
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 56
PROPYLENE OXIDE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1985
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154196 2
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1985
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR PROPYLENE OXIDE
1. SUMMARY
2. PROPERTIES AND ANALYTICAL METHODS
2.1. Identity
2.2. Chemical and physical properties of propylene oxide
2.3. Analytical methods
3. SOURCES IN THE ENVIRONMENT, ENVIRONMENTAL TRANSPORT AND DISTRIBUTION
3.1. Production, uses, disposal of wastes
3.1.1. Production levels and processes
3.1.2. Uses
3.1.3. Disposal of wastes
3.2. Transport and fate in the environment
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1. Occurrence in the environment
4.2. General population exposure
4.3. Occupational exposure
5. KINETICS AND METABOLISM
5.1. Absorption
5.2. Distribution, metabolic transformation, and excretion
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7. EFFECTS ON ANIMALS
7.1. Single exposures
7.1.1. Oral exposure
7.1.2. Skin and eye irritation
7.1.3. Inhalation exposure
7.2. Repeated exposures
7.2.1. Oral exposure
7.2.2. Inhalation exposure
7.3. Mutagenicity and related end-points
7.4. Carcinogenicity
7.4.1. Oral exposure
7.4.2. Inhalation exposure
7.4.3. Subcutaneous exposure
7.5. Effects on reproduction and teratogenicity
8. EFFECTS ON MAN
8.1. Exposure of skin and eyes; skin sensitization
8.2. Accidental inhalation exposure
8.3. Occupational inhalation exposure
8.4. Mortality studies
8.5. Mutagenicity and related end-points
9. EVALUATION OF THE HEALTH RISKS FOR MAN AND EFFECTS OF THE ENVIRONMENT
10. RECOMMENDATIONS FOR FURTHER RESEARCH
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
WHO TASK GROUP ON PROPYLENE OXIDE
Members
Dr R. Bruce, Environmental and Criteria Assessment Office, US
Environmental Protection Agency, Research Triangle Park,
North Carolin, USA (Rapporteur)
Mr T.P. Bwititi, Hazardous Substances and Articles Department,
Ministry of Health, Harare, Zimbabwe
Dr B. Gilbert, CODETEC, University City, Campinas, Brazil
Prof P. Grasso, Robens Institute, University of Surrey,
Guildford, Surrey, United Kingdom
Prof M. Ikeda, Department of Environmental Health, Tohoku
University School of Medicine, Sendai, Japan (Chairman)
Dr T. Lewis, US National Institute for Occupational Safety and
Health, Cincinnati, Ohio, USA
Dr B. Malek, Prague Hygiene Station, Department of Industrial
Hygiene, Prague, Czechoslovakia
Prof N.C. Nayak, Department of Pathology, All-India Institute
of Medical Sciences, New Delhi, India
Prof M. Noweir, Occupational Health Research Centre, High
Institute of Public Health, Alexandria, Egypt (Vice-Chairman)
Dr G.J. Van Esch, Bilthoven, The Netherlands
Members of Other Organizations
Dr A. Berlin, Health and Safety Directorate, Commission of the
European Communities, Luxembourg
Dr R. Steger, International Commission on Occupational Health,
Geneva, Switzerland
Mme M.Th. Van der Venne, Health and Safety Directorate,
Commission of the European Communities, Luxembourg
Observers
Dr E. Longstaff (European Chemical Industry Ecology and
Toxicology Centre), ICI Central Toxicology Laboratory,
Genetic Toxicology Section, Macclesfield, United Kingdom
Dr M. Martens, Institute of Hygiene and Epidemiology, Division
of Toxicology, Brussels, Belgium
Dr W. Moens, Institute of Hygiene and Epidemiology, Division
of Toxicology, Brussels, Belgium
Dr M. Wooder (European Chemical Industry Ecology and
Toxicology Centre), Shell International Petroleum Company,
Health, Safety and Environment Division, London, United
Kingdom
Secretariat
Prof F. Valic, Andrija Stampar School of Public Health,
University of Zagreb, Zagreb, Yugoslavia (Secretary)a
Dr T. Vermeire, National Institute of Public Health and
Environmental Hygiene, Bilthoven, The Netherlands
(Temporary Adviser)
Mr J. Wilbourn, International Agency for Research on Cancer,
Lyons, France
-------------------------------------------------------------------
a IPCS Consultant.
PREFACE
Although only key references essential for the evaluation
of the risks for human health and the environment are cited,
this document is based on a comprehensive search of the
available original scientific literature, while valuable
information has also been obtained from various reviews.
A detailed data profile on propylene oxide can be obtained
from the International Register of Potentially Toxic Chemicals
(UNEP/IRPTC, Palais des Nations, CH-1211 Geneva 10,
Switzerland, telephone number 988400 - 985850).
The document focuses on describing and evaluating the
risks of propylene oxide for human health and the environment.
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of
the environmental health criteria documents, readers are
kindly requested to communicate any errors, which may have
occurred, to the Manager, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda, which will
appear in subsequent volumes.
ENVIRONMENTAL HEALTH CRITERIA FOR PROPYLENE OXIDE
The WHO Task Group for the Environmental Health Criteria
for Propylene Oxide met at the Institute of Hygiene and
Epidemiology, in Brussels, Belgium, on 21-26 October 1985.
Dr@G. Thiers, who opened the meeting, welcomed the
participants on behalf of the host government, and Dr F. Valic
welcomed them on behalf of the heads of the three IPCS
co-sponsoring organizations (ILO/WHO/UNEP). The Group
reviewed and revised the second draft criteria document and
made an evaluation of the health risks of exposure to
propylene oxide.
The efforts of DR T. VERMEIRE, of the NATIONAL INSTITUTE
OF PUBLIC HEALTH AND ENVIRONMENTAL HYGIENE, Bilthoven, the
Netherlands, who was responsible for the preparation of the
draft, and of all who helped in the preparation and the
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this
criteria document was kindly provided by the United States
Department of Health and Human Services, through a contract
from the National Institute of Environmental Health Sciences,
Research Triangle Park, North Carolina, USA - a WHO
Collaborating Centre for Environmental Health Effects.
1. SUMMARY
Propylene oxide is a colourless, highly volatile, and flammable
liquid at room temperature and normal atmospheric pressure. It is
very reactive towards nucleophiles. The compound can be determined
in air, by gas chromatography, with a detection limit of 0.06 µg/m3
and, in food, with a limit of 0.1 mg/kg. Detection limits of
various other methods include 0.3 µg/litre in biological fluids and
30 mg/kg in synthetic materials.
World production of propylene oxide exceeds 2000 kilotonnes per
year, most of which is used as a chemical intermediate. Small
amounts are used for the sterilization of medical equipment and for
the fumigation of foodstuffs. About 0.07% of all the propylene
oxide used is lost to the atmosphere. The compound can be removed
from the atmosphere by slow oxidation and by rain. It evaporates
from water and is not expected to bioaccumulate. Aerobic
biodegradation is slow.
The main route of human exposure is through inhalation at the
workplace. No published data have been found on ambient levels
away from point sources. Eight-hour time-weighted average
occupational exposure levels are normally less than 5 mg/m3.
However, peak exposures of up to 9010 mg/m3 have been recorded.
Analyses of fumigated foodstuffs revealed that the propylene
oxide derivatives, chloropropanols and 1,2-propane-diol, were
present at levels ranging from 4 to 47 mg/kg and 29 to 2000 mg/kg,
respectively. However, in pure lipids, where degradation is
minimal, levels of propylene oxide of over 4000 mg/kg have been
determined. Levels of up to 6260 mg/kg have been found in wrapping
materials.
Available LC50 values for propylene oxide in various fish
species range between 89 and 215 mg/litre for a 96-h exposure.
From in vitro studies, it would appear that propylene oxide
is metabolized by glutathione epoxide transferase to S-(2-hydroxy-
1-propyl)glutathione. It is converted to 1,2-propanediol by epoxide
hydrolase and non-enzymic hydrolysis, but both of these reactions
are slow. The diol can be oxidized to lactic and pyruvic acid.
The oral LD50 has been reported to be 630 mg/kg body weight
for the mouse, 660 mg/kg for the guinea-pig, and from 520 to
1140 mg/kg for the rat. Damage to the stomach mucosa and liver was
observed in rats exposed to such levels.
The 4-h LC50s for the rat and the mouse via inhalation were
9500 and 4100 mg/m3, respectively. At these concentrations,
there was severe eye and nose irritation, laboured breathing, and
central nervous system depression.
With repeated exposure (6 h/day, 5 days/week, for 2 weeks) to
propylene oxide concentrations ranging from 110 - 3400 mg/m3
(rats) and 50 - 1150 mg/m3 (mice), dyspnoea was observed in rats
exposed to 3400 mg/m3 and in mice exposed to 460 mg/m3 and
1150 mg/m3. Reduced activity was observed in both species, and
irregular limb movement was seen in rats. After exposure to
concentrations of propylene oxide of 240, 460, or 1080 mg/m3 for
112 - 218 days (7 h/day, 5 days/week), monkeys and rabbits did not
show any adverse effects, but irritation of the eyes and respiratory
passages was observed in rats and guinea-pigs at 1080 mg/m3.
Internally, changes were found only in the lungs in both species
and consisted of oedema and haemorrhage. In addition, an increase
in lung weight was observed in female guinea-pigs exposed at
460 mg/m3.
A dose-related increase in the incidence of inflammatory
lesions and proliferative lesions in the nasal epithelium was
observed in Fischer 344/N rats and B6C3F1 mice exposed to 470 or
940 mg/m3, for 6 or 7 h per day, 5 days/week, for 2 years.
Similar inflammatory lesions and hyperplasia were also observed at
240 mg/m3 in another study on Wistar rats exposed for 6 h/day,
5 days per week, for 124 weeks. Non-neoplastic effects were not
observed in the nasal mucosa or internal organs of rats exposed to
70 mg/m3.
Axonal dystrophy was observed in the nucleus gracilis in
cynomolgus monkeys, 2 per group, exposed to 237 and 717 mg
propylene oxide/m3 (7 h/day, 5 days/week) for 2 years. One of
two untreated monkeys also showed such changes. The changes in
the treated groups were more pronounced than those in the controls.
A dose-related increase in the incidence of squamous cell
carcinoma of the forestomach was observed in rats treated by gavage
for 112 weeks with 0, 15, or 60 mg propylene oxide/kg body weight
dissolved in salad oil. The numbers of rats affected were 0
(controls), 2, and 19, respectively. When propylene oxide,
dissolved in trycaprylin, was administered subcutaneously to mice,
once a week, for 106 weeks, only local sarcomas were induced. The
numbers of animals exhibiting sarcomas were 0/200 in untreated
controls, 4/200 in tri-caprylin controls, and 3/100, 2/100, 12/100,
and 15/100 in mice treated with 0, 0.1, 0.3, 1.0, and 2.5 mg per
injection, respectively.
The carcinogenicity of propylene oxide inhalation exposure has
been investigated in rats and mice. Two studies have been conducted
on Fischer 344 rats using groups of 80 - 100 animals, for 2 years.
In one study, exposure was to concentrations of 470 and 940 mg/m3,
6 h/day, for 5 days per week, over 103 weeks; in the second, exposure
was to concentrations of 237 - 717 mg propylene oxide/m3, for
7 h/day, 5 days/week, over 2 years. A few adenomas were observed
in the nasal cavity in both studies at the highest level of treatment.
When Wistar rats were treated with 70, 242, or 712 mg/m3, 6 h/day,
5 days per week, for 124 weeks, no nasal tumours were reported, but
a dose-related increase in the incidence of multiple mammary
fibroadenoma was observed. There was no increased incidence of
brain tumours.
In mice, malignant nasal tumours were induced. Groups of 50
B6C3F1 mice of each sex were exposed to 470 and 940 mg/m3
propylene oxide, for 6 h/day, 5 days/week, over 103 weeks. Tumours
in the nasal cavity occurred in both sexes. Haemangiosarcomas were
observed in the nasal cavity of 5 male and 2 female mice at the
higher concentration, and haemangiomas appeared in 5 males and 3
females at the same site. In addition, one squamous cell carcinoma
and one papilloma appeared in high-dose males and 2 adenocarcinomas
in high-dose females, at the same site. No treatment-related
tumours were observed at the lower dose.
No teratogenic or fetotoxic effects were observed when pregnant
New Zealand rabbits were exposed through inhalation to 1190 mg
propylene oxide/m3, for 7 h/day, during days 1 - 19 days 7 - 19
of gestation. An increase in the number of resorptions was found
when Sprague Dawley rats were exposed to 1190 mg propylene oxide/m3,
for 7 h/day, during days 7 - 16 of gestation. Some reduction in
ossification in vertebrae and ribs and wavy ribs were found when
pregnant Sprague Dawley rats were similarly exposed on days 1 - 16
of gestation. When rats were exposed for 3 weeks prior to mating
and on days 1 - 16 of gestation, the numbers of corpora lutea,
implantations per dam, and live fetuses were decreased compared
with those in the other groups.
Propylene oxide is mutagenic to microorganisms and insects and
produced mutations, DNA damage, and chromosomal effects in mammalian
cells in vitro. Negative results in such studies have never been
reported. In vivo, propylene oxide induced a 5-fold increase in
micronuclei in mice, when given intraperitoneally (ip) at a
concentration of 300 mg/kg body weight, but not at 150 or 75 mg/kg
body weight, nor when administered orally. No dominant-lethal
effects were observed, when propylene oxide was administered via
inhalation to male rats at 720 mg/m3 for 5 days prior to mating,
or, when it was administered daily, by the oral route, to male mice
at 50 or 250 mg/kg body weight for 2 weeks prior to mating. No
increase in chromosome aberrations or sister chromatid exchanges in
peripheral lymphocytes were observed in male Cynomolgus monkeys
exposed to 237 or 717 mg propylene oxide/m3 air, for 7 h/day, 5
days per week, for 2 years.
No sperm head abnormalities were detected in mice after
exposure for 7 h/day, for 5 days, to 720 mg propylene oxide/m3 or
in cynomolgus monkeys exposed to 240 or 710 mg/m3 for 2 years.
There are no adequate epidemiological studies to assess the
toxic effects of propylene oxide on man. Taking into account the
body of available data - the alkylating nature of propylene oxide,
the formation of DNA adducts, the positive responses in in vitro
mutagenesis assays, the carcinogenic effects in animals at sites of
entry into the body, and the absence of adequate data on cancer in
human beings - propylene oxide should be considered as a possible
human carcinogen. Therefore, propylene oxide should be regarded,
for practical purposes, as presenting a carcinogenic risk for man,
and levels in the environment should be kept as low as feasible.
2. PROPERTIES AND ANALYTICAL METHODS
2.1. Identity
Structural formula: H O
| / \
H---C---C---C---H
| | |
H H H
Molecular formula: C3H6O
Abbreviation: PO
Common synonyms: 1,2-epoxypropane, methyl ethylene
oxide, methyl oxirane (IUPAC and
CAS name), propene oxide, propylene
epoxide, 1,2-propylene oxide
CAS registry number: 75-56-9
RTECS registry number: TZ2975000
2.2. Chemical and Physical Properties of Propylene Oxide
Propylene oxide is a colourless, highly-volatile liquid at
room temperature and normal atmospheric pressure. It is highly
flammable. The vapour will form an explosive mixture with air.
The substance may polymerize violently. Ring opening occurs in
reactions with nucleophiles, such as water, alcohols, amines,
halides, and sulfhydryl compounds. Propylene oxide is very
reactive, particularly with chlorine, ammonia, strong oxidants,
and acids. Some physical and chemical data on propylene oxide are
given in Table 1.
Conversion factor 1 ppm = 2.37 mg/m3 air at 25 °C
and 101.3 kPa (760 mm Hg)
Table 1. Some physical and chemical data on propylene oxide
-------------------------------------------------------------------
physical state liquid
colour colourless
odour ethereal
odour threshold 20 mg/m3 for perception and
80 - 470 mg/m3 for recognitiona
relative molecular mass 58.08
melting point -104 °C
boiling point 34 °C
water solubility 405 g/litre, 20 °C
log n-octanol-water partition -0.13
coefficient
density 0.83 g/ml, 20 °C
relative vapour density 2.0
vapour pressure 59 kPa (445 mm Hg), 20 °C
flash point -37 °C (open-cup)
flammable limits 2 - 37% by volume in air
-------------------------------------------------------------------
a From: Jacobson et al. (1956) and Hellman & Small (1974).
2.3. Analytical Methods
A summary of methods for the sampling and determination of
propylene oxide in air, water, food, synthetic materials (including,
e.g., medical equipment), and biological media is presented in
Table 2. However, the methods for the determination in water,
synthetic materials, or biological media are not specific for
propylene oxide.
It was proposed that, as for ethylene oxide (Ehrenberg et al.,
1974; Osterman-Golkar et al., 1976, 1983), the determination of the
degree of alkylation of amino acids, and specifically histidine, in
haemoglobin could be used for monitoring the tissue doses of propylene
oxide. Assuming even distribution, dose is defined as the integral
of the calculated concentration of free propylene oxide in the
tissues over a specified period of time (Farmer et al., 1982;
Svensson & Osterman-Golkar, 1984). Methods for the determination
of N3-(2-hydroxypropyl)histidine in rat haemoglobin have been
described using gas chromatography with mass spectrometry (Farmer
et al., 1982) and high-performance liquid chromoatography after
derivatization with fluorescamine (Svensson & Osterman-Golkar,
1984). In rats, the alkylation of haemoglobin was found to
increase linearly with the level of exposure to propylene oxide
vapour, with a detection limit of 0.002 mg alkylated histidine/kg
haemoglobin (Farmer et al., 1982). Human haemoglobin has a life-
span of about 4 months and, therefore, will integrate the dose of
propylene oxide over a long period. The method was applied to
samples from industrial workers by Osterman-Golkar et al. (1984).
A low background level of alkylation was observed, which may limit
the resolving power of detection at average exposures below
0.7 mg/m3. More work is needed to establish the relationship
between propylene oxide exposure and haemoglobin alkylation in
human beings.
Table 2. Sampling, preparation, analysis
---------------------------------------------------------------------------------------------------------
Medium Sampling Analytical method Detection Comments Reference
method limit
---------------------------------------------------------------------------------------------------------
Air adsorption gas chromatography 0.06 µg/m3 suitable for Krost et al.
on Tenax- with mass spectrometric analysis of (1982)
GC, thermal detection ambient air
desorption,
cryofocusing
Air adsorption gas chromatography recommended for NIOSH (1977)
(work- on coconut with flame ionization the range
place) charcoal, detection 25-720 mg/m3;
desorption suitable
by carbon for personal
disulfide and area sampling
Air adsorption on gas chromatography 2 µg/m3 Russell (1975)
Tenax or with flame ionization
Porapak N, detection
thermal
desorption
Water colorimetry using detection the method is Mishmash & Meloan
cadmium iodide after limit in the not specific (1972)
hydrolysis and nmole range
reaction with periodate
in the solvent system
1,2-dimethoxyethane-
water
Water potentiometric ti- the method is not Swan (1954)
tration using hydro- specific
chloric acid after
reaction with sodium
sulfite
---------------------------------------------------------------------------------------------------------
Table 2. (contd.)
---------------------------------------------------------------------------------------------------------
Medium Sampling Analytical method Detection Comments Reference
method limit
---------------------------------------------------------------------------------------------------------
Food extraction gas chromatography 0.1 mg/kg sample size Heuser & Scudamor
by 5:1 with beta-ionization wet weight 5-10 g (1969)
acetone-water detection
by volume
for 24 h
Synthetic distillation titration using hydro- 30 mg/kg sample size 2 g; Gunther (1965)
materials of samples gen bromide in glacial the method is
in monochloro- acetic acid not specific
benzene
into glacial
acetic acid
Biological fluorimetry employing 0.3 mg/litre sample size Nelis & Sinsheime
media alkylation of nicotin- 0.1 ml; direct (1981)
(blood, amide followed by a analysis; inter-
urine) reaction with aceto- ference by other
phenone to a fluor- alkylating agents
escent compound
---------------------------------------------------------------------------------------------------------
3. SOURCES IN THE ENVIRONMENT, ENVIRONMENTAL TRANSPORT AND
DISTRIBUTION
3.1. Production, Uses, Disposal of Wastes
3.1.1. Production levels and processes
Propylene oxide is produced in the USA, western Europe, Japan,
and several other countries. In the USA, production increased from
800 kilotonnes in 1974 to 1020 kilotonnes in 1979. However,
production in 1983 was estimated to be 720 kilotonnes (Bogyo et al.,
1980; Webber, 1984; IARC, 1985). In western Europe, 850 kilotonnes
were produced in 1979 and 810 kilotonnes in 1982; in Japan, 130
kilotonnes were produced in 1974 and 190 kilotonnes in 1982 (IARC,
1976, 1985).
Propylene oxide can be produced by the chlorohydrin process or
by peroxidation. In the first of these processes, 1-chloro-2-
propanol and 2-chloro-1-propanol react with potassium hydroxide or
calcium oxide to form propylene oxide. In the second process,
propylene oxide is synthesized through a catalysed reaction between
propene and tertiary butyl hydroperoxide. Tertiary butyl hydro-
peroxide is prepared by the oxidation of isobutane (WHO, 1978).
Common impurities, that may be present in small amounts include
water, acetic acid, chloride, and aldehydes. Small amounts of
monochloroacetone, 1,2-dichloro-3-propanol, and propylene dichloride
can occur in propylene oxide, produced by the chlorohydrin process
(IARC, 1985).
3.1.2. Uses
Most propylene oxide produced is used as an intermediate in
the production of various chemicals. In order of importance, in
the USA, these chemicals are: polyether polyols for urethanes,
propylene glycol, mainly for polyester fibres, polypropylene
glycol, dipropylene glycol, glycol ethers, glycerin, and
surfactants. Minor quantities are used for the (antimicrobial)
sterilization or (insecticidal) fumigation of medical equipment and
foodstuffs (IARC, 1976; WHO, 1978). Small quantities are also used
in the production of modified food starch and alginate and as a
stabilizer in dichloromethane.
3.1.3. Disposal of wastes
The emission of propylene oxide through process vents appears
to be the most important source of atmospheric pollution. However,
the waste gas can be removed from air by scrubbing, and emission
from liquid wastes can be controlled by incineration. Little, if
any, propylene oxide seems to be released in waste water in the
chlorohydrin process, but an environmental problem is created by
large amounts of by-products such as calcium chloride and chlorinated
organic compounds. No specific solid wastes are associated with the
manufacture of propylene oxide (Bogyo et al., 1980).
3.2. Transport and Fate in the Environment
Propylene oxide enters the environment mainly through
evaporation and in vented gases during production, handling,
storage, transport, and use. Most of the propylene oxide applied
as a sterilant or fumigant will finally enter the atmosphere (Bogyo
et al., 1980). In the USA, in 1981, a total loss to the atmosphere
of almost 600 tonnes of propylene oxide was estimated, or
approximately 0.07% of total production (Storck, 1981; US EPA,
1981).
The major removal of propylene oxide from the atmosphere will
occur rapidly via oxidation by hydroxyl radicals. On the basis of
a theoretical rate constant for this reaction, the atmospheric
residence time of propylene oxide was calculated to be 8.9 days
(Cupitt, 1980), but by analogy with ethylene oxide (WHO, 1985), the
real value is probably an order of magnitude greater. Because of
its high solubility in water, propylene oxide levels in air can
also be reduced via washout by rain (Bogyo et al., 1980). No data
were found concerning the rate of evaporation of propylene oxide
from water. However, because of its lower vapour pressure and high
water solubility, it can be assumed that the rate is slower for
propylene oxide than that for ethylene oxide, for which, under
certain specified conditions, a half-life of 1 h has been
determined (Conway et al., 1983). Chemical degradation in water
via ionic reactions appears a slow process under environmental
conditions. In neutral fresh water, at 25 °C, propylene oxide will
react to form 1,2-propanediol with a half-life of approximately 12
days (Koskikallio & Whalley, 1959). This reaction is acid catalysed.
In marine waters, 1-and 2-halopropanols are also formed. In neutral
marine water, there is a preference for the formation of 1-halopropan-
2-ol (Addy & Parker, 1963). Bogyo et al. (1980) estimated the
relative importance of the reaction of propylene oxide with water
and that with chloride at 25 °C in neutral sea water of 3% salinity.
According to these calculations, approximately 80% of the propylene
oxide present will react to form chloropropanol; the remainder will
react to form 1,2-propanediol. The overall half-life with respect
to these reactions is 4 days.
Microorganisms from the effluent of a biological sanitary
waste treatment plant were found to degrade propylene oxide very
slowly. The biological oxygen demand (BOD) over 5 days was 8% of
the theoretical oxygen demand (Bridié et al., 1979b). In another
study, the biological oxygen demand of microorganisms from activated
sludge was 20% of the chemical oxygen demand (COD) over 4 h, after
1 month of acclimation (Hatfield, 1957). The aerobic bacteria
Nocardia A60 also oxidized propylene oxide after adaptation. It
has been established that the first step in bacterial metabolism is
the conversion of propylene oxide to 1,2-propanediol by epoxide
hydrolase (EC 3.3.2.3), followed by dehydration and oxidation (Bont
et al., 1982).
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1. Occurrence in the Environment
The only available information on atmospheric concentrations of
propylene oxide are estimates of levels in the vicinity of production
plants. The lowest annual average concentration occurring within
20 km of a specific point source was estimated, using dispersion
models, to be less than 4.836 x 10-8 mg/m3 (Anderson, 1983).
No data are available indicating that propylene oxide occurs as a
natural product.
4.2. General Population Exposure
Exposure via food
The residue levels in food after fumigation or sterilization
by propylene oxide will depend on factors similar to those
investigated by Scudamore & Heuser (1971) in their studies on
ethylene oxide. Important factors are: the total amount and
concentration of propylene oxide, the composition of the treatment
mixture, temperature, aeration and storage conditions after
treatment, the type of commodity and its moisture content, pH,
permeability, and particle size, and the method of packaging.
Few residue data exist on propylene oxide. In Japan, propylene
oxide residues of up to several thousands mg/kg wet weight were
measured in a variety of foodstuffs. For example, after 24 h of
aeration, at 37 °C, levels of over 4000 mg/kg were found in lard
and oleic acid (Oguma et al., 1968, 1969). Food wrappings and
containers were also found to contain propylene oxide residues,
after fumigation. Depending on the materials, these levels
fluctuated between 0 and 6260 mg/kg, 3 h after fumigation (Hirashima
et al., 1970). No migration studies were reported.
Propylene oxide can react with water and chloride in commodities
to form 1,2-propanediol and chloropropanol, respectively. In
commercially-fumigated walnut meat, flour, cocoa, glacé cherries,
and glacé citrons, 4 - 47 mg 1-chloro-propan-2-ol/kg wet weight
were measured in the USA (Ragelis et al., 1968). When dehydrated
mashed potatoes were sterilized, residues of 12.1 mg chloropropanol,
mostly 1-chloropropan-2-ol, and 29 mg 1,2-propanediol/kg wet weight
were measured. There was no detectable reaction of propylene oxide
with the starch (Steele & Hadziyev, 1976). Residues of propylene
oxide in packed prunes were no longer detectable, 7 days after
treatment. At this time, over 50% of the propylene oxide added
appeared to be converted to 1,2-propanediol; residue levels of this
product exceeded 2000 mg/kg wet weight for many months (Mestres &
Barrois, 1964). Residues of 1,2-propanediol of 190 - 900 mg/kg wet
weight were reported for flour, when wheat was treated with propylene
oxide, before milling at room temperature. The amount of residue
increased with the moisture content. When the flour was treated
after milling, 1000 mg/kg wet weight was found (Vojnovich &
Pfeifer, 1967).
4.3. Occupational Exposure
Levels of propylene oxide were measured by personal sampling in
8 plants in the Federal Republic of Germany, where alkene oxides
were produced between 1978 and 1980. In each case, the time-
weighted averages were reported to be far below 240 mg/m3, though
higher concentrations were measured for brief periods (Thiess et
al., 1981a).
More detailed results were reported for a propylene oxide-
producing plant in the USA in 1979, where daily, time-weighted
average exposures were found to range from 0.5 to 4.7 mg/m3.
Peak air concentrations in that year were 24 - 9010 mg/m3 (Flores,
1983).
In a factory in Sweden, in 1981, where starches were alkylated
with propylene oxide, the time-weighted average for 5 of the
workers, potentially exposed to the highest levels of propylene
oxide during their work, varied between 1.4 and 28 mg/m3. The
work with propylene oxide occupied 25 - 75% of the total working
time. Short-term exposures of up to 2370 mg/m3 were recorded
for some workers (Pero et al., 1982).
5. KINETICS AND METABOLISM
5.1. Absorption
No experimental data on the absorption of propylene oxide are
available.
5.2. Distribution, Metabolic Transformation, and Excretion
There are no in vivo data on the distribution and metabolism
of propylene oxide. On the basis of in vitro experiments, 2
metabolic pathways have been suggested (Tachizawa et al., 1982).
Propylene oxide was found to be a substrate for rat liver
glutathione epoxide transferase (EC 4.4.1.7), while nonenzymic
conjugation was negligible (Fjellstedt et al., 1973). It was also
observed to be hydrolysed to 1,2-propanediol by epoxide hydrolase
(EC 3.3.2.3) from rat liver microsomes, but at a low rate
(Guengerich & Mason, 1980; Dent & Schnell, 1981). Propylene
oxide was found to be a poor substrate for human liver epoxide
hydrolase (Oesch, 1974). The nonenzymatic hydrolysis to 1,2-
propanediol is rather slow. At 37 °C, the half-life for the
uncatalysed reaction in a neutral medium was found to be 87 h
(Ross, 1950).
Propanediol can be excreted unchanged via the kidneys and can
be oxidized to lactic and pyruvic acid (Ruddick, 1972). The data
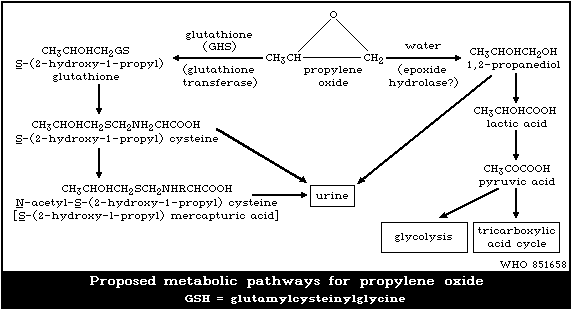
are summarized in Fig. 1.
Propylene oxide is a direct-acting agent, and in vitro
alkylation of DNA deoxynucleosides has been found. A total of
15 different calf thymus DNA adducts of propylene oxide were
detected, which altered 1.3% of the nucleosides in the DNA
molecule (Randerath et al., 1981). Guanosine and, to a lesser
extent, adenosine were alkylated to N7-(2-hydroxypropyl)-
guanosine and N3- or N6-(2-hydroxypropyl)adenosine,
respectively (Lawley & Jarman, 1972; Walles, 1974; Hemminki et
al., 1980). In rats, haemoglobin alkylation was established at
the amino acids, cysteine, valine, and histidine (Farmer et al.,
1982; Svensson & Osterman-Golkar, 1984). Histidine alkylation was
found to increase linearly with the level of vapour exposure.
Exposure of rats for 4 h to 3080 mg/m3 resulted in 10.5 mg
hydroxypropylhistidine/kg haemoglobin (Farmer et al., 1982).
On the basis of the haemoglobin alkylation data of Farmer et
al. (1982), a half-life of approximately 40 min can be calculated
for the elimination of propylene oxide from rat tissues, assuming
100% alveolar absorption and first order kinetics.
 6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
In an acute toxicity test on goldfish (Carassius auratus),
in static fresh water, at 20 °C, pH between 6 and 8, and a
dissolved oxygen content greater than 4 mg/litre, the 24-h LC50
for propylene oxide was 170 mg/litre (Bridié et al., 1979a). In
other static tests, with the fresh water species bluegill sunfish
(Lepomis machrochirus) and mosquito fish (Gambusia affinis),
96-h LC50 values for propylene oxide were 215 and 141 mg/litre,
respectively, at 23 °C. For the common mullet (Mugil cephalus),
a marine fish, a static 96-h LC50 for propylene oxide of 89
mg/litre was determined at 23 °C and a water salinity of 1.5% (no
water analysis was reported) (Crews, 1974). The 24-h LC50 for
1,2-propanediol (a product of propylene oxide) for goldfish was
over 5000 mg/litre (Bridié et al., 1979a). Propylene oxide is very
soluble in aqueous media; the log n-octanol water partition
coefficient was reported to be -0.13 (Radding et al., 1977) and,
therefore, it is not expected to bioaccumulate.
7. EFFECTS ON ANIMALS
7.1. Single Exposures
7.1.1. Oral exposure
In 2 studies, the oral LD50s for the rat were reported to be
1140 mg/kg body weight and 520 mg/kg body weight for males, and
540 mg/kg for females (Smyth et al., 1941; Antonova et al., 1981);
the difference between 16 and 84% mortality was caused by a dose of
only approximately 300 mg/kg body weight, indicating a rather steep
dose-effect relationship. In one of these studies, oral LD50s for
male mice and guinea-pigs were 630 and 660 mg/kg body weight,
respectively (Antonova et al., 1981).
