl
phosphorothioate; S-2-Ethylthioethyl-
dimethyl phosphorothioate; USP 2 571
989; 2-Ethylthioethyl dimethyl
phosphorothioate.
Formulations: EC (250 or 500 g a.i./litre), DSM
(Campbell), Metasystox55 (Bayer),
Mepatox (FCC),
EC (580 g/litre).
Purity: >90%
Impurities: O,O,S-trimethylthiophosphate (maximum of
1.5%)
O-methyl-S-2-(ethylmercapto)-ethylthioph
osphate (maximum of 3.0%)
2-ethylthioethylmercaptan max 0.8%
bis(2-ethylthioethyl)-disulfide (maximum
of 0.8%)
Various ionic components (sulfonium
compounds, organic salts) (total maximum
of 2.5%)
Oligomeric alkyl(thio) phosphates
(maximum of 1.0%)
Water (maximum of 0.1%)
2.2 Physical and chemical properties
Some relevant physical and chemical properties are summarized in
Table 1.
Table 1. Some chemical and physical properties of demeton-S-methyl
Physical state: oily liquid
Colour: pale yellow
Odour: penetrating, reminiscent of leeks
Boiling point 74°C at 6.65 Pa (0.05 mmHg)
92°C at 26.6 Pa (0.20 mmHg)
102°C at 53.2 Pa (0.40 mmHg)
118°C at 133 Pa (1.00 mmHg)
Vapour pressure: 21.3 mPa (1.6 × 10-4 mmHg) at 10°C
63.8 mPa (4.8 × 10-4 mmHg) at 20°C
193 mPa (1.45 × 10-3 mmHg) at 30°C
400 mPa (3.8 × 10-3 mmHg) at 40°C
Relative density at 20°C: 1.21
n-Octanol/water partition
coefficient: log Pow = 1.32
Solubility in water: 3.3 g/litre (at room temperature)
Solubility in organic readily soluble in most organic
solvents: solvents; limited solubility in
petroleum ether
Stability: hydrolysed by alkali and oxidized to the
sulfoxide (oxydemeton-methyl) and
sulfone (demeton-S-methylsulfone)
Half-life in water: 11 days at 37°C
Half-lives at 22°C: 63 days at pH 4
56 days at pH 7
8 days at pH 9
2.3 Conversion factors
1 ppm = 9.42 mg/m3 (at 25°C)
1 mg/m3 = 0.106 ppm
2.4 Analytical methods
Analytical methods for the determination of residues of the
demeton-S-methyl group (i.e. demeton-S-methyl, its sulfoxide and its
sulfone) are either identical or very similar.
Originally, colorimetric methods (i.e. determination of total
phosphorus) were used (FAO/WHO, 1993). Current methods are based on
GLC. In principle, these methods involve an oxidation step, using
potassium permanganate, to produce demeton-S-methylsulfone, which is
then determined with a thermoionic emission detector.
A GLC method (Wagner & Thornton, 1977) is suitable for
determining residues in plants, soil and water. The method is based on
the principle of oxidation described above, with variations depending
on the sample to be analysed. Before the oxidation step, maceration
with acetone is used for samples with a high fat or oil content. The
macerate and water samples are then extracted with chloroform or
dichloromethane. Since the analytical method involves the oxidation to
the sulfone, the determination is of demeton-S-methylsulfone, from
which the demeton-S-methyl residue can be calculated. The method was
used to determine residues in a wide range of crops with a minimum
recovery above 80%. The limit of determination depends upon the sample
and generally lies between 0.01 and 0.2 mg/kg. Another method, based
on similar principles, has been described for oxydemeton-methyl
residues in plant and animal tissues and in soil (Thornton et al.,
1977).
An alternative GLC method for sulfides (including demeton-S-
methyl), sulfoxides and sulfones has been proposed by Hill et al.
(1984), who reported that the use of acetone as a co-solvent during
potassium permanganate oxidation causes unpredictable (from negligible
to complete) loss of demeton-S-methyl. These authors used ethyl
acetate for extraction of organophosphorus compounds from fruit and
vegetable samples (Anonymous, 1977). The extracts were cleaned-up by
chromatography on a column of activated charcoal, magnesium oxide and
Celite. The compounds were eluted from the column using a mixture of
ethyl acetate, acetone and toluene. A mean recovery of >68% (mainly
>80%) was found for a number of organophosphorus sulfides, sulfoxides
and sulfones, that of demeton-S-methyl being 82.4±6.6% (mean ± SD,
n=10) when determined in lettuce.
Wilkins et al. (1985) reported the characterization of nearly
90 organophosphorus sulfides, sulfoxides and sulfones by gas
chromatography and mass spectrometry. Using similar experimental
conditions with three different mass spectrometers, the spectra
produced from a given compound were almost identical.
A TLC method for 10 different organophosphorus insecticides
(including demeton-S-methyl and demeton-S-methylsulfone) was reported
by Funk et al. (1989). This method has a limit of detection of
4-10 ng/spot.
2.5 Formation of derivatives during storage
Storage of "pure" demeton-S-methyl in the dark at room
temperature leads to the formation of sulfonium derivatives. This was
found to be associated with increased intravenous toxicity but not
oral toxicity (Heath & Vandekar, 1957). Hecht (1960) reported that the
oral toxicity in rat did not change if 4- or 24-hour-old aqueous
solutions of demeton-S-methyl were used.
The concentration in a water suspension of 1 g a.i./litre
decreased by about 50 and 75% after 7 and 28 days of storage
(apparently at 37°C), respectively (Hecht, 1960) (see also section
7.1).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrences
Demeton-S-methyl does not occur as a natural product.
3.2 Man-made sources
3.2.1 Production
In 1954, a reaction mixture containing demeton-S-methyl and
demeton-O-methyl (O-2-ethylthioethyl O,O-dimethyl phosphorothioate)
was introduced by Farbenfabrik Bayer AG (now Bayer AG) with the common
name of demeton-methyl. An improved manufacturing process led to the
introduction, in 1957, of demeton-S-methyl by Bayer AG. Information on
the global production is not available. It is formulated as an
emulsifiable concentrate.
3.2.2 Uses
Demeton-S-methyl is a systemic and contact insecticide and
acaricide used to control Acarina, Thysanoptera, Hymenoptera and
Homoptera on cereals, fruits, ornamentals and vegetables. It is
applied as an emulsifiable concentrate formulation mainly as a spray
and usually at a concentration of 0.025% a.i. (FAO/WHO, 1974). It has
been reported that most national registrations for demeton-S-methyl
should be transferred during the next few years to oxydemeton-methyl,
which has similar use and is applied at similar rates (FAO/WHO, 1993).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
The sorption behaviour of demeton-S-methyl and three other
organophosphorus pesticides in natural pond sediments was tested by
Froebe et al. (1989). Two different sediments were used, containing
9.3 and 6.2% organic matter, at pH 5.9 and 6.5, and a specific surface
area of 23.7 and 16.7 m2/g, respectively. The semi-quantitative
mineralogical composition was similar. For the four pesticides, their
sorption coefficients (Kd) followed the same sequence as their
lipophilicity (expressed as n-octanol/water coefficient). The
sorption efficiency for demeton-S-methyl was higher in the sediment
with a lower content of organic matter (Kd of 0.689 and 2.66,
respectively).
4.2 Abiotic and biotic transformation
4.2.1 Hydrolytic degradation
In water at 22°C demeton-S-methyl has a half-life of 63 days at
pH 4, 56 days at pH 7, and 8 days at pH 9. The main reactions are
demethylation in acid medium and hydrolysis of the phosphorus-ester
bond in basic medium (Krohn, 1984) (see also section 2.2).
4.2.2 Photodegradation
Demeton-S-methyl in aqueous solution does not absorb any light at
247 nm or longer wavelengths. Therefore, no direct photo-degradation
of demeton-S-methyl in the environment is to be expected
(Hellpointner, 1990). When solutions of demeton-S-methyl in water
(3.6-3.7 mg/litre) were irradiated for 8 h with a high-pressure
mercury vapour lamp, no photodegradation was detected. However, when
solutions were fortified with humic acid (10 mg/litre), the half-life
of photodegradation was
8 h (the degradation products were not identified). This suggests that
degradation by sensitized or indirect photolysis may also occur in the
environment (Wilmes, 1984).
4.2.3 Degradation in soil
The metabolism of demeton-S-methyl in soil is shown in Fig. 1.
The ability of microbial organisms to biodegrade demeton-S-methyl
sulfoxide was evaluated in a laboratory test and under aerobic
conditions using various species (Nocardia, Arthrobacter,
Corynebacter, Brevibacterium, Bacillus and Pseudomanas) and strains
(Ziegler et al., 1980). Almost all the organisms were able to degrade
the insecticide. The amount of insecticide degraded ranged between 65%
(Arthrobacter roseoparaffineus) and 99% (Pseudomonas putida) after
14 days of incubation. The biodegradation process led to the
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize
the important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DEMETON-S-METHYL
Members
Dr P.J. Abbott, Australia and New Zealand Food Authority
(ANZFA), Canberra, Australia
Dr K. Barabas, Department of Public Health, Albert Szent-Gyorgyi,
University Medical School, Szeged, Hungary
Dr A.L. Black, Woden, ACT, Australia
Professor J.F. Borzelleca, Pharmacology and Toxicology,
Richmond, Virginia, USA
Dr P.J. Campbell, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries and Food, Kings Pool, York,
United Kingdom
Professor L.G. Costa, Department of Environmental Health,
University of Washington, Seattle, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr I. Dewhurst, Mammalian Toxicology Branch, Pesticides Safety
Directorate, Ministry of Agriculture, Fisheries and Food,
Kings Pool, York, United Kingdom
Dr V. Drevenkar, Institute for Medical Research and Occupational
Health, Zagreb, Croatia
Dr W. Erickson, Environmental Fate and Effects Division,
US Environmental Protection Agency, Washington, D.C., USA
Dr A. Finizio, Group of Ecotoxicology, Institute of Agricultural
Entomology, University of Milan, Milan, Italy
Mr K. Garvey, Office of Pesticide Programs (7501C),
US Environmental Protection Agency, Washington, D.C., USA
Dr A.B. Kocialski, Health Effects Division, Office of Pesticide
Programs, US Environmental Protection Agency,
Washington, D.C., USA
Dr A. Moretto, Institute of Occupational Medicine, University
of Padua, Padua, Italy
Professor O. Pelkonen, Department of Pharmacology and
Toxicology, University of Oulu, Oulu, Finland
Dr D. Ray, Medical Research Council Toxicology Unit, University
of Leicester, Leicester, United Kingdom
Dr J.H.M. Temmink, Department of Toxicology, Wageningen
Agricultural University, Wageningen, The Netherlands
Observers
Dr J.W. Adcock, AgrEvo UK Limited, Chesterford Park, Saffron,
Waldon, Essex, United Kingdom
Mr D. Arnold, Environmental Sciences, AgrEvo UK Ltd.,
Chesterford Park, Saffron Waldon, Essex, United Kingdom
Dr E. Bellet, CCII, Overland Park, Kansas, USA
Mr Jan Chart, AMVAC Chemical Corporation, Newport Beach,
California, USA
Dr H. Egli, Novartis Crop Protection AG, Basel, Switzerland
Dr P. Harvey, AgrEvo UK Ltd., Chesterford Park, Saffron Walden,
Essex, United Kingdom
Dr G. Krinke, Novartis Crop Protection AG, Basel, Switzerland
Dr A. McReath, DowElanco Limited, Letcombe Regis, Wantage,
Oxford, United Kingdom
Dr H. Scheffler, Novartis Crop Protection AG, Basel, Switzerland
Dr A.E. Smith, Novartis Crop Protection AG, Basel, Switzerland
Secretariat
Dr L. Harrison, Health and Safety Executive, Bootle, Merseyside,
United Kingdom
Dr J.L. Herrman, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P.G. Jenkins, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr D. McGregor, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
Dr R. Plestina, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr E. Smith, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P. Toft, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DEMETON-S-METHYL
The Core Assessment Group (CAG) of the Joint Meeting on
Pesticides (JMP) met at the Institute for Environment and Health,
Leicester, United Kingdom, from 3 to 8 March 1997. Dr L.L. Smith
welcomed the participants on behalf of the Institute, and
Dr R. Plestina on behalf of the three IPCS cooperating organizations
(UNEP/ILO/WHO). The CAG reviewed and revised the draft monograph and
made an evaluation of the risks for human health and the environment
from exposure to demeton-S-methyl.
The first draft of the monograph was prepared by Dr A. Moretto,
Institute of Occupational Medicine, University of Padua, Italy.
Extensive scientific comments were received following circulation of
the first draft to the IPCS contact points for Environmental Health
Criteria monographs and these comments were incorporated into the
second draft by the Secretariat.
Dr R. Plestina and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively. The efforts of all who helped in the
preparation and finalization of the monograph are gratefully
acknowledged.
ABBREVIATIONS
ACGIH American Conference of Governmental Industrial Hygienists
AChE acetylcholinesterase
ADI acceptable daily intake
a.i. active ingredient
BuChE butyrylcholinesterase
b.w. body weight
ChE cholinesterase
EC50 median effective concentration
GLC gas-liquid chromatography
HID highest ineffective dose
I50 concentration inhibiting 50% of the enzyme activity
i.p. intraperitoneal administration
i.v. intravenous administration
JMPR Joint Meeting on Pesticide Residues
Kd sorption coefficient
LC50 median lethal concentration
LD50 median lethal dose
LED lowest effective dose
LOEC lowest-observed-effect concentration
MRL maximum residue level
NT not tested
NTE neuropathy target esterase
NOAEL no-observed-adverse-effect level
NOEC no-observed-effect concentration
PEC predicted environmental concentration
RBC red blood cell
s.c. subcutaneous
SCE sister chromatid exchange
STS standard type of soil
TER toxicity-exposure ratio
TLC thin layer chromatography
TLV threshold limit value
TWA time-weighted average
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Identity, physical and chemical properties, and analytical
methods
Demeton-S-methyl, a pale yellow oily liquid with a penetrating
odour, is a systemic and contact organophosphate insecticide and
acaricide used to control Acarina, Thysanoptera, Hymenoptera and
Homoptera in fruits, cereals, ornamentals and vegetables. It has a
vapour pressure of 63.8 mPa at 20°C, is readily soluble in most
organic solvents, has a high water solubility of 3.3 g/litre at room
temperature and an octanol-water partition coefficient (log Pow) of
1.32. Demeton-S-methyl is stable in non-aqueous solvents.
Residual and environmental analyses are performed by extraction
with an organic solvent, followed by oxidation to the corresponding
sulfone. Measurement is then performed by gas chromatography, using a
phosphorus-specific detector.
1.1.2 Sources of human and environmental exposure
Prior to 1957, methyl-demeton was marketed as a mixture of
demeton-S-methyl and demeton-O-methyl isomers.
Demeton-S-methyl has been in use since 1957. It is formulated as
an emulsifiable concentrate and used as a spray on cereals, fruits,
ornamentals and vegetables. It is being replaced by oxydemeton-methyl,
which is a plant, soil and mammalian metabolite of demeton-S-methyl.
1.1.3 Environmental transport, distribution and transformation
Hydrolytic degradation of demeton-S-methyl depends on the pH of
the solution; at 22°C the half-life is 63 days at pH 4, 56 days at pH
7 and 8 days at pH 9. In soil, biodegradation is the primary route of
degradation. The half-life of demeton-S-methyl in soil is about 4 h.
However, after 24 h, oxydemeton-methyl still represents 20-30% of the
applied dose of demeton-S-methyl. The sorption coefficient (Kd) of
demeton-S-methyl in soil is 0.68 to 2.66, depending on the soil
composition.
Photolysis is not one of the major mechanisms of degradation of
demeton-S-methyl in the environment.
Metabolism in spring wheat is rapid and similar to that in soil
and mammals.
1.1.4 Environmental levels and human exposure
Primary exposure for the general human population is from
residues of demeton-S-methyl on food crops. The Joint FAO/WHO Meeting
on Pesticide Residues (JMPR) recommended acceptable daily intake (ADI)
is 0.0003 mg/kg body weight. This is a group ADI for demeton-S-methyl,
oxydemeton-methyl and demeton-S-methyl-sulfone, since the routine
analytical methods do not discriminate between these three compounds.
Excessive dermal exposure and absorption of demeton-S-methyl has
caused cholinergic toxicity in workers inadequately protected during
packaging of the concentrate formulation.
When volunteers engaged in a simulated spray activity with a
mixture of demeton-S-methyl and demeton-O-methyl (30 and 70%,
respectively) were exposed to 8.8-27 mg/m3 of the two active
ingredients combined, they experienced no adverse effects on plasma or
erythrocyte cholinesterase activities.
1.1.5 Kinetics and metabolism
Demeton-S-methyl is rapidly and almost completely absorbed from
the intestinal tract of rats and is uniformly (except for high
concentration in erythrocytes) distributed to body tissues. It is
rapidly metabolized and excreted via the urine. Blood concentration
decreases with an initial half-life of about 2 h. About 1% of the oral
dose is present in the body 24 h after treatment. The main metabolic
pathway of demeton-S-methyl in rats is the oxidation of the side
chain leading to the formation of the corresponding sulfoxide
(oxydemeton-methyl) and, to a lesser extent, after further oxidation,
to the sulfone. Another important metabolic route is O-demethylation.
1.1.6 Effects on laboratory animals and in vitro test systems
1.1.6.1 Single exposure
Demeton-S-methyl causes cholinergic toxicity. The LD50 values
for mammals range from 7 to 100 mg/kg body weight, depending on the
route of administration and species.
1.1.6.2 Short-term exposure
An early dietary study showed that rats fed demeton-S-methyl at
50 mg/kg diet had substantially reduced brain and erythrocyte
cholinesterase activity after 26 weeks of exposure. Cholinergic signs
were observed in rats fed 200 mg/kg diet during the first 5 weeks of
exposure.
In a one-year dietary study on dogs, a no-observed-adverse-effect
level (NOAEL) of 1 mg/kg diet (equal to 0.036 mg/kg body weight per
day) was established, based on effects on brain cholinesterase.
1.1.6.3 Long-term exposure
Mice were fed diets containing 0, 1, 15 or 75 mg/kg demeton-S-
methyl for 21 months. The NOAEL was found to be 1 mg/kg diet (equal to
0.24 mg/kg body weight per day) based on inhibition of brain
cholinesterase.
In rats fed diets containing 0, 1, 7 or 50 mg/kg demeton-S-
methyl, the NOAEL, based on inhibition of brain cholinesterase, was
1 mg/kg diet (equal to 0.05 mg/kg body weight per day).
No increased tumour incidence was found in either species.
1.1.6.4 Skin and eye irritation and sensitization
Demeton-S-methyl is a mild skin and eye irritant. Positive
results were obtained with the Magnusson and Klingman maximization
test in guinea-pigs. However, the Buehler epidermal patch test gave no
indication of skin sensitization, suggesting that sensitization should
not be a problem in the practical use of demeton-S-methyl.
1.1.6.5 Reproduction, embryotoxicity and teratogenicity
In a two-generation dietary rat study, demeton-S-methyl caused
reduced viability and body weight of pups (F1b generation only)
at a dose level of 5 mg/kg diet. The NOAEL was 1 mg/kg diet, equal to
0.07 mg/kg body weight per day.
Demeton-S-methyl was neither embryotoxic nor teratogenic in rats
and rabbits.
1.1.6.6 Mutagenicity and related end-points
Demeton-S-methyl induces point mutations in vitro. Chromosomal
effects have been demonstrated in vivo with commercial formulations
only. The available information is insufficient to permit an adequate
assessment of the genotoxic potential of demeton-S-methyl.
1.1.6.7 Delayed neurotoxicity
Demeton-S-methyl caused neither delayed polyneuropathy nor
inhibition of neuropathy target esterase (NTE) when tested in hens at
a level equal to the oral LD50.
1.1.6.8 Toxicity of metabolites
Two plant and mammalian metabolites of demeton-S-methyl
(i.e. oxydemeton-methyl and demeton-S-methylsulfone) are also
commercial pesticides and have been extensively studied. It has been
reported that the toxicological profile of these two compounds does
not significantly differ, either quantitatively or qualitatively, from
that of demeton-S-methyl.
1.1.7 Mechanism of toxicity - mode of action
Demeton-S-methyl is a direct cholinesterase inhibitor, and the
toxicity it causes is related to inhibition of acetylcholinesterase
(AChE) at nerve terminals. AChE inhibited by demeton-S-methyl
reactivates spontaneously with an in vitro half-life of about 1.3 h,
as expected for dimethyl phosphorylated AChE.
1.1.8 Effects on humans
A few cases of acute intoxication with cholinergic syndrome,
following suicide attempts, have been reported. Surviving patients,
including a pregnant woman, did not show delayed effects.
Following careless occupational exposure during packaging of
the commercial formulation, some workers developed cholinergic
toxicity which required pharmacological treatment. Absorption of
demeton-S-methyl was probably through the skin. Similarly, improper
working conditions may have caused excessive dermal absorption during
application of demeton-S-methyl in cotton fields.
1.1.9 Effects on other organisms in the laboratory and field
The 96-h EC50s for green algae range from 8 to 37 mg/litre. The
LC50s for a range of aquatic invertebrates range from 0.004 to
1.3 mg/litre. The toxicity for fish varies, with 96-h LC50 ranging
from 0.59 mg/litre for the rainbow trout to about 40 mg/litre for the
golden orfe, the goldfish and the carp.
The acute oral LD50 for the Japanese quail and the canary is
10-50 mg/kg body weight. In starlings, a single oral dose of 2 mg/kg
body weight caused 20% inhibition of brain AChE 3 h after treatment.
The LC50 of demeton-S-methyl in soil for earthworms is 66 mg/kg
for 14 days. The acute oral and contact LD50 for demeton-S-methyl are
0.21 and 0.6 µg/bee respectively. When used on winter wheat at the
suggested rate, demeton-S-methyl significantly reduced the number of
crop foliage invertebrates (mainly Empididae flies) but not the
number of soil surface entomophagous invertebrates.
1.2 Conclusions
Demeton-S-methyl is a highly toxic (class Ib of the WHO
classification) (WHO, 1996) organophosphorus ester insecticide. The
mechanism of toxicity is that of AChE inhibition at nerve terminals.
Exposure of the general population results mainly from residues
present in crop commodities.
With good work practices, hygienic measures and safety
precautions, the use of demeton-S-methyl during manufacture or
application should not cause adverse effects. Effects due to chronic
exposure are unlikely to occur.
Demeton-S-methyl does not persist in the environment and is not
accumulated by organisms. It has high acute toxicity to aquatic
invertebrates and is toxic to fish and birds, leading to high or
moderate risk factors for these organisms. However, significant field
kills of organisms have not been reported for the compound.
Precautions should be taken to minimize exposure of non-target
organisms (e.g., do not spray over water bodies, minimize exposure by
spray drift).
1.3 Recommendations
For the health and welfare of workers and the general population,
the handling and application of demeton-S-methyl should only be
entrusted to supervised and well-trained operators who follow the
required safety measures and good application practices.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical formula: C6H15O3PS2
Chemical structure:
O
"
CH3CH2SCH2CH2SP(OCH3)2
Relative molecular mass: 230.3
Common name: demeton-S-methyl
CAS chemical name: S-[2-(ethylthio)ethyl] O,O-dimethyl
phosphorothioate
IUPAC name: S-2-ethylthioethyl O,O-dimethyl
phosphorothioate
CAS registry number: 919-86-8
RTECS number: TG1750000
Common synonyms and AI3-24963; BAY 18436; Bayer 18 436;
trade names: Bayer 25/154; Demetox; DEP 836 349;
Duratox; ethanethiol,
2-(ethylthio)-S-ester with O,O-dimethyl
phosphorothioate; HSDB 6410;
Isometasystox; Isomethylsystox;
Metaisoseptox; Metaisosystox; Metasystox
(I); metasystox forte; Metasystox I;
Metasystox J; Metasystox 55; methyl
demeton thioester; methyl isosystox;
methyl-mercaptofos teolery;
methyl-mercaptofos teolovy (USSR);
methylmercaptofostiol (USSR); Mifatox;
O,O-dimethyl S-(2-(ethylthio)
ethylphosphorothioate; O,O-dimethyl
S-ethylmercaptoethyl thiophosphate;
O,O-dimethyl 2-ethylmercaptoethyl
thiophosphate, thiolo isomer;
phosphorothioic acid, O,O-dimethyl
S-(2-(ethylthio)ethyl) ester;
phosphorothioic acid,
S-(2-(ethylthio)ethyl) O,O-dimethyl
ester; S-(2-(ethylthio)ethyl);
dimethyl phosphorothiolate;
S-(2-(Ethylthio)ethyl) O,O-dimethyl
phosphorothioate (8CI)(9CI);
S-(2-(ethylthio)ethyl) O,O-dimethyl
phosphorothioate; S-(2-ethylthio)ethyl)
O,O-dimethyl phosphorothioate;
S-(2-ethylthioetyl)0,0-dimethyl
phosphorothioate; S-2-Ethylthioethyl-
dimethyl phosphorothioate; USP 2 571
989; 2-Ethylthioethyl dimethyl
phosphorothioate.
Formulations: EC (250 or 500 g a.i./litre), DSM
(Campbell), Metasystox55 (Bayer),
Mepatox (FCC),
EC (580 g/litre).
Purity: >90%
Impurities: O,O,S-trimethylthiophosphate (maximum of
1.5%)
O-methyl-S-2-(ethylmercapto)-ethylthioph
osphate (maximum of 3.0%)
2-ethylthioethylmercaptan max 0.8%
bis(2-ethylthioethyl)-disulfide (maximum
of 0.8%)
Various ionic components (sulfonium
compounds, organic salts) (total maximum
of 2.5%)
Oligomeric alkyl(thio) phosphates
(maximum of 1.0%)
Water (maximum of 0.1%)
2.2 Physical and chemical properties
Some relevant physical and chemical properties are summarized in
Table 1.
Table 1. Some chemical and physical properties of demeton-S-methyl
Physical state: oily liquid
Colour: pale yellow
Odour: penetrating, reminiscent of leeks
Boiling point 74°C at 6.65 Pa (0.05 mmHg)
92°C at 26.6 Pa (0.20 mmHg)
102°C at 53.2 Pa (0.40 mmHg)
118°C at 133 Pa (1.00 mmHg)
Vapour pressure: 21.3 mPa (1.6 × 10-4 mmHg) at 10°C
63.8 mPa (4.8 × 10-4 mmHg) at 20°C
193 mPa (1.45 × 10-3 mmHg) at 30°C
400 mPa (3.8 × 10-3 mmHg) at 40°C
Relative density at 20°C: 1.21
n-Octanol/water partition
coefficient: log Pow = 1.32
Solubility in water: 3.3 g/litre (at room temperature)
Solubility in organic readily soluble in most organic
solvents: solvents; limited solubility in
petroleum ether
Stability: hydrolysed by alkali and oxidized to the
sulfoxide (oxydemeton-methyl) and
sulfone (demeton-S-methylsulfone)
Half-life in water: 11 days at 37°C
Half-lives at 22°C: 63 days at pH 4
56 days at pH 7
8 days at pH 9
2.3 Conversion factors
1 ppm = 9.42 mg/m3 (at 25°C)
1 mg/m3 = 0.106 ppm
2.4 Analytical methods
Analytical methods for the determination of residues of the
demeton-S-methyl group (i.e. demeton-S-methyl, its sulfoxide and its
sulfone) are either identical or very similar.
Originally, colorimetric methods (i.e. determination of total
phosphorus) were used (FAO/WHO, 1993). Current methods are based on
GLC. In principle, these methods involve an oxidation step, using
potassium permanganate, to produce demeton-S-methylsulfone, which is
then determined with a thermoionic emission detector.
A GLC method (Wagner & Thornton, 1977) is suitable for
determining residues in plants, soil and water. The method is based on
the principle of oxidation described above, with variations depending
on the sample to be analysed. Before the oxidation step, maceration
with acetone is used for samples with a high fat or oil content. The
macerate and water samples are then extracted with chloroform or
dichloromethane. Since the analytical method involves the oxidation to
the sulfone, the determination is of demeton-S-methylsulfone, from
which the demeton-S-methyl residue can be calculated. The method was
used to determine residues in a wide range of crops with a minimum
recovery above 80%. The limit of determination depends upon the sample
and generally lies between 0.01 and 0.2 mg/kg. Another method, based
on similar principles, has been described for oxydemeton-methyl
residues in plant and animal tissues and in soil (Thornton et al.,
1977).
An alternative GLC method for sulfides (including demeton-S-
methyl), sulfoxides and sulfones has been proposed by Hill et al.
(1984), who reported that the use of acetone as a co-solvent during
potassium permanganate oxidation causes unpredictable (from negligible
to complete) loss of demeton-S-methyl. These authors used ethyl
acetate for extraction of organophosphorus compounds from fruit and
vegetable samples (Anonymous, 1977). The extracts were cleaned-up by
chromatography on a column of activated charcoal, magnesium oxide and
Celite. The compounds were eluted from the column using a mixture of
ethyl acetate, acetone and toluene. A mean recovery of >68% (mainly
>80%) was found for a number of organophosphorus sulfides, sulfoxides
and sulfones, that of demeton-S-methyl being 82.4±6.6% (mean ± SD,
n=10) when determined in lettuce.
Wilkins et al. (1985) reported the characterization of nearly
90 organophosphorus sulfides, sulfoxides and sulfones by gas
chromatography and mass spectrometry. Using similar experimental
conditions with three different mass spectrometers, the spectra
produced from a given compound were almost identical.
A TLC method for 10 different organophosphorus insecticides
(including demeton-S-methyl and demeton-S-methylsulfone) was reported
by Funk et al. (1989). This method has a limit of detection of
4-10 ng/spot.
2.5 Formation of derivatives during storage
Storage of "pure" demeton-S-methyl in the dark at room
temperature leads to the formation of sulfonium derivatives. This was
found to be associated with increased intravenous toxicity but not
oral toxicity (Heath & Vandekar, 1957). Hecht (1960) reported that the
oral toxicity in rat did not change if 4- or 24-hour-old aqueous
solutions of demeton-S-methyl were used.
The concentration in a water suspension of 1 g a.i./litre
decreased by about 50 and 75% after 7 and 28 days of storage
(apparently at 37°C), respectively (Hecht, 1960) (see also section
7.1).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrences
Demeton-S-methyl does not occur as a natural product.
3.2 Man-made sources
3.2.1 Production
In 1954, a reaction mixture containing demeton-S-methyl and
demeton-O-methyl (O-2-ethylthioethyl O,O-dimethyl phosphorothioate)
was introduced by Farbenfabrik Bayer AG (now Bayer AG) with the common
name of demeton-methyl. An improved manufacturing process led to the
introduction, in 1957, of demeton-S-methyl by Bayer AG. Information on
the global production is not available. It is formulated as an
emulsifiable concentrate.
3.2.2 Uses
Demeton-S-methyl is a systemic and contact insecticide and
acaricide used to control Acarina, Thysanoptera, Hymenoptera and
Homoptera on cereals, fruits, ornamentals and vegetables. It is
applied as an emulsifiable concentrate formulation mainly as a spray
and usually at a concentration of 0.025% a.i. (FAO/WHO, 1974). It has
been reported that most national registrations for demeton-S-methyl
should be transferred during the next few years to oxydemeton-methyl,
which has similar use and is applied at similar rates (FAO/WHO, 1993).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
The sorption behaviour of demeton-S-methyl and three other
organophosphorus pesticides in natural pond sediments was tested by
Froebe et al. (1989). Two different sediments were used, containing
9.3 and 6.2% organic matter, at pH 5.9 and 6.5, and a specific surface
area of 23.7 and 16.7 m2/g, respectively. The semi-quantitative
mineralogical composition was similar. For the four pesticides, their
sorption coefficients (Kd) followed the same sequence as their
lipophilicity (expressed as n-octanol/water coefficient). The
sorption efficiency for demeton-S-methyl was higher in the sediment
with a lower content of organic matter (Kd of 0.689 and 2.66,
respectively).
4.2 Abiotic and biotic transformation
4.2.1 Hydrolytic degradation
In water at 22°C demeton-S-methyl has a half-life of 63 days at
pH 4, 56 days at pH 7, and 8 days at pH 9. The main reactions are
demethylation in acid medium and hydrolysis of the phosphorus-ester
bond in basic medium (Krohn, 1984) (see also section 2.2).
4.2.2 Photodegradation
Demeton-S-methyl in aqueous solution does not absorb any light at
247 nm or longer wavelengths. Therefore, no direct photo-degradation
of demeton-S-methyl in the environment is to be expected
(Hellpointner, 1990). When solutions of demeton-S-methyl in water
(3.6-3.7 mg/litre) were irradiated for 8 h with a high-pressure
mercury vapour lamp, no photodegradation was detected. However, when
solutions were fortified with humic acid (10 mg/litre), the half-life
of photodegradation was
8 h (the degradation products were not identified). This suggests that
degradation by sensitized or indirect photolysis may also occur in the
environment (Wilmes, 1984).
4.2.3 Degradation in soil
The metabolism of demeton-S-methyl in soil is shown in Fig. 1.
The ability of microbial organisms to biodegrade demeton-S-methyl
sulfoxide was evaluated in a laboratory test and under aerobic
conditions using various species (Nocardia, Arthrobacter,
Corynebacter, Brevibacterium, Bacillus and Pseudomanas) and strains
(Ziegler et al., 1980). Almost all the organisms were able to degrade
the insecticide. The amount of insecticide degraded ranged between 65%
(Arthrobacter roseoparaffineus) and 99% (Pseudomonas putida) after
14 days of incubation. The biodegradation process led to the
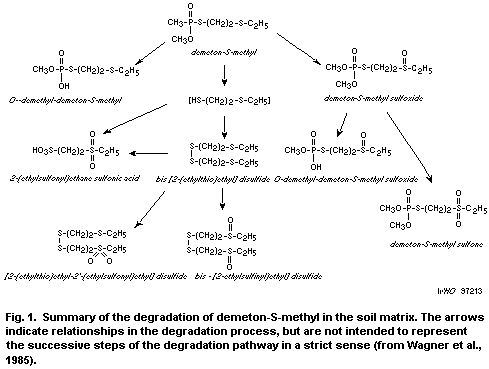 production of several metabolites. In particular, P. putida and
Nocardia sp. during growth were able to metabolize almost completely
2 mmol/litre of demeton-S-methyl sulfoxide (99% and 98% respectively)
within 13 days. Three major metabolites, i.e., O-demethyl-demeton-S-
methyl, demeton-S-methyl sulfoxide and bis[2-ethylsulfinyl)ether]
disulfide, were found to be produced by Pseudomonas, whereas Nocardia
showed different pathways leading to the formation of different
metabolites, i.e., 2-(ethylsulfonyl)ethane sulfonic acid,
demeton-S-methyl sulfone and bis[2-(ethylthio)ethyl] disulfide. In
sterile controls about 48% of the parent compound remained after 20
days of incubation.
In a laboratory study, biodegradation of demeton-S-methyl was
investigated in two different standardized soils (S1, and S2) with
different characteristics particularly in terms of organic matter
content and cation exchange capability (Wagner et al., 1985). The
study was conducted under aerobic and anaerobic conditions with
sterile controls and using 14C-labelled demeton-S-methyl. One day
after the start of the test no parent compound could be detected.
After 63 days under aerobic conditions 54% (S1) and 34% (S2) of the
14C activity applied was eliminated as 14CO2, indicating a higher
activity in the soil with higher organic matter content and cation
exchange capability. Various metabolites were isolated and identified
(Fig. 1). Under anaerobic conditions 0.5% (S1) and 1.1% (S2) of the
14C activity applied was eliminated as 14CO2, and there was a
predominance of the metabolites O-demethyl-demeton-S-methyl and
demeton-S-methyl sulfoxide. The half-lives of demeton-S-methyl were
approximately 5 h in the non-sterile soil and 70 h in the sterile test
control.
4.2.4 Biodegradation in plants
The metabolic behaviour of ethylene-1-14C demeton-S-methyl in
spring wheat (Schirokko variety) was investigated in a greenhouse test
(Wagner & Oehlmann, 1987). The distribution of the 14C radioactivity
in the wheat matrix was determined at 3, 14, 42 and 60 days after
application of demeton-S-methyl during crop stage 0 (flowering), and
the isolated biotransformation products were analysed by spectroscopy.
The composition of the applied spray was 241.5 µCi ethylene-1-14C
demeton-S-methyl (corresponding to about 0.5 kg a.i./hectare as
opposed to a use rate of 0.15 kg a.i./hectare) in 2 ml benzene plus
1 drop emulsifier Np10 plus 20 ml water. The majority of the applied
radioactivity was found in the wheat straw (about 85% of total
radioactivity corresponding to 10.3 mg a.i. equivalents/kg, 60 days
after application), while the amount in the kernels at harvest was
substantially smaller (0.7 mg a.i. equivalents/kg). About 0.5 mg a.i.
equivalents/kg could not be extracted with water and organic solvents,
and 24% of the 14C activity could not be extracted from the straw
using solvents of different polarity. Only a minor portion of the a.i.
was detected 3 days after application. Identified metabolites were
O,O-dimethyl-S-[2-(ethylsulfonyl)-ethyl]-thiophosphate (11.7% of
recovered radioactivity at 60 days), O,O-dimethyl-S-[2-
(ethylsulfonyl)-ethyl]-thiophosphate (9.8% of recovered radioactivity
at 60 days), 2-ethylsulfonyl-ethanesulfonic acid (8.2% of recovered
radioactivity at 60 days), S-(2-(ethylsulfonyl)-ethyl)-thiophosphate
(5.2% of recovered radioactivity at 60 days), 1-(ethylsulfinyl)-2-
(methylsulfinyl)-ethane (8.9% of recovered radioactivity at 60 days),
and 2-ethylsulfinylethanol (5.1% of recovered radioactivity at 60
days). Additional biotransformation products occurred to a minor
extent (<0.2%) in the kernels only.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 General population exposure
Data on residues in crops resulting from the use of demeton-S-
methyl have been summarized by FAO/WHO (1974). Maximum residue limits,
varying from 0.01 to 1 mg/kg, have been recommended for a range of
commodities. These residue limits were previously expressed as
demeton-S-methyl but now as oxydemeton-methyl, and are common for
demeton-S-methyl, oxydemeton-methyl and demeton-S-methylsulfone
(see section 2.4). More updated values referring to the use of
oxydemeton-methyl have been reported by FAO/WHO (1993). Data on
residue levels in meat and milk from cows, and in chickens, eggs and
fish have been reported for oxydemeton-methyl in the same document
but not for demeton-S-methyl. Some data are also available for
demeton-S-methylsulfone (FAO/WHO, 1993). The JMPR recommended a group
ADI of 0-0.003 mg/kg body weight for demeton-S-methyl,
oxydemeton-methyl and demeton-S-methyl sulfone (FAO/WHO, 1990).
5.2 Occupational exposure during manufacture, formulation or use
When Metasystox (30% demeton-S-methyl, 70% demeton-O-methyl) was
sprayed with a hand-held nebulizer, the concentration of the two
active ingredients combined was 8.8-27 mg/m3 of ambient air (Klimmer
& Pfaff, 1955; see also section 8.2.2).
The ACGIH proposed a very conservative TLV/TWA for methyl-demeton
of 0.5 mg/m3 with the "skin" notation (ACGIH, 1993). The "skin"
notation indicates that dermal absorption is likely to occur and
therefore adequate protective equipment should be used.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption, distribution and excretion
Absorption, distribution and excretion of (ethylene-1-14C)-
demeton-S-methyl (93% radiochemical purity) were studied in SD SPF
rats. Male rats (n=5 per group) were given a single oral dose of 0.1,
0.5, 5 or 10 mg/kg body weight or a single intravenous dose of 0.5 or
1 mg/kg body weight of radio-labelled compound. Female rats (n=5)
received a single oral dose of 0.5 mg/kg body weight. Total
radioactivity recovery was 90-100% in each animal. The authors
reported that kinetic parameters did not change with dose or sex;
therefore, only data on male rats given 5 mg/kg orally have been
reported. Absorption after oral administration was rapid (peak blood
concentration was reached within one hour) and almost complete (98-99%
of the administered radioactivity was, in fact, eliminated through the
urine). The blood concentration decreased with a half-life of about
2 h during the first 6 h and then with a half-life of about 6 h for
the next 48 h. The half-life thereafter was even longer. The
radioactivity associated with erythrocytes accounted for almost all of
the blood radioactivity found 24 h or more after dosing. The half-life
of urinary elimination was 2-3 h during the first 24 h and 1.5 days
thereafter. Elimination through faeces and exhaled air accounted for
0.5-2% and about 0.2% of the applied dose, respectively. Except for
erythrocytes, radioactivity was distributed rather uniformly in
various body tissues and organs. At 2, 24 and 48 h after dosing, the
radioactivity remaining in the body was about 60%, 1% and 0.5% of the
administered dose, respectively. At 10 days, radioactivity was almost
undetectable in most organs except in the erythrocytes. In a separate
experiment, whole-body autoradiography indicated some localized
accumulation of radioactivity in the pineal gland, thyroid and some
glands of the genital tract (Cowper's gland, seminal vesicle,
accessory genital gland). When the labelled compound (0.5 mg/kg body
weight) was administered into the duodenum of rats with cannulated
bile ducts, it was shown that about 3% of the radioactivity was
excreted into the bile in the first 24 h (Weber et al., 1978).
6.2 Metabolic transformation
The proposed metabolic pathway of demeton-S-methyl in rats
is shown in Fig. 2. This was derived from the analysis of urine
samples of SD rats given a single oral dose of 5 or 10 mg/kg of
(ethylene-1-14C)-demeton-S-methyl. Urine samples were collected
for 8 or 24 h after dosing and there was a 92% or 96% recovery,
respectively, of the applied dose. The main metabolic route was
oxidation of the side chain leading to the formation of the
corresponding sulfoxide oxydemeton-methyl, and to a lesser extent,
after further oxidation, the sulfone; O-demethylation was also an
important route. Neither glucuronide nor sulfate conjugates were found
(Ecker, 1978; Ecker & Cölln, 1983).
production of several metabolites. In particular, P. putida and
Nocardia sp. during growth were able to metabolize almost completely
2 mmol/litre of demeton-S-methyl sulfoxide (99% and 98% respectively)
within 13 days. Three major metabolites, i.e., O-demethyl-demeton-S-
methyl, demeton-S-methyl sulfoxide and bis[2-ethylsulfinyl)ether]
disulfide, were found to be produced by Pseudomonas, whereas Nocardia
showed different pathways leading to the formation of different
metabolites, i.e., 2-(ethylsulfonyl)ethane sulfonic acid,
demeton-S-methyl sulfone and bis[2-(ethylthio)ethyl] disulfide. In
sterile controls about 48% of the parent compound remained after 20
days of incubation.
In a laboratory study, biodegradation of demeton-S-methyl was
investigated in two different standardized soils (S1, and S2) with
different characteristics particularly in terms of organic matter
content and cation exchange capability (Wagner et al., 1985). The
study was conducted under aerobic and anaerobic conditions with
sterile controls and using 14C-labelled demeton-S-methyl. One day
after the start of the test no parent compound could be detected.
After 63 days under aerobic conditions 54% (S1) and 34% (S2) of the
14C activity applied was eliminated as 14CO2, indicating a higher
activity in the soil with higher organic matter content and cation
exchange capability. Various metabolites were isolated and identified
(Fig. 1). Under anaerobic conditions 0.5% (S1) and 1.1% (S2) of the
14C activity applied was eliminated as 14CO2, and there was a
predominance of the metabolites O-demethyl-demeton-S-methyl and
demeton-S-methyl sulfoxide. The half-lives of demeton-S-methyl were
approximately 5 h in the non-sterile soil and 70 h in the sterile test
control.
4.2.4 Biodegradation in plants
The metabolic behaviour of ethylene-1-14C demeton-S-methyl in
spring wheat (Schirokko variety) was investigated in a greenhouse test
(Wagner & Oehlmann, 1987). The distribution of the 14C radioactivity
in the wheat matrix was determined at 3, 14, 42 and 60 days after
application of demeton-S-methyl during crop stage 0 (flowering), and
the isolated biotransformation products were analysed by spectroscopy.
The composition of the applied spray was 241.5 µCi ethylene-1-14C
demeton-S-methyl (corresponding to about 0.5 kg a.i./hectare as
opposed to a use rate of 0.15 kg a.i./hectare) in 2 ml benzene plus
1 drop emulsifier Np10 plus 20 ml water. The majority of the applied
radioactivity was found in the wheat straw (about 85% of total
radioactivity corresponding to 10.3 mg a.i. equivalents/kg, 60 days
after application), while the amount in the kernels at harvest was
substantially smaller (0.7 mg a.i. equivalents/kg). About 0.5 mg a.i.
equivalents/kg could not be extracted with water and organic solvents,
and 24% of the 14C activity could not be extracted from the straw
using solvents of different polarity. Only a minor portion of the a.i.
was detected 3 days after application. Identified metabolites were
O,O-dimethyl-S-[2-(ethylsulfonyl)-ethyl]-thiophosphate (11.7% of
recovered radioactivity at 60 days), O,O-dimethyl-S-[2-
(ethylsulfonyl)-ethyl]-thiophosphate (9.8% of recovered radioactivity
at 60 days), 2-ethylsulfonyl-ethanesulfonic acid (8.2% of recovered
radioactivity at 60 days), S-(2-(ethylsulfonyl)-ethyl)-thiophosphate
(5.2% of recovered radioactivity at 60 days), 1-(ethylsulfinyl)-2-
(methylsulfinyl)-ethane (8.9% of recovered radioactivity at 60 days),
and 2-ethylsulfinylethanol (5.1% of recovered radioactivity at 60
days). Additional biotransformation products occurred to a minor
extent (<0.2%) in the kernels only.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 General population exposure
Data on residues in crops resulting from the use of demeton-S-
methyl have been summarized by FAO/WHO (1974). Maximum residue limits,
varying from 0.01 to 1 mg/kg, have been recommended for a range of
commodities. These residue limits were previously expressed as
demeton-S-methyl but now as oxydemeton-methyl, and are common for
demeton-S-methyl, oxydemeton-methyl and demeton-S-methylsulfone
(see section 2.4). More updated values referring to the use of
oxydemeton-methyl have been reported by FAO/WHO (1993). Data on
residue levels in meat and milk from cows, and in chickens, eggs and
fish have been reported for oxydemeton-methyl in the same document
but not for demeton-S-methyl. Some data are also available for
demeton-S-methylsulfone (FAO/WHO, 1993). The JMPR recommended a group
ADI of 0-0.003 mg/kg body weight for demeton-S-methyl,
oxydemeton-methyl and demeton-S-methyl sulfone (FAO/WHO, 1990).
5.2 Occupational exposure during manufacture, formulation or use
When Metasystox (30% demeton-S-methyl, 70% demeton-O-methyl) was
sprayed with a hand-held nebulizer, the concentration of the two
active ingredients combined was 8.8-27 mg/m3 of ambient air (Klimmer
& Pfaff, 1955; see also section 8.2.2).
The ACGIH proposed a very conservative TLV/TWA for methyl-demeton
of 0.5 mg/m3 with the "skin" notation (ACGIH, 1993). The "skin"
notation indicates that dermal absorption is likely to occur and
therefore adequate protective equipment should be used.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption, distribution and excretion
Absorption, distribution and excretion of (ethylene-1-14C)-
demeton-S-methyl (93% radiochemical purity) were studied in SD SPF
rats. Male rats (n=5 per group) were given a single oral dose of 0.1,
0.5, 5 or 10 mg/kg body weight or a single intravenous dose of 0.5 or
1 mg/kg body weight of radio-labelled compound. Female rats (n=5)
received a single oral dose of 0.5 mg/kg body weight. Total
radioactivity recovery was 90-100% in each animal. The authors
reported that kinetic parameters did not change with dose or sex;
therefore, only data on male rats given 5 mg/kg orally have been
reported. Absorption after oral administration was rapid (peak blood
concentration was reached within one hour) and almost complete (98-99%
of the administered radioactivity was, in fact, eliminated through the
urine). The blood concentration decreased with a half-life of about
2 h during the first 6 h and then with a half-life of about 6 h for
the next 48 h. The half-life thereafter was even longer. The
radioactivity associated with erythrocytes accounted for almost all of
the blood radioactivity found 24 h or more after dosing. The half-life
of urinary elimination was 2-3 h during the first 24 h and 1.5 days
thereafter. Elimination through faeces and exhaled air accounted for
0.5-2% and about 0.2% of the applied dose, respectively. Except for
erythrocytes, radioactivity was distributed rather uniformly in
various body tissues and organs. At 2, 24 and 48 h after dosing, the
radioactivity remaining in the body was about 60%, 1% and 0.5% of the
administered dose, respectively. At 10 days, radioactivity was almost
undetectable in most organs except in the erythrocytes. In a separate
experiment, whole-body autoradiography indicated some localized
accumulation of radioactivity in the pineal gland, thyroid and some
glands of the genital tract (Cowper's gland, seminal vesicle,
accessory genital gland). When the labelled compound (0.5 mg/kg body
weight) was administered into the duodenum of rats with cannulated
bile ducts, it was shown that about 3% of the radioactivity was
excreted into the bile in the first 24 h (Weber et al., 1978).
6.2 Metabolic transformation
The proposed metabolic pathway of demeton-S-methyl in rats
is shown in Fig. 2. This was derived from the analysis of urine
samples of SD rats given a single oral dose of 5 or 10 mg/kg of
(ethylene-1-14C)-demeton-S-methyl. Urine samples were collected
for 8 or 24 h after dosing and there was a 92% or 96% recovery,
respectively, of the applied dose. The main metabolic route was
oxidation of the side chain leading to the formation of the
corresponding sulfoxide oxydemeton-methyl, and to a lesser extent,
after further oxidation, the sulfone; O-demethylation was also an
important route. Neither glucuronide nor sulfate conjugates were found
(Ecker, 1978; Ecker & Cölln, 1983).
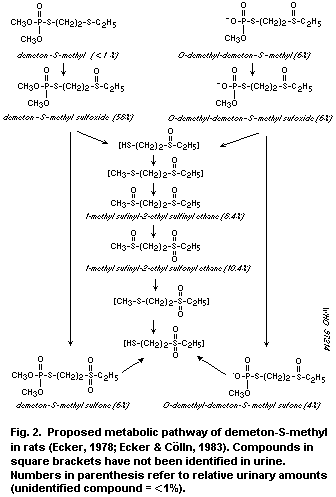 7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Demeton-S-methyl causes cholinergic toxicity. The acute toxicity
data are reported in Table 2.
Heath & Vandekar (1957) and Vandekar (1958) showed a significant
increase in intravenous but not oral toxicity after storage of
demeton-S-methyl ("pure") at room temperature in the dark. This was
associated with the formation of sulfonium derivatives, which have a
lower oral toxicity, possibly because of poor absorption.
Dilution with water also increased the intravenous toxicity, and
this was again associated with the formation of sulfonium derivatives.
The maximum toxicity of a 1 mg/ml suspension kept at 35°C for one day
was about 30 times the initial toxicity (the LD50 decreased from
about 60 to about 2 mg/kg in rats) (Heath & Vandekar, 1957). Similar
results were obtained with commercial Metasystox (70% demeton-O-
methyl, 30% demeton-S-methyl) (see also section 2.2.)
7.1.1 Oral
Following oral dosing with demeton-S-methyl performed on a very
small number of animals (one per dose level), one rabbit given
50 mg/kg died within 2 h, while the dose of 20 mg/kg caused symptoms
but the animal recovered. A cat given 10 mg/kg died after 2 days,
while the dose of 5 mg/kg caused signs that were reversible and
animals recovered (few details given) (Hecht, 1955).
Single oral doses (180 mg/kg) of metasystox, containing 25%
demeton-S-methyl, were administered to seven male buffalo calves
(10-12 months of age). Signs of poisoning appeared 15 to 35 min later.
Two calves were treated repeatedly with atropine (1.5 mg/kg body
weight, i.v.), D-tubocurarine (0.1 mg/kg body weight, i.v.) and
glucose (3-4 mg/kg body weight, i.v.). Another two calves were treated
repeatedly with atropine (1.5 mg/kg body weight, i.v.), gallamine
(1 mg/kg body weight, i.v.) and glucose (3-4 mg/kg body weight, i.v.)
Three calves were not treated. All calves displayed typical
cholinergic signs. Treatments delayed but did not prevent death, which
occurred in about 2 h in calves not treated with antidotes and in
about 23 h in calves treated with antidotes (Mitra et al., 1978).
7.1.2 Inhalation
The LC50 (4 h of exposure) for Wistar rats was found to be 310
and 210 mg/m3 for males and females, respectively (Flucke & Pauluhn,
1983).
Table 2. LD50 of demeton-S-methyl for various species and different routes of administration
Species Sex Observation Route Purity LD50 Vehicle References
(strain) period (mg/kg
(days) body weight)
Mouse M 3 oral ? 17 ? Klimmer & Pfaff (1955)
Mouse ? 7 i.v. ? 7 water Hecht (1960)
Rat F 1 oral "pure" 63 ? Heath & Vandekar (1957)
Vandekar (1958)
Rat M 3 days minimum oral ? 40 ? Klimmer & Pfaff (1955)
Rat ? ? oral ? 35 ? Hecht (1955)
Rat M 14 oral 25% formulation 33 water Klimmer (1964)
(Wistar)
Rat M ? oral 86-89% 57-64 Lutrol 9 Klimmer (1964)
(Sprague-
Dawley)
Rat M & F 14 oral 50% formulation 64-65 water Flucke & Pauluhn (1983)
(Wistar)
Rat M 7 oral 50% formulation 129 water Edson (1960)
(Wistar)
Rat M 14 oral 90% formulation 44 cremophor EL Flucke & Kimmerle (1977)
(Wistar)
Table 2. (con't)
Species Sex Observation Route Purity LD50 Vehicle References
(strain) period (mg/kg
(days) body weight)
Rat F 10 oral ? 80 ethanol (20%) DuBois & Doull (1955)
(Sprague- and DuBois & Plzak (1962)
Dawley) propylene
glycol (80%)
Rat M & F 14 i.p. ? 7.5 ethanol (20%) DuBois & Doull (1955)
(Sprague- and propylene DuBois & Plzak (1962)
Dawley) glycol (80%)
Rat ? ? i.p. formulation 10 water Hecht (1960)
Rat ? ? dermal ? 85 ? Edson (1960)
Rat M 14 dermal 50% formulation 71 none Flucke & Pauluhn (1983)
(Wistar)
Rat F 14 dermal 50% formulation 45 none Flucke & Pauluhn (1983)
(Wistar)
Rat ? 7 dermal technical 100-200 none Hecht (1960)
Rat ? 7 dermal 25% formulation about 10 none Hecht (1960)
Rat F 1 i.v. "pure" 65 ? Heath & Vandekar (1957)
Vandekar (1958)
Table 2. (con't)
Species Sex Observation Route Purity LD50 Vehicle References
(strain) period (mg/kg
(days) body weight)
Guinea- M 10 oral ? 110 ethanol (20%) Du Bois & Doull (1955)
pig and propylene
glycol (80%)
Guinea- M 14 i.p. ? 12.5 ethanol (20%) Du Bois & Plzak (1962)
pig and propylene
glycol (80%)
When rats (n=2) and mice (n=4) were exposed for 1 h to 1, 2.5 or
5 g/m3 of demeton-S-methyl (alcohol solution), all mice died whereas
rats displayed signs but recovered (few experimental details given)
(Hecht, 1955).
Rats (n=20) were exposed for 8 h to nebulized demeton-S-methyl
(0.5 g/m3 of air), which was obtained from a 25% emulsifiable
commercial formulation diluted 1:250 (0.1% final concentration of
active ingredient). None of the animals died or showed overt
cholinergic signs. Erythrocyte cholinesterase activity, determined in
three animals immediately after the end of exposure, was reduced by
70%. When purified active ingredient was used, lower erythrocyte
cholinesterase inhibition (60%) was found under the same experimental
conditions. This was paralleled by an increased rat LD50 (from 10 to
27.5 mg/kg i.p.) (Hecht, 1960).
7.1.3 Dermal
Doses of 20 or 100 mg/kg demeton-S-methyl applied to the shaved
skin of two cats caused death. A dose of 10 mg/kg caused mild signs;
very few details were given (Hecht, 1955).
7.2 Short-term exposure
7.2.1 Rat
Groups of six male rats (strain not specified) were fed
demeton-S-methyl at levels of 0, 50, 100 or 200 mg/kg diet in
the diet (equivalent to 0, 5, 10 and 20 mg/kg body weight per day,
respectively) for 6 months. Cholinergic signs (slight tremors and
fasciculations) were observed at the highest dose-level during the
first 5 weeks of the study. Brain and erythrocyte cholinesterase
activities were reduced at 50 mg/kg diet (by about 80 and 88%,
respectively), 100 mg/kg diet (by about 85 and 92%) and 200 mg/kg diet
(by about 90 and 94%) groups. Body weight gain was depressed at 100
and 200 mg/kg diet. Gross microscopic examination of tissues (liver,
kidney and adrenals) showed no treatment-related changes (Vandekar,
1958).
Groups of 30-day-old female Holtzman rats were fed dietary
levels of 0, 1, 5 or 25 mg/kg diet of demeton-S-methyl (purity not
reported) for 1 week. At termination, serum, liver and brain
acetylcholinesterase (n=3) and liver and serum aliesterase (n=3) with
diethylsuccinate and tributyrin as substrates activities were
measured. Interpolated dietary levels producing 50% inhibition were
15-28 mg/kg diet for acetylcholinesterase, 4-6 mg/kg diet for liver
aliesterase and about 25 mg/kg diet for serum aliesterase, equivalent
to 1.5-2.8, 0.4-0.6 and 2.5 mg/kg body weight per day, respectively
(Su et al., 1971).
7.2.2 Dog
In a one-year study, pure-bred beagle dogs (n=6 animals of each
sex per group) were fed 0, 1, 10 or 100 mg a.i./kg diet (day 1-36) or
50 mg a.i./kg diet (day 37-termination) of demeton-S-methyl (52.2% in
xylene). Haematological, blood biochemical and urinalysis parameters
were determined during pretest period and at months 1, 2, 3, 4, 5, 6,
8, 10, 12. Hearing tests and ophthalmoscopic examinations were
performed once in the pretest period and at months 3, 6 and 12 of
treatment. At termination animals were killed for pathology, and
determination of organ weights, brain cholinesterase activity, hepatic
cytochrome P-450 and triglyceride contents and N-demethylase
activity were carried out.
All animals survived the study. Diarrhoea and vomiting were
observed in all animals, most frequently in the high-dose group: these
animals also showed reduced food consumption before the dose was
reduced to 50 mg/kg diet. The mean daily compound intake was found to
be 0.036, 0.36 and 4.6/1.5 mg/kg body weight at 1, 10 and 100/50 mg/kg
diet, respectively. Body weight was similar in all groups. No
alterations were observed in the hearing test and ophthalmoscopic
examination. Haematological, blood chemistry (excluding cholinesterase
activities) and urinalysis parameters and organ weights at termination
were not significantly altered by any of the treatments. Hepatic
biochemical parameters were not altered by any of the treatments. No
treatment-related gross pathology alterations were found. However,
multifocal slight/moderate atrophy and/or hypertrophy of proximal
renal tubules was demonstrated in three males and three females of the
high-dose group. Plasma cholinesterase activity was reduced as
compared to controls by 20-30% and 5-20% in males and females,
respectively, at 10 mg/kg diet, and by 45-65% (males) and 50-70%
(females) at 50 mg/kg diet. Erythrocyte cholinesterase activity was
reduced by 25-35% and 30-45% in males and females, respectively, at
10 mg/kg diet. A higher inhibition was found at 50 mg/kg diet, where
inhibition was 80-90% and 55-65% in males and females, respectively.
Brain cholinesterase activity was reduced by 25% in males at 10 mg/kg
diet and by 64% (males) and 15% (females) at 50 mg/kg diet. Based on
effects on brain cholinesterase activity, the NOAEL was 1 mg/kg diet,
equal to 0.036 mg/kg body weight per day (Bathe, 1983).
7.3 Long-term exposure
7.3.1 Mouse
A long-term carcinogenicity study was conducted in NMRI mice
(70 animals of each sex per group) that were given demeton-S-methyl
(about 50% in xylene) mixed into the feed with approximately 10 mg/kg
diet of groundnut oil at concentrations of 0, 1, 15 or 75 mg a.i./kg
diet, or xylene (75 mg/kg diet). Groups were subdivided into two
subgroups: one (n=20) was terminated at 12 months, the second one was
terminated at 21 months.
Animals in the high-dose group had a lower (significantly lower
during the first 4 weeks only) food consumption and a reduced (about
10% throughout the study in males only, and at the beginning in
females) body weight. The mean daily intake of demeton-S-methyl was
(males/females): 0.24/0.29, 3.47/4.18, 17.81/20.0 mg/kg body weight
at 1, 15, 75 mg/kg diet, respectively. Clinical signs due to
cholinesterase inhibition were not observed. Mortality at 21 months
was (males/females) 16/30, 13/34, 17/35, 16/35 and 16/32% in the
control, low-, mid-, high-dose and xylene groups, respectively.
Haematological and clinical chemical parameters were not affected by
the treatment except plasma urea (lower than control in the high-dose
males) and plasma and erythrocyte cholinesterase activities. Plasma
cholinesterase activity was significantly decreased in mid- (by
63-74%) and high-dose (by 91-97%) groups. Erythrocyte cholinesterase
activity was only slightly reduced in the mid- and high-dose groups.
Brain cholinesterase activity (n=10 per group) was reduced in
high-dose groups (in males by 70% and in females by 59%) and in the
mid-dose group (in males by 44% and in females by 38%). Histological
examination did not reveal an increased incidence of neoplastic and
non-neoplastic lesions in treated groups. Based on inhibition of brain
cholinesterase, the NOAEL was 1 mg/kg diet, equal to 0.24 mg/kg body
weight per day (Schmidt & Bomhard, 1988).
7.3.2 Rat
Wistar rats (60 animals of each sex per group) were given
demeton-S-methyl (about 50% in xylene mixed into the feed with
approximately 10 mg/kg of groundnut oil) at concentrations of 0, 1, 7
or 50 mg a.i./kg diet, or 50 mg/kg diet of xylene. Groups were
subdivided into two subgroups; one (n=10) was terminated at 12 months,
the second one was terminated at 24 months.
Hair loss (up to 50% of females) and diarrhoea (up to 50% of
males) were observed significantly more frequently in the animals of
the high-dose group. Body weight was reduced in mid-dose males (by
5-10%) and in both males (by 10-20%) and females (by 5%) in the
high-dose group. Food consumption was similar in all groups. The mean
daily intake of demeton-S-methyl was (males/females): 0.05/0.06,
0.31/0.41, 2.59/3.09 mg/kg body weight at 1, 7 and 50 mg/kg diet,
respectively. Mortality at 24 months was (males/females) 12/26, 10/20,
10/20, 4/26 and 8/24% in the control, low-, mid-, high-dose and xylene
groups, respectively. Haematological and clinical chemical parameters,
except for cholinesterases, measured at months 6, 12, 18 and 24, were
unaffected by the treatments. Plasma and erythrocyte cholinesterase
activities, measured at months 3, 6, 12 and 24, were significantly
decreased in groups given 7 mg/kg diet (plasma cholinesterase by
30-56%, erythrocyte cholinesterase by 12-31%) or 50 mg/kg diet (plasma
cholinesterase by 75-92%, erythrocyte cholinesterase by 20-44%). Brain
cholinesterase activity (measured at 12 and 24 months) was reduced in
the high-dose group (by 67-75%) and in the mid-dose group (by 15-47%).
A statistically significant decrease in brain cholinesterase activity
(33%) was observed in males given 1 mg/kg diet at 24 months but not at
12 months. The toxicological significance of this finding is not
clear; it should also be noted that brain cholinesterase activity at
7 mg/kg diet was higher than at 1 mg/kg diet and that plasma
cholinesterase and erythrocyte cholinesterase activities were not
inhibited at either of these dose levels. Histological examination did
not reveal an increased incidence of neoplastic lesions in treated
groups. Increased incidence of retinal atrophy (78% of males, 92% of
females as compared to 36-63% and 61-70% in the other groups) and
keratitis (44% of males, 22% of females as compared to 4-12% and 0-2%,
respectively, in the other groups) was observed at 50 mg/kg diet. The
retinal atrophy mainly affected the fundus, unilaterally or
bilaterally, and occurred in either the inner corneal layer, the inner
and outer layers, or all layers of the retina. Based on inhibition of
brain cholinesterase, the NOAEL was considered to be 1 mg/kg diet,
equal to 0.05 mg/kg body weight per day (Schmidt & Westen, 1988).
7.4 Skin and eye irritation and sensitization
7.4.1 Skin and eye irritation
Demeton-S-methyl (commercial formulation, 50% of active
ingredient) was applied (0.5 ml) to the shaved skin of three New
Zealand white rabbits on a 2.5 × 2.5 cm cellulose dressing for 4 h.
Mild erythema and oedema were observed, which generally disappeared
after 3 days (Flucke & Pauluhn, 1983). A 52.5% solution of
demeton-S-methyl in xylene produced slight skin and eye irritancy in
New Zealand white rabbits, but this was attributed to the solvent
(Thyssen, 1981).
A commercial formulation of demeton-S-methyl (50%) was instilled
(0.1 ml) either undiluted or as a 0.5% aqueous dilution into the
conjunctival sac of one eye of groups (n=3) of New Zealand white
rabbits. Treated eyes were washed with physiological solution after
24 h. No signs of eye irritation were observed in rabbits treated with
the 0.5% aqueous solution. The undiluted formulation caused severe
lacrimation and miosis on application. Mild corneal opacity and
discrete redness and oedema of conjunctivae were observed and
recovered in about 7 days (Flucke & Pauluhn, 1983).
