
Nitrates and nitrites
NITRATES AND NITRITES
International Programme on Chemical Safety
Poisons Information Monograph (Group Monograph) G016
Chemical
1. NAME
1.1 Substances
Nitrates and nitrites of
1.2 Group
Sodium nitrite
Potassium nitrite
Calcium nitrite
Magnesium nitrite
Sodium nitrate
Potassium nitrate
Calcium nitrate
Magnesium nitrate
Ammonium nitrate
1.3 Synonyms
Potassium nitrate: saltpetre; nitre;
Sodium nitrate: Chilean saltpetre; cubic nitre; soda nitre;
Ammonium nitrate: Ammonium salpetre;
Calcium nitrate: Norway salpetre; lime salpetre;
Magnesium nitrate: nitromagnesite (hydrated form,
mineral which is occurs in nature);
Sodium nitrite: erinitrit;
1.4 Identification numbers
1.4.1 CAS number
Sodium nitrate: 7631-99-4
1.4.2 Other numbers
Potassium nitrate: 7757-79-1
Ammonium nitrate: 6484-52-2
Calcium nitrate: 10124-37-5
Calcium nitrate hydrated: 13477-34-4
Magnesium nitrate: 10377-60-3
Magnesium nitrate hydrated: 13446-18-9
Sodium nitrite: 7632-00-0
Potassium nitrite: 7758-09-0
Calcium nitrite: 13780-06-8
Calcium nitrite hydrated: 10031-34-2
Magnesium nitrite: 15070-34-5
Ammonium nitrite: 13446-48-5
RTECS numbers
Sodium nitrate: WC5600000
Potassium nitrate: TT3700000
Ammonium nitrate: BR9050000
Calcium nitrate:
Calcium nitrate hydrated: EW3000000
Magnesium nitrate: OM3750000
Magnesium nitrate hydrated: OM3756000
Sodium nitrite: RA1225000
Potassium nitrite: TT3750000
Ammonium nitrite: RA0770000
UN transportation number
inorganic nitrates: 1477 (class 5.1)
inorganic nitrites: 2627 (class 5.1)
Calcium nitrate: 1454
Sodium nitrate: 1498
Sodium nitrate & Potassium nitrate mixture: 1499
Potassium nitrate: 1486
Potassium nitrate & Sodium nitrite mixture: 1487
Potassium nitrite: 1488
Sodium nitrite: 1500
Ammonium nitrate fertilizer: 2067,
2068,,
2069,,
2070,,
2072 (class 5.1),;
0223 (class 1.1D),;
2071 (class 9)
Ammonium nitrite: 0222 (class 1.1D),;
1942 (class 5.1)
Ammonium nitrate liquid (hot concentrated solution): 2426 (class 5.1)
EEC number
Potassium nitrite: 007-011-00-X
Sodium nitrite: 007-010-00-4
1.5 Brand names/Trade names
1.6 Manufacturers/Importers
2. SUMMARY
2.1 Main risks and target organs
The major acute toxic effect of nitrate and nitrite
poisoning is methaemoglobinaemia. Blood is the target organ.
Methaemoglobin reduces the oxygen-carrying capacity of the
blood and in addition, it shifts the oxyhaemoglobin
dissociation curve to the left interfering with the unloading
of oxygen.
Hypotension and collapse may also occur.
The principal concern with exposure to nitrate is its
biological reduction to reactive and toxic nitrite. Nitrate
itself is rather harmless.
2.2 Summary of clinical effects
Hematological effects include blue-greyish cyanosis that
may appear within a few minutes to 45 minutes or more after
exposure.
Stupor, coma and convulsions in severe poisoning due to
severe hypoxia. Tachycardia, hypotension and collapse may
also occur. Nausea, vomiting and abdominal pain may be
seen.
2.3 Diagnosis
The diagnosis is based on: (i) the clinical presentation
of the patient, mainly a central cyanosis in absence of
cardiac or pulmonary cause; (ii) the brownish colour of blood
and high levels of methaemoglobinaemia (which correlate well
with clinical symptoms); and (iii) circumstances of exposure.
Other relevant laboratory analyses are: arterial blood gases,
acid base balance, nitrates could be measured in urine. The
levels are usually below 150 mg NO3-/day).
2.4 First-aid measures and management principles
Induce emesis and/or gastric lavage if ingestion was
recent.
Administer activated charcoal.
Monitor vital signs, blood pressure, respiration and onset of
cyanosis.
Administer oxygen if there are clinical signs of
methaemoglobinaemia.
Methylene blue is the specific antidote indicated in case of
methaemoglobinemia.
3. PHYSICO-CHEMICAL PROPERTIES
3.1 Origin of substance
Nitrates can be of natural and synthetic origin.
Nitrate is an important metabolite in the biological nitrogen
cycle, produced during nitrification of reduced nitrogen
compounds. It is a natural constituent of soil and
vegetation. Nitrate is also a normal metabolite in
mammals.
Nitrate in soil, ground and surface water derive mainly from
mineralization of soil organic matter; some also from
application of mineral fertilizers.
The major natural deposit of nitrates is that of sodium
nitrate (Chilean saltpetre) in northern Chile.
Nitrates are produced on a large scale from nitric acid made
from ammonia by catalytic oxidation. Nitrates are formed
from the reaction of nitric acid with ammonia or minerals
(e.g. phosphate rock) to give ammonium nitrate and water
soluble salts used as fertilizers.
Nitrite is also a metabolite in the biological nitrogen
cycle; an intermediate in both nitrification and
denitrification. It is also a normal metabolite in
mammals.
Nitrites in commercial use are all of synthetic origin. They
are made mainly by dissolving nitrogen oxides (NO and NO2)
in alkaline solutions.
Nitrites can also be prepared by reduction of nitrates.
3.2 Chemical structure
The structural formula of nitrate is:
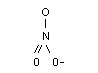 Molecular weight: 62.05
The structural formula of nitrite is: O == N -- O-
Molecular weight: 46.006
3.3 Physical properties
3.3.1 Colour
See individual nitrate compounds in Section 3.3.3
3.3.2 State/form
See individual nitrate compounds in Section 3.3.3
3.3.3 Description
NaNO3: melting point = 308°C; decomposes at
380°C; solubility in H20 = 9225 g/100 mL; soluble in
alcohol; density = 2.26.
KNO3: melting point = 334°C; decomposes at 400°C;
solubility in H20 = 13.3°; insoluble in alcohol;
insoluble in ether; density = 2.109.
NH4NO3: melting point = 170°C; decomposes at 210°C;
solubility in H2O = 118°; solubility in alcohol =
3.820; insoluble in ether; density = 1.72.
Ca(NO3)2: melting point = 561°C; solubility in
water = 12118; solubility in alcohol = 1415; insoluble
in ether.
Ca(NO3)2.3H20: melting point = 51°C
Ca(NO3)2.4H20: melting point = approx.40°C;
decomposes at 132°C; solubility in water = 266°;
soluble in alcohol.
Mg(NO3)2.2H20: melting point = 129°C; soluble in
water; soluble in alcohol.
Mg(NO3)2.6H20: melting point = 89°C; decomposes
at 330°C; solubility in water = 125b; soluble in
alcohol.
NaNO2: melting point = 271°C; decompose at 320°C;
solubility in water = 8215; slightly soluble in water;
solubility in alcohol = 0.320;
KNO2: melting point = 440°C; decomposes at 350°C a;
solubility in water = 281°; soluble in hot
alcohol.
Ca(NO2)2.H2O: melting point = 100°C; solubility
in water= 46°; slightly soluble in alcohol.
Ca(NO2)2.4H2O: solubility in water= 75°; soluble
in alcohol.
Mg(NO2).3H2O: decomposes at 100°C; soluble in
cold water; soluble in alcohol.
Key:
a: decomposition starts at 350°C
b: temperature not specified (cold water)
upper case number denotes temperature (°C)
(Weast, l982)
Sodium nitrate: colourless transparent crystals or
white granules or powder, with saline, slightly bitter
taste, deliquesces in moist air.
Potassium nitrate: colourless transparent prisms or
white, granular or crystalline powder with cooling,
saline pungent taste.
Ammonium nitrate: odourless, transparent, hygroscopic
deliquescent crystals or white granules. Five solid
phases exist at normal pressure. Orthorhombic at room
temperature.
Calcium nitrate: deliquescent, colourless granules.
Evolve heat when dissolved in water.
Magnesium nitrate hexahydrate: colourless, clear,
deliquescent crystals.
Sodium nitrite: white or slightly yellow deliquescent
granules or rods decomposed even by weak acids with
evolution of brown fumes of nitrous anhydride.
Calcium nitrite: white or yellowish deliquescent
hexagonal crystals.
Ammonium nitrite: white/yellowish crystals decomposed
in hot water.
(Budavari, 1996)
The water solutions of nitrates usually have a pH in
the range of 5 to 8. The water solutions of nitrites
are slightly alkaline (pH 9).
3.4 Hazardous characteristics
Under normal conditions, both nitrites (except the
ammonium salt) and nitrates are stable compounds. However,
at higher temperatures they decompose, and may be explosive
at extreme conditions (high temperature and pressure).
Nitrites also decompose in weak acids. Generally, the
presence of chlorides, some metals and organic material
destabilize both nitrates and nitrites. Nitrates form NO and
NO2 upon thermal decomposition, but ammonium nitrate also
forms N2O, N2 and H2O depending on temperature.
Nitrites mainly decompose to N2 and NO. N2O3 can also be
formed. Ammonium nitrite is unstable and decomposes to N2
and H2O. Nitrites oxidize slowly to nitrates when exposed
to air.
Toxic gases can form if buildings, where nitrates or nitrites
are stored, catch fire. If these materials are enclosed or
contaminated with combustible matter, fire may result in an
explosion.
Salts of nitrate are odourless and colourless with a saline
taste. They are generally hygroscopic.
Salts of nitrite are colourless or slightly yellow. They are
generally hygroscopic.
Environmental concerns:
Nitrate is a nutrient for algae and other microorganisms, and
excesses can contribute towards excessive algal growth in
natural waters (eutrophication). Microbial processes in soil
and water transform nitrate to nitrogen gas (N2), a process
known as denitrification. Some nitrous oxide (N2O) a
greenhouse gas, is also formed.
4. USES/HIGH RISK CIRCUMSTANCES OF POISONING
4.1 Uses
4.1.1 Uses
4.1.2 Description
The major use of nitrate is as fertilizer. It
is also used in the manufacture of nitrites, nitrous
oxide, explosives, pyrotechnics, matches, freezing
mixtures and special cements. It is also used as a
colouring and preserving additive to food, for
coagulation of latexes, in the nuclear industry and
for odour (sulphide) and corrosion control in aqueous
systems.
Nitrite is used as a food preservative and colouring
agent, e.g. curing of meat, in the manufacture of
diazo dyes and rubber, in the textile industry and in
photography. Nitrite is also used in analytical and
preparative chemistry, as a corrosion inhibitor and as
an antidote in cyanide poisoning.
4.2 High risk circumstances of poisoning
Accidental exposure:
Accidental addition of nitrates/nitrites to food in mistake
for common salt has resulted in poisoning.
Intentional exposure:
Employees with access to nitrites at work, e.g. laboratory
personnel, have occasionally attempted suicide through
ingestion of nitrite.
Medical exposure:
Mashed carrot widely used to treat infant diarrhoea has
occasionally resulted in intake of toxic amounts of nitrate
(ECETOC, 1988).
Sodium nitrite given intravenously is traditionally used as
an antidote in cyanide poisoning (see Section 5.5).
Other types of exposure:
The major concern of nitrate and nitrite is associated with
intake of food and water. Drinking water contains variable
amounts of nitrate. The statutory limits vary slightly from
country to country, but is usually either maximum 10 mg
NO3--N/L(= 44.3 mg NO3-/L) in the USA or maximum 50 mg
NO3-/L in the EU.
Plants contain nitrate as a normal cell constituent and
vegetables are usually the main dietary source of nitrate.
Normal daily intake varies with dietary customs, 50 to 150 mg
NO3-/day seems typical for a western diet. Vegetarians can
exceed this, with daily intakes of over 300 mg NO3- (Walker,
1990).
Dietary exposure to nitrites is normally very low, commonly
<2 mg NO2-/day and usually <5 mg/day per capita (<0.1
mg/kg/day) (Walker, 1990). Exceptionally, higher levels may
result from microbial reduction of nitrates in hygienically
poor quality well water or in foods rich in nitrates stored
under inappropriate conditions. Bacterial reduction of
nitrate secreted in the saliva and gastric juice is usually
the source of nitrite (Eisenbrand et al., 1980; Mueller et
al., 1986). NAS (1981) estimated that approximately 3.5 mg
NO2- is formed per day in an average adult in the USA. This
process is dependent upon several factors (e.g. nitrate
intake) and varies substantially between individuals.
Several cases have been reported where poisoning has been
wholly or partly ascribed to high nitrate or nitrite intake.
Neonates are at special risk.
