
IMAZALIL JMPR 1977
IDENTITY
Imazalil is a proposed common name of the International Organization
for Standardization (ISO) and a British Standard recommended common
name.
Chemical name
1-(ß-allyloxy-2,4-dichlorophenthyl)imidazole
1-[2-(2,4-dichlorophenyl)-2-(2-propenyloxy)-ethyl] -1H-imidazole
Synonyms
Florasan R (R), Fungaflor (R), R 23979 (imazalil), R 18531
(imazalil nitrate), R 27180 (imazalil sulphate)
Structural formula
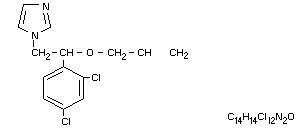 Other information on identity and properties
a) Composition of technical imazalil
The technical material as manufactured contains approximately 97.5-98%
(w/w) imazalil. Detailed information on the impurities in technical
imazalil was supplied to the Joint Meeting (Pattyn, 1976).
b) Physical and chemical properties of imazalil
Physical state: Pure imazalil is a hygroscopic, nearly colourless
liquid. Technical imazalil is a slightly yellowish to brownish oily
liquid.
Molecular weight: 297.18
Freezing point: -10°C - -15°C
Boiling point: -130°C. at 10-3mm Hg
Vapour pressure: 7 × 10-8mm Hg at 20°C; 4 × 10-5mm Hg at 70°C
Refractive index: n 20 1.5643
D
Specific gravity: d23 1.2429
Solubility of imazalil and its salts (g product / 100 ml solvent at
20°C)
solvent imazalil imazalil imazalil
(base) nitrate sulphate
water 0.14 2.38 >50
methanol >50 >100 >50
ethanol >50 31.18 36.3
2-propanol >50 4.57 4.42
exylene >50 0.49 <0.005
toluene >50 0.87 <0.005
n. heptane. 5.41 <0.001 <0.005
benzene >50 <0.005
4-methyl-2-pentanone >50 1.76 0.03
hexane 5.21 <0.005 <0.005
petroleum ether 6.16 <0.005 <0.005
Stability: Imazalil is thermally stable up to approximately 285°C
(Helsen, 1976). It is chemically stable at room
temperature in the absence of light. Decomposition
occurs at elevated temperatures (80°C) and under the
influence of light. The shelf life is more than 2
years. In aqueous solution the product is stable up to
40°C at pHs ranging from 2.4 to 7.0 for at least 8
weeks (Anon., 1975a). When refluxed for 72 hours the
compound remains stable in water and in 1 N sodium
hydroxide, but decomposes in 1 N hydrochloric acid.
c) Physical and chemical properties of imazalil nitrate and sulphate
Physical state: Imazalil nitrate is a slightly beige to brownish
coloured amorphous, non-hygroscopic powder.
Imazalil sulphate is a white to beige crystalline, hygroscopic powder.
Melting range: Imazalil nitrate = 80 - 90°C
Imazalil sulphate 118 - 130°C
Stability: Imazalil nitrate and imazalil sulphate are thermally
stable up to approximately 150°C and 270°C,
respectively.
d) Formulations
Imazalil is used as 20% (w/v) emulsifiable concentrate, 50% (w/w)
wettable powder, and as a seed dressing (also used in combination with
guazatine).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Imazalil in rapidly absorbed, distributed, metabolized and excreted by
rats. Two groups of five male and two groups of five female rats were
given a single 20 mg/kg oral dose of 3H-labelled imazalil sulphate.
Almost 90% of the administered radioactivity was excreted within 96
hours, with approximately equal quantities detected in the urine and
faeces. Tissue residues ranged from 5.4 to 6.1% of the administered
radioactivity 48 hours after dosing and from 1.8 to 3.5% 96 hours
after dosing. The highest levels of radioactivity were found in the
liver, lung and kidneys. Analysis of the urine from dosed animals
indicated that 4% of the tritium was volatile by lyophilization. Thus
tritium exchange apparently occurred to a minor extent. (Heykants at
al., 1975).
Five male rats were given a single oral 20 mg/kg dose of 3H-labelled
imazalil sulphate to study the absorption, distribution, metabolism
and excretion of imazalil in the rat. Approximately 90% of the
administered radioactivity was excreted within 96 hours with
comparable quantities excreted in the urine and faeces. Similar
results were found when a single oral dose of
alpha-(2,4-diochlorophenyl)-1H-imidazole-1-ethanol (a metabolite of
imazalil) was administered. In both these studies, up to approximately
2% of the administered radioactivity was identified as tritiated water
indicating exchange of the tritium label. The relative amount of
tritiated water found in the urine increased with time (Meuldermans et
al., 1977b).
Biotransformation
Studies involving a single 20 mg/kg oral dosing of rats with
3H-labelled imazalil sulphate indicate that extensive metabolism of
imazalil occurs in the rat. In one study,
alpha-(2,4-dichlorophenyl)-1H-imidazole-1-ethanol and
2,4-dichloromandolic acid were identified as the major metabolites in
the urine. In this study 10%, of the radioactivity in the urine and 3%
of the radioactivity in the faeces was identified as unchanged
imazalil (Heykants et al., 1975).
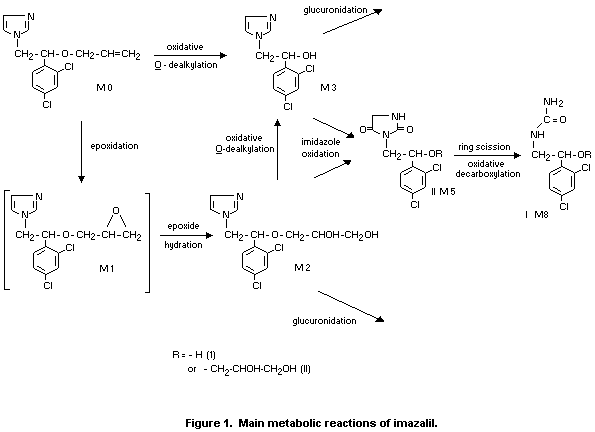
In a more extensive metabolic study, the major metabolites identified
are shown in Figure 1. Several metabolites detected are shown. The
compound shown in brackets in Figure 1 were not identified in the
studies. In contrast to the study described above, no unchanged
imazalil was detected in the urine and only 0.1% of the administered
radioactivity was identified as unchanged imazalil in the faeces.
(Meuldermans al., 1977b).
Tritium labelled imazalil sulphate was incubated with rat liver
homogenates to study the in vitro metabolism of imazalil. The two
metabolites identified were
-(2,4-dichlorophenyl-1-1H-imidazole-1-ethanol and
1- [-(2,4-dichlorophenyl-2-(2,3-dihydroxypropyloxy)ethyl] -1H
-imidazole (Meuldermans et al., 1977a).
Experiments on the biotransformation of imazalil in plants are
described in the section "Fate of residues".
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Groups of male (6/group) and female (10/group) mice were administered
a single dose of imazalil in arachis oil, by gavage, at levels of 10,
40 or 160 mg/kg, to determine dominant lethal effects. Positive
controls received 210 mg/kg cyclophosphamide. Negative controls
received only the oil at the same volume 0.1 ml/10g body weight, as
all other groups. Following dosing each male was mated with 4
untreated females at weekly intervals, for eight consecutive weeks.
The females were caged with single untreated males for a maximum of 5
days. This was repeated for 5 consecutive weeks. No significant
dose-related differences were observed in untreated females and the
negative controls nor in the treated females when the number of dead
and live implants were expressed as a percentage of corpora lutea vs.
total implants (Marsboom, 1976a, 1976b).
Special studies on reproduction
Three separate reproduction studies were carried out using differing
dosing periods. In all three the dietary doses based on active
ingredient were 0, 5, 20 or 80 mg/kg.
In the first study groups of 20 female rats received imazalil on a
non-continuous basis for 3 generations. Prior to and after the period
of organogenesis and throughout lactation the females of the first and
second generations received only the basal laboratory diet. In each of
these generations, the dams received the various levels from day 6
through day 15 of pregnancy. In the third generation, the dams
received the various levels from 3 weeks until 3 months of age and
further from day 1 to 21 after mating. There was a trend toward a
lower number of live births at the 80 mg/kg level in all generations.
No other effects were noted at any other level. No abnormalities were
noted in any foetuses in any generation which were not within the
normal range for the rats used (Marsboom, 19750).
In the second study, rats (20/Sex/group) received the various levels
of imazalil as follows: males for 60 days prior to pairing with
females and through copulation; females for 14 days prior to pairing
all throughout pregnancy. Pregnancy rate was not effected at any level
regardless of treatment of either sex. Test group data for all other
measured or observed parameters were comparable to the control data
(Marsboom, 1977a).
In the third study 20 females/group received the various levels of
imazalil from day 16 of pregnancy through a 3 week lactation period.
No differences in percentage of pregnancies or average duration of
gestation were noted between test and control groups. Average size was
slightly decreased in the high level (9) compared with the control
(11). The percentage of dead foetuses was higher in the 80 mg/kg group
(27%) when compared to the control (4%), 5 mg/kg (0%) and 20 mg/kg,
(1%) groups. No abnormalities were observed in offspring from control
or test groups (Marsboom, 1975a).
Special study on teratology
Groups of female rats (20/Group) were fed imazalil nitrate from days 6
through 15 of pregnancy, at levels of 0, 5, 20 and 80 mg/kg day.
Foetuses were delivered by caesarean section on day 21. There were no
differences between the control group and the test groups in regard to
average number of implantations, number of live or dead foetuses,
distribution of embryos or resorbed foetuses in either uterine horn,
weights or number of pups, or body weight gains, food consumption,
survival, and number of pregnancies among the dams. No abnormalities
were noted among the pups in the control and various test groups with
waved ribs in one litter of the 80 mg/kg group (Marsboom, 1970).
Other information on identity and properties
a) Composition of technical imazalil
The technical material as manufactured contains approximately 97.5-98%
(w/w) imazalil. Detailed information on the impurities in technical
imazalil was supplied to the Joint Meeting (Pattyn, 1976).
b) Physical and chemical properties of imazalil
Physical state: Pure imazalil is a hygroscopic, nearly colourless
liquid. Technical imazalil is a slightly yellowish to brownish oily
liquid.
Molecular weight: 297.18
Freezing point: -10°C - -15°C
Boiling point: -130°C. at 10-3mm Hg
Vapour pressure: 7 × 10-8mm Hg at 20°C; 4 × 10-5mm Hg at 70°C
Refractive index: n 20 1.5643
D
Specific gravity: d23 1.2429
Solubility of imazalil and its salts (g product / 100 ml solvent at
20°C)
solvent imazalil imazalil imazalil
(base) nitrate sulphate
water 0.14 2.38 >50
methanol >50 >100 >50
ethanol >50 31.18 36.3
2-propanol >50 4.57 4.42
exylene >50 0.49 <0.005
toluene >50 0.87 <0.005
n. heptane. 5.41 <0.001 <0.005
benzene >50 <0.005
4-methyl-2-pentanone >50 1.76 0.03
hexane 5.21 <0.005 <0.005
petroleum ether 6.16 <0.005 <0.005
Stability: Imazalil is thermally stable up to approximately 285°C
(Helsen, 1976). It is chemically stable at room
temperature in the absence of light. Decomposition
occurs at elevated temperatures (80°C) and under the
influence of light. The shelf life is more than 2
years. In aqueous solution the product is stable up to
40°C at pHs ranging from 2.4 to 7.0 for at least 8
weeks (Anon., 1975a). When refluxed for 72 hours the
compound remains stable in water and in 1 N sodium
hydroxide, but decomposes in 1 N hydrochloric acid.
c) Physical and chemical properties of imazalil nitrate and sulphate
Physical state: Imazalil nitrate is a slightly beige to brownish
coloured amorphous, non-hygroscopic powder.
Imazalil sulphate is a white to beige crystalline, hygroscopic powder.
Melting range: Imazalil nitrate = 80 - 90°C
Imazalil sulphate 118 - 130°C
Stability: Imazalil nitrate and imazalil sulphate are thermally
stable up to approximately 150°C and 270°C,
respectively.
d) Formulations
Imazalil is used as 20% (w/v) emulsifiable concentrate, 50% (w/w)
wettable powder, and as a seed dressing (also used in combination with
guazatine).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Imazalil in rapidly absorbed, distributed, metabolized and excreted by
rats. Two groups of five male and two groups of five female rats were
given a single 20 mg/kg oral dose of 3H-labelled imazalil sulphate.
Almost 90% of the administered radioactivity was excreted within 96
hours, with approximately equal quantities detected in the urine and
faeces. Tissue residues ranged from 5.4 to 6.1% of the administered
radioactivity 48 hours after dosing and from 1.8 to 3.5% 96 hours
after dosing. The highest levels of radioactivity were found in the
liver, lung and kidneys. Analysis of the urine from dosed animals
indicated that 4% of the tritium was volatile by lyophilization. Thus
tritium exchange apparently occurred to a minor extent. (Heykants at
al., 1975).
Five male rats were given a single oral 20 mg/kg dose of 3H-labelled
imazalil sulphate to study the absorption, distribution, metabolism
and excretion of imazalil in the rat. Approximately 90% of the
administered radioactivity was excreted within 96 hours with
comparable quantities excreted in the urine and faeces. Similar
results were found when a single oral dose of
alpha-(2,4-diochlorophenyl)-1H-imidazole-1-ethanol (a metabolite of
imazalil) was administered. In both these studies, up to approximately
2% of the administered radioactivity was identified as tritiated water
indicating exchange of the tritium label. The relative amount of
tritiated water found in the urine increased with time (Meuldermans et
al., 1977b).
Biotransformation
Studies involving a single 20 mg/kg oral dosing of rats with
3H-labelled imazalil sulphate indicate that extensive metabolism of
imazalil occurs in the rat. In one study,
alpha-(2,4-dichlorophenyl)-1H-imidazole-1-ethanol and
2,4-dichloromandolic acid were identified as the major metabolites in
the urine. In this study 10%, of the radioactivity in the urine and 3%
of the radioactivity in the faeces was identified as unchanged
imazalil (Heykants et al., 1975).
In a more extensive metabolic study, the major metabolites identified
are shown in Figure 1. Several metabolites detected are shown. The
compound shown in brackets in Figure 1 were not identified in the
studies. In contrast to the study described above, no unchanged
imazalil was detected in the urine and only 0.1% of the administered
radioactivity was identified as unchanged imazalil in the faeces.
(Meuldermans al., 1977b).
Tritium labelled imazalil sulphate was incubated with rat liver
homogenates to study the in vitro metabolism of imazalil. The two
metabolites identified were
-(2,4-dichlorophenyl-1-1H-imidazole-1-ethanol and
1- [-(2,4-dichlorophenyl-2-(2,3-dihydroxypropyloxy)ethyl] -1H
-imidazole (Meuldermans et al., 1977a).
Experiments on the biotransformation of imazalil in plants are
described in the section "Fate of residues".
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Groups of male (6/group) and female (10/group) mice were administered
a single dose of imazalil in arachis oil, by gavage, at levels of 10,
40 or 160 mg/kg, to determine dominant lethal effects. Positive
controls received 210 mg/kg cyclophosphamide. Negative controls
received only the oil at the same volume 0.1 ml/10g body weight, as
all other groups. Following dosing each male was mated with 4
untreated females at weekly intervals, for eight consecutive weeks.
The females were caged with single untreated males for a maximum of 5
days. This was repeated for 5 consecutive weeks. No significant
dose-related differences were observed in untreated females and the
negative controls nor in the treated females when the number of dead
and live implants were expressed as a percentage of corpora lutea vs.
total implants (Marsboom, 1976a, 1976b).
Special studies on reproduction
Three separate reproduction studies were carried out using differing
dosing periods. In all three the dietary doses based on active
ingredient were 0, 5, 20 or 80 mg/kg.
In the first study groups of 20 female rats received imazalil on a
non-continuous basis for 3 generations. Prior to and after the period
of organogenesis and throughout lactation the females of the first and
second generations received only the basal laboratory diet. In each of
these generations, the dams received the various levels from day 6
through day 15 of pregnancy. In the third generation, the dams
received the various levels from 3 weeks until 3 months of age and
further from day 1 to 21 after mating. There was a trend toward a
lower number of live births at the 80 mg/kg level in all generations.
No other effects were noted at any other level. No abnormalities were
noted in any foetuses in any generation which were not within the
normal range for the rats used (Marsboom, 19750).
In the second study, rats (20/Sex/group) received the various levels
of imazalil as follows: males for 60 days prior to pairing with
females and through copulation; females for 14 days prior to pairing
all throughout pregnancy. Pregnancy rate was not effected at any level
regardless of treatment of either sex. Test group data for all other
measured or observed parameters were comparable to the control data
(Marsboom, 1977a).
In the third study 20 females/group received the various levels of
imazalil from day 16 of pregnancy through a 3 week lactation period.
No differences in percentage of pregnancies or average duration of
gestation were noted between test and control groups. Average size was
slightly decreased in the high level (9) compared with the control
(11). The percentage of dead foetuses was higher in the 80 mg/kg group
(27%) when compared to the control (4%), 5 mg/kg (0%) and 20 mg/kg,
(1%) groups. No abnormalities were observed in offspring from control
or test groups (Marsboom, 1975a).
Special study on teratology
Groups of female rats (20/Group) were fed imazalil nitrate from days 6
through 15 of pregnancy, at levels of 0, 5, 20 and 80 mg/kg day.
Foetuses were delivered by caesarean section on day 21. There were no
differences between the control group and the test groups in regard to
average number of implantations, number of live or dead foetuses,
distribution of embryos or resorbed foetuses in either uterine horn,
weights or number of pups, or body weight gains, food consumption,
survival, and number of pregnancies among the dams. No abnormalities
were noted among the pups in the control and various test groups with
waved ribs in one litter of the 80 mg/kg group (Marsboom, 1970).
 Acute Toxicity
Species Route Sex LD50 Reference
Rat Oral M 376 Niemegeers, 1973
ip M 288 Niemegeers, 1977
F 155
Dermal M 4200 Van Ravestyn &
F 4800 Marsboom, 1975
Oral* M 374 Niemegeers, 1976
F 362
*(20% EC formulation)
The observed signs of intoxication were tremors, ataxia, sedation,
salivation, hypothermia, lacrimation, righting reflex loss, lung
oedema and clonic closures.
Imazalil was given, as a single dose by gavage, to 5 dogs (3 males and
2 females) at 100 mg/kg and to 3 dogs (1 male and 2 females) at 640
mg/kg. All dogs except one, receiving 160 mg/kg vomited within 15
minutes, and those receiving 640 mg/kg within 10 minutes after dosing.
No mortality or abnormal behavior, except diarrhoea in one 160 mg/kg
dog, occurred during the 7 day observation period. An
LD50 >640 mg/kg was postulated (Niemegeers, 1975),
Short term studies
Rat
Groups of rats (40/sex/group) were fed imazalil nitrate at dietary
levels of 0, 5, 20 and 80 mg/kg day for 14 weeks.
Histological examination revealed no changes which could be attributed
to imazalil in any organs or tissues except the livers of the 80 mg/kg
group, which showed a fatty reaction localized in the midzonal to
centrilobular areas and vacuolization coincident with fatty spots in
Scarlet-red stained sections.
Appearance, behaviour, survival, food consumption, or urinalysis were
not affected at any level. Body weights of the 80 mg/kg group were
slightly depressed.
Hematological and blood chemistry values were within normal limits,
except for an increase in bilirubin in both sexes in the 20 mg/kg
group (P < 0.001) and both sexes in the 80 mg/kg (P < 0.01) group
(Marsboom et al., 1971).
Dog
Groups of dogs (3/sex/group) received technical grade imazalil, in
arachis oil, at levels of 0, 1.25, 5 or 20 mg/kg by capsule 7
days/week for two years. The 20 mg/kg level produced frequent emesis
and frequent and abundant salivation, after compound administration,
in all dogs. Examination of tissues revealed no compound or dose
related effects except for slight ground glass aspect of the
cytoplasma in the centrilobular areas of the liver in the dogs
receiving 5 and 20 mg/kg. Controls and the 1.25 mg/kg dosed dogs
showed a normal and comparable weight gain during the study. At 5
mg/kg, weight gain was slightly lower (P < 0.05). The 20 mg/kg groups
gain was also lower (P < 0.01) than the controls at 24 months. All
other parameters, measured or observed, were within normal limits and
comparable to those of the control group (Marsboom et al., 1977b).
Long term studies
Rat
Groups of rats were fed a 50% wettable powder of imazalil at dietary
concentrations equivalent to 0, 5, 20 and 80 mg/kg/day (active
ingredient) for 6, 12 and 24 months. At 6 months the livers of many 80
mg/kg rats, particularly females, showed centrilobular swelling and
numerous large vacuoles. No evident fatty degeneration was seen but a
tendency to some fatty surcharge was noted. At 12 months these changes
were still present. In addition, livers of the high level group
females exhibited a tendency to more concentrated glycogen in the
centrilobular areas. No other effects were noted which could be
attributed to the compound administration. Except for liver and
kidneys no consistent changes in weight or body weight ratios were
noted in any group at any termination. At 6 months the livers of the
80 mg/kg females were significantly increased in both absolute weight
and ratio. At 12 and 24 months the livers of female were not
significantly different.
In males receiving 20 and 80 mg/kg, the 12 month liver weights and
ratios were increased significantly but not at the 24 month
termination. At 6 and 24 months kidney weights and ratios were
significantly increased in the 80 mg/kg female group. This trend was
present at 12 months but not of statistical significance. Males
receiving this level exhibited, at 6 months, a trend toward increased
kidney weights and a significant increase in their kidney/body weight
ratio. At 12 months male kidney weights were significantly higher than
their control values, but at 24 months this difference was not
evident. Hematological and blood chemistry revealed no consistent
changes, except that at 6 months bilirubin values for the female 20
and 80 mg/kg groups were significantly elevated. This trend continued
throughout the study in the 80 mg/kg females but was not statistically
significant at the 12 and 24 month terminations. Males did not exhibit
this trend at any level during the study. Urinalysis produced nothing
remarkable. The dietary administration of imazalil did not produce a
compound- or dose-related effect on body weight gains, food
consumption, appearance, behaviour or survival rate (Marsboom at al.,
1975b).
COMMENTS
Imazalil is moderately acutely toxic to mammals. Preliminary studies
indicate that it is rapidly absorbed, metabolized and excreted by the
rat. The major metabolites are
alpha-(2,4-dichlorophenyl)-1H-imidazole-l-ethanol and
2,4-dichloromandelic acid. Dominant lethal mutagenic effects were not
evident in male and female mice. No teratological effects were noted
in rats and no reproductive effects were observed in a three
generation rat study. However, survival of pups at weaning was
adversely affected at levels higher than 5 mg/kg. Long-term studies in
the rat and a study in the dog were inadequate because of unresolved
questions concerning rat kidney and dog liver pathology. Because of
these concerns, only a temporary ADI for humans was established for
this compound based on short-term studies.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 5 (mg/kg bw)/day
Dog: 1.25 (mg/kg bw)/day
ESTIMATE OF A TEMPORARY ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.01 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Mode of action
Imazalil is a systemic fungicide from the group of N-substituted
imidazoles. Members of this chemical class affect the cellular
permeability barrier of the yeasts. The fungitoxic action of imazalil
on Penicillium may also involve the inhibition of the cell membrane
functions.
Uptake and membrane effects noted in several studies suggest that
imazalil inhibits ergosterol biosynthesis in fungal cells (Van
Bossche, 1977
Under natural conditions, the probability of the development of
resistant strains of fungi is much lower with the polygenic system of
imazalil than with the oligogenic system of the benzimidazole type
fungicides. Most probably, occurrence of resistant strains to imazalil
under field conditions is an extremely rare phenomenon which must
involve 21 genes on 8 loci, linked with 6 groups (Laville et al.,
1977; van Tuyl, 1977).