7.4.2 Skin sensitization
The skin-sensitizing potential of demeton-S-methyl was assessed
by the Magnusson and Kligman maximization test with Freund's adjuvant
on guinea-pigs (n=20, Bor:SPF, DHPW strain). The concentrations of
demeton-S-methyl (96.3% purity, average of three determinations) used
were: 0.1% for the intra-dermal induction, 10% for the topical
induction and the first challenge, and 1% for the second challenge.
All twenty animals reacted positively to the 1st challenge (controls
4/10), and 16 reacted positively to the 2nd challenge (controls 3/10).
The results indicate that demeton-S-methyl has a skin-sensitizing
potential (Heimann, 1987a).
In another study, the Buehler epidermal patch test was used on
guinea-pigs (n=12, Bor: SPF, DHPW strain). The concentrations of
demeton-S-methyl (95.6% purity, average of three determinations) used
were 10% for topical induction (once a week for 3 weeks) and the first
challenge, and 20% for the second challenge. The results indicate that
demeton-S-methyl does not have a skin-sensitizing potential under
these conditions (Heimann, 1987b).
It is concluded that demeton-S-methyl might have some
skin-sensitizing potential, but this is of no relevance in practice.
7.5 Reproduction, embryotoxicity and teratogenicity
7.5.1 Reproduction
A standard two-generation study (two litters/generation) was
conducted in SPF rats (BOR:WISW) (10 males and 20 females) that were
given demeton-S-methyl at 0, 1, 5 or 25 mg/kg diet (Eiben et al.,
1984). The compound was used as a pre-mix in xylene (about 50%). Rats
in an extra control group were given xylene at 25 mg/kg diet.
In the F0 generation, none of the animals died. No treatment-
related signs were observed in any animal. Body weight gain was
reduced in males (by 10%) and in some females at 25 mg/kg diet. Food
intake was also reduced (by 7%) in high-dose males. Fertility index
was not affected by treatment. At 25 mg/kg diet, the viability of pups
was reduced; it was 89% and 85% in first and second matings,
respectively. Lactation index was also reduced in the high-dose group
(85-92%), the control value being 98-99%. Body weight at birth was
comparable in all groups, but body weight gain was significantly
reduced (by 8-10%) in pups fed 25 mg/kg diet.
In the F1b generation, one female was found dead in the 5-mg/kg
diet group and one in the 25-mg/kg diet group; one male and one female
in one of the 25-mg/kg diet litters also died. Autopsy did not reveal
treatment-related alterations. No treatment-related signs were
observed in any animal. Body weight gain was reduced at times in
low-dose males and consistently in mid- and high-dose and xylene-
treated males when compared to untreated animals. When compared to
xylene-treated animals (which were 5-15% lighter than untreated
animals), however, only males of the high-dose group had a
significantly reduced (by about 15%) body weight gain. Females of the
high-dose and xylene groups had a reduced (by about 10%) body weight,
compared to controls, the former being at times lighter weight than
the latter. Fertility index was not significantly affected. The number
of pups born was reduced in the high-dose group and the viability of
pups was also reduced in the mid- and high-dose groups in a
dose-related manner (82-88% and 47-67% of controls, respectively).
No compound-related malformation was found in animals of any of
the treatment groups. Demeton-S-methyl intake, as calculated in the
F0 generation, was found to be (female data in parentheses): 0.07
(0.08), 0.32 (0.39) and 1.71 (1.90) mg/kg body weight per day in the
low-, mid- and high-dose groups, respectively, and xylene intake was
1.66 (1.96) mg/kg body weight per day. Based on the viability of pups
and body weight in the F1b generation, the NOAEL was 1 mg/kg diet,
equal to 0.07 mg/kg body weight per day (Eiben et al., 1984).
7.5.2 Embroytoxicity and teratogenicity
7.5.2.1 Rat
Groups (n=25) of fertilized female rats (BAY:FB 30 strain) were
given daily (0, 0.3, 1 or 3 mg/kg body weight orally) demeton-S-methyl
(from a 52.6% solution in xylene) dissolved in corn oil from day 6 to
15 of gestation. At day 20 of gestation, pups were delivered by
caesarean section. Fetuses were weighed, sexed, inspected for external
abnormalities and examined for visceral and bone malformations. No
alteration of physical appearance or behaviour was observed in any
group. All animals survived until the caesarean section. Body weight
gain was reduced (by 13%) in the high-dose group. The numbers of live
fetuses and resorptions, fetal weight, number of fetuses with
malformations and number of implants were comparable in all groups. No
treatment-related visceral or skeletal abnormalities were observed
(Renhof, 1985).
7.5.2.2 Rabbit
A formulation of demeton-S-methyl (52.2% a.i. in xylene) was
administered by gavage to mated chinchilla hybrid rabbits (n=15-16) on
gestation days 6 to 18 at dose levels of 0, 3, 6 and 12 mg/kg body
weight per day. Caesarean sections were performed on gestation day 28.
There were no mortalities. In the high-dose group, diarrhoea was
observed in all animals after 4 to 10 days of treatment. Beginning 1
to 2 h after dosing, it persisted for 6 to 24 h. In the high-dose
group, mean food consumption was decreased by 7% during gestation days
6 to 18 and by 17% during gestation days 19 to 24 when compared to
controls. This was associated with decreased mean body weight gain
(-7%). There were no abortions and no relevant differences between
test and control groups in the numbers of implantations per dam, pre-
implantation losses, post-implantation losses, resorptions, living and
dead fetuses or sex ratios. A decrease in mean fetal body weight,
compared to the mean control weight, of 6.6% was observed in the
high-dose group. There was no treatment-related increase in gross,
skeletal or visceral malformations (Becker, 1983).
7.6 Mutagenicity and related end-points
A summary of the studies conducted to assess mutagenicity of
demeton-S-methyl is given in Table 3.
7.6.1 DNA damage and repair
Demeton-S-methyl did not induce DNA damage in the Pol test on
Escherichia coli either with or without metabolic activation.
7.6.2 Mutation
Increased mutation rates were observed in the Ames test and in
the mouse lymphoma forward mutation assay both with and without
metabolic activation.
7.6.3 Chromosomal effects
In in vivo tests, no SCEs were found in the bone marrow of
Chinese hamsters treated with high doses of demeton-S-methyl.
Bone marrow micronucleus and dominant lethal tests on mice
treated with demeton-S-methyl gave negative results. Chromosomal
aberrations were found in the bone marrow of Syrian hamsters treated
with a commercial formulation of demeton-S-methyl.
It is concluded that the available information is insufficient to
permit an adequate assessment of the genotoxic potential of
demeton-S-methyl.
7.7 Delayed neurotoxicity
Adult Leghorn hens (n=20) were given two doses of 100 mg a.i./kg
body weight (approximately equal to the LD50) of demeton-S-methyl
(51.2% in xylene) by gavage. The second dose was given 21 days
after the first one. Positive control animals (n=5) received
tri- ortho-cresyl phosphate (TOCP) (375 mg/kg body weight by gavage).
Animals were pretreated with atropine (100 mg/kg body weight
intramuscularly 10 min before the dose of demeton-S-methyl and
50 mg/kg body weight subcutaneously 6 h later). Surviving animals
received atropine (30 mg/kg s.c.) 24, 30 and 48 h later. At the second
dose, atropine treatment was suspended after 24 h. Hens treated with
demeton-S-methyl had signs of cholinergic toxicity. The recovery
started on day 3 and by day 8 all treated animals, except for one,
were free of signs. After the second dose, the recovery started on day
2 and by day 5 all treated animals were free of signs. One animal died
after the second treatment and surviving animals did not develop
neurological deficits. TOCP-treated animals showed locomotor
Table 3. Studies on mutagenicity of demeton-S-methyl
Test Organism Purity Results LED or HIDa Reference
-S9 +S9
Microorganisms
Pol assay Escherichia coli p3478, 93% - - 10 000 µg/plate Herbold (1983a)
W3110
Reverse Salmonella typhimurium unknown + n.t. 5 µg/plate Hanna & Dyer (1975)
mutation TA1530, TA1535, his C117,
his G46
E. coli WP2, WP2 uvra, unknown + n.t. 5 µg/plate Hanna & Dyer (1975)
CM561, CM571, CM611, WP67,
WP12,
S. typhimurium TA98, TA100, 50.2% ++ 300 µg/plate Herbold (1979)
TA1535, TA1537 (formulation)
S. typhimurium TA98, TA100, >98% ++ 20 µg/plate Herbold (1980a)
TA1535, TA1537
Saccharomyces cerevisiae 53.1% -- 1062 µg/ml Hoorn (1982)
S138 S211ý (formulations
in xylene)
Insects
Recessive Drosophila melanogaster unknown + 80 mg/kg diet Hanna & Dyer (1975)
lethal
Table 3. (con't)
Test Organism Purity Results LED or HIDa Reference
-S9 +S9
Cultured mammalian cells
Mutation, tk Mouse lymphoma L5178Y cells 94% ++ 50 µg/ml Cifone (1984)
locus
Mammals in vivo
Bone marrow NMRI mouse >98% - 2 × 5 mg/kg b.w. Herbold (1980b)
micronucleus oral
SCE in bone Chinese hamster 94% - 20 mg/kg b.w. oral Herbold (1983b)
marrow
Chromosomal Syrian hamster, female 50% + 2 mg/kg b.w. i.p. Dzwonkowska & Hübner (1986)
aberration (commercial)
Dominant NMRO mouse >98% - 5 mg/kg b.w. oral Herbold (1980c)
lethal
a LED = lowest effective dose; HID = highest ineffective dose
impairment beginning on day 10. Histological examination showed
moderate axonal degeneration in peripheral nerves and medulla in
TOCP-treated animals but not in demeton-S-methyl-treated or solvent
control animals (Flucke & Kaliner, 1988).
Neuropathy target esterase (NTE), the target for organophosphate-
induced delayed neuropathy, was not inhibited in hen brain and
spinal cord 1, 2 and 7 days after treatment with demeton-S-methyl
(80 mg a.i./kg body weight) by gavage. Positive controls (TOCP at
100 mg/kg body weight) showed NTE inhibition (> 90%) in both brain
and spinal cord (Flucke & Eben, 1988).
7.8 Toxicity of metabolites
Two plant and mammalian metabolites of demeton-S-methyl (namely
oxydemeton-methyl and demeton-S-methylsulfone) have been studied
extensively, since they are also the active ingredient of commercial
pesticides. According to the JMPR, the toxicity of the two compounds
does not differ substantially, either qualitatively or quantitatively,
from that of demeton-S-methyl (FAO/WHO, 1990).
7.9 Mechanism of toxicity - mode of action
Demeton-S-methyl is a direct cholinesterase inhibitor and
it causes signs and symptoms of the cholinergic syndrome. The
in vitro I50 (30 min, 37°C) for sheep erythrocyte cholinesterase
was 6.5 × 10-5 mol/litre. The I50 of oxydemeton-methyl and
demeton-S-methylsulfone were of the same order of magnitude
(2.7 × 10-5 and 4.3 × 10-5 mol/litre, respectively). The half-life
of recovery of acetylcholinesterase activity after inhibition by
demeton-S-methyl was 1.3 h, as expected from a dimethyl phosphorylated
acetylcholinesterase (Heath & Vandekar, 1957).
The 1973 JMPR (FAO/WHO, 1974) reported that rat brain
cholinesterase was more sensitive to in vitro inhibition by demeton-
S-methyl than by oxydemeton-methyl (I50 values of 9.52 × 10-5 and
1.43 × 10-3 mol/litre, respectively; time of incubation, temperature
and pH not reported). It was also reported that demeton-S-methyl was a
more potent inhibitor of human serum cholinesterase (no details
given).
Data on in vivo inhibition of plasma cholinesterase and
erythrocyte and brain cholinesterase are reported in section 7.2 and
7.3.
7.10 Potentiation
Male Wistar rats (160-180 g body weight) were given trichlorfon
(98.6% purity) and demeton-S-methyl (90% purity) in combination by
gavage. The amount of compound in the mixture was proportional to its
oral LD50 (302 mg/kg body weight for trichlorfon and 44 mg/kg body
weight for demeton-S-methyl) in order to obtain equitoxic doses. The
resulting toxicity was additive (LD50 of the mixture was 223 mg/kg
body weight against expected 173 mg/kg body weight) (Flucke &
Kimmerle, 1977).
Similarly, an additive effect was obtained when demeton-S-methyl
was given in combination with phenamiphos (ethyl-4-(methylthio)
m-tolyl-isopropyl-phosphoroamidate). The experimental LD50 of the
mixture was 55 mg/kg body weight while that estimated from the
individual LD50s was 50 mg/kg body weight (Kimmerle, 1972).
8. EFFECTS ON HUMANS
8.1 General population exposure
A 31-year old woman attempted suicide with an unknown amount
("1 or 2 mouthfuls") of Metasystox I (25% demeton-S-methyl). On
admission to hospital she was comatose, sweating and salivating,
and had pin-point pupils. She stayed in the Intensive Care Unit for 15
days where she was treated with atropine (up to 97 mg per day, 550 mg
in 14 days). Oximes (2-PAM) were only given on day 1 (0.5 + 0.25 +
0.25 g) and there was no apparent clinical improvement. On admission,
plasma and erythrocyte cholinesterase activities were less than 10% of
normal control values. The patient was discharged on the 30th day with
normal plasma cholinesterase values; erythrocyte cholinesterase
activity was still below the normal values (about 65%), but had
recovered a month later (Barr, 1966).
A case of acute poisoning in a 5-month pregnant woman has been
described (Carrington da Costa et al., 1982). A 41-year-old woman
attempted suicide by ingesting Metasystox, which resulted in an
estimated intake of 12 g methyl demeton (it should be noted that in
the report Metasystox was said to contain methyl-demeton and not
oxydemeton-methyl as the commercial name implies). On admission, 3.5 h
after poisoning, blood cholinesterase (it was not specified whether
pseudo- or acetylcholinesterase) was 10% of normal values. The patient
became comatose 12 h after admission. She was treated with atropine,
obidoxime and haemoperfusion 72 h after hospitalization. Artificial
ventilation was required on the 4th day of hospitalization. The
patient was discharged after 24 days and 4 months later she delivered
a healthy female child.
In a fatal suicidal case with a commercial formulation of
demeton-S-methyl, the concentration of the compound was measured in
several organs. It was estimated that death occurred about 6 h after
poisoning. Highest demeton-S-methyl levels were found in the proximal
small intestine (166 mg/kg of tissue); in brain, kidney and muscles,
the levels were 30-80 mg/kg; lowest concentrations were found in liver
(7 mg/kg tissue) and blood (7-16 mg/litre). Metabolites were not
measured (Schludecker & Aderjan, 1988).
In the United Kingdom in 1991 there were five cases of poisoning
by demeton-S-methyl and in 1994 three cases, none of which were fatal
(personal communication by G. Volans, Medical Toxicology Unit, London,
to the IPCS, dated 15 January 1996).
Hegazy (1965) reported three cases of suspected poisoning with
demeton-S-methyl in children (6-14 years of age) exposed in a recently
sprayed field and 1 case after ingestion of contaminated feed.
Symptoms were mild and the patients recovered fully. Serum
cholinesterase determinations gave ambiguous results.
8.2 Occupational exposure
8.2.1 Acute poisoning
A worker inadvertently exposed to demeton-S-methyl (no details)
was monitored for about 100 days. Plasma cholinesterase activity was
always within the normal values of the laboratory. However, if the
activity on day 40 and 100 is considered as the normal value for this
worker, then a 30% inhibition occurred 2-3 days after exposure. The
activity recovered with an half-life of about 10 days. Erythrocyte
cholinesterase activity was below the normal value for about 40 days.
When calculated on the activity of day 100, a 60% inhibition was found
up to day 10. The activity recovered with a half-life of about 35 days
(Lewis et al., 1981).
Two workers in a chemical packaging company, whose job it was to
fill a concentrate of demeton-S-methyl (500 g/litre of xylene) into
one-litre containers using a weight-triggered bottle-filling machine
for 3 to 4 h, were admitted to hospital because of organophosphate
poisoning and were treated with atropine. Cholinesterase measurement
made 14 days after exposure (1 worker) showed inhibited erythrocyte
cholinesterase, but the plasma cholinesterase activity was within the
normal range. In the second worker, whose symptoms lasted for 3 days,
erythrocyte cholinesterase activity was below the normal value 5 weeks
after exposure. These workers wore gloves, overalls and boots, but
frequent spillage was reported. Normal clothing had been left under
the filling apparatus and apparently was contaminated. The filling
system was changed by housing the filling machine in a fume cupboard
and by providing new protective garments and changing room facilities.
One more worker was admitted to the hospital and treated with atropine
because of organophosphate poisoning. This occurred on the morning
after a day spent fitting the infill seal and screwing on the tops
of cans. He had reduced erythrocyte and plasma cholinesterase
(erythrocyte > plasma) activities for several days after exposure.
Another worker showed a sharp drop in erythrocyte and plasma
cholinesterase activities associated with abdominal cramps, which
resolved in about 5 days. Another worker had depressed erythrocyte and
plasma cholinesterases activities without complaining of any adverse
effect. It was concluded that absorption of demeton-S-methyl was
through the skin because of penetration of the protective clothing
used or because this clothing was not worn properly. It should be
noted that the active ingredient was dissolved in xylene, which is
known to attack rubber and plastics, making the penetration of gloves
possible (Jones, 1982).
8.2.2 Effects of short- and long-term exposure
Three volunteers without any protective equipment were exposed
for two consecutive days to Metasystox (30% demeton-S-methyl, 70%
demeton-O-methyl) while spraying with a hand-held nebulizer. Exposure
lasted for 3 and 6 h on the first and second days, respectively. The
concentrations of the active ingredient (isomers not separated) were
8.8-27 mg/m3 of ambient air. Plasma and erythrocyte cholinesterase
activities measured up to 14 days after exposure did not show
significant decreases when compared to pre-exposure values (Klimmer &
Pfaff, 1955).
Volunteers, wearing overalls but not mask protection, were
exposed to metasystox (30% demeton-S-methyl, 70% demeton-O-methyl)
while spraying in a greenhouse. They used the splash method
(0.03-0.05% a.i.), the low volume (0.5% a.i.) or the high volume spray
method (0.05% a.i.) for 5 to 25 min. There was no effect on plasma or
erythrocyte cholinesterase activity measured after exposure (Klimmer &
Pfaff, 1958).
Six workers engaged in hop cultivation using Metasystox I
(reported to contain demeton-O-methyl instead of demeton-S-methyl as
the commercial name implies) were monitored. They sprayed up to
2400 litres of a 0.1% solution (in water) of the insecticide in one
day. Protective clothing and masks were not always used. No
significant inhibition of blood acetyl cholinesterase was observed at
the end of exposure or 1 or 2 days later. One subject, who was exposed
twice, showed a 29% decrease in blood acetyl cholinesterase after the
second exposure. No signs or symptoms were observed in these workers
(Winkler & Arent, 1970).
The medical department of a company reported no adverse effect in
workers employed in the formulation of demeton-S-methyl from 1967 to
1984 (Faul, 1984).
Agricultural workers exposed to demeton-S-methyl for 3
consecutive days were monitored. Pre-and post-exposure urinary levels
of the metabolite dimethyl phosphorothiolate potassium salt (DMPThK),
and plasma and whole blood cholinesterase activities were measured.
Exposed subjects were divided into three groups according to their
job, i.e. mixers (n=7), sprayers (n=6) and others (n=7) not directly
involved in handling the pesticide. Higher levels of DMPThK were
found in mixers, with a medium value of 83 µg/litre and a range of
0-822 µg/litre (neither corrected for creatinine nor for urine
volume). Sprayers had a mean value of 30 µg/litre (limit of detection)
and a range of 0-208 µg/litre; the other subject had a mean value of
30 µg/litre and a range of 0-100 µg/litre. Whole blood cholinesterase
activity was not affected by the exposure, while plasma cholinesterase
activity was slightly (about 10%, statistically significant) reduced
when compared to pre-exposure levels in mixers. However, no
correlation was found between DMPThK levels and plasma cholinesterase
activity (Vasilic et al., 1987).
Hegazy (1965) reported a study of 121 spraymen exposed to
Meta-isosystox during spraying of cotton fields in Egypt. The spraymen
applied Meta-isosystox at a rate of 0.5 litres of concentrate in
400 litres of spray per 4200 m2 mainly by hand-operated knapsack
sprayers, or in a few cases by high-pressure motor-powered sprayers
with a large tank connected by a long hose to a multi-nozzled
spray-boom. Sprayers were not involved in chemical mixing. Workmen
washed exposed body parts with soap and water after spraying. Working
clothes were removed at the end of the day, but the clothes may not
have been washed before re-use. Not all workers used protective
clothing and masks. None of the workers were re-exposed to
Meta-isosystox after the onset of symptoms. Serum cholinesterase
activity estimates were performed within 24 h after the onset of
symptoms in some patients and after the cessation of symptoms in some
others. In most cases they were repeated 2-3 times at various
intervals up to 40 days from the onset of symptoms. In general, the
serum cholinesterase activity in spraymen underwent a marked initial
fall, followed by a rise to above-normal levels after about 30-40
days. Signs and symptoms of toxicity in spraymen occurred after 1-18
days of exposure, with a mean time of 3 days. These consisted of
gastrointestinal disturbances (58% of total), dizziness (23%),
persistent general weakness and fatigue (19%), respiratory
manifestations (16%), headache (16%), sweating, salivation or
lacrimation (12%), tremors of outstretched hands, intention tremors,
ataxia (4.1%), exaggerated superficial and deep reflexes (5.8%),
hiccough (2.5%), muscular fasciculations (2.5%), or had apparently
resolved (30%), at the first determination of serum cholinesterase
activity (Hegazy, 1965).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Aquatic organisms
9.1.1 Algae
Table 4 reports the results obtained when different
formulations of demeton-S-methyl were tested on the green alga
Scenedesmus subspicatus (Heimbach, 1985a, 1990b).
9.1.2 Invertebrates
The acute toxicity of demeton-S-methyl for molluscs and
crustaceans is given in Table 5.
The lowest concentration tested on the water flea Daphnia
magna (10 µg a.i./litre) caused toxic effects (Heimbach, 1985b,c). A
21-day exposure test on water flea reproduction produced a NOEC of
> 5.6 µg a.i./litre (Heimbach, 1990d). A study performed with a
27.3% emulsifiable concentrate formulation (Heimbach, 1985c) yielded
a NOEC of 3.7 µg/litre (equal to 1 µg a.i./litre) and a LOEC of
11.7 µg/litre (equal to 3.1 µg a.i./litre).
Using a commercial formulation of metasystox, the 96-h LC50 for
the lammellibranch mollusc Paphia laterisulca was 2 µg/litre (Akarte
et al., 1986) and that for the freshwater prawn Donax cuneatus was
4 µg/litre (Muley et al., 1987).
9.1.3 Fish
Table 6 indicates the toxicity of demeton-S-methyl for fish.
9.2 Terrestrial organisms
9.2.1 Soil microorganisms
In a study conducted in silty sand soil or loamy silt soil
with doses of demeton-S-methyl up to 5 times those recommended
(2 litres/hectare of a 27% emulsifiable concentrate formulation),
no influence on soil respiration or nitrification in soil was found
(Anderson, 1989; Blumenstock, 1989).
9.2.2 Invertebrates
When demeton-S-methyl was mixed with an artificial soil where
earthworms (Eisenia foetida) were kept for 14 days, the LC50 was
241 mg/kg of dry substrate of the commercial formulation (a 25%
emulsifiable concentrate), corresponding to 60 mg a.i./kg (Heimbach,
1990a).
Table 4. Effect of demeton-S-methyl on the green alga Scenedesmus subspicatusa
Specification of test EC50 (mg a.i./litre) NOEC LOEC Duration Conditions
substance (mg/litre) (mg/litre) (h)
Increase of Growth rate
biomass
Technical 97.3% purity 8 22 1 3 96 pH 7.8 - 8.5
23 °C
EC formulationb 37 >100 18 32 96 pH 7.6 - 10.4
(27.3% a.i.) 23 °C
Pre-solutionc 13 37 1 10 96 pH 7.7 - 8.5
xylene (53.7% a.i.) 22 °C
a From: Heinbach (1985a, 1990b)
b Tests performed with the blank formulation gave the same results as the highest tested concentration.
c No test with the blank pre-solution was performed.
Table 5. Acute toxicity of demeton-S-methyl for molluscs and crustaceans
Species Specification of test Temperature LC50 Duration of Reference
substance (°C) (mg/litre) exposure
(h)
Mollusc commercial formulation ? 0.0042 96 Akarte et al. (1986)
(Paphia laterisulca)
Water flea (Daphnia magna) technical (96.7%) 20 ± 1 >0.1 24 Heimbach (1985b)
0.023 48
pre-solution in xylene 20 ± 1 >0.1 24 Heimbach (1985c)
(53.7%) formulation 0.022 48
Clam (Donax cuneatus) commercial formulation 25-28.5 0.0064 96 Muley et al. (1987)
Prawn (Macrobrachium lamerrii) commercial formulation 27 ± 2 1.3 72 Mary et al. (1986)
Table 6. Toxicity of demeton-S-methyl for fish (96-h exposure)
Species Mass and length Temperature LC50 (mg/litre) Reference
(°C)
Rainbow trout (Onchorhyncus mykiss) 4.0 - 5.5 cm 16 4.5 Grau (1985a)
1.0 - 1.5 g (52.7% of a.i.)
6.4 ± 1.0 cm 15±2 0.59 Grau (1990c)
3.0 ± 0.6 g (69.5% of a.i.)
6.9 ± 1.1 cm 15±2 6.44 Grau (1990a)
3.5 ± 1.6 g (27.3% of a.i.)
Golden orfe (Leuciscus idus melamotus) 6.0 - 7.5 cm 21 43 Grau (1985b)
2.5 - 4.2 g (52.7% of a.i.)
6.4 ± 0.6 cm 21±2 23.2 Grau (1990b)
2.5 ± 0.6 g (27.3% of a.i.)
Goldfish (Carassius auratus) 6 cm 18 20-40 Hermann (1974a)
1.5 g (28.1% of a.i.)
Carp (Cyprinus carpio) 6 cm 18 40-60 Hermann (1974b)
1.6 g (28.1% of a.i.)
Scardinius erythrophthalmus 6 cm 18 30-40 Hermann (1974c)
1.3 g
Cirrhana mrigala (larvae) 51 ± 3 mg 20 1.45 Verma et al. (1984)
Demeton-S-methyl was applied on fields of winter wheat by
fixed-wing aircraft using conventional boom-and-nozzle equipment.
The applied amount was 245 g a.i. per hectare at a volume rate of
20 litres/hectare. Samples of the soil surface and crop foliage fauna
were collected 1-2 times before and 4-5 times after application from a
treated field and from a control untreated field. The number of crop
foliage but not of soil surface entomophagus invertebrates was reduced
soon after application of demeton-S-methyl. Empididai (dance flies)
was the only group to be significantly reduced in numbers by
demeton-S-methyl. Predatory Coleoptera (beatles) (Carabidae and
Staphylinidae), Araneae (spiders) and predatory Diptera (flies)
(except Empididae) were not affected by demeton-S-methyl. Among the
ephytophagus fauna, cereal aphids markedly declined in number soon
after application, but a rapid increase was observed 2 months later,
when numbers of Diptera and Thripidae (thrips) were also
increased. Numbers of entomobryid and sminthurid springtails decreased
soon after the application but were higher than in the control field
2 months later (Shires, 1985).
Demeton-S-methyl was slightly toxic to the predatory mite
Phytoseiulus persimilis since a 70% population reduction was
obtained with a concentration of 0.025% a.i. (Kniehase, 1984).
The contact LD50 for bees has been reported to be 0.60 µg/bee
(Westlake et al. 1985). A field study was conducted on bee
colonies following application of a 0.2% formulation of Metasystox
(at 600 litres/hectare) to field beans (Vicia faba) after bee
foraging had ceased in the evening. Toxicity to bees was assessed by
collecting dead insects on canvas sheets in front of the hive
entrance. Before spraying, the mortality in exposed and control hives
was comparable. Following spraying, there was a mortality rate of 123
bees per hive in the exposed and 90 bees in the controls (prior to
exposure mortality was 98 and 95 respectively). The mortality increase
persisted for 3 days after the application (Bayer, 1970).
9.2.3 Birds
Some oral LD50 values are given in Table 7.
Table 7. Acute oral LD50 values for birds
Species Vehicle Purity LD50 Reference
(mg/kg b.w.)
Japanese quail Cremephor EL 99.7% 44-50 Flucke (1984)
(Coturnix japonica)
Canary ? 99.7% 10-20 Grau (1984)
(Serinus canarius)
Groups of 10 starlings (Sturnus vulgaris) were given (by
gavage) 0, 0.2, 1.5 or 2 mg/kg body weight of demeton-S-methyl
(technical grade) dissolved in corn oil. Serum butyrylcholinesterase
and carboxylesterase and brain cholinesterase activities were measured
at 3 (high-dose only), 6 and 24 h after dosing (n=2 for brain, n=4 for
serum). The highest dose inhibited brain cholinesterase by about 20%,
serum carboxylesterase by about 50% and serum butyrylcholinesterase by
about 80%. The peak effect was at 3 h with some recovery after 24 h.
In vitro treatment of the enzyme preparation with pralidoxine
chloride (2-PAM) caused almost complete reactivation of brain
cholinesterase but had no effect on the other enzymes (Thompson et
al., 1991).
9.2.4 Effects in field
The activities of brain cholinesterase, serum
butyrylcholinesterase and glutamate oxaloacetate transaminase (GOT)
were measured in house sparrows (Passer domesticus) captured 1
(n=7), 2 (n=6) or 3 (n=6) days after spraying wheat fields with
demeton-S-methyl (0.485 litres a.i./hectare from an emulsifiable
concentrate formulation in a xylene solvent base). Liver weight was
also recorded and histological examination of the liver was performed.
Serum butyrylcholinesterase was reduced to 64% of that of controls
(n=5) (statistically not significant) and brain cholinesterase was 82%
of that of controls (statistically significant) for birds trapped on
day 2, but serum GOT was not significantly affected in the exposed
birds. The authors claimed that there was evidence of liver damage
in exposed birds because of a significant increase of binucleation
(3.3-5 vs. 1.9 binucleation/10 fields) and inflammatory cell foci
(more evident after 3 days of exposure) (Tarrant et al., 1992).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
The major metabolites of demeton-S-methyl in plant and mammals
are oxydemeton-methyl and demeton-S-methylsulfone. These two compounds
are also commercial insecticides. Their mechanism of action
is the same as that of demeton-S-methyl (i.e. inhibition of
acetylcholinesterase) and their toxicological profiles have been
reported to be similar to that of demeton-S-methyl (FAO/WHO, 1990).
10.1 Evaluation of human health risks
Sources of exposure of humans to demeton-S-methyl are dietary and
through dermal absorption and inhalation during the manufacture or use
of the compound.
There is very limited information on actual dietary exposure to
demeton-S-methyl.
In an early study, during application of a mixture of demeton-S-
methyl (30%) and its isomer, demeton-O-methyl (70%), an ambient air
concentration of 8.8-27 mg/m3 of the combined isomers was found. This
is much higher than the conservative TLV/TWA for methyl-demeton
proposed by ACGIH (0.5 mg/m3). However, no inhibition of plasma and
erythrocyte cholinesterase activities was found after exposure.
Cases of acute intoxication, which required pharmacological
treatment, have been reported where workers were filling bottles with
a 50% xylene solution of demeton-S-methyl. Skin absorption was
considered the cause of poisoning because of inadequate personal
protection. Agricultural workers exposed to demeton-S-methyl while
mixing or spraying during three consecutive days did not show
significant inhibition of either plasma or erythrocyte cholinesterase.
Agricultural workers exposed to demeton-S-methyl while spraying cotton
for 1-18 days (average of 3 days) showed signs and symptoms of
poisoning. Improper working conditions could not be excluded.
Cases of severe demeton-S-methyl poisoning (suicide attempts)
have been reported. No delayed toxicity developed in the patients who
survived.
No data are available on the effect of repeated human exposure to
demeton-S-methyl.
No reproductive, developmental or carcinogenic effects have been
found in laboratory animals exposed to demeton-S-methyl. NOAEL values
have been based on inhibition of brain acetyl cholinesterase or, in a
rat reproduction study, on reduced pup viability and body weight in
the F1b generation.
The available information is insufficient to permit an adequate
assessment of the genotoxic potential of demeton-S-methyl.
10.2 Evaluation of effects on the environment
The information on utilization and application rates that
has been employed for this risk assessment is derived from the
agricultural use of demeton-S-methyl within the European Union. It
should be possible to extrapolate this assessment to other
agricultural uses at similar application rates elsewhere in the world.
The application rates for demeton-S-methyl can be summarised as
follows: arable (tractor-mounted/drawn hydraulic spray boom
applications), 0.32 kg/ha; top fruit (broadcast air-assisted
applications), 0.49 kg/ha.
The following risk assessment is based on the principle of
calculating toxicity-exposure ratios (TERs) (Fig. 3), which follows
the European and Mediterranean Plant Protection Organisation and
Council of Europe (EPPO/CoE) Environmental Risk Assessment Scheme
model and associated trigger values (EPPO/CoE, 1993a,b).
10.2.1 Aquatic organisms
The main risk to aquatic organisms from the use of demeton-S-
methyl is from spray drift during either arable applications
(0.32 kg/ha) or top fruit air-assisted (0.49 kg/ha). For each of these
risk scenarios, the predicted environmental concentration (PEC) in a
30-cm-deep static surface water body, arising from either arable-based
spray drift at 1 m from the edge of the spray boom or from top fruit
air-assisted spray drift at 3 m from the point of application (both
based on Ganzelmeier et al., 1995), was calculated as follows:
PEC (mg demeton-S-methyl/litre)
= max application rate (kg/ha) × A (% spray drift)
300
where
A = 5 for ground-based hydraulic spray applications 1 m from
edge of boom
or 30 for air-assisted application 3 m from point of application
10.2.1.1 Acute risk
The acute LC/EC50 values for the most sensitive fish was
0.59 mg/litre, for the most sensitive aquatic invertebrate (mollusc)
was 0.0042 mg/litre (0.022 mg/litre for Daphnia), and for the most
sensitive algal species was 8 mg/litre.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Demeton-S-methyl causes cholinergic toxicity. The acute toxicity
data are reported in Table 2.
Heath & Vandekar (1957) and Vandekar (1958) showed a significant
increase in intravenous but not oral toxicity after storage of
demeton-S-methyl ("pure") at room temperature in the dark. This was
associated with the formation of sulfonium derivatives, which have a
lower oral toxicity, possibly because of poor absorption.
Dilution with water also increased the intravenous toxicity, and
this was again associated with the formation of sulfonium derivatives.
The maximum toxicity of a 1 mg/ml suspension kept at 35°C for one day
was about 30 times the initial toxicity (the LD50 decreased from
about 60 to about 2 mg/kg in rats) (Heath & Vandekar, 1957). Similar
results were obtained with commercial Metasystox (70% demeton-O-
methyl, 30% demeton-S-methyl) (see also section 2.2.)
7.1.1 Oral
Following oral dosing with demeton-S-methyl performed on a very
small number of animals (one per dose level), one rabbit given
50 mg/kg died within 2 h, while the dose of 20 mg/kg caused symptoms
but the animal recovered. A cat given 10 mg/kg died after 2 days,
while the dose of 5 mg/kg caused signs that were reversible and
animals recovered (few details given) (Hecht, 1955).
Single oral doses (180 mg/kg) of metasystox, containing 25%
demeton-S-methyl, were administered to seven male buffalo calves
(10-12 months of age). Signs of poisoning appeared 15 to 35 min later.
Two calves were treated repeatedly with atropine (1.5 mg/kg body
weight, i.v.), D-tubocurarine (0.1 mg/kg body weight, i.v.) and
glucose (3-4 mg/kg body weight, i.v.). Another two calves were treated
repeatedly with atropine (1.5 mg/kg body weight, i.v.), gallamine
(1 mg/kg body weight, i.v.) and glucose (3-4 mg/kg body weight, i.v.)
Three calves were not treated. All calves displayed typical
cholinergic signs. Treatments delayed but did not prevent death, which
occurred in about 2 h in calves not treated with antidotes and in
about 23 h in calves treated with antidotes (Mitra et al., 1978).
7.1.2 Inhalation
The LC50 (4 h of exposure) for Wistar rats was found to be 310
and 210 mg/m3 for males and females, respectively (Flucke & Pauluhn,
1983).
Table 2. LD50 of demeton-S-methyl for various species and different routes of administration
Species Sex Observation Route Purity LD50 Vehicle References
(strain) period (mg/kg
(days) body weight)
Mouse M 3 oral ? 17 ? Klimmer & Pfaff (1955)
Mouse ? 7 i.v. ? 7 water Hecht (1960)
Rat F 1 oral "pure" 63 ? Heath & Vandekar (1957)
Vandekar (1958)
Rat M 3 days minimum oral ? 40 ? Klimmer & Pfaff (1955)
Rat ? ? oral ? 35 ? Hecht (1955)
Rat M 14 oral 25% formulation 33 water Klimmer (1964)
(Wistar)
Rat M ? oral 86-89% 57-64 Lutrol 9 Klimmer (1964)
(Sprague-
Dawley)
Rat M & F 14 oral 50% formulation 64-65 water Flucke & Pauluhn (1983)
(Wistar)
Rat M 7 oral 50% formulation 129 water Edson (1960)
(Wistar)
Rat M 14 oral 90% formulation 44 cremophor EL Flucke & Kimmerle (1977)
(Wistar)
Table 2. (con't)
Species Sex Observation Route Purity LD50 Vehicle References
(strain) period (mg/kg
(days) body weight)
Rat F 10 oral ? 80 ethanol (20%) DuBois & Doull (1955)
(Sprague- and DuBois & Plzak (1962)
Dawley) propylene
glycol (80%)
Rat M & F 14 i.p. ? 7.5 ethanol (20%) DuBois & Doull (1955)
(Sprague- and propylene DuBois & Plzak (1962)
Dawley) glycol (80%)
Rat ? ? i.p. formulation 10 water Hecht (1960)
Rat ? ? dermal ? 85 ? Edson (1960)
Rat M 14 dermal 50% formulation 71 none Flucke & Pauluhn (1983)
(Wistar)
Rat F 14 dermal 50% formulation 45 none Flucke & Pauluhn (1983)
(Wistar)
Rat ? 7 dermal technical 100-200 none Hecht (1960)
Rat ? 7 dermal 25% formulation about 10 none Hecht (1960)
Rat F 1 i.v. "pure" 65 ? Heath & Vandekar (1957)
Vandekar (1958)
Table 2. (con't)
Species Sex Observation Route Purity LD50 Vehicle References
(strain) period (mg/kg
(days) body weight)
Guinea- M 10 oral ? 110 ethanol (20%) Du Bois & Doull (1955)
pig and propylene
glycol (80%)
Guinea- M 14 i.p. ? 12.5 ethanol (20%) Du Bois & Plzak (1962)
pig and propylene
glycol (80%)
When rats (n=2) and mice (n=4) were exposed for 1 h to 1, 2.5 or
5 g/m3 of demeton-S-methyl (alcohol solution), all mice died whereas
rats displayed signs but recovered (few experimental details given)
(Hecht, 1955).
Rats (n=20) were exposed for 8 h to nebulized demeton-S-methyl
(0.5 g/m3 of air), which was obtained from a 25% emulsifiable
commercial formulation diluted 1:250 (0.1% final concentration of
active ingredient). None of the animals died or showed overt
cholinergic signs. Erythrocyte cholinesterase activity, determined in
three animals immediately after the end of exposure, was reduced by
70%. When purified active ingredient was used, lower erythrocyte
cholinesterase inhibition (60%) was found under the same experimental
conditions. This was paralleled by an increased rat LD50 (from 10 to
27.5 mg/kg i.p.) (Hecht, 1960).
7.1.3 Dermal
Doses of 20 or 100 mg/kg demeton-S-methyl applied to the shaved
skin of two cats caused death. A dose of 10 mg/kg caused mild signs;
very few details were given (Hecht, 1955).
7.2 Short-term exposure
7.2.1 Rat
Groups of six male rats (strain not specified) were fed
demeton-S-methyl at levels of 0, 50, 100 or 200 mg/kg diet in
the diet (equivalent to 0, 5, 10 and 20 mg/kg body weight per day,
respectively) for 6 months. Cholinergic signs (slight tremors and
fasciculations) were observed at the highest dose-level during the
first 5 weeks of the study. Brain and erythrocyte cholinesterase
activities were reduced at 50 mg/kg diet (by about 80 and 88%,
respectively), 100 mg/kg diet (by about 85 and 92%) and 200 mg/kg diet
(by about 90 and 94%) groups. Body weight gain was depressed at 100
and 200 mg/kg diet. Gross microscopic examination of tissues (liver,
kidney and adrenals) showed no treatment-related changes (Vandekar,
1958).
Groups of 30-day-old female Holtzman rats were fed dietary
levels of 0, 1, 5 or 25 mg/kg diet of demeton-S-methyl (purity not
reported) for 1 week. At termination, serum, liver and brain
acetylcholinesterase (n=3) and liver and serum aliesterase (n=3) with
diethylsuccinate and tributyrin as substrates activities were
measured. Interpolated dietary levels producing 50% inhibition were
15-28 mg/kg diet for acetylcholinesterase, 4-6 mg/kg diet for liver
aliesterase and about 25 mg/kg diet for serum aliesterase, equivalent
to 1.5-2.8, 0.4-0.6 and 2.5 mg/kg body weight per day, respectively
(Su et al., 1971).
7.2.2 Dog
In a one-year study, pure-bred beagle dogs (n=6 animals of each
sex per group) were fed 0, 1, 10 or 100 mg a.i./kg diet (day 1-36) or
50 mg a.i./kg diet (day 37-termination) of demeton-S-methyl (52.2% in
xylene). Haematological, blood biochemical and urinalysis parameters
were determined during pretest period and at months 1, 2, 3, 4, 5, 6,
8, 10, 12. Hearing tests and ophthalmoscopic examinations were
performed once in the pretest period and at months 3, 6 and 12 of
treatment. At termination animals were killed for pathology, and
determination of organ weights, brain cholinesterase activity, hepatic
cytochrome P-450 and triglyceride contents and N-demethylase
activity were carried out.
All animals survived the study. Diarrhoea and vomiting were
observed in all animals, most frequently in the high-dose group: these
animals also showed reduced food consumption before the dose was
reduced to 50 mg/kg diet. The mean daily compound intake was found to
be 0.036, 0.36 and 4.6/1.5 mg/kg body weight at 1, 10 and 100/50 mg/kg
diet, respectively. Body weight was similar in all groups. No
alterations were observed in the hearing test and ophthalmoscopic
examination. Haematological, blood chemistry (excluding cholinesterase
activities) and urinalysis parameters and organ weights at termination
were not significantly altered by any of the treatments. Hepatic
biochemical parameters were not altered by any of the treatments. No
treatment-related gross pathology alterations were found. However,
multifocal slight/moderate atrophy and/or hypertrophy of proximal
renal tubules was demonstrated in three males and three females of the
high-dose group. Plasma cholinesterase activity was reduced as
compared to controls by 20-30% and 5-20% in males and females,
respectively, at 10 mg/kg diet, and by 45-65% (males) and 50-70%
(females) at 50 mg/kg diet. Erythrocyte cholinesterase activity was
reduced by 25-35% and 30-45% in males and females, respectively, at
10 mg/kg diet. A higher inhibition was found at 50 mg/kg diet, where
inhibition was 80-90% and 55-65% in males and females, respectively.
Brain cholinesterase activity was reduced by 25% in males at 10 mg/kg
diet and by 64% (males) and 15% (females) at 50 mg/kg diet. Based on
effects on brain cholinesterase activity, the NOAEL was 1 mg/kg diet,
equal to 0.036 mg/kg body weight per day (Bathe, 1983).
7.3 Long-term exposure
7.3.1 Mouse
A long-term carcinogenicity study was conducted in NMRI mice
(70 animals of each sex per group) that were given demeton-S-methyl
(about 50% in xylene) mixed into the feed with approximately 10 mg/kg
diet of groundnut oil at concentrations of 0, 1, 15 or 75 mg a.i./kg
diet, or xylene (75 mg/kg diet). Groups were subdivided into two
subgroups: one (n=20) was terminated at 12 months, the second one was
terminated at 21 months.
Animals in the high-dose group had a lower (significantly lower
during the first 4 weeks only) food consumption and a reduced (about
10% throughout the study in males only, and at the beginning in
females) body weight. The mean daily intake of demeton-S-methyl was
(males/females): 0.24/0.29, 3.47/4.18, 17.81/20.0 mg/kg body weight
at 1, 15, 75 mg/kg diet, respectively. Clinical signs due to
cholinesterase inhibition were not observed. Mortality at 21 months
was (males/females) 16/30, 13/34, 17/35, 16/35 and 16/32% in the
control, low-, mid-, high-dose and xylene groups, respectively.
Haematological and clinical chemical parameters were not affected by
the treatment except plasma urea (lower than control in the high-dose
males) and plasma and erythrocyte cholinesterase activities. Plasma
cholinesterase activity was significantly decreased in mid- (by
63-74%) and high-dose (by 91-97%) groups. Erythrocyte cholinesterase
activity was only slightly reduced in the mid- and high-dose groups.
Brain cholinesterase activity (n=10 per group) was reduced in
high-dose groups (in males by 70% and in females by 59%) and in the
mid-dose group (in males by 44% and in females by 38%). Histological
examination did not reveal an increased incidence of neoplastic and
non-neoplastic lesions in treated groups. Based on inhibition of brain
cholinesterase, the NOAEL was 1 mg/kg diet, equal to 0.24 mg/kg body
weight per day (Schmidt & Bomhard, 1988).
7.3.2 Rat
Wistar rats (60 animals of each sex per group) were given
demeton-S-methyl (about 50% in xylene mixed into the feed with
approximately 10 mg/kg of groundnut oil) at concentrations of 0, 1, 7
or 50 mg a.i./kg diet, or 50 mg/kg diet of xylene. Groups were
subdivided into two subgroups; one (n=10) was terminated at 12 months,
the second one was terminated at 24 months.
Hair loss (up to 50% of females) and diarrhoea (up to 50% of
males) were observed significantly more frequently in the animals of
the high-dose group. Body weight was reduced in mid-dose males (by
5-10%) and in both males (by 10-20%) and females (by 5%) in the
high-dose group. Food consumption was similar in all groups. The mean
daily intake of demeton-S-methyl was (males/females): 0.05/0.06,
0.31/0.41, 2.59/3.09 mg/kg body weight at 1, 7 and 50 mg/kg diet,
respectively. Mortality at 24 months was (males/females) 12/26, 10/20,
10/20, 4/26 and 8/24% in the control, low-, mid-, high-dose and xylene
groups, respectively. Haematological and clinical chemical parameters,
except for cholinesterases, measured at months 6, 12, 18 and 24, were
unaffected by the treatments. Plasma and erythrocyte cholinesterase
activities, measured at months 3, 6, 12 and 24, were significantly
decreased in groups given 7 mg/kg diet (plasma cholinesterase by
30-56%, erythrocyte cholinesterase by 12-31%) or 50 mg/kg diet (plasma
cholinesterase by 75-92%, erythrocyte cholinesterase by 20-44%). Brain
cholinesterase activity (measured at 12 and 24 months) was reduced in
the high-dose group (by 67-75%) and in the mid-dose group (by 15-47%).
A statistically significant decrease in brain cholinesterase activity
(33%) was observed in males given 1 mg/kg diet at 24 months but not at
12 months. The toxicological significance of this finding is not
clear; it should also be noted that brain cholinesterase activity at
7 mg/kg diet was higher than at 1 mg/kg diet and that plasma
cholinesterase and erythrocyte cholinesterase activities were not
inhibited at either of these dose levels. Histological examination did
not reveal an increased incidence of neoplastic lesions in treated
groups. Increased incidence of retinal atrophy (78% of males, 92% of
females as compared to 36-63% and 61-70% in the other groups) and
keratitis (44% of males, 22% of females as compared to 4-12% and 0-2%,
respectively, in the other groups) was observed at 50 mg/kg diet. The
retinal atrophy mainly affected the fundus, unilaterally or
bilaterally, and occurred in either the inner corneal layer, the inner
and outer layers, or all layers of the retina. Based on inhibition of
brain cholinesterase, the NOAEL was considered to be 1 mg/kg diet,
equal to 0.05 mg/kg body weight per day (Schmidt & Westen, 1988).
7.4 Skin and eye irritation and sensitization
7.4.1 Skin and eye irritation
Demeton-S-methyl (commercial formulation, 50% of active
ingredient) was applied (0.5 ml) to the shaved skin of three New
Zealand white rabbits on a 2.5 × 2.5 cm cellulose dressing for 4 h.
Mild erythema and oedema were observed, which generally disappeared
after 3 days (Flucke & Pauluhn, 1983). A 52.5% solution of
demeton-S-methyl in xylene produced slight skin and eye irritancy in
New Zealand white rabbits, but this was attributed to the solvent
(Thyssen, 1981).
A commercial formulation of demeton-S-methyl (50%) was instilled
(0.1 ml) either undiluted or as a 0.5% aqueous dilution into the
conjunctival sac of one eye of groups (n=3) of New Zealand white
rabbits. Treated eyes were washed with physiological solution after
24 h. No signs of eye irritation were observed in rabbits treated with
the 0.5% aqueous solution. The undiluted formulation caused severe
lacrimation and miosis on application. Mild corneal opacity and
discrete redness and oedema of conjunctivae were observed and
recovered in about 7 days (Flucke & Pauluhn, 1983).
7.4.2 Skin sensitization
The skin-sensitizing potential of demeton-S-methyl was assessed
by the Magnusson and Kligman maximization test with Freund's adjuvant
on guinea-pigs (n=20, Bor:SPF, DHPW strain). The concentrations of
demeton-S-methyl (96.3% purity, average of three determinations) used
were: 0.1% for the intra-dermal induction, 10% for the topical
induction and the first challenge, and 1% for the second challenge.
All twenty animals reacted positively to the 1st challenge (controls
4/10), and 16 reacted positively to the 2nd challenge (controls 3/10).
The results indicate that demeton-S-methyl has a skin-sensitizing
potential (Heimann, 1987a).
In another study, the Buehler epidermal patch test was used on
guinea-pigs (n=12, Bor: SPF, DHPW strain). The concentrations of
demeton-S-methyl (95.6% purity, average of three determinations) used
were 10% for topical induction (once a week for 3 weeks) and the first
challenge, and 20% for the second challenge. The results indicate that
demeton-S-methyl does not have a skin-sensitizing potential under
these conditions (Heimann, 1987b).
It is concluded that demeton-S-methyl might have some
skin-sensitizing potential, but this is of no relevance in practice.
7.5 Reproduction, embryotoxicity and teratogenicity
7.5.1 Reproduction
A standard two-generation study (two litters/generation) was
conducted in SPF rats (BOR:WISW) (10 males and 20 females) that were
given demeton-S-methyl at 0, 1, 5 or 25 mg/kg diet (Eiben et al.,
1984). The compound was used as a pre-mix in xylene (about 50%). Rats
in an extra control group were given xylene at 25 mg/kg diet.
In the F0 generation, none of the animals died. No treatment-
related signs were observed in any animal. Body weight gain was
reduced in males (by 10%) and in some females at 25 mg/kg diet. Food
intake was also reduced (by 7%) in high-dose males. Fertility index
was not affected by treatment. At 25 mg/kg diet, the viability of pups
was reduced; it was 89% and 85% in first and second matings,
respectively. Lactation index was also reduced in the high-dose group
(85-92%), the control value being 98-99%. Body weight at birth was
comparable in all groups, but body weight gain was significantly
reduced (by 8-10%) in pups fed 25 mg/kg diet.
In the F1b generation, one female was found dead in the 5-mg/kg
diet group and one in the 25-mg/kg diet group; one male and one female
in one of the 25-mg/kg diet litters also died. Autopsy did not reveal
treatment-related alterations. No treatment-related signs were
observed in any animal. Body weight gain was reduced at times in
low-dose males and consistently in mid- and high-dose and xylene-
treated males when compared to untreated animals. When compared to
xylene-treated animals (which were 5-15% lighter than untreated
animals), however, only males of the high-dose group had a
significantly reduced (by about 15%) body weight gain. Females of the
high-dose and xylene groups had a reduced (by about 10%) body weight,
compared to controls, the former being at times lighter weight than
the latter. Fertility index was not significantly affected. The number
of pups born was reduced in the high-dose group and the viability of
pups was also reduced in the mid- and high-dose groups in a
dose-related manner (82-88% and 47-67% of controls, respectively).
No compound-related malformation was found in animals of any of
the treatment groups. Demeton-S-methyl intake, as calculated in the
F0 generation, was found to be (female data in parentheses): 0.07
(0.08), 0.32 (0.39) and 1.71 (1.90) mg/kg body weight per day in the
low-, mid- and high-dose groups, respectively, and xylene intake was
1.66 (1.96) mg/kg body weight per day. Based on the viability of pups
and body weight in the F1b generation, the NOAEL was 1 mg/kg diet,
equal to 0.07 mg/kg body weight per day (Eiben et al., 1984).
7.5.2 Embroytoxicity and teratogenicity
7.5.2.1 Rat
Groups (n=25) of fertilized female rats (BAY:FB 30 strain) were
given daily (0, 0.3, 1 or 3 mg/kg body weight orally) demeton-S-methyl
(from a 52.6% solution in xylene) dissolved in corn oil from day 6 to
15 of gestation. At day 20 of gestation, pups were delivered by
caesarean section. Fetuses were weighed, sexed, inspected for external
abnormalities and examined for visceral and bone malformations. No
alteration of physical appearance or behaviour was observed in any
group. All animals survived until the caesarean section. Body weight
gain was reduced (by 13%) in the high-dose group. The numbers of live
fetuses and resorptions, fetal weight, number of fetuses with
malformations and number of implants were comparable in all groups. No
treatment-related visceral or skeletal abnormalities were observed
(Renhof, 1985).
7.5.2.2 Rabbit
A formulation of demeton-S-methyl (52.2% a.i. in xylene) was
administered by gavage to mated chinchilla hybrid rabbits (n=15-16) on
gestation days 6 to 18 at dose levels of 0, 3, 6 and 12 mg/kg body
weight per day. Caesarean sections were performed on gestation day 28.
There were no mortalities. In the high-dose group, diarrhoea was
observed in all animals after 4 to 10 days of treatment. Beginning 1
to 2 h after dosing, it persisted for 6 to 24 h. In the high-dose
group, mean food consumption was decreased by 7% during gestation days
6 to 18 and by 17% during gestation days 19 to 24 when compared to
controls. This was associated with decreased mean body weight gain
(-7%). There were no abortions and no relevant differences between
test and control groups in the numbers of implantations per dam, pre-
implantation losses, post-implantation losses, resorptions, living and
dead fetuses or sex ratios. A decrease in mean fetal body weight,
compared to the mean control weight, of 6.6% was observed in the
high-dose group. There was no treatment-related increase in gross,
skeletal or visceral malformations (Becker, 1983).
7.6 Mutagenicity and related end-points
A summary of the studies conducted to assess mutagenicity of
demeton-S-methyl is given in Table 3.
7.6.1 DNA damage and repair
Demeton-S-methyl did not induce DNA damage in the Pol test on
Escherichia coli either with or without metabolic activation.
7.6.2 Mutation
Increased mutation rates were observed in the Ames test and in
the mouse lymphoma forward mutation assay both with and without
metabolic activation.
7.6.3 Chromosomal effects
In in vivo tests, no SCEs were found in the bone marrow of
Chinese hamsters treated with high doses of demeton-S-methyl.
Bone marrow micronucleus and dominant lethal tests on mice
treated with demeton-S-methyl gave negative results. Chromosomal
aberrations were found in the bone marrow of Syrian hamsters treated
with a commercial formulation of demeton-S-methyl.
It is concluded that the available information is insufficient to
permit an adequate assessment of the genotoxic potential of
demeton-S-methyl.
7.7 Delayed neurotoxicity
Adult Leghorn hens (n=20) were given two doses of 100 mg a.i./kg
body weight (approximately equal to the LD50) of demeton-S-methyl
(51.2% in xylene) by gavage. The second dose was given 21 days
after the first one. Positive control animals (n=5) received
tri- ortho-cresyl phosphate (TOCP) (375 mg/kg body weight by gavage).
Animals were pretreated with atropine (100 mg/kg body weight
intramuscularly 10 min before the dose of demeton-S-methyl and
50 mg/kg body weight subcutaneously 6 h later). Surviving animals
received atropine (30 mg/kg s.c.) 24, 30 and 48 h later. At the second
dose, atropine treatment was suspended after 24 h. Hens treated with
demeton-S-methyl had signs of cholinergic toxicity. The recovery
started on day 3 and by day 8 all treated animals, except for one,
were free of signs. After the second dose, the recovery started on day
2 and by day 5 all treated animals were free of signs. One animal died
after the second treatment and surviving animals did not develop
neurological deficits. TOCP-treated animals showed locomotor
Table 3. Studies on mutagenicity of demeton-S-methyl
Test Organism Purity Results LED or HIDa Reference
-S9 +S9
Microorganisms
Pol assay Escherichia coli p3478, 93% - - 10 000 µg/plate Herbold (1983a)
W3110
Reverse Salmonella typhimurium unknown + n.t. 5 µg/plate Hanna & Dyer (1975)
mutation TA1530, TA1535, his C117,
his G46
E. coli WP2, WP2 uvra, unknown + n.t. 5 µg/plate Hanna & Dyer (1975)
CM561, CM571, CM611, WP67,
WP12,
S. typhimurium TA98, TA100, 50.2% ++ 300 µg/plate Herbold (1979)
TA1535, TA1537 (formulation)
S. typhimurium TA98, TA100, >98% ++ 20 µg/plate Herbold (1980a)
TA1535, TA1537
Saccharomyces cerevisiae 53.1% -- 1062 µg/ml Hoorn (1982)
S138 S211ý (formulations
in xylene)
Insects
Recessive Drosophila melanogaster unknown + 80 mg/kg diet Hanna & Dyer (1975)
lethal
Table 3. (con't)
Test Organism Purity Results LED or HIDa Reference
-S9 +S9
Cultured mammalian cells
Mutation, tk Mouse lymphoma L5178Y cells 94% ++ 50 µg/ml Cifone (1984)
locus
Mammals in vivo
Bone marrow NMRI mouse >98% - 2 × 5 mg/kg b.w. Herbold (1980b)
micronucleus oral
SCE in bone Chinese hamster 94% - 20 mg/kg b.w. oral Herbold (1983b)
marrow
Chromosomal Syrian hamster, female 50% + 2 mg/kg b.w. i.p. Dzwonkowska & Hübner (1986)
aberration (commercial)
Dominant NMRO mouse >98% - 5 mg/kg b.w. oral Herbold (1980c)
lethal
a LED = lowest effective dose; HID = highest ineffective dose
impairment beginning on day 10. Histological examination showed
moderate axonal degeneration in peripheral nerves and medulla in
TOCP-treated animals but not in demeton-S-methyl-treated or solvent
control animals (Flucke & Kaliner, 1988).
Neuropathy target esterase (NTE), the target for organophosphate-
induced delayed neuropathy, was not inhibited in hen brain and
spinal cord 1, 2 and 7 days after treatment with demeton-S-methyl
(80 mg a.i./kg body weight) by gavage. Positive controls (TOCP at
100 mg/kg body weight) showed NTE inhibition (> 90%) in both brain
and spinal cord (Flucke & Eben, 1988).
7.8 Toxicity of metabolites
Two plant and mammalian metabolites of demeton-S-methyl (namely
oxydemeton-methyl and demeton-S-methylsulfone) have been studied
extensively, since they are also the active ingredient of commercial
pesticides. According to the JMPR, the toxicity of the two compounds
does not differ substantially, either qualitatively or quantitatively,
from that of demeton-S-methyl (FAO/WHO, 1990).
7.9 Mechanism of toxicity - mode of action
Demeton-S-methyl is a direct cholinesterase inhibitor and
it causes signs and symptoms of the cholinergic syndrome. The
in vitro I50 (30 min, 37°C) for sheep erythrocyte cholinesterase
was 6.5 × 10-5 mol/litre. The I50 of oxydemeton-methyl and
demeton-S-methylsulfone were of the same order of magnitude
(2.7 × 10-5 and 4.3 × 10-5 mol/litre, respectively). The half-life
of recovery of acetylcholinesterase activity after inhibition by
demeton-S-methyl was 1.3 h, as expected from a dimethyl phosphorylated
acetylcholinesterase (Heath & Vandekar, 1957).
The 1973 JMPR (FAO/WHO, 1974) reported that rat brain
cholinesterase was more sensitive to in vitro inhibition by demeton-
S-methyl than by oxydemeton-methyl (I50 values of 9.52 × 10-5 and
1.43 × 10-3 mol/litre, respectively; time of incubation, temperature
and pH not reported). It was also reported that demeton-S-methyl was a
more potent inhibitor of human serum cholinesterase (no details
given).
Data on in vivo inhibition of plasma cholinesterase and
erythrocyte and brain cholinesterase are reported in section 7.2 and
7.3.
7.10 Potentiation
Male Wistar rats (160-180 g body weight) were given trichlorfon
(98.6% purity) and demeton-S-methyl (90% purity) in combination by
gavage. The amount of compound in the mixture was proportional to its
oral LD50 (302 mg/kg body weight for trichlorfon and 44 mg/kg body
weight for demeton-S-methyl) in order to obtain equitoxic doses. The
resulting toxicity was additive (LD50 of the mixture was 223 mg/kg
body weight against expected 173 mg/kg body weight) (Flucke &
Kimmerle, 1977).
Similarly, an additive effect was obtained when demeton-S-methyl
was given in combination with phenamiphos (ethyl-4-(methylthio)
m-tolyl-isopropyl-phosphoroamidate). The experimental LD50 of the
mixture was 55 mg/kg body weight while that estimated from the
individual LD50s was 50 mg/kg body weight (Kimmerle, 1972).
8. EFFECTS ON HUMANS
8.1 General population exposure
A 31-year old woman attempted suicide with an unknown amount
("1 or 2 mouthfuls") of Metasystox I (25% demeton-S-methyl). On
admission to hospital she was comatose, sweating and salivating,
and had pin-point pupils. She stayed in the Intensive Care Unit for 15
days where she was treated with atropine (up to 97 mg per day, 550 mg
in 14 days). Oximes (2-PAM) were only given on day 1 (0.5 + 0.25 +
0.25 g) and there was no apparent clinical improvement. On admission,
plasma and erythrocyte cholinesterase activities were less than 10% of
normal control values. The patient was discharged on the 30th day with
normal plasma cholinesterase values; erythrocyte cholinesterase
activity was still below the normal values (about 65%), but had
recovered a month later (Barr, 1966).
A case of acute poisoning in a 5-month pregnant woman has been
described (Carrington da Costa et al., 1982). A 41-year-old woman
attempted suicide by ingesting Metasystox, which resulted in an
estimated intake of 12 g methyl demeton (it should be noted that in
the report Metasystox was said to contain methyl-demeton and not
oxydemeton-methyl as the commercial name implies). On admission, 3.5 h
after poisoning, blood cholinesterase (it was not specified whether
pseudo- or acetylcholinesterase) was 10% of normal values. The patient
became comatose 12 h after admission. She was treated with atropine,
obidoxime and haemoperfusion 72 h after hospitalization. Artificial
ventilation was required on the 4th day of hospitalization. The
patient was discharged after 24 days and 4 months later she delivered
a healthy female child.
In a fatal suicidal case with a commercial formulation of
demeton-S-methyl, the concentration of the compound was measured in
several organs. It was estimated that death occurred about 6 h after
poisoning. Highest demeton-S-methyl levels were found in the proximal
small intestine (166 mg/kg of tissue); in brain, kidney and muscles,
the levels were 30-80 mg/kg; lowest concentrations were found in liver
(7 mg/kg tissue) and blood (7-16 mg/litre). Metabolites were not
measured (Schludecker & Aderjan, 1988).
In the United Kingdom in 1991 there were five cases of poisoning
by demeton-S-methyl and in 1994 three cases, none of which were fatal
(personal communication by G. Volans, Medical Toxicology Unit, London,
to the IPCS, dated 15 January 1996).
Hegazy (1965) reported three cases of suspected poisoning with
demeton-S-methyl in children (6-14 years of age) exposed in a recently
sprayed field and 1 case after ingestion of contaminated feed.
Symptoms were mild and the patients recovered fully. Serum
cholinesterase determinations gave ambiguous results.
8.2 Occupational exposure
8.2.1 Acute poisoning
A worker inadvertently exposed to demeton-S-methyl (no details)
was monitored for about 100 days. Plasma cholinesterase activity was
always within the normal values of the laboratory. However, if the
activity on day 40 and 100 is considered as the normal value for this
worker, then a 30% inhibition occurred 2-3 days after exposure. The
activity recovered with an half-life of about 10 days. Erythrocyte
cholinesterase activity was below the normal value for about 40 days.
When calculated on the activity of day 100, a 60% inhibition was found
up to day 10. The activity recovered with a half-life of about 35 days
(Lewis et al., 1981).
Two workers in a chemical packaging company, whose job it was to
fill a concentrate of demeton-S-methyl (500 g/litre of xylene) into
one-litre containers using a weight-triggered bottle-filling machine
for 3 to 4 h, were admitted to hospital because of organophosphate
poisoning and were treated with atropine. Cholinesterase measurement
made 14 days after exposure (1 worker) showed inhibited erythrocyte
cholinesterase, but the plasma cholinesterase activity was within the
normal range. In the second worker, whose symptoms lasted for 3 days,
erythrocyte cholinesterase activity was below the normal value 5 weeks
after exposure. These workers wore gloves, overalls and boots, but
frequent spillage was reported. Normal clothing had been left under
the filling apparatus and apparently was contaminated. The filling
system was changed by housing the filling machine in a fume cupboard
and by providing new protective garments and changing room facilities.
