
Isoniazid
ISONIAZID
International Programme on Chemical Safety
Poisons Information Monograph 288
Pharmaceutical
1. NAME
1.1 Substance
Isoniazid
1.2 Group
Tuberculocidal
Antimycobacterial agent
ATC: JO4A CO1
1.3 Synonyms
INH; INAH; Isoniazidium;
Isonicotinic acid hydrazide;
Isonicotinyl hydrazide;
Isonicotinohydrazide; Pycazide;
Tubazid
1.4 Identification numbers
1.4.1 CAS number
54-85-3
1.4.2 Other numbers
UPDT: 7911
RTECS: NS 175 000
1.5 Main brand names/main trade names
Anidrasona (Hortel, Spain); Bacikoch (Ibys, Spain);
Cemidon (Gayoso Wellcome, Spain); Cin Vis (Vis, Italy);
Dardex (Llorente, Spain); Diazid (Nippon Shinyaky, Japan);
Fimazid (Wasserman, Spain); Hidrafasa (Sabater, Spain);
Hidranic (Spain); Hidranison (Cheminova, Spain); Hidrasolco
(Inibsa, Spain); Hidrasstol (Sur De Espagna, Spain);
Hidrazida (Cronofar, Spain; Rovi, Spain); Hidrulta (Euroulta,
Spain); Hiperazida (Spain); Hydra; Hydronsan (Chugai, Japan);
Idrazil (bracco, Italy); Iscontin (Daiichi, Japan); Iso-
Dexter (Spain); Isotamine (ICN, Canada); Isotinyl (USV,
Australia); Isozid (Saarstickstoff-Fatol, Germany); Kridan
Simple (Cidan, Spain); Laniazid (Lannett, USA); Lefos
(Spain); Lubacida (Spain); Midral (Orravan, Spain); Neoteben
(Bayer, Germany); Nicazide (IFI, Italy); Nicizina
(Farmitalia, Italy); Nicotibina (Lepetit, Argentine);
Nicotibine (Belgique); Nicozid (Piam, Italy); Nidrazid
(Squibb, USA); Panazid (US Products, USA); Pyreazid (Salvat,
Spain); Rimifon (Roche, Belgique; Roche, Canada; Roche,
France, Roche Germany; Roche, Portugal; Roche, Spain; Roche,
Switzerland; Roche, UK); Sumifon (Sumitomo, Japan); Tb-
Phlogin (Heyl, Germany); Tebesium-S (Hefa-Frenon, Germany);
Tibinide (Ferrosan, Sweden); Tibizina (Italy); Zidafimia
(Santos, Spain); Zideluy (Spain).
The following names have been used for multi-ingredient
preparations containing isoniazid-Inapasade (Smith & Nephew
Pharmaceuticals, UK); Isoprodian (Kolassa, Australia;
Saarstickstoff-Fatol, Germany; Saarstickstoff-Fatol, South
Africa); Mynah (Lederle, UK); Pasinah-D (Wander, UK);
Rifamate (Merrell Dow, USA); Rifater (Merrell, UK); Rifinah
(Merrell, UK); Rimactazid (Ciba, UK).
1.6 Main manufacturers/main importers
See section 1.5.
2. SUMMARY
2.1 Main risks and target organs
CNS is the target organ of INH acute toxicity. INH
induces generalized convulsions, coma and metabolic acidosis.
Death may occur from acute respiratory failure or
hypotension.
Liver and peripheral nervous and haematologic systems are the
main target organs of INH chronic toxicity. INH may induce
acute hepatitis, peripheral neuropathy, haemolytic
anaemia.
2.2 Summary of clinical effects
Toxicity appears after a short delay of 0.5 to 4 hours
following ingestion.
Symptoms may include:
- slurred speech, hallucinations, coma
- generalized convulsions, status epilepticus
- respiratory failure, hypotension
- severe metabolic acidosis, fever
- rhabdomyolysis
- gastrointestinal symptoms (nausea, vomiting) are
frequent prior to the onset of convulsions.
Hepatitis, peripheral neuropathy and haemolytic anaemia are
manifestations of chronic toxicity.
2.3 Diagnosis
Severe isoniazid poisoning is characterized by the
clinical triad of: repetitive convulsions not responsive to
usual treatment; metabolic acidosis and coma.
Chronic isoniazid toxicity produces nausea, vomiting,
restlessness, fever and many other signs and symptons. Toxic
hepatitis and hemolytic anemia may be observed, as well as
other haemotological effects and psychosis.
Blood gases, serum electrolytes, glucose and BUN
determinations should be performed. Severe metabolic
disorders may be observed: lactic acidosis, hyperkalaemia,
hypocalcaemia, hyperglycaemia and ketonuria increase in
hepatic and muscle enzymes. Measurement of INH serum levels
are not useful for the clinical management of INH
overdose.
2.4 First-aid measures and management principles
Patients with INH overdose should always be admitted to
an emergency or intensive care unit.
Treatment includes:
Supportive measures:
- control of convulsions by short-acting barbiturates
(thiopental) or benzodiazepines (diazepam)
- protection of airway (intubation) and maintenance of
adequate ventilation (artificial ventilation) if
necessary
- correction of hypotension by plasma expanders and/or
dopamine
- rehydration and correction of metabolic acidosis
(sodium bicarbonate) and electrolyte
abnormalities.
Antidotes: Convulsions should be treated with
intravenous pyridoxine (approximately 1 g
pyridoxine for each gram of INH
ingested).
Elimination: Gastric lavage and oral activated charcoal
are indicated within 3 to 4 hours following
ingestion. Ensure adequate diuresis. The
usefulness of forced diuresis is not
established. Haemodialysis may be considered
in patients unresponsive to supportive
treatment, anticonvulsant drugs and
intravenous pyridoxine.
3. PHYSICO-CHEMICAL PROPERTIES
3.1 Origin of the substance
INH is a chemical synthetic molecule (Meyer and Nelly,
1912), a pyridine derivative of nicotinamide.
3.2 Chemical structure
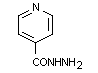 Chemical name: isonicotinic acid hydrazide
Molecular formula: C6H7N3O
Molecular weight: 137.14
3.3 Physical properties
3.3.1 Colour
Colourless.
3.3.2 State/form
3.3.3 Description
INH is a colourless, odourless, white
crystalline powder slowly affected by exposure to air
and to light.
Solubility: 1 g in 8 g water, 1 g in 50 mL alcohol;
slightly soluble in chloroform and very slightly
soluble in ether. A 10% solution has a pH of 6.0 to
8.0.
The solution for parenteral injection is a clear,
colourless liquid. The pH ranges between 5.6 and 6.0
(B.P. injection) or between 6.0 and 7.0 (U.S.P.
injection).
It is recommended that sugars such as glucose,
fructose and sucrose should not be used in INH
preparations because the absorption of the drug is
impaired by the formation of a condensation product.
Sorbitol might be a suitable substitute.
3.4 Other characteristics
3.4.1 Shelf-life of the substance
Three to five years.
3.4.2 Storage conditions
Store in airtight conditions and protect from light.
4. USES
4.1 Indications
4.1.1 Indications
4.1.2 Description
INH is an antimycobacterial agent which is
bactericidal for both extracellular and intracellular
organisms. It is the primary drug for the treatment of
tuberculosis when the disease is caused by
isoniazid-sensitive strains of the M. tuberculosis
(Goodman & Gillman, 1990). In combination with
rifampicin, ethambutol or pyrazinamide, INH is a first
line agent in the treatment of pulmonary and
extrapulmonary tuberculosis. It is a component of all
combined antituberculosis chemotherapy regimens
recommended by WHO.
INH may be used for tuberculosis prophylaxis.
4.2 Therapeutic dosage
4.2.1 Adults
(a) Pulmonary and extrapulmonary tuberculosis
The usual adult dose of INH is a single dose of
5 mg/kg/day up to a maximum of 300 mg daily (PO, IM or
as a slow infusion).
Initial treatment should combine isoniazid
(5 mg/kg/day), rifampicin (10 mg/kg/day) and
pyrazinamide (30 mg/kg/day) for 2 months. In patients
that are known to have been exposed to resistant
organisms, a fourth drug should be added: ethambutol
(20 mg/kg/day) or streptomycin (20 mg/kg/day)
After the initial 2 months, isoniazid and rifampicin
are used in a 9 months regimen. INH should then be
administered as 5 mg/kg/day or 15 mg/kg twice weekly
(to a maximum of 900 mg per dose). In some instances
(lymphadenitis, bone and joint tuberculosis), longer
therapy may be required.
(b) Tuberculosis prophylaxis
300 mg/day orally as a single dose for 6 to 12 months
(c) Dosage in renal failure
Glomerular filtration rate (GFR) > 10 mL/min: no
dosage adjustment
GFR < 10 mL/min: reduce to 66 to 75 % of the normal
dose when used for tuberculosis therapy and stop when
the drug is used for tuberculosis prophylaxis
4.2.2 Children
(a) Pulmonary and extrapulmonary tuberculosis
The usual dosage in infants and children is 10 to 14
mg/kg/day up to a maximum of 300 mg
Initial treatment: Isoniazid, rifampicin and
pyrazinamide for 6 months
After 6th month: isoniazid and rifampicin 3 to 6
months
(b) Pulmonary prophylaxis
10 mg/kg/day orally as a single dose for 1 year
4.3 Contraindications
Known severe adverse reactions or hypersensitivity due
to the drug. Previous hepatitis associated with INH. INH
should not be used in patients with acute liver disease. INH
may precipitate porphyria.
Caution: Convulsions may be precipitated in patients with
epilepsy. Patients at risk of neuropathy should additionally
receive pyridoxine, 10 mg daily. Liver function should be
monitored regularly in patients with previous hepatic
disease.
5. ROUTES OF ENTRY
5.1 Oral
This is the most frequent route of intoxication because
the drug is usually administered orally.
5.2 Inhalation
No data available.
5.3 Dermal
No data available.
5.4 Eye
No data available.
5.5 Parenteral
No case has been reported, but INH intoxication may
occur after parenteral administration.
5.6 Other
No data available.
6. KINETICS
6.1 Absorption by route of exposure
Oral
INH is rapidly and almost completely (90-95%) absorbed from
the gastrointestinal tract. Peak plasma concentrations are
reached within 1 to 2 hours after ingestion. The peak plasma
concentration after a dose of 5 mg/kg is 5 mg/L.
When INH is administered with food, the extent of absorption
and the peak plasma concentration may be reduced (Notterman
et al., 1986).
6.2 Distribution by route of exposure
Protein binding is less than 10 to 15 % (Boxenbaum &
Riegelman, 1974; Benett, 1983).
INH is distributed into all body tissues and fluids (pleural,
ascitic fluids, saliva, CSF) with tissue or fluid levels
similar to serum levels (Kucers & Bennet, 1979). Skin
contains large amounts and acts as a storage depot (Rolson
and Sullivan,1963). INH penetrates well into caseous
lesions.
INH readily crosses the placenta. The concentrations in milk
are approximately equal to plasma maternal
concentrations.
The apparent volume of distribution is 0.6 l/kg (Boxenbaum &
Riegelman, 1974).
6.3 Biological half-life by route of exposure
The plasma half-life in patients with normal renal and
hepatic function is 1 to 4 hours, depending on the rate of
metabolism (see section 6.4.): it is 0.5 to 1.6 hours in fast
acetylators and 2 to 5 hours in slow acetylators (Anderson,
1976; Jeme, 1964; Ellard, 1984). The plasma halflife may be
prolonged to 4.3 hours in patients with impaired hepatic
function (Anderson, 1976) or severe renal impairment
(Bowersox et al., 1973). Plasma half-life may also be
prolonged in acute overdose.
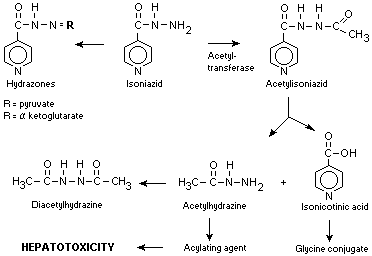
6.4 Metabolism
Metabolic pathway
The major route of isoniazid metabolism is hepatic
acetylation by N-acetyl transferase which produces
acetylisoniazid. The rate of acetylation is genetically
determined. Acetyisoniazid is further hydrolysed to
isonicotinic acid and acetylhydrazine, both of which are
excreted in the urine. Isonicotinic acid is conjugated with
glycine. Acetylhydrazine is further metabolised to
diacetylhydrazine and may be converted by the hepatic
microsomal enzymes to the reactive metabolite (presumed to be
hydrazine) which are thought responsible for INH-induced
hepatotoxicity. Acid labile hydrazones of isoniazid are
formed with a-ketoglutarate and pyruvate, but since these do
not appear to any extent in the blood, they are thought to be
produced in the bladder (Ellard et al., 1972; Russell, 1972;
Boxenbaum & Riegelman, 1974).
Chemical name: isonicotinic acid hydrazide
Molecular formula: C6H7N3O
Molecular weight: 137.14
3.3 Physical properties
3.3.1 Colour
Colourless.
3.3.2 State/form
3.3.3 Description
INH is a colourless, odourless, white
crystalline powder slowly affected by exposure to air
and to light.
Solubility: 1 g in 8 g water, 1 g in 50 mL alcohol;
slightly soluble in chloroform and very slightly
soluble in ether. A 10% solution has a pH of 6.0 to
8.0.
The solution for parenteral injection is a clear,
colourless liquid. The pH ranges between 5.6 and 6.0
(B.P. injection) or between 6.0 and 7.0 (U.S.P.
injection).
It is recommended that sugars such as glucose,
fructose and sucrose should not be used in INH
preparations because the absorption of the drug is
impaired by the formation of a condensation product.
Sorbitol might be a suitable substitute.
3.4 Other characteristics
3.4.1 Shelf-life of the substance
Three to five years.
3.4.2 Storage conditions
Store in airtight conditions and protect from light.
4. USES
4.1 Indications
4.1.1 Indications
4.1.2 Description
INH is an antimycobacterial agent which is
bactericidal for both extracellular and intracellular
organisms. It is the primary drug for the treatment of
tuberculosis when the disease is caused by
isoniazid-sensitive strains of the M. tuberculosis
(Goodman & Gillman, 1990). In combination with
rifampicin, ethambutol or pyrazinamide, INH is a first
line agent in the treatment of pulmonary and
extrapulmonary tuberculosis. It is a component of all
combined antituberculosis chemotherapy regimens
recommended by WHO.
INH may be used for tuberculosis prophylaxis.
4.2 Therapeutic dosage
4.2.1 Adults
(a) Pulmonary and extrapulmonary tuberculosis
The usual adult dose of INH is a single dose of
5 mg/kg/day up to a maximum of 300 mg daily (PO, IM or
as a slow infusion).
Initial treatment should combine isoniazid
(5 mg/kg/day), rifampicin (10 mg/kg/day) and
pyrazinamide (30 mg/kg/day) for 2 months. In patients
that are known to have been exposed to resistant
organisms, a fourth drug should be added: ethambutol
(20 mg/kg/day) or streptomycin (20 mg/kg/day)
After the initial 2 months, isoniazid and rifampicin
are used in a 9 months regimen. INH should then be
administered as 5 mg/kg/day or 15 mg/kg twice weekly
(to a maximum of 900 mg per dose). In some instances
(lymphadenitis, bone and joint tuberculosis), longer
therapy may be required.
(b) Tuberculosis prophylaxis
300 mg/day orally as a single dose for 6 to 12 months
(c) Dosage in renal failure
Glomerular filtration rate (GFR) > 10 mL/min: no
dosage adjustment
GFR < 10 mL/min: reduce to 66 to 75 % of the normal
dose when used for tuberculosis therapy and stop when
the drug is used for tuberculosis prophylaxis
4.2.2 Children
(a) Pulmonary and extrapulmonary tuberculosis
The usual dosage in infants and children is 10 to 14
mg/kg/day up to a maximum of 300 mg
Initial treatment: Isoniazid, rifampicin and
pyrazinamide for 6 months
After 6th month: isoniazid and rifampicin 3 to 6
months
(b) Pulmonary prophylaxis
10 mg/kg/day orally as a single dose for 1 year
4.3 Contraindications
Known severe adverse reactions or hypersensitivity due
to the drug. Previous hepatitis associated with INH. INH
should not be used in patients with acute liver disease. INH
may precipitate porphyria.
Caution: Convulsions may be precipitated in patients with
epilepsy. Patients at risk of neuropathy should additionally
receive pyridoxine, 10 mg daily. Liver function should be
monitored regularly in patients with previous hepatic
disease.
5. ROUTES OF ENTRY
5.1 Oral
This is the most frequent route of intoxication because
the drug is usually administered orally.
5.2 Inhalation
No data available.
5.3 Dermal
No data available.
5.4 Eye
No data available.
5.5 Parenteral
No case has been reported, but INH intoxication may
occur after parenteral administration.
5.6 Other
No data available.
6. KINETICS
6.1 Absorption by route of exposure
Oral
INH is rapidly and almost completely (90-95%) absorbed from
the gastrointestinal tract. Peak plasma concentrations are
reached within 1 to 2 hours after ingestion. The peak plasma
concentration after a dose of 5 mg/kg is 5 mg/L.
When INH is administered with food, the extent of absorption
and the peak plasma concentration may be reduced (Notterman
et al., 1986).
6.2 Distribution by route of exposure
Protein binding is less than 10 to 15 % (Boxenbaum &
Riegelman, 1974; Benett, 1983).
INH is distributed into all body tissues and fluids (pleural,
ascitic fluids, saliva, CSF) with tissue or fluid levels
similar to serum levels (Kucers & Bennet, 1979). Skin
contains large amounts and acts as a storage depot (Rolson
and Sullivan,1963). INH penetrates well into caseous
lesions.
INH readily crosses the placenta. The concentrations in milk
are approximately equal to plasma maternal
concentrations.
The apparent volume of distribution is 0.6 l/kg (Boxenbaum &
Riegelman, 1974).
6.3 Biological half-life by route of exposure
The plasma half-life in patients with normal renal and
hepatic function is 1 to 4 hours, depending on the rate of
metabolism (see section 6.4.): it is 0.5 to 1.6 hours in fast
acetylators and 2 to 5 hours in slow acetylators (Anderson,
1976; Jeme, 1964; Ellard, 1984). The plasma halflife may be
prolonged to 4.3 hours in patients with impaired hepatic
function (Anderson, 1976) or severe renal impairment
(Bowersox et al., 1973). Plasma half-life may also be
prolonged in acute overdose.
6.4 Metabolism
Metabolic pathway
The major route of isoniazid metabolism is hepatic
acetylation by N-acetyl transferase which produces
acetylisoniazid. The rate of acetylation is genetically
determined. Acetyisoniazid is further hydrolysed to
isonicotinic acid and acetylhydrazine, both of which are
excreted in the urine. Isonicotinic acid is conjugated with
glycine. Acetylhydrazine is further metabolised to
diacetylhydrazine and may be converted by the hepatic
microsomal enzymes to the reactive metabolite (presumed to be
hydrazine) which are thought responsible for INH-induced
hepatotoxicity. Acid labile hydrazones of isoniazid are
formed with a-ketoglutarate and pyruvate, but since these do
not appear to any extent in the blood, they are thought to be
produced in the bladder (Ellard et al., 1972; Russell, 1972;
Boxenbaum & Riegelman, 1974).
 Acetylation phenotype
The rate of acetylation is genetically determined and is
subject to individual variations. Although it may be
influenced by age and weight it is usually constant for each
person. Two groups of people can be distinguished: slow
acetylators and fast acetylators.
The phenotype of slow acetylators is an autosomal recessive
trait and results from a relative deficiency of the hepatic
enzyme Nacetyl transferase. The incidence taken from various
sources estimates 45-55% in Americans, 60% in Europeans, 50-
65% in Caucasians, Blacks, South Indians, Mexicans. The
incidence of fast acetylators is 80-90% in Eskimos, Japanese
and Chinese.
Slow acetylators have higher serum levels of INH at a given
dose. Six hours after ingestion of 4 mg/kg INH, plasma
concentrations are 0.8 mg/L in slow acetylators and only 0.2
mg/L in fast acetylators (Meyers et al., 1976). It has also
been suggested that fast acetylators may have a poorer
response to treatment than slow acetylators (Mitchell, 1958;
Ellard, 1984). The rate of isoniazid acetylation does not
appear to alter efficacy when the drug is administered daily
or 2 or 3 times weekly. However, a relationship between rapid
inactivation and poor therapeutic response has been noted in
once-weekly treatments (Lauterburg et al, 1985).
6.5 Elimination and excretion
Kidney
In adults with normal renal function, approximately 50 to 70%
of a 5 mg/kg oral dose is excreted in urine within 24 hours
as unchanged drug and as metabolites. The percentage of the
different compounds excreted varies with the acetylator
phenotype (Kucers & Bennet, 1979).
Acetyl isoniazid Free isoniazid and
and metabolites hydrazone conjugates
Slow acetylators 63% 37%
Fast acetylators 94% 6%
Breast milk
0.75 to 2.3% of the dose is excreted into breast milk in 24
hours. This corresponds to 6-20% of a usual therapeutic
paediatric dose.
Other routes
Small amounts of the drug are excreted in saliva, sputum and
faeces.
7. PHARMACOLOGY AND TOXICOLOGY
7.1 Mode of action
7.1.1 Toxicodynamics
Tonic-clonic seizures and severe metabolic
acidosis are the most common features in INH
overdose.
