
BROMOPROPYLATE
First draft prepared by
A. Takanaka
Biological Safety Research Center, National Institute of Health
Sciences
Tokyo, Japan
EXPLANATION
Bromopropylate was previously evaluated by the Joint Meeting in
1973 when an ADI of 0-0.008 mg/kg bw was allocated (Annex 1,
reference 20 & 21). That meeting recommended further desirable work
as follows: 1) studies to elucidate the effects on survival rate of
rats in long-term feeding studies; 2) long-term studies in a second
animal species; 3) studies on the effects of bromopropylate on the
liver. The results of these studies and additional data were
submitted for the present evaluation. This monograph summarizes the
data that have been reviewed since the previous evaluation and
incorporates relevant studies from the previous monograph.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
Rats
The absorption, distribution and elimination of bromopropylate
were evaluated in rats. [U-14C]phenyl bromopropylate was given
orally at single doses of 0.5 mg/kg bw (at a no-effect level) or 100
mg/kg bw (to produce some pharmacological/toxicological effect) to
groups of 5 male and 5 female rats (Sprague-Dawley Crl: CD [SD] BR).
At the low dose level an additional group received 14 consecutive
daily doses of 0.5 mg/kg bw non-labelled compound (> 95% pure)
followed by a single dose with radiolabelled compound. Under all
three dosing regimens the total recoveries of radioactivity within
168 hours past termination of dosing were 90% and higher. Most of
the dose was eliminated in faeces (males: 85.3-89.3%, females: 52.5-
63.7%). Lower amounts of radioactivity were excreted in the urine
(males: 2.3-3%, females: 21.8-28.7%). Excretion of radioactivity
was essentially complete within 96 hours independent of dose level
or pretreatment. No radioactivity was expired in CO2.
At the low-dose after 168 hours, tissue concentrations were low
with highest values in liver (0.05 µg equivalents of
bromopropylate/g tissue in male or female) and abdominal fat (0.02
or 0.03 µg equivalents of bromopropylate/g tissue in male or
female). At the high dose, the tissue residue pattern was similar,
with highest concentration of radioactivity in the abdominal fat
(equivalent to 3.3 or 8.7 µg/g in male or female). Pre-treatment
with the non-radiolabelled compound did not significantly alter
tissue residues at 168 hours post-dose over those animals receiving
a single administration. It was concluded that excretion rates were
fast, and independent of dose level and pretreatment. The routes of
elimination were sex-dependent. The low tissue residue levels were
observed at 168 hours after administration (Cornelissen & Hopkins,
1991).
Two male and two female rats were each administered by gavage
1.6 mg 14C-labelled bromopropylate. Expired CO2, urine and faeces
were collected for the next 120 hours in 24 hour periods after which
blood and other tissues were taken for analysis. Less than 0.2% of
the activity was found in respired CO2. In males, 90% of the
activity was found in faeces and 6% in urine while in females 55%
appeared in faeces and 33% in urine. About 75% of the activity was
eliminated in 48 hours but after 120 hours, 2.6% (males) and 1.5%
(females) of the administered dose remained in tissues, mainly in
kidneys, liver and fat (Cassidy et al., 1968).
Biotransformation
The metabolic profile of bromopropylate was investigated in
urine, faeces, and tissue extracts from male and female rats. The
study involved single oral administration at a low (0.5 mg/kg bw)
and high dose level (100 mg/kg bw), and repeated oral administration
at the low dose level. Excreta and tissue samples generated in the
absorption, distribution and excretion study were used in this
experiment.
In the urine pools, representing 1.7% to 26.4% of the
administered dose, there were seven distinct metabolite fractions.
The metabolite pattern did not significantly differ between the dose
levels or dose regimen. However, a pronounced sex difference was
observed: the benzilic acid was a minor fraction (10%-14%) in males
but the major fraction (65%-72%) in females. The metabolite pattern
in faeces did not significantly differ between dose levels, dose
regimen and, in contrast to urine, between the sexes. The urinary
and faecal metabolic patterns differed qualitatively: there was a
non-polar fraction in the faeces, corresponding to unchanged
bromopropylate, which represented 14 to 54% of the total faecal
radioactivity.
The metabolite pattern in liver tissue was not sex-dependent
and rather simple with the two major fractions corresponding to
bromopropylate and the benzilic acid. The pattern in kidney tissue
was also not sex-dependent. It contained only one major fraction
accounting for at least 80% of the radioactivity in kidneys and
corresponding to the benzilic acid. The benzilic acid accounted for
at least 70% and 40% of the radioactivity in male and female lung
tissue, respectively. The fat tissue contained unchanged
bromopropylate as a major component representing 40% and 80% of the
radioactivity for males and females, respectively.
Thus, within the tested dose range the metabolism of
bromopropylate in the rat was independent of dose level and dose
regimen. However, a pronounced sex difference was observed in all
groups. The benzilic acid was a minor metabolite in males
(approximately 6% of the dose) and the major metabolite in females
(approximately 23% of the dose). This parallels the sex difference
observed for the route of excretion. The dominant compound in
kidney and lung tissue was the benzilic acid, in fat tissue the
unchanged bromopropylate, and in liver tissue both were present in
substantial amounts (Mathies, 1991).
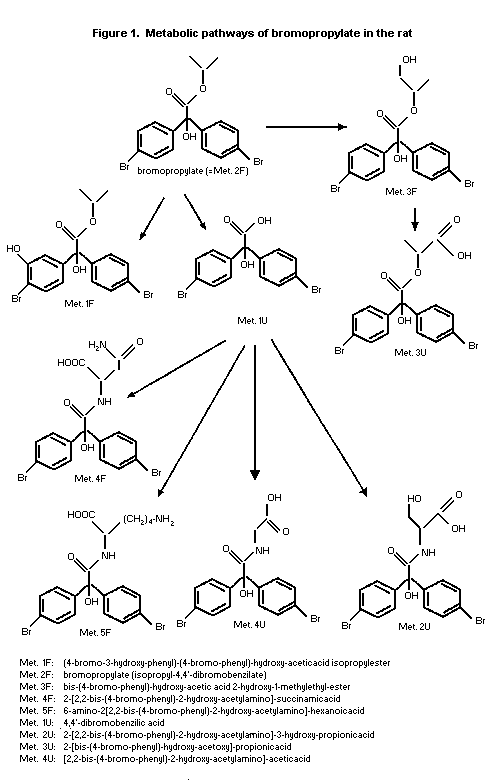
The structures of eight metabolites were identified from urine
and faeces, covering 80% and 72% of the dose for males and females,
respectively. The metabolic pathway is depicted in Figure 1. It is
concluded that bromopropylate was metabolized preferentially by
cleavage of the isopropyl ester and to a minor extent by oxidation
reactions attacking the phenyl ring and the isopropyl group. The
primary product formed after ester cleavage, the benzilic acid, was
subject to subsequent conjugation reactions, leading to a variety of
amino acid conjugates, or was excreted as such. Metabolites formed
after oxidation were 3-hydroxy-benzilate and a propylene glycol
derivative of the parent compound (Schulze-Aurich, 1993).
Effects on enzymes and other biochemical parameters
Mice
Bromopropylate (95% pure) was administered by gavage to male
mice (Tif: MAG f) at daily doses of 0, 10, 50, 150 or 300 mg/kg bw
for 14 days. A dose-related increase in liver weight was associated
with moderate induction of some enzymes known to be involved in
xenobiotic metabolism and with a weak proliferation of smooth
endoplasmic reticulum in hepatocytes. Particularly, cytochrome P-
450, ethoxycoumarin O-deethylase and styrene oxide hydrolase
activities were enhanced at all dose levels; the activity of
cytosolic glutathione S-transferase was increased at the two high
dose levels, while that of UDP-glucuronyl-transferase was only
slightly increased and that of cytosolic trans-stilbene oxide
hydrolase was not affected by treatment. Reversibility studies
demonstrated that the increased liver weights as well as the
investigated biochemical parameters fully reverted to control levels
after 4-week recovery. Moreover, proliferation of smooth
endoplasmic membranes disappeared. The observed biochemical and
morphological response pattern can therefore be interpreted as a
reversible adaptive response to a functional overload (Waechter et
al., 1986).
In a recent study, bromopropylate (94.2% pure) was given to
groups of 6 male mice (Tif:Mag f) at single oral doses of 0, 3, 15,
100 or 300 mg/kg bw for 14 days. Two additional groups received
phenobarbital (40 mg/kg bw, i.p. for 4 consecutive days) or 3-
methylcholanthrene (80 mg/kg bw, i.p. 72 and 48 hours prior to
sacrifice). No symptoms and no compound-related mortalities were
recorded. Body-weight gains were slightly reduced at 300 mg/kg bw.
Liver weights were increased in the high-dose group and in the
groups receiving phenobarbital or 3-methylcholanthrene. In the
liver, microsomal protein contents were increased dose-dependently
in the bromopropylate-treated animals as well as in both groups
receiving the reference compounds. A slight to moderate dose-
dependent increase of the microsomal P-450 content was observed.
 The ethoxycoumarin O-deethylase, ethoxyresorufin O-deethylase,
pentoxyresorufin O-depentylase, and total testosterone hydroxylase
activities were also increased. The results of studies with
monoclonal antibodies against rat liver cytochrome P-450 isozymes of
four different gene families confirmed that bromopropylate shares
properties typical of a moderate phenobarbital-type inducer and
possibly is also a very weak peroxisome proliferator-type inducer.
It was concluded that bromopropylate has the properties of a
phenobarbital-like inducer of cytochrome P-450 in the mouse liver.
In addition, it might act as a very weak peroxisome proliferator.
The increased liver weights at the high-dose and the biochemical
changes were therefore regarded as a reversible adaptation to a
functional overload. At 15 mg/kg bw/day only minimal biochemical
changes were observed and the low dose of 3 mg/kg bw/day was without
any effects (Thomas, 1991).
A DNA-binding study was conducted with mice (Tif: MAG f)
pretreated for two weeks with unlabelled bromopropylate (94.2% pure)
at daily oral doses of 300 mg/kg bw. Radiolabelled [ bis-phenyl-
14C] bromopropylate was then administered by gavage at a dose level
of 300 mg/kg bw. After 24 hours, DNA was isolated from the liver
and purified. No radioactivity was detected. Bromopropylate is
devoid of a genotoxic potential mediated by DNA-binding (Sagelsdorff
& Heuberger, 1990).
Toxicological studies
Acute toxicity studies
Bromopropylate has a low acute toxicity in rats and rabbits.
WHO has classified bromopropylate as unlikely to present acute
hazard in normal use (WHO, 1992). The LD50 and LC50 values are
summarized in Table 1.
Short-term toxicity studies
Rats
Groups of 10 male and 10 female rats received 0, 40, 200, 1000
or 5000 mg bromopropylate/kg bw/day by gavage as a suspension in
0.5% aqueous tragacanth on six days each week for four weeks. The
highest dosage level produced polyuria throughout the 21 days before
the animals were killed and for the first eight days pale mucoid
faeces were produced. The rate of body-weight gain and food intake
were reduced in the 200 and 1000 mg/kg bw/day groups. Rats of the
5000 mg/kg bw/day group developed a relative neutrophilia but the
results of haematological analyses in all groups were normal. The
absolute and relative liver weights were increased in the three
highest dosage groups and cytoplasmic swelling and periportal
Table 1. Results of acute toxicity studies with bromopropylate
Species Sex Route Solvent LD50/LC50 Reference
(vehicle) (mg/kg bw)/
(mg/m3 air)
Mouse1 M oral - 8000 Ueda & Kondo, 1968
Rat1 M,F oral - > 5000 Stenger, 1967
Rat2 M,F oral - 6000 Drake, 1970
Rat2 ? oral - > 23 000 Fancher et al., 1968a,b
Rat3 M,F oral corn oil approx. 5000 Kuhn, 1989a
Rat4 M,F dermal PEG 400 > 4000 Bathe & Sachsse, 1979
Rat5 M,F inhalation ethanol > 4458 Hartmann, 1989
Rabbit4 M,F oral CMC6 > 6000 Ullmann & Sachsse, 1979a
Rabbit3 M,F dermal saline > 2020 Kuhn, 1989b
1 Purity given as Technical.
2 Purity given as Formulation.
3 Purity not given but known to be 90%.
4 90% pure.
5 95% pure.
6 CMC = 2% carboxymethylcellulose in water.
infiltration were seen. Hepatic necrosis occurred at the highest
dosage level. At 40 mg/kg bw/day, the one animal which died during
the test showed similar abnormalities in the liver; others in the
group appeared normal (Paterson, 1967a).
Groups of 12 male and 12 female rats were fed diets containing
0, 100, 300 or 1000 ppm bromopropylate for 90 days. Another group
received diets containing 3000 ppm for 55 days and 4000 ppm for a
further 35 days. At the highest dose, the food intake and rate of
weight gain were below normal and at autopsy the liver, kidney and
testes weights relative to body weight were higher than in controls.
Swelling and loss of basophil material, pigmentation (possibly
lipofuscin) and fatty infiltration were present in hepatocytes.
Mild to severe regressive changes were seen in testes on
histological examination. At 1000 ppm, food intake was slightly
less than in controls although weight gain was similar. Testes were
lighter than in controls and similar histological changes were seen
in the liver. At the two lower levels, smaller numbers of rats
showed loss of basophilic material and cytoplasmic swelling with
focal vacuolation. These changes were considered to be
physiological in that they were attributed to SER hypertrophy, but
no EM studies were carried out to confirm this. The results of
haematological and blood biochemistry studies and of urine analyses
were similar in all groups (Paterson & Drake, 1967).
Bromopropylate (94.2% pure) was applied to the shaved skin of 5
male and 5 female rats (Tif:RAI f) per dose group for 4 weeks on a
5-day per week basis. The daily exposure period was 6 hours. The
compound was given on moistened gauze patches under occlusive
dressing at dose levels of 0, 50, 200 or 1000 mg/kg bw/day. There
was no mortality, no clinical signs, no signs of local irritation
and no body weight or food consumption changes that could be
attributed to treatment. At 1000 mg/kg bw/day, slightly lower
glucose levels and a minimal increase of urea were evident in blood
chemistry, while no changes were seen in haematology. Organ
weights, macro- and microscopic histopathological findings were
similar in all groups. The NOAEL in this study was 200 mg/kg bw/day
in both sexes (Schneider et al., 1989).
Dogs
A group of two male and two female dogs received orally, by
capsule, 1000 mg/kg bw/day of bromopropylate for 30 days. A second
group received 2000 mg/kg bw/day for four days, then 500 mg/kg
bw/day for a further 26 days. Diarrhoea and vomiting occurred in
dogs of both groups. During the first week, food intake and body
weight decreased. The results of haematological examinations were
normal. Determination of organ weights did not indicate any dose-
related changes. In both groups, serum transaminase and alkaline
phosphatase levels were increased by treatment and cytoplasmic
swelling and fine vacuolation of hepatocytes were found. No mature
spermatozoa were found in male animals, but this may have been due
to the age of the animals (Paterson, 1967b).
Four groups of eight male and eight female dogs were fed for
two years on diets containing 30, 100, 250 or 1000 ppm
bromopropylate. A fifth group received 4000 ppm in the diet for
three months. A group of 10 males and 10 females acted as controls.
The highest dosage level produced light-coloured soft stools after
one week and semi-fluid stools thereafter. Food intake was reduced
and weight loss occurred. Two dogs died (after four and six weeks)
and two others were killed because they became cachectic. In these
four animals, increased serum alkaline phosphatase, diminished serum
cholesterol and mild anaemia were found. Haemosiderosis of kupfer
cells, proximal tubular cells of the kidney, the spleen and the bone
marrow, degenerative changes in the distal convoluted tubules of the
kidney, hyperplasia of bone marrow and extra-medullary
haematopoiesis were seen in these animals at autopsy. In the other
animals of the 4000 ppm group killed at three months, accumulation
of haemosiderin in the macrophages of the spleen and bone marrow and
in kupfer cells was observed. Examination of liver by EM showed SER
hypertrophy and an increase in numbers of lysosomes, many of which
contained lamellar structures. Two additional animals of each sex
were fed for nine months on control diets following three months on
4000 ppm diet. Stools returned to normal consistency within a week
and weight gain was normal. Biochemical and haematological
parameters also returned to normal in a short time; animals were
indistinguishable from controls at the time of autopsy. The 1000
ppm diet also produced softening of stools. Although weight gain by
males was normal, females failed to gain weight. Some females
showed slight anaemia on some occasions. The microsomal enzymes,
biphenyl hydroxylases, showed increased activity and this was
associated with an increase in liver weight relative to body weight
at six months and at two years (in females only). SER hypertrophy
was marked at three months and numerous myelin bodies were also
seen. Although at two years the SER hypertrophy was not different
from controls, myelin bodies were still present. An increase in
microsomal enzymes was found in the 250 ppm group together with an
enlarged liver (relative to body weight) at six months but not at
two years. No adverse effects were observed in dogs fed on 30 or
100 ppm diets (Coulston et al., 1970a).
Bromopropylate (95% pure) was administered in the diet at
concentrations of 0, 100, 400 or 2000 ppm to groups of 6 male and 6
female beagle dogs for one year. After completion of the treatment
period, subgroups of 2 animals per sex were kept for one month on
compound-free (control) diet to determine reversibility of potential
toxic effects. There were no compound-related mortalities and no
clinical signs. Body-weight gain was decreased markedly at 2000 ppm
in both sexes and slightly at 400 ppm in females from week 28
onwards. Food consumption was not affected and there were no
ophthalmic, haematological or urinary changes. The only relevant
change in blood chemistry was a slight reversible increase of
alkaline phosphatase activity in the females at 2000 ppm. At the
end of the 12-month treatment period, liver weights were slightly
increased and there was a slight hypertrophy of hepatocytes. These
changes only occurred at 2000 ppm and were not present after the
one-month recovery period. The NOAEL in this study was 100 ppm,
equal to 2.7 mg/kg bw/day (males) and 2.8 mg/kg bw/day (females)
(Gretener et al., 1989).
Long-term toxicity/carcinogenicity studies
Mice
In an oncogenicity study, mice (Charles River CD-1, 50 males
and 50 females per group) were given bromopropylate (98.7% pure) in
the diet during 18 and 20 months for males and females,
respectively, at concentrations of 0, 30, 300 or 1000 ppm. Similar
reduction in body weight was noted for the control and test groups
after 12 months of testing, males losing an average of 6 to 7 grams
and females 2 to 3 grams. This generalized reduction in body weight
occurred following alterations in both the method of feeding and the
standard diet fed. However, no statistically significant
differences in mean body weights were noted for any treatment group.
Meaningful evaluation of body-weight gain cannot be made since body
weights were not measured during the first 6 months of testing.
Negative body-weight gains were recorded for the observation period
for all groups. The mean survival time of males in the 1000 ppm
group was significantly reduced. Significantly increased
mortalities in the 30 and 1000 ppm groups were observed, which were
considered coincidental. No treatment-related findings were seen at
gross pathological examination. Histopathology revealed dose-
related centrilobular hypertrophy of hepatocytes in the two higher
doses. Additional sporadic histopathological findings were not
considered as related to treatment. The compound did not show an
oncogenic potential in this study (Charles et al., 1979).
Bromopropylate (95% pure) was administered to male and female
mice (Tif: MAGf (SPF), hybrids of inbred MIG x NIH mice) in the diet
at levels of 0, 30, 150, 1000 or 3000 ppm (60 males and 60 females
per group) for 24 months. Appearance and behaviour were not
affected by treatment. Lower body-weight gain was recorded for the
3000 ppm group over the first 12 weeks for males and the first 25
weeks for females. Thereafter, mean body weights remained 5-7% and
15-18% lower than control values, for males and females,
respectively. Food consumption was slightly lowered in females in
the 1000 and 3000 ppm groups during the first year. Water
consumption was increased at 3000 ppm. Haematological parameters
were not significantly affected by treatment. Absolute and relative
liver weights were significantly increased in both sexes at 1000 and
3000 ppm. Macroscopic examination revealed dose-related increase of
masses and nodules in the livers of male and female mice from these
two groups. Kidney weights at 3000 ppm were lower than in the
control group. On microscopic examination, hepatocellular
neoplastic lesions, i.e. benign hepatomas and hepatocellular
carcinomas, accounted for most of the macroscopically observed
masses and nodules in the liver. The frequency of these lesions was
significantly increased in both sexes at 1000 and 3000 ppm. Animals
of both sexes of these two groups also showed a frequent hypertrophy
of hepatocytes. The only other compound-related finding was an
increased incidence of reactive hyperplasia of splenic white pulp in
female mice of the high dose group (3000 ppm). The NOAEL in this
study was 150 ppm, equal to 16 mg/kg bw/day in both males and
females (Basler et al., 1990).
Rats
Groups of 50 male and 50 female rats were fed diets containing
0, 15, 30 or 100 ppm bromopropylate for two years. Five male and
five female rats from each group were killed after six months or one
year and autopsies performed. No changes in appearance or behaviour
occurred. Food consumption and weight gain were similar in test and
control animals throughout the test and survival rates were similar
for the first 18 months. Approximately half of the control female
animals alive at 18 months survived to 24 months while only a third
of test animals at 30 and 100 ppm survived over this period. No
alterations attributable to bromopropylate were observed in the
haematological examinations in test animals. No changes in organ
weights or gross or microscopic abnormalities attributable to
ingestion of the pesticide were observed in animals examined at any
time. However, EM studies on the livers of animals of the 100 ppm
group surviving two years showed slightly less glycogen, focal
enlargement of intracrestal space in the mitochondria, focal
dilatation of SER and more prominent lipid accumulations than livers
of control animals. These differences were not considered to be
significant pathological alterations in cell ultrastructure. The
number of tumours and their location were similar in control and
test groups (Coulston et al., 1970b).
Bromopropylate (95% pure) was administered in the diet to rats
(Tif: RAlf, 80 males and 80 females per dose group) at levels of 0,
100, 700 or 5000 ppm. The study duration was 119 weeks for males
and 131 weeks for females. Average intakes of bromopropylate were
3.7, 26 or 217 mg/kg bw/day for males and 4.4, 32 or 270 mg/kg
bw/day for females. In the 5000 ppm group, males maintained mean
body weights 20% lower than control values from 15 weeks onwards,
whereas females showed a progressive divergence, attaining a 37%
difference from control values at 95 weeks. Food consumption was
decreased in males and females at 5000 ppm, and water consumption
was increased in both males and females at 5000 ppm and in males at
700 ppm. Calculated red blood cell indices (MCV, MCH) were
decreased in males (from 80 weeks) and in females (from 27 weeks) at
5000 ppm, suggesting a tendency to microcytosis and hypochromoasia
of red blood cells. Increased prothrombin times were recorded in
males at weeks 12, 27, and 80, and in females at all investigated
weeks at 5000 ppm. Blood samples were collected at 12, 27, 53, 80,
105 and 131 weeks. A non-progressive lowering of blood glucose was
recorded at weeks 12 to 80 for males and at weeks 27, 53 and 80 for
females at 5000 ppm. A hyperproteinemia resulting from
hyperglobulinemia was observed in the males and females at 5000 ppm.
