
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
SUMMARY OF TOXICOLOGICAL DATA OF CERTAIN FOOD ADDITIVES
WHO FOOD ADDITIVES SERIES NO. 12
The data contained in this document were examined by the
Joint FAO/WHO Expert Committee on Food Additives*
Geneva, 18-27 April 1977
Food and Agriculture Organization of the United Nations
World Health Organization
* Twenty-first Report of the Joint FAO/WHO Expert Committee on Food
Additives, Geneva, 1977, WHO Technical Report Series No. 617
XYLITOL
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Schmidt et al. (1964) found that when 14C-xylitol (250 mg) was
administered by intubation to rats, the half live of the resorption of
xylitol is about seven to eight hours. The resorption rate is about
15-20% of that of glucose (Mehnert et al., 1964; Grütte and Rödel,
1975). After the animals had been fed xylitol for 14 days the half
life fell to 4.5 hours (Schmidt et al., 1964; Lang, 1974; Grütte and
Rödel, 1975). The cause of an adaptive elevation of the xylitol
resorption rate from the gut has been ascribed to the intensifying of
the xylitol metabolism (Stein, 1966). Lang (1964) observed in rats a
resorption of 10% of dietary xylitol from the intestinal tract.
In rats 80% of the xylitol is degraded in the liver, the
remaining 20% is metabolized in extrahepatic organs, mainly in the
kidneys (Birnesser et.al., 1973; Schmidt et al., 1964; Lang, 1974).
According to Grütte and Rödel (1975) heart and adipose tissue, islets
of Langerhans in the pancreas, adrenals and brains are also able to
utilize xylitol. Froesch (1975) found that in rats xylitol was fast
converted into glucose and the peripheral metabolism of xylitol
follows only after the converting into glucose and by the influence of
insulin. Five minutes after the injection of 14C-xylitol more than
50% of the 14C is circulating in the blood as glucose. Insulin is not
needed for the uptake of 14C-xylitol, but for the incorporation of
I4C-glucose in the extrahepatic organs (Froesch, 1972, 1975; Schmidt
et al., 1964). The erythrocytes (Bässler and Reinold, 1965), and the
adipose tissue (Opitz, 1967; Yamagata et al., 1967) are able to
metabolize xylitol, although this is neglectable in comparison to the
metabolism in the liver.
Insulin stimulated the uptake of 14C in the adipose tissue and
the diaphragma of the rat, which indicates the transport action on the
from 14C-xylitol derived 14C-glucose. 14C-xylitol disappear
extremely rapidly and the calculated half life is 165 seconds (Froesch
and Jakob, 1974). The converting rate is about the same as that of
sorbitol. The half time is 20 minutes (Froesch, 1975).
Lang (1964) found in urine of the rats practically no xylitol.
In a study he carried out with 14C-xylitol unadapted animals fed
250 mg xylitol/kg body weight excrete 3-8% of the activity in urine
and 61-62% is exhaled, while 6-10% is excreted in faeces and 20-25% is
still found in the animal in a period of 16-24 hours. Amounts of
0.3.-0.4% of the dose were found in the glycogen of the liver and
muscle. The half life of exhalation of 14CO2 after feeding with
xylitol in unadapted animals is 295 minutes, whereas rats which were
adapted for 14 days to xylitol had a half life of 237 minutes (Schmidt
et al., 1964; Lang, 1964).
The rate of expired xylitol in rats had a maximum value of
27 mg xylitol/hour/kg body weight for the adapted animals and
9 mg xylitol/ hour/kg body weight for the unadapted animals (Schmidt
et al., 1964). Schmidt et al. (1964) also found that the activity of
the oxidized xylitol was present for the greatest part as 14CO2 (68%
of the administered dose) in the expired air, the urea present in the
urine provided around 2% of the activity. The adaptive improvement in
the rate of absorption had in all probabilities no effect on the
retention of activity in the carcass. Approximately one quarter of the
14C-dosage administered as xylitol was retained.
Biotransformation
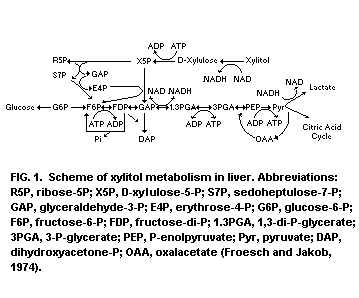
The metabolism of xylitol in rats was studied by Froesch and
Jakob (1974) and Froesch (1975). Xylitol is first oxidized to
D-xylulose by the NAD-xylitol dehydrogenase, causing the NADH/NAD
ratio to increase. The next step is the phosphorylation of D-xylulose
to D-xylulose-5-phosphate by D-xylulose-kinase. D-xylulose-5-phosphate
is an intermediate of the pentose phosphate shunt and it is
metabolized to fructose-6-phosphate and glyceraldehyde phosphate
by this pathway. Three molecules xylitol yield two molecules of
fructose-6-phosphate and one molecule of glyceraldehyde phosphate.
Fructose-6-phosphate can readily be converted to glucose and glycogen;
glyceraldehyde phosphate either to glucose, glycogen or lactate. Most
of the xylitol is rapidly converted to glucose and only small
quantities are converted to lactate (Jakob et al., 1971).
 These biotransformation studies are also supported by Schmidt et
al. (1964), Hörecher (1969), Touster (1974), Stein (1966), Petrich et
al. (1972) and Grütte and Rödel (1975). McCormick and Touster (1957)
found that the metabolism of rat and guinea pig was similar.
Effects on enzymes and other biochemical parameters
Xylitol has especially influence on the activity of the enzymes
of carbohydrates metabolism and the insulin hormone. Both in
experiments with parenteral and oral administration of xylitol a
stimulation of insulin secretion and an elevated serum insulin
concentration are observed in rat, dog and monkey, although the plasma
glucose level slightly increased for a while and then decreased below
the fasting level (Bässler and Prellwitz, 1964; Kuzuya et al., 1966,
1969; Hirata et al., 1967, 1968; Montague et al., 1967; Wilson and
Martin, 1970; Froesch, 1975; Jourdan et al., 1972; Touster, 1974).
Kuzuya et al. (1969) found that the insulin secretion produced by
xylitol in dogs was more pronounced than by glucose. Wilson and Martin
(1970) observed the same effect in dogs, while they found that in
monkeys glucose caused a more marked stimulation of the insulin
secretion than xylitol did. The insulin secretion in dogs is also much
stronger than in man (Hirata et al., 1967). Meng (1974) however did
not find any changes of the insulin level in dogs, i.v. infused with
xylitol. This study included only five animals.
Glycogen production is stimulated by the administration of
xylitol to guinea pigs, rat and perfused rat liver, even when diabetic
animals are used: (McCormick and Touster, 1957; Hosaya et al., 1966;
Touster, 1970; Förster and Hoffman, 1973). Jakob et al. (1971) found
that in liver most of the xylitol administered to the animals was
rapidly converted to glucose and to small quantities of lactate.
Bässler and Reimold (1965) observed a stimulating influence of xylitol
on the production of lactic acid in erythrocytes which was also
noticed with glucose. In perfused rat liver xylitol stimulated the
lactate production and caused an increased lactate to pyruvate ratio
(Jakob et al., 1971; Förster and Hoffmann, 1973; Woods and Krebs,
1973; Woods, 1975). Besides the increased ratio of lactate to
pyruvate Jakob et al. (1971) found also increased ratios of
alpha-glycerophosphate to dihydroxy acetone-P and triosephosphate to
3-P-glycerate. In a study of Hirata et al. (1968) a significant
decrease of blood pyruvic acid level was noticed in normal dogs.
In close relation with the influence of the carbohydrate
metabolism an antiketogenic and antilipolytic effect of xylitol is
also observed (Haydon, 1961; Hosaya and Machiya, 1967; Bässler and
Wagner, 1970; Jakob et al., 1971; Wagner and Bässler, 1971; Birnesser
et al., 1973). As a result of accumulation of alpha-glycerophosphate
and other phosphorylated intermediates a fall in the inorganic
phosphorus and adenine nucleotides was observed (Petrich et al., 1972;
Woods and Krebs, 1973; Woods, 1975). The fall in the inorganic
phosphorus leads to an increased purine catabolism and to a
hyperuricemia in isolated perfused rat liver (Woods and Krebs, 1973;
Woods, 1975). Forster and Hoffmann (1973) found that xylitol
accelerates the biosynthesis of uric acid.
Shima et al. (1970) found a stimulation of testicular protein and
RNA biosynthesis in rats fed xylitol. Staub and Thiessen (1972)
observed increased cholesterol levels in rats fed xylitol.
In experiments with xylitol oxalate formation is observed, but
glucose is reported to have a stronger effect on this formation (Wang
et al., 1975; Oshinsky et al., 1976; Brin and Miller, 1974). Therefore
no clear relationship between oxalate accumulation and xylitol
toxicity is found by Oshinsky et al. (1976) and Wang et al. (1975).
TOXICOLOGICAL STUDIES
Special studies on reproduction
In a reproduction study five groups of 30 male and 60 female rats
of the P-generation were fed 0, 2, 5, 10 and 20% dietary xylitol. The
total amount of carbohydrates was kept constant. In addition 20%
sorbitol and a 20% sucrose group were used as sugar controls. This
study has only been carried out till the F1B-generation. At the
second mating, an abrupt increase in pup mortality occurred in all
groups between four and 12 days. This was caused by a heating failure.
This resulted in loss of too many pups to complete the reproduction
study.
In the animals receiving 5, 10 and 20% xylitol decreased
food-intake and weight gain was recorded. At the first mating, a
slightly higher initial litter size among test groups was
counterbalanced by a slightly higher cumulative pup loss during
lactation. No other effects on the reproduction were noticed in the
F1a-animals receiving xylitol. This study will be repeated (Hummler,
H. 1976a).
Levels of xylitol up to 20% of the diet fed to rats up to four
months had no significant adverse effects. Growth rate was normal.
Adaptation to high dosage was shown to be important. Xylitol had no
effect on the number or size of pups or dams fed xylitol, and the pups
adapted readily to the supplement at 5 and 10% in diet, after weaning,
as instituted following adverse effects with the 20% xylitol level,
initially (Hosoyol and Iitoyo, 1969).
Special studies on carcinogenicity
In a carcinogenicity study 100 female and 100 male mice per group
weighing 24-25 g, were fed 0, 2, 10 and 20% dietary xylitol during
1.5-2 years. The total amounts of carbohydrates were kept constant and
animals receiving sucrose served as sugar control group. Body weight
and food intake were recorded weekly, while with intervals water
consumption was determined. At 26, 52 and 79 weeks and prior to
terminal sacrifice ophthalmoscopy was carried out. After 80 weeks 20
males and 20 females from each group were killed. The mice were killed
when survival within a group reached 20%. Gross pathology was
performed visually and by palpation. Microscopic examination on
adrenals, thyroids, ovaries, liver, spleen, lymph, pituitary gland and
bone marrow and all macroscopically observed lesions suggestive of
neoplasia were routinely carried out for every animal, along with
blood smears.
At the start of the experiment a severe diarrhoea was observed,
therefore a number of adaptation phases with slowly increasing xylitol
concentrations in the diet were necessary. Animals receiving 10% and
20% xylitol showed increased water and food intake. In the animals
receiving 2% and 10% xylitol, 20% sorbitol an increased body weight
gain was observed, whereas the animals receiving 20% xylitol had a
significant lower body weight gain. The mortality of the male mice
receiving 10 and 20% xylitol was elevated. No histopathology and no
details on tumour incidence are available. The study is at the moment
an inadequate carcinogenicity study, which has to be completed first
(Hummler, H., 1976b).
In a long-term toxicity and carcinogenicity study five groups of
75 female and 75 male Sprague-Dawley rats, weighing 120-150 g, were
fed 0, 2, 5, 10 and 20% dietary xylitol. The total amounts of
carbohydrates in the diet were kept constant. Animals receiving 20%
sorbitol and 20% sucrose were used as sugar control groups. Food
intake and body weight were recorded weekly. Water consumption was
recorded during weeks 12, 25, 52 and 78. Ophthalmoscopy, urinalysis,
haematology and biochemistry were performed at 0, 13, 26, 52, 78 and
104 weeks. Urinalyses included pH, specific gravity, protein, reducing
substances, glucose, ketones, bile pigments, urobiligen, haemoglobin
and oxalic acid determinations. Haematology consisted of determination
of PCV, Hb, RBC, WBC, MCHC, MCV, differential count, prothrombin
index, whole blood clotting time. The biochemical estimations were
urea and glucose in plasma, total proteins, protein electrophoresis,
Alk Pase, GPT, GOT, bilirubin, uric acid, cholesterol, lactate, LDH,
alpha-HBDH in serum and total reducing substances, insulin,
xylitol,Na+, K+, Cl- and bicarbonate in blood. After 26 and 52
weeks, five males and five females per group were killed. In the gross
pathology the animals were examined visually and by palpation. Brain,
heart, liver, kidneys, adrenals, pituitary, thyroid, spleen,
testes/ovaries were weighed. These organs and pancreas, thymus,
uterus, cervical and mesenteric lymph nodes, stomach, bone marrow,
ileum, coecum, duodenum, urinary bladder, eye, lungs and any
macroscopically abnormal tissues were histopathologically
investigated. Blood smears of these organs were also studied.
Significant decreased weight gain in animals receiving 10% or 20%
xylitol during the experiment was observed, while there was only
tendency to decreased food intake in animals receiving 20% xylitol at
78 weeks. In rats receiving 20% xylitol, higher water intakes were
recorded during weeks 26 and 52, and in female rats also during week
78.
In male and female rats at 13, 26 and 52 weeks and in female rats
at week 78, receiving 20% xylitol greater volumes of more diluted
urine were excreted. After 26 weeks, the urine from animals receiving
5, 10 and 20% showed decreased specific gravity.
No increase in mortality was noticed in the treated groups,
Except a significant decreased prothrombin index in all male rats
receiving xylitol and female rats receiving 20% xylitol, no
haematological changes were observed at 78 weeks. In biochemistry a
tendency to decreased lactate contents in the blood of animals
receiving 10 and 20% xylitol and at 78 weeks a tendency to increased
uric acid content in male rats of the 20% xylitol group were observed.
No morphological abnormalities in the treated groups differing
significantly from the control group ware observed. The standard
deviation in the insulin determination was too great for making an
evaluation of the results possible.
The study is not yet completed. No details on tumour incidence
are available so far (Hummler, 1976c).
Acute toxicity
LD50
Animal Route mg/kg References
body weight
mouse oral 14 100 Bächtold (1972)
mouse oral 22 000 Pool and Hane (1972)
mouse oral >4 000 Pool and Hane (1970)
mouse oral 25 700 Kieckebuch (1961)
mouse intraperitoneal >2 000 Pool and Hane (1970)
mouse subcutaneous >2 000 Pool and Haue (1970)
(con't)
LD50
Animal Route mg/kg References
body weight
mouse intravenous >4 000 Pool and Hane (1970)
mouse intravenous 22 200 Grütte and Rödel (1915)
mouse intravenous 3 770-9 450 Pazenko (1969)
adult rat oral >4 000 Pool and Hane (1970)
neonatal rat oral > 4 000 Pool and Hane (1970)
rat oral 14 100 Bächtold (1972)
rabbit oral > 2 000 Pool and Hane (1970)
rabbit intravenous 4 000-6 000 Wang et al. (1973)a
a The acute toxicity in rabbits was determined by intravenous
infusion of 87 mg xylitol/kg/minute. The observed LD50 was 4-6 g/kg
body weight. Striking increases of SGOT and serum LDH levels, along
with an increase urine volume were observed (Wang et al., 1973).
Short-term studies
In a subacute toxicity study xylitol was administered to 20
female and 20 male rats per group by daily gastric intubation for a
period of 14 days. Dose levels were 1.25, 2.5, 5.0 and 10.0 g/kg. The
animals receiving 1.25 g xylitol/kg during the first nine days,
received 10.0 g xylitol/kg during the last seven days. In the control
groups animals received 0, 2.5 and 5.0 g sucrose/kg/day. During
treatment (two, five and 14 days) animals were submitted to careful
clinical examinations and blood serum analyses related to hepatic
functions. These analyses were glucose in blood and bilirubin, FFA,
total lipids, triglycerides, cholesterol, G-6-PDH and Alk. Pase, GPT
and GOT in serum. Also body weight and food consumption were recorded.
The animals were sacrificed after two, five and 14 days. Heart, liver,
spleen, kidneys, adrenals, gonads, stomach were weighed. These organs
and jejunum, colon, pancreas and brain were histologically examined in
two and five days treatment groups, while in 14 days treatment group
this examination was only performed on liver. The histological study
was conducted on five males and five females per group.
Except for a decrease of the FFA content in the blood at all
xylitol dose levels, no effects are observed in this study. No
evidence of hepatotoxicity was recorded (Truhaut et al., 1977).
In a 15 days' experiment with rats, sucrose was replaced
respectively by 10 and 20% xylitol. The protein of the diet consisted
of casein, while their carbohydrate allowance consisted of starch and
sugar. In the 10% xylitol dose group no histopathological changes were
observed. However, in the 20% xylitol dose group adverse effects of
the metabolism of the liver cells were noticed. A reduction of the
glycogen and lipids concentrations were observed. The mitotic activity
of the liver cells was sharply reduced to a level five times lower
than in the control rats (Jursons et al., 1974).
In a short-term (13 weeks) study four groups of eight female and
eight male Charles River CD rats weighing 120-150 g, were fed 0, 5, 10
and 20 g dietary xylitol/kg/day. Food and water consumption were
recorded daily and body weight weekly. Haematology, blood glucose and
urinalyses of five male and five female rats of each group were
carried out at four, eight and 12 weeks. Alk. Pase, GOT, bilirubin and
uric acid in serum and BUN of five males and five females per group
were determined at 13 weeks.
The animals, particularly the male rats, showed reduced body
weight gain and food consumption, that were dose dependent. In the 20%
xylitol group BUN was elevated. In treatment groups dose-related
decrease of the absolute heart weight are observed. Except for
transient diarrhoea in a number of treated animals, no other
significant changes were observed. No histopathological lesions
related to xylitol administered were noticed (Swarm and Banziger,
1970).
Rat
Rats fed a cariogenic diet containing 10% xylitol showed no
carious lesions in the first and third molars and only minimal
involvement of the second molars was observed (Grunberg et al., 1973).
Dog
In short-term study five dogs were dosed 10 g xylitol/kg/day
by intravenous infusion during seven weeks. Blood chemistry and
urinalyses were investigated weekly. Plasma glucose content was
reduced to 64 mg % after six weeks. A slight increase in plasma
lactate and a significant increase of SGPT and Alk. Pase was observed.
Also an increased urinary loss was observed. No effect of xylitol on
plasma insulin levels and no oxalate crystals in the kidneys were
observed (Meng, 1974).
A six weeks toxicity experiment with one female and one male dog
(5.5-6 kg) per group was carried out. The concentration was during the
experiment increased from 5% after two weeks to 20%. Food consumption
was recorded twice daily and body weights twice a week. At the end of
the experiment gross pathology was carried out, and brain, heart,
liver, lungs, pituitary, spleen, pancreas, thymus, prostate/uterus,
kidneys, thyroids, adrenals and testes/ovaries were weighed. No
histopathological investigation was carried out.
Except for a tendency to increased liver weights in the xylitol
groups no effects are observed. This study is not an adequate toxicity
study, but has to be seen as preliminary experiment (Hummler, 1974).
In a long-term toxicity study eight female and eight male beagle
dogs per group, weighing 6-9 kg, were fed 0, 2, 5, 10 and 20% dietary
xylitol during two years. The total amounts of carbohydrates were kept
constant. In addition animals receiving 20% sorbitol and 20% sucrose
were used as sugar controls. Body weight and food consumption was
recorded weekly, while water consumption was recorded over five day
periods at the weeks one to four, 9-12, 21-24, 35-38 and 46-49.
Ophthalmoscopy, dental examination and a full neurological examination
of four male and four female dogs were carried out at 13, 26, 39, 50,
64 and 76 weeks. Haematology, biochemistry and urinalyses were carried
out at the beginning of the experiment and at 12, 26, 38, 50, 64 and
76 weeks. The haematology included erythrocyte sedimentation rate,
PCV, Hb, RBC, reticulocyte count, MCHC, MCV, WBC, differential count,
platelet count, prothrombin index, whole blood clotting time. The
biochemistry included determination of total protein, Al, GPT, GOT,
total LDH, alpha-HBDH, bilirubin, uric acid, cholesterol, lactate and
insulin in serum and urea xylitol, glucose concentration and total
reducing substances in plasma. Sodium, potassium, chloride and
bicarbonate were also detected in the blood. Urinalyses consisted of
estimations of Ph, protein, reducing substances, glucose, ketones,
bile pigments, urobiligen and Hb. On completion of 52 weeks, two male
and two female dogs per group were sacrificed for interim study, while
the remaining dogs will be killed after two years' dosing. The weights
of brain, liver, kidneys, pituitary, thyroid, spleen, heart, lungs,
adrenals, ovaries, testes, uterus, thymus, prostate, pancreas were
recorded. Histopathology of these organs and of aorta, trachea, lymph
nodes, gall bladder, urinary bladder, salivary glands, oesophagus,
duodenum, stomach, jenunum, ileum, skin, skeletal muscle, mammary
glands, tongue, eye with optic nerve and sciatic nerve was performed.
The food intake and weight gain of the 20% xylitol group were
increased. A dose-related increase of SAP and SGPT and a decreased
lactate level was noticed, while in the 20% xylitol dose group also
the total serum protein was significantly increased. At 52 weeks a
tendency to increased cholesterol content in serum was observed.
Except a dose related decrease in the pituitary of the xylitol
dose groups no effects on organ weights, in gross necropsy and
histopathology were observed. The number of animals, however, did not
allow a real statistic evaluation (Hummler, 1976d).
Mean values of haematological, biochemical and urinalyses were
calculated from total population of female and male animals.
Significant changes could be looked over by elevated standard
deviation. In the insulin determinations variation was too much for
further conclusion. In view of the mentioned restrictions it can be
stated that this study is not fully adequate, it has to be completed
first.
In a 13 weeks toxicity study two male and two female rhesus
monkeys, weighing 2-3 kg, were dosed twice daily six days a week 0,
1.0, 3.0 and 5.0 g xylitol/kg/day by gastric intubation. The animals
were observed daily for mortality, appearance, behaviour, appetite,
elimination and pharmacotoxic effects.
Body weights were recorded weekly. At 0, 4, 8 and 13 weeks
ophthalmoscopic and neurologic examinations, haematology, clinical
chemistry and urinalyses were performed. Haematology included Ht, Hb,
total and differential leucocyte counts, prothrombin time and
coagulation time. Blood chemistry consisted of blood sugar, BUN, SAP,
SGPT and SGOT estimations. In addition, free and total bilirubin and
uric acid determinations were conducted at four, eight and 13 weeks.
Urinalyses included specific gravity, pH, glucose, ketones, total
protein, bilirubin and microscopic examination of sediment.
Gross-necropsy was carried out on all animals. Spleen, brain,
pituitary, thyroid, heart, lung, liver, kidneys, adrenals, testes,
prostate, ovaries, uterus were weighed. Histopathology of these organs
and thoracic spinal cord, eye, gall bladder, thymus, salivary gland,
stomach, pancreas, small intestine, large intestine, mesenteric lymph
nodes, sciatic nerve, skeletal muscle, urinary bladder, skin, rib,
vertebra, femur, sternum were carried out at 13 weeks.
Soft stool and/or diarrhoea were noted in all treatment groups
intermittently throughout the study and appeared to be dose related.
In the treatment groups a tendency to decreased coagulation time (from
3.16 to 2.40 minutes) was noticed. Brain weight tended to a dose
related decrease in the male animals receiving xylitol. No further
dose related effects were observed in this study (Banziger, 1970).
Long-term studies
A long-term study with mice and studies with rats are reported
under special studies on carcinogenicity and special study on
reproduction.
In a long-term toxicity study xylitol has been administered
orally 100 mg/xylitol/kg to 20 female and 20 male Wistar rats for
11 months and to 15 female and 15 male rats for 24 months. Body
weights were recorded weekly. At termination detailed gross pathology
was performed and a microscopic study was carried out on stomach,
intestines, liver, spleen, pancreas, kidneys, adrenals,
testes/ovaries, uterus/prostate, lungs, heart, brains, salivary
glands, lymph nodes, thyroid, thymus, sphenoid, pituitary, ganglions
of Glasser and femur. The experiment was carried out with two
generations and the fertility was also checked. The results were
compared with 450 control animals kept under similar experimental
conditions.
No malignant tumours were observed in the animals treated with
100 mg xylitol/kg and no stimulation of the growth of tumours was
noticed. According to the author there was no harmful influence on
reproduction and xylitol did not produce any pathological changes in
the animals (Mosinger, 1971).
In this experiment, however, only one very low dose level was
studied and too little animals were used. No control animals running
simultaneously with the test group were used. In addition not enough
details and no control values were shown. No comparison and no
statistical evaluation was possible. For these reasons this study has
to be considered as not adequate.
OBSERVATIONS IN MAN
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Xylitol is absorbed from the intestine very slowly compared to
glucose (Bässler et al., 1962; Dehmel et al., 1967; Förster, 1972).
After adaptation no significant rise of the absorption rate was
observed (Liebau, 1964) and although the absorption increased with
administrated dose of xylitol the percentage of absorption decreased
(Asano et al., 1972). The absorption of xylitol ranged from 49 to 95%
depending on the dose level (Asano et al., 1973; Keller and Froesch,
1972; Müller-Hess et al., 1975). After both oral and intravenous
administration of xylitol subjects showed a fast distribution in the
extracellular compartment and the tissues (Bässler et al., 1962). The
initial fast distribution phase had a half life of about four minutes,
while the apparent half life of elimination was approximately 20
minutes (Dixon and East, 1973). In studies with 14C-xylitol 90% of
C-atoms taken up could be recovered in products and intermediates of
the glycolytic and pentose phosphate pathway (Quadflieg and Brand,
1974; Müller-Hess et al., 1975). The excretion of 14C-activity in
urine is low after oral administration of 14C-xylitol (Bässler et
al., 1962). An excretion of 1-3% in urine is recorded after oral
administration and of 10% after intravenous infusion of xylitol
(Bässler, 1965). In faeces also 1% of xylitol was excreted
(Müller-Hess et al., 1975).
Metabolism
Exogenous xylitol enters the pathway by conversion to D-xylulose
by a nonspecific cytoplasmic polyol dehydrogenase. Phosphorylation
then yield D-xylulose phosphate, the link between the glucuronic acid
and the pentose phosphate pathways; the latter leads to the formation
of glyceraldehyde-3-phosphate and fructose-6-phosphate, intermediate
metabolites of the Embden-Meyerhof (glycolytic) pathway. Thus xylitol
can be metabolized via glucose-6-phosphate to glycogen and pyruvate or
lactate via the citric acid cycle to CO2 (Hollmann, 1967; Bässler,
1965; Förster, 1974; Asakura, 1967; Touster and Shaw, 1962). Xylitol
is mainly metabolized in the liver (80% to glucose only 20%) but a
small amount also in kidney, myocardium, erythrocytes, adrenal, brain,
lungs and adipose tissue (Bässler, 1965; Hollmann, 1967). Exogenous
xylitol can be metabolized in large quantities, intravenously
0.4 gm/kg/hour or 40 g/day orally raises the plasma level to a maximum
of 1.5-16 mg/100 ml (Bässler, 1965). The metabolic rate for xylitol is
identical in both healthy and diabetic or uraemic patients and
patients who suffered from liver diseases (Lang, 1972).
