
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
Toxicological evaluation of certain veterinary drug
residues in food
WHO FOOD ADDITIVES SERIES 39
Prepared by:
The forty-eighth meeting of the Joint FAO/WHO Expert
Committee on Food Additives (JECFA)
World Health Organization, Geneva 1997
CYFLUTHRIN
L. Ritter and M.J. Chappel
Canadian Network of Toxicology Centres
University of Guelph, Guelph, Ontario, Canada
1. Explanation
2. Biological data
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
2.1.2 Biotransformation
2.2 Toxicological studies
2.2.1 Acute toxicity
2.2.2 Short-term toxicity
2.2.3 Long-term toxicity and carcinogenicity
2.2.4 Genotoxicity
2.2.5 Reproductive toxicity
2.2.5.1 Multigeneration reproductive toxicity
2.2.5.2 Developmental toxicity
2.2.6 Special studies on neurotoxicity
2.2.7 Special studies on tumour promotion
2.3 Observations in humans
3. Comments
4. Evaluation
5. References
1. EXPLANATION
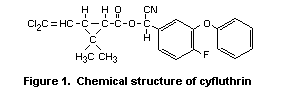
Cyfluthrin (Figure 1) is a synthetic cyano-containing pyrethroid
insecticide. It has a broad spectrum of activity and is used as an
effective ectoparasiticide in industrial settings, as an effective
vector control agent, and in some countries for the protection of
stored products. Cyfluthrin can be formulated as wettable powders,
emulsifiable concentrates, oil-in-water emulsions, oily solutions,
concentrates, and dust formulations. These formulations can be applied
as sprays, dusts, fogs, and aerial sprays. The oil formulations are
commonly used to protect cattle from infestation by flies, lice, and
tabanids. Technical-grade cyfluthrin consists of a mixture of four
diastereomeric pairs of enantiomers (giving rise to eight optical
isomers), which consist of two cis and two trans isomeric pairs.
Cyfluthrin is manufactured with a typical purity of 92% (sum of all
isomers), with six impurities identified chemically.
Cyfluthrin was evaluated by the 1987 Joint FAO/WHO Meeting on
Pesticide Residues (WHO/FAO, 1988), which established an ADI of 0-0.02
mg/kg bw. Cyfluthrin has not previously been evaluated by the Joint
Expert Committee on Food Additives.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
The influence of the formulation vehicle on the rate of
absorption of cyfluthrin was studied in groups of 14 fasted male
Wistar rats given single doses by gavage of 10 mg/kg bw dissolved in
either polyethylene glycol (PEG) 400 or a Cremophor EL:water emulsion.
Two rats from each group were killed 0.5, 1, 2, 4, 6, 16, and 24 h
after treatment. The concentrations of cyfluthrin and its respective
enantiomers were determined in the blood and stomach. When the
compound was emulsified in the Cremophor EL:water solution, absorption
was rapid; the cis isomer was that most frequently detected within
30 min after treatment. Maximum peak blood concentrations occurred
within 1 h of treatment. Cyfluthrin emulsified in PEG 400 was not
detected until 4 h after administration, and peak blood levels were
observed only 6 h after treatment. Examination of the stomach contents
revealed a larger quantity of cyfluthrin in the stomachs of rats
treated with the drug in a PEG 400 emulsion (Eben et al., 1982).
Rats
Four groups of 30 male and 24 female Mura:SPRA (SPF 68 Han) rats
received 14C radiolabelled cyfluthrin as either oral doses of 0.5 or
10 mg/kg bw or intravenous or intraduodenal doses of 0.5 mg/kg bw.
Another group received unlabelled cyfluthrin orally once a day for 14
consecutive days, followed by a single oral dose of 0.5 mg/kg bw
14C-cyfluthrin. Excreta, organs, tissues, and blood samples were
collected at several intervals and assayed for radiolabel.
After oral administration by any schedule, up to 80% of the
labelled cyfluthrin was effectively absorbed by females and about 90%
by males. The half-time for absorption was 30-32 min, and absorption
commenced 13 min after administration. The absorption half-time was
dependent on neither dose, sex, nor pretreatment. Excretion was rapid
and proportional to dose. Less than 2% of radiolabelled drug was
present 48 h after oral administration. No significant pulmonary
excretion pathway exists, as < 0.001% was detected in the expired
gaseous phase as 14CO21. About 98-99% of the orally administered
dose was readily available for renal and faecal excretion, which would
be expected to be eliminated within two days. Males excreted two to
three times more in the urine than in the faeces, whereas the
renal:faecal excretion ratio in females was 1.2-1.7:1 after oral
administration. Consequently, the area under the curve (AUC) is two
times larger for females than males.
Forty-eight hours after the intravenous administration, 93-95% of
the dose was excreted, with a renal:faecal excretion ratio of 2.9:1 in
males and 2.3:1 in females. Therefore, excretion depends somewhat on
the route of administration and on sex. Rats with bile fistulae
excreted one-third of the dose within two days, more than 50% of which
was excreted within 2 h and 90% within 6 h of administration. These
rats excreted about 50% of the recovered radioactivity via the urine
and < 12% via faeces. A proportion of the radiolabelled dose excreted
in bile underwent enterohepatic circulation.
The apparent volume of distribution after the intravenous
treatment was calculated to be 17%, indicating distribution primarily
to the extracellular fluid. Plasma elimination, i.e. distribution to
the tissues, was biphasic, with an initial rapid half-time of 2.1 h
and then a slower, dose- and pretreatment-independent half-time of
21 h. The maximum relative concentration in the plasma was independent
of dose and was observed 2 h after oral administration in male and
female rats.
The residues found in the organs and tissues were influenced by
the route of administration, as the mean relative concentration of
cyfluthrin in the bodies of males and females at sacrifice was lower
after oral administration (0.013) than after intravenous injection
(0.06). Female rats had higher plasma concentrations after oral
administration of the single high or low dose; 48 h after
administration, lower concentrations were detected in the bone and
muscle of animals of each sex and in the testes of males rats. The
sciatic nerve showed a similar relative concentration value, which may
explain the toxic effects observed on the peripheral nervous system.
Higher concentrations were detected in the spleen, adrenal glands,
liver, and plasma of both males and females and in the ovaries. The
renal fatty tissue concentration was about seven times higher after
either oral or intravenous administration, whereas the mean
concentration in brain was significantly lower ( p = 0.0006-0.006)
(Klein et al., 1983).
2.1.2 Biotransformation
Rats
Eight hours after oral administration of 14C-labelled
cyfluthrin at a dose of 10 mg/kg bw to three male Sprague-Dawley rats,
about 60% of the labelled cyfluthrin was eliminated in the urine in
conjugated forms. Conjugates of 4'-hydroxy-3-phenoxyfluorobenzoic acid
(50%) were identified; a second major metabolite was identified after
hydrochloric acid hydrolysis as a conjugate of
3-phenoxy-4-fluorohippuric acid (40%). These metabolites represented
33 and 27% of the administered radiolabel, respectively. A glycine
conjugate constituted 2.5% of the conjugated metabolites (Ecker,
1982).
After intraperitoneal injection of 14C-cyfluthrin to Wistar
(TNO/W 74) albino rats at doses of 1, 5, 10, or 15 mg/kg bw, urine was
collected for three days. It was found that 24-42% of the alpha-cyano
group of cyfluthrin was excreted via the kidneys in the thiocyanate
form (Eben & Thyssen, 1981).
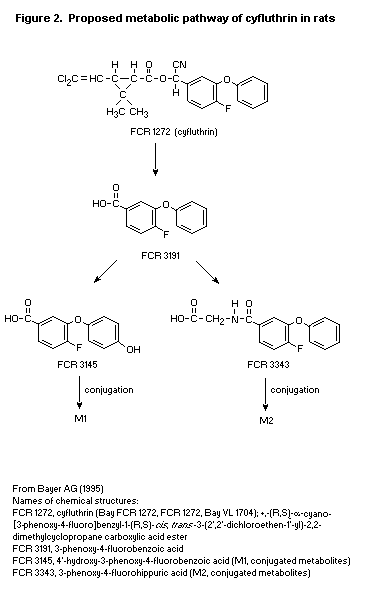
In another study carried out to characterize the complete kinetic
behaviour and metabolic pathway of cyfluthrin in rats, four groups of
five male and five female rats received 14C-cyfluthrin by a protocol
identical to that described by Klein et al. (1983). The initial step
in cyfluthrin biotransformation was ester hydrolysis, giving a
3-phenoxy-4-fluorobenzyl alcohol intermediate and the permethric acid
fraction. The metabolism of permethric acid has been well established
in the rat in studies with chemically similar pyrethroids. After ester
hydrolysis, the 3-phenoxy-4-fluorobenzyl alcohol moiety was oxidized
to the free metabolite 3-phenoxy-4-fluorobenzoic acid. This metabolite
can then either be conjugated with glycine to form
3-phenoxy-4-fluorohippuric acid (a minor metabolite constituting < 3%
of the recovered urinary radiolabel, dependent on neither sex nor
dose) or hydroxylated to give 4'-hydroxy-3-phenoxy-4-fluorobenzoic
acid (conjugates of which account for 41-50% of the total urinary
radiolabel recovered from rats given one or multiple doses of
cyfluthrin at 0.5 mg/kg bw). Females tended to excrete more of this
metabolite as the free form in the faeces than did males. Males and
females at the high dose (10 mg/kg bw) excreted about 35% of the
administered dose as conjugates of
4'-hydroxy-3-phenoxy-4-fluorobenzoic acid, whereas females excreted
about 5% more than males as the free metabolite. After repeated oral
doses of 0.5 mg/kg bw for 14 days, 12-16% of labelled metabolite was
found in the faeces as cyfluthrin, whereas < 1% was found when single
oral doses were administered. After a single high dose of 10 mg/kg bw,
17-19% was recovered in the faeces as parent compound. The authors
concluded that the metabolism of cyfluthrin is slightly dose-dependent
(Ecker, 1983).
The proposed metabolic pathway of cyfluthrin in rats is shown in
Figure 2.
Cattle
A 484-kg lactating Holstein dairy cow (Bos taurus) was given
14C-cyfluthrin orally at a dose of 0.5 mg/kg bw after the evening
milking each day for five consecutive days. Milk was collected in the
morning and evening throughout the study. The cow was sacrificed on
day 6 after initial administration, and blood, major organs, fatty
tissue, and muscle were analysed for radiolabel. The maximum
concentration of radioactivity in the milk was 0.079 mg/litre
cyfluthrin equivalents three days after initial dosing; the level then
declined steadily. Of the radiolabel found in milk, 98% was on
unmetabolized parent compound. The highest levels of residues were
found in the liver (0.622 mg/kg), fat (0.195 mg/kg), and kidney (0.188
mg/kg); brain, skeletal muscle, and myocardium had the lowest levels,
ranging from 0.015 to 0.040 mg/kg. Most of the radiolabel recovered in
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
The influence of the formulation vehicle on the rate of
absorption of cyfluthrin was studied in groups of 14 fasted male
Wistar rats given single doses by gavage of 10 mg/kg bw dissolved in
either polyethylene glycol (PEG) 400 or a Cremophor EL:water emulsion.
Two rats from each group were killed 0.5, 1, 2, 4, 6, 16, and 24 h
after treatment. The concentrations of cyfluthrin and its respective
enantiomers were determined in the blood and stomach. When the
compound was emulsified in the Cremophor EL:water solution, absorption
was rapid; the cis isomer was that most frequently detected within
30 min after treatment. Maximum peak blood concentrations occurred
within 1 h of treatment. Cyfluthrin emulsified in PEG 400 was not
detected until 4 h after administration, and peak blood levels were
observed only 6 h after treatment. Examination of the stomach contents
revealed a larger quantity of cyfluthrin in the stomachs of rats
treated with the drug in a PEG 400 emulsion (Eben et al., 1982).
Rats
Four groups of 30 male and 24 female Mura:SPRA (SPF 68 Han) rats
received 14C radiolabelled cyfluthrin as either oral doses of 0.5 or
10 mg/kg bw or intravenous or intraduodenal doses of 0.5 mg/kg bw.
Another group received unlabelled cyfluthrin orally once a day for 14
consecutive days, followed by a single oral dose of 0.5 mg/kg bw
14C-cyfluthrin. Excreta, organs, tissues, and blood samples were
collected at several intervals and assayed for radiolabel.
After oral administration by any schedule, up to 80% of the
labelled cyfluthrin was effectively absorbed by females and about 90%
by males. The half-time for absorption was 30-32 min, and absorption
commenced 13 min after administration. The absorption half-time was
dependent on neither dose, sex, nor pretreatment. Excretion was rapid
and proportional to dose. Less than 2% of radiolabelled drug was
present 48 h after oral administration. No significant pulmonary
excretion pathway exists, as < 0.001% was detected in the expired
gaseous phase as 14CO21. About 98-99% of the orally administered
dose was readily available for renal and faecal excretion, which would
be expected to be eliminated within two days. Males excreted two to
three times more in the urine than in the faeces, whereas the
renal:faecal excretion ratio in females was 1.2-1.7:1 after oral
administration. Consequently, the area under the curve (AUC) is two
times larger for females than males.
Forty-eight hours after the intravenous administration, 93-95% of
the dose was excreted, with a renal:faecal excretion ratio of 2.9:1 in
males and 2.3:1 in females. Therefore, excretion depends somewhat on
the route of administration and on sex. Rats with bile fistulae
excreted one-third of the dose within two days, more than 50% of which
was excreted within 2 h and 90% within 6 h of administration. These
rats excreted about 50% of the recovered radioactivity via the urine
and < 12% via faeces. A proportion of the radiolabelled dose excreted
in bile underwent enterohepatic circulation.
The apparent volume of distribution after the intravenous
treatment was calculated to be 17%, indicating distribution primarily
to the extracellular fluid. Plasma elimination, i.e. distribution to
the tissues, was biphasic, with an initial rapid half-time of 2.1 h
and then a slower, dose- and pretreatment-independent half-time of
21 h. The maximum relative concentration in the plasma was independent
of dose and was observed 2 h after oral administration in male and
female rats.
The residues found in the organs and tissues were influenced by
the route of administration, as the mean relative concentration of
cyfluthrin in the bodies of males and females at sacrifice was lower
after oral administration (0.013) than after intravenous injection
(0.06). Female rats had higher plasma concentrations after oral
administration of the single high or low dose; 48 h after
administration, lower concentrations were detected in the bone and
muscle of animals of each sex and in the testes of males rats. The
sciatic nerve showed a similar relative concentration value, which may
explain the toxic effects observed on the peripheral nervous system.
Higher concentrations were detected in the spleen, adrenal glands,
liver, and plasma of both males and females and in the ovaries. The
renal fatty tissue concentration was about seven times higher after
either oral or intravenous administration, whereas the mean
concentration in brain was significantly lower ( p = 0.0006-0.006)
(Klein et al., 1983).
2.1.2 Biotransformation
Rats
Eight hours after oral administration of 14C-labelled
cyfluthrin at a dose of 10 mg/kg bw to three male Sprague-Dawley rats,
about 60% of the labelled cyfluthrin was eliminated in the urine in
conjugated forms. Conjugates of 4'-hydroxy-3-phenoxyfluorobenzoic acid
(50%) were identified; a second major metabolite was identified after
hydrochloric acid hydrolysis as a conjugate of
3-phenoxy-4-fluorohippuric acid (40%). These metabolites represented
33 and 27% of the administered radiolabel, respectively. A glycine
conjugate constituted 2.5% of the conjugated metabolites (Ecker,
1982).
After intraperitoneal injection of 14C-cyfluthrin to Wistar
(TNO/W 74) albino rats at doses of 1, 5, 10, or 15 mg/kg bw, urine was
collected for three days. It was found that 24-42% of the alpha-cyano
group of cyfluthrin was excreted via the kidneys in the thiocyanate
form (Eben & Thyssen, 1981).
In another study carried out to characterize the complete kinetic
behaviour and metabolic pathway of cyfluthrin in rats, four groups of
five male and five female rats received 14C-cyfluthrin by a protocol
identical to that described by Klein et al. (1983). The initial step
in cyfluthrin biotransformation was ester hydrolysis, giving a
3-phenoxy-4-fluorobenzyl alcohol intermediate and the permethric acid
fraction. The metabolism of permethric acid has been well established
in the rat in studies with chemically similar pyrethroids. After ester
hydrolysis, the 3-phenoxy-4-fluorobenzyl alcohol moiety was oxidized
to the free metabolite 3-phenoxy-4-fluorobenzoic acid. This metabolite
can then either be conjugated with glycine to form
3-phenoxy-4-fluorohippuric acid (a minor metabolite constituting < 3%
of the recovered urinary radiolabel, dependent on neither sex nor
dose) or hydroxylated to give 4'-hydroxy-3-phenoxy-4-fluorobenzoic
acid (conjugates of which account for 41-50% of the total urinary
radiolabel recovered from rats given one or multiple doses of
cyfluthrin at 0.5 mg/kg bw). Females tended to excrete more of this
metabolite as the free form in the faeces than did males. Males and
females at the high dose (10 mg/kg bw) excreted about 35% of the
administered dose as conjugates of
4'-hydroxy-3-phenoxy-4-fluorobenzoic acid, whereas females excreted
about 5% more than males as the free metabolite. After repeated oral
doses of 0.5 mg/kg bw for 14 days, 12-16% of labelled metabolite was
found in the faeces as cyfluthrin, whereas < 1% was found when single
oral doses were administered. After a single high dose of 10 mg/kg bw,
17-19% was recovered in the faeces as parent compound. The authors
concluded that the metabolism of cyfluthrin is slightly dose-dependent
(Ecker, 1983).
The proposed metabolic pathway of cyfluthrin in rats is shown in
Figure 2.
Cattle
A 484-kg lactating Holstein dairy cow (Bos taurus) was given
14C-cyfluthrin orally at a dose of 0.5 mg/kg bw after the evening
milking each day for five consecutive days. Milk was collected in the
morning and evening throughout the study. The cow was sacrificed on
day 6 after initial administration, and blood, major organs, fatty
tissue, and muscle were analysed for radiolabel. The maximum
concentration of radioactivity in the milk was 0.079 mg/litre
cyfluthrin equivalents three days after initial dosing; the level then
declined steadily. Of the radiolabel found in milk, 98% was on
unmetabolized parent compound. The highest levels of residues were
found in the liver (0.622 mg/kg), fat (0.195 mg/kg), and kidney (0.188
mg/kg); brain, skeletal muscle, and myocardium had the lowest levels,
ranging from 0.015 to 0.040 mg/kg. Most of the radiolabel recovered in
 tissues was on unmetabolized parent compound. Heart and kidney also
contained a metabolite identified as
4-fluoro-3-phenoxybenzenemethanol, and liver also contained
4-fluoro-3-phenoxybenzaldehyde (Shaw et al., 1983).
2.2 Toxicological studies
2.2.1 Acute toxicity
Cyfluthrin induces signs of toxicity in the species that have
been studied that are typical of cyano-containing type-II pyrethroid
poisoning. After oral administration, the symptoms of toxicity include
increased salivation, uncoordinated movements, increased activity and
vocalization, and reduced, laboured breathing. These signs appeared
within 10-60 min after dosing; the survivors usually recovered within
7-10 days (Flucke & Thyssen, 1980). Apathy, straddle-legged gait
(mostly in the rear legs), and reduced sensitivity to external stimuli
were observed after the acute intoxication phase. Rabbits showed signs
of apathy and suppression of appetite, while dogs vomited for up to
5 h after treatment with 50-100 mg/kg bw.
Anti-inflammatory, analgesic, anti-epileptic and sedative agents
and compounds that regulate neuromuscular transmission failed to
provide adequate protection against the oral toxicity of cyfluthrin.
Likewise, calcium, cyanide antidotes and blood pressure regulators had
no antagonistic effects. Tetrazepam, the active ingredient of
Musaril(R), a centrally acting muscle relaxant, increased the oral
LD50 in rats by a factor of 1.6 and delayed the onset of mortality
(Heimann, 1983a); however, the antagonistic effect was weak and did
not provide complete protection. Atropine sulfate at 50 mg/kg bw
combined with methocarbamol at 100 mg/kg bw had a moderate but
incomplete protective effect against the oral toxicity of cyfluthrin.
When one-half of the dose was administered to male rats 20 min, 3 h,
and 24 h after treatment with cyfluthrin, the LD50 increased from
2100 to 3100 mg/kg bw (Watanabe & Iyatomi, 1984).
Sub-additive effects were seen when cyfluthrin was combined in
equitoxic doses with methamidophos, propoxur, dichlorvos, or
fenfluthrin (Heimann, 1983b; Flucke, 1984a,b,c). Krotlinger (1988)
observed a slight super-additive effect after oral administration
simultaneously with the organophosphorous compound omethoate.
The studies on the acute toxicity of cyfluthrin are summarized in
Table 2.
Table 2. Acute toxicity of cyfluthrin to laboratory animals
Species Sex Route Purity LD50 or LC50 Remarks Reference
(%) (mg/kg bw
or mg/m3)
Mouse (NMRI) M Gavage 83.6 291 In PEG 400 Flucke & Thyssen (1980)
Mouse (NMRI) F Gavage 83.6 609 In PEG 400 Flucke & Thyssen (1980)
Rat (Wistar) M Gavage 83.6 1271 Fed, in PEG 400 Flucke & Thyssen (1980)
Rat (Wistar) F Gavage 83.6 869 Fed, in PEG 400 Flucke & Thyssen (1980)
Rat (Wistar) M Gavage 83.6 1189 Fasted, in PEG 400 Flucke & Thyssen (1980)
Rat (Wistar) F Gavage 83.6 590 Fasted, in PEG 400 Flucke & Thyssen (1980)
Rat NR Gavage cis:trans 425 cis:trans isomer Flucke & Thyssen (1981)
55:45 in PEG 400
Rat M Gavage NR 795 Fasted, in PEG 400 Heimann (1984)
Rat M Gavage NR 926 Fasted, in PEG 400 Heimann (1983a) warm)
Rat M Gavage NR 499 Fasted, in xylol Heimann (1983c)
Rat M Gavage NR 505 Fasted, in Heimann (1983c)
isododecan
Rabbit (New M Gavage 83.6 > 1000 In PEG 400 Flucke & Thyssen (1980)
Zealand)
Dog (beagle) M Gavage 83.6 > 100a In PEG 400 Flucke & Thyssen (1980)
Hen (white F Gavage 84-94 5000 In PEG 400 Thyssen et al. (1981)
Leghorn)
Hen (white F Gavage 93.5 > 5000 In Cremophor Sachsse & Zbinden (1985)
Leghorn) EL:dw
Hen (white F Gavage 93.5 4500 In PEG 400 Sachsse & Zbinden (1985)
Leghorn)
Mouse F Oral NR < 100 Fasted, in Heimann (1982)
Cremophor EL:dw
Rat M Oral NR 254 Fasted, in acetone: Heimann (1982)
oil (1:10)
Rat M Oral NR 396 Fasted, in dimethyl Heimann (1982)
sulfoxide
Rat M Oral NR 16.2 Fasted, in Heimann (1982)
Cremophor EL:dw
Rat M Oral NR 500-1000 N-Dimethyl Heimann (1982)
pyrroliodone
Table 2. (continued)
Species Sex Route Purity LD50 or LC50 Remarks Reference
(%) (mg/kg bw
or mg/m3)
Rat M Oral 93 155 Fasted, in acetone: Heimann (1987)
peanut oil
Rat F Oral 93 160 Fasted, in acetone: Heimann (1987)
peanut oil
Dog (beagle) M&F Oral 95 > 100a In Cremophor Hoffmann (1981a)
EL:dw
Sheep M&F Oral NR 1000 50% premix with Hoffmann (1981b)
Wessalon
Rat M Intraperitoneal 83.6 66 In PEG 400 Flucke & Thyssen (1980)
Rat F Intraperitoneal 83.6 104 In PEG 400 Flucke & Thyssen (1980)
Rat M Intraperitoneal NR 20 In Cremophor Heimann (1982)
EL:dw
Rat F Intraperitoneal NR 24 In Cremophor Heimann (1982)
EL:dw
Rat M Intraperitoneal NR 34 In PEG 400 Heimann (1982)
Rats F Intraperitoneal NR 94 In PEG 400 Heimann (1982)
Mouse M Subcutaneous 83.6 > 2500 In PEG 400 Flucke & Thyssen (1980)
Mouse F Subcutaneous 83.6 > 2500 In PEG 400 Flucke & Thyssen (1980)
Rat M Dermal 83.6 > 5000 NR Flucke & Thyssen (1980)
Rat F Dermal 83.6 > 5000 NR Flucke & Thyssen (1980)
Rat M&F Inhalation 83.6 > 1089 In 1:1 ethanol: Flucke & Thyssen (1980)
(1-h) PEG 400
Rat M&F Inhalation 83.6 469-592 In 1:1 ethanol: Flucke & Thyssen (1980)
(4-h) PEG 400
Rat M&F Inhalation cis:trans 180-326 cis:trans isomer in Flucke & Thyssen (1981)
(4-h) 55:45 PEG 400
Rat M&F Inhalation 93 405 PEG 400:ethanol Pauluhn (1987)
(4-h) 1:1
M, male; F, female; NR, not reported; dw, deionized water; PEG, polyethylene glycol
a Vomiting occurred at 50-100 mg/kg, no higher dose used
2.2.2 Short-term toxicity
Mice
Groups of 18 mice of each sex received diets containing 0, 300,
1000, or 3000 mg/kg cyfluthrin in the feed. After one month, 12 rats
of each sex in each group were sacrificed; the remainder were
sacrificed after four weeks of recovery on control diet. Mice at the
highest dose developed ataxia, salivation, and/or emaciation. One
female mouse at 3000 mg/kg feed died. Body-weight gain was depressed
in males at 3000 and in females at 1000 mg/kg feed. Decreased growth
rates were not seen during the recovery period, as all mice recovered
to normal control values. In males at 3000 mg/kg feed, alkaline
phosphatase and blood urea nitrogen levels were slightly increased,
while those of glutamate pyruvate transaminase and cholesterol were
normal throughout. Necropsy after treatment revealed dark-red livers
in some mice at 3000 mg/kg feed; this effect was not observed after
the recovery period. The relative weights of the submaxillary glands
and kidneys were increased in animals at the highest dose, and males
at this dose also had significantly increased relative liver weights.
