
ANNEX 5
A PROCEDURE FOR THE SAFETY EVALUATION OF FLAVOURING SUBSTANCES
I.C. Munro, Ph.D., FRCPath
CanTox, Inc.
Mississauga, Ontario
Canada
This paper was considered at the forty-fourth meeting of the
Joint FAO/WHO Expert Committee on Food Additives. The conclusions of
the Committee relating to its consideration of the paper are provided
in the report, which has been published in the WHO Technical Report
Series. This paper was not endorsed by the Committee, and the opinions
expressed therein are those of the author. Comments on the approach
outlined in the paper are invited, which should be addressed to the
WHO Joint Secretary of JECFA, International Programme on Chemical
Safety, 20, Avenue Appia, World Health Organization CH-1211 Geneva 27,
Switzerland.
ANNEX 5
A PROCEDURE FOR THE SAFETY EVALUATION OF FLAVOURING SUBSTANCES
Table of Contents
1. INTRODUCTION
2. CONCEPTS EMPLOYED IN THE SAFETY EVALUATION OF FLAVOURING
SUBSTANCES
2.1 Exposure to flavouring substances through food
2.2 Structure-activity relationships
2.3 Use of toxicity data
3. INTEGRATED METHOD FOR THE SAFETY EVALUATION OF FLAVOURING
SUBSTANCES
3.1 Structure-activity relationships and metabolic fate
3.2 Integrating information on exposure and toxicity
3.3 Evaluation criteria
3.4 Integrating data on the consumption ratio
4. CONCLUSIONS
5. REFERENCES
APPENDICES
Appendix A Estimating distribution of intakes of food ingredients
across the population
Appendix B Substances in reference database by order of structural
class
1. INTRODUCTION
The objective of this paper is to provide a procedure that can be
used by the Joint FAO/WHO Expert Committee on Food Additives (JECFA)
for the safety evaluation of flavouring substances. The procedure
extends principles and procedures recommended by IECFA in the past and
is consistent with international concepts in safety evaluation. These
principles have been discussed by JECFA (JECFA, 1972; 1974b; 1976a;
1978; 1980a,b; 1982a; 1983a; 1984a; 1986; 1987a,b; 1989; 1990a; 1991a;
1992) and have been recapitulated in a report, which outlines criteria
for the safety evaluation of flavouring substances (WHO, 1987).
This paper takes this previous background into account and
provides a scientific basis for assessing the safety of flavouring
substances used in food by presenting a procedure for evaluating this
large group of compounds in a consistent, timely, and appropriate
manner.
2. CONCEPTS EMPLOYED IN THE SAFETY EVALUATION OF FLAVOURING
SUBSTANCES
Criteria for the safety evaluation of flavouring substances have
been put forward by several national and international authorities.
Included among these are JECFA, a special Task Group convened by the
World Health Organization (WHO, 1987), the Committee of Experts on
Flavoring Substances of the Council of Europe (CE), the Commission of
the European Communities' Scientific Committee for Food (SCF, 1991),
the British Industrial Biological Research Association (BIBRA), and
the Flavor and Extract Manufacturers' Association of the United States
Expert Panel (FEXPAN).
In 1987, WHO published a health criteria document entitled
Principles of the Safety Evaluation of Food Additives and
Contaminants in Food, which contains a discussion of principles
related to the safety evaluation of flavouring substances. This report
recapitulated principles previously stated by JECFA on numerous
occasions (JECFA, 1968; 1972; 1974b; 1976a; 1978; 1980a,b; 1982a;
1983a; 1984a; 1986; 1987a,b; 1989; 1990a; 1991a; 1992). Early on, it
was recognized by JECFA that the safety evaluation of flavouring
substances warranted special consideration in light of use patterns
and typically low levels of human exposure (JECFA, 1972). This view
also has been recognized by the (SCF) and has been recorded in their
document entitled Guidelines for the Evaluation of Flavorings for Use
in Foodstuffs. 1. Chemically, Defined Flavoring Substances
(SCF, 1991).
In addition, several organizations including JECFA (WHO, 1987),
FEXPAN (Woods & Doull, 1991), SCF (1991), and the Council of Europe
(CE, 1974, 1981, 1992) have noted that knowledge of structure-activity
relationships and metabolism plays a key role along with exposure in
the safety evaluation of flavouring substances. In this regard, JECFA
(WHO, 1987) has used structure-activity relationships in evaluating
groups of structurally related flavouring substances in a homologous
series where toxicology studies exist on only one or a few members of
the series. Structure-activity relationships can provide a useful
means of assessing substances that lack toxicity data based on
structurally related substances that have adequate toxicity data.
2.1 Exposure to flavouring substances through food
Flavouring substances are used in processed foods and beverages
to impart desirable organoleptic qualities and to provide the specific
flavour profile traditionally associated with certain food products.
Unlike many substances which are added to food to achieve a
technological purpose, the use of flavouring substances is generally
self-limiting and governed by the flavour intensity required to
provide the necessary organoleptic appeal. Thus, flavouring substances
are used generally in low concentrations resulting in human exposures
that are very low.
Estimates of the intake of flavouring substances by the
population typically have involved the acquisition of data on poundage
used in foods. Between 1970 and 1987, the U.S. National Academy of
Sciences/National Research Council (NAS/NRC) conducted, under contract
with the Food and Drug Administration (FDA), a series of poundage
surveys of substances intentionally added to food (NAS, 1978, 1979,
1984, 1989). These surveys obtained information, both from ingredient
manufacturers and from food processors, on the poundage of each
substance committed to the food supply and on the usual and maximum
levels at which each substance was added to foods in each of a number
of broad food categories.
Estimates of flavouring substance intake in the U.S. have been
performed in two ways. One is to assume that the total poundage
reported in food annually is completely consumed by the total
population. Numerous checks using data from independent sources, such
as imports, show that, in general, the reported poundage in the survey
accounted for only 60% of the total used. Much more detailed and
elaborate analyses (see Appendix A) led to the conclusion that it was
conservative but reasonable to assume that each flavouring substance
is consumed by only 10% of the population. A second method of
calculating intake is to combine data on the level of use in specific
food groups with data on food consumption to calculate the intake of
each flavouring substance. Both methods tend to overestimate human
intakes of flavouring substances because they deal with disappearance,
that is the amount presumed to be used in food, and take no account of
losses and waste during food manufacture, storage, preparation and
consumption.
Estimating intake of flavouring substances based on level of use
data, coupled with data on the consumption of foods, typically leads
to substantial overestimates of intake. This is because intake data
for food commodities are stratified into broad categories of food
products (e.g., baked products). Thus, cardamom, ordinarily used only
in certain types of coffee cakes, would be assumed to be present in
all breads, rolls, cakes and pastries In extreme cases, this may lead
to overestimates of intake which are exaggerated several hundred-fold.
Through a series of detailed studies conducted between 1970 and
1987 (see Appendix A) it has become clear that, while there is at
present no perfect way to estimate intake of flavouring substances,
poundage used provides a reasonable basis for calculating intakes. The
intake data reported in this paper rely on annual poundage used in
food and the estimates of intake reflect the assumptions that: i) the
available survey data accounted for only 60% of production; and ii)
the total amount produced is consumed by 10% of the population. Table
1 presents the intake data for 1323 chemically defined flavouring
substances permitted for use in the U.S. calculated in this fashion.
As can be seen from Table 1, most flavouring substances are consumed
in amounts of less than 1 mg/person/day. The data are taken from the
most recent U.S. NAS/NRC survey (NAS, 1989) of poundage used in food.
For reasons previously stated, it can be assumed that these intake
estimates are overestimated.
Another important factor to consider in the evaluation of human
exposure to flavouring substances is the extent to which flavouring
substances intentionally added to foods also occur naturally in the
food supply. The natural presence of flavouring substances in food is
of course not necessarily indicative of safety. For many flavouring
substances that occur naturally in foods, such natural occurrence,
rather than intentionally added use, is the principal component of
human exposure. The comparison of natural occurrence to intentional
addition has been expressed as the consumption ratio (CR) (Stofberg &
Kirschman, 1985). A CR of greater than one indicates food predominance
(i.e. the flavouring substance is consumed at a higher level from
foods than as an added substance). A CR greater than 10 indicates an
almost insignificant contribution (-10%) of the flavouring substance
as a food additive to the total intake (Stofberg & Grundschober,
1987). Stofberg and Grundschober (1987) calculated that out of 499
flavouring substances, 415 (83%) were food predominant (i.e. CR > 1)
and 309 (62%) made an insignificant contribution to the food supply
(i.e. CR > 10). As can be seen from this analysis, the use of natural
occurrence as part of the safety evaluation provides an important
perspective on the impact of intentional addition of flavouring
substances to foods.
If a flavouring substance is one of the few that for any reason,
including high intake, is of relatively high safety concern, then a
high consumption ratio likely enhances that concern because it
indicates a much larger and uncontrolled exposure from natural
occurrence than from intentional addition. If, on the other hand, a
flavouring substance is one of the vast majority that have few, if
any, safety issues and consequently low inherent concern, then a high
consumption ratio reduces any concern still further because it
indicates that intentionally added use is trivial. Stated in another
way, if the added use of a flavouring substance amounts to less than
10% of its natural occurrence, this would indicate a minimal safety
concern about added use. If added use is < 1% of natural use (i.e. CR
> 100) then the added use can at most be of trivial safety concern.
2.2 Structure-activity relationships
Toxicity is dependent on the chemical structure of a substance,
its pharmacokinetics, and its metabolic reaction pathways. Available
metabolic pathways are usually dose-dependent and, to a large extent,
govern the magnitude of the toxic effect. Therefore, chemical
structure, pharmacokinetics, metabolic fate and dose are key
determinants of toxicity and play a critical role in safety evaluation
of flavouring ingredients.
Table 1. Number of flavouring substancesa within various intake
categories
Intake categoryb Cumulative
(µg/day) No. of flavours frequency
(% of total)
< 0.01 349 26
0.01-0.1 93 33
0.1-1 274 54
1-10 224 71
10-100 204 86
100-1000 111 95
1000-10 000 45 98
10 000-100 000 16 99
100 000+ 7 100
TOTAL 1323
a Chemically defined flavouring substances permitted for use in the
U.S. excluding botanicals.
b Intake data calculated assuming: survey poundage reflects 60% of
actual usage, 10% of population exposed, U.S. population in 1987
was 240 million. Formula: Intake (µg/person/day) = [(annual
flavour usage in µg)‰0.6]‰(24×106 persons × 365 days). Poundage
data from 1987 NAS/NRC survey data.
Refinements to the initial concepts of structure-activity came as
a result of increasing knowledge and confidence in predicting
structure-activity relationships for flavouring substances. This
formed the basis of a paper by Cramer et al. (1978) which, through
the use of a "decision tree" approach, permitted the classification of
flavouring substances into "classes of concern" based on structure and
other considerations, similar in many respects to, but predating
the "Concern Level" concept outlined by the U.S. Food and Drug
Administration in its "Redbook" (FDA, 1982, 1993).
The concept of establishing concern levels also has been
investigated further by BIBRA to evaluate its applicability to food
chemicals more generally (Phillips et al., 1987). This group
evaluated and established concern levels for several food additives,
plastic monomers, as well as flavouring substances. Although they
reported that, in their opinion, the Cramer et al. (1978) decision
tree misclassified a few substances, the decision tree was likely to
be a more realistic approach for predicting toxicity than any other
reported quantitative structure-activity relationship (QSAR)
technique. Thus, there is general consensus, based on the work of
Cramer et al. (1978), the subsequent work by BIBRA (Phillips
et al., 1987), and the fact that the FDA (1982; 1993) uses
structure-activity relationships in defining Concern Levels for food
substances, that structure-activity has a solid basis in science when
applied to substances of simple and closely related structures
encountered at low exposures, a number of which are of known low
toxicity and safe metabolic disposition. This is particularly the case
for all but a very few flavouring substances. As will be discussed
later in this paper, structure-activity relationships play an
important role in the evaluation of flavouring substances.
2.3 Use of toxicity data
Traditional safety evaluation procedures are only partially
applicable to flavouring substances. Traditional approaches to the
safety assessment of food additives typically involve the evaluation
of considerable toxicological data, usually in an amount sufficient
to establish a no-observed-effect-level (NOEL), permitting the
establishment of an acceptable daily intake (ADI). Approximately half
of the flavouring substances currently in use are naturally occurring
simple acids, aldehydes, alcohols and esters. With few exceptions,
these are rapidly metabolized to innocuous end products, the safety of
which is well established or can be assumed from metabolic and
toxicity data on the substance in question or on structurally related
substances. Moreover, the acquisition of extensive toxicity data is
unnecessary for the majority of flavouring substances because
structure-activity relationships can be used as a means of assessing
substances in a homologous series, in which only a few substances have
toxicology data, to determine safety in use. This concept has been
used by JECFA in the evaluation of structurally related flavouring
substances, including the allyl esters, amyl acetate and isoamyl
butyrate, benzyl compounds, citral compounds, alpha- and ß-ionones,
and nonanal and octanal (JECFA, 1967: 1968; 1980a; 1984a,b; 1990a,b;
1991a,b; 1993a,b). In addition, as previously indicated in Table 1,
95% of flavouring substances are consumed at exposure levels less than
1 mg/person/day and in keeping with the safety evaluation procedure
outlined in this paper, only limited toxicological data are required
in such circumstances.
When exposure is extremely low and there are organoleptic
limitations on use levels, a primary consideration is whether there is
a need to establish a numerical ADI for flavouring substances. There
are several reasons why it is not appropriate or necessary to
establish ADIs in the case of the majority of flavouring substances.
ADIs are based on toxicological data and the establishment of a NOEL,
an approach that differs from the concept that a safety evaluation
can be performed in many instances on the basis of exposure and
structure-activity relationships. Moreover, the organoleptic and
gustatory properties of flavouring substances typically limit their
use and, consequently, exposure to them. In addition, because a
majority of flavouring substances occur in nature, there is a long
history of human experience in flavouring substance consumption from
traditional foods. Nearly fifty percent of flavouring substances given
full ADIs by IECFA have consumption ratios greater than 1, indicating
their predominant natural occurrence in food (Stofberg & Grundschober,
1987). The above factors have been noted by WHO (1987) as important in
the evaluation of flavouring substances. It is also evident that very
large safety margins exist for flavouring substances, as evidenced by
the fact that the margin between the NOEL and the per capita intake of
flavouring substances ranges from more than 50 to more than 1 000 000
times for the flavouring substances given full ADIs by JECFA (Table 2).
Table 2. Safety margins between NOELs and per capita daily
exposure for various flavouring substances given full ADIs
by JECFAa
Safety Margin Number of Flavours
<100 1
100 - 1000 4
1000 - 10 000 13
10 000 - 100 000 9
100 000 + 7
TOTAL 34
a JECFA, 1967; 1968; 1970; 1971; 1972; 1974a,b; 1976a.b; 1978;
1980a,b,c; 1981a,b; 1982a,b; 1983a,b; 1984a,b; 1986; 1987a,b;
1989; 1990a,b; 1991a,b; 1992; 1993a.b,c.
The factors discussed above lend support to the argument that it
is unnecessary to establish ADIs for the majority of flavouring
substances. Not establishing a numerical ADI for the great majority of
flavouring substances is also consistent with the concept that
presumed or known metabolism can be used as a basis for safety
evaluation.
3. INTEGRATED METHOD FOR THE SAFETY EVALUATION OF FLAVOURING
SUBSTANCES
In a continuing effort to improve the basis for the safety
evaluation of flavouring substances, this paper presents a procedure
which integrates information on exposure, structure-activity
relationships, metabolic fate and toxicity. It presents a safety
evaluation procedure which allows a determination of the safety of
flavouring substances under conditions of intended use. The key
elements of the safety evaluation procedure are discussed below.
3.1 Structure-activity relationships and metabolic fate
Without exception, flavouring substances are volatile organic
chemicals. The vast majority of flavourings ingredients have simple,
well characterized structures with a single functional group and low
molecular weight (< 300). More than 700 of the 1323 chemically
defined flavouring substances used in food in the U.S. are simple
aliphatic acyclic and alicyclic alcohols, aldehydes, ketones,
carboxylic acids, and related esters, lactones, ketals, and acetals.
Other structural categories include aromatic (e.g., cinnamaldehydes
and amhranilates), heteroaromatic (e.g., pyrazines and pyrroles) and
heterocyclic (e.g., furanones and thiofurans) substances with
characteristic organoleptic properties (e.g., furanones providing a
strawberry note). For most flavouring substances, the structural
differences are small. Incremental changes in carbon chain length and
the position of a functional group or hydrocarbon chain typically
describe the structural variation in groups of related flavouring
substances. These systematic changes in structure provide the basis
for understanding the effect of structure on the chemical and
biological properties of a substance.
Toxicity is dependent on the chemical structure and metabolism of
a substance. The "decision tree" procedure (Cramer et al., 1978)
relies primarily on chemical structure and estimates of total human
intake to assess toxic hazard and to establish priorities for
appropriate testing. The procedure utilizes recognized pathways of
metabolic deactivation and activation, data on toxicity, and the
presence of the substance as a component of traditional foods and as
an endogenous metabolite. Substances are classified according to three
categories:
Class I - Substances of simple chemical structure and efficient
modes of metabolism which would suggest a low order
of oral toxicity (e.g., butyl alcohol or isoamyl
butyrate).
Class II - Contains structures that are intermediate. They
possess structures that are less innocuous than
substances in Class I, but do not contain structural
features suggestive of toxicity like those substances
in Class III. Members of Class II may contain
reactive functional groups (e.g., furfuryl alcohol,
methyl 2-octynoate, and allyl propionate).
Class III - Substances of a chemical structure that permit no
strong initial presumption of safety, or may even
suggest significant toxicity (e.g., 2-phenyl-
3-carbethoxy furan and benzoin).
The decision tree is a tool for classifying flavour ingredients
according to levels of concern. The majority of flavouring substances
fall into Class I because they are simple alcohols, aldehydes,
ketones, acids or their corresponding esters, acetals and ketals that
occur naturally in food and, in many cases, are endogenous substances.
They are rapidly metabolized to innocuous products (e.g., carbon
dioxide, hippuric acid, and acetic acid) by well recognized reactions
catalyzed by cellular enzymes that exhibit high specificity and high
catalytic efficiency (e.g., alcohol dehydrogenase and isovaleryl
coenzyme A dehydrogenase). Substances that do not undergo detoxication
via these highly efficient pathways (e.g., fatty acid pathway and
citric acid cycle) are metabolized by reactions catalyzed by enzymes
of low specificity and relatively low efficiency (e.g., cytochrome
P-450 and glutathione transferase). For some groups of substances
(e.g., branched-chain carboxylic acids, allyl esters, and linear
aliphatic acyclic ketones), metabolic thresholds for intoxication have
been identified (Krasavage et al., 1980; Deisinger et al., 1994;
Jaeschke et al., 1987). The dose range, over which a well-defined
change in metabolic pathway occurs, generally correlates with the dose
range over which a transition occurs from a no-observed-adverse-effect
level to an adverse-effect level. For such groups of substances the
dose range at which this transition occurs is orders of magnitude
greater than the level of exposure from use as flavour ingredients.
Most substances in Class II belong to either of two categories;
one includes substances with functional groups which are similar to,
but somewhat more reactive than functional groups in Class I
(e.g., allyl and alkyne); the other includes substances with more
complex structures than substances in Class I, but that are common
components of food. This category includes heterocyclic substances
(e.g., 4-methylthiazole) and terpene ketones (e.g., carvone).
The majority of the flavouring substances within Class III
include heterocyclic and heteroaromatic substances and cyclic ethers.
Many of the heterocyclic and heteroaromatic substances have sidechains
with reactive functional groups. In a few cases, metabolism may
destroy the heteroaromaticity of the ring system (e.g., furan).
Although metabolism studies have been performed for Class III
flavouring substances with elevated levels of exposure, the metabolic
fate of many substances in this structural class cannot be confidently
predicted. Review of the group of substances in each of the structural
classes indicates that as structural complexity increases (Class I -
III), the number of flavouring substances and the levels of exposure
decrease significantly (Table 3). In all structural classes,
one-quarter or more of the flavouring substances are consumed at
levels below 0.01 µg/day or 0.2 µg/kg bw/day.
Table 3. Number of flavouring substancesa divided by structural class
within various intake categories
Intake Categoryb No. of Flavours (% of Total)
(µg/day)
Class I Class II Class III
<0.01 212 (24) 68 (28) 69 (34)
0.01-0.1 55 (6) 20 (8) 18 (9)
0.1-1 169 (19) 48 (20) 57 (28)
1-10 145 (17) 45 (19) 34 (17)
10-100 147 (17) 39 (16) 18 (9)
100-1000 95 (11) 12 (5) 4 (2)
1000-10 000 34 (4) 9 (4) 2 (1)
10 000-100 000 16 (2) 0 0
100 000+ 5 (0.6) 2 (0.8) 0
TOTAL 878 243 202
a Chemically defined flavouring substances permitted for use in the
U.S. excluding botanicals.
b Intake data calculated assuming: survey poundage reflects 60% of
actual usage, 10% of population exposed, U.S. population in 1987
was 240 million. Formula: Intake (µg/person/day) = [(annual
flavour usage in µg)‰0.6]‰(24×106persons × 365 days). Poundage
data from 1987 NAS/NRC survey data.
3.2 Integrating information on exposure and toxicity
One of the key elements of the safety evaluation procedure is
based on the premise that intake levels can be specified for
flavouring substances that would not present a safety concern. This
paper presents a procedure which provides the scientific underpinnings
for defining toxicologically inconsequential exposures for flavouring
substances and expressing these as human exposure thresholds. The
concept of specifying human exposure thresholds relies on principles
that permit specifying the daily intake of a substance which can be
considered, for practical purposes, as presenting no toxicological
risks (and thus of no health or safety risk to consumers) even in the
absence of specific toxicological data on the substance (Federal
Register, 1993; Munro, 1990; Rulis, 1986; Frawley, 1967). The
concept relies on knowledge of the range of toxicological risks for
structurally related substances and on knowledge regarding the
toxicological potency of relevant classes of chemicals for which good
toxicity data exist. The principles underpinning the establishment of
human exposure thresholds have been embodied in a recent United States
government Federal Register (1993)1 notice emanating from the FDA,
which provides the scientific basis for the conclusion that an
exposure level to indirect food additives can be specified, below
which no risk to public health would likely accrue. This exposure
level has, in turn, been used by FDA to establish a proposed
"threshold of regulation" for indirect food additives which precludes
the need for toxicological evaluation of substances migrating into
food from food-contact articles provided the amount that migrates does
not lead to a dietary level in excess of 500 ppt (equivalent to
1.5 µg/person/day assuming a daily food intake of 3000 g). The FDA has
noted that such a level would result in negligible risk to consumers
even if the substance was shown later to be a carcinogen. This concept
is in keeping with the well-established principle that resources
should be directed to the safety evaluation of substances having high
exposure and therefore greater potential for adverse effects and not
toward substances with trivial exposure. The concept is particularly
applicable to substances of low toxicity and with known or predictable
metabolic fate. The scientific basis for the establishment of human
exposure thresholds and the proposed FDA regulation are discussed
below.
Frawley (1967) initially suggested the concept of establishing
human exposure thresholds. He showed, on the basis of studies
conducted on several well-tested substances, including food additives,
industrial and consumer chemicals, and pesticides that a generic
"no-effect" level could be established that could preclude the need
for toxicity studies and safety evaluation for a majority of
substances intended for use as food packaging materials. Frawley
constructed a reference database of non-tumorigenic endpoints using
220 two-year rodent studies. He presented the NOELs for all 220
compounds. Frawley (1967) reported that if he excluded heavy metals
and pesticides from the analysis, there was no compound in the
remaining database (except for acrylamide) which showed evidence of
chronic toxicity at dietary concentrations of less than 100 ppm.
Application of a typical 100-fold safety factor to the 100 ppm
generalized NOEL would mean that humans could safely consume any of
the materials provided the dietary concentration did not exceed 1 ppm.
Frawley (1967), noting that his database was incomplete, proposed
adding an additional safety factor of 10 which would translate to a
toxicologically insignificant human exposure level of 0.1 ppm in the
diet. Assuming an individual consumes 1500 grams of food per day, an
exposure of 150 µg/person/day (approximately 2.5 µg/kg/day) or less to
a chemical of unknown toxicity would be considered toxicologically
insignificant. According to Frawley, such exposures could be
considered of no safety concern.
More recently, Rulis (1986) conducted a similar analysis of the
FDA's Priority-Based Assessment of Food Additives (PAFA) database
containing 159 compounds with subchronic or chronic toxicity data. He
came to the same conclusion as Frawley (1967). Essentially there is no
risk of toxicity in rodents exposed to certain food additives at
dietary levels of less than 1 mg/kg bw/day or in human terms,
approximately 1 to 10 µg/kg bw/day depending on the safety factor
applied. Even twenty years apart, using different databases, the
toxicologically inconsequential levels proposed by Frawley (1967) and
Rulis (1986) were nearly identical.
Munro (1990) used a database of approximately 350 substances
compiled by Gold et al. (1984, 1989) to develop a human exposure
threshold value to be applied to substances for which no presumption
of safety can be made because of a complete lack of data on metabolism
and potential toxicity. Munro (1990) proposed a threshold of
regulation of up to 1000 ppt for indirect additives which would
translate to a daily intake of 1.5 to 3.0 µg/person/day depending upon
assumptions regarding food intake. The acceptable exposure of
1.5 µg/day is equivalent to that considered by FDA (Federal Register,
1993)1 to present no regulatory concern for a food packaging
material even if later it was determined to be a carcinogen.
1 FDA has proposed a dietary concentration of 500 ppt as the
threshold of regulation for substances used in food-contact
articles. Assuming that an individual consumes 1500 g of solid
food and 1500 g of liquid food per day, this threshold would
equate to a toxicologically inconsequential level of 1.5 µg/day
(Federal Register, 1993).
The work conducted by the FDA (Federal Register, 1993), Frawley
(1967), Rulis (1986) and Munro (1990) is expanded upon in this paper
through the compilation of a large database of reference substances
(Appendix B) from which a distribution of NOELs could be derived for
chemicals of various structural types. The reference database
describes the relationships between exposure, structure and toxicity
for a wide variety of chemicals of divergent structure and it can be
used as a reference point from which to judge the safety of flavouring
substances.
In compiling the database, strict criteria were applied in the
selection of data sets. The objective of the exercise was to identify
as many high quality toxicological studies as possible representing a
variety of toxic endpoints and chemical structures. To accomplish
this, the study types included those typically conducted in
toxicology, such as subchronic, chronic, reproductive and teratology
studies. Short term and acute studies were not included since these
were considered not to be relevant for establishing chronic NOELs. The
database consisted mainly of studies in rodents and rabbits. Very few
studies in dogs and other species were found that met the established
criteria. An evaluation of randomly selected dog and primate studies
indicated that many had too few animals per group to derive a
statistically valid NOEL. Moreover, for many dog studies, a common
endpoint was reduced body weight and/or food consumption which was
due, in many cases, either to palatability problems with the diet or
vomiting. In addition, most studies in dogs and other non-rodent
species were simply too short in duration to be classified as chronic
studies. Only oral studies were included in the database. A further
criterion for inclusion in the database was that a study had to have a
demonstrated lowest-observed-effect level (LOEL) as well as a NOEL,
thus ensuring that the study was rigorous enough to detect toxic
effects. In some instances NOELs were included for studies not
demonstrating a LOEL, and these were substances such as major food
ingredients that were without toxicity at the highest dose tested in
well-conducted studies. It should be noted that the inclusion of such
substances in the database would not bias the database in favour of
higher NOELs since the true NOEL for such substances probably would
exceed the NOEL established from the available studies.
In order to group NOELs for substances with only subchronic
studies with those with chronic studies to derive the cumulative
distribution of NOELs, subchronic NOELs were divided by a factor of
three to approximate the most likely NOEL that would be derived from a
chronic study. This conversion factor is based on research defining
the relationship between subchronic and chronic NOELs. Weil and
McCollister (1963) compared 3 month NOELs with 2 year NOELS for 33
different substances (including pharmaceuticals, pesticides, and food
additives) fed to rats. They found that for most of the compounds
(30), the ratio of the NOELs between subchronic and chronic studies
was 5 or less and more than half of the compounds had a ratio equal to
2 or less. More recently, it has been discovered through further
analysis of more chemical substances, that a more accurate adjustment
factor for extrapolating NOELs derived from subchronic studies to
lifetime was between 2 and 3 (Dourson, personal communication;
Lewis et al., 1990; Beck et al., 1993).
In compiling the database, emphasis was placed on retrieving data
from certain databases known to contain well-validated toxicological
endpoints for a series of well-defined chemical structures. An
exhaustive search was made of compounds evaluated by JECFA. Other
sources included the U.S. Environmental Protection Agency's (EPA)
Integrated Risk Information System (IRIS) on-line database, the
National Toxicology Program (NTP) studies, the Developmental and
Reproductive Toxicity (DART) on-line database from EPA and the U.S.
National Institute of Environmental Health Sciences and the published
literature in general. The data entered into the database included the
name of the chemical, Chemical Abstracts Service Registry Numbers (CAS
No.), structural classification as assessed using the Cramer et al.
(1978) decision tree and the FDA "Redbook", species, sex, route of
administration, dose levels tested, study type, duration, endpoints
reported, LOEL, NOEL and references. In an effort to be conservative
in the construction of the reference database, NOELs selected by the
author(s) of each study were used even though in some cases authors
tended to over-interpret their data. In some instances, it was found
that the stated NOEL may have been based on a misjudgment of an
adverse effect by the author (e.g., physiological versus toxicological
effects) or on artifactual effects (e.g., fetal toxicity as a result
of maternal toxicity). An example of this is isopropyl alcohol, which
has been reported to produce teratogenic effects at very low doses
(0.018 mg/kg) in one study; however, its structure, known metabolism
and other toxicological data provide no evidence for concluding
teratogenicity. Even though scientifically, some of these
author-derived NOELs were not thoroughly substantiated, they were
included in the reference database, thereby increasing the degree of
its conservative nature. NOELs selected by EPA for the IRIS database
were entered without further review. In all, the database consists of
612 substances representing a range of industrial chemicals,
pharmaceuticals, food substances and environmental and consumer
chemicals likely to be encountered in commerce. Since the database was
developed as a reference database for the evaluation of flavouring
substances, all of which are organic chemicals, no organometallic or
inorganic compounds were included in the database. For many of the
substances, more than one NOEL was identified from the literature
resulting from the fact that some substances were tested in more than
one species and sex and/or demonstrated a range of endpoints suitable
for establishing a NOEL. This led, in some cases, to multiple NOELs
for individual substances. In all, the database contains 2944 entries.
In order to correlate chemical structure with toxicity, the
substances in the database were classified into three groups
corresponding to the three structural classes outlined in the
Cramer et al. (1978) decision tree. For each substance, the most
conservative NOEL was selected from the reference database based on
the most sensitive species, sex and endpoint. The cumulative
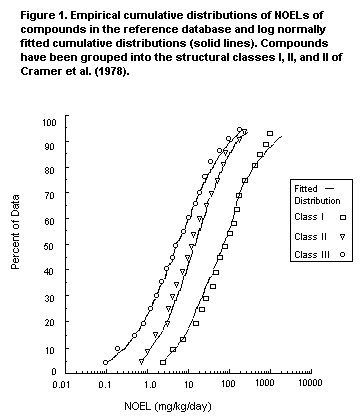
distribution of the NOELs within each class is shown in Figure 1,
along with the lognormal distributions fitted to these data. These
results clearly delineate the effects of structural class on toxicity,
with the median (50th percentile) NOEL decreasing from Class I through
III. Similar differences among structural classes exist in the range
between the 5th and 95th percentile.
The human exposure threshold for each of the structural classes
was calculated from the 5th percentile NOEL. The 5th percentile NOEL
was chosen because this value would provide 95% confidence that any
other substance of unknown toxicity but of the same structural class
as those comprising the reference database would not have a NOEL less
than the 5th percentile for that particular structural class within
the reference database.
The 5th percentile NOELs for each structural class are shown in
Table 4. In converting the 5th percentile NOELs to human exposure
thresholds (Table 4) for the various structural classes, a 100-fold
safety factor was used since such a factor would inherently be applied
in establishing safe intake levels for the substances comprising the
database. The use of such a factor provides a substantive margin of
safety since the human exposure thresholds are based on a large
database of approximately 612 compounds with good supporting
toxicological data. Furthermore, 5th percentile NOELs were used to
calculate the thresholds, providing a more conservative figure than
the arithmetic mean. Moreover, the estimated daily intakes of
flavouring substances to which the human exposure threshold are
compared are greatly overestimated as they represent the "eaters only"
(10%) population. Thus, it is believed that a 100-fold safety factor
provides a wide margin of safety in relating the results of the
analysis of the reference database to flavouring substance exposure.
It is evident from Table 4 that there are substantial differences
in the 5th percentile NOELs for the various structural classes,
indicating an obvious effect of structure on toxic potency.
It is enlightening to compare the human exposure thresholds with
present intakes of chemically defined flavouring substances in the
U.S. As shown in Table 5, it is clear that for nearly all (93 to
97%) flavouring substances used in the U.S., intakes are below the
human exposure threshold for their respective structural class.
Because most flavouring substances possess simple structures and their
metabolism is known or reasonably predictable, it can be concluded
that it is highly improbable that they would present a toxicological
 risk at exposure levels below the human exposure threshold for their
respective structural class. However, even if information on
structural class, metabolic fate and existing toxicity studies were
not available, 743/1323 (56%) of flavouring substances used in the
U.S. are consumed in amounts less than the 1.5 µg/person/day standard
proposed by the FDA (Federal Register, 1993) and Munro (1990). This
indicates that for approximately half of the existing list of 1323
chemically defined flavouring substances permitted for use in the
U.S., exposure can be considered to be trivial.
Table 4. Fifth percentile NOELs and human exposure thresholds for
Cramer et al. (1978) structural classes in the reference
database
5th Percentile NOELs, Human Exposure
(µg/kg bw/day) Threshold,(µg/day)a,b
I 137 2993 1800
II 28 906 540
III 447 147 88
a The human exposure threshold was calculated by multiplying the
5th percentile NOEL by 60 (assuming an individual weighs 60 kg)
and dividing by a safety factor of 100, as discussed in the text.
b Numbers rounded to two (2) significant figures.