Seizures
The precipitating mechanism of the seizures is not
exactly known but it may be related to the INH-induced
deficiency of pyridoxine. INH produces pharmacologic
changes in pyridoxine metabolism (Biehl & Vitter,
1954):
- Increased renal excretion of pyridoxine by
formation of INH-pyridoxine hydrazones
- The hydrazones competitively inhibit pyridoxine
kinase, the activating enzyme that converts
pyridoxine to the physiologically active pyridoxal
phosphate
- Inactivation of the pyridoxal containing enzymes.
The subsequent reduction in pyridoxine and pyridoxal
phosphate inhibits the formation of the inhibitory
neurotransmitter, gamma aminobutyric acid or GABA
(Wood & Peesker, 1972). This reduction in GABA levels
may explain the seizures in patients with INH
poisoning.
Metabolic acidosis
The metabolic acidosis may be related to:
- a lactic acidosis due to the seizures,
- a blockage in the conversion of lactate to
pyruvate,
- an increased metabolism of fatty acids resulting
from an impaired glucose metabolism with
hyperglycaemia and ketonuria (Terman & Teitelbaum,
1970).
Neuropathy
Peripheral neuropathy secondary to chronic exposure is
due to the deficiency of pyridoxine and pyridoxal
phosphate.
Hepatitis
Hepatitis is due to a toxic metabolite of monoacetyl
hydrazine, which binds covalently to liver proteins
(Black et al., 1975). In some patients an allergic
mechanism has also been proposed: acylation of hepatic
macromolecules by acetyl hydrazine may lead to the
release of antigenic macromolecules which induce the
formation of antibodies directed against the liver
(Davies, 1981).
7.1.2 Pharmacodynamics
The exact mechanism of action of INH is not
known. INH may act by inhibition of mycolic acid
synthesis and disruption of the cell wall in
susceptible organisms. Since mycolic acids are unique
to mycobacteria, this action explains the high degree
of selectivity of the antimicrobial activity.
Mutation conferring resistance may occur in
susceptible microorganisms. There is a cross
resistance between INH, rifampicin and ethambutol.
However the simultaneous use of two of these drugs
markedly delays the emergence of resistant mutants
either agent.
7.2 Toxicity
7.2.1 Human data
7.2.1.1 Adults
Acute exposure
Doses of 30 to 40 mg/kg may produce seizures
(Manoguerra, 1980). In adults with prior
seizure disorders, seizures may occur after
ingestion of doses as low as 14 mg/kg.
Doses of 80 to 150 mg/kg produce seizures and
can cause death.
Dose of 150 to 200 mg/kg are often fatal if
not treated (Terman & Teitelbaum, 1970).
Chronic toxicity
Symptoms of chronic toxicity may appear after
therapeutic doses.
7.2.1.2 Children
No data available. See section 7.2.1.1.
7.2.2 Relevant animal data
Species Route Effect & Dose
Rat oral LD50 650 mg/kg
subcutaneous 329 mg/kg
Mouse oral LD 50 176 mg/kg
subcutaneous 160 mg/kg
intramuscular 140 mg/kg
intravenous 149 mg/kg
Dog oral LD 50 150 mg/kg
Rabbit oral LD 50 250 mg/kg
subcutaneous 285 mg/kg
intravenous 94 mg/kg
Guinea Pig oral LD 50 450 mg/kg
subcutaneous 255 mg/kg
(RTECS, 1979)
7.2.3 Relevant in vitro data
Not relevant
7.3 Carcinogenicity
There is no evidence to support carcinogenic effects in
humans. A recent study did not detect an increase in cancer
deaths in a series of 338 women treated with INH for
pulmonary tuberculosis (Meyer et al., 1988). Several other
studies fail to show a carcinogenic effect, including a study
by the US Public Health Service in 25,000 patients followed
up for 9 to 14 years; a study by Scott in 3,842 patients in
the UK; and the studies of Hammond (1967) and Sanders &
Drayer (1979).
7.4 Teratogenicity
INH is classified as category C by Briggs et al. (1986)
and may be used safely during pregnancy. INH and ethambutol
are considered the safest drugs for the treatment of
tuberculosis during pregnancy (Holdiness, 1987; Ludford,
1973).
7.5 Mutagenicity
No data available.
7.6 Interactions
Several drugs may interact with INH:
Drugs which interfere with INH pharmacokinetics:
Aminosalicylic acid, procainamide, propranolol increase INH
serum levels by reduction of the acetylation.
Aluminium-containing antacids decrease the gastrointestinal
absorption of INH.
Pyrazinamide decreases the serum levels of INH.
Drugs which decrease serum levels when used with INH
Cyclosporine, enflurane, folic acid, ketoconazole,
verapamil.
Drugs which increase serum levels when used with INH
Carbamazepine, diazepam, dicoumarol, ethosuximide, phenytoin,
primidone, theophylline.
Other interactions
The concomitant administration of INH and the following drugs
may produce:
- psychiatric responses: tricyclic antidepressants
- hyperglycaemia: chlorpropamide
- agitation or parkinsonian tremor: levodopa
- hypotension: pethidine (meperidine)
- increased incidence of hepatotoxicity: rifampicin
- hypertension, tachycardia, hyperthermia: monoamine
oxidase inhibitors
7.7 Main adverse effects
(Drugdex, 1991)
Peripheral neuropathy and hepatotoxicity are the most
frequently observed adverse effects of INH.
Hepatotoxicity
Asymptomatic elevation of serum aspartate transferase (SGOT)
is noted in 10-20 % of the patients (Scharer and Smith,
1969). This increase is usually transient and the level
returns to normal with continuing therapy. INH should be
discontinued in patients with a transaminase level three
times greater than normal.
Hepatitis with jaundice may occur (0.5 %). In most cases,
hepatitis occurs within the 3 months following the onset of
the treatment. Some factors predisposing to INH
hepatotoxicity include: age, alcohol, rapid acetylator
status, concomitant administration of isoniazid and
rifampicin (Acocella, 1972).
Peripheral neuropathies
Peripheral neuropathy is the most common side effect of INH.
It is secondary to a pyridoxine deficiency. The predisposing
factors are: alcohol, slow acetylator status, diabetes,
malnutrition, pregnancy.
Peripheral neuropathy is dose-related: it is uncommon at
doses below 5 mg/kg and very frequent with doses over 300_mg
daily.
Treatment and prophylaxis are based on administration of
pyridoxine.
Other adverse reactions:
(Drugdex, 1991; Davies, 1981)
Haematologic: disseminated intravascular coagulation
(Roberts, 1976); granulocytosis, eosinophilia, anaemia
(haemolytic, aplastic, sideroblastic, megaloblastic),
thrombocytopenia.
Neurologic: peripheral neuropathy (see 7.7.2),
hypersensitivity meningitis (Goragusi, 1976), dystonias,
encephalopathy, seizures, cerebellar syndromes.
Psychiatric: confusional states, transient memory
impairment, delirium.
Endocrine/metabolic: hyperglycaemia, hypocalcaemia,
porphyria, gynaecomastia.
Gastrointestinal: abdominal pain, acute pancreatitis.
Kidney: nephrotoxicity is a very rare complication, (1 case
of acute renal insufficiency has been described by Traimis,
1981).
Ocular: optic neuropathy (especially when combined therapy
with INH and ethambutol); optic atrophy
Dermatologic: pellagra (due to a nicotinic acid
deficiency), exfoliative dermatitis (one case described by
Rosin, 1982), Steven-Johnson syndrome; cutaneous reactions
(2%), urticaria, angioneurotic oedema, morbilliform
eruptions, purpura, acneiform eruptions,
photosensitivity.
Musculoskeletal: arthritis, arthralgias, systemic lupus
erythematosus; antinuclear antibodies in the serum may
appear, especially in slow acetylators, approximately five
months after the onset of therapy.
8. TOXICOLOGICAL AND BIOMEDICAL INVESTIGATIONS
8.1 Material sampling plan
8.1.1 Sampling and specimen collection
8.1.1.1 Toxicological analyses
Toxic ingredient: tablets, capsules,
liquids, suspect materials
In case of ingestion:
- Vomitus: total amount
- Gastric aspirate: total amount (or gastric
lavage - first portion: 100 mL)
- Blood without additives: 10 mL
- Urine (random specimen): 50 mL
8.1.1.2 Biochemical analyses
Plasma (lithium heparin as
anticoagulant) or serum and urine for
standard biochemical analyses. Whole blood
with anticoagulant and glycolytic inhibitor
(e.g. heparin or fluoride) for lactate
determination.
8.1.1.3 Arterial blood gas analysis
Heparinized arterial blood sample.
8.1.1.4 Haematological analyses
Anticoagulated blood (e.g. EDTA) for
standard haematological analyses and
differential blood picture. Anticoagulated
blood for prothrombin time.
8.1.1.5 Other (unspecified) analyses
No further materials.
8.1.2 Storage of laboratory samples and specimens
8.1.2.1 Toxicological analyses
Serum should be separated and stored
in freezer (-20°C). There is controversy
over the stability of INH and acetylINH in
serum and urine. Some early assays based on
fluorimetry and colourimetry give apparent
loss of INH and acetylINHzid when serum is
stored for longer than 1 to 2 hours, even if
frozen, and it was recommended that protein
be precipitated immediately to ensure
reliable results. However, subsequent
substitution of stronger acids as
precipitating agents release isoniazid from
the proteins to which it becomes bound on
storage. Further investigation shows that
isoniazid and acetylisoniazid are almost
completely lost from serum after one week at
room temperature (acetylINH is hydrolysed to
isonicotinic acid by serum enzymes). Protein
precipitation should therefore be performed
within 12 hours if frozen storage of
separated serum (-20°C) is not possible.
While acetylisoniazid is usually stable in
urine, pronounced decay is sometimes observed
and is therefore thought to be bacterial
(Ellard et al., 1972).
8.1.2.2 Biochemical analyses
No special requirements, but as
usually performed.
8.1.2.3 Arterial blood gas analysis
No special requirements, but as
usually performed.
8.1.2.4 Haematological analyses
Not applicable.
8.1.2.5 Other (unspecified) analyses
Not applicable.
8.1.3 Transport of laboratory samples and specimens
8.1.3.1 Toxicological analyses
No special requirements, but as
usually performed.
8.1.3.2 Biochemical analyses
No special requirements, but as
usually performed.
8.1.3.3 Arterial blood gas analysis
No special requirements, but as
usually performed.
8.1.3.4 Haematological analyses
No special requirements, but as
usually performed.
8.1.3.5 Other (unspecified) analyses
Not applicable.
8.2 Toxicological analyses and their interpretation
8.2.1 Tests on toxic ingredient(s) of material
8.2.1.1 Simple qualitative test(s)
The presence of INH in materials can
be inferred by a number of chemical tests.
Heating of INH (10 mg material) with
anhydrous sodium carbonate (200 mg) releases
pyridine, which is easily detected by its
odour (WHO, 1986).
Several simple colour tests are available,
but results must be interpreted with caution,
since many other drugs give similar
reactions, and the limitations of each test
are given where known.
Nitroprusside test: INH can be reacted with
sodium nitropentacyanoferroate reagent
(freshly prepared equal volume mix of sodium
nitroprusside and 4M NaOH) to give an intense
orange colour which fades slowly (Baselt,
1987; Flanagan et al., 1995).
Nessler's Reagent: Add solid potassium
iodide to a saturated solution of mercuric
chloride until the initial red precipitate
dissolves, then add an equal volume of 10M
NaOH solution. Mix 1 mL of reagent with a
small amount (10 mg) of the material. The
presence of the -NH-NH2 side chain of INH
produces an immediate black colour. A
similar response is given by ortho and para
hydroxyl groups, and by the -NH-NH- group. A
number of other compounds produce a similar
response if heated (Moffat et al., 1986).
UV spectrophotometry: Dissolve a portion of
material in dilute acid (e.g. 0.1M HCl) or
alkali (e.g. 0.1M NaOH) to achieve an
appropriate instrument response. If
necessary, centrifuge or filter the mixture
and analyse the clear supernatant. The
spectrum in aqueous acid gives two equal
9max at 213 and 266 nm (A| = 390); in
alkali 9max is at 272 and 298 nm, but are
not stable.
After dissolving the material in 0.1M HCl,
INH can be coupled at room temperature on
mixing with several reagents to give products
which absorb strongly in the UV region. For
example: 1% trans-cinnamaldehyde in ethanol
(358 nm); 2% vanillin in ethanol / water
(1:3) (380 nm); (Eidus & Harnanansingh,
1971).
There are no commonly-available immunoassay
kits for drug testing which respond to
INH.
Thin layer chromatography may be used for
identification of isoniazid, and may be
either an in-house system or a
commercially-available system such as
Toxi-Lab (Ansys Inc, Irvine, California
92718, USA). Dissolve the material in an
organic solvent such as methanol or
dichloromethane and apply directly to the
plate. Using silica plates without modifiers
and standard systems, the Rf of INH is 0.47
on methanol / concentrated ammonia (100:
1.2), and 0.29 on ethyl acetate / methanol /
ammonia (85:15:6). Acidified iodoplatinate
gives an uncharacteristic dark blue response,
and a positive reaction is obtained with
Dragendorff's with a sensitivity of
approximately 50 ng (Moffat et al.,
1986).
8.2.1.2 Advanced qualitative confirmation test(s)
Gas chromatography can be used after
dissolving the material in a small amount of
organic solvent (e.g. 10 mg in 10 mL
methanol). The retention index for INH is
1650 on OV1, SE30, DB5 or similar phases.
Isothermal analysis may be performed at about
200°C, although peak shape is improved on
temperature-programmed capillary columns.
Flame ionization detection gives adequate
sensitivity. Derivatization of the material
improves chromatography (e.g. treatment of
the dry residue with TFAA at 60°C for 30
minutes (LoDico et al., 1992) or with BSTFA /
1% TMCS for 1 hour at 80°C (Frater-Schröder &
Zbinden, 1975), or with p-chlorobenzaldehyde
in methanol for 30 minutes at room
temperature (Timbrell et al., 1977). Excess
derivatizing reagent is evaporated to
dryness, and the extract reconstituted in a
suitable solvent for GC analysis. The
retention times for the derivatives are
broadly in line with that of underivatized
INH. Characteristic fragmentation on mass
spectrometry is seen, and the most abundant
ions are: INH m/z 78, 106, 51 and 137;
INH-TFA m/z 215, 78, 106 and 146; INH-diTMS
m/z 73, 75, 266, 147 and 117.
HPLC may be used to identify INH in residues
by dissolving a small amount of the suspect
material in the mobile phase, and filtering
if necessary to obtain a clear solution.
Hutchings et al. (1983) performed
chromatography on a Spherisorb nitrile column
with a mobile phase of 0.01 M phosphoric acid
in acetonitrile / water (80:20). Detection
was at 266 nm, and chromatograms showed good
peak shape with separation over 6 min,
without interference from other commonly
prescribed antituberculous drugs.
Alternatively, Hsu & Ho (1989) used a reverse
phase phenyl column with a mobile phase of 10
mM phosphate buffer containing 0.25 mM
tetrabutylammonium phosphate as paired-ion
source (pH 4.1). Good separation was seen
over 20 minutes, with detection at 280 nm.
Additional confirmation of identity may be
obtained by performing a full scan analysis
on the appropriate portion of the HPLC
effluent or incorporating a diode array
detector. El-Yazigi & Yusuf (1991) show
excellent chromatography on a C18 column in a
radial compression (Z) module with a mobile
phase of 10 mM sodium dibasic phosphate (pH
7.0 with phosphoric acid) and methanol (93.5:
6.5); electrochemical detection was at +800
mV.
8.2.1.3 Simple quantitative method(s)
In the methods described below a
known mount of the material is weighed into
an aqueous solution, and centrifuged or
filtered to obtain a clear supernatant for
analysis. Quantitation is performed by
comparison of the response of the material to
the analysis of known amounts of INH prepared
similarly. All methods have adequate
sensitivity for residue analysis, but suffer
interference from compounds of similar
structure.
Colourimetric assays
INH can be reacted with sodium
nitropentacyanoferroate reagent (freshly
prepared equal volume mix of sodium
nitroprusside and 4M NaOH) to give an intense
orange product absorbing at 440 nm. The
colour is unstable, and measurements should
be timed to 2 minutes (Björnesjö & Jarnulf,
1967; Baselt, 1987; Flanagan et al.,
1995).
Chlorpromazine free radical solution
(prepared by treating chlorpromazine with
2-iodoxybenzoic acid in 50% w/v
orthophosphoric acid) absorbs at 530 nm, and
the addition of INH results in quantitative
reduction to colourless chlorpromazine
(El-Brashy & El-Ashry, 1992).
INH in 0.1M HCl can be coupled at room
temperature on mixing with several reagents
to give products which absorb strongly in the
UV region. For example: 1%
trans-cinnamaldehyde in ethanol (358 nm); 2%
vanillin in ethanol / water (1:3) (380 nm)
(Eidus & Harnanansingh, 1971).
Titrimetric determination
INH solution is titrated with
N-bromophthalimide using methyl red
indicator. Alternatively, a known excess of
titrant is added to the INH solution, and the
residual unreacted reagent determined by
iodometric back-titration (El-Brashy &
El-Ashry, 1992).
8.2.1.4 Advanced quantitative method(s)
In the methods described below a
known mount of the material is weighed into
aqueous solution or a suitable organic
solvent. Quantitation is performed by
addition of a suitable internal standard, and
comparison of the response of the material to
the analysis of known amounts of INH prepared
similarly. All methods have adequate
sensitivity for residue analysis.
Gas chromatography can be used directly after
dissolving the material in an organic
solvent. The retention index for INH is 1650
on OV1, SE30, DB5 or similar phases.
Isothermal analysis may be performed at about
200°C, although peak shape is improved on
temperature-programmed capillary columns.
Flame ionization detection gives adequate
sensitivity. Derivatization of the material
improves chromatography (e.g. treatment of
the dry residue with TFAA at 60°C for 30
minutes using phentermine as internal
standard (LoDico et al., 1992), or with
eicosan internal standard and BSTFA / 1% TMCS
for 1 hour at 80°C (Frater-Schröder &
Zbinden, 1975), or with p-chlorobenzaldehyde
in methanol for 30 minutes at room
temperature, followed by addition of
p-bromobenzaldehyde isonicotinyl hydrazone
internal standard (Timbrell et al., 1977).
Excess solvent and derivatizing reagents are
evaporated to dryness, and the extract
reconstituted in a suitable solvent for GC
analysis. The retention times for the
derivatives are broadly in line with that of
underivatized INH. Characteristic
fragmentation is achieved on mass
spectrometry, and the most abundant ions are:
INH m/z 78, 106, 51 and 137; INH-TFAA m/z
215, 78, 106, 146; INH-diTMS m/z 73, 75,
266, 147 and 117.
HPLC may be used to quantify INH in residues,
and two UV methods are highly suitable.
Hutchings et al. (1983) used a Spherisorb
nitrile column with a mobile phase of 0.01 M
phosphoric acid in acetonitrile / water
(80:20). Detection was at 266 nm, and
iproniazid was used as internal standard.
Chromatograms showed good peak shape with
separation over 6 min, without interference
from other commonly prescribed
antituberculous drugs. Alternatively, Hsu &
Ho (1989) used a reverse phase phenyl column
with a mobile phase of 10 mM phosphate buffer
containing 0.25 mM tetrabutyl-ammonium
phosphate as paired-ion source (pH 4.1).
Niacinamide was used as internal standard,
and good separation was seen over 20 minutes,
with detection at 280 nm. Additional
confirmation of identity may be obtained by
performing a full scan analysis on the
appropriate portion of the HPLC effluent or
incorporating a diode array detector.
El-Yazigi & Yusuf (1991) obtained excellent
chromatography on a C18 column in a radial
compression (Z) module with a mobile phase of
10 mM sodium dibasic phosphate solution (pH
7.0 with phosphoric acid) / methanol (93.5:
6.5). Electrochemical detection was at +800
mV, and diphenylcarbazide was used as
internal standard.
8.2.2 Tests for biological specimens
8.2.2.1 Simple qualitative test(s)
There are no commonly-available
immunoassay kits for drug testing which
respond to INH or its metabolites in
biological specimens.
Direct UV methods may be applied to the
detection of INH in gastric contents, but are
not useful for the analysis of other fluids.
Disperse a portion of gastric contents in
dilute acid (e.g. 0.1M HCl) or alkali (e.g.
0.1M NaOH) to achieve an appropriate
instrument response. If necessary,
centrifuge or filter the mixture and analyse
the clear supernatant. The spectrum in
aqueous acid gives 9max at 266 nm (A| =
390); in alkali 9max is at 298 nm.
Thin layer chromatography can be used after
extraction from biological specimens (urine
or gastric contents - 10 - 20 mL) at pH 5 to
7. A polar extraction solvent (e.g.
dichloromethane, ethyl acetate) and
saturation of the aqueous phase with solid
sodium chloride are required to maximize
recovery of INH and metabolites. Treatment of
urine with dilute HCl (15 minutes at room
temperature) greatly increases INH recovery.
The extract is concentrated by evaporation of
the solvent. Thin layer chromatography may
be either an in-house system or a
commercially-available system such as
Toxi-Lab (Ansys Inc, Irvine, California
92718, USA). Using silica plates without
modifiers and the standard methanol /
concentrated ammonia (100: 1.2) system, the
following Rf data are reported by Walubo et
al., 1994. INH 0.55, acetylINH 0.55,
monoacetylhydrazine 0.65, diacetylhydrazine
0.45, hydrazine 0.09. All compounds gave a
positive reaction to acidified iodoplatinate
(dark blue), and to Dragendorff s with a
sensitivity of approximately 50 ng on
plate.