At lethal oral doses, necrosis of the stomach mucosa was
observed in rats. Succinate dehydrogenase (EC 1.3.99.1) activity
was reported to be decreased in the stomach mucosa. The liver
cells showed oedema and fatty changes. In serum, alanine amino-
transferase (EC 2.6.1.2) and aspartate aminotransferase (EC 2.6.1.1)
activity and histamine levels were increased. Kidney function was
disturbed. Mature lymphocytes were reduced in the spleen (Antonova
et al., 1981).
7.1.2. Skin and eye irritation
Solutions of 100 or 200 g propylene oxide/litre water, applied
under a plastic cover on the intact skin of rabbits produced hyper-
aemia, oedema, and finally scars after 6 or more min of exposure.
The intensity of the effects was proportional to the exposure time
(Rowe et al., 1956).
No, or only slight, irritation was observed when undiluted
1,2-propanediol, a possible reaction product of propylene oxide in
water, was applied to the skin of various animals under occlusive
conditions for 24 or 72 h (Davies et al., 1972).
When 5 µl of undiluted propylene oxide was applied once on the
centre of the cornea of rabbits, a severe burn resulted with
necrosis (Weil et al., 1963).
7.1.3. Inhalation exposure
In inhalation studies, the 4-h LC50s for rats and mice were
9500 and 4100 mg/m3, respectively (Jacobson et al., 1956). The
lowest lethal concentration (US NTP, 1984) or the 0.1% mortality
level (Jacobson et al., 1956) for 4-h exposures was approximately
5250 mg/m3 for rats and 900 mg/m3 for mice. In both species,
100% mortality was reached after 4 h of exposure at levels above
17 000 mg/m3 (Jacobson et al., 1956). In rats, 100% mortality
was also reported after a 30-min exposure to 38 000 mg/m3 or a
7-h exposure to 9500 mg/m3 (Rowe et al., 1956).
After inhalation of propylene oxide, rodents showed eye and
nose irritation, nasal discharge, dyspnoea, and depression of the
central nervous system, the severity of which increased with
increasing level and length of the exposure (effect levels not
specified) (Rowe et al., 1956). Gross pathology revealed
distension of the stomach only at lethal concentrations (Jacobson
et al., 1956). No organ damage was observed in rats exposed to
2370 mg/m3 for 7 h, 4740 mg/m3 for 4 h, or 9480 mg/m3 for 0.5 h
(Rowe et al., 1956). In 4 groups, each containing 3 dogs, exposed
for 4 h to propylene oxide concentrations of 3230, 4750, 4810, or
5880 mg/m3, lachrymation, salivation, nasal discharge, and vomiting
occurred. Congestion in the lungs and trachea, oedema of pulmonary
tissues, and necrosis of bronchiolar epithelium were observed at
4810 and 5880 mg/m3. Deaths occurred in the groups exposed to
concentrations of 4750 mg/m3 or more (Jacobson et al., 1956).
7.2. Repeated Exposures
7.2.1. Oral exposure
Propylene oxide was administered in the drinking-water to 4
groups of rats of both sexes at dose levels calculated by the
authors to be 0.52, 5.2, 52, or 520 µg/kg body weight per day, over
a 26-week period. At the highest dose level, polyuria, haematological
abnormalities, lowering of serum-albumin, increased serum-beta-
globulin levels, and increased activity of gastrointestinal mucosal
enzymes were observed. Mild haematological abnormalities occurred
at the next lower dose, while no adverse effects were noted at the
two lowest doses (Antonova et al., 1981).
7.2.2. Inhalation exposure
In range-finding tests for a carcinogenicity study, groups of 5
male and 5 female Fischer 344/N rats were exposed to 0, 110, 230,
460, 1150, or 3400 mg propylene oxide/m3 air for 5 days per week,
and 6 h per day, during a 2-week exposure period. B6C3F1 mice were
exposed for the same period to 0, 50, 110, 230, 460, or 1150 mg/m3.
All animals were necropsied on the 12th day. No pathological
effects were observed. Body weights were not affected. Dyspnoea
and gasping were observed in rats, one of which died at 3400 mg/m3.
Mice showed dyspnoea at 460 and 1150 mg/m3. Both species were
less active at the highest exposure. Rats also showed irregular
limb movements and diarrhoea. After 13 weeks of a similar exposure
regime up to concentration of 1150 mg/m3, rats and mice did not
show any gross- or histopathological effects. Body weights were
reduced at 1150 mg/m3 only (US NTP, 1984).
Groups of 10 - 20 rats, 8 guinea-pigs, 1 or 2 rabbits of both
sexes, and 1 or 2 female Rhesus monkeys were exposed to 0, 240,
460, or 1080 mg propylene oxide/m3 air for 112 - 218 days, for
7 h per day, and 5 days per week. Rabbits and monkeys did not
show any adverse effects with regard to appearance, behaviour,
mortality rate, growth, organ weights, and gross- and histopathology.
Rats showed irritation of the eyes and respiratory passages and an
increased mortality rate due to pneumonia at 1080 mg/m3. Guinea-
pigs also showed irritation of the eyes and respiratory passages at
this exposure level, but no increase in mortality rate. In
addition, the growth of the female guinea-pigs was reduced. Livers
exhibited slight fatty degeneration in male guinea-pigs. The
relative weights of the lungs of the guinea-pigs were slightly, but
significantly, increased in both sexes at 1080 mg/m3, and in
females at 460 mg/m3. It should be noted that control lung
weights were low. Histopathological findings were alveolar
haemorrhages and oedema, and interstitial oedema and hyperaemia in
the lungs of guinea-pigs after 157 days of exposure to 1080 mg/m3.
After 37 - 39 days of exposure to 1080 mg/m3, these histopatho-
logical effects had also been observed in rats (Rowe et al., 1956).
The possibility of the induction of neuropathological effects
by propylene oxide was investigated in groups of 12 male Cynomolgus
monkeys. The animals were exposed to actual concentrations of 0,
237, and 717 mg/m3 propylene oxide, for 7 h per day, 5 days per
week, for 2 years. In 2 monkeys per group, brain, ulnar and
sciatic nerves, and spinal cord were examined histologically after
exposure. No clinical signs were reported. The only treatment-
related neuropathological lesions found were in the medulla
oblongata of the brain. Axonal dystrophy was observed in the
nucleus gracilis in all of the 4 treated monkeys examined, without
any apparent dose-effect relationship. The same lesion was observed
in one of the two control monkeys (Sprinz et al., 1982).
7.3. Mutagenicity and Related End-Points
A summary of mutagenicity tests with positive results is
presented in Table 3. Propylene oxide is mutagenic in bacteria,
fungi, and insects; no such studies with negative results have been
reported.
Table 3. Mutagenic tests for propylene oxide with positive resultsa
---------------------------------------------------------------------------------------------------------
Test description System description Reference
Organism Strain/cell type
---------------------------------------------------------------------------------------------------------
Gene mutations
Forward mutations virus Bacillus subtilis Garro & Phillips (1980)b
phage phi 105
Reverse mutations (base-pair bacterium Escherichia coli WP2 McMahon et al. (1979)c
substitutions) Escherichia coli WP2 McMahon et al. (1979)c
Escherichia coli WP2 Hemminki & Falck (1979)
Escherichia coli WP2 Bootman et al. (1979)c
CM871, CM891
Bacillus subtilis Phillips et al. (1980)
Salmonella typhimurium Wade et al. (1978)
TA 1535, TA 100 McMahon et al. (1979)c;
Bootman et al. (1979)c;
Pfeiffer & Dunkelberg
(1980)d
Reverse mutations bacterium Escherichia coli SD4 Hussain & Osterman-Golkar
(1984)
Forward mutations Klebsiella pneumoniae Voogd et al. (1981)
Reverse mutations fungus Neurospora crassa W40 Kolmark & Giles (1955)
macroconidia
Schizosaccharomyces Heslot (1962)
pombe
Forward mutations fungus Schizosaccharomyces Migliore et al. (1982)c
pombe Pl
Sex-linked recessive lethals insect Drosophila melanogaster Hardin et al. (1983)
Forward mutations on specific mammal Chinese hamster Zamora et al. (1983);
locus (in vitro) ovary cells
---------------------------------------------------------------------------------------------------------
Table 3. (contd.)
-------------------------------------------------------------------------------------------------------------------
Test description System description Reference
Organism Strain/cell type
-------------------------------------------------------------------------------------------------------------------
DNA damage
Single-strand breaks bacterium Bacillus subtilis Phillips et al. (1980)b
mammal (in vitro) rat hepatocytes Sina et al. (1983)
calf thymus DNA Walles (1974)
Chromosome damage
Chromatid gaps and breaks, mammal (in vitro) human lymphocytes Bootman et al. (1979)
Chromosome breaks, fragments
Chromatid gaps, deletions, mammal (in vitro) epithelial rat liver Dean & Hodson-Walker
exchanges cells (1979)c
Micronuclei mammal (ip) mouse polychromatic Bootman et al. (1979)
(2 x 300 mg/kg) erythrocytes
-------------------------------------------------------------------------------------------------------------------
a For details of these studies, see text and data profile (IRPTC, 1984).
b Treatment of isolated DNA in vitro, followed by transformation (bacteria) or transfection (virus).
c A similar or slightly reduced effect after metabolic activation by S9 rat or mouse liver fraction.
d Chloropropanols were also mutagenic, but not as mutagenic as propylene oxide; 1,2-propanediol was not
mutagenic.
Propylene oxide causes single-strand breaks in isolated calf
thymus DNA, probably by alkylation of the phosphodiester bond
(Walles, 1974). Single-strand breaks were observed in the DNA of
isolated rat hepatocytes at concentrations that were not toxic
(Sina et al., 1983).
Propylene oxide caused chromosome aberrations in mammalian
cells in vitro, in particular, chromatid gaps and breaks (Bootman
et al., 1979; Dean & Hodson-Walker, 1979). However, no increases
in chromosome aberrations or sister-chromatid exchanges were found
in peripheral lymphocytes of male Cynomolgus monkeys, after long-
term vapour exposure, in vivo. The animals were exposed in
groups of 12 to concentrations of 0, 237, and 717 mg propylene
oxide/m3 air, for 7 h per day, 5 days per week, for 2 years
(Lynch et al., 1984b).
Two oral doses of up to 500 mg propylene oxide/kg body weight,
in gum tragacanth, administered within 24 h to mice, did not induce
micronuclei in the polychromatic erythrocytes. In contrast, 2
intraperitoneal doses of 300 mg/kg body weight in water, administered
within 24 h, gave a 5-fold increase over controls. No effects were
observed following ip administration of 2 doses of 75 mg/kg or 150
mg/kg (Bootman et al., 1979).
The results of the dominant-lethal assay were negative when
male rats were exposed, for 7 h per day, to propylene oxide vapour
at a concentration of 720 mg/m3, for 5 days prior to mating
(Hardin et al., 1983). A negative result was also obtained in a
dominant lethal assay in which male mice received, once a day, 0,
50, or 250 mg propylene oxide/kg body weight in gum tragacanth, by
gavage, for 2 weeks prior to mating (Bootman et al., 1979).
No increased frequency of abnormal sperm heads was observed,
1 - 9 weeks after exposure of mice for 5 days to propylene oxide
vapour at a concentration of 720 mg/m3, for 7 h per day (Hardin
et al., 1983). Similarly, no increase was observed in the frequency
of abnormal sperm heads in the groups of Cynomolgus monkeys exposed
for 2 years (Lynch et al., 1984c). These monkeys were used by
Sprinz et al. (1982) (section 7.2.2) and Lynch et al. (1984b)
(section 7.3).
7.4. Carcinogenicity
7.4.1. Oral exposure
Groups of 50 female Sprague Dawley rats received 2 doses of
15.0 or 60.0 mg propylene oxide/kg body weight per week, by gavage,
for a total of 112 weeks. The compound was dissolved in salad oil
and administered to fasting rats. Two groups of 50 female rats
served as vehicle controls or untreated controls. There was an
exposure-free period between the 79th and 82nd week because of an
outbreak of pneumonia. The rats were observed up to 150 weeks.
No statistical analysis of the results was reported. Mortality
rates were not affected by the exposures. There was a dose-related
incidence of squamous cell carcinoma of the forestomach. In
addition, one early carcinoma of the forestomach was observed. The
numbers of rats affected were 0, 2, and 19 in animals receiving 0,
15, and 60 mg/kg body weight, respectively. The combined incidences
of hyperkeratosis, hyperplasia, and papillomas were 0, 7, and 17 at
0, 15, and 60 mg/kg body weight, respectively. At the highest dose
level, one adenocarcinoma of the pylorus was also observed. There
was no treatment-related increase of any other tumour type
(Dunkelberg, 1982).
7.4.2. Inhalation exposure
The following studies have been reported by the US NTP (1984).
Groups of 50 Fischer 344/N rats and 50 B6C3F1 mice of each sex were
exposed to average propylene oxide vapour concentrations of 0, 470,
and 940 mg/m3 air, for 6 h per day, 5 days per week, over a 103-
week period. Three accidental over-exposures at the highest
exposure level occurred. The concentrations did not exceed 15 300
mg/m3 for a maximum of 12 min during these periods.
In rats, survival rate was not affected and was over 58% at the
end of the study, at all exposure levels. Growth was slightly
reduced from week 20 onwards. The respiratory epithelium of the
nasal turbinates showed a dose-related increase in the incidence of
suppurative inflammation of the mucosae, hyperplasia, and squamous
cell metaplasia. At 940 mg/m3, 2 out of 50 male and 3 out of 50
female rats had papillary adenomas involving the respiratory
epithelium and the underlying submucosal glands of the nasal
turbinates. No such tumours were observed in controls or low-dose
animals. In the thyroid of the female rats, a dose-related increase
in the combined incidences of C-cell adenomas and carcinomas occurred,
which was significant at 940 mg/m3. The combined incidence of
endometrial stromal polyps and sarcomas was reported to have
increased, at both exposure levels, in a dose-related manner.
According to the authors, the C-cell adenomas and C-cell carcinomas
were not related to treatment. The incidences of polyps and
sarcomas were within the historical control range for the species
tested. In males, an increase in skin keratoacanthomas was
observed, with a statistically-significant positive trend. Induced
non-neoplastic lesions further included testicular atrophy, acinar
cell atrophy in the pancreas of males, cytomegaly in the adrenal
cortex of females, and cystic endometrial hyperplasia (US NTP,
1984).
The survival rate in mice was decreased at 940 mg/m3, from
week 60 onwards and was 58 and 20% for male and female mice,
respectively, at the end of the study, compared with 84 and 76% for
male and female controls. Growth was slightly reduced from week 29
onwards. In the nasal turbinates, a dose-related increased
incidence of inflammation occurred. Squamous cell metaplasia was
observed in 1 male at 470 mg/m3 and 2 females at 940 mg/m3. At
940 mg/m3, haemangiosarcomas were found in the nasal cavity of 5
5 males and 2 females. Haemangiomas were also observed in the
nasal cavity of 5 males and 3 females at this dose level. The
increases in the incidence of haemangiosarcomas in the males and of
haemangiomas in both sexes were statistically-significant.
One squamous cell carcinoma and one papilloma were induced in
the nasal cavity of male mice at 940 mg/m3 and 2 adenocarcinomas
were induced in the nasal cavity of females at 940 mg/m3. None
was observed in controls or low-dose animals. The incidence of
adenocarcinomas of the mammary gland was increased at 940 mg/m3,
but did not exceed the historical control range for the species
tested. Induced non-neoplastic lesions further included ovarian
atrophy and suppurative inflammation, mainly of the uterus and
peritoneum (US NTP, 1984).
In a combined NIOSH toxicity-carcinogenicity study, groups of
80 male Fischer 344 rats were exposed to 237 or 717 mg propylene
oxide/m3, for 7 h per day, 5 days per week, over 2 years. A
control group contained 80 rats. There was an exposure-free period
of 2 weeks in month 16 because of a pulmonary infection, which
contributed to the mortality rate. The mortality rate was
increased at both exposure levels, and the increase was significant
at 717 mg/m3. The mean survival was 96 weeks at this exposure
level, compared with 103 weeks for control rats. Body weights were
reduced from the 2nd week at 717 mg/m3 and from the 39th week at
237 mg/m3. After 2 years, at both concentrations, the relative
weights of lungs, adrenals, and brain were increased and those of
the testes decreased. The only altered biochemical parameters were
increased haemoglobin concentrations in blood at both exposure
levels and increased activities of serum aspartate aminotransferase
(EC 2.6.1.1) and serum sorbitol dehydrogenase (EC 1.1.1.14) at 237
mg/m3. Dose-related increases in the incidence and severity of
inflammatory lesions in the lungs, nasal cavity, trachea, and
middle ear were observed. In the nasal passages, there was a dose-
related increased incidence of complex epithelial hyperplasia that
was significant at 717 mg/m3. Metaplasia was not observed. Two
rats showed nasal-cavity adenomas at 717 mg/m3. At this
concentration, there was also an increased incidence of multifocal
areas of atrophy and degeneration of skeletal muscles. No lesions
were observed in the nerves. The only statistically-significant
neoplastic change observed was an increased incidence of adrenal
phaeochromocytomas at both concentrations. The incidences were
8/78 in controls, 25/78 in the low-dose group, and 22/80 in the
high-dose group (Lynch et al., 1984a).
Another study on the possible toxic and carcinogenic properties
of inhaled propylene oxide was performed on randomly-bred Wistar
rats. Groups of 100 rats of each sex were exposed to propylene
oxide concentrations of 70, 242, or 712 mg/m3, for 6 h per day, 5
days per week, over a period of 123 - 124 weeks. Groups of 100
rats of each sex served as controls. Ten rats per sex and per
group were killed for examination after 12, 18, and 24 months of
exposure. No adverse effects were observed on general health,
behaviour, food intake, biochemistry, urinalysis, and haematology
in comparison with control rats. The mortality rate was increased
at the highest exposure level from week 73, in males, and from week
109, in females, and at the end of the exposure to 242 mg/m3 in
females. Rats of both sexes gained less weight than controls at
712 mg/m3, though the difference compared with controls became
smaller towards the end of the exposure. The relative and absolute
weights of adrenals, spleen, liver, and lungs of males were
increased at 712 mg/m3, but no pathological lesions were observed
in these organs. Male rats showed a lower incidence of pale
exorbital lachrymal glands at this exposure level. In both sexes,
the incidences of basal cell hyperplasia and atrophy of the
olfactory epithelium and the incidence of nest-like infolds of the
respiratory epithelium were increased mainly at the 2 highest
exposure levels. These changes were also noted at interim kills.
The severity of the changes increased only slightly, if at all,
with increasing length of exposure and with age. Squamous
metaplasia was not observed. One rat was found with squamous cell
carcinoma of the nose. In female rats, the incidence of benign
tumours of the mammary glands, mostly fibroadenomas, was increased
at 712 mg/m3 in comparison to both the concurrent controls and
the historical controls. Moreover, the number of females bearing 2
or more mammary fibroadenomas increased in a dose-related manner
from the lowest exposure level onwards when compared with controls.
The increased incidence of tubulopapillary mammary carcinomas at
712 mg/m3 was within the range of historical controls. With the
exclusion of mammary tumours, the total incidence of primary
tumours in females and the number of malignant tumour-bearing rats
of both sexes was increased at the highest exposure level (Reuzel &
Kuper, 1983). Following reports that ethylene oxide had induced
brain tumours in rats, the histopathological investigation of the
brain of the rats was extended. There was no evidence that
propylene oxide induced such tumours (Reuzel & Kuper, 1984).
7.4.3. Subcutaneous exposure
Groups of 100 female NMRI mice received subcutaneous doses of
0.1, 0.3, 1.0, or 2.5 mg propylene oxide per animal in tricaprylin
once a week, for 106 weeks. These groups were compared with 200
controls receiving tricaprylin and 200 untreated controls. In the
second year of exposure, mice were not treated for 11 weeks. Body
weights and survival rates were not affected by treatment. At the
site of injection, a dose-related increase in the incidence of
sarcomas (mainly fibrosarcomas) occurred. The numbers of mice
affected were 0/200 in untreated controls, 4/200 in tricaprylin
controls, and 3/100, 2/100, 12/100, and 15/100 at the 0.1, 0.3,
1.0, and 2.5 mg dose levels, respectively. The increases in
incidence at the 2 highest dose levels were statistically
significant. The first tumour appeared in week 38 (Dunkelberg,
1981).
7.5. Effects on Reproduction and Teratogenicity
When B6C3F1 mice of both sexes were exposed repeatedly for 2
years to propylene oxide at concentrations of 470 and 940 mg/m3
air (section 7.4.2), the incidence of ovarian atrophy was increased
at the higher exposure level (US NTP, 1984). In F344 rats, similarly
exposed, higher incidences of testicular atrophy were found at both
exposure levels, but they were not dose-related (US NTP, 1984).
Decreased relative weights of the testes were reported by Lynch et
al. (1984a), when male F344 rats were exposed repeatedly for 2 years
to propylene oxide at levels of 240 and 720 mg/m3 air.
Sperm head abnormalities were not detected in mice after
exposure for 5 days, 7 h per day, to 720 mg propylene oxide/m3
(Hardin et al., 1983), or in groups of 12 Cynomolgus monkeys,
exposed for 2 years to 240 and 710 mg propylene oxide/m3, for
7 h per day, 5 days per week. In monkeys, at both exposure levels,
sperm counts and sperm motility were reduced, and the sperm drive
range was elevated (Lynch et al., 1984c).
In a reproduction-teratogenicity study, groups of 41 - 44
female Sprague Dawley rats were exposed to 1190 mg propylene
oxide/m3 air, for 7 h per day, during days 7 - 16 of gestation
(Group 1), days 1 - 16 of gestation (Group 2), or for 3 weeks
(5 days/week) before mating and on days 1 - 16 of gestation
(Group 3). A group of 46 rats served as controls. The dams were
sacrificed on day 21. No deaths of dams occurred, their histology
was normal, and the percentage of pregnant rats was not affected by
the exposure. Toxic effects in dams were expressed as decreased
body weights and food consumption and increased relative weights of
kidneys in all exposed groups. The numbers of corpora lutea,
implantations per dam, and live fetuses were lower in group 3,
compared with those in the other groups. In group 1, the number of
resorptions was increased compared with that in group 2. The body
weights and lengths of fetuses were decreased in all exposed groups
compared with controls, but more so in group 3. In group 2,
increases in wavy rib and in reduced ossification, primarily of the
vertebrae and ribs, were observed (Hackett et al., 1982).
Groups of New Zealand rabbits were also exposed to 1190 mg/m3,
for 7 h per day, during days 1 - 19 or days 7 - 19 of gestation.
No evidence of toxicity was observed in the mothers, fetuses, or
embryos, and no developmental defects were noted (Hacket et al.,
1982).
Female rats administered a single oral dose of 260 mg/kg body
weight of propylene oxide showed disturbance of the estrus cycle.
Pregnant female rats administered an identical dose during the
first 2 weeks of gestation exhibited higher embryotoxicity and
lower offspring body weight compared with the controls. Male rats
exposed to a single oral LD50 (520 mg/kg) dose of propylene oxide
showed reduced sperm motility and damage to primary spermatocytes.
When such rats were mated with normal female rats, between 2 and 10
weeks after exposure, 50% of the males were infertile. The fertility
of the first generation was reduced by 23% (Antonova et al., 1981).
8. EFFECTS ON MAN
8.1. Exposure of Skin and Eyes; Skin Sensitization
Accidental exposure of the eyes of 3 persons to propylene
oxide (it was not reported whether this was liquid or vapour)
resulted in alterations in the cornea and conjunctiva (McLauglin,
1946).
Allergic contact dermatitis was diagnosed in 3 cases of
exposure to solutions of propylene oxide (Ketel, 1979; Jensen,
1981). Biopsy on the skin of these patients revealed spongiosis
in the epidermis, oedema in the cutis, and dense perivascular
infiltrates with mononuclear cells.
8.2. Accidental Inhalation Exposure
No data are available.
8.3. Occupational Inhalation Exposure
In the Federal Republic of Germany, 279 employees from 8
plants where alkene oxides were produced or processed, were
examined during 1978. They were employed for an average of 10.8
years. No clinical abnormalities were found that could be related
to exposure to alkene oxides. Propylene oxide levels were measured
by personal sampling over 1 - 10 h, but levels of exposure were not
reported. The workers were exposed to many other chemicals,
including ethylene oxide (Stocker & Thiess, 1979). Because of the
mixed exposure and unavailability of propylene oxide exposure data,
the Task Group was unable to evaluate this study.
8.4. Mortality Studies
In the Federal Republic of Germany, 602 workers were
investigated for mortality over the period 1928-80. The workers
had been employed for at least 6 months in 8 plants producing
ethylene oxide and propylene oxide, the latter produced only since
1959. A subcohort of 351 workers was observed for more than 10
years. Control data came from a styrene plant and from national
statistics. Propylene oxide levels, measured by personal sampling
(number of samples not reported), from 1978 to 1980, were far below
a time-weighted average of 240 mg/m3 over a working shift of 12 h
(Thiess et al., 1981a). Higher levels were measured for brief
periods. The workers were also exposed to many other chemicals,
some of which might be carcinogenic for human beings. There were
56 deaths compared with 76.6 expected. No significant excess of
deaths could be found due to any cause in the cohort of 602 workers.
In the sub-cohort of 351 workers, there was a significant increase
in mortality rate due to kidney disease (3 compared with 0.4
expected). There was 1 death from gall-bladder cancer, 1 death
from urinary-bladder cancer, 1 death from brain cancer, and 1 death
from myeloid leukaemia. The stomach tumours were observed compared
with 1.8 expected (Thiess et al., 1981b). Since the workers were
also exposed to many other chemicals, such as butylene oxide,
epichlorohydrin, dioxan, dichloropropane, and chlorohydrins, the
Task Group was unable to evaluate this study in relation to
propylene oxide.
8.5. Mutagenicity and Related End-Points
In 43 male workers from the cohort of 602 workers discussed in
section 8.4 (Thiess et al., 1981b), no increase in chromosome
aberration rate was found in 2 groups of workers exposed to alkene
oxides for average periods of either 12.5 or 17.6 years; in addition,
workers in the latter group had at least one accidental high exposure
to ethylene oxide. The results were also negative in a group of
workers exposed once for a brief period following an accident. On
the other hand, the aberration rate was significantly elevated in
workers exposed for more than 20 years (Thiess et al., 1981a).
For the reason mentioned in section 8.4, the Task Group was
unable to evaluate this study in relation to propylene oxide.
Unscheduled DNA synthesis, induced in vitro by the mutagen
N-acetoxy-2-acetylaminofluorene, was inhibited in the lymphocytes
of 23 workers from a factory in Sweden, where starch was modified
with propylene oxide. The workers had been exposed for 1 - 20
years. At the time of the study, exposure was generally below a
time-weighted average of 28 mg/m3. However, short-term exposures
of up to 2370 mg/m3 were recorded for some workers (Pero et al.,
1982).
9. EVALUATION OF THE HEALTH RISKS FOR MAN AND EFFECTS ON
THE ENVIRONMENT
Exposure of man to propylene oxide mainly occurs through
inhalation at the work-place.
Data are insufficient to estimate the exposure to propylene
oxide residues in food after fumigation and sterilization. The
main conversion products in foodstuffs are chloropropanols and
1,2-propanediol, which are more persistent than the parent compound
(section 4.2). No adverse effects have been reported due to the
ingestion of propylene oxide and its reaction products in food.
No ambient air monitoring data are available, but the lowest
annual average concentration at distances of up to 20 km from
production plants has been assessed, by modelling, to be less than
4.836 x 10-8 mg/m3(section 4.1). The risk for human health
under such conditions of exposure is likely to be negligible.
Propylene oxide is highly soluble in water but is likely to
evaporate to a great extent. However, no data on the rate of
evaporation are available. In neutral fresh water, propylene
oxide is converted to 1,2-propanediol and, in marine waters, to
halopropanols, but, even in the presence of micro-organisms, these
processes are slow (section 3.2). Because of the low log n-octanol
water-partition coefficient, propylene oxide and its conversion
products are unlikely to bioaccumulate (Table 1). The toxicity of
propylene oxide for aquatic organisms is low. The available LC50s
are above approximately 90 mg/litre (section 6). Thus, the
probability of an adverse impact on the aquatic environment is
considered low.
Eight-hour time-weighted occupational exposure in propylene
oxide production and use is normally below 5 mg/m3, with
occasional peak exposures up to 9000 mg/m3 (section 4.3).
Inhaled propylene oxide is probably readily absorbed,
distributed throughout the body, and rapidly metabolized. The
half-life in rat tissues has been estimated to be 40 min (section
5.2). There are no data on skin absorption.
An aqueous solution of propylene oxide (100 or 200 g/litre)
is irritating to rabbit skin when applied under occlusive cover
(section 7.1.2). In man, corneal and conjunctival damage, and
allergic contact dermatitis have been reported through accidental
exposure to propylene oxide vapour (section 8.1).
The 4-h LC50s for the rat and the mouse were 9500 mg/m3
and 4100 mg/m3, respectively. Dogs exposed by inhalation, once
for 4 h, to a concentration of 3230 - 5880 mg/m3 showed
salivation, lachrymation, nasal discharge, and vomiting. Deaths
were observed at 4750 - 5880 mg/m3 but not at 3230 mg/m3
(section 7.1.3).
On repeated exposure of various species to propylene oxide
vapour, 7 h per day, 5 days per week, for 112 - 218 days, at
concentrations of 0, 240, 460, and 1080 mg/m3, rabbits and
monkeys did not show any adverse effects on appearance, mortality,
growth, and histopathology of internal organs. Rats and guinea-
pigs showed irritation of the respiratory passages and lung damage
histologically at 1080 mg/m3. An increase in lung weight was
observed in female guinea-pigs at concentrations of 460 mg/m3 or
more. No effect was observed in any species at a concentration of
240 mg/m3. A concentration of 3400 mg/m3 administered for 2
weeks, 6 h/day, 5 days per week, resulted in dyspnoea and death in
rats. In the same study, mice showed dyspnoea at 460 and 1150 mg/m3
(section 7.2.2).
Rats and mice exposed for 2 years showed increased incidences
of inflammatory and proliferative lesions of the nasal epithelium
from an exposure level of 470 mg/m3. In rats, the inflammatory
lesions and hyperplasia were also observed at an exposure level of
240 mg/m3, but not at 70 mg/m3 (section 7.4.2).
Depression of the central nervous system, the severity of
which increased with increasing level and length of exposure, was
observed when rats and mice were exposed by inhalation to single
high concentrations of propylene oxide (section 7.1.3). Although
no clinical central nervous system effects were reported when
monkeys were exposed to 237 and 717 mg/m3 for 2 years, some
histopathological changes were observed in the treated animals
(section 7.2.2). It should be noted that these data cannot be
evaluated for estimating hazard for man, since only 2 monkeys were
examined in each of the exposed groups, and the lesion appeared
also in one of the 2 controls.
Summarizing the results of animal studies (excluding
mutagenic, carcinogenic, and reproduction effects) the no-
observed-adverse-effect level for prolonged repeated 6- to 7-h
daily exposure is 70 mg/m3.
Propylene oxide administered by inhalation for 2 years
produced ovarian atrophy in mice at 940 mg/m3. Testicular
atrophy was found at 240 and 720 mg/m3 in rats (section 7.5).
No sperm head abnormalities were detected in mice after
exposure for 5 days to 720 mg/m3 or in Cynomolgus monkeys
exposed to 240 or 710 mg/m3 for 2 years, but sperm count and
motility were reduced, and sperm drive range (time to traverse a
linear path) increased in the monkeys. A reduced sperm motility
and damage to spermatocytes were observed in male rats treated with
an oral LD50 (520 mg/kg body weight) dose. A reduced fertility
was observed when these males were mated between 2 and 10 weeks
after exposure with normal females (sections 7.3, 7.5).
Reduced fetal ossification of vertebrae and ribs and wavy ribs
were observed when pregnant Sprague Dawley rats were exposed to
1190 mg propylene oxide/m3, 7 h per day, on days 1 - 16 of
gestation. When rats were additionally exposed for 3 weeks before
mating, the numbers of corpora lutea, implantations per dam, and
live fetuses were decreased. No teratogenic or fetotoxic effects
were found in New Zealand rabbits exposed to 1190 mg/m3 (section
7.5).
Propylene oxide is mutagenic in microorganisms and insects and
produces DNA damage and chromosomal aberrations in mammalian cells
in vitro. It produced micronuclei in mouse erythrocytes after
parenteral injection of 2 doses of 300 mg/kg body weight but not
after oral exposure. No chromosomal aberrations or sister
chromatid exchanges occurred in monkeys exposed by inhalation to
237 and 717 mg/m3, for 7 h/day, 5 days/week, for 2 years. No
dominant-lethal effects were observed in rats or mice following
inhalation or oral exposure, respectively, in 2 studies (section
7.3). No adequate data on chromosomal effects in human beings are
available.
In studies on the carcinogenicity of propylene oxide in rats
and mice, malignant tumours were mainly observed at the site of
entry into the body: for stomach, at 15 and 60 mg/kg body weight;
for subcutaneous tissues, at 1.0 - 2.5 mg; and, for nasal passages,
at 940 mg/m3. At the dose levels at which these tumours were
induced, propylene oxide produced tissue damage. This damage may
have played a role in the appearance of the tumours. Propylene
oxide also produced an increase in the incidence of multiple benign
mammary tumours in female rats following inhalation exposure
(section 7.4). No adequate epidemiological studies on cancer
incidence in exposed human populations have been carried out.
