
WHO FOOD ADDITIVES SERIES: 52
ALIPHATIC, ALICYCLIC, LINEAR, alpha,beta-UNSATURATED, DI-AND TRIENALS
AND RELATED ALCOHOLS, ACIDS AND ESTERS
First draft prepared by
Professor G. Williams
Department of Environmental Pathology and Toxicology, New York Medical College, Valhalla, New York, USA
1. EVALUATION
1.1 Introduction
The Committee evaluated a group of 26 alpha,beta-unsaturated flavouring agents (see Table 1) by the Procedure for the Safety Evaluation of Flavouring Agents (see Figure 1, Introduction). This group included 12 dienals (Nos 1173, 1175, 1179, 1181, 1182, 1185–1187, 1190, 1195–1197), a trienal (No. 1198), five dienols (Nos 1174, 1180, 1183, 1184 and 1189), a dienoic acid (No. 1176) and seven related esters (Nos 1177, 1178, 1188, 1191–1194), all of which contain unsaturation in the 2,3-position and some of which contain saturation in the 4,5-position. The Committee had evaluated one member of the group, (E,E)-2,4-hexadienoic acid (No. 1176, sorbic acid) in its capacity as an antimicrobial preservative at the seventeenth meeting (Annex 1, reference 32), when an ADI of 0–25 mg/kg bw per day was established.
alpha,beta-Unsaturated aldehydes are formed endogenously by lipid peroxidation of polyunsaturated fatty acids (Frankel et al., 1987) or can be consumed as naturally-occurring constituents of food (Stofberg & Grundschober, 1987; Maarse et al., 1999), and are only consumed to a minor extent as added flavouring agents.
Twenty-one of the 26 flavouring agents in this group of flavouring agents have been reported to occur naturally in food. They have been detected in apples, grapes, broccoli, roast chicken, tea and beer (Maarse et al., 1999).
1.2 Estimated daily intake
The total annual volume of production of the 26 flavouring agents in this group is approximately 1000 kg in Europe (International Organization of the Flavour Industry, 1995) and 1500 kg in the USA (National Academy of Sciences, 1982; Lucas et al., 1999). Approximately 50–60% of the total annual volume of production of the aliphatic linear dienals in Europe and the USA is accounted for by 2-trans,4-trans-decadienal (No. 1190). The estimated combined per capita daily intake of the 12 dienals is approximately 40 µg in Europe and 120 µg in the USA, and the per capita daily intake of 2-trans,4-trans-decadienal is approximately 20 µg in Europe and 70 µg in the USA. The per capita intakes of all the other flavouring agents in the group are in the range of 0.007–24 µg/day (National Academy of Sciences, 1982; International Organization of the Flavour Industry, 1995; Lucas et al., 1999), most values being at the lower end of this range. The daily per capita intake of each agent in Europe and in the USA is reported in Table 1.
Table 1. Summary of results of safety evaluations of aliphatic di- and trienals and related alcohols, acids and estersa,b
|
Flavouring agent |
No. |
CAS No. and structure |
Step A3
Does intake exceed threshold for human intake?c |
Step A4
Is the flavouring agent or are its metabolites endogenous? |
Comments |
Conclusion based on current intake |
|
Structural class I |
|
|
|
|
|
|
|
(E,E)-2,4-Hexadienoic acid |
1176 |
110-44-1
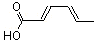 |
No
Europe: ND
USA: 6 |
NR |
See note 3 |
See footnoted |
|
Methyl sorbate |
1177 |
689-89-4
 |
No
Europe: 0.1
USA: ND |
NR |
See note 4 |
No safety concern |
|
Ethyl sorbate |
1178 |
2396-84-1
 |
No
Europe: 59
USA: 3 |
NR |
See note 4 |
No safety concern |
|
2-trans,6-trans-Octadienal |
1182 |
56767-18-1
 |
No
Europe: 0.1
USA: 0.007 |
NR |
See note 1 |
No safety concern |
|
2,6-Nonadien-1-ol |
1184 |
7786-44-9
 |
No
Europe: 2
USA: 1 |
NR |
See note 2 |
No safety concern |
|
Nona-2-trans-6-cis-dienal |
1186 |
557-48-2
 |
No
Europe: 7
USA: 24 |
NR |
See note 1 |
No safety concern |
|
2-trans-6-trans-Nonadienal |
1187 |
17587-33-6
 |
No
Europe: ND
USA: 0.007 |
NR |
See note 1 |
No safety concern |
|
(E,Z)-2,6-Nonadien-1-ol acetate |
1188 |
68555-65-7
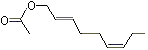 |
No
Europe: ND
USA: 18 |
NR |
See note 4 |
No safety concern |
|
Methyl (E)-2-(Z)-4-decadienoate |
1191 |
4493-42-9
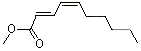 |
No
Europe: ND
USA: 1 |
NR |
See note 4 |
No safety concern |
|
Ethyl trans-2-cis-4-decadienoate |
1192 |
3025-30-7
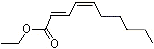 |
No
Europe: 34
USA: 3 |
NR |
See note 4 |
No safety concern |
|
Ethyl 2,4,7-decatrienoate |
1193 |
78417-28-4
 |
No
Europe: ND
USA: 0.4 |
NR |
See note 4 |
No safety concern |
|
Propyl 2,4-decadienoate |
1194 |
84788-08-9
 |
No
Europe: 0.9
USA: ND |
NR |
See note 4 |
No safety concern |
|
2-trans-6-cis-Dodecadienal |
1197 |
21662-13-5
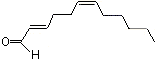 |
No
Europe: 0.6
USA: 0.02 |
NR |
See note1 |
No safety concern |
|
2,4-Pentadienal |
1173 |
764-40-9
 |
No
Europe: 0.1
USA: 0.2 |
Yes. The NOEL of 15 mg/kg body weight per day (National Toxicology Program, 2001) for the related substance trans,trans-2,4-hexadienal is >1 million times the estimated daily intake of 2,4-pentadienal when used as a flavouring agent. |
See note 1 |
No safety concern |
|
(E,E)-2,4-Hexadien-1-ol |
1174 |
111-28-4
 |
No
Europe: ND
USA: 0.4 |
Yes. The NOEL of 15 mg/kg bw per day (National Toxicology Program, 2001) for the related substance trans,trans-2,4-hexadienal is >1million times the estimated daily intake of (E,E)-2,4-hexadien-1-ol when used as a flavouring agent. |
See note 2 |
No safety concern |
|
trans,trans-2,4-Hexadienal |
1175 |
142-83-6
 |
No
Europe: 1
USA: 0.1 |
Yes. The NOEL of 15 mg/kg bw per day (National Toxicology Program, 2001) is >100 000 times the estimated daily intake of trans,trans-2,4-hexadienal when used as a flavouring agent. |
See note 1 |
No safety concern |
|
2,4-Heptadienal |
1179 |
4313-03-5
 |
No
Europe: 4
USA: 23 |
Yes. The NOEL of 15 mg/kg bw per day (National Toxicology Program, 2001) for the related substance trans,trans-2,4-hexadienal is >10 000 times the estimated daily intake of 2,4-heptadienal when used as a flavouring agent. |
See note1 |
No safety concern |
|
(E,E)-2,4-Octadien-1-ol |
1180 |
18409-20-6
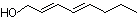 |
No
Europe: ND
USA: 18 |
Yes. The NOEL of 15 mg/kg bw per day (National Toxicology Program, 2001) for the related substance trans,trans-2,4-hexadienal is >10 000 times the estimated daily intake of (E,E)-2,4-octadien-1-ol when used as a flavouring agent. |
See note 2 |
No safety concern |
|
trans,trans-2,4-Octadienal |
1181 |
30361-28-5
 |
No
Europe: 0.7
USA: 0.007 |
Yes. The NOEL of 15 mg/kg bw per day (National Toxicology Program, 2001) for the related substance trans,trans-2,4-hexadienal is >1million times the estimated daily intake of trans,trans-2,4-octadienal when used as a flavouring agent. |
See note1 |
No safety concern |
|
2,4-Nonadien-1-ol |
1183 |
62488-56-6
 |
No
Europe: ND
USA: 26 |
Yes. The NOEL of 33.9 mg/kg bw per day (Damske et al., 1980) for the related substance 2-trans,4-trans-decadienal is >10 000 times the estimated daily intake of 2,4-nonadien-1-ol when used as a flavouring agent. |
See note 2 |
No safety concern |
|
2,4-Nonadienal |
1185 |
6750-03-4
 |
No
Europe: 2
USA: 0.7 |
Yes. The NOEL of 33.9 mg/kg bw per day for the related substance 2-trans,4-trans-decadienal is >1 million times the estimated daily intake of 2,4-nonadienal when used as a flavouring agent. |
See note 1 |
No safety concern |
|
(E,E)-2,4-Decadien-1-ol |
1189 |
18409-21-7
 |
No
Europe: ND
USA: 26 |
Yes. The NOEL of 33.9 mg/kg bw per day (Damske et al., 1980) for the related substance 2-trans,4-trans-decadienal is >10 000 times the estimated daily intake of (E,E)-2,4-decadien-1-ol when used as a flavouring agent. |
See note 2 |
No safety concern |
|
2-trans,4-trans-Decadienal |
1190 |
25152-84-5
 |
No
Europe: 22
USA: 70 |
Yes. The NOEL of 33.9 mg/kg bw per day (Damske et al., 1980) for 2-trans,4-trans-decadienal is >10 000 times the estimated daily intake of 2-trans,4-trans-decadienal when used as a flavouring agent. |
See note 1 |
No safety concern |
|
2,4-Undecadienal |
1195 |
13162-46-4
 |
No
Europe: 4
USA: 0.4 |
Yes. The NOEL of 33.9 mg/kg bw per day (Damske et al., 1980) for the related substance 2-trans,4-trans-decadienal is >100 000 times the estimated daily intake of 2,4-undecadienal when used as a flavouring agent. |
See note 1 |
No safety concern |
|
trans,trans-2,4-Dodecadienal |
1196 |
21662-16-8
 |
No
Europe: 0.7
USA: 0.1 |
Yes. The NOEL of 33.9 mg/kg bw per day (Damske et al., 1980) for the related substance 2-trans,4-trans-decadienal is >1 million times the estimated daily intake of trans,trans-2,4-dodecadienal when used as a flavouring agent. |
See note 1 |
No safety concern |
|
2-trans-4-cis-7-cis-Tridecatrienal |
1198 |
13552-96-0
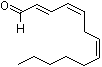 |
No
Europe: 0.3
USA: 0.009 |
Yes. The NOEL of 33 mg/kg bw per day (Edwards, 1973) is >1 million times the estimated daily intake of 2-trans-4-cis-7-cis-tridecatrienal when used as a flavouring agent |
See note 1 |
|
|
CAS: Chemical Abstract Service; ND: No intake data reported; NR: Not required for evaluation because consumption of the substances was determined to be of no safety concern at step A3 of the decision-tree |
|
a |
Step 1: All of the flavouring agents in this group are in structural class I (Cramer et al., 1978) |
|
b |
Step 2: Thirteen flavouring agents (Nos 1176–1178, 1182, 1184, 1186–1188, 1191–1194, and 1197) in this group are expected to be metabolized to innocuous products. The evaluation of these flavouring agents therefore proceeded via the A-side of the decision-tree. The alpha,beta-unsaturated dienals and related alcohols (Nos 1173–1175, 1179–1181, 1183, 1185, 1189, 1190, 1195, 1196, and 1198) in this group cannot be predicted to be metabolized to innocuous products. The evaluation of these 13 flavouring agents therefore proceeded via the B-side of the Procedure |
|
c |
The threshold for human intake for structural class I is 1800 µg per day. All intake values are expressed in µg per day. The combined per capita intakes of flavouring agents in structural class I are 138 µg per day in Europe and 221 µg per day in the USA |
|
d |
An ADI of 0–25 mg/kg bw was established for (E,E)-2,4-hexadienoic acid by the Committee at its seventeenth meeting (Annex 1, reference 32), and this was maintained at the present meeting. Use of the chemical as a flavouring agent is subsumed in the ADI |
|
Notes: |
|
1 |
Oxidized to acids, which may undergo beta-oxidative cleavage and complete alphametabolism via the tricarboxylic acid cycle. Alternately, may undergo glutathione conjugation and excretion as mercapturic acid derivatives |
|
2 |
Oxidized to aldehydes and acids, which metabolize completely in the fatty acid beta-oxidation pathway |
|
3 |
Undergoes beta-oxidative cleavage and complete metabolism via the tricarboxylic acid cycle |
|
4 |
Hydrolysed to corresponding alcohols and acids, followed by complete metabolism in the fatty acid pathway or the tricarboxylic acid cycle |
1.3 Absorption, distribution, metabolism and elimination
In general, aliphatic esters are hydrolysed rapidly to their component alcohols and carboxylic acids by classes of enzymes known as carboxylesterases in the intestinal mucosa. Once hydrolysed, the resulting aliphatic alcohols and carboxylic acids are absorbed into the portal circulation. The unsaturated alcohols are oxidized successively to the corresponding aldehydes and carboxylic acids, which participate in fundamental biochemical pathways, including the fatty acid pathway and tricarboxylic acid cycle (Nelson & Cox, 2000).
It is anticipated that humans will metabolize dienals and trienals by oxidation to the corresponding acids, which may undergo beta-oxidative cleavage and complete metabolism via the tricarboxylic acid cycle. An alternate minor pathway may involve conjugation of the unsaturated aldehyde to glutathione, followed by excretion as the mercapturic acid derivative.
Under conditions of glutathione depletion and oxidative stress, and at high cellular concentrations, alpha,beta-unsaturated aldehydes have been shown to form adducts with DNA nucleotides, to cause cytohistopathology, and to induce apoptosis. However, metabolic evidence indicates that low concentrations of alpha,beta-unsaturated aldehydes are safely metabolized in the high-capacity beta-oxidation pathway or via glutathione conjugation.
1.4 Application of the procedure for the safety evaluation of flavouring agents
|
Step 1. |
In applying the Procedure for the Safety Evaluation of Flavouring Agents to the 26 flavouring agents in this group, the Committee assigned all of them to structural class I (Cramer et al., 1978). |
|
Step 2. |
Thirteen flavouring agents (Nos 1176–1178, 1182, 1184, 1186–1188, 1191–1194, and 1197) in this group are expected to be metabolized to innocuous products. The evaluation of these flavouring agents therefore proceeded via the A-side of the decision-tree. The alpha,beta-unsaturated 2,4-dienals and alcohol precursors (Nos 1173–1175, 1179–1181, 1183, 1185, 1189, 1190, 1195, 1196, and 1198) cannot be predicted to be metabolized to innocuous products and the evaluation of these 13 flavouring agents therefore proceeded via the B-side of the decision-tree. |
|
Step A3. |
The estimated daily per capita intakes in Europe and the USA of the 13 flavouring agents in this group that are metabolized to innocuous products (Nos 1176–1178, 1182, 1184, 1186–1188, 1191–1194, and 1197) are below the threshold for concern for class I (i.e. 1800 µg/day). The Committee concluded that these substances would not be expected to be of safety concern at their currently estimated levels of intake as flavouring agents. |
|
Step B3. |
The estimated daily per capita intakes in Europe and the USA of the remaining 13 flavouring agents in this group that cannot be predicted to be metabolized to innocuous products are also below the threshold of concern for structural class I (1800 mg/day). Accordingly, the evaluation of these 13 agents proceeded to step B4. |
|
Step B4. |
The NOEL of 15 mg/kg bw per day for trans,trans-2,4-hexadienal (No.1175) administered by gavage in a 14-week study in rats (National Toxicology Program, 2001) provides an adequate margin of safety (>100 000) in relation to the known levels of intake of this agent. This NOEL is also appropriate for the structurally related agents 2,4-pentadienal (No. 1173), 2,4-heptadienal (No. 1179), and trans,trans-2,4-octadienal (No. 1181), because these agents are all dienals that will undergo oxidation and subsequent metabolism via similar metabolic pathways. The NOEL for trans,trans-2,4-hexadienal is also appropriate for the structurally related (E,E)-2,4-hexadien-1-ol (No. 1174), and (E,E)-2,4-octadien-1-ol (No. 1180) because these alcohols will be oxidized to the corresponding aldehydes and subsequently undergo metabolism in a similar manner to the dienals. |
The NOEL of 33.9 mg/kg bw per day for 2-trans,4-trans-decadienal (No. 1190), identified from a 14-week study in rats treated by gavage (Damske et al., 1980), provides an adequate margin of safety (>10 000) in relation to the known levels of intake of this agent. The NOEL for 2-trans,4-trans-decadienal is also appropriate for the structurally related substances 2,4-nonadien-1-ol (No. 1183), 2,4-nonadienal (No. 1185), (E,E)-2,4-decadien-1-ol (No. 1189), 2,4-undecadienal (No. 1195) and trans,trans-2,4-dodecadienal (No. 1196), because of their similar metabolic pathways.
For 2-trans-4-cis-7-cis-tridecatrienal (No. 1198), the NOEL of 33 mg/kg bw per day identified from a 4-week study in rats (Edwards, 1973) provides an adequate margin of safety (>1 000 000) in relation to the known levels of intake of this agent.