Effects on enzymes and other biochemical parameters
Oral ingestion of xylitol does not significantly raise the blood
glucose and insulin concentration in normal subjects and diabetics
(Lang, 1972; Bässler et al., 1962; Huthunen et al., 1975), whereas
Müller-Hess et al. (1975) found a small but significant increase in
blood glucose and insulin levels, which were the confirmed findings of
Felber (1976) and Berger et al. (1973). Administration of xylitol
caused an increased hepatic storage of glycogen (compared to glucose)
in both normal and diabetic subjects (Müller-Hess et al., 1975).
After xylitol ingestion increase of serum lactate concentration
and lactate-pyruvate ratio was observed, but to a degree less than
after glucose (Asakura, 1967; Förster, 1972, 1974). Asakura (1967)
also found a marked increase of alpha-dihydroxybutyrate. Complete
metabolism of xylitol produces 35 equivalents of ATP compared to 32
from glucose (Horecker, 1969).
Xylitol caused a fall in the serum free fatty acid level both in
healthy and diabetic subjects (Horecker, 1969; Hötzel, 1971; Pitkänen
and Sahlström, 1968; Förster, 1974). The increased hepatic uptake and
triglyceride synthesis after xylitol administration caused an
elevation of serum triglycerides, but the total serum lipid
concentration was reduced or not influenced (Förster, 1972; Horecker,
1969; Huthunen et al., 1975). The free glycerol concentration in
plasma was also significantly diminished (Müller-Hess et al., 1975).
Xylitol had a marked antiheterogenic action (30 gm xylitol were
adequate). The increased esterification of FFA due to the formation of
alpha-glycero-phosphate leads to a lowered acetyl-CoA concentration
and lowered ketone body formation (Horecker, 1969; Hötzel, 1971;
Förster, 1972). Administration of xylitol caused an inhibition of the
gluconeogenesis from proteins/amino acids. This inhibition lowers the
requirement of energy from fatty acid oxidation in the liver and by
protein-sparing effect the blood urea level and urea excretion were
reduced (Förster, 1972; Lang, 1972). Administration of high daily
doses of xylitol can provoke an increase in the serum uric acid
concentration. This results from an augmented purine biosynthesis, due
to enhanced formation of ribose phosphate (Förster, 1972; Förster et
al., 1972; Förster and Ziege, 1970; Henckenkamp and Zöllner, 1972).
TOXICOLOGICAL STUDIES
In a short-term experiment with eight normal subjects
(21-27 years) receiving orally xylitol over two weeks the fat and uric
acid metabolism was studied. After an initial adaptation phase the
xylitol level was raised from 5 g up to 50 g during seven days. No
significant changes in the serum concentration of triglycerides, FFA,
free glycerol, alpha-lipoproteins, total cholesterol, phosphatides,
aceto-acetate and ß-hydroxybutyrate were observed during xylitol
administration. The serum inorganic phosphate concentration was
elevated during the experiment and decreased again afterwards. After
an initial rise a significant decrease of the serum pyruvate level and
decrease of lactate level from the beginning were observed. The serum
uric acid level was not influenced by a xylitol intake (Mertz et al.,
1972).
After parenteral administration postoperatively of 10% xylitol
solution (1.5 g/kg body weight) Shumer (1971) found a significant
increase of lactic acid, uric acid, bilirubin and Alk.Pase in two
diabetic and two non-diabetic patients. In a short-term experiment he
carried out with two normal volunteers a dosage of 4.5 g/kg during
five days produced significant increased levels of urine uric acid,
SGOT, SAP, bilirubin, lactic acid, and inorganic phosphate in serum.
The levels returned to normal 10 days after cessation of infusion. No
effects were found in the BHN, Ca, cholesterol, glucose, amino-acids
and insulin analyses, urinalyses and haematology were found (Shumer,
1971).
In a short-term study respectively five, 15 and 30 g xylitol were
administered to healthy men during two and three weeks. Blood
chemistry, bilirubin, SGOT, Alk.Pase, uric acid and blood sugar were
normal. No diarrhoea was observed (Asano et al., 1972).
In an oral tolerance test with five persons adapted to xylitol up
to 120 g/day. The initial dose level was 30 g/day and this was raised
with 30 g/day at three day intervals. Liver function tests were normal
throughout the experiment, while there was a transient increase in
plasma lactate and urate. No diarrhoea below 90 g/day was noticed
(Amador and Eisenstein, 1971).
The tolerance of xylitol was studied in 18 male and one female
non-diabetic students aged 21-27 years. The students were given
xylitol for 21 days in increasing dose levels from five up to a
maximum of 75 g/day. After one month of interruption the same group
received xylitol in increasing dose levels from 40 g up to 220 g
again during 21 days (19 students received during the first week
40-100 g/day, 28 during the second week, 100-150 g and six during the
third week 150-220 g). The subjects themselves recorded quantity,
daily division of xylitol intake, number and consistency of bowel
movements as well as general condition and obvious side effects. Body
weights were estimated weekly. At the third day of each experimental
period and seven days after termination, fasting blood sugar analyses
and urinalyses on presence of reducing sugar were carried out. From a
130 g/day dose level diarrhoea was observed when the single doses were
poorly distributed over the day. No other significant effects were
noticed. In a similar study 23 men and three women were given xylitol
or sorbitol. The initial dose was 5 g which was increased to 75 g/day
after 14 days. In addition to the parameters investigated in the first
experiment, xylitol and glucose analyses in 24 hour urine were carried
out. Identically to the first experiment diarrhoea was the only
observed effect (Dubach et al., 1969).
In a two year tolerance study three groups of volunteers (35, 38
and 52) remained on strict diet containing respectively, fructose,
sucrose and xylitol. The average monthly amounts consumed in a varied
assortment of foods were respectively 2.1, 2.2 and 1.5 kg. The highest
daily doses of fructose and xylitol were 200-400 g. Serum samples were
analysed for Na, K, Ca, Mg, inorganic phosphates, ascorbate,
bilirubin, amylase, Alk.Pase, amino-acids, IgA, IgG and IgM. In
addition saliva analyses of IgA, IgG and IgM and amylase were carried
out. The number of occurrences of diarrhoea and flatulence-like
conditions were also scored. Body weights of the volunteers were
recorded weekly. The number of pregnancies in the groups were as
follows; eight in the sucrose, six in the fructose and eight in the
xylitol group.
No significant changes of clinico-chemical parameters in serum
and saliva were observed in the xylitol group. A significant rise in
the occurrence of diarrhoea and flatulence-like conditions was noted
in the xylitol group. All pregnancies, deliveries and infants were
normal.
A series of studies in Turku, Finland showed that after one year
on test in which xylitol replaced sucrose in the diet, dental caries
were reduced in the xylitol group by approximately 90% (Scheinin,
1974).
A more recent study involved 100 human young adults. Subjects
were divided into S-(sucrose) group, and X-(xylitol) group.
Consumption was 4.0 chewing gums per subject per day in the S-group
and 4.5 in the X-group. Frequency of sucrose intake was 4.2 times per
day in the S-group, and 4.9 in the X-group. The caries incidence was
2.92 in the S-group and -1.04 in the X-group. The results show a
profound difference in the caries increment rate between the two
experimental groups (Scheinin et al., 1975).
In an accident that happened in 1969/1970 in Australia, 20
patients who had received Japanese xylitol from two specific batches
by intravenous infusion died with symptoms of severe acidosis, liver
necrosis and renal oxalosis, and in one case oxalate crystals were
also found. The patient with the severest and most dramatic liver
damage had received only 200 g of xylitol. In these xylitol batches a
highly toxic substance was determined by the chicken embryo test.
Coats (1970) identified 2-mercaptoimidazole and 2-hydroxyimidazole in
the xylitol batches. 2-mercaptoimidazole was used as a plasticizer of
the rubber stoppers of the ampoules. All further efforts to obtain
material from the suspect batches from the Japanese firm failed, so
that no explanation for the incidents could be found (Lang, 1974).
In 1971/1972 oxalate crystals were observed in the kidneys and
brain in necropsies performed at the Rafenkrankenhaus in Hamburg on
five patients who had received infusions of xylitol. According to the
doctors responsible for their treatment they died of the severe
conditions from which they had been suffering. These findings gave
rise to extensive discussions as to whether xylitol was involved in
the causation-of oxalosis. However, it is most improbable that the
oxalosis observed in Hamburg was due to a specific toxic action of
xylitol. Further investigation of this subject has to be carried out
(Lang, 1974).
Tolerance studies in diabetes
In addition no harmful effects were observed in a diabetic person
who consumed 65 g xylitol/day during the two years experimental period
(Mäkinen and Scheinin, 1975; Mäkinen, 1976).
Acute tests with xylitol were conducted on 13 recruited adult
diabetics and the following parameters were determined: aceto-acetate,
ß-hydroxy-butyrate, unesterized fatty acid (UFA), free glycerine and
total lipids. A significant drop in concentrations of aceto-acetate
and ß-hydroxybutyrate occurred under administration of xylitol as
compared with the control experiments. UFA behaved similarly. The
concentration of free glycerine also clearly dropped at the end of the
experiment. It can be assumed from the results that the diminished
lipolyserate is the cause of reduced formation of ketone bodies. A
drop in total lipids indicated that hyperlipidemia might possibly also
be favourably influenced by xylitol (Grabner, 1971).
Xylitol in concentration varying from 5 to 20 gm in food was
administered to 23 diabetic persons. Except for diarrhoea in some
cases no harmful side effects were observed. No negative influence on
the diabetie metabolism was observed. Xylitol (25 g) dissolved in tea
caused more frequent diarrhoea and other abdominal side effects in the
subjects (Mellinghof, 1961).
Yamagata et al. (1967) investigated the effects of acute
intravenous infusion of 30 g/90 minutes on blood Sugar, pyruvate and
lactate, and on plasma free fatty acids (FFA) and insulin levels in
six normal and 11 diabetic persons. Xylitol infusion produced no
change of blood sugar in normal and a slight increase in diabetic
patients. Blood lactate showed a significant increase in the diabetic
group but only a slight increase in the normal group. Blood ketone
bodies and plasma FFA decreased in both groups after xylitol
administration. Plasma insulin increased slightly in five of the
normal subjects and in four of the nine diabetic subjects (Yamagata
et al., 1967).
In another experiment xylitol was chronically intravenously
infused to 12 mildly diabetic patients every morning for seven days.
No changes were observed in fasting blood sugar, serum triglycerides,
total cholesterol, serum electrolytes or ketone bodies. However, four
cases indicated the marked decrease of urine sugar excretion during
the experimental period. Xylitol was also given to two diabetic
persons with ketosis and decreased the ketone bodies from 5.1 to
0.2 mg % within four hours (Yamagata et al., 1967).
During two to six weeks 30 g xylitol/day was given orally to 12
stable diabetics. The caloric intake and amount of carbohydrates was
kept constant. Changes in fasting blood sugar, urine sugar, serum
lipids, serum mucoprotein, SGOT and SGPT were studied before, during
and after xylitol administration. In three of the 12 cases urinary
sugar excretion disappeared. A slight impairment of glucose tolerance
and a tendency to decreased SGOT and SGPT levels were observed. In
some cases diarrhoea was noticed, but no other effects of xylitol were
noticed (Yamagata et al., 1967).
Eighteen diabetic children received 30 g dietary xylitol during
four weeks. A significant elevation of the uric acid concentration in
serum was observed, also significant increases of total protein
content and of inorganic phosphorus. In these children no diarrhoea
was noticed (Förster et al., 1977).
REFERENCES
Amador, F. and Eisenstein, A. (1971) The effects of oral xylitol
administration in human subjects. Unpublished data cited by Brin, M.
and Miller, O. N. (1974) The safety of oral xylitol; Sugars in
Nutrition; Ed. Sipple and McNutt, 33, 591-606
Asakura, T. (1967) Non-glycolytic sugar-metabolism in human
erythrocytes 1. Xylitol metabolism, J. Biochem., 62, 184-193
Asano, T., Levitt, M.D. and Goetz, F. C. (1972) Xylitol absorption in
healthy men, Diabetes., 21, suppl. 1, 350-351
Asano, T., Levitt, M.D. and Goetz, F. C. (1973) Xylitol absorption in
healthy men, Diabetes, 22, 279-281
Bächtold, H. P. (1972) Prüfung in 5-Tage-Toxizitätsversuch an Ratten
und Mäusen. Unpublished report from the Research Department of
Hoffmann La Roche Co., submitted to the World Health Organization by
Hoffmann La Roche Co., Basle, Switzerland
Banziger, R. (1970) A thirteen week oral toxicity study of xylitol in
monkeys. Unpublished report of Hazleton Laboratories Inc., submitted
to the World Health Organization by Hoffmann La Roche Co., Basle,
Switzerland
Bässler, K. H. (1965) Biochemie und Stoffwechsel von Xylit (1965)
Dtsch. Lebensm. Rdsd., 61, 171-173
Bässler, K. H. und Prellwitz, W. (1964) Insulin und der
Verteilungsraum von Xylit bei eviscerierten Ratten, Klin.
Wocherschrift, 42, 94-95
Bässler, K. H., Prellwitz, W., Unbehaun, V. und Lang, K. (1962)
Xylitstoffwechsel beim Menschen, Klin. Wschr., 40, 791
Bässler, K. H. und Reimold, W. V. (1965) Lactaatbildung aus Zuckern
und Zuckeralkoholen in Erythrocyten, Klin. Wschr., 43, 169-171
Bässler, K. H. und Wagner, G. (1970) Fettsäuregehalt der Rattenleber
unter verschiedenen Bedingungen der parenteralen Ernährung
Berger, W., Göschke, H., Moppert, J. and Künzli, J. (1973) Insulin
concentration in portal venous and peripheral venous blood in man
following administration of glucose, galactose, xylitol and
tolbutamide, Horm. Metab. Res., 5, 4-8
Birnesser, H., Reinauer, H. and Hollmann, S. (1973) Comparative study
of enzyme activities degrading sorbitol, ribitol, xylitol and
gluconate in guinea-pigs' tissues, Diabetologia, 9, 30-33
Brin, M. and Miller, O. N. (1974) The safety of oral xylitol. Sugars
in Nutrition, Ed. Sipple, H. L. and McNutt, K. W., Acad. Press
Coats, D. A. (1970) Report to the Deutsche Arzeimittelkommission 1970.
Cited by K. Lang (1974) (Unpublished report)
Dehmel, K. H., Förster, H. and Mehnert, H. (1967) Absorption of
xylitol. Int. Symp. on metabolism, physiology and clinical use of
pentoses and pentitols, Hakone, Japan, 1967, 177-181, Ed. Horecker,
B. L. e.a.
Dixon, R. and East, S. (1973) Xylitol levels in plasma following
intravenous and oral administration to man. Unpublished report,
Psycho-Endocrine Centre, St. James's Hospital, Dublin 8, Ireland,
submitted to the World Health Organization by Hoffmann La Roche Co.,
Basle, Switzerland
Dubach, U. C., Feiner, E. and Fargo, E. (1969) Orale Verträglichkeit
von Xylitol bei stoff wechselgesunden Probanden, Schweiz. Med.
Wschr. 99, 190-194
Felber, J.P. (1976) Untersuchung der Wirkung des Xylits auf den
Blutzuckerspiegel beim Menschen, Abstract einer Expertise vom 24-6-76.
Sitzung der Eidg. Ernährung Kommission in Bern
Förster, H. (1972) Grundlagen für die Verwendung der drei
Zuckeraustauschstoffe Fructose, Sobit und Xylit, Med. Ernähr.,
13, 7-15
Förster, H. (1974) Zum Stoffwechsel von Monosacchariden und Polyolen,
Ernährungs. Med., 1, 37-55
Förster, H., Boecker, S. and Ziege, M. (1972) Anstieg der
Konzentration der Serumharnsäure nach akuter und chronische Zufuhr von
Saccharose, Fructose, Sorbitol und Xylit, Med. u. Ernähr.,
13, 193-196
Förster, H., Boecker, S. and Walther, A. (1977) Verwendung von Xylit
als Zuckeraustauschstoff bei diabetischen Kindern, Fortschr. Med.,
95, nr. 2, 99-102
Förster, H. und Hoffmann, H. (1973) Biochemische Überlegungen zur
Verwendung der Kohlenhydrate in der parenteralen Ernährung,
Infusiontherapie, 1, 265-279
Förster, H. und Ziege, M. (1970) Anstieg der
Senumharnsäurekonzentration nach oraler Zufuhr von Fructose, Sorbit
und Xylit, Z. Ernährungswiss., 10, 394-396
Froesch, E. R. (1972) Übersicht über den Haushalt der Betriebstoffe
mit besonderer Berücksichtigung des Stoffwechsels von Glukose,
Fruktose, Sorbit und Xylit und deren therapeutische Verwendbarkeit,
Int. Z. Vit. und Ernährungsforsch., Suppl. 12, 73-86
Froesch, E. R. (1975) Umsatz und Verwertung von Glukose und
Glukoseaustauschstoffen bei Mensch und Tier, Nutr. Metabol., 18
(suppl. 1), 30-44
Froesch, E. R. and Jakob, A. (1974) The metabolism of xylitol. Sugars
in nutrition, Ed. H. L. Sipple and K. W. McNutt, Academic Press,
241-258
Grabner, W., Berger, G., Bergner, D. and Matzkies, F. (1971) Über den
Einfluss des Xylits auf den Fettstoffwechsel von Diabetikern,
Med. Ernährung, 12, 126-128
Grunberg, E., Beskid, G. and Brin, M. (1973) Xylitol and Dental
Caries. Efficacy of xylitol in reducing dental caries in rats,
Internat. J. Vit. Nutr. Res., 43, 227-232
Grütte, F. K. and Rödel, H. (1975) Zur Bedeutung des Zuckeralcohols
Xylit als Zuckeraustauschstoff Ernährungsforschung, 3, nr. 20, 74-79
Haydon, R. K. (1961) The antiketogenic effects of polyhydric alcohols
in rat-liver slices, Biochim. Biophys. Acta., 46, 598-599
Heuckenkamp, P. U. und Züllner, N. (1972) Xylitbalanz während
mehrstündiger infusionen mit konstanten Zufahrraten bei gesunden
Manschen, Klin. Wschr., 50, 1863-1864
Hirata, Y., Fujisawa, M. and Ogushi, T. (1967) Effects of intravenous
injection of xylitol on plasma insulin. Int. Symp. on metabolism,
physiology and clinical use of pentoses and pentitols, Hakone, Japan,
Ed. Horecker, B. L. et al.
Hirata, Y., Fujisawa, M., Sato, H., Asano, T. and Katsuki, S. (1968)
Effects of intravenous injection of xylitol on blood sugar, blood
pyruvic acid and plasma insulin levels in the dog, Z. für dieo
gesamte exptl. Med., 145, 111-119
Hollmann, S. (1967) Pentitol-metabolizing enzymes of the uronic acid
pathway, Int. Symp. on metabolism, physiology and clinical use of
pentoses and pentitols, Hakone, Japan, 97-108
Horecker, B. L. (1969) Stoffwechselwege der Kohlenhydrate: ihre
Regulierung und physiologische Bedeutung. III Stoffwechsel und
physiologische Wirkungen von Xylit, Med. Ernährung., 10, 66-69,
95-98, 127-129
Hosoya, N. and Machiya, T. (1967) Xylitol effect on ketogenesis in
alloxan diabetic rat liver slice, Int. Symp. on metabolism, physiology
and clinical use of pentoses and pentitols, Hakone, Japan, 1967, Ed.
Horecker, B. L. et al.
Hosoya, M., Sakurai, T. and Shimazono, N. (1966) Glycogen and
glycolytic intermediates in Alloxan-diabetic liver slices, VIIth
International Congress of Nutrition, Hamburg
Hosoya, M. and Iitoyo, M. (1969) Effects of xylitol administration on
the development (growth) of rats (cited in Brin and Miller, 1974)),
J. Jap. Soc. Food Nutr., 22, 17-20
Hötzel, D. (1971) Über den Intermediärstoffwechsel von Sorbit, Xylit
und Cyclamaten, Dtsch. Zahnärzl. Z., 26, 1035-1040
Hummler, H. (1974) Orientierende Verträglichkeitsuntersuchungen über 6
Wochen mit Xylit, Sorbit und Saccharose am Hund. Unpublished report
from Huntingdon Research Centre, submitted to the World Health
Organization by Hoffmann La Roche Co., Basle, Switzerland
Hummler, H. (1976a) Reproduktionstoxikologische Untersuchungen mit
Xylit an der Rätte. Unpublished report from Huntingdon Research
Centre, submitted to the World Health Organization by Hoffmann La
Roche and Co., Basle, Switzerland
Hummler, H. (1976b) Cancerogenese Untersuchungen mit Xylit an der
Maus. Unpublished interim report from Huntingdon Research Centre,
submitted to the World Health Organization by Hoffmann La Roche and
Co., Basle, Switzerland
Hummler, H. (1976c) Chronische Toxizitäts- und Carcinogenese-
Untersuchungen mit Xylit an Ratten. Unpublished report from Huntingdon
Research Centre submitted to the World Health Organization by Hoffmann
La Roche and Co., Basle, Switzerland
Hummler, H. (1976d) Chronische Verträglichkeitsprüfung mit Xylit an
Hunden (52 and 78 w.). Unpublished report from Huntingdon Research
Centre, submitted to the World Health Organization by Hoffmann La
Roche and Co., Basle, Switzerland
Huthunen, J. K., Mäkinen, K. K. and Scheinin, A. (1975) Effects of
sucrose, fructose and xylitol diets on glucose, lipid and urate
metabolism, Acta Odont. Scand., 33, suppl. 70, 239-245
Jakob, A., Williamson, J. R. and Asakura, T. (1971) Xylitol metabolism
in perfused rat liver. Interactions with gluconeogenesis and
ketogenesis, J. Biol. Chem., 246, No. 24, 7623-7631
Jourdan, M. H., McDonald, J. and Henderson, J. R. (1972) The effects
of 14C-xylitol, given intravenously, on the serum glucose, insulin
and lipid concentrations of male and female baboons, Nutr. Metabol.,
14, 92-99
Jursons, K. K., Puzaka, J. J., Aalmans, A. R. and Vitolin, S. P.
(1974) Izvestija Akademii Nauh Latvijskoj SSR., 9, 16-22
Keller, U. and Froesch, E. R. (1972) Vergleichende Untersuchungen über
den Stoffwechsel von Xylit, Sorbit und Fruktose beim Menschen,
Schwerz. Med. Wschr., 102, 1017-1022
Kieckebusch, W., Gziem, W. und Lang, K. (1961) Die Verwertbarkeit von
Xylit als Nahrungskohlenhydrat und seine Verträglich, Klin. Wschr.,
39, 447
Kuzyha, T., Kanazawa, Y. and Kosaka, K. (1966) Plasma insulin response
to intravenously administered xylitol in dogs, Metabolism, 15, No.
12, 1149-1152
Kuzuya, T., Kanazawa, Y. and Kosaka, K. (1969) Stimulation of insulin
secretion by xylitol in dogs, Endocrinology, 84, 200-207
Lang, K. (1964) Die ernährungsphysiologischen Eigenschaften von Xylit,
Internat. Z. Vitaminforsch., 34, 117-122
Lang, K. (1972) Xylit - Biochemie und Klinik, Suppl. 13 zur
Z. Ernährungswiss: "Rehabilitation durch Ernährung" Darmstadt 11-15
(symp. CSSR)
Lang, K. (1974) Xylit in der oralen und parenteralen Ernährung Heft
der Schriftenreihe des Bundes für Lebensmittelrecht und
Lebensmittelkund, 75, 21-23
Liebau, K. (1964) Untersuchungen der Resorption des Xylit beim
Menschen Dissertation of K. Liebau, submitted to the World Health
Organization by Hoffmann La Roche Co., Basle, Switzerland (Unpublished
report)
Mäkinen, K. K. (1976) Long-term tolerance of healthy human subjects
to high amounts of xylitol and fructose: general and biochemical
findings. In: Monosaccharides and polyalcohols in nutrition
therapy and diabetics, Ed. G. Ritzel, G. Brubacher, Verlag H.
Huber, Int. J. Vit. Nutr. Res. nr., 15
Mäkinen, K. K. and Scheinin, A. (1975) Effect of the diet on certain
clinico-chemical values of serum, Acta Odont. Scand., 33, suppl.
70, 265-276
McCormick, D. B. and Touster, O. (1957) The conversion in vivo
of xylitol to glycogen via the pentose phosphate pathway, J. Biol.
Chem., 239, 451-461
Mehnert, H., Summa, J. D. and Fürster, H. (1964) Untersuchungen zum
Xylitstoffwechsel bei gesunden, Leberkranken und diabetischen
Personen, Klin. Wschr., 42, 382
Mellinghoff, G. H. (1961) Über die Verwendbarkeit des Xylit als
Ersatzzucker bei Diabetikern, Klin. Wschr., 39, 447
Meng, H. C. (1974) Sugars in parenteral nutrition. Sugars in
nutrition, Ed. H. L. Sipple, K. W. McNutt, Academic Press, 527-566
Mertz, D. P., Kaiser, V., Klöpfer-Zaar, M. and Beisbarth, H. (1972)
Serumkonzentrationen verschiedener Lipide und von Harnsäure während 2
wüchiger Verabreichung von Xylit, Klin. Wschr., 50, 1107-1111
Montague, W., Howell, S. L. and Taylor, K. W. (1967) Pentitols and the
mechanism of insulin release, Nature, 215, 1088-1089
Mosinger, M. (1971) Effects of the long-term oral administration of
xylitol. Unpublished report of Mosinger, M., submitted to the World
Health Organization by Hoffmann La Roche, Basle, Switzerland
Müller-Hess, R., Geser, C. A., Bonjour, J.P., Jequier, E. and Felber,
J.P. (1975) Effects of oral xylitol administration on carbohydrate and
lipid metabolism in normal subjects, Infusionstherapie, 2, 247-252
Opitz, K. (1967) The influence of xylitol and other polyols and sugars
on fat mobilization, Int. Symp. on metabolism, physiology and clinical
use of pentoses and pentitols, Hakone, Japan, Ed. Horecker, B. L. et
al.
Oshinsky, R. J., Wang, Y. M. and Eys, J. v. (1976) Oxalate formation
following xylitol infusion in 4-deoxypyridoxine treated rabbits,
Fed. Proc., 35, 419
Pazenko, A. G. (1969) Voprosy prolilaktiki, diagnostiki i lecenija
zabolevanij organor, Piscevarenija, Kiev, 133
Petrich, Ch., Reinauer, H. and Hollmann, S. (1972) Vergleichende
Aktivitätsmessungen der Enzyme des Glucuronsäure Xylulose-Zyklus
in der Leber, dem epididymalen Fettgewebe der Ratte, in einem
Morris-Hepatoom und einer Fibrocyten-Kultur, Z. Klin. Chem. Klin.
Biochem., 10, 355-358
Pitkänen, E. and Sahlström, K. (1968) Increased excretion of
xylitol after administration of glucorone, lactone and ethanol in
man, Ann. Med. exp. Fenn., 47, 143-150
Pool, W. and Hane, D. (1970) Acute toxicity and dog tolerance testing
of xylitol. Unpublished report from the Research Department of
Hoffmann La Roche Co., submitted to World Health Organisation by
Hoffmann La Roche Co., Basle, Switzerland
Pool, W. and Hane, D. (1972) Acute mouse toxicity of xylitol.
Unpublished report from the Research Department of Hoffmann La Roche
Co., submitted to the World Health Organization by Hoffmann La Roche
Co., Basle, Switzerland
Quadflieg, K. H. and Brand, K. (1974) Xylitol metabolism in human
erythrocytes, Z. Phys. Chemie, 355, 1239
Scheinin, Ulla (1974) Incidence of dental caries among a group of
university students in Turkey, Acta. Odont. Scand., 32, 335-344
Scheinin, Arje., Mäkinen, Kauko K., Tammisalo, Erkki., Rekola, Maarit.