The weights of both the liver and the submaxillary gland returned to
normal after the four-week recovery period, while those of the kidney
remained elevated. Histopathological examination revealed minimally
increased chromatin in the nuclei of hepatocytes of males receiving
1000 mg/kg feed or more (Watanabe et al., 1982a) and in females at
the high dose. Mice receiving 1000 mg/kg feed or more had cytoplasmic
swelling of the glandular epithelium of the submaxillary glands. These
findings all disappeared after the four-week recovery period. The NOEL
was 300 mg/kg feed, equivalent to 45 mg/kg bw per day (Watanabe
et al., 1982b).
Rats
Groups of 20 SPF Wistar albino rats of each sex received doses of
0, 5, 20, or 80 mg/kg bw per day cyfluthrin (purity, 85%) dissolved in
PEG 400 by gavage once daily for four weeks followed by a six-week
observation period. The high dose was lowered to 40 mg/kg bw per day
in weeks 2 and 4 because of severe intoxication. Physical appearance
and behaviour were assessed daily throughout the study, and body
weights were measured at the start of each week. Blood measurements
and urinalysis were performed on five male and five female rats from
each group at the end of the four-week treatment period and at the end
of the six-week observation period.
The control group and rats given doses of 5 or 20 mg/kg bw per
day behaved normally, but rats at the highest dose developed symptoms
of apathy, ruffled coat, dyspnoea, salivation, hyperkinesis, ataxia,
and uncoordinated movements. The symptoms were more pronounced during
weeks 1 and 3 of treatment at the highest dose. Deaths occurred only
in rats at the high dose, 6/20 males dying between treatment days 3
and 21 and one female on day 26; no deaths were noted during the
recovery period. The body-weight gain of male rats at the high dose
was reduced by 10% during week 4 of treatment, but the weights
returned to normal during the recovery period. The body weights and
body-weight gain of females were not affected by treatment.
Haematological parameters were all within the normal ranges, and no
toxicologically relevant, group-specific or dose-related differences
from controls were seen in serum enzyme activities or electrolyte
concentrations. The plasma activity of glutamate pyruvate transaminase
was slightly higher in rats receiving the highest dose than in
controls. Urinalysis and gross and histopathological examination
revealed no treatment-related variations from normal. Likewise, no
differences in blood chemical parameters were seen at the end of the
observation period. The absolute and relative weights of the adrenal
glands were increased in female rats at the end of treatment with the
highest dose, while only the relative weight of the liver was higher
in males at the high dose in comparison with the control animals. The
increase in relative adrenal weights was not accompanied by
histopathological changes. No deaths occurred during the recovery
period. No gross pathological changes related to administration of
cyfluthrin were seen after treatment or recovery, and there were no
signs of delayed neurotoxicity at the end of the recovery period. The
NOEL was 20 mg/kg bw per day (Flucke & Schilde, 1980).
Groups of 18 rats (strain unspecified) of each sex received diets
containing 0, 100, 300, or 1000 mg/kg cyfluthrin for one month, when
12 rats of each sex per group were sacrificed; the remainder were
killed after one month of recovery on control diet. Rats at the
highest dose developed straddled gait, salivation, and/or nervousness
during treatment, but these symptoms disappeared rapidly during the
recovery period. The body-weight gain, food intake, and water
consumption of animals of each sex at 1000 mg/kg feed were depressed
but returned to normal during the recovery period. Urinalysis
indicated a higher incidence of urobilinogen and ketone bodies in rats
at 1000 mg/kg feed, but, again, these signs returned to normal after
the end of treatment. The haematological effects seen after treatment
with 1000 mg/kg feed included a decrease in erythrocyte count,
haematocrit, and haemoglobin content, which also returned to normal
after the four-week recovery period. No significant effects were seen
on liver microsomal enzyme activity. Total protein and blood glucose
levels were significantly decreased in male rats given 300 mg/kg feed.
No effects were seen on macroscopic examination. Histopathologically,
minimal degeneration of single fibres of the sciatic nerve was noted
occasionally after the treatment period. Cytoplasmic swelling,
increasing the size of the glandular epithelium of the submaxillary
glands was observed at the high dose, but these alterations
disappeared after the recovery period. On the basis of the depressed
blood glucose levels in male rats at 300 mg/kg feed, the NOEL was 100
mg/kg feed, equivalent to 5 mg/kg bw (Watanabe et al., 1982a).
Groups of 30 male and 30 female Wistar rats received diets
containing 0, 30, 100, or 300 mg/kg cyfluthrin (purity, 84.2%) diluted
in an oil vehicle (Wessalon S; equivalent to a 50% premix) for three
months. They were observed daily for alterations in appearance and
behaviour, while body weights and food consumption were recorded
weekly. Five male and five female rats from each group were killed
after one and four weeks for interim examinations, including
microsomal enzyme induction assays ( N- and O-demethylase and
cytochrome P450 content). All rats that survived the treatment were
sacrificed at the end of treatment and examined.
Appearance and behaviour were not modified during treatment, even
at the highest dose. No effects were found on body weight, food
consumption, haematological, clinical chemical or urinary parameters,
or mortality rates. Blood sugar and cholesterol levels were within the
normal range throughout treatment. At week 1, a dose-independent
increase in N-demethylase activity was seen in all treated males and
in O-demethylase activity in females at 100 and 300 mg/kg feed; the
activity of enzymes of the cytochrome P450 system was significantly
increased only in males at 300 mg/kg feed at this time. By three
months, no differences were seen in the activities of the microsomal
enzymes in any treated group. No treatment-related effects were seen
on gross or histopathological examination. No toxic effects were seen
at any dose (Loser & Schilde, 1980).
Groups of 28 male and 28 female Sprague-Dawley rats received
diets containing 0, 100, 300, or 1000 mg/kg cyfluthrin (purity, 95%)
incorporated into the powdered diet on a 0.4% maximum clay carrier,
daily for three months. Body weights were recorded weekly and food
consumption and efficiency were determined. After treatment, 20 rats
at each dose were autopsied immediately, and the remaining eight rats
in each group were allowed to recover on a control diet for a
one-month observation period before autopsy. Clinical chemical and
urinary parameters were investigated before each sacrifice.
Only the rats at the highest dose showed signs of intoxication.
Both males and females of this dose showed abnormal gait and excess
salivation, increasing in incidence until the second week of feeding,
after which a gradual decrease was noted, although recovery from this
effect was somewhat delayed in males. By the third month of treatment,
no such symptoms were seen in any group. Mortality was not affected by
treatment. The food consumption and body-weight gains of females and
males at the highest dose were significantly lower than those of the
control group. The body-weight gain depression in females appeared to
recover after cessation of treatment, while that of the males remained
significantly low. Overall feed efficiency did not vary between
groups. The results of urinalysis and haematological examination were
within the normal physiological range and revealed no
treatment-related effects. Biochemical analysis showed that rats at
300 or 1000 mg/kg feed had significantly increased blood urea nitrogen
levels, and males at 1000 mg/kg feed had significantly increased
aspartate aminotransaminase activity. The blood glucose levels were
depressed in males at 300 or 1000 mg/kg feed and in females at 1000
mg/kg feed. All biochemical parameters were within normal limits after
the recovery period. The absolute weights of the liver, heart, and
lung were decreased in male rats at the high dose, and females at this
dose had depressed liver, kidney, adrenal, and ovarian weights. In
both males and females at the high dose, the relative weight of the
submaxillary gland was increased. No other treatment-related gross
pathological effect was observed. Degeneration of single fibres of the
sciatic nerve was noted in 5/20 males and 3/20 females at the high
dose after the three-month treatment period, and in 1/8 males at the
high dose after the one-month recovery. The authors concluded that the
damage was mild and minimal, corresponding to partial loss of the
myelin sheaths of single nerve fibres. The NOEL was 100 mg/kg feed,
equal to 6.2 mg/kg bw per day (Oikawa & Iyatomi, 1983).
Rabbits
In a three-week study of dermal toxicity, groups of three New
Zealand white rabbits of each sex were given dermal applications of 0,
50, or 250 mg/kg bw cyfluthrin (purity, 85%) dissolved in PEG 400 on
the intact or abraded skin. The treatments were applied five times a
week for a contact time of 6 h/day for three weeks, and then the
rabbits were killed and examined. Tissues were collected for
microscopic examination from controls and rabbits at the high dose;
the testes were collected from animals at 50 mg/kg bw per day. No
effects were observed on appearance, behaviour, or haematological,
clinical chemical, or urinary parameters. All rabbits survived the
treatment period. No toxicologically significant effect on body-weight
gain was observed. Gross and histopathological examination showed no
alterations due to treatment with cyfluthrin. The NOEL was 250 mg/kg
bw per day (Flucke & Vogel, 1980).
Dogs
Groups of six male and six female beagle dogs were fed cyfluthrin
(purity, 84.8%) in the diet at concentrations of 0, 65, 200, or 600
mg/kg for six months. No deaths occurred, and the physical appearance
of the dogs remained normal. Tests of reflexes and measurement of body
temperature showed no significant alterations. Five males and six
females receiving the high dose showed signs of uncoordinated, stiff
gait of the hind limbs, persisting for several hours, on several
occasions after week 21. Vomiting after dosing occurred slightly more
often in animals at the high dose than in controls. Diarrhoea occurred
in all groups including controls, but much more frequently in dogs at
the high dose, from the time of initiation of treatment. No effects
were found on food intake or water consumption in the controls or in
dogs at 65 or 200 mg/kg feed, but males at the high dose had slightly
lower food consumption during the first week of treatment. No group
differences in food consumption were observed by the sixth week. Lower
mean body weights were seen in males and females at 200 or 600 mg/kg
feed from week 2 to the end of treatment. Males at 200 and females at
600 mg/kg feed had lower average weight gains between weeks 1 and 26
of treatment, whereas the average body weights of males at the high
dose were similar to those of controls at the end of the feeding
period. No effects were found on ophthalmoscopic, haematological,
clinical chemical, or urinary parameters or on organ weights; no
treatment-related changes were seen grossly or histologically. The
NOEL was 65 mg/kg feed, equal to 2 mg/kg bw per day (Hoffmann &
Kaliner, 1981).
2.2.3 Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female mice SPF strain CF1/W74 mice
received a diet containing 0, 50, 200, or 800 mg/kg cyfluthrin in the
form of a 50% premix with Wessalon S for 23 months. Clinical
examinations were carried out on 10 mice of each sex at each dose 6,
12, 18, and 23 months after the initial administration. After
treatment, five mice of each sex from each group were selected
randomly for analysis of fluoride accumulation in bone and teeth. The
remaining mice were killed and examined grossly, and tissues were
taken for histopathological examination. Neither appearance nor
behaviour was altered in any group. No effects were observed on
mortality, haematological parameters, fluoridation of bones or teeth,
food intake, or water consumption. The body-weight gain of treated
animals was not affected at doses up to and including 200 mg/kg feed,
but mean body-weight gain was significantly reduced in females at the
high dose and males at this dose had a slightly lower body-weight gain
than controls. Plasma alkaline phosphatase activity was increased in
all treated males at the six-month examination; however, at the end of
the study several controls also showed elevated alkaline phosphatase
activity. Alanine aminotransaminase activity was slightly increased in
males at the high dose after 12 and 18 months of treatment. All other
clinical chemical values were within normal ranges. No
treatment-related alterations were seen at autopsy, and the relative
and absolute organ weights were not affected by treatment. Urinalysis
and microscopic examination revealed no evidence of treatment-related
damage at any dose. The incidence and histological type of
non-neoplastic and neoplastic alterations were all within normal
ranges. The NOEL was 200 mg/kg feed, equivalent to 30 mg/kg bw per day
(Suberg & Loser, 1983a).
Rats
Groups of 65 male and 65 female SPF BOR:WISW strain rats received
a diet containing 50, 150, or 450 mg/kg cyfluthrin premixed with
Wessalon S for two years. The control group consisted of 65 rats of
each sex fed untreated diet. Both the control and the test diets were
given ad libitum. All rats were observed twice a day for alterations
in appearance, behaviour, and activity. Body weights were determined
weekly up to week 4, then every other week to conclusion of treatment.
After seven days of treatment, five rats of each sex at each dose were
killed in order to measure mixed-function enzyme induction and
cytochrome P450 content. Clinical tests were conducted on 10 rats of
each sex per dose 6, 12, 18, and 24 months after the start of
treatment. After one and two years, five rats of each sex per dose
were killed in order to determine the fluoride content of their bones
and teeth. Rats that died during treatment and five rats of each sex
per dose killed at one year were examined grossly. All remaining rats
were autopsied and examined grossly and histologically at the end of
treatment.
No treatment-related effects were seen on appearance, behaviour,
activity, or food consumption. There were no treatment-related effects
on body-weight gain at the low dose, but rats at 150 mg/kg feed
appeared initially to have slightly decreased body-weight gain. The
high dose consistently induced significantly decreased body-weight
gain throughout the experiment. No treatment-related effects were seen
on mortality, and no effects were seen on haematological or urinary
parameters, gross appearance, organ weights, or histopathological
manifestations. N-Demethylase activity was increased by 30% in males
and 20% in females after seven days at the high dose, but there were
no effects on O-demethylase activity or P450 enzyme induction.
Induction of the microsomal enzyme N-demethylase in rats at the high
dose is considered not to be of toxicological relevance as no specific
morphological alteration or damage to the liver was seen at
histopathological examination. No significant differences in fluoride
accumulation in bones and teeth were noted at interim sacrifice.
Significant, dose-related differences were found after two years of
treatment, but the biological relevance of this finding was questioned
by the authors, as minimal changes in fluoride accumulation were seen
and the assay method involved large experimental error. The results of
the final necropsy showed isolated but significant differences in
organ weights, which were attributable to dissimilar body weights and
were not dose-related; they were considered to be of little
toxicological significance. The absolute kidney and liver weights were
decreased in rats at the high dose; however, there was no
dose-dependent effect on the relative weights. The incidence of
tumours was not altered by treatment at any dose, and no other
treatment-related alterations were observed histologically. The NOEL
was 50 mg/kg feed, equal to 2 mg/kg bw per day (Suberg & Loser,
1983b).
Dogs
Groups of six beagle dogs of each sex, 22-30 weeks old, were fed
a diet containing 0, 40, 160, or 640 mg/kg cyfluthrin for one year. No
effects on appearance or behaviour were seen in dogs at 40 or 160
mg/kg feed. Two dogs at the highest dose had slight disturbances in
movement, clumsy gait primarily in the hindquarters, and a reluctance
to move. This observation was made only once during weeks 36-37 of the
study. Reflex tests indicated no deviations in any treated animals.
Body temperature, mortality, and feed and water consumption were not
affected by treatment. The body-weight gain of males at the high dose
was slightly lower than that in the other groups; females at this dose
had somewhat higher weight gains than those at other doses. Males at
the high dose had a higher incidence of vomiting, and soft, pasty
faeces were observed more frequently than in controls and in the other
groups. Ophthalmoloscopy, haematology, clinical chemistry, and
urinalysis revealed no treatment-related alterations. At termination
of treatment, all of the dogs were killed and necropsied, and the
tissues of males at the high dose and of controls were evaluated
histologically. No significant treatment-related effects were found at
autopsy. Treatment had no effect on absolute or relative organ
weights, except that the mean spleen weight of females at the high
dose was absolutely and relatively higher than that of female
controls; however, the histopathological appearance was normal in all
treated animals. Microscopic examination of the sciatic nerve showed
no pathological alterations. The NOEL was 160 mg/kg feed, equal to 5.1
mg/kg bw per day (Hoffmann & Schilde, 1983).
2.2.4 Genotoxicity
Cyfluthrin had no mutagenic potential in vivo or in vitro.
The results of assays for genotoxicity with cyfluthrin are summarized
in Table 3.
2.2.5 Reproductive toxicity
2.2.5.1 Multigeneration reproductive toxicity
Rats
Groups of 10 male and 20 female SPF-Cpb rats, five to six weeks
old, received diets containing 0, 50, 150, or 450 mg/kg cyfluthrin as
a 50% premix with Wessalon S in the feed, given ad libitum 105 days
before and throughout mating, gestation, and rearing of pups. The
parameters measured included behaviour, growth, food consumption,
mortality, fertility, viability during gestation and lactation,
development of the young, parental body-weight gain, and male:female
ratio. The study was conducted for three generations, with two litters
per generation. F0 parents produced F1a and F1b litters; F1b
parents produced F2a and F2b litters; and F2b parents produced
F3a and F3b litters. The parameters assessed included fertility,
litter size, fetal birth weight, fetal variability, and parental
weight gain. Gross and histopathological examinations were also
performed on F2b parental animals and F3b pups.
Treatment did not affect appearance, behaviour, or survival,
although one F2a pup receiving 150 mg/kg feed and another receiving
450 mg/kg feed had convulsions. Treatment did not consistently affect
birth weight or litter size at birth. A decrease in pup survival from
birth through five days of lactation was seen in animals from the
F3a and F3b matings at 150 or 450 mg/kg feed, and decreased
survival of pups on days 5-28 of lactation was noted among pups at the
high dose in all matings. Similarly, body weight was consistently
suppressed in F3a and F3b generation rats at 150 and 450 mg/kg
feed during the 28-day lactation period. Necropsy revealed decreased
absolute liver and kidney weights in rats at 150 and 450 mg/kg feed;
this was probably related to the decreased body-weight gain in these
animals. No effect was observed on relative organ weights. The NOEL
was 50 mg/kg feed, equal to 3 mg/kg bw per day (Loser & Eiben, 1983).
Table 3. Results of assays for genotoxicity with cyfluthrin
End-point Test object Concentration Purity (%) Results S9 QA Reference
In vitro
Reverse mutation Salmonella typhimurium TA100, TA98, 20-24 000 µg/plate 83.6 Negative + No Herbold (1980a)
TA1535, TA1537 Negative -
Reverse mutation Salmonella typhimurium TA100, TA98, 5-5000 µg/plate 95.0 Negative + No Nagane et al.
TA1535, TA1537, TA1538 Negative - (1982)
Reverse mutation Salmonella typhimurium TA100, TA98, 10-25 000 µg/plate 95.0 Negative + No Ohta & Moriya
TA1535, TA1537, TA1538 Negative - (1982)
Reverse mutation Escherichia coli B/r WP2 try- hcr- 5-5000 µg/plate 95.0 Negative + No Nagane et al.
Negative - (1982)
Reverse mutation Escherichia coli WP2 hcr 10-25 000 µg/plate 95.0 Negative + No Ohta & Moriya
Negative - (1982)
Reverse mutation Saccharomyces cerevisiae S138, 312.5-10 000 µg/ml 95.0 Negative + No Brusick (1982a,b)
S211a) Negative -
Reverse mutation Saccharomyces cerevisiae D7 625-10 000 µg/ml 95.0 Negative + No Brusick (1982c)
Negative -
Gene mutation Chinese hamster ovary K1-BH4 cells, 3-10 µl/ml 94.7 Negative + Yes Yang & Louie
hprt locus Negative - (1985)
Chromosomal Chinese hamster lung cells 3.3 × 10-5-3.3 × 10-3 93.7 Negative + Yes Sasaki et al. aber
ration mol/litre Negative - (1986)
Chromosomal Human lymphocytes 500-5000 µg/ml 95.1-95.5 Negative + No Herbold (1988)
aberration Negative -
Sister chromatid Chinese hamster ovary cells 3-20 µg/ml Technical Negative + Yes Putman (1985)
exchange Negative -
DNA damage Escherichia coli pol A+ and pol A1- 62.5-1000 µg/plate 95.0 Negative + No Herbold (1981a)
Negative -
DNA damage Bacillus subtilis H17, M45 rec- 200 µg/disc 95.0 Negative + No Nagane et al.(1982)
DNA damage Bacillus subtillus H17, M45 rec- 100-10 000 µg/disc 95.0 Negative + No Ohta & Moriya
(1982)
Unscheduled DNA Male Sprague-Dawley rat primary 17-5000 µg/ml 94.7 Negative Yes Curren (1985)
synthesis hepatocytes
Table 3. (continued)
End-point Test object Concentration Purity (%) Results S9 QA Reference
Mitotic gene Saccharomyces cerevisiae D7 625-10 000 µg/ml 95.0 Negative + No Brusick (1982c)
conversion Negative -
Mitotic crossing Saccharomyces cerevisiae D7 625-10 000 µg/ml 95.0 Negative + No Brusick (1982c)
over Negative -
In vivo
Micronucleus Male and female NMRI mice, 2 × 7.5, 2 × 15 83.6 Negative No Herbold (1980b)
formation bone-marrow erythroblasts mg/kg bw orally
Dominant lethal Male NMRI/ORIG Kisslegg mice 30, 60 mg/kg bw 83.6 Negative No Herbold (1981b)
mutation orally
a S9, 9000 × g fraction of rat liver
2.2.5.2 Developmental toxicity
Rats
Groups of 25 outbred pregnant female Wistar KFM-HAN (SPF) rats
received 0, 1, 3, or 10 mg/kg bw per day cyfluthrin (purity, 93.4%) in
a 1% Cremophor EL solution in drinking-water by gavage on days 6-15 of
gestation. Clinical signs, body weights, and food consumption were
recorded. On day 21 of gestation, females were killed, caesarean
sections performed, and the pups were necropsied. Although the
standard was not specified, the study conduct underwent periodic
inspections by the quality assurance unit of the laboratory.
No effects on mortality were observed. Partial hair loss occurred
in one female at 3 mg/kg bw per day. Body-weight gain was not affected
by the low or middle doses. Rats receiving the high dose had 7.7%
lower corrected mean body-weight gain. Mean food consumption was 14%
lower in rats at the high dose on days 6-11 after mating. Mating,
fertility, and gestation parameters were normal, and no dose-related
difference was seen on examination and necropsy of dams. One dam at
the high dose totally resorbed its litter. Litter size, fetal body
weight, and sex ratio were not affected by treatment. The incidences
of visceral and skeletal anomalies were not increased by treatment in
a dose-dependent manner. No teratogenic effects were seen. As a
maternal maximum tolerated dose was not achieved in this study, the
authors did not report a NOEL (Becker, 1983).
Groups of 25 Bay:FB30 rats received cyfluthrin dissolved in PEG
400 at doses of 0, 3, 10, or 30 mg/kg bw per day by gavage during days
6-15 of gestation. They were mated in a 2:1 ratio with males. The
pregnant females were killed on day 20 of gestation for examination of
their uterine contents. Six rats at 10 or 30 mg/kg bw per day were
reported to have occasional 'high-stepping gait'; at the highest dose,
they showed signs of ataxia and decreased motility. No effects on
maternal weight gain were seen that were attributable to treatment.
Litter size, frequency of resorptions, and placental weights were not
affected by treatment, even at the highest dose. Likewise, no
treatment-related increase in the incidence of stunted growth or bone
anomalies was observed in the fetuses. The author concluded that
cyfluthrin had no specific teratogenic or embryotoxic effect. As 6/25
rats at 10 mg/kg bw per day showed signs of toxicity, the NOEL for
maternal toxicity was 3 mg/kg bw per day; the NOEL for fetal toxicity
was 30 mg/kg bw per day (Schluter, 1982).