Pre-harvest treatments
Imazalil is active against a large number of fungi imperfecti, e.g.
Alternaria spp., Aspergillus, Cercospora, Colletotrichum,
Fusarium, Helminthosporium, Penicillium, Thielaviopsis. Some
fungi of the class "Ascomycetae" are also well controlled, e.g.
Erysiphe spp., Podosphaera, Sphaerotheca, Venturia.
Owing to its high specific activity (4 - 5 g a.i./100 kg seed) against
Helminthosporium spp., Fusarium spp., and Septoria spp.,
imazalil has largely contributed to replacing mercury-containing
cereal seed treatment products (Bartlett and Ballard, 1975). Products
containing imazalil are registered and sold in Sweden, Denmark, The
Netherlands, Italy, France and United Kingdom.
As a foliar spray imazalil is used on ornamentals and cucurbitacae.
Further development work is being carried out and registration for
foliar application against leaf spot diseases in bananas will be
applied for. Sigatoka "Mycosphaerella musicola" and Black Sigatoka
"Mycosphaerella fijiensis var. diformis", have developed
resistance against benzimidazoles in important banana-growing areas.
Imazalil has proved to control both Sigatoka forms and black leaf
streak (Stover, 1976; Melin and al., 1976) under practical conditions
sprayed by aircraft in a spray oil or oil-in-water emulsion.
Registration and tolerances have been applied for in banana-producing
and banana-importing countries.
Post-harvest treatments
a) Citrus
Imazalil has been investigated world-wide as a post-harvest fungicide
because of its effectiveness against benzimidazole-resistant strains
of plant pathogenic fungi. Laville at al. (1977a) contributed to the
development of imazalil as a post-harvest fungicide and stressed the
need for a replacement for benzimidazoles.
Imazalil is not only curative, but also preventive against infections
occurring after treatment during packing, transport and storage until
the fruit reaches the consumer.
At application rates of less than 2 g a.i./tonne of fruit and residue
levels below 2 mg/kg, imazalil treated fruit can be stored for more
than 3 months. Application techniques and exact dosage are facilitated
by the high solubility in water and waxes.
b) Banana
Frossard at al. (1976) and Stover (1976) have reported excellent
results. Imazalil water-soluble powder (imazalil sulphate) was used in
these experiments in water at 500 mg a.i./ka dip or spray. As
resistance to benzimidazoles had already been detected, Frossard et
al. (1976) concluded that there was a need for replacement.
Main uses
The main application fields and registered uses of imazalil are
summarized in Tables 1a and 1b.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The residues of imazalil resulting from supervised trials after
pre-harvest application on cucumbers or gherkins under glass and on
green, unripe bananas are shown in Table 2. Residues in/on cucumbers
are below 0.5 mg/kg, oven when imazalil is used between croppings.
When imazalil in applied as a post-harvest fungicide, residues on
bananas do not exceed 2 mg/kg whole fruit and are below 0.2 mg/kg in
the pulp. Residue data for citrus fruits have shown that imazalil
residues are below 5 mg/kg on whole citrus fruits or on the peel and
below 0.1 mg/kg in the pulp, when imazalil in used in accordance with
good post-harvest practice in packing houses.
The residue levels on citrus fruit largely depend on the amount of
imazalil applied. There was no significant difference between residues
when Valencia oranges were stored up to 32 days at 4°C or 22 - 25°C,
or as a result of different formulations or types of application
(Greenberg and Resnick, 1977). Residue data obtained after
post-harvest application are summarized in Table 3.
TABLE 1a. Main application fields of imazalil
Formulation and mode of Concentration
application a.i.
Ornamentals
Powdery mildew spray 0:15% 30 g/hl
E.C. 20% (300 ppm)
Cucurbits
Powdery mildew spray 0 025% 5g/hl
0.05% 10 g/hl
E.C. 20% (50-100 ppm)
Cereal seed treatment
Leaf stripe (Helminthosporium 8pp) liquid 2.5% a.i. 5 g/100 kg
seed
Septori spp.
Fusarium spp. powder 2.5% a.i. (50 ppm)
Post-harvest treatment of citrus,
bananas, dip 75% W.P. 66g/hl
pome fruit and pears against (500 ppm)
fruit rots
Penicillium spp. spray 75% W.P. 66g/hl
Alternaria spp. (500 ppm)
Diplodia spp. wax 95% techn. 2000 ppm
Thielaviopsia spp. in wax
Colletotrichum musarum
Fusarium moniliforme
Gloeosporium musarum
Foliar treatment bananas
Sigatoka disease spray 50% ULV 150-200 g.
Cercospora muses in oil a.i. per ha.
TABLE 1b. Registrations (existing and applied for) for commercial application of imazalil
Country Crop Application Dosage rate Pre-harvest Limitations
type kg a.i./ha interval, days
Italy ornamentals foliar spray 0.2-0.4 - -
U.K. ornamentals foliar spray 0.2-0.4 - -
France cucurbits foliar spray 0.2-0.4 2 outdoors only
Denmark barley seed dressing 0.005-0.01 - -
Holland cereals seed dressing 0.005-0.01 - -
Sweden cereals seed dressing 0.005-0.01 - -
U.K. cereals seed dressing 0.005-0.01 - -
TABLE 2. Residues of imazalil (base; mg/kg) resulting from pre-harvest treatments
Crop Application
& a) no. rate
Country kg Formulation Residues at interval (days) after last
a.i./ application
ha 0 1 3/4
Cucumbers under
glass NL b)
whole fruit 5 0.3 EC 20% 0.13-0.18 0.11-0.12 0.04-0.09
flesh 0.05-0.08 0.11-0.12 0.04-0.08
peel 0.60-0.85 0.05-0.15 0.04-0.13
whole fruit 5 0.4 EC 20% 0.33-0.42 0.14-0.21 0.05-0.12
flesh 0.16-0.35 0.08-0.22 0.05-0.12
peel 0.98-1.43 0.14-0.53 0.07-0.12
whole fruit 5 0.6 EC 20% 0.33-0.47 0.18-0.30 0.08-0.18
flesh 0.23-0.34 0.18-0.30 0.08-0.18
peel 0.98-1.53 0.15-0.27 0.11-0.19
whole fruit Bc) 3 0.2 EC 20% <0.01-0.07
flesh g/l <0.01-0.04
peel <0.01-0.24
Gherkins under
glass NLd) 7 0.4 EC 20% <0.01-0.01
6 0.6 EC 20% 0.03-0.05
Bananas (green)
BH e) 9 0.25- EC 50% (1-2 days + 10 days transport)
0.5
whole fruit <0.02-0.02
pulp <0.02
TABLE 2 (Continued)
a) trials in: B = Belgium, BH = British Honduras, NL = Netherlands
b) Wynants & Woestenborghs, 1976
c) Nangniot, 1977
d) ten Broeke & Dornseiffen, 1977
e) Anonymous, 1976c,g
TABLE 3. Residues of imazalil (base; mg/kg) resulting from post-harvest treatments
Crop Rate and type of application Residues at interval (weeks) after (last) References
& application
Country a) 0-1 2 3-4 6
Bananas BH 0.5 g/l spray-water (13
(ripened) weeks
after
treatment)
0.64-1.1 Anonymous, 1976c
whole fruit 0.02-0.07
pulp 0.48-1.1 Anonymous, 1976g
whole fruit pre-harvest treatment + 0.5 g/l 0.03-0.15
pulp spray water
Citrus
fruits
not
specified ZA 1 g/kg or 1 wax-prep.?
whole fruit 0.5-0.6 0.5-0.6 0.3-0.5 0.2-0.4 Beijers, 1976
pulp <0.1 <0.1 <0.1 <0.1
peel 2.2-2.6 1.9-2.1 1.0-1.5
not
specified ZA 0.5 g/l 1 aqueous prep.
whole fruit <0.1-0.1 <0.1-0.1 <0.1-0.1 <0.1-0.1 Beijers, 1976
pulp <0.1 <0.1 <0.1 <0.1
peel 0.2-0.5 0.3-0.6 0.1-0.3 0.1-0.3
Grapefruit
whole
fruit IL 0.5-4 g/l aqueous dip 0.7-2.2 Greenberg & Resnick,
0.5-4 g/kg wax spray 1.0-4.0 1977
(25-100 g/l ULV (?) 1.5-8.0)
TABLE 3. (Continued)
Crop Rate and type of application Residues at interval (weeks) after (last) References
& application
Country a) 0-1 2 3-4 6
Grapefruit USA
whole fruit 0.5-1g/kg aqueous dip 0.7-1.0 Kraght, 1977b
0.5-1g/kg aqueous drench 0.8-1.0
1-2g/kg aqueous spray 0.4-0.7
2-4g kg wax spray 0.5-2.2
foamer 0.2%? 0.2
Oranges
whole fruit IL 0.1-1 g/l aqueous dip 0.5-1.4 Greenberg & Resnick,
2.5-8 g/kg wax dip 2.0-11 1977
(Valencia) 2-3 g/kg wax dip 0-5-2.0 1.2-2.5 0.1-3.2
(Shamuti) 1-2 g/kg wax dip 0.6-2.2
whole fruit I 0.5g/l aqueous dip or 0.8-1.6 0.01 Maier-Bode, 1976
pulp 2g/l wax spray 1.9-4.2
peel
Oranges
(Navel)
whole
fruit MA 1g/l aqueous dip or 1.2-1.7 Corpel & Gauthier,
pulp 1-4g/l wax spray 0.01-0.03 1977
peel 1.3-4.3
peel 1g/kg dip See Table Anon.,1975b
4, p. 000
whole
fruit ZA 1.5-3g/kg wax spray 1.4-1.6 0.6-1.2 1.0-2.0 0.6-1.2 Beijers, 1975
1.5-3g/kg aqueous dip 0.1-0.8 0.2-0.8
TABLE 3. (Continued)
Crop Rate and type of application Residues at interval (weeks) after (last) References
& application
Country a) 0-1 2 3-4 6
whole
fruit ZA 0.25-1g/kg wax spray 0.04-0.32 Bliss & Orme,
pulp (time 0.01-0.02 1976
after
treatment
peel unknown) 0.16-0.72
(Navel
whole
fruit USA 0.5g/kg aqueous dip,
drench or spray 0.3-5.2 Kraght, 1977b
1g/kg aqueous dip,
drench or spray 0.6-5.7
2g/kg wax spray 0.04-1.9
4/kg 0.4-3.7
Tangerines USA 0.5-1g/kg aqueous dip,
drench or spray 0.2-0.3 Kraght, 1977b
2-4g/kg wax spray 0.3-2.0
a) trials in
BH = British Honduras
I = Italy
IL = Israel
MA = Morocco
USA = United States of America
ZA = South Africa
FATE OF RESIDUES
In animals
Approximately 44% of a single dose of 20 mg 3H-imazalil per kg to
rats was excreted with the urine within 4 days after treatment and
approximately 46% was excreted with the faeces. Only 3% of the
administered dose was excreted unchanged with the faeces indicating an
almost complete absorption from the gastro-intestinal tract. In
addition to unchanged imazalil (approximately 5% of the dose) the
urine contained at least 4 metabolites, probably formed by
dealkylation. Two of them were identified as
alpha-(2,4-dichlorophenyl)-1H-imidazole-1-ethanol and
2,4-dichloromandelic acid; 48 hours after administration,
concentrations higher than 1 mg/kg were found only in the liver, the
lung and the kidneys. Nearly the same concentrations were found in
male and female rats. Accumulation of imazalil in fatty tissues did
not occur (Heykants at al., 1975). This study is also referred to
previously ("Biochemical aspects").
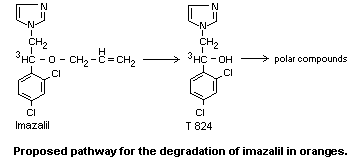
In plants
Fourteen weeks after treatment of oranges with a solution containing
1000 mg/kg (3H-2-ethyl labelled)-imazalil, approximately 50% of the
radioactivity remaining was found in the peel in an organo-soluble
form; 30% was bound to the insoluble residue of the peel. In the flesh
of the fruit 18% of the remaining radioactivity was present as the
metabolite alpha-(2,4-dichlorophenyl)-1H-imidazole-l-ethanol (T 824).
Acute Toxicity
Species Route Sex LD50 Reference
Rat Oral M 376 Niemegeers, 1973
ip M 288 Niemegeers, 1977
F 155
Dermal M 4200 Van Ravestyn &
F 4800 Marsboom, 1975
Oral* M 374 Niemegeers, 1976
F 362
*(20% EC formulation)
The observed signs of intoxication were tremors, ataxia, sedation,
salivation, hypothermia, lacrimation, righting reflex loss, lung
oedema and clonic closures.
Imazalil was given, as a single dose by gavage, to 5 dogs (3 males and
2 females) at 100 mg/kg and to 3 dogs (1 male and 2 females) at 640
mg/kg. All dogs except one, receiving 160 mg/kg vomited within 15
minutes, and those receiving 640 mg/kg within 10 minutes after dosing.
No mortality or abnormal behavior, except diarrhoea in one 160 mg/kg
dog, occurred during the 7 day observation period. An
LD50 >640 mg/kg was postulated (Niemegeers, 1975),
Short term studies
Rat
Groups of rats (40/sex/group) were fed imazalil nitrate at dietary
levels of 0, 5, 20 and 80 mg/kg day for 14 weeks.
Histological examination revealed no changes which could be attributed
to imazalil in any organs or tissues except the livers of the 80 mg/kg
group, which showed a fatty reaction localized in the midzonal to
centrilobular areas and vacuolization coincident with fatty spots in
Scarlet-red stained sections.
Appearance, behaviour, survival, food consumption, or urinalysis were
not affected at any level. Body weights of the 80 mg/kg group were
slightly depressed.
Hematological and blood chemistry values were within normal limits,
except for an increase in bilirubin in both sexes in the 20 mg/kg
group (P < 0.001) and both sexes in the 80 mg/kg (P < 0.01) group
(Marsboom et al., 1971).
Dog
Groups of dogs (3/sex/group) received technical grade imazalil, in
arachis oil, at levels of 0, 1.25, 5 or 20 mg/kg by capsule 7
days/week for two years. The 20 mg/kg level produced frequent emesis
and frequent and abundant salivation, after compound administration,
in all dogs. Examination of tissues revealed no compound or dose
related effects except for slight ground glass aspect of the
cytoplasma in the centrilobular areas of the liver in the dogs
receiving 5 and 20 mg/kg. Controls and the 1.25 mg/kg dosed dogs
showed a normal and comparable weight gain during the study. At 5
mg/kg, weight gain was slightly lower (P < 0.05). The 20 mg/kg groups
gain was also lower (P < 0.01) than the controls at 24 months. All
other parameters, measured or observed, were within normal limits and
comparable to those of the control group (Marsboom et al., 1977b).
Long term studies
Rat
Groups of rats were fed a 50% wettable powder of imazalil at dietary
concentrations equivalent to 0, 5, 20 and 80 mg/kg/day (active
ingredient) for 6, 12 and 24 months. At 6 months the livers of many 80
mg/kg rats, particularly females, showed centrilobular swelling and
numerous large vacuoles. No evident fatty degeneration was seen but a
tendency to some fatty surcharge was noted. At 12 months these changes
were still present. In addition, livers of the high level group
females exhibited a tendency to more concentrated glycogen in the
centrilobular areas. No other effects were noted which could be
attributed to the compound administration. Except for liver and
kidneys no consistent changes in weight or body weight ratios were
noted in any group at any termination. At 6 months the livers of the
80 mg/kg females were significantly increased in both absolute weight
and ratio. At 12 and 24 months the livers of female were not
significantly different.
In males receiving 20 and 80 mg/kg, the 12 month liver weights and
ratios were increased significantly but not at the 24 month
termination. At 6 and 24 months kidney weights and ratios were
significantly increased in the 80 mg/kg female group. This trend was
present at 12 months but not of statistical significance. Males
receiving this level exhibited, at 6 months, a trend toward increased
kidney weights and a significant increase in their kidney/body weight
ratio. At 12 months male kidney weights were significantly higher than
their control values, but at 24 months this difference was not
evident. Hematological and blood chemistry revealed no consistent
changes, except that at 6 months bilirubin values for the female 20
and 80 mg/kg groups were significantly elevated. This trend continued
throughout the study in the 80 mg/kg females but was not statistically
significant at the 12 and 24 month terminations. Males did not exhibit
this trend at any level during the study. Urinalysis produced nothing
remarkable. The dietary administration of imazalil did not produce a
compound- or dose-related effect on body weight gains, food
consumption, appearance, behaviour or survival rate (Marsboom at al.,
1975b).
COMMENTS
Imazalil is moderately acutely toxic to mammals. Preliminary studies
indicate that it is rapidly absorbed, metabolized and excreted by the
rat. The major metabolites are
alpha-(2,4-dichlorophenyl)-1H-imidazole-l-ethanol and
2,4-dichloromandelic acid. Dominant lethal mutagenic effects were not
evident in male and female mice. No teratological effects were noted
in rats and no reproductive effects were observed in a three
generation rat study. However, survival of pups at weaning was
adversely affected at levels higher than 5 mg/kg. Long-term studies in
the rat and a study in the dog were inadequate because of unresolved
questions concerning rat kidney and dog liver pathology. Because of
these concerns, only a temporary ADI for humans was established for
this compound based on short-term studies.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 5 (mg/kg bw)/day
Dog: 1.25 (mg/kg bw)/day
ESTIMATE OF A TEMPORARY ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.01 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Mode of action
Imazalil is a systemic fungicide from the group of N-substituted
imidazoles. Members of this chemical class affect the cellular
permeability barrier of the yeasts. The fungitoxic action of imazalil
on Penicillium may also involve the inhibition of the cell membrane
functions.
Uptake and membrane effects noted in several studies suggest that
imazalil inhibits ergosterol biosynthesis in fungal cells (Van
Bossche, 1977
Under natural conditions, the probability of the development of
resistant strains of fungi is much lower with the polygenic system of
imazalil than with the oligogenic system of the benzimidazole type
fungicides. Most probably, occurrence of resistant strains to imazalil
under field conditions is an extremely rare phenomenon which must
involve 21 genes on 8 loci, linked with 6 groups (Laville et al.,
1977; van Tuyl, 1977).
Pre-harvest treatments
Imazalil is active against a large number of fungi imperfecti, e.g.
Alternaria spp., Aspergillus, Cercospora, Colletotrichum,
Fusarium, Helminthosporium, Penicillium, Thielaviopsis. Some
fungi of the class "Ascomycetae" are also well controlled, e.g.
Erysiphe spp., Podosphaera, Sphaerotheca, Venturia.
Owing to its high specific activity (4 - 5 g a.i./100 kg seed) against
Helminthosporium spp., Fusarium spp., and Septoria spp.,
imazalil has largely contributed to replacing mercury-containing
cereal seed treatment products (Bartlett and Ballard, 1975). Products
containing imazalil are registered and sold in Sweden, Denmark, The
Netherlands, Italy, France and United Kingdom.
As a foliar spray imazalil is used on ornamentals and cucurbitacae.
Further development work is being carried out and registration for
foliar application against leaf spot diseases in bananas will be
applied for. Sigatoka "Mycosphaerella musicola" and Black Sigatoka
"Mycosphaerella fijiensis var. diformis", have developed
resistance against benzimidazoles in important banana-growing areas.
Imazalil has proved to control both Sigatoka forms and black leaf
streak (Stover, 1976; Melin and al., 1976) under practical conditions
sprayed by aircraft in a spray oil or oil-in-water emulsion.
Registration and tolerances have been applied for in banana-producing
and banana-importing countries.
Post-harvest treatments
a) Citrus
Imazalil has been investigated world-wide as a post-harvest fungicide
because of its effectiveness against benzimidazole-resistant strains
of plant pathogenic fungi. Laville at al. (1977a) contributed to the
development of imazalil as a post-harvest fungicide and stressed the
need for a replacement for benzimidazoles.
Imazalil is not only curative, but also preventive against infections
occurring after treatment during packing, transport and storage until
the fruit reaches the consumer.
At application rates of less than 2 g a.i./tonne of fruit and residue
levels below 2 mg/kg, imazalil treated fruit can be stored for more
than 3 months. Application techniques and exact dosage are facilitated
by the high solubility in water and waxes.
b) Banana
Frossard at al. (1976) and Stover (1976) have reported excellent
results. Imazalil water-soluble powder (imazalil sulphate) was used in
these experiments in water at 500 mg a.i./ka dip or spray. As
resistance to benzimidazoles had already been detected, Frossard et
al. (1976) concluded that there was a need for replacement.
Main uses
The main application fields and registered uses of imazalil are
summarized in Tables 1a and 1b.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The residues of imazalil resulting from supervised trials after
pre-harvest application on cucumbers or gherkins under glass and on
green, unripe bananas are shown in Table 2. Residues in/on cucumbers
are below 0.5 mg/kg, oven when imazalil is used between croppings.
When imazalil in applied as a post-harvest fungicide, residues on
bananas do not exceed 2 mg/kg whole fruit and are below 0.2 mg/kg in
the pulp. Residue data for citrus fruits have shown that imazalil
residues are below 5 mg/kg on whole citrus fruits or on the peel and
below 0.1 mg/kg in the pulp, when imazalil in used in accordance with
good post-harvest practice in packing houses.
The residue levels on citrus fruit largely depend on the amount of
imazalil applied. There was no significant difference between residues
when Valencia oranges were stored up to 32 days at 4°C or 22 - 25°C,
or as a result of different formulations or types of application
(Greenberg and Resnick, 1977). Residue data obtained after
post-harvest application are summarized in Table 3.
TABLE 1a. Main application fields of imazalil
Formulation and mode of Concentration
application a.i.
Ornamentals
Powdery mildew spray 0:15% 30 g/hl
E.C. 20% (300 ppm)
Cucurbits
Powdery mildew spray 0 025% 5g/hl
0.05% 10 g/hl
E.C. 20% (50-100 ppm)
Cereal seed treatment
Leaf stripe (Helminthosporium 8pp) liquid 2.5% a.i. 5 g/100 kg
seed
Septori spp.
Fusarium spp. powder 2.5% a.i. (50 ppm)
Post-harvest treatment of citrus,
bananas, dip 75% W.P. 66g/hl
pome fruit and pears against (500 ppm)
fruit rots
Penicillium spp. spray 75% W.P. 66g/hl
Alternaria spp. (500 ppm)
Diplodia spp. wax 95% techn. 2000 ppm
Thielaviopsia spp. in wax
Colletotrichum musarum
Fusarium moniliforme
Gloeosporium musarum
Foliar treatment bananas
Sigatoka disease spray 50% ULV 150-200 g.
Cercospora muses in oil a.i. per ha.
