
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Health and Safety Guide No. 108
CARBON TETRACHLORIDE
HEALTH AND SAFETY GUIDE
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
WORLD HEALTH ORGANIZATION, GENEVA 1998
This is a companion volume to
Environmental Health Criteria 208: Carbon tetrachloride
Published by the World Health Organization for the International
Programme on Chemical Safety (a collaborative programme of the United
Nations Environment Programme, the International Labour Organisation,
and the World Health Organization) and produced within the framework
of the Inter-Organization Programme for the Sound Management of
Chemicals
WORLD HEALTH ORGANIZATION, GENEVA 1998
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
WHO Library Cataloguing in Publication Data
Carbon tetrachloride: health and safety guide.
(Health and safety guide ; no. 108)
1. Carbon tetrachloride - toxicity
2. Environmental exposure
3. Guidelines
I. International Programme on Chemical Safety
II. Series
ISBN 92 4 151108 7 (NLM Classification: QD 305.H5)
ISSN 0259-7268
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1998
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in this
publication do not imply the expression of any opinion whatsoever on
the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
The Federal Ministry for the Environment, Nature Conservation and
Nuclear Safety (Germany) provided financial support for, and undertook
the printing of, this publication
CONTENTS
INTRODUCTION
1. PRODUCT IDENTITY AND USES
1.1. Identity
1.2. Physical and chemical properties
1.3. Analysis
1.4. Production and uses
2. SUMMARY AND EVALUATION
3. CONCLUSIONS
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY
RESPONSE
4.1. Human health hazards, prevention and protection, first aid
4.2. Advice to physicians
4.3. Health surveillance advice
4.4. Explosion and fire hazards, prevention
4.4.1. Explosion and fire hazards
4.4.2. Prevention
4.5. Storage
4.6. Transport
4.7. Disposal
4.8. Spillage
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
6. SUMMARY OF CHEMICAL SAFETY INFORMATION
7. CURRENT REGULATIONS, GUIDELINES AND STANDARDS
7.1. Exposure limit values
7.2. Specific restrictions/requirements
7.2.1. USA
7.2.2. Canada
7.2.3. EEC
7.3. Labelling, packaging and transport
7.3.1. USA
7.3.2. EEC
7.3.3. United Kingdom
BIBLIOGRAPHY
INTRODUCTION
The Environmental Health Criteria (EHC) monographs produced by the
International Programme on Chemical Safety include an assessment of
the effects on the environment and on human health of exposure to a
chemical or combination of chemicals, or physical or biological
agents. They also provide guidelines for setting exposure limits.
The purpose of a Health and Safety Guide is to facilitate the
application of these guidelines in national chemical safety
programmes. The first three sections of a Health and Safety Guide
highlight the relevant technical information in the corresponding EHC.
Section 4 includes advice on preventive and protective measures and
emergency action; health workers should be thoroughly familiar with
the medical information to ensure that they can act efficiently in an
emergency. Within the Guide is a Summary of Chemical Safety
Information which should be readily available, and should be clearly
explained, to all who could come into contact with the chemical. The
section on regulatory information has been extracted from the legal
file of the International Register of Potentially Toxic Chemicals
(IRPTC) and from other United Nations sources.
The target readership includes occupational health services, those in
ministries, governmental agencies, industry, and trade unions who are
involved in the safe use of chemicals and the avoidance of
environmental health hazards, and those wanting more information on
this topic. An attempt has been made to use only terms that will be
familiar to the intended user. However, sections 1 and 2 inevitably
contain some technical terms. A bibliography has been included for
readers who require further background information.
Revision of the information in this Guide will take place in due
course, and the eventual aim is to use standardized terminology.
Comments on any difficulties encountered in using the Guide would be
very helpful and should be addressed to:
The Director
International Programme on Chemical Safety
World Health Organization
1211 Geneva 27
Switzerland
THE INFORMATION IN THIS GUIDE SHOULD BE CONSIDERED AS A
STARTING POINT TO A COMPREHENSIVE HEALTH AND SAFETY PROGRAMME
1. PRODUCT IDENTITY AND USES
1.1 Identity
Chemical formula: CCl4
Chemical structure:
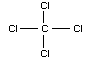 Common name: carbon tetrachloride
Common synonyms: Carbona, carbon chloride,
tetrachloromethane, carbon tet, methane
tetrachloride, perchloromethane,
tetrachlorocarbon
Trade names: Benzinoform, Fasciolin, Freon 10, Halon
104, Tetraform, Tetrafinol
CAS chemical name: tetrachloromethane
CAS registry number: 56-23-5
RTECS registry number: FG 4900000
Conversion factor: 1 ppm = 6.41 mg carbon tetrachloride/m3
air
1 mg carbon tetrachloride/m3 air =
0.156 ppm
at 20°C and 101.3 kPa (760 mmHg).
1.2 Physical and chemical properties
Carbon tetrachloride is a volatile, colourless, clear, heavy
liquid with a characteristic sweet, non-irritant odour. The odour
threshold in water is 0.52 mg/litre, and in air it is >64.1 mg/m3
(10 ppm). Carbon tetrachloride is miscible with most aliphatic
solvents and it is a solvent itself. The solubility in water is low.
It is non-flammable and fairly stable in the presence of air and
light. Decomposition of carbon terachloride forms phosgene, carbon
dioxide and hydrochloric acid. It reacts explosively with aluminium
powder and some other reactive metals, and, in the presence of
peroxides or light, with unsaturated compounds.
The most important physical and chemical properties of carbon
tetrachloride are presented in the Summary of Chemical Safety
Information (section 6).
1.3 Analysis
Several sufficiently sensitive and accurate analytical methods
for determining carbon tetrachloride in air, water and biological
samples are available. The majority of these methods are based on
direct injection into a gas chromatograph or adsorption on activated
charcoal, then desorption or evaporation and subsequent gas
chromatographic detection. For air and water, detection limits of
0.003 µg/m3 and 0.001 µg/litre, respectively, can be achieved.
1.4 Production and uses
Most of the carbon tetrachloride produced is used in the
production of chlorofluorocarbons (CFCs) and other chlorinated
hydrocarbons. The global production of carbon tetrachloride amounted
to 960 000 tonnes in 1987. However, since 1990 the use and
consequently the production of carbon tetrachloride has decreased and
will continue to decrease in future owing to the gradual phase-out,
established by the Montreal Protocol and its amendments, of CFCs and
carbon tetrachloride.
Carbon tetrachloride has been mainly manufactured by the
chlorination of methane or carbon disulfide.
2. SUMMARY AND EVALUATION
Nearly all carbon tetrachloride released to the environment will
ultimately be present in the atmosphere, owing to its volatility.
Since the atmospheric residence time of carbon tetrachloride is long,
it is widely distributed. During the period 1980-1990, atmospheric
levels were around 0.5-1.0 µg/m3. Estimates of atmospheric lifetime
are variable, but 45-50 years is accepted as the most reasonable
value. Carbon tetrachloride contributes both to ozone depletion and to
global warming. It is in general resistant to aerobic biodegradation
but less so to anaerobic. Acclimation increases biodegradation rates.