(1975) Turku sugar studies. XVIII. Incidence of dental caries in
relation to one-year consumption of xylitol chewing gum, Acta.
Odont. Scand., 33, 269-278
Schmidt, B., Fingerhut, N. and Lang, K. (1964) Über den Stoffwechsel
von radioaktiv markiertem Xylit bei der Ratte, Klin. Wschr., 42,
1073-1077
Shima, S., Mitsunaga, M. and Nakao, T. (1970) Effect of xylitol on
testicular protein and RNO biosynthesis of rats, Endocrinol. Jap.,
17, 283-287
Shumer, W. (1971) Adverse effects of xylitol in parenteral
alimentation Metabolism., 20, 345-347
Staub, H. W. and Thiessen, R. (1972) Effect of dietary polyols on
serum cholesterol in rats, Federation Proc., 31, 291
Stein, G. (1966) Adaptive Prozesse im Xylitstoffwechsel und ihre
Auswirkungen Dissertation Mainz, 1966, submitted to the World Health
Organization by Hoffmann La Roche and Co., Basle, Switzerland
(Unpublished report)
Swarm, R. L. and Banziger, R. (1970) A thirteen week oral feeding
study in rats with xylitol (Ro-6-7045). Unpublished report of Hazleton
Laboratories Inc., submitted to the World Health Organization by
Hoffmann La Roche, Basle, Switzerland
Touster, O. (1970) Parenteral nutrition (Proc. on an internat. Symp.
Vanderbilt University, School of Medicine, Nashville) 131-138, Ed.
Meng, H. C., Law, D. H., Thomas, C. C., Publisher Springfield,
Illinois, USA
Touster, O. (1974) The metabolism of polyols. In: Sugars in Nutrition,
Ed. H. L. Sipple, K. W. McNutt, Academic Press, 229-239
Touster, O. and Shaw, D. (1962) Biochemistry of the acyclic polyols,
physiol. Rev., 42, 181-225
Truhaut, R., Coquet, B., Fouillet, X., Galland, I., Guyot, D., Rouaud,
J. L. and Long, D. W. (1977) Subacute toxicity of xylitol in rats.
Personal communication to the World Health Organization (Unpublished
report)
Wagner, G. and Bässler, K. H. (1971) Gibt es Unterschiede in der
antiketogenen Wirkung gebräuhlicher Zucker und Polyalkohole, Medizin
Ernährung., 12, 53-54
Wang, Y. M., King, S. M., Patterson, J. H. and Van Eys, J. van. (1973)
Mechanism of xylitol toxicity in the rabbit, Metabolism, 22, nr.
7, 885-894
Wang, Y. H., Oshinky, R. J., Lantin, E. and Eys, J. van. (1975) An
investigation of oxalate formation from xylitol infusion in rabbits,
Fed. Proc., 35, 920
Wilson, R. B. and Martin, J. M. (1970) Plasma insulin concentrations
in dogs and monkeys after Xylitol, glucose or tolbutamide infusion,
Diabets., 19, 17-22
Woods, H. F. (1975) Hepatic metabolism of pentites, Nutr. Metabol.,
18 (Suppl. 1), 65-78
Woods, H. F. and Krebs, H. A. (1973) Xylitol metabolism in the
isolated perfused rat liver, Biochem. J., 134, 437-443
Yamagata, S., Goto, Y., Ohneda, A., Anzai, M., Kwashima, S., Kikuchi,
J., Chiba, M., Maruhama, Y., Yamauchi, Y. and Toyota, T. (1967)
Clinical application of xylitol in diabetics, Int. Symp. on
metabolism, physiology and clinical use of pentoses and pentitols,
Hakone, Japan, 316-325, Ed. Horecker, B. L. et al.
These biotransformation studies are also supported by Schmidt et
al. (1964), Hörecher (1969), Touster (1974), Stein (1966), Petrich et
al. (1972) and Grütte and Rödel (1975). McCormick and Touster (1957)
found that the metabolism of rat and guinea pig was similar.
Effects on enzymes and other biochemical parameters
Xylitol has especially influence on the activity of the enzymes
of carbohydrates metabolism and the insulin hormone. Both in
experiments with parenteral and oral administration of xylitol a
stimulation of insulin secretion and an elevated serum insulin
concentration are observed in rat, dog and monkey, although the plasma
glucose level slightly increased for a while and then decreased below
the fasting level (Bässler and Prellwitz, 1964; Kuzuya et al., 1966,
1969; Hirata et al., 1967, 1968; Montague et al., 1967; Wilson and
Martin, 1970; Froesch, 1975; Jourdan et al., 1972; Touster, 1974).
Kuzuya et al. (1969) found that the insulin secretion produced by
xylitol in dogs was more pronounced than by glucose. Wilson and Martin
(1970) observed the same effect in dogs, while they found that in
monkeys glucose caused a more marked stimulation of the insulin
secretion than xylitol did. The insulin secretion in dogs is also much
stronger than in man (Hirata et al., 1967). Meng (1974) however did
not find any changes of the insulin level in dogs, i.v. infused with
xylitol. This study included only five animals.
Glycogen production is stimulated by the administration of
xylitol to guinea pigs, rat and perfused rat liver, even when diabetic
animals are used: (McCormick and Touster, 1957; Hosaya et al., 1966;
Touster, 1970; Förster and Hoffman, 1973). Jakob et al. (1971) found
that in liver most of the xylitol administered to the animals was
rapidly converted to glucose and to small quantities of lactate.
Bässler and Reimold (1965) observed a stimulating influence of xylitol
on the production of lactic acid in erythrocytes which was also
noticed with glucose. In perfused rat liver xylitol stimulated the
lactate production and caused an increased lactate to pyruvate ratio
(Jakob et al., 1971; Förster and Hoffmann, 1973; Woods and Krebs,
1973; Woods, 1975). Besides the increased ratio of lactate to
pyruvate Jakob et al. (1971) found also increased ratios of
alpha-glycerophosphate to dihydroxy acetone-P and triosephosphate to
3-P-glycerate. In a study of Hirata et al. (1968) a significant
decrease of blood pyruvic acid level was noticed in normal dogs.
In close relation with the influence of the carbohydrate
metabolism an antiketogenic and antilipolytic effect of xylitol is
also observed (Haydon, 1961; Hosaya and Machiya, 1967; Bässler and
Wagner, 1970; Jakob et al., 1971; Wagner and Bässler, 1971; Birnesser
et al., 1973). As a result of accumulation of alpha-glycerophosphate
and other phosphorylated intermediates a fall in the inorganic
phosphorus and adenine nucleotides was observed (Petrich et al., 1972;
Woods and Krebs, 1973; Woods, 1975). The fall in the inorganic
phosphorus leads to an increased purine catabolism and to a
hyperuricemia in isolated perfused rat liver (Woods and Krebs, 1973;
Woods, 1975). Forster and Hoffmann (1973) found that xylitol
accelerates the biosynthesis of uric acid.
Shima et al. (1970) found a stimulation of testicular protein and
RNA biosynthesis in rats fed xylitol. Staub and Thiessen (1972)
observed increased cholesterol levels in rats fed xylitol.
In experiments with xylitol oxalate formation is observed, but
glucose is reported to have a stronger effect on this formation (Wang
et al., 1975; Oshinsky et al., 1976; Brin and Miller, 1974). Therefore
no clear relationship between oxalate accumulation and xylitol
toxicity is found by Oshinsky et al. (1976) and Wang et al. (1975).
TOXICOLOGICAL STUDIES
Special studies on reproduction
In a reproduction study five groups of 30 male and 60 female rats
of the P-generation were fed 0, 2, 5, 10 and 20% dietary xylitol. The
total amount of carbohydrates was kept constant. In addition 20%
sorbitol and a 20% sucrose group were used as sugar controls. This
study has only been carried out till the F1B-generation. At the
second mating, an abrupt increase in pup mortality occurred in all
groups between four and 12 days. This was caused by a heating failure.
This resulted in loss of too many pups to complete the reproduction
study.
In the animals receiving 5, 10 and 20% xylitol decreased
food-intake and weight gain was recorded. At the first mating, a
slightly higher initial litter size among test groups was
counterbalanced by a slightly higher cumulative pup loss during
lactation. No other effects on the reproduction were noticed in the
F1a-animals receiving xylitol. This study will be repeated (Hummler,
H. 1976a).
Levels of xylitol up to 20% of the diet fed to rats up to four
months had no significant adverse effects. Growth rate was normal.
Adaptation to high dosage was shown to be important. Xylitol had no
effect on the number or size of pups or dams fed xylitol, and the pups
adapted readily to the supplement at 5 and 10% in diet, after weaning,
as instituted following adverse effects with the 20% xylitol level,
initially (Hosoyol and Iitoyo, 1969).
Special studies on carcinogenicity
In a carcinogenicity study 100 female and 100 male mice per group
weighing 24-25 g, were fed 0, 2, 10 and 20% dietary xylitol during
1.5-2 years. The total amounts of carbohydrates were kept constant and
animals receiving sucrose served as sugar control group. Body weight
and food intake were recorded weekly, while with intervals water
consumption was determined. At 26, 52 and 79 weeks and prior to
terminal sacrifice ophthalmoscopy was carried out. After 80 weeks 20
males and 20 females from each group were killed. The mice were killed
when survival within a group reached 20%. Gross pathology was
performed visually and by palpation. Microscopic examination on
adrenals, thyroids, ovaries, liver, spleen, lymph, pituitary gland and
bone marrow and all macroscopically observed lesions suggestive of
neoplasia were routinely carried out for every animal, along with
blood smears.
At the start of the experiment a severe diarrhoea was observed,
therefore a number of adaptation phases with slowly increasing xylitol
concentrations in the diet were necessary. Animals receiving 10% and
20% xylitol showed increased water and food intake. In the animals
receiving 2% and 10% xylitol, 20% sorbitol an increased body weight
gain was observed, whereas the animals receiving 20% xylitol had a
significant lower body weight gain. The mortality of the male mice
receiving 10 and 20% xylitol was elevated. No histopathology and no
details on tumour incidence are available. The study is at the moment
an inadequate carcinogenicity study, which has to be completed first
(Hummler, H., 1976b).
In a long-term toxicity and carcinogenicity study five groups of
75 female and 75 male Sprague-Dawley rats, weighing 120-150 g, were
fed 0, 2, 5, 10 and 20% dietary xylitol. The total amounts of
carbohydrates in the diet were kept constant. Animals receiving 20%
sorbitol and 20% sucrose were used as sugar control groups. Food
intake and body weight were recorded weekly. Water consumption was
recorded during weeks 12, 25, 52 and 78. Ophthalmoscopy, urinalysis,
haematology and biochemistry were performed at 0, 13, 26, 52, 78 and
104 weeks. Urinalyses included pH, specific gravity, protein, reducing
substances, glucose, ketones, bile pigments, urobiligen, haemoglobin
and oxalic acid determinations. Haematology consisted of determination
of PCV, Hb, RBC, WBC, MCHC, MCV, differential count, prothrombin
index, whole blood clotting time. The biochemical estimations were
urea and glucose in plasma, total proteins, protein electrophoresis,
Alk Pase, GPT, GOT, bilirubin, uric acid, cholesterol, lactate, LDH,
alpha-HBDH in serum and total reducing substances, insulin,
xylitol,Na+, K+, Cl- and bicarbonate in blood. After 26 and 52
weeks, five males and five females per group were killed. In the gross
pathology the animals were examined visually and by palpation. Brain,
heart, liver, kidneys, adrenals, pituitary, thyroid, spleen,
testes/ovaries were weighed. These organs and pancreas, thymus,
uterus, cervical and mesenteric lymph nodes, stomach, bone marrow,
ileum, coecum, duodenum, urinary bladder, eye, lungs and any
macroscopically abnormal tissues were histopathologically
investigated. Blood smears of these organs were also studied.
Significant decreased weight gain in animals receiving 10% or 20%
xylitol during the experiment was observed, while there was only
tendency to decreased food intake in animals receiving 20% xylitol at
78 weeks. In rats receiving 20% xylitol, higher water intakes were
recorded during weeks 26 and 52, and in female rats also during week
78.
In male and female rats at 13, 26 and 52 weeks and in female rats
at week 78, receiving 20% xylitol greater volumes of more diluted
urine were excreted. After 26 weeks, the urine from animals receiving
5, 10 and 20% showed decreased specific gravity.
No increase in mortality was noticed in the treated groups,
Except a significant decreased prothrombin index in all male rats
receiving xylitol and female rats receiving 20% xylitol, no
haematological changes were observed at 78 weeks. In biochemistry a
tendency to decreased lactate contents in the blood of animals
receiving 10 and 20% xylitol and at 78 weeks a tendency to increased
uric acid content in male rats of the 20% xylitol group were observed.
No morphological abnormalities in the treated groups differing
significantly from the control group ware observed. The standard
deviation in the insulin determination was too great for making an
evaluation of the results possible.
The study is not yet completed. No details on tumour incidence
are available so far (Hummler, 1976c).
Acute toxicity
LD50
Animal Route mg/kg References
body weight
mouse oral 14 100 Bächtold (1972)
mouse oral 22 000 Pool and Hane (1972)
mouse oral >4 000 Pool and Hane (1970)
mouse oral 25 700 Kieckebuch (1961)
mouse intraperitoneal >2 000 Pool and Hane (1970)
mouse subcutaneous >2 000 Pool and Haue (1970)
(con't)
LD50
Animal Route mg/kg References
body weight
mouse intravenous >4 000 Pool and Hane (1970)
mouse intravenous 22 200 Grütte and Rödel (1915)
mouse intravenous 3 770-9 450 Pazenko (1969)
adult rat oral >4 000 Pool and Hane (1970)
neonatal rat oral > 4 000 Pool and Hane (1970)
rat oral 14 100 Bächtold (1972)
rabbit oral > 2 000 Pool and Hane (1970)
rabbit intravenous 4 000-6 000 Wang et al. (1973)a
a The acute toxicity in rabbits was determined by intravenous
infusion of 87 mg xylitol/kg/minute. The observed LD50 was 4-6 g/kg
body weight. Striking increases of SGOT and serum LDH levels, along
with an increase urine volume were observed (Wang et al., 1973).
Short-term studies
In a subacute toxicity study xylitol was administered to 20
female and 20 male rats per group by daily gastric intubation for a
period of 14 days. Dose levels were 1.25, 2.5, 5.0 and 10.0 g/kg. The
animals receiving 1.25 g xylitol/kg during the first nine days,
received 10.0 g xylitol/kg during the last seven days. In the control
groups animals received 0, 2.5 and 5.0 g sucrose/kg/day. During
treatment (two, five and 14 days) animals were submitted to careful
clinical examinations and blood serum analyses related to hepatic
functions. These analyses were glucose in blood and bilirubin, FFA,
total lipids, triglycerides, cholesterol, G-6-PDH and Alk. Pase, GPT
and GOT in serum. Also body weight and food consumption were recorded.
The animals were sacrificed after two, five and 14 days. Heart, liver,
spleen, kidneys, adrenals, gonads, stomach were weighed. These organs
and jejunum, colon, pancreas and brain were histologically examined in
two and five days treatment groups, while in 14 days treatment group
this examination was only performed on liver. The histological study
was conducted on five males and five females per group.
Except for a decrease of the FFA content in the blood at all
xylitol dose levels, no effects are observed in this study. No
evidence of hepatotoxicity was recorded (Truhaut et al., 1977).
In a 15 days' experiment with rats, sucrose was replaced
respectively by 10 and 20% xylitol. The protein of the diet consisted
of casein, while their carbohydrate allowance consisted of starch and
sugar. In the 10% xylitol dose group no histopathological changes were
observed. However, in the 20% xylitol dose group adverse effects of
the metabolism of the liver cells were noticed. A reduction of the
glycogen and lipids concentrations were observed. The mitotic activity
of the liver cells was sharply reduced to a level five times lower
than in the control rats (Jursons et al., 1974).
In a short-term (13 weeks) study four groups of eight female and
eight male Charles River CD rats weighing 120-150 g, were fed 0, 5, 10
and 20 g dietary xylitol/kg/day. Food and water consumption were
recorded daily and body weight weekly. Haematology, blood glucose and
urinalyses of five male and five female rats of each group were
carried out at four, eight and 12 weeks. Alk. Pase, GOT, bilirubin and
uric acid in serum and BUN of five males and five females per group
were determined at 13 weeks.
The animals, particularly the male rats, showed reduced body
weight gain and food consumption, that were dose dependent. In the 20%
xylitol group BUN was elevated. In treatment groups dose-related
decrease of the absolute heart weight are observed. Except for
transient diarrhoea in a number of treated animals, no other
significant changes were observed. No histopathological lesions
related to xylitol administered were noticed (Swarm and Banziger,
1970).
Rat
Rats fed a cariogenic diet containing 10% xylitol showed no
carious lesions in the first and third molars and only minimal
involvement of the second molars was observed (Grunberg et al., 1973).
Dog
In short-term study five dogs were dosed 10 g xylitol/kg/day
by intravenous infusion during seven weeks. Blood chemistry and
urinalyses were investigated weekly. Plasma glucose content was
reduced to 64 mg % after six weeks. A slight increase in plasma
lactate and a significant increase of SGPT and Alk. Pase was observed.
Also an increased urinary loss was observed. No effect of xylitol on
plasma insulin levels and no oxalate crystals in the kidneys were
observed (Meng, 1974).
A six weeks toxicity experiment with one female and one male dog
(5.5-6 kg) per group was carried out. The concentration was during the
experiment increased from 5% after two weeks to 20%. Food consumption
was recorded twice daily and body weights twice a week. At the end of
the experiment gross pathology was carried out, and brain, heart,
liver, lungs, pituitary, spleen, pancreas, thymus, prostate/uterus,
kidneys, thyroids, adrenals and testes/ovaries were weighed. No
histopathological investigation was carried out.
Except for a tendency to increased liver weights in the xylitol
groups no effects are observed. This study is not an adequate toxicity
study, but has to be seen as preliminary experiment (Hummler, 1974).
In a long-term toxicity study eight female and eight male beagle
dogs per group, weighing 6-9 kg, were fed 0, 2, 5, 10 and 20% dietary
xylitol during two years. The total amounts of carbohydrates were kept
constant. In addition animals receiving 20% sorbitol and 20% sucrose
were used as sugar controls. Body weight and food consumption was
recorded weekly, while water consumption was recorded over five day
periods at the weeks one to four, 9-12, 21-24, 35-38 and 46-49.
Ophthalmoscopy, dental examination and a full neurological examination
of four male and four female dogs were carried out at 13, 26, 39, 50,
64 and 76 weeks. Haematology, biochemistry and urinalyses were carried
out at the beginning of the experiment and at 12, 26, 38, 50, 64 and
76 weeks. The haematology included erythrocyte sedimentation rate,
PCV, Hb, RBC, reticulocyte count, MCHC, MCV, WBC, differential count,
platelet count, prothrombin index, whole blood clotting time. The
biochemistry included determination of total protein, Al, GPT, GOT,
total LDH, alpha-HBDH, bilirubin, uric acid, cholesterol, lactate and
insulin in serum and urea xylitol, glucose concentration and total
reducing substances in plasma. Sodium, potassium, chloride and
bicarbonate were also detected in the blood. Urinalyses consisted of
estimations of Ph, protein, reducing substances, glucose, ketones,
bile pigments, urobiligen and Hb. On completion of 52 weeks, two male
and two female dogs per group were sacrificed for interim study, while
the remaining dogs will be killed after two years' dosing. The weights
of brain, liver, kidneys, pituitary, thyroid, spleen, heart, lungs,
adrenals, ovaries, testes, uterus, thymus, prostate, pancreas were
recorded. Histopathology of these organs and of aorta, trachea, lymph
nodes, gall bladder, urinary bladder, salivary glands, oesophagus,
duodenum, stomach, jenunum, ileum, skin, skeletal muscle, mammary
glands, tongue, eye with optic nerve and sciatic nerve was performed.
The food intake and weight gain of the 20% xylitol group were
increased. A dose-related increase of SAP and SGPT and a decreased
lactate level was noticed, while in the 20% xylitol dose group also
the total serum protein was significantly increased. At 52 weeks a
tendency to increased cholesterol content in serum was observed.
Except a dose related decrease in the pituitary of the xylitol
dose groups no effects on organ weights, in gross necropsy and
histopathology were observed. The number of animals, however, did not
allow a real statistic evaluation (Hummler, 1976d).
Mean values of haematological, biochemical and urinalyses were
calculated from total population of female and male animals.
Significant changes could be looked over by elevated standard
deviation. In the insulin determinations variation was too much for
further conclusion. In view of the mentioned restrictions it can be
stated that this study is not fully adequate, it has to be completed
first.
In a 13 weeks toxicity study two male and two female rhesus
monkeys, weighing 2-3 kg, were dosed twice daily six days a week 0,
1.0, 3.0 and 5.0 g xylitol/kg/day by gastric intubation. The animals
were observed daily for mortality, appearance, behaviour, appetite,
elimination and pharmacotoxic effects.
Body weights were recorded weekly. At 0, 4, 8 and 13 weeks
ophthalmoscopic and neurologic examinations, haematology, clinical
chemistry and urinalyses were performed. Haematology included Ht, Hb,
total and differential leucocyte counts, prothrombin time and
coagulation time. Blood chemistry consisted of blood sugar, BUN, SAP,
SGPT and SGOT estimations. In addition, free and total bilirubin and
uric acid determinations were conducted at four, eight and 13 weeks.
Urinalyses included specific gravity, pH, glucose, ketones, total
protein, bilirubin and microscopic examination of sediment.
Gross-necropsy was carried out on all animals. Spleen, brain,
pituitary, thyroid, heart, lung, liver, kidneys, adrenals, testes,
prostate, ovaries, uterus were weighed. Histopathology of these organs
and thoracic spinal cord, eye, gall bladder, thymus, salivary gland,
stomach, pancreas, small intestine, large intestine, mesenteric lymph
nodes, sciatic nerve, skeletal muscle, urinary bladder, skin, rib,
vertebra, femur, sternum were carried out at 13 weeks.
Soft stool and/or diarrhoea were noted in all treatment groups
intermittently throughout the study and appeared to be dose related.
In the treatment groups a tendency to decreased coagulation time (from
3.16 to 2.40 minutes) was noticed. Brain weight tended to a dose
related decrease in the male animals receiving xylitol. No further
dose related effects were observed in this study (Banziger, 1970).
Long-term studies
A long-term study with mice and studies with rats are reported
under special studies on carcinogenicity and special study on
reproduction.
In a long-term toxicity study xylitol has been administered
orally 100 mg/xylitol/kg to 20 female and 20 male Wistar rats for
11 months and to 15 female and 15 male rats for 24 months. Body
weights were recorded weekly. At termination detailed gross pathology
was performed and a microscopic study was carried out on stomach,
intestines, liver, spleen, pancreas, kidneys, adrenals,
testes/ovaries, uterus/prostate, lungs, heart, brains, salivary
glands, lymph nodes, thyroid, thymus, sphenoid, pituitary, ganglions
of Glasser and femur. The experiment was carried out with two
generations and the fertility was also checked. The results were
compared with 450 control animals kept under similar experimental
conditions.
No malignant tumours were observed in the animals treated with
100 mg xylitol/kg and no stimulation of the growth of tumours was
noticed. According to the author there was no harmful influence on
reproduction and xylitol did not produce any pathological changes in
the animals (Mosinger, 1971).
In this experiment, however, only one very low dose level was
studied and too little animals were used. No control animals running
simultaneously with the test group were used. In addition not enough
details and no control values were shown. No comparison and no
statistical evaluation was possible. For these reasons this study has
to be considered as not adequate.
OBSERVATIONS IN MAN
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Xylitol is absorbed from the intestine very slowly compared to
glucose (Bässler et al., 1962; Dehmel et al., 1967; Förster, 1972).
After adaptation no significant rise of the absorption rate was
observed (Liebau, 1964) and although the absorption increased with
administrated dose of xylitol the percentage of absorption decreased
(Asano et al., 1972). The absorption of xylitol ranged from 49 to 95%
depending on the dose level (Asano et al., 1973; Keller and Froesch,
1972; Müller-Hess et al., 1975). After both oral and intravenous
administration of xylitol subjects showed a fast distribution in the
extracellular compartment and the tissues (Bässler et al., 1962). The
initial fast distribution phase had a half life of about four minutes,
while the apparent half life of elimination was approximately 20
minutes (Dixon and East, 1973). In studies with 14C-xylitol 90% of
C-atoms taken up could be recovered in products and intermediates of
the glycolytic and pentose phosphate pathway (Quadflieg and Brand,
1974; Müller-Hess et al., 1975). The excretion of 14C-activity in
urine is low after oral administration of 14C-xylitol (Bässler et
al., 1962). An excretion of 1-3% in urine is recorded after oral
administration and of 10% after intravenous infusion of xylitol
(Bässler, 1965). In faeces also 1% of xylitol was excreted
(Müller-Hess et al., 1975).
Metabolism
Exogenous xylitol enters the pathway by conversion to D-xylulose
by a nonspecific cytoplasmic polyol dehydrogenase. Phosphorylation
then yield D-xylulose phosphate, the link between the glucuronic acid
and the pentose phosphate pathways; the latter leads to the formation
of glyceraldehyde-3-phosphate and fructose-6-phosphate, intermediate
metabolites of the Embden-Meyerhof (glycolytic) pathway. Thus xylitol
can be metabolized via glucose-6-phosphate to glycogen and pyruvate or
lactate via the citric acid cycle to CO2 (Hollmann, 1967; Bässler,
1965; Förster, 1974; Asakura, 1967; Touster and Shaw, 1962). Xylitol
is mainly metabolized in the liver (80% to glucose only 20%) but a
small amount also in kidney, myocardium, erythrocytes, adrenal, brain,
lungs and adipose tissue (Bässler, 1965; Hollmann, 1967). Exogenous
xylitol can be metabolized in large quantities, intravenously
0.4 gm/kg/hour or 40 g/day orally raises the plasma level to a maximum
of 1.5-16 mg/100 ml (Bässler, 1965). The metabolic rate for xylitol is
identical in both healthy and diabetic or uraemic patients and
patients who suffered from liver diseases (Lang, 1972).
Effects on enzymes and other biochemical parameters
Oral ingestion of xylitol does not significantly raise the blood
glucose and insulin concentration in normal subjects and diabetics
(Lang, 1972; Bässler et al., 1962; Huthunen et al., 1975), whereas
Müller-Hess et al. (1975) found a small but significant increase in
blood glucose and insulin levels, which were the confirmed findings of
Felber (1976) and Berger et al. (1973). Administration of xylitol
caused an increased hepatic storage of glycogen (compared to glucose)
in both normal and diabetic subjects (Müller-Hess et al., 1975).
After xylitol ingestion increase of serum lactate concentration
and lactate-pyruvate ratio was observed, but to a degree less than
after glucose (Asakura, 1967; Förster, 1972, 1974). Asakura (1967)
also found a marked increase of alpha-dihydroxybutyrate. Complete
metabolism of xylitol produces 35 equivalents of ATP compared to 32
from glucose (Horecker, 1969).
Xylitol caused a fall in the serum free fatty acid level both in
healthy and diabetic subjects (Horecker, 1969; Hötzel, 1971; Pitkänen
and Sahlström, 1968; Förster, 1974). The increased hepatic uptake and
triglyceride synthesis after xylitol administration caused an
elevation of serum triglycerides, but the total serum lipid
concentration was reduced or not influenced (Förster, 1972; Horecker,
1969; Huthunen et al., 1975). The free glycerol concentration in
plasma was also significantly diminished (Müller-Hess et al., 1975).