TABLE 1b. Registrations (existing and applied for) for commercial application of imazalil
Country Crop Application Dosage rate Pre-harvest Limitations
type kg a.i./ha interval, days
Italy ornamentals foliar spray 0.2-0.4 - -
U.K. ornamentals foliar spray 0.2-0.4 - -
France cucurbits foliar spray 0.2-0.4 2 outdoors only
Denmark barley seed dressing 0.005-0.01 - -
Holland cereals seed dressing 0.005-0.01 - -
Sweden cereals seed dressing 0.005-0.01 - -
U.K. cereals seed dressing 0.005-0.01 - -
TABLE 2. Residues of imazalil (base; mg/kg) resulting from pre-harvest treatments
Crop Application
& a) no. rate
Country kg Formulation Residues at interval (days) after last
a.i./ application
ha 0 1 3/4
Cucumbers under
glass NL b)
whole fruit 5 0.3 EC 20% 0.13-0.18 0.11-0.12 0.04-0.09
flesh 0.05-0.08 0.11-0.12 0.04-0.08
peel 0.60-0.85 0.05-0.15 0.04-0.13
whole fruit 5 0.4 EC 20% 0.33-0.42 0.14-0.21 0.05-0.12
flesh 0.16-0.35 0.08-0.22 0.05-0.12
peel 0.98-1.43 0.14-0.53 0.07-0.12
whole fruit 5 0.6 EC 20% 0.33-0.47 0.18-0.30 0.08-0.18
flesh 0.23-0.34 0.18-0.30 0.08-0.18
peel 0.98-1.53 0.15-0.27 0.11-0.19
whole fruit Bc) 3 0.2 EC 20% <0.01-0.07
flesh g/l <0.01-0.04
peel <0.01-0.24
Gherkins under
glass NLd) 7 0.4 EC 20% <0.01-0.01
6 0.6 EC 20% 0.03-0.05
Bananas (green)
BH e) 9 0.25- EC 50% (1-2 days + 10 days transport)
0.5
whole fruit <0.02-0.02
pulp <0.02
TABLE 2 (Continued)
a) trials in: B = Belgium, BH = British Honduras, NL = Netherlands
b) Wynants & Woestenborghs, 1976
c) Nangniot, 1977
d) ten Broeke & Dornseiffen, 1977
e) Anonymous, 1976c,g
TABLE 3. Residues of imazalil (base; mg/kg) resulting from post-harvest treatments
Crop Rate and type of application Residues at interval (weeks) after (last) References
& application
Country a) 0-1 2 3-4 6
Bananas BH 0.5 g/l spray-water (13
(ripened) weeks
after
treatment)
0.64-1.1 Anonymous, 1976c
whole fruit 0.02-0.07
pulp 0.48-1.1 Anonymous, 1976g
whole fruit pre-harvest treatment + 0.5 g/l 0.03-0.15
pulp spray water
Citrus
fruits
not
specified ZA 1 g/kg or 1 wax-prep.?
whole fruit 0.5-0.6 0.5-0.6 0.3-0.5 0.2-0.4 Beijers, 1976
pulp <0.1 <0.1 <0.1 <0.1
peel 2.2-2.6 1.9-2.1 1.0-1.5
not
specified ZA 0.5 g/l 1 aqueous prep.
whole fruit <0.1-0.1 <0.1-0.1 <0.1-0.1 <0.1-0.1 Beijers, 1976
pulp <0.1 <0.1 <0.1 <0.1
peel 0.2-0.5 0.3-0.6 0.1-0.3 0.1-0.3
Grapefruit
whole
fruit IL 0.5-4 g/l aqueous dip 0.7-2.2 Greenberg & Resnick,
0.5-4 g/kg wax spray 1.0-4.0 1977
(25-100 g/l ULV (?) 1.5-8.0)
TABLE 3. (Continued)
Crop Rate and type of application Residues at interval (weeks) after (last) References
& application
Country a) 0-1 2 3-4 6
Grapefruit USA
whole fruit 0.5-1g/kg aqueous dip 0.7-1.0 Kraght, 1977b
0.5-1g/kg aqueous drench 0.8-1.0
1-2g/kg aqueous spray 0.4-0.7
2-4g kg wax spray 0.5-2.2
foamer 0.2%? 0.2
Oranges
whole fruit IL 0.1-1 g/l aqueous dip 0.5-1.4 Greenberg & Resnick,
2.5-8 g/kg wax dip 2.0-11 1977
(Valencia) 2-3 g/kg wax dip 0-5-2.0 1.2-2.5 0.1-3.2
(Shamuti) 1-2 g/kg wax dip 0.6-2.2
whole fruit I 0.5g/l aqueous dip or 0.8-1.6 0.01 Maier-Bode, 1976
pulp 2g/l wax spray 1.9-4.2
peel
Oranges
(Navel)
whole
fruit MA 1g/l aqueous dip or 1.2-1.7 Corpel & Gauthier,
pulp 1-4g/l wax spray 0.01-0.03 1977
peel 1.3-4.3
peel 1g/kg dip See Table Anon.,1975b
4, p. 000
whole
fruit ZA 1.5-3g/kg wax spray 1.4-1.6 0.6-1.2 1.0-2.0 0.6-1.2 Beijers, 1975
1.5-3g/kg aqueous dip 0.1-0.8 0.2-0.8
TABLE 3. (Continued)
Crop Rate and type of application Residues at interval (weeks) after (last) References
& application
Country a) 0-1 2 3-4 6
whole
fruit ZA 0.25-1g/kg wax spray 0.04-0.32 Bliss & Orme,
pulp (time 0.01-0.02 1976
after
treatment
peel unknown) 0.16-0.72
(Navel
whole
fruit USA 0.5g/kg aqueous dip,
drench or spray 0.3-5.2 Kraght, 1977b
1g/kg aqueous dip,
drench or spray 0.6-5.7
2g/kg wax spray 0.04-1.9
4/kg 0.4-3.7
Tangerines USA 0.5-1g/kg aqueous dip,
drench or spray 0.2-0.3 Kraght, 1977b
2-4g/kg wax spray 0.3-2.0
a) trials in
BH = British Honduras
I = Italy
IL = Israel
MA = Morocco
USA = United States of America
ZA = South Africa
FATE OF RESIDUES
In animals
Approximately 44% of a single dose of 20 mg 3H-imazalil per kg to
rats was excreted with the urine within 4 days after treatment and
approximately 46% was excreted with the faeces. Only 3% of the
administered dose was excreted unchanged with the faeces indicating an
almost complete absorption from the gastro-intestinal tract. In
addition to unchanged imazalil (approximately 5% of the dose) the
urine contained at least 4 metabolites, probably formed by
dealkylation. Two of them were identified as
alpha-(2,4-dichlorophenyl)-1H-imidazole-1-ethanol and
2,4-dichloromandelic acid; 48 hours after administration,
concentrations higher than 1 mg/kg were found only in the liver, the
lung and the kidneys. Nearly the same concentrations were found in
male and female rats. Accumulation of imazalil in fatty tissues did
not occur (Heykants at al., 1975). This study is also referred to
previously ("Biochemical aspects").
In plants
Fourteen weeks after treatment of oranges with a solution containing
1000 mg/kg (3H-2-ethyl labelled)-imazalil, approximately 50% of the
radioactivity remaining was found in the peel in an organo-soluble
form; 30% was bound to the insoluble residue of the peel. In the flesh
of the fruit 18% of the remaining radioactivity was present as the
metabolite alpha-(2,4-dichlorophenyl)-1H-imidazole-l-ethanol (T 824).
 The polar, hydrophilic fraction containing nearly 50% of the
radioactivity present in the methanol extract of the peel did not
contain tritiated water or other volatile radioactivity. Since
concentrations of radioactivity were identical before and after
lyophilisation of this fraction, it seems that no label loss is to be
expected during metabolism (Anon., 1976a).
A similar test was conducted under conditions closer to commercial
practice. In addition, a more rigorous solvent extraction procedure
was used in order to recover more radioactive material.
As metabolites were expected to be present in very small amounts, the
fruit was treated with 4 times the normal concentration, 2000 mg
a.i./1 aqueous dip for 1 minute. The oranges were stored for 12 weeks
and the distribution of radioactivity in the fruit and the presence of
metabolites were studied during and after this period.
Most of the radioactivity (89%) remained as unchanged imazalil, in
contrast to the above results where considerable degradation was
reported. This was due to the extreme storage conditions used in the
above trial (high temperature and light), which are completely
artificial and not related to commercial practice. No tritium exchange
was detected, thus the tritium labelling was suitable for the purpose
of the study. 99.5% or more of the radioactivity in the
3H-imazalil-treated oranges was solvent-extractable: about 0.5% or
less remained in the insoluble residue. After 12 weeks of storage
approximately 10-12% of the original radioactivity appeared in the
form of an unknown water-soluble compound (Kraght, 1977a).
The distribution of imazalil in/on oranges was determined at intervals
after treatment at 1000 mg/kg (Anon., 1975b). Results are shown in
Table 4.
Gherkins sprayed seven times with imazalil under practical conditions
in a glasshouse were sampled three days after the last application,
then stored at -18°C for 3-7 weeks. Residues in/on the gherkins were
between 0.03 -0.05 mg imazalil/kg and at or below 0.01 mg
T 824-metabolite/kg (ten Broeke and Dornseiffen, 1977).
Barley seeds treated with 3H-imazalil were sown in perlite. After 3
weeks the plants were harvested and the roots and leaves extracted
separately. Large amounts of radioactivity were present in an
unextractable form in both the roots and the leaves. In a methanol
extract of the roots imazalil accounted for 33% of the total
radioactivity and in a toluene extract for 81%. A certain amount of
the methanol-insoluble residue may consist of physically bound
imazalil. Only about 7% or less of the label was the metabolite T 824
(Anon., 1976b).
TABLE 4. Distribution of imazalil in oranges after treatment at 1000 mg/kg
Imazalil, mg/kg, at indicated interval after dipping (Anon., 1975b)
0 weeks 4 weeks 8 weeks 12 weeks
dipped dipped dipped dipped
´ min. 1 min. ´ min. 1 min. ´ min. 1 min ´ min. 1 min.
peel 0.9 2.4 0.51 0.85 2.3 1.7 0.8 1.4
4.0 2.5 0.55 0.49 1.2 1.5 0.7 1.5
pulp n.d. n.d. 0.053 0.047 0.047 0.12 0.10 0.36
0.13 n.d. 0.027 0.062 0.14 0.11 0.09 0.06
n.d. = not determined.
Comments: -degradation of imazalil m orangss is slow;
-half-life is estimated to be 12 to 20 weeks
-only small amounts of imazalil are present in the fruit flesh and part
of this may result from contamination during peeling.
Barley seeds were treated with a dose of 3H-imazalil corresponding
to 10 g a.i./100 kg seed. After germination on water agar for 9 days,
42% of the radioactivity was found in the agar and 37% in the seed
coats. Only 10% of the total radioactivity was present in the roots
and 2% in the leaves of the seedlings.
Barley seeds treated with 3H-imazalil at 10 g a.i./100 kg were also
sown in soil. Plants were harvested after 1 and 3 weeks. Soil and
plant parts were analyzed for radioactivity. Most of the radioactivity
was present in the soil directly around the seed coats, 76% and 29% in
the seed coats. After 3 weeks the green parts of the plants contained
only 6% of the radioactivity which had originally adhered to the
seeds.
In soil
The half-life of imazalil (1 mg/kg in a sandy loam and a clay loam)
was 4-5 months (Anon., 1976e), After a 36 weeks incubation period 6-7%
of the initially applied 3H-imazalil was present as the metabolite
T 824: alpha-(2,4-dichlorophenyl)-1H-imidazole-1-ethanol. About 13%
was found to consist of more polar compounds than imazalil and T 824,
but could not be identified. 9-13% could not be extracted and was
probably due to unchanged imazalil bound to soil constituents. Little
or no loss of label occurred. The results are given in Table 5.
TABLE 5. Extractable and unextractable radioactivity from soils after treatment
with 1 mg/kg 3H-imazalil (mean result from 2 flasks) Anon., 1976e
Radioactivity as% of that initially
applied to soil
Sandy loam Clay loam
Weeks incubation 0 16 36 0 16 36
Methanol extract
(fraction 1) 63 67.5 62 66.5 51 44
Methanol-NaOH extract
(fraction 2) 18 14.5 18.5 17 25.5 30
Unextracted radioactivity 12.5 9 9 8 13 12
Total 93.5 89.5 89.5 91.5 89.5 86
Imazalil in a 20% E.C. formulation was sprayed 1, 4 and 7 times with
14 days interval, directly on the soil of field plots sown with spring
barley. High dosage rates and water volumes were used in order to
obtain detectable residue levels in the soil layers (0.1, 0.4 and 2.8
kg a.i./ha in 4000 1 water/ha). Results at the two lower
concentrations are shown in Table 6.
TABLE 6. Concentration of imazalil in field soil treated with varying
doses of imazalil
(Anon., 1976f; Anon., 1976h)
Dosage rate Depth imazalil (mg/kg) after months
(cm) 0 1 3
untreated 0-10 <0.01 <0.01 <0.01
10-20 <0.01 <0.01 <0.01
1 X 0.1 kg a.i./ha 0-10 0.021 0.019 0.016
10-20 <0.01 <0.01 <0.01
4 X 6.4 kg a.i./ha 0-10 0.066 0.074 0.048
10-20 0.013 0.016 <0.01
7 X 0.4 kg a.i./ha 0-10 0.085 0.14 0.087
10-20 <0.01 0.018 0.017
Comments; - Residue levels were very low in the upper layer of 0-10
cm, 0.085-0.14 mg/kg after 7 applications, although
practically 90% of the 7 × 4000 1/ha spraywash came into
direct contact with the soil.
- There was no leaching of practical significance within 6
months into the soil layer 10-20 cm deep; residues of 0.018
mg/kg were close to the detection limit.
- No accumulation occurred even after 7 sprays at the
highest dosage.
- The half-life for the initial concentration of 0.021 mg/kg
was 6.5 ± 2.5 months.
METHODS OF RESIDUE ANALYSIS
Gas-chromatographic procedures for the determination of imazalil in
and on citrus fruit are described by various authors (Wynants and
Woestenborghs, 1975b) Greenberg and Resnick, 1977, Wynants, 1977a;
Kraght, 1977b)
The methods comprise extraction of imazalil as the free base in
alkaline solution with an organic solvent (e.g. benzene, ethyl acetate
or chloroform). Clean-up can be achieved by repeated extraction in
acidified aqueous and alkaline organic solution. For the final
gas-chromatographic determination 3% OV-17 on Supelcoport in a glass
column (300°C silanized) and an electron capture detector can be used.
Flame-photometric, alkali-flame and electrolytic conductivity
detectors, although less sensitive then ECD, are much more selective
and thus avoid many interferences which may be encountered with the
ECD. The lower limit of determination depends, inter alia, on the
commodity to be analysed and is generally between 0.01 and 0.1 mg/kg.
Recoveries were 80-95%.
Similar methods for the determination of imazalil residues in or on
cucurbitaceae (Wynants and Woestenborghs, 1975a), bananas (Anonymous,
1976d), wheat and wheat-straw (ten Broeke and Dornseiffen, 1976) are
also available.
Recently a new method has been developed for the determination of
imazalil and its metabolite
alpha-(2,4-dichlorophenyl)-1H-imidazole-1-othanol (T824), based on the
above principles but using heptane/isoamyl alcohol (95/5, v/v) for the
extraction. When T 824 is converted by
N,O-bis(trimethylsilyl)acetamide to a silyl ether it can be
quantatively determined by GC with 3% OV-225 on Gas Chrom Q using an
ECD (Wynants, 1977b; ten Broeke and Dornseiffen, 1977).
NATIONAL TOLERANCES
No national tolerances were known to the Meeting.
APPRAISAL
Imazalil is a systemic fungicide which is effective against fungi
imperfecti and some fungi of the class "Ascomycetae". It is also
effective against benzimidazole-resistant strains of plant pathogenic
fungi. It is formulated as a seed dressing for cereals, an
emulsifiable concentrate and a wettable powder. The main pre-harvest
applications for the latter formulations are on bananas and cucurbits:
other uses are being developed. Imazalil is also highly effective
against fungal infections on bananas and citrus fruit after harvest.
It is used in several European countries.
In plants alpha-(2,4-dichlorophenyl)-1H-imidazole-1-ethanol and other
unidentified polar, hydrophilic compounds are the main degradation
products. The half-lives of imazalil on oranges and in soil are 12-20
weeks and 4-5 months respectively. No accumulation in soil was
observed.
Following pre-harvest application in accordance with good agricultural
practice, the maximum residue levels of imazalil in or on cucumbers
and gherkins under glass were below 0.5 mg/kg. Post-harvest
application at recomended dosages resulted in residues of imazalil
below 2 mg/kg on bananas and below 0.2 mg/kg in the pulp. Residues
were below 5 mg/kg on citrus fruit and below 0.1 mg/kg in the peeled
fruit and in wheat straw; residues in/on wheat grain were below 0.01
mg/kg.
Residue levels on citrus fruit largely depend on the dosage applied;
no significant differences in the residue levels on fruits which were
stored for 32 days at 40°C and 22-25°C were noticed.
Gas-chromatographic methods for the determination of imazalil in
various crops, suitable for regulatory purposes, have been elaborated.
RECOMMENDATIONS
The following temporary maximum residue limits are recommended on the
basis of data resulting from supervised trials involving pre- and
post-harvest uses. They refer to imazalil only.
Commodity Temporary limit, Pre- or post-harvest
mg/kg interval (days) on
which recommendations
is based (post-harvest
in brackets)
Citrus fruit
(whole) 5 (0-42)
Bananas
(whole) 2 (0-100)
Cucumbers,
gherkins 0.5 0-1
Bananas
(without peel) 0.2 (0-100)
Citrus fruit
(without peel) 0.1 (0-42)
Wheat straw 0.1
Wheat (grain) 0.01*
* at or about the limit of determination.
FURTHER WORK OR INFORMATION
REQUIRED (by July 1981)
1. Comparative acute toxicity studies of the different salts.
2. Farther long-term studies.
DESIRABLE
1. Pharmacokinetic studies of the different salts.
2. Metabolic studies with imazalil labelled with 14C.
3. Further information on the nature and level of metabolites and
degradation products in plants.
REFERENCES
Anonymous (1975a) "Physico-chemistry of R 18531 -imazalil nitrate,
R 23979 - imazalil, and R 27180 -imazalil sulphate". Janssen
Pharmaceutica; Pre-formulation report no. 496; 750107.
Anonymous (1975b) "Degradation of imazalil in oranges". Biochemical
Department of the Organisch Chemisch Instituut TNO - Utrecht - The
Netherlands; Progress report of project 1.1; 751106.
Anonymous (1976a) "Metabolism of imazalil applied to oranges".
Biochemical Department of the Institute for Organic Chemistry TNO
- Utrecht - The Netherlands; Report of project 1.II; 760224.
Anonymous (1976b) "Metabolism of imazalil in barley plants".
Biochemical Department of the Institute for Organic Chemistry
- Utrecht - The Netherlands; Addition to the first report of project
2; 760521.
Anonymous (1976c) "Testicide residue report - imazalil on bananas".
Jansses Pharmaceutica., 760816.
Anonymous (1976d) "Procedure for imazalil in whole bananas". Janssen
Pharmaceutica; 761009.
Anonymous (1976e) "A study of the fate of imazalil in soil".
Biochemical Department of the Institute for Organic Chemistry TNO
- Utrecht - The Netherlands; Report of Project 4; 761021.
Anonymous (1976f) "Degradation of imazalil in soil under field
conditions". Biochemical Department of the Institute for Organic
Chemistry TNO - Utrecht - The Netherlands; Report of project 5, part
1; 761105. Attached letter of TNO - 770524.
Anonymous (1976g) "Residue summary sheets for imazalil in bananas".
Morse Laboratories, Ltd. - Sacramento - California - U.S.A.; 761129.
Anonymous (1976h) "Degradation of imazalil in soil under field
conditions". Biochemical Department of the Institute for Organic
Chemistry TNO - Utrecht - The Netherlands; Report of project 5, part
2; 761209.
Bartlett, D.H., Ballard, N.E. (1975) "The effectiveness of guazatine
and imazalil as seed treatment fungicides in barley". Proc. 8th Brit.
Insect. Fung. Conf. - 1975.
Beijers, H.P. (1975) "Imazalil residues in citrus samples". SABS
- South Africa; Report no. 0311/8882/M410; 751128.
Beijers, H.P. (1976) "Imazalil residues in citrus samples". SABS Rep.
No. 0311/8960/N400; Pretoria, 10 Nov. 1976.
Bliss, G.W., Orme, J.P.R. (1976) "Certificate of analysis - Analysis
of residues of imazalil in oranges". Huntingdon Research Centre;
Huntingdon - U.K. report VPT/2; 761223.
ten Broeke, R., Dornseiffen, J.W. (1976) "Imazalil residues in wheat
and wheat-straw". Keuringsdienst van Waren - Amsterdam; Rapport
KvW 197/Vondelingenplaat 1976-8; 761020.
ten Broeke, R., Dornseiffen, J.W. (1977) Residues of imazalil and its
metabolite T 824 in gherkins under glass. KvW 203/4.2. (2.1.01) 1976;
July 1977.
Corpel J., Gauthier, M. (1977) "Campagne orange du Marco".
Commissariat a l'energie atomique; Centre d'études nucléaires de
Cadarache; 770407.
Frossard, P., Laville, E., Plaud, G. (1976) "Etude des traitements
fongicides appliques aux bananes aprés récolte; III: Action de
l'imazalil". Fruits 31, 361-364 (1976).
Greenberg, R., Resnick, C., (1977) "A gas-liquid chromatographic
method for determining imazalil residues in citrus fruit"; Pestic.
Sci. 8, 59-64 (1977).
Helsen, W. (1976) "Thermal stability of imazalil and salts". Janssen
Pharmaceutica; memo dated 760813.
Heykants, J., Meuldermans, W., Hurkmans, R. (1975) "On the excretion,
metabolism and tissue distribution of imazalil-3H in the male and
female Wistar rat". Janssen Pharmaceutica; Department of drug
metabolism; 7512.
Kraght, A.J. (1977a) "Metabolism of imazalil applied to
oranges - Metabolite study by tracer technique". Pennwalt
Corporation - Decco Division - U.S.A. 770518.
Kraght, A.J. (1977b) "Residue data of imazalil on citrus". Pennwalt
Corporation - Decco Division - U.S.A.; 770518.
Laville, E., Herding, P.R., Dagan, Y., Rahat, M., Kraght, A.J. (1977)
"Studies on imazalil as potential treatment for control of citrus
fruit decay". Proc. Int. Soc. Citriculture, 1977.
Maier-Bode, H. (1976) "Imazalil residues in oranges after post-harvest
treatment". Rickenbach - Federal Republic of Germany; 760622.
Marsboom, R. (1970) Potential of R18531 for Embryotoxicity and
Teratogenic Effects in Rats Receiving R18531 Orally. Unpublished
report from Janssen Pharmaceutical submitted to the World Health
Organization by Pennwalt Corporation.
Marsboom, R., Vandesteen, R., Herin, V. and Pardoel, L. (1971) Safety
Evaluation of R18531 During 14 Weeks of Oral Treatment to Rats.
Unpublished report from Janssen Pharmaceutical submitted to the World
Health Organization by Pennwalt Corporation.
Marsboom, R. (1975a) Oral Embryotoxicity and Teratogenicity Study in
Wistar Rate During the Peri- and Postnatal Study (Segment III). A
report from Jansses Pharmaceutical submitted to the World Health
Organization by Pennwalt Corporation. (unpublished report)
Marsboom, R., Herin, V., Vandesteene, R. and Pardoell L. (1975b) Oral
Toxicity Study in Wistar Rats (repeated dosage for 6, 12 or 24
months). Unpublished report from Janssen Pharmaceutical submitted to
the World Health Organization by Pennwalt Corporation.
Marshboom, R. (1975c) Oral Three Generation Study in Wistar Rats.
Unpublished report from Janssen Pharmaceutics, submitted to the WorId
Health Organization by Pennwalt Corporation.
Marshboom, R. (1976a) Dominant Lethal Test in Male Mice. Unpublished
report from Janssen Pharmaceutica, submitted to the World Health
Organization by Pennwalt Corporation.
Marsboom, R. (1976b) Dominant Lethal Test in Female Mice (single oral
dose). Unpublished report from Janssen Pharmaceutica, submitted to the
World Health Organization by Pennwalt Corporation.
Marsboom, R. (1977a) Oral Male and Female Fertility Study in Wistar
Rats (Segment I). Unpublished report from Janssen Pharmaceutical
submitted to the World Health Organization by Pennwalt Corporation.
Marsboom, R., Herin, V., Van Desteene, R. and Van Belle. (1977b) Oral
Toxicity Study in Beagle Dogs (repeated dosage for 24 months).
Unpublished report from Janssen Pharmaceutical submitted to the World
Health Organization by Pennwalt Corporation.