Table 5. Flavouring substances within each Cramer et al. (1978)
structural class consumed in amounts below human exposure
thresholds
No. of No. of Flavours
Human Exposure Flavours Under Human
Structural Class Threshold Within Exposure
(µg/day) Structural Threshold (%)
Classa
I 1,800 878 835 (95)
II 540 243 227 (93)
III 88 202 195 (97)
a Adapted from the FEMA flavouring substance database of flavouring
substances permitted for use in the U.S.
3.3 Evaluation criteria
The evaluation criteria proposed for application to flavouring
substances in this paper involve the integration of information on
exposure, structure-activity relationships, metabolism and, as
required, toxicity data. It should be noted that the safety evaluation
criteria outlined below are not intended to be applied to any
flavouring substances with unresolved toxicity problems or to
substances that are presumed or known carcinogens. Such substances
warrant special consideration and must be evaluated using more
traditional methods of safety evaluation. While toxicity data exist on
numerous flavouring substances and can be used as the basis for
evaluation, there are many flavouring substances that lack toxicity
data. The evaluation criteria are intended to provide a means of
evaluating such substances. The criteria incorporate, where the data
permit, the concept that metabolic fate can be predicted on the basis
of presumed structure-activity relationships. The criteria also rely,
in part, on the NOEL reference database which provides a human
exposure threshold for each of the three structural classes of
flavouring substances, as shown in Table 4. The evaluation criteria
also incorporate, where available, toxicity data on flavouring
substances and closely related structural analogues as a basis for
safety evaluation. One of the criteria (number 5 below) incorporates
the concept of a minimum human exposure threshold based on the
1.5 µg/person/ day standard proposed by the FDA (Federal Register,
1993) and Munro (1990). This standard can be applied to those
flavouring substances for which metabolic fate is unknown and cannot
be confidently predicted and for which there are no toxicity data on
the flavouring substance or on a structurally related material from
which to conclude any inference of safety in use.
Flavouring substances that meet one of the five numbered criteria
outlined below can be declared safe for their intended use without
further evaluation:
1. a) The flavouring substance has a simple structure and will be
metabolized and excreted through known detoxication pathways
to innocuous end-products; and
b) the conditions of intended use do not result in an exposure
greater than the human exposure threshold for the relevant
structural class, indicating a low probability of potential
for adverse effects.
2. a) The conditions of intended use result in an exposure that
exceeds the human exposure threshold for the relevant
structural class; however
b) the flavouring substance has a simple structure and will be
metabolized and excreted through known detoxication pathways
to innocuous end-products and it or its metabolites are
endogenous human metabolites with no known biochemical
regulating function.
3. a) The flavouring substance has a simple structure and will be
metabolized and excreted through known detoxication pathways
to innocuous end-products; and
b) the conditions of intended use result in an exposure that
exceeds the human exposure threshold for the relevant
structural class; however
c) toxicity data exist on the flavouring substance which
provide assurance of safety under conditions of intended
use, or there are toxicity data on one or more close
structural relatives which provide a NOEL high enough to
accommodate any perceived difference2 in toxicity between
the flavouring substance and the structurally related
substance having toxicity data.
4. a) The metabolic fate of the flavouring substance cannot be
confidently predicted on the basis of structure; however
b) the conditions of intended use result in an exposure below
the human exposure threshold for the relevant structural
class indicating a low probability of potential for adverse
effects; and
c) there are toxicity data on the substance, or on one or more
structurally related substances with a NOEL at least 10
times greater than the 5th percentile NOEL for the relevant
structural class.
5. a) The metabolic fate of the flavouring substance cannot be
confidently predicted on the basis of structure; however
b) the conditions of intended use result in an exposure below
the human exposure threshold of 1.5 µg/day2, providing
assurance that the substance will be safe under conditions
of intended use.
[NOTE: This criterion accommodates flavouring substances for
which there are limited data on structure-activity and toxicity,
but they would not be considered to present a safety concern
below the human exposure threshold of 1.5 µg/day (Federal
Register, 1993)].
The evaluation criteria listed above identify substances which
are considered safe under conditions of intended use or those that may
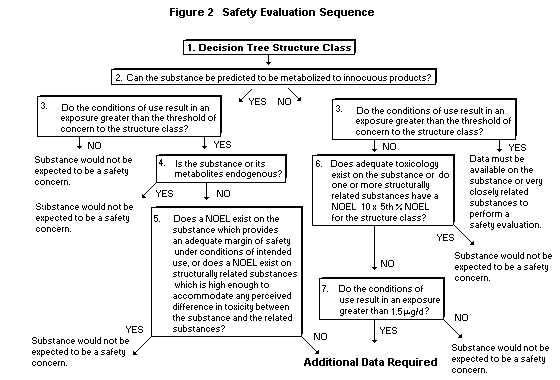
require additional data and further evaluation. Figure 2 presents the
same criteria in the form of an evaluation sequence. The sequence
contains a number of questions on structure, metabolism, exposure data
and toxicity and provides an integrated mechanism to evaluate the
safety of a flavour ingredient. Although the procedure incorporates
relevant toxicity data on a substance or related substances where
available, it does not require them. The procedure identifies those
substances for which additional safety data may be required in order
to perform an adequate safety evaluation.
The effective application of this safety evaluation procedure
depends on a substantial knowledge of toxicology, chemistry,
metabolism, and exposure to flavouring substances. It can be applied
most effectively when groups of structurally related flavouring
substances are evaluated together. In a group evaluation, conclusions
reached on the safety of individual substances are supported by
similar conclusions for structurally related substances. For example,
the results of the evaluation for butyl butyrate should be consistent
with results for other esters formed from aliphatic acyclic linear
saturated alcohols and acids having similar levels of exposure. These
similar substances will pass through the same branch of the safety
evaluation procedure because they fall into the same structural class,
possess similar metabolic fate, and exhibit similar patterns of
exposure from use as flavour ingredients and as components of food.
2 In most instances, groups of structurally related materials have
toxicology data on at least one member of the group, usually the
flavoring substance with the highest poundage. In most cases a
large margin of safety ( i.e., 100- to 1000-fold) exists
between the NOEL and the calculated exposure to the substance
having toxicological data. Such margins of safety would be
expected to accommodate any perceived difference between the
toxicity of a flavouring substance having no toxicological data
and its close structural relative for which a NOEL has been
established.
In the first step of the safety evaluation procedure (Figure 2)
the user must assign a decision tree structure class (Cramer et al.,
1978) to the substance. Following assignment of structure class, a
question on metabolic fate appears. This question identifies those
substances which are anticipated to be efficiently metabolized
to innocuous products (e.g., 1-butanol) versus those which are
transformed to more toxic metabolites (e.g., estragole) or have
limited information on which to predict confidently the metabolic fate
(e.g., 2-phenyl-3-carbethoxy furan).
Once a substance has been sorted according to structure class
and knowledge of metabolic fate, the next question compares the
substance's daily per capita intake ("eaters only") from use as a
flavour ingredient to the human exposure threshold (Table 4) for the
same structure class.
If the substance is metabolized to innocuous products (Step
No. 2) and has an intake less than the human exposure threshold for
the structure class (Step No. 3), the substance is considered safe
(e.g., 1-octanol). If the intake is greater than the human exposure
threshold (Step No. 3) and the substance or its metabolites are
endogenous (Step No. 4), the substance is also considered safe, even
though the intake is greater than the human exposure threshold (e.g.,
butyric acid). If the substance is not endogenous, then the substance
or related substances must have a NOEL (Step No. 5) significantly
greater than the intake of the substance in order to be considered
safe (e.g., citral). If no such data exist or the NOEL is not
significantly greater than the intake for the substance, then
additional data are required in order to complete the safety
evaluation.
If metabolic fate cannot be confidently predicted and the intake
(Step No. 2) is greater than the human exposure threshold (Step
No. 3), additional data on metabolic fate or toxicity on the substance
or structurally related substances are required to complete the safety
evaluation (e.g., dihydro-coumarin). If the intake is less than the
human exposure threshold, the substance or structurally related
substances must have a NOEL significantly greater than the NOEL for
the structure class (Step No. 6) in order for the substance to be
considered safe (e.g., 2-ethyl-4-hydroxy-3(2H)-furanone). If an
adequate toxicity study is not available and the substance has an
intake less than 1.5 µg/day (Step No. 7), the substance is considered
safe (e.g., 3-acetyl-2,5-dimethylthiophene). Otherwise additional data
are required in order to complete the safety evaluation (e.g.,
2-ethylfuran).
risk at exposure levels below the human exposure threshold for their
respective structural class. However, even if information on
structural class, metabolic fate and existing toxicity studies were
not available, 743/1323 (56%) of flavouring substances used in the
U.S. are consumed in amounts less than the 1.5 µg/person/day standard
proposed by the FDA (Federal Register, 1993) and Munro (1990). This
indicates that for approximately half of the existing list of 1323
chemically defined flavouring substances permitted for use in the
U.S., exposure can be considered to be trivial.
Table 4. Fifth percentile NOELs and human exposure thresholds for
Cramer et al. (1978) structural classes in the reference
database
5th Percentile NOELs, Human Exposure
(µg/kg bw/day) Threshold,(µg/day)a,b
I 137 2993 1800
II 28 906 540
III 447 147 88
a The human exposure threshold was calculated by multiplying the
5th percentile NOEL by 60 (assuming an individual weighs 60 kg)
and dividing by a safety factor of 100, as discussed in the text.
b Numbers rounded to two (2) significant figures.
Table 5. Flavouring substances within each Cramer et al. (1978)
structural class consumed in amounts below human exposure
thresholds
No. of No. of Flavours
Human Exposure Flavours Under Human
Structural Class Threshold Within Exposure
(µg/day) Structural Threshold (%)
Classa
I 1,800 878 835 (95)
II 540 243 227 (93)
III 88 202 195 (97)
a Adapted from the FEMA flavouring substance database of flavouring
substances permitted for use in the U.S.
3.3 Evaluation criteria
The evaluation criteria proposed for application to flavouring
substances in this paper involve the integration of information on
exposure, structure-activity relationships, metabolism and, as
required, toxicity data. It should be noted that the safety evaluation
criteria outlined below are not intended to be applied to any
flavouring substances with unresolved toxicity problems or to
substances that are presumed or known carcinogens. Such substances
warrant special consideration and must be evaluated using more
traditional methods of safety evaluation. While toxicity data exist on
numerous flavouring substances and can be used as the basis for
evaluation, there are many flavouring substances that lack toxicity
data. The evaluation criteria are intended to provide a means of
evaluating such substances. The criteria incorporate, where the data
permit, the concept that metabolic fate can be predicted on the basis
of presumed structure-activity relationships. The criteria also rely,
in part, on the NOEL reference database which provides a human
exposure threshold for each of the three structural classes of
flavouring substances, as shown in Table 4. The evaluation criteria
also incorporate, where available, toxicity data on flavouring
substances and closely related structural analogues as a basis for
safety evaluation. One of the criteria (number 5 below) incorporates
the concept of a minimum human exposure threshold based on the
1.5 µg/person/ day standard proposed by the FDA (Federal Register,
1993) and Munro (1990). This standard can be applied to those
flavouring substances for which metabolic fate is unknown and cannot
be confidently predicted and for which there are no toxicity data on
the flavouring substance or on a structurally related material from
which to conclude any inference of safety in use.
Flavouring substances that meet one of the five numbered criteria
outlined below can be declared safe for their intended use without
further evaluation:
1. a) The flavouring substance has a simple structure and will be
metabolized and excreted through known detoxication pathways
to innocuous end-products; and
b) the conditions of intended use do not result in an exposure
greater than the human exposure threshold for the relevant
structural class, indicating a low probability of potential
for adverse effects.
2. a) The conditions of intended use result in an exposure that
exceeds the human exposure threshold for the relevant
structural class; however
b) the flavouring substance has a simple structure and will be
metabolized and excreted through known detoxication pathways
to innocuous end-products and it or its metabolites are
endogenous human metabolites with no known biochemical
regulating function.
3. a) The flavouring substance has a simple structure and will be
metabolized and excreted through known detoxication pathways
to innocuous end-products; and
b) the conditions of intended use result in an exposure that
exceeds the human exposure threshold for the relevant
structural class; however
c) toxicity data exist on the flavouring substance which
provide assurance of safety under conditions of intended
use, or there are toxicity data on one or more close
structural relatives which provide a NOEL high enough to
accommodate any perceived difference2 in toxicity between
the flavouring substance and the structurally related
substance having toxicity data.
4. a) The metabolic fate of the flavouring substance cannot be
confidently predicted on the basis of structure; however
b) the conditions of intended use result in an exposure below
the human exposure threshold for the relevant structural
class indicating a low probability of potential for adverse
effects; and
c) there are toxicity data on the substance, or on one or more
structurally related substances with a NOEL at least 10
times greater than the 5th percentile NOEL for the relevant
structural class.
5. a) The metabolic fate of the flavouring substance cannot be
confidently predicted on the basis of structure; however
b) the conditions of intended use result in an exposure below
the human exposure threshold of 1.5 µg/day2, providing
assurance that the substance will be safe under conditions
of intended use.
[NOTE: This criterion accommodates flavouring substances for
which there are limited data on structure-activity and toxicity,
but they would not be considered to present a safety concern
below the human exposure threshold of 1.5 µg/day (Federal
Register, 1993)].
The evaluation criteria listed above identify substances which
are considered safe under conditions of intended use or those that may
require additional data and further evaluation. Figure 2 presents the
same criteria in the form of an evaluation sequence. The sequence
contains a number of questions on structure, metabolism, exposure data
and toxicity and provides an integrated mechanism to evaluate the
safety of a flavour ingredient. Although the procedure incorporates
relevant toxicity data on a substance or related substances where
available, it does not require them. The procedure identifies those
substances for which additional safety data may be required in order
to perform an adequate safety evaluation.
The effective application of this safety evaluation procedure
depends on a substantial knowledge of toxicology, chemistry,
metabolism, and exposure to flavouring substances. It can be applied
most effectively when groups of structurally related flavouring
substances are evaluated together. In a group evaluation, conclusions
reached on the safety of individual substances are supported by
similar conclusions for structurally related substances. For example,
the results of the evaluation for butyl butyrate should be consistent
with results for other esters formed from aliphatic acyclic linear
saturated alcohols and acids having similar levels of exposure. These
similar substances will pass through the same branch of the safety
evaluation procedure because they fall into the same structural class,
possess similar metabolic fate, and exhibit similar patterns of
exposure from use as flavour ingredients and as components of food.
2 In most instances, groups of structurally related materials have
toxicology data on at least one member of the group, usually the
flavoring substance with the highest poundage. In most cases a
large margin of safety ( i.e., 100- to 1000-fold) exists
between the NOEL and the calculated exposure to the substance
having toxicological data. Such margins of safety would be
expected to accommodate any perceived difference between the
toxicity of a flavouring substance having no toxicological data
and its close structural relative for which a NOEL has been
established.
In the first step of the safety evaluation procedure (Figure 2)
the user must assign a decision tree structure class (Cramer et al.,
1978) to the substance. Following assignment of structure class, a
question on metabolic fate appears. This question identifies those
substances which are anticipated to be efficiently metabolized
to innocuous products (e.g., 1-butanol) versus those which are
transformed to more toxic metabolites (e.g., estragole) or have
limited information on which to predict confidently the metabolic fate
(e.g., 2-phenyl-3-carbethoxy furan).
Once a substance has been sorted according to structure class
and knowledge of metabolic fate, the next question compares the
substance's daily per capita intake ("eaters only") from use as a
flavour ingredient to the human exposure threshold (Table 4) for the
same structure class.
If the substance is metabolized to innocuous products (Step
No. 2) and has an intake less than the human exposure threshold for
the structure class (Step No. 3), the substance is considered safe
(e.g., 1-octanol). If the intake is greater than the human exposure
threshold (Step No. 3) and the substance or its metabolites are
endogenous (Step No. 4), the substance is also considered safe, even
though the intake is greater than the human exposure threshold (e.g.,
butyric acid). If the substance is not endogenous, then the substance
or related substances must have a NOEL (Step No. 5) significantly
greater than the intake of the substance in order to be considered
safe (e.g., citral). If no such data exist or the NOEL is not
significantly greater than the intake for the substance, then
additional data are required in order to complete the safety
evaluation.
If metabolic fate cannot be confidently predicted and the intake
(Step No. 2) is greater than the human exposure threshold (Step
No. 3), additional data on metabolic fate or toxicity on the substance
or structurally related substances are required to complete the safety
evaluation (e.g., dihydro-coumarin). If the intake is less than the
human exposure threshold, the substance or structurally related
substances must have a NOEL significantly greater than the NOEL for
the structure class (Step No. 6) in order for the substance to be
considered safe (e.g., 2-ethyl-4-hydroxy-3(2H)-furanone). If an
adequate toxicity study is not available and the substance has an
intake less than 1.5 µg/day (Step No. 7), the substance is considered
safe (e.g., 3-acetyl-2,5-dimethylthiophene). Otherwise additional data
are required in order to complete the safety evaluation (e.g.,
2-ethylfuran).
 The principal objective of the safety evaluation procedure is to
identify two groups of flavourings substances: i) those substances
whose structure, metabolism, and relevant toxicity data clearly
indicate that the substance would be expected not to be a safety
concern under current conditions of intended use; and ii) those
substances which may require additional data in order to perform an
adequate safety evaluation.
3.4 Integrating data on the consumption ratio
As pointed out by WHO (1987) and SCF (1991), natural occurrence
is no guarantee of safety, but it is important to recognize that the
safety evaluation of added use of flavouring substances needs to be
conducted with an appreciation of the consumption ratio. Clearly, if
added use of flavouring substances results in an exposure that exceeds
that from natural sources, this will increase awareness of the need to
consider carefully overall exposure in the light of existing data on
toxicity and structure-activity relationships. On the other hand, if
the added use is trivial with a consumption ratio of 10 to 100, that
is, it increases total exposure by only 1 to 10%, then this fact needs
to be taken into consideration when applying the criteria outlined
above.
The substances of primary concern are those which, in Figure 2,
receive a "No" answer to Step No. 5, or a "Yes" answer to Step No. 7,
indicating a possible need for additional data and evaluation beyond
that included in the evaluation procedure outlined in this paper. In
such an evaluation, as stated immediately above, the extent of natural
occurrence should be given appropriate weight.
Of much less concern with respect to consumption ratio are those
substances that drop out of further consideration as a result of "No"
answers to Step Nos. 3 or 7, or "Yes" answers to Step Nos. 4 or 6. The
derivation of the thresholds, the estimation of intakes (see Appendix
A), and the special factors applicable to the use of flavours
(volatility, self-limiting use, etc.) build in multiple conservative
assumptions more than adequate to cover additional exposure from
natural occurrence to substances that in any case are of low inherent
concern.
The advances in analytical chemistry in the past 50 years provide
virtual assurance that no flavouring substances of extensive natural
occurrence remain unknown. Those of potential value yet to be
discovered (e.g., as yet unknown components of roast beef, coffee, or
chocolate flavour) are being sought at the ppb and ppt level. This
does not suggest exposures above any of the thresholds discussed in
this paper.
4. CONCLUSIONS
It is neither possible nor necessary to conduct toxicological
studies on all individual flavouring substances used in food. The vast
majority of flavouring substances are members of groups of substances
with common metabolic pathways, and typically, individual members of
such a group display a similar toxicity profile. This is not
surprising in light of the close structural similarity of the various
flavouring substances comprising a chemical group. As demonstrated in
this paper, exposure to flavouring substances is usually low and, in
the majority of cases, below the human exposure threshold values
presented in this paper. This knowledge, coupled with knowledge
regarding structure-activity relationships, metabolic disposition, and
toxicology data, permits the establishment of the proposed paradigm
for safety assessment which provides a solid foundation to assure the
safety of flavouring substances under conditions of intended use.
5. REFERENCES
BECK, B.D., CONOLLY, R.B., DOURSON, M.L., GUTH, D., HATTIS, D.,
KIMMEL, C. & LEWIS, C. (1993). Symposium overview. Improvements in
quantitative noncancer risk assessment. Fund Appl Toxicol.,
20: 1-14.
COUNCIL OF EUROPE (1974). Natural Flavouring Substances, Their
Sources, And Added Artificial Flavouring Substances. Council of
Europe, Strasbourg, France.
COUNCIL OF EUROPE ( 1981 ). Flavouring Substances And Natural Sources
Of Flavourings, Third Edition. Council of Europe, Strasbourg, France.
COUNCIL OF EUROPE (1992). Flavouring Substances and Natural Sources
of Flavourings. Volume I. Chemically-Defined Flavouring Substances,
Fourth Edition. Council of Europe, Maissoneuve, France.
CRAMER, G.M., FORD, R.A. & HALL, R.L. (1978). Estimation of toxic
hazard-A decision tree approach. Food Cosmet Toxicol., 16: 255-276.
DEISINGER, P.J., BOATMAN, R.J. & GUEST, D. (1994). Metabolism of
2-ethylhexanol administered orally and dermally to the female Fischer
344 rat. Xenobiotica, 24: 429-440.
FOOD AND DRUG ADMINISTRATION (1982). Toxicological Principles for the
Safety Assessment of Direct Food Additives and Color Additives Used in
Food. Redbook. U.S. Food and Drug Administration, Bureau of Foods,
Washington, DC.
FOOD AND DRUG ADMINISTRATION (1993). Toxicological Principles for the
Safety Assessment of Direct Food Additives and Color Additives Used in
Food. Redbook II (Draft). U.S. Food and Drug Administration, Center
for Food Safety and Applied Nutrition, Washington, DC.
FEDERAL REGISTER (1993). Food additives; threshold of regulation for
substances used in food-contact articles. 58(195): 52719-52729.
FRAWLEY, J.P. (1967). Scientific evidence and common sense as a basis
for food-packaging regulations. Food Cosmet Toxicol., 5: 293-308.
GOLD, L.S., SAWYER, C.B., MAGAW, R., BACKMAN, G.M., DE VECIANA, M.,
LEVINSON, R., HOOPER, N.K., HAVENDER, W.R., BERNSTEIN, L., PETO, R.,
PIKE, M. & AMES, B.M. (1984). A carcinogenic potency database of the
standardized results of animal bioassays. Environ Health Perspect.,
58: 9-319.
GOLD, L.S., SLONE, T.H. & BERNSTEIN, L. (1989). Summary of
carcinogenic potency and positivity for 492 rodent carcinogens in the
carcinogenic potency database. Environ. Health Perspect., 79: 259-272.
JAESCHKE, H., KLEINWAECHTER, C. & WENDEL, A. (1987). The role of
acrolein in allyl alcohol-induced lipid peroxidation and liver cell
damage in mice. Biochem. Pharmacol., 36: 51-57.
JECFA (1967). Toxicological Evaluation of Some Flavouring Substances
and Non-Nutritive Sweetening Agents. Eleventh Meeting of the Joint
FAO/WHO Expert Committee on Food Additives.
JECFA (1968). Specifications for the Identity and Purity of Food
Additives and their Toxicological Evaluation: Some Flavouring
Substances and Non-nutritive Sweetening Agents. Eleventh Report of the
Joint FAO/WHO Expert Committee on Food Additives, FAO Nutrition
Meetings Report Series No. 44, WHO Technical Report Series No. 383.
JECFA (1970). Toxicological Evaluation of Some Extraction Solvents and
Certain Other Substances. Fourteenth Meeting of the Joint FAO/WHO
Expert Committee on Food Additives.
JECFA (1971). Evaluation of Food Additives. Fourteenth Report of the
Joint FAO/WHO Expert Committee on Food Additives, WHO Technical Report
Series No. 462.
JECFA (1972). Evaluation of Food Additives. Some Enzymes, Modified
Starches, and Certain Other Substances: Toxicological Evaluations and
Specifications and a Review of the Technological Efficacy of Some
Antioxidants. Fifteenth Report of the Joint FAO/WHO Expert Committee
on Food Additives, WHO Technical Report Series No. 488.
JECFA (1974a). Toxicological Evaluation of Certain Food Additives
Including Anticaking Agents, Antimicrobials, Antioxidants, Emulsifiers
and Thickening Agents. Seventeenth Meeting of Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 5.
JECFA (1974b). Toxicological Evaluation of Certain Food Additives with
a Review of General Principles and of Specifications. Seventeenth
Report of the Joint FAO/WHO Expert Committee on Food Additives, WHO
Technical Report Series No. 539.
JECFA (1976a). Evaluation of Certain Food Additives. Twentieth Report
of the Joint FAO/WHO Expert Committee on Food Additives, FAO Nutrition
Meetings Report Series No. 1, WHO Technical Report Series No. 599.
JECFA (1976b). Toxicological Evaluation of Certain Food Additives.
Twentieth Meeting of the Joint FAO/WHO Expert Committee on Food
Additives, WHO Food Additive Series No. 10.
JECFA (1978). Evaluation of Certain Food Additives and Contaminants.
Twenty-second Report of the Joint FAO/WHO Expert Committee on Food
Additives, WHO Technical Report Series No. 631.
JECFA (1980a). Evaluation of Certain Food Additives. Twenty-third
Report of the Joint FAO/WHO Expert Committee on Food Additives. World
Health Organization, Geneva. WHO Technical Report Series No. 648.
JECFA (1980b). Evaluation of Certain Food Additives. Twenty-fourth
Report of the Joint FAO/WHO Expert Committee on Food Additives. World
Health Organization, Geneva. WHO Technical Report Series No. 653.
JECFA (1980c). Toxicological Evaluation of Certain Food Additives.
Twenty-third Meeting of the Joint FAO/WHO Expert Committee on Food
Additives, WHO Food Additive Series No. 14.
JECFA (1981a). Evaluation of Certain Food Additives. Twenty-fifth
Report of the Joint FAO/WHO Expert Committee on Food Additives,
Technical Report Series No. 669.
JECFA (1981b). Toxicological Evaluation of Certain Food Additives.
Twenty-fifth Meeting of the Joint FAO/WHO Expert Committee on Food
Additives, WHO Food Additive Series No. 16.
JECFA (1982a). Evaluation of Certain Food Additives and Contaminants.
Twenty-sixth Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 683.
JECFA (1982b). Toxicological Evaluation of Certain Food Additives.
Twenty-sixth Meeting of the Joint FAO/WHO Expert Committee on Food
Additives, WHO Food Additive Series No. 17.
JECFA (1983a). Evaluation of Certain Food Additives and Contaminants.
Twenty-seventh Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 696.
JECFA (1983b). Toxicological Evaluation of Certain Food Additives and
Contaminants. Twenty-seventh Meeting of the Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 18.
JECFA (1984a). Evaluation of Certain Food Additives and Contaminants.
Twenty-eighth Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 710
JECFA (1984b). Toxicological Evaluation of Certain Food Additives and
Contaminants. Twenty-eighth Meeting of the Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 19.
JECFA (1986). Evaluation of Certain Food Additives and Contaminants.
Twenty-ninth Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 733.
JECFA (1987a). Evaluation of Certain Food Additives and Contaminants.
Thirtieth Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization. Geneva. WHO Technical Report
Series No. 751.
JECFA (1987b). Evaluation of Certain Food Additives and Contaminants.
Thirty-first Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 759.
JECFA (1989). Evaluation of Certain Food Additives and Contaminants.
Thirty-third Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 776.
JECFA (1990a). Evaluation of Certain Food Additives and Contaminants.
Thirty-fifth Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 789.
JECFA (1990b). Toxicological Evaluation of Certain Food Additives and
Contaminants. Thirty-fifth Meeting of the Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 26.
JECFA (1991a). Evaluation of Certain Food Additives and Contaminants.
Thirty-seventh Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 806.
JECFA (1991b). Toxicological Evaluation of Certain Food Additives and
Contaminants. Thirty-seventh Meeting of the Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 28.
JECFA (1992). Evaluation of Certain Food Additives and Naturally
Occurring Contaminants. Thirty-ninth Report of the Joint FAO/WHO
Expert Committee on Food Additives. World Health Organization, Geneva.
WHO Technical Report Series No. 828.
JECFA (1993a). Evaluation of Certain Food Additives and Contaminants.
Forty-first Report of the Joint FAO/WHO Expert Committee on Food
Additives, Technical Report Series No. 837.
JECFA (1993b). Toxicological Evaluation of Certain Food Additives and
Contaminants. Forty-first Meeting of the Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 32.
JECFA (1993c). Toxicological Evaluation of Certain Food Additives and
Naturally Occurring Toxicants. Thirty-Ninth Meeting of the Joint
FAO/WHO Expert Committee on Food Additives, WHO Food Additive Series
No. 30.
KRASAVAGE, W.J., O'DONOGHUE, J.L., DIVINCENZO, G.D. & TERHAAR, C.J.
(1980). The relative neurotoxicity of methyl-n-butyl ketone, n-hexane
and their metabolites. Toxicol. Appl. Pharmacol., 52: 433-441.
LEWIS, S.C., LYNCH, J.R. & NIKIFOROV, A.I. (1990). A new approach to
deriving community exposure guidelines from "no-observed-adverse-
effect levels". Regul. Toxicol. Pharmacol., 11: 314-330.
MUNRO, I.C. (1990). Safety assessment procedures for indirect food
additives: An overview. Regul. Toxicol. Pharmacol., 12: 001-0011.
NAS (1978). Resurvey of the Annual Poundage of Food Chemicals
Generally Recognized as Safe, National Technical Information Service
(NTIS) PB288-081.
NAS (1979). Comprehensive Survey of Industry on the Use of Food
Additives, National Technical Information Service (NTIS) PB80-113418.
NAS (1984). Poundage Update of Food Chemicals, National Technical
Information Service (NTIS) PB84-162148.
NAS (1989). 1987 Poundage and Technical Effects Update of Substances
Added to Food. National Research Council, Washington, DC. Prepared
for: Food and Drug Administration, Washington, D.C. NTIS Report
No. PB91-127266.
PHILLIPS, J.C., PURCHASE, R., WATTS, P. & GANGOLLI, S.D. (1987). An
evaluation of the decision tree approach for assessing priorities for
safety testing of food additives. Food Addit. Contam., 4(2): 109-123.
RULIS, A.M. (1986). De Minimis and the Threshold of Regulation.
In: Felix, C.W. (Ed.) Food Protection Technology. Lewis Publishers
Inc., Chelsea, MI. pp. 29-37.
SCF (1991). Guidelines for the Evaluation of Flavourings for Use in
Foodstuffs: I. Chemically Defined Flavouring Substances. Commission of
the European Communities, Scientific Committee for Food, Brussels,
Belgium.
STOFBERG, J. & KIRSCHMAN, J. (1985). The consumption ratio of
flavouring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23: 857-860.
STOFBERG, J. & GRUNDSCHOBER, F. (1987). Consumption ratio and food
predominance of flavouring materials. Perfumer Flavourist,
12: 27-68.
WEIL, C.S. & McCOLLISTER, D.D. 1963. Safety evaluation of chemicals.
Relationship between short- and long-term feeding studies in designing
an effective toxicity test. Agr. Food Chem., 11(6): 486-491.
WHO (1987). Principles For The Safety Assessment Of Food Additives
And Contaminants In Food. WHO Environmental Health Criteria. World
Health Organization (WHO), International Program on Chemical Safety in
Cooperation with the Joint FAO (Food & Agriculture Organization of the
United Nations), WHO Expert Committee on Food Additives (JECFA),
Geneva, Switzerland, Environmental Health Criteria 70.
WOODS, L.A. & DOULL, J. (1991). GRAS evaluation of flavouring
substances by the Expert Panel of FEMA. Regul. Toxicol. Pharmacol.,
14: 48-58.
ANNEX 5, Appendix A
ESTIMATING DISTRIBUTION OF INTAKES OF FOOD INGREDIENTS ACROSS
THE POPULATION
In response to the Food Additives Amendment of 1958 to the U.S.
Food, Drug and Cosmetic Act, the Flavor and Extract Manufacturers'
Association (FEMA) in 1960 conducted a comprehensive survey of all
flavoring materials then thought or claimed to be in use. Information
included: identity; poundage used annually in food; year of first use;
levels (in ppm) used in major food categories; toxicological data; and
other information related to safety. After evaluation by a panel of
external experts, this and subsequent information was published in a
series of "FEMA GRAS Lists" (Hall and Oser, 1965, 1970; Oser and Hall,
1972; Oser and Ford, 1973ab, 1974, 1975, 1977, 1978, 1979; Oser et al.,
1984, 1985; Burdock et al., 1990; Smith and Ford, 1993).
FEMA determined in 1968 that another survey was needed to monitor
trends and to improve on the previous effort. In addition, the U.S.
Food and Drug Administration (FDA) began to plan to review its
Generally Recognized as Safe (GRAS) lists, and in so doing, made use
of the prior experience of FEMA. FEMA and FDA efforts merged into the
first comprehensive survey of ingredients used in food, conducted by
the National Academy of Sciences/National Research Council (NAS) in
1970 (NAS, 1973) with several subsequent surveys (NAS, 1978, 1979,
1984, 1989).
Emerging from the 1960 and 1970 surveys was a clear indication
that per capita intake of an ingredient (from annual poundage),
although useful for comparison with other ingredients, has a
significant limitation. As an average of intakes by those who consume
foods containing the ingredient ("eaters") and by those who do not
(non-eaters"), it does not represent the intake by the individuals of
concern ( i.e., the "eaters"). This is not the case for ingredients
commonly consumed by almost everyone ( e.g., sugar), but the
difference between the average and "eaters" is significant for other
ingredients consumed only by few people ( e.g. raspberry flavor).
Because of these issues, an alternative method of calculating
intakes was used in the first (1973) NAS report. This method involved
the average level of use of an ingredient in a broad food category
(e.g., baked goods) multiplied by the average daily consumption of
that food category per person. The daily consumption for each category
was obtained by multiplying the average portion size for the category,
obtained from the U.S. Department of Agriculture (USDA) Food
Consumption Survey by the average daily frequency of consumption of
foods in that category over a 14-day survey period. The frequency of
consumption data came from a 12,000+ member panel operated by the
Market Research Corporation of America (MRCA; see note b to Table 1).
Thus the average intake of an ingredient for all relevant food
categories provided the "possible average daily intake" (PADI). These
intakes were often highly exaggerated, as much as a hundred-fold,
because an ingredient used in only a few foods in a food category was
assumed to be at the average level in all foods in the category. Thus,
for example, cardamom, used only in a few coffee cakes, was assumed to
be used in each food in the entire category of baked goods ( i.e.,
all breads, rolls, crackers, and pastries).
To address this issue, another method was developed to give the
probable distribution of intakes for the entire population without
exaggerating intake. This required an informed estimate of the
probability that each food eaten would contain the ingredient of
interest. The first series of such intake estimates used calculation
"A", as shown in Table 1, and the results are summarized in Section
"A" of Table 2.