Rabbits
In the first part of a two-part study, groups of 15 Himalayan
rabbits received cyfluthrin at 0, 3, 10, or 30 mg/kg bw per day in
Cremophor EL as a solution in drinking-water by gavage on days 6-18 of
gestation. The dams were killed on day 29 of gestation. Maternal
appearance and behaviour were not affected. Lower mean fetal and
placental weights were observed, and a higher incidence of
arthrogryposis (persistent flexure or contracture of a joint) was
observed in the nine fetuses of rabbits receiving 10 or 30 mg/kg bw
per day.
Because an embryotoxic effect was observed, a second study was
conducted. Groups of 15 pregnant Himalayan rabbits received cyfluthrin
at 0, 10, or 30 mg/kg bw per day in Cremophor EL as a solution in
drinking-water by gavage during days 6-18 of gestation. The dams were
killed on day 29 of gestation, and the fetuses removed for
examination. Doses up to and including 30 mg/kg bw per day had no
effect on behaviour or appearance. One dam at the high dose died of an
unknown cause on day 26 of gestation. Mean weight gain was affected by
treatment, and the average weight gain of the animals at 30 mg/kg bw
per day was statistically significantly lower than that of controls.
Treatment had no effect on the fertilization rate or gestation or on
embryonic development, including implantation rate, litter size, and
rate of resorptions. The incidence of skeletal anomalies was not
increased at any dose. One case of arthrogryposis occurred in the
control group. There was no indication of embryotoxicity or
teratogenicity. The NOEL for maternal toxicity was 30 mg/kg bw per day
and that for embryotoxicity was 30 mg/kg bw (Schluter, 1981).
Groups of 15 pregnant female Himalayan CHBB:HM rabbits received
cyfluthrin at 0, 5, 15, or 45 mg/kg bw per day by gavage in 0.5%
aqueous Cremophor EL emulsion during days 6-18 of gestation. Dams were
observed for clinical signs and body weight. On day 29 of gestation,
the dams were killed and their fetuses removed by caesarean section
and examined for anomalies. No effects were observed on mortality,
behaviour, appearance, insemination rate, or maternal weight gain. Two
rabbits at the high dose aborted on gestation days 25 and 28, and one
completely resorbed its implants. The authors concluded that the
effects seen at the high dose were treatment-related. Litter size,
placental weights, and fetal body weights were not affected by
treatment. The rate of resorptions appeared to be dose-related.
Although arthrogryposis was seen in rabbits at the high dose, the
event was considered to be spontaneous. The NOEL for maternal toxicity
was 15 mg/kg bw per day and that for embryotoxicity was 45 mg/kg bw
per day (Roetz, 1983).
In a more recent study, cyfluthrin was tested for its embryonic
and teratogenic potential in pregnant chinchilla rabbits (Chb:CH
hybrids, SPF). Groups of 16 pregnant rabbits received 0, 20, 60, or
180 mg/kg bw per day by gavage during days 6-18 of gestation. They
were killed 28 days after mating, and the fetuses were removed by
caesarean section. The study was conducted in accordance with the
specifications of the US FIFRA GLP (40 CFR Part 160), OECD test
guideline 414, and US EPA test guideline Vol. 43 Series 163.83-3
Subdivision F, Hazard Evaluation.
Clinical observations, appearance, and behaviour were not
affected by treatment. No deaths occurred. Food consumption and
body-weight gain were reduced in a dose-dependent manner in animals at
60 and 180 mg/kg bw per day but were significantly increased 24-28
days after mating. A dose-related, statistically significant increase
in post-implantation losses, due to increased embryonic resorptions,
was observed in rabbits given the two higher doses. Necropsy revealed
no significant alteration due to treatment. The sex ratio and fetal
body weights were unaffected by treatment, and no significant
alterations were found in fetal heads or brains. Skeletal alterations
were seen, including ossification of vertebrae, sternebrae, and ribs,
but these findings were considered to be within the normal spontaneous
range. There was no indication of a teratogenic effect. On the basis
of the increased post-implantation losses, the NOEL for maternal and
embryonic toxicity was 20 mg/kg bw per day (Becker & Biedermann,
1992).
2.2.6 Special studies on neurotoxicity
Rats
Fifty male Sprague-Dawley received technical-grade cyfluthrin
(purity, 95%) as a 1.6% solution in PEG 400 by gavage at a dose of 80
mg/kg bw per day on five consecutive days. The dose was reduced to 40
mg/kg bw per day on days 6-14 owing to the onset of mild to severe
symptoms of toxicity. Twenty-five male rats received PEG 400 alone.
All rats were observed for appearance and behaviour throughout
treatment and a recovery period of three months. Ten treated and five
control rats were killed, and their tissues examined histologically on
the days 1 and 5 of treatment and one, two, and three months after
treatment; tissues from two treated and one control males were also
examined at each time point by electron microscopy. The treated rats
showed symptoms of intoxication throughout the dosing period,
including abnormal, straddled gait, slow leg movements, and excess
salivation, but no symptoms were observed five days after treatment.
The body-weight gain of the treated rats was depressed in comparison
with controls throughout treatment but recovered during the
observation period. Histological examination revealed a minimal degree
of axonal degeneration (swelling and demyelination) in single fibres
of the sciatic nerve in 6/8 rats at the first examination, in 3/8 five
days to one month after treatment, and in 2/9 two months after
treatment. A similar lesion was reported in the femoral nerve of one
treated male examined on the fifth day after treatment but was no
longer seen three months later No alterations were reported in any
other nervous or muscular tissue examined. Electron microscopy
revealed dilatation of neurotubules, accompanied by proliferation of
neurofilaments and degeneration of mitochondria, which was maximal one
month after treatment but was not seen after three months. A NOEL was
not identified (Oikawa et al., 1983).
Groups of 15 male and 15 female Wistar TNO/W74 albino rats
received cyfluthrin (purity, 83.3%) emulsified in PEG 400 by gavage
daily on seven days a week for 35 weeks. The doses administered ranged
from 30 to 80 mg/kg bw per day and were varied intermittently, so that
the rats had signs of acute toxicity after each administration.
Clinical observations were recorded daily, while body weights were
recorded weekly. Clinical chemistry was evaluated at the end of the
study, and liver, kidney, adrenal gland, brain, spinal cord, and
sciatic nerve tissues from five treated and five control rats of each
sex were examined microscopically.
The treated group displayed characteristic signs of acute
toxicity throughout the study, including apathy, ruffled coat, and
respiratory disturbances. Increased salivation, tremor, and
uncoordinated gait were observed in some rats immediately after
dosing. No paralysis of the extremities was observed. Treated males
had a 20-25% reduction in body-weight gain, while no effect was seen
on female body-weight gain. Treatment had no effect on clinical
chemical parameters or induction of cytochrome P450 or N- or
O-demethylase activity. The mortality rate was significantly higher
among treated than control males. Gross pathological and microscopic
examination showed no treatment-related pathological alterations in
any organ examined. The weights of the liver and kidney were lower in
treated males, due mostly to their reduced body weight. Although the
authors reported no histological damage to the nervous tissue, rats
that died during the treatment period were not examined, limiting the
usefulness of this study. A NOEL was not identified (Thyssen & Vogel,
1982).
Groups of five male and five female Wistar (SPF-Cpb) rats
received cyfluthrin at 0 or 60 mg/kg bw per day for two weeks by
gavage, and a supplementary group of male rats received doses of 0 or
50 mg/kg bw per day. Modified preparation techniques, i.e. perfusion
of animals with formalin and fixation of the spinal column with cord
in toto, were used to determine the neurotoxic effect of cyfluthrin.
Symptoms of acute toxicity were observed in all treated animals,
including tremor, altered gait, and increased vocalization. Four males
at 60 mg/kg bw per day died between treatment days 5 and 8, but gross
pathologicam examination revealed no specific alterations. The
body-weight gain of females was not affected by treatment, but
decreases were seen for males at 50 or 60 mg/kg bw per day. Small,
fresh brain haemorrhages were seen in all four males that died on
test. The authors concluded that the 'most likely explanation is that
these are the result of a terminal cardiovascular disorder with
necrosis of the vascular walls'. Since this finding was not seen in
control animals, a treatment-related effect could not be ruled out. A
NOEL was not identified (Heimann & Kaliner, 1983).
In a supplementary study, neuromuscular dysfunction was assessed
in the tilting plane test. Groups of 10 male rats received cyfluthrin
dissolved in Cremophor EL as a single oral administration by stomach
tube, at doses of 0, 0.1, 0.3, or 1.0 mg/kg bw in the first experiment
and 0, 0.01, 0.03, or 0.1 in the second. Diazepam was given at a
single oral dose of 5 mg/kg bw as a positive control. The angle at
which the animal started to slide from a tilting plane was evaluated
for each rat 30 min and 2, 5 and 7 h after administration.
At a dose of 0.01 mg/kg bw, cyfluthrin had no effect at any time
interval. At 0.03 mg/kg bw, the slip angle was marginally
significantly ( p < 0.05) reduced, only at 7 h. At 0.1 mg/kg bw
cyfluthrin, the angle was reduced at 5 h but was normal at other times
(Polacek, 1984). In view of the lack of a standardized protocol for
this study, the use of Cremophor EL as a vehicle (which can
substantially enhance the acute toxicity of cyfluthrin), and the
absence of a dose- or time-dependent reduction in slip angle, the
Committee considered this study inappropriate for assessing
neurotoxicity.
Hens
In a study of delayed neurotoxicity, 10 adult laying hens were
given single doses of cyfluthrin in carbowax at 5000 mg/kg bw by
gavage, and a group of 20 hens received the same dose twice at an
interval of one week. Hens given the single dose were observed for
mortality and symptoms of toxicity and were killed 56 days after
treatment; those given two doses were killed 49 days after treatment.
No treatment-related effects on body-weight gain, mortality, or gross
pathological appearance were observed. No symptoms characteristic of
delayed neurotoxicity were observed in hens given the single dose; one
hen given two doses had abnormal appearance and behaviour but
recovered the next day, while another developed ataxia complicated by
decreased activity and was killed in extremis on day 32. As
histopathological examination revealed no lesions in the brain, spinal
cord, or sciatic nerve tissue, characteristic delayed neurotoxicity
was not indicated in this study (Hixon, 1981).
Groups of white Leghorn hens were treated with cyfluthrin
(purity, 85-94%) in PEG 400 by gavage as either a single dose of 1000,
2500, or 5000 mg/kg bw, two doses of 5000 mg/kg bw at a three-week
interval, or daily administration of 5000 mg/kg bw for five
consecutive days. The single or multiple doses of 5000 mg/kg bw
induced noticeable behavioural alterations, episodes of drowsiness,
weight loss, uncoordinated gait, and some deaths. Several surviving
hens showed signs of excitation before recovery. Gross pathological
examination of hens at the high dose revealed spotty, brittle livers
and pale, slightly mottled kidneys. Histopathological examination
revealed minimal damage to the sciatic nerve, including axonal
swelling and fragmentation, proliferation of Schwann cells, and
increased vacuolization of myelin sheaths (Thyssen et al., 1981).
The authors of these two studies concluded that a delayed
neurotoxic effect was not apparent or consistent and indicated that
the histopathological findings were attributable to the normal
background neuropathic alterations seen in hens.
A group of 12 hens received a single oral dose of cyfluthrin in
PEG 400 at 4300 mg/kg bw and were observed for three weeks. A second
group of 16 hens received two doses of 4300 mg/kg bw three weeks apart
by gavage and were allowed to recover for eight weeks. A final group
of 12 hens received doses of 1500 mg/kg bw per day by gavage for five
consecutive days and were allowed to recover for eight weeks. All
animals were autopsied after the recovery periods. Neurotoxic esterase
activity was determined in the brains and spinal cords from five hens
of each group 24, 48, and 72 h and seven days after treatment. The
study was carried out in accordance with the specifications of OECD
GLP and US EPA Pesticide Assessment Guidelines, Subdivision F, Hazard
Evaluation, paragraph 81-7.
All treated hens showed signs of drowsiness and emaciation. One
control hen, two receiving 4300 mg/kg bw per day, and one receiving
1500 mg/kg bw per day died during treatment. One hen at the high dose
and one given multiple low doses were killed in extremis. No symptoms
of delayed-type neurotoxicity were seen in any treated birds. Food
consumption and body weights were not affected by treatment, and no
significant inhibition of neurotoxic esterase activity was noted in
any treated bird examined. No histopathological changes were seen in
hens receiving 1500 mg/kg bw per day cyfluthrin. Histopathological
examination of two hens receiving the high dose, one as a single dose
and one as two doses, showed slight axonal degeneration of single
nerve sites in the sciatic nerve and/or spinal cord (Sachsse, 1986).
Groups of 15 white Leghorn Lohmann strain laying hens were given
cyfluthrin formulated in PEG 400 at a dose of 5000 mg/kg bw per day or
tri-ortho-cresylphosphate as a positive control, orally for three
consecutive days. Three hens from each group were killed 24 h after
the first and second administration to determine neurotoxic esterase
activity; the remaining hens were killed 24 h after the third
administration. Hens receiving cyfluthrin displayed increased
salivation, dyspnoea, ruffled coat, and increased vocalization. Two
hens died after the second dose and six after the third dose. No
inhibition of neurotoxic esterase activity and no characteristic
delayed-type neurotoxicity were seen (Flucke & Eben, 1985).
2.2.7 Special studies on tumour promotion in vitro
As inhibition of intercellular communication is a common property
of known or suspected tumour promotors (Trosko et al., 1981; Chen
et al., 1984; Malcolm et al., 1985), inhibition of cellular
communication may be indicative of tumour-promoting potential.
Cyfluthrin at doses of 5-15 µmol/litre did not inhibit intracellular
communication (metabolic cooperation) in V79 fibroblasts and had no
effect on mutant cell recovery (Warngard & Flodstrom, 1989).
2.3 Observations in humans
The symptoms and signs of acute poisoning resulting from exposure
to different pyrethroids are similar. Clinical analysis of 573 cases
of acute pyrethroid poisoning due to occupational or accidental
exposure revealed symptoms including burning, itching, and tingling
sensations of the skin, which resolved after several hours. Washing
was not an effective treatment. The systemic symptoms included
dizziness, headache, nausea, anorexia, and fatigue; vomiting was most
common in cases due to ingestion of pyrethroids. Although less
frequently reported, tightness of the chest, paraesthesia,
palpitation, blurred vision, and increased sweating were observed in
some cases. Coarse muscular fasciculations were observed in more
serious cases. Convulsions and coma can also result from acute
poisoning with pyrethroids (He et al., 1989).
Several cases of acute occupational exposure to cyfluthrin have
been reported. The most significant effect is pronounced irritation
and burning of the skin and especially mucosal areas (lips, prepuce).
The symptoms usually appear after 1-2 h and last for one or two days.
A stinging sensation was observed 12-24 h after facial and mucosal
contact. A greater risk was associated with exposure to powdered
compound than to liquid formulations. The irritating effect was
probably due to a local action on sensitive nerve pathways and sensory
nerve cells (Flucke, 1979) and was reversible. With proper measures,
such as protective gloves and clothing, training, and avoidance of
dermal contact, occupational exposure and its potential effects can be
minimized (Miksche, 1979; Faul, 1984, 1988; Kollert, 1988).
3. COMMENTS
Cyfluthrin was evaluated by the 1987 Joint FAO/WHO Meeting on
Pesticide Residues (JMPR) (WHO/FAO, 1988), which established an ADI of
0-0.02 mg/kg bw. The same data and several new studies were reviewed
by the present Committee. The studies were performed according to
appropriate standards for study protocol and conduct.
In rats, cyfluthrin is readily absorbed and distributed. About
98% of radiolabelled drug was eliminated in urine and faeces within 48
h after oral administration, with similar amounts eliminated after
intravenous administration. In lactating dairy cattle, orally
administered cyfluthrin was also readily absorbed and distributed. The
concentration of cyfluthrin in milk reached a maximum three days after
initial dosing and then declined steadily. In cattle, the liver,
kidney, and fat contained the highest levels of residues, primarily of
unmetabolized parent compound. The major metabolic transformation is
ester hydrolysis to a 3-phenoxy-4-fluorobenzyl alcohol intermediate
and a permethric acid moiety. After ester hydrolysis, the benzyl
alcohol moiety is oxidized to the free 3-phenoxy-4-fluorobenzoic acid
metabolite, which can be either conjugated with glycine or oxidized to
give 4'-hydroxy-3-phenoxy-4-fluorobenzoic acid.
The acute oral LD50 values in rats ranged from 16 to more than
1000 mg/kg bw, depending on the vehicle used. At lethal or near-lethal
doses, signs typical of the toxicity of this class of pyrethroids were
observed. WHO has classified cyfluthrin as 'moderately hazardous'
(WHO, 1996).
Several short-term studies of the toxicity of orally administered
cyfluthrin were available in which it was tested in rats (four weeks;
three months), mice (four weeks), and dogs (six months), at doses
ranging from 5 to 450 mg/kg bw per day. Cyfluthrin reduced body weight
and increased liver and kidney weights. At lethal or near-lethal
doses, signs of neurotoxicity were observed, including ataxia,
abnormal gait, and increased vocalization. Histological evidence of
limited axonal demyelination of the sciatic nerves was observed at
these doses, which was completely reversed within three months of
cessation of treatment. In separate studies, the NOEL in rats ranged
from 5 mg/kg bw per day on the basis of depressed blood glucose levels
to 20 mg/kg bw per day on the basis of mortality and decreased
body-weight gain. The NOEL in mice was 43 mg/kg bw per day on the
basis of swelling of the submaxillary glandular epithelium. The NOEL
in dogs was equivalent to 2 mg/kg bw per day, on the basis of lowered
mean body weights.
In a three-week study of toxicity, rabbits were treated by dermal
application of cyfluthrin (in polyethylene glycol 400) at 0, 50, or
250 mg/kg bw per day for 6 h per day, five days per week for three
weeks. No toxicological effects were observed at any dose.
In a study in which dogs received cyfluthrin at up to 640 mg/kg
of feed, equal to 23 mg/kg bw per day, for one year, two out of six
dogs at the highest dose exhibited slight disturbances in movement and
gait on one occasion. There was no histopathological evidence of
neurotoxicity. Slight increases in the weights of the spleens of
females were observed at the high dose. The NOEL was therefore 160
mg/kg feed, equal to 5.1 mg/kg bw per day.
In long-term studies of toxicity and carcinogenicity in rats and
mice treated at dietary concentrations of cyfluthrin up to 450 and 800
mg/kg feed (equal to 19 mg/kg bw per day in rats and equivalent to 120
mg/kg bw per day in mice), respectively, the toxic effects were
largely non-specific and were essentially restricted to minor
alterations in body weight, organ weights, and blood biochemical
parameters. The NOELs were 50 mg/kg feed in rats, equal to 2 mg/kg bw
per day, and 200 mg/kg feed in mice, equivalent to 30 mg/kg bw per
day, both on the basis of decreased body-weight gain. Cyfluthrin
treatment was not associated with increased tumorigenesis in either
rats or mice.
In a three-generation study of reproductive toxicity in rats,
cyfluthrin at dietary concentrations of 0, 50, 150, or 450 mg/kg feed
had no consistent effect on birth weight or litter size. A decrease in
viability during lactation was noted in animals of the third
generation at the two higher levels, and weight gain was consistently
depressed in these animals. The NOEL was 50 mg/kg feed, equivalent to
2.5 mg/kg bw per day, on the basis of body-weight depression.
In a study of developmental toxicity, cyfluthrin was not
embryotoxic or teratogenic in rats given doses up to 30 mg/kg bw per
day on gestation days 6-15. The NOEL was 3 mg/kg bw per day for
maternal toxicity on the basis of clinical signs of altered gait and
30 mg/kg bw per day, the highest dose tested, for developmental
effects. The developmental toxicity of cyfluthrin has also been
evaluated in rabbits which received doses of 0, 20, 60, or 180 mg/kg
bw per day by gavage on days 6-18 of gestation. Food consumption and
body-weight gain were reduced and a dose-related increase in
post-implantation losses was observed in the groups at 60 and 180
mg/kg bw per day. The NOEL for maternal and embryotoxicity was 20
mg/kg bw per day on the basis of resorptions. The NOEL for
developmental toxicity was 180 mg/kg bw per day, the highest dose
tested.
Cyfluthrin has been tested for its ability to induce DNA damage,
chromosomal aberrations, and gene mutations in vitro and chromosomal
aberrations in vivo. Negative results were obtained in all of these
studies. These results, in conjunction with those of the studies of
carcinogenicity in rodents, indicate that cyfluthrin is neither
genotoxic nor carcinogenic.
In studies of the neurotoxicity of cyfluthrin in rats, daily oral
doses of 30-80 mg/kg bw in polyethylene glycol 400 for up to 35 weeks
resulted in characteristic signs of acute toxicity, including
salivation, tremor, and abnormal gait. Histological evidence of
limited swelling and fragmentation of myelin was observed only
infrequently, and these signs were completely reversible within three
months of cessation of treatment. Rats were also evaluated in a
neurobehavioural test, the inclined plane test, after administration
of single oral doses of up to 0.1 mg/kg bw cyfluthrin in
polyethoxylated castor oil (Cremophor EL). The results were
inconsistent and not dose-dependent. Hence, the Committee considered
that the results of this test were not useful for assessing
neurotoxicity in this case.
Although cyfluthrin is not an organophosphorus compound, it was
also evaluated for its ability to induce delayed-type neurotoxicity in
several studies in adult hens given single or multiple oral treatments
of up to 5000 mg/kg bw. The only neurological effects observed were
acute behavioural disturbances and minor changes in sciatic nerves,
including Schwann-cell proliferation and vacuolization of the myelin
sheath. Cyfluthrin produced no symptoms of delayed-type neurotoxicity
and no inhibition of neurotoxic target esterase in brain, spinal cord,
or the peripheral nervous system.
The Committee concluded that cyfluthrin does not cause
irreversible neurological damage and that the observed effects on the
nervous system occur only at high doses.
4. EVALUATION
The Committee concluded that the effects most relevant for the
toxicological evaluation of cyfluthrin were those observed in the
long-term study in rats fed the compound in the diet. The NOEL was 50
mg/kg in feed, equal to 2 mg/kg bw per day, on the basis of depression
of body-weight gain. Using this NOEL and a safety factor of 100, the
Committee established an ADI for cyfluthrin of 0-20 µg/kg bw. The
Committee noted that the same value had been established by the JMPR
in 1987.
5. REFERENCES
Bayer AG (1995) Chemical structure of cyfluthrin. Toxicological expert
opinion in accordance with Council Regulation (EEC) 2377/90 and
Directive (EEC) 81/852 as amended by Directive 92/18 (EEC).
Unpublished report from Bayer Institute of Toxicology. Submitted to
WHO by Bayer AG, Leverkeusen, Germany.
Becker, H. (1983) Embryotoxicity (including teratogenicity) study with
FCR 1272 in the rat. Unpublished report No. R 2774 from Research and
Consulting Co. AG, Itingen, Switzerland. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Becker, H. & Biedermann, K. (1992) Embryotocity study (including
teratogenicity) with FCR 1272 in the rabbit. Unpublished report No.
5770 from Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Brusick, D.J. (1982a) Mutagenicity evaluation of FCR 1272 in the
reverse mutation induction assay with Saccharomyces cerevisiae strains
S138 and S211, final report. Unpublished report No. 2200 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Brusick, D.J. (1982b) Evaluation of FCR 1272 in the reverse mutation
induction assay with Saccharomyces cerevisiae strains S138 and S211,
addendum to the final report. Unpublished report No. 2248 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Brusick, D.J. (1982c) Evaluation of FCR 1272 in the induced mitotic
crossing over, reverse mutation and gene conversion assay in
Saccharomyces cerevisiae strain D7. Addendum to final report.
Unpublished report No. 2249 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Chen, T.H., Kavanagh, T.J., Chang, C.C. & Trosko, J.E. (1984)
Inhibition of metabolic cooperation in Chinese hamster V79 cells by
various organic solvents and simple compounds. Cell Biol. Toxicol., 1,
155-171. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Curren, R. (1985) Unscheduled DNA synthesis in rat primary
hepatocytes. Unpublished report No. 701 (Mobay) from Microbiological
Associates, Bethesda, MD, USA. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Eben, A. & Thyssen, J. (1981) Thiocyanate excretion in rats' urine
after intraperitoneal administration of FCR 1272 and decamethrin in
comparable doses and after exposure to defined FCR 1272 concentrations
in the inhalation air. Unpublished report No. 10130 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Eben, A., Heimann, K.G. & Machemer, L. (1982) Comparative study of
rats on absorption of FCR 1272 after single oral administration in
polyethylene glycol 400 or Cremophor EL/water as formulation vehicle.
