
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
TOXICOLOGICAL EVALUATION OF CERTAIN
VETERINARY DRUG RESIDUES IN FOOD
WHO FOOD ADDITIVES SERIES 45
Prepared by the
Fifty-fourth meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)
World Health Organization, Geneva, 2000
TRICHLORFON
First draft prepared by
Dr G. Roberts
Chemical Products Assessment Section, Therapeutic Goods
Administration, Department of Health and Aged Care, Canberra,
Australia
Explanation
Biological data
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Long-term studies of toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multigeneration studies
Developmental toxicity
Special studies
Neurotoxicity
Immunotoxicity
Observations in humans
Comments
Evaluation
References
1. EXPLANATION
Trichlorfon, dimethyl(2,2,2-trichloro-1-hydroxyethyl)phosphonate
or metrifonate, is an organophosphonate insecticide with insecticidal,
acaricidal, and anthelmintic properties. It is used as an insecticide
on food crops and forests. It is given orally, topically, or
parentally for the control of endo- and ectoparasites in and on
animals of various species. The recommended dose for treatment of
cattle orally or with aqueous pour-on, wash, or spray solutions is
50-75 mg/kg bw. Repeated dosing may be necessary. The preparations for
use on horses are similar, but the recommended oral dose is 35 mg/kg
bw. One topical application for use on horses contains febantel.
Humans may be given trichlorfon orally for infestation with
Schistosoma haematobium, and the drug has been studied for use in
the treatment of Alzheimer disease.
Trichlorfon has not previously been evaluated by the Committee.
It was evaluated on three occasions by the Joint FAO/WHO Meeting on
Pesticide Residues (FAO/WHO, 1972a, 1976a, 1979a), which established
an ADI of 0-0.01 mg/kg bw in 1978. An Environmental Health Criteria
publication on trichlorfon has been issued (WHO, 1992).
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
Few studies were available of the kinetics of trichlorfon in
laboratory animals; however, the JMPR reported that trichlorfon is
absorbed, distributed, degraded, and excreted very rapidly in mammals.
After oral administration, radiolabel was detected in the blood of a
cow within 0.5 h. While residues were observed in cattle and sheep
meat within 1 h, the concentrations decreased rapidly thereafter.
Secretion into the milk of cows was seen 6-8 h after treatment, with
peak concentrations at 14-18 h. After intraperitoneal administration
of trichlorfon to rats, 71% of the total dose was eliminated in urine
within 16 h. After oral administration to cows, 66% of the dose was
eliminated within 12 h (FAO/WHO, 1972b).
After [14CH3]trichlorfon was administered by stomach tube to
pregnant guinea-pigs on days 35 and 52 of gestation, distribution to
the main organs was rapid, the highest concentrations being present in
the liver, kidney, and lung. Thirty minutes after dosing, substantial
uptake of radiolabel into the fetus was found, which was more
pronounced after administration at 52 days of gestation, the
concentration in fetal liver equalling that in the placenta at that
time (Berge & Nafstad, 1986; WHO, 1992).
Groups of four healthy volunteers were given trichlorfon at a
single oral dose of 2.5, 5, 7.5, or 15 mg/kg bw in a randomized
double-blind study. Peak plasma concentrations were achieved within 2
h, and the rate of absorption was linear over the dose range. The
plasma half-time was 2-2.5 h in all cases (Aden-Abdi et al., 1990).
Similar results were obtained by Nordgren et al. (1980, 1981) in
patients withschistosomiasis .
2.1.2 Biotransformation
In vitro
In the pH range 5.4-8.0, 0-60% of a dose of trichlorfon was
decomposed in vitro within 2 h (Metcalf et al., 1959). Trichlorfon
underwent dehydrochlorina-tion and rearrangement to form dichlorvos
under alkaline, neutral, or slightly acidic conditions in vitro.
Conversion ceased at pH 5. Studies on inhibition of house fly
acetylcholinesterase and chymotrypsin in vitro suggested that
trichlorfon had little or no inhibitory action at any pH. It was
postulated that the insecticidal action of trichlorfon is due to its
conversion to the active metabolite, dichlorvos (Miyamoto, 1959). The
rate of decomposition of trichlorfon correlated with inhibition of
acetylcholinesterase from bovine erythrocytes (Reiner et al., 1975).
Incubation of trichlorfon in buffer at pH 7.4 in vitro resulted
in decomposition to dichlorvos. Inclusion of the soluble fraction
(105 000 × g) from chicken or cow liver led to faster degradation
and the formation of desmethyl trichlorfon and desmethyl dichlorvos,
but predominantly the latter. Enzymatic pathways were the major routes
of degradation (Akhtar, 1982).
When 32P-trichlorfon at a final concentration of 0.014 mol/L
was incubated with 20% rat brain homogenate for 5 h at 37°C, 18% of
the compound was metabolized to three acidic and one non-acidic
metabolites. Two of the acidic metabolites, monodemethylated
trichlorfon and monomethyl phosphate, represented 37% and 7% of the
total metabolites, respectively. The third acidic metabolite was
thought to be 2,2,2-trichloro-1-hydroxyethyl phosphonic acid and
represented 16% of the metabolites. The non-acidic metabolite was not
identified. The main metabolic pathway in nervous tissue appears to be
hydrolysis of the methyl ester linkages. Trichlorfon irreversibly
inhibits rat brain acetylcholinesterase activity in vitro, and the
presence of acetylcholine offered competitive protection (Hassan et
al., 1965a).
[14CH3]Trichlorfon was incubated with human serum for 3 h at
37.5°C at a concentration of 1 mg/ml. Precipitated protein was
hydrolysed, and the resulting amino acids were separated on an ion
exchanger. The metabolites included dichlorvos, dimethyl phosphate,
and desmethyl trichlorfon. The 14C content of the amino acid
hydrolysate represented approximately 15% of the total radiolabel.
These results suggest cleavage of the methoxy moiety and methylation
of protein (Dedek & Lohs, 1970a).
In vivo
After an intraperitoneal injection of 125 mg/kg bw trichlorfon to
mice, dichlorvos was detected in the brain about 15 min later at a
peak concentration that was less than 1% of the peak brain
concentration of trichlorfon (Nordgren et al., 1978).
Mice were given an oral dose of 6.2 mg 32P-trichlorfon, and
water-extractable metabolites were determined in the whole body. The
percentages of metabolites after 0.5 and 4 h were, respectively,
desmethyl trichlorfon (4.3, 4.0), desmethyl dichlorvos (21, 9.9),
dimethyl hydrogen phosphate (35, 48), methyl hydrogen phosphate (14,
18), phosphoric acid (22, 21), and unknown compounds (3.6, 0) (WHO,
1992).
Sixteen hours after an intraperitoneal dose of 5 mg
32P-trichlorfon to rats, the urinary metabolites found were
trichlorfon (0.7%), dimethyl phosphate (38%), phosphoric acid and
methyl phosphate (0.8%), O-desmethyl trichlorfon (1.4%), and
O-desmethyl dichlorvos (0.8%). The remainder was unidentified. (Bull
& Ridgway, 1969).
When trichlorfon with 14C in the two methyl groups was
administered intraperitoneally to rats at 200 mg/kg bw per day, 28% of
the dose was recovered as 14CO2 in expired air within 24 h. In
urine, 32% was recovered as formate (2-4%), dimethyl phosphate
(20-24%), and unidentified substances within 24 h (Hassan & Zayed,
1965).
After intravenous or intraperitoneal administration of
[14CH3]trichlorfon to male rats, unextractable label was found
principally in the liver but also in other organs. The data suggest
that methylation of proteins occurs in vivo (Dedek & Lohs,1970b).
[32P]Trichlorfon was administered intraperitoneally to rats at
100 mg/kg bw per day in saline, and 75-85% of the administered dose
was recovered in urine collected for 24 h. The three metabolites found
were acidic: monomethyl phosphate accounted for 20-30%, dimethyl
phosphate for 60-70%, and an unidentified metabolite for 10%.
Monodemethylated trichlorfon is likely to be formed as an
intermediate, before loss of trichloroethanol as the glucuronide and
subsequent formation of monomethyl phosphate (Hassan et al., 1965b).
[32P]Trichlorfon was injected intravenously to dogs at a dose
of 150 mg/kg bw, and urine was collected for 48 h. Less than 1% of the
dose was recovered as unchanged compound, with approximately 65% as
the glucuronic acid conjugate of trichloroethanol (Arthur & Casida,
1957).
Patients with schistosomiasis received oral doses of 7.5-10 mg/kg
bw trichlorfon on two occasions 14 days apart. Their plasma
concentrations of trichlorfon peaked between 1 to 2 h, and the
concentrations of dichlorvos represented about 1% of parent drug
(Nordgren et al., 1980, 1981).
The main metabolites of trichlorfon in mammals were desmethyl
trichlorfon, desmethyl dichlorvos, dimethyl hydrogen phosphate, methyl
dihydrogen phosphate, and phosphoric acid. Thus, the main degradation
routes of trichlorfon are demethylation, P-C bond cleavage, and ester
hydrolysis via dichlorvos. The proposed metabolic pathways for
trichlorfon, as identified by WHO (1992), are reproduced in Figure 1.
2.2 Toxicological studies
2.2.1 Acute toxicity
The results of studies of the acute toxicity of trichlorofon are
shown in Table 1. The acute toxicity of trichlorfon in rats and mice
was similar when it was administered orally, intraperitoneally, or
subcutaneously. It was less toxic to rats and rabbits after dermal
application than when given by the other routes. The acute toxicity of
trichlorfon is due to inhibition of acetylcholinesterase activity at
nerve endings, leading to accumulation of endogenous acetylcholine.
The signs of toxicity are sweating, salivation, diarrhoea,
bronchorrhoea, bradycardia, bronchoconstriction, muscle
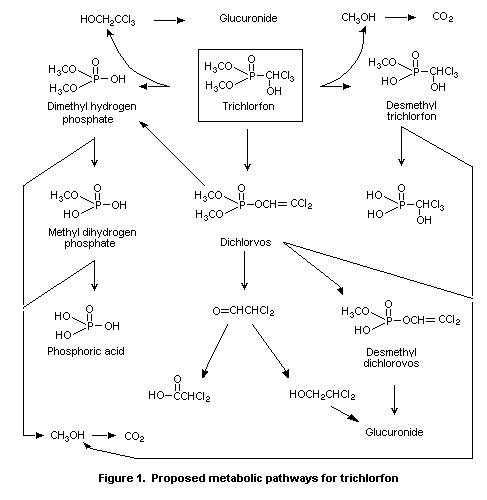 Table 1. Results of studies of the acute toxicity of trichlorfon
Species Sex Route LD50 References
(mg/kg bw)
Mouse M&F Oral 616-950 Klimmer (1955); Mihail
(1978); FAO/WHO (1972b);
Hirakawa (1983a); Renhof
(1997)
Mouse M&F Intraperitoneal 344-600 Dubois & Cotter (1955);
Hirakawa (1983a); WHO
(1992)
Mouse M&F Subcutaneous 267360 FAO/WHO (1972b); Hirakawa
(1983a)
Mouse M&F Intravenous 354-370 Renhof (1997)
Mouse M&F Dermal > 5000 Hirakawa (1983a)
Rat M&F Oral 136-660 Dubois & Cotter (1955);
Klimmer (1955); Kimmerle
(1970); FAO/WHO (1972b);
Crawford & Anderson (1973);
Mihail (1978); Hirakawa
(1983b); Heimann (1985);
WHO (1992); Renhof (1997)
Rat M&F Intraperitoneal 156-485 Dubois & Cotter (1955);
Arthur & Casida (1957);
FAO/WHO (1972b); Crawford &
Anderson (1973); Hirakawa
(1983b)
Rat M&F Subcutaneous 290-400 FAO/WHO (1972b);
Hirakawa (1983b)
Rat M Intravenous 184 Kimmerle (1970)
Rat M&F Inhalation LC50, > 533 Kimmerle (1975a)
mg/m3
Rat M&F Dermal 2800-> 5000 Hirakawa (1983b); Mihail
(1978); WHO (1992)
Guinea-pig M&F Intraperitoneal 300 Dubois & Cotter (1955);
WHO (1992)
Rabbit Oral 160 FAO/WHO (1972b)
Rabbit M Dermal 5000 WHO (1992)
Dog M oral > 300-420 Mihail (1978); WHO (1992);
Kawauchi (1997)
fasciculations, and coma. The primary cause of death is respiratory
failure. After sublethal doses, animals recover very rapidly,
suggesting that trichlorfon is detoxified faster than many other
organophosphorus compounds (WHO, 1992).
In two separate studies, six New Zealand white rabbits were
treated dermally with trichlorfon for 24 h on intact and abraded skin.
No irritation was seen after 24, 48, or 72 h. Further animals received
trichlorfon in the eye for 5 min ( n = 5) or 24 h ( n = 3). Severe
redness was seen up to 72 h after dosing for either length of time.
Slight to moderate chemosis was also observed up to 48-72 h after
dosing in both groups. The effects did not persist to day 7, and no
effect was seen in the cornea or iris. The compound is a slight ocular
irritant (Thyssen, 1981; Bond, 1986).
Trichlorfon was assessed for skin sensitizing potential in eight
male BOR: DHPW guinea-pigs by the open epicutaneous test. Dermal
induction was carried out 5 days a week for 4 weeks with 0, 1, 3, or
10% trichlorfon, and dermal challenge was conducted with 0.3, 1, 3, or
10 % trichlorfon 4, 6, or 8 weeks after induction. No skin reactions
were seen in animals induced with 1% trichlorfon, but approximately
half the animals induced with 3 or 10% trichlorfon showed skin
reactions when challenged with each concentration of trichlorfon. The
compound was therefore considered to have skin sensitizing potential
(Mihail, 1986).
2.2.2 Short-term studies of toxicity and carcinogenicity
Mice
Trichlorfon (purity, 98.2%) was administered to groups of 15
Charles River CD-1 mice of each sex at a dietary concentration of 0,
100, 300, 900, or 2700 mg/kg for 8 weeks. There were no deaths or
drug-related clinical signs, and body weights were not affected. Food
consumption was not affected in males, but appeared to have been
increased by 18-32% in females. The report suggests that the latter
finding was due to increased wastage by the treated female mice.
Cholinesterase activity in plasma and erythrocytes, measured during
weeks 4, 6, and 8, was not significantly affected at the lowest dose
but was dose-dependently inhibited at higher doses; the maximum
inhibition was by about 70% for plasma and 50% for erythrocyte enzyme.
Brain acetylcholines-terase activity at the end of the study was
dose-dependently inhibited at doses > 300 mg/kg of diet, by up to
68%. Gross necropsy revealed no remarkable changes. Haematology, serum
biochemistry, urinary anlysis, organ weighing, and histopathology were
not performed (Hayes, 1985).
Groups of 10 male and 10 female B6C3F1 mice were given
trichlorfon (purity unknown) in the diet at a concentration of 0, 62,
180, 560, 1700, or 5000 mg/kg for 13 weeks. The results were reported
in abstract form only. All but one male at 5000 mg/kg of diet and one
female at 1666 mg/kg of diet survived. The body weights of mice at
5000 mg/kg of diet were reduced. Plasma and erythrocyte cholinesterase
activities were decreased in a dose-related manner, while motor
activity and grip strength were reduced at 1700 and 5000 mg/kg of
diet. The absolute and relative weights of the liver, kidney, and
spleen were increased in mice at 1700 and 5000 mg/kg of diet. The
neurotoxic effects and changes in organ weight were not accompanied by
pathological effects (Chan & Peters, 1989; WHO, 1992).
Rats
Groups of 10 male and 10 female rats were exposed for 6 h/day for
3 weeks (total, 15 exposures) to an atmosphere containing trichlorfon
(purity unknown) at a concentration of 0, 12.7, 35.4, or 103.5
mg/m3. The health of the animals at 103.5 mg/m3 was affected, but
details were not provided. Body-weight gain was unaffected, and
haematological, clinical chemical, and urinary parameters measured at
the end of the study were similar to those of controls. Plasma,
erythrocyte, and brain cholinesterase activities were inhibited in
males given 103.5 mg/m3 and in females given 35.4 and 103.5 mg/m3.
Gross and histopathological examinations showed no remarkable changes,
the only finding at autopsy being increased spleen weights in males at
35.4 and 103.5 mg/m3 (Kimmerle, 1975b; WHO,1992).
Groups of 12 male and 12 female Crj: CD SD rats were given
trichlorfon (purity, 100%) at a dose of 0, 25, 50, or 100 mg/kg bw per
day by gavage for 30 days. Further groups of 10 rats of each sex were
treated and then allowed a 2-week recovery period. Additional treated
groups of 20 rats of each sex were used to determine the profiles of
the drug in blood after dosing on days 1, 15, and 30. The peak
concentrations of the individual enantiomers of trichlorfon and
dichlorvos were dose-related and were reached by about 1 h, declining
rapidly to become undetectable by 24 h. The concentrations of each
enantiomer were roughly similar in males, while that of the
(-)-enantiomer was generally higher than that of the (+)enantiomer in
females; this was reflected in higher total drug concentrations in
females. The concentrations of dichlorvos in blood represented only a
small fraction of those of trichlorfon and were again higher in female
rats. There was no evidence of accumulation in blood.
The signs of toxicity included tremor, decreased locomotor
activity, salivation, bradypnoea, and prone position in many females
given 100 mg/kg bw per day, in one or two females given 50 mg/kg bw
per day, and in males given 100 mg/kg bw per day. There were no deaths
and no effects on body weight, food consumption, or ophthalmological
or urinary parameters. Slight decreases in erythrocyte counts and
haematocrit were seen in females at 100 mg/kg bw per day when compared
with controls at the end of treatment, but these values were not
significantly different from those of controls at the end of the
recovery period. Animals at 100 mg/kg bw per day had increased blood
concentrations of cholesterol, triglycerides, and phospholipids
(females), and increased activity of lactic dehydrogenase (males). At
autopsy, the livers of animals at 50 and 100 mg/kg bw per day were
heavier and/or enlarged and showed hypertrophy of centrilobular
hepatocytes. The weight of the kidney was increased in males at 100
mg/kg bw per day. The NOEL was 25 mg/kg bw per day (Toyoshi, 1996).
Groups of seven Wistar rats of each sex were given trichlorfon
(purity unknown) in the diet to provide a dose of 0, 1, 5, 10, 30, 50,
or 100 mg/kg bw per day for 12 weeks. The highest dose inhibited
cholinesterase activity in all tissues examined (erythrocytes, serum,
brain, heart, liver, and skeletal muscle), but the extent of the
inhibition differed. Nevertheless, no changes were detected in
nocturnal behaviour, reaction to external stimuli, conjunctival or
pinna reflexes, or the avoidance reflex to painful stimuli. No
significant histological alterations were observed (reviewed in WHO,
1992).
Groups of 10 male and 10 female Fischer rats were given
trichlorfon (purity unknown) in the diet at a concentration of 0, 62,
180, 560, 1700, or 5000 mg/kg for 13 weeks. The results were reported
in abstract form only. All rats survived. The body weights of those at
5000 mg/kg of diet were reduced. Plasma and erythrocyte cholinesterase
activities were lowered in a dose-related manner, while motor activity
and grip strength were reduced at 1700 and 5000 mg/kg of diet. The
absolute and relative weights of the liver, kidney, and spleen were
increased in animals at 1700 and 5000 mg/kg of diet. The neurotoxic
effects and changes in organ weights were not accompanied by
pathological effects (Chan & Peters, 1989; WHO, 1992).
Groups of 13 rats of each sex were fed diets containing
trichlorfon (purity unknown) at a concentration of 0, 20, 100, or 300
mg/kg for 16 weeks. No effects were observed on growth, behaviour,
food consumption, organ weights, or gross or microscopic appearance of
the tissues. At 300 mg/kg of diet, plasma and erythrocyte
cholinesterase activity was depressed by 20-30% in males and females,
while that of brain cholinesterase was depressed by 20% in females
only. Salivary gland cholinesterase activity was not inhibited. At 100
mg/kg of diet, a slight depression of plasma cholinesterase activity
was observed (Doull & Rehfuss, 1956; WHO, 1992).
Groups of 10 male Wistar albino rats were fed diets containing
trichlorfon (purity unknown) at 0, 1, 5, 25, or 125 mg/kg for 16
weeks. Erythrocyte cholinesterase activity was not depressed at any
dose, and no effects were seen on food consumption, growth, or gross
appearance of the tissues (reviewed in WHO, 1992).
Guinea-pigs
After oral administration of trichlorfon (purity unknown) at 100
mg/kg bw per day for 60 days to guinea-pigs, the haemoglobin
concentration but not the haematocrit was decreased. The activity of
serum cholinesterase was decreased by 50%, and that of alkaline
phosphatase was increased (reviewed in WHO, 1992).
Rabbits
Groups of five New Zealand white rabbits of each sex received
dermal applications of 0, 100, 300, or 1000 mg/kg bw per day
trichlorfon (purity, 99%) onto shaved skin. The drug was left on the
skin of restrained animals for 6 h/day, 5 days/week for 3 weeks. The
appearance and behaviour of the rabbits were unchanged, and there were
no deaths. Body weight, food consumption, and the skin at the
application sites were unaffected. There were no meaningful changes in
haematological, clinical chemical, or urinary parameters at the end of
the study. Plasma, erythrocyte, and brain cholinesterase activities
were inhibited by 20-30% in animals at 300 and 1000 mg/kg bw per day.
At autopsy, no treatment-related alterations in liver microsomal
enzyme activities, organ weights, or histological appearance were seen
(Heimann & Wood, 1987).
Dogs
Four dogs were fed diets containing trichlorfon (purity unknown)
for 12 weeks at a concentration of 20, 100, 300, or 500 mg/kg and were
then left for a further 4 weeks on unmedicated diet. All of the dogs
remained healthy and maintained their body weight throughout the
study. After 12 weeks of treatment, the erythrocyte and plasma
cholinesterase activities of animals at 300 and 500 mg/kg of diet were
decreased by 40-60% when compared with the concentrations before
treatment, and the activity in plasma was inhibited by 10% in animals
at 100 mg/kg of diet. On cessation of treatment, the enzyme levels
recovered within 2-4 weeks (Doull & Vaughn, 1958; FAO/WHO, 1972b).
Four dogs were fed diets containing trichlorfon (purity unknown)
at a concentration of 0, 50, 200, or 500 mg/kg for 12 weeks. Plasma
and erythrocyte cholinesterase activities were depressed within 2
weeks in animals at 500 mg/kg of diet, and the effect peaked at 6-8
weeks, at which time the inhibition was about 60% in plasma and 45% in
erythrocytes. A gradual increase in plasma cholinesterase activity was
then seen, to 45% inhibition at termination, even though the compound
was still present in the feed. At 200 mg/kg of diet, the
cholinesterase activity was slightly decreased but was within the
control range. The enzyme activities were restored 6 weeks after
treatment ceased (Williams et al., 1959; FAO/WHO, 1972b).
Groups of three male and three female beagles were given
trichlorfon (purity, 99.2%) in capsules at a dose of 0, 5, 15, or 45
mg/kg bw per day for 3 months. A further three animals of each sex
were given the vehicle or the high dose for 3 months and then allowed
to recover for 2 weeks. The blood concentrations of the enantiomers of
trichlorfon and dichlorvos were measured after dosing on days 1, 30,
and 90. Peak concentrations were achieved within 1 h, the
(-)-enantiomer being detected at 10-100-fold higher concentrations
than the (+)-enantiomer and dichlorvos. Disappearance from blood was
rapid, and the compounds were virtually undetectable by 7 h. The drug
did not accumulate in blood.
No deaths were observed in any group, and no meaningful effects
were seen on mean body weight, food intake, or in ophthalmological,
electrocardiographic, or auditory parameters. Dogs receiving 15 mg/kg
bw per day or more showed tremor, decreased locomotor activity, and
crouching, while at 45 mg/kg bw per day, ataxic gait, lateral
position, vomiting, salivation, licking, liquid, loose, or bloody
stools, and wasting were observed. The results of blood chemistry and
urinary analysis were unremarkable. Haemoglobin, haematocrit, and
erythrocyte count were slightly but progressively decreased during
treatment in males given 45 mg/kg bw per day. It was reported, with no
supporting data, that erythrocyte cholinesterase activity was
inhibited at 15 and 45 mg/kg bw per day. One dog at 45 mg/kg bw per
day lost weight, and at autopsy was found to have decreased thymus
weight with lymphocyte depletion in the cortex, small and immature
testes and prostate, and oligospermia in small epididymides. Similar
thymic changes were observed in one female at 45 mg/kg bw per day. All
of the effects were reversible on cessation of treatment. The NOEL was
5 mg/kg bw per day (Suzuki, 1996).
Monkeys
Groups of five rhesus monkeys of each sex were given trichlorfon
(purity, 98.7%) at a dose of 0, 0.1, or 0.2 mg/kg bw per day in
sterile water by oral intubation for 26 weeks in order to establish an
NOEL for inhibition of erythrocyte cholinesterase activity. There were
no deaths and no effects on appearance, behaviour, nutritional state,
feed or water consumption, or body-weight gain. Haematological tests
showed no changes. No compound-related effects on the liver or kidney
were seen in clinical chemical or urinary analyses, on gross
pathology, or in organ weights. At 0.2 mg/kg bw per day, small
increases and decreases in erythrocyte cholinesterase activity
(< 20%) were seen, which were within the range of biological
variation. The NOEL was 0.2 mg/kg bw per day, the highest dose tested
(Hoffman et al., 1988; WHO, 1992).
2.2.3 Long-term studies of toxicity and carcinogenicity
Mice
Three studies of carcinogenicity were performed with groups of 30
AB/Jena mice that received an oral dose of 0 or 30 mg/kg bw twice
weekly, an intraperitoneal dose of 0 or 28 mg/kg bw per day, or a
dermal application of 0 or 0.25 ml of a 1% solution, providing 2.5 mg
trichlorfon per animal. Dosing was continued for 75 weeks, and the
studies were terminated after 80 weeks. The treated animals showed
reduced body-weight gain and survival, but the tumour incidences were
not increased (Teichmann & Hauschild, 1978; FAO/WHO, 1979b).
Groups of 60 male and 60 female Charles River CD-1 mice were
given trichlorfon (purity unknown) in the diet at a concentration of
0, 100, 300, or 1000 mg/kg for 90 weeks, except for males at the
highest dose which were treated for 82 weeks. These doses were
equivalent to 15, 45, and 150 mg/kg bw per day. Inhibition of
body-weight gain was observed in females given 300 or 1000 mg/kg of
diet, and cholinesterase activity was depressed in animals of each sex
at 1000 mg/kg of diet. No significant microscopic alterations were
reported, and the tumour incidences were not treatment-related
(reviewed in WHO, 1992).
Groups of 50 Crl:CD-1(ICR)BR mice of each sex were given
trichlorfon (purity, 98.2%) in the diet at a concentration of 0, 280,
890, or 2700 mg/kg for 104 weeks, equal to 49, 160, and 510 mg/kg bw
per day for males and 66, 240, and 750 mg/kg bw per day for females.
Increased incidences of urine-stained fur, 'ear lesions' (males), and
vaginal discharge (females) were observed. The body weights of treated
females were increased at various times during the study, but a
consistent effect was observed only at the high dose. There were no
effects on survival, food intake, or haematological end-points. Plasma
and erythrocyte cholinesterase activities were depressed by 20-74% in
the groups given 890 and 2700 mg/kg of diet and in females given
280 mg/kg of diet. Brain cholinesterase activity was inhibited by
20-70% at all doses in a dose-related manner. The weights of the liver
were increased in females at the intermediate and high doses, but this
effect was not accompanied by microscopic changes. Pathological
examination revealed no treatment-related alterations (Hayes, 1988;
WHO, 1992).
Rats
von Gibel and co-workers undertook a series of studies in Wistar
rats (von Gibel et al.,1971, 1973; Stieglitz et al., 1974), which were
given trichlorfon three times per week orally at 30 mg/kg bw, twice
per week by subcutaneous injection at 30 mg/kg bw, twice per week
orally at 15 mg/kg bw, or twice per week by intraperitoneal injection
of 15 mg/kg bw. Treatment was continued for the lifetime of the
animals. The groups given 30 mg/kg bw were reported to have higher
incidences of liver necrosis, liver cirrhosis, and forestomach
papillomas than controls. The lower doses caused hyperplasia of the
blood-forming elements of bone marrow, extraosseal metaplasia in the
liver and spleen, and hepatotoxicity and carcinogenicity. JMPR
(FAO/WHO, 1972b, 1976b) and the International Agency for Research on
Cancer (IARC, 1983) concluded that these studies were difficult to
interpret because of lack of information on the methods and results
and the amalgamation of tumour types. Also, the findings contradicted
those of other studies. Therefore, little use could be made of these
studies .
Groups of 25 male and 25 female Sprague-Dawley rats were fed
diets containing trichlorfon (purity unknown) at a concentration of 0,
50, 250, 500, or 1000 mg/kg for 17 months for males and for 24 months
for females. These doses were equivalent to 2.5, 12, 25, and 50 mg/kg
bw per day. Survival was reduced at 1000 mg/kg of diet, and the
body-weight gain of males at this dose was retarded. Cholinesterase
activity was depressed in serum, erythrocytes, and submaxillary gland
but not in the brain of rats at 1000 mg/kg of diet; at 500 mg/kg of
diet, only serum enzyme activity was inhibited. Female rats fed 500 or
1000 mg/kg of diet had no primary follicules and had primitive ova,
while male rats fed 1000 mg/kg of diet showed depression of
spermatogenesis. Necrotizing arteritis was observed at the two highest
concentrations. The frequencies of mammary tumours in females, in
ascending order of dose, were 14, 8, 20, 21, and 25%. The time to
appearance of these tumours was reduced in a dose-related manner
(reviewed in FAO/WHO, 1972b).
Groups of 25 male and 50 female Sprague-Dawley rats were fed
diets containing trichlorfon (purity unknown) at a concentration of 0,
100, 200, or 400 mg/kg for 18 months, equivalent to 5, 10, and 20
mg/kg bw per day. A significant increase in the mortality rate in all
groups led to premature termination of the study at 70 weeks. Serum
and erythrocyte cholinesterase activities were inhibited at 400 mg/kg
of diet. Cystic granular alterations of the ovaries were seen at 200
and 400 mg/kg of diet, and ovarian atrophy, absence of primary
follicules, and primitive ova were noted at 400 mg/kg of diet. Benign
mammary tumours were found in 8% of controls, 11% of females at 200
mg/kg of diet, and 15% of females at 400 mg/kg of diet, with no change
in the time of their appearance. These results and those of the study
described above were considered to indicate that trichlorfon enhances
certain normal ageing processes, particularly in reproductive tissues
(reviewed in FAO/WHO, 1972b).
Groups of 50 FB30 rats of each sex were fed diets containing
trichlorfon (purity, 95.3%) at a concentration of 50, 250, 500, or
1000 mg/kg for 2 years, equivalent to 2.5, 12.5, 25, and 50 mg/kg bw
per day. The control group comprised 100 males and 100 females. No
changes in survival, general behaviour, appearance, body-weight gain,
or food consumption were reported. No significant differences in blood
count or urinary parameters were reported in samples obtained during
the final 3 weeks of the study. Tests of liver function showed an
increase in 'serum dehydrogenase' activity in males at 50 mg/kg of
diet and in females at 50 and 500 mg/kg of diet. Serum protein
concentration and alanine and aspartate aminotransferase activities
were unchanged. Acetylcholinesterase activity in 'total blood' was
decreased by approximately 20% at 1000 mg/kg of diet in week 90. No
macroscopic evidence of treatment-related changes was reported at
necropsy. Liver weights were significantly increased in males at 250
and 1000 mg/kg of diet, and spleen weights were significantly
increased in females at 50 and 1000 mg/kg of diet. Histological
examination was performed on five male and five female rats at 1000
mg/kg of diet and on three male and three female control animals. The
ovaries of an additional 15 treated and 30 control animals were
examined, as previous studies had indicated a possible effect. The
findings, including tumour incidences, were unrelated to treatment
(Lorke & Loser, 1966; Grundmann & Hobik, 1966; FAO/WHO, 1972b).
Groups of 30 male and 35 female albino rats received an oral dose
of 0 or 30 mg/kg bw or an intraperitoneal dose of 0 or 12 mg/kg bw of
trichlorfon (purity unknown) twice per week. Dosing was continued for
90 weeks, and the studies were terminated after 118 weeks. The treated
animals showed reduced survival, but the tumour incidences were not
increased (Teichmann et al., 1978; FAO/WHO, 1979b).
Trichlorfon (purity, 98.6-99.1%) was fed in the diet to groups of
75 Long-Evans rats of each sex at a concentration of 0, 100, 300, or
1000 mg/kg, equivalent to 5, 15, and 50 mg/kg bw per day. The animals
were maintained on their respective diets for 90 days and then mated
within groups. The resulting F1 litters were reared to 21 days
post partum, at which time 75 offspring of each sex per group were
selected to continue on the experiment. The F0 and selected F1
rats were treated for 2 years. There was no effect on fertility,
reproduction, food intake, body-weight gain, or mortality rate in
either generation. Plasma cholinesterase activity was depressed by
30-60% in both generations at 1000 mg/kg of diet at most times during
the study. Erythrocyte cholinesterase activity was reduced by 30-60%
in both generations given 1000 mg/kg of diet, at most sampling times
in females and on some occasions in males. Brain cholinesterase
activity was significantly lower (40%) in females given 1000 mg/kg of
diet and was 25% lower in F1 males. No histological changes
attributable to treatment were found (Rosenblum, 1981; Griffin et al.,
1982).
Groups of 50 Fischer 344 rats of each sex were given diets
containing trichlorfon (purity, 98.8%) for 2 years at a mean measured
time-weighted average concentration of 0, 92, 270, or 1500 mg/kg. The
nominal concentrations were 0, 100, 300, and, at the highest
concentration, 1000 mg/kg of diet for 27 weeks, 1250 mg/kg of diet for
5 weeks, 1500 mg/kg of diet for 8 weeks, and 1750 mg/kg of diet for 65
weeks. These doses are equal to 4.5, 13, and 76 mg/kg bw per day for
males and 5.8, 17, and 94 mg/kg bw per day for females. Satellite
groups of 20 animals of each sex in the control and high-dose groups
were killed after 1 year. The only noteworthy clinical signs were
paleness and hunched backs in males and rough coat in females at 1750
mg/kg of diet. Ophthalmic parameters were normal, and the mortality
rate was not affected. Females at 1750 mg/kg of diet showed slightly
decreased feed consumption and body-weight gain during the first 66
weeks, while the body weight of males at this dose was lower than that
of controls during the final 25 weeks of the study.
Hypercholesterolaemia occurred in males at 300 and 1750 mg/kg of diet
and in females at 1750 mg/kg of diet. Plasma, erythrocyte, and brain
cholinesterase activities were depressed by 20-40% at the highest
dose. Erythrocyte counts, haemoglobin concentration, and haematocrit
were slightly decreased at 1750 mg/kg of diet, but this anaemic
response had recovered in females by the end of the study. Urinary
parameters were not affected. At 1750 mg/kg of diet, the liver weights
were increased in males and females and the spleen weights were
increased in males, but with no associated pathological changes. The
kidney weights and the incidence of granular kidneys were increased at
1750 mg/kg of diet, corresponding to a slight enhancement in chronic
(age-related) nephropathy. The pathological changes included
hyperplasia of the upper small intestine and gastritis of the
non-glandular stomach in animals at 300 and 1750 mg/kg of diet and an
increased incidence of chronic lung inflammation in females at 1750
mg/kg of diet. The incidence of adrenal phaeochromocytomas was
increased in males at 1750 mg/kg of diet but was within the historical
control range. The NOEL was 92 mg/kg of diet, equal to 4.5 mg/kg bw
per day (Hayes, 1989; WHO, 1992).
Groups of 50 Fischer 344 rats of each sex were given diets
containing trichlorfon (purity, 98.5%) for 2 years at a nominal
concentration of 0 or 2500 mg/kg, equal to 130 mg/kg bw per day for
males and 160 mg/kg bw per day for females. Satellite groups of 20
animals of each sex per dose were killed after 1 year. This study was
initiated to ensure that trichlorfon had been tested at the maximum
tolerated dose. The body weights of treated animals were 10-15% lower
than those of controls throughout the study. Treated animals had
reduced food consumption during about the first half of the study, but
thereafter it was comparable to that of controls. The mortality rate
and ophthalmoscopic parameters were unaffected. The clinical signs in
treated animals included urine staining and enlarged abdomen in
animals of each sex and pale eyes and rough coat in males. Decreased
erythrocyte count, haemoglobin concentration, and haematocrit were
noted in treated rats but had recovered in males by the end of 2
years. Hypercholesterolaemia was seen in treated groups throughout the
study. The activities of gamma-glutamine transferase, alanine and
aspartate aminotransferases, and alkaline phosphatase in blood were
increased in treated males at various times but not at termination.
Urinary parameters were not affected. Statistically significant,
40-70% depressions in plasma, erythrocyte, and brain cholinesterase
activities were observed in animals of each sex at all times studied.
The liver and kidney weights were increased in treated groups,
and the lung weights were increased in females after 2 years; after 1
year, only changes in liver and kidney were reported. Histological
examination revealed duodenal hyperplasia, gastritis of the
non-glandular stomach, myodegeneration of the stomach tunica
muscularis, pulmonary inflammation and hyperplasia, hepatocellular
cytoplasmic vacuolation, focal hepatocellular hyperplasia associated
with sinusoidal dilatation and cystic degeneration (males),
nasolachrymal duct inflammation (females), chronic nephropathy
(females), and renal tubular hyperplasia. Non-statistically
significant increases in the incidences of renal tubular adenomas in
males and mononuclear-cell leukaemia in females were within the
historical control range. The increased incidences of
alveolar/bronchiolar adenomas in males and of carcinomas in females
were greater than the recorded historical control means, but the
numbers were small and did not attain statistical significance
(Christenson, 1990).
An increased incidence of tumours was thus detected in two
studies in rats conducted in the same laboratory. The increased
incidence of mammary tumours in females was slight, and the tumours
were benign. The strain used was Sprague-Dawley, which has a
relatively high, variable background incidence of mammary tumours
(Haseman et al., 1986). The lack of confirmation of this finding in
several studies in other species indicates that the evidence for the
carcinogenicity of trichlorfon in animals is limited.
Hamsters
A group of 23 male and 25 female Syrian golden hamsters received
trichlorfon (purity unknown) at a weekly intraperitoneal dose of 0 or
20 mg/kg bw for 90 weeks, and the study was terminated after 100
weeks. The treated animals showed reduced survival and body-weight
gain, but the tumour incidences were not increased (Teichmann &
Schmidt, 1978; FAO/WHO, 1979).
Dogs
Trichlorfon (purity unknown) was added to the diet of groups of
one male and one female beagle at a concentration of 50, 250, 500, or
1000 mg/kg, equivalent to 1.2, 6.2, 12, and 25 mg/kg bw per day, for 1
year. Few or no data on individual animals were provided. No effect on
food consumption or weight gain was reported. Serum and erythrocyte
cholinesterase activities were significantly depressed (by 40-50% of
control values) at 500 and 1000 mg/kg of diet. Brain
acetylcholinesterase activity was depressed by 40% in animals at
1000 mg/kg of diet. The relative spleen weights were increased and
showed marked congestion and apparent lymphoid tissue atrophy at
1000 mg/kg of diet and to a lesser extent at other doses. A slight
increase in the severity of foci of chronic inflammatory cells in the
liver and a decrease in spermatogenesis were seen at 1000 mg/kg of
diet. Hyperplastic adrenal cortical nodules recorded as 'mild' were
seen in all treated groups but not in controls. Owing to the small
number of animals in each group, however, the significance of this
finding is questionable (Doull et al., 1962; WHO, 1992).
Groups of four beagles of each sex were given diets containing
trichlorfon (purity, 95.3-98.3%) at a concentration of 0, 50, 200,
800, or 3200 mg/kg, equivalent to 1.2, 5, 20, and 80 mg/kg bw per day
in a study designed to last 4 years. All males at 3200 mg/kg of diet
died within the first year, and only one female at this dose survived
4 years. At this dose, the animals showed muscular twitching, severe
abdominal cramps, and salivation. The males in particular quickly
became emaciated. Autopsy of the animals that died during the
experiment showed brittle livers of a 'yellowish or loam' colour. Two
females and three males at 800 mg/kg of diet died during the first
year. The mortality rates at the two lower doses were similar to those
of controls, and the appearance and behaviour of the animals were
normal. Animals at higher doses were weakened and sick, and lower body
weights were recorded for surviving animals at 800 mg/kg of diet
during the latter stages and for the surviving female at 3200 mg/kg of
diet throughout the study. The effects on food consumption were
similar to those on body weight. Haematological parameters for treated
dogs were within the normal range. Elevated serum activities of
alanine and aspartate aminotransferases were noted in females at 3200
mg/kg of diet at 2 years but not at 4 years. No treatment-related
effects were found on urinary analysis. Cholinesterase activity in
whole blood was not inhibited at 50 mg/kg of diet; at 200 mg/kg of
diet, a 30% depression was seen at 1 month but the level rose again at
4 months. The group at 200 mg/kg of diet also showed inhibited
activity (15-20%) in plasma and erythrocytes throughout the study. At
800 and 3200 mg/kg of diet, dose-related depressions of 30-80% in
cholinesterase activity in whole blood, plasma, and erythrocytes were
found. The female at 3200 mg/kg of diet which survived to 4 years had
an enlarged, swollen liver, an enlarged spleen and adrenals, and small
ovaries. Dogs fed 800 mg/kg of diet had increased spleen and adrenal
weights and decreased testis weight. There were no histopathological
changes that were considered to be related to treatment. The NOEL was
50 mg/kg of diet, equivalent to 1.2 mg/kg bw per day (Loser, 1970;
Spicer & Payne, 1971; FAO/WHO, 1972b).
Monkeys
Groups of five male and five female rhesus monkeys (Macaca
mulatta) were given aqueous solutions of trichlorfon (purity,
98.6-99.1%) by gavage at a dose of 0, 0.2, 1, or 5 mg/kg bw per day, 6
days/week for 10 years. Two animals at 5 mg/kg bw per day showed
transient pupillary constriction, and one had muscular fasciculations
during the first month only; diarrhoea and soft stools were noted
frequently. At this dose, the body weights were lower towards the end
of the study, and there was a tendency to lower erythrocyte count,
haemoglobin concentration, and haematocrit at various sampling times.
No treatment-related effects on the mortality rate or clinical
chemical or urinary parameters were recorded. Plasma, erythrocyte, and
brain cholinesterase activities were inhibited by 30-80% in animals at
1 and 5 mg/kg bw per day, while males at 0.2 mg/kg bw per day showed
slight cholinesterase inhibition (16%) only in erythrocytes, which was
considered not to be biologically significant. Liver morphology in
biopsy samples taken during the first 3 years was unchanged. Gross and
microscopic examination of tissues showed no changes related to
treatment (Griffin, 1988; WHO, 1992).
2.2.4 Genotoxicity
The results of genotoxicity assays with trichlorfon are
summarized in Table 2. Other studies, evaluated by IARC (1983) and WHO
(1992), showed similar findings. The inconsistencies in a number of
tests has been suggested to be due to the variable purity of the
materials tested (Dreist & Mihail, 1990).
2.2.5 Reproductive toxicity
(a) Multigeneration studies
Trichlorfon (purity, 98.3%) was administered to groups of 10 male
and 20 female FB 30 rats in the diet at a concentration of 0, 100,
300, 1000, or 3000 mg/kg for three generations with two litters per
generation. Females in the F0 generation at 1000 and 3000 mg/kg of
diet had significantly depressed weight gain. The size of F1a
litters was reduced at doses > 1000 mg/kg of diet, while pup growth
and survival during lactation were impaired at 3000 mg/kg of diet. The
F1b mating resulted in lower pregnancy rates at 1000 and 3000 mg/kg
of diet. Litter size and pup growth and survival were also reduced at
3000 mg/kg of diet, and all animals in this group died before mating
to produce the F2 generation. During the remainder of the study, no
effects were found on fertility or on litter size or weight. The
F2a, F2b, F3a, and F3b groups given 1000 mg/kg of diet all had
reduced body-weight gain during the lactation period. No fetal
malformations were found in any group. Examination of the F0, F1b,
and F2b adults revealed no gross or microscopic alterations. The
NOEL was 300 mg/kg of diet, equivalent to 30 mg/kg bw per day (Loser,
1969; Spicer & Urwin, 1971; WHO, 1992).
(b) Developmental toxicity
Mice
A single dose of 360 mg/kg bw trichlorfon (purity unspecified),
injected intraperitoneally into 33 AS/Jena mice on day 1 of gestation,
was embryotoxic. The frequency of post-implantation loss was increased
with single doses of 60, 120, or 240 mg/kg bw on day 9 (13-15 mice per
group) and with 120 or 240 mg/kg bw on days 1-7 (25-30 mice per
group), and was most pronounced with 240 mg/kg bw on days 7-14 (23
mice). There were no severe malformations (Scheufler, 1975; WHO,
1992).
CD-1 mice were given trichlorfon (purity unspecified) by gavage
at a dose of 0, 300, 400, 500, or 600 mg/kg bw per day on days 6-10
(3-25 females per group) or 10-14 (4-21 females per group) of
gestation and were killed on day 18. The food intake and weight gain
of dams were depressed at all doses but with no dose-response
relationship. Fetal body weight was lower at doses > 400 mg/kg bw
per day, while the frequency of fetal malformations (cleft palate) was
slightly increased only in the groups treated with 500 or 600 mg/kg bw
per day during days 10-14 of gestation. The highest dose, 600 mg/kg bw
per day, was also administered by gavage on day 8 or on days 8-10,
10-12, or 12-14 of gestation (4-28 females per group). An increase in
the frequency of cleft palate was seen only in the last group. The
NOEL was 300 mg/kg bw per day (Staples & Goulding, 1979; WHO, 1992).
Trichlorfon (purity, > 98%) was given by gavage to CD-1 mice at
a dose of 0, 200, 300, or 400 mg/kg bw per day on days 7-16 of
gestation (15-24 females per group), and the animals were killed 1 day
after the last dose. The two higher doses increased the mortality
rate, and all doses decreased the weight gain of dams. The number of
live fetuses and fetal weight were decreased at 400 mg/kg bw per day.
Delayed development of fetuses was found, as indicated by reduced
calcification in a number of skeletal areas, rib variations, and
increased incidences of enlarged renal pelves in treated groups
(Courtney et al., 1986).
Table 2. Summary of results of tests for genotoxicity with trichlorfon
End-point Test object Concentration Result Reference
or dose (maximum)
In vitro
Interaction Determination of 160 mg/kg bw i.p. Positive Dedek et al.
with DNA 7-methylguanine (1976)
in mouse urine
Determination of 120 mg/kg bw i.p. Positive Dedek (1981)
7-methylguanine
in mouse liver and
kidney
Gene mutation B. subtilis NIG17, 0.3 mg per disc -S9 Negative Inukai & Iyatomi
(rec) NIG45 (1977)
P. mirabilis 10 mg per spot -S9 Positive Adler et al. (1976)
PG273, PG713
B. subtilis H17, NR -S9 Negative Shirasu et al.
M45 (1976)
B. subtilis H17, 2 mg per disc -S9 Positive Shirasu et al.
M45 (1979)
S. typhimurium 10 mg/disc ?S9 Positive Jones et al. (1984)
Reverse S. typhimurium 5 mg per plate ±S9 Positive Byeon et al. (1976)
mutation TA100
TA98, TA1535, 5 mg per plate ±S9 Negative
TA1538
TA98, TA100, 0.5 mg per plate ±S9 Negative Inukai & Iyatomi
TA1535, TA1537 (1977)
Table 2. (continued)
End-point Test object Concentration Result Reference
or dose (maximum)
TA98, TA100 ~ 8.5 mg per plate Positive Batzinger &
±S9 Bueding (1977)
TA100, TA1535 10 mg per plate ±S9 Negative Zeiger et al. (1987)
TA98, TA100 2 mg per plate ±S9 Negative Diril et al. (1990)
TA1535, TA1536, NR -S9 Negative Shirasu et al.
TA1537, TA1538, (1976)
E. coli WP2, WP2hcr
TA1535, TA1536, 2 mg per disc -S9 Negative Carere et al.
TA1537, TA1538 (1978a,b)
TA100, E. coli 20 mg per plate ±S9 Positive Shirasu et al.
WP2hcr (1979); Moriya et
TA98, TA1535, 20 mg per plate ±S9 Negative al. (1983)
TA1537, TA1538
TA100, TA104 25 mg per plate ±S9 Positive Barrueco et al.
TA97, TA98, 25 mg per plate ±S9 Negative (1991)
TA1535
TA98, TA1537 5 mg per plate±S9 Negative Watabe (1997)
TA100, TA1535, 5 mg per plate±S9 Positive
E, coli WP2uvrA
S. cerevisiae NR -S9 Negative Guerzoni et al.
632/4, 632/1b, (1976)
814/18b
Table 2. (continued)
End-point Test object Concentration Result Reference
or dose (maximum)
Reverse S. cerevisiae 10 mg per ml ±S9 Negative Hoorn (1983)
mutation S138, S211a
S. cerevisiae D7 40 mg per ml ±S9 Positive Jones et al.
(1984)
Mitotic crossing S. cerevisiae D7 40 mg per ml ±S9 Positive Jones et al.
over, gene (1984)
conversion
Forward S. coelicolor 2 mg per disc -S9 Positive Carere et al.
mutation (1978a,b)
S pombe SP-198 30 mg per ml ±S9 Positive Gilot-Delhalle et
al. (1983)
V79 cells 200 mg per ml -S9 Negative Aquilina et al. (1984)
L5178Y cells 200 µg per ml -S9 Positive Witterland
600 µg per ml +S9 Positive (1984); Jones et
al. (1984)
DNA damage E coli pol+/- 10 mg per plate ±S9 Negative Herbold (1984)
E. coli (SOS NR ±S9 Negative Xu & Schurr
chromotest) (1990)
Unscheduled EUE cells 1000 mg per ml -S9 Positive Aquilina et al.
DNA synthesis (1984)
Primary rat 50 µg per ml -S9 Negative Myhr (1983)
hepatocytes
Table 2. (continued)
End-point Test object Concentration Result Reference
or dose (maximum)
Sister chromatid V79 cells 80 µg per ml -S9 Positive Chen et al.
exchange 60 µg per ml +S9 Positive (1981, 1982)
CHO cells 100 µg per ml -S9 Positive Jones et al.
2 mg per ml +S9 Positive (1984); Putman
(1987)
Chromosomal Don-6 cells 250 mg per ml -S9 Positive Sasaki et al.
damage (1980)
Human lymphocytes 30 mg per ml -S9 Positive Herbold (1986)
3000 mg per ml +S9 Positive
In vivo
Reverse Host-mediated Batzinger &
mutation assay in mice Bueding (1977)
S. typhimurium 200 mg/kg bw orally Negative
TA98
S. typhimurium 200 mg/kg bw orally Positive
TA100
Recessive lethal Drosophila 4.5 mg/kg bw Negative Benes & Sram
mutation melanogaster (1969); Brzheskiy
(1973); Lamb
(1977)
Sister chromatid Chinese hamster 300 mg/kg bw orally Negative Volkner (1987)
exchange bone marrow
Table 2. (continued)
End-point Test object Concentration Result Reference
or dose (maximum)
Chromosomal Micronucleus 2 x 312 mg/kg bw i.p. Negative Paik & Lee
damage formation in mouse 2 x 250 mg/kg bw Negative (1977); Herbold
bone marrow orally (1979a); Jones
2 x 400 mg/kg bw Negative et al. (1984);
orally Herbold (1997)
400 mg/kg bw orally Negative
400 mg/kg bw orally Negative
of (+)-enantiomer
600 mg/kg bw orally Positive
of (-)-enantiomer
Metaphase analysis 400 mg/kg bw orally Positive Kurinnyi (1975);
in mouse 10 mg/kg bw i.p. Positive Moutschen-Dahmen
bone marrow 100 mg/kg bw i.p. Negative et al.
0.5 mg/ml in drinking, Negative (1981);
water 5 days/week, Degraeve et al.
7 weeks (1982, 1984);
405 mg/kg bw i.p. Negative Nehes et al.
(1982)
Metaphase analysis 250 mg/kg bw i.p. Negative Dzwonkowska &
in hamster Hubner (1986)
bone marrow
Metaphase analysis 250 mg/kg bw orally Negative Bootman &
in rat bone Hodson-Walker
marrow (1987)
Table 2. (continued)
End-point Test object Concentration Result Reference
or dose (maximum)
Metaphase analysis 1.5 mg/ml in drinking, Positive Bulsiewicz et al.
in mouse water 50-100 days (1976);
spermatogonia 100 mg/kg bw i.p. Negative Moutschen-Dahmen
and spermatocytes 0.5 mg/ml in drinking, Negative et al.
water 5 days/week, (1981);
7 weeks Degraeve et al.
100 mg/kg bw i.p. Negative (1982, 1984);
Herbold (1992)
Dominant lethal 100 mg/kg bw i.p. Negative Epstein et al.
mutation in mice 0.5 mg/ml in (1972); Dedek
drinking-water, Negative et al. (1975) ;
5 days/week, Fischer et al.
7 weeks (1977); Herbold
280 mg/kg bw i.p. Negative (1979b,c);
405 mg/kg bw i.p. Negative Becker &
405 mg/kg bw i.p. Positive Schoneich
250 mg/kg bw orally Negative (1980);
NR Negative Moutschen-Dahmen
405 mg/kg bw i.p. Positive et al. (1981);
54 mg/kg bw per day, Positive Degraeve et al.
3 weeks i.p. (1982, 1984);
WHO (1992)
NR, not reported; S9, metabolic activation; i.p., intraperitoneally
Rats
Trichlorfon (purity unspecified) was administered by gavage to
groups of 11 female Wistar rats at a single dose of 80 mg/kg bw on day
9 or 13 of gestation or to 10 rats at a dose of 8 mg/kg bw per day
daily throughout gestation. The animals were killed on day 19 of
gestation. The frequencies of post-implantation deaths and fetal
malformations such as exencephaly and non-closing eyelids were
increased at the single dose on day 13. The other treatments had no
effect on development (Martson & Voronina, 1976; WHO, 1992).
Trichlorfon (purity, 98.5%) was administered in the diet to
groups of 9-26 female CD rats on days 6-15 of gestation, to give an
intake of 0, 76, 140, 380, 430, or 520 mg/kg bw per day, with necropsy
on day 21. At the two highest doses, maternal food intake and body
weight and fetal weights were reduced, while the number of fetal
deaths was increased at 430 mg/kg bw per day. Major external and
skeletal malformations were found in fetuses at 430 and 520 mg/kg bw
per day and relatively minor skeletal changes at 380 mg/kg bw per day.
The predominant malformations were alterations of the skull and
central nervous system (exencephaly, meningocoele, hydrocephaly), the
limbs (syndactyly, markedly shortened radii and ulnae, missing
digits), micrognathia, cleft palate, facial haematomas, generalized
oedema, and great vessel defects. The minor skeletal alterations were
mainly doubled vertebral centra, wavy ribs, fenestrated supraoccipital
bones. and umbilical hernia. The NOEL was 140 mg/kg bw per day
(Staples et al., 1976; WHO,1992).
Trichlorfon (purity, 98.5%) was given by gavage at a dose of 0,
50, 75, 150, 200, or 250 mg/kg bw per day to groups of 9-30 female CD
rats on days 6-15 of gestation. Deaths occurred among dams at 150,
200, and 250 mg/kg bw per day, with overt signs of toxicity at the
highest dose. The survivors were killed on day 21 of gestation. Fetal
body weights were reduced significantly at doses > 75 mg/kg bw per
day, but the incidence of malformations was unaffected. The NOEL was
50 mg/kg bw per day (Staples et al., 1976).
Trichlorfon (purity unspecified) was given at a dose of 480 mg/kg
bw per day to 34 CD rats by gavage on days 6-15 of gestation. Lower
fetal body weight, a higher fetal mortality rate, and malformations
such as generalized oedema, herniation of the brain, hydrocephaly,
micrognathia, cleft palate, and skeletal alterations were seen. A
single gavage dose of 480 mg/kg bw on day 8 or 10 of gestation to 14
females per group decreased maternal food consumption but had no
adverse effect on fetal development (Staples & Goulding, 1979; WHO,
1992).
Groups of 25 female Long-Evans rats were given trichlorfon
(purity, 98.4%) by gavage at a dose of 0, 10, 30, or 100 mg/kg bw per
day on days 6-16 of gestation and were killed on day 20. There were no
deaths, but diarrhoea was observed in some animals at 100 mg/kg bw per
day. Fetal development was not affected. The NOEL for maternal
toxicity was 30 mg/kg bw per day (Machemer, 1979a; WHO, 1992).
Trichlorfon (purity, > 98%) was given by gavage to CD rats at a
dose of 0, 50, 100, or 200 mg/kg bw per day on days 7-19 (10-20
females per group) or 8-20 of gestation (15-18 females per group).
The animals were killed 1 day after the last dose. The highest dose
increased the mortality rate and decreased the weight gain of the
dams. Delayed development of fetuses was found, as indicated by
reduced calcification in a number of skeletal areas, but no
abnormalities were noted (Courtney et al., 1986).
Groups of 33 female Charles River Crl:CD BR rats were given diets
containing trichlorfon (purity, 99%) at a concentration of 0, 500,
1100, or 2500 mg/kg on days 6-15 of gestation. Body-weight gain was
slightly reduced among animals at the highest dose. Analysis of
cholinesterase activity in five animals per group killed on day 16 of
gestation showed inhibition of plasma, erythrocyte, and brain enzymes.
When the remaining dams were killed on day 20 of gestation, only brain
acetylcholinesterase activity was significantly inhibited. No effect
was seen on the numbers of resorptions, live fetuses, fetal body
weight, or malformation frequency. Increased incidences of delayed
ossification and curved, wavy, or bulbous ribs were seen at 2500 mg/kg
of diet. While fetal brain acetylcholinesterase activity was lower in
all treated groups, there was no dose-response relationship (Kowalski
et al., 1987; WHO, 1992).
Hamsters
Groups of 5-30 female golden hamsters received trichlorfon
(purity unspecified) at a dose of 0, 100, 200, 300, or 400 mg/kg bw
per day by gavage on days 7-11 of gestation, and the survivors were
killed on day 15. Three of 30 hamsters at the highest dose died, and
the signs of cholinesterase inhibition were dose-related at doses >
200 mg/kg bw per day. Maternal food consumption and body-weight gain
and fetal body weight were decreased at 300 and 400 mg/kg bw per day.
Fetuses at the highest dose showed an increased frequency of stunting
malformations, mostly consisting of oedema, cleft palate, patagium,
and fused ribs. A single dose of 400 mg/kg bw per day by gavage given
on day 8 of gestation to 16 females reduced maternal food consumption
and increased the fetal death rate but had no meaningful effect on
fetal development. The NOEL was 100 mg/kg bw per day (Staples &
Goulding, 1979; WHO, 1992).