Taking into account the body of available data - the
alkylating nature of propylene oxide, the formation of DNA
adducts, the positive responses in in vitro mutagenesis assays,
the carcinogenic effects in animals at the sites of entry into the
body, and the absence of adequate data on cancer in human beings -
propylene oxide should be considered as a possible human carcinogen.
Thus, for practical purposes, propylene oxide should be regarded as
if it presented a carcinogenic risk for man, and levels in the
environment should be kept as low as feasible.
10. RECOMMENDATIONS FOR FURTHER RESEARCH
1. A thorough detailed study on the absorption, distribution,
and metabolism of propylene oxide should be conducted.
2. Biological indicators should be developed that quantify both
the incidence and severity of human exposure.
3. The epidemiological studies indicating an increased risk of
cancer in workers exposed to propylene oxide in combination
with other chemicals suggest that additional studies should
be conducted on populations whose exposure has been primarily
to propylene oxide. The adequate quantification of past
exposures should be a part of these studies.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
An International Agency for Research on Cancer Working Group
(IARC, 1985) evaluated the carcinogenicity of propylene oxide and
concluded that:
"There is sufficient evidence for the carcinogenicity of
propylene oxide to experimental animals; there is inadequate
evidence for its carcinogenicity to humans. It is noted that,
in the absence of adequate data in humans, it is reasonable,
for practical purposes, to regard chemicals for which there is
sufficient evidence of carcinogenicity in experimental animals
as if they represented a carcinogenic risk to humans."
REFERENCES
ADDY, J.K. & PARKER, R. (1963) The mechanism of epoxide
reactions. V. The reaction of 1,2-epoxypropane with chloride
ions in water under neutral and acidic conditions. J. Chem.
Soc., 915-921.
ANDERSON, G.E. (1983) Human exposure to atmospheric
concentrations of selected chemicals. Vol. II, Research
Triangle Park, North Carolina, US Environmental Protection
Agency, pp. 574-601 (Prepared by Systems Applications, Inc.,
San Rafael, California, No. SA1/EF81-156R2).
ANTONOVA, V.I., ZOMMER, E.A., KUZNETSOVA, A.D., & PETROVA,
N.A. (1981) [Toxicology of propylene oxide and regulation
for surface water.] Gig. i Sanit., 46: 76-79 (in Russian).
BOGYO, D.A., LANDE, S.S., MEYLAND, W.M., HOWARD, P.H., &
SANTODONATO, J. (1980) Investigation of selected potential
environmental contaminants: Epoxides, Syracuse, New York,
Centre for Chemical Hazard Assessment, Syracuse Research
Corporation (Prepared for US EPA) (US EPA 560/11-80-005, PB
80-183197).
BONT, J.A.M. DE, DIJKEN, J.P. VAN, & GINKEL, K.G. VAN (1982)
The metabolism of 1,2-propanediol by the propylene oxide
utilizing bacterium Nocardia 60. Biochem. Biophys. Acta, 714:
465-470.
BOOTMAN, J., LODGE, D.C., & WHALLEY, H.E. (1979) Mutagenic
activity of propylene oxide in bacterial and mammalian
systems. Mutat. Res., 67: 101-112.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979a) The acute
toxicity of some petrochemicals to goldfish. Water Res., 13:
623-626.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) BOD and
COD of some petrochemicals. Water Res., 13: 627-630.
CALLEMAN, C.J., EHRENBERG, L., JANSSON, B., OSTERMAN-GOLKAR,
S., SEGERBACK, D., SVENSSON, K., & WACHTMEISTER, C.A. (1978)
Monitoring and risk assessment by means of alkyl groups in
haemoglobin in persons occupationally exposed to ethylene
oxide. J. environ. Pathol. Toxicol., 2: 247-442.
CONWAY, R.A., WAGGY, G.T., SPIEGEL, M.H., & BERGLUND, R.L.
(1983) Environmental fate and effects of ethylene oxide.
Environ. Sci. Technol., 17: 107-112.
COOKSON, M.J., SIMS, P., & GROVER, P.L. (1971) Mutagenicity
of epoxides of polycyclic hydrocarbons correlates with
carcinogenicity of parent hydrocarbons. Nature (New Biol.),
234: 186-187.
CREWS, R.C. (1974) Effects of propylene oxide on selected
species of fishes, Florida, Air Force Armament Center, Eglin
Air Force Base (US NTIS AD/A003637).
CUPITT, L.T. (1980) Fate of toxic and hazardous materials in
the environment, Research Trianlge Park, North Carolina, US
Environmental Protection Agency, Environmental Sciences
Laboratory, Office of Research and Development (US EPA
600/3-80-084, PB 80-221948).
DAVIES, R.E., HARPER, K.H., & KYNOCH, S.R. (1972)
Inter-species variation in dermal reactivity. J. Soc. Cosmet.
Chem., 23: 371-382.
DEAN, B.J. & HODSON-WALKER, G. (1979) An in vitro chromosome
assay using cultured rat liver cells. Mutat. Res., 64: 329-337.
DENT, J.G. & SCHNELL, S.R. (1981) Inhibition of microsomal
membrane-bound and purified epoxide hydrolase by C2-C8
1,2-alkene oxides. Biochem. Pharmacol., 30: 1712-1714.
DUNKELBERG, H. (1981) [Carcinogenic activity of ethylene
oxide and its reaction products 2-chloroethanol,
2-bromoethanol, ethylene glycol, and diethylene glycol. I.
Carcinogenicity of ethylene oxide in comparison with
1,2-propylene oxide after subcutaneous administration in
mice.] Zbl. Bakt. Hyg. (I. Abt. Orig. B), 174: 383-404 (in
German).
DUNKELBERG, H. (1982) Carcinogenicity of ethylene oxide and
1,2-propylene oxide upon intragastric administration to rats.
Br. J. Cancer, 46: 924-933.
EHRENBERG, L., HIESCHE, K.D., OSTERMAN-GOLKAR, S., & WENNBERG,
I. (1974) Evaluation of genetic risks of alkylating agents:
Tissue doses in the mouse from air contaminated with ethylene
oxide. Mutat. Res., 24: 83-103.
FAO/WHO JOINT EXPERT COMMITTEE ON FOOD ADDITIVES (1974) WHO
Food Add. Ser., 5: 491-497 (Toxicological Monographs arising
from the Seventeenth Report of the Joint FAO/WHO Expert
Committee on Food Additives).
FARMER, P.B., GORF, S.M., & BAILEY, E. (1982) Determination
of hydroxypropylhistidine in haemoglobin as a measure of
exposure to propylene oxide using high resolution gas
chromatography mass spectrometry. Biomed. Mass Spectrometr.,
9: 69-71.
FEDERAL REGISTER (1971) Paragraph 121.1076, propylene oxide,
subpart D, food additives, Washington DC, US Environmental
Protection Agency, Food and Drug Administration, p. 33.
FJELLSTEDT, T.A., ALLEN, R.H., DUNCAN, B.K., & JAKOBY, W.B.
(1973) Enzymatic conjugation of epoxides with glutathione. J.
Biol. Chem., 248: 3702-3707.
FLORES, G.H. (1983) Reducing plant pollution exposure:
controlling exposure to alkene oxides. Chem. Eng. News, 79:
39-43.
GARRO, A.J. & PHILLIPS, R.A. (1980) Detection of
mutagen-induced lesions in isolated DNA by marker rescue of
Bacillus subtilis phage stopb105. Mutat. Res., 73: 1-13.
GUENGERICH, F.P. & MASON, P.S. (1980) Alcohol dehydrogenase-
coupled spectrophotometric assay of epoxide hydratase
activity. Anal. Biochem., 104: 445-451.
GUNTHER, D.A. (1965) Determination of adsorbed ethylene and
propylene oxides by distillation and titration. Anal. Chem.,
37: 1172-1173.
HACKETT, P.L., BROWN, M.G., BUSCHBOM, R.L., CLARK, M.L.,
MILLER, R.A., MUSIC, R.L., ROWE, S.E., SCHIRMER, R.E., &
SIKOV, M.R. (1982) Teratogenic study of ethylene and
propylene oxide and N-butyl acetate, Richland, Washington,
Batelle Pacific Northwest Laboratories (Prepared for NIOSH)
(PB 83-258038).
HARDIN, B.D., SCHULER, R.L., MCGINNIS, P.M., NIEMEIER, R.W., &
SMITH, R.J. (1983) Evaluation of propylene oxide for
mutagenic activity in 3 in vivo test systems. Mutat. Res.,
117: 337-344.
HATFIELD, R. (1957) Biological oxidation of some organic
compounds. Ind. Eng. Chem., 49: 192-196.
HELLMAN, T.M. & SMALL, F.H. (1974) Characterization of the
odour properties of 101 petrochemicals using sensory method.
J. Air Pollut. Contr. Assoc., 24: 979-982.
HEMMINKI, K. & FALCK, K. (1979) Correlation of mutagenicity
and 4-(p-nitrobenzyl)-pyridine alkylation by epoxides.
Toxicol. Lett., 4: 103-106.
HEMMINKI, K., PAASIVIRTA, J., KURKIRINNE, T., & VIRKKI, L.
(1980) Alkylation products of DNA bases by simple epoxides.
Chem.-biol. Interact., 30: 259-270.
HESLOT, H. (1962) Etude quantitative de réversions
biochimiques induites chez la levure Schizosaccharomyces pombe
par des radiations et des substances radiomimétiques. Abh.
Dtsch. Akad. Wiss. Berlin, Kl. Med., 1: 193-228.
HEUSER, S.G. & SCUDAMORE, K.A. (1969) Determination of
fumigant residues in cereals and other foodstuffs: A multi-
detection scheme for gas chromatography of solvent extracts.
J. Sci. Food Agric., 20: 566-572.
HIRASHIMA, T., OGUMA, T., HOSOGAI, Y., & FUJII, S. (1970)
[Studies on gaseous antimicrobial agents. III. Determination
of propylene oxide residue in food wrappings and containers.]
J. Food Hyg. Soc. Jpn, 11: 161-163 (in Japanese).
HUSSAIN, S. & OSTERMAN-GOLKAR, S. (1984) Dose-response
relationships for mutations induced in E. coli by some model
compounds. Hereditas, 101: 57-68.
IARC (1976) Cadmium, nickel, some epoxides, miscellaneous
industrial chemicals and general considerations on volatile
anaesthetics, Lyons, International Agency for Research on
Cancer, 293 pp (IARC Monographs on the Evaluation of
Carcinogenic Risk of Chemicals to Man, Vol. 11).
IARC (1985) Allyl compounds, aldehydes, epoxides, and
peroxides, Lyons, International Agency for Research on Cancer
(Monographs on the Evaluation of Carcinogenic Risk of
Chemicals to Man, Vol. 36).
IRPTC (1984) Data profile on propylene oxide, Geneva,
Switzerland, International Register on Potentially Toxic
Chemicals, United Nations Environment Programme.
JACOBSON, K.H., HACKLEY, E.B., & FEINSILVER, L. (1956) The
toxicity of inhaled ethylene oxide and propylene oxide
vapours. Arch. ind. Health, 13: 237-244.
JENSEN, O. (1981) Contact allergy to propylene oxide and
isopropyl alcohol in a skin desinfectant swab. Contact
Dermatit., 7: 148-150.
KETEL, W.G. VAN (1979) Contact dermatitis from propylene
oxide. Contact Dermatit., 5: 191-192.
KOLMARK, H.G. & GILES, N.H. (1955) Comparative studies of
monoepoxides as inducers of reverse mutations in Neurospora.
Genetics, 40: 890-902.
KOSKIKALLIO, J. & WHALLEY, E. (1959) Effect of pressure on
the spontaneous and the base-catalysed hydrolyses of epoxides.
Can. J. Chem., 37: 783-787.
KROST, K.J., PELLIZZARI, E.D., WALBURN, S.G., & HUBBARD, S.A.
(1982) Collection and analysis of hazardous organic
emissions. Anal. Chem., 54: 810-817.
LAWLEY, P.D. & JARMAN, M. (1972) Alylation by propylene
oxide of deoxyribonucleic acid, adenine, guanosine, and
deoxyguanylic acid. Biochem. J., 126: 893-900.
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., BURG, J.R., GROTH,
D.H., KHAN, A., ACKERMAN, L.J., & COCKRELL, B.Y. (1984a)
Carcinogenic and toxicologic effects of inhaled ethylene oxide
and propylene oxide in F344 rats. Toxicol. appl. Pharmacol.,
76(1): 69-84.
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., BURG, J.R., GULATI,
D.K., KAUR, P., & SABHARWAL, P.S. (1984b) Sister-chromatid
exchanges and chromosome aberrations in lymphocytes from
monkeys exposed to ethylene oxide and propylene oxide by
inhalation. Toxicol. appl. Pharmacol., 76(1): 85-95.
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., LAL, J.B., BURG,
J.R., GULATI, D.K., ZAVOS, P.M., & SABHARWAL, P.S. (1984c)
Toxic and mutagenic effects of inhaled ethylene oxide and
propylene oxide on spermatogenic functions in monkeys.
Toxicologist, 3: 60.
MCLAUGHLIN, R.S. (1946) Chemical burns of the human cornea.
Am. J. Ophthalmol., 29: 1355-1362.
MCMAHON, R.E., CLINE, J.C., & THOMPSON, C.Z. (1979) Assay of
855 test chemicals in ten tester strains using a new
modification of the Ames test for bacterial mutagens. Cancer
Res., 39: 682-693.
MESTRES, R. & BARROIS, C. (1964) Recherche et dosage de
l'oxyde de propylène et du propylène-glycol dans les fruits
par chromatographie gazeuse directe. Bull. Soc. Pharm.
Montpellier, 24: 47-63.
MIGLIORE, L., ROSSI, A.M., & LOPRIENO, N. (1982) Mutagenic
action of structurally-related alkene oxides on Schizosac-
charomyces pombe: The influence, "in vitro", of mouse liver
metabolizing system. Mutat. Res., 102: 425-437.
MISHMASH, E.H. & MELOAN, C.E. (1972) Indirect
spectrophotometric determination of nanomale quantities of
oxiranes. Anal. Chem., 44: 835-836.
NELIS, H.J.C.F. & SINSHEIMER, J.E. (1981) A sensitive
fluorimetric procedure for the determination of aliphatic
epoxides under physiological conditions. Anal. Biochem., 115:
151-157.
NIOSH (1977) Manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, US Department of Health, Education and
Welfare, National Institute for Occupational Safety and
Health, Vol. 2, pp. S75-1 - S75-8.
OESCH, F. (1974) Purification and specificity of a human
microsomal epoxide hydratase. Biochem. J., 139: 77-88.
OGUMA, T., HOSOGAI, Y., FUJII, S., & KAWASHIRO, I. (1968)
[Gaseous antimicrobial agents. I. Determination of propylene
oxide residues in foods.] J. Food Hyg. Soc. Jpn, 9: 395-398
(in Japanese).
OGUMA, T., HOSOGAI, Y., & FUJII, S. (1969) [Studies on
gaseous antimicrobial agents. II. Determination of propylene
oxide residue in fats and oils.] J. Food Hyg. Soc. Jpn, 10:
37-39 (in Japanese).
OSTERMAN-GOLKAR, S., EHRENBERG, L., SEGERBACK, D., &
HALLSTROM, I. (1976) Evaluation of genetic risks of
alkylating agents. II. Haemoglobin as a dose monitor. Mutat.
Res., 34: 1-10.
OSTERMAN-GOLKAR, S., FARMER, P.B., SEGERBACK, D., BAILEY, E.,
CALLEMAN, C.J., SVENSSON, K., & EHRENBERG, L. (1983)
Dosimetry of ethylene oxide in the rat by quantitation of
alkylated histidine in haemoglobin. Teratog. Carcinog.
Mutagen., 3: 395-405.
OSTERMAN-GOLKAR, S., BAILEY, E., FARMER, P.B., GORF, S.M., &
LAMB, J.H. (1984) Monitoring exposure to propylene oxide
through the determination of haemoglobin alkylation. Scand. J.
Work environ. Health, 10: 99-102.
PERO, R.W., BRYNGELSSON, T., WIDEGREN, B., HOGSTEDT, B., &
WELINDER, H. (1982) A reduced capacity for unscheduled DNA
synthesis in lymphocytes from individuals exposed to propylene
oxide and ethylene oxide. Mutat. Res., 104: 193-200.
PFEIFFER, E.H. & DUNKELBERG, H. (1980) Mutagenicity of
ethylene oxide and propylene oxide and of the glycols and
halohydrins formed from them during the fumigation of
foodstuffs. Food Cosmet. Toxicol., 18: 115-118.
PHILLIPS, R.A., ZAHLER, S.A., & GARRO, A.J. (1980) Detection
of mutagen-induced lesions in isolated DNA using a new
Bacillus subtilis transformation-based assay. Mutat. Res., 74:
267-281.
RADDING, S.B., LUI, D.H., JOHNSON, H.L., & MILL, T. (1977)
Review of the environmental fate of selected chemicals,
Washington DC, US Environmental Protection Agency, Office of
Toxic Substances (US EPA 560/5-77-003, PB 267121).
RAGELIS, E.P., FISHER, B.S., KLIMECK, B.A., & JOHNSON, C.
(1968) Isolation and determination of chlorohydrins in foods
fumigated with ethylene oxide or with propylene oxide. J.
Assoc. Offic. Agric. Chem., 51: 709-715.
RANDERATH, K., REDDY, M.V., & GUPTA, R.C. (1981) 32P-
labelling test for DNA damage. Proc. Natl Acad. Sci., 78:
6126-6129.
REUZEL, P.G.J. & KUPER, C.F. (1983) Chronic (28-month)
inhalation toxicity/carcinogenicity study of 1,2-propylene
oxide in rats. Final Report, Zeist, The Netherland, TNO,
Division of Nutrition and Food Research (Report No.
V82.215/280853).
REUZEL, P.G.J. & KUPER, C.F. (1984) Chronic (28-month)
inhalation toxicity/carcinogenicity study of 1,2-propylene
oxide in rats, Zeist, The Netherland, TNO, Division of
Nutrition and Food Research (Addendum to Report No.
V82.215/280853).
ROSS, W.C.J. (1950) The reactions of certain epoxides in
aqueous solutions. J. Chem. Soc., 2257-2271.
ROWE, V.K., HOLLINGSWORTH, R.L., OYEN, F., MCCOLLISTER, D.D.,
& SPENCER, H.C. (1956) Toxicity of propylene oxide deter-
mined on experimental animals. Arch. ind. Health, 13: 228-236.
RUDDICK, J.A. (1972) Toxicology, metabolism, and biochemis-
try of 1,2-propanediol. Toxicol. appl. Pharmacol., 21: 102-111.
RUSSELL, J.W. (1975) Analyses of air pollutants using
sampling tubes and gas chromatography. Environ. Sci. Technol.,
9: 1175-1178.
SCUDAMORE, K.A. & HEUSER, S.G. (1971) Ethylene oxide and its
persistent reaction products in wheat flour and other
commodities: Residues from fumigation or sterilization, and
effects of processing. Pestic. Sci., 2: 80-91.
SINA, J.F., BEAN, C.L., DYSART, G.R., TAYLOR, V.I., & BRADLEY,
M.O. (1983) Evaluation of the alkaline elution/rat
hepatocyte assay as a predictor of carcinogenic/mutagenic
potential. Mutat. Res., 113: 357-391.
SMYTH, H.F., SEATON, J., & FISHER, L. (1941) The single dose
toxicity of some glycols and derivatives. J. ind. Hyg.
Toxicol., 23: 259-268.
SNELLINGS, W.M., WEIL, C.S., & MARONPOT, R.R. (1984) A
two-year inhalation study of the carcinogenic potential of
ethylene oxide in Fischer 344 rats. Toxicol. appl. Pharmacol.,
75: 105-117.
SPRINZ, H., MATZKE, H., & CARTER, J. (1982) Neuropatho-
logical evaluation of monkeys exposed to ethylene and
propylene oxide, Kansas City, Missouri, Midwest Research
Institute (Prepared for NIOSH) (PB 83-134817).
STEELE, L. & HADZIYEV, D. (1976) Sterilization of dehydrated
potato granules with propylene oxide. Z. Lebensm.
Unters.-Forsch., 162: 387-399.
STOCKER, W.G. & THIESS, A.M. (1979) Morbidity study on
workers exposed to ethylene oxide/propylene oxide. In: The 7th
Medichem Congress, Gera, 11-15 September, 1979.
STORCK, W.J. (1981) Output of big-volume chemicals fell in
1980. Chem. Eng. News, 59: 36-39.
SVENSSON, K. & OSTERMAN-GOLKAR, S. (1984) Kinetics of
metabolism of propene and covalent binding to macromolecules
in the mouse. Toxicol. appl. Pharmacol., 73: 363-372.
SWAN, J.D. (1954) Determination of epoxides with sodium
sulfite. Anal. Chem., 26: 878-880.
TACHIZAWA, H., MACDONALD, T.L., & NEAL, R.A. (1982) Rat
liver microsomal metabolism of propyl halides. Mol.
Pharmacol., 22: 745-751.
THIESS, A.M., SCHWEGLER, H., FLEIG, I., & STOCKER, W.G.
(1981a) Mutagenicity study of workers exposed to alkylene
oxides (ethylene oxide/propylene oxide) and derivatives. J.
occup. Med., 23: 343-347.
THIESS, A.M., FRENZEL-BEYME, R., LINK, R., & STOCKER, W.H.
(1981b) Mortality study on employees exposed to alkylene
oxides (ethylene oxide/propylene oxide) and their derivatives.
In: Proceedings of the International Symposium on Prevention
of Occupational Cancer, Helsinki, Finland, pp. 249-259.
US EPA (1981) Human exposure to atmospheric concentrations
of selected chemicals, Research Triangle Park, North Carolina,
US Environmental Protection Agency, Office of Air Quality
Planning and Standards (US NTIS PB 84-102540, PB 83-265249).
US NTP (1984) Toxicology and carcinogenesis studies of
propylene oxide in F344/N rats and B6C3F1 mice (inhalation
studies), Research Triangle Park, North Carolina, National
Toxicology Program (NTP 83-020) (NIH Publication No. 84-2523).
VOJNOVICH, C. & PFEIFER, V.F. (1967) Reducing the microbial
population of flour during milling. Cereal Sci. Today, 12:
54-60.
VOOGD, C.E., STEL, J.J. VAN DE, & JACOBS, J.J.J.A.A. (1981)
The mutagenic action of aliphatic epoxides. Mutat. Res., 89:
269-282.
WADE, D.R., AIRY, S.C., & SINSHEIMER, J.E. (1978)
Mutagenicity of aliphatic epoxides. Mutat. Res., 58: 217-223.
WALLES, S.A. (1974) The influence of some alkylating agents
on the structure of DNA in vitro . Chem.-biol. Interact., 9:
97-103.
WEBBER, D. (1984) Basic chemical output returns to growth.
Top 50 chemical products. Chem. Eng. News, May 7: 8-10.
WEIL, C.S., CONDRA, N., HAUN, C., & STRIEGEL, J.A. (1963)
Experimental carcinogenicity and acute toxicity of
representative epoxides. Am. Ind. Hyg. Assoc. J., 24: 305-325.
WHO (1978) Environmental health problems associated with the
manufacture and uses of synthetic organic chemicals, Geneva,
World Health Organization (Report No. HCS/78.2).
WHO (1985) EHC 55: Ethylene oxide, Geneva, World Health
Organization, 79 pp.
ZAMORA, P.O., BENSON, J.M., LI, A.P., & BROOKS, A.L. (1983)
Evaluation of an exposure system using cells grown on collagen
gels for detecting highly-volatile mutagens in the CHO/HGPRT
mutation assay. Environ. Mutagen., 5: 795-801.
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
In an acute toxicity test on goldfish (Carassius auratus),
in static fresh water, at 20 °C, pH between 6 and 8, and a
dissolved oxygen content greater than 4 mg/litre, the 24-h LC50
for propylene oxide was 170 mg/litre (Bridié et al., 1979a). In
other static tests, with the fresh water species bluegill sunfish
(Lepomis machrochirus) and mosquito fish (Gambusia affinis),
96-h LC50 values for propylene oxide were 215 and 141 mg/litre,
respectively, at 23 °C. For the common mullet (Mugil cephalus),
a marine fish, a static 96-h LC50 for propylene oxide of 89
mg/litre was determined at 23 °C and a water salinity of 1.5% (no
water analysis was reported) (Crews, 1974). The 24-h LC50 for
1,2-propanediol (a product of propylene oxide) for goldfish was
over 5000 mg/litre (Bridié et al., 1979a). Propylene oxide is very
soluble in aqueous media; the log n-octanol water partition
coefficient was reported to be -0.13 (Radding et al., 1977) and,
therefore, it is not expected to bioaccumulate.
7. EFFECTS ON ANIMALS
7.1. Single Exposures
7.1.1. Oral exposure
In 2 studies, the oral LD50s for the rat were reported to be
1140 mg/kg body weight and 520 mg/kg body weight for males, and
540 mg/kg for females (Smyth et al., 1941; Antonova et al., 1981);
the difference between 16 and 84% mortality was caused by a dose of
only approximately 300 mg/kg body weight, indicating a rather steep
dose-effect relationship. In one of these studies, oral LD50s for
male mice and guinea-pigs were 630 and 660 mg/kg body weight,
respectively (Antonova et al., 1981).
At lethal oral doses, necrosis of the stomach mucosa was
observed in rats. Succinate dehydrogenase (EC 1.3.99.1) activity
was reported to be decreased in the stomach mucosa. The liver
cells showed oedema and fatty changes. In serum, alanine amino-
transferase (EC 2.6.1.2) and aspartate aminotransferase (EC 2.6.1.1)
activity and histamine levels were increased. Kidney function was
disturbed. Mature lymphocytes were reduced in the spleen (Antonova
et al., 1981).
7.1.2. Skin and eye irritation
Solutions of 100 or 200 g propylene oxide/litre water, applied
under a plastic cover on the intact skin of rabbits produced hyper-
aemia, oedema, and finally scars after 6 or more min of exposure.
The intensity of the effects was proportional to the exposure time
(Rowe et al., 1956).
No, or only slight, irritation was observed when undiluted
1,2-propanediol, a possible reaction product of propylene oxide in
water, was applied to the skin of various animals under occlusive
conditions for 24 or 72 h (Davies et al., 1972).
When 5 µl of undiluted propylene oxide was applied once on the
centre of the cornea of rabbits, a severe burn resulted with
necrosis (Weil et al., 1963).
7.1.3. Inhalation exposure
In inhalation studies, the 4-h LC50s for rats and mice were
9500 and 4100 mg/m3, respectively (Jacobson et al., 1956). The
lowest lethal concentration (US NTP, 1984) or the 0.1% mortality
level (Jacobson et al., 1956) for 4-h exposures was approximately
5250 mg/m3 for rats and 900 mg/m3 for mice. In both species,
100% mortality was reached after 4 h of exposure at levels above
17 000 mg/m3 (Jacobson et al., 1956). In rats, 100% mortality
was also reported after a 30-min exposure to 38 000 mg/m3 or a
7-h exposure to 9500 mg/m3 (Rowe et al., 1956).
After inhalation of propylene oxide, rodents showed eye and
nose irritation, nasal discharge, dyspnoea, and depression of the
central nervous system, the severity of which increased with
increasing level and length of the exposure (effect levels not
specified) (Rowe et al., 1956). Gross pathology revealed
distension of the stomach only at lethal concentrations (Jacobson
et al., 1956). No organ damage was observed in rats exposed to
2370 mg/m3 for 7 h, 4740 mg/m3 for 4 h, or 9480 mg/m3 for 0.5 h
(Rowe et al., 1956). In 4 groups, each containing 3 dogs, exposed
for 4 h to propylene oxide concentrations of 3230, 4750, 4810, or
5880 mg/m3, lachrymation, salivation, nasal discharge, and vomiting
occurred. Congestion in the lungs and trachea, oedema of pulmonary
tissues, and necrosis of bronchiolar epithelium were observed at
4810 and 5880 mg/m3. Deaths occurred in the groups exposed to
concentrations of 4750 mg/m3 or more (Jacobson et al., 1956).
7.2. Repeated Exposures
7.2.1. Oral exposure
Propylene oxide was administered in the drinking-water to 4
groups of rats of both sexes at dose levels calculated by the
authors to be 0.52, 5.2, 52, or 520 µg/kg body weight per day, over
a 26-week period. At the highest dose level, polyuria, haematological
abnormalities, lowering of serum-albumin, increased serum-beta-
globulin levels, and increased activity of gastrointestinal mucosal
enzymes were observed. Mild haematological abnormalities occurred
at the next lower dose, while no adverse effects were noted at the
two lowest doses (Antonova et al., 1981).
7.2.2. Inhalation exposure
In range-finding tests for a carcinogenicity study, groups of 5
male and 5 female Fischer 344/N rats were exposed to 0, 110, 230,
460, 1150, or 3400 mg propylene oxide/m3 air for 5 days per week,
and 6 h per day, during a 2-week exposure period. B6C3F1 mice were
exposed for the same period to 0, 50, 110, 230, 460, or 1150 mg/m3.
All animals were necropsied on the 12th day. No pathological
effects were observed. Body weights were not affected. Dyspnoea
and gasping were observed in rats, one of which died at 3400 mg/m3.
Mice showed dyspnoea at 460 and 1150 mg/m3. Both species were
less active at the highest exposure. Rats also showed irregular
limb movements and diarrhoea. After 13 weeks of a similar exposure
regime up to concentration of 1150 mg/m3, rats and mice did not
show any gross- or histopathological effects. Body weights were
reduced at 1150 mg/m3 only (US NTP, 1984).
Groups of 10 - 20 rats, 8 guinea-pigs, 1 or 2 rabbits of both
sexes, and 1 or 2 female Rhesus monkeys were exposed to 0, 240,
460, or 1080 mg propylene oxide/m3 air for 112 - 218 days, for
7 h per day, and 5 days per week. Rabbits and monkeys did not
show any adverse effects with regard to appearance, behaviour,
mortality rate, growth, organ weights, and gross- and histopathology.
Rats showed irritation of the eyes and respiratory passages and an
increased mortality rate due to pneumonia at 1080 mg/m3. Guinea-
pigs also showed irritation of the eyes and respiratory passages at
this exposure level, but no increase in mortality rate. In
addition, the growth of the female guinea-pigs was reduced. Livers
exhibited slight fatty degeneration in male guinea-pigs. The
relative weights of the lungs of the guinea-pigs were slightly, but
significantly, increased in both sexes at 1080 mg/m3, and in
females at 460 mg/m3. It should be noted that control lung
weights were low. Histopathological findings were alveolar
haemorrhages and oedema, and interstitial oedema and hyperaemia in
the lungs of guinea-pigs after 157 days of exposure to 1080 mg/m3.
After 37 - 39 days of exposure to 1080 mg/m3, these histopatho-
logical effects had also been observed in rats (Rowe et al., 1956).
The possibility of the induction of neuropathological effects
by propylene oxide was investigated in groups of 12 male Cynomolgus
monkeys. The animals were exposed to actual concentrations of 0,
237, and 717 mg/m3 propylene oxide, for 7 h per day, 5 days per
week, for 2 years. In 2 monkeys per group, brain, ulnar and
sciatic nerves, and spinal cord were examined histologically after
exposure. No clinical signs were reported. The only treatment-
related neuropathological lesions found were in the medulla
oblongata of the brain. Axonal dystrophy was observed in the
nucleus gracilis in all of the 4 treated monkeys examined, without
any apparent dose-effect relationship. The same lesion was observed
in one of the two control monkeys (Sprinz et al., 1982).
7.3. Mutagenicity and Related End-Points
A summary of mutagenicity tests with positive results is
presented in Table 3. Propylene oxide is mutagenic in bacteria,
fungi, and insects; no such studies with negative results have been
reported.
Table 3. Mutagenic tests for propylene oxide with positive resultsa
---------------------------------------------------------------------------------------------------------
Test description System description Reference
Organism Strain/cell type
---------------------------------------------------------------------------------------------------------
Gene mutations
Forward mutations virus Bacillus subtilis Garro & Phillips (1980)b
phage phi 105
Reverse mutations (base-pair bacterium Escherichia coli WP2 McMahon et al. (1979)c
substitutions) Escherichia coli WP2 McMahon et al. (1979)c
Escherichia coli WP2 Hemminki & Falck (1979)
Escherichia coli WP2 Bootman et al. (1979)c
CM871, CM891
Bacillus subtilis Phillips et al. (1980)
Salmonella typhimurium Wade et al. (1978)
TA 1535, TA 100 McMahon et al. (1979)c;
Bootman et al. (1979)c;
Pfeiffer & Dunkelberg
(1980)d
Reverse mutations bacterium Escherichia coli SD4 Hussain & Osterman-Golkar
(1984)
Forward mutations Klebsiella pneumoniae Voogd et al. (1981)
Reverse mutations fungus Neurospora crassa W40 Kolmark & Giles (1955)
macroconidia
Schizosaccharomyces Heslot (1962)
pombe
Forward mutations fungus Schizosaccharomyces Migliore et al. (1982)c
pombe Pl
Sex-linked recessive lethals insect Drosophila melanogaster Hardin et al. (1983)
Forward mutations on specific mammal Chinese hamster Zamora et al. (1983);
locus (in vitro) ovary cells
---------------------------------------------------------------------------------------------------------
Table 3. (contd.)
