
Pesticide residues in food 2001
First draft prepared by
Roland Solecki
Pesticides and Biocides Division, Federal Institute for Health Protection of Consumers and Veterinary Medicine, Berlin, Germany
Imidacloprid, 1[(6-chloro-3-pyridinyl)methyl]-N-nitro-2-imidazolidinimine, is a new neonicotinoid insecticide which has become an important pest control agent on many crops. The neonicotinoid insecticides are related to nicotine in their structure and action at the nicotinic acetylcholine receptor (Casida, 1998). Imidacloprid is most active against suckling insects because of their unique plant-systemic and translaminar properties. Imidacloprid poisoning in the American cockroach, Periplaneta americana, is characterized, in sequence, by loss of leg strength, leg tremors, body shaking and death. Neurophysiological studies have confirmed that imidacloprid is an agonist at the postsynaptic nicotinic acetylcholine receptor of insects. It appears to act like acetylcholine, by exciting specific nerve cells. There is also some evidence, however, that imidacloprid has multiple agonist and antagonist effects on neuronal nicotinic acetylcholine receptor channels of clonal rat phaeochromocytoma cells (Nagata et al., 1998).
The selective toxicity of imidacloprid to insects and not to mammals is attributed to differences in the binding affinity or potency at the nicotinic acetylcholine receptor (Chao & Casida, 1997). The specificity of nicotinoid insecticides appears to be related to receptor subtype, function, neuronal region and developmental stage, and their metabolic lability leads to bioactivated toxicants such as desnitro-imidacloprid in mammals. The selectivity also depends in large part on major structural differences in the neuronal nicotinic acetylcholine receptor binding sites of mammals and insects (Casida, 1998). The selectivity of the chloronicotyl compounds for insects as opposed to mammals can be partly explained by differences in the ionization of the pyrolidine nitrogen. Imidacloprid is poorly ionized in neutral media, in contrast to nicotine, and thus passes easily through insect lipophilic barriers (Sone et al., 1994; Roe et al., 1999). Imidacloprid has not previously been evaluated by the JMPR.
(a) Absorption, distribution and excretion
Imidacloprid labelled with 14C in either the methylene or the imidazolidine ring was administered in a physiological saline solution at concentrations of 0.1–2 mg/l to a total of 50 male and 20 female rats. Groups of five male and five female rats were given a single dose of 1 mg/kg bw intravenously or a single dose of 1 or 20 mg/kg bw orally. Other groups were given 14 doses of of the non-radiolabelled compound at 1 mg/kg bw orally once per day and, 24 h after the last dose, a single oral dose of 1 mg/kg bw as the radiolabelled compound. Radiolabel was determined in plasma and excreta as a function of time and, after sacrifice 48 h after treatment, the concentration of total radiolabel was determined in organs and tissues. A further group of five male rats was given a single oral dose of radiolabelled compound at 20 mg/kg bw, and 14CO2 was measured over 48 h. In an additional test, four groups of five male rats were given a single oral dose of 20 mg/kg bw, killed after 40 min, 1.5, 3 and 6 h, and total radiolabel was determined as a function of time in single organs. A final group of five male rats with bile-duct fistulas was given a single intraduodenal dose of 1 mg/kg bw in order to determine the amount of total radiolabel absorbed and to measure the rate and extent of biliary excretion. The design of the study complied with good laboratory practice (GLP).
After oral administration of 1 or 20 mg/kg bw of [pyridinyl-14C-methylene]imidacloprid, the radiolabel was extensively absorbed from the intestinal lumen and readily distributed from the plasma into the body. The radiolabel was also readily eliminated. After intravenous administration of 1 mg/kg bw, about 92% of the recovered radiolabel was excreted in urine and faeces within 48 h. Most of the radiolabel was excreted via the kidneys, with an average ratio in urine:faeces of 4:1. After oral administration, about 96% of the dose was excreted in urine and faeces within 48 h. No difference was found between female and male rats. More than 90% of the renal radiolabel was excreted during 24 h after dosage. The average residual radiolabel in the body, excluding the gastrointestinal tract, at sacrifice was about 0.5%, and that in the gastrointestinal tract was about 0.06% of the administered dose. The rats with bile-duct fistulas excreted only 4.7% of the administered dose with faeces, 56% in urine and about 36% with bile. These findings indicate the existence of enterohepatic circulation of the radiolabel. Significant amounts of radiolabel were not excreted in expired air (CO2). The elimination of total radiolabel from the plasma could be approximated by a combination of two exponential terms, from which elimination half-lives were calculated. The two half-lives varied from 2.6–3.6 to 26–118 h, respectively (Klein, 1987a).
The distribution of [pyridinyl-14C-methylene]imidacloprid was investigated in male rats by conventional whole-body autoradiography on X-ray film in a study conducted according to GLP. The rats were given a single oral dose of 20 mg/kg bw, and the distribution of radiolabel was visualized at 1, 2, 4, 24 and 48 h after treatment. For visualization of the initial distribution, one animal was injected intravenously with 20 mg/kg bw and killed 5 min later.
The radiolabel was readily absorbed and rapidly distributed to the tissues and organs. The pattern of distribution showed that the radiolabel could readily permeate tissues: With the exception of fatty tissues, the central nervous system and the mineral part of bone, blackening on the autoradiogram was seen on all other parts of the body 5 min after intravenous injection and 1 h after oral dosage. Higher concentrations were seen later in the thyroid and adrenals, but, after 24 h, all other organs and tissues showed only small amounts of radiolabel. The high degree of blackening over the kidney during the first 24 h is a reflection of the high rate of renal excretion of the administered compound. The concentration of radiolabel decreased in organs and tissues with time after administration. The concentrations in the fatty tissues and in the central nervous system were very low throughout the study (Klein, 1987b).
Imidacloprid labelled with 14C in the 4 and 5 positions of the imidazolidine ring was administered orally to male and female rats at a dose of 1 mg/kg bw and additionally to male rats at a dose of 150 mg/kg bw, in a study conducted according to GLP. Radiolabel was determined in excreta and in plasma as a function of time, and single organs and tissues were assayed for total radiolabel at sacrifice 48 h after treatment. The biokinetics of [imidazolidine-4,5-14C]imidacloprid was similar to that of methylene-14C-labelled compound. The administered radiolabel was extensively absorbed from the intestinal lumen and rapidly distributed in the body. Excretion was rapid, and the renal route predominated (Klein & Brauner, 1991).
The nitroso metabolite of imidacloprid, 1-(6-chloro-3-pyridylmethyl)-N-nitroso(imidazoli-din-2-ylidene)amine (WAK 3839), has been identified as a minor constituent of edible plant commodities but was not found in the excreta of rats in the studies described above. Absorption of imidacloprid and WAK 3839 began immediately after oral administration of 1 mg/kg bw. A comparison of the curves of concentration–time in plasma suggested that the absorption was similarly extensive, and no significant differences in terminal half-times were found after administration of imidacloprid (38 h) and WAK 3839 (47 h). WAK 3839 was eliminated slightly faster, and the concentrations of total radiolabel in the organs were lower than with the parent compound. After administration of a single high dose of imidacloprid, 150 mg/kg bw, to male rats, the ratio of excretion of total radiolabel was the same as after the low dose. Pretreatment with repeated doses also did not affect the pattern of excretion (Klein, 1990).
In the biokinetics study of Klein (1987a), urine was collected separately from each rat under cool conditions, at intervals of 0–4, 4–8, 8–24 and 24–48 h, and faeces were collected at intervals of 0–24 and 24–48 h after treatment. The metabolites were extracted from faeces, further isolated by preparative high-performance liquid chromatography (HPLC) and then purified by other HPLC methods. The metabolites were identified by chromatographic comparison with authentic reference compounds in at least two independent chromatographic systems or by 1H-nuclear magnetic resonance and mass spectroscopic techniques.
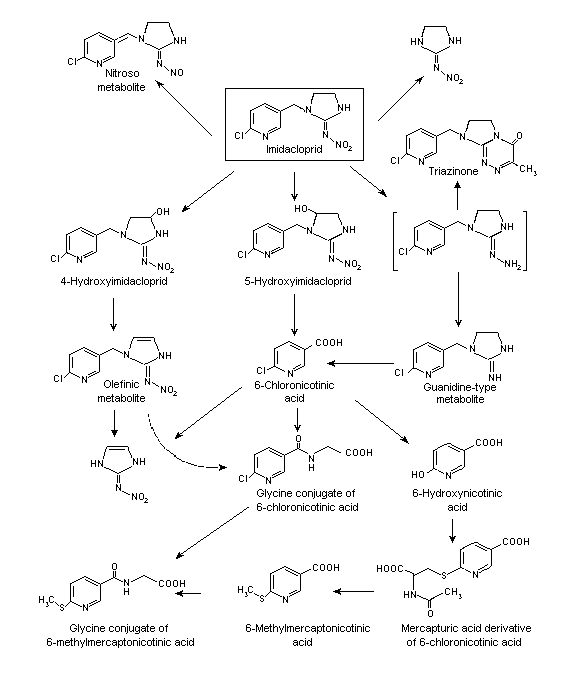
A proposed metabolic pathway of imidacloprid in rats is shown in Figure 1. After administration of low doses, no relevant sex dependence was seen in the excretion pattern of the compound or in the metabolic profiles in excreta. All the identified metabolites were found at each dose group and in both sexes. The main metabolites were 6-chloronicotinic acid and its glycine conjugate, which were found only in urine. The amounts of monohydroxylated (5-hydroxy-imidacloprid) and olefinic metabolites were similar to those of unchanged parent compound. All other biotransformation products were quantitatively of minor significance.