The Committee noted that 2,4-trans-hexadienal (No. 1175) induced forestomach hyperplasia and squamous cell tumours in rats and mice of each sex. This is a common finding in National Toxicology Program bioassays in which a high concentration of an irritating material suspended in corn oil is delivered by gavage into the forestomach every day for 2 years.
Trans,trans-2,4-hexadienal gave positive results in some tests for genotoxicity in vitro, but was inactive in tests carried out in vivo. Thus, this substance may be genotoxic under some conditions, but this is not believed to be the basis for its effects in the rodent forestomach. There was evidence of injury to the forestomach epithelium attributable to exposure and this is believed to be the primary cause of the development of neoplasia. Mice and rats in the bioassays developed forestomach hyperplasia following corn oil gavage, and a low incidence of adenomas was observed in mice, reflecting the sensitivity of the forestomach to irritation. The forestomach was the only site of increased neoplasia in treated animals.
An IARC Working Group concluded that when evaluating the relevance for human cancer of the induction of forestomach tumours in rodents, the experimental conditions of exposure should be considered. The conditions of exposure during oral administration are unusual in that physical effects may cause high local concentrations of test substances in the forestomach and prolonged exposure of the epithelium. Agents that only produce tumours of the forestomach in rodents after prolonged treatment, through non-DNA-reactive mechanisms, may be of less concern to humans since human exposure to such agents would need to surpass time-integrated dose thresholds in order to elicit the carcinogenic response.
Therefore, the Committee concluded that the appearance of forestomach tumours in the 2-year bioassays in rodents treated by gavage in which trans, trans-2,4-hexadienal was administered at a high concentration is of no toxicological relevance to humans.
Table 1 summarizes the evaluations of the 26 alpha,beta-unsaturated flavouring agents in this group.
1.5 Consideration of combined intakes from use as flavouring agents
Although the flavouring agents evaluated in this group are not converted to a common metabolite, they are subject to conjugation with reduced glutathione (GSH). Accordingly, simultaneous consumption of the alpha,beta-unsaturated aldehydes, at sufficiently high concentrations, could theoretically deplete concentrations of GSH, resulting in lipid peroxidation. However, under normal conditions, concentrations of intracellular replenishable GSH (approximately 1–10 mmol/l) are sufficient to detoxify the quantities of alpha,beta-unsaturated aldehydes being ingested as flavouring agents (Armstrong, 1987; 1991). Additionally, since the alpha,beta-unsaturated aldehydes provide similar flavouring characteristics, it is unlikely that all foods containing these flavouring agents would be consumed concurrently on a daily basis. Therefore, at the levels of alpha,beta-unsaturated aldehydes used as flavouring agents, and in consideration of the constant replenishment of GSH by biosynthesis, the Committee considered that the combined intake of these flavouring agents does not present a safety concern.
The estimated current intake of (E,E)-2,4-hexadienoic acid (No. 1176, sorbic acid) (0.1 µg/kg bw per day) from its use as a flavouring agent is below the individual ADI (0–25 mg/kg bw) established previously by the Committee (Annex 1, reference 33).
1.6 Consideration of secondary components
Ten members of this group of flavouring agents (Nos 1179, 1180, 1183, 1185, 1189–1192, 1196 and 1198) have minimum assay values of <95%. Information on the safety of the secondary components of these 10 compounds is summarized in Annex 6 (Summary of the safety evaluation of secondary components of flavouring agents with minimum assay values of <95%). In all cases, the secondary components were expected to share the same metabolic fate as the primary flavouring agents (Nos 1179, 1180, 1189, 1190 and 1196), or have metabolites that are substrates for the fatty acid cycle, which are subsequently excreted as carbon dioxide and water (Nos 1183, 1185, 1191, 1192, and 1198). One of the secondary components of No. 1185 (2,4-nonadien-1-ol) was evaluated at the present meeting, while two of the secondary components of No. 1190 (acetone and isopropanol) were evaluated at the fifty-first meeting. None of the secondary components was considered to present a safety concern at current levels of intake. The other secondary component of No. 1185 (2,4-nonen-1-ol) has not been previously evaluated; however, it is expected to be oxidized and completely metabolized in the fatty acid cycle. On this basis, 2,4-nonen-1-ol was considered not to present a safety concern at current intake levels.
1.7 Conclusions
The Committee retained the previously established ADI of 0–25 mg/kg bw for (E,E)-2,4-hexadienoic acid (No. 1176). The Committee concluded that none of the flavouring agents in this group would pose a safety concern at the currently estimated levels of intake. It was noted that other data on the toxicity and metabolism of the flavouring agents in the group were consistent with the results of the safety evaluation.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
This monograph summarizes the key data relevant to the safety evaluation of 26 aliphatic, alicyclic, linear alpha,beta-unsaturated, di- and trienals and related alcohols, acids and esters used as flavouring agents (see Table 1).
2.2 Additional considerations on intake
As shown in Table 2, 21 of the 26 substances in this group have been reported to occur naturally in foods. Lower dienal homologues (C5 to C9) have been detected in apples, grapes, broccoli, chicken, tea and beer (Maarse et al., 1999). Higher homologues (>C9) have been detected in roasted products such as peanuts and chicken (Maarse et al., 1999). In plants, aliphatic dienals are formed from polyunsaturated fatty acids by the action of lipooxygenases (Almosnino & Belin, 1991; Andrianarison et al., 1991). Quantitative data on natural occurrence and consumption ratios have been reported for 2,4-pentadienal (No. 1173), trans,trans-2,4-hexadienal (No. 1175), 2,4-heptadienal (No. 1179), trans,trans-2,4-octadienal (No. 1181), 2,6-nonadien-1-ol (No. 1184), 2,4-nonadienal (No. 1185), nona-2-trans-6-cis-dienal (No. 1186); 2-trans,4-trans-decadienal (No. 1190), ethyl trans-2-cis-4-decadienoate (No. 1192), and 2,4-undecadienal (No. 1195) and demonstrate that consumption of these agents occurs predominantly from traditional foods (i.e. con-sumption ratio >1) (Stofberg & Kirschman, 1985; Stofberg & Grundschober, 1987) (see Table 2).
Table 2. Annual volumes of production of aliphatic, alicyclic, linear, alpha,beta-unsaturated, di- and trienals and related alcohols, acids and esters used as flavouring agents in Europe and the USA
|
Flavouring agent (No.) |
Most recent annual volume (kg)a |
Intakeb ("eaters only") |
Annual volume in naturally occurring foods (kg)c |
Consumption ratiod |
|
µg/day |
µg/kg bw per day |
|
2,4-Pentadienal (1173) |
|
Europe |
1 |
0.1 |
0.002 |
|
|
|
USAe |
0.9 |
0.2 |
0.003 |
4 |
4 |
|
(E,E)-2,4-Hexadien-1-ol (1174) |
|
Europe |
ND |
ND |
ND |
|
|
|
USAf |
2 |
0.4 |
0.006 |
+ |
NA |
|
trans,trans-2,4-Hexadienal (1175) |
|
Europe |
8 |
1 |
0.02 |
|
|
|
USA |
1 |
0.1 |
0.002 |
1 |
1 |
|
(E,E)-2,4-Hexadienoic acid (1176) |
|
Europe |
ND |
ND |
ND |
|
|
|
USAf,g |
37 |
6 |
0.1 |
+ |
NA |
|
Methyl sorbate (1177) |
|
Europe |
0.8 |
0.1 |
0.002 |
|
|
|
USA |
ND |
ND |
ND |
+ |
NA |
|
Ethyl sorbate (1178) |
|
Europe |
411 |
59 |
1 |
|
|
|
USA |
22 |
3 |
0.05 |
+ |
NA |
|
2,4-Heptadienal (1179) |
|
Europe |
25 |
4 |
0.06 |
|
|
|
USA |
177 |
23 |
0.4 |
872 |
5 |
|
(E,E)-2,4-Octadien-1-ol (1180) |
|
Europe |
ND |
ND |
ND |
|
|
|
USAf |
100 |
18 |
0.3 |
- |
NA |
|
trans,trans-2,4-Octadienal (1181) |
|
Europeh |
5 |
0.7 |
0.01 |
|
|
|
USA |
0.05 |
0.007 |
0.0001 |
0.7 |
14 |
|
2-trans,6-trans-Octadienal (1182) |
|
Europe |
1 |
0.1 |
0.002 |
|
|
|
USA |
0.05 |
0.007 |
0.0001 |
- |
NA |
|
2,4-Nonadien-1-ol (1183) |
|
Europe |
ND |
ND |
ND |
|
|
|
USAf |
150 |
26 |
0.4 |
+ |
NA |
|
2,6-Nonadien-1-ol (1184) |
|
Europe |
16 |
2 |
0.04 |
|
|
|
USA |
8 |
1 |
0.02 |
50 |
6 |
|
2,4-Nonadienal (1185) |
|
Europe |
12 |
2 |
0.03 |
|
|
|
USA |
5 |
0.7 |
0.01 |
189 |
38 |
|
Nona-2-trans-6-cis-dienal (1186) |
|
Europe |
50 |
7 |
0.1 |
|
|
|
USA |
181 |
24 |
0.4 |
3265 |
18 |
|
2-trans-6-trans-Nonadienal (1187) |
|
Europe |
ND |
ND |
ND |
|
|
|
USA |
0.05 |
0.007 |
0.0001 |
+ |
NA |
|
(E,Z)-2,6-Nonadien-1-ol acetate (1188) |
|
Europe |
ND |
ND |
ND |
|
|
|
USAf |
100 |
18 |
0.3 |
- |
NA |
|
(E,E)-2,4-Decadien-1-ol (1189) |
|
Europe |
ND |
ND |
ND |
|
|
|
USAf |
150 |
26 |
0.4 |
+ |
NA |
|
2-trans,4-trans-Decadienal (1190) |
|
Europe |
154 |
22 |
0.4 |
|
|
|
USA |
531 |
70 |
1 |
33414 |
63 |
|
Methyl (E)-2-(Z)-4-decadienoate (1191) |
|
Europe |
ND |
ND |
ND |
|
|
|
USAf |
4.5 |
1 |
0.01 |
+ |
NA |
|
Ethyl trans-2-cis-4-decadienoate (1192) |
|
Europe |
241 |
34 |
0.6 |
|
|
|
USA |
20 |
3 |
0.04 |
455 |
23 |
|
Ethyl 2,4,7-decatrienoate (1193) |
|
Europe |
ND |
ND |
ND |
|
|
|
USAf |
2.4 |
0.4 |
0.007 |
+ |
NA |
|
Propyl 2,4-decadienoate (1194) |
|
Europeh |
6 |
0.9 |
0.01 |
|
|
|
USA |
ND |
ND |
ND |
- |
NA |
|
2,4-Undecadienal (1195) |
|
Europeh |
26 |
4 |
0.06 |
|
|
|
USA |
3 |
0.4 |
0.007 |
4 |
1 |
|
trans,trans-2,4-Dodecadienal (1196) |
|
Europe |
5 |
0.7 |
0.01 |
|
|
|
USA |
0.9 |
0.1 |
0.002 |
- |
NA |
|
2-trans-6-cis-Dodecadienal (1197) |
|
Europe |
4 |
0.6 |
0.01 |
|
|
|
USAe |
0.1 |
0.02 |
0.0003 |
+ |
NA |
|
2-trans-4-cis-7-cis-Tridecatrienal (1198) |
|
Europe |
2 |
0.3 |
0.005 |
|
|
|
USAe |
0.05 |
0.009 |
0.0001 |
+ |
NA |
|
Total |
|
Europe |
968 |
|
|
|
|
|
USA |
1 496 |
|
|
|
|
|
NA, not available; ND, no intake data reported; +, reported to occur naturally in foods (Maarse et al., 1999), but no quantitative data; -, not reported to occur naturally in foods |
|
a |
From International Organization of the Flavour Industry (1995) and Lucas et al. (1999) or National Academy of Sciences (1982) |
|
b |
Intake (µg/person per day) was calculated as follows: |
| |
[(annual volume, kg) × (1 × 109 µg/kg)]/[population × survey correction factor × 365 days], where population |
| |
(10%, "eaters only") =32 × 106 for Europe and 26 × 106 for the USA; where correction factor =0.6 for Europe and USA National Academy of Sciences surveys and 0.8 for the Lucas et al. USA survey representing the assumption that only 60% and 80% of the annual flavour volume, respectively, was reported in the poundage surveys (International Organization of the Flavour Industry, 1995; Lucas et al., 1999; National Academy of Sciences, 1982) |
| |
Intake (µg/kg bw per day) was calculated as follows: |
| |
[(µg/person per day)/body weight], where body weight =60 kg. Slight variations may occur from rounding Quantitative data for the United States reported by Stofberg & Grundschober (1987) |
|
d |
The consumption ratio is calculated as follows: |
| |
(annual consumption via food, kg)/(most recent reported volume as a flavouring agent, kg) |
|
e |
Annual volume reported in previous USA surveys (National Academy of Sciences, 1982) |
|
f |
The volume cited is the anticipated annual volume, which was the maximum amount of flavouring agent estimated to be used annually by the manufacturer at the time the material was proposed for flavour use. Subsequent national surveys (National Academy of Sciences, 1970, 1982 or 1987; Lucas et al., 1999) revealed no reported use of the substance as a flavouring agent |
|
g |
The annual volume reported in the most recent poundage survey (Lucas et al., 1999) reflects the use of (E,E)-2.4-hexadienoic acid use as a microbial agent, and not as a flavouring agent because 100% of the annual volume reported in the survey in 1995 survey was reported under the technical effect F01(anti-microbial agent). Therefore, the volume listed here is the anticipated annual volume reported by the manufacturer at the time the material was proposed for use as a flavouring agent, and more accurately represents its use as a flavouring agent in the USA. The Committee evaluated (E,E)-2,4-hexadienoic acid in 1973 in its capacity as a antimicrobial preservative and assigned an ADI of 0–25 mg/kg bw (Annex 1, reference 33) |
|
h |
The volume cited is the annual volume reported for an isomer of the compound |
The dienal with the highest annual production volume, 2-trans,4-trans-decadienal, is ubiquitous in the food supply. It has been identified in approximately 90 foods, primarily citrus fruit and fruit oils at concentrations of up to 500 ppm (Maarse et al., 1999). The combined daily per capita intake for some foods and essential oils derived from foods that contain high concentrations of 2-trans,4-trans-decadienal was calculated to be approximately 354 µg/person per day (see Table 3). The highest intake of 2-trans,4-trans-decadienal occurs from foods that are high in fat, such as chicken (214 µg/person per day), butter (118 µg/person per day) and potato chips (11 µg/person per day). The consumption ratio calculated for 2-trans,4-trans-decadienal is approximately 63, indicating that exposure occurs predominately from the consumption of traditional foods and essential oils (i.e. consumption ratio >1) (Stofberg & Kirschman, 1985; Stofberg & Grundschober, 1987; Lucas et al., 1999; Maarse et al., 1999) (see Tables 2 and 3).
Table 3. Consumption of 2-trans,4-trans-decadienal from foods and essential oils in the USA
|
Food |
Annual consumption of this food in the USA (kg/year) |
Concentration of
2-trans,4-trans-decadienal in food (mg/kg food)3 |
Annual consumption of
2-trans,4-trans-decadienal via this food in the USA (kg)4 |
|
Beer |
20 000 000 0001 |
0.0025 |
50 |
|
Potato |
10 000 000 0001 |
0.025 |
250 |
|
Chicken |
5 796 000 0001 |
3.5 |
20 286 |
|
Orange juice |
3 680 000 0001 |
0.07 |
258 |
|
Butter (heated) |
460 000 0001 |
24.4 |
11 224 |
|
Potato chips |
437 000 0001 |
2.3 |
1 005 |
|
Lemon peel oil |
690 0001 |
220 |
152 |
|
Orange peel oil |
585 1342 |
300 |
176 |
|
Tangerine peel oil |
27 1252 |
500 |
14 |
|
Total |
|
|
33 415 |
|
Flavouring agent |
Annual consumption via food in the USA (kg) |
Annual consumption as added flavouring agent in the USA (kg)2 |
Consumption ratio5 |
|
2,4-Decadienal |
33 415 |
531 |
63 |
|
1 |
From Stofberg & Grundschober (1987) |
|
2 |
From Lucas et al. (1999) |
|
3 |
From Maarse et al. (1999) |
|
4 |
Annual consumption of this food in the USA (kg/year) × concentration of 2-trans,4-trans-decadienal in this food (mg/kg food) × (1 × 10-6kg/mg) =annual consumption of 2-trans,4-trans-decadienal via this food in the USA (kg) |
|
5 |
The consumption ratio is calculated as follows: |
| |
(annual consumption via food, kg)/(most recent reported volume as flavouring agent, kg) |
In addition to being found in food and flavourings, aliphatic linear 2-alkenals such as 2-hexenal1 and 2,4-alkadienals such as 2-trans,4-trans-decadienal are also produced endogenously in animals as products of lipid peroxidation of polyunsaturated fatty acids (Frankel et al., 1987). In this manner, peroxidation of linoleic acid (9,12-octadecadienoic acid) or linoleic esters yields 2-trans,4-trans-decadienal and peroxidation of 12,15-octadecadienoic acid or its related esters yields 2-hexenal.