In Table 2, Column 1 ("Substance") lists the substances selected
to test and improve the more refined method. In Section A, nutmeg and
mace were chosen, among other reasons because they are not grown
domestically in the U.S. and import data, therefore, provide a firm
external check on pounds used. Furthermore, more than half of all
nutmeg is consumed in four foods: bologna, pork sausage, frankfurters,
and doughnuts. It thus presented an opportunity to see how heavy
consumers of those foods would skew total intake distribution. The
flavor ingredient 4-(p-hydroxyphenyl)butan-2-one was chosen because it
is used in, and occurs naturally, almost entirely in only one flavor,
raspberry flavor, and is far less widely consumed than chocolate,
vanilla, or citrus flavors. Thus, it is a good example of an uncommon
ingredient consumed by those who particularly like the few foods in
which it is used (Hall, 1975).
Column 2 [" per capita (External Check)"] lists per capita
intake derived from import data, product manufacturer surveys, or
tariff reports, all independent of the NAS survey to serve as an
external check. Column 3 (" per capita [NAS(lbs)/0.6]") lists the
per capita intake from the poundage reported in the NAS survey
assumed to be 60% complete. Further research (see references to NRC
surveys) showed this to be generally a valid assumption, but with
certain qualifications. Ingredients such as sugar, corn syrup, and
salt, could not be fully or reliably reported by bakers and other
small food processors, whereas the flavor industry reported more
fully, and the 60% assumption, while used, usually resulted in a
poundage per capita overestimate.
For Section A, Column 4 ["50th Percentile (Total Panel)"]
presents the total panel 50th percentile for each substance, and
Column 4a ("50th Percentile 3 S.D. Total Panel"), the 50th percentile
plus 3 standard deviations. Column 4b ["very high (Total Panel)"]
shows the so-called "very high intake". This is not a realistic
figure, because no person could consume all foods using an ingredient
at the highest level for every food in every major category. Note
particularly, also, the earlier discussion of the exaggeration that
arises from assuming that an ingredient used only in a few foods is
used in all foods in a major food category. The "very high intake"
serves simply as a wholly unreachable and therefore unrealistic value.
Given the remaining and inevitable uncertainties due to possible
errors in estimations, and possible non-food uses, the agreement
between the total panel 50th percentile (Section A, Column 4), the NRC
per capita, (Column 3), and the per capita from external sources
(Column 2), is entirely satisfactory. However, calculation "A" did
not provide data on only those who actually consumed the substance
("eaters only"), and the statistical treatment was not as solidly
based as was desirable. This led to an improved calculation, "B", as
shown in Table 3. Table 2, Section B, presents the results of these
calculations.
The substances shown in Column 1 of Section B were selected, as
discussed in the referenced reports themselves, by the FDA or the NAS
committee (NAS, 1976). An improved method of calculation was used, as
described below, which provides the total sample mean (Column 4 ["Mean
(Total Panel)"]) as a check against the per capita data in Columns 2
and 3.
The external per capita figure for calcium propionate is twice
the total panel mean, probably due primarily to usage by many small
bakeries not included in the survey, and also to the high wastage of
bread. The slightly smaller discrepancy for MSG may well reflect
several added sources of error. Even so, comparison of the total panel
mean with the per capita data from the NAS survey and the external
checks shows satisfactory agreement. Food consumption surveys have
significant and well-recognized sources of error in addition to those
affecting the NRC surveys, and it seems unlikely that it is practical
to expect intake estimates derived by different means to correspond
more closely than within a factor of two.
For Section B, Column 4 ["Mean (Total Panel)"] provides the total
panel mean, Column 4a ["Mean (Eaters Only)"] the "eaters only" mean
age, 2 and over and Column 4b ["S.D. (Eaters Only)"], the standard
deviation for "eaters only".
The procedure in calculation B does not eliminate all sources of
error. Intake is still calculated from "disappearance figures"
( i.e, the levels introduced into food during processing, not the
lower levels actually consumed). Thus, ignoring the often large losses
due, for example, to volatilization or leaching in processing,
preparation and storage result in exaggerated estimates of intake. In
the other direction, heavy (frequent) eaters will often eat larger
portion sizes, resulting in a tendency to underestimate higher
intakes. The result overall, however, remains highly satisfactory.
Table 1 Calculation "A"
Step Operation Result
For each specific food (SF)a e.g. bologna,
within a broad food category, eg., meats,
obtain from MRCAb the
1 total number of eatings of each SF by the total
panel, in two weeks
14 days × 12,337 (persons in panel) = mean frequency of eating, p/d
×
2 mean portion size in gc = mean consumption of SF, g/p/d
×
3 probability of SF containing ingredient "I"d = probable mean consumption of SF
containing I g/p/d
×
4 usual use level of "I" in ppm of major food = 50th percentile of probable intake of I
category containing SF ‰ 1,000e from SF in mg/p/d
×
5 90th percentile MRCA frequency of eating = 90th percentile of probable intake of I
50th percentile MRCA frequency of eating from SF in mg/p/d
6 & 7 sum 4 and 5 separately across all SFs in that = 50th and 90th percentiles of probable
major food category intake of I from that major food category
Table 1 (cont'd) Calculation "A"
Step Operation Result
8 fit a curve, assuming normal distribution, to = 50th percentile and standard deviation
the points determined in 6 and 7 and calculate (SD) for each major food category
9 repeat steps 1 through 8 and sum across all = 50th percentile and SD in mg/p/d
food categories probable total intake
10 repeat steps 4 and following, using "average = "very high intake"
maximum use level" from the survey for each
major food category
a Specific food (SF), in this context, denotes a narrow category of very similar foods serving
the same dietary and market niches (e.g. all bologna, all cola drinks). The eatings of each SF
include in one total for each SF all eatings of similar brands or home-prepared versions of the
same SF. There were approximately 10,000 such SFs.
b The Market Research Corporation of America (MRCA) is a private market research company that
surveys usage of food products and components It uses a nationally representative panel and
records for each panel member the number of servings of each specific loud for each day of a
two-week period. The panelists' reporting periods are distributed evenly throughout the year. The
1973 NAS report used data from the third Menu Census (1967-1968) involving 12,871 individuals.
The dataused in calculations "A" and "B" came from the Fourth Menu Census (1972-1973) involving
12,337 individuals.
c The USDA Food Consumption Survey (USDA, 1965)
d Obtained from a consensus panel of experts knowledgeable about the ingredient and the food.
e From NAS, 1973
Table 2 Distribution of MRCA 14-Day Average Daily Intakes (in mg/person/day)a of Selected Food Ingredients
(1) (2) (3) (4) (4a) (4b) (5)
"High Eaters
Section Substance per capita per capita Mean Mean plus "Very High" Only"
(External [NAS (lbs.)/0.6] (Total Panel) 3 S.D.b (Total Panel) [NAS
Check) Total Panel (lbs.)/0.6]×10
A Nutmeg 23.6 10.3 15.7 51.3 71.1 103
Nutmeg Oil 1.2 1.0 2.0 5.7 7.1 10
A Mace 3.3 2.8 3.3 8.6 14.4 28
Mace, Oil NAc 0.26 0.19 0.67 0.46 2.6
Mace, Oleoresin NA 0.070 0.13 0.39 0.41 0.70
A 4-p-Hydroxyphenyl 0.048 0.086 0.041 0.16 0.37 0.86
Butan-2-one
Table 2 (cont'd).
(1) (2) (3) (4) (4a) (4b) (5)
"High Eaters
Section Substance per capita per capita Mean Mean plus "Very High" Only"
(External [NAS (lbs.)/0.6] (Total Panel) 3 S.D.b (Total Panel) [NAS
Check) Total Panel (lbs.)/0.6]×10
Mean Mean S.D.
(Total Panel) (Eaters Only) (Eaters Only)
B Calcium Propionate 92 33 44.8 45.1 20.7 330
Sodium Propionate NA 16 33,2 33.3 14.7 160
B Carob Bean Gum NA 26 35.6 36.1 35.0 260
Chondrus Extract 19.3 13 10.6 11.1 18.5 130
B Mono- and diglycerides NA 727 884 885 467 7,270
Monosodium Glutamate 186 194 98.8 99.0 88.5 1,940
(MSG)
a See text for explanation of columns.
b S.D. = Standard Deviation.
Table 3 Calculation "B"
1 From the MRCA survey, obtain the total
number of eatings of each SF, by each panel
member, each day over the 14 - day period
×
2 USDA mean portion size, for that person's = quantity of SF in g for that
age group and the relevant major food person, that day
category
×
3 weighted mean of usual use level of I in = quantity of I in mg/d, that
ppm ‰ 1,000 person, that day, if all of
the SF contained I
×
4 probability that particular SF contains I = expected intake of I for
that person for that day,
mg/d
5 Repeat steps 1 through 4 for that person for = expected intake of I for
each of the 14 days that person for 14 days,
mg
6 ‰ 14 = average daily intake over
14 days for that person,
g/p/d
7 tabulate all intakes and compute = total panel mean intake of I
mg/p/d
8 discard all persons with zero intake for the = "eaters only" mean and SD
14 day period, tabulate all "eaters only" of for I, mg/p/d
I, age 2 and over, and compute
Unfortunately, this more refined approach, while successful, is
extremely elaborate, tedious, and expensive. The MRCA panel is
expensive to maintain and operate, and the data are correspondingly
costly. The approach requires extensive input from a broadly-based,
necessarily large and well-informed panel of expert technologists.
Such an effort can only be justified in those few cases where it is
necessary to obtain the distribution of intakes with maximum accuracy.
Given the wide safety margins food ingredients typically enjoy, and
the uncertainties and assumptions inherent in interpreting
toxicological data, such effort is rarely necessary. A much more
appropriate, but quick and easy, method of estimating intake was
needed, so long as it was conservative, but not excessively so. This
led Rulis and others in FDA (Rulis et al., 1984; Rulis, 1987) to
test and adopt the assumption that, except for an unusually narrow
margin of safety or specific concern, the per capita "eaters only"
intake could safely and conservatively be assumed - even for heavy
eaters - simply by multiplying the best available estimate of per
capita intake by ten (Column 5 ["High Eaters Only"]). Comparison of
Column 5 with Column 4b, for Section B, makes clear that in every case
the: "High Eaters Only" ( per capita × 10) estimate far exceeds the
"Eaters Only mean plus 3 standard deviations", yet in no case are the
estimates so high as to be unusable.
With the special factors surrounding the use of flavoring
ingredients (i.e., completeness of the surveys, typical volatility
with consequent loss in processing, and self-limiting nature) the
procedure in Column 5 is practical but highly conservative. It has
been widely used in other publications (FDA, 1982, 1993; Easterday
et al., 1993). It can be used safely in all instances except when a
very narrow margin of safety requires maximally precise data for both
toxicological effects and exposure.
Recently, still more sophisticated methods for analyzing USDA
Food Consumption Survey data have become available under license for
those applications in which the need for relatively high precision of
estimates justifies the cost (Helmbach, 1994). In nearly all instances
where broadly- based ingredient disappearance data are available,
per capita × 10, in actuality, a very high "eaters only" estimate,
remains the approach that is by far the simplest and least expensive,
while being conservative but realistic.
REFERENCES
Burdock, G.A., Wagner, B.M., Smith, R.L., Munro, I.C. and Newberne,
P.M. 1990. Recent progress in the consideration of flavoring
ingredients under the food additives amendment. 15. GRAS substances.
A list of flavoring ingredient substances considered generally
recognized as safe by the Flavor & Extract Manufacturers' Association
Expert Panel. Food Technol 44(2):78, 80, 82, 84, & 86.
Easterday, O.D., Ford, R.A., Hall, R.L., Stofberg, J., Cadby, P. and
Grundschober, F. 1992. A Flavor Priority Ranking System, Acceptance
and Internationalization. In: Finley, J.W., Robinson, S.F. and
Armstrong, D.J. (Eds.) Food Safety Assessment. American Chemical
Society, ACS Symposium Series No. 484.
FDA. 1982. Toxicological Principles for the Safety Assessment of
Direct Food Additives and Color Additives Used in Food. Redbook. U.S.
Food and Drug Administration, Bureau of Foods, Washington, DC.
FDA. 1993. Toxicological Principles for the Safety Assessment of
Direct Food Additives and Color Additives Used in Food. Redbook II
(Draft). U.S. Food and Drug Administration, Center for Food Safety and
Applied Nutrition, Washington, DC.
Hall, R.L. and Oser, B.L. 1965. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment. III. GRAS
substances. Food Technol 19(2):151-197.
Hall, R.L. and Oser, B.L. 1970. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment. IV. GRAS
substances. Food Technol 24(5):25-28, 30-32, 34.
Hall, R.L. 1975. Unpublished data from personal communication to the
NAS/NRC Committee on GRAS List Survey - Phase III, and to the FEMA.
Heimbach, J.T. 1994. Personal communication re: TAS-DIET from J.T.
Helmbach, Technical Assessment Systems, Inc., Washington, D.C.
NAS. 1973. A comprehensive Survey on the Use of Food Chemicals
Generally Recognized as Safe, National Technical Information Service
(NTIS) PB-221949.
NAS. 1976. Estimating Distribution of Daily Intakes of Certain GRAS
Substances. National Research Council, Washington, DC. Prepared for:
Food and Drug Administration, Washington, DC. December 1976. National
Technical Information Service (NTIS) PB-299-381.
NAS. 1978. Resurvey of the Annual Poundage of Food Chemicals Generally
Recognized as Safe, National Technical Information Service (NTIS)
PB288-081.
NAS. 1979. Comprehensive Survey of Industry on the Use of Food
Additives, National Technical Information Service (NTIS) PB80-113418.
NAS. 1984. Poundage Update of Food Chemicals, National Technical
Information Service (NTIS) PB84-162148.
NAS. 1989. 1987 Poundage and Technical Effects Update of Substances
Added to Food. National Research Council, Washington, DC. Prepared
for: Food and Drug Administration, Washington, D.C. NTIS Report
No. PB91-127266.
Oser, B.L. and Ford, R.A. 1973a. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 6. GRAS
substances. Food Technol 27(1):64-67.
Oser, B.L. and Ford, R.A. 1973b. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 7. GRAS
substances. Food Technol 27(11):56-57.
Oser, B.L. and Ford, R.A. 1974. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 8. GRAS
substances. Food Technol 28(9):76-80.
Oser, B.L. and Ford, R.A. 1975. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 9. GRAS
substances. Food Technol 29(8):70-72.
Oser, B.L. and Ford, R.A. 1977. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 10. GRAS
substances. Food Technol 31(1):65-74.
Oser, B.L. and Ford, R.A. 1978. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 11. GRAS
substances. Food Technol 32(2):60-70.
Oser, B.L. and Ford, R.A. 1979. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 12. GRAS
substances. Food Technol 33(7):65-73.
Oser, B.L. and Hall, R.L. 1972. Recent progress in the consideration
of flavoring Ingredients under the Food Additives Amendment 5. GRAS
substances. Food Technol 26(5):35-42.
Oser, B.L. and Hall, R.L. 1977. Criteria employed by the Expert Panel
of FEMA for the GRAS evaluation of flavoring substances. Food Cosmet
Toxicol 15:457-466.
Oser, B.L., Ford, R.A. and Bernard, B.K. 1984. Recent Progress in the
consideration of flavoring ingredients under the Food Additives
Amendment 13. GRAS substances. Food Technol 38(10):66-89.
Oser, B.L., Well, C.S., Woods, L.A. and Bernard, B.K. 1985. Recent
progress in the consideration of flavoring ingredients under the Food
Additives Amendment 14. GRAS substances. Food Technol 39(11):108-117.
Rulis, A.M., Hattan, D.G., and Morgenroth, V.H.,III. 1984. FDA's
priority-based assessment of food additives. I. Preliminary results.
Reg Toxicol Pharm 4:37-56.
Rulis, A.M. 1987. Safety assurance margins for food additives
currently in use. Reg Toxicol Pharm 7:160-168.
Smith, R.L. and Ford, R.A. 1993. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 16. GRAS
substances. Food Technol-June 1993, pp. 104-117.
ANNEX 5 Appendix B
SUBSTANCES IN REFERENCE DATABASE BY ORDER OF STRUCTURAL CLASS
Cramer et al. (1978) Structural Class I
1 acetic acid
2 acetoin
3 acetone
4 adipic acid
5 allura red AC
6 aminoundecanoic acid, 11-
7 ascorbic acid
8 ascorbic acid, 1-
9 benzaldehyde
10 benzoic acid
11 benzyl acetate
12 benzyl alcohol
13 bis(2-ethylhexyl)phthalate
14 brilliant black BN
15 butanediol, 1,3-
16 butanol, 2-
17 butanol, n-
18 butyl benzyl phthalate
19 butylated hydroxyanisole
20 butyrolactone, gamma-
21 calcium cyclamate
22 calcium formate
23 calcium stearoyl lactylate
24 carotenoic acid, beta-apo-8'-, methyl ester
25 cinnamaldehyde
26 citral
27 citranaxanthin
28 cumene
29 di(2-ethylhexyl)adipate
30 dibutyl phthalate
31 diethyl phthalate
32 diethylene glycol
33 diethylene glycol monoethyl ether
34 dimethoxane
35 dimethyl terephthalate
36 dimethylcarbonate
37 dimethyldicarbonate
38 dimethylphenol, 2,4-
39 dimethylphenol, 2,6-
40 dimethylphenol, 3,4-
41 dioctyl sodium sulfosuccinate
42 disodium 5'-guanylate
43 disodium 5'-inosinate
44 dodecyl gallate
45 ethanol
46 ethyl acetate
47 ethyl acrylate
48 ethyl butyrate
49 ethyl ether
50 ethyl formate
51 ethyl glycol monomethyl ether
52 ethyl heptanoate
53 ethyl nonanoate
54 ethyl-1-hexanol, 2-
55 ethylbenzene
56 ethylbutyric acid, 2-
57 ethylene glycol
58 ethylhexanoic acid, 2-
59 ethylphthalyl ethylglycolate
60 eugenol
61 FD & C blue No. 1
62 FD & C blue no. 2
63 formaldehyde
64 fumaric acid
65 geranyl acetate
66 glutamate, monosodium
67 glutamic acid hydrochloride
68 glutamic acid, 1-
69 glycerol
70 glyceryl tribenzoate
71 hexenal, 2- (trans)
72 hexylresorcinol, 4-
73 hydroquinone
74 hydroxybenzoic acid butyl ester, p-
75 hydroxybenzoic acid ethyl ester, p-
76 hydroxybenzoic acid methyl ester, p-
77 hydroxybenzoic acid propyl ester, p-
78 hydroxybenzyl acetone, p-
79 inosine monophosphate
80 ionone
81 isoamyl butyrate
82 isoamyl salicylate
83 isobutyl alcohol
84 isomaltitol
85 isopropyl alcohol
86 isovaleric acid
87 lactitol
88 limonene, d-
89 lithocholic acid
90 malonaldehyde, sodium salt
91 mannitol
92 mannitol, d-
93 menthol
94 methanol
95 methyl ethyl carbonate
96 methyl methacrylate
97 methyl salicylate
98 methyl-1-phenylpentan-2-ol, 4-
99 methylenebis, 2,2'-
100 methylphenol, 3-
101 methylphenylcarbinyl acetate
102 myrcene, beta-
103 nonalactone, gamma-
104 octyl acetate
105 octyl gallate
106 oleylamine
107 oxalic acid
108 phenol
109 phenoxyethanol
110 phenyl-1-propanol, 2-
111 phenylalanine
112 potassium sorbate
113 propyl gallate
114 propylene glycol
115 resorcinol
116 retinol
117 riboflavin
118 sodium benzoate
119 sodium erythorbate
120 sodium lauryl glyceryl ether sulfonate
121 sodium lauryl trioxyethylene sulfate
122 sodium stearoyl lactylate
123 sorbic acid
124 stearyl tartrate
125 styrene
126 sucrose monopalmitate
127 sucrose monostearate
128 tartaric acid
129 tertiary butyl hydroquinone
130 tocopherol, alpha-
131 tolualdehyde
132 toluene
133 triethylene glycol
134 triethylene glycol monomethyl ether
135 trimethylamine
136 undecalactone, gamma-
137 vanillin
138 xylitol
Cramer et al. (1978) Structural Class II
1 acrylic acid
2 allyl alcohol
3 allyl heptanoate
4 allyl hexanoate
5 butylated hydroxytoluene
6 caffeine
7 carotene, beta-
8 carvone
9 carvone, d-
10 diacetyl
11 diallyl phthalate
12 diketopiperazine
13 ethyl maltol
14 ethyl vanillin
15 ethylhexyl phthalate, mono-2
16 etretinate
17 fenthion
18 furfural
19 isobornyl acetate
20 isophorone
21 maltol
22 methyl amyl ketone
23 methyl anthranilate
24 piperidine
25 piperonal
26 propargyl alcohol
27 pyridine
28 thujone
Cramer et al, (1978) Structural Class III
1 (1-naphthyl)ethylene--diamine dihydro chloride, N-
2 (2-chloroethyl)trimethyl-ammonium chloride
3 (8-beta)N-cyclohexyl-6-methyl- 1 -(1 -methyl
ethyl)ergoline-8 -carboxamide
4 (chloroacetyl)-acetanilide, 4'-
5 1,1'-(2,2,2-trichloroethylidene)bis(4-chloro)-benzene
6 11-oxo-11H-pyrido(2,1-b)quinazoline-2-carboxylic acid
7 2(2,4,5-trichlorophenoxy) propionic acid
8 2-(2-methyl--4-chlorophenoxy)propionic acid
9 4-(2-methyl-4-chlorophenoxy) butyric acid
10 acenaphthene
11 acephate
12 acesulfame potassium
13 acetaminophen
14 acetoacetamide
15 acetoacetamide-N-sulfonic acid
16 acetochlor
17 acetonitrile
18 acifluorin sodium
19 acrylamide
20 acrylonitrile
21 alachlor
22 albendazole
23 albendazole sulfoxide
24 aldicarb
25 aldicarb sulfone
26 alinidine hydrobromide
27 allyl isovalerate
28 amaranth
29 ameltolide
30 ametryn
31 amino-4-ethoxy-acetanilide, 3-
32 amino-4-nitrophenol, 2-
33 amino-5-nitrophenol, 2-
34 aminophenol, p-
35 amitraz
36 ammonium carmine
37 amphetamine sulfate, dl-
38 ampicillin trihydrate
39 anethole, trans-
40 anilazine
41 anisidine hydrochloride, p-
42 anthranilic acid
43 arochlor 1254
44 aspartame
45 asulam
46 atrazine
47 avermectin B1
48 azaperone
49 azinphos methyl
50 azorubine
51 azuletil (KT1-32)
52 baythroid
53 benomyl
54 bentazon
55 benzofuran
56 benzoic acid, 2-[[[[N-(4-methoxy-6-methyl
-1,2,3-triazin-2-yl)-N-methylamino]carbonyl]
amino]sulfonyl] methyl ester
57 benzoin
58 benzyl violet 4B
59 benzyl-p-chlorophenol, o-
60 betaxolol
61 bidrin
62 biphenthrin
63 biphenyl, 1,1-
64 biphenylamine hydrochloride, 2-
65 bis(2-chloro-1-methyl-ethyl)ether Technical Grade
66 bis(2-chloroisopropyl)ether
67 bisphenol A
68 bromoacetic acid, 2-
69 bromodichloromethane
70 bromomethane
71 bromoxynil
72 bromoxynil octanoate
73 brown FK
74 butyl chloride, n-
75 butylate
76 calcium cyanamide
77 canthaxanthin
78 caprolactam
79 captafol
80 captan
81 carazolol
82 carbaryl
83 carbendazim
84 carbofuran
85 carbon tetrachloride
86 carbosulfan
87 carboxin
88 carmoisine
89 chloramben
90 chlordane
91 chlorendic acid
92 chlorimuron-ethyl
93 chloro-p-toluidine, 3-
94 chloroacetic acid
95 chloroaniline hydrochloride, p-
96 chlorobenzene
97 chlorobenzilate
98 chlorodibromomethane
99 chloroform
100 chlorofructose, 6-
101 chloronaphthalene, beta-
102 chlorophenol, 2-
103 chloropropylate
104 chlorothalonil
105 chlorotoluene, o-
106 chlorpheniramine maleate
107 chlorpromazine
108 chlorsulfuron
109 chlorthal dimethyl
110 chocolate brown HT
111 C.I. Acid Orange 12
112 C.I. Acid Orange 3
113 C.I. Acid Red 18
114 C.I. Disperse Blue 1
115 C.I. acid red 14
116 C.I. disperse yellow 3
117 C.I. pigment red 23
118 C.I. solvent yellow 14
119 cimetidine
120 clofentezine
121 clonitralid
122 closantel
123 codeine
124 coumaphos
125 coumarin
126 crufomate
127 curcumin
128 cyclodextrin, beta-
129 cyclohexylamine
130 cyclophosphamide
131 cyhalothrin
132 cypermethrin
133 cyromazine
134 daminozide
135 decabromodiphenyl oxide
136 deoxyquinine
137 diamino-2,2'-stilbenedisulfonic acid, 4,4'-, disodium sa
138 diaminophenol dihydrochloride, 2,4-
139 diazinon
140 dibenzo-p-dioxin
141 dibromobenzene, 1,4-
142 dicamba
143 dichloro-2-propanol, 1,3-
144 dichloro-p-phenylenediamine, 2,6-
145 dichlorobenzene, 1,2-
146 dichlorobenzene, 1,4-
147 dichlorobenzilic acid
148 dichlorodifluoromethane
149 dichloroethane, 1,1-
150 dichloroethane, 1,2-
151 dichloroethylene, 1,1-
152 dichloroethylene, trans- 1,2-
153 dichloromethane
154 dichlorophenol, 2,4-
155 dichlorophenoxyacetic acid, 2,4-
156 dichloropropanol, 2,3-
157 dichloropropene, 1,3-
158 dichlorvos
159 dicofol
160 dieldrin
161 diethyldithiocarbamate
162 diethylthiourea, N,N'-
163 difenzoquat
164 diflubenzuron
165 dihydroavermectin-B1 a, 22,23-
166 dihydroavermectin-B1 b, 22,23-
167 dihydrocoumarin, 3,4-
168 diiodomethyl p-tolyl sulfone
169 dimethipin
170 dimethoate
171 dimethoxyaniline hydrochloride, 2,4-
172 dimethoxybenzidine-4,4'-diisocyanate, 3,3'-
173 dimethyl hydrogen phosphite
174 dimethyl methylphosphonate
175 dimethyl morpholinophosphoramidate
176 dimethylaniline, N,N'-
177 dinitrobenzene, m-
178 dinitrotoluene, 2,4-
179 dinocap
180 dinoseb
181 diphenamid
182 diphenhydramine hydrochloride
183 diphenylamine
184 diphenylhydantoin, 5,5'-
185 diphenylhydantoin, 5,5-
186 diquat
187 disulfoton
188 dithiobiurea, 2,5-
189 diuron
190 dodecylguanidine acetate, n-
191 EDTA, disodium
192 endothall
193 ephedrine sulfate
194 epichlorohydrin
195 erythromycin stearate
196 erythrosine
197 ethalfuralin
198 ethephon
199 ethion
200 ethyl dipropylthiocarbamate, s-
201 ethyl p-nitrophenyl phenylphosphorothioate
202 ethylene chlorohydrin
203 ethylene thiourea
204 ethylmethylphenylglycidate
205 fast green FCF
206 febantel
207 fenamiphos
208 fenbendazole
209 fenchlorphos
210 fluometuron
211 fluoranthene
212 fluorene
213 fluridone
214 flurprimidol
215 flutolanil
216 fluvalinate
217 folpet
218 fonofos
219 fosetyl-al
220 glufosinate-ammonium
221 glyphosate
222 haloxyfop-methyl
223 HC blue no. 1
224 HC blue no. 2
225 HC yellow 4
226 heptachlor
227 heplachlor epoxide
228 hexabromobenzene
229 hexachlorobenzene
230 hexachlorobutadiene
231 hexachlorocyclohexane, gamma-
232 hexachlorocyclopentadiene
233 hexachloroethane
234 hexachlorophene
235 hexahydro-1,3,5-trinitro-1,3,5-triazine
236 hexamethylenetetramine
237 hexazinone
238 hexythiazox
239 hydralazine
240 hydrazobenzene
241 hydrochlorothiazide
242 hydroxypropyl methanethiolsulfonate, 2-
243 hydroxyquinoline, 8-
244 imazalil
245 imazaquin
246 imazethapyr
247 iodinated glycerol
248 ipazilide
249 iprodione
250 ipronidazole
251 isopropalin
252 isoxaben
253 jervine
254 lactofen
255 lansoprazole
256 levamisole
257 linamarin
258 linuron
259 londax
260 malaoxon
261 malathion
262 maleic anhydride
263 maleic hydrazine
264 manidipine hydrochloride
265 melamine
266 mepiquat chloride
267 mercaptobenzothiazole, 2-
268 merphos
269 merphos oxide
270 meso-2,3-dimercaptosuccinic acid
271 metalaxyl
272 methamidophos
273 methidathion
274 methomyl
275 methoxychlor
276 methoxypsoralen, 8-
277 methyl carbamate
278 methyl ethyl ketoxime
279 methyl parathion
280 methyl-4-chlorophenoxyacetic acid, 2-
281 methyl-N-methylanthranilate
282 methyldopa sesquthydrate, alpha-
283 methylolacrylamide, N-
284 metolachlor
285 metribuzin
286 metsulfuron methyl
287 mexacarbate
288 miporamicin
289 mirex
290 molinate
291 monodiethanolamine salt of riboflavin-5'-phosphate
292 monuron
293 myclobutanil
294 naled
295 nalidixic acid
296 naphthoxy acetic acid, 2-
297 napropamide
298 natamycin
299 nitro-o-toluidine, 5-
300 nitro-p-phenylenediamine, 2-
301 nitroaniline, p-
302 nitroanthranilic acid, 4-
303 nitrofurantoin
304 nitrofurazone
305 nitroguanidine
306 nitronaphthalene, 1-
307 nitrosodiphenylamine, N-
308 norflurazon
309 ochratoxin A
310 octabromodiphenyl ether
311 octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine
312 olaquindox
313 oryzalin
314 oxadiazon
315 oxamyl
316 oxfendazole
317 oxyfluorfen
318 oxytetracycline
319 oxytetracycline hydrochloride
320 oxythioquinox
321 paclobutrazol
322 paraquat
323 parathion
324 patulin
325 pendimethalin
326 penicillin VK
327 pentabromodiphenyl ether
328 pentachloroethane
329 pentachloronitrobenzene
330 pentachlorophenol
331 pentaerythritol tetranitrate
332 permethrin
333 phenformin
334 phenyl-2-naphthylamine, n-
335 phenyl-3-methyl-5-pyrazolone, 1-
336 phenylbutazone
337 phenylenediamine, m-
338 phenylephrine hydrochloride
339 phosmet
340 phosphamidon
341 photodieldrin
342 phthalamide
343 phthalic anhydride
344 picloram
345 pilsicainide hydrochloride
346 pirimiphos-methyl
347 pravadoline
348 probenecid
349 prochloraz
350 proflavine monohydrochloride hemihydrate
351 promethazine hydrochloride
352 prometon
353 prometryn
354 pronamide
355 propachlor
356 propanil
357 propargite
358 propazine
359 propham
360 propiconazole
361 propiverine hydrochloride (P-4)
362 propoxur
363 propylene dichloride
364 pydrin
365 pyrazinamide
366 pyrene
367 pyrimidinone
368 quercetin
369 quinalphos
370 quinine hydrochloride
371 quinofop ethyl
372 red 2G
373 reserpine
374 resmethrin
375 thudamine 6G
376 ronidazole
377 rotenone
378 saccharin
379 sethoxydim
380 simazine
381 sodium 2,2-dichloropropionate
382 sodium cyclamate
383 sodium fluoroacetate
384 sodium naphthionate
385 sorbitan monolaurate
386 sorbitan monostearate
387 succinic anhydride
388 sucrose acetate isobutyrate
389 sulfadimidine
390 sulfisuxazole
391 sulfolene, 3-
392 sunset yellow FCF
393 suplatast tosilate
394 tebuthiuron
395 terbacil
396 terbutryn
397 tert-butyl-2-chlorophenol, 4-
398 tetrachlorobenzene, 1,2,4,5-
399 tetrachloroethane, 1,1,1,2-
400 tetrachloroethane, 1,1,2,2-
401 tetrachloroethylene
402 tetrachlorophenol, 2,3,4,6-
403 tetrachloropyridine, 2,3,5,6-
404 tetrachlorvinphos
405 tetracycline hydrochloride
406 tetraethyldithiopyrophosphate
407 tetraethylthiourea disulfide
408 tetrahydrocannabinol, delta-9-
409 tetrakis(hydroxymethyl)phosphonium chloride (THPC)
410 tetrakis(hydroxymethyl)phosphonium sulphate (THPS)
411 thiabendazole
412 thiameturon methyl
413 thiobencarb
414 thiophanate-methyl
415 thiram
416 tocopheryl acetate, dl-alpha-
417 toluenediamine hydrochloride, 2,6-
418 toluenediamine sulfate, 2,5-
419 tralomethrin
420 trenbolone acetate
421 trenbolone hydroxide, 17-alpha-
422 triadimefon
423 triallate
424 triamterene
425 tribromomethane
426 trichloroacetonitrile
427 trichlorobenzene, 1,2,4-
428 trichloroethane, 1,1,2-
429 trichloroethane, 1,1,1-
430 trichloroethylene
431 trichloroethylene, 1,1,2-
432 trichlorogalactosucrose
433 trichlorophenol, 2,4,5-
434 trichlorophenoxyacetic acid, 2,4,5-
435 tridiphane
436 triethylene tetramine dihydrochloride
437 trifluralin
438 trimethylaniline, 2,4,5-
439 trinitrotoluene, 2,4,6-
440 tris-(2-chloroethyl) phosphate
441 trisulfuron
442 tylosin
443 vinclozolin
444 vinyl chloride
445 zatosetron maleate
446 zearalenone
447 zeranol
The principal objective of the safety evaluation procedure is to
identify two groups of flavourings substances: i) those substances
whose structure, metabolism, and relevant toxicity data clearly
indicate that the substance would be expected not to be a safety
concern under current conditions of intended use; and ii) those
substances which may require additional data in order to perform an
adequate safety evaluation.