4.3 Occupationally exposed population
Workers in the fertilizer and explosives industries may
be exposed to nitrate through inhalation of dusts containing
nitrate salts. Dust can dissolve in sweat and expose skin to
concentrated solutions of the salts. This also applies to
farmers, although they are only periodically exposed.
5. ROUTES OF ENTRY
5.1 Oral
Oral intake of nitrate and nitrite in food and drinking
water is the major route of entry (see Section 4.5). Suicidal
attempts by ingestion of sodium nitrite tablets are
reported.
5.2 Inhalation
The body can take up nitrate and nitrite from inhaled
dust, e.g. from fertilizers.
Nitrogen oxides are transformed to nitrate/nitrite in the
lung (Yoshida & Kasama, 1987; Saul & Archer, 1983), but this
exposure gives only 1.3 mg NO3-/day (NAS, 1981).
5.3 Dermal
There is no information available on inorganic nitrate
and nitrite absorption through intact skin. Absorption could
take place through skin damaged by extensive burns (Harris et
al., 1979), but no analytical data have confirmed this type
of absorption.
5.4 Eye
Unknown.
5.5 Parenteral
Sodium nitrite given intravenously is traditionally used
in the treatment of cyanide poisoning in conjunction with
sodium thiosulphate in doses of 300 mg for adults (= 200 mg
NO2). For children under 25 kg body weight doses of 10
mg/kg (= 7 mg NO2/kg) are regarded as safe treatment. Half
the dose may be repeated within 24 to 48 hours (Klaassen et
al., 1986).
5.6 Other
No data available.
6. KINETICS
6.1 Absorption by route of exposure
Nitrate and nitrite given orally are absorbed and
transferred to the blood in the upper part of the
gastrointestinal tract.
Abundant pectin in the food may delay absorption which may
then occur lower down in the intestine, with possible
increased risk for microbial transformation of nitrate into
nitrite.
6.2 Distribution by route of exposure
Regardless of route of exposure, nitrate and nitrite are
rapidly transferred into the blood. Nitrite is gradually
oxidized to nitrate which is readily distributed into most
body fluids (urine, saliva, gastric juice, sweat, ileostomy
fluid). Distribution of nitrate into plasma, erythrocytes,
saliva and urine following an oral dose of sodium nitrate has
been demonstrated by Cortas & Wakid (1991).
6.3 Biological half-life by route of exposure
Wagner et al. (1983) showed the half-life in the body
for an oral dose of nitrate to be approximately 5 hours. As
blood absorption depends on food matrix (see Section 6.1) and
route of exposure, and as larger doses may increase the
urinary excretion rate, the biological half-life for both
nitrate and nitrite should be expected to be 3 to 8
hours.
Nitrate does not accumulate in the body.
6.4 Metabolism
Where bacteria are present and the environment can be
anaerobic, nitrate can be reduced to nitrite. The main site
for this reaction is mouth and stomach, but nitrite formation
in the lower intestine and in the bladder (urinary infection)
may also be of some toxicological importance.
Nitrite may be further reduced to nitrogen by bacteria under
some conditions. In blood, nitrite transforms haemoglobin to
methaemoglobin and is simultaneously oxidized to nitrate.
Normally methaemoglobin gradually reverts to haemoglobin
through enzymatic reactions.
Nitrite has vasodilating properties, probably through
transformation into nitric oxide (NO) or a NO-containing
molecule acting as a signal factor for smooth muscle
relaxation.
Nitrite easily transforms into a nitrosating agent in an
acidic environment and can react with a variety of compounds,
e.g. ascorbic acid, amines, amides.
Nitrosation can also be mediated by bacteria, e.g. in the
stomach. Some reaction products are carcinogenic (e.g. most
nitrosoamines and amides.
6.5 Elimination by route of exposure
Approximately 60% of oral nitrate is excreted in urine
(Wagner et al., 1983). The fate of the rest is not
completely known, but bacterial or endogenous metabolism
probably accounts for the remainder. A minor part is
excreted in sweat. See also Section 6.4.
7. TOXICOLOGY
7.1 Mode of action
The toxicology of nitrate and nitrite in humans and
animals has been thoroughly reviewed in monographs by WHO
(1985), ECETOC (1988), BIBRA (1990a, b) and Walker (1990).
Literature in Russian is reviewed by UNEP (1982a; 1982b).
There are no reports that suggest that nitrate as such has
toxicological effects. The main toxicological concern is
associated with its conversion to nitrite before or after
reaching the human body (see Section 6.4).
The major acute toxic effect from nitrite is development of
methaemoglobinaemia, a condition where more than 10% of the
haemoglobin is transformed into methaemoglobin. When the
conversion exceeds 70% the condition can be fatal.
Nitrite may also cause sudden fall in blood pressure due to
its vasodilating properties.
These effects are reversible.
The major concern of possible long-term effects of exposure
to nitrate and nitrite is associated with formation of
nitroso compounds, many of which are carcinogenic. This
formation may take place wherever nitrite and nitrosable
compounds are present, but it is favoured by acidic
conditions or the presence of some bacteria. The
gastrointestinal tract and especially the stomach is regarded
as the main formation site, but nitrosation reactions can
also take place in an infected urinary bladder.
Nitrosation reactions also occur elsewhere in the body as a
result of endogenous formation of nitric oxide and nitrite,
but the relative contribution of this source is presently
unknown.
7.2 Toxicity
7.2.1 Human data
7.2.1.1 Adults
The lethal oral dose of potassium
nitrate for an adult has been estimated to be
between 4 and 30 g (about 40 to 300 mg NO3-
kg).
It has been reported that adults have
tolerated large doses of nitrate as sodium
and ammonium salt (> 100 mg NO3-/kg) in
some cases repeated for several days for
medical or experimental purposes with only
minor effects in some subjects (light
methaemoglobinemia, diarrhoea, vomiting).
Death and severe effects of nitrate
ingestion are generally associated with
doses above 10 g NO3-.
Doses between 2 and 9 g NO3- have been
reported to cause methaemoglobinemia. These
values correspond to 33 to 150 mg NO3-/kg
(Walker, 1990).
The lethal oral dose of nitrite for adults
has been variously reported to be between
0.7 and 6 g NO2- (approximately 10 to
100 mg NO2-/kg) (WHO, l985; Corre &
Breimer, 1979; Fassett, 1973; De Beer et al.,
1975). Lower doses may apply for children
(especially neonates), the elderly and
people with certain enzyme deficiencies.
The broad range is due to the wide
variability in individual sensitivity
illustrated by the following examples:
Gowans (1990) reported a fatal case of a
17-year-old nurse who probably ingested 1 g
sodium nitrite (= 670 mg NO2-) as a
tablet. In contrast, an adult survived
without lasting problems after ingestion of
9.7 g NO2- (as sodium nitrite) (Vetter,
1951).
Human volunteers given sodium nitrite
intravenously produced a maximum
methaemoglobin level of 7% after a dose of
2.7 mg NO2-/kg and 30% after a dose of 8
mg/kg (Kiese & Weger, 1969). (This indicates
a lethal dose within the range reported above
(ECETOC, 1988)).
The first symptoms of oral nitrite poisoning
develop within 15 to 45 minutes (ECETOC,
1988).
7.2.1.2 Children
Neonates are at special risk for
high nitrate and nitrite levels as their
enzyme system for regeneration of
haemoglobin is not fully developed. Special
care should therefore be taken referring to
the values stated in Section 7.2.1.1.
Most clinical case data refers to neonates
developing methaemoglobinemia after drinking
water or water-based formulations with high
nitrate or nitrite content. The great
majority of cases (well-water
methaemoglobinemia) occurred when nitrate
levels in drinking water exceeded 100 mg
NO3-/L (Bockman & Bryson, 1989) It is
generally acknowledged that water nitrate
content of 50 mg/L is safe even for
neonates. Assuming normal liquid intake of
150 mL/kg/day by neonates, nitrate intake of
7.5 mg NO3-/kg/day is safe.
Experiments indicate that even twice the
amount is safe, but at a higher intake can
cause methaemoglobinemia.
Cases of methaemoglobinemia have also been
reported due to feeding babies vegetable
preparations where nitrate has been converted
to nitrite through bacterial
action.
7.2.2 Relevant animal data
Animal experiments are difficult to evaluate
and use for assessing the toxicity of nitrate in man
because the toxic doses of nitrate and nitrite depend
upon competing processes, the rates of which are not
necessarily the same in man and animals.
Thus the kinetics of nitrite formation from nitrate,
and the rates of haemoglobin regeneration, are not
necessarily comparable between animals and humans
(ECETOC, 1988). This is especially so for ruminants
where ingested nitrate is reduced in the rumen.
LD50 values for nitrate in rodents varies between 1.2
and 6.6 g NO3-/kg.
WHO (1974) and JECFA (1980) considered 365 mg NO3-kg
as the highest daily dose over lifetime without
adverse effects in rats. On this basis the ADI (given
by WHO/FAO) is set (see section (7.2.5).
LD50 values for nitrite in rodents varies between 57
to 157 mg NO2-/kg.
WHO (1974) concluded from long-term studies, that the
level causing no toxicological effect was less than
100 mg
NaNO2-/kg/day (=67 mg NO2-/kg/day).
7.2.3 Relevant in vitro data
No data available on acute toxicity.
7.2.4 Workplace standards
Exposure is usually to dust, so regulations for
dust in the working environment apply.
7.2.5 Acceptable daily intake (ADI)
The acceptable daily intake for nitrates (total
for potassium and sodium nitrate) is up to 5 mg/kg,
which corresponds to maximum 3.65 mg NO3-/kg
(FAO/WHO, 1985). Walker (1990) suggests this value be
changed to 18.5 mg NO3-/kg, based on a more recent
study.
The guideline value given by WHO (1984) for maximum
concentration of nitrate in drinking water is 10 mg
nitrate (as N) /L (= 44.3 mg NO3-/L) (WHO,
1984).
The acceptable daily intake for nitrites (total for
potassium and sodium nitrite) is up to 0.2 mg/kg,
which corresponds to maximum 0.13 mg NO2-/kg
(FAO/WHO, 1985). Walker (1990) suggests this value be
changed to 0.07 mg NO2-/kg based on a more recent
study. These values are not applicable to
neonates.
WHO makes no specific recommendations for maximum
nitrite concentration in drinking water, but in the
European Union the statutory maximum concentration is
0.1 mg NO2-/L (EEC, 1980).
Nitrate or nitrite should not be added to baby food.
Vegetables known to have a very high nitrate content
(e.g. spinach) should be avoided in baby food
preparations.
7.3 Carcinogenicity
There is no evidence that nitrate or nitrite as such
cause cancer in animals (ECETOC, 1988). However, a causative
connection between nitrate/nitrite and cancer through the
formation of N-nitroso compounds is suspected.
The role of nitrate and nitrite in the etiology of cancer in
humans, especially gastric cancer, is addressed in numerous
studies which are reviewed and discussed by Walker (1990),
Forman et al. (1989), ECETOC (1988), IARC (1987) and WHO
(1985). Included are also epidemiological studies seeking to
find correlation between frequency of cancer and nitrate
intake with food and water. Evidence from these sources does
not support the hypothesis of a straightforward cause and
effect association between nitrate exposure and cancer risk
(Forman, 1989).
7.4 Teratogenicity
Studies relating congenital malformations and cardio-
vascular effects to nitrate levels in drinking water have not
produced consistent results (WHO, 1985; Black, 1989). Studies
with mice given nitrite (up to 1 g/L) in drinking water gave
no evidence for teratogenic or mutagenic effects on the
fetuses (Shimada, 1989).
7.5 Mutagenicity
Nitrates show no mutagenic activity in microbial tests
under aerobic conditions. Activity has been reported under
anaerobic conditions, probably due to reduction of nitrate
into nitrite. Mutagenic activity in vivo of high doses of
nitrate is difficult to evaluate because of the possibility
of chemical reduction.
Nitrite is mutagenic in a number of in vitro assays against
microorganisms or cultured mammalian cells. Mutagenic effects
were also observed in an in vivo and in vitro experiment
using Syrian hamsters. In vivo assays have been equivocal,
both positive and negative results having been reported
(Walker, 1990).
7.6 Interactions
Methaemoglobinemia can also result from several other
chemical compounds; e.g. Acetanilide, o-Aminophenol, p-
Aminophenol, Aniline, Dimethylaniline, Hydroxylamine, p-
Nitroaniline, Nitrobenzene, Nitro-glycerine and Amylnitrite
(Clayton & Clayton, 1981). Cases have also been reported due
to the use and overdose of some medicines, e.g. benzocaine,
dapsone. There should thus be potential for synergism
between methaemoglobin-forming substances and nitrite, but we
are not aware of any studies on this topic.
