WHO FOOD ADDITIVES SERIES 46:Pulegone and related substances
First draft prepared by
G.J.A. Speijers
Section on Public Health, Centre for Substances and Risk Assessment,
National Institute of Public Health and the Environment, Bilthoven, Netherlands
1. EVALUATION
1.1 Introduction
The Committee evaluated six flavouring agents using the Procedure for the Safety Evaluation of Flavouring Agents (see Figure): pulegone (No. 753), isopulegone (No. 754), isopulegol (No. 755), the acetate ester of isopulegol (No. 756), an unsaturated analogue of pulegone, para-mentha-1,4(8)-dien-3-one (No. 757), and a principal metabolite of pulegone, menthofuran (No. 758) (Table 1). With the exception of the metabolite, menthofuran, these substances contain a 3-menthyl-carbon skeleton (a 2-isopropyl-5-methyl-3-cyclohexyl derivative). Isopulegone, isopulegol, and isopulegyl acetate contain an isopropenyl side-chain, while pulegone and para-mentha-1,4(8)-dien-3-one contain an isopropylidene side-chain. None of these flavouring agents has been evaluated previously by the Committee.
Table 1. Summary of the results of safety evaluations of pulegone and five related flavouring agentsa
|
Flavouring agent |
No. |
CAS No. and structure |
Step B3b
Does intake exceed the threshold for human intake? |
Step B4
Adequate NOEL for substance or related substance? |
Conclusion based on current intake |
|
Structural class I |
|
Isopulegol |
755 |
89-79-2
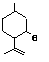
|
No
Europe: 6
USA: 7 |
Yes. The NOEL of 0.44 mg/kg bw per day for pulegone in a 90-day study (Spindler& Madsen, 1992) is > 1000 times the estimated daily intake of isopulegol when used as a flavouring agent. |
No safety concern |
|
Isopulegyl acetate |
756 |
57576-09-7
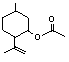
|
No
Europe: 0.4
USA: 1 |
Yes. The NOEL of 0.44 mg/kg bw per day for pulegone in a 90-day study (Spindler & Maqdsen, 1992) is >
10 000 times the estimated daily intake of isopulegyl acetate when used as a flavouring agent. |
No safety concern |
|
Structural class II |
|
Pulegone |
753 |
89-82-7
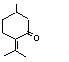
|
No
Europe: 2
USA: 2 |
Yes. The NOEL of 0.44 mg/kg bw per day in a 90-day study (Spindler & Madsen, 1992) is >
10 000 times the estimated daily intake of pulegone when used as a flavouring
agent. |
No safety concern |
|
Isopulegone |
754 |
29606-79-9
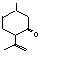 |
No
Europe: 1
USA: 0.01 |
Yes. The NOEL of 0.44 mg/kg bw per day for pulegone in a 90-day study (Spindler & Madsen, 1992) is >
10 000 times the estimated daily intake of isopulegone when used as a flavouring agent. |
No safety concern |
|
para-Mentha-1,4(8)-dien-3-one |
757 |
491-09-8
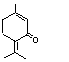
|
No
Europe: 2
USA: 0.01 |
Yes. The NOEL of 0.44 mg/kg bw per day for pulegone in a 90-day study (Spindler & Madsen, 1992) is >
10 000 times the estimated daily intake of para-mentha-1,4(8)-dien-3-one when used as a flavouring agent. |
No safety concern |
|
Menthofuran |
758 |
494-09-8
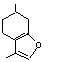
|
No
Europe: 13
USA: 25 |
Yes. The NOEL of 0.44 mg/kg bw per day for pulegone in a 90-day study (Spindler & Madsen, 1992) is 1000 times the estimated daily intake of menthofuran when used as a flavouring agent. |
No safety concern |
CAS, Chemical Abstracts Service
a
Step 2: None of the substances in this group is expected to be metabolized to innocuous products.
b
The thresholds for human intake are 1800 µg/day for structural class I and 540 µg/day for class II. All intake values are expressed in µg/day.
Of the six flavouring agents reviewed, only isopulegyl acetate has not been found to occur naturally in food. The other agents occur in several plant and fruit juices and in oils such as peppermint and pennyroyal oil. Isopulegol has been found in citrus peel oils, cognac, rum, and lemon balm. Isopulegone has a minty, herbaceous aroma and has been detected in ginger and buchu oil. Mentha-1,4(8)-dien-3-one has been detected in orange and grapefruit juices (Stofberg & Kirschman, 1985; Stofberg & Grundschober, 1987; Maarse et al., 1996).
1.2 Estimated daily intake
The total annual production volume of isopulegone derivatives, pulegone derivatives, and menthofuran is 250 kg in Europe (International Organization of the Flavor Industry, 1995) and 180 kg in the USA (National Academy of Sciences, 1987; Lucas et al., 1999). The flavouring agents produced in the highest volumes are menthofuran (No. 758) (170 kg in Europe and 95 kg in the USA) and isopulegol (No. 755) (50 kg in Europe and 45 kg in the USA). These two flavouring agents account for > 90% of the use of this group of substances in both Europe and the USA. The estimated intakes of menthofuran based on the reported annual volumes are approximately 25 µg/person per day in Europe and 13 µg/person per day in the USA. For isopulegol, the corresponding figures are 7 µg/person per day in Europe and 6 µg/person per day in the USA. The estimated intakes of the other substances in this group are 2 µg/person per day or less in both Europe and the USA. The intake values for each substance are reported in Table 2.
Table 2. Annual volume and estimated per capita intake of isopulegone, pulegone, and related substances in Europe and the United States
|
Substance (No.) |
Most recent annual volume (kg)a |
Intakeb |
Annual volume in naturally occurring foods (kg)c |
Consumption ratiod |
|
µg/day |
µg/kg bw per day |
|
Isopulegone (754) |
|
|
|
+ |
NA |
|
Europe |
0.1 |
0.01 |
0.0002 |
|
|
|
USA |
10 |
1 |
0.02 |
|
|
|
Isopulegol (755) |
|
|
|
+ |
NA |
|
Europe |
50 |
7 |
0.1 |
|
|
|
USA |
45 |
6 |
0.1 |
|
|
|
Isopulegyl acetate (756) |
|
|
– |
NA |
|
|
Europe |
8 |
1 |
0.02 |
|
|
|
USA |
3 |
0.4 |
0.01 |
|
|
|
Pulegone (753) |
|
|
|
4600 |
350 |
|
Europe |
15 |
2 |
0.04 |
|
|
|
USA |
13 |
12 |
0.03 |
|
|
|
paraMentha-1,4(8)-dien-3-one (757) |
|
|
224 |
2200 |
|
|
Europe |
0.1 |
0.01 |
0.0002 |
|
|
|
USA |
15 |
2 |
0.03 |
|
|
|
Menthofuran (758) |
|
|
|
5520 |
58 |
|
Europe |
174 |
25 |
0.4 |
|
|
|
USA |
95 |
13 |
0.2 |
|
|
|
Total |
|
|
|
|
|
|
Europe |
250 |
|
|
|
|
|
USA |
180 |
|
|
|
|
NA, not applicable; +, reported to occur naturally in foods (Maarse et al., 1996), but no quantitative data; –, not reported to occur naturally in foods
a
From International Organization of the Flavour Industry (1995) and Lucas (1999)
b
Intake (µg/person per day) calculated as follows: [(annual volume, kg) ´ (1 ´ 109 µg/kg) / (population ´ survey correction factor ´ 365 days)], where population (10%, ‘eaters only’) = 32 ´ 106 for Europe and 26 ´ 106 for the USA. The correction factor = 0.6 for Europe and 0.8 for the USA, representing the assumption that only 60% and 80% of the annual volume of the flavour, respectively, was reported in the poundage surveys (International Organization of the Flavor Industry, 1995; Lucas et al., 1999). Intake (µg/kg bw per day) calculated as follows: [(µg/person per day)/body weight], where body weight = 60 kg. Slight variations may occur from rounding.