3.4 Integrating data on the consumption ratio
As pointed out by WHO (1987) and SCF (1991), natural occurrence
is no guarantee of safety, but it is important to recognize that the
safety evaluation of added use of flavouring substances needs to be
conducted with an appreciation of the consumption ratio. Clearly, if
added use of flavouring substances results in an exposure that exceeds
that from natural sources, this will increase awareness of the need to
consider carefully overall exposure in the light of existing data on
toxicity and structure-activity relationships. On the other hand, if
the added use is trivial with a consumption ratio of 10 to 100, that
is, it increases total exposure by only 1 to 10%, then this fact needs
to be taken into consideration when applying the criteria outlined
above.
The substances of primary concern are those which, in Figure 2,
receive a "No" answer to Step No. 5, or a "Yes" answer to Step No. 7,
indicating a possible need for additional data and evaluation beyond
that included in the evaluation procedure outlined in this paper. In
such an evaluation, as stated immediately above, the extent of natural
occurrence should be given appropriate weight.
Of much less concern with respect to consumption ratio are those
substances that drop out of further consideration as a result of "No"
answers to Step Nos. 3 or 7, or "Yes" answers to Step Nos. 4 or 6. The
derivation of the thresholds, the estimation of intakes (see Appendix
A), and the special factors applicable to the use of flavours
(volatility, self-limiting use, etc.) build in multiple conservative
assumptions more than adequate to cover additional exposure from
natural occurrence to substances that in any case are of low inherent
concern.
The advances in analytical chemistry in the past 50 years provide
virtual assurance that no flavouring substances of extensive natural
occurrence remain unknown. Those of potential value yet to be
discovered (e.g., as yet unknown components of roast beef, coffee, or
chocolate flavour) are being sought at the ppb and ppt level. This
does not suggest exposures above any of the thresholds discussed in
this paper.
4. CONCLUSIONS
It is neither possible nor necessary to conduct toxicological
studies on all individual flavouring substances used in food. The vast
majority of flavouring substances are members of groups of substances
with common metabolic pathways, and typically, individual members of
such a group display a similar toxicity profile. This is not
surprising in light of the close structural similarity of the various
flavouring substances comprising a chemical group. As demonstrated in
this paper, exposure to flavouring substances is usually low and, in
the majority of cases, below the human exposure threshold values
presented in this paper. This knowledge, coupled with knowledge
regarding structure-activity relationships, metabolic disposition, and
toxicology data, permits the establishment of the proposed paradigm
for safety assessment which provides a solid foundation to assure the
safety of flavouring substances under conditions of intended use.
5. REFERENCES
BECK, B.D., CONOLLY, R.B., DOURSON, M.L., GUTH, D., HATTIS, D.,
KIMMEL, C. & LEWIS, C. (1993). Symposium overview. Improvements in
quantitative noncancer risk assessment. Fund Appl Toxicol.,
20: 1-14.
COUNCIL OF EUROPE (1974). Natural Flavouring Substances, Their
Sources, And Added Artificial Flavouring Substances. Council of
Europe, Strasbourg, France.
COUNCIL OF EUROPE ( 1981 ). Flavouring Substances And Natural Sources
Of Flavourings, Third Edition. Council of Europe, Strasbourg, France.
COUNCIL OF EUROPE (1992). Flavouring Substances and Natural Sources
of Flavourings. Volume I. Chemically-Defined Flavouring Substances,
Fourth Edition. Council of Europe, Maissoneuve, France.
CRAMER, G.M., FORD, R.A. & HALL, R.L. (1978). Estimation of toxic
hazard-A decision tree approach. Food Cosmet Toxicol., 16: 255-276.
DEISINGER, P.J., BOATMAN, R.J. & GUEST, D. (1994). Metabolism of
2-ethylhexanol administered orally and dermally to the female Fischer
344 rat. Xenobiotica, 24: 429-440.
FOOD AND DRUG ADMINISTRATION (1982). Toxicological Principles for the
Safety Assessment of Direct Food Additives and Color Additives Used in
Food. Redbook. U.S. Food and Drug Administration, Bureau of Foods,
Washington, DC.
FOOD AND DRUG ADMINISTRATION (1993). Toxicological Principles for the
Safety Assessment of Direct Food Additives and Color Additives Used in
Food. Redbook II (Draft). U.S. Food and Drug Administration, Center
for Food Safety and Applied Nutrition, Washington, DC.
FEDERAL REGISTER (1993). Food additives; threshold of regulation for
substances used in food-contact articles. 58(195): 52719-52729.
FRAWLEY, J.P. (1967). Scientific evidence and common sense as a basis
for food-packaging regulations. Food Cosmet Toxicol., 5: 293-308.
GOLD, L.S., SAWYER, C.B., MAGAW, R., BACKMAN, G.M., DE VECIANA, M.,
LEVINSON, R., HOOPER, N.K., HAVENDER, W.R., BERNSTEIN, L., PETO, R.,
PIKE, M. & AMES, B.M. (1984). A carcinogenic potency database of the
standardized results of animal bioassays. Environ Health Perspect.,
58: 9-319.
GOLD, L.S., SLONE, T.H. & BERNSTEIN, L. (1989). Summary of
carcinogenic potency and positivity for 492 rodent carcinogens in the
carcinogenic potency database. Environ. Health Perspect., 79: 259-272.
JAESCHKE, H., KLEINWAECHTER, C. & WENDEL, A. (1987). The role of
acrolein in allyl alcohol-induced lipid peroxidation and liver cell
damage in mice. Biochem. Pharmacol., 36: 51-57.
JECFA (1967). Toxicological Evaluation of Some Flavouring Substances
and Non-Nutritive Sweetening Agents. Eleventh Meeting of the Joint
FAO/WHO Expert Committee on Food Additives.
JECFA (1968). Specifications for the Identity and Purity of Food
Additives and their Toxicological Evaluation: Some Flavouring
Substances and Non-nutritive Sweetening Agents. Eleventh Report of the
Joint FAO/WHO Expert Committee on Food Additives, FAO Nutrition
Meetings Report Series No. 44, WHO Technical Report Series No. 383.
JECFA (1970). Toxicological Evaluation of Some Extraction Solvents and
Certain Other Substances. Fourteenth Meeting of the Joint FAO/WHO
Expert Committee on Food Additives.
JECFA (1971). Evaluation of Food Additives. Fourteenth Report of the
Joint FAO/WHO Expert Committee on Food Additives, WHO Technical Report
Series No. 462.
JECFA (1972). Evaluation of Food Additives. Some Enzymes, Modified
Starches, and Certain Other Substances: Toxicological Evaluations and
Specifications and a Review of the Technological Efficacy of Some
Antioxidants. Fifteenth Report of the Joint FAO/WHO Expert Committee
on Food Additives, WHO Technical Report Series No. 488.
JECFA (1974a). Toxicological Evaluation of Certain Food Additives
Including Anticaking Agents, Antimicrobials, Antioxidants, Emulsifiers
and Thickening Agents. Seventeenth Meeting of Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 5.
JECFA (1974b). Toxicological Evaluation of Certain Food Additives with
a Review of General Principles and of Specifications. Seventeenth
Report of the Joint FAO/WHO Expert Committee on Food Additives, WHO
Technical Report Series No. 539.
JECFA (1976a). Evaluation of Certain Food Additives. Twentieth Report
of the Joint FAO/WHO Expert Committee on Food Additives, FAO Nutrition
Meetings Report Series No. 1, WHO Technical Report Series No. 599.
JECFA (1976b). Toxicological Evaluation of Certain Food Additives.
Twentieth Meeting of the Joint FAO/WHO Expert Committee on Food
Additives, WHO Food Additive Series No. 10.
JECFA (1978). Evaluation of Certain Food Additives and Contaminants.
Twenty-second Report of the Joint FAO/WHO Expert Committee on Food
Additives, WHO Technical Report Series No. 631.
JECFA (1980a). Evaluation of Certain Food Additives. Twenty-third
Report of the Joint FAO/WHO Expert Committee on Food Additives. World
Health Organization, Geneva. WHO Technical Report Series No. 648.
JECFA (1980b). Evaluation of Certain Food Additives. Twenty-fourth
Report of the Joint FAO/WHO Expert Committee on Food Additives. World
Health Organization, Geneva. WHO Technical Report Series No. 653.
JECFA (1980c). Toxicological Evaluation of Certain Food Additives.
Twenty-third Meeting of the Joint FAO/WHO Expert Committee on Food
Additives, WHO Food Additive Series No. 14.
JECFA (1981a). Evaluation of Certain Food Additives. Twenty-fifth
Report of the Joint FAO/WHO Expert Committee on Food Additives,
Technical Report Series No. 669.
JECFA (1981b). Toxicological Evaluation of Certain Food Additives.
Twenty-fifth Meeting of the Joint FAO/WHO Expert Committee on Food
Additives, WHO Food Additive Series No. 16.
JECFA (1982a). Evaluation of Certain Food Additives and Contaminants.
Twenty-sixth Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 683.
JECFA (1982b). Toxicological Evaluation of Certain Food Additives.
Twenty-sixth Meeting of the Joint FAO/WHO Expert Committee on Food
Additives, WHO Food Additive Series No. 17.
JECFA (1983a). Evaluation of Certain Food Additives and Contaminants.
Twenty-seventh Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 696.
JECFA (1983b). Toxicological Evaluation of Certain Food Additives and
Contaminants. Twenty-seventh Meeting of the Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 18.
JECFA (1984a). Evaluation of Certain Food Additives and Contaminants.
Twenty-eighth Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 710
JECFA (1984b). Toxicological Evaluation of Certain Food Additives and
Contaminants. Twenty-eighth Meeting of the Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 19.
JECFA (1986). Evaluation of Certain Food Additives and Contaminants.
Twenty-ninth Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 733.
JECFA (1987a). Evaluation of Certain Food Additives and Contaminants.
Thirtieth Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization. Geneva. WHO Technical Report
Series No. 751.
JECFA (1987b). Evaluation of Certain Food Additives and Contaminants.
Thirty-first Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 759.
JECFA (1989). Evaluation of Certain Food Additives and Contaminants.
Thirty-third Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 776.
JECFA (1990a). Evaluation of Certain Food Additives and Contaminants.
Thirty-fifth Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 789.
JECFA (1990b). Toxicological Evaluation of Certain Food Additives and
Contaminants. Thirty-fifth Meeting of the Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 26.
JECFA (1991a). Evaluation of Certain Food Additives and Contaminants.
Thirty-seventh Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 806.
JECFA (1991b). Toxicological Evaluation of Certain Food Additives and
Contaminants. Thirty-seventh Meeting of the Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 28.
JECFA (1992). Evaluation of Certain Food Additives and Naturally
Occurring Contaminants. Thirty-ninth Report of the Joint FAO/WHO
Expert Committee on Food Additives. World Health Organization, Geneva.
WHO Technical Report Series No. 828.
JECFA (1993a). Evaluation of Certain Food Additives and Contaminants.
Forty-first Report of the Joint FAO/WHO Expert Committee on Food
Additives, Technical Report Series No. 837.
JECFA (1993b). Toxicological Evaluation of Certain Food Additives and
Contaminants. Forty-first Meeting of the Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 32.
JECFA (1993c). Toxicological Evaluation of Certain Food Additives and
Naturally Occurring Toxicants. Thirty-Ninth Meeting of the Joint
FAO/WHO Expert Committee on Food Additives, WHO Food Additive Series
No. 30.
KRASAVAGE, W.J., O'DONOGHUE, J.L., DIVINCENZO, G.D. & TERHAAR, C.J.
(1980). The relative neurotoxicity of methyl-n-butyl ketone, n-hexane
and their metabolites. Toxicol. Appl. Pharmacol., 52: 433-441.
LEWIS, S.C., LYNCH, J.R. & NIKIFOROV, A.I. (1990). A new approach to
deriving community exposure guidelines from "no-observed-adverse-
effect levels". Regul. Toxicol. Pharmacol., 11: 314-330.
MUNRO, I.C. (1990). Safety assessment procedures for indirect food
additives: An overview. Regul. Toxicol. Pharmacol., 12: 001-0011.
NAS (1978). Resurvey of the Annual Poundage of Food Chemicals
Generally Recognized as Safe, National Technical Information Service
(NTIS) PB288-081.
NAS (1979). Comprehensive Survey of Industry on the Use of Food
Additives, National Technical Information Service (NTIS) PB80-113418.
NAS (1984). Poundage Update of Food Chemicals, National Technical
Information Service (NTIS) PB84-162148.
NAS (1989). 1987 Poundage and Technical Effects Update of Substances
Added to Food. National Research Council, Washington, DC. Prepared
for: Food and Drug Administration, Washington, D.C. NTIS Report
No. PB91-127266.
PHILLIPS, J.C., PURCHASE, R., WATTS, P. & GANGOLLI, S.D. (1987). An
evaluation of the decision tree approach for assessing priorities for
safety testing of food additives. Food Addit. Contam., 4(2): 109-123.
RULIS, A.M. (1986). De Minimis and the Threshold of Regulation.
In: Felix, C.W. (Ed.) Food Protection Technology. Lewis Publishers
Inc., Chelsea, MI. pp. 29-37.
SCF (1991). Guidelines for the Evaluation of Flavourings for Use in
Foodstuffs: I. Chemically Defined Flavouring Substances. Commission of
the European Communities, Scientific Committee for Food, Brussels,
Belgium.
STOFBERG, J. & KIRSCHMAN, J. (1985). The consumption ratio of
flavouring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23: 857-860.
STOFBERG, J. & GRUNDSCHOBER, F. (1987). Consumption ratio and food
predominance of flavouring materials. Perfumer Flavourist,
12: 27-68.
WEIL, C.S. & McCOLLISTER, D.D. 1963. Safety evaluation of chemicals.
Relationship between short- and long-term feeding studies in designing
an effective toxicity test. Agr. Food Chem., 11(6): 486-491.
WHO (1987). Principles For The Safety Assessment Of Food Additives
And Contaminants In Food. WHO Environmental Health Criteria. World
Health Organization (WHO), International Program on Chemical Safety in
Cooperation with the Joint FAO (Food & Agriculture Organization of the
United Nations), WHO Expert Committee on Food Additives (JECFA),
Geneva, Switzerland, Environmental Health Criteria 70.
WOODS, L.A. & DOULL, J. (1991). GRAS evaluation of flavouring
substances by the Expert Panel of FEMA. Regul. Toxicol. Pharmacol.,
14: 48-58.
ANNEX 5, Appendix A
ESTIMATING DISTRIBUTION OF INTAKES OF FOOD INGREDIENTS ACROSS
THE POPULATION
In response to the Food Additives Amendment of 1958 to the U.S.
Food, Drug and Cosmetic Act, the Flavor and Extract Manufacturers'
Association (FEMA) in 1960 conducted a comprehensive survey of all
flavoring materials then thought or claimed to be in use. Information
included: identity; poundage used annually in food; year of first use;
levels (in ppm) used in major food categories; toxicological data; and
other information related to safety. After evaluation by a panel of
external experts, this and subsequent information was published in a
series of "FEMA GRAS Lists" (Hall and Oser, 1965, 1970; Oser and Hall,
1972; Oser and Ford, 1973ab, 1974, 1975, 1977, 1978, 1979; Oser et al.,
1984, 1985; Burdock et al., 1990; Smith and Ford, 1993).
FEMA determined in 1968 that another survey was needed to monitor
trends and to improve on the previous effort. In addition, the U.S.
Food and Drug Administration (FDA) began to plan to review its
Generally Recognized as Safe (GRAS) lists, and in so doing, made use
of the prior experience of FEMA. FEMA and FDA efforts merged into the
first comprehensive survey of ingredients used in food, conducted by
the National Academy of Sciences/National Research Council (NAS) in
1970 (NAS, 1973) with several subsequent surveys (NAS, 1978, 1979,
1984, 1989).
Emerging from the 1960 and 1970 surveys was a clear indication
that per capita intake of an ingredient (from annual poundage),
although useful for comparison with other ingredients, has a
significant limitation. As an average of intakes by those who consume
foods containing the ingredient ("eaters") and by those who do not
(non-eaters"), it does not represent the intake by the individuals of
concern ( i.e., the "eaters"). This is not the case for ingredients
commonly consumed by almost everyone ( e.g., sugar), but the
difference between the average and "eaters" is significant for other
ingredients consumed only by few people ( e.g. raspberry flavor).
Because of these issues, an alternative method of calculating
intakes was used in the first (1973) NAS report. This method involved
the average level of use of an ingredient in a broad food category
(e.g., baked goods) multiplied by the average daily consumption of
that food category per person. The daily consumption for each category
was obtained by multiplying the average portion size for the category,
obtained from the U.S. Department of Agriculture (USDA) Food
Consumption Survey by the average daily frequency of consumption of
foods in that category over a 14-day survey period. The frequency of
consumption data came from a 12,000+ member panel operated by the
Market Research Corporation of America (MRCA; see note b to Table 1).
Thus the average intake of an ingredient for all relevant food
categories provided the "possible average daily intake" (PADI). These
intakes were often highly exaggerated, as much as a hundred-fold,
because an ingredient used in only a few foods in a food category was
assumed to be at the average level in all foods in the category. Thus,
for example, cardamom, used only in a few coffee cakes, was assumed to
be used in each food in the entire category of baked goods ( i.e.,
all breads, rolls, crackers, and pastries).
To address this issue, another method was developed to give the
probable distribution of intakes for the entire population without
exaggerating intake. This required an informed estimate of the
probability that each food eaten would contain the ingredient of
interest. The first series of such intake estimates used calculation
"A", as shown in Table 1, and the results are summarized in Section
"A" of Table 2.
In Table 2, Column 1 ("Substance") lists the substances selected
to test and improve the more refined method. In Section A, nutmeg and
mace were chosen, among other reasons because they are not grown
domestically in the U.S. and import data, therefore, provide a firm
external check on pounds used. Furthermore, more than half of all
nutmeg is consumed in four foods: bologna, pork sausage, frankfurters,
and doughnuts. It thus presented an opportunity to see how heavy
consumers of those foods would skew total intake distribution. The
flavor ingredient 4-(p-hydroxyphenyl)butan-2-one was chosen because it
is used in, and occurs naturally, almost entirely in only one flavor,
raspberry flavor, and is far less widely consumed than chocolate,
vanilla, or citrus flavors. Thus, it is a good example of an uncommon
ingredient consumed by those who particularly like the few foods in
which it is used (Hall, 1975).
Column 2 [" per capita (External Check)"] lists per capita
intake derived from import data, product manufacturer surveys, or
tariff reports, all independent of the NAS survey to serve as an
external check. Column 3 (" per capita [NAS(lbs)/0.6]") lists the
per capita intake from the poundage reported in the NAS survey
assumed to be 60% complete. Further research (see references to NRC
surveys) showed this to be generally a valid assumption, but with
certain qualifications. Ingredients such as sugar, corn syrup, and
salt, could not be fully or reliably reported by bakers and other
small food processors, whereas the flavor industry reported more
fully, and the 60% assumption, while used, usually resulted in a
poundage per capita overestimate.
For Section A, Column 4 ["50th Percentile (Total Panel)"]
presents the total panel 50th percentile for each substance, and
Column 4a ("50th Percentile 3 S.D. Total Panel"), the 50th percentile
plus 3 standard deviations. Column 4b ["very high (Total Panel)"]
shows the so-called "very high intake". This is not a realistic
figure, because no person could consume all foods using an ingredient
at the highest level for every food in every major category. Note
particularly, also, the earlier discussion of the exaggeration that
arises from assuming that an ingredient used only in a few foods is
used in all foods in a major food category. The "very high intake"
serves simply as a wholly unreachable and therefore unrealistic value.
Given the remaining and inevitable uncertainties due to possible
errors in estimations, and possible non-food uses, the agreement
between the total panel 50th percentile (Section A, Column 4), the NRC
per capita, (Column 3), and the per capita from external sources
(Column 2), is entirely satisfactory. However, calculation "A" did
not provide data on only those who actually consumed the substance
("eaters only"), and the statistical treatment was not as solidly
based as was desirable. This led to an improved calculation, "B", as
shown in Table 3. Table 2, Section B, presents the results of these
calculations.
The substances shown in Column 1 of Section B were selected, as
discussed in the referenced reports themselves, by the FDA or the NAS
committee (NAS, 1976). An improved method of calculation was used, as
described below, which provides the total sample mean (Column 4 ["Mean
(Total Panel)"]) as a check against the per capita data in Columns 2
and 3.
The external per capita figure for calcium propionate is twice
the total panel mean, probably due primarily to usage by many small
bakeries not included in the survey, and also to the high wastage of
bread. The slightly smaller discrepancy for MSG may well reflect
several added sources of error. Even so, comparison of the total panel
mean with the per capita data from the NAS survey and the external
checks shows satisfactory agreement. Food consumption surveys have
significant and well-recognized sources of error in addition to those
affecting the NRC surveys, and it seems unlikely that it is practical
to expect intake estimates derived by different means to correspond
more closely than within a factor of two.
For Section B, Column 4 ["Mean (Total Panel)"] provides the total
panel mean, Column 4a ["Mean (Eaters Only)"] the "eaters only" mean
age, 2 and over and Column 4b ["S.D. (Eaters Only)"], the standard
deviation for "eaters only".
The procedure in calculation B does not eliminate all sources of
error. Intake is still calculated from "disappearance figures"
( i.e, the levels introduced into food during processing, not the
lower levels actually consumed). Thus, ignoring the often large losses
due, for example, to volatilization or leaching in processing,
preparation and storage result in exaggerated estimates of intake. In
the other direction, heavy (frequent) eaters will often eat larger
portion sizes, resulting in a tendency to underestimate higher
intakes. The result overall, however, remains highly satisfactory.
Table 1 Calculation "A"
Step Operation Result
For each specific food (SF)a e.g. bologna,
within a broad food category, eg., meats,
obtain from MRCAb the
1 total number of eatings of each SF by the total
panel, in two weeks
14 days × 12,337 (persons in panel) = mean frequency of eating, p/d
×
2 mean portion size in gc = mean consumption of SF, g/p/d
×
3 probability of SF containing ingredient "I"d = probable mean consumption of SF
containing I g/p/d
×
4 usual use level of "I" in ppm of major food = 50th percentile of probable intake of I
category containing SF ‰ 1,000e from SF in mg/p/d
×
5 90th percentile MRCA frequency of eating = 90th percentile of probable intake of I
50th percentile MRCA frequency of eating from SF in mg/p/d
6 & 7 sum 4 and 5 separately across all SFs in that = 50th and 90th percentiles of probable
major food category intake of I from that major food category
Table 1 (cont'd) Calculation "A"
Step Operation Result
8 fit a curve, assuming normal distribution, to = 50th percentile and standard deviation
the points determined in 6 and 7 and calculate (SD) for each major food category
9 repeat steps 1 through 8 and sum across all = 50th percentile and SD in mg/p/d
food categories probable total intake
10 repeat steps 4 and following, using "average = "very high intake"
maximum use level" from the survey for each
major food category
a Specific food (SF), in this context, denotes a narrow category of very similar foods serving
the same dietary and market niches (e.g. all bologna, all cola drinks). The eatings of each SF
include in one total for each SF all eatings of similar brands or home-prepared versions of the
same SF. There were approximately 10,000 such SFs.
b The Market Research Corporation of America (MRCA) is a private market research company that
surveys usage of food products and components It uses a nationally representative panel and
records for each panel member the number of servings of each specific loud for each day of a
two-week period. The panelists' reporting periods are distributed evenly throughout the year. The
1973 NAS report used data from the third Menu Census (1967-1968) involving 12,871 individuals.
The dataused in calculations "A" and "B" came from the Fourth Menu Census (1972-1973) involving
12,337 individuals.
c The USDA Food Consumption Survey (USDA, 1965)
d Obtained from a consensus panel of experts knowledgeable about the ingredient and the food.
e From NAS, 1973
Table 2 Distribution of MRCA 14-Day Average Daily Intakes (in mg/person/day)a of Selected Food Ingredients
(1) (2) (3) (4) (4a) (4b) (5)
"High Eaters
Section Substance per capita per capita Mean Mean plus "Very High" Only"
(External [NAS (lbs.)/0.6] (Total Panel) 3 S.D.b (Total Panel) [NAS
Check) Total Panel (lbs.)/0.6]×10
A Nutmeg 23.6 10.3 15.7 51.3 71.1 103
Nutmeg Oil 1.2 1.0 2.0 5.7 7.1 10
A Mace 3.3 2.8 3.3 8.6 14.4 28
Mace, Oil NAc 0.26 0.19 0.67 0.46 2.6
Mace, Oleoresin NA 0.070 0.13 0.39 0.41 0.70
A 4-p-Hydroxyphenyl 0.048 0.086 0.041 0.16 0.37 0.86
Butan-2-one
Table 2 (cont'd).
(1) (2) (3) (4) (4a) (4b) (5)
"High Eaters
Section Substance per capita per capita Mean Mean plus "Very High" Only"
(External [NAS (lbs.)/0.6] (Total Panel) 3 S.D.b (Total Panel) [NAS
Check) Total Panel (lbs.)/0.6]×10
Mean Mean S.D.
(Total Panel) (Eaters Only) (Eaters Only)
B Calcium Propionate 92 33 44.8 45.1 20.7 330
Sodium Propionate NA 16 33,2 33.3 14.7 160
B Carob Bean Gum NA 26 35.6 36.1 35.0 260
Chondrus Extract 19.3 13 10.6 11.1 18.5 130
B Mono- and diglycerides NA 727 884 885 467 7,270
Monosodium Glutamate 186 194 98.8 99.0 88.5 1,940
(MSG)
a See text for explanation of columns.
b S.D. = Standard Deviation.
Table 3 Calculation "B"
1 From the MRCA survey, obtain the total
number of eatings of each SF, by each panel
member, each day over the 14 - day period
×
2 USDA mean portion size, for that person's = quantity of SF in g for that
age group and the relevant major food person, that day
category
×
3 weighted mean of usual use level of I in = quantity of I in mg/d, that
ppm ‰ 1,000 person, that day, if all of
the SF contained I
×
4 probability that particular SF contains I = expected intake of I for
that person for that day,
mg/d
5 Repeat steps 1 through 4 for that person for = expected intake of I for
each of the 14 days that person for 14 days,
mg
6 ‰ 14 = average daily intake over
14 days for that person,
g/p/d
7 tabulate all intakes and compute = total panel mean intake of I
mg/p/d
8 discard all persons with zero intake for the = "eaters only" mean and SD
14 day period, tabulate all "eaters only" of for I, mg/p/d
I, age 2 and over, and compute
Unfortunately, this more refined approach, while successful, is
extremely elaborate, tedious, and expensive. The MRCA panel is
expensive to maintain and operate, and the data are correspondingly
costly. The approach requires extensive input from a broadly-based,
necessarily large and well-informed panel of expert technologists.
Such an effort can only be justified in those few cases where it is
necessary to obtain the distribution of intakes with maximum accuracy.
Given the wide safety margins food ingredients typically enjoy, and
the uncertainties and assumptions inherent in interpreting
toxicological data, such effort is rarely necessary. A much more
appropriate, but quick and easy, method of estimating intake was
needed, so long as it was conservative, but not excessively so. This
led Rulis and others in FDA (Rulis et al., 1984; Rulis, 1987) to
test and adopt the assumption that, except for an unusually narrow
margin of safety or specific concern, the per capita "eaters only"
intake could safely and conservatively be assumed - even for heavy
eaters - simply by multiplying the best available estimate of per
capita intake by ten (Column 5 ["High Eaters Only"]). Comparison of
Column 5 with Column 4b, for Section B, makes clear that in every case
the: "High Eaters Only" ( per capita × 10) estimate far exceeds the
"Eaters Only mean plus 3 standard deviations", yet in no case are the
estimates so high as to be unusable.
With the special factors surrounding the use of flavoring
ingredients (i.e., completeness of the surveys, typical volatility
with consequent loss in processing, and self-limiting nature) the
procedure in Column 5 is practical but highly conservative. It has
been widely used in other publications (FDA, 1982, 1993; Easterday
et al., 1993). It can be used safely in all instances except when a
very narrow margin of safety requires maximally precise data for both
toxicological effects and exposure.
Recently, still more sophisticated methods for analyzing USDA
Food Consumption Survey data have become available under license for
those applications in which the need for relatively high precision of
estimates justifies the cost (Helmbach, 1994). In nearly all instances
where broadly- based ingredient disappearance data are available,
per capita × 10, in actuality, a very high "eaters only" estimate,
remains the approach that is by far the simplest and least expensive,
while being conservative but realistic.
REFERENCES
Burdock, G.A., Wagner, B.M., Smith, R.L., Munro, I.C. and Newberne,
P.M. 1990. Recent progress in the consideration of flavoring
ingredients under the food additives amendment. 15. GRAS substances.
A list of flavoring ingredient substances considered generally
recognized as safe by the Flavor & Extract Manufacturers' Association
Expert Panel. Food Technol 44(2):78, 80, 82, 84, & 86.
Easterday, O.D., Ford, R.A., Hall, R.L., Stofberg, J., Cadby, P. and
Grundschober, F. 1992. A Flavor Priority Ranking System, Acceptance
and Internationalization. In: Finley, J.W., Robinson, S.F. and
Armstrong, D.J. (Eds.) Food Safety Assessment. American Chemical
Society, ACS Symposium Series No. 484.
FDA. 1982. Toxicological Principles for the Safety Assessment of
Direct Food Additives and Color Additives Used in Food. Redbook. U.S.
Food and Drug Administration, Bureau of Foods, Washington, DC.
FDA. 1993. Toxicological Principles for the Safety Assessment of
Direct Food Additives and Color Additives Used in Food. Redbook II
(Draft). U.S. Food and Drug Administration, Center for Food Safety and
Applied Nutrition, Washington, DC.
Hall, R.L. and Oser, B.L. 1965. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment. III. GRAS
substances. Food Technol 19(2):151-197.
Hall, R.L. and Oser, B.L. 1970. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment. IV. GRAS
substances. Food Technol 24(5):25-28, 30-32, 34.
Hall, R.L. 1975. Unpublished data from personal communication to the
NAS/NRC Committee on GRAS List Survey - Phase III, and to the FEMA.
Heimbach, J.T. 1994. Personal communication re: TAS-DIET from J.T.
Helmbach, Technical Assessment Systems, Inc., Washington, D.C.
NAS. 1973. A comprehensive Survey on the Use of Food Chemicals
Generally Recognized as Safe, National Technical Information Service
(NTIS) PB-221949.
NAS. 1976. Estimating Distribution of Daily Intakes of Certain GRAS
Substances. National Research Council, Washington, DC. Prepared for:
Food and Drug Administration, Washington, DC. December 1976. National
Technical Information Service (NTIS) PB-299-381.
NAS. 1978. Resurvey of the Annual Poundage of Food Chemicals Generally
Recognized as Safe, National Technical Information Service (NTIS)
PB288-081.
NAS. 1979. Comprehensive Survey of Industry on the Use of Food
Additives, National Technical Information Service (NTIS) PB80-113418.
NAS. 1984. Poundage Update of Food Chemicals, National Technical
Information Service (NTIS) PB84-162148.
NAS. 1989. 1987 Poundage and Technical Effects Update of Substances
Added to Food. National Research Council, Washington, DC. Prepared
for: Food and Drug Administration, Washington, D.C. NTIS Report
No. PB91-127266.
Oser, B.L. and Ford, R.A. 1973a. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 6. GRAS
substances. Food Technol 27(1):64-67.
Oser, B.L. and Ford, R.A. 1973b. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 7. GRAS
substances. Food Technol 27(11):56-57.
Oser, B.L. and Ford, R.A. 1974. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 8. GRAS
substances. Food Technol 28(9):76-80.
Oser, B.L. and Ford, R.A. 1975. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 9. GRAS
substances. Food Technol 29(8):70-72.
Oser, B.L. and Ford, R.A. 1977. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 10. GRAS
substances. Food Technol 31(1):65-74.
Oser, B.L. and Ford, R.A. 1978. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 11. GRAS
substances. Food Technol 32(2):60-70.
Oser, B.L. and Ford, R.A. 1979. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 12. GRAS
substances. Food Technol 33(7):65-73.
Oser, B.L. and Hall, R.L. 1972. Recent progress in the consideration
of flavoring Ingredients under the Food Additives Amendment 5. GRAS
substances. Food Technol 26(5):35-42.
Oser, B.L. and Hall, R.L. 1977. Criteria employed by the Expert Panel
of FEMA for the GRAS evaluation of flavoring substances. Food Cosmet
Toxicol 15:457-466.
Oser, B.L., Ford, R.A. and Bernard, B.K. 1984. Recent Progress in the
consideration of flavoring ingredients under the Food Additives
Amendment 13. GRAS substances. Food Technol 38(10):66-89.
Oser, B.L., Well, C.S., Woods, L.A. and Bernard, B.K. 1985. Recent
progress in the consideration of flavoring ingredients under the Food
Additives Amendment 14. GRAS substances. Food Technol 39(11):108-117.
Rulis, A.M., Hattan, D.G., and Morgenroth, V.H.,III. 1984. FDA's
priority-based assessment of food additives. I. Preliminary results.
Reg Toxicol Pharm 4:37-56.
Rulis, A.M. 1987. Safety assurance margins for food additives
currently in use. Reg Toxicol Pharm 7:160-168.
Smith, R.L. and Ford, R.A. 1993. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 16. GRAS
substances. Food Technol-June 1993, pp. 104-117.