AcetylINH can be detected in urine by mixing
1 mL with an equal volume of 0.5M pH 6 buffer
(87.7 mL potassium dihydrophosphate and 12.3
mL potassium hydrophosphate). Addition of 1
mL freshly prepared 20% potassium cyanide is
followed by 4 mL 12.5% freshly prepared
chloramine T. After 2 minutes, 5 mL of
acetone is added and a cherry pink colour is
formed. Increased sensitivity can be
obtained by first acetylating INH by shaking
the urine with one drop of acetic anhydride,
followed by one drop of 7M NaOH, and
proceeding as above. Chlorpromazine
interferes (Eidus et al., 1973).
Identification of toxic amounts of INH in
urine or serum by spectrophotometry can be
achieved by coupling with chromogenic
reagents. There is interference from
pyrazinamide and iproniazid. For serum, a
protein free filtrate should be prepared
(e.g. with 2 volumes of methanol or
acetonitrile, and the organic solvent
evaporated off), and the supernatant
analysed. For colourimetry, the supernatant
is mixed with 2M acetic acid followed by
equal volumes of 2% sodium nitroprusside and
4M NaOH (freshly prepared). The yellow
colour has maximum absorbance at 440 nm
(Björnesjö & Jarnulf, 1967; Baselt, 1987).
Alternatively, for UV spectrophotometry, the
supernatant is mixed with 0.04%
trans-cinnamaldehyde solution in absolute
ethanol. Absorbance is measured at 340 nm
(Eidus & Harnanansingh, 1971;
1974).
8.2.2.2 Advanced qualitative confirmation test(s)
Gas chromatography can be used for
identification of INH and metabolites in
urine after extraction into a polar organic
solvent (e.g methylene chloride or ethyl
acetate) from a pH adjusted to 4 to 7 using a
phosphate buffer. Some methods utilize
salting-out of the drug and metabolites with
solid sodium sulphate. Urine is generally
incubated at room temperature for 15 minutes
with 0.1 M HCl to hydrolyze acid-labile
hydrazone metabolites back to INH prior to
analysis. Methods have insufficient
sensitivity for serum analysis.
The Retention Index for INH is 1650 on OV1,
SE30, DB5 or similar phases, and for
acetylINH is 1950. Isothermal analysis may
be performed at about 200°C, although peak
shape is improved on temperature-programmed
capillary columns. Flame ionization
detection gives poor sensitivity (2 to 5 ng
on column), which is improved only slightly
by nitrogen-phosphorus detection (Timbrell et
al., 1977). Derivatization improves
chromatography (e.g. treatment of the dry
extracted residue with TFAA at 60°C for 30
min using phentermine as internal standard
(LoDico et al., 1992), or with BSTFA / 1%
TMCS for 1 hour at 80°C (Frater-Schröder &
Zbinden, 1975). The retention times for the
derivatives are broadly in line with that of
underivatized INH. Characteristic
fragmentation is achieved on mass
spectrometry without the need for
derivatization, and the most abundant ions
are: INH m/z 78, 106, 51 and 137; acetyl
INH m/z 179, 106, 137, and 78.
Fragmentation for the derivatives are:
TFAA-INH m/z 215, 78, 106 and 146,
diTMS-INH m/z 73, 75, 266, 147 and 117,
and diTMS-acetylINH 73, 75, 308, 147 and
132.
HPLC is more suited to the identification of
INH and acetylINH. Several approaches have
been taken isolate INH and acetylINH from
serum. Preparation of a protein-free
filtrate using an centrifugal filter device
(e.g. Amicon Centrifree) such as is commonly
used for the analysis of unbound drugs in TDM
provides clean extracts (Svensson et al.,
1985; Kohno et al., 1991). Altrnatively,
salting out with sodium chloride is required
if solvent extraction (e.g. dichloromethane /
n-butanol (7:3) at neutral or basic pH) is to
be used (Hutchings et al., 1983).
Precipitation of proteins with solid ammonium
sulphate and phosphoric acid, followed by
extraction into acetonitrile has also been
reported (Hsu & Ho, 1989). Urine is
generally incubated at room temperature for
15 minutes with 0.1 M HCl to hydrolyze
acid-labile hydrazone metabolites back to INH
prior to analysis (Svensson et al., 1985;
Khono et al., 1991).
Two UV methods are highly suitable. Hutchings
et al. (1983) used a Spherisorb nitrile
column with a mobile phase of 0.01 M
phosphoric acid in acetonitrile / water
(80:20). Detection was at 266 nm.
Chromatograms showed good peak shape with
separation over 6 min, without interference
from other commonly prescribed
antituberculous drugs. Alternatively, Hsu &
Ho (1989) used a reverse phase phenyl column
with a mobile phase of 10 mM phosphate buffer
containing 0.25 mM tetrabutylammonium
phosphate as paired-ion source (pH 4.1).
Good separation was seen over 20 minutes, and
detection was at 280 nm. El-Yazigi & Yusuf
(1991) obtained excellent chromatography on a
C18 column in a radial compression (Z) module
with a mobile phase of 10 mM sodium dibasic
phosphate solution (pH 7.0 with phosphoric
acid) and methanol (93.5: 6.5).
Electrochemical detection was at +800 mV, and
monoacetylhydrazine and hydrazine were also
seen. Additional confirmation of identity
may be obtained by performing a full scan
analysis on the appropriate portion of the
HPLC effluent or incorporating a diode array
detector.
8.2.2.3 Simple quantitative method(s)
Quantitative colourimetric,
spectrophotometric and fluorimetric analysis
of INH in biological fluids are widely used.
Some methods additionally quantify acetylINH.
Urine must be treated with dilute
hydrochloric acid (15 min room temperature)
to ensure conversion of acid labile
hydrazones back to INH, to reflect urinary
excretion reliably since these compounds are
thought to be produced in the bladder.
Measurement of INH is not affected by
administration of pyridoxine antidote, and
although INH pyridoxine hydrazone can be seen
in urine, it is also produced post-renally
(Russell, 1972). In all methods,
quantitation is performed by comparison to
the analysis of known amounts of drug
prepared in a similar matrix to the sample
and processed similarly. Note section
8.1.2.1 regarding sample preservation.
AcetylINH can be quantified in urine by
mixing 1 mL of the neutralized acidic
hydrolysate (above) with an equal volume of
0.5M pH 6 buffer (87.7 mL potassium
dihydrophosphate and 12.3 mL potassium
hydrophosphate). Addition of 1 mL freshly
prepared 20% potassium cyanide is followed by
4 mL 12.5% freshly prepared chloramine T.
After 2 minutes, 5 mL of acetone is added and
the pink colour monitored at 550 nm. INH can
also be determined by first acetylating INH
by shaking the acidic urine with one drop of
acetic anhydride, followed by one drop of 7M
NaOH, neutralizing and proceeding as above.
The method gives sufficient sensitivity to
determine acetylation status, and the colour
obtained by the INH and acetylINH
measurements can be compared visually if a
spectrophotometer is not available. Glucose
may reduce recovery by 20%; chlorpromazine
interferes (Eidus et al., 1973).
Colourimetry after derivative formation is
suitable for toxic concentrations of INH in
plasma. After protein precipitation (1 mL
sample with 2 mL water and 1 mL 20%
metaphosphoric acid), the supernatant (2 mL)
is mixed with 1 mL 2M acetic acid and 1 mL
chromogenic reagent (equal volumes of 2%
sodium nitroprusside and 4M NaOH, freshly
prepared). The colour intensity is measured
at 440 nm at 2 minutes, and each sample
should have a reagent blank since the colour
is unstable. Sensitivity is 5 mg/L, and
there is some interference from pyrazinamide
and iproniazid, and from p-aminosalicylate at
very high concentrations (Björnesjö &
Jarnulf, 1967; Baselt, 1987).
UV spectrophotometry after derivative
formation is suitable for therapeutic
concentrations of INH in serum. INH is
extracted from 3 mL serum with 1 drop 4M NaOH
and 3.2 g ammonium sulphate into 20 mL
purified butanol / dichloromethane (3:7) for
30 min. The filtered organic phase is
re-extracted into 1 mL 0.1M HCl. 0.5 mL of
the acid phase is mixed with 0.15 mL 0.04%
trans-cinnamaldehyde solution in absolute
ethanol. Absorbance is measured at 340 nm;
sensitivity is 0.5 mg/L. There is some
interference from pyrazinamide and iproniazid
(Eidus & Harnanansingh, 1971; 1974).
Fluorimetry is useful for therapeutic INH
concentrations. Both assays described
calculate the acetylINH concentration by
difference after hydrolyzing it to INH. The
most sensitive is that described by Ioannou
(1988). Proteins are removed from serum (100
µL) by acetonitrile precipitation (200 µL).
Supernatant (100 µL) is reacted with 100 µL
scandium oxide (10 mM pH 1.1 with sodium
hydroxide) and 50 µL
2-hydroxy-1-naphthaldehyde (10 mM in
acetonitrile) in an ultrasonic bath for 10
min. 2 mL working buffer (50:50 acetonitrile
and 0.1M sodium acetate with 1%
hydroxylammonium chloride, pH 6.3) is added.
The complex formed between scandium and the
hydrazone is strongly fluorescent; excitation
430 nm, emission 510 nm. The author
describes both a kinetic procedure over 1
minute, or an end point reaction at 10 min.
Sensitivity is 0.01 mg/L. Hydrolysis of
acetylINH in a second portion of supernatant
is performed by addition of 20 µL M HCl
heated to 80 °C for 1 hour. The acid is
neutralized with 20 µL M NaOH, and INH
determined as above. No interferences are
known.
The earlier method of Miceli & Olson (1982)
is still widely used and is described here
since it uses more readily available
chemicals. Protein-free supernate is
prepared by addition of 1 mL 10% TCAA to 200
µL serum. INH is measured in one aliquot,
and is converted to a non-reactive azide in a
second aliquot by addition of 100 µL 0.5%
sodium nitrite solution, followed by 5%
ammonium sulphamate (100 µL) to destroy
excess nitrite. The acetylINH is then
hydrolysed to INH by heating with 100 µL 6M
HCl at 80 °C for 1 hour. The extract is
neutralized with 100 µL 6M NaOH. Both
aliquots are then analysed for INH by forming
a hydrazone with 0.5 mL salicylaldehyde
reagent (combine 100 µL salicylaldehyde in 3
mL ethanol with 13.67 g sodium acetate
trihydrate and 2.1 mL 10M NaOH in 250 mL
water). The pH is adjusted to 4 (0.5 M HCl
or NaOH) and left for 15 minutes. Excess
aldehyde is removed by adding 1 mL bisulphite
solution (13.1 g sodium acetate trihydrate,
0.38g sodium bisulphite, 4.15 mL 10M NaOH in
250 mL water), and pH adjusted to 5.7. Add
100 µL 10% ascorbic acid reducing agent, heat
at 50°C for 10 min, and then extract with 1.5
mL iso-butanol. Fluorescence is measured
(386 nm excitation, 462 nm emission) in the
upper organic layer. Sensitivity is 0.02
mg/L, without interference from other
commonly prescribed antitubercular drugs
(Olson et al., 1977; Miceli & Olson,
1982).
Two groups describe schemes for the
measurement of INH, acetylINH, mono- and
di-acetylhydrazine, isonicotinic acid and
isonicotinylglycine in serum and urine using
differential extractions and both
colourimetric and fluorimetric procedures
with good sensitivity (Ellard et al., 1972;
Boxenbaum & Riegelman, 1974).
8.2.2.4 Advanced quantitative method(s)
A number of HPLC methods are
suitable for quantitative analysis of INH and
acetylINH in serum and urine. Older methods
were complicated by poor chromatography
despite derivatization, and those which aim
to detect other antituberculous drugs are
encumbered by multiple extractions or
multiple chromatography of the same sample
extract, or by gradient elution systems.
Quantitation is performed by comparison to
known amounts of INH and acetylINH in
matrix-matched samples extracted under
similar conditions. Several approaches have
been taken to recover INH and acetylINH from
serum. Preparation of a protein-free
filtrate using an centrifugal filter device
(e.g. Amicon Centrifree) such as is commonly
used for the analysis of unbound drugs in TDM
provides clean extracts (Svensson et al.,
1985; Kohno et al., 1991). Alternatively,
salting-out with sodium chloride is required
if solvent extraction (e.g. dichloromethane /
n-butanol (7:3) at neutral or basic pH) is to
be used (Hutchings et al., 1983).
Precipitation of proteins with solid ammonium
sulphate and phosphoric acid, followed by
extraction into acetonitrile has also been
reported (Hsu & Ho, 1989). Urine is
generally incubated at room temperature for
15 minutes with 0.1 M HCl to hydrolyze
acid-labile hydrazones back to INH prior to
analysis in order to reflect urinary
excretion reliably since these compounds are
thought to be produced in the bladder
(Svensson et al., 1985; Khono et al.,
1991).
The best of the UV methods is that of
Hutchings et al., (1983) which uses
iproniazid as internal standard.
Chromatography was performed at ambient
temperature on a Spherisorb nitrile column
with a mobile phase of 0.01 M phosphoric acid
in acetonitrile / water (80:20). Detection
was at 266 nm. Chromatograms showed good
peak shape with separation over 6 minutes,
without interference from other commonly
prescribed antituberculous drugs. The
sensitivity was 0.02 mg/L using a 1 mL
sample. Two other useful UV methods are
described. Hsu & Ho (1989) used niacinamide
as internal standard, with a reverse phase
phenyl column. The mobile phase was 10 mM
phosphate buffer containing 0.25 mM
tetrabutylammonium phosphate as paired-ion
source (pH 4.1). Good separation was seen
over 20 minutes. Detection was at 280 nm,
with a sensitivity of 0.5 mg/L using a 1 mL
sample. Svensson et al (1985) used UV
detection at 270 mn to detect
propionylderivatives of INH and acetylINH.
The derivatives form easily by incubation of
the protein free extract for 10 minutes at
ambient temperature with propionic anhydride
in phosphoric acid. Chromatography was
performed on an Ultrasphere ion-pair reverse
phase column using a mobile phase of 10 mM
sodium dihydrogen phosphate (pH 3 with
phosphoric acid) with 1 mM dodecylsulphate
and 25% acetonitrile. Separation was
achieved over 6 minutes with a sensitivity of
0.2 mg/L from a 1 mL sample.
Kohno et al. (1991) report a fluorimetric
assay with post column deivatization. INH
and metabolites were separated well by
reverse phase ion-exchange chromatography
(C18 column). The mobile phase was 0.067 M
phosphate buffer (pH 6.98) with 3 mM hydrogen
peroxide as fluorogenic agent and 5 mM
butanesulfonate as hydrophobic ion-exchanger.
Detection was at 415 nm emission with 317 nm
excitation, and the sensitivity 0.2 mg/L.
AcetylINH, isonicotinic acid and
isonicotinylglycine were also detected. The
requirement for a 160°C coil/heater module
detracts from its routine application.
El-Yazigi & Yusuf (1991) report a highly
sensitive method for analysis of
underivatized INH and acetylINH using
electrochemical detection with
diphenylcarbazide internal standard.
Excellent chromatography was produced on a
C18 column in a radial compression (Z) module
with a mobile phase of 10 mM sodium dibasic
phosphate solution (pH 7.0 with phosphoric
acid) and methanol (93.5: 6.5). Detection
was at +800 mV, with a sensitivity of 0.1
mg/L on a 100 µL sample. Monoacetylhydrazine
and hydrazine were also seen.
Gas chromatography is not particularly useful
for quantitation of INH in routine clinical
settings. Flame ionization detection gives
poor sensitivity even after derivative
formation (1 mL of serum gives a sensitivity
of only 30 mg/L; Frater-Schröder & Zbinden,
1975), and nitrogen-phosphorus detection is
not much more successful (Timbrell et al.,
1977). Compounds must first be extracted
into a polar organic solvent (e.g. methylene
chloride or ethyl acetate) from a pH adjusted
to 4 to 7. Some methods utilize salting-out
of the drug and metabolites with solid sodium
sulphate. Urine is generally incubated at
room temperature for 15 minutes with 0.1 M
HCl to hydrolyze acid-labile hydrazone
metabolites back to INH prior to analysis.
Some methods are only suited to the analysis
of intact INH (LoDico et al., 1992; Stewart
et al., 1995), or are limited by sample
volume to urine (Timbrell et al., 1977).
Later methods utilize mass spectrometry but
often require deuterated internal standards,
or use tedious differential extraction and
double-derivatization procedures. These
methods have high specificity and are
designed to analyse hydrazine metabolites in
addition to INH and acetylINH, for use in
metabolic and pharmacokinetic research (see
for example, Lauterberg et al.,
1981).
8.2.2.5 Other dedicated method(s)
Not applicable.
8.2.3 Interpretation of toxicological analyses
Specific identification of INH in cases of
severe intoxication where the history is not clear, is
useful to rule out other causes of seizures, confirm
intoxication and ensure appropriate treatment. There
is considerable variation in individual response to a
given serum INH concentration, in both therapeutic and
toxic effects. In overdose, contribution to the
overall clinical picture can be made by co-ingested
medications, the amount of toxic metabolites produced,
the degree of tissue distribution, underlying medical
conditions, presence of infective agents etc. The
rapid distribution kinetics of INH, and relatively
short elimination half-life (1 to 2 hours in fast, and
3 to 5 hours in slow acetylators) means that serum
concentrations fall sharply, and can be only a
fraction (20%) of the peak within 5 or 6 hours.
Measurement is not therefore particularly helpful in
the routine management of the poisoned patient, but
may be considered if symptoms are unusually severe or
prolonged. Case reports with serum concentrations
include the following: Brown, 1972; Watson et al.,
1981; Parish & Brownstein, 1986; Siefkin et al., 1987;
Curnani et al., 1992. However, much of the published
data should be interpreted with caution since authors
have employed different analytical methodologies, and
attention to sample storage is often not considered
(see 8.1.2.1). As a guide, the following table shows
typical concentrations of INH in serum.
mg/L µmol/L
Single oral dose 15 mg/kg (Weber & Hein, 1979)
INH peak 1 hr rapid acetylators 10 - 20 73 - 146
INH peak 1 hr slow acetylators 15 - 25 110 - 183
INH 5 hr rapid acetylators 0.5 - 2.5 3 - 18
INH 5 hr slow acetylators 8 - 12 58 - 88
AcetylINH peak 5 hr rapid acetylators 8 - 12 44 - 67
AcetylINH peak 5 hr slow acetylators 3 - 5 22 -36
Toxicity apparent (nausea, dizziness, >30 >219
visual disturbance)
Potentially fatal (intractable seizures, coma, >50 >365
respiratory distress, metabolic acidosis)
Highest reported concentration in survivor 710 5183
(Watson et al., 1981)
Measurement of INH is not affected by administration
of pyridoxine antidote, and although INH pyridoxine
hydrazone can be seen in urine, it is produced
post-renally (Russell, 1972).
8.3 Biomedical investigations and their interpretation
8.3.1 Biochemical analysis
8.3.1.1 Blood, plasma or serum
- Sodium, potassium, calcium
- Alanine aminotransferase, aspartate
aminotransferase
- gamma-Glutamyltransferrase, alkaline
phosphatase, bilirubin (total and direct),
creatine kinase
- Creatinine (urea), protein
- Glucose, lactate
- Optional: ß-hydroxybutyrate
- Dedicated analysis: pyridoxine
8.3.1.2 Urine
Qualitative testing for glucose,
ketone bodies, sediment, haeme
proteins
8.3.1.3 Other fluids
No dedicated test
8.3.2 Arterial blood gas analyses
pH, pCO2, pO2, actual HCO3-
concentration, base excess, O2-saturation
8.3.3 Haematological Analyses
Count of red and white blood cells, platelets
Haemoglobin, haematocrit
Differential blood picture
Prothrombin time
8.3.4 Interpretation of biomedical investigations
In severe cases of INH poisoning metabolic
acidosis occurs. It is partially caused by elevation
of lactic acid, which cannot be metabolized into
pyruvate as NAD+ production is blocked by INH. The
presence of seizures will contribute to the acidosis,
and cause rhabdomyolysis with elevation in CK and the
appearance of haemeproteinuia. Disseminated
intravascular coagulation may result from
rhabdomyolysis. Liver disease may develop especially
in case of concomitant treatment with carbamazepine,
rifampicin, and phenobarbital. Activity of
transaminases will be elevated as well as bilirubin
concentration. Coagulation profile is changed
likewise. Hyperglycaemia may occur as well as
ketonuria. Renal insufficiency may develop and cause
uraemia. Proteinuria was observed occasionally.
Osmolal as well as anion gap may be enlarged. In
chronic poisoning anaemia may occur as well as
leukocytosis and eosinophilia. Phenytoin metabolism
is inhibited by INH.
8.4 Other biomedical (diagnostic) investigations and their
interpretation
In case of coma, EEG monitoring is recommended.
8.5 Overall Interpretation of Toxicological Analyses & Biomedical
Investigations
Specific identification of INH in cases of intoxication
with seizures is useful to rule out other causes, confirm
intoxication and ensure appropriate treatment. Presumptive
tests on toxic ingredients of materials can be performed by
colourimetric, spectrophotometric or thin layer
chromatographic techniques. High performance liquid
chromatography or gas chromatography of INH are much more
specific.
Measurement of serum concentrations of INH may be useful in
cases where symptoms are particularly severe. INH and
acetylINH are unstable in serum at room temperature, and
unless serum can be stored frozen, the serum proteins should
be precipitated (e.g. with 2 volumes 10% trichloroacetic
acid) as soon as possible. Urine must be treated with dilute
hydrochloric acid (15 minutes at room temperature) to ensure
conversion of acid labile hydrazones back to INH, to reflect
urinary excretion reliably since these compounds are thought
to be produced in the bladder. Measurement of INH is not
affected by administration of pyridoxine antidote, and
although INH pyridoxine hydrazone can be seen in urine, it is
also produced post-renally.