c
Quantitative data from Stofberg & Grundschober (1987)
d
Calculated as follows: (annual consumption in food, kg)/(most recently reported volume as a flavouring substance, kg)
1.3 Absorption, metabolism, and elimination
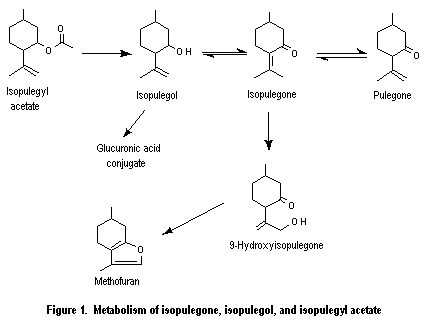
Isopulegone (No. 754) is predicted to be rapidly absorbed and metabolized in vivo, mainly by reduction, to yield isopulegol (No. 755) and undergoes reversible isomerization to pulegone (No. 753). Isopulegol, which may also be formed by hydrolysis of the corresponding acetate ester (No. 756), is predicted to be conjugated with glucuronic acid and excreted in the urine (Thomassen et al., 1991). Pulegone (No. 753) and para-mentha-1,4(8)-dien-3-one (No. 757) are either reduced to the corresponding alcohols and excreted or undergo allylic oxidation to yield the corresponding 9-hydroxy derivatives. In the case of pulegone (No. 753), the 9-hydroxy derivative cyclizes to yield menthofuran (No. 758) as the principal metabolite.
The metabolic pathway involving conversion of pulegone (No. 753) to menthofuran (No. 758) is considered to be a significant source of toxic products. Menthofuran is a proximate hepatotoxic agent that is transformed via an epoxide intermediate to the ultimate toxic agent, 8-pulegone aldehyde. This gamma-ketoenal has been shown to bind covalently to mouse, rat, and human liver microsomes, and the binding parallels the hepatotoxicity of menthofuran in these species. para-Mentha-1,4(8)-dien-3-one (No. 757) is presumed to participate in the same pathway, since its effects are similar to those of pulegone, but there was no direct evidence of the mechanism of toxicity of this compound. Other major routes of metabolism can be considered to be detoxication pathways.
Low concentrations of pulegone, menthofuran and their metabolites, menthofuran epoxide and the gamma-ketoenal, are conjugated with glutathione and glucuronic acid (Gordon et al., 1982; Thomassen et al., 1991; Oishi & Nelson, 1993). Metabolism of these substances may lead to formation of a reactive metabolite, glutathione depletion, and, eventually, hepatotoxicity at levels of intake of 100 mg/kg bw or more. Because these compounds may undergo metabolic bioactivation, the evaluation of their safety was based on a comparison with available data on toxicity (Steps B3–B4), although the estimated daily per capita intakes would not be sufficient to result in appreciable depletion of hepatic glutathione.
1.4 Application of the Procedure for the Safety Evaluation of Flavouring Agents
|
Step 1. |
In applying the Procedure for the Safety Evaluation of Flavouring Agents to the above-mentioned substances, the Committee assigned isopulegol (No. 755) and isopulegyl acetate (No. 756) to structural class I (Cramer et al., 1978); isopulegone (No. 754), pulegone (No. 753), and para-mentha-1,4(8)-diene-3-one (No. 757), which are monocycloalkenones, to structural class II and menthofuran (No. 758), a heterocyclic compound that is a common component of food, to structural class II. |
|
Step 2. |
At current levels of intake, none of the six flavouring agents would be expected to saturate the available metabolic detoxication pathways, but they are not completely metabolized to innocuous products. Since pulegone and related substances are metabolized, in part, to reactive metabolites, their evaluation proceeded via the right-hand side of the decision-tree . |
|
Step B3. |
The daily per capita intakes of all the substances in this group are below the threshold for human intake for each class (class l, 1800 µg; class ll, 540 µg). Accordingly, evaluation of these substances proceeded to step B4. |
|
Step B4. |
The lack of toxicity of pulegone at low levels of intake was demonstrated in a 90-day study in rats fed peppermint oil that contained 1.1% pulegone. The NOEL of 40 mg/kg bw per day for nephropathy associated with hyaline droplets at a higher dose (Spindler & Madsen, 1992) corresponds to a NOEL of 0.44 mg/kg bw per day (26 mg/person per day) for pulegone. This NOEL is > 10 000 times the intake of 0.033 µg/kg bw per day from use of pulegone as a flavouring agent. Since pulegone is metabolized to menthofuran, data on pulegone can be used to evaluate the safety of menthofuran, although the latter was about three times more hepatotoxic after single doses (Gordon et al., 1982). Isopulegone was less hepatotoxic than pulegone after single doses. The NOEL of 0.44 mg/kg bw per day for pulegone in the 90-day study is > 1000 times the daily intake of 0.4 µg/kg bw per day from use of menthofuran as a flavouring agent. Isopulegone, isopulegol, and isopulegyl acetate are expected to be partly metabolized to menthofuran. Even if these compounds are assumed to be metabolized to menthofuran to the same extent as pulegone, however, the NOEL for pulegone is > 10 000 times the daily intake from use of isopulegone and isopulegyl acetate and is > 1000 times the daily intake from use of isopulegol as a flavouring agent. |
Table 1 summarizes the evaluation of pulegone and five related substances.
1.5 Consideration of combined intakes
In the unlikely event that all foods containing isopulegol and isopulegyl acetate were consumed concurrently on a daily basis, the estimated combined intake would not exceed the threshold for human intake for structural class I (1800 µg/person per day). In the unlikely event that all foods containing isopulegone, pulegone, para-mentha-1,4(8)-diene-3-one, and menthofuran were consumed concurrently on a daily basis, the estimated combined intake would not exceed the threshold for human intake for class II (540 µg/person per day). Furthermore, there is an adequate safety margin between the estimated combined intake of all six substances (approximately 40 µg/person per day) and the NOEL for pulegone.
1.6 Conclusions
The Committee concluded that the safety of the substances in this group would not raise concern at the current estimated levels of intake. Other data on toxicity, including the results of short-term studies of toxicity and studies of the genotoxicity of pulegone and related compounds, were consistent with the results of the safety evaluation.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
This monograph summarizes the key data relevant to evaluating the safety of isopulegone (No. 754), its corresponding alcohol (No. 755) and acetate ester (No. 756), pulegone (No. 753), para-mentha-1,4(8)-dien-3-one (No. 757), and a principal metabolite of pulegone, menthofuran (No. 758) (see Table 1 and Figure 1). All of these substances except the acetate ester are components of various mint oils and are metabolically interrelated. The first three substances contain a 2-isopropenyl-5-methyl cyclohexyl carbon skeleton, while pulegone and para-mentha-1,4(8)-dien-3-one are 2-isopropylidene-5-methylcyclohexanone derivatives. Menthofuran contains a furan ring fused to a cyclohexane ring.
2.2 Additional considerations on intake
Of the six substances in this group of flavouring agents, only isopulegyl acetate has not been found to occur naturally in food. Isopulegone and pulegone derivatives have a minty, herbaceous aroma. With the exception of the acetate ester (No. 756), all the members of this group occur in peppermint oil, pennyroyal oil, and citrus peel oil. Isopulegone has also been been detected in ginger and buchu oil. Isopulegol has been found in cognac, rum, and lemon balm and at concentrations up to 4000 mg/kg in orange-peel oil. Pulegone and menthofuran occur naturally in a variety of other foods such as tea, beans, and oregano (Maarse et al., 1996). The intakes of pulegone and menthofuran from consumption of peppermint oil are > 100 and > 25 times, respectively, the intake from their use as flavouring substances. Mentha-1,4(8)-dien-3-one (No. 757) has been detected in orange and grapefruit juice (Maarse et al., 1996), and its intake from consumption of these oils is > 2000 times its intake from use as a flavouring substance (Stofberg & Kirschman, 1985; Stofberg & Grundschober, 1987; Table 2).