Xylitol had a marked antiheterogenic action (30 gm xylitol were
adequate). The increased esterification of FFA due to the formation of
alpha-glycero-phosphate leads to a lowered acetyl-CoA concentration
and lowered ketone body formation (Horecker, 1969; Hötzel, 1971;
Förster, 1972). Administration of xylitol caused an inhibition of the
gluconeogenesis from proteins/amino acids. This inhibition lowers the
requirement of energy from fatty acid oxidation in the liver and by
protein-sparing effect the blood urea level and urea excretion were
reduced (Förster, 1972; Lang, 1972). Administration of high daily
doses of xylitol can provoke an increase in the serum uric acid
concentration. This results from an augmented purine biosynthesis, due
to enhanced formation of ribose phosphate (Förster, 1972; Förster et
al., 1972; Förster and Ziege, 1970; Henckenkamp and Zöllner, 1972).
TOXICOLOGICAL STUDIES
In a short-term experiment with eight normal subjects
(21-27 years) receiving orally xylitol over two weeks the fat and uric
acid metabolism was studied. After an initial adaptation phase the
xylitol level was raised from 5 g up to 50 g during seven days. No
significant changes in the serum concentration of triglycerides, FFA,
free glycerol, alpha-lipoproteins, total cholesterol, phosphatides,
aceto-acetate and ß-hydroxybutyrate were observed during xylitol
administration. The serum inorganic phosphate concentration was
elevated during the experiment and decreased again afterwards. After
an initial rise a significant decrease of the serum pyruvate level and
decrease of lactate level from the beginning were observed. The serum
uric acid level was not influenced by a xylitol intake (Mertz et al.,
1972).
After parenteral administration postoperatively of 10% xylitol
solution (1.5 g/kg body weight) Shumer (1971) found a significant
increase of lactic acid, uric acid, bilirubin and Alk.Pase in two
diabetic and two non-diabetic patients. In a short-term experiment he
carried out with two normal volunteers a dosage of 4.5 g/kg during
five days produced significant increased levels of urine uric acid,
SGOT, SAP, bilirubin, lactic acid, and inorganic phosphate in serum.
The levels returned to normal 10 days after cessation of infusion. No
effects were found in the BHN, Ca, cholesterol, glucose, amino-acids
and insulin analyses, urinalyses and haematology were found (Shumer,
1971).
In a short-term study respectively five, 15 and 30 g xylitol were
administered to healthy men during two and three weeks. Blood
chemistry, bilirubin, SGOT, Alk.Pase, uric acid and blood sugar were
normal. No diarrhoea was observed (Asano et al., 1972).
In an oral tolerance test with five persons adapted to xylitol up
to 120 g/day. The initial dose level was 30 g/day and this was raised
with 30 g/day at three day intervals. Liver function tests were normal
throughout the experiment, while there was a transient increase in
plasma lactate and urate. No diarrhoea below 90 g/day was noticed
(Amador and Eisenstein, 1971).
The tolerance of xylitol was studied in 18 male and one female
non-diabetic students aged 21-27 years. The students were given
xylitol for 21 days in increasing dose levels from five up to a
maximum of 75 g/day. After one month of interruption the same group
received xylitol in increasing dose levels from 40 g up to 220 g
again during 21 days (19 students received during the first week
40-100 g/day, 28 during the second week, 100-150 g and six during the
third week 150-220 g). The subjects themselves recorded quantity,
daily division of xylitol intake, number and consistency of bowel
movements as well as general condition and obvious side effects. Body
weights were estimated weekly. At the third day of each experimental
period and seven days after termination, fasting blood sugar analyses
and urinalyses on presence of reducing sugar were carried out. From a
130 g/day dose level diarrhoea was observed when the single doses were
poorly distributed over the day. No other significant effects were
noticed. In a similar study 23 men and three women were given xylitol
or sorbitol. The initial dose was 5 g which was increased to 75 g/day
after 14 days. In addition to the parameters investigated in the first
experiment, xylitol and glucose analyses in 24 hour urine were carried
out. Identically to the first experiment diarrhoea was the only
observed effect (Dubach et al., 1969).
In a two year tolerance study three groups of volunteers (35, 38
and 52) remained on strict diet containing respectively, fructose,
sucrose and xylitol. The average monthly amounts consumed in a varied
assortment of foods were respectively 2.1, 2.2 and 1.5 kg. The highest
daily doses of fructose and xylitol were 200-400 g. Serum samples were
analysed for Na, K, Ca, Mg, inorganic phosphates, ascorbate,
bilirubin, amylase, Alk.Pase, amino-acids, IgA, IgG and IgM. In
addition saliva analyses of IgA, IgG and IgM and amylase were carried
out. The number of occurrences of diarrhoea and flatulence-like
conditions were also scored. Body weights of the volunteers were
recorded weekly. The number of pregnancies in the groups were as
follows; eight in the sucrose, six in the fructose and eight in the
xylitol group.
No significant changes of clinico-chemical parameters in serum
and saliva were observed in the xylitol group. A significant rise in
the occurrence of diarrhoea and flatulence-like conditions was noted
in the xylitol group. All pregnancies, deliveries and infants were
normal.
A series of studies in Turku, Finland showed that after one year
on test in which xylitol replaced sucrose in the diet, dental caries
were reduced in the xylitol group by approximately 90% (Scheinin,
1974).
A more recent study involved 100 human young adults. Subjects
were divided into S-(sucrose) group, and X-(xylitol) group.
Consumption was 4.0 chewing gums per subject per day in the S-group
and 4.5 in the X-group. Frequency of sucrose intake was 4.2 times per
day in the S-group, and 4.9 in the X-group. The caries incidence was
2.92 in the S-group and -1.04 in the X-group. The results show a
profound difference in the caries increment rate between the two
experimental groups (Scheinin et al., 1975).
In an accident that happened in 1969/1970 in Australia, 20
patients who had received Japanese xylitol from two specific batches
by intravenous infusion died with symptoms of severe acidosis, liver
necrosis and renal oxalosis, and in one case oxalate crystals were
also found. The patient with the severest and most dramatic liver
damage had received only 200 g of xylitol. In these xylitol batches a
highly toxic substance was determined by the chicken embryo test.
Coats (1970) identified 2-mercaptoimidazole and 2-hydroxyimidazole in
the xylitol batches. 2-mercaptoimidazole was used as a plasticizer of
the rubber stoppers of the ampoules. All further efforts to obtain
material from the suspect batches from the Japanese firm failed, so
that no explanation for the incidents could be found (Lang, 1974).
In 1971/1972 oxalate crystals were observed in the kidneys and
brain in necropsies performed at the Rafenkrankenhaus in Hamburg on
five patients who had received infusions of xylitol. According to the
doctors responsible for their treatment they died of the severe
conditions from which they had been suffering. These findings gave
rise to extensive discussions as to whether xylitol was involved in
the causation-of oxalosis. However, it is most improbable that the
oxalosis observed in Hamburg was due to a specific toxic action of
xylitol. Further investigation of this subject has to be carried out
(Lang, 1974).
Tolerance studies in diabetes
In addition no harmful effects were observed in a diabetic person
who consumed 65 g xylitol/day during the two years experimental period
(Mäkinen and Scheinin, 1975; Mäkinen, 1976).
Acute tests with xylitol were conducted on 13 recruited adult
diabetics and the following parameters were determined: aceto-acetate,
ß-hydroxy-butyrate, unesterized fatty acid (UFA), free glycerine and
total lipids. A significant drop in concentrations of aceto-acetate
and ß-hydroxybutyrate occurred under administration of xylitol as
compared with the control experiments. UFA behaved similarly. The
concentration of free glycerine also clearly dropped at the end of the
experiment. It can be assumed from the results that the diminished
lipolyserate is the cause of reduced formation of ketone bodies. A
drop in total lipids indicated that hyperlipidemia might possibly also
be favourably influenced by xylitol (Grabner, 1971).
Xylitol in concentration varying from 5 to 20 gm in food was
administered to 23 diabetic persons. Except for diarrhoea in some
cases no harmful side effects were observed. No negative influence on
the diabetie metabolism was observed. Xylitol (25 g) dissolved in tea
caused more frequent diarrhoea and other abdominal side effects in the
subjects (Mellinghof, 1961).
Yamagata et al. (1967) investigated the effects of acute
intravenous infusion of 30 g/90 minutes on blood Sugar, pyruvate and
lactate, and on plasma free fatty acids (FFA) and insulin levels in
six normal and 11 diabetic persons. Xylitol infusion produced no
change of blood sugar in normal and a slight increase in diabetic
patients. Blood lactate showed a significant increase in the diabetic
group but only a slight increase in the normal group. Blood ketone
bodies and plasma FFA decreased in both groups after xylitol
administration. Plasma insulin increased slightly in five of the
normal subjects and in four of the nine diabetic subjects (Yamagata
et al., 1967).
In another experiment xylitol was chronically intravenously
infused to 12 mildly diabetic patients every morning for seven days.
No changes were observed in fasting blood sugar, serum triglycerides,
total cholesterol, serum electrolytes or ketone bodies. However, four
cases indicated the marked decrease of urine sugar excretion during
the experimental period. Xylitol was also given to two diabetic
persons with ketosis and decreased the ketone bodies from 5.1 to
0.2 mg % within four hours (Yamagata et al., 1967).
During two to six weeks 30 g xylitol/day was given orally to 12
stable diabetics. The caloric intake and amount of carbohydrates was
kept constant. Changes in fasting blood sugar, urine sugar, serum
lipids, serum mucoprotein, SGOT and SGPT were studied before, during
and after xylitol administration. In three of the 12 cases urinary
sugar excretion disappeared. A slight impairment of glucose tolerance
and a tendency to decreased SGOT and SGPT levels were observed. In
some cases diarrhoea was noticed, but no other effects of xylitol were
noticed (Yamagata et al., 1967).
Eighteen diabetic children received 30 g dietary xylitol during
four weeks. A significant elevation of the uric acid concentration in
serum was observed, also significant increases of total protein
content and of inorganic phosphorus. In these children no diarrhoea
was noticed (Förster et al., 1977).
REFERENCES
Amador, F. and Eisenstein, A. (1971) The effects of oral xylitol
administration in human subjects. Unpublished data cited by Brin, M.
and Miller, O. N. (1974) The safety of oral xylitol; Sugars in
Nutrition; Ed. Sipple and McNutt, 33, 591-606
Asakura, T. (1967) Non-glycolytic sugar-metabolism in human
erythrocytes 1. Xylitol metabolism, J. Biochem., 62, 184-193
Asano, T., Levitt, M.D. and Goetz, F. C. (1972) Xylitol absorption in
healthy men, Diabetes., 21, suppl. 1, 350-351
Asano, T., Levitt, M.D. and Goetz, F. C. (1973) Xylitol absorption in
healthy men, Diabetes, 22, 279-281
Bächtold, H. P. (1972) Prüfung in 5-Tage-Toxizitätsversuch an Ratten
und Mäusen. Unpublished report from the Research Department of
Hoffmann La Roche Co., submitted to the World Health Organization by
Hoffmann La Roche Co., Basle, Switzerland
Banziger, R. (1970) A thirteen week oral toxicity study of xylitol in
monkeys. Unpublished report of Hazleton Laboratories Inc., submitted
to the World Health Organization by Hoffmann La Roche Co., Basle,
Switzerland
Bässler, K. H. (1965) Biochemie und Stoffwechsel von Xylit (1965)
Dtsch. Lebensm. Rdsd., 61, 171-173
Bässler, K. H. und Prellwitz, W. (1964) Insulin und der
Verteilungsraum von Xylit bei eviscerierten Ratten, Klin.
Wocherschrift, 42, 94-95
Bässler, K. H., Prellwitz, W., Unbehaun, V. und Lang, K. (1962)
Xylitstoffwechsel beim Menschen, Klin. Wschr., 40, 791
Bässler, K. H. und Reimold, W. V. (1965) Lactaatbildung aus Zuckern
und Zuckeralkoholen in Erythrocyten, Klin. Wschr., 43, 169-171
Bässler, K. H. und Wagner, G. (1970) Fettsäuregehalt der Rattenleber
unter verschiedenen Bedingungen der parenteralen Ernährung
Berger, W., Göschke, H., Moppert, J. and Künzli, J. (1973) Insulin
concentration in portal venous and peripheral venous blood in man
following administration of glucose, galactose, xylitol and
tolbutamide, Horm. Metab. Res., 5, 4-8
Birnesser, H., Reinauer, H. and Hollmann, S. (1973) Comparative study
of enzyme activities degrading sorbitol, ribitol, xylitol and
gluconate in guinea-pigs' tissues, Diabetologia, 9, 30-33
Brin, M. and Miller, O. N. (1974) The safety of oral xylitol. Sugars
in Nutrition, Ed. Sipple, H. L. and McNutt, K. W., Acad. Press
Coats, D. A. (1970) Report to the Deutsche Arzeimittelkommission 1970.
Cited by K. Lang (1974) (Unpublished report)
Dehmel, K. H., Förster, H. and Mehnert, H. (1967) Absorption of
xylitol. Int. Symp. on metabolism, physiology and clinical use of
pentoses and pentitols, Hakone, Japan, 1967, 177-181, Ed. Horecker,
B. L. e.a.
Dixon, R. and East, S. (1973) Xylitol levels in plasma following
intravenous and oral administration to man. Unpublished report,
Psycho-Endocrine Centre, St. James's Hospital, Dublin 8, Ireland,
submitted to the World Health Organization by Hoffmann La Roche Co.,
Basle, Switzerland
Dubach, U. C., Feiner, E. and Fargo, E. (1969) Orale Verträglichkeit
von Xylitol bei stoff wechselgesunden Probanden, Schweiz. Med.
Wschr. 99, 190-194
Felber, J.P. (1976) Untersuchung der Wirkung des Xylits auf den
Blutzuckerspiegel beim Menschen, Abstract einer Expertise vom 24-6-76.
Sitzung der Eidg. Ernährung Kommission in Bern
Förster, H. (1972) Grundlagen für die Verwendung der drei
Zuckeraustauschstoffe Fructose, Sobit und Xylit, Med. Ernähr.,
13, 7-15
Förster, H. (1974) Zum Stoffwechsel von Monosacchariden und Polyolen,
Ernährungs. Med., 1, 37-55
Förster, H., Boecker, S. and Ziege, M. (1972) Anstieg der
Konzentration der Serumharnsäure nach akuter und chronische Zufuhr von
Saccharose, Fructose, Sorbitol und Xylit, Med. u. Ernähr.,
13, 193-196
Förster, H., Boecker, S. and Walther, A. (1977) Verwendung von Xylit
als Zuckeraustauschstoff bei diabetischen Kindern, Fortschr. Med.,
95, nr. 2, 99-102
Förster, H. und Hoffmann, H. (1973) Biochemische Überlegungen zur
Verwendung der Kohlenhydrate in der parenteralen Ernährung,
Infusiontherapie, 1, 265-279
Förster, H. und Ziege, M. (1970) Anstieg der
Senumharnsäurekonzentration nach oraler Zufuhr von Fructose, Sorbit
und Xylit, Z. Ernährungswiss., 10, 394-396
Froesch, E. R. (1972) Übersicht über den Haushalt der Betriebstoffe
mit besonderer Berücksichtigung des Stoffwechsels von Glukose,
Fruktose, Sorbit und Xylit und deren therapeutische Verwendbarkeit,
Int. Z. Vit. und Ernährungsforsch., Suppl. 12, 73-86
Froesch, E. R. (1975) Umsatz und Verwertung von Glukose und
Glukoseaustauschstoffen bei Mensch und Tier, Nutr. Metabol., 18
(suppl. 1), 30-44
Froesch, E. R. and Jakob, A. (1974) The metabolism of xylitol. Sugars
in nutrition, Ed. H. L. Sipple and K. W. McNutt, Academic Press,
241-258
Grabner, W., Berger, G., Bergner, D. and Matzkies, F. (1971) Über den
Einfluss des Xylits auf den Fettstoffwechsel von Diabetikern,
Med. Ernährung, 12, 126-128
Grunberg, E., Beskid, G. and Brin, M. (1973) Xylitol and Dental
Caries. Efficacy of xylitol in reducing dental caries in rats,
Internat. J. Vit. Nutr. Res., 43, 227-232
Grütte, F. K. and Rödel, H. (1975) Zur Bedeutung des Zuckeralcohols
Xylit als Zuckeraustauschstoff Ernährungsforschung, 3, nr. 20, 74-79
Haydon, R. K. (1961) The antiketogenic effects of polyhydric alcohols
in rat-liver slices, Biochim. Biophys. Acta., 46, 598-599
Heuckenkamp, P. U. und Züllner, N. (1972) Xylitbalanz während
mehrstündiger infusionen mit konstanten Zufahrraten bei gesunden
Manschen, Klin. Wschr., 50, 1863-1864
Hirata, Y., Fujisawa, M. and Ogushi, T. (1967) Effects of intravenous
injection of xylitol on plasma insulin. Int. Symp. on metabolism,
physiology and clinical use of pentoses and pentitols, Hakone, Japan,
Ed. Horecker, B. L. et al.
Hirata, Y., Fujisawa, M., Sato, H., Asano, T. and Katsuki, S. (1968)
Effects of intravenous injection of xylitol on blood sugar, blood
pyruvic acid and plasma insulin levels in the dog, Z. für dieo
gesamte exptl. Med., 145, 111-119
Hollmann, S. (1967) Pentitol-metabolizing enzymes of the uronic acid
pathway, Int. Symp. on metabolism, physiology and clinical use of
pentoses and pentitols, Hakone, Japan, 97-108
Horecker, B. L. (1969) Stoffwechselwege der Kohlenhydrate: ihre
Regulierung und physiologische Bedeutung. III Stoffwechsel und
physiologische Wirkungen von Xylit, Med. Ernährung., 10, 66-69,
95-98, 127-129
Hosoya, N. and Machiya, T. (1967) Xylitol effect on ketogenesis in
alloxan diabetic rat liver slice, Int. Symp. on metabolism, physiology
and clinical use of pentoses and pentitols, Hakone, Japan, 1967, Ed.
Horecker, B. L. et al.
Hosoya, M., Sakurai, T. and Shimazono, N. (1966) Glycogen and
glycolytic intermediates in Alloxan-diabetic liver slices, VIIth
International Congress of Nutrition, Hamburg
Hosoya, M. and Iitoyo, M. (1969) Effects of xylitol administration on
the development (growth) of rats (cited in Brin and Miller, 1974)),
J. Jap. Soc. Food Nutr., 22, 17-20
Hötzel, D. (1971) Über den Intermediärstoffwechsel von Sorbit, Xylit
und Cyclamaten, Dtsch. Zahnärzl. Z., 26, 1035-1040
Hummler, H. (1974) Orientierende Verträglichkeitsuntersuchungen über 6
Wochen mit Xylit, Sorbit und Saccharose am Hund. Unpublished report
from Huntingdon Research Centre, submitted to the World Health
Organization by Hoffmann La Roche Co., Basle, Switzerland
Hummler, H. (1976a) Reproduktionstoxikologische Untersuchungen mit
Xylit an der Rätte. Unpublished report from Huntingdon Research
Centre, submitted to the World Health Organization by Hoffmann La
Roche and Co., Basle, Switzerland
Hummler, H. (1976b) Cancerogenese Untersuchungen mit Xylit an der
Maus. Unpublished interim report from Huntingdon Research Centre,
submitted to the World Health Organization by Hoffmann La Roche and
Co., Basle, Switzerland
Hummler, H. (1976c) Chronische Toxizitäts- und Carcinogenese-
Untersuchungen mit Xylit an Ratten. Unpublished report from Huntingdon
Research Centre submitted to the World Health Organization by Hoffmann
La Roche and Co., Basle, Switzerland
Hummler, H. (1976d) Chronische Verträglichkeitsprüfung mit Xylit an
Hunden (52 and 78 w.). Unpublished report from Huntingdon Research
Centre, submitted to the World Health Organization by Hoffmann La
Roche and Co., Basle, Switzerland
Huthunen, J. K., Mäkinen, K. K. and Scheinin, A. (1975) Effects of
sucrose, fructose and xylitol diets on glucose, lipid and urate
metabolism, Acta Odont. Scand., 33, suppl. 70, 239-245
Jakob, A., Williamson, J. R. and Asakura, T. (1971) Xylitol metabolism
in perfused rat liver. Interactions with gluconeogenesis and
ketogenesis, J. Biol. Chem., 246, No. 24, 7623-7631
Jourdan, M. H., McDonald, J. and Henderson, J. R. (1972) The effects
of 14C-xylitol, given intravenously, on the serum glucose, insulin
and lipid concentrations of male and female baboons, Nutr. Metabol.,
14, 92-99
Jursons, K. K., Puzaka, J. J., Aalmans, A. R. and Vitolin, S. P.
(1974) Izvestija Akademii Nauh Latvijskoj SSR., 9, 16-22
Keller, U. and Froesch, E. R. (1972) Vergleichende Untersuchungen über
den Stoffwechsel von Xylit, Sorbit und Fruktose beim Menschen,
Schwerz. Med. Wschr., 102, 1017-1022
Kieckebusch, W., Gziem, W. und Lang, K. (1961) Die Verwertbarkeit von
Xylit als Nahrungskohlenhydrat und seine Verträglich, Klin. Wschr.,
39, 447
Kuzyha, T., Kanazawa, Y. and Kosaka, K. (1966) Plasma insulin response
to intravenously administered xylitol in dogs, Metabolism, 15, No.
12, 1149-1152
Kuzuya, T., Kanazawa, Y. and Kosaka, K. (1969) Stimulation of insulin
secretion by xylitol in dogs, Endocrinology, 84, 200-207
Lang, K. (1964) Die ernährungsphysiologischen Eigenschaften von Xylit,
Internat. Z. Vitaminforsch., 34, 117-122
Lang, K. (1972) Xylit - Biochemie und Klinik, Suppl. 13 zur
Z. Ernährungswiss: "Rehabilitation durch Ernährung" Darmstadt 11-15
(symp. CSSR)
Lang, K. (1974) Xylit in der oralen und parenteralen Ernährung Heft
der Schriftenreihe des Bundes für Lebensmittelrecht und
Lebensmittelkund, 75, 21-23
Liebau, K. (1964) Untersuchungen der Resorption des Xylit beim
Menschen Dissertation of K. Liebau, submitted to the World Health
Organization by Hoffmann La Roche Co., Basle, Switzerland (Unpublished
report)
Mäkinen, K. K. (1976) Long-term tolerance of healthy human subjects
to high amounts of xylitol and fructose: general and biochemical
findings. In: Monosaccharides and polyalcohols in nutrition
therapy and diabetics, Ed. G. Ritzel, G. Brubacher, Verlag H.
Huber, Int. J. Vit. Nutr. Res. nr., 15
Mäkinen, K. K. and Scheinin, A. (1975) Effect of the diet on certain
clinico-chemical values of serum, Acta Odont. Scand., 33, suppl.
70, 265-276
McCormick, D. B. and Touster, O. (1957) The conversion in vivo
of xylitol to glycogen via the pentose phosphate pathway, J. Biol.
Chem., 239, 451-461
Mehnert, H., Summa, J. D. and Fürster, H. (1964) Untersuchungen zum
Xylitstoffwechsel bei gesunden, Leberkranken und diabetischen
Personen, Klin. Wschr., 42, 382
Mellinghoff, G. H. (1961) Über die Verwendbarkeit des Xylit als
Ersatzzucker bei Diabetikern, Klin. Wschr., 39, 447
Meng, H. C. (1974) Sugars in parenteral nutrition. Sugars in
nutrition, Ed. H. L. Sipple, K. W. McNutt, Academic Press, 527-566
Mertz, D. P., Kaiser, V., Klöpfer-Zaar, M. and Beisbarth, H. (1972)
Serumkonzentrationen verschiedener Lipide und von Harnsäure während 2
wüchiger Verabreichung von Xylit, Klin. Wschr., 50, 1107-1111
Montague, W., Howell, S. L. and Taylor, K. W. (1967) Pentitols and the
mechanism of insulin release, Nature, 215, 1088-1089
Mosinger, M. (1971) Effects of the long-term oral administration of
xylitol. Unpublished report of Mosinger, M., submitted to the World
Health Organization by Hoffmann La Roche, Basle, Switzerland
Müller-Hess, R., Geser, C. A., Bonjour, J.P., Jequier, E. and Felber,
J.P. (1975) Effects of oral xylitol administration on carbohydrate and
lipid metabolism in normal subjects, Infusionstherapie, 2, 247-252
Opitz, K. (1967) The influence of xylitol and other polyols and sugars
on fat mobilization, Int. Symp. on metabolism, physiology and clinical
use of pentoses and pentitols, Hakone, Japan, Ed. Horecker, B. L. et
al.
Oshinsky, R. J., Wang, Y. M. and Eys, J. v. (1976) Oxalate formation
following xylitol infusion in 4-deoxypyridoxine treated rabbits,
Fed. Proc., 35, 419
Pazenko, A. G. (1969) Voprosy prolilaktiki, diagnostiki i lecenija
zabolevanij organor, Piscevarenija, Kiev, 133
Petrich, Ch., Reinauer, H. and Hollmann, S. (1972) Vergleichende
Aktivitätsmessungen der Enzyme des Glucuronsäure Xylulose-Zyklus
in der Leber, dem epididymalen Fettgewebe der Ratte, in einem
Morris-Hepatoom und einer Fibrocyten-Kultur, Z. Klin. Chem. Klin.
Biochem., 10, 355-358
Pitkänen, E. and Sahlström, K. (1968) Increased excretion of
xylitol after administration of glucorone, lactone and ethanol in
man, Ann. Med. exp. Fenn., 47, 143-150
Pool, W. and Hane, D. (1970) Acute toxicity and dog tolerance testing
of xylitol. Unpublished report from the Research Department of
Hoffmann La Roche Co., submitted to World Health Organisation by
Hoffmann La Roche Co., Basle, Switzerland
Pool, W. and Hane, D. (1972) Acute mouse toxicity of xylitol.
Unpublished report from the Research Department of Hoffmann La Roche
Co., submitted to the World Health Organization by Hoffmann La Roche
Co., Basle, Switzerland
Quadflieg, K. H. and Brand, K. (1974) Xylitol metabolism in human
erythrocytes, Z. Phys. Chemie, 355, 1239
Scheinin, Ulla (1974) Incidence of dental caries among a group of
university students in Turkey, Acta. Odont. Scand., 32, 335-344
Scheinin, Arje., Mäkinen, Kauko K., Tammisalo, Erkki., Rekola, Maarit.
(1975) Turku sugar studies. XVIII. Incidence of dental caries in
relation to one-year consumption of xylitol chewing gum, Acta.
Odont. Scand., 33, 269-278
Schmidt, B., Fingerhut, N. and Lang, K. (1964) Über den Stoffwechsel
von radioaktiv markiertem Xylit bei der Ratte, Klin. Wschr., 42,
1073-1077
Shima, S., Mitsunaga, M. and Nakao, T. (1970) Effect of xylitol on
testicular protein and RNO biosynthesis of rats, Endocrinol. Jap.,
17, 283-287
Shumer, W. (1971) Adverse effects of xylitol in parenteral
alimentation Metabolism., 20, 345-347
Staub, H. W. and Thiessen, R. (1972) Effect of dietary polyols on
serum cholesterol in rats, Federation Proc., 31, 291
Stein, G. (1966) Adaptive Prozesse im Xylitstoffwechsel und ihre
Auswirkungen Dissertation Mainz, 1966, submitted to the World Health
Organization by Hoffmann La Roche and Co., Basle, Switzerland
(Unpublished report)
Swarm, R. L. and Banziger, R. (1970) A thirteen week oral feeding
study in rats with xylitol (Ro-6-7045). Unpublished report of Hazleton
Laboratories Inc., submitted to the World Health Organization by
Hoffmann La Roche, Basle, Switzerland
Touster, O. (1970) Parenteral nutrition (Proc. on an internat. Symp.