Although the octanol-water partition coefficient indicates moderate
potential for bioaccumulation, short tissue lifetime reduces this
tendency.
In water, reports have indicated levels of less than 10 ng/litre
in the ocean and generally less than 1 µg/litre in fresh water, but
much higher values close to release sites. Levels of up to 60 µg/kg
have been recorded in foods processed with carbon tetrachloride, but
this practice has now ceased.
The general population is exposed to carbon tetrachloride mainly
via air. On the basis of the reported concentrations in ambient air,
foodstuffs and drinking-water, a daily carbon tetrachloride intake of
around 1 µg/kg body weight has been estimated. This estimate is
probably rather high for the present day, because the use of carbon
tetrachloride as a fumigant of grain has stopped and the carbon
tetrachloride values reported for food and used in the calculation
were especially those found in fatty and grain-based foods. Values of
0.1 to 0.27 µg/kg body weight for daily exposure of the general
population have been reported elsewhere. Exposure to higher levels of
carbon tetrachloride can occur in the workplace as a result of
accidental spillage.
Carbon tetrachloride is well absorbed from the gastrointestinal
and respiratory tract in animals and humans. Dermal absorption of
liquid carbon tetrachloride is possible, but dermal absorption of the
vapour is slow.
Carbon tetrachloride is distributed throughout the whole body,
with highest concentrations in liver, brain, kidney, muscle, fat and
blood. The parent compound is eliminated primarily in exhaled air,
while minimal amounts are excreted in the faeces and urine.
The first step in the biotransformation of carbon tetrachloride
is catalysed by cytochrome P-450 enzymes, leading to the formation of
the reactive trichloromethyl radical. Oxidative biotransformation is
the most important pathway in the elimination of the radical, forming
the even more reactive trichloromethylperoxyl radical, which can react
further to form phosgene. Phosgene may be detoxified by reaction with
water to produce carbon dioxide or with glutathione or cysteine.
Formation of chloroform and dichlorocarbene occurs under anaerobic
conditions.
Covalent binding to macromolecules and lipid peroxidation occur
via metabolic intermediates of carbon tetrachloride.
The liver and kidney are target organs for carbon tetrachloride
toxicity. The severity of the effects on the liver depends on a number
of factors such as species susceptibility, route and mode of exposure,
diet or co-exposure to other compounds, in particular ethanol.
Furthermore, it appears that pretreatment with various compounds, such
as phenobarbital and vitamin A, enhances hepatotoxicity, while other
compounds, such as vitamin E, reduce the hepatotoxic action of carbon
tetrachloride.
Moderate irritation after dermal application was seen on the
skins of rabbits and guinea-pigs, and there was a mild reaction after
application into the rabbit eye.
The lowest LD50 of 2391 mg/kg body weight (14-day period) was
reported in a study on dogs involving intraperitoneal administration.
In rats the LD50 values ranged from 2821 to 10 054 mg/kg body weight.
In a 12-week oral study on rats (5 days/week), the
no-observed-adverse-effect level (NOAEL) was 1 mg/kg body weight. The
lowest-observed-adverse-effect level (LOAEL) reported in this study
was 10 mg/kg body weight, showing a slight, but significant increase
in sorbitol dehydrogenase (SDH) activity and mild hepatic
centrilobular vacuolization. A similar NOAEL of 1.2 mg/kg body weight
(5 days/ week) was found in a 90-day oral study on mice, with a LOAEL
of 12 mg/kg body weight, where hepatotoxicity occurred.
When rats were exposed to carbon tetrachloride by inhalation for
approximately 6 months, 5 days/week, 7 h/day, a NOAEL of 32 mg/m3 was
reported. The LOAEL, based on changes in the liver morphology, was
reported to be 63 mg/m3. In another 90-day study on rats, a NOAEL of
6.1 mg/m3 was found after continuous exposure to carbon
tetrachloride. The lowest exposure level of 32 mg/m3 (the lowest
concentration studied) in a 2-year inhalation study on rats caused
marginal effects.
The only oral long-term toxicity study available was a 2-year
study in rats, which were exposed to 0, 80 or 200 mg carbon
tetrachloride/kg feed. Owing to chronic respiratory disease in all
animals beginning at 14 months, which resulted in increased mortality,
the results reported upon necropsy at 2 years are inadequate for a
health risk evaluation.
It was concluded that carbon tetrachloride can induce embryotoxic
and embryolethal effects, but only at doses that are maternally toxic,
as observed in inhalation studies in rats and mice. Carbon
tetrachloride is not teratogenic.
Many genotoxicity assays have been conducted with carbon
tetrachloride. On the basis of available data, carbon tetrachloride
can be considered as a non-genotoxic compound.
Carbon tetrachloride induces hepatomas and hepatocellular
carcinomas in mice and rats. The doses inducing hepatic tumours are
higher than those inducing cell toxicity.
In humans, acute symptoms after carbon tetrachloride exposure are
independent of the route of intake and are characterized by
gastrointestinal and neurological symptoms, such as nausea, vomiting,
headache, dizziness, dyspnoea and death. Liver damage appears after 24
h or more. Kidney damage is evident often only 2 to 3 weeks following
the poisoning.
Epidemiological studies have not established an association
between carbon tetrachloride exposure and increased risk of mortality,
neoplasia or liver disease. Some studies have suggested an association
with increased risk of non-Hodgkin's lymphoma and, in one study, with
mortality and liver cirrhosis. However, not all of these studies
pinpointed specific exposure to carbon tetrachloride, and the
statistical associations were not strong.
In general carbon tetrachloride appears to be of low toxicity to
bacteria, protozoa and algae; the lowest toxic concentration reported
was for methanogenic bacteria with an IC50 of 6.4 mg/litre. For
aquatic invertebrates acute LC50 values range from 28 to > 770
mg/litre. In freshwater fish the lowest acute LC50 value of 13
mg/litre was found in the golden orfe (Leuciscus idus melanotus),
and for marine species an LC50 value of 50 mg/litre was reported for
the dab (Limanda limanda). Carbon tetrachloride appears to be more
toxic to embryo-larval stages of fish and amphibians than to adults.
The common bullfrog (Rana catesbeiara) is the most susceptible
species, the LC50 being 0.92 mg/litre (fertilization to 4 days after
hatching).
The available data indicate that hepatic tumours are induced by a
non- genotoxic mechanism, and it therefore seems acceptable to develop
a tolerable daily intake (TDI) and a tolerable daily concentration in
air (TC) for carbon tetrachloride.
On the basis of the study of Bruckner et al. (1986), in which a
NOAEL of 1 mg/kg body weight was observed in a 12-week oral study on
rats, and incorporating a conversion factor of 5/7 for daily dosing
and applying an uncertainty factor of 500 (100 for inter- and
intraspecies variation, 10 for duration of the study, and modifying
factor 0.5 because it was a bolus study), a TDI of 1.42 µg/kg body
weight is obtained.