8. TOXICOLOGICAL ANALYSES/INVESTIGATIONS
8.1 Material sampling plan
8.1.1 Sampling and specimen collection
8.1.1.1 Toxicological analyses
8.1.1.2 Biomedical analyses
8.1.1.3 Arterial blood gas analysis
8.1.1.4 Haematological analyses
8.1.1.5 Other (unspecified) analyses
8.1.2 Storage of laboratory samples and specimens
8.1.2.1 Toxicological analyses
8.1.2.2 Biomedical analyses
8.1.2.3 Arterial blood gas analysis
8.1.2.4 Haematological analyses
8.1.2.5 Other (unspecified) analyses
8.1.3 Transport of laboratory samples and specimens
8.1.3.1 Toxicological analyses
8.1.3.2 Biomedical analyses
8.1.3.3 Arterial blood gas analysis
8.1.3.4 Haematological analyses
8.1.3.5 Other (unspecified) analyses
8.2 Toxicological Analyses and Their Interpretation
8.2.1 Tests on toxic ingredient(s) of material
8.2.1.1 Simple Qualitative Test(s)
8.2.1.2 Advanced Qualitative Confirmation Test(s)
8.2.1.3 Simple Quantitative Method(s)
8.2.1.4 Advanced Quantitative Method(s)
8.2.2 Tests for biological specimens
8.2.2.1 Simple Qualitative Test(s)
8.2.2.2 Advanced Qualitative Confirmation Test(s)
8.2.2.3 Simple Quantitative Method(s)
8.2.2.4 Advanced Quantitative Method(s)
8.2.2.5 Other Dedicated Method(s)
8.2.3 Interpretation of toxicological analyses
8.3 Biomedical investigations and their interpretation
8.3.1 Biochemical analysis
8.3.1.1 Blood, plasma or serum
8.3.1.2 Urine
8.3.1.3 Other fluids
8.3.2 Arterial blood gas analyses
8.3.3 Haematological analyses
8.3.4 Interpretation of biomedical investigations
8.4 Other biomedical (diagnostic) investigations and their
interpretation
8.5 Overall Interpretation of all toxicological analyses and
toxicological investigations
Sample collection
Arterial blood sampling reveals a characteristic
chocolate-brown colour. Methaemoglobin concentrations can be
quantified by spectrophotometry and should be measured
immediately.
Biochemical analysis
Total haemoglobin, blood count.
Serum electrolytes, especially potassium.
Acid-base balance.
Arterial blood gases.
Urine analysis.
Toxicological analysis
The most relevant investigation is methaemoglobin
concentration which correlates well with symptoms and should
be monitored according to the clinical condition.
8.6 References
9. CLINICAL EFFECTS
9.1 Acute poisoning
9.1.1 Ingestion
Ingestion is the major route of exposure. The
first symptoms may appear within 10 to 45 minutes.
Methaemoglobinaemia is the principal and constant
feature of nitrate/nitrite poisoning.
Clinical symptoms may include: nausea, vomiting,
abdominal pain, headache, dizziness, fall in blood
pressure, tachycardia, collapse, bluish-grey cyanosis,
hyperventilation, stupor, convulsions, coma and
death.
9.1.2 Inhalation
No data available.
9.1.3 Skin exposure
No information available on inorganic nitrate
and nitrite absorption through intact skin, although
absorption may take place through skin damaged by
burning (Harris et al., 1979) (see Section
5.3).
9.1.4 Eye contact
No data available.
9.1.5 Parenteral exposure
Lethal methaemoglobineamia developed after the
intravenous injection of 450 mg of sodium nitrite in a
17 month-old child after acute cyanide poisoning
(Berlin et al., 1985) had been mistakenly
diagnosed.
Administration of 600 mg to an adult for the treatment
of cyanide toxicity resulted in a methaemoglobin level
of 58% (van Heijst et al., 1987).
9.1.6 Other
No data available.
9.2 Chronic poisoning
9.2.1 Ingestion
No data available.
9.2.2 Inhalation
No data available.
9.2.3 Skin exposure
No data available.
9.2.4 Eye contact
No data available.
9.2.5 Parenteral exposure
No data available.
9.2.6 Other
No data available.
9.3 Course, prognosis, cause of death
In mild cases, gastrointestinal symptoms and
asymptomatic cyanosis dominate the clinical presentation. In
severe cases coma and death can occur in the first hour due
to hypoxia (severe methaemoglobinaemia) and circulatory
collapse. In case of parenteral administration the onset of
methaemoglobinaemia is immediate.
Prognosis is usually good if adequate treatment is provided.
Death due to nitrates and nitrites have resulted from large
suicidal ingestions, ingestion of contaminated food,
industrial accidents and ingestion of contaminated well water
in neonates (Harris et al., 1979; Gosselin et al., 1984;
Johnson et al., 1987; Donovan, 1990).
9.4 Systematic description of clinical effects
9.4.1 Cardiovascular
Acute: Nitrite produces relaxation of smooth
muscle, especially in veins, but also in coronary and
peripheral arteries. Venous pooling in the lower
extremities leads to a decreased cardiac preload and
output inducing hypotension and thus ischemia of vital
organs. Hypotension and syncope induced by large
doses of nitrite is due initially to the pooling of
blood in dilated post-arteriolar vessels, notably
venules and even large veins. This vasodilation is
not blocked by atropine or by any recognized drug.
Reflex tachycardia is the rule but a vasovagal reflex
may induce transient bradycardia just before complete
collapse (Gosselin et al., 1984; Donovan, 1990). This
collapse can occur from marked vasodilation, decreased
cardiac output and vital organ anoxia. Arythmias have
been reported (Gowans, 1990) Electrographic changes of
hyperkalemia (peaked T waves) have been reported by
Sporer and Mayer (1991) in a 37-year-old man who had
ingested saltpeter (potassium nitrate).
9.4.2 Respiratory
Acute: Tachypnea and hyperventilation may
occur. Cyanosis is due to methaemoglobinaemia.
9.4.3 Neurological
9.4.3.1 Central Nervous System (CNS)
Headache, dizziness, restlessness,
agitation and confusion are common in
moderate poisoning. In severe cases, stupor,
convulsions and coma can occur as a result of
cerebral anoxia.
9.4.3.2 Peripheral nervous system
No data available.
9.4.3.3 Autonomic nervous system
No data available.
9.4.3.4 Skeletal and smooth muscle
No data available.
9.4.4 Gastro-intestinal
Acute: Nausea, vomiting and abdominal pain are
usually the first symptoms.
9.4.5 Hepatic
No data available.
9.4.6 Urinary
9.4.6.1 Renal
No data available.
9.4.6.2 Other
No data available.
9.4.7 Endocrine and reproductive systems
No data available.
9.4.8 Dermatological
Acute: Blue-grey cyanosis is due to
methaemoglobinaemia.
9.4.9 Eye, ear, nose, throat: local effects
No data available.
9.4.10 Haematological
Acute: Methaemoglobinaemia is the principal
and constant feature of acute nitrate and nitrite
poisoning. Methaemoglobin is haemoglobin in which the
iron has been oxidized to the ferric state, Fe3+,
rendering it incapable of oxygen transport.
Methaemoglobin exerts its toxicity in two ways: (a) it
reduces the oxygen-carrying capacity of the blood; (b)
in addition, it shifts the oxyhaemoglobin dissociation
curve to the left, interfering with the unloading of
oxygen (Donovan, 1990; Goldfrank, 1990).
In mild cases, slate grey cyanosis may be visible only
in the lips and mucous membranes.
The appearance of cyanosis also depends on the total
haemoglobin, oxygen saturation, skin pigmentation, and
ambient lighting.
Severe methaemoglobinemia exceeding 70% may be
associated with fatal outcome.
Methaemoglobin levels correlate well with symptoms in
most cases (Hall et al., 1986):
0-3% Normal level
3-10% No clinical symptoms
10-15% None or slate grey cutaneous
coloration "chocolate brown" blood
15-20% Generalized blue-grey cyanosis,
usually asymptomatic
20-45% Headache, fatigue, dizziness,
exercise intolerance, syncope
45-55% Increasing CNS depression
55-65% Coma, seizures, cardiac failure,
cardiac arrhythmias, metabolic
acidosis
> 65% High incidence of mortality
9.4.11 Immunological
No data available.
9.4.12 Metabolic
9.4.12.1 Acid-base disturbances
Acute: Metabolic acidosis secondary
to tissue hypoxia may be observed in moderate
and severe poisoning (Gosselin et al., 1984;
Hall et al., 1986).
9.4.12.2 Fluid and electrolyte disturbances
Significant hyperkalemia has been
observed in one patient after the ingestion
of potassium nitrate (saltpeter) (Sporer and
Mayer, 1991), due to the high potassium
intake.
9.4.12.3 Others
No data available.
9.4.13 Allergic reactions
No data available.
9.4.14 Other clinical effects
No data available.
9.4.15 Special risks
Nitrate or nitrite poisoning during pregnancy
has not been reported.
The nitrate content of mothers' milk, and in milk in
general, is low: below 10 mg NO3-/L (Wettig et al.,
1988; Geissler et al., 1991).
Human red cells deficient in glucose-6-phosphate
dehydrogenase are more sensitive to the
methaemoglobin-generating activities of nitrite than
normal red cells (Gosselin et al., 1984). Patients
with congenital NADPH Methb Reductase deficiency are
also particularly susceptible to
nitrates/nitrites.
Neonates are particularly sensitive to methaemoglobin
induced by nitrates and nitrites due to their
transient deficiency in methaemoglobin reductase,
their low levels of erythrocyte NADH and the greater
susceptibility of haemoglobin F (foetal haemoglobin)
to oxidation (Lukens, 1987; Walley and Flanagan,
1987). Near-adult levels of methaemoglobin reductase
and haemoglobin A are reached by 4 months of age
(Keating et al., 1973; Donovan, 1990).
9.5 Others
No data available.
9.6 Summary
10. MANAGEMENT
10.1 General principles
Patients with severe acute nitrate or nitrite poisoning
should be admitted to an intensive care unit because rapid
deterioration can occur. Monitor vital signs as respiration,
blood pressure and the onset of cyanosis. Monitor acid-base
balance and arterial blood gases. Administer oxygen and
artificial respiration if necessary.
Symptomatic measures, especially oxygen therapy; gastric
lavage or emesis; oral activated charcoal; antidotes for
methaemoglobinemia - methylene blue; or exchange transfusion
may be considered in severe cases if methylene blue
fails.
10.2 Life supportive procedures and symptomatic treatment
Supportive measures include treatment of respiratory
failure, shock, acid-base disturbances and convulsions.
Oxygen therapy is indicated if there are clinical signs of
methaemoglobinemia.
10.3 Decontamination
Ingestion: gastric lavage is indicated in recent
ingestion up to four hours. Although activated charcoal is
not less effective in adsorbing nitrite than other
methaemoglobin-inducing organic compounds, it could be
administered per os. If an oro-or naso-gastric tube is in
place, administer activated charcoal through the tube after
the lavage.
Skin: take off contaminated clothes. Wash skin with copious
amounts of water.
10.4 Enhanced elimination
Data on the use of exchange transfusion for removing
nitrates and nitrites from the blood are limited (Harris et
al., 1979). The advantages of this mode of therapy is the
rapid reduction of circulating methaemoglobin levels and the
removal of some of the offending agents. Exchange
transfusion may be useful for methylene blue failures or for
patients with known G6PD or NADPH methaemoglobin reductase
deficiencies (Harris et al., 1979; Harrison, 1977).
No data indicating the benefit of forced diuresis,
haemodialysis or haemoperfusion are available.
10.5 Antidote
10.5.1 Adults
Methylene blue (tetramethyl thionine chloride)
is the specific antidote indicated in
methaemoglobinaemia induced by nitrates and nitrites.
It is effective but may have significant side effects
if used inappropriately (see end of this section).
Treatment with methylene blue is indicated in
symptomatic patients and when methaemoglobinemia
levels are greater than 30% (Curry, 1982; Hall et al.,
1986; Donovan, 1990). The initial dosage is 1 to 2
mg/kg or 0.1 to 0.2 mL/kg of a 1% solution,
administered intravenously over 5 to 10 minutes.
Clinical improvement and clearing of cyanosis occur
within 1 to 2 hours. Methaemoglobin levels should be
monitored 1 hour after the infusion. If the patient
remains symptomatic and the level is still high, a
second dose may be given (Harris et al., 1979; Hall et
al., 1986; Donovan, 1990).
A single case of methaemoglobinemia treated with a
continuous methylene blue infusion has been reported
by Wilson (1976), who described a case of a 36-year-
old man who had ingested two sodium nitrite tablets.
On admission he had cyanosis and respiratory distress.
The patient was treated with oxygen and an infusion of
20 mL of methylene blue (1% Solution) in 500 mL of
dextrose/saline (4%: N/5) given over four hours with
total recovery.
Methylene blue will be ineffective in reversing
methaemoglobinemia and may produce hemolytic anemia in
patients with glucose-6-phosphate dehydrogenase
deficiency as this enzyme is essential for the
generation of NADPH in the hexose monophosphate
shunt.
Without NADPH methylene blue cannot act as a reducing
agent in the transformation of methaemoglobin to
haemoglobin (Goldfrank, 1990).
Ascorbic acid has been mentioned as an alternative
therapy but according to most authors its reducing
effects are too slow to have significant benefits
(Harris et al., 1979; Hall et al., 1986; Donovan,
1990).