Vanderbilt University, School of Medicine, Nashville) 131-138, Ed.
Meng, H. C., Law, D. H., Thomas, C. C., Publisher Springfield,
Illinois, USA
Touster, O. (1974) The metabolism of polyols. In: Sugars in Nutrition,
Ed. H. L. Sipple, K. W. McNutt, Academic Press, 229-239
Touster, O. and Shaw, D. (1962) Biochemistry of the acyclic polyols,
physiol. Rev., 42, 181-225
Truhaut, R., Coquet, B., Fouillet, X., Galland, I., Guyot, D., Rouaud,
J. L. and Long, D. W. (1977) Subacute toxicity of xylitol in rats.
Personal communication to the World Health Organization (Unpublished
report)
Wagner, G. and Bässler, K. H. (1971) Gibt es Unterschiede in der
antiketogenen Wirkung gebräuhlicher Zucker und Polyalkohole, Medizin
Ernährung., 12, 53-54
Wang, Y. M., King, S. M., Patterson, J. H. and Van Eys, J. van. (1973)
Mechanism of xylitol toxicity in the rabbit, Metabolism, 22, nr.
7, 885-894
Wang, Y. H., Oshinky, R. J., Lantin, E. and Eys, J. van. (1975) An
investigation of oxalate formation from xylitol infusion in rabbits,
Fed. Proc., 35, 920
Wilson, R. B. and Martin, J. M. (1970) Plasma insulin concentrations
in dogs and monkeys after Xylitol, glucose or tolbutamide infusion,
Diabets., 19, 17-22
Woods, H. F. (1975) Hepatic metabolism of pentites, Nutr. Metabol.,
18 (Suppl. 1), 65-78
Woods, H. F. and Krebs, H. A. (1973) Xylitol metabolism in the
isolated perfused rat liver, Biochem. J., 134, 437-443
Yamagata, S., Goto, Y., Ohneda, A., Anzai, M., Kwashima, S., Kikuchi,
J., Chiba, M., Maruhama, Y., Yamauchi, Y. and Toyota, T. (1967)
Clinical application of xylitol in diabetics, Int. Symp. on
metabolism, physiology and clinical use of pentoses and pentitols,
Hakone, Japan, 316-325, Ed. Horecker, B. L. et al.
See Also:
Toxicological Abbreviations
Xylitol (WHO Food Additives Series 13)
Xylitol (WHO Food Additives Series 18)
XYLITOL (JECFA Evaluation)
 These biotransformation studies are also supported by Schmidt et
al. (1964), Hörecher (1969), Touster (1974), Stein (1966), Petrich et
al. (1972) and Grütte and Rödel (1975). McCormick and Touster (1957)
found that the metabolism of rat and guinea pig was similar.
Effects on enzymes and other biochemical parameters
Xylitol has especially influence on the activity of the enzymes
of carbohydrates metabolism and the insulin hormone. Both in
experiments with parenteral and oral administration of xylitol a
stimulation of insulin secretion and an elevated serum insulin
concentration are observed in rat, dog and monkey, although the plasma
glucose level slightly increased for a while and then decreased below
the fasting level (Bässler and Prellwitz, 1964; Kuzuya et al., 1966,
1969; Hirata et al., 1967, 1968; Montague et al., 1967; Wilson and
Martin, 1970; Froesch, 1975; Jourdan et al., 1972; Touster, 1974).
Kuzuya et al. (1969) found that the insulin secretion produced by
xylitol in dogs was more pronounced than by glucose. Wilson and Martin
(1970) observed the same effect in dogs, while they found that in
monkeys glucose caused a more marked stimulation of the insulin
secretion than xylitol did. The insulin secretion in dogs is also much
stronger than in man (Hirata et al., 1967). Meng (1974) however did
not find any changes of the insulin level in dogs, i.v. infused with
xylitol. This study included only five animals.
Glycogen production is stimulated by the administration of
xylitol to guinea pigs, rat and perfused rat liver, even when diabetic
animals are used: (McCormick and Touster, 1957; Hosaya et al., 1966;
Touster, 1970; Förster and Hoffman, 1973). Jakob et al. (1971) found
that in liver most of the xylitol administered to the animals was
rapidly converted to glucose and to small quantities of lactate.
Bässler and Reimold (1965) observed a stimulating influence of xylitol
on the production of lactic acid in erythrocytes which was also
noticed with glucose. In perfused rat liver xylitol stimulated the
lactate production and caused an increased lactate to pyruvate ratio
(Jakob et al., 1971; Förster and Hoffmann, 1973; Woods and Krebs,
1973; Woods, 1975). Besides the increased ratio of lactate to
pyruvate Jakob et al. (1971) found also increased ratios of
alpha-glycerophosphate to dihydroxy acetone-P and triosephosphate to
3-P-glycerate. In a study of Hirata et al. (1968) a significant
decrease of blood pyruvic acid level was noticed in normal dogs.
In close relation with the influence of the carbohydrate
metabolism an antiketogenic and antilipolytic effect of xylitol is
also observed (Haydon, 1961; Hosaya and Machiya, 1967; Bässler and
Wagner, 1970; Jakob et al., 1971; Wagner and Bässler, 1971; Birnesser
et al., 1973). As a result of accumulation of alpha-glycerophosphate
and other phosphorylated intermediates a fall in the inorganic
phosphorus and adenine nucleotides was observed (Petrich et al., 1972;
Woods and Krebs, 1973; Woods, 1975). The fall in the inorganic
phosphorus leads to an increased purine catabolism and to a
hyperuricemia in isolated perfused rat liver (Woods and Krebs, 1973;
Woods, 1975). Forster and Hoffmann (1973) found that xylitol
accelerates the biosynthesis of uric acid.
Shima et al. (1970) found a stimulation of testicular protein and
RNA biosynthesis in rats fed xylitol. Staub and Thiessen (1972)
observed increased cholesterol levels in rats fed xylitol.
In experiments with xylitol oxalate formation is observed, but
glucose is reported to have a stronger effect on this formation (Wang
et al., 1975; Oshinsky et al., 1976; Brin and Miller, 1974). Therefore
no clear relationship between oxalate accumulation and xylitol
toxicity is found by Oshinsky et al. (1976) and Wang et al. (1975).
TOXICOLOGICAL STUDIES
Special studies on reproduction
In a reproduction study five groups of 30 male and 60 female rats
of the P-generation were fed 0, 2, 5, 10 and 20% dietary xylitol. The
total amount of carbohydrates was kept constant. In addition 20%
sorbitol and a 20% sucrose group were used as sugar controls. This
study has only been carried out till the F1B-generation. At the
second mating, an abrupt increase in pup mortality occurred in all
groups between four and 12 days. This was caused by a heating failure.
This resulted in loss of too many pups to complete the reproduction
study.
In the animals receiving 5, 10 and 20% xylitol decreased
food-intake and weight gain was recorded. At the first mating, a
slightly higher initial litter size among test groups was
counterbalanced by a slightly higher cumulative pup loss during
lactation. No other effects on the reproduction were noticed in the
F1a-animals receiving xylitol. This study will be repeated (Hummler,
H. 1976a).
Levels of xylitol up to 20% of the diet fed to rats up to four
months had no significant adverse effects. Growth rate was normal.
Adaptation to high dosage was shown to be important. Xylitol had no
effect on the number or size of pups or dams fed xylitol, and the pups
adapted readily to the supplement at 5 and 10% in diet, after weaning,
as instituted following adverse effects with the 20% xylitol level,
initially (Hosoyol and Iitoyo, 1969).
Special studies on carcinogenicity
In a carcinogenicity study 100 female and 100 male mice per group
weighing 24-25 g, were fed 0, 2, 10 and 20% dietary xylitol during
1.5-2 years. The total amounts of carbohydrates were kept constant and
animals receiving sucrose served as sugar control group. Body weight
and food intake were recorded weekly, while with intervals water
consumption was determined. At 26, 52 and 79 weeks and prior to
terminal sacrifice ophthalmoscopy was carried out. After 80 weeks 20
males and 20 females from each group were killed. The mice were killed
when survival within a group reached 20%. Gross pathology was
performed visually and by palpation. Microscopic examination on
adrenals, thyroids, ovaries, liver, spleen, lymph, pituitary gland and
bone marrow and all macroscopically observed lesions suggestive of
neoplasia were routinely carried out for every animal, along with
blood smears.
At the start of the experiment a severe diarrhoea was observed,
therefore a number of adaptation phases with slowly increasing xylitol
concentrations in the diet were necessary. Animals receiving 10% and
20% xylitol showed increased water and food intake. In the animals
receiving 2% and 10% xylitol, 20% sorbitol an increased body weight
gain was observed, whereas the animals receiving 20% xylitol had a
significant lower body weight gain. The mortality of the male mice
receiving 10 and 20% xylitol was elevated. No histopathology and no
details on tumour incidence are available. The study is at the moment
an inadequate carcinogenicity study, which has to be completed first
(Hummler, H., 1976b).
In a long-term toxicity and carcinogenicity study five groups of
75 female and 75 male Sprague-Dawley rats, weighing 120-150 g, were
fed 0, 2, 5, 10 and 20% dietary xylitol. The total amounts of
carbohydrates in the diet were kept constant. Animals receiving 20%
sorbitol and 20% sucrose were used as sugar control groups. Food
intake and body weight were recorded weekly. Water consumption was
recorded during weeks 12, 25, 52 and 78. Ophthalmoscopy, urinalysis,
haematology and biochemistry were performed at 0, 13, 26, 52, 78 and
104 weeks. Urinalyses included pH, specific gravity, protein, reducing
substances, glucose, ketones, bile pigments, urobiligen, haemoglobin
and oxalic acid determinations. Haematology consisted of determination
of PCV, Hb, RBC, WBC, MCHC, MCV, differential count, prothrombin
index, whole blood clotting time. The biochemical estimations were
urea and glucose in plasma, total proteins, protein electrophoresis,
Alk Pase, GPT, GOT, bilirubin, uric acid, cholesterol, lactate, LDH,
alpha-HBDH in serum and total reducing substances, insulin,
xylitol,Na+, K+, Cl- and bicarbonate in blood. After 26 and 52
weeks, five males and five females per group were killed. In the gross
pathology the animals were examined visually and by palpation. Brain,
heart, liver, kidneys, adrenals, pituitary, thyroid, spleen,
testes/ovaries were weighed. These organs and pancreas, thymus,
uterus, cervical and mesenteric lymph nodes, stomach, bone marrow,
ileum, coecum, duodenum, urinary bladder, eye, lungs and any
macroscopically abnormal tissues were histopathologically
investigated. Blood smears of these organs were also studied.
Significant decreased weight gain in animals receiving 10% or 20%
xylitol during the experiment was observed, while there was only
tendency to decreased food intake in animals receiving 20% xylitol at
78 weeks. In rats receiving 20% xylitol, higher water intakes were
recorded during weeks 26 and 52, and in female rats also during week
78.
In male and female rats at 13, 26 and 52 weeks and in female rats
at week 78, receiving 20% xylitol greater volumes of more diluted
urine were excreted. After 26 weeks, the urine from animals receiving
5, 10 and 20% showed decreased specific gravity.
No increase in mortality was noticed in the treated groups,
Except a significant decreased prothrombin index in all male rats
receiving xylitol and female rats receiving 20% xylitol, no
haematological changes were observed at 78 weeks. In biochemistry a
tendency to decreased lactate contents in the blood of animals
receiving 10 and 20% xylitol and at 78 weeks a tendency to increased
uric acid content in male rats of the 20% xylitol group were observed.
No morphological abnormalities in the treated groups differing
significantly from the control group ware observed. The standard
deviation in the insulin determination was too great for making an
evaluation of the results possible.
The study is not yet completed. No details on tumour incidence
are available so far (Hummler, 1976c).
Acute toxicity
LD50
Animal Route mg/kg References
body weight
mouse oral 14 100 Bächtold (1972)
mouse oral 22 000 Pool and Hane (1972)
mouse oral >4 000 Pool and Hane (1970)
mouse oral 25 700 Kieckebuch (1961)
mouse intraperitoneal >2 000 Pool and Hane (1970)
mouse subcutaneous >2 000 Pool and Haue (1970)
(con't)
LD50
Animal Route mg/kg References
body weight
mouse intravenous >4 000 Pool and Hane (1970)
mouse intravenous 22 200 Grütte and Rödel (1915)
mouse intravenous 3 770-9 450 Pazenko (1969)
adult rat oral >4 000 Pool and Hane (1970)
neonatal rat oral > 4 000 Pool and Hane (1970)
rat oral 14 100 Bächtold (1972)
rabbit oral > 2 000 Pool and Hane (1970)
rabbit intravenous 4 000-6 000 Wang et al. (1973)a
a The acute toxicity in rabbits was determined by intravenous
infusion of 87 mg xylitol/kg/minute. The observed LD50 was 4-6 g/kg
body weight. Striking increases of SGOT and serum LDH levels, along
with an increase urine volume were observed (Wang et al., 1973).
Short-term studies
In a subacute toxicity study xylitol was administered to 20
female and 20 male rats per group by daily gastric intubation for a
period of 14 days. Dose levels were 1.25, 2.5, 5.0 and 10.0 g/kg. The
animals receiving 1.25 g xylitol/kg during the first nine days,
received 10.0 g xylitol/kg during the last seven days. In the control
groups animals received 0, 2.5 and 5.0 g sucrose/kg/day. During
treatment (two, five and 14 days) animals were submitted to careful
clinical examinations and blood serum analyses related to hepatic
functions. These analyses were glucose in blood and bilirubin, FFA,
total lipids, triglycerides, cholesterol, G-6-PDH and Alk. Pase, GPT
and GOT in serum. Also body weight and food consumption were recorded.
The animals were sacrificed after two, five and 14 days. Heart, liver,
spleen, kidneys, adrenals, gonads, stomach were weighed. These organs
and jejunum, colon, pancreas and brain were histologically examined in
two and five days treatment groups, while in 14 days treatment group
this examination was only performed on liver. The histological study
was conducted on five males and five females per group.
Except for a decrease of the FFA content in the blood at all
xylitol dose levels, no effects are observed in this study. No
evidence of hepatotoxicity was recorded (Truhaut et al., 1977).
In a 15 days' experiment with rats, sucrose was replaced
respectively by 10 and 20% xylitol. The protein of the diet consisted
of casein, while their carbohydrate allowance consisted of starch and
sugar. In the 10% xylitol dose group no histopathological changes were
observed. However, in the 20% xylitol dose group adverse effects of
the metabolism of the liver cells were noticed. A reduction of the
glycogen and lipids concentrations were observed. The mitotic activity
of the liver cells was sharply reduced to a level five times lower
than in the control rats (Jursons et al., 1974).
In a short-term (13 weeks) study four groups of eight female and
eight male Charles River CD rats weighing 120-150 g, were fed 0, 5, 10
and 20 g dietary xylitol/kg/day. Food and water consumption were
recorded daily and body weight weekly. Haematology, blood glucose and
urinalyses of five male and five female rats of each group were
carried out at four, eight and 12 weeks. Alk. Pase, GOT, bilirubin and
uric acid in serum and BUN of five males and five females per group
were determined at 13 weeks.
The animals, particularly the male rats, showed reduced body
weight gain and food consumption, that were dose dependent. In the 20%
xylitol group BUN was elevated. In treatment groups dose-related
decrease of the absolute heart weight are observed. Except for
transient diarrhoea in a number of treated animals, no other
significant changes were observed. No histopathological lesions
related to xylitol administered were noticed (Swarm and Banziger,
1970).
Rat
Rats fed a cariogenic diet containing 10% xylitol showed no
carious lesions in the first and third molars and only minimal
involvement of the second molars was observed (Grunberg et al., 1973).
Dog
In short-term study five dogs were dosed 10 g xylitol/kg/day
by intravenous infusion during seven weeks. Blood chemistry and
urinalyses were investigated weekly. Plasma glucose content was
reduced to 64 mg % after six weeks. A slight increase in plasma
lactate and a significant increase of SGPT and Alk. Pase was observed.
Also an increased urinary loss was observed. No effect of xylitol on
plasma insulin levels and no oxalate crystals in the kidneys were
observed (Meng, 1974).
A six weeks toxicity experiment with one female and one male dog
(5.5-6 kg) per group was carried out. The concentration was during the
experiment increased from 5% after two weeks to 20%. Food consumption
was recorded twice daily and body weights twice a week. At the end of
the experiment gross pathology was carried out, and brain, heart,
liver, lungs, pituitary, spleen, pancreas, thymus, prostate/uterus,
kidneys, thyroids, adrenals and testes/ovaries were weighed. No
histopathological investigation was carried out.
Except for a tendency to increased liver weights in the xylitol
groups no effects are observed. This study is not an adequate toxicity
study, but has to be seen as preliminary experiment (Hummler, 1974).
In a long-term toxicity study eight female and eight male beagle
dogs per group, weighing 6-9 kg, were fed 0, 2, 5, 10 and 20% dietary
xylitol during two years. The total amounts of carbohydrates were kept
constant. In addition animals receiving 20% sorbitol and 20% sucrose
were used as sugar controls. Body weight and food consumption was
recorded weekly, while water consumption was recorded over five day
periods at the weeks one to four, 9-12, 21-24, 35-38 and 46-49.
Ophthalmoscopy, dental examination and a full neurological examination
of four male and four female dogs were carried out at 13, 26, 39, 50,
64 and 76 weeks. Haematology, biochemistry and urinalyses were carried
out at the beginning of the experiment and at 12, 26, 38, 50, 64 and
76 weeks. The haematology included erythrocyte sedimentation rate,
PCV, Hb, RBC, reticulocyte count, MCHC, MCV, WBC, differential count,
platelet count, prothrombin index, whole blood clotting time. The
biochemistry included determination of total protein, Al, GPT, GOT,
total LDH, alpha-HBDH, bilirubin, uric acid, cholesterol, lactate and
insulin in serum and urea xylitol, glucose concentration and total
reducing substances in plasma. Sodium, potassium, chloride and
bicarbonate were also detected in the blood. Urinalyses consisted of
estimations of Ph, protein, reducing substances, glucose, ketones,
bile pigments, urobiligen and Hb. On completion of 52 weeks, two male
and two female dogs per group were sacrificed for interim study, while
the remaining dogs will be killed after two years' dosing. The weights
of brain, liver, kidneys, pituitary, thyroid, spleen, heart, lungs,
adrenals, ovaries, testes, uterus, thymus, prostate, pancreas were
recorded. Histopathology of these organs and of aorta, trachea, lymph
nodes, gall bladder, urinary bladder, salivary glands, oesophagus,
duodenum, stomach, jenunum, ileum, skin, skeletal muscle, mammary
glands, tongue, eye with optic nerve and sciatic nerve was performed.
The food intake and weight gain of the 20% xylitol group were
increased. A dose-related increase of SAP and SGPT and a decreased
lactate level was noticed, while in the 20% xylitol dose group also
the total serum protein was significantly increased. At 52 weeks a
tendency to increased cholesterol content in serum was observed.
Except a dose related decrease in the pituitary of the xylitol
dose groups no effects on organ weights, in gross necropsy and
histopathology were observed. The number of animals, however, did not
allow a real statistic evaluation (Hummler, 1976d).
Mean values of haematological, biochemical and urinalyses were
calculated from total population of female and male animals.
Significant changes could be looked over by elevated standard
deviation. In the insulin determinations variation was too much for
further conclusion. In view of the mentioned restrictions it can be
stated that this study is not fully adequate, it has to be completed
first.
In a 13 weeks toxicity study two male and two female rhesus
monkeys, weighing 2-3 kg, were dosed twice daily six days a week 0,
1.0, 3.0 and 5.0 g xylitol/kg/day by gastric intubation. The animals
were observed daily for mortality, appearance, behaviour, appetite,
elimination and pharmacotoxic effects.
Body weights were recorded weekly. At 0, 4, 8 and 13 weeks
ophthalmoscopic and neurologic examinations, haematology, clinical
chemistry and urinalyses were performed. Haematology included Ht, Hb,
total and differential leucocyte counts, prothrombin time and
coagulation time. Blood chemistry consisted of blood sugar, BUN, SAP,
SGPT and SGOT estimations. In addition, free and total bilirubin and
uric acid determinations were conducted at four, eight and 13 weeks.
Urinalyses included specific gravity, pH, glucose, ketones, total
protein, bilirubin and microscopic examination of sediment.
Gross-necropsy was carried out on all animals. Spleen, brain,
pituitary, thyroid, heart, lung, liver, kidneys, adrenals, testes,
prostate, ovaries, uterus were weighed. Histopathology of these organs
and thoracic spinal cord, eye, gall bladder, thymus, salivary gland,
stomach, pancreas, small intestine, large intestine, mesenteric lymph
nodes, sciatic nerve, skeletal muscle, urinary bladder, skin, rib,
vertebra, femur, sternum were carried out at 13 weeks.
Soft stool and/or diarrhoea were noted in all treatment groups
intermittently throughout the study and appeared to be dose related.
In the treatment groups a tendency to decreased coagulation time (from
3.16 to 2.40 minutes) was noticed. Brain weight tended to a dose
related decrease in the male animals receiving xylitol. No further
dose related effects were observed in this study (Banziger, 1970).
Long-term studies
A long-term study with mice and studies with rats are reported
under special studies on carcinogenicity and special study on
reproduction.
In a long-term toxicity study xylitol has been administered
orally 100 mg/xylitol/kg to 20 female and 20 male Wistar rats for
11 months and to 15 female and 15 male rats for 24 months. Body
weights were recorded weekly. At termination detailed gross pathology
was performed and a microscopic study was carried out on stomach,
intestines, liver, spleen, pancreas, kidneys, adrenals,
testes/ovaries, uterus/prostate, lungs, heart, brains, salivary
glands, lymph nodes, thyroid, thymus, sphenoid, pituitary, ganglions
of Glasser and femur. The experiment was carried out with two
generations and the fertility was also checked. The results were
compared with 450 control animals kept under similar experimental
conditions.
No malignant tumours were observed in the animals treated with
100 mg xylitol/kg and no stimulation of the growth of tumours was
noticed. According to the author there was no harmful influence on
reproduction and xylitol did not produce any pathological changes in
the animals (Mosinger, 1971).
In this experiment, however, only one very low dose level was
studied and too little animals were used. No control animals running
simultaneously with the test group were used. In addition not enough
details and no control values were shown. No comparison and no
statistical evaluation was possible. For these reasons this study has
to be considered as not adequate.
OBSERVATIONS IN MAN
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Xylitol is absorbed from the intestine very slowly compared to
glucose (Bässler et al., 1962; Dehmel et al., 1967; Förster, 1972).
After adaptation no significant rise of the absorption rate was
observed (Liebau, 1964) and although the absorption increased with
administrated dose of xylitol the percentage of absorption decreased
(Asano et al., 1972). The absorption of xylitol ranged from 49 to 95%
depending on the dose level (Asano et al., 1973; Keller and Froesch,
1972; Müller-Hess et al., 1975). After both oral and intravenous
administration of xylitol subjects showed a fast distribution in the
extracellular compartment and the tissues (Bässler et al., 1962). The
initial fast distribution phase had a half life of about four minutes,
while the apparent half life of elimination was approximately 20
minutes (Dixon and East, 1973). In studies with 14C-xylitol 90% of
C-atoms taken up could be recovered in products and intermediates of
the glycolytic and pentose phosphate pathway (Quadflieg and Brand,
1974; Müller-Hess et al., 1975). The excretion of 14C-activity in
urine is low after oral administration of 14C-xylitol (Bässler et
al., 1962). An excretion of 1-3% in urine is recorded after oral
administration and of 10% after intravenous infusion of xylitol
(Bässler, 1965). In faeces also 1% of xylitol was excreted
(Müller-Hess et al., 1975).
Metabolism
Exogenous xylitol enters the pathway by conversion to D-xylulose
by a nonspecific cytoplasmic polyol dehydrogenase. Phosphorylation
then yield D-xylulose phosphate, the link between the glucuronic acid
and the pentose phosphate pathways; the latter leads to the formation
of glyceraldehyde-3-phosphate and fructose-6-phosphate, intermediate
metabolites of the Embden-Meyerhof (glycolytic) pathway. Thus xylitol
can be metabolized via glucose-6-phosphate to glycogen and pyruvate or
lactate via the citric acid cycle to CO2 (Hollmann, 1967; Bässler,
1965; Förster, 1974; Asakura, 1967; Touster and Shaw, 1962). Xylitol
is mainly metabolized in the liver (80% to glucose only 20%) but a
small amount also in kidney, myocardium, erythrocytes, adrenal, brain,
lungs and adipose tissue (Bässler, 1965; Hollmann, 1967). Exogenous
xylitol can be metabolized in large quantities, intravenously
0.4 gm/kg/hour or 40 g/day orally raises the plasma level to a maximum
of 1.5-16 mg/100 ml (Bässler, 1965). The metabolic rate for xylitol is
identical in both healthy and diabetic or uraemic patients and
patients who suffered from liver diseases (Lang, 1972).
Effects on enzymes and other biochemical parameters
Oral ingestion of xylitol does not significantly raise the blood
glucose and insulin concentration in normal subjects and diabetics
(Lang, 1972; Bässler et al., 1962; Huthunen et al., 1975), whereas
Müller-Hess et al. (1975) found a small but significant increase in
blood glucose and insulin levels, which were the confirmed findings of
Felber (1976) and Berger et al. (1973). Administration of xylitol
caused an increased hepatic storage of glycogen (compared to glucose)
in both normal and diabetic subjects (Müller-Hess et al., 1975).
After xylitol ingestion increase of serum lactate concentration
and lactate-pyruvate ratio was observed, but to a degree less than
after glucose (Asakura, 1967; Förster, 1972, 1974). Asakura (1967)
also found a marked increase of alpha-dihydroxybutyrate. Complete
metabolism of xylitol produces 35 equivalents of ATP compared to 32
from glucose (Horecker, 1969).
Xylitol caused a fall in the serum free fatty acid level both in
healthy and diabetic subjects (Horecker, 1969; Hötzel, 1971; Pitkänen
and Sahlström, 1968; Förster, 1974). The increased hepatic uptake and
triglyceride synthesis after xylitol administration caused an
elevation of serum triglycerides, but the total serum lipid
concentration was reduced or not influenced (Förster, 1972; Horecker,
1969; Huthunen et al., 1975). The free glycerol concentration in
plasma was also significantly diminished (Müller-Hess et al., 1975).
Xylitol had a marked antiheterogenic action (30 gm xylitol were
adequate). The increased esterification of FFA due to the formation of
alpha-glycero-phosphate leads to a lowered acetyl-CoA concentration
and lowered ketone body formation (Horecker, 1969; Hötzel, 1971;
Förster, 1972). Administration of xylitol caused an inhibition of the
gluconeogenesis from proteins/amino acids. This inhibition lowers the
requirement of energy from fatty acid oxidation in the liver and by
protein-sparing effect the blood urea level and urea excretion were
reduced (Förster, 1972; Lang, 1972). Administration of high daily
doses of xylitol can provoke an increase in the serum uric acid
concentration. This results from an augmented purine biosynthesis, due
to enhanced formation of ribose phosphate (Förster, 1972; Förster et
al., 1972; Förster and Ziege, 1970; Henckenkamp and Zöllner, 1972).