On the basis of a 90-day inhalation study on rats (Prendergast et
al., 1967), in which a NOAEL of 6.1 mg/m3 was reported, a TC of 6.1
µg/m3 was calculated using the factors 7/24 and 5/7 to convert to
continuous exposure and an uncertainty factor of 1000 (100 for inter-
and intraspecies variation and 10 for the duration of the study). This
TC corresponds to a TDI of 0.85 µg/kg body weight.
Comparing the estimated upper limit of prevailing human daily
intake of 0.2 µg/kg body weight with the lowest TDI value (0.85 µg/kg
body weight), the conclusion can be drawn that the currently
prevailing exposure of the general population to carbon tetrachloride
from all sources is unlikely to cause excessive intake of the
chemical.
In general, the risk to aquatic organisms from carbon
tetrachloride is low. However, it may present a risk to embryo-larval
stages at, or near, sites of industrial discharges or spills.
3. CONCLUSIONS
The general population is generally exposed to only low levels of
carbon tetrachloride via air, drinking-water and food (total daily
uptake is estimated to be 0.2 µg/kg body weight; see chapter 2).
Carbon tetrachloride can induce embryotoxic and embryolethal
effects at maternally toxic doses, but it is not teratogenic. The
liver is the target organ for carbon-tetrachloride-induced toxicity.
Carbon tetrachloride can produce hepatic tumours at dose levels that
are higher than those producing toxic effects in the liver. The weight
of evidence indicates that carbon tetrachloride has no genotoxic
properties. On the basis of this fact and the fact that the induced
hepatoxicity appears to be of major importance for its
carcinogenicity, carbon tetrachloride can be considered as a compound
with carcinogenic properties. A tolerable daily intake (TDI) of 0.85
µg/kg body weight and a tolerable daily concentration (TC) in air of
6.1 µg/m3 have been derived.
Because carbon tetrachloride does not remain in water, due to its
high volatility and low solubility, it is likely that it will present
a risk only to the embryo-larval stages of some sensitive amphibians
at times of industrial discharges or spills.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY
RESPONSE
4.1 Human health hazards, prevention and protection, first aid
The human health hazards associated with exposure to carbon
tetrachloride, together with preventive and protective measures, and
first-aid recommendations, are listed in the Summary of Chemical
Safety Information in section 6.
4.2 Advice to physicians
If carbon tetrachloride has been ingested, vomiting should not be
induced. The patient should drink water to delay absorption, but not
oil or milk. If the patient has been exposed to carbon tetrachloride
vapour he should immediately be moved to fresh air (or given
artificial respiration) and kept under observation. Special attention
must be paid to the use of alcoholic beverages in combination with
exposure to carbon tetrachloride, because the toxic effects are
enhanced by ingestion of alcohol.
4.3 Health surveillance advice
Workers frequently exposed to carbon tetrachloride should be
examined periodically and appropriate measures should be taken.
Replacement and periodic examinations should include appropriate tests
for liver and kidney functions, and special attention should be given
to any history of alcoholism. In all cases of accidental exposure a
medical practitioner should be immediately consulted.
4.4 Explosion and fire hazards, prevention
4.4.1 Explosion and fire hazards
Carbon tetrachloride vapour is invisible, heavier than air and
spreads along the ground. Carbon tetrachloride is non-flammable, but
it can generate phosgene and similar toxic gases when heated to high
temperatures or when involved in a fire. Carbon tetrachloride reacts
explosively when mixed with unsaturated compounds in the presence of
peroxides or light.
Carbon tetrachloride reacts vigorously or explosively with
chemically active metals (lithium, potassium, barium, aluminium,
magnesium, zinc and uranium) or fluorine. Violent reactions occur
between carbon tetrachloride and N,N-dimethylacetamide or
N,N-dimethylformamide in the presence of iron. Explosions have been
reported with carbon tetrachloride and aluminium alkyls, boranes and
carbaboranes, calcium disilicide, calcium hypochlorite, chlorine
trifluoride, allyl alcohol, ethylene, liquid oxygen, nitrogen dioxide
and silanes.
4.4.2 Prevention
If large closed containers with carbon tetrachloride are exposed
to heat or fire, they must be kept cool by spraying with water.
Work with carbon tetrachloride should be carried out with
adequate ventilation. Breathing the vapour and skin contact should be
avoided. Chemical protective clothing, masks and gloves made from
materials that provide a high degree of permeation resistance and eye
protection should be used. Note that rubber is not a suitable
protective material since carbon tetrachloride migrates through it.
4.5 Storage
Carbon tetrachloride should be stored in labelled, airtight
containers in a well-ventilated place protected from light and at a
temperature below 30°C. It must be stored separated from chemically
active metals.
4.6 Transport
In case of accident, stop the engine. Notify police and fire
brigade immediately, keep public away from danger area, mark roads and
warn other road users. Do not smoke, do not use naked lights and keep
upwind.
In case of spillage or fire, the advice given in sections 4.8 and
4.4, respectively, should be followed.
In case of poisoning, the advice in the Summary of Chemical
Safety Information should be followed.
4.7 Disposal
Small quantities of carbon tetrachloride may be disposed of by
evaporation in a fume cupboard or in a safe, open area. Incineration
is not recommended due to the non-flammability of carbon tetrachloride
and to the formation of phosgene, hydrogen chloride and other toxic
gases on heating.
4.8 Spillage
In case of spillage of carbon tetrachloride, ensure personel
protection (protective clothing, safety goggles, rubber gloves and
respiratory protective device) and carefully shut off leaks. Adsorb
the spilt carbon tetrachloride in earth, sand or inert absorbent and
remove to a safe place. Prevent liquid from entering sewers, basements
and workpits because the vapour may create a toxic atmosphere.
If carbon tetrachloride has entered a water course or sewer or if
it has contaminated soil or vegetation, the police should be warned.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
In view of the toxicity of carbon tetrachloride for embryo-larval
stages of some aquatic organisms, it may present a hazard to these
organisms at, or near, sites of industrial discharges or spills.
Contamination of the environment can be minimized by proper
methods of storage, handling, transport and protection.
In case of spillage, the methods recommended in section 4.8
should be used.
6. SUMMARY OF CHEMICAL SAFETY INFORMATION
This summary should be easily available to all health workers
concerned with, and users of, carbon tetrachloride. It should be
displayed at, or near, entrances to areas where there is potential
exposure to carbon tetrachloride, and on processing equipment and
containers. The summary should be translated into the appropriate
language(s). All persons potentially exposed to the chemical should
also have the instructions in the summary clearly explained.
SUMMARY OF CHEMICAL SAFETY INFORMATION
Carbon tetrachloride
CCl4; CAS Registry No. 56-23-5
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Melting point (°C) -22.92 Carbon tetrachloride is a clear, volatile,
Boiling point (°C) 76.72 colourless, heavy liquid, with a characteristic
Relative molecular mass 153.8 sweet, non-irritant odour. Although non-flammable,
Density (20 °C) 1.59 g/ml it decomposes in fire or in heat, giving off
Ignition temperature (°C) >1000 toxic fumes (phosgene and hydrochloric acid).