One more worker was admitted to the hospital and treated with atropine
because of organophosphate poisoning. This occurred on the morning
after a day spent fitting the infill seal and screwing on the tops
of cans. He had reduced erythrocyte and plasma cholinesterase
(erythrocyte > plasma) activities for several days after exposure.
Another worker showed a sharp drop in erythrocyte and plasma
cholinesterase activities associated with abdominal cramps, which
resolved in about 5 days. Another worker had depressed erythrocyte and
plasma cholinesterases activities without complaining of any adverse
effect. It was concluded that absorption of demeton-S-methyl was
through the skin because of penetration of the protective clothing
used or because this clothing was not worn properly. It should be
noted that the active ingredient was dissolved in xylene, which is
known to attack rubber and plastics, making the penetration of gloves
possible (Jones, 1982).
8.2.2 Effects of short- and long-term exposure
Three volunteers without any protective equipment were exposed
for two consecutive days to Metasystox (30% demeton-S-methyl, 70%
demeton-O-methyl) while spraying with a hand-held nebulizer. Exposure
lasted for 3 and 6 h on the first and second days, respectively. The
concentrations of the active ingredient (isomers not separated) were
8.8-27 mg/m3 of ambient air. Plasma and erythrocyte cholinesterase
activities measured up to 14 days after exposure did not show
significant decreases when compared to pre-exposure values (Klimmer &
Pfaff, 1955).
Volunteers, wearing overalls but not mask protection, were
exposed to metasystox (30% demeton-S-methyl, 70% demeton-O-methyl)
while spraying in a greenhouse. They used the splash method
(0.03-0.05% a.i.), the low volume (0.5% a.i.) or the high volume spray
method (0.05% a.i.) for 5 to 25 min. There was no effect on plasma or
erythrocyte cholinesterase activity measured after exposure (Klimmer &
Pfaff, 1958).
Six workers engaged in hop cultivation using Metasystox I
(reported to contain demeton-O-methyl instead of demeton-S-methyl as
the commercial name implies) were monitored. They sprayed up to
2400 litres of a 0.1% solution (in water) of the insecticide in one
day. Protective clothing and masks were not always used. No
significant inhibition of blood acetyl cholinesterase was observed at
the end of exposure or 1 or 2 days later. One subject, who was exposed
twice, showed a 29% decrease in blood acetyl cholinesterase after the
second exposure. No signs or symptoms were observed in these workers
(Winkler & Arent, 1970).
The medical department of a company reported no adverse effect in
workers employed in the formulation of demeton-S-methyl from 1967 to
1984 (Faul, 1984).
Agricultural workers exposed to demeton-S-methyl for 3
consecutive days were monitored. Pre-and post-exposure urinary levels
of the metabolite dimethyl phosphorothiolate potassium salt (DMPThK),
and plasma and whole blood cholinesterase activities were measured.
Exposed subjects were divided into three groups according to their
job, i.e. mixers (n=7), sprayers (n=6) and others (n=7) not directly
involved in handling the pesticide. Higher levels of DMPThK were
found in mixers, with a medium value of 83 µg/litre and a range of
0-822 µg/litre (neither corrected for creatinine nor for urine
volume). Sprayers had a mean value of 30 µg/litre (limit of detection)
and a range of 0-208 µg/litre; the other subject had a mean value of
30 µg/litre and a range of 0-100 µg/litre. Whole blood cholinesterase
activity was not affected by the exposure, while plasma cholinesterase
activity was slightly (about 10%, statistically significant) reduced
when compared to pre-exposure levels in mixers. However, no
correlation was found between DMPThK levels and plasma cholinesterase
activity (Vasilic et al., 1987).
Hegazy (1965) reported a study of 121 spraymen exposed to
Meta-isosystox during spraying of cotton fields in Egypt. The spraymen
applied Meta-isosystox at a rate of 0.5 litres of concentrate in
400 litres of spray per 4200 m2 mainly by hand-operated knapsack
sprayers, or in a few cases by high-pressure motor-powered sprayers
with a large tank connected by a long hose to a multi-nozzled
spray-boom. Sprayers were not involved in chemical mixing. Workmen
washed exposed body parts with soap and water after spraying. Working
clothes were removed at the end of the day, but the clothes may not
have been washed before re-use. Not all workers used protective
clothing and masks. None of the workers were re-exposed to
Meta-isosystox after the onset of symptoms. Serum cholinesterase
activity estimates were performed within 24 h after the onset of
symptoms in some patients and after the cessation of symptoms in some
others. In most cases they were repeated 2-3 times at various
intervals up to 40 days from the onset of symptoms. In general, the
serum cholinesterase activity in spraymen underwent a marked initial
fall, followed by a rise to above-normal levels after about 30-40
days. Signs and symptoms of toxicity in spraymen occurred after 1-18
days of exposure, with a mean time of 3 days. These consisted of
gastrointestinal disturbances (58% of total), dizziness (23%),
persistent general weakness and fatigue (19%), respiratory
manifestations (16%), headache (16%), sweating, salivation or
lacrimation (12%), tremors of outstretched hands, intention tremors,
ataxia (4.1%), exaggerated superficial and deep reflexes (5.8%),
hiccough (2.5%), muscular fasciculations (2.5%), or had apparently
resolved (30%), at the first determination of serum cholinesterase
activity (Hegazy, 1965).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Aquatic organisms
9.1.1 Algae
Table 4 reports the results obtained when different
formulations of demeton-S-methyl were tested on the green alga
Scenedesmus subspicatus (Heimbach, 1985a, 1990b).
9.1.2 Invertebrates
The acute toxicity of demeton-S-methyl for molluscs and
crustaceans is given in Table 5.
The lowest concentration tested on the water flea Daphnia
magna (10 µg a.i./litre) caused toxic effects (Heimbach, 1985b,c). A
21-day exposure test on water flea reproduction produced a NOEC of
> 5.6 µg a.i./litre (Heimbach, 1990d). A study performed with a
27.3% emulsifiable concentrate formulation (Heimbach, 1985c) yielded
a NOEC of 3.7 µg/litre (equal to 1 µg a.i./litre) and a LOEC of
11.7 µg/litre (equal to 3.1 µg a.i./litre).
Using a commercial formulation of metasystox, the 96-h LC50 for
the lammellibranch mollusc Paphia laterisulca was 2 µg/litre (Akarte
et al., 1986) and that for the freshwater prawn Donax cuneatus was
4 µg/litre (Muley et al., 1987).
9.1.3 Fish
Table 6 indicates the toxicity of demeton-S-methyl for fish.
9.2 Terrestrial organisms
9.2.1 Soil microorganisms
In a study conducted in silty sand soil or loamy silt soil
with doses of demeton-S-methyl up to 5 times those recommended
(2 litres/hectare of a 27% emulsifiable concentrate formulation),
no influence on soil respiration or nitrification in soil was found
(Anderson, 1989; Blumenstock, 1989).
9.2.2 Invertebrates
When demeton-S-methyl was mixed with an artificial soil where
earthworms (Eisenia foetida) were kept for 14 days, the LC50 was
241 mg/kg of dry substrate of the commercial formulation (a 25%
emulsifiable concentrate), corresponding to 60 mg a.i./kg (Heimbach,
1990a).
Table 4. Effect of demeton-S-methyl on the green alga Scenedesmus subspicatusa
Specification of test EC50 (mg a.i./litre) NOEC LOEC Duration Conditions
substance (mg/litre) (mg/litre) (h)
Increase of Growth rate
biomass
Technical 97.3% purity 8 22 1 3 96 pH 7.8 - 8.5
23 °C
EC formulationb 37 >100 18 32 96 pH 7.6 - 10.4
(27.3% a.i.) 23 °C
Pre-solutionc 13 37 1 10 96 pH 7.7 - 8.5
xylene (53.7% a.i.) 22 °C
a From: Heinbach (1985a, 1990b)
b Tests performed with the blank formulation gave the same results as the highest tested concentration.
c No test with the blank pre-solution was performed.
Table 5. Acute toxicity of demeton-S-methyl for molluscs and crustaceans
Species Specification of test Temperature LC50 Duration of Reference
substance (°C) (mg/litre) exposure
(h)
Mollusc commercial formulation ? 0.0042 96 Akarte et al. (1986)
(Paphia laterisulca)
Water flea (Daphnia magna) technical (96.7%) 20 ± 1 >0.1 24 Heimbach (1985b)
0.023 48
pre-solution in xylene 20 ± 1 >0.1 24 Heimbach (1985c)
(53.7%) formulation 0.022 48
Clam (Donax cuneatus) commercial formulation 25-28.5 0.0064 96 Muley et al. (1987)
Prawn (Macrobrachium lamerrii) commercial formulation 27 ± 2 1.3 72 Mary et al. (1986)
Table 6. Toxicity of demeton-S-methyl for fish (96-h exposure)
Species Mass and length Temperature LC50 (mg/litre) Reference
(°C)
Rainbow trout (Onchorhyncus mykiss) 4.0 - 5.5 cm 16 4.5 Grau (1985a)
1.0 - 1.5 g (52.7% of a.i.)
6.4 ± 1.0 cm 15±2 0.59 Grau (1990c)
3.0 ± 0.6 g (69.5% of a.i.)
6.9 ± 1.1 cm 15±2 6.44 Grau (1990a)
3.5 ± 1.6 g (27.3% of a.i.)
Golden orfe (Leuciscus idus melamotus) 6.0 - 7.5 cm 21 43 Grau (1985b)
2.5 - 4.2 g (52.7% of a.i.)
6.4 ± 0.6 cm 21±2 23.2 Grau (1990b)
2.5 ± 0.6 g (27.3% of a.i.)
Goldfish (Carassius auratus) 6 cm 18 20-40 Hermann (1974a)
1.5 g (28.1% of a.i.)
Carp (Cyprinus carpio) 6 cm 18 40-60 Hermann (1974b)
1.6 g (28.1% of a.i.)
Scardinius erythrophthalmus 6 cm 18 30-40 Hermann (1974c)
1.3 g
Cirrhana mrigala (larvae) 51 ± 3 mg 20 1.45 Verma et al. (1984)
Demeton-S-methyl was applied on fields of winter wheat by
fixed-wing aircraft using conventional boom-and-nozzle equipment.
The applied amount was 245 g a.i. per hectare at a volume rate of
20 litres/hectare. Samples of the soil surface and crop foliage fauna
were collected 1-2 times before and 4-5 times after application from a
treated field and from a control untreated field. The number of crop
foliage but not of soil surface entomophagus invertebrates was reduced
soon after application of demeton-S-methyl. Empididai (dance flies)
was the only group to be significantly reduced in numbers by
demeton-S-methyl. Predatory Coleoptera (beatles) (Carabidae and
Staphylinidae), Araneae (spiders) and predatory Diptera (flies)
(except Empididae) were not affected by demeton-S-methyl. Among the
ephytophagus fauna, cereal aphids markedly declined in number soon
after application, but a rapid increase was observed 2 months later,
when numbers of Diptera and Thripidae (thrips) were also
increased. Numbers of entomobryid and sminthurid springtails decreased
soon after the application but were higher than in the control field
2 months later (Shires, 1985).
Demeton-S-methyl was slightly toxic to the predatory mite
Phytoseiulus persimilis since a 70% population reduction was
obtained with a concentration of 0.025% a.i. (Kniehase, 1984).
The contact LD50 for bees has been reported to be 0.60 µg/bee
(Westlake et al. 1985). A field study was conducted on bee
colonies following application of a 0.2% formulation of Metasystox
(at 600 litres/hectare) to field beans (Vicia faba) after bee
foraging had ceased in the evening. Toxicity to bees was assessed by
collecting dead insects on canvas sheets in front of the hive
entrance. Before spraying, the mortality in exposed and control hives
was comparable. Following spraying, there was a mortality rate of 123
bees per hive in the exposed and 90 bees in the controls (prior to
exposure mortality was 98 and 95 respectively). The mortality increase
persisted for 3 days after the application (Bayer, 1970).
9.2.3 Birds
Some oral LD50 values are given in Table 7.
Table 7. Acute oral LD50 values for birds
Species Vehicle Purity LD50 Reference
(mg/kg b.w.)
Japanese quail Cremephor EL 99.7% 44-50 Flucke (1984)
(Coturnix japonica)
Canary ? 99.7% 10-20 Grau (1984)
(Serinus canarius)
Groups of 10 starlings (Sturnus vulgaris) were given (by
gavage) 0, 0.2, 1.5 or 2 mg/kg body weight of demeton-S-methyl
(technical grade) dissolved in corn oil. Serum butyrylcholinesterase
and carboxylesterase and brain cholinesterase activities were measured
at 3 (high-dose only), 6 and 24 h after dosing (n=2 for brain, n=4 for
serum). The highest dose inhibited brain cholinesterase by about 20%,
serum carboxylesterase by about 50% and serum butyrylcholinesterase by
about 80%. The peak effect was at 3 h with some recovery after 24 h.
In vitro treatment of the enzyme preparation with pralidoxine
chloride (2-PAM) caused almost complete reactivation of brain
cholinesterase but had no effect on the other enzymes (Thompson et
al., 1991).
9.2.4 Effects in field
The activities of brain cholinesterase, serum
butyrylcholinesterase and glutamate oxaloacetate transaminase (GOT)
were measured in house sparrows (Passer domesticus) captured 1
(n=7), 2 (n=6) or 3 (n=6) days after spraying wheat fields with
demeton-S-methyl (0.485 litres a.i./hectare from an emulsifiable
concentrate formulation in a xylene solvent base). Liver weight was
also recorded and histological examination of the liver was performed.
Serum butyrylcholinesterase was reduced to 64% of that of controls
(n=5) (statistically not significant) and brain cholinesterase was 82%
of that of controls (statistically significant) for birds trapped on
day 2, but serum GOT was not significantly affected in the exposed
birds. The authors claimed that there was evidence of liver damage
in exposed birds because of a significant increase of binucleation
(3.3-5 vs. 1.9 binucleation/10 fields) and inflammatory cell foci
(more evident after 3 days of exposure) (Tarrant et al., 1992).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
The major metabolites of demeton-S-methyl in plant and mammals
are oxydemeton-methyl and demeton-S-methylsulfone. These two compounds
are also commercial insecticides. Their mechanism of action
is the same as that of demeton-S-methyl (i.e. inhibition of
acetylcholinesterase) and their toxicological profiles have been
reported to be similar to that of demeton-S-methyl (FAO/WHO, 1990).
10.1 Evaluation of human health risks
Sources of exposure of humans to demeton-S-methyl are dietary and
through dermal absorption and inhalation during the manufacture or use
of the compound.
There is very limited information on actual dietary exposure to
demeton-S-methyl.
In an early study, during application of a mixture of demeton-S-
methyl (30%) and its isomer, demeton-O-methyl (70%), an ambient air
concentration of 8.8-27 mg/m3 of the combined isomers was found. This
is much higher than the conservative TLV/TWA for methyl-demeton
proposed by ACGIH (0.5 mg/m3). However, no inhibition of plasma and
erythrocyte cholinesterase activities was found after exposure.
Cases of acute intoxication, which required pharmacological
treatment, have been reported where workers were filling bottles with
a 50% xylene solution of demeton-S-methyl. Skin absorption was
considered the cause of poisoning because of inadequate personal
protection. Agricultural workers exposed to demeton-S-methyl while
mixing or spraying during three consecutive days did not show
significant inhibition of either plasma or erythrocyte cholinesterase.
Agricultural workers exposed to demeton-S-methyl while spraying cotton
for 1-18 days (average of 3 days) showed signs and symptoms of
poisoning. Improper working conditions could not be excluded.
Cases of severe demeton-S-methyl poisoning (suicide attempts)
have been reported. No delayed toxicity developed in the patients who
survived.
No data are available on the effect of repeated human exposure to
demeton-S-methyl.
No reproductive, developmental or carcinogenic effects have been
found in laboratory animals exposed to demeton-S-methyl. NOAEL values
have been based on inhibition of brain acetyl cholinesterase or, in a
rat reproduction study, on reduced pup viability and body weight in
the F1b generation.
The available information is insufficient to permit an adequate
assessment of the genotoxic potential of demeton-S-methyl.
10.2 Evaluation of effects on the environment
The information on utilization and application rates that
has been employed for this risk assessment is derived from the
agricultural use of demeton-S-methyl within the European Union. It
should be possible to extrapolate this assessment to other
agricultural uses at similar application rates elsewhere in the world.
The application rates for demeton-S-methyl can be summarised as
follows: arable (tractor-mounted/drawn hydraulic spray boom
applications), 0.32 kg/ha; top fruit (broadcast air-assisted
applications), 0.49 kg/ha.
The following risk assessment is based on the principle of
calculating toxicity-exposure ratios (TERs) (Fig. 3), which follows
the European and Mediterranean Plant Protection Organisation and
Council of Europe (EPPO/CoE) Environmental Risk Assessment Scheme
model and associated trigger values (EPPO/CoE, 1993a,b).
10.2.1 Aquatic organisms
The main risk to aquatic organisms from the use of demeton-S-
methyl is from spray drift during either arable applications
(0.32 kg/ha) or top fruit air-assisted (0.49 kg/ha). For each of these
risk scenarios, the predicted environmental concentration (PEC) in a
30-cm-deep static surface water body, arising from either arable-based
spray drift at 1 m from the edge of the spray boom or from top fruit
air-assisted spray drift at 3 m from the point of application (both
based on Ganzelmeier et al., 1995), was calculated as follows:
PEC (mg demeton-S-methyl/litre)
= max application rate (kg/ha) × A (% spray drift)
300
where
A = 5 for ground-based hydraulic spray applications 1 m from
edge of boom
or 30 for air-assisted application 3 m from point of application
10.2.1.1 Acute risk
The acute LC/EC50 values for the most sensitive fish was
0.59 mg/litre, for the most sensitive aquatic invertebrate (mollusc)
was 0.0042 mg/litre (0.022 mg/litre for Daphnia), and for the most
sensitive algal species was 8 mg/litre.
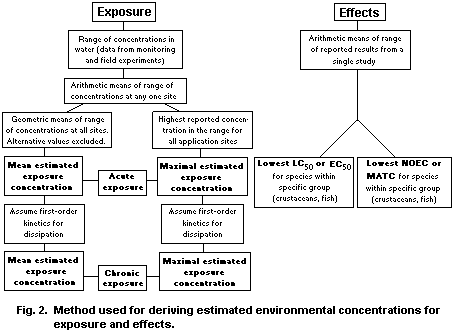 (a) Spray drift from ground-based applications
The acute PEC for spray drift (1 m from the edge of the spray
boom into a 30-cm-deep static water body at the maximum application
rate (see PEC assumptions above) is 0.005 mg/litre. Therefore, the
TERs based on this PEC and the above LC/EC50 toxicity values are:
fish, 110; aquatic invertebrates, 0.8 (4.1 for Daphnia); and algae,
1500. Based on the EPPO/CoE risk assessment scheme for aquatic
organisms, these TERs indicate a low acute risk to fish and algae.
However, for aquatic invertebrates the TER is less than 10, indicating
a potential risk to aquatic invertebrates (both molluscs and
arthropods). In such risk situations the use of a "no-spray"
restriction zone next to surface waters may reduce the risk to such
aquatic invertebrates. For example, arable spray drift at 5 m from the
edge of boom is 0.6% (Ganzelmeier et. al., 1995). Based on this 5 m
drift data, the PEC is 0.0006 mg/litre and results in a 5 m TER of 7
for molluscs and 37 for Daphnia. The TER for the most sensitive
invertebrate (mollusc) is still below the EPPO trigger of 10,
indicating a borderline risk. However, the Daphnia 5 m TER, which is
above 10, indicates that the use of a 5 m "no-spray" restriction zone
next to surface waters would help reduce the acute risk to aquatic
invertebrates.
(b) Spray drift from broadcast air-assisted top fruit applications
The acute PEC for spray drift (3 m from the point of application
into a 30-cm-deep static water body at the maximum application rate
(see PEC assumptions above) is 0.049 mg/litre. Therefore, the TERs
based on this PEC and the above LC/EC50 toxicity values are: fish,
12; aquatic invertebrates, 0.09 (0.4 for Daphnia); and algae, 163.
Based on the EPPO/CoE risk assessment scheme for aquatic organisms,
these TERs indicate a low acute risk to fish and algae. However, for
aquatic invertebrates the TER is less than 1 indicating a high risk to
aquatic invertebrates (both molluscs and arthropods). In such risk
situations the use of a "no-spray" restriction zone next to surface
waters may reduce the risk to such aquatic invertebrates. For example,
spray drift from broadcast air-assisted applications to top fruit at
15 m from point of application is 6.0% (Ganzelmeier et al., 1995).
Based on this 15 m drift data, the PEC is 0.006 mg/litre and results
in a 15 m TER of 0.7 for molluscs and 3.4 for Daphnia. These TERs at
15 m for the most sensitive invertebrates are still below the EPPO
trigger of 10, indicating a potential risk. Therefore, even with a 15
"no-spray" restriction zone next to surface waters, there is still a
potential risk to aquatic invertebrates from the broadcast
air-assisted use of demeton-S-methyl. Table 8 summarizes the acute
TERs for demeton-S-methyl to aquatic organisms at 1 m for arable and
3 m for broadcast air-assisted applications.
Table 8. Acute toxicity-exposure ratios (TER) for aquatic organisms at 1 m for arable and 3 m for broadcast air-assisted
applications
Species LC/EC50 PEC (mg/litre) at 1 m PEC (mg/litre) at 3 m TER TER
(mg/litre) (arable) (air assisted) (arable) (air assisted)
Fish 0.59 0.005 0.049 110 12
(Oncorhynchus mykiss)
Aquatic invertebrate 0.0042 0.005 0.049 0.8 0.09
(Paphia laterisulca)
Aquatic invertebrate 0.022 0.005 0.049 4.1 0.4
(Daphnia magna)
Alga 8.0 0.005 0.049 1500 163
(Scenedesmus subspicatus)
10.2.1.2 Chronic risk
No chronic toxicity data are available for fish. However, a
chronic NOEC of 0.0056 mg/litre has been reported for Daphnia
magna. There are no data reporting the persistence or degradation of
demeton-S-methyl in water, and so a chronic PEC could not be derived.
Therefore, owing to the lack of data on chronic fish toxicity and
environmental fate in water, it is not possible to assess the chronic
risk to either fish or aquatic invertebrates. There are also no data
available on either the toxicity of demeton-S-methyl to sediment-
dwelling invertebrates or fate of demeton-S-methyl in aquatic
sediments. Therefore, the risk to sediment-dwelling invertebrates
could also not be assessed. No fish bioaccumulation data are
available, but, as the log Pow is 1.3 (i.e. < 3), the risk of
bioaccumulation in fish should be low.
10.2.2 Terrestrial organisms
Vertebrates are likely to be exposed to demeton-S-methyl from
either grazing on treated vegetation or consuming contaminated
insects. For this risk assessment, typical application rates of
0.32 kg/ha are used for ground spray application on arable crops and
0.49 kg/ha for application by air-assisted spraying for fruit.
10.2.2.1 Birds
The lowest reported acute oral LD50 for birds is 10 mg/kg body
weight for the canary. Dietary data are not available.
Indicator birds for use in the risk assessment are:
* Greylag goose (Anser anser), as a grazing species, with a body
weight of 3 kg and total daily food consumption of 900 g
vegetation (dry weight) (Owen, 1975)
* Blue tit (Parus caeruleus), as an insectivorous species, with a
body weight of 11 g and total daily food consumption of 8.23 g
(dry weight) (Kenaga, 1973)
a) Grazing birds
Initial residues on short grass or cereal shoots are estimated to
be 35.8 mg/kg dry weight (based on 112 × application rate) arising
from application at 0.32 kg/ha to arable crops (EPPO/CoE, 1993a,b) and
55 mg/kg from application to fruit at a rate of 0.49 kg/ha. This gives
an estimated total oral intake for the goose of 32.2 mg and 49.5 mg
for the two application rates assuming that the goose ate exclusively
food contaminated at this level. This is equivalent to a daily intake
of 10.7 and 16.5 mg/kg body weight, respectively. TERs can be
calculated as follows:
End-point LD50/LC50 Application rate Predicted TER
(mg/kg b.w.) (kg/ha) concentration
in food (mg/kg)
Bird acute 10 0.32 (arable) 35.8 0.93
oral (Canary) 0.49 (fruit) 55 0.61
The calculated TER values fall well below the EPPO/CoE trigger
values for concern (TER <10) and indicate a high risk to grazing
birds.
b) Insectivorous birds
Initial residues on small insects are estimated to be 9.3 mg/kg
dry weight (based on 29 × application rate) arising from application
at 0.32 kg/ha to arable crops (EPPO/CoE, 1993a,b) and 14.2 mg/kg from
application to fruit at a rate of 0.49 kg/ha. This gives an estimated
total oral intake for the blue tit of 0.08 mg and 0.12 mg for the two
application rates assuming that the blue tit ate exclusively food
contaminated at this level. This is equivalent to a daily intake of
6.94 and 10.6 mg/kg body weight, respectively. TERs can be calculated
as follows:
End-point LD50/LC50 Application rate Predicted TER
(mg/kg b.w.) (kg/ha) concentration
in food (mg/kg)
Bird acute 10 0.32 (arable) 9.3 1.44
oral (Canary) 0.49 (fruit) 14.2 0.94
The TERs for acute toxicity to insectivorous birds are
substantially less than the trigger value of <10, indicating high
acute risk to these birds.
10.2.2.2 Mammals
The lowest reported acute oral LD50 for laboratory mammals is
63 mg/kg body weight for the rat.
Indicator mammals for use in the risk assessment will be:
* Rabbit (Oryctolagus cuniculus), as a grazing mammal, with a
body weight of 1200 g and a total daily food consumption of 500 g
vegetation (dry weight) (Ross, personal communication to the
IPCS).
* Shrew (Sorex araneus), as an insectivorous mammal, with a body
weight of 18 g and a total daily food consumption of 18 g
(Churchfield, 1986)
a) Grazing mammals
Initial residues on short grass or cereal shoots are estimated to
be 35.8 mg/kg dry weight (based on 112 × application rate) arising
from application at 0.32 kg/ha to arable crops (EPPO/CoE, 1993a,b) and
at 55 mg/kg from application to fruit at a rate of 0.49 kg/ha. This
gives an estimated total oral intake for the rabbit of 17.9 mg and
27.4 mg for the two application rates, assuming that the rabbit ate
exclusively food contaminated at this level. This is equivalent to a
daily intake of 15 and 23 mg/kg body weight, respectively. TERs can be
calculated as follows:
End-point LD50 Application rate Predicted TER
(test (mg/kg b.w.) (kg/ha) concentration
species) in food (mg/kg)
Acute oral 63 0.32 (arable) 17.9 4.20
(Rat) 0.49 (fruit) 27.5 2.76
Since the TERs are < 10, this indicates a high risk to grazing
mammals.
b) Insectivorous mammals
Initial residues on large insects are estimated to be 0.86 mg/kg
dry weight (based on 2.7 × application rate) arising from application
at 0.32 kg/ha to arable crops (EPPO/CoE, 1993a,b) and 1.32 mg/kg from
application to fruit at a rate of 0.49 kg/ha. This gives an estimated
total oral intake for the shrew of 0.016 mg and 0.024 mg for the two
application rates, assuming that the shrew ate exclusively food
contaminated at this level. This is equivalent to a daily intake of
0.86 and 1.32 mg/kg body weight, respectively. TERs can be calculated
as follows:
End-point LD50 Application rate Predicted TER
(mg/kg b.w.) (kg/ha) concentration
in food (mg/kg)
Mammal 63 (Rat) 0.32 (arable) 0.86 72.9
acute oral 0.49 (fruit) 1.32 47.6
These TERs fall outside the trigger for high risk for
insectivorous mammals but within the range for medium risk.
10.2.2.3 Bees
The reported contact toxicity to bees gives an LD50 of
0.26 µg/bee. Using application rates at 0.32 and 0.49 kg/ha for
cereals and fruit respectively, hazard quotients are calculated to be
1231 and 1885 (application (g/ha)/LD50 (µg/bee)). The trigger for
concern is >50 (EPPO/CoE, 1993a,b) and, therefore, there is
substantial concern for exposed bees. The compound should not be
applied to flowering plants, and exposure of flying bees should be
avoided.
10.2.2.4 Earthworms
Earthworms are likely to be exposed to demeton-S-methyl. Based on
a soil depth of 5 cm and a soil density of 1.5 g/cm3, the soil PEC
would be 0.43 mg/kg assuming even distribution in the medium. The
reported LC50 for earthworms (Eisenia foetida) is 60 mg/kg
soil giving a TER of 140.6. As this is above the trigger value of
10 (EPPO/CoE, 1993a,b), the acute risk to earthworms is very low.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
Demeton-S-methyl causes acute cholinergic toxicity in humans and
laboratory animals. Carcinogenic, reproductive and developmental
effects have not been found in laboratory animals. Effects due to
chronic human exposure are unlikely to occur. Exposure of the general
population may only occur through food residues. However, the levels
of exposure are unlikely to cause adverse effects.
Given the high acute toxicity, as determined in a number of test
species and the known cases of human poisoning, it can be concluded
that the risk of occupational poisoning with demeton-S-methyl exists
when adequate protective measures and good practices are not adopted.
Therefore, handling and application of demeton-S-methyl should only be
performed by supervised and trained workers who must adhere to good
application practices and adopt adequate safety measures.
Demeton-S-methyl does not persist in the environment and is not
accumulated by organisms. It has high acute toxicity to aquatic
invertebrates and is toxic to fish and birds, leading to high or
moderate risk factors for these organisms. However, significant field
kills of organisms have not been reported for the compound.
Precautions should be taken to minimize exposure of non-target
organisms (e.g., do not spray over water bodies, minimize exposure by
spray drift).
In view of the negative results obtained in developmental and
carcinogenicity studies, it is felt that further clarification of the
genotoxic potential of demeton-S-methyl is not necessary.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Demeton-S-methyl was classified as highly hazardous (class Ib) by
WHO (1996).
Demeton-S-methyl was evaluated by the Joint FAO/WHO Meeting on
Pesticide Residues (JMPR) in 1972, 1973, 1979, 1982, 1984, 1989, 1992.
The 1989 Meeting (FAO/WHO, 1990) evaluated demeton-S-methyl,
oxydemeton-methyl and demeton-S-methylsulfone and set the following
NOAEL levels:
demeton-S-methyl: mouse: 1 mg/kg diet (equal to
0.24 mg/kg body weight per day)
rat: 1 mg/kg diet (equal to
0.05 mg/kg body weight per day)
dog: 1 mg/kg diet (equal to 0.036 mg/kg
body weight per day)
oxydemeton-methyl: mouse: 30 mg/kg diet (equal to
0.03 mg/kg body weight per day)
rat: 0.57 mg/kg diet (equal to
0.03 mg/kg body weight per day)
dog: 0.125 mg/kg body weight per day
demeton-S-methylsulfone: rat: 1 mg/litre in drinking water
(equal to 0.06 mg/kg body weight per
day)
dog: 10 mg/kg diet (equal to 0.36 mg/kg
body weight per day)
The estimated ADI for demeton-S-methyl was established at
0-0.0003 mg/kg body weight. This ADI applies to demeton-S-methyl
alone or in combination with oxydemeton-methyl and/or demeton-S-
methylsulfone because residues are determined after oxidation and are
expressed as demeton-S-methyl. The 1992 JMPR reviewed the residue data
on these three related compounds. Updated figures were available only
for oxydemeton-methyl, which is replacing demeton-S-methyl in most
registrations. The Meeting decided therefore to change the definition
of the residues to "the sum of demeton-S-methyl, oxydemeton-methyl and
demeton-S-methylsulfone expressed as oxydemeton-methyl". The MRLs
varied from 0.05 to 1 mg/kg commodity and have been obtained from
supervised trials on oxydemeton-methyl rather than on
demeton-S-methyl.
Demeton-S-methyl has not been evaluated by the International
Agency for Research on Cancer (IARC).
REFERENCES
ACGIH (1993) 1993-1994 Threshold limit values for chemical substances
and physical agents. Cincinnati, Ohio, American Conference of
Governmental Industrial Hygienist.
Akarte SR, Rao KR, & Kulkarni DA (1986) Acute toxicity of Metasystox
to an estuarine lamellibranch mollusc, Paphia laterisulca, from
Ratnagiri coast, India. Int J Ecol Environ Sci, 12: 125-129.
Anderson JPE (1989) Influence of the commercial product Metasystox (i)
on soil respiration after amendment with glucose. Leverkusen, Germany,
Bayer AG (Unpublished report No. AJO/78289).
Anonymous (1977) Determination of residues of organophosphorus
pesticides in fruits and vegetables. Analyst, 102: 858-868.
Barr AM (1966) Further experience in the treatment of severe organic
phosphate poisoning. Med J. Aust, 1: 490-492.
Bathe R (1983) 12-month oral (feeding) toxicity study with E 154
in Beagle dogs. Leverkusen, Germany, Bayer AG (Unpublished report
No. 2542).
Bayer (1970) Testing the effects of Metasystox i and Metasystox R on
bees. Leverkusen, Germany, Bayer AG (Unpublished report).
Becker H (1983) Embryotoxicity and teratogenicity study on E 154 in
rabbits. Leverkusen, Germany, Bayer AG (Unpublished report No. 2422).
Blumenstock I (1989) Influence of the commercial product Metasystox
(I) on nitrification in soils. Leverkusen, Germany, Bayer AG
(Unpublished report No. BSI/78189).
Carrington da Costa RB, Maul ER, Pimentel J, Goncalves JS, Rebelo A,
Oliveira LC, & Rebelo ASC (1982) A case of acute poisoning by methyl
demeton in a female 5 months pregnant. Arch Toxicol, 5(Suppl):
202-204.
Churchfield S (1986) Shrews. Oswestry, A. Nelson (The Mammal Society
series).
Cifone MA (1984) Mutagenicity evaluation of E154 (C.N. demeton-S-
methyl) in the mouse lymphoma forward mutation assay. Leverkusen,
Germany, Bayer AG (Unpublished report No. 2789).
DuBois KP & Doull J (1955) The acute toxicity of P=O Meta-systox, P=O
Metasystox sulphoxide and P=O Meta-systox sulphone to mammals.
Leverkusen, Germany, Bayer AG (Unpublished report).
DuBois KP & Plzak G (1962) The acute toxicity and anticholinesterase
action of 0,0-dimethyl-S-ethyl-2-sulphinylethylphosphorothioate
(Meta-systox R) and related compounds. Toxicol Appl Pharmacol,
4: 621-630.
Dzwonkowska A & Hübner H (1986) Induction of chromosomal aberration in
the Syrian hamster by insecticides tested in vivo. Arch Toxicol,
58: 152-156.
Ecker W (1978) Biotransformation of (ethylene-1-14C) demeton-S-methyl
in rats. Leverkusen, Germany, Bayer AG (Unpublished report No. 7458).
Ecker W & Cölln R (1983) Biotransformation of demeton-S-methyl in
rats. Leverkusen, Germany, Bayer AG (Unpublished report No. 11431).
Edson EF (1960) Agriculture chemicals - Applied toxicology of
pesticides. Pharm J, 185: 361-367.
Eiben R, Gröning P, & Löser E (1984) Generation study with rats
(two generations). Leverkusen, Germany, Bayer AG (Unpublished report
No. 13135).
EPPO/CoE (European & Mediterranean Plant Protection Organisation/
Council of Europe) (1993a) Decision making schemes for the
environmental risk assessment of plant protection products.
OEPP/EPPO Bull, 23: 1-54.
EPPO/CoE (European & Mediterranean Plant Protection Organisation/
Council of Europe) (1993b) Decision making schemes for the
environmental risk assessment of plant protection products.
OEPP/EPPO Bull, 24: 1-87.
FAO/WHO (1974) Demeton-S-methyl and related compounds. In: 1973
Evaluations of some pesticides in food. Geneva, World Health
Organization, pp 195-240 (WHO Pesticide Residue Series No. 3).
FAO/WHO (1990) Demeton-S-methyl and related compounds. In: Pesticides
residues in food - 1989. Evaluations: Part II - Toxicology. Rome, Food
and Agriculture Organization of the United Nations, pp 49-74 (FAO
Plant Production and Protection Paper 100/2).
FAO/WHO (1993) Demeton-S-methyl. In: Pesticides residues in food -
1992. Evaluations: Part I - Residues. Rome, Food and Agriculture
Organization of the United Nations, p 287 (FAO Plant Production and
Protection Paper 118).
Faul J (1984) [E 154 = 25/154 (demeton-S-methyl; Metasystox i-active
ingredient] Leverkusen, Germany, Bayer AG (Unpublished report)
(in German).
Flucke W (1984) [Tests on acute oral toxicity to quail (Coturnix
coturnix japonica).] Leverkusen, Germany, Bayer AG (Unpublished
report No. 13119) (in German).
Flucke W & Eben A (1988) E154 50 VL (C.N. demeton-S-methyl): Study of
effect on neuropathy target esterase (NTE) after oral administration
to the hen. Leverkusen, Germany, Bayer AG (Unpublished report
No. 17264).
Flucke W & Kaliner G (1988) E 154 50 VL (C.N. demeton-S-methyl): Study
for acute delayed neurotoxicity after oral administration to the hen.
Leverkusen, Germany, Bayer AG (Unpublished report No. 16920).
Flucke W & Kimmerle G (1977) Trichlorfon (Dipterex active ingredient),
demeton-S-methyl (Metasystox (I) active ingredient): Study for acute
toxicity after simultaneous administration of both active ingredients.
Leverkusen, Germany, Bayer AG (Unpublished report No. 7066).
Flucke W & Pauluhn J (1983) Metaisosystox 50 EC: Study for
formulation toxicity. Leverkusen, Germany, Bayer AG (Unpublished
report No. 11770).
Froebe Z, Drevenkar V, Stengl B, & Juracic M (1989) Sorption behaviour
of some organophosphorus pesticides in natural sediments. Toxicol
Environ Chem, 19: 69-82.
Funk W, Cleres L, Pitzer H, & Donnevert G (1989) Organophosphorus
insecticides: Quantitative HPTLC determination and characterization.
J Planar Chromatogr, 2: 285-289.
Ganzelmeier IH, Rautmann D, Spangenberg R, Streloke M, Herrmann M,
Wenzelburger HJ, & Walter HF (1995) Studies on the spray drift of
plant protection products: Results of a test program carried out
throughout the Federal Republic of Germany. Oxford, London, Edinburgh,
Melbourne, Blackwell Scientific Publications.
Grau R (1984) [Oral toxicity to female bird/canary (Serinus
canarius).] Leverkusen, Germany, Bayer AG (Unpublished report
No. VK-243) (in German).
Grau R (1985a) Fish toxicity (Metaisosystox 50% pre-solution).
Leverkusen, Germany, Bayer AG (Unpublished report No. FF-177).
Grau R (1985b) Fish toxicity (Metaisosystox 50% pre-solution, golden
orfe). Leverkusen, Germany, Bayer AG (Unpublished report No. FO-818).
Grau R (1990a) [Acute toxicity of Metaisosystox EC 25 to rainbow trout
(Oncorhynchus mykiss) using flow test.] Leverkusen, Germany, Bayer
AG (Unpublished report No. FF-285) (in German).
Grau R (1990b) [Acute toxicity of Metasystox EC 25 to golden orfe
(Leuciscus idus melanotus) using flow test.] Leverkusen, Germany,
Bayer AG (Unpublished report No. FO-1222) (in German).
Grau R (1990c) [Toxicity of Metasystox VL 70 to rainbow trout
(O ncorhynchus mykiss) with extensive exposure (21 days).]
Leverkusen, Germany, Bayer AG (Unpublished report No. FF-287)
(in German).
Hanna PJ & Dyer KF (1975) Mutagenicity of organophosphorus compounds
in bacteria and Drosophila. Mutat Res, 28: 405-420.
Heath DF & Vandekar M (1957) Some spontaneous reactions of
O,O-dimethyl S-ethylthioethyl phosphorothioate and related compounds
in water and on storage, and their effects on the toxicological
properties of the compounds. Biochem J, 67: 187-201.
Hecht G (1955) [Comparison tests of preparation 25/154, R 2170
and M 3/158.] Leverkusen, Germany, Bayer AG (Unpublished report)
(in German).
Hecht G (1960) [Toxicological investigations on Metasystox (i)
presentation given in Tokyo, Korinkaku.] Leverkusen, Germany, Bayer AG
(unpublished report) (in German).
Hegazy MR (1965) Poisoning by meta-isosystox in spraymen and in
accidentally exposed patients. Br J Ind Med, 22: 230-235.
Heimann KG (1987a) E 154 techn. (C.N. demeton-S-methyl): Study for
skin-sensitizing effect on guinea pigs. Leverkusen, Germany, Bayer AG
(Unpublished report No. 15896).
Heimann G (1987b) E 154 techn. (C.N. demeton-S-methyl): Study for
skin-sensitizing effect on guinea pigs in the epicutaneous test.
Leverkusen, Germany, Bayer AG (unpublished report No. 16106).
Heimbach F (1985a) Growth inhibition of green algae (Scenedesmus
subspicatus) caused by demeton-S-methyl (techn.). Leverkusen,
Germany, Bayer AG (Unpublished report No. HBF/AL11).
Heimbach F (1985b) Acute toxicity of demeton-S-methyl (techn.) to
water fleas. Leverkusen, Germany, Bayer AG (Unpublished report
No. HBF/DM 47).
Heimbach F (1985c) [Acute toxicity of demeton-S-methyl 50 VL to
water fleas.] Leverkusen, Germany, Bayer AG (Unpublished report
No. HBF/DM 48) (in German).
Heimbach F (1990a) Toxicity of Metasystox (I) to earthworms.
Leverkusen, Germany, Bayer AG (Unpublished report No. HBF/RG 120).
Heimbach F (1990b) Growth inhibition of green algae (Scenedesmus
subspicatus) by Metasystox (I). Leverkusen, Germany, Bayer AG
(Unpublished report No. HBF/AL 74).
Heimbach F (1990c) Influence of Metasystox (I) on the reproduction
rate of water fleas. Leverkusen, Germany, Bayer AG (Unpublished
report No. HBF/RDM 25).
Heimbach F (1990d) Influence of demeton-S-methyl VL 70 on the
reproduction rate of water fleas. Leverkusen, Germany, Bayer AG
(Unpublished report No. HBF/RDM 27).
Hellpointner E (1990) Assessment of the environmental half-life of
the direct photodegradation of demeton-S-methyl in water. Leverkusen,
Germany, Bayer AG (Unpublished report No. PF3446).
Herbold B (1979) E 25/154 Salmonella/microsome test to evaluate for
point mutation. Leverkusen, Germany, Bayer AG (Unpublished report
No. 8393).
Herbold B (1980a) E 25/154 Salmonella/microsome test to evaluate for
point mutation. Leverkusen, Germany, Bayer AG (Unpublished report
No. 8809).
Herbold B (1980b) E 25/154 micronucleus test on the mouse to evaluate
for mutagenic effect. Leverkusen, Germany, Bayer AG (Unpublished
report No. 8932).
Herbold B (1980c) E 25/154 (demeton-S-methyl) dominant lethal study on
male mouse to test for mutagenic effects. Leverkusen, Germany, Bayer
AG (Unpublished report No. 9336).
Herbold B (1983a) E 154 (demeton-S-methyl) pol test on E. coli to
evaluate for DNA damage. Leverkusen, Germany, Bayer AG (Unpublished
report No. 12152).
Herbold B (1983b) E 154 (demeton-S-methyl) sister chromatid exchange
in the bone marrow of the Chinese hamster in vivo to evaluate for
harmful effect on DNA. Leverkusen, Germany, Bayer AG (Unpublished
report No. 12323).
Hermann G (1974a) [Fish toxicity of Metasystox (I) to goldfish.]
Leverkusen, Germany, Bayer AG (Unpublished report No. FG-112)
(in German).
Hermann G (1974b) [Fish toxicity of Metasystox (I) to carp.]
Leverkusen, Germany, Bayer AG (Unpublished report No. FK-28)
(in German).
Hermann G (1974c) [Fish toxicity of Metasystox (I) to rudd.]
Leverkusen, Germany, Bayer AG (Unpublished report No. FR-29)
(in German).
Hill ARC, Wilkins JPG, Findlay NRI, & Lontay KEM (1984)
Organophosphorus sulphides, sulphoxides and sulphones - Part I:
Determination of residues in fruit and vegetables by gas-liquid
chromatography. Analyst, 109: 483-487.
Hoorn AJW (1982) Mutagenicity evaluation of E 154 50 VL (in xylene)
in the reverse mutation induction assay with Saccharomyces
cerevisiae strains S138 and S211a. Leverkusen, Germany, Bayer AG
(Unpublished report No. 2372).
Jones RD (1982) Organophosphorus poisoning at a chemical packaging
company. Br J Ind Med, 39: 377-381.
Kenaga E (1973) Factors to be considered in the evaluation of the
toxicity of pesticides to birds in the environment. Environ Qual Saf,
2: 166-181.
Kimmerle G (1972) Acute toxicity of SRA 3886 in combination with S 276
and with E 154 to rats. Leverkusen, Germany, Bayer AG (Unpublished
report No. 3438).
Klimmer OR (1964) [Determination of acute oral toxicity of Metasystox
(i).] Leverkusen, Germany, Bayer AG (Unpublished report) (in German).
Klimmer OR & Pfaff W (1955) [Toxicity tests on the new contact
insecticide O,O-dimethyl-thiophosphoric acid-O/ -S-ethylester
("Metasystox").] Arzneimittel-Forschung, 5: 584-587 (in German).
Klimmer R & Pfaff W (1958) [Toxicity tests during application of the
systemic insecticide O,O-dimethyl-(ethyl-thioethyl)-thiophosphoric
acid-ester.] Arzneimittel-Forschung, 8: 265-269 (in German).
Kniehase U (1984) [Effects of Metasystox (I) 250 EC on mite
(Phytoseiulus persimilis).] Leverkusen, Germany, Bayer AG
(Unpublished report No. ZDE/PP 4/84) (in German).
Krohn J (1984) Fate/behaviour of crop protection products in water:
demeton-S-methyl. Leverkusen, Germany, Bayer AG (Unpublished report
No. M2421).
Lewis PJ, Lowing RK, & Gomopertz D (1981) Automated discrete kinetic
method for erythrocyte acetylcholinesterase and plasma cholinesterase.
Clin Chem, 27: 926-929.
Mary ASR, Nagabhushanam R, & Sarojini R (1986) Toxicity evaluation of
organophosphorus and chlorinated hydrocarbon pesticides in freshwater
prawn Macrobrachium lamerrii. J Environ Biol, 7: 189-195.
Mitra PK, Sud SC, & Bahga HS (1978) Acute oral toxicity of Metasystox
in buffalo calves. Indian J Exp Biol, 16: 813-815.
Muley DV, Akarte SR, Kulkarni DA, & Rao KR (1987) Acute toxicity of
Metasystox to wedge clam, Donax cuneatus, from West Coast. Fish
Technol, 24: 27-30.
Owen M (1975) The management of grass swards for captive wildfowl. Int
Zoo Year Book, 16: 135-138.
Pandita TK (1983) Mutagenic studies on the insecticide Metasystox-R
with different genetic systems. Mutat Res, 124: 97-102.
Renhof M (1985) E 154 (C.N. demeton-S-methyl): Study for embryotoxic
effect on rats after oral administration. Leverkusen, Germany, Bayer
AG (Unpublished report No. 13237).
Schludecker D & Aderjan R (1988) [Chemical-toxicological results of a
suicidal poisoning with demeton-S-methyl.] Z Rechtsmed, 101:55-60
(in German).
Schmid E (1984) Degradation of demeton-S-methyl in soil. Leverkusen,
Germany, Bayer AG (Unpublished report No. Im1428).
Schmid ER (1986) Degradation of demeton-S-methyl in soil at a
concentration of 3 ppm under anaerobic conditions. Leverkusen,
Germany, Bayer AG (Unpublished report No. IM1579).
Schmidt WM & Bomhard E (1988) Study for carcinogenicity in NMRI mice
(administration in diet for up to 21 months). Leverkusen, Germany,
Bayer AG (Unpublished report No. 16812).
Schmidt WM & Westen H (1988) Study for chronic toxicity and
carcinogenicity in Wistar rats (administration in diet for up to 2
years). Leverkusen, Germany, Bayer AG (Unpublished report No. 16888).
Shires SW (1985) Effects of aerial applications of cypermethrin and
demeton-S-methyl on non-target arthropods of wheat. Ecotoxicol Environ
Saf, 10: 1-11.
Stone HM (1961) The anticholinesterase activity of demeton-S-methyl.
NZ J Sci, 4: 78-90.
Su MQ, Kinoshita FK, Frawley JP, & DuBois KP (1971) Comparative
inhibition of aliesterases and cholinesterase in rats fed eighteen
organophosphorus insecticides. Toxicol Appl Pharmacol, 20: 241-249.
Tarrant KA, Thompson HM, & Hardy AR (1992) Biochemical and
histological effect of the aphicide demeton-S-methyl on house sparrows
(Passer domesticus) under field conditions. Bull Environ Contam
Toxicol, 48: 360-366.
Thompson HM, Walker CH, & Hardy AR (1991) Changes in activity of avian
serum esterases following exposure to organophosphorus insecticides.
Arch Environ Contam Toxicol, 20: 514-518.
Thornton JS, Olson TJ, & Wagner K (1977) Determination of residues
of Metasystox-R and metabolite in plant and animal tissues and soil.
J Agric Food Chem, 25: 573-576.
Thyssen J (1981) [Tests on skin and mucous-irritating effects of
demeton-S-methyl.] Leverkusen, Germany, Bayer AG (Unpublished report)
(in German).
Vandekar M (1958) The toxic properties of demeton-methyl
("Metasystox") and some related compounds. Brit J Ind Med,
15: 158-167.
Vasilic Z, Drevenkar V, Froebe Z, Stengl B, & Tkalcevic B (1987) The
metabolites of organophosphorus pesticides in urine as an indicator of
occupational exposure. Toxicol Environ Chem, 14:11-127.
Verma SP, Tonk IP, Gupta AK, & Saxena M (1984) Evaluation of an
application factor for determining the safe concentration of
agricultural and industrial chemicals. Water Res, 18(1): 111-115.
Wagner K & Oehlmann L (1987) Studies on the metabolic behaviour of
demeton-S-methyl in wheat. Leverkusen, Germany, Bayer AG (Unpublished
report No. PF2701).
Wagner K & Thornton JS (1977) Method for the gas-chromatographic
determination of Metasystox (i) and Metasystox R residues in plants,
soil and water. Pflanzenschutz Nachr Bayer, 30: 1-17.
Wagner K, Schütz U, & Oehlmann L (1985) Degradation of Metasystox-(i)
in soil under aerobic, anaerobic and sterile test conditions.
Leverkusen, Germany, Bayer AG (Unpublished report No. PF2565).
Weber H, Patzschke K, & Wagner LA (1978) (14C), demeton-S-methyl
(Metasystox in active ingredient)/Biokinetic studies on rats.
Leverkusen, Germany, Bayer AG (Unpublished report No. 7491).
Westlake GE, Hardy AR, & Stevenson JH (1985) Effects of storage and
pesticide treatments on honey bee brain acetyl cholinesterase
activities. Bull Environ Contam Toxicol, 34: 668-675.
WHO (1996) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1996-1997. Geneva, World Health
Organization, International Programme on Chemical Safety
(WHO/PCS/96.3).
Wilkins JPG, Hill ARC, & Lee DF (1985) Organophosphorus sulphides,
sulphoxide and sulphones - Part 2: Characterisation by gas
chromatography-mass spectrometry. Analyst, 110: 1045-1051.
Wilmes R (1984) Orientating light stability: demeton-S-methyl.
Leverkusen, Germany, Bayer AG (Unpublished report No. IM1408).
Winkler KO & Arent H (1970) Medical observation of personnel occupied
with spraying in hop cultivation during the application of the organic
phosphoric acid esters mevinphos and demeton-S-methyl (Metasystox i).
Leverkusen, Germany, Bayer AG (Unpublished report No. 24618).
Xue SZ, Ding XJ, & Ding Y (1985) Clinical observation and comparison
of the effectiveness of several oxime cholinesterase reactivators.
Scand J Work Environ Health, 11(Suppl 4): 46-48.
Ziegler W, Engelhardt G, Wallnöfer PR, Oehlmann L, & Wagner K (1980)
Degradation of demeton-S-methyl sulfoxide (Metasystox R) by soil
microorganisms. J Agric Food Chem, 28: 1102-1106.
RÉSUMÉ ET ÉVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Identité, propriétés physiques et chimiques
Le déméton-S-méthyl se présente sous la forme d'un liquide
huileux jaune pâle à l'odeur pénétrante. C'est un organophosphoré
agissant par la voie générale et par contact qui est utilisé comme
insecticide et acaricide sur les fruits, les légumes, les céréales et
les plantes ornementales pour lutter contre Acarina, Thysanoptera,
Hymenoptera et Homoptera. Sa tension de vapeur est de 63,8 mPa à
20°C et il est facilement soluble dans la plupart des solvants
organiques. Il est très soluble dans l'eau (3,3 g/litre à la
température ambiante) et son coefficient de partage entre l'octanol et
l'eau (log Pow) est de 1,32. Le déméton-S-méthyl est stable dans les
solvants non aqueux.
Pour la recherche et le dosage des résidus et l'analyse des
échantillons prélevés dans l'environnement, on procède à une
extraction au moyen d'un solvant organique, suivie d'une oxydation de
la sulfone correspondante. Le dosage s'effectue ensuite par
chromatographie en phase gazeuse avec un détecteur spécifique du
phosphore.
1.2 Sources d'exposition humaine et environnementale
Jusqu'en 1957, le déméton-méthyl a été commercialisé sous la
forme d'un mélange de deux isomères, le déméton-S-méthyl et le
déméton-O-méthyl. La formulation commerciale est un concentré
émulsionnable que l'on utilise en pulvérisations sur les céréales, les
fruits, les légumes et les plantes ornementales. On est en train de le
remplacer par l'oxydéméton-méthyl qui est un métabolite produit par
les plantes et les mammifères ou qui résulte du séjour du
déméton-S-méthyl dans le sol.
1.3 Transport, distribution et transformation dans l'environnement
La décomposition par hydrolyse du déméton-S-méthyl dépend du pH
de la solution. A 22°C, sa demi-vie est de 56 jours à pH 7 et de 8
jours à pH 9. Dans le sol, sa voie de décomposition principale est la
biodégradation, avec une demi-vie d'environ 4 h. Au bout de 24 h, il y
a encore dans le sol une proportion d'oxydéméton-méthyl qui représente
20 à 30% de la dose initiale de déméton-S-méthyl. Le coefficient de
sorption dans le sol (Kd) du déméton-S-méthyl est de 0,68 à 2,66,
selon la composition du sol.
Dans l'environnement, l'une des principales voies de dégradation
du composé est la photolyse. La métabolisation par le blé de printemps
est rapide et elle analogue à celle qui se produit dans le sol et chez
les mammifères.
1.4 Niveaux d'exposition environnementale et humaine
La population générale est principalement exposée au
déméton-S-méthyl par l'intermédiaire des résidus qui subsistent sur
les cultures vivrières. La réunion conjointe FAO/OMS sur les résidus
de pesticides (JMPR) a recommandé une dose journalière admissible
(DJA) de 0,0003 mg/kg de poids corporel. Il s'agit d'une DJA
collective qui englobe le déméton-S-méthyl, l'oxydéméton-méthyl et la
déméton-S-méthylsulfone, étant donné que les méthodes habituelles
d'analyse ne distinguent pas ces différents composés. Il est arrivé
qu'une exposition excessive au déméton-S-méthyl par la voie
transcutanée entraîne une intoxication de type cholinergique chez des
ouvriers mal protégés qui procédaient au conditionnement du concentré.
En revanche, des volontaires qui s'étaient prêtés à l'épandage
simulé d'un mélange de déméton-S-méthyl et de déméton-O-méthyl (30 et
70% respectivement) et avaient été exposés à des concentrations de 8,8
à 27 mg/m3 des deux matières actives, n'ont présenté aucune réduction
de leur activité cholinestérasique plasmatique ou érythrocytaire.
1.5 Cinétique et métabolisme
Chez le rat, le déméton-S-méthyl est rapidement et presque
complètement résorbé au niveau de l'intestin et il se répartit
uniformément dans les tissus (exception faite des hématies dans
lesquelles il se concentre plus fortement). Il est rapidement
métabolisé et excrété dans les urines. Sa concentration sanguine
diminue avec une demi-vie qui est initialement de 2 h environ. Au
bout de 24 h, il reste environ 1% de la dose dans l'organisme. La
principale voie métabolique du déméton-S-méthyl consiste en une
oxydation de la chaîne latérale qui conduit à la formation du
sulfoxyde correspondant (oxydéméton-méthyl) et, dans une moindre
proportion, après une oxydation plus poussée, à la sulfone.
L'O-déméthylation constitue une autre voie métabolique importante.
1.6 Effets sur les animaux de laboratoire et les systèmes d'épreuve
in vitro
1.6.1 Exposition unique
Le déméton-S-méthyl provoque des intoxications de type
cholinergique. Chez les mammifères, la DL50 varie de 7 à 100 mg/kg
p.c. selon la voie d'administration et l'espèce.
1.6.2 Exposition de courte durée
Une des premières études, comportant une exposition par la voie
alimentaire, a montré que des rats qui recevaient le composé à raison
de 50 mg/kg de nourriture, avaient une activité cholonestérasique
cérébrale sensiblement réduite au bout de 26 semaines de ce régime.
Des signes d'intoxication de type cholinergique ont été observés au
bout de 5 semaines de traitement chez des rats recevant 200 mg de
composé par kg de nourriture.
Lors d'une étude d'alimentation d'un an effectuée sur des chiens,
on a obtenu une dose sans effet nocif observable (NOAEL), basée sur
les effets cholinergiques, de 1 mg/kg de nourriture, soit l'équivalent
quotidien de 0,036 mg/kg de poids corporel.
1.6.3 Exposition de longue durée
Des souris ont reçu pendant 21 mois des rations contenant
respectivement 0, 1, 15 ou 75 mg de déméton-S-méthyl par kg de
nourriture. La NOAEL, a été trouvée égale à 1 mg/kg de nourriture,
soit l'équivalent quotidien de 0,24 mg/kg de poids corporel en prenant
comme base l'inhibition de la cholinesterase cérébrale.
Chez des rats ayant reçu une alimentation contenant
respectivement 0, 1, 7 ou 50 mg/kg de déméton-S-méthyl, la NOAEL,
basée sur l'inhibition de la cholinestérase cérébrale, s'est révélée
égale à 1 mg/kg de nourriture, soit l'équivalent quotidien de
0,05 mg/kg p.c.
Chez aucune espèce il n'a été constaté d'augmentation de
l'incidence des tumeurs.
1.6.4 Irritation et sensibilisation cutanée ou oculaire
Le déméton-S-méthyl est légèrement irritant pour l'oeil et la
peau. Le test de Magnusson et Klingman a donné des résultats positifs
chez des cobayes. En revanche, le test de sensibilisation de Buehler
(application d'un timbre cutané) n'a pas fourni d'indice d'une
sensibilisation cutanée, ce qui donne à penser que, dans la pratique,
l'usage du déméton-S-méthyl ne devrait pas poser de problème de
sensibilisation.
1.6.5 Effets sur la reproduction, embryotoxicité et tératogénicité
Lors d'une étude sur deux générations de rats, au cours de
laquelle les animaux ont été exposés au composé par la voie
alimentaire, on a constaté une réduction de la viabilité et du poids
corporel des ratons (uniquement la génération F1b) à la dose de
5 mg/kg de nourriture. La NOAEL était de 1 mg/kg de nourriture, soit
l'équivalent quotidien de 0,07 mg/kg de poids corporel.
Le déméton-S-méthyl ne s'est révélé ni embryotoxique, ni
tératogène chez le rat et le lapin.
1.6.6 Mutagénicité et points apparentés d'aboutissement des effets
toxiques
Le déméton-S-méthyl produit des mutations ponctuelles
in vitro. On n' a mis en évidence des effets chromosomiques
in vivo qu'avec des formulations du commerce. Les données
disponibles sont insuffisantes pour que l'on puisse procéder à une
évaluation satisfaisante du pouvoir génotoxique de ce composé.
1.6.7 Neurotoxicité retardée
Le déméton-S-méthyl n'a produit ni polyneuropathie, ni inhibition
de l'estérase cible correspondante (NTE) lorsqu'on l'a administré à
des poules en dose égale à la DL50 par voie orale.
1.6.8 Toxicité des métabolites
Deux métabolites du déméton-S-méthyl produits par des
plantes et des mammifères, à savoir l'oxydéméton-méthyl et la
déméton-S-méthylsulfone, sont également des pesticides du commerce et
ont été très largement étudiés. Il ressort de ces travaux que le
profil toxicologique de ces composés ne diffère pas sensiblement,
quantitativement ou qualitativement, de celui du déméton-S-méthyl.
1.7 Mécanisme de la toxicité - Mode d'action
Le déméton-S-méthyl est un inhibiteur direct de la cholinestérase
et sa toxicité est liée au fait qu'il inhibe l'acétylcholinestérase
(AChE) au niveau des terminaisons nerveuses. Une fois inhibée par le
déméton-S-méthyl, l'acétylcholinestérase se réactive spontanément avec
une demi-vie in vitro de 1,3 h environ, comme on peut s'y attendre
pour une AChE diméthylphosphorylée.
1.8 Effets sur l'homme
On a signalé quelques cas d'intoxication aiguë avec syndrome
cholinergique, consécutifs à des tentatives de suicide. Aucun effet
retardé n'a été observé chez les survivants, dont une femme enceinte.
A la suite d'une exposition subie par négligence lors du
conditionnement de formulations du commerce, quelques employés ont
présenté des troubles d'origine cholinergique qui ont nécessité un
traitement pharmacologique. Le composé avait probablement pénétré par
la voie transcutanée. De même, il est possible que des conditions de
travail laissant à désirer aient entraîné une absorption excessive de
déméton-S-méthyl au cours de l'épandage de ce composé sur des champs
de coton.
1.9 Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Pour les algues vertes, la CE50 à 96 h va de 8 à 37 mg/litre. On
a mesuré une CL50 allant de 0,004 à 1,3 mg/litre chez une série
d'invertébrés aquatiques. Chez les poisson, la toxicité est variable,
avec une CL50 à 96 h qui peut aller de 0,59 mg/litre pour la truite
arcen-ciel à environ 40 mg/litre pour l'orfe, le cyprin doré et la
carpe.
Chez la caille japonaise et le canari, on a obtenu une DL50
(effet aigu, voie orale) de 10-50 mg/kg de poids corporel. Chez des
étourneaux, une dose unique par voie orale de 2 mg/kg p.c. a provoqué
une inhibition de l'acétylcholinestérase cérébrale 3 h après le
traitement.
Pour les lombrics terricoles, la CL50 du déméton-S-méthyl est de
66 mg/kg sur 14 jours. Pour les abeilles, on a obtenu des valeurs de
la DL50 de contact et par voie orale (effet aigu) respectivement
égales à 0,21 et 0,6 par insecte. En traitant du blé d'hiver à la dose
recommandée, on a constaté une réduction sensible du nombre
d'invertébrés présents sur les feuilles (principalement des diptères
du genre Empididae) sans effet sur les invertébrés entomophages
présents à la surface du sol.
2. Conclusions
Le déméton-S-méthyl est un insecticide organophosphoré fortement
toxique (classe Ib de la classification de l'OMS) (OMS, 1966). Son
action toxique s'explique par l'inhibition de l'acétylcholinestérase
au niveau des terminaisons nerveuses. L'exposition de la population
générale résulte principalement des résidus présents dans les denrées
alimentaires provenant des cultures traitées.
Moyennant de bonnes pratiques agricoles et le respect des règles
d'hygiène et de sécurité, la manipulation du composé lors de sa
fabrication ou de son épandage ne devrait entraîner aucun effet
indésirable. Des effets résultant d'une exposition chronique sont
improbables.
Le déméton-S-méthyl ne persiste pas dans l'environnement et il ne
s'accumule pas chez les êtres vivants. Il est très toxique pour les
invertébrés aquatiques et présente une toxicité notable pour les
poissons et les oiseaux, aussi doit on considérer que pour ces
organismes, le facteur de risque est élevé ou modéré. Néanmoins, on
n'a pas signalé de mortalité importante dans la nature du fait de ce
composé. Des précautions sont à prendre pour réduire au minimum
l'exposition des organismes non visés (par ex. ne pas le pulvériser
sur des étendues d'eau et veiller à ce que les gouttelettes
d'insecticide ne soient pas entraînées vers les zones à respecter).
3. Recommandations
Afin de protéger la santé et le bien-être des travailleurs et de
la population dans son ensemble, il ne faut confier la manipulation et
l'épandage du déméton-S-méthyl qu'à des opérateurs dûment formés et
encadrés, qui auront le souci de respecter les mesures de sécurité et
d'épandre le produit selon les règles.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
1.1 Identidad, propiedades físicas y químicas, y métodos analíticos
El demeton-S-metilo, un líquido oleoso de color amarillo pálido y
olor penetrante, es un insecticida y acaricida organofosfatado
sistémico y de contacto que se utiliza para combatir Acarina,
Thysanoptera y Homóptera en frutas, cereales, plantas ornamentales
y hortalizas. Tiene una presión de vapor de 63,8 mPa a 20°C, es
fácilmente soluble en la mayoría de los disolventes orgánicos, tiene
una gran solubilidad en agua, de 3,3 g/litro a temperatura ambiente, y
un coeficiente de reparto octanol/agua (log Pow) de 1,32. El
demeton-S-metilo es estable en disolventes no acuosos.
Los análisis de residuos y ambientales se realizan por extracción
con un disolvente orgánico y oxidación a la sulfona correspondiente.
Las mediciones se efectúan por cromatografía de gases utilizando un
detector específico del fósforo.
1.2 Fuentes de exposición humana y ambiental
Antes de 1957, el metildemeton se comercializaba como mezcla de
los isómeros demeton-S-metilo y demeton-O-metilo.