Unpublished report No. 1632 from Bayer Institute of Pharmacokinetics.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Ecker, W. (1982) Biotransformation of (F-phenyl-UL-14C)-cyfluthrin;
characterization and preliminary identification of metabolites.
Unpublished report No. 1632 from Bayer Institute of Pharmacokinetics.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Ecker, W. (1983) [Fluorobenzene ring-UL-14C]FCR 1272;
[fluorobenzene-UL-14C]Cyfluthrin: Metabolism part of the general
metabolism studies in the rat. Unpublished report No. 2059 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Faul, J. (1984) Cyfluthrin (FCR 1272). Unpublished letter report of 2
July 1984 from Bayer Institute of Toxicology. Submitted to WHO by
Bayer AG, Leverkeusen, Germany.
Faul, J. (1988) Medical data on employees in cyfluthrin formulation.
Letter of 28 March 1988 from Bayer Medical Department. Submitted to
WHO by Bayer AG, Leverkeusen, Germany.
Flucke, W. (1979) Irritant effects after work with FCR 1272.
Memorandum dated 8 October 1979 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Flucke, W. (1984a) FCR 1272 (cyfluthrin); BOQ 5812315 (propoxur);
study for combination toxicity. Unpublished report No. 12544 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Flucke, W. (1984b) FCR 1272 (cyfluthrin); DDVP (dichlorvos); study for
combination toxicity. Unpublished report No. 12567 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Flucke, W. (1984c) FCR 1272 (cyfluthrin); NAK 1654 (fenfluthrin);
study for combination toxicity. Unpublished report No. 12572 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Flucke, W. & Eben, A. (1985) FCR 1272. Study for effect on the
neurotoxic target enzyme (NTE) with the chicken (Gallus
domesticus). Unpublished report No. 13821 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Flucke, W. & Schilde, B. (1980) FCR 1272. Subacute oral toxicity study
on rats. Unpublished report No. 9039 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Flucke, W. & Thyssen, J. (1980) FCR 1272. Acute toxicity studies.
Unpublished report No. 8800 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Flucke, W. & Thyssen, J. (1981) FCR 1272 ( cis:trans isomer ratio =
55:45); acute toxicity study. Unpublished report No. 9673 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Flucke, W. & Vogel, O. (1980) FCR 1272. Subacute dermal toxicity study
on rabbits. Unpublished report No. 8928 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
He, F.S., Wang, S.G., Liu, L.H., Liu, L.H., Chen, S.Y., Zhang, Z.X. &
Sun, J.X. (1989) Clinical manifestation and diagnosis of acute
pyrethroid poisoning. Arch. Toxicol., 63, 54-58.
Heimann, K.G. (1982) FCR 1272; comparative tests for acute toxicity
with various formulation aids. Unpublished report No. 10931 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Heimann, K.G. (1983a) Test to determine antidote effect against FCR
1272 toxicity in rats. Unpublished report No. 11854 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Heimann, K.G. (1983b) Cyfluthrin and methamidophos study for
combination toxicity. Unpublished report No. 12003 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Heimann, K.G. (1983c) Determination of acute toxicity (LD50), letter
report of 22 February 1983. Unpublished report from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Heimann, K.G. (1984) Determination of acute toxicity (LD50), letter
report of 5 September 1984. Unpublished report from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Heimann, K.G. (1987) FCR 1272 (c.n. cyfluthrin); study for acute oral
toxicity to rats (formulation acetone and peanut oil). Unpublished
report No. 10931 from Bayer Institute of Toxicology. Submitted to WHO
by Bayer AG, Leverkeusen, Germany.
Heimann, K.G. & Kaliner, G. (1983) FCR 1272. Study for neurotoxic
effect on rats after subacute oral administration (with addendum).
Unpublished reports Nos 12338 and 12338a from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Herbold, B. (1980a) FCR 1272. Salmonella/microsome test for detection
of point-mutagenic effects. Unpublished report No. 9273 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Herbold, B. (1980b) Micronucleus test on mouse to evaluate FCR 1272
for mutagenic potential. Unpublished report No. 9435 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Herbold, B. (1981a) FCR 1272 cyfluthrin. Pol A1-test on E. coli to
evaluate effects for DNA damage. Unpublished report No. 10450 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Herbold, B. (1981b) Dominant lethal test on male mouse to evaluate FCR
1272 for mutagenic potential. Unpublished report No. 9678 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Herbold, B. (1988) FCR 1272 (c.n. cfluthrin). In vitro cytogenetic
study with human lymphocytes for the detection of induced clastogenic
effects. Report No. 17358 from Bayer Institsute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Hixon, E.J. (1981) Investigative neurotoxicity studies in hens.
Unpublished report No. 165 from Mobay Chemical Corporation, Corporate
Toxicology Department. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Hoffmann, K. (1981a) FCR 1272 (Cyfluthrin); acute oral toxicity to
dogs. Unpublished report No. T6010889 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Hoffmann, K. (1981b) FCR 1272. Acute toxicity to sheep after oral
administration. Unpublished report No. 9750 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Hoffmann, K. & Kaliner, G. (1981) FCR 1272. Chronic toxicity study on
dogs (six-month feeding experiment). Unpublished report No. 9991 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Hoffmann, K. & Schilde, B. (1983) FCR 1272 (Cyfluthrin). Chronic
toxicity to dogs on oral administration (12 months feeding study).
Unpublished report No. 11983 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Klein, O., Weber, H. & Suwelak, D. (1983) Biokinetic part of the
general metabolism studies in the rat. Unpublished report No. 11872
from Bayer Institute of Pharmacokinetics. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Kollert, W. (1988) Medical data on workers employed in cyfluthrin
production and formulation/registration of the active ingredient in
Brazil. Letter report of 2 March 1988. Unpublished report from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Krotlinger, F. (1988) E6876 and FCR 1272 (c.n. omethoate, cyfluthrin);
study for combination toxicity on rats. Unpublished report No. 16968
from Bayer Institute of Pharmacokinetics. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Loser, E. & Eiben, R. (1983) FCR 1272. Multigeneration study on rats.
Unpublished report No. 11870 from Bayer Institute of Pharmacokinetics.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Loser, E. & Schilde; B. (1980) FCR 1272. Subchronic toxicity study on
rats. Unpublished report No. 9386 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Malcolm, A.R., Mills, L.J. & Mckenna, E.J. (1985) Effects of phorbol
myristate acetate, phorbol dibutyrate, ethanol, dimethylsulfoxide,
phenol and seven metabolites of phenol on metabolic cooperation
between Chinese hamster V79 lung fibroblasts. Cell Biol. Toxicol., 1,
269-284. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Miksche, L. (1979) Symptoms of irritation when working with FCR 1272.
Unpublished report of 2 August 1979 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Nagane, M., Hatanaka, J. & Iyatomi, A. (1982) FCR 1272. Mutagenicity
test on bacterial system. Unpublished report No. 213 from Nihon
Tokushu Noyaku Seiko K.K. Agricultural Chemicals Institute, Japan.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Ohta, T. & Moriya, M. (1982) FCR 1272; Microbial mutagenicity study.
Unpublished report dated 17 May 1982 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Oikawa, K., Iyatomi, A. & Watanabe, M. (1983) FCR 1272. Special
toxicological study - morphological effects on the nervous system of
rats. Unpublished report dated 30 June 1983 from Nihon Tokushu Noyaku
Seiko K.K. Agricultural Chemicals Institute, Japan, and St Marianna
Medical College, Department of Pathology. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Pauluhn, J. (1987) FCR 1272; study of the acute inhalation toxicity to
rats using OECD guideline No. 403. Unpublished report No. 15612 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Polacek, I. (1984) Study of FCR 1272 on neuromuscular dysfunction in
the tilting plane test on rats. Unpublished report No. R2896 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Putman, D. (1985) Sister chromatid exchange assay in Chinese hamster
ovary (CHO) cells. Test article Baythroid (FCR 1272), technical
cyfluthrin. Unpublished report No. 693 (Mobay) from Microbiological
Associates, Bethesda, MD, USA. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Roetz, R. (1983) FCR 1272 [Cyfluthrin]. Study for embryotoxic effects
on rabbits after oral administration. Unpublished report No. 11855
from Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Sacchse, K. (1986) Acute delayed neurotoxicity study with FCR 1272
(common name cyfluthrin) in the hen. Unpublished report No. 3690 from
Research and Consulting Company AG, Itingen, Switzerland. Submitted to
WHO by Bayer AG, Leverkeusen, Germany.
Sacchse, K. & Zbinden, K. (1985) Acute oral toxicity study with FCR
1272 in the hen. Unpublished report No. R3622 from Research and
Consulting Company AG, Itingen, Switzerland. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Sasaki, Y., Imanishi, H., Watanabe, M. & Ohta, T. (1986) Cyfluthrin:
In vitro cytogenetics test. Unpublished report from Kodaira
Laboratories, Institute of Environmental Toxicology, Japan. Submitted
to WHO by Bayer AG, Leverkeusen, Germany.
Schluter, G. (1981) FCR 1272 [Cyfluthrin]. Study of embryotoxic and/or
teratogenic effects after oral administration to rabbits. Unpublished
report No. 10170 from Bayer Institute of Toxicology. Submitted to WHO
by Bayer AG, Leverkeusen, Germany.
Schluter, G. (1982) FCR 1272 [Cyfluthrin]. Evaluation for embryotoxic
and teratogenic effects on orally dosed rats. Unpublished report No.
10562 from Bayer Institute of Toxicology. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Shaw, H.R., Ayers, J.E. & McCann, S.A. (1983) Metabolism of Baythroid
in a dairy cow. Unpublished report No. 86043 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Suberg, H. & Loser, E. (1983a) FCR 1272 (Cyfluthrin). Chronic toxicity
study on mice (feeding study over 23 months). Unpublished report No.
12035 from Bayer Institute of Toxicology. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Suberg, H. & Loser, E. (1983b) FCR 1272 (Cyfluthrin). Chronic toxicity
study on rats. Unpublished report No. 11949 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Thyssen, J. & Vogel, O. (1982) FCR 1272. Study for nerve damage effect
on the rat after 5-months oral application. Unpublished report No.
10705 from Bayer Institute of Toxicology. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Thyssen, J., Kaliner, G. & Groning, P. (1981) Neurotoxicity studies on
hens. Unpublished report No. 9753 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Trosko, J.E., Yotti, L.P., Dawson, B. & Chang, C.C. (1981) In vitro
assay for tumor promoters. In: Stich, H.F. & San, R.H.C., eds,
Short-term Tests for Chemical Carcinogens, New York, Springer-Verlag,
pp. 420-427. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Warngard, L. & Flodstrom, S. (1989) Effects of tetradecanoyl phorbol
acetate, pyrethroids and DDT in the V79. Cell Biol. Toxicol., 5,
67-75. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Watanabe, M. & Iyatomi, A. (1984) FCR 1272; antidotal test.
Unpublished report No. 271 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Watanabe, M., Hatanaka, J., Uwanuma, Y., Itoh, H. & Iyatomi, A.
(1982a) FCR 1272; short-term toxicity tests on rats (4-week feeding
and 4-week recovery tests). Unpublished report No. 215 from Bayer
Institute of Pharmacokinetics. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Watanabe, M., Hatanaka, J., Uwanuma, Y., Itoh, H. & Iyatomi, A.
(1982b) FCR 1272; short-term toxicity tests on mice (4-week feeding
and 4-week recovery tests). Unpublished report No. 221 from Bayer
Institute of Pharmacokinetics. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.
WHO/FAO (1988) Pesticide Residues in Food - 1987 Report. FAO Plant
Production and Protection Paper 84, Rome.
Yang, L. & Louie, A. (1985) CHO/HGPRT mutation assay in the presence
and absence of exogenous metabolic activation. Test article Baythroid
(FCR 1272), technical cyfluthrin. Unpublished report No. 694 from
(Mobay) Microbiological Associates, Bethesda, MD, USA. Submitted to
WHO by Bayer AG, Leverkeusen, Germany.
tissues was on unmetabolized parent compound. Heart and kidney also
contained a metabolite identified as
4-fluoro-3-phenoxybenzenemethanol, and liver also contained
4-fluoro-3-phenoxybenzaldehyde (Shaw et al., 1983).
2.2 Toxicological studies
2.2.1 Acute toxicity
Cyfluthrin induces signs of toxicity in the species that have
been studied that are typical of cyano-containing type-II pyrethroid
poisoning. After oral administration, the symptoms of toxicity include
increased salivation, uncoordinated movements, increased activity and
vocalization, and reduced, laboured breathing. These signs appeared
within 10-60 min after dosing; the survivors usually recovered within
7-10 days (Flucke & Thyssen, 1980). Apathy, straddle-legged gait
(mostly in the rear legs), and reduced sensitivity to external stimuli
were observed after the acute intoxication phase. Rabbits showed signs
of apathy and suppression of appetite, while dogs vomited for up to
5 h after treatment with 50-100 mg/kg bw.
Anti-inflammatory, analgesic, anti-epileptic and sedative agents
and compounds that regulate neuromuscular transmission failed to
provide adequate protection against the oral toxicity of cyfluthrin.
Likewise, calcium, cyanide antidotes and blood pressure regulators had
no antagonistic effects. Tetrazepam, the active ingredient of
Musaril(R), a centrally acting muscle relaxant, increased the oral
LD50 in rats by a factor of 1.6 and delayed the onset of mortality
(Heimann, 1983a); however, the antagonistic effect was weak and did
not provide complete protection. Atropine sulfate at 50 mg/kg bw
combined with methocarbamol at 100 mg/kg bw had a moderate but
incomplete protective effect against the oral toxicity of cyfluthrin.
When one-half of the dose was administered to male rats 20 min, 3 h,
and 24 h after treatment with cyfluthrin, the LD50 increased from
2100 to 3100 mg/kg bw (Watanabe & Iyatomi, 1984).
Sub-additive effects were seen when cyfluthrin was combined in
equitoxic doses with methamidophos, propoxur, dichlorvos, or
fenfluthrin (Heimann, 1983b; Flucke, 1984a,b,c). Krotlinger (1988)
observed a slight super-additive effect after oral administration
simultaneously with the organophosphorous compound omethoate.
The studies on the acute toxicity of cyfluthrin are summarized in
Table 2.
Table 2. Acute toxicity of cyfluthrin to laboratory animals
Species Sex Route Purity LD50 or LC50 Remarks Reference
(%) (mg/kg bw
or mg/m3)
Mouse (NMRI) M Gavage 83.6 291 In PEG 400 Flucke & Thyssen (1980)
Mouse (NMRI) F Gavage 83.6 609 In PEG 400 Flucke & Thyssen (1980)
Rat (Wistar) M Gavage 83.6 1271 Fed, in PEG 400 Flucke & Thyssen (1980)
Rat (Wistar) F Gavage 83.6 869 Fed, in PEG 400 Flucke & Thyssen (1980)
Rat (Wistar) M Gavage 83.6 1189 Fasted, in PEG 400 Flucke & Thyssen (1980)
Rat (Wistar) F Gavage 83.6 590 Fasted, in PEG 400 Flucke & Thyssen (1980)
Rat NR Gavage cis:trans 425 cis:trans isomer Flucke & Thyssen (1981)
55:45 in PEG 400
Rat M Gavage NR 795 Fasted, in PEG 400 Heimann (1984)
Rat M Gavage NR 926 Fasted, in PEG 400 Heimann (1983a) warm)
Rat M Gavage NR 499 Fasted, in xylol Heimann (1983c)
Rat M Gavage NR 505 Fasted, in Heimann (1983c)
isododecan
Rabbit (New M Gavage 83.6 > 1000 In PEG 400 Flucke & Thyssen (1980)
Zealand)
Dog (beagle) M Gavage 83.6 > 100a In PEG 400 Flucke & Thyssen (1980)
Hen (white F Gavage 84-94 5000 In PEG 400 Thyssen et al. (1981)
Leghorn)
Hen (white F Gavage 93.5 > 5000 In Cremophor Sachsse & Zbinden (1985)
Leghorn) EL:dw
Hen (white F Gavage 93.5 4500 In PEG 400 Sachsse & Zbinden (1985)
Leghorn)
Mouse F Oral NR < 100 Fasted, in Heimann (1982)
Cremophor EL:dw
Rat M Oral NR 254 Fasted, in acetone: Heimann (1982)
oil (1:10)
Rat M Oral NR 396 Fasted, in dimethyl Heimann (1982)
sulfoxide
Rat M Oral NR 16.2 Fasted, in Heimann (1982)
Cremophor EL:dw
Rat M Oral NR 500-1000 N-Dimethyl Heimann (1982)
pyrroliodone
Table 2. (continued)
Species Sex Route Purity LD50 or LC50 Remarks Reference
(%) (mg/kg bw
or mg/m3)
Rat M Oral 93 155 Fasted, in acetone: Heimann (1987)
peanut oil
Rat F Oral 93 160 Fasted, in acetone: Heimann (1987)
peanut oil
Dog (beagle) M&F Oral 95 > 100a In Cremophor Hoffmann (1981a)
EL:dw
Sheep M&F Oral NR 1000 50% premix with Hoffmann (1981b)
Wessalon
Rat M Intraperitoneal 83.6 66 In PEG 400 Flucke & Thyssen (1980)
Rat F Intraperitoneal 83.6 104 In PEG 400 Flucke & Thyssen (1980)
Rat M Intraperitoneal NR 20 In Cremophor Heimann (1982)
EL:dw
Rat F Intraperitoneal NR 24 In Cremophor Heimann (1982)
EL:dw
Rat M Intraperitoneal NR 34 In PEG 400 Heimann (1982)
Rats F Intraperitoneal NR 94 In PEG 400 Heimann (1982)
Mouse M Subcutaneous 83.6 > 2500 In PEG 400 Flucke & Thyssen (1980)
Mouse F Subcutaneous 83.6 > 2500 In PEG 400 Flucke & Thyssen (1980)
Rat M Dermal 83.6 > 5000 NR Flucke & Thyssen (1980)
Rat F Dermal 83.6 > 5000 NR Flucke & Thyssen (1980)
Rat M&F Inhalation 83.6 > 1089 In 1:1 ethanol: Flucke & Thyssen (1980)
(1-h) PEG 400
Rat M&F Inhalation 83.6 469-592 In 1:1 ethanol: Flucke & Thyssen (1980)
(4-h) PEG 400
Rat M&F Inhalation cis:trans 180-326 cis:trans isomer in Flucke & Thyssen (1981)
(4-h) 55:45 PEG 400
Rat M&F Inhalation 93 405 PEG 400:ethanol Pauluhn (1987)
(4-h) 1:1
M, male; F, female; NR, not reported; dw, deionized water; PEG, polyethylene glycol
a Vomiting occurred at 50-100 mg/kg, no higher dose used
2.2.2 Short-term toxicity
Mice
Groups of 18 mice of each sex received diets containing 0, 300,
1000, or 3000 mg/kg cyfluthrin in the feed. After one month, 12 rats
of each sex in each group were sacrificed; the remainder were
sacrificed after four weeks of recovery on control diet. Mice at the
highest dose developed ataxia, salivation, and/or emaciation. One
female mouse at 3000 mg/kg feed died. Body-weight gain was depressed
in males at 3000 and in females at 1000 mg/kg feed. Decreased growth
rates were not seen during the recovery period, as all mice recovered
to normal control values. In males at 3000 mg/kg feed, alkaline
phosphatase and blood urea nitrogen levels were slightly increased,
while those of glutamate pyruvate transaminase and cholesterol were
normal throughout. Necropsy after treatment revealed dark-red livers
in some mice at 3000 mg/kg feed; this effect was not observed after
the recovery period. The relative weights of the submaxillary glands
and kidneys were increased in animals at the highest dose, and males
at this dose also had significantly increased relative liver weights.
The weights of both the liver and the submaxillary gland returned to
normal after the four-week recovery period, while those of the kidney
remained elevated. Histopathological examination revealed minimally
increased chromatin in the nuclei of hepatocytes of males receiving
1000 mg/kg feed or more (Watanabe et al., 1982a) and in females at
the high dose. Mice receiving 1000 mg/kg feed or more had cytoplasmic
swelling of the glandular epithelium of the submaxillary glands. These
findings all disappeared after the four-week recovery period. The NOEL
was 300 mg/kg feed, equivalent to 45 mg/kg bw per day (Watanabe
et al., 1982b).
Rats
Groups of 20 SPF Wistar albino rats of each sex received doses of
0, 5, 20, or 80 mg/kg bw per day cyfluthrin (purity, 85%) dissolved in
PEG 400 by gavage once daily for four weeks followed by a six-week
observation period. The high dose was lowered to 40 mg/kg bw per day
in weeks 2 and 4 because of severe intoxication. Physical appearance
and behaviour were assessed daily throughout the study, and body
weights were measured at the start of each week. Blood measurements
and urinalysis were performed on five male and five female rats from
each group at the end of the four-week treatment period and at the end
of the six-week observation period.
The control group and rats given doses of 5 or 20 mg/kg bw per
day behaved normally, but rats at the highest dose developed symptoms
of apathy, ruffled coat, dyspnoea, salivation, hyperkinesis, ataxia,
and uncoordinated movements. The symptoms were more pronounced during
weeks 1 and 3 of treatment at the highest dose. Deaths occurred only
in rats at the high dose, 6/20 males dying between treatment days 3
and 21 and one female on day 26; no deaths were noted during the
recovery period. The body-weight gain of male rats at the high dose
was reduced by 10% during week 4 of treatment, but the weights
returned to normal during the recovery period. The body weights and
body-weight gain of females were not affected by treatment.
Haematological parameters were all within the normal ranges, and no
toxicologically relevant, group-specific or dose-related differences
from controls were seen in serum enzyme activities or electrolyte
concentrations. The plasma activity of glutamate pyruvate transaminase
was slightly higher in rats receiving the highest dose than in
controls. Urinalysis and gross and histopathological examination
revealed no treatment-related variations from normal. Likewise, no
differences in blood chemical parameters were seen at the end of the
observation period. The absolute and relative weights of the adrenal
glands were increased in female rats at the end of treatment with the
highest dose, while only the relative weight of the liver was higher
in males at the high dose in comparison with the control animals. The
increase in relative adrenal weights was not accompanied by
histopathological changes. No deaths occurred during the recovery
period. No gross pathological changes related to administration of
cyfluthrin were seen after treatment or recovery, and there were no
signs of delayed neurotoxicity at the end of the recovery period. The
NOEL was 20 mg/kg bw per day (Flucke & Schilde, 1980).
Groups of 18 rats (strain unspecified) of each sex received diets
containing 0, 100, 300, or 1000 mg/kg cyfluthrin for one month, when
12 rats of each sex per group were sacrificed; the remainder were
killed after one month of recovery on control diet. Rats at the
highest dose developed straddled gait, salivation, and/or nervousness
during treatment, but these symptoms disappeared rapidly during the
recovery period. The body-weight gain, food intake, and water
consumption of animals of each sex at 1000 mg/kg feed were depressed
but returned to normal during the recovery period. Urinalysis
indicated a higher incidence of urobilinogen and ketone bodies in rats
at 1000 mg/kg feed, but, again, these signs returned to normal after
the end of treatment. The haematological effects seen after treatment
with 1000 mg/kg feed included a decrease in erythrocyte count,
haematocrit, and haemoglobin content, which also returned to normal
after the four-week recovery period. No significant effects were seen
on liver microsomal enzyme activity. Total protein and blood glucose
levels were significantly decreased in male rats given 300 mg/kg feed.
No effects were seen on macroscopic examination. Histopathologically,
minimal degeneration of single fibres of the sciatic nerve was noted
occasionally after the treatment period. Cytoplasmic swelling,
increasing the size of the glandular epithelium of the submaxillary
glands was observed at the high dose, but these alterations
disappeared after the recovery period. On the basis of the depressed
blood glucose levels in male rats at 300 mg/kg feed, the NOEL was 100
mg/kg feed, equivalent to 5 mg/kg bw (Watanabe et al., 1982a).
Groups of 30 male and 30 female Wistar rats received diets
containing 0, 30, 100, or 300 mg/kg cyfluthrin (purity, 84.2%) diluted
in an oil vehicle (Wessalon S; equivalent to a 50% premix) for three
months. They were observed daily for alterations in appearance and
behaviour, while body weights and food consumption were recorded
weekly. Five male and five female rats from each group were killed
after one and four weeks for interim examinations, including
microsomal enzyme induction assays ( N- and O-demethylase and
cytochrome P450 content). All rats that survived the treatment were
sacrificed at the end of treatment and examined.