Females of the 700 ppm group showed a similar trend, but to a lesser
degree between weeks 27 and 105. Higher levels of cholesterol and
phospholipids were recorded at week 53 for males and on all
occasions for females at 5000 ppm. In the 700 ppm group, high
levels of cholesterol were observed in females at weeks 12, 27 and
80. Also, high levels of phospholipids were recorded in females at
12 and 80 weeks. Both sexes of the 5000 ppm group showed a marked
increase in gamma glutamyl transpeptidase activity at each
investigation.
At the interim sacrifices during weeks 53 and 105 and at the
termination of the study, relative liver weight for males and
females of the 5000 ppm group were significantly increased. In
addition, the relative liver weights for rats at 700 ppm were
significantly increased at week 53 for females and at termination
for males. Significantly increased relative kidney weights were
recorded for males and females of the 5000 ppm group at week 53 and
for females at week 105. Relative thyroid gland weights in males at
700 and 5000 ppm were significantly increased at week 53 and the
relative weight in males at 5000 ppm was also increased at
termination of the study. Macroscopic examination revealed an
increased number of rats with edema of the testes and an increased
number of females with liver cysts at 5000 ppm. In
histopathological examinations, increased incidences of focal
hepatocellular hypertrophy and fatty changes and pigmentation of
hepatocytes were also observed in both sexes at 700 and 5000 ppm.
Incidences of biliary cysts and cholangiofibrosis were increased in
females at 5000 ppm. Occurrence of lung alveolar foam cells were
increased in females at 5000 ppm. An increase in the number of rats
with testicular tubular atrophy and an associated edema and nodular
hyperplasia (Leydig cells) was observed at 5000 ppm. Numbers of
rats with follicular cystic dilatation and thyroid gland hyperplasia
were increased in both sexes at 5000 ppm. Incidence of rats with
thyroid gland adenoma was increased at 5000 ppm in males. The NOAEL
in this study was 100 ppm (equal to 3.7 mg/kg bw/day for males and
4.4 mg/kg bw/day for females) (Basler et al., 1989).
Reproduction studies
Rats
Groups of 20 male and 20 female rats in the first generation
and groups of 25 male and 25 female rats in the second and third
generations were used in a three-generation test (two litters being
produced in each generation) and fed diets containing 0, 2.5 or 5
ppm bromopropylate. In a second test, groups of 25 male and 25
female rats received diets containing 0, 30 or 100 ppm
bromopropylate. Throughout the tests no abnormalities attributable
to bromopropylate were found in the reproductive physiology of male
or female rats and there was no evidence of gross abnormalities in
the offspring throughout the studies. The number of young in each
litter and their growth and survival were normal. The weights of
liver, kidneys and spleen were comparable with controls in the rats
of the F3b generation whose parents received 2.5 or 5 ppm diets
while spleen and liver weights of the F3b young of parents fed 30
or 100 ppm diets were slightly higher than controls. No
histological abnormalities were found in these animals at any dosage
level (Coulston et al., 1971).
In a two-generation reproduction study, rats (Sprague-Dawley-
served, Tif:RAI f) were administered bromopropylate (93.5% pure) in
the diet at concentrations of 0, 165, 750 or 2250 ppm (28
rats/sex/dose level). Approximate daily intakes of bromopropylate
were 12, 54 or 170 mg/kg bw for parental males and 16, 72 or 218
mg/kg bw for parental females. The administration started 10 weeks
prior to mating of the F0 parents and was continued throughout the
mating, lactation and weaning periods of the F1 and up to weaning
of the F2 generation.
F0 parental animals showed reduced body-weight gain and food
consumption at 2250 ppm and, to a lesser extent, in the 750 ppm
group. The females of the F1 parental generation showed some
depression in food consumption at 750 and 2250 ppm prior to mating
and only at 2250 ppm during lactation. Histological changes
consisted of a dose-related slight to severe hepatocellular
enlargement at 750 ppm and 2250 ppm corresponding to liver weight
increases in the F1 animals; in addition, some of the males in the
high-dose group showed hepatocyte necrosis. Reproductive parameters
(concerning mating, fertility, pregnancy, parturition and rearing)
were not affected by the treatment. With respect to the offspring,
the F1 pups of the 2250 ppm group showed an increased mortality
rate. For both F1 and F2 young rats of the 2250 ppm group, a
diminished body-weight gain was recorded from weaning onwards.
Exploratory locomotion was depressed in the young F1 rats at 750
and 2250 ppm and in the F2 rats at 750 ppm only.
Histopathological examination of the F1 rats revealed changes
in the liver, as indicated by slight to severe hypertrophy of
hepatocytes in about two-thirds of the males and females at 750 ppm
and in all animals at 2250 ppm. In the males, necrosis (8/24 at
2250 ppm) or other structural alterations of hepatocytes (11/23 at
750 ppm, 22/24 at 2250 ppm) were noted. Parallel to these results,
absolute as well as relative liver weights were found to be
increased in a "positive trend" from the control to the high
concentration. The lowest mean daily compound intakes during the
various study periods were calculated to be approximately 9, 40 or
128 mg/kg body weight. The NOAEL in this study was 165 ppm (equal
to 9 mg/kg bw/day) (Fritz et al., 1986).
Dogs
Groups of three male and three female dogs were fed diets
containing 0, 30 or 100 ppm bromopropylate. After an unstated time,
the females were mated with males of the same group. All matings
resulted in pregnancy and delivery of normally sized litters. The
only deformed pup was found in a control litter. All others were
normal in appearance, behaviour and development. Increases in the
body weight of the pups in most of the litters of groups receiving
bromopropylate were similar to those of control pups. The reduced
growth rate of one litter of the 100 ppm group was attributed to the
results of poor maternal care (Coulston et al., 1970a; Coulston &
Benitz, 1972).
Special studies on embryo/fetotoxicity
Rats
Bromopropylate (90% pure) was administered orally by gavage to
three groups of pregnant rats (CRL: COBS CD[SD]BR, 26 females/group)
at doses of 0, 50, 300 or 700 mg/kg bw once daily during days 6
through 15 of gestation. A control group of 26 female rats received
an equivalent volume (10 ml/kg bw) of aqueous 3% corn starch with
0.5% Tween 80. All surviving animals were sacrificed on day 20 of
presumed gestation. Evaluations were conducted on clinical signs,
maternal body weights, food consumption, necropsy and skeletal
abnormalities. Compound-related effects were noted at 300 and 700
mg/kg bw/day. These changes included: an increased incidence of
salivation and alopecia accompanied by skin irritation; transient
body-weight loss and slight reductions in daily food consumption at
700 mg/kg bw/day and of body-weight gain at 300 mg/kg bw/day; slight
decreases in mean male and female fetal weight at 700 mg/kg bw/day;
and an increased incidence of skeletal variations of fully formed
14th ribs and rudimentary 14th ribs at 300 and 700 mg/kg bw/day and
in forepaw metacarpals not ossified at 700 mg/kg bw/day. The fetal
findings were considered to be slight treatment-related effects
associated with the compound-induced maternal toxicity. There was
no evidence of embryo/fetotoxicity or teratogenicity at doses up to
and including 700 mg/kg bw/day. The maternal NOAEL in this study
was 50 mg/kg bw/day (Singh & Yau, 1990).
Rabbits
Bromopropylate (90% pure) was administered orally by gavage to
three groups of pregnant rabbits (New Zeeland white, 20
females/group) at doses of 0, 20, 60, or 120 mg/kg bw once daily
during gestation days 7 through 19. A control group of 20 pregnant
rabbits received an equivalent volume (5 ml/kg bw) of the vehicle.
All surviving animals were sacrificed on day 29 of presumed
gestation. Evaluations were conducted on clinical signs, maternal
body weights, food consumption, necropsy, reproductive parameters,
fetal weights and fetal gross, visceral and skeletal observations.
Treatment-related maternal effects were observed at 60 and 120 mg/kg
bw/day. These changes included: mortality/moribundity of two
animals at 120 mg/kg bw/day; clinical signs of lethargy at 120 mg/kg
bw/day and anogenital staining at 60 and 120 mg/kg bw/day;
reductions in mean body weight at 120 mg/kg bw/day, mean body-weight
gain at 60 and 120 mg/kg bw/day, and reduction of food consumption
at 120 mg/kg bw/day; and the necropsy finding of distended stomach
with viscous/gelatinous material at 120 mg/kg bw/day. There was no
evidence of embryotoxicity, fetotoxicity or teratogenicity at any
dose level. The NOAEL for maternal toxicity was 20 mg/kg bw/day
(Sing et al., 1990).
Special studies on genotoxicity
The results of genotoxicity studies conducted with bacteria,
mammalian cells in vitro and with intact animals in vivo are
presented in Tables 2a, b and c. In all studies, bromopropylate was
devoid of mutagenic potential.
Special studies on inhibition of cell-cell communication and liver
tumour promotion
Bromopropylate was investigated in vitro for its ability to
inhibit gap junctional intercellular communication in the Chinese
hamster fibroblast cell V79 metabolic cooperation assay and in the
scrape-loading/dye-transfer assay in WBF344 rat liver epithelial
cells, and in vivo altered hepatic foci assay and hepatic drug
metabolizing activity in male Sprague-Dawley rats. Technical grade
bromopropylate (94% pure) induced a dose-dependent increase in the
recovery of 6-thioguanine-resistant mutants within the same dose
range as reported earlier for the analytical grade (Wärngard et
al., 1985). In the scrape-loading assay, intercellular
communication was abolished at non-cytotoxic concentrations both
with technical and analytical grade bromopropylate. There was a
gradual decrease in dye transfer from the lowest concentration (20
µM) up to the dose inducing complete blockade of dye transfer.
Bromopropylate was shown to cause hepatomegaly and induction of
cytochrome P-450b-related O-dealkylase enzyme activities in hepatic
microsomes when fed to rats at 1000 ppm for 11 weeks, indicating a
phenobarbital-type induction of hepatic metabolism.
The rats were initiated by partial (2/3) hepatectomy and 24
hours later received a single intraperitoneal dose of 30 mg
nitrosodiethylamine/kg bw. From week 2 after initiation until
termination at week 13, the animals were fed a diet containing 1000
ppm bromopropylate. A group of non-hepatectomized, non-
diethylnitrosamine-treated rats was also fed the bromopropylate-
containing food. Appropriate control groups received the control
diet. Bromopropylate enhanced the incidence of GGT-positive foci
and the percentage of liver occupied by foci's tissue in initiated
rats (Flodström et al., 1990).
Table 2a. Mutagenicity tests in bacteria in vitro
Test Test object S-9 mix Concentrations Purity Results Reference
Escherichia coli WP2hcr +/- 10-5000 µg/plate 99.7% - Shirasu et al., 1979
Escherichia coli B/r WP2 try- 500-2000 µg/disc ? - Toyoshima et al., 1977
SD-4
Bacillus subtilis rec+(H17) 20-2000 µg/disc 99.7% - Shirasu et al., 1977
rec-(M45)
Bacillus subtilis rec+(H17) 50-2000 µg/disc ? - Toyoshima et al., 1977
rec-(M45)
Salmonella TA98 +/- 10-5000 µg/plate 99.7% - Shirasu et al., 1979
typhimurium TA100 +/- -
TA1535 +/- -
TA1537 +/- -
TA1538 +/- -
TA98 +/- 313-5000 µg/0.1 ml 94.2% - Deparade, 1989
TA100 +/- -
TA1535 +/- -
TA1537 +/- -
Table 2b. Mutagenicity tests in mammalian cells in vitro
Test Test object Concentrations Purity Results Reference
Chromosomal Chinese hamster 3.13-25 µg/ml Batch op. - Strasser, 1990
aberrations ovary cells with 406162
and without S-9 94.2%
mix
DNA repair human fibroblasts 0.5-60 µg/ml Batch op. - Meyer & Puri,
(UDS) 406162 1987
95%
DNA repair rat hepatocytes 0.4-40 µg/ml Batch op. - Hertner & Puri,
(UDS) 406162 1987
95%
Table 2c. Mutagenicity tests in mammalian cells in vivo
Test Test object Dose levels Purity Results Reference
Chromosomal Mouse five times and batch P 43/78 - Hool & Müller,
aberrations spermatocytes 3000 mg/kg 90% 1989a
within 10 days
Chromosomal Mouse five times and batch P 43/78 - Hool & Müller,
aberrations spermatogonia 3000 mg/kg on 90% 1989b
five consecutive
days
Nucleus Chinese 2500, 5000 and batch P 43/78 - Hool et al.,
anomaly test hamster, 10 000 mg/kg 90% 1980
bone marrow twice on two
consecutive days
Dominant Male mice single oral doses batch Mg. - Hool & Müller,
lethal test of 0, 1100 or 68/77 1981
3300 mg/kg 91.5%
Special studies on skin and eye irritation
A primary eye irritation test on bromopropylate (90% pure) was
performed by using 3 male and 3 female New Zeeland white rabbits.
Irritation observed after the application of 100 mg of undiluted
test material into the conjunctival sac consisted of reversible
redness, chemosis and discharge in all rabbits and iridal
involvement in one rabbit. The compound was given a descriptive
rating "mildly irritant" in non-washed eyes and in washed eyes, with
maximum average irritation scores of 9.3 and 3.3, respectively.
However, the mean values of the scores calculated from the 24, 48
and 72 hour readings over all tested animals were 0.07 for iris
lesions, 0.6 for redness, and 0.3 for edema of conjunctiva.
According to the EEC Directive 83/467 the test substance is
therefore classified as "non-irritant" to eyes (Kuhn, 1989c).
A primary skin irritation test on bromopropylate (90% pure) was
performed using 3 male and 3 female New Zeeland white rabbits.
Irritation observed on the skin after the application of 500 mg of
test material moistened with 0.4 ml of saline consisted of grade 1
erythema in 5 out of 6 rabbits at the 1 hour reading time. The
compound was given a descriptive rating "slight irritant". However,
the mean values of the scores calculated from the 24, 48 and 72 hour
readings over all tested animals were 0 for erythema and 0 for
edema. According to the EEC Directive 83/467 the test substance is
therefore classified as "non-irritant" to skin (Kuhn, 1989d).
Special studies on skin sensitization
A sensitization assay with bromopropylate (90% pure) using
Freund's complete adjuvant during the induction period (optimization
test) was conducted at a concentration of 0.1% in propylene glycol
as vehicle in guinea-pigs (Pirbright white strain, 10 males and 10
females/group). Significant differences between the test group
(14/20) and vehicle-treated controls (4/20) were seen only after
intradermal challenge application, i.e. when the skin barrier was
intentionally by-passed. No difference between the test (0/19) and
the control (0/20) groups was seen after epidermal challenge
application. It was therefore concluded that, in artificially
sensitized guinea-pigs, exposure of intact skin does not provoke
contact dermatitis (Ullmann & Sachsse, 1979b).
Observations in humans
No information available.
COMMENTS
After oral administration of [U-14C]phenyl bromopropylate,
most of the radioactivity was eliminated in the faeces, with lower
amounts of radioactivity excreted in the urine. The routes of
elimination were sex-dependent.
Bromopropylate was metabolized preferentially by cleavage of
the isopropyl ester, and to a minor extent by oxidation reactions
attacking the phenyl ring and the isopropyl group.
Bromopropylate has the properties of a phenobarbital-like
inducer of cytochrome P-450 in the mouse liver. In a DNA-binding
study conducted with mice, no radioactivity was detectable in liver
DNA, indicating that bromopropylate is devoid of genotoxic potential
in this tissue.
Bromopropylate has a low acute toxicity in rats and rabbits.
WHO has classified bromopropylate as unlikely to present acute
hazard in normal use.
In a one-year study in dogs at dietary concentrations of 0,
100, 400 or 2000 ppm, the NOAEL was 100 ppm (equal to 2.7 mg/kg
bw/day), based on depressed body-weight gain at 400 ppm and above.
In a study in mice using dietary concentrations of 0, 30, 150,
1000 or 3000 ppm for 24 months, the NOAEL was 150 ppm (equal to 16
mg/kg bw/day), based on increased absolute and relative liver weight
and hepatocellular neoplastic lesions at 1000 ppm and above.
Long-term toxicity/carcinogenicity studies in rats were
reviewed. The study considered by the 1973 Joint Meeting was found
to be unacceptable. In a new study at dietary concentrations of 0,
100, 700 or 5000 ppm, the NOAEL was 100 ppm (equal to 3.7 mg/kg
bw/day), based on increased water consumption and increased relative
liver and thyroid weights at 700 ppm and above. Increased
incidences of focal hepatocellular hypertrophy and fatty changes and
pigmentation of hepatocytes were also observed at 700 ppm and above.
In a reproduction study in rats using dietary concentrations of
0, 165, 750 or 2250 ppm, the NOAEL was 165 ppm (equal to 9 mg/kg
bw/day), based on increased liver weight and hypertrophy of
hepatocytes in F1 animals at 750 ppm and above.
Teratogenicity studies were conducted with rats and rabbits.
In the study in rats at doses of 0, 50, 300 or 700 mg/kg bw/day,
depressed maternal body-weight gain and an increased incidence of
skeletal variations of fully formed 14th ribs and rudimentary 14th
ribs were recorded at 300 mg/kg bw/day and above. The maternal
NOAEL in this study was 50 mg/kg bw/day. There was no evidence of
embryo/fetotoxicity or teratogenicity. In the study in rabbits at
doses of 0, 20, 60, or 120 mg/kg bw/day, mean body-weight gain was
depressed at 60 mg/kg bw/day. The NOAEL for maternal toxicity was
20 mg/kg bw/day, and no embryo/fetotoxic or teratogenic effects were
found.
After reviewing the available genotoxicity data, the Meeting
concluded that bromopropylate was not genotoxic.
An ADI was established based on the NOAEL of 2.7 mg/kg bw/day
in the one-year study in dogs, using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 150 ppm, equal to 16 mg/kg bw/day (two-year study)
Rat: 100 ppm, equal to 3.7 mg/kg bw/day (two-year study)
Dog: 100 ppm, equal to 2.7 mg/kg bw/day (one-year study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Basler, W., Hunter, B., Gretener, P., Froehlich, E., Faccini, J.M.,
Christen, P., Seewald, W., Dieterle, R. & Gfeller, W. (1989). GS
19'851 tech.: Lifetime carcinogenicity and chronic toxicity study in
rats. Proj. No: 831417. Unpublished report dated October 26, 1989
from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Basler, W., Hunter, B., Gretener, P., Froehlich, E., Krinke, G.,
Christen, P., Dieterle, R. & Gfeller, W. (1990). GS 19'851 tech.:
Lifetime carcinogenicity study in mice. Proj. No: 850334.
Unpublished report dated February 22, 1990 from Ciba-Geigy Ltd.,
Stein, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Bathe, R. & Sachsse, K. (1979). GS 19'851 tech.: Acute dermal LD50
in the rat. Proj. No: 790969. Unpublished report dated October 9,
1979 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Cassidy, J.E., Mattson, A., Cullen, T., & Min, B. (1968) The
metabolic fate of 14C GS 19851 administered to a cow by capsules.
Unpublished report from Geigy Chemical Corporation, Ardsley, N.Y.
Charles, J.M., Stevens, J.T., Sumner, D.D., Barnett, J.W. & Ellis,
J.F. (1979). GS 19'851 tech.: Carcinogenicity evaluation in albino
mice. Proj. No: 62206726. Unpublished report dated July 25, 1979
from Ciba-Geigy Corp., Greensboro, NC/USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland .
Cornelissen K. & Hopkins R. (1991). [U-14C]phenyl GS 19'851:
Absorption, distribution, and excretion in the rat. Proj. No: 6453-
380/151. Unpublished report dated April 3, 1991 from Hazleton UK,
Harrogate. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Coulston, F., Fabian, R.J., Abraham, R., & Benitz, K.F. (1970a) Two-
year safety evaluation of isopropyl 4,4'-dibromobenzilate in beagle
dogs. Unpublished report from Institute of Experimental Pathology
and Toxicology, Albany Medical College, Albany, N.Y. Submitted by
Geigy Chemical Corporation.
Coulston, F., Fabian R.J., & Benitz, K.F. (1970b) Two-year safety
evaluation study of isopropyl 4,4'-dibromobenzilate in albino rats
by dietary feeding. Unpublished report from Institute of
Experimental Pathology and Toxicology, Albany Medical College,
Albany, N.Y. Submitted by Geigy Chemical Corporation.
Coulston, F., Le Fevre, R., & Fabian, R. (1971) Three generation
study with GS 19 851 in albino rats. Unpublished report from the
Institute of Experimental Pathology and Toxicology, Albany Medical
College, Albany, N.Y. Submitted by Geigy Chemical Corporation.
Coulston F. & Benitz, K.F. (1972) Supplemental data on studies made
with GS 19 851 in beagle dogs. Unpublished report from the Institute
of Experimental Pathology and Toxicology, Albany Medical College,
Albany, N.Y. Submitted by Geigy Chemical Corporation.
Deparade E. (1989). GS 19'851 tech.: Salmonella/mammalian-
microsome mutagenicity test. Proj. No: 881537. Unpublished report
dated May 19, 1989 from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Drake, J.C. (1970) A 2443 A - Acute median lethal dose in rats.
Unpublished report from Geigy (U.K.) Ltd., Stamford Lodge, Wilmslow,
England.
Fancher, O.E., Schoenig, G., & Keplinger, M.L. (1968a) Acute
toxicity studies on GS 19 851 2 E (GA-494)- IBT No. A 6194.
Unpublished report from the Industrial Bio-Test Laboratories
Incorporated, Northbrook, Illinois. Submitted by Geigy Chemical
Corporation.
Fancher, O.E., Schoenig, G., & Keplinger, M.L. (1968b) Acute
toxicity studies on GS 19 851 25 W (GA-481) IBT No. A 6196.
Unpublished report from the Industrial Bio-Test Laboratories
Incorporated, Northbrook, Illinois. Submitted by Geigy Chemical
Corporation.
Flodström, S., Hemming, H., Wärngard, L. & Ahlborg, U.G. (1990).
Promotion of altered hepatic foci development in rat liver,
cytochrome P450 enzyme induction and inhibition of cell-cell
communication by DDT and some structurally related organohalogen
pesticides. Carcinogenesis, 11(8): 1413-1417.
Fritz, H., Suter, P., Zak, F., Malinowski, W. & Hess, R. (1986). GS
19'851 tech.: Two-generation reproduction toxicity study in rats.
Proj. No: 831523. Unpublished report dated December 1986 from Ciba-
Geigy Ltd., Stein, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Gretener, P., Froehlich, E., Malinowski, W., Christen, P. & Gfeller,
W. (1989). GS 19'851 tech.: 12-month chronic dietary toxicity study
in beagle dogs. Proj. No: 860018. Unpublished report dated May 11,
1989 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Hartmann, H.R. (1989). GS 19'851 tech.: Acute inhalation toxicity in
the rat. Proj. No: 881279. Unpublished report dated April 11, 1989
from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Hertner, Th. & Puri, E. (1987). GS 19'851 tech.: Autoradiographic
DNA-repair test on rat hepatocytes (OECD-CONFORM). Proj. No: 861631.