El demeton-S-metilo se utiliza desde 1957. Su presentación es la
de un concentrado emulsionable y se aplica por rociamiento a los
cereales, frutas, plantas ornamentales y hortalizas. Se está
sustituyendo por el oxidemetonmetilo, que es un metabolito del
demeton-S-metilo producido en las plantas, el suelo y los mamíferos.
1.3 Transporte, distribución y transformación en el medio ambiente
La degradación hidrolítica del demeton-S-metilo depende del pH de
la solución; a 22°C la semivida es de 63 días con un pH de 4, de 56
días con un pH de 7 y de 8 días con un pH de 9. La principal vía de
degradación en el suelo es la biodegradación. La semivida del
demeton-S-metilo en el suelo es de unas 4 horas. Sin embargo, al cabo
de 24 horas, el oxidemetonmetilo representa aún el 20-30 % de la dosis
aplicada de demeton-S-metilo. El coeficiente de sorción (Kd) del
demeton-S-metilo en el suelo oscila entre 0,68 y 2,66, dependiendo de
la composición del suelo.
La fotolisis no es uno de los principales mecanismos de
degradación del demeton-S-metilo en el medio ambiente.
Su metabolismo en el trigo de primavera es rápido y similar al
que se produce en el suelo y en los mamíferos.
1.4 Niveles ambientales y exposición humana
La exposición primaria de la población humana en general se
produce a través de los residuos de demeton-S-metilo en los cultivos
alimentarios. En la reunión conjunta FAO/OMS sobre residuos de
plaguicidas (JMPR) se recomendó una ingesta diaria admisible (IDA) de
0,0003 mg/kg de peso corporal. Se trata de una IDA colectiva para el
demeton-S-metilo, el oxidemetonmetilo y el demeton-S-metilsulfona, ya
que los métodos habituales de análisis no diferencian entre estos tres
compuestos.
La exposición excesiva al demeton-S-metilo y su absorción cutánea
produjeron toxicidad colinérgica en trabajadores insuficientemente
protegidos durante el envasado de la formulación concentrada.
Tras haber expuesto a voluntarios participantes en una simulación
de rociamiento con una mezcla de demeton-S-metilo y demeton-O-metilo
(30 y 70% respectivamente) a 8,8-27 mg/m3 de ambos componentes
activos combinados, no se observaron efectos adversos en la actividad
de la colinesterasa en el plasma ni en los eritrocitos.
1.5 Cinética y metabolismo
El demeton-S-metilo se absorbe rápida y casi completamente por el
tracto intestinal en las ratas y se distribuye uniformemente a los
tejidos (excepto una concentración elevada en los eritrocitos). Se
metaboliza rápidamente y se excreta por orina. La concentración en la
sangre disminuye, con una semivida inicial de unas 2 horas.
Aproximadamente el 1% de la dosis oral sigue presente en el organismo
24 horas después del tratamiento. La principal ruta metabólica del
demeton-S-metilo en las ratas es la oxidación de la cadena lateral,
que da lugar a la formación del sulfóxido correspondiente
(oxidemetonmetilo) y, en menor medida, a la sulfona, tras una
oxidación mayor. Otra ruta metabólica importante es la O-demetilación.
1.6 Efectos en mamíferos de laboratorio y en sistemas de ensayo
in vitro
1.6.1 Exposición única
El demeton-S-metilo produce toxicidad colinérgica. Los valores de
la DL50 para los mamíferos oscilan entre 7 y 100 mg/kg de peso
corporal, dependiendo de la vía de administración y de la especie.
1.6.2 Exposición breve
Un estudio alimentario inicial reveló que las ratas alimentadas
con 50 mg de demeton-S-metilo por kg de la dieta habían sufrido una
reducción notable de la actividad de la colinesterasa cerebral y
eritrocitaria tras una exposición de 26 semanas. Se observaron
síntomas colinérgicos en ratas alimentadas con 200 mg/kg de la dieta
durante las 5 primeras semanas de exposición.
En un estudio alimentario de un año de duración efectuado en
perros se estableció un nivel sin efectos adversos observados (NOAEL)
de 1 mg/kg de la dieta (equivalente a 0,036 mg/kg de peso corporal por
día) sobre la base de los efectos de la colinesterasa cerebral.
1.6.3 Exposición prolongada
Se administró durante 21 meses a ratones una dieta que contenía
0, 1, 15 ó 75 mg/kg de demeton-S-metilo. El NOAEL, determinado sobre
la base de la inhibición de la colinesterasa cerebral, fue de 1 mg/kg
de la dieta (equivalente a 0,24 mg/kg de peso corporal por día).
En ratas cuya dieta contenía 0, 1, 7 ó 50 mg/kg de demeton-S-metilo,
el NOAEL, determinado sobre la bases de la inhibición de la
colinesterasa cerebral, fue de 1 mg/kg de la dieta (equivalente a
0,05 mg/kg de peso corporal por día).
La incidencia de tumores no aumentó en ninguna de esas especies.
1.6.4 Irritación y sensibilización de la piel y los ojos
El demeton-S-metilo es un irritante cutáneo y ocular leve. La
prueba de maximización de Magnusson y Klingman dio resultados
positivos en cobayos. Sin embargo, la prueba del parche cutáneo de
Buehler no mostró indicios de sensibilización cutánea, lo que parece
indicar que el uso práctico del demeton-S-metilo no debería acarrear
problemas de sensibilización.
1.6.5 Reproducción, embriotoxicidad y teratogenicidad
En un estudio alimentario llevado a cabo en dos generaciones de
ratas, se observó una reducción de la viabilidad y del peso corporal
de los cachorros (generación F1b solamente) con una dosis de 5 mg de
demeton-S-metilo por kg de la dieta. El NOAEL fue de 1 mg/kg de la
dieta, equivalente a 0,07 mg/kg de peso corporal por día.
El demeton-S-metilo no resultó embriotóxico ni teratogénico en
ratas ni en conejos.
1.6.6 Mutagenicidad y parámetros conexos
El demeton-S-metilo produce mutaciones puntuales in vitro. Se han
demostrado efectos cromosómicos in vivo con formulaciones comerciales
solamente. La información disponible es insuficiente para realizar una
evaluación adecuada del potencial genotóxico del demeton-S-metilo.
1.6.7 Neurotoxicidad retardada
El demeton-S-metilo no causó polineuropatías retardadas ni
inhibición de la Esterasa Diana de Neuropatía en pruebas con gallinas
a un nivel igual a la DL50 oral.
1.6.8 Toxicidad de los metabolitos
Las plantas y los mamíferos producen dos metabolitos del
demeton-S-metilo (el oxidemetonmetilo y el demeton-S-metilsulfona) que
también son plaguicidas comerciales y han sido ampliamente estudiados.
Se ha señalado de que el perfil toxicológico de estos dos compuestos
no difiere de manera significativa, cuantitativa ni cualitativamente,
del demeton-S-metilo.
1.7 Mecanismo de toxicidad: modalidad de acción
El demeton-S-metilo es un inhibidor indirecto de la colinesterasa
y su toxicidad que está relacionada con la inhibición de la
acetilcolinesterasa en las terminales nerviosas. La
acetilcolinesterasa inhibida por el demeton-S-metilo se reactiva
espontáneamente, con una semivida in vitro de aproximadamente
1,3 horas, como cabe esperar en la acetilcolinesterasa dimetil
fosforilada.
1.8 Efectos en el ser humano
Se han notificado varios casos de intoxicación aguda con
síndrome colinérgico tras intentos de suicidio. Los pacientes que
sobrevivieron, inclusive una mujer embarazada, no presentaron efectos
retardados.
Tras una exposición laboral por inatención durante el envasado de
la formulación comercial, algunos trabajadores desarrollaron una
toxicidad colinérgica que precisó tratamiento farmacológico. El
demeton-S-metilo se absorbió probablemente a través de la piel.
Análogamente, condiciones de trabajo inapropiadas podrían haber sido
la causa de una absorción cutánea excesiva durante la aplicación del
demeton-S-metilo en algodonales.
1.9 Efectos en otros organismos en el laboratorio y sobre el terreno
Las CE50 96-h para las algas verdes se sitúan entre 8 y
37 mg/litro. Las CL50 para diversos invertebrados acuáticos se
encuentran entre 0,004 y 1,3 mg/litro. La toxicidad para los peces
varía; la CL50 96-h oscila entre 0,59 mg/litro para la trucha
arcoiris y unos 40 mg/litro para Leuciscus idus, Carassius auratus
y la carpa.
La DL50 oral aguda para la codorniz japonesa y el canario es de
10-50 mg/kg de peso corporal. En el estornino, una dosis oral única de
2 mg/kg de peso corporal produjo inhibición del 20% de la AChE
cerebral 3 horas después del tratamiento.
La CL50 del demeton-S-metilo en el suelo para las lombrices
de tierra es de 66 mg/kg para un período de 14 días. La DL50 aguda
oral y de contacto del demeton-S-metilo fue de 0,21 y 0,6 g/abeja
respectivamente. Después de haber sido aplicado al trigo de invierno
según la proporción propuesta, el demeton-S-metilo redujo
significativamente el número de invertebrados en el follaje de los
cultivos (principalmente Empididae), pero no el número de
invertebrados entomófagos de la superficie del suelo.
2. Conclusiones
El demeton-S-metilo es un éster organofósforado sumamente tóxico
(pertenece a la clase Ib de la clasificación de la OMS) (OMS, 1996)
utilizado como insecticida. La acción tóxica consiste en la inhibición
de la acetilcolinesterasa en las terminales nerviosas. La exposición
de la población en general se produce principalmente a causa de los
residuos presentes en los productos agrícolas.
Con buenas prácticas laborales y medidas de higiene y de
seguridad, el demeton-S-metilo no debería producir efectos adversos
durante la fabricación o la aplicación. Es poco probable que aparezcan
efectos debidos a la exposición crónica.
El demeton-S-metilo no es persistente en el medio ambiente y no
se acumula en los organismos. Tiene una toxicidad aguda elevada para
los invertebrados acuáticos y es tóxico para los peces y las aves,
llevando aparejados factores de riesgo agudo o moderado elevados para
esos organismos. Sin embargo, no se han comunicado muertes masivas
significativas de organismos en el campo a causa de este compuesto.
Habría que tomar precauciones para reducir al mínimo la exposición de
organismos no combatidos (por ejemplo, no rociar sobre masas de agua y
reducir al mínimo la exposición por desviación del rociado).
3. Recomendaciones
Para la salud y el bienestar de los trabajadores y del público en
general, el manejo y la aplicación del demeton-S-metilo deberían
encomendarse exclusivamente a personal bien adiestrado y supervisado
que aplique las medidas de seguridad necesarias y buenas prácticas de
aplicación.
(a) Spray drift from ground-based applications
The acute PEC for spray drift (1 m from the edge of the spray
boom into a 30-cm-deep static water body at the maximum application
rate (see PEC assumptions above) is 0.005 mg/litre. Therefore, the
TERs based on this PEC and the above LC/EC50 toxicity values are:
fish, 110; aquatic invertebrates, 0.8 (4.1 for Daphnia); and algae,
1500. Based on the EPPO/CoE risk assessment scheme for aquatic
organisms, these TERs indicate a low acute risk to fish and algae.
However, for aquatic invertebrates the TER is less than 10, indicating
a potential risk to aquatic invertebrates (both molluscs and
arthropods). In such risk situations the use of a "no-spray"
restriction zone next to surface waters may reduce the risk to such
aquatic invertebrates. For example, arable spray drift at 5 m from the
edge of boom is 0.6% (Ganzelmeier et. al., 1995). Based on this 5 m
drift data, the PEC is 0.0006 mg/litre and results in a 5 m TER of 7
for molluscs and 37 for Daphnia. The TER for the most sensitive
invertebrate (mollusc) is still below the EPPO trigger of 10,
indicating a borderline risk. However, the Daphnia 5 m TER, which is
above 10, indicates that the use of a 5 m "no-spray" restriction zone
next to surface waters would help reduce the acute risk to aquatic
invertebrates.
(b) Spray drift from broadcast air-assisted top fruit applications
The acute PEC for spray drift (3 m from the point of application
into a 30-cm-deep static water body at the maximum application rate
(see PEC assumptions above) is 0.049 mg/litre. Therefore, the TERs
based on this PEC and the above LC/EC50 toxicity values are: fish,
12; aquatic invertebrates, 0.09 (0.4 for Daphnia); and algae, 163.
Based on the EPPO/CoE risk assessment scheme for aquatic organisms,
these TERs indicate a low acute risk to fish and algae. However, for
aquatic invertebrates the TER is less than 1 indicating a high risk to
aquatic invertebrates (both molluscs and arthropods). In such risk
situations the use of a "no-spray" restriction zone next to surface
waters may reduce the risk to such aquatic invertebrates. For example,
spray drift from broadcast air-assisted applications to top fruit at
15 m from point of application is 6.0% (Ganzelmeier et al., 1995).
Based on this 15 m drift data, the PEC is 0.006 mg/litre and results
in a 15 m TER of 0.7 for molluscs and 3.4 for Daphnia. These TERs at
15 m for the most sensitive invertebrates are still below the EPPO
trigger of 10, indicating a potential risk. Therefore, even with a 15
"no-spray" restriction zone next to surface waters, there is still a
potential risk to aquatic invertebrates from the broadcast
air-assisted use of demeton-S-methyl. Table 8 summarizes the acute
TERs for demeton-S-methyl to aquatic organisms at 1 m for arable and
3 m for broadcast air-assisted applications.
Table 8. Acute toxicity-exposure ratios (TER) for aquatic organisms at 1 m for arable and 3 m for broadcast air-assisted
applications
Species LC/EC50 PEC (mg/litre) at 1 m PEC (mg/litre) at 3 m TER TER
(mg/litre) (arable) (air assisted) (arable) (air assisted)
Fish 0.59 0.005 0.049 110 12
(Oncorhynchus mykiss)
Aquatic invertebrate 0.0042 0.005 0.049 0.8 0.09
(Paphia laterisulca)
Aquatic invertebrate 0.022 0.005 0.049 4.1 0.4
(Daphnia magna)
Alga 8.0 0.005 0.049 1500 163
(Scenedesmus subspicatus)
10.2.1.2 Chronic risk
No chronic toxicity data are available for fish. However, a
chronic NOEC of 0.0056 mg/litre has been reported for Daphnia
magna. There are no data reporting the persistence or degradation of
demeton-S-methyl in water, and so a chronic PEC could not be derived.
Therefore, owing to the lack of data on chronic fish toxicity and
environmental fate in water, it is not possible to assess the chronic
risk to either fish or aquatic invertebrates. There are also no data
available on either the toxicity of demeton-S-methyl to sediment-
dwelling invertebrates or fate of demeton-S-methyl in aquatic
sediments. Therefore, the risk to sediment-dwelling invertebrates
could also not be assessed. No fish bioaccumulation data are
available, but, as the log Pow is 1.3 (i.e. < 3), the risk of
bioaccumulation in fish should be low.
10.2.2 Terrestrial organisms
Vertebrates are likely to be exposed to demeton-S-methyl from
either grazing on treated vegetation or consuming contaminated
insects. For this risk assessment, typical application rates of
0.32 kg/ha are used for ground spray application on arable crops and
0.49 kg/ha for application by air-assisted spraying for fruit.
10.2.2.1 Birds
The lowest reported acute oral LD50 for birds is 10 mg/kg body
weight for the canary. Dietary data are not available.
Indicator birds for use in the risk assessment are:
* Greylag goose (Anser anser), as a grazing species, with a body
weight of 3 kg and total daily food consumption of 900 g
vegetation (dry weight) (Owen, 1975)
* Blue tit (Parus caeruleus), as an insectivorous species, with a
body weight of 11 g and total daily food consumption of 8.23 g
(dry weight) (Kenaga, 1973)
a) Grazing birds
Initial residues on short grass or cereal shoots are estimated to
be 35.8 mg/kg dry weight (based on 112 × application rate) arising
from application at 0.32 kg/ha to arable crops (EPPO/CoE, 1993a,b) and
55 mg/kg from application to fruit at a rate of 0.49 kg/ha. This gives
an estimated total oral intake for the goose of 32.2 mg and 49.5 mg
for the two application rates assuming that the goose ate exclusively
food contaminated at this level. This is equivalent to a daily intake
of 10.7 and 16.5 mg/kg body weight, respectively. TERs can be
calculated as follows:
End-point LD50/LC50 Application rate Predicted TER
(mg/kg b.w.) (kg/ha) concentration
in food (mg/kg)
Bird acute 10 0.32 (arable) 35.8 0.93
oral (Canary) 0.49 (fruit) 55 0.61
The calculated TER values fall well below the EPPO/CoE trigger
values for concern (TER <10) and indicate a high risk to grazing
birds.
b) Insectivorous birds
Initial residues on small insects are estimated to be 9.3 mg/kg
dry weight (based on 29 × application rate) arising from application
at 0.32 kg/ha to arable crops (EPPO/CoE, 1993a,b) and 14.2 mg/kg from
application to fruit at a rate of 0.49 kg/ha. This gives an estimated
total oral intake for the blue tit of 0.08 mg and 0.12 mg for the two
application rates assuming that the blue tit ate exclusively food
contaminated at this level. This is equivalent to a daily intake of
6.94 and 10.6 mg/kg body weight, respectively. TERs can be calculated
as follows:
End-point LD50/LC50 Application rate Predicted TER
(mg/kg b.w.) (kg/ha) concentration
in food (mg/kg)
Bird acute 10 0.32 (arable) 9.3 1.44
oral (Canary) 0.49 (fruit) 14.2 0.94
The TERs for acute toxicity to insectivorous birds are
substantially less than the trigger value of <10, indicating high
acute risk to these birds.
10.2.2.2 Mammals
The lowest reported acute oral LD50 for laboratory mammals is
63 mg/kg body weight for the rat.
Indicator mammals for use in the risk assessment will be:
* Rabbit (Oryctolagus cuniculus), as a grazing mammal, with a
body weight of 1200 g and a total daily food consumption of 500 g
vegetation (dry weight) (Ross, personal communication to the
IPCS).
* Shrew (Sorex araneus), as an insectivorous mammal, with a body
weight of 18 g and a total daily food consumption of 18 g
(Churchfield, 1986)
a) Grazing mammals
Initial residues on short grass or cereal shoots are estimated to
be 35.8 mg/kg dry weight (based on 112 × application rate) arising
from application at 0.32 kg/ha to arable crops (EPPO/CoE, 1993a,b) and
at 55 mg/kg from application to fruit at a rate of 0.49 kg/ha. This
gives an estimated total oral intake for the rabbit of 17.9 mg and
27.4 mg for the two application rates, assuming that the rabbit ate
exclusively food contaminated at this level. This is equivalent to a
daily intake of 15 and 23 mg/kg body weight, respectively. TERs can be
calculated as follows:
End-point LD50 Application rate Predicted TER
(test (mg/kg b.w.) (kg/ha) concentration
species) in food (mg/kg)
Acute oral 63 0.32 (arable) 17.9 4.20
(Rat) 0.49 (fruit) 27.5 2.76
Since the TERs are < 10, this indicates a high risk to grazing
mammals.
b) Insectivorous mammals
Initial residues on large insects are estimated to be 0.86 mg/kg
dry weight (based on 2.7 × application rate) arising from application
at 0.32 kg/ha to arable crops (EPPO/CoE, 1993a,b) and 1.32 mg/kg from
application to fruit at a rate of 0.49 kg/ha. This gives an estimated
total oral intake for the shrew of 0.016 mg and 0.024 mg for the two
application rates, assuming that the shrew ate exclusively food
contaminated at this level. This is equivalent to a daily intake of
0.86 and 1.32 mg/kg body weight, respectively. TERs can be calculated
as follows:
End-point LD50 Application rate Predicted TER
(mg/kg b.w.) (kg/ha) concentration
in food (mg/kg)
Mammal 63 (Rat) 0.32 (arable) 0.86 72.9
acute oral 0.49 (fruit) 1.32 47.6
These TERs fall outside the trigger for high risk for
insectivorous mammals but within the range for medium risk.
10.2.2.3 Bees
The reported contact toxicity to bees gives an LD50 of
0.26 µg/bee. Using application rates at 0.32 and 0.49 kg/ha for
cereals and fruit respectively, hazard quotients are calculated to be
1231 and 1885 (application (g/ha)/LD50 (µg/bee)). The trigger for
concern is >50 (EPPO/CoE, 1993a,b) and, therefore, there is
substantial concern for exposed bees. The compound should not be
applied to flowering plants, and exposure of flying bees should be
avoided.
10.2.2.4 Earthworms
Earthworms are likely to be exposed to demeton-S-methyl. Based on
a soil depth of 5 cm and a soil density of 1.5 g/cm3, the soil PEC
would be 0.43 mg/kg assuming even distribution in the medium. The
reported LC50 for earthworms (Eisenia foetida) is 60 mg/kg
soil giving a TER of 140.6. As this is above the trigger value of
10 (EPPO/CoE, 1993a,b), the acute risk to earthworms is very low.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
Demeton-S-methyl causes acute cholinergic toxicity in humans and
laboratory animals. Carcinogenic, reproductive and developmental
effects have not been found in laboratory animals. Effects due to
chronic human exposure are unlikely to occur. Exposure of the general
population may only occur through food residues. However, the levels
of exposure are unlikely to cause adverse effects.
Given the high acute toxicity, as determined in a number of test
species and the known cases of human poisoning, it can be concluded
that the risk of occupational poisoning with demeton-S-methyl exists
when adequate protective measures and good practices are not adopted.
Therefore, handling and application of demeton-S-methyl should only be
performed by supervised and trained workers who must adhere to good
application practices and adopt adequate safety measures.
Demeton-S-methyl does not persist in the environment and is not
accumulated by organisms. It has high acute toxicity to aquatic
invertebrates and is toxic to fish and birds, leading to high or
moderate risk factors for these organisms. However, significant field
kills of organisms have not been reported for the compound.
Precautions should be taken to minimize exposure of non-target
organisms (e.g., do not spray over water bodies, minimize exposure by
spray drift).
In view of the negative results obtained in developmental and
carcinogenicity studies, it is felt that further clarification of the
genotoxic potential of demeton-S-methyl is not necessary.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Demeton-S-methyl was classified as highly hazardous (class Ib) by
WHO (1996).
Demeton-S-methyl was evaluated by the Joint FAO/WHO Meeting on
Pesticide Residues (JMPR) in 1972, 1973, 1979, 1982, 1984, 1989, 1992.
The 1989 Meeting (FAO/WHO, 1990) evaluated demeton-S-methyl,
oxydemeton-methyl and demeton-S-methylsulfone and set the following
NOAEL levels:
demeton-S-methyl: mouse: 1 mg/kg diet (equal to
0.24 mg/kg body weight per day)
rat: 1 mg/kg diet (equal to
0.05 mg/kg body weight per day)
dog: 1 mg/kg diet (equal to 0.036 mg/kg
body weight per day)
oxydemeton-methyl: mouse: 30 mg/kg diet (equal to
0.03 mg/kg body weight per day)
rat: 0.57 mg/kg diet (equal to
0.03 mg/kg body weight per day)
dog: 0.125 mg/kg body weight per day
demeton-S-methylsulfone: rat: 1 mg/litre in drinking water
(equal to 0.06 mg/kg body weight per
day)
dog: 10 mg/kg diet (equal to 0.36 mg/kg
body weight per day)
The estimated ADI for demeton-S-methyl was established at
0-0.0003 mg/kg body weight. This ADI applies to demeton-S-methyl
alone or in combination with oxydemeton-methyl and/or demeton-S-
methylsulfone because residues are determined after oxidation and are
expressed as demeton-S-methyl. The 1992 JMPR reviewed the residue data
on these three related compounds. Updated figures were available only
for oxydemeton-methyl, which is replacing demeton-S-methyl in most
registrations. The Meeting decided therefore to change the definition
of the residues to "the sum of demeton-S-methyl, oxydemeton-methyl and
demeton-S-methylsulfone expressed as oxydemeton-methyl". The MRLs
varied from 0.05 to 1 mg/kg commodity and have been obtained from
supervised trials on oxydemeton-methyl rather than on
demeton-S-methyl.
Demeton-S-methyl has not been evaluated by the International
Agency for Research on Cancer (IARC).
REFERENCES
ACGIH (1993) 1993-1994 Threshold limit values for chemical substances
and physical agents. Cincinnati, Ohio, American Conference of
Governmental Industrial Hygienist.
Akarte SR, Rao KR, & Kulkarni DA (1986) Acute toxicity of Metasystox
to an estuarine lamellibranch mollusc, Paphia laterisulca, from
Ratnagiri coast, India. Int J Ecol Environ Sci, 12: 125-129.
Anderson JPE (1989) Influence of the commercial product Metasystox (i)
on soil respiration after amendment with glucose. Leverkusen, Germany,
Bayer AG (Unpublished report No. AJO/78289).
Anonymous (1977) Determination of residues of organophosphorus
pesticides in fruits and vegetables. Analyst, 102: 858-868.
Barr AM (1966) Further experience in the treatment of severe organic
phosphate poisoning. Med J. Aust, 1: 490-492.
Bathe R (1983) 12-month oral (feeding) toxicity study with E 154
in Beagle dogs. Leverkusen, Germany, Bayer AG (Unpublished report
No. 2542).
Bayer (1970) Testing the effects of Metasystox i and Metasystox R on
bees. Leverkusen, Germany, Bayer AG (Unpublished report).
Becker H (1983) Embryotoxicity and teratogenicity study on E 154 in
rabbits. Leverkusen, Germany, Bayer AG (Unpublished report No. 2422).
Blumenstock I (1989) Influence of the commercial product Metasystox
(I) on nitrification in soils. Leverkusen, Germany, Bayer AG
(Unpublished report No. BSI/78189).
Carrington da Costa RB, Maul ER, Pimentel J, Goncalves JS, Rebelo A,
Oliveira LC, & Rebelo ASC (1982) A case of acute poisoning by methyl
demeton in a female 5 months pregnant. Arch Toxicol, 5(Suppl):
202-204.
Churchfield S (1986) Shrews. Oswestry, A. Nelson (The Mammal Society
series).
Cifone MA (1984) Mutagenicity evaluation of E154 (C.N. demeton-S-
methyl) in the mouse lymphoma forward mutation assay. Leverkusen,
Germany, Bayer AG (Unpublished report No. 2789).
DuBois KP & Doull J (1955) The acute toxicity of P=O Meta-systox, P=O
Metasystox sulphoxide and P=O Meta-systox sulphone to mammals.
Leverkusen, Germany, Bayer AG (Unpublished report).
DuBois KP & Plzak G (1962) The acute toxicity and anticholinesterase
action of 0,0-dimethyl-S-ethyl-2-sulphinylethylphosphorothioate
(Meta-systox R) and related compounds. Toxicol Appl Pharmacol,
4: 621-630.
Dzwonkowska A & Hübner H (1986) Induction of chromosomal aberration in
the Syrian hamster by insecticides tested in vivo. Arch Toxicol,
58: 152-156.
Ecker W (1978) Biotransformation of (ethylene-1-14C) demeton-S-methyl
in rats. Leverkusen, Germany, Bayer AG (Unpublished report No. 7458).
Ecker W & Cölln R (1983) Biotransformation of demeton-S-methyl in
rats. Leverkusen, Germany, Bayer AG (Unpublished report No. 11431).
Edson EF (1960) Agriculture chemicals - Applied toxicology of
pesticides. Pharm J, 185: 361-367.
Eiben R, Gröning P, & Löser E (1984) Generation study with rats
(two generations). Leverkusen, Germany, Bayer AG (Unpublished report
No. 13135).
EPPO/CoE (European & Mediterranean Plant Protection Organisation/
Council of Europe) (1993a) Decision making schemes for the
environmental risk assessment of plant protection products.
OEPP/EPPO Bull, 23: 1-54.
EPPO/CoE (European & Mediterranean Plant Protection Organisation/
Council of Europe) (1993b) Decision making schemes for the
environmental risk assessment of plant protection products.
OEPP/EPPO Bull, 24: 1-87.
FAO/WHO (1974) Demeton-S-methyl and related compounds. In: 1973
Evaluations of some pesticides in food. Geneva, World Health
Organization, pp 195-240 (WHO Pesticide Residue Series No. 3).
FAO/WHO (1990) Demeton-S-methyl and related compounds. In: Pesticides
residues in food - 1989. Evaluations: Part II - Toxicology. Rome, Food
and Agriculture Organization of the United Nations, pp 49-74 (FAO
Plant Production and Protection Paper 100/2).
FAO/WHO (1993) Demeton-S-methyl. In: Pesticides residues in food -
1992. Evaluations: Part I - Residues. Rome, Food and Agriculture
Organization of the United Nations, p 287 (FAO Plant Production and
Protection Paper 118).
Faul J (1984) [E 154 = 25/154 (demeton-S-methyl; Metasystox i-active
ingredient] Leverkusen, Germany, Bayer AG (Unpublished report)
(in German).
Flucke W (1984) [Tests on acute oral toxicity to quail (Coturnix
coturnix japonica).] Leverkusen, Germany, Bayer AG (Unpublished
report No. 13119) (in German).
Flucke W & Eben A (1988) E154 50 VL (C.N. demeton-S-methyl): Study of
effect on neuropathy target esterase (NTE) after oral administration
to the hen. Leverkusen, Germany, Bayer AG (Unpublished report
No. 17264).
Flucke W & Kaliner G (1988) E 154 50 VL (C.N. demeton-S-methyl): Study
for acute delayed neurotoxicity after oral administration to the hen.
Leverkusen, Germany, Bayer AG (Unpublished report No. 16920).
Flucke W & Kimmerle G (1977) Trichlorfon (Dipterex active ingredient),
demeton-S-methyl (Metasystox (I) active ingredient): Study for acute
toxicity after simultaneous administration of both active ingredients.
Leverkusen, Germany, Bayer AG (Unpublished report No. 7066).
Flucke W & Pauluhn J (1983) Metaisosystox 50 EC: Study for
formulation toxicity. Leverkusen, Germany, Bayer AG (Unpublished
report No. 11770).
Froebe Z, Drevenkar V, Stengl B, & Juracic M (1989) Sorption behaviour
of some organophosphorus pesticides in natural sediments. Toxicol
Environ Chem, 19: 69-82.
Funk W, Cleres L, Pitzer H, & Donnevert G (1989) Organophosphorus
insecticides: Quantitative HPTLC determination and characterization.
J Planar Chromatogr, 2: 285-289.
Ganzelmeier IH, Rautmann D, Spangenberg R, Streloke M, Herrmann M,
Wenzelburger HJ, & Walter HF (1995) Studies on the spray drift of
plant protection products: Results of a test program carried out
throughout the Federal Republic of Germany. Oxford, London, Edinburgh,
Melbourne, Blackwell Scientific Publications.
Grau R (1984) [Oral toxicity to female bird/canary (Serinus
canarius).] Leverkusen, Germany, Bayer AG (Unpublished report
No. VK-243) (in German).
Grau R (1985a) Fish toxicity (Metaisosystox 50% pre-solution).
Leverkusen, Germany, Bayer AG (Unpublished report No. FF-177).
Grau R (1985b) Fish toxicity (Metaisosystox 50% pre-solution, golden
orfe). Leverkusen, Germany, Bayer AG (Unpublished report No. FO-818).
Grau R (1990a) [Acute toxicity of Metaisosystox EC 25 to rainbow trout
(Oncorhynchus mykiss) using flow test.] Leverkusen, Germany, Bayer
AG (Unpublished report No. FF-285) (in German).
Grau R (1990b) [Acute toxicity of Metasystox EC 25 to golden orfe
(Leuciscus idus melanotus) using flow test.] Leverkusen, Germany,
Bayer AG (Unpublished report No. FO-1222) (in German).
Grau R (1990c) [Toxicity of Metasystox VL 70 to rainbow trout
(O ncorhynchus mykiss) with extensive exposure (21 days).]
Leverkusen, Germany, Bayer AG (Unpublished report No. FF-287)
(in German).
Hanna PJ & Dyer KF (1975) Mutagenicity of organophosphorus compounds
in bacteria and Drosophila. Mutat Res, 28: 405-420.
Heath DF & Vandekar M (1957) Some spontaneous reactions of
O,O-dimethyl S-ethylthioethyl phosphorothioate and related compounds
in water and on storage, and their effects on the toxicological
properties of the compounds. Biochem J, 67: 187-201.
Hecht G (1955) [Comparison tests of preparation 25/154, R 2170
and M 3/158.] Leverkusen, Germany, Bayer AG (Unpublished report)
(in German).
Hecht G (1960) [Toxicological investigations on Metasystox (i)
presentation given in Tokyo, Korinkaku.] Leverkusen, Germany, Bayer AG
(unpublished report) (in German).
Hegazy MR (1965) Poisoning by meta-isosystox in spraymen and in
accidentally exposed patients. Br J Ind Med, 22: 230-235.
Heimann KG (1987a) E 154 techn. (C.N. demeton-S-methyl): Study for
skin-sensitizing effect on guinea pigs. Leverkusen, Germany, Bayer AG
(Unpublished report No. 15896).
Heimann G (1987b) E 154 techn. (C.N. demeton-S-methyl): Study for
skin-sensitizing effect on guinea pigs in the epicutaneous test.
Leverkusen, Germany, Bayer AG (unpublished report No. 16106).
Heimbach F (1985a) Growth inhibition of green algae (Scenedesmus
subspicatus) caused by demeton-S-methyl (techn.). Leverkusen,
Germany, Bayer AG (Unpublished report No. HBF/AL11).
Heimbach F (1985b) Acute toxicity of demeton-S-methyl (techn.) to
water fleas. Leverkusen, Germany, Bayer AG (Unpublished report
No. HBF/DM 47).
Heimbach F (1985c) [Acute toxicity of demeton-S-methyl 50 VL to
water fleas.] Leverkusen, Germany, Bayer AG (Unpublished report
No. HBF/DM 48) (in German).
Heimbach F (1990a) Toxicity of Metasystox (I) to earthworms.
Leverkusen, Germany, Bayer AG (Unpublished report No. HBF/RG 120).
Heimbach F (1990b) Growth inhibition of green algae (Scenedesmus
subspicatus) by Metasystox (I). Leverkusen, Germany, Bayer AG
(Unpublished report No. HBF/AL 74).
Heimbach F (1990c) Influence of Metasystox (I) on the reproduction
rate of water fleas. Leverkusen, Germany, Bayer AG (Unpublished
report No. HBF/RDM 25).
Heimbach F (1990d) Influence of demeton-S-methyl VL 70 on the
reproduction rate of water fleas. Leverkusen, Germany, Bayer AG
(Unpublished report No. HBF/RDM 27).
Hellpointner E (1990) Assessment of the environmental half-life of
the direct photodegradation of demeton-S-methyl in water. Leverkusen,
Germany, Bayer AG (Unpublished report No. PF3446).
Herbold B (1979) E 25/154 Salmonella/microsome test to evaluate for
point mutation. Leverkusen, Germany, Bayer AG (Unpublished report
No. 8393).
Herbold B (1980a) E 25/154 Salmonella/microsome test to evaluate for
point mutation. Leverkusen, Germany, Bayer AG (Unpublished report
No. 8809).
Herbold B (1980b) E 25/154 micronucleus test on the mouse to evaluate
for mutagenic effect. Leverkusen, Germany, Bayer AG (Unpublished
report No. 8932).
Herbold B (1980c) E 25/154 (demeton-S-methyl) dominant lethal study on
male mouse to test for mutagenic effects. Leverkusen, Germany, Bayer
AG (Unpublished report No. 9336).
Herbold B (1983a) E 154 (demeton-S-methyl) pol test on E. coli to
evaluate for DNA damage. Leverkusen, Germany, Bayer AG (Unpublished
report No. 12152).
Herbold B (1983b) E 154 (demeton-S-methyl) sister chromatid exchange
in the bone marrow of the Chinese hamster in vivo to evaluate for
harmful effect on DNA. Leverkusen, Germany, Bayer AG (Unpublished
report No. 12323).
Hermann G (1974a) [Fish toxicity of Metasystox (I) to goldfish.]
Leverkusen, Germany, Bayer AG (Unpublished report No. FG-112)
(in German).
Hermann G (1974b) [Fish toxicity of Metasystox (I) to carp.]
Leverkusen, Germany, Bayer AG (Unpublished report No. FK-28)
(in German).
Hermann G (1974c) [Fish toxicity of Metasystox (I) to rudd.]
Leverkusen, Germany, Bayer AG (Unpublished report No. FR-29)
(in German).
Hill ARC, Wilkins JPG, Findlay NRI, & Lontay KEM (1984)
Organophosphorus sulphides, sulphoxides and sulphones - Part I:
Determination of residues in fruit and vegetables by gas-liquid
chromatography. Analyst, 109: 483-487.
Hoorn AJW (1982) Mutagenicity evaluation of E 154 50 VL (in xylene)
in the reverse mutation induction assay with Saccharomyces
cerevisiae strains S138 and S211a. Leverkusen, Germany, Bayer AG
(Unpublished report No. 2372).
Jones RD (1982) Organophosphorus poisoning at a chemical packaging
company. Br J Ind Med, 39: 377-381.
Kenaga E (1973) Factors to be considered in the evaluation of the
toxicity of pesticides to birds in the environment. Environ Qual Saf,
2: 166-181.
Kimmerle G (1972) Acute toxicity of SRA 3886 in combination with S 276
and with E 154 to rats. Leverkusen, Germany, Bayer AG (Unpublished
report No. 3438).
Klimmer OR (1964) [Determination of acute oral toxicity of Metasystox
(i).] Leverkusen, Germany, Bayer AG (Unpublished report) (in German).
Klimmer OR & Pfaff W (1955) [Toxicity tests on the new contact
insecticide O,O-dimethyl-thiophosphoric acid-O/ -S-ethylester
("Metasystox").] Arzneimittel-Forschung, 5: 584-587 (in German).
Klimmer R & Pfaff W (1958) [Toxicity tests during application of the
systemic insecticide O,O-dimethyl-(ethyl-thioethyl)-thiophosphoric
acid-ester.] Arzneimittel-Forschung, 8: 265-269 (in German).
Kniehase U (1984) [Effects of Metasystox (I) 250 EC on mite
(Phytoseiulus persimilis).] Leverkusen, Germany, Bayer AG
(Unpublished report No. ZDE/PP 4/84) (in German).
Krohn J (1984) Fate/behaviour of crop protection products in water:
demeton-S-methyl. Leverkusen, Germany, Bayer AG (Unpublished report
No. M2421).
Lewis PJ, Lowing RK, & Gomopertz D (1981) Automated discrete kinetic
method for erythrocyte acetylcholinesterase and plasma cholinesterase.
Clin Chem, 27: 926-929.
Mary ASR, Nagabhushanam R, & Sarojini R (1986) Toxicity evaluation of
organophosphorus and chlorinated hydrocarbon pesticides in freshwater
prawn Macrobrachium lamerrii. J Environ Biol, 7: 189-195.
Mitra PK, Sud SC, & Bahga HS (1978) Acute oral toxicity of Metasystox
in buffalo calves. Indian J Exp Biol, 16: 813-815.
Muley DV, Akarte SR, Kulkarni DA, & Rao KR (1987) Acute toxicity of
Metasystox to wedge clam, Donax cuneatus, from West Coast. Fish
Technol, 24: 27-30.
Owen M (1975) The management of grass swards for captive wildfowl. Int
Zoo Year Book, 16: 135-138.
Pandita TK (1983) Mutagenic studies on the insecticide Metasystox-R
with different genetic systems. Mutat Res, 124: 97-102.
Renhof M (1985) E 154 (C.N. demeton-S-methyl): Study for embryotoxic
effect on rats after oral administration. Leverkusen, Germany, Bayer
AG (Unpublished report No. 13237).
Schludecker D & Aderjan R (1988) [Chemical-toxicological results of a
suicidal poisoning with demeton-S-methyl.] Z Rechtsmed, 101:55-60
(in German).
Schmid E (1984) Degradation of demeton-S-methyl in soil. Leverkusen,
Germany, Bayer AG (Unpublished report No. Im1428).
Schmid ER (1986) Degradation of demeton-S-methyl in soil at a
concentration of 3 ppm under anaerobic conditions. Leverkusen,
Germany, Bayer AG (Unpublished report No. IM1579).
Schmidt WM & Bomhard E (1988) Study for carcinogenicity in NMRI mice
(administration in diet for up to 21 months). Leverkusen, Germany,
Bayer AG (Unpublished report No. 16812).
Schmidt WM & Westen H (1988) Study for chronic toxicity and
carcinogenicity in Wistar rats (administration in diet for up to 2
years). Leverkusen, Germany, Bayer AG (Unpublished report No. 16888).
Shires SW (1985) Effects of aerial applications of cypermethrin and
demeton-S-methyl on non-target arthropods of wheat. Ecotoxicol Environ
Saf, 10: 1-11.
Stone HM (1961) The anticholinesterase activity of demeton-S-methyl.
NZ J Sci, 4: 78-90.
Su MQ, Kinoshita FK, Frawley JP, & DuBois KP (1971) Comparative
inhibition of aliesterases and cholinesterase in rats fed eighteen
organophosphorus insecticides. Toxicol Appl Pharmacol, 20: 241-249.
Tarrant KA, Thompson HM, & Hardy AR (1992) Biochemical and
histological effect of the aphicide demeton-S-methyl on house sparrows
(Passer domesticus) under field conditions. Bull Environ Contam
Toxicol, 48: 360-366.
Thompson HM, Walker CH, & Hardy AR (1991) Changes in activity of avian
serum esterases following exposure to organophosphorus insecticides.
Arch Environ Contam Toxicol, 20: 514-518.
Thornton JS, Olson TJ, & Wagner K (1977) Determination of residues
of Metasystox-R and metabolite in plant and animal tissues and soil.
J Agric Food Chem, 25: 573-576.
Thyssen J (1981) [Tests on skin and mucous-irritating effects of
demeton-S-methyl.] Leverkusen, Germany, Bayer AG (Unpublished report)
(in German).
Vandekar M (1958) The toxic properties of demeton-methyl
("Metasystox") and some related compounds. Brit J Ind Med,
15: 158-167.
Vasilic Z, Drevenkar V, Froebe Z, Stengl B, & Tkalcevic B (1987) The
metabolites of organophosphorus pesticides in urine as an indicator of
occupational exposure. Toxicol Environ Chem, 14:11-127.
Verma SP, Tonk IP, Gupta AK, & Saxena M (1984) Evaluation of an
application factor for determining the safe concentration of
agricultural and industrial chemicals. Water Res, 18(1): 111-115.
Wagner K & Oehlmann L (1987) Studies on the metabolic behaviour of
demeton-S-methyl in wheat. Leverkusen, Germany, Bayer AG (Unpublished
report No. PF2701).
Wagner K & Thornton JS (1977) Method for the gas-chromatographic
determination of Metasystox (i) and Metasystox R residues in plants,
soil and water. Pflanzenschutz Nachr Bayer, 30: 1-17.
Wagner K, Schütz U, & Oehlmann L (1985) Degradation of Metasystox-(i)
in soil under aerobic, anaerobic and sterile test conditions.
Leverkusen, Germany, Bayer AG (Unpublished report No. PF2565).
Weber H, Patzschke K, & Wagner LA (1978) (14C), demeton-S-methyl
(Metasystox in active ingredient)/Biokinetic studies on rats.
Leverkusen, Germany, Bayer AG (Unpublished report No. 7491).
Westlake GE, Hardy AR, & Stevenson JH (1985) Effects of storage and
pesticide treatments on honey bee brain acetyl cholinesterase
activities. Bull Environ Contam Toxicol, 34: 668-675.
WHO (1996) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1996-1997. Geneva, World Health
Organization, International Programme on Chemical Safety
(WHO/PCS/96.3).
Wilkins JPG, Hill ARC, & Lee DF (1985) Organophosphorus sulphides,
sulphoxide and sulphones - Part 2: Characterisation by gas
chromatography-mass spectrometry. Analyst, 110: 1045-1051.
Wilmes R (1984) Orientating light stability: demeton-S-methyl.
Leverkusen, Germany, Bayer AG (Unpublished report No. IM1408).
Winkler KO & Arent H (1970) Medical observation of personnel occupied
with spraying in hop cultivation during the application of the organic
phosphoric acid esters mevinphos and demeton-S-methyl (Metasystox i).
Leverkusen, Germany, Bayer AG (Unpublished report No. 24618).
Xue SZ, Ding XJ, & Ding Y (1985) Clinical observation and comparison
of the effectiveness of several oxime cholinesterase reactivators.
Scand J Work Environ Health, 11(Suppl 4): 46-48.
Ziegler W, Engelhardt G, Wallnöfer PR, Oehlmann L, & Wagner K (1980)
Degradation of demeton-S-methyl sulfoxide (Metasystox R) by soil
microorganisms. J Agric Food Chem, 28: 1102-1106.
RÉSUMÉ ET ÉVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Identité, propriétés physiques et chimiques
Le déméton-S-méthyl se présente sous la forme d'un liquide
huileux jaune pâle à l'odeur pénétrante. C'est un organophosphoré
agissant par la voie générale et par contact qui est utilisé comme
insecticide et acaricide sur les fruits, les légumes, les céréales et
les plantes ornementales pour lutter contre Acarina, Thysanoptera,
Hymenoptera et Homoptera. Sa tension de vapeur est de 63,8 mPa à
20°C et il est facilement soluble dans la plupart des solvants
organiques. Il est très soluble dans l'eau (3,3 g/litre à la
température ambiante) et son coefficient de partage entre l'octanol et
l'eau (log Pow) est de 1,32. Le déméton-S-méthyl est stable dans les
solvants non aqueux.
Pour la recherche et le dosage des résidus et l'analyse des
échantillons prélevés dans l'environnement, on procède à une
extraction au moyen d'un solvant organique, suivie d'une oxydation de
la sulfone correspondante. Le dosage s'effectue ensuite par
chromatographie en phase gazeuse avec un détecteur spécifique du
phosphore.
1.2 Sources d'exposition humaine et environnementale
Jusqu'en 1957, le déméton-méthyl a été commercialisé sous la
forme d'un mélange de deux isomères, le déméton-S-méthyl et le
déméton-O-méthyl. La formulation commerciale est un concentré
émulsionnable que l'on utilise en pulvérisations sur les céréales, les
fruits, les légumes et les plantes ornementales. On est en train de le
remplacer par l'oxydéméton-méthyl qui est un métabolite produit par
les plantes et les mammifères ou qui résulte du séjour du
déméton-S-méthyl dans le sol.
1.3 Transport, distribution et transformation dans l'environnement
La décomposition par hydrolyse du déméton-S-méthyl dépend du pH
de la solution. A 22°C, sa demi-vie est de 56 jours à pH 7 et de 8
jours à pH 9. Dans le sol, sa voie de décomposition principale est la
biodégradation, avec une demi-vie d'environ 4 h. Au bout de 24 h, il y
a encore dans le sol une proportion d'oxydéméton-méthyl qui représente
20 à 30% de la dose initiale de déméton-S-méthyl. Le coefficient de
sorption dans le sol (Kd) du déméton-S-méthyl est de 0,68 à 2,66,
selon la composition du sol.
Dans l'environnement, l'une des principales voies de dégradation
du composé est la photolyse. La métabolisation par le blé de printemps
est rapide et elle analogue à celle qui se produit dans le sol et chez
les mammifères.
1.4 Niveaux d'exposition environnementale et humaine
La population générale est principalement exposée au
déméton-S-méthyl par l'intermédiaire des résidus qui subsistent sur
les cultures vivrières. La réunion conjointe FAO/OMS sur les résidus
de pesticides (JMPR) a recommandé une dose journalière admissible
(DJA) de 0,0003 mg/kg de poids corporel. Il s'agit d'une DJA
collective qui englobe le déméton-S-méthyl, l'oxydéméton-méthyl et la
déméton-S-méthylsulfone, étant donné que les méthodes habituelles
d'analyse ne distinguent pas ces différents composés. Il est arrivé
qu'une exposition excessive au déméton-S-méthyl par la voie
transcutanée entraîne une intoxication de type cholinergique chez des
ouvriers mal protégés qui procédaient au conditionnement du concentré.
En revanche, des volontaires qui s'étaient prêtés à l'épandage
simulé d'un mélange de déméton-S-méthyl et de déméton-O-méthyl (30 et
70% respectivement) et avaient été exposés à des concentrations de 8,8
à 27 mg/m3 des deux matières actives, n'ont présenté aucune réduction
de leur activité cholinestérasique plasmatique ou érythrocytaire.
1.5 Cinétique et métabolisme
Chez le rat, le déméton-S-méthyl est rapidement et presque
complètement résorbé au niveau de l'intestin et il se répartit
uniformément dans les tissus (exception faite des hématies dans
lesquelles il se concentre plus fortement). Il est rapidement
métabolisé et excrété dans les urines. Sa concentration sanguine
diminue avec une demi-vie qui est initialement de 2 h environ. Au
bout de 24 h, il reste environ 1% de la dose dans l'organisme. La
principale voie métabolique du déméton-S-méthyl consiste en une
oxydation de la chaîne latérale qui conduit à la formation du
sulfoxyde correspondant (oxydéméton-méthyl) et, dans une moindre
proportion, après une oxydation plus poussée, à la sulfone.
L'O-déméthylation constitue une autre voie métabolique importante.
1.6 Effets sur les animaux de laboratoire et les systèmes d'épreuve
in vitro
1.6.1 Exposition unique
Le déméton-S-méthyl provoque des intoxications de type
cholinergique. Chez les mammifères, la DL50 varie de 7 à 100 mg/kg
p.c. selon la voie d'administration et l'espèce.
1.6.2 Exposition de courte durée
Une des premières études, comportant une exposition par la voie
alimentaire, a montré que des rats qui recevaient le composé à raison
de 50 mg/kg de nourriture, avaient une activité cholonestérasique
cérébrale sensiblement réduite au bout de 26 semaines de ce régime.
Des signes d'intoxication de type cholinergique ont été observés au
bout de 5 semaines de traitement chez des rats recevant 200 mg de
composé par kg de nourriture.
Lors d'une étude d'alimentation d'un an effectuée sur des chiens,
on a obtenu une dose sans effet nocif observable (NOAEL), basée sur
les effets cholinergiques, de 1 mg/kg de nourriture, soit l'équivalent
quotidien de 0,036 mg/kg de poids corporel.
1.6.3 Exposition de longue durée
Des souris ont reçu pendant 21 mois des rations contenant
respectivement 0, 1, 15 ou 75 mg de déméton-S-méthyl par kg de
nourriture. La NOAEL, a été trouvée égale à 1 mg/kg de nourriture,
soit l'équivalent quotidien de 0,24 mg/kg de poids corporel en prenant
comme base l'inhibition de la cholinesterase cérébrale.
Chez des rats ayant reçu une alimentation contenant
respectivement 0, 1, 7 ou 50 mg/kg de déméton-S-méthyl, la NOAEL,
basée sur l'inhibition de la cholinestérase cérébrale, s'est révélée
égale à 1 mg/kg de nourriture, soit l'équivalent quotidien de
0,05 mg/kg p.c.
Chez aucune espèce il n'a été constaté d'augmentation de
l'incidence des tumeurs.
1.6.4 Irritation et sensibilisation cutanée ou oculaire
Le déméton-S-méthyl est légèrement irritant pour l'oeil et la
peau. Le test de Magnusson et Klingman a donné des résultats positifs
chez des cobayes. En revanche, le test de sensibilisation de Buehler
(application d'un timbre cutané) n'a pas fourni d'indice d'une
sensibilisation cutanée, ce qui donne à penser que, dans la pratique,
l'usage du déméton-S-méthyl ne devrait pas poser de problème de
sensibilisation.
1.6.5 Effets sur la reproduction, embryotoxicité et tératogénicité
Lors d'une étude sur deux générations de rats, au cours de
laquelle les animaux ont été exposés au composé par la voie
alimentaire, on a constaté une réduction de la viabilité et du poids
corporel des ratons (uniquement la génération F1b) à la dose de
5 mg/kg de nourriture. La NOAEL était de 1 mg/kg de nourriture, soit
l'équivalent quotidien de 0,07 mg/kg de poids corporel.
Le déméton-S-méthyl ne s'est révélé ni embryotoxique, ni
tératogène chez le rat et le lapin.
1.6.6 Mutagénicité et points apparentés d'aboutissement des effets
toxiques
Le déméton-S-méthyl produit des mutations ponctuelles
in vitro. On n' a mis en évidence des effets chromosomiques
in vivo qu'avec des formulations du commerce. Les données
disponibles sont insuffisantes pour que l'on puisse procéder à une
évaluation satisfaisante du pouvoir génotoxique de ce composé.
1.6.7 Neurotoxicité retardée
Le déméton-S-méthyl n'a produit ni polyneuropathie, ni inhibition
de l'estérase cible correspondante (NTE) lorsqu'on l'a administré à
des poules en dose égale à la DL50 par voie orale.
1.6.8 Toxicité des métabolites
Deux métabolites du déméton-S-méthyl produits par des
plantes et des mammifères, à savoir l'oxydéméton-méthyl et la
déméton-S-méthylsulfone, sont également des pesticides du commerce et
ont été très largement étudiés. Il ressort de ces travaux que le
profil toxicologique de ces composés ne diffère pas sensiblement,
quantitativement ou qualitativement, de celui du déméton-S-méthyl.
1.7 Mécanisme de la toxicité - Mode d'action
Le déméton-S-méthyl est un inhibiteur direct de la cholinestérase
et sa toxicité est liée au fait qu'il inhibe l'acétylcholinestérase
(AChE) au niveau des terminaisons nerveuses. Une fois inhibée par le
déméton-S-méthyl, l'acétylcholinestérase se réactive spontanément avec
une demi-vie in vitro de 1,3 h environ, comme on peut s'y attendre
pour une AChE diméthylphosphorylée.
1.8 Effets sur l'homme
On a signalé quelques cas d'intoxication aiguë avec syndrome
cholinergique, consécutifs à des tentatives de suicide. Aucun effet
retardé n'a été observé chez les survivants, dont une femme enceinte.
A la suite d'une exposition subie par négligence lors du
conditionnement de formulations du commerce, quelques employés ont
présenté des troubles d'origine cholinergique qui ont nécessité un
traitement pharmacologique. Le composé avait probablement pénétré par
la voie transcutanée. De même, il est possible que des conditions de
travail laissant à désirer aient entraîné une absorption excessive de
déméton-S-méthyl au cours de l'épandage de ce composé sur des champs
de coton.
1.9 Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Pour les algues vertes, la CE50 à 96 h va de 8 à 37 mg/litre. On
a mesuré une CL50 allant de 0,004 à 1,3 mg/litre chez une série
d'invertébrés aquatiques. Chez les poisson, la toxicité est variable,
avec une CL50 à 96 h qui peut aller de 0,59 mg/litre pour la truite
arcen-ciel à environ 40 mg/litre pour l'orfe, le cyprin doré et la
carpe.
Chez la caille japonaise et le canari, on a obtenu une DL50
(effet aigu, voie orale) de 10-50 mg/kg de poids corporel. Chez des
étourneaux, une dose unique par voie orale de 2 mg/kg p.c. a provoqué
une inhibition de l'acétylcholinestérase cérébrale 3 h après le
traitement.
Pour les lombrics terricoles, la CL50 du déméton-S-méthyl est de
66 mg/kg sur 14 jours. Pour les abeilles, on a obtenu des valeurs de
la DL50 de contact et par voie orale (effet aigu) respectivement
égales à 0,21 et 0,6 par insecte. En traitant du blé d'hiver à la dose
recommandée, on a constaté une réduction sensible du nombre
d'invertébrés présents sur les feuilles (principalement des diptères
du genre Empididae) sans effet sur les invertébrés entomophages
présents à la surface du sol.
2. Conclusions
Le déméton-S-méthyl est un insecticide organophosphoré fortement
toxique (classe Ib de la classification de l'OMS) (OMS, 1966). Son
action toxique s'explique par l'inhibition de l'acétylcholinestérase
au niveau des terminaisons nerveuses. L'exposition de la population
générale résulte principalement des résidus présents dans les denrées
alimentaires provenant des cultures traitées.
Moyennant de bonnes pratiques agricoles et le respect des règles
d'hygiène et de sécurité, la manipulation du composé lors de sa
fabrication ou de son épandage ne devrait entraîner aucun effet
indésirable. Des effets résultant d'une exposition chronique sont
improbables.
Le déméton-S-méthyl ne persiste pas dans l'environnement et il ne
s'accumule pas chez les êtres vivants. Il est très toxique pour les
invertébrés aquatiques et présente une toxicité notable pour les
poissons et les oiseaux, aussi doit on considérer que pour ces
organismes, le facteur de risque est élevé ou modéré. Néanmoins, on
n'a pas signalé de mortalité importante dans la nature du fait de ce
composé. Des précautions sont à prendre pour réduire au minimum
l'exposition des organismes non visés (par ex. ne pas le pulvériser
sur des étendues d'eau et veiller à ce que les gouttelettes
d'insecticide ne soient pas entraînées vers les zones à respecter).
3. Recommandations
Afin de protéger la santé et le bien-être des travailleurs et de
la population dans son ensemble, il ne faut confier la manipulation et
l'épandage du déméton-S-méthyl qu'à des opérateurs dûment formés et
encadrés, qui auront le souci de respecter les mesures de sécurité et
d'épandre le produit selon les règles.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
1.1 Identidad, propiedades físicas y químicas, y métodos analíticos
El demeton-S-metilo, un líquido oleoso de color amarillo pálido y
olor penetrante, es un insecticida y acaricida organofosfatado
sistémico y de contacto que se utiliza para combatir Acarina,
Thysanoptera y Homóptera en frutas, cereales, plantas ornamentales
y hortalizas. Tiene una presión de vapor de 63,8 mPa a 20°C, es
fácilmente soluble en la mayoría de los disolventes orgánicos, tiene
una gran solubilidad en agua, de 3,3 g/litro a temperatura ambiente, y
un coeficiente de reparto octanol/agua (log Pow) de 1,32. El
demeton-S-metilo es estable en disolventes no acuosos.
Los análisis de residuos y ambientales se realizan por extracción
con un disolvente orgánico y oxidación a la sulfona correspondiente.
Las mediciones se efectúan por cromatografía de gases utilizando un
detector específico del fósforo.
1.2 Fuentes de exposición humana y ambiental
Antes de 1957, el metildemeton se comercializaba como mezcla de
los isómeros demeton-S-metilo y demeton-O-metilo.
El demeton-S-metilo se utiliza desde 1957. Su presentación es la
de un concentrado emulsionable y se aplica por rociamiento a los
cereales, frutas, plantas ornamentales y hortalizas. Se está
sustituyendo por el oxidemetonmetilo, que es un metabolito del
demeton-S-metilo producido en las plantas, el suelo y los mamíferos.
1.3 Transporte, distribución y transformación en el medio ambiente
La degradación hidrolítica del demeton-S-metilo depende del pH de
la solución; a 22°C la semivida es de 63 días con un pH de 4, de 56
días con un pH de 7 y de 8 días con un pH de 9. La principal vía de
degradación en el suelo es la biodegradación. La semivida del
demeton-S-metilo en el suelo es de unas 4 horas. Sin embargo, al cabo
de 24 horas, el oxidemetonmetilo representa aún el 20-30 % de la dosis
aplicada de demeton-S-metilo. El coeficiente de sorción (Kd) del
demeton-S-metilo en el suelo oscila entre 0,68 y 2,66, dependiendo de
la composición del suelo.
La fotolisis no es uno de los principales mecanismos de
degradación del demeton-S-metilo en el medio ambiente.
Su metabolismo en el trigo de primavera es rápido y similar al
que se produce en el suelo y en los mamíferos.
1.4 Niveles ambientales y exposición humana
La exposición primaria de la población humana en general se
produce a través de los residuos de demeton-S-metilo en los cultivos
alimentarios. En la reunión conjunta FAO/OMS sobre residuos de
plaguicidas (JMPR) se recomendó una ingesta diaria admisible (IDA) de
0,0003 mg/kg de peso corporal. Se trata de una IDA colectiva para el
demeton-S-metilo, el oxidemetonmetilo y el demeton-S-metilsulfona, ya
que los métodos habituales de análisis no diferencian entre estos tres
compuestos.
La exposición excesiva al demeton-S-metilo y su absorción cutánea
produjeron toxicidad colinérgica en trabajadores insuficientemente
protegidos durante el envasado de la formulación concentrada.
Tras haber expuesto a voluntarios participantes en una simulación
de rociamiento con una mezcla de demeton-S-metilo y demeton-O-metilo
(30 y 70% respectivamente) a 8,8-27 mg/m3 de ambos componentes
activos combinados, no se observaron efectos adversos en la actividad
de la colinesterasa en el plasma ni en los eritrocitos.
1.5 Cinética y metabolismo
El demeton-S-metilo se absorbe rápida y casi completamente por el
tracto intestinal en las ratas y se distribuye uniformemente a los
tejidos (excepto una concentración elevada en los eritrocitos). Se
metaboliza rápidamente y se excreta por orina. La concentración en la
sangre disminuye, con una semivida inicial de unas 2 horas.
Aproximadamente el 1% de la dosis oral sigue presente en el organismo
24 horas después del tratamiento. La principal ruta metabólica del
demeton-S-metilo en las ratas es la oxidación de la cadena lateral,
que da lugar a la formación del sulfóxido correspondiente
(oxidemetonmetilo) y, en menor medida, a la sulfona, tras una
oxidación mayor. Otra ruta metabólica importante es la O-demetilación.
1.6 Efectos en mamíferos de laboratorio y en sistemas de ensayo
in vitro
1.6.1 Exposición única
El demeton-S-metilo produce toxicidad colinérgica. Los valores de
la DL50 para los mamíferos oscilan entre 7 y 100 mg/kg de peso
corporal, dependiendo de la vía de administración y de la especie.
1.6.2 Exposición breve
Un estudio alimentario inicial reveló que las ratas alimentadas
con 50 mg de demeton-S-metilo por kg de la dieta habían sufrido una
reducción notable de la actividad de la colinesterasa cerebral y
eritrocitaria tras una exposición de 26 semanas. Se observaron
síntomas colinérgicos en ratas alimentadas con 200 mg/kg de la dieta
durante las 5 primeras semanas de exposición.
En un estudio alimentario de un año de duración efectuado en
perros se estableció un nivel sin efectos adversos observados (NOAEL)
de 1 mg/kg de la dieta (equivalente a 0,036 mg/kg de peso corporal por
día) sobre la base de los efectos de la colinesterasa cerebral.
1.6.3 Exposición prolongada
Se administró durante 21 meses a ratones una dieta que contenía
0, 1, 15 ó 75 mg/kg de demeton-S-metilo. El NOAEL, determinado sobre
la base de la inhibición de la colinesterasa cerebral, fue de 1 mg/kg
de la dieta (equivalente a 0,24 mg/kg de peso corporal por día).
En ratas cuya dieta contenía 0, 1, 7 ó 50 mg/kg de demeton-S-metilo,
el NOAEL, determinado sobre la bases de la inhibición de la
colinesterasa cerebral, fue de 1 mg/kg de la dieta (equivalente a
0,05 mg/kg de peso corporal por día).
La incidencia de tumores no aumentó en ninguna de esas especies.
1.6.4 Irritación y sensibilización de la piel y los ojos
El demeton-S-metilo es un irritante cutáneo y ocular leve. La
prueba de maximización de Magnusson y Klingman dio resultados
positivos en cobayos. Sin embargo, la prueba del parche cutáneo de
Buehler no mostró indicios de sensibilización cutánea, lo que parece
indicar que el uso práctico del demeton-S-metilo no debería acarrear
problemas de sensibilización.
1.6.5 Reproducción, embriotoxicidad y teratogenicidad
En un estudio alimentario llevado a cabo en dos generaciones de
ratas, se observó una reducción de la viabilidad y del peso corporal
de los cachorros (generación F1b solamente) con una dosis de 5 mg de
demeton-S-metilo por kg de la dieta. El NOAEL fue de 1 mg/kg de la
dieta, equivalente a 0,07 mg/kg de peso corporal por día.
El demeton-S-metilo no resultó embriotóxico ni teratogénico en
ratas ni en conejos.
1.6.6 Mutagenicidad y parámetros conexos
El demeton-S-metilo produce mutaciones puntuales in vitro. Se han
demostrado efectos cromosómicos in vivo con formulaciones comerciales
solamente. La información disponible es insuficiente para realizar una
evaluación adecuada del potencial genotóxico del demeton-S-metilo.
1.6.7 Neurotoxicidad retardada
El demeton-S-metilo no causó polineuropatías retardadas ni
inhibición de la Esterasa Diana de Neuropatía en pruebas con gallinas
a un nivel igual a la DL50 oral.
1.6.8 Toxicidad de los metabolitos
Las plantas y los mamíferos producen dos metabolitos del
demeton-S-metilo (el oxidemetonmetilo y el demeton-S-metilsulfona) que
también son plaguicidas comerciales y han sido ampliamente estudiados.
Se ha señalado de que el perfil toxicológico de estos dos compuestos
no difiere de manera significativa, cuantitativa ni cualitativamente,
del demeton-S-metilo.
1.7 Mecanismo de toxicidad: modalidad de acción
El demeton-S-metilo es un inhibidor indirecto de la colinesterasa
y su toxicidad que está relacionada con la inhibición de la
acetilcolinesterasa en las terminales nerviosas. La
acetilcolinesterasa inhibida por el demeton-S-metilo se reactiva
espontáneamente, con una semivida in vitro de aproximadamente
1,3 horas, como cabe esperar en la acetilcolinesterasa dimetil
fosforilada.
1.8 Efectos en el ser humano
Se han notificado varios casos de intoxicación aguda con
síndrome colinérgico tras intentos de suicidio. Los pacientes que
sobrevivieron, inclusive una mujer embarazada, no presentaron efectos
retardados.
Tras una exposición laboral por inatención durante el envasado de
la formulación comercial, algunos trabajadores desarrollaron una
toxicidad colinérgica que precisó tratamiento farmacológico. El
demeton-S-metilo se absorbió probablemente a través de la piel.