Appearance and behaviour were not modified during treatment, even
at the highest dose. No effects were found on body weight, food
consumption, haematological, clinical chemical or urinary parameters,
or mortality rates. Blood sugar and cholesterol levels were within the
normal range throughout treatment. At week 1, a dose-independent
increase in N-demethylase activity was seen in all treated males and
in O-demethylase activity in females at 100 and 300 mg/kg feed; the
activity of enzymes of the cytochrome P450 system was significantly
increased only in males at 300 mg/kg feed at this time. By three
months, no differences were seen in the activities of the microsomal
enzymes in any treated group. No treatment-related effects were seen
on gross or histopathological examination. No toxic effects were seen
at any dose (Loser & Schilde, 1980).
Groups of 28 male and 28 female Sprague-Dawley rats received
diets containing 0, 100, 300, or 1000 mg/kg cyfluthrin (purity, 95%)
incorporated into the powdered diet on a 0.4% maximum clay carrier,
daily for three months. Body weights were recorded weekly and food
consumption and efficiency were determined. After treatment, 20 rats
at each dose were autopsied immediately, and the remaining eight rats
in each group were allowed to recover on a control diet for a
one-month observation period before autopsy. Clinical chemical and
urinary parameters were investigated before each sacrifice.
Only the rats at the highest dose showed signs of intoxication.
Both males and females of this dose showed abnormal gait and excess
salivation, increasing in incidence until the second week of feeding,
after which a gradual decrease was noted, although recovery from this
effect was somewhat delayed in males. By the third month of treatment,
no such symptoms were seen in any group. Mortality was not affected by
treatment. The food consumption and body-weight gains of females and
males at the highest dose were significantly lower than those of the
control group. The body-weight gain depression in females appeared to
recover after cessation of treatment, while that of the males remained
significantly low. Overall feed efficiency did not vary between
groups. The results of urinalysis and haematological examination were
within the normal physiological range and revealed no
treatment-related effects. Biochemical analysis showed that rats at
300 or 1000 mg/kg feed had significantly increased blood urea nitrogen
levels, and males at 1000 mg/kg feed had significantly increased
aspartate aminotransaminase activity. The blood glucose levels were
depressed in males at 300 or 1000 mg/kg feed and in females at 1000
mg/kg feed. All biochemical parameters were within normal limits after
the recovery period. The absolute weights of the liver, heart, and
lung were decreased in male rats at the high dose, and females at this
dose had depressed liver, kidney, adrenal, and ovarian weights. In
both males and females at the high dose, the relative weight of the
submaxillary gland was increased. No other treatment-related gross
pathological effect was observed. Degeneration of single fibres of the
sciatic nerve was noted in 5/20 males and 3/20 females at the high
dose after the three-month treatment period, and in 1/8 males at the
high dose after the one-month recovery. The authors concluded that the
damage was mild and minimal, corresponding to partial loss of the
myelin sheaths of single nerve fibres. The NOEL was 100 mg/kg feed,
equal to 6.2 mg/kg bw per day (Oikawa & Iyatomi, 1983).
Rabbits
In a three-week study of dermal toxicity, groups of three New
Zealand white rabbits of each sex were given dermal applications of 0,
50, or 250 mg/kg bw cyfluthrin (purity, 85%) dissolved in PEG 400 on
the intact or abraded skin. The treatments were applied five times a
week for a contact time of 6 h/day for three weeks, and then the
rabbits were killed and examined. Tissues were collected for
microscopic examination from controls and rabbits at the high dose;
the testes were collected from animals at 50 mg/kg bw per day. No
effects were observed on appearance, behaviour, or haematological,
clinical chemical, or urinary parameters. All rabbits survived the
treatment period. No toxicologically significant effect on body-weight
gain was observed. Gross and histopathological examination showed no
alterations due to treatment with cyfluthrin. The NOEL was 250 mg/kg
bw per day (Flucke & Vogel, 1980).
Dogs
Groups of six male and six female beagle dogs were fed cyfluthrin
(purity, 84.8%) in the diet at concentrations of 0, 65, 200, or 600
mg/kg for six months. No deaths occurred, and the physical appearance
of the dogs remained normal. Tests of reflexes and measurement of body
temperature showed no significant alterations. Five males and six
females receiving the high dose showed signs of uncoordinated, stiff
gait of the hind limbs, persisting for several hours, on several
occasions after week 21. Vomiting after dosing occurred slightly more
often in animals at the high dose than in controls. Diarrhoea occurred
in all groups including controls, but much more frequently in dogs at
the high dose, from the time of initiation of treatment. No effects
were found on food intake or water consumption in the controls or in
dogs at 65 or 200 mg/kg feed, but males at the high dose had slightly
lower food consumption during the first week of treatment. No group
differences in food consumption were observed by the sixth week. Lower
mean body weights were seen in males and females at 200 or 600 mg/kg
feed from week 2 to the end of treatment. Males at 200 and females at
600 mg/kg feed had lower average weight gains between weeks 1 and 26
of treatment, whereas the average body weights of males at the high
dose were similar to those of controls at the end of the feeding
period. No effects were found on ophthalmoscopic, haematological,
clinical chemical, or urinary parameters or on organ weights; no
treatment-related changes were seen grossly or histologically. The
NOEL was 65 mg/kg feed, equal to 2 mg/kg bw per day (Hoffmann &
Kaliner, 1981).
2.2.3 Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female mice SPF strain CF1/W74 mice
received a diet containing 0, 50, 200, or 800 mg/kg cyfluthrin in the
form of a 50% premix with Wessalon S for 23 months. Clinical
examinations were carried out on 10 mice of each sex at each dose 6,
12, 18, and 23 months after the initial administration. After
treatment, five mice of each sex from each group were selected
randomly for analysis of fluoride accumulation in bone and teeth. The
remaining mice were killed and examined grossly, and tissues were
taken for histopathological examination. Neither appearance nor
behaviour was altered in any group. No effects were observed on
mortality, haematological parameters, fluoridation of bones or teeth,
food intake, or water consumption. The body-weight gain of treated
animals was not affected at doses up to and including 200 mg/kg feed,
but mean body-weight gain was significantly reduced in females at the
high dose and males at this dose had a slightly lower body-weight gain
than controls. Plasma alkaline phosphatase activity was increased in
all treated males at the six-month examination; however, at the end of
the study several controls also showed elevated alkaline phosphatase
activity. Alanine aminotransaminase activity was slightly increased in
males at the high dose after 12 and 18 months of treatment. All other
clinical chemical values were within normal ranges. No
treatment-related alterations were seen at autopsy, and the relative
and absolute organ weights were not affected by treatment. Urinalysis
and microscopic examination revealed no evidence of treatment-related
damage at any dose. The incidence and histological type of
non-neoplastic and neoplastic alterations were all within normal
ranges. The NOEL was 200 mg/kg feed, equivalent to 30 mg/kg bw per day
(Suberg & Loser, 1983a).
Rats
Groups of 65 male and 65 female SPF BOR:WISW strain rats received
a diet containing 50, 150, or 450 mg/kg cyfluthrin premixed with
Wessalon S for two years. The control group consisted of 65 rats of
each sex fed untreated diet. Both the control and the test diets were
given ad libitum. All rats were observed twice a day for alterations
in appearance, behaviour, and activity. Body weights were determined
weekly up to week 4, then every other week to conclusion of treatment.
After seven days of treatment, five rats of each sex at each dose were
killed in order to measure mixed-function enzyme induction and
cytochrome P450 content. Clinical tests were conducted on 10 rats of
each sex per dose 6, 12, 18, and 24 months after the start of
treatment. After one and two years, five rats of each sex per dose
were killed in order to determine the fluoride content of their bones
and teeth. Rats that died during treatment and five rats of each sex
per dose killed at one year were examined grossly. All remaining rats
were autopsied and examined grossly and histologically at the end of
treatment.
No treatment-related effects were seen on appearance, behaviour,
activity, or food consumption. There were no treatment-related effects
on body-weight gain at the low dose, but rats at 150 mg/kg feed
appeared initially to have slightly decreased body-weight gain. The
high dose consistently induced significantly decreased body-weight
gain throughout the experiment. No treatment-related effects were seen
on mortality, and no effects were seen on haematological or urinary
parameters, gross appearance, organ weights, or histopathological
manifestations. N-Demethylase activity was increased by 30% in males
and 20% in females after seven days at the high dose, but there were
no effects on O-demethylase activity or P450 enzyme induction.
Induction of the microsomal enzyme N-demethylase in rats at the high
dose is considered not to be of toxicological relevance as no specific
morphological alteration or damage to the liver was seen at
histopathological examination. No significant differences in fluoride
accumulation in bones and teeth were noted at interim sacrifice.
Significant, dose-related differences were found after two years of
treatment, but the biological relevance of this finding was questioned
by the authors, as minimal changes in fluoride accumulation were seen
and the assay method involved large experimental error. The results of
the final necropsy showed isolated but significant differences in
organ weights, which were attributable to dissimilar body weights and
were not dose-related; they were considered to be of little
toxicological significance. The absolute kidney and liver weights were
decreased in rats at the high dose; however, there was no
dose-dependent effect on the relative weights. The incidence of
tumours was not altered by treatment at any dose, and no other
treatment-related alterations were observed histologically. The NOEL
was 50 mg/kg feed, equal to 2 mg/kg bw per day (Suberg & Loser,
1983b).
Dogs
Groups of six beagle dogs of each sex, 22-30 weeks old, were fed
a diet containing 0, 40, 160, or 640 mg/kg cyfluthrin for one year. No
effects on appearance or behaviour were seen in dogs at 40 or 160
mg/kg feed. Two dogs at the highest dose had slight disturbances in
movement, clumsy gait primarily in the hindquarters, and a reluctance
to move. This observation was made only once during weeks 36-37 of the
study. Reflex tests indicated no deviations in any treated animals.
Body temperature, mortality, and feed and water consumption were not
affected by treatment. The body-weight gain of males at the high dose
was slightly lower than that in the other groups; females at this dose
had somewhat higher weight gains than those at other doses. Males at
the high dose had a higher incidence of vomiting, and soft, pasty
faeces were observed more frequently than in controls and in the other
groups. Ophthalmoloscopy, haematology, clinical chemistry, and
urinalysis revealed no treatment-related alterations. At termination
of treatment, all of the dogs were killed and necropsied, and the
tissues of males at the high dose and of controls were evaluated
histologically. No significant treatment-related effects were found at
autopsy. Treatment had no effect on absolute or relative organ
weights, except that the mean spleen weight of females at the high
dose was absolutely and relatively higher than that of female
controls; however, the histopathological appearance was normal in all
treated animals. Microscopic examination of the sciatic nerve showed
no pathological alterations. The NOEL was 160 mg/kg feed, equal to 5.1
mg/kg bw per day (Hoffmann & Schilde, 1983).
2.2.4 Genotoxicity
Cyfluthrin had no mutagenic potential in vivo or in vitro.
The results of assays for genotoxicity with cyfluthrin are summarized
in Table 3.
2.2.5 Reproductive toxicity
2.2.5.1 Multigeneration reproductive toxicity
Rats
Groups of 10 male and 20 female SPF-Cpb rats, five to six weeks
old, received diets containing 0, 50, 150, or 450 mg/kg cyfluthrin as
a 50% premix with Wessalon S in the feed, given ad libitum 105 days
before and throughout mating, gestation, and rearing of pups. The
parameters measured included behaviour, growth, food consumption,
mortality, fertility, viability during gestation and lactation,
development of the young, parental body-weight gain, and male:female
ratio. The study was conducted for three generations, with two litters
per generation. F0 parents produced F1a and F1b litters; F1b
parents produced F2a and F2b litters; and F2b parents produced
F3a and F3b litters. The parameters assessed included fertility,
litter size, fetal birth weight, fetal variability, and parental
weight gain. Gross and histopathological examinations were also
performed on F2b parental animals and F3b pups.
Treatment did not affect appearance, behaviour, or survival,
although one F2a pup receiving 150 mg/kg feed and another receiving
450 mg/kg feed had convulsions. Treatment did not consistently affect
birth weight or litter size at birth. A decrease in pup survival from
birth through five days of lactation was seen in animals from the
F3a and F3b matings at 150 or 450 mg/kg feed, and decreased
survival of pups on days 5-28 of lactation was noted among pups at the
high dose in all matings. Similarly, body weight was consistently
suppressed in F3a and F3b generation rats at 150 and 450 mg/kg
feed during the 28-day lactation period. Necropsy revealed decreased
absolute liver and kidney weights in rats at 150 and 450 mg/kg feed;
this was probably related to the decreased body-weight gain in these
animals. No effect was observed on relative organ weights. The NOEL
was 50 mg/kg feed, equal to 3 mg/kg bw per day (Loser & Eiben, 1983).
Table 3. Results of assays for genotoxicity with cyfluthrin
End-point Test object Concentration Purity (%) Results S9 QA Reference
In vitro
Reverse mutation Salmonella typhimurium TA100, TA98, 20-24 000 µg/plate 83.6 Negative + No Herbold (1980a)
TA1535, TA1537 Negative -
Reverse mutation Salmonella typhimurium TA100, TA98, 5-5000 µg/plate 95.0 Negative + No Nagane et al.
TA1535, TA1537, TA1538 Negative - (1982)
Reverse mutation Salmonella typhimurium TA100, TA98, 10-25 000 µg/plate 95.0 Negative + No Ohta & Moriya
TA1535, TA1537, TA1538 Negative - (1982)
Reverse mutation Escherichia coli B/r WP2 try- hcr- 5-5000 µg/plate 95.0 Negative + No Nagane et al.
Negative - (1982)
Reverse mutation Escherichia coli WP2 hcr 10-25 000 µg/plate 95.0 Negative + No Ohta & Moriya
Negative - (1982)
Reverse mutation Saccharomyces cerevisiae S138, 312.5-10 000 µg/ml 95.0 Negative + No Brusick (1982a,b)
S211a) Negative -
Reverse mutation Saccharomyces cerevisiae D7 625-10 000 µg/ml 95.0 Negative + No Brusick (1982c)
Negative -
Gene mutation Chinese hamster ovary K1-BH4 cells, 3-10 µl/ml 94.7 Negative + Yes Yang & Louie
hprt locus Negative - (1985)
Chromosomal Chinese hamster lung cells 3.3 × 10-5-3.3 × 10-3 93.7 Negative + Yes Sasaki et al. aber
ration mol/litre Negative - (1986)
Chromosomal Human lymphocytes 500-5000 µg/ml 95.1-95.5 Negative + No Herbold (1988)
aberration Negative -
Sister chromatid Chinese hamster ovary cells 3-20 µg/ml Technical Negative + Yes Putman (1985)
exchange Negative -
DNA damage Escherichia coli pol A+ and pol A1- 62.5-1000 µg/plate 95.0 Negative + No Herbold (1981a)
Negative -
DNA damage Bacillus subtilis H17, M45 rec- 200 µg/disc 95.0 Negative + No Nagane et al.(1982)
DNA damage Bacillus subtillus H17, M45 rec- 100-10 000 µg/disc 95.0 Negative + No Ohta & Moriya
(1982)
Unscheduled DNA Male Sprague-Dawley rat primary 17-5000 µg/ml 94.7 Negative Yes Curren (1985)
synthesis hepatocytes
Table 3. (continued)
End-point Test object Concentration Purity (%) Results S9 QA Reference
Mitotic gene Saccharomyces cerevisiae D7 625-10 000 µg/ml 95.0 Negative + No Brusick (1982c)
conversion Negative -
Mitotic crossing Saccharomyces cerevisiae D7 625-10 000 µg/ml 95.0 Negative + No Brusick (1982c)
over Negative -
In vivo
Micronucleus Male and female NMRI mice, 2 × 7.5, 2 × 15 83.6 Negative No Herbold (1980b)
formation bone-marrow erythroblasts mg/kg bw orally
Dominant lethal Male NMRI/ORIG Kisslegg mice 30, 60 mg/kg bw 83.6 Negative No Herbold (1981b)
mutation orally
a S9, 9000 × g fraction of rat liver
2.2.5.2 Developmental toxicity
Rats
Groups of 25 outbred pregnant female Wistar KFM-HAN (SPF) rats
received 0, 1, 3, or 10 mg/kg bw per day cyfluthrin (purity, 93.4%) in
a 1% Cremophor EL solution in drinking-water by gavage on days 6-15 of
gestation. Clinical signs, body weights, and food consumption were
recorded. On day 21 of gestation, females were killed, caesarean
sections performed, and the pups were necropsied. Although the
standard was not specified, the study conduct underwent periodic
inspections by the quality assurance unit of the laboratory.
No effects on mortality were observed. Partial hair loss occurred
in one female at 3 mg/kg bw per day. Body-weight gain was not affected
by the low or middle doses. Rats receiving the high dose had 7.7%
lower corrected mean body-weight gain. Mean food consumption was 14%
lower in rats at the high dose on days 6-11 after mating. Mating,
fertility, and gestation parameters were normal, and no dose-related
difference was seen on examination and necropsy of dams. One dam at
the high dose totally resorbed its litter. Litter size, fetal body
weight, and sex ratio were not affected by treatment. The incidences
of visceral and skeletal anomalies were not increased by treatment in
a dose-dependent manner. No teratogenic effects were seen. As a
maternal maximum tolerated dose was not achieved in this study, the
authors did not report a NOEL (Becker, 1983).
Groups of 25 Bay:FB30 rats received cyfluthrin dissolved in PEG
400 at doses of 0, 3, 10, or 30 mg/kg bw per day by gavage during days
6-15 of gestation. They were mated in a 2:1 ratio with males. The
pregnant females were killed on day 20 of gestation for examination of
their uterine contents. Six rats at 10 or 30 mg/kg bw per day were
reported to have occasional 'high-stepping gait'; at the highest dose,
they showed signs of ataxia and decreased motility. No effects on
maternal weight gain were seen that were attributable to treatment.
Litter size, frequency of resorptions, and placental weights were not
affected by treatment, even at the highest dose. Likewise, no
treatment-related increase in the incidence of stunted growth or bone
anomalies was observed in the fetuses. The author concluded that
cyfluthrin had no specific teratogenic or embryotoxic effect. As 6/25
rats at 10 mg/kg bw per day showed signs of toxicity, the NOEL for
maternal toxicity was 3 mg/kg bw per day; the NOEL for fetal toxicity
was 30 mg/kg bw per day (Schluter, 1982).
Rabbits
In the first part of a two-part study, groups of 15 Himalayan
rabbits received cyfluthrin at 0, 3, 10, or 30 mg/kg bw per day in
Cremophor EL as a solution in drinking-water by gavage on days 6-18 of
gestation. The dams were killed on day 29 of gestation. Maternal
appearance and behaviour were not affected. Lower mean fetal and
placental weights were observed, and a higher incidence of
arthrogryposis (persistent flexure or contracture of a joint) was
observed in the nine fetuses of rabbits receiving 10 or 30 mg/kg bw
per day.
Because an embryotoxic effect was observed, a second study was
conducted. Groups of 15 pregnant Himalayan rabbits received cyfluthrin
at 0, 10, or 30 mg/kg bw per day in Cremophor EL as a solution in
drinking-water by gavage during days 6-18 of gestation. The dams were
killed on day 29 of gestation, and the fetuses removed for
examination. Doses up to and including 30 mg/kg bw per day had no
effect on behaviour or appearance. One dam at the high dose died of an
unknown cause on day 26 of gestation. Mean weight gain was affected by
treatment, and the average weight gain of the animals at 30 mg/kg bw
per day was statistically significantly lower than that of controls.
Treatment had no effect on the fertilization rate or gestation or on
embryonic development, including implantation rate, litter size, and
rate of resorptions. The incidence of skeletal anomalies was not
increased at any dose. One case of arthrogryposis occurred in the
control group. There was no indication of embryotoxicity or
teratogenicity. The NOEL for maternal toxicity was 30 mg/kg bw per day
and that for embryotoxicity was 30 mg/kg bw (Schluter, 1981).
Groups of 15 pregnant female Himalayan CHBB:HM rabbits received
cyfluthrin at 0, 5, 15, or 45 mg/kg bw per day by gavage in 0.5%
aqueous Cremophor EL emulsion during days 6-18 of gestation. Dams were
observed for clinical signs and body weight. On day 29 of gestation,
the dams were killed and their fetuses removed by caesarean section
and examined for anomalies. No effects were observed on mortality,
behaviour, appearance, insemination rate, or maternal weight gain. Two
rabbits at the high dose aborted on gestation days 25 and 28, and one
completely resorbed its implants. The authors concluded that the
effects seen at the high dose were treatment-related. Litter size,
placental weights, and fetal body weights were not affected by
treatment. The rate of resorptions appeared to be dose-related.
Although arthrogryposis was seen in rabbits at the high dose, the
event was considered to be spontaneous. The NOEL for maternal toxicity
was 15 mg/kg bw per day and that for embryotoxicity was 45 mg/kg bw
per day (Roetz, 1983).
In a more recent study, cyfluthrin was tested for its embryonic
and teratogenic potential in pregnant chinchilla rabbits (Chb:CH
hybrids, SPF). Groups of 16 pregnant rabbits received 0, 20, 60, or
180 mg/kg bw per day by gavage during days 6-18 of gestation. They
were killed 28 days after mating, and the fetuses were removed by
caesarean section. The study was conducted in accordance with the
specifications of the US FIFRA GLP (40 CFR Part 160), OECD test
guideline 414, and US EPA test guideline Vol. 43 Series 163.83-3
Subdivision F, Hazard Evaluation.
Clinical observations, appearance, and behaviour were not
affected by treatment. No deaths occurred. Food consumption and
body-weight gain were reduced in a dose-dependent manner in animals at
60 and 180 mg/kg bw per day but were significantly increased 24-28
days after mating. A dose-related, statistically significant increase
in post-implantation losses, due to increased embryonic resorptions,
was observed in rabbits given the two higher doses. Necropsy revealed
no significant alteration due to treatment. The sex ratio and fetal
body weights were unaffected by treatment, and no significant
alterations were found in fetal heads or brains. Skeletal alterations
were seen, including ossification of vertebrae, sternebrae, and ribs,
but these findings were considered to be within the normal spontaneous
range. There was no indication of a teratogenic effect. On the basis
of the increased post-implantation losses, the NOEL for maternal and
embryonic toxicity was 20 mg/kg bw per day (Becker & Biedermann,
1992).
2.2.6 Special studies on neurotoxicity
Rats
Fifty male Sprague-Dawley received technical-grade cyfluthrin
(purity, 95%) as a 1.6% solution in PEG 400 by gavage at a dose of 80
mg/kg bw per day on five consecutive days. The dose was reduced to 40
mg/kg bw per day on days 6-14 owing to the onset of mild to severe
symptoms of toxicity. Twenty-five male rats received PEG 400 alone.
All rats were observed for appearance and behaviour throughout
treatment and a recovery period of three months. Ten treated and five
control rats were killed, and their tissues examined histologically on
the days 1 and 5 of treatment and one, two, and three months after
treatment; tissues from two treated and one control males were also
examined at each time point by electron microscopy. The treated rats
showed symptoms of intoxication throughout the dosing period,
including abnormal, straddled gait, slow leg movements, and excess
salivation, but no symptoms were observed five days after treatment.
The body-weight gain of the treated rats was depressed in comparison
with controls throughout treatment but recovered during the
observation period. Histological examination revealed a minimal degree
of axonal degeneration (swelling and demyelination) in single fibres
of the sciatic nerve in 6/8 rats at the first examination, in 3/8 five
days to one month after treatment, and in 2/9 two months after
treatment. A similar lesion was reported in the femoral nerve of one
treated male examined on the fifth day after treatment but was no
longer seen three months later No alterations were reported in any
other nervous or muscular tissue examined. Electron microscopy
revealed dilatation of neurotubules, accompanied by proliferation of
neurofilaments and degeneration of mitochondria, which was maximal one
month after treatment but was not seen after three months. A NOEL was
not identified (Oikawa et al., 1983).
Groups of 15 male and 15 female Wistar TNO/W74 albino rats
received cyfluthrin (purity, 83.3%) emulsified in PEG 400 by gavage
daily on seven days a week for 35 weeks. The doses administered ranged
from 30 to 80 mg/kg bw per day and were varied intermittently, so that
the rats had signs of acute toxicity after each administration.
Clinical observations were recorded daily, while body weights were
recorded weekly. Clinical chemistry was evaluated at the end of the
study, and liver, kidney, adrenal gland, brain, spinal cord, and
sciatic nerve tissues from five treated and five control rats of each
sex were examined microscopically.
The treated group displayed characteristic signs of acute
toxicity throughout the study, including apathy, ruffled coat, and
respiratory disturbances. Increased salivation, tremor, and
uncoordinated gait were observed in some rats immediately after
dosing. No paralysis of the extremities was observed. Treated males
had a 20-25% reduction in body-weight gain, while no effect was seen
on female body-weight gain. Treatment had no effect on clinical
chemical parameters or induction of cytochrome P450 or N- or
O-demethylase activity. The mortality rate was significantly higher
among treated than control males. Gross pathological and microscopic
examination showed no treatment-related pathological alterations in
any organ examined. The weights of the liver and kidney were lower in
treated males, due mostly to their reduced body weight. Although the
authors reported no histological damage to the nervous tissue, rats
that died during the treatment period were not examined, limiting the
usefulness of this study. A NOEL was not identified (Thyssen & Vogel,
1982).