2.3 Biological data
2.3.1.1 Ester hydrolysis
Isopulegyl acetate (No. 756) is expected to be hydrolysed to isopulegol (No. 755) and acetic acid (Figure 1). Unless sterically hindered, aliphatic esters are hydrolysed to their component alcohols and carboxylic acids (Leegwater & van Straten, 1974; Butterworth et al., 1975; Grundschober, 1977; Longland et al., 1977; Heymann, 1980; Anders, 1989; Voet & Voet, 1990). The hydrolysis of esters in relation to application of the Procedure for the Safety Evaluation of Flavouring Agents was reviewed by the Committee at its fifty-first meeting (Annex 1, reference 137).
2.3.1.2 Absorption and metabolism
Isopulegone (No. 754), isopulegol (No. 755), and isopulegyl acetate (No. 756)
Isopulegone (No. 754) is expected mainly to be reduced to isopulegol (No. 755), oxidized at the allylic position to yield 9-hydroxyisopulegone, and isomerized to pulegone (No. 753) (Figure 1). The major metabolite of isopulegone in liver microsomes from phenobarbital-treated rats was 9-hydroxyisopulegone, which is formed by allylic hydroxylation (oxidation) of the isopropenyl side-chain. 9-Hydroxyisopulegone cyclizes in part to menthofuran (No. 758) but at a slower rate than 9-hydroxypulegone. Thus, the relative formation of menthofuran from isopulegone is less than that of pulegone in vitro (Madyastha & Raj, 1990).
The metabolic fate of isopulegol may be predicted, by analogy with the known biotransformation of other cyclohexanols, to occur primarily by conjugation and excretion as a glucuronide and to a lesser extent by further oxidation. The intermediate isopulegone epoxide was more hepatotoxic than pulegone epoxide (Gordon et al., 1982), but the hepatotoxicity of both compounds is thought to be due to their metabolism to menthofuran, which is more acutely hepatotoxic (see below). Cyclohexanol, for example, is conjugated with glucuronic acid and excreted as the glucuronide or oxidized to cyclohexanediol (Elliott et al., 1959). Similarly, in humans, (–)-menthol, the saturated form of isopulegol, is primarily conjugated with glucuronic acid and excreted in the urine (Quick, 1928; Eisenberg et al., 1955; Atzl et al., 1972; White et al., 1987).
Pulegone (No. 753
), para-mentha-1,4(8)-dien-3-one (No. 757), and menthofuran (No. 758)
The metabolic fate of pulegone has been studied in vitro and in vivo. Most of the studies were performed with the toxic R(+)-stereoisomer ((+)-pulegone), found naturally in pennyroyal oil, but the S(–)-isomer has been shown to be metabolized by the same pathways (Madyastha & Gaikwad, 1998). Conjugated metabolites of pulegone (which accounted for 3% of the radiolabel excreted in bile) are predicted to be excreted rapidly into the bile of rats after oral administration. The glucuronides were two times more abundant than the glutathione conjugate, and the most abundant biliary metabolites were glucuronides of hydroxylated pulegone and pulegol.
Pulegone is extensively metabolized (Figure 2), primarily to menthofuran and para-mentha-1,4(8)-dien-3-one (piperitenone), both of which are further metabolized (Nelson et al., 1992a). The tertiary ring carbon (C5) is hydroxylated to yield 5-hydroxypulegone, which then dehydrates to para-mentha-1,4(8)-dien-3-one (Madyastha & Raj, 1993). para-Mentha-1,4(8)-dien-3-one undergoes further ring and side-chain oxidation to yield a series of hydroxylated derivatives. In the predominant pathway, which eventually leads to reactive and unreactive metabolites, the isopropylidene substituent of pulegone undergoes regiospecific allylic oxidation to yield 9-hydroxypulegone, which then cyclizes to form menthofuran (Gordon et al., 1987; Madyastha & Raj, 1993). The subsequent metabolism of menthofuran is discussed below.
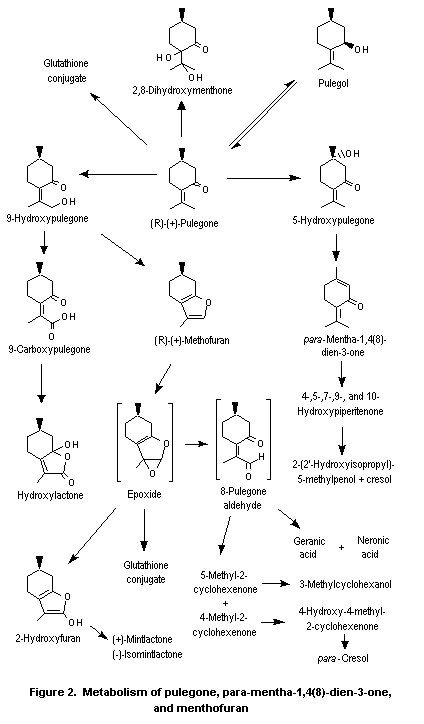
In minor pathways, the exocyclic alkene of pulegone is oxidized, presumably through the epoxide intermediate (Nelson et al., 1992b), to yield 2,8-dihydroxy-menthone (Moorthy et al., 1989a; Madyastha & Raj, 1993). Pulegone is also reduced to pulegol (Madyastha et al., 1985; Moorthy et al., 1989a; Madyastha & Raj, 1993) and rearranged to isopulegone (Gordon et al., 1987), probably through a free-radical intermediate (McClanahan et al., 1988). In addition to cyclizing to yield menthofuran, 9-hydroxypulegone is oxidized in a secondary detoxication pathway to 9-carboxy-pulegone (5-methyl-2-(1-methyl-1-carboxyethylidene)cyclohexanone) which partly cyclizes to a hydroxylactone or undergoes oxidation and hydration to yield polar hydroxyacids, which are excreted primarily in the urine (Moorthy et al., 1989a; Madyastha & Raj, 1993; Figure 2).
After oral administration to rats, para-mentha-1,4(8)-dien-3-one was extensively hydroxylated, and urinary metabolites were isolated which were derived from hydroxylation at the 4, 5, 7, and 10 positions. The only other saturated carbon available for hydroxylation is in the 9 position, the allylic position of the isopropylidene group syn to the ketone. This oxidation product was not isolated, but the cyclization product from hydroxylation at this position, dehydromenthofuran, was isolated and found to be unstable. Both para-mentha-1,4(8)-dien-3-one and menthofuran are thought to form the ultimate hepatotoxin gamma-ketoenal, as discussed below. para-Mentha-1,4(8)-dien-3-one is also metabolized to para-cresol and 2-(2´-hydroxy-isopropyl)-5-methylphenol. The finding of para-cresol among the metabolites supports the hypothesis of the formation of the gamma-ketoenal. The 4- and 5-hydroxypiperitenone metabolites are then dehydrated to form 2-isopropenyl-5-methylphenol, which, after hydration, would give the observed urinary metabolite, 2-(2-hydroxyispropyl)-5-methylphenol. While the precise quantities of these metabolites were not given, all were formed in appreciable amounts, representing 5–12% of the administered dose (Madyastha & Gaikwad, 1999). It was reported previously that para-mentha-1,4(8)-dien-3-one and hydroxy-para-mentha-1,4(8)-dien-3-one were found in the urine after oral administration of pulegone to rats (Madyastha & Raj, 1993). These metabolites arise from reduction of the exocyclic double bond of para-mentha-1,4(8)-dien-3-one followed by hydroxylation at the 7 position.
The second major metabolite of pulegone is menthofuran. Menthofuran is converted to a variety of stable, polar metabolites and also to a reactive metabolite, a gamma-ketoenal (8-pulegone aldehyde; Figure 2), which is thought to be the primary cause of the hepatotoxicity of menthofuran. Two stable, polar metabolites, geranic acid and neranic acid, arise from ring cleavage of this reactive ketoenal (Madyastha & Raj, 1992, 1993) by mechanisms not yet fully understood. All the other metabolites, and perhaps these two as well, apparently arise from initial epoxidation of menthofuran followed by rearrangement directly to the gamma-ketoenal, 2(Z)-(2’-keto-4’-methylcyclohexylidene)propanal (8-pulegone aldehyde), and indirectly through 2-hydroxymenthofuran.