Melin, Ph., Plaud, G., Tezenas du Moricel, H., Laville, E. (1976)
"Action do l'imazalil sur le niveau d'infestation et l'état
d'évolution de la cercosporiose". Fruits 31 (10)t 599-602 (1976).
Meuldermans, W., Swysen, E., Lauwers, W., Heykrantz, Jr. (1977a) An
in-vitro Study of Imazalil - 3H in the Rat. Unpublished report from
Janssen Pharmaceutica, submitted to the World Health Organization by
Pennwalt Corporation.
Meuldermans, W., Hurkmans, J., Lanwers, W., and Heykants, J. (1977b)
The Excretion and Biotransformation of Imazalil and One of Its
Metabolites (R14821) in the Wistar Rat. Unpublished report from
Janssen Pharmaceutical Department of Drug Metabolism and Analytical
Department, submitted to the World Health Organization by Pennwalt
Corporation.
Nangniot, P. (1977) "Teneurs résiduaires an imazalil dans des
concombers". Centre de Recherche de
Phytophaxmacie - Gembloux - Belgique; 770428.
Niemegeers, G.J.E. (1973) Acute Oral Toxicity in Rats. Unpublished
report from Janssen Pharmaceutica, submitted to the World Health
Organization by Pennwalt Corporation.
Niemegeers, C.J.E. (1976) Acute Oral Toxicity of R23979 - 20% E.C. in
Rats. Unpublished report from Janssen Pharmaceutica, submitted to the
World Health Organization by Pennwalt Corporation.
Niemegeers, G.J.E. (1977) Acute Intraperitoneal Toxicity of R23979 in
Wistar Rats. Unpublished report from Janssen Pharmaceutica, submitted
to the World Health Organization by the Pennwalt Corporation.
Pattyn, W. (1976) "Purity of R23979 -imazalil technical". Janssen
Pharmaceutica; Chemical Quality Control Department; 761001.
Stover, R.H. (1976) "Imazalil efficacy data". Vining C. Dunlap
Laboratories of the United Fruit Company in Honduras; 761102.
Attachment: Stover, R.H., Dickson, J.D. "Leaf sport of bananas caused
by Mycosphaerella musicola: Methods of measuring spotting prevalence
and severity". Tropical Agriculture 47, 289-302. (1970)
Van den Bossche, H. (1977) Studies on the mode of action of imazalil.
Janssen Pharmaceutica; 770425. Unpublished.
Van Ravestyn, O. and Marsboom, R. (1975) Acute Dermal Toxicity in Rats
(single dosage). Unpublished report from Janssen Pharmaceutical
submitted to the World Health Organization by Pennwalt Corporation.
Van Tuyl, J.Y. (1977) Genetics of fungal resistance to systemic
fungioides. Mededelinger
Landborwhogeschool - Wageningen - Netherlands; 77-2.
Wynants, J, Woestenborghs, R. (1975a) "A gas-chromatographic assay
method for imazalil in cucumbers". Janssen Pharmaceutics; 7512.
Wynants, J., Woestenborghs, R. (1975b) "A gas-chromatographic assay
method for imazalil in citrus fruit". Janssen Pharmaceutics; 7512.
Wynants, J., Woestenborghs, R. (1976) "Residue analysis of imazalil in
cucumbers". Janssen (1976) Pharmaceutics; 7601.
Wynants J. (1977a) "Chromatographic assay methods of imazalil in
citrus fruit". Proc. Int. Soc. Citriculture, 1977, vol. H: 38.
Wynants J. (1977b) "Residue detection method for imazalil and its
metabolite T824". Janssen Pharmaceutics; provisional report; 770420.
The polar, hydrophilic fraction containing nearly 50% of the
radioactivity present in the methanol extract of the peel did not
contain tritiated water or other volatile radioactivity. Since
concentrations of radioactivity were identical before and after
lyophilisation of this fraction, it seems that no label loss is to be
expected during metabolism (Anon., 1976a).
A similar test was conducted under conditions closer to commercial
practice. In addition, a more rigorous solvent extraction procedure
was used in order to recover more radioactive material.
As metabolites were expected to be present in very small amounts, the
fruit was treated with 4 times the normal concentration, 2000 mg
a.i./1 aqueous dip for 1 minute. The oranges were stored for 12 weeks
and the distribution of radioactivity in the fruit and the presence of
metabolites were studied during and after this period.
Most of the radioactivity (89%) remained as unchanged imazalil, in
contrast to the above results where considerable degradation was
reported. This was due to the extreme storage conditions used in the
above trial (high temperature and light), which are completely
artificial and not related to commercial practice. No tritium exchange
was detected, thus the tritium labelling was suitable for the purpose
of the study. 99.5% or more of the radioactivity in the
3H-imazalil-treated oranges was solvent-extractable: about 0.5% or
less remained in the insoluble residue. After 12 weeks of storage
approximately 10-12% of the original radioactivity appeared in the
form of an unknown water-soluble compound (Kraght, 1977a).
The distribution of imazalil in/on oranges was determined at intervals
after treatment at 1000 mg/kg (Anon., 1975b). Results are shown in
Table 4.
Gherkins sprayed seven times with imazalil under practical conditions
in a glasshouse were sampled three days after the last application,
then stored at -18°C for 3-7 weeks. Residues in/on the gherkins were
between 0.03 -0.05 mg imazalil/kg and at or below 0.01 mg
T 824-metabolite/kg (ten Broeke and Dornseiffen, 1977).
Barley seeds treated with 3H-imazalil were sown in perlite. After 3
weeks the plants were harvested and the roots and leaves extracted
separately. Large amounts of radioactivity were present in an
unextractable form in both the roots and the leaves. In a methanol
extract of the roots imazalil accounted for 33% of the total
radioactivity and in a toluene extract for 81%. A certain amount of
the methanol-insoluble residue may consist of physically bound
imazalil. Only about 7% or less of the label was the metabolite T 824
(Anon., 1976b).
TABLE 4. Distribution of imazalil in oranges after treatment at 1000 mg/kg
Imazalil, mg/kg, at indicated interval after dipping (Anon., 1975b)
0 weeks 4 weeks 8 weeks 12 weeks
dipped dipped dipped dipped
´ min. 1 min. ´ min. 1 min. ´ min. 1 min ´ min. 1 min.
peel 0.9 2.4 0.51 0.85 2.3 1.7 0.8 1.4
4.0 2.5 0.55 0.49 1.2 1.5 0.7 1.5
pulp n.d. n.d. 0.053 0.047 0.047 0.12 0.10 0.36
0.13 n.d. 0.027 0.062 0.14 0.11 0.09 0.06
n.d. = not determined.
Comments: -degradation of imazalil m orangss is slow;
-half-life is estimated to be 12 to 20 weeks
-only small amounts of imazalil are present in the fruit flesh and part
of this may result from contamination during peeling.
Barley seeds were treated with a dose of 3H-imazalil corresponding
to 10 g a.i./100 kg seed. After germination on water agar for 9 days,
42% of the radioactivity was found in the agar and 37% in the seed
coats. Only 10% of the total radioactivity was present in the roots
and 2% in the leaves of the seedlings.
Barley seeds treated with 3H-imazalil at 10 g a.i./100 kg were also
sown in soil. Plants were harvested after 1 and 3 weeks. Soil and
plant parts were analyzed for radioactivity. Most of the radioactivity
was present in the soil directly around the seed coats, 76% and 29% in
the seed coats. After 3 weeks the green parts of the plants contained
only 6% of the radioactivity which had originally adhered to the
seeds.
In soil
The half-life of imazalil (1 mg/kg in a sandy loam and a clay loam)
was 4-5 months (Anon., 1976e), After a 36 weeks incubation period 6-7%
of the initially applied 3H-imazalil was present as the metabolite
T 824: alpha-(2,4-dichlorophenyl)-1H-imidazole-1-ethanol. About 13%
was found to consist of more polar compounds than imazalil and T 824,
but could not be identified. 9-13% could not be extracted and was
probably due to unchanged imazalil bound to soil constituents. Little
or no loss of label occurred. The results are given in Table 5.
TABLE 5. Extractable and unextractable radioactivity from soils after treatment
with 1 mg/kg 3H-imazalil (mean result from 2 flasks) Anon., 1976e
Radioactivity as% of that initially
applied to soil
Sandy loam Clay loam
Weeks incubation 0 16 36 0 16 36
Methanol extract
(fraction 1) 63 67.5 62 66.5 51 44
Methanol-NaOH extract
(fraction 2) 18 14.5 18.5 17 25.5 30
Unextracted radioactivity 12.5 9 9 8 13 12
Total 93.5 89.5 89.5 91.5 89.5 86
Imazalil in a 20% E.C. formulation was sprayed 1, 4 and 7 times with
14 days interval, directly on the soil of field plots sown with spring
barley. High dosage rates and water volumes were used in order to
obtain detectable residue levels in the soil layers (0.1, 0.4 and 2.8
kg a.i./ha in 4000 1 water/ha). Results at the two lower
concentrations are shown in Table 6.
TABLE 6. Concentration of imazalil in field soil treated with varying
doses of imazalil
(Anon., 1976f; Anon., 1976h)
Dosage rate Depth imazalil (mg/kg) after months
(cm) 0 1 3
untreated 0-10 <0.01 <0.01 <0.01
10-20 <0.01 <0.01 <0.01
1 X 0.1 kg a.i./ha 0-10 0.021 0.019 0.016
10-20 <0.01 <0.01 <0.01
4 X 6.4 kg a.i./ha 0-10 0.066 0.074 0.048
10-20 0.013 0.016 <0.01
7 X 0.4 kg a.i./ha 0-10 0.085 0.14 0.087
10-20 <0.01 0.018 0.017
Comments; - Residue levels were very low in the upper layer of 0-10
cm, 0.085-0.14 mg/kg after 7 applications, although
practically 90% of the 7 × 4000 1/ha spraywash came into
direct contact with the soil.
- There was no leaching of practical significance within 6
months into the soil layer 10-20 cm deep; residues of 0.018
mg/kg were close to the detection limit.
- No accumulation occurred even after 7 sprays at the
highest dosage.
- The half-life for the initial concentration of 0.021 mg/kg
was 6.5 ± 2.5 months.
METHODS OF RESIDUE ANALYSIS
Gas-chromatographic procedures for the determination of imazalil in
and on citrus fruit are described by various authors (Wynants and
Woestenborghs, 1975b) Greenberg and Resnick, 1977, Wynants, 1977a;
Kraght, 1977b)
The methods comprise extraction of imazalil as the free base in
alkaline solution with an organic solvent (e.g. benzene, ethyl acetate
or chloroform). Clean-up can be achieved by repeated extraction in
acidified aqueous and alkaline organic solution. For the final
gas-chromatographic determination 3% OV-17 on Supelcoport in a glass
column (300°C silanized) and an electron capture detector can be used.
Flame-photometric, alkali-flame and electrolytic conductivity
detectors, although less sensitive then ECD, are much more selective
and thus avoid many interferences which may be encountered with the
ECD. The lower limit of determination depends, inter alia, on the
commodity to be analysed and is generally between 0.01 and 0.1 mg/kg.
Recoveries were 80-95%.
Similar methods for the determination of imazalil residues in or on
cucurbitaceae (Wynants and Woestenborghs, 1975a), bananas (Anonymous,
1976d), wheat and wheat-straw (ten Broeke and Dornseiffen, 1976) are
also available.
Recently a new method has been developed for the determination of
imazalil and its metabolite
alpha-(2,4-dichlorophenyl)-1H-imidazole-1-othanol (T824), based on the
above principles but using heptane/isoamyl alcohol (95/5, v/v) for the
extraction. When T 824 is converted by
N,O-bis(trimethylsilyl)acetamide to a silyl ether it can be
quantatively determined by GC with 3% OV-225 on Gas Chrom Q using an
ECD (Wynants, 1977b; ten Broeke and Dornseiffen, 1977).
NATIONAL TOLERANCES
No national tolerances were known to the Meeting.
APPRAISAL
Imazalil is a systemic fungicide which is effective against fungi
imperfecti and some fungi of the class "Ascomycetae". It is also
effective against benzimidazole-resistant strains of plant pathogenic
fungi. It is formulated as a seed dressing for cereals, an
emulsifiable concentrate and a wettable powder. The main pre-harvest
applications for the latter formulations are on bananas and cucurbits:
other uses are being developed. Imazalil is also highly effective
against fungal infections on bananas and citrus fruit after harvest.
It is used in several European countries.
In plants alpha-(2,4-dichlorophenyl)-1H-imidazole-1-ethanol and other
unidentified polar, hydrophilic compounds are the main degradation
products. The half-lives of imazalil on oranges and in soil are 12-20
weeks and 4-5 months respectively. No accumulation in soil was
observed.
Following pre-harvest application in accordance with good agricultural
practice, the maximum residue levels of imazalil in or on cucumbers
and gherkins under glass were below 0.5 mg/kg. Post-harvest
application at recomended dosages resulted in residues of imazalil
below 2 mg/kg on bananas and below 0.2 mg/kg in the pulp. Residues
were below 5 mg/kg on citrus fruit and below 0.1 mg/kg in the peeled
fruit and in wheat straw; residues in/on wheat grain were below 0.01
mg/kg.
Residue levels on citrus fruit largely depend on the dosage applied;
no significant differences in the residue levels on fruits which were
stored for 32 days at 40°C and 22-25°C were noticed.
Gas-chromatographic methods for the determination of imazalil in
various crops, suitable for regulatory purposes, have been elaborated.
RECOMMENDATIONS
The following temporary maximum residue limits are recommended on the
basis of data resulting from supervised trials involving pre- and
post-harvest uses. They refer to imazalil only.
Commodity Temporary limit, Pre- or post-harvest
mg/kg interval (days) on
which recommendations
is based (post-harvest
in brackets)
Citrus fruit
(whole) 5 (0-42)
Bananas
(whole) 2 (0-100)
Cucumbers,
gherkins 0.5 0-1
Bananas
(without peel) 0.2 (0-100)
Citrus fruit
(without peel) 0.1 (0-42)
Wheat straw 0.1
Wheat (grain) 0.01*
* at or about the limit of determination.
FURTHER WORK OR INFORMATION
REQUIRED (by July 1981)
1. Comparative acute toxicity studies of the different salts.
2. Farther long-term studies.
DESIRABLE
1. Pharmacokinetic studies of the different salts.
2. Metabolic studies with imazalil labelled with 14C.
3. Further information on the nature and level of metabolites and
degradation products in plants.
REFERENCES
Anonymous (1975a) "Physico-chemistry of R 18531 -imazalil nitrate,
R 23979 - imazalil, and R 27180 -imazalil sulphate". Janssen
Pharmaceutica; Pre-formulation report no. 496; 750107.
Anonymous (1975b) "Degradation of imazalil in oranges". Biochemical
Department of the Organisch Chemisch Instituut TNO - Utrecht - The
Netherlands; Progress report of project 1.1; 751106.
Anonymous (1976a) "Metabolism of imazalil applied to oranges".
Biochemical Department of the Institute for Organic Chemistry TNO
- Utrecht - The Netherlands; Report of project 1.II; 760224.
Anonymous (1976b) "Metabolism of imazalil in barley plants".
Biochemical Department of the Institute for Organic Chemistry
- Utrecht - The Netherlands; Addition to the first report of project
2; 760521.
Anonymous (1976c) "Testicide residue report - imazalil on bananas".
Jansses Pharmaceutica., 760816.
Anonymous (1976d) "Procedure for imazalil in whole bananas". Janssen
Pharmaceutica; 761009.
Anonymous (1976e) "A study of the fate of imazalil in soil".
Biochemical Department of the Institute for Organic Chemistry TNO
- Utrecht - The Netherlands; Report of Project 4; 761021.
Anonymous (1976f) "Degradation of imazalil in soil under field
conditions". Biochemical Department of the Institute for Organic
Chemistry TNO - Utrecht - The Netherlands; Report of project 5, part
1; 761105. Attached letter of TNO - 770524.
Anonymous (1976g) "Residue summary sheets for imazalil in bananas".
Morse Laboratories, Ltd. - Sacramento - California - U.S.A.; 761129.
Anonymous (1976h) "Degradation of imazalil in soil under field
conditions". Biochemical Department of the Institute for Organic
Chemistry TNO - Utrecht - The Netherlands; Report of project 5, part
2; 761209.
Bartlett, D.H., Ballard, N.E. (1975) "The effectiveness of guazatine
and imazalil as seed treatment fungicides in barley". Proc. 8th Brit.
Insect. Fung. Conf. - 1975.
Beijers, H.P. (1975) "Imazalil residues in citrus samples". SABS
- South Africa; Report no. 0311/8882/M410; 751128.
Beijers, H.P. (1976) "Imazalil residues in citrus samples". SABS Rep.
No. 0311/8960/N400; Pretoria, 10 Nov. 1976.
Bliss, G.W., Orme, J.P.R. (1976) "Certificate of analysis - Analysis
of residues of imazalil in oranges". Huntingdon Research Centre;
Huntingdon - U.K. report VPT/2; 761223.
ten Broeke, R., Dornseiffen, J.W. (1976) "Imazalil residues in wheat
and wheat-straw". Keuringsdienst van Waren - Amsterdam; Rapport
KvW 197/Vondelingenplaat 1976-8; 761020.
ten Broeke, R., Dornseiffen, J.W. (1977) Residues of imazalil and its
metabolite T 824 in gherkins under glass. KvW 203/4.2. (2.1.01) 1976;
July 1977.
Corpel J., Gauthier, M. (1977) "Campagne orange du Marco".
Commissariat a l'energie atomique; Centre d'études nucléaires de
Cadarache; 770407.
Frossard, P., Laville, E., Plaud, G. (1976) "Etude des traitements
fongicides appliques aux bananes aprés récolte; III: Action de
l'imazalil". Fruits 31, 361-364 (1976).
Greenberg, R., Resnick, C., (1977) "A gas-liquid chromatographic
method for determining imazalil residues in citrus fruit"; Pestic.
Sci. 8, 59-64 (1977).
Helsen, W. (1976) "Thermal stability of imazalil and salts". Janssen
Pharmaceutica; memo dated 760813.
Heykants, J., Meuldermans, W., Hurkmans, R. (1975) "On the excretion,
metabolism and tissue distribution of imazalil-3H in the male and
female Wistar rat". Janssen Pharmaceutica; Department of drug
metabolism; 7512.
Kraght, A.J. (1977a) "Metabolism of imazalil applied to
oranges - Metabolite study by tracer technique". Pennwalt
Corporation - Decco Division - U.S.A. 770518.
Kraght, A.J. (1977b) "Residue data of imazalil on citrus". Pennwalt
Corporation - Decco Division - U.S.A.; 770518.
Laville, E., Herding, P.R., Dagan, Y., Rahat, M., Kraght, A.J. (1977)
"Studies on imazalil as potential treatment for control of citrus
fruit decay". Proc. Int. Soc. Citriculture, 1977.
Maier-Bode, H. (1976) "Imazalil residues in oranges after post-harvest
treatment". Rickenbach - Federal Republic of Germany; 760622.
Marsboom, R. (1970) Potential of R18531 for Embryotoxicity and
Teratogenic Effects in Rats Receiving R18531 Orally. Unpublished
report from Janssen Pharmaceutical submitted to the World Health
Organization by Pennwalt Corporation.
Marsboom, R., Vandesteen, R., Herin, V. and Pardoel, L. (1971) Safety
Evaluation of R18531 During 14 Weeks of Oral Treatment to Rats.
Unpublished report from Janssen Pharmaceutical submitted to the World
Health Organization by Pennwalt Corporation.
Marsboom, R. (1975a) Oral Embryotoxicity and Teratogenicity Study in
Wistar Rate During the Peri- and Postnatal Study (Segment III). A
report from Jansses Pharmaceutical submitted to the World Health
Organization by Pennwalt Corporation. (unpublished report)
Marsboom, R., Herin, V., Vandesteene, R. and Pardoell L. (1975b) Oral
Toxicity Study in Wistar Rats (repeated dosage for 6, 12 or 24
months). Unpublished report from Janssen Pharmaceutical submitted to
the World Health Organization by Pennwalt Corporation.
Marshboom, R. (1975c) Oral Three Generation Study in Wistar Rats.
Unpublished report from Janssen Pharmaceutics, submitted to the WorId
Health Organization by Pennwalt Corporation.
Marshboom, R. (1976a) Dominant Lethal Test in Male Mice. Unpublished
report from Janssen Pharmaceutica, submitted to the World Health
Organization by Pennwalt Corporation.
Marsboom, R. (1976b) Dominant Lethal Test in Female Mice (single oral
dose). Unpublished report from Janssen Pharmaceutica, submitted to the
World Health Organization by Pennwalt Corporation.
Marsboom, R. (1977a) Oral Male and Female Fertility Study in Wistar
Rats (Segment I). Unpublished report from Janssen Pharmaceutical
submitted to the World Health Organization by Pennwalt Corporation.
Marsboom, R., Herin, V., Van Desteene, R. and Van Belle. (1977b) Oral
Toxicity Study in Beagle Dogs (repeated dosage for 24 months).
Unpublished report from Janssen Pharmaceutical submitted to the World
Health Organization by Pennwalt Corporation.
Melin, Ph., Plaud, G., Tezenas du Moricel, H., Laville, E. (1976)
"Action do l'imazalil sur le niveau d'infestation et l'état
d'évolution de la cercosporiose". Fruits 31 (10)t 599-602 (1976).
Meuldermans, W., Swysen, E., Lauwers, W., Heykrantz, Jr. (1977a) An
in-vitro Study of Imazalil - 3H in the Rat. Unpublished report from
Janssen Pharmaceutica, submitted to the World Health Organization by
Pennwalt Corporation.
Meuldermans, W., Hurkmans, J., Lanwers, W., and Heykants, J. (1977b)
The Excretion and Biotransformation of Imazalil and One of Its
Metabolites (R14821) in the Wistar Rat. Unpublished report from
Janssen Pharmaceutical Department of Drug Metabolism and Analytical
Department, submitted to the World Health Organization by Pennwalt
Corporation.
Nangniot, P. (1977) "Teneurs résiduaires an imazalil dans des
concombers". Centre de Recherche de
Phytophaxmacie - Gembloux - Belgique; 770428.
Niemegeers, G.J.E. (1973) Acute Oral Toxicity in Rats. Unpublished
report from Janssen Pharmaceutica, submitted to the World Health
Organization by Pennwalt Corporation.
Niemegeers, C.J.E. (1976) Acute Oral Toxicity of R23979 - 20% E.C. in
Rats. Unpublished report from Janssen Pharmaceutica, submitted to the
World Health Organization by Pennwalt Corporation.
Niemegeers, G.J.E. (1977) Acute Intraperitoneal Toxicity of R23979 in
Wistar Rats. Unpublished report from Janssen Pharmaceutica, submitted
to the World Health Organization by the Pennwalt Corporation.
Pattyn, W. (1976) "Purity of R23979 -imazalil technical". Janssen
Pharmaceutica; Chemical Quality Control Department; 761001.
Stover, R.H. (1976) "Imazalil efficacy data". Vining C. Dunlap
Laboratories of the United Fruit Company in Honduras; 761102.
Attachment: Stover, R.H., Dickson, J.D. "Leaf sport of bananas caused
by Mycosphaerella musicola: Methods of measuring spotting prevalence
and severity". Tropical Agriculture 47, 289-302. (1970)
Van den Bossche, H. (1977) Studies on the mode of action of imazalil.
Janssen Pharmaceutica; 770425. Unpublished.
Van Ravestyn, O. and Marsboom, R. (1975) Acute Dermal Toxicity in Rats
(single dosage). Unpublished report from Janssen Pharmaceutical
submitted to the World Health Organization by Pennwalt Corporation.