Análogamente, condiciones de trabajo inapropiadas podrían haber sido
la causa de una absorción cutánea excesiva durante la aplicación del
demeton-S-metilo en algodonales.
1.9 Efectos en otros organismos en el laboratorio y sobre el terreno
Las CE50 96-h para las algas verdes se sitúan entre 8 y
37 mg/litro. Las CL50 para diversos invertebrados acuáticos se
encuentran entre 0,004 y 1,3 mg/litro. La toxicidad para los peces
varía; la CL50 96-h oscila entre 0,59 mg/litro para la trucha
arcoiris y unos 40 mg/litro para Leuciscus idus, Carassius auratus
y la carpa.
La DL50 oral aguda para la codorniz japonesa y el canario es de
10-50 mg/kg de peso corporal. En el estornino, una dosis oral única de
2 mg/kg de peso corporal produjo inhibición del 20% de la AChE
cerebral 3 horas después del tratamiento.
La CL50 del demeton-S-metilo en el suelo para las lombrices
de tierra es de 66 mg/kg para un período de 14 días. La DL50 aguda
oral y de contacto del demeton-S-metilo fue de 0,21 y 0,6 g/abeja
respectivamente. Después de haber sido aplicado al trigo de invierno
según la proporción propuesta, el demeton-S-metilo redujo
significativamente el número de invertebrados en el follaje de los
cultivos (principalmente Empididae), pero no el número de
invertebrados entomófagos de la superficie del suelo.
2. Conclusiones
El demeton-S-metilo es un éster organofósforado sumamente tóxico
(pertenece a la clase Ib de la clasificación de la OMS) (OMS, 1996)
utilizado como insecticida. La acción tóxica consiste en la inhibición
de la acetilcolinesterasa en las terminales nerviosas. La exposición
de la población en general se produce principalmente a causa de los
residuos presentes en los productos agrícolas.
Con buenas prácticas laborales y medidas de higiene y de
seguridad, el demeton-S-metilo no debería producir efectos adversos
durante la fabricación o la aplicación. Es poco probable que aparezcan
efectos debidos a la exposición crónica.
El demeton-S-metilo no es persistente en el medio ambiente y no
se acumula en los organismos. Tiene una toxicidad aguda elevada para
los invertebrados acuáticos y es tóxico para los peces y las aves,
llevando aparejados factores de riesgo agudo o moderado elevados para
esos organismos. Sin embargo, no se han comunicado muertes masivas
significativas de organismos en el campo a causa de este compuesto.
Habría que tomar precauciones para reducir al mínimo la exposición de
organismos no combatidos (por ejemplo, no rociar sobre masas de agua y
reducir al mínimo la exposición por desviación del rociado).
3. Recomendaciones
Para la salud y el bienestar de los trabajadores y del público en
general, el manejo y la aplicación del demeton-S-metilo deberían
encomendarse exclusivamente a personal bien adiestrado y supervisado
que aplique las medidas de seguridad necesarias y buenas prácticas de
aplicación.
 150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize
the important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DEMETON-S-METHYL
Members
Dr P.J. Abbott, Australia and New Zealand Food Authority
(ANZFA), Canberra, Australia
Dr K. Barabas, Department of Public Health, Albert Szent-Gyorgyi,
University Medical School, Szeged, Hungary
Dr A.L. Black, Woden, ACT, Australia
Professor J.F. Borzelleca, Pharmacology and Toxicology,
Richmond, Virginia, USA
Dr P.J. Campbell, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries and Food, Kings Pool, York,
United Kingdom
Professor L.G. Costa, Department of Environmental Health,
University of Washington, Seattle, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr I. Dewhurst, Mammalian Toxicology Branch, Pesticides Safety
Directorate, Ministry of Agriculture, Fisheries and Food,
Kings Pool, York, United Kingdom
Dr V. Drevenkar, Institute for Medical Research and Occupational
Health, Zagreb, Croatia
Dr W. Erickson, Environmental Fate and Effects Division,
US Environmental Protection Agency, Washington, D.C., USA
Dr A. Finizio, Group of Ecotoxicology, Institute of Agricultural
Entomology, University of Milan, Milan, Italy
Mr K. Garvey, Office of Pesticide Programs (7501C),
US Environmental Protection Agency, Washington, D.C., USA
Dr A.B. Kocialski, Health Effects Division, Office of Pesticide
Programs, US Environmental Protection Agency,
Washington, D.C., USA
Dr A. Moretto, Institute of Occupational Medicine, University
of Padua, Padua, Italy
Professor O. Pelkonen, Department of Pharmacology and
Toxicology, University of Oulu, Oulu, Finland
Dr D. Ray, Medical Research Council Toxicology Unit, University
of Leicester, Leicester, United Kingdom
Dr J.H.M. Temmink, Department of Toxicology, Wageningen
Agricultural University, Wageningen, The Netherlands
Observers
Dr J.W. Adcock, AgrEvo UK Limited, Chesterford Park, Saffron,
Waldon, Essex, United Kingdom
Mr D. Arnold, Environmental Sciences, AgrEvo UK Ltd.,
Chesterford Park, Saffron Waldon, Essex, United Kingdom
Dr E. Bellet, CCII, Overland Park, Kansas, USA
Mr Jan Chart, AMVAC Chemical Corporation, Newport Beach,
California, USA
Dr H. Egli, Novartis Crop Protection AG, Basel, Switzerland
Dr P. Harvey, AgrEvo UK Ltd., Chesterford Park, Saffron Walden,
Essex, United Kingdom
Dr G. Krinke, Novartis Crop Protection AG, Basel, Switzerland
Dr A. McReath, DowElanco Limited, Letcombe Regis, Wantage,
Oxford, United Kingdom
Dr H. Scheffler, Novartis Crop Protection AG, Basel, Switzerland
Dr A.E. Smith, Novartis Crop Protection AG, Basel, Switzerland
Secretariat
Dr L. Harrison, Health and Safety Executive, Bootle, Merseyside,
United Kingdom
Dr J.L. Herrman, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P.G. Jenkins, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr D. McGregor, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
Dr R. Plestina, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr E. Smith, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P. Toft, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DEMETON-S-METHYL
The Core Assessment Group (CAG) of the Joint Meeting on
Pesticides (JMP) met at the Institute for Environment and Health,
Leicester, United Kingdom, from 3 to 8 March 1997. Dr L.L. Smith
welcomed the participants on behalf of the Institute, and
Dr R. Plestina on behalf of the three IPCS cooperating organizations
(UNEP/ILO/WHO). The CAG reviewed and revised the draft monograph and
made an evaluation of the risks for human health and the environment
from exposure to demeton-S-methyl.
The first draft of the monograph was prepared by Dr A. Moretto,
Institute of Occupational Medicine, University of Padua, Italy.
Extensive scientific comments were received following circulation of
the first draft to the IPCS contact points for Environmental Health
Criteria monographs and these comments were incorporated into the
second draft by the Secretariat.
Dr R. Plestina and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively. The efforts of all who helped in the
preparation and finalization of the monograph are gratefully
acknowledged.
ABBREVIATIONS
ACGIH American Conference of Governmental Industrial Hygienists
AChE acetylcholinesterase
ADI acceptable daily intake
a.i. active ingredient
BuChE butyrylcholinesterase
b.w. body weight
ChE cholinesterase
EC50 median effective concentration
GLC gas-liquid chromatography
HID highest ineffective dose
I50 concentration inhibiting 50% of the enzyme activity
i.p. intraperitoneal administration
i.v. intravenous administration
JMPR Joint Meeting on Pesticide Residues
Kd sorption coefficient
LC50 median lethal concentration
LD50 median lethal dose
LED lowest effective dose
LOEC lowest-observed-effect concentration
MRL maximum residue level
NT not tested
NTE neuropathy target esterase
NOAEL no-observed-adverse-effect level
NOEC no-observed-effect concentration
PEC predicted environmental concentration
RBC red blood cell
s.c. subcutaneous
SCE sister chromatid exchange
STS standard type of soil
TER toxicity-exposure ratio
TLC thin layer chromatography
TLV threshold limit value
TWA time-weighted average
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Identity, physical and chemical properties, and analytical
methods
Demeton-S-methyl, a pale yellow oily liquid with a penetrating
odour, is a systemic and contact organophosphate insecticide and
acaricide used to control Acarina, Thysanoptera, Hymenoptera and
Homoptera in fruits, cereals, ornamentals and vegetables. It has a
vapour pressure of 63.8 mPa at 20°C, is readily soluble in most
organic solvents, has a high water solubility of 3.3 g/litre at room
temperature and an octanol-water partition coefficient (log Pow) of
1.32. Demeton-S-methyl is stable in non-aqueous solvents.
Residual and environmental analyses are performed by extraction
with an organic solvent, followed by oxidation to the corresponding
sulfone. Measurement is then performed by gas chromatography, using a
phosphorus-specific detector.
1.1.2 Sources of human and environmental exposure
Prior to 1957, methyl-demeton was marketed as a mixture of
demeton-S-methyl and demeton-O-methyl isomers.
Demeton-S-methyl has been in use since 1957. It is formulated as
an emulsifiable concentrate and used as a spray on cereals, fruits,
ornamentals and vegetables. It is being replaced by oxydemeton-methyl,
which is a plant, soil and mammalian metabolite of demeton-S-methyl.
1.1.3 Environmental transport, distribution and transformation
Hydrolytic degradation of demeton-S-methyl depends on the pH of
the solution; at 22°C the half-life is 63 days at pH 4, 56 days at pH
7 and 8 days at pH 9. In soil, biodegradation is the primary route of
degradation. The half-life of demeton-S-methyl in soil is about 4 h.
However, after 24 h, oxydemeton-methyl still represents 20-30% of the
applied dose of demeton-S-methyl. The sorption coefficient (Kd) of
demeton-S-methyl in soil is 0.68 to 2.66, depending on the soil
composition.
Photolysis is not one of the major mechanisms of degradation of
demeton-S-methyl in the environment.
Metabolism in spring wheat is rapid and similar to that in soil
and mammals.
1.1.4 Environmental levels and human exposure
Primary exposure for the general human population is from
residues of demeton-S-methyl on food crops. The Joint FAO/WHO Meeting
on Pesticide Residues (JMPR) recommended acceptable daily intake (ADI)
is 0.0003 mg/kg body weight. This is a group ADI for demeton-S-methyl,
oxydemeton-methyl and demeton-S-methyl-sulfone, since the routine
analytical methods do not discriminate between these three compounds.
Excessive dermal exposure and absorption of demeton-S-methyl has
caused cholinergic toxicity in workers inadequately protected during
packaging of the concentrate formulation.
When volunteers engaged in a simulated spray activity with a
mixture of demeton-S-methyl and demeton-O-methyl (30 and 70%,
respectively) were exposed to 8.8-27 mg/m3 of the two active
ingredients combined, they experienced no adverse effects on plasma or
erythrocyte cholinesterase activities.
1.1.5 Kinetics and metabolism
Demeton-S-methyl is rapidly and almost completely absorbed from
the intestinal tract of rats and is uniformly (except for high
concentration in erythrocytes) distributed to body tissues. It is
rapidly metabolized and excreted via the urine. Blood concentration
decreases with an initial half-life of about 2 h. About 1% of the oral
dose is present in the body 24 h after treatment. The main metabolic
pathway of demeton-S-methyl in rats is the oxidation of the side
chain leading to the formation of the corresponding sulfoxide
(oxydemeton-methyl) and, to a lesser extent, after further oxidation,
to the sulfone. Another important metabolic route is O-demethylation.
1.1.6 Effects on laboratory animals and in vitro test systems
1.1.6.1 Single exposure
Demeton-S-methyl causes cholinergic toxicity. The LD50 values
for mammals range from 7 to 100 mg/kg body weight, depending on the
route of administration and species.
1.1.6.2 Short-term exposure
An early dietary study showed that rats fed demeton-S-methyl at
50 mg/kg diet had substantially reduced brain and erythrocyte
cholinesterase activity after 26 weeks of exposure. Cholinergic signs
were observed in rats fed 200 mg/kg diet during the first 5 weeks of
exposure.
In a one-year dietary study on dogs, a no-observed-adverse-effect
level (NOAEL) of 1 mg/kg diet (equal to 0.036 mg/kg body weight per
day) was established, based on effects on brain cholinesterase.
1.1.6.3 Long-term exposure
Mice were fed diets containing 0, 1, 15 or 75 mg/kg demeton-S-
methyl for 21 months. The NOAEL was found to be 1 mg/kg diet (equal to
0.24 mg/kg body weight per day) based on inhibition of brain
cholinesterase.
In rats fed diets containing 0, 1, 7 or 50 mg/kg demeton-S-
methyl, the NOAEL, based on inhibition of brain cholinesterase, was
1 mg/kg diet (equal to 0.05 mg/kg body weight per day).
No increased tumour incidence was found in either species.
1.1.6.4 Skin and eye irritation and sensitization
Demeton-S-methyl is a mild skin and eye irritant. Positive
results were obtained with the Magnusson and Klingman maximization
test in guinea-pigs. However, the Buehler epidermal patch test gave no
indication of skin sensitization, suggesting that sensitization should
not be a problem in the practical use of demeton-S-methyl.
1.1.6.5 Reproduction, embryotoxicity and teratogenicity
In a two-generation dietary rat study, demeton-S-methyl caused
reduced viability and body weight of pups (F1b generation only)
at a dose level of 5 mg/kg diet. The NOAEL was 1 mg/kg diet, equal to
0.07 mg/kg body weight per day.
Demeton-S-methyl was neither embryotoxic nor teratogenic in rats
and rabbits.
1.1.6.6 Mutagenicity and related end-points
Demeton-S-methyl induces point mutations in vitro. Chromosomal
effects have been demonstrated in vivo with commercial formulations
only. The available information is insufficient to permit an adequate
assessment of the genotoxic potential of demeton-S-methyl.
1.1.6.7 Delayed neurotoxicity
Demeton-S-methyl caused neither delayed polyneuropathy nor
inhibition of neuropathy target esterase (NTE) when tested in hens at
a level equal to the oral LD50.
1.1.6.8 Toxicity of metabolites
Two plant and mammalian metabolites of demeton-S-methyl
(i.e. oxydemeton-methyl and demeton-S-methylsulfone) are also
commercial pesticides and have been extensively studied. It has been
reported that the toxicological profile of these two compounds does
not significantly differ, either quantitatively or qualitatively, from
that of demeton-S-methyl.
1.1.7 Mechanism of toxicity - mode of action
Demeton-S-methyl is a direct cholinesterase inhibitor, and the
toxicity it causes is related to inhibition of acetylcholinesterase
(AChE) at nerve terminals. AChE inhibited by demeton-S-methyl
reactivates spontaneously with an in vitro half-life of about 1.3 h,
as expected for dimethyl phosphorylated AChE.
1.1.8 Effects on humans
A few cases of acute intoxication with cholinergic syndrome,
following suicide attempts, have been reported. Surviving patients,
including a pregnant woman, did not show delayed effects.
Following careless occupational exposure during packaging of
the commercial formulation, some workers developed cholinergic
toxicity which required pharmacological treatment. Absorption of
demeton-S-methyl was probably through the skin. Similarly, improper
working conditions may have caused excessive dermal absorption during
application of demeton-S-methyl in cotton fields.
1.1.9 Effects on other organisms in the laboratory and field
The 96-h EC50s for green algae range from 8 to 37 mg/litre. The
LC50s for a range of aquatic invertebrates range from 0.004 to
1.3 mg/litre. The toxicity for fish varies, with 96-h LC50 ranging
from 0.59 mg/litre for the rainbow trout to about 40 mg/litre for the
golden orfe, the goldfish and the carp.
The acute oral LD50 for the Japanese quail and the canary is
10-50 mg/kg body weight. In starlings, a single oral dose of 2 mg/kg
body weight caused 20% inhibition of brain AChE 3 h after treatment.
The LC50 of demeton-S-methyl in soil for earthworms is 66 mg/kg
for 14 days. The acute oral and contact LD50 for demeton-S-methyl are
0.21 and 0.6 µg/bee respectively. When used on winter wheat at the
suggested rate, demeton-S-methyl significantly reduced the number of
crop foliage invertebrates (mainly Empididae flies) but not the
number of soil surface entomophagous invertebrates.
1.2 Conclusions
Demeton-S-methyl is a highly toxic (class Ib of the WHO
classification) (WHO, 1996) organophosphorus ester insecticide. The
mechanism of toxicity is that of AChE inhibition at nerve terminals.
Exposure of the general population results mainly from residues
present in crop commodities.
With good work practices, hygienic measures and safety
precautions, the use of demeton-S-methyl during manufacture or
application should not cause adverse effects. Effects due to chronic
exposure are unlikely to occur.
Demeton-S-methyl does not persist in the environment and is not
accumulated by organisms. It has high acute toxicity to aquatic
invertebrates and is toxic to fish and birds, leading to high or
moderate risk factors for these organisms. However, significant field
kills of organisms have not been reported for the compound.
Precautions should be taken to minimize exposure of non-target
organisms (e.g., do not spray over water bodies, minimize exposure by
spray drift).
1.3 Recommendations
For the health and welfare of workers and the general population,
the handling and application of demeton-S-methyl should only be
entrusted to supervised and well-trained operators who follow the
required safety measures and good application practices.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical formula: C6H15O3PS2
Chemical structure:
O
"
CH3CH2SCH2CH2SP(OCH3)2
Relative molecular mass: 230.3
Common name: demeton-S-methyl
CAS chemical name: S-[2-(ethylthio)ethyl] O,O-dimethyl
phosphorothioate
IUPAC name: S-2-ethylthioethyl O,O-dimethyl
phosphorothioate
CAS registry number:
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize
the important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DEMETON-S-METHYL
Members
Dr P.J. Abbott, Australia and New Zealand Food Authority
(ANZFA), Canberra, Australia
Dr K. Barabas, Department of Public Health, Albert Szent-Gyorgyi,
University Medical School, Szeged, Hungary
Dr A.L. Black, Woden, ACT, Australia
Professor J.F. Borzelleca, Pharmacology and Toxicology,
Richmond, Virginia, USA
Dr P.J. Campbell, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries and Food, Kings Pool, York,
United Kingdom
Professor L.G. Costa, Department of Environmental Health,
University of Washington, Seattle, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr I. Dewhurst, Mammalian Toxicology Branch, Pesticides Safety
Directorate, Ministry of Agriculture, Fisheries and Food,
Kings Pool, York, United Kingdom
Dr V. Drevenkar, Institute for Medical Research and Occupational
Health, Zagreb, Croatia
Dr W. Erickson, Environmental Fate and Effects Division,
US Environmental Protection Agency, Washington, D.C., USA
Dr A. Finizio, Group of Ecotoxicology, Institute of Agricultural
Entomology, University of Milan, Milan, Italy
Mr K. Garvey, Office of Pesticide Programs (7501C),
US Environmental Protection Agency, Washington, D.C., USA
Dr A.B. Kocialski, Health Effects Division, Office of Pesticide
Programs, US Environmental Protection Agency,
Washington, D.C., USA
Dr A. Moretto, Institute of Occupational Medicine, University
of Padua, Padua, Italy
Professor O. Pelkonen, Department of Pharmacology and
Toxicology, University of Oulu, Oulu, Finland
Dr D. Ray, Medical Research Council Toxicology Unit, University
of Leicester, Leicester, United Kingdom
Dr J.H.M. Temmink, Department of Toxicology, Wageningen
Agricultural University, Wageningen, The Netherlands
Observers
Dr J.W. Adcock, AgrEvo UK Limited, Chesterford Park, Saffron,
Waldon, Essex, United Kingdom
Mr D. Arnold, Environmental Sciences, AgrEvo UK Ltd.,
Chesterford Park, Saffron Waldon, Essex, United Kingdom
Dr E. Bellet, CCII, Overland Park, Kansas, USA
Mr Jan Chart, AMVAC Chemical Corporation, Newport Beach,
California, USA
Dr H. Egli, Novartis Crop Protection AG, Basel, Switzerland
Dr P. Harvey, AgrEvo UK Ltd., Chesterford Park, Saffron Walden,
Essex, United Kingdom
Dr G. Krinke, Novartis Crop Protection AG, Basel, Switzerland
Dr A. McReath, DowElanco Limited, Letcombe Regis, Wantage,
Oxford, United Kingdom
Dr H. Scheffler, Novartis Crop Protection AG, Basel, Switzerland
Dr A.E. Smith, Novartis Crop Protection AG, Basel, Switzerland
Secretariat
Dr L. Harrison, Health and Safety Executive, Bootle, Merseyside,
United Kingdom
Dr J.L. Herrman, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P.G. Jenkins, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr D. McGregor, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
Dr R. Plestina, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr E. Smith, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P. Toft, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DEMETON-S-METHYL
The Core Assessment Group (CAG) of the Joint Meeting on
Pesticides (JMP) met at the Institute for Environment and Health,
Leicester, United Kingdom, from 3 to 8 March 1997. Dr L.L. Smith
welcomed the participants on behalf of the Institute, and
Dr R. Plestina on behalf of the three IPCS cooperating organizations
(UNEP/ILO/WHO). The CAG reviewed and revised the draft monograph and
made an evaluation of the risks for human health and the environment
from exposure to demeton-S-methyl.
The first draft of the monograph was prepared by Dr A. Moretto,
Institute of Occupational Medicine, University of Padua, Italy.
Extensive scientific comments were received following circulation of
the first draft to the IPCS contact points for Environmental Health
Criteria monographs and these comments were incorporated into the
second draft by the Secretariat.
Dr R. Plestina and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively. The efforts of all who helped in the
preparation and finalization of the monograph are gratefully
acknowledged.
ABBREVIATIONS
ACGIH American Conference of Governmental Industrial Hygienists
AChE acetylcholinesterase
ADI acceptable daily intake
a.i. active ingredient
BuChE butyrylcholinesterase
b.w. body weight
ChE cholinesterase
EC50 median effective concentration
GLC gas-liquid chromatography
HID highest ineffective dose
I50 concentration inhibiting 50% of the enzyme activity
i.p. intraperitoneal administration
i.v. intravenous administration
JMPR Joint Meeting on Pesticide Residues
Kd sorption coefficient
LC50 median lethal concentration
LD50 median lethal dose
LED lowest effective dose
LOEC lowest-observed-effect concentration
MRL maximum residue level
NT not tested
NTE neuropathy target esterase
NOAEL no-observed-adverse-effect level
NOEC no-observed-effect concentration
PEC predicted environmental concentration
RBC red blood cell
s.c. subcutaneous
SCE sister chromatid exchange
STS standard type of soil
TER toxicity-exposure ratio
TLC thin layer chromatography
TLV threshold limit value
TWA time-weighted average
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Identity, physical and chemical properties, and analytical
methods
Demeton-S-methyl, a pale yellow oily liquid with a penetrating
odour, is a systemic and contact organophosphate insecticide and
acaricide used to control Acarina, Thysanoptera, Hymenoptera and
Homoptera in fruits, cereals, ornamentals and vegetables. It has a
vapour pressure of 63.8 mPa at 20°C, is readily soluble in most
organic solvents, has a high water solubility of 3.3 g/litre at room
temperature and an octanol-water partition coefficient (log Pow) of
1.32. Demeton-S-methyl is stable in non-aqueous solvents.
Residual and environmental analyses are performed by extraction
with an organic solvent, followed by oxidation to the corresponding
sulfone. Measurement is then performed by gas chromatography, using a
phosphorus-specific detector.
1.1.2 Sources of human and environmental exposure
Prior to 1957, methyl-demeton was marketed as a mixture of
demeton-S-methyl and demeton-O-methyl isomers.
Demeton-S-methyl has been in use since 1957. It is formulated as
an emulsifiable concentrate and used as a spray on cereals, fruits,
ornamentals and vegetables. It is being replaced by oxydemeton-methyl,
which is a plant, soil and mammalian metabolite of demeton-S-methyl.
1.1.3 Environmental transport, distribution and transformation
Hydrolytic degradation of demeton-S-methyl depends on the pH of
the solution; at 22°C the half-life is 63 days at pH 4, 56 days at pH
7 and 8 days at pH 9. In soil, biodegradation is the primary route of
degradation. The half-life of demeton-S-methyl in soil is about 4 h.
However, after 24 h, oxydemeton-methyl still represents 20-30% of the
applied dose of demeton-S-methyl. The sorption coefficient (Kd) of
demeton-S-methyl in soil is 0.68 to 2.66, depending on the soil
composition.
Photolysis is not one of the major mechanisms of degradation of
demeton-S-methyl in the environment.
Metabolism in spring wheat is rapid and similar to that in soil
and mammals.
1.1.4 Environmental levels and human exposure
Primary exposure for the general human population is from
residues of demeton-S-methyl on food crops. The Joint FAO/WHO Meeting
on Pesticide Residues (JMPR) recommended acceptable daily intake (ADI)
is 0.0003 mg/kg body weight. This is a group ADI for demeton-S-methyl,
oxydemeton-methyl and demeton-S-methyl-sulfone, since the routine
analytical methods do not discriminate between these three compounds.
Excessive dermal exposure and absorption of demeton-S-methyl has
caused cholinergic toxicity in workers inadequately protected during
packaging of the concentrate formulation.
When volunteers engaged in a simulated spray activity with a
mixture of demeton-S-methyl and demeton-O-methyl (30 and 70%,
respectively) were exposed to 8.8-27 mg/m3 of the two active
ingredients combined, they experienced no adverse effects on plasma or
erythrocyte cholinesterase activities.
1.1.5 Kinetics and metabolism
Demeton-S-methyl is rapidly and almost completely absorbed from
the intestinal tract of rats and is uniformly (except for high
concentration in erythrocytes) distributed to body tissues. It is
rapidly metabolized and excreted via the urine. Blood concentration
decreases with an initial half-life of about 2 h. About 1% of the oral
dose is present in the body 24 h after treatment. The main metabolic
pathway of demeton-S-methyl in rats is the oxidation of the side
chain leading to the formation of the corresponding sulfoxide
(oxydemeton-methyl) and, to a lesser extent, after further oxidation,
to the sulfone. Another important metabolic route is O-demethylation.
1.1.6 Effects on laboratory animals and in vitro test systems
1.1.6.1 Single exposure
Demeton-S-methyl causes cholinergic toxicity. The LD50 values
for mammals range from 7 to 100 mg/kg body weight, depending on the
route of administration and species.
1.1.6.2 Short-term exposure
An early dietary study showed that rats fed demeton-S-methyl at
50 mg/kg diet had substantially reduced brain and erythrocyte
cholinesterase activity after 26 weeks of exposure. Cholinergic signs
were observed in rats fed 200 mg/kg diet during the first 5 weeks of
exposure.
In a one-year dietary study on dogs, a no-observed-adverse-effect
level (NOAEL) of 1 mg/kg diet (equal to 0.036 mg/kg body weight per
day) was established, based on effects on brain cholinesterase.
1.1.6.3 Long-term exposure
Mice were fed diets containing 0, 1, 15 or 75 mg/kg demeton-S-
methyl for 21 months. The NOAEL was found to be 1 mg/kg diet (equal to
0.24 mg/kg body weight per day) based on inhibition of brain
cholinesterase.
In rats fed diets containing 0, 1, 7 or 50 mg/kg demeton-S-
methyl, the NOAEL, based on inhibition of brain cholinesterase, was
1 mg/kg diet (equal to 0.05 mg/kg body weight per day).
No increased tumour incidence was found in either species.
1.1.6.4 Skin and eye irritation and sensitization
Demeton-S-methyl is a mild skin and eye irritant. Positive
results were obtained with the Magnusson and Klingman maximization
test in guinea-pigs. However, the Buehler epidermal patch test gave no
indication of skin sensitization, suggesting that sensitization should
not be a problem in the practical use of demeton-S-methyl.
1.1.6.5 Reproduction, embryotoxicity and teratogenicity
In a two-generation dietary rat study, demeton-S-methyl caused
reduced viability and body weight of pups (F1b generation only)
at a dose level of 5 mg/kg diet. The NOAEL was 1 mg/kg diet, equal to
0.07 mg/kg body weight per day.
Demeton-S-methyl was neither embryotoxic nor teratogenic in rats
and rabbits.
1.1.6.6 Mutagenicity and related end-points
Demeton-S-methyl induces point mutations in vitro. Chromosomal
effects have been demonstrated in vivo with commercial formulations
only. The available information is insufficient to permit an adequate
assessment of the genotoxic potential of demeton-S-methyl.
1.1.6.7 Delayed neurotoxicity
Demeton-S-methyl caused neither delayed polyneuropathy nor
inhibition of neuropathy target esterase (NTE) when tested in hens at
a level equal to the oral LD50.
1.1.6.8 Toxicity of metabolites
Two plant and mammalian metabolites of demeton-S-methyl
(i.e. oxydemeton-methyl and demeton-S-methylsulfone) are also
commercial pesticides and have been extensively studied. It has been
reported that the toxicological profile of these two compounds does
not significantly differ, either quantitatively or qualitatively, from
that of demeton-S-methyl.
1.1.7 Mechanism of toxicity - mode of action
Demeton-S-methyl is a direct cholinesterase inhibitor, and the
toxicity it causes is related to inhibition of acetylcholinesterase
(AChE) at nerve terminals. AChE inhibited by demeton-S-methyl
reactivates spontaneously with an in vitro half-life of about 1.3 h,
as expected for dimethyl phosphorylated AChE.
1.1.8 Effects on humans
A few cases of acute intoxication with cholinergic syndrome,
following suicide attempts, have been reported. Surviving patients,
including a pregnant woman, did not show delayed effects.
Following careless occupational exposure during packaging of
the commercial formulation, some workers developed cholinergic
toxicity which required pharmacological treatment. Absorption of
demeton-S-methyl was probably through the skin. Similarly, improper
working conditions may have caused excessive dermal absorption during
application of demeton-S-methyl in cotton fields.
1.1.9 Effects on other organisms in the laboratory and field
The 96-h EC50s for green algae range from 8 to 37 mg/litre. The
LC50s for a range of aquatic invertebrates range from 0.004 to
1.3 mg/litre. The toxicity for fish varies, with 96-h LC50 ranging
from 0.59 mg/litre for the rainbow trout to about 40 mg/litre for the
golden orfe, the goldfish and the carp.
The acute oral LD50 for the Japanese quail and the canary is
10-50 mg/kg body weight. In starlings, a single oral dose of 2 mg/kg
body weight caused 20% inhibition of brain AChE 3 h after treatment.
The LC50 of demeton-S-methyl in soil for earthworms is 66 mg/kg
for 14 days. The acute oral and contact LD50 for demeton-S-methyl are
0.21 and 0.6 µg/bee respectively. When used on winter wheat at the
suggested rate, demeton-S-methyl significantly reduced the number of
crop foliage invertebrates (mainly Empididae flies) but not the
number of soil surface entomophagous invertebrates.
1.2 Conclusions
Demeton-S-methyl is a highly toxic (class Ib of the WHO
classification) (WHO, 1996) organophosphorus ester insecticide. The
mechanism of toxicity is that of AChE inhibition at nerve terminals.
Exposure of the general population results mainly from residues
present in crop commodities.
With good work practices, hygienic measures and safety
precautions, the use of demeton-S-methyl during manufacture or
application should not cause adverse effects. Effects due to chronic
exposure are unlikely to occur.
Demeton-S-methyl does not persist in the environment and is not
accumulated by organisms. It has high acute toxicity to aquatic
invertebrates and is toxic to fish and birds, leading to high or
moderate risk factors for these organisms. However, significant field
kills of organisms have not been reported for the compound.
Precautions should be taken to minimize exposure of non-target
organisms (e.g., do not spray over water bodies, minimize exposure by
spray drift).
1.3 Recommendations
For the health and welfare of workers and the general population,
the handling and application of demeton-S-methyl should only be
entrusted to supervised and well-trained operators who follow the
required safety measures and good application practices.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical formula: C6H15O3PS2
Chemical structure:
O
"
CH3CH2SCH2CH2SP(OCH3)2
Relative molecular mass: 230.3
Common name: demeton-S-methyl
CAS chemical name: S-[2-(ethylthio)ethyl] O,O-dimethyl
phosphorothioate
IUPAC name: S-2-ethylthioethyl O,O-dimethyl
phosphorothioate
CAS registry number:  production of several metabolites. In particular, P. putida and
Nocardia sp. during growth were able to metabolize almost completely
2 mmol/litre of demeton-S-methyl sulfoxide (99% and 98% respectively)
within 13 days. Three major metabolites, i.e., O-demethyl-demeton-S-
methyl, demeton-S-methyl sulfoxide and bis[2-ethylsulfinyl)ether]
disulfide, were found to be produced by Pseudomonas, whereas Nocardia
showed different pathways leading to the formation of different
metabolites, i.e., 2-(ethylsulfonyl)ethane sulfonic acid,
demeton-S-methyl sulfone and bis[2-(ethylthio)ethyl] disulfide. In
sterile controls about 48% of the parent compound remained after 20
days of incubation.
In a laboratory study, biodegradation of demeton-S-methyl was
investigated in two different standardized soils (S1, and S2) with
different characteristics particularly in terms of organic matter
content and cation exchange capability (Wagner et al., 1985). The
study was conducted under aerobic and anaerobic conditions with
sterile controls and using 14C-labelled demeton-S-methyl. One day
after the start of the test no parent compound could be detected.
After 63 days under aerobic conditions 54% (S1) and 34% (S2) of the
14C activity applied was eliminated as 14CO2, indicating a higher
activity in the soil with higher organic matter content and cation
exchange capability. Various metabolites were isolated and identified
(Fig. 1). Under anaerobic conditions 0.5% (S1) and 1.1% (S2) of the
14C activity applied was eliminated as 14CO2, and there was a
predominance of the metabolites O-demethyl-demeton-S-methyl and
demeton-S-methyl sulfoxide. The half-lives of demeton-S-methyl were
approximately 5 h in the non-sterile soil and 70 h in the sterile test
control.
4.2.4 Biodegradation in plants
The metabolic behaviour of ethylene-1-14C demeton-S-methyl in
spring wheat (Schirokko variety) was investigated in a greenhouse test
(Wagner & Oehlmann, 1987). The distribution of the 14C radioactivity
in the wheat matrix was determined at 3, 14, 42 and 60 days after
application of demeton-S-methyl during crop stage 0 (flowering), and
the isolated biotransformation products were analysed by spectroscopy.
The composition of the applied spray was 241.5 µCi ethylene-1-14C
demeton-S-methyl (corresponding to about 0.5 kg a.i./hectare as
opposed to a use rate of 0.15 kg a.i./hectare) in 2 ml benzene plus
1 drop emulsifier Np10 plus 20 ml water. The majority of the applied
radioactivity was found in the wheat straw (about 85% of total
radioactivity corresponding to 10.3 mg a.i. equivalents/kg, 60 days
after application), while the amount in the kernels at harvest was
substantially smaller (0.7 mg a.i. equivalents/kg). About 0.5 mg a.i.
equivalents/kg could not be extracted with water and organic solvents,
and 24% of the 14C activity could not be extracted from the straw
using solvents of different polarity. Only a minor portion of the a.i.
was detected 3 days after application. Identified metabolites were
O,O-dimethyl-S-[2-(ethylsulfonyl)-ethyl]-thiophosphate (11.7% of
recovered radioactivity at 60 days), O,O-dimethyl-S-[2-
(ethylsulfonyl)-ethyl]-thiophosphate (9.8% of recovered radioactivity
at 60 days), 2-ethylsulfonyl-ethanesulfonic acid (8.2% of recovered
radioactivity at 60 days), S-(2-(ethylsulfonyl)-ethyl)-thiophosphate
(5.2% of recovered radioactivity at 60 days), 1-(ethylsulfinyl)-2-
(methylsulfinyl)-ethane (8.9% of recovered radioactivity at 60 days),
and 2-ethylsulfinylethanol (5.1% of recovered radioactivity at 60
days). Additional biotransformation products occurred to a minor
extent (<0.2%) in the kernels only.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 General population exposure
Data on residues in crops resulting from the use of demeton-S-
methyl have been summarized by FAO/WHO (1974). Maximum residue limits,
varying from 0.01 to 1 mg/kg, have been recommended for a range of
commodities. These residue limits were previously expressed as
demeton-S-methyl but now as oxydemeton-methyl, and are common for
demeton-S-methyl, oxydemeton-methyl and demeton-S-methylsulfone
(see section 2.4). More updated values referring to the use of
oxydemeton-methyl have been reported by FAO/WHO (1993). Data on
residue levels in meat and milk from cows, and in chickens, eggs and
fish have been reported for oxydemeton-methyl in the same document
but not for demeton-S-methyl. Some data are also available for
demeton-S-methylsulfone (FAO/WHO, 1993). The JMPR recommended a group
ADI of 0-0.003 mg/kg body weight for demeton-S-methyl,
oxydemeton-methyl and demeton-S-methyl sulfone (FAO/WHO, 1990).
5.2 Occupational exposure during manufacture, formulation or use
When Metasystox (30% demeton-S-methyl, 70% demeton-O-methyl) was
sprayed with a hand-held nebulizer, the concentration of the two
active ingredients combined was 8.8-27 mg/m3 of ambient air (Klimmer
& Pfaff, 1955; see also section 8.2.2).
The ACGIH proposed a very conservative TLV/TWA for methyl-demeton
of 0.5 mg/m3 with the "skin" notation (ACGIH, 1993). The "skin"
notation indicates that dermal absorption is likely to occur and
therefore adequate protective equipment should be used.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption, distribution and excretion
Absorption, distribution and excretion of (ethylene-1-14C)-
demeton-S-methyl (93% radiochemical purity) were studied in SD SPF
rats. Male rats (n=5 per group) were given a single oral dose of 0.1,
0.5, 5 or 10 mg/kg body weight or a single intravenous dose of 0.5 or
1 mg/kg body weight of radio-labelled compound. Female rats (n=5)
received a single oral dose of 0.5 mg/kg body weight. Total
radioactivity recovery was 90-100% in each animal. The authors
reported that kinetic parameters did not change with dose or sex;
therefore, only data on male rats given 5 mg/kg orally have been
reported. Absorption after oral administration was rapid (peak blood
concentration was reached within one hour) and almost complete (98-99%
of the administered radioactivity was, in fact, eliminated through the
urine). The blood concentration decreased with a half-life of about
2 h during the first 6 h and then with a half-life of about 6 h for
the next 48 h. The half-life thereafter was even longer. The
radioactivity associated with erythrocytes accounted for almost all of
the blood radioactivity found 24 h or more after dosing. The half-life
of urinary elimination was 2-3 h during the first 24 h and 1.5 days
thereafter. Elimination through faeces and exhaled air accounted for
0.5-2% and about 0.2% of the applied dose, respectively. Except for
erythrocytes, radioactivity was distributed rather uniformly in
various body tissues and organs. At 2, 24 and 48 h after dosing, the
radioactivity remaining in the body was about 60%, 1% and 0.5% of the
administered dose, respectively. At 10 days, radioactivity was almost
undetectable in most organs except in the erythrocytes. In a separate
experiment, whole-body autoradiography indicated some localized
accumulation of radioactivity in the pineal gland, thyroid and some
glands of the genital tract (Cowper's gland, seminal vesicle,
accessory genital gland). When the labelled compound (0.5 mg/kg body
weight) was administered into the duodenum of rats with cannulated
bile ducts, it was shown that about 3% of the radioactivity was
excreted into the bile in the first 24 h (Weber et al., 1978).
6.2 Metabolic transformation
The proposed metabolic pathway of demeton-S-methyl in rats
is shown in Fig. 2. This was derived from the analysis of urine
samples of SD rats given a single oral dose of 5 or 10 mg/kg of
(ethylene-1-14C)-demeton-S-methyl. Urine samples were collected
for 8 or 24 h after dosing and there was a 92% or 96% recovery,
respectively, of the applied dose. The main metabolic route was
oxidation of the side chain leading to the formation of the
corresponding sulfoxide oxydemeton-methyl, and to a lesser extent,
after further oxidation, the sulfone; O-demethylation was also an
important route. Neither glucuronide nor sulfate conjugates were found
(Ecker, 1978; Ecker & Cölln, 1983).
production of several metabolites. In particular, P. putida and
Nocardia sp. during growth were able to metabolize almost completely
2 mmol/litre of demeton-S-methyl sulfoxide (99% and 98% respectively)
within 13 days. Three major metabolites, i.e., O-demethyl-demeton-S-
methyl, demeton-S-methyl sulfoxide and bis[2-ethylsulfinyl)ether]
disulfide, were found to be produced by Pseudomonas, whereas Nocardia
showed different pathways leading to the formation of different
metabolites, i.e., 2-(ethylsulfonyl)ethane sulfonic acid,
demeton-S-methyl sulfone and bis[2-(ethylthio)ethyl] disulfide. In
sterile controls about 48% of the parent compound remained after 20
days of incubation.
In a laboratory study, biodegradation of demeton-S-methyl was
investigated in two different standardized soils (S1, and S2) with
different characteristics particularly in terms of organic matter
content and cation exchange capability (Wagner et al., 1985). The
study was conducted under aerobic and anaerobic conditions with
sterile controls and using 14C-labelled demeton-S-methyl. One day
after the start of the test no parent compound could be detected.
After 63 days under aerobic conditions 54% (S1) and 34% (S2) of the
14C activity applied was eliminated as 14CO2, indicating a higher
activity in the soil with higher organic matter content and cation
exchange capability. Various metabolites were isolated and identified
(Fig. 1). Under anaerobic conditions 0.5% (S1) and 1.1% (S2) of the
14C activity applied was eliminated as 14CO2, and there was a
predominance of the metabolites O-demethyl-demeton-S-methyl and
demeton-S-methyl sulfoxide. The half-lives of demeton-S-methyl were
approximately 5 h in the non-sterile soil and 70 h in the sterile test
control.
4.2.4 Biodegradation in plants
The metabolic behaviour of ethylene-1-14C demeton-S-methyl in
spring wheat (Schirokko variety) was investigated in a greenhouse test
(Wagner & Oehlmann, 1987). The distribution of the 14C radioactivity
in the wheat matrix was determined at 3, 14, 42 and 60 days after
application of demeton-S-methyl during crop stage 0 (flowering), and
the isolated biotransformation products were analysed by spectroscopy.
The composition of the applied spray was 241.5 µCi ethylene-1-14C
demeton-S-methyl (corresponding to about 0.5 kg a.i./hectare as
opposed to a use rate of 0.15 kg a.i./hectare) in 2 ml benzene plus
1 drop emulsifier Np10 plus 20 ml water. The majority of the applied
radioactivity was found in the wheat straw (about 85% of total
radioactivity corresponding to 10.3 mg a.i. equivalents/kg, 60 days
after application), while the amount in the kernels at harvest was
substantially smaller (0.7 mg a.i. equivalents/kg). About 0.5 mg a.i.
equivalents/kg could not be extracted with water and organic solvents,
and 24% of the 14C activity could not be extracted from the straw
using solvents of different polarity. Only a minor portion of the a.i.
was detected 3 days after application. Identified metabolites were
O,O-dimethyl-S-[2-(ethylsulfonyl)-ethyl]-thiophosphate (11.7% of
recovered radioactivity at 60 days), O,O-dimethyl-S-[2-
(ethylsulfonyl)-ethyl]-thiophosphate (9.8% of recovered radioactivity
at 60 days), 2-ethylsulfonyl-ethanesulfonic acid (8.2% of recovered
radioactivity at 60 days), S-(2-(ethylsulfonyl)-ethyl)-thiophosphate
(5.2% of recovered radioactivity at 60 days), 1-(ethylsulfinyl)-2-
(methylsulfinyl)-ethane (8.9% of recovered radioactivity at 60 days),
and 2-ethylsulfinylethanol (5.1% of recovered radioactivity at 60
days). Additional biotransformation products occurred to a minor
extent (<0.2%) in the kernels only.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 General population exposure
Data on residues in crops resulting from the use of demeton-S-
methyl have been summarized by FAO/WHO (1974). Maximum residue limits,
varying from 0.01 to 1 mg/kg, have been recommended for a range of
commodities. These residue limits were previously expressed as
demeton-S-methyl but now as oxydemeton-methyl, and are common for
demeton-S-methyl, oxydemeton-methyl and demeton-S-methylsulfone
(see section 2.4). More updated values referring to the use of
oxydemeton-methyl have been reported by FAO/WHO (1993). Data on
residue levels in meat and milk from cows, and in chickens, eggs and
fish have been reported for oxydemeton-methyl in the same document
but not for demeton-S-methyl. Some data are also available for
demeton-S-methylsulfone (FAO/WHO, 1993). The JMPR recommended a group
ADI of 0-0.003 mg/kg body weight for demeton-S-methyl,
oxydemeton-methyl and demeton-S-methyl sulfone (FAO/WHO, 1990).
5.2 Occupational exposure during manufacture, formulation or use
When Metasystox (30% demeton-S-methyl, 70% demeton-O-methyl) was
sprayed with a hand-held nebulizer, the concentration of the two
active ingredients combined was 8.8-27 mg/m3 of ambient air (Klimmer
& Pfaff, 1955; see also section 8.2.2).
The ACGIH proposed a very conservative TLV/TWA for methyl-demeton
of 0.5 mg/m3 with the "skin" notation (ACGIH, 1993). The "skin"
notation indicates that dermal absorption is likely to occur and
therefore adequate protective equipment should be used.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption, distribution and excretion
Absorption, distribution and excretion of (ethylene-1-14C)-
demeton-S-methyl (93% radiochemical purity) were studied in SD SPF
rats. Male rats (n=5 per group) were given a single oral dose of 0.1,
0.5, 5 or 10 mg/kg body weight or a single intravenous dose of 0.5 or
1 mg/kg body weight of radio-labelled compound. Female rats (n=5)
received a single oral dose of 0.5 mg/kg body weight. Total
radioactivity recovery was 90-100% in each animal. The authors
reported that kinetic parameters did not change with dose or sex;
therefore, only data on male rats given 5 mg/kg orally have been
reported. Absorption after oral administration was rapid (peak blood
concentration was reached within one hour) and almost complete (98-99%
of the administered radioactivity was, in fact, eliminated through the
urine). The blood concentration decreased with a half-life of about
2 h during the first 6 h and then with a half-life of about 6 h for
the next 48 h. The half-life thereafter was even longer. The
radioactivity associated with erythrocytes accounted for almost all of
the blood radioactivity found 24 h or more after dosing. The half-life
of urinary elimination was 2-3 h during the first 24 h and 1.5 days
thereafter. Elimination through faeces and exhaled air accounted for
0.5-2% and about 0.2% of the applied dose, respectively. Except for
erythrocytes, radioactivity was distributed rather uniformly in
various body tissues and organs. At 2, 24 and 48 h after dosing, the
radioactivity remaining in the body was about 60%, 1% and 0.5% of the
administered dose, respectively. At 10 days, radioactivity was almost
undetectable in most organs except in the erythrocytes. In a separate
experiment, whole-body autoradiography indicated some localized
accumulation of radioactivity in the pineal gland, thyroid and some
glands of the genital tract (Cowper's gland, seminal vesicle,
accessory genital gland). When the labelled compound (0.5 mg/kg body
weight) was administered into the duodenum of rats with cannulated
bile ducts, it was shown that about 3% of the radioactivity was
excreted into the bile in the first 24 h (Weber et al., 1978).
6.2 Metabolic transformation
The proposed metabolic pathway of demeton-S-methyl in rats
is shown in Fig. 2. This was derived from the analysis of urine
samples of SD rats given a single oral dose of 5 or 10 mg/kg of
(ethylene-1-14C)-demeton-S-methyl. Urine samples were collected
for 8 or 24 h after dosing and there was a 92% or 96% recovery,
respectively, of the applied dose. The main metabolic route was
oxidation of the side chain leading to the formation of the
corresponding sulfoxide oxydemeton-methyl, and to a lesser extent,
after further oxidation, the sulfone; O-demethylation was also an
important route. Neither glucuronide nor sulfate conjugates were found
(Ecker, 1978; Ecker & Cölln, 1983).
 7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Demeton-S-methyl causes cholinergic toxicity. The acute toxicity
data are reported in Table 2.
Heath & Vandekar (1957) and Vandekar (1958) showed a significant
increase in intravenous but not oral toxicity after storage of
demeton-S-methyl ("pure") at room temperature in the dark. This was
associated with the formation of sulfonium derivatives, which have a
lower oral toxicity, possibly because of poor absorption.
Dilution with water also increased the intravenous toxicity, and
this was again associated with the formation of sulfonium derivatives.
The maximum toxicity of a 1 mg/ml suspension kept at 35°C for one day
was about 30 times the initial toxicity (the LD50 decreased from
about 60 to about 2 mg/kg in rats) (Heath & Vandekar, 1957). Similar
results were obtained with commercial Metasystox (70% demeton-O-
methyl, 30% demeton-S-methyl) (see also section 2.2.)
7.1.1 Oral
Following oral dosing with demeton-S-methyl performed on a very
small number of animals (one per dose level), one rabbit given
50 mg/kg died within 2 h, while the dose of 20 mg/kg caused symptoms
but the animal recovered. A cat given 10 mg/kg died after 2 days,
while the dose of 5 mg/kg caused signs that were reversible and
animals recovered (few details given) (Hecht, 1955).
Single oral doses (180 mg/kg) of metasystox, containing 25%
demeton-S-methyl, were administered to seven male buffalo calves
(10-12 months of age). Signs of poisoning appeared 15 to 35 min later.
Two calves were treated repeatedly with atropine (1.5 mg/kg body
weight, i.v.), D-tubocurarine (0.1 mg/kg body weight, i.v.) and
glucose (3-4 mg/kg body weight, i.v.). Another two calves were treated
repeatedly with atropine (1.5 mg/kg body weight, i.v.), gallamine
(1 mg/kg body weight, i.v.) and glucose (3-4 mg/kg body weight, i.v.)
Three calves were not treated. All calves displayed typical
cholinergic signs. Treatments delayed but did not prevent death, which
occurred in about 2 h in calves not treated with antidotes and in
about 23 h in calves treated with antidotes (Mitra et al., 1978).
7.1.2 Inhalation
The LC50 (4 h of exposure) for Wistar rats was found to be 310
and 210 mg/m3 for males and females, respectively (Flucke & Pauluhn,
1983).
Table 2. LD50 of demeton-S-methyl for various species and different routes of administration
Species Sex Observation Route Purity LD50 Vehicle References
(strain) period (mg/kg
(days) body weight)
Mouse M 3 oral ? 17 ? Klimmer & Pfaff (1955)
Mouse ? 7 i.v. ? 7 water Hecht (1960)
Rat F 1 oral "pure" 63 ? Heath & Vandekar (1957)
Vandekar (1958)
Rat M 3 days minimum oral ? 40 ? Klimmer & Pfaff (1955)
Rat ? ? oral ? 35 ? Hecht (1955)
Rat M 14 oral 25% formulation 33 water Klimmer (1964)
(Wistar)
Rat M ? oral 86-89% 57-64 Lutrol 9 Klimmer (1964)
(Sprague-
Dawley)
Rat M & F 14 oral 50% formulation 64-65 water Flucke & Pauluhn (1983)
(Wistar)
Rat M 7 oral 50% formulation 129 water Edson (1960)
(Wistar)
Rat M 14 oral 90% formulation 44 cremophor EL Flucke & Kimmerle (1977)
(Wistar)
Table 2. (con't)
Species Sex Observation Route Purity LD50 Vehicle References
(strain) period (mg/kg
(days) body weight)
Rat F 10 oral ? 80 ethanol (20%) DuBois & Doull (1955)
(Sprague- and DuBois & Plzak (1962)
Dawley) propylene
glycol (80%)
Rat M & F 14 i.p. ? 7.5 ethanol (20%) DuBois & Doull (1955)
(Sprague- and propylene DuBois & Plzak (1962)
Dawley) glycol (80%)
Rat ? ? i.p. formulation 10 water Hecht (1960)
Rat ? ? dermal ? 85 ? Edson (1960)
Rat M 14 dermal 50% formulation 71 none Flucke & Pauluhn (1983)
(Wistar)
Rat F 14 dermal 50% formulation 45 none Flucke & Pauluhn (1983)
(Wistar)
Rat ? 7 dermal technical 100-200 none Hecht (1960)
Rat ? 7 dermal 25% formulation about 10 none Hecht (1960)
Rat F 1 i.v. "pure" 65 ? Heath & Vandekar (1957)
Vandekar (1958)
Table 2. (con't)
Species Sex Observation Route Purity LD50 Vehicle References
(strain) period (mg/kg
(days) body weight)
Guinea- M 10 oral ? 110 ethanol (20%) Du Bois & Doull (1955)
pig and propylene
glycol (80%)
Guinea- M 14 i.p. ? 12.5 ethanol (20%) Du Bois & Plzak (1962)
pig and propylene
glycol (80%)
When rats (n=2) and mice (n=4) were exposed for 1 h to 1, 2.5 or
5 g/m3 of demeton-S-methyl (alcohol solution), all mice died whereas
rats displayed signs but recovered (few experimental details given)
(Hecht, 1955).
Rats (n=20) were exposed for 8 h to nebulized demeton-S-methyl
(0.5 g/m3 of air), which was obtained from a 25% emulsifiable
commercial formulation diluted 1:250 (0.1% final concentration of
active ingredient). None of the animals died or showed overt
cholinergic signs. Erythrocyte cholinesterase activity, determined in
three animals immediately after the end of exposure, was reduced by
70%. When purified active ingredient was used, lower erythrocyte
cholinesterase inhibition (60%) was found under the same experimental
conditions. This was paralleled by an increased rat LD50 (from 10 to
27.5 mg/kg i.p.) (Hecht, 1960).
7.1.3 Dermal
Doses of 20 or 100 mg/kg demeton-S-methyl applied to the shaved
skin of two cats caused death. A dose of 10 mg/kg caused mild signs;
very few details were given (Hecht, 1955).
7.2 Short-term exposure
7.2.1 Rat
Groups of six male rats (strain not specified) were fed
demeton-S-methyl at levels of 0, 50, 100 or 200 mg/kg diet in
the diet (equivalent to 0, 5, 10 and 20 mg/kg body weight per day,
respectively) for 6 months. Cholinergic signs (slight tremors and
fasciculations) were observed at the highest dose-level during the
first 5 weeks of the study. Brain and erythrocyte cholinesterase
activities were reduced at 50 mg/kg diet (by about 80 and 88%,
respectively), 100 mg/kg diet (by about 85 and 92%) and 200 mg/kg diet
(by about 90 and 94%) groups. Body weight gain was depressed at 100
and 200 mg/kg diet. Gross microscopic examination of tissues (liver,
kidney and adrenals) showed no treatment-related changes (Vandekar,
1958).
Groups of 30-day-old female Holtzman rats were fed dietary
levels of 0, 1, 5 or 25 mg/kg diet of demeton-S-methyl (purity not
reported) for 1 week. At termination, serum, liver and brain
acetylcholinesterase (n=3) and liver and serum aliesterase (n=3) with
diethylsuccinate and tributyrin as substrates activities were
measured. Interpolated dietary levels producing 50% inhibition were
15-28 mg/kg diet for acetylcholinesterase, 4-6 mg/kg diet for liver
aliesterase and about 25 mg/kg diet for serum aliesterase, equivalent
to 1.5-2.8, 0.4-0.6 and 2.5 mg/kg body weight per day, respectively
(Su et al., 1971).
7.2.2 Dog
In a one-year study, pure-bred beagle dogs (n=6 animals of each
sex per group) were fed 0, 1, 10 or 100 mg a.i./kg diet (day 1-36) or
50 mg a.i./kg diet (day 37-termination) of demeton-S-methyl (52.2% in
xylene). Haematological, blood biochemical and urinalysis parameters
were determined during pretest period and at months 1, 2, 3, 4, 5, 6,
8, 10, 12. Hearing tests and ophthalmoscopic examinations were
performed once in the pretest period and at months 3, 6 and 12 of
treatment. At termination animals were killed for pathology, and
determination of organ weights, brain cholinesterase activity, hepatic
cytochrome P-450 and triglyceride contents and N-demethylase
activity were carried out.
All animals survived the study. Diarrhoea and vomiting were
observed in all animals, most frequently in the high-dose group: these
animals also showed reduced food consumption before the dose was
reduced to 50 mg/kg diet. The mean daily compound intake was found to
be 0.036, 0.36 and 4.6/1.5 mg/kg body weight at 1, 10 and 100/50 mg/kg
diet, respectively. Body weight was similar in all groups. No
alterations were observed in the hearing test and ophthalmoscopic
examination. Haematological, blood chemistry (excluding cholinesterase
activities) and urinalysis parameters and organ weights at termination
were not significantly altered by any of the treatments. Hepatic
biochemical parameters were not altered by any of the treatments. No
treatment-related gross pathology alterations were found. However,
multifocal slight/moderate atrophy and/or hypertrophy of proximal
renal tubules was demonstrated in three males and three females of the
high-dose group. Plasma cholinesterase activity was reduced as
compared to controls by 20-30% and 5-20% in males and females,
respectively, at 10 mg/kg diet, and by 45-65% (males) and 50-70%
(females) at 50 mg/kg diet. Erythrocyte cholinesterase activity was
reduced by 25-35% and 30-45% in males and females, respectively, at
10 mg/kg diet. A higher inhibition was found at 50 mg/kg diet, where
inhibition was 80-90% and 55-65% in males and females, respectively.
Brain cholinesterase activity was reduced by 25% in males at 10 mg/kg
diet and by 64% (males) and 15% (females) at 50 mg/kg diet. Based on
effects on brain cholinesterase activity, the NOAEL was 1 mg/kg diet,
equal to 0.036 mg/kg body weight per day (Bathe, 1983).
7.3 Long-term exposure
7.3.1 Mouse
A long-term carcinogenicity study was conducted in NMRI mice
(70 animals of each sex per group) that were given demeton-S-methyl
(about 50% in xylene) mixed into the feed with approximately 10 mg/kg
diet of groundnut oil at concentrations of 0, 1, 15 or 75 mg a.i./kg
diet, or xylene (75 mg/kg diet). Groups were subdivided into two
subgroups: one (n=20) was terminated at 12 months, the second one was
terminated at 21 months.
Animals in the high-dose group had a lower (significantly lower
during the first 4 weeks only) food consumption and a reduced (about
10% throughout the study in males only, and at the beginning in
females) body weight. The mean daily intake of demeton-S-methyl was
(males/females): 0.24/0.29, 3.47/4.18, 17.81/20.0 mg/kg body weight
at 1, 15, 75 mg/kg diet, respectively. Clinical signs due to
cholinesterase inhibition were not observed. Mortality at 21 months
was (males/females) 16/30, 13/34, 17/35, 16/35 and 16/32% in the
control, low-, mid-, high-dose and xylene groups, respectively.
Haematological and clinical chemical parameters were not affected by
the treatment except plasma urea (lower than control in the high-dose
males) and plasma and erythrocyte cholinesterase activities. Plasma
cholinesterase activity was significantly decreased in mid- (by
63-74%) and high-dose (by 91-97%) groups. Erythrocyte cholinesterase
activity was only slightly reduced in the mid- and high-dose groups.
Brain cholinesterase activity (n=10 per group) was reduced in
high-dose groups (in males by 70% and in females by 59%) and in the
mid-dose group (in males by 44% and in females by 38%). Histological
examination did not reveal an increased incidence of neoplastic and
non-neoplastic lesions in treated groups. Based on inhibition of brain
cholinesterase, the NOAEL was 1 mg/kg diet, equal to 0.24 mg/kg body
weight per day (Schmidt & Bomhard, 1988).
7.3.2 Rat
Wistar rats (60 animals of each sex per group) were given
demeton-S-methyl (about 50% in xylene mixed into the feed with
approximately 10 mg/kg of groundnut oil) at concentrations of 0, 1, 7
or 50 mg a.i./kg diet, or 50 mg/kg diet of xylene. Groups were
subdivided into two subgroups; one (n=10) was terminated at 12 months,
the second one was terminated at 24 months.
Hair loss (up to 50% of females) and diarrhoea (up to 50% of
males) were observed significantly more frequently in the animals of
the high-dose group. Body weight was reduced in mid-dose males (by
5-10%) and in both males (by 10-20%) and females (by 5%) in the
high-dose group. Food consumption was similar in all groups. The mean
daily intake of demeton-S-methyl was (males/females): 0.05/0.06,
0.31/0.41, 2.59/3.09 mg/kg body weight at 1, 7 and 50 mg/kg diet,
respectively. Mortality at 24 months was (males/females) 12/26, 10/20,
10/20, 4/26 and 8/24% in the control, low-, mid-, high-dose and xylene
groups, respectively. Haematological and clinical chemical parameters,
except for cholinesterases, measured at months 6, 12, 18 and 24, were
unaffected by the treatments. Plasma and erythrocyte cholinesterase
activities, measured at months 3, 6, 12 and 24, were significantly
decreased in groups given 7 mg/kg diet (plasma cholinesterase by
30-56%, erythrocyte cholinesterase by 12-31%) or 50 mg/kg diet (plasma
cholinesterase by 75-92%, erythrocyte cholinesterase by 20-44%). Brain
cholinesterase activity (measured at 12 and 24 months) was reduced in
the high-dose group (by 67-75%) and in the mid-dose group (by 15-47%).
A statistically significant decrease in brain cholinesterase activity
(33%) was observed in males given 1 mg/kg diet at 24 months but not at
12 months. The toxicological significance of this finding is not
clear; it should also be noted that brain cholinesterase activity at
7 mg/kg diet was higher than at 1 mg/kg diet and that plasma
cholinesterase and erythrocyte cholinesterase activities were not
inhibited at either of these dose levels. Histological examination did
not reveal an increased incidence of neoplastic lesions in treated
groups. Increased incidence of retinal atrophy (78% of males, 92% of
females as compared to 36-63% and 61-70% in the other groups) and
keratitis (44% of males, 22% of females as compared to 4-12% and 0-2%,
respectively, in the other groups) was observed at 50 mg/kg diet. The
retinal atrophy mainly affected the fundus, unilaterally or
bilaterally, and occurred in either the inner corneal layer, the inner
and outer layers, or all layers of the retina. Based on inhibition of
brain cholinesterase, the NOAEL was considered to be 1 mg/kg diet,
equal to 0.05 mg/kg body weight per day (Schmidt & Westen, 1988).
7.4 Skin and eye irritation and sensitization
7.4.1 Skin and eye irritation
Demeton-S-methyl (commercial formulation, 50% of active
ingredient) was applied (0.5 ml) to the shaved skin of three New
Zealand white rabbits on a 2.5 × 2.5 cm cellulose dressing for 4 h.
Mild erythema and oedema were observed, which generally disappeared
after 3 days (Flucke & Pauluhn, 1983). A 52.5% solution of
demeton-S-methyl in xylene produced slight skin and eye irritancy in
New Zealand white rabbits, but this was attributed to the solvent
(Thyssen, 1981).
A commercial formulation of demeton-S-methyl (50%) was instilled
(0.1 ml) either undiluted or as a 0.5% aqueous dilution into the
conjunctival sac of one eye of groups (n=3) of New Zealand white
rabbits. Treated eyes were washed with physiological solution after
24 h. No signs of eye irritation were observed in rabbits treated with
the 0.5% aqueous solution. The undiluted formulation caused severe
lacrimation and miosis on application. Mild corneal opacity and
discrete redness and oedema of conjunctivae were observed and
recovered in about 7 days (Flucke & Pauluhn, 1983).
7.4.2 Skin sensitization
The skin-sensitizing potential of demeton-S-methyl was assessed
by the Magnusson and Kligman maximization test with Freund's adjuvant
on guinea-pigs (n=20, Bor:SPF, DHPW strain). The concentrations of
demeton-S-methyl (96.3% purity, average of three determinations) used
were: 0.1% for the intra-dermal induction, 10% for the topical
induction and the first challenge, and 1% for the second challenge.
All twenty animals reacted positively to the 1st challenge (controls
4/10), and 16 reacted positively to the 2nd challenge (controls 3/10).
The results indicate that demeton-S-methyl has a skin-sensitizing
potential (Heimann, 1987a).
In another study, the Buehler epidermal patch test was used on
guinea-pigs (n=12, Bor: SPF, DHPW strain). The concentrations of
demeton-S-methyl (95.6% purity, average of three determinations) used
were 10% for topical induction (once a week for 3 weeks) and the first
challenge, and 20% for the second challenge. The results indicate that
demeton-S-methyl does not have a skin-sensitizing potential under
these conditions (Heimann, 1987b).
It is concluded that demeton-S-methyl might have some
skin-sensitizing potential, but this is of no relevance in practice.
7.5 Reproduction, embryotoxicity and teratogenicity
7.5.1 Reproduction
A standard two-generation study (two litters/generation) was
conducted in SPF rats (BOR:WISW) (10 males and 20 females) that were
given demeton-S-methyl at 0, 1, 5 or 25 mg/kg diet (Eiben et al.,
1984). The compound was used as a pre-mix in xylene (about 50%). Rats
in an extra control group were given xylene at 25 mg/kg diet.
In the F0 generation, none of the animals died. No treatment-
related signs were observed in any animal. Body weight gain was
reduced in males (by 10%) and in some females at 25 mg/kg diet. Food
intake was also reduced (by 7%) in high-dose males. Fertility index
was not affected by treatment. At 25 mg/kg diet, the viability of pups
was reduced; it was 89% and 85% in first and second matings,
respectively. Lactation index was also reduced in the high-dose group
(85-92%), the control value being 98-99%. Body weight at birth was
comparable in all groups, but body weight gain was significantly
reduced (by 8-10%) in pups fed 25 mg/kg diet.