TOXICOLOGICAL STUDIES
In a short-term experiment with eight normal subjects
(21-27 years) receiving orally xylitol over two weeks the fat and uric
acid metabolism was studied. After an initial adaptation phase the
xylitol level was raised from 5 g up to 50 g during seven days. No
significant changes in the serum concentration of triglycerides, FFA,
free glycerol, alpha-lipoproteins, total cholesterol, phosphatides,
aceto-acetate and ß-hydroxybutyrate were observed during xylitol
administration. The serum inorganic phosphate concentration was
elevated during the experiment and decreased again afterwards. After
an initial rise a significant decrease of the serum pyruvate level and
decrease of lactate level from the beginning were observed. The serum
uric acid level was not influenced by a xylitol intake (Mertz et al.,
1972).
After parenteral administration postoperatively of 10% xylitol
solution (1.5 g/kg body weight) Shumer (1971) found a significant
increase of lactic acid, uric acid, bilirubin and Alk.Pase in two
diabetic and two non-diabetic patients. In a short-term experiment he
carried out with two normal volunteers a dosage of 4.5 g/kg during
five days produced significant increased levels of urine uric acid,
SGOT, SAP, bilirubin, lactic acid, and inorganic phosphate in serum.
The levels returned to normal 10 days after cessation of infusion. No
effects were found in the BHN, Ca, cholesterol, glucose, amino-acids
and insulin analyses, urinalyses and haematology were found (Shumer,
1971).
In a short-term study respectively five, 15 and 30 g xylitol were
administered to healthy men during two and three weeks. Blood
chemistry, bilirubin, SGOT, Alk.Pase, uric acid and blood sugar were
normal. No diarrhoea was observed (Asano et al., 1972).
In an oral tolerance test with five persons adapted to xylitol up
to 120 g/day. The initial dose level was 30 g/day and this was raised
with 30 g/day at three day intervals. Liver function tests were normal
throughout the experiment, while there was a transient increase in
plasma lactate and urate. No diarrhoea below 90 g/day was noticed
(Amador and Eisenstein, 1971).
The tolerance of xylitol was studied in 18 male and one female
non-diabetic students aged 21-27 years. The students were given
xylitol for 21 days in increasing dose levels from five up to a
maximum of 75 g/day. After one month of interruption the same group
received xylitol in increasing dose levels from 40 g up to 220 g
again during 21 days (19 students received during the first week
40-100 g/day, 28 during the second week, 100-150 g and six during the
third week 150-220 g). The subjects themselves recorded quantity,
daily division of xylitol intake, number and consistency of bowel
movements as well as general condition and obvious side effects. Body
weights were estimated weekly. At the third day of each experimental
period and seven days after termination, fasting blood sugar analyses
and urinalyses on presence of reducing sugar were carried out. From a
130 g/day dose level diarrhoea was observed when the single doses were
poorly distributed over the day. No other significant effects were
noticed. In a similar study 23 men and three women were given xylitol
or sorbitol. The initial dose was 5 g which was increased to 75 g/day
after 14 days. In addition to the parameters investigated in the first
experiment, xylitol and glucose analyses in 24 hour urine were carried
out. Identically to the first experiment diarrhoea was the only
observed effect (Dubach et al., 1969).
In a two year tolerance study three groups of volunteers (35, 38
and 52) remained on strict diet containing respectively, fructose,
sucrose and xylitol. The average monthly amounts consumed in a varied
assortment of foods were respectively 2.1, 2.2 and 1.5 kg. The highest
daily doses of fructose and xylitol were 200-400 g. Serum samples were
analysed for Na, K, Ca, Mg, inorganic phosphates, ascorbate,
bilirubin, amylase, Alk.Pase, amino-acids, IgA, IgG and IgM. In
addition saliva analyses of IgA, IgG and IgM and amylase were carried
out. The number of occurrences of diarrhoea and flatulence-like
conditions were also scored. Body weights of the volunteers were
recorded weekly. The number of pregnancies in the groups were as
follows; eight in the sucrose, six in the fructose and eight in the
xylitol group.
No significant changes of clinico-chemical parameters in serum
and saliva were observed in the xylitol group. A significant rise in
the occurrence of diarrhoea and flatulence-like conditions was noted
in the xylitol group. All pregnancies, deliveries and infants were
normal.
A series of studies in Turku, Finland showed that after one year
on test in which xylitol replaced sucrose in the diet, dental caries
were reduced in the xylitol group by approximately 90% (Scheinin,
1974).
A more recent study involved 100 human young adults. Subjects
were divided into S-(sucrose) group, and X-(xylitol) group.
Consumption was 4.0 chewing gums per subject per day in the S-group
and 4.5 in the X-group. Frequency of sucrose intake was 4.2 times per
day in the S-group, and 4.9 in the X-group. The caries incidence was
2.92 in the S-group and -1.04 in the X-group. The results show a
profound difference in the caries increment rate between the two
experimental groups (Scheinin et al., 1975).
In an accident that happened in 1969/1970 in Australia, 20
patients who had received Japanese xylitol from two specific batches
by intravenous infusion died with symptoms of severe acidosis, liver
necrosis and renal oxalosis, and in one case oxalate crystals were
also found. The patient with the severest and most dramatic liver
damage had received only 200 g of xylitol. In these xylitol batches a
highly toxic substance was determined by the chicken embryo test.
Coats (1970) identified 2-mercaptoimidazole and 2-hydroxyimidazole in
the xylitol batches. 2-mercaptoimidazole was used as a plasticizer of
the rubber stoppers of the ampoules. All further efforts to obtain
material from the suspect batches from the Japanese firm failed, so
that no explanation for the incidents could be found (Lang, 1974).
In 1971/1972 oxalate crystals were observed in the kidneys and
brain in necropsies performed at the Rafenkrankenhaus in Hamburg on
five patients who had received infusions of xylitol. According to the
doctors responsible for their treatment they died of the severe
conditions from which they had been suffering. These findings gave
rise to extensive discussions as to whether xylitol was involved in
the causation-of oxalosis. However, it is most improbable that the
oxalosis observed in Hamburg was due to a specific toxic action of
xylitol. Further investigation of this subject has to be carried out
(Lang, 1974).
Tolerance studies in diabetes
In addition no harmful effects were observed in a diabetic person
who consumed 65 g xylitol/day during the two years experimental period
(Mäkinen and Scheinin, 1975; Mäkinen, 1976).
Acute tests with xylitol were conducted on 13 recruited adult
diabetics and the following parameters were determined: aceto-acetate,
ß-hydroxy-butyrate, unesterized fatty acid (UFA), free glycerine and
total lipids. A significant drop in concentrations of aceto-acetate
and ß-hydroxybutyrate occurred under administration of xylitol as
compared with the control experiments. UFA behaved similarly. The
concentration of free glycerine also clearly dropped at the end of the
experiment. It can be assumed from the results that the diminished
lipolyserate is the cause of reduced formation of ketone bodies. A
drop in total lipids indicated that hyperlipidemia might possibly also
be favourably influenced by xylitol (Grabner, 1971).
Xylitol in concentration varying from 5 to 20 gm in food was
administered to 23 diabetic persons. Except for diarrhoea in some
cases no harmful side effects were observed. No negative influence on
the diabetie metabolism was observed. Xylitol (25 g) dissolved in tea
caused more frequent diarrhoea and other abdominal side effects in the
subjects (Mellinghof, 1961).
Yamagata et al. (1967) investigated the effects of acute
intravenous infusion of 30 g/90 minutes on blood Sugar, pyruvate and
lactate, and on plasma free fatty acids (FFA) and insulin levels in
six normal and 11 diabetic persons. Xylitol infusion produced no
change of blood sugar in normal and a slight increase in diabetic
patients. Blood lactate showed a significant increase in the diabetic
group but only a slight increase in the normal group. Blood ketone
bodies and plasma FFA decreased in both groups after xylitol
administration. Plasma insulin increased slightly in five of the
normal subjects and in four of the nine diabetic subjects (Yamagata
et al., 1967).
In another experiment xylitol was chronically intravenously
infused to 12 mildly diabetic patients every morning for seven days.
No changes were observed in fasting blood sugar, serum triglycerides,
total cholesterol, serum electrolytes or ketone bodies. However, four
cases indicated the marked decrease of urine sugar excretion during
the experimental period. Xylitol was also given to two diabetic
persons with ketosis and decreased the ketone bodies from 5.1 to
0.2 mg % within four hours (Yamagata et al., 1967).
During two to six weeks 30 g xylitol/day was given orally to 12
stable diabetics. The caloric intake and amount of carbohydrates was
kept constant. Changes in fasting blood sugar, urine sugar, serum
lipids, serum mucoprotein, SGOT and SGPT were studied before, during
and after xylitol administration. In three of the 12 cases urinary
sugar excretion disappeared. A slight impairment of glucose tolerance
and a tendency to decreased SGOT and SGPT levels were observed. In
some cases diarrhoea was noticed, but no other effects of xylitol were
noticed (Yamagata et al., 1967).
Eighteen diabetic children received 30 g dietary xylitol during
four weeks. A significant elevation of the uric acid concentration in
serum was observed, also significant increases of total protein
content and of inorganic phosphorus. In these children no diarrhoea
was noticed (Förster et al., 1977).
REFERENCES
Amador, F. and Eisenstein, A. (1971) The effects of oral xylitol
administration in human subjects. Unpublished data cited by Brin, M.
and Miller, O. N. (1974) The safety of oral xylitol; Sugars in
Nutrition; Ed. Sipple and McNutt, 33, 591-606
Asakura, T. (1967) Non-glycolytic sugar-metabolism in human
erythrocytes 1. Xylitol metabolism, J. Biochem., 62, 184-193
Asano, T., Levitt, M.D. and Goetz, F. C. (1972) Xylitol absorption in
healthy men, Diabetes., 21, suppl. 1, 350-351
Asano, T., Levitt, M.D. and Goetz, F. C. (1973) Xylitol absorption in
healthy men, Diabetes, 22, 279-281
Bächtold, H. P. (1972) Prüfung in 5-Tage-Toxizitätsversuch an Ratten
und Mäusen. Unpublished report from the Research Department of
Hoffmann La Roche Co., submitted to the World Health Organization by
Hoffmann La Roche Co., Basle, Switzerland
Banziger, R. (1970) A thirteen week oral toxicity study of xylitol in
monkeys. Unpublished report of Hazleton Laboratories Inc., submitted
to the World Health Organization by Hoffmann La Roche Co., Basle,
Switzerland
Bässler, K. H. (1965) Biochemie und Stoffwechsel von Xylit (1965)
Dtsch. Lebensm. Rdsd., 61, 171-173
Bässler, K. H. und Prellwitz, W. (1964) Insulin und der
Verteilungsraum von Xylit bei eviscerierten Ratten, Klin.
Wocherschrift, 42, 94-95
Bässler, K. H., Prellwitz, W., Unbehaun, V. und Lang, K. (1962)
Xylitstoffwechsel beim Menschen, Klin. Wschr., 40, 791
Bässler, K. H. und Reimold, W. V. (1965) Lactaatbildung aus Zuckern
und Zuckeralkoholen in Erythrocyten, Klin. Wschr., 43, 169-171
Bässler, K. H. und Wagner, G. (1970) Fettsäuregehalt der Rattenleber
unter verschiedenen Bedingungen der parenteralen Ernährung
Berger, W., Göschke, H., Moppert, J. and Künzli, J. (1973) Insulin
concentration in portal venous and peripheral venous blood in man
following administration of glucose, galactose, xylitol and
tolbutamide, Horm. Metab. Res., 5, 4-8
Birnesser, H., Reinauer, H. and Hollmann, S. (1973) Comparative study
of enzyme activities degrading sorbitol, ribitol, xylitol and
gluconate in guinea-pigs' tissues, Diabetologia, 9, 30-33
Brin, M. and Miller, O. N. (1974) The safety of oral xylitol. Sugars
in Nutrition, Ed. Sipple, H. L. and McNutt, K. W., Acad. Press
Coats, D. A. (1970) Report to the Deutsche Arzeimittelkommission 1970.
Cited by K. Lang (1974) (Unpublished report)
Dehmel, K. H., Förster, H. and Mehnert, H. (1967) Absorption of
xylitol. Int. Symp. on metabolism, physiology and clinical use of
pentoses and pentitols, Hakone, Japan, 1967, 177-181, Ed. Horecker,
B. L. e.a.
Dixon, R. and East, S. (1973) Xylitol levels in plasma following
intravenous and oral administration to man. Unpublished report,
Psycho-Endocrine Centre, St. James's Hospital, Dublin 8, Ireland,
submitted to the World Health Organization by Hoffmann La Roche Co.,
Basle, Switzerland
Dubach, U. C., Feiner, E. and Fargo, E. (1969) Orale Verträglichkeit
von Xylitol bei stoff wechselgesunden Probanden, Schweiz. Med.
Wschr. 99, 190-194
Felber, J.P. (1976) Untersuchung der Wirkung des Xylits auf den
Blutzuckerspiegel beim Menschen, Abstract einer Expertise vom 24-6-76.
Sitzung der Eidg. Ernährung Kommission in Bern
Förster, H. (1972) Grundlagen für die Verwendung der drei
Zuckeraustauschstoffe Fructose, Sobit und Xylit, Med. Ernähr.,
13, 7-15
Förster, H. (1974) Zum Stoffwechsel von Monosacchariden und Polyolen,
Ernährungs. Med., 1, 37-55
Förster, H., Boecker, S. and Ziege, M. (1972) Anstieg der
Konzentration der Serumharnsäure nach akuter und chronische Zufuhr von
Saccharose, Fructose, Sorbitol und Xylit, Med. u. Ernähr.,
13, 193-196
Förster, H., Boecker, S. and Walther, A. (1977) Verwendung von Xylit
als Zuckeraustauschstoff bei diabetischen Kindern, Fortschr. Med.,
95, nr. 2, 99-102
Förster, H. und Hoffmann, H. (1973) Biochemische Überlegungen zur
Verwendung der Kohlenhydrate in der parenteralen Ernährung,
Infusiontherapie, 1, 265-279
Förster, H. und Ziege, M. (1970) Anstieg der
Senumharnsäurekonzentration nach oraler Zufuhr von Fructose, Sorbit
und Xylit, Z. Ernährungswiss., 10, 394-396
Froesch, E. R. (1972) Übersicht über den Haushalt der Betriebstoffe
mit besonderer Berücksichtigung des Stoffwechsels von Glukose,
Fruktose, Sorbit und Xylit und deren therapeutische Verwendbarkeit,
Int. Z. Vit. und Ernährungsforsch., Suppl. 12, 73-86
Froesch, E. R. (1975) Umsatz und Verwertung von Glukose und
Glukoseaustauschstoffen bei Mensch und Tier, Nutr. Metabol., 18
(suppl. 1), 30-44
Froesch, E. R. and Jakob, A. (1974) The metabolism of xylitol. Sugars
in nutrition, Ed. H. L. Sipple and K. W. McNutt, Academic Press,
241-258
Grabner, W., Berger, G., Bergner, D. and Matzkies, F. (1971) Über den
Einfluss des Xylits auf den Fettstoffwechsel von Diabetikern,
Med. Ernährung, 12, 126-128
Grunberg, E., Beskid, G. and Brin, M. (1973) Xylitol and Dental
Caries. Efficacy of xylitol in reducing dental caries in rats,
Internat. J. Vit. Nutr. Res., 43, 227-232
Grütte, F. K. and Rödel, H. (1975) Zur Bedeutung des Zuckeralcohols
Xylit als Zuckeraustauschstoff Ernährungsforschung, 3, nr. 20, 74-79
Haydon, R. K. (1961) The antiketogenic effects of polyhydric alcohols
in rat-liver slices, Biochim. Biophys. Acta., 46, 598-599
Heuckenkamp, P. U. und Züllner, N. (1972) Xylitbalanz während
mehrstündiger infusionen mit konstanten Zufahrraten bei gesunden
Manschen, Klin. Wschr., 50, 1863-1864
Hirata, Y., Fujisawa, M. and Ogushi, T. (1967) Effects of intravenous
injection of xylitol on plasma insulin. Int. Symp. on metabolism,
physiology and clinical use of pentoses and pentitols, Hakone, Japan,
Ed. Horecker, B. L. et al.
Hirata, Y., Fujisawa, M., Sato, H., Asano, T. and Katsuki, S. (1968)
Effects of intravenous injection of xylitol on blood sugar, blood
pyruvic acid and plasma insulin levels in the dog, Z. für dieo
gesamte exptl. Med., 145, 111-119
Hollmann, S. (1967) Pentitol-metabolizing enzymes of the uronic acid
pathway, Int. Symp. on metabolism, physiology and clinical use of
pentoses and pentitols, Hakone, Japan, 97-108
Horecker, B. L. (1969) Stoffwechselwege der Kohlenhydrate: ihre
Regulierung und physiologische Bedeutung. III Stoffwechsel und
physiologische Wirkungen von Xylit, Med. Ernährung., 10, 66-69,
95-98, 127-129
Hosoya, N. and Machiya, T. (1967) Xylitol effect on ketogenesis in
alloxan diabetic rat liver slice, Int. Symp. on metabolism, physiology
and clinical use of pentoses and pentitols, Hakone, Japan, 1967, Ed.
Horecker, B. L. et al.
Hosoya, M., Sakurai, T. and Shimazono, N. (1966) Glycogen and
glycolytic intermediates in Alloxan-diabetic liver slices, VIIth
International Congress of Nutrition, Hamburg
Hosoya, M. and Iitoyo, M. (1969) Effects of xylitol administration on
the development (growth) of rats (cited in Brin and Miller, 1974)),
J. Jap. Soc. Food Nutr., 22, 17-20
Hötzel, D. (1971) Über den Intermediärstoffwechsel von Sorbit, Xylit
und Cyclamaten, Dtsch. Zahnärzl. Z., 26, 1035-1040
Hummler, H. (1974) Orientierende Verträglichkeitsuntersuchungen über 6
Wochen mit Xylit, Sorbit und Saccharose am Hund. Unpublished report
from Huntingdon Research Centre, submitted to the World Health
Organization by Hoffmann La Roche Co., Basle, Switzerland
Hummler, H. (1976a) Reproduktionstoxikologische Untersuchungen mit
Xylit an der Rätte. Unpublished report from Huntingdon Research
Centre, submitted to the World Health Organization by Hoffmann La
Roche and Co., Basle, Switzerland
Hummler, H. (1976b) Cancerogenese Untersuchungen mit Xylit an der
Maus. Unpublished interim report from Huntingdon Research Centre,
submitted to the World Health Organization by Hoffmann La Roche and
Co., Basle, Switzerland
Hummler, H. (1976c) Chronische Toxizitäts- und Carcinogenese-
Untersuchungen mit Xylit an Ratten. Unpublished report from Huntingdon
Research Centre submitted to the World Health Organization by Hoffmann
La Roche and Co., Basle, Switzerland
Hummler, H. (1976d) Chronische Verträglichkeitsprüfung mit Xylit an
Hunden (52 and 78 w.). Unpublished report from Huntingdon Research
Centre, submitted to the World Health Organization by Hoffmann La
Roche and Co., Basle, Switzerland
Huthunen, J. K., Mäkinen, K. K. and Scheinin, A. (1975) Effects of
sucrose, fructose and xylitol diets on glucose, lipid and urate
metabolism, Acta Odont. Scand., 33, suppl. 70, 239-245
Jakob, A., Williamson, J. R. and Asakura, T. (1971) Xylitol metabolism
in perfused rat liver. Interactions with gluconeogenesis and
ketogenesis, J. Biol. Chem., 246, No. 24, 7623-7631
Jourdan, M. H., McDonald, J. and Henderson, J. R. (1972) The effects
of 14C-xylitol, given intravenously, on the serum glucose, insulin
and lipid concentrations of male and female baboons, Nutr. Metabol.,
14, 92-99
Jursons, K. K., Puzaka, J. J., Aalmans, A. R. and Vitolin, S. P.
(1974) Izvestija Akademii Nauh Latvijskoj SSR., 9, 16-22
Keller, U. and Froesch, E. R. (1972) Vergleichende Untersuchungen über
den Stoffwechsel von Xylit, Sorbit und Fruktose beim Menschen,
Schwerz. Med. Wschr., 102, 1017-1022
Kieckebusch, W., Gziem, W. und Lang, K. (1961) Die Verwertbarkeit von
Xylit als Nahrungskohlenhydrat und seine Verträglich, Klin. Wschr.,
39, 447
Kuzyha, T., Kanazawa, Y. and Kosaka, K. (1966) Plasma insulin response
to intravenously administered xylitol in dogs, Metabolism, 15, No.
12, 1149-1152
Kuzuya, T., Kanazawa, Y. and Kosaka, K. (1969) Stimulation of insulin
secretion by xylitol in dogs, Endocrinology, 84, 200-207
Lang, K. (1964) Die ernährungsphysiologischen Eigenschaften von Xylit,
Internat. Z. Vitaminforsch., 34, 117-122
Lang, K. (1972) Xylit - Biochemie und Klinik, Suppl. 13 zur
Z. Ernährungswiss: "Rehabilitation durch Ernährung" Darmstadt 11-15
(symp. CSSR)
Lang, K. (1974) Xylit in der oralen und parenteralen Ernährung Heft
der Schriftenreihe des Bundes für Lebensmittelrecht und
Lebensmittelkund, 75, 21-23
Liebau, K. (1964) Untersuchungen der Resorption des Xylit beim
Menschen Dissertation of K. Liebau, submitted to the World Health
Organization by Hoffmann La Roche Co., Basle, Switzerland (Unpublished
report)
Mäkinen, K. K. (1976) Long-term tolerance of healthy human subjects
to high amounts of xylitol and fructose: general and biochemical
findings. In: Monosaccharides and polyalcohols in nutrition
therapy and diabetics, Ed. G. Ritzel, G. Brubacher, Verlag H.
Huber, Int. J. Vit. Nutr. Res. nr., 15
Mäkinen, K. K. and Scheinin, A. (1975) Effect of the diet on certain
clinico-chemical values of serum, Acta Odont. Scand., 33, suppl.
70, 265-276
McCormick, D. B. and Touster, O. (1957) The conversion in vivo
of xylitol to glycogen via the pentose phosphate pathway, J. Biol.
Chem., 239, 451-461
Mehnert, H., Summa, J. D. and Fürster, H. (1964) Untersuchungen zum
Xylitstoffwechsel bei gesunden, Leberkranken und diabetischen
Personen, Klin. Wschr., 42, 382
Mellinghoff, G. H. (1961) Über die Verwendbarkeit des Xylit als
Ersatzzucker bei Diabetikern, Klin. Wschr., 39, 447
Meng, H. C. (1974) Sugars in parenteral nutrition. Sugars in
nutrition, Ed. H. L. Sipple, K. W. McNutt, Academic Press, 527-566
Mertz, D. P., Kaiser, V., Klöpfer-Zaar, M. and Beisbarth, H. (1972)
Serumkonzentrationen verschiedener Lipide und von Harnsäure während 2
wüchiger Verabreichung von Xylit, Klin. Wschr., 50, 1107-1111
Montague, W., Howell, S. L. and Taylor, K. W. (1967) Pentitols and the
mechanism of insulin release, Nature, 215, 1088-1089
Mosinger, M. (1971) Effects of the long-term oral administration of
xylitol. Unpublished report of Mosinger, M., submitted to the World
Health Organization by Hoffmann La Roche, Basle, Switzerland
Müller-Hess, R., Geser, C. A., Bonjour, J.P., Jequier, E. and Felber,
J.P. (1975) Effects of oral xylitol administration on carbohydrate and
lipid metabolism in normal subjects, Infusionstherapie, 2, 247-252
Opitz, K. (1967) The influence of xylitol and other polyols and sugars
on fat mobilization, Int. Symp. on metabolism, physiology and clinical
use of pentoses and pentitols, Hakone, Japan, Ed. Horecker, B. L. et
al.
Oshinsky, R. J., Wang, Y. M. and Eys, J. v. (1976) Oxalate formation
following xylitol infusion in 4-deoxypyridoxine treated rabbits,
Fed. Proc., 35, 419
Pazenko, A. G. (1969) Voprosy prolilaktiki, diagnostiki i lecenija
zabolevanij organor, Piscevarenija, Kiev, 133
Petrich, Ch., Reinauer, H. and Hollmann, S. (1972) Vergleichende
Aktivitätsmessungen der Enzyme des Glucuronsäure Xylulose-Zyklus
in der Leber, dem epididymalen Fettgewebe der Ratte, in einem
Morris-Hepatoom und einer Fibrocyten-Kultur, Z. Klin. Chem. Klin.
Biochem., 10, 355-358
Pitkänen, E. and Sahlström, K. (1968) Increased excretion of
xylitol after administration of glucorone, lactone and ethanol in
man, Ann. Med. exp. Fenn., 47, 143-150
Pool, W. and Hane, D. (1970) Acute toxicity and dog tolerance testing
of xylitol. Unpublished report from the Research Department of
Hoffmann La Roche Co., submitted to World Health Organisation by
Hoffmann La Roche Co., Basle, Switzerland
Pool, W. and Hane, D. (1972) Acute mouse toxicity of xylitol.
Unpublished report from the Research Department of Hoffmann La Roche
Co., submitted to the World Health Organization by Hoffmann La Roche
Co., Basle, Switzerland
Quadflieg, K. H. and Brand, K. (1974) Xylitol metabolism in human
erythrocytes, Z. Phys. Chemie, 355, 1239
Scheinin, Ulla (1974) Incidence of dental caries among a group of
university students in Turkey, Acta. Odont. Scand., 32, 335-344
Scheinin, Arje., Mäkinen, Kauko K., Tammisalo, Erkki., Rekola, Maarit.
(1975) Turku sugar studies. XVIII. Incidence of dental caries in
relation to one-year consumption of xylitol chewing gum, Acta.
Odont. Scand., 33, 269-278
Schmidt, B., Fingerhut, N. and Lang, K. (1964) Über den Stoffwechsel
von radioaktiv markiertem Xylit bei der Ratte, Klin. Wschr., 42,
1073-1077
Shima, S., Mitsunaga, M. and Nakao, T. (1970) Effect of xylitol on
testicular protein and RNO biosynthesis of rats, Endocrinol. Jap.,
17, 283-287
Shumer, W. (1971) Adverse effects of xylitol in parenteral
alimentation Metabolism., 20, 345-347
Staub, H. W. and Thiessen, R. (1972) Effect of dietary polyols on
serum cholesterol in rats, Federation Proc., 31, 291
Stein, G. (1966) Adaptive Prozesse im Xylitstoffwechsel und ihre
Auswirkungen Dissertation Mainz, 1966, submitted to the World Health
Organization by Hoffmann La Roche and Co., Basle, Switzerland
(Unpublished report)
Swarm, R. L. and Banziger, R. (1970) A thirteen week oral feeding
study in rats with xylitol (Ro-6-7045). Unpublished report of Hazleton
Laboratories Inc., submitted to the World Health Organization by
Hoffmann La Roche, Basle, Switzerland
Touster, O. (1970) Parenteral nutrition (Proc. on an internat. Symp.
Vanderbilt University, School of Medicine, Nashville) 131-138, Ed.
Meng, H. C., Law, D. H., Thomas, C. C., Publisher Springfield,
Illinois, USA
Touster, O. (1974) The metabolism of polyols. In: Sugars in Nutrition,
Ed. H. L. Sipple, K. W. McNutt, Academic Press, 229-239
Touster, O. and Shaw, D. (1962) Biochemistry of the acyclic polyols,
physiol. Rev., 42, 181-225
Truhaut, R., Coquet, B., Fouillet, X., Galland, I., Guyot, D., Rouaud,
J. L. and Long, D. W. (1977) Subacute toxicity of xylitol in rats.
Personal communication to the World Health Organization (Unpublished
report)
Wagner, G. and Bässler, K. H. (1971) Gibt es Unterschiede in der
antiketogenen Wirkung gebräuhlicher Zucker und Polyalkohole, Medizin
Ernährung., 12, 53-54
Wang, Y. M., King, S. M., Patterson, J. H. and Van Eys, J. van. (1973)
Mechanism of xylitol toxicity in the rabbit, Metabolism, 22, nr.