Figure 1. Proposed metabolic pathway of imidacloprid in rats
Two main routes of metabolism responsible for the degradation of imidacloprid were identified. The first is oxidative cleavage, yielding 6-chloronicotinic acid, which is conjugated with glycine to form a hippuric acid-type conjugate. These two metabolites together represented most of the identified metabolites, or about 30% of the recovered radiolabel. Of minor importance in terms of quantity is dechlorination of the pyridinyl moiety, producing the 6-hydroxy nicotinic acid and its methylmercapturic acid derivative, probably as a degradation product of a glutathione conjugate. The 6-methylmercapto nicotinic acid conjugated with glycine, and the glycine conjugate constituted 5.6% of the recovered radiolabel. The second important biodegradation step starts with hydroxylation of the imidazolidine ring at the 4 or 5 position, and about 16% of the recovered radiolabel was identified as the sum of 4- and 5-hydroxy imidacloprid. The loss of water yields the olefinic compound. These biotransformation products and the unchanged parent compound were excreted in urine and faeces, while the guanidine compound was a less important metabolite and was eliminated only in faeces.
The high excretion rate of the parent compound (average, 14%) indicates rapid passage through the body, as was confirmed by the elimination of > 90% of the recovered radiolabel within 24 h after administration (Klein & Karl, 1990).
The distribution of the metabolites in liver and kidney at various times after a single oral dose was investigated in rats under the same test conditions as used by Klein (1987a). The metabolites were extracted from lyophilized organs with water and methanol, further purified by HPLC and thin-layer chromatography and identified by comparative HPLC with authentic reference compounds in at least two independent chromatographic systems and also by mass and 1H-nuclear magnetic resonance spectroscopic techniques. The metabolites found in the kidney were identical to those identified in urine. Triazinone was not found in the excreta and may have undergone further biodegradation before elimination via the kidney or the bile. The relative amounts of those biotransformation products formed by oxidative mechanisms (e.g. 6-chloronicotinic acid) increased in the liver during the test period. In kidney, the relative amount of the more polar compounds decreased with time (6-chloronicotinic acid and its glycine conjugate), while the amounts of the olefinic metabolite and the mono-hydroxylated derivative 4-hydroxyimidacloprid showed a relative increase. The proportion of the parent compound decreased slowly as it was metabolized (Karl & Klein, 1992).
The metabolic pattern of imidacloprid and its nitroso metabolite in excreta was investigated in male rats after administration of a single oral dose of 1 mg/kg bw of imidacloprid or the nitroso compound and after administration of a high dose of 150 mg/kg bw imidacloprid. After the low dose of imidacloprid, about 82% of the renal radiolabel was detected. The main renal metabolite (about 30%) was the glycine conjugate of 6-chloronicotinic acid. The parent compound was present at about 12%, the two monohydroxylated biotransformation products (4-hydroxy and 5-hydroxyimidacloprid) at about 19% and the olefinic metabolite at about 11%. 6-Chloronicotinic acid represented 7.9% of the renal radiolabel. No nitroso compound was formed under these conditions. In the faeces, imidacloprid, the olefinic metabolite, 6-chloronicotinic acid and its glycine conjugate were identified. These findings are in good agreement with those reported by Klein & Karl (1990).
Few metabolites were found in the urine of male rats given the nitroso compound orally. Besides unchanged nitroso compound, only 8% of the renal radiolabel was attributable to the guanidine-type metabolite, some of which was also found in faeces. This finding indicates that the metabolism of the nitroso compound is completely different from that of its parent compound. In order to investigate whether the nitroso compound is formed as a biotransformation product of imidacloprid in vivo, a high single oral dose of 150 mg/kg bw imidacloprid was given to male rats. No nitroso metabolite was formed. In the urine of rats given a diet containing 1800 ppm of imidacloprid for 1 year and then one oral dose of [methylene-14C]imidacloprid, 9.3% of the radiolabel was attributable to the nitroso metabolite, corresponding to 6.8% of the administered dose. The nitroso compound is therefore formed in vivo in rats after long-term intake of imidacloprid. In order to confirm this finding, a direct isotope dilution analysis was conducted with the urine of these rats and also with the urine of mice that had been fed a diet containing 2000 ppm of imidacloprid for about 1 year. Both analyses clearly demonstrated the presence of the nitroso compound in the urine (Klein, 1990).
The results of studies on the acute toxicity of imidacloprid are summarized in Table 1. The methods used in these studies complied with OECD guidelines and GLP.
Table 1. Acute toxicity of imidacloprid
|
Species |
Strain |
Sex |
Route |
LD50 |
LC50 |
Purity |
Reference |
|
Rat |
Bor:WISW |
Male |
Oral |
420 |
|
94.2 |
Bomann (1989a) |
|
|
|
Female |
|
450–480 |
|
|
|
|
Rat |
Bor:WISW |
Male |
Oral |
640 |
|
96.0 |
Bomann (1991a) |
|
|
|
Female |
|
650 |
|
|
|
|
Rat |
Bor:WISW |
Male |
Oral |
500 |
|
94.3 |
Bomann (1991b) |
|
|
|
Female |
|
380 |
|
|
|
|
Mouse |
Bor:NMRI |
Male |
Oral |
130 |
|
94.2 |
Bomann (1989b) |
|
|
|
Female |
|
170 |
|
|
|
|
Rat |
Bor:WISW |
Male |
Percutaneous |
> 5000 |
|
94.2 |
Krötlinger (1989) |
|
|
|
Female |
|
> 5000 |
|
|
|
|
Rat |
Bor:WISW |
Male |
Inhalation (aerosol, 4 h) |
|
> 69 |
95.3 |
Pauluhn (1988a) |
|
|
|
Female |
|
|
> 69 |
|
|
|
Rat |
Bor:WISW |
Male |
Inhalation (dust, 4 h) |
|
> 5300 |
95.3 |
Pauluhn (1988a) |
|
|
|
Female |
|
|
> 5300 |
|
|
|
Rat |
Bor:WISW |
Male |
Intraperitoneal |
160–170 |
|
94.2 |
Krötlinger (1990) |
Imidacloprid given orally as a single dose was moderately toxic to rats (LD50, 380–650 mg/kg bw) and mice (LD50, 130–170 mg/kg bw). Behavioural and respiratory signs, disturbances of motility, narrowed palpebral fissures, transient trembling and spasms were seen in rats and mice treated orally at doses > 200 mg/kg bw and > 71 mg/kg bw, respectively. The clinical signs were reversed within 6 days. Imidacloprid given intraperitoneally showed moderate to low acute toxicity in rats, the signs being similar to those after oral administration. Very little toxicity was seen after acute dermal application. The LC50 for a single exposure to an aerosol could not be determined exactly, as rats tolerated inhalation for 4 h of the maximum concentration of dust that could be produced technically (0.069 mg/l of air) without signs or deaths.
Imidacloprid (purity, 94.2%) did not irritate the eyes or skin of HC New Zealand white rabbits (Pauluhn, 1988b,c) and did not sensitize the skin of DHPW guinea-pigs (Ohta, 1988).
(b) Short-term studies of toxicity
Mice
Groups of 10 male and 10 female Charles-River B6C3F1 mice were given diets containing imidacloprid (purity, 92.8%) at a concentration of 0, 120, 600 or 3000 ppm for up to 107 days, equivalent to 17, 86 and 430 mg/kg bw per day. The study did not comply with GLP.
Seven males and seven females at 3000 ppm died; furthermore, several animals displayed a poor general condition, and rough coats were observed frequently. Body-weight gain was reduced and food consumption was increased in males at 600 ppm and males and females at 3000 ppm. At this dose, clinical chemical tests showed significantly decreased urea and cholesterol concentrations in males and lowered alanine aminotransferase activity and glucose concentration in females. Alkaline phosphatase activity was significantly increased in both sexes at 3000 ppm and in females at 120 and 600 ppm. Differences in the weights of the liver, heart, spleen, kidneys, testes and adrenals were observed at 3000 ppm. The NOAEL was 120 ppm, equivalent to 17 mg/kg bw per day (Eiben, 1988a).
Rats
Groups of 10 male and 10 female Wistar rats [Bor:WISW(SPF-Cpb)] received diets containing imidacloprid (purity, 92.8%) at a concentration of 0, 120, 600 or 3000 ppm for up to 98 days, equal to 11, 57 and 410 mg/kg bw per day for males and 15, 78 and 510 mg/kg bw per day for females. The study did not comply with GLP.
Food intake was increased in animals at 3000 ppm, and body-weight gain was decreased at 600 and 3000 ppm. Significantly elevated alkaline phosphatase activity and depressed glucose concentration were found in males and females at 3000 ppm, and the males also showed a reduced cholesterol concentration. Degenerative histological lesions in the epithelium of the testicular tubules were seen in five of 10 males at 3000 ppm, and multifocal group cell necrosis was diagnosed in the liver of one male at this dietary concentration. The NOAEL was 120 ppm, equal to 11 mg/kg bw per day (Eiben, 1988b).