In the F1b generation, one female was found dead in the 5-mg/kg
diet group and one in the 25-mg/kg diet group; one male and one female
in one of the 25-mg/kg diet litters also died. Autopsy did not reveal
treatment-related alterations. No treatment-related signs were
observed in any animal. Body weight gain was reduced at times in
low-dose males and consistently in mid- and high-dose and xylene-
treated males when compared to untreated animals. When compared to
xylene-treated animals (which were 5-15% lighter than untreated
animals), however, only males of the high-dose group had a
significantly reduced (by about 15%) body weight gain. Females of the
high-dose and xylene groups had a reduced (by about 10%) body weight,
compared to controls, the former being at times lighter weight than
the latter. Fertility index was not significantly affected. The number
of pups born was reduced in the high-dose group and the viability of
pups was also reduced in the mid- and high-dose groups in a
dose-related manner (82-88% and 47-67% of controls, respectively).
No compound-related malformation was found in animals of any of
the treatment groups. Demeton-S-methyl intake, as calculated in the
F0 generation, was found to be (female data in parentheses): 0.07
(0.08), 0.32 (0.39) and 1.71 (1.90) mg/kg body weight per day in the
low-, mid- and high-dose groups, respectively, and xylene intake was
1.66 (1.96) mg/kg body weight per day. Based on the viability of pups
and body weight in the F1b generation, the NOAEL was 1 mg/kg diet,
equal to 0.07 mg/kg body weight per day (Eiben et al., 1984).
7.5.2 Embroytoxicity and teratogenicity
7.5.2.1 Rat
Groups (n=25) of fertilized female rats (BAY:FB 30 strain) were
given daily (0, 0.3, 1 or 3 mg/kg body weight orally) demeton-S-methyl
(from a 52.6% solution in xylene) dissolved in corn oil from day 6 to
15 of gestation. At day 20 of gestation, pups were delivered by
caesarean section. Fetuses were weighed, sexed, inspected for external
abnormalities and examined for visceral and bone malformations. No
alteration of physical appearance or behaviour was observed in any
group. All animals survived until the caesarean section. Body weight
gain was reduced (by 13%) in the high-dose group. The numbers of live
fetuses and resorptions, fetal weight, number of fetuses with
malformations and number of implants were comparable in all groups. No
treatment-related visceral or skeletal abnormalities were observed
(Renhof, 1985).
7.5.2.2 Rabbit
A formulation of demeton-S-methyl (52.2% a.i. in xylene) was
administered by gavage to mated chinchilla hybrid rabbits (n=15-16) on
gestation days 6 to 18 at dose levels of 0, 3, 6 and 12 mg/kg body
weight per day. Caesarean sections were performed on gestation day 28.
There were no mortalities. In the high-dose group, diarrhoea was
observed in all animals after 4 to 10 days of treatment. Beginning 1
to 2 h after dosing, it persisted for 6 to 24 h. In the high-dose
group, mean food consumption was decreased by 7% during gestation days
6 to 18 and by 17% during gestation days 19 to 24 when compared to
controls. This was associated with decreased mean body weight gain
(-7%). There were no abortions and no relevant differences between
test and control groups in the numbers of implantations per dam, pre-
implantation losses, post-implantation losses, resorptions, living and
dead fetuses or sex ratios. A decrease in mean fetal body weight,
compared to the mean control weight, of 6.6% was observed in the
high-dose group. There was no treatment-related increase in gross,
skeletal or visceral malformations (Becker, 1983).
7.6 Mutagenicity and related end-points
A summary of the studies conducted to assess mutagenicity of
demeton-S-methyl is given in Table 3.
7.6.1 DNA damage and repair
Demeton-S-methyl did not induce DNA damage in the Pol test on
Escherichia coli either with or without metabolic activation.
7.6.2 Mutation
Increased mutation rates were observed in the Ames test and in
the mouse lymphoma forward mutation assay both with and without
metabolic activation.
7.6.3 Chromosomal effects
In in vivo tests, no SCEs were found in the bone marrow of
Chinese hamsters treated with high doses of demeton-S-methyl.
Bone marrow micronucleus and dominant lethal tests on mice
treated with demeton-S-methyl gave negative results. Chromosomal
aberrations were found in the bone marrow of Syrian hamsters treated
with a commercial formulation of demeton-S-methyl.
It is concluded that the available information is insufficient to
permit an adequate assessment of the genotoxic potential of
demeton-S-methyl.
7.7 Delayed neurotoxicity
Adult Leghorn hens (n=20) were given two doses of 100 mg a.i./kg
body weight (approximately equal to the LD50) of demeton-S-methyl
(51.2% in xylene) by gavage. The second dose was given 21 days
after the first one. Positive control animals (n=5) received
tri- ortho-cresyl phosphate (TOCP) (375 mg/kg body weight by gavage).
Animals were pretreated with atropine (100 mg/kg body weight
intramuscularly 10 min before the dose of demeton-S-methyl and
50 mg/kg body weight subcutaneously 6 h later). Surviving animals
received atropine (30 mg/kg s.c.) 24, 30 and 48 h later. At the second
dose, atropine treatment was suspended after 24 h. Hens treated with
demeton-S-methyl had signs of cholinergic toxicity. The recovery
started on day 3 and by day 8 all treated animals, except for one,
were free of signs. After the second dose, the recovery started on day
2 and by day 5 all treated animals were free of signs. One animal died
after the second treatment and surviving animals did not develop
neurological deficits. TOCP-treated animals showed locomotor
Table 3. Studies on mutagenicity of demeton-S-methyl
Test Organism Purity Results LED or HIDa Reference
-S9 +S9
Microorganisms
Pol assay Escherichia coli p3478, 93% - - 10 000 µg/plate Herbold (1983a)
W3110
Reverse Salmonella typhimurium unknown + n.t. 5 µg/plate Hanna & Dyer (1975)
mutation TA1530, TA1535, his C117,
his G46
E. coli WP2, WP2 uvra, unknown + n.t. 5 µg/plate Hanna & Dyer (1975)
CM561, CM571, CM611, WP67,
WP12,
S. typhimurium TA98, TA100, 50.2% ++ 300 µg/plate Herbold (1979)
TA1535, TA1537 (formulation)
S. typhimurium TA98, TA100, >98% ++ 20 µg/plate Herbold (1980a)
TA1535, TA1537
Saccharomyces cerevisiae 53.1% -- 1062 µg/ml Hoorn (1982)
S138 S211ý (formulations
in xylene)
Insects
Recessive Drosophila melanogaster unknown + 80 mg/kg diet Hanna & Dyer (1975)
lethal
Table 3. (con't)
Test Organism Purity Results LED or HIDa Reference
-S9 +S9
Cultured mammalian cells
Mutation, tk Mouse lymphoma L5178Y cells 94% ++ 50 µg/ml Cifone (1984)
locus
Mammals in vivo
Bone marrow NMRI mouse >98% - 2 × 5 mg/kg b.w. Herbold (1980b)
micronucleus oral
SCE in bone Chinese hamster 94% - 20 mg/kg b.w. oral Herbold (1983b)
marrow
Chromosomal Syrian hamster, female 50% + 2 mg/kg b.w. i.p. Dzwonkowska & Hübner (1986)
aberration (commercial)
Dominant NMRO mouse >98% - 5 mg/kg b.w. oral Herbold (1980c)
lethal
a LED = lowest effective dose; HID = highest ineffective dose
impairment beginning on day 10. Histological examination showed
moderate axonal degeneration in peripheral nerves and medulla in
TOCP-treated animals but not in demeton-S-methyl-treated or solvent
control animals (Flucke & Kaliner, 1988).
Neuropathy target esterase (NTE), the target for organophosphate-
induced delayed neuropathy, was not inhibited in hen brain and
spinal cord 1, 2 and 7 days after treatment with demeton-S-methyl
(80 mg a.i./kg body weight) by gavage. Positive controls (TOCP at
100 mg/kg body weight) showed NTE inhibition (> 90%) in both brain
and spinal cord (Flucke & Eben, 1988).
7.8 Toxicity of metabolites
Two plant and mammalian metabolites of demeton-S-methyl (namely
oxydemeton-methyl and demeton-S-methylsulfone) have been studied
extensively, since they are also the active ingredient of commercial
pesticides. According to the JMPR, the toxicity of the two compounds
does not differ substantially, either qualitatively or quantitatively,
from that of demeton-S-methyl (FAO/WHO, 1990).
7.9 Mechanism of toxicity - mode of action
Demeton-S-methyl is a direct cholinesterase inhibitor and
it causes signs and symptoms of the cholinergic syndrome. The
in vitro I50 (30 min, 37°C) for sheep erythrocyte cholinesterase
was 6.5 × 10-5 mol/litre. The I50 of oxydemeton-methyl and
demeton-S-methylsulfone were of the same order of magnitude
(2.7 × 10-5 and 4.3 × 10-5 mol/litre, respectively). The half-life
of recovery of acetylcholinesterase activity after inhibition by
demeton-S-methyl was 1.3 h, as expected from a dimethyl phosphorylated
acetylcholinesterase (Heath & Vandekar, 1957).
The 1973 JMPR (FAO/WHO, 1974) reported that rat brain
cholinesterase was more sensitive to in vitro inhibition by demeton-
S-methyl than by oxydemeton-methyl (I50 values of 9.52 × 10-5 and
1.43 × 10-3 mol/litre, respectively; time of incubation, temperature
and pH not reported). It was also reported that demeton-S-methyl was a
more potent inhibitor of human serum cholinesterase (no details
given).
Data on in vivo inhibition of plasma cholinesterase and
erythrocyte and brain cholinesterase are reported in section 7.2 and
7.3.
7.10 Potentiation
Male Wistar rats (160-180 g body weight) were given trichlorfon
(98.6% purity) and demeton-S-methyl (90% purity) in combination by
gavage. The amount of compound in the mixture was proportional to its
oral LD50 (302 mg/kg body weight for trichlorfon and 44 mg/kg body
weight for demeton-S-methyl) in order to obtain equitoxic doses. The
resulting toxicity was additive (LD50 of the mixture was 223 mg/kg
body weight against expected 173 mg/kg body weight) (Flucke &
Kimmerle, 1977).
Similarly, an additive effect was obtained when demeton-S-methyl
was given in combination with phenamiphos (ethyl-4-(methylthio)
m-tolyl-isopropyl-phosphoroamidate). The experimental LD50 of the
mixture was 55 mg/kg body weight while that estimated from the
individual LD50s was 50 mg/kg body weight (Kimmerle, 1972).
8. EFFECTS ON HUMANS
8.1 General population exposure
A 31-year old woman attempted suicide with an unknown amount
("1 or 2 mouthfuls") of Metasystox I (25% demeton-S-methyl). On
admission to hospital she was comatose, sweating and salivating,
and had pin-point pupils. She stayed in the Intensive Care Unit for 15
days where she was treated with atropine (up to 97 mg per day, 550 mg
in 14 days). Oximes (2-PAM) were only given on day 1 (0.5 + 0.25 +
0.25 g) and there was no apparent clinical improvement. On admission,
plasma and erythrocyte cholinesterase activities were less than 10% of
normal control values. The patient was discharged on the 30th day with
normal plasma cholinesterase values; erythrocyte cholinesterase
activity was still below the normal values (about 65%), but had
recovered a month later (Barr, 1966).
A case of acute poisoning in a 5-month pregnant woman has been
described (Carrington da Costa et al., 1982). A 41-year-old woman
attempted suicide by ingesting Metasystox, which resulted in an
estimated intake of 12 g methyl demeton (it should be noted that in
the report Metasystox was said to contain methyl-demeton and not
oxydemeton-methyl as the commercial name implies). On admission, 3.5 h
after poisoning, blood cholinesterase (it was not specified whether
pseudo- or acetylcholinesterase) was 10% of normal values. The patient
became comatose 12 h after admission. She was treated with atropine,
obidoxime and haemoperfusion 72 h after hospitalization. Artificial
ventilation was required on the 4th day of hospitalization. The
patient was discharged after 24 days and 4 months later she delivered
a healthy female child.
In a fatal suicidal case with a commercial formulation of
demeton-S-methyl, the concentration of the compound was measured in
several organs. It was estimated that death occurred about 6 h after
poisoning. Highest demeton-S-methyl levels were found in the proximal
small intestine (166 mg/kg of tissue); in brain, kidney and muscles,
the levels were 30-80 mg/kg; lowest concentrations were found in liver
(7 mg/kg tissue) and blood (7-16 mg/litre). Metabolites were not
measured (Schludecker & Aderjan, 1988).
In the United Kingdom in 1991 there were five cases of poisoning
by demeton-S-methyl and in 1994 three cases, none of which were fatal
(personal communication by G. Volans, Medical Toxicology Unit, London,
to the IPCS, dated 15 January 1996).
Hegazy (1965) reported three cases of suspected poisoning with
demeton-S-methyl in children (6-14 years of age) exposed in a recently
sprayed field and 1 case after ingestion of contaminated feed.
Symptoms were mild and the patients recovered fully. Serum
cholinesterase determinations gave ambiguous results.
8.2 Occupational exposure
8.2.1 Acute poisoning
A worker inadvertently exposed to demeton-S-methyl (no details)
was monitored for about 100 days. Plasma cholinesterase activity was
always within the normal values of the laboratory. However, if the
activity on day 40 and 100 is considered as the normal value for this
worker, then a 30% inhibition occurred 2-3 days after exposure. The
activity recovered with an half-life of about 10 days. Erythrocyte
cholinesterase activity was below the normal value for about 40 days.
When calculated on the activity of day 100, a 60% inhibition was found
up to day 10. The activity recovered with a half-life of about 35 days
(Lewis et al., 1981).
Two workers in a chemical packaging company, whose job it was to
fill a concentrate of demeton-S-methyl (500 g/litre of xylene) into
one-litre containers using a weight-triggered bottle-filling machine
for 3 to 4 h, were admitted to hospital because of organophosphate
poisoning and were treated with atropine. Cholinesterase measurement
made 14 days after exposure (1 worker) showed inhibited erythrocyte
cholinesterase, but the plasma cholinesterase activity was within the
normal range. In the second worker, whose symptoms lasted for 3 days,
erythrocyte cholinesterase activity was below the normal value 5 weeks
after exposure. These workers wore gloves, overalls and boots, but
frequent spillage was reported. Normal clothing had been left under
the filling apparatus and apparently was contaminated. The filling
system was changed by housing the filling machine in a fume cupboard
and by providing new protective garments and changing room facilities.
One more worker was admitted to the hospital and treated with atropine
because of organophosphate poisoning. This occurred on the morning
after a day spent fitting the infill seal and screwing on the tops
of cans. He had reduced erythrocyte and plasma cholinesterase
(erythrocyte > plasma) activities for several days after exposure.
Another worker showed a sharp drop in erythrocyte and plasma
cholinesterase activities associated with abdominal cramps, which
resolved in about 5 days. Another worker had depressed erythrocyte and
plasma cholinesterases activities without complaining of any adverse
effect. It was concluded that absorption of demeton-S-methyl was
through the skin because of penetration of the protective clothing
used or because this clothing was not worn properly. It should be
noted that the active ingredient was dissolved in xylene, which is
known to attack rubber and plastics, making the penetration of gloves
possible (Jones, 1982).
8.2.2 Effects of short- and long-term exposure
Three volunteers without any protective equipment were exposed
for two consecutive days to Metasystox (30% demeton-S-methyl, 70%
demeton-O-methyl) while spraying with a hand-held nebulizer. Exposure
lasted for 3 and 6 h on the first and second days, respectively. The
concentrations of the active ingredient (isomers not separated) were
8.8-27 mg/m3 of ambient air. Plasma and erythrocyte cholinesterase
activities measured up to 14 days after exposure did not show
significant decreases when compared to pre-exposure values (Klimmer &
Pfaff, 1955).
Volunteers, wearing overalls but not mask protection, were
exposed to metasystox (30% demeton-S-methyl, 70% demeton-O-methyl)
while spraying in a greenhouse. They used the splash method
(0.03-0.05% a.i.), the low volume (0.5% a.i.) or the high volume spray
method (0.05% a.i.) for 5 to 25 min. There was no effect on plasma or
erythrocyte cholinesterase activity measured after exposure (Klimmer &
Pfaff, 1958).
Six workers engaged in hop cultivation using Metasystox I
(reported to contain demeton-O-methyl instead of demeton-S-methyl as
the commercial name implies) were monitored. They sprayed up to
2400 litres of a 0.1% solution (in water) of the insecticide in one
day. Protective clothing and masks were not always used. No
significant inhibition of blood acetyl cholinesterase was observed at
the end of exposure or 1 or 2 days later. One subject, who was exposed
twice, showed a 29% decrease in blood acetyl cholinesterase after the
second exposure. No signs or symptoms were observed in these workers
(Winkler & Arent, 1970).
The medical department of a company reported no adverse effect in
workers employed in the formulation of demeton-S-methyl from 1967 to
1984 (Faul, 1984).
Agricultural workers exposed to demeton-S-methyl for 3
consecutive days were monitored. Pre-and post-exposure urinary levels
of the metabolite dimethyl phosphorothiolate potassium salt (DMPThK),
and plasma and whole blood cholinesterase activities were measured.
Exposed subjects were divided into three groups according to their
job, i.e. mixers (n=7), sprayers (n=6) and others (n=7) not directly
involved in handling the pesticide. Higher levels of DMPThK were
found in mixers, with a medium value of 83 µg/litre and a range of
0-822 µg/litre (neither corrected for creatinine nor for urine
volume). Sprayers had a mean value of 30 µg/litre (limit of detection)
and a range of 0-208 µg/litre; the other subject had a mean value of
30 µg/litre and a range of 0-100 µg/litre. Whole blood cholinesterase
activity was not affected by the exposure, while plasma cholinesterase
activity was slightly (about 10%, statistically significant) reduced
when compared to pre-exposure levels in mixers. However, no
correlation was found between DMPThK levels and plasma cholinesterase
activity (Vasilic et al., 1987).
Hegazy (1965) reported a study of 121 spraymen exposed to
Meta-isosystox during spraying of cotton fields in Egypt. The spraymen
applied Meta-isosystox at a rate of 0.5 litres of concentrate in
400 litres of spray per 4200 m2 mainly by hand-operated knapsack
sprayers, or in a few cases by high-pressure motor-powered sprayers
with a large tank connected by a long hose to a multi-nozzled
spray-boom. Sprayers were not involved in chemical mixing. Workmen
washed exposed body parts with soap and water after spraying. Working
clothes were removed at the end of the day, but the clothes may not
have been washed before re-use. Not all workers used protective
clothing and masks. None of the workers were re-exposed to
Meta-isosystox after the onset of symptoms. Serum cholinesterase
activity estimates were performed within 24 h after the onset of
symptoms in some patients and after the cessation of symptoms in some
others. In most cases they were repeated 2-3 times at various
intervals up to 40 days from the onset of symptoms. In general, the
serum cholinesterase activity in spraymen underwent a marked initial
fall, followed by a rise to above-normal levels after about 30-40
days. Signs and symptoms of toxicity in spraymen occurred after 1-18
days of exposure, with a mean time of 3 days. These consisted of
gastrointestinal disturbances (58% of total), dizziness (23%),
persistent general weakness and fatigue (19%), respiratory
manifestations (16%), headache (16%), sweating, salivation or
lacrimation (12%), tremors of outstretched hands, intention tremors,
ataxia (4.1%), exaggerated superficial and deep reflexes (5.8%),
hiccough (2.5%), muscular fasciculations (2.5%), or had apparently
resolved (30%), at the first determination of serum cholinesterase
activity (Hegazy, 1965).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Aquatic organisms
9.1.1 Algae
Table 4 reports the results obtained when different
formulations of demeton-S-methyl were tested on the green alga
Scenedesmus subspicatus (Heimbach, 1985a, 1990b).
9.1.2 Invertebrates
The acute toxicity of demeton-S-methyl for molluscs and
crustaceans is given in Table 5.
The lowest concentration tested on the water flea Daphnia
magna (10 µg a.i./litre) caused toxic effects (Heimbach, 1985b,c). A
21-day exposure test on water flea reproduction produced a NOEC of
> 5.6 µg a.i./litre (Heimbach, 1990d). A study performed with a
27.3% emulsifiable concentrate formulation (Heimbach, 1985c) yielded
a NOEC of 3.7 µg/litre (equal to 1 µg a.i./litre) and a LOEC of
11.7 µg/litre (equal to 3.1 µg a.i./litre).
Using a commercial formulation of metasystox, the 96-h LC50 for
the lammellibranch mollusc Paphia laterisulca was 2 µg/litre (Akarte
et al., 1986) and that for the freshwater prawn Donax cuneatus was
4 µg/litre (Muley et al., 1987).
9.1.3 Fish
Table 6 indicates the toxicity of demeton-S-methyl for fish.
9.2 Terrestrial organisms
9.2.1 Soil microorganisms
In a study conducted in silty sand soil or loamy silt soil
with doses of demeton-S-methyl up to 5 times those recommended
(2 litres/hectare of a 27% emulsifiable concentrate formulation),
no influence on soil respiration or nitrification in soil was found
(Anderson, 1989; Blumenstock, 1989).
9.2.2 Invertebrates
When demeton-S-methyl was mixed with an artificial soil where
earthworms (Eisenia foetida) were kept for 14 days, the LC50 was
241 mg/kg of dry substrate of the commercial formulation (a 25%
emulsifiable concentrate), corresponding to 60 mg a.i./kg (Heimbach,
1990a).
Table 4. Effect of demeton-S-methyl on the green alga Scenedesmus subspicatusa
Specification of test EC50 (mg a.i./litre) NOEC LOEC Duration Conditions
substance (mg/litre) (mg/litre) (h)
Increase of Growth rate
biomass
Technical 97.3% purity 8 22 1 3 96 pH 7.8 - 8.5
23 °C
EC formulationb 37 >100 18 32 96 pH 7.6 - 10.4
(27.3% a.i.) 23 °C
Pre-solutionc 13 37 1 10 96 pH 7.7 - 8.5
xylene (53.7% a.i.) 22 °C
a From: Heinbach (1985a, 1990b)
b Tests performed with the blank formulation gave the same results as the highest tested concentration.
c No test with the blank pre-solution was performed.
Table 5. Acute toxicity of demeton-S-methyl for molluscs and crustaceans
Species Specification of test Temperature LC50 Duration of Reference
substance (°C) (mg/litre) exposure
(h)
Mollusc commercial formulation ? 0.0042 96 Akarte et al. (1986)
(Paphia laterisulca)
Water flea (Daphnia magna) technical (96.7%) 20 ± 1 >0.1 24 Heimbach (1985b)
0.023 48
pre-solution in xylene 20 ± 1 >0.1 24 Heimbach (1985c)
(53.7%) formulation 0.022 48
Clam (Donax cuneatus) commercial formulation 25-28.5 0.0064 96 Muley et al. (1987)
Prawn (Macrobrachium lamerrii) commercial formulation 27 ± 2 1.3 72 Mary et al. (1986)
Table 6. Toxicity of demeton-S-methyl for fish (96-h exposure)
Species Mass and length Temperature LC50 (mg/litre) Reference
(°C)
Rainbow trout (Onchorhyncus mykiss) 4.0 - 5.5 cm 16 4.5 Grau (1985a)
1.0 - 1.5 g (52.7% of a.i.)
6.4 ± 1.0 cm 15±2 0.59 Grau (1990c)
3.0 ± 0.6 g (69.5% of a.i.)
6.9 ± 1.1 cm 15±2 6.44 Grau (1990a)
3.5 ± 1.6 g (27.3% of a.i.)
Golden orfe (Leuciscus idus melamotus) 6.0 - 7.5 cm 21 43 Grau (1985b)
2.5 - 4.2 g (52.7% of a.i.)
6.4 ± 0.6 cm 21±2 23.2 Grau (1990b)
2.5 ± 0.6 g (27.3% of a.i.)
Goldfish (Carassius auratus) 6 cm 18 20-40 Hermann (1974a)
1.5 g (28.1% of a.i.)
Carp (Cyprinus carpio) 6 cm 18 40-60 Hermann (1974b)
1.6 g (28.1% of a.i.)
Scardinius erythrophthalmus 6 cm 18 30-40 Hermann (1974c)
1.3 g
Cirrhana mrigala (larvae) 51 ± 3 mg 20 1.45 Verma et al. (1984)
Demeton-S-methyl was applied on fields of winter wheat by
fixed-wing aircraft using conventional boom-and-nozzle equipment.
The applied amount was 245 g a.i. per hectare at a volume rate of
20 litres/hectare. Samples of the soil surface and crop foliage fauna
were collected 1-2 times before and 4-5 times after application from a
treated field and from a control untreated field. The number of crop
foliage but not of soil surface entomophagus invertebrates was reduced
soon after application of demeton-S-methyl. Empididai (dance flies)
was the only group to be significantly reduced in numbers by
demeton-S-methyl. Predatory Coleoptera (beatles) (Carabidae and
Staphylinidae), Araneae (spiders) and predatory Diptera (flies)
(except Empididae) were not affected by demeton-S-methyl. Among the
ephytophagus fauna, cereal aphids markedly declined in number soon
after application, but a rapid increase was observed 2 months later,
when numbers of Diptera and Thripidae (thrips) were also
increased. Numbers of entomobryid and sminthurid springtails decreased
soon after the application but were higher than in the control field
2 months later (Shires, 1985).
Demeton-S-methyl was slightly toxic to the predatory mite
Phytoseiulus persimilis since a 70% population reduction was
obtained with a concentration of 0.025% a.i. (Kniehase, 1984).
The contact LD50 for bees has been reported to be 0.60 µg/bee
(Westlake et al. 1985). A field study was conducted on bee
colonies following application of a 0.2% formulation of Metasystox
(at 600 litres/hectare) to field beans (Vicia faba) after bee
foraging had ceased in the evening. Toxicity to bees was assessed by
collecting dead insects on canvas sheets in front of the hive
entrance. Before spraying, the mortality in exposed and control hives
was comparable. Following spraying, there was a mortality rate of 123
bees per hive in the exposed and 90 bees in the controls (prior to
exposure mortality was 98 and 95 respectively). The mortality increase
persisted for 3 days after the application (Bayer, 1970).
9.2.3 Birds
Some oral LD50 values are given in Table 7.
Table 7. Acute oral LD50 values for birds
Species Vehicle Purity LD50 Reference
(mg/kg b.w.)
Japanese quail Cremephor EL 99.7% 44-50 Flucke (1984)
(Coturnix japonica)
Canary ? 99.7% 10-20 Grau (1984)
(Serinus canarius)
Groups of 10 starlings (Sturnus vulgaris) were given (by
gavage) 0, 0.2, 1.5 or 2 mg/kg body weight of demeton-S-methyl
(technical grade) dissolved in corn oil. Serum butyrylcholinesterase
and carboxylesterase and brain cholinesterase activities were measured
at 3 (high-dose only), 6 and 24 h after dosing (n=2 for brain, n=4 for
serum). The highest dose inhibited brain cholinesterase by about 20%,
serum carboxylesterase by about 50% and serum butyrylcholinesterase by
about 80%. The peak effect was at 3 h with some recovery after 24 h.
In vitro treatment of the enzyme preparation with pralidoxine
chloride (2-PAM) caused almost complete reactivation of brain
cholinesterase but had no effect on the other enzymes (Thompson et
al., 1991).
9.2.4 Effects in field
The activities of brain cholinesterase, serum
butyrylcholinesterase and glutamate oxaloacetate transaminase (GOT)
were measured in house sparrows (Passer domesticus) captured 1
(n=7), 2 (n=6) or 3 (n=6) days after spraying wheat fields with
demeton-S-methyl (0.485 litres a.i./hectare from an emulsifiable
concentrate formulation in a xylene solvent base). Liver weight was
also recorded and histological examination of the liver was performed.
Serum butyrylcholinesterase was reduced to 64% of that of controls
(n=5) (statistically not significant) and brain cholinesterase was 82%
of that of controls (statistically significant) for birds trapped on
day 2, but serum GOT was not significantly affected in the exposed
birds. The authors claimed that there was evidence of liver damage
in exposed birds because of a significant increase of binucleation
(3.3-5 vs. 1.9 binucleation/10 fields) and inflammatory cell foci
(more evident after 3 days of exposure) (Tarrant et al., 1992).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
The major metabolites of demeton-S-methyl in plant and mammals
are oxydemeton-methyl and demeton-S-methylsulfone. These two compounds
are also commercial insecticides. Their mechanism of action
is the same as that of demeton-S-methyl (i.e. inhibition of
acetylcholinesterase) and their toxicological profiles have been
reported to be similar to that of demeton-S-methyl (FAO/WHO, 1990).
10.1 Evaluation of human health risks
Sources of exposure of humans to demeton-S-methyl are dietary and
through dermal absorption and inhalation during the manufacture or use
of the compound.
There is very limited information on actual dietary exposure to
demeton-S-methyl.
In an early study, during application of a mixture of demeton-S-
methyl (30%) and its isomer, demeton-O-methyl (70%), an ambient air
concentration of 8.8-27 mg/m3 of the combined isomers was found. This
is much higher than the conservative TLV/TWA for methyl-demeton
proposed by ACGIH (0.5 mg/m3). However, no inhibition of plasma and
erythrocyte cholinesterase activities was found after exposure.
Cases of acute intoxication, which required pharmacological
treatment, have been reported where workers were filling bottles with
a 50% xylene solution of demeton-S-methyl. Skin absorption was
considered the cause of poisoning because of inadequate personal
protection. Agricultural workers exposed to demeton-S-methyl while
mixing or spraying during three consecutive days did not show
significant inhibition of either plasma or erythrocyte cholinesterase.
Agricultural workers exposed to demeton-S-methyl while spraying cotton
for 1-18 days (average of 3 days) showed signs and symptoms of
poisoning. Improper working conditions could not be excluded.
Cases of severe demeton-S-methyl poisoning (suicide attempts)
have been reported. No delayed toxicity developed in the patients who
survived.
No data are available on the effect of repeated human exposure to
demeton-S-methyl.
No reproductive, developmental or carcinogenic effects have been
found in laboratory animals exposed to demeton-S-methyl. NOAEL values
have been based on inhibition of brain acetyl cholinesterase or, in a
rat reproduction study, on reduced pup viability and body weight in
the F1b generation.
The available information is insufficient to permit an adequate
assessment of the genotoxic potential of demeton-S-methyl.
10.2 Evaluation of effects on the environment
The information on utilization and application rates that
has been employed for this risk assessment is derived from the
agricultural use of demeton-S-methyl within the European Union. It
should be possible to extrapolate this assessment to other
agricultural uses at similar application rates elsewhere in the world.
The application rates for demeton-S-methyl can be summarised as
follows: arable (tractor-mounted/drawn hydraulic spray boom
applications), 0.32 kg/ha; top fruit (broadcast air-assisted
applications), 0.49 kg/ha.
The following risk assessment is based on the principle of
calculating toxicity-exposure ratios (TERs) (Fig. 3), which follows
the European and Mediterranean Plant Protection Organisation and
Council of Europe (EPPO/CoE) Environmental Risk Assessment Scheme
model and associated trigger values (EPPO/CoE, 1993a,b).
10.2.1 Aquatic organisms
The main risk to aquatic organisms from the use of demeton-S-
methyl is from spray drift during either arable applications
(0.32 kg/ha) or top fruit air-assisted (0.49 kg/ha). For each of these
risk scenarios, the predicted environmental concentration (PEC) in a
30-cm-deep static surface water body, arising from either arable-based
spray drift at 1 m from the edge of the spray boom or from top fruit
air-assisted spray drift at 3 m from the point of application (both
based on Ganzelmeier et al., 1995), was calculated as follows:
PEC (mg demeton-S-methyl/litre)
= max application rate (kg/ha) × A (% spray drift)
300
where
A = 5 for ground-based hydraulic spray applications 1 m from
edge of boom
or 30 for air-assisted application 3 m from point of application
10.2.1.1 Acute risk
The acute LC/EC50 values for the most sensitive fish was
0.59 mg/litre, for the most sensitive aquatic invertebrate (mollusc)
was 0.0042 mg/litre (0.022 mg/litre for Daphnia), and for the most
sensitive algal species was 8 mg/litre.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Demeton-S-methyl causes cholinergic toxicity. The acute toxicity
data are reported in Table 2.
Heath & Vandekar (1957) and Vandekar (1958) showed a significant
increase in intravenous but not oral toxicity after storage of
demeton-S-methyl ("pure") at room temperature in the dark. This was
associated with the formation of sulfonium derivatives, which have a
lower oral toxicity, possibly because of poor absorption.
Dilution with water also increased the intravenous toxicity, and
this was again associated with the formation of sulfonium derivatives.
The maximum toxicity of a 1 mg/ml suspension kept at 35°C for one day
was about 30 times the initial toxicity (the LD50 decreased from
about 60 to about 2 mg/kg in rats) (Heath & Vandekar, 1957). Similar
results were obtained with commercial Metasystox (70% demeton-O-
methyl, 30% demeton-S-methyl) (see also section 2.2.)
7.1.1 Oral
Following oral dosing with demeton-S-methyl performed on a very
small number of animals (one per dose level), one rabbit given
50 mg/kg died within 2 h, while the dose of 20 mg/kg caused symptoms
but the animal recovered. A cat given 10 mg/kg died after 2 days,
while the dose of 5 mg/kg caused signs that were reversible and
animals recovered (few details given) (Hecht, 1955).
Single oral doses (180 mg/kg) of metasystox, containing 25%
demeton-S-methyl, were administered to seven male buffalo calves
(10-12 months of age). Signs of poisoning appeared 15 to 35 min later.
Two calves were treated repeatedly with atropine (1.5 mg/kg body
weight, i.v.), D-tubocurarine (0.1 mg/kg body weight, i.v.) and
glucose (3-4 mg/kg body weight, i.v.). Another two calves were treated
repeatedly with atropine (1.5 mg/kg body weight, i.v.), gallamine
(1 mg/kg body weight, i.v.) and glucose (3-4 mg/kg body weight, i.v.)
Three calves were not treated. All calves displayed typical
cholinergic signs. Treatments delayed but did not prevent death, which
occurred in about 2 h in calves not treated with antidotes and in
about 23 h in calves treated with antidotes (Mitra et al., 1978).
7.1.2 Inhalation
The LC50 (4 h of exposure) for Wistar rats was found to be 310
and 210 mg/m3 for males and females, respectively (Flucke & Pauluhn,
1983).
Table 2. LD50 of demeton-S-methyl for various species and different routes of administration
Species Sex Observation Route Purity LD50 Vehicle References
(strain) period (mg/kg
(days) body weight)
Mouse M 3 oral ? 17 ? Klimmer & Pfaff (1955)
Mouse ? 7 i.v. ? 7 water Hecht (1960)
Rat F 1 oral "pure" 63 ? Heath & Vandekar (1957)
Vandekar (1958)
Rat M 3 days minimum oral ? 40 ? Klimmer & Pfaff (1955)
Rat ? ? oral ? 35 ? Hecht (1955)
Rat M 14 oral 25% formulation 33 water Klimmer (1964)
(Wistar)
Rat M ? oral 86-89% 57-64 Lutrol 9 Klimmer (1964)
(Sprague-
Dawley)
Rat M & F 14 oral 50% formulation 64-65 water Flucke & Pauluhn (1983)
(Wistar)
Rat M 7 oral 50% formulation 129 water Edson (1960)
(Wistar)
Rat M 14 oral 90% formulation 44 cremophor EL Flucke & Kimmerle (1977)
(Wistar)
Table 2. (con't)
Species Sex Observation Route Purity LD50 Vehicle References
(strain) period (mg/kg
(days) body weight)
Rat F 10 oral ? 80 ethanol (20%) DuBois & Doull (1955)
(Sprague- and DuBois & Plzak (1962)
Dawley) propylene
glycol (80%)
Rat M & F 14 i.p. ? 7.5 ethanol (20%) DuBois & Doull (1955)
(Sprague- and propylene DuBois & Plzak (1962)
Dawley) glycol (80%)
Rat ? ? i.p. formulation 10 water Hecht (1960)
Rat ? ? dermal ? 85 ? Edson (1960)
Rat M 14 dermal 50% formulation 71 none Flucke & Pauluhn (1983)
(Wistar)
Rat F 14 dermal 50% formulation 45 none Flucke & Pauluhn (1983)
(Wistar)
Rat ? 7 dermal technical 100-200 none Hecht (1960)
Rat ? 7 dermal 25% formulation about 10 none Hecht (1960)
Rat F 1 i.v. "pure" 65 ? Heath & Vandekar (1957)
Vandekar (1958)
Table 2. (con't)
Species Sex Observation Route Purity LD50 Vehicle References
(strain) period (mg/kg
(days) body weight)
Guinea- M 10 oral ? 110 ethanol (20%) Du Bois & Doull (1955)
pig and propylene
glycol (80%)
Guinea- M 14 i.p. ? 12.5 ethanol (20%) Du Bois & Plzak (1962)
pig and propylene
glycol (80%)
When rats (n=2) and mice (n=4) were exposed for 1 h to 1, 2.5 or
5 g/m3 of demeton-S-methyl (alcohol solution), all mice died whereas
rats displayed signs but recovered (few experimental details given)
(Hecht, 1955).
Rats (n=20) were exposed for 8 h to nebulized demeton-S-methyl
(0.5 g/m3 of air), which was obtained from a 25% emulsifiable
commercial formulation diluted 1:250 (0.1% final concentration of
active ingredient). None of the animals died or showed overt
cholinergic signs. Erythrocyte cholinesterase activity, determined in
three animals immediately after the end of exposure, was reduced by
70%. When purified active ingredient was used, lower erythrocyte
cholinesterase inhibition (60%) was found under the same experimental
conditions. This was paralleled by an increased rat LD50 (from 10 to
27.5 mg/kg i.p.) (Hecht, 1960).
7.1.3 Dermal
Doses of 20 or 100 mg/kg demeton-S-methyl applied to the shaved
skin of two cats caused death. A dose of 10 mg/kg caused mild signs;
very few details were given (Hecht, 1955).
7.2 Short-term exposure
7.2.1 Rat
Groups of six male rats (strain not specified) were fed
demeton-S-methyl at levels of 0, 50, 100 or 200 mg/kg diet in
the diet (equivalent to 0, 5, 10 and 20 mg/kg body weight per day,
respectively) for 6 months. Cholinergic signs (slight tremors and
fasciculations) were observed at the highest dose-level during the
first 5 weeks of the study. Brain and erythrocyte cholinesterase
activities were reduced at 50 mg/kg diet (by about 80 and 88%,
respectively), 100 mg/kg diet (by about 85 and 92%) and 200 mg/kg diet
(by about 90 and 94%) groups. Body weight gain was depressed at 100
and 200 mg/kg diet. Gross microscopic examination of tissues (liver,
kidney and adrenals) showed no treatment-related changes (Vandekar,
1958).
Groups of 30-day-old female Holtzman rats were fed dietary
levels of 0, 1, 5 or 25 mg/kg diet of demeton-S-methyl (purity not
reported) for 1 week. At termination, serum, liver and brain
acetylcholinesterase (n=3) and liver and serum aliesterase (n=3) with
diethylsuccinate and tributyrin as substrates activities were
measured. Interpolated dietary levels producing 50% inhibition were
15-28 mg/kg diet for acetylcholinesterase, 4-6 mg/kg diet for liver
aliesterase and about 25 mg/kg diet for serum aliesterase, equivalent
to 1.5-2.8, 0.4-0.6 and 2.5 mg/kg body weight per day, respectively
(Su et al., 1971).
7.2.2 Dog
In a one-year study, pure-bred beagle dogs (n=6 animals of each
sex per group) were fed 0, 1, 10 or 100 mg a.i./kg diet (day 1-36) or
50 mg a.i./kg diet (day 37-termination) of demeton-S-methyl (52.2% in
xylene). Haematological, blood biochemical and urinalysis parameters
were determined during pretest period and at months 1, 2, 3, 4, 5, 6,
8, 10, 12. Hearing tests and ophthalmoscopic examinations were
performed once in the pretest period and at months 3, 6 and 12 of
treatment. At termination animals were killed for pathology, and
determination of organ weights, brain cholinesterase activity, hepatic
cytochrome P-450 and triglyceride contents and N-demethylase
activity were carried out.
All animals survived the study. Diarrhoea and vomiting were
observed in all animals, most frequently in the high-dose group: these
animals also showed reduced food consumption before the dose was
reduced to 50 mg/kg diet. The mean daily compound intake was found to
be 0.036, 0.36 and 4.6/1.5 mg/kg body weight at 1, 10 and 100/50 mg/kg
diet, respectively. Body weight was similar in all groups. No
alterations were observed in the hearing test and ophthalmoscopic
examination. Haematological, blood chemistry (excluding cholinesterase
activities) and urinalysis parameters and organ weights at termination
were not significantly altered by any of the treatments. Hepatic
biochemical parameters were not altered by any of the treatments. No
treatment-related gross pathology alterations were found. However,
multifocal slight/moderate atrophy and/or hypertrophy of proximal
renal tubules was demonstrated in three males and three females of the
high-dose group. Plasma cholinesterase activity was reduced as
compared to controls by 20-30% and 5-20% in males and females,
respectively, at 10 mg/kg diet, and by 45-65% (males) and 50-70%
(females) at 50 mg/kg diet. Erythrocyte cholinesterase activity was
reduced by 25-35% and 30-45% in males and females, respectively, at
10 mg/kg diet. A higher inhibition was found at 50 mg/kg diet, where
inhibition was 80-90% and 55-65% in males and females, respectively.
Brain cholinesterase activity was reduced by 25% in males at 10 mg/kg
diet and by 64% (males) and 15% (females) at 50 mg/kg diet. Based on
effects on brain cholinesterase activity, the NOAEL was 1 mg/kg diet,
equal to 0.036 mg/kg body weight per day (Bathe, 1983).
7.3 Long-term exposure
7.3.1 Mouse
A long-term carcinogenicity study was conducted in NMRI mice
(70 animals of each sex per group) that were given demeton-S-methyl
(about 50% in xylene) mixed into the feed with approximately 10 mg/kg
diet of groundnut oil at concentrations of 0, 1, 15 or 75 mg a.i./kg
diet, or xylene (75 mg/kg diet). Groups were subdivided into two
subgroups: one (n=20) was terminated at 12 months, the second one was
terminated at 21 months.
Animals in the high-dose group had a lower (significantly lower
during the first 4 weeks only) food consumption and a reduced (about
10% throughout the study in males only, and at the beginning in
females) body weight. The mean daily intake of demeton-S-methyl was
(males/females): 0.24/0.29, 3.47/4.18, 17.81/20.0 mg/kg body weight
at 1, 15, 75 mg/kg diet, respectively. Clinical signs due to
cholinesterase inhibition were not observed. Mortality at 21 months
was (males/females) 16/30, 13/34, 17/35, 16/35 and 16/32% in the
control, low-, mid-, high-dose and xylene groups, respectively.
Haematological and clinical chemical parameters were not affected by
the treatment except plasma urea (lower than control in the high-dose
males) and plasma and erythrocyte cholinesterase activities. Plasma
cholinesterase activity was significantly decreased in mid- (by
63-74%) and high-dose (by 91-97%) groups. Erythrocyte cholinesterase
activity was only slightly reduced in the mid- and high-dose groups.
Brain cholinesterase activity (n=10 per group) was reduced in
high-dose groups (in males by 70% and in females by 59%) and in the
mid-dose group (in males by 44% and in females by 38%). Histological
examination did not reveal an increased incidence of neoplastic and
non-neoplastic lesions in treated groups. Based on inhibition of brain
cholinesterase, the NOAEL was 1 mg/kg diet, equal to 0.24 mg/kg body
weight per day (Schmidt & Bomhard, 1988).
7.3.2 Rat
Wistar rats (60 animals of each sex per group) were given
demeton-S-methyl (about 50% in xylene mixed into the feed with
approximately 10 mg/kg of groundnut oil) at concentrations of 0, 1, 7
or 50 mg a.i./kg diet, or 50 mg/kg diet of xylene. Groups were
subdivided into two subgroups; one (n=10) was terminated at 12 months,
the second one was terminated at 24 months.
Hair loss (up to 50% of females) and diarrhoea (up to 50% of
males) were observed significantly more frequently in the animals of
the high-dose group. Body weight was reduced in mid-dose males (by
5-10%) and in both males (by 10-20%) and females (by 5%) in the
high-dose group. Food consumption was similar in all groups. The mean
daily intake of demeton-S-methyl was (males/females): 0.05/0.06,
0.31/0.41, 2.59/3.09 mg/kg body weight at 1, 7 and 50 mg/kg diet,
respectively. Mortality at 24 months was (males/females) 12/26, 10/20,
10/20, 4/26 and 8/24% in the control, low-, mid-, high-dose and xylene
groups, respectively. Haematological and clinical chemical parameters,
except for cholinesterases, measured at months 6, 12, 18 and 24, were
unaffected by the treatments. Plasma and erythrocyte cholinesterase
activities, measured at months 3, 6, 12 and 24, were significantly
decreased in groups given 7 mg/kg diet (plasma cholinesterase by
30-56%, erythrocyte cholinesterase by 12-31%) or 50 mg/kg diet (plasma
cholinesterase by 75-92%, erythrocyte cholinesterase by 20-44%). Brain
cholinesterase activity (measured at 12 and 24 months) was reduced in
the high-dose group (by 67-75%) and in the mid-dose group (by 15-47%).
A statistically significant decrease in brain cholinesterase activity
(33%) was observed in males given 1 mg/kg diet at 24 months but not at
12 months. The toxicological significance of this finding is not
clear; it should also be noted that brain cholinesterase activity at
7 mg/kg diet was higher than at 1 mg/kg diet and that plasma
cholinesterase and erythrocyte cholinesterase activities were not
inhibited at either of these dose levels. Histological examination did
not reveal an increased incidence of neoplastic lesions in treated
groups. Increased incidence of retinal atrophy (78% of males, 92% of
females as compared to 36-63% and 61-70% in the other groups) and
keratitis (44% of males, 22% of females as compared to 4-12% and 0-2%,
respectively, in the other groups) was observed at 50 mg/kg diet. The
retinal atrophy mainly affected the fundus, unilaterally or
bilaterally, and occurred in either the inner corneal layer, the inner
and outer layers, or all layers of the retina. Based on inhibition of
brain cholinesterase, the NOAEL was considered to be 1 mg/kg diet,
equal to 0.05 mg/kg body weight per day (Schmidt & Westen, 1988).
7.4 Skin and eye irritation and sensitization
7.4.1 Skin and eye irritation
Demeton-S-methyl (commercial formulation, 50% of active
ingredient) was applied (0.5 ml) to the shaved skin of three New
Zealand white rabbits on a 2.5 × 2.5 cm cellulose dressing for 4 h.
Mild erythema and oedema were observed, which generally disappeared
after 3 days (Flucke & Pauluhn, 1983). A 52.5% solution of
demeton-S-methyl in xylene produced slight skin and eye irritancy in
New Zealand white rabbits, but this was attributed to the solvent
(Thyssen, 1981).
A commercial formulation of demeton-S-methyl (50%) was instilled
(0.1 ml) either undiluted or as a 0.5% aqueous dilution into the
conjunctival sac of one eye of groups (n=3) of New Zealand white
rabbits. Treated eyes were washed with physiological solution after
24 h. No signs of eye irritation were observed in rabbits treated with
the 0.5% aqueous solution. The undiluted formulation caused severe
lacrimation and miosis on application. Mild corneal opacity and
discrete redness and oedema of conjunctivae were observed and
recovered in about 7 days (Flucke & Pauluhn, 1983).
7.4.2 Skin sensitization
The skin-sensitizing potential of demeton-S-methyl was assessed
by the Magnusson and Kligman maximization test with Freund's adjuvant
on guinea-pigs (n=20, Bor:SPF, DHPW strain). The concentrations of
demeton-S-methyl (96.3% purity, average of three determinations) used
were: 0.1% for the intra-dermal induction, 10% for the topical
induction and the first challenge, and 1% for the second challenge.
All twenty animals reacted positively to the 1st challenge (controls
4/10), and 16 reacted positively to the 2nd challenge (controls 3/10).
The results indicate that demeton-S-methyl has a skin-sensitizing
potential (Heimann, 1987a).
In another study, the Buehler epidermal patch test was used on
guinea-pigs (n=12, Bor: SPF, DHPW strain). The concentrations of
demeton-S-methyl (95.6% purity, average of three determinations) used
were 10% for topical induction (once a week for 3 weeks) and the first
challenge, and 20% for the second challenge. The results indicate that
demeton-S-methyl does not have a skin-sensitizing potential under
these conditions (Heimann, 1987b).
It is concluded that demeton-S-methyl might have some
skin-sensitizing potential, but this is of no relevance in practice.
7.5 Reproduction, embryotoxicity and teratogenicity
7.5.1 Reproduction
A standard two-generation study (two litters/generation) was
conducted in SPF rats (BOR:WISW) (10 males and 20 females) that were
given demeton-S-methyl at 0, 1, 5 or 25 mg/kg diet (Eiben et al.,
1984). The compound was used as a pre-mix in xylene (about 50%). Rats
in an extra control group were given xylene at 25 mg/kg diet.
In the F0 generation, none of the animals died. No treatment-
related signs were observed in any animal. Body weight gain was
reduced in males (by 10%) and in some females at 25 mg/kg diet. Food
intake was also reduced (by 7%) in high-dose males. Fertility index
was not affected by treatment. At 25 mg/kg diet, the viability of pups
was reduced; it was 89% and 85% in first and second matings,
respectively. Lactation index was also reduced in the high-dose group
(85-92%), the control value being 98-99%. Body weight at birth was
comparable in all groups, but body weight gain was significantly
reduced (by 8-10%) in pups fed 25 mg/kg diet.
In the F1b generation, one female was found dead in the 5-mg/kg
diet group and one in the 25-mg/kg diet group; one male and one female
in one of the 25-mg/kg diet litters also died. Autopsy did not reveal
treatment-related alterations. No treatment-related signs were
observed in any animal. Body weight gain was reduced at times in
low-dose males and consistently in mid- and high-dose and xylene-
treated males when compared to untreated animals. When compared to
xylene-treated animals (which were 5-15% lighter than untreated
animals), however, only males of the high-dose group had a
significantly reduced (by about 15%) body weight gain. Females of the
high-dose and xylene groups had a reduced (by about 10%) body weight,
compared to controls, the former being at times lighter weight than
the latter. Fertility index was not significantly affected. The number
of pups born was reduced in the high-dose group and the viability of
pups was also reduced in the mid- and high-dose groups in a
dose-related manner (82-88% and 47-67% of controls, respectively).
No compound-related malformation was found in animals of any of
the treatment groups. Demeton-S-methyl intake, as calculated in the
F0 generation, was found to be (female data in parentheses): 0.07
(0.08), 0.32 (0.39) and 1.71 (1.90) mg/kg body weight per day in the
low-, mid- and high-dose groups, respectively, and xylene intake was
1.66 (1.96) mg/kg body weight per day. Based on the viability of pups
and body weight in the F1b generation, the NOAEL was 1 mg/kg diet,
equal to 0.07 mg/kg body weight per day (Eiben et al., 1984).
7.5.2 Embroytoxicity and teratogenicity
7.5.2.1 Rat
Groups (n=25) of fertilized female rats (BAY:FB 30 strain) were
given daily (0, 0.3, 1 or 3 mg/kg body weight orally) demeton-S-methyl
(from a 52.6% solution in xylene) dissolved in corn oil from day 6 to
15 of gestation. At day 20 of gestation, pups were delivered by
caesarean section. Fetuses were weighed, sexed, inspected for external
abnormalities and examined for visceral and bone malformations. No
alteration of physical appearance or behaviour was observed in any
group. All animals survived until the caesarean section. Body weight
gain was reduced (by 13%) in the high-dose group. The numbers of live
fetuses and resorptions, fetal weight, number of fetuses with
malformations and number of implants were comparable in all groups. No
treatment-related visceral or skeletal abnormalities were observed
(Renhof, 1985).
7.5.2.2 Rabbit
A formulation of demeton-S-methyl (52.2% a.i. in xylene) was
administered by gavage to mated chinchilla hybrid rabbits (n=15-16) on
gestation days 6 to 18 at dose levels of 0, 3, 6 and 12 mg/kg body
weight per day. Caesarean sections were performed on gestation day 28.
There were no mortalities. In the high-dose group, diarrhoea was
observed in all animals after 4 to 10 days of treatment. Beginning 1
to 2 h after dosing, it persisted for 6 to 24 h. In the high-dose
group, mean food consumption was decreased by 7% during gestation days
6 to 18 and by 17% during gestation days 19 to 24 when compared to
controls. This was associated with decreased mean body weight gain
(-7%). There were no abortions and no relevant differences between
test and control groups in the numbers of implantations per dam, pre-
implantation losses, post-implantation losses, resorptions, living and
dead fetuses or sex ratios. A decrease in mean fetal body weight,
compared to the mean control weight, of 6.6% was observed in the
high-dose group. There was no treatment-related increase in gross,
skeletal or visceral malformations (Becker, 1983).
7.6 Mutagenicity and related end-points
A summary of the studies conducted to assess mutagenicity of
demeton-S-methyl is given in Table 3.
7.6.1 DNA damage and repair
Demeton-S-methyl did not induce DNA damage in the Pol test on
Escherichia coli either with or without metabolic activation.
7.6.2 Mutation
Increased mutation rates were observed in the Ames test and in
the mouse lymphoma forward mutation assay both with and without
metabolic activation.
7.6.3 Chromosomal effects
In in vivo tests, no SCEs were found in the bone marrow of
Chinese hamsters treated with high doses of demeton-S-methyl.
Bone marrow micronucleus and dominant lethal tests on mice
treated with demeton-S-methyl gave negative results. Chromosomal
aberrations were found in the bone marrow of Syrian hamsters treated
with a commercial formulation of demeton-S-methyl.
It is concluded that the available information is insufficient to
permit an adequate assessment of the genotoxic potential of
demeton-S-methyl.
7.7 Delayed neurotoxicity
Adult Leghorn hens (n=20) were given two doses of 100 mg a.i./kg
body weight (approximately equal to the LD50) of demeton-S-methyl
(51.2% in xylene) by gavage. The second dose was given 21 days
after the first one. Positive control animals (n=5) received
tri- ortho-cresyl phosphate (TOCP) (375 mg/kg body weight by gavage).
Animals were pretreated with atropine (100 mg/kg body weight
intramuscularly 10 min before the dose of demeton-S-methyl and
50 mg/kg body weight subcutaneously 6 h later). Surviving animals
received atropine (30 mg/kg s.c.) 24, 30 and 48 h later. At the second
dose, atropine treatment was suspended after 24 h. Hens treated with
demeton-S-methyl had signs of cholinergic toxicity. The recovery
started on day 3 and by day 8 all treated animals, except for one,
were free of signs. After the second dose, the recovery started on day
2 and by day 5 all treated animals were free of signs. One animal died
after the second treatment and surviving animals did not develop
neurological deficits. TOCP-treated animals showed locomotor
Table 3. Studies on mutagenicity of demeton-S-methyl
Test Organism Purity Results LED or HIDa Reference
-S9 +S9
Microorganisms
Pol assay Escherichia coli p3478, 93% - - 10 000 µg/plate Herbold (1983a)
W3110
Reverse Salmonella typhimurium unknown + n.t. 5 µg/plate Hanna & Dyer (1975)
mutation TA1530, TA1535, his C117,
his G46
E. coli WP2, WP2 uvra, unknown + n.t. 5 µg/plate Hanna & Dyer (1975)
CM561, CM571, CM611, WP67,
WP12,
S. typhimurium TA98, TA100, 50.2% ++ 300 µg/plate Herbold (1979)
TA1535, TA1537 (formulation)
S. typhimurium TA98, TA100, >98% ++ 20 µg/plate Herbold (1980a)
TA1535, TA1537
Saccharomyces cerevisiae 53.1% -- 1062 µg/ml Hoorn (1982)
S138 S211ý (formulations
in xylene)
Insects
Recessive Drosophila melanogaster unknown + 80 mg/kg diet Hanna & Dyer (1975)
lethal
Table 3. (con't)
Test Organism Purity Results LED or HIDa Reference
-S9 +S9
Cultured mammalian cells
Mutation, tk Mouse lymphoma L5178Y cells 94% ++ 50 µg/ml Cifone (1984)
locus
Mammals in vivo
Bone marrow NMRI mouse >98% - 2 × 5 mg/kg b.w. Herbold (1980b)
micronucleus oral
SCE in bone Chinese hamster 94% - 20 mg/kg b.w. oral Herbold (1983b)
marrow
Chromosomal Syrian hamster, female 50% + 2 mg/kg b.w. i.p. Dzwonkowska & Hübner (1986)
aberration (commercial)
Dominant NMRO mouse >98% - 5 mg/kg b.w. oral Herbold (1980c)
lethal
a LED = lowest effective dose; HID = highest ineffective dose
impairment beginning on day 10. Histological examination showed
moderate axonal degeneration in peripheral nerves and medulla in
TOCP-treated animals but not in demeton-S-methyl-treated or solvent
control animals (Flucke & Kaliner, 1988).
Neuropathy target esterase (NTE), the target for organophosphate-
induced delayed neuropathy, was not inhibited in hen brain and
spinal cord 1, 2 and 7 days after treatment with demeton-S-methyl
(80 mg a.i./kg body weight) by gavage. Positive controls (TOCP at
100 mg/kg body weight) showed NTE inhibition (> 90%) in both brain
and spinal cord (Flucke & Eben, 1988).
7.8 Toxicity of metabolites
Two plant and mammalian metabolites of demeton-S-methyl (namely
oxydemeton-methyl and demeton-S-methylsulfone) have been studied
extensively, since they are also the active ingredient of commercial
pesticides. According to the JMPR, the toxicity of the two compounds
does not differ substantially, either qualitatively or quantitatively,
from that of demeton-S-methyl (FAO/WHO, 1990).
7.9 Mechanism of toxicity - mode of action
Demeton-S-methyl is a direct cholinesterase inhibitor and
it causes signs and symptoms of the cholinergic syndrome. The
in vitro I50 (30 min, 37°C) for sheep erythrocyte cholinesterase
was 6.5 × 10-5 mol/litre. The I50 of oxydemeton-methyl and
demeton-S-methylsulfone were of the same order of magnitude
(2.7 × 10-5 and 4.3 × 10-5 mol/litre, respectively). The half-life
of recovery of acetylcholinesterase activity after inhibition by
demeton-S-methyl was 1.3 h, as expected from a dimethyl phosphorylated
acetylcholinesterase (Heath & Vandekar, 1957).
The 1973 JMPR (FAO/WHO, 1974) reported that rat brain
cholinesterase was more sensitive to in vitro inhibition by demeton-
S-methyl than by oxydemeton-methyl (I50 values of 9.52 × 10-5 and
1.43 × 10-3 mol/litre, respectively; time of incubation, temperature
and pH not reported). It was also reported that demeton-S-methyl was a
more potent inhibitor of human serum cholinesterase (no details
given).
Data on in vivo inhibition of plasma cholinesterase and
erythrocyte and brain cholinesterase are reported in section 7.2 and
7.3.
7.10 Potentiation
Male Wistar rats (160-180 g body weight) were given trichlorfon
(98.6% purity) and demeton-S-methyl (90% purity) in combination by
gavage. The amount of compound in the mixture was proportional to its
oral LD50 (302 mg/kg body weight for trichlorfon and 44 mg/kg body
weight for demeton-S-methyl) in order to obtain equitoxic doses. The
resulting toxicity was additive (LD50 of the mixture was 223 mg/kg
body weight against expected 173 mg/kg body weight) (Flucke &
Kimmerle, 1977).
Similarly, an additive effect was obtained when demeton-S-methyl
was given in combination with phenamiphos (ethyl-4-(methylthio)
m-tolyl-isopropyl-phosphoroamidate). The experimental LD50 of the
mixture was 55 mg/kg body weight while that estimated from the
individual LD50s was 50 mg/kg body weight (Kimmerle, 1972).
8. EFFECTS ON HUMANS
8.1 General population exposure
A 31-year old woman attempted suicide with an unknown amount
("1 or 2 mouthfuls") of Metasystox I (25% demeton-S-methyl). On
admission to hospital she was comatose, sweating and salivating,
and had pin-point pupils. She stayed in the Intensive Care Unit for 15
days where she was treated with atropine (up to 97 mg per day, 550 mg
in 14 days). Oximes (2-PAM) were only given on day 1 (0.5 + 0.25 +
0.25 g) and there was no apparent clinical improvement. On admission,
plasma and erythrocyte cholinesterase activities were less than 10% of
normal control values. The patient was discharged on the 30th day with
normal plasma cholinesterase values; erythrocyte cholinesterase
activity was still below the normal values (about 65%), but had
recovered a month later (Barr, 1966).
A case of acute poisoning in a 5-month pregnant woman has been
described (Carrington da Costa et al., 1982). A 41-year-old woman
attempted suicide by ingesting Metasystox, which resulted in an
estimated intake of 12 g methyl demeton (it should be noted that in
the report Metasystox was said to contain methyl-demeton and not
oxydemeton-methyl as the commercial name implies). On admission, 3.5 h
after poisoning, blood cholinesterase (it was not specified whether
pseudo- or acetylcholinesterase) was 10% of normal values. The patient
became comatose 12 h after admission. She was treated with atropine,
obidoxime and haemoperfusion 72 h after hospitalization. Artificial
ventilation was required on the 4th day of hospitalization. The
patient was discharged after 24 days and 4 months later she delivered
a healthy female child.
In a fatal suicidal case with a commercial formulation of
demeton-S-methyl, the concentration of the compound was measured in
several organs. It was estimated that death occurred about 6 h after
poisoning. Highest demeton-S-methyl levels were found in the proximal
small intestine (166 mg/kg of tissue); in brain, kidney and muscles,
the levels were 30-80 mg/kg; lowest concentrations were found in liver
(7 mg/kg tissue) and blood (7-16 mg/litre). Metabolites were not
measured (Schludecker & Aderjan, 1988).
In the United Kingdom in 1991 there were five cases of poisoning
by demeton-S-methyl and in 1994 three cases, none of which were fatal
(personal communication by G. Volans, Medical Toxicology Unit, London,
to the IPCS, dated 15 January 1996).
Hegazy (1965) reported three cases of suspected poisoning with
demeton-S-methyl in children (6-14 years of age) exposed in a recently
sprayed field and 1 case after ingestion of contaminated feed.
Symptoms were mild and the patients recovered fully. Serum
cholinesterase determinations gave ambiguous results.
8.2 Occupational exposure
8.2.1 Acute poisoning
A worker inadvertently exposed to demeton-S-methyl (no details)
was monitored for about 100 days. Plasma cholinesterase activity was
always within the normal values of the laboratory. However, if the
activity on day 40 and 100 is considered as the normal value for this
worker, then a 30% inhibition occurred 2-3 days after exposure. The
activity recovered with an half-life of about 10 days. Erythrocyte
cholinesterase activity was below the normal value for about 40 days.
When calculated on the activity of day 100, a 60% inhibition was found
up to day 10. The activity recovered with a half-life of about 35 days
(Lewis et al., 1981).
Two workers in a chemical packaging company, whose job it was to
fill a concentrate of demeton-S-methyl (500 g/litre of xylene) into
one-litre containers using a weight-triggered bottle-filling machine
for 3 to 4 h, were admitted to hospital because of organophosphate
poisoning and were treated with atropine. Cholinesterase measurement
made 14 days after exposure (1 worker) showed inhibited erythrocyte
cholinesterase, but the plasma cholinesterase activity was within the
normal range. In the second worker, whose symptoms lasted for 3 days,
erythrocyte cholinesterase activity was below the normal value 5 weeks
after exposure. These workers wore gloves, overalls and boots, but
frequent spillage was reported. Normal clothing had been left under
the filling apparatus and apparently was contaminated. The filling
system was changed by housing the filling machine in a fume cupboard
and by providing new protective garments and changing room facilities.
One more worker was admitted to the hospital and treated with atropine
because of organophosphate poisoning. This occurred on the morning
after a day spent fitting the infill seal and screwing on the tops
of cans. He had reduced erythrocyte and plasma cholinesterase
(erythrocyte > plasma) activities for several days after exposure.
Another worker showed a sharp drop in erythrocyte and plasma
cholinesterase activities associated with abdominal cramps, which
resolved in about 5 days. Another worker had depressed erythrocyte and
plasma cholinesterases activities without complaining of any adverse
effect. It was concluded that absorption of demeton-S-methyl was
through the skin because of penetration of the protective clothing
used or because this clothing was not worn properly. It should be
noted that the active ingredient was dissolved in xylene, which is
known to attack rubber and plastics, making the penetration of gloves
possible (Jones, 1982).
8.2.2 Effects of short- and long-term exposure
Three volunteers without any protective equipment were exposed
for two consecutive days to Metasystox (30% demeton-S-methyl, 70%
demeton-O-methyl) while spraying with a hand-held nebulizer. Exposure
lasted for 3 and 6 h on the first and second days, respectively. The
concentrations of the active ingredient (isomers not separated) were
8.8-27 mg/m3 of ambient air. Plasma and erythrocyte cholinesterase
activities measured up to 14 days after exposure did not show
significant decreases when compared to pre-exposure values (Klimmer &
Pfaff, 1955).
Volunteers, wearing overalls but not mask protection, were
exposed to metasystox (30% demeton-S-methyl, 70% demeton-O-methyl)
while spraying in a greenhouse. They used the splash method
(0.03-0.05% a.i.), the low volume (0.5% a.i.) or the high volume spray
method (0.05% a.i.) for 5 to 25 min. There was no effect on plasma or
erythrocyte cholinesterase activity measured after exposure (Klimmer &
Pfaff, 1958).
Six workers engaged in hop cultivation using Metasystox I
(reported to contain demeton-O-methyl instead of demeton-S-methyl as
the commercial name implies) were monitored. They sprayed up to
2400 litres of a 0.1% solution (in water) of the insecticide in one
day. Protective clothing and masks were not always used. No
significant inhibition of blood acetyl cholinesterase was observed at
the end of exposure or 1 or 2 days later. One subject, who was exposed
twice, showed a 29% decrease in blood acetyl cholinesterase after the
second exposure. No signs or symptoms were observed in these workers
(Winkler & Arent, 1970).
The medical department of a company reported no adverse effect in
workers employed in the formulation of demeton-S-methyl from 1967 to
1984 (Faul, 1984).