White and Weiss (1991) state that the total dose of
methylene blue should not exceed 7 mg/kg. Methylene
blue therapy may have significant side effects (non
specific): precordial pain, dysnea, restlessness and
methemoglobinemia.
10.5.2 Children
No data available.
10.6 Management discussion
Patients with mild cyanosis but without symptomatic
evidence of hypoxia and methaemoglobin concentrations of less
than 25% will only require close observation and supplemental
oxygen (Hall et al., 1986; Goldfrank 1990).
Lloyd (1992) states that toxic methaemoglobinaemia that is
not life-threatening is best treated by preventing further
administration of the offending chemical and allowing normal
metabolic pathways to reduce the methaemoglobin with high
flow oxygen treatment as a valuable adjunct to management.
Levels of 20 to 30% of methaemoglobin resolve spontaneously
in two to three days with no further effects. Severe
methaemoglobinaemia of toxic or hereditary etiology should be
treated with one to two mg/kg of methylene blue intravenously
as a 1% solution in saline. It acts as a co-factor in the
alternate methaemoglobin reduction pathways involving
NADPH.
11. ILLUSTRATIVE CASES
11.1 Case reports from the literature
Gowans (1990) reported a 17-year-old dental nurse who
was admitted to the casualty department with central
cyanosis, tachycardia, tachypnea and systolic blood pressure
of 90 mmHg. Fifteen minutes after admission, she vomited,
aspirated and suffered a respiratory arrest. She was
intubated and ventilated. Her blood sample was a chocolate
brown color. Forty five minutes after admission, methylene
blue was administered intravenously (2 mg/kg over l0
minutes), however fifteen minutes later her blood pressure
was unrecordable, a series of cardiac arrhythmias ensued and
despite the insertion of a temporary pacing wire together
with standard resuscitation measures she failed to recover
and was certified dead two hours after admission. Biochemical
analysis after death revealed that the level of
methaemoglobinemia was 35%, implying a much higher level on
admission to hospital and the serum nitrite ion level was 13
mg/L. In this case the dental nurse almost certainly
obtained the tablet (s) of 1 g sodium nitrite from the
practice in which she was employed.
Kaplan et al. (1990) reported a group of ten patients
suffering from moderate to severe methaemoglobinemia after
accidental intoxication with sodium nitrite mistaken for
table salt. One patient died in the casualty department but
the other nine recovered rapidly after appropriate therapy
with methylene blue and ascorbic acid. The two patients most
severely affected who survived methaemoglobin levels of 79%
and 71%, respectively, required a repeated dose of methylene
blue after two hours. All patients improved dramatically and
there were no after-effects.
Keating et al. (1973) reported a case of a two-week-old black
male infant who was taken to the Emergency Room because his
grandmother had noted that his lips and nail beds had "dark
color". In the previous 24 hours he had consumed 500 mL of
carrot juice. Physical examination revealed an alert,
irritable infant with marked cyanosis of the nail and lips.
Methaemoglobin level was 9 g/l00 mL, representing 60% the
total haemoglobin. Methylene blue, 1 mg/kg body weight, was
given intravenously and the patient's colour improved
promptly. Subsequent methaemoglobin determinations 1 and 12
hours later revealed 0.9 and 0 g/100 mL, respectively. The
carrot juice fed to the infant was found to contain large
quantities of both nitrates and nitrites, 525 ppm and 775
ppm, respectively.
Johnson et al. (1987) described a fatal outcome in a
two-month-old female infant fed with a powdered formula mixed
with well water. For the one-month checkup the mother noted
blueness around the infant's mouth and of the feet and hands,
some trouble in breathing, and occasional diarrhea and
vomiting. The infant was given progressively larger amounts
of the powdered formula prepared with well water, until she
began to vomit, and had severe diarrhea and severe cyanosis.
The parents rushed her to their physician who gave her oxygen
for 15 minutes, however the infant did not improve and was
referred to a hospital in another town 33 miles distant for
further treatment. The infant stopped breathing during the
trip, she was given cardiopulmonary resuscitation after
arrival at the hospital but could not be resuscitated. The
infant's blood was noted to be a chocolate brown colour. The
well water at the farm was found to have a concentration of
about 664 mg NO3-/L.
12. ADDITIONAL INFORMATION
12.1 Specific preventive measures
Because the consumption of well water with chemical or
bacterial contamination may have serious consequences
especially for infants, such wells should be tested
periodically to ensure their safety, and compliance with
national regulations or WHO recommendations for potable
water.
Vegetables of known high nitrate content (carrots, spinach,
beets, cabbage) might be restricted in infants under four
months of age (Keating et al., 1973).
Sodium nitrites tablets (1 g) widely used in the medical and
dental profession to prevent rusting of instruments should be
recognized as a potent poison and should be kept in secure
storage (Gowans, 1990).
12.2 Other
No other data.
13. REFERENCES
Berlin G, Brodin B, Hilden JO. (1985) Acute dapsone
intoxication: A case treated with continuous infusion of Methylene
Blue, forced diuresis and plasma exchange. Clinical Toxicology
22: 6 537-548
BIBRA (1990a) (British Industrial Biological Research
Association). Toxicity profile: Nitrates. BIBRA Information Dept.,
London.
BIBRA (1990b) (British Industrial Biological Research
Association). Toxicity profile: Nitrites. BIBRA Information Dept.,
London.
Black CA (1989) Reducing American exposure to nitrate, nitrite
and nitroso compounds: The national network to prevent birth
defects proposal. Comments from CAST 1989-1. Council for
Agricultural Science and Technology, Ames, Iowa.
Bockman OC & Bryson DD (1989) Well-water methaemoglobinemia: The
bacterial factor. In: Wheeler D. Richardson ML. & Brigdes
J.:Watershed 89. The Future for Water Quality in Europe. Vol II.
Pergamon Press. Oxford pp 239-44.
Budavari S ed. (1996) The Merck Index: an encyclopedia of
chemicals, drugs, and biologicals, Rahway, New Jersey, Merck and
Co. Inc.
Clayton GD & Clayton FE eds (1981) Patty's Industrial Hygiene and
Toxicology. Vol 2A. Toxicology. Wiley-Interscience, New York, pp.
2416.
Corre WJ & Breimer T (1979) Nitrate and nitrite in vegetables.
Literature survey N 39 Centre for Agricultural Publishing and
Documentation. Wageningen.
Cortas NK & Wakid NW (1991) Pharmacokinetic aspects of inorganic
nitrate ingestion in man. Pharmacol Toxicol, 68: 192-195.
Curry S (1982) Methaemoglobinemia. Ann Emerg Med 11:
214-221.
De Beer J, Heyndrickx A & Timperman J (1975) Suicidal poisoning
by nitrite. Eur J Toxicol, 8: 247-251.
Donovan JW (1990) Nitrates, Nitrites and other Sources of
Methaemoglobinemia In Haddad & Winchester (Eds) Clinical
management of poisoning and drug overdose. WB Saunders,
Philadelphia, pp 1419-1431.
ECETOC (European Chemical Industry Ecology and Toxicology Centre)
(1988) Nitrate and drinking water. Technical Report N 27.
Brussels.
EEC (1980) Council Directive relating to the quality of water
intended for human consumption. Off J Eur Comm, N L 229/11.
Eisenbrand G, Spiegelhalder B, Preussmann R (1980) Nitrate and
nitrite in saliva. Oncology, 37: 227-231.
Ellenhorn M & Barceloux D (1988) Nitrates, Nitrites and
Methaemoglobinemia. In Medical Toxicology. Diagnosis and Treatment
of Human Poisoning New York, Elsevier Science Publishing Company
Inc 1st ed., pp 844-852.
FAO/WHO (1985). FAO/WHO Food Additives Data Systems. Evaluations
by the Joint FAO/WHO Expert Committee on Food Additives 1956-1984
FAO Food and Nutrition Paper 30/Rev Rome.
Fassett DW (1973) Nitrates and nitrites. In: Toxicants occurring
naturally in foods. 2nd ed., pp 7-25. NAS, Washington.
Forman D (1989) Are nitrates a significant risk factor in human
cancer? Canc Sur, 8: 443-458.
Forman D, Shuker D, Franks LM eds (1989) Cancer Surveys, Vol. 8
Nitrate, nitrite and nitroso compounds in human cancer. Oxford
University Press.
Gao L & Guo-YS (1991) Acute nitrate poisoning: a report of 80
cases (letter) Am J Emerg Med, 9(2): 200-201.
Geissler C, Steinhöfel O, Ulbrich M (1991) Zum Nitratgehalt in
der Milch. Arch Anim Nutr, Berlin, 41: 649-656.
Goldfrank's Toxicological Emergencies (1990) Appleton & Lange 4th
edition, pps 209-210, 391-396, 397-399.
Goldstein GM & Doull J (197l) Treatment of nitrite induced
methaemoglobinemia with hyperbaric oxygen. Proc Soc Exp Biol Med
l38: l37-l39.
Gosselin RE, Smith RP, Hodge HC et al (l984) Nitrite. In Clinical
Toxicology of Commercial Products, 5th ed. Williams & Wilkins,
Baltimore, II, pp 314-319.
Gowans WJ (1990) Fatal methaemoglobinemia in a dental nurse. A
case of sodium nitrite poisoning. Br J Gen Pract, 40:
470-471.
Hall AH, Kulig KW, Rumack BH (1986) Drug and chemical-induced
methaemoglobinemia: Clinical features and management. Med Toxicol
1: 253-260.
Harris JC, Rumack BH, Peterson RG, McGuire BM (1979)
Methaemoglobinemia resulting from absorption of nitrates. JAMA
242: 2869-2871.
Harrison MR (1977) Toxic methaemoglobinemia. Anaesthesia, 32(3):
270-272.
IARC (1987) (International Agency for Research on Cancer) Bartsch
H, O'Neill IK & Schulte-Hermann R eds. The relevance of N-nitroso
compounds to human cancer. Exposures and mechanisms. IARC Sci
Publ N 84.
JECFA (1980) 23rd Report of the Joint FAO/WHO Expert Committee on
Food Additives. WHO Technical Report Series N 648.
Johnson CJ, Bonrud PA, Dosh TL, Kilness AW, Senger KA, Busch DC,
Meyer MR (1987) Fatal outcome of methaemoglobinaemia in an
infant. JAMA, 257(20): 2796-2797.
Kaplan A, Smith C, Promnitz DA, Joffe BI, Seftel AB (199O)
Methaemoglobinemia due to accidental sodium nitrite poisoning.
Report of l0 cases. S Afr Med J, 77(6): 300-301.
Keating JP, Lell ME, Strauss AW, Zarkowsky H, Smith GE (1973)
Infantile methaemoglobinemia caused by carrot juice. N Engl J
Med, 288: 824-826.
Kiese M & Weger N (1969) Formation of ferrihaemoglobin with
aminophenols in the human for the treatment of cyanide poisoning.
Eur J Pharmacol, 7: 97-105.
Kirsch IR & Cohen HJ (1980) Heinz-body hemolytic anemia from the
use of methylene blue in neonates. J Pediatr, 96: 272-278.
Klaassen CD, Amdur MO, Doull J eds (1986) Casarett and Doull's
Toxicology. The basic science of poisons. 3rd ed., Macmillan Publ.
Comp., New York.
Lloyd CJ (1992) Chemically induced methaemoglobinaemia in a
neonate. Br J Oral & Maxillofacial Surg, 30: 63-65.
Lukens JN (1987) The legacy of well-water methaemoglobinaemia.
JAMA, 257: 2793-2795.
Mueller RL, Hagel HJ, Wild H, Ruppin H & Domschke W (1986)
Nitrate and nitrite in normal gastric juice. Oncology, 43:
50-53.
NAS (National Academy of Science) (1981) The health effects of
nitrate, nitrite and N-nitroso compounds. Assembly of Life
Sciences. National Academy Press, Washington.
Saul RL & Archer MC (1983) Nitrate formation in rats exposed to
nitrogen dioxide. Toxicol Appl Pharmacol, 67: 284-291.
Shimada T (1989) Lack of teratogenic and mutagenic effects of
nitrite on mouse fetuses. Arch Environ Health, 44: 59-63.
Silber R, Pernas JC, Gonzalez H (1985) Methaemoglobinemia in
small infants. Arch argent pediatr, 83(4): 230-232.
Smith RP & Olson MV (1973) Drug-induced methemoglobinemia. Semin.
Hematol. 10:253-68.
Sporer KA & Mayer AP (1991) Saltpeter ingestion. Am J Emerg Med,
9(2): 164-165.
UNEP (United Nations Environment Programme) (1982a) Nitrates.
Series in Scientific Reviews of Soviet Literature on Toxicity and
Hazards of Chemicals, No. 21. Izmerov NF, Ed.
UNEP (United Nations Environment Programme) (1982b) Nitrites.
Series in Scientific Reviews of Soviet Literature on Toxicity and
Hazards of Chemicals, No. 22. Izmerov NF, Ed.
van Heijst ANP, Douze JMC, van Kesteren RG (1987) Therapeutic
problems in cyanide poisoning. Clin Toxicol, 25: 383-398.