-------------------------------------------------------------------------------------------------------------------
Test description System description Reference
Organism Strain/cell type
-------------------------------------------------------------------------------------------------------------------
DNA damage
Single-strand breaks bacterium Bacillus subtilis Phillips et al. (1980)b
mammal (in vitro) rat hepatocytes Sina et al. (1983)
calf thymus DNA Walles (1974)
Chromosome damage
Chromatid gaps and breaks, mammal (in vitro) human lymphocytes Bootman et al. (1979)
Chromosome breaks, fragments
Chromatid gaps, deletions, mammal (in vitro) epithelial rat liver Dean & Hodson-Walker
exchanges cells (1979)c
Micronuclei mammal (ip) mouse polychromatic Bootman et al. (1979)
(2 x 300 mg/kg) erythrocytes
-------------------------------------------------------------------------------------------------------------------
a For details of these studies, see text and data profile (IRPTC, 1984).
b Treatment of isolated DNA in vitro, followed by transformation (bacteria) or transfection (virus).
c A similar or slightly reduced effect after metabolic activation by S9 rat or mouse liver fraction.
d Chloropropanols were also mutagenic, but not as mutagenic as propylene oxide; 1,2-propanediol was not
mutagenic.
Propylene oxide causes single-strand breaks in isolated calf
thymus DNA, probably by alkylation of the phosphodiester bond
(Walles, 1974). Single-strand breaks were observed in the DNA of
isolated rat hepatocytes at concentrations that were not toxic
(Sina et al., 1983).
Propylene oxide caused chromosome aberrations in mammalian
cells in vitro, in particular, chromatid gaps and breaks (Bootman
et al., 1979; Dean & Hodson-Walker, 1979). However, no increases
in chromosome aberrations or sister-chromatid exchanges were found
in peripheral lymphocytes of male Cynomolgus monkeys, after long-
term vapour exposure, in vivo. The animals were exposed in
groups of 12 to concentrations of 0, 237, and 717 mg propylene
oxide/m3 air, for 7 h per day, 5 days per week, for 2 years
(Lynch et al., 1984b).
Two oral doses of up to 500 mg propylene oxide/kg body weight,
in gum tragacanth, administered within 24 h to mice, did not induce
micronuclei in the polychromatic erythrocytes. In contrast, 2
intraperitoneal doses of 300 mg/kg body weight in water, administered
within 24 h, gave a 5-fold increase over controls. No effects were
observed following ip administration of 2 doses of 75 mg/kg or 150
mg/kg (Bootman et al., 1979).
The results of the dominant-lethal assay were negative when
male rats were exposed, for 7 h per day, to propylene oxide vapour
at a concentration of 720 mg/m3, for 5 days prior to mating
(Hardin et al., 1983). A negative result was also obtained in a
dominant lethal assay in which male mice received, once a day, 0,
50, or 250 mg propylene oxide/kg body weight in gum tragacanth, by
gavage, for 2 weeks prior to mating (Bootman et al., 1979).
No increased frequency of abnormal sperm heads was observed,
1 - 9 weeks after exposure of mice for 5 days to propylene oxide
vapour at a concentration of 720 mg/m3, for 7 h per day (Hardin
et al., 1983). Similarly, no increase was observed in the frequency
of abnormal sperm heads in the groups of Cynomolgus monkeys exposed
for 2 years (Lynch et al., 1984c). These monkeys were used by
Sprinz et al. (1982) (section 7.2.2) and Lynch et al. (1984b)
(section 7.3).
7.4. Carcinogenicity
7.4.1. Oral exposure
Groups of 50 female Sprague Dawley rats received 2 doses of
15.0 or 60.0 mg propylene oxide/kg body weight per week, by gavage,
for a total of 112 weeks. The compound was dissolved in salad oil
and administered to fasting rats. Two groups of 50 female rats
served as vehicle controls or untreated controls. There was an
exposure-free period between the 79th and 82nd week because of an
outbreak of pneumonia. The rats were observed up to 150 weeks.
No statistical analysis of the results was reported. Mortality
rates were not affected by the exposures. There was a dose-related
incidence of squamous cell carcinoma of the forestomach. In
addition, one early carcinoma of the forestomach was observed. The
numbers of rats affected were 0, 2, and 19 in animals receiving 0,
15, and 60 mg/kg body weight, respectively. The combined incidences
of hyperkeratosis, hyperplasia, and papillomas were 0, 7, and 17 at
0, 15, and 60 mg/kg body weight, respectively. At the highest dose
level, one adenocarcinoma of the pylorus was also observed. There
was no treatment-related increase of any other tumour type
(Dunkelberg, 1982).
7.4.2. Inhalation exposure
The following studies have been reported by the US NTP (1984).
Groups of 50 Fischer 344/N rats and 50 B6C3F1 mice of each sex were
exposed to average propylene oxide vapour concentrations of 0, 470,
and 940 mg/m3 air, for 6 h per day, 5 days per week, over a 103-
week period. Three accidental over-exposures at the highest
exposure level occurred. The concentrations did not exceed 15 300
mg/m3 for a maximum of 12 min during these periods.
In rats, survival rate was not affected and was over 58% at the
end of the study, at all exposure levels. Growth was slightly
reduced from week 20 onwards. The respiratory epithelium of the
nasal turbinates showed a dose-related increase in the incidence of
suppurative inflammation of the mucosae, hyperplasia, and squamous
cell metaplasia. At 940 mg/m3, 2 out of 50 male and 3 out of 50
female rats had papillary adenomas involving the respiratory
epithelium and the underlying submucosal glands of the nasal
turbinates. No such tumours were observed in controls or low-dose
animals. In the thyroid of the female rats, a dose-related increase
in the combined incidences of C-cell adenomas and carcinomas occurred,
which was significant at 940 mg/m3. The combined incidence of
endometrial stromal polyps and sarcomas was reported to have
increased, at both exposure levels, in a dose-related manner.
According to the authors, the C-cell adenomas and C-cell carcinomas
were not related to treatment. The incidences of polyps and
sarcomas were within the historical control range for the species
tested. In males, an increase in skin keratoacanthomas was
observed, with a statistically-significant positive trend. Induced
non-neoplastic lesions further included testicular atrophy, acinar
cell atrophy in the pancreas of males, cytomegaly in the adrenal
cortex of females, and cystic endometrial hyperplasia (US NTP,
1984).
The survival rate in mice was decreased at 940 mg/m3, from
week 60 onwards and was 58 and 20% for male and female mice,
respectively, at the end of the study, compared with 84 and 76% for
male and female controls. Growth was slightly reduced from week 29
onwards. In the nasal turbinates, a dose-related increased
incidence of inflammation occurred. Squamous cell metaplasia was
observed in 1 male at 470 mg/m3 and 2 females at 940 mg/m3. At
940 mg/m3, haemangiosarcomas were found in the nasal cavity of 5
5 males and 2 females. Haemangiomas were also observed in the
nasal cavity of 5 males and 3 females at this dose level. The
increases in the incidence of haemangiosarcomas in the males and of
haemangiomas in both sexes were statistically-significant.
One squamous cell carcinoma and one papilloma were induced in
the nasal cavity of male mice at 940 mg/m3 and 2 adenocarcinomas
were induced in the nasal cavity of females at 940 mg/m3. None
was observed in controls or low-dose animals. The incidence of
adenocarcinomas of the mammary gland was increased at 940 mg/m3,
but did not exceed the historical control range for the species
tested. Induced non-neoplastic lesions further included ovarian
atrophy and suppurative inflammation, mainly of the uterus and
peritoneum (US NTP, 1984).
In a combined NIOSH toxicity-carcinogenicity study, groups of
80 male Fischer 344 rats were exposed to 237 or 717 mg propylene
oxide/m3, for 7 h per day, 5 days per week, over 2 years. A
control group contained 80 rats. There was an exposure-free period
of 2 weeks in month 16 because of a pulmonary infection, which
contributed to the mortality rate. The mortality rate was
increased at both exposure levels, and the increase was significant
at 717 mg/m3. The mean survival was 96 weeks at this exposure
level, compared with 103 weeks for control rats. Body weights were
reduced from the 2nd week at 717 mg/m3 and from the 39th week at
237 mg/m3. After 2 years, at both concentrations, the relative
weights of lungs, adrenals, and brain were increased and those of
the testes decreased. The only altered biochemical parameters were
increased haemoglobin concentrations in blood at both exposure
levels and increased activities of serum aspartate aminotransferase
(EC 2.6.1.1) and serum sorbitol dehydrogenase (EC 1.1.1.14) at 237
mg/m3. Dose-related increases in the incidence and severity of
inflammatory lesions in the lungs, nasal cavity, trachea, and
middle ear were observed. In the nasal passages, there was a dose-
related increased incidence of complex epithelial hyperplasia that
was significant at 717 mg/m3. Metaplasia was not observed. Two
rats showed nasal-cavity adenomas at 717 mg/m3. At this
concentration, there was also an increased incidence of multifocal
areas of atrophy and degeneration of skeletal muscles. No lesions
were observed in the nerves. The only statistically-significant
neoplastic change observed was an increased incidence of adrenal
phaeochromocytomas at both concentrations. The incidences were
8/78 in controls, 25/78 in the low-dose group, and 22/80 in the
high-dose group (Lynch et al., 1984a).
Another study on the possible toxic and carcinogenic properties
of inhaled propylene oxide was performed on randomly-bred Wistar
rats. Groups of 100 rats of each sex were exposed to propylene
oxide concentrations of 70, 242, or 712 mg/m3, for 6 h per day, 5
days per week, over a period of 123 - 124 weeks. Groups of 100
rats of each sex served as controls. Ten rats per sex and per
group were killed for examination after 12, 18, and 24 months of
exposure. No adverse effects were observed on general health,
behaviour, food intake, biochemistry, urinalysis, and haematology
in comparison with control rats. The mortality rate was increased
at the highest exposure level from week 73, in males, and from week
109, in females, and at the end of the exposure to 242 mg/m3 in
females. Rats of both sexes gained less weight than controls at
712 mg/m3, though the difference compared with controls became
smaller towards the end of the exposure. The relative and absolute
weights of adrenals, spleen, liver, and lungs of males were
increased at 712 mg/m3, but no pathological lesions were observed
in these organs. Male rats showed a lower incidence of pale
exorbital lachrymal glands at this exposure level. In both sexes,
the incidences of basal cell hyperplasia and atrophy of the
olfactory epithelium and the incidence of nest-like infolds of the
respiratory epithelium were increased mainly at the 2 highest
exposure levels. These changes were also noted at interim kills.
The severity of the changes increased only slightly, if at all,
with increasing length of exposure and with age. Squamous
metaplasia was not observed. One rat was found with squamous cell
carcinoma of the nose. In female rats, the incidence of benign
tumours of the mammary glands, mostly fibroadenomas, was increased
at 712 mg/m3 in comparison to both the concurrent controls and
the historical controls. Moreover, the number of females bearing 2
or more mammary fibroadenomas increased in a dose-related manner
from the lowest exposure level onwards when compared with controls.
The increased incidence of tubulopapillary mammary carcinomas at
712 mg/m3 was within the range of historical controls. With the
exclusion of mammary tumours, the total incidence of primary
tumours in females and the number of malignant tumour-bearing rats
of both sexes was increased at the highest exposure level (Reuzel &
Kuper, 1983). Following reports that ethylene oxide had induced
brain tumours in rats, the histopathological investigation of the
brain of the rats was extended. There was no evidence that
propylene oxide induced such tumours (Reuzel & Kuper, 1984).
7.4.3. Subcutaneous exposure
Groups of 100 female NMRI mice received subcutaneous doses of
0.1, 0.3, 1.0, or 2.5 mg propylene oxide per animal in tricaprylin
once a week, for 106 weeks. These groups were compared with 200
controls receiving tricaprylin and 200 untreated controls. In the
second year of exposure, mice were not treated for 11 weeks. Body
weights and survival rates were not affected by treatment. At the
site of injection, a dose-related increase in the incidence of
sarcomas (mainly fibrosarcomas) occurred. The numbers of mice
affected were 0/200 in untreated controls, 4/200 in tricaprylin
controls, and 3/100, 2/100, 12/100, and 15/100 at the 0.1, 0.3,
1.0, and 2.5 mg dose levels, respectively. The increases in
incidence at the 2 highest dose levels were statistically
significant. The first tumour appeared in week 38 (Dunkelberg,
1981).
7.5. Effects on Reproduction and Teratogenicity
When B6C3F1 mice of both sexes were exposed repeatedly for 2
years to propylene oxide at concentrations of 470 and 940 mg/m3
air (section 7.4.2), the incidence of ovarian atrophy was increased
at the higher exposure level (US NTP, 1984). In F344 rats, similarly
exposed, higher incidences of testicular atrophy were found at both
exposure levels, but they were not dose-related (US NTP, 1984).
Decreased relative weights of the testes were reported by Lynch et
al. (1984a), when male F344 rats were exposed repeatedly for 2 years
to propylene oxide at levels of 240 and 720 mg/m3 air.
Sperm head abnormalities were not detected in mice after
exposure for 5 days, 7 h per day, to 720 mg propylene oxide/m3
(Hardin et al., 1983), or in groups of 12 Cynomolgus monkeys,
exposed for 2 years to 240 and 710 mg propylene oxide/m3, for
7 h per day, 5 days per week. In monkeys, at both exposure levels,
sperm counts and sperm motility were reduced, and the sperm drive
range was elevated (Lynch et al., 1984c).
In a reproduction-teratogenicity study, groups of 41 - 44
female Sprague Dawley rats were exposed to 1190 mg propylene
oxide/m3 air, for 7 h per day, during days 7 - 16 of gestation
(Group 1), days 1 - 16 of gestation (Group 2), or for 3 weeks
(5 days/week) before mating and on days 1 - 16 of gestation
(Group 3). A group of 46 rats served as controls. The dams were
sacrificed on day 21. No deaths of dams occurred, their histology
was normal, and the percentage of pregnant rats was not affected by
the exposure. Toxic effects in dams were expressed as decreased
body weights and food consumption and increased relative weights of
kidneys in all exposed groups. The numbers of corpora lutea,
implantations per dam, and live fetuses were lower in group 3,
compared with those in the other groups. In group 1, the number of
resorptions was increased compared with that in group 2. The body
weights and lengths of fetuses were decreased in all exposed groups
compared with controls, but more so in group 3. In group 2,
increases in wavy rib and in reduced ossification, primarily of the
vertebrae and ribs, were observed (Hackett et al., 1982).
Groups of New Zealand rabbits were also exposed to 1190 mg/m3,
for 7 h per day, during days 1 - 19 or days 7 - 19 of gestation.
No evidence of toxicity was observed in the mothers, fetuses, or
embryos, and no developmental defects were noted (Hacket et al.,
1982).
Female rats administered a single oral dose of 260 mg/kg body
weight of propylene oxide showed disturbance of the estrus cycle.
Pregnant female rats administered an identical dose during the
first 2 weeks of gestation exhibited higher embryotoxicity and
lower offspring body weight compared with the controls. Male rats
exposed to a single oral LD50 (520 mg/kg) dose of propylene oxide
showed reduced sperm motility and damage to primary spermatocytes.
When such rats were mated with normal female rats, between 2 and 10
weeks after exposure, 50% of the males were infertile. The fertility
of the first generation was reduced by 23% (Antonova et al., 1981).
8. EFFECTS ON MAN
8.1. Exposure of Skin and Eyes; Skin Sensitization
Accidental exposure of the eyes of 3 persons to propylene
oxide (it was not reported whether this was liquid or vapour)
resulted in alterations in the cornea and conjunctiva (McLauglin,
1946).
Allergic contact dermatitis was diagnosed in 3 cases of
exposure to solutions of propylene oxide (Ketel, 1979; Jensen,
1981). Biopsy on the skin of these patients revealed spongiosis
in the epidermis, oedema in the cutis, and dense perivascular
infiltrates with mononuclear cells.
8.2. Accidental Inhalation Exposure
No data are available.
8.3. Occupational Inhalation Exposure
In the Federal Republic of Germany, 279 employees from 8
plants where alkene oxides were produced or processed, were
examined during 1978. They were employed for an average of 10.8
years. No clinical abnormalities were found that could be related
to exposure to alkene oxides. Propylene oxide levels were measured
by personal sampling over 1 - 10 h, but levels of exposure were not
reported. The workers were exposed to many other chemicals,
including ethylene oxide (Stocker & Thiess, 1979). Because of the
mixed exposure and unavailability of propylene oxide exposure data,
the Task Group was unable to evaluate this study.
8.4. Mortality Studies
In the Federal Republic of Germany, 602 workers were
investigated for mortality over the period 1928-80. The workers
had been employed for at least 6 months in 8 plants producing
ethylene oxide and propylene oxide, the latter produced only since
1959. A subcohort of 351 workers was observed for more than 10
years. Control data came from a styrene plant and from national
statistics. Propylene oxide levels, measured by personal sampling
(number of samples not reported), from 1978 to 1980, were far below
a time-weighted average of 240 mg/m3 over a working shift of 12 h
(Thiess et al., 1981a). Higher levels were measured for brief
periods. The workers were also exposed to many other chemicals,
some of which might be carcinogenic for human beings. There were
56 deaths compared with 76.6 expected. No significant excess of
deaths could be found due to any cause in the cohort of 602 workers.
In the sub-cohort of 351 workers, there was a significant increase
in mortality rate due to kidney disease (3 compared with 0.4
expected). There was 1 death from gall-bladder cancer, 1 death
from urinary-bladder cancer, 1 death from brain cancer, and 1 death
from myeloid leukaemia. The stomach tumours were observed compared
with 1.8 expected (Thiess et al., 1981b). Since the workers were
also exposed to many other chemicals, such as butylene oxide,
epichlorohydrin, dioxan, dichloropropane, and chlorohydrins, the
Task Group was unable to evaluate this study in relation to
propylene oxide.
8.5. Mutagenicity and Related End-Points
In 43 male workers from the cohort of 602 workers discussed in
section 8.4 (Thiess et al., 1981b), no increase in chromosome
aberration rate was found in 2 groups of workers exposed to alkene
oxides for average periods of either 12.5 or 17.6 years; in addition,
workers in the latter group had at least one accidental high exposure
to ethylene oxide. The results were also negative in a group of
workers exposed once for a brief period following an accident. On
the other hand, the aberration rate was significantly elevated in
workers exposed for more than 20 years (Thiess et al., 1981a).
For the reason mentioned in section 8.4, the Task Group was
unable to evaluate this study in relation to propylene oxide.
Unscheduled DNA synthesis, induced in vitro by the mutagen
N-acetoxy-2-acetylaminofluorene, was inhibited in the lymphocytes
of 23 workers from a factory in Sweden, where starch was modified
with propylene oxide. The workers had been exposed for 1 - 20
years. At the time of the study, exposure was generally below a
time-weighted average of 28 mg/m3. However, short-term exposures
of up to 2370 mg/m3 were recorded for some workers (Pero et al.,
1982).
9. EVALUATION OF THE HEALTH RISKS FOR MAN AND EFFECTS ON
THE ENVIRONMENT
Exposure of man to propylene oxide mainly occurs through
inhalation at the work-place.
Data are insufficient to estimate the exposure to propylene
oxide residues in food after fumigation and sterilization. The
main conversion products in foodstuffs are chloropropanols and
1,2-propanediol, which are more persistent than the parent compound
(section 4.2). No adverse effects have been reported due to the
ingestion of propylene oxide and its reaction products in food.
No ambient air monitoring data are available, but the lowest
annual average concentration at distances of up to 20 km from
production plants has been assessed, by modelling, to be less than
4.836 x 10-8 mg/m3(section 4.1). The risk for human health
under such conditions of exposure is likely to be negligible.
Propylene oxide is highly soluble in water but is likely to
evaporate to a great extent. However, no data on the rate of
evaporation are available. In neutral fresh water, propylene
oxide is converted to 1,2-propanediol and, in marine waters, to
halopropanols, but, even in the presence of micro-organisms, these
processes are slow (section 3.2). Because of the low log n-octanol
water-partition coefficient, propylene oxide and its conversion
products are unlikely to bioaccumulate (Table 1). The toxicity of
propylene oxide for aquatic organisms is low. The available LC50s
are above approximately 90 mg/litre (section 6). Thus, the
probability of an adverse impact on the aquatic environment is
considered low.
Eight-hour time-weighted occupational exposure in propylene
oxide production and use is normally below 5 mg/m3, with
occasional peak exposures up to 9000 mg/m3 (section 4.3).
Inhaled propylene oxide is probably readily absorbed,
distributed throughout the body, and rapidly metabolized. The
half-life in rat tissues has been estimated to be 40 min (section
5.2). There are no data on skin absorption.
An aqueous solution of propylene oxide (100 or 200 g/litre)
is irritating to rabbit skin when applied under occlusive cover
(section 7.1.2). In man, corneal and conjunctival damage, and
allergic contact dermatitis have been reported through accidental
exposure to propylene oxide vapour (section 8.1).
The 4-h LC50s for the rat and the mouse were 9500 mg/m3
and 4100 mg/m3, respectively. Dogs exposed by inhalation, once
for 4 h, to a concentration of 3230 - 5880 mg/m3 showed
salivation, lachrymation, nasal discharge, and vomiting. Deaths
were observed at 4750 - 5880 mg/m3 but not at 3230 mg/m3
(section 7.1.3).
On repeated exposure of various species to propylene oxide
vapour, 7 h per day, 5 days per week, for 112 - 218 days, at
concentrations of 0, 240, 460, and 1080 mg/m3, rabbits and
monkeys did not show any adverse effects on appearance, mortality,
growth, and histopathology of internal organs. Rats and guinea-
pigs showed irritation of the respiratory passages and lung damage
histologically at 1080 mg/m3. An increase in lung weight was
observed in female guinea-pigs at concentrations of 460 mg/m3 or
more. No effect was observed in any species at a concentration of
240 mg/m3. A concentration of 3400 mg/m3 administered for 2
weeks, 6 h/day, 5 days per week, resulted in dyspnoea and death in
rats. In the same study, mice showed dyspnoea at 460 and 1150 mg/m3
(section 7.2.2).
Rats and mice exposed for 2 years showed increased incidences
of inflammatory and proliferative lesions of the nasal epithelium
from an exposure level of 470 mg/m3. In rats, the inflammatory
lesions and hyperplasia were also observed at an exposure level of
240 mg/m3, but not at 70 mg/m3 (section 7.4.2).
Depression of the central nervous system, the severity of
which increased with increasing level and length of exposure, was
observed when rats and mice were exposed by inhalation to single
high concentrations of propylene oxide (section 7.1.3). Although
no clinical central nervous system effects were reported when
monkeys were exposed to 237 and 717 mg/m3 for 2 years, some
histopathological changes were observed in the treated animals
(section 7.2.2). It should be noted that these data cannot be
evaluated for estimating hazard for man, since only 2 monkeys were
examined in each of the exposed groups, and the lesion appeared
also in one of the 2 controls.
Summarizing the results of animal studies (excluding
mutagenic, carcinogenic, and reproduction effects) the no-
observed-adverse-effect level for prolonged repeated 6- to 7-h
daily exposure is 70 mg/m3.
Propylene oxide administered by inhalation for 2 years
produced ovarian atrophy in mice at 940 mg/m3. Testicular
atrophy was found at 240 and 720 mg/m3 in rats (section 7.5).
No sperm head abnormalities were detected in mice after
exposure for 5 days to 720 mg/m3 or in Cynomolgus monkeys
exposed to 240 or 710 mg/m3 for 2 years, but sperm count and
motility were reduced, and sperm drive range (time to traverse a
linear path) increased in the monkeys. A reduced sperm motility
and damage to spermatocytes were observed in male rats treated with
an oral LD50 (520 mg/kg body weight) dose. A reduced fertility
was observed when these males were mated between 2 and 10 weeks
after exposure with normal females (sections 7.3, 7.5).
Reduced fetal ossification of vertebrae and ribs and wavy ribs
were observed when pregnant Sprague Dawley rats were exposed to
1190 mg propylene oxide/m3, 7 h per day, on days 1 - 16 of
gestation. When rats were additionally exposed for 3 weeks before
mating, the numbers of corpora lutea, implantations per dam, and
live fetuses were decreased. No teratogenic or fetotoxic effects
were found in New Zealand rabbits exposed to 1190 mg/m3 (section
7.5).
Propylene oxide is mutagenic in microorganisms and insects and
produces DNA damage and chromosomal aberrations in mammalian cells
in vitro. It produced micronuclei in mouse erythrocytes after
parenteral injection of 2 doses of 300 mg/kg body weight but not
after oral exposure. No chromosomal aberrations or sister
chromatid exchanges occurred in monkeys exposed by inhalation to
237 and 717 mg/m3, for 7 h/day, 5 days/week, for 2 years. No
dominant-lethal effects were observed in rats or mice following
inhalation or oral exposure, respectively, in 2 studies (section
7.3). No adequate data on chromosomal effects in human beings are
available.
In studies on the carcinogenicity of propylene oxide in rats
and mice, malignant tumours were mainly observed at the site of
entry into the body: for stomach, at 15 and 60 mg/kg body weight;
for subcutaneous tissues, at 1.0 - 2.5 mg; and, for nasal passages,
at 940 mg/m3. At the dose levels at which these tumours were
induced, propylene oxide produced tissue damage. This damage may
have played a role in the appearance of the tumours. Propylene
oxide also produced an increase in the incidence of multiple benign
mammary tumours in female rats following inhalation exposure
(section 7.4). No adequate epidemiological studies on cancer
incidence in exposed human populations have been carried out.
Taking into account the body of available data - the
alkylating nature of propylene oxide, the formation of DNA
adducts, the positive responses in in vitro mutagenesis assays,
the carcinogenic effects in animals at the sites of entry into the
body, and the absence of adequate data on cancer in human beings -
propylene oxide should be considered as a possible human carcinogen.
Thus, for practical purposes, propylene oxide should be regarded as
if it presented a carcinogenic risk for man, and levels in the
environment should be kept as low as feasible.
10. RECOMMENDATIONS FOR FURTHER RESEARCH
1. A thorough detailed study on the absorption, distribution,
and metabolism of propylene oxide should be conducted.
2. Biological indicators should be developed that quantify both
the incidence and severity of human exposure.
3. The epidemiological studies indicating an increased risk of
cancer in workers exposed to propylene oxide in combination
with other chemicals suggest that additional studies should
be conducted on populations whose exposure has been primarily
to propylene oxide. The adequate quantification of past
exposures should be a part of these studies.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
An International Agency for Research on Cancer Working Group
(IARC, 1985) evaluated the carcinogenicity of propylene oxide and
concluded that:
"There is sufficient evidence for the carcinogenicity of
propylene oxide to experimental animals; there is inadequate
evidence for its carcinogenicity to humans. It is noted that,
in the absence of adequate data in humans, it is reasonable,
for practical purposes, to regard chemicals for which there is
sufficient evidence of carcinogenicity in experimental animals
as if they represented a carcinogenic risk to humans."
REFERENCES
ADDY, J.K. & PARKER, R. (1963) The mechanism of epoxide
reactions. V. The reaction of 1,2-epoxypropane with chloride
ions in water under neutral and acidic conditions. J. Chem.
Soc., 915-921.
ANDERSON, G.E. (1983) Human exposure to atmospheric
concentrations of selected chemicals. Vol. II, Research
Triangle Park, North Carolina, US Environmental Protection
Agency, pp. 574-601 (Prepared by Systems Applications, Inc.,
San Rafael, California, No. SA1/EF81-156R2).
ANTONOVA, V.I., ZOMMER, E.A., KUZNETSOVA, A.D., & PETROVA,
N.A. (1981) [Toxicology of propylene oxide and regulation
for surface water.] Gig. i Sanit., 46: 76-79 (in Russian).
BOGYO, D.A., LANDE, S.S., MEYLAND, W.M., HOWARD, P.H., &
SANTODONATO, J. (1980) Investigation of selected potential
environmental contaminants: Epoxides, Syracuse, New York,
Centre for Chemical Hazard Assessment, Syracuse Research
Corporation (Prepared for US EPA) (US EPA 560/11-80-005, PB
80-183197).
BONT, J.A.M. DE, DIJKEN, J.P. VAN, & GINKEL, K.G. VAN (1982)
The metabolism of 1,2-propanediol by the propylene oxide
utilizing bacterium Nocardia 60. Biochem. Biophys. Acta, 714:
465-470.
BOOTMAN, J., LODGE, D.C., & WHALLEY, H.E. (1979) Mutagenic
activity of propylene oxide in bacterial and mammalian
systems. Mutat. Res., 67: 101-112.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979a) The acute
toxicity of some petrochemicals to goldfish. Water Res., 13:
623-626.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) BOD and
COD of some petrochemicals. Water Res., 13: 627-630.
CALLEMAN, C.J., EHRENBERG, L., JANSSON, B., OSTERMAN-GOLKAR,
S., SEGERBACK, D., SVENSSON, K., & WACHTMEISTER, C.A. (1978)
Monitoring and risk assessment by means of alkyl groups in
haemoglobin in persons occupationally exposed to ethylene
oxide. J. environ. Pathol. Toxicol., 2: 247-442.
CONWAY, R.A., WAGGY, G.T., SPIEGEL, M.H., & BERGLUND, R.L.
(1983) Environmental fate and effects of ethylene oxide.
Environ. Sci. Technol., 17: 107-112.
COOKSON, M.J., SIMS, P., & GROVER, P.L. (1971) Mutagenicity
of epoxides of polycyclic hydrocarbons correlates with
carcinogenicity of parent hydrocarbons. Nature (New Biol.),
234: 186-187.
CREWS, R.C. (1974) Effects of propylene oxide on selected
species of fishes, Florida, Air Force Armament Center, Eglin
Air Force Base (US NTIS AD/A003637).
CUPITT, L.T. (1980) Fate of toxic and hazardous materials in
the environment, Research Trianlge Park, North Carolina, US
Environmental Protection Agency, Environmental Sciences
Laboratory, Office of Research and Development (US EPA
600/3-80-084, PB 80-221948).
DAVIES, R.E., HARPER, K.H., & KYNOCH, S.R. (1972)
Inter-species variation in dermal reactivity. J. Soc. Cosmet.
Chem., 23: 371-382.
DEAN, B.J. & HODSON-WALKER, G. (1979) An in vitro chromosome
assay using cultured rat liver cells. Mutat. Res., 64: 329-337.
DENT, J.G. & SCHNELL, S.R. (1981) Inhibition of microsomal
membrane-bound and purified epoxide hydrolase by C2-C8
1,2-alkene oxides. Biochem. Pharmacol., 30: 1712-1714.
DUNKELBERG, H. (1981) [Carcinogenic activity of ethylene
oxide and its reaction products 2-chloroethanol,
2-bromoethanol, ethylene glycol, and diethylene glycol. I.
Carcinogenicity of ethylene oxide in comparison with
1,2-propylene oxide after subcutaneous administration in
mice.] Zbl. Bakt. Hyg. (I. Abt. Orig. B), 174: 383-404 (in
German).
DUNKELBERG, H. (1982) Carcinogenicity of ethylene oxide and
1,2-propylene oxide upon intragastric administration to rats.
Br. J. Cancer, 46: 924-933.
EHRENBERG, L., HIESCHE, K.D., OSTERMAN-GOLKAR, S., & WENNBERG,
I. (1974) Evaluation of genetic risks of alkylating agents:
Tissue doses in the mouse from air contaminated with ethylene
oxide. Mutat. Res., 24: 83-103.
FAO/WHO JOINT EXPERT COMMITTEE ON FOOD ADDITIVES (1974) WHO
Food Add. Ser., 5: 491-497 (Toxicological Monographs arising
from the Seventeenth Report of the Joint FAO/WHO Expert
Committee on Food Additives).
FARMER, P.B., GORF, S.M., & BAILEY, E. (1982) Determination
of hydroxypropylhistidine in haemoglobin as a measure of
exposure to propylene oxide using high resolution gas
chromatography mass spectrometry. Biomed. Mass Spectrometr.,
9: 69-71.
FEDERAL REGISTER (1971) Paragraph 121.1076, propylene oxide,
subpart D, food additives, Washington DC, US Environmental
Protection Agency, Food and Drug Administration, p. 33.
FJELLSTEDT, T.A., ALLEN, R.H., DUNCAN, B.K., & JAKOBY, W.B.
(1973) Enzymatic conjugation of epoxides with glutathione. J.
Biol. Chem., 248: 3702-3707.
FLORES, G.H. (1983) Reducing plant pollution exposure:
controlling exposure to alkene oxides. Chem. Eng. News, 79:
39-43.
GARRO, A.J. & PHILLIPS, R.A. (1980) Detection of
mutagen-induced lesions in isolated DNA by marker rescue of
Bacillus subtilis phage stopb105. Mutat. Res., 73: 1-13.
GUENGERICH, F.P. & MASON, P.S. (1980) Alcohol dehydrogenase-
coupled spectrophotometric assay of epoxide hydratase
activity. Anal. Biochem., 104: 445-451.
GUNTHER, D.A. (1965) Determination of adsorbed ethylene and
propylene oxides by distillation and titration. Anal. Chem.,
37: 1172-1173.