ANNEX 5 Appendix B
SUBSTANCES IN REFERENCE DATABASE BY ORDER OF STRUCTURAL CLASS
Cramer et al. (1978) Structural Class I
1 acetic acid
2 acetoin
3 acetone
4 adipic acid
5 allura red AC
6 aminoundecanoic acid, 11-
7 ascorbic acid
8 ascorbic acid, 1-
9 benzaldehyde
10 benzoic acid
11 benzyl acetate
12 benzyl alcohol
13 bis(2-ethylhexyl)phthalate
14 brilliant black BN
15 butanediol, 1,3-
16 butanol, 2-
17 butanol, n-
18 butyl benzyl phthalate
19 butylated hydroxyanisole
20 butyrolactone, gamma-
21 calcium cyclamate
22 calcium formate
23 calcium stearoyl lactylate
24 carotenoic acid, beta-apo-8'-, methyl ester
25 cinnamaldehyde
26 citral
27 citranaxanthin
28 cumene
29 di(2-ethylhexyl)adipate
30 dibutyl phthalate
31 diethyl phthalate
32 diethylene glycol
33 diethylene glycol monoethyl ether
34 dimethoxane
35 dimethyl terephthalate
36 dimethylcarbonate
37 dimethyldicarbonate
38 dimethylphenol, 2,4-
39 dimethylphenol, 2,6-
40 dimethylphenol, 3,4-
41 dioctyl sodium sulfosuccinate
42 disodium 5'-guanylate
43 disodium 5'-inosinate
44 dodecyl gallate
45 ethanol
46 ethyl acetate
47 ethyl acrylate
48 ethyl butyrate
49 ethyl ether
50 ethyl formate
51 ethyl glycol monomethyl ether
52 ethyl heptanoate
53 ethyl nonanoate
54 ethyl-1-hexanol, 2-
55 ethylbenzene
56 ethylbutyric acid, 2-
57 ethylene glycol
58 ethylhexanoic acid, 2-
59 ethylphthalyl ethylglycolate
60 eugenol
61 FD & C blue No. 1
62 FD & C blue no. 2
63 formaldehyde
64 fumaric acid
65 geranyl acetate
66 glutamate, monosodium
67 glutamic acid hydrochloride
68 glutamic acid, 1-
69 glycerol
70 glyceryl tribenzoate
71 hexenal, 2- (trans)
72 hexylresorcinol, 4-
73 hydroquinone
74 hydroxybenzoic acid butyl ester, p-
75 hydroxybenzoic acid ethyl ester, p-
76 hydroxybenzoic acid methyl ester, p-
77 hydroxybenzoic acid propyl ester, p-
78 hydroxybenzyl acetone, p-
79 inosine monophosphate
80 ionone
81 isoamyl butyrate
82 isoamyl salicylate
83 isobutyl alcohol
84 isomaltitol
85 isopropyl alcohol
86 isovaleric acid
87 lactitol
88 limonene, d-
89 lithocholic acid
90 malonaldehyde, sodium salt
91 mannitol
92 mannitol, d-
93 menthol
94 methanol
95 methyl ethyl carbonate
96 methyl methacrylate
97 methyl salicylate
98 methyl-1-phenylpentan-2-ol, 4-
99 methylenebis, 2,2'-
100 methylphenol, 3-
101 methylphenylcarbinyl acetate
102 myrcene, beta-
103 nonalactone, gamma-
104 octyl acetate
105 octyl gallate
106 oleylamine
107 oxalic acid
108 phenol
109 phenoxyethanol
110 phenyl-1-propanol, 2-
111 phenylalanine
112 potassium sorbate
113 propyl gallate
114 propylene glycol
115 resorcinol
116 retinol
117 riboflavin
118 sodium benzoate
119 sodium erythorbate
120 sodium lauryl glyceryl ether sulfonate
121 sodium lauryl trioxyethylene sulfate
122 sodium stearoyl lactylate
123 sorbic acid
124 stearyl tartrate
125 styrene
126 sucrose monopalmitate
127 sucrose monostearate
128 tartaric acid
129 tertiary butyl hydroquinone
130 tocopherol, alpha-
131 tolualdehyde
132 toluene
133 triethylene glycol
134 triethylene glycol monomethyl ether
135 trimethylamine
136 undecalactone, gamma-
137 vanillin
138 xylitol
Cramer et al. (1978) Structural Class II
1 acrylic acid
2 allyl alcohol
3 allyl heptanoate
4 allyl hexanoate
5 butylated hydroxytoluene
6 caffeine
7 carotene, beta-
8 carvone
9 carvone, d-
10 diacetyl
11 diallyl phthalate
12 diketopiperazine
13 ethyl maltol
14 ethyl vanillin
15 ethylhexyl phthalate, mono-2
16 etretinate
17 fenthion
18 furfural
19 isobornyl acetate
20 isophorone
21 maltol
22 methyl amyl ketone
23 methyl anthranilate
24 piperidine
25 piperonal
26 propargyl alcohol
27 pyridine
28 thujone
Cramer et al, (1978) Structural Class III
1 (1-naphthyl)ethylene--diamine dihydro chloride, N-
2 (2-chloroethyl)trimethyl-ammonium chloride
3 (8-beta)N-cyclohexyl-6-methyl- 1 -(1 -methyl
ethyl)ergoline-8 -carboxamide
4 (chloroacetyl)-acetanilide, 4'-
5 1,1'-(2,2,2-trichloroethylidene)bis(4-chloro)-benzene
6 11-oxo-11H-pyrido(2,1-b)quinazoline-2-carboxylic acid
7 2(2,4,5-trichlorophenoxy) propionic acid
8 2-(2-methyl--4-chlorophenoxy)propionic acid
9 4-(2-methyl-4-chlorophenoxy) butyric acid
10 acenaphthene
11 acephate
12 acesulfame potassium
13 acetaminophen
14 acetoacetamide
15 acetoacetamide-N-sulfonic acid
16 acetochlor
17 acetonitrile
18 acifluorin sodium
19 acrylamide
20 acrylonitrile
21 alachlor
22 albendazole
23 albendazole sulfoxide
24 aldicarb
25 aldicarb sulfone
26 alinidine hydrobromide
27 allyl isovalerate
28 amaranth
29 ameltolide
30 ametryn
31 amino-4-ethoxy-acetanilide, 3-
32 amino-4-nitrophenol, 2-
33 amino-5-nitrophenol, 2-
34 aminophenol, p-
35 amitraz
36 ammonium carmine
37 amphetamine sulfate, dl-
38 ampicillin trihydrate
39 anethole, trans-
40 anilazine
41 anisidine hydrochloride, p-
42 anthranilic acid
43 arochlor 1254
44 aspartame
45 asulam
46 atrazine
47 avermectin B1
48 azaperone
49 azinphos methyl
50 azorubine
51 azuletil (KT1-32)
52 baythroid
53 benomyl
54 bentazon
55 benzofuran
56 benzoic acid, 2-[[[[N-(4-methoxy-6-methyl
-1,2,3-triazin-2-yl)-N-methylamino]carbonyl]
amino]sulfonyl] methyl ester
57 benzoin
58 benzyl violet 4B
59 benzyl-p-chlorophenol, o-
60 betaxolol
61 bidrin
62 biphenthrin
63 biphenyl, 1,1-
64 biphenylamine hydrochloride, 2-
65 bis(2-chloro-1-methyl-ethyl)ether Technical Grade
66 bis(2-chloroisopropyl)ether
67 bisphenol A
68 bromoacetic acid, 2-
69 bromodichloromethane
70 bromomethane
71 bromoxynil
72 bromoxynil octanoate
73 brown FK
74 butyl chloride, n-
75 butylate
76 calcium cyanamide
77 canthaxanthin
78 caprolactam
79 captafol
80 captan
81 carazolol
82 carbaryl
83 carbendazim
84 carbofuran
85 carbon tetrachloride
86 carbosulfan
87 carboxin
88 carmoisine
89 chloramben
90 chlordane
91 chlorendic acid
92 chlorimuron-ethyl
93 chloro-p-toluidine, 3-
94 chloroacetic acid
95 chloroaniline hydrochloride, p-
96 chlorobenzene
97 chlorobenzilate
98 chlorodibromomethane
99 chloroform
100 chlorofructose, 6-
101 chloronaphthalene, beta-
102 chlorophenol, 2-
103 chloropropylate
104 chlorothalonil
105 chlorotoluene, o-
106 chlorpheniramine maleate
107 chlorpromazine
108 chlorsulfuron
109 chlorthal dimethyl
110 chocolate brown HT
111 C.I. Acid Orange 12
112 C.I. Acid Orange 3
113 C.I. Acid Red 18
114 C.I. Disperse Blue 1
115 C.I. acid red 14
116 C.I. disperse yellow 3
117 C.I. pigment red 23
118 C.I. solvent yellow 14
119 cimetidine
120 clofentezine
121 clonitralid
122 closantel
123 codeine
124 coumaphos
125 coumarin
126 crufomate
127 curcumin
128 cyclodextrin, beta-
129 cyclohexylamine
130 cyclophosphamide
131 cyhalothrin
132 cypermethrin
133 cyromazine
134 daminozide
135 decabromodiphenyl oxide
136 deoxyquinine
137 diamino-2,2'-stilbenedisulfonic acid, 4,4'-, disodium sa
138 diaminophenol dihydrochloride, 2,4-
139 diazinon
140 dibenzo-p-dioxin
141 dibromobenzene, 1,4-
142 dicamba
143 dichloro-2-propanol, 1,3-
144 dichloro-p-phenylenediamine, 2,6-
145 dichlorobenzene, 1,2-
146 dichlorobenzene, 1,4-
147 dichlorobenzilic acid
148 dichlorodifluoromethane
149 dichloroethane, 1,1-
150 dichloroethane, 1,2-
151 dichloroethylene, 1,1-
152 dichloroethylene, trans- 1,2-
153 dichloromethane
154 dichlorophenol, 2,4-
155 dichlorophenoxyacetic acid, 2,4-
156 dichloropropanol, 2,3-
157 dichloropropene, 1,3-
158 dichlorvos
159 dicofol
160 dieldrin
161 diethyldithiocarbamate
162 diethylthiourea, N,N'-
163 difenzoquat
164 diflubenzuron
165 dihydroavermectin-B1 a, 22,23-
166 dihydroavermectin-B1 b, 22,23-
167 dihydrocoumarin, 3,4-
168 diiodomethyl p-tolyl sulfone
169 dimethipin
170 dimethoate
171 dimethoxyaniline hydrochloride, 2,4-
172 dimethoxybenzidine-4,4'-diisocyanate, 3,3'-
173 dimethyl hydrogen phosphite
174 dimethyl methylphosphonate
175 dimethyl morpholinophosphoramidate
176 dimethylaniline, N,N'-
177 dinitrobenzene, m-
178 dinitrotoluene, 2,4-
179 dinocap
180 dinoseb
181 diphenamid
182 diphenhydramine hydrochloride
183 diphenylamine
184 diphenylhydantoin, 5,5'-
185 diphenylhydantoin, 5,5-
186 diquat
187 disulfoton
188 dithiobiurea, 2,5-
189 diuron
190 dodecylguanidine acetate, n-
191 EDTA, disodium
192 endothall
193 ephedrine sulfate
194 epichlorohydrin
195 erythromycin stearate
196 erythrosine
197 ethalfuralin
198 ethephon
199 ethion
200 ethyl dipropylthiocarbamate, s-
201 ethyl p-nitrophenyl phenylphosphorothioate
202 ethylene chlorohydrin
203 ethylene thiourea
204 ethylmethylphenylglycidate
205 fast green FCF
206 febantel
207 fenamiphos
208 fenbendazole
209 fenchlorphos
210 fluometuron
211 fluoranthene
212 fluorene
213 fluridone
214 flurprimidol
215 flutolanil
216 fluvalinate
217 folpet
218 fonofos
219 fosetyl-al
220 glufosinate-ammonium
221 glyphosate
222 haloxyfop-methyl
223 HC blue no. 1
224 HC blue no. 2
225 HC yellow 4
226 heptachlor
227 heplachlor epoxide
228 hexabromobenzene
229 hexachlorobenzene
230 hexachlorobutadiene
231 hexachlorocyclohexane, gamma-
232 hexachlorocyclopentadiene
233 hexachloroethane
234 hexachlorophene
235 hexahydro-1,3,5-trinitro-1,3,5-triazine
236 hexamethylenetetramine
237 hexazinone
238 hexythiazox
239 hydralazine
240 hydrazobenzene
241 hydrochlorothiazide
242 hydroxypropyl methanethiolsulfonate, 2-
243 hydroxyquinoline, 8-
244 imazalil
245 imazaquin
246 imazethapyr
247 iodinated glycerol
248 ipazilide
249 iprodione
250 ipronidazole
251 isopropalin
252 isoxaben
253 jervine
254 lactofen
255 lansoprazole
256 levamisole
257 linamarin
258 linuron
259 londax
260 malaoxon
261 malathion
262 maleic anhydride
263 maleic hydrazine
264 manidipine hydrochloride
265 melamine
266 mepiquat chloride
267 mercaptobenzothiazole, 2-
268 merphos
269 merphos oxide
270 meso-2,3-dimercaptosuccinic acid
271 metalaxyl
272 methamidophos
273 methidathion
274 methomyl
275 methoxychlor
276 methoxypsoralen, 8-
277 methyl carbamate
278 methyl ethyl ketoxime
279 methyl parathion
280 methyl-4-chlorophenoxyacetic acid, 2-
281 methyl-N-methylanthranilate
282 methyldopa sesquthydrate, alpha-
283 methylolacrylamide, N-
284 metolachlor
285 metribuzin
286 metsulfuron methyl
287 mexacarbate
288 miporamicin
289 mirex
290 molinate
291 monodiethanolamine salt of riboflavin-5'-phosphate
292 monuron
293 myclobutanil
294 naled
295 nalidixic acid
296 naphthoxy acetic acid, 2-
297 napropamide
298 natamycin
299 nitro-o-toluidine, 5-
300 nitro-p-phenylenediamine, 2-
301 nitroaniline, p-
302 nitroanthranilic acid, 4-
303 nitrofurantoin
304 nitrofurazone
305 nitroguanidine
306 nitronaphthalene, 1-
307 nitrosodiphenylamine, N-
308 norflurazon
309 ochratoxin A
310 octabromodiphenyl ether
311 octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine
312 olaquindox
313 oryzalin
314 oxadiazon
315 oxamyl
316 oxfendazole
317 oxyfluorfen
318 oxytetracycline
319 oxytetracycline hydrochloride
320 oxythioquinox
321 paclobutrazol
322 paraquat
323 parathion
324 patulin
325 pendimethalin
326 penicillin VK
327 pentabromodiphenyl ether
328 pentachloroethane
329 pentachloronitrobenzene
330 pentachlorophenol
331 pentaerythritol tetranitrate
332 permethrin
333 phenformin
334 phenyl-2-naphthylamine, n-
335 phenyl-3-methyl-5-pyrazolone, 1-
336 phenylbutazone
337 phenylenediamine, m-
338 phenylephrine hydrochloride
339 phosmet
340 phosphamidon
341 photodieldrin
342 phthalamide
343 phthalic anhydride
344 picloram
345 pilsicainide hydrochloride
346 pirimiphos-methyl
347 pravadoline
348 probenecid
349 prochloraz
350 proflavine monohydrochloride hemihydrate
351 promethazine hydrochloride
352 prometon
353 prometryn
354 pronamide
355 propachlor
356 propanil
357 propargite
358 propazine
359 propham
360 propiconazole
361 propiverine hydrochloride (P-4)
362 propoxur
363 propylene dichloride
364 pydrin
365 pyrazinamide
366 pyrene
367 pyrimidinone
368 quercetin
369 quinalphos
370 quinine hydrochloride
371 quinofop ethyl
372 red 2G
373 reserpine
374 resmethrin
375 thudamine 6G
376 ronidazole
377 rotenone
378 saccharin
379 sethoxydim
380 simazine
381 sodium 2,2-dichloropropionate
382 sodium cyclamate
383 sodium fluoroacetate
384 sodium naphthionate
385 sorbitan monolaurate
386 sorbitan monostearate
387 succinic anhydride
388 sucrose acetate isobutyrate
389 sulfadimidine
390 sulfisuxazole
391 sulfolene, 3-
392 sunset yellow FCF
393 suplatast tosilate
394 tebuthiuron
395 terbacil
396 terbutryn
397 tert-butyl-2-chlorophenol, 4-
398 tetrachlorobenzene, 1,2,4,5-
399 tetrachloroethane, 1,1,1,2-
400 tetrachloroethane, 1,1,2,2-
401 tetrachloroethylene
402 tetrachlorophenol, 2,3,4,6-
403 tetrachloropyridine, 2,3,5,6-
404 tetrachlorvinphos
405 tetracycline hydrochloride
406 tetraethyldithiopyrophosphate
407 tetraethylthiourea disulfide
408 tetrahydrocannabinol, delta-9-
409 tetrakis(hydroxymethyl)phosphonium chloride (THPC)
410 tetrakis(hydroxymethyl)phosphonium sulphate (THPS)
411 thiabendazole
412 thiameturon methyl
413 thiobencarb
414 thiophanate-methyl
415 thiram
416 tocopheryl acetate, dl-alpha-
417 toluenediamine hydrochloride, 2,6-
418 toluenediamine sulfate, 2,5-
419 tralomethrin
420 trenbolone acetate
421 trenbolone hydroxide, 17-alpha-
422 triadimefon
423 triallate
424 triamterene
425 tribromomethane
426 trichloroacetonitrile
427 trichlorobenzene, 1,2,4-
428 trichloroethane, 1,1,2-
429 trichloroethane, 1,1,1-
430 trichloroethylene
431 trichloroethylene, 1,1,2-
432 trichlorogalactosucrose
433 trichlorophenol, 2,4,5-
434 trichlorophenoxyacetic acid, 2,4,5-
435 tridiphane
436 triethylene tetramine dihydrochloride
437 trifluralin
438 trimethylaniline, 2,4,5-
439 trinitrotoluene, 2,4,6-
440 tris-(2-chloroethyl) phosphate
441 trisulfuron
442 tylosin
443 vinclozolin
444 vinyl chloride
445 zatosetron maleate
446 zearalenone
447 zeranol
 risk at exposure levels below the human exposure threshold for their
respective structural class. However, even if information on
structural class, metabolic fate and existing toxicity studies were
not available, 743/1323 (56%) of flavouring substances used in the
U.S. are consumed in amounts less than the 1.5 µg/person/day standard
proposed by the FDA (Federal Register, 1993) and Munro (1990). This
indicates that for approximately half of the existing list of 1323
chemically defined flavouring substances permitted for use in the
U.S., exposure can be considered to be trivial.
Table 4. Fifth percentile NOELs and human exposure thresholds for
Cramer et al. (1978) structural classes in the reference
database
5th Percentile NOELs, Human Exposure
(µg/kg bw/day) Threshold,(µg/day)a,b
I 137 2993 1800
II 28 906 540
III 447 147 88
a The human exposure threshold was calculated by multiplying the
5th percentile NOEL by 60 (assuming an individual weighs 60 kg)
and dividing by a safety factor of 100, as discussed in the text.
b Numbers rounded to two (2) significant figures.
Table 5. Flavouring substances within each Cramer et al. (1978)
structural class consumed in amounts below human exposure
thresholds
No. of No. of Flavours
Human Exposure Flavours Under Human
Structural Class Threshold Within Exposure
(µg/day) Structural Threshold (%)
Classa
I 1,800 878 835 (95)
II 540 243 227 (93)
III 88 202 195 (97)
a Adapted from the FEMA flavouring substance database of flavouring
substances permitted for use in the U.S.
3.3 Evaluation criteria
The evaluation criteria proposed for application to flavouring
substances in this paper involve the integration of information on
exposure, structure-activity relationships, metabolism and, as
required, toxicity data. It should be noted that the safety evaluation
criteria outlined below are not intended to be applied to any
flavouring substances with unresolved toxicity problems or to
substances that are presumed or known carcinogens. Such substances
warrant special consideration and must be evaluated using more
traditional methods of safety evaluation. While toxicity data exist on
numerous flavouring substances and can be used as the basis for
evaluation, there are many flavouring substances that lack toxicity
data. The evaluation criteria are intended to provide a means of
evaluating such substances. The criteria incorporate, where the data
permit, the concept that metabolic fate can be predicted on the basis
of presumed structure-activity relationships. The criteria also rely,
in part, on the NOEL reference database which provides a human
exposure threshold for each of the three structural classes of
flavouring substances, as shown in Table 4. The evaluation criteria
also incorporate, where available, toxicity data on flavouring
substances and closely related structural analogues as a basis for
safety evaluation. One of the criteria (number 5 below) incorporates
the concept of a minimum human exposure threshold based on the
1.5 µg/person/ day standard proposed by the FDA (Federal Register,
1993) and Munro (1990). This standard can be applied to those
flavouring substances for which metabolic fate is unknown and cannot
be confidently predicted and for which there are no toxicity data on
the flavouring substance or on a structurally related material from
which to conclude any inference of safety in use.
Flavouring substances that meet one of the five numbered criteria
outlined below can be declared safe for their intended use without
further evaluation:
1. a) The flavouring substance has a simple structure and will be
metabolized and excreted through known detoxication pathways
to innocuous end-products; and
b) the conditions of intended use do not result in an exposure
greater than the human exposure threshold for the relevant
structural class, indicating a low probability of potential
for adverse effects.
2. a) The conditions of intended use result in an exposure that
exceeds the human exposure threshold for the relevant
structural class; however
b) the flavouring substance has a simple structure and will be
metabolized and excreted through known detoxication pathways
to innocuous end-products and it or its metabolites are
endogenous human metabolites with no known biochemical
regulating function.
3. a) The flavouring substance has a simple structure and will be
metabolized and excreted through known detoxication pathways
to innocuous end-products; and
b) the conditions of intended use result in an exposure that
exceeds the human exposure threshold for the relevant
structural class; however
c) toxicity data exist on the flavouring substance which
provide assurance of safety under conditions of intended
use, or there are toxicity data on one or more close
structural relatives which provide a NOEL high enough to
accommodate any perceived difference2 in toxicity between
the flavouring substance and the structurally related
substance having toxicity data.
4. a) The metabolic fate of the flavouring substance cannot be
confidently predicted on the basis of structure; however
b) the conditions of intended use result in an exposure below
the human exposure threshold for the relevant structural
class indicating a low probability of potential for adverse
effects; and
c) there are toxicity data on the substance, or on one or more
structurally related substances with a NOEL at least 10
times greater than the 5th percentile NOEL for the relevant
structural class.
5. a) The metabolic fate of the flavouring substance cannot be
confidently predicted on the basis of structure; however
b) the conditions of intended use result in an exposure below
the human exposure threshold of 1.5 µg/day2, providing
assurance that the substance will be safe under conditions
of intended use.
[NOTE: This criterion accommodates flavouring substances for
which there are limited data on structure-activity and toxicity,
but they would not be considered to present a safety concern
below the human exposure threshold of 1.5 µg/day (Federal
Register, 1993)].
The evaluation criteria listed above identify substances which
are considered safe under conditions of intended use or those that may
require additional data and further evaluation. Figure 2 presents the
same criteria in the form of an evaluation sequence. The sequence
contains a number of questions on structure, metabolism, exposure data
and toxicity and provides an integrated mechanism to evaluate the
safety of a flavour ingredient. Although the procedure incorporates
relevant toxicity data on a substance or related substances where
available, it does not require them. The procedure identifies those
substances for which additional safety data may be required in order
to perform an adequate safety evaluation.
The effective application of this safety evaluation procedure
depends on a substantial knowledge of toxicology, chemistry,
metabolism, and exposure to flavouring substances. It can be applied
most effectively when groups of structurally related flavouring
substances are evaluated together. In a group evaluation, conclusions
reached on the safety of individual substances are supported by
similar conclusions for structurally related substances. For example,
the results of the evaluation for butyl butyrate should be consistent
with results for other esters formed from aliphatic acyclic linear
saturated alcohols and acids having similar levels of exposure. These
similar substances will pass through the same branch of the safety
evaluation procedure because they fall into the same structural class,
possess similar metabolic fate, and exhibit similar patterns of
exposure from use as flavour ingredients and as components of food.
2 In most instances, groups of structurally related materials have
toxicology data on at least one member of the group, usually the
flavoring substance with the highest poundage. In most cases a
large margin of safety ( i.e., 100- to 1000-fold) exists
between the NOEL and the calculated exposure to the substance
having toxicological data. Such margins of safety would be
expected to accommodate any perceived difference between the
toxicity of a flavouring substance having no toxicological data
and its close structural relative for which a NOEL has been
established.
In the first step of the safety evaluation procedure (Figure 2)
the user must assign a decision tree structure class (Cramer et al.,
1978) to the substance. Following assignment of structure class, a
question on metabolic fate appears. This question identifies those
substances which are anticipated to be efficiently metabolized
to innocuous products (e.g., 1-butanol) versus those which are
transformed to more toxic metabolites (e.g., estragole) or have
limited information on which to predict confidently the metabolic fate
(e.g., 2-phenyl-3-carbethoxy furan).
Once a substance has been sorted according to structure class
and knowledge of metabolic fate, the next question compares the
substance's daily per capita intake ("eaters only") from use as a
flavour ingredient to the human exposure threshold (Table 4) for the
same structure class.
If the substance is metabolized to innocuous products (Step
No. 2) and has an intake less than the human exposure threshold for
the structure class (Step No. 3), the substance is considered safe
(e.g., 1-octanol). If the intake is greater than the human exposure
threshold (Step No. 3) and the substance or its metabolites are
endogenous (Step No. 4), the substance is also considered safe, even
though the intake is greater than the human exposure threshold (e.g.,
butyric acid). If the substance is not endogenous, then the substance
or related substances must have a NOEL (Step No. 5) significantly
greater than the intake of the substance in order to be considered
safe (e.g., citral). If no such data exist or the NOEL is not
significantly greater than the intake for the substance, then
additional data are required in order to complete the safety
evaluation.
If metabolic fate cannot be confidently predicted and the intake
(Step No. 2) is greater than the human exposure threshold (Step
No. 3), additional data on metabolic fate or toxicity on the substance
or structurally related substances are required to complete the safety
evaluation (e.g., dihydro-coumarin). If the intake is less than the
human exposure threshold, the substance or structurally related
substances must have a NOEL significantly greater than the NOEL for
the structure class (Step No. 6) in order for the substance to be
considered safe (e.g., 2-ethyl-4-hydroxy-3(2H)-furanone). If an
adequate toxicity study is not available and the substance has an
intake less than 1.5 µg/day (Step No. 7), the substance is considered
safe (e.g., 3-acetyl-2,5-dimethylthiophene). Otherwise additional data
are required in order to complete the safety evaluation (e.g.,
2-ethylfuran).
risk at exposure levels below the human exposure threshold for their
respective structural class. However, even if information on
structural class, metabolic fate and existing toxicity studies were
not available, 743/1323 (56%) of flavouring substances used in the
U.S. are consumed in amounts less than the 1.5 µg/person/day standard
proposed by the FDA (Federal Register, 1993) and Munro (1990). This
indicates that for approximately half of the existing list of 1323
chemically defined flavouring substances permitted for use in the
U.S., exposure can be considered to be trivial.
Table 4. Fifth percentile NOELs and human exposure thresholds for
Cramer et al. (1978) structural classes in the reference
database
5th Percentile NOELs, Human Exposure
(µg/kg bw/day) Threshold,(µg/day)a,b
I 137 2993 1800
II 28 906 540
III 447 147 88
a The human exposure threshold was calculated by multiplying the
5th percentile NOEL by 60 (assuming an individual weighs 60 kg)
and dividing by a safety factor of 100, as discussed in the text.
b Numbers rounded to two (2) significant figures.
Table 5. Flavouring substances within each Cramer et al. (1978)
structural class consumed in amounts below human exposure
thresholds
No. of No. of Flavours
Human Exposure Flavours Under Human
Structural Class Threshold Within Exposure
(µg/day) Structural Threshold (%)
Classa
I 1,800 878 835 (95)
II 540 243 227 (93)
III 88 202 195 (97)
a Adapted from the FEMA flavouring substance database of flavouring
substances permitted for use in the U.S.
3.3 Evaluation criteria
The evaluation criteria proposed for application to flavouring
substances in this paper involve the integration of information on
exposure, structure-activity relationships, metabolism and, as
required, toxicity data. It should be noted that the safety evaluation
criteria outlined below are not intended to be applied to any
flavouring substances with unresolved toxicity problems or to
substances that are presumed or known carcinogens. Such substances
warrant special consideration and must be evaluated using more
traditional methods of safety evaluation. While toxicity data exist on
numerous flavouring substances and can be used as the basis for
evaluation, there are many flavouring substances that lack toxicity
data. The evaluation criteria are intended to provide a means of
evaluating such substances. The criteria incorporate, where the data
permit, the concept that metabolic fate can be predicted on the basis
of presumed structure-activity relationships. The criteria also rely,
in part, on the NOEL reference database which provides a human
exposure threshold for each of the three structural classes of
flavouring substances, as shown in Table 4. The evaluation criteria
also incorporate, where available, toxicity data on flavouring
substances and closely related structural analogues as a basis for
safety evaluation. One of the criteria (number 5 below) incorporates
the concept of a minimum human exposure threshold based on the
1.5 µg/person/ day standard proposed by the FDA (Federal Register,
1993) and Munro (1990). This standard can be applied to those
flavouring substances for which metabolic fate is unknown and cannot
be confidently predicted and for which there are no toxicity data on
the flavouring substance or on a structurally related material from
which to conclude any inference of safety in use.
Flavouring substances that meet one of the five numbered criteria
outlined below can be declared safe for their intended use without
further evaluation:
1. a) The flavouring substance has a simple structure and will be
metabolized and excreted through known detoxication pathways
to innocuous end-products; and
b) the conditions of intended use do not result in an exposure
greater than the human exposure threshold for the relevant
structural class, indicating a low probability of potential
for adverse effects.
2. a) The conditions of intended use result in an exposure that
exceeds the human exposure threshold for the relevant
structural class; however
b) the flavouring substance has a simple structure and will be
metabolized and excreted through known detoxication pathways
to innocuous end-products and it or its metabolites are
endogenous human metabolites with no known biochemical
regulating function.
3. a) The flavouring substance has a simple structure and will be
metabolized and excreted through known detoxication pathways
to innocuous end-products; and
b) the conditions of intended use result in an exposure that
exceeds the human exposure threshold for the relevant
structural class; however
c) toxicity data exist on the flavouring substance which
provide assurance of safety under conditions of intended
use, or there are toxicity data on one or more close
structural relatives which provide a NOEL high enough to
accommodate any perceived difference2 in toxicity between
the flavouring substance and the structurally related
substance having toxicity data.
4. a) The metabolic fate of the flavouring substance cannot be
confidently predicted on the basis of structure; however
b) the conditions of intended use result in an exposure below
the human exposure threshold for the relevant structural
class indicating a low probability of potential for adverse
effects; and
c) there are toxicity data on the substance, or on one or more
structurally related substances with a NOEL at least 10
times greater than the 5th percentile NOEL for the relevant
structural class.
5. a) The metabolic fate of the flavouring substance cannot be
confidently predicted on the basis of structure; however
b) the conditions of intended use result in an exposure below
the human exposure threshold of 1.5 µg/day2, providing
assurance that the substance will be safe under conditions
of intended use.
[NOTE: This criterion accommodates flavouring substances for
which there are limited data on structure-activity and toxicity,
but they would not be considered to present a safety concern
below the human exposure threshold of 1.5 µg/day (Federal
Register, 1993)].
The evaluation criteria listed above identify substances which
are considered safe under conditions of intended use or those that may
require additional data and further evaluation. Figure 2 presents the
same criteria in the form of an evaluation sequence. The sequence
contains a number of questions on structure, metabolism, exposure data
and toxicity and provides an integrated mechanism to evaluate the
safety of a flavour ingredient. Although the procedure incorporates
relevant toxicity data on a substance or related substances where
available, it does not require them. The procedure identifies those
substances for which additional safety data may be required in order
to perform an adequate safety evaluation.
The effective application of this safety evaluation procedure
depends on a substantial knowledge of toxicology, chemistry,
metabolism, and exposure to flavouring substances. It can be applied
most effectively when groups of structurally related flavouring
substances are evaluated together. In a group evaluation, conclusions
reached on the safety of individual substances are supported by
similar conclusions for structurally related substances. For example,
the results of the evaluation for butyl butyrate should be consistent
with results for other esters formed from aliphatic acyclic linear
saturated alcohols and acids having similar levels of exposure. These
similar substances will pass through the same branch of the safety
evaluation procedure because they fall into the same structural class,
possess similar metabolic fate, and exhibit similar patterns of
exposure from use as flavour ingredients and as components of food.
2 In most instances, groups of structurally related materials have
toxicology data on at least one member of the group, usually the
flavoring substance with the highest poundage. In most cases a
large margin of safety ( i.e., 100- to 1000-fold) exists
between the NOEL and the calculated exposure to the substance
having toxicological data. Such margins of safety would be
expected to accommodate any perceived difference between the
toxicity of a flavouring substance having no toxicological data
and its close structural relative for which a NOEL has been
established.
In the first step of the safety evaluation procedure (Figure 2)
the user must assign a decision tree structure class (Cramer et al.,
1978) to the substance. Following assignment of structure class, a
question on metabolic fate appears. This question identifies those
substances which are anticipated to be efficiently metabolized
to innocuous products (e.g., 1-butanol) versus those which are
transformed to more toxic metabolites (e.g., estragole) or have
limited information on which to predict confidently the metabolic fate
(e.g., 2-phenyl-3-carbethoxy furan).
Once a substance has been sorted according to structure class
and knowledge of metabolic fate, the next question compares the
substance's daily per capita intake ("eaters only") from use as a
flavour ingredient to the human exposure threshold (Table 4) for the
same structure class.
If the substance is metabolized to innocuous products (Step
No. 2) and has an intake less than the human exposure threshold for
the structure class (Step No. 3), the substance is considered safe
(e.g., 1-octanol). If the intake is greater than the human exposure
threshold (Step No. 3) and the substance or its metabolites are
endogenous (Step No. 4), the substance is also considered safe, even
though the intake is greater than the human exposure threshold (e.g.,
butyric acid). If the substance is not endogenous, then the substance
or related substances must have a NOEL (Step No. 5) significantly
greater than the intake of the substance in order to be considered
safe (e.g., citral). If no such data exist or the NOEL is not
significantly greater than the intake for the substance, then
additional data are required in order to complete the safety
evaluation.
If metabolic fate cannot be confidently predicted and the intake
(Step No. 2) is greater than the human exposure threshold (Step
No. 3), additional data on metabolic fate or toxicity on the substance
or structurally related substances are required to complete the safety
evaluation (e.g., dihydro-coumarin). If the intake is less than the
human exposure threshold, the substance or structurally related
substances must have a NOEL significantly greater than the NOEL for
the structure class (Step No. 6) in order for the substance to be
considered safe (e.g., 2-ethyl-4-hydroxy-3(2H)-furanone). If an
adequate toxicity study is not available and the substance has an
intake less than 1.5 µg/day (Step No. 7), the substance is considered
safe (e.g., 3-acetyl-2,5-dimethylthiophene). Otherwise additional data
are required in order to complete the safety evaluation (e.g.,
2-ethylfuran).
 The principal objective of the safety evaluation procedure is to
identify two groups of flavourings substances: i) those substances
whose structure, metabolism, and relevant toxicity data clearly
indicate that the substance would be expected not to be a safety
concern under current conditions of intended use; and ii) those
substances which may require additional data in order to perform an
adequate safety evaluation.