Unpublished report dated September 18, 1987 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hool, G. & Müller, D. (1980a). GS 19'861 tech.: Chromosome studies
in male germinal epithelium mouse (test for mutagenic effects on
spermatocytes). Proj. No: 790980. Unpublished report dated September
16, 1980, supplemented July 19, 1988 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hool, G. & Müller, D. (1980b). GS 19'851 tech.: Chromosome studies
in male germinal epithelium (test for mutagenic effects on
spermatogonia). Proj. No: 790979. Unpublished report dated June 17,
1980, supplemented July 19, 1988 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hool, G. & Müller, D. (1981). GS 19'851 tech.: Dominant lethal study
mouse (test for cytotoxic or mutagenic effects on male germinal
cells). Proj. No: 782203. Unpublished report dated February 24,
1981, supplemented July 19, 1988 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hool, G., Langauer, M. & Müller, D. (1980). GS 19'851 tech.: Nucleus
anomaly test in somatic interphase nuclei Chinese hamster (test for
mutagenic effects on bone marrow cells). Proj No.: 790981.
Unpublished report dated August 11, 1980, supplemented July 19, 1988
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Kuhn, J.O. (1989a). GS 19'851 tech.: Acute oral toxicity study in
rats. Proj. No: 6031-89. Unpublished report dated July 11, 1989 from
Stillmeadow Inc., Houston, TX/USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Kuhn, J.O. (1989b). GS 19'851 tech.: Acute dermal toxicity study in
rabbits. Proj. No: 6032-89. Unpublished report dated April, 11, 1989
from Stillmeadow, Inc., Houston, TX/USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Kuhn, J.O. (1989c). GS 19'851 tech.: Primary eye irritation study in
rabbits. Proj. No: 6033-89. Unpublished report dated March 30, 1989
from Stillmeadow, Inc., Houston, TX/USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Kuhn, J.O. (1989d). GS 19'851 tech.: Primary dermal irritation study
in rabbits. Proj. No: 603489. Unpublished report dated April 4, 1989
from Stillmeadow, Inc., Houston, TX/USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Mathies, P. (1991). The metabolite profiles in urine, faeces and
tissue extracts of rats after administration of [U-14C]phenyl GS
19'851. Proj. No: 17/91. Unpublished report dated June 28, 1991 from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Meyer, A. & Puri, E. (1987). GS 19'851 tech.: Autoradiographic DNA
repair test on human fibroblasts (OECD-CONFORM). Proj. No: 861632.
Unpublished report dated September 10, 1987, supplemented June 21,
1988 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Paterson, R.A. & Drake, J.C. (1967) GS 19 851 13-week oral toxicity
study in rats. Final report - Amendment to the report. Unpublished
from Geigy (U.K.) Ltd., Stamford Lodge, Wilmslow, England.
Paterson, R.A. (1967a) GS 19 851 28-day oral toxicity study in rats.
Final report. Unpublished, from Geigy (U.K.) Ltd., Stamford Lodge,
Wilmslow, England.
Paterson, R.A. (1967b) GS 19 851 30-day oral toxicity study in dogs.
Final report. Unpublished, from Geigy (U.K.) Ltd., Stamford Lodge,
Wilmslow, England.
Sagelsdorff P. & Heuberger B. (1990). GS 19'851 tech.: Investigation
of the potential for DNA binding. Proj. No: PS32-03901. Unpublished
report dated November 2, 1990 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Schneider, M., Gretener, P., Froehlich, E., Beri, J., Malinowski, W.
& Basler, W., (1989). GS 19'851 tech.: 28-Day repeated dose dermal
toxicity study in the rat. Proj. No: 891009. Unpublished report
dated December 11, 1989 from Ciba-Geigy Ltd., Stein, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Schulze-Aurich, J. (1993). The metabolism of [U-14C]Phenyl GS
19'851 in the rat. Proj. No. 07PM02. Unpublished report in
preparation from Ciba-Geigy Ltd., Stein, Switzerland. To be
submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Shirasu, Y., Moriya, M. & Koyashiki, R. (1979). Mutagenicity study
with phenisobromolate in bacteria. English translation of the
unpublished report dated November 22, 1979 from The Institute of
Environmental Toxicology, Japan. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Singh, A.R. & Yau, E.T. (1990). GS 19'851 tech.: A teratology
(segment ll) study in rats. Proj. No: MIN 892112/Tox./Path. Report
No.: 89131. Unpublished report dated May 25, 1990 from Ciba-Geigy
Corp., Summit, NJ/USA. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Singh, A.R., Hazelette, J.R. & Yau, E.T. (1990). GS 19'851 tech.: A
teratology (segment ll) study in rabbits. Proj. No: MIN
892014/Tox./Path. Report No.: 89162. Unpublished report dated June
18, 1990 from Ciba-Geigy Corp. Summit, NJ/USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Stenger, E.G. (1967) Akute Toxizität, Ratte per os, Nr. 295/14217.
Unpublished report from J.R. Geigy Ltd., Basle, Switzerland.
Strasser F. (1990). GS 19'851 tech.: Chromosome studies on Chinese
hamster ovary cell line CCL 61 in vitro. Proj. No: 891515.
Unpublished report dated July 4, 1990 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Thomas, H. (1991). Inductive effects of GS 19'851 on mouse liver
cytochromes P-450 and associated monooxygenase activities. Proj. No:
CB 90/28 BY. Unpublished report dated September 16, 1991 from Ciba-
Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Toyoshima, S., Fujita, H. & Satoh, R. (1977). GS 19'851 tech.:
Recombination assay and reversion test. Unpublished report dated
February 15, 1977 from Chemotherapy Dept., The Institute of Pharma-
chemistry, Keio University and Japan Experimental Medical Research
Inst. Co, Ltd. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Ueda, K. & Kondo, T. (1968) Report on experiment of acute toxicity.
Unpublished report from Tokyo Dental College, Hygienics Laboratory,
Tokyo, Japan. Submitted by Geigy Chemical Corporation.
Ullmann, L. & Sachsse, K. (1979a). GS 19'851 tech.: Acute oral LD50
in the rabbit. Proj. No: 790968. Unpublished report dated September
5, 1979 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Ullmann, L. & Sachsse, K. (1979b). GS 19'851 tech.: Skin sensitizing
(contact allergenic) effect in guinea pigs. Proj. No: 790972.
Unpublished report dated September 5, 1979 from Ciba-Geigy Ltd.,
Stein, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Waechter, F., Bentley, P. & Staeubli, W. (1986). The effect of GS
19'851 on mouse liver drug metabolizing enzymes. Unpublished report
dated September 19, 1986 from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Wärngard, L., Flodström, S., Ljungquist, S. & Ahlborg, U.G. (1985).
Inhibition of metabolic cooperation in Chinese hamster lung
fibroblast cells (V79) in culture by various DDT-analogs. Arch.
Environ. Contam. Toxicol., 14: 541-546.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
The ethoxycoumarin O-deethylase, ethoxyresorufin O-deethylase,
pentoxyresorufin O-depentylase, and total testosterone hydroxylase
activities were also increased. The results of studies with
monoclonal antibodies against rat liver cytochrome P-450 isozymes of
four different gene families confirmed that bromopropylate shares
properties typical of a moderate phenobarbital-type inducer and
possibly is also a very weak peroxisome proliferator-type inducer.
It was concluded that bromopropylate has the properties of a
phenobarbital-like inducer of cytochrome P-450 in the mouse liver.
In addition, it might act as a very weak peroxisome proliferator.
The increased liver weights at the high-dose and the biochemical
changes were therefore regarded as a reversible adaptation to a
functional overload. At 15 mg/kg bw/day only minimal biochemical
changes were observed and the low dose of 3 mg/kg bw/day was without
any effects (Thomas, 1991).
A DNA-binding study was conducted with mice (Tif: MAG f)
pretreated for two weeks with unlabelled bromopropylate (94.2% pure)
at daily oral doses of 300 mg/kg bw. Radiolabelled [ bis-phenyl-
14C] bromopropylate was then administered by gavage at a dose level
of 300 mg/kg bw. After 24 hours, DNA was isolated from the liver
and purified. No radioactivity was detected. Bromopropylate is
devoid of a genotoxic potential mediated by DNA-binding (Sagelsdorff
& Heuberger, 1990).
Toxicological studies
Acute toxicity studies
Bromopropylate has a low acute toxicity in rats and rabbits.
WHO has classified bromopropylate as unlikely to present acute
hazard in normal use (WHO, 1992). The LD50 and LC50 values are
summarized in Table 1.
Short-term toxicity studies
Rats
Groups of 10 male and 10 female rats received 0, 40, 200, 1000
or 5000 mg bromopropylate/kg bw/day by gavage as a suspension in
0.5% aqueous tragacanth on six days each week for four weeks. The
highest dosage level produced polyuria throughout the 21 days before
the animals were killed and for the first eight days pale mucoid
faeces were produced. The rate of body-weight gain and food intake
were reduced in the 200 and 1000 mg/kg bw/day groups. Rats of the
5000 mg/kg bw/day group developed a relative neutrophilia but the
results of haematological analyses in all groups were normal. The
absolute and relative liver weights were increased in the three
highest dosage groups and cytoplasmic swelling and periportal
Table 1. Results of acute toxicity studies with bromopropylate
Species Sex Route Solvent LD50/LC50 Reference
(vehicle) (mg/kg bw)/
(mg/m3 air)
Mouse1 M oral - 8000 Ueda & Kondo, 1968
Rat1 M,F oral - > 5000 Stenger, 1967
Rat2 M,F oral - 6000 Drake, 1970
Rat2 ? oral - > 23 000 Fancher et al., 1968a,b
Rat3 M,F oral corn oil approx. 5000 Kuhn, 1989a
Rat4 M,F dermal PEG 400 > 4000 Bathe & Sachsse, 1979
Rat5 M,F inhalation ethanol > 4458 Hartmann, 1989
Rabbit4 M,F oral CMC6 > 6000 Ullmann & Sachsse, 1979a
Rabbit3 M,F dermal saline > 2020 Kuhn, 1989b
1 Purity given as Technical.
2 Purity given as Formulation.
3 Purity not given but known to be 90%.
4 90% pure.
5 95% pure.
6 CMC = 2% carboxymethylcellulose in water.
infiltration were seen. Hepatic necrosis occurred at the highest
dosage level. At 40 mg/kg bw/day, the one animal which died during
the test showed similar abnormalities in the liver; others in the
group appeared normal (Paterson, 1967a).
Groups of 12 male and 12 female rats were fed diets containing
0, 100, 300 or 1000 ppm bromopropylate for 90 days. Another group
received diets containing 3000 ppm for 55 days and 4000 ppm for a
further 35 days. At the highest dose, the food intake and rate of
weight gain were below normal and at autopsy the liver, kidney and
testes weights relative to body weight were higher than in controls.
Swelling and loss of basophil material, pigmentation (possibly
lipofuscin) and fatty infiltration were present in hepatocytes.
Mild to severe regressive changes were seen in testes on
histological examination. At 1000 ppm, food intake was slightly
less than in controls although weight gain was similar. Testes were
lighter than in controls and similar histological changes were seen
in the liver. At the two lower levels, smaller numbers of rats
showed loss of basophilic material and cytoplasmic swelling with
focal vacuolation. These changes were considered to be
physiological in that they were attributed to SER hypertrophy, but
no EM studies were carried out to confirm this. The results of
haematological and blood biochemistry studies and of urine analyses
were similar in all groups (Paterson & Drake, 1967).
Bromopropylate (94.2% pure) was applied to the shaved skin of 5
male and 5 female rats (Tif:RAI f) per dose group for 4 weeks on a
5-day per week basis. The daily exposure period was 6 hours. The
compound was given on moistened gauze patches under occlusive
dressing at dose levels of 0, 50, 200 or 1000 mg/kg bw/day. There
was no mortality, no clinical signs, no signs of local irritation
and no body weight or food consumption changes that could be
attributed to treatment. At 1000 mg/kg bw/day, slightly lower
glucose levels and a minimal increase of urea were evident in blood
chemistry, while no changes were seen in haematology. Organ
weights, macro- and microscopic histopathological findings were
similar in all groups. The NOAEL in this study was 200 mg/kg bw/day
in both sexes (Schneider et al., 1989).
Dogs
A group of two male and two female dogs received orally, by
capsule, 1000 mg/kg bw/day of bromopropylate for 30 days. A second
group received 2000 mg/kg bw/day for four days, then 500 mg/kg
bw/day for a further 26 days. Diarrhoea and vomiting occurred in
dogs of both groups. During the first week, food intake and body
weight decreased. The results of haematological examinations were
normal. Determination of organ weights did not indicate any dose-
related changes. In both groups, serum transaminase and alkaline
phosphatase levels were increased by treatment and cytoplasmic
swelling and fine vacuolation of hepatocytes were found. No mature
spermatozoa were found in male animals, but this may have been due
to the age of the animals (Paterson, 1967b).
Four groups of eight male and eight female dogs were fed for
two years on diets containing 30, 100, 250 or 1000 ppm
bromopropylate. A fifth group received 4000 ppm in the diet for
three months. A group of 10 males and 10 females acted as controls.
The highest dosage level produced light-coloured soft stools after
one week and semi-fluid stools thereafter. Food intake was reduced
and weight loss occurred. Two dogs died (after four and six weeks)
and two others were killed because they became cachectic. In these
four animals, increased serum alkaline phosphatase, diminished serum
cholesterol and mild anaemia were found. Haemosiderosis of kupfer
cells, proximal tubular cells of the kidney, the spleen and the bone
marrow, degenerative changes in the distal convoluted tubules of the
kidney, hyperplasia of bone marrow and extra-medullary
haematopoiesis were seen in these animals at autopsy. In the other
animals of the 4000 ppm group killed at three months, accumulation
of haemosiderin in the macrophages of the spleen and bone marrow and
in kupfer cells was observed. Examination of liver by EM showed SER
hypertrophy and an increase in numbers of lysosomes, many of which
contained lamellar structures. Two additional animals of each sex
were fed for nine months on control diets following three months on
4000 ppm diet. Stools returned to normal consistency within a week
and weight gain was normal. Biochemical and haematological
parameters also returned to normal in a short time; animals were
indistinguishable from controls at the time of autopsy. The 1000
ppm diet also produced softening of stools. Although weight gain by
males was normal, females failed to gain weight. Some females
showed slight anaemia on some occasions. The microsomal enzymes,
biphenyl hydroxylases, showed increased activity and this was
associated with an increase in liver weight relative to body weight
at six months and at two years (in females only). SER hypertrophy
was marked at three months and numerous myelin bodies were also
seen. Although at two years the SER hypertrophy was not different
from controls, myelin bodies were still present. An increase in
microsomal enzymes was found in the 250 ppm group together with an
enlarged liver (relative to body weight) at six months but not at
two years. No adverse effects were observed in dogs fed on 30 or
100 ppm diets (Coulston et al., 1970a).
Bromopropylate (95% pure) was administered in the diet at
concentrations of 0, 100, 400 or 2000 ppm to groups of 6 male and 6
female beagle dogs for one year. After completion of the treatment
period, subgroups of 2 animals per sex were kept for one month on
compound-free (control) diet to determine reversibility of potential
toxic effects. There were no compound-related mortalities and no
clinical signs. Body-weight gain was decreased markedly at 2000 ppm
in both sexes and slightly at 400 ppm in females from week 28
onwards. Food consumption was not affected and there were no
ophthalmic, haematological or urinary changes. The only relevant
change in blood chemistry was a slight reversible increase of
alkaline phosphatase activity in the females at 2000 ppm. At the
end of the 12-month treatment period, liver weights were slightly
increased and there was a slight hypertrophy of hepatocytes. These
changes only occurred at 2000 ppm and were not present after the
one-month recovery period. The NOAEL in this study was 100 ppm,
equal to 2.7 mg/kg bw/day (males) and 2.8 mg/kg bw/day (females)
(Gretener et al., 1989).
Long-term toxicity/carcinogenicity studies
Mice
In an oncogenicity study, mice (Charles River CD-1, 50 males
and 50 females per group) were given bromopropylate (98.7% pure) in
the diet during 18 and 20 months for males and females,
respectively, at concentrations of 0, 30, 300 or 1000 ppm. Similar
reduction in body weight was noted for the control and test groups
after 12 months of testing, males losing an average of 6 to 7 grams
and females 2 to 3 grams. This generalized reduction in body weight
occurred following alterations in both the method of feeding and the
standard diet fed. However, no statistically significant
differences in mean body weights were noted for any treatment group.
Meaningful evaluation of body-weight gain cannot be made since body
weights were not measured during the first 6 months of testing.
Negative body-weight gains were recorded for the observation period
for all groups. The mean survival time of males in the 1000 ppm
group was significantly reduced. Significantly increased
mortalities in the 30 and 1000 ppm groups were observed, which were
considered coincidental. No treatment-related findings were seen at
gross pathological examination. Histopathology revealed dose-
related centrilobular hypertrophy of hepatocytes in the two higher
doses. Additional sporadic histopathological findings were not
considered as related to treatment. The compound did not show an
oncogenic potential in this study (Charles et al., 1979).
Bromopropylate (95% pure) was administered to male and female
mice (Tif: MAGf (SPF), hybrids of inbred MIG x NIH mice) in the diet
at levels of 0, 30, 150, 1000 or 3000 ppm (60 males and 60 females
per group) for 24 months. Appearance and behaviour were not
affected by treatment. Lower body-weight gain was recorded for the
3000 ppm group over the first 12 weeks for males and the first 25
weeks for females. Thereafter, mean body weights remained 5-7% and
15-18% lower than control values, for males and females,
respectively. Food consumption was slightly lowered in females in
the 1000 and 3000 ppm groups during the first year. Water
consumption was increased at 3000 ppm. Haematological parameters
were not significantly affected by treatment. Absolute and relative
liver weights were significantly increased in both sexes at 1000 and
3000 ppm. Macroscopic examination revealed dose-related increase of
masses and nodules in the livers of male and female mice from these
two groups. Kidney weights at 3000 ppm were lower than in the
control group. On microscopic examination, hepatocellular
neoplastic lesions, i.e. benign hepatomas and hepatocellular
carcinomas, accounted for most of the macroscopically observed
masses and nodules in the liver. The frequency of these lesions was
significantly increased in both sexes at 1000 and 3000 ppm. Animals
of both sexes of these two groups also showed a frequent hypertrophy
of hepatocytes. The only other compound-related finding was an
increased incidence of reactive hyperplasia of splenic white pulp in
female mice of the high dose group (3000 ppm). The NOAEL in this
study was 150 ppm, equal to 16 mg/kg bw/day in both males and
females (Basler et al., 1990).
Rats
Groups of 50 male and 50 female rats were fed diets containing
0, 15, 30 or 100 ppm bromopropylate for two years. Five male and
five female rats from each group were killed after six months or one
year and autopsies performed. No changes in appearance or behaviour
occurred. Food consumption and weight gain were similar in test and
control animals throughout the test and survival rates were similar
for the first 18 months. Approximately half of the control female
animals alive at 18 months survived to 24 months while only a third
of test animals at 30 and 100 ppm survived over this period. No
alterations attributable to bromopropylate were observed in the
haematological examinations in test animals. No changes in organ
weights or gross or microscopic abnormalities attributable to
ingestion of the pesticide were observed in animals examined at any
time. However, EM studies on the livers of animals of the 100 ppm
group surviving two years showed slightly less glycogen, focal
enlargement of intracrestal space in the mitochondria, focal
dilatation of SER and more prominent lipid accumulations than livers
of control animals. These differences were not considered to be
significant pathological alterations in cell ultrastructure. The
number of tumours and their location were similar in control and
test groups (Coulston et al., 1970b).
Bromopropylate (95% pure) was administered in the diet to rats
(Tif: RAlf, 80 males and 80 females per dose group) at levels of 0,
100, 700 or 5000 ppm. The study duration was 119 weeks for males
and 131 weeks for females. Average intakes of bromopropylate were
3.7, 26 or 217 mg/kg bw/day for males and 4.4, 32 or 270 mg/kg
bw/day for females. In the 5000 ppm group, males maintained mean
body weights 20% lower than control values from 15 weeks onwards,
whereas females showed a progressive divergence, attaining a 37%
difference from control values at 95 weeks. Food consumption was
decreased in males and females at 5000 ppm, and water consumption
was increased in both males and females at 5000 ppm and in males at
700 ppm. Calculated red blood cell indices (MCV, MCH) were
decreased in males (from 80 weeks) and in females (from 27 weeks) at
5000 ppm, suggesting a tendency to microcytosis and hypochromoasia
of red blood cells. Increased prothrombin times were recorded in
males at weeks 12, 27, and 80, and in females at all investigated
weeks at 5000 ppm. Blood samples were collected at 12, 27, 53, 80,
105 and 131 weeks. A non-progressive lowering of blood glucose was
recorded at weeks 12 to 80 for males and at weeks 27, 53 and 80 for
females at 5000 ppm. A hyperproteinemia resulting from
hyperglobulinemia was observed in the males and females at 5000 ppm.
Females of the 700 ppm group showed a similar trend, but to a lesser
degree between weeks 27 and 105. Higher levels of cholesterol and
phospholipids were recorded at week 53 for males and on all
occasions for females at 5000 ppm. In the 700 ppm group, high
levels of cholesterol were observed in females at weeks 12, 27 and
80. Also, high levels of phospholipids were recorded in females at
12 and 80 weeks. Both sexes of the 5000 ppm group showed a marked
increase in gamma glutamyl transpeptidase activity at each
investigation.
At the interim sacrifices during weeks 53 and 105 and at the
termination of the study, relative liver weight for males and
females of the 5000 ppm group were significantly increased. In
addition, the relative liver weights for rats at 700 ppm were
significantly increased at week 53 for females and at termination
for males. Significantly increased relative kidney weights were
recorded for males and females of the 5000 ppm group at week 53 and
for females at week 105. Relative thyroid gland weights in males at
700 and 5000 ppm were significantly increased at week 53 and the
relative weight in males at 5000 ppm was also increased at
termination of the study. Macroscopic examination revealed an
increased number of rats with edema of the testes and an increased
number of females with liver cysts at 5000 ppm. In
histopathological examinations, increased incidences of focal
hepatocellular hypertrophy and fatty changes and pigmentation of
hepatocytes were also observed in both sexes at 700 and 5000 ppm.
Incidences of biliary cysts and cholangiofibrosis were increased in
females at 5000 ppm. Occurrence of lung alveolar foam cells were
increased in females at 5000 ppm. An increase in the number of rats
with testicular tubular atrophy and an associated edema and nodular
hyperplasia (Leydig cells) was observed at 5000 ppm. Numbers of
rats with follicular cystic dilatation and thyroid gland hyperplasia
were increased in both sexes at 5000 ppm. Incidence of rats with
thyroid gland adenoma was increased at 5000 ppm in males. The NOAEL
in this study was 100 ppm (equal to 3.7 mg/kg bw/day for males and
4.4 mg/kg bw/day for females) (Basler et al., 1989).