Groups of five male and five female Wistar (SPF-Cpb) rats
received cyfluthrin at 0 or 60 mg/kg bw per day for two weeks by
gavage, and a supplementary group of male rats received doses of 0 or
50 mg/kg bw per day. Modified preparation techniques, i.e. perfusion
of animals with formalin and fixation of the spinal column with cord
in toto, were used to determine the neurotoxic effect of cyfluthrin.
Symptoms of acute toxicity were observed in all treated animals,
including tremor, altered gait, and increased vocalization. Four males
at 60 mg/kg bw per day died between treatment days 5 and 8, but gross
pathologicam examination revealed no specific alterations. The
body-weight gain of females was not affected by treatment, but
decreases were seen for males at 50 or 60 mg/kg bw per day. Small,
fresh brain haemorrhages were seen in all four males that died on
test. The authors concluded that the 'most likely explanation is that
these are the result of a terminal cardiovascular disorder with
necrosis of the vascular walls'. Since this finding was not seen in
control animals, a treatment-related effect could not be ruled out. A
NOEL was not identified (Heimann & Kaliner, 1983).
In a supplementary study, neuromuscular dysfunction was assessed
in the tilting plane test. Groups of 10 male rats received cyfluthrin
dissolved in Cremophor EL as a single oral administration by stomach
tube, at doses of 0, 0.1, 0.3, or 1.0 mg/kg bw in the first experiment
and 0, 0.01, 0.03, or 0.1 in the second. Diazepam was given at a
single oral dose of 5 mg/kg bw as a positive control. The angle at
which the animal started to slide from a tilting plane was evaluated
for each rat 30 min and 2, 5 and 7 h after administration.
At a dose of 0.01 mg/kg bw, cyfluthrin had no effect at any time
interval. At 0.03 mg/kg bw, the slip angle was marginally
significantly ( p < 0.05) reduced, only at 7 h. At 0.1 mg/kg bw
cyfluthrin, the angle was reduced at 5 h but was normal at other times
(Polacek, 1984). In view of the lack of a standardized protocol for
this study, the use of Cremophor EL as a vehicle (which can
substantially enhance the acute toxicity of cyfluthrin), and the
absence of a dose- or time-dependent reduction in slip angle, the
Committee considered this study inappropriate for assessing
neurotoxicity.
Hens
In a study of delayed neurotoxicity, 10 adult laying hens were
given single doses of cyfluthrin in carbowax at 5000 mg/kg bw by
gavage, and a group of 20 hens received the same dose twice at an
interval of one week. Hens given the single dose were observed for
mortality and symptoms of toxicity and were killed 56 days after
treatment; those given two doses were killed 49 days after treatment.
No treatment-related effects on body-weight gain, mortality, or gross
pathological appearance were observed. No symptoms characteristic of
delayed neurotoxicity were observed in hens given the single dose; one
hen given two doses had abnormal appearance and behaviour but
recovered the next day, while another developed ataxia complicated by
decreased activity and was killed in extremis on day 32. As
histopathological examination revealed no lesions in the brain, spinal
cord, or sciatic nerve tissue, characteristic delayed neurotoxicity
was not indicated in this study (Hixon, 1981).
Groups of white Leghorn hens were treated with cyfluthrin
(purity, 85-94%) in PEG 400 by gavage as either a single dose of 1000,
2500, or 5000 mg/kg bw, two doses of 5000 mg/kg bw at a three-week
interval, or daily administration of 5000 mg/kg bw for five
consecutive days. The single or multiple doses of 5000 mg/kg bw
induced noticeable behavioural alterations, episodes of drowsiness,
weight loss, uncoordinated gait, and some deaths. Several surviving
hens showed signs of excitation before recovery. Gross pathological
examination of hens at the high dose revealed spotty, brittle livers
and pale, slightly mottled kidneys. Histopathological examination
revealed minimal damage to the sciatic nerve, including axonal
swelling and fragmentation, proliferation of Schwann cells, and
increased vacuolization of myelin sheaths (Thyssen et al., 1981).
The authors of these two studies concluded that a delayed
neurotoxic effect was not apparent or consistent and indicated that
the histopathological findings were attributable to the normal
background neuropathic alterations seen in hens.
A group of 12 hens received a single oral dose of cyfluthrin in
PEG 400 at 4300 mg/kg bw and were observed for three weeks. A second
group of 16 hens received two doses of 4300 mg/kg bw three weeks apart
by gavage and were allowed to recover for eight weeks. A final group
of 12 hens received doses of 1500 mg/kg bw per day by gavage for five
consecutive days and were allowed to recover for eight weeks. All
animals were autopsied after the recovery periods. Neurotoxic esterase
activity was determined in the brains and spinal cords from five hens
of each group 24, 48, and 72 h and seven days after treatment. The
study was carried out in accordance with the specifications of OECD
GLP and US EPA Pesticide Assessment Guidelines, Subdivision F, Hazard
Evaluation, paragraph 81-7.
All treated hens showed signs of drowsiness and emaciation. One
control hen, two receiving 4300 mg/kg bw per day, and one receiving
1500 mg/kg bw per day died during treatment. One hen at the high dose
and one given multiple low doses were killed in extremis. No symptoms
of delayed-type neurotoxicity were seen in any treated birds. Food
consumption and body weights were not affected by treatment, and no
significant inhibition of neurotoxic esterase activity was noted in
any treated bird examined. No histopathological changes were seen in
hens receiving 1500 mg/kg bw per day cyfluthrin. Histopathological
examination of two hens receiving the high dose, one as a single dose
and one as two doses, showed slight axonal degeneration of single
nerve sites in the sciatic nerve and/or spinal cord (Sachsse, 1986).
Groups of 15 white Leghorn Lohmann strain laying hens were given
cyfluthrin formulated in PEG 400 at a dose of 5000 mg/kg bw per day or
tri-ortho-cresylphosphate as a positive control, orally for three
consecutive days. Three hens from each group were killed 24 h after
the first and second administration to determine neurotoxic esterase
activity; the remaining hens were killed 24 h after the third
administration. Hens receiving cyfluthrin displayed increased
salivation, dyspnoea, ruffled coat, and increased vocalization. Two
hens died after the second dose and six after the third dose. No
inhibition of neurotoxic esterase activity and no characteristic
delayed-type neurotoxicity were seen (Flucke & Eben, 1985).
2.2.7 Special studies on tumour promotion in vitro
As inhibition of intercellular communication is a common property
of known or suspected tumour promotors (Trosko et al., 1981; Chen
et al., 1984; Malcolm et al., 1985), inhibition of cellular
communication may be indicative of tumour-promoting potential.
Cyfluthrin at doses of 5-15 µmol/litre did not inhibit intracellular
communication (metabolic cooperation) in V79 fibroblasts and had no
effect on mutant cell recovery (Warngard & Flodstrom, 1989).
2.3 Observations in humans
The symptoms and signs of acute poisoning resulting from exposure
to different pyrethroids are similar. Clinical analysis of 573 cases
of acute pyrethroid poisoning due to occupational or accidental
exposure revealed symptoms including burning, itching, and tingling
sensations of the skin, which resolved after several hours. Washing
was not an effective treatment. The systemic symptoms included
dizziness, headache, nausea, anorexia, and fatigue; vomiting was most
common in cases due to ingestion of pyrethroids. Although less
frequently reported, tightness of the chest, paraesthesia,
palpitation, blurred vision, and increased sweating were observed in
some cases. Coarse muscular fasciculations were observed in more
serious cases. Convulsions and coma can also result from acute
poisoning with pyrethroids (He et al., 1989).
Several cases of acute occupational exposure to cyfluthrin have
been reported. The most significant effect is pronounced irritation
and burning of the skin and especially mucosal areas (lips, prepuce).
The symptoms usually appear after 1-2 h and last for one or two days.
A stinging sensation was observed 12-24 h after facial and mucosal
contact. A greater risk was associated with exposure to powdered
compound than to liquid formulations. The irritating effect was
probably due to a local action on sensitive nerve pathways and sensory
nerve cells (Flucke, 1979) and was reversible. With proper measures,
such as protective gloves and clothing, training, and avoidance of
dermal contact, occupational exposure and its potential effects can be
minimized (Miksche, 1979; Faul, 1984, 1988; Kollert, 1988).
3. COMMENTS
Cyfluthrin was evaluated by the 1987 Joint FAO/WHO Meeting on
Pesticide Residues (JMPR) (WHO/FAO, 1988), which established an ADI of
0-0.02 mg/kg bw. The same data and several new studies were reviewed
by the present Committee. The studies were performed according to
appropriate standards for study protocol and conduct.
In rats, cyfluthrin is readily absorbed and distributed. About
98% of radiolabelled drug was eliminated in urine and faeces within 48
h after oral administration, with similar amounts eliminated after
intravenous administration. In lactating dairy cattle, orally
administered cyfluthrin was also readily absorbed and distributed. The
concentration of cyfluthrin in milk reached a maximum three days after
initial dosing and then declined steadily. In cattle, the liver,
kidney, and fat contained the highest levels of residues, primarily of
unmetabolized parent compound. The major metabolic transformation is
ester hydrolysis to a 3-phenoxy-4-fluorobenzyl alcohol intermediate
and a permethric acid moiety. After ester hydrolysis, the benzyl
alcohol moiety is oxidized to the free 3-phenoxy-4-fluorobenzoic acid
metabolite, which can be either conjugated with glycine or oxidized to
give 4'-hydroxy-3-phenoxy-4-fluorobenzoic acid.
The acute oral LD50 values in rats ranged from 16 to more than
1000 mg/kg bw, depending on the vehicle used. At lethal or near-lethal
doses, signs typical of the toxicity of this class of pyrethroids were
observed. WHO has classified cyfluthrin as 'moderately hazardous'
(WHO, 1996).
Several short-term studies of the toxicity of orally administered
cyfluthrin were available in which it was tested in rats (four weeks;
three months), mice (four weeks), and dogs (six months), at doses
ranging from 5 to 450 mg/kg bw per day. Cyfluthrin reduced body weight
and increased liver and kidney weights. At lethal or near-lethal
doses, signs of neurotoxicity were observed, including ataxia,
abnormal gait, and increased vocalization. Histological evidence of
limited axonal demyelination of the sciatic nerves was observed at
these doses, which was completely reversed within three months of
cessation of treatment. In separate studies, the NOEL in rats ranged
from 5 mg/kg bw per day on the basis of depressed blood glucose levels
to 20 mg/kg bw per day on the basis of mortality and decreased
body-weight gain. The NOEL in mice was 43 mg/kg bw per day on the
basis of swelling of the submaxillary glandular epithelium. The NOEL
in dogs was equivalent to 2 mg/kg bw per day, on the basis of lowered
mean body weights.
In a three-week study of toxicity, rabbits were treated by dermal
application of cyfluthrin (in polyethylene glycol 400) at 0, 50, or
250 mg/kg bw per day for 6 h per day, five days per week for three
weeks. No toxicological effects were observed at any dose.
In a study in which dogs received cyfluthrin at up to 640 mg/kg
of feed, equal to 23 mg/kg bw per day, for one year, two out of six
dogs at the highest dose exhibited slight disturbances in movement and
gait on one occasion. There was no histopathological evidence of
neurotoxicity. Slight increases in the weights of the spleens of
females were observed at the high dose. The NOEL was therefore 160
mg/kg feed, equal to 5.1 mg/kg bw per day.
In long-term studies of toxicity and carcinogenicity in rats and
mice treated at dietary concentrations of cyfluthrin up to 450 and 800
mg/kg feed (equal to 19 mg/kg bw per day in rats and equivalent to 120
mg/kg bw per day in mice), respectively, the toxic effects were
largely non-specific and were essentially restricted to minor
alterations in body weight, organ weights, and blood biochemical
parameters. The NOELs were 50 mg/kg feed in rats, equal to 2 mg/kg bw
per day, and 200 mg/kg feed in mice, equivalent to 30 mg/kg bw per
day, both on the basis of decreased body-weight gain. Cyfluthrin
treatment was not associated with increased tumorigenesis in either
rats or mice.
In a three-generation study of reproductive toxicity in rats,
cyfluthrin at dietary concentrations of 0, 50, 150, or 450 mg/kg feed
had no consistent effect on birth weight or litter size. A decrease in
viability during lactation was noted in animals of the third
generation at the two higher levels, and weight gain was consistently
depressed in these animals. The NOEL was 50 mg/kg feed, equivalent to
2.5 mg/kg bw per day, on the basis of body-weight depression.
In a study of developmental toxicity, cyfluthrin was not
embryotoxic or teratogenic in rats given doses up to 30 mg/kg bw per
day on gestation days 6-15. The NOEL was 3 mg/kg bw per day for
maternal toxicity on the basis of clinical signs of altered gait and
30 mg/kg bw per day, the highest dose tested, for developmental
effects. The developmental toxicity of cyfluthrin has also been
evaluated in rabbits which received doses of 0, 20, 60, or 180 mg/kg
bw per day by gavage on days 6-18 of gestation. Food consumption and
body-weight gain were reduced and a dose-related increase in
post-implantation losses was observed in the groups at 60 and 180
mg/kg bw per day. The NOEL for maternal and embryotoxicity was 20
mg/kg bw per day on the basis of resorptions. The NOEL for
developmental toxicity was 180 mg/kg bw per day, the highest dose
tested.
Cyfluthrin has been tested for its ability to induce DNA damage,
chromosomal aberrations, and gene mutations in vitro and chromosomal
aberrations in vivo. Negative results were obtained in all of these
studies. These results, in conjunction with those of the studies of
carcinogenicity in rodents, indicate that cyfluthrin is neither
genotoxic nor carcinogenic.
In studies of the neurotoxicity of cyfluthrin in rats, daily oral
doses of 30-80 mg/kg bw in polyethylene glycol 400 for up to 35 weeks
resulted in characteristic signs of acute toxicity, including
salivation, tremor, and abnormal gait. Histological evidence of
limited swelling and fragmentation of myelin was observed only
infrequently, and these signs were completely reversible within three
months of cessation of treatment. Rats were also evaluated in a
neurobehavioural test, the inclined plane test, after administration
of single oral doses of up to 0.1 mg/kg bw cyfluthrin in
polyethoxylated castor oil (Cremophor EL). The results were
inconsistent and not dose-dependent. Hence, the Committee considered
that the results of this test were not useful for assessing
neurotoxicity in this case.
Although cyfluthrin is not an organophosphorus compound, it was
also evaluated for its ability to induce delayed-type neurotoxicity in
several studies in adult hens given single or multiple oral treatments
of up to 5000 mg/kg bw. The only neurological effects observed were
acute behavioural disturbances and minor changes in sciatic nerves,
including Schwann-cell proliferation and vacuolization of the myelin
sheath. Cyfluthrin produced no symptoms of delayed-type neurotoxicity
and no inhibition of neurotoxic target esterase in brain, spinal cord,
or the peripheral nervous system.
The Committee concluded that cyfluthrin does not cause
irreversible neurological damage and that the observed effects on the
nervous system occur only at high doses.
4. EVALUATION
The Committee concluded that the effects most relevant for the
toxicological evaluation of cyfluthrin were those observed in the
long-term study in rats fed the compound in the diet. The NOEL was 50
mg/kg in feed, equal to 2 mg/kg bw per day, on the basis of depression
of body-weight gain. Using this NOEL and a safety factor of 100, the
Committee established an ADI for cyfluthrin of 0-20 µg/kg bw. The
Committee noted that the same value had been established by the JMPR
in 1987.
5. REFERENCES
Bayer AG (1995) Chemical structure of cyfluthrin. Toxicological expert
opinion in accordance with Council Regulation (EEC) 2377/90 and
Directive (EEC) 81/852 as amended by Directive 92/18 (EEC).
Unpublished report from Bayer Institute of Toxicology. Submitted to
WHO by Bayer AG, Leverkeusen, Germany.
Becker, H. (1983) Embryotoxicity (including teratogenicity) study with
FCR 1272 in the rat. Unpublished report No. R 2774 from Research and
Consulting Co. AG, Itingen, Switzerland. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Becker, H. & Biedermann, K. (1992) Embryotocity study (including
teratogenicity) with FCR 1272 in the rabbit. Unpublished report No.
5770 from Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Brusick, D.J. (1982a) Mutagenicity evaluation of FCR 1272 in the
reverse mutation induction assay with Saccharomyces cerevisiae strains
S138 and S211, final report. Unpublished report No. 2200 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Brusick, D.J. (1982b) Evaluation of FCR 1272 in the reverse mutation
induction assay with Saccharomyces cerevisiae strains S138 and S211,
addendum to the final report. Unpublished report No. 2248 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Brusick, D.J. (1982c) Evaluation of FCR 1272 in the induced mitotic
crossing over, reverse mutation and gene conversion assay in
Saccharomyces cerevisiae strain D7. Addendum to final report.
Unpublished report No. 2249 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Chen, T.H., Kavanagh, T.J., Chang, C.C. & Trosko, J.E. (1984)
Inhibition of metabolic cooperation in Chinese hamster V79 cells by
various organic solvents and simple compounds. Cell Biol. Toxicol., 1,
155-171. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Curren, R. (1985) Unscheduled DNA synthesis in rat primary
hepatocytes. Unpublished report No. 701 (Mobay) from Microbiological
Associates, Bethesda, MD, USA. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Eben, A. & Thyssen, J. (1981) Thiocyanate excretion in rats' urine
after intraperitoneal administration of FCR 1272 and decamethrin in
comparable doses and after exposure to defined FCR 1272 concentrations
in the inhalation air. Unpublished report No. 10130 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Eben, A., Heimann, K.G. & Machemer, L. (1982) Comparative study of
rats on absorption of FCR 1272 after single oral administration in
polyethylene glycol 400 or Cremophor EL/water as formulation vehicle.
Unpublished report No. 1632 from Bayer Institute of Pharmacokinetics.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Ecker, W. (1982) Biotransformation of (F-phenyl-UL-14C)-cyfluthrin;
characterization and preliminary identification of metabolites.
Unpublished report No. 1632 from Bayer Institute of Pharmacokinetics.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Ecker, W. (1983) [Fluorobenzene ring-UL-14C]FCR 1272;
[fluorobenzene-UL-14C]Cyfluthrin: Metabolism part of the general
metabolism studies in the rat. Unpublished report No. 2059 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Faul, J. (1984) Cyfluthrin (FCR 1272). Unpublished letter report of 2
July 1984 from Bayer Institute of Toxicology. Submitted to WHO by
Bayer AG, Leverkeusen, Germany.
Faul, J. (1988) Medical data on employees in cyfluthrin formulation.
Letter of 28 March 1988 from Bayer Medical Department. Submitted to
WHO by Bayer AG, Leverkeusen, Germany.
Flucke, W. (1979) Irritant effects after work with FCR 1272.
Memorandum dated 8 October 1979 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Flucke, W. (1984a) FCR 1272 (cyfluthrin); BOQ 5812315 (propoxur);
study for combination toxicity. Unpublished report No. 12544 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Flucke, W. (1984b) FCR 1272 (cyfluthrin); DDVP (dichlorvos); study for
combination toxicity. Unpublished report No. 12567 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Flucke, W. (1984c) FCR 1272 (cyfluthrin); NAK 1654 (fenfluthrin);
study for combination toxicity. Unpublished report No. 12572 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Flucke, W. & Eben, A. (1985) FCR 1272. Study for effect on the
neurotoxic target enzyme (NTE) with the chicken (Gallus
domesticus). Unpublished report No. 13821 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Flucke, W. & Schilde, B. (1980) FCR 1272. Subacute oral toxicity study
on rats. Unpublished report No. 9039 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Flucke, W. & Thyssen, J. (1980) FCR 1272. Acute toxicity studies.
Unpublished report No. 8800 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Flucke, W. & Thyssen, J. (1981) FCR 1272 ( cis:trans isomer ratio =
55:45); acute toxicity study. Unpublished report No. 9673 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Flucke, W. & Vogel, O. (1980) FCR 1272. Subacute dermal toxicity study
on rabbits. Unpublished report No. 8928 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
He, F.S., Wang, S.G., Liu, L.H., Liu, L.H., Chen, S.Y., Zhang, Z.X. &
Sun, J.X. (1989) Clinical manifestation and diagnosis of acute
pyrethroid poisoning. Arch. Toxicol., 63, 54-58.
Heimann, K.G. (1982) FCR 1272; comparative tests for acute toxicity
with various formulation aids. Unpublished report No. 10931 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Heimann, K.G. (1983a) Test to determine antidote effect against FCR
1272 toxicity in rats. Unpublished report No. 11854 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Heimann, K.G. (1983b) Cyfluthrin and methamidophos study for
combination toxicity. Unpublished report No. 12003 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Heimann, K.G. (1983c) Determination of acute toxicity (LD50), letter
report of 22 February 1983. Unpublished report from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Heimann, K.G. (1984) Determination of acute toxicity (LD50), letter
report of 5 September 1984. Unpublished report from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Heimann, K.G. (1987) FCR 1272 (c.n. cyfluthrin); study for acute oral
toxicity to rats (formulation acetone and peanut oil). Unpublished
report No. 10931 from Bayer Institute of Toxicology. Submitted to WHO
by Bayer AG, Leverkeusen, Germany.
Heimann, K.G. & Kaliner, G. (1983) FCR 1272. Study for neurotoxic
effect on rats after subacute oral administration (with addendum).
Unpublished reports Nos 12338 and 12338a from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Herbold, B. (1980a) FCR 1272. Salmonella/microsome test for detection
of point-mutagenic effects. Unpublished report No. 9273 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Herbold, B. (1980b) Micronucleus test on mouse to evaluate FCR 1272
for mutagenic potential. Unpublished report No. 9435 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Herbold, B. (1981a) FCR 1272 cyfluthrin. Pol A1-test on E. coli to
evaluate effects for DNA damage. Unpublished report No. 10450 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Herbold, B. (1981b) Dominant lethal test on male mouse to evaluate FCR
1272 for mutagenic potential. Unpublished report No. 9678 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Herbold, B. (1988) FCR 1272 (c.n. cfluthrin). In vitro cytogenetic
study with human lymphocytes for the detection of induced clastogenic
effects. Report No. 17358 from Bayer Institsute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Hixon, E.J. (1981) Investigative neurotoxicity studies in hens.
Unpublished report No. 165 from Mobay Chemical Corporation, Corporate
Toxicology Department. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Hoffmann, K. (1981a) FCR 1272 (Cyfluthrin); acute oral toxicity to
dogs. Unpublished report No. T6010889 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Hoffmann, K. (1981b) FCR 1272. Acute toxicity to sheep after oral
administration. Unpublished report No. 9750 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Hoffmann, K. & Kaliner, G. (1981) FCR 1272. Chronic toxicity study on
dogs (six-month feeding experiment). Unpublished report No. 9991 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Hoffmann, K. & Schilde, B. (1983) FCR 1272 (Cyfluthrin). Chronic
toxicity to dogs on oral administration (12 months feeding study).
Unpublished report No. 11983 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Klein, O., Weber, H. & Suwelak, D. (1983) Biokinetic part of the
general metabolism studies in the rat. Unpublished report No. 11872
from Bayer Institute of Pharmacokinetics. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Kollert, W. (1988) Medical data on workers employed in cyfluthrin
production and formulation/registration of the active ingredient in
Brazil. Letter report of 2 March 1988. Unpublished report from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Krotlinger, F. (1988) E6876 and FCR 1272 (c.n. omethoate, cyfluthrin);
study for combination toxicity on rats. Unpublished report No. 16968
from Bayer Institute of Pharmacokinetics. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Loser, E. & Eiben, R. (1983) FCR 1272. Multigeneration study on rats.
Unpublished report No. 11870 from Bayer Institute of Pharmacokinetics.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Loser, E. & Schilde; B. (1980) FCR 1272. Subchronic toxicity study on
rats. Unpublished report No. 9386 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Malcolm, A.R., Mills, L.J. & Mckenna, E.J. (1985) Effects of phorbol
myristate acetate, phorbol dibutyrate, ethanol, dimethylsulfoxide,
phenol and seven metabolites of phenol on metabolic cooperation
between Chinese hamster V79 lung fibroblasts. Cell Biol. Toxicol., 1,
269-284. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Miksche, L. (1979) Symptoms of irritation when working with FCR 1272.
Unpublished report of 2 August 1979 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Nagane, M., Hatanaka, J. & Iyatomi, A. (1982) FCR 1272. Mutagenicity
test on bacterial system. Unpublished report No. 213 from Nihon
Tokushu Noyaku Seiko K.K. Agricultural Chemicals Institute, Japan.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Ohta, T. & Moriya, M. (1982) FCR 1272; Microbial mutagenicity study.