Sensitive fluorescence methods can be applied to the
quantitative analysis of INH and its acetyl metabolite in
serum by differential analysis. Colourimetric and
spectrophotometric methods are less sensitive and less
specific. The degree of specificity is highly
method-dependent. Chromatographic measurement of INH and
metabolites in biological materials is possible after
extraction into a polar organic solvent or precipitation of
proteins. Metabolites (particularly acetylINH, and the
hydrazine derivatives) are seen by most advanced techniques.
Qualitative analysis is easily performed by thin layer
chromatography. High performance liquid chromatography
allows for both qualitative and quantitative analysis, and UV
detection gives adequate sensitivity for most applications.
Gas chromatography and gas chromatography / mass spectrometry
methods usually require derivatization and are rarely used
for clinical analysis.
Typical concentrations of INH in serum are:
mg/L µmol/L
Single oral dose 15 mg/kg (Weber & Hein, 1979)
INH peak 1 hr rapid acetylators 10 - 20 73 - 146
INH peak 1 hr slow acetylators 15 - 25 110 - 183
INH 5 hr rapid acetylators 0.5 - 2.5 3 - 18
INH 5 hr slow acetylators 8 - 12 58 - 88
AcetylINH peak 5 hr rapid acetylators 8 - 12 44 - 67
AcetylINH peak 5 hr slow acetylators 3 - 5 22 -36
Toxicity apparent (nausea, dizziness, visual disturbance) >30 >219
Potentially fatal (intractable seizures, coma, >50 >365
respiratory distress, metabolic acidosis)
Highest reported concentration in survivor 710 5183
(Watson et al., 1981)
INH toxicity manifests as metabolic acidosis, repetitive
refractive convulsions, and coma. Blood gases, pH,
coagulation and hepatic function, renal status, muscle
enzymes and electrolytes should be monitored in the usual
manner.
9. CLINICAL EFFECTS
9.1 Acute poisoning
9.1.1 Ingestion
Toxic manifestations usually appear after a
delay of 1 to 2 hours but they may occur from 30
minutes up to 7 hours following ingestion. The higher
the dose, the shorter the delay of onset of
symptoms.
The first manifestations include: nausea, vomiting,
blurred vision, coloured lights, spots, dizziness,
slurred speech.
A second phase follows rapidly, including severe grand
mal seizures, respiratory distress, coma and severe
metabolic acidosis.
Signs and symptoms may include: fever, lethargy,
stupor, coma, tonicclonic seizures, respiratory
depression, respiratory distress during seizures,
vomiting, nausea, abdominal pain, tachycardia,
hypotension.
9.1.2 Inhalation
Not relevant.
9.1.3 Skin exposure
Not relevant.
9.1.4 Eye contact
Not relevant.
9.1.5 Parenteral exposure
The clinical course is similar to that observed
after ingestion. The delay of onset of symptoms may be
shorter, depending on the dose and the rate of
injection.
9.1.6 Other
No data available.
9.2 Chronic poisoning
9.2.1 Ingestion
Chronic overdose of isoniazid may induce
similar toxicity similar to that of acute poisoning.
Chronic administration may also induce several adverse
effects (see section 7.7). Hepatitis, peripheral
neuropathy are the most frequent manifestations of
chronic ingestion.
9.2.2 Inhalation
Not relevant.
9.2.3 Skin exposure
Not relevant.
9.2.4 Eye contact
Not relevant.
9.2.5 Parenteral exposure
Similar to acute poisoning.
9.2.6 Other
No data available.
9.3 Course, prognosis, cause of death
Course: acute overdose results in seizures associated
with metabolic acidosis, coma and respiratory distress. The
onset of seizure may occur from a few minutes to 3 to 5 hours
post-ingestion
Prognosis: the prognosis depends on the ingested dose and
on the promptness of the treatment. With adequate treatment
and in the absence of complications the prognosis is usually
good after 24-48 hours.
Cause of death: at the early stage (1 to 12 hours) of the
intoxication, death is due to respiratory distress and/or
cardiac arrest (acidosis, hypoxemia) as a result of
seizures.
Deaths due to acute hepatitis, acute pancreatitis, postanoxic
coma have also been reported.
9.4 Systematic description of clinical effects
9.4.1 Cardiovascular
Acute:
- tachycardia and hypotension are common
- cardiac arrest may occur and is due to severe
hypoxemia (respiratory distress) and/or metabolic
acidosis
- no direct cardiotoxicity of INH has been reported
Chronic: no data available.
9.4.2 Respiratory
Acute:
- Respiratory depression with resultant hypoxemia and
cyanosis is frequent during seizures or intractable
grand mal seizures
- aspiration pneumonia may occur
- hypoventilation with Kussmaul-type respiration may
be observed between the periods of seizure
activity
Chronic: no data available.
9.4.3 Neurological
9.4.3.1 Central Nervous System (CNS)
Acute:
- dizziness, slurred speech, stupor,
hallucinations, coma
- tonic-clonic seizures
- areflexia, Babinski sign
Chronic:
Adverse CNS effects following chronic therapy
are rare but may include: stupor, euphoria,
dizziness, memory impairment, ataxia,
cerebellar syndromes, encephalopathy,
seizures (usually dose dependent), psychosis
(Shazma, 1979).
9.4.3.2 Peripheral nervous system
Acute: No data available.
Chronic:
- peripheral neuropathy occurs in about 20 %
of the patients receiving 6 mg/kg/day INH
without supplemental pyridoxine.
Predisposing factors are: slow
acetylation, alcohol, diabetes,
malnutrition. The neuropathy occurs from 3
to 35 weeks after the start of therapy. It
is usually reversible with high doses of
pyridoxine (100 to 200 mg/day).
Administration of small doses of
pyridoxine is recommended in order to
prevent the neuropathy. Symptoms include:
cramps, leg pains, weakness of distal
extremities, decreased tendon
reflexes.
- optic neuropathy (Kass, 1957) has been
reported
- trigeminal neuropathy (Kay, 1972) has been
noted.
9.4.3.3 Autonomic nervous system
Acute: fever is frequently noted as
a result of seizures.
Chronic: fever has been noted in about 1 to
2% of patients during the course of
treatment. This fever is not a prodrome to
other complications.
9.4.3.4 Skeletal and smooth muscle
Acute: rhabdomyolysis is a potential
complication of seizures.
Chronic: rhabdomyolysis may also be seen
after therapeutic dose (Caskright, 1989).
Arthritis, arthralgias and bilateral
shoulder-hand syndrome have also been
reported.
9.4.4 Gastrointestinal
Acute: Nausea, vomiting, abdominal pain are
often the initial presentation (Brown, 1972).
Chronic: Nausea, vomiting, abdominal pain may occur
(10% of the patients treated). Acute pancreatitis has
also been reported (Larsen, 1962).
9.4.5 Hepatic
Acute: Mild hepatic dysfunction with elevation
of transaminases has been observed in a 7-year-old
child after ingestion of 125 mg/kg INH (Oclavski,
1988).
Chronic: An asymptomatic increase of transaminases
occur in 10 to 20% of patients within the first 2
months of therapy (Smith and Sharer, 1969); Byrd,
1972; Kester, 1971).
Hepatitis with jaundice is less common and occurs in
0.1% of the patients treated (Garibaldi, 1972; Maddrey
and Boitmatt, 1973). The clinical presentation is
similar to viral hepatitis.
Fulminant hepatitis has been reported by Pessayre et
al. (1977) in 6 patients, 6 to 10 days after starting
Rifampicin and INH.
9.4.6 Urinary
9.4.6.1 Renal
Acute: Direct INH nephrotoxicity is
not recognized, however, albuminuria and
oliguria progressing to anuria have been
noted in severe poisoning and are probably
secondary to seizure activity and
rhabdomyolysis.
Chronic: Nephrotoxicity is very rare.
Nephrosis may occur when INH is used in
combination with other antituberculosis
agents. One case of acute renal
insufficiency (acute renal nephritis) has
been reported (Trainis, 1981) in a patient
treated for 4 months with INH
alone.
9.4.6.2 Other
No data available.
9.4.7 Endocrine and reproductive systems
Acute: No data available.
Chronic: Gynaecomastia - Bergogne-Berezin (1976) has
reported a case of a 52-yearold man, a slow
acetylator, receiving 10 mg/kg/day INH for 4 months
who developed bilateral gynaecomastia despite a normal
hormonal balance.
9.4.8 Dermatological
Acute: no data available.
Chronic: isoniazid may produce a great variety of
cutaneous effects (Meyler & Peck, 1976):
- atrophic striae may occur after prolonged
therapy
- pruritus (50 % of patients)
- morbilliform eruptions
- pellagra-like syndrome in alcoholic patients
- exfoliative dermatitis has been reported in a
75-year-old man (Rosin & Krig, 1982)
- Stevens Johnson syndrome may occur (Drugdex, 1991)
- acneiform eruptions
- photosensitivity
- syndrome similar to lupus erythematosus
9.4.9 Eye, ear, nose, throat: local effects
Acute: No data available.
Chronic: Optic neuritis with a disturbance of colour
vision and/or decreased visual acuity have been
reported (Kass, 1957; Garrett, 1985; Karmon, 1979).
Optic atrophy may follow optic neuropathy.
9.4.10 Haematological
Acute: Leukocytosis may be observed.
Chronic: Several haematological disturbances may be
observed during INH therapy.
Anaemia (haemolytic, sideroblastic, aplastic,
megaloblastic), agranulocytosis, eosinophilia,
thrombocytopenia; disseminated intravascular
coagulation (Stuart & Roberts, 1976); lymphadenopathy
due to hypersensitivity reactions has been
reported.
9.4.11 Immunological
Acute: no data available.
Chronic: systemic lupus erythematosus with
polyarthralgias, fever, skin rash, lymphadenopathy,
hepatosplenomegaly, pleural and pericardial effusions,
haemolytic anaemia has been reported (Hothersall,
1986; Rothfield, 1971; Gaulier, 1972; Greenberg, 1972;
Grunwald, 1982). LE cells may be found and
antinuclear antibodies are found in 5-33% of treated
patients (Rothfield, 1971).
A positive direct Coombs test has been reported in
patients with haemolytic anaemia (Robinson,
1969).
9.4.12 Metabolic
9.4.12.1 Acidbase disturbances
Severe metabolic acidosis with
increased anion gap is common. This acidosis
is due to an increase of lactate secondary to
anoxia following seizures and to impaired
metabolic conversion of lactate to
pyruvate.
9.4.12.2 Fluid and electrolyte disturbances
Hyperkalaemia has been reported
with high doses of INH (Hanster, 1979) but
hypokalaemia is more frequently observed
(Rouge et al., 1983; Parish & Brownstein,
1986).
Hypocalcaemia has been reported, especially
in Asian patients (Perry, 1982).
9.4.12.3 Others
Hypo- or hyperthermia; hypo- or
hyperglycaemia has been observed.
Acute hyperglycaemia with ketonuria and
glycosuria have been reported.
Chronic: elevation of bilirubin, alkaline
phosphatase, liver transaminases and LDH may
be seen with INH therapy (Mitchell, 1976;
Beaudry, 1974; Black et al., 1975).
Low serum folate levels have been reported.
9.4.13 Allergic reactions
Acute: no data available.
Chronic: one case of hypersensitivity-type reaction
resulting in subconjunctival haemorrhage, optic
neuritis, iridoplegia has been reported (Ahmad-Clark,
1967).
Lymphadenopathy attributed to a hypersensitivity
reaction has also been reported (< 1%).
One case of hypersensitivity meningitis has been
reported (Garageisi, 1976) in a 27-yearold man on
prophylactic INH therapy.
9.4.14 Other clinical effects
Acute: no data available.
Chronic: porphyria may be precipitated by INH.
9.4.15 Special risks
Acute: no data available.
Chronic: 0.75 to 2.3% of the dose is excreted into
breast milk in 24 hours. This corresponds to 6 to 20%
of an usual therapeutic paediatric dose. INH is
classified as category C by Briggs et al. (1986) and
may be used safely during pregnancy.
9.5 Other
No data available.
9.6 Summary
10. MANAGEMENT
10.1 General principles
Patients with INH overdose should always be admitted to
an emergency or intensive care unit. Patients who are
asymptomatic 6 hours after ingestion are unlikely to develop
complications. Monitor vital signs (ECG, BP,
respiration).
Treatment depends on the dose ingested, the symptomatology
and the delay following ingestion. It includes:
- Early gastric lavage after control of seizures and
protection of airway
- Oral activated charcoal
- Supportive treatment:
- control seizures with diazepam and or pentothal
- correct metabolic acidosis by infusion of sodium
bicarbonate solution
- manage respiratory failure by oxygen and artificial
ventilation
- correct hypotension and shock by plasma expanders
and/or dopamine
- Antidote: administration of pyridoxine. Intravenous: 1 g
pyridoxine for each 1 g INH ingested. If the dose
ingested is unknown, initial administration of pyridoxine
may be 5 g intravenously in severly poisoned patients, and
repeated until seizures are under control (see Section
10.6.1).
10.2 Life supportive procedures and symptomatic/specific
treatment
Supportive care with early artificial ventilation,
administration of pyridoxine and anticonvulsant drugs are
indicated in severe poisoning with seizures.
Observation and monitoring
Systematically monitor vital signs, ECG, blood pressure,
respiration and diuresis.
Immediate venous access is indicated for alkalinization, drug
injection and hydration.
Seizures
Treat seizures with intravenous diazepam: initial dose
5 to 10 mg IV and increase the dose if needed.
Artificial ventilation should be performed if respiratory
depression occurs.
Correct pyridoxine deficiency with intravenous pyridoxine.
Recent reports suggest that administration of 1 g pyridoxine
for each gram of isoniazid ingested, abolishes isoniazid-
induced seizure activity (Wasan, 1981; Marbrough, 1983;
Kurtz, 1970). See section 10.6.
Correct severe metabolic acidosis by administration of IV
sodium bicarbonate.
If refractory seizures occur after diazepam and pyridoxine,
barbiturates may be indicated (thiopental 2.5% 5 mg/kg).
EEG monitoring in order to confirm the cessation of cerebral
seizure activity.
Acidosis
The acidosis may be severe with a pH < 7.2
Control of seizures using anticonvulsant drugs (diazepam,
thiopental) and pyridoxine may resolve the acidosis without
the use of IV sodium bicarbonate.
Sodium bicarbonate should be used if the pH is below 7.2
(molar bicarbonate infusion via venous a catheter at a dose
of 1 to 3 mEq/kg).
Monitor arterial blood gases as a guide to therapy.
Respiratory depression
Respiratory failure may occur during seizures, and is common
after the use of anticonvulsant drugs.Therefore, protection
of airway with cuffed endotracheal intubation and artificial
ventilation are indicated in severe intoxications with
seizures.
If aspiration pneumonia has occurred, appropriate treatment
should be instituted.
Hypotension, shock
Correct metabolic acidosis.
Administer fluids (crystalloids or plasma expander solutions)
and dopamine with central monitoring.
Rhabdomyolysis
Perform urine alkalinization and maintain adequate diuresis
by infusion of fluids and mannitol.
10.3 Decontamination
Emesis may be useful in very recent ingestion, but its
risk with impending seizures should be considered very
carefully.
Gastric lavage is indicated in recent ingestion.
In severe cases of acute poisoning, first control seizures
and protect airway by tracheal intubation before performing
gastric lavage.
Oral activated charcoal reduces the absorption and the
toxicity of isoniazid. One dose (50 g) should be given at
the end of the gastric lavage and repeated every 4-6
hours.
The usefulness of cathartics has not been established.
10.4 Enhanced elimination
Diuresis:
4 to 27% of INH is eliminated as free drug in the urine.
Conclusive evidence of efficacy of forced diuresis is not
established although some authors suggests that forced
diuresis enhances INH elimination (Corger, 1976; Terman &
Teitelbaum, 1970; Brown, 1972; Sievers, 1975)
Forced diuresis is therefore not recommended but maintain
adequate urine output.
Dialysis:
Peritoneal dialysis and haemodialysis have been used (Maher,
1967, Cocco, 1963; Brown, 1972; Orlowski et al., 1988).
INH is a small, watersoluble molecule which is poorly protein
bound and distributes in a small volume (0.6 l/kg)). Based
on these properties, INH would efficiently be removed from
the body by dialysis procedures.
However, the relative merits of peritoneal clearance and
total body clearance are not clear. Therefore, haemodialysis
should be reserved for the patients who do not respond to
correct supportive care and adequate doses of pyridoxine.
Because pyridoxine is also dialyzed, the dose may require
adjustment.
Haemoperfusion: No data available. available.
Exchange transfusion: one exchange transfusion has been
performed in a 19-month-old child (Katz, 1956). However,
considering INH kinetics, this procedure is not
recommended.
10.5 Antidote treatment
10.5.1 Adults
INH induces pharmacologic changes in
pyridoxine metabolism (see section 7.1.1.) and
pyridoxine is used as antidote.
Pure pyridoxine (vitamin B6) is recommended as the
antidote for INH poisoning. In situations where this
is not available and a combination vitamins B1, B6 and
B12 is used, there is a risk of anaphylactoid
reactions if vitamin B1 (thiamine) is given in doses
of more than 1 g. Pyridoxine may be given in smaller
aliquots every 10 minutes until the pyridoxine
deficiency is corrected.
Acute poisoning:
Correction of pyridoxine deficiency contributes to the
control of seizures and the correction of metabolic
acidosis (Wason, 1981; Parish & Brownstein, 1986;
Sievers, 1982; Coger, 1976; Yarbrough, 1983)
In acute ingestion of more than 80 mg/Kg,
administration of IV pyridoxine should be considered,
even in asymptomatic patients
The recommended dose is 1 g of pyridoxine for each
gram of isoniazid ingested. Administer 5 g IV in the
first minutes in severely symptomatic patients with
seizures and acidosis, and repeat administration until
seizures are controlled (Poisindex). If the dose
ingested is known follow the same protocol.
Chronic poisoning:
Treatment of isoniazid-induced neuropathy is effected
with pyridoxine 100 to 200 mg daily and withdrawal of
INH when possible.
For the prevention of isoniazid-induced neuropathy: 10
mg pyridoxine daily is adequate in high risk patients
(Katcher, 1982). Higher doses could interfere with
antibacterial efficacy and are unnecessary.
10.5.2 Children
See 10.6.1.
10.6 Management discussion
Asymptomatic patients with suspected isoniazid
poisoning should be monitored for at least 6 hours.
Give an intravenous bolus of pyridoxine (5 g in adults, 1 g
in children) as soon as isoniazid toxicity is suspected. The
total dose of pyridoxine required is 1 g per gram of INH
ingested.
Potentiate the antidotal (anticonvulsant) effects of
pyridoxine with diazepam.
Administration of sodium bicarbonate should be reserved to
cases with severe acidosis (pH < 7.2).
After pyridoxine treatment and supportive care, gastric
lavage and administration of activated charcoal may be
performed.
Blood samples should be sent for monitoring of biological
parameters.
Ensure adequate diuresis. Forced diuresis is not useful.
Dialysis is only indicated in the most severe cases
unresponsive to adequate supportive care, anticonvulsant
drugs and pyridoxine therapy.
Rhabdomyolysis, neuropathy and coagulopathy may occur and
should be treated appropriately.
11. ILLUSTRATIVE CASES
11.1 Case reports from literature
Wason (1981) reported 5 cases of isoniazid poisoning
treated with IV pyridoxine at a dose of 1 g per gram of INH
ingested. The doses ingested ranged from 4 to 25 g (mean 204
mg/kg). The delay before admission was 1 hour in all cases.
Initial serum levels ranged from 26 to 128 mg/L. Grand mal
seizures had occurred in every patient before admission and
continued after adequate doses of diazepam or phenytoin.
After pyridoxine therapy, none of the 5 patients developed
recurrent seizures and metabolic acidosis resolved. All
patients recovered.
Parish & Brownstein (1986) reported the cases of an acute
poisoning with 6 g of INH in a 14 year-old girl and an acute
overdosage in a 8 year-old boy treated for several months
before admission. Both had developed seizures before
admission. Treatment with IV pyridoxine and diazepam was
quickly successful.
Orlowski et al. (1988) reported the case of a 7-year-old
child who had ingested 125 mg/kg of INH and had persistent
metabolic acidosis and coma after 6 g of pyridoxine. A
five-hour haemodialysis was performed 11.5 hours after
ingestion. At the end of dialysis the patient was fully
conscious and free of seizure activity.
Goldin (1987) reported 2 cases of acute ingestion in patients
under prophylactic treatment. One patient (13 years old)
responded to pyridoxine. The other patient (30 years old) was
relatively refractory to initial pyridoxine and other
supportive therapy.
Hartemann et al. (1983) described a case in a 32-month-old
girl with acute poisoning (190 mg/kg) with generalized
seizures. Intravenous pyridoxine was successful and she
recovered.
Rubin (1983) reported a case of chronic ingestion of INH 400
mg/day in a 7 year-old child who developed seizures and
vomiting. A decline in his mental alertness was also
observed; pyridoxine 500 mg IV was administered with
improvement after 2 hours.
12. ADDITIONAL INFORMATION
12.1 Specific preventive measures
Toxic effects can be minimized by prophylactic therapy
with pyyridoxine and careful surveillance of the patient
(Goodman & Gillman, 1990).
12.2 Other
No data available.
13. REFERENCES
Anderson PO (1976) Drugs and breast feeding a review. Drug
Intel Clin Pharm, 11: 208
Baselt RC (1987) Analytical Procedures for Therapeutic Dug
Monitoring and Emergency Toxicology. 2nd ed. California,
Biomedical Publications, pp145-146.