Reproduction studies
Rats
Groups of 20 male and 20 female rats in the first generation
and groups of 25 male and 25 female rats in the second and third
generations were used in a three-generation test (two litters being
produced in each generation) and fed diets containing 0, 2.5 or 5
ppm bromopropylate. In a second test, groups of 25 male and 25
female rats received diets containing 0, 30 or 100 ppm
bromopropylate. Throughout the tests no abnormalities attributable
to bromopropylate were found in the reproductive physiology of male
or female rats and there was no evidence of gross abnormalities in
the offspring throughout the studies. The number of young in each
litter and their growth and survival were normal. The weights of
liver, kidneys and spleen were comparable with controls in the rats
of the F3b generation whose parents received 2.5 or 5 ppm diets
while spleen and liver weights of the F3b young of parents fed 30
or 100 ppm diets were slightly higher than controls. No
histological abnormalities were found in these animals at any dosage
level (Coulston et al., 1971).
In a two-generation reproduction study, rats (Sprague-Dawley-
served, Tif:RAI f) were administered bromopropylate (93.5% pure) in
the diet at concentrations of 0, 165, 750 or 2250 ppm (28
rats/sex/dose level). Approximate daily intakes of bromopropylate
were 12, 54 or 170 mg/kg bw for parental males and 16, 72 or 218
mg/kg bw for parental females. The administration started 10 weeks
prior to mating of the F0 parents and was continued throughout the
mating, lactation and weaning periods of the F1 and up to weaning
of the F2 generation.
F0 parental animals showed reduced body-weight gain and food
consumption at 2250 ppm and, to a lesser extent, in the 750 ppm
group. The females of the F1 parental generation showed some
depression in food consumption at 750 and 2250 ppm prior to mating
and only at 2250 ppm during lactation. Histological changes
consisted of a dose-related slight to severe hepatocellular
enlargement at 750 ppm and 2250 ppm corresponding to liver weight
increases in the F1 animals; in addition, some of the males in the
high-dose group showed hepatocyte necrosis. Reproductive parameters
(concerning mating, fertility, pregnancy, parturition and rearing)
were not affected by the treatment. With respect to the offspring,
the F1 pups of the 2250 ppm group showed an increased mortality
rate. For both F1 and F2 young rats of the 2250 ppm group, a
diminished body-weight gain was recorded from weaning onwards.
Exploratory locomotion was depressed in the young F1 rats at 750
and 2250 ppm and in the F2 rats at 750 ppm only.
Histopathological examination of the F1 rats revealed changes
in the liver, as indicated by slight to severe hypertrophy of
hepatocytes in about two-thirds of the males and females at 750 ppm
and in all animals at 2250 ppm. In the males, necrosis (8/24 at
2250 ppm) or other structural alterations of hepatocytes (11/23 at
750 ppm, 22/24 at 2250 ppm) were noted. Parallel to these results,
absolute as well as relative liver weights were found to be
increased in a "positive trend" from the control to the high
concentration. The lowest mean daily compound intakes during the
various study periods were calculated to be approximately 9, 40 or
128 mg/kg body weight. The NOAEL in this study was 165 ppm (equal
to 9 mg/kg bw/day) (Fritz et al., 1986).
Dogs
Groups of three male and three female dogs were fed diets
containing 0, 30 or 100 ppm bromopropylate. After an unstated time,
the females were mated with males of the same group. All matings
resulted in pregnancy and delivery of normally sized litters. The
only deformed pup was found in a control litter. All others were
normal in appearance, behaviour and development. Increases in the
body weight of the pups in most of the litters of groups receiving
bromopropylate were similar to those of control pups. The reduced
growth rate of one litter of the 100 ppm group was attributed to the
results of poor maternal care (Coulston et al., 1970a; Coulston &
Benitz, 1972).
Special studies on embryo/fetotoxicity
Rats
Bromopropylate (90% pure) was administered orally by gavage to
three groups of pregnant rats (CRL: COBS CD[SD]BR, 26 females/group)
at doses of 0, 50, 300 or 700 mg/kg bw once daily during days 6
through 15 of gestation. A control group of 26 female rats received
an equivalent volume (10 ml/kg bw) of aqueous 3% corn starch with
0.5% Tween 80. All surviving animals were sacrificed on day 20 of
presumed gestation. Evaluations were conducted on clinical signs,
maternal body weights, food consumption, necropsy and skeletal
abnormalities. Compound-related effects were noted at 300 and 700
mg/kg bw/day. These changes included: an increased incidence of
salivation and alopecia accompanied by skin irritation; transient
body-weight loss and slight reductions in daily food consumption at
700 mg/kg bw/day and of body-weight gain at 300 mg/kg bw/day; slight
decreases in mean male and female fetal weight at 700 mg/kg bw/day;
and an increased incidence of skeletal variations of fully formed
14th ribs and rudimentary 14th ribs at 300 and 700 mg/kg bw/day and
in forepaw metacarpals not ossified at 700 mg/kg bw/day. The fetal
findings were considered to be slight treatment-related effects
associated with the compound-induced maternal toxicity. There was
no evidence of embryo/fetotoxicity or teratogenicity at doses up to
and including 700 mg/kg bw/day. The maternal NOAEL in this study
was 50 mg/kg bw/day (Singh & Yau, 1990).
Rabbits
Bromopropylate (90% pure) was administered orally by gavage to
three groups of pregnant rabbits (New Zeeland white, 20
females/group) at doses of 0, 20, 60, or 120 mg/kg bw once daily
during gestation days 7 through 19. A control group of 20 pregnant
rabbits received an equivalent volume (5 ml/kg bw) of the vehicle.
All surviving animals were sacrificed on day 29 of presumed
gestation. Evaluations were conducted on clinical signs, maternal
body weights, food consumption, necropsy, reproductive parameters,
fetal weights and fetal gross, visceral and skeletal observations.
Treatment-related maternal effects were observed at 60 and 120 mg/kg
bw/day. These changes included: mortality/moribundity of two
animals at 120 mg/kg bw/day; clinical signs of lethargy at 120 mg/kg
bw/day and anogenital staining at 60 and 120 mg/kg bw/day;
reductions in mean body weight at 120 mg/kg bw/day, mean body-weight
gain at 60 and 120 mg/kg bw/day, and reduction of food consumption
at 120 mg/kg bw/day; and the necropsy finding of distended stomach
with viscous/gelatinous material at 120 mg/kg bw/day. There was no
evidence of embryotoxicity, fetotoxicity or teratogenicity at any
dose level. The NOAEL for maternal toxicity was 20 mg/kg bw/day
(Sing et al., 1990).
Special studies on genotoxicity
The results of genotoxicity studies conducted with bacteria,
mammalian cells in vitro and with intact animals in vivo are
presented in Tables 2a, b and c. In all studies, bromopropylate was
devoid of mutagenic potential.
Special studies on inhibition of cell-cell communication and liver
tumour promotion
Bromopropylate was investigated in vitro for its ability to
inhibit gap junctional intercellular communication in the Chinese
hamster fibroblast cell V79 metabolic cooperation assay and in the
scrape-loading/dye-transfer assay in WBF344 rat liver epithelial
cells, and in vivo altered hepatic foci assay and hepatic drug
metabolizing activity in male Sprague-Dawley rats. Technical grade
bromopropylate (94% pure) induced a dose-dependent increase in the
recovery of 6-thioguanine-resistant mutants within the same dose
range as reported earlier for the analytical grade (Wärngard et
al., 1985). In the scrape-loading assay, intercellular
communication was abolished at non-cytotoxic concentrations both
with technical and analytical grade bromopropylate. There was a
gradual decrease in dye transfer from the lowest concentration (20
µM) up to the dose inducing complete blockade of dye transfer.
Bromopropylate was shown to cause hepatomegaly and induction of
cytochrome P-450b-related O-dealkylase enzyme activities in hepatic
microsomes when fed to rats at 1000 ppm for 11 weeks, indicating a
phenobarbital-type induction of hepatic metabolism.
The rats were initiated by partial (2/3) hepatectomy and 24
hours later received a single intraperitoneal dose of 30 mg
nitrosodiethylamine/kg bw. From week 2 after initiation until
termination at week 13, the animals were fed a diet containing 1000
ppm bromopropylate. A group of non-hepatectomized, non-
diethylnitrosamine-treated rats was also fed the bromopropylate-
containing food. Appropriate control groups received the control
diet. Bromopropylate enhanced the incidence of GGT-positive foci
and the percentage of liver occupied by foci's tissue in initiated
rats (Flodström et al., 1990).
Table 2a. Mutagenicity tests in bacteria in vitro
Test Test object S-9 mix Concentrations Purity Results Reference
Escherichia coli WP2hcr +/- 10-5000 µg/plate 99.7% - Shirasu et al., 1979
Escherichia coli B/r WP2 try- 500-2000 µg/disc ? - Toyoshima et al., 1977
SD-4
Bacillus subtilis rec+(H17) 20-2000 µg/disc 99.7% - Shirasu et al., 1977
rec-(M45)
Bacillus subtilis rec+(H17) 50-2000 µg/disc ? - Toyoshima et al., 1977
rec-(M45)
Salmonella TA98 +/- 10-5000 µg/plate 99.7% - Shirasu et al., 1979
typhimurium TA100 +/- -
TA1535 +/- -
TA1537 +/- -
TA1538 +/- -
TA98 +/- 313-5000 µg/0.1 ml 94.2% - Deparade, 1989
TA100 +/- -
TA1535 +/- -
TA1537 +/- -
Table 2b. Mutagenicity tests in mammalian cells in vitro
Test Test object Concentrations Purity Results Reference
Chromosomal Chinese hamster 3.13-25 µg/ml Batch op. - Strasser, 1990
aberrations ovary cells with 406162
and without S-9 94.2%
mix
DNA repair human fibroblasts 0.5-60 µg/ml Batch op. - Meyer & Puri,
(UDS) 406162 1987
95%
DNA repair rat hepatocytes 0.4-40 µg/ml Batch op. - Hertner & Puri,
(UDS) 406162 1987
95%
Table 2c. Mutagenicity tests in mammalian cells in vivo
Test Test object Dose levels Purity Results Reference
Chromosomal Mouse five times and batch P 43/78 - Hool & Müller,
aberrations spermatocytes 3000 mg/kg 90% 1989a
within 10 days
Chromosomal Mouse five times and batch P 43/78 - Hool & Müller,
aberrations spermatogonia 3000 mg/kg on 90% 1989b
five consecutive
days
Nucleus Chinese 2500, 5000 and batch P 43/78 - Hool et al.,
anomaly test hamster, 10 000 mg/kg 90% 1980
bone marrow twice on two
consecutive days
Dominant Male mice single oral doses batch Mg. - Hool & Müller,
lethal test of 0, 1100 or 68/77 1981
3300 mg/kg 91.5%
Special studies on skin and eye irritation
A primary eye irritation test on bromopropylate (90% pure) was
performed by using 3 male and 3 female New Zeeland white rabbits.
Irritation observed after the application of 100 mg of undiluted
test material into the conjunctival sac consisted of reversible
redness, chemosis and discharge in all rabbits and iridal
involvement in one rabbit. The compound was given a descriptive
rating "mildly irritant" in non-washed eyes and in washed eyes, with
maximum average irritation scores of 9.3 and 3.3, respectively.
However, the mean values of the scores calculated from the 24, 48
and 72 hour readings over all tested animals were 0.07 for iris
lesions, 0.6 for redness, and 0.3 for edema of conjunctiva.
According to the EEC Directive 83/467 the test substance is
therefore classified as "non-irritant" to eyes (Kuhn, 1989c).
A primary skin irritation test on bromopropylate (90% pure) was
performed using 3 male and 3 female New Zeeland white rabbits.
Irritation observed on the skin after the application of 500 mg of
test material moistened with 0.4 ml of saline consisted of grade 1
erythema in 5 out of 6 rabbits at the 1 hour reading time. The
compound was given a descriptive rating "slight irritant". However,
the mean values of the scores calculated from the 24, 48 and 72 hour
readings over all tested animals were 0 for erythema and 0 for
edema. According to the EEC Directive 83/467 the test substance is
therefore classified as "non-irritant" to skin (Kuhn, 1989d).
Special studies on skin sensitization
A sensitization assay with bromopropylate (90% pure) using
Freund's complete adjuvant during the induction period (optimization
test) was conducted at a concentration of 0.1% in propylene glycol
as vehicle in guinea-pigs (Pirbright white strain, 10 males and 10
females/group). Significant differences between the test group
(14/20) and vehicle-treated controls (4/20) were seen only after
intradermal challenge application, i.e. when the skin barrier was
intentionally by-passed. No difference between the test (0/19) and
the control (0/20) groups was seen after epidermal challenge
application. It was therefore concluded that, in artificially
sensitized guinea-pigs, exposure of intact skin does not provoke
contact dermatitis (Ullmann & Sachsse, 1979b).
Observations in humans
No information available.
COMMENTS
After oral administration of [U-14C]phenyl bromopropylate,
most of the radioactivity was eliminated in the faeces, with lower
amounts of radioactivity excreted in the urine. The routes of
elimination were sex-dependent.
Bromopropylate was metabolized preferentially by cleavage of
the isopropyl ester, and to a minor extent by oxidation reactions
attacking the phenyl ring and the isopropyl group.
Bromopropylate has the properties of a phenobarbital-like
inducer of cytochrome P-450 in the mouse liver. In a DNA-binding
study conducted with mice, no radioactivity was detectable in liver
DNA, indicating that bromopropylate is devoid of genotoxic potential
in this tissue.
Bromopropylate has a low acute toxicity in rats and rabbits.
WHO has classified bromopropylate as unlikely to present acute
hazard in normal use.
In a one-year study in dogs at dietary concentrations of 0,
100, 400 or 2000 ppm, the NOAEL was 100 ppm (equal to 2.7 mg/kg
bw/day), based on depressed body-weight gain at 400 ppm and above.
In a study in mice using dietary concentrations of 0, 30, 150,
1000 or 3000 ppm for 24 months, the NOAEL was 150 ppm (equal to 16
mg/kg bw/day), based on increased absolute and relative liver weight
and hepatocellular neoplastic lesions at 1000 ppm and above.
Long-term toxicity/carcinogenicity studies in rats were
reviewed. The study considered by the 1973 Joint Meeting was found
to be unacceptable. In a new study at dietary concentrations of 0,
100, 700 or 5000 ppm, the NOAEL was 100 ppm (equal to 3.7 mg/kg
bw/day), based on increased water consumption and increased relative
liver and thyroid weights at 700 ppm and above. Increased
incidences of focal hepatocellular hypertrophy and fatty changes and
pigmentation of hepatocytes were also observed at 700 ppm and above.
In a reproduction study in rats using dietary concentrations of
0, 165, 750 or 2250 ppm, the NOAEL was 165 ppm (equal to 9 mg/kg
bw/day), based on increased liver weight and hypertrophy of
hepatocytes in F1 animals at 750 ppm and above.
Teratogenicity studies were conducted with rats and rabbits.
In the study in rats at doses of 0, 50, 300 or 700 mg/kg bw/day,
depressed maternal body-weight gain and an increased incidence of
skeletal variations of fully formed 14th ribs and rudimentary 14th
ribs were recorded at 300 mg/kg bw/day and above. The maternal
NOAEL in this study was 50 mg/kg bw/day. There was no evidence of
embryo/fetotoxicity or teratogenicity. In the study in rabbits at
doses of 0, 20, 60, or 120 mg/kg bw/day, mean body-weight gain was
depressed at 60 mg/kg bw/day. The NOAEL for maternal toxicity was
20 mg/kg bw/day, and no embryo/fetotoxic or teratogenic effects were
found.
After reviewing the available genotoxicity data, the Meeting
concluded that bromopropylate was not genotoxic.
An ADI was established based on the NOAEL of 2.7 mg/kg bw/day
in the one-year study in dogs, using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 150 ppm, equal to 16 mg/kg bw/day (two-year study)
Rat: 100 ppm, equal to 3.7 mg/kg bw/day (two-year study)
Dog: 100 ppm, equal to 2.7 mg/kg bw/day (one-year study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Basler, W., Hunter, B., Gretener, P., Froehlich, E., Faccini, J.M.,
Christen, P., Seewald, W., Dieterle, R. & Gfeller, W. (1989). GS
19'851 tech.: Lifetime carcinogenicity and chronic toxicity study in
rats. Proj. No: 831417. Unpublished report dated October 26, 1989
from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Basler, W., Hunter, B., Gretener, P., Froehlich, E., Krinke, G.,
Christen, P., Dieterle, R. & Gfeller, W. (1990). GS 19'851 tech.:
Lifetime carcinogenicity study in mice. Proj. No: 850334.
Unpublished report dated February 22, 1990 from Ciba-Geigy Ltd.,
Stein, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Bathe, R. & Sachsse, K. (1979). GS 19'851 tech.: Acute dermal LD50
in the rat. Proj. No: 790969. Unpublished report dated October 9,
1979 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Cassidy, J.E., Mattson, A., Cullen, T., & Min, B. (1968) The
metabolic fate of 14C GS 19851 administered to a cow by capsules.
Unpublished report from Geigy Chemical Corporation, Ardsley, N.Y.
Charles, J.M., Stevens, J.T., Sumner, D.D., Barnett, J.W. & Ellis,
J.F. (1979). GS 19'851 tech.: Carcinogenicity evaluation in albino
mice. Proj. No: 62206726. Unpublished report dated July 25, 1979
from Ciba-Geigy Corp., Greensboro, NC/USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland .
Cornelissen K. & Hopkins R. (1991). [U-14C]phenyl GS 19'851:
Absorption, distribution, and excretion in the rat. Proj. No: 6453-
380/151. Unpublished report dated April 3, 1991 from Hazleton UK,
Harrogate. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Coulston, F., Fabian, R.J., Abraham, R., & Benitz, K.F. (1970a) Two-
year safety evaluation of isopropyl 4,4'-dibromobenzilate in beagle
dogs. Unpublished report from Institute of Experimental Pathology
and Toxicology, Albany Medical College, Albany, N.Y. Submitted by
Geigy Chemical Corporation.
Coulston, F., Fabian R.J., & Benitz, K.F. (1970b) Two-year safety
evaluation study of isopropyl 4,4'-dibromobenzilate in albino rats
by dietary feeding. Unpublished report from Institute of
Experimental Pathology and Toxicology, Albany Medical College,
Albany, N.Y. Submitted by Geigy Chemical Corporation.
Coulston, F., Le Fevre, R., & Fabian, R. (1971) Three generation
study with GS 19 851 in albino rats. Unpublished report from the
Institute of Experimental Pathology and Toxicology, Albany Medical
College, Albany, N.Y. Submitted by Geigy Chemical Corporation.
Coulston F. & Benitz, K.F. (1972) Supplemental data on studies made
with GS 19 851 in beagle dogs. Unpublished report from the Institute
of Experimental Pathology and Toxicology, Albany Medical College,
Albany, N.Y. Submitted by Geigy Chemical Corporation.
Deparade E. (1989). GS 19'851 tech.: Salmonella/mammalian-
microsome mutagenicity test. Proj. No: 881537. Unpublished report
dated May 19, 1989 from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Drake, J.C. (1970) A 2443 A - Acute median lethal dose in rats.
Unpublished report from Geigy (U.K.) Ltd., Stamford Lodge, Wilmslow,
England.
Fancher, O.E., Schoenig, G., & Keplinger, M.L. (1968a) Acute
toxicity studies on GS 19 851 2 E (GA-494)- IBT No. A 6194.
Unpublished report from the Industrial Bio-Test Laboratories
Incorporated, Northbrook, Illinois. Submitted by Geigy Chemical
Corporation.
Fancher, O.E., Schoenig, G., & Keplinger, M.L. (1968b) Acute
toxicity studies on GS 19 851 25 W (GA-481) IBT No. A 6196.
Unpublished report from the Industrial Bio-Test Laboratories
Incorporated, Northbrook, Illinois. Submitted by Geigy Chemical
Corporation.
Flodström, S., Hemming, H., Wärngard, L. & Ahlborg, U.G. (1990).
Promotion of altered hepatic foci development in rat liver,
cytochrome P450 enzyme induction and inhibition of cell-cell
communication by DDT and some structurally related organohalogen
pesticides. Carcinogenesis, 11(8): 1413-1417.
Fritz, H., Suter, P., Zak, F., Malinowski, W. & Hess, R. (1986). GS
19'851 tech.: Two-generation reproduction toxicity study in rats.
Proj. No: 831523. Unpublished report dated December 1986 from Ciba-
Geigy Ltd., Stein, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Gretener, P., Froehlich, E., Malinowski, W., Christen, P. & Gfeller,
W. (1989). GS 19'851 tech.: 12-month chronic dietary toxicity study
in beagle dogs. Proj. No: 860018. Unpublished report dated May 11,
1989 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Hartmann, H.R. (1989). GS 19'851 tech.: Acute inhalation toxicity in
the rat. Proj. No: 881279. Unpublished report dated April 11, 1989
from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Hertner, Th. & Puri, E. (1987). GS 19'851 tech.: Autoradiographic
DNA-repair test on rat hepatocytes (OECD-CONFORM). Proj. No: 861631.
Unpublished report dated September 18, 1987 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hool, G. & Müller, D. (1980a). GS 19'861 tech.: Chromosome studies
in male germinal epithelium mouse (test for mutagenic effects on
spermatocytes). Proj. No: 790980. Unpublished report dated September
16, 1980, supplemented July 19, 1988 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hool, G. & Müller, D. (1980b). GS 19'851 tech.: Chromosome studies
in male germinal epithelium (test for mutagenic effects on
spermatogonia). Proj. No: 790979. Unpublished report dated June 17,
1980, supplemented July 19, 1988 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hool, G. & Müller, D. (1981). GS 19'851 tech.: Dominant lethal study
mouse (test for cytotoxic or mutagenic effects on male germinal
cells). Proj. No: 782203. Unpublished report dated February 24,
1981, supplemented July 19, 1988 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hool, G., Langauer, M. & Müller, D. (1980). GS 19'851 tech.: Nucleus
anomaly test in somatic interphase nuclei Chinese hamster (test for
mutagenic effects on bone marrow cells). Proj No.: 790981.
Unpublished report dated August 11, 1980, supplemented July 19, 1988
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Kuhn, J.O. (1989a). GS 19'851 tech.: Acute oral toxicity study in
rats. Proj. No: 6031-89. Unpublished report dated July 11, 1989 from
Stillmeadow Inc., Houston, TX/USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Kuhn, J.O. (1989b). GS 19'851 tech.: Acute dermal toxicity study in
rabbits. Proj. No: 6032-89. Unpublished report dated April, 11, 1989
from Stillmeadow, Inc., Houston, TX/USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Kuhn, J.O. (1989c). GS 19'851 tech.: Primary eye irritation study in
rabbits. Proj. No: 6033-89. Unpublished report dated March 30, 1989
from Stillmeadow, Inc., Houston, TX/USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Kuhn, J.O. (1989d). GS 19'851 tech.: Primary dermal irritation study
in rabbits. Proj. No: 603489. Unpublished report dated April 4, 1989
from Stillmeadow, Inc., Houston, TX/USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Mathies, P. (1991). The metabolite profiles in urine, faeces and
tissue extracts of rats after administration of [U-14C]phenyl GS
19'851. Proj. No: 17/91. Unpublished report dated June 28, 1991 from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Meyer, A. & Puri, E. (1987). GS 19'851 tech.: Autoradiographic DNA
repair test on human fibroblasts (OECD-CONFORM). Proj. No: 861632.