3.4 Integrating data on the consumption ratio
As pointed out by WHO (1987) and SCF (1991), natural occurrence
is no guarantee of safety, but it is important to recognize that the
safety evaluation of added use of flavouring substances needs to be
conducted with an appreciation of the consumption ratio. Clearly, if
added use of flavouring substances results in an exposure that exceeds
that from natural sources, this will increase awareness of the need to
consider carefully overall exposure in the light of existing data on
toxicity and structure-activity relationships. On the other hand, if
the added use is trivial with a consumption ratio of 10 to 100, that
is, it increases total exposure by only 1 to 10%, then this fact needs
to be taken into consideration when applying the criteria outlined
above.
The substances of primary concern are those which, in Figure 2,
receive a "No" answer to Step No. 5, or a "Yes" answer to Step No. 7,
indicating a possible need for additional data and evaluation beyond
that included in the evaluation procedure outlined in this paper. In
such an evaluation, as stated immediately above, the extent of natural
occurrence should be given appropriate weight.
Of much less concern with respect to consumption ratio are those
substances that drop out of further consideration as a result of "No"
answers to Step Nos. 3 or 7, or "Yes" answers to Step Nos. 4 or 6. The
derivation of the thresholds, the estimation of intakes (see Appendix
A), and the special factors applicable to the use of flavours
(volatility, self-limiting use, etc.) build in multiple conservative
assumptions more than adequate to cover additional exposure from
natural occurrence to substances that in any case are of low inherent
concern.
The advances in analytical chemistry in the past 50 years provide
virtual assurance that no flavouring substances of extensive natural
occurrence remain unknown. Those of potential value yet to be
discovered (e.g., as yet unknown components of roast beef, coffee, or
chocolate flavour) are being sought at the ppb and ppt level. This
does not suggest exposures above any of the thresholds discussed in
this paper.
4. CONCLUSIONS
It is neither possible nor necessary to conduct toxicological
studies on all individual flavouring substances used in food. The vast
majority of flavouring substances are members of groups of substances
with common metabolic pathways, and typically, individual members of
such a group display a similar toxicity profile. This is not
surprising in light of the close structural similarity of the various
flavouring substances comprising a chemical group. As demonstrated in
this paper, exposure to flavouring substances is usually low and, in
the majority of cases, below the human exposure threshold values
presented in this paper. This knowledge, coupled with knowledge
regarding structure-activity relationships, metabolic disposition, and
toxicology data, permits the establishment of the proposed paradigm
for safety assessment which provides a solid foundation to assure the
safety of flavouring substances under conditions of intended use.
5. REFERENCES
BECK, B.D., CONOLLY, R.B., DOURSON, M.L., GUTH, D., HATTIS, D.,
KIMMEL, C. & LEWIS, C. (1993). Symposium overview. Improvements in
quantitative noncancer risk assessment. Fund Appl Toxicol.,
20: 1-14.
COUNCIL OF EUROPE (1974). Natural Flavouring Substances, Their
Sources, And Added Artificial Flavouring Substances. Council of
Europe, Strasbourg, France.
COUNCIL OF EUROPE ( 1981 ). Flavouring Substances And Natural Sources
Of Flavourings, Third Edition. Council of Europe, Strasbourg, France.
COUNCIL OF EUROPE (1992). Flavouring Substances and Natural Sources
of Flavourings. Volume I. Chemically-Defined Flavouring Substances,
Fourth Edition. Council of Europe, Maissoneuve, France.
CRAMER, G.M., FORD, R.A. & HALL, R.L. (1978). Estimation of toxic
hazard-A decision tree approach. Food Cosmet Toxicol., 16: 255-276.
DEISINGER, P.J., BOATMAN, R.J. & GUEST, D. (1994). Metabolism of
2-ethylhexanol administered orally and dermally to the female Fischer
344 rat. Xenobiotica, 24: 429-440.
FOOD AND DRUG ADMINISTRATION (1982). Toxicological Principles for the
Safety Assessment of Direct Food Additives and Color Additives Used in
Food. Redbook. U.S. Food and Drug Administration, Bureau of Foods,
Washington, DC.
FOOD AND DRUG ADMINISTRATION (1993). Toxicological Principles for the
Safety Assessment of Direct Food Additives and Color Additives Used in
Food. Redbook II (Draft). U.S. Food and Drug Administration, Center
for Food Safety and Applied Nutrition, Washington, DC.
FEDERAL REGISTER (1993). Food additives; threshold of regulation for
substances used in food-contact articles. 58(195): 52719-52729.
FRAWLEY, J.P. (1967). Scientific evidence and common sense as a basis
for food-packaging regulations. Food Cosmet Toxicol., 5: 293-308.
GOLD, L.S., SAWYER, C.B., MAGAW, R., BACKMAN, G.M., DE VECIANA, M.,
LEVINSON, R., HOOPER, N.K., HAVENDER, W.R., BERNSTEIN, L., PETO, R.,
PIKE, M. & AMES, B.M. (1984). A carcinogenic potency database of the
standardized results of animal bioassays. Environ Health Perspect.,
58: 9-319.
GOLD, L.S., SLONE, T.H. & BERNSTEIN, L. (1989). Summary of
carcinogenic potency and positivity for 492 rodent carcinogens in the
carcinogenic potency database. Environ. Health Perspect., 79: 259-272.
JAESCHKE, H., KLEINWAECHTER, C. & WENDEL, A. (1987). The role of
acrolein in allyl alcohol-induced lipid peroxidation and liver cell
damage in mice. Biochem. Pharmacol., 36: 51-57.
JECFA (1967). Toxicological Evaluation of Some Flavouring Substances
and Non-Nutritive Sweetening Agents. Eleventh Meeting of the Joint
FAO/WHO Expert Committee on Food Additives.
JECFA (1968). Specifications for the Identity and Purity of Food
Additives and their Toxicological Evaluation: Some Flavouring
Substances and Non-nutritive Sweetening Agents. Eleventh Report of the
Joint FAO/WHO Expert Committee on Food Additives, FAO Nutrition
Meetings Report Series No. 44, WHO Technical Report Series No. 383.
JECFA (1970). Toxicological Evaluation of Some Extraction Solvents and
Certain Other Substances. Fourteenth Meeting of the Joint FAO/WHO
Expert Committee on Food Additives.
JECFA (1971). Evaluation of Food Additives. Fourteenth Report of the
Joint FAO/WHO Expert Committee on Food Additives, WHO Technical Report
Series No. 462.
JECFA (1972). Evaluation of Food Additives. Some Enzymes, Modified
Starches, and Certain Other Substances: Toxicological Evaluations and
Specifications and a Review of the Technological Efficacy of Some
Antioxidants. Fifteenth Report of the Joint FAO/WHO Expert Committee
on Food Additives, WHO Technical Report Series No. 488.
JECFA (1974a). Toxicological Evaluation of Certain Food Additives
Including Anticaking Agents, Antimicrobials, Antioxidants, Emulsifiers
and Thickening Agents. Seventeenth Meeting of Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 5.
JECFA (1974b). Toxicological Evaluation of Certain Food Additives with
a Review of General Principles and of Specifications. Seventeenth
Report of the Joint FAO/WHO Expert Committee on Food Additives, WHO
Technical Report Series No. 539.
JECFA (1976a). Evaluation of Certain Food Additives. Twentieth Report
of the Joint FAO/WHO Expert Committee on Food Additives, FAO Nutrition
Meetings Report Series No. 1, WHO Technical Report Series No. 599.
JECFA (1976b). Toxicological Evaluation of Certain Food Additives.
Twentieth Meeting of the Joint FAO/WHO Expert Committee on Food
Additives, WHO Food Additive Series No. 10.
JECFA (1978). Evaluation of Certain Food Additives and Contaminants.
Twenty-second Report of the Joint FAO/WHO Expert Committee on Food
Additives, WHO Technical Report Series No. 631.
JECFA (1980a). Evaluation of Certain Food Additives. Twenty-third
Report of the Joint FAO/WHO Expert Committee on Food Additives. World
Health Organization, Geneva. WHO Technical Report Series No. 648.
JECFA (1980b). Evaluation of Certain Food Additives. Twenty-fourth
Report of the Joint FAO/WHO Expert Committee on Food Additives. World
Health Organization, Geneva. WHO Technical Report Series No. 653.
JECFA (1980c). Toxicological Evaluation of Certain Food Additives.
Twenty-third Meeting of the Joint FAO/WHO Expert Committee on Food
Additives, WHO Food Additive Series No. 14.
JECFA (1981a). Evaluation of Certain Food Additives. Twenty-fifth
Report of the Joint FAO/WHO Expert Committee on Food Additives,
Technical Report Series No. 669.
JECFA (1981b). Toxicological Evaluation of Certain Food Additives.
Twenty-fifth Meeting of the Joint FAO/WHO Expert Committee on Food
Additives, WHO Food Additive Series No. 16.
JECFA (1982a). Evaluation of Certain Food Additives and Contaminants.
Twenty-sixth Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 683.
JECFA (1982b). Toxicological Evaluation of Certain Food Additives.
Twenty-sixth Meeting of the Joint FAO/WHO Expert Committee on Food
Additives, WHO Food Additive Series No. 17.
JECFA (1983a). Evaluation of Certain Food Additives and Contaminants.
Twenty-seventh Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 696.
JECFA (1983b). Toxicological Evaluation of Certain Food Additives and
Contaminants. Twenty-seventh Meeting of the Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 18.
JECFA (1984a). Evaluation of Certain Food Additives and Contaminants.
Twenty-eighth Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 710
JECFA (1984b). Toxicological Evaluation of Certain Food Additives and
Contaminants. Twenty-eighth Meeting of the Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 19.
JECFA (1986). Evaluation of Certain Food Additives and Contaminants.
Twenty-ninth Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 733.
JECFA (1987a). Evaluation of Certain Food Additives and Contaminants.
Thirtieth Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization. Geneva. WHO Technical Report
Series No. 751.
JECFA (1987b). Evaluation of Certain Food Additives and Contaminants.
Thirty-first Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 759.
JECFA (1989). Evaluation of Certain Food Additives and Contaminants.
Thirty-third Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 776.
JECFA (1990a). Evaluation of Certain Food Additives and Contaminants.
Thirty-fifth Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 789.
JECFA (1990b). Toxicological Evaluation of Certain Food Additives and
Contaminants. Thirty-fifth Meeting of the Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 26.
JECFA (1991a). Evaluation of Certain Food Additives and Contaminants.
Thirty-seventh Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 806.
JECFA (1991b). Toxicological Evaluation of Certain Food Additives and
Contaminants. Thirty-seventh Meeting of the Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 28.
JECFA (1992). Evaluation of Certain Food Additives and Naturally
Occurring Contaminants. Thirty-ninth Report of the Joint FAO/WHO
Expert Committee on Food Additives. World Health Organization, Geneva.
WHO Technical Report Series No. 828.
JECFA (1993a). Evaluation of Certain Food Additives and Contaminants.
Forty-first Report of the Joint FAO/WHO Expert Committee on Food
Additives, Technical Report Series No. 837.
JECFA (1993b). Toxicological Evaluation of Certain Food Additives and
Contaminants. Forty-first Meeting of the Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 32.
JECFA (1993c). Toxicological Evaluation of Certain Food Additives and
Naturally Occurring Toxicants. Thirty-Ninth Meeting of the Joint
FAO/WHO Expert Committee on Food Additives, WHO Food Additive Series
No. 30.
KRASAVAGE, W.J., O'DONOGHUE, J.L., DIVINCENZO, G.D. & TERHAAR, C.J.
(1980). The relative neurotoxicity of methyl-n-butyl ketone, n-hexane
and their metabolites. Toxicol. Appl. Pharmacol., 52: 433-441.
LEWIS, S.C., LYNCH, J.R. & NIKIFOROV, A.I. (1990). A new approach to
deriving community exposure guidelines from "no-observed-adverse-
effect levels". Regul. Toxicol. Pharmacol., 11: 314-330.
MUNRO, I.C. (1990). Safety assessment procedures for indirect food
additives: An overview. Regul. Toxicol. Pharmacol., 12: 001-0011.
NAS (1978). Resurvey of the Annual Poundage of Food Chemicals
Generally Recognized as Safe, National Technical Information Service
(NTIS) PB288-081.
NAS (1979). Comprehensive Survey of Industry on the Use of Food
Additives, National Technical Information Service (NTIS) PB80-113418.
NAS (1984). Poundage Update of Food Chemicals, National Technical
Information Service (NTIS) PB84-162148.
NAS (1989). 1987 Poundage and Technical Effects Update of Substances
Added to Food. National Research Council, Washington, DC. Prepared
for: Food and Drug Administration, Washington, D.C. NTIS Report
No. PB91-127266.
PHILLIPS, J.C., PURCHASE, R., WATTS, P. & GANGOLLI, S.D. (1987). An
evaluation of the decision tree approach for assessing priorities for
safety testing of food additives. Food Addit. Contam., 4(2): 109-123.
RULIS, A.M. (1986). De Minimis and the Threshold of Regulation.
In: Felix, C.W. (Ed.) Food Protection Technology. Lewis Publishers
Inc., Chelsea, MI. pp. 29-37.
SCF (1991). Guidelines for the Evaluation of Flavourings for Use in
Foodstuffs: I. Chemically Defined Flavouring Substances. Commission of
the European Communities, Scientific Committee for Food, Brussels,
Belgium.
STOFBERG, J. & KIRSCHMAN, J. (1985). The consumption ratio of
flavouring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23: 857-860.
STOFBERG, J. & GRUNDSCHOBER, F. (1987). Consumption ratio and food
predominance of flavouring materials. Perfumer Flavourist,
12: 27-68.
WEIL, C.S. & McCOLLISTER, D.D. 1963. Safety evaluation of chemicals.
Relationship between short- and long-term feeding studies in designing
an effective toxicity test. Agr. Food Chem., 11(6): 486-491.
WHO (1987). Principles For The Safety Assessment Of Food Additives
And Contaminants In Food. WHO Environmental Health Criteria. World
Health Organization (WHO), International Program on Chemical Safety in
Cooperation with the Joint FAO (Food & Agriculture Organization of the
United Nations), WHO Expert Committee on Food Additives (JECFA),
Geneva, Switzerland, Environmental Health Criteria 70.
WOODS, L.A. & DOULL, J. (1991). GRAS evaluation of flavouring
substances by the Expert Panel of FEMA. Regul. Toxicol. Pharmacol.,
14: 48-58.
ANNEX 5, Appendix A
ESTIMATING DISTRIBUTION OF INTAKES OF FOOD INGREDIENTS ACROSS
THE POPULATION
In response to the Food Additives Amendment of 1958 to the U.S.
Food, Drug and Cosmetic Act, the Flavor and Extract Manufacturers'
Association (FEMA) in 1960 conducted a comprehensive survey of all
flavoring materials then thought or claimed to be in use. Information
included: identity; poundage used annually in food; year of first use;
levels (in ppm) used in major food categories; toxicological data; and
other information related to safety. After evaluation by a panel of
external experts, this and subsequent information was published in a
series of "FEMA GRAS Lists" (Hall and Oser, 1965, 1970; Oser and Hall,
1972; Oser and Ford, 1973ab, 1974, 1975, 1977, 1978, 1979; Oser et al.,
1984, 1985; Burdock et al., 1990; Smith and Ford, 1993).
FEMA determined in 1968 that another survey was needed to monitor
trends and to improve on the previous effort. In addition, the U.S.
Food and Drug Administration (FDA) began to plan to review its
Generally Recognized as Safe (GRAS) lists, and in so doing, made use
of the prior experience of FEMA. FEMA and FDA efforts merged into the
first comprehensive survey of ingredients used in food, conducted by
the National Academy of Sciences/National Research Council (NAS) in
1970 (NAS, 1973) with several subsequent surveys (NAS, 1978, 1979,
1984, 1989).
Emerging from the 1960 and 1970 surveys was a clear indication
that per capita intake of an ingredient (from annual poundage),
although useful for comparison with other ingredients, has a
significant limitation. As an average of intakes by those who consume
foods containing the ingredient ("eaters") and by those who do not
(non-eaters"), it does not represent the intake by the individuals of
concern ( i.e., the "eaters"). This is not the case for ingredients
commonly consumed by almost everyone ( e.g., sugar), but the
difference between the average and "eaters" is significant for other
ingredients consumed only by few people ( e.g. raspberry flavor).
Because of these issues, an alternative method of calculating
intakes was used in the first (1973) NAS report. This method involved
the average level of use of an ingredient in a broad food category
(e.g., baked goods) multiplied by the average daily consumption of
that food category per person. The daily consumption for each category
was obtained by multiplying the average portion size for the category,
obtained from the U.S. Department of Agriculture (USDA) Food
Consumption Survey by the average daily frequency of consumption of
foods in that category over a 14-day survey period. The frequency of
consumption data came from a 12,000+ member panel operated by the
Market Research Corporation of America (MRCA; see note b to Table 1).
Thus the average intake of an ingredient for all relevant food
categories provided the "possible average daily intake" (PADI). These
intakes were often highly exaggerated, as much as a hundred-fold,
because an ingredient used in only a few foods in a food category was
assumed to be at the average level in all foods in the category. Thus,
for example, cardamom, used only in a few coffee cakes, was assumed to
be used in each food in the entire category of baked goods ( i.e.,
all breads, rolls, crackers, and pastries).
To address this issue, another method was developed to give the
probable distribution of intakes for the entire population without
exaggerating intake. This required an informed estimate of the
probability that each food eaten would contain the ingredient of
interest. The first series of such intake estimates used calculation
"A", as shown in Table 1, and the results are summarized in Section
"A" of Table 2.
In Table 2, Column 1 ("Substance") lists the substances selected
to test and improve the more refined method. In Section A, nutmeg and
mace were chosen, among other reasons because they are not grown
domestically in the U.S. and import data, therefore, provide a firm
external check on pounds used. Furthermore, more than half of all
nutmeg is consumed in four foods: bologna, pork sausage, frankfurters,
and doughnuts. It thus presented an opportunity to see how heavy
consumers of those foods would skew total intake distribution. The
flavor ingredient 4-(p-hydroxyphenyl)butan-2-one was chosen because it
is used in, and occurs naturally, almost entirely in only one flavor,
raspberry flavor, and is far less widely consumed than chocolate,
vanilla, or citrus flavors. Thus, it is a good example of an uncommon
ingredient consumed by those who particularly like the few foods in
which it is used (Hall, 1975).
Column 2 [" per capita (External Check)"] lists per capita
intake derived from import data, product manufacturer surveys, or
tariff reports, all independent of the NAS survey to serve as an
external check. Column 3 (" per capita [NAS(lbs)/0.6]") lists the
per capita intake from the poundage reported in the NAS survey
assumed to be 60% complete. Further research (see references to NRC
surveys) showed this to be generally a valid assumption, but with
certain qualifications. Ingredients such as sugar, corn syrup, and
salt, could not be fully or reliably reported by bakers and other
small food processors, whereas the flavor industry reported more
fully, and the 60% assumption, while used, usually resulted in a
poundage per capita overestimate.
For Section A, Column 4 ["50th Percentile (Total Panel)"]
presents the total panel 50th percentile for each substance, and
Column 4a ("50th Percentile 3 S.D. Total Panel"), the 50th percentile
plus 3 standard deviations. Column 4b ["very high (Total Panel)"]
shows the so-called "very high intake". This is not a realistic
figure, because no person could consume all foods using an ingredient
at the highest level for every food in every major category. Note
particularly, also, the earlier discussion of the exaggeration that
arises from assuming that an ingredient used only in a few foods is
used in all foods in a major food category. The "very high intake"
serves simply as a wholly unreachable and therefore unrealistic value.
Given the remaining and inevitable uncertainties due to possible
errors in estimations, and possible non-food uses, the agreement
between the total panel 50th percentile (Section A, Column 4), the NRC
per capita, (Column 3), and the per capita from external sources
(Column 2), is entirely satisfactory. However, calculation "A" did
not provide data on only those who actually consumed the substance
("eaters only"), and the statistical treatment was not as solidly
based as was desirable. This led to an improved calculation, "B", as
shown in Table 3. Table 2, Section B, presents the results of these
calculations.
The substances shown in Column 1 of Section B were selected, as
discussed in the referenced reports themselves, by the FDA or the NAS
committee (NAS, 1976). An improved method of calculation was used, as
described below, which provides the total sample mean (Column 4 ["Mean
(Total Panel)"]) as a check against the per capita data in Columns 2
and 3.
The external per capita figure for calcium propionate is twice
the total panel mean, probably due primarily to usage by many small
bakeries not included in the survey, and also to the high wastage of
bread. The slightly smaller discrepancy for MSG may well reflect
several added sources of error. Even so, comparison of the total panel
mean with the per capita data from the NAS survey and the external
checks shows satisfactory agreement. Food consumption surveys have
significant and well-recognized sources of error in addition to those
affecting the NRC surveys, and it seems unlikely that it is practical
to expect intake estimates derived by different means to correspond
more closely than within a factor of two.
For Section B, Column 4 ["Mean (Total Panel)"] provides the total
panel mean, Column 4a ["Mean (Eaters Only)"] the "eaters only" mean
age, 2 and over and Column 4b ["S.D. (Eaters Only)"], the standard
deviation for "eaters only".
The procedure in calculation B does not eliminate all sources of
error. Intake is still calculated from "disappearance figures"
( i.e, the levels introduced into food during processing, not the
lower levels actually consumed). Thus, ignoring the often large losses
due, for example, to volatilization or leaching in processing,
preparation and storage result in exaggerated estimates of intake. In
the other direction, heavy (frequent) eaters will often eat larger
portion sizes, resulting in a tendency to underestimate higher
intakes. The result overall, however, remains highly satisfactory.
Table 1 Calculation "A"
Step Operation Result
For each specific food (SF)a e.g. bologna,
within a broad food category, eg., meats,
obtain from MRCAb the
1 total number of eatings of each SF by the total
panel, in two weeks
14 days × 12,337 (persons in panel) = mean frequency of eating, p/d
×
2 mean portion size in gc = mean consumption of SF, g/p/d
×
3 probability of SF containing ingredient "I"d = probable mean consumption of SF
containing I g/p/d
×
4 usual use level of "I" in ppm of major food = 50th percentile of probable intake of I
category containing SF ‰ 1,000e from SF in mg/p/d
×
5 90th percentile MRCA frequency of eating = 90th percentile of probable intake of I
50th percentile MRCA frequency of eating from SF in mg/p/d
6 & 7 sum 4 and 5 separately across all SFs in that = 50th and 90th percentiles of probable
major food category intake of I from that major food category
Table 1 (cont'd) Calculation "A"
Step Operation Result
8 fit a curve, assuming normal distribution, to = 50th percentile and standard deviation
the points determined in 6 and 7 and calculate (SD) for each major food category
9 repeat steps 1 through 8 and sum across all = 50th percentile and SD in mg/p/d
food categories probable total intake
10 repeat steps 4 and following, using "average = "very high intake"
maximum use level" from the survey for each
major food category
a Specific food (SF), in this context, denotes a narrow category of very similar foods serving
the same dietary and market niches (e.g. all bologna, all cola drinks). The eatings of each SF
include in one total for each SF all eatings of similar brands or home-prepared versions of the
same SF. There were approximately 10,000 such SFs.
b The Market Research Corporation of America (MRCA) is a private market research company that
surveys usage of food products and components It uses a nationally representative panel and
records for each panel member the number of servings of each specific loud for each day of a
two-week period. The panelists' reporting periods are distributed evenly throughout the year. The
1973 NAS report used data from the third Menu Census (1967-1968) involving 12,871 individuals.
The dataused in calculations "A" and "B" came from the Fourth Menu Census (1972-1973) involving
12,337 individuals.
c The USDA Food Consumption Survey (USDA, 1965)
d Obtained from a consensus panel of experts knowledgeable about the ingredient and the food.
e From NAS, 1973
Table 2 Distribution of MRCA 14-Day Average Daily Intakes (in mg/person/day)a of Selected Food Ingredients
(1) (2) (3) (4) (4a) (4b) (5)
"High Eaters
Section Substance per capita per capita Mean Mean plus "Very High" Only"
(External [NAS (lbs.)/0.6] (Total Panel) 3 S.D.b (Total Panel) [NAS
Check) Total Panel (lbs.)/0.6]×10
A Nutmeg 23.6 10.3 15.7 51.3 71.1 103
Nutmeg Oil 1.2 1.0 2.0 5.7 7.1 10
A Mace 3.3 2.8 3.3 8.6 14.4 28
Mace, Oil NAc 0.26 0.19 0.67 0.46 2.6
Mace, Oleoresin NA 0.070 0.13 0.39 0.41 0.70
A 4-p-Hydroxyphenyl 0.048 0.086 0.041 0.16 0.37 0.86
Butan-2-one
Table 2 (cont'd).
(1) (2) (3) (4) (4a) (4b) (5)
"High Eaters
Section Substance per capita per capita Mean Mean plus "Very High" Only"
(External [NAS (lbs.)/0.6] (Total Panel) 3 S.D.b (Total Panel) [NAS
Check) Total Panel (lbs.)/0.6]×10
Mean Mean S.D.
(Total Panel) (Eaters Only) (Eaters Only)
B Calcium Propionate 92 33 44.8 45.1 20.7 330
Sodium Propionate NA 16 33,2 33.3 14.7 160
B Carob Bean Gum NA 26 35.6 36.1 35.0 260
Chondrus Extract 19.3 13 10.6 11.1 18.5 130
B Mono- and diglycerides NA 727 884 885 467 7,270
Monosodium Glutamate 186 194 98.8 99.0 88.5 1,940
(MSG)
a See text for explanation of columns.
b S.D. = Standard Deviation.
Table 3 Calculation "B"
1 From the MRCA survey, obtain the total
number of eatings of each SF, by each panel
member, each day over the 14 - day period
×
2 USDA mean portion size, for that person's = quantity of SF in g for that
age group and the relevant major food person, that day
category
×
3 weighted mean of usual use level of I in = quantity of I in mg/d, that
ppm ‰ 1,000 person, that day, if all of
the SF contained I
×
4 probability that particular SF contains I = expected intake of I for
that person for that day,
mg/d
5 Repeat steps 1 through 4 for that person for = expected intake of I for
each of the 14 days that person for 14 days,
mg
6 ‰ 14 = average daily intake over
14 days for that person,
g/p/d
7 tabulate all intakes and compute = total panel mean intake of I
mg/p/d
8 discard all persons with zero intake for the = "eaters only" mean and SD
14 day period, tabulate all "eaters only" of for I, mg/p/d
I, age 2 and over, and compute
Unfortunately, this more refined approach, while successful, is
extremely elaborate, tedious, and expensive. The MRCA panel is
expensive to maintain and operate, and the data are correspondingly
costly. The approach requires extensive input from a broadly-based,
necessarily large and well-informed panel of expert technologists.
Such an effort can only be justified in those few cases where it is
necessary to obtain the distribution of intakes with maximum accuracy.
Given the wide safety margins food ingredients typically enjoy, and
the uncertainties and assumptions inherent in interpreting
toxicological data, such effort is rarely necessary. A much more
appropriate, but quick and easy, method of estimating intake was
needed, so long as it was conservative, but not excessively so. This
led Rulis and others in FDA (Rulis et al., 1984; Rulis, 1987) to
test and adopt the assumption that, except for an unusually narrow
margin of safety or specific concern, the per capita "eaters only"
intake could safely and conservatively be assumed - even for heavy
eaters - simply by multiplying the best available estimate of per
capita intake by ten (Column 5 ["High Eaters Only"]). Comparison of
Column 5 with Column 4b, for Section B, makes clear that in every case
the: "High Eaters Only" ( per capita × 10) estimate far exceeds the
"Eaters Only mean plus 3 standard deviations", yet in no case are the
estimates so high as to be unusable.
With the special factors surrounding the use of flavoring
ingredients (i.e., completeness of the surveys, typical volatility
with consequent loss in processing, and self-limiting nature) the
procedure in Column 5 is practical but highly conservative. It has
been widely used in other publications (FDA, 1982, 1993; Easterday
et al., 1993). It can be used safely in all instances except when a
very narrow margin of safety requires maximally precise data for both
toxicological effects and exposure.
Recently, still more sophisticated methods for analyzing USDA
Food Consumption Survey data have become available under license for
those applications in which the need for relatively high precision of
estimates justifies the cost (Helmbach, 1994). In nearly all instances
where broadly- based ingredient disappearance data are available,
per capita × 10, in actuality, a very high "eaters only" estimate,
remains the approach that is by far the simplest and least expensive,
while being conservative but realistic.
REFERENCES
Burdock, G.A., Wagner, B.M., Smith, R.L., Munro, I.C. and Newberne,
P.M. 1990. Recent progress in the consideration of flavoring
ingredients under the food additives amendment. 15. GRAS substances.
A list of flavoring ingredient substances considered generally
recognized as safe by the Flavor & Extract Manufacturers' Association
Expert Panel. Food Technol 44(2):78, 80, 82, 84, & 86.
Easterday, O.D., Ford, R.A., Hall, R.L., Stofberg, J., Cadby, P. and
Grundschober, F. 1992. A Flavor Priority Ranking System, Acceptance
and Internationalization. In: Finley, J.W., Robinson, S.F. and
Armstrong, D.J. (Eds.) Food Safety Assessment. American Chemical
Society, ACS Symposium Series No. 484.
FDA. 1982. Toxicological Principles for the Safety Assessment of
Direct Food Additives and Color Additives Used in Food. Redbook. U.S.
Food and Drug Administration, Bureau of Foods, Washington, DC.
FDA. 1993. Toxicological Principles for the Safety Assessment of
Direct Food Additives and Color Additives Used in Food. Redbook II
(Draft). U.S. Food and Drug Administration, Center for Food Safety and
Applied Nutrition, Washington, DC.
Hall, R.L. and Oser, B.L. 1965. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment. III. GRAS
substances. Food Technol 19(2):151-197.
Hall, R.L. and Oser, B.L. 1970. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment. IV. GRAS
substances. Food Technol 24(5):25-28, 30-32, 34.
Hall, R.L. 1975. Unpublished data from personal communication to the
NAS/NRC Committee on GRAS List Survey - Phase III, and to the FEMA.
Heimbach, J.T. 1994. Personal communication re: TAS-DIET from J.T.
Helmbach, Technical Assessment Systems, Inc., Washington, D.C.
NAS. 1973. A comprehensive Survey on the Use of Food Chemicals
Generally Recognized as Safe, National Technical Information Service
(NTIS) PB-221949.
NAS. 1976. Estimating Distribution of Daily Intakes of Certain GRAS
Substances. National Research Council, Washington, DC. Prepared for:
Food and Drug Administration, Washington, DC. December 1976. National
Technical Information Service (NTIS) PB-299-381.
NAS. 1978. Resurvey of the Annual Poundage of Food Chemicals Generally
Recognized as Safe, National Technical Information Service (NTIS)
PB288-081.
NAS. 1979. Comprehensive Survey of Industry on the Use of Food
Additives, National Technical Information Service (NTIS) PB80-113418.
NAS. 1984. Poundage Update of Food Chemicals, National Technical
Information Service (NTIS) PB84-162148.
NAS. 1989. 1987 Poundage and Technical Effects Update of Substances
Added to Food. National Research Council, Washington, DC. Prepared
for: Food and Drug Administration, Washington, D.C. NTIS Report
No. PB91-127266.
Oser, B.L. and Ford, R.A. 1973a. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 6. GRAS
substances. Food Technol 27(1):64-67.
Oser, B.L. and Ford, R.A. 1973b. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 7. GRAS
substances. Food Technol 27(11):56-57.
Oser, B.L. and Ford, R.A. 1974. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 8. GRAS
substances. Food Technol 28(9):76-80.
Oser, B.L. and Ford, R.A. 1975. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 9. GRAS
substances. Food Technol 29(8):70-72.
Oser, B.L. and Ford, R.A. 1977. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 10. GRAS
substances. Food Technol 31(1):65-74.
Oser, B.L. and Ford, R.A. 1978. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 11. GRAS
substances. Food Technol 32(2):60-70.
Oser, B.L. and Ford, R.A. 1979. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 12. GRAS
substances. Food Technol 33(7):65-73.
Oser, B.L. and Hall, R.L. 1972. Recent progress in the consideration
of flavoring Ingredients under the Food Additives Amendment 5. GRAS
substances. Food Technol 26(5):35-42.
Oser, B.L. and Hall, R.L. 1977. Criteria employed by the Expert Panel
of FEMA for the GRAS evaluation of flavoring substances. Food Cosmet
Toxicol 15:457-466.
Oser, B.L., Ford, R.A. and Bernard, B.K. 1984. Recent Progress in the
consideration of flavoring ingredients under the Food Additives
Amendment 13. GRAS substances. Food Technol 38(10):66-89.
Oser, B.L., Well, C.S., Woods, L.A. and Bernard, B.K. 1985. Recent
progress in the consideration of flavoring ingredients under the Food
Additives Amendment 14. GRAS substances. Food Technol 39(11):108-117.
Rulis, A.M., Hattan, D.G., and Morgenroth, V.H.,III. 1984. FDA's
priority-based assessment of food additives. I. Preliminary results.
Reg Toxicol Pharm 4:37-56.
Rulis, A.M. 1987. Safety assurance margins for food additives
currently in use. Reg Toxicol Pharm 7:160-168.
Smith, R.L. and Ford, R.A. 1993. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 16. GRAS
substances. Food Technol-June 1993, pp. 104-117.