Evidence for the formation of the menthofuran epoxide was provided by isolation of the thioether that would be formed by reaction of the epoxide with glutathione (Oishi & Nelson, 1993) after oral administration of menthofuran to rats. Evidence for the formation of the 2-hydroxymenthofuran comes from its isolation in vitro after incubation of menthofuran with human cytochrome P450 isozymes (Khojasteh-Bakht et al., 1999) and from isolation of (+)-mintlactone and (–)-isomintlactone, which are rearrangement products of 2-hydroxymenthofuran, in vitro (Nelson et al., 1992b; Thomassen et al., 1992; Khojasteh-Bakht et al., 1999) and in vivo after oral dosing of rats with menthofuran (Oishi & Nelson, 1993). In the last study, the mintlactones were shown to be further converted to a ketoacid, which was excreted as a glucuronide conjugate (Figure 2).
The reactive intermediate, gamma-ketoenal (8-pulegone aldehyde), has not been detected in vivo but has been trapped with semicarbazide in vitro from mouse liver microsomes incubated with pulegone (McClanahan et al., 1989) or menthofuran (Thomassen et al., 1992) and from rat liver microsomes incubated with menthofuran (Madyastha & Raj, 1990). The rate of formation of 8-pulegone aldehyde in mouse, rat, and human hepatic microsomes, with menthofuran as the substrate (Thomassen et al., 1992) is 5–10 times faster than when pulegone is used as the substrate (McClanahan et al., 1989), providing support for the proposal that this gamma-ketoenal is formed from pulegone via menthofuran. Evidence that 8-pulegone aldehyde is the ultimate toxicant is presented below.
8-Pulegone aldehyde is not only converted to mintlactones and subsequent metabolites but can also be detoxicated by conversion to 5-methyl-2-cyclohexenone or 4-methyl-2-cyclohexenone (Figure 2). 5-Methyl-2-cyclohexenone is converted to 3-methylcyclohexanol through the intermediate 3-methylcyclohexanone (Madyastha & Raj, 1992), and 4-methyl-2-cyclohexenone is ultimately converted to para-cresol (Madyastha & Raj, 1990, 1991, 1992, 1993; Figure 2). Both are then converted to benzoic acid (Madyastha & Raj, 1992).
Conjugates of pulegone, menthofuran, and other metabolites with glucuronic acid and glutathione, as well as mixed glutathionyl glucuronide conjugates have been detected in the bile of rats given pulegone or menthofuran (Thomassen et al., 1991).
2.3.1 Toxicological studies
2.3.1.1 Acute toxicity
LD50 values in rats treated orally have been reported for three of the six substances in this group: isopulegol (No. 755; 936 mg/kg bw; Lynch, 1971), isopulegyl acetate (No. 756; > 5000 mg/kg bw; Russell, 1973), and the R(+)-isomer of pulegone (No. 753; 470 mg/kg bw; Moreno, 1975). The acute toxicity of the S(–)- and R(+)-isomers of pulegone was compared in mice treated by intraperitoneal injection; the S(–)-isomer was significantly less toxic (4/10 deaths at 600 mg/kg bw) than the R(+)-isomer (5/10 deaths at 400 mg/kg bw) (Gordon et al., 1982). Thus, the racemic mixture would be expected to be less acutely toxic than the R(+) isomer.
Pennyroyal oil, an essential oil derived from the leaves of Mentha pulegium, consists of 62–97% of R(+)-pulegone (Grundschober, 1979) and has been eaten by humans for centuries, mainly for its supposed abortifacient properties (Gunby, 1979), although its effectiveness, at least in the absence of severe toxicity, has been questioned (Centers for Disease Control, 1978). In several cases, ingestion of > 10 ml of pennyroyal oil resulted in moderate to severe toxicity, and ingestion of > 15 ml (approximately 250 mg/kg bw for a 60-kg woman) resulted in death (Anderson et al., 1996). The ingestion of large doses of pennyroyal oil by humans is followed by massive centrilobular necrosis, pulmonary oedema, internal bleeding, and weight loss (Sullivan et al., 1979). These effects have been attributed to the pulegone content of the oil, and similar effects have been produced after intraperitoneal injection of either pennyroyal oil or pulegone in mice (see below; Gordon et al., 1982). In one case of fatal poisoning, the blood of the patient at autopsy contained both pulegone (at 18 ng/ml) and the proximate toxicant menthofuran (at 1 ng/ml) (Anderson et al., 1996).
While no LD50 values have been reported after oral administration of the other three substances, their acute toxicity has been investigated. In a comparison of the toxicity of the components of pennyroyal oil, isopulegol was not toxic at 600 mg/kg bw, R(+)-pulegone caused 9/16 deaths at 400 mg/kg bw, isopulegone caused 3/13 deaths at 500 mg/kg bw and 3/5 at 600 mg/kg bw, para-mentha-1,4(8)-dien-3-one caused 1/10 deaths at 500 mg/kg bw and 2/10 at 600 mg/kg bw, and menthofuran caused 5/15 deaths at 200 mg/kg bw and 10/16 at 300 mg/kg bw. These results indicate that isopulegone and para-mentha-1,4(8)-dien-3-one are significantly less toxic than R(+)- pulegone and that menthofuran is significantly more toxic. The main effects of R(+)- pulegone, isopulegone, para-mentha-1,4(8)-dien-3-one, and menthofuran in this study were hepatic centrilobular necrosis and, to a lesser extent, bronchiolar necrosis. R(+)- pulegone also caused a decrease in glutathione concentration. Pretreatment with diethyl maleate, in order to deplete glutathione, increased the toxicity of R(+)- pulegone. Isopulegyl acetate was not tested since it is not a known constituent of pennyroyal oil (Gordon et al., 1982).
R(+)-Pulegone administered orally to rats by gavage at a dose of 400 mg/kg bw per day for up to 5 days decreased liver microsomal cytochrome P450 activity and haem, increased the serum activity of alanine aminotransferase, and decreased the activities of glucose-6-phosphatase and aminopyrine N-demethylase (Moorthy et al., 1989b). Administration of R(+)-pulegone at a dose of 100 mg/kg bw per day caused an increase in alanine aminotransferase activity, but no other effects were seen. Pretreatment with phenobarbital or diethyl maleate increased the toxicity, while pretreatment with 3-methylcholanthrene or piperonyl butoxide completely protected the animals from toxicity. Similarly, menthofuran administered by oral gavage to rats at doses of 100–400 mg/kg bw per day produced a dose-related increase in alanine aminotransferase activity and decreased activities of glucose-6-phosphatase and aminopyrine N-demethylase. The effects at 100 mg/kg bw per day were minimal; pretreatment with phenobarbital increased the toxicity, but 3-methylcholanthrene had no effect (Madyastha & Raj, 1994).
2.3.1.2 Short-term studies of toxicity
The results of short-term studies of the toxicity of isopulegone, pulegone, and structurally related substances are presented in Table 3 and summarized below.
Table 3. Results of short-term studies of toxicity with isopulegone, pulegone and structurally related substances used as flavouring substances
|
Substance |
No. |
Species; sex |
No. of test groupsa/ no. per test groupb |
Route |
Duration |
NOEL (mg/kg bw per day) |
Reference |
|
Isopulegol |
755 |
Rats; M |
2/3–4 |
food |
14 days |
250 |
Imaizumi et al. (1985) |
|
Pulegone |
753 |
Rats; M, F |
3/20 |
gavage |
28 days |
20 |
Thorup et al. (1983a) |
|
Pulegone |
753 |
Rats; M |
2/3–4 |
food |
14 days |
250 |
Imaizumi et al. (1985) |
|
Pulegone |
753 |
Rat; F |
1/28 |
Oral |
28 days |
< 160 |
Mølck et al. (1998) |
|
Peppermint oil
(1.7% pulegone) |
|
Rats; M, F |
3/20 |
gavage |
28 days |
10 (0.17 for pulegone) |
Thorup et al. (1983b) |
|
Peppermint oil
(1–2% pulegone) |
|
Rats; M |
3/12 |
gavage |
5 weeks |
500 (5–10 for pulegone) |
Mengs & Stotzem (1989) |
|
Peppermint oil
(1–2% pulegone) |
|
Dogs; M, F |
2/6 |
gavage |
5 weeks |
125 (1.25–2.5 for pulegone) |
Mengs & Stotzem (1989) |
|
Peppermint oil
(1.1% pulegone) |
|
Rats; M, F |
3/14 |
gavage |
90 days |
40 (0.4 for pulegone) |
Spindler & Madsen (1992) |
|
Menthofuran |
758 |
Rats; M, F |
1/5 |
food |
14 days |
23c |
Van Miller & Weaver (1987) |
M, male; F, female
a
Total number of test groups does not include control animals.
b
Total number per test group includes both male and female animals.
c
Study performed wuth either a single dose or multiple doses that had no adverse effect; the value is therefore the highest dose tested.