Unpublished report dated September 10, 1987, supplemented June 21,
1988 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Paterson, R.A. & Drake, J.C. (1967) GS 19 851 13-week oral toxicity
study in rats. Final report - Amendment to the report. Unpublished
from Geigy (U.K.) Ltd., Stamford Lodge, Wilmslow, England.
Paterson, R.A. (1967a) GS 19 851 28-day oral toxicity study in rats.
Final report. Unpublished, from Geigy (U.K.) Ltd., Stamford Lodge,
Wilmslow, England.
Paterson, R.A. (1967b) GS 19 851 30-day oral toxicity study in dogs.
Final report. Unpublished, from Geigy (U.K.) Ltd., Stamford Lodge,
Wilmslow, England.
Sagelsdorff P. & Heuberger B. (1990). GS 19'851 tech.: Investigation
of the potential for DNA binding. Proj. No: PS32-03901. Unpublished
report dated November 2, 1990 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Schneider, M., Gretener, P., Froehlich, E., Beri, J., Malinowski, W.
& Basler, W., (1989). GS 19'851 tech.: 28-Day repeated dose dermal
toxicity study in the rat. Proj. No: 891009. Unpublished report
dated December 11, 1989 from Ciba-Geigy Ltd., Stein, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Schulze-Aurich, J. (1993). The metabolism of [U-14C]Phenyl GS
19'851 in the rat. Proj. No. 07PM02. Unpublished report in
preparation from Ciba-Geigy Ltd., Stein, Switzerland. To be
submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Shirasu, Y., Moriya, M. & Koyashiki, R. (1979). Mutagenicity study
with phenisobromolate in bacteria. English translation of the
unpublished report dated November 22, 1979 from The Institute of
Environmental Toxicology, Japan. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Singh, A.R. & Yau, E.T. (1990). GS 19'851 tech.: A teratology
(segment ll) study in rats. Proj. No: MIN 892112/Tox./Path. Report
No.: 89131. Unpublished report dated May 25, 1990 from Ciba-Geigy
Corp., Summit, NJ/USA. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Singh, A.R., Hazelette, J.R. & Yau, E.T. (1990). GS 19'851 tech.: A
teratology (segment ll) study in rabbits. Proj. No: MIN
892014/Tox./Path. Report No.: 89162. Unpublished report dated June
18, 1990 from Ciba-Geigy Corp. Summit, NJ/USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Stenger, E.G. (1967) Akute Toxizität, Ratte per os, Nr. 295/14217.
Unpublished report from J.R. Geigy Ltd., Basle, Switzerland.
Strasser F. (1990). GS 19'851 tech.: Chromosome studies on Chinese
hamster ovary cell line CCL 61 in vitro. Proj. No: 891515.
Unpublished report dated July 4, 1990 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Thomas, H. (1991). Inductive effects of GS 19'851 on mouse liver
cytochromes P-450 and associated monooxygenase activities. Proj. No:
CB 90/28 BY. Unpublished report dated September 16, 1991 from Ciba-
Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Toyoshima, S., Fujita, H. & Satoh, R. (1977). GS 19'851 tech.:
Recombination assay and reversion test. Unpublished report dated
February 15, 1977 from Chemotherapy Dept., The Institute of Pharma-
chemistry, Keio University and Japan Experimental Medical Research
Inst. Co, Ltd. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Ueda, K. & Kondo, T. (1968) Report on experiment of acute toxicity.
Unpublished report from Tokyo Dental College, Hygienics Laboratory,
Tokyo, Japan. Submitted by Geigy Chemical Corporation.
Ullmann, L. & Sachsse, K. (1979a). GS 19'851 tech.: Acute oral LD50
in the rabbit. Proj. No: 790968. Unpublished report dated September
5, 1979 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Ullmann, L. & Sachsse, K. (1979b). GS 19'851 tech.: Skin sensitizing
(contact allergenic) effect in guinea pigs. Proj. No: 790972.
Unpublished report dated September 5, 1979 from Ciba-Geigy Ltd.,
Stein, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Waechter, F., Bentley, P. & Staeubli, W. (1986). The effect of GS
19'851 on mouse liver drug metabolizing enzymes. Unpublished report
dated September 19, 1986 from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Wärngard, L., Flodström, S., Ljungquist, S. & Ahlborg, U.G. (1985).
Inhibition of metabolic cooperation in Chinese hamster lung
fibroblast cells (V79) in culture by various DDT-analogs. Arch.
Environ. Contam. Toxicol., 14: 541-546.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
 The ethoxycoumarin O-deethylase, ethoxyresorufin O-deethylase,
pentoxyresorufin O-depentylase, and total testosterone hydroxylase
activities were also increased. The results of studies with
monoclonal antibodies against rat liver cytochrome P-450 isozymes of
four different gene families confirmed that bromopropylate shares
properties typical of a moderate phenobarbital-type inducer and
possibly is also a very weak peroxisome proliferator-type inducer.
It was concluded that bromopropylate has the properties of a
phenobarbital-like inducer of cytochrome P-450 in the mouse liver.
In addition, it might act as a very weak peroxisome proliferator.
The increased liver weights at the high-dose and the biochemical
changes were therefore regarded as a reversible adaptation to a
functional overload. At 15 mg/kg bw/day only minimal biochemical
changes were observed and the low dose of 3 mg/kg bw/day was without
any effects (Thomas, 1991).
A DNA-binding study was conducted with mice (Tif: MAG f)
pretreated for two weeks with unlabelled bromopropylate (94.2% pure)
at daily oral doses of 300 mg/kg bw. Radiolabelled [ bis-phenyl-
14C] bromopropylate was then administered by gavage at a dose level
of 300 mg/kg bw. After 24 hours, DNA was isolated from the liver
and purified. No radioactivity was detected. Bromopropylate is
devoid of a genotoxic potential mediated by DNA-binding (Sagelsdorff
& Heuberger, 1990).
Toxicological studies
Acute toxicity studies
Bromopropylate has a low acute toxicity in rats and rabbits.
WHO has classified bromopropylate as unlikely to present acute
hazard in normal use (WHO, 1992). The LD50 and LC50 values are
summarized in Table 1.
Short-term toxicity studies
Rats
Groups of 10 male and 10 female rats received 0, 40, 200, 1000
or 5000 mg bromopropylate/kg bw/day by gavage as a suspension in
0.5% aqueous tragacanth on six days each week for four weeks. The
highest dosage level produced polyuria throughout the 21 days before
the animals were killed and for the first eight days pale mucoid
faeces were produced. The rate of body-weight gain and food intake
were reduced in the 200 and 1000 mg/kg bw/day groups. Rats of the
5000 mg/kg bw/day group developed a relative neutrophilia but the
results of haematological analyses in all groups were normal. The
absolute and relative liver weights were increased in the three
highest dosage groups and cytoplasmic swelling and periportal
Table 1. Results of acute toxicity studies with bromopropylate
Species Sex Route Solvent LD50/LC50 Reference
(vehicle) (mg/kg bw)/
(mg/m3 air)
Mouse1 M oral - 8000 Ueda & Kondo, 1968
Rat1 M,F oral - > 5000 Stenger, 1967
Rat2 M,F oral - 6000 Drake, 1970
Rat2 ? oral - > 23 000 Fancher et al., 1968a,b
Rat3 M,F oral corn oil approx. 5000 Kuhn, 1989a
Rat4 M,F dermal PEG 400 > 4000 Bathe & Sachsse, 1979
Rat5 M,F inhalation ethanol > 4458 Hartmann, 1989
Rabbit4 M,F oral CMC6 > 6000 Ullmann & Sachsse, 1979a
Rabbit3 M,F dermal saline > 2020 Kuhn, 1989b
1 Purity given as Technical.
2 Purity given as Formulation.
3 Purity not given but known to be 90%.
4 90% pure.
5 95% pure.
6 CMC = 2% carboxymethylcellulose in water.
infiltration were seen. Hepatic necrosis occurred at the highest
dosage level. At 40 mg/kg bw/day, the one animal which died during
the test showed similar abnormalities in the liver; others in the
group appeared normal (Paterson, 1967a).
Groups of 12 male and 12 female rats were fed diets containing
0, 100, 300 or 1000 ppm bromopropylate for 90 days. Another group
received diets containing 3000 ppm for 55 days and 4000 ppm for a
further 35 days. At the highest dose, the food intake and rate of
weight gain were below normal and at autopsy the liver, kidney and
testes weights relative to body weight were higher than in controls.
Swelling and loss of basophil material, pigmentation (possibly
lipofuscin) and fatty infiltration were present in hepatocytes.
Mild to severe regressive changes were seen in testes on
histological examination. At 1000 ppm, food intake was slightly
less than in controls although weight gain was similar. Testes were
lighter than in controls and similar histological changes were seen
in the liver. At the two lower levels, smaller numbers of rats
showed loss of basophilic material and cytoplasmic swelling with
focal vacuolation. These changes were considered to be
physiological in that they were attributed to SER hypertrophy, but
no EM studies were carried out to confirm this. The results of
haematological and blood biochemistry studies and of urine analyses
were similar in all groups (Paterson & Drake, 1967).
Bromopropylate (94.2% pure) was applied to the shaved skin of 5
male and 5 female rats (Tif:RAI f) per dose group for 4 weeks on a
5-day per week basis. The daily exposure period was 6 hours. The
compound was given on moistened gauze patches under occlusive
dressing at dose levels of 0, 50, 200 or 1000 mg/kg bw/day. There
was no mortality, no clinical signs, no signs of local irritation
and no body weight or food consumption changes that could be
attributed to treatment. At 1000 mg/kg bw/day, slightly lower
glucose levels and a minimal increase of urea were evident in blood
chemistry, while no changes were seen in haematology. Organ
weights, macro- and microscopic histopathological findings were
similar in all groups. The NOAEL in this study was 200 mg/kg bw/day
in both sexes (Schneider et al., 1989).
Dogs
A group of two male and two female dogs received orally, by
capsule, 1000 mg/kg bw/day of bromopropylate for 30 days. A second
group received 2000 mg/kg bw/day for four days, then 500 mg/kg
bw/day for a further 26 days. Diarrhoea and vomiting occurred in
dogs of both groups. During the first week, food intake and body
weight decreased. The results of haematological examinations were
normal. Determination of organ weights did not indicate any dose-
related changes. In both groups, serum transaminase and alkaline
phosphatase levels were increased by treatment and cytoplasmic
swelling and fine vacuolation of hepatocytes were found. No mature
spermatozoa were found in male animals, but this may have been due
to the age of the animals (Paterson, 1967b).
Four groups of eight male and eight female dogs were fed for
two years on diets containing 30, 100, 250 or 1000 ppm
bromopropylate. A fifth group received 4000 ppm in the diet for
three months. A group of 10 males and 10 females acted as controls.
The highest dosage level produced light-coloured soft stools after
one week and semi-fluid stools thereafter. Food intake was reduced
and weight loss occurred. Two dogs died (after four and six weeks)
and two others were killed because they became cachectic. In these
four animals, increased serum alkaline phosphatase, diminished serum
cholesterol and mild anaemia were found. Haemosiderosis of kupfer
cells, proximal tubular cells of the kidney, the spleen and the bone
marrow, degenerative changes in the distal convoluted tubules of the
kidney, hyperplasia of bone marrow and extra-medullary
haematopoiesis were seen in these animals at autopsy. In the other
animals of the 4000 ppm group killed at three months, accumulation
of haemosiderin in the macrophages of the spleen and bone marrow and
in kupfer cells was observed. Examination of liver by EM showed SER
hypertrophy and an increase in numbers of lysosomes, many of which
contained lamellar structures. Two additional animals of each sex
were fed for nine months on control diets following three months on
4000 ppm diet. Stools returned to normal consistency within a week
and weight gain was normal. Biochemical and haematological
parameters also returned to normal in a short time; animals were
indistinguishable from controls at the time of autopsy. The 1000
ppm diet also produced softening of stools. Although weight gain by
males was normal, females failed to gain weight. Some females
showed slight anaemia on some occasions. The microsomal enzymes,
biphenyl hydroxylases, showed increased activity and this was
associated with an increase in liver weight relative to body weight
at six months and at two years (in females only). SER hypertrophy
was marked at three months and numerous myelin bodies were also
seen. Although at two years the SER hypertrophy was not different
from controls, myelin bodies were still present. An increase in
microsomal enzymes was found in the 250 ppm group together with an
enlarged liver (relative to body weight) at six months but not at
two years. No adverse effects were observed in dogs fed on 30 or
100 ppm diets (Coulston et al., 1970a).
Bromopropylate (95% pure) was administered in the diet at
concentrations of 0, 100, 400 or 2000 ppm to groups of 6 male and 6
female beagle dogs for one year. After completion of the treatment
period, subgroups of 2 animals per sex were kept for one month on
compound-free (control) diet to determine reversibility of potential
toxic effects. There were no compound-related mortalities and no
clinical signs. Body-weight gain was decreased markedly at 2000 ppm
in both sexes and slightly at 400 ppm in females from week 28
onwards. Food consumption was not affected and there were no
ophthalmic, haematological or urinary changes. The only relevant
change in blood chemistry was a slight reversible increase of
alkaline phosphatase activity in the females at 2000 ppm. At the
end of the 12-month treatment period, liver weights were slightly
increased and there was a slight hypertrophy of hepatocytes. These
changes only occurred at 2000 ppm and were not present after the
one-month recovery period. The NOAEL in this study was 100 ppm,
equal to 2.7 mg/kg bw/day (males) and 2.8 mg/kg bw/day (females)
(Gretener et al., 1989).
Long-term toxicity/carcinogenicity studies
Mice
In an oncogenicity study, mice (Charles River CD-1, 50 males
and 50 females per group) were given bromopropylate (98.7% pure) in
the diet during 18 and 20 months for males and females,
respectively, at concentrations of 0, 30, 300 or 1000 ppm. Similar
reduction in body weight was noted for the control and test groups
after 12 months of testing, males losing an average of 6 to 7 grams
and females 2 to 3 grams. This generalized reduction in body weight
occurred following alterations in both the method of feeding and the
standard diet fed. However, no statistically significant
differences in mean body weights were noted for any treatment group.
Meaningful evaluation of body-weight gain cannot be made since body
weights were not measured during the first 6 months of testing.
Negative body-weight gains were recorded for the observation period
for all groups. The mean survival time of males in the 1000 ppm
group was significantly reduced. Significantly increased
mortalities in the 30 and 1000 ppm groups were observed, which were
considered coincidental. No treatment-related findings were seen at
gross pathological examination. Histopathology revealed dose-
related centrilobular hypertrophy of hepatocytes in the two higher
doses. Additional sporadic histopathological findings were not
considered as related to treatment. The compound did not show an
oncogenic potential in this study (Charles et al., 1979).
Bromopropylate (95% pure) was administered to male and female
mice (Tif: MAGf (SPF), hybrids of inbred MIG x NIH mice) in the diet
at levels of 0, 30, 150, 1000 or 3000 ppm (60 males and 60 females
per group) for 24 months. Appearance and behaviour were not
affected by treatment. Lower body-weight gain was recorded for the
3000 ppm group over the first 12 weeks for males and the first 25
weeks for females. Thereafter, mean body weights remained 5-7% and
15-18% lower than control values, for males and females,
respectively. Food consumption was slightly lowered in females in
the 1000 and 3000 ppm groups during the first year. Water
consumption was increased at 3000 ppm. Haematological parameters
were not significantly affected by treatment. Absolute and relative
liver weights were significantly increased in both sexes at 1000 and
3000 ppm. Macroscopic examination revealed dose-related increase of
masses and nodules in the livers of male and female mice from these
two groups. Kidney weights at 3000 ppm were lower than in the
control group. On microscopic examination, hepatocellular
neoplastic lesions, i.e. benign hepatomas and hepatocellular
carcinomas, accounted for most of the macroscopically observed
masses and nodules in the liver. The frequency of these lesions was
significantly increased in both sexes at 1000 and 3000 ppm. Animals
of both sexes of these two groups also showed a frequent hypertrophy
of hepatocytes. The only other compound-related finding was an
increased incidence of reactive hyperplasia of splenic white pulp in
female mice of the high dose group (3000 ppm). The NOAEL in this
study was 150 ppm, equal to 16 mg/kg bw/day in both males and
females (Basler et al., 1990).
Rats
Groups of 50 male and 50 female rats were fed diets containing
0, 15, 30 or 100 ppm bromopropylate for two years. Five male and
five female rats from each group were killed after six months or one
year and autopsies performed. No changes in appearance or behaviour
occurred. Food consumption and weight gain were similar in test and
control animals throughout the test and survival rates were similar
for the first 18 months. Approximately half of the control female
animals alive at 18 months survived to 24 months while only a third
of test animals at 30 and 100 ppm survived over this period. No
alterations attributable to bromopropylate were observed in the
haematological examinations in test animals. No changes in organ
weights or gross or microscopic abnormalities attributable to
ingestion of the pesticide were observed in animals examined at any
time. However, EM studies on the livers of animals of the 100 ppm
group surviving two years showed slightly less glycogen, focal
enlargement of intracrestal space in the mitochondria, focal
dilatation of SER and more prominent lipid accumulations than livers
of control animals. These differences were not considered to be
significant pathological alterations in cell ultrastructure. The
number of tumours and their location were similar in control and
test groups (Coulston et al., 1970b).
Bromopropylate (95% pure) was administered in the diet to rats
(Tif: RAlf, 80 males and 80 females per dose group) at levels of 0,
100, 700 or 5000 ppm. The study duration was 119 weeks for males
and 131 weeks for females. Average intakes of bromopropylate were
3.7, 26 or 217 mg/kg bw/day for males and 4.4, 32 or 270 mg/kg
bw/day for females. In the 5000 ppm group, males maintained mean
body weights 20% lower than control values from 15 weeks onwards,
whereas females showed a progressive divergence, attaining a 37%
difference from control values at 95 weeks. Food consumption was
decreased in males and females at 5000 ppm, and water consumption
was increased in both males and females at 5000 ppm and in males at
700 ppm. Calculated red blood cell indices (MCV, MCH) were
decreased in males (from 80 weeks) and in females (from 27 weeks) at
5000 ppm, suggesting a tendency to microcytosis and hypochromoasia
of red blood cells. Increased prothrombin times were recorded in
males at weeks 12, 27, and 80, and in females at all investigated
weeks at 5000 ppm. Blood samples were collected at 12, 27, 53, 80,
105 and 131 weeks. A non-progressive lowering of blood glucose was
recorded at weeks 12 to 80 for males and at weeks 27, 53 and 80 for
females at 5000 ppm. A hyperproteinemia resulting from
hyperglobulinemia was observed in the males and females at 5000 ppm.
Females of the 700 ppm group showed a similar trend, but to a lesser
degree between weeks 27 and 105. Higher levels of cholesterol and
phospholipids were recorded at week 53 for males and on all
occasions for females at 5000 ppm. In the 700 ppm group, high
levels of cholesterol were observed in females at weeks 12, 27 and
80. Also, high levels of phospholipids were recorded in females at
12 and 80 weeks. Both sexes of the 5000 ppm group showed a marked
increase in gamma glutamyl transpeptidase activity at each
investigation.
At the interim sacrifices during weeks 53 and 105 and at the
termination of the study, relative liver weight for males and
females of the 5000 ppm group were significantly increased. In
addition, the relative liver weights for rats at 700 ppm were
significantly increased at week 53 for females and at termination
for males. Significantly increased relative kidney weights were
recorded for males and females of the 5000 ppm group at week 53 and
for females at week 105. Relative thyroid gland weights in males at
700 and 5000 ppm were significantly increased at week 53 and the
relative weight in males at 5000 ppm was also increased at
termination of the study. Macroscopic examination revealed an
increased number of rats with edema of the testes and an increased
number of females with liver cysts at 5000 ppm. In
histopathological examinations, increased incidences of focal
hepatocellular hypertrophy and fatty changes and pigmentation of
hepatocytes were also observed in both sexes at 700 and 5000 ppm.
Incidences of biliary cysts and cholangiofibrosis were increased in
females at 5000 ppm. Occurrence of lung alveolar foam cells were
increased in females at 5000 ppm. An increase in the number of rats
with testicular tubular atrophy and an associated edema and nodular
hyperplasia (Leydig cells) was observed at 5000 ppm. Numbers of
rats with follicular cystic dilatation and thyroid gland hyperplasia
were increased in both sexes at 5000 ppm. Incidence of rats with
thyroid gland adenoma was increased at 5000 ppm in males. The NOAEL
in this study was 100 ppm (equal to 3.7 mg/kg bw/day for males and
4.4 mg/kg bw/day for females) (Basler et al., 1989).
Reproduction studies
Rats
Groups of 20 male and 20 female rats in the first generation
and groups of 25 male and 25 female rats in the second and third
generations were used in a three-generation test (two litters being
produced in each generation) and fed diets containing 0, 2.5 or 5
ppm bromopropylate. In a second test, groups of 25 male and 25
female rats received diets containing 0, 30 or 100 ppm
bromopropylate. Throughout the tests no abnormalities attributable
to bromopropylate were found in the reproductive physiology of male
or female rats and there was no evidence of gross abnormalities in
the offspring throughout the studies. The number of young in each
litter and their growth and survival were normal. The weights of
liver, kidneys and spleen were comparable with controls in the rats
of the F3b generation whose parents received 2.5 or 5 ppm diets
while spleen and liver weights of the F3b young of parents fed 30
or 100 ppm diets were slightly higher than controls. No
histological abnormalities were found in these animals at any dosage
level (Coulston et al., 1971).
In a two-generation reproduction study, rats (Sprague-Dawley-
served, Tif:RAI f) were administered bromopropylate (93.5% pure) in
the diet at concentrations of 0, 165, 750 or 2250 ppm (28
rats/sex/dose level). Approximate daily intakes of bromopropylate
were 12, 54 or 170 mg/kg bw for parental males and 16, 72 or 218
mg/kg bw for parental females. The administration started 10 weeks
prior to mating of the F0 parents and was continued throughout the
mating, lactation and weaning periods of the F1 and up to weaning
of the F2 generation.
F0 parental animals showed reduced body-weight gain and food
consumption at 2250 ppm and, to a lesser extent, in the 750 ppm
group. The females of the F1 parental generation showed some
depression in food consumption at 750 and 2250 ppm prior to mating
and only at 2250 ppm during lactation. Histological changes
consisted of a dose-related slight to severe hepatocellular
enlargement at 750 ppm and 2250 ppm corresponding to liver weight
increases in the F1 animals; in addition, some of the males in the
high-dose group showed hepatocyte necrosis. Reproductive parameters
(concerning mating, fertility, pregnancy, parturition and rearing)
were not affected by the treatment. With respect to the offspring,
the F1 pups of the 2250 ppm group showed an increased mortality
rate. For both F1 and F2 young rats of the 2250 ppm group, a
diminished body-weight gain was recorded from weaning onwards.
Exploratory locomotion was depressed in the young F1 rats at 750
and 2250 ppm and in the F2 rats at 750 ppm only.