2.3 Biological data
2.3.1 Biochemical data
(a) Hydrolysis
In general, aliphatic esters (Nos 1177, 1178, 1188, 1191–1194) are rapidly hydrolysed to their component alcohols and carboxylic acids by classes of enzymes known as carboxylesterases (see Figure 1). The most important carboxylesterases, B-esterases, catalyse the hydrolysis of esters prior to entering systemic circulation. Although these enzymes are present in most mammalian tissues, they predominate in the hepatocytes (Anders, 1989; Graffner-Nordberg et al., 1998). The substrate specificity of B-carboxylesterase isoenzymes has been correlated with the structure of the alcohol and the carboxylic acid components (e.g. R and R’, see Figure 1) (Heymann, 1980).
Figure 1. Hydrolysis of aliphatic esters
Studies of hydrolysis of various aliphatic esters have been performed in vitro with carboxylesterases isolated from pig and rat liver (Arndt & Krisch, 1973; Junge & Heymann, 1979) as well as in simulated pancreatic fluid, simulated gastric fluid, and preparations of intestinal mucosa (Leegwater & van Straten, 1974; Butterworth et al., 1975; Grundschober, 1977; Longland et al., 1977; Graffner-Nordberg et al., 1998). Select isoenzymes exhibit an increase in substrate affinity (lower Km) and maximal velocity (Vmax) as the length of the carbon chain of either the alcohol or carboxylic acid component of the substrate increases. Results of studies of hydrolysis in vitro indicate that the rate of hydrolysis of straight-chain esters is approximately 100 times faster than the rate of hydrolysis of branched-chain esters (Drake et al., 1975).
An experiment was conducted in which a series of aliphatic flavouring esters (50 µmol/l) were incubated in artificial pancreatic fluid (50 ml) at 37 °C in vitro (Buck & Renwick, 2000). In the presence of pancreatin, the half-lives (based upon the loss of parent ester) for geranyl formate, geranyl acetate, geranyl butyrate and neryl acetate were rapid, and approximated 0.1, 0.2, 0.03, and 0.2 min, respectively (Buck & Renwick, 2000). These results confirm the expected rapid hydrolysis of aliphatic esters to their corresponding alcohols and acids.
Once hydrolysed, the resulting aliphatic alcohols and carboxylic acids are readily absorbed and metabolized in well-recognized biochemical pathways.
(b) Absorption, distribution, and excretion
The alpha,beta-unsaturated aldehydes are rapidly absorbed, distributed and excreted in the urine, and to a lesser extent in the faeces. Groups of 10 male Wistar albino rats were given a single dose of 100 mg/kg bw of the structurally related substance trans-2-nonenal2 or trans-2-pentenalfn3 in unheated olive oil by gavage. The control group received only the unheated olive oil. Analyses by proton nuclear magnetic resonance spectroscopy (1H-NMR) conducted on urine samples collected before and after administration of the test materials confirmed that the aldehydes are absorbed from the gastrointestinal tract into the systemic circulation and excreted in the urine as C3 mercapturate conjugates within 24 h. Trace amounts of trans-2-nonenal were detected in the faeces. Similar results were obtained from the rats given trans-2-pentenal (Grootveld et al., 1998).
Studies with 14C-labelled (E,E)-2,4-hexadienoic acid (No. 1176 ) in rats (Fingerhut et al., 1962) and mice (Westoo, 1964) show that the acid is absorbed, completely metabolized, and excreted primarily in expired air as 14C-labelled CO2 14CO2) and water. Female mice given a single oral dose of either 40 or 8000 mg [1-14C](E,E)-2,4-hexadienoic acid/kg bw excreted 77–85% of the dose as expired 14CO2 within 4 days. Approximately 88% of the 14CO2 was recovered within the first 24 h. Between 4% and 5% of the original dose was excreted in the urine as (E,E)-muconic acid4 (i.e. (E,E)-2,4-hexadienedioic acid) and unchanged (E,E)-2,4-hexadienoic acid, respectively, accounting for 0.4% and 0.7% of the total radioactivity present in the urine collected over the first 24 h. Only about 1% of the dose of 40 mg/kg bw was recovered in the faeces (Westoo, 1964). When [1-14C](E,E)-2,4-hexadienoic acid was administered to rats at a dose of between 61 and 1213 mg/kg bw, 85% was excreted as exhaled 14CO2 within 10 h, regardless of dose. In the same period of time, approximately 2% of the radiolabel was detected in the urine. (E,E)-Muconic acid and unchanged (E,E)-2,4-hexadienoic acid were not detected (Fingerhut et al., 1962).
(c) Metabolism
In general, unsaturated alcohols are efficiently oxidized to unsaturated aldehydes and acids by alcohol dehydrogenase and these products are completely metabolized in the fatty acid beta-oxidation pathway, regardless of the position of unsaturation on the carbon chain (Nelson & Cox, 2000). alpha,beta-Unsaturated aldehydes also may react with GSH in a Michael-type addition. At high doses, depletion of cellular levels of GSH could theoretically lead to oxidative stress (Schulz et al., 2000).
(d) Enzymatic conversion of aliphatic alcohols, aldehydes and acids
Isoenzyme mixtures of oxidized/reduced nicotinamide adenine dinucleotide (NAD+/NADH)-dependent alcohol dehydrogenase obtained from human liver microsomes catalyse the oxidation of aliphatic unsaturated alcohols (Pietruszko et al., 1973). There is a correlation between increasing carbon chain length (C1 to C6) of the alcohol substrate and increasing binding affinity (lower Km) of alcohol dehydrogenase. However, maximum rates of reaction (higher Vmax) of oxidation were essentially constant, regardless of the structure of the alcohol (Klesov et al., 1977).
Similarly, aldehyde dehydrogenase present predominantly in the hepatic cytosol (Lame & Segall, 1986) exhibits broad specificity for the oxidation of aliphatic and aromatic aldehydes (Feldman & Weiner, 1972) and demonstrates higher catalytic activity in vitro for higher molecular weight, and more lipophilic aldehydes (Nakayasu et al., 1978). The alpha,beta-unsaturated aldehyde, trans-2-hexenal, is readily oxidized to trans-2-hexenoic acid in mouse hepatic cytosol fractions (Lame & Segall, 1986) and in isoenzymes of rat aldehyde dehydrogenase present in mitochondrial, cytosolic, and microsomal fractions (Mitchell & Petersen, 1987). The molybdenum-containing enzymes xanthine oxidase and aldehyde oxidase also catalyse the oxidation of a wide range of aldehydes (Beedham, 1988).
The resulting linear alpha,beta-unsaturated acids are intermediates in the normal oxidation of saturated fatty acids and as such participate in normal fatty acid metabolism. In this pathway, the acid is first condensed with (CoA) (Nelson & Cox, 2000). The resulting trans-2,3-unsaturated CoA ester (trans-Delta2-enoyl CoA) is converted to the 3-ketothioester, which undergoes beta-cleavage to yield an acetyl CoA fragment and a new thioester reduced by two carbons.
Cleavage of acetyl CoA units will continue along the carbon chain until the position of unsaturation is reached. If the unsaturation begins at an odd-numbered carbon, acetyl CoA fragmentation will eventually yield a Delta3-enoyl CoA, which cannot enter the fatty acid cycle until it is isomerized to the trans-Delta2-enoyl CoA by enoyl CoA isomerase. If unsaturation begins at an even-numbered carbon, acetyl CoA fragmentation yields a Delta2-enoyl CoA product, which is a substrate for further fatty acid oxidation. If the regiochemistry of the double bond is "cis", it is isomerized to the "trans" double bond by the action of 3-hydroxyacyl CoA epimerase before entering the fatty acid oxidation pathway. Even numbered carbon acids continue to be cleaved to acetyl CoA but odd numbered carbon acids yield acetyl CoA and propionyl CoA. Acetyl CoA enters the citric acid cycle directly, whereas propionyl CoA is transformed into succinyl CoA, which then enters the citric acid cycle (Nelson & Cox, 2000).
Studies with [1-14C](E,E)-2,4-hexadienoic acid (No. 1176) in rats (Fingerhut et al., 1962) and mice (Westoo, 1964) show that the carboxylic acid is absorbed, completely metabolized, and excreted primarily as carbon dioxide and water. In addition, small amounts of the beta-oxidation product (E,E)-2,4-hexadienedioic acid (E,E-muconic acid) were detected in the urine of mice. On the basis of these data, it is concluded that alpha,beta-unsaturated acids participate in normal fatty acid metabolism.
(e) Glutathione conjugation, oxidative stress, lipid peroxidation and apoptosis
The cellular formation and fate of alpha,beta-unsaturated aldehydes has been directly linked to lipid peroxidation and a phenomenon known as oxidative stress. Oxidative stress results when free radicals (oxygen, O2, and hydroxyl, OH) react with proteins, polypeptides, RNA and DNA bases, and particularly polyunsaturated fatty acid chains of phospholipids in cell membranes. Under cell homeostasis, high intracellular concentrations (1–10 mmol/l) of glutathione (GSH) are found in mammalian cells (Armstrong, 1987; 1991). GSH is a major antioxidant present in all animal cells, and its reactive thiol function rapidly scavenges these unstable free radicals. However, under conditions of oxidative stress in which GSH depletion has occurred (i.e. low ratio of reduced glutathione (GSH) to oxidized glutathione (GSSG)), free radicals are available to react with polyunsaturated fatty acids, leading to a variety of fragmentation products including 2-butenal, trans-2-hexenal, 4-hydroxy-2-nonenal, and 2,4-decadienal. In addition to forming reactive unsaturated aldehydes, lipid peroxidation disturbs the structural integrity of the lipid bilayer, compromising cell permeability. This leads to membrane leakage, Na+influx, K+efflux, and influx of water leading to cytotoxic oedema. Additionally, aldehyde fragmentation products induce apoptotic cell death during oxidative stress (Esterbauer et al., 1991; Eckl et al., 1993; Dianzani, 1998).
(f) Glutathione conjugation
Regardless of the source, alpha,beta-unsaturated aldehydes may conjugate with GSH directly or undergo allylic hydroxylation to yield 4-hydroxyalkenals (Esterbauer et al., 1982) that also may conjugate with GSH (Esterbauer et al., 1975). Studies on the metabolism in vivo of trans-2-alkenals show that they are partly metabolized by the addition of GSH across their electrophillic carbon–carbon double bonds in a Michael-type addition (Grootveld et al., 1998). alpha,beta-Unsaturated aldehydes for which beta-oxidation is not facile are mainly conjugated with GSH. The mercapturic acid conjugate was the major urinary excretion product isolated from rats given a single intraperitoneal dose of 100 mg/kg bw of (E)-2-propyl 2,4-pentadienoic acid5 (Kassahun et al., 1991). Conjugated dienals and, to a lesser extent, dienoic acids, conjugate with GSH and eventually are excreted as the mercapturic acid derivatives (Boyland & Chasseaud, 1967).
The GSH redox cycle maintains adequate levels of GSH in animal cells (Nelson & Cox, 2000; Schulz et al., 2000) and is a major intracellular mechanism involved in the detoxication of alpha,beta-unsaturated aldehydes (Reed et al., 1986). The addition of GSH across the electrophilic carbon–carbon double bond is catalysed by the enzyme glutathione S-transferase, but can also occur in a non-enzymatic reaction (Eisenbrand et al., 1995; Grootveld et al., 1998). Cultured rat hepatocytes that are rich in GSH and glutathione S-transferase have been shown to metabolize greater amounts of 2-alkenals than human lymphoblastoid cells (Namalva cells) (Eisenbrand et al., 1995). Low levels of GSH, glutathione S-transferase, and deactivating enzymes make the human lymphoblastoid cells more susceptible to the cytotoxic effects of 2-alkenals. In both cell types, the consumption of alkenals was directly related to the depletion of intracellular GSH (Eisenbrand et al., 1995). A 75% decrease in the levels of liver GSH occurred when male rats were given the structurally related trans,trans-muconaldehyde6 by intraperitoneal injection at a dose of 36 µmol/kg bw (Witz, 1989). Additionally, the report that the presence of GSH reduces the cytotoxicity of alpha,beta-unsaturated aldehydes in Salmonella typhimurium TA104 in vitro provides additional evidence that GSH conjugation plays an important role in the detoxication process (Marnett et al., 1985).
(g) Oxidative stress
The effectiveness of the GSH detoxication pathway for alpha,beta-unsaturated aldehydes hinges on the ability of the cell to maintain the equilibrium between its pro-oxidant and antioxidant systems (Nelson & Cox, 2000). A decrease in the concentrations of antioxidants or an increase in the production of reactive oxygen species (e.g. O2, H2O2, OH) can lead to oxidative stress, a condition in which the cell is unable to maintain the level of reactive oxygen species below a toxic threshold (Schulz et al., 2000). During periods of oxidative stress, the ratio of GSH to GSSG decreases owing to loss of GSH and accumulation of GSSG. The low levels of cellular GSH render the detoxication pathway inefficient and allow for increased interaction between the alpha,beta-unsaturated aldehydes and cellular components (proteins and DNA) eventually leading to cytotoxicity and apoptosis (Eder et al., 1993). In cultured rat hepatocytes and human lymphoblastoid cells treated with various 2-alkenals, DNA adducts were detectable after intracellular concentrations of GSH were reduced to approximately 20% of pre-treatment levels. Before the incubation of Namalva cells and rat hepatocytes with 2-hexenal at 37 °C, concentrations of GSH were measured to be approximately 1.6 and 80 nmol/2 × 106 cells in each cell line, respectively. After the 1-h incubation, concentrations of GSH were reduced to approximately 10% of the control values (Eisenbrand et al., 1995).
Owing to the nature of the GSH detoxication pathway, studies of genotoxicity that use high concentrations of alpha,beta-unsaturated aldehydes are likely to produce effects consistent with oxidative stress. It is anticipated that GSH will be rapidly depleted in cells exposed to high doses of alpha,beta-unsaturated aldehydes, leading to the formation of protein and DNA adducts and eventually to cellular damage and decreased cell viability.
(h) Lipid peroxidation and formation of alpha,beta-unsaturated aldehydes
Under conditions of oxidative stress, lipid peroxidation of polyunsaturated fatty acids, particularly those in phospholipids, results in the production of alpha,beta-unsaturated aldehydes in vivo. In this pathway, abstraction of a diallylic hydrogen atom from a polyunsaturated fatty acid (e.g. the C11 hydrogen of 9,12-octadecadeinoic acid, linoleic acid) and subsequent rearrangement yields a hydroperoxide intermediate. The unstable hydroperoxide readily degrades to an alkoxy radical that is prone to undergo either beta-scission or hydrogen abstraction. beta-Scission yields a shortened, conjugated, unsaturated aldehyde. Given the low concentrations of cellular GSH during oxidative stress, aldehydes formed during lipid peroxidation become available to react with proteins and DNA nucleotides to yield adducts (Loureiro et al., 2000) and exert cytotoxic effects, including apoptotic cell death (Kirichenko et al., 1996; Kruman et al., 1997; Celli et al., 1998; Liu et al., 2000).
Studies using fluorescence spectroscopy revealed that alpha,beta-unsaturated aldehydes, whether formed endogenously or resulting from exogenous exposure, react with DNA to form adducts (Frankel et al., 1987; Eder et al., 1993; Cadet et al., 1999; National Toxicology Program, 2001). 2-trans,4-trans-Decadienal (No. 1190; concentration, 4 mmol/l), an important product of lipid peroxidation, has been shown to react with 2’-deoxyadenosine in calf thymus DNA to produce low concentrations of of five etheno adducts: (1,N6-etheno-2’-deoxyadenosine; 1-[3-(2-dexoy-beta-D-erythro-pentofuranosyl)-3H-imidazo[2,1-i]purin-7-yl]-1,2,3-octanetriol; 1-[3-(2-dexoy-beta-D-erythro-pentofuranosyl)-3H-imidazo[2,1-i]purin-7-yl]-1,2-heptanediol; 1-[3-(2-dexoy-beta-D-erythro-pentofuranosyl)-3H-imidazo[2,1-i]purin-7-yl]-hexanol and 1-[3-(2-dexoy-beta-D-erythro-pentofuranosyl)-3H-imidazo[2,1-i]purin-7-yl]-2,3-epoxy-1-octanol). The variety, not the volume of adducts produced, is related to the cytotoxicity of 2-trans,4-trans-decadienal (Carvalho et al., 1998, 2000).
In addition to direct reaction with DNA, alpha,beta-unsaturated aldehydes act to induce DNA fragmentation through apoptosis. Recent experiments (Ji et al., 2001) indicate that depletion of GSH by alpha,beta-unsaturated aldehydes (4-hydroxy-2-nonenal), progressing in a dose- and time-dependent manner, induces poly-ADP-ribose polymerase (PARP) cleavage and DNA fragmentation. In one of the pathways of apoptosis, depletion of GSH results in the release of mitochondrial cytochrome c to the cytosol where a cascade of cytosolic cysteine proteases (i.e. caspases) is activated. Activation of caspase-3 causes cleavage of cellular proteins and PARP leading to DNA fragmentation and subsequent cell death (Liu et al., 1996; Li et al., 1997; Zou et al., 1997; Green & Reed, 1998; Cain et al., 1999).
2.3.2 Toxicological studies
Typically, toxicological studies in the monograph are organized according to duration (i.e. short-term, long-term, and carcinogenicity), flavouring agent and then species. However, in order to group the studies performed by the National Toxicology Program, the short-term studies of toxicity and studies of carcinogenicity will be discussed in the section on long-term studies (see 2.3.2.(c)) in the sequence in which they were conducted.