Groups of 10 male and 10 female Wistar rats [Bor:WISW(SPF-Cpb)] received diets containing imidacloprid (purity, 95.3%) at a concentration of 0, 150, 600 or 2400 ppm for up to 96 days, equal to 14, 61 and 300 mg/kg bw per day for males and 20, 83 and 420 mg/kg bw per day for females. Satellite groups consisting of 10 male and 10 female rats received the test substance at a concentration of 0 or 2400 ppm over the same period, and, to study the reversibility of any effects, control diet was provided during the subsequent 4-week post-treatment observation period. The study was conducted according to GLP.
In animals at 2400 ppm, feed intake was increased during treatment and recovery. Reduced body-weight gain was observed in males at 600 ppm and in females at 2400 ppm. Slightly longer thromboplastin times and depressed thrombocyte counts were found at 2400 ppm, both of which were only partially reversible. Elevated alkaline phosphatase and alanine aminotransferase activities and depressed protein, albumin, cholesterol and triglyceride concentrations were found in males and females at 2400 ppm. Depressed protein concentrations were also found in males at 150 and 600 ppm. Males at 2400 ppm showed increased incidences of cellular necroses, round-cell infiltration, swollen cellular nuclei and cytoplasmic lesions in the liver, but these hepatotoxic effects were reversible within the subsequent 4-week post-treatment observation period. The NOAEL was 150 ppm, equal to 14 mg/kg bw per day (Eiben & Rinke, 1989).
Imidacloprid (purity, 95.3%) was administered in dust form through the nose only to groups of 10 male and 10 female Wistar rats [Bor:WISW(SPF-Cpb)] at analytically determined concentrations of 0, 20, 110 and 500 mg/m3 on 5 consecutive days for 6 h/day. The respirability of the test substance at the highest concentration was relatively low; about 20% of the administered mass of the substance was in the form of particles < 5 mg. The study was conducted according to GLP.
Body-weight gain was slightly decreased at the two higher concentrations. Elevated O-demethylase and N-demethylase activities were found in a liver homogenate from animals at 110 mg/m3 air. The NOAEL was 20 mg/ m3 air (Pauluhn, 1988a).
Imidacloprid (purity, 95.3%) was administered in dust form through the nose only to groups of 10 male and 10 female Wistar rats [Bor:WISW(SPF-Cpb)] at analytically determined concentrations of 0, 5.5, 30 and 190 mg/m3 for 6 h/day, 5 days per week for 4 weeks. The study was conducted according to GLP.
Body-weight gain was decreased in males at the two higher concentrations. Increased mixed-function oxidase activities were found in liver homogenate from females at these concentrations and in males at 190 mg/m3 air. Increased alanine aminotransferase and glutamate dehydrogenase activities were seen in both sexes at the highest concentration, and alanine aminotransferase activity was increased in females at 30 mg/m3 air. The females had increased alkaline phosphatase activity at the two higher concentrations and increased liver weights at 190 mg/m3 of air. The serum alpha1-globulin fraction was reduced in both sexes at the two higher concentrations. At 190 mg/m3 of air, blood coagulation time was increased in females and thrombocyte counts were depressed in males. The NOAEL was 5.5 mg/ m3 air (Pauluhn, 1988d)
Rabbits
Imidacloprid (purity, 95.0%), mixed to a paste in a physiological saline solution containing 2% Cremophor EL, was applied to the shorn dorsal and flank skin of groups of five male and five female HC New Zealand white rabbits at a dose of 0 or 1000 mg/kg bw for 6 h/day for 15 days. The study was conducted according to GLP. No treatment-related effects were observed. The NOAEL was 1000 mg/kg bw per day at the limit dose tested (Flucke, 1990).
Dogs
Groups of two male and two female pure-bred beagle dogs received diets containing imidacloprid (purity, 92.8%) at a concentration of 0, 200, 1000 or 5000 ppm for up to 28 days, providing average intakes of 0, 7.3, 31 and 49 mg/kg bw per day for males and females combined. The study was not conducted according to GLP.
Two animals at 5000 ppm died, and the other two were killed in moribund condition. The symptoms observed were ataxia, tremor and occasional vomiting. Reductions in food consumption were recorded at the two higher concentrations, and body weight was markedly reduced at 5000 ppm. The results of hearing tests, ophthalmic examinations, urine analyses and haematological examiinations indicated no treatment-related changes at any dietary concentration. Cytochrome P450 activity in the liver was slightly increased in males and females at 1000 ppm. The triiodothyronine concentration in plasma was slightly decreased in one male and one female at 5000 ppm. The weight of the liver was increased in one female at 1000 ppm; one male at this concentration showed slight hepatocellular hypertrophy with slight pigmentation of Kupffer cells, and another had minimal follicular atrophy of the thyroid. The NOAEL was 200 ppm, equal to 7.3 mg/kg bw per day (Bloch et al., 1987).
Groups of four male and four female beagles [Bor:Beag] were given diets containing imidacloprid (purity, 95.3%) at a concentration of 0, 200, 600 or 1800/1200 ppm for 13 weeks. The concentration of active ingredient at the highest level was reduced at week 4 because of low food intake. The study was conducted according to GLP.
The food consumption of animals at 600 ppm was reduced. At higher concentrations, the animals showed a clinically emaciated state and transient trembling. Body-weight gain was reduced at 1800 ppm, but, once the active ingredient concentration had been reduced to 1200 ppm, a trend to normalization was apparent. The NOAEL was 200 ppm, equal to 7.5 mg/kg bw per day (Ruf, 1990).
Groups of four male and four female pure-bred beagles were given diets containing imidacloprid (purity, 94.9%) at a concentration of 0, 200, 500 or 1250/2500 ppm for 52 weeks. The concentration of 1250 ppm was increased from week 17 onwards. The mean intakes of imidacloprid were equal to 0, 6.1, 15 and 41/72 mg/kg bw per day for males and females combined. The study was conducted according to GLP.
Slight, temporary reductions in food intake were seen in both sexes at 1250 ppm and at the increased concentration of 2500 ppm. At 1250/2500 ppm, clinical chemical examination revealed an increased plasma cholesterol concentration in females after 13 and 26 weeks, increased hepatic cytochrome P450 activity and slightly increased liver weights in males and females after 52 weeks. The NOAEL was 500 ppm, equal to 15 mg/kg bw per day (Allen et al., 1989).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female Charles-River B6C3F1 mice received diets containing imidacloprid (purity, 95.3%) at a concentration of 0, 100, 330 or 1000 ppm for 24 months. In a supplemental study to determine the maximum tolerated dose, groups of 50 male and female mice were given diets containing imidacloprid (purity, 90.0%) at a concentration of 0 or 2000 ppm for 24 months. Ten additional mice of each sex were included per dose in both studies for interim sacrifice after 12 months of treatment. The mean intake of imidacloprid was equal to 20, 66, 210 and 410 mg/kg bw per day for males and 30, 100, 270 and 420 mg/kg bw per day for females. The study was conducted according to GLP.
Food intake was decreased by 24% in females at 2000 ppm, and water intake was decreased in males and females at this concentration. Mice at 1000 and 2000 ppm had significantly, dose-related reduced weight gains, particularly during the latter half of the study. At 2000 ppm, lower leukocyte counts were found in both sexes. Reduced blood cholesterol concentrations were observed at 2000 ppm after 52 weeks. An increased incidence of low-grade periacinar hepatic-cell hypertrophy was found in males at 2000 ppm. The brains of animals at the highest concentration showed more mineralization of the thalamus than in the control groups. There was no evidence of a carcinogenic effect. The NOAEL was 330 ppm, equal to 66 mg/kg bw per day (Watta-Gebert, 1991a,b).
Rats
Groups of 50 male and 50 female Wistar rats [Bor:WISW (SPF-Cpb)] received diets containing imidacloprid (purity, 94.3–95.3%; mixed batch) at a concentration of 0, 100, 300 or 900 ppm for 24 months. In a supplemental study to determine the maximum tolerated dose, groups of 50 male and female Wistar rats were given diets containing imidacloprid at a concentration of 0 or 1800 ppm for 24 months. Ten additional rats of each sex were included per dose in each study for interim sacrifice after 12 months of treatment. The mean intake of imidacloprid was equal to 5.7, 17, 51 and 100 mg/kg bw per day for males and 7.6, 25, 73 and 140 mg/kg bw per day for females. The study was conducted according to GLP.
Water intake was reduced by 13% in females at 1800 ppm. Reduced weight gain was noted in males and females at 900 ppm, the decreases reaching a maximum of 11–12% at 1800 ppm. The absolute weights of the liver and kidney in females and of the liver in males were reduced after 12 months at 900 ppm. Histological assessment of these organs afforded no evidence of treatment-related lesions. An increased incidence of mineralization in the colloid of the thyroid gland follicles, in comparison with the incidences of this lesion in controls in previous studies, was determined in males at concentrations > 300 ppm and in females at 900 ppm. At 1800 ppm, fewer colloid aggregations and parafollicular hyperplasia were observed. There was no evidence of a carcinogenic effect. The NOAEL was 100 ppm, equal to 5.7 mg/kg bw per day (Eiben, 1991; Eiben & Kaliner, 1991).
Imidacloprid was investigated in an adequate range of assays for genotoxicity in vitro and in vivo (Table 2).