Water solubility (25 °C) 785 mg/litre Owing to its low conductivity, vapour
Vapour pressure (0 °C) 4.4 kPa electrostatic charges may be generated through
Vapour pressure (20 °C) 12.2 kPa flow, movement etc. It reacts violently with
Vapour density (101.3 kPa; 0 °C) 5.3 kg/m3 chemically active metals (such as aluminium,
n-octanol-water partition coefficient (log Pow) 2.64 magnesium, sodium, lithium, potassium, iron, zinc).
Henry's law constant (24.8°C) 365 kJ/mole It reacts explosively when mixed with unsaturated
Flash point (°C) none compounds in the presence of peroxides or light.
Explosive limits none
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
SKIN: redness, pain, blisters; may be Protective gloves and clothing Remove contaminated clothing and wash skin
absorbed with plenty of water; obtain medical attention
EYES: redness, pain Safety goggles or face shield in combination Wash the eyes with plenty of water or neutral
with breathing protection (organic filter) saline solution for several minutes (remove
contact lenses if possible) or blow out with
an air stream; obtain medical attention
INHALATION: dizziness, drowsiness, Apply ventilation, use in an exhaust hood or Remove victim to fresh air, apply artificial
headache, nausea, unconsciousness use personal breathing protection (organic respiration if indicated; obtain medical
filter) attention or move if necessary to hospital
SUMMARY OF CHEMICAL SAFETY INFORMATION (continued)
INGESTION: abdominal pain, diarrhoea, Do not eat, drink, chew or smoke during Rinse mouth; do not induce vomiting, let victim
dizziness, drowsiness, nausea, work; do not keep food in areas with drink water and refer for medical attention
vomiting, unconsciousness potential exposure; keep out of reach of
children
ENVIRONMENT: may present a hazard to Minimize contamination of water, soil and
embryo-larval stages of some aquatic atmosphere by proper methods of storage,
organisms at discharges or spills handling, transport and waste disposal
SPILLAGE STORAGE FIRE AND EXPLOSION
Ensure personal protection; shut off Store separately from chemically active CCl4 is not combustible, but gives off
leaks if without risk; collect metals in a cool place; do not store in irritating toxic fumes in a fire. All types
leaking liquid in closed containers; aluminium containers; ventilate along of extinguishing agents can be used when
absorb spilt carbon tetrachloride in the floor there is a fire in the direct environment;
earth, sand or inert absorbent and in case of fire, keep containers cool by
remove to a safe place; prevent entry spraying with water
into a sewer
WASTE DISPOSAL NATIONAL INFORMATION
Incineration is not recommended due to National occupational exposure limit:
non-flammability and formation of
phosgene, hydrogen chloride and other National Poison Control Centre:
toxic gases on heating
7. CURRENT REGULATIONS, GUIDELINES AND STANDARDS
The information in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file. Its intention is to give the reader an overview of current
regulations, guidelines and standards.
Regulatory decisions about chemicals, taken in a certain country,
can only be fully understood within the framework of the legislation
of that country. Furthermore, the regulations and guidelines of all
countries are subject to change and should always be verified with the
appropriate regulatory authorities before application.
7.1 Exposure limit values
Some exposure limit values are given in the table. When no
effective date appears in the IRPTC legal file, the year of the
reference from which the data are taken is indicated by (r).
7.2 Specific restrictions/requirements
7.2.1 USA
In the USA carbon tetrachloride and any mixture containing it is
banned as a hazardous product because it posesses such a degree of
hazard that adequate cautionary labelling cannot be written and public
health can only be served by keeping it out of interstate commerce.
This does not apply to unavoidable manufacturing residues in other
chemicals if any reasonable use does not result in atmospheric
concentrations of more than 63 mg/m3 (reference date, 1982).
7.2.2 Canada
In Canada it is illegal to sell, advertise or import products
that consist of or contain carbon tetrachloride where it is packaged
as a consumer product (effective date, 1971).
7.2.3 EEC
a) In case of exposure to carbon tetrachloride, Member States shall,
in addition to other general measures, ensure: 1) medical
surveillance of workers prior to and/or during exposure; 2)
access for workers to their individual and anonymus collective
results. The general measures are in order to keep exposure as
low as reasonably practicable (effective date, 1983).
b) Carbon tetrachloride must not form part of the composition of
cosmetic products. The marketing of cosmetic products containing
the substance is prohibited (effective date, 1988).
CURRENT REGULATIONS, GUIDELINES AND STANDARDS
Exposure limit values
Medium Specification Country/organization Exposure limit description Value Effective date
AIR Occupational Australia Threshold limit value (TLV) 1983 (r)
- Time-weighted average (TWA) 30 mg/m3
- Short-term exposure limit (STEL) 125 mg/m3
Belgium Threshold limit value (TLV) 1984 (r)
- Time-weighted average (TWA) 30 mg/m3
Canada Threshold limit value (TLV) 1980
- Time-weighted average (TWA) 30 mg/m3
Finland Maximum permissible concentration 1982 (r)
- Time-weighted average (TWA) 33 mg/m3
- Short-term exposure limit (STEL) 66 mg/m3
(15 min)
Germany Maximum acceptable concentration 1987 (r)
- Short-term exposure limit (STEL) 20 mg/m3
Hungary Maximum limit 10 mg/m3 1988
Italy Threshold limit value (TLV) 65 mg/m3 1978 (r)
Japan - Threshold limit value (TLV) 31 mg/m3 1991
(Skin adsorption)
The Netherlands Maximum limit (MXL) 1986 (r)
- Time-weighted average (TWA) 12.6 mg/m3
Poland Maximum permissible concentration 1982 (r)
- Time-weighted average (TWA) 20 mg/m3
- Short-term exposure limit (30 min TWA) 100 mg/m3
CURRENT REGULATIONS, GUIDELINES AND STANDARDS (continued)
Medium Specification Country/organization Exposure limit description Value Effective date
Romania Maximum permissible concentration 1975 (r)
- Time-weighted average (TWA) 50 mg/m3
- Ceiling limit value (CLV) 100 mg/m3
Russia Time-weighted average (TWA) 20 mg/m3 1988
Sweden Hygienic limit value (HLV) 1988
- Time-weighted average (TWA) 13 mg/m3
- Short-term exposure limit (STEL) 19 mg/m3
(15-min TWA)
Switzerland Maximum acceptable concentration 1987 (r)
- Time-weighted average (TWA) 30 mg/m3
United Kingdom Time-weighted average (TWA) 65 mg/m3 1987 (r)
- Short-term exposure limit (STEL) 130 mg/m3
(10-min TWA)
USA/ACGIH Threshold limit value (TLV) 1987 (r)
- Time-weighted average (TWA) 31 mg/m3
- Short-term exposure limit (STEL) 63 mg/m3
USA Permissible exposure limit (PEL) 1974
- Time-weighted average (TWA) 63 mg/m3
- Ceiling limit value (CLV) 160 mg/m3
c) Carbon tetrachloride may not be used in ornamental objects
intended to produce light or colour effects by means of different
phases, for example in ornamental lamps and ashtrays (effective
date, 1987)
7.3 Labelling, packaging and transport
7.3.1 USA
In the USA it is permitted to use carbon tetrachloride as a
component of adhesives in articles intended for use in packaging,
transporting or holding food (reference date, 1981).