Histopathological examination of the F1 rats revealed changes
in the liver, as indicated by slight to severe hypertrophy of
hepatocytes in about two-thirds of the males and females at 750 ppm
and in all animals at 2250 ppm. In the males, necrosis (8/24 at
2250 ppm) or other structural alterations of hepatocytes (11/23 at
750 ppm, 22/24 at 2250 ppm) were noted. Parallel to these results,
absolute as well as relative liver weights were found to be
increased in a "positive trend" from the control to the high
concentration. The lowest mean daily compound intakes during the
various study periods were calculated to be approximately 9, 40 or
128 mg/kg body weight. The NOAEL in this study was 165 ppm (equal
to 9 mg/kg bw/day) (Fritz et al., 1986).
Dogs
Groups of three male and three female dogs were fed diets
containing 0, 30 or 100 ppm bromopropylate. After an unstated time,
the females were mated with males of the same group. All matings
resulted in pregnancy and delivery of normally sized litters. The
only deformed pup was found in a control litter. All others were
normal in appearance, behaviour and development. Increases in the
body weight of the pups in most of the litters of groups receiving
bromopropylate were similar to those of control pups. The reduced
growth rate of one litter of the 100 ppm group was attributed to the
results of poor maternal care (Coulston et al., 1970a; Coulston &
Benitz, 1972).
Special studies on embryo/fetotoxicity
Rats
Bromopropylate (90% pure) was administered orally by gavage to
three groups of pregnant rats (CRL: COBS CD[SD]BR, 26 females/group)
at doses of 0, 50, 300 or 700 mg/kg bw once daily during days 6
through 15 of gestation. A control group of 26 female rats received
an equivalent volume (10 ml/kg bw) of aqueous 3% corn starch with
0.5% Tween 80. All surviving animals were sacrificed on day 20 of
presumed gestation. Evaluations were conducted on clinical signs,
maternal body weights, food consumption, necropsy and skeletal
abnormalities. Compound-related effects were noted at 300 and 700
mg/kg bw/day. These changes included: an increased incidence of
salivation and alopecia accompanied by skin irritation; transient
body-weight loss and slight reductions in daily food consumption at
700 mg/kg bw/day and of body-weight gain at 300 mg/kg bw/day; slight
decreases in mean male and female fetal weight at 700 mg/kg bw/day;
and an increased incidence of skeletal variations of fully formed
14th ribs and rudimentary 14th ribs at 300 and 700 mg/kg bw/day and
in forepaw metacarpals not ossified at 700 mg/kg bw/day. The fetal
findings were considered to be slight treatment-related effects
associated with the compound-induced maternal toxicity. There was
no evidence of embryo/fetotoxicity or teratogenicity at doses up to
and including 700 mg/kg bw/day. The maternal NOAEL in this study
was 50 mg/kg bw/day (Singh & Yau, 1990).
Rabbits
Bromopropylate (90% pure) was administered orally by gavage to
three groups of pregnant rabbits (New Zeeland white, 20
females/group) at doses of 0, 20, 60, or 120 mg/kg bw once daily
during gestation days 7 through 19. A control group of 20 pregnant
rabbits received an equivalent volume (5 ml/kg bw) of the vehicle.
All surviving animals were sacrificed on day 29 of presumed
gestation. Evaluations were conducted on clinical signs, maternal
body weights, food consumption, necropsy, reproductive parameters,
fetal weights and fetal gross, visceral and skeletal observations.
Treatment-related maternal effects were observed at 60 and 120 mg/kg
bw/day. These changes included: mortality/moribundity of two
animals at 120 mg/kg bw/day; clinical signs of lethargy at 120 mg/kg
bw/day and anogenital staining at 60 and 120 mg/kg bw/day;
reductions in mean body weight at 120 mg/kg bw/day, mean body-weight
gain at 60 and 120 mg/kg bw/day, and reduction of food consumption
at 120 mg/kg bw/day; and the necropsy finding of distended stomach
with viscous/gelatinous material at 120 mg/kg bw/day. There was no
evidence of embryotoxicity, fetotoxicity or teratogenicity at any
dose level. The NOAEL for maternal toxicity was 20 mg/kg bw/day
(Sing et al., 1990).
Special studies on genotoxicity
The results of genotoxicity studies conducted with bacteria,
mammalian cells in vitro and with intact animals in vivo are
presented in Tables 2a, b and c. In all studies, bromopropylate was
devoid of mutagenic potential.
Special studies on inhibition of cell-cell communication and liver
tumour promotion
Bromopropylate was investigated in vitro for its ability to
inhibit gap junctional intercellular communication in the Chinese
hamster fibroblast cell V79 metabolic cooperation assay and in the
scrape-loading/dye-transfer assay in WBF344 rat liver epithelial
cells, and in vivo altered hepatic foci assay and hepatic drug
metabolizing activity in male Sprague-Dawley rats. Technical grade
bromopropylate (94% pure) induced a dose-dependent increase in the
recovery of 6-thioguanine-resistant mutants within the same dose
range as reported earlier for the analytical grade (Wärngard et
al., 1985). In the scrape-loading assay, intercellular
communication was abolished at non-cytotoxic concentrations both
with technical and analytical grade bromopropylate. There was a
gradual decrease in dye transfer from the lowest concentration (20
µM) up to the dose inducing complete blockade of dye transfer.
Bromopropylate was shown to cause hepatomegaly and induction of
cytochrome P-450b-related O-dealkylase enzyme activities in hepatic
microsomes when fed to rats at 1000 ppm for 11 weeks, indicating a
phenobarbital-type induction of hepatic metabolism.
The rats were initiated by partial (2/3) hepatectomy and 24
hours later received a single intraperitoneal dose of 30 mg
nitrosodiethylamine/kg bw. From week 2 after initiation until
termination at week 13, the animals were fed a diet containing 1000
ppm bromopropylate. A group of non-hepatectomized, non-
diethylnitrosamine-treated rats was also fed the bromopropylate-
containing food. Appropriate control groups received the control
diet. Bromopropylate enhanced the incidence of GGT-positive foci
and the percentage of liver occupied by foci's tissue in initiated
rats (Flodström et al., 1990).
Table 2a. Mutagenicity tests in bacteria in vitro
Test Test object S-9 mix Concentrations Purity Results Reference
Escherichia coli WP2hcr +/- 10-5000 µg/plate 99.7% - Shirasu et al., 1979
Escherichia coli B/r WP2 try- 500-2000 µg/disc ? - Toyoshima et al., 1977
SD-4
Bacillus subtilis rec+(H17) 20-2000 µg/disc 99.7% - Shirasu et al., 1977
rec-(M45)
Bacillus subtilis rec+(H17) 50-2000 µg/disc ? - Toyoshima et al., 1977
rec-(M45)
Salmonella TA98 +/- 10-5000 µg/plate 99.7% - Shirasu et al., 1979
typhimurium TA100 +/- -
TA1535 +/- -
TA1537 +/- -
TA1538 +/- -
TA98 +/- 313-5000 µg/0.1 ml 94.2% - Deparade, 1989
TA100 +/- -
TA1535 +/- -
TA1537 +/- -
Table 2b. Mutagenicity tests in mammalian cells in vitro
Test Test object Concentrations Purity Results Reference
Chromosomal Chinese hamster 3.13-25 µg/ml Batch op. - Strasser, 1990
aberrations ovary cells with 406162
and without S-9 94.2%
mix
DNA repair human fibroblasts 0.5-60 µg/ml Batch op. - Meyer & Puri,
(UDS) 406162 1987
95%
DNA repair rat hepatocytes 0.4-40 µg/ml Batch op. - Hertner & Puri,
(UDS) 406162 1987
95%
Table 2c. Mutagenicity tests in mammalian cells in vivo
Test Test object Dose levels Purity Results Reference
Chromosomal Mouse five times and batch P 43/78 - Hool & Müller,
aberrations spermatocytes 3000 mg/kg 90% 1989a
within 10 days
Chromosomal Mouse five times and batch P 43/78 - Hool & Müller,
aberrations spermatogonia 3000 mg/kg on 90% 1989b
five consecutive
days
Nucleus Chinese 2500, 5000 and batch P 43/78 - Hool et al.,
anomaly test hamster, 10 000 mg/kg 90% 1980
bone marrow twice on two
consecutive days
Dominant Male mice single oral doses batch Mg. - Hool & Müller,
lethal test of 0, 1100 or 68/77 1981
3300 mg/kg 91.5%
Special studies on skin and eye irritation
A primary eye irritation test on bromopropylate (90% pure) was
performed by using 3 male and 3 female New Zeeland white rabbits.
Irritation observed after the application of 100 mg of undiluted
test material into the conjunctival sac consisted of reversible
redness, chemosis and discharge in all rabbits and iridal
involvement in one rabbit. The compound was given a descriptive
rating "mildly irritant" in non-washed eyes and in washed eyes, with
maximum average irritation scores of 9.3 and 3.3, respectively.
However, the mean values of the scores calculated from the 24, 48
and 72 hour readings over all tested animals were 0.07 for iris
lesions, 0.6 for redness, and 0.3 for edema of conjunctiva.
According to the EEC Directive 83/467 the test substance is
therefore classified as "non-irritant" to eyes (Kuhn, 1989c).
A primary skin irritation test on bromopropylate (90% pure) was
performed using 3 male and 3 female New Zeeland white rabbits.
Irritation observed on the skin after the application of 500 mg of
test material moistened with 0.4 ml of saline consisted of grade 1
erythema in 5 out of 6 rabbits at the 1 hour reading time. The
compound was given a descriptive rating "slight irritant". However,
the mean values of the scores calculated from the 24, 48 and 72 hour
readings over all tested animals were 0 for erythema and 0 for
edema. According to the EEC Directive 83/467 the test substance is
therefore classified as "non-irritant" to skin (Kuhn, 1989d).
Special studies on skin sensitization
A sensitization assay with bromopropylate (90% pure) using
Freund's complete adjuvant during the induction period (optimization
test) was conducted at a concentration of 0.1% in propylene glycol
as vehicle in guinea-pigs (Pirbright white strain, 10 males and 10
females/group). Significant differences between the test group
(14/20) and vehicle-treated controls (4/20) were seen only after
intradermal challenge application, i.e. when the skin barrier was
intentionally by-passed. No difference between the test (0/19) and
the control (0/20) groups was seen after epidermal challenge
application. It was therefore concluded that, in artificially
sensitized guinea-pigs, exposure of intact skin does not provoke
contact dermatitis (Ullmann & Sachsse, 1979b).
Observations in humans
No information available.
COMMENTS
After oral administration of [U-14C]phenyl bromopropylate,
most of the radioactivity was eliminated in the faeces, with lower
amounts of radioactivity excreted in the urine. The routes of
elimination were sex-dependent.
Bromopropylate was metabolized preferentially by cleavage of
the isopropyl ester, and to a minor extent by oxidation reactions
attacking the phenyl ring and the isopropyl group.
Bromopropylate has the properties of a phenobarbital-like
inducer of cytochrome P-450 in the mouse liver. In a DNA-binding
study conducted with mice, no radioactivity was detectable in liver
DNA, indicating that bromopropylate is devoid of genotoxic potential
in this tissue.
Bromopropylate has a low acute toxicity in rats and rabbits.
WHO has classified bromopropylate as unlikely to present acute
hazard in normal use.
In a one-year study in dogs at dietary concentrations of 0,
100, 400 or 2000 ppm, the NOAEL was 100 ppm (equal to 2.7 mg/kg
bw/day), based on depressed body-weight gain at 400 ppm and above.
In a study in mice using dietary concentrations of 0, 30, 150,
1000 or 3000 ppm for 24 months, the NOAEL was 150 ppm (equal to 16
mg/kg bw/day), based on increased absolute and relative liver weight
and hepatocellular neoplastic lesions at 1000 ppm and above.
Long-term toxicity/carcinogenicity studies in rats were
reviewed. The study considered by the 1973 Joint Meeting was found
to be unacceptable. In a new study at dietary concentrations of 0,
100, 700 or 5000 ppm, the NOAEL was 100 ppm (equal to 3.7 mg/kg
bw/day), based on increased water consumption and increased relative
liver and thyroid weights at 700 ppm and above. Increased
incidences of focal hepatocellular hypertrophy and fatty changes and
pigmentation of hepatocytes were also observed at 700 ppm and above.
In a reproduction study in rats using dietary concentrations of
0, 165, 750 or 2250 ppm, the NOAEL was 165 ppm (equal to 9 mg/kg
bw/day), based on increased liver weight and hypertrophy of
hepatocytes in F1 animals at 750 ppm and above.
Teratogenicity studies were conducted with rats and rabbits.
In the study in rats at doses of 0, 50, 300 or 700 mg/kg bw/day,
depressed maternal body-weight gain and an increased incidence of
skeletal variations of fully formed 14th ribs and rudimentary 14th
ribs were recorded at 300 mg/kg bw/day and above. The maternal
NOAEL in this study was 50 mg/kg bw/day. There was no evidence of
embryo/fetotoxicity or teratogenicity. In the study in rabbits at
doses of 0, 20, 60, or 120 mg/kg bw/day, mean body-weight gain was
depressed at 60 mg/kg bw/day. The NOAEL for maternal toxicity was
20 mg/kg bw/day, and no embryo/fetotoxic or teratogenic effects were
found.
After reviewing the available genotoxicity data, the Meeting
concluded that bromopropylate was not genotoxic.
An ADI was established based on the NOAEL of 2.7 mg/kg bw/day
in the one-year study in dogs, using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 150 ppm, equal to 16 mg/kg bw/day (two-year study)
Rat: 100 ppm, equal to 3.7 mg/kg bw/day (two-year study)
Dog: 100 ppm, equal to 2.7 mg/kg bw/day (one-year study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Basler, W., Hunter, B., Gretener, P., Froehlich, E., Faccini, J.M.,
Christen, P., Seewald, W., Dieterle, R. & Gfeller, W. (1989). GS
19'851 tech.: Lifetime carcinogenicity and chronic toxicity study in
rats. Proj. No: 831417. Unpublished report dated October 26, 1989
from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Basler, W., Hunter, B., Gretener, P., Froehlich, E., Krinke, G.,
Christen, P., Dieterle, R. & Gfeller, W. (1990). GS 19'851 tech.:
Lifetime carcinogenicity study in mice. Proj. No: 850334.
Unpublished report dated February 22, 1990 from Ciba-Geigy Ltd.,
Stein, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Bathe, R. & Sachsse, K. (1979). GS 19'851 tech.: Acute dermal LD50
in the rat. Proj. No: 790969. Unpublished report dated October 9,
1979 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Cassidy, J.E., Mattson, A., Cullen, T., & Min, B. (1968) The
metabolic fate of 14C GS 19851 administered to a cow by capsules.
Unpublished report from Geigy Chemical Corporation, Ardsley, N.Y.
Charles, J.M., Stevens, J.T., Sumner, D.D., Barnett, J.W. & Ellis,
J.F. (1979). GS 19'851 tech.: Carcinogenicity evaluation in albino
mice. Proj. No: 62206726. Unpublished report dated July 25, 1979
from Ciba-Geigy Corp., Greensboro, NC/USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland .
Cornelissen K. & Hopkins R. (1991). [U-14C]phenyl GS 19'851:
Absorption, distribution, and excretion in the rat. Proj. No: 6453-
380/151. Unpublished report dated April 3, 1991 from Hazleton UK,
Harrogate. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Coulston, F., Fabian, R.J., Abraham, R., & Benitz, K.F. (1970a) Two-
year safety evaluation of isopropyl 4,4'-dibromobenzilate in beagle
dogs. Unpublished report from Institute of Experimental Pathology
and Toxicology, Albany Medical College, Albany, N.Y. Submitted by
Geigy Chemical Corporation.
Coulston, F., Fabian R.J., & Benitz, K.F. (1970b) Two-year safety
evaluation study of isopropyl 4,4'-dibromobenzilate in albino rats
by dietary feeding. Unpublished report from Institute of
Experimental Pathology and Toxicology, Albany Medical College,
Albany, N.Y. Submitted by Geigy Chemical Corporation.
Coulston, F., Le Fevre, R., & Fabian, R. (1971) Three generation
study with GS 19 851 in albino rats. Unpublished report from the
Institute of Experimental Pathology and Toxicology, Albany Medical
College, Albany, N.Y. Submitted by Geigy Chemical Corporation.
Coulston F. & Benitz, K.F. (1972) Supplemental data on studies made
with GS 19 851 in beagle dogs. Unpublished report from the Institute
of Experimental Pathology and Toxicology, Albany Medical College,
Albany, N.Y. Submitted by Geigy Chemical Corporation.
Deparade E. (1989). GS 19'851 tech.: Salmonella/mammalian-
microsome mutagenicity test. Proj. No: 881537. Unpublished report
dated May 19, 1989 from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Drake, J.C. (1970) A 2443 A - Acute median lethal dose in rats.
Unpublished report from Geigy (U.K.) Ltd., Stamford Lodge, Wilmslow,
England.
Fancher, O.E., Schoenig, G., & Keplinger, M.L. (1968a) Acute
toxicity studies on GS 19 851 2 E (GA-494)- IBT No. A 6194.
Unpublished report from the Industrial Bio-Test Laboratories
Incorporated, Northbrook, Illinois. Submitted by Geigy Chemical
Corporation.
Fancher, O.E., Schoenig, G., & Keplinger, M.L. (1968b) Acute
toxicity studies on GS 19 851 25 W (GA-481) IBT No. A 6196.
Unpublished report from the Industrial Bio-Test Laboratories
Incorporated, Northbrook, Illinois. Submitted by Geigy Chemical
Corporation.
Flodström, S., Hemming, H., Wärngard, L. & Ahlborg, U.G. (1990).
Promotion of altered hepatic foci development in rat liver,
cytochrome P450 enzyme induction and inhibition of cell-cell
communication by DDT and some structurally related organohalogen
pesticides. Carcinogenesis, 11(8): 1413-1417.
Fritz, H., Suter, P., Zak, F., Malinowski, W. & Hess, R. (1986). GS
19'851 tech.: Two-generation reproduction toxicity study in rats.
Proj. No: 831523. Unpublished report dated December 1986 from Ciba-
Geigy Ltd., Stein, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Gretener, P., Froehlich, E., Malinowski, W., Christen, P. & Gfeller,
W. (1989). GS 19'851 tech.: 12-month chronic dietary toxicity study
in beagle dogs. Proj. No: 860018. Unpublished report dated May 11,
1989 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Hartmann, H.R. (1989). GS 19'851 tech.: Acute inhalation toxicity in
the rat. Proj. No: 881279. Unpublished report dated April 11, 1989
from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Hertner, Th. & Puri, E. (1987). GS 19'851 tech.: Autoradiographic
DNA-repair test on rat hepatocytes (OECD-CONFORM). Proj. No: 861631.
Unpublished report dated September 18, 1987 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hool, G. & Müller, D. (1980a). GS 19'861 tech.: Chromosome studies
in male germinal epithelium mouse (test for mutagenic effects on
spermatocytes). Proj. No: 790980. Unpublished report dated September
16, 1980, supplemented July 19, 1988 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hool, G. & Müller, D. (1980b). GS 19'851 tech.: Chromosome studies
in male germinal epithelium (test for mutagenic effects on
spermatogonia). Proj. No: 790979. Unpublished report dated June 17,
1980, supplemented July 19, 1988 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hool, G. & Müller, D. (1981). GS 19'851 tech.: Dominant lethal study
mouse (test for cytotoxic or mutagenic effects on male germinal
cells). Proj. No: 782203. Unpublished report dated February 24,
1981, supplemented July 19, 1988 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hool, G., Langauer, M. & Müller, D. (1980). GS 19'851 tech.: Nucleus
anomaly test in somatic interphase nuclei Chinese hamster (test for
mutagenic effects on bone marrow cells). Proj No.: 790981.
Unpublished report dated August 11, 1980, supplemented July 19, 1988
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Kuhn, J.O. (1989a). GS 19'851 tech.: Acute oral toxicity study in
rats. Proj. No: 6031-89. Unpublished report dated July 11, 1989 from
Stillmeadow Inc., Houston, TX/USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Kuhn, J.O. (1989b). GS 19'851 tech.: Acute dermal toxicity study in
rabbits. Proj. No: 6032-89. Unpublished report dated April, 11, 1989
from Stillmeadow, Inc., Houston, TX/USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Kuhn, J.O. (1989c). GS 19'851 tech.: Primary eye irritation study in
rabbits. Proj. No: 6033-89. Unpublished report dated March 30, 1989
from Stillmeadow, Inc., Houston, TX/USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Kuhn, J.O. (1989d). GS 19'851 tech.: Primary dermal irritation study
in rabbits. Proj. No: 603489. Unpublished report dated April 4, 1989
from Stillmeadow, Inc., Houston, TX/USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Mathies, P. (1991). The metabolite profiles in urine, faeces and
tissue extracts of rats after administration of [U-14C]phenyl GS
19'851. Proj. No: 17/91. Unpublished report dated June 28, 1991 from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Meyer, A. & Puri, E. (1987). GS 19'851 tech.: Autoradiographic DNA
repair test on human fibroblasts (OECD-CONFORM). Proj. No: 861632.
Unpublished report dated September 10, 1987, supplemented June 21,
1988 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Paterson, R.A. & Drake, J.C. (1967) GS 19 851 13-week oral toxicity
study in rats. Final report - Amendment to the report. Unpublished
from Geigy (U.K.) Ltd., Stamford Lodge, Wilmslow, England.
Paterson, R.A. (1967a) GS 19 851 28-day oral toxicity study in rats.
Final report. Unpublished, from Geigy (U.K.) Ltd., Stamford Lodge,
Wilmslow, England.
Paterson, R.A. (1967b) GS 19 851 30-day oral toxicity study in dogs.
Final report. Unpublished, from Geigy (U.K.) Ltd., Stamford Lodge,
Wilmslow, England.
Sagelsdorff P. & Heuberger B. (1990). GS 19'851 tech.: Investigation
of the potential for DNA binding. Proj. No: PS32-03901. Unpublished
report dated November 2, 1990 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Schneider, M., Gretener, P., Froehlich, E., Beri, J., Malinowski, W.
& Basler, W., (1989). GS 19'851 tech.: 28-Day repeated dose dermal
toxicity study in the rat. Proj. No: 891009. Unpublished report
dated December 11, 1989 from Ciba-Geigy Ltd., Stein, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Schulze-Aurich, J. (1993). The metabolism of [U-14C]Phenyl GS
19'851 in the rat. Proj. No. 07PM02. Unpublished report in
preparation from Ciba-Geigy Ltd., Stein, Switzerland. To be
submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Shirasu, Y., Moriya, M. & Koyashiki, R. (1979). Mutagenicity study
with phenisobromolate in bacteria. English translation of the
unpublished report dated November 22, 1979 from The Institute of
Environmental Toxicology, Japan. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Singh, A.R. & Yau, E.T. (1990). GS 19'851 tech.: A teratology
(segment ll) study in rats. Proj. No: MIN 892112/Tox./Path. Report
No.: 89131. Unpublished report dated May 25, 1990 from Ciba-Geigy
Corp., Summit, NJ/USA. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Singh, A.R., Hazelette, J.R. & Yau, E.T. (1990). GS 19'851 tech.: A
teratology (segment ll) study in rabbits. Proj. No: MIN
892014/Tox./Path. Report No.: 89162. Unpublished report dated June
18, 1990 from Ciba-Geigy Corp. Summit, NJ/USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Stenger, E.G. (1967) Akute Toxizität, Ratte per os, Nr. 295/14217.