Isopulegol (No. 754)
Groups of three or four male Wistar rats were maintained on diets containing 0, 0.5, or 1% isopulegol for 14 days. The relative weight of the liver and the concentrations of cholesterol and triglyceride were significantly increased at 1% isopulegol (Imaizumi et al., 1985). The concentration of 0.5% was calculated (Food & Drug Administration, 1993) to provide an average daily intake of 250 mg/kg bw per day.
Pulegone (No. 753)
R(+)-Pulegone was administered orally to groups of 10 male and 10 female Wistar SPF rats by gavage in soya bean oil at a dose of 0, 20, 80, or 160 mg/kg bw per day for 28 days. The animals were observed twice daily, and body weight and food and water consumption were measured weekly. Dose-dependent atonia was onbserved after a few days. The water consumption of rats at the highest dose was reduced, and the body-weight gain of animals at the highest dose was reduced by 20% and that of rats at the intermediate dose by 10%. Haematological examinations and blood chemical determinations perfomed on eight animals of each sex on day 21 or 22 of dosing showed that animals at the highest dose had a significant, dose-dependent decrease in plasma creatinine concentration and an increased number of neurophil granulocytes. At necropsy, the rats at the highest dose had distended stomachs. As the terminal weights of the body and organs were significantly decreased in all treated rats, the relative weights were not taken into consideration. The histopathological changes included vacuolization of hepatocytes, mainly around the central vein, at the two higher doses, which the authors considered to represent an adaptive response. Dose-related alterations in the brain, which appeared as ‘cyst-like spaces’ in the white matter, were reported at the two higher doses (Thorup et al., 1983a). No cellular reaction was found in the surrounding tissue, and special staining revealed no demyelination (Olsen & Thorup, 1984). The authors noted that the alteration resembled the neuropathy induced in rats by hexachlorophene. The NOEL was 20 mg/kg bw per day.
Similar effects were reported from the same laboratory in a study that followed the same protocol, when peppermint oil containing 1–3% R(+)-pulegone was given by gavage to provide a dose of 0, 10, 40, or 100 mg/kg bw per day to groups of 10 male and of10 female Wistar SPF rats for 28 days. No differences in body weight or food consumption were found between treated and control groups. A slight, non-significant increase in water consumption was reported in all treated groups. Haematological examinations, blood chemical determinations, and urine analysis revealed normal values. The only significant histopathological change was the appearance of ‘cyst-like spaces’ in the white matter of the cerebellum in animals at the two higher doses, but there were no obvious clinical sugns of encephalopathy (Thorup et al., 1983b; Olsen & Thorup, 1984).
A similar study was conducted to confirm the presence of the ‘cyst-like spaces’ in the white matter. When pulegone was given orally by gavage to groups of 28 female Wistar SPF rats at a dose of 160 mg/kg bw per day for 28 days, the animals showed slackness, depression, and significantly decreased food consumption and body weight (p < 0.001). Blood chemical examinations performed on day 27 or 28 of dosing revealed increased plasma glucose concentration, increased alkaline phosphatase activity, a non-significant increase in alanine aminotransferase activity, and a decreased plasma creatinine concentration. Significantly increased absolute (p < 0.1) and relative (p < 0.05) weights of the liver were also observed, but there were no significant histopathological findings in the liver, and no ‘cyst-like spaces’ were observed in the white matter of the cerebellum, with or without fixation of the tissue by perfusion. The authors concluded that the discrepancies in the findings of these studies may have been due to impurities in the test substance or to a change in the genetic constitution of the animals. The ‘cyst like spaces’ may have been artefacts arising from inadequate tissue fixation procedures (Mølck et al., 1998).
Peppermint oil containing 1–2% pulegone was administered to groups of three beagle dogs of each sex at a dose of 25 or 125 mg/kg bw per day or to groups of 12 male Wistar rats at a dose of 20, 150, or 500 mg/kg bw per day, by gavage for 5 weeks. The animals were inspected daily for clinical signs; body weight and food consumption were recorded weekly; haematological, blood biochemical, and urinary parameters were measured before treatment and during week 5; and histological examination was conducted at termination. The rats showed no effects on general health, behaviour, or body weight, and the hematological and urinary parameters were normal. Histological examination revealed no specific pathological lesions. A reduction in triglyceride concentration in rats at the high dose was attributed to decreased food consumption. Similar results were found for dogs, except that males at the high dose had slightly, non-significantly increased alkaline phosphatase activity and urea concentrations. These increases were considered to be of no toxicological relevance (Mengs & Stotzem, 1989).
Pulegone was added to the diets of groups of three or four male Wistar rats at a concentration of 0, 0.5, or 1% for 14 days. The food intake and body-weight gain of animals at the high dose were decreased, and the relative liver weight and triglyceride concentrations were significantly increased (Imaizumi et al., 1985). The concentration of 0.5% was calculated (Food & Drug Administration, 1993) to provide an average daily intake of 250 mg/kg bw.
Peppermint oil containing 1.1% pulegone was administered to groups of 14 male and 14 female Wistar rats by oral gavage in soya bean oil at a dose of 0, 10, 40, or 100 mg/kg bw per day for 90 days. Body weights and food and water consumption were measured weekly; no differences were found between treated and control animals. Haematological examinations and blood chemical determinations perfomed on 10 animals of each sex on days 30 and 86 of dosing showed normal values. Animals at the low and intermediate doses showed no effects, but male rats at the high dose had nephropathy, in the form of hyaline droplets. The authors concluded that this effect was an early manifestation of sex- and species-specific nephropathy due to the appearance of alpha-2-microglobulin in the kidney. ‘Cyst-like spaces’ in the cerebellum were reported in animals at the high dose, but there were no other signs of encephalopathy (Spindler & Madsen, 1992). As this effect was not reproduced in the 28-day study in which animals were given pulegone at 160 mg/kg bw per day, an NOEL for peppermint oil of 40 mg/kg bw per day could be identified, which corres-ponds to an NOEL for pulegone of 0.44 mg/kg bw per day. Nevertheless, it is ques-tionable whether the effects at the high dose are relevant in terms of human risk.
Menthofuran (No. 758)
In a screening test for toxicity, menthofuran was added to the diet of rats at a concentration resulting in an average daily intake of 23 mg/kg bw for 14 days. No effects on body-weight gain, food consumption, liver or kidney weights, or gross histological appearance of the liver and kidney were seen (Van Miller & Weaver, 1987).
2.3.1.2 genotoxicity
Assays for genotoxicity have been performed with pulegone (No. 753) and menthofuran (No. 758) (Table 4). Pulegone did not induce reverse mutation in Salmonella typhimurium strain TA1537, TA1535, TA100, TA98, or TA97, with or without metabolic activation, at concentrations up to 800 µg/plate (Andersen & Jensen, 1984). Neither substance was mutagenic in S. typhimurium strains TA100 and TA98 at concentrations up to 1000 µg/plate, with or without metabolic activation (Nelson & Dybing, 1998). In a study of the insecticidal properties of mint oils, concentrations of pulegone in excess of the LD50 value for Drosophila larvae (0.17 µL)l induced a slight increase in the frequency of wing mutations (mosaic spots) over that induced by control solutions (Franzios et al., 1997).