Table 2. Studies of the genotoxicity of imidacloprid
|
End-point |
Test object |
Concentration or dose |
Purity |
Result |
Reference |
|
In vitro |
|||||
|
Reverse mutation |
S. typhimurium TA1535, TA100, TA1537, TA98 |
< 12 000 µg/plate |
95.0 |
Negativea |
Herbold (1989a) |
|
Reverse mutation |
S. typhimurium TA1535, TA100, TA1537, TA98 |
< 5000 µg/plate |
96.0 |
Negativea |
Herbold (1991) |
|
Reverse mutation |
S. typhimurium TA1535, TA100, TA1537, TA98 |
< 5000 µg/plate |
96.3 |
Negativea |
Herbold (1991) |
|
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 |
< 5000 µg/plate |
97.4 |
Negativea |
Herbold (1992) |
|
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537; E. coli WP2/uvrA |
< 5000 µg/plate |
93.7 |
Negative |
Watanabe (1991a) |
|
DNA damage |
B. subtilis H17 (rec+), M45 (rec–) |
< 5000 µg/plate |
94.7 |
Negative |
Watanabe (1990a) |
|
Point mutation |
hprt locus of Chinese hamster ovary cells |
< 120, 1200 µg/ml |
95.2 |
Negativea |
Lehn (1989a) |
|
Mitotic recombination |
Saccharomyces cerevisiae D7 |
< 10 000 µg/ml |
95.3 |
Negative |
Herbold (1988a) |
|
Unscheduled DNA synthesis |
Rat primary hepatocytes |
5.0–750 µg/ml |
95.2 |
Negative |
Cifone (1988) |
|
Sister chromatid exchange |
Chinese hamster ovary cells |
< 5000 µg/ml |
95.2 |
Positivea |
Taalman (1988) |
|
Sister chromatid exchange |
Chinese hamster ovary cells |
< 1200 µg/ml |
95.2 |
Negativea |
Putman & Morris |
|
Chromosomal aberrations |
Human lymphocytes |
< 5200 µg/ml |
95.2 |
Positiveb |
Herbold (1989b) |
|
In vivo |
|||||
|
Micronucleus formation |
Chinese hamster bone marrow |
2000 mg/kg bw |
94.6 |
Negative |
Herbold (1989c) |
|
Micronucleus formation |
Mouse |
80 mg/kg bw |
95.3 |
Negative |
Herbold (1988b) |
|
Sister chromatid exchange |
Chinese hamster bone marrow |
500, 1000, 2000 mg/kg bw |
95.0 |
Negative |
Herbold (1989d) |
|
Cytogenetic damage |
Mouse germ cells |
80 mg/kg bw |
94.1 |
Negative |
Völker (1990) |
a
With and without metabolic activationb
With metabolic activationTests for reverse mutation in Salmonella typhimurium and for point mutations at the hprt locus in Chinese hamster ovary cells gave negative results. Weak induction of sister chromatid exchange was found in one test in Chinese hamster ovary cells in vitro but not in vivo. Tests for mitotic recombination, DNA damage in the rec assay and unscheduled DNA synthesis also gave negative results. In human lymphocyte cultures, a slight increase in the chromosomal aberration rate was observed in the range of cytotoxic concentrations in the absence of metabolic activation; no unequivocal findings could be established with metabolic activation. The effect of damaged cellular components on the chromosomes must be considered in assessing this effect. As the results of all tests for chromosomal damage in vivo (micronucleus formation and cytogenetic effects in bone marrow and spermatogonia) were negative, the significance of the clastogenic effect of imidacloprid in human lymphocyte cultures is questionable.
Rats
Groups of Wistar [KfM: Wist] rats received diets containing technical-grade imidacloprid (purity, 94.4–95.3%; mixed batch) at a concentration of 0, 100, 250 or 700 ppm, equivalent to 6.6, 17 and 47 mg/kg bw per day, for 84 days before mating and throughout mating, gestation and lactation of the F1a and F1b litters. The F1 parent animals were selected after weaning of the F1b litters on day 21 post partum. The diets were fed for 105 days before breeding of the F2a and F2b litters. Each group of the F0 parent generation consisted of 30 male and 30 female rats, and each group of the F1 parent generation consisted of 26 males and 26 females. The study was conducted according to GLP.
At 700 ppm, reduced food consumption and depressed body-weight gain were observed in F0 males, reduced food intake in F0 and F1 females and reduced body-weight gain in F0 females. Increased cytochrome P450, O-demethylase and N-demethylase activities were found in males at 700 ppm and increased O-demethylase activities in F1 females at 250 and 700 ppm. Reduced body weights and body-weight gains were observed in all generations (F1a, F1b, F2a and F2b) at 700 ppm during lactation. The NOAEL for parental effects was 100 ppm, equivalent to 6.6 mg/kg bw per day, and the NOAEL for reproductive effects was 250 ppm, equivalent to 17 mg/kg bw per day (Suter et al., 1990).
Rats
Imidacloprid (purity, 94.2%) was administered by gavage to groups of 25 mated female Wistar [KfM: Wist] rats at a dose of 0, 10, 30 or 100 mg/kg bw per day on days 6–15 post coitum. A standard volume of 10 ml/kg bw with daily adjustment to the actual body weight was used. The rats were killed on day 21 post coitum, and the fetuses were removed surgically. The study was conducted according to GLP.
Dose-related reductions in food consumption and body-weight gain were observed in dams at 30 and 100 mg/kg bw per day. Skeletal examination showed a slightly increased incidence of wavy ribs at 100 mg/kg bw per day. The NOAEL for maternal effects was 10 mg/kg bw per day, and the NOAEL for developmental effects was 30 mg/kg bw per day (Becker et al., 1988a).
Rabbits
Imidacloprid (purity, 94.2%) was administered by gavage to groups of 16 female chinchilla [Kfm:CHIN] rabbits at a dose of 0, 8, 24 or 72 mg/kg bw per day on days 6–18 post coitum. A standard volume of 4 ml/kg bw with daily adjustment to the actual body weight was used. The rabbits were killed on day 28 post coitum, and the fetuses were removed surgically. The study was conducted according to GLP.
Decreased food intake and body-weight gain were observed at doses > 24 mg/kg bw per day, and an increased mortality rate was observed at 72 mg/kg bw per day, two females dying on days 18 and 19 post coitum. A further female in this group aborted on day 26 post coitum, and two females showed total resorption at terminal necropsy. The does at 72 mg/kg bw per day had slightly increased post-implantation loss when only does with live fetuses at termination were considered, which increased to a high loss (32.5%) when females with total resorption or abortion were included. The body weights of the fetuses were reduced, and the incidence of fetuses with retarded ossification was increased at 72 mg/kg bw per day. The NOAEL for maternal effects was 8 mg/kg bw per day, and that for developmental effects was 24 mg/kg bw per day (Becker et al., 1988b).
Rats
Imidacloprid (purity, 97.6–98.8%) was administered by gavage to groups of 18 male and 18 female fasted Sprague-Dawley [Sas:CD(SD)BR] rats at a single dose of 0, 42, 150 or 310 mg/kg bw. The test substance was suspended in 0.5% (w/v) methylcellulose with 0.4% (w/v) Tween 80 in deionized water and was administered in a volume of 10 ml/kg bw. In a supplemental study, imidacloprid was administered to 12 female rats by gavage at an analytically confirmed dose of 0 or 20 mg/kg bw. Behavioural tests were administered on day 0 of treatment at the time of the peak plasma concentration.
Four males and 10 females at the highest dose died, either on the day of treatment or the next day. These deaths were attributed to treatment. A dose-related increase in the incidence and severity of clinical signs was seen, with treatment-related effects in males at 150 or 310 mg/kg bw and in females at the highest dose. In males at 150 mg/kg bw, the effects were limited to tremors and nasal staining, while males at the highest dose also had uncoordinated gait, decreased activity and urine staining and were cool to touch. The treatment-related effects in females at the highest dose consisted of tremors, uncoordinated gait, decreased activity, increased reactivity and red nasal staining. Clinical signs of toxicity were generally observed on day 0 and resolved in surviving males and females within 1–5 days after treatment.
Treatment-related effects in a ‘functional observational battery’ were observed in males and females at the two higher doses. These consisted of an increased incidence of recumbency, tremors and nasal staining in males and tremors in females at 150 mg/kg bw and numerous treatment-related effects in males and females at 310 mg/kg bw, consistent with the lethality of this dose within 24 h after treatment. All the toxic effects had resolved in surviving animals by the next observation period, 7 days after treatment. A dose-related decrease in a measure of motor and locomotor activity was observed in both sexes, with reduced activity in males at the two higher doses and in females at all three doses on day 0. As the slightly reduced motor activity in females at 42 mg/kg bw was comparable to that seen before treatment and also 14 days after treatment, this reduction was considered not to be an adverse effect. Habituation was not affected. All clinical signs and neurobehavioural effects showed complete reversal within 7 days of treatment at sublethal doses.
Animals at 150 mg/kg bw showed a decrease in serum triglyceride concentration, and animals that survived the highest dose had decreased serum potassium and cholesterol concentrations (females) and decreased serum alanine aminotransferase activity (males and females). Haematological changes were found in females at the highest dose. The NOAEL was 42 mg/kg bw (Sheets, 1994a).