ANNEX 5 Appendix B
SUBSTANCES IN REFERENCE DATABASE BY ORDER OF STRUCTURAL CLASS
Cramer et al. (1978) Structural Class I
1 acetic acid
2 acetoin
3 acetone
4 adipic acid
5 allura red AC
6 aminoundecanoic acid, 11-
7 ascorbic acid
8 ascorbic acid, 1-
9 benzaldehyde
10 benzoic acid
11 benzyl acetate
12 benzyl alcohol
13 bis(2-ethylhexyl)phthalate
14 brilliant black BN
15 butanediol, 1,3-
16 butanol, 2-
17 butanol, n-
18 butyl benzyl phthalate
19 butylated hydroxyanisole
20 butyrolactone, gamma-
21 calcium cyclamate
22 calcium formate
23 calcium stearoyl lactylate
24 carotenoic acid, beta-apo-8'-, methyl ester
25 cinnamaldehyde
26 citral
27 citranaxanthin
28 cumene
29 di(2-ethylhexyl)adipate
30 dibutyl phthalate
31 diethyl phthalate
32 diethylene glycol
33 diethylene glycol monoethyl ether
34 dimethoxane
35 dimethyl terephthalate
36 dimethylcarbonate
37 dimethyldicarbonate
38 dimethylphenol, 2,4-
39 dimethylphenol, 2,6-
40 dimethylphenol, 3,4-
41 dioctyl sodium sulfosuccinate
42 disodium 5'-guanylate
43 disodium 5'-inosinate
44 dodecyl gallate
45 ethanol
46 ethyl acetate
47 ethyl acrylate
48 ethyl butyrate
49 ethyl ether
50 ethyl formate
51 ethyl glycol monomethyl ether
52 ethyl heptanoate
53 ethyl nonanoate
54 ethyl-1-hexanol, 2-
55 ethylbenzene
56 ethylbutyric acid, 2-
57 ethylene glycol
58 ethylhexanoic acid, 2-
59 ethylphthalyl ethylglycolate
60 eugenol
61 FD & C blue No. 1
62 FD & C blue no. 2
63 formaldehyde
64 fumaric acid
65 geranyl acetate
66 glutamate, monosodium
67 glutamic acid hydrochloride
68 glutamic acid, 1-
69 glycerol
70 glyceryl tribenzoate
71 hexenal, 2- (trans)
72 hexylresorcinol, 4-
73 hydroquinone
74 hydroxybenzoic acid butyl ester, p-
75 hydroxybenzoic acid ethyl ester, p-
76 hydroxybenzoic acid methyl ester, p-
77 hydroxybenzoic acid propyl ester, p-
78 hydroxybenzyl acetone, p-
79 inosine monophosphate
80 ionone
81 isoamyl butyrate
82 isoamyl salicylate
83 isobutyl alcohol
84 isomaltitol
85 isopropyl alcohol
86 isovaleric acid
87 lactitol
88 limonene, d-
89 lithocholic acid
90 malonaldehyde, sodium salt
91 mannitol
92 mannitol, d-
93 menthol
94 methanol
95 methyl ethyl carbonate
96 methyl methacrylate
97 methyl salicylate
98 methyl-1-phenylpentan-2-ol, 4-
99 methylenebis, 2,2'-
100 methylphenol, 3-
101 methylphenylcarbinyl acetate
102 myrcene, beta-
103 nonalactone, gamma-
104 octyl acetate
105 octyl gallate
106 oleylamine
107 oxalic acid
108 phenol
109 phenoxyethanol
110 phenyl-1-propanol, 2-
111 phenylalanine
112 potassium sorbate
113 propyl gallate
114 propylene glycol
115 resorcinol
116 retinol
117 riboflavin
118 sodium benzoate
119 sodium erythorbate
120 sodium lauryl glyceryl ether sulfonate
121 sodium lauryl trioxyethylene sulfate
122 sodium stearoyl lactylate
123 sorbic acid
124 stearyl tartrate
125 styrene
126 sucrose monopalmitate
127 sucrose monostearate
128 tartaric acid
129 tertiary butyl hydroquinone
130 tocopherol, alpha-
131 tolualdehyde
132 toluene
133 triethylene glycol
134 triethylene glycol monomethyl ether
135 trimethylamine
136 undecalactone, gamma-
137 vanillin
138 xylitol
Cramer et al. (1978) Structural Class II
1 acrylic acid
2 allyl alcohol
3 allyl heptanoate
4 allyl hexanoate
5 butylated hydroxytoluene
6 caffeine
7 carotene, beta-
8 carvone
9 carvone, d-
10 diacetyl
11 diallyl phthalate
12 diketopiperazine
13 ethyl maltol
14 ethyl vanillin
15 ethylhexyl phthalate, mono-2
16 etretinate
17 fenthion
18 furfural
19 isobornyl acetate
20 isophorone
21 maltol
22 methyl amyl ketone
23 methyl anthranilate
24 piperidine
25 piperonal
26 propargyl alcohol
27 pyridine
28 thujone
Cramer et al, (1978) Structural Class III
1 (1-naphthyl)ethylene--diamine dihydro chloride, N-
2 (2-chloroethyl)trimethyl-ammonium chloride
3 (8-beta)N-cyclohexyl-6-methyl- 1 -(1 -methyl
ethyl)ergoline-8 -carboxamide
4 (chloroacetyl)-acetanilide, 4'-
5 1,1'-(2,2,2-trichloroethylidene)bis(4-chloro)-benzene
6 11-oxo-11H-pyrido(2,1-b)quinazoline-2-carboxylic acid
7 2(2,4,5-trichlorophenoxy) propionic acid
8 2-(2-methyl--4-chlorophenoxy)propionic acid
9 4-(2-methyl-4-chlorophenoxy) butyric acid
10 acenaphthene
11 acephate
12 acesulfame potassium
13 acetaminophen
14 acetoacetamide
15 acetoacetamide-N-sulfonic acid
16 acetochlor
17 acetonitrile
18 acifluorin sodium
19 acrylamide
20 acrylonitrile
21 alachlor
22 albendazole
23 albendazole sulfoxide
24 aldicarb
25 aldicarb sulfone
26 alinidine hydrobromide
27 allyl isovalerate
28 amaranth
29 ameltolide
30 ametryn
31 amino-4-ethoxy-acetanilide, 3-
32 amino-4-nitrophenol, 2-
33 amino-5-nitrophenol, 2-
34 aminophenol, p-
35 amitraz
36 ammonium carmine
37 amphetamine sulfate, dl-
38 ampicillin trihydrate
39 anethole, trans-
40 anilazine
41 anisidine hydrochloride, p-
42 anthranilic acid
43 arochlor 1254
44 aspartame
45 asulam
46 atrazine
47 avermectin B1
48 azaperone
49 azinphos methyl
50 azorubine
51 azuletil (KT1-32)
52 baythroid
53 benomyl
54 bentazon
55 benzofuran
56 benzoic acid, 2-[[[[N-(4-methoxy-6-methyl
-1,2,3-triazin-2-yl)-N-methylamino]carbonyl]
amino]sulfonyl] methyl ester
57 benzoin
58 benzyl violet 4B
59 benzyl-p-chlorophenol, o-
60 betaxolol
61 bidrin
62 biphenthrin
63 biphenyl, 1,1-
64 biphenylamine hydrochloride, 2-
65 bis(2-chloro-1-methyl-ethyl)ether Technical Grade
66 bis(2-chloroisopropyl)ether
67 bisphenol A
68 bromoacetic acid, 2-
69 bromodichloromethane
70 bromomethane
71 bromoxynil
72 bromoxynil octanoate
73 brown FK
74 butyl chloride, n-
75 butylate
76 calcium cyanamide
77 canthaxanthin
78 caprolactam
79 captafol
80 captan
81 carazolol
82 carbaryl
83 carbendazim
84 carbofuran
85 carbon tetrachloride
86 carbosulfan
87 carboxin
88 carmoisine
89 chloramben
90 chlordane
91 chlorendic acid
92 chlorimuron-ethyl
93 chloro-p-toluidine, 3-
94 chloroacetic acid
95 chloroaniline hydrochloride, p-
96 chlorobenzene
97 chlorobenzilate
98 chlorodibromomethane
99 chloroform
100 chlorofructose, 6-
101 chloronaphthalene, beta-
102 chlorophenol, 2-
103 chloropropylate
104 chlorothalonil
105 chlorotoluene, o-
106 chlorpheniramine maleate
107 chlorpromazine
108 chlorsulfuron
109 chlorthal dimethyl
110 chocolate brown HT
111 C.I. Acid Orange 12
112 C.I. Acid Orange 3
113 C.I. Acid Red 18
114 C.I. Disperse Blue 1
115 C.I. acid red 14
116 C.I. disperse yellow 3
117 C.I. pigment red 23
118 C.I. solvent yellow 14
119 cimetidine
120 clofentezine
121 clonitralid
122 closantel
123 codeine
124 coumaphos
125 coumarin
126 crufomate
127 curcumin
128 cyclodextrin, beta-
129 cyclohexylamine
130 cyclophosphamide
131 cyhalothrin
132 cypermethrin
133 cyromazine
134 daminozide
135 decabromodiphenyl oxide
136 deoxyquinine
137 diamino-2,2'-stilbenedisulfonic acid, 4,4'-, disodium sa
138 diaminophenol dihydrochloride, 2,4-
139 diazinon
140 dibenzo-p-dioxin
141 dibromobenzene, 1,4-
142 dicamba
143 dichloro-2-propanol, 1,3-
144 dichloro-p-phenylenediamine, 2,6-
145 dichlorobenzene, 1,2-
146 dichlorobenzene, 1,4-
147 dichlorobenzilic acid
148 dichlorodifluoromethane
149 dichloroethane, 1,1-
150 dichloroethane, 1,2-
151 dichloroethylene, 1,1-
152 dichloroethylene, trans- 1,2-
153 dichloromethane
154 dichlorophenol, 2,4-
155 dichlorophenoxyacetic acid, 2,4-
156 dichloropropanol, 2,3-
157 dichloropropene, 1,3-
158 dichlorvos
159 dicofol
160 dieldrin
161 diethyldithiocarbamate
162 diethylthiourea, N,N'-
163 difenzoquat
164 diflubenzuron
165 dihydroavermectin-B1 a, 22,23-
166 dihydroavermectin-B1 b, 22,23-
167 dihydrocoumarin, 3,4-
168 diiodomethyl p-tolyl sulfone
169 dimethipin
170 dimethoate
171 dimethoxyaniline hydrochloride, 2,4-
172 dimethoxybenzidine-4,4'-diisocyanate, 3,3'-
173 dimethyl hydrogen phosphite
174 dimethyl methylphosphonate
175 dimethyl morpholinophosphoramidate
176 dimethylaniline, N,N'-
177 dinitrobenzene, m-
178 dinitrotoluene, 2,4-
179 dinocap
180 dinoseb
181 diphenamid
182 diphenhydramine hydrochloride
183 diphenylamine
184 diphenylhydantoin, 5,5'-
185 diphenylhydantoin, 5,5-
186 diquat
187 disulfoton
188 dithiobiurea, 2,5-
189 diuron
190 dodecylguanidine acetate, n-
191 EDTA, disodium
192 endothall
193 ephedrine sulfate
194 epichlorohydrin
195 erythromycin stearate
196 erythrosine
197 ethalfuralin
198 ethephon
199 ethion
200 ethyl dipropylthiocarbamate, s-
201 ethyl p-nitrophenyl phenylphosphorothioate
202 ethylene chlorohydrin
203 ethylene thiourea
204 ethylmethylphenylglycidate
205 fast green FCF
206 febantel
207 fenamiphos
208 fenbendazole
209 fenchlorphos
210 fluometuron
211 fluoranthene
212 fluorene
213 fluridone
214 flurprimidol
215 flutolanil
216 fluvalinate
217 folpet
218 fonofos
219 fosetyl-al
220 glufosinate-ammonium
221 glyphosate
222 haloxyfop-methyl
223 HC blue no. 1
224 HC blue no. 2
225 HC yellow 4
226 heptachlor
227 heplachlor epoxide
228 hexabromobenzene
229 hexachlorobenzene
230 hexachlorobutadiene
231 hexachlorocyclohexane, gamma-
232 hexachlorocyclopentadiene
233 hexachloroethane
234 hexachlorophene
235 hexahydro-1,3,5-trinitro-1,3,5-triazine
236 hexamethylenetetramine
237 hexazinone
238 hexythiazox
239 hydralazine
240 hydrazobenzene
241 hydrochlorothiazide
242 hydroxypropyl methanethiolsulfonate, 2-
243 hydroxyquinoline, 8-
244 imazalil
245 imazaquin
246 imazethapyr
247 iodinated glycerol
248 ipazilide
249 iprodione
250 ipronidazole
251 isopropalin
252 isoxaben
253 jervine
254 lactofen
255 lansoprazole
256 levamisole
257 linamarin
258 linuron
259 londax
260 malaoxon
261 malathion
262 maleic anhydride
263 maleic hydrazine
264 manidipine hydrochloride
265 melamine
266 mepiquat chloride
267 mercaptobenzothiazole, 2-
268 merphos
269 merphos oxide
270 meso-2,3-dimercaptosuccinic acid
271 metalaxyl
272 methamidophos
273 methidathion
274 methomyl
275 methoxychlor
276 methoxypsoralen, 8-
277 methyl carbamate
278 methyl ethyl ketoxime
279 methyl parathion
280 methyl-4-chlorophenoxyacetic acid, 2-
281 methyl-N-methylanthranilate
282 methyldopa sesquthydrate, alpha-
283 methylolacrylamide, N-
284 metolachlor
285 metribuzin
286 metsulfuron methyl
287 mexacarbate
288 miporamicin
289 mirex
290 molinate
291 monodiethanolamine salt of riboflavin-5'-phosphate
292 monuron
293 myclobutanil
294 naled
295 nalidixic acid
296 naphthoxy acetic acid, 2-
297 napropamide
298 natamycin
299 nitro-o-toluidine, 5-
300 nitro-p-phenylenediamine, 2-
301 nitroaniline, p-
302 nitroanthranilic acid, 4-
303 nitrofurantoin
304 nitrofurazone
305 nitroguanidine
306 nitronaphthalene, 1-
307 nitrosodiphenylamine, N-
308 norflurazon
309 ochratoxin A
310 octabromodiphenyl ether
311 octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine
312 olaquindox
313 oryzalin
314 oxadiazon
315 oxamyl
316 oxfendazole
317 oxyfluorfen
318 oxytetracycline
319 oxytetracycline hydrochloride
320 oxythioquinox
321 paclobutrazol
322 paraquat
323 parathion
324 patulin
325 pendimethalin
326 penicillin VK
327 pentabromodiphenyl ether
328 pentachloroethane
329 pentachloronitrobenzene
330 pentachlorophenol
331 pentaerythritol tetranitrate
332 permethrin
333 phenformin
334 phenyl-2-naphthylamine, n-
335 phenyl-3-methyl-5-pyrazolone, 1-
336 phenylbutazone
337 phenylenediamine, m-
338 phenylephrine hydrochloride
339 phosmet
340 phosphamidon
341 photodieldrin
342 phthalamide
343 phthalic anhydride
344 picloram
345 pilsicainide hydrochloride
346 pirimiphos-methyl
347 pravadoline
348 probenecid
349 prochloraz
350 proflavine monohydrochloride hemihydrate
351 promethazine hydrochloride
352 prometon
353 prometryn
354 pronamide
355 propachlor
356 propanil
357 propargite
358 propazine
359 propham
360 propiconazole
361 propiverine hydrochloride (P-4)
362 propoxur
363 propylene dichloride
364 pydrin
365 pyrazinamide
366 pyrene
367 pyrimidinone
368 quercetin
369 quinalphos
370 quinine hydrochloride
371 quinofop ethyl
372 red 2G
373 reserpine
374 resmethrin
375 thudamine 6G
376 ronidazole
377 rotenone
378 saccharin
379 sethoxydim
380 simazine
381 sodium 2,2-dichloropropionate
382 sodium cyclamate
383 sodium fluoroacetate
384 sodium naphthionate
385 sorbitan monolaurate
386 sorbitan monostearate
387 succinic anhydride
388 sucrose acetate isobutyrate
389 sulfadimidine
390 sulfisuxazole
391 sulfolene, 3-
392 sunset yellow FCF
393 suplatast tosilate
394 tebuthiuron
395 terbacil
396 terbutryn
397 tert-butyl-2-chlorophenol, 4-
398 tetrachlorobenzene, 1,2,4,5-
399 tetrachloroethane, 1,1,1,2-
400 tetrachloroethane, 1,1,2,2-
401 tetrachloroethylene
402 tetrachlorophenol, 2,3,4,6-
403 tetrachloropyridine, 2,3,5,6-
404 tetrachlorvinphos
405 tetracycline hydrochloride
406 tetraethyldithiopyrophosphate
407 tetraethylthiourea disulfide
408 tetrahydrocannabinol, delta-9-
409 tetrakis(hydroxymethyl)phosphonium chloride (THPC)
410 tetrakis(hydroxymethyl)phosphonium sulphate (THPS)
411 thiabendazole
412 thiameturon methyl
413 thiobencarb
414 thiophanate-methyl
415 thiram
416 tocopheryl acetate, dl-alpha-
417 toluenediamine hydrochloride, 2,6-
418 toluenediamine sulfate, 2,5-
419 tralomethrin
420 trenbolone acetate
421 trenbolone hydroxide, 17-alpha-
422 triadimefon
423 triallate
424 triamterene
425 tribromomethane
426 trichloroacetonitrile
427 trichlorobenzene, 1,2,4-
428 trichloroethane, 1,1,2-
429 trichloroethane, 1,1,1-
430 trichloroethylene
431 trichloroethylene, 1,1,2-
432 trichlorogalactosucrose
433 trichlorophenol, 2,4,5-
434 trichlorophenoxyacetic acid, 2,4,5-
435 tridiphane
436 triethylene tetramine dihydrochloride
437 trifluralin
438 trimethylaniline, 2,4,5-
439 trinitrotoluene, 2,4,6-
440 tris-(2-chloroethyl) phosphate
441 trisulfuron
442 tylosin
443 vinclozolin
444 vinyl chloride
445 zatosetron maleate
446 zearalenone
447 zeranol
The principal objective of the safety evaluation procedure is to
identify two groups of flavourings substances: i) those substances
whose structure, metabolism, and relevant toxicity data clearly
indicate that the substance would be expected not to be a safety
concern under current conditions of intended use; and ii) those
substances which may require additional data in order to perform an
adequate safety evaluation.
3.4 Integrating data on the consumption ratio
As pointed out by WHO (1987) and SCF (1991), natural occurrence
is no guarantee of safety, but it is important to recognize that the
safety evaluation of added use of flavouring substances needs to be
conducted with an appreciation of the consumption ratio. Clearly, if
added use of flavouring substances results in an exposure that exceeds
that from natural sources, this will increase awareness of the need to
consider carefully overall exposure in the light of existing data on
toxicity and structure-activity relationships. On the other hand, if
the added use is trivial with a consumption ratio of 10 to 100, that
is, it increases total exposure by only 1 to 10%, then this fact needs
to be taken into consideration when applying the criteria outlined
above.
The substances of primary concern are those which, in Figure 2,
receive a "No" answer to Step No. 5, or a "Yes" answer to Step No. 7,
indicating a possible need for additional data and evaluation beyond
that included in the evaluation procedure outlined in this paper. In
such an evaluation, as stated immediately above, the extent of natural
occurrence should be given appropriate weight.
Of much less concern with respect to consumption ratio are those
substances that drop out of further consideration as a result of "No"
answers to Step Nos. 3 or 7, or "Yes" answers to Step Nos. 4 or 6. The
derivation of the thresholds, the estimation of intakes (see Appendix
A), and the special factors applicable to the use of flavours
(volatility, self-limiting use, etc.) build in multiple conservative
assumptions more than adequate to cover additional exposure from
natural occurrence to substances that in any case are of low inherent
concern.
The advances in analytical chemistry in the past 50 years provide
virtual assurance that no flavouring substances of extensive natural
occurrence remain unknown. Those of potential value yet to be
discovered (e.g., as yet unknown components of roast beef, coffee, or
chocolate flavour) are being sought at the ppb and ppt level. This
does not suggest exposures above any of the thresholds discussed in
this paper.
4. CONCLUSIONS
It is neither possible nor necessary to conduct toxicological
studies on all individual flavouring substances used in food. The vast
majority of flavouring substances are members of groups of substances
with common metabolic pathways, and typically, individual members of
such a group display a similar toxicity profile. This is not
surprising in light of the close structural similarity of the various
flavouring substances comprising a chemical group. As demonstrated in
this paper, exposure to flavouring substances is usually low and, in
the majority of cases, below the human exposure threshold values
presented in this paper. This knowledge, coupled with knowledge
regarding structure-activity relationships, metabolic disposition, and
toxicology data, permits the establishment of the proposed paradigm
for safety assessment which provides a solid foundation to assure the
safety of flavouring substances under conditions of intended use.
5. REFERENCES
BECK, B.D., CONOLLY, R.B., DOURSON, M.L., GUTH, D., HATTIS, D.,
KIMMEL, C. & LEWIS, C. (1993). Symposium overview. Improvements in
quantitative noncancer risk assessment. Fund Appl Toxicol.,
20: 1-14.
COUNCIL OF EUROPE (1974). Natural Flavouring Substances, Their
Sources, And Added Artificial Flavouring Substances. Council of
Europe, Strasbourg, France.
COUNCIL OF EUROPE ( 1981 ). Flavouring Substances And Natural Sources
Of Flavourings, Third Edition. Council of Europe, Strasbourg, France.
COUNCIL OF EUROPE (1992). Flavouring Substances and Natural Sources
of Flavourings. Volume I. Chemically-Defined Flavouring Substances,
Fourth Edition. Council of Europe, Maissoneuve, France.
CRAMER, G.M., FORD, R.A. & HALL, R.L. (1978). Estimation of toxic
hazard-A decision tree approach. Food Cosmet Toxicol., 16: 255-276.
DEISINGER, P.J., BOATMAN, R.J. & GUEST, D. (1994). Metabolism of
2-ethylhexanol administered orally and dermally to the female Fischer
344 rat. Xenobiotica, 24: 429-440.
FOOD AND DRUG ADMINISTRATION (1982). Toxicological Principles for the
Safety Assessment of Direct Food Additives and Color Additives Used in
Food. Redbook. U.S. Food and Drug Administration, Bureau of Foods,
Washington, DC.
FOOD AND DRUG ADMINISTRATION (1993). Toxicological Principles for the
Safety Assessment of Direct Food Additives and Color Additives Used in
Food. Redbook II (Draft). U.S. Food and Drug Administration, Center
for Food Safety and Applied Nutrition, Washington, DC.
FEDERAL REGISTER (1993). Food additives; threshold of regulation for
substances used in food-contact articles. 58(195): 52719-52729.
FRAWLEY, J.P. (1967). Scientific evidence and common sense as a basis
for food-packaging regulations. Food Cosmet Toxicol., 5: 293-308.
GOLD, L.S., SAWYER, C.B., MAGAW, R., BACKMAN, G.M., DE VECIANA, M.,
LEVINSON, R., HOOPER, N.K., HAVENDER, W.R., BERNSTEIN, L., PETO, R.,
PIKE, M. & AMES, B.M. (1984). A carcinogenic potency database of the
standardized results of animal bioassays. Environ Health Perspect.,
58: 9-319.
GOLD, L.S., SLONE, T.H. & BERNSTEIN, L. (1989). Summary of
carcinogenic potency and positivity for 492 rodent carcinogens in the
carcinogenic potency database. Environ. Health Perspect., 79: 259-272.
JAESCHKE, H., KLEINWAECHTER, C. & WENDEL, A. (1987). The role of
acrolein in allyl alcohol-induced lipid peroxidation and liver cell
damage in mice. Biochem. Pharmacol., 36: 51-57.
JECFA (1967). Toxicological Evaluation of Some Flavouring Substances
and Non-Nutritive Sweetening Agents. Eleventh Meeting of the Joint
FAO/WHO Expert Committee on Food Additives.
JECFA (1968). Specifications for the Identity and Purity of Food
Additives and their Toxicological Evaluation: Some Flavouring
Substances and Non-nutritive Sweetening Agents. Eleventh Report of the
Joint FAO/WHO Expert Committee on Food Additives, FAO Nutrition
Meetings Report Series No. 44, WHO Technical Report Series No. 383.
JECFA (1970). Toxicological Evaluation of Some Extraction Solvents and
Certain Other Substances. Fourteenth Meeting of the Joint FAO/WHO
Expert Committee on Food Additives.
JECFA (1971). Evaluation of Food Additives. Fourteenth Report of the
Joint FAO/WHO Expert Committee on Food Additives, WHO Technical Report
Series No. 462.
JECFA (1972). Evaluation of Food Additives. Some Enzymes, Modified
Starches, and Certain Other Substances: Toxicological Evaluations and
Specifications and a Review of the Technological Efficacy of Some
Antioxidants. Fifteenth Report of the Joint FAO/WHO Expert Committee
on Food Additives, WHO Technical Report Series No. 488.
JECFA (1974a). Toxicological Evaluation of Certain Food Additives
Including Anticaking Agents, Antimicrobials, Antioxidants, Emulsifiers
and Thickening Agents. Seventeenth Meeting of Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 5.
JECFA (1974b). Toxicological Evaluation of Certain Food Additives with
a Review of General Principles and of Specifications. Seventeenth
Report of the Joint FAO/WHO Expert Committee on Food Additives, WHO
Technical Report Series No. 539.
JECFA (1976a). Evaluation of Certain Food Additives. Twentieth Report
of the Joint FAO/WHO Expert Committee on Food Additives, FAO Nutrition
Meetings Report Series No. 1, WHO Technical Report Series No. 599.
JECFA (1976b). Toxicological Evaluation of Certain Food Additives.
Twentieth Meeting of the Joint FAO/WHO Expert Committee on Food
Additives, WHO Food Additive Series No. 10.
JECFA (1978). Evaluation of Certain Food Additives and Contaminants.
Twenty-second Report of the Joint FAO/WHO Expert Committee on Food
Additives, WHO Technical Report Series No. 631.
JECFA (1980a). Evaluation of Certain Food Additives. Twenty-third
Report of the Joint FAO/WHO Expert Committee on Food Additives. World
Health Organization, Geneva. WHO Technical Report Series No. 648.
JECFA (1980b). Evaluation of Certain Food Additives. Twenty-fourth
Report of the Joint FAO/WHO Expert Committee on Food Additives. World
Health Organization, Geneva. WHO Technical Report Series No. 653.
JECFA (1980c). Toxicological Evaluation of Certain Food Additives.
Twenty-third Meeting of the Joint FAO/WHO Expert Committee on Food
Additives, WHO Food Additive Series No. 14.
JECFA (1981a). Evaluation of Certain Food Additives. Twenty-fifth
Report of the Joint FAO/WHO Expert Committee on Food Additives,
Technical Report Series No. 669.
JECFA (1981b). Toxicological Evaluation of Certain Food Additives.
Twenty-fifth Meeting of the Joint FAO/WHO Expert Committee on Food
Additives, WHO Food Additive Series No. 16.
JECFA (1982a). Evaluation of Certain Food Additives and Contaminants.
Twenty-sixth Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 683.
JECFA (1982b). Toxicological Evaluation of Certain Food Additives.
Twenty-sixth Meeting of the Joint FAO/WHO Expert Committee on Food
Additives, WHO Food Additive Series No. 17.
JECFA (1983a). Evaluation of Certain Food Additives and Contaminants.
Twenty-seventh Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 696.
JECFA (1983b). Toxicological Evaluation of Certain Food Additives and
Contaminants. Twenty-seventh Meeting of the Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 18.
JECFA (1984a). Evaluation of Certain Food Additives and Contaminants.
Twenty-eighth Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 710
JECFA (1984b). Toxicological Evaluation of Certain Food Additives and
Contaminants. Twenty-eighth Meeting of the Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 19.
JECFA (1986). Evaluation of Certain Food Additives and Contaminants.
Twenty-ninth Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 733.
JECFA (1987a). Evaluation of Certain Food Additives and Contaminants.
Thirtieth Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization. Geneva. WHO Technical Report
Series No. 751.
JECFA (1987b). Evaluation of Certain Food Additives and Contaminants.
Thirty-first Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 759.
JECFA (1989). Evaluation of Certain Food Additives and Contaminants.
Thirty-third Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 776.
JECFA (1990a). Evaluation of Certain Food Additives and Contaminants.
Thirty-fifth Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 789.
JECFA (1990b). Toxicological Evaluation of Certain Food Additives and
Contaminants. Thirty-fifth Meeting of the Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 26.
JECFA (1991a). Evaluation of Certain Food Additives and Contaminants.
Thirty-seventh Report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva. WHO Technical Report
Series No. 806.
JECFA (1991b). Toxicological Evaluation of Certain Food Additives and
Contaminants. Thirty-seventh Meeting of the Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 28.
JECFA (1992). Evaluation of Certain Food Additives and Naturally
Occurring Contaminants. Thirty-ninth Report of the Joint FAO/WHO
Expert Committee on Food Additives. World Health Organization, Geneva.
WHO Technical Report Series No. 828.
JECFA (1993a). Evaluation of Certain Food Additives and Contaminants.
Forty-first Report of the Joint FAO/WHO Expert Committee on Food
Additives, Technical Report Series No. 837.
JECFA (1993b). Toxicological Evaluation of Certain Food Additives and
Contaminants. Forty-first Meeting of the Joint FAO/WHO Expert
Committee on Food Additives, WHO Food Additive Series No. 32.
JECFA (1993c). Toxicological Evaluation of Certain Food Additives and
Naturally Occurring Toxicants. Thirty-Ninth Meeting of the Joint
FAO/WHO Expert Committee on Food Additives, WHO Food Additive Series
No. 30.
KRASAVAGE, W.J., O'DONOGHUE, J.L., DIVINCENZO, G.D. & TERHAAR, C.J.
(1980). The relative neurotoxicity of methyl-n-butyl ketone, n-hexane
and their metabolites. Toxicol. Appl. Pharmacol., 52: 433-441.
LEWIS, S.C., LYNCH, J.R. & NIKIFOROV, A.I. (1990). A new approach to
deriving community exposure guidelines from "no-observed-adverse-
effect levels". Regul. Toxicol. Pharmacol., 11: 314-330.
MUNRO, I.C. (1990). Safety assessment procedures for indirect food
additives: An overview. Regul. Toxicol. Pharmacol., 12: 001-0011.
NAS (1978). Resurvey of the Annual Poundage of Food Chemicals
Generally Recognized as Safe, National Technical Information Service
(NTIS) PB288-081.
NAS (1979). Comprehensive Survey of Industry on the Use of Food
Additives, National Technical Information Service (NTIS) PB80-113418.
NAS (1984). Poundage Update of Food Chemicals, National Technical
Information Service (NTIS) PB84-162148.
NAS (1989). 1987 Poundage and Technical Effects Update of Substances
Added to Food. National Research Council, Washington, DC. Prepared
for: Food and Drug Administration, Washington, D.C. NTIS Report
No. PB91-127266.
PHILLIPS, J.C., PURCHASE, R., WATTS, P. & GANGOLLI, S.D. (1987). An
evaluation of the decision tree approach for assessing priorities for
safety testing of food additives. Food Addit. Contam., 4(2): 109-123.
RULIS, A.M. (1986). De Minimis and the Threshold of Regulation.
In: Felix, C.W. (Ed.) Food Protection Technology. Lewis Publishers
Inc., Chelsea, MI. pp. 29-37.
SCF (1991). Guidelines for the Evaluation of Flavourings for Use in
Foodstuffs: I. Chemically Defined Flavouring Substances. Commission of
the European Communities, Scientific Committee for Food, Brussels,
Belgium.
STOFBERG, J. & KIRSCHMAN, J. (1985). The consumption ratio of
flavouring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23: 857-860.
STOFBERG, J. & GRUNDSCHOBER, F. (1987). Consumption ratio and food
predominance of flavouring materials. Perfumer Flavourist,
12: 27-68.
WEIL, C.S. & McCOLLISTER, D.D. 1963. Safety evaluation of chemicals.
Relationship between short- and long-term feeding studies in designing
an effective toxicity test. Agr. Food Chem., 11(6): 486-491.
WHO (1987). Principles For The Safety Assessment Of Food Additives
And Contaminants In Food. WHO Environmental Health Criteria. World
Health Organization (WHO), International Program on Chemical Safety in
Cooperation with the Joint FAO (Food & Agriculture Organization of the
United Nations), WHO Expert Committee on Food Additives (JECFA),
Geneva, Switzerland, Environmental Health Criteria 70.
WOODS, L.A. & DOULL, J. (1991). GRAS evaluation of flavouring
substances by the Expert Panel of FEMA. Regul. Toxicol. Pharmacol.,
14: 48-58.
ANNEX 5, Appendix A
ESTIMATING DISTRIBUTION OF INTAKES OF FOOD INGREDIENTS ACROSS
THE POPULATION
In response to the Food Additives Amendment of 1958 to the U.S.
Food, Drug and Cosmetic Act, the Flavor and Extract Manufacturers'
Association (FEMA) in 1960 conducted a comprehensive survey of all
flavoring materials then thought or claimed to be in use. Information
included: identity; poundage used annually in food; year of first use;
levels (in ppm) used in major food categories; toxicological data; and
other information related to safety. After evaluation by a panel of
external experts, this and subsequent information was published in a
series of "FEMA GRAS Lists" (Hall and Oser, 1965, 1970; Oser and Hall,
1972; Oser and Ford, 1973ab, 1974, 1975, 1977, 1978, 1979; Oser et al.,
1984, 1985; Burdock et al., 1990; Smith and Ford, 1993).
FEMA determined in 1968 that another survey was needed to monitor
trends and to improve on the previous effort. In addition, the U.S.
Food and Drug Administration (FDA) began to plan to review its
Generally Recognized as Safe (GRAS) lists, and in so doing, made use
of the prior experience of FEMA. FEMA and FDA efforts merged into the
first comprehensive survey of ingredients used in food, conducted by
the National Academy of Sciences/National Research Council (NAS) in
1970 (NAS, 1973) with several subsequent surveys (NAS, 1978, 1979,
1984, 1989).
Emerging from the 1960 and 1970 surveys was a clear indication
that per capita intake of an ingredient (from annual poundage),
although useful for comparison with other ingredients, has a
significant limitation. As an average of intakes by those who consume
foods containing the ingredient ("eaters") and by those who do not
(non-eaters"), it does not represent the intake by the individuals of
concern ( i.e., the "eaters"). This is not the case for ingredients
commonly consumed by almost everyone ( e.g., sugar), but the
difference between the average and "eaters" is significant for other
ingredients consumed only by few people ( e.g. raspberry flavor).
Because of these issues, an alternative method of calculating
intakes was used in the first (1973) NAS report. This method involved
the average level of use of an ingredient in a broad food category
(e.g., baked goods) multiplied by the average daily consumption of
that food category per person. The daily consumption for each category
was obtained by multiplying the average portion size for the category,
obtained from the U.S. Department of Agriculture (USDA) Food
Consumption Survey by the average daily frequency of consumption of
foods in that category over a 14-day survey period. The frequency of
consumption data came from a 12,000+ member panel operated by the
Market Research Corporation of America (MRCA; see note b to Table 1).
Thus the average intake of an ingredient for all relevant food
categories provided the "possible average daily intake" (PADI). These
intakes were often highly exaggerated, as much as a hundred-fold,
because an ingredient used in only a few foods in a food category was
assumed to be at the average level in all foods in the category. Thus,
for example, cardamom, used only in a few coffee cakes, was assumed to
be used in each food in the entire category of baked goods ( i.e.,
all breads, rolls, crackers, and pastries).