Table 4. Results of studies of genotoxicity with isopulegone, pulegone, and structurally related substances used as flavouring agents
|
No. |
Substance |
End-point |
Test system |
Concentration |
Result |
Reference |
|
753 |
Pulegone |
Reverse mutation |
S. typhimurium TA97, TA98, TA100, TA1535, TA1537 |
Ł 800 mg/platea |
Negative |
Andersen & Jensen (1984) |
|
753 |
Pulegone |
Reverse mutation |
S. typhimurium TA98, TA1537 |
1000 mg/platea |
Negative |
Nelson & Dybing (1998) |
|
753 |
Pulegone |
Wing spot mutation |
D. melanogaster |
0.2 µL |
Weakly positive |
Franzios et al. (1997) |
|
|
Pennyroyl oil (75.7% pulegone) |
Wing spot mutation |
D. melanogaster |
2.1 µL (9.8 µmol pulegone) |
Negative |
Franzios et al. (1997) |
|
758 |
Menthofuran |
Reverse mutation |
S. typhimurium TA98, TA100 |
1000 mg/platea |
Negative |
Nelson & Dybing (1998) |
a
With and without metabolic activation
2.3.1.4 Immunotoxicity
In a screening study for immunotoxicity, mice were treated with isopulegol (No. 755) at doses up to 500 mg/kg bw per day orally for 5 days. No effects were seen on body weight, lymphoid organ weight or cellularity, or in functional tests for humoral and cell-mediated immunity (Vollmuth et al., 1989).
2.3.1.5 Mechanisms of toxicity
The mechanisms by which pulegone (No. 753) and its proximate hepatotoxicant, menthofuran (No. 758), exert toxic effects have been studied extensively both in vitro and in vivo, presumably because of the use and abuse of pennyroyal oil. Pulegone has been shown to be the active constituent of pennyroyal oil, and menthofuran produced the same toxic effects as pulegone after intraperitoneal injection to mice (Gordon et al., 1982). These effects are similar to those reported in humans after ingestion of pennyroyal oil (Anderson et al., 1996).
In rats given R(+)-pulegone by intraperitoneal injection at a dose of 300 mg/kg bw, the livers showed dilatation of the central veins and distension of sinusoidal spaces 6 h after treatment and centrilobular necrosis beginning at 12 h. Electron microscopic examination after 24 h revealed degeneration of the endoplasmic reticulum, swelling of mitochondria, and nuclear changes (Moorthy et al., 1991a). It has been suggested that pulegone metabolites specifically deactivate cytochrome P450 isozymes by modifying the prosthetic haem or apoprotein of the enzyme (Madyastha et al., 1985; Moorthy et al., 1991b). In human liver microsomes, menthofuran specifically inhibited CYP2A6, and adducts with this enzyme have been isolated (Khojasteh-Bakht et al., 1998).
A comparison of the pharmacokinetics of pulegone and menthofuran after intraperitoneal administration to mice showed that a significant amount of the hepatotoxicity of pulegone could be accounted for by the formation of menthofuran (Thomassen et al., 1988), which has been named as the proximate hepatotoxin. Pulegone is metabolized primarily to para-mentha-1,4(8)-dien-3-one (No. 757) and menthofuran (No. 758) (Figure 1). para-Mentha-1,4(8)-dien-3-one is a double alpha, beta-unsaturated ketone and would be expected to be biologically active, although there is no evidence to support this hypothesis other than the fact that the toxic effects of this metabolite are similar, but less severe, than those of pulegone (Gordon et al., 1982). In contrast, menthofuran is converted to the reactive gamma-ketoenal, 8-pulegone aldehyde (Figure 1), which has been trapped in vitro with semicarbazide (McClanahan et al., 1989; Madyastha & Raj, 1990; Thomassen et al., 1992).
Binding of radiolabel to macromolecules was demonstrated after incubation of [14C]pulegone with rat liver microsomes (Madyastha & Moorthy, 1989) and with mouse liver, lung, and kidney proteins, the binding being greater in liver than in lung and that in lung being similar to binding in the kidney. The degree of binding to liver protein paralleled the degree of hepatotoxicity (McClanahan et al., 1989). In all cases, treatment with semicarbazide decreased the binding, clearly indicating that 8-pulegone aldehyde is the ultimate toxicant. A similar pattern of covalent binding in liver, lung, and kidney was seen after intraperitoneal administration to mice of [14C]menthofuran, and the degree of binding in rat, mouse, and human liver microsomes was similar (Thomassen et al., 1992).
While there is good evidence that 8-pulegone aldehyde is the ultimate toxicant, there is also evidence that this metabolite of menthofuran accounts for only some of the toxicity of pulegone. It has been proposed that para-cresol formed both from menthofuran and from para-mentha-1,4(8)-dien-3-one (Figure 1) also contributes to the toxicity (Madyastha & Raj, 1991; Thompson et al, 1994; Madyastha & Gaikwad, 1999). This is unlikely, however, since only a relatively small amount of para-cresol (< 15% of the dose) is formed from para-mentha-1,4(8)-dien-3-one (Madyastha & Gaikwad, 1999), para-cresol did not have the same type of toxic effects in rats or mice after 28 days of oral administration (National Toxicology Program, 1992) or in rats after 90 days (Environmental Protection Agency, 1988), and the NOELs in these studies are of the same order of magnitude as that for pulegone itself.
The role of cytochrome P450 in the hepatotoxicity of pulegone after intraperitoneal administration to mice was demonstrated by the finding that a variety of inhibitors of these enzymes decreased the toxicity (Gordon et al., 1987; Mizutani et al., 1987; Moorthy et al., 1989b). Mizutani et al. (1987) found no increase in hepatotoxicity when mice were pretreated with phenobarbital; however, others reported greater toxicity in phenobarbital-pretreated mice (Gordon et al., 1987). It has also been reported that pretreatment with phenobarbital significantly enhanced the toxicity of pulegone administered orally to rats (Moorthy et al., 1989b). Similarly, phenobarbital pretreatment enhanced the hepatotoxicity of orally administered menthofuran in rats (Madyastha & Raj, 1994). Thus, the oxidative process enhances the hepatotoxicity of both pulegone and menthofuran, as would be expected, since pulegone is converted to menthofuran by 8-hydroxypulegone (Figure 1) and the reactive 8-pulegone aldehyde is the ultimate toxicant. Evidence that pulegone is oxidized to 8-hydroxypulegone via a free radical mechanism, as proposed by McClanahan et al. (1988) is provided by the finding that C-phycocyanin, a free-radical scavenger, decreased the hepatotoxicity of pulegone after intraperitoneal administration to rats (Vadiraja et al., 1998).
Glutathione has also been shown to play a role in the detoxication of pulegone. Pulegone administered intraperitoneally to mice decreased the plasma concentration of glutathione, and pretreatment with diethyl maleate (to decrease glutathione levels) enhanced the toxicity of pulegone but not that of menthofuran (Gordon et al., 1982; Thomassen et al., 1990). glutathione has been shown to react with menthofuran epoxide, the precursor to 8-pulegone aldehyde (Oishi & Nelson, 1993). Isolation of glutathione conjugates, including a novel mixed glutathionyl–glucuronide conjugate, from the bile of rats treated intraperitoenally with pulegone led to the proposal that glutathione conjugation plays a major role in detoxication of the cytochrome P450-bioactivated pulegone metabolite (i.e. menthofuran or the gamma-ketoenal) (Thomassen et al., 1991).
There is considerable evidence that the reactive metabolite of pulegone found in animals also exists in humans, resulting in the formation of menthofuran and other reactive and unreactive polar metabolites, which may be readily excreted. For example, menthofuran was detected in the blood in a case of poisoning with pennyroyal (Anderson et al.,1996). When menthofuran is given at high doses, it is a proximate hepatotoxic substance; however, if the concentration of toxic metabolites of pulegone is not sufficient to deplete hepatocellular concentrations of glutathione (5–10 mmol/L) (Sies et al., 1983; Armstrong, 1987), hepatotoxicity may not be observed. Therefore, the concentrations of pulegone and its reactive metabolites from use of pulegone as a flavour ingredient are not sufficient to deplete the hepatocellular concentration of glutathione.