Agricultural workers exposed to demeton-S-methyl for 3
consecutive days were monitored. Pre-and post-exposure urinary levels
of the metabolite dimethyl phosphorothiolate potassium salt (DMPThK),
and plasma and whole blood cholinesterase activities were measured.
Exposed subjects were divided into three groups according to their
job, i.e. mixers (n=7), sprayers (n=6) and others (n=7) not directly
involved in handling the pesticide. Higher levels of DMPThK were
found in mixers, with a medium value of 83 µg/litre and a range of
0-822 µg/litre (neither corrected for creatinine nor for urine
volume). Sprayers had a mean value of 30 µg/litre (limit of detection)
and a range of 0-208 µg/litre; the other subject had a mean value of
30 µg/litre and a range of 0-100 µg/litre. Whole blood cholinesterase
activity was not affected by the exposure, while plasma cholinesterase
activity was slightly (about 10%, statistically significant) reduced
when compared to pre-exposure levels in mixers. However, no
correlation was found between DMPThK levels and plasma cholinesterase
activity (Vasilic et al., 1987).
Hegazy (1965) reported a study of 121 spraymen exposed to
Meta-isosystox during spraying of cotton fields in Egypt. The spraymen
applied Meta-isosystox at a rate of 0.5 litres of concentrate in
400 litres of spray per 4200 m2 mainly by hand-operated knapsack
sprayers, or in a few cases by high-pressure motor-powered sprayers
with a large tank connected by a long hose to a multi-nozzled
spray-boom. Sprayers were not involved in chemical mixing. Workmen
washed exposed body parts with soap and water after spraying. Working
clothes were removed at the end of the day, but the clothes may not
have been washed before re-use. Not all workers used protective
clothing and masks. None of the workers were re-exposed to
Meta-isosystox after the onset of symptoms. Serum cholinesterase
activity estimates were performed within 24 h after the onset of
symptoms in some patients and after the cessation of symptoms in some
others. In most cases they were repeated 2-3 times at various
intervals up to 40 days from the onset of symptoms. In general, the
serum cholinesterase activity in spraymen underwent a marked initial
fall, followed by a rise to above-normal levels after about 30-40
days. Signs and symptoms of toxicity in spraymen occurred after 1-18
days of exposure, with a mean time of 3 days. These consisted of
gastrointestinal disturbances (58% of total), dizziness (23%),
persistent general weakness and fatigue (19%), respiratory
manifestations (16%), headache (16%), sweating, salivation or
lacrimation (12%), tremors of outstretched hands, intention tremors,
ataxia (4.1%), exaggerated superficial and deep reflexes (5.8%),
hiccough (2.5%), muscular fasciculations (2.5%), or had apparently
resolved (30%), at the first determination of serum cholinesterase
activity (Hegazy, 1965).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Aquatic organisms
9.1.1 Algae
Table 4 reports the results obtained when different
formulations of demeton-S-methyl were tested on the green alga
Scenedesmus subspicatus (Heimbach, 1985a, 1990b).
9.1.2 Invertebrates
The acute toxicity of demeton-S-methyl for molluscs and
crustaceans is given in Table 5.
The lowest concentration tested on the water flea Daphnia
magna (10 µg a.i./litre) caused toxic effects (Heimbach, 1985b,c). A
21-day exposure test on water flea reproduction produced a NOEC of
> 5.6 µg a.i./litre (Heimbach, 1990d). A study performed with a
27.3% emulsifiable concentrate formulation (Heimbach, 1985c) yielded
a NOEC of 3.7 µg/litre (equal to 1 µg a.i./litre) and a LOEC of
11.7 µg/litre (equal to 3.1 µg a.i./litre).
Using a commercial formulation of metasystox, the 96-h LC50 for
the lammellibranch mollusc Paphia laterisulca was 2 µg/litre (Akarte
et al., 1986) and that for the freshwater prawn Donax cuneatus was
4 µg/litre (Muley et al., 1987).
9.1.3 Fish
Table 6 indicates the toxicity of demeton-S-methyl for fish.
9.2 Terrestrial organisms
9.2.1 Soil microorganisms
In a study conducted in silty sand soil or loamy silt soil
with doses of demeton-S-methyl up to 5 times those recommended
(2 litres/hectare of a 27% emulsifiable concentrate formulation),
no influence on soil respiration or nitrification in soil was found
(Anderson, 1989; Blumenstock, 1989).
9.2.2 Invertebrates
When demeton-S-methyl was mixed with an artificial soil where
earthworms (Eisenia foetida) were kept for 14 days, the LC50 was
241 mg/kg of dry substrate of the commercial formulation (a 25%
emulsifiable concentrate), corresponding to 60 mg a.i./kg (Heimbach,
1990a).
Table 4. Effect of demeton-S-methyl on the green alga Scenedesmus subspicatusa
Specification of test EC50 (mg a.i./litre) NOEC LOEC Duration Conditions
substance (mg/litre) (mg/litre) (h)
Increase of Growth rate
biomass
Technical 97.3% purity 8 22 1 3 96 pH 7.8 - 8.5
23 °C
EC formulationb 37 >100 18 32 96 pH 7.6 - 10.4
(27.3% a.i.) 23 °C
Pre-solutionc 13 37 1 10 96 pH 7.7 - 8.5
xylene (53.7% a.i.) 22 °C
a From: Heinbach (1985a, 1990b)
b Tests performed with the blank formulation gave the same results as the highest tested concentration.
c No test with the blank pre-solution was performed.
Table 5. Acute toxicity of demeton-S-methyl for molluscs and crustaceans
Species Specification of test Temperature LC50 Duration of Reference
substance (°C) (mg/litre) exposure
(h)
Mollusc commercial formulation ? 0.0042 96 Akarte et al. (1986)
(Paphia laterisulca)
Water flea (Daphnia magna) technical (96.7%) 20 ± 1 >0.1 24 Heimbach (1985b)
0.023 48
pre-solution in xylene 20 ± 1 >0.1 24 Heimbach (1985c)
(53.7%) formulation 0.022 48
Clam (Donax cuneatus) commercial formulation 25-28.5 0.0064 96 Muley et al. (1987)
Prawn (Macrobrachium lamerrii) commercial formulation 27 ± 2 1.3 72 Mary et al. (1986)
Table 6. Toxicity of demeton-S-methyl for fish (96-h exposure)
Species Mass and length Temperature LC50 (mg/litre) Reference
(°C)
Rainbow trout (Onchorhyncus mykiss) 4.0 - 5.5 cm 16 4.5 Grau (1985a)
1.0 - 1.5 g (52.7% of a.i.)
6.4 ± 1.0 cm 15±2 0.59 Grau (1990c)
3.0 ± 0.6 g (69.5% of a.i.)
6.9 ± 1.1 cm 15±2 6.44 Grau (1990a)
3.5 ± 1.6 g (27.3% of a.i.)
Golden orfe (Leuciscus idus melamotus) 6.0 - 7.5 cm 21 43 Grau (1985b)
2.5 - 4.2 g (52.7% of a.i.)
6.4 ± 0.6 cm 21±2 23.2 Grau (1990b)
2.5 ± 0.6 g (27.3% of a.i.)
Goldfish (Carassius auratus) 6 cm 18 20-40 Hermann (1974a)
1.5 g (28.1% of a.i.)
Carp (Cyprinus carpio) 6 cm 18 40-60 Hermann (1974b)
1.6 g (28.1% of a.i.)
Scardinius erythrophthalmus 6 cm 18 30-40 Hermann (1974c)
1.3 g
Cirrhana mrigala (larvae) 51 ± 3 mg 20 1.45 Verma et al. (1984)
Demeton-S-methyl was applied on fields of winter wheat by
fixed-wing aircraft using conventional boom-and-nozzle equipment.
The applied amount was 245 g a.i. per hectare at a volume rate of
20 litres/hectare. Samples of the soil surface and crop foliage fauna
were collected 1-2 times before and 4-5 times after application from a
treated field and from a control untreated field. The number of crop
foliage but not of soil surface entomophagus invertebrates was reduced
soon after application of demeton-S-methyl. Empididai (dance flies)
was the only group to be significantly reduced in numbers by
demeton-S-methyl. Predatory Coleoptera (beatles) (Carabidae and
Staphylinidae), Araneae (spiders) and predatory Diptera (flies)
(except Empididae) were not affected by demeton-S-methyl. Among the
ephytophagus fauna, cereal aphids markedly declined in number soon
after application, but a rapid increase was observed 2 months later,
when numbers of Diptera and Thripidae (thrips) were also
increased. Numbers of entomobryid and sminthurid springtails decreased
soon after the application but were higher than in the control field
2 months later (Shires, 1985).
Demeton-S-methyl was slightly toxic to the predatory mite
Phytoseiulus persimilis since a 70% population reduction was
obtained with a concentration of 0.025% a.i. (Kniehase, 1984).
The contact LD50 for bees has been reported to be 0.60 µg/bee
(Westlake et al. 1985). A field study was conducted on bee
colonies following application of a 0.2% formulation of Metasystox
(at 600 litres/hectare) to field beans (Vicia faba) after bee
foraging had ceased in the evening. Toxicity to bees was assessed by
collecting dead insects on canvas sheets in front of the hive
entrance. Before spraying, the mortality in exposed and control hives
was comparable. Following spraying, there was a mortality rate of 123
bees per hive in the exposed and 90 bees in the controls (prior to
exposure mortality was 98 and 95 respectively). The mortality increase
persisted for 3 days after the application (Bayer, 1970).
9.2.3 Birds
Some oral LD50 values are given in Table 7.
Table 7. Acute oral LD50 values for birds
Species Vehicle Purity LD50 Reference
(mg/kg b.w.)
Japanese quail Cremephor EL 99.7% 44-50 Flucke (1984)
(Coturnix japonica)
Canary ? 99.7% 10-20 Grau (1984)
(Serinus canarius)
Groups of 10 starlings (Sturnus vulgaris) were given (by
gavage) 0, 0.2, 1.5 or 2 mg/kg body weight of demeton-S-methyl
(technical grade) dissolved in corn oil. Serum butyrylcholinesterase
and carboxylesterase and brain cholinesterase activities were measured
at 3 (high-dose only), 6 and 24 h after dosing (n=2 for brain, n=4 for
serum). The highest dose inhibited brain cholinesterase by about 20%,
serum carboxylesterase by about 50% and serum butyrylcholinesterase by
about 80%. The peak effect was at 3 h with some recovery after 24 h.
In vitro treatment of the enzyme preparation with pralidoxine
chloride (2-PAM) caused almost complete reactivation of brain
cholinesterase but had no effect on the other enzymes (Thompson et
al., 1991).
9.2.4 Effects in field
The activities of brain cholinesterase, serum
butyrylcholinesterase and glutamate oxaloacetate transaminase (GOT)
were measured in house sparrows (Passer domesticus) captured 1
(n=7), 2 (n=6) or 3 (n=6) days after spraying wheat fields with
demeton-S-methyl (0.485 litres a.i./hectare from an emulsifiable
concentrate formulation in a xylene solvent base). Liver weight was
also recorded and histological examination of the liver was performed.
Serum butyrylcholinesterase was reduced to 64% of that of controls
(n=5) (statistically not significant) and brain cholinesterase was 82%
of that of controls (statistically significant) for birds trapped on
day 2, but serum GOT was not significantly affected in the exposed
birds. The authors claimed that there was evidence of liver damage
in exposed birds because of a significant increase of binucleation
(3.3-5 vs. 1.9 binucleation/10 fields) and inflammatory cell foci
(more evident after 3 days of exposure) (Tarrant et al., 1992).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
The major metabolites of demeton-S-methyl in plant and mammals
are oxydemeton-methyl and demeton-S-methylsulfone. These two compounds
are also commercial insecticides. Their mechanism of action
is the same as that of demeton-S-methyl (i.e. inhibition of
acetylcholinesterase) and their toxicological profiles have been
reported to be similar to that of demeton-S-methyl (FAO/WHO, 1990).
10.1 Evaluation of human health risks
Sources of exposure of humans to demeton-S-methyl are dietary and
through dermal absorption and inhalation during the manufacture or use
of the compound.
There is very limited information on actual dietary exposure to
demeton-S-methyl.
In an early study, during application of a mixture of demeton-S-
methyl (30%) and its isomer, demeton-O-methyl (70%), an ambient air
concentration of 8.8-27 mg/m3 of the combined isomers was found. This
is much higher than the conservative TLV/TWA for methyl-demeton
proposed by ACGIH (0.5 mg/m3). However, no inhibition of plasma and
erythrocyte cholinesterase activities was found after exposure.
Cases of acute intoxication, which required pharmacological
treatment, have been reported where workers were filling bottles with
a 50% xylene solution of demeton-S-methyl. Skin absorption was
considered the cause of poisoning because of inadequate personal
protection. Agricultural workers exposed to demeton-S-methyl while
mixing or spraying during three consecutive days did not show
significant inhibition of either plasma or erythrocyte cholinesterase.
Agricultural workers exposed to demeton-S-methyl while spraying cotton
for 1-18 days (average of 3 days) showed signs and symptoms of
poisoning. Improper working conditions could not be excluded.
Cases of severe demeton-S-methyl poisoning (suicide attempts)
have been reported. No delayed toxicity developed in the patients who
survived.
No data are available on the effect of repeated human exposure to
demeton-S-methyl.
No reproductive, developmental or carcinogenic effects have been
found in laboratory animals exposed to demeton-S-methyl. NOAEL values
have been based on inhibition of brain acetyl cholinesterase or, in a
rat reproduction study, on reduced pup viability and body weight in
the F1b generation.
The available information is insufficient to permit an adequate
assessment of the genotoxic potential of demeton-S-methyl.
10.2 Evaluation of effects on the environment
The information on utilization and application rates that
has been employed for this risk assessment is derived from the
agricultural use of demeton-S-methyl within the European Union. It
should be possible to extrapolate this assessment to other
agricultural uses at similar application rates elsewhere in the world.
The application rates for demeton-S-methyl can be summarised as
follows: arable (tractor-mounted/drawn hydraulic spray boom
applications), 0.32 kg/ha; top fruit (broadcast air-assisted
applications), 0.49 kg/ha.
The following risk assessment is based on the principle of
calculating toxicity-exposure ratios (TERs) (Fig. 3), which follows
the European and Mediterranean Plant Protection Organisation and
Council of Europe (EPPO/CoE) Environmental Risk Assessment Scheme
model and associated trigger values (EPPO/CoE, 1993a,b).
10.2.1 Aquatic organisms
The main risk to aquatic organisms from the use of demeton-S-
methyl is from spray drift during either arable applications
(0.32 kg/ha) or top fruit air-assisted (0.49 kg/ha). For each of these
risk scenarios, the predicted environmental concentration (PEC) in a
30-cm-deep static surface water body, arising from either arable-based
spray drift at 1 m from the edge of the spray boom or from top fruit
air-assisted spray drift at 3 m from the point of application (both
based on Ganzelmeier et al., 1995), was calculated as follows:
PEC (mg demeton-S-methyl/litre)
= max application rate (kg/ha) × A (% spray drift)
300
where
A = 5 for ground-based hydraulic spray applications 1 m from
edge of boom
or 30 for air-assisted application 3 m from point of application
10.2.1.1 Acute risk
The acute LC/EC50 values for the most sensitive fish was
0.59 mg/litre, for the most sensitive aquatic invertebrate (mollusc)
was 0.0042 mg/litre (0.022 mg/litre for Daphnia), and for the most
sensitive algal species was 8 mg/litre.
 (a) Spray drift from ground-based applications
The acute PEC for spray drift (1 m from the edge of the spray
boom into a 30-cm-deep static water body at the maximum application
rate (see PEC assumptions above) is 0.005 mg/litre. Therefore, the
TERs based on this PEC and the above LC/EC50 toxicity values are:
fish, 110; aquatic invertebrates, 0.8 (4.1 for Daphnia); and algae,
1500. Based on the EPPO/CoE risk assessment scheme for aquatic
organisms, these TERs indicate a low acute risk to fish and algae.
However, for aquatic invertebrates the TER is less than 10, indicating
a potential risk to aquatic invertebrates (both molluscs and
arthropods). In such risk situations the use of a "no-spray"
restriction zone next to surface waters may reduce the risk to such
aquatic invertebrates. For example, arable spray drift at 5 m from the
edge of boom is 0.6% (Ganzelmeier et. al., 1995). Based on this 5 m
drift data, the PEC is 0.0006 mg/litre and results in a 5 m TER of 7
for molluscs and 37 for Daphnia. The TER for the most sensitive
invertebrate (mollusc) is still below the EPPO trigger of 10,
indicating a borderline risk. However, the Daphnia 5 m TER, which is
above 10, indicates that the use of a 5 m "no-spray" restriction zone
next to surface waters would help reduce the acute risk to aquatic
invertebrates.
(b) Spray drift from broadcast air-assisted top fruit applications
The acute PEC for spray drift (3 m from the point of application
into a 30-cm-deep static water body at the maximum application rate
(see PEC assumptions above) is 0.049 mg/litre. Therefore, the TERs
based on this PEC and the above LC/EC50 toxicity values are: fish,
12; aquatic invertebrates, 0.09 (0.4 for Daphnia); and algae, 163.
Based on the EPPO/CoE risk assessment scheme for aquatic organisms,
these TERs indicate a low acute risk to fish and algae. However, for
aquatic invertebrates the TER is less than 1 indicating a high risk to
aquatic invertebrates (both molluscs and arthropods). In such risk
situations the use of a "no-spray" restriction zone next to surface
waters may reduce the risk to such aquatic invertebrates. For example,
spray drift from broadcast air-assisted applications to top fruit at
15 m from point of application is 6.0% (Ganzelmeier et al., 1995).
Based on this 15 m drift data, the PEC is 0.006 mg/litre and results
in a 15 m TER of 0.7 for molluscs and 3.4 for Daphnia. These TERs at
15 m for the most sensitive invertebrates are still below the EPPO
trigger of 10, indicating a potential risk. Therefore, even with a 15
"no-spray" restriction zone next to surface waters, there is still a
potential risk to aquatic invertebrates from the broadcast
air-assisted use of demeton-S-methyl. Table 8 summarizes the acute
TERs for demeton-S-methyl to aquatic organisms at 1 m for arable and
3 m for broadcast air-assisted applications.
Table 8. Acute toxicity-exposure ratios (TER) for aquatic organisms at 1 m for arable and 3 m for broadcast air-assisted
applications
Species LC/EC50 PEC (mg/litre) at 1 m PEC (mg/litre) at 3 m TER TER
(mg/litre) (arable) (air assisted) (arable) (air assisted)
Fish 0.59 0.005 0.049 110 12
(Oncorhynchus mykiss)
Aquatic invertebrate 0.0042 0.005 0.049 0.8 0.09
(Paphia laterisulca)
Aquatic invertebrate 0.022 0.005 0.049 4.1 0.4
(Daphnia magna)
Alga 8.0 0.005 0.049 1500 163
(Scenedesmus subspicatus)
10.2.1.2 Chronic risk
No chronic toxicity data are available for fish. However, a
chronic NOEC of 0.0056 mg/litre has been reported for Daphnia
magna. There are no data reporting the persistence or degradation of
demeton-S-methyl in water, and so a chronic PEC could not be derived.
Therefore, owing to the lack of data on chronic fish toxicity and
environmental fate in water, it is not possible to assess the chronic
risk to either fish or aquatic invertebrates. There are also no data
available on either the toxicity of demeton-S-methyl to sediment-
dwelling invertebrates or fate of demeton-S-methyl in aquatic
sediments. Therefore, the risk to sediment-dwelling invertebrates
could also not be assessed. No fish bioaccumulation data are
available, but, as the log Pow is 1.3 (i.e. < 3), the risk of
bioaccumulation in fish should be low.
10.2.2 Terrestrial organisms
Vertebrates are likely to be exposed to demeton-S-methyl from
either grazing on treated vegetation or consuming contaminated
insects. For this risk assessment, typical application rates of
0.32 kg/ha are used for ground spray application on arable crops and
0.49 kg/ha for application by air-assisted spraying for fruit.
10.2.2.1 Birds
The lowest reported acute oral LD50 for birds is 10 mg/kg body
weight for the canary. Dietary data are not available.
Indicator birds for use in the risk assessment are:
* Greylag goose (Anser anser), as a grazing species, with a body
weight of 3 kg and total daily food consumption of 900 g
vegetation (dry weight) (Owen, 1975)
* Blue tit (Parus caeruleus), as an insectivorous species, with a
body weight of 11 g and total daily food consumption of 8.23 g
(dry weight) (Kenaga, 1973)
a) Grazing birds
Initial residues on short grass or cereal shoots are estimated to
be 35.8 mg/kg dry weight (based on 112 × application rate) arising
from application at 0.32 kg/ha to arable crops (EPPO/CoE, 1993a,b) and
55 mg/kg from application to fruit at a rate of 0.49 kg/ha. This gives
an estimated total oral intake for the goose of 32.2 mg and 49.5 mg
for the two application rates assuming that the goose ate exclusively
food contaminated at this level. This is equivalent to a daily intake
of 10.7 and 16.5 mg/kg body weight, respectively. TERs can be
calculated as follows:
End-point LD50/LC50 Application rate Predicted TER
(mg/kg b.w.) (kg/ha) concentration
in food (mg/kg)
Bird acute 10 0.32 (arable) 35.8 0.93
oral (Canary) 0.49 (fruit) 55 0.61
The calculated TER values fall well below the EPPO/CoE trigger
values for concern (TER <10) and indicate a high risk to grazing
birds.
b) Insectivorous birds
Initial residues on small insects are estimated to be 9.3 mg/kg
dry weight (based on 29 × application rate) arising from application
at 0.32 kg/ha to arable crops (EPPO/CoE, 1993a,b) and 14.2 mg/kg from
application to fruit at a rate of 0.49 kg/ha. This gives an estimated
total oral intake for the blue tit of 0.08 mg and 0.12 mg for the two
application rates assuming that the blue tit ate exclusively food
contaminated at this level. This is equivalent to a daily intake of
6.94 and 10.6 mg/kg body weight, respectively. TERs can be calculated
as follows:
End-point LD50/LC50 Application rate Predicted TER
(mg/kg b.w.) (kg/ha) concentration
in food (mg/kg)
Bird acute 10 0.32 (arable) 9.3 1.44
oral (Canary) 0.49 (fruit) 14.2 0.94
The TERs for acute toxicity to insectivorous birds are
substantially less than the trigger value of <10, indicating high
acute risk to these birds.
10.2.2.2 Mammals
The lowest reported acute oral LD50 for laboratory mammals is
63 mg/kg body weight for the rat.
Indicator mammals for use in the risk assessment will be:
* Rabbit (Oryctolagus cuniculus), as a grazing mammal, with a
body weight of 1200 g and a total daily food consumption of 500 g
vegetation (dry weight) (Ross, personal communication to the
IPCS).
* Shrew (Sorex araneus), as an insectivorous mammal, with a body
weight of 18 g and a total daily food consumption of 18 g
(Churchfield, 1986)
a) Grazing mammals
Initial residues on short grass or cereal shoots are estimated to
be 35.8 mg/kg dry weight (based on 112 × application rate) arising
from application at 0.32 kg/ha to arable crops (EPPO/CoE, 1993a,b) and
at 55 mg/kg from application to fruit at a rate of 0.49 kg/ha. This
gives an estimated total oral intake for the rabbit of 17.9 mg and
27.4 mg for the two application rates, assuming that the rabbit ate
exclusively food contaminated at this level. This is equivalent to a
daily intake of 15 and 23 mg/kg body weight, respectively. TERs can be
calculated as follows:
End-point LD50 Application rate Predicted TER
(test (mg/kg b.w.) (kg/ha) concentration
species) in food (mg/kg)
Acute oral 63 0.32 (arable) 17.9 4.20
(Rat) 0.49 (fruit) 27.5 2.76
Since the TERs are < 10, this indicates a high risk to grazing
mammals.
b) Insectivorous mammals
Initial residues on large insects are estimated to be 0.86 mg/kg
dry weight (based on 2.7 × application rate) arising from application
at 0.32 kg/ha to arable crops (EPPO/CoE, 1993a,b) and 1.32 mg/kg from
application to fruit at a rate of 0.49 kg/ha. This gives an estimated
total oral intake for the shrew of 0.016 mg and 0.024 mg for the two
application rates, assuming that the shrew ate exclusively food
contaminated at this level. This is equivalent to a daily intake of
0.86 and 1.32 mg/kg body weight, respectively. TERs can be calculated
as follows:
End-point LD50 Application rate Predicted TER
(mg/kg b.w.) (kg/ha) concentration
in food (mg/kg)
Mammal 63 (Rat) 0.32 (arable) 0.86 72.9
acute oral 0.49 (fruit) 1.32 47.6
These TERs fall outside the trigger for high risk for
insectivorous mammals but within the range for medium risk.
10.2.2.3 Bees
The reported contact toxicity to bees gives an LD50 of
0.26 µg/bee. Using application rates at 0.32 and 0.49 kg/ha for
cereals and fruit respectively, hazard quotients are calculated to be
1231 and 1885 (application (g/ha)/LD50 (µg/bee)). The trigger for
concern is >50 (EPPO/CoE, 1993a,b) and, therefore, there is
substantial concern for exposed bees. The compound should not be
applied to flowering plants, and exposure of flying bees should be
avoided.
10.2.2.4 Earthworms
Earthworms are likely to be exposed to demeton-S-methyl. Based on
a soil depth of 5 cm and a soil density of 1.5 g/cm3, the soil PEC
would be 0.43 mg/kg assuming even distribution in the medium. The
reported LC50 for earthworms (Eisenia foetida) is 60 mg/kg
soil giving a TER of 140.6. As this is above the trigger value of
10 (EPPO/CoE, 1993a,b), the acute risk to earthworms is very low.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
Demeton-S-methyl causes acute cholinergic toxicity in humans and
laboratory animals. Carcinogenic, reproductive and developmental
effects have not been found in laboratory animals. Effects due to
chronic human exposure are unlikely to occur. Exposure of the general
population may only occur through food residues. However, the levels
of exposure are unlikely to cause adverse effects.
Given the high acute toxicity, as determined in a number of test
species and the known cases of human poisoning, it can be concluded
that the risk of occupational poisoning with demeton-S-methyl exists
when adequate protective measures and good practices are not adopted.
Therefore, handling and application of demeton-S-methyl should only be
performed by supervised and trained workers who must adhere to good
application practices and adopt adequate safety measures.
Demeton-S-methyl does not persist in the environment and is not
accumulated by organisms. It has high acute toxicity to aquatic
invertebrates and is toxic to fish and birds, leading to high or
moderate risk factors for these organisms. However, significant field
kills of organisms have not been reported for the compound.
Precautions should be taken to minimize exposure of non-target
organisms (e.g., do not spray over water bodies, minimize exposure by
spray drift).
In view of the negative results obtained in developmental and
carcinogenicity studies, it is felt that further clarification of the
genotoxic potential of demeton-S-methyl is not necessary.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Demeton-S-methyl was classified as highly hazardous (class Ib) by
WHO (1996).
Demeton-S-methyl was evaluated by the Joint FAO/WHO Meeting on
Pesticide Residues (JMPR) in 1972, 1973, 1979, 1982, 1984, 1989, 1992.
The 1989 Meeting (FAO/WHO, 1990) evaluated demeton-S-methyl,
oxydemeton-methyl and demeton-S-methylsulfone and set the following
NOAEL levels:
demeton-S-methyl: mouse: 1 mg/kg diet (equal to
0.24 mg/kg body weight per day)
rat: 1 mg/kg diet (equal to
0.05 mg/kg body weight per day)
dog: 1 mg/kg diet (equal to 0.036 mg/kg
body weight per day)
oxydemeton-methyl: mouse: 30 mg/kg diet (equal to
0.03 mg/kg body weight per day)
rat: 0.57 mg/kg diet (equal to
0.03 mg/kg body weight per day)
dog: 0.125 mg/kg body weight per day
demeton-S-methylsulfone: rat: 1 mg/litre in drinking water
(equal to 0.06 mg/kg body weight per
day)
dog: 10 mg/kg diet (equal to 0.36 mg/kg
body weight per day)
The estimated ADI for demeton-S-methyl was established at
0-0.0003 mg/kg body weight. This ADI applies to demeton-S-methyl
alone or in combination with oxydemeton-methyl and/or demeton-S-
methylsulfone because residues are determined after oxidation and are
expressed as demeton-S-methyl. The 1992 JMPR reviewed the residue data
on these three related compounds. Updated figures were available only
for oxydemeton-methyl, which is replacing demeton-S-methyl in most
registrations. The Meeting decided therefore to change the definition
of the residues to "the sum of demeton-S-methyl, oxydemeton-methyl and
demeton-S-methylsulfone expressed as oxydemeton-methyl". The MRLs
varied from 0.05 to 1 mg/kg commodity and have been obtained from
supervised trials on oxydemeton-methyl rather than on
demeton-S-methyl.
Demeton-S-methyl has not been evaluated by the International
Agency for Research on Cancer (IARC).
REFERENCES
ACGIH (1993) 1993-1994 Threshold limit values for chemical substances
and physical agents. Cincinnati, Ohio, American Conference of
Governmental Industrial Hygienist.
Akarte SR, Rao KR, & Kulkarni DA (1986) Acute toxicity of Metasystox
to an estuarine lamellibranch mollusc, Paphia laterisulca, from
Ratnagiri coast, India. Int J Ecol Environ Sci, 12: 125-129.
Anderson JPE (1989) Influence of the commercial product Metasystox (i)
on soil respiration after amendment with glucose. Leverkusen, Germany,
Bayer AG (Unpublished report No. AJO/78289).
Anonymous (1977) Determination of residues of organophosphorus
pesticides in fruits and vegetables. Analyst, 102: 858-868.
Barr AM (1966) Further experience in the treatment of severe organic
phosphate poisoning. Med J. Aust, 1: 490-492.
Bathe R (1983) 12-month oral (feeding) toxicity study with E 154
in Beagle dogs. Leverkusen, Germany, Bayer AG (Unpublished report
No. 2542).
Bayer (1970) Testing the effects of Metasystox i and Metasystox R on
bees. Leverkusen, Germany, Bayer AG (Unpublished report).
Becker H (1983) Embryotoxicity and teratogenicity study on E 154 in
rabbits. Leverkusen, Germany, Bayer AG (Unpublished report No. 2422).
Blumenstock I (1989) Influence of the commercial product Metasystox
(I) on nitrification in soils. Leverkusen, Germany, Bayer AG
(Unpublished report No. BSI/78189).
Carrington da Costa RB, Maul ER, Pimentel J, Goncalves JS, Rebelo A,
Oliveira LC, & Rebelo ASC (1982) A case of acute poisoning by methyl
demeton in a female 5 months pregnant. Arch Toxicol, 5(Suppl):
202-204.
Churchfield S (1986) Shrews. Oswestry, A. Nelson (The Mammal Society
series).
Cifone MA (1984) Mutagenicity evaluation of E154 (C.N. demeton-S-
methyl) in the mouse lymphoma forward mutation assay. Leverkusen,
Germany, Bayer AG (Unpublished report No. 2789).
DuBois KP & Doull J (1955) The acute toxicity of P=O Meta-systox, P=O
Metasystox sulphoxide and P=O Meta-systox sulphone to mammals.
Leverkusen, Germany, Bayer AG (Unpublished report).
DuBois KP & Plzak G (1962) The acute toxicity and anticholinesterase
action of 0,0-dimethyl-S-ethyl-2-sulphinylethylphosphorothioate
(Meta-systox R) and related compounds. Toxicol Appl Pharmacol,
4: 621-630.
Dzwonkowska A & Hübner H (1986) Induction of chromosomal aberration in
the Syrian hamster by insecticides tested in vivo. Arch Toxicol,
58: 152-156.
Ecker W (1978) Biotransformation of (ethylene-1-14C) demeton-S-methyl
in rats. Leverkusen, Germany, Bayer AG (Unpublished report No. 7458).
Ecker W & Cölln R (1983) Biotransformation of demeton-S-methyl in
rats. Leverkusen, Germany, Bayer AG (Unpublished report No. 11431).
Edson EF (1960) Agriculture chemicals - Applied toxicology of
pesticides. Pharm J, 185: 361-367.
Eiben R, Gröning P, & Löser E (1984) Generation study with rats
(two generations). Leverkusen, Germany, Bayer AG (Unpublished report
No. 13135).
EPPO/CoE (European & Mediterranean Plant Protection Organisation/
Council of Europe) (1993a) Decision making schemes for the
environmental risk assessment of plant protection products.
OEPP/EPPO Bull, 23: 1-54.
EPPO/CoE (European & Mediterranean Plant Protection Organisation/
Council of Europe) (1993b) Decision making schemes for the
environmental risk assessment of plant protection products.
OEPP/EPPO Bull, 24: 1-87.
FAO/WHO (1974) Demeton-S-methyl and related compounds. In: 1973
Evaluations of some pesticides in food. Geneva, World Health
Organization, pp 195-240 (WHO Pesticide Residue Series No. 3).
FAO/WHO (1990) Demeton-S-methyl and related compounds. In: Pesticides
residues in food - 1989. Evaluations: Part II - Toxicology. Rome, Food
and Agriculture Organization of the United Nations, pp 49-74 (FAO
Plant Production and Protection Paper 100/2).
FAO/WHO (1993) Demeton-S-methyl. In: Pesticides residues in food -
1992. Evaluations: Part I - Residues. Rome, Food and Agriculture
Organization of the United Nations, p 287 (FAO Plant Production and
Protection Paper 118).
Faul J (1984) [E 154 = 25/154 (demeton-S-methyl; Metasystox i-active
ingredient] Leverkusen, Germany, Bayer AG (Unpublished report)
(in German).
Flucke W (1984) [Tests on acute oral toxicity to quail (Coturnix
coturnix japonica).] Leverkusen, Germany, Bayer AG (Unpublished
report No. 13119) (in German).
Flucke W & Eben A (1988) E154 50 VL (C.N. demeton-S-methyl): Study of
effect on neuropathy target esterase (NTE) after oral administration
to the hen. Leverkusen, Germany, Bayer AG (Unpublished report
No. 17264).
Flucke W & Kaliner G (1988) E 154 50 VL (C.N. demeton-S-methyl): Study
for acute delayed neurotoxicity after oral administration to the hen.
Leverkusen, Germany, Bayer AG (Unpublished report No. 16920).
Flucke W & Kimmerle G (1977) Trichlorfon (Dipterex active ingredient),
demeton-S-methyl (Metasystox (I) active ingredient): Study for acute
toxicity after simultaneous administration of both active ingredients.
Leverkusen, Germany, Bayer AG (Unpublished report No. 7066).
Flucke W & Pauluhn J (1983) Metaisosystox 50 EC: Study for
formulation toxicity. Leverkusen, Germany, Bayer AG (Unpublished
report No. 11770).
Froebe Z, Drevenkar V, Stengl B, & Juracic M (1989) Sorption behaviour
of some organophosphorus pesticides in natural sediments. Toxicol
Environ Chem, 19: 69-82.
Funk W, Cleres L, Pitzer H, & Donnevert G (1989) Organophosphorus
insecticides: Quantitative HPTLC determination and characterization.
J Planar Chromatogr, 2: 285-289.
Ganzelmeier IH, Rautmann D, Spangenberg R, Streloke M, Herrmann M,
Wenzelburger HJ, & Walter HF (1995) Studies on the spray drift of
plant protection products: Results of a test program carried out
throughout the Federal Republic of Germany. Oxford, London, Edinburgh,
Melbourne, Blackwell Scientific Publications.
Grau R (1984) [Oral toxicity to female bird/canary (Serinus
canarius).] Leverkusen, Germany, Bayer AG (Unpublished report
No. VK-243) (in German).
Grau R (1985a) Fish toxicity (Metaisosystox 50% pre-solution).
Leverkusen, Germany, Bayer AG (Unpublished report No. FF-177).
Grau R (1985b) Fish toxicity (Metaisosystox 50% pre-solution, golden
orfe). Leverkusen, Germany, Bayer AG (Unpublished report No. FO-818).
Grau R (1990a) [Acute toxicity of Metaisosystox EC 25 to rainbow trout
(Oncorhynchus mykiss) using flow test.] Leverkusen, Germany, Bayer
AG (Unpublished report No. FF-285) (in German).
Grau R (1990b) [Acute toxicity of Metasystox EC 25 to golden orfe
(Leuciscus idus melanotus) using flow test.] Leverkusen, Germany,
Bayer AG (Unpublished report No. FO-1222) (in German).
Grau R (1990c) [Toxicity of Metasystox VL 70 to rainbow trout
(O ncorhynchus mykiss) with extensive exposure (21 days).]
Leverkusen, Germany, Bayer AG (Unpublished report No. FF-287)
(in German).
Hanna PJ & Dyer KF (1975) Mutagenicity of organophosphorus compounds
in bacteria and Drosophila. Mutat Res, 28: 405-420.
Heath DF & Vandekar M (1957) Some spontaneous reactions of
O,O-dimethyl S-ethylthioethyl phosphorothioate and related compounds
in water and on storage, and their effects on the toxicological
properties of the compounds. Biochem J, 67: 187-201.
Hecht G (1955) [Comparison tests of preparation 25/154, R 2170
and M 3/158.] Leverkusen, Germany, Bayer AG (Unpublished report)
(in German).
Hecht G (1960) [Toxicological investigations on Metasystox (i)
presentation given in Tokyo, Korinkaku.] Leverkusen, Germany, Bayer AG
(unpublished report) (in German).
Hegazy MR (1965) Poisoning by meta-isosystox in spraymen and in
accidentally exposed patients. Br J Ind Med, 22: 230-235.
Heimann KG (1987a) E 154 techn. (C.N. demeton-S-methyl): Study for
skin-sensitizing effect on guinea pigs. Leverkusen, Germany, Bayer AG
(Unpublished report No. 15896).
Heimann G (1987b) E 154 techn. (C.N. demeton-S-methyl): Study for
skin-sensitizing effect on guinea pigs in the epicutaneous test.
Leverkusen, Germany, Bayer AG (unpublished report No. 16106).
Heimbach F (1985a) Growth inhibition of green algae (Scenedesmus
subspicatus) caused by demeton-S-methyl (techn.). Leverkusen,
Germany, Bayer AG (Unpublished report No. HBF/AL11).
Heimbach F (1985b) Acute toxicity of demeton-S-methyl (techn.) to
water fleas. Leverkusen, Germany, Bayer AG (Unpublished report
No. HBF/DM 47).
Heimbach F (1985c) [Acute toxicity of demeton-S-methyl 50 VL to
water fleas.] Leverkusen, Germany, Bayer AG (Unpublished report
No. HBF/DM 48) (in German).
Heimbach F (1990a) Toxicity of Metasystox (I) to earthworms.
Leverkusen, Germany, Bayer AG (Unpublished report No. HBF/RG 120).
Heimbach F (1990b) Growth inhibition of green algae (Scenedesmus
subspicatus) by Metasystox (I). Leverkusen, Germany, Bayer AG
(Unpublished report No. HBF/AL 74).
Heimbach F (1990c) Influence of Metasystox (I) on the reproduction
rate of water fleas. Leverkusen, Germany, Bayer AG (Unpublished
report No. HBF/RDM 25).
Heimbach F (1990d) Influence of demeton-S-methyl VL 70 on the
reproduction rate of water fleas. Leverkusen, Germany, Bayer AG
(Unpublished report No. HBF/RDM 27).
Hellpointner E (1990) Assessment of the environmental half-life of
the direct photodegradation of demeton-S-methyl in water. Leverkusen,
Germany, Bayer AG (Unpublished report No. PF3446).
Herbold B (1979) E 25/154 Salmonella/microsome test to evaluate for
point mutation. Leverkusen, Germany, Bayer AG (Unpublished report
No. 8393).
Herbold B (1980a) E 25/154 Salmonella/microsome test to evaluate for
point mutation. Leverkusen, Germany, Bayer AG (Unpublished report
No. 8809).
Herbold B (1980b) E 25/154 micronucleus test on the mouse to evaluate
for mutagenic effect. Leverkusen, Germany, Bayer AG (Unpublished
report No. 8932).
Herbold B (1980c) E 25/154 (demeton-S-methyl) dominant lethal study on
male mouse to test for mutagenic effects. Leverkusen, Germany, Bayer
AG (Unpublished report No. 9336).
Herbold B (1983a) E 154 (demeton-S-methyl) pol test on E. coli to
evaluate for DNA damage. Leverkusen, Germany, Bayer AG (Unpublished
report No. 12152).
Herbold B (1983b) E 154 (demeton-S-methyl) sister chromatid exchange
in the bone marrow of the Chinese hamster in vivo to evaluate for
harmful effect on DNA. Leverkusen, Germany, Bayer AG (Unpublished
report No. 12323).
Hermann G (1974a) [Fish toxicity of Metasystox (I) to goldfish.]
Leverkusen, Germany, Bayer AG (Unpublished report No. FG-112)
(in German).
Hermann G (1974b) [Fish toxicity of Metasystox (I) to carp.]
Leverkusen, Germany, Bayer AG (Unpublished report No. FK-28)
(in German).
Hermann G (1974c) [Fish toxicity of Metasystox (I) to rudd.]
Leverkusen, Germany, Bayer AG (Unpublished report No. FR-29)
(in German).
Hill ARC, Wilkins JPG, Findlay NRI, & Lontay KEM (1984)
Organophosphorus sulphides, sulphoxides and sulphones - Part I:
Determination of residues in fruit and vegetables by gas-liquid
chromatography. Analyst, 109: 483-487.
Hoorn AJW (1982) Mutagenicity evaluation of E 154 50 VL (in xylene)
in the reverse mutation induction assay with Saccharomyces
cerevisiae strains S138 and S211a. Leverkusen, Germany, Bayer AG
(Unpublished report No. 2372).
Jones RD (1982) Organophosphorus poisoning at a chemical packaging
company. Br J Ind Med, 39: 377-381.
Kenaga E (1973) Factors to be considered in the evaluation of the
toxicity of pesticides to birds in the environment. Environ Qual Saf,
2: 166-181.
Kimmerle G (1972) Acute toxicity of SRA 3886 in combination with S 276
and with E 154 to rats. Leverkusen, Germany, Bayer AG (Unpublished
report No. 3438).
Klimmer OR (1964) [Determination of acute oral toxicity of Metasystox
(i).] Leverkusen, Germany, Bayer AG (Unpublished report) (in German).
Klimmer OR & Pfaff W (1955) [Toxicity tests on the new contact
insecticide O,O-dimethyl-thiophosphoric acid-O/ -S-ethylester
("Metasystox").] Arzneimittel-Forschung, 5: 584-587 (in German).
Klimmer R & Pfaff W (1958) [Toxicity tests during application of the
systemic insecticide O,O-dimethyl-(ethyl-thioethyl)-thiophosphoric
acid-ester.] Arzneimittel-Forschung, 8: 265-269 (in German).
Kniehase U (1984) [Effects of Metasystox (I) 250 EC on mite
(Phytoseiulus persimilis).] Leverkusen, Germany, Bayer AG
(Unpublished report No. ZDE/PP 4/84) (in German).
Krohn J (1984) Fate/behaviour of crop protection products in water:
demeton-S-methyl. Leverkusen, Germany, Bayer AG (Unpublished report
No. M2421).
Lewis PJ, Lowing RK, & Gomopertz D (1981) Automated discrete kinetic
method for erythrocyte acetylcholinesterase and plasma cholinesterase.
Clin Chem, 27: 926-929.
Mary ASR, Nagabhushanam R, & Sarojini R (1986) Toxicity evaluation of
organophosphorus and chlorinated hydrocarbon pesticides in freshwater
prawn Macrobrachium lamerrii. J Environ Biol, 7: 189-195.
Mitra PK, Sud SC, & Bahga HS (1978) Acute oral toxicity of Metasystox
in buffalo calves. Indian J Exp Biol, 16: 813-815.
Muley DV, Akarte SR, Kulkarni DA, & Rao KR (1987) Acute toxicity of
Metasystox to wedge clam, Donax cuneatus, from West Coast. Fish
Technol, 24: 27-30.
Owen M (1975) The management of grass swards for captive wildfowl. Int
Zoo Year Book, 16: 135-138.
Pandita TK (1983) Mutagenic studies on the insecticide Metasystox-R
with different genetic systems. Mutat Res, 124: 97-102.
Renhof M (1985) E 154 (C.N. demeton-S-methyl): Study for embryotoxic
effect on rats after oral administration. Leverkusen, Germany, Bayer
AG (Unpublished report No. 13237).
Schludecker D & Aderjan R (1988) [Chemical-toxicological results of a
suicidal poisoning with demeton-S-methyl.] Z Rechtsmed, 101:55-60
(in German).
Schmid E (1984) Degradation of demeton-S-methyl in soil. Leverkusen,
Germany, Bayer AG (Unpublished report No. Im1428).
Schmid ER (1986) Degradation of demeton-S-methyl in soil at a
concentration of 3 ppm under anaerobic conditions. Leverkusen,
Germany, Bayer AG (Unpublished report No. IM1579).
Schmidt WM & Bomhard E (1988) Study for carcinogenicity in NMRI mice
(administration in diet for up to 21 months). Leverkusen, Germany,
Bayer AG (Unpublished report No. 16812).
Schmidt WM & Westen H (1988) Study for chronic toxicity and
carcinogenicity in Wistar rats (administration in diet for up to 2
years). Leverkusen, Germany, Bayer AG (Unpublished report No. 16888).
Shires SW (1985) Effects of aerial applications of cypermethrin and
demeton-S-methyl on non-target arthropods of wheat. Ecotoxicol Environ
Saf, 10: 1-11.
Stone HM (1961) The anticholinesterase activity of demeton-S-methyl.
NZ J Sci, 4: 78-90.
Su MQ, Kinoshita FK, Frawley JP, & DuBois KP (1971) Comparative
inhibition of aliesterases and cholinesterase in rats fed eighteen
organophosphorus insecticides. Toxicol Appl Pharmacol, 20: 241-249.
Tarrant KA, Thompson HM, & Hardy AR (1992) Biochemical and
histological effect of the aphicide demeton-S-methyl on house sparrows
(Passer domesticus) under field conditions. Bull Environ Contam
Toxicol, 48: 360-366.
Thompson HM, Walker CH, & Hardy AR (1991) Changes in activity of avian
serum esterases following exposure to organophosphorus insecticides.
Arch Environ Contam Toxicol, 20: 514-518.
Thornton JS, Olson TJ, & Wagner K (1977) Determination of residues
of Metasystox-R and metabolite in plant and animal tissues and soil.
J Agric Food Chem, 25: 573-576.
Thyssen J (1981) [Tests on skin and mucous-irritating effects of
demeton-S-methyl.] Leverkusen, Germany, Bayer AG (Unpublished report)
(in German).
Vandekar M (1958) The toxic properties of demeton-methyl
("Metasystox") and some related compounds. Brit J Ind Med,
15: 158-167.
Vasilic Z, Drevenkar V, Froebe Z, Stengl B, & Tkalcevic B (1987) The
metabolites of organophosphorus pesticides in urine as an indicator of
occupational exposure. Toxicol Environ Chem, 14:11-127.
Verma SP, Tonk IP, Gupta AK, & Saxena M (1984) Evaluation of an
application factor for determining the safe concentration of
agricultural and industrial chemicals. Water Res, 18(1): 111-115.
Wagner K & Oehlmann L (1987) Studies on the metabolic behaviour of
demeton-S-methyl in wheat. Leverkusen, Germany, Bayer AG (Unpublished
report No. PF2701).
Wagner K & Thornton JS (1977) Method for the gas-chromatographic
determination of Metasystox (i) and Metasystox R residues in plants,
soil and water. Pflanzenschutz Nachr Bayer, 30: 1-17.
Wagner K, Schütz U, & Oehlmann L (1985) Degradation of Metasystox-(i)
in soil under aerobic, anaerobic and sterile test conditions.
Leverkusen, Germany, Bayer AG (Unpublished report No. PF2565).
Weber H, Patzschke K, & Wagner LA (1978) (14C), demeton-S-methyl
(Metasystox in active ingredient)/Biokinetic studies on rats.
Leverkusen, Germany, Bayer AG (Unpublished report No. 7491).
Westlake GE, Hardy AR, & Stevenson JH (1985) Effects of storage and
pesticide treatments on honey bee brain acetyl cholinesterase
activities. Bull Environ Contam Toxicol, 34: 668-675.
WHO (1996) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1996-1997. Geneva, World Health
Organization, International Programme on Chemical Safety
(WHO/PCS/96.3).
Wilkins JPG, Hill ARC, & Lee DF (1985) Organophosphorus sulphides,
sulphoxide and sulphones - Part 2: Characterisation by gas
chromatography-mass spectrometry. Analyst, 110: 1045-1051.
Wilmes R (1984) Orientating light stability: demeton-S-methyl.
Leverkusen, Germany, Bayer AG (Unpublished report No. IM1408).
Winkler KO & Arent H (1970) Medical observation of personnel occupied
with spraying in hop cultivation during the application of the organic
phosphoric acid esters mevinphos and demeton-S-methyl (Metasystox i).
Leverkusen, Germany, Bayer AG (Unpublished report No. 24618).
Xue SZ, Ding XJ, & Ding Y (1985) Clinical observation and comparison
of the effectiveness of several oxime cholinesterase reactivators.
Scand J Work Environ Health, 11(Suppl 4): 46-48.
Ziegler W, Engelhardt G, Wallnöfer PR, Oehlmann L, & Wagner K (1980)
Degradation of demeton-S-methyl sulfoxide (Metasystox R) by soil
microorganisms. J Agric Food Chem, 28: 1102-1106.
RÉSUMÉ ET ÉVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Identité, propriétés physiques et chimiques
Le déméton-S-méthyl se présente sous la forme d'un liquide
huileux jaune pâle à l'odeur pénétrante. C'est un organophosphoré
agissant par la voie générale et par contact qui est utilisé comme
insecticide et acaricide sur les fruits, les légumes, les céréales et
les plantes ornementales pour lutter contre Acarina, Thysanoptera,
Hymenoptera et Homoptera. Sa tension de vapeur est de 63,8 mPa à
20°C et il est facilement soluble dans la plupart des solvants
organiques. Il est très soluble dans l'eau (3,3 g/litre à la
température ambiante) et son coefficient de partage entre l'octanol et
l'eau (log Pow) est de 1,32. Le déméton-S-méthyl est stable dans les
solvants non aqueux.
Pour la recherche et le dosage des résidus et l'analyse des
échantillons prélevés dans l'environnement, on procède à une
extraction au moyen d'un solvant organique, suivie d'une oxydation de
la sulfone correspondante. Le dosage s'effectue ensuite par
chromatographie en phase gazeuse avec un détecteur spécifique du
phosphore.
1.2 Sources d'exposition humaine et environnementale
Jusqu'en 1957, le déméton-méthyl a été commercialisé sous la
forme d'un mélange de deux isomères, le déméton-S-méthyl et le
déméton-O-méthyl. La formulation commerciale est un concentré
émulsionnable que l'on utilise en pulvérisations sur les céréales, les
fruits, les légumes et les plantes ornementales. On est en train de le
remplacer par l'oxydéméton-méthyl qui est un métabolite produit par
les plantes et les mammifères ou qui résulte du séjour du
déméton-S-méthyl dans le sol.
1.3 Transport, distribution et transformation dans l'environnement
La décomposition par hydrolyse du déméton-S-méthyl dépend du pH
de la solution. A 22°C, sa demi-vie est de 56 jours à pH 7 et de 8
jours à pH 9. Dans le sol, sa voie de décomposition principale est la
biodégradation, avec une demi-vie d'environ 4 h. Au bout de 24 h, il y
a encore dans le sol une proportion d'oxydéméton-méthyl qui représente
20 à 30% de la dose initiale de déméton-S-méthyl. Le coefficient de
sorption dans le sol (Kd) du déméton-S-méthyl est de 0,68 à 2,66,
selon la composition du sol.
Dans l'environnement, l'une des principales voies de dégradation
du composé est la photolyse. La métabolisation par le blé de printemps
est rapide et elle analogue à celle qui se produit dans le sol et chez
les mammifères.
1.4 Niveaux d'exposition environnementale et humaine
La population générale est principalement exposée au
déméton-S-méthyl par l'intermédiaire des résidus qui subsistent sur
les cultures vivrières. La réunion conjointe FAO/OMS sur les résidus
de pesticides (JMPR) a recommandé une dose journalière admissible
(DJA) de 0,0003 mg/kg de poids corporel. Il s'agit d'une DJA
collective qui englobe le déméton-S-méthyl, l'oxydéméton-méthyl et la
déméton-S-méthylsulfone, étant donné que les méthodes habituelles
d'analyse ne distinguent pas ces différents composés. Il est arrivé
qu'une exposition excessive au déméton-S-méthyl par la voie
transcutanée entraîne une intoxication de type cholinergique chez des
ouvriers mal protégés qui procédaient au conditionnement du concentré.
En revanche, des volontaires qui s'étaient prêtés à l'épandage
simulé d'un mélange de déméton-S-méthyl et de déméton-O-méthyl (30 et
70% respectivement) et avaient été exposés à des concentrations de 8,8
à 27 mg/m3 des deux matières actives, n'ont présenté aucune réduction
de leur activité cholinestérasique plasmatique ou érythrocytaire.
1.5 Cinétique et métabolisme
Chez le rat, le déméton-S-méthyl est rapidement et presque
complètement résorbé au niveau de l'intestin et il se répartit
uniformément dans les tissus (exception faite des hématies dans
lesquelles il se concentre plus fortement). Il est rapidement
métabolisé et excrété dans les urines. Sa concentration sanguine
diminue avec une demi-vie qui est initialement de 2 h environ. Au
bout de 24 h, il reste environ 1% de la dose dans l'organisme. La
principale voie métabolique du déméton-S-méthyl consiste en une
oxydation de la chaîne latérale qui conduit à la formation du
sulfoxyde correspondant (oxydéméton-méthyl) et, dans une moindre
proportion, après une oxydation plus poussée, à la sulfone.
L'O-déméthylation constitue une autre voie métabolique importante.
1.6 Effets sur les animaux de laboratoire et les systèmes d'épreuve
in vitro
1.6.1 Exposition unique
Le déméton-S-méthyl provoque des intoxications de type
cholinergique. Chez les mammifères, la DL50 varie de 7 à 100 mg/kg
p.c. selon la voie d'administration et l'espèce.
1.6.2 Exposition de courte durée
Une des premières études, comportant une exposition par la voie
alimentaire, a montré que des rats qui recevaient le composé à raison
de 50 mg/kg de nourriture, avaient une activité cholonestérasique
cérébrale sensiblement réduite au bout de 26 semaines de ce régime.
Des signes d'intoxication de type cholinergique ont été observés au
bout de 5 semaines de traitement chez des rats recevant 200 mg de
composé par kg de nourriture.
Lors d'une étude d'alimentation d'un an effectuée sur des chiens,
on a obtenu une dose sans effet nocif observable (NOAEL), basée sur
les effets cholinergiques, de 1 mg/kg de nourriture, soit l'équivalent
quotidien de 0,036 mg/kg de poids corporel.
1.6.3 Exposition de longue durée
Des souris ont reçu pendant 21 mois des rations contenant
respectivement 0, 1, 15 ou 75 mg de déméton-S-méthyl par kg de
nourriture. La NOAEL, a été trouvée égale à 1 mg/kg de nourriture,
soit l'équivalent quotidien de 0,24 mg/kg de poids corporel en prenant
comme base l'inhibition de la cholinesterase cérébrale.
Chez des rats ayant reçu une alimentation contenant
respectivement 0, 1, 7 ou 50 mg/kg de déméton-S-méthyl, la NOAEL,
basée sur l'inhibition de la cholinestérase cérébrale, s'est révélée
égale à 1 mg/kg de nourriture, soit l'équivalent quotidien de
0,05 mg/kg p.c.
Chez aucune espèce il n'a été constaté d'augmentation de
l'incidence des tumeurs.
1.6.4 Irritation et sensibilisation cutanée ou oculaire
Le déméton-S-méthyl est légèrement irritant pour l'oeil et la
peau. Le test de Magnusson et Klingman a donné des résultats positifs
chez des cobayes. En revanche, le test de sensibilisation de Buehler
(application d'un timbre cutané) n'a pas fourni d'indice d'une
sensibilisation cutanée, ce qui donne à penser que, dans la pratique,
l'usage du déméton-S-méthyl ne devrait pas poser de problème de
sensibilisation.
1.6.5 Effets sur la reproduction, embryotoxicité et tératogénicité
Lors d'une étude sur deux générations de rats, au cours de
laquelle les animaux ont été exposés au composé par la voie
alimentaire, on a constaté une réduction de la viabilité et du poids
corporel des ratons (uniquement la génération F1b) à la dose de
5 mg/kg de nourriture. La NOAEL était de 1 mg/kg de nourriture, soit
l'équivalent quotidien de 0,07 mg/kg de poids corporel.
Le déméton-S-méthyl ne s'est révélé ni embryotoxique, ni
tératogène chez le rat et le lapin.
1.6.6 Mutagénicité et points apparentés d'aboutissement des effets
toxiques
Le déméton-S-méthyl produit des mutations ponctuelles
in vitro. On n' a mis en évidence des effets chromosomiques
in vivo qu'avec des formulations du commerce. Les données
disponibles sont insuffisantes pour que l'on puisse procéder à une
évaluation satisfaisante du pouvoir génotoxique de ce composé.
1.6.7 Neurotoxicité retardée
Le déméton-S-méthyl n'a produit ni polyneuropathie, ni inhibition
de l'estérase cible correspondante (NTE) lorsqu'on l'a administré à
des poules en dose égale à la DL50 par voie orale.
1.6.8 Toxicité des métabolites
Deux métabolites du déméton-S-méthyl produits par des
plantes et des mammifères, à savoir l'oxydéméton-méthyl et la
déméton-S-méthylsulfone, sont également des pesticides du commerce et
ont été très largement étudiés. Il ressort de ces travaux que le
profil toxicologique de ces composés ne diffère pas sensiblement,
quantitativement ou qualitativement, de celui du déméton-S-méthyl.
1.7 Mécanisme de la toxicité - Mode d'action
Le déméton-S-méthyl est un inhibiteur direct de la cholinestérase
et sa toxicité est liée au fait qu'il inhibe l'acétylcholinestérase
(AChE) au niveau des terminaisons nerveuses. Une fois inhibée par le
déméton-S-méthyl, l'acétylcholinestérase se réactive spontanément avec
une demi-vie in vitro de 1,3 h environ, comme on peut s'y attendre
pour une AChE diméthylphosphorylée.
1.8 Effets sur l'homme
On a signalé quelques cas d'intoxication aiguë avec syndrome
cholinergique, consécutifs à des tentatives de suicide. Aucun effet
retardé n'a été observé chez les survivants, dont une femme enceinte.
A la suite d'une exposition subie par négligence lors du
conditionnement de formulations du commerce, quelques employés ont
présenté des troubles d'origine cholinergique qui ont nécessité un
traitement pharmacologique. Le composé avait probablement pénétré par
la voie transcutanée. De même, il est possible que des conditions de
travail laissant à désirer aient entraîné une absorption excessive de
déméton-S-méthyl au cours de l'épandage de ce composé sur des champs
de coton.
1.9 Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Pour les algues vertes, la CE50 à 96 h va de 8 à 37 mg/litre. On
a mesuré une CL50 allant de 0,004 à 1,3 mg/litre chez une série
d'invertébrés aquatiques. Chez les poisson, la toxicité est variable,
avec une CL50 à 96 h qui peut aller de 0,59 mg/litre pour la truite
arcen-ciel à environ 40 mg/litre pour l'orfe, le cyprin doré et la
carpe.
Chez la caille japonaise et le canari, on a obtenu une DL50
(effet aigu, voie orale) de 10-50 mg/kg de poids corporel. Chez des
étourneaux, une dose unique par voie orale de 2 mg/kg p.c. a provoqué
une inhibition de l'acétylcholinestérase cérébrale 3 h après le
traitement.
Pour les lombrics terricoles, la CL50 du déméton-S-méthyl est de
66 mg/kg sur 14 jours. Pour les abeilles, on a obtenu des valeurs de
la DL50 de contact et par voie orale (effet aigu) respectivement
égales à 0,21 et 0,6 par insecte. En traitant du blé d'hiver à la dose
recommandée, on a constaté une réduction sensible du nombre
d'invertébrés présents sur les feuilles (principalement des diptères
du genre Empididae) sans effet sur les invertébrés entomophages
présents à la surface du sol.
2. Conclusions
Le déméton-S-méthyl est un insecticide organophosphoré fortement
toxique (classe Ib de la classification de l'OMS) (OMS, 1966). Son
action toxique s'explique par l'inhibition de l'acétylcholinestérase
au niveau des terminaisons nerveuses. L'exposition de la population
générale résulte principalement des résidus présents dans les denrées
alimentaires provenant des cultures traitées.
Moyennant de bonnes pratiques agricoles et le respect des règles
d'hygiène et de sécurité, la manipulation du composé lors de sa
fabrication ou de son épandage ne devrait entraîner aucun effet
indésirable. Des effets résultant d'une exposition chronique sont
improbables.
Le déméton-S-méthyl ne persiste pas dans l'environnement et il ne
s'accumule pas chez les êtres vivants. Il est très toxique pour les
invertébrés aquatiques et présente une toxicité notable pour les
poissons et les oiseaux, aussi doit on considérer que pour ces
organismes, le facteur de risque est élevé ou modéré. Néanmoins, on
n'a pas signalé de mortalité importante dans la nature du fait de ce
composé. Des précautions sont à prendre pour réduire au minimum
l'exposition des organismes non visés (par ex. ne pas le pulvériser
sur des étendues d'eau et veiller à ce que les gouttelettes
d'insecticide ne soient pas entraînées vers les zones à respecter).
3. Recommandations
Afin de protéger la santé et le bien-être des travailleurs et de
la population dans son ensemble, il ne faut confier la manipulation et
l'épandage du déméton-S-méthyl qu'à des opérateurs dûment formés et
encadrés, qui auront le souci de respecter les mesures de sécurité et
d'épandre le produit selon les règles.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
1.1 Identidad, propiedades físicas y químicas, y métodos analíticos
El demeton-S-metilo, un líquido oleoso de color amarillo pálido y
olor penetrante, es un insecticida y acaricida organofosfatado
sistémico y de contacto que se utiliza para combatir Acarina,
Thysanoptera y Homóptera en frutas, cereales, plantas ornamentales
y hortalizas. Tiene una presión de vapor de 63,8 mPa a 20°C, es
fácilmente soluble en la mayoría de los disolventes orgánicos, tiene
una gran solubilidad en agua, de 3,3 g/litro a temperatura ambiente, y
un coeficiente de reparto octanol/agua (log Pow) de 1,32. El
demeton-S-metilo es estable en disolventes no acuosos.
Los análisis de residuos y ambientales se realizan por extracción
con un disolvente orgánico y oxidación a la sulfona correspondiente.
Las mediciones se efectúan por cromatografía de gases utilizando un
detector específico del fósforo.
1.2 Fuentes de exposición humana y ambiental
Antes de 1957, el metildemeton se comercializaba como mezcla de
los isómeros demeton-S-metilo y demeton-O-metilo.
El demeton-S-metilo se utiliza desde 1957. Su presentación es la
de un concentrado emulsionable y se aplica por rociamiento a los
cereales, frutas, plantas ornamentales y hortalizas. Se está
sustituyendo por el oxidemetonmetilo, que es un metabolito del
demeton-S-metilo producido en las plantas, el suelo y los mamíferos.
1.3 Transporte, distribución y transformación en el medio ambiente
La degradación hidrolítica del demeton-S-metilo depende del pH de
la solución; a 22°C la semivida es de 63 días con un pH de 4, de 56
días con un pH de 7 y de 8 días con un pH de 9. La principal vía de
degradación en el suelo es la biodegradación. La semivida del
demeton-S-metilo en el suelo es de unas 4 horas. Sin embargo, al cabo
de 24 horas, el oxidemetonmetilo representa aún el 20-30 % de la dosis
aplicada de demeton-S-metilo. El coeficiente de sorción (Kd) del
demeton-S-metilo en el suelo oscila entre 0,68 y 2,66, dependiendo de
la composición del suelo.
La fotolisis no es uno de los principales mecanismos de
degradación del demeton-S-metilo en el medio ambiente.
Su metabolismo en el trigo de primavera es rápido y similar al
que se produce en el suelo y en los mamíferos.
1.4 Niveles ambientales y exposición humana
La exposición primaria de la población humana en general se
produce a través de los residuos de demeton-S-metilo en los cultivos
alimentarios. En la reunión conjunta FAO/OMS sobre residuos de
plaguicidas (JMPR) se recomendó una ingesta diaria admisible (IDA) de
0,0003 mg/kg de peso corporal. Se trata de una IDA colectiva para el
demeton-S-metilo, el oxidemetonmetilo y el demeton-S-metilsulfona, ya
que los métodos habituales de análisis no diferencian entre estos tres
compuestos.
La exposición excesiva al demeton-S-metilo y su absorción cutánea
produjeron toxicidad colinérgica en trabajadores insuficientemente
protegidos durante el envasado de la formulación concentrada.
Tras haber expuesto a voluntarios participantes en una simulación
de rociamiento con una mezcla de demeton-S-metilo y demeton-O-metilo
(30 y 70% respectivamente) a 8,8-27 mg/m3 de ambos componentes
activos combinados, no se observaron efectos adversos en la actividad
de la colinesterasa en el plasma ni en los eritrocitos.
1.5 Cinética y metabolismo
El demeton-S-metilo se absorbe rápida y casi completamente por el
tracto intestinal en las ratas y se distribuye uniformemente a los
tejidos (excepto una concentración elevada en los eritrocitos). Se
metaboliza rápidamente y se excreta por orina. La concentración en la
sangre disminuye, con una semivida inicial de unas 2 horas.
Aproximadamente el 1% de la dosis oral sigue presente en el organismo
24 horas después del tratamiento. La principal ruta metabólica del
demeton-S-metilo en las ratas es la oxidación de la cadena lateral,
que da lugar a la formación del sulfóxido correspondiente
(oxidemetonmetilo) y, en menor medida, a la sulfona, tras una
oxidación mayor. Otra ruta metabólica importante es la O-demetilación.