HACKETT, P.L., BROWN, M.G., BUSCHBOM, R.L., CLARK, M.L.,
MILLER, R.A., MUSIC, R.L., ROWE, S.E., SCHIRMER, R.E., &
SIKOV, M.R. (1982) Teratogenic study of ethylene and
propylene oxide and N-butyl acetate, Richland, Washington,
Batelle Pacific Northwest Laboratories (Prepared for NIOSH)
(PB 83-258038).
HARDIN, B.D., SCHULER, R.L., MCGINNIS, P.M., NIEMEIER, R.W., &
SMITH, R.J. (1983) Evaluation of propylene oxide for
mutagenic activity in 3 in vivo test systems. Mutat. Res.,
117: 337-344.
HATFIELD, R. (1957) Biological oxidation of some organic
compounds. Ind. Eng. Chem., 49: 192-196.
HELLMAN, T.M. & SMALL, F.H. (1974) Characterization of the
odour properties of 101 petrochemicals using sensory method.
J. Air Pollut. Contr. Assoc., 24: 979-982.
HEMMINKI, K. & FALCK, K. (1979) Correlation of mutagenicity
and 4-(p-nitrobenzyl)-pyridine alkylation by epoxides.
Toxicol. Lett., 4: 103-106.
HEMMINKI, K., PAASIVIRTA, J., KURKIRINNE, T., & VIRKKI, L.
(1980) Alkylation products of DNA bases by simple epoxides.
Chem.-biol. Interact., 30: 259-270.
HESLOT, H. (1962) Etude quantitative de réversions
biochimiques induites chez la levure Schizosaccharomyces pombe
par des radiations et des substances radiomimétiques. Abh.
Dtsch. Akad. Wiss. Berlin, Kl. Med., 1: 193-228.
HEUSER, S.G. & SCUDAMORE, K.A. (1969) Determination of
fumigant residues in cereals and other foodstuffs: A multi-
detection scheme for gas chromatography of solvent extracts.
J. Sci. Food Agric., 20: 566-572.
HIRASHIMA, T., OGUMA, T., HOSOGAI, Y., & FUJII, S. (1970)
[Studies on gaseous antimicrobial agents. III. Determination
of propylene oxide residue in food wrappings and containers.]
J. Food Hyg. Soc. Jpn, 11: 161-163 (in Japanese).
HUSSAIN, S. & OSTERMAN-GOLKAR, S. (1984) Dose-response
relationships for mutations induced in E. coli by some model
compounds. Hereditas, 101: 57-68.
IARC (1976) Cadmium, nickel, some epoxides, miscellaneous
industrial chemicals and general considerations on volatile
anaesthetics, Lyons, International Agency for Research on
Cancer, 293 pp (IARC Monographs on the Evaluation of
Carcinogenic Risk of Chemicals to Man, Vol. 11).
IARC (1985) Allyl compounds, aldehydes, epoxides, and
peroxides, Lyons, International Agency for Research on Cancer
(Monographs on the Evaluation of Carcinogenic Risk of
Chemicals to Man, Vol. 36).
IRPTC (1984) Data profile on propylene oxide, Geneva,
Switzerland, International Register on Potentially Toxic
Chemicals, United Nations Environment Programme.
JACOBSON, K.H., HACKLEY, E.B., & FEINSILVER, L. (1956) The
toxicity of inhaled ethylene oxide and propylene oxide
vapours. Arch. ind. Health, 13: 237-244.
JENSEN, O. (1981) Contact allergy to propylene oxide and
isopropyl alcohol in a skin desinfectant swab. Contact
Dermatit., 7: 148-150.
KETEL, W.G. VAN (1979) Contact dermatitis from propylene
oxide. Contact Dermatit., 5: 191-192.
KOLMARK, H.G. & GILES, N.H. (1955) Comparative studies of
monoepoxides as inducers of reverse mutations in Neurospora.
Genetics, 40: 890-902.
KOSKIKALLIO, J. & WHALLEY, E. (1959) Effect of pressure on
the spontaneous and the base-catalysed hydrolyses of epoxides.
Can. J. Chem., 37: 783-787.
KROST, K.J., PELLIZZARI, E.D., WALBURN, S.G., & HUBBARD, S.A.
(1982) Collection and analysis of hazardous organic
emissions. Anal. Chem., 54: 810-817.
LAWLEY, P.D. & JARMAN, M. (1972) Alylation by propylene
oxide of deoxyribonucleic acid, adenine, guanosine, and
deoxyguanylic acid. Biochem. J., 126: 893-900.
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., BURG, J.R., GROTH,
D.H., KHAN, A., ACKERMAN, L.J., & COCKRELL, B.Y. (1984a)
Carcinogenic and toxicologic effects of inhaled ethylene oxide
and propylene oxide in F344 rats. Toxicol. appl. Pharmacol.,
76(1): 69-84.
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., BURG, J.R., GULATI,
D.K., KAUR, P., & SABHARWAL, P.S. (1984b) Sister-chromatid
exchanges and chromosome aberrations in lymphocytes from
monkeys exposed to ethylene oxide and propylene oxide by
inhalation. Toxicol. appl. Pharmacol., 76(1): 85-95.
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., LAL, J.B., BURG,
J.R., GULATI, D.K., ZAVOS, P.M., & SABHARWAL, P.S. (1984c)
Toxic and mutagenic effects of inhaled ethylene oxide and
propylene oxide on spermatogenic functions in monkeys.
Toxicologist, 3: 60.
MCLAUGHLIN, R.S. (1946) Chemical burns of the human cornea.
Am. J. Ophthalmol., 29: 1355-1362.
MCMAHON, R.E., CLINE, J.C., & THOMPSON, C.Z. (1979) Assay of
855 test chemicals in ten tester strains using a new
modification of the Ames test for bacterial mutagens. Cancer
Res., 39: 682-693.
MESTRES, R. & BARROIS, C. (1964) Recherche et dosage de
l'oxyde de propylène et du propylène-glycol dans les fruits
par chromatographie gazeuse directe. Bull. Soc. Pharm.
Montpellier, 24: 47-63.
MIGLIORE, L., ROSSI, A.M., & LOPRIENO, N. (1982) Mutagenic
action of structurally-related alkene oxides on Schizosac-
charomyces pombe: The influence, "in vitro", of mouse liver
metabolizing system. Mutat. Res., 102: 425-437.
MISHMASH, E.H. & MELOAN, C.E. (1972) Indirect
spectrophotometric determination of nanomale quantities of
oxiranes. Anal. Chem., 44: 835-836.
NELIS, H.J.C.F. & SINSHEIMER, J.E. (1981) A sensitive
fluorimetric procedure for the determination of aliphatic
epoxides under physiological conditions. Anal. Biochem., 115:
151-157.
NIOSH (1977) Manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, US Department of Health, Education and
Welfare, National Institute for Occupational Safety and
Health, Vol. 2, pp. S75-1 - S75-8.
OESCH, F. (1974) Purification and specificity of a human
microsomal epoxide hydratase. Biochem. J., 139: 77-88.
OGUMA, T., HOSOGAI, Y., FUJII, S., & KAWASHIRO, I. (1968)
[Gaseous antimicrobial agents. I. Determination of propylene
oxide residues in foods.] J. Food Hyg. Soc. Jpn, 9: 395-398
(in Japanese).
OGUMA, T., HOSOGAI, Y., & FUJII, S. (1969) [Studies on
gaseous antimicrobial agents. II. Determination of propylene
oxide residue in fats and oils.] J. Food Hyg. Soc. Jpn, 10:
37-39 (in Japanese).
OSTERMAN-GOLKAR, S., EHRENBERG, L., SEGERBACK, D., &
HALLSTROM, I. (1976) Evaluation of genetic risks of
alkylating agents. II. Haemoglobin as a dose monitor. Mutat.
Res., 34: 1-10.
OSTERMAN-GOLKAR, S., FARMER, P.B., SEGERBACK, D., BAILEY, E.,
CALLEMAN, C.J., SVENSSON, K., & EHRENBERG, L. (1983)
Dosimetry of ethylene oxide in the rat by quantitation of
alkylated histidine in haemoglobin. Teratog. Carcinog.
Mutagen., 3: 395-405.
OSTERMAN-GOLKAR, S., BAILEY, E., FARMER, P.B., GORF, S.M., &
LAMB, J.H. (1984) Monitoring exposure to propylene oxide
through the determination of haemoglobin alkylation. Scand. J.
Work environ. Health, 10: 99-102.
PERO, R.W., BRYNGELSSON, T., WIDEGREN, B., HOGSTEDT, B., &
WELINDER, H. (1982) A reduced capacity for unscheduled DNA
synthesis in lymphocytes from individuals exposed to propylene
oxide and ethylene oxide. Mutat. Res., 104: 193-200.
PFEIFFER, E.H. & DUNKELBERG, H. (1980) Mutagenicity of
ethylene oxide and propylene oxide and of the glycols and
halohydrins formed from them during the fumigation of
foodstuffs. Food Cosmet. Toxicol., 18: 115-118.
PHILLIPS, R.A., ZAHLER, S.A., & GARRO, A.J. (1980) Detection
of mutagen-induced lesions in isolated DNA using a new
Bacillus subtilis transformation-based assay. Mutat. Res., 74:
267-281.
RADDING, S.B., LUI, D.H., JOHNSON, H.L., & MILL, T. (1977)
Review of the environmental fate of selected chemicals,
Washington DC, US Environmental Protection Agency, Office of
Toxic Substances (US EPA 560/5-77-003, PB 267121).
RAGELIS, E.P., FISHER, B.S., KLIMECK, B.A., & JOHNSON, C.
(1968) Isolation and determination of chlorohydrins in foods
fumigated with ethylene oxide or with propylene oxide. J.
Assoc. Offic. Agric. Chem., 51: 709-715.
RANDERATH, K., REDDY, M.V., & GUPTA, R.C. (1981) 32P-
labelling test for DNA damage. Proc. Natl Acad. Sci., 78:
6126-6129.
REUZEL, P.G.J. & KUPER, C.F. (1983) Chronic (28-month)
inhalation toxicity/carcinogenicity study of 1,2-propylene
oxide in rats. Final Report, Zeist, The Netherland, TNO,
Division of Nutrition and Food Research (Report No.
V82.215/280853).
REUZEL, P.G.J. & KUPER, C.F. (1984) Chronic (28-month)
inhalation toxicity/carcinogenicity study of 1,2-propylene
oxide in rats, Zeist, The Netherland, TNO, Division of
Nutrition and Food Research (Addendum to Report No.
V82.215/280853).
ROSS, W.C.J. (1950) The reactions of certain epoxides in
aqueous solutions. J. Chem. Soc., 2257-2271.
ROWE, V.K., HOLLINGSWORTH, R.L., OYEN, F., MCCOLLISTER, D.D.,
& SPENCER, H.C. (1956) Toxicity of propylene oxide deter-
mined on experimental animals. Arch. ind. Health, 13: 228-236.
RUDDICK, J.A. (1972) Toxicology, metabolism, and biochemis-
try of 1,2-propanediol. Toxicol. appl. Pharmacol., 21: 102-111.
RUSSELL, J.W. (1975) Analyses of air pollutants using
sampling tubes and gas chromatography. Environ. Sci. Technol.,
9: 1175-1178.
SCUDAMORE, K.A. & HEUSER, S.G. (1971) Ethylene oxide and its
persistent reaction products in wheat flour and other
commodities: Residues from fumigation or sterilization, and
effects of processing. Pestic. Sci., 2: 80-91.
SINA, J.F., BEAN, C.L., DYSART, G.R., TAYLOR, V.I., & BRADLEY,
M.O. (1983) Evaluation of the alkaline elution/rat
hepatocyte assay as a predictor of carcinogenic/mutagenic
potential. Mutat. Res., 113: 357-391.
SMYTH, H.F., SEATON, J., & FISHER, L. (1941) The single dose
toxicity of some glycols and derivatives. J. ind. Hyg.
Toxicol., 23: 259-268.
SNELLINGS, W.M., WEIL, C.S., & MARONPOT, R.R. (1984) A
two-year inhalation study of the carcinogenic potential of
ethylene oxide in Fischer 344 rats. Toxicol. appl. Pharmacol.,
75: 105-117.
SPRINZ, H., MATZKE, H., & CARTER, J. (1982) Neuropatho-
logical evaluation of monkeys exposed to ethylene and
propylene oxide, Kansas City, Missouri, Midwest Research
Institute (Prepared for NIOSH) (PB 83-134817).
STEELE, L. & HADZIYEV, D. (1976) Sterilization of dehydrated
potato granules with propylene oxide. Z. Lebensm.
Unters.-Forsch., 162: 387-399.
STOCKER, W.G. & THIESS, A.M. (1979) Morbidity study on
workers exposed to ethylene oxide/propylene oxide. In: The 7th
Medichem Congress, Gera, 11-15 September, 1979.
STORCK, W.J. (1981) Output of big-volume chemicals fell in
1980. Chem. Eng. News, 59: 36-39.
SVENSSON, K. & OSTERMAN-GOLKAR, S. (1984) Kinetics of
metabolism of propene and covalent binding to macromolecules
in the mouse. Toxicol. appl. Pharmacol., 73: 363-372.
SWAN, J.D. (1954) Determination of epoxides with sodium
sulfite. Anal. Chem., 26: 878-880.
TACHIZAWA, H., MACDONALD, T.L., & NEAL, R.A. (1982) Rat
liver microsomal metabolism of propyl halides. Mol.
Pharmacol., 22: 745-751.
THIESS, A.M., SCHWEGLER, H., FLEIG, I., & STOCKER, W.G.
(1981a) Mutagenicity study of workers exposed to alkylene
oxides (ethylene oxide/propylene oxide) and derivatives. J.
occup. Med., 23: 343-347.
THIESS, A.M., FRENZEL-BEYME, R., LINK, R., & STOCKER, W.H.
(1981b) Mortality study on employees exposed to alkylene
oxides (ethylene oxide/propylene oxide) and their derivatives.
In: Proceedings of the International Symposium on Prevention
of Occupational Cancer, Helsinki, Finland, pp. 249-259.
US EPA (1981) Human exposure to atmospheric concentrations
of selected chemicals, Research Triangle Park, North Carolina,
US Environmental Protection Agency, Office of Air Quality
Planning and Standards (US NTIS PB 84-102540, PB 83-265249).
US NTP (1984) Toxicology and carcinogenesis studies of
propylene oxide in F344/N rats and B6C3F1 mice (inhalation
studies), Research Triangle Park, North Carolina, National
Toxicology Program (NTP 83-020) (NIH Publication No. 84-2523).
VOJNOVICH, C. & PFEIFER, V.F. (1967) Reducing the microbial
population of flour during milling. Cereal Sci. Today, 12:
54-60.
VOOGD, C.E., STEL, J.J. VAN DE, & JACOBS, J.J.J.A.A. (1981)
The mutagenic action of aliphatic epoxides. Mutat. Res., 89:
269-282.
WADE, D.R., AIRY, S.C., & SINSHEIMER, J.E. (1978)
Mutagenicity of aliphatic epoxides. Mutat. Res., 58: 217-223.
WALLES, S.A. (1974) The influence of some alkylating agents
on the structure of DNA in vitro . Chem.-biol. Interact., 9:
97-103.
WEBBER, D. (1984) Basic chemical output returns to growth.
Top 50 chemical products. Chem. Eng. News, May 7: 8-10.
WEIL, C.S., CONDRA, N., HAUN, C., & STRIEGEL, J.A. (1963)
Experimental carcinogenicity and acute toxicity of
representative epoxides. Am. Ind. Hyg. Assoc. J., 24: 305-325.
WHO (1978) Environmental health problems associated with the
manufacture and uses of synthetic organic chemicals, Geneva,
World Health Organization (Report No. HCS/78.2).
WHO (1985) EHC 55: Ethylene oxide, Geneva, World Health
Organization, 79 pp.
ZAMORA, P.O., BENSON, J.M., LI, A.P., & BROOKS, A.L. (1983)
Evaluation of an exposure system using cells grown on collagen
gels for detecting highly-volatile mutagens in the CHO/HGPRT
mutation assay. Environ. Mutagen., 5: 795-801.
 6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
In an acute toxicity test on goldfish (Carassius auratus),
in static fresh water, at 20 °C, pH between 6 and 8, and a
dissolved oxygen content greater than 4 mg/litre, the 24-h LC50
for propylene oxide was 170 mg/litre (Bridié et al., 1979a). In
other static tests, with the fresh water species bluegill sunfish
(Lepomis machrochirus) and mosquito fish (Gambusia affinis),
96-h LC50 values for propylene oxide were 215 and 141 mg/litre,
respectively, at 23 °C. For the common mullet (Mugil cephalus),
a marine fish, a static 96-h LC50 for propylene oxide of 89
mg/litre was determined at 23 °C and a water salinity of 1.5% (no
water analysis was reported) (Crews, 1974). The 24-h LC50 for
1,2-propanediol (a product of propylene oxide) for goldfish was
over 5000 mg/litre (Bridié et al., 1979a). Propylene oxide is very
soluble in aqueous media; the log n-octanol water partition
coefficient was reported to be -0.13 (Radding et al., 1977) and,
therefore, it is not expected to bioaccumulate.
7. EFFECTS ON ANIMALS
7.1. Single Exposures
7.1.1. Oral exposure
In 2 studies, the oral LD50s for the rat were reported to be
1140 mg/kg body weight and 520 mg/kg body weight for males, and
540 mg/kg for females (Smyth et al., 1941; Antonova et al., 1981);
the difference between 16 and 84% mortality was caused by a dose of
only approximately 300 mg/kg body weight, indicating a rather steep
dose-effect relationship. In one of these studies, oral LD50s for
male mice and guinea-pigs were 630 and 660 mg/kg body weight,
respectively (Antonova et al., 1981).
At lethal oral doses, necrosis of the stomach mucosa was
observed in rats. Succinate dehydrogenase (EC 1.3.99.1) activity
was reported to be decreased in the stomach mucosa. The liver
cells showed oedema and fatty changes. In serum, alanine amino-
transferase (EC 2.6.1.2) and aspartate aminotransferase (EC 2.6.1.1)
activity and histamine levels were increased. Kidney function was
disturbed. Mature lymphocytes were reduced in the spleen (Antonova
et al., 1981).
7.1.2. Skin and eye irritation
Solutions of 100 or 200 g propylene oxide/litre water, applied
under a plastic cover on the intact skin of rabbits produced hyper-
aemia, oedema, and finally scars after 6 or more min of exposure.
The intensity of the effects was proportional to the exposure time
(Rowe et al., 1956).
No, or only slight, irritation was observed when undiluted
1,2-propanediol, a possible reaction product of propylene oxide in
water, was applied to the skin of various animals under occlusive
conditions for 24 or 72 h (Davies et al., 1972).
When 5 µl of undiluted propylene oxide was applied once on the
centre of the cornea of rabbits, a severe burn resulted with
necrosis (Weil et al., 1963).
7.1.3. Inhalation exposure
In inhalation studies, the 4-h LC50s for rats and mice were
9500 and 4100 mg/m3, respectively (Jacobson et al., 1956). The
lowest lethal concentration (US NTP, 1984) or the 0.1% mortality
level (Jacobson et al., 1956) for 4-h exposures was approximately
5250 mg/m3 for rats and 900 mg/m3 for mice. In both species,
100% mortality was reached after 4 h of exposure at levels above
17 000 mg/m3 (Jacobson et al., 1956). In rats, 100% mortality
was also reported after a 30-min exposure to 38 000 mg/m3 or a
7-h exposure to 9500 mg/m3 (Rowe et al., 1956).
After inhalation of propylene oxide, rodents showed eye and
nose irritation, nasal discharge, dyspnoea, and depression of the
central nervous system, the severity of which increased with
increasing level and length of the exposure (effect levels not
specified) (Rowe et al., 1956). Gross pathology revealed
distension of the stomach only at lethal concentrations (Jacobson
et al., 1956). No organ damage was observed in rats exposed to
2370 mg/m3 for 7 h, 4740 mg/m3 for 4 h, or 9480 mg/m3 for 0.5 h
(Rowe et al., 1956). In 4 groups, each containing 3 dogs, exposed
for 4 h to propylene oxide concentrations of 3230, 4750, 4810, or
5880 mg/m3, lachrymation, salivation, nasal discharge, and vomiting
occurred. Congestion in the lungs and trachea, oedema of pulmonary
tissues, and necrosis of bronchiolar epithelium were observed at
4810 and 5880 mg/m3. Deaths occurred in the groups exposed to
concentrations of 4750 mg/m3 or more (Jacobson et al., 1956).
7.2. Repeated Exposures
7.2.1. Oral exposure
Propylene oxide was administered in the drinking-water to 4
groups of rats of both sexes at dose levels calculated by the
authors to be 0.52, 5.2, 52, or 520 µg/kg body weight per day, over
a 26-week period. At the highest dose level, polyuria, haematological
abnormalities, lowering of serum-albumin, increased serum-beta-
globulin levels, and increased activity of gastrointestinal mucosal
enzymes were observed. Mild haematological abnormalities occurred
at the next lower dose, while no adverse effects were noted at the
two lowest doses (Antonova et al., 1981).
7.2.2. Inhalation exposure
In range-finding tests for a carcinogenicity study, groups of 5
male and 5 female Fischer 344/N rats were exposed to 0, 110, 230,
460, 1150, or 3400 mg propylene oxide/m3 air for 5 days per week,
and 6 h per day, during a 2-week exposure period. B6C3F1 mice were
exposed for the same period to 0, 50, 110, 230, 460, or 1150 mg/m3.
All animals were necropsied on the 12th day. No pathological
effects were observed. Body weights were not affected. Dyspnoea
and gasping were observed in rats, one of which died at 3400 mg/m3.
Mice showed dyspnoea at 460 and 1150 mg/m3. Both species were
less active at the highest exposure. Rats also showed irregular
limb movements and diarrhoea. After 13 weeks of a similar exposure
regime up to concentration of 1150 mg/m3, rats and mice did not
show any gross- or histopathological effects. Body weights were
reduced at 1150 mg/m3 only (US NTP, 1984).
Groups of 10 - 20 rats, 8 guinea-pigs, 1 or 2 rabbits of both
sexes, and 1 or 2 female Rhesus monkeys were exposed to 0, 240,
460, or 1080 mg propylene oxide/m3 air for 112 - 218 days, for
7 h per day, and 5 days per week. Rabbits and monkeys did not
show any adverse effects with regard to appearance, behaviour,
mortality rate, growth, organ weights, and gross- and histopathology.
Rats showed irritation of the eyes and respiratory passages and an
increased mortality rate due to pneumonia at 1080 mg/m3. Guinea-
pigs also showed irritation of the eyes and respiratory passages at
this exposure level, but no increase in mortality rate. In
addition, the growth of the female guinea-pigs was reduced. Livers
exhibited slight fatty degeneration in male guinea-pigs. The
relative weights of the lungs of the guinea-pigs were slightly, but
significantly, increased in both sexes at 1080 mg/m3, and in
females at 460 mg/m3. It should be noted that control lung
weights were low. Histopathological findings were alveolar
haemorrhages and oedema, and interstitial oedema and hyperaemia in
the lungs of guinea-pigs after 157 days of exposure to 1080 mg/m3.
After 37 - 39 days of exposure to 1080 mg/m3, these histopatho-
logical effects had also been observed in rats (Rowe et al., 1956).
The possibility of the induction of neuropathological effects
by propylene oxide was investigated in groups of 12 male Cynomolgus
monkeys. The animals were exposed to actual concentrations of 0,
237, and 717 mg/m3 propylene oxide, for 7 h per day, 5 days per
week, for 2 years. In 2 monkeys per group, brain, ulnar and
sciatic nerves, and spinal cord were examined histologically after
exposure. No clinical signs were reported. The only treatment-
related neuropathological lesions found were in the medulla
oblongata of the brain. Axonal dystrophy was observed in the
nucleus gracilis in all of the 4 treated monkeys examined, without
any apparent dose-effect relationship. The same lesion was observed
in one of the two control monkeys (Sprinz et al., 1982).
7.3. Mutagenicity and Related End-Points
A summary of mutagenicity tests with positive results is
presented in Table 3. Propylene oxide is mutagenic in bacteria,
fungi, and insects; no such studies with negative results have been
reported.
Table 3. Mutagenic tests for propylene oxide with positive resultsa
---------------------------------------------------------------------------------------------------------
Test description System description Reference
Organism Strain/cell type
---------------------------------------------------------------------------------------------------------
Gene mutations
Forward mutations virus Bacillus subtilis Garro & Phillips (1980)b
phage phi 105
Reverse mutations (base-pair bacterium Escherichia coli WP2 McMahon et al. (1979)c
substitutions) Escherichia coli WP2 McMahon et al. (1979)c
Escherichia coli WP2 Hemminki & Falck (1979)
Escherichia coli WP2 Bootman et al. (1979)c
CM871, CM891
Bacillus subtilis Phillips et al. (1980)
Salmonella typhimurium Wade et al. (1978)
TA 1535, TA 100 McMahon et al. (1979)c;
Bootman et al. (1979)c;
Pfeiffer & Dunkelberg
(1980)d
Reverse mutations bacterium Escherichia coli SD4 Hussain & Osterman-Golkar
(1984)
Forward mutations Klebsiella pneumoniae Voogd et al. (1981)
Reverse mutations fungus Neurospora crassa W40 Kolmark & Giles (1955)
macroconidia
Schizosaccharomyces Heslot (1962)
pombe
Forward mutations fungus Schizosaccharomyces Migliore et al. (1982)c
pombe Pl
Sex-linked recessive lethals insect Drosophila melanogaster Hardin et al. (1983)
Forward mutations on specific mammal Chinese hamster Zamora et al. (1983);
locus (in vitro) ovary cells
---------------------------------------------------------------------------------------------------------
Table 3. (contd.)
-------------------------------------------------------------------------------------------------------------------
Test description System description Reference
Organism Strain/cell type
-------------------------------------------------------------------------------------------------------------------
DNA damage
Single-strand breaks bacterium Bacillus subtilis Phillips et al. (1980)b
mammal (in vitro) rat hepatocytes Sina et al. (1983)
calf thymus DNA Walles (1974)
Chromosome damage
Chromatid gaps and breaks, mammal (in vitro) human lymphocytes Bootman et al. (1979)
Chromosome breaks, fragments
Chromatid gaps, deletions, mammal (in vitro) epithelial rat liver Dean & Hodson-Walker
exchanges cells (1979)c
Micronuclei mammal (ip) mouse polychromatic Bootman et al. (1979)
(2 x 300 mg/kg) erythrocytes
-------------------------------------------------------------------------------------------------------------------
a For details of these studies, see text and data profile (IRPTC, 1984).
b Treatment of isolated DNA in vitro, followed by transformation (bacteria) or transfection (virus).
c A similar or slightly reduced effect after metabolic activation by S9 rat or mouse liver fraction.
d Chloropropanols were also mutagenic, but not as mutagenic as propylene oxide; 1,2-propanediol was not
mutagenic.
Propylene oxide causes single-strand breaks in isolated calf
thymus DNA, probably by alkylation of the phosphodiester bond
(Walles, 1974). Single-strand breaks were observed in the DNA of
isolated rat hepatocytes at concentrations that were not toxic
(Sina et al., 1983).
Propylene oxide caused chromosome aberrations in mammalian
cells in vitro, in particular, chromatid gaps and breaks (Bootman
et al., 1979; Dean & Hodson-Walker, 1979). However, no increases
in chromosome aberrations or sister-chromatid exchanges were found
in peripheral lymphocytes of male Cynomolgus monkeys, after long-
term vapour exposure, in vivo. The animals were exposed in
groups of 12 to concentrations of 0, 237, and 717 mg propylene
oxide/m3 air, for 7 h per day, 5 days per week, for 2 years
(Lynch et al., 1984b).
Two oral doses of up to 500 mg propylene oxide/kg body weight,
in gum tragacanth, administered within 24 h to mice, did not induce
micronuclei in the polychromatic erythrocytes. In contrast, 2
intraperitoneal doses of 300 mg/kg body weight in water, administered
within 24 h, gave a 5-fold increase over controls. No effects were
observed following ip administration of 2 doses of 75 mg/kg or 150
mg/kg (Bootman et al., 1979).
The results of the dominant-lethal assay were negative when
male rats were exposed, for 7 h per day, to propylene oxide vapour
at a concentration of 720 mg/m3, for 5 days prior to mating
(Hardin et al., 1983). A negative result was also obtained in a
dominant lethal assay in which male mice received, once a day, 0,
50, or 250 mg propylene oxide/kg body weight in gum tragacanth, by
gavage, for 2 weeks prior to mating (Bootman et al., 1979).
No increased frequency of abnormal sperm heads was observed,
1 - 9 weeks after exposure of mice for 5 days to propylene oxide
vapour at a concentration of 720 mg/m3, for 7 h per day (Hardin
et al., 1983). Similarly, no increase was observed in the frequency
of abnormal sperm heads in the groups of Cynomolgus monkeys exposed
for 2 years (Lynch et al., 1984c). These monkeys were used by
Sprinz et al. (1982) (section 7.2.2) and Lynch et al. (1984b)
(section 7.3).
7.4. Carcinogenicity
7.4.1. Oral exposure
Groups of 50 female Sprague Dawley rats received 2 doses of
15.0 or 60.0 mg propylene oxide/kg body weight per week, by gavage,
for a total of 112 weeks. The compound was dissolved in salad oil
and administered to fasting rats. Two groups of 50 female rats
served as vehicle controls or untreated controls. There was an
exposure-free period between the 79th and 82nd week because of an
outbreak of pneumonia. The rats were observed up to 150 weeks.
No statistical analysis of the results was reported. Mortality
rates were not affected by the exposures. There was a dose-related
incidence of squamous cell carcinoma of the forestomach. In
addition, one early carcinoma of the forestomach was observed. The
numbers of rats affected were 0, 2, and 19 in animals receiving 0,
15, and 60 mg/kg body weight, respectively. The combined incidences
of hyperkeratosis, hyperplasia, and papillomas were 0, 7, and 17 at
0, 15, and 60 mg/kg body weight, respectively. At the highest dose
level, one adenocarcinoma of the pylorus was also observed. There
was no treatment-related increase of any other tumour type
(Dunkelberg, 1982).
7.4.2. Inhalation exposure
The following studies have been reported by the US NTP (1984).
Groups of 50 Fischer 344/N rats and 50 B6C3F1 mice of each sex were
exposed to average propylene oxide vapour concentrations of 0, 470,
and 940 mg/m3 air, for 6 h per day, 5 days per week, over a 103-
week period. Three accidental over-exposures at the highest
exposure level occurred. The concentrations did not exceed 15 300
mg/m3 for a maximum of 12 min during these periods.
In rats, survival rate was not affected and was over 58% at the
end of the study, at all exposure levels. Growth was slightly
reduced from week 20 onwards. The respiratory epithelium of the
nasal turbinates showed a dose-related increase in the incidence of
suppurative inflammation of the mucosae, hyperplasia, and squamous
cell metaplasia. At 940 mg/m3, 2 out of 50 male and 3 out of 50
female rats had papillary adenomas involving the respiratory
epithelium and the underlying submucosal glands of the nasal
turbinates. No such tumours were observed in controls or low-dose
animals. In the thyroid of the female rats, a dose-related increase
in the combined incidences of C-cell adenomas and carcinomas occurred,
which was significant at 940 mg/m3. The combined incidence of
endometrial stromal polyps and sarcomas was reported to have
increased, at both exposure levels, in a dose-related manner.
According to the authors, the C-cell adenomas and C-cell carcinomas
were not related to treatment. The incidences of polyps and
sarcomas were within the historical control range for the species
tested. In males, an increase in skin keratoacanthomas was
observed, with a statistically-significant positive trend. Induced
non-neoplastic lesions further included testicular atrophy, acinar
cell atrophy in the pancreas of males, cytomegaly in the adrenal
cortex of females, and cystic endometrial hyperplasia (US NTP,
1984).
The survival rate in mice was decreased at 940 mg/m3, from
week 60 onwards and was 58 and 20% for male and female mice,
respectively, at the end of the study, compared with 84 and 76% for
male and female controls. Growth was slightly reduced from week 29
onwards. In the nasal turbinates, a dose-related increased
incidence of inflammation occurred. Squamous cell metaplasia was
observed in 1 male at 470 mg/m3 and 2 females at 940 mg/m3. At
940 mg/m3, haemangiosarcomas were found in the nasal cavity of 5
5 males and 2 females. Haemangiomas were also observed in the
nasal cavity of 5 males and 3 females at this dose level. The
increases in the incidence of haemangiosarcomas in the males and of
haemangiomas in both sexes were statistically-significant.
One squamous cell carcinoma and one papilloma were induced in
the nasal cavity of male mice at 940 mg/m3 and 2 adenocarcinomas
were induced in the nasal cavity of females at 940 mg/m3. None
was observed in controls or low-dose animals. The incidence of
adenocarcinomas of the mammary gland was increased at 940 mg/m3,
but did not exceed the historical control range for the species
tested. Induced non-neoplastic lesions further included ovarian
atrophy and suppurative inflammation, mainly of the uterus and
peritoneum (US NTP, 1984).