Guinea-pigs
Groups of 10 white female guinea-pigs were given six doses of 100
mg/kg bw per day trichlorfon (purity, 97%) by gavage on days 36-38 and
51-53 of gestation. Seven females served as controls, and all animals
were allowed to deliver naturally. The numbers of abortions and
stillborn fetuses were increased and fetal body weight was decreased
in the treated group. The pups of treated dams developed trembling and
locomotor disturbances. At autopsy on postnatal day 2-3, the total
weights of the brain, cerebellum, medulla, cerebral cortex,
hippocampus, thalamus, and colliculi were decreased. The cerebellum
showed reduction of the external granular and molecular layers, with
regional absence of Purkinje cells. The activities of the
neurotransmitter enzymes choline acetyltransferase and glutamate
decarboxylase in the cerebellum were depressed when compared with
controls (Berge et al., 1986; WHO, 1992).
Rabbits
Groups of 15 female Himalayan rabbits were given trichlorfon
(purity, 98.4%) by gavage at a dose of 0, 5, 15, or 45 mg/kg bw per
day on days 6-18 of gestation and were killed on day 29. Body-weight
gain was reduced in treated groups, and two abortions occurred at 45
mg/kg bw per day. Fetal development was not affected (Machemer, 1979b;
WHO, 1992).
Groups of 20 female American Dutch rabbits were given trichlorfon
(purity, 99%) by gavage at a dose of 0, 10, 35, or 110 mg/kg bw per
day on days 6-18 of gestation and were killed on day 28. The highest
dose was not well tolerated and resulted in deaths, overt signs of
toxicity, and reduced body-weight gain. At 35 mg/kg bw per day, there
were also signs of toxicity, one death, and one abortion. Erythrocyte
but not plasma cholinesterase activity was inhibited on day 19 of
gestation in animals at 35 and 110 mg/kg bw per day, while on day 28
only brain cholinesterase activity was inhibited at these doses. At
110 mg/kg bw per day, there was a slight increase in the number of
resorptions, slight decreases in fetal and placental weights, and
delayed ossification. The frequency of fetal abnormalities was not
increased. The NOELs were 10 mg/kg bw per day for maternal toxicity
and 35 mg/kg bw per day for embryo- and fetal toxicity (Clemens et
al., 1990; WHO, 1992).
Pigs
Several outbreaks of congenital tremor in piglets have been
described in herds in which the sows had been treated with trichlorfon
preparations between day 45 and day 63 of gestation. Clinically, the
syndome was characterized by ataxia, tremor, pronounced hypoplasia of
the cerebellum, and a reduction in the size of the spinal cord (WHO,
1992). This syndrome was reproduced experimentaly in sows given diets
providing one to four doses of 50-100 mg/kg bw per day 55-98 days
after conception. The offspring had a high incidence of overt
neurological signs and cerebellar hypoplasia. Histological examination
revealed patchy loss of Purkinje cells in the cerebellum. Postnatally,
the rate of increase of brain weight was similar to that of controls,
but normal weights had not been achieved up to 35 days after birth
(Knox et al., 1978; Pope et al., 1986; Berge et al., 1987a,b; WHO,
1992).
2.2.6 Special studies
(a) Neurotoxicity
The results of these studies are summarized in Table 3. Like
other organophosphonates, trichlorfon has neurotoxic potential. Rats
given a dose of about 200 mg/kg bw per day for 13 weeks showed
decreased locomotor activity and loss of coordination, whereas rats
given a dose of 30 mg/kg bw per day for 3 weeks showed increased
locomotion and decreased learning ability and nerve conduction
velocity. Numerous studies in hens consistently demonstrated the
neurotoxicity of single doses of trichlorfon. Leg weakness, impaired
walking ability, and decreased activity were seen immediately after
treatment. These effects are considered to be secondary to inhibition
of cholinesterase activity and to be generally unrelated to damage to
the nervous tissues. Delayed neurotoxicity is usually detected some
weeks after dosing and is associated with degeneration of nervous
tissue and marked inhibition of neuropathy target esterase activity.
Neuropathy target esterase is a protein which is located specifically
in neurons, but its biological function, other than as a target for
neuropathic organophosphates, is unknown. The toxicity of an agent can
be predicted by its relative ability to interact with either
acetylcholinesterase to produce cholinergic effects or neuropathy
target esterase to produce delayed polyneuropathy (Ray, 1998). The
studies gave no evidence of delayed neuropathy in chickens. In a
monkey given a single oral dose of trichlorfon at 250 mg/kg bw per
day, nerve conduction was impaired 4 weeks after treatment, and there
was histological evidence of demyelination of nerves and axonal
degeneration.
(b) Immunotoxicity
Groups of four female BALB/cByJ and C57BL/6J mice were given
drinking-water containing trichlorfon at 1.75 mg/ml for 14 days.
Measurement of a variety of responses to immunization with influenza
virus indicated that immune function was not impaired (Reiss et al.,
1987; WHO, 1992).
2.3 Observations in humans
Wegner (1970) reviewed data on over 6000 people, obtained mostly
in South Africa and South America, who had been treated with
trichlorfon for various intestinal and body parasites (Ankylostoma,
Ascaris, Strongyloides, Trichocephalus, and Schistosoma), at doses
varying from 5 to 70 mg/kg bw per day for up to 12 days. A dose of 7.5
mg/kg bw given two to four times at 2-week intervals was considered to
be optimal, as the side-effects observed were less severe than at
other dosages. The symptoms included depression of cholinesterase
activity, weakness, nausea, diarrhoea, and abdominal pain. A dose of
24 mg/kg bw caused more severe symptoms, including tachycardia,
salivation, colic pain, vomiting, nausea, fatigue, tremors, and
sweating. The effects were not cumulative, and spontaneous recovery
was rapid in all cases. Spermatogenesis (size and shape of sperm)
appeared to be affected in a few cases. Inhibition of cholinesterase
activity was seen at all doses (FAO/WHO, 1972b; WHO, 1992).
Reduced erythrocyte and plasma cholinesterase activities have
been reported in groups of patients with various parasitic
infestations given trichlorfon orally at doses of 5-12.5 mg/kg bw. The
drug was reported to be well tolerated with few clinical symptoms
related to treatment. The symptoms that were observed were cholinergic
in nature and were usually found at doses > 10 mg/kg bw.
Cholinesterase activity recovered to pretreatment levels within 4-15
weeks after cessation of treatment (Abdel Aal et al., 1970; Plestina
et al., 1972; Jewsbury, 1981).
In a clinical trial, 10 male and 10 female patients with
Alzheimer disease were given oral doses of trichlorfon for up to 30
weeks according to the following dose regimen: 2.5 mg/kg bw at week 0,
5 mg/kg bw in week 1, 7.5 mg/kg bw in weeks 6 and 7, 15 mg/kg bw in
weeks 8 and 9, and the 'best weekly dose' in weeks 18-30. The 'best
dose' was defined as that associated with the greatest improvement for
each patient. This small trial indicated general improvement in a
variety of functional deficits associated with Alzheimer disease.
Side-effects were reported at doses > 5 mg/kg bw, which included
nausea, vomiting, and diarrhoea. Plasma cholinesterase activity was
inhibited by 45-60% at all doses, and erythrocyte acetylcholinesterase
activity was inhibited by 30-70% at doses > 5 mg/kg bw with no
concomitant clinical findings. Measurement of acetylcholinesterase
activity in the cerebrospinal fluid from two patients 24 h after a
dose of 5 mg/kg bw revealed inhibition of 37 and 47% below
pretreatment levels (Becker et al., 1990).
In a prospective, double-blind, randomized study, four parallel
groups of 119-121 patients with Alzheimer disease received oral doses
of placebo or trichlorfon once daily. An initial loading dose of 0,
0.5, 0.9, or 2.0 mg/kg bw per day was given during weeks 0-2 in order
to achieve steady-state inhibition of cholinesterase activity more
quickly, and this was followed during weeks 3-10 by a dose of 0, 0.2,
0.3, or 0.65 mg/kg bw per day. The intermediate and high doses were
found to improve cognitive function, but the low dose had equivocal
effects. Abdominal pain, diarrhoea, flatulence, nausea, and leg cramps
were the most commonly reported side-effects and were most severe in
the patients given the high dose. Erythrocyte acetylcholinesterase
activity was inhibited in a dose-related manner (Table 4). The initial
dose of 0.5 mg/kg bw per day for 2 weeks inhibited erythrocyte
acetylcholinesterase activity by 29%, and subsequent administration of
0.2 mg/kg bw per day for 10 weeks maintained the inhibition at 30-37%,
with no increase with duration of dosing. Since this dose enhanced
inhibition over that caused by the initial dose by only 8%, an
insignificant change, the Committee considered that the NOEL for
inhibition of acetylcholinesterase activity was 0.2 mg/kg bw per day
(Cummings et al., 1998).
Table 3. Summary of studies of neurotoxicity with trichlorfon
Species Treatment Results Reference
Rats 200 mg/kg bw per day Electrophysiological changes Averbrook &
i.p. for 5-15 days indicating enhanced excitability Anderson (1983)
in sciatic nerve. No pathological
changes in nerves
Rats 30 mg/kg bw per day Increased locomotion, decrease Lehotzky (1982)
orally for 3 weeks in rotorod performance, learning
ability, and nerve conduction
velocity
Rats 0, 85, 438, or 2275 Rats given 2275 ppm had stained Sheets &
ppm in the diet for 13 fur, reduced motor and locomotor Hamilton (1995)
weeks activity, slightly uncoordinated
righting response (males only),
30-80% inhibition of plasma,
erythrocyte, and brain
cholinesterase activity, and
demyelination in spinal nerve roots.
At 438 ppm, only plasma and
erythrocyte cholinesterase activity
was inhibited (20-30%), and no
behavioural or biochemical
evidence of neurotoxicity was
seen at 85 ppm (6 mg/kg bw
per day)
Chicken Single subcutaneous Leg weakness for 1-2 days, no Witter & Gaines
dose of 90 mg/kg bw delayed paralysis (1963)
Chicken Single dose of 25-500 Birds that survived the acute Lorke &
mg/kg bw orally or toxicity of trichlorfon showed no Kimmerle (1966)
75-500 mg/kg bw i.p. signs of neurotoxicity over
6-10 weeks
Chicken 100-5000 ppm in Body weight loss at > 500 ppm. Lorke &
feed for 30 days Blood cholinesterase activity Kimmerle (1966)
decreased by 40-60% at all Hobik (1967);
doses but no signs of FAO/WHO
neurotoxicity and no (1972)
degeneration in nervous tissue.
Table 3. (continued)
Species Treatment Results Reference
Chicken Single subcutaneous One hen died 1.5 h after dosing; Hierons &
dose of 200 mg/kg bw the lone survivor was killed 24 h Johnson (1978)
after dosing, when NTE was
inhibited by 46% in brain and
17% in spinal cord.
Chicken Single doses of 50, Dose-related deaths after oral Olajos et al.
100 mg/kg bw orally, doses only. All treatments had (1979)
50, 100, 200 mg/kg acute effects; ataxia from 11-17
bw subcutaneously, days after dosing. Brain NTE
or 200 +100 mg/kg bw decreased by 40-60% at high
subcutaneously 3 subcutaneous doses but by only
days apart about 20% at <100 mg/kg bw.
Mild degenerative changes seen
in cerebellum, brainstem, and
striatum at higher doses.
Chicken 185 mg/kg bw orally, Acute signs of intoxication but Thyssen et al.
survivors given 167 no pathological changes in (1982)
mg/kg bw 21 days nervous tissue
later
Chicken Single subcutaneous Marked cholinesterase inhibition Slott &
dose of 100 or 300 in plasma, brain, and spinal cord Ecobichon
mg/kg bw (50-60%) but no inhibition of NTE (1984)
in brain or spinal chord
Chicken 100 mg/kg bw Plasma, brain, and spinal cord Slott &
subcutaneously every cholinesterase inhibited by Ecobichon
72 h for 6 doses 10-60%, and walking ability (1984)
slightly impaired. No inhibition
of NTE in brain or spinal cord
and only slight oedematous
effects on nervous tissue
Chicken 3, 9, or 18 mg/kg bw Signs of ataxia and decreased Hayes & Ramm
per day for 3 months activity at 18 mg/kg bw per day (1987)
by gavage but no effect on locomotor activity.
Blood cholinesterase activity
decreased in all groups but
nervous tissue unremarkable.
Table 3. (continued)
Species Treatment Results Reference
Chicken Single gavage dose Acute signs of intoxication, Bomann &
of 340 mg/kg bw, 220 deaths, and marked inhibition Kaliner
mg/kg bw (90%) of brain cholinesterase (1996)
(-)-enantiomer, activity with each compound.
or 400 mg/kg bw < 50% NTE inhibition in brain,
(+)-enantiomer spinal cord, and sciatic nerve; no
changes in gait or nervous tissue
Monkey Single dose of Weakness of lower extremities Shiraishi et al.
(Macaca 250 mg/kg bw by on day 24 and nerve conduction (1983)
fuscata) gavage defects by day 28. On day 37,
demyelination of sural and tibial
nerves with increased axonal
degeneration
NTE, neuropathy target esterase
Table 4. Mean inhibition of erythrocyte acetylcholinesterase
activity (%) in patients with Alzheimer disease
given trichlorfon orally
Time (week) Dose of trichlorfon (mg/kg bw per day)
0-2 0 0.5 0.9 2.0
3-10 0 0.2 0.3 0.65
2 1.1 ± 14 29 ± 12 49 ± 12 71 ± 82
8 0.1 ± 21 37 ± 13 52 ±12 68 ± 95
12 1.0 ± 24 34 ± 14 52 ± 10 72 ± 24
A position paper (Bayer AG, 1999) reported that the results of
clinical trials involving over 4000 treated patients show that
trichlorfon at the recommended dose of 0.5-0.9 mg/kg bw is 'a safe and
effective treatment' for Alzheimer disease. A dose-response
relationship was found between the dose of trichlorfon and the
incidence of muscle weakness in placebo-controlled trials, and the
increased risk for muscular weakness was statistically significant at
doses > 1.25 mg/kg bw. The recommended dose of 0.5-0.9 mg/kg bw
resulted in incidences of 0.6% for generalized weakness or myasthenia
(0.2% with placebo) and 4.3% for fatigue or asthenia (3.3% with
placebo). The muscular weakness was reported to be reversible on
discontinuation of therapy.
Acute poisoning of humans with trichlorfon has been reported to
manifest as delayed neurotoxicity, severe polyneuritis, and
polyneuropathy 2-3 weeks after ingestion. These diagnoses were based
on observations of weakness, loss of feeling in the extremities, and
difficulty in walking, accompanied by muscular atrophy, myalgia, and
were related to motor nerve damage (Pollingher et al., 1973; Akimov et
al., 1975; Eljasz & Kryzyszton-Przekop, 1975; Fukuhara et al., 1977;
Shiraishi et al., 1977; Hierons & Johnson, 1978; Shiraishi et al.,
1982; Niedziella et al., 1985; De Freitas et al., 1990). After
reviewing the literature, Johnson (1981) concluded that only doses of
trichlorfon that exceed the lethal dose but are survived by the victim
because of treatment are likely to result in a level of inhibition of
neuropathy target esterase activity at which delayed neurotoxicity
would be expected. Development of neuropathy after repeated exposure
to lower doses was considered to be unlikely (WHO, 1992).
Five persons exposed to trichlorfon, two while attempting suicide
and three occupationally, showed increased incidences of chromosomal
breaks and stable chromosomal rearrangements in cultured lymphocytes.
The effect was observed immediately after exposure and 1 month later,
but not 6 months later (Bao et al., 1974). Seventeen workers involved
in the production of trichlorfon-containing products for at least 6
months had increased incidences of chromatid breaks in their
lymphocytes when compared with an unexposed control group, but the
incidences were lower than those in a control group of factory workers
who had had no direct exposure to pesticides (Kiraly et al., 1979).
3. COMMENTS
The Committee considered the results of studies on the
toxicokinetics, metabolism, and acute toxicity of trichlorfon,
short-term and long-term studies of toxicity, and studies of
genotoxicity, reproductive and developmental toxicity, neurotoxicity,
and immune function, and studies in humans. Most of the available
studies were relatively old, and many were published reports which
lacked raw data or data on individual animals. Such studies are
difficult to evaluate, and most could not be assessed independently.
Unpublished reports of studies conducted during the 1980s and 1990s
contained sufficient detail and were carried out according to
appropriate standards for study protocol and conduct.
The absorption of trichlorfon is rapid in all species tested,
including humans, irrespective of the route of administration. Peak
blood concentrations were achieved within 1-2 h but decreased quickly
thereafter; the half-time of trichlorfon in human plasma is
approximately 2 h. It is widely distributed. Trichlorfon was detected
in the milk of lactating cows, and the compound and its metabolites
were found in fetal tissue in treated guinea-pigs. Trichlorfon
undergoes conversion to dichlorvos via a dehydrochlorination reaction
that occurs spontaneously at pH values above 5.5. Although little
dichlorvos was recovered, it is generally believed to be responsible
for the anticholinesterase effects of trichlorfon. The metabolism of
trichlorfon in mammals also occurs through O-demethylation and
cleavage of phosphorus-carbon bonds. Therefore, the major metabolites
are desmethyl trichlorfon, desmethyl dichlorvos, dimethyl hydrogen
phosphate, methyl dihydrogen phosphate, and phosphoric acid.
Trichlorfon and its metabolites are excreted primarily in the urine.
Trichlorfon is moderately toxic when given as a single oral dose,
the LD50 values being 620-950 mg/kg bw in mice, 140-660 mg/kg bw in
rats, 160 mg/kg bw in rabbits, and 300-420 mg/kg bw in dogs. The signs
of toxicity were indicative of inhibition of acetylcholinesterase
activity, and the cause of death was usually respiratory failure. The
rapid recovery observed after sublethal doses suggests that
trichlorfon is readily detoxified.
Trichlorfon was given orally to mice at doses of 10-750 mg/kg bw
per day, to rats at doses of 0.05-250 mg/kg bw per day, and to dogs at
doses of 0.5-45 mg/kg bw per day for periods up to 16 weeks. The
weights of the liver, kidney, and spleen were increased at doses of
250 mg/kg bw per day and above in mice and at doses of 50 mg/kg bw per
day and above in rats, but these changes were usually not associated
with other evidence of toxicity, except in one study in rats in which
hypertrophy of centrilobular hepatocytes and biochemical changes
suggestive of altered lipid metabolism were seen. These effects
occurred at doses which also induced significant signs of cholinergic
intoxication. Slight anaemia was observed in one study in rats at 100
mg/kg bw per day and in one study in dogs at 45 mg/kg bw per day. One
dog out of three given trichlorfon at a dose of 45 mg/kg bw per day
had small, immature testes and prostate and oligospermia, but the
animal had also lost weight and appeared to be severely stressed by
the treatment. The NOELs were 15 mg/kg bw per day for inhibition of
cholinesterase activity in brain and erythrocytes in mice, 5 mg/kg bw
per day for inhibition in brain and erythrocytes in rats, and 5 mg/kg
bw per day for inhibition in in erythrocytes in dogs.
In two studies of up to 2 years' duration, mice were given oral
doses of 15-750 mg/kg bw per day. Body-weight gain was impaired at
doses of 30 mg/kg bw per day and above; the mean weight of the liver
was increased at a dose of 240 mg/kg bw per day in one study, but no
pathological alterations were found. The NOEL for inhibition of brain
and erythrocyte cholinesterase activity was 49 mg/kg bw per day. The
incidences of tumours were unaffected by treatment. Neither study was
suitable for identifying a NOEL.
Six studies of up to 2 years' duration were conducted in which
rats were given trichlorfon in the diet at concentrations providing
doses of 2.5-160 mg/kg bw per day. The weights of the liver and spleen
were increased in three studies at doses of 50 mg/kg bw per day and
above. Hypercholesterolaemia, duodenal hyperplasia, and gastritis of
the non-glandular stomach were seen at 13 mg/kg bw per day and above.
Anaemia, enhancement of age-related nephropathy, increased renal
tubular hyperplasia, myodegeneration of the stomach tunica muscularis,
pulmonary inflammation, and hyperplasia were observed at doses of 75
mg/kg bw per day and above. Other observations included ovarian
atrophy at 20 mg/kg bw per day and above, depression of
spermatogenesis at 50 mg/kg bw per day, and a slight increase in the
incidence of benign mammary tumours in females with a reduction in the
time to appearance at doses of 20 mg/kg bw per day and above. The NOEL
for inhibition of brain and erythrocyte acetylcholinesterase activity
was 13 mg/kg bw per day. The incidence of mammary tumours was
increased in female Sprague-Dawley rats in two studies. The increases
were slight, the tumours were benign, this strain of rats has a
relatively high and variable background incidence of mammary tumours,
and the finding could not be confirmed in several other studies in
various species. The Committee concluded that trichlorfon does not
have carcinogenic potential in rats. The overall NOEL in rats was 4.5
mg/kg bw per day on the basis of an increased cholesterol
concentration and pathological changes to the gastrointestinal tract.
In two studies of 1 and 4 years' duration, dogs were given
trichlorfon orally at doses of 1.3-80 mg/kg bw per day. Deaths
occurred at doses of 20 mg/kg bw per day and above, and signs of
hepatocellular damage and increased severity of inflammation were seen
in the liver at doses of 25 mg/kg bw per day and above. In animals
given 20-25 mg/kg bw per day, the weight of the spleen was increased,
with atrophy of lymphoid tissue; furthermore, small ovaries, reduced
testis weight, and decreased spermatogenesis were seen. 'Blood
cholinesterase' activity was inhibited at doses of 5 mg/kg bw per day
and above. The overall NOEL in dogs was 1.3 mg/kg bw per day on the
basis of inhibition of cholinesterase activity.
Oral administration of trichlorfon to rhesus monkeys at a dose of
0, 0.2, 1, or 5 mg/kg bw per day for 10 years resulted in clinical
signs of toxicity indicative of cholinesterase inhibition, reduced
body-weight gain, and anaemia at the highest dose. Inhibition of
plasma, erythrocyte, and brain cholinesterase activities was seen at
the two higher doses. In a separate 6-month study in rhesus monkeys,
erythrocyte acetylcholinesterase activity was not depressed at 0.2
mg/kg bw per day, the highest dose tested. The NOEL in monkeys was 0.2
mg/kg bw per day on the basis of inhibition of brain and erythrocyte
acetylcholinesterase activity.
Trichlorfon has been tested in a wide range of assays for
genotoxicity and DNA damaging activity, with considerable variation in
the results. Trichlorfon induced point mutations in microorganisms and
cultured mammalian cells, DNA damage in microorganisms, and
chromosomal aberrations and sister chromosome exchange in cultured
mammalian cells. The results of tests for point mutation in
Drosophila melanogaster and for sister chromatid exchange in bone
marrow were clearly negative. Although positive results were obtained
in a few assays for chromosomal damage in bone marrow and in the germ
cells of male animals exposed at near-lethal doses, the results of
most studies of micronucleus formation, metaphase alterations, and
dominant lethal mutations were negative. Since the tests conducted
in vivo produced mostly negative results when trichlorfon was
administered orally, the Committee considered that the weight of
evidence indicates that trichlorfon is unlikely to represent a
genotoxic risk.
Trichlorfon was given to rats at a dose of 0, 10, 30 100, or 300
mg/kg bw per day in a three-generation study of reproductive toxicity,
with two litters per generation. The body-weight gain of F0 dams at
100 and 300 mg/kg bw per day was depressed. The slight effects on the
gonads reported in the studies of general toxicity were not
consistently reflected in this study. The pregnancy rate of rats of
the F1b generation at doses of 100 mg/kg bw per day and above was
reduced, but the fertility of other generations was unaffected. The
litter size of dams at 300 mg/kg bw per day was reduced and all of the
offspring died, but reproductive parameters were unaffected at lower
doses. The NOEL was 30 mg/kg bw per day in adults, on the basis of
reduced body-weight gain, and 100 mg/kg bw per day for reproductive
effects, on the basis of reduced litter size and the death of
offspring.
Two studies of developmental toxicity were conducted in mice
given oral doses of 200-600 mg/kg bw per day. Dams at 200 mg/kg bw per
day showed reduced body-weight gain, and higher doses led to an
increased mortality rate. The effects of these maternally toxic doses
included increased embryotoxicity, decreased fetal weight, and delayed
fetal development. The incidence of cleft palate was slightly
increased at doses of 500 and 600 mg/kg bw per day. NOELs were not
identified for maternal toxicity or fetotoxicity.
Studies of developmental toxicity were conducted in rats given
oral doses of 8-520 mg/kg bw per day. In three studies, maternal
toxicity in the form of reduced body-weight gain and/or an increased
mortality rate was observed at doses of 150 mg/kg bw per day and
above. The NOEL for maternal toxicity was 75 mg/kg bw per day. In the
same studies, the incidences of fetal malformations such as
morphological alterations of the skull, central nervous system, and
limbs, micrognathia, cleft palate, facial haematomas, generalized
oedema, and defects in major blood vessels were increased at doses of
430 mg/kg bw per day and above. At 380 mg/kg bw per day, minor
skeletal changes were observed. Reduced fetal body weight was seen at
75 mg/kg bw per day. In three other studies, the incidence of fetal
malformations was unaffected. The NOEL for developmental effects was
50 mg/kg bw per day on the basis of fetotoxicity.
Hamsters given trichlorfon orally at a dose of 0, 200, 300, or
400 mg/kg bw per day during gestation showed signs of cholinesterase
inhibition, and depressed body-weight gain was seen at the two higher
doses. The NOEL for maternal toxicity was 100 mg/kg bw per day. Fetal
body weight was decreased at the two higher doses, and an increased
incidence of fetal stunting was seen at the highest dose. The NOEL for
developmental effects was 200 mg/kg bw per day.
Guinea-pigs given trichlorfon at an oral dose of 100 mg/kg bw per
day for 6 days during gestation had abortions and stillborn fetuses,
and the offspring showed reduced body weight and brain weight,
locomotor disturbances, and morphological and biochemical alterations
in the brain.
In two studies of developmental toxicity in rabbits, oral doses
of 5-110 mg/kg bw per day were given. Overt toxicity and inhibition of
erythrocyte acetylcholinesterase activity were observed in dams given
doses of 35 mg/kg bw per day and above, and body-weight gain was
affected at all doses. The highest dose, 110 mg/kg bw per day,
slightly increased the rate of resorptions and retarded fetal
development, but no fetal abnormalities were found. A NOEL for
maternal toxicity was not identified. The NOEL for developmental
effects was 45 mg/kg bw per day on the basis of toxicity to embryos
and fetuses.
Several outbreaks of congenital tremor with cerebellar hypoplasia
were reported in piglets of sows that had been treated with
trichlorfon, and the syndrome was subsequently reproduced
experimentally. The piglets of sows treated with trichlorfon at doses
of 50-100 mg/kg bw per day during appropriate periods of gestation
showed neurological signs and hypoplasia and loss of Purkinje cells in
the cerebellum.
Rats given a dose equivalent to 200 mg/kg bw per day for 13 weeks
showed decreased locomotor activity and loss of coordination. In
another study in rats, a dose of 30 mg/kg bw per day for 3 weeks
increased locomotor activity and decreased learning ability and nerve
conduction velocity.
The results of numerous studies in hens consistently demonstrated
the acute neurotoxicity of trichlorfon. Delayed neurotoxicity,
associated with degeneration of nervous tissue and marked inhibition
of neuropathy target esterase, was not seen in hens. A monkey given a
single oral dose of 250 mg/kg bw showed impaired nerve conduction 4
weeks after treatment and histological evidence of demyelination of
nerves and axonal degeneration.
Trichlorfon has been used as an anthelmintic agent in humans.
Oral doses of up to 10 mg/kg bw given on two to four occasions were
well tolerated, with few clinical symptoms. A dose of 24 mg/kg bw
caused significant cholinergic symptoms, but the effects were not
cumulative. In a few cases, spermatogenesis appeared to have been
impaired. The results of clinical trials with trichlorfon in the
treatment of Alzheimer disease showed dose-related but reversible
muscle weakness. The recommended dose of 0.5-0.9 mg/kg bw resulted in
a small increase in the frequency of generalized weakness and fatigue,
while doses of 1.25 mg/kg bw and higher produced significant weakness.
Dose-related inhibition of erythrocyte acetylcholinesterase activity
was also observed in these studies.
In 121 subjects, an initial dose of 0.5 mg/kg bw per day for 2
weeks inhibited erythrocyte acetylcholinesterase activity by 29%.
Subsequent administration of a dose of 0.2 mg/kg bw per day for
10 weeks maintained the inhibition of erythrocyte acetylcholinesterase
activity at levels between 30 and 37%, with no increase with duration
of dosing. Since this dose enhanced the inhibition caused by the
initial dose by only 8%, an insignificant change, the Committee
concluded that the NOEL was 0.2 mg/kg bw per day.
In some cases of severe human poisoning with trichlorfon,
weakness and loss of feeling in the extremities, difficulty in
walking, muscular atrophy, and motor nerve damage have been observed.
In many of these cases, the doses might have been lethal in the
absence of medical intervention. The Committee concluded that
extremely high doses of trichlorfon would be required to achieve the
level of inhibition of neuropathy target esterase associated with
delayed neurotoxicity.
4. EVALUATION
The Committee concluded that inhibition of acetylcholinesterase
activity was the most relevant end-point for establishing an ADI. The
most appropriate NOEL was 0.2 mg/kg bw per day for inhibition of
erythrocyte acetylcholinesterase activity in humans treated orally. A
safety factor of 10 was applied to this figure, giving an ADI of 0-20
µg/kg bw.
5. REFERENCES
Abdel Aal, A.M.A., El-Hawary, M.F.S., Kamel, H., Abdel-Khalek, M.K. &
El-Diwany, K.M. (1970) Blood cholinesterases, hepatic renal and
haemopoietic functionsin children receiving repeated doses of
'Dipterex'. J. Egypt. Med. Assoc., 53, 265-271.
Aden-Abdi, Y., Villen, T., Ericsson, O., Gustafsson, L.L. &
Dahl-Puustinen, M.L. (1990) Metrifonate in healthy volunteers:
Interrelationship between pharmacokinetic properties,
cholinesterase inhibition and side effects. Bull. World Health
Organ., 68, 731-736.
Adler, B., Braun, R., Schöneich, J. & Böhme, H. (1976)
Repair-defective mutants of Proteus mirabilis as a prescreening
system for the detection of potential carcinogens. Biol. Zbl.,
95, 463- 469.
Akhtar, M.H. (1982) Fate of trichlorfon in buffer and soluble fraction
(105 000 g) from cow and chicken liver homogenates. J. Agric.
Food Chem., 30, 551-554.
Akimov, G.A., Buchko, V.M. & Kremleva, R.V. (1975) Neurological
disorders in chlorophos poisoning. Klin. Med., 5, 65-69
( Pestic. Abstr., 75-2380).
Aquilina, G., Benigni, R., Bignami, M., Calcagnile, A., Dogliotti, E.,
Falcone, E. & Carere, A. (1984) Genotoxic activity of dichlorvos,
trichlorfon, and dichloroacetaldehyde. Pestic. Sci., 15,
439-442.
Arthur, B.W. & Casida, J.E. (1957) Metabolism and selectivity of
O,O-dimethyl 2,2,2-trichloro-1-hydroxyethylphosphonate and its
acetyl and vinyl derivatives. J. Agric. Food Chem., 5, 186-192.
Averbook, B.J. & Anderson, R.J. (1983) Electrophysiological changes
associated with chronic administration of organophosphates.
Arch. Toxicol., 52, 167-172.
Bao, T.V., Szabo, I., Ruzicska, P. & Czeizel, A. (1974) Chromosome
aberrations in patients suffering acute organic phosphate
insecticide intoxication. Humangenetik, 24, 33-57.
Barrueco, C., Herrera, A. & De La Pena, E. (1991) Mutagenic evaluation
of trichlorfon using different assay methods with Salmonella
typhimurium. Mutagenesis, 6, 71-76.
Batzinger, R.P. & Bueding, E. (1977) Mutagenic activities in vitro
and in vivo of five antischistosomal compounds. J. Pharmacol.
Exp. Ther., 200, 1-9.
Bayer AG (1999) Pro Mem (metrifonate) and muscle weakness. Unpublished
position paper from Bayer AG. Submitted to WHO by Bayer AG,
Germany.
Becker, K. & Schöneich, J. (1980) Dominant lethal test with
trichlorphon in male mice. Mutat. Res., 74, 224.
Becker, R.E., Colliver, J., Elble, R., Feldman, E., Giacobini, E.,
Kumar, V., Markwell, S., Moriearty, P., Parks, R., Shillcutt,
S.D., Unni, L., Vicari, S., Womack, C. & Zec, R.F. (1990) Effects
of metrifonate, a long-acting cholinesterase inhibitor, in
Alzheimer disease: Report of an open trial. Drug Dev. Res., 19,
425-434.
Benes, V. & Sram, R. (1969) Mutagenic activity of some pesticides in
Drosophila melanogaster. Ind. Med., 38, 50-52.
Berge, G.N. & Nafstad, I. (1986) Distribution and placental transfer
of trichlorfon in guinea-pigs. Arch. Toxicol., 59, 26-29.
Berge, G.N., Nafstad, I. & Fonnum, F. (1986) Prenatal effects of
trichlorfon on the guinea-pig brain. Arch. Toxicol., 59, 30-35.
Berge, G.N., Fonnum, F. & Brodal, P. (1987a) Neurotoxic effects of
prenatal trichlorfon administration in pigs. Acta Vet. Scand.,
28, 321-332.
Berge, G.N., Fonnum, F., Soli, N.E. & Sognen, E. (1987b)
Neurotoxicological examination of the piglet brain after prenatal
and postnatal exposure to trichlorfon. Acta Vet. Scand., 28,
313-320.
Bomann, W. & Kaliner, G. (1996) BAY a 9826 (metrifonate) and BAY z
7216, BAY z 7217. Study for delayed neurotoxicity following acute
oral administration to hens. Unpublished report no PH 25171 from
Bayer, Wuppertal, Germany. Submitted to WHO by Bayer AG, Germany.
Bond, G.P. (1986) Primary dermal irritation of technical trichlorfon
(Dylox) in albino rabbits. Unpublished report no 751 from Mobay
Corporation, USA. Submitted to WHO by Bayer AG, Germany.
Bootman, J. & Hodson-Walker, G. (1987) L13/59: Investigation of
effects on bone marrow chromosomes of the rat after acute oral
administration. Unpublished report no R4221 from Life Sciences
Research, United Kingdom. Submitted to WHO by Bayer AG, Germany.
Brzheskiy, V.V. (1973) [Induction of mutations in Drosophila
melanogaster.] Med. Parazitol. Parazit. Bolez., 42, 703-706
(in Russian).
Bull, D.L. & Ridgway, R.L. (1969) Metabolism of trichlorfon in animals
and plants. J. Agric. Food Chem., 17, 837-841.
Bulsiewicz, H., Rozewicka, L., Januszewska, H. & Bajko, J. (1976)
Aberrations of meiotic chromosomes induced in mice with
insecticides. Folia Morphol. (Warsaw), 35, 361-368.
Byeon, W.H., Hyun, H.H. & Lee, S.Y. (1976) Salmonella/microsomal
enzyme activation system. Mutagenicity of pesticides in the
Salmonella/microsome system. Korean J. Microbiol., 14,
128-134.
Carere, A., Ortali, V.A., Cardamone, G. & Morpurgo, G. (1978a)
Mutagenicity of dichlorvos and other structurally related
pesticides in Salmonella and Streptomyces. Chem.-Biol.
Interactions, 22, 297-308.
Carere, A., Ortali, V.A., Cardamone, G., Torracca, A.M. & Raschetti,
R. (1978b) Microbiological mutagenicity studies of pesticides
in vitro. Mutat. Res., 57, 277-286.
Chan, P.C. & Peters, A.C. (1989) Thirteen-week subchronic dosed feed
study of trichlorfon in Fischer rats and B6C3F1 mice. Proc.
Am. Assoc. Cancer Res., 30, 137.
Chen, H.H., Hsueh, J.L., Sirianni, S.R. & Huang, C.C. (1981) Induction
of sister chromatid exchanges and cell cycle delay in cultured
mammalian cells treated with eight organophosphorus pesticides.
Mutat. Res., 88, 307-316.
Chen, H.H., Sirianni, S.R. & Huang, C.C. (1982) Sister chromatid
exchanges in Chinese hamster cells treated with seventeen
organophosphorus compounds in the presence of a metabolic
activation system. Environ. Mutag., 4, 621-624.
Christenson, W.R. (1990) A combined chronic toxicity/oncogenicity
study of technical grade trichlorfon (Dylox) with rats.
Unpublished report no 5388 from Mobay Corporation, USA. Submitted
to WHO by Bayer AG, Germany.
Clemens, G.R., Troup, C.M. & Hartnagel, R.E., Jr (1990) Teratology
study in the rabbit with Dylox technical (Trichlorfon).
Unpublished report no MTD0134 from Miles Laboratories, USA.
Submitted to WHO by Bayer AG, Germany.
Courtney, K.D., Andrews, J.E. & Springer, J. (1986) Assessment of
teratogenic potential of trichlorfon in mice and rats.
J. Environ. Sci. Health, B21, 207-227.
Crawford, C.R. & Anderson, R.H. (1973) The acute oral and
intraperitoneal toxicity of five trichlorfon technical samples to
rats. Unpublished report no 37204 from Baychem Corporation.
Submitted to WHO by Bayer AG, Germany.
Cummings, J.L., Cyrus, P.A., Bieber, F., Mas, J., Orazem, J. &
Gulanski, B. (1998) Metrifonate treatment of the cognitive
deficits of Alzheimer's disease. Neurology, 50, 1214-1221.
De Freitas, M.R.G., Chimelli, L., Nascimento, O.J.M., Cincinatus, D.,
Marques, H.A. & Nevares, M.T. (1990) [Polyneuropathy induced by
trichlorfon. Report of a case with electrophysiology and
histopathology in the sural nerve.] Arq. NeuroPsiquiatr., 48,
515-519 (in Spanish).
Dedek, W. (1981) Guanine N7-alkylation in mice in vivo by
metrifonate -- Discussion of possible genotoxic risk in mammals.
Acta Pharmacol. Toxicol., 49 (Suppl. V), 40-50.
Dedek, W. & Lohs, K. (1970a) [Alkylating effect of trichlorfon in
mammals. I. Studies in vitro in human serum with
14C-trichlorfon.] Z. Naturforsch., B25, 94-96 (in German).
Dedek, W. & Lohs, K. (1970b) [Allkylkating effect of trichlorfon in
mammals. Distribution of 14C in organs and liver proteins of
rats after administration of 14Ctrichlorfon.]
Z. Naturforsch.,B25, 1110-1113 (in German).
Dedek, W., Scheufler, H., & Fischer, G.W. (1975) [Mutagenicity of
desmethyltrichlorfon in the dominant lethal test in the mouse.]
Arch. Toxicol., 33, 163-168 (in German).
Dedek, W., Lohs, K., Fischer, G.W. & Schmidt, R. (1976) Alkylation of
guanine in mice in vivo by organophosphorus insecticides. I.
Trichlorfone and butonate. Pestic. Biochem. Physiol., 6,
101-110.
Degraeve, N., Chollet, M.-C., Moutschen, J., Moutschen-Damen, M.,
Gilot-Delhalle, J. & Colizzi A. (1982) Genetic and cytogenetic
effects of chronic treatments with organophosphorous
insecticides. Mutat. Res., 97, 179-180.
Degraeve, N., Chollet, M.-C. & Moutschen, J. (1984) Cytogenetic and
genetic effects of subchronic treatments with organophosphorus
insecticides. Arch. Toxicol., 56, 66-67.
Diril, N., Sumer, S. & Izbirak, A. (1990) [A survey on the mutagenic
effects of some organophosphorous insecticides in the
Salmonella/microsome test system.] Doga Muhendislik Cevre
Bilimleri, 14, 272-279 (in Turkish).
Doull, J. & Rehfuss, P.A. (1956) The effects of diets containing
Dipterex on rats. Unpublished report No. 1376 from Department of
Pharmacology, University of Chicago, USA. Submitted to WHO by
Bayer AG, Germany.
Doull, J. & Vaughn, G. (1958) Measurement of the safe dietary level of
Dipterex for dogs. Unpublished report No. 2105 from Department of
Pharmacology, University of Chicago, USA. Submitted to WHO by
Bayer AG, Germany.
Doull, J., Root, M., Vesselinovitch, D., Meskauskas, J., & Fitch, F.
(1962) Chronic oral toxicity of Dylox to male and female dogs.
Unpublished report No. 8644 from Department of Pharmacology and
Pathology, University of Chicago, USA. Submitted to WHO by Bayer
AG, Germany.
Dreist, M. & Mihail, F.L. (1990) L 13/59 (trichlorfon). Synoptic
evaluation of the toxicological data. Unpublished report no 20033
from Bayer, WuppertalElberfeld, Germany. Submitted to WHO by
Bayer AG, Germany.
Dubois, K.P. & Cotter, G.J. (1955) Studies on the toxicity and
mechanism of action of dipterex. Arch. Ind. Health, 11, 53-60.
Dzwonkowska, A. & Hubner, H. (1986) Induction of chromosomal
aberrations in the Syrian hamster by insecticides tested
in vivo. Arch. Toxicol., 58, 152-156.
Eljasz, L. & Kryzyszton-Przekop, T. (1975) [Polyneuropathy as a result
of foschlor poisoning.] Pol. Tyg. Lek., 30, 1841-1842 ( Pest.
Abstr. 76-1688) (in Polish).
Epstein, S.S., Arnold, E., Andrea, J., Bass, W. & Bishop, Y. (1972)
Detection of chemical mutagens by the dominant lethal assay in
the mouse. Toxicol. Appl. Pharmacol., 23, 288-325.
FAO/WHO (1972a) Pesticide residues in food. Report of the 1971
Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues. FAO Agricultural Series, No. 88; WHO Technical Report
Series, No. 502, Rome/Geneva.
FAO/WHO (1972b) 1971 Evaluations of some pesticide residues in
food. AEP-1971/M/13; WHO Pesticide Residues Series, No. 5,
Rome/Geneva.
FAO/WHO (1976a) Pesticide residues in food. Report of the 1975
Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on
Pesticide Residues. FAO Plant Production and Protection Series,
No. 1; WHO Technical Report Series, No. 592, Rome/Geneva.
FAO/WHO (1976b) 1975 Evaluations of some pesticide residues in
food. AEP-1975/M/9/1; WHO Pesticide Residues Series, No. 1,
Rome/Geneva.
FAO/WHO (1979a) Pesticide residues in food--1978. Report of the
Joint Meeting of the FAO Panel of Experts on Pesticide
Residues and Environment and the WHO Expert Group on
Pesticide Residues. FAO Plant Production and Protection Paper
15, Rome.
FAO/WHO (1979b) Pesticide residues in food: 1978 Evaluations. FAO
Plant Production and Protection Paper 15 Suppl., Rome.
Fischer, G.W., Schneider, P. & Scheufler, H. (1977) [Mutagenicity of
dichloroacetaldehyde and methyl-2,2-dichloro-1,1-dihydroxyethane
phosphonate, possible trichlorfon metabolites.] Chem.-Biol.
Interactions, 19, 205-213 (in German).
Fukuhara, N., Hoshi, M. & Mori, S. (1977) Core/targetoid fibres and
multiple cytoplasmic bodies in organophosphate neuropathy.
Acta Neuropathol., 40, 137-144.
von Gibel, W., Lohs, K., Wildner, G.P. & Ziebarth, D. (1971) [Animal
experimental studies on the hepatotoxic and carcinogenic activity
of organophosphorus compounds.] Arch. Geschwülstforsch., 37,
303-312 (in German).
von Gibel, W., Lohs, K., Wildner, G.P., Ziebarth, D. & Stieglitz, R.
(1973) [Carcinogenicity, haematoxicity and hepatotoxicity of
organophosphorus compounds.] Arch. Geschwülstforsch., 41,
311-328 (in German).
Gilot-Delhalle, J., Colizzi, A., Moutschen, J. & Moutschen-Dahmen, M.
(1983) Mutagenicity of some organophosphorus compounds at the
ade6 locus of Schizosaccharomyces pombe. Mutat. Res., 117,
139-148.
Griffin, T.B. (1988) Safety evaluation and tumorigenesis of
trichlorfon in rhesus monkeys: A ten-year study. Unpublished
report from White Sands Research Center, USA. Submitted to WHO by
Bayer AG, Germany.
Griffin, T.B., Coulston, F. & Stein, A.A. (1982) Trichlorfon.
Tumorigenicity study in the rat (2 generation). Unpublished
report from White Sands Research Center, USA. Submitted to WHO by
Bayer AG, Germany.
Grundmann, E. & Hobik, H.P. (1966) Bay 15922. 2 year feeding
experiment on rats. Histology. Unpublished report addendum from
Bayer, Wuppertal-Elberfeld. Submitted to WHO by Bayer AG,
Germany.
Guerzoni, M.E., Del Cupolo, L. & Ponti, I. (1976) [Mutagenic activity
of pesticides.] Riv. Sci. Techn. Alim. Nutr. Um., 6, 161-165
(in Italian).
Haseman, J.K., Winbush, J.S. & O'donnell, M.R. (1986) Use of dual
control groups to estimate false positive rates in laboratory
animal carcinogenicity studies. Fundam. Appl. Toxicol., 7,
573-584.
Hassan, A. & Zayed, S.M.A.D. (1965) Metabolism of organophosphorus
insecticides. III. Fate of the methyl groups of Dipterex
in vivo. Can. J. Biochem., 43, 1271-1275.
Hassan, A., Zayed, S.M.A.D. & Abdel-Hamid, F.M. (1965a) Metabolism of
organophosphorus insecticides. II. Metabolism of
O,O-dimethyl-2,2,2-trichloro-1hydroxyethyl phosphonate (Dipterex)
in mammalian nervous tissue and kinetics involved in its reaction
with acetyl cholinesterase. Can J. Biochem., 43, 1263-1269.
Hassan, A., Zayed, S.M.A.D. & Hashish, S. (1965b) Metabolism of
organophosphorus insecticides. VI. Mechanism of detoxification of
Dipterex in the rat. Biochem. Pharmacol., 14, 1692-1694.
Hayes, R.H. (1985) Pilot study on trichlorfon with mice. Unpublished
report No. 656 from Mobay Corporation, USA. Submitted to WHO by
Bayer AG, Germany.
Hayes, R.H. (1988) Oncogenicity study of technical grade trichlorfon
with mice. Unpublished report No. 1039 from Mobay Corporation,
USA. Submitted to WHO by Bayer AG, Germany.
Hayes, R.H. (1989) Chronic toxicity/oncogenicity study of technical
grade trichlorfon (Dylox) with rats. Unpublished report No. 1108
from Mobay Corporation, USA. Submitted to WHO by Bayer AG,
Germany.
Hayes, R.H. & Ramm, W.W. (1987) Subchronic delayed neurotoxicity study
of trichlorfon technical (Dylox) with hens. Unpublished report
No. 939 from Mobay Corporation, USA. Submitted to WHO by Bayer
AG, Germany.
Heimann, K.G. (1985) [Dipterex (L 13/59, techn.), determination of
acute toxicity (LD50).] Unpublished letter from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany (in German).
Heimann, K.G. & Wood, C.M. (1987) Trichlorfon technical. Study of
subacute dermal toxicity to rabbits. Unpublished report No. R
4150 from Bayer, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Germany.
Herbold, B. (1979a) L 13/59. Micronucleus test in the mouse for
evaluation of mutagenicity. Unpublished report No. 8505 from
Bayer, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Germany (in German).
Herbold, B. (1979b) [Appraisal of dominant lethal test on trichlorfon
(Dipterex).] Unpublished report from Martin Luther Universitat,
Germany. Submitted to WHO by Bayer AG, Germany (in German).
Herbold, B. (1979c) L 13/59. Dominant lethal study on male mouse to
test for mutagenic effects. Unpublished report No. 8745 from
Bayer, Wuppertal, Germany. Submitted to WHO by Bayer AG, Germany.
Herbold, B. (1984) [L 13/59. Pol test in E. coli to investigate DNA
damage effects.] Unpublished report No. 12434 from Bayer,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Germany (in
German).
Herbold, B. (1986) L 13/59. Cytogenetic study with human lymphocyte
cultures in vitro to evaluate for harmful effects on
chromosomes. Unpublished report No. 14837 from Bayer, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Germany.
Herbold, B. (1992) L 13/59. In vivo cytogenetic study of the
spermatogonia in Chinese hamster to evaluate for induced
clastogenic effects. Unpublished report No. 21688 from Bayer,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Germany.
Herbold, B. (1997) BAY a 9826, BAY z 7216, BAY z 7217 Micronucleus
tests on the mouse. Unpublished report No. PH 26329 from Bayer,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Germany.
Hierons, R. & Johnson, M.K. (1978) Clinical and toxicological
investigations of a case of delayed neuropathy in man after acute
poisoning by an organophosphorus pesticide. Arch. Toxicol., 40,
279-284.
Hirakawa, T. (1983a) Acute toxicity study of trichlorfon on mice by
oral, intraperitoneal, subcutaneous and percutaneous route.
Unpublished report ID 17258 from Bozo Research Centre, Japan.
Submitted to WHO by Bayer AG, Germany.
Hirakawa, T. (1983b) Acute toxicity study of trichlorfon on rats by
oral, intraperitoneal, subcutaneous and percutaneous route.
Unpublished report ID 17259 from Bozo Research Centre, Japan.
Submitted to WHO by Bayer AG, Germany.
Hobik, H.P. (1967) [Histological investigation of spinal cord and
sciatic nerves from neurotoxicity experiemnts in hens dosed with
Dipterex.] Unpublished report no 446 from Bayer, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Germany (in German).
Hoffmann, K., Ruf, J. & Mager, H. (1988) [Chronic toxicity in rhesus
monkeys dosed oraqlly for 26 weeks (gavage).] Unpublished study
No. 16545 from Bayer, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Germany (in German).
Hoorn, A.J.W. (1983) Mutagenicity evaluation of L 13/59 in the reverse
mutation induction assay with Saccharomyces cerevisiae strains
S138 and S211alpha. Unpublished report No. R2540 from Litton
Bionetics, Netherlands. Submitted to WHO by Bayer AG, Germany.
IARC (1983) Trichlorfon. In: IARC Monographs on the Evaluation of
the Carcinogenic Risk of Chemicals to Humans, Vol. 30,
Miscellaneous pesticides, Lyon, International Agency for
Research on Cancer, pp. 207-231.
IARC (1987) IARC Monographs on the Evaluation of the Carcinogenic
Risk of Chemicals to Humans, Suppl. 7, Overall Evaluations
of Carcinogenicity: An Updating of IARC Monographs Volumes 1
to 42, Lyon, International Agency for Research on Cancer,
p. 73.
Inukai, H. & Iyatomi, A. (1977) Trichlorfon. Mutagenicity test on
bacterial systems. Unpublished report No. 87 from Nitokuno
Agricultural Chemicals Institute, Japan. Submitted to WHO by
Bayer AG, Germany.
Jewsbury, J.M. (1981) Metrifonate in schistosomiasis therapy and
prophylaxis. Acta Pharmacol. Toxicol., 49 (Suppl. V),123-130.
Johnson, M.K. (1981) Delayed neurotoxicity -- Do trichlorphon and/or
dichlorvos cause delayed neuropathy in man or in test animals?
Acta Pharmacol. Toxicol., 49 (Suppl. V), 87-98.
Jones, D.C.L., Simmon, V.F., Mortelmans, K.E., Mitchell, A.D., Evans,
E.L., Jotz, M.M., Riccio, E.S., Robinson, D.E. & Kirkhart, B.A.
(1984) In vitro and in vivo mutagenicity studies of
environmental chemicals. Report from SRI International, USA, for
Environmental Protection Agency (EPA-600/1-84-003). National
Technical Information Service, USA.
Kawauchi, S. (1997) BAY a 9826 single oral administration toxicity
study in dogs. Unpublished report No. R 6836 from Experimental
Biomedical Research, Japan. Submitted to WHO by Bayer AG,
Germany.
Kimmerle, G. (1970) Metrifonat (Bayer 2349), toxicological
investigations. Unpublished report No. 2142 from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Kimmerle, G. (1975a) L 13/59, acute inhalation toxicity study on rats.
Unpublished report No. 5581 from Bayer, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Germany.
Kimmerle, G. (1975b) L 13/59, subacute inhalation toxicity study on
rats. Unpublished report No. 5582 from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Klimmer, O.R. (1955) [Final report on the new insecticide Bayer L
13/59.] Unpublished report from Pharmakologisches Institut, Bonn,
Germany. Submitted to WHO by Bayer AG, Germany (in German).
Kiraly, J., Szentesi, I., Ruzicska, M. & Czeize, A. (1979) Chromosome
studies in workers producing organophosphate insecticides.
Arch. Environ. Contam. Toxicol., 8, 309-319.
Knox, B., Askaa, J., Basse, A., Bitsch, V., Eskildsen, M., Mandrup,
M., Ottosen, H.E., Overby, E., Pedersen, K.B. & Rasmussen, F.
(1978) Congenital ataxia and tremor with cerebellar hypoplasia in
piglets borne by sows treated with Neguvon(R) vet (Metrifonate,
Trichlorfon) during pregnancy. Nord. Vet. Med., 30, 538-545.
Kowalski, R.L., Clemens, G.R., Bare, J.J. & Hartnagel, R.E., Jr (1987)
A teratology study with Dylox (R), technical (Trichlorfon) in the
rat. Unpublished report No. MTD0017 from Miles Laboratories, USA.
Submitted to WHO by Bayer AG, Germany.
Kurinnyi, A.I. (1975) Comparative study of the cytogenetic effect of
certain organophosphorus pesticides. Genetika, 11, 64-69.
Lamb, M.J. (1977) Mutagenicity tests with metrifonate in
Drosophila melanogaster. Mutat. Res., 56, 157-162.
Lehotzky, K. (1982) Effect of pesticides on central and peripheral
nervous system function in rats. Neurobehav. Toxicol.
Teratol., 4, 665-669.
Lorke, D & Kimmerle, G. (1966) Neurotoxicity studies on hens with
Dipterex active ingredient. Unpublished report from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Lorke, D. & Loser, E. (1966) Chronic toxicological studies on rats.
Unpublished report No. 19292 from Bayer, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Germany.
Loser, E. (1969) BAY 15922 Generation study on rats. Unpublished
report No. 1195 from Bayer, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Germany.
Loser, E. (1970) Bay 15922. Chronic toxicological studies on dogs.
Unpublished report No. 2467 from Bayer, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Germany.
Machemer, L. (1979a) [L 13/59 (trichlorfon): Evaluation for
embryotoxic and teratogenic effects on orally dosed rats.]
Unpublished report No. 8400 from Bayer,Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Germany (in German).
Machemer, L. (1979b) [L 13/59 (trichlorfon): Evaluation for
embryotoxic and teratogenic effects on orally doses rabbits.]
Unpublished report No. 8430 from Bayer,Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Germany (in German).
Martson, L.V. & Voronina, V.M. (1976) Experimental study of the effect
of a series of phosphoroorganic pesticides (dipterex and imidan)
on embryogenesis. Environ. Health Perspectives, 13, 121-125.
Metcalf, R.L., Fukuto, T.R. & March, R.B. (1959) Toxic action of
Dipterex and DDVP to the house fly. J. Econ. Entomol., 52,
44-49.
Mihail, F. (1978) [L 13/59 (Dipterex active ingredient), acute
toxicological studies.] Unpublished report No. 7619 from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany (in German).
Mihail, F. (1986) L 13/59 (trichlorfon): Study for skin-sensitizing
effect on guineapigs in the open epicutaneous test. Unpublished
report No. 14348 from Bayer, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Germany.
Miyamoto, J. (1959) Non-enzymatic conversion of Dipterex into DDVP and
their inhibitory action on enzymes. Botyu-Kagaku, 24, 130-137.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Moutschen-Dahmen, J., Moutschen-Dahmen, M. & Degraeve, N. (1981)
Metrifonate and dichlorvos: Cytogenetic investigations.
Acta Pharmacol. Toxicol., 49 (Suppl. V), 29-39.
Myhr, B.C. (1983) Evaluation of L 13/59 trichlorfon in the primary rat
hepatocyte unscheduled DNA synthesis assay. Unpublished report
No. R2687 from Litton Bionetics, USA. Submitted to WHO by Bayer
AG, Germany.
Nehes, M., Selypes, A. & Scheufler, H. (1982)[ Mutagenicity of
trichlorfon and dimethoate on mouse bone marrow cells in vivo].
Wissensch. Z. Univ. Halle, 31, 57- 62 (in German).
Niedziella, S.W., Göpel, W. & Banzhaf, E. (1985) [Acute alkylphosphate
poisoning (trichlorphon) with delayed polyneuropathy syndrome.]
Z. Ges. Inn. Med., 40, 237-239 (in German).
Nordgren, I., Bergström, M., Holmstedt, B. & Sandoz, M. (1978)
Transformation and action of metrifonate. Arch. Toxicol., 41,
31-41.
Nordgren, I., Holmstedt, B., Bengtsson, E. & Finkel, Y. (1980) Plasma
levels of metrifonate and dichlorvos during treatment of
schistosomiasis with Bilarcil. Am. J. Trop. Med. Hyg., 29,
426-430.
Nordgren, I., Bengtsson, E., Holmstedt, B. & Pettersson, B.M. (1981)
Levels of metrifonate and dichlorvos in plasma and erythrocytes
during treatment of schistosomiasis with Bilarcil. Acta
Pharmacol. Toxicol., 49 (Suppl. V), 79-86.
Olajos, E.J., Rosenblum, I., Coulston, F. & Strominger, N. (1979) The
dose response relationship of trichlorfon neurotoxicity in hens.
Ecotoxicol. Environ. Saf., 3, 245-255.
Paik, S.G. & Lee, S.Y. (1977) Genetic effects of pesticides in the
mammalian cells. 1. Induction of micronucleus. Korean J.
Zool., 20, 19-28.
Plestina, R., Davis, A. & Bailey, D.R. (1972) Effect of metrifonate on
blood cholinesterases in children during treatment of
schistosomiasis. Bull. World Health Organ., 46, 747-759.
Pollingher, B., Cozma, V. & Oprisan, C. (1973) Clinical and
electromyographic study of two cases of severe polyneuritis
following acute poisoning with Neguvon (organophosphoric
pesticide). Electroencephalogr. Clin. Neurophysiol., 35, 433
( Pestic. Abstr., 75-0322).
Pope, A.M., Heavner, J.E., Guarnieri, J.A. & Knobloch, C.P. (1986)
Trichlorfoninduced congenital cerebellar hypoplasia in neonatal
pigs. J. Am. Vet. Med. Assoc., 189, 781-783.