3. REFERENCES
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics by the kidney. In: Hutson, D.H., Caldwell, J. & Paulson, G.D., eds, Intermediary Xenobiotic Metabolism in Animals, New York : Taylor & Francis, pp. 81–97.
Andersen, P.H. & Jensen, N.J. (1984) Mutagenic investigation of peppermint oil in the Salmonella/mammalian-microsome test. Mutat. Res., 138, 17–20.
Anderson, I.B., Mullen, W.H., Meeker, J.E., Khojasteh-Bakht, S.C., Oishi, S., Nelson, S.D. & Blanc, P.D. (1996) Pennyroyal toxicity: Measurement of toxic metabolite levels in two cases and review of the literature. Ann. Intern. Med., 124, 726–734.
Armstrong, R.N. (1987) Enzyme catalyzed detoxication reactions: Mechanisms and stereochemistry. CRC Crit. Rev. Biochem., 22, 39–88.
Atzl, G., Bertl, M., Daxenbichler, G. & Gleispach, H. (1972) Determination of etheral oils from the urine by gas–liquid chromatography. Chromatographia, 5, 250–255.
Butterworth, K.R., Carpanini, G.B., Gaunt, I.F., Grasso, P. & Lloyd, A.G. (1975) A new approach to the evaluation of the safety of flavouring esters. Proc. Br. Phil. Soc., 54, 268–269.
Centers for Disease Control (1978) Fatality and illness associated with consumption of pennyroyal oil—Colorado. Morbid. Mortal. Wkly Rep., 27, 512–513.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic hazard—A decision tree approach. Food Cosmet. Toxicol., 16, 255–276.
Eisenberg, F., Field, J.B. & Stetten, D. (1955) Studies on glucuronide conjugation in man. Arch. Biochem. Biophys., 59, 297–299.
Elliott, T.H., Parke, D.V. & Williams, R.T. (1959) Studies in detoxication. The metabolism of cyclo[(14)]hexane and its derivatives. Biochem. J., 72, 193–200.
Environmental Protection Agency (1988) Subchronic Toxicity of para-Cresol in Sprague-Dawley Rats: MBA Chemical No. 25. EPA/PB88-195292, Research Triangle Park, North Carolina, USA.
Food & Drug Administration (1993) Priority-based Assessment of Food Additives (PAFA) Database. Center for Food Safety and Applied Nutrition, Washington DC, USA, p. 58.
Franzios, G., Mirotsou, M., Hatziapostolou, E., Kral, J., Scouras, Z.G. & Mavragani-Tsipidou, P. (1997) Insecticidal and genotoxic activities of mint essential oils. J. Agric. Food Chem., 45, 2690–2694.
Gordon, W.P., Forte, A.J., McMurtry, R.J., Gal, H. & Nelson, S.D. (1982) Hepatotoxicity and pulmonary toxicity of pennyroyal oil and its constituents terpenes in the mouse. Toxicol. Appl. Pharmacol., 65, 413–424.
Gordon, W.P., Huitric, A.C., Seth, C.L., McClanahan, R.H. & Nelson, S.D. (1987) The metabolism of the abortifacient terpene, (R)-(+)-pulegone, to a proximate toxin, menthofuran. Drug Metab. Disposition, 15, 589–594.
Grundschober, F. (1977) Toxicological assessment of flavoring esters. Toxicology, 8, 387–390.
Grundschober, F. (1979) Literature review of pulegone. Perfum. Flavorist, 4, 15–17.
Gunby, P. (1979) Plant known for centuries still causes problems today. J. Am. Med. Assoc., 243, 1355–1366.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B., ed., Enzymatic Basis of Detoxication, 2nd Ed., New York: Academic Press, pp. 291–323.
Imaizumi, K., Hanada, K., Mawartari, K. & Sugano, M. (1985) Effect of essential oils on the concentration of serum lipids and apolipoproteins in rats. J. Agric. Biol. Chem., 49, 2795–2796.
International Organization of the Flavour Industry (1995) European Inquiry on Volume of Use. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Khojasteh-Bakht, S.G., Koenigs, L.L.. Peter, R.M., Trager, W.F. & Nelson, S.D. (1998) (R)-(+)-Menthofuran Is a potent, mechanism-based inactivator of human liver cytochrome P450 2A6. Drug Metab. Disposition, 26, 701–704.
Khojasteh-Bakht, S.G., Chen, W., Koenigs, L.L., Peter, R.M. & Nelson, S.D. (1999) Metabolism of (R)-(+)-pulegone and (R)-(+)-menthofuran by human liver cytochrome P-450s: Evidence for formation of a furan epoxide. Drug Metab. Disposition, 27, 574–580
Leegwater, D.C. & van Straten, S. (1974) In vitro study on the hydrolysis of twenty-six organic esters by pancreatin. Unpublished report from Central Institute for Nutrition and Food Research, Zeist, Netherlands. Submitted to WHO by the Flavor and Extract Manufacturers’ Association of the United States, Washington DC, USA.
Longland, R.C., Shilling, W.H. & Gangolli, S.D. (1977) The hydrolysis of flavouring esters by artificial gastrointestinal juices and rat tissue preparations. Toxicology, 8, 197–204.
Lucas, C., Putnam, J., Hallagan J. & the Flavor Ingredients Committee (1999) 1995 Poundage and Technical Effects Update Survey, Flavor and Extract Manufacturers’ Association of the United States, Washington DC, USA.
Lynch, T.A. (1971) Acute dermal toxicity—Albino rabbits. Unpublished report to the Research Institute for Fragrance Materials, Arlington Research Laboratory, Inc., Plain City, Iowa, USA. Submitted to WHO by the Flavor and Extract Manufacturers’ Association of the United States, Washington DC, USA.
Maarse, H., Visscher, C.A., Willemsens, L.C., Nijssen, L.M. & Boelens, M.H., eds (1996) Volatile Components in Food, Suppl. 5 to the 6th Edition, Zeist, TNO Nutrition and Food Research.
Madyastha, K.M. & Gaikwad, N.W. (1998) Metabolic fate of S-(–) pulegone in rat. Xenobiotica, 28, 723–734.
Madyastha, K.M. & Gaikwad, N.W. (1999) Metabolic disposition of a monoterpene ketone, piperitenone, in rats: Evidence for the formation of a known toxin, p-cresol. Drug Metab. Disposition, 27, 74–80.
Madyastha, K.M. & Moorthy, B. (1989) Pulegone mediated hepatotoxicity: Evidence for covalent binding of R-(+)-C14-pulegone to microsomal proteins in vitro. Chem.–Biol. Interactions, 72, 325–333.
Madyastha, K.M. & Raj, C.P. (1990) Biotransformation of R-(+)-pulegone and menthofuran in vitro: Chemical basis for toxicity. Biochem. Biophys. Res. Commun., 173, 1086–1092.
Madyastha, K.M. & Raj, C.P. (1991) Evidence for the formation of a known toxin, p-cresol, from menthofuran. Biochem. Biophys. Res. Commun., 177, 440–446.
Madyastha, K.M. & Raj, C.P. (1992) Metabolic fate of menthofuran in rats: Novel oxidative pathways. Drug Metab. Disposition, 20, 295–301.
Madyastha, K.M. & Raj, C.P. (1993) Studies on the metabolism of a monoterpene ketone, R-(+)-pulegone—A hepatotoxin in rat: Isolation and characterization of new metabolites. Xenobiotica, 23, 509–518.
Madyastha, K.M. & Raj, C.P. (1994) Effects of menthofuran, a monoterpene furan on rat liver microsomal enzymes, in vivo. Toxicology, 89, 119–125.
Madyastha, P., Moorthy, B., Vaidyanathan & Madyastha, K.M. (1985) In vivo and in vitro destruction of rat liver cytochrome P-450 by a monoterpene ketone, pulegone. Biochem. Biophys. Res. Commun., 128, 921–927.