(a) Acute toxicity
Oral LD50 values have been reported for 11 of the 26 substances in this group. Six of the aliphatic, alicyclic, linear, alpha,beta-unsaturated, di- and trienals and related alcohols, acids, and esters used as flavouring agents (Nos 1175, 1176, 1179, 1186, and 1193) have oral LD50 values in the range from 300 to 12 500 mg/kg bw in rats (Deuel et al., 1954; Smyth et al., 1954; de Groot et al., 1974; Moreno, 1976, 1980; Uchida et al., 1985; Driscoll, 1996). Three oral LD50 values were reported for trans,trans-2,4-hexadienal (No. 1175) in the range of 300 to <5000 mg/kg bw in rats (Smyth et al., 1954; de Groot et al., 1974; Moreno, 1980).
In mice, oral LD50 values for Nos 1177, 1178, 1184, 1194, 1197 and 1198 are in the range of 1000 to >8000 mg/kg bw (Sparfel et al., 1968; Pellmont, 1971, 1977; Edwards, 1973; Moreno, 1978), demonstrating that the acute oral toxicity of aliphatic, alicyclic, linear, alpha,beta-unsaturated, di- and trienals and related alcohols, acids and esters is low (see Table 4).
Table 4. Studies of acute oral toxicity with aliphatic di- and trienals and alcohols, acids and esters
|
No. |
Flavouring agent |
Species; sexa |
LD50
(mg/kg bw) |
Reference |
|
1175 |
trans,trans-2,4-Hexadienal |
Rat; M |
300 |
Moreno (1980) |
|
1175 |
trans,trans-2,4-Hexadienal |
Rat; M,F |
730 |
Smyth et al. (1954) |
|
1175 |
trans,trans-2,4-Hexadienal |
Rat; M,F |
<5 000b |
DeGroot et al. (1974) |
|
1176 |
(E,E)-2,4-Hexadienoic acid |
Rat; F |
9 600 |
Uchida et al. (1985) |
|
1176 |
(E,E)-2,4-Hexadienoic acid |
Rat; M,F |
10 500 |
Deuel et al. (1954) |
|
1176 |
(E,E)-2,4-Hexadienoic acid |
Rat; M |
12 500 |
Uchida et al. (1985) |
|
1177 |
Methyl sorbate |
Mouse; NR |
5 600 |
Pellmont (1977) |
|
1178 |
Ethyl sorbate |
Mouse; F |
>8 000 |
Sparfel et al. (1968) |
|
1179 |
2,4-Heptadienal |
Rat; M |
1 150 |
Moreno (1980) |
|
1184 |
2,6-Nonadien-1-ol |
Mouse; NR |
>5 000 |
Moreno (1978) |
|
1186 |
Nona-2-trans-6-cis-dienal |
Rat; NR |
>5 000 |
Moreno (1976) |
|
1193 |
Ethyl 2,4,7-decatrienoate |
Rat; M,F |
>2 000 |
Driscoll (1996) |
|
1194 |
Propyl 2,4-decadienoate |
Mouse; NR |
1 000 |
Pellmont (1971) |
|
1197 |
2-trans-6-cis-Dodecadienal |
Mouse; NR |
5 000 |
Edwards (1973) |
|
1198 |
2-trans-4-cis-7-cis-Tridecatrienal |
Mouse; NR |
5 000 |
Edwards (1973) |
|
a |
M, Male; F, Female; NR, Not reported |
|
b |
does not represent a true LD50 as this is the only dose evaluated at which a 100% mortality was reported |
(b) Short-term studies of toxicity
(i) trans,trans-2,4-hexadienal (no. 1175)
Rats
Groups of five male and five female rats were given trans,trans-2,4-hexadienal at a dose of 0, 0.75, or 7.5 mg/kg bw per day by gavage in corn oil, 6 days per week for 14 days. Individual body weights, and food and water consumption recorded after weeks 1 and 2 showed no difference between animals in treated and control groups. Concentrations of haemoglobin determined at day 14 were not significantly different from those observed in control groups. At necropsy, macroscopic examination did not reveal any abnormalities related to treatment. Significantly increased relative liver weights were reported in males at 0.75 mg/kg bw per day, but not at 7.5 mg/kg bw per day. The increased liver weight was not accompanied by any histopathological findings in the liver or kidneys. Consequently, the NOEL for trans,trans-2,4-hexadienal was 7.5 mg /kg bw per day, the highest dose tested (de Groot et al., 1974).
Groups of 24 male and 24 female Charles River rats were fed diets containing trans,trans-2,4-hexadienal at a dose of 0 or 2.23 mg/kg bw per day, 7 days a week, for 13 weeks. Concurrent groups of controls were maintained. All animals were observed daily for mortality and signs of toxicity throughout the study. Haematological evaluations, blood chemistry and urine analyses were conducted at weeks 6 and 12. Compared with the control group, transient decreases in blood concentrations of glucose and increases in blood concentrations of urea nitrogen were reported in males and females, respectively, at week 6, but not at week 12. Weekly measurements of body weights and food consumption revealed no significant differences between animals in treated and control groups. At necropsy, gross and histopathological examinations revealed no lesions that could be associated with the test material. Organ weights did not differ between animals in treated and control groups. The authors reported that the NOEL for 2,4-hexadienal was 2.23 mg/kg bw per day (Mecler & Craig, 1980).
(ii) (E,E)-2,4-Hexadienoic acid (No. 1176)
Rats
Groups of five male and five female Sherman rats were fed diets containing 0, 0.5, 1, 2, 4, or 8% (E,E)-2,4-hexadienoic acid (sorbic acid) for a period of 13 weeks. These concentrations correspond to daily intakes of 0, 320, 630, 1260, 2480, and 5060 mg/ bw, respectively. All animals survived until termination of the study and no differences in body-weight gain and food use were reported between the treated animals and controls at any dose. At necropsy, histological examination did not reveal any abnormal findings. A slight increase in relative liver weight was reported at 8% (E,E)-2,4-hexadienoic acid when compared with controls. However, this was not accompanied by histological changes in the liver at any dose. No treatment-related effects were reported in the animals given 4% (E,E)-2,4-hexadienoic acid (2480 mg/kg bw per day) (Deuel et al., 1954).
Dogs
In a 13-week study, a group of two male and one female puppies (breed not specified) was fed a diet containing 4% (E,E)-2,4-hexadienoic acid. No differences in food consumption and body-weight gain were reported between the treated and control animals throughout the study. At termination, histopathological examination did not reveal any treatment-related lesions. The authors concluded that a diet containing 4% (E,E)-2,4-hexadienoic acid, which corresponds to a daily intake of 1333 mg/kg bw (Food and Drug Administration, 1993), does not cause adverse effects in dogs (Deuel et al., 1954).
(iii) 2-trans,4-trans-Decadienal (No. 1190)
Mice
Groups of five male and five female B6C3F1 mice were given 2-trans,4-trans-decadienal at a dose of 0, 45, 133, 400, 1200, or 3600 mg/kg bw per day by gavage in corn oil, 5 days per week, for 17 days. All animals in the group receiving a dose of 3600 mg/kg bw per day and one male and female receiving a dose of 1200 mg/kg bw per day died before the study was completed. A significant decrease in body-weight gain was reported in males and in females at 1200 mg/kg bw per day. Treatment-related clinical effects included diarrhoea, and lethargy, reported in all animals. Ruffled fur and a thin appearance were reported in males and females at 1200 and 3600 mg/kg bw per day, while ataxia and abnormal breathing were reported only at the highest dose (3600 mg/kg bw per day). No treatment-related clinical signs of toxicity or effects on organ weights were reported at any dose <400 mg/kg bw per day. At necropsy, gross pathological evaluation and microscopic evaluation of the forestomach revealed ulceration in males and females at 1200 mg/kg bw per day. The NOEL for 2-trans,4-trans-decadienal was 400 mg/kg bw per day in mice on the basis of no treatment-related effects at this dose (National Toxicology Program, 1997).
Groups of 10 male and 10 female B6C3F1 mice were given 2-trans,4-trans-decadienal at a dose of 0, 50, 100, 200, 400, or 800 mg/kg bw per day by gavage in corn oil, 5 days per week, for 13 weeks. 2-trans,4-trans-Decadienal did not have any effects on the survival of the treated animals at any dose. A lower rate of body-weight gain was reported in males at 800 mg/kg bw per day compared with the controls. At doses of 400 and 800 mg/kg bw per day, increased salivation after treatment was reported in animals of both sexes. Increased salivation was first noticed during week 7 of the study and continued intermittently until week 10. Lethargy was observed only in females at 200, 400, and 800 mg/kg bw per day at week 12. No treatment-related differences were reported in haematology or organ weights at any dose. Histological evaluation revealed minimal or mild epithelial hyperplasia, inflammation and oedema in the forestomach of treated animals in the groups receiving a dose of 200 (males only), 400, and 800 mg/kg bw per day. On the basis of these findings, the NOEL for 2-trans,4-trans-decadienal was 100 and 200 mg/kg bw per day for male and female B6C3F1 mice, respectively (National Toxicology Program, 1997).
Rats
Groups of five male and five female Fischer 344/N rats were given 2-trans,4-trans-decadienal at a dose of 0, 45, 133, 400, 1200, or 3600 mg/kg bw per day by gavage in corn oil, 5 days per week, for 17 days. All animals in the group receiving a dose of 3600 mg/kg bw per day died before the study was completed; however, no early deaths were reported at any other dose. A statistically significant decrease in body-weight gain was reported at 1200 mg/kg bw per day in both males and females relative to that of the controls. Diarrhoea was reported in both sexes at 1200 mg/kg bw per day. At necropsy, gross pathological evaluation and microscopic evaluation of the forestomach revealed ulceration in both sexes also at 1200 mg/kg bw per day. The NOEL for 2-trans,4-trans-decadienal was 400 mg/kg bw per day in Fischer 344/N rats, on the basis of absence of treatment-related effects at this dose (National Toxicology Program, 1997).
Groups of 10 male and 10 female Fischer 344/N rats were given 2-trans,4-trans-decadienal at a dose of 0, 50, 100, 200, 400, or 800 mg/kg bw per day by gavage in corn oil, 5 days per week, for 13 weeks. There were no treatment-related effects on the survival of the rats at any dose. Rats of both sexes at 200, 400, and 800 mg/kg bw per day showed a lower rate of body-weight gain throughout the study relative to that of the controls. Salivation was reported before and after administration of 2-trans,4-trans-decadienal in both sexes at 200, 400, and 800 mg/kg bw per day, but only sporadically and only after treatment at 50 and 100 mg/kg bw per day. The severity of the salivation was reported to increase with dose. Towards the end of the study, salivation became less prevalent at the lower doses. Lethargy after treatment was also reported at 200, 400, and 800 mg/kg bw per day, beginning at week 7 and continuing until the end of the study. Lethargy occurred sporadically in rats at the lower doses (i.e. 50 and 100 mg/kg bw per day). Although the severity of the lethargy was reported to be dose-dependent, the rats recovered within minutes and appeared normal at all other times. At necropsy, histological evaluation revealed minimal or mild epithelial hyperplasia in the forestomachs of male and female rats at 400 and 800 mg/kg bw per day . Since salivation and lethargy were sporadic at the lower doses (i.e. 50 and 100 mg/kg bw per day) and the rats recovered from both within minutes, the NOEL for 2-trans,4-trans-decadienal in this 13-week study in rats was 100 mg/kg bw per day (National Toxicology Program, 1997).
Groups of six male and six female Charles River rats were fed diets containing 2-trans,4-trans-decadienal at concentrations providing a daily dose of 0, 3.39, 10.70, or 33.90 mg/kg bw per day for 13 weeks. Concurrent groups of controls were maintained. Daily observations were made for mortality and general signs of toxicity. Weekly measurements of body weights and food consumption revealed no significant differences between treated animals and controls. At week 6, a decrease in concentration of haemoglobin and in erythrocyte volume fraction in females was reported at doses of 10.70 and 33.90 mg/kg bw per day. These values were comparable to those of the controls at week 12. At necropsy, histopathologic evaluations performed on major tissues from all animals receiving the highest dose and from half of the controls, and of livers and kidneys from all study animals revealed no treatment-related effects. Final body and organ weights of the treated animals did not differ significantly from those of the controls at any dose (Damske et al., 1980).
(iv) 2-trans-6-cis-dodecadienal (no. 1197) and 2-trans-4-cis-7-cis-tridecatrienal (No. 1198)
Rats
Groups of six male and six female rats were given diets containing a mixture of 2-trans-6-cis-dodecadienal and 2-trans-4-cis-7-cis-tridecatrienal microencapsulated in maltodextrin at a concentration of 0.2, 0.4, 1.0, 2.0, 4.0, 10.0, or 20.0 ppm of 2-trans-6-cis-dodecadienal, plus 3.2, 6.4, 16, 32, 64, 160, or 320 ppm of 2-trans-4-cis-7-cis-tridecatrienal, for 4 weeks. On the basis of feed consumption, the highest concentrations were reported to provide intakes of 1.93 and 2.06 mg 2-trans-6-cis-dodecadienal/kg bw per day for males and females respectively, and 30.9 and 33mg 2-trans-4-cis-7-cis-tridecatrienal/kg bw per day for males and females, respectively. An additional group of 12 rats was fed a control diet containing maltodextrin only. No significant effects on body-weight gain, food consumption or food utilization, organ weights, clinical chemistry or macroscopic pathology were reported in either sex at any dose. A histopathological examination conducted on animals receiving the highest dose revealed only non-treatment-related lesions. The NOELs were 2.06 and 33 mg/kg bw per day for trans-2-cis-6-dodecadienal and trans-2-cis-4-cis-7-tridecatrienal, respectively (Edwards, 1973).
The results of short-term and long-term studies of toxicity and carinogenicity are summarized in Table 7.
(c) Long-term studies of toxicity and carcinogenicity
(i) trans,trans-2,4-Hexadienal (No. 1175)
Mice
In a 16-day study, groups of five male and five female B6C3F1 mice were given trans, trans-2,4-hexadienal at a dose of 0, 3, 9, 27, 80, or 240 mg/kg bw per day, by gavage in corn oil, 5 days per week for a total of 12 doses. Observations were made twice daily and body weights and clinical findings were recorded at the start, on day 8, and at the end of the study. A 20% mortality rate, and clinical signs of toxicity including lethargy, ruffled fur, and convulsions, as well as significant weight loss limited to female mice were reported at 240 mg/kg bw per day. No deaths or clinical signs of toxicity were reported in any other treated groups. No treatment-related differences in organ weights were reported at any dose. At necroscopy, gross pathological evaluation revealed ulceration and/or necrosis of the forestomach in all mice treated with trans,trans-2,4-hexadienal at a dose of 240 mg/kg bw per day and mild epithelial hyperplasia and hyperkeratosis in both sexes at a dose of 80 mg/kg bw per day. The NOEL for trans, trans-2,4-hexadienal was 27 mg/kg bw per day in male and female mice (National Toxicology Program, 2001).
Groups of 10 male and 10 female B6C3F1 mice were given trans,trans-2,4-hexadienal at a dose of 0, 7.5, 15, 30, 60, or 120 mg/kg bw per day by gavage in corn oil, 5 days per week, for 14 weeks. The animals were observed twice daily for general health and behaviour and clinical findings were recorded weekly. At termination, necropsies were performed on all treated animals and complete histopathological examinations were undertaken for animals receiving the highest dose and for the controls. No treatment-related effects on survival or body-weight gain were reported in either sex at any dose. The deaths of three males during the study were attributed to dosing accidents. Salivation was reported during week 7 in males at 60 and 120 mg/kg bw per day and in females at 120 mg/kg bw per day. Anal wetness was observed in these same groups at week 9 or 10 of the study. No treatment-related or biologically significant differences were reported in haematology between treated mice and controls at any dose. The absolute and relative liver weights of both sexes (male, p <0.05; female, p <0.01) at 60 mg/kg bw per day and relative liver weights of all treated females were significantly (p <0.01) greater than those of the controls. Additionally, significantly increased absolute (p <0.05) and relative (60 mg/kg bw per day, p <0.05; 120 mg/kg bw per day, p <0.01) kidney weights were reported in males at 60 and 120 mg/kg bw per day. An increase in the incidence of minimal to mild epithelial hyperplasia was reported in the forestomach of females in the groups receiving a dose of 120 mg/kg bw, as compared with controls; however, this was not associated with appreciable inflammation, or basal cell proliferation. Compared with that in controls, incidence of minimal to mild necrosis of the olfactory epithelium was significantly elevated in mice of both sexes receiving a dose of 120 mg/kg bw per day, whereas a significant increase in the incidence of olfactory epithelial atrophy was limited to males at this dose. The NOEL for trans, trans-2,4-hexadienal was 30 mg/kg bw per day in male B6C3F1 mice in this study. A NOEL cannot be identified for female B6C3F1 mice owing to the increased relative liver weights observed at all doses (National Toxicology Program, 2001).