Vetter J (1951) Die neurozirkulatorische Dystonie der
Netzhautgefasse. Gleichzeitig ein Bericht uber eine schwere
Vergiftung mit Natriumnitrit. Klin Mbl Augenheilk, 118: 165.
Wagner DA, Schultz DS, Deen WN, Young VR & Tannenbaum SR (1983)
Metabolic fate of an oral dose of 15N-labeled nitrate in humans:
Effect of diet supplementation with ascorbic acid. Canc Res, 43:
1921-1925.
Walker R (1990) Nitrates, nitrites and N-nitrosocompounds: A
review of the occurrence in food and diet and the toxicological
implications. Food Add Cont, 7: 717-768.
Walley T & Flanagan M (1987) Nitrite induced methaemoglobinemia.
Postgrad Med J, 63: 643-644.
Weast RC ed (1982) CRC Handbook of chemistry and physics, 63rd
edition. CRC Press Inc., Florida.
Wettig K, Proksch A, Broschinski L, Diener W, Fischer G & Namaschk
A (1988) Die Nahrug, 32: 301-303.
White CD & Weiss LD (1991) Varying presentations of
methemoglobinemia:two cases. J. Emerg. Med. 9 (Suppl.):45-9
Whitwan JG, Taylor AR, White JM (1979) Potential hazard of
methylene blue. Anaesthesia, 34: 181-182.
WHO (1974) Toxicological evaluation of some food additives
including anticaking agents, antimicrobials, antioxidants,
emulsifiers and thickening agents. WHO Food Additive Series, N 5,
Geneva.
WHO (1984) Guidelines for drinking water quality. Vol I,
Recommendations, Geneva.
WHO (1985) Health hazards from nitrates in drinking-water,
Copenhagen.
Wilson L (1976) Accidental sodium nitrite ingestion (letter). Med
J Aust, 1: 505.
Yoshida K & Kasama K (1987) Biotransformation of nitric oxide.
Environ Health Persp, 73: 201-206.
14. AUTHOR, REVIEWER, DATE, COMPLETE ADDRESS
Authors: Oluf Chr. Bockman & Tom Granli
(sections Norsk Hydro Research Centre
1, 3, 4, P.O. Box 2560
5, 6, 7) 3901 Porsgrunm
Norway
Tel: 47-3-562000
Fax: 47-2-562327
Date: October 1991
Author: María Cristina Alonzo
Centro de Informacion y Asesoramiento Toxicologico
Hospital de Clinicas - Piso 7
Av. Italia s/n
Montevideo
Uruguay
Tel: 598-2-804000
Fax: 598-2-470300
Date January 1992
Peer Review: Newcastle-upon-Tyne, United Kingdom,
February 1992
Peer Review Berlin, October 1995.
Finalised IPCS, Septeber 1996
Editor: M.Ruse (IPCS, May, 1999)
Molecular weight: 62.05
The structural formula of nitrite is: O == N -- O-
Molecular weight: 46.006
3.3 Physical properties
3.3.1 Colour
See individual nitrate compounds in Section 3.3.3
3.3.2 State/form
See individual nitrate compounds in Section 3.3.3
3.3.3 Description
NaNO3: melting point = 308°C; decomposes at
380°C; solubility in H20 = 9225 g/100 mL; soluble in
alcohol; density = 2.26.
KNO3: melting point = 334°C; decomposes at 400°C;
solubility in H20 = 13.3°; insoluble in alcohol;
insoluble in ether; density = 2.109.
NH4NO3: melting point = 170°C; decomposes at 210°C;
solubility in H2O = 118°; solubility in alcohol =
3.820; insoluble in ether; density = 1.72.
Ca(NO3)2: melting point = 561°C; solubility in
water = 12118; solubility in alcohol = 1415; insoluble
in ether.
Ca(NO3)2.3H20: melting point = 51°C
Ca(NO3)2.4H20: melting point = approx.40°C;
decomposes at 132°C; solubility in water = 266°;
soluble in alcohol.
Mg(NO3)2.2H20: melting point = 129°C; soluble in
water; soluble in alcohol.
Mg(NO3)2.6H20: melting point = 89°C; decomposes
at 330°C; solubility in water = 125b; soluble in
alcohol.
NaNO2: melting point = 271°C; decompose at 320°C;
solubility in water = 8215; slightly soluble in water;
solubility in alcohol = 0.320;
KNO2: melting point = 440°C; decomposes at 350°C a;
solubility in water = 281°; soluble in hot
alcohol.
Ca(NO2)2.H2O: melting point = 100°C; solubility
in water= 46°; slightly soluble in alcohol.
Ca(NO2)2.4H2O: solubility in water= 75°; soluble
in alcohol.
Mg(NO2).3H2O: decomposes at 100°C; soluble in
cold water; soluble in alcohol.
Key:
a: decomposition starts at 350°C
b: temperature not specified (cold water)
upper case number denotes temperature (°C)
(Weast, l982)
Sodium nitrate: colourless transparent crystals or
white granules or powder, with saline, slightly bitter
taste, deliquesces in moist air.
Potassium nitrate: colourless transparent prisms or
white, granular or crystalline powder with cooling,
saline pungent taste.
Ammonium nitrate: odourless, transparent, hygroscopic
deliquescent crystals or white granules. Five solid
phases exist at normal pressure. Orthorhombic at room
temperature.
Calcium nitrate: deliquescent, colourless granules.
Evolve heat when dissolved in water.
Magnesium nitrate hexahydrate: colourless, clear,
deliquescent crystals.
Sodium nitrite: white or slightly yellow deliquescent
granules or rods decomposed even by weak acids with
evolution of brown fumes of nitrous anhydride.
Calcium nitrite: white or yellowish deliquescent
hexagonal crystals.
Ammonium nitrite: white/yellowish crystals decomposed
in hot water.
(Budavari, 1996)
The water solutions of nitrates usually have a pH in
the range of 5 to 8. The water solutions of nitrites
are slightly alkaline (pH 9).
3.4 Hazardous characteristics
Under normal conditions, both nitrites (except the
ammonium salt) and nitrates are stable compounds. However,
at higher temperatures they decompose, and may be explosive
at extreme conditions (high temperature and pressure).
Nitrites also decompose in weak acids. Generally, the
presence of chlorides, some metals and organic material
destabilize both nitrates and nitrites. Nitrates form NO and
NO2 upon thermal decomposition, but ammonium nitrate also
forms N2O, N2 and H2O depending on temperature.
Nitrites mainly decompose to N2 and NO. N2O3 can also be
formed. Ammonium nitrite is unstable and decomposes to N2
and H2O. Nitrites oxidize slowly to nitrates when exposed
to air.
Toxic gases can form if buildings, where nitrates or nitrites
are stored, catch fire. If these materials are enclosed or
contaminated with combustible matter, fire may result in an
explosion.
Salts of nitrate are odourless and colourless with a saline
taste. They are generally hygroscopic.
Salts of nitrite are colourless or slightly yellow. They are
generally hygroscopic.
Environmental concerns:
Nitrate is a nutrient for algae and other microorganisms, and
excesses can contribute towards excessive algal growth in
natural waters (eutrophication). Microbial processes in soil
and water transform nitrate to nitrogen gas (N2), a process
known as denitrification. Some nitrous oxide (N2O) a
greenhouse gas, is also formed.
4. USES/HIGH RISK CIRCUMSTANCES OF POISONING
4.1 Uses
4.1.1 Uses
4.1.2 Description
The major use of nitrate is as fertilizer. It
is also used in the manufacture of nitrites, nitrous
oxide, explosives, pyrotechnics, matches, freezing
mixtures and special cements. It is also used as a
colouring and preserving additive to food, for
coagulation of latexes, in the nuclear industry and
for odour (sulphide) and corrosion control in aqueous
systems.
Nitrite is used as a food preservative and colouring
agent, e.g. curing of meat, in the manufacture of
diazo dyes and rubber, in the textile industry and in
photography. Nitrite is also used in analytical and
preparative chemistry, as a corrosion inhibitor and as
an antidote in cyanide poisoning.
4.2 High risk circumstances of poisoning
Accidental exposure:
Accidental addition of nitrates/nitrites to food in mistake
for common salt has resulted in poisoning.
Intentional exposure:
Employees with access to nitrites at work, e.g. laboratory
personnel, have occasionally attempted suicide through
ingestion of nitrite.
Medical exposure:
Mashed carrot widely used to treat infant diarrhoea has
occasionally resulted in intake of toxic amounts of nitrate
(ECETOC, 1988).
Sodium nitrite given intravenously is traditionally used as
an antidote in cyanide poisoning (see Section 5.5).
Other types of exposure:
The major concern of nitrate and nitrite is associated with
intake of food and water. Drinking water contains variable
amounts of nitrate. The statutory limits vary slightly from
country to country, but is usually either maximum 10 mg
NO3--N/L(= 44.3 mg NO3-/L) in the USA or maximum 50 mg
NO3-/L in the EU.
Plants contain nitrate as a normal cell constituent and
vegetables are usually the main dietary source of nitrate.
Normal daily intake varies with dietary customs, 50 to 150 mg
NO3-/day seems typical for a western diet. Vegetarians can
exceed this, with daily intakes of over 300 mg NO3- (Walker,
1990).
Dietary exposure to nitrites is normally very low, commonly
<2 mg NO2-/day and usually <5 mg/day per capita (<0.1
mg/kg/day) (Walker, 1990). Exceptionally, higher levels may
result from microbial reduction of nitrates in hygienically
poor quality well water or in foods rich in nitrates stored
under inappropriate conditions. Bacterial reduction of
nitrate secreted in the saliva and gastric juice is usually
the source of nitrite (Eisenbrand et al., 1980; Mueller et
al., 1986). NAS (1981) estimated that approximately 3.5 mg
NO2- is formed per day in an average adult in the USA. This
process is dependent upon several factors (e.g. nitrate
intake) and varies substantially between individuals.
Several cases have been reported where poisoning has been
wholly or partly ascribed to high nitrate or nitrite intake.
Neonates are at special risk.
4.3 Occupationally exposed population
Workers in the fertilizer and explosives industries may
be exposed to nitrate through inhalation of dusts containing
nitrate salts. Dust can dissolve in sweat and expose skin to
concentrated solutions of the salts. This also applies to
farmers, although they are only periodically exposed.
5. ROUTES OF ENTRY
5.1 Oral
Oral intake of nitrate and nitrite in food and drinking
water is the major route of entry (see Section 4.5). Suicidal
attempts by ingestion of sodium nitrite tablets are
reported.
5.2 Inhalation
The body can take up nitrate and nitrite from inhaled
dust, e.g. from fertilizers.
Nitrogen oxides are transformed to nitrate/nitrite in the
lung (Yoshida & Kasama, 1987; Saul & Archer, 1983), but this
exposure gives only 1.3 mg NO3-/day (NAS, 1981).
5.3 Dermal
There is no information available on inorganic nitrate
and nitrite absorption through intact skin. Absorption could
take place through skin damaged by extensive burns (Harris et
al., 1979), but no analytical data have confirmed this type
of absorption.
5.4 Eye
Unknown.
5.5 Parenteral
Sodium nitrite given intravenously is traditionally used
in the treatment of cyanide poisoning in conjunction with
sodium thiosulphate in doses of 300 mg for adults (= 200 mg
NO2). For children under 25 kg body weight doses of 10
mg/kg (= 7 mg NO2/kg) are regarded as safe treatment. Half
the dose may be repeated within 24 to 48 hours (Klaassen et
al., 1986).
5.6 Other
No data available.
6. KINETICS
6.1 Absorption by route of exposure
Nitrate and nitrite given orally are absorbed and
transferred to the blood in the upper part of the
gastrointestinal tract.
Abundant pectin in the food may delay absorption which may
then occur lower down in the intestine, with possible
increased risk for microbial transformation of nitrate into
nitrite.
6.2 Distribution by route of exposure
Regardless of route of exposure, nitrate and nitrite are
rapidly transferred into the blood. Nitrite is gradually
oxidized to nitrate which is readily distributed into most
body fluids (urine, saliva, gastric juice, sweat, ileostomy
fluid). Distribution of nitrate into plasma, erythrocytes,
saliva and urine following an oral dose of sodium nitrate has
been demonstrated by Cortas & Wakid (1991).
6.3 Biological half-life by route of exposure
Wagner et al. (1983) showed the half-life in the body
for an oral dose of nitrate to be approximately 5 hours. As
blood absorption depends on food matrix (see Section 6.1) and
route of exposure, and as larger doses may increase the
urinary excretion rate, the biological half-life for both
nitrate and nitrite should be expected to be 3 to 8
hours.
Nitrate does not accumulate in the body.
6.4 Metabolism
Where bacteria are present and the environment can be
anaerobic, nitrate can be reduced to nitrite. The main site
for this reaction is mouth and stomach, but nitrite formation
in the lower intestine and in the bladder (urinary infection)
may also be of some toxicological importance.
Nitrite may be further reduced to nitrogen by bacteria under
some conditions. In blood, nitrite transforms haemoglobin to
methaemoglobin and is simultaneously oxidized to nitrate.