In a combined NIOSH toxicity-carcinogenicity study, groups of
80 male Fischer 344 rats were exposed to 237 or 717 mg propylene
oxide/m3, for 7 h per day, 5 days per week, over 2 years. A
control group contained 80 rats. There was an exposure-free period
of 2 weeks in month 16 because of a pulmonary infection, which
contributed to the mortality rate. The mortality rate was
increased at both exposure levels, and the increase was significant
at 717 mg/m3. The mean survival was 96 weeks at this exposure
level, compared with 103 weeks for control rats. Body weights were
reduced from the 2nd week at 717 mg/m3 and from the 39th week at
237 mg/m3. After 2 years, at both concentrations, the relative
weights of lungs, adrenals, and brain were increased and those of
the testes decreased. The only altered biochemical parameters were
increased haemoglobin concentrations in blood at both exposure
levels and increased activities of serum aspartate aminotransferase
(EC 2.6.1.1) and serum sorbitol dehydrogenase (EC 1.1.1.14) at 237
mg/m3. Dose-related increases in the incidence and severity of
inflammatory lesions in the lungs, nasal cavity, trachea, and
middle ear were observed. In the nasal passages, there was a dose-
related increased incidence of complex epithelial hyperplasia that
was significant at 717 mg/m3. Metaplasia was not observed. Two
rats showed nasal-cavity adenomas at 717 mg/m3. At this
concentration, there was also an increased incidence of multifocal
areas of atrophy and degeneration of skeletal muscles. No lesions
were observed in the nerves. The only statistically-significant
neoplastic change observed was an increased incidence of adrenal
phaeochromocytomas at both concentrations. The incidences were
8/78 in controls, 25/78 in the low-dose group, and 22/80 in the
high-dose group (Lynch et al., 1984a).
Another study on the possible toxic and carcinogenic properties
of inhaled propylene oxide was performed on randomly-bred Wistar
rats. Groups of 100 rats of each sex were exposed to propylene
oxide concentrations of 70, 242, or 712 mg/m3, for 6 h per day, 5
days per week, over a period of 123 - 124 weeks. Groups of 100
rats of each sex served as controls. Ten rats per sex and per
group were killed for examination after 12, 18, and 24 months of
exposure. No adverse effects were observed on general health,
behaviour, food intake, biochemistry, urinalysis, and haematology
in comparison with control rats. The mortality rate was increased
at the highest exposure level from week 73, in males, and from week
109, in females, and at the end of the exposure to 242 mg/m3 in
females. Rats of both sexes gained less weight than controls at
712 mg/m3, though the difference compared with controls became
smaller towards the end of the exposure. The relative and absolute
weights of adrenals, spleen, liver, and lungs of males were
increased at 712 mg/m3, but no pathological lesions were observed
in these organs. Male rats showed a lower incidence of pale
exorbital lachrymal glands at this exposure level. In both sexes,
the incidences of basal cell hyperplasia and atrophy of the
olfactory epithelium and the incidence of nest-like infolds of the
respiratory epithelium were increased mainly at the 2 highest
exposure levels. These changes were also noted at interim kills.
The severity of the changes increased only slightly, if at all,
with increasing length of exposure and with age. Squamous
metaplasia was not observed. One rat was found with squamous cell
carcinoma of the nose. In female rats, the incidence of benign
tumours of the mammary glands, mostly fibroadenomas, was increased
at 712 mg/m3 in comparison to both the concurrent controls and
the historical controls. Moreover, the number of females bearing 2
or more mammary fibroadenomas increased in a dose-related manner
from the lowest exposure level onwards when compared with controls.
The increased incidence of tubulopapillary mammary carcinomas at
712 mg/m3 was within the range of historical controls. With the
exclusion of mammary tumours, the total incidence of primary
tumours in females and the number of malignant tumour-bearing rats
of both sexes was increased at the highest exposure level (Reuzel &
Kuper, 1983). Following reports that ethylene oxide had induced
brain tumours in rats, the histopathological investigation of the
brain of the rats was extended. There was no evidence that
propylene oxide induced such tumours (Reuzel & Kuper, 1984).
7.4.3. Subcutaneous exposure
Groups of 100 female NMRI mice received subcutaneous doses of
0.1, 0.3, 1.0, or 2.5 mg propylene oxide per animal in tricaprylin
once a week, for 106 weeks. These groups were compared with 200
controls receiving tricaprylin and 200 untreated controls. In the
second year of exposure, mice were not treated for 11 weeks. Body
weights and survival rates were not affected by treatment. At the
site of injection, a dose-related increase in the incidence of
sarcomas (mainly fibrosarcomas) occurred. The numbers of mice
affected were 0/200 in untreated controls, 4/200 in tricaprylin
controls, and 3/100, 2/100, 12/100, and 15/100 at the 0.1, 0.3,
1.0, and 2.5 mg dose levels, respectively. The increases in
incidence at the 2 highest dose levels were statistically
significant. The first tumour appeared in week 38 (Dunkelberg,
1981).
7.5. Effects on Reproduction and Teratogenicity
When B6C3F1 mice of both sexes were exposed repeatedly for 2
years to propylene oxide at concentrations of 470 and 940 mg/m3
air (section 7.4.2), the incidence of ovarian atrophy was increased
at the higher exposure level (US NTP, 1984). In F344 rats, similarly
exposed, higher incidences of testicular atrophy were found at both
exposure levels, but they were not dose-related (US NTP, 1984).
Decreased relative weights of the testes were reported by Lynch et
al. (1984a), when male F344 rats were exposed repeatedly for 2 years
to propylene oxide at levels of 240 and 720 mg/m3 air.
Sperm head abnormalities were not detected in mice after
exposure for 5 days, 7 h per day, to 720 mg propylene oxide/m3
(Hardin et al., 1983), or in groups of 12 Cynomolgus monkeys,
exposed for 2 years to 240 and 710 mg propylene oxide/m3, for
7 h per day, 5 days per week. In monkeys, at both exposure levels,
sperm counts and sperm motility were reduced, and the sperm drive
range was elevated (Lynch et al., 1984c).
In a reproduction-teratogenicity study, groups of 41 - 44
female Sprague Dawley rats were exposed to 1190 mg propylene
oxide/m3 air, for 7 h per day, during days 7 - 16 of gestation
(Group 1), days 1 - 16 of gestation (Group 2), or for 3 weeks
(5 days/week) before mating and on days 1 - 16 of gestation
(Group 3). A group of 46 rats served as controls. The dams were
sacrificed on day 21. No deaths of dams occurred, their histology
was normal, and the percentage of pregnant rats was not affected by
the exposure. Toxic effects in dams were expressed as decreased
body weights and food consumption and increased relative weights of
kidneys in all exposed groups. The numbers of corpora lutea,
implantations per dam, and live fetuses were lower in group 3,
compared with those in the other groups. In group 1, the number of
resorptions was increased compared with that in group 2. The body
weights and lengths of fetuses were decreased in all exposed groups
compared with controls, but more so in group 3. In group 2,
increases in wavy rib and in reduced ossification, primarily of the
vertebrae and ribs, were observed (Hackett et al., 1982).
Groups of New Zealand rabbits were also exposed to 1190 mg/m3,
for 7 h per day, during days 1 - 19 or days 7 - 19 of gestation.
No evidence of toxicity was observed in the mothers, fetuses, or
embryos, and no developmental defects were noted (Hacket et al.,
1982).
Female rats administered a single oral dose of 260 mg/kg body
weight of propylene oxide showed disturbance of the estrus cycle.
Pregnant female rats administered an identical dose during the
first 2 weeks of gestation exhibited higher embryotoxicity and
lower offspring body weight compared with the controls. Male rats
exposed to a single oral LD50 (520 mg/kg) dose of propylene oxide
showed reduced sperm motility and damage to primary spermatocytes.
When such rats were mated with normal female rats, between 2 and 10
weeks after exposure, 50% of the males were infertile. The fertility
of the first generation was reduced by 23% (Antonova et al., 1981).
8. EFFECTS ON MAN
8.1. Exposure of Skin and Eyes; Skin Sensitization
Accidental exposure of the eyes of 3 persons to propylene
oxide (it was not reported whether this was liquid or vapour)
resulted in alterations in the cornea and conjunctiva (McLauglin,
1946).
Allergic contact dermatitis was diagnosed in 3 cases of
exposure to solutions of propylene oxide (Ketel, 1979; Jensen,
1981). Biopsy on the skin of these patients revealed spongiosis
in the epidermis, oedema in the cutis, and dense perivascular
infiltrates with mononuclear cells.
8.2. Accidental Inhalation Exposure
No data are available.
8.3. Occupational Inhalation Exposure
In the Federal Republic of Germany, 279 employees from 8
plants where alkene oxides were produced or processed, were
examined during 1978. They were employed for an average of 10.8
years. No clinical abnormalities were found that could be related
to exposure to alkene oxides. Propylene oxide levels were measured
by personal sampling over 1 - 10 h, but levels of exposure were not
reported. The workers were exposed to many other chemicals,
including ethylene oxide (Stocker & Thiess, 1979). Because of the
mixed exposure and unavailability of propylene oxide exposure data,
the Task Group was unable to evaluate this study.
8.4. Mortality Studies
In the Federal Republic of Germany, 602 workers were
investigated for mortality over the period 1928-80. The workers
had been employed for at least 6 months in 8 plants producing
ethylene oxide and propylene oxide, the latter produced only since
1959. A subcohort of 351 workers was observed for more than 10
years. Control data came from a styrene plant and from national
statistics. Propylene oxide levels, measured by personal sampling
(number of samples not reported), from 1978 to 1980, were far below
a time-weighted average of 240 mg/m3 over a working shift of 12 h
(Thiess et al., 1981a). Higher levels were measured for brief
periods. The workers were also exposed to many other chemicals,
some of which might be carcinogenic for human beings. There were
56 deaths compared with 76.6 expected. No significant excess of
deaths could be found due to any cause in the cohort of 602 workers.
In the sub-cohort of 351 workers, there was a significant increase
in mortality rate due to kidney disease (3 compared with 0.4
expected). There was 1 death from gall-bladder cancer, 1 death
from urinary-bladder cancer, 1 death from brain cancer, and 1 death
from myeloid leukaemia. The stomach tumours were observed compared
with 1.8 expected (Thiess et al., 1981b). Since the workers were
also exposed to many other chemicals, such as butylene oxide,
epichlorohydrin, dioxan, dichloropropane, and chlorohydrins, the
Task Group was unable to evaluate this study in relation to
propylene oxide.
8.5. Mutagenicity and Related End-Points
In 43 male workers from the cohort of 602 workers discussed in
section 8.4 (Thiess et al., 1981b), no increase in chromosome
aberration rate was found in 2 groups of workers exposed to alkene
oxides for average periods of either 12.5 or 17.6 years; in addition,
workers in the latter group had at least one accidental high exposure
to ethylene oxide. The results were also negative in a group of
workers exposed once for a brief period following an accident. On
the other hand, the aberration rate was significantly elevated in
workers exposed for more than 20 years (Thiess et al., 1981a).
For the reason mentioned in section 8.4, the Task Group was
unable to evaluate this study in relation to propylene oxide.
Unscheduled DNA synthesis, induced in vitro by the mutagen
N-acetoxy-2-acetylaminofluorene, was inhibited in the lymphocytes
of 23 workers from a factory in Sweden, where starch was modified
with propylene oxide. The workers had been exposed for 1 - 20
years. At the time of the study, exposure was generally below a
time-weighted average of 28 mg/m3. However, short-term exposures
of up to 2370 mg/m3 were recorded for some workers (Pero et al.,
1982).
9. EVALUATION OF THE HEALTH RISKS FOR MAN AND EFFECTS ON
THE ENVIRONMENT
Exposure of man to propylene oxide mainly occurs through
inhalation at the work-place.
Data are insufficient to estimate the exposure to propylene
oxide residues in food after fumigation and sterilization. The
main conversion products in foodstuffs are chloropropanols and
1,2-propanediol, which are more persistent than the parent compound
(section 4.2). No adverse effects have been reported due to the
ingestion of propylene oxide and its reaction products in food.
No ambient air monitoring data are available, but the lowest
annual average concentration at distances of up to 20 km from
production plants has been assessed, by modelling, to be less than
4.836 x 10-8 mg/m3(section 4.1). The risk for human health
under such conditions of exposure is likely to be negligible.
Propylene oxide is highly soluble in water but is likely to
evaporate to a great extent. However, no data on the rate of
evaporation are available. In neutral fresh water, propylene
oxide is converted to 1,2-propanediol and, in marine waters, to
halopropanols, but, even in the presence of micro-organisms, these
processes are slow (section 3.2). Because of the low log n-octanol
water-partition coefficient, propylene oxide and its conversion
products are unlikely to bioaccumulate (Table 1). The toxicity of
propylene oxide for aquatic organisms is low. The available LC50s
are above approximately 90 mg/litre (section 6). Thus, the
probability of an adverse impact on the aquatic environment is
considered low.
Eight-hour time-weighted occupational exposure in propylene
oxide production and use is normally below 5 mg/m3, with
occasional peak exposures up to 9000 mg/m3 (section 4.3).
Inhaled propylene oxide is probably readily absorbed,
distributed throughout the body, and rapidly metabolized. The
half-life in rat tissues has been estimated to be 40 min (section
5.2). There are no data on skin absorption.
An aqueous solution of propylene oxide (100 or 200 g/litre)
is irritating to rabbit skin when applied under occlusive cover
(section 7.1.2). In man, corneal and conjunctival damage, and
allergic contact dermatitis have been reported through accidental
exposure to propylene oxide vapour (section 8.1).
The 4-h LC50s for the rat and the mouse were 9500 mg/m3
and 4100 mg/m3, respectively. Dogs exposed by inhalation, once
for 4 h, to a concentration of 3230 - 5880 mg/m3 showed
salivation, lachrymation, nasal discharge, and vomiting. Deaths
were observed at 4750 - 5880 mg/m3 but not at 3230 mg/m3
(section 7.1.3).
On repeated exposure of various species to propylene oxide
vapour, 7 h per day, 5 days per week, for 112 - 218 days, at
concentrations of 0, 240, 460, and 1080 mg/m3, rabbits and
monkeys did not show any adverse effects on appearance, mortality,
growth, and histopathology of internal organs. Rats and guinea-
pigs showed irritation of the respiratory passages and lung damage
histologically at 1080 mg/m3. An increase in lung weight was
observed in female guinea-pigs at concentrations of 460 mg/m3 or
more. No effect was observed in any species at a concentration of
240 mg/m3. A concentration of 3400 mg/m3 administered for 2
weeks, 6 h/day, 5 days per week, resulted in dyspnoea and death in
rats. In the same study, mice showed dyspnoea at 460 and 1150 mg/m3
(section 7.2.2).
Rats and mice exposed for 2 years showed increased incidences
of inflammatory and proliferative lesions of the nasal epithelium
from an exposure level of 470 mg/m3. In rats, the inflammatory
lesions and hyperplasia were also observed at an exposure level of
240 mg/m3, but not at 70 mg/m3 (section 7.4.2).
Depression of the central nervous system, the severity of
which increased with increasing level and length of exposure, was
observed when rats and mice were exposed by inhalation to single
high concentrations of propylene oxide (section 7.1.3). Although
no clinical central nervous system effects were reported when
monkeys were exposed to 237 and 717 mg/m3 for 2 years, some
histopathological changes were observed in the treated animals
(section 7.2.2). It should be noted that these data cannot be
evaluated for estimating hazard for man, since only 2 monkeys were
examined in each of the exposed groups, and the lesion appeared
also in one of the 2 controls.
Summarizing the results of animal studies (excluding
mutagenic, carcinogenic, and reproduction effects) the no-
observed-adverse-effect level for prolonged repeated 6- to 7-h
daily exposure is 70 mg/m3.
Propylene oxide administered by inhalation for 2 years
produced ovarian atrophy in mice at 940 mg/m3. Testicular
atrophy was found at 240 and 720 mg/m3 in rats (section 7.5).
No sperm head abnormalities were detected in mice after
exposure for 5 days to 720 mg/m3 or in Cynomolgus monkeys
exposed to 240 or 710 mg/m3 for 2 years, but sperm count and
motility were reduced, and sperm drive range (time to traverse a
linear path) increased in the monkeys. A reduced sperm motility
and damage to spermatocytes were observed in male rats treated with
an oral LD50 (520 mg/kg body weight) dose. A reduced fertility
was observed when these males were mated between 2 and 10 weeks
after exposure with normal females (sections 7.3, 7.5).
Reduced fetal ossification of vertebrae and ribs and wavy ribs
were observed when pregnant Sprague Dawley rats were exposed to
1190 mg propylene oxide/m3, 7 h per day, on days 1 - 16 of
gestation. When rats were additionally exposed for 3 weeks before
mating, the numbers of corpora lutea, implantations per dam, and
live fetuses were decreased. No teratogenic or fetotoxic effects
were found in New Zealand rabbits exposed to 1190 mg/m3 (section
7.5).
Propylene oxide is mutagenic in microorganisms and insects and
produces DNA damage and chromosomal aberrations in mammalian cells
in vitro. It produced micronuclei in mouse erythrocytes after
parenteral injection of 2 doses of 300 mg/kg body weight but not
after oral exposure. No chromosomal aberrations or sister
chromatid exchanges occurred in monkeys exposed by inhalation to
237 and 717 mg/m3, for 7 h/day, 5 days/week, for 2 years. No
dominant-lethal effects were observed in rats or mice following
inhalation or oral exposure, respectively, in 2 studies (section
7.3). No adequate data on chromosomal effects in human beings are
available.
In studies on the carcinogenicity of propylene oxide in rats
and mice, malignant tumours were mainly observed at the site of
entry into the body: for stomach, at 15 and 60 mg/kg body weight;
for subcutaneous tissues, at 1.0 - 2.5 mg; and, for nasal passages,
at 940 mg/m3. At the dose levels at which these tumours were
induced, propylene oxide produced tissue damage. This damage may
have played a role in the appearance of the tumours. Propylene
oxide also produced an increase in the incidence of multiple benign
mammary tumours in female rats following inhalation exposure
(section 7.4). No adequate epidemiological studies on cancer
incidence in exposed human populations have been carried out.
Taking into account the body of available data - the
alkylating nature of propylene oxide, the formation of DNA
adducts, the positive responses in in vitro mutagenesis assays,
the carcinogenic effects in animals at the sites of entry into the
body, and the absence of adequate data on cancer in human beings -
propylene oxide should be considered as a possible human carcinogen.
Thus, for practical purposes, propylene oxide should be regarded as
if it presented a carcinogenic risk for man, and levels in the
environment should be kept as low as feasible.
10. RECOMMENDATIONS FOR FURTHER RESEARCH
1. A thorough detailed study on the absorption, distribution,
and metabolism of propylene oxide should be conducted.
2. Biological indicators should be developed that quantify both
the incidence and severity of human exposure.
3. The epidemiological studies indicating an increased risk of
cancer in workers exposed to propylene oxide in combination
with other chemicals suggest that additional studies should
be conducted on populations whose exposure has been primarily
to propylene oxide. The adequate quantification of past
exposures should be a part of these studies.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
An International Agency for Research on Cancer Working Group
(IARC, 1985) evaluated the carcinogenicity of propylene oxide and
concluded that:
"There is sufficient evidence for the carcinogenicity of
propylene oxide to experimental animals; there is inadequate
evidence for its carcinogenicity to humans. It is noted that,
in the absence of adequate data in humans, it is reasonable,
for practical purposes, to regard chemicals for which there is
sufficient evidence of carcinogenicity in experimental animals
as if they represented a carcinogenic risk to humans."
REFERENCES
ADDY, J.K. & PARKER, R. (1963) The mechanism of epoxide
reactions. V. The reaction of 1,2-epoxypropane with chloride
ions in water under neutral and acidic conditions. J. Chem.
Soc., 915-921.
ANDERSON, G.E. (1983) Human exposure to atmospheric
concentrations of selected chemicals. Vol. II, Research
Triangle Park, North Carolina, US Environmental Protection
Agency, pp. 574-601 (Prepared by Systems Applications, Inc.,
San Rafael, California, No. SA1/EF81-156R2).
ANTONOVA, V.I., ZOMMER, E.A., KUZNETSOVA, A.D., & PETROVA,
N.A. (1981) [Toxicology of propylene oxide and regulation
for surface water.] Gig. i Sanit., 46: 76-79 (in Russian).
BOGYO, D.A., LANDE, S.S., MEYLAND, W.M., HOWARD, P.H., &
SANTODONATO, J. (1980) Investigation of selected potential
environmental contaminants: Epoxides, Syracuse, New York,
Centre for Chemical Hazard Assessment, Syracuse Research
Corporation (Prepared for US EPA) (US EPA 560/11-80-005, PB
80-183197).
BONT, J.A.M. DE, DIJKEN, J.P. VAN, & GINKEL, K.G. VAN (1982)
The metabolism of 1,2-propanediol by the propylene oxide
utilizing bacterium Nocardia 60. Biochem. Biophys. Acta, 714:
465-470.
BOOTMAN, J., LODGE, D.C., & WHALLEY, H.E. (1979) Mutagenic
activity of propylene oxide in bacterial and mammalian
systems. Mutat. Res., 67: 101-112.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979a) The acute
toxicity of some petrochemicals to goldfish. Water Res., 13:
623-626.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) BOD and
COD of some petrochemicals. Water Res., 13: 627-630.
CALLEMAN, C.J., EHRENBERG, L., JANSSON, B., OSTERMAN-GOLKAR,
S., SEGERBACK, D., SVENSSON, K., & WACHTMEISTER, C.A. (1978)
Monitoring and risk assessment by means of alkyl groups in
haemoglobin in persons occupationally exposed to ethylene
oxide. J. environ. Pathol. Toxicol., 2: 247-442.
CONWAY, R.A., WAGGY, G.T., SPIEGEL, M.H., & BERGLUND, R.L.
(1983) Environmental fate and effects of ethylene oxide.
Environ. Sci. Technol., 17: 107-112.
COOKSON, M.J., SIMS, P., & GROVER, P.L. (1971) Mutagenicity
of epoxides of polycyclic hydrocarbons correlates with
carcinogenicity of parent hydrocarbons. Nature (New Biol.),
234: 186-187.
CREWS, R.C. (1974) Effects of propylene oxide on selected
species of fishes, Florida, Air Force Armament Center, Eglin
Air Force Base (US NTIS AD/A003637).
CUPITT, L.T. (1980) Fate of toxic and hazardous materials in
the environment, Research Trianlge Park, North Carolina, US
Environmental Protection Agency, Environmental Sciences
Laboratory, Office of Research and Development (US EPA
600/3-80-084, PB 80-221948).
DAVIES, R.E., HARPER, K.H., & KYNOCH, S.R. (1972)
Inter-species variation in dermal reactivity. J. Soc. Cosmet.
Chem., 23: 371-382.
DEAN, B.J. & HODSON-WALKER, G. (1979) An in vitro chromosome
assay using cultured rat liver cells. Mutat. Res., 64: 329-337.
DENT, J.G. & SCHNELL, S.R. (1981) Inhibition of microsomal
membrane-bound and purified epoxide hydrolase by C2-C8
1,2-alkene oxides. Biochem. Pharmacol., 30: 1712-1714.
DUNKELBERG, H. (1981) [Carcinogenic activity of ethylene
oxide and its reaction products 2-chloroethanol,
2-bromoethanol, ethylene glycol, and diethylene glycol. I.
Carcinogenicity of ethylene oxide in comparison with
1,2-propylene oxide after subcutaneous administration in
mice.] Zbl. Bakt. Hyg. (I. Abt. Orig. B), 174: 383-404 (in
German).
DUNKELBERG, H. (1982) Carcinogenicity of ethylene oxide and
1,2-propylene oxide upon intragastric administration to rats.
Br. J. Cancer, 46: 924-933.
EHRENBERG, L., HIESCHE, K.D., OSTERMAN-GOLKAR, S., & WENNBERG,
I. (1974) Evaluation of genetic risks of alkylating agents:
Tissue doses in the mouse from air contaminated with ethylene
oxide. Mutat. Res., 24: 83-103.
FAO/WHO JOINT EXPERT COMMITTEE ON FOOD ADDITIVES (1974) WHO
Food Add. Ser., 5: 491-497 (Toxicological Monographs arising
from the Seventeenth Report of the Joint FAO/WHO Expert
Committee on Food Additives).
FARMER, P.B., GORF, S.M., & BAILEY, E. (1982) Determination
of hydroxypropylhistidine in haemoglobin as a measure of
exposure to propylene oxide using high resolution gas
chromatography mass spectrometry. Biomed. Mass Spectrometr.,
9: 69-71.
FEDERAL REGISTER (1971) Paragraph 121.1076, propylene oxide,
subpart D, food additives, Washington DC, US Environmental
Protection Agency, Food and Drug Administration, p. 33.
FJELLSTEDT, T.A., ALLEN, R.H., DUNCAN, B.K., & JAKOBY, W.B.
(1973) Enzymatic conjugation of epoxides with glutathione. J.
Biol. Chem., 248: 3702-3707.
FLORES, G.H. (1983) Reducing plant pollution exposure:
controlling exposure to alkene oxides. Chem. Eng. News, 79:
39-43.
GARRO, A.J. & PHILLIPS, R.A. (1980) Detection of
mutagen-induced lesions in isolated DNA by marker rescue of
Bacillus subtilis phage stopb105. Mutat. Res., 73: 1-13.
GUENGERICH, F.P. & MASON, P.S. (1980) Alcohol dehydrogenase-
coupled spectrophotometric assay of epoxide hydratase
activity. Anal. Biochem., 104: 445-451.
GUNTHER, D.A. (1965) Determination of adsorbed ethylene and
propylene oxides by distillation and titration. Anal. Chem.,
37: 1172-1173.
HACKETT, P.L., BROWN, M.G., BUSCHBOM, R.L., CLARK, M.L.,
MILLER, R.A., MUSIC, R.L., ROWE, S.E., SCHIRMER, R.E., &
SIKOV, M.R. (1982) Teratogenic study of ethylene and
propylene oxide and N-butyl acetate, Richland, Washington,
Batelle Pacific Northwest Laboratories (Prepared for NIOSH)
(PB 83-258038).
HARDIN, B.D., SCHULER, R.L., MCGINNIS, P.M., NIEMEIER, R.W., &
SMITH, R.J. (1983) Evaluation of propylene oxide for
mutagenic activity in 3 in vivo test systems. Mutat. Res.,
117: 337-344.
HATFIELD, R. (1957) Biological oxidation of some organic
compounds. Ind. Eng. Chem., 49: 192-196.
HELLMAN, T.M. & SMALL, F.H. (1974) Characterization of the
odour properties of 101 petrochemicals using sensory method.
J. Air Pollut. Contr. Assoc., 24: 979-982.
HEMMINKI, K. & FALCK, K. (1979) Correlation of mutagenicity
and 4-(p-nitrobenzyl)-pyridine alkylation by epoxides.
Toxicol. Lett., 4: 103-106.
HEMMINKI, K., PAASIVIRTA, J., KURKIRINNE, T., & VIRKKI, L.
(1980) Alkylation products of DNA bases by simple epoxides.
Chem.-biol. Interact., 30: 259-270.
HESLOT, H. (1962) Etude quantitative de réversions
biochimiques induites chez la levure Schizosaccharomyces pombe
par des radiations et des substances radiomimétiques. Abh.
Dtsch. Akad. Wiss. Berlin, Kl. Med., 1: 193-228.
HEUSER, S.G. & SCUDAMORE, K.A. (1969) Determination of
fumigant residues in cereals and other foodstuffs: A multi-
detection scheme for gas chromatography of solvent extracts.
J. Sci. Food Agric., 20: 566-572.
HIRASHIMA, T., OGUMA, T., HOSOGAI, Y., & FUJII, S. (1970)
[Studies on gaseous antimicrobial agents. III. Determination
of propylene oxide residue in food wrappings and containers.]
J. Food Hyg. Soc. Jpn, 11: 161-163 (in Japanese).
HUSSAIN, S. & OSTERMAN-GOLKAR, S. (1984) Dose-response
relationships for mutations induced in E. coli by some model
compounds. Hereditas, 101: 57-68.
IARC (1976) Cadmium, nickel, some epoxides, miscellaneous
industrial chemicals and general considerations on volatile
anaesthetics, Lyons, International Agency for Research on
Cancer, 293 pp (IARC Monographs on the Evaluation of
Carcinogenic Risk of Chemicals to Man, Vol. 11).
IARC (1985) Allyl compounds, aldehydes, epoxides, and
peroxides, Lyons, International Agency for Research on Cancer
(Monographs on the Evaluation of Carcinogenic Risk of
Chemicals to Man, Vol. 36).
IRPTC (1984) Data profile on propylene oxide, Geneva,
Switzerland, International Register on Potentially Toxic
Chemicals, United Nations Environment Programme.
JACOBSON, K.H., HACKLEY, E.B., & FEINSILVER, L. (1956) The
toxicity of inhaled ethylene oxide and propylene oxide
vapours. Arch. ind. Health, 13: 237-244.
JENSEN, O. (1981) Contact allergy to propylene oxide and
isopropyl alcohol in a skin desinfectant swab. Contact
Dermatit., 7: 148-150.
KETEL, W.G. VAN (1979) Contact dermatitis from propylene
oxide. Contact Dermatit., 5: 191-192.
KOLMARK, H.G. & GILES, N.H. (1955) Comparative studies of
monoepoxides as inducers of reverse mutations in Neurospora.
Genetics, 40: 890-902.
KOSKIKALLIO, J. & WHALLEY, E. (1959) Effect of pressure on
the spontaneous and the base-catalysed hydrolyses of epoxides.
Can. J. Chem., 37: 783-787.
KROST, K.J., PELLIZZARI, E.D., WALBURN, S.G., & HUBBARD, S.A.
(1982) Collection and analysis of hazardous organic
emissions. Anal. Chem., 54: 810-817.
LAWLEY, P.D. & JARMAN, M. (1972) Alylation by propylene
oxide of deoxyribonucleic acid, adenine, guanosine, and
deoxyguanylic acid. Biochem. J., 126: 893-900.
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., BURG, J.R., GROTH,
D.H., KHAN, A., ACKERMAN, L.J., & COCKRELL, B.Y. (1984a)
Carcinogenic and toxicologic effects of inhaled ethylene oxide
and propylene oxide in F344 rats. Toxicol. appl. Pharmacol.,
76(1): 69-84.
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., BURG, J.R., GULATI,
D.K., KAUR, P., & SABHARWAL, P.S. (1984b) Sister-chromatid
exchanges and chromosome aberrations in lymphocytes from
monkeys exposed to ethylene oxide and propylene oxide by
inhalation. Toxicol. appl. Pharmacol., 76(1): 85-95.
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., LAL, J.B., BURG,
J.R., GULATI, D.K., ZAVOS, P.M., & SABHARWAL, P.S. (1984c)
Toxic and mutagenic effects of inhaled ethylene oxide and
propylene oxide on spermatogenic functions in monkeys.
Toxicologist, 3: 60.
MCLAUGHLIN, R.S. (1946) Chemical burns of the human cornea.
Am. J. Ophthalmol., 29: 1355-1362.
MCMAHON, R.E., CLINE, J.C., & THOMPSON, C.Z. (1979) Assay of
855 test chemicals in ten tester strains using a new
modification of the Ames test for bacterial mutagens. Cancer
Res., 39: 682-693.
MESTRES, R. & BARROIS, C. (1964) Recherche et dosage de
l'oxyde de propylène et du propylène-glycol dans les fruits
par chromatographie gazeuse directe. Bull. Soc. Pharm.
Montpellier, 24: 47-63.
MIGLIORE, L., ROSSI, A.M., & LOPRIENO, N. (1982) Mutagenic
action of structurally-related alkene oxides on Schizosac-
charomyces pombe: The influence, "in vitro", of mouse liver
metabolizing system. Mutat. Res., 102: 425-437.
MISHMASH, E.H. & MELOAN, C.E. (1972) Indirect
spectrophotometric determination of nanomale quantities of
oxiranes. Anal. Chem., 44: 835-836.
NELIS, H.J.C.F. & SINSHEIMER, J.E. (1981) A sensitive
fluorimetric procedure for the determination of aliphatic
epoxides under physiological conditions. Anal. Biochem., 115:
151-157.
NIOSH (1977) Manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, US Department of Health, Education and
Welfare, National Institute for Occupational Safety and
Health, Vol. 2, pp. S75-1 - S75-8.
OESCH, F. (1974) Purification and specificity of a human
microsomal epoxide hydratase. Biochem. J., 139: 77-88.
OGUMA, T., HOSOGAI, Y., FUJII, S., & KAWASHIRO, I. (1968)
[Gaseous antimicrobial agents. I. Determination of propylene
oxide residues in foods.] J. Food Hyg. Soc. Jpn, 9: 395-398
(in Japanese).
OGUMA, T., HOSOGAI, Y., & FUJII, S. (1969) [Studies on
gaseous antimicrobial agents. II. Determination of propylene
oxide residue in fats and oils.] J. Food Hyg. Soc. Jpn, 10:
37-39 (in Japanese).
OSTERMAN-GOLKAR, S., EHRENBERG, L., SEGERBACK, D., &
HALLSTROM, I. (1976) Evaluation of genetic risks of
alkylating agents. II. Haemoglobin as a dose monitor. Mutat.
Res., 34: 1-10.
OSTERMAN-GOLKAR, S., FARMER, P.B., SEGERBACK, D., BAILEY, E.,
CALLEMAN, C.J., SVENSSON, K., & EHRENBERG, L. (1983)
Dosimetry of ethylene oxide in the rat by quantitation of
alkylated histidine in haemoglobin. Teratog. Carcinog.
Mutagen., 3: 395-405.