Groups of 18 male and 18 female Fischer 344 CDF/BR rats were given diets containing imidacloprid (purity, 97.6–98.8%) at a concentration of 0, 140, 960 or 3000 ppm for 13 weeks, equal to 0, 9.3, 63 and 200 mg/kg bw per day for males and 0, 10, 69 and 210 mg/kg bw per day for females. Twelve rats of each sex per dietary level were used for neurobehavioural evaluation and half of them for neuropathological examination, and six rats of each sex per group were used for observation of clinical effects at interim sacrifice.
Body weight and food consumption were reduced by treatment at the two higher concentrations in males and females. In the ‘functional observational battery’, treatment-related effects were seen in males at the highest concentration but not in treated females. Motor activity was not affected in males or females at any concentration. The NOAEL was 140 ppm, equal to 9.3 mg/kg bw per day (Sheets, 1994b).
Several metabolites of imidacloprid were tested for acute toxicity by oral administration to rats and for their ability to induce point mutations in S. typhimurium.
The results of studies on the acute toxicity of imidacloprid metabolites are summarized in Table 3. The methods used complied with OECD guidelines and GLP. The metabolites showed moderate acute toxicity after oral administrations, the clinical signs being mydriasis, abnormal gait, sedation, abnormal respiration, salivation and tremor.
Table 3. Acute toxicity of metabolites of imidacloprid given orally to specific pathogen-free rodents
|
Metabolite |
Species |
Sex |
LD50 |
Purity |
Reference |
|
1-(6-Chloro-3-pyridylmethyl)-2-imidazolidinone |
Rat |
Male |
4080 |
99.9 |
Ohta (1991a) |
|
Female |
1820 |
|
|
||
|
1-(6-Chloro-3-pyridylmethyl)-N-nitro(4-imidazolin-2-ylidene)amine (olefinic metabolite) |
Rat |
Male |
3500 |
98.0 |
Ohta (1991b) |
|
Female |
1100 |
|
|
||
|
1-(6-Chloro-3-pyridylmethyl)-N-nitroso(imidazolidin-2-ylideneamine (nitroso metabolite) |
Rat |
Male |
1980 |
98.1 |
Ohta (1991c) |
|
Female |
3560 |
|
|
||
|
1-(6-Chloro-3-pyridylmethyl)-N-nitroso(imidazolidin-2-ylideneamine (nitroso metabolite) |
Rat |
Male |
> 600 |
Not reported |
Nakazato (1988a) |
|
Female |
> 600 |
|
|
||
|
1-(6-Chloro-3-pyridylmethyl)-N-nitroso(imidazolidin-2-ylideneamine (nitroso metabolite) |
Mouse |
Male |
200 |
Not reported |
Nakazato (1988b) |
|
Female |
200 |
|
|
||
|
1-(6-Chloro-3-pyridylmethyl)imidazolidin-2-ylideneamine |
Rat |
Male |
300 |
87.0 |
Nakazato (1991) |
|
Female |
280 |
|
|
Groups of 15 male and 15 female Wistar rats [Bor:WISW (SPF-Cpb)] received an unlimited supply of drinking-water containing nitroso metabolite at a concentration of 0, 100, 300 or 1000 ppm for 12 weeks, providing mean intakes of 13, 35 and 110 mg/kg bw per day for males and 13, 39 and 120 mg/kg bw per day for females. Water intake was decreased in the groups at 1000 ppm. At 300 ppm, higher lymphocyte counts and lower numbers of polymorphonuclear cells were observed. The NOAEL was 100 ppm, equal to 13 mg/kg bw per day (Krötlinger, 1992).
All tests for genotoxicity with imidacloprid metabolites in vitro and in vivo gave negative results (Table 4).
Table 4. Studies of the genotoxicity of metabolites of imidacloprid
|
Metabolitea |
End-point |
Test object |
Concentration or dose |
Purity |
Result |
Reference |
|
In vitro |
|
|
|
|
|
|
|
Metabolite 1 |
Reverse mutation |
S. typhimurium TA1535, TA100, TA1537, TA98; E. coli WP2uvrA |
< 5000 µg/plate |
99.9 |
Negative |
Watanabe (1991b) |
|
Metabolite 2 |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537; E. coli WP2uvrA |
< 5000 µg/plate |
98.0 |
Negative |
Ohta (1991d) |
|
Metabolite 3 |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537; E. coli WP2uvrA |
< 2500 µg/plate |
87.0 |
Negative |
Watanabe (1991c) |
|
Metabolite 4 |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537; E. coli WP2uvrA |
< 5000 µg/plate |
98.3 |
Negative |
Watanabe (1990b) |
|
Metabolite 4 |
Point mutation |
hprt locus of Chinese hamster ovary cells |
< 2000 µg/ml |
94.3 |
Negativea |
Lehn (1989b) |
|
Metabolite 4 |
Point mutation |
hprt locus of V79 Chinese hamster cells |
< 2000 µg/ml |
98.9 |
Negativea |
Lehn (1989c) |
|
Metabolite 4 |
DNA damage |
B. subtilis H17 (rec+), M45 (rec–) |
< 2000 µg/plate |
98.1 |
Negative |
Watanabe (1991d) |
|
Metabolite 4 |
Unscheduled DNA synthesis |
Rat primary hepatocytes |
5.0–750 µg/ml |
98.9 |
Negative |
Fautz (1989) |
|
Metabolite 4 |
Chromosomal aberrations |
V79 Chinese hamster cells |
< 1000 µg/ml |
98.9 |
Negativea |
Heidemann |
|
Metabolite 4 |
Chromosomal aberrations |
V79 Chinese hamster cells |
1.0–0.25 mg/ml |
NR |
Negativea |
Usami (1988a) |
|
In vivo |
|
|
|
|
|
|
|
Metabolite 4 |
Micronucleus formation |
Male BDF1 mice |
0, 40, 80, 160 mg/kg bw orally |
96.4 |
Negative |
Usami (1988b) |
|
Metabolite 4 |
Micronucleus formation |
Male BDF1 mice |
20, 40, 80 mg/kg bw intraperitoneally |
96.4 |
Negative |
Usami (1988c) |
|
Metabolite 4 |
Micronucleus formation |
Mice |
100 mg/kg bw orally |
98.9 |
Negative |
Herbold (1989e) |
|
Metabolite 4 |
Micronucleus formation |
Mice |
50 mg/kg bw orally |
98.9 |
Negative |
Herbold (1989f) |
NR, not reported
a
Metabolite 1, 1-(6-chloro-3-pyridylmethyl)-2-imidazolidinone; metabolite 2, 1-(6-chloro-3-pyridylmethyl)-N-nitro(4-imidazolin-2-ylidene)amine (olefin metabolite); metabolite 3, 1-(6-chloro-3-pyridylmethyl)imidazolidi-2-ylideneamine; metabolite 4, 1-(6-chloro-3-pyridylmethyl)-N-nitroso(imidazolidin-2-ylidene)amine (nitroso metabolite)Periodic examinations at the medical department of Bayer AG showed no adverse health effects in employees handling imidacloprid during the production of the active ingredient and formulations (Faul, 1996). In experimental biological testing and field tests with imidacloprid formulations, no adverse effects on the health of operators or workers were reported. No epidemiological studies on exposure of the general population to imidacloprid were available.
Mild cases of contact dermatitis have been reported in pet owners after use of a veterinary formulation of imidacloprid (Advantage®). The effect appeared to be due to constituents of the product that are not present in plant protection formulations. No data on symptoms of poisoning or clinical signs were available. A 4-year-old child who ingested four rodlets containing 50 mg of imidacloprid per rodlet, corresponding to about 10 mg/kg bw, showed no signs of poisoning or adverse health effects (Steffens, 2000).
Imidacloprid is rapidly and almost completely absorbed (> 92%) from the gastrointestinal tract of rats, and is eliminated from the organism rapidly and completely, with no indication of bioaccumulation of the parent compound or its metabolites. On average, 75% of an administered dose was excreted in the urine and the remainder in the faeces. Most of that in the faeces originated from biliary excretion. Peak plasma concentrations of radiolabel were reached within approximately 2.5 h. The radiolabel was rapidly distributed from the intravascular space to the peripheral tissues and organs; the concentrations in tissues after 48 h were very low. Imidacloprid penetrated the blood–brain barrier to only a very limited extent.
The metabolism of imidacloprid in rats was rapid, and the amount of unchanged parent compound represented 10–16% of a given dose. The main urinary metabolites were 6-chloronicotinic acid and its glycine conjugate and the two corresponding imidazolidine ring-containing biotransformation products. The two monohydroxylated metabolites (5-hydroxyimidacloprid) and (4-hydroxyimidacloprid) and an unsaturated compound were also detected in urine.
Imidacloprid given orally as a single dose was moderately toxic to rats (LD50, 380–650 mg/kg bw) and mice (LD50, 130–170 mg/kg bw). Behavioural and respiratory signs, disturbances of motility, narrowed palpebral fissures, transient trembling and spasms were seen in rats and mice treated orally at doses > 200 mg/kg bw and > 71 mg/kg bw, respectively. The clinical signs were reversed within 6 days. Imidacloprid given intraperitoneally showed moderate to low acute toxicity in rats, the signs being similar to those after oral administration. Very little toxicity was seen after acute dermal application. The LC50 for acute exposure to an aerosol could not be determined exactly, as rats tolerated inhalation for 4 h of the maximum concentration of dust that could be produced technically (0.069 mg/l of air) without signs or deaths. Imidacloprid did not irritate the skin or eyes of rabbits and did not sensitize the skin of guinea-pigs in a maximization test.