7.3.2 EEC
Carbon tetrachloride is considered to be a harmful substance.
Member States shall ensure that dangerous preparations (solvents) are
not placed on the market unless their packages and fastenings and
labels comply with the EEC requirements (effective date, 1984).
7.3.3 United Kingdom
Labelling of road tankers: toxic substance (emergency action
code, 2Z) (effective date, 1979).
BIBLIOGRAPHY
CEC/IPCS (1994) International Chemical Safety Card 0024: Carbon
tetrachloride. Luxembourg, Commission of the European Communities.
Dutch Chemical Industry Association (1994) Chemical safety sheets.
Samson Chemical Publishers.
IARC (1979) Some halogenated hydrocarbons. Lyon, International Agency
for Research on Cancer. (IARC Monographs on the evaluation of
carcinogenic risk of chemicals to humans, volume 20).
IRPTC Data Profile (legal file) on carbon terachloride. In: Database
of ECDIN (via DIMDI), field 'Standards and Regulations'. Geneva,
International Registry of Potentially Toxic Chemicals.
Walsh D (1989) Chemical Safety Data Sheets, Volume 1. Royal Society of
Chemistry, England.
WHO (in press) Environmental Health Criteria 208: Carbon
tetrachloride. Geneva, World Health Organization.
Common name: carbon tetrachloride
Common synonyms: Carbona, carbon chloride,
tetrachloromethane, carbon tet, methane
tetrachloride, perchloromethane,
tetrachlorocarbon
Trade names: Benzinoform, Fasciolin, Freon 10, Halon
104, Tetraform, Tetrafinol
CAS chemical name: tetrachloromethane
CAS registry number: 56-23-5
RTECS registry number: FG 4900000
Conversion factor: 1 ppm = 6.41 mg carbon tetrachloride/m3
air
1 mg carbon tetrachloride/m3 air =
0.156 ppm
at 20°C and 101.3 kPa (760 mmHg).
1.2 Physical and chemical properties
Carbon tetrachloride is a volatile, colourless, clear, heavy
liquid with a characteristic sweet, non-irritant odour. The odour
threshold in water is 0.52 mg/litre, and in air it is >64.1 mg/m3
(10 ppm). Carbon tetrachloride is miscible with most aliphatic
solvents and it is a solvent itself. The solubility in water is low.
It is non-flammable and fairly stable in the presence of air and
light. Decomposition of carbon terachloride forms phosgene, carbon
dioxide and hydrochloric acid. It reacts explosively with aluminium
powder and some other reactive metals, and, in the presence of
peroxides or light, with unsaturated compounds.
The most important physical and chemical properties of carbon
tetrachloride are presented in the Summary of Chemical Safety
Information (section 6).
1.3 Analysis
Several sufficiently sensitive and accurate analytical methods
for determining carbon tetrachloride in air, water and biological
samples are available. The majority of these methods are based on
direct injection into a gas chromatograph or adsorption on activated
charcoal, then desorption or evaporation and subsequent gas
chromatographic detection. For air and water, detection limits of
0.003 µg/m3 and 0.001 µg/litre, respectively, can be achieved.
1.4 Production and uses
Most of the carbon tetrachloride produced is used in the
production of chlorofluorocarbons (CFCs) and other chlorinated
hydrocarbons. The global production of carbon tetrachloride amounted
to 960 000 tonnes in 1987. However, since 1990 the use and
consequently the production of carbon tetrachloride has decreased and
will continue to decrease in future owing to the gradual phase-out,
established by the Montreal Protocol and its amendments, of CFCs and
carbon tetrachloride.
Carbon tetrachloride has been mainly manufactured by the
chlorination of methane or carbon disulfide.
2. SUMMARY AND EVALUATION
Nearly all carbon tetrachloride released to the environment will
ultimately be present in the atmosphere, owing to its volatility.
Since the atmospheric residence time of carbon tetrachloride is long,
it is widely distributed. During the period 1980-1990, atmospheric
levels were around 0.5-1.0 µg/m3. Estimates of atmospheric lifetime
are variable, but 45-50 years is accepted as the most reasonable
value. Carbon tetrachloride contributes both to ozone depletion and to
global warming. It is in general resistant to aerobic biodegradation
but less so to anaerobic. Acclimation increases biodegradation rates.
Although the octanol-water partition coefficient indicates moderate
potential for bioaccumulation, short tissue lifetime reduces this
tendency.
In water, reports have indicated levels of less than 10 ng/litre
in the ocean and generally less than 1 µg/litre in fresh water, but
much higher values close to release sites. Levels of up to 60 µg/kg
have been recorded in foods processed with carbon tetrachloride, but
this practice has now ceased.
The general population is exposed to carbon tetrachloride mainly
via air. On the basis of the reported concentrations in ambient air,
foodstuffs and drinking-water, a daily carbon tetrachloride intake of
around 1 µg/kg body weight has been estimated. This estimate is
probably rather high for the present day, because the use of carbon
tetrachloride as a fumigant of grain has stopped and the carbon
tetrachloride values reported for food and used in the calculation
were especially those found in fatty and grain-based foods. Values of
0.1 to 0.27 µg/kg body weight for daily exposure of the general
population have been reported elsewhere. Exposure to higher levels of
carbon tetrachloride can occur in the workplace as a result of
accidental spillage.
Carbon tetrachloride is well absorbed from the gastrointestinal
and respiratory tract in animals and humans. Dermal absorption of
liquid carbon tetrachloride is possible, but dermal absorption of the
vapour is slow.
Carbon tetrachloride is distributed throughout the whole body,
with highest concentrations in liver, brain, kidney, muscle, fat and
blood. The parent compound is eliminated primarily in exhaled air,
while minimal amounts are excreted in the faeces and urine.
The first step in the biotransformation of carbon tetrachloride
is catalysed by cytochrome P-450 enzymes, leading to the formation of
the reactive trichloromethyl radical. Oxidative biotransformation is
the most important pathway in the elimination of the radical, forming
the even more reactive trichloromethylperoxyl radical, which can react
further to form phosgene. Phosgene may be detoxified by reaction with
water to produce carbon dioxide or with glutathione or cysteine.
Formation of chloroform and dichlorocarbene occurs under anaerobic
conditions.
Covalent binding to macromolecules and lipid peroxidation occur
via metabolic intermediates of carbon tetrachloride.
The liver and kidney are target organs for carbon tetrachloride
toxicity. The severity of the effects on the liver depends on a number
of factors such as species susceptibility, route and mode of exposure,
diet or co-exposure to other compounds, in particular ethanol.