7, 885-894
Wang, Y. H., Oshinky, R. J., Lantin, E. and Eys, J. van. (1975) An
investigation of oxalate formation from xylitol infusion in rabbits,
Fed. Proc., 35, 920
Wilson, R. B. and Martin, J. M. (1970) Plasma insulin concentrations
in dogs and monkeys after Xylitol, glucose or tolbutamide infusion,
Diabets., 19, 17-22
Woods, H. F. (1975) Hepatic metabolism of pentites, Nutr. Metabol.,
18 (Suppl. 1), 65-78
Woods, H. F. and Krebs, H. A. (1973) Xylitol metabolism in the
isolated perfused rat liver, Biochem. J., 134, 437-443
Yamagata, S., Goto, Y., Ohneda, A., Anzai, M., Kwashima, S., Kikuchi,
J., Chiba, M., Maruhama, Y., Yamauchi, Y. and Toyota, T. (1967)
Clinical application of xylitol in diabetics, Int. Symp. on
metabolism, physiology and clinical use of pentoses and pentitols,
Hakone, Japan, 316-325, Ed. Horecker, B. L. et al.
These biotransformation studies are also supported by Schmidt et
al. (1964), Hörecher (1969), Touster (1974), Stein (1966), Petrich et
al. (1972) and Grütte and Rödel (1975). McCormick and Touster (1957)
found that the metabolism of rat and guinea pig was similar.
Effects on enzymes and other biochemical parameters
Xylitol has especially influence on the activity of the enzymes
of carbohydrates metabolism and the insulin hormone. Both in
experiments with parenteral and oral administration of xylitol a
stimulation of insulin secretion and an elevated serum insulin
concentration are observed in rat, dog and monkey, although the plasma
glucose level slightly increased for a while and then decreased below
the fasting level (Bässler and Prellwitz, 1964; Kuzuya et al., 1966,
1969; Hirata et al., 1967, 1968; Montague et al., 1967; Wilson and
Martin, 1970; Froesch, 1975; Jourdan et al., 1972; Touster, 1974).
Kuzuya et al. (1969) found that the insulin secretion produced by
xylitol in dogs was more pronounced than by glucose. Wilson and Martin
(1970) observed the same effect in dogs, while they found that in
monkeys glucose caused a more marked stimulation of the insulin
secretion than xylitol did. The insulin secretion in dogs is also much
stronger than in man (Hirata et al., 1967). Meng (1974) however did
not find any changes of the insulin level in dogs, i.v. infused with
xylitol. This study included only five animals.
Glycogen production is stimulated by the administration of
xylitol to guinea pigs, rat and perfused rat liver, even when diabetic
animals are used: (McCormick and Touster, 1957; Hosaya et al., 1966;
Touster, 1970; Förster and Hoffman, 1973). Jakob et al. (1971) found
that in liver most of the xylitol administered to the animals was
rapidly converted to glucose and to small quantities of lactate.
Bässler and Reimold (1965) observed a stimulating influence of xylitol
on the production of lactic acid in erythrocytes which was also
noticed with glucose. In perfused rat liver xylitol stimulated the
lactate production and caused an increased lactate to pyruvate ratio
(Jakob et al., 1971; Förster and Hoffmann, 1973; Woods and Krebs,
1973; Woods, 1975). Besides the increased ratio of lactate to
pyruvate Jakob et al. (1971) found also increased ratios of
alpha-glycerophosphate to dihydroxy acetone-P and triosephosphate to
3-P-glycerate. In a study of Hirata et al. (1968) a significant
decrease of blood pyruvic acid level was noticed in normal dogs.
In close relation with the influence of the carbohydrate
metabolism an antiketogenic and antilipolytic effect of xylitol is
also observed (Haydon, 1961; Hosaya and Machiya, 1967; Bässler and
Wagner, 1970; Jakob et al., 1971; Wagner and Bässler, 1971; Birnesser
et al., 1973). As a result of accumulation of alpha-glycerophosphate
and other phosphorylated intermediates a fall in the inorganic
phosphorus and adenine nucleotides was observed (Petrich et al., 1972;
Woods and Krebs, 1973; Woods, 1975). The fall in the inorganic
phosphorus leads to an increased purine catabolism and to a
hyperuricemia in isolated perfused rat liver (Woods and Krebs, 1973;
Woods, 1975). Forster and Hoffmann (1973) found that xylitol
accelerates the biosynthesis of uric acid.
Shima et al. (1970) found a stimulation of testicular protein and
RNA biosynthesis in rats fed xylitol. Staub and Thiessen (1972)
observed increased cholesterol levels in rats fed xylitol.
In experiments with xylitol oxalate formation is observed, but
glucose is reported to have a stronger effect on this formation (Wang
et al., 1975; Oshinsky et al., 1976; Brin and Miller, 1974). Therefore
no clear relationship between oxalate accumulation and xylitol
toxicity is found by Oshinsky et al. (1976) and Wang et al. (1975).
TOXICOLOGICAL STUDIES
Special studies on reproduction
In a reproduction study five groups of 30 male and 60 female rats
of the P-generation were fed 0, 2, 5, 10 and 20% dietary xylitol. The
total amount of carbohydrates was kept constant. In addition 20%
sorbitol and a 20% sucrose group were used as sugar controls. This
study has only been carried out till the F1B-generation. At the
second mating, an abrupt increase in pup mortality occurred in all
groups between four and 12 days. This was caused by a heating failure.
This resulted in loss of too many pups to complete the reproduction
study.
In the animals receiving 5, 10 and 20% xylitol decreased
food-intake and weight gain was recorded. At the first mating, a
slightly higher initial litter size among test groups was
counterbalanced by a slightly higher cumulative pup loss during
lactation. No other effects on the reproduction were noticed in the
F1a-animals receiving xylitol. This study will be repeated (Hummler,
H. 1976a).
Levels of xylitol up to 20% of the diet fed to rats up to four
months had no significant adverse effects. Growth rate was normal.
Adaptation to high dosage was shown to be important. Xylitol had no
effect on the number or size of pups or dams fed xylitol, and the pups
adapted readily to the supplement at 5 and 10% in diet, after weaning,
as instituted following adverse effects with the 20% xylitol level,
initially (Hosoyol and Iitoyo, 1969).
Special studies on carcinogenicity
In a carcinogenicity study 100 female and 100 male mice per group
weighing 24-25 g, were fed 0, 2, 10 and 20% dietary xylitol during
1.5-2 years. The total amounts of carbohydrates were kept constant and
animals receiving sucrose served as sugar control group. Body weight
and food intake were recorded weekly, while with intervals water
consumption was determined. At 26, 52 and 79 weeks and prior to
terminal sacrifice ophthalmoscopy was carried out. After 80 weeks 20
males and 20 females from each group were killed. The mice were killed
when survival within a group reached 20%. Gross pathology was
performed visually and by palpation. Microscopic examination on
adrenals, thyroids, ovaries, liver, spleen, lymph, pituitary gland and
bone marrow and all macroscopically observed lesions suggestive of
neoplasia were routinely carried out for every animal, along with
blood smears.
At the start of the experiment a severe diarrhoea was observed,
therefore a number of adaptation phases with slowly increasing xylitol
concentrations in the diet were necessary. Animals receiving 10% and
20% xylitol showed increased water and food intake. In the animals
receiving 2% and 10% xylitol, 20% sorbitol an increased body weight
gain was observed, whereas the animals receiving 20% xylitol had a
significant lower body weight gain. The mortality of the male mice
receiving 10 and 20% xylitol was elevated. No histopathology and no
details on tumour incidence are available. The study is at the moment
an inadequate carcinogenicity study, which has to be completed first
(Hummler, H., 1976b).
In a long-term toxicity and carcinogenicity study five groups of
75 female and 75 male Sprague-Dawley rats, weighing 120-150 g, were
fed 0, 2, 5, 10 and 20% dietary xylitol. The total amounts of
carbohydrates in the diet were kept constant. Animals receiving 20%
sorbitol and 20% sucrose were used as sugar control groups. Food
intake and body weight were recorded weekly. Water consumption was
recorded during weeks 12, 25, 52 and 78. Ophthalmoscopy, urinalysis,
haematology and biochemistry were performed at 0, 13, 26, 52, 78 and
104 weeks. Urinalyses included pH, specific gravity, protein, reducing
substances, glucose, ketones, bile pigments, urobiligen, haemoglobin
and oxalic acid determinations. Haematology consisted of determination
of PCV, Hb, RBC, WBC, MCHC, MCV, differential count, prothrombin
index, whole blood clotting time. The biochemical estimations were
urea and glucose in plasma, total proteins, protein electrophoresis,
Alk Pase, GPT, GOT, bilirubin, uric acid, cholesterol, lactate, LDH,
alpha-HBDH in serum and total reducing substances, insulin,
xylitol,Na+, K+, Cl- and bicarbonate in blood. After 26 and 52
weeks, five males and five females per group were killed. In the gross
pathology the animals were examined visually and by palpation. Brain,
heart, liver, kidneys, adrenals, pituitary, thyroid, spleen,
testes/ovaries were weighed. These organs and pancreas, thymus,
uterus, cervical and mesenteric lymph nodes, stomach, bone marrow,
ileum, coecum, duodenum, urinary bladder, eye, lungs and any
macroscopically abnormal tissues were histopathologically
investigated. Blood smears of these organs were also studied.
Significant decreased weight gain in animals receiving 10% or 20%
xylitol during the experiment was observed, while there was only
tendency to decreased food intake in animals receiving 20% xylitol at
78 weeks. In rats receiving 20% xylitol, higher water intakes were
recorded during weeks 26 and 52, and in female rats also during week
78.
In male and female rats at 13, 26 and 52 weeks and in female rats
at week 78, receiving 20% xylitol greater volumes of more diluted
urine were excreted. After 26 weeks, the urine from animals receiving
5, 10 and 20% showed decreased specific gravity.
No increase in mortality was noticed in the treated groups,
Except a significant decreased prothrombin index in all male rats
receiving xylitol and female rats receiving 20% xylitol, no
haematological changes were observed at 78 weeks. In biochemistry a
tendency to decreased lactate contents in the blood of animals
receiving 10 and 20% xylitol and at 78 weeks a tendency to increased
uric acid content in male rats of the 20% xylitol group were observed.
No morphological abnormalities in the treated groups differing
significantly from the control group ware observed. The standard
deviation in the insulin determination was too great for making an
evaluation of the results possible.
The study is not yet completed. No details on tumour incidence
are available so far (Hummler, 1976c).
Acute toxicity
LD50
Animal Route mg/kg References
body weight
mouse oral 14 100 Bächtold (1972)
mouse oral 22 000 Pool and Hane (1972)
mouse oral >4 000 Pool and Hane (1970)
mouse oral 25 700 Kieckebuch (1961)
mouse intraperitoneal >2 000 Pool and Hane (1970)
mouse subcutaneous >2 000 Pool and Haue (1970)
(con't)
LD50
Animal Route mg/kg References
body weight
mouse intravenous >4 000 Pool and Hane (1970)
mouse intravenous 22 200 Grütte and Rödel (1915)
mouse intravenous 3 770-9 450 Pazenko (1969)
adult rat oral >4 000 Pool and Hane (1970)
neonatal rat oral > 4 000 Pool and Hane (1970)
rat oral 14 100 Bächtold (1972)
rabbit oral > 2 000 Pool and Hane (1970)
rabbit intravenous 4 000-6 000 Wang et al. (1973)a
a The acute toxicity in rabbits was determined by intravenous
infusion of 87 mg xylitol/kg/minute. The observed LD50 was 4-6 g/kg
body weight. Striking increases of SGOT and serum LDH levels, along
with an increase urine volume were observed (Wang et al., 1973).
Short-term studies
In a subacute toxicity study xylitol was administered to 20
female and 20 male rats per group by daily gastric intubation for a
period of 14 days. Dose levels were 1.25, 2.5, 5.0 and 10.0 g/kg. The
animals receiving 1.25 g xylitol/kg during the first nine days,
received 10.0 g xylitol/kg during the last seven days. In the control
groups animals received 0, 2.5 and 5.0 g sucrose/kg/day. During
treatment (two, five and 14 days) animals were submitted to careful
clinical examinations and blood serum analyses related to hepatic
functions. These analyses were glucose in blood and bilirubin, FFA,
total lipids, triglycerides, cholesterol, G-6-PDH and Alk. Pase, GPT
and GOT in serum. Also body weight and food consumption were recorded.
The animals were sacrificed after two, five and 14 days. Heart, liver,
spleen, kidneys, adrenals, gonads, stomach were weighed. These organs
and jejunum, colon, pancreas and brain were histologically examined in
two and five days treatment groups, while in 14 days treatment group
this examination was only performed on liver. The histological study
was conducted on five males and five females per group.
Except for a decrease of the FFA content in the blood at all
xylitol dose levels, no effects are observed in this study. No
evidence of hepatotoxicity was recorded (Truhaut et al., 1977).
In a 15 days' experiment with rats, sucrose was replaced
respectively by 10 and 20% xylitol. The protein of the diet consisted
of casein, while their carbohydrate allowance consisted of starch and
sugar. In the 10% xylitol dose group no histopathological changes were
observed. However, in the 20% xylitol dose group adverse effects of
the metabolism of the liver cells were noticed. A reduction of the
glycogen and lipids concentrations were observed. The mitotic activity
of the liver cells was sharply reduced to a level five times lower
than in the control rats (Jursons et al., 1974).
In a short-term (13 weeks) study four groups of eight female and
eight male Charles River CD rats weighing 120-150 g, were fed 0, 5, 10
and 20 g dietary xylitol/kg/day. Food and water consumption were
recorded daily and body weight weekly. Haematology, blood glucose and
urinalyses of five male and five female rats of each group were
carried out at four, eight and 12 weeks. Alk. Pase, GOT, bilirubin and
uric acid in serum and BUN of five males and five females per group
were determined at 13 weeks.
The animals, particularly the male rats, showed reduced body
weight gain and food consumption, that were dose dependent. In the 20%
xylitol group BUN was elevated. In treatment groups dose-related
decrease of the absolute heart weight are observed. Except for
transient diarrhoea in a number of treated animals, no other
significant changes were observed. No histopathological lesions
related to xylitol administered were noticed (Swarm and Banziger,
1970).
Rat
Rats fed a cariogenic diet containing 10% xylitol showed no
carious lesions in the first and third molars and only minimal
involvement of the second molars was observed (Grunberg et al., 1973).
Dog
In short-term study five dogs were dosed 10 g xylitol/kg/day
by intravenous infusion during seven weeks. Blood chemistry and
urinalyses were investigated weekly. Plasma glucose content was
reduced to 64 mg % after six weeks. A slight increase in plasma
lactate and a significant increase of SGPT and Alk. Pase was observed.
Also an increased urinary loss was observed. No effect of xylitol on
plasma insulin levels and no oxalate crystals in the kidneys were
observed (Meng, 1974).
A six weeks toxicity experiment with one female and one male dog
(5.5-6 kg) per group was carried out. The concentration was during the
experiment increased from 5% after two weeks to 20%. Food consumption
was recorded twice daily and body weights twice a week. At the end of
the experiment gross pathology was carried out, and brain, heart,
liver, lungs, pituitary, spleen, pancreas, thymus, prostate/uterus,
kidneys, thyroids, adrenals and testes/ovaries were weighed. No
histopathological investigation was carried out.
Except for a tendency to increased liver weights in the xylitol
groups no effects are observed. This study is not an adequate toxicity
study, but has to be seen as preliminary experiment (Hummler, 1974).
In a long-term toxicity study eight female and eight male beagle
dogs per group, weighing 6-9 kg, were fed 0, 2, 5, 10 and 20% dietary
xylitol during two years. The total amounts of carbohydrates were kept
constant. In addition animals receiving 20% sorbitol and 20% sucrose
were used as sugar controls. Body weight and food consumption was
recorded weekly, while water consumption was recorded over five day
periods at the weeks one to four, 9-12, 21-24, 35-38 and 46-49.
Ophthalmoscopy, dental examination and a full neurological examination
of four male and four female dogs were carried out at 13, 26, 39, 50,
64 and 76 weeks. Haematology, biochemistry and urinalyses were carried
out at the beginning of the experiment and at 12, 26, 38, 50, 64 and
76 weeks. The haematology included erythrocyte sedimentation rate,
PCV, Hb, RBC, reticulocyte count, MCHC, MCV, WBC, differential count,
platelet count, prothrombin index, whole blood clotting time. The
biochemistry included determination of total protein, Al, GPT, GOT,
total LDH, alpha-HBDH, bilirubin, uric acid, cholesterol, lactate and
insulin in serum and urea xylitol, glucose concentration and total
reducing substances in plasma. Sodium, potassium, chloride and
bicarbonate were also detected in the blood. Urinalyses consisted of
estimations of Ph, protein, reducing substances, glucose, ketones,
bile pigments, urobiligen and Hb. On completion of 52 weeks, two male
and two female dogs per group were sacrificed for interim study, while
the remaining dogs will be killed after two years' dosing. The weights
of brain, liver, kidneys, pituitary, thyroid, spleen, heart, lungs,
adrenals, ovaries, testes, uterus, thymus, prostate, pancreas were
recorded. Histopathology of these organs and of aorta, trachea, lymph
nodes, gall bladder, urinary bladder, salivary glands, oesophagus,
duodenum, stomach, jenunum, ileum, skin, skeletal muscle, mammary
glands, tongue, eye with optic nerve and sciatic nerve was performed.
The food intake and weight gain of the 20% xylitol group were
increased. A dose-related increase of SAP and SGPT and a decreased
lactate level was noticed, while in the 20% xylitol dose group also
the total serum protein was significantly increased. At 52 weeks a
tendency to increased cholesterol content in serum was observed.
Except a dose related decrease in the pituitary of the xylitol
dose groups no effects on organ weights, in gross necropsy and
histopathology were observed. The number of animals, however, did not
allow a real statistic evaluation (Hummler, 1976d).
Mean values of haematological, biochemical and urinalyses were
calculated from total population of female and male animals.
Significant changes could be looked over by elevated standard
deviation. In the insulin determinations variation was too much for
further conclusion. In view of the mentioned restrictions it can be
stated that this study is not fully adequate, it has to be completed
first.
In a 13 weeks toxicity study two male and two female rhesus
monkeys, weighing 2-3 kg, were dosed twice daily six days a week 0,
1.0, 3.0 and 5.0 g xylitol/kg/day by gastric intubation. The animals
were observed daily for mortality, appearance, behaviour, appetite,
elimination and pharmacotoxic effects.
Body weights were recorded weekly. At 0, 4, 8 and 13 weeks
ophthalmoscopic and neurologic examinations, haematology, clinical
chemistry and urinalyses were performed. Haematology included Ht, Hb,
total and differential leucocyte counts, prothrombin time and
coagulation time. Blood chemistry consisted of blood sugar, BUN, SAP,
SGPT and SGOT estimations. In addition, free and total bilirubin and
uric acid determinations were conducted at four, eight and 13 weeks.
Urinalyses included specific gravity, pH, glucose, ketones, total
protein, bilirubin and microscopic examination of sediment.
Gross-necropsy was carried out on all animals. Spleen, brain,
pituitary, thyroid, heart, lung, liver, kidneys, adrenals, testes,
prostate, ovaries, uterus were weighed. Histopathology of these organs
and thoracic spinal cord, eye, gall bladder, thymus, salivary gland,
stomach, pancreas, small intestine, large intestine, mesenteric lymph
nodes, sciatic nerve, skeletal muscle, urinary bladder, skin, rib,
vertebra, femur, sternum were carried out at 13 weeks.
Soft stool and/or diarrhoea were noted in all treatment groups
intermittently throughout the study and appeared to be dose related.
In the treatment groups a tendency to decreased coagulation time (from
3.16 to 2.40 minutes) was noticed. Brain weight tended to a dose
related decrease in the male animals receiving xylitol. No further
dose related effects were observed in this study (Banziger, 1970).
Long-term studies
A long-term study with mice and studies with rats are reported
under special studies on carcinogenicity and special study on
reproduction.
In a long-term toxicity study xylitol has been administered
orally 100 mg/xylitol/kg to 20 female and 20 male Wistar rats for
11 months and to 15 female and 15 male rats for 24 months. Body
weights were recorded weekly. At termination detailed gross pathology
was performed and a microscopic study was carried out on stomach,
intestines, liver, spleen, pancreas, kidneys, adrenals,
testes/ovaries, uterus/prostate, lungs, heart, brains, salivary
glands, lymph nodes, thyroid, thymus, sphenoid, pituitary, ganglions
of Glasser and femur. The experiment was carried out with two
generations and the fertility was also checked. The results were
compared with 450 control animals kept under similar experimental
conditions.
No malignant tumours were observed in the animals treated with
100 mg xylitol/kg and no stimulation of the growth of tumours was
noticed. According to the author there was no harmful influence on
reproduction and xylitol did not produce any pathological changes in
the animals (Mosinger, 1971).
In this experiment, however, only one very low dose level was
studied and too little animals were used. No control animals running
simultaneously with the test group were used. In addition not enough
details and no control values were shown. No comparison and no
statistical evaluation was possible. For these reasons this study has
to be considered as not adequate.
OBSERVATIONS IN MAN
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Xylitol is absorbed from the intestine very slowly compared to
glucose (Bässler et al., 1962; Dehmel et al., 1967; Förster, 1972).
After adaptation no significant rise of the absorption rate was
observed (Liebau, 1964) and although the absorption increased with
administrated dose of xylitol the percentage of absorption decreased
(Asano et al., 1972). The absorption of xylitol ranged from 49 to 95%
depending on the dose level (Asano et al., 1973; Keller and Froesch,
1972; Müller-Hess et al., 1975). After both oral and intravenous
administration of xylitol subjects showed a fast distribution in the
extracellular compartment and the tissues (Bässler et al., 1962). The
initial fast distribution phase had a half life of about four minutes,
while the apparent half life of elimination was approximately 20
minutes (Dixon and East, 1973). In studies with 14C-xylitol 90% of
C-atoms taken up could be recovered in products and intermediates of
the glycolytic and pentose phosphate pathway (Quadflieg and Brand,
1974; Müller-Hess et al., 1975). The excretion of 14C-activity in
urine is low after oral administration of 14C-xylitol (Bässler et
al., 1962). An excretion of 1-3% in urine is recorded after oral
administration and of 10% after intravenous infusion of xylitol
(Bässler, 1965). In faeces also 1% of xylitol was excreted
(Müller-Hess et al., 1975).
Metabolism
Exogenous xylitol enters the pathway by conversion to D-xylulose
by a nonspecific cytoplasmic polyol dehydrogenase. Phosphorylation
then yield D-xylulose phosphate, the link between the glucuronic acid
and the pentose phosphate pathways; the latter leads to the formation
of glyceraldehyde-3-phosphate and fructose-6-phosphate, intermediate
metabolites of the Embden-Meyerhof (glycolytic) pathway. Thus xylitol
can be metabolized via glucose-6-phosphate to glycogen and pyruvate or
lactate via the citric acid cycle to CO2 (Hollmann, 1967; Bässler,
1965; Förster, 1974; Asakura, 1967; Touster and Shaw, 1962). Xylitol
is mainly metabolized in the liver (80% to glucose only 20%) but a
small amount also in kidney, myocardium, erythrocytes, adrenal, brain,
lungs and adipose tissue (Bässler, 1965; Hollmann, 1967). Exogenous
xylitol can be metabolized in large quantities, intravenously
0.4 gm/kg/hour or 40 g/day orally raises the plasma level to a maximum
of 1.5-16 mg/100 ml (Bässler, 1965). The metabolic rate for xylitol is
identical in both healthy and diabetic or uraemic patients and
patients who suffered from liver diseases (Lang, 1972).
Effects on enzymes and other biochemical parameters
Oral ingestion of xylitol does not significantly raise the blood
glucose and insulin concentration in normal subjects and diabetics
(Lang, 1972; Bässler et al., 1962; Huthunen et al., 1975), whereas
Müller-Hess et al. (1975) found a small but significant increase in
blood glucose and insulin levels, which were the confirmed findings of
Felber (1976) and Berger et al. (1973). Administration of xylitol
caused an increased hepatic storage of glycogen (compared to glucose)
in both normal and diabetic subjects (Müller-Hess et al., 1975).
After xylitol ingestion increase of serum lactate concentration
and lactate-pyruvate ratio was observed, but to a degree less than
after glucose (Asakura, 1967; Förster, 1972, 1974). Asakura (1967)
also found a marked increase of alpha-dihydroxybutyrate. Complete
metabolism of xylitol produces 35 equivalents of ATP compared to 32
from glucose (Horecker, 1969).
Xylitol caused a fall in the serum free fatty acid level both in
healthy and diabetic subjects (Horecker, 1969; Hötzel, 1971; Pitkänen
and Sahlström, 1968; Förster, 1974). The increased hepatic uptake and
triglyceride synthesis after xylitol administration caused an
elevation of serum triglycerides, but the total serum lipid
concentration was reduced or not influenced (Förster, 1972; Horecker,
1969; Huthunen et al., 1975). The free glycerol concentration in
plasma was also significantly diminished (Müller-Hess et al., 1975).
Xylitol had a marked antiheterogenic action (30 gm xylitol were
adequate). The increased esterification of FFA due to the formation of
alpha-glycero-phosphate leads to a lowered acetyl-CoA concentration
and lowered ketone body formation (Horecker, 1969; Hötzel, 1971;
Förster, 1972). Administration of xylitol caused an inhibition of the
gluconeogenesis from proteins/amino acids. This inhibition lowers the
requirement of energy from fatty acid oxidation in the liver and by
protein-sparing effect the blood urea level and urea excretion were
reduced (Förster, 1972; Lang, 1972). Administration of high daily
doses of xylitol can provoke an increase in the serum uric acid
concentration. This results from an augmented purine biosynthesis, due
to enhanced formation of ribose phosphate (Förster, 1972; Förster et
al., 1972; Förster and Ziege, 1970; Henckenkamp and Zöllner, 1972).
TOXICOLOGICAL STUDIES
In a short-term experiment with eight normal subjects
(21-27 years) receiving orally xylitol over two weeks the fat and uric
acid metabolism was studied. After an initial adaptation phase the
xylitol level was raised from 5 g up to 50 g during seven days. No
significant changes in the serum concentration of triglycerides, FFA,
free glycerol, alpha-lipoproteins, total cholesterol, phosphatides,
aceto-acetate and ß-hydroxybutyrate were observed during xylitol
administration. The serum inorganic phosphate concentration was
elevated during the experiment and decreased again afterwards. After
an initial rise a significant decrease of the serum pyruvate level and
decrease of lactate level from the beginning were observed. The serum
uric acid level was not influenced by a xylitol intake (Mertz et al.,
1972).
After parenteral administration postoperatively of 10% xylitol
solution (1.5 g/kg body weight) Shumer (1971) found a significant
increase of lactic acid, uric acid, bilirubin and Alk.Pase in two
diabetic and two non-diabetic patients. In a short-term experiment he
carried out with two normal volunteers a dosage of 4.5 g/kg during
five days produced significant increased levels of urine uric acid,
SGOT, SAP, bilirubin, lactic acid, and inorganic phosphate in serum.
The levels returned to normal 10 days after cessation of infusion. No
effects were found in the BHN, Ca, cholesterol, glucose, amino-acids
and insulin analyses, urinalyses and haematology were found (Shumer,
1971).
In a short-term study respectively five, 15 and 30 g xylitol were
administered to healthy men during two and three weeks. Blood
chemistry, bilirubin, SGOT, Alk.Pase, uric acid and blood sugar were
normal. No diarrhoea was observed (Asano et al., 1972).