McClanahan, R.H., Huitric, A.C., Pearson, P.G., Desper, J.C. & Nelson, S.D. (1988) Evidence for a cytochrome P-450 catalysed allylic rearrangement with double bond topomerization, J. Am. Chem. Soc., 110, 1979–1981.
McClanahan, R.H., Thomassen, D., Slattery, J.T. & Nelson, S.D.(1989) Metabolic activation of (R)-(+)-pulegone to a reactive enonal that covalently binds to mouse liver proteins. Chem. Res. Toxicol., 2, 349–355.
Mengs, U. & Stotzem, C.D. (1989) Toxicological evaluation of peppermint oil in rodents and dogs. Med. Sci. Res., 17, 499–500.
Mizutani, T., Nomura, H., Nakanishi, K. & Fujita, S. (1987) Effects of drug metabolism modifiers on pulegone-induced hepatotoxicity in mice. Res. Commun. Chem. Pathol. Pharmacol., 58, 75–83.
Mølck, A.-M., Poulsen, M., Tingard Lauridsen, S. & Olsen, P. (1998) Lack of histological cerebellar changes in Wistar rats given pulegone for 28 days. Comparison of immersion and perfusion fixation. Toxicol. Lett., 95, 117–122.
Moorthy, B., Madyastha, P. & Madyastha, K.M. (1989a) Metabolism of a monoterpene ketone, R-(+)-pulegone a hepatotoxin in rat. Xenobiotica, 19, 217–224.
Moorthy, B., Madyastha, P. & Madyastha, M. (1989b) Hepatotoxicity of pulegone in rats: Its effects on microsomal enzymes, in vivo. Toxicology, 55, 327–337.
Moorthy, B., Vijayasarathi, S.K., Basu, A. & Madyastha, K.M. (1991a) Biochemical, histopathological and ultrastructural changes in rat liver induced by R-(+)-pulegone, a monoterpene ketone. Toxicol. Environ. Chem., 33, 121–131.
Moorthy, B., Madyastha, P. & Madyastha, K.M. (1991b) Destruction of rat liver microsomal cytochrome P-450 in vitro by a monoterpene ketone, pulegone—A hepatotoxin. Indian J. Chem., 30, 138–146.
Moreno, O.M. (1975) Acute oral toxicity in rats and dermal toxicity in rabbits. Unpublished report to Research Institute for Fragrance Materials, MB Research Laboratories, Inc., Spinnerstown, Pennsylvania, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
National Academy of Sciences (1987) Evaluating the Safety of Food Chemicals, Washington DC.
National Toxicology Program (1992) Toxicity Studies of Cresols in F344/N Rats and B6C3F1 Mice (Feed Studies), NTP-TOX 9, Research Triangle Park, North Carolina, USA.
Nelson, S. & Dybing, E. (1998) Unpublished study. Submitted to WHO by the Flavor and Extract Manufacturers’ Association of the United States, Washington DC, USA.
Nelson, S., McClanahan, R.H., Knebel, N., Thomassen, D., Gordon, W.P. & Oishi, S. (1992a) The metabolism of (R)-(+)-pulegone, a toxic monoterpene. Environ. Sci. Res., 44, 287–296.
Nelson, S.D., McClanahan, R.H., Thomassen, D., Gordon, W.P. & Knebel, N. (1992b) Investigations of mechanisms of reactive metabolite formation from (R)-(+)-pulegone. Xenobiotica, 22, 1157–1164.
Oishi, S. & Nelson, S.D. (1993) Metabolic disposition of menthofuran in rat: Identification of urinary metabolites. Department of Medical Chemistry, University of Washington, Seattle, Washington, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Olsen, P. & Thorup, I. (1984) Neurotoxicity in rats dosed with peppermint oil and pulegone. Arch. Toxicol., Suppl. 7, 408–409.
Quick, A.J. (1928) Quantitative studies of beta-oxidation. IV. The metabolism of conjugated glycuronic acids. J. Biol. Chem., 80, 535–541.
Russell, T.J. (1973) Acute oral toxicity and acute dermal toxicity. Unpublished report to the Research Institute for Fragrance Materials, Toxicological Resources, East Millstone, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Sies, H., Brigelius, R. & Akerboom, R.P.M. (1983) Intrahepatic glutathione status. In: Larson, A., Holmgren, A., Orrenius, S. & Mannervik, B., eds, Functions of Glutathione: Biochemical, Physiological, Toxicological and Clinical Aspects, New York: Raven Press, pp. 51–64.
Spindler, P. & Madsen, C. (1992) Subchronic toxicity study of peppermint oil in rats. Toxicol. Lett., 62, 215–220.
Stofberg, J. & Grundschober, F. (1987) Consumption ratio and food predominance of flavoring materials. Perfum. Flavorist, 12, 27.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of flavoring materials: A mechanism for setting priorities for safety evaluation. Food Chem. Toxicol., 23, 857–860.
Sullivan, J.B., Rumack, B.H., Thomas, H., Peterson, R.G. & Brysch, P. (1979) Pennyroyal oil poisoning and hepatotoxicity. J. Am. Med. Assoc., 242, 2873–2874.
Thomassen, D., Slattery, J.T. & Nelson, S.D. (1988) Contribution of menthofuran to the hepatotoxicity of pulegone: Assessment based on matched area under the curve and on matched time course. J. Pharmacol. Exp. Ther., 224, 825.
Thomassen, D., Slattery, J.T. & Nelson, S.D. (1990) Menthofuran-dependent and -independent aspects of pulegone hepatotoxicity: Roles of glutathione. J. Pharmacol. Exp. Ther., 253, 567.
Thomassen, D., Pearson, P.G., Slattery, J.T. & Nelson, S.D. (1991) Partial characterization of biliary metabolites of pulegone by tandem mass spectrometry. Drug Metab. Disposition, 19, 997.
Thomassen, D., Knebel, N., Slattery, J.T., McClanahan, R.H. & Nelson, S.D. (1992) Reactive intermediates in the oxidation of menthofuran by cytochrome P-450. Chem. Res. Toxicol., 5, 123–130.
Thompson, D.C., Perera, K., Fisher, R. & Brendel, K. (1994) Cresol isomers: Comparison of toxic potency in rat liver slices. Toxicol. Appl. Pharmacol., 125, 51–58.
Thorup, I., Wuertzen, J., Carstensen, J. & Olsen, P. (1983a) Short-term toxicity study in rats dosed with pulegone and menthol. Toxicol. Lett., 19, 207–210.
Thorup, I., Wuertzen, J., Carstensen, J. & Olsen, P. (1983b) Short-term toxicity study in rats dosed with peppermint oil. Toxicol. Lett., 19, 211–215.
Vadiraja, B.B., Gaikwad, N.W. & Madyastha, K.M. (1998) Hepatoprotective effect of C-phycocyanin: Protection for carbon tetrachloride and R-(+)-pulegone-mediated hepatotoxicity in rats. Biochem. Biophys. Res. Commun., 249, 428–431.
Van Miller, J.P. & Weaver, E.V. (1987) Fourteen-day dietary minimum toxicity screen (MTS) in albino rats. Unpublished report to the Flavor and Extract Manufacturers’ Association, Bushy Run Research Center, Export, Pennsylvania, USA. Submitted to WHO by the Flavor and Extract Manufacturers’ Association of the United States, Washington DC, USA.
Voet, D. & Voet, J.G. (1990) Metabolism in the citric acid cycle. In: Biochemistry, New York: John Wiley & Sons, pp. 507–525.
Vollmuth, T.A., Heck, J.D., Ratajczak, H.V. & Thomas, P.T. (1989) Immunotoxicity assessment of flavoring ingredients using a rapid and economical screen. Toxicologist, 9, 206.
White, D.A., Thompson, S.P., Wilson, C.G. & Bell, G.D. (1987) A pharmacokinetic comparison of two delayed-release peppermint oil preparations, Colpermin and Mintec, for treatment of the irritable bowel syndrome. Int. J. Pharm., 40, 151–155.
See Also:
Toxicological Abbreviations
![]()