Furthermore, it appears that pretreatment with various compounds, such
as phenobarbital and vitamin A, enhances hepatotoxicity, while other
compounds, such as vitamin E, reduce the hepatotoxic action of carbon
tetrachloride.
Moderate irritation after dermal application was seen on the
skins of rabbits and guinea-pigs, and there was a mild reaction after
application into the rabbit eye.
The lowest LD50 of 2391 mg/kg body weight (14-day period) was
reported in a study on dogs involving intraperitoneal administration.
In rats the LD50 values ranged from 2821 to 10 054 mg/kg body weight.
In a 12-week oral study on rats (5 days/week), the
no-observed-adverse-effect level (NOAEL) was 1 mg/kg body weight. The
lowest-observed-adverse-effect level (LOAEL) reported in this study
was 10 mg/kg body weight, showing a slight, but significant increase
in sorbitol dehydrogenase (SDH) activity and mild hepatic
centrilobular vacuolization. A similar NOAEL of 1.2 mg/kg body weight
(5 days/ week) was found in a 90-day oral study on mice, with a LOAEL
of 12 mg/kg body weight, where hepatotoxicity occurred.
When rats were exposed to carbon tetrachloride by inhalation for
approximately 6 months, 5 days/week, 7 h/day, a NOAEL of 32 mg/m3 was
reported. The LOAEL, based on changes in the liver morphology, was
reported to be 63 mg/m3. In another 90-day study on rats, a NOAEL of
6.1 mg/m3 was found after continuous exposure to carbon
tetrachloride. The lowest exposure level of 32 mg/m3 (the lowest
concentration studied) in a 2-year inhalation study on rats caused
marginal effects.
The only oral long-term toxicity study available was a 2-year
study in rats, which were exposed to 0, 80 or 200 mg carbon
tetrachloride/kg feed. Owing to chronic respiratory disease in all
animals beginning at 14 months, which resulted in increased mortality,
the results reported upon necropsy at 2 years are inadequate for a
health risk evaluation.
It was concluded that carbon tetrachloride can induce embryotoxic
and embryolethal effects, but only at doses that are maternally toxic,
as observed in inhalation studies in rats and mice. Carbon
tetrachloride is not teratogenic.
Many genotoxicity assays have been conducted with carbon
tetrachloride. On the basis of available data, carbon tetrachloride
can be considered as a non-genotoxic compound.
Carbon tetrachloride induces hepatomas and hepatocellular
carcinomas in mice and rats. The doses inducing hepatic tumours are
higher than those inducing cell toxicity.
In humans, acute symptoms after carbon tetrachloride exposure are
independent of the route of intake and are characterized by
gastrointestinal and neurological symptoms, such as nausea, vomiting,
headache, dizziness, dyspnoea and death. Liver damage appears after 24
h or more. Kidney damage is evident often only 2 to 3 weeks following
the poisoning.
Epidemiological studies have not established an association
between carbon tetrachloride exposure and increased risk of mortality,
neoplasia or liver disease. Some studies have suggested an association
with increased risk of non-Hodgkin's lymphoma and, in one study, with
mortality and liver cirrhosis. However, not all of these studies
pinpointed specific exposure to carbon tetrachloride, and the
statistical associations were not strong.
In general carbon tetrachloride appears to be of low toxicity to
bacteria, protozoa and algae; the lowest toxic concentration reported
was for methanogenic bacteria with an IC50 of 6.4 mg/litre. For
aquatic invertebrates acute LC50 values range from 28 to > 770
mg/litre. In freshwater fish the lowest acute LC50 value of 13
mg/litre was found in the golden orfe (Leuciscus idus melanotus),
and for marine species an LC50 value of 50 mg/litre was reported for
the dab (Limanda limanda). Carbon tetrachloride appears to be more
toxic to embryo-larval stages of fish and amphibians than to adults.
The common bullfrog (Rana catesbeiara) is the most susceptible
species, the LC50 being 0.92 mg/litre (fertilization to 4 days after
hatching).
The available data indicate that hepatic tumours are induced by a
non- genotoxic mechanism, and it therefore seems acceptable to develop
a tolerable daily intake (TDI) and a tolerable daily concentration in
air (TC) for carbon tetrachloride.
On the basis of the study of Bruckner et al. (1986), in which a
NOAEL of 1 mg/kg body weight was observed in a 12-week oral study on
rats, and incorporating a conversion factor of 5/7 for daily dosing
and applying an uncertainty factor of 500 (100 for inter- and
intraspecies variation, 10 for duration of the study, and modifying
factor 0.5 because it was a bolus study), a TDI of 1.42 µg/kg body
weight is obtained.
On the basis of a 90-day inhalation study on rats (Prendergast et
al., 1967), in which a NOAEL of 6.1 mg/m3 was reported, a TC of 6.1
µg/m3 was calculated using the factors 7/24 and 5/7 to convert to
continuous exposure and an uncertainty factor of 1000 (100 for inter-
and intraspecies variation and 10 for the duration of the study). This
TC corresponds to a TDI of 0.85 µg/kg body weight.
Comparing the estimated upper limit of prevailing human daily
intake of 0.2 µg/kg body weight with the lowest TDI value (0.85 µg/kg
body weight), the conclusion can be drawn that the currently
prevailing exposure of the general population to carbon tetrachloride
from all sources is unlikely to cause excessive intake of the
chemical.
In general, the risk to aquatic organisms from carbon
tetrachloride is low. However, it may present a risk to embryo-larval
stages at, or near, sites of industrial discharges or spills.
3. CONCLUSIONS
The general population is generally exposed to only low levels of
carbon tetrachloride via air, drinking-water and food (total daily
uptake is estimated to be 0.2 µg/kg body weight; see chapter 2).
Carbon tetrachloride can induce embryotoxic and embryolethal
effects at maternally toxic doses, but it is not teratogenic. The
liver is the target organ for carbon-tetrachloride-induced toxicity.
Carbon tetrachloride can produce hepatic tumours at dose levels that
are higher than those producing toxic effects in the liver. The weight
of evidence indicates that carbon tetrachloride has no genotoxic
properties. On the basis of this fact and the fact that the induced
hepatoxicity appears to be of major importance for its
carcinogenicity, carbon tetrachloride can be considered as a compound
with carcinogenic properties. A tolerable daily intake (TDI) of 0.85
µg/kg body weight and a tolerable daily concentration (TC) in air of
6.1 µg/m3 have been derived.
Because carbon tetrachloride does not remain in water, due to its
high volatility and low solubility, it is likely that it will present
a risk only to the embryo-larval stages of some sensitive amphibians
at times of industrial discharges or spills.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY
RESPONSE
4.1 Human health hazards, prevention and protection, first aid
The human health hazards associated with exposure to carbon
tetrachloride, together with preventive and protective measures, and
first-aid recommendations, are listed in the Summary of Chemical
Safety Information in section 6.