Normally methaemoglobin gradually reverts to haemoglobin
through enzymatic reactions.
Nitrite has vasodilating properties, probably through
transformation into nitric oxide (NO) or a NO-containing
molecule acting as a signal factor for smooth muscle
relaxation.
Nitrite easily transforms into a nitrosating agent in an
acidic environment and can react with a variety of compounds,
e.g. ascorbic acid, amines, amides.
Nitrosation can also be mediated by bacteria, e.g. in the
stomach. Some reaction products are carcinogenic (e.g. most
nitrosoamines and amides.
6.5 Elimination by route of exposure
Approximately 60% of oral nitrate is excreted in urine
(Wagner et al., 1983). The fate of the rest is not
completely known, but bacterial or endogenous metabolism
probably accounts for the remainder. A minor part is
excreted in sweat. See also Section 6.4.
7. TOXICOLOGY
7.1 Mode of action
The toxicology of nitrate and nitrite in humans and
animals has been thoroughly reviewed in monographs by WHO
(1985), ECETOC (1988), BIBRA (1990a, b) and Walker (1990).
Literature in Russian is reviewed by UNEP (1982a; 1982b).
There are no reports that suggest that nitrate as such has
toxicological effects. The main toxicological concern is
associated with its conversion to nitrite before or after
reaching the human body (see Section 6.4).
The major acute toxic effect from nitrite is development of
methaemoglobinaemia, a condition where more than 10% of the
haemoglobin is transformed into methaemoglobin. When the
conversion exceeds 70% the condition can be fatal.
Nitrite may also cause sudden fall in blood pressure due to
its vasodilating properties.
These effects are reversible.
The major concern of possible long-term effects of exposure
to nitrate and nitrite is associated with formation of
nitroso compounds, many of which are carcinogenic. This
formation may take place wherever nitrite and nitrosable
compounds are present, but it is favoured by acidic
conditions or the presence of some bacteria. The
gastrointestinal tract and especially the stomach is regarded
as the main formation site, but nitrosation reactions can
also take place in an infected urinary bladder.
Nitrosation reactions also occur elsewhere in the body as a
result of endogenous formation of nitric oxide and nitrite,
but the relative contribution of this source is presently
unknown.
7.2 Toxicity
7.2.1 Human data
7.2.1.1 Adults
The lethal oral dose of potassium
nitrate for an adult has been estimated to be
between 4 and 30 g (about 40 to 300 mg NO3-
kg).
It has been reported that adults have
tolerated large doses of nitrate as sodium
and ammonium salt (> 100 mg NO3-/kg) in
some cases repeated for several days for
medical or experimental purposes with only
minor effects in some subjects (light
methaemoglobinemia, diarrhoea, vomiting).
Death and severe effects of nitrate
ingestion are generally associated with
doses above 10 g NO3-.
Doses between 2 and 9 g NO3- have been
reported to cause methaemoglobinemia. These
values correspond to 33 to 150 mg NO3-/kg
(Walker, 1990).
The lethal oral dose of nitrite for adults
has been variously reported to be between
0.7 and 6 g NO2- (approximately 10 to
100 mg NO2-/kg) (WHO, l985; Corre &
Breimer, 1979; Fassett, 1973; De Beer et al.,
1975). Lower doses may apply for children
(especially neonates), the elderly and
people with certain enzyme deficiencies.
The broad range is due to the wide
variability in individual sensitivity
illustrated by the following examples:
Gowans (1990) reported a fatal case of a
17-year-old nurse who probably ingested 1 g
sodium nitrite (= 670 mg NO2-) as a
tablet. In contrast, an adult survived
without lasting problems after ingestion of
9.7 g NO2- (as sodium nitrite) (Vetter,
1951).
Human volunteers given sodium nitrite
intravenously produced a maximum
methaemoglobin level of 7% after a dose of
2.7 mg NO2-/kg and 30% after a dose of 8
mg/kg (Kiese & Weger, 1969). (This indicates
a lethal dose within the range reported above
(ECETOC, 1988)).
The first symptoms of oral nitrite poisoning
develop within 15 to 45 minutes (ECETOC,
1988).
7.2.1.2 Children
Neonates are at special risk for
high nitrate and nitrite levels as their
enzyme system for regeneration of
haemoglobin is not fully developed. Special
care should therefore be taken referring to
the values stated in Section 7.2.1.1.
Most clinical case data refers to neonates
developing methaemoglobinemia after drinking
water or water-based formulations with high
nitrate or nitrite content. The great
majority of cases (well-water
methaemoglobinemia) occurred when nitrate
levels in drinking water exceeded 100 mg
NO3-/L (Bockman & Bryson, 1989) It is
generally acknowledged that water nitrate
content of 50 mg/L is safe even for
neonates. Assuming normal liquid intake of
150 mL/kg/day by neonates, nitrate intake of
7.5 mg NO3-/kg/day is safe.
Experiments indicate that even twice the
amount is safe, but at a higher intake can
cause methaemoglobinemia.
Cases of methaemoglobinemia have also been
reported due to feeding babies vegetable
preparations where nitrate has been converted
to nitrite through bacterial
action.
7.2.2 Relevant animal data
Animal experiments are difficult to evaluate
and use for assessing the toxicity of nitrate in man
because the toxic doses of nitrate and nitrite depend
upon competing processes, the rates of which are not
necessarily the same in man and animals.
Thus the kinetics of nitrite formation from nitrate,
and the rates of haemoglobin regeneration, are not
necessarily comparable between animals and humans
(ECETOC, 1988). This is especially so for ruminants
where ingested nitrate is reduced in the rumen.
LD50 values for nitrate in rodents varies between 1.2
and 6.6 g NO3-/kg.
WHO (1974) and JECFA (1980) considered 365 mg NO3-kg
as the highest daily dose over lifetime without
adverse effects in rats. On this basis the ADI (given
by WHO/FAO) is set (see section (7.2.5).
LD50 values for nitrite in rodents varies between 57
to 157 mg NO2-/kg.
WHO (1974) concluded from long-term studies, that the
level causing no toxicological effect was less than
100 mg
NaNO2-/kg/day (=67 mg NO2-/kg/day).
7.2.3 Relevant in vitro data
No data available on acute toxicity.
7.2.4 Workplace standards
Exposure is usually to dust, so regulations for
dust in the working environment apply.
7.2.5 Acceptable daily intake (ADI)
The acceptable daily intake for nitrates (total
for potassium and sodium nitrate) is up to 5 mg/kg,
which corresponds to maximum 3.65 mg NO3-/kg
(FAO/WHO, 1985). Walker (1990) suggests this value be
changed to 18.5 mg NO3-/kg, based on a more recent
study.
The guideline value given by WHO (1984) for maximum
concentration of nitrate in drinking water is 10 mg
nitrate (as N) /L (= 44.3 mg NO3-/L) (WHO,
1984).
The acceptable daily intake for nitrites (total for
potassium and sodium nitrite) is up to 0.2 mg/kg,
which corresponds to maximum 0.13 mg NO2-/kg
(FAO/WHO, 1985). Walker (1990) suggests this value be
changed to 0.07 mg NO2-/kg based on a more recent
study. These values are not applicable to
neonates.
WHO makes no specific recommendations for maximum
nitrite concentration in drinking water, but in the
European Union the statutory maximum concentration is
0.1 mg NO2-/L (EEC, 1980).
Nitrate or nitrite should not be added to baby food.
Vegetables known to have a very high nitrate content
(e.g. spinach) should be avoided in baby food
preparations.
7.3 Carcinogenicity
There is no evidence that nitrate or nitrite as such
cause cancer in animals (ECETOC, 1988). However, a causative
connection between nitrate/nitrite and cancer through the
formation of N-nitroso compounds is suspected.
The role of nitrate and nitrite in the etiology of cancer in
humans, especially gastric cancer, is addressed in numerous
studies which are reviewed and discussed by Walker (1990),
Forman et al. (1989), ECETOC (1988), IARC (1987) and WHO
(1985). Included are also epidemiological studies seeking to
find correlation between frequency of cancer and nitrate
intake with food and water. Evidence from these sources does
not support the hypothesis of a straightforward cause and
effect association between nitrate exposure and cancer risk
(Forman, 1989).
7.4 Teratogenicity
Studies relating congenital malformations and cardio-
vascular effects to nitrate levels in drinking water have not
produced consistent results (WHO, 1985; Black, 1989). Studies
with mice given nitrite (up to 1 g/L) in drinking water gave
no evidence for teratogenic or mutagenic effects on the
fetuses (Shimada, 1989).
7.5 Mutagenicity
Nitrates show no mutagenic activity in microbial tests
under aerobic conditions. Activity has been reported under
anaerobic conditions, probably due to reduction of nitrate
into nitrite. Mutagenic activity in vivo of high doses of
nitrate is difficult to evaluate because of the possibility
of chemical reduction.
Nitrite is mutagenic in a number of in vitro assays against
microorganisms or cultured mammalian cells. Mutagenic effects
were also observed in an in vivo and in vitro experiment
using Syrian hamsters. In vivo assays have been equivocal,
both positive and negative results having been reported
(Walker, 1990).
7.6 Interactions
Methaemoglobinemia can also result from several other
chemical compounds; e.g. Acetanilide, o-Aminophenol, p-
Aminophenol, Aniline, Dimethylaniline, Hydroxylamine, p-
Nitroaniline, Nitrobenzene, Nitro-glycerine and Amylnitrite
(Clayton & Clayton, 1981). Cases have also been reported due
to the use and overdose of some medicines, e.g. benzocaine,
dapsone. There should thus be potential for synergism
between methaemoglobin-forming substances and nitrite, but we
are not aware of any studies on this topic.
8. TOXICOLOGICAL ANALYSES/INVESTIGATIONS
8.1 Material sampling plan
8.1.1 Sampling and specimen collection
8.1.1.1 Toxicological analyses
8.1.1.2 Biomedical analyses
8.1.1.3 Arterial blood gas analysis
8.1.1.4 Haematological analyses
8.1.1.5 Other (unspecified) analyses
8.1.2 Storage of laboratory samples and specimens
8.1.2.1 Toxicological analyses
8.1.2.2 Biomedical analyses
8.1.2.3 Arterial blood gas analysis
8.1.2.4 Haematological analyses
8.1.2.5 Other (unspecified) analyses
8.1.3 Transport of laboratory samples and specimens
8.1.3.1 Toxicological analyses
8.1.3.2 Biomedical analyses
8.1.3.3 Arterial blood gas analysis
8.1.3.4 Haematological analyses
8.1.3.5 Other (unspecified) analyses
8.2 Toxicological Analyses and Their Interpretation
8.2.1 Tests on toxic ingredient(s) of material
8.2.1.1 Simple Qualitative Test(s)
8.2.1.2 Advanced Qualitative Confirmation Test(s)
8.2.1.3 Simple Quantitative Method(s)
8.2.1.4 Advanced Quantitative Method(s)
8.2.2 Tests for biological specimens
8.2.2.1 Simple Qualitative Test(s)
8.2.2.2 Advanced Qualitative Confirmation Test(s)
8.2.2.3 Simple Quantitative Method(s)
8.2.2.4 Advanced Quantitative Method(s)
8.2.2.5 Other Dedicated Method(s)
8.2.3 Interpretation of toxicological analyses
8.3 Biomedical investigations and their interpretation
8.3.1 Biochemical analysis
8.3.1.1 Blood, plasma or serum
8.3.1.2 Urine
8.3.1.3 Other fluids
8.3.2 Arterial blood gas analyses
8.3.3 Haematological analyses
8.3.4 Interpretation of biomedical investigations
8.4 Other biomedical (diagnostic) investigations and their
interpretation
8.5 Overall Interpretation of all toxicological analyses and
toxicological investigations
Sample collection
Arterial blood sampling reveals a characteristic
chocolate-brown colour. Methaemoglobin concentrations can be
quantified by spectrophotometry and should be measured
immediately.
Biochemical analysis
Total haemoglobin, blood count.
Serum electrolytes, especially potassium.
Acid-base balance.
Arterial blood gases.
Urine analysis.
Toxicological analysis
The most relevant investigation is methaemoglobin
concentration which correlates well with symptoms and should
be monitored according to the clinical condition.
8.6 References
9. CLINICAL EFFECTS
9.1 Acute poisoning
9.1.1 Ingestion
Ingestion is the major route of exposure. The
first symptoms may appear within 10 to 45 minutes.
Methaemoglobinaemia is the principal and constant
feature of nitrate/nitrite poisoning.
Clinical symptoms may include: nausea, vomiting,
abdominal pain, headache, dizziness, fall in blood
pressure, tachycardia, collapse, bluish-grey cyanosis,
hyperventilation, stupor, convulsions, coma and
death.
9.1.2 Inhalation
No data available.
9.1.3 Skin exposure
No information available on inorganic nitrate
and nitrite absorption through intact skin, although
absorption may take place through skin damaged by
burning (Harris et al., 1979) (see Section
5.3).
9.1.4 Eye contact
No data available.