Putman, D.L. (1987) Sister chromatid exchange assay in Chinese hamster
ovary (CHO) cells. Unpublished report No. 826 from
Microbiological Associates, USA. Submitted to WHO by Bayer AG,
Germany.
Ray, E.R. (1998) Chronic effects of low level exposure to
anticholinesterases -- A mechanistic review. Toxicol. Lett.,
102-103, 527-533.
Reiner, E., Krauthacker, B., Simeon, V. & Skrinjaric-Spoljar, M.
(1975) Mechanism of inhibition in vitro of mammalian
acetylcholinesterase and cholinesterase in solutions of
O,O-dimethyl-2,2,2-trichloro-1-hydroxyethyl phosphonate
(trichlorphon). Biochem. Pharmacol., 24, 717-722.
Reiss, C.S., Herrman, J.M. & Hopkins, R.E., II (1987) Effect of
antihelminthic treatment on the immune response of mice.
Lab. Anim. Sci., 37, 773-775.
Renhof, M. (1997) BAY a 9826: Acute toxicity in the mouse and rat
after oral administration and in the mouse after intravenous
administration. Unpublished report No. 25832 from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Rosenblum, I. (1981) A two-generation, two-year feeding study of
trichlorfon in the rat. Unpublished report No. 2142 from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Sasaki, M., Sugimura, K., Yoshida, M. A. & Abe, S. (1980) Cytogenetic
effects of 60 chemicals on cultured human and Chinese hamster
cells. Kromosomo, 20, 574-584.
Scheufler, H. (1975) [Effect of relatively high doses of dimethoate
and trichlorfon on embryogenesis in mice.] Biol. Rundsch., 13,
238-240 (in German).
Sheets, L.P. & Hamilton, B.F. (1995) A subchronic dietary
neurotoxicity screening study with technical grade trichlorfon
(Dylox, Dipterex) in Fischer 344 rats. Unpublished report No.
MRC-00284 from Bayer Corp., USA. Submitted to WHO by Bayer AG,
Germany.
Shiraishi, S., Goto, I., Yamashita, Y., Ohnishi, A. & Nagao, H. (1977)
Dipterex polyneuropathy. Neurol. Med., 6, 34-38.
Shiraishi, S., Inoue, N. & Murai, Y. (1982) Dipterex poisoning.
J. Univ. Occup. Environ. Health, 4, 177-183.
Shiraishi, S., Inoue, N., Murai, Y., Onishi, A., & Noda, S. (1983)
Dipterex (trichlorfon) poisoning -- Clinical and pathological
studies in human and monkeys. J. Univ. Occup. Environ.
Health, 5 (Suppl.), 125-132.
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A. & Kada, T. (1976)
Mutagenicity screening of pesticides in the microbial system.
Mutat. Res., 40, 19-30.
Shirasu, Y., Moriya, M. & Koyashiki, R. (1979) Trichlorfon.
Mutagenicity test on bacterial systems. Unpublished report from
Institute of Environmental Toxicology, Japan. Submitted to WHO by
Bayer AG, Germany.
Slott, V. & Ecobichon, D.J. (1984) An acute and subacute neurotoxicity
assessment of trichlorfon. Can. J. Physiol. Pharmacol., 62,
513-518.
Spicer, E.J.F. & Payne, S. (1971) Pathology report of Bay 15922 4-year
dog study. Unpublished report from Huntingdon Research Centre,
United Kingdom. Submitted to WHO by Bayer AG, Germany.
Spicer, E.J.F. & Urwin, C (1971) Pathology report of Bay 15922
Generation experiment in rats. Unpublished report from Huntingdon
Research Centre, England. Submitted to WHO by Bayer AG, Germany.
Staples, R.E. & Goulding, E.H. (1979) Dipterex teratogenicity in the
rat, hamster and mouse when given by gavage. Environ. Health
Perspectives, 30, 105-113.
Staples, R.E., Kellam, R.G. & Haseman, J.K. (1976) Developmental
toxicity in the rat after ingestion or gavage of organophosphate
pesticides (dipterex, imidan) during pregnancy. Environ.
Health Perspectives, 13, 133-140.
Stieglitz, R., Gibel, W., Werner, W. & Stobbe, H. (1974) Experimental
study on haematotoxic and leukaemogenic effects of trichlorophone
and dimethoate. Acta Haematol., 52, 70-76.
Suzuki, T. (1996) Three-month repeated oral administration toxicity
study and recovery test of BAY a 9826 in dogs. Unpublished report
No. R 6641 from JBC, Gifu Research Laboratory, Japan. Submitted
to WHO by Bayer AG, Germany.
Teichmann, B. & Hauschild, F. (1978) [Test of O,O-dimethyl
(1-hydroxy-2,2,2trichloroethyl)phosphonate (trichlorfon) for
carcinogenicity in mice after oral (oesophageal-gastric gavage)
and dermal application.] Arch. Geschwulstforsch., 48, 301-307
(in German).
Teichmann, B. & Schmidt, A. (1978) [Test of O,O-dimethyl
(1-hydroxy-2,2,2trichlorethyl)phosphonate (trichlorphon) for
carcinogenicity in Syrian golden hamsters ( Mesocricetus
auratus Waterhouse) after intraperitoneal Injection.]
Arch. Geschwulstforsch., 48, 718-721 (in German).
Teichmann, B., Hauschild, F. & Eckelmann, A. (1978) [Test of
O,O-dimethyl(1hydroxy-2,2,2-trichlorethyl)phosphonate
(trichlorphon) for carcinogenicity in rats after oral
(oesophageal-gastric gavage) and intraperitoneal Injection.]
Arch. Geschwulstforsch., 48, 112-119.
Thyssen, J. (1981) L 13/59 (Dipterex active ingredient). Tests for
skin and eye irritation. Unpublished report from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Thyssen, J., Kaliner, G. & Eben, A. (1982) [Trichlorfon, Schmelzware
Japan, neurotoxicity studies in hens.] Unpublished report No.
10811 from Bayer, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Germany (in German).
Toyoshi, T. (1996) One-month repeated oral administration toxicity
study and recovery test of BAY a 9826 in rats. Unpublished report
No. R 6642 from JBC, Gifu Research Laboratory, Japan. Submitted
to WHO by Bayer AG, Germany.
Völkner, W. (1987) Sister chromatid exchanges (SCEs) in Chinese
hamster bone marrow cells. Unpublished report No. R4079 from
Technical University of Darmstadt, Germany. Submitted to WHO by
Bayer AG, Germany.
Watabe, H. (1997) A reverse mutation assay of metrifonate using
bacteria. Unpublished report No. R 6837 from Mitsubishi Yuka
Bio-Clinical Labs, Japan. Submitted to WHO by Bayer AG, Germany.
Wegner, D. (1970) Bilarcil (Bay 2349) Clinical experience 1960-1969
(survey of the literature). Unpublished report No. 2225 from
Bayer, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Germany.
WHO (1992) Trichlorfon (Environmental Health Criteria 132). Geneva,
World Health Organization.
Williams, M.W., Fuyat, H.N. & Fitzhugh, O.G. (1959) The subacute
toxicity of four organic phosphates to dogs. Toxicol. Appl.
Pharmacol., 1, 1-7.
Witter, R.F. & Gaines, T. B. (1963) Relationship between depression of
brain or plasma cholinesterase and paralysis in chickens caused
by certain organic phosphorus compounds. Biochem. Pharmacol.,
12, 1377-1386.
Witterland, W.F. (1984) Mutagenicity evaluation of L 13/59 in the
mouse lymphoma forward mutation assay. Unpublished report No.
R2824 from Litton Bionetics, Netherlands. Submitted to WHO by
Bayer AG, Germany.
Xu, H.H. & Schurr, K.M. (1990) Genotoxicity of 22 pesticides in
microtitration SOS Chromotest. Toxicol. Assess. Int. J., 5,
1-14.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T., Mortelmans, K. &
Speck, W. (1987) Salmonella mutagenicity tests: III Results from
the testing of 255 chemicals. Environ. Mutag., 9 (Suppl. 9),
1-109.
Table 1. Results of studies of the acute toxicity of trichlorfon
Species Sex Route LD50 References
(mg/kg bw)
Mouse M&F Oral 616-950 Klimmer (1955); Mihail
(1978); FAO/WHO (1972b);
Hirakawa (1983a); Renhof
(1997)
Mouse M&F Intraperitoneal 344-600 Dubois & Cotter (1955);
Hirakawa (1983a); WHO
(1992)
Mouse M&F Subcutaneous 267360 FAO/WHO (1972b); Hirakawa
(1983a)
Mouse M&F Intravenous 354-370 Renhof (1997)
Mouse M&F Dermal > 5000 Hirakawa (1983a)
Rat M&F Oral 136-660 Dubois & Cotter (1955);
Klimmer (1955); Kimmerle
(1970); FAO/WHO (1972b);
Crawford & Anderson (1973);
Mihail (1978); Hirakawa
(1983b); Heimann (1985);
WHO (1992); Renhof (1997)
Rat M&F Intraperitoneal 156-485 Dubois & Cotter (1955);
Arthur & Casida (1957);
FAO/WHO (1972b); Crawford &
Anderson (1973); Hirakawa
(1983b)
Rat M&F Subcutaneous 290-400 FAO/WHO (1972b);
Hirakawa (1983b)
Rat M Intravenous 184 Kimmerle (1970)
Rat M&F Inhalation LC50, > 533 Kimmerle (1975a)
mg/m3
Rat M&F Dermal 2800-> 5000 Hirakawa (1983b); Mihail
(1978); WHO (1992)
Guinea-pig M&F Intraperitoneal 300 Dubois & Cotter (1955);
WHO (1992)
Rabbit Oral 160 FAO/WHO (1972b)
Rabbit M Dermal 5000 WHO (1992)
Dog M oral > 300-420 Mihail (1978); WHO (1992);
Kawauchi (1997)
fasciculations, and coma. The primary cause of death is respiratory
failure. After sublethal doses, animals recover very rapidly,
suggesting that trichlorfon is detoxified faster than many other
organophosphorus compounds (WHO, 1992).
In two separate studies, six New Zealand white rabbits were
treated dermally with trichlorfon for 24 h on intact and abraded skin.
No irritation was seen after 24, 48, or 72 h. Further animals received
trichlorfon in the eye for 5 min ( n = 5) or 24 h ( n = 3). Severe
redness was seen up to 72 h after dosing for either length of time.
Slight to moderate chemosis was also observed up to 48-72 h after
dosing in both groups. The effects did not persist to day 7, and no
effect was seen in the cornea or iris. The compound is a slight ocular
irritant (Thyssen, 1981; Bond, 1986).
Trichlorfon was assessed for skin sensitizing potential in eight
male BOR: DHPW guinea-pigs by the open epicutaneous test. Dermal
induction was carried out 5 days a week for 4 weeks with 0, 1, 3, or
10% trichlorfon, and dermal challenge was conducted with 0.3, 1, 3, or
10 % trichlorfon 4, 6, or 8 weeks after induction. No skin reactions
were seen in animals induced with 1% trichlorfon, but approximately
half the animals induced with 3 or 10% trichlorfon showed skin
reactions when challenged with each concentration of trichlorfon. The
compound was therefore considered to have skin sensitizing potential
(Mihail, 1986).
2.2.2 Short-term studies of toxicity and carcinogenicity
Mice
Trichlorfon (purity, 98.2%) was administered to groups of 15
Charles River CD-1 mice of each sex at a dietary concentration of 0,
100, 300, 900, or 2700 mg/kg for 8 weeks. There were no deaths or
drug-related clinical signs, and body weights were not affected. Food
consumption was not affected in males, but appeared to have been
increased by 18-32% in females. The report suggests that the latter
finding was due to increased wastage by the treated female mice.
Cholinesterase activity in plasma and erythrocytes, measured during
weeks 4, 6, and 8, was not significantly affected at the lowest dose
but was dose-dependently inhibited at higher doses; the maximum
inhibition was by about 70% for plasma and 50% for erythrocyte enzyme.
Brain acetylcholines-terase activity at the end of the study was
dose-dependently inhibited at doses > 300 mg/kg of diet, by up to
68%. Gross necropsy revealed no remarkable changes. Haematology, serum
biochemistry, urinary anlysis, organ weighing, and histopathology were
not performed (Hayes, 1985).
Groups of 10 male and 10 female B6C3F1 mice were given
trichlorfon (purity unknown) in the diet at a concentration of 0, 62,
180, 560, 1700, or 5000 mg/kg for 13 weeks. The results were reported
in abstract form only. All but one male at 5000 mg/kg of diet and one
female at 1666 mg/kg of diet survived. The body weights of mice at
5000 mg/kg of diet were reduced. Plasma and erythrocyte cholinesterase
activities were decreased in a dose-related manner, while motor
activity and grip strength were reduced at 1700 and 5000 mg/kg of
diet. The absolute and relative weights of the liver, kidney, and
spleen were increased in mice at 1700 and 5000 mg/kg of diet. The
neurotoxic effects and changes in organ weight were not accompanied by
pathological effects (Chan & Peters, 1989; WHO, 1992).
Rats
Groups of 10 male and 10 female rats were exposed for 6 h/day for
3 weeks (total, 15 exposures) to an atmosphere containing trichlorfon
(purity unknown) at a concentration of 0, 12.7, 35.4, or 103.5
mg/m3. The health of the animals at 103.5 mg/m3 was affected, but
details were not provided. Body-weight gain was unaffected, and
haematological, clinical chemical, and urinary parameters measured at
the end of the study were similar to those of controls. Plasma,
erythrocyte, and brain cholinesterase activities were inhibited in
males given 103.5 mg/m3 and in females given 35.4 and 103.5 mg/m3.
Gross and histopathological examinations showed no remarkable changes,
the only finding at autopsy being increased spleen weights in males at
35.4 and 103.5 mg/m3 (Kimmerle, 1975b; WHO,1992).
Groups of 12 male and 12 female Crj: CD SD rats were given
trichlorfon (purity, 100%) at a dose of 0, 25, 50, or 100 mg/kg bw per
day by gavage for 30 days. Further groups of 10 rats of each sex were
treated and then allowed a 2-week recovery period. Additional treated
groups of 20 rats of each sex were used to determine the profiles of
the drug in blood after dosing on days 1, 15, and 30. The peak
concentrations of the individual enantiomers of trichlorfon and
dichlorvos were dose-related and were reached by about 1 h, declining
rapidly to become undetectable by 24 h. The concentrations of each
enantiomer were roughly similar in males, while that of the
(-)-enantiomer was generally higher than that of the (+)enantiomer in
females; this was reflected in higher total drug concentrations in
females. The concentrations of dichlorvos in blood represented only a
small fraction of those of trichlorfon and were again higher in female
rats. There was no evidence of accumulation in blood.
The signs of toxicity included tremor, decreased locomotor
activity, salivation, bradypnoea, and prone position in many females
given 100 mg/kg bw per day, in one or two females given 50 mg/kg bw
per day, and in males given 100 mg/kg bw per day. There were no deaths
and no effects on body weight, food consumption, or ophthalmological
or urinary parameters. Slight decreases in erythrocyte counts and
haematocrit were seen in females at 100 mg/kg bw per day when compared
with controls at the end of treatment, but these values were not
significantly different from those of controls at the end of the
recovery period. Animals at 100 mg/kg bw per day had increased blood
concentrations of cholesterol, triglycerides, and phospholipids
(females), and increased activity of lactic dehydrogenase (males). At
autopsy, the livers of animals at 50 and 100 mg/kg bw per day were
heavier and/or enlarged and showed hypertrophy of centrilobular
hepatocytes. The weight of the kidney was increased in males at 100
mg/kg bw per day. The NOEL was 25 mg/kg bw per day (Toyoshi, 1996).
Groups of seven Wistar rats of each sex were given trichlorfon
(purity unknown) in the diet to provide a dose of 0, 1, 5, 10, 30, 50,
or 100 mg/kg bw per day for 12 weeks. The highest dose inhibited
cholinesterase activity in all tissues examined (erythrocytes, serum,
brain, heart, liver, and skeletal muscle), but the extent of the
inhibition differed. Nevertheless, no changes were detected in
nocturnal behaviour, reaction to external stimuli, conjunctival or
pinna reflexes, or the avoidance reflex to painful stimuli. No
significant histological alterations were observed (reviewed in WHO,
1992).
Groups of 10 male and 10 female Fischer rats were given
trichlorfon (purity unknown) in the diet at a concentration of 0, 62,
180, 560, 1700, or 5000 mg/kg for 13 weeks. The results were reported
in abstract form only. All rats survived. The body weights of those at
5000 mg/kg of diet were reduced. Plasma and erythrocyte cholinesterase
activities were lowered in a dose-related manner, while motor activity
and grip strength were reduced at 1700 and 5000 mg/kg of diet. The
absolute and relative weights of the liver, kidney, and spleen were
increased in animals at 1700 and 5000 mg/kg of diet. The neurotoxic
effects and changes in organ weights were not accompanied by
pathological effects (Chan & Peters, 1989; WHO, 1992).
Groups of 13 rats of each sex were fed diets containing
trichlorfon (purity unknown) at a concentration of 0, 20, 100, or 300
mg/kg for 16 weeks. No effects were observed on growth, behaviour,
food consumption, organ weights, or gross or microscopic appearance of
the tissues. At 300 mg/kg of diet, plasma and erythrocyte
cholinesterase activity was depressed by 20-30% in males and females,
while that of brain cholinesterase was depressed by 20% in females
only. Salivary gland cholinesterase activity was not inhibited. At 100
mg/kg of diet, a slight depression of plasma cholinesterase activity
was observed (Doull & Rehfuss, 1956; WHO, 1992).
Groups of 10 male Wistar albino rats were fed diets containing
trichlorfon (purity unknown) at 0, 1, 5, 25, or 125 mg/kg for 16
weeks. Erythrocyte cholinesterase activity was not depressed at any
dose, and no effects were seen on food consumption, growth, or gross
appearance of the tissues (reviewed in WHO, 1992).
Guinea-pigs
After oral administration of trichlorfon (purity unknown) at 100
mg/kg bw per day for 60 days to guinea-pigs, the haemoglobin
concentration but not the haematocrit was decreased. The activity of
serum cholinesterase was decreased by 50%, and that of alkaline
phosphatase was increased (reviewed in WHO, 1992).
Rabbits
Groups of five New Zealand white rabbits of each sex received
dermal applications of 0, 100, 300, or 1000 mg/kg bw per day
trichlorfon (purity, 99%) onto shaved skin. The drug was left on the
skin of restrained animals for 6 h/day, 5 days/week for 3 weeks. The
appearance and behaviour of the rabbits were unchanged, and there were
no deaths. Body weight, food consumption, and the skin at the
application sites were unaffected. There were no meaningful changes in
haematological, clinical chemical, or urinary parameters at the end of
the study. Plasma, erythrocyte, and brain cholinesterase activities
were inhibited by 20-30% in animals at 300 and 1000 mg/kg bw per day.
At autopsy, no treatment-related alterations in liver microsomal
enzyme activities, organ weights, or histological appearance were seen
(Heimann & Wood, 1987).
Dogs
Four dogs were fed diets containing trichlorfon (purity unknown)
for 12 weeks at a concentration of 20, 100, 300, or 500 mg/kg and were
then left for a further 4 weeks on unmedicated diet. All of the dogs
remained healthy and maintained their body weight throughout the
study. After 12 weeks of treatment, the erythrocyte and plasma
cholinesterase activities of animals at 300 and 500 mg/kg of diet were
decreased by 40-60% when compared with the concentrations before
treatment, and the activity in plasma was inhibited by 10% in animals
at 100 mg/kg of diet. On cessation of treatment, the enzyme levels
recovered within 2-4 weeks (Doull & Vaughn, 1958; FAO/WHO, 1972b).
Four dogs were fed diets containing trichlorfon (purity unknown)
at a concentration of 0, 50, 200, or 500 mg/kg for 12 weeks. Plasma
and erythrocyte cholinesterase activities were depressed within 2
weeks in animals at 500 mg/kg of diet, and the effect peaked at 6-8
weeks, at which time the inhibition was about 60% in plasma and 45% in
erythrocytes. A gradual increase in plasma cholinesterase activity was
then seen, to 45% inhibition at termination, even though the compound
was still present in the feed. At 200 mg/kg of diet, the
cholinesterase activity was slightly decreased but was within the
control range. The enzyme activities were restored 6 weeks after
treatment ceased (Williams et al., 1959; FAO/WHO, 1972b).
Groups of three male and three female beagles were given
trichlorfon (purity, 99.2%) in capsules at a dose of 0, 5, 15, or 45
mg/kg bw per day for 3 months. A further three animals of each sex
were given the vehicle or the high dose for 3 months and then allowed
to recover for 2 weeks. The blood concentrations of the enantiomers of
trichlorfon and dichlorvos were measured after dosing on days 1, 30,
and 90. Peak concentrations were achieved within 1 h, the
(-)-enantiomer being detected at 10-100-fold higher concentrations
than the (+)-enantiomer and dichlorvos. Disappearance from blood was
rapid, and the compounds were virtually undetectable by 7 h. The drug
did not accumulate in blood.
No deaths were observed in any group, and no meaningful effects
were seen on mean body weight, food intake, or in ophthalmological,
electrocardiographic, or auditory parameters. Dogs receiving 15 mg/kg
bw per day or more showed tremor, decreased locomotor activity, and
crouching, while at 45 mg/kg bw per day, ataxic gait, lateral
position, vomiting, salivation, licking, liquid, loose, or bloody
stools, and wasting were observed. The results of blood chemistry and
urinary analysis were unremarkable. Haemoglobin, haematocrit, and
erythrocyte count were slightly but progressively decreased during
treatment in males given 45 mg/kg bw per day. It was reported, with no
supporting data, that erythrocyte cholinesterase activity was
inhibited at 15 and 45 mg/kg bw per day. One dog at 45 mg/kg bw per
day lost weight, and at autopsy was found to have decreased thymus
weight with lymphocyte depletion in the cortex, small and immature
testes and prostate, and oligospermia in small epididymides. Similar
thymic changes were observed in one female at 45 mg/kg bw per day. All
of the effects were reversible on cessation of treatment. The NOEL was
5 mg/kg bw per day (Suzuki, 1996).
Monkeys
Groups of five rhesus monkeys of each sex were given trichlorfon
(purity, 98.7%) at a dose of 0, 0.1, or 0.2 mg/kg bw per day in
sterile water by oral intubation for 26 weeks in order to establish an
NOEL for inhibition of erythrocyte cholinesterase activity. There were
no deaths and no effects on appearance, behaviour, nutritional state,
feed or water consumption, or body-weight gain. Haematological tests
showed no changes. No compound-related effects on the liver or kidney
were seen in clinical chemical or urinary analyses, on gross
pathology, or in organ weights. At 0.2 mg/kg bw per day, small
increases and decreases in erythrocyte cholinesterase activity
(< 20%) were seen, which were within the range of biological
variation. The NOEL was 0.2 mg/kg bw per day, the highest dose tested
(Hoffman et al., 1988; WHO, 1992).
2.2.3 Long-term studies of toxicity and carcinogenicity
Mice
Three studies of carcinogenicity were performed with groups of 30
AB/Jena mice that received an oral dose of 0 or 30 mg/kg bw twice
weekly, an intraperitoneal dose of 0 or 28 mg/kg bw per day, or a
dermal application of 0 or 0.25 ml of a 1% solution, providing 2.5 mg
trichlorfon per animal. Dosing was continued for 75 weeks, and the
studies were terminated after 80 weeks. The treated animals showed
reduced body-weight gain and survival, but the tumour incidences were
not increased (Teichmann & Hauschild, 1978; FAO/WHO, 1979b).
Groups of 60 male and 60 female Charles River CD-1 mice were
given trichlorfon (purity unknown) in the diet at a concentration of
0, 100, 300, or 1000 mg/kg for 90 weeks, except for males at the
highest dose which were treated for 82 weeks. These doses were
equivalent to 15, 45, and 150 mg/kg bw per day. Inhibition of
body-weight gain was observed in females given 300 or 1000 mg/kg of
diet, and cholinesterase activity was depressed in animals of each sex
at 1000 mg/kg of diet. No significant microscopic alterations were
reported, and the tumour incidences were not treatment-related
(reviewed in WHO, 1992).
Groups of 50 Crl:CD-1(ICR)BR mice of each sex were given
trichlorfon (purity, 98.2%) in the diet at a concentration of 0, 280,
890, or 2700 mg/kg for 104 weeks, equal to 49, 160, and 510 mg/kg bw
per day for males and 66, 240, and 750 mg/kg bw per day for females.
Increased incidences of urine-stained fur, 'ear lesions' (males), and
vaginal discharge (females) were observed. The body weights of treated
females were increased at various times during the study, but a
consistent effect was observed only at the high dose. There were no
effects on survival, food intake, or haematological end-points. Plasma
and erythrocyte cholinesterase activities were depressed by 20-74% in
the groups given 890 and 2700 mg/kg of diet and in females given
280 mg/kg of diet. Brain cholinesterase activity was inhibited by
20-70% at all doses in a dose-related manner. The weights of the liver
were increased in females at the intermediate and high doses, but this
effect was not accompanied by microscopic changes. Pathological
examination revealed no treatment-related alterations (Hayes, 1988;
WHO, 1992).
Rats
von Gibel and co-workers undertook a series of studies in Wistar
rats (von Gibel et al.,1971, 1973; Stieglitz et al., 1974), which were
given trichlorfon three times per week orally at 30 mg/kg bw, twice
per week by subcutaneous injection at 30 mg/kg bw, twice per week
orally at 15 mg/kg bw, or twice per week by intraperitoneal injection
of 15 mg/kg bw. Treatment was continued for the lifetime of the
animals. The groups given 30 mg/kg bw were reported to have higher
incidences of liver necrosis, liver cirrhosis, and forestomach
papillomas than controls. The lower doses caused hyperplasia of the
blood-forming elements of bone marrow, extraosseal metaplasia in the
liver and spleen, and hepatotoxicity and carcinogenicity. JMPR
(FAO/WHO, 1972b, 1976b) and the International Agency for Research on
Cancer (IARC, 1983) concluded that these studies were difficult to
interpret because of lack of information on the methods and results
and the amalgamation of tumour types. Also, the findings contradicted
those of other studies. Therefore, little use could be made of these
studies .
Groups of 25 male and 25 female Sprague-Dawley rats were fed
diets containing trichlorfon (purity unknown) at a concentration of 0,
50, 250, 500, or 1000 mg/kg for 17 months for males and for 24 months
for females. These doses were equivalent to 2.5, 12, 25, and 50 mg/kg
bw per day. Survival was reduced at 1000 mg/kg of diet, and the
body-weight gain of males at this dose was retarded. Cholinesterase
activity was depressed in serum, erythrocytes, and submaxillary gland
but not in the brain of rats at 1000 mg/kg of diet; at 500 mg/kg of
diet, only serum enzyme activity was inhibited. Female rats fed 500 or
1000 mg/kg of diet had no primary follicules and had primitive ova,
while male rats fed 1000 mg/kg of diet showed depression of
spermatogenesis. Necrotizing arteritis was observed at the two highest
concentrations. The frequencies of mammary tumours in females, in
ascending order of dose, were 14, 8, 20, 21, and 25%. The time to
appearance of these tumours was reduced in a dose-related manner
(reviewed in FAO/WHO, 1972b).
Groups of 25 male and 50 female Sprague-Dawley rats were fed
diets containing trichlorfon (purity unknown) at a concentration of 0,
100, 200, or 400 mg/kg for 18 months, equivalent to 5, 10, and 20
mg/kg bw per day. A significant increase in the mortality rate in all
groups led to premature termination of the study at 70 weeks. Serum
and erythrocyte cholinesterase activities were inhibited at 400 mg/kg
of diet. Cystic granular alterations of the ovaries were seen at 200
and 400 mg/kg of diet, and ovarian atrophy, absence of primary
follicules, and primitive ova were noted at 400 mg/kg of diet. Benign
mammary tumours were found in 8% of controls, 11% of females at 200
mg/kg of diet, and 15% of females at 400 mg/kg of diet, with no change
in the time of their appearance. These results and those of the study
described above were considered to indicate that trichlorfon enhances
certain normal ageing processes, particularly in reproductive tissues
(reviewed in FAO/WHO, 1972b).
Groups of 50 FB30 rats of each sex were fed diets containing
trichlorfon (purity, 95.3%) at a concentration of 50, 250, 500, or
1000 mg/kg for 2 years, equivalent to 2.5, 12.5, 25, and 50 mg/kg bw
per day. The control group comprised 100 males and 100 females. No
changes in survival, general behaviour, appearance, body-weight gain,
or food consumption were reported. No significant differences in blood
count or urinary parameters were reported in samples obtained during
the final 3 weeks of the study. Tests of liver function showed an
increase in 'serum dehydrogenase' activity in males at 50 mg/kg of
diet and in females at 50 and 500 mg/kg of diet. Serum protein
concentration and alanine and aspartate aminotransferase activities
were unchanged. Acetylcholinesterase activity in 'total blood' was
decreased by approximately 20% at 1000 mg/kg of diet in week 90. No
macroscopic evidence of treatment-related changes was reported at
necropsy. Liver weights were significantly increased in males at 250
and 1000 mg/kg of diet, and spleen weights were significantly
increased in females at 50 and 1000 mg/kg of diet. Histological
examination was performed on five male and five female rats at 1000
mg/kg of diet and on three male and three female control animals. The
ovaries of an additional 15 treated and 30 control animals were
examined, as previous studies had indicated a possible effect. The
findings, including tumour incidences, were unrelated to treatment
(Lorke & Loser, 1966; Grundmann & Hobik, 1966; FAO/WHO, 1972b).
Groups of 30 male and 35 female albino rats received an oral dose
of 0 or 30 mg/kg bw or an intraperitoneal dose of 0 or 12 mg/kg bw of
trichlorfon (purity unknown) twice per week. Dosing was continued for
90 weeks, and the studies were terminated after 118 weeks. The treated
animals showed reduced survival, but the tumour incidences were not
increased (Teichmann et al., 1978; FAO/WHO, 1979b).
Trichlorfon (purity, 98.6-99.1%) was fed in the diet to groups of
75 Long-Evans rats of each sex at a concentration of 0, 100, 300, or
1000 mg/kg, equivalent to 5, 15, and 50 mg/kg bw per day. The animals
were maintained on their respective diets for 90 days and then mated
within groups. The resulting F1 litters were reared to 21 days
post partum, at which time 75 offspring of each sex per group were
selected to continue on the experiment. The F0 and selected F1
rats were treated for 2 years. There was no effect on fertility,
reproduction, food intake, body-weight gain, or mortality rate in
either generation. Plasma cholinesterase activity was depressed by
30-60% in both generations at 1000 mg/kg of diet at most times during
the study. Erythrocyte cholinesterase activity was reduced by 30-60%
in both generations given 1000 mg/kg of diet, at most sampling times
in females and on some occasions in males. Brain cholinesterase
activity was significantly lower (40%) in females given 1000 mg/kg of
diet and was 25% lower in F1 males. No histological changes
attributable to treatment were found (Rosenblum, 1981; Griffin et al.,
1982).
Groups of 50 Fischer 344 rats of each sex were given diets
containing trichlorfon (purity, 98.8%) for 2 years at a mean measured
time-weighted average concentration of 0, 92, 270, or 1500 mg/kg. The
nominal concentrations were 0, 100, 300, and, at the highest
concentration, 1000 mg/kg of diet for 27 weeks, 1250 mg/kg of diet for
5 weeks, 1500 mg/kg of diet for 8 weeks, and 1750 mg/kg of diet for 65
weeks. These doses are equal to 4.5, 13, and 76 mg/kg bw per day for
males and 5.8, 17, and 94 mg/kg bw per day for females. Satellite
groups of 20 animals of each sex in the control and high-dose groups
were killed after 1 year. The only noteworthy clinical signs were
paleness and hunched backs in males and rough coat in females at 1750
mg/kg of diet. Ophthalmic parameters were normal, and the mortality
rate was not affected. Females at 1750 mg/kg of diet showed slightly
decreased feed consumption and body-weight gain during the first 66
weeks, while the body weight of males at this dose was lower than that
of controls during the final 25 weeks of the study.
Hypercholesterolaemia occurred in males at 300 and 1750 mg/kg of diet
and in females at 1750 mg/kg of diet. Plasma, erythrocyte, and brain
cholinesterase activities were depressed by 20-40% at the highest
dose. Erythrocyte counts, haemoglobin concentration, and haematocrit
were slightly decreased at 1750 mg/kg of diet, but this anaemic
response had recovered in females by the end of the study. Urinary
parameters were not affected. At 1750 mg/kg of diet, the liver weights
were increased in males and females and the spleen weights were
increased in males, but with no associated pathological changes. The
kidney weights and the incidence of granular kidneys were increased at
1750 mg/kg of diet, corresponding to a slight enhancement in chronic
(age-related) nephropathy. The pathological changes included
hyperplasia of the upper small intestine and gastritis of the
non-glandular stomach in animals at 300 and 1750 mg/kg of diet and an
increased incidence of chronic lung inflammation in females at 1750
mg/kg of diet. The incidence of adrenal phaeochromocytomas was
increased in males at 1750 mg/kg of diet but was within the historical
control range. The NOEL was 92 mg/kg of diet, equal to 4.5 mg/kg bw
per day (Hayes, 1989; WHO, 1992).
Groups of 50 Fischer 344 rats of each sex were given diets
containing trichlorfon (purity, 98.5%) for 2 years at a nominal
concentration of 0 or 2500 mg/kg, equal to 130 mg/kg bw per day for
males and 160 mg/kg bw per day for females. Satellite groups of 20
animals of each sex per dose were killed after 1 year. This study was
initiated to ensure that trichlorfon had been tested at the maximum
tolerated dose. The body weights of treated animals were 10-15% lower
than those of controls throughout the study. Treated animals had
reduced food consumption during about the first half of the study, but
thereafter it was comparable to that of controls. The mortality rate
and ophthalmoscopic parameters were unaffected. The clinical signs in
treated animals included urine staining and enlarged abdomen in
animals of each sex and pale eyes and rough coat in males. Decreased
erythrocyte count, haemoglobin concentration, and haematocrit were
noted in treated rats but had recovered in males by the end of 2
years. Hypercholesterolaemia was seen in treated groups throughout the
study. The activities of gamma-glutamine transferase, alanine and
aspartate aminotransferases, and alkaline phosphatase in blood were
increased in treated males at various times but not at termination.
Urinary parameters were not affected. Statistically significant,
40-70% depressions in plasma, erythrocyte, and brain cholinesterase
activities were observed in animals of each sex at all times studied.
The liver and kidney weights were increased in treated groups,
and the lung weights were increased in females after 2 years; after 1
year, only changes in liver and kidney were reported. Histological
examination revealed duodenal hyperplasia, gastritis of the
non-glandular stomach, myodegeneration of the stomach tunica
muscularis, pulmonary inflammation and hyperplasia, hepatocellular
cytoplasmic vacuolation, focal hepatocellular hyperplasia associated
with sinusoidal dilatation and cystic degeneration (males),
nasolachrymal duct inflammation (females), chronic nephropathy
(females), and renal tubular hyperplasia. Non-statistically
significant increases in the incidences of renal tubular adenomas in
males and mononuclear-cell leukaemia in females were within the
historical control range. The increased incidences of
alveolar/bronchiolar adenomas in males and of carcinomas in females
were greater than the recorded historical control means, but the
numbers were small and did not attain statistical significance
(Christenson, 1990).
An increased incidence of tumours was thus detected in two
studies in rats conducted in the same laboratory. The increased
incidence of mammary tumours in females was slight, and the tumours
were benign. The strain used was Sprague-Dawley, which has a
relatively high, variable background incidence of mammary tumours
(Haseman et al., 1986). The lack of confirmation of this finding in
several studies in other species indicates that the evidence for the
carcinogenicity of trichlorfon in animals is limited.
Hamsters
A group of 23 male and 25 female Syrian golden hamsters received
trichlorfon (purity unknown) at a weekly intraperitoneal dose of 0 or
20 mg/kg bw for 90 weeks, and the study was terminated after 100
weeks. The treated animals showed reduced survival and body-weight
gain, but the tumour incidences were not increased (Teichmann &
Schmidt, 1978; FAO/WHO, 1979).
Dogs
Trichlorfon (purity unknown) was added to the diet of groups of
one male and one female beagle at a concentration of 50, 250, 500, or
1000 mg/kg, equivalent to 1.2, 6.2, 12, and 25 mg/kg bw per day, for 1
year. Few or no data on individual animals were provided. No effect on
food consumption or weight gain was reported. Serum and erythrocyte
cholinesterase activities were significantly depressed (by 40-50% of
control values) at 500 and 1000 mg/kg of diet. Brain
acetylcholinesterase activity was depressed by 40% in animals at
1000 mg/kg of diet. The relative spleen weights were increased and
showed marked congestion and apparent lymphoid tissue atrophy at
1000 mg/kg of diet and to a lesser extent at other doses. A slight
increase in the severity of foci of chronic inflammatory cells in the
liver and a decrease in spermatogenesis were seen at 1000 mg/kg of
diet. Hyperplastic adrenal cortical nodules recorded as 'mild' were
seen in all treated groups but not in controls. Owing to the small
number of animals in each group, however, the significance of this
finding is questionable (Doull et al., 1962; WHO, 1992).
Groups of four beagles of each sex were given diets containing
trichlorfon (purity, 95.3-98.3%) at a concentration of 0, 50, 200,
800, or 3200 mg/kg, equivalent to 1.2, 5, 20, and 80 mg/kg bw per day
in a study designed to last 4 years. All males at 3200 mg/kg of diet
died within the first year, and only one female at this dose survived
4 years. At this dose, the animals showed muscular twitching, severe
abdominal cramps, and salivation. The males in particular quickly
became emaciated. Autopsy of the animals that died during the
experiment showed brittle livers of a 'yellowish or loam' colour. Two
females and three males at 800 mg/kg of diet died during the first
year. The mortality rates at the two lower doses were similar to those
of controls, and the appearance and behaviour of the animals were
normal. Animals at higher doses were weakened and sick, and lower body
weights were recorded for surviving animals at 800 mg/kg of diet
during the latter stages and for the surviving female at 3200 mg/kg of
diet throughout the study. The effects on food consumption were
similar to those on body weight. Haematological parameters for treated
dogs were within the normal range. Elevated serum activities of
alanine and aspartate aminotransferases were noted in females at 3200
mg/kg of diet at 2 years but not at 4 years. No treatment-related
effects were found on urinary analysis. Cholinesterase activity in
whole blood was not inhibited at 50 mg/kg of diet; at 200 mg/kg of
diet, a 30% depression was seen at 1 month but the level rose again at
4 months. The group at 200 mg/kg of diet also showed inhibited
activity (15-20%) in plasma and erythrocytes throughout the study. At
800 and 3200 mg/kg of diet, dose-related depressions of 30-80% in
cholinesterase activity in whole blood, plasma, and erythrocytes were
found. The female at 3200 mg/kg of diet which survived to 4 years had
an enlarged, swollen liver, an enlarged spleen and adrenals, and small
ovaries. Dogs fed 800 mg/kg of diet had increased spleen and adrenal
weights and decreased testis weight. There were no histopathological
changes that were considered to be related to treatment. The NOEL was
50 mg/kg of diet, equivalent to 1.2 mg/kg bw per day (Loser, 1970;
Spicer & Payne, 1971; FAO/WHO, 1972b).
Monkeys
Groups of five male and five female rhesus monkeys (Macaca
mulatta) were given aqueous solutions of trichlorfon (purity,
98.6-99.1%) by gavage at a dose of 0, 0.2, 1, or 5 mg/kg bw per day, 6
days/week for 10 years. Two animals at 5 mg/kg bw per day showed
transient pupillary constriction, and one had muscular fasciculations
during the first month only; diarrhoea and soft stools were noted
frequently. At this dose, the body weights were lower towards the end
of the study, and there was a tendency to lower erythrocyte count,
haemoglobin concentration, and haematocrit at various sampling times.
No treatment-related effects on the mortality rate or clinical
chemical or urinary parameters were recorded. Plasma, erythrocyte, and
brain cholinesterase activities were inhibited by 30-80% in animals at
1 and 5 mg/kg bw per day, while males at 0.2 mg/kg bw per day showed
slight cholinesterase inhibition (16%) only in erythrocytes, which was
considered not to be biologically significant. Liver morphology in
biopsy samples taken during the first 3 years was unchanged. Gross and
microscopic examination of tissues showed no changes related to
treatment (Griffin, 1988; WHO, 1992).
2.2.4 Genotoxicity
The results of genotoxicity assays with trichlorfon are
summarized in Table 2. Other studies, evaluated by IARC (1983) and WHO
(1992), showed similar findings. The inconsistencies in a number of
tests has been suggested to be due to the variable purity of the
materials tested (Dreist & Mihail, 1990).
2.2.5 Reproductive toxicity
(a) Multigeneration studies
Trichlorfon (purity, 98.3%) was administered to groups of 10 male
and 20 female FB 30 rats in the diet at a concentration of 0, 100,
300, 1000, or 3000 mg/kg for three generations with two litters per
generation. Females in the F0 generation at 1000 and 3000 mg/kg of
diet had significantly depressed weight gain. The size of F1a
litters was reduced at doses > 1000 mg/kg of diet, while pup growth
and survival during lactation were impaired at 3000 mg/kg of diet. The
F1b mating resulted in lower pregnancy rates at 1000 and 3000 mg/kg
of diet. Litter size and pup growth and survival were also reduced at
3000 mg/kg of diet, and all animals in this group died before mating
to produce the F2 generation. During the remainder of the study, no
effects were found on fertility or on litter size or weight. The
F2a, F2b, F3a, and F3b groups given 1000 mg/kg of diet all had
reduced body-weight gain during the lactation period. No fetal
malformations were found in any group. Examination of the F0, F1b,
and F2b adults revealed no gross or microscopic alterations. The
NOEL was 300 mg/kg of diet, equivalent to 30 mg/kg bw per day (Loser,
1969; Spicer & Urwin, 1971; WHO, 1992).
(b) Developmental toxicity
Mice
A single dose of 360 mg/kg bw trichlorfon (purity unspecified),
injected intraperitoneally into 33 AS/Jena mice on day 1 of gestation,
was embryotoxic. The frequency of post-implantation loss was increased
with single doses of 60, 120, or 240 mg/kg bw on day 9 (13-15 mice per
group) and with 120 or 240 mg/kg bw on days 1-7 (25-30 mice per
group), and was most pronounced with 240 mg/kg bw on days 7-14 (23
mice). There were no severe malformations (Scheufler, 1975; WHO,
1992).
CD-1 mice were given trichlorfon (purity unspecified) by gavage
at a dose of 0, 300, 400, 500, or 600 mg/kg bw per day on days 6-10
(3-25 females per group) or 10-14 (4-21 females per group) of
gestation and were killed on day 18. The food intake and weight gain
of dams were depressed at all doses but with no dose-response
relationship. Fetal body weight was lower at doses > 400 mg/kg bw
per day, while the frequency of fetal malformations (cleft palate) was
slightly increased only in the groups treated with 500 or 600 mg/kg bw
per day during days 10-14 of gestation. The highest dose, 600 mg/kg bw
per day, was also administered by gavage on day 8 or on days 8-10,
10-12, or 12-14 of gestation (4-28 females per group). An increase in
the frequency of cleft palate was seen only in the last group. The
NOEL was 300 mg/kg bw per day (Staples & Goulding, 1979; WHO, 1992).
Trichlorfon (purity, > 98%) was given by gavage to CD-1 mice at
a dose of 0, 200, 300, or 400 mg/kg bw per day on days 7-16 of
gestation (15-24 females per group), and the animals were killed 1 day
after the last dose. The two higher doses increased the mortality
rate, and all doses decreased the weight gain of dams. The number of
live fetuses and fetal weight were decreased at 400 mg/kg bw per day.
Delayed development of fetuses was found, as indicated by reduced
calcification in a number of skeletal areas, rib variations, and
increased incidences of enlarged renal pelves in treated groups
(Courtney et al., 1986).
Table 2. Summary of results of tests for genotoxicity with trichlorfon
End-point Test object Concentration Result Reference
or dose (maximum)
In vitro
Interaction Determination of 160 mg/kg bw i.p. Positive Dedek et al.
with DNA 7-methylguanine (1976)
in mouse urine
Determination of 120 mg/kg bw i.p. Positive Dedek (1981)
7-methylguanine
in mouse liver and
kidney
Gene mutation B. subtilis NIG17, 0.3 mg per disc -S9 Negative Inukai & Iyatomi
(rec) NIG45 (1977)
P. mirabilis 10 mg per spot -S9 Positive Adler et al. (1976)
PG273, PG713
B. subtilis H17, NR -S9 Negative Shirasu et al.
M45 (1976)
B. subtilis H17, 2 mg per disc -S9 Positive Shirasu et al.
M45 (1979)
S. typhimurium 10 mg/disc ?S9 Positive Jones et al. (1984)
Reverse S. typhimurium 5 mg per plate ±S9 Positive Byeon et al. (1976)
mutation TA100
TA98, TA1535, 5 mg per plate ±S9 Negative
TA1538
TA98, TA100, 0.5 mg per plate ±S9 Negative Inukai & Iyatomi
TA1535, TA1537 (1977)
Table 2. (continued)
End-point Test object Concentration Result Reference
or dose (maximum)
TA98, TA100 ~ 8.5 mg per plate Positive Batzinger &
±S9 Bueding (1977)
TA100, TA1535 10 mg per plate ±S9 Negative Zeiger et al. (1987)
TA98, TA100 2 mg per plate ±S9 Negative Diril et al. (1990)
TA1535, TA1536, NR -S9 Negative Shirasu et al.
TA1537, TA1538, (1976)
E. coli WP2, WP2hcr
TA1535, TA1536, 2 mg per disc -S9 Negative Carere et al.
TA1537, TA1538 (1978a,b)
TA100, E. coli 20 mg per plate ±S9 Positive Shirasu et al.
WP2hcr (1979); Moriya et
TA98, TA1535, 20 mg per plate ±S9 Negative al. (1983)
TA1537, TA1538
TA100, TA104 25 mg per plate ±S9 Positive Barrueco et al.
TA97, TA98, 25 mg per plate ±S9 Negative (1991)
TA1535
TA98, TA1537 5 mg per plate±S9 Negative Watabe (1997)
TA100, TA1535, 5 mg per plate±S9 Positive
E, coli WP2uvrA
S. cerevisiae NR -S9 Negative Guerzoni et al.
632/4, 632/1b, (1976)
814/18b
Table 2. (continued)
End-point Test object Concentration Result Reference
or dose (maximum)
Reverse S. cerevisiae 10 mg per ml ±S9 Negative Hoorn (1983)
mutation S138, S211a
S. cerevisiae D7 40 mg per ml ±S9 Positive Jones et al.
(1984)
Mitotic crossing S. cerevisiae D7 40 mg per ml ±S9 Positive Jones et al.
over, gene (1984)
conversion
Forward S. coelicolor 2 mg per disc -S9 Positive Carere et al.
mutation (1978a,b)
S pombe SP-198 30 mg per ml ±S9 Positive Gilot-Delhalle et
al. (1983)
V79 cells 200 mg per ml -S9 Negative Aquilina et al. (1984)
L5178Y cells 200 µg per ml -S9 Positive Witterland
600 µg per ml +S9 Positive (1984); Jones et
al. (1984)
DNA damage E coli pol+/- 10 mg per plate ±S9 Negative Herbold (1984)
E. coli (SOS NR ±S9 Negative Xu & Schurr
chromotest) (1990)
Unscheduled EUE cells 1000 mg per ml -S9 Positive Aquilina et al.
DNA synthesis (1984)
Primary rat 50 µg per ml -S9 Negative Myhr (1983)
hepatocytes
Table 2. (continued)
End-point Test object Concentration Result Reference
or dose (maximum)
Sister chromatid V79 cells 80 µg per ml -S9 Positive Chen et al.
exchange 60 µg per ml +S9 Positive (1981, 1982)
CHO cells 100 µg per ml -S9 Positive Jones et al.
2 mg per ml +S9 Positive (1984); Putman
(1987)
Chromosomal Don-6 cells 250 mg per ml -S9 Positive Sasaki et al.
damage (1980)
Human lymphocytes 30 mg per ml -S9 Positive Herbold (1986)
3000 mg per ml +S9 Positive
In vivo
Reverse Host-mediated Batzinger &
mutation assay in mice Bueding (1977)
S. typhimurium 200 mg/kg bw orally Negative
TA98
S. typhimurium 200 mg/kg bw orally Positive
TA100
Recessive lethal Drosophila 4.5 mg/kg bw Negative Benes & Sram
mutation melanogaster (1969); Brzheskiy
(1973); Lamb
(1977)
Sister chromatid Chinese hamster 300 mg/kg bw orally Negative Volkner (1987)
exchange bone marrow
Table 2. (continued)
End-point Test object Concentration Result Reference
or dose (maximum)
Chromosomal Micronucleus 2 x 312 mg/kg bw i.p. Negative Paik & Lee
damage formation in mouse 2 x 250 mg/kg bw Negative (1977); Herbold
bone marrow orally (1979a); Jones
2 x 400 mg/kg bw Negative et al. (1984);
orally Herbold (1997)
400 mg/kg bw orally Negative
400 mg/kg bw orally Negative
of (+)-enantiomer
600 mg/kg bw orally Positive
of (-)-enantiomer
Metaphase analysis 400 mg/kg bw orally Positive Kurinnyi (1975);
in mouse 10 mg/kg bw i.p. Positive Moutschen-Dahmen
bone marrow 100 mg/kg bw i.p. Negative et al.
0.5 mg/ml in drinking, Negative (1981);
water 5 days/week, Degraeve et al.
7 weeks (1982, 1984);
405 mg/kg bw i.p. Negative Nehes et al.
(1982)
Metaphase analysis 250 mg/kg bw i.p. Negative Dzwonkowska &
in hamster Hubner (1986)
bone marrow
Metaphase analysis 250 mg/kg bw orally Negative Bootman &
in rat bone Hodson-Walker
marrow (1987)
Table 2. (continued)
End-point Test object Concentration Result Reference
or dose (maximum)
Metaphase analysis 1.5 mg/ml in drinking, Positive Bulsiewicz et al.
in mouse water 50-100 days (1976);
spermatogonia 100 mg/kg bw i.p. Negative Moutschen-Dahmen
and spermatocytes 0.5 mg/ml in drinking, Negative et al.
water 5 days/week, (1981);
7 weeks Degraeve et al.
100 mg/kg bw i.p. Negative (1982, 1984);
Herbold (1992)
Dominant lethal 100 mg/kg bw i.p. Negative Epstein et al.
mutation in mice 0.5 mg/ml in (1972); Dedek
drinking-water, Negative et al. (1975) ;
5 days/week, Fischer et al.
7 weeks (1977); Herbold
280 mg/kg bw i.p. Negative (1979b,c);
405 mg/kg bw i.p. Negative Becker &
405 mg/kg bw i.p. Positive Schoneich
250 mg/kg bw orally Negative (1980);
NR Negative Moutschen-Dahmen
405 mg/kg bw i.p. Positive et al. (1981);
54 mg/kg bw per day, Positive Degraeve et al.
3 weeks i.p. (1982, 1984);
WHO (1992)
NR, not reported; S9, metabolic activation; i.p., intraperitoneally
Rats
Trichlorfon (purity unspecified) was administered by gavage to
groups of 11 female Wistar rats at a single dose of 80 mg/kg bw on day
9 or 13 of gestation or to 10 rats at a dose of 8 mg/kg bw per day
daily throughout gestation. The animals were killed on day 19 of
gestation. The frequencies of post-implantation deaths and fetal
malformations such as exencephaly and non-closing eyelids were
increased at the single dose on day 13. The other treatments had no
effect on development (Martson & Voronina, 1976; WHO, 1992).
Trichlorfon (purity, 98.5%) was administered in the diet to
groups of 9-26 female CD rats on days 6-15 of gestation, to give an
intake of 0, 76, 140, 380, 430, or 520 mg/kg bw per day, with necropsy
on day 21. At the two highest doses, maternal food intake and body
weight and fetal weights were reduced, while the number of fetal
deaths was increased at 430 mg/kg bw per day. Major external and
skeletal malformations were found in fetuses at 430 and 520 mg/kg bw
per day and relatively minor skeletal changes at 380 mg/kg bw per day.
The predominant malformations were alterations of the skull and
central nervous system (exencephaly, meningocoele, hydrocephaly), the
limbs (syndactyly, markedly shortened radii and ulnae, missing
digits), micrognathia, cleft palate, facial haematomas, generalized
oedema, and great vessel defects. The minor skeletal alterations were
mainly doubled vertebral centra, wavy ribs, fenestrated supraoccipital
bones. and umbilical hernia. The NOEL was 140 mg/kg bw per day
(Staples et al., 1976; WHO,1992).
Trichlorfon (purity, 98.5%) was given by gavage at a dose of 0,
50, 75, 150, 200, or 250 mg/kg bw per day to groups of 9-30 female CD
rats on days 6-15 of gestation. Deaths occurred among dams at 150,
200, and 250 mg/kg bw per day, with overt signs of toxicity at the
highest dose. The survivors were killed on day 21 of gestation. Fetal
body weights were reduced significantly at doses > 75 mg/kg bw per
day, but the incidence of malformations was unaffected. The NOEL was
50 mg/kg bw per day (Staples et al., 1976).
Trichlorfon (purity unspecified) was given at a dose of 480 mg/kg
bw per day to 34 CD rats by gavage on days 6-15 of gestation. Lower
fetal body weight, a higher fetal mortality rate, and malformations
such as generalized oedema, herniation of the brain, hydrocephaly,
micrognathia, cleft palate, and skeletal alterations were seen. A
single gavage dose of 480 mg/kg bw on day 8 or 10 of gestation to 14
females per group decreased maternal food consumption but had no
adverse effect on fetal development (Staples & Goulding, 1979; WHO,
1992).
Groups of 25 female Long-Evans rats were given trichlorfon
(purity, 98.4%) by gavage at a dose of 0, 10, 30, or 100 mg/kg bw per
day on days 6-16 of gestation and were killed on day 20. There were no
deaths, but diarrhoea was observed in some animals at 100 mg/kg bw per
day. Fetal development was not affected. The NOEL for maternal
toxicity was 30 mg/kg bw per day (Machemer, 1979a; WHO, 1992).
Trichlorfon (purity, > 98%) was given by gavage to CD rats at a
dose of 0, 50, 100, or 200 mg/kg bw per day on days 7-19 (10-20
females per group) or 8-20 of gestation (15-18 females per group).
The animals were killed 1 day after the last dose. The highest dose
increased the mortality rate and decreased the weight gain of the
dams. Delayed development of fetuses was found, as indicated by
reduced calcification in a number of skeletal areas, but no
abnormalities were noted (Courtney et al., 1986).
Groups of 33 female Charles River Crl:CD BR rats were given diets
containing trichlorfon (purity, 99%) at a concentration of 0, 500,
1100, or 2500 mg/kg on days 6-15 of gestation. Body-weight gain was
slightly reduced among animals at the highest dose. Analysis of
cholinesterase activity in five animals per group killed on day 16 of
gestation showed inhibition of plasma, erythrocyte, and brain enzymes.
When the remaining dams were killed on day 20 of gestation, only brain
acetylcholinesterase activity was significantly inhibited. No effect
was seen on the numbers of resorptions, live fetuses, fetal body
weight, or malformation frequency. Increased incidences of delayed
ossification and curved, wavy, or bulbous ribs were seen at 2500 mg/kg
of diet. While fetal brain acetylcholinesterase activity was lower in
all treated groups, there was no dose-response relationship (Kowalski
et al., 1987; WHO, 1992).
Hamsters
Groups of 5-30 female golden hamsters received trichlorfon
(purity unspecified) at a dose of 0, 100, 200, 300, or 400 mg/kg bw
per day by gavage on days 7-11 of gestation, and the survivors were
killed on day 15. Three of 30 hamsters at the highest dose died, and
the signs of cholinesterase inhibition were dose-related at doses >
200 mg/kg bw per day. Maternal food consumption and body-weight gain
and fetal body weight were decreased at 300 and 400 mg/kg bw per day.
Fetuses at the highest dose showed an increased frequency of stunting
malformations, mostly consisting of oedema, cleft palate, patagium,
and fused ribs. A single dose of 400 mg/kg bw per day by gavage given
on day 8 of gestation to 16 females reduced maternal food consumption
and increased the fetal death rate but had no meaningful effect on
fetal development. The NOEL was 100 mg/kg bw per day (Staples &
Goulding, 1979; WHO, 1992).
Guinea-pigs
Groups of 10 white female guinea-pigs were given six doses of 100
mg/kg bw per day trichlorfon (purity, 97%) by gavage on days 36-38 and
51-53 of gestation. Seven females served as controls, and all animals
were allowed to deliver naturally. The numbers of abortions and
stillborn fetuses were increased and fetal body weight was decreased
in the treated group. The pups of treated dams developed trembling and
locomotor disturbances. At autopsy on postnatal day 2-3, the total
weights of the brain, cerebellum, medulla, cerebral cortex,
hippocampus, thalamus, and colliculi were decreased. The cerebellum
showed reduction of the external granular and molecular layers, with
regional absence of Purkinje cells. The activities of the
neurotransmitter enzymes choline acetyltransferase and glutamate
decarboxylase in the cerebellum were depressed when compared with
controls (Berge et al., 1986; WHO, 1992).
Rabbits
Groups of 15 female Himalayan rabbits were given trichlorfon
(purity, 98.4%) by gavage at a dose of 0, 5, 15, or 45 mg/kg bw per
day on days 6-18 of gestation and were killed on day 29. Body-weight
gain was reduced in treated groups, and two abortions occurred at 45
mg/kg bw per day. Fetal development was not affected (Machemer, 1979b;
WHO, 1992).
Groups of 20 female American Dutch rabbits were given trichlorfon
(purity, 99%) by gavage at a dose of 0, 10, 35, or 110 mg/kg bw per
day on days 6-18 of gestation and were killed on day 28. The highest
dose was not well tolerated and resulted in deaths, overt signs of
toxicity, and reduced body-weight gain. At 35 mg/kg bw per day, there
were also signs of toxicity, one death, and one abortion. Erythrocyte
but not plasma cholinesterase activity was inhibited on day 19 of
gestation in animals at 35 and 110 mg/kg bw per day, while on day 28
only brain cholinesterase activity was inhibited at these doses. At
110 mg/kg bw per day, there was a slight increase in the number of
resorptions, slight decreases in fetal and placental weights, and
delayed ossification. The frequency of fetal abnormalities was not
increased. The NOELs were 10 mg/kg bw per day for maternal toxicity
and 35 mg/kg bw per day for embryo- and fetal toxicity (Clemens et
al., 1990; WHO, 1992).