Biehl JP & Vilter RW (1954) Effects of isoniazid on pyridoxine
metabolism. JAMA, 156: 1549.
Björnesjö KB & Jarnulf B (1967) Determination of isonicotinic
acid hydrazide in blood serum. Scand J Clin Lab Invest, 20:
39-40.
Black M, Mitchell JR, Hyman PD et al. (1975) Isoniazidassociated
hepatitis in 114 patients. Gastroenterology, 69 (2): 289302
Bowersox DW et al. (1973) Isoniazid dosage in patients with renal
failure. N Engl J Med, 289: 84
Boxenbaum HG & Riegelman S (1974) Determination of isoniazid and
metabolites and biological fluids. J Pharm Sci, 63: 1191-1197
Brown CV (1972) Acute isoniazid poisoning. Am Rev Resp Dis, 105:
206216
Curnani A, Chawla R, Kundra P, Bhattacharya A (1992) Acute
isoniazid poisoning. Anesthesia, 47: 781-783.
Davies DM (1981) Textbook of adverse drug reactions, 2nd ed.
Oxford University Press, New York
DRUGDEX (1991) Micromedex
Eidus L, Harnanansingh AMT (1971) A more sensitive
spectrophotometric method for determination of isoniazid in serum
or plasma. Clin Chem, 17: 492-494.
Eidus L, Harnanansingh AMT (1974) In: Sunshine I (ed).
Methodology for Analytical Toxicology. Cleveland, Ohio, CRC Press,
pp200-201.
Eidus L, Varughese P, Hodgkin MM, Hsu AHE, McRae KB (1973)
Simplification of isoniazid phenotyping procedure to promote its
application in the chemotherapy of tuberculosis. Bull World
Health Org, 49: 507-516.
El-Brashy AM & El-Ashry SM (1992) Colourimetric and titrimetric
assay of isoniazid. J Pharmaceut Biomed Anal, 10: 421-426.
Ellard GA (1984) The potential clinical significance of the
isoniazid acetylator phenotype in the treatment of pulmonary
tuberculosis. Tubercle, 65: 211227
Ellard GA, Gammon PT, Wallace SM (1972) The determination of
isoniazid and its metabolites acetylisoniazid,
monoacetylhydrazine, diacetylhydrazine, isonicotinic acid and
isonicotinylglycine in serum and urine. Biochem J, 126:
449-458.
El-Yazigi A & Yusuf A (1991) An expedient liquid chromatographic
micromeasurement of isoniazid in plasma by use of electrochemical
detection. Therapeutic Drug Monitor, 13: 254-259.
Flanagan RJ, Braithwaite RA, Brown SS, Widdop B, deWolff FA (1995)
Basic Analytical Toxicology. Geneva, WHO, pp157-158.
Frater-Schröder M, Zbinden G (1975) A specific rapid gas
chromatographic assay for the determination of isoniazid
N-acetylation: observation in rats with induced constant urine
flow. Biochem Med, 14: 274-284.
Goodman LS & Gilman A (1990) The pharmacological basis of
therapeutics. 8th ed. New York
Hartemann E, Barre M, Frederich M (1983) Etat de mal convulsif
par intoxication accidentelle ą l'isoniazide. Pediatrie, 38 (1):
4345
Hsu K-Y & Ho Y (1989) Determination of isoniazid
methanesulphonate and its metabolites in rabbit blood by
high-performance liquid chromatography. J Chromatogr, 493:
305-312.
Hutchings A, Monie RD, Spragg B, Routledge PA (1983)
High-performance liquid chromatographic analysis of isoniazid and
acetylisoniazid in biological fluids. J Chromatogr, 277:
385-390.
Ioannou PC (1988) A more simple, rapid and sensitive fluorimetric
method for the determination of isoniazid and acetylisoniazid in
serum. Application for acetylator phenotyping. Clin Chim Acta,
175: 175-182.
Kohno H, Kubo H, Furukawa K, Yoshino N, Nishikawa T (1991)
Fluorimetric determination of isoniazid and its metabolites in
urine by high performance liquid chromatography using in-line
derivatization. Therapeutic Drug Monitor, 13: 428-432.
Kucers A & Bennet N (1979) The use of antibiotics, 3rd Ed. William
Heinemann Medical Books Ltd, London
Lauterberg BH, Smith CV, Mitchell JR (1981) Determination of
isoniazid and its hydrazine metabolites, acetylisoniazid,
acetylhydrazine, and diacetylhydrazine in human plasma by gas
chromatography-mass spectrometry. J Chromatogr, 224: 431-438.
LoDico CP, Levine BS, Goldberger BA, Capla YH (1992) Distribution
of isoniazid in an overdose death. J Analyt Toxicol, 16:
57-59.
Manoguerra AS (1980) Acute isoniazid toxicity. Clin Toxicol, 16:
407408
Meyers FH, Jawetz E, Goldfien A (1976) Review of medical
pharmacology, 5th ed. Lange Medical Publications
Meyler L & Peck HM (1976) Drug induced diseases. Vol. 4, ed.
Excerpta Medica, Amsterdam
Miceli JN & Olson WA (1982) In Sunshine I & Jatlow PI eds.
Methodology for Analytical Toxicology Volume II. Cleveland, Ohio,
CRC Press, pp 117-118.
Moffat AC, Jackson JV, Moss MS, Widdop B eds. (1986) Clarke's
Isolation and Identification of Drugs. London, Pharmaceutical
Press.
Olson WA, Dayton PG, Israili ZH, Pruitt AW (1977)
Spectrophotofluorimetric assay for isoniazid and acetylisoniazid
in plasma adapted to pediatric studies. Clin Chem, 23:
745-748.
Orlowski JP, Paganini EP, Pippenger CE (1988) Treatment of a
potentially lethal dose isoniazide ingestion. Annals Emerg Med,
17: 7376
Parish RA & Brownstein D (1986) Emergency department management
of children with acute isoniazid poisoning. Paediatric Emergency
Care, 2 (2): 8890
Pessayre D, Bentata M, Degott C et al. (1977) Isoniazidrifampin
fulminant hepatitis Gastroenterology, 72: 284289
Rouge P, Cougot P, Virenque C (1983) Intoxication volontaire par
l'isoniazide. Cahiers d'Anesthésiologie, 31 (4): 389390
RTECS (1979) Volume 1. US Department of Health and human
services. Ed. Richard J. Lewis Sr. and Rodger L. Tatken
Russell DW (1972) Low circulating levels of acid-labile
hydrazones after oral administration of isonicotinic acid
hydrazine. Clin Chim Acta, 41: 163-168.
Siefkin AD, Albertson TE, Corbett MG (1987) Isoniazid overdose:
pharmacokinetics of oral charcoal in treatment. Hum Toxicol, 6:
497501
Stewart MF, Freemont AJ, Richardson T (1995) Fatal isoniazid
poisoning. Ann Clin Biochem, 32: 229-231.
Svensson J-O, Muchtar A, Ericsson O (1985) Ion-pair
high-performance liquid chromatographic determination of isoniazid
and acetylisoniazid in plasma and urine. J Chromatogr, 341:
193-197.
Terman DS & Teitelbaum DT (1970) Isoniazid self poisoning.
Neurology, 20: 299304
Timbrell JA, Wright JM, Smith CM (1977) Determination of
hydrazine metabolites of isoniazid in human urine by gas
chromatography. J Chromatogr, 138: 165-172.
Walubo A, Smith P, Folb P (1994) Comprehensive assay for
pyrizinamide, rifampicin and isoniazid with its hydrazine
metabolites in human plasma by column liquid chromatography. J
Chromatogr, 658: 391-396.
Watson S, Lacouture PG, Lovejoy FH (1981) Single highdose
pyridoxine treatment for isoniazid overdose. JAMA, 246:
11021104
Weber WW & Hein DW (1979) Clinical pharmacokinetics of isoniazid.
Clin Pharmacokinetics, 4: 401-422.
WHO (1986) Basic Tests for Pharmaceutical Substances. Geneva,
WHO, p96.
Wood JD & Peesker SJ (1972) A correlation between changes in GABA
metabolism and isonicotinic acid hydrazine-induced seizures.
Brain Res, 45: 489-498.
14. AUTHOR(S), REVIEWER(S), DATE(S) (INCLUDING UPDATES, COMPLETE
ADDRESS(ES)
Authors: Dr O.J. Kasilo
Drug & Toxicology Information Service (DaTIS)
Department of Pharmacy
University of Zimbabwe Medical School
P.O. Box A 172 Avondale
Harare
Zimbabwe
Dr CFB Nhachi
Department of Clinical Pharmacology and Toxicology
University of Zimbabwe Medical School
P.O. Box A 172 Avondale
Harare
Zimbabwe
Tel: 273-4-7902333 or 791631 ext. 117/172
Fax: 263-4-303 292
Date: September 1989
Co-Authors: Drs M. Dahlet, F. Flesch, A. Jaeger
Service de Réanimation Médicale et Centre AntiPoisons
Hōpitaux Universitaires
Hōpital Civil
67091 Strasbourg Cedex
France
Tel: 33-88161144
Fax: 33-88161330
Date: January 1992
Peer
Review: Newcastle-upon-Tyne, United Kingdom, February 1992.
Author
Section 8: Dr Sheila Dawling
Center for Clinical Toxicology
Vanderbilt University Medical Center
501 Oxford House
1161 21st Avenue South
Nashville, TN 37232-4632
United States of America
Tel: 1-615-9360760
Fax: 1-615-9360756
E-mail: sheila.dawling@mcmail.vanderbilt.edu
Date: March 1998
Editor: Mrs J. Duménil
International Programme on Chemical Safety
Geneva
Date: May 1999
Acetylation phenotype
The rate of acetylation is genetically determined and is
subject to individual variations. Although it may be
influenced by age and weight it is usually constant for each
person. Two groups of people can be distinguished: slow
acetylators and fast acetylators.
The phenotype of slow acetylators is an autosomal recessive
trait and results from a relative deficiency of the hepatic
enzyme Nacetyl transferase. The incidence taken from various
sources estimates 45-55% in Americans, 60% in Europeans, 50-
65% in Caucasians, Blacks, South Indians, Mexicans. The
incidence of fast acetylators is 80-90% in Eskimos, Japanese
and Chinese.
Slow acetylators have higher serum levels of INH at a given
dose. Six hours after ingestion of 4 mg/kg INH, plasma
concentrations are 0.8 mg/L in slow acetylators and only 0.2
mg/L in fast acetylators (Meyers et al., 1976). It has also
been suggested that fast acetylators may have a poorer
response to treatment than slow acetylators (Mitchell, 1958;
Ellard, 1984). The rate of isoniazid acetylation does not
appear to alter efficacy when the drug is administered daily
or 2 or 3 times weekly. However, a relationship between rapid
inactivation and poor therapeutic response has been noted in
once-weekly treatments (Lauterburg et al, 1985).
6.5 Elimination and excretion
Kidney
In adults with normal renal function, approximately 50 to 70%
of a 5 mg/kg oral dose is excreted in urine within 24 hours
as unchanged drug and as metabolites. The percentage of the
different compounds excreted varies with the acetylator
phenotype (Kucers & Bennet, 1979).
Acetyl isoniazid Free isoniazid and
and metabolites hydrazone conjugates
Slow acetylators 63% 37%
Fast acetylators 94% 6%
Breast milk
0.75 to 2.3% of the dose is excreted into breast milk in 24
hours. This corresponds to 6-20% of a usual therapeutic
paediatric dose.
Other routes
Small amounts of the drug are excreted in saliva, sputum and
faeces.
7. PHARMACOLOGY AND TOXICOLOGY
7.1 Mode of action
7.1.1 Toxicodynamics
Tonic-clonic seizures and severe metabolic
acidosis are the most common features in INH
overdose.
Seizures
The precipitating mechanism of the seizures is not
exactly known but it may be related to the INH-induced
deficiency of pyridoxine. INH produces pharmacologic
changes in pyridoxine metabolism (Biehl & Vitter,
1954):
- Increased renal excretion of pyridoxine by
formation of INH-pyridoxine hydrazones
- The hydrazones competitively inhibit pyridoxine
kinase, the activating enzyme that converts
pyridoxine to the physiologically active pyridoxal
phosphate
- Inactivation of the pyridoxal containing enzymes.
The subsequent reduction in pyridoxine and pyridoxal
phosphate inhibits the formation of the inhibitory
neurotransmitter, gamma aminobutyric acid or GABA
(Wood & Peesker, 1972). This reduction in GABA levels
may explain the seizures in patients with INH
poisoning.
Metabolic acidosis
The metabolic acidosis may be related to:
- a lactic acidosis due to the seizures,
- a blockage in the conversion of lactate to
pyruvate,
- an increased metabolism of fatty acids resulting
from an impaired glucose metabolism with
hyperglycaemia and ketonuria (Terman & Teitelbaum,
1970).
Neuropathy
Peripheral neuropathy secondary to chronic exposure is
due to the deficiency of pyridoxine and pyridoxal
phosphate.
Hepatitis
Hepatitis is due to a toxic metabolite of monoacetyl
hydrazine, which binds covalently to liver proteins
(Black et al., 1975). In some patients an allergic
mechanism has also been proposed: acylation of hepatic
macromolecules by acetyl hydrazine may lead to the
release of antigenic macromolecules which induce the
formation of antibodies directed against the liver
(Davies, 1981).
7.1.2 Pharmacodynamics
The exact mechanism of action of INH is not
known. INH may act by inhibition of mycolic acid
synthesis and disruption of the cell wall in
susceptible organisms. Since mycolic acids are unique
to mycobacteria, this action explains the high degree
of selectivity of the antimicrobial activity.
Mutation conferring resistance may occur in
susceptible microorganisms. There is a cross
resistance between INH, rifampicin and ethambutol.
However the simultaneous use of two of these drugs
markedly delays the emergence of resistant mutants
either agent.
7.2 Toxicity
7.2.1 Human data
7.2.1.1 Adults
Acute exposure
Doses of 30 to 40 mg/kg may produce seizures
(Manoguerra, 1980). In adults with prior
seizure disorders, seizures may occur after
ingestion of doses as low as 14 mg/kg.
Doses of 80 to 150 mg/kg produce seizures and
can cause death.
Dose of 150 to 200 mg/kg are often fatal if
not treated (Terman & Teitelbaum, 1970).
Chronic toxicity
Symptoms of chronic toxicity may appear after
therapeutic doses.
7.2.1.2 Children
No data available. See section 7.2.1.1.
7.2.2 Relevant animal data
Species Route Effect & Dose
Rat oral LD50 650 mg/kg
subcutaneous 329 mg/kg
Mouse oral LD 50 176 mg/kg
subcutaneous 160 mg/kg
intramuscular 140 mg/kg
intravenous 149 mg/kg
Dog oral LD 50 150 mg/kg
Rabbit oral LD 50 250 mg/kg
subcutaneous 285 mg/kg
intravenous 94 mg/kg
Guinea Pig oral LD 50 450 mg/kg
subcutaneous 255 mg/kg
(RTECS, 1979)
7.2.3 Relevant in vitro data
Not relevant
7.3 Carcinogenicity
There is no evidence to support carcinogenic effects in
humans. A recent study did not detect an increase in cancer
deaths in a series of 338 women treated with INH for
pulmonary tuberculosis (Meyer et al., 1988). Several other
studies fail to show a carcinogenic effect, including a study
by the US Public Health Service in 25,000 patients followed
up for 9 to 14 years; a study by Scott in 3,842 patients in
the UK; and the studies of Hammond (1967) and Sanders &
Drayer (1979).
7.4 Teratogenicity
INH is classified as category C by Briggs et al. (1986)
and may be used safely during pregnancy. INH and ethambutol
are considered the safest drugs for the treatment of
tuberculosis during pregnancy (Holdiness, 1987; Ludford,
1973).
7.5 Mutagenicity
No data available.
7.6 Interactions
Several drugs may interact with INH:
Drugs which interfere with INH pharmacokinetics:
Aminosalicylic acid, procainamide, propranolol increase INH
serum levels by reduction of the acetylation.
Aluminium-containing antacids decrease the gastrointestinal
absorption of INH.
Pyrazinamide decreases the serum levels of INH.
Drugs which decrease serum levels when used with INH
Cyclosporine, enflurane, folic acid, ketoconazole,
verapamil.
Drugs which increase serum levels when used with INH
Carbamazepine, diazepam, dicoumarol, ethosuximide, phenytoin,
primidone, theophylline.
Other interactions
The concomitant administration of INH and the following drugs
may produce:
- psychiatric responses: tricyclic antidepressants
- hyperglycaemia: chlorpropamide
- agitation or parkinsonian tremor: levodopa
- hypotension: pethidine (meperidine)
- increased incidence of hepatotoxicity: rifampicin
- hypertension, tachycardia, hyperthermia: monoamine
oxidase inhibitors
7.7 Main adverse effects
(Drugdex, 1991)
Peripheral neuropathy and hepatotoxicity are the most
frequently observed adverse effects of INH.
Hepatotoxicity
Asymptomatic elevation of serum aspartate transferase (SGOT)
is noted in 10-20 % of the patients (Scharer and Smith,
1969). This increase is usually transient and the level
returns to normal with continuing therapy. INH should be
discontinued in patients with a transaminase level three
times greater than normal.
Hepatitis with jaundice may occur (0.5 %). In most cases,
hepatitis occurs within the 3 months following the onset of
the treatment. Some factors predisposing to INH
hepatotoxicity include: age, alcohol, rapid acetylator
status, concomitant administration of isoniazid and
rifampicin (Acocella, 1972).
Peripheral neuropathies
Peripheral neuropathy is the most common side effect of INH.
It is secondary to a pyridoxine deficiency. The predisposing
factors are: alcohol, slow acetylator status, diabetes,
malnutrition, pregnancy.
Peripheral neuropathy is dose-related: it is uncommon at
doses below 5 mg/kg and very frequent with doses over 300_mg
daily.
Treatment and prophylaxis are based on administration of
pyridoxine.
Other adverse reactions:
(Drugdex, 1991; Davies, 1981)
Haematologic: disseminated intravascular coagulation
(Roberts, 1976); granulocytosis, eosinophilia, anaemia
(haemolytic, aplastic, sideroblastic, megaloblastic),
thrombocytopenia.
Neurologic: peripheral neuropathy (see 7.7.2),
hypersensitivity meningitis (Goragusi, 1976), dystonias,
encephalopathy, seizures, cerebellar syndromes.
Psychiatric: confusional states, transient memory
impairment, delirium.
Endocrine/metabolic: hyperglycaemia, hypocalcaemia,
porphyria, gynaecomastia.
Gastrointestinal: abdominal pain, acute pancreatitis.
Kidney: nephrotoxicity is a very rare complication, (1 case
of acute renal insufficiency has been described by Traimis,
1981).
Ocular: optic neuropathy (especially when combined therapy
with INH and ethambutol); optic atrophy
Dermatologic: pellagra (due to a nicotinic acid
deficiency), exfoliative dermatitis (one case described by
Rosin, 1982), Steven-Johnson syndrome; cutaneous reactions
(2%), urticaria, angioneurotic oedema, morbilliform
eruptions, purpura, acneiform eruptions,
photosensitivity.
Musculoskeletal: arthritis, arthralgias, systemic lupus
erythematosus; antinuclear antibodies in the serum may
appear, especially in slow acetylators, approximately five
months after the onset of therapy.
8. TOXICOLOGICAL AND BIOMEDICAL INVESTIGATIONS
8.1 Material sampling plan
8.1.1 Sampling and specimen collection
8.1.1.1 Toxicological analyses
Toxic ingredient: tablets, capsules,
liquids, suspect materials
In case of ingestion:
- Vomitus: total amount
- Gastric aspirate: total amount (or gastric
lavage - first portion: 100 mL)
- Blood without additives: 10 mL
- Urine (random specimen): 50 mL
8.1.1.2 Biochemical analyses
Plasma (lithium heparin as
anticoagulant) or serum and urine for
standard biochemical analyses. Whole blood
with anticoagulant and glycolytic inhibitor
(e.g. heparin or fluoride) for lactate
determination.
8.1.1.3 Arterial blood gas analysis
Heparinized arterial blood sample.
8.1.1.4 Haematological analyses
Anticoagulated blood (e.g. EDTA) for
standard haematological analyses and
differential blood picture. Anticoagulated
blood for prothrombin time.
8.1.1.5 Other (unspecified) analyses
No further materials.
8.1.2 Storage of laboratory samples and specimens
8.1.2.1 Toxicological analyses
Serum should be separated and stored
in freezer (-20°C). There is controversy
over the stability of INH and acetylINH in
serum and urine. Some early assays based on
fluorimetry and colourimetry give apparent
loss of INH and acetylINHzid when serum is
stored for longer than 1 to 2 hours, even if
frozen, and it was recommended that protein
be precipitated immediately to ensure
reliable results. However, subsequent
substitution of stronger acids as
precipitating agents release isoniazid from
the proteins to which it becomes bound on
storage. Further investigation shows that
isoniazid and acetylisoniazid are almost
completely lost from serum after one week at
room temperature (acetylINH is hydrolysed to
isonicotinic acid by serum enzymes). Protein
precipitation should therefore be performed
within 12 hours if frozen storage of
separated serum (-20°C) is not possible.
While acetylisoniazid is usually stable in
urine, pronounced decay is sometimes observed
and is therefore thought to be bacterial
(Ellard et al., 1972).
8.1.2.2 Biochemical analyses
No special requirements, but as
usually performed.
8.1.2.3 Arterial blood gas analysis
No special requirements, but as
usually performed.
8.1.2.4 Haematological analyses
Not applicable.