OSTERMAN-GOLKAR, S., BAILEY, E., FARMER, P.B., GORF, S.M., &
LAMB, J.H. (1984) Monitoring exposure to propylene oxide
through the determination of haemoglobin alkylation. Scand. J.
Work environ. Health, 10: 99-102.
PERO, R.W., BRYNGELSSON, T., WIDEGREN, B., HOGSTEDT, B., &
WELINDER, H. (1982) A reduced capacity for unscheduled DNA
synthesis in lymphocytes from individuals exposed to propylene
oxide and ethylene oxide. Mutat. Res., 104: 193-200.
PFEIFFER, E.H. & DUNKELBERG, H. (1980) Mutagenicity of
ethylene oxide and propylene oxide and of the glycols and
halohydrins formed from them during the fumigation of
foodstuffs. Food Cosmet. Toxicol., 18: 115-118.
PHILLIPS, R.A., ZAHLER, S.A., & GARRO, A.J. (1980) Detection
of mutagen-induced lesions in isolated DNA using a new
Bacillus subtilis transformation-based assay. Mutat. Res., 74:
267-281.
RADDING, S.B., LUI, D.H., JOHNSON, H.L., & MILL, T. (1977)
Review of the environmental fate of selected chemicals,
Washington DC, US Environmental Protection Agency, Office of
Toxic Substances (US EPA 560/5-77-003, PB 267121).
RAGELIS, E.P., FISHER, B.S., KLIMECK, B.A., & JOHNSON, C.
(1968) Isolation and determination of chlorohydrins in foods
fumigated with ethylene oxide or with propylene oxide. J.
Assoc. Offic. Agric. Chem., 51: 709-715.
RANDERATH, K., REDDY, M.V., & GUPTA, R.C. (1981) 32P-
labelling test for DNA damage. Proc. Natl Acad. Sci., 78:
6126-6129.
REUZEL, P.G.J. & KUPER, C.F. (1983) Chronic (28-month)
inhalation toxicity/carcinogenicity study of 1,2-propylene
oxide in rats. Final Report, Zeist, The Netherland, TNO,
Division of Nutrition and Food Research (Report No.
V82.215/280853).
REUZEL, P.G.J. & KUPER, C.F. (1984) Chronic (28-month)
inhalation toxicity/carcinogenicity study of 1,2-propylene
oxide in rats, Zeist, The Netherland, TNO, Division of
Nutrition and Food Research (Addendum to Report No.
V82.215/280853).
ROSS, W.C.J. (1950) The reactions of certain epoxides in
aqueous solutions. J. Chem. Soc., 2257-2271.
ROWE, V.K., HOLLINGSWORTH, R.L., OYEN, F., MCCOLLISTER, D.D.,
& SPENCER, H.C. (1956) Toxicity of propylene oxide deter-
mined on experimental animals. Arch. ind. Health, 13: 228-236.
RUDDICK, J.A. (1972) Toxicology, metabolism, and biochemis-
try of 1,2-propanediol. Toxicol. appl. Pharmacol., 21: 102-111.
RUSSELL, J.W. (1975) Analyses of air pollutants using
sampling tubes and gas chromatography. Environ. Sci. Technol.,
9: 1175-1178.
SCUDAMORE, K.A. & HEUSER, S.G. (1971) Ethylene oxide and its
persistent reaction products in wheat flour and other
commodities: Residues from fumigation or sterilization, and
effects of processing. Pestic. Sci., 2: 80-91.
SINA, J.F., BEAN, C.L., DYSART, G.R., TAYLOR, V.I., & BRADLEY,
M.O. (1983) Evaluation of the alkaline elution/rat
hepatocyte assay as a predictor of carcinogenic/mutagenic
potential. Mutat. Res., 113: 357-391.
SMYTH, H.F., SEATON, J., & FISHER, L. (1941) The single dose
toxicity of some glycols and derivatives. J. ind. Hyg.
Toxicol., 23: 259-268.
SNELLINGS, W.M., WEIL, C.S., & MARONPOT, R.R. (1984) A
two-year inhalation study of the carcinogenic potential of
ethylene oxide in Fischer 344 rats. Toxicol. appl. Pharmacol.,
75: 105-117.
SPRINZ, H., MATZKE, H., & CARTER, J. (1982) Neuropatho-
logical evaluation of monkeys exposed to ethylene and
propylene oxide, Kansas City, Missouri, Midwest Research
Institute (Prepared for NIOSH) (PB 83-134817).
STEELE, L. & HADZIYEV, D. (1976) Sterilization of dehydrated
potato granules with propylene oxide. Z. Lebensm.
Unters.-Forsch., 162: 387-399.
STOCKER, W.G. & THIESS, A.M. (1979) Morbidity study on
workers exposed to ethylene oxide/propylene oxide. In: The 7th
Medichem Congress, Gera, 11-15 September, 1979.
STORCK, W.J. (1981) Output of big-volume chemicals fell in
1980. Chem. Eng. News, 59: 36-39.
SVENSSON, K. & OSTERMAN-GOLKAR, S. (1984) Kinetics of
metabolism of propene and covalent binding to macromolecules
in the mouse. Toxicol. appl. Pharmacol., 73: 363-372.
SWAN, J.D. (1954) Determination of epoxides with sodium
sulfite. Anal. Chem., 26: 878-880.
TACHIZAWA, H., MACDONALD, T.L., & NEAL, R.A. (1982) Rat
liver microsomal metabolism of propyl halides. Mol.
Pharmacol., 22: 745-751.
THIESS, A.M., SCHWEGLER, H., FLEIG, I., & STOCKER, W.G.
(1981a) Mutagenicity study of workers exposed to alkylene
oxides (ethylene oxide/propylene oxide) and derivatives. J.
occup. Med., 23: 343-347.
THIESS, A.M., FRENZEL-BEYME, R., LINK, R., & STOCKER, W.H.
(1981b) Mortality study on employees exposed to alkylene
oxides (ethylene oxide/propylene oxide) and their derivatives.
In: Proceedings of the International Symposium on Prevention
of Occupational Cancer, Helsinki, Finland, pp. 249-259.
US EPA (1981) Human exposure to atmospheric concentrations
of selected chemicals, Research Triangle Park, North Carolina,
US Environmental Protection Agency, Office of Air Quality
Planning and Standards (US NTIS PB 84-102540, PB 83-265249).
US NTP (1984) Toxicology and carcinogenesis studies of
propylene oxide in F344/N rats and B6C3F1 mice (inhalation
studies), Research Triangle Park, North Carolina, National
Toxicology Program (NTP 83-020) (NIH Publication No. 84-2523).
VOJNOVICH, C. & PFEIFER, V.F. (1967) Reducing the microbial
population of flour during milling. Cereal Sci. Today, 12:
54-60.
VOOGD, C.E., STEL, J.J. VAN DE, & JACOBS, J.J.J.A.A. (1981)
The mutagenic action of aliphatic epoxides. Mutat. Res., 89:
269-282.
WADE, D.R., AIRY, S.C., & SINSHEIMER, J.E. (1978)
Mutagenicity of aliphatic epoxides. Mutat. Res., 58: 217-223.
WALLES, S.A. (1974) The influence of some alkylating agents
on the structure of DNA in vitro . Chem.-biol. Interact., 9:
97-103.
WEBBER, D. (1984) Basic chemical output returns to growth.
Top 50 chemical products. Chem. Eng. News, May 7: 8-10.
WEIL, C.S., CONDRA, N., HAUN, C., & STRIEGEL, J.A. (1963)
Experimental carcinogenicity and acute toxicity of
representative epoxides. Am. Ind. Hyg. Assoc. J., 24: 305-325.
WHO (1978) Environmental health problems associated with the
manufacture and uses of synthetic organic chemicals, Geneva,
World Health Organization (Report No. HCS/78.2).
WHO (1985) EHC 55: Ethylene oxide, Geneva, World Health
Organization, 79 pp.
ZAMORA, P.O., BENSON, J.M., LI, A.P., & BROOKS, A.L. (1983)
Evaluation of an exposure system using cells grown on collagen
gels for detecting highly-volatile mutagens in the CHO/HGPRT
mutation assay. Environ. Mutagen., 5: 795-801.
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
In an acute toxicity test on goldfish (Carassius auratus),
in static fresh water, at 20 °C, pH between 6 and 8, and a
dissolved oxygen content greater than 4 mg/litre, the 24-h LC50
for propylene oxide was 170 mg/litre (Bridié et al., 1979a). In
other static tests, with the fresh water species bluegill sunfish
(Lepomis machrochirus) and mosquito fish (Gambusia affinis),
96-h LC50 values for propylene oxide were 215 and 141 mg/litre,
respectively, at 23 °C. For the common mullet (Mugil cephalus),
a marine fish, a static 96-h LC50 for propylene oxide of 89
mg/litre was determined at 23 °C and a water salinity of 1.5% (no
water analysis was reported) (Crews, 1974). The 24-h LC50 for
1,2-propanediol (a product of propylene oxide) for goldfish was
over 5000 mg/litre (Bridié et al., 1979a). Propylene oxide is very
soluble in aqueous media; the log n-octanol water partition
coefficient was reported to be -0.13 (Radding et al., 1977) and,
therefore, it is not expected to bioaccumulate.
7. EFFECTS ON ANIMALS
7.1. Single Exposures
7.1.1. Oral exposure
In 2 studies, the oral LD50s for the rat were reported to be
1140 mg/kg body weight and 520 mg/kg body weight for males, and
540 mg/kg for females (Smyth et al., 1941; Antonova et al., 1981);
the difference between 16 and 84% mortality was caused by a dose of
only approximately 300 mg/kg body weight, indicating a rather steep
dose-effect relationship. In one of these studies, oral LD50s for
male mice and guinea-pigs were 630 and 660 mg/kg body weight,
respectively (Antonova et al., 1981).
At lethal oral doses, necrosis of the stomach mucosa was
observed in rats. Succinate dehydrogenase (EC 1.3.99.1) activity
was reported to be decreased in the stomach mucosa. The liver
cells showed oedema and fatty changes. In serum, alanine amino-
transferase (EC 2.6.1.2) and aspartate aminotransferase (EC 2.6.1.1)
activity and histamine levels were increased. Kidney function was
disturbed. Mature lymphocytes were reduced in the spleen (Antonova
et al., 1981).
7.1.2. Skin and eye irritation
Solutions of 100 or 200 g propylene oxide/litre water, applied
under a plastic cover on the intact skin of rabbits produced hyper-
aemia, oedema, and finally scars after 6 or more min of exposure.
The intensity of the effects was proportional to the exposure time
(Rowe et al., 1956).
No, or only slight, irritation was observed when undiluted
1,2-propanediol, a possible reaction product of propylene oxide in
water, was applied to the skin of various animals under occlusive
conditions for 24 or 72 h (Davies et al., 1972).
When 5 µl of undiluted propylene oxide was applied once on the
centre of the cornea of rabbits, a severe burn resulted with
necrosis (Weil et al., 1963).
7.1.3. Inhalation exposure
In inhalation studies, the 4-h LC50s for rats and mice were
9500 and 4100 mg/m3, respectively (Jacobson et al., 1956). The
lowest lethal concentration (US NTP, 1984) or the 0.1% mortality
level (Jacobson et al., 1956) for 4-h exposures was approximately
5250 mg/m3 for rats and 900 mg/m3 for mice. In both species,
100% mortality was reached after 4 h of exposure at levels above
17 000 mg/m3 (Jacobson et al., 1956). In rats, 100% mortality
was also reported after a 30-min exposure to 38 000 mg/m3 or a
7-h exposure to 9500 mg/m3 (Rowe et al., 1956).
After inhalation of propylene oxide, rodents showed eye and
nose irritation, nasal discharge, dyspnoea, and depression of the
central nervous system, the severity of which increased with
increasing level and length of the exposure (effect levels not
specified) (Rowe et al., 1956). Gross pathology revealed
distension of the stomach only at lethal concentrations (Jacobson
et al., 1956). No organ damage was observed in rats exposed to
2370 mg/m3 for 7 h, 4740 mg/m3 for 4 h, or 9480 mg/m3 for 0.5 h
(Rowe et al., 1956). In 4 groups, each containing 3 dogs, exposed
for 4 h to propylene oxide concentrations of 3230, 4750, 4810, or
5880 mg/m3, lachrymation, salivation, nasal discharge, and vomiting
occurred. Congestion in the lungs and trachea, oedema of pulmonary
tissues, and necrosis of bronchiolar epithelium were observed at
4810 and 5880 mg/m3. Deaths occurred in the groups exposed to
concentrations of 4750 mg/m3 or more (Jacobson et al., 1956).
7.2. Repeated Exposures
7.2.1. Oral exposure
Propylene oxide was administered in the drinking-water to 4
groups of rats of both sexes at dose levels calculated by the
authors to be 0.52, 5.2, 52, or 520 µg/kg body weight per day, over
a 26-week period. At the highest dose level, polyuria, haematological
abnormalities, lowering of serum-albumin, increased serum-beta-
globulin levels, and increased activity of gastrointestinal mucosal
enzymes were observed. Mild haematological abnormalities occurred
at the next lower dose, while no adverse effects were noted at the
two lowest doses (Antonova et al., 1981).
7.2.2. Inhalation exposure
In range-finding tests for a carcinogenicity study, groups of 5
male and 5 female Fischer 344/N rats were exposed to 0, 110, 230,
460, 1150, or 3400 mg propylene oxide/m3 air for 5 days per week,
and 6 h per day, during a 2-week exposure period. B6C3F1 mice were
exposed for the same period to 0, 50, 110, 230, 460, or 1150 mg/m3.
All animals were necropsied on the 12th day. No pathological
effects were observed. Body weights were not affected. Dyspnoea
and gasping were observed in rats, one of which died at 3400 mg/m3.
Mice showed dyspnoea at 460 and 1150 mg/m3. Both species were
less active at the highest exposure. Rats also showed irregular
limb movements and diarrhoea. After 13 weeks of a similar exposure
regime up to concentration of 1150 mg/m3, rats and mice did not
show any gross- or histopathological effects. Body weights were
reduced at 1150 mg/m3 only (US NTP, 1984).
Groups of 10 - 20 rats, 8 guinea-pigs, 1 or 2 rabbits of both
sexes, and 1 or 2 female Rhesus monkeys were exposed to 0, 240,
460, or 1080 mg propylene oxide/m3 air for 112 - 218 days, for
7 h per day, and 5 days per week. Rabbits and monkeys did not
show any adverse effects with regard to appearance, behaviour,
mortality rate, growth, organ weights, and gross- and histopathology.
Rats showed irritation of the eyes and respiratory passages and an
increased mortality rate due to pneumonia at 1080 mg/m3. Guinea-
pigs also showed irritation of the eyes and respiratory passages at
this exposure level, but no increase in mortality rate. In
addition, the growth of the female guinea-pigs was reduced. Livers
exhibited slight fatty degeneration in male guinea-pigs. The
relative weights of the lungs of the guinea-pigs were slightly, but
significantly, increased in both sexes at 1080 mg/m3, and in
females at 460 mg/m3. It should be noted that control lung
weights were low. Histopathological findings were alveolar
haemorrhages and oedema, and interstitial oedema and hyperaemia in
the lungs of guinea-pigs after 157 days of exposure to 1080 mg/m3.
After 37 - 39 days of exposure to 1080 mg/m3, these histopatho-
logical effects had also been observed in rats (Rowe et al., 1956).
The possibility of the induction of neuropathological effects
by propylene oxide was investigated in groups of 12 male Cynomolgus
monkeys. The animals were exposed to actual concentrations of 0,
237, and 717 mg/m3 propylene oxide, for 7 h per day, 5 days per
week, for 2 years. In 2 monkeys per group, brain, ulnar and
sciatic nerves, and spinal cord were examined histologically after
exposure. No clinical signs were reported. The only treatment-
related neuropathological lesions found were in the medulla
oblongata of the brain. Axonal dystrophy was observed in the
nucleus gracilis in all of the 4 treated monkeys examined, without
any apparent dose-effect relationship. The same lesion was observed
in one of the two control monkeys (Sprinz et al., 1982).
7.3. Mutagenicity and Related End-Points
A summary of mutagenicity tests with positive results is
presented in Table 3. Propylene oxide is mutagenic in bacteria,
fungi, and insects; no such studies with negative results have been
reported.
Table 3. Mutagenic tests for propylene oxide with positive resultsa
---------------------------------------------------------------------------------------------------------
Test description System description Reference
Organism Strain/cell type
---------------------------------------------------------------------------------------------------------
Gene mutations
Forward mutations virus Bacillus subtilis Garro & Phillips (1980)b
phage phi 105
Reverse mutations (base-pair bacterium Escherichia coli WP2 McMahon et al. (1979)c
substitutions) Escherichia coli WP2 McMahon et al. (1979)c
Escherichia coli WP2 Hemminki & Falck (1979)
Escherichia coli WP2 Bootman et al. (1979)c
CM871, CM891
Bacillus subtilis Phillips et al. (1980)
Salmonella typhimurium Wade et al. (1978)
TA 1535, TA 100 McMahon et al. (1979)c;
Bootman et al. (1979)c;
Pfeiffer & Dunkelberg
(1980)d
Reverse mutations bacterium Escherichia coli SD4 Hussain & Osterman-Golkar
(1984)
Forward mutations Klebsiella pneumoniae Voogd et al. (1981)
Reverse mutations fungus Neurospora crassa W40 Kolmark & Giles (1955)
macroconidia
Schizosaccharomyces Heslot (1962)
pombe
Forward mutations fungus Schizosaccharomyces Migliore et al. (1982)c
pombe Pl
Sex-linked recessive lethals insect Drosophila melanogaster Hardin et al. (1983)
Forward mutations on specific mammal Chinese hamster Zamora et al. (1983);
locus (in vitro) ovary cells
---------------------------------------------------------------------------------------------------------
Table 3. (contd.)
-------------------------------------------------------------------------------------------------------------------
Test description System description Reference
Organism Strain/cell type
-------------------------------------------------------------------------------------------------------------------
DNA damage
Single-strand breaks bacterium Bacillus subtilis Phillips et al. (1980)b
mammal (in vitro) rat hepatocytes Sina et al. (1983)
calf thymus DNA Walles (1974)
Chromosome damage
Chromatid gaps and breaks, mammal (in vitro) human lymphocytes Bootman et al. (1979)
Chromosome breaks, fragments
Chromatid gaps, deletions, mammal (in vitro) epithelial rat liver Dean & Hodson-Walker
exchanges cells (1979)c
Micronuclei mammal (ip) mouse polychromatic Bootman et al. (1979)
(2 x 300 mg/kg) erythrocytes
-------------------------------------------------------------------------------------------------------------------
a For details of these studies, see text and data profile (IRPTC, 1984).
b Treatment of isolated DNA in vitro, followed by transformation (bacteria) or transfection (virus).
c A similar or slightly reduced effect after metabolic activation by S9 rat or mouse liver fraction.
d Chloropropanols were also mutagenic, but not as mutagenic as propylene oxide; 1,2-propanediol was not
mutagenic.
Propylene oxide causes single-strand breaks in isolated calf
thymus DNA, probably by alkylation of the phosphodiester bond
(Walles, 1974). Single-strand breaks were observed in the DNA of
isolated rat hepatocytes at concentrations that were not toxic
(Sina et al., 1983).
Propylene oxide caused chromosome aberrations in mammalian
cells in vitro, in particular, chromatid gaps and breaks (Bootman
et al., 1979; Dean & Hodson-Walker, 1979). However, no increases
in chromosome aberrations or sister-chromatid exchanges were found
in peripheral lymphocytes of male Cynomolgus monkeys, after long-
term vapour exposure, in vivo. The animals were exposed in
groups of 12 to concentrations of 0, 237, and 717 mg propylene
oxide/m3 air, for 7 h per day, 5 days per week, for 2 years
(Lynch et al., 1984b).
Two oral doses of up to 500 mg propylene oxide/kg body weight,
in gum tragacanth, administered within 24 h to mice, did not induce
micronuclei in the polychromatic erythrocytes. In contrast, 2
intraperitoneal doses of 300 mg/kg body weight in water, administered
within 24 h, gave a 5-fold increase over controls. No effects were
observed following ip administration of 2 doses of 75 mg/kg or 150
mg/kg (Bootman et al., 1979).
The results of the dominant-lethal assay were negative when
male rats were exposed, for 7 h per day, to propylene oxide vapour
at a concentration of 720 mg/m3, for 5 days prior to mating
(Hardin et al., 1983). A negative result was also obtained in a
dominant lethal assay in which male mice received, once a day, 0,
50, or 250 mg propylene oxide/kg body weight in gum tragacanth, by
gavage, for 2 weeks prior to mating (Bootman et al., 1979).
No increased frequency of abnormal sperm heads was observed,
1 - 9 weeks after exposure of mice for 5 days to propylene oxide
vapour at a concentration of 720 mg/m3, for 7 h per day (Hardin
et al., 1983). Similarly, no increase was observed in the frequency
of abnormal sperm heads in the groups of Cynomolgus monkeys exposed
for 2 years (Lynch et al., 1984c). These monkeys were used by
Sprinz et al. (1982) (section 7.2.2) and Lynch et al. (1984b)
(section 7.3).
7.4. Carcinogenicity
7.4.1. Oral exposure
Groups of 50 female Sprague Dawley rats received 2 doses of
15.0 or 60.0 mg propylene oxide/kg body weight per week, by gavage,
for a total of 112 weeks. The compound was dissolved in salad oil
and administered to fasting rats. Two groups of 50 female rats
served as vehicle controls or untreated controls. There was an
exposure-free period between the 79th and 82nd week because of an
outbreak of pneumonia. The rats were observed up to 150 weeks.
No statistical analysis of the results was reported. Mortality
rates were not affected by the exposures. There was a dose-related
incidence of squamous cell carcinoma of the forestomach. In
addition, one early carcinoma of the forestomach was observed. The
numbers of rats affected were 0, 2, and 19 in animals receiving 0,
15, and 60 mg/kg body weight, respectively. The combined incidences
of hyperkeratosis, hyperplasia, and papillomas were 0, 7, and 17 at
0, 15, and 60 mg/kg body weight, respectively. At the highest dose
level, one adenocarcinoma of the pylorus was also observed. There
was no treatment-related increase of any other tumour type
(Dunkelberg, 1982).
7.4.2. Inhalation exposure
The following studies have been reported by the US NTP (1984).
Groups of 50 Fischer 344/N rats and 50 B6C3F1 mice of each sex were
exposed to average propylene oxide vapour concentrations of 0, 470,
and 940 mg/m3 air, for 6 h per day, 5 days per week, over a 103-
week period. Three accidental over-exposures at the highest
exposure level occurred. The concentrations did not exceed 15 300
mg/m3 for a maximum of 12 min during these periods.
In rats, survival rate was not affected and was over 58% at the
end of the study, at all exposure levels. Growth was slightly
reduced from week 20 onwards. The respiratory epithelium of the
nasal turbinates showed a dose-related increase in the incidence of
suppurative inflammation of the mucosae, hyperplasia, and squamous
cell metaplasia. At 940 mg/m3, 2 out of 50 male and 3 out of 50
female rats had papillary adenomas involving the respiratory
epithelium and the underlying submucosal glands of the nasal
turbinates. No such tumours were observed in controls or low-dose
animals. In the thyroid of the female rats, a dose-related increase
in the combined incidences of C-cell adenomas and carcinomas occurred,
which was significant at 940 mg/m3. The combined incidence of
endometrial stromal polyps and sarcomas was reported to have
increased, at both exposure levels, in a dose-related manner.
According to the authors, the C-cell adenomas and C-cell carcinomas
were not related to treatment. The incidences of polyps and
sarcomas were within the historical control range for the species
tested. In males, an increase in skin keratoacanthomas was
observed, with a statistically-significant positive trend. Induced
non-neoplastic lesions further included testicular atrophy, acinar
cell atrophy in the pancreas of males, cytomegaly in the adrenal
cortex of females, and cystic endometrial hyperplasia (US NTP,
1984).
The survival rate in mice was decreased at 940 mg/m3, from
week 60 onwards and was 58 and 20% for male and female mice,
respectively, at the end of the study, compared with 84 and 76% for
male and female controls. Growth was slightly reduced from week 29
onwards. In the nasal turbinates, a dose-related increased
incidence of inflammation occurred. Squamous cell metaplasia was
observed in 1 male at 470 mg/m3 and 2 females at 940 mg/m3. At
940 mg/m3, haemangiosarcomas were found in the nasal cavity of 5
5 males and 2 females. Haemangiomas were also observed in the
nasal cavity of 5 males and 3 females at this dose level. The
increases in the incidence of haemangiosarcomas in the males and of
haemangiomas in both sexes were statistically-significant.
One squamous cell carcinoma and one papilloma were induced in
the nasal cavity of male mice at 940 mg/m3 and 2 adenocarcinomas
were induced in the nasal cavity of females at 940 mg/m3. None
was observed in controls or low-dose animals. The incidence of
adenocarcinomas of the mammary gland was increased at 940 mg/m3,
but did not exceed the historical control range for the species
tested. Induced non-neoplastic lesions further included ovarian
atrophy and suppurative inflammation, mainly of the uterus and
peritoneum (US NTP, 1984).
In a combined NIOSH toxicity-carcinogenicity study, groups of
80 male Fischer 344 rats were exposed to 237 or 717 mg propylene
oxide/m3, for 7 h per day, 5 days per week, over 2 years. A
control group contained 80 rats. There was an exposure-free period
of 2 weeks in month 16 because of a pulmonary infection, which
contributed to the mortality rate. The mortality rate was
increased at both exposure levels, and the increase was significant
at 717 mg/m3. The mean survival was 96 weeks at this exposure
level, compared with 103 weeks for control rats. Body weights were
reduced from the 2nd week at 717 mg/m3 and from the 39th week at
237 mg/m3. After 2 years, at both concentrations, the relative
weights of lungs, adrenals, and brain were increased and those of
the testes decreased. The only altered biochemical parameters were
increased haemoglobin concentrations in blood at both exposure
levels and increased activities of serum aspartate aminotransferase
(EC 2.6.1.1) and serum sorbitol dehydrogenase (EC 1.1.1.14) at 237
mg/m3. Dose-related increases in the incidence and severity of
inflammatory lesions in the lungs, nasal cavity, trachea, and
middle ear were observed. In the nasal passages, there was a dose-
related increased incidence of complex epithelial hyperplasia that
was significant at 717 mg/m3. Metaplasia was not observed. Two
rats showed nasal-cavity adenomas at 717 mg/m3. At this
concentration, there was also an increased incidence of multifocal
areas of atrophy and degeneration of skeletal muscles. No lesions
were observed in the nerves. The only statistically-significant
neoplastic change observed was an increased incidence of adrenal
phaeochromocytomas at both concentrations. The incidences were
8/78 in controls, 25/78 in the low-dose group, and 22/80 in the
high-dose group (Lynch et al., 1984a).
Another study on the possible toxic and carcinogenic properties
of inhaled propylene oxide was performed on randomly-bred Wistar
rats. Groups of 100 rats of each sex were exposed to propylene
oxide concentrations of 70, 242, or 712 mg/m3, for 6 h per day, 5
days per week, over a period of 123 - 124 weeks. Groups of 100
rats of each sex served as controls. Ten rats per sex and per
group were killed for examination after 12, 18, and 24 months of
exposure. No adverse effects were observed on general health,
behaviour, food intake, biochemistry, urinalysis, and haematology
in comparison with control rats. The mortality rate was increased
at the highest exposure level from week 73, in males, and from week
109, in females, and at the end of the exposure to 242 mg/m3 in
females. Rats of both sexes gained less weight than controls at
712 mg/m3, though the difference compared with controls became
smaller towards the end of the exposure. The relative and absolute
weights of adrenals, spleen, liver, and lungs of males were
increased at 712 mg/m3, but no pathological lesions were observed
in these organs. Male rats showed a lower incidence of pale
exorbital lachrymal glands at this exposure level. In both sexes,
the incidences of basal cell hyperplasia and atrophy of the
olfactory epithelium and the incidence of nest-like infolds of the
respiratory epithelium were increased mainly at the 2 highest
exposure levels. These changes were also noted at interim kills.
The severity of the changes increased only slightly, if at all,
with increasing length of exposure and with age. Squamous
metaplasia was not observed. One rat was found with squamous cell
carcinoma of the nose. In female rats, the incidence of benign
tumours of the mammary glands, mostly fibroadenomas, was increased
at 712 mg/m3 in comparison to both the concurrent controls and
the historical controls. Moreover, the number of females bearing 2
or more mammary fibroadenomas increased in a dose-related manner
from the lowest exposure level onwards when compared with controls.
The increased incidence of tubulopapillary mammary carcinomas at
712 mg/m3 was within the range of historical controls. With the
exclusion of mammary tumours, the total incidence of primary
tumours in females and the number of malignant tumour-bearing rats
of both sexes was increased at the highest exposure level (Reuzel &
Kuper, 1983). Following reports that ethylene oxide had induced
brain tumours in rats, the histopathological investigation of the
brain of the rats was extended. There was no evidence that
propylene oxide induced such tumours (Reuzel & Kuper, 1984).
7.4.3. Subcutaneous exposure
Groups of 100 female NMRI mice received subcutaneous doses of
0.1, 0.3, 1.0, or 2.5 mg propylene oxide per animal in tricaprylin
once a week, for 106 weeks. These groups were compared with 200
controls receiving tricaprylin and 200 untreated controls. In the
second year of exposure, mice were not treated for 11 weeks. Body
weights and survival rates were not affected by treatment. At the
site of injection, a dose-related increase in the incidence of
sarcomas (mainly fibrosarcomas) occurred. The numbers of mice
affected were 0/200 in untreated controls, 4/200 in tricaprylin
controls, and 3/100, 2/100, 12/100, and 15/100 at the 0.1, 0.3,
1.0, and 2.5 mg dose levels, respectively. The increases in
incidence at the 2 highest dose levels were statistically
significant. The first tumour appeared in week 38 (Dunkelberg,
1981).
7.5. Effects on Reproduction and Teratogenicity
When B6C3F1 mice of both sexes were exposed repeatedly for 2
years to propylene oxide at concentrations of 470 and 940 mg/m3
air (section 7.4.2), the incidence of ovarian atrophy was increased
at the higher exposure level (US NTP, 1984). In F344 rats, similarly
exposed, higher incidences of testicular atrophy were found at both
exposure levels, but they were not dose-related (US NTP, 1984).
Decreased relative weights of the testes were reported by Lynch et
al. (1984a), when male F344 rats were exposed repeatedly for 2 years
to propylene oxide at levels of 240 and 720 mg/m3 air.
Sperm head abnormalities were not detected in mice after
exposure for 5 days, 7 h per day, to 720 mg propylene oxide/m3
(Hardin et al., 1983), or in groups of 12 Cynomolgus monkeys,
exposed for 2 years to 240 and 710 mg propylene oxide/m3, for
7 h per day, 5 days per week. In monkeys, at both exposure levels,
sperm counts and sperm motility were reduced, and the sperm drive
range was elevated (Lynch et al., 1984c).
In a reproduction-teratogenicity study, groups of 41 - 44
female Sprague Dawley rats were exposed to 1190 mg propylene
oxide/m3 air, for 7 h per day, during days 7 - 16 of gestation
(Group 1), days 1 - 16 of gestation (Group 2), or for 3 weeks
(5 days/week) before mating and on days 1 - 16 of gestation
(Group 3). A group of 46 rats served as controls. The dams were
sacrificed on day 21. No deaths of dams occurred, their histology
was normal, and the percentage of pregnant rats was not affected by
the exposure. Toxic effects in dams were expressed as decreased
body weights and food consumption and increased relative weights of
kidneys in all exposed groups. The numbers of corpora lutea,
implantations per dam, and live fetuses were lower in group 3,
compared with those in the other groups. In group 1, the number of
resorptions was increased compared with that in group 2. The body
weights and lengths of fetuses were decreased in all exposed groups
compared with controls, but more so in group 3. In group 2,
increases in wavy rib and in reduced ossification, primarily of the
vertebrae and ribs, were observed (Hackett et al., 1982).
Groups of New Zealand rabbits were also exposed to 1190 mg/m3,
for 7 h per day, during days 1 - 19 or days 7 - 19 of gestation.
No evidence of toxicity was observed in the mothers, fetuses, or
embryos, and no developmental defects were noted (Hacket et al.,
1982).
Female rats administered a single oral dose of 260 mg/kg body
weight of propylene oxide showed disturbance of the estrus cycle.
Pregnant female rats administered an identical dose during the
first 2 weeks of gestation exhibited higher embryotoxicity and
lower offspring body weight compared with the controls. Male rats
exposed to a single oral LD50 (520 mg/kg) dose of propylene oxide
showed reduced sperm motility and damage to primary spermatocytes.
When such rats were mated with normal female rats, between 2 and 10
weeks after exposure, 50% of the males were infertile. The fertility
of the first generation was reduced by 23% (Antonova et al., 1981).
8. EFFECTS ON MAN
8.1. Exposure of Skin and Eyes; Skin Sensitization
Accidental exposure of the eyes of 3 persons to propylene
oxide (it was not reported whether this was liquid or vapour)
resulted in alterations in the cornea and conjunctiva (McLauglin,
1946).
Allergic contact dermatitis was diagnosed in 3 cases of
exposure to solutions of propylene oxide (Ketel, 1979; Jensen,
1981). Biopsy on the skin of these patients revealed spongiosis
in the epidermis, oedema in the cutis, and dense perivascular
infiltrates with mononuclear cells.