Pigs
Several outbreaks of congenital tremor in piglets have been
described in herds in which the sows had been treated with trichlorfon
preparations between day 45 and day 63 of gestation. Clinically, the
syndome was characterized by ataxia, tremor, pronounced hypoplasia of
the cerebellum, and a reduction in the size of the spinal cord (WHO,
1992). This syndrome was reproduced experimentaly in sows given diets
providing one to four doses of 50-100 mg/kg bw per day 55-98 days
after conception. The offspring had a high incidence of overt
neurological signs and cerebellar hypoplasia. Histological examination
revealed patchy loss of Purkinje cells in the cerebellum. Postnatally,
the rate of increase of brain weight was similar to that of controls,
but normal weights had not been achieved up to 35 days after birth
(Knox et al., 1978; Pope et al., 1986; Berge et al., 1987a,b; WHO,
1992).
2.2.6 Special studies
(a) Neurotoxicity
The results of these studies are summarized in Table 3. Like
other organophosphonates, trichlorfon has neurotoxic potential. Rats
given a dose of about 200 mg/kg bw per day for 13 weeks showed
decreased locomotor activity and loss of coordination, whereas rats
given a dose of 30 mg/kg bw per day for 3 weeks showed increased
locomotion and decreased learning ability and nerve conduction
velocity. Numerous studies in hens consistently demonstrated the
neurotoxicity of single doses of trichlorfon. Leg weakness, impaired
walking ability, and decreased activity were seen immediately after
treatment. These effects are considered to be secondary to inhibition
of cholinesterase activity and to be generally unrelated to damage to
the nervous tissues. Delayed neurotoxicity is usually detected some
weeks after dosing and is associated with degeneration of nervous
tissue and marked inhibition of neuropathy target esterase activity.
Neuropathy target esterase is a protein which is located specifically
in neurons, but its biological function, other than as a target for
neuropathic organophosphates, is unknown. The toxicity of an agent can
be predicted by its relative ability to interact with either
acetylcholinesterase to produce cholinergic effects or neuropathy
target esterase to produce delayed polyneuropathy (Ray, 1998). The
studies gave no evidence of delayed neuropathy in chickens. In a
monkey given a single oral dose of trichlorfon at 250 mg/kg bw per
day, nerve conduction was impaired 4 weeks after treatment, and there
was histological evidence of demyelination of nerves and axonal
degeneration.
(b) Immunotoxicity
Groups of four female BALB/cByJ and C57BL/6J mice were given
drinking-water containing trichlorfon at 1.75 mg/ml for 14 days.
Measurement of a variety of responses to immunization with influenza
virus indicated that immune function was not impaired (Reiss et al.,
1987; WHO, 1992).
2.3 Observations in humans
Wegner (1970) reviewed data on over 6000 people, obtained mostly
in South Africa and South America, who had been treated with
trichlorfon for various intestinal and body parasites (Ankylostoma,
Ascaris, Strongyloides, Trichocephalus, and Schistosoma), at doses
varying from 5 to 70 mg/kg bw per day for up to 12 days. A dose of 7.5
mg/kg bw given two to four times at 2-week intervals was considered to
be optimal, as the side-effects observed were less severe than at
other dosages. The symptoms included depression of cholinesterase
activity, weakness, nausea, diarrhoea, and abdominal pain. A dose of
24 mg/kg bw caused more severe symptoms, including tachycardia,
salivation, colic pain, vomiting, nausea, fatigue, tremors, and
sweating. The effects were not cumulative, and spontaneous recovery
was rapid in all cases. Spermatogenesis (size and shape of sperm)
appeared to be affected in a few cases. Inhibition of cholinesterase
activity was seen at all doses (FAO/WHO, 1972b; WHO, 1992).
Reduced erythrocyte and plasma cholinesterase activities have
been reported in groups of patients with various parasitic
infestations given trichlorfon orally at doses of 5-12.5 mg/kg bw. The
drug was reported to be well tolerated with few clinical symptoms
related to treatment. The symptoms that were observed were cholinergic
in nature and were usually found at doses > 10 mg/kg bw.
Cholinesterase activity recovered to pretreatment levels within 4-15
weeks after cessation of treatment (Abdel Aal et al., 1970; Plestina
et al., 1972; Jewsbury, 1981).
In a clinical trial, 10 male and 10 female patients with
Alzheimer disease were given oral doses of trichlorfon for up to 30
weeks according to the following dose regimen: 2.5 mg/kg bw at week 0,
5 mg/kg bw in week 1, 7.5 mg/kg bw in weeks 6 and 7, 15 mg/kg bw in
weeks 8 and 9, and the 'best weekly dose' in weeks 18-30. The 'best
dose' was defined as that associated with the greatest improvement for
each patient. This small trial indicated general improvement in a
variety of functional deficits associated with Alzheimer disease.
Side-effects were reported at doses > 5 mg/kg bw, which included
nausea, vomiting, and diarrhoea. Plasma cholinesterase activity was
inhibited by 45-60% at all doses, and erythrocyte acetylcholinesterase
activity was inhibited by 30-70% at doses > 5 mg/kg bw with no
concomitant clinical findings. Measurement of acetylcholinesterase
activity in the cerebrospinal fluid from two patients 24 h after a
dose of 5 mg/kg bw revealed inhibition of 37 and 47% below
pretreatment levels (Becker et al., 1990).
In a prospective, double-blind, randomized study, four parallel
groups of 119-121 patients with Alzheimer disease received oral doses
of placebo or trichlorfon once daily. An initial loading dose of 0,
0.5, 0.9, or 2.0 mg/kg bw per day was given during weeks 0-2 in order
to achieve steady-state inhibition of cholinesterase activity more
quickly, and this was followed during weeks 3-10 by a dose of 0, 0.2,
0.3, or 0.65 mg/kg bw per day. The intermediate and high doses were
found to improve cognitive function, but the low dose had equivocal
effects. Abdominal pain, diarrhoea, flatulence, nausea, and leg cramps
were the most commonly reported side-effects and were most severe in
the patients given the high dose. Erythrocyte acetylcholinesterase
activity was inhibited in a dose-related manner (Table 4). The initial
dose of 0.5 mg/kg bw per day for 2 weeks inhibited erythrocyte
acetylcholinesterase activity by 29%, and subsequent administration of
0.2 mg/kg bw per day for 10 weeks maintained the inhibition at 30-37%,
with no increase with duration of dosing. Since this dose enhanced
inhibition over that caused by the initial dose by only 8%, an
insignificant change, the Committee considered that the NOEL for
inhibition of acetylcholinesterase activity was 0.2 mg/kg bw per day
(Cummings et al., 1998).
Table 3. Summary of studies of neurotoxicity with trichlorfon
Species Treatment Results Reference
Rats 200 mg/kg bw per day Electrophysiological changes Averbrook &
i.p. for 5-15 days indicating enhanced excitability Anderson (1983)
in sciatic nerve. No pathological
changes in nerves
Rats 30 mg/kg bw per day Increased locomotion, decrease Lehotzky (1982)
orally for 3 weeks in rotorod performance, learning
ability, and nerve conduction
velocity
Rats 0, 85, 438, or 2275 Rats given 2275 ppm had stained Sheets &
ppm in the diet for 13 fur, reduced motor and locomotor Hamilton (1995)
weeks activity, slightly uncoordinated
righting response (males only),
30-80% inhibition of plasma,
erythrocyte, and brain
cholinesterase activity, and
demyelination in spinal nerve roots.
At 438 ppm, only plasma and
erythrocyte cholinesterase activity
was inhibited (20-30%), and no
behavioural or biochemical
evidence of neurotoxicity was
seen at 85 ppm (6 mg/kg bw
per day)
Chicken Single subcutaneous Leg weakness for 1-2 days, no Witter & Gaines
dose of 90 mg/kg bw delayed paralysis (1963)
Chicken Single dose of 25-500 Birds that survived the acute Lorke &
mg/kg bw orally or toxicity of trichlorfon showed no Kimmerle (1966)
75-500 mg/kg bw i.p. signs of neurotoxicity over
6-10 weeks
Chicken 100-5000 ppm in Body weight loss at > 500 ppm. Lorke &
feed for 30 days Blood cholinesterase activity Kimmerle (1966)
decreased by 40-60% at all Hobik (1967);
doses but no signs of FAO/WHO
neurotoxicity and no (1972)
degeneration in nervous tissue.
Table 3. (continued)
Species Treatment Results Reference
Chicken Single subcutaneous One hen died 1.5 h after dosing; Hierons &
dose of 200 mg/kg bw the lone survivor was killed 24 h Johnson (1978)
after dosing, when NTE was
inhibited by 46% in brain and
17% in spinal cord.
Chicken Single doses of 50, Dose-related deaths after oral Olajos et al.
100 mg/kg bw orally, doses only. All treatments had (1979)
50, 100, 200 mg/kg acute effects; ataxia from 11-17
bw subcutaneously, days after dosing. Brain NTE
or 200 +100 mg/kg bw decreased by 40-60% at high
subcutaneously 3 subcutaneous doses but by only
days apart about 20% at <100 mg/kg bw.
Mild degenerative changes seen
in cerebellum, brainstem, and
striatum at higher doses.
Chicken 185 mg/kg bw orally, Acute signs of intoxication but Thyssen et al.
survivors given 167 no pathological changes in (1982)
mg/kg bw 21 days nervous tissue
later
Chicken Single subcutaneous Marked cholinesterase inhibition Slott &
dose of 100 or 300 in plasma, brain, and spinal cord Ecobichon
mg/kg bw (50-60%) but no inhibition of NTE (1984)
in brain or spinal chord
Chicken 100 mg/kg bw Plasma, brain, and spinal cord Slott &
subcutaneously every cholinesterase inhibited by Ecobichon
72 h for 6 doses 10-60%, and walking ability (1984)
slightly impaired. No inhibition
of NTE in brain or spinal cord
and only slight oedematous
effects on nervous tissue
Chicken 3, 9, or 18 mg/kg bw Signs of ataxia and decreased Hayes & Ramm
per day for 3 months activity at 18 mg/kg bw per day (1987)
by gavage but no effect on locomotor activity.
Blood cholinesterase activity
decreased in all groups but
nervous tissue unremarkable.
Table 3. (continued)
Species Treatment Results Reference
Chicken Single gavage dose Acute signs of intoxication, Bomann &
of 340 mg/kg bw, 220 deaths, and marked inhibition Kaliner
mg/kg bw (90%) of brain cholinesterase (1996)
(-)-enantiomer, activity with each compound.
or 400 mg/kg bw < 50% NTE inhibition in brain,
(+)-enantiomer spinal cord, and sciatic nerve; no
changes in gait or nervous tissue
Monkey Single dose of Weakness of lower extremities Shiraishi et al.
(Macaca 250 mg/kg bw by on day 24 and nerve conduction (1983)
fuscata) gavage defects by day 28. On day 37,
demyelination of sural and tibial
nerves with increased axonal
degeneration
NTE, neuropathy target esterase
Table 4. Mean inhibition of erythrocyte acetylcholinesterase
activity (%) in patients with Alzheimer disease
given trichlorfon orally
Time (week) Dose of trichlorfon (mg/kg bw per day)
0-2 0 0.5 0.9 2.0
3-10 0 0.2 0.3 0.65
2 1.1 ± 14 29 ± 12 49 ± 12 71 ± 82
8 0.1 ± 21 37 ± 13 52 ±12 68 ± 95
12 1.0 ± 24 34 ± 14 52 ± 10 72 ± 24
A position paper (Bayer AG, 1999) reported that the results of
clinical trials involving over 4000 treated patients show that
trichlorfon at the recommended dose of 0.5-0.9 mg/kg bw is 'a safe and
effective treatment' for Alzheimer disease. A dose-response
relationship was found between the dose of trichlorfon and the
incidence of muscle weakness in placebo-controlled trials, and the
increased risk for muscular weakness was statistically significant at
doses > 1.25 mg/kg bw. The recommended dose of 0.5-0.9 mg/kg bw
resulted in incidences of 0.6% for generalized weakness or myasthenia
(0.2% with placebo) and 4.3% for fatigue or asthenia (3.3% with
placebo). The muscular weakness was reported to be reversible on
discontinuation of therapy.
Acute poisoning of humans with trichlorfon has been reported to
manifest as delayed neurotoxicity, severe polyneuritis, and
polyneuropathy 2-3 weeks after ingestion. These diagnoses were based
on observations of weakness, loss of feeling in the extremities, and
difficulty in walking, accompanied by muscular atrophy, myalgia, and
were related to motor nerve damage (Pollingher et al., 1973; Akimov et
al., 1975; Eljasz & Kryzyszton-Przekop, 1975; Fukuhara et al., 1977;
Shiraishi et al., 1977; Hierons & Johnson, 1978; Shiraishi et al.,
1982; Niedziella et al., 1985; De Freitas et al., 1990). After
reviewing the literature, Johnson (1981) concluded that only doses of
trichlorfon that exceed the lethal dose but are survived by the victim
because of treatment are likely to result in a level of inhibition of
neuropathy target esterase activity at which delayed neurotoxicity
would be expected. Development of neuropathy after repeated exposure
to lower doses was considered to be unlikely (WHO, 1992).
Five persons exposed to trichlorfon, two while attempting suicide
and three occupationally, showed increased incidences of chromosomal
breaks and stable chromosomal rearrangements in cultured lymphocytes.
The effect was observed immediately after exposure and 1 month later,
but not 6 months later (Bao et al., 1974). Seventeen workers involved
in the production of trichlorfon-containing products for at least 6
months had increased incidences of chromatid breaks in their
lymphocytes when compared with an unexposed control group, but the
incidences were lower than those in a control group of factory workers
who had had no direct exposure to pesticides (Kiraly et al., 1979).
3. COMMENTS
The Committee considered the results of studies on the
toxicokinetics, metabolism, and acute toxicity of trichlorfon,
short-term and long-term studies of toxicity, and studies of
genotoxicity, reproductive and developmental toxicity, neurotoxicity,
and immune function, and studies in humans. Most of the available
studies were relatively old, and many were published reports which
lacked raw data or data on individual animals. Such studies are
difficult to evaluate, and most could not be assessed independently.
Unpublished reports of studies conducted during the 1980s and 1990s
contained sufficient detail and were carried out according to
appropriate standards for study protocol and conduct.
The absorption of trichlorfon is rapid in all species tested,
including humans, irrespective of the route of administration. Peak
blood concentrations were achieved within 1-2 h but decreased quickly
thereafter; the half-time of trichlorfon in human plasma is
approximately 2 h. It is widely distributed. Trichlorfon was detected
in the milk of lactating cows, and the compound and its metabolites
were found in fetal tissue in treated guinea-pigs. Trichlorfon
undergoes conversion to dichlorvos via a dehydrochlorination reaction
that occurs spontaneously at pH values above 5.5. Although little
dichlorvos was recovered, it is generally believed to be responsible
for the anticholinesterase effects of trichlorfon. The metabolism of
trichlorfon in mammals also occurs through O-demethylation and
cleavage of phosphorus-carbon bonds. Therefore, the major metabolites
are desmethyl trichlorfon, desmethyl dichlorvos, dimethyl hydrogen
phosphate, methyl dihydrogen phosphate, and phosphoric acid.
Trichlorfon and its metabolites are excreted primarily in the urine.
Trichlorfon is moderately toxic when given as a single oral dose,
the LD50 values being 620-950 mg/kg bw in mice, 140-660 mg/kg bw in
rats, 160 mg/kg bw in rabbits, and 300-420 mg/kg bw in dogs. The signs
of toxicity were indicative of inhibition of acetylcholinesterase
activity, and the cause of death was usually respiratory failure. The
rapid recovery observed after sublethal doses suggests that
trichlorfon is readily detoxified.
Trichlorfon was given orally to mice at doses of 10-750 mg/kg bw
per day, to rats at doses of 0.05-250 mg/kg bw per day, and to dogs at
doses of 0.5-45 mg/kg bw per day for periods up to 16 weeks. The
weights of the liver, kidney, and spleen were increased at doses of
250 mg/kg bw per day and above in mice and at doses of 50 mg/kg bw per
day and above in rats, but these changes were usually not associated
with other evidence of toxicity, except in one study in rats in which
hypertrophy of centrilobular hepatocytes and biochemical changes
suggestive of altered lipid metabolism were seen. These effects
occurred at doses which also induced significant signs of cholinergic
intoxication. Slight anaemia was observed in one study in rats at 100
mg/kg bw per day and in one study in dogs at 45 mg/kg bw per day. One
dog out of three given trichlorfon at a dose of 45 mg/kg bw per day
had small, immature testes and prostate and oligospermia, but the
animal had also lost weight and appeared to be severely stressed by
the treatment. The NOELs were 15 mg/kg bw per day for inhibition of
cholinesterase activity in brain and erythrocytes in mice, 5 mg/kg bw
per day for inhibition in brain and erythrocytes in rats, and 5 mg/kg
bw per day for inhibition in in erythrocytes in dogs.
In two studies of up to 2 years' duration, mice were given oral
doses of 15-750 mg/kg bw per day. Body-weight gain was impaired at
doses of 30 mg/kg bw per day and above; the mean weight of the liver
was increased at a dose of 240 mg/kg bw per day in one study, but no
pathological alterations were found. The NOEL for inhibition of brain
and erythrocyte cholinesterase activity was 49 mg/kg bw per day. The
incidences of tumours were unaffected by treatment. Neither study was
suitable for identifying a NOEL.
Six studies of up to 2 years' duration were conducted in which
rats were given trichlorfon in the diet at concentrations providing
doses of 2.5-160 mg/kg bw per day. The weights of the liver and spleen
were increased in three studies at doses of 50 mg/kg bw per day and
above. Hypercholesterolaemia, duodenal hyperplasia, and gastritis of
the non-glandular stomach were seen at 13 mg/kg bw per day and above.
Anaemia, enhancement of age-related nephropathy, increased renal
tubular hyperplasia, myodegeneration of the stomach tunica muscularis,
pulmonary inflammation, and hyperplasia were observed at doses of 75
mg/kg bw per day and above. Other observations included ovarian
atrophy at 20 mg/kg bw per day and above, depression of
spermatogenesis at 50 mg/kg bw per day, and a slight increase in the
incidence of benign mammary tumours in females with a reduction in the
time to appearance at doses of 20 mg/kg bw per day and above. The NOEL
for inhibition of brain and erythrocyte acetylcholinesterase activity
was 13 mg/kg bw per day. The incidence of mammary tumours was
increased in female Sprague-Dawley rats in two studies. The increases
were slight, the tumours were benign, this strain of rats has a
relatively high and variable background incidence of mammary tumours,
and the finding could not be confirmed in several other studies in
various species. The Committee concluded that trichlorfon does not
have carcinogenic potential in rats. The overall NOEL in rats was 4.5
mg/kg bw per day on the basis of an increased cholesterol
concentration and pathological changes to the gastrointestinal tract.
In two studies of 1 and 4 years' duration, dogs were given
trichlorfon orally at doses of 1.3-80 mg/kg bw per day. Deaths
occurred at doses of 20 mg/kg bw per day and above, and signs of
hepatocellular damage and increased severity of inflammation were seen
in the liver at doses of 25 mg/kg bw per day and above. In animals
given 20-25 mg/kg bw per day, the weight of the spleen was increased,
with atrophy of lymphoid tissue; furthermore, small ovaries, reduced
testis weight, and decreased spermatogenesis were seen. 'Blood
cholinesterase' activity was inhibited at doses of 5 mg/kg bw per day
and above. The overall NOEL in dogs was 1.3 mg/kg bw per day on the
basis of inhibition of cholinesterase activity.
Oral administration of trichlorfon to rhesus monkeys at a dose of
0, 0.2, 1, or 5 mg/kg bw per day for 10 years resulted in clinical
signs of toxicity indicative of cholinesterase inhibition, reduced
body-weight gain, and anaemia at the highest dose. Inhibition of
plasma, erythrocyte, and brain cholinesterase activities was seen at
the two higher doses. In a separate 6-month study in rhesus monkeys,
erythrocyte acetylcholinesterase activity was not depressed at 0.2
mg/kg bw per day, the highest dose tested. The NOEL in monkeys was 0.2
mg/kg bw per day on the basis of inhibition of brain and erythrocyte
acetylcholinesterase activity.
Trichlorfon has been tested in a wide range of assays for
genotoxicity and DNA damaging activity, with considerable variation in
the results. Trichlorfon induced point mutations in microorganisms and
cultured mammalian cells, DNA damage in microorganisms, and
chromosomal aberrations and sister chromosome exchange in cultured
mammalian cells. The results of tests for point mutation in
Drosophila melanogaster and for sister chromatid exchange in bone
marrow were clearly negative. Although positive results were obtained
in a few assays for chromosomal damage in bone marrow and in the germ
cells of male animals exposed at near-lethal doses, the results of
most studies of micronucleus formation, metaphase alterations, and
dominant lethal mutations were negative. Since the tests conducted
in vivo produced mostly negative results when trichlorfon was
administered orally, the Committee considered that the weight of
evidence indicates that trichlorfon is unlikely to represent a
genotoxic risk.
Trichlorfon was given to rats at a dose of 0, 10, 30 100, or 300
mg/kg bw per day in a three-generation study of reproductive toxicity,
with two litters per generation. The body-weight gain of F0 dams at
100 and 300 mg/kg bw per day was depressed. The slight effects on the
gonads reported in the studies of general toxicity were not
consistently reflected in this study. The pregnancy rate of rats of
the F1b generation at doses of 100 mg/kg bw per day and above was
reduced, but the fertility of other generations was unaffected. The
litter size of dams at 300 mg/kg bw per day was reduced and all of the
offspring died, but reproductive parameters were unaffected at lower
doses. The NOEL was 30 mg/kg bw per day in adults, on the basis of
reduced body-weight gain, and 100 mg/kg bw per day for reproductive
effects, on the basis of reduced litter size and the death of
offspring.
Two studies of developmental toxicity were conducted in mice
given oral doses of 200-600 mg/kg bw per day. Dams at 200 mg/kg bw per
day showed reduced body-weight gain, and higher doses led to an
increased mortality rate. The effects of these maternally toxic doses
included increased embryotoxicity, decreased fetal weight, and delayed
fetal development. The incidence of cleft palate was slightly
increased at doses of 500 and 600 mg/kg bw per day. NOELs were not
identified for maternal toxicity or fetotoxicity.
Studies of developmental toxicity were conducted in rats given
oral doses of 8-520 mg/kg bw per day. In three studies, maternal
toxicity in the form of reduced body-weight gain and/or an increased
mortality rate was observed at doses of 150 mg/kg bw per day and
above. The NOEL for maternal toxicity was 75 mg/kg bw per day. In the
same studies, the incidences of fetal malformations such as
morphological alterations of the skull, central nervous system, and
limbs, micrognathia, cleft palate, facial haematomas, generalized
oedema, and defects in major blood vessels were increased at doses of
430 mg/kg bw per day and above. At 380 mg/kg bw per day, minor
skeletal changes were observed. Reduced fetal body weight was seen at
75 mg/kg bw per day. In three other studies, the incidence of fetal
malformations was unaffected. The NOEL for developmental effects was
50 mg/kg bw per day on the basis of fetotoxicity.
Hamsters given trichlorfon orally at a dose of 0, 200, 300, or
400 mg/kg bw per day during gestation showed signs of cholinesterase
inhibition, and depressed body-weight gain was seen at the two higher
doses. The NOEL for maternal toxicity was 100 mg/kg bw per day. Fetal
body weight was decreased at the two higher doses, and an increased
incidence of fetal stunting was seen at the highest dose. The NOEL for
developmental effects was 200 mg/kg bw per day.
Guinea-pigs given trichlorfon at an oral dose of 100 mg/kg bw per
day for 6 days during gestation had abortions and stillborn fetuses,
and the offspring showed reduced body weight and brain weight,
locomotor disturbances, and morphological and biochemical alterations
in the brain.
In two studies of developmental toxicity in rabbits, oral doses
of 5-110 mg/kg bw per day were given. Overt toxicity and inhibition of
erythrocyte acetylcholinesterase activity were observed in dams given
doses of 35 mg/kg bw per day and above, and body-weight gain was
affected at all doses. The highest dose, 110 mg/kg bw per day,
slightly increased the rate of resorptions and retarded fetal
development, but no fetal abnormalities were found. A NOEL for
maternal toxicity was not identified. The NOEL for developmental
effects was 45 mg/kg bw per day on the basis of toxicity to embryos
and fetuses.
Several outbreaks of congenital tremor with cerebellar hypoplasia
were reported in piglets of sows that had been treated with
trichlorfon, and the syndrome was subsequently reproduced
experimentally. The piglets of sows treated with trichlorfon at doses
of 50-100 mg/kg bw per day during appropriate periods of gestation
showed neurological signs and hypoplasia and loss of Purkinje cells in
the cerebellum.
Rats given a dose equivalent to 200 mg/kg bw per day for 13 weeks
showed decreased locomotor activity and loss of coordination. In
another study in rats, a dose of 30 mg/kg bw per day for 3 weeks
increased locomotor activity and decreased learning ability and nerve
conduction velocity.
The results of numerous studies in hens consistently demonstrated
the acute neurotoxicity of trichlorfon. Delayed neurotoxicity,
associated with degeneration of nervous tissue and marked inhibition
of neuropathy target esterase, was not seen in hens. A monkey given a
single oral dose of 250 mg/kg bw showed impaired nerve conduction 4
weeks after treatment and histological evidence of demyelination of
nerves and axonal degeneration.
Trichlorfon has been used as an anthelmintic agent in humans.
Oral doses of up to 10 mg/kg bw given on two to four occasions were
well tolerated, with few clinical symptoms. A dose of 24 mg/kg bw
caused significant cholinergic symptoms, but the effects were not
cumulative. In a few cases, spermatogenesis appeared to have been
impaired. The results of clinical trials with trichlorfon in the
treatment of Alzheimer disease showed dose-related but reversible
muscle weakness. The recommended dose of 0.5-0.9 mg/kg bw resulted in
a small increase in the frequency of generalized weakness and fatigue,
while doses of 1.25 mg/kg bw and higher produced significant weakness.
Dose-related inhibition of erythrocyte acetylcholinesterase activity
was also observed in these studies.
In 121 subjects, an initial dose of 0.5 mg/kg bw per day for 2
weeks inhibited erythrocyte acetylcholinesterase activity by 29%.
Subsequent administration of a dose of 0.2 mg/kg bw per day for
10 weeks maintained the inhibition of erythrocyte acetylcholinesterase
activity at levels between 30 and 37%, with no increase with duration
of dosing. Since this dose enhanced the inhibition caused by the
initial dose by only 8%, an insignificant change, the Committee
concluded that the NOEL was 0.2 mg/kg bw per day.
In some cases of severe human poisoning with trichlorfon,
weakness and loss of feeling in the extremities, difficulty in
walking, muscular atrophy, and motor nerve damage have been observed.
In many of these cases, the doses might have been lethal in the
absence of medical intervention. The Committee concluded that
extremely high doses of trichlorfon would be required to achieve the
level of inhibition of neuropathy target esterase associated with
delayed neurotoxicity.
4. EVALUATION
The Committee concluded that inhibition of acetylcholinesterase
activity was the most relevant end-point for establishing an ADI. The
most appropriate NOEL was 0.2 mg/kg bw per day for inhibition of
erythrocyte acetylcholinesterase activity in humans treated orally. A
safety factor of 10 was applied to this figure, giving an ADI of 0-20
µg/kg bw.
5. REFERENCES
Abdel Aal, A.M.A., El-Hawary, M.F.S., Kamel, H., Abdel-Khalek, M.K. &
El-Diwany, K.M. (1970) Blood cholinesterases, hepatic renal and
haemopoietic functionsin children receiving repeated doses of
'Dipterex'. J. Egypt. Med. Assoc., 53, 265-271.
Aden-Abdi, Y., Villen, T., Ericsson, O., Gustafsson, L.L. &
Dahl-Puustinen, M.L. (1990) Metrifonate in healthy volunteers:
Interrelationship between pharmacokinetic properties,
cholinesterase inhibition and side effects. Bull. World Health
Organ., 68, 731-736.
Adler, B., Braun, R., Schöneich, J. & Böhme, H. (1976)
Repair-defective mutants of Proteus mirabilis as a prescreening
system for the detection of potential carcinogens. Biol. Zbl.,
95, 463- 469.
Akhtar, M.H. (1982) Fate of trichlorfon in buffer and soluble fraction
(105 000 g) from cow and chicken liver homogenates. J. Agric.
Food Chem., 30, 551-554.
Akimov, G.A., Buchko, V.M. & Kremleva, R.V. (1975) Neurological
disorders in chlorophos poisoning. Klin. Med., 5, 65-69
( Pestic. Abstr., 75-2380).
Aquilina, G., Benigni, R., Bignami, M., Calcagnile, A., Dogliotti, E.,
Falcone, E. & Carere, A. (1984) Genotoxic activity of dichlorvos,
trichlorfon, and dichloroacetaldehyde. Pestic. Sci., 15,
439-442.
Arthur, B.W. & Casida, J.E. (1957) Metabolism and selectivity of
O,O-dimethyl 2,2,2-trichloro-1-hydroxyethylphosphonate and its
acetyl and vinyl derivatives. J. Agric. Food Chem., 5, 186-192.
Averbook, B.J. & Anderson, R.J. (1983) Electrophysiological changes
associated with chronic administration of organophosphates.
Arch. Toxicol., 52, 167-172.
Bao, T.V., Szabo, I., Ruzicska, P. & Czeizel, A. (1974) Chromosome
aberrations in patients suffering acute organic phosphate
insecticide intoxication. Humangenetik, 24, 33-57.
Barrueco, C., Herrera, A. & De La Pena, E. (1991) Mutagenic evaluation
of trichlorfon using different assay methods with Salmonella
typhimurium. Mutagenesis, 6, 71-76.
Batzinger, R.P. & Bueding, E. (1977) Mutagenic activities in vitro
and in vivo of five antischistosomal compounds. J. Pharmacol.
Exp. Ther., 200, 1-9.
Bayer AG (1999) Pro Mem (metrifonate) and muscle weakness. Unpublished
position paper from Bayer AG. Submitted to WHO by Bayer AG,
Germany.
Becker, K. & Schöneich, J. (1980) Dominant lethal test with
trichlorphon in male mice. Mutat. Res., 74, 224.
Becker, R.E., Colliver, J., Elble, R., Feldman, E., Giacobini, E.,
Kumar, V., Markwell, S., Moriearty, P., Parks, R., Shillcutt,
S.D., Unni, L., Vicari, S., Womack, C. & Zec, R.F. (1990) Effects
of metrifonate, a long-acting cholinesterase inhibitor, in
Alzheimer disease: Report of an open trial. Drug Dev. Res., 19,
425-434.
Benes, V. & Sram, R. (1969) Mutagenic activity of some pesticides in
Drosophila melanogaster. Ind. Med., 38, 50-52.
Berge, G.N. & Nafstad, I. (1986) Distribution and placental transfer
of trichlorfon in guinea-pigs. Arch. Toxicol., 59, 26-29.
Berge, G.N., Nafstad, I. & Fonnum, F. (1986) Prenatal effects of
trichlorfon on the guinea-pig brain. Arch. Toxicol., 59, 30-35.
Berge, G.N., Fonnum, F. & Brodal, P. (1987a) Neurotoxic effects of
prenatal trichlorfon administration in pigs. Acta Vet. Scand.,
28, 321-332.
Berge, G.N., Fonnum, F., Soli, N.E. & Sognen, E. (1987b)
Neurotoxicological examination of the piglet brain after prenatal
and postnatal exposure to trichlorfon. Acta Vet. Scand., 28,
313-320.
Bomann, W. & Kaliner, G. (1996) BAY a 9826 (metrifonate) and BAY z
7216, BAY z 7217. Study for delayed neurotoxicity following acute
oral administration to hens. Unpublished report no PH 25171 from
Bayer, Wuppertal, Germany. Submitted to WHO by Bayer AG, Germany.
Bond, G.P. (1986) Primary dermal irritation of technical trichlorfon
(Dylox) in albino rabbits. Unpublished report no 751 from Mobay
Corporation, USA. Submitted to WHO by Bayer AG, Germany.
Bootman, J. & Hodson-Walker, G. (1987) L13/59: Investigation of
effects on bone marrow chromosomes of the rat after acute oral
administration. Unpublished report no R4221 from Life Sciences
Research, United Kingdom. Submitted to WHO by Bayer AG, Germany.
Brzheskiy, V.V. (1973) [Induction of mutations in Drosophila
melanogaster.] Med. Parazitol. Parazit. Bolez., 42, 703-706
(in Russian).
Bull, D.L. & Ridgway, R.L. (1969) Metabolism of trichlorfon in animals
and plants. J. Agric. Food Chem., 17, 837-841.
Bulsiewicz, H., Rozewicka, L., Januszewska, H. & Bajko, J. (1976)
Aberrations of meiotic chromosomes induced in mice with
insecticides. Folia Morphol. (Warsaw), 35, 361-368.
Byeon, W.H., Hyun, H.H. & Lee, S.Y. (1976) Salmonella/microsomal
enzyme activation system. Mutagenicity of pesticides in the
Salmonella/microsome system. Korean J. Microbiol., 14,
128-134.
Carere, A., Ortali, V.A., Cardamone, G. & Morpurgo, G. (1978a)
Mutagenicity of dichlorvos and other structurally related
pesticides in Salmonella and Streptomyces. Chem.-Biol.
Interactions, 22, 297-308.
Carere, A., Ortali, V.A., Cardamone, G., Torracca, A.M. & Raschetti,
R. (1978b) Microbiological mutagenicity studies of pesticides
in vitro. Mutat. Res., 57, 277-286.
Chan, P.C. & Peters, A.C. (1989) Thirteen-week subchronic dosed feed
study of trichlorfon in Fischer rats and B6C3F1 mice. Proc.
Am. Assoc. Cancer Res., 30, 137.
Chen, H.H., Hsueh, J.L., Sirianni, S.R. & Huang, C.C. (1981) Induction
of sister chromatid exchanges and cell cycle delay in cultured
mammalian cells treated with eight organophosphorus pesticides.
Mutat. Res., 88, 307-316.
Chen, H.H., Sirianni, S.R. & Huang, C.C. (1982) Sister chromatid
exchanges in Chinese hamster cells treated with seventeen
organophosphorus compounds in the presence of a metabolic
activation system. Environ. Mutag., 4, 621-624.
Christenson, W.R. (1990) A combined chronic toxicity/oncogenicity
study of technical grade trichlorfon (Dylox) with rats.
Unpublished report no 5388 from Mobay Corporation, USA. Submitted
to WHO by Bayer AG, Germany.
Clemens, G.R., Troup, C.M. & Hartnagel, R.E., Jr (1990) Teratology
study in the rabbit with Dylox technical (Trichlorfon).
Unpublished report no MTD0134 from Miles Laboratories, USA.
Submitted to WHO by Bayer AG, Germany.
Courtney, K.D., Andrews, J.E. & Springer, J. (1986) Assessment of
teratogenic potential of trichlorfon in mice and rats.
J. Environ. Sci. Health, B21, 207-227.
Crawford, C.R. & Anderson, R.H. (1973) The acute oral and
intraperitoneal toxicity of five trichlorfon technical samples to
rats. Unpublished report no 37204 from Baychem Corporation.
Submitted to WHO by Bayer AG, Germany.
Cummings, J.L., Cyrus, P.A., Bieber, F., Mas, J., Orazem, J. &
Gulanski, B. (1998) Metrifonate treatment of the cognitive
deficits of Alzheimer's disease. Neurology, 50, 1214-1221.
De Freitas, M.R.G., Chimelli, L., Nascimento, O.J.M., Cincinatus, D.,
Marques, H.A. & Nevares, M.T. (1990) [Polyneuropathy induced by
trichlorfon. Report of a case with electrophysiology and
histopathology in the sural nerve.] Arq. NeuroPsiquiatr., 48,
515-519 (in Spanish).
Dedek, W. (1981) Guanine N7-alkylation in mice in vivo by
metrifonate -- Discussion of possible genotoxic risk in mammals.
Acta Pharmacol. Toxicol., 49 (Suppl. V), 40-50.
Dedek, W. & Lohs, K. (1970a) [Alkylating effect of trichlorfon in
mammals. I. Studies in vitro in human serum with
14C-trichlorfon.] Z. Naturforsch., B25, 94-96 (in German).
Dedek, W. & Lohs, K. (1970b) [Allkylkating effect of trichlorfon in
mammals. Distribution of 14C in organs and liver proteins of
rats after administration of 14Ctrichlorfon.]
Z. Naturforsch.,B25, 1110-1113 (in German).
Dedek, W., Scheufler, H., & Fischer, G.W. (1975) [Mutagenicity of
desmethyltrichlorfon in the dominant lethal test in the mouse.]
Arch. Toxicol., 33, 163-168 (in German).
Dedek, W., Lohs, K., Fischer, G.W. & Schmidt, R. (1976) Alkylation of
guanine in mice in vivo by organophosphorus insecticides. I.
Trichlorfone and butonate. Pestic. Biochem. Physiol., 6,
101-110.
Degraeve, N., Chollet, M.-C., Moutschen, J., Moutschen-Damen, M.,
Gilot-Delhalle, J. & Colizzi A. (1982) Genetic and cytogenetic
effects of chronic treatments with organophosphorous
insecticides. Mutat. Res., 97, 179-180.
Degraeve, N., Chollet, M.-C. & Moutschen, J. (1984) Cytogenetic and
genetic effects of subchronic treatments with organophosphorus
insecticides. Arch. Toxicol., 56, 66-67.
Diril, N., Sumer, S. & Izbirak, A. (1990) [A survey on the mutagenic
effects of some organophosphorous insecticides in the
Salmonella/microsome test system.] Doga Muhendislik Cevre
Bilimleri, 14, 272-279 (in Turkish).
Doull, J. & Rehfuss, P.A. (1956) The effects of diets containing
Dipterex on rats. Unpublished report No. 1376 from Department of
Pharmacology, University of Chicago, USA. Submitted to WHO by
Bayer AG, Germany.
Doull, J. & Vaughn, G. (1958) Measurement of the safe dietary level of
Dipterex for dogs. Unpublished report No. 2105 from Department of
Pharmacology, University of Chicago, USA. Submitted to WHO by
Bayer AG, Germany.
Doull, J., Root, M., Vesselinovitch, D., Meskauskas, J., & Fitch, F.
(1962) Chronic oral toxicity of Dylox to male and female dogs.
Unpublished report No. 8644 from Department of Pharmacology and
Pathology, University of Chicago, USA. Submitted to WHO by Bayer
AG, Germany.
Dreist, M. & Mihail, F.L. (1990) L 13/59 (trichlorfon). Synoptic
evaluation of the toxicological data. Unpublished report no 20033
from Bayer, WuppertalElberfeld, Germany. Submitted to WHO by
Bayer AG, Germany.
Dubois, K.P. & Cotter, G.J. (1955) Studies on the toxicity and
mechanism of action of dipterex. Arch. Ind. Health, 11, 53-60.
Dzwonkowska, A. & Hubner, H. (1986) Induction of chromosomal
aberrations in the Syrian hamster by insecticides tested
in vivo. Arch. Toxicol., 58, 152-156.
Eljasz, L. & Kryzyszton-Przekop, T. (1975) [Polyneuropathy as a result
of foschlor poisoning.] Pol. Tyg. Lek., 30, 1841-1842 ( Pest.
Abstr. 76-1688) (in Polish).
Epstein, S.S., Arnold, E., Andrea, J., Bass, W. & Bishop, Y. (1972)
Detection of chemical mutagens by the dominant lethal assay in
the mouse. Toxicol. Appl. Pharmacol., 23, 288-325.
FAO/WHO (1972a) Pesticide residues in food. Report of the 1971
Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues. FAO Agricultural Series, No. 88; WHO Technical Report
Series, No. 502, Rome/Geneva.
FAO/WHO (1972b) 1971 Evaluations of some pesticide residues in
food. AEP-1971/M/13; WHO Pesticide Residues Series, No. 5,
Rome/Geneva.
FAO/WHO (1976a) Pesticide residues in food. Report of the 1975
Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on
Pesticide Residues. FAO Plant Production and Protection Series,
No. 1; WHO Technical Report Series, No. 592, Rome/Geneva.
FAO/WHO (1976b) 1975 Evaluations of some pesticide residues in
food. AEP-1975/M/9/1; WHO Pesticide Residues Series, No. 1,
Rome/Geneva.
FAO/WHO (1979a) Pesticide residues in food--1978. Report of the
Joint Meeting of the FAO Panel of Experts on Pesticide
Residues and Environment and the WHO Expert Group on
Pesticide Residues. FAO Plant Production and Protection Paper
15, Rome.
FAO/WHO (1979b) Pesticide residues in food: 1978 Evaluations. FAO
Plant Production and Protection Paper 15 Suppl., Rome.
Fischer, G.W., Schneider, P. & Scheufler, H. (1977) [Mutagenicity of
dichloroacetaldehyde and methyl-2,2-dichloro-1,1-dihydroxyethane
phosphonate, possible trichlorfon metabolites.] Chem.-Biol.
Interactions, 19, 205-213 (in German).
Fukuhara, N., Hoshi, M. & Mori, S. (1977) Core/targetoid fibres and
multiple cytoplasmic bodies in organophosphate neuropathy.
Acta Neuropathol., 40, 137-144.
von Gibel, W., Lohs, K., Wildner, G.P. & Ziebarth, D. (1971) [Animal
experimental studies on the hepatotoxic and carcinogenic activity
of organophosphorus compounds.] Arch. Geschwülstforsch., 37,
303-312 (in German).
von Gibel, W., Lohs, K., Wildner, G.P., Ziebarth, D. & Stieglitz, R.
(1973) [Carcinogenicity, haematoxicity and hepatotoxicity of
organophosphorus compounds.] Arch. Geschwülstforsch., 41,
311-328 (in German).
Gilot-Delhalle, J., Colizzi, A., Moutschen, J. & Moutschen-Dahmen, M.
(1983) Mutagenicity of some organophosphorus compounds at the
ade6 locus of Schizosaccharomyces pombe. Mutat. Res., 117,
139-148.
Griffin, T.B. (1988) Safety evaluation and tumorigenesis of
trichlorfon in rhesus monkeys: A ten-year study. Unpublished
report from White Sands Research Center, USA. Submitted to WHO by
Bayer AG, Germany.
Griffin, T.B., Coulston, F. & Stein, A.A. (1982) Trichlorfon.
Tumorigenicity study in the rat (2 generation). Unpublished
report from White Sands Research Center, USA. Submitted to WHO by
Bayer AG, Germany.
Grundmann, E. & Hobik, H.P. (1966) Bay 15922. 2 year feeding
experiment on rats. Histology. Unpublished report addendum from
Bayer, Wuppertal-Elberfeld. Submitted to WHO by Bayer AG,
Germany.
Guerzoni, M.E., Del Cupolo, L. & Ponti, I. (1976) [Mutagenic activity
of pesticides.] Riv. Sci. Techn. Alim. Nutr. Um., 6, 161-165
(in Italian).
Haseman, J.K., Winbush, J.S. & O'donnell, M.R. (1986) Use of dual
control groups to estimate false positive rates in laboratory
animal carcinogenicity studies. Fundam. Appl. Toxicol., 7,
573-584.
Hassan, A. & Zayed, S.M.A.D. (1965) Metabolism of organophosphorus
insecticides. III. Fate of the methyl groups of Dipterex
in vivo. Can. J. Biochem., 43, 1271-1275.
Hassan, A., Zayed, S.M.A.D. & Abdel-Hamid, F.M. (1965a) Metabolism of
organophosphorus insecticides. II. Metabolism of
O,O-dimethyl-2,2,2-trichloro-1hydroxyethyl phosphonate (Dipterex)
in mammalian nervous tissue and kinetics involved in its reaction
with acetyl cholinesterase. Can J. Biochem., 43, 1263-1269.
Hassan, A., Zayed, S.M.A.D. & Hashish, S. (1965b) Metabolism of
organophosphorus insecticides. VI. Mechanism of detoxification of
Dipterex in the rat. Biochem. Pharmacol., 14, 1692-1694.
Hayes, R.H. (1985) Pilot study on trichlorfon with mice. Unpublished
report No. 656 from Mobay Corporation, USA. Submitted to WHO by
Bayer AG, Germany.
Hayes, R.H. (1988) Oncogenicity study of technical grade trichlorfon
with mice. Unpublished report No. 1039 from Mobay Corporation,
USA. Submitted to WHO by Bayer AG, Germany.
Hayes, R.H. (1989) Chronic toxicity/oncogenicity study of technical
grade trichlorfon (Dylox) with rats. Unpublished report No. 1108
from Mobay Corporation, USA. Submitted to WHO by Bayer AG,
Germany.
Hayes, R.H. & Ramm, W.W. (1987) Subchronic delayed neurotoxicity study
of trichlorfon technical (Dylox) with hens. Unpublished report
No. 939 from Mobay Corporation, USA. Submitted to WHO by Bayer
AG, Germany.
Heimann, K.G. (1985) [Dipterex (L 13/59, techn.), determination of
acute toxicity (LD50).] Unpublished letter from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany (in German).
Heimann, K.G. & Wood, C.M. (1987) Trichlorfon technical. Study of
subacute dermal toxicity to rabbits. Unpublished report No. R
4150 from Bayer, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Germany.
Herbold, B. (1979a) L 13/59. Micronucleus test in the mouse for
evaluation of mutagenicity. Unpublished report No. 8505 from
Bayer, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Germany (in German).
Herbold, B. (1979b) [Appraisal of dominant lethal test on trichlorfon
(Dipterex).] Unpublished report from Martin Luther Universitat,
Germany. Submitted to WHO by Bayer AG, Germany (in German).
Herbold, B. (1979c) L 13/59. Dominant lethal study on male mouse to
test for mutagenic effects. Unpublished report No. 8745 from
Bayer, Wuppertal, Germany. Submitted to WHO by Bayer AG, Germany.
Herbold, B. (1984) [L 13/59. Pol test in E. coli to investigate DNA
damage effects.] Unpublished report No. 12434 from Bayer,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Germany (in
German).
Herbold, B. (1986) L 13/59. Cytogenetic study with human lymphocyte
cultures in vitro to evaluate for harmful effects on
chromosomes. Unpublished report No. 14837 from Bayer, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Germany.
Herbold, B. (1992) L 13/59. In vivo cytogenetic study of the
spermatogonia in Chinese hamster to evaluate for induced
clastogenic effects. Unpublished report No. 21688 from Bayer,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Germany.
Herbold, B. (1997) BAY a 9826, BAY z 7216, BAY z 7217 Micronucleus
tests on the mouse. Unpublished report No. PH 26329 from Bayer,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Germany.
Hierons, R. & Johnson, M.K. (1978) Clinical and toxicological
investigations of a case of delayed neuropathy in man after acute
poisoning by an organophosphorus pesticide. Arch. Toxicol., 40,
279-284.
Hirakawa, T. (1983a) Acute toxicity study of trichlorfon on mice by
oral, intraperitoneal, subcutaneous and percutaneous route.
Unpublished report ID 17258 from Bozo Research Centre, Japan.
Submitted to WHO by Bayer AG, Germany.
Hirakawa, T. (1983b) Acute toxicity study of trichlorfon on rats by
oral, intraperitoneal, subcutaneous and percutaneous route.
Unpublished report ID 17259 from Bozo Research Centre, Japan.
Submitted to WHO by Bayer AG, Germany.
Hobik, H.P. (1967) [Histological investigation of spinal cord and
sciatic nerves from neurotoxicity experiemnts in hens dosed with
Dipterex.] Unpublished report no 446 from Bayer, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Germany (in German).
Hoffmann, K., Ruf, J. & Mager, H. (1988) [Chronic toxicity in rhesus
monkeys dosed oraqlly for 26 weeks (gavage).] Unpublished study
No. 16545 from Bayer, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Germany (in German).
Hoorn, A.J.W. (1983) Mutagenicity evaluation of L 13/59 in the reverse
mutation induction assay with Saccharomyces cerevisiae strains
S138 and S211alpha. Unpublished report No. R2540 from Litton
Bionetics, Netherlands. Submitted to WHO by Bayer AG, Germany.
IARC (1983) Trichlorfon. In: IARC Monographs on the Evaluation of
the Carcinogenic Risk of Chemicals to Humans, Vol. 30,
Miscellaneous pesticides, Lyon, International Agency for
Research on Cancer, pp. 207-231.
IARC (1987) IARC Monographs on the Evaluation of the Carcinogenic
Risk of Chemicals to Humans, Suppl. 7, Overall Evaluations
of Carcinogenicity: An Updating of IARC Monographs Volumes 1
to 42, Lyon, International Agency for Research on Cancer,
p. 73.
Inukai, H. & Iyatomi, A. (1977) Trichlorfon. Mutagenicity test on
bacterial systems. Unpublished report No. 87 from Nitokuno
Agricultural Chemicals Institute, Japan. Submitted to WHO by
Bayer AG, Germany.
Jewsbury, J.M. (1981) Metrifonate in schistosomiasis therapy and
prophylaxis. Acta Pharmacol. Toxicol., 49 (Suppl. V),123-130.
Johnson, M.K. (1981) Delayed neurotoxicity -- Do trichlorphon and/or
dichlorvos cause delayed neuropathy in man or in test animals?
Acta Pharmacol. Toxicol., 49 (Suppl. V), 87-98.
Jones, D.C.L., Simmon, V.F., Mortelmans, K.E., Mitchell, A.D., Evans,
E.L., Jotz, M.M., Riccio, E.S., Robinson, D.E. & Kirkhart, B.A.
(1984) In vitro and in vivo mutagenicity studies of
environmental chemicals. Report from SRI International, USA, for
Environmental Protection Agency (EPA-600/1-84-003). National
Technical Information Service, USA.
Kawauchi, S. (1997) BAY a 9826 single oral administration toxicity
study in dogs. Unpublished report No. R 6836 from Experimental
Biomedical Research, Japan. Submitted to WHO by Bayer AG,
Germany.
Kimmerle, G. (1970) Metrifonat (Bayer 2349), toxicological
investigations. Unpublished report No. 2142 from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Kimmerle, G. (1975a) L 13/59, acute inhalation toxicity study on rats.
Unpublished report No. 5581 from Bayer, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Germany.
Kimmerle, G. (1975b) L 13/59, subacute inhalation toxicity study on
rats. Unpublished report No. 5582 from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Klimmer, O.R. (1955) [Final report on the new insecticide Bayer L
13/59.] Unpublished report from Pharmakologisches Institut, Bonn,
Germany. Submitted to WHO by Bayer AG, Germany (in German).
Kiraly, J., Szentesi, I., Ruzicska, M. & Czeize, A. (1979) Chromosome
studies in workers producing organophosphate insecticides.
Arch. Environ. Contam. Toxicol., 8, 309-319.
Knox, B., Askaa, J., Basse, A., Bitsch, V., Eskildsen, M., Mandrup,
M., Ottosen, H.E., Overby, E., Pedersen, K.B. & Rasmussen, F.
(1978) Congenital ataxia and tremor with cerebellar hypoplasia in
piglets borne by sows treated with Neguvon(R) vet (Metrifonate,
Trichlorfon) during pregnancy. Nord. Vet. Med., 30, 538-545.
Kowalski, R.L., Clemens, G.R., Bare, J.J. & Hartnagel, R.E., Jr (1987)
A teratology study with Dylox (R), technical (Trichlorfon) in the
rat. Unpublished report No. MTD0017 from Miles Laboratories, USA.
Submitted to WHO by Bayer AG, Germany.
Kurinnyi, A.I. (1975) Comparative study of the cytogenetic effect of
certain organophosphorus pesticides. Genetika, 11, 64-69.
Lamb, M.J. (1977) Mutagenicity tests with metrifonate in
Drosophila melanogaster. Mutat. Res., 56, 157-162.
Lehotzky, K. (1982) Effect of pesticides on central and peripheral
nervous system function in rats. Neurobehav. Toxicol.
Teratol., 4, 665-669.
Lorke, D & Kimmerle, G. (1966) Neurotoxicity studies on hens with
Dipterex active ingredient. Unpublished report from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Lorke, D. & Loser, E. (1966) Chronic toxicological studies on rats.
Unpublished report No. 19292 from Bayer, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Germany.
Loser, E. (1969) BAY 15922 Generation study on rats. Unpublished
report No. 1195 from Bayer, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Germany.
Loser, E. (1970) Bay 15922. Chronic toxicological studies on dogs.
Unpublished report No. 2467 from Bayer, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Germany.
Machemer, L. (1979a) [L 13/59 (trichlorfon): Evaluation for
embryotoxic and teratogenic effects on orally dosed rats.]
Unpublished report No. 8400 from Bayer,Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Germany (in German).
Machemer, L. (1979b) [L 13/59 (trichlorfon): Evaluation for
embryotoxic and teratogenic effects on orally doses rabbits.]
Unpublished report No. 8430 from Bayer,Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Germany (in German).
Martson, L.V. & Voronina, V.M. (1976) Experimental study of the effect
of a series of phosphoroorganic pesticides (dipterex and imidan)
on embryogenesis. Environ. Health Perspectives, 13, 121-125.
Metcalf, R.L., Fukuto, T.R. & March, R.B. (1959) Toxic action of
Dipterex and DDVP to the house fly. J. Econ. Entomol., 52,
44-49.
Mihail, F. (1978) [L 13/59 (Dipterex active ingredient), acute
toxicological studies.] Unpublished report No. 7619 from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany (in German).
Mihail, F. (1986) L 13/59 (trichlorfon): Study for skin-sensitizing
effect on guineapigs in the open epicutaneous test. Unpublished
report No. 14348 from Bayer, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Germany.
Miyamoto, J. (1959) Non-enzymatic conversion of Dipterex into DDVP and
their inhibitory action on enzymes. Botyu-Kagaku, 24, 130-137.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Moutschen-Dahmen, J., Moutschen-Dahmen, M. & Degraeve, N. (1981)
Metrifonate and dichlorvos: Cytogenetic investigations.
Acta Pharmacol. Toxicol., 49 (Suppl. V), 29-39.
Myhr, B.C. (1983) Evaluation of L 13/59 trichlorfon in the primary rat
hepatocyte unscheduled DNA synthesis assay. Unpublished report
No. R2687 from Litton Bionetics, USA. Submitted to WHO by Bayer
AG, Germany.
Nehes, M., Selypes, A. & Scheufler, H. (1982)[ Mutagenicity of
trichlorfon and dimethoate on mouse bone marrow cells in vivo].
Wissensch. Z. Univ. Halle, 31, 57- 62 (in German).
Niedziella, S.W., Göpel, W. & Banzhaf, E. (1985) [Acute alkylphosphate
poisoning (trichlorphon) with delayed polyneuropathy syndrome.]
Z. Ges. Inn. Med., 40, 237-239 (in German).
Nordgren, I., Bergström, M., Holmstedt, B. & Sandoz, M. (1978)
Transformation and action of metrifonate. Arch. Toxicol., 41,
31-41.
Nordgren, I., Holmstedt, B., Bengtsson, E. & Finkel, Y. (1980) Plasma
levels of metrifonate and dichlorvos during treatment of
schistosomiasis with Bilarcil. Am. J. Trop. Med. Hyg., 29,
426-430.
Nordgren, I., Bengtsson, E., Holmstedt, B. & Pettersson, B.M. (1981)
Levels of metrifonate and dichlorvos in plasma and erythrocytes
during treatment of schistosomiasis with Bilarcil. Acta
Pharmacol. Toxicol., 49 (Suppl. V), 79-86.
Olajos, E.J., Rosenblum, I., Coulston, F. & Strominger, N. (1979) The
dose response relationship of trichlorfon neurotoxicity in hens.
Ecotoxicol. Environ. Saf., 3, 245-255.
Paik, S.G. & Lee, S.Y. (1977) Genetic effects of pesticides in the
mammalian cells. 1. Induction of micronucleus. Korean J.
Zool., 20, 19-28.
Plestina, R., Davis, A. & Bailey, D.R. (1972) Effect of metrifonate on
blood cholinesterases in children during treatment of
schistosomiasis. Bull. World Health Organ., 46, 747-759.
Pollingher, B., Cozma, V. & Oprisan, C. (1973) Clinical and
electromyographic study of two cases of severe polyneuritis
following acute poisoning with Neguvon (organophosphoric
pesticide). Electroencephalogr. Clin. Neurophysiol., 35, 433
( Pestic. Abstr., 75-0322).
Pope, A.M., Heavner, J.E., Guarnieri, J.A. & Knobloch, C.P. (1986)
Trichlorfoninduced congenital cerebellar hypoplasia in neonatal
pigs. J. Am. Vet. Med. Assoc., 189, 781-783.
Putman, D.L. (1987) Sister chromatid exchange assay in Chinese hamster
ovary (CHO) cells. Unpublished report No. 826 from
Microbiological Associates, USA. Submitted to WHO by Bayer AG,
Germany.
Ray, E.R. (1998) Chronic effects of low level exposure to
anticholinesterases -- A mechanistic review. Toxicol. Lett.,
102-103, 527-533.
Reiner, E., Krauthacker, B., Simeon, V. & Skrinjaric-Spoljar, M.
(1975) Mechanism of inhibition in vitro of mammalian
acetylcholinesterase and cholinesterase in solutions of
O,O-dimethyl-2,2,2-trichloro-1-hydroxyethyl phosphonate
(trichlorphon). Biochem. Pharmacol., 24, 717-722.
Reiss, C.S., Herrman, J.M. & Hopkins, R.E., II (1987) Effect of
antihelminthic treatment on the immune response of mice.
Lab. Anim. Sci., 37, 773-775.
Renhof, M. (1997) BAY a 9826: Acute toxicity in the mouse and rat
after oral administration and in the mouse after intravenous
administration. Unpublished report No. 25832 from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Rosenblum, I. (1981) A two-generation, two-year feeding study of
trichlorfon in the rat. Unpublished report No. 2142 from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Sasaki, M., Sugimura, K., Yoshida, M. A. & Abe, S. (1980) Cytogenetic
effects of 60 chemicals on cultured human and Chinese hamster
cells. Kromosomo, 20, 574-584.
Scheufler, H. (1975) [Effect of relatively high doses of dimethoate
and trichlorfon on embryogenesis in mice.] Biol. Rundsch., 13,
238-240 (in German).
Sheets, L.P. & Hamilton, B.F. (1995) A subchronic dietary
neurotoxicity screening study with technical grade trichlorfon
(Dylox, Dipterex) in Fischer 344 rats. Unpublished report No.
MRC-00284 from Bayer Corp., USA. Submitted to WHO by Bayer AG,
Germany.
Shiraishi, S., Goto, I., Yamashita, Y., Ohnishi, A. & Nagao, H. (1977)
Dipterex polyneuropathy. Neurol. Med., 6, 34-38.
Shiraishi, S., Inoue, N. & Murai, Y. (1982) Dipterex poisoning.
J. Univ. Occup. Environ. Health, 4, 177-183.
Shiraishi, S., Inoue, N., Murai, Y., Onishi, A., & Noda, S. (1983)
Dipterex (trichlorfon) poisoning -- Clinical and pathological
studies in human and monkeys. J. Univ. Occup. Environ.
Health, 5 (Suppl.), 125-132.
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A. & Kada, T. (1976)
Mutagenicity screening of pesticides in the microbial system.
Mutat. Res., 40, 19-30.