Unpublished report from J.R. Geigy Ltd., Basle, Switzerland.
Strasser F. (1990). GS 19'851 tech.: Chromosome studies on Chinese
hamster ovary cell line CCL 61 in vitro. Proj. No: 891515.
Unpublished report dated July 4, 1990 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Thomas, H. (1991). Inductive effects of GS 19'851 on mouse liver
cytochromes P-450 and associated monooxygenase activities. Proj. No:
CB 90/28 BY. Unpublished report dated September 16, 1991 from Ciba-
Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Toyoshima, S., Fujita, H. & Satoh, R. (1977). GS 19'851 tech.:
Recombination assay and reversion test. Unpublished report dated
February 15, 1977 from Chemotherapy Dept., The Institute of Pharma-
chemistry, Keio University and Japan Experimental Medical Research
Inst. Co, Ltd. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Ueda, K. & Kondo, T. (1968) Report on experiment of acute toxicity.
Unpublished report from Tokyo Dental College, Hygienics Laboratory,
Tokyo, Japan. Submitted by Geigy Chemical Corporation.
Ullmann, L. & Sachsse, K. (1979a). GS 19'851 tech.: Acute oral LD50
in the rabbit. Proj. No: 790968. Unpublished report dated September
5, 1979 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Ullmann, L. & Sachsse, K. (1979b). GS 19'851 tech.: Skin sensitizing
(contact allergenic) effect in guinea pigs. Proj. No: 790972.
Unpublished report dated September 5, 1979 from Ciba-Geigy Ltd.,
Stein, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Waechter, F., Bentley, P. & Staeubli, W. (1986). The effect of GS
19'851 on mouse liver drug metabolizing enzymes. Unpublished report
dated September 19, 1986 from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Wärngard, L., Flodström, S., Ljungquist, S. & Ahlborg, U.G. (1985).
Inhibition of metabolic cooperation in Chinese hamster lung
fibroblast cells (V79) in culture by various DDT-analogs. Arch.
Environ. Contam. Toxicol., 14: 541-546.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
The ethoxycoumarin O-deethylase, ethoxyresorufin O-deethylase,
pentoxyresorufin O-depentylase, and total testosterone hydroxylase
activities were also increased. The results of studies with
monoclonal antibodies against rat liver cytochrome P-450 isozymes of
four different gene families confirmed that bromopropylate shares
properties typical of a moderate phenobarbital-type inducer and
possibly is also a very weak peroxisome proliferator-type inducer.
It was concluded that bromopropylate has the properties of a
phenobarbital-like inducer of cytochrome P-450 in the mouse liver.
In addition, it might act as a very weak peroxisome proliferator.
The increased liver weights at the high-dose and the biochemical
changes were therefore regarded as a reversible adaptation to a
functional overload. At 15 mg/kg bw/day only minimal biochemical
changes were observed and the low dose of 3 mg/kg bw/day was without
any effects (Thomas, 1991).
A DNA-binding study was conducted with mice (Tif: MAG f)
pretreated for two weeks with unlabelled bromopropylate (94.2% pure)
at daily oral doses of 300 mg/kg bw. Radiolabelled [ bis-phenyl-
14C] bromopropylate was then administered by gavage at a dose level
of 300 mg/kg bw. After 24 hours, DNA was isolated from the liver
and purified. No radioactivity was detected. Bromopropylate is
devoid of a genotoxic potential mediated by DNA-binding (Sagelsdorff
& Heuberger, 1990).
Toxicological studies
Acute toxicity studies
Bromopropylate has a low acute toxicity in rats and rabbits.
WHO has classified bromopropylate as unlikely to present acute
hazard in normal use (WHO, 1992). The LD50 and LC50 values are
summarized in Table 1.
Short-term toxicity studies
Rats
Groups of 10 male and 10 female rats received 0, 40, 200, 1000
or 5000 mg bromopropylate/kg bw/day by gavage as a suspension in
0.5% aqueous tragacanth on six days each week for four weeks. The
highest dosage level produced polyuria throughout the 21 days before
the animals were killed and for the first eight days pale mucoid
faeces were produced. The rate of body-weight gain and food intake
were reduced in the 200 and 1000 mg/kg bw/day groups. Rats of the
5000 mg/kg bw/day group developed a relative neutrophilia but the
results of haematological analyses in all groups were normal. The
absolute and relative liver weights were increased in the three
highest dosage groups and cytoplasmic swelling and periportal
Table 1. Results of acute toxicity studies with bromopropylate
Species Sex Route Solvent LD50/LC50 Reference
(vehicle) (mg/kg bw)/
(mg/m3 air)
Mouse1 M oral - 8000 Ueda & Kondo, 1968
Rat1 M,F oral - > 5000 Stenger, 1967
Rat2 M,F oral - 6000 Drake, 1970
Rat2 ? oral - > 23 000 Fancher et al., 1968a,b
Rat3 M,F oral corn oil approx. 5000 Kuhn, 1989a
Rat4 M,F dermal PEG 400 > 4000 Bathe & Sachsse, 1979
Rat5 M,F inhalation ethanol > 4458 Hartmann, 1989
Rabbit4 M,F oral CMC6 > 6000 Ullmann & Sachsse, 1979a
Rabbit3 M,F dermal saline > 2020 Kuhn, 1989b
1 Purity given as Technical.
2 Purity given as Formulation.
3 Purity not given but known to be 90%.
4 90% pure.
5 95% pure.
6 CMC = 2% carboxymethylcellulose in water.
infiltration were seen. Hepatic necrosis occurred at the highest
dosage level. At 40 mg/kg bw/day, the one animal which died during
the test showed similar abnormalities in the liver; others in the
group appeared normal (Paterson, 1967a).
Groups of 12 male and 12 female rats were fed diets containing
0, 100, 300 or 1000 ppm bromopropylate for 90 days. Another group
received diets containing 3000 ppm for 55 days and 4000 ppm for a
further 35 days. At the highest dose, the food intake and rate of
weight gain were below normal and at autopsy the liver, kidney and
testes weights relative to body weight were higher than in controls.
Swelling and loss of basophil material, pigmentation (possibly
lipofuscin) and fatty infiltration were present in hepatocytes.
Mild to severe regressive changes were seen in testes on
histological examination. At 1000 ppm, food intake was slightly
less than in controls although weight gain was similar. Testes were
lighter than in controls and similar histological changes were seen
in the liver. At the two lower levels, smaller numbers of rats
showed loss of basophilic material and cytoplasmic swelling with
focal vacuolation. These changes were considered to be
physiological in that they were attributed to SER hypertrophy, but
no EM studies were carried out to confirm this. The results of
haematological and blood biochemistry studies and of urine analyses
were similar in all groups (Paterson & Drake, 1967).
Bromopropylate (94.2% pure) was applied to the shaved skin of 5
male and 5 female rats (Tif:RAI f) per dose group for 4 weeks on a
5-day per week basis. The daily exposure period was 6 hours. The
compound was given on moistened gauze patches under occlusive
dressing at dose levels of 0, 50, 200 or 1000 mg/kg bw/day. There
was no mortality, no clinical signs, no signs of local irritation
and no body weight or food consumption changes that could be
attributed to treatment. At 1000 mg/kg bw/day, slightly lower
glucose levels and a minimal increase of urea were evident in blood
chemistry, while no changes were seen in haematology. Organ
weights, macro- and microscopic histopathological findings were
similar in all groups. The NOAEL in this study was 200 mg/kg bw/day
in both sexes (Schneider et al., 1989).
Dogs
A group of two male and two female dogs received orally, by
capsule, 1000 mg/kg bw/day of bromopropylate for 30 days. A second
group received 2000 mg/kg bw/day for four days, then 500 mg/kg
bw/day for a further 26 days. Diarrhoea and vomiting occurred in
dogs of both groups. During the first week, food intake and body
weight decreased. The results of haematological examinations were
normal. Determination of organ weights did not indicate any dose-
related changes. In both groups, serum transaminase and alkaline
phosphatase levels were increased by treatment and cytoplasmic
swelling and fine vacuolation of hepatocytes were found. No mature
spermatozoa were found in male animals, but this may have been due
to the age of the animals (Paterson, 1967b).
Four groups of eight male and eight female dogs were fed for
two years on diets containing 30, 100, 250 or 1000 ppm
bromopropylate. A fifth group received 4000 ppm in the diet for
three months. A group of 10 males and 10 females acted as controls.
The highest dosage level produced light-coloured soft stools after
one week and semi-fluid stools thereafter. Food intake was reduced
and weight loss occurred. Two dogs died (after four and six weeks)
and two others were killed because they became cachectic. In these
four animals, increased serum alkaline phosphatase, diminished serum
cholesterol and mild anaemia were found. Haemosiderosis of kupfer
cells, proximal tubular cells of the kidney, the spleen and the bone
marrow, degenerative changes in the distal convoluted tubules of the
kidney, hyperplasia of bone marrow and extra-medullary
haematopoiesis were seen in these animals at autopsy. In the other
animals of the 4000 ppm group killed at three months, accumulation
of haemosiderin in the macrophages of the spleen and bone marrow and
in kupfer cells was observed. Examination of liver by EM showed SER
hypertrophy and an increase in numbers of lysosomes, many of which
contained lamellar structures. Two additional animals of each sex
were fed for nine months on control diets following three months on
4000 ppm diet. Stools returned to normal consistency within a week
and weight gain was normal. Biochemical and haematological
parameters also returned to normal in a short time; animals were
indistinguishable from controls at the time of autopsy. The 1000
ppm diet also produced softening of stools. Although weight gain by
males was normal, females failed to gain weight. Some females
showed slight anaemia on some occasions. The microsomal enzymes,
biphenyl hydroxylases, showed increased activity and this was
associated with an increase in liver weight relative to body weight
at six months and at two years (in females only). SER hypertrophy
was marked at three months and numerous myelin bodies were also
seen. Although at two years the SER hypertrophy was not different
from controls, myelin bodies were still present. An increase in
microsomal enzymes was found in the 250 ppm group together with an
enlarged liver (relative to body weight) at six months but not at
two years. No adverse effects were observed in dogs fed on 30 or
100 ppm diets (Coulston et al., 1970a).
Bromopropylate (95% pure) was administered in the diet at
concentrations of 0, 100, 400 or 2000 ppm to groups of 6 male and 6
female beagle dogs for one year. After completion of the treatment
period, subgroups of 2 animals per sex were kept for one month on
compound-free (control) diet to determine reversibility of potential
toxic effects. There were no compound-related mortalities and no
clinical signs. Body-weight gain was decreased markedly at 2000 ppm
in both sexes and slightly at 400 ppm in females from week 28
onwards. Food consumption was not affected and there were no
ophthalmic, haematological or urinary changes. The only relevant
change in blood chemistry was a slight reversible increase of
alkaline phosphatase activity in the females at 2000 ppm. At the
end of the 12-month treatment period, liver weights were slightly
increased and there was a slight hypertrophy of hepatocytes. These
changes only occurred at 2000 ppm and were not present after the
one-month recovery period. The NOAEL in this study was 100 ppm,
equal to 2.7 mg/kg bw/day (males) and 2.8 mg/kg bw/day (females)
(Gretener et al., 1989).
Long-term toxicity/carcinogenicity studies
Mice
In an oncogenicity study, mice (Charles River CD-1, 50 males
and 50 females per group) were given bromopropylate (98.7% pure) in
the diet during 18 and 20 months for males and females,
respectively, at concentrations of 0, 30, 300 or 1000 ppm. Similar
reduction in body weight was noted for the control and test groups
after 12 months of testing, males losing an average of 6 to 7 grams
and females 2 to 3 grams. This generalized reduction in body weight
occurred following alterations in both the method of feeding and the
standard diet fed. However, no statistically significant
differences in mean body weights were noted for any treatment group.
Meaningful evaluation of body-weight gain cannot be made since body
weights were not measured during the first 6 months of testing.
Negative body-weight gains were recorded for the observation period
for all groups. The mean survival time of males in the 1000 ppm
group was significantly reduced. Significantly increased
mortalities in the 30 and 1000 ppm groups were observed, which were
considered coincidental. No treatment-related findings were seen at
gross pathological examination. Histopathology revealed dose-
related centrilobular hypertrophy of hepatocytes in the two higher
doses. Additional sporadic histopathological findings were not
considered as related to treatment. The compound did not show an
oncogenic potential in this study (Charles et al., 1979).
Bromopropylate (95% pure) was administered to male and female
mice (Tif: MAGf (SPF), hybrids of inbred MIG x NIH mice) in the diet
at levels of 0, 30, 150, 1000 or 3000 ppm (60 males and 60 females
per group) for 24 months. Appearance and behaviour were not
affected by treatment. Lower body-weight gain was recorded for the
3000 ppm group over the first 12 weeks for males and the first 25
weeks for females. Thereafter, mean body weights remained 5-7% and
15-18% lower than control values, for males and females,
respectively. Food consumption was slightly lowered in females in
the 1000 and 3000 ppm groups during the first year. Water
consumption was increased at 3000 ppm. Haematological parameters
were not significantly affected by treatment. Absolute and relative
liver weights were significantly increased in both sexes at 1000 and
3000 ppm. Macroscopic examination revealed dose-related increase of
masses and nodules in the livers of male and female mice from these
two groups. Kidney weights at 3000 ppm were lower than in the
control group. On microscopic examination, hepatocellular
neoplastic lesions, i.e. benign hepatomas and hepatocellular
carcinomas, accounted for most of the macroscopically observed
masses and nodules in the liver. The frequency of these lesions was
significantly increased in both sexes at 1000 and 3000 ppm. Animals
of both sexes of these two groups also showed a frequent hypertrophy
of hepatocytes. The only other compound-related finding was an
increased incidence of reactive hyperplasia of splenic white pulp in
female mice of the high dose group (3000 ppm). The NOAEL in this
study was 150 ppm, equal to 16 mg/kg bw/day in both males and
females (Basler et al., 1990).
Rats
Groups of 50 male and 50 female rats were fed diets containing
0, 15, 30 or 100 ppm bromopropylate for two years. Five male and
five female rats from each group were killed after six months or one
year and autopsies performed. No changes in appearance or behaviour
occurred. Food consumption and weight gain were similar in test and
control animals throughout the test and survival rates were similar
for the first 18 months. Approximately half of the control female
animals alive at 18 months survived to 24 months while only a third
of test animals at 30 and 100 ppm survived over this period. No
alterations attributable to bromopropylate were observed in the
haematological examinations in test animals. No changes in organ
weights or gross or microscopic abnormalities attributable to
ingestion of the pesticide were observed in animals examined at any
time. However, EM studies on the livers of animals of the 100 ppm
group surviving two years showed slightly less glycogen, focal
enlargement of intracrestal space in the mitochondria, focal
dilatation of SER and more prominent lipid accumulations than livers
of control animals. These differences were not considered to be
significant pathological alterations in cell ultrastructure. The
number of tumours and their location were similar in control and
test groups (Coulston et al., 1970b).
Bromopropylate (95% pure) was administered in the diet to rats
(Tif: RAlf, 80 males and 80 females per dose group) at levels of 0,
100, 700 or 5000 ppm. The study duration was 119 weeks for males
and 131 weeks for females. Average intakes of bromopropylate were
3.7, 26 or 217 mg/kg bw/day for males and 4.4, 32 or 270 mg/kg
bw/day for females. In the 5000 ppm group, males maintained mean
body weights 20% lower than control values from 15 weeks onwards,
whereas females showed a progressive divergence, attaining a 37%
difference from control values at 95 weeks. Food consumption was
decreased in males and females at 5000 ppm, and water consumption
was increased in both males and females at 5000 ppm and in males at
700 ppm. Calculated red blood cell indices (MCV, MCH) were
decreased in males (from 80 weeks) and in females (from 27 weeks) at
5000 ppm, suggesting a tendency to microcytosis and hypochromoasia
of red blood cells. Increased prothrombin times were recorded in
males at weeks 12, 27, and 80, and in females at all investigated
weeks at 5000 ppm. Blood samples were collected at 12, 27, 53, 80,
105 and 131 weeks. A non-progressive lowering of blood glucose was
recorded at weeks 12 to 80 for males and at weeks 27, 53 and 80 for
females at 5000 ppm. A hyperproteinemia resulting from
hyperglobulinemia was observed in the males and females at 5000 ppm.
Females of the 700 ppm group showed a similar trend, but to a lesser
degree between weeks 27 and 105. Higher levels of cholesterol and
phospholipids were recorded at week 53 for males and on all
occasions for females at 5000 ppm. In the 700 ppm group, high
levels of cholesterol were observed in females at weeks 12, 27 and
80. Also, high levels of phospholipids were recorded in females at
12 and 80 weeks. Both sexes of the 5000 ppm group showed a marked
increase in gamma glutamyl transpeptidase activity at each
investigation.
At the interim sacrifices during weeks 53 and 105 and at the
termination of the study, relative liver weight for males and
females of the 5000 ppm group were significantly increased. In
addition, the relative liver weights for rats at 700 ppm were
significantly increased at week 53 for females and at termination
for males. Significantly increased relative kidney weights were
recorded for males and females of the 5000 ppm group at week 53 and
for females at week 105. Relative thyroid gland weights in males at
700 and 5000 ppm were significantly increased at week 53 and the
relative weight in males at 5000 ppm was also increased at
termination of the study. Macroscopic examination revealed an
increased number of rats with edema of the testes and an increased
number of females with liver cysts at 5000 ppm. In
histopathological examinations, increased incidences of focal
hepatocellular hypertrophy and fatty changes and pigmentation of
hepatocytes were also observed in both sexes at 700 and 5000 ppm.
Incidences of biliary cysts and cholangiofibrosis were increased in
females at 5000 ppm. Occurrence of lung alveolar foam cells were
increased in females at 5000 ppm. An increase in the number of rats
with testicular tubular atrophy and an associated edema and nodular
hyperplasia (Leydig cells) was observed at 5000 ppm. Numbers of
rats with follicular cystic dilatation and thyroid gland hyperplasia
were increased in both sexes at 5000 ppm. Incidence of rats with
thyroid gland adenoma was increased at 5000 ppm in males. The NOAEL
in this study was 100 ppm (equal to 3.7 mg/kg bw/day for males and
4.4 mg/kg bw/day for females) (Basler et al., 1989).
Reproduction studies
Rats
Groups of 20 male and 20 female rats in the first generation
and groups of 25 male and 25 female rats in the second and third
generations were used in a three-generation test (two litters being
produced in each generation) and fed diets containing 0, 2.5 or 5
ppm bromopropylate. In a second test, groups of 25 male and 25
female rats received diets containing 0, 30 or 100 ppm
bromopropylate. Throughout the tests no abnormalities attributable
to bromopropylate were found in the reproductive physiology of male
or female rats and there was no evidence of gross abnormalities in
the offspring throughout the studies. The number of young in each
litter and their growth and survival were normal. The weights of
liver, kidneys and spleen were comparable with controls in the rats
of the F3b generation whose parents received 2.5 or 5 ppm diets
while spleen and liver weights of the F3b young of parents fed 30
or 100 ppm diets were slightly higher than controls. No
histological abnormalities were found in these animals at any dosage
level (Coulston et al., 1971).
In a two-generation reproduction study, rats (Sprague-Dawley-
served, Tif:RAI f) were administered bromopropylate (93.5% pure) in
the diet at concentrations of 0, 165, 750 or 2250 ppm (28
rats/sex/dose level). Approximate daily intakes of bromopropylate
were 12, 54 or 170 mg/kg bw for parental males and 16, 72 or 218
mg/kg bw for parental females. The administration started 10 weeks
prior to mating of the F0 parents and was continued throughout the
mating, lactation and weaning periods of the F1 and up to weaning
of the F2 generation.
F0 parental animals showed reduced body-weight gain and food
consumption at 2250 ppm and, to a lesser extent, in the 750 ppm
group. The females of the F1 parental generation showed some
depression in food consumption at 750 and 2250 ppm prior to mating
and only at 2250 ppm during lactation. Histological changes
consisted of a dose-related slight to severe hepatocellular
enlargement at 750 ppm and 2250 ppm corresponding to liver weight
increases in the F1 animals; in addition, some of the males in the
high-dose group showed hepatocyte necrosis. Reproductive parameters
(concerning mating, fertility, pregnancy, parturition and rearing)
were not affected by the treatment. With respect to the offspring,
the F1 pups of the 2250 ppm group showed an increased mortality
rate. For both F1 and F2 young rats of the 2250 ppm group, a
diminished body-weight gain was recorded from weaning onwards.
Exploratory locomotion was depressed in the young F1 rats at 750
and 2250 ppm and in the F2 rats at 750 ppm only.
Histopathological examination of the F1 rats revealed changes
in the liver, as indicated by slight to severe hypertrophy of
hepatocytes in about two-thirds of the males and females at 750 ppm
and in all animals at 2250 ppm. In the males, necrosis (8/24 at
2250 ppm) or other structural alterations of hepatocytes (11/23 at
750 ppm, 22/24 at 2250 ppm) were noted. Parallel to these results,
absolute as well as relative liver weights were found to be
increased in a "positive trend" from the control to the high
concentration. The lowest mean daily compound intakes during the
various study periods were calculated to be approximately 9, 40 or
128 mg/kg body weight. The NOAEL in this study was 165 ppm (equal
to 9 mg/kg bw/day) (Fritz et al., 1986).
Dogs
Groups of three male and three female dogs were fed diets
containing 0, 30 or 100 ppm bromopropylate. After an unstated time,
the females were mated with males of the same group. All matings
resulted in pregnancy and delivery of normally sized litters. The
only deformed pup was found in a control litter. All others were
normal in appearance, behaviour and development. Increases in the
body weight of the pups in most of the litters of groups receiving
bromopropylate were similar to those of control pups. The reduced
growth rate of one litter of the 100 ppm group was attributed to the
results of poor maternal care (Coulston et al., 1970a; Coulston &
Benitz, 1972).
Special studies on embryo/fetotoxicity
Rats
Bromopropylate (90% pure) was administered orally by gavage to
three groups of pregnant rats (CRL: COBS CD[SD]BR, 26 females/group)
at doses of 0, 50, 300 or 700 mg/kg bw once daily during days 6
through 15 of gestation. A control group of 26 female rats received
an equivalent volume (10 ml/kg bw) of aqueous 3% corn starch with
0.5% Tween 80. All surviving animals were sacrificed on day 20 of
presumed gestation. Evaluations were conducted on clinical signs,
maternal body weights, food consumption, necropsy and skeletal
abnormalities. Compound-related effects were noted at 300 and 700
mg/kg bw/day. These changes included: an increased incidence of
salivation and alopecia accompanied by skin irritation; transient
body-weight loss and slight reductions in daily food consumption at
700 mg/kg bw/day and of body-weight gain at 300 mg/kg bw/day; slight
decreases in mean male and female fetal weight at 700 mg/kg bw/day;
and an increased incidence of skeletal variations of fully formed
14th ribs and rudimentary 14th ribs at 300 and 700 mg/kg bw/day and
in forepaw metacarpals not ossified at 700 mg/kg bw/day. The fetal
findings were considered to be slight treatment-related effects
associated with the compound-induced maternal toxicity. There was
no evidence of embryo/fetotoxicity or teratogenicity at doses up to
and including 700 mg/kg bw/day. The maternal NOAEL in this study
was 50 mg/kg bw/day (Singh & Yau, 1990).