8.1.2.5 Other (unspecified) analyses
Not applicable.
8.1.3 Transport of laboratory samples and specimens
8.1.3.1 Toxicological analyses
No special requirements, but as
usually performed.
8.1.3.2 Biochemical analyses
No special requirements, but as
usually performed.
8.1.3.3 Arterial blood gas analysis
No special requirements, but as
usually performed.
8.1.3.4 Haematological analyses
No special requirements, but as
usually performed.
8.1.3.5 Other (unspecified) analyses
Not applicable.
8.2 Toxicological analyses and their interpretation
8.2.1 Tests on toxic ingredient(s) of material
8.2.1.1 Simple qualitative test(s)
The presence of INH in materials can
be inferred by a number of chemical tests.
Heating of INH (10 mg material) with
anhydrous sodium carbonate (200 mg) releases
pyridine, which is easily detected by its
odour (WHO, 1986).
Several simple colour tests are available,
but results must be interpreted with caution,
since many other drugs give similar
reactions, and the limitations of each test
are given where known.
Nitroprusside test: INH can be reacted with
sodium nitropentacyanoferroate reagent
(freshly prepared equal volume mix of sodium
nitroprusside and 4M NaOH) to give an intense
orange colour which fades slowly (Baselt,
1987; Flanagan et al., 1995).
Nessler's Reagent: Add solid potassium
iodide to a saturated solution of mercuric
chloride until the initial red precipitate
dissolves, then add an equal volume of 10M
NaOH solution. Mix 1 mL of reagent with a
small amount (10 mg) of the material. The
presence of the -NH-NH2 side chain of INH
produces an immediate black colour. A
similar response is given by ortho and para
hydroxyl groups, and by the -NH-NH- group. A
number of other compounds produce a similar
response if heated (Moffat et al., 1986).
UV spectrophotometry: Dissolve a portion of
material in dilute acid (e.g. 0.1M HCl) or
alkali (e.g. 0.1M NaOH) to achieve an
appropriate instrument response. If
necessary, centrifuge or filter the mixture
and analyse the clear supernatant. The
spectrum in aqueous acid gives two equal
9max at 213 and 266 nm (A| = 390); in
alkali 9max is at 272 and 298 nm, but are
not stable.
After dissolving the material in 0.1M HCl,
INH can be coupled at room temperature on
mixing with several reagents to give products
which absorb strongly in the UV region. For
example: 1% trans-cinnamaldehyde in ethanol
(358 nm); 2% vanillin in ethanol / water
(1:3) (380 nm); (Eidus & Harnanansingh,
1971).
There are no commonly-available immunoassay
kits for drug testing which respond to
INH.
Thin layer chromatography may be used for
identification of isoniazid, and may be
either an in-house system or a
commercially-available system such as
Toxi-Lab (Ansys Inc, Irvine, California
92718, USA). Dissolve the material in an
organic solvent such as methanol or
dichloromethane and apply directly to the
plate. Using silica plates without modifiers
and standard systems, the Rf of INH is 0.47
on methanol / concentrated ammonia (100:
1.2), and 0.29 on ethyl acetate / methanol /
ammonia (85:15:6). Acidified iodoplatinate
gives an uncharacteristic dark blue response,
and a positive reaction is obtained with
Dragendorff's with a sensitivity of
approximately 50 ng (Moffat et al.,
1986).
8.2.1.2 Advanced qualitative confirmation test(s)
Gas chromatography can be used after
dissolving the material in a small amount of
organic solvent (e.g. 10 mg in 10 mL
methanol). The retention index for INH is
1650 on OV1, SE30, DB5 or similar phases.
Isothermal analysis may be performed at about
200°C, although peak shape is improved on
temperature-programmed capillary columns.
Flame ionization detection gives adequate
sensitivity. Derivatization of the material
improves chromatography (e.g. treatment of
the dry residue with TFAA at 60°C for 30
minutes (LoDico et al., 1992) or with BSTFA /
1% TMCS for 1 hour at 80°C (Frater-Schröder &
Zbinden, 1975), or with p-chlorobenzaldehyde
in methanol for 30 minutes at room
temperature (Timbrell et al., 1977). Excess
derivatizing reagent is evaporated to
dryness, and the extract reconstituted in a
suitable solvent for GC analysis. The
retention times for the derivatives are
broadly in line with that of underivatized
INH. Characteristic fragmentation on mass
spectrometry is seen, and the most abundant
ions are: INH m/z 78, 106, 51 and 137;
INH-TFA m/z 215, 78, 106 and 146; INH-diTMS
m/z 73, 75, 266, 147 and 117.
HPLC may be used to identify INH in residues
by dissolving a small amount of the suspect
material in the mobile phase, and filtering
if necessary to obtain a clear solution.
Hutchings et al. (1983) performed
chromatography on a Spherisorb nitrile column
with a mobile phase of 0.01 M phosphoric acid
in acetonitrile / water (80:20). Detection
was at 266 nm, and chromatograms showed good
peak shape with separation over 6 min,
without interference from other commonly
prescribed antituberculous drugs.
Alternatively, Hsu & Ho (1989) used a reverse
phase phenyl column with a mobile phase of 10
mM phosphate buffer containing 0.25 mM
tetrabutylammonium phosphate as paired-ion
source (pH 4.1). Good separation was seen
over 20 minutes, with detection at 280 nm.
Additional confirmation of identity may be
obtained by performing a full scan analysis
on the appropriate portion of the HPLC
effluent or incorporating a diode array
detector. El-Yazigi & Yusuf (1991) show
excellent chromatography on a C18 column in a
radial compression (Z) module with a mobile
phase of 10 mM sodium dibasic phosphate (pH
7.0 with phosphoric acid) and methanol (93.5:
6.5); electrochemical detection was at +800
mV.
8.2.1.3 Simple quantitative method(s)
In the methods described below a
known mount of the material is weighed into
an aqueous solution, and centrifuged or
filtered to obtain a clear supernatant for
analysis. Quantitation is performed by
comparison of the response of the material to
the analysis of known amounts of INH prepared
similarly. All methods have adequate
sensitivity for residue analysis, but suffer
interference from compounds of similar
structure.
Colourimetric assays
INH can be reacted with sodium
nitropentacyanoferroate reagent (freshly
prepared equal volume mix of sodium
nitroprusside and 4M NaOH) to give an intense
orange product absorbing at 440 nm. The
colour is unstable, and measurements should
be timed to 2 minutes (Björnesjö & Jarnulf,
1967; Baselt, 1987; Flanagan et al.,
1995).
Chlorpromazine free radical solution
(prepared by treating chlorpromazine with
2-iodoxybenzoic acid in 50% w/v
orthophosphoric acid) absorbs at 530 nm, and
the addition of INH results in quantitative
reduction to colourless chlorpromazine
(El-Brashy & El-Ashry, 1992).
INH in 0.1M HCl can be coupled at room
temperature on mixing with several reagents
to give products which absorb strongly in the
UV region. For example: 1%
trans-cinnamaldehyde in ethanol (358 nm); 2%
vanillin in ethanol / water (1:3) (380 nm)
(Eidus & Harnanansingh, 1971).
Titrimetric determination
INH solution is titrated with
N-bromophthalimide using methyl red
indicator. Alternatively, a known excess of
titrant is added to the INH solution, and the
residual unreacted reagent determined by
iodometric back-titration (El-Brashy &
El-Ashry, 1992).
8.2.1.4 Advanced quantitative method(s)
In the methods described below a
known mount of the material is weighed into
aqueous solution or a suitable organic
solvent. Quantitation is performed by
addition of a suitable internal standard, and
comparison of the response of the material to
the analysis of known amounts of INH prepared
similarly. All methods have adequate
sensitivity for residue analysis.
Gas chromatography can be used directly after
dissolving the material in an organic
solvent. The retention index for INH is 1650
on OV1, SE30, DB5 or similar phases.
Isothermal analysis may be performed at about
200°C, although peak shape is improved on
temperature-programmed capillary columns.
Flame ionization detection gives adequate
sensitivity. Derivatization of the material
improves chromatography (e.g. treatment of
the dry residue with TFAA at 60°C for 30
minutes using phentermine as internal
standard (LoDico et al., 1992), or with
eicosan internal standard and BSTFA / 1% TMCS
for 1 hour at 80°C (Frater-Schröder &
Zbinden, 1975), or with p-chlorobenzaldehyde
in methanol for 30 minutes at room
temperature, followed by addition of
p-bromobenzaldehyde isonicotinyl hydrazone
internal standard (Timbrell et al., 1977).
Excess solvent and derivatizing reagents are
evaporated to dryness, and the extract
reconstituted in a suitable solvent for GC
analysis. The retention times for the
derivatives are broadly in line with that of
underivatized INH. Characteristic
fragmentation is achieved on mass
spectrometry, and the most abundant ions are:
INH m/z 78, 106, 51 and 137; INH-TFAA m/z
215, 78, 106, 146; INH-diTMS m/z 73, 75,
266, 147 and 117.
HPLC may be used to quantify INH in residues,
and two UV methods are highly suitable.
Hutchings et al. (1983) used a Spherisorb
nitrile column with a mobile phase of 0.01 M
phosphoric acid in acetonitrile / water
(80:20). Detection was at 266 nm, and
iproniazid was used as internal standard.
Chromatograms showed good peak shape with
separation over 6 min, without interference
from other commonly prescribed
antituberculous drugs. Alternatively, Hsu &
Ho (1989) used a reverse phase phenyl column
with a mobile phase of 10 mM phosphate buffer
containing 0.25 mM tetrabutyl-ammonium
phosphate as paired-ion source (pH 4.1).
Niacinamide was used as internal standard,
and good separation was seen over 20 minutes,
with detection at 280 nm. Additional
confirmation of identity may be obtained by
performing a full scan analysis on the
appropriate portion of the HPLC effluent or
incorporating a diode array detector.
El-Yazigi & Yusuf (1991) obtained excellent
chromatography on a C18 column in a radial
compression (Z) module with a mobile phase of
10 mM sodium dibasic phosphate solution (pH
7.0 with phosphoric acid) / methanol (93.5:
6.5). Electrochemical detection was at +800
mV, and diphenylcarbazide was used as
internal standard.
8.2.2 Tests for biological specimens
8.2.2.1 Simple qualitative test(s)
There are no commonly-available
immunoassay kits for drug testing which
respond to INH or its metabolites in
biological specimens.
Direct UV methods may be applied to the
detection of INH in gastric contents, but are
not useful for the analysis of other fluids.
Disperse a portion of gastric contents in
dilute acid (e.g. 0.1M HCl) or alkali (e.g.
0.1M NaOH) to achieve an appropriate
instrument response. If necessary,
centrifuge or filter the mixture and analyse
the clear supernatant. The spectrum in
aqueous acid gives 9max at 266 nm (A| =
390); in alkali 9max is at 298 nm.
Thin layer chromatography can be used after
extraction from biological specimens (urine
or gastric contents - 10 - 20 mL) at pH 5 to
7. A polar extraction solvent (e.g.
dichloromethane, ethyl acetate) and
saturation of the aqueous phase with solid
sodium chloride are required to maximize
recovery of INH and metabolites. Treatment of
urine with dilute HCl (15 minutes at room
temperature) greatly increases INH recovery.
The extract is concentrated by evaporation of
the solvent. Thin layer chromatography may
be either an in-house system or a
commercially-available system such as
Toxi-Lab (Ansys Inc, Irvine, California
92718, USA). Using silica plates without
modifiers and the standard methanol /
concentrated ammonia (100: 1.2) system, the
following Rf data are reported by Walubo et
al., 1994. INH 0.55, acetylINH 0.55,
monoacetylhydrazine 0.65, diacetylhydrazine
0.45, hydrazine 0.09. All compounds gave a
positive reaction to acidified iodoplatinate
(dark blue), and to Dragendorff s with a
sensitivity of approximately 50 ng on
plate.
AcetylINH can be detected in urine by mixing
1 mL with an equal volume of 0.5M pH 6 buffer
(87.7 mL potassium dihydrophosphate and 12.3
mL potassium hydrophosphate). Addition of 1
mL freshly prepared 20% potassium cyanide is
followed by 4 mL 12.5% freshly prepared
chloramine T. After 2 minutes, 5 mL of
acetone is added and a cherry pink colour is
formed. Increased sensitivity can be
obtained by first acetylating INH by shaking
the urine with one drop of acetic anhydride,
followed by one drop of 7M NaOH, and
proceeding as above. Chlorpromazine
interferes (Eidus et al., 1973).
Identification of toxic amounts of INH in
urine or serum by spectrophotometry can be
achieved by coupling with chromogenic
reagents. There is interference from
pyrazinamide and iproniazid. For serum, a
protein free filtrate should be prepared
(e.g. with 2 volumes of methanol or
acetonitrile, and the organic solvent
evaporated off), and the supernatant
analysed. For colourimetry, the supernatant
is mixed with 2M acetic acid followed by
equal volumes of 2% sodium nitroprusside and
4M NaOH (freshly prepared). The yellow
colour has maximum absorbance at 440 nm
(Björnesjö & Jarnulf, 1967; Baselt, 1987).
Alternatively, for UV spectrophotometry, the
supernatant is mixed with 0.04%
trans-cinnamaldehyde solution in absolute
ethanol. Absorbance is measured at 340 nm
(Eidus & Harnanansingh, 1971;
1974).
8.2.2.2 Advanced qualitative confirmation test(s)
Gas chromatography can be used for
identification of INH and metabolites in
urine after extraction into a polar organic
solvent (e.g methylene chloride or ethyl
acetate) from a pH adjusted to 4 to 7 using a
phosphate buffer. Some methods utilize
salting-out of the drug and metabolites with
solid sodium sulphate. Urine is generally
incubated at room temperature for 15 minutes
with 0.1 M HCl to hydrolyze acid-labile
hydrazone metabolites back to INH prior to
analysis. Methods have insufficient
sensitivity for serum analysis.
The Retention Index for INH is 1650 on OV1,
SE30, DB5 or similar phases, and for
acetylINH is 1950. Isothermal analysis may
be performed at about 200°C, although peak
shape is improved on temperature-programmed
capillary columns. Flame ionization
detection gives poor sensitivity (2 to 5 ng
on column), which is improved only slightly
by nitrogen-phosphorus detection (Timbrell et
al., 1977). Derivatization improves
chromatography (e.g. treatment of the dry
extracted residue with TFAA at 60°C for 30
min using phentermine as internal standard
(LoDico et al., 1992), or with BSTFA / 1%
TMCS for 1 hour at 80°C (Frater-Schröder &
Zbinden, 1975). The retention times for the
derivatives are broadly in line with that of
underivatized INH. Characteristic
fragmentation is achieved on mass
spectrometry without the need for
derivatization, and the most abundant ions
are: INH m/z 78, 106, 51 and 137; acetyl
INH m/z 179, 106, 137, and 78.
Fragmentation for the derivatives are:
TFAA-INH m/z 215, 78, 106 and 146,
diTMS-INH m/z 73, 75, 266, 147 and 117,
and diTMS-acetylINH 73, 75, 308, 147 and
132.
HPLC is more suited to the identification of
INH and acetylINH. Several approaches have
been taken isolate INH and acetylINH from
serum. Preparation of a protein-free
filtrate using an centrifugal filter device
(e.g. Amicon Centrifree) such as is commonly
used for the analysis of unbound drugs in TDM
provides clean extracts (Svensson et al.,
1985; Kohno et al., 1991). Altrnatively,
salting out with sodium chloride is required
if solvent extraction (e.g. dichloromethane /
n-butanol (7:3) at neutral or basic pH) is to
be used (Hutchings et al., 1983).
Precipitation of proteins with solid ammonium
sulphate and phosphoric acid, followed by
extraction into acetonitrile has also been
reported (Hsu & Ho, 1989). Urine is
generally incubated at room temperature for
15 minutes with 0.1 M HCl to hydrolyze
acid-labile hydrazone metabolites back to INH
prior to analysis (Svensson et al., 1985;
Khono et al., 1991).
Two UV methods are highly suitable. Hutchings
et al. (1983) used a Spherisorb nitrile
column with a mobile phase of 0.01 M
phosphoric acid in acetonitrile / water
(80:20). Detection was at 266 nm.
Chromatograms showed good peak shape with
separation over 6 min, without interference
from other commonly prescribed
antituberculous drugs. Alternatively, Hsu &
Ho (1989) used a reverse phase phenyl column
with a mobile phase of 10 mM phosphate buffer
containing 0.25 mM tetrabutylammonium
phosphate as paired-ion source (pH 4.1).
Good separation was seen over 20 minutes, and
detection was at 280 nm. El-Yazigi & Yusuf
(1991) obtained excellent chromatography on a
C18 column in a radial compression (Z) module
with a mobile phase of 10 mM sodium dibasic
phosphate solution (pH 7.0 with phosphoric
acid) and methanol (93.5: 6.5).
Electrochemical detection was at +800 mV, and
monoacetylhydrazine and hydrazine were also
seen. Additional confirmation of identity
may be obtained by performing a full scan
analysis on the appropriate portion of the
HPLC effluent or incorporating a diode array
detector.
8.2.2.3 Simple quantitative method(s)
Quantitative colourimetric,
spectrophotometric and fluorimetric analysis
of INH in biological fluids are widely used.
Some methods additionally quantify acetylINH.
Urine must be treated with dilute
hydrochloric acid (15 min room temperature)
to ensure conversion of acid labile
hydrazones back to INH, to reflect urinary
excretion reliably since these compounds are
thought to be produced in the bladder.
Measurement of INH is not affected by
administration of pyridoxine antidote, and
although INH pyridoxine hydrazone can be seen
in urine, it is also produced post-renally
(Russell, 1972). In all methods,
quantitation is performed by comparison to
the analysis of known amounts of drug
prepared in a similar matrix to the sample
and processed similarly. Note section
8.1.2.1 regarding sample preservation.
AcetylINH can be quantified in urine by
mixing 1 mL of the neutralized acidic
hydrolysate (above) with an equal volume of
0.5M pH 6 buffer (87.7 mL potassium
dihydrophosphate and 12.3 mL potassium
hydrophosphate). Addition of 1 mL freshly
prepared 20% potassium cyanide is followed by
4 mL 12.5% freshly prepared chloramine T.
After 2 minutes, 5 mL of acetone is added and
the pink colour monitored at 550 nm. INH can
also be determined by first acetylating INH
by shaking the acidic urine with one drop of
acetic anhydride, followed by one drop of 7M
NaOH, neutralizing and proceeding as above.
The method gives sufficient sensitivity to
determine acetylation status, and the colour
obtained by the INH and acetylINH
measurements can be compared visually if a
spectrophotometer is not available. Glucose
may reduce recovery by 20%; chlorpromazine
interferes (Eidus et al., 1973).
Colourimetry after derivative formation is
suitable for toxic concentrations of INH in
plasma. After protein precipitation (1 mL
sample with 2 mL water and 1 mL 20%
metaphosphoric acid), the supernatant (2 mL)
is mixed with 1 mL 2M acetic acid and 1 mL
chromogenic reagent (equal volumes of 2%
sodium nitroprusside and 4M NaOH, freshly
prepared). The colour intensity is measured
at 440 nm at 2 minutes, and each sample
should have a reagent blank since the colour
is unstable. Sensitivity is 5 mg/L, and
there is some interference from pyrazinamide
and iproniazid, and from p-aminosalicylate at
very high concentrations (Björnesjö &
Jarnulf, 1967; Baselt, 1987).
UV spectrophotometry after derivative
formation is suitable for therapeutic
concentrations of INH in serum. INH is
extracted from 3 mL serum with 1 drop 4M NaOH
and 3.2 g ammonium sulphate into 20 mL
purified butanol / dichloromethane (3:7) for
30 min. The filtered organic phase is
re-extracted into 1 mL 0.1M HCl. 0.5 mL of
the acid phase is mixed with 0.15 mL 0.04%
trans-cinnamaldehyde solution in absolute
ethanol. Absorbance is measured at 340 nm;
sensitivity is 0.5 mg/L. There is some
interference from pyrazinamide and iproniazid
(Eidus & Harnanansingh, 1971; 1974).
Fluorimetry is useful for therapeutic INH
concentrations. Both assays described
calculate the acetylINH concentration by
difference after hydrolyzing it to INH. The
most sensitive is that described by Ioannou
(1988). Proteins are removed from serum (100
µL) by acetonitrile precipitation (200 µL).
Supernatant (100 µL) is reacted with 100 µL
scandium oxide (10 mM pH 1.1 with sodium
hydroxide) and 50 µL
2-hydroxy-1-naphthaldehyde (10 mM in
acetonitrile) in an ultrasonic bath for 10
min. 2 mL working buffer (50:50 acetonitrile
and 0.1M sodium acetate with 1%
hydroxylammonium chloride, pH 6.3) is added.
The complex formed between scandium and the
hydrazone is strongly fluorescent; excitation
430 nm, emission 510 nm. The author
describes both a kinetic procedure over 1
minute, or an end point reaction at 10 min.
Sensitivity is 0.01 mg/L. Hydrolysis of
acetylINH in a second portion of supernatant
is performed by addition of 20 µL M HCl
heated to 80 °C for 1 hour. The acid is
neutralized with 20 µL M NaOH, and INH
determined as above. No interferences are
known.