Shirasu, Y., Moriya, M. & Koyashiki, R. (1979) Trichlorfon.
Mutagenicity test on bacterial systems. Unpublished report from
Institute of Environmental Toxicology, Japan. Submitted to WHO by
Bayer AG, Germany.
Slott, V. & Ecobichon, D.J. (1984) An acute and subacute neurotoxicity
assessment of trichlorfon. Can. J. Physiol. Pharmacol., 62,
513-518.
Spicer, E.J.F. & Payne, S. (1971) Pathology report of Bay 15922 4-year
dog study. Unpublished report from Huntingdon Research Centre,
United Kingdom. Submitted to WHO by Bayer AG, Germany.
Spicer, E.J.F. & Urwin, C (1971) Pathology report of Bay 15922
Generation experiment in rats. Unpublished report from Huntingdon
Research Centre, England. Submitted to WHO by Bayer AG, Germany.
Staples, R.E. & Goulding, E.H. (1979) Dipterex teratogenicity in the
rat, hamster and mouse when given by gavage. Environ. Health
Perspectives, 30, 105-113.
Staples, R.E., Kellam, R.G. & Haseman, J.K. (1976) Developmental
toxicity in the rat after ingestion or gavage of organophosphate
pesticides (dipterex, imidan) during pregnancy. Environ.
Health Perspectives, 13, 133-140.
Stieglitz, R., Gibel, W., Werner, W. & Stobbe, H. (1974) Experimental
study on haematotoxic and leukaemogenic effects of trichlorophone
and dimethoate. Acta Haematol., 52, 70-76.
Suzuki, T. (1996) Three-month repeated oral administration toxicity
study and recovery test of BAY a 9826 in dogs. Unpublished report
No. R 6641 from JBC, Gifu Research Laboratory, Japan. Submitted
to WHO by Bayer AG, Germany.
Teichmann, B. & Hauschild, F. (1978) [Test of O,O-dimethyl
(1-hydroxy-2,2,2trichloroethyl)phosphonate (trichlorfon) for
carcinogenicity in mice after oral (oesophageal-gastric gavage)
and dermal application.] Arch. Geschwulstforsch., 48, 301-307
(in German).
Teichmann, B. & Schmidt, A. (1978) [Test of O,O-dimethyl
(1-hydroxy-2,2,2trichlorethyl)phosphonate (trichlorphon) for
carcinogenicity in Syrian golden hamsters ( Mesocricetus
auratus Waterhouse) after intraperitoneal Injection.]
Arch. Geschwulstforsch., 48, 718-721 (in German).
Teichmann, B., Hauschild, F. & Eckelmann, A. (1978) [Test of
O,O-dimethyl(1hydroxy-2,2,2-trichlorethyl)phosphonate
(trichlorphon) for carcinogenicity in rats after oral
(oesophageal-gastric gavage) and intraperitoneal Injection.]
Arch. Geschwulstforsch., 48, 112-119.
Thyssen, J. (1981) L 13/59 (Dipterex active ingredient). Tests for
skin and eye irritation. Unpublished report from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Thyssen, J., Kaliner, G. & Eben, A. (1982) [Trichlorfon, Schmelzware
Japan, neurotoxicity studies in hens.] Unpublished report No.
10811 from Bayer, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Germany (in German).
Toyoshi, T. (1996) One-month repeated oral administration toxicity
study and recovery test of BAY a 9826 in rats. Unpublished report
No. R 6642 from JBC, Gifu Research Laboratory, Japan. Submitted
to WHO by Bayer AG, Germany.
Völkner, W. (1987) Sister chromatid exchanges (SCEs) in Chinese
hamster bone marrow cells. Unpublished report No. R4079 from
Technical University of Darmstadt, Germany. Submitted to WHO by
Bayer AG, Germany.
Watabe, H. (1997) A reverse mutation assay of metrifonate using
bacteria. Unpublished report No. R 6837 from Mitsubishi Yuka
Bio-Clinical Labs, Japan. Submitted to WHO by Bayer AG, Germany.
Wegner, D. (1970) Bilarcil (Bay 2349) Clinical experience 1960-1969
(survey of the literature). Unpublished report No. 2225 from
Bayer, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Germany.
WHO (1992) Trichlorfon (Environmental Health Criteria 132). Geneva,
World Health Organization.
Williams, M.W., Fuyat, H.N. & Fitzhugh, O.G. (1959) The subacute
toxicity of four organic phosphates to dogs. Toxicol. Appl.
Pharmacol., 1, 1-7.
Witter, R.F. & Gaines, T. B. (1963) Relationship between depression of
brain or plasma cholinesterase and paralysis in chickens caused
by certain organic phosphorus compounds. Biochem. Pharmacol.,
12, 1377-1386.
Witterland, W.F. (1984) Mutagenicity evaluation of L 13/59 in the
mouse lymphoma forward mutation assay. Unpublished report No.
R2824 from Litton Bionetics, Netherlands. Submitted to WHO by
Bayer AG, Germany.
Xu, H.H. & Schurr, K.M. (1990) Genotoxicity of 22 pesticides in
microtitration SOS Chromotest. Toxicol. Assess. Int. J., 5,
1-14.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T., Mortelmans, K. &
Speck, W. (1987) Salmonella mutagenicity tests: III Results from
the testing of 255 chemicals. Environ. Mutag., 9 (Suppl. 9),
1-109.
 Table 1. Results of studies of the acute toxicity of trichlorfon
Species Sex Route LD50 References
(mg/kg bw)
Mouse M&F Oral 616-950 Klimmer (1955); Mihail
(1978); FAO/WHO (1972b);
Hirakawa (1983a); Renhof
(1997)
Mouse M&F Intraperitoneal 344-600 Dubois & Cotter (1955);
Hirakawa (1983a); WHO
(1992)
Mouse M&F Subcutaneous 267360 FAO/WHO (1972b); Hirakawa
(1983a)
Mouse M&F Intravenous 354-370 Renhof (1997)
Mouse M&F Dermal > 5000 Hirakawa (1983a)
Rat M&F Oral 136-660 Dubois & Cotter (1955);
Klimmer (1955); Kimmerle
(1970); FAO/WHO (1972b);
Crawford & Anderson (1973);
Mihail (1978); Hirakawa
(1983b); Heimann (1985);
WHO (1992); Renhof (1997)
Rat M&F Intraperitoneal 156-485 Dubois & Cotter (1955);
Arthur & Casida (1957);
FAO/WHO (1972b); Crawford &
Anderson (1973); Hirakawa
(1983b)
Rat M&F Subcutaneous 290-400 FAO/WHO (1972b);
Hirakawa (1983b)
Rat M Intravenous 184 Kimmerle (1970)
Rat M&F Inhalation LC50, > 533 Kimmerle (1975a)
mg/m3
Rat M&F Dermal 2800-> 5000 Hirakawa (1983b); Mihail
(1978); WHO (1992)
Guinea-pig M&F Intraperitoneal 300 Dubois & Cotter (1955);
WHO (1992)
Rabbit Oral 160 FAO/WHO (1972b)
Rabbit M Dermal 5000 WHO (1992)
Dog M oral > 300-420 Mihail (1978); WHO (1992);
Kawauchi (1997)
fasciculations, and coma. The primary cause of death is respiratory
failure. After sublethal doses, animals recover very rapidly,
suggesting that trichlorfon is detoxified faster than many other
organophosphorus compounds (WHO, 1992).
In two separate studies, six New Zealand white rabbits were
treated dermally with trichlorfon for 24 h on intact and abraded skin.
No irritation was seen after 24, 48, or 72 h. Further animals received
trichlorfon in the eye for 5 min ( n = 5) or 24 h ( n = 3). Severe
redness was seen up to 72 h after dosing for either length of time.
Slight to moderate chemosis was also observed up to 48-72 h after
dosing in both groups. The effects did not persist to day 7, and no
effect was seen in the cornea or iris. The compound is a slight ocular
irritant (Thyssen, 1981; Bond, 1986).
Trichlorfon was assessed for skin sensitizing potential in eight
male BOR: DHPW guinea-pigs by the open epicutaneous test. Dermal
induction was carried out 5 days a week for 4 weeks with 0, 1, 3, or
10% trichlorfon, and dermal challenge was conducted with 0.3, 1, 3, or
10 % trichlorfon 4, 6, or 8 weeks after induction. No skin reactions
were seen in animals induced with 1% trichlorfon, but approximately
half the animals induced with 3 or 10% trichlorfon showed skin
reactions when challenged with each concentration of trichlorfon. The
compound was therefore considered to have skin sensitizing potential
(Mihail, 1986).
2.2.2 Short-term studies of toxicity and carcinogenicity
Mice
Trichlorfon (purity, 98.2%) was administered to groups of 15
Charles River CD-1 mice of each sex at a dietary concentration of 0,
100, 300, 900, or 2700 mg/kg for 8 weeks. There were no deaths or
drug-related clinical signs, and body weights were not affected. Food
consumption was not affected in males, but appeared to have been
increased by 18-32% in females. The report suggests that the latter
finding was due to increased wastage by the treated female mice.
Cholinesterase activity in plasma and erythrocytes, measured during
weeks 4, 6, and 8, was not significantly affected at the lowest dose
but was dose-dependently inhibited at higher doses; the maximum
inhibition was by about 70% for plasma and 50% for erythrocyte enzyme.
Brain acetylcholines-terase activity at the end of the study was
dose-dependently inhibited at doses > 300 mg/kg of diet, by up to
68%. Gross necropsy revealed no remarkable changes. Haematology, serum
biochemistry, urinary anlysis, organ weighing, and histopathology were
not performed (Hayes, 1985).
Groups of 10 male and 10 female B6C3F1 mice were given
trichlorfon (purity unknown) in the diet at a concentration of 0, 62,
180, 560, 1700, or 5000 mg/kg for 13 weeks. The results were reported
in abstract form only. All but one male at 5000 mg/kg of diet and one
female at 1666 mg/kg of diet survived. The body weights of mice at
5000 mg/kg of diet were reduced. Plasma and erythrocyte cholinesterase
activities were decreased in a dose-related manner, while motor
activity and grip strength were reduced at 1700 and 5000 mg/kg of
diet. The absolute and relative weights of the liver, kidney, and
spleen were increased in mice at 1700 and 5000 mg/kg of diet. The
neurotoxic effects and changes in organ weight were not accompanied by
pathological effects (Chan & Peters, 1989; WHO, 1992).
Rats
Groups of 10 male and 10 female rats were exposed for 6 h/day for
3 weeks (total, 15 exposures) to an atmosphere containing trichlorfon
(purity unknown) at a concentration of 0, 12.7, 35.4, or 103.5
mg/m3. The health of the animals at 103.5 mg/m3 was affected, but
details were not provided. Body-weight gain was unaffected, and
haematological, clinical chemical, and urinary parameters measured at
the end of the study were similar to those of controls. Plasma,
erythrocyte, and brain cholinesterase activities were inhibited in
males given 103.5 mg/m3 and in females given 35.4 and 103.5 mg/m3.
Gross and histopathological examinations showed no remarkable changes,
the only finding at autopsy being increased spleen weights in males at
35.4 and 103.5 mg/m3 (Kimmerle, 1975b; WHO,1992).
Groups of 12 male and 12 female Crj: CD SD rats were given
trichlorfon (purity, 100%) at a dose of 0, 25, 50, or 100 mg/kg bw per
day by gavage for 30 days. Further groups of 10 rats of each sex were
treated and then allowed a 2-week recovery period. Additional treated
groups of 20 rats of each sex were used to determine the profiles of
the drug in blood after dosing on days 1, 15, and 30. The peak
concentrations of the individual enantiomers of trichlorfon and
dichlorvos were dose-related and were reached by about 1 h, declining
rapidly to become undetectable by 24 h. The concentrations of each
enantiomer were roughly similar in males, while that of the
(-)-enantiomer was generally higher than that of the (+)enantiomer in
females; this was reflected in higher total drug concentrations in
females. The concentrations of dichlorvos in blood represented only a
small fraction of those of trichlorfon and were again higher in female
rats. There was no evidence of accumulation in blood.
The signs of toxicity included tremor, decreased locomotor
activity, salivation, bradypnoea, and prone position in many females
given 100 mg/kg bw per day, in one or two females given 50 mg/kg bw
per day, and in males given 100 mg/kg bw per day. There were no deaths
and no effects on body weight, food consumption, or ophthalmological
or urinary parameters. Slight decreases in erythrocyte counts and
haematocrit were seen in females at 100 mg/kg bw per day when compared
with controls at the end of treatment, but these values were not
significantly different from those of controls at the end of the
recovery period. Animals at 100 mg/kg bw per day had increased blood
concentrations of cholesterol, triglycerides, and phospholipids
(females), and increased activity of lactic dehydrogenase (males). At
autopsy, the livers of animals at 50 and 100 mg/kg bw per day were
heavier and/or enlarged and showed hypertrophy of centrilobular
hepatocytes. The weight of the kidney was increased in males at 100
mg/kg bw per day. The NOEL was 25 mg/kg bw per day (Toyoshi, 1996).
Groups of seven Wistar rats of each sex were given trichlorfon
(purity unknown) in the diet to provide a dose of 0, 1, 5, 10, 30, 50,
or 100 mg/kg bw per day for 12 weeks. The highest dose inhibited
cholinesterase activity in all tissues examined (erythrocytes, serum,
brain, heart, liver, and skeletal muscle), but the extent of the
inhibition differed. Nevertheless, no changes were detected in
nocturnal behaviour, reaction to external stimuli, conjunctival or
pinna reflexes, or the avoidance reflex to painful stimuli. No
significant histological alterations were observed (reviewed in WHO,
1992).
Groups of 10 male and 10 female Fischer rats were given
trichlorfon (purity unknown) in the diet at a concentration of 0, 62,
180, 560, 1700, or 5000 mg/kg for 13 weeks. The results were reported
in abstract form only. All rats survived. The body weights of those at
5000 mg/kg of diet were reduced. Plasma and erythrocyte cholinesterase
activities were lowered in a dose-related manner, while motor activity
and grip strength were reduced at 1700 and 5000 mg/kg of diet. The
absolute and relative weights of the liver, kidney, and spleen were
increased in animals at 1700 and 5000 mg/kg of diet. The neurotoxic
effects and changes in organ weights were not accompanied by
pathological effects (Chan & Peters, 1989; WHO, 1992).
Groups of 13 rats of each sex were fed diets containing
trichlorfon (purity unknown) at a concentration of 0, 20, 100, or 300
mg/kg for 16 weeks. No effects were observed on growth, behaviour,
food consumption, organ weights, or gross or microscopic appearance of
the tissues. At 300 mg/kg of diet, plasma and erythrocyte
cholinesterase activity was depressed by 20-30% in males and females,
while that of brain cholinesterase was depressed by 20% in females
only. Salivary gland cholinesterase activity was not inhibited. At 100
mg/kg of diet, a slight depression of plasma cholinesterase activity
was observed (Doull & Rehfuss, 1956; WHO, 1992).
Groups of 10 male Wistar albino rats were fed diets containing
trichlorfon (purity unknown) at 0, 1, 5, 25, or 125 mg/kg for 16
weeks. Erythrocyte cholinesterase activity was not depressed at any
dose, and no effects were seen on food consumption, growth, or gross
appearance of the tissues (reviewed in WHO, 1992).
Guinea-pigs
After oral administration of trichlorfon (purity unknown) at 100
mg/kg bw per day for 60 days to guinea-pigs, the haemoglobin
concentration but not the haematocrit was decreased. The activity of
serum cholinesterase was decreased by 50%, and that of alkaline
phosphatase was increased (reviewed in WHO, 1992).
Rabbits
Groups of five New Zealand white rabbits of each sex received
dermal applications of 0, 100, 300, or 1000 mg/kg bw per day
trichlorfon (purity, 99%) onto shaved skin. The drug was left on the
skin of restrained animals for 6 h/day, 5 days/week for 3 weeks. The
appearance and behaviour of the rabbits were unchanged, and there were
no deaths. Body weight, food consumption, and the skin at the
application sites were unaffected. There were no meaningful changes in
haematological, clinical chemical, or urinary parameters at the end of
the study. Plasma, erythrocyte, and brain cholinesterase activities
were inhibited by 20-30% in animals at 300 and 1000 mg/kg bw per day.
At autopsy, no treatment-related alterations in liver microsomal
enzyme activities, organ weights, or histological appearance were seen
(Heimann & Wood, 1987).
Dogs
Four dogs were fed diets containing trichlorfon (purity unknown)
for 12 weeks at a concentration of 20, 100, 300, or 500 mg/kg and were
then left for a further 4 weeks on unmedicated diet. All of the dogs
remained healthy and maintained their body weight throughout the
study. After 12 weeks of treatment, the erythrocyte and plasma
cholinesterase activities of animals at 300 and 500 mg/kg of diet were
decreased by 40-60% when compared with the concentrations before
treatment, and the activity in plasma was inhibited by 10% in animals
at 100 mg/kg of diet. On cessation of treatment, the enzyme levels
recovered within 2-4 weeks (Doull & Vaughn, 1958; FAO/WHO, 1972b).
Four dogs were fed diets containing trichlorfon (purity unknown)
at a concentration of 0, 50, 200, or 500 mg/kg for 12 weeks. Plasma
and erythrocyte cholinesterase activities were depressed within 2
weeks in animals at 500 mg/kg of diet, and the effect peaked at 6-8
weeks, at which time the inhibition was about 60% in plasma and 45% in
erythrocytes. A gradual increase in plasma cholinesterase activity was
then seen, to 45% inhibition at termination, even though the compound
was still present in the feed. At 200 mg/kg of diet, the
cholinesterase activity was slightly decreased but was within the
control range. The enzyme activities were restored 6 weeks after
treatment ceased (Williams et al., 1959; FAO/WHO, 1972b).
Groups of three male and three female beagles were given
trichlorfon (purity, 99.2%) in capsules at a dose of 0, 5, 15, or 45
mg/kg bw per day for 3 months. A further three animals of each sex
were given the vehicle or the high dose for 3 months and then allowed
to recover for 2 weeks. The blood concentrations of the enantiomers of
trichlorfon and dichlorvos were measured after dosing on days 1, 30,
and 90. Peak concentrations were achieved within 1 h, the
(-)-enantiomer being detected at 10-100-fold higher concentrations
than the (+)-enantiomer and dichlorvos. Disappearance from blood was
rapid, and the compounds were virtually undetectable by 7 h. The drug
did not accumulate in blood.
No deaths were observed in any group, and no meaningful effects
were seen on mean body weight, food intake, or in ophthalmological,
electrocardiographic, or auditory parameters. Dogs receiving 15 mg/kg
bw per day or more showed tremor, decreased locomotor activity, and
crouching, while at 45 mg/kg bw per day, ataxic gait, lateral
position, vomiting, salivation, licking, liquid, loose, or bloody
stools, and wasting were observed. The results of blood chemistry and
urinary analysis were unremarkable. Haemoglobin, haematocrit, and
erythrocyte count were slightly but progressively decreased during
treatment in males given 45 mg/kg bw per day. It was reported, with no
supporting data, that erythrocyte cholinesterase activity was
inhibited at 15 and 45 mg/kg bw per day. One dog at 45 mg/kg bw per
day lost weight, and at autopsy was found to have decreased thymus
weight with lymphocyte depletion in the cortex, small and immature
testes and prostate, and oligospermia in small epididymides. Similar
thymic changes were observed in one female at 45 mg/kg bw per day. All
of the effects were reversible on cessation of treatment. The NOEL was
5 mg/kg bw per day (Suzuki, 1996).
Monkeys
Groups of five rhesus monkeys of each sex were given trichlorfon
(purity, 98.7%) at a dose of 0, 0.1, or 0.2 mg/kg bw per day in
sterile water by oral intubation for 26 weeks in order to establish an
NOEL for inhibition of erythrocyte cholinesterase activity. There were
no deaths and no effects on appearance, behaviour, nutritional state,
feed or water consumption, or body-weight gain. Haematological tests
showed no changes. No compound-related effects on the liver or kidney
were seen in clinical chemical or urinary analyses, on gross
pathology, or in organ weights. At 0.2 mg/kg bw per day, small
increases and decreases in erythrocyte cholinesterase activity
(< 20%) were seen, which were within the range of biological
variation. The NOEL was 0.2 mg/kg bw per day, the highest dose tested
(Hoffman et al., 1988; WHO, 1992).
2.2.3 Long-term studies of toxicity and carcinogenicity
Mice
Three studies of carcinogenicity were performed with groups of 30
AB/Jena mice that received an oral dose of 0 or 30 mg/kg bw twice
weekly, an intraperitoneal dose of 0 or 28 mg/kg bw per day, or a
dermal application of 0 or 0.25 ml of a 1% solution, providing 2.5 mg
trichlorfon per animal. Dosing was continued for 75 weeks, and the
studies were terminated after 80 weeks. The treated animals showed
reduced body-weight gain and survival, but the tumour incidences were
not increased (Teichmann & Hauschild, 1978; FAO/WHO, 1979b).
Groups of 60 male and 60 female Charles River CD-1 mice were
given trichlorfon (purity unknown) in the diet at a concentration of
0, 100, 300, or 1000 mg/kg for 90 weeks, except for males at the
highest dose which were treated for 82 weeks. These doses were
equivalent to 15, 45, and 150 mg/kg bw per day. Inhibition of
body-weight gain was observed in females given 300 or 1000 mg/kg of
diet, and cholinesterase activity was depressed in animals of each sex
at 1000 mg/kg of diet. No significant microscopic alterations were
reported, and the tumour incidences were not treatment-related
(reviewed in WHO, 1992).
Groups of 50 Crl:CD-1(ICR)BR mice of each sex were given
trichlorfon (purity, 98.2%) in the diet at a concentration of 0, 280,
890, or 2700 mg/kg for 104 weeks, equal to 49, 160, and 510 mg/kg bw
per day for males and 66, 240, and 750 mg/kg bw per day for females.
Increased incidences of urine-stained fur, 'ear lesions' (males), and
vaginal discharge (females) were observed. The body weights of treated
females were increased at various times during the study, but a
consistent effect was observed only at the high dose. There were no
effects on survival, food intake, or haematological end-points. Plasma
and erythrocyte cholinesterase activities were depressed by 20-74% in
the groups given 890 and 2700 mg/kg of diet and in females given
280 mg/kg of diet. Brain cholinesterase activity was inhibited by
20-70% at all doses in a dose-related manner. The weights of the liver
were increased in females at the intermediate and high doses, but this
effect was not accompanied by microscopic changes. Pathological
examination revealed no treatment-related alterations (Hayes, 1988;
WHO, 1992).
Rats
von Gibel and co-workers undertook a series of studies in Wistar
rats (von Gibel et al.,1971, 1973; Stieglitz et al., 1974), which were
given trichlorfon three times per week orally at 30 mg/kg bw, twice
per week by subcutaneous injection at 30 mg/kg bw, twice per week
orally at 15 mg/kg bw, or twice per week by intraperitoneal injection
of 15 mg/kg bw. Treatment was continued for the lifetime of the
animals. The groups given 30 mg/kg bw were reported to have higher
incidences of liver necrosis, liver cirrhosis, and forestomach
papillomas than controls. The lower doses caused hyperplasia of the
blood-forming elements of bone marrow, extraosseal metaplasia in the
liver and spleen, and hepatotoxicity and carcinogenicity. JMPR
(FAO/WHO, 1972b, 1976b) and the International Agency for Research on
Cancer (IARC, 1983) concluded that these studies were difficult to
interpret because of lack of information on the methods and results
and the amalgamation of tumour types. Also, the findings contradicted
those of other studies. Therefore, little use could be made of these
studies .
Groups of 25 male and 25 female Sprague-Dawley rats were fed
diets containing trichlorfon (purity unknown) at a concentration of 0,
50, 250, 500, or 1000 mg/kg for 17 months for males and for 24 months
for females. These doses were equivalent to 2.5, 12, 25, and 50 mg/kg
bw per day. Survival was reduced at 1000 mg/kg of diet, and the
body-weight gain of males at this dose was retarded. Cholinesterase
activity was depressed in serum, erythrocytes, and submaxillary gland
but not in the brain of rats at 1000 mg/kg of diet; at 500 mg/kg of
diet, only serum enzyme activity was inhibited. Female rats fed 500 or
1000 mg/kg of diet had no primary follicules and had primitive ova,
while male rats fed 1000 mg/kg of diet showed depression of
spermatogenesis. Necrotizing arteritis was observed at the two highest
concentrations. The frequencies of mammary tumours in females, in
ascending order of dose, were 14, 8, 20, 21, and 25%. The time to
appearance of these tumours was reduced in a dose-related manner
(reviewed in FAO/WHO, 1972b).
Groups of 25 male and 50 female Sprague-Dawley rats were fed
diets containing trichlorfon (purity unknown) at a concentration of 0,
100, 200, or 400 mg/kg for 18 months, equivalent to 5, 10, and 20
mg/kg bw per day. A significant increase in the mortality rate in all
groups led to premature termination of the study at 70 weeks. Serum
and erythrocyte cholinesterase activities were inhibited at 400 mg/kg
of diet. Cystic granular alterations of the ovaries were seen at 200
and 400 mg/kg of diet, and ovarian atrophy, absence of primary
follicules, and primitive ova were noted at 400 mg/kg of diet. Benign
mammary tumours were found in 8% of controls, 11% of females at 200
mg/kg of diet, and 15% of females at 400 mg/kg of diet, with no change
in the time of their appearance. These results and those of the study
described above were considered to indicate that trichlorfon enhances
certain normal ageing processes, particularly in reproductive tissues
(reviewed in FAO/WHO, 1972b).
Groups of 50 FB30 rats of each sex were fed diets containing
trichlorfon (purity, 95.3%) at a concentration of 50, 250, 500, or
1000 mg/kg for 2 years, equivalent to 2.5, 12.5, 25, and 50 mg/kg bw
per day. The control group comprised 100 males and 100 females. No
changes in survival, general behaviour, appearance, body-weight gain,
or food consumption were reported. No significant differences in blood
count or urinary parameters were reported in samples obtained during
the final 3 weeks of the study. Tests of liver function showed an
increase in 'serum dehydrogenase' activity in males at 50 mg/kg of
diet and in females at 50 and 500 mg/kg of diet. Serum protein
concentration and alanine and aspartate aminotransferase activities
were unchanged. Acetylcholinesterase activity in 'total blood' was
decreased by approximately 20% at 1000 mg/kg of diet in week 90. No
macroscopic evidence of treatment-related changes was reported at
necropsy. Liver weights were significantly increased in males at 250
and 1000 mg/kg of diet, and spleen weights were significantly
increased in females at 50 and 1000 mg/kg of diet. Histological
examination was performed on five male and five female rats at 1000
mg/kg of diet and on three male and three female control animals. The
ovaries of an additional 15 treated and 30 control animals were
examined, as previous studies had indicated a possible effect. The
findings, including tumour incidences, were unrelated to treatment
(Lorke & Loser, 1966; Grundmann & Hobik, 1966; FAO/WHO, 1972b).
Groups of 30 male and 35 female albino rats received an oral dose
of 0 or 30 mg/kg bw or an intraperitoneal dose of 0 or 12 mg/kg bw of
trichlorfon (purity unknown) twice per week. Dosing was continued for
90 weeks, and the studies were terminated after 118 weeks. The treated
animals showed reduced survival, but the tumour incidences were not
increased (Teichmann et al., 1978; FAO/WHO, 1979b).
Trichlorfon (purity, 98.6-99.1%) was fed in the diet to groups of
75 Long-Evans rats of each sex at a concentration of 0, 100, 300, or
1000 mg/kg, equivalent to 5, 15, and 50 mg/kg bw per day. The animals
were maintained on their respective diets for 90 days and then mated
within groups. The resulting F1 litters were reared to 21 days
post partum, at which time 75 offspring of each sex per group were
selected to continue on the experiment. The F0 and selected F1
rats were treated for 2 years. There was no effect on fertility,
reproduction, food intake, body-weight gain, or mortality rate in
either generation. Plasma cholinesterase activity was depressed by
30-60% in both generations at 1000 mg/kg of diet at most times during
the study. Erythrocyte cholinesterase activity was reduced by 30-60%
in both generations given 1000 mg/kg of diet, at most sampling times
in females and on some occasions in males. Brain cholinesterase
activity was significantly lower (40%) in females given 1000 mg/kg of
diet and was 25% lower in F1 males. No histological changes
attributable to treatment were found (Rosenblum, 1981; Griffin et al.,
1982).
Groups of 50 Fischer 344 rats of each sex were given diets
containing trichlorfon (purity, 98.8%) for 2 years at a mean measured
time-weighted average concentration of 0, 92, 270, or 1500 mg/kg. The
nominal concentrations were 0, 100, 300, and, at the highest
concentration, 1000 mg/kg of diet for 27 weeks, 1250 mg/kg of diet for
5 weeks, 1500 mg/kg of diet for 8 weeks, and 1750 mg/kg of diet for 65
weeks. These doses are equal to 4.5, 13, and 76 mg/kg bw per day for
males and 5.8, 17, and 94 mg/kg bw per day for females. Satellite
groups of 20 animals of each sex in the control and high-dose groups
were killed after 1 year. The only noteworthy clinical signs were
paleness and hunched backs in males and rough coat in females at 1750
mg/kg of diet. Ophthalmic parameters were normal, and the mortality
rate was not affected. Females at 1750 mg/kg of diet showed slightly
decreased feed consumption and body-weight gain during the first 66
weeks, while the body weight of males at this dose was lower than that
of controls during the final 25 weeks of the study.
Hypercholesterolaemia occurred in males at 300 and 1750 mg/kg of diet
and in females at 1750 mg/kg of diet. Plasma, erythrocyte, and brain
cholinesterase activities were depressed by 20-40% at the highest
dose. Erythrocyte counts, haemoglobin concentration, and haematocrit
were slightly decreased at 1750 mg/kg of diet, but this anaemic
response had recovered in females by the end of the study. Urinary
parameters were not affected. At 1750 mg/kg of diet, the liver weights
were increased in males and females and the spleen weights were
increased in males, but with no associated pathological changes. The
kidney weights and the incidence of granular kidneys were increased at
1750 mg/kg of diet, corresponding to a slight enhancement in chronic
(age-related) nephropathy. The pathological changes included
hyperplasia of the upper small intestine and gastritis of the
non-glandular stomach in animals at 300 and 1750 mg/kg of diet and an
increased incidence of chronic lung inflammation in females at 1750
mg/kg of diet. The incidence of adrenal phaeochromocytomas was
increased in males at 1750 mg/kg of diet but was within the historical
control range. The NOEL was 92 mg/kg of diet, equal to 4.5 mg/kg bw
per day (Hayes, 1989; WHO, 1992).
Groups of 50 Fischer 344 rats of each sex were given diets
containing trichlorfon (purity, 98.5%) for 2 years at a nominal
concentration of 0 or 2500 mg/kg, equal to 130 mg/kg bw per day for
males and 160 mg/kg bw per day for females. Satellite groups of 20
animals of each sex per dose were killed after 1 year. This study was
initiated to ensure that trichlorfon had been tested at the maximum
tolerated dose. The body weights of treated animals were 10-15% lower
than those of controls throughout the study. Treated animals had
reduced food consumption during about the first half of the study, but
thereafter it was comparable to that of controls. The mortality rate
and ophthalmoscopic parameters were unaffected. The clinical signs in
treated animals included urine staining and enlarged abdomen in
animals of each sex and pale eyes and rough coat in males. Decreased
erythrocyte count, haemoglobin concentration, and haematocrit were
noted in treated rats but had recovered in males by the end of 2
years. Hypercholesterolaemia was seen in treated groups throughout the
study. The activities of gamma-glutamine transferase, alanine and
aspartate aminotransferases, and alkaline phosphatase in blood were
increased in treated males at various times but not at termination.
Urinary parameters were not affected. Statistically significant,
40-70% depressions in plasma, erythrocyte, and brain cholinesterase
activities were observed in animals of each sex at all times studied.
The liver and kidney weights were increased in treated groups,
and the lung weights were increased in females after 2 years; after 1
year, only changes in liver and kidney were reported. Histological
examination revealed duodenal hyperplasia, gastritis of the
non-glandular stomach, myodegeneration of the stomach tunica
muscularis, pulmonary inflammation and hyperplasia, hepatocellular
cytoplasmic vacuolation, focal hepatocellular hyperplasia associated
with sinusoidal dilatation and cystic degeneration (males),
nasolachrymal duct inflammation (females), chronic nephropathy
(females), and renal tubular hyperplasia. Non-statistically
significant increases in the incidences of renal tubular adenomas in
males and mononuclear-cell leukaemia in females were within the
historical control range. The increased incidences of
alveolar/bronchiolar adenomas in males and of carcinomas in females
were greater than the recorded historical control means, but the
numbers were small and did not attain statistical significance
(Christenson, 1990).
An increased incidence of tumours was thus detected in two
studies in rats conducted in the same laboratory. The increased
incidence of mammary tumours in females was slight, and the tumours
were benign. The strain used was Sprague-Dawley, which has a
relatively high, variable background incidence of mammary tumours
(Haseman et al., 1986). The lack of confirmation of this finding in
several studies in other species indicates that the evidence for the
carcinogenicity of trichlorfon in animals is limited.
Hamsters
A group of 23 male and 25 female Syrian golden hamsters received
trichlorfon (purity unknown) at a weekly intraperitoneal dose of 0 or
20 mg/kg bw for 90 weeks, and the study was terminated after 100
weeks. The treated animals showed reduced survival and body-weight
gain, but the tumour incidences were not increased (Teichmann &
Schmidt, 1978; FAO/WHO, 1979).
Dogs
Trichlorfon (purity unknown) was added to the diet of groups of
one male and one female beagle at a concentration of 50, 250, 500, or
1000 mg/kg, equivalent to 1.2, 6.2, 12, and 25 mg/kg bw per day, for 1
year. Few or no data on individual animals were provided. No effect on
food consumption or weight gain was reported. Serum and erythrocyte
cholinesterase activities were significantly depressed (by 40-50% of
control values) at 500 and 1000 mg/kg of diet. Brain
acetylcholinesterase activity was depressed by 40% in animals at
1000 mg/kg of diet. The relative spleen weights were increased and
showed marked congestion and apparent lymphoid tissue atrophy at
1000 mg/kg of diet and to a lesser extent at other doses. A slight
increase in the severity of foci of chronic inflammatory cells in the
liver and a decrease in spermatogenesis were seen at 1000 mg/kg of
diet. Hyperplastic adrenal cortical nodules recorded as 'mild' were
seen in all treated groups but not in controls. Owing to the small
number of animals in each group, however, the significance of this
finding is questionable (Doull et al., 1962; WHO, 1992).
Groups of four beagles of each sex were given diets containing
trichlorfon (purity, 95.3-98.3%) at a concentration of 0, 50, 200,
800, or 3200 mg/kg, equivalent to 1.2, 5, 20, and 80 mg/kg bw per day
in a study designed to last 4 years. All males at 3200 mg/kg of diet
died within the first year, and only one female at this dose survived
4 years. At this dose, the animals showed muscular twitching, severe
abdominal cramps, and salivation. The males in particular quickly
became emaciated. Autopsy of the animals that died during the
experiment showed brittle livers of a 'yellowish or loam' colour. Two
females and three males at 800 mg/kg of diet died during the first
year. The mortality rates at the two lower doses were similar to those
of controls, and the appearance and behaviour of the animals were
normal. Animals at higher doses were weakened and sick, and lower body
weights were recorded for surviving animals at 800 mg/kg of diet
during the latter stages and for the surviving female at 3200 mg/kg of
diet throughout the study. The effects on food consumption were
similar to those on body weight. Haematological parameters for treated
dogs were within the normal range. Elevated serum activities of
alanine and aspartate aminotransferases were noted in females at 3200
mg/kg of diet at 2 years but not at 4 years. No treatment-related
effects were found on urinary analysis. Cholinesterase activity in
whole blood was not inhibited at 50 mg/kg of diet; at 200 mg/kg of
diet, a 30% depression was seen at 1 month but the level rose again at
4 months. The group at 200 mg/kg of diet also showed inhibited
activity (15-20%) in plasma and erythrocytes throughout the study. At
800 and 3200 mg/kg of diet, dose-related depressions of 30-80% in
cholinesterase activity in whole blood, plasma, and erythrocytes were
found. The female at 3200 mg/kg of diet which survived to 4 years had
an enlarged, swollen liver, an enlarged spleen and adrenals, and small
ovaries. Dogs fed 800 mg/kg of diet had increased spleen and adrenal
weights and decreased testis weight. There were no histopathological
changes that were considered to be related to treatment. The NOEL was
50 mg/kg of diet, equivalent to 1.2 mg/kg bw per day (Loser, 1970;
Spicer & Payne, 1971; FAO/WHO, 1972b).
Monkeys
Groups of five male and five female rhesus monkeys (Macaca
mulatta) were given aqueous solutions of trichlorfon (purity,
98.6-99.1%) by gavage at a dose of 0, 0.2, 1, or 5 mg/kg bw per day, 6
days/week for 10 years. Two animals at 5 mg/kg bw per day showed
transient pupillary constriction, and one had muscular fasciculations
during the first month only; diarrhoea and soft stools were noted
frequently. At this dose, the body weights were lower towards the end
of the study, and there was a tendency to lower erythrocyte count,
haemoglobin concentration, and haematocrit at various sampling times.
No treatment-related effects on the mortality rate or clinical
chemical or urinary parameters were recorded. Plasma, erythrocyte, and
brain cholinesterase activities were inhibited by 30-80% in animals at
1 and 5 mg/kg bw per day, while males at 0.2 mg/kg bw per day showed
slight cholinesterase inhibition (16%) only in erythrocytes, which was
considered not to be biologically significant. Liver morphology in
biopsy samples taken during the first 3 years was unchanged. Gross and
microscopic examination of tissues showed no changes related to
treatment (Griffin, 1988; WHO, 1992).
2.2.4 Genotoxicity
The results of genotoxicity assays with trichlorfon are
summarized in Table 2. Other studies, evaluated by IARC (1983) and WHO
(1992), showed similar findings. The inconsistencies in a number of
tests has been suggested to be due to the variable purity of the
materials tested (Dreist & Mihail, 1990).
2.2.5 Reproductive toxicity
(a) Multigeneration studies
Trichlorfon (purity, 98.3%) was administered to groups of 10 male
and 20 female FB 30 rats in the diet at a concentration of 0, 100,
300, 1000, or 3000 mg/kg for three generations with two litters per
generation. Females in the F0 generation at 1000 and 3000 mg/kg of
diet had significantly depressed weight gain. The size of F1a
litters was reduced at doses > 1000 mg/kg of diet, while pup growth
and survival during lactation were impaired at 3000 mg/kg of diet. The
F1b mating resulted in lower pregnancy rates at 1000 and 3000 mg/kg
of diet. Litter size and pup growth and survival were also reduced at
3000 mg/kg of diet, and all animals in this group died before mating
to produce the F2 generation. During the remainder of the study, no
effects were found on fertility or on litter size or weight. The
F2a, F2b, F3a, and F3b groups given 1000 mg/kg of diet all had
reduced body-weight gain during the lactation period. No fetal
malformations were found in any group. Examination of the F0, F1b,
and F2b adults revealed no gross or microscopic alterations. The
NOEL was 300 mg/kg of diet, equivalent to 30 mg/kg bw per day (Loser,
1969; Spicer & Urwin, 1971; WHO, 1992).
(b) Developmental toxicity
Mice
A single dose of 360 mg/kg bw trichlorfon (purity unspecified),
injected intraperitoneally into 33 AS/Jena mice on day 1 of gestation,
was embryotoxic. The frequency of post-implantation loss was increased
with single doses of 60, 120, or 240 mg/kg bw on day 9 (13-15 mice per
group) and with 120 or 240 mg/kg bw on days 1-7 (25-30 mice per
group), and was most pronounced with 240 mg/kg bw on days 7-14 (23
mice). There were no severe malformations (Scheufler, 1975; WHO,
1992).
CD-1 mice were given trichlorfon (purity unspecified) by gavage
at a dose of 0, 300, 400, 500, or 600 mg/kg bw per day on days 6-10
(3-25 females per group) or 10-14 (4-21 females per group) of
gestation and were killed on day 18. The food intake and weight gain
of dams were depressed at all doses but with no dose-response
relationship. Fetal body weight was lower at doses > 400 mg/kg bw
per day, while the frequency of fetal malformations (cleft palate) was
slightly increased only in the groups treated with 500 or 600 mg/kg bw
per day during days 10-14 of gestation. The highest dose, 600 mg/kg bw
per day, was also administered by gavage on day 8 or on days 8-10,
10-12, or 12-14 of gestation (4-28 females per group). An increase in
the frequency of cleft palate was seen only in the last group. The
NOEL was 300 mg/kg bw per day (Staples & Goulding, 1979; WHO, 1992).
Trichlorfon (purity, > 98%) was given by gavage to CD-1 mice at
a dose of 0, 200, 300, or 400 mg/kg bw per day on days 7-16 of
gestation (15-24 females per group), and the animals were killed 1 day
after the last dose. The two higher doses increased the mortality
rate, and all doses decreased the weight gain of dams. The number of
live fetuses and fetal weight were decreased at 400 mg/kg bw per day.
Delayed development of fetuses was found, as indicated by reduced
calcification in a number of skeletal areas, rib variations, and
increased incidences of enlarged renal pelves in treated groups
(Courtney et al., 1986).
Table 2. Summary of results of tests for genotoxicity with trichlorfon
End-point Test object Concentration Result Reference
or dose (maximum)
In vitro
Interaction Determination of 160 mg/kg bw i.p. Positive Dedek et al.
with DNA 7-methylguanine (1976)
in mouse urine
Determination of 120 mg/kg bw i.p. Positive Dedek (1981)
7-methylguanine
in mouse liver and
kidney
Gene mutation B. subtilis NIG17, 0.3 mg per disc -S9 Negative Inukai & Iyatomi
(rec) NIG45 (1977)
P. mirabilis 10 mg per spot -S9 Positive Adler et al. (1976)
PG273, PG713
B. subtilis H17, NR -S9 Negative Shirasu et al.
M45 (1976)
B. subtilis H17, 2 mg per disc -S9 Positive Shirasu et al.
M45 (1979)
S. typhimurium 10 mg/disc ?S9 Positive Jones et al. (1984)
Reverse S. typhimurium 5 mg per plate ±S9 Positive Byeon et al. (1976)
mutation TA100
TA98, TA1535, 5 mg per plate ±S9 Negative
TA1538
TA98, TA100, 0.5 mg per plate ±S9 Negative Inukai & Iyatomi
TA1535, TA1537 (1977)
Table 2. (continued)
End-point Test object Concentration Result Reference
or dose (maximum)
TA98, TA100 ~ 8.5 mg per plate Positive Batzinger &
±S9 Bueding (1977)
TA100, TA1535 10 mg per plate ±S9 Negative Zeiger et al. (1987)
TA98, TA100 2 mg per plate ±S9 Negative Diril et al. (1990)
TA1535, TA1536, NR -S9 Negative Shirasu et al.
TA1537, TA1538, (1976)
E. coli WP2, WP2hcr
TA1535, TA1536, 2 mg per disc -S9 Negative Carere et al.
TA1537, TA1538 (1978a,b)
TA100, E. coli 20 mg per plate ±S9 Positive Shirasu et al.
WP2hcr (1979); Moriya et
TA98, TA1535, 20 mg per plate ±S9 Negative al. (1983)
TA1537, TA1538
TA100, TA104 25 mg per plate ±S9 Positive Barrueco et al.
TA97, TA98, 25 mg per plate ±S9 Negative (1991)
TA1535
TA98, TA1537 5 mg per plate±S9 Negative Watabe (1997)
TA100, TA1535, 5 mg per plate±S9 Positive
E, coli WP2uvrA
S. cerevisiae NR -S9 Negative Guerzoni et al.
632/4, 632/1b, (1976)
814/18b
Table 2. (continued)
End-point Test object Concentration Result Reference
or dose (maximum)
Reverse S. cerevisiae 10 mg per ml ±S9 Negative Hoorn (1983)
mutation S138, S211a
S. cerevisiae D7 40 mg per ml ±S9 Positive Jones et al.
(1984)
Mitotic crossing S. cerevisiae D7 40 mg per ml ±S9 Positive Jones et al.
over, gene (1984)
conversion
Forward S. coelicolor 2 mg per disc -S9 Positive Carere et al.
mutation (1978a,b)
S pombe SP-198 30 mg per ml ±S9 Positive Gilot-Delhalle et
al. (1983)
V79 cells 200 mg per ml -S9 Negative Aquilina et al. (1984)
L5178Y cells 200 µg per ml -S9 Positive Witterland
600 µg per ml +S9 Positive (1984); Jones et
al. (1984)
DNA damage E coli pol+/- 10 mg per plate ±S9 Negative Herbold (1984)
E. coli (SOS NR ±S9 Negative Xu & Schurr
chromotest) (1990)
Unscheduled EUE cells 1000 mg per ml -S9 Positive Aquilina et al.
DNA synthesis (1984)
Primary rat 50 µg per ml -S9 Negative Myhr (1983)
hepatocytes
Table 2. (continued)
End-point Test object Concentration Result Reference
or dose (maximum)
Sister chromatid V79 cells 80 µg per ml -S9 Positive Chen et al.
exchange 60 µg per ml +S9 Positive (1981, 1982)
CHO cells 100 µg per ml -S9 Positive Jones et al.
2 mg per ml +S9 Positive (1984); Putman
(1987)
Chromosomal Don-6 cells 250 mg per ml -S9 Positive Sasaki et al.
damage (1980)
Human lymphocytes 30 mg per ml -S9 Positive Herbold (1986)
3000 mg per ml +S9 Positive
In vivo
Reverse Host-mediated Batzinger &
mutation assay in mice Bueding (1977)
S. typhimurium 200 mg/kg bw orally Negative
TA98
S. typhimurium 200 mg/kg bw orally Positive
TA100
Recessive lethal Drosophila 4.5 mg/kg bw Negative Benes & Sram
mutation melanogaster (1969); Brzheskiy
(1973); Lamb
(1977)
Sister chromatid Chinese hamster 300 mg/kg bw orally Negative Volkner (1987)
exchange bone marrow
Table 2. (continued)
End-point Test object Concentration Result Reference
or dose (maximum)
Chromosomal Micronucleus 2 x 312 mg/kg bw i.p. Negative Paik & Lee
damage formation in mouse 2 x 250 mg/kg bw Negative (1977); Herbold
bone marrow orally (1979a); Jones
2 x 400 mg/kg bw Negative et al. (1984);
orally Herbold (1997)
400 mg/kg bw orally Negative
400 mg/kg bw orally Negative
of (+)-enantiomer
600 mg/kg bw orally Positive
of (-)-enantiomer
Metaphase analysis 400 mg/kg bw orally Positive Kurinnyi (1975);
in mouse 10 mg/kg bw i.p. Positive Moutschen-Dahmen
bone marrow 100 mg/kg bw i.p. Negative et al.
0.5 mg/ml in drinking, Negative (1981);
water 5 days/week, Degraeve et al.
7 weeks (1982, 1984);
405 mg/kg bw i.p. Negative Nehes et al.
(1982)
Metaphase analysis 250 mg/kg bw i.p. Negative Dzwonkowska &
in hamster Hubner (1986)
bone marrow
Metaphase analysis 250 mg/kg bw orally Negative Bootman &
in rat bone Hodson-Walker
marrow (1987)
Table 2. (continued)
End-point Test object Concentration Result Reference
or dose (maximum)
Metaphase analysis 1.5 mg/ml in drinking, Positive Bulsiewicz et al.
in mouse water 50-100 days (1976);
spermatogonia 100 mg/kg bw i.p. Negative Moutschen-Dahmen
and spermatocytes 0.5 mg/ml in drinking, Negative et al.
water 5 days/week, (1981);
7 weeks Degraeve et al.
100 mg/kg bw i.p. Negative (1982, 1984);
Herbold (1992)
Dominant lethal 100 mg/kg bw i.p. Negative Epstein et al.
mutation in mice 0.5 mg/ml in (1972); Dedek
drinking-water, Negative et al. (1975) ;
5 days/week, Fischer et al.
7 weeks (1977); Herbold
280 mg/kg bw i.p. Negative (1979b,c);
405 mg/kg bw i.p. Negative Becker &
405 mg/kg bw i.p. Positive Schoneich
250 mg/kg bw orally Negative (1980);
NR Negative Moutschen-Dahmen
405 mg/kg bw i.p. Positive et al. (1981);
54 mg/kg bw per day, Positive Degraeve et al.
3 weeks i.p. (1982, 1984);
WHO (1992)
NR, not reported; S9, metabolic activation; i.p., intraperitoneally
Rats
Trichlorfon (purity unspecified) was administered by gavage to
groups of 11 female Wistar rats at a single dose of 80 mg/kg bw on day
9 or 13 of gestation or to 10 rats at a dose of 8 mg/kg bw per day
daily throughout gestation. The animals were killed on day 19 of
gestation. The frequencies of post-implantation deaths and fetal
malformations such as exencephaly and non-closing eyelids were
increased at the single dose on day 13. The other treatments had no
effect on development (Martson & Voronina, 1976; WHO, 1992).
Trichlorfon (purity, 98.5%) was administered in the diet to
groups of 9-26 female CD rats on days 6-15 of gestation, to give an
intake of 0, 76, 140, 380, 430, or 520 mg/kg bw per day, with necropsy
on day 21. At the two highest doses, maternal food intake and body
weight and fetal weights were reduced, while the number of fetal
deaths was increased at 430 mg/kg bw per day. Major external and
skeletal malformations were found in fetuses at 430 and 520 mg/kg bw
per day and relatively minor skeletal changes at 380 mg/kg bw per day.
The predominant malformations were alterations of the skull and
central nervous system (exencephaly, meningocoele, hydrocephaly), the
limbs (syndactyly, markedly shortened radii and ulnae, missing
digits), micrognathia, cleft palate, facial haematomas, generalized
oedema, and great vessel defects. The minor skeletal alterations were
mainly doubled vertebral centra, wavy ribs, fenestrated supraoccipital
bones. and umbilical hernia. The NOEL was 140 mg/kg bw per day
(Staples et al., 1976; WHO,1992).
Trichlorfon (purity, 98.5%) was given by gavage at a dose of 0,
50, 75, 150, 200, or 250 mg/kg bw per day to groups of 9-30 female CD
rats on days 6-15 of gestation. Deaths occurred among dams at 150,
200, and 250 mg/kg bw per day, with overt signs of toxicity at the
highest dose. The survivors were killed on day 21 of gestation. Fetal
body weights were reduced significantly at doses > 75 mg/kg bw per
day, but the incidence of malformations was unaffected. The NOEL was
50 mg/kg bw per day (Staples et al., 1976).
Trichlorfon (purity unspecified) was given at a dose of 480 mg/kg
bw per day to 34 CD rats by gavage on days 6-15 of gestation. Lower
fetal body weight, a higher fetal mortality rate, and malformations
such as generalized oedema, herniation of the brain, hydrocephaly,
micrognathia, cleft palate, and skeletal alterations were seen. A
single gavage dose of 480 mg/kg bw on day 8 or 10 of gestation to 14
females per group decreased maternal food consumption but had no
adverse effect on fetal development (Staples & Goulding, 1979; WHO,
1992).
Groups of 25 female Long-Evans rats were given trichlorfon
(purity, 98.4%) by gavage at a dose of 0, 10, 30, or 100 mg/kg bw per
day on days 6-16 of gestation and were killed on day 20. There were no
deaths, but diarrhoea was observed in some animals at 100 mg/kg bw per
day. Fetal development was not affected. The NOEL for maternal
toxicity was 30 mg/kg bw per day (Machemer, 1979a; WHO, 1992).
Trichlorfon (purity, > 98%) was given by gavage to CD rats at a
dose of 0, 50, 100, or 200 mg/kg bw per day on days 7-19 (10-20
females per group) or 8-20 of gestation (15-18 females per group).
The animals were killed 1 day after the last dose. The highest dose
increased the mortality rate and decreased the weight gain of the
dams. Delayed development of fetuses was found, as indicated by
reduced calcification in a number of skeletal areas, but no
abnormalities were noted (Courtney et al., 1986).
Groups of 33 female Charles River Crl:CD BR rats were given diets
containing trichlorfon (purity, 99%) at a concentration of 0, 500,
1100, or 2500 mg/kg on days 6-15 of gestation. Body-weight gain was
slightly reduced among animals at the highest dose. Analysis of
cholinesterase activity in five animals per group killed on day 16 of
gestation showed inhibition of plasma, erythrocyte, and brain enzymes.
When the remaining dams were killed on day 20 of gestation, only brain
acetylcholinesterase activity was significantly inhibited. No effect
was seen on the numbers of resorptions, live fetuses, fetal body
weight, or malformation frequency. Increased incidences of delayed
ossification and curved, wavy, or bulbous ribs were seen at 2500 mg/kg
of diet. While fetal brain acetylcholinesterase activity was lower in
all treated groups, there was no dose-response relationship (Kowalski
et al., 1987; WHO, 1992).
Hamsters
Groups of 5-30 female golden hamsters received trichlorfon
(purity unspecified) at a dose of 0, 100, 200, 300, or 400 mg/kg bw
per day by gavage on days 7-11 of gestation, and the survivors were
killed on day 15. Three of 30 hamsters at the highest dose died, and
the signs of cholinesterase inhibition were dose-related at doses >
200 mg/kg bw per day. Maternal food consumption and body-weight gain
and fetal body weight were decreased at 300 and 400 mg/kg bw per day.
Fetuses at the highest dose showed an increased frequency of stunting
malformations, mostly consisting of oedema, cleft palate, patagium,
and fused ribs. A single dose of 400 mg/kg bw per day by gavage given
on day 8 of gestation to 16 females reduced maternal food consumption
and increased the fetal death rate but had no meaningful effect on
fetal development. The NOEL was 100 mg/kg bw per day (Staples &
Goulding, 1979; WHO, 1992).
Guinea-pigs
Groups of 10 white female guinea-pigs were given six doses of 100
mg/kg bw per day trichlorfon (purity, 97%) by gavage on days 36-38 and
51-53 of gestation. Seven females served as controls, and all animals
were allowed to deliver naturally. The numbers of abortions and
stillborn fetuses were increased and fetal body weight was decreased
in the treated group. The pups of treated dams developed trembling and
locomotor disturbances. At autopsy on postnatal day 2-3, the total
weights of the brain, cerebellum, medulla, cerebral cortex,
hippocampus, thalamus, and colliculi were decreased. The cerebellum
showed reduction of the external granular and molecular layers, with
regional absence of Purkinje cells. The activities of the
neurotransmitter enzymes choline acetyltransferase and glutamate
decarboxylase in the cerebellum were depressed when compared with
controls (Berge et al., 1986; WHO, 1992).
Rabbits
Groups of 15 female Himalayan rabbits were given trichlorfon
(purity, 98.4%) by gavage at a dose of 0, 5, 15, or 45 mg/kg bw per
day on days 6-18 of gestation and were killed on day 29. Body-weight
gain was reduced in treated groups, and two abortions occurred at 45
mg/kg bw per day. Fetal development was not affected (Machemer, 1979b;
WHO, 1992).
Groups of 20 female American Dutch rabbits were given trichlorfon
(purity, 99%) by gavage at a dose of 0, 10, 35, or 110 mg/kg bw per
day on days 6-18 of gestation and were killed on day 28. The highest
dose was not well tolerated and resulted in deaths, overt signs of
toxicity, and reduced body-weight gain. At 35 mg/kg bw per day, there
were also signs of toxicity, one death, and one abortion. Erythrocyte
but not plasma cholinesterase activity was inhibited on day 19 of
gestation in animals at 35 and 110 mg/kg bw per day, while on day 28
only brain cholinesterase activity was inhibited at these doses. At
110 mg/kg bw per day, there was a slight increase in the number of
resorptions, slight decreases in fetal and placental weights, and
delayed ossification. The frequency of fetal abnormalities was not
increased. The NOELs were 10 mg/kg bw per day for maternal toxicity
and 35 mg/kg bw per day for embryo- and fetal toxicity (Clemens et
al., 1990; WHO, 1992).
Pigs
Several outbreaks of congenital tremor in piglets have been
described in herds in which the sows had been treated with trichlorfon
preparations between day 45 and day 63 of gestation. Clinically, the
syndome was characterized by ataxia, tremor, pronounced hypoplasia of
the cerebellum, and a reduction in the size of the spinal cord (WHO,
1992). This syndrome was reproduced experimentaly in sows given diets
providing one to four doses of 50-100 mg/kg bw per day 55-98 days
after conception. The offspring had a high incidence of overt
neurological signs and cerebellar hypoplasia. Histological examination
revealed patchy loss of Purkinje cells in the cerebellum. Postnatally,
the rate of increase of brain weight was similar to that of controls,
but normal weights had not been achieved up to 35 days after birth
(Knox et al., 1978; Pope et al., 1986; Berge et al., 1987a,b; WHO,
1992).
2.2.6 Special studies
(a) Neurotoxicity
The results of these studies are summarized in Table 3. Like
other organophosphonates, trichlorfon has neurotoxic potential. Rats
given a dose of about 200 mg/kg bw per day for 13 weeks showed
decreased locomotor activity and loss of coordination, whereas rats
given a dose of 30 mg/kg bw per day for 3 weeks showed increased
locomotion and decreased learning ability and nerve conduction
velocity. Numerous studies in hens consistently demonstrated the
neurotoxicity of single doses of trichlorfon. Leg weakness, impaired
walking ability, and decreased activity were seen immediately after
treatment. These effects are considered to be secondary to inhibition
of cholinesterase activity and to be generally unrelated to damage to
the nervous tissues. Delayed neurotoxicity is usually detected some
weeks after dosing and is associated with degeneration of nervous
tissue and marked inhibition of neuropathy target esterase activity.
Neuropathy target esterase is a protein which is located specifically
in neurons, but its biological function, other than as a target for
neuropathic organophosphates, is unknown. The toxicity of an agent can
be predicted by its relative ability to interact with either
acetylcholinesterase to produce cholinergic effects or neuropathy
target esterase to produce delayed polyneuropathy (Ray, 1998). The
studies gave no evidence of delayed neuropathy in chickens. In a
monkey given a single oral dose of trichlorfon at 250 mg/kg bw per
day, nerve conduction was impaired 4 weeks after treatment, and there
was histological evidence of demyelination of nerves and axonal
degeneration.
(b) Immunotoxicity
Groups of four female BALB/cByJ and C57BL/6J mice were given
drinking-water containing trichlorfon at 1.75 mg/ml for 14 days.
Measurement of a variety of responses to immunization with influenza
virus indicated that immune function was not impaired (Reiss et al.,
1987; WHO, 1992).