Van Tuyl, J.Y. (1977) Genetics of fungal resistance to systemic
fungioides. Mededelinger
Landborwhogeschool - Wageningen - Netherlands; 77-2.
Wynants, J, Woestenborghs, R. (1975a) "A gas-chromatographic assay
method for imazalil in cucumbers". Janssen Pharmaceutics; 7512.
Wynants, J., Woestenborghs, R. (1975b) "A gas-chromatographic assay
method for imazalil in citrus fruit". Janssen Pharmaceutics; 7512.
Wynants, J., Woestenborghs, R. (1976) "Residue analysis of imazalil in
cucumbers". Janssen (1976) Pharmaceutics; 7601.
Wynants J. (1977a) "Chromatographic assay methods of imazalil in
citrus fruit". Proc. Int. Soc. Citriculture, 1977, vol. H: 38.
Wynants J. (1977b) "Residue detection method for imazalil and its
metabolite T824". Janssen Pharmaceutics; provisional report; 770420.
 Other information on identity and properties
a) Composition of technical imazalil
The technical material as manufactured contains approximately 97.5-98%
(w/w) imazalil. Detailed information on the impurities in technical
imazalil was supplied to the Joint Meeting (Pattyn, 1976).
b) Physical and chemical properties of imazalil
Physical state: Pure imazalil is a hygroscopic, nearly colourless
liquid. Technical imazalil is a slightly yellowish to brownish oily
liquid.
Molecular weight: 297.18
Freezing point: -10°C - -15°C
Boiling point: -130°C. at 10-3mm Hg
Vapour pressure: 7 × 10-8mm Hg at 20°C; 4 × 10-5mm Hg at 70°C
Refractive index: n 20 1.5643
D
Specific gravity: d23 1.2429
Solubility of imazalil and its salts (g product / 100 ml solvent at
20°C)
solvent imazalil imazalil imazalil
(base) nitrate sulphate
water 0.14 2.38 >50
methanol >50 >100 >50
ethanol >50 31.18 36.3
2-propanol >50 4.57 4.42
exylene >50 0.49 <0.005
toluene >50 0.87 <0.005
n. heptane. 5.41 <0.001 <0.005
benzene >50 <0.005
4-methyl-2-pentanone >50 1.76 0.03
hexane 5.21 <0.005 <0.005
petroleum ether 6.16 <0.005 <0.005
Stability: Imazalil is thermally stable up to approximately 285°C
(Helsen, 1976). It is chemically stable at room
temperature in the absence of light. Decomposition
occurs at elevated temperatures (80°C) and under the
influence of light. The shelf life is more than 2
years. In aqueous solution the product is stable up to
40°C at pHs ranging from 2.4 to 7.0 for at least 8
weeks (Anon., 1975a). When refluxed for 72 hours the
compound remains stable in water and in 1 N sodium
hydroxide, but decomposes in 1 N hydrochloric acid.
c) Physical and chemical properties of imazalil nitrate and sulphate
Physical state: Imazalil nitrate is a slightly beige to brownish
coloured amorphous, non-hygroscopic powder.
Imazalil sulphate is a white to beige crystalline, hygroscopic powder.
Melting range: Imazalil nitrate = 80 - 90°C
Imazalil sulphate 118 - 130°C
Stability: Imazalil nitrate and imazalil sulphate are thermally
stable up to approximately 150°C and 270°C,
respectively.
d) Formulations
Imazalil is used as 20% (w/v) emulsifiable concentrate, 50% (w/w)
wettable powder, and as a seed dressing (also used in combination with
guazatine).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Imazalil in rapidly absorbed, distributed, metabolized and excreted by
rats. Two groups of five male and two groups of five female rats were
given a single 20 mg/kg oral dose of 3H-labelled imazalil sulphate.
Almost 90% of the administered radioactivity was excreted within 96
hours, with approximately equal quantities detected in the urine and
faeces. Tissue residues ranged from 5.4 to 6.1% of the administered
radioactivity 48 hours after dosing and from 1.8 to 3.5% 96 hours
after dosing. The highest levels of radioactivity were found in the
liver, lung and kidneys. Analysis of the urine from dosed animals
indicated that 4% of the tritium was volatile by lyophilization. Thus
tritium exchange apparently occurred to a minor extent. (Heykants at
al., 1975).
Five male rats were given a single oral 20 mg/kg dose of 3H-labelled
imazalil sulphate to study the absorption, distribution, metabolism
and excretion of imazalil in the rat. Approximately 90% of the
administered radioactivity was excreted within 96 hours with
comparable quantities excreted in the urine and faeces. Similar
results were found when a single oral dose of
alpha-(2,4-diochlorophenyl)-1H-imidazole-1-ethanol (a metabolite of
imazalil) was administered. In both these studies, up to approximately
2% of the administered radioactivity was identified as tritiated water
indicating exchange of the tritium label. The relative amount of
tritiated water found in the urine increased with time (Meuldermans et
al., 1977b).
Biotransformation
Studies involving a single 20 mg/kg oral dosing of rats with
3H-labelled imazalil sulphate indicate that extensive metabolism of
imazalil occurs in the rat. In one study,
alpha-(2,4-dichlorophenyl)-1H-imidazole-1-ethanol and
2,4-dichloromandolic acid were identified as the major metabolites in
the urine. In this study 10%, of the radioactivity in the urine and 3%
of the radioactivity in the faeces was identified as unchanged
imazalil (Heykants et al., 1975).
In a more extensive metabolic study, the major metabolites identified
are shown in Figure 1. Several metabolites detected are shown. The
compound shown in brackets in Figure 1 were not identified in the
studies. In contrast to the study described above, no unchanged
imazalil was detected in the urine and only 0.1% of the administered
radioactivity was identified as unchanged imazalil in the faeces.
(Meuldermans al., 1977b).
Tritium labelled imazalil sulphate was incubated with rat liver
homogenates to study the in vitro metabolism of imazalil. The two
metabolites identified were
-(2,4-dichlorophenyl-1-1H-imidazole-1-ethanol and
1- [-(2,4-dichlorophenyl-2-(2,3-dihydroxypropyloxy)ethyl] -1H
-imidazole (Meuldermans et al., 1977a).
Experiments on the biotransformation of imazalil in plants are
described in the section "Fate of residues".
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Groups of male (6/group) and female (10/group) mice were administered
a single dose of imazalil in arachis oil, by gavage, at levels of 10,
40 or 160 mg/kg, to determine dominant lethal effects. Positive
controls received 210 mg/kg cyclophosphamide. Negative controls
received only the oil at the same volume 0.1 ml/10g body weight, as
all other groups. Following dosing each male was mated with 4
untreated females at weekly intervals, for eight consecutive weeks.
The females were caged with single untreated males for a maximum of 5
days. This was repeated for 5 consecutive weeks. No significant
dose-related differences were observed in untreated females and the
negative controls nor in the treated females when the number of dead
and live implants were expressed as a percentage of corpora lutea vs.
total implants (Marsboom, 1976a, 1976b).
Special studies on reproduction
Three separate reproduction studies were carried out using differing
dosing periods. In all three the dietary doses based on active
ingredient were 0, 5, 20 or 80 mg/kg.
In the first study groups of 20 female rats received imazalil on a
non-continuous basis for 3 generations. Prior to and after the period
of organogenesis and throughout lactation the females of the first and
second generations received only the basal laboratory diet. In each of
these generations, the dams received the various levels from day 6
through day 15 of pregnancy. In the third generation, the dams
received the various levels from 3 weeks until 3 months of age and
further from day 1 to 21 after mating. There was a trend toward a
lower number of live births at the 80 mg/kg level in all generations.
No other effects were noted at any other level. No abnormalities were
noted in any foetuses in any generation which were not within the
normal range for the rats used (Marsboom, 19750).
In the second study, rats (20/Sex/group) received the various levels
of imazalil as follows: males for 60 days prior to pairing with
females and through copulation; females for 14 days prior to pairing
all throughout pregnancy. Pregnancy rate was not effected at any level
regardless of treatment of either sex. Test group data for all other
measured or observed parameters were comparable to the control data
(Marsboom, 1977a).
In the third study 20 females/group received the various levels of
imazalil from day 16 of pregnancy through a 3 week lactation period.
No differences in percentage of pregnancies or average duration of
gestation were noted between test and control groups. Average size was
slightly decreased in the high level (9) compared with the control
(11). The percentage of dead foetuses was higher in the 80 mg/kg group
(27%) when compared to the control (4%), 5 mg/kg (0%) and 20 mg/kg,
(1%) groups. No abnormalities were observed in offspring from control
or test groups (Marsboom, 1975a).
Special study on teratology
Groups of female rats (20/Group) were fed imazalil nitrate from days 6
through 15 of pregnancy, at levels of 0, 5, 20 and 80 mg/kg day.
Foetuses were delivered by caesarean section on day 21. There were no
differences between the control group and the test groups in regard to
average number of implantations, number of live or dead foetuses,
distribution of embryos or resorbed foetuses in either uterine horn,
weights or number of pups, or body weight gains, food consumption,
survival, and number of pregnancies among the dams. No abnormalities
were noted among the pups in the control and various test groups with
waved ribs in one litter of the 80 mg/kg group (Marsboom, 1970).
Other information on identity and properties
a) Composition of technical imazalil
The technical material as manufactured contains approximately 97.5-98%
(w/w) imazalil. Detailed information on the impurities in technical
imazalil was supplied to the Joint Meeting (Pattyn, 1976).
b) Physical and chemical properties of imazalil
Physical state: Pure imazalil is a hygroscopic, nearly colourless
liquid. Technical imazalil is a slightly yellowish to brownish oily
liquid.
Molecular weight: 297.18
Freezing point: -10°C - -15°C
Boiling point: -130°C. at 10-3mm Hg
Vapour pressure: 7 × 10-8mm Hg at 20°C; 4 × 10-5mm Hg at 70°C
Refractive index: n 20 1.5643
D
Specific gravity: d23 1.2429
Solubility of imazalil and its salts (g product / 100 ml solvent at
20°C)
solvent imazalil imazalil imazalil
(base) nitrate sulphate
water 0.14 2.38 >50
methanol >50 >100 >50
ethanol >50 31.18 36.3
2-propanol >50 4.57 4.42
exylene >50 0.49 <0.005
toluene >50 0.87 <0.005
n. heptane. 5.41 <0.001 <0.005
benzene >50 <0.005
4-methyl-2-pentanone >50 1.76 0.03
hexane 5.21 <0.005 <0.005
petroleum ether 6.16 <0.005 <0.005
Stability: Imazalil is thermally stable up to approximately 285°C
(Helsen, 1976). It is chemically stable at room
temperature in the absence of light. Decomposition
occurs at elevated temperatures (80°C) and under the
influence of light. The shelf life is more than 2
years. In aqueous solution the product is stable up to
40°C at pHs ranging from 2.4 to 7.0 for at least 8
weeks (Anon., 1975a). When refluxed for 72 hours the
compound remains stable in water and in 1 N sodium
hydroxide, but decomposes in 1 N hydrochloric acid.
c) Physical and chemical properties of imazalil nitrate and sulphate
Physical state: Imazalil nitrate is a slightly beige to brownish
coloured amorphous, non-hygroscopic powder.
Imazalil sulphate is a white to beige crystalline, hygroscopic powder.
Melting range: Imazalil nitrate = 80 - 90°C
Imazalil sulphate 118 - 130°C
Stability: Imazalil nitrate and imazalil sulphate are thermally
stable up to approximately 150°C and 270°C,
respectively.
d) Formulations
Imazalil is used as 20% (w/v) emulsifiable concentrate, 50% (w/w)
wettable powder, and as a seed dressing (also used in combination with
guazatine).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Imazalil in rapidly absorbed, distributed, metabolized and excreted by
rats. Two groups of five male and two groups of five female rats were
given a single 20 mg/kg oral dose of 3H-labelled imazalil sulphate.
Almost 90% of the administered radioactivity was excreted within 96
hours, with approximately equal quantities detected in the urine and
faeces. Tissue residues ranged from 5.4 to 6.1% of the administered
radioactivity 48 hours after dosing and from 1.8 to 3.5% 96 hours
after dosing. The highest levels of radioactivity were found in the
liver, lung and kidneys. Analysis of the urine from dosed animals
indicated that 4% of the tritium was volatile by lyophilization. Thus
tritium exchange apparently occurred to a minor extent. (Heykants at
al., 1975).
Five male rats were given a single oral 20 mg/kg dose of 3H-labelled
imazalil sulphate to study the absorption, distribution, metabolism
and excretion of imazalil in the rat. Approximately 90% of the
administered radioactivity was excreted within 96 hours with
comparable quantities excreted in the urine and faeces. Similar
results were found when a single oral dose of
alpha-(2,4-diochlorophenyl)-1H-imidazole-1-ethanol (a metabolite of
imazalil) was administered. In both these studies, up to approximately
2% of the administered radioactivity was identified as tritiated water
indicating exchange of the tritium label. The relative amount of
tritiated water found in the urine increased with time (Meuldermans et
al., 1977b).
Biotransformation
Studies involving a single 20 mg/kg oral dosing of rats with
3H-labelled imazalil sulphate indicate that extensive metabolism of
imazalil occurs in the rat. In one study,
alpha-(2,4-dichlorophenyl)-1H-imidazole-1-ethanol and
2,4-dichloromandolic acid were identified as the major metabolites in
the urine. In this study 10%, of the radioactivity in the urine and 3%
of the radioactivity in the faeces was identified as unchanged
imazalil (Heykants et al., 1975).
In a more extensive metabolic study, the major metabolites identified
are shown in Figure 1. Several metabolites detected are shown. The
compound shown in brackets in Figure 1 were not identified in the
studies. In contrast to the study described above, no unchanged
imazalil was detected in the urine and only 0.1% of the administered
radioactivity was identified as unchanged imazalil in the faeces.
(Meuldermans al., 1977b).
Tritium labelled imazalil sulphate was incubated with rat liver
homogenates to study the in vitro metabolism of imazalil. The two
metabolites identified were
-(2,4-dichlorophenyl-1-1H-imidazole-1-ethanol and
1- [-(2,4-dichlorophenyl-2-(2,3-dihydroxypropyloxy)ethyl] -1H
-imidazole (Meuldermans et al., 1977a).
Experiments on the biotransformation of imazalil in plants are
described in the section "Fate of residues".
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Groups of male (6/group) and female (10/group) mice were administered
a single dose of imazalil in arachis oil, by gavage, at levels of 10,
40 or 160 mg/kg, to determine dominant lethal effects. Positive
controls received 210 mg/kg cyclophosphamide. Negative controls
received only the oil at the same volume 0.1 ml/10g body weight, as
all other groups. Following dosing each male was mated with 4
untreated females at weekly intervals, for eight consecutive weeks.
The females were caged with single untreated males for a maximum of 5
days. This was repeated for 5 consecutive weeks. No significant
dose-related differences were observed in untreated females and the
negative controls nor in the treated females when the number of dead
and live implants were expressed as a percentage of corpora lutea vs.
total implants (Marsboom, 1976a, 1976b).
Special studies on reproduction
Three separate reproduction studies were carried out using differing
dosing periods. In all three the dietary doses based on active
ingredient were 0, 5, 20 or 80 mg/kg.
In the first study groups of 20 female rats received imazalil on a
non-continuous basis for 3 generations. Prior to and after the period
of organogenesis and throughout lactation the females of the first and
second generations received only the basal laboratory diet. In each of
these generations, the dams received the various levels from day 6
through day 15 of pregnancy. In the third generation, the dams
received the various levels from 3 weeks until 3 months of age and
further from day 1 to 21 after mating. There was a trend toward a
lower number of live births at the 80 mg/kg level in all generations.
No other effects were noted at any other level. No abnormalities were
noted in any foetuses in any generation which were not within the
normal range for the rats used (Marsboom, 19750).
In the second study, rats (20/Sex/group) received the various levels
of imazalil as follows: males for 60 days prior to pairing with
females and through copulation; females for 14 days prior to pairing
all throughout pregnancy. Pregnancy rate was not effected at any level
regardless of treatment of either sex. Test group data for all other
measured or observed parameters were comparable to the control data
(Marsboom, 1977a).
In the third study 20 females/group received the various levels of
imazalil from day 16 of pregnancy through a 3 week lactation period.
No differences in percentage of pregnancies or average duration of
gestation were noted between test and control groups. Average size was
slightly decreased in the high level (9) compared with the control
(11). The percentage of dead foetuses was higher in the 80 mg/kg group
(27%) when compared to the control (4%), 5 mg/kg (0%) and 20 mg/kg,
(1%) groups. No abnormalities were observed in offspring from control
or test groups (Marsboom, 1975a).
Special study on teratology
Groups of female rats (20/Group) were fed imazalil nitrate from days 6
through 15 of pregnancy, at levels of 0, 5, 20 and 80 mg/kg day.
Foetuses were delivered by caesarean section on day 21. There were no
differences between the control group and the test groups in regard to
average number of implantations, number of live or dead foetuses,
distribution of embryos or resorbed foetuses in either uterine horn,
weights or number of pups, or body weight gains, food consumption,
survival, and number of pregnancies among the dams. No abnormalities
were noted among the pups in the control and various test groups with
waved ribs in one litter of the 80 mg/kg group (Marsboom, 1970).
 Acute Toxicity
Species Route Sex LD50 Reference
Rat Oral M 376 Niemegeers, 1973
ip M 288 Niemegeers, 1977
F 155
Dermal M 4200 Van Ravestyn &
F 4800 Marsboom, 1975
Oral* M 374 Niemegeers, 1976
F 362
*(20% EC formulation)
The observed signs of intoxication were tremors, ataxia, sedation,
salivation, hypothermia, lacrimation, righting reflex loss, lung
oedema and clonic closures.
Imazalil was given, as a single dose by gavage, to 5 dogs (3 males and
2 females) at 100 mg/kg and to 3 dogs (1 male and 2 females) at 640
mg/kg. All dogs except one, receiving 160 mg/kg vomited within 15
minutes, and those receiving 640 mg/kg within 10 minutes after dosing.
No mortality or abnormal behavior, except diarrhoea in one 160 mg/kg
dog, occurred during the 7 day observation period. An
LD50 >640 mg/kg was postulated (Niemegeers, 1975),
Short term studies
Rat
Groups of rats (40/sex/group) were fed imazalil nitrate at dietary
levels of 0, 5, 20 and 80 mg/kg day for 14 weeks.
Histological examination revealed no changes which could be attributed
to imazalil in any organs or tissues except the livers of the 80 mg/kg
group, which showed a fatty reaction localized in the midzonal to
centrilobular areas and vacuolization coincident with fatty spots in
Scarlet-red stained sections.
Appearance, behaviour, survival, food consumption, or urinalysis were
not affected at any level. Body weights of the 80 mg/kg group were
slightly depressed.
Hematological and blood chemistry values were within normal limits,
except for an increase in bilirubin in both sexes in the 20 mg/kg
group (P < 0.001) and both sexes in the 80 mg/kg (P < 0.01) group
(Marsboom et al., 1971).
Dog
Groups of dogs (3/sex/group) received technical grade imazalil, in
arachis oil, at levels of 0, 1.25, 5 or 20 mg/kg by capsule 7
days/week for two years. The 20 mg/kg level produced frequent emesis
and frequent and abundant salivation, after compound administration,
in all dogs. Examination of tissues revealed no compound or dose
related effects except for slight ground glass aspect of the
cytoplasma in the centrilobular areas of the liver in the dogs
receiving 5 and 20 mg/kg. Controls and the 1.25 mg/kg dosed dogs
showed a normal and comparable weight gain during the study. At 5
mg/kg, weight gain was slightly lower (P < 0.05). The 20 mg/kg groups
gain was also lower (P < 0.01) than the controls at 24 months. All
other parameters, measured or observed, were within normal limits and
comparable to those of the control group (Marsboom et al., 1977b).
Long term studies
Rat
Groups of rats were fed a 50% wettable powder of imazalil at dietary
concentrations equivalent to 0, 5, 20 and 80 mg/kg/day (active
ingredient) for 6, 12 and 24 months. At 6 months the livers of many 80
mg/kg rats, particularly females, showed centrilobular swelling and
numerous large vacuoles. No evident fatty degeneration was seen but a
tendency to some fatty surcharge was noted. At 12 months these changes
were still present. In addition, livers of the high level group
females exhibited a tendency to more concentrated glycogen in the
centrilobular areas. No other effects were noted which could be
attributed to the compound administration. Except for liver and
kidneys no consistent changes in weight or body weight ratios were
noted in any group at any termination. At 6 months the livers of the
80 mg/kg females were significantly increased in both absolute weight
and ratio. At 12 and 24 months the livers of female were not
significantly different.
In males receiving 20 and 80 mg/kg, the 12 month liver weights and
ratios were increased significantly but not at the 24 month
termination. At 6 and 24 months kidney weights and ratios were
significantly increased in the 80 mg/kg female group. This trend was
present at 12 months but not of statistical significance. Males
receiving this level exhibited, at 6 months, a trend toward increased
kidney weights and a significant increase in their kidney/body weight
ratio. At 12 months male kidney weights were significantly higher than
their control values, but at 24 months this difference was not
evident. Hematological and blood chemistry revealed no consistent
changes, except that at 6 months bilirubin values for the female 20
and 80 mg/kg groups were significantly elevated. This trend continued
throughout the study in the 80 mg/kg females but was not statistically
significant at the 12 and 24 month terminations. Males did not exhibit
this trend at any level during the study. Urinalysis produced nothing
remarkable. The dietary administration of imazalil did not produce a
compound- or dose-related effect on body weight gains, food
consumption, appearance, behaviour or survival rate (Marsboom at al.,
1975b).
COMMENTS
Imazalil is moderately acutely toxic to mammals. Preliminary studies
indicate that it is rapidly absorbed, metabolized and excreted by the
rat. The major metabolites are
alpha-(2,4-dichlorophenyl)-1H-imidazole-l-ethanol and
2,4-dichloromandelic acid. Dominant lethal mutagenic effects were not
evident in male and female mice. No teratological effects were noted
in rats and no reproductive effects were observed in a three
generation rat study. However, survival of pups at weaning was
adversely affected at levels higher than 5 mg/kg. Long-term studies in
the rat and a study in the dog were inadequate because of unresolved
questions concerning rat kidney and dog liver pathology. Because of
these concerns, only a temporary ADI for humans was established for
this compound based on short-term studies.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 5 (mg/kg bw)/day
Dog: 1.25 (mg/kg bw)/day
ESTIMATE OF A TEMPORARY ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.01 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Mode of action
Imazalil is a systemic fungicide from the group of N-substituted
imidazoles. Members of this chemical class affect the cellular
permeability barrier of the yeasts. The fungitoxic action of imazalil
on Penicillium may also involve the inhibition of the cell membrane
functions.
Uptake and membrane effects noted in several studies suggest that
imazalil inhibits ergosterol biosynthesis in fungal cells (Van
Bossche, 1977
Under natural conditions, the probability of the development of
resistant strains of fungi is much lower with the polygenic system of
imazalil than with the oligogenic system of the benzimidazole type
fungicides. Most probably, occurrence of resistant strains to imazalil
under field conditions is an extremely rare phenomenon which must
involve 21 genes on 8 loci, linked with 6 groups (Laville et al.,
1977; van Tuyl, 1977).
Pre-harvest treatments
Imazalil is active against a large number of fungi imperfecti, e.g.
Alternaria spp., Aspergillus, Cercospora, Colletotrichum,
Fusarium, Helminthosporium, Penicillium, Thielaviopsis. Some
fungi of the class "Ascomycetae" are also well controlled, e.g.
Erysiphe spp., Podosphaera, Sphaerotheca, Venturia.
Owing to its high specific activity (4 - 5 g a.i./100 kg seed) against
Helminthosporium spp., Fusarium spp., and Septoria spp.,
imazalil has largely contributed to replacing mercury-containing
cereal seed treatment products (Bartlett and Ballard, 1975). Products
containing imazalil are registered and sold in Sweden, Denmark, The
Netherlands, Italy, France and United Kingdom.