Reduced body-weight gain was the most sensitive toxicological end-point in mice at doses > 86 mg/kg bw per day, in rats at doses > 30 mg/kg bw per day, in rabbits at doses > 24 mg/kg bw per day and in dogs at doses > 22 mg/kg bw per day. Furthermore, decreased body-weight gain was the main effect during lactation in rat pups of dams given doses > 6.6 mg/kg bw per day. In some studies, the lower body weights were accompanied by a simultaneous increase in feed intake.
The liver was the other main target organ after repeated administration of imidacloprid to mice, rats and dogs at doses > 410 mg/kg bw per day, > 17 mg/kg bw per day and > 31 mg/kg bw per day, respectively. The spectrum of changes observed ranged from induction of hepatic microsomal enzymes through disturbances of hepatic function to histologically apparent damage to the organ. The initial sign of an effect on the liver was increased activity of cytochrome P450 enzymes, accompanied by slight hepatocellular hypertrophy and necrosis, swollen cell nuclei, round-cell infiltration and increased liver weight. Changes in blood cholesterol, triglyceride, protein and albumin concentrations as well as increased activities of alanine aminotransferase, alkaline phosphatase and galactodehydrogenase in plasma were also observed.
Slight follicular atrophy of the thyroid gland and slightly depressed triiodothyronine in plasma were found at the highest dietary concentration, providing an average intake of 49 mg/kg bw per day, in a short-term study in dogs. However, these effects were not found in the 1-year study in dogs given a dietary concentration providing an average intake of 72 mg/kg bw per day. An increased incidence of mineralization was seen in the colloid of thyroid gland follicles in a long-term study in rats given a dietary concentration equal to 17 mg/kg bw per day, although the plasma concentrations of thyroid hormones remained unchanged. The NOAEL in this study was 5.7 mg/kg bw per day.
No evidence of a carcinogenic effect of imidacloprid was found in either mice or rats in the long-term studies of dietary administration.
Imidacloprid gave negative results in an adequate range of assays for genotoxicity in vitro and in vivo. Weak induction of sister chromatid exchange was found in one test with Chinese hamster ovary cells in vitro, but not in vivo.
The Meeting concluded that imidacloprid is unlikely to be genotoxic or to pose a carcinogenic risk to humans.
Imidacloprid was investigated for reproductive toxicity in a two-generation study in rats and in studies of developmental toxicity in rats and rabbits. Reproductive behaviour and outcome were not affected. An increased incidence of wavy ribs was observed at a maternally toxic dose of 30 mg/kg bw per day. Reduced body weights and retarded ossification were found in rabbit fetuses at a maternally toxic dose of 24 mg/kg bw per day. The Meeting concluded that imidacloprid has no teratogenic potential.
Several metabolites were tested for acute toxicity by oral administration to rats and for their ability to induce point mutations in Salmonella typhimurium. They were found to be less acutely toxic than the parent compound, and no indications of genotoxic potential were found. In a 12-week study in rats in which a nitroso metabolite was administered in drinking-water, the NOAEL was 100 ppm, equal to 13 mg/kg bw per day, on the basis of increased lymphocyte counts and reduced polymorphonuclear cell counts.
In a study of acute neurotoxicity in rats, clinical signs and effects on motor and locomotor activity and in a ‘functional observational battery’ were observed at doses > 150 mg/kg bw 1 day after application. Complete recovery was observed within 7 days. The NOAEL was 42 mg/kg bw. In a 13-week study of neurotoxicity in rats, the NOAEL of 140 ppm, equal to 9.3 mg/kg bw per day, was based on reduced body-weight gain and food consumption at doses > 960 ppm, equal to 63 mg/kg bw per day. Behavioural effects were observed only in the ‘functional observational battery’ in males at 3000 ppm, equal to 200 mg/kg bw per day.
The results of periodic examinations of employees exposed to imidacloprid showed no adverse health effects. No epidemiological studies of the effects of imidacloprid and no information on symptoms of poisoning or clinical signs were available. A 4-year-old child who ingested about 10 mg/kg bw of a veterinary preparation of imidacloprid showed no signs of poisoning or adverse health effects.
The Meeting concluded that the existing database was adequate to characterize the potential hazards of imidacloprid to fetuses, infants and children.
The Meeting established an ADI of 0–0.06 mg/kg bw on the basis of the NOAEL for effects on the thyroid gland of 100 ppm, equal to 5.7 mg/kg bw per day, in the long-term study of toxicity and carcinogenicity in rats and a safety factor of 100. This ADI is supported by the NOAEL for effects on the liver in parental animals in the multigeneration study of reproductive toxicity.
The Meeting established an acute reference dose of 0.4 mg/kg bw on the basis of the NOAEL of 42 mg/kg bw in the study of acute neurotoxicity in rats and a safety factor of 100.
Levels relevant to risk assessment
|
Species |
Study |
Effect |
NOAEL |
LOAEL |
|
Mouse |
2-year studies of toxicity and carcinogenicitya |
Toxicity |
330 ppm, equal to 66 mg/kg bw per day |
1000 ppm, equal to 210 mg/kg bw per day |
|
Carcinogenicity |
1000 ppm, equal to 210 mg/kg bw per day b |
– |
||
|
Rat |
2-year studies of toxicity and carcinogenicitya |
Toxicity |
100 ppm, equal to 5.7 mg/kg bw per day |
300 ppm, equal to 17 mg/kg bw per day |
|
Carcinogenicity |
300 ppm, equal to 100 mg/kg bw per dayb |
– |
||
|
Two-generation study of reproductive toxicitya |
Parental toxicity |
100 ppm, equivalent to 6.6 mg/kg bw per day |
250 ppm, equivalent to 17 mg/kg bw per day |
|
|
Offspring toxicity |
250 ppm, equivalent to 17 mg/kg bw per day |
700 ppm, equivalent to 47 mg/kg bw per day |
||
|
Developmental toxicityc |
Maternal toxicity |
10 mg/kg bw per day |
30 mg/kg bw per day |
|
|
Embryo- and feto-toxicity |
30 mg/kg bw per day |
100 mg/kg bw per day |
||
|
Acute neurotoxicityb,c |
42 mg/kg bw per day |
150 mg/kg bw per day |
||
|
Rabbit |
Developmental toxicityc |
Maternal toxicity |
8 mg/kg bw per day |
24 mg/kg bw per day |
|
Embryo- and feto-toxicity |
24 mg/kg bw per day |
72 mg/kg bw per day |
||
|
Dog |
13- and 52-week studies of toxicitya,d |
|
500 ppm, equal to 15 mg/kg bw per day (52-week study) |
600 ppm, equivalent to22 mg/kg bw per day (13-week study) |
a
Dietb
Highest dose testedc
Gavaged
Two or more studies combinedEstimate of acceptable daily intake for humans
0–0.06 mg/kg bw
Estimate of acute reference dose
0.4 mg/kg bw
Studies that would provide information useful for continued evaluation of the compound
Further observations in humans
List of end-points relevant for setting guidance values for dietary and non-dietary exposure
|
Absorption, distribution, excretion and metabolism in mammals |
|
|
Rate and extent of oral absorption |
Extensively absorbed (95%) on basis of urinary and biliary excretion |
|
Distribution |
Uniformly and rapidly distributed, highest residues (after 48 h) in liver, kidney, lung, and skin |
|
Potential for accumulation |
No evidence of accumulation |
|
Rate and extent of excretion |
Rapidly and completely (~25% in faeces, 75% in urine within 48 h) |
|
Metabolism in animals |
Extensively metabolized by oxidative cleavage, hydroxylation, conjugation |
|
Toxicologically significant compounds (animals, plants, and environment) |
Parent compound and metabolites, including plant nitroso metabolite, 1-(6-chloro-3-pyridylmethyl)-N-nitroso(imidazolidin-2-ylidene)amine |
|
Acute toxicity |
|
|
Rat, LD50, oral |
380-650 mg/kg bw |
|
Rabbit, LD50, dermal |
> 5000 mg/kg bw |
|
Rat, LC50, inhalation |
> 0.69 mg/l of air (4 h, nose only) |
|
Skin irritation |
Not irritating |
|
Eye irritation |
Not irritating |
|
Skin sensitization |
Not sensitizing (Magnussen and Kligman) |
|
Short-term toxicity |
|
|
Target / critical effect |
Decreased body-weight gain, liver; thyroid |
|
Lowest relevant oral NOAEL / NOEL |
52 weeks, dog: 500 ppm (15 mg/kg bw per day) |
|
Genotoxicity |
No potential for genotoxicity |
|
Long-term toxicity and carcinogenicity |
|
|
Target/critical effect |
Decreased body-weight gain, liver; thyroid |
|
Lowest relevant NOAEL / NOEL |
2 years, rat: 100 ppm (5.7 mg/kg bw per day) |
|
Carcinogenicity |
No potential for carcinogenicity |
|
Reproductive toxicity |
|
|
Reproduction target / critical effect |
Decreased body-weight gain in pups during lactation at parentally toxic doses |
|
Lowest relevant reproductive NOAEL |
250 ppm (17 mg/kg bw per day) |
|
Developmental target / critical effect |
Reduced body weight, increased incidence of retarded ossification at maternally toxic doses |
|
Lowest relevant developmental NOAEL |
Rabbit: 24 mg/kg bw per day |
|
Neurotoxicity |
Clinical signs and neurobehavioural effects ascribed to acute cholinergic toxicity; short-term effects related to general toxicity |
|
NOAEL (acute neurotoxicity) |
42 mg/kg bw |
|
NOAEL (short-term study of neurotoxicity) |
140 ppm (9.3 mg/kg bw per day) |
|
Other toxicological studies |
Studies of kinetics and metabolism, acute toxicity, short-term effects, and mutagenicity of the plant nitroso-metabolite revealed no evidence of particular risk |
|
Human data |
No evidence of adverse effects |
|
Summary |
Value |
Study |
Safety factor |
|
ADI |
0–0.06 mg/kg bw |
Rat, 2 years |
100 |
|
Acute reference dose |
0.4 mg/kg bw |
Rat, acute neurotoxicity |
100 |
Allen, T.R., Frei, T., Luetkemeier, H., Vogel, O., Biedermann, K. & Wilson, J. (1989) 52-week oral toxicity (feeding) study with NTN 33893 technical in the dog. Unpublished report from Research & Consulting Company AG, report No. R 4856, dated 19 October 1989, GLP, amendment No. R 4856A, dated 3 March 1992. Submitted to WHO by Bayer AG, Mannheim, Germany.