2.3 Observations in humans
Wegner (1970) reviewed data on over 6000 people, obtained mostly
in South Africa and South America, who had been treated with
trichlorfon for various intestinal and body parasites (Ankylostoma,
Ascaris, Strongyloides, Trichocephalus, and Schistosoma), at doses
varying from 5 to 70 mg/kg bw per day for up to 12 days. A dose of 7.5
mg/kg bw given two to four times at 2-week intervals was considered to
be optimal, as the side-effects observed were less severe than at
other dosages. The symptoms included depression of cholinesterase
activity, weakness, nausea, diarrhoea, and abdominal pain. A dose of
24 mg/kg bw caused more severe symptoms, including tachycardia,
salivation, colic pain, vomiting, nausea, fatigue, tremors, and
sweating. The effects were not cumulative, and spontaneous recovery
was rapid in all cases. Spermatogenesis (size and shape of sperm)
appeared to be affected in a few cases. Inhibition of cholinesterase
activity was seen at all doses (FAO/WHO, 1972b; WHO, 1992).
Reduced erythrocyte and plasma cholinesterase activities have
been reported in groups of patients with various parasitic
infestations given trichlorfon orally at doses of 5-12.5 mg/kg bw. The
drug was reported to be well tolerated with few clinical symptoms
related to treatment. The symptoms that were observed were cholinergic
in nature and were usually found at doses > 10 mg/kg bw.
Cholinesterase activity recovered to pretreatment levels within 4-15
weeks after cessation of treatment (Abdel Aal et al., 1970; Plestina
et al., 1972; Jewsbury, 1981).
In a clinical trial, 10 male and 10 female patients with
Alzheimer disease were given oral doses of trichlorfon for up to 30
weeks according to the following dose regimen: 2.5 mg/kg bw at week 0,
5 mg/kg bw in week 1, 7.5 mg/kg bw in weeks 6 and 7, 15 mg/kg bw in
weeks 8 and 9, and the 'best weekly dose' in weeks 18-30. The 'best
dose' was defined as that associated with the greatest improvement for
each patient. This small trial indicated general improvement in a
variety of functional deficits associated with Alzheimer disease.
Side-effects were reported at doses > 5 mg/kg bw, which included
nausea, vomiting, and diarrhoea. Plasma cholinesterase activity was
inhibited by 45-60% at all doses, and erythrocyte acetylcholinesterase
activity was inhibited by 30-70% at doses > 5 mg/kg bw with no
concomitant clinical findings. Measurement of acetylcholinesterase
activity in the cerebrospinal fluid from two patients 24 h after a
dose of 5 mg/kg bw revealed inhibition of 37 and 47% below
pretreatment levels (Becker et al., 1990).
In a prospective, double-blind, randomized study, four parallel
groups of 119-121 patients with Alzheimer disease received oral doses
of placebo or trichlorfon once daily. An initial loading dose of 0,
0.5, 0.9, or 2.0 mg/kg bw per day was given during weeks 0-2 in order
to achieve steady-state inhibition of cholinesterase activity more
quickly, and this was followed during weeks 3-10 by a dose of 0, 0.2,
0.3, or 0.65 mg/kg bw per day. The intermediate and high doses were
found to improve cognitive function, but the low dose had equivocal
effects. Abdominal pain, diarrhoea, flatulence, nausea, and leg cramps
were the most commonly reported side-effects and were most severe in
the patients given the high dose. Erythrocyte acetylcholinesterase
activity was inhibited in a dose-related manner (Table 4). The initial
dose of 0.5 mg/kg bw per day for 2 weeks inhibited erythrocyte
acetylcholinesterase activity by 29%, and subsequent administration of
0.2 mg/kg bw per day for 10 weeks maintained the inhibition at 30-37%,
with no increase with duration of dosing. Since this dose enhanced
inhibition over that caused by the initial dose by only 8%, an
insignificant change, the Committee considered that the NOEL for
inhibition of acetylcholinesterase activity was 0.2 mg/kg bw per day
(Cummings et al., 1998).
Table 3. Summary of studies of neurotoxicity with trichlorfon
Species Treatment Results Reference
Rats 200 mg/kg bw per day Electrophysiological changes Averbrook &
i.p. for 5-15 days indicating enhanced excitability Anderson (1983)
in sciatic nerve. No pathological
changes in nerves
Rats 30 mg/kg bw per day Increased locomotion, decrease Lehotzky (1982)
orally for 3 weeks in rotorod performance, learning
ability, and nerve conduction
velocity
Rats 0, 85, 438, or 2275 Rats given 2275 ppm had stained Sheets &
ppm in the diet for 13 fur, reduced motor and locomotor Hamilton (1995)
weeks activity, slightly uncoordinated
righting response (males only),
30-80% inhibition of plasma,
erythrocyte, and brain
cholinesterase activity, and
demyelination in spinal nerve roots.
At 438 ppm, only plasma and
erythrocyte cholinesterase activity
was inhibited (20-30%), and no
behavioural or biochemical
evidence of neurotoxicity was
seen at 85 ppm (6 mg/kg bw
per day)
Chicken Single subcutaneous Leg weakness for 1-2 days, no Witter & Gaines
dose of 90 mg/kg bw delayed paralysis (1963)
Chicken Single dose of 25-500 Birds that survived the acute Lorke &
mg/kg bw orally or toxicity of trichlorfon showed no Kimmerle (1966)
75-500 mg/kg bw i.p. signs of neurotoxicity over
6-10 weeks
Chicken 100-5000 ppm in Body weight loss at > 500 ppm. Lorke &
feed for 30 days Blood cholinesterase activity Kimmerle (1966)
decreased by 40-60% at all Hobik (1967);
doses but no signs of FAO/WHO
neurotoxicity and no (1972)
degeneration in nervous tissue.
Table 3. (continued)
Species Treatment Results Reference
Chicken Single subcutaneous One hen died 1.5 h after dosing; Hierons &
dose of 200 mg/kg bw the lone survivor was killed 24 h Johnson (1978)
after dosing, when NTE was
inhibited by 46% in brain and
17% in spinal cord.
Chicken Single doses of 50, Dose-related deaths after oral Olajos et al.
100 mg/kg bw orally, doses only. All treatments had (1979)
50, 100, 200 mg/kg acute effects; ataxia from 11-17
bw subcutaneously, days after dosing. Brain NTE
or 200 +100 mg/kg bw decreased by 40-60% at high
subcutaneously 3 subcutaneous doses but by only
days apart about 20% at <100 mg/kg bw.
Mild degenerative changes seen
in cerebellum, brainstem, and
striatum at higher doses.
Chicken 185 mg/kg bw orally, Acute signs of intoxication but Thyssen et al.
survivors given 167 no pathological changes in (1982)
mg/kg bw 21 days nervous tissue
later
Chicken Single subcutaneous Marked cholinesterase inhibition Slott &
dose of 100 or 300 in plasma, brain, and spinal cord Ecobichon
mg/kg bw (50-60%) but no inhibition of NTE (1984)
in brain or spinal chord
Chicken 100 mg/kg bw Plasma, brain, and spinal cord Slott &
subcutaneously every cholinesterase inhibited by Ecobichon
72 h for 6 doses 10-60%, and walking ability (1984)
slightly impaired. No inhibition
of NTE in brain or spinal cord
and only slight oedematous
effects on nervous tissue
Chicken 3, 9, or 18 mg/kg bw Signs of ataxia and decreased Hayes & Ramm
per day for 3 months activity at 18 mg/kg bw per day (1987)
by gavage but no effect on locomotor activity.
Blood cholinesterase activity
decreased in all groups but
nervous tissue unremarkable.
Table 3. (continued)
Species Treatment Results Reference
Chicken Single gavage dose Acute signs of intoxication, Bomann &
of 340 mg/kg bw, 220 deaths, and marked inhibition Kaliner
mg/kg bw (90%) of brain cholinesterase (1996)
(-)-enantiomer, activity with each compound.
or 400 mg/kg bw < 50% NTE inhibition in brain,
(+)-enantiomer spinal cord, and sciatic nerve; no
changes in gait or nervous tissue
Monkey Single dose of Weakness of lower extremities Shiraishi et al.
(Macaca 250 mg/kg bw by on day 24 and nerve conduction (1983)
fuscata) gavage defects by day 28. On day 37,
demyelination of sural and tibial
nerves with increased axonal
degeneration
NTE, neuropathy target esterase
Table 4. Mean inhibition of erythrocyte acetylcholinesterase
activity (%) in patients with Alzheimer disease
given trichlorfon orally
Time (week) Dose of trichlorfon (mg/kg bw per day)
0-2 0 0.5 0.9 2.0
3-10 0 0.2 0.3 0.65
2 1.1 ± 14 29 ± 12 49 ± 12 71 ± 82
8 0.1 ± 21 37 ± 13 52 ±12 68 ± 95
12 1.0 ± 24 34 ± 14 52 ± 10 72 ± 24
A position paper (Bayer AG, 1999) reported that the results of
clinical trials involving over 4000 treated patients show that
trichlorfon at the recommended dose of 0.5-0.9 mg/kg bw is 'a safe and
effective treatment' for Alzheimer disease. A dose-response
relationship was found between the dose of trichlorfon and the
incidence of muscle weakness in placebo-controlled trials, and the
increased risk for muscular weakness was statistically significant at
doses > 1.25 mg/kg bw. The recommended dose of 0.5-0.9 mg/kg bw
resulted in incidences of 0.6% for generalized weakness or myasthenia
(0.2% with placebo) and 4.3% for fatigue or asthenia (3.3% with
placebo). The muscular weakness was reported to be reversible on
discontinuation of therapy.
Acute poisoning of humans with trichlorfon has been reported to
manifest as delayed neurotoxicity, severe polyneuritis, and
polyneuropathy 2-3 weeks after ingestion. These diagnoses were based
on observations of weakness, loss of feeling in the extremities, and
difficulty in walking, accompanied by muscular atrophy, myalgia, and
were related to motor nerve damage (Pollingher et al., 1973; Akimov et
al., 1975; Eljasz & Kryzyszton-Przekop, 1975; Fukuhara et al., 1977;
Shiraishi et al., 1977; Hierons & Johnson, 1978; Shiraishi et al.,
1982; Niedziella et al., 1985; De Freitas et al., 1990). After
reviewing the literature, Johnson (1981) concluded that only doses of
trichlorfon that exceed the lethal dose but are survived by the victim
because of treatment are likely to result in a level of inhibition of
neuropathy target esterase activity at which delayed neurotoxicity
would be expected. Development of neuropathy after repeated exposure
to lower doses was considered to be unlikely (WHO, 1992).
Five persons exposed to trichlorfon, two while attempting suicide
and three occupationally, showed increased incidences of chromosomal
breaks and stable chromosomal rearrangements in cultured lymphocytes.
The effect was observed immediately after exposure and 1 month later,
but not 6 months later (Bao et al., 1974). Seventeen workers involved
in the production of trichlorfon-containing products for at least 6
months had increased incidences of chromatid breaks in their
lymphocytes when compared with an unexposed control group, but the
incidences were lower than those in a control group of factory workers
who had had no direct exposure to pesticides (Kiraly et al., 1979).
3. COMMENTS
The Committee considered the results of studies on the
toxicokinetics, metabolism, and acute toxicity of trichlorfon,
short-term and long-term studies of toxicity, and studies of
genotoxicity, reproductive and developmental toxicity, neurotoxicity,
and immune function, and studies in humans. Most of the available
studies were relatively old, and many were published reports which
lacked raw data or data on individual animals. Such studies are
difficult to evaluate, and most could not be assessed independently.
Unpublished reports of studies conducted during the 1980s and 1990s
contained sufficient detail and were carried out according to
appropriate standards for study protocol and conduct.
The absorption of trichlorfon is rapid in all species tested,
including humans, irrespective of the route of administration. Peak
blood concentrations were achieved within 1-2 h but decreased quickly
thereafter; the half-time of trichlorfon in human plasma is
approximately 2 h. It is widely distributed. Trichlorfon was detected
in the milk of lactating cows, and the compound and its metabolites
were found in fetal tissue in treated guinea-pigs. Trichlorfon
undergoes conversion to dichlorvos via a dehydrochlorination reaction
that occurs spontaneously at pH values above 5.5. Although little
dichlorvos was recovered, it is generally believed to be responsible
for the anticholinesterase effects of trichlorfon. The metabolism of
trichlorfon in mammals also occurs through O-demethylation and
cleavage of phosphorus-carbon bonds. Therefore, the major metabolites
are desmethyl trichlorfon, desmethyl dichlorvos, dimethyl hydrogen
phosphate, methyl dihydrogen phosphate, and phosphoric acid.
Trichlorfon and its metabolites are excreted primarily in the urine.
Trichlorfon is moderately toxic when given as a single oral dose,
the LD50 values being 620-950 mg/kg bw in mice, 140-660 mg/kg bw in
rats, 160 mg/kg bw in rabbits, and 300-420 mg/kg bw in dogs. The signs
of toxicity were indicative of inhibition of acetylcholinesterase
activity, and the cause of death was usually respiratory failure. The
rapid recovery observed after sublethal doses suggests that
trichlorfon is readily detoxified.
Trichlorfon was given orally to mice at doses of 10-750 mg/kg bw
per day, to rats at doses of 0.05-250 mg/kg bw per day, and to dogs at
doses of 0.5-45 mg/kg bw per day for periods up to 16 weeks. The
weights of the liver, kidney, and spleen were increased at doses of
250 mg/kg bw per day and above in mice and at doses of 50 mg/kg bw per
day and above in rats, but these changes were usually not associated
with other evidence of toxicity, except in one study in rats in which
hypertrophy of centrilobular hepatocytes and biochemical changes
suggestive of altered lipid metabolism were seen. These effects
occurred at doses which also induced significant signs of cholinergic
intoxication. Slight anaemia was observed in one study in rats at 100
mg/kg bw per day and in one study in dogs at 45 mg/kg bw per day. One
dog out of three given trichlorfon at a dose of 45 mg/kg bw per day
had small, immature testes and prostate and oligospermia, but the
animal had also lost weight and appeared to be severely stressed by
the treatment. The NOELs were 15 mg/kg bw per day for inhibition of
cholinesterase activity in brain and erythrocytes in mice, 5 mg/kg bw
per day for inhibition in brain and erythrocytes in rats, and 5 mg/kg
bw per day for inhibition in in erythrocytes in dogs.
In two studies of up to 2 years' duration, mice were given oral
doses of 15-750 mg/kg bw per day. Body-weight gain was impaired at
doses of 30 mg/kg bw per day and above; the mean weight of the liver
was increased at a dose of 240 mg/kg bw per day in one study, but no
pathological alterations were found. The NOEL for inhibition of brain
and erythrocyte cholinesterase activity was 49 mg/kg bw per day. The
incidences of tumours were unaffected by treatment. Neither study was
suitable for identifying a NOEL.
Six studies of up to 2 years' duration were conducted in which
rats were given trichlorfon in the diet at concentrations providing
doses of 2.5-160 mg/kg bw per day. The weights of the liver and spleen
were increased in three studies at doses of 50 mg/kg bw per day and
above. Hypercholesterolaemia, duodenal hyperplasia, and gastritis of
the non-glandular stomach were seen at 13 mg/kg bw per day and above.
Anaemia, enhancement of age-related nephropathy, increased renal
tubular hyperplasia, myodegeneration of the stomach tunica muscularis,
pulmonary inflammation, and hyperplasia were observed at doses of 75
mg/kg bw per day and above. Other observations included ovarian
atrophy at 20 mg/kg bw per day and above, depression of
spermatogenesis at 50 mg/kg bw per day, and a slight increase in the
incidence of benign mammary tumours in females with a reduction in the
time to appearance at doses of 20 mg/kg bw per day and above. The NOEL
for inhibition of brain and erythrocyte acetylcholinesterase activity
was 13 mg/kg bw per day. The incidence of mammary tumours was
increased in female Sprague-Dawley rats in two studies. The increases
were slight, the tumours were benign, this strain of rats has a
relatively high and variable background incidence of mammary tumours,
and the finding could not be confirmed in several other studies in
various species. The Committee concluded that trichlorfon does not
have carcinogenic potential in rats. The overall NOEL in rats was 4.5
mg/kg bw per day on the basis of an increased cholesterol
concentration and pathological changes to the gastrointestinal tract.
In two studies of 1 and 4 years' duration, dogs were given
trichlorfon orally at doses of 1.3-80 mg/kg bw per day. Deaths
occurred at doses of 20 mg/kg bw per day and above, and signs of
hepatocellular damage and increased severity of inflammation were seen
in the liver at doses of 25 mg/kg bw per day and above. In animals
given 20-25 mg/kg bw per day, the weight of the spleen was increased,
with atrophy of lymphoid tissue; furthermore, small ovaries, reduced
testis weight, and decreased spermatogenesis were seen. 'Blood
cholinesterase' activity was inhibited at doses of 5 mg/kg bw per day
and above. The overall NOEL in dogs was 1.3 mg/kg bw per day on the
basis of inhibition of cholinesterase activity.
Oral administration of trichlorfon to rhesus monkeys at a dose of
0, 0.2, 1, or 5 mg/kg bw per day for 10 years resulted in clinical
signs of toxicity indicative of cholinesterase inhibition, reduced
body-weight gain, and anaemia at the highest dose. Inhibition of
plasma, erythrocyte, and brain cholinesterase activities was seen at
the two higher doses. In a separate 6-month study in rhesus monkeys,
erythrocyte acetylcholinesterase activity was not depressed at 0.2
mg/kg bw per day, the highest dose tested. The NOEL in monkeys was 0.2
mg/kg bw per day on the basis of inhibition of brain and erythrocyte
acetylcholinesterase activity.
Trichlorfon has been tested in a wide range of assays for
genotoxicity and DNA damaging activity, with considerable variation in
the results. Trichlorfon induced point mutations in microorganisms and
cultured mammalian cells, DNA damage in microorganisms, and
chromosomal aberrations and sister chromosome exchange in cultured
mammalian cells. The results of tests for point mutation in
Drosophila melanogaster and for sister chromatid exchange in bone
marrow were clearly negative. Although positive results were obtained
in a few assays for chromosomal damage in bone marrow and in the germ
cells of male animals exposed at near-lethal doses, the results of
most studies of micronucleus formation, metaphase alterations, and
dominant lethal mutations were negative. Since the tests conducted
in vivo produced mostly negative results when trichlorfon was
administered orally, the Committee considered that the weight of
evidence indicates that trichlorfon is unlikely to represent a
genotoxic risk.
Trichlorfon was given to rats at a dose of 0, 10, 30 100, or 300
mg/kg bw per day in a three-generation study of reproductive toxicity,
with two litters per generation. The body-weight gain of F0 dams at
100 and 300 mg/kg bw per day was depressed. The slight effects on the
gonads reported in the studies of general toxicity were not
consistently reflected in this study. The pregnancy rate of rats of
the F1b generation at doses of 100 mg/kg bw per day and above was
reduced, but the fertility of other generations was unaffected. The
litter size of dams at 300 mg/kg bw per day was reduced and all of the
offspring died, but reproductive parameters were unaffected at lower
doses. The NOEL was 30 mg/kg bw per day in adults, on the basis of
reduced body-weight gain, and 100 mg/kg bw per day for reproductive
effects, on the basis of reduced litter size and the death of
offspring.
Two studies of developmental toxicity were conducted in mice
given oral doses of 200-600 mg/kg bw per day. Dams at 200 mg/kg bw per
day showed reduced body-weight gain, and higher doses led to an
increased mortality rate. The effects of these maternally toxic doses
included increased embryotoxicity, decreased fetal weight, and delayed
fetal development. The incidence of cleft palate was slightly
increased at doses of 500 and 600 mg/kg bw per day. NOELs were not
identified for maternal toxicity or fetotoxicity.
Studies of developmental toxicity were conducted in rats given
oral doses of 8-520 mg/kg bw per day. In three studies, maternal
toxicity in the form of reduced body-weight gain and/or an increased
mortality rate was observed at doses of 150 mg/kg bw per day and
above. The NOEL for maternal toxicity was 75 mg/kg bw per day. In the
same studies, the incidences of fetal malformations such as
morphological alterations of the skull, central nervous system, and
limbs, micrognathia, cleft palate, facial haematomas, generalized
oedema, and defects in major blood vessels were increased at doses of
430 mg/kg bw per day and above. At 380 mg/kg bw per day, minor
skeletal changes were observed. Reduced fetal body weight was seen at
75 mg/kg bw per day. In three other studies, the incidence of fetal
malformations was unaffected. The NOEL for developmental effects was
50 mg/kg bw per day on the basis of fetotoxicity.
Hamsters given trichlorfon orally at a dose of 0, 200, 300, or
400 mg/kg bw per day during gestation showed signs of cholinesterase
inhibition, and depressed body-weight gain was seen at the two higher
doses. The NOEL for maternal toxicity was 100 mg/kg bw per day. Fetal
body weight was decreased at the two higher doses, and an increased
incidence of fetal stunting was seen at the highest dose. The NOEL for
developmental effects was 200 mg/kg bw per day.
Guinea-pigs given trichlorfon at an oral dose of 100 mg/kg bw per
day for 6 days during gestation had abortions and stillborn fetuses,
and the offspring showed reduced body weight and brain weight,
locomotor disturbances, and morphological and biochemical alterations
in the brain.
In two studies of developmental toxicity in rabbits, oral doses
of 5-110 mg/kg bw per day were given. Overt toxicity and inhibition of
erythrocyte acetylcholinesterase activity were observed in dams given
doses of 35 mg/kg bw per day and above, and body-weight gain was
affected at all doses. The highest dose, 110 mg/kg bw per day,
slightly increased the rate of resorptions and retarded fetal
development, but no fetal abnormalities were found. A NOEL for
maternal toxicity was not identified. The NOEL for developmental
effects was 45 mg/kg bw per day on the basis of toxicity to embryos
and fetuses.
Several outbreaks of congenital tremor with cerebellar hypoplasia
were reported in piglets of sows that had been treated with
trichlorfon, and the syndrome was subsequently reproduced
experimentally. The piglets of sows treated with trichlorfon at doses
of 50-100 mg/kg bw per day during appropriate periods of gestation
showed neurological signs and hypoplasia and loss of Purkinje cells in
the cerebellum.
Rats given a dose equivalent to 200 mg/kg bw per day for 13 weeks
showed decreased locomotor activity and loss of coordination. In
another study in rats, a dose of 30 mg/kg bw per day for 3 weeks
increased locomotor activity and decreased learning ability and nerve
conduction velocity.
The results of numerous studies in hens consistently demonstrated
the acute neurotoxicity of trichlorfon. Delayed neurotoxicity,
associated with degeneration of nervous tissue and marked inhibition
of neuropathy target esterase, was not seen in hens. A monkey given a
single oral dose of 250 mg/kg bw showed impaired nerve conduction 4
weeks after treatment and histological evidence of demyelination of
nerves and axonal degeneration.
Trichlorfon has been used as an anthelmintic agent in humans.
Oral doses of up to 10 mg/kg bw given on two to four occasions were
well tolerated, with few clinical symptoms. A dose of 24 mg/kg bw
caused significant cholinergic symptoms, but the effects were not
cumulative. In a few cases, spermatogenesis appeared to have been
impaired. The results of clinical trials with trichlorfon in the
treatment of Alzheimer disease showed dose-related but reversible
muscle weakness. The recommended dose of 0.5-0.9 mg/kg bw resulted in
a small increase in the frequency of generalized weakness and fatigue,
while doses of 1.25 mg/kg bw and higher produced significant weakness.
Dose-related inhibition of erythrocyte acetylcholinesterase activity
was also observed in these studies.
In 121 subjects, an initial dose of 0.5 mg/kg bw per day for 2
weeks inhibited erythrocyte acetylcholinesterase activity by 29%.
Subsequent administration of a dose of 0.2 mg/kg bw per day for
10 weeks maintained the inhibition of erythrocyte acetylcholinesterase
activity at levels between 30 and 37%, with no increase with duration
of dosing. Since this dose enhanced the inhibition caused by the
initial dose by only 8%, an insignificant change, the Committee
concluded that the NOEL was 0.2 mg/kg bw per day.
In some cases of severe human poisoning with trichlorfon,
weakness and loss of feeling in the extremities, difficulty in
walking, muscular atrophy, and motor nerve damage have been observed.
In many of these cases, the doses might have been lethal in the
absence of medical intervention. The Committee concluded that
extremely high doses of trichlorfon would be required to achieve the
level of inhibition of neuropathy target esterase associated with
delayed neurotoxicity.
4. EVALUATION
The Committee concluded that inhibition of acetylcholinesterase
activity was the most relevant end-point for establishing an ADI. The
most appropriate NOEL was 0.2 mg/kg bw per day for inhibition of
erythrocyte acetylcholinesterase activity in humans treated orally. A
safety factor of 10 was applied to this figure, giving an ADI of 0-20
µg/kg bw.
5. REFERENCES
Abdel Aal, A.M.A., El-Hawary, M.F.S., Kamel, H., Abdel-Khalek, M.K. &
El-Diwany, K.M. (1970) Blood cholinesterases, hepatic renal and
haemopoietic functionsin children receiving repeated doses of
'Dipterex'. J. Egypt. Med. Assoc., 53, 265-271.
Aden-Abdi, Y., Villen, T., Ericsson, O., Gustafsson, L.L. &
Dahl-Puustinen, M.L. (1990) Metrifonate in healthy volunteers:
Interrelationship between pharmacokinetic properties,
cholinesterase inhibition and side effects. Bull. World Health
Organ., 68, 731-736.
Adler, B., Braun, R., Schöneich, J. & Böhme, H. (1976)
Repair-defective mutants of Proteus mirabilis as a prescreening
system for the detection of potential carcinogens. Biol. Zbl.,
95, 463- 469.
Akhtar, M.H. (1982) Fate of trichlorfon in buffer and soluble fraction
(105 000 g) from cow and chicken liver homogenates. J. Agric.
Food Chem., 30, 551-554.
Akimov, G.A., Buchko, V.M. & Kremleva, R.V. (1975) Neurological
disorders in chlorophos poisoning. Klin. Med., 5, 65-69
( Pestic. Abstr., 75-2380).
Aquilina, G., Benigni, R., Bignami, M., Calcagnile, A., Dogliotti, E.,
Falcone, E. & Carere, A. (1984) Genotoxic activity of dichlorvos,
trichlorfon, and dichloroacetaldehyde. Pestic. Sci., 15,
439-442.
Arthur, B.W. & Casida, J.E. (1957) Metabolism and selectivity of
O,O-dimethyl 2,2,2-trichloro-1-hydroxyethylphosphonate and its
acetyl and vinyl derivatives. J. Agric. Food Chem., 5, 186-192.
Averbook, B.J. & Anderson, R.J. (1983) Electrophysiological changes
associated with chronic administration of organophosphates.
Arch. Toxicol., 52, 167-172.
Bao, T.V., Szabo, I., Ruzicska, P. & Czeizel, A. (1974) Chromosome
aberrations in patients suffering acute organic phosphate
insecticide intoxication. Humangenetik, 24, 33-57.
Barrueco, C., Herrera, A. & De La Pena, E. (1991) Mutagenic evaluation
of trichlorfon using different assay methods with Salmonella
typhimurium. Mutagenesis, 6, 71-76.
Batzinger, R.P. & Bueding, E. (1977) Mutagenic activities in vitro
and in vivo of five antischistosomal compounds. J. Pharmacol.
Exp. Ther., 200, 1-9.
Bayer AG (1999) Pro Mem (metrifonate) and muscle weakness. Unpublished
position paper from Bayer AG. Submitted to WHO by Bayer AG,
Germany.
Becker, K. & Schöneich, J. (1980) Dominant lethal test with
trichlorphon in male mice. Mutat. Res., 74, 224.
Becker, R.E., Colliver, J., Elble, R., Feldman, E., Giacobini, E.,
Kumar, V., Markwell, S., Moriearty, P., Parks, R., Shillcutt,
S.D., Unni, L., Vicari, S., Womack, C. & Zec, R.F. (1990) Effects
of metrifonate, a long-acting cholinesterase inhibitor, in
Alzheimer disease: Report of an open trial. Drug Dev. Res., 19,
425-434.
Benes, V. & Sram, R. (1969) Mutagenic activity of some pesticides in
Drosophila melanogaster. Ind. Med., 38, 50-52.
Berge, G.N. & Nafstad, I. (1986) Distribution and placental transfer
of trichlorfon in guinea-pigs. Arch. Toxicol., 59, 26-29.
Berge, G.N., Nafstad, I. & Fonnum, F. (1986) Prenatal effects of
trichlorfon on the guinea-pig brain. Arch. Toxicol., 59, 30-35.
Berge, G.N., Fonnum, F. & Brodal, P. (1987a) Neurotoxic effects of
prenatal trichlorfon administration in pigs. Acta Vet. Scand.,
28, 321-332.
Berge, G.N., Fonnum, F., Soli, N.E. & Sognen, E. (1987b)
Neurotoxicological examination of the piglet brain after prenatal
and postnatal exposure to trichlorfon. Acta Vet. Scand., 28,
313-320.
Bomann, W. & Kaliner, G. (1996) BAY a 9826 (metrifonate) and BAY z
7216, BAY z 7217. Study for delayed neurotoxicity following acute
oral administration to hens. Unpublished report no PH 25171 from
Bayer, Wuppertal, Germany. Submitted to WHO by Bayer AG, Germany.
Bond, G.P. (1986) Primary dermal irritation of technical trichlorfon
(Dylox) in albino rabbits. Unpublished report no 751 from Mobay
Corporation, USA. Submitted to WHO by Bayer AG, Germany.
Bootman, J. & Hodson-Walker, G. (1987) L13/59: Investigation of
effects on bone marrow chromosomes of the rat after acute oral
administration. Unpublished report no R4221 from Life Sciences
Research, United Kingdom. Submitted to WHO by Bayer AG, Germany.
Brzheskiy, V.V. (1973) [Induction of mutations in Drosophila
melanogaster.] Med. Parazitol. Parazit. Bolez., 42, 703-706
(in Russian).
Bull, D.L. & Ridgway, R.L. (1969) Metabolism of trichlorfon in animals
and plants. J. Agric. Food Chem., 17, 837-841.
Bulsiewicz, H., Rozewicka, L., Januszewska, H. & Bajko, J. (1976)
Aberrations of meiotic chromosomes induced in mice with
insecticides. Folia Morphol. (Warsaw), 35, 361-368.
Byeon, W.H., Hyun, H.H. & Lee, S.Y. (1976) Salmonella/microsomal
enzyme activation system. Mutagenicity of pesticides in the
Salmonella/microsome system. Korean J. Microbiol., 14,
128-134.
Carere, A., Ortali, V.A., Cardamone, G. & Morpurgo, G. (1978a)
Mutagenicity of dichlorvos and other structurally related
pesticides in Salmonella and Streptomyces. Chem.-Biol.
Interactions, 22, 297-308.
Carere, A., Ortali, V.A., Cardamone, G., Torracca, A.M. & Raschetti,
R. (1978b) Microbiological mutagenicity studies of pesticides
in vitro. Mutat. Res., 57, 277-286.
Chan, P.C. & Peters, A.C. (1989) Thirteen-week subchronic dosed feed
study of trichlorfon in Fischer rats and B6C3F1 mice. Proc.
Am. Assoc. Cancer Res., 30, 137.
Chen, H.H., Hsueh, J.L., Sirianni, S.R. & Huang, C.C. (1981) Induction
of sister chromatid exchanges and cell cycle delay in cultured
mammalian cells treated with eight organophosphorus pesticides.
Mutat. Res., 88, 307-316.
Chen, H.H., Sirianni, S.R. & Huang, C.C. (1982) Sister chromatid
exchanges in Chinese hamster cells treated with seventeen
organophosphorus compounds in the presence of a metabolic
activation system. Environ. Mutag., 4, 621-624.
Christenson, W.R. (1990) A combined chronic toxicity/oncogenicity
study of technical grade trichlorfon (Dylox) with rats.
Unpublished report no 5388 from Mobay Corporation, USA. Submitted
to WHO by Bayer AG, Germany.
Clemens, G.R., Troup, C.M. & Hartnagel, R.E., Jr (1990) Teratology
study in the rabbit with Dylox technical (Trichlorfon).
Unpublished report no MTD0134 from Miles Laboratories, USA.
Submitted to WHO by Bayer AG, Germany.
Courtney, K.D., Andrews, J.E. & Springer, J. (1986) Assessment of
teratogenic potential of trichlorfon in mice and rats.
J. Environ. Sci. Health, B21, 207-227.
Crawford, C.R. & Anderson, R.H. (1973) The acute oral and
intraperitoneal toxicity of five trichlorfon technical samples to
rats. Unpublished report no 37204 from Baychem Corporation.
Submitted to WHO by Bayer AG, Germany.
Cummings, J.L., Cyrus, P.A., Bieber, F., Mas, J., Orazem, J. &
Gulanski, B. (1998) Metrifonate treatment of the cognitive
deficits of Alzheimer's disease. Neurology, 50, 1214-1221.
De Freitas, M.R.G., Chimelli, L., Nascimento, O.J.M., Cincinatus, D.,
Marques, H.A. & Nevares, M.T. (1990) [Polyneuropathy induced by
trichlorfon. Report of a case with electrophysiology and
histopathology in the sural nerve.] Arq. NeuroPsiquiatr., 48,
515-519 (in Spanish).
Dedek, W. (1981) Guanine N7-alkylation in mice in vivo by
metrifonate -- Discussion of possible genotoxic risk in mammals.
Acta Pharmacol. Toxicol., 49 (Suppl. V), 40-50.
Dedek, W. & Lohs, K. (1970a) [Alkylating effect of trichlorfon in
mammals. I. Studies in vitro in human serum with
14C-trichlorfon.] Z. Naturforsch., B25, 94-96 (in German).
Dedek, W. & Lohs, K. (1970b) [Allkylkating effect of trichlorfon in
mammals. Distribution of 14C in organs and liver proteins of
rats after administration of 14Ctrichlorfon.]
Z. Naturforsch.,B25, 1110-1113 (in German).
Dedek, W., Scheufler, H., & Fischer, G.W. (1975) [Mutagenicity of
desmethyltrichlorfon in the dominant lethal test in the mouse.]
Arch. Toxicol., 33, 163-168 (in German).
Dedek, W., Lohs, K., Fischer, G.W. & Schmidt, R. (1976) Alkylation of
guanine in mice in vivo by organophosphorus insecticides. I.
Trichlorfone and butonate. Pestic. Biochem. Physiol., 6,
101-110.
Degraeve, N., Chollet, M.-C., Moutschen, J., Moutschen-Damen, M.,
Gilot-Delhalle, J. & Colizzi A. (1982) Genetic and cytogenetic
effects of chronic treatments with organophosphorous
insecticides. Mutat. Res., 97, 179-180.
Degraeve, N., Chollet, M.-C. & Moutschen, J. (1984) Cytogenetic and
genetic effects of subchronic treatments with organophosphorus
insecticides. Arch. Toxicol., 56, 66-67.
Diril, N., Sumer, S. & Izbirak, A. (1990) [A survey on the mutagenic
effects of some organophosphorous insecticides in the
Salmonella/microsome test system.] Doga Muhendislik Cevre
Bilimleri, 14, 272-279 (in Turkish).
Doull, J. & Rehfuss, P.A. (1956) The effects of diets containing
Dipterex on rats. Unpublished report No. 1376 from Department of
Pharmacology, University of Chicago, USA. Submitted to WHO by
Bayer AG, Germany.
Doull, J. & Vaughn, G. (1958) Measurement of the safe dietary level of
Dipterex for dogs. Unpublished report No. 2105 from Department of
Pharmacology, University of Chicago, USA. Submitted to WHO by
Bayer AG, Germany.
Doull, J., Root, M., Vesselinovitch, D., Meskauskas, J., & Fitch, F.
(1962) Chronic oral toxicity of Dylox to male and female dogs.
Unpublished report No. 8644 from Department of Pharmacology and
Pathology, University of Chicago, USA. Submitted to WHO by Bayer
AG, Germany.
Dreist, M. & Mihail, F.L. (1990) L 13/59 (trichlorfon). Synoptic
evaluation of the toxicological data. Unpublished report no 20033
from Bayer, WuppertalElberfeld, Germany. Submitted to WHO by
Bayer AG, Germany.
Dubois, K.P. & Cotter, G.J. (1955) Studies on the toxicity and
mechanism of action of dipterex. Arch. Ind. Health, 11, 53-60.
Dzwonkowska, A. & Hubner, H. (1986) Induction of chromosomal
aberrations in the Syrian hamster by insecticides tested
in vivo. Arch. Toxicol., 58, 152-156.
Eljasz, L. & Kryzyszton-Przekop, T. (1975) [Polyneuropathy as a result
of foschlor poisoning.] Pol. Tyg. Lek., 30, 1841-1842 ( Pest.
Abstr. 76-1688) (in Polish).
Epstein, S.S., Arnold, E., Andrea, J., Bass, W. & Bishop, Y. (1972)
Detection of chemical mutagens by the dominant lethal assay in
the mouse. Toxicol. Appl. Pharmacol., 23, 288-325.
FAO/WHO (1972a) Pesticide residues in food. Report of the 1971
Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues. FAO Agricultural Series, No. 88; WHO Technical Report
Series, No. 502, Rome/Geneva.
FAO/WHO (1972b) 1971 Evaluations of some pesticide residues in
food. AEP-1971/M/13; WHO Pesticide Residues Series, No. 5,
Rome/Geneva.
FAO/WHO (1976a) Pesticide residues in food. Report of the 1975
Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on
Pesticide Residues. FAO Plant Production and Protection Series,
No. 1; WHO Technical Report Series, No. 592, Rome/Geneva.
FAO/WHO (1976b) 1975 Evaluations of some pesticide residues in
food. AEP-1975/M/9/1; WHO Pesticide Residues Series, No. 1,
Rome/Geneva.
FAO/WHO (1979a) Pesticide residues in food--1978. Report of the
Joint Meeting of the FAO Panel of Experts on Pesticide
Residues and Environment and the WHO Expert Group on
Pesticide Residues. FAO Plant Production and Protection Paper
15, Rome.
FAO/WHO (1979b) Pesticide residues in food: 1978 Evaluations. FAO
Plant Production and Protection Paper 15 Suppl., Rome.
Fischer, G.W., Schneider, P. & Scheufler, H. (1977) [Mutagenicity of
dichloroacetaldehyde and methyl-2,2-dichloro-1,1-dihydroxyethane
phosphonate, possible trichlorfon metabolites.] Chem.-Biol.
Interactions, 19, 205-213 (in German).
Fukuhara, N., Hoshi, M. & Mori, S. (1977) Core/targetoid fibres and
multiple cytoplasmic bodies in organophosphate neuropathy.
Acta Neuropathol., 40, 137-144.
von Gibel, W., Lohs, K., Wildner, G.P. & Ziebarth, D. (1971) [Animal
experimental studies on the hepatotoxic and carcinogenic activity
of organophosphorus compounds.] Arch. Geschwülstforsch., 37,
303-312 (in German).
von Gibel, W., Lohs, K., Wildner, G.P., Ziebarth, D. & Stieglitz, R.
(1973) [Carcinogenicity, haematoxicity and hepatotoxicity of
organophosphorus compounds.] Arch. Geschwülstforsch., 41,
311-328 (in German).
Gilot-Delhalle, J., Colizzi, A., Moutschen, J. & Moutschen-Dahmen, M.
(1983) Mutagenicity of some organophosphorus compounds at the
ade6 locus of Schizosaccharomyces pombe. Mutat. Res., 117,
139-148.
Griffin, T.B. (1988) Safety evaluation and tumorigenesis of
trichlorfon in rhesus monkeys: A ten-year study. Unpublished
report from White Sands Research Center, USA. Submitted to WHO by
Bayer AG, Germany.
Griffin, T.B., Coulston, F. & Stein, A.A. (1982) Trichlorfon.
Tumorigenicity study in the rat (2 generation). Unpublished
report from White Sands Research Center, USA. Submitted to WHO by
Bayer AG, Germany.
Grundmann, E. & Hobik, H.P. (1966) Bay 15922. 2 year feeding
experiment on rats. Histology. Unpublished report addendum from
Bayer, Wuppertal-Elberfeld. Submitted to WHO by Bayer AG,
Germany.
Guerzoni, M.E., Del Cupolo, L. & Ponti, I. (1976) [Mutagenic activity
of pesticides.] Riv. Sci. Techn. Alim. Nutr. Um., 6, 161-165
(in Italian).
Haseman, J.K., Winbush, J.S. & O'donnell, M.R. (1986) Use of dual
control groups to estimate false positive rates in laboratory
animal carcinogenicity studies. Fundam. Appl. Toxicol., 7,
573-584.
Hassan, A. & Zayed, S.M.A.D. (1965) Metabolism of organophosphorus
insecticides. III. Fate of the methyl groups of Dipterex
in vivo. Can. J. Biochem., 43, 1271-1275.
Hassan, A., Zayed, S.M.A.D. & Abdel-Hamid, F.M. (1965a) Metabolism of
organophosphorus insecticides. II. Metabolism of
O,O-dimethyl-2,2,2-trichloro-1hydroxyethyl phosphonate (Dipterex)
in mammalian nervous tissue and kinetics involved in its reaction
with acetyl cholinesterase. Can J. Biochem., 43, 1263-1269.
Hassan, A., Zayed, S.M.A.D. & Hashish, S. (1965b) Metabolism of
organophosphorus insecticides. VI. Mechanism of detoxification of
Dipterex in the rat. Biochem. Pharmacol., 14, 1692-1694.
Hayes, R.H. (1985) Pilot study on trichlorfon with mice. Unpublished
report No. 656 from Mobay Corporation, USA. Submitted to WHO by
Bayer AG, Germany.
Hayes, R.H. (1988) Oncogenicity study of technical grade trichlorfon
with mice. Unpublished report No. 1039 from Mobay Corporation,
USA. Submitted to WHO by Bayer AG, Germany.
Hayes, R.H. (1989) Chronic toxicity/oncogenicity study of technical
grade trichlorfon (Dylox) with rats. Unpublished report No. 1108
from Mobay Corporation, USA. Submitted to WHO by Bayer AG,
Germany.
Hayes, R.H. & Ramm, W.W. (1987) Subchronic delayed neurotoxicity study
of trichlorfon technical (Dylox) with hens. Unpublished report
No. 939 from Mobay Corporation, USA. Submitted to WHO by Bayer
AG, Germany.
Heimann, K.G. (1985) [Dipterex (L 13/59, techn.), determination of
acute toxicity (LD50).] Unpublished letter from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany (in German).
Heimann, K.G. & Wood, C.M. (1987) Trichlorfon technical. Study of
subacute dermal toxicity to rabbits. Unpublished report No. R
4150 from Bayer, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Germany.
Herbold, B. (1979a) L 13/59. Micronucleus test in the mouse for
evaluation of mutagenicity. Unpublished report No. 8505 from
Bayer, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Germany (in German).
Herbold, B. (1979b) [Appraisal of dominant lethal test on trichlorfon
(Dipterex).] Unpublished report from Martin Luther Universitat,
Germany. Submitted to WHO by Bayer AG, Germany (in German).
Herbold, B. (1979c) L 13/59. Dominant lethal study on male mouse to
test for mutagenic effects. Unpublished report No. 8745 from
Bayer, Wuppertal, Germany. Submitted to WHO by Bayer AG, Germany.
Herbold, B. (1984) [L 13/59. Pol test in E. coli to investigate DNA
damage effects.] Unpublished report No. 12434 from Bayer,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Germany (in
German).
Herbold, B. (1986) L 13/59. Cytogenetic study with human lymphocyte
cultures in vitro to evaluate for harmful effects on
chromosomes. Unpublished report No. 14837 from Bayer, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Germany.
Herbold, B. (1992) L 13/59. In vivo cytogenetic study of the
spermatogonia in Chinese hamster to evaluate for induced
clastogenic effects. Unpublished report No. 21688 from Bayer,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Germany.
Herbold, B. (1997) BAY a 9826, BAY z 7216, BAY z 7217 Micronucleus
tests on the mouse. Unpublished report No. PH 26329 from Bayer,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Germany.
Hierons, R. & Johnson, M.K. (1978) Clinical and toxicological
investigations of a case of delayed neuropathy in man after acute
poisoning by an organophosphorus pesticide. Arch. Toxicol., 40,
279-284.
Hirakawa, T. (1983a) Acute toxicity study of trichlorfon on mice by
oral, intraperitoneal, subcutaneous and percutaneous route.
Unpublished report ID 17258 from Bozo Research Centre, Japan.
Submitted to WHO by Bayer AG, Germany.
Hirakawa, T. (1983b) Acute toxicity study of trichlorfon on rats by
oral, intraperitoneal, subcutaneous and percutaneous route.
Unpublished report ID 17259 from Bozo Research Centre, Japan.
Submitted to WHO by Bayer AG, Germany.
Hobik, H.P. (1967) [Histological investigation of spinal cord and
sciatic nerves from neurotoxicity experiemnts in hens dosed with
Dipterex.] Unpublished report no 446 from Bayer, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Germany (in German).
Hoffmann, K., Ruf, J. & Mager, H. (1988) [Chronic toxicity in rhesus
monkeys dosed oraqlly for 26 weeks (gavage).] Unpublished study
No. 16545 from Bayer, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Germany (in German).
Hoorn, A.J.W. (1983) Mutagenicity evaluation of L 13/59 in the reverse
mutation induction assay with Saccharomyces cerevisiae strains
S138 and S211alpha. Unpublished report No. R2540 from Litton
Bionetics, Netherlands. Submitted to WHO by Bayer AG, Germany.
IARC (1983) Trichlorfon. In: IARC Monographs on the Evaluation of
the Carcinogenic Risk of Chemicals to Humans, Vol. 30,
Miscellaneous pesticides, Lyon, International Agency for
Research on Cancer, pp. 207-231.
IARC (1987) IARC Monographs on the Evaluation of the Carcinogenic
Risk of Chemicals to Humans, Suppl. 7, Overall Evaluations
of Carcinogenicity: An Updating of IARC Monographs Volumes 1
to 42, Lyon, International Agency for Research on Cancer,
p. 73.
Inukai, H. & Iyatomi, A. (1977) Trichlorfon. Mutagenicity test on
bacterial systems. Unpublished report No. 87 from Nitokuno
Agricultural Chemicals Institute, Japan. Submitted to WHO by
Bayer AG, Germany.
Jewsbury, J.M. (1981) Metrifonate in schistosomiasis therapy and
prophylaxis. Acta Pharmacol. Toxicol., 49 (Suppl. V),123-130.
Johnson, M.K. (1981) Delayed neurotoxicity -- Do trichlorphon and/or
dichlorvos cause delayed neuropathy in man or in test animals?
Acta Pharmacol. Toxicol., 49 (Suppl. V), 87-98.
Jones, D.C.L., Simmon, V.F., Mortelmans, K.E., Mitchell, A.D., Evans,
E.L., Jotz, M.M., Riccio, E.S., Robinson, D.E. & Kirkhart, B.A.
(1984) In vitro and in vivo mutagenicity studies of
environmental chemicals. Report from SRI International, USA, for
Environmental Protection Agency (EPA-600/1-84-003). National
Technical Information Service, USA.
Kawauchi, S. (1997) BAY a 9826 single oral administration toxicity
study in dogs. Unpublished report No. R 6836 from Experimental
Biomedical Research, Japan. Submitted to WHO by Bayer AG,
Germany.
Kimmerle, G. (1970) Metrifonat (Bayer 2349), toxicological
investigations. Unpublished report No. 2142 from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Kimmerle, G. (1975a) L 13/59, acute inhalation toxicity study on rats.
Unpublished report No. 5581 from Bayer, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Germany.
Kimmerle, G. (1975b) L 13/59, subacute inhalation toxicity study on
rats. Unpublished report No. 5582 from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Klimmer, O.R. (1955) [Final report on the new insecticide Bayer L
13/59.] Unpublished report from Pharmakologisches Institut, Bonn,
Germany. Submitted to WHO by Bayer AG, Germany (in German).
Kiraly, J., Szentesi, I., Ruzicska, M. & Czeize, A. (1979) Chromosome
studies in workers producing organophosphate insecticides.
Arch. Environ. Contam. Toxicol., 8, 309-319.
Knox, B., Askaa, J., Basse, A., Bitsch, V., Eskildsen, M., Mandrup,
M., Ottosen, H.E., Overby, E., Pedersen, K.B. & Rasmussen, F.
(1978) Congenital ataxia and tremor with cerebellar hypoplasia in
piglets borne by sows treated with Neguvon(R) vet (Metrifonate,
Trichlorfon) during pregnancy. Nord. Vet. Med., 30, 538-545.
Kowalski, R.L., Clemens, G.R., Bare, J.J. & Hartnagel, R.E., Jr (1987)
A teratology study with Dylox (R), technical (Trichlorfon) in the
rat. Unpublished report No. MTD0017 from Miles Laboratories, USA.
Submitted to WHO by Bayer AG, Germany.
Kurinnyi, A.I. (1975) Comparative study of the cytogenetic effect of
certain organophosphorus pesticides. Genetika, 11, 64-69.
Lamb, M.J. (1977) Mutagenicity tests with metrifonate in
Drosophila melanogaster. Mutat. Res., 56, 157-162.
Lehotzky, K. (1982) Effect of pesticides on central and peripheral
nervous system function in rats. Neurobehav. Toxicol.
Teratol., 4, 665-669.
Lorke, D & Kimmerle, G. (1966) Neurotoxicity studies on hens with
Dipterex active ingredient. Unpublished report from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Lorke, D. & Loser, E. (1966) Chronic toxicological studies on rats.
Unpublished report No. 19292 from Bayer, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Germany.
Loser, E. (1969) BAY 15922 Generation study on rats. Unpublished
report No. 1195 from Bayer, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Germany.
Loser, E. (1970) Bay 15922. Chronic toxicological studies on dogs.
Unpublished report No. 2467 from Bayer, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Germany.
Machemer, L. (1979a) [L 13/59 (trichlorfon): Evaluation for
embryotoxic and teratogenic effects on orally dosed rats.]
Unpublished report No. 8400 from Bayer,Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Germany (in German).
Machemer, L. (1979b) [L 13/59 (trichlorfon): Evaluation for
embryotoxic and teratogenic effects on orally doses rabbits.]
Unpublished report No. 8430 from Bayer,Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Germany (in German).
Martson, L.V. & Voronina, V.M. (1976) Experimental study of the effect
of a series of phosphoroorganic pesticides (dipterex and imidan)
on embryogenesis. Environ. Health Perspectives, 13, 121-125.
Metcalf, R.L., Fukuto, T.R. & March, R.B. (1959) Toxic action of
Dipterex and DDVP to the house fly. J. Econ. Entomol., 52,
44-49.
Mihail, F. (1978) [L 13/59 (Dipterex active ingredient), acute
toxicological studies.] Unpublished report No. 7619 from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany (in German).
Mihail, F. (1986) L 13/59 (trichlorfon): Study for skin-sensitizing
effect on guineapigs in the open epicutaneous test. Unpublished
report No. 14348 from Bayer, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Germany.
Miyamoto, J. (1959) Non-enzymatic conversion of Dipterex into DDVP and
their inhibitory action on enzymes. Botyu-Kagaku, 24, 130-137.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Moutschen-Dahmen, J., Moutschen-Dahmen, M. & Degraeve, N. (1981)
Metrifonate and dichlorvos: Cytogenetic investigations.
Acta Pharmacol. Toxicol., 49 (Suppl. V), 29-39.
Myhr, B.C. (1983) Evaluation of L 13/59 trichlorfon in the primary rat
hepatocyte unscheduled DNA synthesis assay. Unpublished report
No. R2687 from Litton Bionetics, USA. Submitted to WHO by Bayer
AG, Germany.
Nehes, M., Selypes, A. & Scheufler, H. (1982)[ Mutagenicity of
trichlorfon and dimethoate on mouse bone marrow cells in vivo].
Wissensch. Z. Univ. Halle, 31, 57- 62 (in German).
Niedziella, S.W., Göpel, W. & Banzhaf, E. (1985) [Acute alkylphosphate
poisoning (trichlorphon) with delayed polyneuropathy syndrome.]
Z. Ges. Inn. Med., 40, 237-239 (in German).
Nordgren, I., Bergström, M., Holmstedt, B. & Sandoz, M. (1978)
Transformation and action of metrifonate. Arch. Toxicol., 41,
31-41.
Nordgren, I., Holmstedt, B., Bengtsson, E. & Finkel, Y. (1980) Plasma
levels of metrifonate and dichlorvos during treatment of
schistosomiasis with Bilarcil. Am. J. Trop. Med. Hyg., 29,
426-430.
Nordgren, I., Bengtsson, E., Holmstedt, B. & Pettersson, B.M. (1981)
Levels of metrifonate and dichlorvos in plasma and erythrocytes
during treatment of schistosomiasis with Bilarcil. Acta
Pharmacol. Toxicol., 49 (Suppl. V), 79-86.
Olajos, E.J., Rosenblum, I., Coulston, F. & Strominger, N. (1979) The
dose response relationship of trichlorfon neurotoxicity in hens.
Ecotoxicol. Environ. Saf., 3, 245-255.
Paik, S.G. & Lee, S.Y. (1977) Genetic effects of pesticides in the
mammalian cells. 1. Induction of micronucleus. Korean J.
Zool., 20, 19-28.
Plestina, R., Davis, A. & Bailey, D.R. (1972) Effect of metrifonate on
blood cholinesterases in children during treatment of
schistosomiasis. Bull. World Health Organ., 46, 747-759.
Pollingher, B., Cozma, V. & Oprisan, C. (1973) Clinical and
electromyographic study of two cases of severe polyneuritis
following acute poisoning with Neguvon (organophosphoric
pesticide). Electroencephalogr. Clin. Neurophysiol., 35, 433
( Pestic. Abstr., 75-0322).
Pope, A.M., Heavner, J.E., Guarnieri, J.A. & Knobloch, C.P. (1986)
Trichlorfoninduced congenital cerebellar hypoplasia in neonatal
pigs. J. Am. Vet. Med. Assoc., 189, 781-783.
Putman, D.L. (1987) Sister chromatid exchange assay in Chinese hamster
ovary (CHO) cells. Unpublished report No. 826 from
Microbiological Associates, USA. Submitted to WHO by Bayer AG,
Germany.
Ray, E.R. (1998) Chronic effects of low level exposure to
anticholinesterases -- A mechanistic review. Toxicol. Lett.,
102-103, 527-533.
Reiner, E., Krauthacker, B., Simeon, V. & Skrinjaric-Spoljar, M.
(1975) Mechanism of inhibition in vitro of mammalian
acetylcholinesterase and cholinesterase in solutions of
O,O-dimethyl-2,2,2-trichloro-1-hydroxyethyl phosphonate
(trichlorphon). Biochem. Pharmacol., 24, 717-722.
Reiss, C.S., Herrman, J.M. & Hopkins, R.E., II (1987) Effect of
antihelminthic treatment on the immune response of mice.
Lab. Anim. Sci., 37, 773-775.
Renhof, M. (1997) BAY a 9826: Acute toxicity in the mouse and rat
after oral administration and in the mouse after intravenous
administration. Unpublished report No. 25832 from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Rosenblum, I. (1981) A two-generation, two-year feeding study of
trichlorfon in the rat. Unpublished report No. 2142 from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Sasaki, M., Sugimura, K., Yoshida, M. A. & Abe, S. (1980) Cytogenetic
effects of 60 chemicals on cultured human and Chinese hamster
cells. Kromosomo, 20, 574-584.
Scheufler, H. (1975) [Effect of relatively high doses of dimethoate
and trichlorfon on embryogenesis in mice.] Biol. Rundsch., 13,
238-240 (in German).
Sheets, L.P. & Hamilton, B.F. (1995) A subchronic dietary
neurotoxicity screening study with technical grade trichlorfon
(Dylox, Dipterex) in Fischer 344 rats. Unpublished report No.
MRC-00284 from Bayer Corp., USA. Submitted to WHO by Bayer AG,
Germany.
Shiraishi, S., Goto, I., Yamashita, Y., Ohnishi, A. & Nagao, H. (1977)
Dipterex polyneuropathy. Neurol. Med., 6, 34-38.
Shiraishi, S., Inoue, N. & Murai, Y. (1982) Dipterex poisoning.
J. Univ. Occup. Environ. Health, 4, 177-183.
Shiraishi, S., Inoue, N., Murai, Y., Onishi, A., & Noda, S. (1983)
Dipterex (trichlorfon) poisoning -- Clinical and pathological
studies in human and monkeys. J. Univ. Occup. Environ.
Health, 5 (Suppl.), 125-132.
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A. & Kada, T. (1976)
Mutagenicity screening of pesticides in the microbial system.
Mutat. Res., 40, 19-30.
Shirasu, Y., Moriya, M. & Koyashiki, R. (1979) Trichlorfon.
Mutagenicity test on bacterial systems. Unpublished report from
Institute of Environmental Toxicology, Japan. Submitted to WHO by
Bayer AG, Germany.
Slott, V. & Ecobichon, D.J. (1984) An acute and subacute neurotoxicity
assessment of trichlorfon. Can. J. Physiol. Pharmacol., 62,
513-518.
Spicer, E.J.F. & Payne, S. (1971) Pathology report of Bay 15922 4-year
dog study. Unpublished report from Huntingdon Research Centre,
United Kingdom. Submitted to WHO by Bayer AG, Germany.
Spicer, E.J.F. & Urwin, C (1971) Pathology report of Bay 15922
Generation experiment in rats. Unpublished report from Huntingdon
Research Centre, England. Submitted to WHO by Bayer AG, Germany.
Staples, R.E. & Goulding, E.H. (1979) Dipterex teratogenicity in the
rat, hamster and mouse when given by gavage. Environ. Health
Perspectives, 30, 105-113.
Staples, R.E., Kellam, R.G. & Haseman, J.K. (1976) Developmental
toxicity in the rat after ingestion or gavage of organophosphate
pesticides (dipterex, imidan) during pregnancy. Environ.
Health Perspectives, 13, 133-140.
Stieglitz, R., Gibel, W., Werner, W. & Stobbe, H. (1974) Experimental
study on haematotoxic and leukaemogenic effects of trichlorophone
and dimethoate. Acta Haematol., 52, 70-76.
Suzuki, T. (1996) Three-month repeated oral administration toxicity
study and recovery test of BAY a 9826 in dogs. Unpublished report
No. R 6641 from JBC, Gifu Research Laboratory, Japan. Submitted
to WHO by Bayer AG, Germany.
Teichmann, B. & Hauschild, F. (1978) [Test of O,O-dimethyl
(1-hydroxy-2,2,2trichloroethyl)phosphonate (trichlorfon) for
carcinogenicity in mice after oral (oesophageal-gastric gavage)
and dermal application.] Arch. Geschwulstforsch., 48, 301-307
(in German).
Teichmann, B. & Schmidt, A. (1978) [Test of O,O-dimethyl
(1-hydroxy-2,2,2trichlorethyl)phosphonate (trichlorphon) for
carcinogenicity in Syrian golden hamsters ( Mesocricetus
auratus Waterhouse) after intraperitoneal Injection.]
Arch. Geschwulstforsch., 48, 718-721 (in German).
Teichmann, B., Hauschild, F. & Eckelmann, A. (1978) [Test of
O,O-dimethyl(1hydroxy-2,2,2-trichlorethyl)phosphonate
(trichlorphon) for carcinogenicity in rats after oral
(oesophageal-gastric gavage) and intraperitoneal Injection.]