Unpublished report dated 17 May 1982 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Oikawa, K., Iyatomi, A. & Watanabe, M. (1983) FCR 1272. Special
toxicological study - morphological effects on the nervous system of
rats. Unpublished report dated 30 June 1983 from Nihon Tokushu Noyaku
Seiko K.K. Agricultural Chemicals Institute, Japan, and St Marianna
Medical College, Department of Pathology. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Pauluhn, J. (1987) FCR 1272; study of the acute inhalation toxicity to
rats using OECD guideline No. 403. Unpublished report No. 15612 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Polacek, I. (1984) Study of FCR 1272 on neuromuscular dysfunction in
the tilting plane test on rats. Unpublished report No. R2896 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Putman, D. (1985) Sister chromatid exchange assay in Chinese hamster
ovary (CHO) cells. Test article Baythroid (FCR 1272), technical
cyfluthrin. Unpublished report No. 693 (Mobay) from Microbiological
Associates, Bethesda, MD, USA. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Roetz, R. (1983) FCR 1272 [Cyfluthrin]. Study for embryotoxic effects
on rabbits after oral administration. Unpublished report No. 11855
from Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Sacchse, K. (1986) Acute delayed neurotoxicity study with FCR 1272
(common name cyfluthrin) in the hen. Unpublished report No. 3690 from
Research and Consulting Company AG, Itingen, Switzerland. Submitted to
WHO by Bayer AG, Leverkeusen, Germany.
Sacchse, K. & Zbinden, K. (1985) Acute oral toxicity study with FCR
1272 in the hen. Unpublished report No. R3622 from Research and
Consulting Company AG, Itingen, Switzerland. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Sasaki, Y., Imanishi, H., Watanabe, M. & Ohta, T. (1986) Cyfluthrin:
In vitro cytogenetics test. Unpublished report from Kodaira
Laboratories, Institute of Environmental Toxicology, Japan. Submitted
to WHO by Bayer AG, Leverkeusen, Germany.
Schluter, G. (1981) FCR 1272 [Cyfluthrin]. Study of embryotoxic and/or
teratogenic effects after oral administration to rabbits. Unpublished
report No. 10170 from Bayer Institute of Toxicology. Submitted to WHO
by Bayer AG, Leverkeusen, Germany.
Schluter, G. (1982) FCR 1272 [Cyfluthrin]. Evaluation for embryotoxic
and teratogenic effects on orally dosed rats. Unpublished report No.
10562 from Bayer Institute of Toxicology. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Shaw, H.R., Ayers, J.E. & McCann, S.A. (1983) Metabolism of Baythroid
in a dairy cow. Unpublished report No. 86043 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Suberg, H. & Loser, E. (1983a) FCR 1272 (Cyfluthrin). Chronic toxicity
study on mice (feeding study over 23 months). Unpublished report No.
12035 from Bayer Institute of Toxicology. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Suberg, H. & Loser, E. (1983b) FCR 1272 (Cyfluthrin). Chronic toxicity
study on rats. Unpublished report No. 11949 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Thyssen, J. & Vogel, O. (1982) FCR 1272. Study for nerve damage effect
on the rat after 5-months oral application. Unpublished report No.
10705 from Bayer Institute of Toxicology. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Thyssen, J., Kaliner, G. & Groning, P. (1981) Neurotoxicity studies on
hens. Unpublished report No. 9753 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Trosko, J.E., Yotti, L.P., Dawson, B. & Chang, C.C. (1981) In vitro
assay for tumor promoters. In: Stich, H.F. & San, R.H.C., eds,
Short-term Tests for Chemical Carcinogens, New York, Springer-Verlag,
pp. 420-427. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Warngard, L. & Flodstrom, S. (1989) Effects of tetradecanoyl phorbol
acetate, pyrethroids and DDT in the V79. Cell Biol. Toxicol., 5,
67-75. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Watanabe, M. & Iyatomi, A. (1984) FCR 1272; antidotal test.
Unpublished report No. 271 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Watanabe, M., Hatanaka, J., Uwanuma, Y., Itoh, H. & Iyatomi, A.
(1982a) FCR 1272; short-term toxicity tests on rats (4-week feeding
and 4-week recovery tests). Unpublished report No. 215 from Bayer
Institute of Pharmacokinetics. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Watanabe, M., Hatanaka, J., Uwanuma, Y., Itoh, H. & Iyatomi, A.
(1982b) FCR 1272; short-term toxicity tests on mice (4-week feeding
and 4-week recovery tests). Unpublished report No. 221 from Bayer
Institute of Pharmacokinetics. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.
WHO/FAO (1988) Pesticide Residues in Food - 1987 Report. FAO Plant
Production and Protection Paper 84, Rome.
Yang, L. & Louie, A. (1985) CHO/HGPRT mutation assay in the presence
and absence of exogenous metabolic activation. Test article Baythroid
(FCR 1272), technical cyfluthrin. Unpublished report No. 694 from
(Mobay) Microbiological Associates, Bethesda, MD, USA. Submitted to
WHO by Bayer AG, Leverkeusen, Germany.
See Also:
Toxicological Abbreviations
Cyfluthrin (ICSC)
CYFLUTHRIN (JECFA Evaluation)
Cyfluthrin (Pesticide residues in food: 1987 evaluations Part II Toxicology)
Cyfluthrin (UKPID)
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
The influence of the formulation vehicle on the rate of
absorption of cyfluthrin was studied in groups of 14 fasted male
Wistar rats given single doses by gavage of 10 mg/kg bw dissolved in
either polyethylene glycol (PEG) 400 or a Cremophor EL:water emulsion.
Two rats from each group were killed 0.5, 1, 2, 4, 6, 16, and 24 h
after treatment. The concentrations of cyfluthrin and its respective
enantiomers were determined in the blood and stomach. When the
compound was emulsified in the Cremophor EL:water solution, absorption
was rapid; the cis isomer was that most frequently detected within
30 min after treatment. Maximum peak blood concentrations occurred
within 1 h of treatment. Cyfluthrin emulsified in PEG 400 was not
detected until 4 h after administration, and peak blood levels were
observed only 6 h after treatment. Examination of the stomach contents
revealed a larger quantity of cyfluthrin in the stomachs of rats
treated with the drug in a PEG 400 emulsion (Eben et al., 1982).
Rats
Four groups of 30 male and 24 female Mura:SPRA (SPF 68 Han) rats
received 14C radiolabelled cyfluthrin as either oral doses of 0.5 or
10 mg/kg bw or intravenous or intraduodenal doses of 0.5 mg/kg bw.
Another group received unlabelled cyfluthrin orally once a day for 14
consecutive days, followed by a single oral dose of 0.5 mg/kg bw
14C-cyfluthrin. Excreta, organs, tissues, and blood samples were
collected at several intervals and assayed for radiolabel.
After oral administration by any schedule, up to 80% of the
labelled cyfluthrin was effectively absorbed by females and about 90%
by males. The half-time for absorption was 30-32 min, and absorption
commenced 13 min after administration. The absorption half-time was
dependent on neither dose, sex, nor pretreatment. Excretion was rapid
and proportional to dose. Less than 2% of radiolabelled drug was
present 48 h after oral administration. No significant pulmonary
excretion pathway exists, as < 0.001% was detected in the expired
gaseous phase as 14CO21. About 98-99% of the orally administered
dose was readily available for renal and faecal excretion, which would
be expected to be eliminated within two days. Males excreted two to
three times more in the urine than in the faeces, whereas the
renal:faecal excretion ratio in females was 1.2-1.7:1 after oral
administration. Consequently, the area under the curve (AUC) is two
times larger for females than males.
Forty-eight hours after the intravenous administration, 93-95% of
the dose was excreted, with a renal:faecal excretion ratio of 2.9:1 in
males and 2.3:1 in females. Therefore, excretion depends somewhat on
the route of administration and on sex. Rats with bile fistulae
excreted one-third of the dose within two days, more than 50% of which
was excreted within 2 h and 90% within 6 h of administration. These
rats excreted about 50% of the recovered radioactivity via the urine
and < 12% via faeces. A proportion of the radiolabelled dose excreted
in bile underwent enterohepatic circulation.
The apparent volume of distribution after the intravenous
treatment was calculated to be 17%, indicating distribution primarily
to the extracellular fluid. Plasma elimination, i.e. distribution to
the tissues, was biphasic, with an initial rapid half-time of 2.1 h
and then a slower, dose- and pretreatment-independent half-time of
21 h. The maximum relative concentration in the plasma was independent
of dose and was observed 2 h after oral administration in male and
female rats.
The residues found in the organs and tissues were influenced by
the route of administration, as the mean relative concentration of
cyfluthrin in the bodies of males and females at sacrifice was lower
after oral administration (0.013) than after intravenous injection
(0.06). Female rats had higher plasma concentrations after oral
administration of the single high or low dose; 48 h after
administration, lower concentrations were detected in the bone and
muscle of animals of each sex and in the testes of males rats. The
sciatic nerve showed a similar relative concentration value, which may
explain the toxic effects observed on the peripheral nervous system.
Higher concentrations were detected in the spleen, adrenal glands,
liver, and plasma of both males and females and in the ovaries. The
renal fatty tissue concentration was about seven times higher after
either oral or intravenous administration, whereas the mean
concentration in brain was significantly lower ( p = 0.0006-0.006)
(Klein et al., 1983).
2.1.2 Biotransformation
Rats
Eight hours after oral administration of 14C-labelled
cyfluthrin at a dose of 10 mg/kg bw to three male Sprague-Dawley rats,
about 60% of the labelled cyfluthrin was eliminated in the urine in
conjugated forms. Conjugates of 4'-hydroxy-3-phenoxyfluorobenzoic acid
(50%) were identified; a second major metabolite was identified after
hydrochloric acid hydrolysis as a conjugate of
3-phenoxy-4-fluorohippuric acid (40%). These metabolites represented
33 and 27% of the administered radiolabel, respectively. A glycine
conjugate constituted 2.5% of the conjugated metabolites (Ecker,
1982).
After intraperitoneal injection of 14C-cyfluthrin to Wistar
(TNO/W 74) albino rats at doses of 1, 5, 10, or 15 mg/kg bw, urine was
collected for three days. It was found that 24-42% of the alpha-cyano
group of cyfluthrin was excreted via the kidneys in the thiocyanate
form (Eben & Thyssen, 1981).
In another study carried out to characterize the complete kinetic
behaviour and metabolic pathway of cyfluthrin in rats, four groups of
five male and five female rats received 14C-cyfluthrin by a protocol
identical to that described by Klein et al. (1983). The initial step
in cyfluthrin biotransformation was ester hydrolysis, giving a
3-phenoxy-4-fluorobenzyl alcohol intermediate and the permethric acid
fraction. The metabolism of permethric acid has been well established
in the rat in studies with chemically similar pyrethroids. After ester
hydrolysis, the 3-phenoxy-4-fluorobenzyl alcohol moiety was oxidized
to the free metabolite 3-phenoxy-4-fluorobenzoic acid. This metabolite
can then either be conjugated with glycine to form
3-phenoxy-4-fluorohippuric acid (a minor metabolite constituting < 3%
of the recovered urinary radiolabel, dependent on neither sex nor
dose) or hydroxylated to give 4'-hydroxy-3-phenoxy-4-fluorobenzoic
acid (conjugates of which account for 41-50% of the total urinary
radiolabel recovered from rats given one or multiple doses of
cyfluthrin at 0.5 mg/kg bw). Females tended to excrete more of this
metabolite as the free form in the faeces than did males. Males and
females at the high dose (10 mg/kg bw) excreted about 35% of the
administered dose as conjugates of
4'-hydroxy-3-phenoxy-4-fluorobenzoic acid, whereas females excreted
about 5% more than males as the free metabolite. After repeated oral
doses of 0.5 mg/kg bw for 14 days, 12-16% of labelled metabolite was
found in the faeces as cyfluthrin, whereas < 1% was found when single
oral doses were administered. After a single high dose of 10 mg/kg bw,
17-19% was recovered in the faeces as parent compound. The authors
concluded that the metabolism of cyfluthrin is slightly dose-dependent
(Ecker, 1983).
The proposed metabolic pathway of cyfluthrin in rats is shown in
Figure 2.
Cattle
A 484-kg lactating Holstein dairy cow (Bos taurus) was given
14C-cyfluthrin orally at a dose of 0.5 mg/kg bw after the evening
milking each day for five consecutive days. Milk was collected in the
morning and evening throughout the study. The cow was sacrificed on
day 6 after initial administration, and blood, major organs, fatty
tissue, and muscle were analysed for radiolabel. The maximum
concentration of radioactivity in the milk was 0.079 mg/litre
cyfluthrin equivalents three days after initial dosing; the level then
declined steadily. Of the radiolabel found in milk, 98% was on
unmetabolized parent compound. The highest levels of residues were
found in the liver (0.622 mg/kg), fat (0.195 mg/kg), and kidney (0.188
mg/kg); brain, skeletal muscle, and myocardium had the lowest levels,
ranging from 0.015 to 0.040 mg/kg. Most of the radiolabel recovered in
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
The influence of the formulation vehicle on the rate of
absorption of cyfluthrin was studied in groups of 14 fasted male
Wistar rats given single doses by gavage of 10 mg/kg bw dissolved in
either polyethylene glycol (PEG) 400 or a Cremophor EL:water emulsion.
Two rats from each group were killed 0.5, 1, 2, 4, 6, 16, and 24 h
after treatment. The concentrations of cyfluthrin and its respective
enantiomers were determined in the blood and stomach. When the
compound was emulsified in the Cremophor EL:water solution, absorption
was rapid; the cis isomer was that most frequently detected within
30 min after treatment. Maximum peak blood concentrations occurred
within 1 h of treatment. Cyfluthrin emulsified in PEG 400 was not
detected until 4 h after administration, and peak blood levels were
observed only 6 h after treatment. Examination of the stomach contents
revealed a larger quantity of cyfluthrin in the stomachs of rats
treated with the drug in a PEG 400 emulsion (Eben et al., 1982).
Rats
Four groups of 30 male and 24 female Mura:SPRA (SPF 68 Han) rats
received 14C radiolabelled cyfluthrin as either oral doses of 0.5 or
10 mg/kg bw or intravenous or intraduodenal doses of 0.5 mg/kg bw.
Another group received unlabelled cyfluthrin orally once a day for 14
consecutive days, followed by a single oral dose of 0.5 mg/kg bw
14C-cyfluthrin. Excreta, organs, tissues, and blood samples were
collected at several intervals and assayed for radiolabel.
After oral administration by any schedule, up to 80% of the
labelled cyfluthrin was effectively absorbed by females and about 90%
by males. The half-time for absorption was 30-32 min, and absorption
commenced 13 min after administration. The absorption half-time was
dependent on neither dose, sex, nor pretreatment. Excretion was rapid
and proportional to dose. Less than 2% of radiolabelled drug was
present 48 h after oral administration. No significant pulmonary
excretion pathway exists, as < 0.001% was detected in the expired
gaseous phase as 14CO21. About 98-99% of the orally administered
dose was readily available for renal and faecal excretion, which would
be expected to be eliminated within two days. Males excreted two to
three times more in the urine than in the faeces, whereas the
renal:faecal excretion ratio in females was 1.2-1.7:1 after oral
administration. Consequently, the area under the curve (AUC) is two
times larger for females than males.
Forty-eight hours after the intravenous administration, 93-95% of
the dose was excreted, with a renal:faecal excretion ratio of 2.9:1 in
males and 2.3:1 in females. Therefore, excretion depends somewhat on
the route of administration and on sex. Rats with bile fistulae
excreted one-third of the dose within two days, more than 50% of which
was excreted within 2 h and 90% within 6 h of administration. These
rats excreted about 50% of the recovered radioactivity via the urine
and < 12% via faeces. A proportion of the radiolabelled dose excreted
in bile underwent enterohepatic circulation.
The apparent volume of distribution after the intravenous
treatment was calculated to be 17%, indicating distribution primarily
to the extracellular fluid. Plasma elimination, i.e. distribution to
the tissues, was biphasic, with an initial rapid half-time of 2.1 h
and then a slower, dose- and pretreatment-independent half-time of
21 h. The maximum relative concentration in the plasma was independent
of dose and was observed 2 h after oral administration in male and
female rats.
The residues found in the organs and tissues were influenced by
the route of administration, as the mean relative concentration of
cyfluthrin in the bodies of males and females at sacrifice was lower
after oral administration (0.013) than after intravenous injection
(0.06). Female rats had higher plasma concentrations after oral
administration of the single high or low dose; 48 h after
administration, lower concentrations were detected in the bone and
muscle of animals of each sex and in the testes of males rats. The
sciatic nerve showed a similar relative concentration value, which may
explain the toxic effects observed on the peripheral nervous system.
Higher concentrations were detected in the spleen, adrenal glands,
liver, and plasma of both males and females and in the ovaries. The
renal fatty tissue concentration was about seven times higher after
either oral or intravenous administration, whereas the mean
concentration in brain was significantly lower ( p = 0.0006-0.006)
(Klein et al., 1983).
2.1.2 Biotransformation
Rats
Eight hours after oral administration of 14C-labelled
cyfluthrin at a dose of 10 mg/kg bw to three male Sprague-Dawley rats,
about 60% of the labelled cyfluthrin was eliminated in the urine in
conjugated forms. Conjugates of 4'-hydroxy-3-phenoxyfluorobenzoic acid
(50%) were identified; a second major metabolite was identified after
hydrochloric acid hydrolysis as a conjugate of
3-phenoxy-4-fluorohippuric acid (40%). These metabolites represented
33 and 27% of the administered radiolabel, respectively. A glycine
conjugate constituted 2.5% of the conjugated metabolites (Ecker,
1982).
After intraperitoneal injection of 14C-cyfluthrin to Wistar
(TNO/W 74) albino rats at doses of 1, 5, 10, or 15 mg/kg bw, urine was
collected for three days. It was found that 24-42% of the alpha-cyano
group of cyfluthrin was excreted via the kidneys in the thiocyanate
form (Eben & Thyssen, 1981).
In another study carried out to characterize the complete kinetic
behaviour and metabolic pathway of cyfluthrin in rats, four groups of
five male and five female rats received 14C-cyfluthrin by a protocol
identical to that described by Klein et al. (1983). The initial step
in cyfluthrin biotransformation was ester hydrolysis, giving a
3-phenoxy-4-fluorobenzyl alcohol intermediate and the permethric acid
fraction. The metabolism of permethric acid has been well established
in the rat in studies with chemically similar pyrethroids. After ester
hydrolysis, the 3-phenoxy-4-fluorobenzyl alcohol moiety was oxidized
to the free metabolite 3-phenoxy-4-fluorobenzoic acid. This metabolite
can then either be conjugated with glycine to form
3-phenoxy-4-fluorohippuric acid (a minor metabolite constituting < 3%
of the recovered urinary radiolabel, dependent on neither sex nor
dose) or hydroxylated to give 4'-hydroxy-3-phenoxy-4-fluorobenzoic
acid (conjugates of which account for 41-50% of the total urinary
radiolabel recovered from rats given one or multiple doses of
cyfluthrin at 0.5 mg/kg bw). Females tended to excrete more of this
metabolite as the free form in the faeces than did males. Males and
females at the high dose (10 mg/kg bw) excreted about 35% of the
administered dose as conjugates of
4'-hydroxy-3-phenoxy-4-fluorobenzoic acid, whereas females excreted
about 5% more than males as the free metabolite. After repeated oral
doses of 0.5 mg/kg bw for 14 days, 12-16% of labelled metabolite was
found in the faeces as cyfluthrin, whereas < 1% was found when single
oral doses were administered. After a single high dose of 10 mg/kg bw,
17-19% was recovered in the faeces as parent compound. The authors
concluded that the metabolism of cyfluthrin is slightly dose-dependent
(Ecker, 1983).
The proposed metabolic pathway of cyfluthrin in rats is shown in
Figure 2.
Cattle
A 484-kg lactating Holstein dairy cow (Bos taurus) was given
14C-cyfluthrin orally at a dose of 0.5 mg/kg bw after the evening
milking each day for five consecutive days. Milk was collected in the
morning and evening throughout the study. The cow was sacrificed on
day 6 after initial administration, and blood, major organs, fatty
tissue, and muscle were analysed for radiolabel. The maximum
concentration of radioactivity in the milk was 0.079 mg/litre
cyfluthrin equivalents three days after initial dosing; the level then
declined steadily. Of the radiolabel found in milk, 98% was on
unmetabolized parent compound. The highest levels of residues were
found in the liver (0.622 mg/kg), fat (0.195 mg/kg), and kidney (0.188
mg/kg); brain, skeletal muscle, and myocardium had the lowest levels,
ranging from 0.015 to 0.040 mg/kg. Most of the radiolabel recovered in
 tissues was on unmetabolized parent compound. Heart and kidney also
contained a metabolite identified as
4-fluoro-3-phenoxybenzenemethanol, and liver also contained
4-fluoro-3-phenoxybenzaldehyde (Shaw et al., 1983).
2.2 Toxicological studies
2.2.1 Acute toxicity
Cyfluthrin induces signs of toxicity in the species that have
been studied that are typical of cyano-containing type-II pyrethroid
poisoning. After oral administration, the symptoms of toxicity include
increased salivation, uncoordinated movements, increased activity and
vocalization, and reduced, laboured breathing. These signs appeared
within 10-60 min after dosing; the survivors usually recovered within
7-10 days (Flucke & Thyssen, 1980). Apathy, straddle-legged gait
(mostly in the rear legs), and reduced sensitivity to external stimuli
were observed after the acute intoxication phase. Rabbits showed signs
of apathy and suppression of appetite, while dogs vomited for up to
5 h after treatment with 50-100 mg/kg bw.
Anti-inflammatory, analgesic, anti-epileptic and sedative agents
and compounds that regulate neuromuscular transmission failed to
provide adequate protection against the oral toxicity of cyfluthrin.
Likewise, calcium, cyanide antidotes and blood pressure regulators had
no antagonistic effects. Tetrazepam, the active ingredient of
Musaril(R), a centrally acting muscle relaxant, increased the oral
LD50 in rats by a factor of 1.6 and delayed the onset of mortality
(Heimann, 1983a); however, the antagonistic effect was weak and did
not provide complete protection. Atropine sulfate at 50 mg/kg bw
combined with methocarbamol at 100 mg/kg bw had a moderate but
incomplete protective effect against the oral toxicity of cyfluthrin.
When one-half of the dose was administered to male rats 20 min, 3 h,
and 24 h after treatment with cyfluthrin, the LD50 increased from
2100 to 3100 mg/kg bw (Watanabe & Iyatomi, 1984).
Sub-additive effects were seen when cyfluthrin was combined in
equitoxic doses with methamidophos, propoxur, dichlorvos, or
fenfluthrin (Heimann, 1983b; Flucke, 1984a,b,c). Krotlinger (1988)
observed a slight super-additive effect after oral administration
simultaneously with the organophosphorous compound omethoate.
The studies on the acute toxicity of cyfluthrin are summarized in
Table 2.