Groups of 50 male and 50 female B6C3F1 mice were given trans, trans-2,4-hexadienal at a dose of 0, 30, 60, or 120 mg/kg bw per day by gavage in corn oil, 5 days per week for 104 weeks. Animals were observed twice daily for mortality and body weights and clinical findings were recorded every 4 weeks for the duration of the study. Survival and mean body weights of all treated animals were similar to those of the controls throughout the study. No treatment-related clinical findings were observed at any dose. A statistically significant increase in the incidence of epithelial hyperplasia was reported in males at 120 mg/kg bw per day (p <0.01) and in females at 60 and 120 mg/kg bw per day (p <0.05, at both doses) (see Table 5). A significant increase in the incidence of squamous cell papillomas and combined incidence of squamous cell papilloma or carcinoma of the forestomach also were reported in males at 120 mg/kg bw per day and in females at 60 and 120 mg/kg bw per day. At the highest dose (120 mg/kg bw per day), a statistically significant increase (p <0.01) in the incidence of squamous cell carcinomas of the forestomach was reported in females (National Toxicology Program, 2001).
Table 5. Incidences of lesions of the forestomach in B6C3F1 mice given trans,trans-2,4-hexadienal by gavage
|
Dose (mg/kg bw) |
|
0 (Vehicle control) |
30 |
60 |
120 |
|
Males |
|
|
|
|
|
Squamous epithelium hyperplasia (%) |
14/50 (28%) |
7/50 (14%) |
9/50 (18%) |
26/50(52%)a |
|
Squamous cell papilloma incidenceb (%) |
2/50 (4%) |
4/50 (8%) |
5/50 (10%) |
8/50 (16%)c |
|
Combined squamous cell papilloma or carcinoma incidenced (%) |
2/50 (4%) |
4/50 (8%) |
5/50 (10%) |
10/50 (20%) |
|
Females |
|
|
|
|
|
Squamous epithelium hyperplasia (%) |
4/50 (8%) |
8/49 (16%) |
12/50 (24%)e |
31/50 (62%)a |
|
Squamous cell papilloma incidencef (%) |
2/50 (4%) |
2/49 (4%) |
11/50 (22%)g |
13/50 (26%)h |
|
Squamous cell carcinoma incidencei (%) |
0/50 (0%) |
0/49 (0%) |
0/50 (0%) |
7/50 (14%)a |
|
Combined squamous cell papilloma or carcinoma incidencej (%) |
2/50 (4%) |
2/49 (4%) |
11/50 (22%) |
18/50 (36%) |
|
From National Toxicology Program (2001) |
|
a |
p <0.01 |
|
b |
Historical incidence for 2-year studies with controls given NTP-2000 diet (mean ±standard deviation): 10/659 (1.8% ±1.9%), range, 06%; with corn oil vehicle controls given NIH-07 diet 19/464 (4.1% ±1.7%), range 2–6% |
|
c |
p =0.035 |
|
d |
Historical incidence for NTP-2000: 11/659 (2.0% ±2.0%), range, 06%; for NIH-07 diet: 22/464 (4.7 ±2.0%), range 2–8% |
|
e |
Significantly different (p <0.05) from the vehicle control group by the Poly-3 test |
|
f |
Historical incidence for NTP-2000: 9/659 (1.4% ±2.0%), range, 0–6%; for NIH-07 diet: 19/463 (4.1% ±3.5%), range 0–10% |
|
g |
p =0.006 |
|
h |
p <0.001 |
|
i |
Historical incidence for NTP-2000: 1/659 (0.2% ±0.6%), range, 0–2%; for NIH-07 diet: 0/463 |
|
j |
Historical incidence for NTP-2000: 9/659 (1.4% ±2.0%), range, 0–6%; for NIH-07 diet: 19/463 (4.1% ±3.5%), range 0–10% |
|
Note: The NTP-2000 diet contains less protein and more fibre and fat than NIH-07 diet previously used in 2-year studies conducted by the National Toxicology Program |
On the basis of these findings, the National Toxicology Program report concluded, "there was clear evidence of carcinogenic activity of 2,4-hexadienal in male and female B6C3F1 mice based on increased incidences of squamous cell neoplasms of the forestomach" (National Toxicology Program, 2001).
Rats
In a 16-day study, groups of five male and five female Fischer 344/N rats were given trans,trans-2,4-hexadienal at a dose of 0, 3, 9, 27, 80, or 240 mg/ bw per day by gavage in corn oil, 5 days per week for a total of 12 doses. Observations were made twice daily and body weights and clinical findings were recorded at the start, on day 8, and at the end of the study. At the highest dose (240 mg/kg bw per day), clinical signs of toxicity included diarrhoea, ataxia, lethargy, and anal and eye discharge in males, and lethargy, paleness, and abnormal breathing in females. Furthermore, 60% mortality occurred in both sexes at this dose. No mortality or clinical signs of toxicity were reported at any other dose. Significantly reduced body-weight gain (p <0.01) was reported for males and females at 240 mg/kg bw per day as compared with the controls. Increased liver weights were reported for females at 240 mg/kg bw per day. At necropsy, gross pathological evaluation revealed necrosis and ulceration of the forestomach in most rats of both sexes at 240 mg/kg bw per day, and mild to moderate epithelial hyperplasia of the forestomach at 80 mg/kg bw per day. No treatment-related effects were seen in males or females at 27 mg/kg bw per day (National Toxicology Program, 2001).
In a 14-week study, groups of 10 male and 10 female Fischer 344/N rats were given trans,trans-2,4-hexadienal at a dose of 0, 7.5, 15, 30, 60, or 120 mg/kg bw per day by gavage in corn oil, 5 days per week, for a total of 70 doses. The animals were observed twice daily for general health and behaviour and clinical findings were recorded weekly. At termination, necropsies were performed on all treated animals and complete histopathology was performed on animals in groups receiving the highest dose and the control group. All animals were survived the duration of the study. In comparison to the controls, final mean body weights and body-weight gains were significantly reduced in male rats at 30, 60 and 120 mg/kg bw per day. With the exception of increased salivation reported in males and females at 30 and 120 mg/kg bw per day during week 4 and only in the 120 mg/kg bw per day group thereafter, no other signs of clinical toxicity were observed. Increased incidences of mild to moderate forestomach epithelial hyperplasia, accompanied by degeneration and acute inflammation, were reported in males and females at 120 mg/kg bw per day as compared with controls. Males receiving the highest dose (120 mg/kg bw per day) demonstrated a significant increase in the incidence of olfactory epithelial atrophy, osteofibrosis, and exudate of the nose. No biologically significant changes in organ weights were observed at any dose. Although statistically significant variations were reported in haematological and clinical chemistry values for treated animals as compared with controls, most were considered to be not related to treatment, as they were minor, sporadic and not dose-dependent. The NOEL for trans,trans-2,4-hexadienal was 15 and 60 mg/kg bw per day in male and female rats, respectively (National Toxicology Program, 2001).
Groups of 50 male and 50 female Fischer 344/N rats were given trans,trans-2,4-hexadienal at a dose of 0, 22.5, 45, or 90 mg/kg bw per day by gavage in corn oil, 5 days per week, for 104 weeks. Animals were observed twice daily for mortality and body weights and clinical findings were recorded every 4 weeks throughout the study. Survival of all treated rats was similar to that of the controls. After week 27, the mean body weights of males at 90 mg/kg bw per day were lower than those of the controls. No treatment-related clinical findings were reported at any dose. There were statistically significant (p <0.01) increases in the incidence of mild to moderate forestomach epithelial hyperplasia at all doses in both sexes (see Table 6). Significant increases in the incidence of squamous cell papillomas of the forestomach were reported in males and females at 45 and 90 mg/kg bw per day per day, as compared with the controls. A statistically significant (p <0.01) increase in the combined incidence of squamous cell papilloma or carcinoma was reported in males at 45 and 90 mg/kg bw per day. There was no statistically significant increase in the incidence of squamous cell carcinomas of the forestomach in either males or females at any dose (National Toxicology Program, 2001).
Table 6. Incidences of lesions of the forestomach in Fischer 344/N rats given trans,trans-2,4-hexadienal by gavage
|
Dose (mg/kg bw) |
|
0 (Vehicle control) |
22.5 |
45 |
90 |
|
Males |
|
|
|
|
|
Epithelium hyperplasia (%) |
3/50 (6%) |
19/50 (38%)a |
42/50 (84%)a |
50/50 (100%)a |
|
Squamous cell papilloma incidenceb (%) |
0/50 (0%) |
3/50 (6%) |
10/50 (20%)c |
29/50 (58%)c |
|
Combined squamous cell papilloma or carcinoma incidenceb (%) |
0/50 (0%) |
3/50 (6%) |
11/50 (22%)a |
29/50 (58%)a |
|
Females |
|
|
|
|
|
Epithelium hyperplasia, incidence (%) |
2/50 (4%) |
16/50 (32%)a |
37/50 (74%)a |
41/50 (82%)a |
|
Squamous cell papilloma incidenced (%) |
0/50 (0%) |
1/50 (2%) |
5/50 (10%)e |
17/50 (34%)c |
|
From National Toxicology Program (2001) |
|
a |
p <0.01 |
|
b |
Historical incidence for 2-year studies with controls given NTP-2000 diet (mean ± standard deviation): 2/609 (0.3 ±0.7%), range 0–2%; with corn-oil vehicle controls given NIH-07 diet: 2/402 (0.5 ±0.9%), range 0–2% |
|
c |
p <0.001 |
|
d |
Historical incidence for NTP-2000: 0/659; for NIH-07 diet: 2/401 (0.5 ±0.9%); range, 0–2% |
|
e |
p =0.031 |
|
Note: The NTP-2000 diet contains less protein and more fibre and fat than NIH-07 diet previously used in 2-year studies conducted by the National Toxicology Program |
On the basis of these findings, the National Toxicology Program report concluded "there was clear evidence of carcinogenic activity of 2,4-hexadienal in male and female F344/N rats based on increased incidences of squamous cell neoplasms of the forestomach" (National Toxicology Program, 2001).
Forestomach effects in rodents
The occurrence of forestomach hyperplasia and squamous cell tumours in rodents is common in bioassay studies by the National Toxicology Program in which a high concentration of an irritating material in corn oil is delivered daily by gavage into the forestomach for 2 years. High concentrations of aldehydes (e.g. malonaldehyde, furfural, benzaldehyde and trans,trans-2,4-hexadienal (National Toxicology Program, 1988, 1990, 1993, 2001, respectively) and other irritating substances (e.g. dihydrocoumarin, coumarin (National Toxicology Program, 1990, 1992, respectively)) delivered in corn oil by gavage are consistently associated with these phenomena in the forestomach of rodents.
Trans,trans-2,4-Hexadienal produced some positive results in short-term tests for genotoxicity in vitro, but was inactive in tests in vivo (section 2.3.2 (d)). Thus, although it may be genotoxic under some conditions, this is not believed to be the basis for its effects in the rodent forestomach. There was evidence of treatment-related injury to the forestomach epithelium and this is believed to be the primary cause of the neoplastic development. In the bioassays, development of hyperplasia in mice and rats receiving test substance by gavage in corn oil, and a low incidence of adenoma observed in mice reflect the sensitivity of the forestomach to irritation. The forestomach was the only site at which in increased incidence of neoplasia was observed in treated animals.
The relevance of the development of forestomach tumours in rodents to potential carcinogenic targets in humans has been the subject of much investigation (Grice, 1988; Wester & Kroes, 1988; Clayson et al., 1990). An International Agency for Research on Cancer Working Group (IARC, 2003) concluded that in order to evaluate the relevance of the induction of forestomach tumours in rodents to cancer in humans, the exposure conditions used in these experiments have to be considered. The exposure conditions during oral administration are unusual (particularly if dosing is effected by gavage) in that physical effects may result in high local concentrations of test substances in the forestomach and prolonged exposure of the epithelial tissue. Agents that only produce tumours in the forestomach in rodents after prolonged treatment and via mechanisms that do not involve reaction with DNA may be of less relevance to humans, since human exposure to such agents would need to surpass time-integrated dose thresholds in order to elicit the carcinogenic response.
Therefore, the appearance of these lesions in the 2-year bioassay in rodents given trans,trans-2,4-hexadienal at a high concentration by gavage has no relevance to humans, given that the results are due to the irritating effect of high bolus doses of trans,trans-2,4-hexadienal delivered to the contact site (the forestomach) by gavage and not the effects of systemic concentrations in the whole animal. Moreover, human exposure is limited to consumption of low concentrations of aldehyde in the diet.
(ii) (E,E)-2,4-hexadienoic acid (sorbic acid) (no. 1176)
Mice
Groups of ASH/CS1 mice (48 males and 50 females) were fed diets containing 0, 1, 5, or 10% (E,E)-2,4-hexadienoic acid, corresponding to daily intakes of approximately 1400, 7500, and 15 000 mg/kg bw per day, respectively (Food & Drug Administration, 1993), for 80 weeks. Body weights were recorded at the beginning of the study and at varying unspecified intervals up to week 74. At termination, all surviving mice were killed and necropsied. Macroscopic examinations were conducted on the major organs and tissue samples were preserved for microscopic examination. The administration of (E,E)-2,4-hexadienoic acid had no effect on mortality. At necropsy, significant reductions in weight gain were reported in males receiving diet containing 5% (E,E)-2,4-hexadienoic acid and in males and females receiving containing 10% (E,E)-2,4-hexadienoic acid. No statistically significant haematological differences were reported between animals in the treated and control groups at any dose. The authors did not consider the increased relative kidney weights in the animals given the diet containing 5% or 10% E,E)-2,4-hexadienoic acid to be treatment-related, because histological examination revealed that the incidences of kidney lesions in the treated animals were significantly lower than those in the controls. In spite of the high doses and prolonged treatment, lower incidences of histological changes (e.g. early degenerative changes, chronic inflammation, and hyperplastic nodules) were reported in the livers of the treated mice as compared with the controls. On the basis of this finding, the authors concluded that the increased relative liver weights observed in all groups of treated animals were due to an increase in metabolic demand and not to treatment-related toxicity. These results indicate that (E,E)-2,4-hexadienoic acid is not carcinogenic in mice when given in the diet at concentrations of up to 10% for 80 weeks. No treatment-related effects were reported in the animals given diet containing 1% (E,E)-2,4-hexadienoic acid (Hendy et al., 1976).
Rats
Groups of 48 male and 48 female Wistar rats were fed diets containing 0, 1.5, or 10% (E,E)-2,4-hexadienoic acid for 2 years. This corresponds to approximate daily intakes of 0, 750, and 5000 mg/kg bw per day, respectively (Food & Drug Administration, 1993). Body weight, food consumption and water consumption were recorded initially, after 1 month and at 3-month intervals thereafter. Animals were observed daily for general health and behaviour. (E,E)-2,4-Hexadienoic acid did not have any effect on the rate of mortality of the treated rats. A significant, but slight decrease in body-weight gain was reported in both sexes in the group receiving diet containing 10% (E,E)-2,4-hexadienoic acid. No consistent differences were observed in food consumption between treated and control animals. No treatment-related effects were observed in the haematological examinations, serum analyses, studies of renal function or histopathological examinations. At the higher concentration (10% (E,E)-2,4-hexadienoic acid), increased relative liver weights were reported in males and females, and increased relative kidney weights were reported in females. The increased liver weights were not accompanied by any histological findings, and therefore were attributed by the authors to an increase in metabolic demand resulting from the presence of high concentrations of fatty acids. In many cases, tumours occurred with the same frequency in controls and treated animals, or were solely confined to the control group without comparable findings in the treated animals. Tumours identified in animals at the lower dose (1.5% in the diet) were not observed in animals at the higher dose (10% in the diet). Consequently, the authors concluded that tumours at lower dose were not related to treatment. Overall, these results indicate that no carcinogenic effects are associated with the administration of a diet containing up to 10% (E,E)-2,4-hexadienoic acid, equivalent to approximately 5000 mg/kg bw per day. The NOEL for (E,E)-2,4-hexadienoic acid was 1.5%, equivalent to approximately 750 mg/kg bw per day in rats (Gaunt et al., 1975).
Two groups of six male rats were given the potassium salt of (E,E)-2,4-hexadienoic acid at 0.1% in the diet or 0.3% in the drinking-water, for 60 weeks. This corresponds to daily intakes of 50 and 300 mg/kg bw per day, respectively (Food & Drug Administration, 1993). Concurrent groups of controls were maintained. No treatment-related changes were reported in the overall health and behaviour of the rats throughout the study. With the exception of scattered, small white nodules on the surface of livers observed in some rats given drinking-water containing potassium (E,E)-2,4-hexadienoic acid, exploratory laparotomy performed on all surviving rats at week 65 and at the end of the study did not reveal the presence of liver tumours or any other treatment-related effects. Subsequent histological examination confirmed the lesions to be inflammatory in nature and non-neoplastic. At necropsy, conducted when animals died or at week 100, no treatment-related tumours were detected in either group of rats. The authors concluded that potassium (E,E)-2,4-hexadienoic acid administered continuously at a dose of 50 mg/kg bw per day in the diet or 300 mg/kg bw per day in the drinking-water for 60 weeks does not induce any toxic or carcinogenic effects in male rats (Dickens et al., 1968).
Long-term studies with (E,E)-2,4-hexadienoic acid have typically involved administration of diets containing >5% of the test material (Deuel et al., 1954; Gaunt et al., 1975; Hendy et al., 1976). According to protocols of the National Toxicology Program, the maximum level for any addition to the food is generally accepted to be 5%. Exposure to test materials in the diet at levels >5% are known to cause nutritional alterations in the diet that can impact upon evaluation of the chronic toxicity and carcinogenicity of the test material.
The results of short-term and long-term studies of toxicity and carinogenicity are summarized in Table 7.