8.2. Accidental Inhalation Exposure
No data are available.
8.3. Occupational Inhalation Exposure
In the Federal Republic of Germany, 279 employees from 8
plants where alkene oxides were produced or processed, were
examined during 1978. They were employed for an average of 10.8
years. No clinical abnormalities were found that could be related
to exposure to alkene oxides. Propylene oxide levels were measured
by personal sampling over 1 - 10 h, but levels of exposure were not
reported. The workers were exposed to many other chemicals,
including ethylene oxide (Stocker & Thiess, 1979). Because of the
mixed exposure and unavailability of propylene oxide exposure data,
the Task Group was unable to evaluate this study.
8.4. Mortality Studies
In the Federal Republic of Germany, 602 workers were
investigated for mortality over the period 1928-80. The workers
had been employed for at least 6 months in 8 plants producing
ethylene oxide and propylene oxide, the latter produced only since
1959. A subcohort of 351 workers was observed for more than 10
years. Control data came from a styrene plant and from national
statistics. Propylene oxide levels, measured by personal sampling
(number of samples not reported), from 1978 to 1980, were far below
a time-weighted average of 240 mg/m3 over a working shift of 12 h
(Thiess et al., 1981a). Higher levels were measured for brief
periods. The workers were also exposed to many other chemicals,
some of which might be carcinogenic for human beings. There were
56 deaths compared with 76.6 expected. No significant excess of
deaths could be found due to any cause in the cohort of 602 workers.
In the sub-cohort of 351 workers, there was a significant increase
in mortality rate due to kidney disease (3 compared with 0.4
expected). There was 1 death from gall-bladder cancer, 1 death
from urinary-bladder cancer, 1 death from brain cancer, and 1 death
from myeloid leukaemia. The stomach tumours were observed compared
with 1.8 expected (Thiess et al., 1981b). Since the workers were
also exposed to many other chemicals, such as butylene oxide,
epichlorohydrin, dioxan, dichloropropane, and chlorohydrins, the
Task Group was unable to evaluate this study in relation to
propylene oxide.
8.5. Mutagenicity and Related End-Points
In 43 male workers from the cohort of 602 workers discussed in
section 8.4 (Thiess et al., 1981b), no increase in chromosome
aberration rate was found in 2 groups of workers exposed to alkene
oxides for average periods of either 12.5 or 17.6 years; in addition,
workers in the latter group had at least one accidental high exposure
to ethylene oxide. The results were also negative in a group of
workers exposed once for a brief period following an accident. On
the other hand, the aberration rate was significantly elevated in
workers exposed for more than 20 years (Thiess et al., 1981a).
For the reason mentioned in section 8.4, the Task Group was
unable to evaluate this study in relation to propylene oxide.
Unscheduled DNA synthesis, induced in vitro by the mutagen
N-acetoxy-2-acetylaminofluorene, was inhibited in the lymphocytes
of 23 workers from a factory in Sweden, where starch was modified
with propylene oxide. The workers had been exposed for 1 - 20
years. At the time of the study, exposure was generally below a
time-weighted average of 28 mg/m3. However, short-term exposures
of up to 2370 mg/m3 were recorded for some workers (Pero et al.,
1982).
9. EVALUATION OF THE HEALTH RISKS FOR MAN AND EFFECTS ON
THE ENVIRONMENT
Exposure of man to propylene oxide mainly occurs through
inhalation at the work-place.
Data are insufficient to estimate the exposure to propylene
oxide residues in food after fumigation and sterilization. The
main conversion products in foodstuffs are chloropropanols and
1,2-propanediol, which are more persistent than the parent compound
(section 4.2). No adverse effects have been reported due to the
ingestion of propylene oxide and its reaction products in food.
No ambient air monitoring data are available, but the lowest
annual average concentration at distances of up to 20 km from
production plants has been assessed, by modelling, to be less than
4.836 x 10-8 mg/m3(section 4.1). The risk for human health
under such conditions of exposure is likely to be negligible.
Propylene oxide is highly soluble in water but is likely to
evaporate to a great extent. However, no data on the rate of
evaporation are available. In neutral fresh water, propylene
oxide is converted to 1,2-propanediol and, in marine waters, to
halopropanols, but, even in the presence of micro-organisms, these
processes are slow (section 3.2). Because of the low log n-octanol
water-partition coefficient, propylene oxide and its conversion
products are unlikely to bioaccumulate (Table 1). The toxicity of
propylene oxide for aquatic organisms is low. The available LC50s
are above approximately 90 mg/litre (section 6). Thus, the
probability of an adverse impact on the aquatic environment is
considered low.
Eight-hour time-weighted occupational exposure in propylene
oxide production and use is normally below 5 mg/m3, with
occasional peak exposures up to 9000 mg/m3 (section 4.3).
Inhaled propylene oxide is probably readily absorbed,
distributed throughout the body, and rapidly metabolized. The
half-life in rat tissues has been estimated to be 40 min (section
5.2). There are no data on skin absorption.
An aqueous solution of propylene oxide (100 or 200 g/litre)
is irritating to rabbit skin when applied under occlusive cover
(section 7.1.2). In man, corneal and conjunctival damage, and
allergic contact dermatitis have been reported through accidental
exposure to propylene oxide vapour (section 8.1).
The 4-h LC50s for the rat and the mouse were 9500 mg/m3
and 4100 mg/m3, respectively. Dogs exposed by inhalation, once
for 4 h, to a concentration of 3230 - 5880 mg/m3 showed
salivation, lachrymation, nasal discharge, and vomiting. Deaths
were observed at 4750 - 5880 mg/m3 but not at 3230 mg/m3
(section 7.1.3).
On repeated exposure of various species to propylene oxide
vapour, 7 h per day, 5 days per week, for 112 - 218 days, at
concentrations of 0, 240, 460, and 1080 mg/m3, rabbits and
monkeys did not show any adverse effects on appearance, mortality,
growth, and histopathology of internal organs. Rats and guinea-
pigs showed irritation of the respiratory passages and lung damage
histologically at 1080 mg/m3. An increase in lung weight was
observed in female guinea-pigs at concentrations of 460 mg/m3 or
more. No effect was observed in any species at a concentration of
240 mg/m3. A concentration of 3400 mg/m3 administered for 2
weeks, 6 h/day, 5 days per week, resulted in dyspnoea and death in
rats. In the same study, mice showed dyspnoea at 460 and 1150 mg/m3
(section 7.2.2).
Rats and mice exposed for 2 years showed increased incidences
of inflammatory and proliferative lesions of the nasal epithelium
from an exposure level of 470 mg/m3. In rats, the inflammatory
lesions and hyperplasia were also observed at an exposure level of
240 mg/m3, but not at 70 mg/m3 (section 7.4.2).
Depression of the central nervous system, the severity of
which increased with increasing level and length of exposure, was
observed when rats and mice were exposed by inhalation to single
high concentrations of propylene oxide (section 7.1.3). Although
no clinical central nervous system effects were reported when
monkeys were exposed to 237 and 717 mg/m3 for 2 years, some
histopathological changes were observed in the treated animals
(section 7.2.2). It should be noted that these data cannot be
evaluated for estimating hazard for man, since only 2 monkeys were
examined in each of the exposed groups, and the lesion appeared
also in one of the 2 controls.
Summarizing the results of animal studies (excluding
mutagenic, carcinogenic, and reproduction effects) the no-
observed-adverse-effect level for prolonged repeated 6- to 7-h
daily exposure is 70 mg/m3.
Propylene oxide administered by inhalation for 2 years
produced ovarian atrophy in mice at 940 mg/m3. Testicular
atrophy was found at 240 and 720 mg/m3 in rats (section 7.5).
No sperm head abnormalities were detected in mice after
exposure for 5 days to 720 mg/m3 or in Cynomolgus monkeys
exposed to 240 or 710 mg/m3 for 2 years, but sperm count and
motility were reduced, and sperm drive range (time to traverse a
linear path) increased in the monkeys. A reduced sperm motility
and damage to spermatocytes were observed in male rats treated with
an oral LD50 (520 mg/kg body weight) dose. A reduced fertility
was observed when these males were mated between 2 and 10 weeks
after exposure with normal females (sections 7.3, 7.5).
Reduced fetal ossification of vertebrae and ribs and wavy ribs
were observed when pregnant Sprague Dawley rats were exposed to
1190 mg propylene oxide/m3, 7 h per day, on days 1 - 16 of
gestation. When rats were additionally exposed for 3 weeks before
mating, the numbers of corpora lutea, implantations per dam, and
live fetuses were decreased. No teratogenic or fetotoxic effects
were found in New Zealand rabbits exposed to 1190 mg/m3 (section
7.5).
Propylene oxide is mutagenic in microorganisms and insects and
produces DNA damage and chromosomal aberrations in mammalian cells
in vitro. It produced micronuclei in mouse erythrocytes after
parenteral injection of 2 doses of 300 mg/kg body weight but not
after oral exposure. No chromosomal aberrations or sister
chromatid exchanges occurred in monkeys exposed by inhalation to
237 and 717 mg/m3, for 7 h/day, 5 days/week, for 2 years. No
dominant-lethal effects were observed in rats or mice following
inhalation or oral exposure, respectively, in 2 studies (section
7.3). No adequate data on chromosomal effects in human beings are
available.
In studies on the carcinogenicity of propylene oxide in rats
and mice, malignant tumours were mainly observed at the site of
entry into the body: for stomach, at 15 and 60 mg/kg body weight;
for subcutaneous tissues, at 1.0 - 2.5 mg; and, for nasal passages,
at 940 mg/m3. At the dose levels at which these tumours were
induced, propylene oxide produced tissue damage. This damage may
have played a role in the appearance of the tumours. Propylene
oxide also produced an increase in the incidence of multiple benign
mammary tumours in female rats following inhalation exposure
(section 7.4). No adequate epidemiological studies on cancer
incidence in exposed human populations have been carried out.
Taking into account the body of available data - the
alkylating nature of propylene oxide, the formation of DNA
adducts, the positive responses in in vitro mutagenesis assays,
the carcinogenic effects in animals at the sites of entry into the
body, and the absence of adequate data on cancer in human beings -
propylene oxide should be considered as a possible human carcinogen.
Thus, for practical purposes, propylene oxide should be regarded as
if it presented a carcinogenic risk for man, and levels in the
environment should be kept as low as feasible.
10. RECOMMENDATIONS FOR FURTHER RESEARCH
1. A thorough detailed study on the absorption, distribution,
and metabolism of propylene oxide should be conducted.
2. Biological indicators should be developed that quantify both
the incidence and severity of human exposure.
3. The epidemiological studies indicating an increased risk of
cancer in workers exposed to propylene oxide in combination
with other chemicals suggest that additional studies should
be conducted on populations whose exposure has been primarily
to propylene oxide. The adequate quantification of past
exposures should be a part of these studies.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
An International Agency for Research on Cancer Working Group
(IARC, 1985) evaluated the carcinogenicity of propylene oxide and
concluded that:
"There is sufficient evidence for the carcinogenicity of
propylene oxide to experimental animals; there is inadequate
evidence for its carcinogenicity to humans. It is noted that,
in the absence of adequate data in humans, it is reasonable,
for practical purposes, to regard chemicals for which there is
sufficient evidence of carcinogenicity in experimental animals
as if they represented a carcinogenic risk to humans."
REFERENCES
ADDY, J.K. & PARKER, R. (1963) The mechanism of epoxide
reactions. V. The reaction of 1,2-epoxypropane with chloride
ions in water under neutral and acidic conditions. J. Chem.
Soc., 915-921.
ANDERSON, G.E. (1983) Human exposure to atmospheric
concentrations of selected chemicals. Vol. II, Research
Triangle Park, North Carolina, US Environmental Protection
Agency, pp. 574-601 (Prepared by Systems Applications, Inc.,
San Rafael, California, No. SA1/EF81-156R2).
ANTONOVA, V.I., ZOMMER, E.A., KUZNETSOVA, A.D., & PETROVA,
N.A. (1981) [Toxicology of propylene oxide and regulation
for surface water.] Gig. i Sanit., 46: 76-79 (in Russian).
BOGYO, D.A., LANDE, S.S., MEYLAND, W.M., HOWARD, P.H., &
SANTODONATO, J. (1980) Investigation of selected potential
environmental contaminants: Epoxides, Syracuse, New York,
Centre for Chemical Hazard Assessment, Syracuse Research
Corporation (Prepared for US EPA) (US EPA 560/11-80-005, PB
80-183197).
BONT, J.A.M. DE, DIJKEN, J.P. VAN, & GINKEL, K.G. VAN (1982)
The metabolism of 1,2-propanediol by the propylene oxide
utilizing bacterium Nocardia 60. Biochem. Biophys. Acta, 714:
465-470.
BOOTMAN, J., LODGE, D.C., & WHALLEY, H.E. (1979) Mutagenic
activity of propylene oxide in bacterial and mammalian
systems. Mutat. Res., 67: 101-112.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979a) The acute
toxicity of some petrochemicals to goldfish. Water Res., 13:
623-626.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) BOD and
COD of some petrochemicals. Water Res., 13: 627-630.
CALLEMAN, C.J., EHRENBERG, L., JANSSON, B., OSTERMAN-GOLKAR,
S., SEGERBACK, D., SVENSSON, K., & WACHTMEISTER, C.A. (1978)
Monitoring and risk assessment by means of alkyl groups in
haemoglobin in persons occupationally exposed to ethylene
oxide. J. environ. Pathol. Toxicol., 2: 247-442.
CONWAY, R.A., WAGGY, G.T., SPIEGEL, M.H., & BERGLUND, R.L.
(1983) Environmental fate and effects of ethylene oxide.
Environ. Sci. Technol., 17: 107-112.
COOKSON, M.J., SIMS, P., & GROVER, P.L. (1971) Mutagenicity
of epoxides of polycyclic hydrocarbons correlates with
carcinogenicity of parent hydrocarbons. Nature (New Biol.),
234: 186-187.
CREWS, R.C. (1974) Effects of propylene oxide on selected
species of fishes, Florida, Air Force Armament Center, Eglin
Air Force Base (US NTIS AD/A003637).
CUPITT, L.T. (1980) Fate of toxic and hazardous materials in
the environment, Research Trianlge Park, North Carolina, US
Environmental Protection Agency, Environmental Sciences
Laboratory, Office of Research and Development (US EPA
600/3-80-084, PB 80-221948).
DAVIES, R.E., HARPER, K.H., & KYNOCH, S.R. (1972)
Inter-species variation in dermal reactivity. J. Soc. Cosmet.
Chem., 23: 371-382.
DEAN, B.J. & HODSON-WALKER, G. (1979) An in vitro chromosome
assay using cultured rat liver cells. Mutat. Res., 64: 329-337.
DENT, J.G. & SCHNELL, S.R. (1981) Inhibition of microsomal
membrane-bound and purified epoxide hydrolase by C2-C8
1,2-alkene oxides. Biochem. Pharmacol., 30: 1712-1714.
DUNKELBERG, H. (1981) [Carcinogenic activity of ethylene
oxide and its reaction products 2-chloroethanol,
2-bromoethanol, ethylene glycol, and diethylene glycol. I.
Carcinogenicity of ethylene oxide in comparison with
1,2-propylene oxide after subcutaneous administration in
mice.] Zbl. Bakt. Hyg. (I. Abt. Orig. B), 174: 383-404 (in
German).
DUNKELBERG, H. (1982) Carcinogenicity of ethylene oxide and
1,2-propylene oxide upon intragastric administration to rats.
Br. J. Cancer, 46: 924-933.
EHRENBERG, L., HIESCHE, K.D., OSTERMAN-GOLKAR, S., & WENNBERG,
I. (1974) Evaluation of genetic risks of alkylating agents:
Tissue doses in the mouse from air contaminated with ethylene
oxide. Mutat. Res., 24: 83-103.
FAO/WHO JOINT EXPERT COMMITTEE ON FOOD ADDITIVES (1974) WHO
Food Add. Ser., 5: 491-497 (Toxicological Monographs arising
from the Seventeenth Report of the Joint FAO/WHO Expert
Committee on Food Additives).
FARMER, P.B., GORF, S.M., & BAILEY, E. (1982) Determination
of hydroxypropylhistidine in haemoglobin as a measure of
exposure to propylene oxide using high resolution gas
chromatography mass spectrometry. Biomed. Mass Spectrometr.,
9: 69-71.
FEDERAL REGISTER (1971) Paragraph 121.1076, propylene oxide,
subpart D, food additives, Washington DC, US Environmental
Protection Agency, Food and Drug Administration, p. 33.
FJELLSTEDT, T.A., ALLEN, R.H., DUNCAN, B.K., & JAKOBY, W.B.
(1973) Enzymatic conjugation of epoxides with glutathione. J.
Biol. Chem., 248: 3702-3707.
FLORES, G.H. (1983) Reducing plant pollution exposure:
controlling exposure to alkene oxides. Chem. Eng. News, 79:
39-43.
GARRO, A.J. & PHILLIPS, R.A. (1980) Detection of
mutagen-induced lesions in isolated DNA by marker rescue of
Bacillus subtilis phage stopb105. Mutat. Res., 73: 1-13.
GUENGERICH, F.P. & MASON, P.S. (1980) Alcohol dehydrogenase-
coupled spectrophotometric assay of epoxide hydratase
activity. Anal. Biochem., 104: 445-451.
GUNTHER, D.A. (1965) Determination of adsorbed ethylene and
propylene oxides by distillation and titration. Anal. Chem.,
37: 1172-1173.
HACKETT, P.L., BROWN, M.G., BUSCHBOM, R.L., CLARK, M.L.,
MILLER, R.A., MUSIC, R.L., ROWE, S.E., SCHIRMER, R.E., &
SIKOV, M.R. (1982) Teratogenic study of ethylene and
propylene oxide and N-butyl acetate, Richland, Washington,
Batelle Pacific Northwest Laboratories (Prepared for NIOSH)
(PB 83-258038).
HARDIN, B.D., SCHULER, R.L., MCGINNIS, P.M., NIEMEIER, R.W., &
SMITH, R.J. (1983) Evaluation of propylene oxide for
mutagenic activity in 3 in vivo test systems. Mutat. Res.,
117: 337-344.
HATFIELD, R. (1957) Biological oxidation of some organic
compounds. Ind. Eng. Chem., 49: 192-196.
HELLMAN, T.M. & SMALL, F.H. (1974) Characterization of the
odour properties of 101 petrochemicals using sensory method.
J. Air Pollut. Contr. Assoc., 24: 979-982.
HEMMINKI, K. & FALCK, K. (1979) Correlation of mutagenicity
and 4-(p-nitrobenzyl)-pyridine alkylation by epoxides.
Toxicol. Lett., 4: 103-106.
HEMMINKI, K., PAASIVIRTA, J., KURKIRINNE, T., & VIRKKI, L.
(1980) Alkylation products of DNA bases by simple epoxides.
Chem.-biol. Interact., 30: 259-270.
HESLOT, H. (1962) Etude quantitative de réversions
biochimiques induites chez la levure Schizosaccharomyces pombe
par des radiations et des substances radiomimétiques. Abh.
Dtsch. Akad. Wiss. Berlin, Kl. Med., 1: 193-228.
HEUSER, S.G. & SCUDAMORE, K.A. (1969) Determination of
fumigant residues in cereals and other foodstuffs: A multi-
detection scheme for gas chromatography of solvent extracts.
J. Sci. Food Agric., 20: 566-572.
HIRASHIMA, T., OGUMA, T., HOSOGAI, Y., & FUJII, S. (1970)
[Studies on gaseous antimicrobial agents. III. Determination
of propylene oxide residue in food wrappings and containers.]
J. Food Hyg. Soc. Jpn, 11: 161-163 (in Japanese).
HUSSAIN, S. & OSTERMAN-GOLKAR, S. (1984) Dose-response
relationships for mutations induced in E. coli by some model
compounds. Hereditas, 101: 57-68.
IARC (1976) Cadmium, nickel, some epoxides, miscellaneous
industrial chemicals and general considerations on volatile
anaesthetics, Lyons, International Agency for Research on
Cancer, 293 pp (IARC Monographs on the Evaluation of
Carcinogenic Risk of Chemicals to Man, Vol. 11).
IARC (1985) Allyl compounds, aldehydes, epoxides, and
peroxides, Lyons, International Agency for Research on Cancer
(Monographs on the Evaluation of Carcinogenic Risk of
Chemicals to Man, Vol. 36).
IRPTC (1984) Data profile on propylene oxide, Geneva,
Switzerland, International Register on Potentially Toxic
Chemicals, United Nations Environment Programme.
JACOBSON, K.H., HACKLEY, E.B., & FEINSILVER, L. (1956) The
toxicity of inhaled ethylene oxide and propylene oxide
vapours. Arch. ind. Health, 13: 237-244.
JENSEN, O. (1981) Contact allergy to propylene oxide and
isopropyl alcohol in a skin desinfectant swab. Contact
Dermatit., 7: 148-150.
KETEL, W.G. VAN (1979) Contact dermatitis from propylene
oxide. Contact Dermatit., 5: 191-192.
KOLMARK, H.G. & GILES, N.H. (1955) Comparative studies of
monoepoxides as inducers of reverse mutations in Neurospora.
Genetics, 40: 890-902.
KOSKIKALLIO, J. & WHALLEY, E. (1959) Effect of pressure on
the spontaneous and the base-catalysed hydrolyses of epoxides.
Can. J. Chem., 37: 783-787.
KROST, K.J., PELLIZZARI, E.D., WALBURN, S.G., & HUBBARD, S.A.
(1982) Collection and analysis of hazardous organic
emissions. Anal. Chem., 54: 810-817.
LAWLEY, P.D. & JARMAN, M. (1972) Alylation by propylene
oxide of deoxyribonucleic acid, adenine, guanosine, and
deoxyguanylic acid. Biochem. J., 126: 893-900.
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., BURG, J.R., GROTH,
D.H., KHAN, A., ACKERMAN, L.J., & COCKRELL, B.Y. (1984a)
Carcinogenic and toxicologic effects of inhaled ethylene oxide
and propylene oxide in F344 rats. Toxicol. appl. Pharmacol.,
76(1): 69-84.
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., BURG, J.R., GULATI,
D.K., KAUR, P., & SABHARWAL, P.S. (1984b) Sister-chromatid
exchanges and chromosome aberrations in lymphocytes from
monkeys exposed to ethylene oxide and propylene oxide by
inhalation. Toxicol. appl. Pharmacol., 76(1): 85-95.
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., LAL, J.B., BURG,
J.R., GULATI, D.K., ZAVOS, P.M., & SABHARWAL, P.S. (1984c)
Toxic and mutagenic effects of inhaled ethylene oxide and
propylene oxide on spermatogenic functions in monkeys.
Toxicologist, 3: 60.
MCLAUGHLIN, R.S. (1946) Chemical burns of the human cornea.
Am. J. Ophthalmol., 29: 1355-1362.
MCMAHON, R.E., CLINE, J.C., & THOMPSON, C.Z. (1979) Assay of
855 test chemicals in ten tester strains using a new
modification of the Ames test for bacterial mutagens. Cancer
Res., 39: 682-693.
MESTRES, R. & BARROIS, C. (1964) Recherche et dosage de
l'oxyde de propylène et du propylène-glycol dans les fruits
par chromatographie gazeuse directe. Bull. Soc. Pharm.
Montpellier, 24: 47-63.
MIGLIORE, L., ROSSI, A.M., & LOPRIENO, N. (1982) Mutagenic
action of structurally-related alkene oxides on Schizosac-
charomyces pombe: The influence, "in vitro", of mouse liver
metabolizing system. Mutat. Res., 102: 425-437.
MISHMASH, E.H. & MELOAN, C.E. (1972) Indirect
spectrophotometric determination of nanomale quantities of
oxiranes. Anal. Chem., 44: 835-836.
NELIS, H.J.C.F. & SINSHEIMER, J.E. (1981) A sensitive
fluorimetric procedure for the determination of aliphatic
epoxides under physiological conditions. Anal. Biochem., 115:
151-157.
NIOSH (1977) Manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, US Department of Health, Education and
Welfare, National Institute for Occupational Safety and
Health, Vol. 2, pp. S75-1 - S75-8.
OESCH, F. (1974) Purification and specificity of a human
microsomal epoxide hydratase. Biochem. J., 139: 77-88.
OGUMA, T., HOSOGAI, Y., FUJII, S., & KAWASHIRO, I. (1968)
[Gaseous antimicrobial agents. I. Determination of propylene
oxide residues in foods.] J. Food Hyg. Soc. Jpn, 9: 395-398
(in Japanese).
OGUMA, T., HOSOGAI, Y., & FUJII, S. (1969) [Studies on
gaseous antimicrobial agents. II. Determination of propylene
oxide residue in fats and oils.] J. Food Hyg. Soc. Jpn, 10:
37-39 (in Japanese).
OSTERMAN-GOLKAR, S., EHRENBERG, L., SEGERBACK, D., &
HALLSTROM, I. (1976) Evaluation of genetic risks of
alkylating agents. II. Haemoglobin as a dose monitor. Mutat.
Res., 34: 1-10.
OSTERMAN-GOLKAR, S., FARMER, P.B., SEGERBACK, D., BAILEY, E.,
CALLEMAN, C.J., SVENSSON, K., & EHRENBERG, L. (1983)
Dosimetry of ethylene oxide in the rat by quantitation of
alkylated histidine in haemoglobin. Teratog. Carcinog.
Mutagen., 3: 395-405.
OSTERMAN-GOLKAR, S., BAILEY, E., FARMER, P.B., GORF, S.M., &
LAMB, J.H. (1984) Monitoring exposure to propylene oxide
through the determination of haemoglobin alkylation. Scand. J.
Work environ. Health, 10: 99-102.
PERO, R.W., BRYNGELSSON, T., WIDEGREN, B., HOGSTEDT, B., &
WELINDER, H. (1982) A reduced capacity for unscheduled DNA
synthesis in lymphocytes from individuals exposed to propylene
oxide and ethylene oxide. Mutat. Res., 104: 193-200.
PFEIFFER, E.H. & DUNKELBERG, H. (1980) Mutagenicity of
ethylene oxide and propylene oxide and of the glycols and
halohydrins formed from them during the fumigation of
foodstuffs. Food Cosmet. Toxicol., 18: 115-118.
PHILLIPS, R.A., ZAHLER, S.A., & GARRO, A.J. (1980) Detection
of mutagen-induced lesions in isolated DNA using a new
Bacillus subtilis transformation-based assay. Mutat. Res., 74:
267-281.
RADDING, S.B., LUI, D.H., JOHNSON, H.L., & MILL, T. (1977)
Review of the environmental fate of selected chemicals,
Washington DC, US Environmental Protection Agency, Office of
Toxic Substances (US EPA 560/5-77-003, PB 267121).
RAGELIS, E.P., FISHER, B.S., KLIMECK, B.A., & JOHNSON, C.
(1968) Isolation and determination of chlorohydrins in foods
fumigated with ethylene oxide or with propylene oxide. J.
Assoc. Offic. Agric. Chem., 51: 709-715.
RANDERATH, K., REDDY, M.V., & GUPTA, R.C. (1981) 32P-
labelling test for DNA damage. Proc. Natl Acad. Sci., 78:
6126-6129.
REUZEL, P.G.J. & KUPER, C.F. (1983) Chronic (28-month)
inhalation toxicity/carcinogenicity study of 1,2-propylene
oxide in rats. Final Report, Zeist, The Netherland, TNO,
Division of Nutrition and Food Research (Report No.
V82.215/280853).
REUZEL, P.G.J. & KUPER, C.F. (1984) Chronic (28-month)
inhalation toxicity/carcinogenicity study of 1,2-propylene
oxide in rats, Zeist, The Netherland, TNO, Division of
Nutrition and Food Research (Addendum to Report No.
V82.215/280853).
ROSS, W.C.J. (1950) The reactions of certain epoxides in
aqueous solutions. J. Chem. Soc., 2257-2271.
ROWE, V.K., HOLLINGSWORTH, R.L., OYEN, F., MCCOLLISTER, D.D.,
& SPENCER, H.C. (1956) Toxicity of propylene oxide deter-
mined on experimental animals. Arch. ind. Health, 13: 228-236.
RUDDICK, J.A. (1972) Toxicology, metabolism, and biochemis-
try of 1,2-propanediol. Toxicol. appl. Pharmacol., 21: 102-111.
RUSSELL, J.W. (1975) Analyses of air pollutants using
sampling tubes and gas chromatography. Environ. Sci. Technol.,
9: 1175-1178.
SCUDAMORE, K.A. & HEUSER, S.G. (1971) Ethylene oxide and its
persistent reaction products in wheat flour and other
commodities: Residues from fumigation or sterilization, and
effects of processing. Pestic. Sci., 2: 80-91.
SINA, J.F., BEAN, C.L., DYSART, G.R., TAYLOR, V.I., & BRADLEY,
M.O. (1983) Evaluation of the alkaline elution/rat
hepatocyte assay as a predictor of carcinogenic/mutagenic
potential. Mutat. Res., 113: 357-391.
SMYTH, H.F., SEATON, J., & FISHER, L. (1941) The single dose
toxicity of some glycols and derivatives. J. ind. Hyg.
Toxicol., 23: 259-268.
SNELLINGS, W.M., WEIL, C.S., & MARONPOT, R.R. (1984) A
two-year inhalation study of the carcinogenic potential of
ethylene oxide in Fischer 344 rats. Toxicol. appl. Pharmacol.,
75: 105-117.
SPRINZ, H., MATZKE, H., & CARTER, J. (1982) Neuropatho-
logical evaluation of monkeys exposed to ethylene and
propylene oxide, Kansas City, Missouri, Midwest Research
Institute (Prepared for NIOSH) (PB 83-134817).
STEELE, L. & HADZIYEV, D. (1976) Sterilization of dehydrated
potato granules with propylene oxide. Z. Lebensm.
Unters.-Forsch., 162: 387-399.
STOCKER, W.G. & THIESS, A.M. (1979) Morbidity study on
workers exposed to ethylene oxide/propylene oxide. In: The 7th
Medichem Congress, Gera, 11-15 September, 1979.
STORCK, W.J. (1981) Output of big-volume chemicals fell in
1980. Chem. Eng. News, 59: 36-39.
SVENSSON, K. & OSTERMAN-GOLKAR, S. (1984) Kinetics of
metabolism of propene and covalent binding to macromolecules
in the mouse. Toxicol. appl. Pharmacol., 73: 363-372.
SWAN, J.D. (1954) Determination of epoxides with sodium
sulfite. Anal. Chem., 26: 878-880.
TACHIZAWA, H., MACDONALD, T.L., & NEAL, R.A. (1982) Rat
liver microsomal metabolism of propyl halides. Mol.
Pharmacol., 22: 745-751.
THIESS, A.M., SCHWEGLER, H., FLEIG, I., & STOCKER, W.G.
(1981a) Mutagenicity study of workers exposed to alkylene
oxides (ethylene oxide/propylene oxide) and derivatives. J.
occup. Med., 23: 343-347.
THIESS, A.M., FRENZEL-BEYME, R., LINK, R., & STOCKER, W.H.
(1981b) Mortality study on employees exposed to alkylene
oxides (ethylene oxide/propylene oxide) and their derivatives.
In: Proceedings of the International Symposium on Prevention
of Occupational Cancer, Helsinki, Finland, pp. 249-259.
US EPA (1981) Human exposure to atmospheric concentrations
of selected chemicals, Research Triangle Park, North Carolina,
US Environmental Protection Agency, Office of Air Quality
Planning and Standards (US NTIS PB 84-102540, PB 83-265249).
US NTP (1984) Toxicology and carcinogenesis studies of
propylene oxide in F344/N rats and B6C3F1 mice (inhalation
studies), Research Triangle Park, North Carolina, National
Toxicology Program (NTP 83-020) (NIH Publication No. 84-2523).
VOJNOVICH, C. & PFEIFER, V.F. (1967) Reducing the microbial
population of flour during milling. Cereal Sci. Today, 12:
54-60.
VOOGD, C.E., STEL, J.J. VAN DE, & JACOBS, J.J.J.A.A. (1981)
The mutagenic action of aliphatic epoxides. Mutat. Res., 89:
269-282.
WADE, D.R., AIRY, S.C., & SINSHEIMER, J.E. (1978)
Mutagenicity of aliphatic epoxides. Mutat. Res., 58: 217-223.
WALLES, S.A. (1974) The influence of some alkylating agents
on the structure of DNA in vitro . Chem.-biol. Interact., 9:
97-103.
WEBBER, D. (1984) Basic chemical output returns to growth.
Top 50 chemical products. Chem. Eng. News, May 7: 8-10.
WEIL, C.S., CONDRA, N., HAUN, C., & STRIEGEL, J.A. (1963)
Experimental carcinogenicity and acute toxicity of
representative epoxides. Am. Ind. Hyg. Assoc. J., 24: 305-325.
WHO (1978) Environmental health problems associated with the
manufacture and uses of synthetic organic chemicals, Geneva,
World Health Organization (Report No. HCS/78.2).
WHO (1985) EHC 55: Ethylene oxide, Geneva, World Health
Organization, 79 pp.
ZAMORA, P.O., BENSON, J.M., LI, A.P., & BROOKS, A.L. (1983)
Evaluation of an exposure system using cells grown on collagen
gels for detecting highly-volatile mutagens in the CHO/HGPRT
mutation assay. Environ. Mutagen., 5: 795-801.