Arch. Geschwulstforsch., 48, 112-119.
Thyssen, J. (1981) L 13/59 (Dipterex active ingredient). Tests for
skin and eye irritation. Unpublished report from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Thyssen, J., Kaliner, G. & Eben, A. (1982) [Trichlorfon, Schmelzware
Japan, neurotoxicity studies in hens.] Unpublished report No.
10811 from Bayer, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Germany (in German).
Toyoshi, T. (1996) One-month repeated oral administration toxicity
study and recovery test of BAY a 9826 in rats. Unpublished report
No. R 6642 from JBC, Gifu Research Laboratory, Japan. Submitted
to WHO by Bayer AG, Germany.
Völkner, W. (1987) Sister chromatid exchanges (SCEs) in Chinese
hamster bone marrow cells. Unpublished report No. R4079 from
Technical University of Darmstadt, Germany. Submitted to WHO by
Bayer AG, Germany.
Watabe, H. (1997) A reverse mutation assay of metrifonate using
bacteria. Unpublished report No. R 6837 from Mitsubishi Yuka
Bio-Clinical Labs, Japan. Submitted to WHO by Bayer AG, Germany.
Wegner, D. (1970) Bilarcil (Bay 2349) Clinical experience 1960-1969
(survey of the literature). Unpublished report No. 2225 from
Bayer, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Germany.
WHO (1992) Trichlorfon (Environmental Health Criteria 132). Geneva,
World Health Organization.
Williams, M.W., Fuyat, H.N. & Fitzhugh, O.G. (1959) The subacute
toxicity of four organic phosphates to dogs. Toxicol. Appl.
Pharmacol., 1, 1-7.
Witter, R.F. & Gaines, T. B. (1963) Relationship between depression of
brain or plasma cholinesterase and paralysis in chickens caused
by certain organic phosphorus compounds. Biochem. Pharmacol.,
12, 1377-1386.
Witterland, W.F. (1984) Mutagenicity evaluation of L 13/59 in the
mouse lymphoma forward mutation assay. Unpublished report No.
R2824 from Litton Bionetics, Netherlands. Submitted to WHO by
Bayer AG, Germany.
Xu, H.H. & Schurr, K.M. (1990) Genotoxicity of 22 pesticides in
microtitration SOS Chromotest. Toxicol. Assess. Int. J., 5,
1-14.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T., Mortelmans, K. &
Speck, W. (1987) Salmonella mutagenicity tests: III Results from
the testing of 255 chemicals. Environ. Mutag., 9 (Suppl. 9),
1-109.
Table 1. Results of studies of the acute toxicity of trichlorfon
Species Sex Route LD50 References
(mg/kg bw)
Mouse M&F Oral 616-950 Klimmer (1955); Mihail
(1978); FAO/WHO (1972b);
Hirakawa (1983a); Renhof
(1997)
Mouse M&F Intraperitoneal 344-600 Dubois & Cotter (1955);
Hirakawa (1983a); WHO
(1992)
Mouse M&F Subcutaneous 267360 FAO/WHO (1972b); Hirakawa
(1983a)
Mouse M&F Intravenous 354-370 Renhof (1997)
Mouse M&F Dermal > 5000 Hirakawa (1983a)
Rat M&F Oral 136-660 Dubois & Cotter (1955);
Klimmer (1955); Kimmerle
(1970); FAO/WHO (1972b);
Crawford & Anderson (1973);
Mihail (1978); Hirakawa
(1983b); Heimann (1985);
WHO (1992); Renhof (1997)
Rat M&F Intraperitoneal 156-485 Dubois & Cotter (1955);
Arthur & Casida (1957);
FAO/WHO (1972b); Crawford &
Anderson (1973); Hirakawa
(1983b)
Rat M&F Subcutaneous 290-400 FAO/WHO (1972b);
Hirakawa (1983b)
Rat M Intravenous 184 Kimmerle (1970)
Rat M&F Inhalation LC50, > 533 Kimmerle (1975a)
mg/m3
Rat M&F Dermal 2800-> 5000 Hirakawa (1983b); Mihail
(1978); WHO (1992)
Guinea-pig M&F Intraperitoneal 300 Dubois & Cotter (1955);
WHO (1992)
Rabbit Oral 160 FAO/WHO (1972b)
Rabbit M Dermal 5000 WHO (1992)
Dog M oral > 300-420 Mihail (1978); WHO (1992);
Kawauchi (1997)
fasciculations, and coma. The primary cause of death is respiratory
failure. After sublethal doses, animals recover very rapidly,
suggesting that trichlorfon is detoxified faster than many other
organophosphorus compounds (WHO, 1992).
In two separate studies, six New Zealand white rabbits were
treated dermally with trichlorfon for 24 h on intact and abraded skin.
No irritation was seen after 24, 48, or 72 h. Further animals received
trichlorfon in the eye for 5 min ( n = 5) or 24 h ( n = 3). Severe
redness was seen up to 72 h after dosing for either length of time.
Slight to moderate chemosis was also observed up to 48-72 h after
dosing in both groups. The effects did not persist to day 7, and no
effect was seen in the cornea or iris. The compound is a slight ocular
irritant (Thyssen, 1981; Bond, 1986).
Trichlorfon was assessed for skin sensitizing potential in eight
male BOR: DHPW guinea-pigs by the open epicutaneous test. Dermal
induction was carried out 5 days a week for 4 weeks with 0, 1, 3, or
10% trichlorfon, and dermal challenge was conducted with 0.3, 1, 3, or
10 % trichlorfon 4, 6, or 8 weeks after induction. No skin reactions
were seen in animals induced with 1% trichlorfon, but approximately
half the animals induced with 3 or 10% trichlorfon showed skin
reactions when challenged with each concentration of trichlorfon. The
compound was therefore considered to have skin sensitizing potential
(Mihail, 1986).
2.2.2 Short-term studies of toxicity and carcinogenicity
Mice
Trichlorfon (purity, 98.2%) was administered to groups of 15
Charles River CD-1 mice of each sex at a dietary concentration of 0,
100, 300, 900, or 2700 mg/kg for 8 weeks. There were no deaths or
drug-related clinical signs, and body weights were not affected. Food
consumption was not affected in males, but appeared to have been
increased by 18-32% in females. The report suggests that the latter
finding was due to increased wastage by the treated female mice.
Cholinesterase activity in plasma and erythrocytes, measured during
weeks 4, 6, and 8, was not significantly affected at the lowest dose
but was dose-dependently inhibited at higher doses; the maximum
inhibition was by about 70% for plasma and 50% for erythrocyte enzyme.
Brain acetylcholines-terase activity at the end of the study was
dose-dependently inhibited at doses > 300 mg/kg of diet, by up to
68%. Gross necropsy revealed no remarkable changes. Haematology, serum
biochemistry, urinary anlysis, organ weighing, and histopathology were
not performed (Hayes, 1985).
Groups of 10 male and 10 female B6C3F1 mice were given
trichlorfon (purity unknown) in the diet at a concentration of 0, 62,
180, 560, 1700, or 5000 mg/kg for 13 weeks. The results were reported
in abstract form only. All but one male at 5000 mg/kg of diet and one
female at 1666 mg/kg of diet survived. The body weights of mice at
5000 mg/kg of diet were reduced. Plasma and erythrocyte cholinesterase
activities were decreased in a dose-related manner, while motor
activity and grip strength were reduced at 1700 and 5000 mg/kg of
diet. The absolute and relative weights of the liver, kidney, and
spleen were increased in mice at 1700 and 5000 mg/kg of diet. The
neurotoxic effects and changes in organ weight were not accompanied by
pathological effects (Chan & Peters, 1989; WHO, 1992).
Rats
Groups of 10 male and 10 female rats were exposed for 6 h/day for
3 weeks (total, 15 exposures) to an atmosphere containing trichlorfon
(purity unknown) at a concentration of 0, 12.7, 35.4, or 103.5
mg/m3. The health of the animals at 103.5 mg/m3 was affected, but
details were not provided. Body-weight gain was unaffected, and
haematological, clinical chemical, and urinary parameters measured at
the end of the study were similar to those of controls. Plasma,
erythrocyte, and brain cholinesterase activities were inhibited in
males given 103.5 mg/m3 and in females given 35.4 and 103.5 mg/m3.
Gross and histopathological examinations showed no remarkable changes,
the only finding at autopsy being increased spleen weights in males at
35.4 and 103.5 mg/m3 (Kimmerle, 1975b; WHO,1992).
Groups of 12 male and 12 female Crj: CD SD rats were given
trichlorfon (purity, 100%) at a dose of 0, 25, 50, or 100 mg/kg bw per
day by gavage for 30 days. Further groups of 10 rats of each sex were
treated and then allowed a 2-week recovery period. Additional treated
groups of 20 rats of each sex were used to determine the profiles of
the drug in blood after dosing on days 1, 15, and 30. The peak
concentrations of the individual enantiomers of trichlorfon and
dichlorvos were dose-related and were reached by about 1 h, declining
rapidly to become undetectable by 24 h. The concentrations of each
enantiomer were roughly similar in males, while that of the
(-)-enantiomer was generally higher than that of the (+)enantiomer in
females; this was reflected in higher total drug concentrations in
females. The concentrations of dichlorvos in blood represented only a
small fraction of those of trichlorfon and were again higher in female
rats. There was no evidence of accumulation in blood.
The signs of toxicity included tremor, decreased locomotor
activity, salivation, bradypnoea, and prone position in many females
given 100 mg/kg bw per day, in one or two females given 50 mg/kg bw
per day, and in males given 100 mg/kg bw per day. There were no deaths
and no effects on body weight, food consumption, or ophthalmological
or urinary parameters. Slight decreases in erythrocyte counts and
haematocrit were seen in females at 100 mg/kg bw per day when compared
with controls at the end of treatment, but these values were not
significantly different from those of controls at the end of the
recovery period. Animals at 100 mg/kg bw per day had increased blood
concentrations of cholesterol, triglycerides, and phospholipids
(females), and increased activity of lactic dehydrogenase (males). At
autopsy, the livers of animals at 50 and 100 mg/kg bw per day were
heavier and/or enlarged and showed hypertrophy of centrilobular
hepatocytes. The weight of the kidney was increased in males at 100
mg/kg bw per day. The NOEL was 25 mg/kg bw per day (Toyoshi, 1996).
Groups of seven Wistar rats of each sex were given trichlorfon
(purity unknown) in the diet to provide a dose of 0, 1, 5, 10, 30, 50,
or 100 mg/kg bw per day for 12 weeks. The highest dose inhibited
cholinesterase activity in all tissues examined (erythrocytes, serum,
brain, heart, liver, and skeletal muscle), but the extent of the
inhibition differed. Nevertheless, no changes were detected in
nocturnal behaviour, reaction to external stimuli, conjunctival or
pinna reflexes, or the avoidance reflex to painful stimuli. No
significant histological alterations were observed (reviewed in WHO,
1992).
Groups of 10 male and 10 female Fischer rats were given
trichlorfon (purity unknown) in the diet at a concentration of 0, 62,
180, 560, 1700, or 5000 mg/kg for 13 weeks. The results were reported
in abstract form only. All rats survived. The body weights of those at
5000 mg/kg of diet were reduced. Plasma and erythrocyte cholinesterase
activities were lowered in a dose-related manner, while motor activity
and grip strength were reduced at 1700 and 5000 mg/kg of diet. The
absolute and relative weights of the liver, kidney, and spleen were
increased in animals at 1700 and 5000 mg/kg of diet. The neurotoxic
effects and changes in organ weights were not accompanied by
pathological effects (Chan & Peters, 1989; WHO, 1992).
Groups of 13 rats of each sex were fed diets containing
trichlorfon (purity unknown) at a concentration of 0, 20, 100, or 300
mg/kg for 16 weeks. No effects were observed on growth, behaviour,
food consumption, organ weights, or gross or microscopic appearance of
the tissues. At 300 mg/kg of diet, plasma and erythrocyte
cholinesterase activity was depressed by 20-30% in males and females,
while that of brain cholinesterase was depressed by 20% in females
only. Salivary gland cholinesterase activity was not inhibited. At 100
mg/kg of diet, a slight depression of plasma cholinesterase activity
was observed (Doull & Rehfuss, 1956; WHO, 1992).
Groups of 10 male Wistar albino rats were fed diets containing
trichlorfon (purity unknown) at 0, 1, 5, 25, or 125 mg/kg for 16
weeks. Erythrocyte cholinesterase activity was not depressed at any
dose, and no effects were seen on food consumption, growth, or gross
appearance of the tissues (reviewed in WHO, 1992).
Guinea-pigs
After oral administration of trichlorfon (purity unknown) at 100
mg/kg bw per day for 60 days to guinea-pigs, the haemoglobin
concentration but not the haematocrit was decreased. The activity of
serum cholinesterase was decreased by 50%, and that of alkaline
phosphatase was increased (reviewed in WHO, 1992).
Rabbits
Groups of five New Zealand white rabbits of each sex received
dermal applications of 0, 100, 300, or 1000 mg/kg bw per day
trichlorfon (purity, 99%) onto shaved skin. The drug was left on the
skin of restrained animals for 6 h/day, 5 days/week for 3 weeks. The
appearance and behaviour of the rabbits were unchanged, and there were
no deaths. Body weight, food consumption, and the skin at the
application sites were unaffected. There were no meaningful changes in
haematological, clinical chemical, or urinary parameters at the end of
the study. Plasma, erythrocyte, and brain cholinesterase activities
were inhibited by 20-30% in animals at 300 and 1000 mg/kg bw per day.
At autopsy, no treatment-related alterations in liver microsomal
enzyme activities, organ weights, or histological appearance were seen
(Heimann & Wood, 1987).
Dogs
Four dogs were fed diets containing trichlorfon (purity unknown)
for 12 weeks at a concentration of 20, 100, 300, or 500 mg/kg and were
then left for a further 4 weeks on unmedicated diet. All of the dogs
remained healthy and maintained their body weight throughout the
study. After 12 weeks of treatment, the erythrocyte and plasma
cholinesterase activities of animals at 300 and 500 mg/kg of diet were
decreased by 40-60% when compared with the concentrations before
treatment, and the activity in plasma was inhibited by 10% in animals
at 100 mg/kg of diet. On cessation of treatment, the enzyme levels
recovered within 2-4 weeks (Doull & Vaughn, 1958; FAO/WHO, 1972b).
Four dogs were fed diets containing trichlorfon (purity unknown)
at a concentration of 0, 50, 200, or 500 mg/kg for 12 weeks. Plasma
and erythrocyte cholinesterase activities were depressed within 2
weeks in animals at 500 mg/kg of diet, and the effect peaked at 6-8
weeks, at which time the inhibition was about 60% in plasma and 45% in
erythrocytes. A gradual increase in plasma cholinesterase activity was
then seen, to 45% inhibition at termination, even though the compound
was still present in the feed. At 200 mg/kg of diet, the
cholinesterase activity was slightly decreased but was within the
control range. The enzyme activities were restored 6 weeks after
treatment ceased (Williams et al., 1959; FAO/WHO, 1972b).
Groups of three male and three female beagles were given
trichlorfon (purity, 99.2%) in capsules at a dose of 0, 5, 15, or 45
mg/kg bw per day for 3 months. A further three animals of each sex
were given the vehicle or the high dose for 3 months and then allowed
to recover for 2 weeks. The blood concentrations of the enantiomers of
trichlorfon and dichlorvos were measured after dosing on days 1, 30,
and 90. Peak concentrations were achieved within 1 h, the
(-)-enantiomer being detected at 10-100-fold higher concentrations
than the (+)-enantiomer and dichlorvos. Disappearance from blood was
rapid, and the compounds were virtually undetectable by 7 h. The drug
did not accumulate in blood.
No deaths were observed in any group, and no meaningful effects
were seen on mean body weight, food intake, or in ophthalmological,
electrocardiographic, or auditory parameters. Dogs receiving 15 mg/kg
bw per day or more showed tremor, decreased locomotor activity, and
crouching, while at 45 mg/kg bw per day, ataxic gait, lateral
position, vomiting, salivation, licking, liquid, loose, or bloody
stools, and wasting were observed. The results of blood chemistry and
urinary analysis were unremarkable. Haemoglobin, haematocrit, and
erythrocyte count were slightly but progressively decreased during
treatment in males given 45 mg/kg bw per day. It was reported, with no
supporting data, that erythrocyte cholinesterase activity was
inhibited at 15 and 45 mg/kg bw per day. One dog at 45 mg/kg bw per
day lost weight, and at autopsy was found to have decreased thymus
weight with lymphocyte depletion in the cortex, small and immature
testes and prostate, and oligospermia in small epididymides. Similar
thymic changes were observed in one female at 45 mg/kg bw per day. All
of the effects were reversible on cessation of treatment. The NOEL was
5 mg/kg bw per day (Suzuki, 1996).
Monkeys
Groups of five rhesus monkeys of each sex were given trichlorfon
(purity, 98.7%) at a dose of 0, 0.1, or 0.2 mg/kg bw per day in
sterile water by oral intubation for 26 weeks in order to establish an
NOEL for inhibition of erythrocyte cholinesterase activity. There were
no deaths and no effects on appearance, behaviour, nutritional state,
feed or water consumption, or body-weight gain. Haematological tests
showed no changes. No compound-related effects on the liver or kidney
were seen in clinical chemical or urinary analyses, on gross
pathology, or in organ weights. At 0.2 mg/kg bw per day, small
increases and decreases in erythrocyte cholinesterase activity
(< 20%) were seen, which were within the range of biological
variation. The NOEL was 0.2 mg/kg bw per day, the highest dose tested
(Hoffman et al., 1988; WHO, 1992).
2.2.3 Long-term studies of toxicity and carcinogenicity
Mice
Three studies of carcinogenicity were performed with groups of 30
AB/Jena mice that received an oral dose of 0 or 30 mg/kg bw twice
weekly, an intraperitoneal dose of 0 or 28 mg/kg bw per day, or a
dermal application of 0 or 0.25 ml of a 1% solution, providing 2.5 mg
trichlorfon per animal. Dosing was continued for 75 weeks, and the
studies were terminated after 80 weeks. The treated animals showed
reduced body-weight gain and survival, but the tumour incidences were
not increased (Teichmann & Hauschild, 1978; FAO/WHO, 1979b).
Groups of 60 male and 60 female Charles River CD-1 mice were
given trichlorfon (purity unknown) in the diet at a concentration of
0, 100, 300, or 1000 mg/kg for 90 weeks, except for males at the
highest dose which were treated for 82 weeks. These doses were
equivalent to 15, 45, and 150 mg/kg bw per day. Inhibition of
body-weight gain was observed in females given 300 or 1000 mg/kg of
diet, and cholinesterase activity was depressed in animals of each sex
at 1000 mg/kg of diet. No significant microscopic alterations were
reported, and the tumour incidences were not treatment-related
(reviewed in WHO, 1992).
Groups of 50 Crl:CD-1(ICR)BR mice of each sex were given
trichlorfon (purity, 98.2%) in the diet at a concentration of 0, 280,
890, or 2700 mg/kg for 104 weeks, equal to 49, 160, and 510 mg/kg bw
per day for males and 66, 240, and 750 mg/kg bw per day for females.
Increased incidences of urine-stained fur, 'ear lesions' (males), and
vaginal discharge (females) were observed. The body weights of treated
females were increased at various times during the study, but a
consistent effect was observed only at the high dose. There were no
effects on survival, food intake, or haematological end-points. Plasma
and erythrocyte cholinesterase activities were depressed by 20-74% in
the groups given 890 and 2700 mg/kg of diet and in females given
280 mg/kg of diet. Brain cholinesterase activity was inhibited by
20-70% at all doses in a dose-related manner. The weights of the liver
were increased in females at the intermediate and high doses, but this
effect was not accompanied by microscopic changes. Pathological
examination revealed no treatment-related alterations (Hayes, 1988;
WHO, 1992).
Rats
von Gibel and co-workers undertook a series of studies in Wistar
rats (von Gibel et al.,1971, 1973; Stieglitz et al., 1974), which were
given trichlorfon three times per week orally at 30 mg/kg bw, twice
per week by subcutaneous injection at 30 mg/kg bw, twice per week
orally at 15 mg/kg bw, or twice per week by intraperitoneal injection
of 15 mg/kg bw. Treatment was continued for the lifetime of the
animals. The groups given 30 mg/kg bw were reported to have higher
incidences of liver necrosis, liver cirrhosis, and forestomach
papillomas than controls. The lower doses caused hyperplasia of the
blood-forming elements of bone marrow, extraosseal metaplasia in the
liver and spleen, and hepatotoxicity and carcinogenicity. JMPR
(FAO/WHO, 1972b, 1976b) and the International Agency for Research on
Cancer (IARC, 1983) concluded that these studies were difficult to
interpret because of lack of information on the methods and results
and the amalgamation of tumour types. Also, the findings contradicted
those of other studies. Therefore, little use could be made of these
studies .
Groups of 25 male and 25 female Sprague-Dawley rats were fed
diets containing trichlorfon (purity unknown) at a concentration of 0,
50, 250, 500, or 1000 mg/kg for 17 months for males and for 24 months
for females. These doses were equivalent to 2.5, 12, 25, and 50 mg/kg
bw per day. Survival was reduced at 1000 mg/kg of diet, and the
body-weight gain of males at this dose was retarded. Cholinesterase
activity was depressed in serum, erythrocytes, and submaxillary gland
but not in the brain of rats at 1000 mg/kg of diet; at 500 mg/kg of
diet, only serum enzyme activity was inhibited. Female rats fed 500 or
1000 mg/kg of diet had no primary follicules and had primitive ova,
while male rats fed 1000 mg/kg of diet showed depression of
spermatogenesis. Necrotizing arteritis was observed at the two highest
concentrations. The frequencies of mammary tumours in females, in
ascending order of dose, were 14, 8, 20, 21, and 25%. The time to
appearance of these tumours was reduced in a dose-related manner
(reviewed in FAO/WHO, 1972b).
Groups of 25 male and 50 female Sprague-Dawley rats were fed
diets containing trichlorfon (purity unknown) at a concentration of 0,
100, 200, or 400 mg/kg for 18 months, equivalent to 5, 10, and 20
mg/kg bw per day. A significant increase in the mortality rate in all
groups led to premature termination of the study at 70 weeks. Serum
and erythrocyte cholinesterase activities were inhibited at 400 mg/kg
of diet. Cystic granular alterations of the ovaries were seen at 200
and 400 mg/kg of diet, and ovarian atrophy, absence of primary
follicules, and primitive ova were noted at 400 mg/kg of diet. Benign
mammary tumours were found in 8% of controls, 11% of females at 200
mg/kg of diet, and 15% of females at 400 mg/kg of diet, with no change
in the time of their appearance. These results and those of the study
described above were considered to indicate that trichlorfon enhances
certain normal ageing processes, particularly in reproductive tissues
(reviewed in FAO/WHO, 1972b).
Groups of 50 FB30 rats of each sex were fed diets containing
trichlorfon (purity, 95.3%) at a concentration of 50, 250, 500, or
1000 mg/kg for 2 years, equivalent to 2.5, 12.5, 25, and 50 mg/kg bw
per day. The control group comprised 100 males and 100 females. No
changes in survival, general behaviour, appearance, body-weight gain,
or food consumption were reported. No significant differences in blood
count or urinary parameters were reported in samples obtained during
the final 3 weeks of the study. Tests of liver function showed an
increase in 'serum dehydrogenase' activity in males at 50 mg/kg of
diet and in females at 50 and 500 mg/kg of diet. Serum protein
concentration and alanine and aspartate aminotransferase activities
were unchanged. Acetylcholinesterase activity in 'total blood' was
decreased by approximately 20% at 1000 mg/kg of diet in week 90. No
macroscopic evidence of treatment-related changes was reported at
necropsy. Liver weights were significantly increased in males at 250
and 1000 mg/kg of diet, and spleen weights were significantly
increased in females at 50 and 1000 mg/kg of diet. Histological
examination was performed on five male and five female rats at 1000
mg/kg of diet and on three male and three female control animals. The
ovaries of an additional 15 treated and 30 control animals were
examined, as previous studies had indicated a possible effect. The
findings, including tumour incidences, were unrelated to treatment
(Lorke & Loser, 1966; Grundmann & Hobik, 1966; FAO/WHO, 1972b).
Groups of 30 male and 35 female albino rats received an oral dose
of 0 or 30 mg/kg bw or an intraperitoneal dose of 0 or 12 mg/kg bw of
trichlorfon (purity unknown) twice per week. Dosing was continued for
90 weeks, and the studies were terminated after 118 weeks. The treated
animals showed reduced survival, but the tumour incidences were not
increased (Teichmann et al., 1978; FAO/WHO, 1979b).
Trichlorfon (purity, 98.6-99.1%) was fed in the diet to groups of
75 Long-Evans rats of each sex at a concentration of 0, 100, 300, or
1000 mg/kg, equivalent to 5, 15, and 50 mg/kg bw per day. The animals
were maintained on their respective diets for 90 days and then mated
within groups. The resulting F1 litters were reared to 21 days
post partum, at which time 75 offspring of each sex per group were
selected to continue on the experiment. The F0 and selected F1
rats were treated for 2 years. There was no effect on fertility,
reproduction, food intake, body-weight gain, or mortality rate in
either generation. Plasma cholinesterase activity was depressed by
30-60% in both generations at 1000 mg/kg of diet at most times during
the study. Erythrocyte cholinesterase activity was reduced by 30-60%
in both generations given 1000 mg/kg of diet, at most sampling times
in females and on some occasions in males. Brain cholinesterase
activity was significantly lower (40%) in females given 1000 mg/kg of
diet and was 25% lower in F1 males. No histological changes
attributable to treatment were found (Rosenblum, 1981; Griffin et al.,
1982).
Groups of 50 Fischer 344 rats of each sex were given diets
containing trichlorfon (purity, 98.8%) for 2 years at a mean measured
time-weighted average concentration of 0, 92, 270, or 1500 mg/kg. The
nominal concentrations were 0, 100, 300, and, at the highest
concentration, 1000 mg/kg of diet for 27 weeks, 1250 mg/kg of diet for
5 weeks, 1500 mg/kg of diet for 8 weeks, and 1750 mg/kg of diet for 65
weeks. These doses are equal to 4.5, 13, and 76 mg/kg bw per day for
males and 5.8, 17, and 94 mg/kg bw per day for females. Satellite
groups of 20 animals of each sex in the control and high-dose groups
were killed after 1 year. The only noteworthy clinical signs were
paleness and hunched backs in males and rough coat in females at 1750
mg/kg of diet. Ophthalmic parameters were normal, and the mortality
rate was not affected. Females at 1750 mg/kg of diet showed slightly
decreased feed consumption and body-weight gain during the first 66
weeks, while the body weight of males at this dose was lower than that
of controls during the final 25 weeks of the study.
Hypercholesterolaemia occurred in males at 300 and 1750 mg/kg of diet
and in females at 1750 mg/kg of diet. Plasma, erythrocyte, and brain
cholinesterase activities were depressed by 20-40% at the highest
dose. Erythrocyte counts, haemoglobin concentration, and haematocrit
were slightly decreased at 1750 mg/kg of diet, but this anaemic
response had recovered in females by the end of the study. Urinary
parameters were not affected. At 1750 mg/kg of diet, the liver weights
were increased in males and females and the spleen weights were
increased in males, but with no associated pathological changes. The
kidney weights and the incidence of granular kidneys were increased at
1750 mg/kg of diet, corresponding to a slight enhancement in chronic
(age-related) nephropathy. The pathological changes included
hyperplasia of the upper small intestine and gastritis of the
non-glandular stomach in animals at 300 and 1750 mg/kg of diet and an
increased incidence of chronic lung inflammation in females at 1750
mg/kg of diet. The incidence of adrenal phaeochromocytomas was
increased in males at 1750 mg/kg of diet but was within the historical
control range. The NOEL was 92 mg/kg of diet, equal to 4.5 mg/kg bw
per day (Hayes, 1989; WHO, 1992).
Groups of 50 Fischer 344 rats of each sex were given diets
containing trichlorfon (purity, 98.5%) for 2 years at a nominal
concentration of 0 or 2500 mg/kg, equal to 130 mg/kg bw per day for
males and 160 mg/kg bw per day for females. Satellite groups of 20
animals of each sex per dose were killed after 1 year. This study was
initiated to ensure that trichlorfon had been tested at the maximum
tolerated dose. The body weights of treated animals were 10-15% lower
than those of controls throughout the study. Treated animals had
reduced food consumption during about the first half of the study, but
thereafter it was comparable to that of controls. The mortality rate
and ophthalmoscopic parameters were unaffected. The clinical signs in
treated animals included urine staining and enlarged abdomen in
animals of each sex and pale eyes and rough coat in males. Decreased
erythrocyte count, haemoglobin concentration, and haematocrit were
noted in treated rats but had recovered in males by the end of 2
years. Hypercholesterolaemia was seen in treated groups throughout the
study. The activities of gamma-glutamine transferase, alanine and
aspartate aminotransferases, and alkaline phosphatase in blood were
increased in treated males at various times but not at termination.
Urinary parameters were not affected. Statistically significant,
40-70% depressions in plasma, erythrocyte, and brain cholinesterase
activities were observed in animals of each sex at all times studied.
The liver and kidney weights were increased in treated groups,
and the lung weights were increased in females after 2 years; after 1
year, only changes in liver and kidney were reported. Histological
examination revealed duodenal hyperplasia, gastritis of the
non-glandular stomach, myodegeneration of the stomach tunica
muscularis, pulmonary inflammation and hyperplasia, hepatocellular
cytoplasmic vacuolation, focal hepatocellular hyperplasia associated
with sinusoidal dilatation and cystic degeneration (males),
nasolachrymal duct inflammation (females), chronic nephropathy
(females), and renal tubular hyperplasia. Non-statistically
significant increases in the incidences of renal tubular adenomas in
males and mononuclear-cell leukaemia in females were within the
historical control range. The increased incidences of
alveolar/bronchiolar adenomas in males and of carcinomas in females
were greater than the recorded historical control means, but the
numbers were small and did not attain statistical significance
(Christenson, 1990).
An increased incidence of tumours was thus detected in two
studies in rats conducted in the same laboratory. The increased
incidence of mammary tumours in females was slight, and the tumours
were benign. The strain used was Sprague-Dawley, which has a
relatively high, variable background incidence of mammary tumours
(Haseman et al., 1986). The lack of confirmation of this finding in
several studies in other species indicates that the evidence for the
carcinogenicity of trichlorfon in animals is limited.
Hamsters
A group of 23 male and 25 female Syrian golden hamsters received
trichlorfon (purity unknown) at a weekly intraperitoneal dose of 0 or
20 mg/kg bw for 90 weeks, and the study was terminated after 100
weeks. The treated animals showed reduced survival and body-weight
gain, but the tumour incidences were not increased (Teichmann &
Schmidt, 1978; FAO/WHO, 1979).
Dogs
Trichlorfon (purity unknown) was added to the diet of groups of
one male and one female beagle at a concentration of 50, 250, 500, or
1000 mg/kg, equivalent to 1.2, 6.2, 12, and 25 mg/kg bw per day, for 1
year. Few or no data on individual animals were provided. No effect on
food consumption or weight gain was reported. Serum and erythrocyte
cholinesterase activities were significantly depressed (by 40-50% of
control values) at 500 and 1000 mg/kg of diet. Brain
acetylcholinesterase activity was depressed by 40% in animals at
1000 mg/kg of diet. The relative spleen weights were increased and
showed marked congestion and apparent lymphoid tissue atrophy at
1000 mg/kg of diet and to a lesser extent at other doses. A slight
increase in the severity of foci of chronic inflammatory cells in the
liver and a decrease in spermatogenesis were seen at 1000 mg/kg of
diet. Hyperplastic adrenal cortical nodules recorded as 'mild' were
seen in all treated groups but not in controls. Owing to the small
number of animals in each group, however, the significance of this
finding is questionable (Doull et al., 1962; WHO, 1992).
Groups of four beagles of each sex were given diets containing
trichlorfon (purity, 95.3-98.3%) at a concentration of 0, 50, 200,
800, or 3200 mg/kg, equivalent to 1.2, 5, 20, and 80 mg/kg bw per day
in a study designed to last 4 years. All males at 3200 mg/kg of diet
died within the first year, and only one female at this dose survived
4 years. At this dose, the animals showed muscular twitching, severe
abdominal cramps, and salivation. The males in particular quickly
became emaciated. Autopsy of the animals that died during the
experiment showed brittle livers of a 'yellowish or loam' colour. Two
females and three males at 800 mg/kg of diet died during the first
year. The mortality rates at the two lower doses were similar to those
of controls, and the appearance and behaviour of the animals were
normal. Animals at higher doses were weakened and sick, and lower body
weights were recorded for surviving animals at 800 mg/kg of diet
during the latter stages and for the surviving female at 3200 mg/kg of
diet throughout the study. The effects on food consumption were
similar to those on body weight. Haematological parameters for treated
dogs were within the normal range. Elevated serum activities of
alanine and aspartate aminotransferases were noted in females at 3200
mg/kg of diet at 2 years but not at 4 years. No treatment-related
effects were found on urinary analysis. Cholinesterase activity in
whole blood was not inhibited at 50 mg/kg of diet; at 200 mg/kg of
diet, a 30% depression was seen at 1 month but the level rose again at
4 months. The group at 200 mg/kg of diet also showed inhibited
activity (15-20%) in plasma and erythrocytes throughout the study. At
800 and 3200 mg/kg of diet, dose-related depressions of 30-80% in
cholinesterase activity in whole blood, plasma, and erythrocytes were
found. The female at 3200 mg/kg of diet which survived to 4 years had
an enlarged, swollen liver, an enlarged spleen and adrenals, and small
ovaries. Dogs fed 800 mg/kg of diet had increased spleen and adrenal
weights and decreased testis weight. There were no histopathological
changes that were considered to be related to treatment. The NOEL was
50 mg/kg of diet, equivalent to 1.2 mg/kg bw per day (Loser, 1970;
Spicer & Payne, 1971; FAO/WHO, 1972b).
Monkeys
Groups of five male and five female rhesus monkeys (Macaca
mulatta) were given aqueous solutions of trichlorfon (purity,
98.6-99.1%) by gavage at a dose of 0, 0.2, 1, or 5 mg/kg bw per day, 6
days/week for 10 years. Two animals at 5 mg/kg bw per day showed
transient pupillary constriction, and one had muscular fasciculations
during the first month only; diarrhoea and soft stools were noted
frequently. At this dose, the body weights were lower towards the end
of the study, and there was a tendency to lower erythrocyte count,
haemoglobin concentration, and haematocrit at various sampling times.
No treatment-related effects on the mortality rate or clinical
chemical or urinary parameters were recorded. Plasma, erythrocyte, and
brain cholinesterase activities were inhibited by 30-80% in animals at
1 and 5 mg/kg bw per day, while males at 0.2 mg/kg bw per day showed
slight cholinesterase inhibition (16%) only in erythrocytes, which was
considered not to be biologically significant. Liver morphology in
biopsy samples taken during the first 3 years was unchanged. Gross and
microscopic examination of tissues showed no changes related to
treatment (Griffin, 1988; WHO, 1992).
2.2.4 Genotoxicity
The results of genotoxicity assays with trichlorfon are
summarized in Table 2. Other studies, evaluated by IARC (1983) and WHO
(1992), showed similar findings. The inconsistencies in a number of
tests has been suggested to be due to the variable purity of the
materials tested (Dreist & Mihail, 1990).
2.2.5 Reproductive toxicity
(a) Multigeneration studies
Trichlorfon (purity, 98.3%) was administered to groups of 10 male
and 20 female FB 30 rats in the diet at a concentration of 0, 100,
300, 1000, or 3000 mg/kg for three generations with two litters per
generation. Females in the F0 generation at 1000 and 3000 mg/kg of
diet had significantly depressed weight gain. The size of F1a
litters was reduced at doses > 1000 mg/kg of diet, while pup growth
and survival during lactation were impaired at 3000 mg/kg of diet. The
F1b mating resulted in lower pregnancy rates at 1000 and 3000 mg/kg
of diet. Litter size and pup growth and survival were also reduced at
3000 mg/kg of diet, and all animals in this group died before mating
to produce the F2 generation. During the remainder of the study, no
effects were found on fertility or on litter size or weight. The
F2a, F2b, F3a, and F3b groups given 1000 mg/kg of diet all had
reduced body-weight gain during the lactation period. No fetal
malformations were found in any group. Examination of the F0, F1b,
and F2b adults revealed no gross or microscopic alterations. The
NOEL was 300 mg/kg of diet, equivalent to 30 mg/kg bw per day (Loser,
1969; Spicer & Urwin, 1971; WHO, 1992).
(b) Developmental toxicity
Mice
A single dose of 360 mg/kg bw trichlorfon (purity unspecified),
injected intraperitoneally into 33 AS/Jena mice on day 1 of gestation,
was embryotoxic. The frequency of post-implantation loss was increased
with single doses of 60, 120, or 240 mg/kg bw on day 9 (13-15 mice per
group) and with 120 or 240 mg/kg bw on days 1-7 (25-30 mice per
group), and was most pronounced with 240 mg/kg bw on days 7-14 (23
mice). There were no severe malformations (Scheufler, 1975; WHO,
1992).
CD-1 mice were given trichlorfon (purity unspecified) by gavage
at a dose of 0, 300, 400, 500, or 600 mg/kg bw per day on days 6-10
(3-25 females per group) or 10-14 (4-21 females per group) of
gestation and were killed on day 18. The food intake and weight gain
of dams were depressed at all doses but with no dose-response
relationship. Fetal body weight was lower at doses > 400 mg/kg bw
per day, while the frequency of fetal malformations (cleft palate) was
slightly increased only in the groups treated with 500 or 600 mg/kg bw
per day during days 10-14 of gestation. The highest dose, 600 mg/kg bw
per day, was also administered by gavage on day 8 or on days 8-10,
10-12, or 12-14 of gestation (4-28 females per group). An increase in
the frequency of cleft palate was seen only in the last group. The
NOEL was 300 mg/kg bw per day (Staples & Goulding, 1979; WHO, 1992).
Trichlorfon (purity, > 98%) was given by gavage to CD-1 mice at
a dose of 0, 200, 300, or 400 mg/kg bw per day on days 7-16 of
gestation (15-24 females per group), and the animals were killed 1 day
after the last dose. The two higher doses increased the mortality
rate, and all doses decreased the weight gain of dams. The number of
live fetuses and fetal weight were decreased at 400 mg/kg bw per day.
Delayed development of fetuses was found, as indicated by reduced
calcification in a number of skeletal areas, rib variations, and
increased incidences of enlarged renal pelves in treated groups
(Courtney et al., 1986).
Table 2. Summary of results of tests for genotoxicity with trichlorfon
End-point Test object Concentration Result Reference
or dose (maximum)
In vitro
Interaction Determination of 160 mg/kg bw i.p. Positive Dedek et al.
with DNA 7-methylguanine (1976)
in mouse urine
Determination of 120 mg/kg bw i.p. Positive Dedek (1981)
7-methylguanine
in mouse liver and
kidney
Gene mutation B. subtilis NIG17, 0.3 mg per disc -S9 Negative Inukai & Iyatomi
(rec) NIG45 (1977)
P. mirabilis 10 mg per spot -S9 Positive Adler et al. (1976)
PG273, PG713
B. subtilis H17, NR -S9 Negative Shirasu et al.
M45 (1976)
B. subtilis H17, 2 mg per disc -S9 Positive Shirasu et al.
M45 (1979)
S. typhimurium 10 mg/disc ?S9 Positive Jones et al. (1984)
Reverse S. typhimurium 5 mg per plate ±S9 Positive Byeon et al. (1976)
mutation TA100
TA98, TA1535, 5 mg per plate ±S9 Negative
TA1538
TA98, TA100, 0.5 mg per plate ±S9 Negative Inukai & Iyatomi
TA1535, TA1537 (1977)
Table 2. (continued)
End-point Test object Concentration Result Reference
or dose (maximum)
TA98, TA100 ~ 8.5 mg per plate Positive Batzinger &
±S9 Bueding (1977)
TA100, TA1535 10 mg per plate ±S9 Negative Zeiger et al. (1987)
TA98, TA100 2 mg per plate ±S9 Negative Diril et al. (1990)
TA1535, TA1536, NR -S9 Negative Shirasu et al.
TA1537, TA1538, (1976)
E. coli WP2, WP2hcr
TA1535, TA1536, 2 mg per disc -S9 Negative Carere et al.
TA1537, TA1538 (1978a,b)
TA100, E. coli 20 mg per plate ±S9 Positive Shirasu et al.
WP2hcr (1979); Moriya et
TA98, TA1535, 20 mg per plate ±S9 Negative al. (1983)
TA1537, TA1538
TA100, TA104 25 mg per plate ±S9 Positive Barrueco et al.
TA97, TA98, 25 mg per plate ±S9 Negative (1991)
TA1535
TA98, TA1537 5 mg per plate±S9 Negative Watabe (1997)
TA100, TA1535, 5 mg per plate±S9 Positive
E, coli WP2uvrA
S. cerevisiae NR -S9 Negative Guerzoni et al.
632/4, 632/1b, (1976)
814/18b
Table 2. (continued)
End-point Test object Concentration Result Reference
or dose (maximum)
Reverse S. cerevisiae 10 mg per ml ±S9 Negative Hoorn (1983)
mutation S138, S211a
S. cerevisiae D7 40 mg per ml ±S9 Positive Jones et al.
(1984)
Mitotic crossing S. cerevisiae D7 40 mg per ml ±S9 Positive Jones et al.
over, gene (1984)
conversion
Forward S. coelicolor 2 mg per disc -S9 Positive Carere et al.
mutation (1978a,b)
S pombe SP-198 30 mg per ml ±S9 Positive Gilot-Delhalle et
al. (1983)
V79 cells 200 mg per ml -S9 Negative Aquilina et al. (1984)
L5178Y cells 200 µg per ml -S9 Positive Witterland
600 µg per ml +S9 Positive (1984); Jones et
al. (1984)
DNA damage E coli pol+/- 10 mg per plate ±S9 Negative Herbold (1984)
E. coli (SOS NR ±S9 Negative Xu & Schurr
chromotest) (1990)
Unscheduled EUE cells 1000 mg per ml -S9 Positive Aquilina et al.
DNA synthesis (1984)
Primary rat 50 µg per ml -S9 Negative Myhr (1983)
hepatocytes
Table 2. (continued)
End-point Test object Concentration Result Reference
or dose (maximum)
Sister chromatid V79 cells 80 µg per ml -S9 Positive Chen et al.
exchange 60 µg per ml +S9 Positive (1981, 1982)
CHO cells 100 µg per ml -S9 Positive Jones et al.
2 mg per ml +S9 Positive (1984); Putman
(1987)
Chromosomal Don-6 cells 250 mg per ml -S9 Positive Sasaki et al.
damage (1980)
Human lymphocytes 30 mg per ml -S9 Positive Herbold (1986)
3000 mg per ml +S9 Positive
In vivo
Reverse Host-mediated Batzinger &
mutation assay in mice Bueding (1977)
S. typhimurium 200 mg/kg bw orally Negative
TA98
S. typhimurium 200 mg/kg bw orally Positive
TA100
Recessive lethal Drosophila 4.5 mg/kg bw Negative Benes & Sram
mutation melanogaster (1969); Brzheskiy
(1973); Lamb
(1977)
Sister chromatid Chinese hamster 300 mg/kg bw orally Negative Volkner (1987)
exchange bone marrow
Table 2. (continued)
End-point Test object Concentration Result Reference
or dose (maximum)
Chromosomal Micronucleus 2 x 312 mg/kg bw i.p. Negative Paik & Lee
damage formation in mouse 2 x 250 mg/kg bw Negative (1977); Herbold
bone marrow orally (1979a); Jones
2 x 400 mg/kg bw Negative et al. (1984);
orally Herbold (1997)
400 mg/kg bw orally Negative
400 mg/kg bw orally Negative
of (+)-enantiomer
600 mg/kg bw orally Positive
of (-)-enantiomer
Metaphase analysis 400 mg/kg bw orally Positive Kurinnyi (1975);
in mouse 10 mg/kg bw i.p. Positive Moutschen-Dahmen
bone marrow 100 mg/kg bw i.p. Negative et al.
0.5 mg/ml in drinking, Negative (1981);
water 5 days/week, Degraeve et al.
7 weeks (1982, 1984);
405 mg/kg bw i.p. Negative Nehes et al.
(1982)
Metaphase analysis 250 mg/kg bw i.p. Negative Dzwonkowska &
in hamster Hubner (1986)
bone marrow
Metaphase analysis 250 mg/kg bw orally Negative Bootman &
in rat bone Hodson-Walker
marrow (1987)
Table 2. (continued)
End-point Test object Concentration Result Reference
or dose (maximum)
Metaphase analysis 1.5 mg/ml in drinking, Positive Bulsiewicz et al.
in mouse water 50-100 days (1976);
spermatogonia 100 mg/kg bw i.p. Negative Moutschen-Dahmen
and spermatocytes 0.5 mg/ml in drinking, Negative et al.
water 5 days/week, (1981);
7 weeks Degraeve et al.
100 mg/kg bw i.p. Negative (1982, 1984);
Herbold (1992)
Dominant lethal 100 mg/kg bw i.p. Negative Epstein et al.
mutation in mice 0.5 mg/ml in (1972); Dedek
drinking-water, Negative et al. (1975) ;
5 days/week, Fischer et al.
7 weeks (1977); Herbold
280 mg/kg bw i.p. Negative (1979b,c);
405 mg/kg bw i.p. Negative Becker &
405 mg/kg bw i.p. Positive Schoneich
250 mg/kg bw orally Negative (1980);
NR Negative Moutschen-Dahmen
405 mg/kg bw i.p. Positive et al. (1981);
54 mg/kg bw per day, Positive Degraeve et al.
3 weeks i.p. (1982, 1984);
WHO (1992)
NR, not reported; S9, metabolic activation; i.p., intraperitoneally
Rats
Trichlorfon (purity unspecified) was administered by gavage to
groups of 11 female Wistar rats at a single dose of 80 mg/kg bw on day
9 or 13 of gestation or to 10 rats at a dose of 8 mg/kg bw per day
daily throughout gestation. The animals were killed on day 19 of
gestation. The frequencies of post-implantation deaths and fetal
malformations such as exencephaly and non-closing eyelids were
increased at the single dose on day 13. The other treatments had no
effect on development (Martson & Voronina, 1976; WHO, 1992).
Trichlorfon (purity, 98.5%) was administered in the diet to
groups of 9-26 female CD rats on days 6-15 of gestation, to give an
intake of 0, 76, 140, 380, 430, or 520 mg/kg bw per day, with necropsy
on day 21. At the two highest doses, maternal food intake and body
weight and fetal weights were reduced, while the number of fetal
deaths was increased at 430 mg/kg bw per day. Major external and
skeletal malformations were found in fetuses at 430 and 520 mg/kg bw
per day and relatively minor skeletal changes at 380 mg/kg bw per day.
The predominant malformations were alterations of the skull and
central nervous system (exencephaly, meningocoele, hydrocephaly), the
limbs (syndactyly, markedly shortened radii and ulnae, missing
digits), micrognathia, cleft palate, facial haematomas, generalized
oedema, and great vessel defects. The minor skeletal alterations were
mainly doubled vertebral centra, wavy ribs, fenestrated supraoccipital
bones. and umbilical hernia. The NOEL was 140 mg/kg bw per day
(Staples et al., 1976; WHO,1992).
Trichlorfon (purity, 98.5%) was given by gavage at a dose of 0,
50, 75, 150, 200, or 250 mg/kg bw per day to groups of 9-30 female CD
rats on days 6-15 of gestation. Deaths occurred among dams at 150,
200, and 250 mg/kg bw per day, with overt signs of toxicity at the
highest dose. The survivors were killed on day 21 of gestation. Fetal
body weights were reduced significantly at doses > 75 mg/kg bw per
day, but the incidence of malformations was unaffected. The NOEL was
50 mg/kg bw per day (Staples et al., 1976).
Trichlorfon (purity unspecified) was given at a dose of 480 mg/kg
bw per day to 34 CD rats by gavage on days 6-15 of gestation. Lower
fetal body weight, a higher fetal mortality rate, and malformations
such as generalized oedema, herniation of the brain, hydrocephaly,
micrognathia, cleft palate, and skeletal alterations were seen. A
single gavage dose of 480 mg/kg bw on day 8 or 10 of gestation to 14
females per group decreased maternal food consumption but had no
adverse effect on fetal development (Staples & Goulding, 1979; WHO,
1992).
Groups of 25 female Long-Evans rats were given trichlorfon
(purity, 98.4%) by gavage at a dose of 0, 10, 30, or 100 mg/kg bw per
day on days 6-16 of gestation and were killed on day 20. There were no
deaths, but diarrhoea was observed in some animals at 100 mg/kg bw per
day. Fetal development was not affected. The NOEL for maternal
toxicity was 30 mg/kg bw per day (Machemer, 1979a; WHO, 1992).
Trichlorfon (purity, > 98%) was given by gavage to CD rats at a
dose of 0, 50, 100, or 200 mg/kg bw per day on days 7-19 (10-20
females per group) or 8-20 of gestation (15-18 females per group).
The animals were killed 1 day after the last dose. The highest dose
increased the mortality rate and decreased the weight gain of the
dams. Delayed development of fetuses was found, as indicated by
reduced calcification in a number of skeletal areas, but no
abnormalities were noted (Courtney et al., 1986).
Groups of 33 female Charles River Crl:CD BR rats were given diets
containing trichlorfon (purity, 99%) at a concentration of 0, 500,
1100, or 2500 mg/kg on days 6-15 of gestation. Body-weight gain was
slightly reduced among animals at the highest dose. Analysis of
cholinesterase activity in five animals per group killed on day 16 of
gestation showed inhibition of plasma, erythrocyte, and brain enzymes.
When the remaining dams were killed on day 20 of gestation, only brain
acetylcholinesterase activity was significantly inhibited. No effect
was seen on the numbers of resorptions, live fetuses, fetal body
weight, or malformation frequency. Increased incidences of delayed
ossification and curved, wavy, or bulbous ribs were seen at 2500 mg/kg
of diet. While fetal brain acetylcholinesterase activity was lower in
all treated groups, there was no dose-response relationship (Kowalski
et al., 1987; WHO, 1992).
Hamsters
Groups of 5-30 female golden hamsters received trichlorfon
(purity unspecified) at a dose of 0, 100, 200, 300, or 400 mg/kg bw
per day by gavage on days 7-11 of gestation, and the survivors were
killed on day 15. Three of 30 hamsters at the highest dose died, and
the signs of cholinesterase inhibition were dose-related at doses >
200 mg/kg bw per day. Maternal food consumption and body-weight gain
and fetal body weight were decreased at 300 and 400 mg/kg bw per day.
Fetuses at the highest dose showed an increased frequency of stunting
malformations, mostly consisting of oedema, cleft palate, patagium,
and fused ribs. A single dose of 400 mg/kg bw per day by gavage given
on day 8 of gestation to 16 females reduced maternal food consumption
and increased the fetal death rate but had no meaningful effect on
fetal development. The NOEL was 100 mg/kg bw per day (Staples &
Goulding, 1979; WHO, 1992).
Guinea-pigs
Groups of 10 white female guinea-pigs were given six doses of 100
mg/kg bw per day trichlorfon (purity, 97%) by gavage on days 36-38 and
51-53 of gestation. Seven females served as controls, and all animals
were allowed to deliver naturally. The numbers of abortions and
stillborn fetuses were increased and fetal body weight was decreased
in the treated group. The pups of treated dams developed trembling and
locomotor disturbances. At autopsy on postnatal day 2-3, the total
weights of the brain, cerebellum, medulla, cerebral cortex,
hippocampus, thalamus, and colliculi were decreased. The cerebellum
showed reduction of the external granular and molecular layers, with
regional absence of Purkinje cells. The activities of the
neurotransmitter enzymes choline acetyltransferase and glutamate
decarboxylase in the cerebellum were depressed when compared with
controls (Berge et al., 1986; WHO, 1992).
Rabbits
Groups of 15 female Himalayan rabbits were given trichlorfon
(purity, 98.4%) by gavage at a dose of 0, 5, 15, or 45 mg/kg bw per
day on days 6-18 of gestation and were killed on day 29. Body-weight
gain was reduced in treated groups, and two abortions occurred at 45
mg/kg bw per day. Fetal development was not affected (Machemer, 1979b;
WHO, 1992).
Groups of 20 female American Dutch rabbits were given trichlorfon
(purity, 99%) by gavage at a dose of 0, 10, 35, or 110 mg/kg bw per
day on days 6-18 of gestation and were killed on day 28. The highest
dose was not well tolerated and resulted in deaths, overt signs of
toxicity, and reduced body-weight gain. At 35 mg/kg bw per day, there
were also signs of toxicity, one death, and one abortion. Erythrocyte
but not plasma cholinesterase activity was inhibited on day 19 of
gestation in animals at 35 and 110 mg/kg bw per day, while on day 28
only brain cholinesterase activity was inhibited at these doses. At
110 mg/kg bw per day, there was a slight increase in the number of
resorptions, slight decreases in fetal and placental weights, and
delayed ossification. The frequency of fetal abnormalities was not
increased. The NOELs were 10 mg/kg bw per day for maternal toxicity
and 35 mg/kg bw per day for embryo- and fetal toxicity (Clemens et
al., 1990; WHO, 1992).
Pigs
Several outbreaks of congenital tremor in piglets have been
described in herds in which the sows had been treated with trichlorfon
preparations between day 45 and day 63 of gestation. Clinically, the
syndome was characterized by ataxia, tremor, pronounced hypoplasia of
the cerebellum, and a reduction in the size of the spinal cord (WHO,
1992). This syndrome was reproduced experimentaly in sows given diets
providing one to four doses of 50-100 mg/kg bw per day 55-98 days
after conception. The offspring had a high incidence of overt
neurological signs and cerebellar hypoplasia. Histological examination
revealed patchy loss of Purkinje cells in the cerebellum. Postnatally,
the rate of increase of brain weight was similar to that of controls,
but normal weights had not been achieved up to 35 days after birth
(Knox et al., 1978; Pope et al., 1986; Berge et al., 1987a,b; WHO,
1992).
2.2.6 Special studies
(a) Neurotoxicity
The results of these studies are summarized in Table 3. Like
other organophosphonates, trichlorfon has neurotoxic potential. Rats
given a dose of about 200 mg/kg bw per day for 13 weeks showed
decreased locomotor activity and loss of coordination, whereas rats
given a dose of 30 mg/kg bw per day for 3 weeks showed increased
locomotion and decreased learning ability and nerve conduction
velocity. Numerous studies in hens consistently demonstrated the
neurotoxicity of single doses of trichlorfon. Leg weakness, impaired
walking ability, and decreased activity were seen immediately after
treatment. These effects are considered to be secondary to inhibition
of cholinesterase activity and to be generally unrelated to damage to
the nervous tissues. Delayed neurotoxicity is usually detected some
weeks after dosing and is associated with degeneration of nervous
tissue and marked inhibition of neuropathy target esterase activity.
Neuropathy target esterase is a protein which is located specifically
in neurons, but its biological function, other than as a target for
neuropathic organophosphates, is unknown. The toxicity of an agent can
be predicted by its relative ability to interact with either
acetylcholinesterase to produce cholinergic effects or neuropathy
target esterase to produce delayed polyneuropathy (Ray, 1998). The
studies gave no evidence of delayed neuropathy in chickens. In a
monkey given a single oral dose of trichlorfon at 250 mg/kg bw per
day, nerve conduction was impaired 4 weeks after treatment, and there
was histological evidence of demyelination of nerves and axonal
degeneration.
(b) Immunotoxicity
Groups of four female BALB/cByJ and C57BL/6J mice were given
drinking-water containing trichlorfon at 1.75 mg/ml for 14 days.
Measurement of a variety of responses to immunization with influenza
virus indicated that immune function was not impaired (Reiss et al.,
1987; WHO, 1992).
2.3 Observations in humans
Wegner (1970) reviewed data on over 6000 people, obtained mostly
in South Africa and South America, who had been treated with
trichlorfon for various intestinal and body parasites (Ankylostoma,
Ascaris, Strongyloides, Trichocephalus, and Schistosoma), at doses
varying from 5 to 70 mg/kg bw per day for up to 12 days. A dose of 7.5
mg/kg bw given two to four times at 2-week intervals was considered to
be optimal, as the side-effects observed were less severe than at
other dosages. The symptoms included depression of cholinesterase
activity, weakness, nausea, diarrhoea, and abdominal pain. A dose of
24 mg/kg bw caused more severe symptoms, including tachycardia,
salivation, colic pain, vomiting, nausea, fatigue, tremors, and
sweating. The effects were not cumulative, and spontaneous recovery
was rapid in all cases. Spermatogenesis (size and shape of sperm)
appeared to be affected in a few cases. Inhibition of cholinesterase
activity was seen at all doses (FAO/WHO, 1972b; WHO, 1992).
Reduced erythrocyte and plasma cholinesterase activities have
been reported in groups of patients with various parasitic
infestations given trichlorfon orally at doses of 5-12.5 mg/kg bw. The
drug was reported to be well tolerated with few clinical symptoms
related to treatment. The symptoms that were observed were cholinergic
in nature and were usually found at doses > 10 mg/kg bw.
Cholinesterase activity recovered to pretreatment levels within 4-15
weeks after cessation of treatment (Abdel Aal et al., 1970; Plestina
et al., 1972; Jewsbury, 1981).
In a clinical trial, 10 male and 10 female patients with
Alzheimer disease were given oral doses of trichlorfon for up to 30
weeks according to the following dose regimen: 2.5 mg/kg bw at week 0,
5 mg/kg bw in week 1, 7.5 mg/kg bw in weeks 6 and 7, 15 mg/kg bw in
weeks 8 and 9, and the 'best weekly dose' in weeks 18-30. The 'best
dose' was defined as that associated with the greatest improvement for
each patient. This small trial indicated general improvement in a
variety of functional deficits associated with Alzheimer disease.
Side-effects were reported at doses > 5 mg/kg bw, which included
nausea, vomiting, and diarrhoea. Plasma cholinesterase activity was
inhibited by 45-60% at all doses, and erythrocyte acetylcholinesterase
activity was inhibited by 30-70% at doses > 5 mg/kg bw with no
concomitant clinical findings. Measurement of acetylcholinesterase
activity in the cerebrospinal fluid from two patients 24 h after a
dose of 5 mg/kg bw revealed inhibition of 37 and 47% below
pretreatment levels (Becker et al., 1990).