Table 7. Results of short-term and long-term studies of toxicity and carinogenicity with aliphatic, alicyclic, linear, alpha,beta-unsaturated, di- and trienals and related alcohols, acids and esters
|
No. |
Flavouring agent |
Species; sex |
No. of test groupsa/ no. per groupb |
Route |
Duration |
NOEL (mg/kg bw per day) |
Reference |
|
Short-term studies of toxicity |
|
1175 |
trans,trans-2,4-Hexadienal |
Rat; M,F |
2/10 |
Gavage |
14 days |
7.5c |
de Groot et al. (1974) |
|
1175 |
trans,trans-2,4-Hexadienal |
Rat; M,F |
1/48 |
Diet |
90 days |
2.23c |
Mecler and Craig (1980) |
|
1176 |
(E,E)-2,4-Hexadienoic acid |
Rat; M,F |
5/10 |
Diet |
90 days |
2480 |
Deuel et al. (1954) |
|
1176 |
(E,E)-2,4-Hexadienoic acid |
Dog; M,F |
1/3 |
Diet |
90 days |
1333c |
Deuel et al. (1954) |
|
1190 |
2-trans,4-trans-Decadienal |
Mouse; M,F |
5/10 |
Gavage |
17 days |
400 |
National Toxicology Program (1997) |
|
1190 |
2-trans,4-trans-Decadienal |
Mouse; M,F |
5/20 |
Gavage |
90 days |
100 (M)
200 (F) |
National Toxicology Program (1997) |
|
1190 |
2-trans,4-trans-Decadienal |
Rat; M,F |
5/10 |
Gavage |
17 days |
400 |
National Toxicology Program (1997) |
|
1190 |
2-trans,4-trans-Decadienal |
Rat; M,F |
5/20 |
Gavage |
90 days |
100 |
National Toxicology Program (1997) |
|
1190 |
2-trans,4-trans-Decadienal |
Rat; M,F |
3/12 |
Diet |
90 days |
33.9 |
Damske et al. (1980) |
|
1197 |
2-trans-6-cis-Dodecadienal |
Rat; M,F |
7/12 |
Diet |
28 days |
2.06d |
Edwards (1973) |
|
1198 |
2-trans-4-cis-7-cis-Tridecatrienal |
Rat; M,F |
7/12 |
Diet |
28 days |
33d |
Edwards (1973) |
|
Long-term studies of toxicity and carcinogenicitye |
|
1175 |
trans,trans-2,4-Hexadienal |
Mouse; M,F |
5/10 |
Gavage |
16 days |
27 |
National Toxicology Program (2001) |
|
1175 |
trans,trans-2,4-Hexadienal |
Mouse; M,F |
5/20 |
Gavage |
98 days |
30 (M)
NE (F) |
National Toxicology Program (2001) |
|
1175 |
trans,trans-2,4-Hexadienal |
Mouse; M,F |
3/100 |
Gavage |
728 days |
60 (M)
30 (F) |
National Toxicology Program (2001) |
|
1175 |
trans,trans-2,4-Hexadienal |
Rat; M,F |
5/10 |
Gavage |
16 days |
27 |
National ToxicologyProgram (2001) |
|
1175 |
trans,trans-2,4-Hexadienal |
Rat; M,F |
5/20 |
Gavage |
98 days |
15 (M)
60 (F) |
National Toxicology Program (2001) |
|
1175 |
trans,trans-2,4-Hexadienal |
Rat; M,F |
3/100 |
Gavage |
728 days |
NE |
National Toxicology Program (2001) |
|
1176 |
(E,E)-2,4-Hexadienoic acid |
Mouse; M,F |
3/98 |
Diet |
560 days |
1400 |
Hendy et al. (1976) |
|
1176 |
(E,E)-2,4-Hexadienoic acid |
Rat; M,F |
2/96 |
Diet |
730 days |
750 |
Gaunt et al. (1975) |
|
1176 |
(E,E)-2,4-Hexadienoic acid, potassium salt |
Rat; M |
1/6 |
Drinking-water |
420 days |
300c |
Dickens et al., (1968) |
|
1176 |
(E,E)-2,4-Hexadienoic acid, potassium salt |
Rat; M |
1/6 |
Diet |
420 days |
50c |
Dickens et al. (1968) |
|
M, male; F, female; NE, not established |
|
a |
Total number of test groups does not include control animals |
|
b |
Total number per test group includes both male and female animals |
|
c |
Study performed with either a single dose or multiple doses that produced no adverse effect. The value is therefore not a true NOEL, but is the highest dose tested that produced no adverse effects. The actual NOEL may be higher |
|
d |
The substance was administered as a component of a mixture |
|
e |
In the interest of preserving the sequential integrity, the short-term studies published by the National Toxicology Program are presented in this section |
(d) Genotoxicity
(i) In vitro
Testing for genotoxicity in vitro has been performed with six (Nos 1175, 1176, 1185, 1186, 1190 and 1193) representative members of the group of aliphatic, alicyclic, linear, alpha,beta-unsaturated, di- and trienals and related alcohols, acids and esters used as flavouring agents (see Table 8).
Table 8. Results of studies of genotoxicity with aliphatic, alicyclic, linear, di- and trienals and related alcohols, acids and esters
|
No. |
Flavouring agent |
End-point |
Test object |
Dose or concentration |
Results |
Reference |
|
In vitro |
|
1175 |
trans,trans-2,4-Hexadienal |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, and TA1537 |
3 mmol/plate
(288 µg/plate)a |
Negativeb,c |
Florin et al. (1980) |
|
1175 |
trans,trans-2,4-Hexadienal |
Reverse mutation |
S. typhimurium TA104 |
<1 µmol/plate
(96 µg/plate)a |
Positived,e |
Marnett et al. (1985) |
|
1175 |
trans,trans-2,4-Hexadienal |
Reverse mutation |
S. typhimurium TA104 |
>5 µmol/plate
(>481 µg/plate)a |
Positived,e,f |
Marnett et al. (1985) |
|
1175 |
trans,trans-2,4-Hexadienal |
Reverse mutation |
S. typhimurium TA102 |
Not reported |
Negatived,e |
Marnett et al. (1985) |
|
1175 |
trans,trans-2,4-Hexadienal |
Reverse mutation |
S. typhimurium TA100 |
0.01–0.4 µl/plate
(8.95–358 µg/plate)g |
Positivee,h,i |
Eder et al. (1992) |
|
1175 |
trans,trans-2,4-Hexadienal |
Reverse mutation |
S. typhimurium TA100 |
0.01–0.75 µl/plate
(8.95–671.3 µg/plate)g |
Positiveh,i,j |
Eder et al. (1992) |
|
1175 |
trans,trans-2,4-Hexadienal |
Reverse mutation |
S. typhimurium TA100 |
0.005–0.4 µl/plate
(4.48–358 µg/plate)g |
Positivee,k,l |
Eder et al. (1992) |
|
1175 |
trans,trans-2,4-Hexadienal |
Reverse mutation |
S. typhimurium TA100 |
0.005–0.4 µl/plate
(4.48–358 µg/plate)g |
Positivej,k,l |
Eder et al. (1992) |
|
1175 |
trans,trans-2,4-Hexadienal |
Reverse mutation |
S. typhimurium TA1535, TA98 |
<1 500 µg/plate |
Negativej |
National Toxicology Program (2001) |
|
1175 |
trans,trans-2,4-Hexadienal |
Reverse mutation |
S. typhimurium TA98 |
<150 µg/plate |
Negativee |
National Toxicology Program (2001) |
|
1175 |
trans,trans-2,4-Hexadienal |
Reverse mutation |
S. typhimurium TA1535 |
<166 µg/plate |
Negativee |
National Toxicology Program (2001) |
|
1175 |
trans,trans-2,4-Hexadienal |
Reverse mutation |
S. typhimurium TA100 |
<333 µg/plate |
Positivee,m |
National Toxicology Program (2001) |
|
1175 |
trans,trans-2,4-Hexadienal |
Reverse mutation |
S. typhimurium TA100 |
<1 500 µg/plate |
Positivej |
National Toxicology Program (2001) |
|
1175 |
trans,trans-2,4-Hexadienal |
SOS chromotest |
E. coli PQ37 and PQ243 |
<590 nmol
(57 µg/plate)a |
Negative |
Eder et al. (1992) |
|
1175 |
trans,trans-2,4-Hexadienal |
SOS chromotest |
E. coli PQ37 |
Not reported |
Positiven |
Eder et al. (1993) |
|
1175 |
trans,trans-2,4-Hexadienal |
DNA strand breaks |
L1210 mouse leukaemia cells |
20 µmol/ml
(1 923 µg/ml)a |
Negative |
Eder et al. (1993) |
|
300, 500 µmol/ml
(28 839, 48 065 µg/ml)a |
Positiveo |
Schlatter et al. (1992) |
|
1176 |
2,4-Hexadienoic acid, potassium salt |
Cell cycle alterations |
V79 Chinese hamster cells |
<2 500 µg/ml |
Positivep |
Schlatter et al. (1992) |
|
1176 |
2,4-Hexadienoic acid, sodium salt |
Cell cycle alterations |
V79 Chinese hamster cells |
<2 500 µg/ml |
Positivep,q |
Marnett et al. (1985) |
|
1185 |
2,4-Nonadienal |
Reverse mutation |
S. typhimurium TA104 |
<0.4 µmol/plate
(<55 µg/plate)r |
Negatived,e |
Marnett et al. (1985) |
|
1185 |
2,4-Nonadienal |
Reverse mutation |
S. typhimurium TA102 |
Not reported |
Negatived,e |
Eder et al. (1993) |
|
1185 |
2,4-Nonadienal |
SOS chromotest |
E. coli PQ37 |
Not reported |
Negative |
Eder et al. (1993) |
|
1185 |
2,4-Nonadienal |
DNA strand breaks |
L1210 mouse leukaemia cells |
400 µmol/ml
(55 284 µg/ml)r |
Negativeo |
Eder et al. (1992) |
|
500 µmol/ml
(69 105 µg/ml)r |
Positive |
Eder et al. (1992) |
|
1186 |
Nona-2-trans-6-cis-dienal |
Reverse mutation |
S. typhimurium TA100 |
0.01–0.1 µl/plate
(8.6–86 µg/plate)s |
Negativee,h,i |
Eder et al. (1992) |
|
1186 |
Nona-2-trans-6-cis-dienal |
Reverse mutation |
S. typhimurium TA100 |
0.005–0.4 µl/plate
(4.3–344 µg/plate)s |
Negativee,k,l |
Eder et al. (1992) |
|
1186 |
Nona-2-trans-6-cis-dienal |
Reverse mutation |
S. typhimurium TA100 |
0.005–0.25 µl/plate
(4.3–344 µg/plate)s |
Negativej,k,l |
Eder et al. (1992) |
|
1186 |
Nona-2-trans-6-cis-dienal |
SOS chromotest |
E. coli PQ37 and PQ243 |
<80 nmol
(11 µg/plate)r |
Negative |
Eder et al. (1992) |
|
1186 |
Nona-2-trans-6-cis-dienal |
Sister chromatid exchange |
Human lymphoblastoid Namalva cell line |
0–10 µmol/l
(0–1.38 µg/ml)r |
Negative |
Dittberner et al. (1995) |
|
20–40 µmol/l
(2.8–5.5 µg/ml)r |
Positive |
|
1186 |
Nona-2-trans-6-cis-dienal |
Sister chromatid exchange |
Primary human blood lymphocytes |
0–10 µmol/l
(0–1.38 µg/ml)r |
Negative |
Dittberner et al. (1995) |
|
20–50 µmol/l
(2.8–6.9 µg/ml)r |
Positive |
|
1186 |
Nona-2-trans-6-cis-dienal |
Structural chromosomal aberration test |
Human lymphoblastoid Namalva cell line |
5–40 µmol/l
(0.69–5.5 µg/ml)r |
Positive |
Dittberner et al. (1995) |
|
1186 |
Nona-2-trans-6-cis-dienal |
Structural chromosomal aberration test |
Primary human blood lymphocytes |
0–40 µmol/l
(0–5.5 µg/ml)r |
Negative |
Dittberner et al. (1995) |
|
1186 |
Nona-2-trans-6-cis-dienal |
Numerical chromosomal aberration test |
Primary human blood lymphocytes |
0–20 µmol/l
(0–2.76 µg/ml)r |
Negative |
Dittberner et al. (1995) |
| |
|
|
|
40 µmol/l
(5.5 µg/ml)r |
Positive |
|
|
1186 |
Nona-2-trans-6-cis-dienal |
Micronucleus formation |
Primary human blood lymphocytes |
0–10 µmol/l
(0–1.38 µg/ml)r |
Negative |
Dittberner et al. (1995) |
|
20–50 µmol/l
(2.76–6.9 µg/ml)r |
Positive |
|
1186 |
Nona-2-trans-6-cis-dienal |
Micronucleus formation |
Human lymphoblastoid Namalva cell line |
0–20 µmol/l
(0–2.76 µg/ml)r |
Negative |
Dittberner et al. (1995) |
|
40–50 µmol/l
(5.5–6.9 µg/ml)r |
Positive |
|
1190 |
2-trans,4-trans-Decadienal |
Reverse mutation |
S. typhimurium TA97, TA98, TA100, TA102, TA104 and TA1535 |
<333 µg/plate |
Negativec |
National Toxicology Program (1997) |
|
1193 |
Ethyl 2,4,7-decatrienoate |
Reverse mutation |
S. typhimurium TA100, TA1535, TA1538, TA98 and TA1537 |
1.5–5 000 µg/plate |
Negativec |
Thompson (1996) |
|
In vivo |
|
1175 |
trans,trans-2,4-Hexadienal |
Micronucleus formation |
Mouse |
40, 80, 120, or 160 mg/kg |
Inconclusivet |
National Toxicology Program (2001) |
|
1175 |
trans,trans-2,4-Hexadienal |
Micronucleus formation |
Mouse |
7.5, 15, 30, 60, or 120 mg/kg |
Negativeu |
National Toxicology Program (2001) |
|
1175 |
trans,trans-2,4-Hexadienal |
Micronucleus formation |
Rat |
50, 100, 150, or 200 mg/kg |
Inconclusivev |
National Toxicology Program (2001) |
|
1176 |
2,4-Hexadienoic acid, potassium salt |
Somatic mutation and recombination |
Drosophila melanogaster |
3.75 mg/ml |
Negativew |
Schlatter et al. (1992) |
|
1176 |
2,4-Hexadienoic acid, sodium salt |
Somatic mutation and recombination |
Drosophila melanogaster |
3.75 mg/ml |
Negativew |
Schlatter et al. (1992) |
|
1176 |
2,4-Hexadienoic acid |
Chromosome aberration |
Mouse |
15 mg/kg bw |
Positivex,y |
Banerjee & Giri (1986) |
|
1176 |
2,4-Hexadienoic acid |
Micronucleus formation |
Mouse |
2.5, 20 mg/kg bw |
Negativev |
Mukherjee et al. (1988) |
|
150 mg/kg bw |
Positivev |
|
1176 |
2,4-Hexadienoic acid |
Sister chromatid exchange |
Mouse |
25 to 50 mg/kg bw |
Negativev |
Mukherjee et al. (1988) |
|
75, 100, or 150 mg/kg bw |
Positivev |
|
1190 |
2-trans,4-trans-Decadienal |
Micronucleus formation |
Mouse |
50, 100, 200, 400, or 800 mg/kg |
Negativez |
National Toxicology Program (1997) |
Notes to Table 8.
|
a |
Calculated using relative molecular mass =96.13 |
|
b |
Spot test method |
|
c |
With and without metabolic activation |
|
d |
Liquid preincubation |
|
e |
Without metabolic activation |
|
f |
Addition of 10 mmol/l glutathione |
|
g |
Calculated using density =0.896 g/ml (Sigma-Aldrich) |
|
h |
Standard bacterial density |
|
i |
30-min preincubationj With metabolic activation |
|
k |
Three-fold bacterial cell density |
|
l |
90-min preincubation |
|
m |
Positive in 1 of 2 testing centres |
|
n |
With ethanol as solvent instead of dimethylsulfoxide |
|
o |
Results demonstrated in the presence of cytotoxicty |
|
p |
Pattern of positive effects is suggestive of weak aneugenic activity |
|
q |
Positive effects observed only with stored solutions (28-days old) |
|
r |
Calculated using relative molecular mass =138.21 |
|
s |
Calculated using specific gravity =0.850–0.870 g/ml (Food and Chemicals Codex, 1996) |
|
t |
Administered three times by intraperitoneal injection at 24-h intervals |
|
u |
Administered by gavage for 14 weeks |
|
v |
Administered as a single intraperitoneal injection |
|
w |
Administered orally |
|
x |
Administered by gavage for 30 days |
|
y |
Positive effects limited to spindle activity; no effects observed on structural chromosome aberrations |
|
z |
Administered by injection |
Testing for mutagenicity of alpha,beta-unsaturated aldehydes in bacterial systems is problematic due to their high bacterial toxicity. The cytotoxicity of these substances is believed to arise from their interactions with protein sulfhydryl and amino groups (Marnett et al., 1985; Eder et al., 1992). Standard assays for mutagenicity in S. typhimurium were uniformly negative, but in modified assays results have been predominantly positive.