4.2 Advice to physicians
If carbon tetrachloride has been ingested, vomiting should not be
induced. The patient should drink water to delay absorption, but not
oil or milk. If the patient has been exposed to carbon tetrachloride
vapour he should immediately be moved to fresh air (or given
artificial respiration) and kept under observation. Special attention
must be paid to the use of alcoholic beverages in combination with
exposure to carbon tetrachloride, because the toxic effects are
enhanced by ingestion of alcohol.
4.3 Health surveillance advice
Workers frequently exposed to carbon tetrachloride should be
examined periodically and appropriate measures should be taken.
Replacement and periodic examinations should include appropriate tests
for liver and kidney functions, and special attention should be given
to any history of alcoholism. In all cases of accidental exposure a
medical practitioner should be immediately consulted.
4.4 Explosion and fire hazards, prevention
4.4.1 Explosion and fire hazards
Carbon tetrachloride vapour is invisible, heavier than air and
spreads along the ground. Carbon tetrachloride is non-flammable, but
it can generate phosgene and similar toxic gases when heated to high
temperatures or when involved in a fire. Carbon tetrachloride reacts
explosively when mixed with unsaturated compounds in the presence of
peroxides or light.
Carbon tetrachloride reacts vigorously or explosively with
chemically active metals (lithium, potassium, barium, aluminium,
magnesium, zinc and uranium) or fluorine. Violent reactions occur
between carbon tetrachloride and N,N-dimethylacetamide or
N,N-dimethylformamide in the presence of iron. Explosions have been
reported with carbon tetrachloride and aluminium alkyls, boranes and
carbaboranes, calcium disilicide, calcium hypochlorite, chlorine
trifluoride, allyl alcohol, ethylene, liquid oxygen, nitrogen dioxide
and silanes.
4.4.2 Prevention
If large closed containers with carbon tetrachloride are exposed
to heat or fire, they must be kept cool by spraying with water.
Work with carbon tetrachloride should be carried out with
adequate ventilation. Breathing the vapour and skin contact should be
avoided. Chemical protective clothing, masks and gloves made from
materials that provide a high degree of permeation resistance and eye
protection should be used. Note that rubber is not a suitable
protective material since carbon tetrachloride migrates through it.
4.5 Storage
Carbon tetrachloride should be stored in labelled, airtight
containers in a well-ventilated place protected from light and at a
temperature below 30°C. It must be stored separated from chemically
active metals.
4.6 Transport
In case of accident, stop the engine. Notify police and fire
brigade immediately, keep public away from danger area, mark roads and
warn other road users. Do not smoke, do not use naked lights and keep
upwind.
In case of spillage or fire, the advice given in sections 4.8 and
4.4, respectively, should be followed.
In case of poisoning, the advice in the Summary of Chemical
Safety Information should be followed.
4.7 Disposal
Small quantities of carbon tetrachloride may be disposed of by
evaporation in a fume cupboard or in a safe, open area. Incineration
is not recommended due to the non-flammability of carbon tetrachloride
and to the formation of phosgene, hydrogen chloride and other toxic
gases on heating.
4.8 Spillage
In case of spillage of carbon tetrachloride, ensure personel
protection (protective clothing, safety goggles, rubber gloves and
respiratory protective device) and carefully shut off leaks. Adsorb
the spilt carbon tetrachloride in earth, sand or inert absorbent and
remove to a safe place. Prevent liquid from entering sewers, basements
and workpits because the vapour may create a toxic atmosphere.
If carbon tetrachloride has entered a water course or sewer or if
it has contaminated soil or vegetation, the police should be warned.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
In view of the toxicity of carbon tetrachloride for embryo-larval
stages of some aquatic organisms, it may present a hazard to these
organisms at, or near, sites of industrial discharges or spills.
Contamination of the environment can be minimized by proper
methods of storage, handling, transport and protection.
In case of spillage, the methods recommended in section 4.8
should be used.
6. SUMMARY OF CHEMICAL SAFETY INFORMATION
This summary should be easily available to all health workers
concerned with, and users of, carbon tetrachloride. It should be
displayed at, or near, entrances to areas where there is potential
exposure to carbon tetrachloride, and on processing equipment and
containers. The summary should be translated into the appropriate
language(s). All persons potentially exposed to the chemical should
also have the instructions in the summary clearly explained.
SUMMARY OF CHEMICAL SAFETY INFORMATION
Carbon tetrachloride
CCl4; CAS Registry No. 56-23-5
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Melting point (°C) -22.92 Carbon tetrachloride is a clear, volatile,
Boiling point (°C) 76.72 colourless, heavy liquid, with a characteristic
Relative molecular mass 153.8 sweet, non-irritant odour. Although non-flammable,
Density (20 °C) 1.59 g/ml it decomposes in fire or in heat, giving off
Ignition temperature (°C) >1000 toxic fumes (phosgene and hydrochloric acid).
Water solubility (25 °C) 785 mg/litre Owing to its low conductivity, vapour
Vapour pressure (0 °C) 4.4 kPa electrostatic charges may be generated through
Vapour pressure (20 °C) 12.2 kPa flow, movement etc. It reacts violently with
Vapour density (101.3 kPa; 0 °C) 5.3 kg/m3 chemically active metals (such as aluminium,
n-octanol-water partition coefficient (log Pow) 2.64 magnesium, sodium, lithium, potassium, iron, zinc).
Henry's law constant (24.8°C) 365 kJ/mole It reacts explosively when mixed with unsaturated
Flash point (°C) none compounds in the presence of peroxides or light.
Explosive limits none
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
SKIN: redness, pain, blisters; may be Protective gloves and clothing Remove contaminated clothing and wash skin
absorbed with plenty of water; obtain medical attention
EYES: redness, pain Safety goggles or face shield in combination Wash the eyes with plenty of water or neutral
with breathing protection (organic filter) saline solution for several minutes (remove
contact lenses if possible) or blow out with
an air stream; obtain medical attention
INHALATION: dizziness, drowsiness, Apply ventilation, use in an exhaust hood or Remove victim to fresh air, apply artificial
headache, nausea, unconsciousness use personal breathing protection (organic respiration if indicated; obtain medical
filter) attention or move if necessary to hospital
SUMMARY OF CHEMICAL SAFETY INFORMATION (continued)
INGESTION: abdominal pain, diarrhoea, Do not eat, drink, chew or smoke during Rinse mouth; do not induce vomiting, let victim
dizziness, drowsiness, nausea, work; do not keep food in areas with drink water and refer for medical attention
vomiting, unconsciousness potential exposure; keep out of reach of
children
ENVIRONMENT: may present a hazard to Minimize contamination of water, soil and
embryo-larval stages of some aquatic atmosphere by proper methods of storage,
organisms at discharges or spills handling, transport and waste disposal
SPILLAGE STORAGE FIRE AND EXPLOSION
Ensure personal protection; shut off Store separately from chemically active CCl4 is not combustible, but gives off
leaks if without risk; collect metals in a cool place; do not store in irritating toxic fumes in a fire. All types
leaking liquid in closed containers; aluminium containers; ventilate along of extinguishing agents can be used when
absorb spilt carbon tetrachloride in the floor there is a fire in the direct environment;
earth, sand or inert absorbent and in case of fire, keep containers cool by
remove to a safe place; prevent entry spraying with water
into a sewer
WASTE DISPOSAL NATIONAL INFORMATION
Incineration is not recommended due to National occupational exposure limit:
non-flammability and formation of
phosgene, hydrogen chloride and other National Poison Control Centre:
toxic gases on heating
7. CURRENT REGULATIONS, GUIDELINES AND STANDARDS
The information in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file. Its intention is to give the reader an overview of current
regulations, guidelines and standards.