In a prospective, double-blind, randomized study, four parallel
groups of 119-121 patients with Alzheimer disease received oral doses
of placebo or trichlorfon once daily. An initial loading dose of 0,
0.5, 0.9, or 2.0 mg/kg bw per day was given during weeks 0-2 in order
to achieve steady-state inhibition of cholinesterase activity more
quickly, and this was followed during weeks 3-10 by a dose of 0, 0.2,
0.3, or 0.65 mg/kg bw per day. The intermediate and high doses were
found to improve cognitive function, but the low dose had equivocal
effects. Abdominal pain, diarrhoea, flatulence, nausea, and leg cramps
were the most commonly reported side-effects and were most severe in
the patients given the high dose. Erythrocyte acetylcholinesterase
activity was inhibited in a dose-related manner (Table 4). The initial
dose of 0.5 mg/kg bw per day for 2 weeks inhibited erythrocyte
acetylcholinesterase activity by 29%, and subsequent administration of
0.2 mg/kg bw per day for 10 weeks maintained the inhibition at 30-37%,
with no increase with duration of dosing. Since this dose enhanced
inhibition over that caused by the initial dose by only 8%, an
insignificant change, the Committee considered that the NOEL for
inhibition of acetylcholinesterase activity was 0.2 mg/kg bw per day
(Cummings et al., 1998).
Table 3. Summary of studies of neurotoxicity with trichlorfon
Species Treatment Results Reference
Rats 200 mg/kg bw per day Electrophysiological changes Averbrook &
i.p. for 5-15 days indicating enhanced excitability Anderson (1983)
in sciatic nerve. No pathological
changes in nerves
Rats 30 mg/kg bw per day Increased locomotion, decrease Lehotzky (1982)
orally for 3 weeks in rotorod performance, learning
ability, and nerve conduction
velocity
Rats 0, 85, 438, or 2275 Rats given 2275 ppm had stained Sheets &
ppm in the diet for 13 fur, reduced motor and locomotor Hamilton (1995)
weeks activity, slightly uncoordinated
righting response (males only),
30-80% inhibition of plasma,
erythrocyte, and brain
cholinesterase activity, and
demyelination in spinal nerve roots.
At 438 ppm, only plasma and
erythrocyte cholinesterase activity
was inhibited (20-30%), and no
behavioural or biochemical
evidence of neurotoxicity was
seen at 85 ppm (6 mg/kg bw
per day)
Chicken Single subcutaneous Leg weakness for 1-2 days, no Witter & Gaines
dose of 90 mg/kg bw delayed paralysis (1963)
Chicken Single dose of 25-500 Birds that survived the acute Lorke &
mg/kg bw orally or toxicity of trichlorfon showed no Kimmerle (1966)
75-500 mg/kg bw i.p. signs of neurotoxicity over
6-10 weeks
Chicken 100-5000 ppm in Body weight loss at > 500 ppm. Lorke &
feed for 30 days Blood cholinesterase activity Kimmerle (1966)
decreased by 40-60% at all Hobik (1967);
doses but no signs of FAO/WHO
neurotoxicity and no (1972)
degeneration in nervous tissue.
Table 3. (continued)
Species Treatment Results Reference
Chicken Single subcutaneous One hen died 1.5 h after dosing; Hierons &
dose of 200 mg/kg bw the lone survivor was killed 24 h Johnson (1978)
after dosing, when NTE was
inhibited by 46% in brain and
17% in spinal cord.
Chicken Single doses of 50, Dose-related deaths after oral Olajos et al.
100 mg/kg bw orally, doses only. All treatments had (1979)
50, 100, 200 mg/kg acute effects; ataxia from 11-17
bw subcutaneously, days after dosing. Brain NTE
or 200 +100 mg/kg bw decreased by 40-60% at high
subcutaneously 3 subcutaneous doses but by only
days apart about 20% at <100 mg/kg bw.
Mild degenerative changes seen
in cerebellum, brainstem, and
striatum at higher doses.
Chicken 185 mg/kg bw orally, Acute signs of intoxication but Thyssen et al.
survivors given 167 no pathological changes in (1982)
mg/kg bw 21 days nervous tissue
later
Chicken Single subcutaneous Marked cholinesterase inhibition Slott &
dose of 100 or 300 in plasma, brain, and spinal cord Ecobichon
mg/kg bw (50-60%) but no inhibition of NTE (1984)
in brain or spinal chord
Chicken 100 mg/kg bw Plasma, brain, and spinal cord Slott &
subcutaneously every cholinesterase inhibited by Ecobichon
72 h for 6 doses 10-60%, and walking ability (1984)
slightly impaired. No inhibition
of NTE in brain or spinal cord
and only slight oedematous
effects on nervous tissue
Chicken 3, 9, or 18 mg/kg bw Signs of ataxia and decreased Hayes & Ramm
per day for 3 months activity at 18 mg/kg bw per day (1987)
by gavage but no effect on locomotor activity.
Blood cholinesterase activity
decreased in all groups but
nervous tissue unremarkable.
Table 3. (continued)
Species Treatment Results Reference
Chicken Single gavage dose Acute signs of intoxication, Bomann &
of 340 mg/kg bw, 220 deaths, and marked inhibition Kaliner
mg/kg bw (90%) of brain cholinesterase (1996)
(-)-enantiomer, activity with each compound.
or 400 mg/kg bw < 50% NTE inhibition in brain,
(+)-enantiomer spinal cord, and sciatic nerve; no
changes in gait or nervous tissue
Monkey Single dose of Weakness of lower extremities Shiraishi et al.
(Macaca 250 mg/kg bw by on day 24 and nerve conduction (1983)
fuscata) gavage defects by day 28. On day 37,
demyelination of sural and tibial
nerves with increased axonal
degeneration
NTE, neuropathy target esterase
Table 4. Mean inhibition of erythrocyte acetylcholinesterase
activity (%) in patients with Alzheimer disease
given trichlorfon orally
Time (week) Dose of trichlorfon (mg/kg bw per day)
0-2 0 0.5 0.9 2.0
3-10 0 0.2 0.3 0.65
2 1.1 ± 14 29 ± 12 49 ± 12 71 ± 82
8 0.1 ± 21 37 ± 13 52 ±12 68 ± 95
12 1.0 ± 24 34 ± 14 52 ± 10 72 ± 24
A position paper (Bayer AG, 1999) reported that the results of
clinical trials involving over 4000 treated patients show that
trichlorfon at the recommended dose of 0.5-0.9 mg/kg bw is 'a safe and
effective treatment' for Alzheimer disease. A dose-response
relationship was found between the dose of trichlorfon and the
incidence of muscle weakness in placebo-controlled trials, and the
increased risk for muscular weakness was statistically significant at
doses > 1.25 mg/kg bw. The recommended dose of 0.5-0.9 mg/kg bw
resulted in incidences of 0.6% for generalized weakness or myasthenia
(0.2% with placebo) and 4.3% for fatigue or asthenia (3.3% with
placebo). The muscular weakness was reported to be reversible on
discontinuation of therapy.
Acute poisoning of humans with trichlorfon has been reported to
manifest as delayed neurotoxicity, severe polyneuritis, and
polyneuropathy 2-3 weeks after ingestion. These diagnoses were based
on observations of weakness, loss of feeling in the extremities, and
difficulty in walking, accompanied by muscular atrophy, myalgia, and
were related to motor nerve damage (Pollingher et al., 1973; Akimov et
al., 1975; Eljasz & Kryzyszton-Przekop, 1975; Fukuhara et al., 1977;
Shiraishi et al., 1977; Hierons & Johnson, 1978; Shiraishi et al.,
1982; Niedziella et al., 1985; De Freitas et al., 1990). After
reviewing the literature, Johnson (1981) concluded that only doses of
trichlorfon that exceed the lethal dose but are survived by the victim
because of treatment are likely to result in a level of inhibition of
neuropathy target esterase activity at which delayed neurotoxicity
would be expected. Development of neuropathy after repeated exposure
to lower doses was considered to be unlikely (WHO, 1992).
Five persons exposed to trichlorfon, two while attempting suicide
and three occupationally, showed increased incidences of chromosomal
breaks and stable chromosomal rearrangements in cultured lymphocytes.
The effect was observed immediately after exposure and 1 month later,
but not 6 months later (Bao et al., 1974). Seventeen workers involved
in the production of trichlorfon-containing products for at least 6
months had increased incidences of chromatid breaks in their
lymphocytes when compared with an unexposed control group, but the
incidences were lower than those in a control group of factory workers
who had had no direct exposure to pesticides (Kiraly et al., 1979).
3. COMMENTS
The Committee considered the results of studies on the
toxicokinetics, metabolism, and acute toxicity of trichlorfon,
short-term and long-term studies of toxicity, and studies of
genotoxicity, reproductive and developmental toxicity, neurotoxicity,
and immune function, and studies in humans. Most of the available
studies were relatively old, and many were published reports which
lacked raw data or data on individual animals. Such studies are
difficult to evaluate, and most could not be assessed independently.
Unpublished reports of studies conducted during the 1980s and 1990s
contained sufficient detail and were carried out according to
appropriate standards for study protocol and conduct.
The absorption of trichlorfon is rapid in all species tested,
including humans, irrespective of the route of administration. Peak
blood concentrations were achieved within 1-2 h but decreased quickly
thereafter; the half-time of trichlorfon in human plasma is
approximately 2 h. It is widely distributed. Trichlorfon was detected
in the milk of lactating cows, and the compound and its metabolites
were found in fetal tissue in treated guinea-pigs. Trichlorfon
undergoes conversion to dichlorvos via a dehydrochlorination reaction
that occurs spontaneously at pH values above 5.5. Although little
dichlorvos was recovered, it is generally believed to be responsible
for the anticholinesterase effects of trichlorfon. The metabolism of
trichlorfon in mammals also occurs through O-demethylation and
cleavage of phosphorus-carbon bonds. Therefore, the major metabolites
are desmethyl trichlorfon, desmethyl dichlorvos, dimethyl hydrogen
phosphate, methyl dihydrogen phosphate, and phosphoric acid.
Trichlorfon and its metabolites are excreted primarily in the urine.
Trichlorfon is moderately toxic when given as a single oral dose,
the LD50 values being 620-950 mg/kg bw in mice, 140-660 mg/kg bw in
rats, 160 mg/kg bw in rabbits, and 300-420 mg/kg bw in dogs. The signs
of toxicity were indicative of inhibition of acetylcholinesterase
activity, and the cause of death was usually respiratory failure. The
rapid recovery observed after sublethal doses suggests that
trichlorfon is readily detoxified.
Trichlorfon was given orally to mice at doses of 10-750 mg/kg bw
per day, to rats at doses of 0.05-250 mg/kg bw per day, and to dogs at
doses of 0.5-45 mg/kg bw per day for periods up to 16 weeks. The
weights of the liver, kidney, and spleen were increased at doses of
250 mg/kg bw per day and above in mice and at doses of 50 mg/kg bw per
day and above in rats, but these changes were usually not associated
with other evidence of toxicity, except in one study in rats in which
hypertrophy of centrilobular hepatocytes and biochemical changes
suggestive of altered lipid metabolism were seen. These effects
occurred at doses which also induced significant signs of cholinergic
intoxication. Slight anaemia was observed in one study in rats at 100
mg/kg bw per day and in one study in dogs at 45 mg/kg bw per day. One
dog out of three given trichlorfon at a dose of 45 mg/kg bw per day
had small, immature testes and prostate and oligospermia, but the
animal had also lost weight and appeared to be severely stressed by
the treatment. The NOELs were 15 mg/kg bw per day for inhibition of
cholinesterase activity in brain and erythrocytes in mice, 5 mg/kg bw
per day for inhibition in brain and erythrocytes in rats, and 5 mg/kg
bw per day for inhibition in in erythrocytes in dogs.
In two studies of up to 2 years' duration, mice were given oral
doses of 15-750 mg/kg bw per day. Body-weight gain was impaired at
doses of 30 mg/kg bw per day and above; the mean weight of the liver
was increased at a dose of 240 mg/kg bw per day in one study, but no
pathological alterations were found. The NOEL for inhibition of brain
and erythrocyte cholinesterase activity was 49 mg/kg bw per day. The
incidences of tumours were unaffected by treatment. Neither study was
suitable for identifying a NOEL.
Six studies of up to 2 years' duration were conducted in which
rats were given trichlorfon in the diet at concentrations providing
doses of 2.5-160 mg/kg bw per day. The weights of the liver and spleen
were increased in three studies at doses of 50 mg/kg bw per day and
above. Hypercholesterolaemia, duodenal hyperplasia, and gastritis of
the non-glandular stomach were seen at 13 mg/kg bw per day and above.
Anaemia, enhancement of age-related nephropathy, increased renal
tubular hyperplasia, myodegeneration of the stomach tunica muscularis,
pulmonary inflammation, and hyperplasia were observed at doses of 75
mg/kg bw per day and above. Other observations included ovarian
atrophy at 20 mg/kg bw per day and above, depression of
spermatogenesis at 50 mg/kg bw per day, and a slight increase in the
incidence of benign mammary tumours in females with a reduction in the
time to appearance at doses of 20 mg/kg bw per day and above. The NOEL
for inhibition of brain and erythrocyte acetylcholinesterase activity
was 13 mg/kg bw per day. The incidence of mammary tumours was
increased in female Sprague-Dawley rats in two studies. The increases
were slight, the tumours were benign, this strain of rats has a
relatively high and variable background incidence of mammary tumours,
and the finding could not be confirmed in several other studies in
various species. The Committee concluded that trichlorfon does not
have carcinogenic potential in rats. The overall NOEL in rats was 4.5
mg/kg bw per day on the basis of an increased cholesterol
concentration and pathological changes to the gastrointestinal tract.
In two studies of 1 and 4 years' duration, dogs were given
trichlorfon orally at doses of 1.3-80 mg/kg bw per day. Deaths
occurred at doses of 20 mg/kg bw per day and above, and signs of
hepatocellular damage and increased severity of inflammation were seen
in the liver at doses of 25 mg/kg bw per day and above. In animals
given 20-25 mg/kg bw per day, the weight of the spleen was increased,
with atrophy of lymphoid tissue; furthermore, small ovaries, reduced
testis weight, and decreased spermatogenesis were seen. 'Blood
cholinesterase' activity was inhibited at doses of 5 mg/kg bw per day
and above. The overall NOEL in dogs was 1.3 mg/kg bw per day on the
basis of inhibition of cholinesterase activity.
Oral administration of trichlorfon to rhesus monkeys at a dose of
0, 0.2, 1, or 5 mg/kg bw per day for 10 years resulted in clinical
signs of toxicity indicative of cholinesterase inhibition, reduced
body-weight gain, and anaemia at the highest dose. Inhibition of
plasma, erythrocyte, and brain cholinesterase activities was seen at
the two higher doses. In a separate 6-month study in rhesus monkeys,
erythrocyte acetylcholinesterase activity was not depressed at 0.2
mg/kg bw per day, the highest dose tested. The NOEL in monkeys was 0.2
mg/kg bw per day on the basis of inhibition of brain and erythrocyte
acetylcholinesterase activity.
Trichlorfon has been tested in a wide range of assays for
genotoxicity and DNA damaging activity, with considerable variation in
the results. Trichlorfon induced point mutations in microorganisms and
cultured mammalian cells, DNA damage in microorganisms, and
chromosomal aberrations and sister chromosome exchange in cultured
mammalian cells. The results of tests for point mutation in
Drosophila melanogaster and for sister chromatid exchange in bone
marrow were clearly negative. Although positive results were obtained
in a few assays for chromosomal damage in bone marrow and in the germ
cells of male animals exposed at near-lethal doses, the results of
most studies of micronucleus formation, metaphase alterations, and
dominant lethal mutations were negative. Since the tests conducted
in vivo produced mostly negative results when trichlorfon was
administered orally, the Committee considered that the weight of
evidence indicates that trichlorfon is unlikely to represent a
genotoxic risk.
Trichlorfon was given to rats at a dose of 0, 10, 30 100, or 300
mg/kg bw per day in a three-generation study of reproductive toxicity,
with two litters per generation. The body-weight gain of F0 dams at
100 and 300 mg/kg bw per day was depressed. The slight effects on the
gonads reported in the studies of general toxicity were not
consistently reflected in this study. The pregnancy rate of rats of
the F1b generation at doses of 100 mg/kg bw per day and above was
reduced, but the fertility of other generations was unaffected. The
litter size of dams at 300 mg/kg bw per day was reduced and all of the
offspring died, but reproductive parameters were unaffected at lower
doses. The NOEL was 30 mg/kg bw per day in adults, on the basis of
reduced body-weight gain, and 100 mg/kg bw per day for reproductive
effects, on the basis of reduced litter size and the death of
offspring.
Two studies of developmental toxicity were conducted in mice
given oral doses of 200-600 mg/kg bw per day. Dams at 200 mg/kg bw per
day showed reduced body-weight gain, and higher doses led to an
increased mortality rate. The effects of these maternally toxic doses
included increased embryotoxicity, decreased fetal weight, and delayed
fetal development. The incidence of cleft palate was slightly
increased at doses of 500 and 600 mg/kg bw per day. NOELs were not
identified for maternal toxicity or fetotoxicity.
Studies of developmental toxicity were conducted in rats given
oral doses of 8-520 mg/kg bw per day. In three studies, maternal
toxicity in the form of reduced body-weight gain and/or an increased
mortality rate was observed at doses of 150 mg/kg bw per day and
above. The NOEL for maternal toxicity was 75 mg/kg bw per day. In the
same studies, the incidences of fetal malformations such as
morphological alterations of the skull, central nervous system, and
limbs, micrognathia, cleft palate, facial haematomas, generalized
oedema, and defects in major blood vessels were increased at doses of
430 mg/kg bw per day and above. At 380 mg/kg bw per day, minor
skeletal changes were observed. Reduced fetal body weight was seen at
75 mg/kg bw per day. In three other studies, the incidence of fetal
malformations was unaffected. The NOEL for developmental effects was
50 mg/kg bw per day on the basis of fetotoxicity.
Hamsters given trichlorfon orally at a dose of 0, 200, 300, or
400 mg/kg bw per day during gestation showed signs of cholinesterase
inhibition, and depressed body-weight gain was seen at the two higher
doses. The NOEL for maternal toxicity was 100 mg/kg bw per day. Fetal
body weight was decreased at the two higher doses, and an increased
incidence of fetal stunting was seen at the highest dose. The NOEL for
developmental effects was 200 mg/kg bw per day.
Guinea-pigs given trichlorfon at an oral dose of 100 mg/kg bw per
day for 6 days during gestation had abortions and stillborn fetuses,
and the offspring showed reduced body weight and brain weight,
locomotor disturbances, and morphological and biochemical alterations
in the brain.
In two studies of developmental toxicity in rabbits, oral doses
of 5-110 mg/kg bw per day were given. Overt toxicity and inhibition of
erythrocyte acetylcholinesterase activity were observed in dams given
doses of 35 mg/kg bw per day and above, and body-weight gain was
affected at all doses. The highest dose, 110 mg/kg bw per day,
slightly increased the rate of resorptions and retarded fetal
development, but no fetal abnormalities were found. A NOEL for
maternal toxicity was not identified. The NOEL for developmental
effects was 45 mg/kg bw per day on the basis of toxicity to embryos
and fetuses.
Several outbreaks of congenital tremor with cerebellar hypoplasia
were reported in piglets of sows that had been treated with
trichlorfon, and the syndrome was subsequently reproduced
experimentally. The piglets of sows treated with trichlorfon at doses
of 50-100 mg/kg bw per day during appropriate periods of gestation
showed neurological signs and hypoplasia and loss of Purkinje cells in
the cerebellum.
Rats given a dose equivalent to 200 mg/kg bw per day for 13 weeks
showed decreased locomotor activity and loss of coordination. In
another study in rats, a dose of 30 mg/kg bw per day for 3 weeks
increased locomotor activity and decreased learning ability and nerve
conduction velocity.
The results of numerous studies in hens consistently demonstrated
the acute neurotoxicity of trichlorfon. Delayed neurotoxicity,
associated with degeneration of nervous tissue and marked inhibition
of neuropathy target esterase, was not seen in hens. A monkey given a
single oral dose of 250 mg/kg bw showed impaired nerve conduction 4
weeks after treatment and histological evidence of demyelination of
nerves and axonal degeneration.
Trichlorfon has been used as an anthelmintic agent in humans.
Oral doses of up to 10 mg/kg bw given on two to four occasions were
well tolerated, with few clinical symptoms. A dose of 24 mg/kg bw
caused significant cholinergic symptoms, but the effects were not
cumulative. In a few cases, spermatogenesis appeared to have been
impaired. The results of clinical trials with trichlorfon in the
treatment of Alzheimer disease showed dose-related but reversible
muscle weakness. The recommended dose of 0.5-0.9 mg/kg bw resulted in
a small increase in the frequency of generalized weakness and fatigue,
while doses of 1.25 mg/kg bw and higher produced significant weakness.
Dose-related inhibition of erythrocyte acetylcholinesterase activity
was also observed in these studies.
In 121 subjects, an initial dose of 0.5 mg/kg bw per day for 2
weeks inhibited erythrocyte acetylcholinesterase activity by 29%.
Subsequent administration of a dose of 0.2 mg/kg bw per day for
10 weeks maintained the inhibition of erythrocyte acetylcholinesterase
activity at levels between 30 and 37%, with no increase with duration
of dosing. Since this dose enhanced the inhibition caused by the
initial dose by only 8%, an insignificant change, the Committee
concluded that the NOEL was 0.2 mg/kg bw per day.
In some cases of severe human poisoning with trichlorfon,
weakness and loss of feeling in the extremities, difficulty in
walking, muscular atrophy, and motor nerve damage have been observed.
In many of these cases, the doses might have been lethal in the
absence of medical intervention. The Committee concluded that
extremely high doses of trichlorfon would be required to achieve the
level of inhibition of neuropathy target esterase associated with
delayed neurotoxicity.
4. EVALUATION
The Committee concluded that inhibition of acetylcholinesterase
activity was the most relevant end-point for establishing an ADI. The
most appropriate NOEL was 0.2 mg/kg bw per day for inhibition of
erythrocyte acetylcholinesterase activity in humans treated orally. A
safety factor of 10 was applied to this figure, giving an ADI of 0-20
µg/kg bw.
5. REFERENCES
Abdel Aal, A.M.A., El-Hawary, M.F.S., Kamel, H., Abdel-Khalek, M.K. &
El-Diwany, K.M. (1970) Blood cholinesterases, hepatic renal and
haemopoietic functionsin children receiving repeated doses of
'Dipterex'. J. Egypt. Med. Assoc., 53, 265-271.
Aden-Abdi, Y., Villen, T., Ericsson, O., Gustafsson, L.L. &
Dahl-Puustinen, M.L. (1990) Metrifonate in healthy volunteers:
Interrelationship between pharmacokinetic properties,
cholinesterase inhibition and side effects. Bull. World Health
Organ., 68, 731-736.
Adler, B., Braun, R., Schöneich, J. & Böhme, H. (1976)
Repair-defective mutants of Proteus mirabilis as a prescreening
system for the detection of potential carcinogens. Biol. Zbl.,
95, 463- 469.
Akhtar, M.H. (1982) Fate of trichlorfon in buffer and soluble fraction
(105 000 g) from cow and chicken liver homogenates. J. Agric.
Food Chem., 30, 551-554.
Akimov, G.A., Buchko, V.M. & Kremleva, R.V. (1975) Neurological
disorders in chlorophos poisoning. Klin. Med., 5, 65-69
( Pestic. Abstr., 75-2380).
Aquilina, G., Benigni, R., Bignami, M., Calcagnile, A., Dogliotti, E.,
Falcone, E. & Carere, A. (1984) Genotoxic activity of dichlorvos,
trichlorfon, and dichloroacetaldehyde. Pestic. Sci., 15,
439-442.
Arthur, B.W. & Casida, J.E. (1957) Metabolism and selectivity of
O,O-dimethyl 2,2,2-trichloro-1-hydroxyethylphosphonate and its
acetyl and vinyl derivatives. J. Agric. Food Chem., 5, 186-192.
Averbook, B.J. & Anderson, R.J. (1983) Electrophysiological changes
associated with chronic administration of organophosphates.
Arch. Toxicol., 52, 167-172.
Bao, T.V., Szabo, I., Ruzicska, P. & Czeizel, A. (1974) Chromosome
aberrations in patients suffering acute organic phosphate
insecticide intoxication. Humangenetik, 24, 33-57.
Barrueco, C., Herrera, A. & De La Pena, E. (1991) Mutagenic evaluation
of trichlorfon using different assay methods with Salmonella
typhimurium. Mutagenesis, 6, 71-76.
Batzinger, R.P. & Bueding, E. (1977) Mutagenic activities in vitro
and in vivo of five antischistosomal compounds. J. Pharmacol.
Exp. Ther., 200, 1-9.
Bayer AG (1999) Pro Mem (metrifonate) and muscle weakness. Unpublished
position paper from Bayer AG. Submitted to WHO by Bayer AG,
Germany.
Becker, K. & Schöneich, J. (1980) Dominant lethal test with
trichlorphon in male mice. Mutat. Res., 74, 224.
Becker, R.E., Colliver, J., Elble, R., Feldman, E., Giacobini, E.,
Kumar, V., Markwell, S., Moriearty, P., Parks, R., Shillcutt,
S.D., Unni, L., Vicari, S., Womack, C. & Zec, R.F. (1990) Effects
of metrifonate, a long-acting cholinesterase inhibitor, in
Alzheimer disease: Report of an open trial. Drug Dev. Res., 19,
425-434.
Benes, V. & Sram, R. (1969) Mutagenic activity of some pesticides in
Drosophila melanogaster. Ind. Med., 38, 50-52.
Berge, G.N. & Nafstad, I. (1986) Distribution and placental transfer
of trichlorfon in guinea-pigs. Arch. Toxicol., 59, 26-29.
Berge, G.N., Nafstad, I. & Fonnum, F. (1986) Prenatal effects of
trichlorfon on the guinea-pig brain. Arch. Toxicol., 59, 30-35.
Berge, G.N., Fonnum, F. & Brodal, P. (1987a) Neurotoxic effects of
prenatal trichlorfon administration in pigs. Acta Vet. Scand.,
28, 321-332.
Berge, G.N., Fonnum, F., Soli, N.E. & Sognen, E. (1987b)
Neurotoxicological examination of the piglet brain after prenatal
and postnatal exposure to trichlorfon. Acta Vet. Scand., 28,
313-320.
Bomann, W. & Kaliner, G. (1996) BAY a 9826 (metrifonate) and BAY z
7216, BAY z 7217. Study for delayed neurotoxicity following acute
oral administration to hens. Unpublished report no PH 25171 from
Bayer, Wuppertal, Germany. Submitted to WHO by Bayer AG, Germany.
Bond, G.P. (1986) Primary dermal irritation of technical trichlorfon
(Dylox) in albino rabbits. Unpublished report no 751 from Mobay
Corporation, USA. Submitted to WHO by Bayer AG, Germany.
Bootman, J. & Hodson-Walker, G. (1987) L13/59: Investigation of
effects on bone marrow chromosomes of the rat after acute oral
administration. Unpublished report no R4221 from Life Sciences
Research, United Kingdom. Submitted to WHO by Bayer AG, Germany.
Brzheskiy, V.V. (1973) [Induction of mutations in Drosophila
melanogaster.] Med. Parazitol. Parazit. Bolez., 42, 703-706
(in Russian).
Bull, D.L. & Ridgway, R.L. (1969) Metabolism of trichlorfon in animals
and plants. J. Agric. Food Chem., 17, 837-841.
Bulsiewicz, H., Rozewicka, L., Januszewska, H. & Bajko, J. (1976)
Aberrations of meiotic chromosomes induced in mice with
insecticides. Folia Morphol. (Warsaw), 35, 361-368.
Byeon, W.H., Hyun, H.H. & Lee, S.Y. (1976) Salmonella/microsomal
enzyme activation system. Mutagenicity of pesticides in the
Salmonella/microsome system. Korean J. Microbiol., 14,
128-134.
Carere, A., Ortali, V.A., Cardamone, G. & Morpurgo, G. (1978a)
Mutagenicity of dichlorvos and other structurally related
pesticides in Salmonella and Streptomyces. Chem.-Biol.
Interactions, 22, 297-308.
Carere, A., Ortali, V.A., Cardamone, G., Torracca, A.M. & Raschetti,
R. (1978b) Microbiological mutagenicity studies of pesticides
in vitro. Mutat. Res., 57, 277-286.
Chan, P.C. & Peters, A.C. (1989) Thirteen-week subchronic dosed feed
study of trichlorfon in Fischer rats and B6C3F1 mice. Proc.
Am. Assoc. Cancer Res., 30, 137.
Chen, H.H., Hsueh, J.L., Sirianni, S.R. & Huang, C.C. (1981) Induction
of sister chromatid exchanges and cell cycle delay in cultured
mammalian cells treated with eight organophosphorus pesticides.
Mutat. Res., 88, 307-316.
Chen, H.H., Sirianni, S.R. & Huang, C.C. (1982) Sister chromatid
exchanges in Chinese hamster cells treated with seventeen
organophosphorus compounds in the presence of a metabolic
activation system. Environ. Mutag., 4, 621-624.
Christenson, W.R. (1990) A combined chronic toxicity/oncogenicity
study of technical grade trichlorfon (Dylox) with rats.
Unpublished report no 5388 from Mobay Corporation, USA. Submitted
to WHO by Bayer AG, Germany.
Clemens, G.R., Troup, C.M. & Hartnagel, R.E., Jr (1990) Teratology
study in the rabbit with Dylox technical (Trichlorfon).
Unpublished report no MTD0134 from Miles Laboratories, USA.
Submitted to WHO by Bayer AG, Germany.
Courtney, K.D., Andrews, J.E. & Springer, J. (1986) Assessment of
teratogenic potential of trichlorfon in mice and rats.
J. Environ. Sci. Health, B21, 207-227.
Crawford, C.R. & Anderson, R.H. (1973) The acute oral and
intraperitoneal toxicity of five trichlorfon technical samples to
rats. Unpublished report no 37204 from Baychem Corporation.
Submitted to WHO by Bayer AG, Germany.
Cummings, J.L., Cyrus, P.A., Bieber, F., Mas, J., Orazem, J. &
Gulanski, B. (1998) Metrifonate treatment of the cognitive
deficits of Alzheimer's disease. Neurology, 50, 1214-1221.
De Freitas, M.R.G., Chimelli, L., Nascimento, O.J.M., Cincinatus, D.,
Marques, H.A. & Nevares, M.T. (1990) [Polyneuropathy induced by
trichlorfon. Report of a case with electrophysiology and
histopathology in the sural nerve.] Arq. NeuroPsiquiatr., 48,
515-519 (in Spanish).
Dedek, W. (1981) Guanine N7-alkylation in mice in vivo by
metrifonate -- Discussion of possible genotoxic risk in mammals.
Acta Pharmacol. Toxicol., 49 (Suppl. V), 40-50.
Dedek, W. & Lohs, K. (1970a) [Alkylating effect of trichlorfon in
mammals. I. Studies in vitro in human serum with
14C-trichlorfon.] Z. Naturforsch., B25, 94-96 (in German).
Dedek, W. & Lohs, K. (1970b) [Allkylkating effect of trichlorfon in
mammals. Distribution of 14C in organs and liver proteins of
rats after administration of 14Ctrichlorfon.]
Z. Naturforsch.,B25, 1110-1113 (in German).
Dedek, W., Scheufler, H., & Fischer, G.W. (1975) [Mutagenicity of
desmethyltrichlorfon in the dominant lethal test in the mouse.]
Arch. Toxicol., 33, 163-168 (in German).
Dedek, W., Lohs, K., Fischer, G.W. & Schmidt, R. (1976) Alkylation of
guanine in mice in vivo by organophosphorus insecticides. I.
Trichlorfone and butonate. Pestic. Biochem. Physiol., 6,
101-110.
Degraeve, N., Chollet, M.-C., Moutschen, J., Moutschen-Damen, M.,
Gilot-Delhalle, J. & Colizzi A. (1982) Genetic and cytogenetic
effects of chronic treatments with organophosphorous
insecticides. Mutat. Res., 97, 179-180.
Degraeve, N., Chollet, M.-C. & Moutschen, J. (1984) Cytogenetic and
genetic effects of subchronic treatments with organophosphorus
insecticides. Arch. Toxicol., 56, 66-67.
Diril, N., Sumer, S. & Izbirak, A. (1990) [A survey on the mutagenic
effects of some organophosphorous insecticides in the
Salmonella/microsome test system.] Doga Muhendislik Cevre
Bilimleri, 14, 272-279 (in Turkish).
Doull, J. & Rehfuss, P.A. (1956) The effects of diets containing
Dipterex on rats. Unpublished report No. 1376 from Department of
Pharmacology, University of Chicago, USA. Submitted to WHO by
Bayer AG, Germany.
Doull, J. & Vaughn, G. (1958) Measurement of the safe dietary level of
Dipterex for dogs. Unpublished report No. 2105 from Department of
Pharmacology, University of Chicago, USA. Submitted to WHO by
Bayer AG, Germany.
Doull, J., Root, M., Vesselinovitch, D., Meskauskas, J., & Fitch, F.
(1962) Chronic oral toxicity of Dylox to male and female dogs.
Unpublished report No. 8644 from Department of Pharmacology and
Pathology, University of Chicago, USA. Submitted to WHO by Bayer
AG, Germany.
Dreist, M. & Mihail, F.L. (1990) L 13/59 (trichlorfon). Synoptic
evaluation of the toxicological data. Unpublished report no 20033
from Bayer, WuppertalElberfeld, Germany. Submitted to WHO by
Bayer AG, Germany.
Dubois, K.P. & Cotter, G.J. (1955) Studies on the toxicity and
mechanism of action of dipterex. Arch. Ind. Health, 11, 53-60.
Dzwonkowska, A. & Hubner, H. (1986) Induction of chromosomal
aberrations in the Syrian hamster by insecticides tested
in vivo. Arch. Toxicol., 58, 152-156.
Eljasz, L. & Kryzyszton-Przekop, T. (1975) [Polyneuropathy as a result
of foschlor poisoning.] Pol. Tyg. Lek., 30, 1841-1842 ( Pest.
Abstr. 76-1688) (in Polish).
Epstein, S.S., Arnold, E., Andrea, J., Bass, W. & Bishop, Y. (1972)
Detection of chemical mutagens by the dominant lethal assay in
the mouse. Toxicol. Appl. Pharmacol., 23, 288-325.
FAO/WHO (1972a) Pesticide residues in food. Report of the 1971
Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues. FAO Agricultural Series, No. 88; WHO Technical Report
Series, No. 502, Rome/Geneva.
FAO/WHO (1972b) 1971 Evaluations of some pesticide residues in
food. AEP-1971/M/13; WHO Pesticide Residues Series, No. 5,
Rome/Geneva.
FAO/WHO (1976a) Pesticide residues in food. Report of the 1975
Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on
Pesticide Residues. FAO Plant Production and Protection Series,
No. 1; WHO Technical Report Series, No. 592, Rome/Geneva.
FAO/WHO (1976b) 1975 Evaluations of some pesticide residues in
food. AEP-1975/M/9/1; WHO Pesticide Residues Series, No. 1,
Rome/Geneva.
FAO/WHO (1979a) Pesticide residues in food--1978. Report of the
Joint Meeting of the FAO Panel of Experts on Pesticide
Residues and Environment and the WHO Expert Group on
Pesticide Residues. FAO Plant Production and Protection Paper
15, Rome.
FAO/WHO (1979b) Pesticide residues in food: 1978 Evaluations. FAO
Plant Production and Protection Paper 15 Suppl., Rome.
Fischer, G.W., Schneider, P. & Scheufler, H. (1977) [Mutagenicity of
dichloroacetaldehyde and methyl-2,2-dichloro-1,1-dihydroxyethane
phosphonate, possible trichlorfon metabolites.] Chem.-Biol.
Interactions, 19, 205-213 (in German).
Fukuhara, N., Hoshi, M. & Mori, S. (1977) Core/targetoid fibres and
multiple cytoplasmic bodies in organophosphate neuropathy.
Acta Neuropathol., 40, 137-144.
von Gibel, W., Lohs, K., Wildner, G.P. & Ziebarth, D. (1971) [Animal
experimental studies on the hepatotoxic and carcinogenic activity
of organophosphorus compounds.] Arch. Geschwülstforsch., 37,
303-312 (in German).
von Gibel, W., Lohs, K., Wildner, G.P., Ziebarth, D. & Stieglitz, R.
(1973) [Carcinogenicity, haematoxicity and hepatotoxicity of
organophosphorus compounds.] Arch. Geschwülstforsch., 41,
311-328 (in German).
Gilot-Delhalle, J., Colizzi, A., Moutschen, J. & Moutschen-Dahmen, M.
(1983) Mutagenicity of some organophosphorus compounds at the
ade6 locus of Schizosaccharomyces pombe. Mutat. Res., 117,
139-148.
Griffin, T.B. (1988) Safety evaluation and tumorigenesis of
trichlorfon in rhesus monkeys: A ten-year study. Unpublished
report from White Sands Research Center, USA. Submitted to WHO by
Bayer AG, Germany.
Griffin, T.B., Coulston, F. & Stein, A.A. (1982) Trichlorfon.
Tumorigenicity study in the rat (2 generation). Unpublished
report from White Sands Research Center, USA. Submitted to WHO by
Bayer AG, Germany.
Grundmann, E. & Hobik, H.P. (1966) Bay 15922. 2 year feeding
experiment on rats. Histology. Unpublished report addendum from
Bayer, Wuppertal-Elberfeld. Submitted to WHO by Bayer AG,
Germany.
Guerzoni, M.E., Del Cupolo, L. & Ponti, I. (1976) [Mutagenic activity
of pesticides.] Riv. Sci. Techn. Alim. Nutr. Um., 6, 161-165
(in Italian).
Haseman, J.K., Winbush, J.S. & O'donnell, M.R. (1986) Use of dual
control groups to estimate false positive rates in laboratory
animal carcinogenicity studies. Fundam. Appl. Toxicol., 7,
573-584.
Hassan, A. & Zayed, S.M.A.D. (1965) Metabolism of organophosphorus
insecticides. III. Fate of the methyl groups of Dipterex
in vivo. Can. J. Biochem., 43, 1271-1275.
Hassan, A., Zayed, S.M.A.D. & Abdel-Hamid, F.M. (1965a) Metabolism of
organophosphorus insecticides. II. Metabolism of
O,O-dimethyl-2,2,2-trichloro-1hydroxyethyl phosphonate (Dipterex)
in mammalian nervous tissue and kinetics involved in its reaction
with acetyl cholinesterase. Can J. Biochem., 43, 1263-1269.
Hassan, A., Zayed, S.M.A.D. & Hashish, S. (1965b) Metabolism of
organophosphorus insecticides. VI. Mechanism of detoxification of
Dipterex in the rat. Biochem. Pharmacol., 14, 1692-1694.
Hayes, R.H. (1985) Pilot study on trichlorfon with mice. Unpublished
report No. 656 from Mobay Corporation, USA. Submitted to WHO by
Bayer AG, Germany.
Hayes, R.H. (1988) Oncogenicity study of technical grade trichlorfon
with mice. Unpublished report No. 1039 from Mobay Corporation,
USA. Submitted to WHO by Bayer AG, Germany.
Hayes, R.H. (1989) Chronic toxicity/oncogenicity study of technical
grade trichlorfon (Dylox) with rats. Unpublished report No. 1108
from Mobay Corporation, USA. Submitted to WHO by Bayer AG,
Germany.
Hayes, R.H. & Ramm, W.W. (1987) Subchronic delayed neurotoxicity study
of trichlorfon technical (Dylox) with hens. Unpublished report
No. 939 from Mobay Corporation, USA. Submitted to WHO by Bayer
AG, Germany.
Heimann, K.G. (1985) [Dipterex (L 13/59, techn.), determination of
acute toxicity (LD50).] Unpublished letter from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany (in German).
Heimann, K.G. & Wood, C.M. (1987) Trichlorfon technical. Study of
subacute dermal toxicity to rabbits. Unpublished report No. R
4150 from Bayer, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Germany.
Herbold, B. (1979a) L 13/59. Micronucleus test in the mouse for
evaluation of mutagenicity. Unpublished report No. 8505 from
Bayer, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Germany (in German).
Herbold, B. (1979b) [Appraisal of dominant lethal test on trichlorfon
(Dipterex).] Unpublished report from Martin Luther Universitat,
Germany. Submitted to WHO by Bayer AG, Germany (in German).
Herbold, B. (1979c) L 13/59. Dominant lethal study on male mouse to
test for mutagenic effects. Unpublished report No. 8745 from
Bayer, Wuppertal, Germany. Submitted to WHO by Bayer AG, Germany.
Herbold, B. (1984) [L 13/59. Pol test in E. coli to investigate DNA
damage effects.] Unpublished report No. 12434 from Bayer,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Germany (in
German).
Herbold, B. (1986) L 13/59. Cytogenetic study with human lymphocyte
cultures in vitro to evaluate for harmful effects on
chromosomes. Unpublished report No. 14837 from Bayer, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Germany.
Herbold, B. (1992) L 13/59. In vivo cytogenetic study of the
spermatogonia in Chinese hamster to evaluate for induced
clastogenic effects. Unpublished report No. 21688 from Bayer,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Germany.
Herbold, B. (1997) BAY a 9826, BAY z 7216, BAY z 7217 Micronucleus
tests on the mouse. Unpublished report No. PH 26329 from Bayer,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Germany.
Hierons, R. & Johnson, M.K. (1978) Clinical and toxicological
investigations of a case of delayed neuropathy in man after acute
poisoning by an organophosphorus pesticide. Arch. Toxicol., 40,
279-284.
Hirakawa, T. (1983a) Acute toxicity study of trichlorfon on mice by
oral, intraperitoneal, subcutaneous and percutaneous route.
Unpublished report ID 17258 from Bozo Research Centre, Japan.
Submitted to WHO by Bayer AG, Germany.
Hirakawa, T. (1983b) Acute toxicity study of trichlorfon on rats by
oral, intraperitoneal, subcutaneous and percutaneous route.
Unpublished report ID 17259 from Bozo Research Centre, Japan.
Submitted to WHO by Bayer AG, Germany.
Hobik, H.P. (1967) [Histological investigation of spinal cord and
sciatic nerves from neurotoxicity experiemnts in hens dosed with
Dipterex.] Unpublished report no 446 from Bayer, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Germany (in German).
Hoffmann, K., Ruf, J. & Mager, H. (1988) [Chronic toxicity in rhesus
monkeys dosed oraqlly for 26 weeks (gavage).] Unpublished study
No. 16545 from Bayer, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Germany (in German).
Hoorn, A.J.W. (1983) Mutagenicity evaluation of L 13/59 in the reverse
mutation induction assay with Saccharomyces cerevisiae strains
S138 and S211alpha. Unpublished report No. R2540 from Litton
Bionetics, Netherlands. Submitted to WHO by Bayer AG, Germany.
IARC (1983) Trichlorfon. In: IARC Monographs on the Evaluation of
the Carcinogenic Risk of Chemicals to Humans, Vol. 30,
Miscellaneous pesticides, Lyon, International Agency for
Research on Cancer, pp. 207-231.
IARC (1987) IARC Monographs on the Evaluation of the Carcinogenic
Risk of Chemicals to Humans, Suppl. 7, Overall Evaluations
of Carcinogenicity: An Updating of IARC Monographs Volumes 1
to 42, Lyon, International Agency for Research on Cancer,
p. 73.
Inukai, H. & Iyatomi, A. (1977) Trichlorfon. Mutagenicity test on
bacterial systems. Unpublished report No. 87 from Nitokuno
Agricultural Chemicals Institute, Japan. Submitted to WHO by
Bayer AG, Germany.
Jewsbury, J.M. (1981) Metrifonate in schistosomiasis therapy and
prophylaxis. Acta Pharmacol. Toxicol., 49 (Suppl. V),123-130.
Johnson, M.K. (1981) Delayed neurotoxicity -- Do trichlorphon and/or
dichlorvos cause delayed neuropathy in man or in test animals?
Acta Pharmacol. Toxicol., 49 (Suppl. V), 87-98.
Jones, D.C.L., Simmon, V.F., Mortelmans, K.E., Mitchell, A.D., Evans,
E.L., Jotz, M.M., Riccio, E.S., Robinson, D.E. & Kirkhart, B.A.
(1984) In vitro and in vivo mutagenicity studies of
environmental chemicals. Report from SRI International, USA, for
Environmental Protection Agency (EPA-600/1-84-003). National
Technical Information Service, USA.
Kawauchi, S. (1997) BAY a 9826 single oral administration toxicity
study in dogs. Unpublished report No. R 6836 from Experimental
Biomedical Research, Japan. Submitted to WHO by Bayer AG,
Germany.
Kimmerle, G. (1970) Metrifonat (Bayer 2349), toxicological
investigations. Unpublished report No. 2142 from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Kimmerle, G. (1975a) L 13/59, acute inhalation toxicity study on rats.
Unpublished report No. 5581 from Bayer, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Germany.
Kimmerle, G. (1975b) L 13/59, subacute inhalation toxicity study on
rats. Unpublished report No. 5582 from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Klimmer, O.R. (1955) [Final report on the new insecticide Bayer L
13/59.] Unpublished report from Pharmakologisches Institut, Bonn,
Germany. Submitted to WHO by Bayer AG, Germany (in German).
Kiraly, J., Szentesi, I., Ruzicska, M. & Czeize, A. (1979) Chromosome
studies in workers producing organophosphate insecticides.
Arch. Environ. Contam. Toxicol., 8, 309-319.
Knox, B., Askaa, J., Basse, A., Bitsch, V., Eskildsen, M., Mandrup,
M., Ottosen, H.E., Overby, E., Pedersen, K.B. & Rasmussen, F.
(1978) Congenital ataxia and tremor with cerebellar hypoplasia in
piglets borne by sows treated with Neguvon(R) vet (Metrifonate,
Trichlorfon) during pregnancy. Nord. Vet. Med., 30, 538-545.
Kowalski, R.L., Clemens, G.R., Bare, J.J. & Hartnagel, R.E., Jr (1987)
A teratology study with Dylox (R), technical (Trichlorfon) in the
rat. Unpublished report No. MTD0017 from Miles Laboratories, USA.
Submitted to WHO by Bayer AG, Germany.
Kurinnyi, A.I. (1975) Comparative study of the cytogenetic effect of
certain organophosphorus pesticides. Genetika, 11, 64-69.
Lamb, M.J. (1977) Mutagenicity tests with metrifonate in
Drosophila melanogaster. Mutat. Res., 56, 157-162.
Lehotzky, K. (1982) Effect of pesticides on central and peripheral
nervous system function in rats. Neurobehav. Toxicol.
Teratol., 4, 665-669.
Lorke, D & Kimmerle, G. (1966) Neurotoxicity studies on hens with
Dipterex active ingredient. Unpublished report from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Lorke, D. & Loser, E. (1966) Chronic toxicological studies on rats.
Unpublished report No. 19292 from Bayer, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Germany.
Loser, E. (1969) BAY 15922 Generation study on rats. Unpublished
report No. 1195 from Bayer, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Germany.
Loser, E. (1970) Bay 15922. Chronic toxicological studies on dogs.
Unpublished report No. 2467 from Bayer, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Germany.
Machemer, L. (1979a) [L 13/59 (trichlorfon): Evaluation for
embryotoxic and teratogenic effects on orally dosed rats.]
Unpublished report No. 8400 from Bayer,Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Germany (in German).
Machemer, L. (1979b) [L 13/59 (trichlorfon): Evaluation for
embryotoxic and teratogenic effects on orally doses rabbits.]
Unpublished report No. 8430 from Bayer,Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Germany (in German).
Martson, L.V. & Voronina, V.M. (1976) Experimental study of the effect
of a series of phosphoroorganic pesticides (dipterex and imidan)
on embryogenesis. Environ. Health Perspectives, 13, 121-125.
Metcalf, R.L., Fukuto, T.R. & March, R.B. (1959) Toxic action of
Dipterex and DDVP to the house fly. J. Econ. Entomol., 52,
44-49.
Mihail, F. (1978) [L 13/59 (Dipterex active ingredient), acute
toxicological studies.] Unpublished report No. 7619 from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany (in German).
Mihail, F. (1986) L 13/59 (trichlorfon): Study for skin-sensitizing
effect on guineapigs in the open epicutaneous test. Unpublished
report No. 14348 from Bayer, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Germany.
Miyamoto, J. (1959) Non-enzymatic conversion of Dipterex into DDVP and
their inhibitory action on enzymes. Botyu-Kagaku, 24, 130-137.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Moutschen-Dahmen, J., Moutschen-Dahmen, M. & Degraeve, N. (1981)
Metrifonate and dichlorvos: Cytogenetic investigations.
Acta Pharmacol. Toxicol., 49 (Suppl. V), 29-39.
Myhr, B.C. (1983) Evaluation of L 13/59 trichlorfon in the primary rat
hepatocyte unscheduled DNA synthesis assay. Unpublished report
No. R2687 from Litton Bionetics, USA. Submitted to WHO by Bayer
AG, Germany.
Nehes, M., Selypes, A. & Scheufler, H. (1982)[ Mutagenicity of
trichlorfon and dimethoate on mouse bone marrow cells in vivo].
Wissensch. Z. Univ. Halle, 31, 57- 62 (in German).
Niedziella, S.W., Göpel, W. & Banzhaf, E. (1985) [Acute alkylphosphate
poisoning (trichlorphon) with delayed polyneuropathy syndrome.]
Z. Ges. Inn. Med., 40, 237-239 (in German).
Nordgren, I., Bergström, M., Holmstedt, B. & Sandoz, M. (1978)
Transformation and action of metrifonate. Arch. Toxicol., 41,
31-41.
Nordgren, I., Holmstedt, B., Bengtsson, E. & Finkel, Y. (1980) Plasma
levels of metrifonate and dichlorvos during treatment of
schistosomiasis with Bilarcil. Am. J. Trop. Med. Hyg., 29,
426-430.
Nordgren, I., Bengtsson, E., Holmstedt, B. & Pettersson, B.M. (1981)
Levels of metrifonate and dichlorvos in plasma and erythrocytes
during treatment of schistosomiasis with Bilarcil. Acta
Pharmacol. Toxicol., 49 (Suppl. V), 79-86.
Olajos, E.J., Rosenblum, I., Coulston, F. & Strominger, N. (1979) The
dose response relationship of trichlorfon neurotoxicity in hens.
Ecotoxicol. Environ. Saf., 3, 245-255.
Paik, S.G. & Lee, S.Y. (1977) Genetic effects of pesticides in the
mammalian cells. 1. Induction of micronucleus. Korean J.
Zool., 20, 19-28.
Plestina, R., Davis, A. & Bailey, D.R. (1972) Effect of metrifonate on
blood cholinesterases in children during treatment of
schistosomiasis. Bull. World Health Organ., 46, 747-759.
Pollingher, B., Cozma, V. & Oprisan, C. (1973) Clinical and
electromyographic study of two cases of severe polyneuritis
following acute poisoning with Neguvon (organophosphoric
pesticide). Electroencephalogr. Clin. Neurophysiol., 35, 433
( Pestic. Abstr., 75-0322).
Pope, A.M., Heavner, J.E., Guarnieri, J.A. & Knobloch, C.P. (1986)
Trichlorfoninduced congenital cerebellar hypoplasia in neonatal
pigs. J. Am. Vet. Med. Assoc., 189, 781-783.
Putman, D.L. (1987) Sister chromatid exchange assay in Chinese hamster
ovary (CHO) cells. Unpublished report No. 826 from
Microbiological Associates, USA. Submitted to WHO by Bayer AG,
Germany.
Ray, E.R. (1998) Chronic effects of low level exposure to
anticholinesterases -- A mechanistic review. Toxicol. Lett.,
102-103, 527-533.
Reiner, E., Krauthacker, B., Simeon, V. & Skrinjaric-Spoljar, M.
(1975) Mechanism of inhibition in vitro of mammalian
acetylcholinesterase and cholinesterase in solutions of
O,O-dimethyl-2,2,2-trichloro-1-hydroxyethyl phosphonate
(trichlorphon). Biochem. Pharmacol., 24, 717-722.
Reiss, C.S., Herrman, J.M. & Hopkins, R.E., II (1987) Effect of
antihelminthic treatment on the immune response of mice.
Lab. Anim. Sci., 37, 773-775.
Renhof, M. (1997) BAY a 9826: Acute toxicity in the mouse and rat
after oral administration and in the mouse after intravenous
administration. Unpublished report No. 25832 from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Rosenblum, I. (1981) A two-generation, two-year feeding study of
trichlorfon in the rat. Unpublished report No. 2142 from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Sasaki, M., Sugimura, K., Yoshida, M. A. & Abe, S. (1980) Cytogenetic
effects of 60 chemicals on cultured human and Chinese hamster
cells. Kromosomo, 20, 574-584.
Scheufler, H. (1975) [Effect of relatively high doses of dimethoate
and trichlorfon on embryogenesis in mice.] Biol. Rundsch., 13,
238-240 (in German).
Sheets, L.P. & Hamilton, B.F. (1995) A subchronic dietary
neurotoxicity screening study with technical grade trichlorfon
(Dylox, Dipterex) in Fischer 344 rats. Unpublished report No.
MRC-00284 from Bayer Corp., USA. Submitted to WHO by Bayer AG,
Germany.
Shiraishi, S., Goto, I., Yamashita, Y., Ohnishi, A. & Nagao, H. (1977)
Dipterex polyneuropathy. Neurol. Med., 6, 34-38.
Shiraishi, S., Inoue, N. & Murai, Y. (1982) Dipterex poisoning.
J. Univ. Occup. Environ. Health, 4, 177-183.
Shiraishi, S., Inoue, N., Murai, Y., Onishi, A., & Noda, S. (1983)
Dipterex (trichlorfon) poisoning -- Clinical and pathological
studies in human and monkeys. J. Univ. Occup. Environ.
Health, 5 (Suppl.), 125-132.
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A. & Kada, T. (1976)
Mutagenicity screening of pesticides in the microbial system.
Mutat. Res., 40, 19-30.
Shirasu, Y., Moriya, M. & Koyashiki, R. (1979) Trichlorfon.
Mutagenicity test on bacterial systems. Unpublished report from
Institute of Environmental Toxicology, Japan. Submitted to WHO by
Bayer AG, Germany.
Slott, V. & Ecobichon, D.J. (1984) An acute and subacute neurotoxicity
assessment of trichlorfon. Can. J. Physiol. Pharmacol., 62,
513-518.
Spicer, E.J.F. & Payne, S. (1971) Pathology report of Bay 15922 4-year
dog study. Unpublished report from Huntingdon Research Centre,
United Kingdom. Submitted to WHO by Bayer AG, Germany.
Spicer, E.J.F. & Urwin, C (1971) Pathology report of Bay 15922
Generation experiment in rats. Unpublished report from Huntingdon
Research Centre, England. Submitted to WHO by Bayer AG, Germany.
Staples, R.E. & Goulding, E.H. (1979) Dipterex teratogenicity in the
rat, hamster and mouse when given by gavage. Environ. Health
Perspectives, 30, 105-113.
Staples, R.E., Kellam, R.G. & Haseman, J.K. (1976) Developmental
toxicity in the rat after ingestion or gavage of organophosphate
pesticides (dipterex, imidan) during pregnancy. Environ.
Health Perspectives, 13, 133-140.
Stieglitz, R., Gibel, W., Werner, W. & Stobbe, H. (1974) Experimental
study on haematotoxic and leukaemogenic effects of trichlorophone
and dimethoate. Acta Haematol., 52, 70-76.
Suzuki, T. (1996) Three-month repeated oral administration toxicity
study and recovery test of BAY a 9826 in dogs. Unpublished report
No. R 6641 from JBC, Gifu Research Laboratory, Japan. Submitted
to WHO by Bayer AG, Germany.
Teichmann, B. & Hauschild, F. (1978) [Test of O,O-dimethyl
(1-hydroxy-2,2,2trichloroethyl)phosphonate (trichlorfon) for
carcinogenicity in mice after oral (oesophageal-gastric gavage)
and dermal application.] Arch. Geschwulstforsch., 48, 301-307
(in German).
Teichmann, B. & Schmidt, A. (1978) [Test of O,O-dimethyl
(1-hydroxy-2,2,2trichlorethyl)phosphonate (trichlorphon) for
carcinogenicity in Syrian golden hamsters ( Mesocricetus
auratus Waterhouse) after intraperitoneal Injection.]
Arch. Geschwulstforsch., 48, 718-721 (in German).
Teichmann, B., Hauschild, F. & Eckelmann, A. (1978) [Test of
O,O-dimethyl(1hydroxy-2,2,2-trichlorethyl)phosphonate
(trichlorphon) for carcinogenicity in rats after oral
(oesophageal-gastric gavage) and intraperitoneal Injection.]
Arch. Geschwulstforsch., 48, 112-119.
Thyssen, J. (1981) L 13/59 (Dipterex active ingredient). Tests for
skin and eye irritation. Unpublished report from Bayer,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Germany.
Thyssen, J., Kaliner, G. & Eben, A. (1982) [Trichlorfon, Schmelzware
Japan, neurotoxicity studies in hens.] Unpublished report No.
10811 from Bayer, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Germany (in German).
Toyoshi, T. (1996) One-month repeated oral administration toxicity
study and recovery test of BAY a 9826 in rats. Unpublished report
No. R 6642 from JBC, Gifu Research Laboratory, Japan. Submitted
to WHO by Bayer AG, Germany.
Völkner, W. (1987) Sister chromatid exchanges (SCEs) in Chinese
hamster bone marrow cells. Unpublished report No. R4079 from
Technical University of Darmstadt, Germany. Submitted to WHO by
Bayer AG, Germany.
Watabe, H. (1997) A reverse mutation assay of metrifonate using
bacteria. Unpublished report No. R 6837 from Mitsubishi Yuka
Bio-Clinical Labs, Japan. Submitted to WHO by Bayer AG, Germany.
Wegner, D. (1970) Bilarcil (Bay 2349) Clinical experience 1960-1969
(survey of the literature). Unpublished report No. 2225 from
Bayer, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Germany.
WHO (1992) Trichlorfon (Environmental Health Criteria 132). Geneva,
World Health Organization.
Williams, M.W., Fuyat, H.N. & Fitzhugh, O.G. (1959) The subacute
toxicity of four organic phosphates to dogs. Toxicol. Appl.
Pharmacol., 1, 1-7.
Witter, R.F. & Gaines, T. B. (1963) Relationship between depression of
brain or plasma cholinesterase and paralysis in chickens caused
by certain organic phosphorus compounds. Biochem. Pharmacol.,
12, 1377-1386.
Witterland, W.F. (1984) Mutagenicity evaluation of L 13/59 in the
mouse lymphoma forward mutation assay. Unpublished report No.
R2824 from Litton Bionetics, Netherlands. Submitted to WHO by
Bayer AG, Germany.
Xu, H.H. & Schurr, K.M. (1990) Genotoxicity of 22 pesticides in
microtitration SOS Chromotest. Toxicol. Assess. Int. J., 5,
1-14.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T., Mortelmans, K. &
Speck, W. (1987) Salmonella mutagenicity tests: III Results from
the testing of 255 chemicals. Environ. Mutag., 9 (Suppl. 9),
1-109.