Becker, H., Vogel, W. & Terrier, C. (1988a) Embryotoxicity study (including teratogenicity) with NTN 33893 technical in the rabbit. Unpublished report from Research & Consulting Company AG, report No. R 4583, dated 24 November 1988, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Becker, H., Vogel, W. & Terrier, C. (1988b) Embryotoxicity study (including teratogenicity) with NTN 33893 technical in the rat. Unpublished report from Research & Consulting Company AG, report No. R 4582, dated 24 November 1988, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Bloch, I., Frei, T., Luetkemeiner, H., Vogel, W. & Wilson, J. (1987) 28-day oral range-finding toxicity (feeding) study with NTN 33893 tech. in the dog. Unpublished report from Research & Consulting Company AG, report No. R 4196, dated 9 October 1987, non-GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Bomann, W. (1989a) NTN 33893—Study for acute oral toxicity to rats. Unpublished report from Bayer AG, report No. 18594, dated 15 December 1989, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Bomann, W. (1989b) NTN 33893—Study for acute oral toxicity to mice. Unpublished report from Bayer AG, report No. 18593, dated 15 December 1989, GLP, unpublished. Submitted to WHO by Bayer AG, Mannheim, Germany.
Bomann, W. (1991a) NTN 33893 AMP (proposed c.n. imidacloprid)—Study for acute oral toxicity to rats. Unpublished report from Bayer AG, report No. 20591, dated 3 September 1991, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Bomann, W. (1991b) NTN 33893 CNS (c.n. imidacloprid (proposed))—Study for acute oral toxicity in rats. Unpublished report from Bayer AG, report No. 20637, dated 20 September 1991, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Casida, J.E. (1998) Cholinergic insecticide toxicology. Crisp Data Base, National Institutes of Health, National Institute of Environmental Health Sciences, Washongton DC, USA. Submitted to WHO by Bayer AG, Mannheim, Germany.
Chao, S.L. & Casida, J.E. (1997) Interaction of imidacloprid metabolites and analogs with the nicotinic acetylcholine receptor of mouse brain in relation to toxicity. Pest. Biochem. Physiol., 58, 77–88.
Cifone, M.A. (1988) Mutagenicity test on NTN 33893 in the rat primary hepatocyte unscheduled DNA synthesis assay. Unpublished report from Bayer AG, report No. R 4631, dated 21 December 1988, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Eiben, R. (1988a) NTN 33893—Pilot range finding study for cancerogenesis study on B6C3F1 mice (one hundred seven day feeding study). Unpublished report from Bayer AG, report No. 17280, dated 24 October 1988, non-GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Eiben, R. (1988b) NTN 33893—Pilot range finding study for a chronic toxicity study on Wistar rats (ninety-eight day feeding study). Unpublished report from Bayer AG, report No. 17279, dated 24 October 1988, non-GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Eiben, R. (1991) NTN 33893 (proposed common name: imidacloprid)—Chronic toxicity and cancerogenicity studies on Wistar rats (administration in food over 24 months)—Supplementary MTD study for two-year study T 1023699). Unpublished report from Bayer AG, report No. 20541, dated 19 August 1991, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Eiben, R. & Kaliner, G. (1991) NTN 33893 (proposed common name: imidacloprid)—Chronic toxicity and cancerogenicity studies on Wistar rats (administration in food over 24 months). Unpublished report from Bayer AG, report No. 19925, dated 25 January 1991, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Eiben, R. & Rinke, M. (1989) NTN 33893: Subchronic toxicity study on Wistar-rats (administration in the feed for 96 days). Unpublished report from Bayer AG, report No. 18187, dated 14 July 1989. Submitted to WHO by Bayer AG, Mannheim, Germany.
Faul, J. (1996) NTN 33893—Occupational medical experience. Unpublished report from Bayer AG, Medical Department, dated March 1996. Submitted to WHO by Bayer AG, Mannheim, Germany.
Fautz, R. (1989) Unscheduled DNA synthesis in primary hepatocytes of male rats in vitro with WAK 3839. Unpublished report from Research & Consulting Company AG, report No. R 4746, dated 24 April 1989, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Flucke, W. (1990) NTN 33893 techn.—Study for subacute dermal toxicity in the rabbit. Unpublished report from Bayer AG, report No. 19152, dated 11 June 1990, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Heidemann, A. (1989) Chromosome aberration assay in Chinese hamster V79 cells in vitro with WAK 3839. Unpublished report from Research & Consulting Company AG, report No. R 4849, dated 27 September 1989, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Herbold, B. (1988a) NTN 33893—Test on S. cerevisiae D7 to evaluate for induction of mitotic recombination. Unpublished report from Bayer AG, report No. 16832, dated 27 June 1988, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Herbold, B. (1988b) NTN 33893—Micronucleus test on the mouse to evaluate for clastogenic effect. Unpublished report from Bayer AG, report No. 16837, dated 27 June 1988, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Herbold, B. (1989a) NTN 33893—Salmonella/microsome test to evaluate for point mutagenic effect. Unpublished report from Bayer AG, report No. 17577, dated 6 January 1989, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Herbold, B. (1989b) NTN 33893—In vitro cytogenetic study with human lymphocytes for the detection of induced clastogenic effects. Unpublished report from Bayer AG, report No. 18092, dated 16 June 1989, GLP, addendum report No. 18092A, dated 24 August 1989. Submitted to WHO by Bayer AG, Mannheim, Germany.
Herbold, B. (1989c) NTN 33893—In vivo cytogenetic study of the bone marrow in Chinese hamster to evaluate for induced clastogenic effects. Unpublished report from Bayer AG, report No. 18557, dated 24 November 1989, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Herbold, B. (1989d) NTN 33893—Sister chromatid exchange in bone marrow of Chinese hamster in vivo. Unpublished report from Bayer AG, report No. 18093, dated 16 June 1989, GLP, addendum report No. 18093A, dated 23 November 1993. Submitted to WHO by Bayer AG, Mannheim, Germany.
Herbold, B. (1989e) WAK 3839—Micronucleus test on the mouse after oral application. Unpublished report from Bayer AG, report No. 18406, dated 3 October 1989, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Herbold, B. (1989f) WAK 3839 or NTN 37571—Micronucleus test on the mouse after intraperitoneal application. Bayer AG, report No. 18407, dated 3 October 1989, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Herbold, B. (1991) NTN 33893 AMP—Salmonella/microsome test. Unpublished report from Bayer AG, report No. 20090, dated 22 March 1991, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Herbold, B. (1992) NTN 33893 AMP W—Salmonella/microsome test. Unpublished report from Bayer AG, report No. 21775, dated 19 October 1992, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Karl, W. & Klein, O. (1992) [Pyridinyl-14C-methyl] imidacloprid: Distribution of the metabolites in some organs at different times following single oral administration to rats. Unpublished report from Bayer AG, report No. PF 3635, dated 12 March 1992, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Klein, O. (1987a) [14C]-NTN 33893: Biokinetic part of the ‘General metabolism study’ in the rat. Unpublished report from Bayer AG, report No. PF2889, dated 9 November 1987, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Klein, O. (1987b) [14C]-NTN 33893: Investigations on the distribution of the total radioactivity in the rat by whole body autoradiography. Unpublished report from Bayer AG, report No. PF2891, dated 12 October 1987, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Klein, O. (1990) Imidacloprid—WAK 3839: Comparison of biokinetic behaviour and metabolism in the rat following single oral dosage and investigation of the metabolism after chronic feeding of imidacloprid to rats and mice. Unpublished report from Bayer AG, report No. PF3432, dated 17 July 1990, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Klein, O. & Brauner A (1991) [Imidazolidine-4,5-14C] imidacloprid: Investigation of the biokinetic behaviour and metabolism in the rat. Unpublished report from Bayer AG, report No. PF3629, dated 11 January 1991, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Klein, O. & Karl, W. (1990) Methylene [14C] imidacloprid: Metabolism part of the general metabolism study in the rat. Unpublished report from Bayer AG, report No. PF 3316, dated 30 January 1990, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Krötlinger, F. (1989) NTN 33893 (c.n. imidacloprid (proposed))—Study for acute dermal toxicity to rats. Unpublished report from Bayer AG, report No. 18532, dated 15 November 1989, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Krötlinger, F. (1990) NTN 33893 (c.n. imidacloprid [proposed]—Study for acute intraperitoneal toxicity in rats. Unpublished report from Bayer AG, report No. 19245, dated 19 July 1990, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Krötlinger, F. (1992) WAK 3839—Subchronic toxicological study on rats (twelve week administration in drinking water). Unpublished report from Bayer AG, report No. 21140, dated 2 March 1992, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Lehn, H. (1989a) NTN 33893—Mutagenicity study for the detection of induced forward mutations in the CHO-HGPRT assay in vitro. Unpublished report from Bayer AG, report No. 17578, dated 6 January 1989, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Lehn, H. (1989b) WAK 3839—Mutagenicity study for the detection of induced forward mutations in the CHO-HGPRT assay in vitro. Unpublished report from Bayer AG, report No. 17757, dated 22 February 1989, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Lehn, H. (1989c) WAK 3839—Mutagenicity study for the detection of induced forward mutations in the V79-HGPRT assay in vitro. Unpublished report from Bayer AG, report No. 18281, dated 15 August 1989, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Nagata, K., Song, J.H., Shono, T. & Narahashi, T. (1998) Modulation of the neuronal nicotinic acetylcholine receptor-channel by the nitromethylene heterocycle imidacloprid. J. Pharmacol. Exp. Ther., 285, 731–738.