Regulatory decisions about chemicals, taken in a certain country,
can only be fully understood within the framework of the legislation
of that country. Furthermore, the regulations and guidelines of all
countries are subject to change and should always be verified with the
appropriate regulatory authorities before application.
7.1 Exposure limit values
Some exposure limit values are given in the table. When no
effective date appears in the IRPTC legal file, the year of the
reference from which the data are taken is indicated by (r).
7.2 Specific restrictions/requirements
7.2.1 USA
In the USA carbon tetrachloride and any mixture containing it is
banned as a hazardous product because it posesses such a degree of
hazard that adequate cautionary labelling cannot be written and public
health can only be served by keeping it out of interstate commerce.
This does not apply to unavoidable manufacturing residues in other
chemicals if any reasonable use does not result in atmospheric
concentrations of more than 63 mg/m3 (reference date, 1982).
7.2.2 Canada
In Canada it is illegal to sell, advertise or import products
that consist of or contain carbon tetrachloride where it is packaged
as a consumer product (effective date, 1971).
7.2.3 EEC
a) In case of exposure to carbon tetrachloride, Member States shall,
in addition to other general measures, ensure: 1) medical
surveillance of workers prior to and/or during exposure; 2)
access for workers to their individual and anonymus collective
results. The general measures are in order to keep exposure as
low as reasonably practicable (effective date, 1983).
b) Carbon tetrachloride must not form part of the composition of
cosmetic products. The marketing of cosmetic products containing
the substance is prohibited (effective date, 1988).
CURRENT REGULATIONS, GUIDELINES AND STANDARDS
Exposure limit values
Medium Specification Country/organization Exposure limit description Value Effective date
AIR Occupational Australia Threshold limit value (TLV) 1983 (r)
- Time-weighted average (TWA) 30 mg/m3
- Short-term exposure limit (STEL) 125 mg/m3
Belgium Threshold limit value (TLV) 1984 (r)
- Time-weighted average (TWA) 30 mg/m3
Canada Threshold limit value (TLV) 1980
- Time-weighted average (TWA) 30 mg/m3
Finland Maximum permissible concentration 1982 (r)
- Time-weighted average (TWA) 33 mg/m3
- Short-term exposure limit (STEL) 66 mg/m3
(15 min)
Germany Maximum acceptable concentration 1987 (r)
- Short-term exposure limit (STEL) 20 mg/m3
Hungary Maximum limit 10 mg/m3 1988
Italy Threshold limit value (TLV) 65 mg/m3 1978 (r)
Japan - Threshold limit value (TLV) 31 mg/m3 1991
(Skin adsorption)
The Netherlands Maximum limit (MXL) 1986 (r)
- Time-weighted average (TWA) 12.6 mg/m3
Poland Maximum permissible concentration 1982 (r)
- Time-weighted average (TWA) 20 mg/m3
- Short-term exposure limit (30 min TWA) 100 mg/m3
CURRENT REGULATIONS, GUIDELINES AND STANDARDS (continued)
Medium Specification Country/organization Exposure limit description Value Effective date
Romania Maximum permissible concentration 1975 (r)
- Time-weighted average (TWA) 50 mg/m3
- Ceiling limit value (CLV) 100 mg/m3
Russia Time-weighted average (TWA) 20 mg/m3 1988
Sweden Hygienic limit value (HLV) 1988
- Time-weighted average (TWA) 13 mg/m3
- Short-term exposure limit (STEL) 19 mg/m3
(15-min TWA)
Switzerland Maximum acceptable concentration 1987 (r)
- Time-weighted average (TWA) 30 mg/m3
United Kingdom Time-weighted average (TWA) 65 mg/m3 1987 (r)
- Short-term exposure limit (STEL) 130 mg/m3
(10-min TWA)
USA/ACGIH Threshold limit value (TLV) 1987 (r)
- Time-weighted average (TWA) 31 mg/m3
- Short-term exposure limit (STEL) 63 mg/m3
USA Permissible exposure limit (PEL) 1974
- Time-weighted average (TWA) 63 mg/m3
- Ceiling limit value (CLV) 160 mg/m3
c) Carbon tetrachloride may not be used in ornamental objects
intended to produce light or colour effects by means of different
phases, for example in ornamental lamps and ashtrays (effective
date, 1987)
7.3 Labelling, packaging and transport
7.3.1 USA
In the USA it is permitted to use carbon tetrachloride as a
component of adhesives in articles intended for use in packaging,
transporting or holding food (reference date, 1981).
7.3.2 EEC
Carbon tetrachloride is considered to be a harmful substance.
Member States shall ensure that dangerous preparations (solvents) are
not placed on the market unless their packages and fastenings and
labels comply with the EEC requirements (effective date, 1984).
7.3.3 United Kingdom
Labelling of road tankers: toxic substance (emergency action
code, 2Z) (effective date, 1979).
BIBLIOGRAPHY
CEC/IPCS (1994) International Chemical Safety Card 0024: Carbon
tetrachloride. Luxembourg, Commission of the European Communities.
Dutch Chemical Industry Association (1994) Chemical safety sheets.
Samson Chemical Publishers.
IARC (1979) Some halogenated hydrocarbons. Lyon, International Agency
for Research on Cancer. (IARC Monographs on the evaluation of
carcinogenic risk of chemicals to humans, volume 20).
IRPTC Data Profile (legal file) on carbon terachloride. In: Database
of ECDIN (via DIMDI), field 'Standards and Regulations'. Geneva,
International Registry of Potentially Toxic Chemicals.
Walsh D (1989) Chemical Safety Data Sheets, Volume 1. Royal Society of
Chemistry, England.
WHO (in press) Environmental Health Criteria 208: Carbon
tetrachloride. Geneva, World Health Organization.
 Common name: carbon tetrachloride
Common synonyms: Carbona, carbon chloride,
tetrachloromethane, carbon tet, methane
tetrachloride, perchloromethane,
tetrachlorocarbon
Trade names: Benzinoform, Fasciolin, Freon 10, Halon
104, Tetraform, Tetrafinol
CAS chemical name: tetrachloromethane
CAS registry number:
Common name: carbon tetrachloride
Common synonyms: Carbona, carbon chloride,
tetrachloromethane, carbon tet, methane
tetrachloride, perchloromethane,
tetrachlorocarbon
Trade names: Benzinoform, Fasciolin, Freon 10, Halon
104, Tetraform, Tetrafinol
CAS chemical name: tetrachloromethane
CAS registry number: 