In standard assays for mutagenicity in S. typhimurium, trans,trans-2,4-hexadienal was not mutagenic in the standard tester strains TA102 (Marnett et al., 1985), TA98, TA1535 (Florin et al., 1980; National Toxicology Program, 2001), TA100 and TA1537 (Florin et al., 1980; Marnett et al., 1985; National Toxicology Program, 2001) when tested at concentrations of up to 1500 µg/plate, with and without metabolic activation.
In modified assays for mutagenicity using liquid preincubation protocols (i.e. addition of a GSH chase at the end of a 20-min incubation with TA104, or a 90-min preincubation with a three-fold increase in cell density with TA100), significant increases in reverse mutations in the absence of metabolic activation were reported when S. typhimurium strains TA104 and TA100 were incubated with trans,trans-2,4-hexadienal at a concentration of up to 481 µg/plate (Marnett et al., 1985; Eder et al., 1992). Cytotoxicity was observed at concentrations of >96 µg/plate and greater. Strain TA104 contains a nonsense mutation (TAA) at the site of reversion and is much more sensitive to carbonyl mutagenesis than standard strains of S. typhimurium. Also, increased TA104 sensitivity is related to the incorporation of the pKM101 plasmid, which encodes for an error-prone DNA polymerase involved in bypass-replication of lesions (Marnett et al., 1985). Owing to the increased susceptibility of TA104 to cytotoxicity, GSH was incorporated into the S. typhimurium assay for mutagenicity. Consequently, the maximum non-toxic dose of trans,trans-2,4-hexadienal tested increased from 96 to >481 µg/plate after the addition of 10 mmol/l of reduced GSH at the end of the preincubation period; however, its mutagenic potential remained unaltered. The authors proposed that the addition of GSH reduced toxicity by preventing excess aldehyde, present after incubation, from reacting with protein sulfhydryl groups. No evidence of mutagenicity was reported when trans,trans-2,4-hexadienal was incubated with TA102, a strain that contains the uvrB gene that encodes for an error-free DNA excision repair (Marnett et al., 1985).
Other changes in methodology have been used to evaluate mutagenic potential in the presence of significant cytotoxicity. In preincubation assays for mutagenicity in S. typhimurium strain TA100, alpha,beta-unsaturated aldehydes were incubated with the standard density of bacterial cells (Eder et al., 1992, 1993; National Toxicology Program, 2001) or with a density of bacterial cells of three times more than the standard (Eder et al., 1992; 1993). Under normal conditions involving a 30-min preincubation, and a standard density of bacterial cells, the high cytotoxicity exerted by simple linear aldehydes may limit the detection of mutagenic responses (e.g. pentenal, hexenal, heptenal). However, at three times the standard density and an increased preincubation time of 90 min, incubation of pentenal, hexenal, or hexadienal, with or without metabolic activation, produced a frequency of spontaneous reversion of at least twice that observed under standard conditions. Under the specified conditions, results obtained using S. typhimurium strain TA100 were found to be consistent with reports that hexanal and hexadienal are mutagenic in tester strain TA104 in the presence of GSH (Marnett et al., 1985). Among the aldehydes investigated, increased cytotoxicity and mutagenicity correlated with increased lipophilicity. The effect of detoxication in the presence of metabolic activation was indicated by a shift to higher, non-cytotoxic doses and higher peak frequencies of revertants.
Negative results were reported with Escherichia coli strains PQ37 and PQ243 (SOS chromotest) incubated in the presence of trans,trans-2,4-hexadienal or nona-2-trans-6-cis-dienal at concentrations of up to 57 and 11 µg/plate, respectively (Eder et al., 1992).
Negative results were reported in assays for mutagenicity when S. typhimurium strains TA97, TA98, TA100, TA102, TA104, TA1535, TA1537 and TA1538 were incubated with up to 55 µg 2,4-nonadienal/plate (Marnett et al., 1985), up to 344 µg nona-2-trans-6-cis-dienal/plate (Eder et al., 1992), up to 333 µg of 2-trans,4-trans-decadienal/plate (National Toxicology Program, 1997), or up to 5000 µg of ethyl 2,4,7-decatrienoate/plate (Thompson, 1996), with and without metabolic activation.
In an assay for alkaline elution, trans,trans-2,4-hexadienal and 2,4-nonadienal induced single strand breaks. An increase in single strand breaks in DNA was also observed when trans,trans-2,4-hexadienal at the slightly toxic concentration of 28.8 mg/ml was incubated with L1210 mouse leukaemia cells (4 ml at 106cells/ml). 2,4-Nonadienal produced single strand breaks at the highly toxic concentration of 69.1 mg/ml. In an investigation of the reactions occurring between the compound and the components of DNA (nucleosides and nucleotides), trans,trans-2,4-hexadienal was found to form the DNA adduct 1,2-cyclic deoxyguanosine and, to a smaller extent, 7,8-cyclic guanosine adducts. The authors concluded that trans,trans-2,4-hexadienal and 2,4-nonadienal may induce strand breaks either by direct interaction with DNA, or by programmed cell death, mediated by endonucleolytic enzymes (Eder et al., 1993).
A study investigated the ability of alpha,beta-unsaturated aldehydes to induce sister chromatid exchanges, numerical and structural chromosomal aberrations, and micronuclei in cultured cells that were low in GSH and detoxication enzymes (i.e. human blood lymphocytes and Namalva cell lines) (Dittberner et al., 1995). When nona-2-trans-6-cis-dienal at a concentration of 5–50 µmol/l was incubated with cultured human lymphocyte and Namalva cells, the number of sister chromatid exchanges was increased at concentrations of 20 µmol/l (2.8 µg/ml)7 and above. The number of structural chromosomal aberrations in human blood lymphocytes did not change over the concentration range of 0–40 µmol/l (5.5 µg/ml) 8. In Namalva cells, that contain lower concentrations of GSH and detoxication enzymes, an increase in chromosomal aberrations was reported at concentrations of nona-2-trans-6-cis-dienal as low as 5 µmol/l (0.69 µg/ml) 8. The incidence of micronuclei in blood lymphocytes and Namalva cells was increased at a minimum concentration of 20 µmol/l (2.8 µg/ml)8 and 40 µmol/l (5.5 µg/ml) 8, respectively. The authors noted that nona-2-trans-6-cis-dienal exhibited severe toxicity at concentrations of 50 µmol/l and above. No attempts were made to assess at what concentrations lysosomal breakdown occurred in the assays for sister chromatid exchanges and numerical and structural chromosomal aberrations. It has been previously established that increases in the incidence of sister chromatid exchanges and numerical and structural chromosomal aberrations near or at observable levels of cytotoxicity may reflect secondary effects resulting from lysosome breakdown and release of DNAase (Zajac-Kaye & Ts’o, 1984; Bradley et al., 1987).
(ii) In vivo
Testing for the genotoxic potential of sodium and potassium 2,4-hexadienoic acid in somatic cells of Drosophila melanogaster after a 48-h feeding, yielded negative results at respective concentrations of 3.35 and 3.75 mg/ml (Schlatter et al., 1992).
Tests for formation of micronuclei were conducted to investigate the ability of trans,trans-2,4-hexadienal to induce chromosomal damage in polychromatic erythrocytes from bone marrow of B6C3F1 mice and Fischer 344/N rats. The animals were given trans,trans-2,4-hexadienal by intraperitoneal injection at doses ranging from 40 to 160 mg/kg bw per day for mice and 50 to 200 mg/kg bw per day for rats. Although the numbers of micronucleated polychromatic erythrocytes per 1000 polychromatic erythrocytes reported in the trend analyses were significant (mouse, p =0.024; rat, p =0.017), the results were judged to be inconclusive as none of the mean values obtained for the individual treated groups were greater than the mean value for the controls (National Toxicology Program, 2001). Micronucleus tests conducted in the peripheral blood normochromatic erythrocytes of mice after administration of trans,trans-2,4-hexadienal (7.5–120 mg/kg bw per day) (National Toxicology Program, 2001) and 2-trans,4-trans-decadienal (50–800 mg/kg bw per day) by gavage for 14 weeks, yielded negative results (National Toxicology Program, 1997).
The potential genotoxicity of repeated doses of 2,4-hexadienoic acid was investigated in a test for chromosomal aberrations in mouse bone marrow. Groups of 10 Swiss albino male mice were given 2,4-hexadienoic acid at a daily dose of 0 (control) or 15 mg/kg bw by gavage for 30 days. Although there was an increase in the mitotic index, there was no significant increase in structural chromosomal aberrations as compared with the control group (Banerjee & Giri, 1986).
In a later study, groups of eight Swiss albino male mice were given 2,4-hexadienoic acid in a single intraperitoneal injection containing a dose of 0, 25, 50, 75, 100, or 150 mg/kg bw (Mukherjee et al., 1988). A significant increase in sister chromatid exchanges (p <0.05) was observed in animals receiving 2,4-hexadienoic acid at a dose of >75 mg/kg bw per day. In a concurrent assay, groups of four mice were sacrificed at 24 h or 48 h, and the incidence of micronucleated cells per 500 polychromatic erythrocytes was recorded per animal, after administration of 2,4-hexadienoic acid at an acute intraperitoneal dose of 0, 2.5, 20, or 150 mg/kg bw. Micronucleated polychromatic erythrocytes were significantly increased (p <0.05) at the highest dose evaluated (150 mg/kg bw). It is important to note that the positive findings in vivo resulted from intraperitoneal administration, which has no relevance to human consumption of flavouring agents. In studies using administration by gavage, there was no genotoxic activity.
Discussion of data on genotoxicity
Testing for mutagenicity of alpha,beta-unsaturated aldehydes in standard S. typhimurium assays using a variety of strains (TA97, TA98, TA100, TA102, TA104, TA1535, TA1537 and TA1538) have shown no evidence of mutagenicity (Florin et al., 1980; National Toxicology Program, 1997, 2001). However, alternative protocols have been developed to reduce the cytotoxicity of alpha,beta-unsaturated aldehydes. In these studies, positive results were reported in modified assays using preincubation conditions conducive to depletion of metabolic detoxication path-ways (Eder et al., 1992; 1993). Evidence for genotoxicity also was reported in other assays (sister chromatid exchanges, numerical and structural chromosomal aberrations, formation of micronuclei) performed in cultured cells low in detoxication capacity (Namalva cells and human lymphocytes) (Dittberner et al., 1995). The relatively high concentrations of alpha,beta-unsaturated aldehydes (20–40 µmol/l) used in these studies caused single-strand breaks in DNA, but no cross-linking. The conditions of the experiments (high concentrations of aldehyde in cell lines poor in detoxication capacity) provided an opportunity for either direct interaction of alpha,beta-unsaturated aldehydes with DNA or indirect formation of DNA adducts, due to oxidative stress. It is now well recognized that high concentrations of alpha,beta-unsaturated aldehydes deplete GSH, leading to release of nucleocytolytic enzymes that produce DNA fragmentation, cellular damage and apoptosis (see 2.3.1(c)). However, evidence also has indicated that at low concentrations, such as those resulting from intake of flavouring substances, alpha,beta-unsaturated aldehydes are rapidly metabolized in the high capacity beta-oxidation pathway. There is no convincing evidence that alpha,beta-unsaturated aldehydes exhibit a significant genotoxic potential in vivo after oral administration.
(e) Reproductive toxicity
(i) (E,E)-2,4-hexadienoic acid (sorbic acid; no. 1176)
Groups of Sprague-Dawley rats aged 90 days were mated after being fed a diet of Purina laboratory chow containing 10% (E,E)-2,4-hexadienoic acid for 60 days. The offspring of this parent generation were also mated at age 90 days after being fed the diet containing 10% (E,E)-2,4-hexadienoic acid for 70 days. Body weights of individual animals from the parent generation were recorded at the beginning of the study and after 30, 60, 90, and 120 days of feeding. Body weights of individual animals from the F1 generation were recorded at 40, 70, and 120 days of feeding. Throughout the study, general appearance, behaviour, and food consumption were not affected by the diet. Increased ratios of liver weight to body weight were reported in all groups as compared with the corresponding controls, with the exception of the treated females from the F1 generation. No treatment-related effects on reproduction were reported. At the termination of the feeding studies, livers from randomly selected controls and treated rats were removed to study metabolism in liver homogenates. No significant differences in the oxygen consumption of liver homogenates were reported between the treated animals from the parent generation and the controls. In the F1 generation, however, the oxygen consumption of the liver homogenates was significantly different from that of the controls at the 95% level. In this generation, the female controls had the highest rate of oxygen consumption while the male controls had the lowest. This is not toxicologically significant because female rats are known to have a higher metabolic rate than males. The rates of consumption of the treated males and females in the F1 generation were almost identical. The authors concluded that the diet containing 10% (E,E)-2,4-hexadienoic acid did not cause any significant treatment-related effects in rats (Demaree et al., 1955).
Mechanism of action
The mechanism by which hepatomas were induced in mice given diets containing high concentrations of 2,4-hexadienoic acid (Ishizawa et al., 1980) was studied by Tsuchiya & Yamaha (1984). Ether extracts of acidic components of intestinal contents from groups of male Jcl/ICR mice maintained for 1 year on a diet containing 15% 2,4-hexadienoic acid were shown to be mutagenic in S. typhimurium strain TA98. Also, the test substance produced hepatomegaly and a 40% reduction in concentrations of GSH in the liver. Owing to the unorthodox procedure used to determine the carcinogenicity of 2,4-hexadienoic acid in this study and in the mechanistic study, the results of this study cannot be considered informative; the daily intake of diet containing 15% 2,4-hexadienoic acid is three times greater than the generally accepted maximum level (5%) for addition to food according to the protocols of the National Toxicology Program. Test materials administered at levels >5% can cause nutritional alterations in the diet that can impact upon the evaluation of carcinogenicity.
3. SECONDARY COMPONENTS
Ten members (Nos 1179,1180, 1183, 1185, 1189–1192, 1196, 1198) of this group of flavouring agents have minumum assay values of <95%. Some of the secondary components of the flavouring agents are themselves flavouring agents that were evaluated by the Committee at its previous meetings. None of the secondary components were considered to present a safety concern (see Annex 6).
4. REFERENCES
Almosnino, A.M. & Belin, J.M. (1991) Apple pomace: An enzyme system for producing aroma compounds from polyunsaturated fatty acids. Biotechnol. Lett. 13, 893–898.
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics by the kidney. In: Intermediary Xenobiotic Metabolism in Animals, ed. G.D. Paulson, Taylor and Francis, New York, USA, pp. 81–97.
Andrianarison, R-H., Rabinovitch-Chable, H. & Beneytout, J.L. (1991) Oxodiene formation during the vicia sativa lipoxygenase-catalysed reaction: occurrence of dioxygenase and fatty acid lyase activities associated in a single protein. Biochem. Biophys. Res. Commun. 180, 1002–1009.
Armstrong, R. (1987) Enzyme-catalyzed detoxication reactions: Mechanisms and stereochemistry. CRC Crit. Rev. Biochem. 22, 39–88.
Armstrong, R. (1991) Glutathione S-transferases: Reaction mechanism, structure, and function. Chem. Res. Toxicol., 4, 131–140.
Arndt, R. & Krisch, K. (1973) Catalytic properties of an unspecific carboxylesterase (E1) from rat-liver microsomes. Eur. J. Biochem. 36, 129–134.
Banerjee, T.S. & Giri, A.K. (1986) Effects of sorbic acid and sorbic acid-nitrite in vivo on bone marrow chromosomes of mice. Toxicol. Lett. 31, 101–106.
Beedham, C. (1988) Molybdenum hydroxylases. In Metabolism of Xenobiotics, ed. J. Caldwell, Taylor and Francis, London, UK, pp. 21–58.
Boyland, E. & Chasseaud, L.F. (1967) Enzyme-catalysed conjugations of glutathione with unsaturated compounds. Biochem. J. 104, 95–102.
Bradley, M.O., Taylor, V.I., Armstrong, M.J. & Galloway, S.M. (1987) Relationships among cytotoxicity, lysosomal breakdown, chromosome aberrations, and DNA double-strand breaks. Mutat. Res. 189, 69–79.
Buck, N.R. & Renwick, A.G. (2000) The hydrolysis of cinnamyl and furfuryl esters. Unpublished report. Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Butterworth, K.R., Carpanini, F.M.B., Gaunt, I.F., Grasso, P. & Lloyd, A.G. (1975) A new approach to the evaluation of the safety of flavouring esters. Br. J. Pharmacol., 54, 268.
Cadet, J., Carvalho, V.M., Onuki, J., Douki, T., Medeiros, M.H.G. & Di Mascio, P. (1999) Purine DNA adducts of 4,5-dioxovaleric acid and 2,4-decadienal. IARC Scientific Publication, 150, 103-113.
Cain, K., Brown, D.G., Langlais, C. & Cohen, G.M. (1999) Caspase activation involves the formation of the aposome, a large (approximately 700kDa) caspase-activating complex. J. Biol. Chem., 274, 22686-22692.
Carvalho, V.M., Di Mascio, P., de Arruda Campos, I.P., Douki, T., Cadet, J. & Medeiros, M.H.G. (1998) Formation of 1,N6-etheno-2’deoxyadenosine adducts by trans,trans-2,4-decadienal. Chem. Res. Toxicol., 11, 1042-1047.
Carvalho, V.M., Asahara, F., Di Mascio, P., de Arruda Campos, I.P., Cadet, J. & Medeiros, M.H.G. (2000) Novel 1,N6-etheno-2’-deoxyadenosine adducts from lipid peroxidation products. Chem. Res. Toxicol., 13, 397-405.