Nakazato, Y. (1988a) NTN 37571—Oral acute toxicity study on rats. Unpublished report from Nihon Tokushu Noyaku Seizo report No. RS89007, dated 19 January 1988, non-GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Nakazato, Y. (1988b) NTN 37571—Acute toxicity study on mice. Unpublished report from Nihon Tokushu Noyaku Seizo report No. RS88038, dated 19 October 1988, non-GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Nakazato, Y. (1991) NTN 38014—Acute oral toxicity study on rats. Unpublished report from Nihon Tokushu Noyaku Seizo Report No. RA91018, dated 18 March 1991, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Ohta, K. (1988) NTN 33893 technical—Study for skin sensitising effects on guinea pigs (maximisation test). Unpublished report from Bayer AG, report No. 16533, dated 15 March 1988, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Ohta, K. (1991a) NTN 33519—Acute oral toxicity study on rats. Unpublished report from Nihon Bayer Agrochem K.K. report No. RA91023, dated 31 May 1991, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Ohta, K. (1991b) NTN 35884—Acute oral toxicity study on rats. Unpublished report from Nihon Tokushu Noyaku Seizo report No. RA91039, dated 29 November 1991, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Ohta, K. (1991c) WAK 3839—Acute oral toxicity study on rats. Unpublished report from Nihon Tokushu Noyaku Seizo report No. RA91017, dated 11 March 1991, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Ohta, K. (1991d) NTN 35884—Reverse mutation assay (Salmonella typhimurium and Escherichia coli). Unpublished report from Nihon Tokushu Noyaku Seizo report No. RA91040, dated 29 November 1991, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Pauluhn, J. (1988a) NTN 33893—Study for acute inhalation toxicity in the rat in accordance with OECD Guideline No. 403. Unpublished report from Bayer AG, report No. 16777, dated 6 June1988, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Pauluhn, J. (1988b) NTN 33893—Study for irritant/corrosive potential on the skin (rabbit) according to OECD Guideline No. 404. Unpublished report from Bayer AG, report No. 16455, dated 25 February 1988, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Pauluhn, J. (1988c) NTN 33893—Study for irritant/corrosive potential on the eye (rabbit) according to OECD Guideline No. 405. Unpublished report from Bayer AG, report No. 16456, dated 25 February 1988, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Pauluhn, J. (1988d) NTN 33893—Subacute inhalation toxicity study on the rat according to OECD Guideline No. 412. Unpublished report from Bayer AG, report No. 18199, dated 18 July 1988. GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Putman, D.L. & Morris, M.J. (1989) Sister chromatid exchange assay in Chinese hamster ovary cells. Unpublished report from Microbiological Associates Inc., report No. 1149, dated 12 September 1989, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Roe, R.L., Hodgson, E. & Roe, R.M. (1999) Pesticides In: Marquardt, H., Schäfer, S.G., McCellan,.R.O. & Welsch, F., eds, Toxicology, San Diego: Academic Press, pp. 663–697.
Ruf, J. (1990) NTN 33893 technical—Subchronic toxicity study on dogs in oral administration (13-week feeding study). Unpublished report from Bayer AG, report No. 18732, dated 2 February 1990, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Sheets, L.P. (1994a) An acute oral neurotoxicity screening study with technical grade imidacloprid (NTN 33893) in rats. Unpublished report from Miles Inc. report No. MOB7221, dated 16 February 1994, GLP, supplement report No. MOB7221, dated 7 June 1994. Submitted to WHO by Bayer AG, Mannheim, Germany.
Sheets, L.P. (1994b) A subchronic dietary neurotoxicity screening study with technical grade imidacloprid (NTN 33893) in Fischer 344 rats. Unpublished report from Miles Inc., report No. MOB7331, dated 13 June 1994, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Sone, S., Nagata, K., Tsuboi, S. & Shono, T. (1994) Toxic symptoms and neural effect of a new class of insecticide, imidacloprid, on the American cockroach, Periplaneta americana (L.). J. Pest. Sci., 19, 69–72.
Steffens, W. (2000) NTN 33983—Personal communication from Bayer AG, Medical Department, dated 26 October 2000. Submitted to WHO by Bayer AG, Mannheim, Germany.
Suter, P., Vogel, W., Wilson, T. & Terrier, C. (1990) Multiple generation reproduction study with NTN 33893 technical in rats. Unpublished report from Research & Consulting Company AG, report No. R 5097, dated 21 June 1990, GLP, amendment report No. R 5097A, dated 21 November 1990, amendment report No. R 5097B, dated 3 March 1992. Submitted to WHO by Bayer AG, Mannheim, Germany.
Taalmann, R.D.F.M. (1988) Clastogenic evaluation of NTN 33893 in an in vitro cytogenetic assay measuring sister chromatid exchange in Chinese hamster ovary (CHO) cells. Unpublished report from Hazleton Biotechnologies, report No. R 4407, dated 21 April 1988, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Usami, M. (1988a) NTN 37571—In vitro cytogenetic assay measuring chromosome aberrations in CHO-K1 cells—A pilot study. Unpublished report from Nihon Tokushu Noyaku Seizo, report No. RP88008, dated 5 November 1988, non-GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Usami, M. (1988b) NTN 37571—Micronucleus test on the mice after oral treatment—Pilot study. Unpublished report from Nihon Tokushu Noyaku Seizo, report No. RS88040, dated 29 November 1988, non-GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Usami, M. (1988c) NTN 37571—Micronucleus test on the mice after oral treatment—Pilot study. Unpublished report from Nihon Tokushu Noyaku Seizo, report No. RS88041, dated 29 November 1988, non-GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Völker, W. (1990) Mouse germ-cell cytogenetic assay with NTN 33893. Unpublished report from Cytotest Cell Research GmbH, report No. R 5063, dated 22 May 1990, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Watanabe, M. (1990a) NTN 33893—Rec-assay with spores in the bacterial system. Unpublished report from Nihon Bayer report No. RA90016, dated 18 June 1990, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Watanabe, M. (1990b) WAK 3839—Reverse mutation assay (Salmonella typhimurium and Escherichia coli). Unpublished report from Nihon Tokushu Noyaku Seizo report No. RA90035, dated 26 November 1990, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Watanabe, M. (1991a) NTN 33893—Reverse mutation assay (Salmonella thyphimurum and Escherichia coli). Unpublished report from Nihon Bayer report No. RA91002, dated 17 January 1991, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Watanabe, M. (1991b) NTN 33519—Reverse mutation assay (Salmonella typhimurium and Escherichia coli). Unpublished report from Nihon Bayer Agrochem K.K. report No. RA91024, dated 22 July 1991, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Watanabe, M. (1991c) WAK 3839—Rec-assay with spores in the bacterial system. Unpublished report from Nihon Tokushu Noyaku Seizo report No. RA91015, dated 01 March 1991, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Watanabe, M. (1991d) NTN 38014—Reverse mutation assay (Salmonella typhimurium and Escherichia coli). Unpublished report from Nihon Tokushu Noyaku Seizo report No. RA91019, dated 29 March 1991 GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Watta-Gebert, B. (1991a) NTN 33893 (proposed common name imidacloprid)—Carcinogenicity study on B6C3F1 mice (administration in the food for 24 months). Unpublished report from Bayer AG, report No. 19931, dated 28 January 1991, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
Watta-Gebert, B. (1991b) NTN 33893 (proposed common name: imidacloprid)—Carcinogenicity study in B6C3F1 mice (supplementary MTD testing for Study 5025710 with administration in diet over a 24-month period). Unpublished report from Bayer AG, report No. 20769, dated 24 October 1991, GLP. Submitted to WHO by Bayer AG, Mannheim, Germany.
See Also:
Toxicological Abbreviations
Imidacloprid (ICSC)