As a foliar spray imazalil is used on ornamentals and cucurbitacae.
Further development work is being carried out and registration for
foliar application against leaf spot diseases in bananas will be
applied for. Sigatoka "Mycosphaerella musicola" and Black Sigatoka
"Mycosphaerella fijiensis var. diformis", have developed
resistance against benzimidazoles in important banana-growing areas.
Imazalil has proved to control both Sigatoka forms and black leaf
streak (Stover, 1976; Melin and al., 1976) under practical conditions
sprayed by aircraft in a spray oil or oil-in-water emulsion.
Registration and tolerances have been applied for in banana-producing
and banana-importing countries.
Post-harvest treatments
a) Citrus
Imazalil has been investigated world-wide as a post-harvest fungicide
because of its effectiveness against benzimidazole-resistant strains
of plant pathogenic fungi. Laville at al. (1977a) contributed to the
development of imazalil as a post-harvest fungicide and stressed the
need for a replacement for benzimidazoles.
Imazalil is not only curative, but also preventive against infections
occurring after treatment during packing, transport and storage until
the fruit reaches the consumer.
At application rates of less than 2 g a.i./tonne of fruit and residue
levels below 2 mg/kg, imazalil treated fruit can be stored for more
than 3 months. Application techniques and exact dosage are facilitated
by the high solubility in water and waxes.
b) Banana
Frossard at al. (1976) and Stover (1976) have reported excellent
results. Imazalil water-soluble powder (imazalil sulphate) was used in
these experiments in water at 500 mg a.i./ka dip or spray. As
resistance to benzimidazoles had already been detected, Frossard et
al. (1976) concluded that there was a need for replacement.
Main uses
The main application fields and registered uses of imazalil are
summarized in Tables 1a and 1b.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The residues of imazalil resulting from supervised trials after
pre-harvest application on cucumbers or gherkins under glass and on
green, unripe bananas are shown in Table 2. Residues in/on cucumbers
are below 0.5 mg/kg, oven when imazalil is used between croppings.
When imazalil in applied as a post-harvest fungicide, residues on
bananas do not exceed 2 mg/kg whole fruit and are below 0.2 mg/kg in
the pulp. Residue data for citrus fruits have shown that imazalil
residues are below 5 mg/kg on whole citrus fruits or on the peel and
below 0.1 mg/kg in the pulp, when imazalil in used in accordance with
good post-harvest practice in packing houses.
The residue levels on citrus fruit largely depend on the amount of
imazalil applied. There was no significant difference between residues
when Valencia oranges were stored up to 32 days at 4°C or 22 - 25°C,
or as a result of different formulations or types of application
(Greenberg and Resnick, 1977). Residue data obtained after
post-harvest application are summarized in Table 3.
TABLE 1a. Main application fields of imazalil
Formulation and mode of Concentration
application a.i.
Ornamentals
Powdery mildew spray 0:15% 30 g/hl
E.C. 20% (300 ppm)
Cucurbits
Powdery mildew spray 0 025% 5g/hl
0.05% 10 g/hl
E.C. 20% (50-100 ppm)
Cereal seed treatment
Leaf stripe (Helminthosporium 8pp) liquid 2.5% a.i. 5 g/100 kg
seed
Septori spp.
Fusarium spp. powder 2.5% a.i. (50 ppm)
Post-harvest treatment of citrus,
bananas, dip 75% W.P. 66g/hl
pome fruit and pears against (500 ppm)
fruit rots
Penicillium spp. spray 75% W.P. 66g/hl
Alternaria spp. (500 ppm)
Diplodia spp. wax 95% techn. 2000 ppm
Thielaviopsia spp. in wax
Colletotrichum musarum
Fusarium moniliforme
Gloeosporium musarum
Foliar treatment bananas
Sigatoka disease spray 50% ULV 150-200 g.
Cercospora muses in oil a.i. per ha.
TABLE 1b. Registrations (existing and applied for) for commercial application of imazalil
Country Crop Application Dosage rate Pre-harvest Limitations
type kg a.i./ha interval, days
Italy ornamentals foliar spray 0.2-0.4 - -
U.K. ornamentals foliar spray 0.2-0.4 - -
France cucurbits foliar spray 0.2-0.4 2 outdoors only
Denmark barley seed dressing 0.005-0.01 - -
Holland cereals seed dressing 0.005-0.01 - -
Sweden cereals seed dressing 0.005-0.01 - -
U.K. cereals seed dressing 0.005-0.01 - -
TABLE 2. Residues of imazalil (base; mg/kg) resulting from pre-harvest treatments
Crop Application
& a) no. rate
Country kg Formulation Residues at interval (days) after last
a.i./ application
ha 0 1 3/4
Cucumbers under
glass NL b)
whole fruit 5 0.3 EC 20% 0.13-0.18 0.11-0.12 0.04-0.09
flesh 0.05-0.08 0.11-0.12 0.04-0.08
peel 0.60-0.85 0.05-0.15 0.04-0.13
whole fruit 5 0.4 EC 20% 0.33-0.42 0.14-0.21 0.05-0.12
flesh 0.16-0.35 0.08-0.22 0.05-0.12
peel 0.98-1.43 0.14-0.53 0.07-0.12
whole fruit 5 0.6 EC 20% 0.33-0.47 0.18-0.30 0.08-0.18
flesh 0.23-0.34 0.18-0.30 0.08-0.18
peel 0.98-1.53 0.15-0.27 0.11-0.19
whole fruit Bc) 3 0.2 EC 20% <0.01-0.07
flesh g/l <0.01-0.04
peel <0.01-0.24
Gherkins under
glass NLd) 7 0.4 EC 20% <0.01-0.01
6 0.6 EC 20% 0.03-0.05
Bananas (green)
BH e) 9 0.25- EC 50% (1-2 days + 10 days transport)
0.5
whole fruit <0.02-0.02
pulp <0.02
TABLE 2 (Continued)
a) trials in: B = Belgium, BH = British Honduras, NL = Netherlands
b) Wynants & Woestenborghs, 1976
c) Nangniot, 1977
d) ten Broeke & Dornseiffen, 1977
e) Anonymous, 1976c,g
TABLE 3. Residues of imazalil (base; mg/kg) resulting from post-harvest treatments
Crop Rate and type of application Residues at interval (weeks) after (last) References
& application
Country a) 0-1 2 3-4 6
Bananas BH 0.5 g/l spray-water (13
(ripened) weeks
after
treatment)
0.64-1.1 Anonymous, 1976c
whole fruit 0.02-0.07
pulp 0.48-1.1 Anonymous, 1976g
whole fruit pre-harvest treatment + 0.5 g/l 0.03-0.15
pulp spray water
Citrus
fruits
not
specified ZA 1 g/kg or 1 wax-prep.?
whole fruit 0.5-0.6 0.5-0.6 0.3-0.5 0.2-0.4 Beijers, 1976
pulp <0.1 <0.1 <0.1 <0.1
peel 2.2-2.6 1.9-2.1 1.0-1.5
not
specified ZA 0.5 g/l 1 aqueous prep.
whole fruit <0.1-0.1 <0.1-0.1 <0.1-0.1 <0.1-0.1 Beijers, 1976
pulp <0.1 <0.1 <0.1 <0.1
peel 0.2-0.5 0.3-0.6 0.1-0.3 0.1-0.3
Grapefruit
whole
fruit IL 0.5-4 g/l aqueous dip 0.7-2.2 Greenberg & Resnick,
0.5-4 g/kg wax spray 1.0-4.0 1977
(25-100 g/l ULV (?) 1.5-8.0)
TABLE 3. (Continued)
Crop Rate and type of application Residues at interval (weeks) after (last) References
& application
Country a) 0-1 2 3-4 6
Grapefruit USA
whole fruit 0.5-1g/kg aqueous dip 0.7-1.0 Kraght, 1977b
0.5-1g/kg aqueous drench 0.8-1.0
1-2g/kg aqueous spray 0.4-0.7
2-4g kg wax spray 0.5-2.2
foamer 0.2%? 0.2
Oranges
whole fruit IL 0.1-1 g/l aqueous dip 0.5-1.4 Greenberg & Resnick,
2.5-8 g/kg wax dip 2.0-11 1977
(Valencia) 2-3 g/kg wax dip 0-5-2.0 1.2-2.5 0.1-3.2
(Shamuti) 1-2 g/kg wax dip 0.6-2.2
whole fruit I 0.5g/l aqueous dip or 0.8-1.6 0.01 Maier-Bode, 1976
pulp 2g/l wax spray 1.9-4.2
peel
Oranges
(Navel)
whole
fruit MA 1g/l aqueous dip or 1.2-1.7 Corpel & Gauthier,
pulp 1-4g/l wax spray 0.01-0.03 1977
peel 1.3-4.3
peel 1g/kg dip See Table Anon.,1975b
4, p. 000
whole
fruit ZA 1.5-3g/kg wax spray 1.4-1.6 0.6-1.2 1.0-2.0 0.6-1.2 Beijers, 1975
1.5-3g/kg aqueous dip 0.1-0.8 0.2-0.8
TABLE 3. (Continued)
Crop Rate and type of application Residues at interval (weeks) after (last) References
& application
Country a) 0-1 2 3-4 6
whole
fruit ZA 0.25-1g/kg wax spray 0.04-0.32 Bliss & Orme,
pulp (time 0.01-0.02 1976
after
treatment
peel unknown) 0.16-0.72
(Navel
whole
fruit USA 0.5g/kg aqueous dip,
drench or spray 0.3-5.2 Kraght, 1977b
1g/kg aqueous dip,
drench or spray 0.6-5.7
2g/kg wax spray 0.04-1.9
4/kg 0.4-3.7
Tangerines USA 0.5-1g/kg aqueous dip,
drench or spray 0.2-0.3 Kraght, 1977b
2-4g/kg wax spray 0.3-2.0
a) trials in
BH = British Honduras
I = Italy
IL = Israel
MA = Morocco
USA = United States of America
ZA = South Africa
FATE OF RESIDUES
In animals
Approximately 44% of a single dose of 20 mg 3H-imazalil per kg to
rats was excreted with the urine within 4 days after treatment and
approximately 46% was excreted with the faeces. Only 3% of the
administered dose was excreted unchanged with the faeces indicating an
almost complete absorption from the gastro-intestinal tract. In
addition to unchanged imazalil (approximately 5% of the dose) the
urine contained at least 4 metabolites, probably formed by
dealkylation. Two of them were identified as
alpha-(2,4-dichlorophenyl)-1H-imidazole-1-ethanol and
2,4-dichloromandelic acid; 48 hours after administration,
concentrations higher than 1 mg/kg were found only in the liver, the
lung and the kidneys. Nearly the same concentrations were found in
male and female rats. Accumulation of imazalil in fatty tissues did
not occur (Heykants at al., 1975). This study is also referred to
previously ("Biochemical aspects").
In plants
Fourteen weeks after treatment of oranges with a solution containing
1000 mg/kg (3H-2-ethyl labelled)-imazalil, approximately 50% of the
radioactivity remaining was found in the peel in an organo-soluble
form; 30% was bound to the insoluble residue of the peel. In the flesh
of the fruit 18% of the remaining radioactivity was present as the
metabolite alpha-(2,4-dichlorophenyl)-1H-imidazole-l-ethanol (T 824).
Acute Toxicity
Species Route Sex LD50 Reference
Rat Oral M 376 Niemegeers, 1973
ip M 288 Niemegeers, 1977
F 155
Dermal M 4200 Van Ravestyn &
F 4800 Marsboom, 1975
Oral* M 374 Niemegeers, 1976
F 362
*(20% EC formulation)
The observed signs of intoxication were tremors, ataxia, sedation,
salivation, hypothermia, lacrimation, righting reflex loss, lung
oedema and clonic closures.
Imazalil was given, as a single dose by gavage, to 5 dogs (3 males and
2 females) at 100 mg/kg and to 3 dogs (1 male and 2 females) at 640
mg/kg. All dogs except one, receiving 160 mg/kg vomited within 15
minutes, and those receiving 640 mg/kg within 10 minutes after dosing.
No mortality or abnormal behavior, except diarrhoea in one 160 mg/kg
dog, occurred during the 7 day observation period. An
LD50 >640 mg/kg was postulated (Niemegeers, 1975),
Short term studies
Rat
Groups of rats (40/sex/group) were fed imazalil nitrate at dietary
levels of 0, 5, 20 and 80 mg/kg day for 14 weeks.
Histological examination revealed no changes which could be attributed
to imazalil in any organs or tissues except the livers of the 80 mg/kg
group, which showed a fatty reaction localized in the midzonal to
centrilobular areas and vacuolization coincident with fatty spots in
Scarlet-red stained sections.
Appearance, behaviour, survival, food consumption, or urinalysis were
not affected at any level. Body weights of the 80 mg/kg group were
slightly depressed.
Hematological and blood chemistry values were within normal limits,
except for an increase in bilirubin in both sexes in the 20 mg/kg
group (P < 0.001) and both sexes in the 80 mg/kg (P < 0.01) group
(Marsboom et al., 1971).
Dog
Groups of dogs (3/sex/group) received technical grade imazalil, in
arachis oil, at levels of 0, 1.25, 5 or 20 mg/kg by capsule 7
days/week for two years. The 20 mg/kg level produced frequent emesis
and frequent and abundant salivation, after compound administration,
in all dogs. Examination of tissues revealed no compound or dose
related effects except for slight ground glass aspect of the
cytoplasma in the centrilobular areas of the liver in the dogs
receiving 5 and 20 mg/kg. Controls and the 1.25 mg/kg dosed dogs
showed a normal and comparable weight gain during the study. At 5
mg/kg, weight gain was slightly lower (P < 0.05). The 20 mg/kg groups
gain was also lower (P < 0.01) than the controls at 24 months. All
other parameters, measured or observed, were within normal limits and
comparable to those of the control group (Marsboom et al., 1977b).
Long term studies
Rat
Groups of rats were fed a 50% wettable powder of imazalil at dietary
concentrations equivalent to 0, 5, 20 and 80 mg/kg/day (active
ingredient) for 6, 12 and 24 months. At 6 months the livers of many 80
mg/kg rats, particularly females, showed centrilobular swelling and
numerous large vacuoles. No evident fatty degeneration was seen but a
tendency to some fatty surcharge was noted. At 12 months these changes
were still present. In addition, livers of the high level group
females exhibited a tendency to more concentrated glycogen in the
centrilobular areas. No other effects were noted which could be
attributed to the compound administration. Except for liver and
kidneys no consistent changes in weight or body weight ratios were
noted in any group at any termination. At 6 months the livers of the
80 mg/kg females were significantly increased in both absolute weight
and ratio. At 12 and 24 months the livers of female were not
significantly different.
In males receiving 20 and 80 mg/kg, the 12 month liver weights and
ratios were increased significantly but not at the 24 month
termination. At 6 and 24 months kidney weights and ratios were
significantly increased in the 80 mg/kg female group. This trend was
present at 12 months but not of statistical significance. Males
receiving this level exhibited, at 6 months, a trend toward increased
kidney weights and a significant increase in their kidney/body weight
ratio. At 12 months male kidney weights were significantly higher than
their control values, but at 24 months this difference was not
evident. Hematological and blood chemistry revealed no consistent
changes, except that at 6 months bilirubin values for the female 20
and 80 mg/kg groups were significantly elevated. This trend continued
throughout the study in the 80 mg/kg females but was not statistically
significant at the 12 and 24 month terminations. Males did not exhibit
this trend at any level during the study. Urinalysis produced nothing
remarkable. The dietary administration of imazalil did not produce a
compound- or dose-related effect on body weight gains, food
consumption, appearance, behaviour or survival rate (Marsboom at al.,
1975b).
COMMENTS
Imazalil is moderately acutely toxic to mammals. Preliminary studies
indicate that it is rapidly absorbed, metabolized and excreted by the
rat. The major metabolites are
alpha-(2,4-dichlorophenyl)-1H-imidazole-l-ethanol and
2,4-dichloromandelic acid. Dominant lethal mutagenic effects were not
evident in male and female mice. No teratological effects were noted
in rats and no reproductive effects were observed in a three
generation rat study. However, survival of pups at weaning was
adversely affected at levels higher than 5 mg/kg. Long-term studies in
the rat and a study in the dog were inadequate because of unresolved
questions concerning rat kidney and dog liver pathology. Because of
these concerns, only a temporary ADI for humans was established for
this compound based on short-term studies.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 5 (mg/kg bw)/day
Dog: 1.25 (mg/kg bw)/day
ESTIMATE OF A TEMPORARY ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.01 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Mode of action
Imazalil is a systemic fungicide from the group of N-substituted
imidazoles. Members of this chemical class affect the cellular
permeability barrier of the yeasts. The fungitoxic action of imazalil
on Penicillium may also involve the inhibition of the cell membrane
functions.
Uptake and membrane effects noted in several studies suggest that
imazalil inhibits ergosterol biosynthesis in fungal cells (Van
Bossche, 1977
Under natural conditions, the probability of the development of
resistant strains of fungi is much lower with the polygenic system of
imazalil than with the oligogenic system of the benzimidazole type
fungicides. Most probably, occurrence of resistant strains to imazalil
under field conditions is an extremely rare phenomenon which must
involve 21 genes on 8 loci, linked with 6 groups (Laville et al.,
1977; van Tuyl, 1977).
Pre-harvest treatments
Imazalil is active against a large number of fungi imperfecti, e.g.
Alternaria spp., Aspergillus, Cercospora, Colletotrichum,
Fusarium, Helminthosporium, Penicillium, Thielaviopsis. Some
fungi of the class "Ascomycetae" are also well controlled, e.g.
Erysiphe spp., Podosphaera, Sphaerotheca, Venturia.
Owing to its high specific activity (4 - 5 g a.i./100 kg seed) against
Helminthosporium spp., Fusarium spp., and Septoria spp.,
imazalil has largely contributed to replacing mercury-containing
cereal seed treatment products (Bartlett and Ballard, 1975). Products
containing imazalil are registered and sold in Sweden, Denmark, The
Netherlands, Italy, France and United Kingdom.
As a foliar spray imazalil is used on ornamentals and cucurbitacae.
Further development work is being carried out and registration for
foliar application against leaf spot diseases in bananas will be
applied for. Sigatoka "Mycosphaerella musicola" and Black Sigatoka
"Mycosphaerella fijiensis var. diformis", have developed
resistance against benzimidazoles in important banana-growing areas.
Imazalil has proved to control both Sigatoka forms and black leaf
streak (Stover, 1976; Melin and al., 1976) under practical conditions
sprayed by aircraft in a spray oil or oil-in-water emulsion.
Registration and tolerances have been applied for in banana-producing
and banana-importing countries.
Post-harvest treatments
a) Citrus
Imazalil has been investigated world-wide as a post-harvest fungicide
because of its effectiveness against benzimidazole-resistant strains
of plant pathogenic fungi. Laville at al. (1977a) contributed to the
development of imazalil as a post-harvest fungicide and stressed the
need for a replacement for benzimidazoles.
Imazalil is not only curative, but also preventive against infections
occurring after treatment during packing, transport and storage until
the fruit reaches the consumer.
At application rates of less than 2 g a.i./tonne of fruit and residue
levels below 2 mg/kg, imazalil treated fruit can be stored for more
than 3 months. Application techniques and exact dosage are facilitated
by the high solubility in water and waxes.
b) Banana
Frossard at al. (1976) and Stover (1976) have reported excellent
results. Imazalil water-soluble powder (imazalil sulphate) was used in
these experiments in water at 500 mg a.i./ka dip or spray. As
resistance to benzimidazoles had already been detected, Frossard et
al. (1976) concluded that there was a need for replacement.
Main uses
The main application fields and registered uses of imazalil are
summarized in Tables 1a and 1b.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The residues of imazalil resulting from supervised trials after
pre-harvest application on cucumbers or gherkins under glass and on
green, unripe bananas are shown in Table 2. Residues in/on cucumbers
are below 0.5 mg/kg, oven when imazalil is used between croppings.
When imazalil in applied as a post-harvest fungicide, residues on
bananas do not exceed 2 mg/kg whole fruit and are below 0.2 mg/kg in
the pulp. Residue data for citrus fruits have shown that imazalil
residues are below 5 mg/kg on whole citrus fruits or on the peel and
below 0.1 mg/kg in the pulp, when imazalil in used in accordance with
good post-harvest practice in packing houses.
The residue levels on citrus fruit largely depend on the amount of
imazalil applied. There was no significant difference between residues
when Valencia oranges were stored up to 32 days at 4°C or 22 - 25°C,
or as a result of different formulations or types of application
(Greenberg and Resnick, 1977). Residue data obtained after
post-harvest application are summarized in Table 3.
TABLE 1a. Main application fields of imazalil
Formulation and mode of Concentration
application a.i.
Ornamentals
Powdery mildew spray 0:15% 30 g/hl
E.C. 20% (300 ppm)
Cucurbits
Powdery mildew spray 0 025% 5g/hl
0.05% 10 g/hl
E.C. 20% (50-100 ppm)
Cereal seed treatment
Leaf stripe (Helminthosporium 8pp) liquid 2.5% a.i. 5 g/100 kg
seed
Septori spp.
Fusarium spp. powder 2.5% a.i. (50 ppm)
Post-harvest treatment of citrus,
bananas, dip 75% W.P. 66g/hl
pome fruit and pears against (500 ppm)
fruit rots
Penicillium spp. spray 75% W.P. 66g/hl
Alternaria spp. (500 ppm)
Diplodia spp. wax 95% techn. 2000 ppm
Thielaviopsia spp. in wax
Colletotrichum musarum
Fusarium moniliforme
Gloeosporium musarum
Foliar treatment bananas
Sigatoka disease spray 50% ULV 150-200 g.
Cercospora muses in oil a.i. per ha.