In an oral tolerance test with five persons adapted to xylitol up
to 120 g/day. The initial dose level was 30 g/day and this was raised
with 30 g/day at three day intervals. Liver function tests were normal
throughout the experiment, while there was a transient increase in
plasma lactate and urate. No diarrhoea below 90 g/day was noticed
(Amador and Eisenstein, 1971).
The tolerance of xylitol was studied in 18 male and one female
non-diabetic students aged 21-27 years. The students were given
xylitol for 21 days in increasing dose levels from five up to a
maximum of 75 g/day. After one month of interruption the same group
received xylitol in increasing dose levels from 40 g up to 220 g
again during 21 days (19 students received during the first week
40-100 g/day, 28 during the second week, 100-150 g and six during the
third week 150-220 g). The subjects themselves recorded quantity,
daily division of xylitol intake, number and consistency of bowel
movements as well as general condition and obvious side effects. Body
weights were estimated weekly. At the third day of each experimental
period and seven days after termination, fasting blood sugar analyses
and urinalyses on presence of reducing sugar were carried out. From a
130 g/day dose level diarrhoea was observed when the single doses were
poorly distributed over the day. No other significant effects were
noticed. In a similar study 23 men and three women were given xylitol
or sorbitol. The initial dose was 5 g which was increased to 75 g/day
after 14 days. In addition to the parameters investigated in the first
experiment, xylitol and glucose analyses in 24 hour urine were carried
out. Identically to the first experiment diarrhoea was the only
observed effect (Dubach et al., 1969).
In a two year tolerance study three groups of volunteers (35, 38
and 52) remained on strict diet containing respectively, fructose,
sucrose and xylitol. The average monthly amounts consumed in a varied
assortment of foods were respectively 2.1, 2.2 and 1.5 kg. The highest
daily doses of fructose and xylitol were 200-400 g. Serum samples were
analysed for Na, K, Ca, Mg, inorganic phosphates, ascorbate,
bilirubin, amylase, Alk.Pase, amino-acids, IgA, IgG and IgM. In
addition saliva analyses of IgA, IgG and IgM and amylase were carried
out. The number of occurrences of diarrhoea and flatulence-like
conditions were also scored. Body weights of the volunteers were
recorded weekly. The number of pregnancies in the groups were as
follows; eight in the sucrose, six in the fructose and eight in the
xylitol group.
No significant changes of clinico-chemical parameters in serum
and saliva were observed in the xylitol group. A significant rise in
the occurrence of diarrhoea and flatulence-like conditions was noted
in the xylitol group. All pregnancies, deliveries and infants were
normal.
A series of studies in Turku, Finland showed that after one year
on test in which xylitol replaced sucrose in the diet, dental caries
were reduced in the xylitol group by approximately 90% (Scheinin,
1974).
A more recent study involved 100 human young adults. Subjects
were divided into S-(sucrose) group, and X-(xylitol) group.
Consumption was 4.0 chewing gums per subject per day in the S-group
and 4.5 in the X-group. Frequency of sucrose intake was 4.2 times per
day in the S-group, and 4.9 in the X-group. The caries incidence was
2.92 in the S-group and -1.04 in the X-group. The results show a
profound difference in the caries increment rate between the two
experimental groups (Scheinin et al., 1975).
In an accident that happened in 1969/1970 in Australia, 20
patients who had received Japanese xylitol from two specific batches
by intravenous infusion died with symptoms of severe acidosis, liver
necrosis and renal oxalosis, and in one case oxalate crystals were
also found. The patient with the severest and most dramatic liver
damage had received only 200 g of xylitol. In these xylitol batches a
highly toxic substance was determined by the chicken embryo test.
Coats (1970) identified 2-mercaptoimidazole and 2-hydroxyimidazole in
the xylitol batches. 2-mercaptoimidazole was used as a plasticizer of
the rubber stoppers of the ampoules. All further efforts to obtain
material from the suspect batches from the Japanese firm failed, so
that no explanation for the incidents could be found (Lang, 1974).
In 1971/1972 oxalate crystals were observed in the kidneys and
brain in necropsies performed at the Rafenkrankenhaus in Hamburg on
five patients who had received infusions of xylitol. According to the
doctors responsible for their treatment they died of the severe
conditions from which they had been suffering. These findings gave
rise to extensive discussions as to whether xylitol was involved in
the causation-of oxalosis. However, it is most improbable that the
oxalosis observed in Hamburg was due to a specific toxic action of
xylitol. Further investigation of this subject has to be carried out
(Lang, 1974).
Tolerance studies in diabetes
In addition no harmful effects were observed in a diabetic person
who consumed 65 g xylitol/day during the two years experimental period
(Mäkinen and Scheinin, 1975; Mäkinen, 1976).
Acute tests with xylitol were conducted on 13 recruited adult
diabetics and the following parameters were determined: aceto-acetate,
ß-hydroxy-butyrate, unesterized fatty acid (UFA), free glycerine and
total lipids. A significant drop in concentrations of aceto-acetate
and ß-hydroxybutyrate occurred under administration of xylitol as
compared with the control experiments. UFA behaved similarly. The
concentration of free glycerine also clearly dropped at the end of the
experiment. It can be assumed from the results that the diminished
lipolyserate is the cause of reduced formation of ketone bodies. A
drop in total lipids indicated that hyperlipidemia might possibly also
be favourably influenced by xylitol (Grabner, 1971).
Xylitol in concentration varying from 5 to 20 gm in food was
administered to 23 diabetic persons. Except for diarrhoea in some
cases no harmful side effects were observed. No negative influence on
the diabetie metabolism was observed. Xylitol (25 g) dissolved in tea
caused more frequent diarrhoea and other abdominal side effects in the
subjects (Mellinghof, 1961).
Yamagata et al. (1967) investigated the effects of acute
intravenous infusion of 30 g/90 minutes on blood Sugar, pyruvate and
lactate, and on plasma free fatty acids (FFA) and insulin levels in
six normal and 11 diabetic persons. Xylitol infusion produced no
change of blood sugar in normal and a slight increase in diabetic
patients. Blood lactate showed a significant increase in the diabetic
group but only a slight increase in the normal group. Blood ketone
bodies and plasma FFA decreased in both groups after xylitol
administration. Plasma insulin increased slightly in five of the
normal subjects and in four of the nine diabetic subjects (Yamagata
et al., 1967).
In another experiment xylitol was chronically intravenously
infused to 12 mildly diabetic patients every morning for seven days.
No changes were observed in fasting blood sugar, serum triglycerides,
total cholesterol, serum electrolytes or ketone bodies. However, four
cases indicated the marked decrease of urine sugar excretion during
the experimental period. Xylitol was also given to two diabetic
persons with ketosis and decreased the ketone bodies from 5.1 to
0.2 mg % within four hours (Yamagata et al., 1967).
During two to six weeks 30 g xylitol/day was given orally to 12
stable diabetics. The caloric intake and amount of carbohydrates was
kept constant. Changes in fasting blood sugar, urine sugar, serum
lipids, serum mucoprotein, SGOT and SGPT were studied before, during
and after xylitol administration. In three of the 12 cases urinary
sugar excretion disappeared. A slight impairment of glucose tolerance
and a tendency to decreased SGOT and SGPT levels were observed. In
some cases diarrhoea was noticed, but no other effects of xylitol were
noticed (Yamagata et al., 1967).
Eighteen diabetic children received 30 g dietary xylitol during
four weeks. A significant elevation of the uric acid concentration in
serum was observed, also significant increases of total protein
content and of inorganic phosphorus. In these children no diarrhoea
was noticed (Förster et al., 1977).
REFERENCES
Amador, F. and Eisenstein, A. (1971) The effects of oral xylitol
administration in human subjects. Unpublished data cited by Brin, M.
and Miller, O. N. (1974) The safety of oral xylitol; Sugars in
Nutrition; Ed. Sipple and McNutt, 33, 591-606
Asakura, T. (1967) Non-glycolytic sugar-metabolism in human
erythrocytes 1. Xylitol metabolism, J. Biochem., 62, 184-193
Asano, T., Levitt, M.D. and Goetz, F. C. (1972) Xylitol absorption in
healthy men, Diabetes., 21, suppl. 1, 350-351
Asano, T., Levitt, M.D. and Goetz, F. C. (1973) Xylitol absorption in
healthy men, Diabetes, 22, 279-281
Bächtold, H. P. (1972) Prüfung in 5-Tage-Toxizitätsversuch an Ratten
und Mäusen. Unpublished report from the Research Department of
Hoffmann La Roche Co., submitted to the World Health Organization by
Hoffmann La Roche Co., Basle, Switzerland
Banziger, R. (1970) A thirteen week oral toxicity study of xylitol in
monkeys. Unpublished report of Hazleton Laboratories Inc., submitted
to the World Health Organization by Hoffmann La Roche Co., Basle,
Switzerland
Bässler, K. H. (1965) Biochemie und Stoffwechsel von Xylit (1965)
Dtsch. Lebensm. Rdsd., 61, 171-173
Bässler, K. H. und Prellwitz, W. (1964) Insulin und der
Verteilungsraum von Xylit bei eviscerierten Ratten, Klin.
Wocherschrift, 42, 94-95
Bässler, K. H., Prellwitz, W., Unbehaun, V. und Lang, K. (1962)
Xylitstoffwechsel beim Menschen, Klin. Wschr., 40, 791
Bässler, K. H. und Reimold, W. V. (1965) Lactaatbildung aus Zuckern
und Zuckeralkoholen in Erythrocyten, Klin. Wschr., 43, 169-171
Bässler, K. H. und Wagner, G. (1970) Fettsäuregehalt der Rattenleber
unter verschiedenen Bedingungen der parenteralen Ernährung
Berger, W., Göschke, H., Moppert, J. and Künzli, J. (1973) Insulin
concentration in portal venous and peripheral venous blood in man
following administration of glucose, galactose, xylitol and
tolbutamide, Horm. Metab. Res., 5, 4-8
Birnesser, H., Reinauer, H. and Hollmann, S. (1973) Comparative study
of enzyme activities degrading sorbitol, ribitol, xylitol and
gluconate in guinea-pigs' tissues, Diabetologia, 9, 30-33
Brin, M. and Miller, O. N. (1974) The safety of oral xylitol. Sugars
in Nutrition, Ed. Sipple, H. L. and McNutt, K. W., Acad. Press
Coats, D. A. (1970) Report to the Deutsche Arzeimittelkommission 1970.
Cited by K. Lang (1974) (Unpublished report)
Dehmel, K. H., Förster, H. and Mehnert, H. (1967) Absorption of
xylitol. Int. Symp. on metabolism, physiology and clinical use of
pentoses and pentitols, Hakone, Japan, 1967, 177-181, Ed. Horecker,
B. L. e.a.
Dixon, R. and East, S. (1973) Xylitol levels in plasma following
intravenous and oral administration to man. Unpublished report,
Psycho-Endocrine Centre, St. James's Hospital, Dublin 8, Ireland,
submitted to the World Health Organization by Hoffmann La Roche Co.,
Basle, Switzerland
Dubach, U. C., Feiner, E. and Fargo, E. (1969) Orale Verträglichkeit
von Xylitol bei stoff wechselgesunden Probanden, Schweiz. Med.
Wschr. 99, 190-194
Felber, J.P. (1976) Untersuchung der Wirkung des Xylits auf den
Blutzuckerspiegel beim Menschen, Abstract einer Expertise vom 24-6-76.
Sitzung der Eidg. Ernährung Kommission in Bern
Förster, H. (1972) Grundlagen für die Verwendung der drei
Zuckeraustauschstoffe Fructose, Sobit und Xylit, Med. Ernähr.,
13, 7-15
Förster, H. (1974) Zum Stoffwechsel von Monosacchariden und Polyolen,
Ernährungs. Med., 1, 37-55
Förster, H., Boecker, S. and Ziege, M. (1972) Anstieg der
Konzentration der Serumharnsäure nach akuter und chronische Zufuhr von
Saccharose, Fructose, Sorbitol und Xylit, Med. u. Ernähr.,
13, 193-196
Förster, H., Boecker, S. and Walther, A. (1977) Verwendung von Xylit
als Zuckeraustauschstoff bei diabetischen Kindern, Fortschr. Med.,
95, nr. 2, 99-102
Förster, H. und Hoffmann, H. (1973) Biochemische Überlegungen zur
Verwendung der Kohlenhydrate in der parenteralen Ernährung,
Infusiontherapie, 1, 265-279
Förster, H. und Ziege, M. (1970) Anstieg der
Senumharnsäurekonzentration nach oraler Zufuhr von Fructose, Sorbit
und Xylit, Z. Ernährungswiss., 10, 394-396
Froesch, E. R. (1972) Übersicht über den Haushalt der Betriebstoffe
mit besonderer Berücksichtigung des Stoffwechsels von Glukose,
Fruktose, Sorbit und Xylit und deren therapeutische Verwendbarkeit,
Int. Z. Vit. und Ernährungsforsch., Suppl. 12, 73-86
Froesch, E. R. (1975) Umsatz und Verwertung von Glukose und
Glukoseaustauschstoffen bei Mensch und Tier, Nutr. Metabol., 18
(suppl. 1), 30-44
Froesch, E. R. and Jakob, A. (1974) The metabolism of xylitol. Sugars
in nutrition, Ed. H. L. Sipple and K. W. McNutt, Academic Press,
241-258
Grabner, W., Berger, G., Bergner, D. and Matzkies, F. (1971) Über den
Einfluss des Xylits auf den Fettstoffwechsel von Diabetikern,
Med. Ernährung, 12, 126-128
Grunberg, E., Beskid, G. and Brin, M. (1973) Xylitol and Dental
Caries. Efficacy of xylitol in reducing dental caries in rats,
Internat. J. Vit. Nutr. Res., 43, 227-232
Grütte, F. K. and Rödel, H. (1975) Zur Bedeutung des Zuckeralcohols
Xylit als Zuckeraustauschstoff Ernährungsforschung, 3, nr. 20, 74-79
Haydon, R. K. (1961) The antiketogenic effects of polyhydric alcohols
in rat-liver slices, Biochim. Biophys. Acta., 46, 598-599
Heuckenkamp, P. U. und Züllner, N. (1972) Xylitbalanz während
mehrstündiger infusionen mit konstanten Zufahrraten bei gesunden
Manschen, Klin. Wschr., 50, 1863-1864
Hirata, Y., Fujisawa, M. and Ogushi, T. (1967) Effects of intravenous
injection of xylitol on plasma insulin. Int. Symp. on metabolism,
physiology and clinical use of pentoses and pentitols, Hakone, Japan,
Ed. Horecker, B. L. et al.
Hirata, Y., Fujisawa, M., Sato, H., Asano, T. and Katsuki, S. (1968)
Effects of intravenous injection of xylitol on blood sugar, blood
pyruvic acid and plasma insulin levels in the dog, Z. für dieo
gesamte exptl. Med., 145, 111-119
Hollmann, S. (1967) Pentitol-metabolizing enzymes of the uronic acid
pathway, Int. Symp. on metabolism, physiology and clinical use of
pentoses and pentitols, Hakone, Japan, 97-108
Horecker, B. L. (1969) Stoffwechselwege der Kohlenhydrate: ihre
Regulierung und physiologische Bedeutung. III Stoffwechsel und
physiologische Wirkungen von Xylit, Med. Ernährung., 10, 66-69,
95-98, 127-129
Hosoya, N. and Machiya, T. (1967) Xylitol effect on ketogenesis in
alloxan diabetic rat liver slice, Int. Symp. on metabolism, physiology
and clinical use of pentoses and pentitols, Hakone, Japan, 1967, Ed.
Horecker, B. L. et al.
Hosoya, M., Sakurai, T. and Shimazono, N. (1966) Glycogen and
glycolytic intermediates in Alloxan-diabetic liver slices, VIIth
International Congress of Nutrition, Hamburg
Hosoya, M. and Iitoyo, M. (1969) Effects of xylitol administration on
the development (growth) of rats (cited in Brin and Miller, 1974)),
J. Jap. Soc. Food Nutr., 22, 17-20
Hötzel, D. (1971) Über den Intermediärstoffwechsel von Sorbit, Xylit
und Cyclamaten, Dtsch. Zahnärzl. Z., 26, 1035-1040
Hummler, H. (1974) Orientierende Verträglichkeitsuntersuchungen über 6
Wochen mit Xylit, Sorbit und Saccharose am Hund. Unpublished report
from Huntingdon Research Centre, submitted to the World Health
Organization by Hoffmann La Roche Co., Basle, Switzerland
Hummler, H. (1976a) Reproduktionstoxikologische Untersuchungen mit
Xylit an der Rätte. Unpublished report from Huntingdon Research
Centre, submitted to the World Health Organization by Hoffmann La
Roche and Co., Basle, Switzerland
Hummler, H. (1976b) Cancerogenese Untersuchungen mit Xylit an der
Maus. Unpublished interim report from Huntingdon Research Centre,
submitted to the World Health Organization by Hoffmann La Roche and
Co., Basle, Switzerland
Hummler, H. (1976c) Chronische Toxizitäts- und Carcinogenese-
Untersuchungen mit Xylit an Ratten. Unpublished report from Huntingdon
Research Centre submitted to the World Health Organization by Hoffmann
La Roche and Co., Basle, Switzerland
Hummler, H. (1976d) Chronische Verträglichkeitsprüfung mit Xylit an
Hunden (52 and 78 w.). Unpublished report from Huntingdon Research
Centre, submitted to the World Health Organization by Hoffmann La
Roche and Co., Basle, Switzerland
Huthunen, J. K., Mäkinen, K. K. and Scheinin, A. (1975) Effects of
sucrose, fructose and xylitol diets on glucose, lipid and urate
metabolism, Acta Odont. Scand., 33, suppl. 70, 239-245
Jakob, A., Williamson, J. R. and Asakura, T. (1971) Xylitol metabolism
in perfused rat liver. Interactions with gluconeogenesis and
ketogenesis, J. Biol. Chem., 246, No. 24, 7623-7631
Jourdan, M. H., McDonald, J. and Henderson, J. R. (1972) The effects
of 14C-xylitol, given intravenously, on the serum glucose, insulin
and lipid concentrations of male and female baboons, Nutr. Metabol.,
14, 92-99
Jursons, K. K., Puzaka, J. J., Aalmans, A. R. and Vitolin, S. P.
(1974) Izvestija Akademii Nauh Latvijskoj SSR., 9, 16-22
Keller, U. and Froesch, E. R. (1972) Vergleichende Untersuchungen über
den Stoffwechsel von Xylit, Sorbit und Fruktose beim Menschen,
Schwerz. Med. Wschr., 102, 1017-1022
Kieckebusch, W., Gziem, W. und Lang, K. (1961) Die Verwertbarkeit von
Xylit als Nahrungskohlenhydrat und seine Verträglich, Klin. Wschr.,
39, 447
Kuzyha, T., Kanazawa, Y. and Kosaka, K. (1966) Plasma insulin response
to intravenously administered xylitol in dogs, Metabolism, 15, No.
12, 1149-1152
Kuzuya, T., Kanazawa, Y. and Kosaka, K. (1969) Stimulation of insulin
secretion by xylitol in dogs, Endocrinology, 84, 200-207
Lang, K. (1964) Die ernährungsphysiologischen Eigenschaften von Xylit,
Internat. Z. Vitaminforsch., 34, 117-122
Lang, K. (1972) Xylit - Biochemie und Klinik, Suppl. 13 zur
Z. Ernährungswiss: "Rehabilitation durch Ernährung" Darmstadt 11-15
(symp. CSSR)
Lang, K. (1974) Xylit in der oralen und parenteralen Ernährung Heft
der Schriftenreihe des Bundes für Lebensmittelrecht und
Lebensmittelkund, 75, 21-23
Liebau, K. (1964) Untersuchungen der Resorption des Xylit beim
Menschen Dissertation of K. Liebau, submitted to the World Health
Organization by Hoffmann La Roche Co., Basle, Switzerland (Unpublished
report)
Mäkinen, K. K. (1976) Long-term tolerance of healthy human subjects
to high amounts of xylitol and fructose: general and biochemical
findings. In: Monosaccharides and polyalcohols in nutrition
therapy and diabetics, Ed. G. Ritzel, G. Brubacher, Verlag H.
Huber, Int. J. Vit. Nutr. Res. nr., 15
Mäkinen, K. K. and Scheinin, A. (1975) Effect of the diet on certain
clinico-chemical values of serum, Acta Odont. Scand., 33, suppl.
70, 265-276
McCormick, D. B. and Touster, O. (1957) The conversion in vivo
of xylitol to glycogen via the pentose phosphate pathway, J. Biol.
Chem., 239, 451-461
Mehnert, H., Summa, J. D. and Fürster, H. (1964) Untersuchungen zum
Xylitstoffwechsel bei gesunden, Leberkranken und diabetischen
Personen, Klin. Wschr., 42, 382
Mellinghoff, G. H. (1961) Über die Verwendbarkeit des Xylit als
Ersatzzucker bei Diabetikern, Klin. Wschr., 39, 447
Meng, H. C. (1974) Sugars in parenteral nutrition. Sugars in
nutrition, Ed. H. L. Sipple, K. W. McNutt, Academic Press, 527-566
Mertz, D. P., Kaiser, V., Klöpfer-Zaar, M. and Beisbarth, H. (1972)
Serumkonzentrationen verschiedener Lipide und von Harnsäure während 2
wüchiger Verabreichung von Xylit, Klin. Wschr., 50, 1107-1111
Montague, W., Howell, S. L. and Taylor, K. W. (1967) Pentitols and the
mechanism of insulin release, Nature, 215, 1088-1089
Mosinger, M. (1971) Effects of the long-term oral administration of
xylitol. Unpublished report of Mosinger, M., submitted to the World
Health Organization by Hoffmann La Roche, Basle, Switzerland
Müller-Hess, R., Geser, C. A., Bonjour, J.P., Jequier, E. and Felber,
J.P. (1975) Effects of oral xylitol administration on carbohydrate and
lipid metabolism in normal subjects, Infusionstherapie, 2, 247-252
Opitz, K. (1967) The influence of xylitol and other polyols and sugars
on fat mobilization, Int. Symp. on metabolism, physiology and clinical
use of pentoses and pentitols, Hakone, Japan, Ed. Horecker, B. L. et
al.
Oshinsky, R. J., Wang, Y. M. and Eys, J. v. (1976) Oxalate formation
following xylitol infusion in 4-deoxypyridoxine treated rabbits,
Fed. Proc., 35, 419
Pazenko, A. G. (1969) Voprosy prolilaktiki, diagnostiki i lecenija
zabolevanij organor, Piscevarenija, Kiev, 133
Petrich, Ch., Reinauer, H. and Hollmann, S. (1972) Vergleichende
Aktivitätsmessungen der Enzyme des Glucuronsäure Xylulose-Zyklus
in der Leber, dem epididymalen Fettgewebe der Ratte, in einem
Morris-Hepatoom und einer Fibrocyten-Kultur, Z. Klin. Chem. Klin.
Biochem., 10, 355-358
Pitkänen, E. and Sahlström, K. (1968) Increased excretion of
xylitol after administration of glucorone, lactone and ethanol in
man, Ann. Med. exp. Fenn., 47, 143-150
Pool, W. and Hane, D. (1970) Acute toxicity and dog tolerance testing
of xylitol. Unpublished report from the Research Department of
Hoffmann La Roche Co., submitted to World Health Organisation by
Hoffmann La Roche Co., Basle, Switzerland
Pool, W. and Hane, D. (1972) Acute mouse toxicity of xylitol.
Unpublished report from the Research Department of Hoffmann La Roche
Co., submitted to the World Health Organization by Hoffmann La Roche
Co., Basle, Switzerland
Quadflieg, K. H. and Brand, K. (1974) Xylitol metabolism in human
erythrocytes, Z. Phys. Chemie, 355, 1239
Scheinin, Ulla (1974) Incidence of dental caries among a group of
university students in Turkey, Acta. Odont. Scand., 32, 335-344
Scheinin, Arje., Mäkinen, Kauko K., Tammisalo, Erkki., Rekola, Maarit.
(1975) Turku sugar studies. XVIII. Incidence of dental caries in
relation to one-year consumption of xylitol chewing gum, Acta.
Odont. Scand., 33, 269-278
Schmidt, B., Fingerhut, N. and Lang, K. (1964) Über den Stoffwechsel
von radioaktiv markiertem Xylit bei der Ratte, Klin. Wschr., 42,
1073-1077
Shima, S., Mitsunaga, M. and Nakao, T. (1970) Effect of xylitol on
testicular protein and RNO biosynthesis of rats, Endocrinol. Jap.,
17, 283-287
Shumer, W. (1971) Adverse effects of xylitol in parenteral
alimentation Metabolism., 20, 345-347
Staub, H. W. and Thiessen, R. (1972) Effect of dietary polyols on
serum cholesterol in rats, Federation Proc., 31, 291
Stein, G. (1966) Adaptive Prozesse im Xylitstoffwechsel und ihre
Auswirkungen Dissertation Mainz, 1966, submitted to the World Health
Organization by Hoffmann La Roche and Co., Basle, Switzerland
(Unpublished report)
Swarm, R. L. and Banziger, R. (1970) A thirteen week oral feeding
study in rats with xylitol (Ro-6-7045). Unpublished report of Hazleton
Laboratories Inc., submitted to the World Health Organization by
Hoffmann La Roche, Basle, Switzerland
Touster, O. (1970) Parenteral nutrition (Proc. on an internat. Symp.
Vanderbilt University, School of Medicine, Nashville) 131-138, Ed.
Meng, H. C., Law, D. H., Thomas, C. C., Publisher Springfield,
Illinois, USA
Touster, O. (1974) The metabolism of polyols. In: Sugars in Nutrition,
Ed. H. L. Sipple, K. W. McNutt, Academic Press, 229-239
Touster, O. and Shaw, D. (1962) Biochemistry of the acyclic polyols,
physiol. Rev., 42, 181-225
Truhaut, R., Coquet, B., Fouillet, X., Galland, I., Guyot, D., Rouaud,
J. L. and Long, D. W. (1977) Subacute toxicity of xylitol in rats.
Personal communication to the World Health Organization (Unpublished
report)
Wagner, G. and Bässler, K. H. (1971) Gibt es Unterschiede in der
antiketogenen Wirkung gebräuhlicher Zucker und Polyalkohole, Medizin
Ernährung., 12, 53-54
Wang, Y. M., King, S. M., Patterson, J. H. and Van Eys, J. van. (1973)
Mechanism of xylitol toxicity in the rabbit, Metabolism, 22, nr.
7, 885-894
Wang, Y. H., Oshinky, R. J., Lantin, E. and Eys, J. van. (1975) An
investigation of oxalate formation from xylitol infusion in rabbits,
Fed. Proc., 35, 920
Wilson, R. B. and Martin, J. M. (1970) Plasma insulin concentrations
in dogs and monkeys after Xylitol, glucose or tolbutamide infusion,
Diabets., 19, 17-22
Woods, H. F. (1975) Hepatic metabolism of pentites, Nutr. Metabol.,
18 (Suppl. 1), 65-78
Woods, H. F. and Krebs, H. A. (1973) Xylitol metabolism in the
isolated perfused rat liver, Biochem. J., 134, 437-443
Yamagata, S., Goto, Y., Ohneda, A., Anzai, M., Kwashima, S., Kikuchi,
J., Chiba, M., Maruhama, Y., Yamauchi, Y. and Toyota, T. (1967)
Clinical application of xylitol in diabetics, Int. Symp. on
metabolism, physiology and clinical use of pentoses and pentitols,
Hakone, Japan, 316-325, Ed. Horecker, B. L. et al.