9.1.5 Parenteral exposure
Lethal methaemoglobineamia developed after the
intravenous injection of 450 mg of sodium nitrite in a
17 month-old child after acute cyanide poisoning
(Berlin et al., 1985) had been mistakenly
diagnosed.
Administration of 600 mg to an adult for the treatment
of cyanide toxicity resulted in a methaemoglobin level
of 58% (van Heijst et al., 1987).
9.1.6 Other
No data available.
9.2 Chronic poisoning
9.2.1 Ingestion
No data available.
9.2.2 Inhalation
No data available.
9.2.3 Skin exposure
No data available.
9.2.4 Eye contact
No data available.
9.2.5 Parenteral exposure
No data available.
9.2.6 Other
No data available.
9.3 Course, prognosis, cause of death
In mild cases, gastrointestinal symptoms and
asymptomatic cyanosis dominate the clinical presentation. In
severe cases coma and death can occur in the first hour due
to hypoxia (severe methaemoglobinaemia) and circulatory
collapse. In case of parenteral administration the onset of
methaemoglobinaemia is immediate.
Prognosis is usually good if adequate treatment is provided.
Death due to nitrates and nitrites have resulted from large
suicidal ingestions, ingestion of contaminated food,
industrial accidents and ingestion of contaminated well water
in neonates (Harris et al., 1979; Gosselin et al., 1984;
Johnson et al., 1987; Donovan, 1990).
9.4 Systematic description of clinical effects
9.4.1 Cardiovascular
Acute: Nitrite produces relaxation of smooth
muscle, especially in veins, but also in coronary and
peripheral arteries. Venous pooling in the lower
extremities leads to a decreased cardiac preload and
output inducing hypotension and thus ischemia of vital
organs. Hypotension and syncope induced by large
doses of nitrite is due initially to the pooling of
blood in dilated post-arteriolar vessels, notably
venules and even large veins. This vasodilation is
not blocked by atropine or by any recognized drug.
Reflex tachycardia is the rule but a vasovagal reflex
may induce transient bradycardia just before complete
collapse (Gosselin et al., 1984; Donovan, 1990). This
collapse can occur from marked vasodilation, decreased
cardiac output and vital organ anoxia. Arythmias have
been reported (Gowans, 1990) Electrographic changes of
hyperkalemia (peaked T waves) have been reported by
Sporer and Mayer (1991) in a 37-year-old man who had
ingested saltpeter (potassium nitrate).
9.4.2 Respiratory
Acute: Tachypnea and hyperventilation may
occur. Cyanosis is due to methaemoglobinaemia.
9.4.3 Neurological
9.4.3.1 Central Nervous System (CNS)
Headache, dizziness, restlessness,
agitation and confusion are common in
moderate poisoning. In severe cases, stupor,
convulsions and coma can occur as a result of
cerebral anoxia.
9.4.3.2 Peripheral nervous system
No data available.
9.4.3.3 Autonomic nervous system
No data available.
9.4.3.4 Skeletal and smooth muscle
No data available.
9.4.4 Gastro-intestinal
Acute: Nausea, vomiting and abdominal pain are
usually the first symptoms.
9.4.5 Hepatic
No data available.
9.4.6 Urinary
9.4.6.1 Renal
No data available.
9.4.6.2 Other
No data available.
9.4.7 Endocrine and reproductive systems
No data available.
9.4.8 Dermatological
Acute: Blue-grey cyanosis is due to
methaemoglobinaemia.
9.4.9 Eye, ear, nose, throat: local effects
No data available.
9.4.10 Haematological
Acute: Methaemoglobinaemia is the principal
and constant feature of acute nitrate and nitrite
poisoning. Methaemoglobin is haemoglobin in which the
iron has been oxidized to the ferric state, Fe3+,
rendering it incapable of oxygen transport.
Methaemoglobin exerts its toxicity in two ways: (a) it
reduces the oxygen-carrying capacity of the blood; (b)
in addition, it shifts the oxyhaemoglobin dissociation
curve to the left, interfering with the unloading of
oxygen (Donovan, 1990; Goldfrank, 1990).
In mild cases, slate grey cyanosis may be visible only
in the lips and mucous membranes.
The appearance of cyanosis also depends on the total
haemoglobin, oxygen saturation, skin pigmentation, and
ambient lighting.
Severe methaemoglobinemia exceeding 70% may be
associated with fatal outcome.
Methaemoglobin levels correlate well with symptoms in
most cases (Hall et al., 1986):
0-3% Normal level
3-10% No clinical symptoms
10-15% None or slate grey cutaneous
coloration "chocolate brown" blood
15-20% Generalized blue-grey cyanosis,
usually asymptomatic
20-45% Headache, fatigue, dizziness,
exercise intolerance, syncope
45-55% Increasing CNS depression
55-65% Coma, seizures, cardiac failure,
cardiac arrhythmias, metabolic
acidosis
> 65% High incidence of mortality
9.4.11 Immunological
No data available.
9.4.12 Metabolic
9.4.12.1 Acid-base disturbances
Acute: Metabolic acidosis secondary
to tissue hypoxia may be observed in moderate
and severe poisoning (Gosselin et al., 1984;
Hall et al., 1986).
9.4.12.2 Fluid and electrolyte disturbances
Significant hyperkalemia has been
observed in one patient after the ingestion
of potassium nitrate (saltpeter) (Sporer and
Mayer, 1991), due to the high potassium
intake.
9.4.12.3 Others
No data available.
9.4.13 Allergic reactions
No data available.
9.4.14 Other clinical effects
No data available.
9.4.15 Special risks
Nitrate or nitrite poisoning during pregnancy
has not been reported.
The nitrate content of mothers' milk, and in milk in
general, is low: below 10 mg NO3-/L (Wettig et al.,
1988; Geissler et al., 1991).
Human red cells deficient in glucose-6-phosphate
dehydrogenase are more sensitive to the
methaemoglobin-generating activities of nitrite than
normal red cells (Gosselin et al., 1984). Patients
with congenital NADPH Methb Reductase deficiency are
also particularly susceptible to
nitrates/nitrites.
Neonates are particularly sensitive to methaemoglobin
induced by nitrates and nitrites due to their
transient deficiency in methaemoglobin reductase,
their low levels of erythrocyte NADH and the greater
susceptibility of haemoglobin F (foetal haemoglobin)
to oxidation (Lukens, 1987; Walley and Flanagan,
1987). Near-adult levels of methaemoglobin reductase
and haemoglobin A are reached by 4 months of age
(Keating et al., 1973; Donovan, 1990).
9.5 Others
No data available.
9.6 Summary
10. MANAGEMENT
10.1 General principles
Patients with severe acute nitrate or nitrite poisoning
should be admitted to an intensive care unit because rapid
deterioration can occur. Monitor vital signs as respiration,
blood pressure and the onset of cyanosis. Monitor acid-base
balance and arterial blood gases. Administer oxygen and
artificial respiration if necessary.
Symptomatic measures, especially oxygen therapy; gastric
lavage or emesis; oral activated charcoal; antidotes for
methaemoglobinemia - methylene blue; or exchange transfusion
may be considered in severe cases if methylene blue
fails.
10.2 Life supportive procedures and symptomatic treatment
Supportive measures include treatment of respiratory
failure, shock, acid-base disturbances and convulsions.
Oxygen therapy is indicated if there are clinical signs of
methaemoglobinemia.
10.3 Decontamination
Ingestion: gastric lavage is indicated in recent
ingestion up to four hours. Although activated charcoal is
not less effective in adsorbing nitrite than other
methaemoglobin-inducing organic compounds, it could be
administered per os. If an oro-or naso-gastric tube is in
place, administer activated charcoal through the tube after
the lavage.
Skin: take off contaminated clothes. Wash skin with copious
amounts of water.
10.4 Enhanced elimination
Data on the use of exchange transfusion for removing
nitrates and nitrites from the blood are limited (Harris et
al., 1979). The advantages of this mode of therapy is the
rapid reduction of circulating methaemoglobin levels and the
removal of some of the offending agents. Exchange
transfusion may be useful for methylene blue failures or for
patients with known G6PD or NADPH methaemoglobin reductase
deficiencies (Harris et al., 1979; Harrison, 1977).
No data indicating the benefit of forced diuresis,
haemodialysis or haemoperfusion are available.
10.5 Antidote
10.5.1 Adults
Methylene blue (tetramethyl thionine chloride)
is the specific antidote indicated in
methaemoglobinaemia induced by nitrates and nitrites.
It is effective but may have significant side effects
if used inappropriately (see end of this section).
Treatment with methylene blue is indicated in
symptomatic patients and when methaemoglobinemia
levels are greater than 30% (Curry, 1982; Hall et al.,
1986; Donovan, 1990). The initial dosage is 1 to 2
mg/kg or 0.1 to 0.2 mL/kg of a 1% solution,
administered intravenously over 5 to 10 minutes.
Clinical improvement and clearing of cyanosis occur
within 1 to 2 hours. Methaemoglobin levels should be
monitored 1 hour after the infusion. If the patient
remains symptomatic and the level is still high, a
second dose may be given (Harris et al., 1979; Hall et
al., 1986; Donovan, 1990).
A single case of methaemoglobinemia treated with a
continuous methylene blue infusion has been reported
by Wilson (1976), who described a case of a 36-year-
old man who had ingested two sodium nitrite tablets.
On admission he had cyanosis and respiratory distress.
The patient was treated with oxygen and an infusion of
20 mL of methylene blue (1% Solution) in 500 mL of
dextrose/saline (4%: N/5) given over four hours with
total recovery.
Methylene blue will be ineffective in reversing
methaemoglobinemia and may produce hemolytic anemia in
patients with glucose-6-phosphate dehydrogenase
deficiency as this enzyme is essential for the
generation of NADPH in the hexose monophosphate
shunt.
Without NADPH methylene blue cannot act as a reducing
agent in the transformation of methaemoglobin to
haemoglobin (Goldfrank, 1990).
Ascorbic acid has been mentioned as an alternative
therapy but according to most authors its reducing
effects are too slow to have significant benefits
(Harris et al., 1979; Hall et al., 1986; Donovan,
1990).
White and Weiss (1991) state that the total dose of
methylene blue should not exceed 7 mg/kg. Methylene
blue therapy may have significant side effects (non
specific): precordial pain, dysnea, restlessness and
methemoglobinemia.
10.5.2 Children
No data available.
10.6 Management discussion
Patients with mild cyanosis but without symptomatic
evidence of hypoxia and methaemoglobin concentrations of less
than 25% will only require close observation and supplemental
oxygen (Hall et al., 1986; Goldfrank 1990).
Lloyd (1992) states that toxic methaemoglobinaemia that is
not life-threatening is best treated by preventing further
administration of the offending chemical and allowing normal
metabolic pathways to reduce the methaemoglobin with high
flow oxygen treatment as a valuable adjunct to management.
Levels of 20 to 30% of methaemoglobin resolve spontaneously
in two to three days with no further effects. Severe
methaemoglobinaemia of toxic or hereditary etiology should be
treated with one to two mg/kg of methylene blue intravenously
as a 1% solution in saline. It acts as a co-factor in the
alternate methaemoglobin reduction pathways involving
NADPH.
11. ILLUSTRATIVE CASES
11.1 Case reports from the literature
Gowans (1990) reported a 17-year-old dental nurse who
was admitted to the casualty department with central
cyanosis, tachycardia, tachypnea and systolic blood pressure
of 90 mmHg. Fifteen minutes after admission, she vomited,
aspirated and suffered a respiratory arrest. She was
intubated and ventilated. Her blood sample was a chocolate
brown color. Forty five minutes after admission, methylene
blue was administered intravenously (2 mg/kg over l0
minutes), however fifteen minutes later her blood pressure
was unrecordable, a series of cardiac arrhythmias ensued and
despite the insertion of a temporary pacing wire together
with standard resuscitation measures she failed to recover
and was certified dead two hours after admission. Biochemical
analysis after death revealed that the level of
methaemoglobinemia was 35%, implying a much higher level on
admission to hospital and the serum nitrite ion level was 13
mg/L. In this case the dental nurse almost certainly
obtained the tablet (s) of 1 g sodium nitrite from the
practice in which she was employed.
Kaplan et al. (1990) reported a group of ten patients
suffering from moderate to severe methaemoglobinemia after
accidental intoxication with sodium nitrite mistaken for
table salt. One patient died in the casualty department but
the other nine recovered rapidly after appropriate therapy
with methylene blue and ascorbic acid. The two patients most
severely affected who survived methaemoglobin levels of 79%
and 71%, respectively, required a repeated dose of methylene
blue after two hours. All patients improved dramatically and
there were no after-effects.
Keating et al. (1973) reported a case of a two-week-old black
male infant who was taken to the Emergency Room because his
grandmother had noted that his lips and nail beds had "dark
color". In the previous 24 hours he had consumed 500 mL of
carrot juice. Physical examination revealed an alert,
irritable infant with marked cyanosis of the nail and lips.