To address this issue, another method was developed to give the
probable distribution of intakes for the entire population without
exaggerating intake. This required an informed estimate of the
probability that each food eaten would contain the ingredient of
interest. The first series of such intake estimates used calculation
"A", as shown in Table 1, and the results are summarized in Section
"A" of Table 2.
In Table 2, Column 1 ("Substance") lists the substances selected
to test and improve the more refined method. In Section A, nutmeg and
mace were chosen, among other reasons because they are not grown
domestically in the U.S. and import data, therefore, provide a firm
external check on pounds used. Furthermore, more than half of all
nutmeg is consumed in four foods: bologna, pork sausage, frankfurters,
and doughnuts. It thus presented an opportunity to see how heavy
consumers of those foods would skew total intake distribution. The
flavor ingredient 4-(p-hydroxyphenyl)butan-2-one was chosen because it
is used in, and occurs naturally, almost entirely in only one flavor,
raspberry flavor, and is far less widely consumed than chocolate,
vanilla, or citrus flavors. Thus, it is a good example of an uncommon
ingredient consumed by those who particularly like the few foods in
which it is used (Hall, 1975).
Column 2 [" per capita (External Check)"] lists per capita
intake derived from import data, product manufacturer surveys, or
tariff reports, all independent of the NAS survey to serve as an
external check. Column 3 (" per capita [NAS(lbs)/0.6]") lists the
per capita intake from the poundage reported in the NAS survey
assumed to be 60% complete. Further research (see references to NRC
surveys) showed this to be generally a valid assumption, but with
certain qualifications. Ingredients such as sugar, corn syrup, and
salt, could not be fully or reliably reported by bakers and other
small food processors, whereas the flavor industry reported more
fully, and the 60% assumption, while used, usually resulted in a
poundage per capita overestimate.
For Section A, Column 4 ["50th Percentile (Total Panel)"]
presents the total panel 50th percentile for each substance, and
Column 4a ("50th Percentile 3 S.D. Total Panel"), the 50th percentile
plus 3 standard deviations. Column 4b ["very high (Total Panel)"]
shows the so-called "very high intake". This is not a realistic
figure, because no person could consume all foods using an ingredient
at the highest level for every food in every major category. Note
particularly, also, the earlier discussion of the exaggeration that
arises from assuming that an ingredient used only in a few foods is
used in all foods in a major food category. The "very high intake"
serves simply as a wholly unreachable and therefore unrealistic value.
Given the remaining and inevitable uncertainties due to possible
errors in estimations, and possible non-food uses, the agreement
between the total panel 50th percentile (Section A, Column 4), the NRC
per capita, (Column 3), and the per capita from external sources
(Column 2), is entirely satisfactory. However, calculation "A" did
not provide data on only those who actually consumed the substance
("eaters only"), and the statistical treatment was not as solidly
based as was desirable. This led to an improved calculation, "B", as
shown in Table 3. Table 2, Section B, presents the results of these
calculations.
The substances shown in Column 1 of Section B were selected, as
discussed in the referenced reports themselves, by the FDA or the NAS
committee (NAS, 1976). An improved method of calculation was used, as
described below, which provides the total sample mean (Column 4 ["Mean
(Total Panel)"]) as a check against the per capita data in Columns 2
and 3.
The external per capita figure for calcium propionate is twice
the total panel mean, probably due primarily to usage by many small
bakeries not included in the survey, and also to the high wastage of
bread. The slightly smaller discrepancy for MSG may well reflect
several added sources of error. Even so, comparison of the total panel
mean with the per capita data from the NAS survey and the external
checks shows satisfactory agreement. Food consumption surveys have
significant and well-recognized sources of error in addition to those
affecting the NRC surveys, and it seems unlikely that it is practical
to expect intake estimates derived by different means to correspond
more closely than within a factor of two.
For Section B, Column 4 ["Mean (Total Panel)"] provides the total
panel mean, Column 4a ["Mean (Eaters Only)"] the "eaters only" mean
age, 2 and over and Column 4b ["S.D. (Eaters Only)"], the standard
deviation for "eaters only".
The procedure in calculation B does not eliminate all sources of
error. Intake is still calculated from "disappearance figures"
( i.e, the levels introduced into food during processing, not the
lower levels actually consumed). Thus, ignoring the often large losses
due, for example, to volatilization or leaching in processing,
preparation and storage result in exaggerated estimates of intake. In
the other direction, heavy (frequent) eaters will often eat larger
portion sizes, resulting in a tendency to underestimate higher
intakes. The result overall, however, remains highly satisfactory.
Table 1 Calculation "A"
Step Operation Result
For each specific food (SF)a e.g. bologna,
within a broad food category, eg., meats,
obtain from MRCAb the
1 total number of eatings of each SF by the total
panel, in two weeks
14 days × 12,337 (persons in panel) = mean frequency of eating, p/d
×
2 mean portion size in gc = mean consumption of SF, g/p/d
×
3 probability of SF containing ingredient "I"d = probable mean consumption of SF
containing I g/p/d
×
4 usual use level of "I" in ppm of major food = 50th percentile of probable intake of I
category containing SF ‰ 1,000e from SF in mg/p/d
×
5 90th percentile MRCA frequency of eating = 90th percentile of probable intake of I
50th percentile MRCA frequency of eating from SF in mg/p/d
6 & 7 sum 4 and 5 separately across all SFs in that = 50th and 90th percentiles of probable
major food category intake of I from that major food category
Table 1 (cont'd) Calculation "A"
Step Operation Result
8 fit a curve, assuming normal distribution, to = 50th percentile and standard deviation
the points determined in 6 and 7 and calculate (SD) for each major food category
9 repeat steps 1 through 8 and sum across all = 50th percentile and SD in mg/p/d
food categories probable total intake
10 repeat steps 4 and following, using "average = "very high intake"
maximum use level" from the survey for each
major food category
a Specific food (SF), in this context, denotes a narrow category of very similar foods serving
the same dietary and market niches (e.g. all bologna, all cola drinks). The eatings of each SF
include in one total for each SF all eatings of similar brands or home-prepared versions of the
same SF. There were approximately 10,000 such SFs.
b The Market Research Corporation of America (MRCA) is a private market research company that
surveys usage of food products and components It uses a nationally representative panel and
records for each panel member the number of servings of each specific loud for each day of a
two-week period. The panelists' reporting periods are distributed evenly throughout the year. The
1973 NAS report used data from the third Menu Census (1967-1968) involving 12,871 individuals.
The dataused in calculations "A" and "B" came from the Fourth Menu Census (1972-1973) involving
12,337 individuals.
c The USDA Food Consumption Survey (USDA, 1965)
d Obtained from a consensus panel of experts knowledgeable about the ingredient and the food.
e From NAS, 1973
Table 2 Distribution of MRCA 14-Day Average Daily Intakes (in mg/person/day)a of Selected Food Ingredients
(1) (2) (3) (4) (4a) (4b) (5)
"High Eaters
Section Substance per capita per capita Mean Mean plus "Very High" Only"
(External [NAS (lbs.)/0.6] (Total Panel) 3 S.D.b (Total Panel) [NAS
Check) Total Panel (lbs.)/0.6]×10
A Nutmeg 23.6 10.3 15.7 51.3 71.1 103
Nutmeg Oil 1.2 1.0 2.0 5.7 7.1 10
A Mace 3.3 2.8 3.3 8.6 14.4 28
Mace, Oil NAc 0.26 0.19 0.67 0.46 2.6
Mace, Oleoresin NA 0.070 0.13 0.39 0.41 0.70
A 4-p-Hydroxyphenyl 0.048 0.086 0.041 0.16 0.37 0.86
Butan-2-one
Table 2 (cont'd).
(1) (2) (3) (4) (4a) (4b) (5)
"High Eaters
Section Substance per capita per capita Mean Mean plus "Very High" Only"
(External [NAS (lbs.)/0.6] (Total Panel) 3 S.D.b (Total Panel) [NAS
Check) Total Panel (lbs.)/0.6]×10
Mean Mean S.D.
(Total Panel) (Eaters Only) (Eaters Only)
B Calcium Propionate 92 33 44.8 45.1 20.7 330
Sodium Propionate NA 16 33,2 33.3 14.7 160
B Carob Bean Gum NA 26 35.6 36.1 35.0 260
Chondrus Extract 19.3 13 10.6 11.1 18.5 130
B Mono- and diglycerides NA 727 884 885 467 7,270
Monosodium Glutamate 186 194 98.8 99.0 88.5 1,940
(MSG)
a See text for explanation of columns.
b S.D. = Standard Deviation.
Table 3 Calculation "B"
1 From the MRCA survey, obtain the total
number of eatings of each SF, by each panel
member, each day over the 14 - day period
×
2 USDA mean portion size, for that person's = quantity of SF in g for that
age group and the relevant major food person, that day
category
×
3 weighted mean of usual use level of I in = quantity of I in mg/d, that
ppm ‰ 1,000 person, that day, if all of
the SF contained I
×
4 probability that particular SF contains I = expected intake of I for
that person for that day,
mg/d
5 Repeat steps 1 through 4 for that person for = expected intake of I for
each of the 14 days that person for 14 days,
mg
6 ‰ 14 = average daily intake over
14 days for that person,
g/p/d
7 tabulate all intakes and compute = total panel mean intake of I
mg/p/d
8 discard all persons with zero intake for the = "eaters only" mean and SD
14 day period, tabulate all "eaters only" of for I, mg/p/d
I, age 2 and over, and compute
Unfortunately, this more refined approach, while successful, is
extremely elaborate, tedious, and expensive. The MRCA panel is
expensive to maintain and operate, and the data are correspondingly
costly. The approach requires extensive input from a broadly-based,
necessarily large and well-informed panel of expert technologists.
Such an effort can only be justified in those few cases where it is
necessary to obtain the distribution of intakes with maximum accuracy.
Given the wide safety margins food ingredients typically enjoy, and
the uncertainties and assumptions inherent in interpreting
toxicological data, such effort is rarely necessary. A much more
appropriate, but quick and easy, method of estimating intake was
needed, so long as it was conservative, but not excessively so. This
led Rulis and others in FDA (Rulis et al., 1984; Rulis, 1987) to
test and adopt the assumption that, except for an unusually narrow
margin of safety or specific concern, the per capita "eaters only"
intake could safely and conservatively be assumed - even for heavy
eaters - simply by multiplying the best available estimate of per
capita intake by ten (Column 5 ["High Eaters Only"]). Comparison of
Column 5 with Column 4b, for Section B, makes clear that in every case
the: "High Eaters Only" ( per capita × 10) estimate far exceeds the
"Eaters Only mean plus 3 standard deviations", yet in no case are the
estimates so high as to be unusable.
With the special factors surrounding the use of flavoring
ingredients (i.e., completeness of the surveys, typical volatility
with consequent loss in processing, and self-limiting nature) the
procedure in Column 5 is practical but highly conservative. It has
been widely used in other publications (FDA, 1982, 1993; Easterday
et al., 1993). It can be used safely in all instances except when a
very narrow margin of safety requires maximally precise data for both
toxicological effects and exposure.
Recently, still more sophisticated methods for analyzing USDA
Food Consumption Survey data have become available under license for
those applications in which the need for relatively high precision of
estimates justifies the cost (Helmbach, 1994). In nearly all instances
where broadly- based ingredient disappearance data are available,
per capita × 10, in actuality, a very high "eaters only" estimate,
remains the approach that is by far the simplest and least expensive,
while being conservative but realistic.
REFERENCES
Burdock, G.A., Wagner, B.M., Smith, R.L., Munro, I.C. and Newberne,
P.M. 1990. Recent progress in the consideration of flavoring
ingredients under the food additives amendment. 15. GRAS substances.
A list of flavoring ingredient substances considered generally
recognized as safe by the Flavor & Extract Manufacturers' Association
Expert Panel. Food Technol 44(2):78, 80, 82, 84, & 86.
Easterday, O.D., Ford, R.A., Hall, R.L., Stofberg, J., Cadby, P. and
Grundschober, F. 1992. A Flavor Priority Ranking System, Acceptance
and Internationalization. In: Finley, J.W., Robinson, S.F. and
Armstrong, D.J. (Eds.) Food Safety Assessment. American Chemical
Society, ACS Symposium Series No. 484.
FDA. 1982. Toxicological Principles for the Safety Assessment of
Direct Food Additives and Color Additives Used in Food. Redbook. U.S.
Food and Drug Administration, Bureau of Foods, Washington, DC.
FDA. 1993. Toxicological Principles for the Safety Assessment of
Direct Food Additives and Color Additives Used in Food. Redbook II
(Draft). U.S. Food and Drug Administration, Center for Food Safety and
Applied Nutrition, Washington, DC.
Hall, R.L. and Oser, B.L. 1965. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment. III. GRAS
substances. Food Technol 19(2):151-197.
Hall, R.L. and Oser, B.L. 1970. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment. IV. GRAS
substances. Food Technol 24(5):25-28, 30-32, 34.
Hall, R.L. 1975. Unpublished data from personal communication to the
NAS/NRC Committee on GRAS List Survey - Phase III, and to the FEMA.
Heimbach, J.T. 1994. Personal communication re: TAS-DIET from J.T.
Helmbach, Technical Assessment Systems, Inc., Washington, D.C.
NAS. 1973. A comprehensive Survey on the Use of Food Chemicals
Generally Recognized as Safe, National Technical Information Service
(NTIS) PB-221949.
NAS. 1976. Estimating Distribution of Daily Intakes of Certain GRAS
Substances. National Research Council, Washington, DC. Prepared for:
Food and Drug Administration, Washington, DC. December 1976. National
Technical Information Service (NTIS) PB-299-381.
NAS. 1978. Resurvey of the Annual Poundage of Food Chemicals Generally
Recognized as Safe, National Technical Information Service (NTIS)
PB288-081.
NAS. 1979. Comprehensive Survey of Industry on the Use of Food
Additives, National Technical Information Service (NTIS) PB80-113418.
NAS. 1984. Poundage Update of Food Chemicals, National Technical
Information Service (NTIS) PB84-162148.
NAS. 1989. 1987 Poundage and Technical Effects Update of Substances
Added to Food. National Research Council, Washington, DC. Prepared
for: Food and Drug Administration, Washington, D.C. NTIS Report
No. PB91-127266.
Oser, B.L. and Ford, R.A. 1973a. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 6. GRAS
substances. Food Technol 27(1):64-67.
Oser, B.L. and Ford, R.A. 1973b. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 7. GRAS
substances. Food Technol 27(11):56-57.
Oser, B.L. and Ford, R.A. 1974. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 8. GRAS
substances. Food Technol 28(9):76-80.
Oser, B.L. and Ford, R.A. 1975. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 9. GRAS
substances. Food Technol 29(8):70-72.
Oser, B.L. and Ford, R.A. 1977. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 10. GRAS
substances. Food Technol 31(1):65-74.
Oser, B.L. and Ford, R.A. 1978. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 11. GRAS
substances. Food Technol 32(2):60-70.
Oser, B.L. and Ford, R.A. 1979. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 12. GRAS
substances. Food Technol 33(7):65-73.
Oser, B.L. and Hall, R.L. 1972. Recent progress in the consideration
of flavoring Ingredients under the Food Additives Amendment 5. GRAS
substances. Food Technol 26(5):35-42.
Oser, B.L. and Hall, R.L. 1977. Criteria employed by the Expert Panel
of FEMA for the GRAS evaluation of flavoring substances. Food Cosmet
Toxicol 15:457-466.
Oser, B.L., Ford, R.A. and Bernard, B.K. 1984. Recent Progress in the
consideration of flavoring ingredients under the Food Additives
Amendment 13. GRAS substances. Food Technol 38(10):66-89.
Oser, B.L., Well, C.S., Woods, L.A. and Bernard, B.K. 1985. Recent
progress in the consideration of flavoring ingredients under the Food
Additives Amendment 14. GRAS substances. Food Technol 39(11):108-117.
Rulis, A.M., Hattan, D.G., and Morgenroth, V.H.,III. 1984. FDA's
priority-based assessment of food additives. I. Preliminary results.
Reg Toxicol Pharm 4:37-56.
Rulis, A.M. 1987. Safety assurance margins for food additives
currently in use. Reg Toxicol Pharm 7:160-168.
Smith, R.L. and Ford, R.A. 1993. Recent progress in the consideration
of flavoring ingredients under the Food Additives Amendment 16. GRAS
substances. Food Technol-June 1993, pp. 104-117.
ANNEX 5 Appendix B
SUBSTANCES IN REFERENCE DATABASE BY ORDER OF STRUCTURAL CLASS
Cramer et al. (1978) Structural Class I
1 acetic acid
2 acetoin
3 acetone
4 adipic acid
5 allura red AC
6 aminoundecanoic acid, 11-
7 ascorbic acid
8 ascorbic acid, 1-
9 benzaldehyde
10 benzoic acid
11 benzyl acetate
12 benzyl alcohol
13 bis(2-ethylhexyl)phthalate
14 brilliant black BN
15 butanediol, 1,3-
16 butanol, 2-
17 butanol, n-
18 butyl benzyl phthalate
19 butylated hydroxyanisole
20 butyrolactone, gamma-
21 calcium cyclamate
22 calcium formate
23 calcium stearoyl lactylate
24 carotenoic acid, beta-apo-8'-, methyl ester
25 cinnamaldehyde
26 citral
27 citranaxanthin
28 cumene
29 di(2-ethylhexyl)adipate
30 dibutyl phthalate
31 diethyl phthalate
32 diethylene glycol
33 diethylene glycol monoethyl ether
34 dimethoxane
35 dimethyl terephthalate
36 dimethylcarbonate
37 dimethyldicarbonate
38 dimethylphenol, 2,4-
39 dimethylphenol, 2,6-
40 dimethylphenol, 3,4-
41 dioctyl sodium sulfosuccinate
42 disodium 5'-guanylate
43 disodium 5'-inosinate
44 dodecyl gallate
45 ethanol
46 ethyl acetate
47 ethyl acrylate
48 ethyl butyrate
49 ethyl ether
50 ethyl formate
51 ethyl glycol monomethyl ether
52 ethyl heptanoate
53 ethyl nonanoate
54 ethyl-1-hexanol, 2-
55 ethylbenzene
56 ethylbutyric acid, 2-
57 ethylene glycol
58 ethylhexanoic acid, 2-
59 ethylphthalyl ethylglycolate
60 eugenol
61 FD & C blue No. 1
62 FD & C blue no. 2
63 formaldehyde
64 fumaric acid
65 geranyl acetate
66 glutamate, monosodium
67 glutamic acid hydrochloride
68 glutamic acid, 1-
69 glycerol
70 glyceryl tribenzoate
71 hexenal, 2- (trans)
72 hexylresorcinol, 4-
73 hydroquinone
74 hydroxybenzoic acid butyl ester, p-
75 hydroxybenzoic acid ethyl ester, p-
76 hydroxybenzoic acid methyl ester, p-
77 hydroxybenzoic acid propyl ester, p-
78 hydroxybenzyl acetone, p-
79 inosine monophosphate
80 ionone
81 isoamyl butyrate
82 isoamyl salicylate
83 isobutyl alcohol
84 isomaltitol
85 isopropyl alcohol
86 isovaleric acid
87 lactitol
88 limonene, d-
89 lithocholic acid
90 malonaldehyde, sodium salt
91 mannitol
92 mannitol, d-
93 menthol
94 methanol
95 methyl ethyl carbonate
96 methyl methacrylate
97 methyl salicylate
98 methyl-1-phenylpentan-2-ol, 4-
99 methylenebis, 2,2'-
100 methylphenol, 3-
101 methylphenylcarbinyl acetate
102 myrcene, beta-
103 nonalactone, gamma-
104 octyl acetate
105 octyl gallate
106 oleylamine
107 oxalic acid
108 phenol
109 phenoxyethanol
110 phenyl-1-propanol, 2-
111 phenylalanine
112 potassium sorbate
113 propyl gallate
114 propylene glycol
115 resorcinol
116 retinol
117 riboflavin
118 sodium benzoate
119 sodium erythorbate
120 sodium lauryl glyceryl ether sulfonate
121 sodium lauryl trioxyethylene sulfate
122 sodium stearoyl lactylate
123 sorbic acid
124 stearyl tartrate
125 styrene
126 sucrose monopalmitate
127 sucrose monostearate
128 tartaric acid
129 tertiary butyl hydroquinone
130 tocopherol, alpha-
131 tolualdehyde
132 toluene
133 triethylene glycol
134 triethylene glycol monomethyl ether
135 trimethylamine
136 undecalactone, gamma-
137 vanillin
138 xylitol
Cramer et al. (1978) Structural Class II
1 acrylic acid
2 allyl alcohol
3 allyl heptanoate
4 allyl hexanoate
5 butylated hydroxytoluene
6 caffeine
7 carotene, beta-
8 carvone
9 carvone, d-
10 diacetyl
11 diallyl phthalate
12 diketopiperazine
13 ethyl maltol
14 ethyl vanillin
15 ethylhexyl phthalate, mono-2
16 etretinate
17 fenthion
18 furfural
19 isobornyl acetate
20 isophorone
21 maltol
22 methyl amyl ketone
23 methyl anthranilate
24 piperidine
25 piperonal
26 propargyl alcohol
27 pyridine
28 thujone
Cramer et al, (1978) Structural Class III
1 (1-naphthyl)ethylene--diamine dihydro chloride, N-
2 (2-chloroethyl)trimethyl-ammonium chloride
3 (8-beta)N-cyclohexyl-6-methyl- 1 -(1 -methyl
ethyl)ergoline-8 -carboxamide
4 (chloroacetyl)-acetanilide, 4'-
5 1,1'-(2,2,2-trichloroethylidene)bis(4-chloro)-benzene
6 11-oxo-11H-pyrido(2,1-b)quinazoline-2-carboxylic acid
7 2(2,4,5-trichlorophenoxy) propionic acid
8 2-(2-methyl--4-chlorophenoxy)propionic acid
9 4-(2-methyl-4-chlorophenoxy) butyric acid
10 acenaphthene
11 acephate
12 acesulfame potassium
13 acetaminophen
14 acetoacetamide
15 acetoacetamide-N-sulfonic acid
16 acetochlor
17 acetonitrile
18 acifluorin sodium
19 acrylamide
20 acrylonitrile
21 alachlor
22 albendazole
23 albendazole sulfoxide
24 aldicarb
25 aldicarb sulfone
26 alinidine hydrobromide
27 allyl isovalerate
28 amaranth
29 ameltolide
30 ametryn
31 amino-4-ethoxy-acetanilide, 3-
32 amino-4-nitrophenol, 2-
33 amino-5-nitrophenol, 2-
34 aminophenol, p-
35 amitraz
36 ammonium carmine
37 amphetamine sulfate, dl-
38 ampicillin trihydrate
39 anethole, trans-
40 anilazine
41 anisidine hydrochloride, p-
42 anthranilic acid
43 arochlor 1254
44 aspartame
45 asulam
46 atrazine
47 avermectin B1
48 azaperone
49 azinphos methyl
50 azorubine
51 azuletil (KT1-32)
52 baythroid
53 benomyl
54 bentazon
55 benzofuran
56 benzoic acid, 2-[[[[N-(4-methoxy-6-methyl
-1,2,3-triazin-2-yl)-N-methylamino]carbonyl]
amino]sulfonyl] methyl ester
57 benzoin
58 benzyl violet 4B
59 benzyl-p-chlorophenol, o-
60 betaxolol
61 bidrin
62 biphenthrin
63 biphenyl, 1,1-
64 biphenylamine hydrochloride, 2-
65 bis(2-chloro-1-methyl-ethyl)ether Technical Grade
66 bis(2-chloroisopropyl)ether
67 bisphenol A
68 bromoacetic acid, 2-
69 bromodichloromethane
70 bromomethane
71 bromoxynil
72 bromoxynil octanoate
73 brown FK
74 butyl chloride, n-
75 butylate
76 calcium cyanamide
77 canthaxanthin
78 caprolactam
79 captafol
80 captan
81 carazolol
82 carbaryl
83 carbendazim
84 carbofuran
85 carbon tetrachloride
86 carbosulfan
87 carboxin
88 carmoisine
89 chloramben
90 chlordane
91 chlorendic acid
92 chlorimuron-ethyl
93 chloro-p-toluidine, 3-
94 chloroacetic acid
95 chloroaniline hydrochloride, p-
96 chlorobenzene
97 chlorobenzilate
98 chlorodibromomethane
99 chloroform
100 chlorofructose, 6-
101 chloronaphthalene, beta-
102 chlorophenol, 2-
103 chloropropylate
104 chlorothalonil
105 chlorotoluene, o-
106 chlorpheniramine maleate
107 chlorpromazine
108 chlorsulfuron
109 chlorthal dimethyl
110 chocolate brown HT
111 C.I. Acid Orange 12
112 C.I. Acid Orange 3
113 C.I. Acid Red 18
114 C.I. Disperse Blue 1
115 C.I. acid red 14
116 C.I. disperse yellow 3
117 C.I. pigment red 23
118 C.I. solvent yellow 14
119 cimetidine
120 clofentezine
121 clonitralid
122 closantel
123 codeine
124 coumaphos
125 coumarin
126 crufomate
127 curcumin
128 cyclodextrin, beta-
129 cyclohexylamine
130 cyclophosphamide
131 cyhalothrin
132 cypermethrin
133 cyromazine
134 daminozide
135 decabromodiphenyl oxide
136 deoxyquinine
137 diamino-2,2'-stilbenedisulfonic acid, 4,4'-, disodium sa
138 diaminophenol dihydrochloride, 2,4-
139 diazinon
140 dibenzo-p-dioxin
141 dibromobenzene, 1,4-
142 dicamba
143 dichloro-2-propanol, 1,3-
144 dichloro-p-phenylenediamine, 2,6-
145 dichlorobenzene, 1,2-
146 dichlorobenzene, 1,4-
147 dichlorobenzilic acid
148 dichlorodifluoromethane
149 dichloroethane, 1,1-
150 dichloroethane, 1,2-
151 dichloroethylene, 1,1-
152 dichloroethylene, trans- 1,2-
153 dichloromethane
154 dichlorophenol, 2,4-
155 dichlorophenoxyacetic acid, 2,4-
156 dichloropropanol, 2,3-
157 dichloropropene, 1,3-
158 dichlorvos
159 dicofol
160 dieldrin
161 diethyldithiocarbamate
162 diethylthiourea, N,N'-
163 difenzoquat
164 diflubenzuron
165 dihydroavermectin-B1 a, 22,23-
166 dihydroavermectin-B1 b, 22,23-
167 dihydrocoumarin, 3,4-
168 diiodomethyl p-tolyl sulfone
169 dimethipin
170 dimethoate
171 dimethoxyaniline hydrochloride, 2,4-
172 dimethoxybenzidine-4,4'-diisocyanate, 3,3'-
173 dimethyl hydrogen phosphite
174 dimethyl methylphosphonate
175 dimethyl morpholinophosphoramidate
176 dimethylaniline, N,N'-
177 dinitrobenzene, m-
178 dinitrotoluene, 2,4-
179 dinocap
180 dinoseb
181 diphenamid
182 diphenhydramine hydrochloride
183 diphenylamine
184 diphenylhydantoin, 5,5'-
185 diphenylhydantoin, 5,5-
186 diquat
187 disulfoton
188 dithiobiurea, 2,5-
189 diuron
190 dodecylguanidine acetate, n-
191 EDTA, disodium
192 endothall
193 ephedrine sulfate
194 epichlorohydrin
195 erythromycin stearate
196 erythrosine
197 ethalfuralin
198 ethephon
199 ethion
200 ethyl dipropylthiocarbamate, s-
201 ethyl p-nitrophenyl phenylphosphorothioate
202 ethylene chlorohydrin
203 ethylene thiourea
204 ethylmethylphenylglycidate
205 fast green FCF
206 febantel
207 fenamiphos
208 fenbendazole
209 fenchlorphos
210 fluometuron
211 fluoranthene
212 fluorene
213 fluridone
214 flurprimidol
215 flutolanil
216 fluvalinate
217 folpet
218 fonofos
219 fosetyl-al
220 glufosinate-ammonium
221 glyphosate
222 haloxyfop-methyl
223 HC blue no. 1
224 HC blue no. 2
225 HC yellow 4
226 heptachlor
227 heplachlor epoxide
228 hexabromobenzene
229 hexachlorobenzene
230 hexachlorobutadiene
231 hexachlorocyclohexane, gamma-
232 hexachlorocyclopentadiene
233 hexachloroethane
234 hexachlorophene
235 hexahydro-1,3,5-trinitro-1,3,5-triazine
236 hexamethylenetetramine
237 hexazinone
238 hexythiazox
239 hydralazine
240 hydrazobenzene
241 hydrochlorothiazide
242 hydroxypropyl methanethiolsulfonate, 2-
243 hydroxyquinoline, 8-
244 imazalil
245 imazaquin
246 imazethapyr
247 iodinated glycerol
248 ipazilide
249 iprodione
250 ipronidazole
251 isopropalin
252 isoxaben
253 jervine
254 lactofen
255 lansoprazole
256 levamisole
257 linamarin
258 linuron
259 londax
260 malaoxon
261 malathion
262 maleic anhydride
263 maleic hydrazine
264 manidipine hydrochloride
265 melamine
266 mepiquat chloride
267 mercaptobenzothiazole, 2-
268 merphos
269 merphos oxide
270 meso-2,3-dimercaptosuccinic acid
271 metalaxyl
272 methamidophos
273 methidathion
274 methomyl
275 methoxychlor
276 methoxypsoralen, 8-
277 methyl carbamate
278 methyl ethyl ketoxime
279 methyl parathion
280 methyl-4-chlorophenoxyacetic acid, 2-
281 methyl-N-methylanthranilate
282 methyldopa sesquthydrate, alpha-
283 methylolacrylamide, N-
284 metolachlor
285 metribuzin
286 metsulfuron methyl
287 mexacarbate
288 miporamicin
289 mirex
290 molinate
291 monodiethanolamine salt of riboflavin-5'-phosphate
292 monuron
293 myclobutanil
294 naled
295 nalidixic acid
296 naphthoxy acetic acid, 2-
297 napropamide
298 natamycin
299 nitro-o-toluidine, 5-
300 nitro-p-phenylenediamine, 2-
301 nitroaniline, p-
302 nitroanthranilic acid, 4-
303 nitrofurantoin
304 nitrofurazone
305 nitroguanidine
306 nitronaphthalene, 1-
307 nitrosodiphenylamine, N-
308 norflurazon
309 ochratoxin A
310 octabromodiphenyl ether
311 octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine
312 olaquindox
313 oryzalin
314 oxadiazon
315 oxamyl
316 oxfendazole
317 oxyfluorfen
318 oxytetracycline
319 oxytetracycline hydrochloride
320 oxythioquinox
321 paclobutrazol
322 paraquat
323 parathion
324 patulin
325 pendimethalin
326 penicillin VK
327 pentabromodiphenyl ether
328 pentachloroethane
329 pentachloronitrobenzene
330 pentachlorophenol
331 pentaerythritol tetranitrate
332 permethrin
333 phenformin
334 phenyl-2-naphthylamine, n-
335 phenyl-3-methyl-5-pyrazolone, 1-
336 phenylbutazone
337 phenylenediamine, m-
338 phenylephrine hydrochloride
339 phosmet
340 phosphamidon
341 photodieldrin
342 phthalamide
343 phthalic anhydride
344 picloram
345 pilsicainide hydrochloride
346 pirimiphos-methyl
347 pravadoline
348 probenecid
349 prochloraz
350 proflavine monohydrochloride hemihydrate
351 promethazine hydrochloride
352 prometon
353 prometryn
354 pronamide
355 propachlor
356 propanil
357 propargite
358 propazine
359 propham
360 propiconazole
361 propiverine hydrochloride (P-4)
362 propoxur
363 propylene dichloride
364 pydrin
365 pyrazinamide
366 pyrene
367 pyrimidinone
368 quercetin
369 quinalphos
370 quinine hydrochloride
371 quinofop ethyl
372 red 2G
373 reserpine
374 resmethrin
375 thudamine 6G
376 ronidazole
377 rotenone
378 saccharin
379 sethoxydim
380 simazine
381 sodium 2,2-dichloropropionate
382 sodium cyclamate
383 sodium fluoroacetate
384 sodium naphthionate
385 sorbitan monolaurate
386 sorbitan monostearate
387 succinic anhydride
388 sucrose acetate isobutyrate
389 sulfadimidine
390 sulfisuxazole
391 sulfolene, 3-
392 sunset yellow FCF
393 suplatast tosilate
394 tebuthiuron
395 terbacil
396 terbutryn
397 tert-butyl-2-chlorophenol, 4-
398 tetrachlorobenzene, 1,2,4,5-
399 tetrachloroethane, 1,1,1,2-
400 tetrachloroethane, 1,1,2,2-
401 tetrachloroethylene
402 tetrachlorophenol, 2,3,4,6-
403 tetrachloropyridine, 2,3,5,6-
404 tetrachlorvinphos
405 tetracycline hydrochloride
406 tetraethyldithiopyrophosphate
407 tetraethylthiourea disulfide
408 tetrahydrocannabinol, delta-9-
409 tetrakis(hydroxymethyl)phosphonium chloride (THPC)
410 tetrakis(hydroxymethyl)phosphonium sulphate (THPS)
411 thiabendazole
412 thiameturon methyl
413 thiobencarb
414 thiophanate-methyl
415 thiram
416 tocopheryl acetate, dl-alpha-
417 toluenediamine hydrochloride, 2,6-
418 toluenediamine sulfate, 2,5-
419 tralomethrin
420 trenbolone acetate
421 trenbolone hydroxide, 17-alpha-
422 triadimefon
423 triallate
424 triamterene
425 tribromomethane
426 trichloroacetonitrile
427 trichlorobenzene, 1,2,4-
428 trichloroethane, 1,1,2-
429 trichloroethane, 1,1,1-
430 trichloroethylene
431 trichloroethylene, 1,1,2-
432 trichlorogalactosucrose
433 trichlorophenol, 2,4,5-
434 trichlorophenoxyacetic acid, 2,4,5-
435 tridiphane
436 triethylene tetramine dihydrochloride
437 trifluralin
438 trimethylaniline, 2,4,5-
439 trinitrotoluene, 2,4,6-
440 tris-(2-chloroethyl) phosphate
441 trisulfuron
442 tylosin
443 vinclozolin
444 vinyl chloride
445 zatosetron maleate
446 zearalenone
447 zeranol