TABLE 1b. Registrations (existing and applied for) for commercial application of imazalil
Country Crop Application Dosage rate Pre-harvest Limitations
type kg a.i./ha interval, days
Italy ornamentals foliar spray 0.2-0.4 - -
U.K. ornamentals foliar spray 0.2-0.4 - -
France cucurbits foliar spray 0.2-0.4 2 outdoors only
Denmark barley seed dressing 0.005-0.01 - -
Holland cereals seed dressing 0.005-0.01 - -
Sweden cereals seed dressing 0.005-0.01 - -
U.K. cereals seed dressing 0.005-0.01 - -
TABLE 2. Residues of imazalil (base; mg/kg) resulting from pre-harvest treatments
Crop Application
& a) no. rate
Country kg Formulation Residues at interval (days) after last
a.i./ application
ha 0 1 3/4
Cucumbers under
glass NL b)
whole fruit 5 0.3 EC 20% 0.13-0.18 0.11-0.12 0.04-0.09
flesh 0.05-0.08 0.11-0.12 0.04-0.08
peel 0.60-0.85 0.05-0.15 0.04-0.13
whole fruit 5 0.4 EC 20% 0.33-0.42 0.14-0.21 0.05-0.12
flesh 0.16-0.35 0.08-0.22 0.05-0.12
peel 0.98-1.43 0.14-0.53 0.07-0.12
whole fruit 5 0.6 EC 20% 0.33-0.47 0.18-0.30 0.08-0.18
flesh 0.23-0.34 0.18-0.30 0.08-0.18
peel 0.98-1.53 0.15-0.27 0.11-0.19
whole fruit Bc) 3 0.2 EC 20% <0.01-0.07
flesh g/l <0.01-0.04
peel <0.01-0.24
Gherkins under
glass NLd) 7 0.4 EC 20% <0.01-0.01
6 0.6 EC 20% 0.03-0.05
Bananas (green)
BH e) 9 0.25- EC 50% (1-2 days + 10 days transport)
0.5
whole fruit <0.02-0.02
pulp <0.02
TABLE 2 (Continued)
a) trials in: B = Belgium, BH = British Honduras, NL = Netherlands
b) Wynants & Woestenborghs, 1976
c) Nangniot, 1977
d) ten Broeke & Dornseiffen, 1977
e) Anonymous, 1976c,g
TABLE 3. Residues of imazalil (base; mg/kg) resulting from post-harvest treatments
Crop Rate and type of application Residues at interval (weeks) after (last) References
& application
Country a) 0-1 2 3-4 6
Bananas BH 0.5 g/l spray-water (13
(ripened) weeks
after
treatment)
0.64-1.1 Anonymous, 1976c
whole fruit 0.02-0.07
pulp 0.48-1.1 Anonymous, 1976g
whole fruit pre-harvest treatment + 0.5 g/l 0.03-0.15
pulp spray water
Citrus
fruits
not
specified ZA 1 g/kg or 1 wax-prep.?
whole fruit 0.5-0.6 0.5-0.6 0.3-0.5 0.2-0.4 Beijers, 1976
pulp <0.1 <0.1 <0.1 <0.1
peel 2.2-2.6 1.9-2.1 1.0-1.5
not
specified ZA 0.5 g/l 1 aqueous prep.
whole fruit <0.1-0.1 <0.1-0.1 <0.1-0.1 <0.1-0.1 Beijers, 1976
pulp <0.1 <0.1 <0.1 <0.1
peel 0.2-0.5 0.3-0.6 0.1-0.3 0.1-0.3
Grapefruit
whole
fruit IL 0.5-4 g/l aqueous dip 0.7-2.2 Greenberg & Resnick,
0.5-4 g/kg wax spray 1.0-4.0 1977
(25-100 g/l ULV (?) 1.5-8.0)
TABLE 3. (Continued)
Crop Rate and type of application Residues at interval (weeks) after (last) References
& application
Country a) 0-1 2 3-4 6
Grapefruit USA
whole fruit 0.5-1g/kg aqueous dip 0.7-1.0 Kraght, 1977b
0.5-1g/kg aqueous drench 0.8-1.0
1-2g/kg aqueous spray 0.4-0.7
2-4g kg wax spray 0.5-2.2
foamer 0.2%? 0.2
Oranges
whole fruit IL 0.1-1 g/l aqueous dip 0.5-1.4 Greenberg & Resnick,
2.5-8 g/kg wax dip 2.0-11 1977
(Valencia) 2-3 g/kg wax dip 0-5-2.0 1.2-2.5 0.1-3.2
(Shamuti) 1-2 g/kg wax dip 0.6-2.2
whole fruit I 0.5g/l aqueous dip or 0.8-1.6 0.01 Maier-Bode, 1976
pulp 2g/l wax spray 1.9-4.2
peel
Oranges
(Navel)
whole
fruit MA 1g/l aqueous dip or 1.2-1.7 Corpel & Gauthier,
pulp 1-4g/l wax spray 0.01-0.03 1977
peel 1.3-4.3
peel 1g/kg dip See Table Anon.,1975b
4, p. 000
whole
fruit ZA 1.5-3g/kg wax spray 1.4-1.6 0.6-1.2 1.0-2.0 0.6-1.2 Beijers, 1975
1.5-3g/kg aqueous dip 0.1-0.8 0.2-0.8
TABLE 3. (Continued)
Crop Rate and type of application Residues at interval (weeks) after (last) References
& application
Country a) 0-1 2 3-4 6
whole
fruit ZA 0.25-1g/kg wax spray 0.04-0.32 Bliss & Orme,
pulp (time 0.01-0.02 1976
after
treatment
peel unknown) 0.16-0.72
(Navel
whole
fruit USA 0.5g/kg aqueous dip,
drench or spray 0.3-5.2 Kraght, 1977b
1g/kg aqueous dip,
drench or spray 0.6-5.7
2g/kg wax spray 0.04-1.9
4/kg 0.4-3.7
Tangerines USA 0.5-1g/kg aqueous dip,
drench or spray 0.2-0.3 Kraght, 1977b
2-4g/kg wax spray 0.3-2.0
a) trials in
BH = British Honduras
I = Italy
IL = Israel
MA = Morocco
USA = United States of America
ZA = South Africa
FATE OF RESIDUES
In animals
Approximately 44% of a single dose of 20 mg 3H-imazalil per kg to
rats was excreted with the urine within 4 days after treatment and
approximately 46% was excreted with the faeces. Only 3% of the
administered dose was excreted unchanged with the faeces indicating an
almost complete absorption from the gastro-intestinal tract. In
addition to unchanged imazalil (approximately 5% of the dose) the
urine contained at least 4 metabolites, probably formed by
dealkylation. Two of them were identified as
alpha-(2,4-dichlorophenyl)-1H-imidazole-1-ethanol and
2,4-dichloromandelic acid; 48 hours after administration,
concentrations higher than 1 mg/kg were found only in the liver, the
lung and the kidneys. Nearly the same concentrations were found in
male and female rats. Accumulation of imazalil in fatty tissues did
not occur (Heykants at al., 1975). This study is also referred to
previously ("Biochemical aspects").
In plants
Fourteen weeks after treatment of oranges with a solution containing
1000 mg/kg (3H-2-ethyl labelled)-imazalil, approximately 50% of the
radioactivity remaining was found in the peel in an organo-soluble
form; 30% was bound to the insoluble residue of the peel. In the flesh
of the fruit 18% of the remaining radioactivity was present as the
metabolite alpha-(2,4-dichlorophenyl)-1H-imidazole-l-ethanol (T 824).
 The polar, hydrophilic fraction containing nearly 50% of the
radioactivity present in the methanol extract of the peel did not
contain tritiated water or other volatile radioactivity. Since
concentrations of radioactivity were identical before and after
lyophilisation of this fraction, it seems that no label loss is to be
expected during metabolism (Anon., 1976a).
A similar test was conducted under conditions closer to commercial
practice. In addition, a more rigorous solvent extraction procedure
was used in order to recover more radioactive material.
As metabolites were expected to be present in very small amounts, the
fruit was treated with 4 times the normal concentration, 2000 mg
a.i./1 aqueous dip for 1 minute. The oranges were stored for 12 weeks
and the distribution of radioactivity in the fruit and the presence of
metabolites were studied during and after this period.
Most of the radioactivity (89%) remained as unchanged imazalil, in
contrast to the above results where considerable degradation was
reported. This was due to the extreme storage conditions used in the
above trial (high temperature and light), which are completely
artificial and not related to commercial practice. No tritium exchange
was detected, thus the tritium labelling was suitable for the purpose
of the study. 99.5% or more of the radioactivity in the
3H-imazalil-treated oranges was solvent-extractable: about 0.5% or
less remained in the insoluble residue. After 12 weeks of storage
approximately 10-12% of the original radioactivity appeared in the
form of an unknown water-soluble compound (Kraght, 1977a).
The distribution of imazalil in/on oranges was determined at intervals
after treatment at 1000 mg/kg (Anon., 1975b). Results are shown in
Table 4.
Gherkins sprayed seven times with imazalil under practical conditions
in a glasshouse were sampled three days after the last application,
then stored at -18°C for 3-7 weeks. Residues in/on the gherkins were
between 0.03 -0.05 mg imazalil/kg and at or below 0.01 mg
T 824-metabolite/kg (ten Broeke and Dornseiffen, 1977).
Barley seeds treated with 3H-imazalil were sown in perlite. After 3
weeks the plants were harvested and the roots and leaves extracted
separately. Large amounts of radioactivity were present in an
unextractable form in both the roots and the leaves. In a methanol
extract of the roots imazalil accounted for 33% of the total
radioactivity and in a toluene extract for 81%. A certain amount of
the methanol-insoluble residue may consist of physically bound
imazalil. Only about 7% or less of the label was the metabolite T 824
(Anon., 1976b).
TABLE 4. Distribution of imazalil in oranges after treatment at 1000 mg/kg
Imazalil, mg/kg, at indicated interval after dipping (Anon., 1975b)
0 weeks 4 weeks 8 weeks 12 weeks
dipped dipped dipped dipped
´ min. 1 min. ´ min. 1 min. ´ min. 1 min ´ min. 1 min.
peel 0.9 2.4 0.51 0.85 2.3 1.7 0.8 1.4
4.0 2.5 0.55 0.49 1.2 1.5 0.7 1.5
pulp n.d. n.d. 0.053 0.047 0.047 0.12 0.10 0.36
0.13 n.d. 0.027 0.062 0.14 0.11 0.09 0.06
n.d. = not determined.
Comments: -degradation of imazalil m orangss is slow;
-half-life is estimated to be 12 to 20 weeks
-only small amounts of imazalil are present in the fruit flesh and part
of this may result from contamination during peeling.
Barley seeds were treated with a dose of 3H-imazalil corresponding
to 10 g a.i./100 kg seed. After germination on water agar for 9 days,
42% of the radioactivity was found in the agar and 37% in the seed
coats. Only 10% of the total radioactivity was present in the roots
and 2% in the leaves of the seedlings.
Barley seeds treated with 3H-imazalil at 10 g a.i./100 kg were also
sown in soil. Plants were harvested after 1 and 3 weeks. Soil and
plant parts were analyzed for radioactivity. Most of the radioactivity
was present in the soil directly around the seed coats, 76% and 29% in
the seed coats. After 3 weeks the green parts of the plants contained
only 6% of the radioactivity which had originally adhered to the
seeds.
In soil
The half-life of imazalil (1 mg/kg in a sandy loam and a clay loam)
was 4-5 months (Anon., 1976e), After a 36 weeks incubation period 6-7%
of the initially applied 3H-imazalil was present as the metabolite
T 824: alpha-(2,4-dichlorophenyl)-1H-imidazole-1-ethanol. About 13%
was found to consist of more polar compounds than imazalil and T 824,
but could not be identified. 9-13% could not be extracted and was
probably due to unchanged imazalil bound to soil constituents. Little
or no loss of label occurred. The results are given in Table 5.
TABLE 5. Extractable and unextractable radioactivity from soils after treatment
with 1 mg/kg 3H-imazalil (mean result from 2 flasks) Anon., 1976e
Radioactivity as% of that initially
applied to soil
Sandy loam Clay loam
Weeks incubation 0 16 36 0 16 36
Methanol extract
(fraction 1) 63 67.5 62 66.5 51 44
Methanol-NaOH extract
(fraction 2) 18 14.5 18.5 17 25.5 30
Unextracted radioactivity 12.5 9 9 8 13 12
Total 93.5 89.5 89.5 91.5 89.5 86
Imazalil in a 20% E.C. formulation was sprayed 1, 4 and 7 times with
14 days interval, directly on the soil of field plots sown with spring
barley. High dosage rates and water volumes were used in order to
obtain detectable residue levels in the soil layers (0.1, 0.4 and 2.8
kg a.i./ha in 4000 1 water/ha). Results at the two lower
concentrations are shown in Table 6.
TABLE 6. Concentration of imazalil in field soil treated with varying
doses of imazalil
(Anon., 1976f; Anon., 1976h)
Dosage rate Depth imazalil (mg/kg) after months
(cm) 0 1 3
untreated 0-10 <0.01 <0.01 <0.01
10-20 <0.01 <0.01 <0.01
1 X 0.1 kg a.i./ha 0-10 0.021 0.019 0.016
10-20 <0.01 <0.01 <0.01
4 X 6.4 kg a.i./ha 0-10 0.066 0.074 0.048
10-20 0.013 0.016 <0.01
7 X 0.4 kg a.i./ha 0-10 0.085 0.14 0.087
10-20 <0.01 0.018 0.017
Comments; - Residue levels were very low in the upper layer of 0-10
cm, 0.085-0.14 mg/kg after 7 applications, although
practically 90% of the 7 × 4000 1/ha spraywash came into
direct contact with the soil.
- There was no leaching of practical significance within 6
months into the soil layer 10-20 cm deep; residues of 0.018
mg/kg were close to the detection limit.
- No accumulation occurred even after 7 sprays at the
highest dosage.
- The half-life for the initial concentration of 0.021 mg/kg
was 6.5 ± 2.5 months.
METHODS OF RESIDUE ANALYSIS
Gas-chromatographic procedures for the determination of imazalil in
and on citrus fruit are described by various authors (Wynants and
Woestenborghs, 1975b) Greenberg and Resnick, 1977, Wynants, 1977a;
Kraght, 1977b)
The methods comprise extraction of imazalil as the free base in
alkaline solution with an organic solvent (e.g. benzene, ethyl acetate
or chloroform). Clean-up can be achieved by repeated extraction in
acidified aqueous and alkaline organic solution. For the final
gas-chromatographic determination 3% OV-17 on Supelcoport in a glass
column (300°C silanized) and an electron capture detector can be used.
Flame-photometric, alkali-flame and electrolytic conductivity
detectors, although less sensitive then ECD, are much more selective
and thus avoid many interferences which may be encountered with the
ECD. The lower limit of determination depends, inter alia, on the
commodity to be analysed and is generally between 0.01 and 0.1 mg/kg.
Recoveries were 80-95%.
Similar methods for the determination of imazalil residues in or on
cucurbitaceae (Wynants and Woestenborghs, 1975a), bananas (Anonymous,
1976d), wheat and wheat-straw (ten Broeke and Dornseiffen, 1976) are
also available.
Recently a new method has been developed for the determination of
imazalil and its metabolite
alpha-(2,4-dichlorophenyl)-1H-imidazole-1-othanol (T824), based on the
above principles but using heptane/isoamyl alcohol (95/5, v/v) for the
extraction. When T 824 is converted by
N,O-bis(trimethylsilyl)acetamide to a silyl ether it can be
quantatively determined by GC with 3% OV-225 on Gas Chrom Q using an
ECD (Wynants, 1977b; ten Broeke and Dornseiffen, 1977).
NATIONAL TOLERANCES
No national tolerances were known to the Meeting.
APPRAISAL
Imazalil is a systemic fungicide which is effective against fungi
imperfecti and some fungi of the class "Ascomycetae". It is also
effective against benzimidazole-resistant strains of plant pathogenic
fungi. It is formulated as a seed dressing for cereals, an
emulsifiable concentrate and a wettable powder. The main pre-harvest
applications for the latter formulations are on bananas and cucurbits:
other uses are being developed. Imazalil is also highly effective
against fungal infections on bananas and citrus fruit after harvest.
It is used in several European countries.
In plants alpha-(2,4-dichlorophenyl)-1H-imidazole-1-ethanol and other
unidentified polar, hydrophilic compounds are the main degradation
products. The half-lives of imazalil on oranges and in soil are 12-20
weeks and 4-5 months respectively. No accumulation in soil was
observed.
Following pre-harvest application in accordance with good agricultural
practice, the maximum residue levels of imazalil in or on cucumbers
and gherkins under glass were below 0.5 mg/kg. Post-harvest
application at recomended dosages resulted in residues of imazalil
below 2 mg/kg on bananas and below 0.2 mg/kg in the pulp. Residues
were below 5 mg/kg on citrus fruit and below 0.1 mg/kg in the peeled
fruit and in wheat straw; residues in/on wheat grain were below 0.01
mg/kg.
Residue levels on citrus fruit largely depend on the dosage applied;
no significant differences in the residue levels on fruits which were
stored for 32 days at 40°C and 22-25°C were noticed.
Gas-chromatographic methods for the determination of imazalil in
various crops, suitable for regulatory purposes, have been elaborated.
RECOMMENDATIONS
The following temporary maximum residue limits are recommended on the
basis of data resulting from supervised trials involving pre- and
post-harvest uses. They refer to imazalil only.
Commodity Temporary limit, Pre- or post-harvest
mg/kg interval (days) on
which recommendations
is based (post-harvest
in brackets)
Citrus fruit
(whole) 5 (0-42)
Bananas
(whole) 2 (0-100)
Cucumbers,
gherkins 0.5 0-1
Bananas
(without peel) 0.2 (0-100)
Citrus fruit
(without peel) 0.1 (0-42)
Wheat straw 0.1
Wheat (grain) 0.01*
* at or about the limit of determination.
FURTHER WORK OR INFORMATION
REQUIRED (by July 1981)
1. Comparative acute toxicity studies of the different salts.
2. Farther long-term studies.
DESIRABLE
1. Pharmacokinetic studies of the different salts.
2. Metabolic studies with imazalil labelled with 14C.
3. Further information on the nature and level of metabolites and
degradation products in plants.
REFERENCES
Anonymous (1975a) "Physico-chemistry of R 18531 -imazalil nitrate,
R 23979 - imazalil, and R 27180 -imazalil sulphate". Janssen
Pharmaceutica; Pre-formulation report no. 496; 750107.
Anonymous (1975b) "Degradation of imazalil in oranges". Biochemical
Department of the Organisch Chemisch Instituut TNO - Utrecht - The
Netherlands; Progress report of project 1.1; 751106.
Anonymous (1976a) "Metabolism of imazalil applied to oranges".
Biochemical Department of the Institute for Organic Chemistry TNO
- Utrecht - The Netherlands; Report of project 1.II; 760224.
Anonymous (1976b) "Metabolism of imazalil in barley plants".
Biochemical Department of the Institute for Organic Chemistry
- Utrecht - The Netherlands; Addition to the first report of project
2; 760521.
Anonymous (1976c) "Testicide residue report - imazalil on bananas".
Jansses Pharmaceutica., 760816.
Anonymous (1976d) "Procedure for imazalil in whole bananas". Janssen
Pharmaceutica; 761009.
Anonymous (1976e) "A study of the fate of imazalil in soil".
Biochemical Department of the Institute for Organic Chemistry TNO
- Utrecht - The Netherlands; Report of Project 4; 761021.
Anonymous (1976f) "Degradation of imazalil in soil under field
conditions". Biochemical Department of the Institute for Organic
Chemistry TNO - Utrecht - The Netherlands; Report of project 5, part
1; 761105. Attached letter of TNO - 770524.
Anonymous (1976g) "Residue summary sheets for imazalil in bananas".
Morse Laboratories, Ltd. - Sacramento - California - U.S.A.; 761129.
Anonymous (1976h) "Degradation of imazalil in soil under field
conditions". Biochemical Department of the Institute for Organic
Chemistry TNO - Utrecht - The Netherlands; Report of project 5, part
2; 761209.
Bartlett, D.H., Ballard, N.E. (1975) "The effectiveness of guazatine
and imazalil as seed treatment fungicides in barley". Proc. 8th Brit.
Insect. Fung. Conf. - 1975.
Beijers, H.P. (1975) "Imazalil residues in citrus samples". SABS
- South Africa; Report no. 0311/8882/M410; 751128.
Beijers, H.P. (1976) "Imazalil residues in citrus samples". SABS Rep.
No. 0311/8960/N400; Pretoria, 10 Nov. 1976.
Bliss, G.W., Orme, J.P.R. (1976) "Certificate of analysis - Analysis
of residues of imazalil in oranges". Huntingdon Research Centre;
Huntingdon - U.K. report VPT/2; 761223.
ten Broeke, R., Dornseiffen, J.W. (1976) "Imazalil residues in wheat
and wheat-straw". Keuringsdienst van Waren - Amsterdam; Rapport
KvW 197/Vondelingenplaat 1976-8; 761020.
ten Broeke, R., Dornseiffen, J.W. (1977) Residues of imazalil and its
metabolite T 824 in gherkins under glass. KvW 203/4.2. (2.1.01) 1976;
July 1977.
Corpel J., Gauthier, M. (1977) "Campagne orange du Marco".
Commissariat a l'energie atomique; Centre d'études nucléaires de
Cadarache; 770407.
Frossard, P., Laville, E., Plaud, G. (1976) "Etude des traitements
fongicides appliques aux bananes aprés récolte; III: Action de
l'imazalil". Fruits 31, 361-364 (1976).
Greenberg, R., Resnick, C., (1977) "A gas-liquid chromatographic
method for determining imazalil residues in citrus fruit"; Pestic.
Sci. 8, 59-64 (1977).
Helsen, W. (1976) "Thermal stability of imazalil and salts". Janssen
Pharmaceutica; memo dated 760813.
Heykants, J., Meuldermans, W., Hurkmans, R. (1975) "On the excretion,
metabolism and tissue distribution of imazalil-3H in the male and
female Wistar rat". Janssen Pharmaceutica; Department of drug
metabolism; 7512.
Kraght, A.J. (1977a) "Metabolism of imazalil applied to
oranges - Metabolite study by tracer technique". Pennwalt
Corporation - Decco Division - U.S.A. 770518.
Kraght, A.J. (1977b) "Residue data of imazalil on citrus". Pennwalt
Corporation - Decco Division - U.S.A.; 770518.
Laville, E., Herding, P.R., Dagan, Y., Rahat, M., Kraght, A.J. (1977)
"Studies on imazalil as potential treatment for control of citrus
fruit decay". Proc. Int. Soc. Citriculture, 1977.
Maier-Bode, H. (1976) "Imazalil residues in oranges after post-harvest
treatment". Rickenbach - Federal Republic of Germany; 760622.
Marsboom, R. (1970) Potential of R18531 for Embryotoxicity and
Teratogenic Effects in Rats Receiving R18531 Orally. Unpublished
report from Janssen Pharmaceutical submitted to the World Health
Organization by Pennwalt Corporation.
Marsboom, R., Vandesteen, R., Herin, V. and Pardoel, L. (1971) Safety
Evaluation of R18531 During 14 Weeks of Oral Treatment to Rats.
Unpublished report from Janssen Pharmaceutical submitted to the World
Health Organization by Pennwalt Corporation.
Marsboom, R. (1975a) Oral Embryotoxicity and Teratogenicity Study in
Wistar Rate During the Peri- and Postnatal Study (Segment III). A
report from Jansses Pharmaceutical submitted to the World Health
Organization by Pennwalt Corporation. (unpublished report)
Marsboom, R., Herin, V., Vandesteene, R. and Pardoell L. (1975b) Oral
Toxicity Study in Wistar Rats (repeated dosage for 6, 12 or 24
months). Unpublished report from Janssen Pharmaceutical submitted to
the World Health Organization by Pennwalt Corporation.
Marshboom, R. (1975c) Oral Three Generation Study in Wistar Rats.
Unpublished report from Janssen Pharmaceutics, submitted to the WorId
Health Organization by Pennwalt Corporation.
Marshboom, R. (1976a) Dominant Lethal Test in Male Mice. Unpublished
report from Janssen Pharmaceutica, submitted to the World Health
Organization by Pennwalt Corporation.
Marsboom, R. (1976b) Dominant Lethal Test in Female Mice (single oral
dose). Unpublished report from Janssen Pharmaceutica, submitted to the
World Health Organization by Pennwalt Corporation.
Marsboom, R. (1977a) Oral Male and Female Fertility Study in Wistar
Rats (Segment I). Unpublished report from Janssen Pharmaceutical
submitted to the World Health Organization by Pennwalt Corporation.
Marsboom, R., Herin, V., Van Desteene, R. and Van Belle. (1977b) Oral
Toxicity Study in Beagle Dogs (repeated dosage for 24 months).
Unpublished report from Janssen Pharmaceutical submitted to the World
Health Organization by Pennwalt Corporation.
Melin, Ph., Plaud, G., Tezenas du Moricel, H., Laville, E. (1976)
"Action do l'imazalil sur le niveau d'infestation et l'état
d'évolution de la cercosporiose". Fruits 31 (10)t 599-602 (1976).
Meuldermans, W., Swysen, E., Lauwers, W., Heykrantz, Jr. (1977a) An
in-vitro Study of Imazalil - 3H in the Rat. Unpublished report from
Janssen Pharmaceutica, submitted to the World Health Organization by
Pennwalt Corporation.
Meuldermans, W., Hurkmans, J., Lanwers, W., and Heykants, J. (1977b)
The Excretion and Biotransformation of Imazalil and One of Its
Metabolites (R14821) in the Wistar Rat. Unpublished report from
Janssen Pharmaceutical Department of Drug Metabolism and Analytical
Department, submitted to the World Health Organization by Pennwalt
Corporation.