Methaemoglobin level was 9 g/l00 mL, representing 60% the
total haemoglobin. Methylene blue, 1 mg/kg body weight, was
given intravenously and the patient's colour improved
promptly. Subsequent methaemoglobin determinations 1 and 12
hours later revealed 0.9 and 0 g/100 mL, respectively. The
carrot juice fed to the infant was found to contain large
quantities of both nitrates and nitrites, 525 ppm and 775
ppm, respectively.
Johnson et al. (1987) described a fatal outcome in a
two-month-old female infant fed with a powdered formula mixed
with well water. For the one-month checkup the mother noted
blueness around the infant's mouth and of the feet and hands,
some trouble in breathing, and occasional diarrhea and
vomiting. The infant was given progressively larger amounts
of the powdered formula prepared with well water, until she
began to vomit, and had severe diarrhea and severe cyanosis.
The parents rushed her to their physician who gave her oxygen
for 15 minutes, however the infant did not improve and was
referred to a hospital in another town 33 miles distant for
further treatment. The infant stopped breathing during the
trip, she was given cardiopulmonary resuscitation after
arrival at the hospital but could not be resuscitated. The
infant's blood was noted to be a chocolate brown colour. The
well water at the farm was found to have a concentration of
about 664 mg NO3-/L.
12. ADDITIONAL INFORMATION
12.1 Specific preventive measures
Because the consumption of well water with chemical or
bacterial contamination may have serious consequences
especially for infants, such wells should be tested
periodically to ensure their safety, and compliance with
national regulations or WHO recommendations for potable
water.
Vegetables of known high nitrate content (carrots, spinach,
beets, cabbage) might be restricted in infants under four
months of age (Keating et al., 1973).
Sodium nitrites tablets (1 g) widely used in the medical and
dental profession to prevent rusting of instruments should be
recognized as a potent poison and should be kept in secure
storage (Gowans, 1990).
12.2 Other
No other data.
13. REFERENCES
Berlin G, Brodin B, Hilden JO. (1985) Acute dapsone
intoxication: A case treated with continuous infusion of Methylene
Blue, forced diuresis and plasma exchange. Clinical Toxicology
22: 6 537-548
BIBRA (1990a) (British Industrial Biological Research
Association). Toxicity profile: Nitrates. BIBRA Information Dept.,
London.
BIBRA (1990b) (British Industrial Biological Research
Association). Toxicity profile: Nitrites. BIBRA Information Dept.,
London.
Black CA (1989) Reducing American exposure to nitrate, nitrite
and nitroso compounds: The national network to prevent birth
defects proposal. Comments from CAST 1989-1. Council for
Agricultural Science and Technology, Ames, Iowa.
Bockman OC & Bryson DD (1989) Well-water methaemoglobinemia: The
bacterial factor. In: Wheeler D. Richardson ML. & Brigdes
J.:Watershed 89. The Future for Water Quality in Europe. Vol II.
Pergamon Press. Oxford pp 239-44.
Budavari S ed. (1996) The Merck Index: an encyclopedia of
chemicals, drugs, and biologicals, Rahway, New Jersey, Merck and
Co. Inc.
Clayton GD & Clayton FE eds (1981) Patty's Industrial Hygiene and
Toxicology. Vol 2A. Toxicology. Wiley-Interscience, New York, pp.
2416.
Corre WJ & Breimer T (1979) Nitrate and nitrite in vegetables.
Literature survey N 39 Centre for Agricultural Publishing and
Documentation. Wageningen.
Cortas NK & Wakid NW (1991) Pharmacokinetic aspects of inorganic
nitrate ingestion in man. Pharmacol Toxicol, 68: 192-195.
Curry S (1982) Methaemoglobinemia. Ann Emerg Med 11:
214-221.
De Beer J, Heyndrickx A & Timperman J (1975) Suicidal poisoning
by nitrite. Eur J Toxicol, 8: 247-251.
Donovan JW (1990) Nitrates, Nitrites and other Sources of
Methaemoglobinemia In Haddad & Winchester (Eds) Clinical
management of poisoning and drug overdose. WB Saunders,
Philadelphia, pp 1419-1431.
ECETOC (European Chemical Industry Ecology and Toxicology Centre)
(1988) Nitrate and drinking water. Technical Report N 27.
Brussels.
EEC (1980) Council Directive relating to the quality of water
intended for human consumption. Off J Eur Comm, N L 229/11.
Eisenbrand G, Spiegelhalder B, Preussmann R (1980) Nitrate and
nitrite in saliva. Oncology, 37: 227-231.
Ellenhorn M & Barceloux D (1988) Nitrates, Nitrites and
Methaemoglobinemia. In Medical Toxicology. Diagnosis and Treatment
of Human Poisoning New York, Elsevier Science Publishing Company
Inc 1st ed., pp 844-852.
FAO/WHO (1985). FAO/WHO Food Additives Data Systems. Evaluations
by the Joint FAO/WHO Expert Committee on Food Additives 1956-1984
FAO Food and Nutrition Paper 30/Rev Rome.
Fassett DW (1973) Nitrates and nitrites. In: Toxicants occurring
naturally in foods. 2nd ed., pp 7-25. NAS, Washington.
Forman D (1989) Are nitrates a significant risk factor in human
cancer? Canc Sur, 8: 443-458.
Forman D, Shuker D, Franks LM eds (1989) Cancer Surveys, Vol. 8
Nitrate, nitrite and nitroso compounds in human cancer. Oxford
University Press.
Gao L & Guo-YS (1991) Acute nitrate poisoning: a report of 80
cases (letter) Am J Emerg Med, 9(2): 200-201.
Geissler C, Steinhöfel O, Ulbrich M (1991) Zum Nitratgehalt in
der Milch. Arch Anim Nutr, Berlin, 41: 649-656.
Goldfrank's Toxicological Emergencies (1990) Appleton & Lange 4th
edition, pps 209-210, 391-396, 397-399.
Goldstein GM & Doull J (197l) Treatment of nitrite induced
methaemoglobinemia with hyperbaric oxygen. Proc Soc Exp Biol Med
l38: l37-l39.
Gosselin RE, Smith RP, Hodge HC et al (l984) Nitrite. In Clinical
Toxicology of Commercial Products, 5th ed. Williams & Wilkins,
Baltimore, II, pp 314-319.
Gowans WJ (1990) Fatal methaemoglobinemia in a dental nurse. A
case of sodium nitrite poisoning. Br J Gen Pract, 40:
470-471.
Hall AH, Kulig KW, Rumack BH (1986) Drug and chemical-induced
methaemoglobinemia: Clinical features and management. Med Toxicol
1: 253-260.
Harris JC, Rumack BH, Peterson RG, McGuire BM (1979)
Methaemoglobinemia resulting from absorption of nitrates. JAMA
242: 2869-2871.
Harrison MR (1977) Toxic methaemoglobinemia. Anaesthesia, 32(3):
270-272.
IARC (1987) (International Agency for Research on Cancer) Bartsch
H, O'Neill IK & Schulte-Hermann R eds. The relevance of N-nitroso
compounds to human cancer. Exposures and mechanisms. IARC Sci
Publ N 84.
JECFA (1980) 23rd Report of the Joint FAO/WHO Expert Committee on
Food Additives. WHO Technical Report Series N 648.
Johnson CJ, Bonrud PA, Dosh TL, Kilness AW, Senger KA, Busch DC,
Meyer MR (1987) Fatal outcome of methaemoglobinaemia in an
infant. JAMA, 257(20): 2796-2797.
Kaplan A, Smith C, Promnitz DA, Joffe BI, Seftel AB (199O)
Methaemoglobinemia due to accidental sodium nitrite poisoning.
Report of l0 cases. S Afr Med J, 77(6): 300-301.
Keating JP, Lell ME, Strauss AW, Zarkowsky H, Smith GE (1973)
Infantile methaemoglobinemia caused by carrot juice. N Engl J
Med, 288: 824-826.
Kiese M & Weger N (1969) Formation of ferrihaemoglobin with
aminophenols in the human for the treatment of cyanide poisoning.
Eur J Pharmacol, 7: 97-105.
Kirsch IR & Cohen HJ (1980) Heinz-body hemolytic anemia from the
use of methylene blue in neonates. J Pediatr, 96: 272-278.
Klaassen CD, Amdur MO, Doull J eds (1986) Casarett and Doull's
Toxicology. The basic science of poisons. 3rd ed., Macmillan Publ.
Comp., New York.
Lloyd CJ (1992) Chemically induced methaemoglobinaemia in a
neonate. Br J Oral & Maxillofacial Surg, 30: 63-65.
Lukens JN (1987) The legacy of well-water methaemoglobinaemia.
JAMA, 257: 2793-2795.
Mueller RL, Hagel HJ, Wild H, Ruppin H & Domschke W (1986)
Nitrate and nitrite in normal gastric juice. Oncology, 43:
50-53.
NAS (National Academy of Science) (1981) The health effects of
nitrate, nitrite and N-nitroso compounds. Assembly of Life
Sciences. National Academy Press, Washington.
Saul RL & Archer MC (1983) Nitrate formation in rats exposed to
nitrogen dioxide. Toxicol Appl Pharmacol, 67: 284-291.
Shimada T (1989) Lack of teratogenic and mutagenic effects of
nitrite on mouse fetuses. Arch Environ Health, 44: 59-63.
Silber R, Pernas JC, Gonzalez H (1985) Methaemoglobinemia in
small infants. Arch argent pediatr, 83(4): 230-232.
Smith RP & Olson MV (1973) Drug-induced methemoglobinemia. Semin.
Hematol. 10:253-68.
Sporer KA & Mayer AP (1991) Saltpeter ingestion. Am J Emerg Med,
9(2): 164-165.
UNEP (United Nations Environment Programme) (1982a) Nitrates.
Series in Scientific Reviews of Soviet Literature on Toxicity and
Hazards of Chemicals, No. 21. Izmerov NF, Ed.
UNEP (United Nations Environment Programme) (1982b) Nitrites.
Series in Scientific Reviews of Soviet Literature on Toxicity and
Hazards of Chemicals, No. 22. Izmerov NF, Ed.
van Heijst ANP, Douze JMC, van Kesteren RG (1987) Therapeutic
problems in cyanide poisoning. Clin Toxicol, 25: 383-398.
Vetter J (1951) Die neurozirkulatorische Dystonie der
Netzhautgefasse. Gleichzeitig ein Bericht uber eine schwere
Vergiftung mit Natriumnitrit. Klin Mbl Augenheilk, 118: 165.
Wagner DA, Schultz DS, Deen WN, Young VR & Tannenbaum SR (1983)
Metabolic fate of an oral dose of 15N-labeled nitrate in humans:
Effect of diet supplementation with ascorbic acid. Canc Res, 43:
1921-1925.
Walker R (1990) Nitrates, nitrites and N-nitrosocompounds: A
review of the occurrence in food and diet and the toxicological
implications. Food Add Cont, 7: 717-768.
Walley T & Flanagan M (1987) Nitrite induced methaemoglobinemia.
Postgrad Med J, 63: 643-644.
Weast RC ed (1982) CRC Handbook of chemistry and physics, 63rd
edition. CRC Press Inc., Florida.
Wettig K, Proksch A, Broschinski L, Diener W, Fischer G & Namaschk
A (1988) Die Nahrug, 32: 301-303.
White CD & Weiss LD (1991) Varying presentations of
methemoglobinemia:two cases. J. Emerg. Med. 9 (Suppl.):45-9
Whitwan JG, Taylor AR, White JM (1979) Potential hazard of
methylene blue. Anaesthesia, 34: 181-182.
WHO (1974) Toxicological evaluation of some food additives
including anticaking agents, antimicrobials, antioxidants,
emulsifiers and thickening agents. WHO Food Additive Series, N 5,
Geneva.
WHO (1984) Guidelines for drinking water quality. Vol I,
Recommendations, Geneva.
WHO (1985) Health hazards from nitrates in drinking-water,
Copenhagen.
Wilson L (1976) Accidental sodium nitrite ingestion (letter). Med
J Aust, 1: 505.
Yoshida K & Kasama K (1987) Biotransformation of nitric oxide.
Environ Health Persp, 73: 201-206.
14. AUTHOR, REVIEWER, DATE, COMPLETE ADDRESS
Authors: Oluf Chr. Bockman & Tom Granli
(sections Norsk Hydro Research Centre
1, 3, 4, P.O. Box 2560
5, 6, 7) 3901 Porsgrunm
Norway
Tel: 47-3-562000
Fax: 47-2-562327
Date: October 1991
Author: María Cristina Alonzo
Centro de Informacion y Asesoramiento Toxicologico
Hospital de Clinicas - Piso 7
Av. Italia s/n
Montevideo
Uruguay
Tel: 598-2-804000
Fax: 598-2-470300
Date January 1992
Peer Review: Newcastle-upon-Tyne, United Kingdom,
February 1992
Peer Review Berlin, October 1995.
Finalised IPCS, Septeber 1996
Editor: M.Ruse (IPCS, May, 1999)
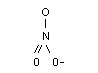 Molecular weight: 62.05
Molecular weight: 62.05
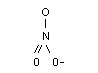 Molecular weight: 62.05
Molecular weight: 62.05