Rabbits
Bromopropylate (90% pure) was administered orally by gavage to
three groups of pregnant rabbits (New Zeeland white, 20
females/group) at doses of 0, 20, 60, or 120 mg/kg bw once daily
during gestation days 7 through 19. A control group of 20 pregnant
rabbits received an equivalent volume (5 ml/kg bw) of the vehicle.
All surviving animals were sacrificed on day 29 of presumed
gestation. Evaluations were conducted on clinical signs, maternal
body weights, food consumption, necropsy, reproductive parameters,
fetal weights and fetal gross, visceral and skeletal observations.
Treatment-related maternal effects were observed at 60 and 120 mg/kg
bw/day. These changes included: mortality/moribundity of two
animals at 120 mg/kg bw/day; clinical signs of lethargy at 120 mg/kg
bw/day and anogenital staining at 60 and 120 mg/kg bw/day;
reductions in mean body weight at 120 mg/kg bw/day, mean body-weight
gain at 60 and 120 mg/kg bw/day, and reduction of food consumption
at 120 mg/kg bw/day; and the necropsy finding of distended stomach
with viscous/gelatinous material at 120 mg/kg bw/day. There was no
evidence of embryotoxicity, fetotoxicity or teratogenicity at any
dose level. The NOAEL for maternal toxicity was 20 mg/kg bw/day
(Sing et al., 1990).
Special studies on genotoxicity
The results of genotoxicity studies conducted with bacteria,
mammalian cells in vitro and with intact animals in vivo are
presented in Tables 2a, b and c. In all studies, bromopropylate was
devoid of mutagenic potential.
Special studies on inhibition of cell-cell communication and liver
tumour promotion
Bromopropylate was investigated in vitro for its ability to
inhibit gap junctional intercellular communication in the Chinese
hamster fibroblast cell V79 metabolic cooperation assay and in the
scrape-loading/dye-transfer assay in WBF344 rat liver epithelial
cells, and in vivo altered hepatic foci assay and hepatic drug
metabolizing activity in male Sprague-Dawley rats. Technical grade
bromopropylate (94% pure) induced a dose-dependent increase in the
recovery of 6-thioguanine-resistant mutants within the same dose
range as reported earlier for the analytical grade (Wärngard et
al., 1985). In the scrape-loading assay, intercellular
communication was abolished at non-cytotoxic concentrations both
with technical and analytical grade bromopropylate. There was a
gradual decrease in dye transfer from the lowest concentration (20
µM) up to the dose inducing complete blockade of dye transfer.
Bromopropylate was shown to cause hepatomegaly and induction of
cytochrome P-450b-related O-dealkylase enzyme activities in hepatic
microsomes when fed to rats at 1000 ppm for 11 weeks, indicating a
phenobarbital-type induction of hepatic metabolism.
The rats were initiated by partial (2/3) hepatectomy and 24
hours later received a single intraperitoneal dose of 30 mg
nitrosodiethylamine/kg bw. From week 2 after initiation until
termination at week 13, the animals were fed a diet containing 1000
ppm bromopropylate. A group of non-hepatectomized, non-
diethylnitrosamine-treated rats was also fed the bromopropylate-
containing food. Appropriate control groups received the control
diet. Bromopropylate enhanced the incidence of GGT-positive foci
and the percentage of liver occupied by foci's tissue in initiated
rats (Flodström et al., 1990).
Table 2a. Mutagenicity tests in bacteria in vitro
Test Test object S-9 mix Concentrations Purity Results Reference
Escherichia coli WP2hcr +/- 10-5000 µg/plate 99.7% - Shirasu et al., 1979
Escherichia coli B/r WP2 try- 500-2000 µg/disc ? - Toyoshima et al., 1977
SD-4
Bacillus subtilis rec+(H17) 20-2000 µg/disc 99.7% - Shirasu et al., 1977
rec-(M45)
Bacillus subtilis rec+(H17) 50-2000 µg/disc ? - Toyoshima et al., 1977
rec-(M45)
Salmonella TA98 +/- 10-5000 µg/plate 99.7% - Shirasu et al., 1979
typhimurium TA100 +/- -
TA1535 +/- -
TA1537 +/- -
TA1538 +/- -
TA98 +/- 313-5000 µg/0.1 ml 94.2% - Deparade, 1989
TA100 +/- -
TA1535 +/- -
TA1537 +/- -
Table 2b. Mutagenicity tests in mammalian cells in vitro
Test Test object Concentrations Purity Results Reference
Chromosomal Chinese hamster 3.13-25 µg/ml Batch op. - Strasser, 1990
aberrations ovary cells with 406162
and without S-9 94.2%
mix
DNA repair human fibroblasts 0.5-60 µg/ml Batch op. - Meyer & Puri,
(UDS) 406162 1987
95%
DNA repair rat hepatocytes 0.4-40 µg/ml Batch op. - Hertner & Puri,
(UDS) 406162 1987
95%
Table 2c. Mutagenicity tests in mammalian cells in vivo
Test Test object Dose levels Purity Results Reference
Chromosomal Mouse five times and batch P 43/78 - Hool & Müller,
aberrations spermatocytes 3000 mg/kg 90% 1989a
within 10 days
Chromosomal Mouse five times and batch P 43/78 - Hool & Müller,
aberrations spermatogonia 3000 mg/kg on 90% 1989b
five consecutive
days
Nucleus Chinese 2500, 5000 and batch P 43/78 - Hool et al.,
anomaly test hamster, 10 000 mg/kg 90% 1980
bone marrow twice on two
consecutive days
Dominant Male mice single oral doses batch Mg. - Hool & Müller,
lethal test of 0, 1100 or 68/77 1981
3300 mg/kg 91.5%
Special studies on skin and eye irritation
A primary eye irritation test on bromopropylate (90% pure) was
performed by using 3 male and 3 female New Zeeland white rabbits.
Irritation observed after the application of 100 mg of undiluted
test material into the conjunctival sac consisted of reversible
redness, chemosis and discharge in all rabbits and iridal
involvement in one rabbit. The compound was given a descriptive
rating "mildly irritant" in non-washed eyes and in washed eyes, with
maximum average irritation scores of 9.3 and 3.3, respectively.
However, the mean values of the scores calculated from the 24, 48
and 72 hour readings over all tested animals were 0.07 for iris
lesions, 0.6 for redness, and 0.3 for edema of conjunctiva.
According to the EEC Directive 83/467 the test substance is
therefore classified as "non-irritant" to eyes (Kuhn, 1989c).
A primary skin irritation test on bromopropylate (90% pure) was
performed using 3 male and 3 female New Zeeland white rabbits.
Irritation observed on the skin after the application of 500 mg of
test material moistened with 0.4 ml of saline consisted of grade 1
erythema in 5 out of 6 rabbits at the 1 hour reading time. The
compound was given a descriptive rating "slight irritant". However,
the mean values of the scores calculated from the 24, 48 and 72 hour
readings over all tested animals were 0 for erythema and 0 for
edema. According to the EEC Directive 83/467 the test substance is
therefore classified as "non-irritant" to skin (Kuhn, 1989d).
Special studies on skin sensitization
A sensitization assay with bromopropylate (90% pure) using
Freund's complete adjuvant during the induction period (optimization
test) was conducted at a concentration of 0.1% in propylene glycol
as vehicle in guinea-pigs (Pirbright white strain, 10 males and 10
females/group). Significant differences between the test group
(14/20) and vehicle-treated controls (4/20) were seen only after
intradermal challenge application, i.e. when the skin barrier was
intentionally by-passed. No difference between the test (0/19) and
the control (0/20) groups was seen after epidermal challenge
application. It was therefore concluded that, in artificially
sensitized guinea-pigs, exposure of intact skin does not provoke
contact dermatitis (Ullmann & Sachsse, 1979b).
Observations in humans
No information available.
COMMENTS
After oral administration of [U-14C]phenyl bromopropylate,
most of the radioactivity was eliminated in the faeces, with lower
amounts of radioactivity excreted in the urine. The routes of
elimination were sex-dependent.
Bromopropylate was metabolized preferentially by cleavage of
the isopropyl ester, and to a minor extent by oxidation reactions
attacking the phenyl ring and the isopropyl group.
Bromopropylate has the properties of a phenobarbital-like
inducer of cytochrome P-450 in the mouse liver. In a DNA-binding
study conducted with mice, no radioactivity was detectable in liver
DNA, indicating that bromopropylate is devoid of genotoxic potential
in this tissue.
Bromopropylate has a low acute toxicity in rats and rabbits.
WHO has classified bromopropylate as unlikely to present acute
hazard in normal use.
In a one-year study in dogs at dietary concentrations of 0,
100, 400 or 2000 ppm, the NOAEL was 100 ppm (equal to 2.7 mg/kg
bw/day), based on depressed body-weight gain at 400 ppm and above.
In a study in mice using dietary concentrations of 0, 30, 150,
1000 or 3000 ppm for 24 months, the NOAEL was 150 ppm (equal to 16
mg/kg bw/day), based on increased absolute and relative liver weight
and hepatocellular neoplastic lesions at 1000 ppm and above.
Long-term toxicity/carcinogenicity studies in rats were
reviewed. The study considered by the 1973 Joint Meeting was found
to be unacceptable. In a new study at dietary concentrations of 0,
100, 700 or 5000 ppm, the NOAEL was 100 ppm (equal to 3.7 mg/kg
bw/day), based on increased water consumption and increased relative
liver and thyroid weights at 700 ppm and above. Increased
incidences of focal hepatocellular hypertrophy and fatty changes and
pigmentation of hepatocytes were also observed at 700 ppm and above.
In a reproduction study in rats using dietary concentrations of
0, 165, 750 or 2250 ppm, the NOAEL was 165 ppm (equal to 9 mg/kg
bw/day), based on increased liver weight and hypertrophy of
hepatocytes in F1 animals at 750 ppm and above.
Teratogenicity studies were conducted with rats and rabbits.
In the study in rats at doses of 0, 50, 300 or 700 mg/kg bw/day,
depressed maternal body-weight gain and an increased incidence of
skeletal variations of fully formed 14th ribs and rudimentary 14th
ribs were recorded at 300 mg/kg bw/day and above. The maternal
NOAEL in this study was 50 mg/kg bw/day. There was no evidence of
embryo/fetotoxicity or teratogenicity. In the study in rabbits at
doses of 0, 20, 60, or 120 mg/kg bw/day, mean body-weight gain was
depressed at 60 mg/kg bw/day. The NOAEL for maternal toxicity was
20 mg/kg bw/day, and no embryo/fetotoxic or teratogenic effects were
found.
After reviewing the available genotoxicity data, the Meeting
concluded that bromopropylate was not genotoxic.
An ADI was established based on the NOAEL of 2.7 mg/kg bw/day
in the one-year study in dogs, using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 150 ppm, equal to 16 mg/kg bw/day (two-year study)
Rat: 100 ppm, equal to 3.7 mg/kg bw/day (two-year study)
Dog: 100 ppm, equal to 2.7 mg/kg bw/day (one-year study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Basler, W., Hunter, B., Gretener, P., Froehlich, E., Faccini, J.M.,
Christen, P., Seewald, W., Dieterle, R. & Gfeller, W. (1989). GS
19'851 tech.: Lifetime carcinogenicity and chronic toxicity study in
rats. Proj. No: 831417. Unpublished report dated October 26, 1989
from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Basler, W., Hunter, B., Gretener, P., Froehlich, E., Krinke, G.,
Christen, P., Dieterle, R. & Gfeller, W. (1990). GS 19'851 tech.:
Lifetime carcinogenicity study in mice. Proj. No: 850334.
Unpublished report dated February 22, 1990 from Ciba-Geigy Ltd.,
Stein, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Bathe, R. & Sachsse, K. (1979). GS 19'851 tech.: Acute dermal LD50
in the rat. Proj. No: 790969. Unpublished report dated October 9,
1979 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Cassidy, J.E., Mattson, A., Cullen, T., & Min, B. (1968) The
metabolic fate of 14C GS 19851 administered to a cow by capsules.
Unpublished report from Geigy Chemical Corporation, Ardsley, N.Y.
Charles, J.M., Stevens, J.T., Sumner, D.D., Barnett, J.W. & Ellis,
J.F. (1979). GS 19'851 tech.: Carcinogenicity evaluation in albino
mice. Proj. No: 62206726. Unpublished report dated July 25, 1979
from Ciba-Geigy Corp., Greensboro, NC/USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland .
Cornelissen K. & Hopkins R. (1991). [U-14C]phenyl GS 19'851:
Absorption, distribution, and excretion in the rat. Proj. No: 6453-
380/151. Unpublished report dated April 3, 1991 from Hazleton UK,
Harrogate. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Coulston, F., Fabian, R.J., Abraham, R., & Benitz, K.F. (1970a) Two-
year safety evaluation of isopropyl 4,4'-dibromobenzilate in beagle
dogs. Unpublished report from Institute of Experimental Pathology
and Toxicology, Albany Medical College, Albany, N.Y. Submitted by
Geigy Chemical Corporation.
Coulston, F., Fabian R.J., & Benitz, K.F. (1970b) Two-year safety
evaluation study of isopropyl 4,4'-dibromobenzilate in albino rats
by dietary feeding. Unpublished report from Institute of
Experimental Pathology and Toxicology, Albany Medical College,
Albany, N.Y. Submitted by Geigy Chemical Corporation.
Coulston, F., Le Fevre, R., & Fabian, R. (1971) Three generation
study with GS 19 851 in albino rats. Unpublished report from the
Institute of Experimental Pathology and Toxicology, Albany Medical
College, Albany, N.Y. Submitted by Geigy Chemical Corporation.
Coulston F. & Benitz, K.F. (1972) Supplemental data on studies made
with GS 19 851 in beagle dogs. Unpublished report from the Institute
of Experimental Pathology and Toxicology, Albany Medical College,
Albany, N.Y. Submitted by Geigy Chemical Corporation.
Deparade E. (1989). GS 19'851 tech.: Salmonella/mammalian-
microsome mutagenicity test. Proj. No: 881537. Unpublished report
dated May 19, 1989 from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Drake, J.C. (1970) A 2443 A - Acute median lethal dose in rats.
Unpublished report from Geigy (U.K.) Ltd., Stamford Lodge, Wilmslow,
England.
Fancher, O.E., Schoenig, G., & Keplinger, M.L. (1968a) Acute
toxicity studies on GS 19 851 2 E (GA-494)- IBT No. A 6194.
Unpublished report from the Industrial Bio-Test Laboratories
Incorporated, Northbrook, Illinois. Submitted by Geigy Chemical
Corporation.
Fancher, O.E., Schoenig, G., & Keplinger, M.L. (1968b) Acute
toxicity studies on GS 19 851 25 W (GA-481) IBT No. A 6196.
Unpublished report from the Industrial Bio-Test Laboratories
Incorporated, Northbrook, Illinois. Submitted by Geigy Chemical
Corporation.
Flodström, S., Hemming, H., Wärngard, L. & Ahlborg, U.G. (1990).
Promotion of altered hepatic foci development in rat liver,
cytochrome P450 enzyme induction and inhibition of cell-cell
communication by DDT and some structurally related organohalogen
pesticides. Carcinogenesis, 11(8): 1413-1417.
Fritz, H., Suter, P., Zak, F., Malinowski, W. & Hess, R. (1986). GS
19'851 tech.: Two-generation reproduction toxicity study in rats.
Proj. No: 831523. Unpublished report dated December 1986 from Ciba-
Geigy Ltd., Stein, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Gretener, P., Froehlich, E., Malinowski, W., Christen, P. & Gfeller,
W. (1989). GS 19'851 tech.: 12-month chronic dietary toxicity study
in beagle dogs. Proj. No: 860018. Unpublished report dated May 11,
1989 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Hartmann, H.R. (1989). GS 19'851 tech.: Acute inhalation toxicity in
the rat. Proj. No: 881279. Unpublished report dated April 11, 1989
from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Hertner, Th. & Puri, E. (1987). GS 19'851 tech.: Autoradiographic
DNA-repair test on rat hepatocytes (OECD-CONFORM). Proj. No: 861631.
Unpublished report dated September 18, 1987 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hool, G. & Müller, D. (1980a). GS 19'861 tech.: Chromosome studies
in male germinal epithelium mouse (test for mutagenic effects on
spermatocytes). Proj. No: 790980. Unpublished report dated September
16, 1980, supplemented July 19, 1988 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hool, G. & Müller, D. (1980b). GS 19'851 tech.: Chromosome studies
in male germinal epithelium (test for mutagenic effects on
spermatogonia). Proj. No: 790979. Unpublished report dated June 17,
1980, supplemented July 19, 1988 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hool, G. & Müller, D. (1981). GS 19'851 tech.: Dominant lethal study
mouse (test for cytotoxic or mutagenic effects on male germinal
cells). Proj. No: 782203. Unpublished report dated February 24,
1981, supplemented July 19, 1988 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hool, G., Langauer, M. & Müller, D. (1980). GS 19'851 tech.: Nucleus
anomaly test in somatic interphase nuclei Chinese hamster (test for
mutagenic effects on bone marrow cells). Proj No.: 790981.
Unpublished report dated August 11, 1980, supplemented July 19, 1988
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Kuhn, J.O. (1989a). GS 19'851 tech.: Acute oral toxicity study in
rats. Proj. No: 6031-89. Unpublished report dated July 11, 1989 from
Stillmeadow Inc., Houston, TX/USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Kuhn, J.O. (1989b). GS 19'851 tech.: Acute dermal toxicity study in
rabbits. Proj. No: 6032-89. Unpublished report dated April, 11, 1989
from Stillmeadow, Inc., Houston, TX/USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Kuhn, J.O. (1989c). GS 19'851 tech.: Primary eye irritation study in
rabbits. Proj. No: 6033-89. Unpublished report dated March 30, 1989
from Stillmeadow, Inc., Houston, TX/USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Kuhn, J.O. (1989d). GS 19'851 tech.: Primary dermal irritation study
in rabbits. Proj. No: 603489. Unpublished report dated April 4, 1989
from Stillmeadow, Inc., Houston, TX/USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Mathies, P. (1991). The metabolite profiles in urine, faeces and
tissue extracts of rats after administration of [U-14C]phenyl GS
19'851. Proj. No: 17/91. Unpublished report dated June 28, 1991 from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Meyer, A. & Puri, E. (1987). GS 19'851 tech.: Autoradiographic DNA
repair test on human fibroblasts (OECD-CONFORM). Proj. No: 861632.
Unpublished report dated September 10, 1987, supplemented June 21,
1988 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Paterson, R.A. & Drake, J.C. (1967) GS 19 851 13-week oral toxicity
study in rats. Final report - Amendment to the report. Unpublished
from Geigy (U.K.) Ltd., Stamford Lodge, Wilmslow, England.
Paterson, R.A. (1967a) GS 19 851 28-day oral toxicity study in rats.
Final report. Unpublished, from Geigy (U.K.) Ltd., Stamford Lodge,
Wilmslow, England.
Paterson, R.A. (1967b) GS 19 851 30-day oral toxicity study in dogs.
Final report. Unpublished, from Geigy (U.K.) Ltd., Stamford Lodge,
Wilmslow, England.
Sagelsdorff P. & Heuberger B. (1990). GS 19'851 tech.: Investigation
of the potential for DNA binding. Proj. No: PS32-03901. Unpublished
report dated November 2, 1990 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Schneider, M., Gretener, P., Froehlich, E., Beri, J., Malinowski, W.
& Basler, W., (1989). GS 19'851 tech.: 28-Day repeated dose dermal
toxicity study in the rat. Proj. No: 891009. Unpublished report
dated December 11, 1989 from Ciba-Geigy Ltd., Stein, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Schulze-Aurich, J. (1993). The metabolism of [U-14C]Phenyl GS
19'851 in the rat. Proj. No. 07PM02. Unpublished report in
preparation from Ciba-Geigy Ltd., Stein, Switzerland. To be
submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Shirasu, Y., Moriya, M. & Koyashiki, R. (1979). Mutagenicity study
with phenisobromolate in bacteria. English translation of the
unpublished report dated November 22, 1979 from The Institute of
Environmental Toxicology, Japan. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Singh, A.R. & Yau, E.T. (1990). GS 19'851 tech.: A teratology
(segment ll) study in rats. Proj. No: MIN 892112/Tox./Path. Report
No.: 89131. Unpublished report dated May 25, 1990 from Ciba-Geigy
Corp., Summit, NJ/USA. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Singh, A.R., Hazelette, J.R. & Yau, E.T. (1990). GS 19'851 tech.: A
teratology (segment ll) study in rabbits. Proj. No: MIN
892014/Tox./Path. Report No.: 89162. Unpublished report dated June
18, 1990 from Ciba-Geigy Corp. Summit, NJ/USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Stenger, E.G. (1967) Akute Toxizität, Ratte per os, Nr. 295/14217.
Unpublished report from J.R. Geigy Ltd., Basle, Switzerland.
Strasser F. (1990). GS 19'851 tech.: Chromosome studies on Chinese
hamster ovary cell line CCL 61 in vitro. Proj. No: 891515.
Unpublished report dated July 4, 1990 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Thomas, H. (1991). Inductive effects of GS 19'851 on mouse liver
cytochromes P-450 and associated monooxygenase activities. Proj. No:
CB 90/28 BY. Unpublished report dated September 16, 1991 from Ciba-
Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Toyoshima, S., Fujita, H. & Satoh, R. (1977). GS 19'851 tech.:
Recombination assay and reversion test. Unpublished report dated
February 15, 1977 from Chemotherapy Dept., The Institute of Pharma-
chemistry, Keio University and Japan Experimental Medical Research
Inst. Co, Ltd. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Ueda, K. & Kondo, T. (1968) Report on experiment of acute toxicity.
Unpublished report from Tokyo Dental College, Hygienics Laboratory,
Tokyo, Japan. Submitted by Geigy Chemical Corporation.
Ullmann, L. & Sachsse, K. (1979a). GS 19'851 tech.: Acute oral LD50
in the rabbit. Proj. No: 790968. Unpublished report dated September
5, 1979 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Ullmann, L. & Sachsse, K. (1979b). GS 19'851 tech.: Skin sensitizing
(contact allergenic) effect in guinea pigs. Proj. No: 790972.
Unpublished report dated September 5, 1979 from Ciba-Geigy Ltd.,
Stein, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Waechter, F., Bentley, P. & Staeubli, W. (1986). The effect of GS
19'851 on mouse liver drug metabolizing enzymes. Unpublished report
dated September 19, 1986 from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Wärngard, L., Flodström, S., Ljungquist, S. & Ahlborg, U.G. (1985).
Inhibition of metabolic cooperation in Chinese hamster lung
fibroblast cells (V79) in culture by various DDT-analogs. Arch.
Environ. Contam. Toxicol., 14: 541-546.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