The earlier method of Miceli & Olson (1982)
is still widely used and is described here
since it uses more readily available
chemicals. Protein-free supernate is
prepared by addition of 1 mL 10% TCAA to 200
µL serum. INH is measured in one aliquot,
and is converted to a non-reactive azide in a
second aliquot by addition of 100 µL 0.5%
sodium nitrite solution, followed by 5%
ammonium sulphamate (100 µL) to destroy
excess nitrite. The acetylINH is then
hydrolysed to INH by heating with 100 µL 6M
HCl at 80 °C for 1 hour. The extract is
neutralized with 100 µL 6M NaOH. Both
aliquots are then analysed for INH by forming
a hydrazone with 0.5 mL salicylaldehyde
reagent (combine 100 µL salicylaldehyde in 3
mL ethanol with 13.67 g sodium acetate
trihydrate and 2.1 mL 10M NaOH in 250 mL
water). The pH is adjusted to 4 (0.5 M HCl
or NaOH) and left for 15 minutes. Excess
aldehyde is removed by adding 1 mL bisulphite
solution (13.1 g sodium acetate trihydrate,
0.38g sodium bisulphite, 4.15 mL 10M NaOH in
250 mL water), and pH adjusted to 5.7. Add
100 µL 10% ascorbic acid reducing agent, heat
at 50°C for 10 min, and then extract with 1.5
mL iso-butanol. Fluorescence is measured
(386 nm excitation, 462 nm emission) in the
upper organic layer. Sensitivity is 0.02
mg/L, without interference from other
commonly prescribed antitubercular drugs
(Olson et al., 1977; Miceli & Olson,
1982).
Two groups describe schemes for the
measurement of INH, acetylINH, mono- and
di-acetylhydrazine, isonicotinic acid and
isonicotinylglycine in serum and urine using
differential extractions and both
colourimetric and fluorimetric procedures
with good sensitivity (Ellard et al., 1972;
Boxenbaum & Riegelman, 1974).
8.2.2.4 Advanced quantitative method(s)
A number of HPLC methods are
suitable for quantitative analysis of INH and
acetylINH in serum and urine. Older methods
were complicated by poor chromatography
despite derivatization, and those which aim
to detect other antituberculous drugs are
encumbered by multiple extractions or
multiple chromatography of the same sample
extract, or by gradient elution systems.
Quantitation is performed by comparison to
known amounts of INH and acetylINH in
matrix-matched samples extracted under
similar conditions. Several approaches have
been taken to recover INH and acetylINH from
serum. Preparation of a protein-free
filtrate using an centrifugal filter device
(e.g. Amicon Centrifree) such as is commonly
used for the analysis of unbound drugs in TDM
provides clean extracts (Svensson et al.,
1985; Kohno et al., 1991). Alternatively,
salting-out with sodium chloride is required
if solvent extraction (e.g. dichloromethane /
n-butanol (7:3) at neutral or basic pH) is to
be used (Hutchings et al., 1983).
Precipitation of proteins with solid ammonium
sulphate and phosphoric acid, followed by
extraction into acetonitrile has also been
reported (Hsu & Ho, 1989). Urine is
generally incubated at room temperature for
15 minutes with 0.1 M HCl to hydrolyze
acid-labile hydrazones back to INH prior to
analysis in order to reflect urinary
excretion reliably since these compounds are
thought to be produced in the bladder
(Svensson et al., 1985; Khono et al.,
1991).
The best of the UV methods is that of
Hutchings et al., (1983) which uses
iproniazid as internal standard.
Chromatography was performed at ambient
temperature on a Spherisorb nitrile column
with a mobile phase of 0.01 M phosphoric acid
in acetonitrile / water (80:20). Detection
was at 266 nm. Chromatograms showed good
peak shape with separation over 6 minutes,
without interference from other commonly
prescribed antituberculous drugs. The
sensitivity was 0.02 mg/L using a 1 mL
sample. Two other useful UV methods are
described. Hsu & Ho (1989) used niacinamide
as internal standard, with a reverse phase
phenyl column. The mobile phase was 10 mM
phosphate buffer containing 0.25 mM
tetrabutylammonium phosphate as paired-ion
source (pH 4.1). Good separation was seen
over 20 minutes. Detection was at 280 nm,
with a sensitivity of 0.5 mg/L using a 1 mL
sample. Svensson et al (1985) used UV
detection at 270 mn to detect
propionylderivatives of INH and acetylINH.
The derivatives form easily by incubation of
the protein free extract for 10 minutes at
ambient temperature with propionic anhydride
in phosphoric acid. Chromatography was
performed on an Ultrasphere ion-pair reverse
phase column using a mobile phase of 10 mM
sodium dihydrogen phosphate (pH 3 with
phosphoric acid) with 1 mM dodecylsulphate
and 25% acetonitrile. Separation was
achieved over 6 minutes with a sensitivity of
0.2 mg/L from a 1 mL sample.
Kohno et al. (1991) report a fluorimetric
assay with post column deivatization. INH
and metabolites were separated well by
reverse phase ion-exchange chromatography
(C18 column). The mobile phase was 0.067 M
phosphate buffer (pH 6.98) with 3 mM hydrogen
peroxide as fluorogenic agent and 5 mM
butanesulfonate as hydrophobic ion-exchanger.
Detection was at 415 nm emission with 317 nm
excitation, and the sensitivity 0.2 mg/L.
AcetylINH, isonicotinic acid and
isonicotinylglycine were also detected. The
requirement for a 160°C coil/heater module
detracts from its routine application.
El-Yazigi & Yusuf (1991) report a highly
sensitive method for analysis of
underivatized INH and acetylINH using
electrochemical detection with
diphenylcarbazide internal standard.
Excellent chromatography was produced on a
C18 column in a radial compression (Z) module
with a mobile phase of 10 mM sodium dibasic
phosphate solution (pH 7.0 with phosphoric
acid) and methanol (93.5: 6.5). Detection
was at +800 mV, with a sensitivity of 0.1
mg/L on a 100 µL sample. Monoacetylhydrazine
and hydrazine were also seen.
Gas chromatography is not particularly useful
for quantitation of INH in routine clinical
settings. Flame ionization detection gives
poor sensitivity even after derivative
formation (1 mL of serum gives a sensitivity
of only 30 mg/L; Frater-Schröder & Zbinden,
1975), and nitrogen-phosphorus detection is
not much more successful (Timbrell et al.,
1977). Compounds must first be extracted
into a polar organic solvent (e.g. methylene
chloride or ethyl acetate) from a pH adjusted
to 4 to 7. Some methods utilize salting-out
of the drug and metabolites with solid sodium
sulphate. Urine is generally incubated at
room temperature for 15 minutes with 0.1 M
HCl to hydrolyze acid-labile hydrazone
metabolites back to INH prior to analysis.
Some methods are only suited to the analysis
of intact INH (LoDico et al., 1992; Stewart
et al., 1995), or are limited by sample
volume to urine (Timbrell et al., 1977).
Later methods utilize mass spectrometry but
often require deuterated internal standards,
or use tedious differential extraction and
double-derivatization procedures. These
methods have high specificity and are
designed to analyse hydrazine metabolites in
addition to INH and acetylINH, for use in
metabolic and pharmacokinetic research (see
for example, Lauterberg et al.,
1981).
8.2.2.5 Other dedicated method(s)
Not applicable.
8.2.3 Interpretation of toxicological analyses
Specific identification of INH in cases of
severe intoxication where the history is not clear, is
useful to rule out other causes of seizures, confirm
intoxication and ensure appropriate treatment. There
is considerable variation in individual response to a
given serum INH concentration, in both therapeutic and
toxic effects. In overdose, contribution to the
overall clinical picture can be made by co-ingested
medications, the amount of toxic metabolites produced,
the degree of tissue distribution, underlying medical
conditions, presence of infective agents etc. The
rapid distribution kinetics of INH, and relatively
short elimination half-life (1 to 2 hours in fast, and
3 to 5 hours in slow acetylators) means that serum
concentrations fall sharply, and can be only a
fraction (20%) of the peak within 5 or 6 hours.
Measurement is not therefore particularly helpful in
the routine management of the poisoned patient, but
may be considered if symptoms are unusually severe or
prolonged. Case reports with serum concentrations
include the following: Brown, 1972; Watson et al.,
1981; Parish & Brownstein, 1986; Siefkin et al., 1987;
Curnani et al., 1992. However, much of the published
data should be interpreted with caution since authors
have employed different analytical methodologies, and
attention to sample storage is often not considered
(see 8.1.2.1). As a guide, the following table shows
typical concentrations of INH in serum.
mg/L µmol/L
Single oral dose 15 mg/kg (Weber & Hein, 1979)
INH peak 1 hr rapid acetylators 10 - 20 73 - 146
INH peak 1 hr slow acetylators 15 - 25 110 - 183
INH 5 hr rapid acetylators 0.5 - 2.5 3 - 18
INH 5 hr slow acetylators 8 - 12 58 - 88
AcetylINH peak 5 hr rapid acetylators 8 - 12 44 - 67
AcetylINH peak 5 hr slow acetylators 3 - 5 22 -36
Toxicity apparent (nausea, dizziness, >30 >219
visual disturbance)
Potentially fatal (intractable seizures, coma, >50 >365
respiratory distress, metabolic acidosis)
Highest reported concentration in survivor 710 5183
(Watson et al., 1981)
Measurement of INH is not affected by administration
of pyridoxine antidote, and although INH pyridoxine
hydrazone can be seen in urine, it is produced
post-renally (Russell, 1972).
8.3 Biomedical investigations and their interpretation
8.3.1 Biochemical analysis
8.3.1.1 Blood, plasma or serum
- Sodium, potassium, calcium
- Alanine aminotransferase, aspartate
aminotransferase
- gamma-Glutamyltransferrase, alkaline
phosphatase, bilirubin (total and direct),
creatine kinase
- Creatinine (urea), protein
- Glucose, lactate
- Optional: ß-hydroxybutyrate
- Dedicated analysis: pyridoxine
8.3.1.2 Urine
Qualitative testing for glucose,
ketone bodies, sediment, haeme
proteins
8.3.1.3 Other fluids
No dedicated test
8.3.2 Arterial blood gas analyses
pH, pCO2, pO2, actual HCO3-
concentration, base excess, O2-saturation
8.3.3 Haematological Analyses
Count of red and white blood cells, platelets
Haemoglobin, haematocrit
Differential blood picture
Prothrombin time
8.3.4 Interpretation of biomedical investigations
In severe cases of INH poisoning metabolic
acidosis occurs. It is partially caused by elevation
of lactic acid, which cannot be metabolized into
pyruvate as NAD+ production is blocked by INH. The
presence of seizures will contribute to the acidosis,
and cause rhabdomyolysis with elevation in CK and the
appearance of haemeproteinuia. Disseminated
intravascular coagulation may result from
rhabdomyolysis. Liver disease may develop especially
in case of concomitant treatment with carbamazepine,
rifampicin, and phenobarbital. Activity of
transaminases will be elevated as well as bilirubin
concentration. Coagulation profile is changed
likewise. Hyperglycaemia may occur as well as
ketonuria. Renal insufficiency may develop and cause
uraemia. Proteinuria was observed occasionally.
Osmolal as well as anion gap may be enlarged. In
chronic poisoning anaemia may occur as well as
leukocytosis and eosinophilia. Phenytoin metabolism
is inhibited by INH.
8.4 Other biomedical (diagnostic) investigations and their
interpretation
In case of coma, EEG monitoring is recommended.
8.5 Overall Interpretation of Toxicological Analyses & Biomedical
Investigations
Specific identification of INH in cases of intoxication
with seizures is useful to rule out other causes, confirm
intoxication and ensure appropriate treatment. Presumptive
tests on toxic ingredients of materials can be performed by
colourimetric, spectrophotometric or thin layer
chromatographic techniques. High performance liquid
chromatography or gas chromatography of INH are much more
specific.
Measurement of serum concentrations of INH may be useful in
cases where symptoms are particularly severe. INH and
acetylINH are unstable in serum at room temperature, and
unless serum can be stored frozen, the serum proteins should
be precipitated (e.g. with 2 volumes 10% trichloroacetic
acid) as soon as possible. Urine must be treated with dilute
hydrochloric acid (15 minutes at room temperature) to ensure
conversion of acid labile hydrazones back to INH, to reflect
urinary excretion reliably since these compounds are thought
to be produced in the bladder. Measurement of INH is not
affected by administration of pyridoxine antidote, and
although INH pyridoxine hydrazone can be seen in urine, it is
also produced post-renally.
Sensitive fluorescence methods can be applied to the
quantitative analysis of INH and its acetyl metabolite in
serum by differential analysis. Colourimetric and
spectrophotometric methods are less sensitive and less
specific. The degree of specificity is highly
method-dependent. Chromatographic measurement of INH and
metabolites in biological materials is possible after
extraction into a polar organic solvent or precipitation of
proteins. Metabolites (particularly acetylINH, and the
hydrazine derivatives) are seen by most advanced techniques.
Qualitative analysis is easily performed by thin layer
chromatography. High performance liquid chromatography
allows for both qualitative and quantitative analysis, and UV
detection gives adequate sensitivity for most applications.
Gas chromatography and gas chromatography / mass spectrometry
methods usually require derivatization and are rarely used
for clinical analysis.
Typical concentrations of INH in serum are:
mg/L µmol/L
Single oral dose 15 mg/kg (Weber & Hein, 1979)
INH peak 1 hr rapid acetylators 10 - 20 73 - 146
INH peak 1 hr slow acetylators 15 - 25 110 - 183
INH 5 hr rapid acetylators 0.5 - 2.5 3 - 18
INH 5 hr slow acetylators 8 - 12 58 - 88
AcetylINH peak 5 hr rapid acetylators 8 - 12 44 - 67
AcetylINH peak 5 hr slow acetylators 3 - 5 22 -36
Toxicity apparent (nausea, dizziness, visual disturbance) >30 >219
Potentially fatal (intractable seizures, coma, >50 >365
respiratory distress, metabolic acidosis)
Highest reported concentration in survivor 710 5183
(Watson et al., 1981)
INH toxicity manifests as metabolic acidosis, repetitive
refractive convulsions, and coma. Blood gases, pH,
coagulation and hepatic function, renal status, muscle
enzymes and electrolytes should be monitored in the usual
manner.
9. CLINICAL EFFECTS
9.1 Acute poisoning
9.1.1 Ingestion
Toxic manifestations usually appear after a
delay of 1 to 2 hours but they may occur from 30
minutes up to 7 hours following ingestion. The higher
the dose, the shorter the delay of onset of
symptoms.
The first manifestations include: nausea, vomiting,
blurred vision, coloured lights, spots, dizziness,
slurred speech.
A second phase follows rapidly, including severe grand
mal seizures, respiratory distress, coma and severe
metabolic acidosis.
Signs and symptoms may include: fever, lethargy,
stupor, coma, tonicclonic seizures, respiratory
depression, respiratory distress during seizures,
vomiting, nausea, abdominal pain, tachycardia,
hypotension.
9.1.2 Inhalation
Not relevant.
9.1.3 Skin exposure
Not relevant.
9.1.4 Eye contact
Not relevant.
9.1.5 Parenteral exposure
The clinical course is similar to that observed
after ingestion. The delay of onset of symptoms may be
shorter, depending on the dose and the rate of
injection.
9.1.6 Other
No data available.
9.2 Chronic poisoning
9.2.1 Ingestion
Chronic overdose of isoniazid may induce
similar toxicity similar to that of acute poisoning.
Chronic administration may also induce several adverse
effects (see section 7.7). Hepatitis, peripheral
neuropathy are the most frequent manifestations of
chronic ingestion.
9.2.2 Inhalation
Not relevant.
9.2.3 Skin exposure
Not relevant.
9.2.4 Eye contact
Not relevant.
9.2.5 Parenteral exposure
Similar to acute poisoning.
9.2.6 Other
No data available.
9.3 Course, prognosis, cause of death
Course: acute overdose results in seizures associated
with metabolic acidosis, coma and respiratory distress. The
onset of seizure may occur from a few minutes to 3 to 5 hours
post-ingestion
Prognosis: the prognosis depends on the ingested dose and
on the promptness of the treatment. With adequate treatment
and in the absence of complications the prognosis is usually
good after 24-48 hours.
Cause of death: at the early stage (1 to 12 hours) of the
intoxication, death is due to respiratory distress and/or
cardiac arrest (acidosis, hypoxemia) as a result of
seizures.
Deaths due to acute hepatitis, acute pancreatitis, postanoxic
coma have also been reported.
9.4 Systematic description of clinical effects
9.4.1 Cardiovascular
Acute:
- tachycardia and hypotension are common
- cardiac arrest may occur and is due to severe
hypoxemia (respiratory distress) and/or metabolic
acidosis
- no direct cardiotoxicity of INH has been reported
Chronic: no data available.
9.4.2 Respiratory
Acute:
- Respiratory depression with resultant hypoxemia and
cyanosis is frequent during seizures or intractable
grand mal seizures
- aspiration pneumonia may occur
- hypoventilation with Kussmaul-type respiration may
be observed between the periods of seizure
activity
Chronic: no data available.
9.4.3 Neurological
9.4.3.1 Central Nervous System (CNS)
Acute:
- dizziness, slurred speech, stupor,
hallucinations, coma
- tonic-clonic seizures
- areflexia, Babinski sign
Chronic:
Adverse CNS effects following chronic therapy
are rare but may include: stupor, euphoria,
dizziness, memory impairment, ataxia,
cerebellar syndromes, encephalopathy,
seizures (usually dose dependent), psychosis
(Shazma, 1979).
9.4.3.2 Peripheral nervous system
Acute: No data available.
Chronic:
- peripheral neuropathy occurs in about 20 %
of the patients receiving 6 mg/kg/day INH
without supplemental pyridoxine.
Predisposing factors are: slow
acetylation, alcohol, diabetes,
malnutrition. The neuropathy occurs from 3
to 35 weeks after the start of therapy. It
is usually reversible with high doses of
pyridoxine (100 to 200 mg/day).
Administration of small doses of
pyridoxine is recommended in order to
prevent the neuropathy. Symptoms include:
cramps, leg pains, weakness of distal
extremities, decreased tendon
reflexes.
- optic neuropathy (Kass, 1957) has been
reported
- trigeminal neuropathy (Kay, 1972) has been
noted.
9.4.3.3 Autonomic nervous system
Acute: fever is frequently noted as
a result of seizures.
Chronic: fever has been noted in about 1 to
2% of patients during the course of
treatment. This fever is not a prodrome to
other complications.
9.4.3.4 Skeletal and smooth muscle
Acute: rhabdomyolysis is a potential
complication of seizures.
Chronic: rhabdomyolysis may also be seen
after therapeutic dose (Caskright, 1989).
Arthritis, arthralgias and bilateral
shoulder-hand syndrome have also been
reported.
9.4.4 Gastrointestinal
Acute: Nausea, vomiting, abdominal pain are
often the initial presentation (Brown, 1972).
Chronic: Nausea, vomiting, abdominal pain may occur
(10% of the patients treated). Acute pancreatitis has
also been reported (Larsen, 1962).
9.4.5 Hepatic
Acute: Mild hepatic dysfunction with elevation
of transaminases has been observed in a 7-year-old
child after ingestion of 125 mg/kg INH (Oclavski,
1988).
Chronic: An asymptomatic increase of transaminases
occur in 10 to 20% of patients within the first 2
months of therapy (Smith and Sharer, 1969); Byrd,
1972; Kester, 1971).
Hepatitis with jaundice is less common and occurs in
0.1% of the patients treated (Garibaldi, 1972; Maddrey
and Boitmatt, 1973). The clinical presentation is
similar to viral hepatitis.
Fulminant hepatitis has been reported by Pessayre et
al. (1977) in 6 patients, 6 to 10 days after starting
Rifampicin and INH.
9.4.6 Urinary
9.4.6.1 Renal
Acute: Direct INH nephrotoxicity is
not recognized, however, albuminuria and
oliguria progressing to anuria have been
noted in severe poisoning and are probably
secondary to seizure activity and
rhabdomyolysis.
Chronic: Nephrotoxicity is very rare.
Nephrosis may occur when INH is used in
combination with other antituberculosis
agents. One case of acute renal
insufficiency (acute renal nephritis) has
been reported (Trainis, 1981) in a patient
treated for 4 months with INH
alone.
9.4.6.2 Other
No data available.
9.4.7 Endocrine and reproductive systems
Acute: No data available.
Chronic: Gynaecomastia - Bergogne-Berezin (1976) has
reported a case of a 52-yearold man, a slow
acetylator, receiving 10 mg/kg/day INH for 4 months
who developed bilateral gynaecomastia despite a normal
hormonal balance.
9.4.8 Dermatological
Acute: no data available.
Chronic: isoniazid may produce a great variety of
cutaneous effects (Meyler & Peck, 1976):
- atrophic striae may occur after prolonged
therapy
- pruritus (50 % of patients)
- morbilliform eruptions
- pellagra-like syndrome in alcoholic patients
- exfoliative dermatitis has been reported in a
75-year-old man (Rosin & Krig, 1982)
- Stevens Johnson syndrome may occur (Drugdex, 1991)
- acneiform eruptions
- photosensitivity
- syndrome similar to lupus erythematosus
9.4.9 Eye, ear, nose, throat: local effects
Acute: No data available.
Chronic: Optic neuritis with a disturbance of colour
vision and/or decreased visual acuity have been
reported (Kass, 1957; Garrett, 1985; Karmon, 1979).
Optic atrophy may follow optic neuropathy.
9.4.10 Haematological
Acute: Leukocytosis may be observed.
Chronic: Several haematological disturbances may be
observed during INH therapy.
Anaemia (haemolytic, sideroblastic, aplastic,
megaloblastic), agranulocytosis, eosinophilia,
thrombocytopenia; disseminated intravascular
coagulation (Stuart & Roberts, 1976); lymphadenopathy
due to hypersensitivity reactions has been
reported.
9.4.11 Immunological
Acute: no data available.