Celli, A., Que, F.G., Gores, G.J. & LaRusso, N.F. (1998) Glutathione depletion is associated with decreased Bcl-2 expression and increased apoptosis in cholangiocytes. Am. J. Physiol., 275, G749-G757.
Clayson, D.B., Iverson, F., Nera, E.A. & Lok, E. (1990) The significance of induced forestomach tumors. Ann. Rev. Pharmacol. Toxicol., 30, 441-463.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic hazard—a decision tree approach. Food Cosmet. Toxicol. 16, 255-276.
Damske, D.R., Mecler, F.J., Beliles, R.P. & Liverman, J.L. (1980) 90-Day toxicity study in rats. 2,4-Decadienal. Unpublished report. Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
de Groot, A.P., Spanjers, M.T., van der Heijden, C.A. & Engel, C. (1974) Acute and sub-acute oral toxicity studies in rats with five flavour compounds. Unpublished report. Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Demaree, G.E., Sjogren, D.W., McCashland, B.W. & Cosgrove, F.P. (1955) Preliminary studies on the effect of feeding sorbic acid upon the growth, reproduction, and cellular metabolism of albino rats. J. Am. Pharm. Assoc., 44, 619-621.
Deuel, H.J., Alfin-Slater, R., Weil, C.S. & Smyth Jr., H.F. (1954) Sorbic acid as a fungistatic agent for foods. I. Harmlessness of sorbic acid as a dietary component. Food Res., 19, 1-12.
Dianzani, M.U. (1998) 4-Hydroxynonenal and cell signalling. Free Radical Res., 28, 553-560.
Dickens, F., Jones, H.E.H. & Waynforth, H.B. (1968) Further tests on the carcinogenicity of sorbic acid in the rat. Br. J. Cancer, 22, 762-768.
Dittberner, U., Eisenbrand, G. & Zankl, H. (1995) Genotoxic effects of the alpha, beta-unsaturated aldehydes 2-trans-butenal, 2-trans-hexenal and 2-trans,6-cis-nonadienal. Mutat. Res., 335, 259-265.
Drake, J.J.-P., Gaunt, I.F., Butterworth, K.R., Hooson, J., Hardy, J. & Gangolli, S.D. (1975) Short-term toxicity of isoamyl salicylate in rats. Food Cosmet. Tox., 13, 185-193.
Driscoll, R. (1996) Acute oral toxicity (limit test) in the rat. Unpublished report. Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Eckl, P.M., Ortner, A. & Esterbauer, H. (1993) Genotoxic properties of 4-hydroxyalkenals and analogous aldehydes. Mutat. Res., 290, 183-192.
Eder, E., Deininger, C., Neudecker, T. & Deininger, D. (1992) Mutagenicity of beta-alkyl substituted acrolein congerners in the Salmonella typhimurium strain TA100 and genotoxicity testing in the SOS chromotest. Environ. Mol. Mutagen., 19, 338-345.
Eder, E., Scheckenbach, S., Deininger, C. & Hoffman, C. (1993) The possible role of alpha,beta-unsaturated carbonyl compounds in mutagenesis and carcinogenesis. Toxicol. Lett., 67, 87-103.
Edwards, K.B. (1973) Biological evaluation of 2,6-dodecadienal and 2,4,7-tridecatrienal. 4-week feeding study in rats. Unpublished report. Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Eisenbrand, G., Schuhmacher, J. & Golzer, P. (1995) The influence of glutathione and detoxifying enzymes on DNA damage induced by 2-alkenals in primary rat hepatocytes and human lymphoblastoid cells. Chem. Res. Toxicol., 8, 40-46.
Esterbauer, H., Zollner, H. & Scholz, N. (1975) Reaction of glutathione with conjugated carbonyls. Zeitschrift Fur Naturforschung, 30, 466-473.
Esterbauer, H., Cheeseman, K.H., Dianzani, M.U., Poli, G. & Slater, T.F. (1982) Separation and characterization of the aldehydic products of lipid peroxidation stimulated by NDP-Fe2+ in rat liver microsomes. Biochem. J., 208, 129-140.
Esterbauer, H., Schaur, R.J. & Zollner, H. (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biol. Med., 11, 81-128.
Food Chemicals Codex (1996) Food and Chemicals Codex, 4th Ed., Washington DC: National Academy Press.
Food and Drug Administration (1993) Priority-based assessment of food additives (PAFA) database center for food safety and applied nutrition.
Feldman, R.I. & Weiner, H. (1972) Horse liver aldehyde dehydrogenase. J. Biol. Chem., 247, 260-266.
Fingerhut, M., Schmidt, B. & Lang, K. (1962) Uber den stoffwechsel der 1-14C-sorbinsaure. Biochemische Zeitschrift, 336, 118-125.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening of tobacco smoke constituents for mutagenicity using the Ames test. Toxicol., 18, 219-232.
Frankel, E.N., Neff, W.E., Brooks, D.D. & Fujimoto, K. (1987) Fluorescence formation from the interaction of DNA with lipid oxidation degradation products. Biochim. Biophys. Acta, 919, 239-244.
Gaunt, I.F., Butterworth, K.R., Hardy, J. & Gangolli, S.D. (1975) Long-term toxicity of sorbic acid in the rat. Food Cosmet. Toxicol., 13, 31-45.
Graffner-Nordberg, M., Karin, S., Anders, T. & Anders, H. (1998) Synthesis and enzymes hydrolysis of esters, constituting simple models of soft drugs. Chem. Pharm. Bull., 46, 591-601.
Green, D.R. & Reed, J.C. (1998) Mitochondria and apoptosis. Science, 281, 1309-1312.
Grice, H.C. (1988) Safety evaluation of butylated hydroxyanisole from the perspective of effects on forestomach and oesophageal squamous epithelium. Food Chem. Toxicol., 26, 717-723.
Grootveld, M., Atherton, M.D., Sheerin, A.N., Hawkes, J., Blake, D.R. & Richens, T.E. (1998) In vivo absorption, metabolism, and urinary excretion of alpha,beta-unsaturated aldehydes in experimental animals. J. Clin. Investig., 101, 1210-1218.
Grundschober, F. (1977) Toxicological assessment of flavouring esters. Toxicol., 8, 387-390.
Hendy, R.J., Hardy, J., Gaunt, I.F., Kiss, I.S. & Butterworth, K.R. (1976) Long-term toxicity studies of sorbic acid in mice. Food Cosmet. Toxicol., 14, 381-386.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B., ed., Enzymatic Basis of Detoxication, 2nd Ed., Vol. II, New York: Academic Press, pp. 291-323.
International Organization of the Flavor Industry (1995). European inquiry on volume use. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
IARC (2003) Predictive value of rodent forestomach and gastric neuroendocrine tumours in evaluation carcinogenic risks to humans. IARC Technical Publication, 39, Lyon: IARC-Press.
Ishizawa, K., Shibuya, K., Gon, S. & Aiso, K. (1980) Poisonous effect of the sorbic acid on the mouse fed in long term with its high concentration. J. Tokyo Kasei Gakuin College, 20, 83-97. Cited in: Tsuchiya & Yamaha (1984).
Ji, C., Amarnath, V., Pietenpol, J.A. & Marnett, L.J. (2001) 4-Hydroxynonenal induces apoptosis via caspase-3 activation and cytochrome c release. Chem. Res. Toxicol., 14, 1090-1096.
Junge, W. & Heymann, E. (1979) Characterization of the isoenzymes of pig-liver esterase. 2. Kinetic studies. Eur. J. Biochem., 95, 519-525.
Kassahun, K., Farrell, K. & Abbott, F. (1991) Identification and characterization of the glutathione and N-acetylcysteine conjugates of (E)-2-propyl-2,4-pentadienoic acid, a toxic metabolite of valporic acid, in rats and humans. Drug Metab. Dispos. 19, 525-235.
Kirichenko, A., Li, L., Morandi, M.T. & Holian, A. (1996) 4-Hydroxy-2-nonenal-protein adducts and apoptosis in murine lung cells after acute ozone exposure. Toxicol. App. Pharmacol., 141, 416-424.
Klesov, A.A., Lang, L.G., Sytkowski, A.J. & Vallee, B.L. (1977) Translation of: Unusual nature of the substrate specificity of alcohol dehydrogenases of different origins. Bioorganicheskaya Khimiya, 3, 1141-1144.
Kruman, I., Bruce-Keller, A.J., Bredesen, D., Waeg, G. & Mattson, M.P. (1997) Evidence that 4-hydroxynonenal mediates oxidative stress-induced neuronal apoptosis. J. Neurosci., 17, 5089-5100.
Lame, M.W. & Segall, H.J. (1986) Metabolism of the pyrrolizidine alkaloid metabolite trans-4-hydroxy-2-hexenal by mouse liver aldehyde dehydrogenases. Toxicol. App. Pharmacol., 82, 94-103.
Leegwater, D.C. & van Straten, S. (1974) In vitro study on the hydrolysis of twenty-six organic esters by pancreatin. Unpublished. Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Li, P., Nijhawan, D., Budihardjo, I., Srinivasula, S.M., Ahmad, M., Alnemri, E.S. & Wang, X. (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91, 479-489.
Liu, W., Kato, M., Akhand, A.A., Hayakawa, A., Suzuki, H., Miyata, T., Kurokawa, K., Hotta, Y., Ishikawa, N. & Nakashima, I. (2000) 4-Hydroxynonenal induces a cellular redox status-related activation of the caspase cascade for apoptotic cell death. J. Cell Sci. 113, 635–641.
Liu, X., Kim, C.N., Yang, J., Jemmerson, R. & Wang, X. (1996) Induction of apoptic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86, 147–157.
Longland, R.C., Shilling, W.H. & Gangolli, S.D. (1977) The hydrolysis of flavouring esters by artificial gastrointestinal juices and rat tissue preparations. Toxicol. 8, 197–204.
Loureiro, A.P.M., Di Mascio, P., Gomes, O.F. & Medeiros, M. H.G. (2000) trans,trans-2,4-Decadienal-Induced 1,N2-etheno-2’-dexoyguanosine adduct formation. Chem. Res. Toxicol. 13, 601–609.
Lucas, C.D., Putnam, J.M. & Hallagan, J.B. (1999) Flavor and Extract Manufacturers Association of the United States 1995 Poundage and Technical Effects Update Survey, Washington DC: Flavor and Extract Manufacturers Association of the United States.
Maarse, H., Visscher, C.A., Willemsens, L.C. & Boelens, M.H. (1999). Volatile Components In Food—Qualitative And Quantitative Data. Zeist: Centraal Instituut Voor Voedingsonderzioek TNO.
Marnett, L.J., Hurd, H.K., Hollstein, M.C., Levin, D.E., Esterbauer, H. & Ames, B.N. (1985) Naturally occurring carbonyl compounds are mutagens in Salmonella tester strain TA104. Mutat. Res., 148, 25–34.
Mecler, F.J. & Craig, D.K. (1980) Three-month toxicity study in rats. Hexadienal. Unpublished report. Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Mitchell, D.Y. & Petersen, D.R. (1987) The oxidation of alpha,beta-unsaturated aldehydic products of lipid peroxidation by rat liver aldehyde dehydrogenases. Toxicol. App. Pharmacol., 87, 403–410.
Moreno, O.M. (1976) Acute toxicity studies in rats, mice, rabbits and guinea pigs. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Moreno, O.M. (1978) Acute toxicity studies in rats, mice, rabbits, and guinea pigs Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Moreno, O.M. (1980) Acute toxicity studies. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Mukherjee, A., Giri, A.K., Talukder, G. & Sharma, A. (1988) Sister chromatid exchanges and micronuclei formations induced by sorbic acid and sorbic acid-nitrite in vivo in mice. Toxicol Lett., 42, 47–53.
Nakayasu, H., Mihra, K. & Sato, R. (1978) Purification and properties of a membrane-bound aldehyde dehydrogenase from rat liver microsomes. Biochem. Biophys. Res. Commun. 83(2), 697–703.
National Academy of Sciences (1982) Evaluating the Safety of Food Chemicals. Washington DC, USA.
Nelson, D.L. & Cox, M.M. (2000) Lehninger: Principles of Biochemistry. New York: Worth Publishers, Inc.
National Toxicology Program (1993) Toxicology and carcinogenesis studies of benzyl acetate (CAS 140-11-4) in F344/N rats and B6C3F1 mice (feed studies). Report No. NTP TR 431.
National Toxicology Program (1997) Final report on subchronic toxicity studies of 2,4-decadienal administered by gavage to F344/N rats and B6C3F1 mice and cellular and genetoxic toxicology tables. Study Nos. 93022.01–93022.02.
National Toxicology Program (2000) Report on carcinogens, 9th Ed., National Toxicology Program (http://ehis.niehs.nih.gov/roc/, accessed April 30, 2002).
National Toxicology Program (2001) Draft report: Toxicology and carcinogenesis studies of 2,4-hexadienal in F344/N rats and B6C3F1 mice (gavage studies). Report No. NTP TR 509.
Pellmont, B. (1971) Acute toxicity studies. Unpublished report. Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Pellmont, B. (1977) Acute toxicity studies. Unpublished. Private communication to the Flavor and Extract Manufacturers Association (FEMA). Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Pietruszko, R., Crawford, K. & Lester, D. (1973) Comparison of substrate specificity of alcohol dehydrogenases from human liver, horse liver, and yeast towards saturated and 2-enoic alcohols and aldehydes. Arch. Bioch. Biophys., 159, 50–60.
Reed, D.J., Fariss, M.W. & Pascoe, G.A. (1986) Mechanisms of chemical toxicity and cellular protection systems. Fundam. App. Toxicol., 6, 591–597.
Schulz, J., Lindenau, J., Seyfried, J. & Dichgans, J. (2000) Glutathione, oxidative stress and neurodegeneration. Eur. J. Biochem., 267, 4904–4911.
Smyth, H.F., Carpenter, C.P., Weil, C. S. & Pozzani, U.C. (1954) Range-finding toxicity data. List V. Arch. Indust. Hyg., 10, 61–68.
Sparfel, L., Lafille, C. & LeReste, S. (1968) Toxicological tests of some sorbic acid derivatives. Paris: CR Academy of Science, D266, 1080–1081.
Stofberg, J. & Grundschober, F. (1987) Consumption ratio and food predominance of flavoring materials. Perfumer Flavorist, 12, 27.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of flavoring materials: A mechanism for setting priorities for safety evaluation. Food Chem. Toxicol. 23, 857–860.
Thompson, P.W. (1996) Reverse mutation assay "Ames Test" using Salmonella Typhimurium. Unpublished. Unpublished. Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Tsuchiya, T. & Yamaha, T. (1984) Depletion of the reduced glutathione level in the liver and production of the mutagens in the intestine in the mice inducing hepatoma by feeding on a high level dose of sorbic acid. Mut. Res., 130, 267–272.
Uchida, O., Naito, K., Yasuhara, K., Owba, S., Sato, C., Shimonuri, S., Kobayashi, K. & Tobe, M. (1985) Studies on the acute oral toxicity of dehydroacetic acid, sorbic acid and their combination compound in rats. Bull. National Inst. Hyg. Sci., Tokyo, 103, 166–171.
Wester, P.W. & Kroes, R. (1988) Forestomach carcinogens: Pathology and relevance to man. Toxicol. Pathol., 16, 165–171.
Westoo, B. (1964) On the metabolism of sorbic acid in the mouse. Acta Chemica Scandinavica, 18, 1373–1378.
Witz, G. (1989) Biological Interactions of alpha,beta-unsaturated aldehydes. Free Radical Biol. Med., 7, 333–349.
Zajac-Kaye, M. & Ts’o, P.O.P. (1984) DNAase I encapsulated in liposomes can induce neoplastic transformation of Syrian hamster embryo cells in culture. Cell, 39, 427– 437.
Zou, H., Henzel, W.J., Liu, X., Lutschg, A. & Wang, X. (1997) Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation for caspase-3. Cell, 90, 405–413.
ENDNOTES:
 2-Hexenal.
2-Hexenal. trans-2-Nonenal.
trans-2-Nonenal.  trans-2-Pentenal.
trans-2-Pentenal.  (E,E)-2,4-Hexadienedioic acid.
(E,E)-2,4-Hexadienedioic acid.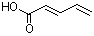 (E)-2-Propyl 2,4-pentadienoic acid.
(E)-2-Propyl 2,4-pentadienoic acid. 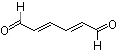 trans,trans-Muconaldehyde.
trans,trans-Muconaldehyde. - (no footnote text)
- Calculated using relative molecular mass =138.21.







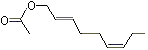
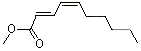
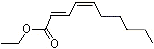







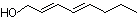










 2-Hexenal.
2-Hexenal. trans-2-Nonenal.
trans-2-Nonenal.  trans-2-Pentenal.
trans-2-Pentenal.  (E,E)-2,4-Hexadienedioic acid.
(E,E)-2,4-Hexadienedioic acid.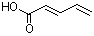 (E)-2-Propyl 2,4-pentadienoic acid.
(E)-2-Propyl 2,4-pentadienoic acid. 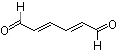 trans,trans-Muconaldehyde.
trans,trans-Muconaldehyde.