Nangniot, P. (1977) "Teneurs résiduaires an imazalil dans des
concombers". Centre de Recherche de
Phytophaxmacie - Gembloux - Belgique; 770428.
Niemegeers, G.J.E. (1973) Acute Oral Toxicity in Rats. Unpublished
report from Janssen Pharmaceutica, submitted to the World Health
Organization by Pennwalt Corporation.
Niemegeers, C.J.E. (1976) Acute Oral Toxicity of R23979 - 20% E.C. in
Rats. Unpublished report from Janssen Pharmaceutica, submitted to the
World Health Organization by Pennwalt Corporation.
Niemegeers, G.J.E. (1977) Acute Intraperitoneal Toxicity of R23979 in
Wistar Rats. Unpublished report from Janssen Pharmaceutica, submitted
to the World Health Organization by the Pennwalt Corporation.
Pattyn, W. (1976) "Purity of R23979 -imazalil technical". Janssen
Pharmaceutica; Chemical Quality Control Department; 761001.
Stover, R.H. (1976) "Imazalil efficacy data". Vining C. Dunlap
Laboratories of the United Fruit Company in Honduras; 761102.
Attachment: Stover, R.H., Dickson, J.D. "Leaf sport of bananas caused
by Mycosphaerella musicola: Methods of measuring spotting prevalence
and severity". Tropical Agriculture 47, 289-302. (1970)
Van den Bossche, H. (1977) Studies on the mode of action of imazalil.
Janssen Pharmaceutica; 770425. Unpublished.
Van Ravestyn, O. and Marsboom, R. (1975) Acute Dermal Toxicity in Rats
(single dosage). Unpublished report from Janssen Pharmaceutical
submitted to the World Health Organization by Pennwalt Corporation.
Van Tuyl, J.Y. (1977) Genetics of fungal resistance to systemic
fungioides. Mededelinger
Landborwhogeschool - Wageningen - Netherlands; 77-2.
Wynants, J, Woestenborghs, R. (1975a) "A gas-chromatographic assay
method for imazalil in cucumbers". Janssen Pharmaceutics; 7512.
Wynants, J., Woestenborghs, R. (1975b) "A gas-chromatographic assay
method for imazalil in citrus fruit". Janssen Pharmaceutics; 7512.
Wynants, J., Woestenborghs, R. (1976) "Residue analysis of imazalil in
cucumbers". Janssen (1976) Pharmaceutics; 7601.
Wynants J. (1977a) "Chromatographic assay methods of imazalil in
citrus fruit". Proc. Int. Soc. Citriculture, 1977, vol. H: 38.
Wynants J. (1977b) "Residue detection method for imazalil and its
metabolite T824". Janssen Pharmaceutics; provisional report; 770420.
The polar, hydrophilic fraction containing nearly 50% of the
radioactivity present in the methanol extract of the peel did not
contain tritiated water or other volatile radioactivity. Since
concentrations of radioactivity were identical before and after
lyophilisation of this fraction, it seems that no label loss is to be
expected during metabolism (Anon., 1976a).
A similar test was conducted under conditions closer to commercial
practice. In addition, a more rigorous solvent extraction procedure
was used in order to recover more radioactive material.
As metabolites were expected to be present in very small amounts, the
fruit was treated with 4 times the normal concentration, 2000 mg
a.i./1 aqueous dip for 1 minute. The oranges were stored for 12 weeks
and the distribution of radioactivity in the fruit and the presence of
metabolites were studied during and after this period.
Most of the radioactivity (89%) remained as unchanged imazalil, in
contrast to the above results where considerable degradation was
reported. This was due to the extreme storage conditions used in the
above trial (high temperature and light), which are completely
artificial and not related to commercial practice. No tritium exchange
was detected, thus the tritium labelling was suitable for the purpose
of the study. 99.5% or more of the radioactivity in the
3H-imazalil-treated oranges was solvent-extractable: about 0.5% or
less remained in the insoluble residue. After 12 weeks of storage
approximately 10-12% of the original radioactivity appeared in the
form of an unknown water-soluble compound (Kraght, 1977a).
The distribution of imazalil in/on oranges was determined at intervals
after treatment at 1000 mg/kg (Anon., 1975b). Results are shown in
Table 4.
Gherkins sprayed seven times with imazalil under practical conditions
in a glasshouse were sampled three days after the last application,
then stored at -18°C for 3-7 weeks. Residues in/on the gherkins were
between 0.03 -0.05 mg imazalil/kg and at or below 0.01 mg
T 824-metabolite/kg (ten Broeke and Dornseiffen, 1977).
Barley seeds treated with 3H-imazalil were sown in perlite. After 3
weeks the plants were harvested and the roots and leaves extracted
separately. Large amounts of radioactivity were present in an
unextractable form in both the roots and the leaves. In a methanol
extract of the roots imazalil accounted for 33% of the total
radioactivity and in a toluene extract for 81%. A certain amount of
the methanol-insoluble residue may consist of physically bound
imazalil. Only about 7% or less of the label was the metabolite T 824
(Anon., 1976b).
TABLE 4. Distribution of imazalil in oranges after treatment at 1000 mg/kg
Imazalil, mg/kg, at indicated interval after dipping (Anon., 1975b)
0 weeks 4 weeks 8 weeks 12 weeks
dipped dipped dipped dipped
´ min. 1 min. ´ min. 1 min. ´ min. 1 min ´ min. 1 min.
peel 0.9 2.4 0.51 0.85 2.3 1.7 0.8 1.4
4.0 2.5 0.55 0.49 1.2 1.5 0.7 1.5
pulp n.d. n.d. 0.053 0.047 0.047 0.12 0.10 0.36
0.13 n.d. 0.027 0.062 0.14 0.11 0.09 0.06
n.d. = not determined.
Comments: -degradation of imazalil m orangss is slow;
-half-life is estimated to be 12 to 20 weeks
-only small amounts of imazalil are present in the fruit flesh and part
of this may result from contamination during peeling.
Barley seeds were treated with a dose of 3H-imazalil corresponding
to 10 g a.i./100 kg seed. After germination on water agar for 9 days,
42% of the radioactivity was found in the agar and 37% in the seed
coats. Only 10% of the total radioactivity was present in the roots
and 2% in the leaves of the seedlings.
Barley seeds treated with 3H-imazalil at 10 g a.i./100 kg were also
sown in soil. Plants were harvested after 1 and 3 weeks. Soil and
plant parts were analyzed for radioactivity. Most of the radioactivity
was present in the soil directly around the seed coats, 76% and 29% in
the seed coats. After 3 weeks the green parts of the plants contained
only 6% of the radioactivity which had originally adhered to the
seeds.
In soil
The half-life of imazalil (1 mg/kg in a sandy loam and a clay loam)
was 4-5 months (Anon., 1976e), After a 36 weeks incubation period 6-7%
of the initially applied 3H-imazalil was present as the metabolite
T 824: alpha-(2,4-dichlorophenyl)-1H-imidazole-1-ethanol. About 13%
was found to consist of more polar compounds than imazalil and T 824,
but could not be identified. 9-13% could not be extracted and was
probably due to unchanged imazalil bound to soil constituents. Little
or no loss of label occurred. The results are given in Table 5.
TABLE 5. Extractable and unextractable radioactivity from soils after treatment
with 1 mg/kg 3H-imazalil (mean result from 2 flasks) Anon., 1976e
Radioactivity as% of that initially
applied to soil
Sandy loam Clay loam
Weeks incubation 0 16 36 0 16 36
Methanol extract
(fraction 1) 63 67.5 62 66.5 51 44
Methanol-NaOH extract
(fraction 2) 18 14.5 18.5 17 25.5 30
Unextracted radioactivity 12.5 9 9 8 13 12
Total 93.5 89.5 89.5 91.5 89.5 86
Imazalil in a 20% E.C. formulation was sprayed 1, 4 and 7 times with
14 days interval, directly on the soil of field plots sown with spring
barley. High dosage rates and water volumes were used in order to
obtain detectable residue levels in the soil layers (0.1, 0.4 and 2.8
kg a.i./ha in 4000 1 water/ha). Results at the two lower
concentrations are shown in Table 6.
TABLE 6. Concentration of imazalil in field soil treated with varying
doses of imazalil
(Anon., 1976f; Anon., 1976h)
Dosage rate Depth imazalil (mg/kg) after months
(cm) 0 1 3
untreated 0-10 <0.01 <0.01 <0.01
10-20 <0.01 <0.01 <0.01
1 X 0.1 kg a.i./ha 0-10 0.021 0.019 0.016
10-20 <0.01 <0.01 <0.01
4 X 6.4 kg a.i./ha 0-10 0.066 0.074 0.048
10-20 0.013 0.016 <0.01
7 X 0.4 kg a.i./ha 0-10 0.085 0.14 0.087
10-20 <0.01 0.018 0.017
Comments; - Residue levels were very low in the upper layer of 0-10
cm, 0.085-0.14 mg/kg after 7 applications, although
practically 90% of the 7 × 4000 1/ha spraywash came into
direct contact with the soil.
- There was no leaching of practical significance within 6
months into the soil layer 10-20 cm deep; residues of 0.018
mg/kg were close to the detection limit.
- No accumulation occurred even after 7 sprays at the
highest dosage.
- The half-life for the initial concentration of 0.021 mg/kg
was 6.5 ± 2.5 months.
METHODS OF RESIDUE ANALYSIS
Gas-chromatographic procedures for the determination of imazalil in
and on citrus fruit are described by various authors (Wynants and
Woestenborghs, 1975b) Greenberg and Resnick, 1977, Wynants, 1977a;
Kraght, 1977b)
The methods comprise extraction of imazalil as the free base in
alkaline solution with an organic solvent (e.g. benzene, ethyl acetate
or chloroform). Clean-up can be achieved by repeated extraction in
acidified aqueous and alkaline organic solution. For the final
gas-chromatographic determination 3% OV-17 on Supelcoport in a glass
column (300°C silanized) and an electron capture detector can be used.
Flame-photometric, alkali-flame and electrolytic conductivity
detectors, although less sensitive then ECD, are much more selective
and thus avoid many interferences which may be encountered with the
ECD. The lower limit of determination depends, inter alia, on the
commodity to be analysed and is generally between 0.01 and 0.1 mg/kg.
Recoveries were 80-95%.
Similar methods for the determination of imazalil residues in or on
cucurbitaceae (Wynants and Woestenborghs, 1975a), bananas (Anonymous,
1976d), wheat and wheat-straw (ten Broeke and Dornseiffen, 1976) are
also available.
Recently a new method has been developed for the determination of
imazalil and its metabolite
alpha-(2,4-dichlorophenyl)-1H-imidazole-1-othanol (T824), based on the
above principles but using heptane/isoamyl alcohol (95/5, v/v) for the
extraction. When T 824 is converted by
N,O-bis(trimethylsilyl)acetamide to a silyl ether it can be
quantatively determined by GC with 3% OV-225 on Gas Chrom Q using an
ECD (Wynants, 1977b; ten Broeke and Dornseiffen, 1977).
NATIONAL TOLERANCES
No national tolerances were known to the Meeting.
APPRAISAL
Imazalil is a systemic fungicide which is effective against fungi
imperfecti and some fungi of the class "Ascomycetae". It is also
effective against benzimidazole-resistant strains of plant pathogenic
fungi. It is formulated as a seed dressing for cereals, an
emulsifiable concentrate and a wettable powder. The main pre-harvest
applications for the latter formulations are on bananas and cucurbits:
other uses are being developed. Imazalil is also highly effective
against fungal infections on bananas and citrus fruit after harvest.
It is used in several European countries.
In plants alpha-(2,4-dichlorophenyl)-1H-imidazole-1-ethanol and other
unidentified polar, hydrophilic compounds are the main degradation
products. The half-lives of imazalil on oranges and in soil are 12-20
weeks and 4-5 months respectively. No accumulation in soil was
observed.
Following pre-harvest application in accordance with good agricultural
practice, the maximum residue levels of imazalil in or on cucumbers
and gherkins under glass were below 0.5 mg/kg. Post-harvest
application at recomended dosages resulted in residues of imazalil
below 2 mg/kg on bananas and below 0.2 mg/kg in the pulp. Residues
were below 5 mg/kg on citrus fruit and below 0.1 mg/kg in the peeled
fruit and in wheat straw; residues in/on wheat grain were below 0.01
mg/kg.
Residue levels on citrus fruit largely depend on the dosage applied;
no significant differences in the residue levels on fruits which were
stored for 32 days at 40°C and 22-25°C were noticed.
Gas-chromatographic methods for the determination of imazalil in
various crops, suitable for regulatory purposes, have been elaborated.
RECOMMENDATIONS
The following temporary maximum residue limits are recommended on the
basis of data resulting from supervised trials involving pre- and
post-harvest uses. They refer to imazalil only.
Commodity Temporary limit, Pre- or post-harvest
mg/kg interval (days) on
which recommendations
is based (post-harvest
in brackets)
Citrus fruit
(whole) 5 (0-42)
Bananas
(whole) 2 (0-100)
Cucumbers,
gherkins 0.5 0-1
Bananas
(without peel) 0.2 (0-100)
Citrus fruit
(without peel) 0.1 (0-42)
Wheat straw 0.1
Wheat (grain) 0.01*
* at or about the limit of determination.
FURTHER WORK OR INFORMATION
REQUIRED (by July 1981)
1. Comparative acute toxicity studies of the different salts.
2. Farther long-term studies.
DESIRABLE
1. Pharmacokinetic studies of the different salts.
2. Metabolic studies with imazalil labelled with 14C.
3. Further information on the nature and level of metabolites and
degradation products in plants.
REFERENCES
Anonymous (1975a) "Physico-chemistry of R 18531 -imazalil nitrate,
R 23979 - imazalil, and R 27180 -imazalil sulphate". Janssen
Pharmaceutica; Pre-formulation report no. 496; 750107.
Anonymous (1975b) "Degradation of imazalil in oranges". Biochemical
Department of the Organisch Chemisch Instituut TNO - Utrecht - The
Netherlands; Progress report of project 1.1; 751106.
Anonymous (1976a) "Metabolism of imazalil applied to oranges".
Biochemical Department of the Institute for Organic Chemistry TNO
- Utrecht - The Netherlands; Report of project 1.II; 760224.
Anonymous (1976b) "Metabolism of imazalil in barley plants".
Biochemical Department of the Institute for Organic Chemistry
- Utrecht - The Netherlands; Addition to the first report of project
2; 760521.
Anonymous (1976c) "Testicide residue report - imazalil on bananas".
Jansses Pharmaceutica., 760816.
Anonymous (1976d) "Procedure for imazalil in whole bananas". Janssen
Pharmaceutica; 761009.
Anonymous (1976e) "A study of the fate of imazalil in soil".
Biochemical Department of the Institute for Organic Chemistry TNO
- Utrecht - The Netherlands; Report of Project 4; 761021.
Anonymous (1976f) "Degradation of imazalil in soil under field
conditions". Biochemical Department of the Institute for Organic
Chemistry TNO - Utrecht - The Netherlands; Report of project 5, part
1; 761105. Attached letter of TNO - 770524.
Anonymous (1976g) "Residue summary sheets for imazalil in bananas".
Morse Laboratories, Ltd. - Sacramento - California - U.S.A.; 761129.
Anonymous (1976h) "Degradation of imazalil in soil under field
conditions". Biochemical Department of the Institute for Organic
Chemistry TNO - Utrecht - The Netherlands; Report of project 5, part
2; 761209.
Bartlett, D.H., Ballard, N.E. (1975) "The effectiveness of guazatine
and imazalil as seed treatment fungicides in barley". Proc. 8th Brit.
Insect. Fung. Conf. - 1975.
Beijers, H.P. (1975) "Imazalil residues in citrus samples". SABS
- South Africa; Report no. 0311/8882/M410; 751128.
Beijers, H.P. (1976) "Imazalil residues in citrus samples". SABS Rep.
No. 0311/8960/N400; Pretoria, 10 Nov. 1976.
Bliss, G.W., Orme, J.P.R. (1976) "Certificate of analysis - Analysis
of residues of imazalil in oranges". Huntingdon Research Centre;
Huntingdon - U.K. report VPT/2; 761223.
ten Broeke, R., Dornseiffen, J.W. (1976) "Imazalil residues in wheat
and wheat-straw". Keuringsdienst van Waren - Amsterdam; Rapport
KvW 197/Vondelingenplaat 1976-8; 761020.
ten Broeke, R., Dornseiffen, J.W. (1977) Residues of imazalil and its
metabolite T 824 in gherkins under glass. KvW 203/4.2. (2.1.01) 1976;
July 1977.
Corpel J., Gauthier, M. (1977) "Campagne orange du Marco".
Commissariat a l'energie atomique; Centre d'études nucléaires de
Cadarache; 770407.
Frossard, P., Laville, E., Plaud, G. (1976) "Etude des traitements
fongicides appliques aux bananes aprés récolte; III: Action de
l'imazalil". Fruits 31, 361-364 (1976).
Greenberg, R., Resnick, C., (1977) "A gas-liquid chromatographic
method for determining imazalil residues in citrus fruit"; Pestic.
Sci. 8, 59-64 (1977).
Helsen, W. (1976) "Thermal stability of imazalil and salts". Janssen
Pharmaceutica; memo dated 760813.
Heykants, J., Meuldermans, W., Hurkmans, R. (1975) "On the excretion,
metabolism and tissue distribution of imazalil-3H in the male and
female Wistar rat". Janssen Pharmaceutica; Department of drug
metabolism; 7512.
Kraght, A.J. (1977a) "Metabolism of imazalil applied to
oranges - Metabolite study by tracer technique". Pennwalt
Corporation - Decco Division - U.S.A. 770518.
Kraght, A.J. (1977b) "Residue data of imazalil on citrus". Pennwalt
Corporation - Decco Division - U.S.A.; 770518.
Laville, E., Herding, P.R., Dagan, Y., Rahat, M., Kraght, A.J. (1977)
"Studies on imazalil as potential treatment for control of citrus
fruit decay". Proc. Int. Soc. Citriculture, 1977.
Maier-Bode, H. (1976) "Imazalil residues in oranges after post-harvest
treatment". Rickenbach - Federal Republic of Germany; 760622.
Marsboom, R. (1970) Potential of R18531 for Embryotoxicity and
Teratogenic Effects in Rats Receiving R18531 Orally. Unpublished
report from Janssen Pharmaceutical submitted to the World Health
Organization by Pennwalt Corporation.
Marsboom, R., Vandesteen, R., Herin, V. and Pardoel, L. (1971) Safety
Evaluation of R18531 During 14 Weeks of Oral Treatment to Rats.
Unpublished report from Janssen Pharmaceutical submitted to the World
Health Organization by Pennwalt Corporation.
Marsboom, R. (1975a) Oral Embryotoxicity and Teratogenicity Study in
Wistar Rate During the Peri- and Postnatal Study (Segment III). A
report from Jansses Pharmaceutical submitted to the World Health
Organization by Pennwalt Corporation. (unpublished report)
Marsboom, R., Herin, V., Vandesteene, R. and Pardoell L. (1975b) Oral
Toxicity Study in Wistar Rats (repeated dosage for 6, 12 or 24
months). Unpublished report from Janssen Pharmaceutical submitted to
the World Health Organization by Pennwalt Corporation.
Marshboom, R. (1975c) Oral Three Generation Study in Wistar Rats.
Unpublished report from Janssen Pharmaceutics, submitted to the WorId
Health Organization by Pennwalt Corporation.
Marshboom, R. (1976a) Dominant Lethal Test in Male Mice. Unpublished
report from Janssen Pharmaceutica, submitted to the World Health
Organization by Pennwalt Corporation.
Marsboom, R. (1976b) Dominant Lethal Test in Female Mice (single oral
dose). Unpublished report from Janssen Pharmaceutica, submitted to the
World Health Organization by Pennwalt Corporation.
Marsboom, R. (1977a) Oral Male and Female Fertility Study in Wistar
Rats (Segment I). Unpublished report from Janssen Pharmaceutical
submitted to the World Health Organization by Pennwalt Corporation.
Marsboom, R., Herin, V., Van Desteene, R. and Van Belle. (1977b) Oral
Toxicity Study in Beagle Dogs (repeated dosage for 24 months).
Unpublished report from Janssen Pharmaceutical submitted to the World
Health Organization by Pennwalt Corporation.
Melin, Ph., Plaud, G., Tezenas du Moricel, H., Laville, E. (1976)
"Action do l'imazalil sur le niveau d'infestation et l'état
d'évolution de la cercosporiose". Fruits 31 (10)t 599-602 (1976).
Meuldermans, W., Swysen, E., Lauwers, W., Heykrantz, Jr. (1977a) An
in-vitro Study of Imazalil - 3H in the Rat. Unpublished report from
Janssen Pharmaceutica, submitted to the World Health Organization by
Pennwalt Corporation.
Meuldermans, W., Hurkmans, J., Lanwers, W., and Heykants, J. (1977b)
The Excretion and Biotransformation of Imazalil and One of Its
Metabolites (R14821) in the Wistar Rat. Unpublished report from
Janssen Pharmaceutical Department of Drug Metabolism and Analytical
Department, submitted to the World Health Organization by Pennwalt
Corporation.
Nangniot, P. (1977) "Teneurs résiduaires an imazalil dans des
concombers". Centre de Recherche de
Phytophaxmacie - Gembloux - Belgique; 770428.
Niemegeers, G.J.E. (1973) Acute Oral Toxicity in Rats. Unpublished
report from Janssen Pharmaceutica, submitted to the World Health
Organization by Pennwalt Corporation.
Niemegeers, C.J.E. (1976) Acute Oral Toxicity of R23979 - 20% E.C. in
Rats. Unpublished report from Janssen Pharmaceutica, submitted to the
World Health Organization by Pennwalt Corporation.
Niemegeers, G.J.E. (1977) Acute Intraperitoneal Toxicity of R23979 in
Wistar Rats. Unpublished report from Janssen Pharmaceutica, submitted
to the World Health Organization by the Pennwalt Corporation.
Pattyn, W. (1976) "Purity of R23979 -imazalil technical". Janssen
Pharmaceutica; Chemical Quality Control Department; 761001.
Stover, R.H. (1976) "Imazalil efficacy data". Vining C. Dunlap
Laboratories of the United Fruit Company in Honduras; 761102.
Attachment: Stover, R.H., Dickson, J.D. "Leaf sport of bananas caused
by Mycosphaerella musicola: Methods of measuring spotting prevalence
and severity". Tropical Agriculture 47, 289-302. (1970)
Van den Bossche, H. (1977) Studies on the mode of action of imazalil.
Janssen Pharmaceutica; 770425. Unpublished.
Van Ravestyn, O. and Marsboom, R. (1975) Acute Dermal Toxicity in Rats
(single dosage). Unpublished report from Janssen Pharmaceutical
submitted to the World Health Organization by Pennwalt Corporation.
Van Tuyl, J.Y. (1977) Genetics of fungal resistance to systemic
fungioides. Mededelinger
Landborwhogeschool - Wageningen - Netherlands; 77-2.
Wynants, J, Woestenborghs, R. (1975a) "A gas-chromatographic assay
method for imazalil in cucumbers". Janssen Pharmaceutics; 7512.
Wynants, J., Woestenborghs, R. (1975b) "A gas-chromatographic assay
method for imazalil in citrus fruit". Janssen Pharmaceutics; 7512.
Wynants, J., Woestenborghs, R. (1976) "Residue analysis of imazalil in
cucumbers". Janssen (1976) Pharmaceutics; 7601.
Wynants J. (1977a) "Chromatographic assay methods of imazalil in
citrus fruit". Proc. Int. Soc. Citriculture, 1977, vol. H: 38.
Wynants J. (1977b) "Residue detection method for imazalil and its
metabolite T824". Janssen Pharmaceutics; provisional report; 770420.