Chronic: systemic lupus erythematosus with
polyarthralgias, fever, skin rash, lymphadenopathy,
hepatosplenomegaly, pleural and pericardial effusions,
haemolytic anaemia has been reported (Hothersall,
1986; Rothfield, 1971; Gaulier, 1972; Greenberg, 1972;
Grunwald, 1982). LE cells may be found and
antinuclear antibodies are found in 5-33% of treated
patients (Rothfield, 1971).
A positive direct Coombs test has been reported in
patients with haemolytic anaemia (Robinson,
1969).
9.4.12 Metabolic
9.4.12.1 Acidbase disturbances
Severe metabolic acidosis with
increased anion gap is common. This acidosis
is due to an increase of lactate secondary to
anoxia following seizures and to impaired
metabolic conversion of lactate to
pyruvate.
9.4.12.2 Fluid and electrolyte disturbances
Hyperkalaemia has been reported
with high doses of INH (Hanster, 1979) but
hypokalaemia is more frequently observed
(Rouge et al., 1983; Parish & Brownstein,
1986).
Hypocalcaemia has been reported, especially
in Asian patients (Perry, 1982).
9.4.12.3 Others
Hypo- or hyperthermia; hypo- or
hyperglycaemia has been observed.
Acute hyperglycaemia with ketonuria and
glycosuria have been reported.
Chronic: elevation of bilirubin, alkaline
phosphatase, liver transaminases and LDH may
be seen with INH therapy (Mitchell, 1976;
Beaudry, 1974; Black et al., 1975).
Low serum folate levels have been reported.
9.4.13 Allergic reactions
Acute: no data available.
Chronic: one case of hypersensitivity-type reaction
resulting in subconjunctival haemorrhage, optic
neuritis, iridoplegia has been reported (Ahmad-Clark,
1967).
Lymphadenopathy attributed to a hypersensitivity
reaction has also been reported (< 1%).
One case of hypersensitivity meningitis has been
reported (Garageisi, 1976) in a 27-yearold man on
prophylactic INH therapy.
9.4.14 Other clinical effects
Acute: no data available.
Chronic: porphyria may be precipitated by INH.
9.4.15 Special risks
Acute: no data available.
Chronic: 0.75 to 2.3% of the dose is excreted into
breast milk in 24 hours. This corresponds to 6 to 20%
of an usual therapeutic paediatric dose. INH is
classified as category C by Briggs et al. (1986) and
may be used safely during pregnancy.
9.5 Other
No data available.
9.6 Summary
10. MANAGEMENT
10.1 General principles
Patients with INH overdose should always be admitted to
an emergency or intensive care unit. Patients who are
asymptomatic 6 hours after ingestion are unlikely to develop
complications. Monitor vital signs (ECG, BP,
respiration).
Treatment depends on the dose ingested, the symptomatology
and the delay following ingestion. It includes:
- Early gastric lavage after control of seizures and
protection of airway
- Oral activated charcoal
- Supportive treatment:
- control seizures with diazepam and or pentothal
- correct metabolic acidosis by infusion of sodium
bicarbonate solution
- manage respiratory failure by oxygen and artificial
ventilation
- correct hypotension and shock by plasma expanders
and/or dopamine
- Antidote: administration of pyridoxine. Intravenous: 1 g
pyridoxine for each 1 g INH ingested. If the dose
ingested is unknown, initial administration of pyridoxine
may be 5 g intravenously in severly poisoned patients, and
repeated until seizures are under control (see Section
10.6.1).
10.2 Life supportive procedures and symptomatic/specific
treatment
Supportive care with early artificial ventilation,
administration of pyridoxine and anticonvulsant drugs are
indicated in severe poisoning with seizures.
Observation and monitoring
Systematically monitor vital signs, ECG, blood pressure,
respiration and diuresis.
Immediate venous access is indicated for alkalinization, drug
injection and hydration.
Seizures
Treat seizures with intravenous diazepam: initial dose
5 to 10 mg IV and increase the dose if needed.
Artificial ventilation should be performed if respiratory
depression occurs.
Correct pyridoxine deficiency with intravenous pyridoxine.
Recent reports suggest that administration of 1 g pyridoxine
for each gram of isoniazid ingested, abolishes isoniazid-
induced seizure activity (Wasan, 1981; Marbrough, 1983;
Kurtz, 1970). See section 10.6.
Correct severe metabolic acidosis by administration of IV
sodium bicarbonate.
If refractory seizures occur after diazepam and pyridoxine,
barbiturates may be indicated (thiopental 2.5% 5 mg/kg).
EEG monitoring in order to confirm the cessation of cerebral
seizure activity.
Acidosis
The acidosis may be severe with a pH < 7.2
Control of seizures using anticonvulsant drugs (diazepam,
thiopental) and pyridoxine may resolve the acidosis without
the use of IV sodium bicarbonate.
Sodium bicarbonate should be used if the pH is below 7.2
(molar bicarbonate infusion via venous a catheter at a dose
of 1 to 3 mEq/kg).
Monitor arterial blood gases as a guide to therapy.
Respiratory depression
Respiratory failure may occur during seizures, and is common
after the use of anticonvulsant drugs.Therefore, protection
of airway with cuffed endotracheal intubation and artificial
ventilation are indicated in severe intoxications with
seizures.
If aspiration pneumonia has occurred, appropriate treatment
should be instituted.
Hypotension, shock
Correct metabolic acidosis.
Administer fluids (crystalloids or plasma expander solutions)
and dopamine with central monitoring.
Rhabdomyolysis
Perform urine alkalinization and maintain adequate diuresis
by infusion of fluids and mannitol.
10.3 Decontamination
Emesis may be useful in very recent ingestion, but its
risk with impending seizures should be considered very
carefully.
Gastric lavage is indicated in recent ingestion.
In severe cases of acute poisoning, first control seizures
and protect airway by tracheal intubation before performing
gastric lavage.
Oral activated charcoal reduces the absorption and the
toxicity of isoniazid. One dose (50 g) should be given at
the end of the gastric lavage and repeated every 4-6
hours.
The usefulness of cathartics has not been established.
10.4 Enhanced elimination
Diuresis:
4 to 27% of INH is eliminated as free drug in the urine.
Conclusive evidence of efficacy of forced diuresis is not
established although some authors suggests that forced
diuresis enhances INH elimination (Corger, 1976; Terman &
Teitelbaum, 1970; Brown, 1972; Sievers, 1975)
Forced diuresis is therefore not recommended but maintain
adequate urine output.
Dialysis:
Peritoneal dialysis and haemodialysis have been used (Maher,
1967, Cocco, 1963; Brown, 1972; Orlowski et al., 1988).
INH is a small, watersoluble molecule which is poorly protein
bound and distributes in a small volume (0.6 l/kg)). Based
on these properties, INH would efficiently be removed from
the body by dialysis procedures.
However, the relative merits of peritoneal clearance and
total body clearance are not clear. Therefore, haemodialysis
should be reserved for the patients who do not respond to
correct supportive care and adequate doses of pyridoxine.
Because pyridoxine is also dialyzed, the dose may require
adjustment.
Haemoperfusion: No data available. available.
Exchange transfusion: one exchange transfusion has been
performed in a 19-month-old child (Katz, 1956). However,
considering INH kinetics, this procedure is not
recommended.
10.5 Antidote treatment
10.5.1 Adults
INH induces pharmacologic changes in
pyridoxine metabolism (see section 7.1.1.) and
pyridoxine is used as antidote.
Pure pyridoxine (vitamin B6) is recommended as the
antidote for INH poisoning. In situations where this
is not available and a combination vitamins B1, B6 and
B12 is used, there is a risk of anaphylactoid
reactions if vitamin B1 (thiamine) is given in doses
of more than 1 g. Pyridoxine may be given in smaller
aliquots every 10 minutes until the pyridoxine
deficiency is corrected.
Acute poisoning:
Correction of pyridoxine deficiency contributes to the
control of seizures and the correction of metabolic
acidosis (Wason, 1981; Parish & Brownstein, 1986;
Sievers, 1982; Coger, 1976; Yarbrough, 1983)
In acute ingestion of more than 80 mg/Kg,
administration of IV pyridoxine should be considered,
even in asymptomatic patients
The recommended dose is 1 g of pyridoxine for each
gram of isoniazid ingested. Administer 5 g IV in the
first minutes in severely symptomatic patients with
seizures and acidosis, and repeat administration until
seizures are controlled (Poisindex). If the dose
ingested is known follow the same protocol.
Chronic poisoning:
Treatment of isoniazid-induced neuropathy is effected
with pyridoxine 100 to 200 mg daily and withdrawal of
INH when possible.
For the prevention of isoniazid-induced neuropathy: 10
mg pyridoxine daily is adequate in high risk patients
(Katcher, 1982). Higher doses could interfere with
antibacterial efficacy and are unnecessary.
10.5.2 Children
See 10.6.1.
10.6 Management discussion
Asymptomatic patients with suspected isoniazid
poisoning should be monitored for at least 6 hours.
Give an intravenous bolus of pyridoxine (5 g in adults, 1 g
in children) as soon as isoniazid toxicity is suspected. The
total dose of pyridoxine required is 1 g per gram of INH
ingested.
Potentiate the antidotal (anticonvulsant) effects of
pyridoxine with diazepam.
Administration of sodium bicarbonate should be reserved to
cases with severe acidosis (pH < 7.2).
After pyridoxine treatment and supportive care, gastric
lavage and administration of activated charcoal may be
performed.
Blood samples should be sent for monitoring of biological
parameters.
Ensure adequate diuresis. Forced diuresis is not useful.
Dialysis is only indicated in the most severe cases
unresponsive to adequate supportive care, anticonvulsant
drugs and pyridoxine therapy.
Rhabdomyolysis, neuropathy and coagulopathy may occur and
should be treated appropriately.
11. ILLUSTRATIVE CASES
11.1 Case reports from literature
Wason (1981) reported 5 cases of isoniazid poisoning
treated with IV pyridoxine at a dose of 1 g per gram of INH
ingested. The doses ingested ranged from 4 to 25 g (mean 204
mg/kg). The delay before admission was 1 hour in all cases.
Initial serum levels ranged from 26 to 128 mg/L. Grand mal
seizures had occurred in every patient before admission and
continued after adequate doses of diazepam or phenytoin.
After pyridoxine therapy, none of the 5 patients developed
recurrent seizures and metabolic acidosis resolved. All
patients recovered.
Parish & Brownstein (1986) reported the cases of an acute
poisoning with 6 g of INH in a 14 year-old girl and an acute
overdosage in a 8 year-old boy treated for several months
before admission. Both had developed seizures before
admission. Treatment with IV pyridoxine and diazepam was
quickly successful.
Orlowski et al. (1988) reported the case of a 7-year-old
child who had ingested 125 mg/kg of INH and had persistent
metabolic acidosis and coma after 6 g of pyridoxine. A
five-hour haemodialysis was performed 11.5 hours after
ingestion. At the end of dialysis the patient was fully
conscious and free of seizure activity.
Goldin (1987) reported 2 cases of acute ingestion in patients
under prophylactic treatment. One patient (13 years old)
responded to pyridoxine. The other patient (30 years old) was
relatively refractory to initial pyridoxine and other
supportive therapy.
Hartemann et al. (1983) described a case in a 32-month-old
girl with acute poisoning (190 mg/kg) with generalized
seizures. Intravenous pyridoxine was successful and she
recovered.
Rubin (1983) reported a case of chronic ingestion of INH 400
mg/day in a 7 year-old child who developed seizures and
vomiting. A decline in his mental alertness was also
observed; pyridoxine 500 mg IV was administered with
improvement after 2 hours.
12. ADDITIONAL INFORMATION
12.1 Specific preventive measures
Toxic effects can be minimized by prophylactic therapy
with pyyridoxine and careful surveillance of the patient
(Goodman & Gillman, 1990).
12.2 Other
No data available.
13. REFERENCES
Anderson PO (1976) Drugs and breast feeding a review. Drug
Intel Clin Pharm, 11: 208
Baselt RC (1987) Analytical Procedures for Therapeutic Dug
Monitoring and Emergency Toxicology. 2nd ed. California,
Biomedical Publications, pp145-146.
Biehl JP & Vilter RW (1954) Effects of isoniazid on pyridoxine
metabolism. JAMA, 156: 1549.
Björnesjö KB & Jarnulf B (1967) Determination of isonicotinic
acid hydrazide in blood serum. Scand J Clin Lab Invest, 20:
39-40.
Black M, Mitchell JR, Hyman PD et al. (1975) Isoniazidassociated
hepatitis in 114 patients. Gastroenterology, 69 (2): 289302
Bowersox DW et al. (1973) Isoniazid dosage in patients with renal
failure. N Engl J Med, 289: 84
Boxenbaum HG & Riegelman S (1974) Determination of isoniazid and
metabolites and biological fluids. J Pharm Sci, 63: 1191-1197
Brown CV (1972) Acute isoniazid poisoning. Am Rev Resp Dis, 105:
206216
Curnani A, Chawla R, Kundra P, Bhattacharya A (1992) Acute
isoniazid poisoning. Anesthesia, 47: 781-783.
Davies DM (1981) Textbook of adverse drug reactions, 2nd ed.
Oxford University Press, New York
DRUGDEX (1991) Micromedex
Eidus L, Harnanansingh AMT (1971) A more sensitive
spectrophotometric method for determination of isoniazid in serum
or plasma. Clin Chem, 17: 492-494.
Eidus L, Harnanansingh AMT (1974) In: Sunshine I (ed).
Methodology for Analytical Toxicology. Cleveland, Ohio, CRC Press,
pp200-201.
Eidus L, Varughese P, Hodgkin MM, Hsu AHE, McRae KB (1973)
Simplification of isoniazid phenotyping procedure to promote its
application in the chemotherapy of tuberculosis. Bull World
Health Org, 49: 507-516.
El-Brashy AM & El-Ashry SM (1992) Colourimetric and titrimetric
assay of isoniazid. J Pharmaceut Biomed Anal, 10: 421-426.
Ellard GA (1984) The potential clinical significance of the
isoniazid acetylator phenotype in the treatment of pulmonary
tuberculosis. Tubercle, 65: 211227
Ellard GA, Gammon PT, Wallace SM (1972) The determination of
isoniazid and its metabolites acetylisoniazid,
monoacetylhydrazine, diacetylhydrazine, isonicotinic acid and
isonicotinylglycine in serum and urine. Biochem J, 126:
449-458.
El-Yazigi A & Yusuf A (1991) An expedient liquid chromatographic
micromeasurement of isoniazid in plasma by use of electrochemical
detection. Therapeutic Drug Monitor, 13: 254-259.
Flanagan RJ, Braithwaite RA, Brown SS, Widdop B, deWolff FA (1995)
Basic Analytical Toxicology. Geneva, WHO, pp157-158.
Frater-Schröder M, Zbinden G (1975) A specific rapid gas
chromatographic assay for the determination of isoniazid
N-acetylation: observation in rats with induced constant urine
flow. Biochem Med, 14: 274-284.
Goodman LS & Gilman A (1990) The pharmacological basis of
therapeutics. 8th ed. New York
Hartemann E, Barre M, Frederich M (1983) Etat de mal convulsif
par intoxication accidentelle ą l'isoniazide. Pediatrie, 38 (1):
4345
Hsu K-Y & Ho Y (1989) Determination of isoniazid
methanesulphonate and its metabolites in rabbit blood by
high-performance liquid chromatography. J Chromatogr, 493:
305-312.
Hutchings A, Monie RD, Spragg B, Routledge PA (1983)
High-performance liquid chromatographic analysis of isoniazid and
acetylisoniazid in biological fluids. J Chromatogr, 277:
385-390.
Ioannou PC (1988) A more simple, rapid and sensitive fluorimetric
method for the determination of isoniazid and acetylisoniazid in
serum. Application for acetylator phenotyping. Clin Chim Acta,
175: 175-182.
Kohno H, Kubo H, Furukawa K, Yoshino N, Nishikawa T (1991)
Fluorimetric determination of isoniazid and its metabolites in
urine by high performance liquid chromatography using in-line
derivatization. Therapeutic Drug Monitor, 13: 428-432.
Kucers A & Bennet N (1979) The use of antibiotics, 3rd Ed. William
Heinemann Medical Books Ltd, London
Lauterberg BH, Smith CV, Mitchell JR (1981) Determination of
isoniazid and its hydrazine metabolites, acetylisoniazid,
acetylhydrazine, and diacetylhydrazine in human plasma by gas
chromatography-mass spectrometry. J Chromatogr, 224: 431-438.
LoDico CP, Levine BS, Goldberger BA, Capla YH (1992) Distribution
of isoniazid in an overdose death. J Analyt Toxicol, 16:
57-59.
Manoguerra AS (1980) Acute isoniazid toxicity. Clin Toxicol, 16:
407408
Meyers FH, Jawetz E, Goldfien A (1976) Review of medical
pharmacology, 5th ed. Lange Medical Publications
Meyler L & Peck HM (1976) Drug induced diseases. Vol. 4, ed.
Excerpta Medica, Amsterdam
Miceli JN & Olson WA (1982) In Sunshine I & Jatlow PI eds.
Methodology for Analytical Toxicology Volume II. Cleveland, Ohio,
CRC Press, pp 117-118.
Moffat AC, Jackson JV, Moss MS, Widdop B eds. (1986) Clarke's
Isolation and Identification of Drugs. London, Pharmaceutical
Press.
Olson WA, Dayton PG, Israili ZH, Pruitt AW (1977)
Spectrophotofluorimetric assay for isoniazid and acetylisoniazid
in plasma adapted to pediatric studies. Clin Chem, 23:
745-748.
Orlowski JP, Paganini EP, Pippenger CE (1988) Treatment of a
potentially lethal dose isoniazide ingestion. Annals Emerg Med,
17: 7376
Parish RA & Brownstein D (1986) Emergency department management
of children with acute isoniazid poisoning. Paediatric Emergency
Care, 2 (2): 8890
Pessayre D, Bentata M, Degott C et al. (1977) Isoniazidrifampin
fulminant hepatitis Gastroenterology, 72: 284289
Rouge P, Cougot P, Virenque C (1983) Intoxication volontaire par
l'isoniazide. Cahiers d'Anesthésiologie, 31 (4): 389390
RTECS (1979) Volume 1. US Department of Health and human
services. Ed. Richard J. Lewis Sr. and Rodger L. Tatken
Russell DW (1972) Low circulating levels of acid-labile
hydrazones after oral administration of isonicotinic acid
hydrazine. Clin Chim Acta, 41: 163-168.
Siefkin AD, Albertson TE, Corbett MG (1987) Isoniazid overdose:
pharmacokinetics of oral charcoal in treatment. Hum Toxicol, 6:
497501
Stewart MF, Freemont AJ, Richardson T (1995) Fatal isoniazid
poisoning. Ann Clin Biochem, 32: 229-231.
Svensson J-O, Muchtar A, Ericsson O (1985) Ion-pair
high-performance liquid chromatographic determination of isoniazid
and acetylisoniazid in plasma and urine. J Chromatogr, 341:
193-197.
Terman DS & Teitelbaum DT (1970) Isoniazid self poisoning.
Neurology, 20: 299304
Timbrell JA, Wright JM, Smith CM (1977) Determination of
hydrazine metabolites of isoniazid in human urine by gas
chromatography. J Chromatogr, 138: 165-172.
Walubo A, Smith P, Folb P (1994) Comprehensive assay for
pyrizinamide, rifampicin and isoniazid with its hydrazine
metabolites in human plasma by column liquid chromatography. J
Chromatogr, 658: 391-396.
Watson S, Lacouture PG, Lovejoy FH (1981) Single highdose
pyridoxine treatment for isoniazid overdose. JAMA, 246:
11021104
Weber WW & Hein DW (1979) Clinical pharmacokinetics of isoniazid.
Clin Pharmacokinetics, 4: 401-422.
WHO (1986) Basic Tests for Pharmaceutical Substances. Geneva,
WHO, p96.
Wood JD & Peesker SJ (1972) A correlation between changes in GABA
metabolism and isonicotinic acid hydrazine-induced seizures.
Brain Res, 45: 489-498.
14. AUTHOR(S), REVIEWER(S), DATE(S) (INCLUDING UPDATES, COMPLETE
ADDRESS(ES)
Authors: Dr O.J. Kasilo
Drug & Toxicology Information Service (DaTIS)
Department of Pharmacy
University of Zimbabwe Medical School
P.O. Box A 172 Avondale
Harare
Zimbabwe
Dr CFB Nhachi
Department of Clinical Pharmacology and Toxicology
University of Zimbabwe Medical School
P.O. Box A 172 Avondale
Harare
Zimbabwe
Tel: 273-4-7902333 or 791631 ext. 117/172
Fax: 263-4-303 292
Date: September 1989
Co-Authors: Drs M. Dahlet, F. Flesch, A. Jaeger
Service de Réanimation Médicale et Centre AntiPoisons
Hōpitaux Universitaires
Hōpital Civil
67091 Strasbourg Cedex
France
Tel: 33-88161144
Fax: 33-88161330
Date: January 1992
Peer
Review: Newcastle-upon-Tyne, United Kingdom, February 1992.
Author
Section 8: Dr Sheila Dawling
Center for Clinical Toxicology
Vanderbilt University Medical Center
501 Oxford House
1161 21st Avenue South
Nashville, TN 37232-4632
United States of America
Tel: 1-615-9360760
Fax: 1-615-9360756
E-mail: sheila.dawling@mcmail.vanderbilt.edu
Date: March 1998
Editor: Mrs J. Duménil
International Programme on Chemical Safety
Geneva
Date: May 1999

 Acetylation phenotype
Acetylation phenotype

 Acetylation phenotype
Acetylation phenotype