1.6 Efectos en mamíferos de laboratorio y en sistemas de ensayo
in vitro
1.6.1 Exposición única
El demeton-S-metilo produce toxicidad colinérgica. Los valores de
la DL50 para los mamíferos oscilan entre 7 y 100 mg/kg de peso
corporal, dependiendo de la vía de administración y de la especie.
1.6.2 Exposición breve
Un estudio alimentario inicial reveló que las ratas alimentadas
con 50 mg de demeton-S-metilo por kg de la dieta habían sufrido una
reducción notable de la actividad de la colinesterasa cerebral y
eritrocitaria tras una exposición de 26 semanas. Se observaron
síntomas colinérgicos en ratas alimentadas con 200 mg/kg de la dieta
durante las 5 primeras semanas de exposición.
En un estudio alimentario de un año de duración efectuado en
perros se estableció un nivel sin efectos adversos observados (NOAEL)
de 1 mg/kg de la dieta (equivalente a 0,036 mg/kg de peso corporal por
día) sobre la base de los efectos de la colinesterasa cerebral.
1.6.3 Exposición prolongada
Se administró durante 21 meses a ratones una dieta que contenía
0, 1, 15 ó 75 mg/kg de demeton-S-metilo. El NOAEL, determinado sobre
la base de la inhibición de la colinesterasa cerebral, fue de 1 mg/kg
de la dieta (equivalente a 0,24 mg/kg de peso corporal por día).
En ratas cuya dieta contenía 0, 1, 7 ó 50 mg/kg de demeton-S-metilo,
el NOAEL, determinado sobre la bases de la inhibición de la
colinesterasa cerebral, fue de 1 mg/kg de la dieta (equivalente a
0,05 mg/kg de peso corporal por día).
La incidencia de tumores no aumentó en ninguna de esas especies.
1.6.4 Irritación y sensibilización de la piel y los ojos
El demeton-S-metilo es un irritante cutáneo y ocular leve. La
prueba de maximización de Magnusson y Klingman dio resultados
positivos en cobayos. Sin embargo, la prueba del parche cutáneo de
Buehler no mostró indicios de sensibilización cutánea, lo que parece
indicar que el uso práctico del demeton-S-metilo no debería acarrear
problemas de sensibilización.
1.6.5 Reproducción, embriotoxicidad y teratogenicidad
En un estudio alimentario llevado a cabo en dos generaciones de
ratas, se observó una reducción de la viabilidad y del peso corporal
de los cachorros (generación F1b solamente) con una dosis de 5 mg de
demeton-S-metilo por kg de la dieta. El NOAEL fue de 1 mg/kg de la
dieta, equivalente a 0,07 mg/kg de peso corporal por día.
El demeton-S-metilo no resultó embriotóxico ni teratogénico en
ratas ni en conejos.
1.6.6 Mutagenicidad y parámetros conexos
El demeton-S-metilo produce mutaciones puntuales in vitro. Se han
demostrado efectos cromosómicos in vivo con formulaciones comerciales
solamente. La información disponible es insuficiente para realizar una
evaluación adecuada del potencial genotóxico del demeton-S-metilo.
1.6.7 Neurotoxicidad retardada
El demeton-S-metilo no causó polineuropatías retardadas ni
inhibición de la Esterasa Diana de Neuropatía en pruebas con gallinas
a un nivel igual a la DL50 oral.
1.6.8 Toxicidad de los metabolitos
Las plantas y los mamíferos producen dos metabolitos del
demeton-S-metilo (el oxidemetonmetilo y el demeton-S-metilsulfona) que
también son plaguicidas comerciales y han sido ampliamente estudiados.
Se ha señalado de que el perfil toxicológico de estos dos compuestos
no difiere de manera significativa, cuantitativa ni cualitativamente,
del demeton-S-metilo.
1.7 Mecanismo de toxicidad: modalidad de acción
El demeton-S-metilo es un inhibidor indirecto de la colinesterasa
y su toxicidad que está relacionada con la inhibición de la
acetilcolinesterasa en las terminales nerviosas. La
acetilcolinesterasa inhibida por el demeton-S-metilo se reactiva
espontáneamente, con una semivida in vitro de aproximadamente
1,3 horas, como cabe esperar en la acetilcolinesterasa dimetil
fosforilada.
1.8 Efectos en el ser humano
Se han notificado varios casos de intoxicación aguda con
síndrome colinérgico tras intentos de suicidio. Los pacientes que
sobrevivieron, inclusive una mujer embarazada, no presentaron efectos
retardados.
Tras una exposición laboral por inatención durante el envasado de
la formulación comercial, algunos trabajadores desarrollaron una
toxicidad colinérgica que precisó tratamiento farmacológico. El
demeton-S-metilo se absorbió probablemente a través de la piel.
Análogamente, condiciones de trabajo inapropiadas podrían haber sido
la causa de una absorción cutánea excesiva durante la aplicación del
demeton-S-metilo en algodonales.
1.9 Efectos en otros organismos en el laboratorio y sobre el terreno
Las CE50 96-h para las algas verdes se sitúan entre 8 y
37 mg/litro. Las CL50 para diversos invertebrados acuáticos se
encuentran entre 0,004 y 1,3 mg/litro. La toxicidad para los peces
varía; la CL50 96-h oscila entre 0,59 mg/litro para la trucha
arcoiris y unos 40 mg/litro para Leuciscus idus, Carassius auratus
y la carpa.
La DL50 oral aguda para la codorniz japonesa y el canario es de
10-50 mg/kg de peso corporal. En el estornino, una dosis oral única de
2 mg/kg de peso corporal produjo inhibición del 20% de la AChE
cerebral 3 horas después del tratamiento.
La CL50 del demeton-S-metilo en el suelo para las lombrices
de tierra es de 66 mg/kg para un período de 14 días. La DL50 aguda
oral y de contacto del demeton-S-metilo fue de 0,21 y 0,6 g/abeja
respectivamente. Después de haber sido aplicado al trigo de invierno
según la proporción propuesta, el demeton-S-metilo redujo
significativamente el número de invertebrados en el follaje de los
cultivos (principalmente Empididae), pero no el número de
invertebrados entomófagos de la superficie del suelo.
2. Conclusiones
El demeton-S-metilo es un éster organofósforado sumamente tóxico
(pertenece a la clase Ib de la clasificación de la OMS) (OMS, 1996)
utilizado como insecticida. La acción tóxica consiste en la inhibición
de la acetilcolinesterasa en las terminales nerviosas. La exposición
de la población en general se produce principalmente a causa de los
residuos presentes en los productos agrícolas.
Con buenas prácticas laborales y medidas de higiene y de
seguridad, el demeton-S-metilo no debería producir efectos adversos
durante la fabricación o la aplicación. Es poco probable que aparezcan
efectos debidos a la exposición crónica.
El demeton-S-metilo no es persistente en el medio ambiente y no
se acumula en los organismos. Tiene una toxicidad aguda elevada para
los invertebrados acuáticos y es tóxico para los peces y las aves,
llevando aparejados factores de riesgo agudo o moderado elevados para
esos organismos. Sin embargo, no se han comunicado muertes masivas
significativas de organismos en el campo a causa de este compuesto.
Habría que tomar precauciones para reducir al mínimo la exposición de
organismos no combatidos (por ejemplo, no rociar sobre masas de agua y
reducir al mínimo la exposición por desviación del rociado).
3. Recomendaciones
Para la salud y el bienestar de los trabajadores y del público en
general, el manejo y la aplicación del demeton-S-metilo deberían
encomendarse exclusivamente a personal bien adiestrado y supervisado
que aplique las medidas de seguridad necesarias y buenas prácticas de
aplicación.
(a) Spray drift from ground-based applications
The acute PEC for spray drift (1 m from the edge of the spray
boom into a 30-cm-deep static water body at the maximum application
rate (see PEC assumptions above) is 0.005 mg/litre. Therefore, the
TERs based on this PEC and the above LC/EC50 toxicity values are:
fish, 110; aquatic invertebrates, 0.8 (4.1 for Daphnia); and algae,
1500. Based on the EPPO/CoE risk assessment scheme for aquatic
organisms, these TERs indicate a low acute risk to fish and algae.
However, for aquatic invertebrates the TER is less than 10, indicating
a potential risk to aquatic invertebrates (both molluscs and
arthropods). In such risk situations the use of a "no-spray"
restriction zone next to surface waters may reduce the risk to such
aquatic invertebrates. For example, arable spray drift at 5 m from the
edge of boom is 0.6% (Ganzelmeier et. al., 1995). Based on this 5 m
drift data, the PEC is 0.0006 mg/litre and results in a 5 m TER of 7
for molluscs and 37 for Daphnia. The TER for the most sensitive
invertebrate (mollusc) is still below the EPPO trigger of 10,
indicating a borderline risk. However, the Daphnia 5 m TER, which is
above 10, indicates that the use of a 5 m "no-spray" restriction zone
next to surface waters would help reduce the acute risk to aquatic
invertebrates.
(b) Spray drift from broadcast air-assisted top fruit applications
The acute PEC for spray drift (3 m from the point of application
into a 30-cm-deep static water body at the maximum application rate
(see PEC assumptions above) is 0.049 mg/litre. Therefore, the TERs
based on this PEC and the above LC/EC50 toxicity values are: fish,
12; aquatic invertebrates, 0.09 (0.4 for Daphnia); and algae, 163.
Based on the EPPO/CoE risk assessment scheme for aquatic organisms,
these TERs indicate a low acute risk to fish and algae. However, for
aquatic invertebrates the TER is less than 1 indicating a high risk to
aquatic invertebrates (both molluscs and arthropods). In such risk
situations the use of a "no-spray" restriction zone next to surface
waters may reduce the risk to such aquatic invertebrates. For example,
spray drift from broadcast air-assisted applications to top fruit at
15 m from point of application is 6.0% (Ganzelmeier et al., 1995).
Based on this 15 m drift data, the PEC is 0.006 mg/litre and results
in a 15 m TER of 0.7 for molluscs and 3.4 for Daphnia. These TERs at
15 m for the most sensitive invertebrates are still below the EPPO
trigger of 10, indicating a potential risk. Therefore, even with a 15
"no-spray" restriction zone next to surface waters, there is still a
potential risk to aquatic invertebrates from the broadcast
air-assisted use of demeton-S-methyl. Table 8 summarizes the acute
TERs for demeton-S-methyl to aquatic organisms at 1 m for arable and
3 m for broadcast air-assisted applications.
Table 8. Acute toxicity-exposure ratios (TER) for aquatic organisms at 1 m for arable and 3 m for broadcast air-assisted
applications
Species LC/EC50 PEC (mg/litre) at 1 m PEC (mg/litre) at 3 m TER TER
(mg/litre) (arable) (air assisted) (arable) (air assisted)
Fish 0.59 0.005 0.049 110 12
(Oncorhynchus mykiss)
Aquatic invertebrate 0.0042 0.005 0.049 0.8 0.09
(Paphia laterisulca)
Aquatic invertebrate 0.022 0.005 0.049 4.1 0.4
(Daphnia magna)
Alga 8.0 0.005 0.049 1500 163
(Scenedesmus subspicatus)
10.2.1.2 Chronic risk
No chronic toxicity data are available for fish. However, a
chronic NOEC of 0.0056 mg/litre has been reported for Daphnia
magna. There are no data reporting the persistence or degradation of
demeton-S-methyl in water, and so a chronic PEC could not be derived.
Therefore, owing to the lack of data on chronic fish toxicity and
environmental fate in water, it is not possible to assess the chronic
risk to either fish or aquatic invertebrates. There are also no data
available on either the toxicity of demeton-S-methyl to sediment-
dwelling invertebrates or fate of demeton-S-methyl in aquatic
sediments. Therefore, the risk to sediment-dwelling invertebrates
could also not be assessed. No fish bioaccumulation data are
available, but, as the log Pow is 1.3 (i.e. < 3), the risk of
bioaccumulation in fish should be low.
10.2.2 Terrestrial organisms
Vertebrates are likely to be exposed to demeton-S-methyl from
either grazing on treated vegetation or consuming contaminated
insects. For this risk assessment, typical application rates of
0.32 kg/ha are used for ground spray application on arable crops and
0.49 kg/ha for application by air-assisted spraying for fruit.
10.2.2.1 Birds
The lowest reported acute oral LD50 for birds is 10 mg/kg body
weight for the canary. Dietary data are not available.
Indicator birds for use in the risk assessment are:
* Greylag goose (Anser anser), as a grazing species, with a body
weight of 3 kg and total daily food consumption of 900 g
vegetation (dry weight) (Owen, 1975)
* Blue tit (Parus caeruleus), as an insectivorous species, with a
body weight of 11 g and total daily food consumption of 8.23 g
(dry weight) (Kenaga, 1973)
a) Grazing birds
Initial residues on short grass or cereal shoots are estimated to
be 35.8 mg/kg dry weight (based on 112 × application rate) arising
from application at 0.32 kg/ha to arable crops (EPPO/CoE, 1993a,b) and
55 mg/kg from application to fruit at a rate of 0.49 kg/ha. This gives
an estimated total oral intake for the goose of 32.2 mg and 49.5 mg
for the two application rates assuming that the goose ate exclusively
food contaminated at this level. This is equivalent to a daily intake
of 10.7 and 16.5 mg/kg body weight, respectively. TERs can be
calculated as follows:
End-point LD50/LC50 Application rate Predicted TER
(mg/kg b.w.) (kg/ha) concentration
in food (mg/kg)
Bird acute 10 0.32 (arable) 35.8 0.93
oral (Canary) 0.49 (fruit) 55 0.61
The calculated TER values fall well below the EPPO/CoE trigger
values for concern (TER <10) and indicate a high risk to grazing
birds.
b) Insectivorous birds
Initial residues on small insects are estimated to be 9.3 mg/kg
dry weight (based on 29 × application rate) arising from application
at 0.32 kg/ha to arable crops (EPPO/CoE, 1993a,b) and 14.2 mg/kg from
application to fruit at a rate of 0.49 kg/ha. This gives an estimated
total oral intake for the blue tit of 0.08 mg and 0.12 mg for the two
application rates assuming that the blue tit ate exclusively food
contaminated at this level. This is equivalent to a daily intake of
6.94 and 10.6 mg/kg body weight, respectively. TERs can be calculated
as follows:
End-point LD50/LC50 Application rate Predicted TER
(mg/kg b.w.) (kg/ha) concentration
in food (mg/kg)
Bird acute 10 0.32 (arable) 9.3 1.44
oral (Canary) 0.49 (fruit) 14.2 0.94
The TERs for acute toxicity to insectivorous birds are
substantially less than the trigger value of <10, indicating high
acute risk to these birds.
10.2.2.2 Mammals
The lowest reported acute oral LD50 for laboratory mammals is
63 mg/kg body weight for the rat.
Indicator mammals for use in the risk assessment will be:
* Rabbit (Oryctolagus cuniculus), as a grazing mammal, with a
body weight of 1200 g and a total daily food consumption of 500 g
vegetation (dry weight) (Ross, personal communication to the
IPCS).
* Shrew (Sorex araneus), as an insectivorous mammal, with a body
weight of 18 g and a total daily food consumption of 18 g
(Churchfield, 1986)
a) Grazing mammals
Initial residues on short grass or cereal shoots are estimated to
be 35.8 mg/kg dry weight (based on 112 × application rate) arising
from application at 0.32 kg/ha to arable crops (EPPO/CoE, 1993a,b) and
at 55 mg/kg from application to fruit at a rate of 0.49 kg/ha. This
gives an estimated total oral intake for the rabbit of 17.9 mg and
27.4 mg for the two application rates, assuming that the rabbit ate
exclusively food contaminated at this level. This is equivalent to a
daily intake of 15 and 23 mg/kg body weight, respectively. TERs can be
calculated as follows:
End-point LD50 Application rate Predicted TER
(test (mg/kg b.w.) (kg/ha) concentration
species) in food (mg/kg)
Acute oral 63 0.32 (arable) 17.9 4.20
(Rat) 0.49 (fruit) 27.5 2.76
Since the TERs are < 10, this indicates a high risk to grazing
mammals.
b) Insectivorous mammals
Initial residues on large insects are estimated to be 0.86 mg/kg
dry weight (based on 2.7 × application rate) arising from application
at 0.32 kg/ha to arable crops (EPPO/CoE, 1993a,b) and 1.32 mg/kg from
application to fruit at a rate of 0.49 kg/ha. This gives an estimated
total oral intake for the shrew of 0.016 mg and 0.024 mg for the two
application rates, assuming that the shrew ate exclusively food
contaminated at this level. This is equivalent to a daily intake of
0.86 and 1.32 mg/kg body weight, respectively. TERs can be calculated
as follows:
End-point LD50 Application rate Predicted TER
(mg/kg b.w.) (kg/ha) concentration
in food (mg/kg)
Mammal 63 (Rat) 0.32 (arable) 0.86 72.9
acute oral 0.49 (fruit) 1.32 47.6
These TERs fall outside the trigger for high risk for
insectivorous mammals but within the range for medium risk.
10.2.2.3 Bees
The reported contact toxicity to bees gives an LD50 of
0.26 µg/bee. Using application rates at 0.32 and 0.49 kg/ha for
cereals and fruit respectively, hazard quotients are calculated to be
1231 and 1885 (application (g/ha)/LD50 (µg/bee)). The trigger for
concern is >50 (EPPO/CoE, 1993a,b) and, therefore, there is
substantial concern for exposed bees. The compound should not be
applied to flowering plants, and exposure of flying bees should be
avoided.
10.2.2.4 Earthworms
Earthworms are likely to be exposed to demeton-S-methyl. Based on
a soil depth of 5 cm and a soil density of 1.5 g/cm3, the soil PEC
would be 0.43 mg/kg assuming even distribution in the medium. The
reported LC50 for earthworms (Eisenia foetida) is 60 mg/kg
soil giving a TER of 140.6. As this is above the trigger value of
10 (EPPO/CoE, 1993a,b), the acute risk to earthworms is very low.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
Demeton-S-methyl causes acute cholinergic toxicity in humans and
laboratory animals. Carcinogenic, reproductive and developmental
effects have not been found in laboratory animals. Effects due to
chronic human exposure are unlikely to occur. Exposure of the general
population may only occur through food residues. However, the levels
of exposure are unlikely to cause adverse effects.
Given the high acute toxicity, as determined in a number of test
species and the known cases of human poisoning, it can be concluded
that the risk of occupational poisoning with demeton-S-methyl exists
when adequate protective measures and good practices are not adopted.
Therefore, handling and application of demeton-S-methyl should only be
performed by supervised and trained workers who must adhere to good
application practices and adopt adequate safety measures.
Demeton-S-methyl does not persist in the environment and is not
accumulated by organisms. It has high acute toxicity to aquatic
invertebrates and is toxic to fish and birds, leading to high or
moderate risk factors for these organisms. However, significant field
kills of organisms have not been reported for the compound.
Precautions should be taken to minimize exposure of non-target
organisms (e.g., do not spray over water bodies, minimize exposure by
spray drift).
In view of the negative results obtained in developmental and
carcinogenicity studies, it is felt that further clarification of the
genotoxic potential of demeton-S-methyl is not necessary.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Demeton-S-methyl was classified as highly hazardous (class Ib) by
WHO (1996).
Demeton-S-methyl was evaluated by the Joint FAO/WHO Meeting on
Pesticide Residues (JMPR) in 1972, 1973, 1979, 1982, 1984, 1989, 1992.
The 1989 Meeting (FAO/WHO, 1990) evaluated demeton-S-methyl,
oxydemeton-methyl and demeton-S-methylsulfone and set the following
NOAEL levels:
demeton-S-methyl: mouse: 1 mg/kg diet (equal to
0.24 mg/kg body weight per day)
rat: 1 mg/kg diet (equal to
0.05 mg/kg body weight per day)
dog: 1 mg/kg diet (equal to 0.036 mg/kg
body weight per day)
oxydemeton-methyl: mouse: 30 mg/kg diet (equal to
0.03 mg/kg body weight per day)
rat: 0.57 mg/kg diet (equal to
0.03 mg/kg body weight per day)
dog: 0.125 mg/kg body weight per day
demeton-S-methylsulfone: rat: 1 mg/litre in drinking water
(equal to 0.06 mg/kg body weight per
day)
dog: 10 mg/kg diet (equal to 0.36 mg/kg
body weight per day)
The estimated ADI for demeton-S-methyl was established at
0-0.0003 mg/kg body weight. This ADI applies to demeton-S-methyl
alone or in combination with oxydemeton-methyl and/or demeton-S-
methylsulfone because residues are determined after oxidation and are
expressed as demeton-S-methyl. The 1992 JMPR reviewed the residue data
on these three related compounds. Updated figures were available only
for oxydemeton-methyl, which is replacing demeton-S-methyl in most
registrations. The Meeting decided therefore to change the definition
of the residues to "the sum of demeton-S-methyl, oxydemeton-methyl and
demeton-S-methylsulfone expressed as oxydemeton-methyl". The MRLs
varied from 0.05 to 1 mg/kg commodity and have been obtained from
supervised trials on oxydemeton-methyl rather than on
demeton-S-methyl.
Demeton-S-methyl has not been evaluated by the International
Agency for Research on Cancer (IARC).
REFERENCES
ACGIH (1993) 1993-1994 Threshold limit values for chemical substances
and physical agents. Cincinnati, Ohio, American Conference of
Governmental Industrial Hygienist.
Akarte SR, Rao KR, & Kulkarni DA (1986) Acute toxicity of Metasystox
to an estuarine lamellibranch mollusc, Paphia laterisulca, from
Ratnagiri coast, India. Int J Ecol Environ Sci, 12: 125-129.
Anderson JPE (1989) Influence of the commercial product Metasystox (i)
on soil respiration after amendment with glucose. Leverkusen, Germany,
Bayer AG (Unpublished report No. AJO/78289).
Anonymous (1977) Determination of residues of organophosphorus
pesticides in fruits and vegetables. Analyst, 102: 858-868.
Barr AM (1966) Further experience in the treatment of severe organic
phosphate poisoning. Med J. Aust, 1: 490-492.
Bathe R (1983) 12-month oral (feeding) toxicity study with E 154
in Beagle dogs. Leverkusen, Germany, Bayer AG (Unpublished report
No. 2542).
Bayer (1970) Testing the effects of Metasystox i and Metasystox R on
bees. Leverkusen, Germany, Bayer AG (Unpublished report).
Becker H (1983) Embryotoxicity and teratogenicity study on E 154 in
rabbits. Leverkusen, Germany, Bayer AG (Unpublished report No. 2422).
Blumenstock I (1989) Influence of the commercial product Metasystox
(I) on nitrification in soils. Leverkusen, Germany, Bayer AG
(Unpublished report No. BSI/78189).
Carrington da Costa RB, Maul ER, Pimentel J, Goncalves JS, Rebelo A,
Oliveira LC, & Rebelo ASC (1982) A case of acute poisoning by methyl
demeton in a female 5 months pregnant. Arch Toxicol, 5(Suppl):
202-204.
Churchfield S (1986) Shrews. Oswestry, A. Nelson (The Mammal Society
series).
Cifone MA (1984) Mutagenicity evaluation of E154 (C.N. demeton-S-
methyl) in the mouse lymphoma forward mutation assay. Leverkusen,
Germany, Bayer AG (Unpublished report No. 2789).
DuBois KP & Doull J (1955) The acute toxicity of P=O Meta-systox, P=O
Metasystox sulphoxide and P=O Meta-systox sulphone to mammals.
Leverkusen, Germany, Bayer AG (Unpublished report).
DuBois KP & Plzak G (1962) The acute toxicity and anticholinesterase
action of 0,0-dimethyl-S-ethyl-2-sulphinylethylphosphorothioate
(Meta-systox R) and related compounds. Toxicol Appl Pharmacol,
4: 621-630.
Dzwonkowska A & Hübner H (1986) Induction of chromosomal aberration in
the Syrian hamster by insecticides tested in vivo. Arch Toxicol,
58: 152-156.
Ecker W (1978) Biotransformation of (ethylene-1-14C) demeton-S-methyl
in rats. Leverkusen, Germany, Bayer AG (Unpublished report No. 7458).
Ecker W & Cölln R (1983) Biotransformation of demeton-S-methyl in
rats. Leverkusen, Germany, Bayer AG (Unpublished report No. 11431).
Edson EF (1960) Agriculture chemicals - Applied toxicology of
pesticides. Pharm J, 185: 361-367.
Eiben R, Gröning P, & Löser E (1984) Generation study with rats
(two generations). Leverkusen, Germany, Bayer AG (Unpublished report
No. 13135).
EPPO/CoE (European & Mediterranean Plant Protection Organisation/
Council of Europe) (1993a) Decision making schemes for the
environmental risk assessment of plant protection products.
OEPP/EPPO Bull, 23: 1-54.
EPPO/CoE (European & Mediterranean Plant Protection Organisation/
Council of Europe) (1993b) Decision making schemes for the
environmental risk assessment of plant protection products.
OEPP/EPPO Bull, 24: 1-87.
FAO/WHO (1974) Demeton-S-methyl and related compounds. In: 1973
Evaluations of some pesticides in food. Geneva, World Health
Organization, pp 195-240 (WHO Pesticide Residue Series No. 3).
FAO/WHO (1990) Demeton-S-methyl and related compounds. In: Pesticides
residues in food - 1989. Evaluations: Part II - Toxicology. Rome, Food
and Agriculture Organization of the United Nations, pp 49-74 (FAO
Plant Production and Protection Paper 100/2).
FAO/WHO (1993) Demeton-S-methyl. In: Pesticides residues in food -
1992. Evaluations: Part I - Residues. Rome, Food and Agriculture
Organization of the United Nations, p 287 (FAO Plant Production and
Protection Paper 118).
Faul J (1984) [E 154 = 25/154 (demeton-S-methyl; Metasystox i-active
ingredient] Leverkusen, Germany, Bayer AG (Unpublished report)
(in German).
Flucke W (1984) [Tests on acute oral toxicity to quail (Coturnix
coturnix japonica).] Leverkusen, Germany, Bayer AG (Unpublished
report No. 13119) (in German).
Flucke W & Eben A (1988) E154 50 VL (C.N. demeton-S-methyl): Study of
effect on neuropathy target esterase (NTE) after oral administration
to the hen. Leverkusen, Germany, Bayer AG (Unpublished report
No. 17264).
Flucke W & Kaliner G (1988) E 154 50 VL (C.N. demeton-S-methyl): Study
for acute delayed neurotoxicity after oral administration to the hen.
Leverkusen, Germany, Bayer AG (Unpublished report No. 16920).
Flucke W & Kimmerle G (1977) Trichlorfon (Dipterex active ingredient),
demeton-S-methyl (Metasystox (I) active ingredient): Study for acute
toxicity after simultaneous administration of both active ingredients.
Leverkusen, Germany, Bayer AG (Unpublished report No. 7066).
Flucke W & Pauluhn J (1983) Metaisosystox 50 EC: Study for
formulation toxicity. Leverkusen, Germany, Bayer AG (Unpublished
report No. 11770).
Froebe Z, Drevenkar V, Stengl B, & Juracic M (1989) Sorption behaviour
of some organophosphorus pesticides in natural sediments. Toxicol
Environ Chem, 19: 69-82.
Funk W, Cleres L, Pitzer H, & Donnevert G (1989) Organophosphorus
insecticides: Quantitative HPTLC determination and characterization.
J Planar Chromatogr, 2: 285-289.
Ganzelmeier IH, Rautmann D, Spangenberg R, Streloke M, Herrmann M,
Wenzelburger HJ, & Walter HF (1995) Studies on the spray drift of
plant protection products: Results of a test program carried out
throughout the Federal Republic of Germany. Oxford, London, Edinburgh,
Melbourne, Blackwell Scientific Publications.
Grau R (1984) [Oral toxicity to female bird/canary (Serinus
canarius).] Leverkusen, Germany, Bayer AG (Unpublished report
No. VK-243) (in German).
Grau R (1985a) Fish toxicity (Metaisosystox 50% pre-solution).
Leverkusen, Germany, Bayer AG (Unpublished report No. FF-177).
Grau R (1985b) Fish toxicity (Metaisosystox 50% pre-solution, golden
orfe). Leverkusen, Germany, Bayer AG (Unpublished report No. FO-818).
Grau R (1990a) [Acute toxicity of Metaisosystox EC 25 to rainbow trout
(Oncorhynchus mykiss) using flow test.] Leverkusen, Germany, Bayer
AG (Unpublished report No. FF-285) (in German).
Grau R (1990b) [Acute toxicity of Metasystox EC 25 to golden orfe
(Leuciscus idus melanotus) using flow test.] Leverkusen, Germany,
Bayer AG (Unpublished report No. FO-1222) (in German).
Grau R (1990c) [Toxicity of Metasystox VL 70 to rainbow trout
(O ncorhynchus mykiss) with extensive exposure (21 days).]
Leverkusen, Germany, Bayer AG (Unpublished report No. FF-287)
(in German).
Hanna PJ & Dyer KF (1975) Mutagenicity of organophosphorus compounds
in bacteria and Drosophila. Mutat Res, 28: 405-420.
Heath DF & Vandekar M (1957) Some spontaneous reactions of
O,O-dimethyl S-ethylthioethyl phosphorothioate and related compounds
in water and on storage, and their effects on the toxicological
properties of the compounds. Biochem J, 67: 187-201.
Hecht G (1955) [Comparison tests of preparation 25/154, R 2170
and M 3/158.] Leverkusen, Germany, Bayer AG (Unpublished report)
(in German).
Hecht G (1960) [Toxicological investigations on Metasystox (i)
presentation given in Tokyo, Korinkaku.] Leverkusen, Germany, Bayer AG
(unpublished report) (in German).
Hegazy MR (1965) Poisoning by meta-isosystox in spraymen and in
accidentally exposed patients. Br J Ind Med, 22: 230-235.
Heimann KG (1987a) E 154 techn. (C.N. demeton-S-methyl): Study for
skin-sensitizing effect on guinea pigs. Leverkusen, Germany, Bayer AG
(Unpublished report No. 15896).
Heimann G (1987b) E 154 techn. (C.N. demeton-S-methyl): Study for
skin-sensitizing effect on guinea pigs in the epicutaneous test.
Leverkusen, Germany, Bayer AG (unpublished report No. 16106).
Heimbach F (1985a) Growth inhibition of green algae (Scenedesmus
subspicatus) caused by demeton-S-methyl (techn.). Leverkusen,
Germany, Bayer AG (Unpublished report No. HBF/AL11).
Heimbach F (1985b) Acute toxicity of demeton-S-methyl (techn.) to
water fleas. Leverkusen, Germany, Bayer AG (Unpublished report
No. HBF/DM 47).
Heimbach F (1985c) [Acute toxicity of demeton-S-methyl 50 VL to
water fleas.] Leverkusen, Germany, Bayer AG (Unpublished report
No. HBF/DM 48) (in German).
Heimbach F (1990a) Toxicity of Metasystox (I) to earthworms.
Leverkusen, Germany, Bayer AG (Unpublished report No. HBF/RG 120).
Heimbach F (1990b) Growth inhibition of green algae (Scenedesmus
subspicatus) by Metasystox (I). Leverkusen, Germany, Bayer AG
(Unpublished report No. HBF/AL 74).
Heimbach F (1990c) Influence of Metasystox (I) on the reproduction
rate of water fleas. Leverkusen, Germany, Bayer AG (Unpublished
report No. HBF/RDM 25).
Heimbach F (1990d) Influence of demeton-S-methyl VL 70 on the
reproduction rate of water fleas. Leverkusen, Germany, Bayer AG
(Unpublished report No. HBF/RDM 27).
Hellpointner E (1990) Assessment of the environmental half-life of
the direct photodegradation of demeton-S-methyl in water. Leverkusen,
Germany, Bayer AG (Unpublished report No. PF3446).
Herbold B (1979) E 25/154 Salmonella/microsome test to evaluate for
point mutation. Leverkusen, Germany, Bayer AG (Unpublished report
No. 8393).
Herbold B (1980a) E 25/154 Salmonella/microsome test to evaluate for
point mutation. Leverkusen, Germany, Bayer AG (Unpublished report
No. 8809).
Herbold B (1980b) E 25/154 micronucleus test on the mouse to evaluate
for mutagenic effect. Leverkusen, Germany, Bayer AG (Unpublished
report No. 8932).
Herbold B (1980c) E 25/154 (demeton-S-methyl) dominant lethal study on
male mouse to test for mutagenic effects. Leverkusen, Germany, Bayer
AG (Unpublished report No. 9336).
Herbold B (1983a) E 154 (demeton-S-methyl) pol test on E. coli to
evaluate for DNA damage. Leverkusen, Germany, Bayer AG (Unpublished
report No. 12152).
Herbold B (1983b) E 154 (demeton-S-methyl) sister chromatid exchange
in the bone marrow of the Chinese hamster in vivo to evaluate for
harmful effect on DNA. Leverkusen, Germany, Bayer AG (Unpublished
report No. 12323).
Hermann G (1974a) [Fish toxicity of Metasystox (I) to goldfish.]
Leverkusen, Germany, Bayer AG (Unpublished report No. FG-112)
(in German).
Hermann G (1974b) [Fish toxicity of Metasystox (I) to carp.]
Leverkusen, Germany, Bayer AG (Unpublished report No. FK-28)
(in German).
Hermann G (1974c) [Fish toxicity of Metasystox (I) to rudd.]
Leverkusen, Germany, Bayer AG (Unpublished report No. FR-29)
(in German).
Hill ARC, Wilkins JPG, Findlay NRI, & Lontay KEM (1984)
Organophosphorus sulphides, sulphoxides and sulphones - Part I:
Determination of residues in fruit and vegetables by gas-liquid
chromatography. Analyst, 109: 483-487.
Hoorn AJW (1982) Mutagenicity evaluation of E 154 50 VL (in xylene)
in the reverse mutation induction assay with Saccharomyces
cerevisiae strains S138 and S211a. Leverkusen, Germany, Bayer AG
(Unpublished report No. 2372).
Jones RD (1982) Organophosphorus poisoning at a chemical packaging
company. Br J Ind Med, 39: 377-381.
Kenaga E (1973) Factors to be considered in the evaluation of the
toxicity of pesticides to birds in the environment. Environ Qual Saf,
2: 166-181.
Kimmerle G (1972) Acute toxicity of SRA 3886 in combination with S 276
and with E 154 to rats. Leverkusen, Germany, Bayer AG (Unpublished
report No. 3438).
Klimmer OR (1964) [Determination of acute oral toxicity of Metasystox
(i).] Leverkusen, Germany, Bayer AG (Unpublished report) (in German).
Klimmer OR & Pfaff W (1955) [Toxicity tests on the new contact
insecticide O,O-dimethyl-thiophosphoric acid-O/ -S-ethylester
("Metasystox").] Arzneimittel-Forschung, 5: 584-587 (in German).
Klimmer R & Pfaff W (1958) [Toxicity tests during application of the
systemic insecticide O,O-dimethyl-(ethyl-thioethyl)-thiophosphoric
acid-ester.] Arzneimittel-Forschung, 8: 265-269 (in German).
Kniehase U (1984) [Effects of Metasystox (I) 250 EC on mite
(Phytoseiulus persimilis).] Leverkusen, Germany, Bayer AG
(Unpublished report No. ZDE/PP 4/84) (in German).
Krohn J (1984) Fate/behaviour of crop protection products in water:
demeton-S-methyl. Leverkusen, Germany, Bayer AG (Unpublished report
No. M2421).
Lewis PJ, Lowing RK, & Gomopertz D (1981) Automated discrete kinetic
method for erythrocyte acetylcholinesterase and plasma cholinesterase.
Clin Chem, 27: 926-929.
Mary ASR, Nagabhushanam R, & Sarojini R (1986) Toxicity evaluation of
organophosphorus and chlorinated hydrocarbon pesticides in freshwater
prawn Macrobrachium lamerrii. J Environ Biol, 7: 189-195.
Mitra PK, Sud SC, & Bahga HS (1978) Acute oral toxicity of Metasystox
in buffalo calves. Indian J Exp Biol, 16: 813-815.
Muley DV, Akarte SR, Kulkarni DA, & Rao KR (1987) Acute toxicity of
Metasystox to wedge clam, Donax cuneatus, from West Coast. Fish
Technol, 24: 27-30.
Owen M (1975) The management of grass swards for captive wildfowl. Int
Zoo Year Book, 16: 135-138.
Pandita TK (1983) Mutagenic studies on the insecticide Metasystox-R
with different genetic systems. Mutat Res, 124: 97-102.
Renhof M (1985) E 154 (C.N. demeton-S-methyl): Study for embryotoxic
effect on rats after oral administration. Leverkusen, Germany, Bayer
AG (Unpublished report No. 13237).
Schludecker D & Aderjan R (1988) [Chemical-toxicological results of a
suicidal poisoning with demeton-S-methyl.] Z Rechtsmed, 101:55-60
(in German).
Schmid E (1984) Degradation of demeton-S-methyl in soil. Leverkusen,
Germany, Bayer AG (Unpublished report No. Im1428).
Schmid ER (1986) Degradation of demeton-S-methyl in soil at a
concentration of 3 ppm under anaerobic conditions. Leverkusen,
Germany, Bayer AG (Unpublished report No. IM1579).
Schmidt WM & Bomhard E (1988) Study for carcinogenicity in NMRI mice
(administration in diet for up to 21 months). Leverkusen, Germany,
Bayer AG (Unpublished report No. 16812).
Schmidt WM & Westen H (1988) Study for chronic toxicity and
carcinogenicity in Wistar rats (administration in diet for up to 2
years). Leverkusen, Germany, Bayer AG (Unpublished report No. 16888).
Shires SW (1985) Effects of aerial applications of cypermethrin and
demeton-S-methyl on non-target arthropods of wheat. Ecotoxicol Environ
Saf, 10: 1-11.
Stone HM (1961) The anticholinesterase activity of demeton-S-methyl.
NZ J Sci, 4: 78-90.
Su MQ, Kinoshita FK, Frawley JP, & DuBois KP (1971) Comparative
inhibition of aliesterases and cholinesterase in rats fed eighteen
organophosphorus insecticides. Toxicol Appl Pharmacol, 20: 241-249.
Tarrant KA, Thompson HM, & Hardy AR (1992) Biochemical and
histological effect of the aphicide demeton-S-methyl on house sparrows
(Passer domesticus) under field conditions. Bull Environ Contam
Toxicol, 48: 360-366.
Thompson HM, Walker CH, & Hardy AR (1991) Changes in activity of avian
serum esterases following exposure to organophosphorus insecticides.
Arch Environ Contam Toxicol, 20: 514-518.
Thornton JS, Olson TJ, & Wagner K (1977) Determination of residues
of Metasystox-R and metabolite in plant and animal tissues and soil.
J Agric Food Chem, 25: 573-576.
Thyssen J (1981) [Tests on skin and mucous-irritating effects of
demeton-S-methyl.] Leverkusen, Germany, Bayer AG (Unpublished report)
(in German).
Vandekar M (1958) The toxic properties of demeton-methyl
("Metasystox") and some related compounds. Brit J Ind Med,
15: 158-167.
Vasilic Z, Drevenkar V, Froebe Z, Stengl B, & Tkalcevic B (1987) The
metabolites of organophosphorus pesticides in urine as an indicator of
occupational exposure. Toxicol Environ Chem, 14:11-127.
Verma SP, Tonk IP, Gupta AK, & Saxena M (1984) Evaluation of an
application factor for determining the safe concentration of
agricultural and industrial chemicals. Water Res, 18(1): 111-115.
Wagner K & Oehlmann L (1987) Studies on the metabolic behaviour of
demeton-S-methyl in wheat. Leverkusen, Germany, Bayer AG (Unpublished
report No. PF2701).
Wagner K & Thornton JS (1977) Method for the gas-chromatographic
determination of Metasystox (i) and Metasystox R residues in plants,
soil and water. Pflanzenschutz Nachr Bayer, 30: 1-17.
Wagner K, Schütz U, & Oehlmann L (1985) Degradation of Metasystox-(i)
in soil under aerobic, anaerobic and sterile test conditions.
Leverkusen, Germany, Bayer AG (Unpublished report No. PF2565).
Weber H, Patzschke K, & Wagner LA (1978) (14C), demeton-S-methyl
(Metasystox in active ingredient)/Biokinetic studies on rats.
Leverkusen, Germany, Bayer AG (Unpublished report No. 7491).
Westlake GE, Hardy AR, & Stevenson JH (1985) Effects of storage and
pesticide treatments on honey bee brain acetyl cholinesterase
activities. Bull Environ Contam Toxicol, 34: 668-675.
WHO (1996) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1996-1997. Geneva, World Health
Organization, International Programme on Chemical Safety
(WHO/PCS/96.3).
Wilkins JPG, Hill ARC, & Lee DF (1985) Organophosphorus sulphides,
sulphoxide and sulphones - Part 2: Characterisation by gas
chromatography-mass spectrometry. Analyst, 110: 1045-1051.
Wilmes R (1984) Orientating light stability: demeton-S-methyl.
Leverkusen, Germany, Bayer AG (Unpublished report No. IM1408).
Winkler KO & Arent H (1970) Medical observation of personnel occupied
with spraying in hop cultivation during the application of the organic
phosphoric acid esters mevinphos and demeton-S-methyl (Metasystox i).
Leverkusen, Germany, Bayer AG (Unpublished report No. 24618).
Xue SZ, Ding XJ, & Ding Y (1985) Clinical observation and comparison
of the effectiveness of several oxime cholinesterase reactivators.
Scand J Work Environ Health, 11(Suppl 4): 46-48.
Ziegler W, Engelhardt G, Wallnöfer PR, Oehlmann L, & Wagner K (1980)
Degradation of demeton-S-methyl sulfoxide (Metasystox R) by soil
microorganisms. J Agric Food Chem, 28: 1102-1106.
RÉSUMÉ ET ÉVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Identité, propriétés physiques et chimiques
Le déméton-S-méthyl se présente sous la forme d'un liquide
huileux jaune pâle à l'odeur pénétrante. C'est un organophosphoré
agissant par la voie générale et par contact qui est utilisé comme
insecticide et acaricide sur les fruits, les légumes, les céréales et
les plantes ornementales pour lutter contre Acarina, Thysanoptera,
Hymenoptera et Homoptera. Sa tension de vapeur est de 63,8 mPa à
20°C et il est facilement soluble dans la plupart des solvants
organiques. Il est très soluble dans l'eau (3,3 g/litre à la
température ambiante) et son coefficient de partage entre l'octanol et
l'eau (log Pow) est de 1,32. Le déméton-S-méthyl est stable dans les
solvants non aqueux.
Pour la recherche et le dosage des résidus et l'analyse des
échantillons prélevés dans l'environnement, on procède à une
extraction au moyen d'un solvant organique, suivie d'une oxydation de
la sulfone correspondante. Le dosage s'effectue ensuite par
chromatographie en phase gazeuse avec un détecteur spécifique du
phosphore.
1.2 Sources d'exposition humaine et environnementale
Jusqu'en 1957, le déméton-méthyl a été commercialisé sous la
forme d'un mélange de deux isomères, le déméton-S-méthyl et le
déméton-O-méthyl. La formulation commerciale est un concentré
émulsionnable que l'on utilise en pulvérisations sur les céréales, les
fruits, les légumes et les plantes ornementales. On est en train de le
remplacer par l'oxydéméton-méthyl qui est un métabolite produit par
les plantes et les mammifères ou qui résulte du séjour du
déméton-S-méthyl dans le sol.
1.3 Transport, distribution et transformation dans l'environnement
La décomposition par hydrolyse du déméton-S-méthyl dépend du pH
de la solution. A 22°C, sa demi-vie est de 56 jours à pH 7 et de 8
jours à pH 9. Dans le sol, sa voie de décomposition principale est la
biodégradation, avec une demi-vie d'environ 4 h. Au bout de 24 h, il y
a encore dans le sol une proportion d'oxydéméton-méthyl qui représente
20 à 30% de la dose initiale de déméton-S-méthyl. Le coefficient de
sorption dans le sol (Kd) du déméton-S-méthyl est de 0,68 à 2,66,
selon la composition du sol.
Dans l'environnement, l'une des principales voies de dégradation
du composé est la photolyse. La métabolisation par le blé de printemps
est rapide et elle analogue à celle qui se produit dans le sol et chez
les mammifères.
1.4 Niveaux d'exposition environnementale et humaine
La population générale est principalement exposée au
déméton-S-méthyl par l'intermédiaire des résidus qui subsistent sur
les cultures vivrières. La réunion conjointe FAO/OMS sur les résidus
de pesticides (JMPR) a recommandé une dose journalière admissible
(DJA) de 0,0003 mg/kg de poids corporel. Il s'agit d'une DJA
collective qui englobe le déméton-S-méthyl, l'oxydéméton-méthyl et la
déméton-S-méthylsulfone, étant donné que les méthodes habituelles
d'analyse ne distinguent pas ces différents composés. Il est arrivé
qu'une exposition excessive au déméton-S-méthyl par la voie
transcutanée entraîne une intoxication de type cholinergique chez des
ouvriers mal protégés qui procédaient au conditionnement du concentré.
En revanche, des volontaires qui s'étaient prêtés à l'épandage
simulé d'un mélange de déméton-S-méthyl et de déméton-O-méthyl (30 et
70% respectivement) et avaient été exposés à des concentrations de 8,8
à 27 mg/m3 des deux matières actives, n'ont présenté aucune réduction
de leur activité cholinestérasique plasmatique ou érythrocytaire.
1.5 Cinétique et métabolisme
Chez le rat, le déméton-S-méthyl est rapidement et presque
complètement résorbé au niveau de l'intestin et il se répartit
uniformément dans les tissus (exception faite des hématies dans
lesquelles il se concentre plus fortement). Il est rapidement
métabolisé et excrété dans les urines. Sa concentration sanguine
diminue avec une demi-vie qui est initialement de 2 h environ. Au
bout de 24 h, il reste environ 1% de la dose dans l'organisme. La
principale voie métabolique du déméton-S-méthyl consiste en une
oxydation de la chaîne latérale qui conduit à la formation du
sulfoxyde correspondant (oxydéméton-méthyl) et, dans une moindre
proportion, après une oxydation plus poussée, à la sulfone.
L'O-déméthylation constitue une autre voie métabolique importante.
1.6 Effets sur les animaux de laboratoire et les systèmes d'épreuve
in vitro
1.6.1 Exposition unique
Le déméton-S-méthyl provoque des intoxications de type
cholinergique. Chez les mammifères, la DL50 varie de 7 à 100 mg/kg
p.c. selon la voie d'administration et l'espèce.
1.6.2 Exposition de courte durée
Une des premières études, comportant une exposition par la voie
alimentaire, a montré que des rats qui recevaient le composé à raison
de 50 mg/kg de nourriture, avaient une activité cholonestérasique
cérébrale sensiblement réduite au bout de 26 semaines de ce régime.
Des signes d'intoxication de type cholinergique ont été observés au
bout de 5 semaines de traitement chez des rats recevant 200 mg de
composé par kg de nourriture.
Lors d'une étude d'alimentation d'un an effectuée sur des chiens,
on a obtenu une dose sans effet nocif observable (NOAEL), basée sur
les effets cholinergiques, de 1 mg/kg de nourriture, soit l'équivalent
quotidien de 0,036 mg/kg de poids corporel.
1.6.3 Exposition de longue durée
Des souris ont reçu pendant 21 mois des rations contenant
respectivement 0, 1, 15 ou 75 mg de déméton-S-méthyl par kg de
nourriture. La NOAEL, a été trouvée égale à 1 mg/kg de nourriture,
soit l'équivalent quotidien de 0,24 mg/kg de poids corporel en prenant
comme base l'inhibition de la cholinesterase cérébrale.
Chez des rats ayant reçu une alimentation contenant
respectivement 0, 1, 7 ou 50 mg/kg de déméton-S-méthyl, la NOAEL,
basée sur l'inhibition de la cholinestérase cérébrale, s'est révélée
égale à 1 mg/kg de nourriture, soit l'équivalent quotidien de
0,05 mg/kg p.c.
Chez aucune espèce il n'a été constaté d'augmentation de
l'incidence des tumeurs.
1.6.4 Irritation et sensibilisation cutanée ou oculaire
Le déméton-S-méthyl est légèrement irritant pour l'oeil et la
peau. Le test de Magnusson et Klingman a donné des résultats positifs
chez des cobayes. En revanche, le test de sensibilisation de Buehler
(application d'un timbre cutané) n'a pas fourni d'indice d'une
sensibilisation cutanée, ce qui donne à penser que, dans la pratique,
l'usage du déméton-S-méthyl ne devrait pas poser de problème de
sensibilisation.
1.6.5 Effets sur la reproduction, embryotoxicité et tératogénicité
Lors d'une étude sur deux générations de rats, au cours de
laquelle les animaux ont été exposés au composé par la voie
alimentaire, on a constaté une réduction de la viabilité et du poids
corporel des ratons (uniquement la génération F1b) à la dose de
5 mg/kg de nourriture. La NOAEL était de 1 mg/kg de nourriture, soit
l'équivalent quotidien de 0,07 mg/kg de poids corporel.
Le déméton-S-méthyl ne s'est révélé ni embryotoxique, ni
tératogène chez le rat et le lapin.
1.6.6 Mutagénicité et points apparentés d'aboutissement des effets
toxiques
Le déméton-S-méthyl produit des mutations ponctuelles
in vitro. On n' a mis en évidence des effets chromosomiques
in vivo qu'avec des formulations du commerce. Les données
disponibles sont insuffisantes pour que l'on puisse procéder à une
évaluation satisfaisante du pouvoir génotoxique de ce composé.
1.6.7 Neurotoxicité retardée
Le déméton-S-méthyl n'a produit ni polyneuropathie, ni inhibition
de l'estérase cible correspondante (NTE) lorsqu'on l'a administré à
des poules en dose égale à la DL50 par voie orale.
1.6.8 Toxicité des métabolites
Deux métabolites du déméton-S-méthyl produits par des
plantes et des mammifères, à savoir l'oxydéméton-méthyl et la
déméton-S-méthylsulfone, sont également des pesticides du commerce et
ont été très largement étudiés. Il ressort de ces travaux que le
profil toxicologique de ces composés ne diffère pas sensiblement,
quantitativement ou qualitativement, de celui du déméton-S-méthyl.
1.7 Mécanisme de la toxicité - Mode d'action
Le déméton-S-méthyl est un inhibiteur direct de la cholinestérase
et sa toxicité est liée au fait qu'il inhibe l'acétylcholinestérase
(AChE) au niveau des terminaisons nerveuses. Une fois inhibée par le
déméton-S-méthyl, l'acétylcholinestérase se réactive spontanément avec
une demi-vie in vitro de 1,3 h environ, comme on peut s'y attendre
pour une AChE diméthylphosphorylée.
1.8 Effets sur l'homme
On a signalé quelques cas d'intoxication aiguë avec syndrome
cholinergique, consécutifs à des tentatives de suicide. Aucun effet
retardé n'a été observé chez les survivants, dont une femme enceinte.
A la suite d'une exposition subie par négligence lors du
conditionnement de formulations du commerce, quelques employés ont
présenté des troubles d'origine cholinergique qui ont nécessité un
traitement pharmacologique. Le composé avait probablement pénétré par
la voie transcutanée. De même, il est possible que des conditions de
travail laissant à désirer aient entraîné une absorption excessive de
déméton-S-méthyl au cours de l'épandage de ce composé sur des champs
de coton.
1.9 Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Pour les algues vertes, la CE50 à 96 h va de 8 à 37 mg/litre. On
a mesuré une CL50 allant de 0,004 à 1,3 mg/litre chez une série
d'invertébrés aquatiques. Chez les poisson, la toxicité est variable,
avec une CL50 à 96 h qui peut aller de 0,59 mg/litre pour la truite
arcen-ciel à environ 40 mg/litre pour l'orfe, le cyprin doré et la
carpe.
Chez la caille japonaise et le canari, on a obtenu une DL50
(effet aigu, voie orale) de 10-50 mg/kg de poids corporel. Chez des
étourneaux, une dose unique par voie orale de 2 mg/kg p.c. a provoqué
une inhibition de l'acétylcholinestérase cérébrale 3 h après le
traitement.
Pour les lombrics terricoles, la CL50 du déméton-S-méthyl est de
66 mg/kg sur 14 jours. Pour les abeilles, on a obtenu des valeurs de
la DL50 de contact et par voie orale (effet aigu) respectivement
égales à 0,21 et 0,6 par insecte. En traitant du blé d'hiver à la dose
recommandée, on a constaté une réduction sensible du nombre
d'invertébrés présents sur les feuilles (principalement des diptères
du genre Empididae) sans effet sur les invertébrés entomophages
présents à la surface du sol.
2. Conclusions
Le déméton-S-méthyl est un insecticide organophosphoré fortement
toxique (classe Ib de la classification de l'OMS) (OMS, 1966). Son
action toxique s'explique par l'inhibition de l'acétylcholinestérase
au niveau des terminaisons nerveuses. L'exposition de la population
générale résulte principalement des résidus présents dans les denrées
alimentaires provenant des cultures traitées.
Moyennant de bonnes pratiques agricoles et le respect des règles
d'hygiène et de sécurité, la manipulation du composé lors de sa
fabrication ou de son épandage ne devrait entraîner aucun effet
indésirable. Des effets résultant d'une exposition chronique sont
improbables.
Le déméton-S-méthyl ne persiste pas dans l'environnement et il ne
s'accumule pas chez les êtres vivants. Il est très toxique pour les
invertébrés aquatiques et présente une toxicité notable pour les
poissons et les oiseaux, aussi doit on considérer que pour ces
organismes, le facteur de risque est élevé ou modéré. Néanmoins, on
n'a pas signalé de mortalité importante dans la nature du fait de ce
composé. Des précautions sont à prendre pour réduire au minimum
l'exposition des organismes non visés (par ex. ne pas le pulvériser
sur des étendues d'eau et veiller à ce que les gouttelettes
d'insecticide ne soient pas entraînées vers les zones à respecter).
3. Recommandations
Afin de protéger la santé et le bien-être des travailleurs et de
la population dans son ensemble, il ne faut confier la manipulation et
l'épandage du déméton-S-méthyl qu'à des opérateurs dûment formés et
encadrés, qui auront le souci de respecter les mesures de sécurité et
d'épandre le produit selon les règles.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
1.1 Identidad, propiedades físicas y químicas, y métodos analíticos
El demeton-S-metilo, un líquido oleoso de color amarillo pálido y
olor penetrante, es un insecticida y acaricida organofosfatado
sistémico y de contacto que se utiliza para combatir Acarina,
Thysanoptera y Homóptera en frutas, cereales, plantas ornamentales
y hortalizas. Tiene una presión de vapor de 63,8 mPa a 20°C, es
fácilmente soluble en la mayoría de los disolventes orgánicos, tiene
una gran solubilidad en agua, de 3,3 g/litro a temperatura ambiente, y
un coeficiente de reparto octanol/agua (log Pow) de 1,32. El
demeton-S-metilo es estable en disolventes no acuosos.
Los análisis de residuos y ambientales se realizan por extracción
con un disolvente orgánico y oxidación a la sulfona correspondiente.
Las mediciones se efectúan por cromatografía de gases utilizando un
detector específico del fósforo.
1.2 Fuentes de exposición humana y ambiental
Antes de 1957, el metildemeton se comercializaba como mezcla de
los isómeros demeton-S-metilo y demeton-O-metilo.
El demeton-S-metilo se utiliza desde 1957. Su presentación es la
de un concentrado emulsionable y se aplica por rociamiento a los
cereales, frutas, plantas ornamentales y hortalizas. Se está
sustituyendo por el oxidemetonmetilo, que es un metabolito del
demeton-S-metilo producido en las plantas, el suelo y los mamíferos.
1.3 Transporte, distribución y transformación en el medio ambiente
La degradación hidrolítica del demeton-S-metilo depende del pH de
la solución; a 22°C la semivida es de 63 días con un pH de 4, de 56
días con un pH de 7 y de 8 días con un pH de 9. La principal vía de
degradación en el suelo es la biodegradación. La semivida del
demeton-S-metilo en el suelo es de unas 4 horas. Sin embargo, al cabo
de 24 horas, el oxidemetonmetilo representa aún el 20-30 % de la dosis
aplicada de demeton-S-metilo. El coeficiente de sorción (Kd) del
demeton-S-metilo en el suelo oscila entre 0,68 y 2,66, dependiendo de
la composición del suelo.
La fotolisis no es uno de los principales mecanismos de
degradación del demeton-S-metilo en el medio ambiente.
Su metabolismo en el trigo de primavera es rápido y similar al
que se produce en el suelo y en los mamíferos.
1.4 Niveles ambientales y exposición humana
La exposición primaria de la población humana en general se
produce a través de los residuos de demeton-S-metilo en los cultivos
alimentarios. En la reunión conjunta FAO/OMS sobre residuos de
plaguicidas (JMPR) se recomendó una ingesta diaria admisible (IDA) de
0,0003 mg/kg de peso corporal. Se trata de una IDA colectiva para el
demeton-S-metilo, el oxidemetonmetilo y el demeton-S-metilsulfona, ya
que los métodos habituales de análisis no diferencian entre estos tres
compuestos.
La exposición excesiva al demeton-S-metilo y su absorción cutánea
produjeron toxicidad colinérgica en trabajadores insuficientemente
protegidos durante el envasado de la formulación concentrada.
Tras haber expuesto a voluntarios participantes en una simulación
de rociamiento con una mezcla de demeton-S-metilo y demeton-O-metilo
(30 y 70% respectivamente) a 8,8-27 mg/m3 de ambos componentes
activos combinados, no se observaron efectos adversos en la actividad
de la colinesterasa en el plasma ni en los eritrocitos.
1.5 Cinética y metabolismo
El demeton-S-metilo se absorbe rápida y casi completamente por el
tracto intestinal en las ratas y se distribuye uniformemente a los
tejidos (excepto una concentración elevada en los eritrocitos). Se
metaboliza rápidamente y se excreta por orina. La concentración en la
sangre disminuye, con una semivida inicial de unas 2 horas.
Aproximadamente el 1% de la dosis oral sigue presente en el organismo
24 horas después del tratamiento. La principal ruta metabólica del
demeton-S-metilo en las ratas es la oxidación de la cadena lateral,
que da lugar a la formación del sulfóxido correspondiente
(oxidemetonmetilo) y, en menor medida, a la sulfona, tras una
oxidación mayor. Otra ruta metabólica importante es la O-demetilación.
1.6 Efectos en mamíferos de laboratorio y en sistemas de ensayo
in vitro
1.6.1 Exposición única
El demeton-S-metilo produce toxicidad colinérgica. Los valores de
la DL50 para los mamíferos oscilan entre 7 y 100 mg/kg de peso
corporal, dependiendo de la vía de administración y de la especie.
1.6.2 Exposición breve
Un estudio alimentario inicial reveló que las ratas alimentadas
con 50 mg de demeton-S-metilo por kg de la dieta habían sufrido una
reducción notable de la actividad de la colinesterasa cerebral y
eritrocitaria tras una exposición de 26 semanas. Se observaron
síntomas colinérgicos en ratas alimentadas con 200 mg/kg de la dieta
durante las 5 primeras semanas de exposición.
En un estudio alimentario de un año de duración efectuado en
perros se estableció un nivel sin efectos adversos observados (NOAEL)
de 1 mg/kg de la dieta (equivalente a 0,036 mg/kg de peso corporal por
día) sobre la base de los efectos de la colinesterasa cerebral.
1.6.3 Exposición prolongada
Se administró durante 21 meses a ratones una dieta que contenía
0, 1, 15 ó 75 mg/kg de demeton-S-metilo. El NOAEL, determinado sobre
la base de la inhibición de la colinesterasa cerebral, fue de 1 mg/kg
de la dieta (equivalente a 0,24 mg/kg de peso corporal por día).
En ratas cuya dieta contenía 0, 1, 7 ó 50 mg/kg de demeton-S-metilo,
el NOAEL, determinado sobre la bases de la inhibición de la
colinesterasa cerebral, fue de 1 mg/kg de la dieta (equivalente a
0,05 mg/kg de peso corporal por día).
La incidencia de tumores no aumentó en ninguna de esas especies.
1.6.4 Irritación y sensibilización de la piel y los ojos
El demeton-S-metilo es un irritante cutáneo y ocular leve. La
prueba de maximización de Magnusson y Klingman dio resultados
positivos en cobayos. Sin embargo, la prueba del parche cutáneo de
Buehler no mostró indicios de sensibilización cutánea, lo que parece
indicar que el uso práctico del demeton-S-metilo no debería acarrear
problemas de sensibilización.
1.6.5 Reproducción, embriotoxicidad y teratogenicidad
En un estudio alimentario llevado a cabo en dos generaciones de
ratas, se observó una reducción de la viabilidad y del peso corporal
de los cachorros (generación F1b solamente) con una dosis de 5 mg de
demeton-S-metilo por kg de la dieta. El NOAEL fue de 1 mg/kg de la
dieta, equivalente a 0,07 mg/kg de peso corporal por día.
El demeton-S-metilo no resultó embriotóxico ni teratogénico en
ratas ni en conejos.
1.6.6 Mutagenicidad y parámetros conexos
El demeton-S-metilo produce mutaciones puntuales in vitro. Se han
demostrado efectos cromosómicos in vivo con formulaciones comerciales
solamente. La información disponible es insuficiente para realizar una
evaluación adecuada del potencial genotóxico del demeton-S-metilo.
1.6.7 Neurotoxicidad retardada
El demeton-S-metilo no causó polineuropatías retardadas ni
inhibición de la Esterasa Diana de Neuropatía en pruebas con gallinas
a un nivel igual a la DL50 oral.
1.6.8 Toxicidad de los metabolitos
Las plantas y los mamíferos producen dos metabolitos del
demeton-S-metilo (el oxidemetonmetilo y el demeton-S-metilsulfona) que
también son plaguicidas comerciales y han sido ampliamente estudiados.
Se ha señalado de que el perfil toxicológico de estos dos compuestos
no difiere de manera significativa, cuantitativa ni cualitativamente,
del demeton-S-metilo.
1.7 Mecanismo de toxicidad: modalidad de acción
El demeton-S-metilo es un inhibidor indirecto de la colinesterasa
y su toxicidad que está relacionada con la inhibición de la
acetilcolinesterasa en las terminales nerviosas. La
acetilcolinesterasa inhibida por el demeton-S-metilo se reactiva
espontáneamente, con una semivida in vitro de aproximadamente
1,3 horas, como cabe esperar en la acetilcolinesterasa dimetil
fosforilada.
1.8 Efectos en el ser humano
Se han notificado varios casos de intoxicación aguda con
síndrome colinérgico tras intentos de suicidio. Los pacientes que
sobrevivieron, inclusive una mujer embarazada, no presentaron efectos
retardados.
Tras una exposición laboral por inatención durante el envasado de
la formulación comercial, algunos trabajadores desarrollaron una
toxicidad colinérgica que precisó tratamiento farmacológico. El
demeton-S-metilo se absorbió probablemente a través de la piel.
Análogamente, condiciones de trabajo inapropiadas podrían haber sido
la causa de una absorción cutánea excesiva durante la aplicación del
demeton-S-metilo en algodonales.
1.9 Efectos en otros organismos en el laboratorio y sobre el terreno
Las CE50 96-h para las algas verdes se sitúan entre 8 y
37 mg/litro. Las CL50 para diversos invertebrados acuáticos se
encuentran entre 0,004 y 1,3 mg/litro. La toxicidad para los peces
varía; la CL50 96-h oscila entre 0,59 mg/litro para la trucha
arcoiris y unos 40 mg/litro para Leuciscus idus, Carassius auratus
y la carpa.
La DL50 oral aguda para la codorniz japonesa y el canario es de
10-50 mg/kg de peso corporal. En el estornino, una dosis oral única de
2 mg/kg de peso corporal produjo inhibición del 20% de la AChE
cerebral 3 horas después del tratamiento.
La CL50 del demeton-S-metilo en el suelo para las lombrices
de tierra es de 66 mg/kg para un período de 14 días. La DL50 aguda
oral y de contacto del demeton-S-metilo fue de 0,21 y 0,6 g/abeja
respectivamente. Después de haber sido aplicado al trigo de invierno
según la proporción propuesta, el demeton-S-metilo redujo
significativamente el número de invertebrados en el follaje de los
cultivos (principalmente Empididae), pero no el número de
invertebrados entomófagos de la superficie del suelo.
2. Conclusiones
El demeton-S-metilo es un éster organofósforado sumamente tóxico
(pertenece a la clase Ib de la clasificación de la OMS) (OMS, 1996)
utilizado como insecticida. La acción tóxica consiste en la inhibición
de la acetilcolinesterasa en las terminales nerviosas. La exposición
de la población en general se produce principalmente a causa de los
residuos presentes en los productos agrícolas.
Con buenas prácticas laborales y medidas de higiene y de
seguridad, el demeton-S-metilo no debería producir efectos adversos
durante la fabricación o la aplicación. Es poco probable que aparezcan
efectos debidos a la exposición crónica.
El demeton-S-metilo no es persistente en el medio ambiente y no
se acumula en los organismos. Tiene una toxicidad aguda elevada para
los invertebrados acuáticos y es tóxico para los peces y las aves,
llevando aparejados factores de riesgo agudo o moderado elevados para
esos organismos. Sin embargo, no se han comunicado muertes masivas
significativas de organismos en el campo a causa de este compuesto.
Habría que tomar precauciones para reducir al mínimo la exposición de
organismos no combatidos (por ejemplo, no rociar sobre masas de agua y
reducir al mínimo la exposición por desviación del rociado).
3. Recomendaciones
Para la salud y el bienestar de los trabajadores y del público en
general, el manejo y la aplicación del demeton-S-metilo deberían
encomendarse exclusivamente a personal bien adiestrado y supervisado
que aplique las medidas de seguridad necesarias y buenas prácticas de
aplicación.