Table 2. Acute toxicity of cyfluthrin to laboratory animals
Species Sex Route Purity LD50 or LC50 Remarks Reference
(%) (mg/kg bw
or mg/m3)
Mouse (NMRI) M Gavage 83.6 291 In PEG 400 Flucke & Thyssen (1980)
Mouse (NMRI) F Gavage 83.6 609 In PEG 400 Flucke & Thyssen (1980)
Rat (Wistar) M Gavage 83.6 1271 Fed, in PEG 400 Flucke & Thyssen (1980)
Rat (Wistar) F Gavage 83.6 869 Fed, in PEG 400 Flucke & Thyssen (1980)
Rat (Wistar) M Gavage 83.6 1189 Fasted, in PEG 400 Flucke & Thyssen (1980)
Rat (Wistar) F Gavage 83.6 590 Fasted, in PEG 400 Flucke & Thyssen (1980)
Rat NR Gavage cis:trans 425 cis:trans isomer Flucke & Thyssen (1981)
55:45 in PEG 400
Rat M Gavage NR 795 Fasted, in PEG 400 Heimann (1984)
Rat M Gavage NR 926 Fasted, in PEG 400 Heimann (1983a) warm)
Rat M Gavage NR 499 Fasted, in xylol Heimann (1983c)
Rat M Gavage NR 505 Fasted, in Heimann (1983c)
isododecan
Rabbit (New M Gavage 83.6 > 1000 In PEG 400 Flucke & Thyssen (1980)
Zealand)
Dog (beagle) M Gavage 83.6 > 100a In PEG 400 Flucke & Thyssen (1980)
Hen (white F Gavage 84-94 5000 In PEG 400 Thyssen et al. (1981)
Leghorn)
Hen (white F Gavage 93.5 > 5000 In Cremophor Sachsse & Zbinden (1985)
Leghorn) EL:dw
Hen (white F Gavage 93.5 4500 In PEG 400 Sachsse & Zbinden (1985)
Leghorn)
Mouse F Oral NR < 100 Fasted, in Heimann (1982)
Cremophor EL:dw
Rat M Oral NR 254 Fasted, in acetone: Heimann (1982)
oil (1:10)
Rat M Oral NR 396 Fasted, in dimethyl Heimann (1982)
sulfoxide
Rat M Oral NR 16.2 Fasted, in Heimann (1982)
Cremophor EL:dw
Rat M Oral NR 500-1000 N-Dimethyl Heimann (1982)
pyrroliodone
Table 2. (continued)
Species Sex Route Purity LD50 or LC50 Remarks Reference
(%) (mg/kg bw
or mg/m3)
Rat M Oral 93 155 Fasted, in acetone: Heimann (1987)
peanut oil
Rat F Oral 93 160 Fasted, in acetone: Heimann (1987)
peanut oil
Dog (beagle) M&F Oral 95 > 100a In Cremophor Hoffmann (1981a)
EL:dw
Sheep M&F Oral NR 1000 50% premix with Hoffmann (1981b)
Wessalon
Rat M Intraperitoneal 83.6 66 In PEG 400 Flucke & Thyssen (1980)
Rat F Intraperitoneal 83.6 104 In PEG 400 Flucke & Thyssen (1980)
Rat M Intraperitoneal NR 20 In Cremophor Heimann (1982)
EL:dw
Rat F Intraperitoneal NR 24 In Cremophor Heimann (1982)
EL:dw
Rat M Intraperitoneal NR 34 In PEG 400 Heimann (1982)
Rats F Intraperitoneal NR 94 In PEG 400 Heimann (1982)
Mouse M Subcutaneous 83.6 > 2500 In PEG 400 Flucke & Thyssen (1980)
Mouse F Subcutaneous 83.6 > 2500 In PEG 400 Flucke & Thyssen (1980)
Rat M Dermal 83.6 > 5000 NR Flucke & Thyssen (1980)
Rat F Dermal 83.6 > 5000 NR Flucke & Thyssen (1980)
Rat M&F Inhalation 83.6 > 1089 In 1:1 ethanol: Flucke & Thyssen (1980)
(1-h) PEG 400
Rat M&F Inhalation 83.6 469-592 In 1:1 ethanol: Flucke & Thyssen (1980)
(4-h) PEG 400
Rat M&F Inhalation cis:trans 180-326 cis:trans isomer in Flucke & Thyssen (1981)
(4-h) 55:45 PEG 400
Rat M&F Inhalation 93 405 PEG 400:ethanol Pauluhn (1987)
(4-h) 1:1
M, male; F, female; NR, not reported; dw, deionized water; PEG, polyethylene glycol
a Vomiting occurred at 50-100 mg/kg, no higher dose used
2.2.2 Short-term toxicity
Mice
Groups of 18 mice of each sex received diets containing 0, 300,
1000, or 3000 mg/kg cyfluthrin in the feed. After one month, 12 rats
of each sex in each group were sacrificed; the remainder were
sacrificed after four weeks of recovery on control diet. Mice at the
highest dose developed ataxia, salivation, and/or emaciation. One
female mouse at 3000 mg/kg feed died. Body-weight gain was depressed
in males at 3000 and in females at 1000 mg/kg feed. Decreased growth
rates were not seen during the recovery period, as all mice recovered
to normal control values. In males at 3000 mg/kg feed, alkaline
phosphatase and blood urea nitrogen levels were slightly increased,
while those of glutamate pyruvate transaminase and cholesterol were
normal throughout. Necropsy after treatment revealed dark-red livers
in some mice at 3000 mg/kg feed; this effect was not observed after
the recovery period. The relative weights of the submaxillary glands
and kidneys were increased in animals at the highest dose, and males
at this dose also had significantly increased relative liver weights.
The weights of both the liver and the submaxillary gland returned to
normal after the four-week recovery period, while those of the kidney
remained elevated. Histopathological examination revealed minimally
increased chromatin in the nuclei of hepatocytes of males receiving
1000 mg/kg feed or more (Watanabe et al., 1982a) and in females at
the high dose. Mice receiving 1000 mg/kg feed or more had cytoplasmic
swelling of the glandular epithelium of the submaxillary glands. These
findings all disappeared after the four-week recovery period. The NOEL
was 300 mg/kg feed, equivalent to 45 mg/kg bw per day (Watanabe
et al., 1982b).
Rats
Groups of 20 SPF Wistar albino rats of each sex received doses of
0, 5, 20, or 80 mg/kg bw per day cyfluthrin (purity, 85%) dissolved in
PEG 400 by gavage once daily for four weeks followed by a six-week
observation period. The high dose was lowered to 40 mg/kg bw per day
in weeks 2 and 4 because of severe intoxication. Physical appearance
and behaviour were assessed daily throughout the study, and body
weights were measured at the start of each week. Blood measurements
and urinalysis were performed on five male and five female rats from
each group at the end of the four-week treatment period and at the end
of the six-week observation period.
The control group and rats given doses of 5 or 20 mg/kg bw per
day behaved normally, but rats at the highest dose developed symptoms
of apathy, ruffled coat, dyspnoea, salivation, hyperkinesis, ataxia,
and uncoordinated movements. The symptoms were more pronounced during
weeks 1 and 3 of treatment at the highest dose. Deaths occurred only
in rats at the high dose, 6/20 males dying between treatment days 3
and 21 and one female on day 26; no deaths were noted during the
recovery period. The body-weight gain of male rats at the high dose
was reduced by 10% during week 4 of treatment, but the weights
returned to normal during the recovery period. The body weights and
body-weight gain of females were not affected by treatment.
Haematological parameters were all within the normal ranges, and no
toxicologically relevant, group-specific or dose-related differences
from controls were seen in serum enzyme activities or electrolyte
concentrations. The plasma activity of glutamate pyruvate transaminase
was slightly higher in rats receiving the highest dose than in
controls. Urinalysis and gross and histopathological examination
revealed no treatment-related variations from normal. Likewise, no
differences in blood chemical parameters were seen at the end of the
observation period. The absolute and relative weights of the adrenal
glands were increased in female rats at the end of treatment with the
highest dose, while only the relative weight of the liver was higher
in males at the high dose in comparison with the control animals. The
increase in relative adrenal weights was not accompanied by
histopathological changes. No deaths occurred during the recovery
period. No gross pathological changes related to administration of
cyfluthrin were seen after treatment or recovery, and there were no
signs of delayed neurotoxicity at the end of the recovery period. The
NOEL was 20 mg/kg bw per day (Flucke & Schilde, 1980).
Groups of 18 rats (strain unspecified) of each sex received diets
containing 0, 100, 300, or 1000 mg/kg cyfluthrin for one month, when
12 rats of each sex per group were sacrificed; the remainder were
killed after one month of recovery on control diet. Rats at the
highest dose developed straddled gait, salivation, and/or nervousness
during treatment, but these symptoms disappeared rapidly during the
recovery period. The body-weight gain, food intake, and water
consumption of animals of each sex at 1000 mg/kg feed were depressed
but returned to normal during the recovery period. Urinalysis
indicated a higher incidence of urobilinogen and ketone bodies in rats
at 1000 mg/kg feed, but, again, these signs returned to normal after
the end of treatment. The haematological effects seen after treatment
with 1000 mg/kg feed included a decrease in erythrocyte count,
haematocrit, and haemoglobin content, which also returned to normal
after the four-week recovery period. No significant effects were seen
on liver microsomal enzyme activity. Total protein and blood glucose
levels were significantly decreased in male rats given 300 mg/kg feed.
No effects were seen on macroscopic examination. Histopathologically,
minimal degeneration of single fibres of the sciatic nerve was noted
occasionally after the treatment period. Cytoplasmic swelling,
increasing the size of the glandular epithelium of the submaxillary
glands was observed at the high dose, but these alterations
disappeared after the recovery period. On the basis of the depressed
blood glucose levels in male rats at 300 mg/kg feed, the NOEL was 100
mg/kg feed, equivalent to 5 mg/kg bw (Watanabe et al., 1982a).
Groups of 30 male and 30 female Wistar rats received diets
containing 0, 30, 100, or 300 mg/kg cyfluthrin (purity, 84.2%) diluted
in an oil vehicle (Wessalon S; equivalent to a 50% premix) for three
months. They were observed daily for alterations in appearance and
behaviour, while body weights and food consumption were recorded
weekly. Five male and five female rats from each group were killed
after one and four weeks for interim examinations, including
microsomal enzyme induction assays ( N- and O-demethylase and
cytochrome P450 content). All rats that survived the treatment were
sacrificed at the end of treatment and examined.
Appearance and behaviour were not modified during treatment, even
at the highest dose. No effects were found on body weight, food
consumption, haematological, clinical chemical or urinary parameters,
or mortality rates. Blood sugar and cholesterol levels were within the
normal range throughout treatment. At week 1, a dose-independent
increase in N-demethylase activity was seen in all treated males and
in O-demethylase activity in females at 100 and 300 mg/kg feed; the
activity of enzymes of the cytochrome P450 system was significantly
increased only in males at 300 mg/kg feed at this time. By three
months, no differences were seen in the activities of the microsomal
enzymes in any treated group. No treatment-related effects were seen
on gross or histopathological examination. No toxic effects were seen
at any dose (Loser & Schilde, 1980).
Groups of 28 male and 28 female Sprague-Dawley rats received
diets containing 0, 100, 300, or 1000 mg/kg cyfluthrin (purity, 95%)
incorporated into the powdered diet on a 0.4% maximum clay carrier,
daily for three months. Body weights were recorded weekly and food
consumption and efficiency were determined. After treatment, 20 rats
at each dose were autopsied immediately, and the remaining eight rats
in each group were allowed to recover on a control diet for a
one-month observation period before autopsy. Clinical chemical and
urinary parameters were investigated before each sacrifice.
Only the rats at the highest dose showed signs of intoxication.
Both males and females of this dose showed abnormal gait and excess
salivation, increasing in incidence until the second week of feeding,
after which a gradual decrease was noted, although recovery from this
effect was somewhat delayed in males. By the third month of treatment,
no such symptoms were seen in any group. Mortality was not affected by
treatment. The food consumption and body-weight gains of females and
males at the highest dose were significantly lower than those of the
control group. The body-weight gain depression in females appeared to
recover after cessation of treatment, while that of the males remained
significantly low. Overall feed efficiency did not vary between
groups. The results of urinalysis and haematological examination were
within the normal physiological range and revealed no
treatment-related effects. Biochemical analysis showed that rats at
300 or 1000 mg/kg feed had significantly increased blood urea nitrogen
levels, and males at 1000 mg/kg feed had significantly increased
aspartate aminotransaminase activity. The blood glucose levels were
depressed in males at 300 or 1000 mg/kg feed and in females at 1000
mg/kg feed. All biochemical parameters were within normal limits after
the recovery period. The absolute weights of the liver, heart, and
lung were decreased in male rats at the high dose, and females at this
dose had depressed liver, kidney, adrenal, and ovarian weights. In
both males and females at the high dose, the relative weight of the
submaxillary gland was increased. No other treatment-related gross
pathological effect was observed. Degeneration of single fibres of the
sciatic nerve was noted in 5/20 males and 3/20 females at the high
dose after the three-month treatment period, and in 1/8 males at the
high dose after the one-month recovery. The authors concluded that the
damage was mild and minimal, corresponding to partial loss of the
myelin sheaths of single nerve fibres. The NOEL was 100 mg/kg feed,
equal to 6.2 mg/kg bw per day (Oikawa & Iyatomi, 1983).
Rabbits
In a three-week study of dermal toxicity, groups of three New
Zealand white rabbits of each sex were given dermal applications of 0,
50, or 250 mg/kg bw cyfluthrin (purity, 85%) dissolved in PEG 400 on
the intact or abraded skin. The treatments were applied five times a
week for a contact time of 6 h/day for three weeks, and then the
rabbits were killed and examined. Tissues were collected for
microscopic examination from controls and rabbits at the high dose;
the testes were collected from animals at 50 mg/kg bw per day. No
effects were observed on appearance, behaviour, or haematological,
clinical chemical, or urinary parameters. All rabbits survived the
treatment period. No toxicologically significant effect on body-weight
gain was observed. Gross and histopathological examination showed no
alterations due to treatment with cyfluthrin. The NOEL was 250 mg/kg
bw per day (Flucke & Vogel, 1980).
Dogs
Groups of six male and six female beagle dogs were fed cyfluthrin
(purity, 84.8%) in the diet at concentrations of 0, 65, 200, or 600
mg/kg for six months. No deaths occurred, and the physical appearance
of the dogs remained normal. Tests of reflexes and measurement of body
temperature showed no significant alterations. Five males and six
females receiving the high dose showed signs of uncoordinated, stiff
gait of the hind limbs, persisting for several hours, on several
occasions after week 21. Vomiting after dosing occurred slightly more
often in animals at the high dose than in controls. Diarrhoea occurred
in all groups including controls, but much more frequently in dogs at
the high dose, from the time of initiation of treatment. No effects
were found on food intake or water consumption in the controls or in
dogs at 65 or 200 mg/kg feed, but males at the high dose had slightly
lower food consumption during the first week of treatment. No group
differences in food consumption were observed by the sixth week. Lower
mean body weights were seen in males and females at 200 or 600 mg/kg
feed from week 2 to the end of treatment. Males at 200 and females at
600 mg/kg feed had lower average weight gains between weeks 1 and 26
of treatment, whereas the average body weights of males at the high
dose were similar to those of controls at the end of the feeding
period. No effects were found on ophthalmoscopic, haematological,
clinical chemical, or urinary parameters or on organ weights; no
treatment-related changes were seen grossly or histologically. The
NOEL was 65 mg/kg feed, equal to 2 mg/kg bw per day (Hoffmann &
Kaliner, 1981).
2.2.3 Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female mice SPF strain CF1/W74 mice
received a diet containing 0, 50, 200, or 800 mg/kg cyfluthrin in the
form of a 50% premix with Wessalon S for 23 months. Clinical
examinations were carried out on 10 mice of each sex at each dose 6,
12, 18, and 23 months after the initial administration. After
treatment, five mice of each sex from each group were selected
randomly for analysis of fluoride accumulation in bone and teeth. The
remaining mice were killed and examined grossly, and tissues were
taken for histopathological examination. Neither appearance nor
behaviour was altered in any group. No effects were observed on
mortality, haematological parameters, fluoridation of bones or teeth,
food intake, or water consumption. The body-weight gain of treated
animals was not affected at doses up to and including 200 mg/kg feed,
but mean body-weight gain was significantly reduced in females at the
high dose and males at this dose had a slightly lower body-weight gain
than controls. Plasma alkaline phosphatase activity was increased in
all treated males at the six-month examination; however, at the end of
the study several controls also showed elevated alkaline phosphatase
activity. Alanine aminotransaminase activity was slightly increased in
males at the high dose after 12 and 18 months of treatment. All other
clinical chemical values were within normal ranges. No
treatment-related alterations were seen at autopsy, and the relative
and absolute organ weights were not affected by treatment. Urinalysis
and microscopic examination revealed no evidence of treatment-related
damage at any dose. The incidence and histological type of
non-neoplastic and neoplastic alterations were all within normal
ranges. The NOEL was 200 mg/kg feed, equivalent to 30 mg/kg bw per day
(Suberg & Loser, 1983a).
Rats
Groups of 65 male and 65 female SPF BOR:WISW strain rats received
a diet containing 50, 150, or 450 mg/kg cyfluthrin premixed with
Wessalon S for two years. The control group consisted of 65 rats of
each sex fed untreated diet. Both the control and the test diets were
given ad libitum. All rats were observed twice a day for alterations
in appearance, behaviour, and activity. Body weights were determined
weekly up to week 4, then every other week to conclusion of treatment.
After seven days of treatment, five rats of each sex at each dose were
killed in order to measure mixed-function enzyme induction and
cytochrome P450 content. Clinical tests were conducted on 10 rats of
each sex per dose 6, 12, 18, and 24 months after the start of
treatment. After one and two years, five rats of each sex per dose
were killed in order to determine the fluoride content of their bones
and teeth. Rats that died during treatment and five rats of each sex
per dose killed at one year were examined grossly. All remaining rats
were autopsied and examined grossly and histologically at the end of
treatment.
No treatment-related effects were seen on appearance, behaviour,
activity, or food consumption. There were no treatment-related effects
on body-weight gain at the low dose, but rats at 150 mg/kg feed
appeared initially to have slightly decreased body-weight gain. The
high dose consistently induced significantly decreased body-weight
gain throughout the experiment. No treatment-related effects were seen
on mortality, and no effects were seen on haematological or urinary
parameters, gross appearance, organ weights, or histopathological
manifestations. N-Demethylase activity was increased by 30% in males
and 20% in females after seven days at the high dose, but there were
no effects on O-demethylase activity or P450 enzyme induction.
Induction of the microsomal enzyme N-demethylase in rats at the high
dose is considered not to be of toxicological relevance as no specific
morphological alteration or damage to the liver was seen at
histopathological examination. No significant differences in fluoride
accumulation in bones and teeth were noted at interim sacrifice.
Significant, dose-related differences were found after two years of
treatment, but the biological relevance of this finding was questioned
by the authors, as minimal changes in fluoride accumulation were seen
and the assay method involved large experimental error. The results of
the final necropsy showed isolated but significant differences in
organ weights, which were attributable to dissimilar body weights and
were not dose-related; they were considered to be of little
toxicological significance. The absolute kidney and liver weights were
decreased in rats at the high dose; however, there was no
dose-dependent effect on the relative weights. The incidence of
tumours was not altered by treatment at any dose, and no other
treatment-related alterations were observed histologically. The NOEL
was 50 mg/kg feed, equal to 2 mg/kg bw per day (Suberg & Loser,
1983b).
Dogs
Groups of six beagle dogs of each sex, 22-30 weeks old, were fed
a diet containing 0, 40, 160, or 640 mg/kg cyfluthrin for one year. No
effects on appearance or behaviour were seen in dogs at 40 or 160
mg/kg feed. Two dogs at the highest dose had slight disturbances in
movement, clumsy gait primarily in the hindquarters, and a reluctance
to move. This observation was made only once during weeks 36-37 of the
study. Reflex tests indicated no deviations in any treated animals.
Body temperature, mortality, and feed and water consumption were not
affected by treatment. The body-weight gain of males at the high dose
was slightly lower than that in the other groups; females at this dose
had somewhat higher weight gains than those at other doses. Males at
the high dose had a higher incidence of vomiting, and soft, pasty
faeces were observed more frequently than in controls and in the other
groups. Ophthalmoloscopy, haematology, clinical chemistry, and
urinalysis revealed no treatment-related alterations. At termination
of treatment, all of the dogs were killed and necropsied, and the
tissues of males at the high dose and of controls were evaluated
histologically. No significant treatment-related effects were found at
autopsy. Treatment had no effect on absolute or relative organ
weights, except that the mean spleen weight of females at the high
dose was absolutely and relatively higher than that of female
controls; however, the histopathological appearance was normal in all
treated animals. Microscopic examination of the sciatic nerve showed
no pathological alterations. The NOEL was 160 mg/kg feed, equal to 5.1
mg/kg bw per day (Hoffmann & Schilde, 1983).
2.2.4 Genotoxicity
Cyfluthrin had no mutagenic potential in vivo or in vitro.
The results of assays for genotoxicity with cyfluthrin are summarized
in Table 3.
2.2.5 Reproductive toxicity
2.2.5.1 Multigeneration reproductive toxicity
Rats
Groups of 10 male and 20 female SPF-Cpb rats, five to six weeks
old, received diets containing 0, 50, 150, or 450 mg/kg cyfluthrin as
a 50% premix with Wessalon S in the feed, given ad libitum 105 days
before and throughout mating, gestation, and rearing of pups. The
parameters measured included behaviour, growth, food consumption,
mortality, fertility, viability during gestation and lactation,
development of the young, parental body-weight gain, and male:female
ratio. The study was conducted for three generations, with two litters
per generation. F0 parents produced F1a and F1b litters; F1b
parents produced F2a and F2b litters; and F2b parents produced
F3a and F3b litters. The parameters assessed included fertility,
litter size, fetal birth weight, fetal variability, and parental
weight gain. Gross and histopathological examinations were also
performed on F2b parental animals and F3b pups.
Treatment did not affect appearance, behaviour, or survival,
although one F2a pup receiving 150 mg/kg feed and another receiving
450 mg/kg feed had convulsions. Treatment did not consistently affect
birth weight or litter size at birth. A decrease in pup survival from
birth through five days of lactation was seen in animals from the
F3a and F3b matings at 150 or 450 mg/kg feed, and decreased
survival of pups on days 5-28 of lactation was noted among pups at the
high dose in all matings. Similarly, body weight was consistently
suppressed in F3a and F3b generation rats at 150 and 450 mg/kg
feed during the 28-day lactation period. Necropsy revealed decreased
absolute liver and kidney weights in rats at 150 and 450 mg/kg feed;
this was probably related to the decreased body-weight gain in these
animals. No effect was observed on relative organ weights. The NOEL
was 50 mg/kg feed, equal to 3 mg/kg bw per day (Loser & Eiben, 1983).
Table 3. Results of assays for genotoxicity with cyfluthrin
End-point Test object Concentration Purity (%) Results S9 QA Reference
In vitro
Reverse mutation Salmonella typhimurium TA100, TA98, 20-24 000 µg/plate 83.6 Negative + No Herbold (1980a)
TA1535, TA1537 Negative -
Reverse mutation Salmonella typhimurium TA100, TA98, 5-5000 µg/plate 95.0 Negative + No Nagane et al.
TA1535, TA1537, TA1538 Negative - (1982)
Reverse mutation Salmonella typhimurium TA100, TA98, 10-25 000 µg/plate 95.0 Negative + No Ohta & Moriya
TA1535, TA1537, TA1538 Negative - (1982)
Reverse mutation Escherichia coli B/r WP2 try- hcr- 5-5000 µg/plate 95.0 Negative + No Nagane et al.
Negative - (1982)
Reverse mutation Escherichia coli WP2 hcr 10-25 000 µg/plate 95.0 Negative + No Ohta & Moriya
Negative - (1982)
Reverse mutation Saccharomyces cerevisiae S138, 312.5-10 000 µg/ml 95.0 Negative + No Brusick (1982a,b)
S211a) Negative -
Reverse mutation Saccharomyces cerevisiae D7 625-10 000 µg/ml 95.0 Negative + No Brusick (1982c)
Negative -
Gene mutation Chinese hamster ovary K1-BH4 cells, 3-10 µl/ml 94.7 Negative + Yes Yang & Louie
hprt locus Negative - (1985)
Chromosomal Chinese hamster lung cells 3.3 × 10-5-3.3 × 10-3 93.7 Negative + Yes Sasaki et al. aber
ration mol/litre Negative - (1986)
Chromosomal Human lymphocytes 500-5000 µg/ml 95.1-95.5 Negative + No Herbold (1988)
aberration Negative -
Sister chromatid Chinese hamster ovary cells 3-20 µg/ml Technical Negative + Yes Putman (1985)
exchange Negative -
DNA damage Escherichia coli pol A+ and pol A1- 62.5-1000 µg/plate 95.0 Negative + No Herbold (1981a)
Negative -
DNA damage Bacillus subtilis H17, M45 rec- 200 µg/disc 95.0 Negative + No Nagane et al.(1982)
DNA damage Bacillus subtillus H17, M45 rec- 100-10 000 µg/disc 95.0 Negative + No Ohta & Moriya
(1982)
Unscheduled DNA Male Sprague-Dawley rat primary 17-5000 µg/ml 94.7 Negative Yes Curren (1985)
synthesis hepatocytes
Table 3. (continued)
End-point Test object Concentration Purity (%) Results S9 QA Reference
Mitotic gene Saccharomyces cerevisiae D7 625-10 000 µg/ml 95.0 Negative + No Brusick (1982c)
conversion Negative -
Mitotic crossing Saccharomyces cerevisiae D7 625-10 000 µg/ml 95.0 Negative + No Brusick (1982c)
over Negative -
In vivo
Micronucleus Male and female NMRI mice, 2 × 7.5, 2 × 15 83.6 Negative No Herbold (1980b)
formation bone-marrow erythroblasts mg/kg bw orally
Dominant lethal Male NMRI/ORIG Kisslegg mice 30, 60 mg/kg bw 83.6 Negative No Herbold (1981b)
mutation orally
a S9, 9000 × g fraction of rat liver
2.2.5.2 Developmental toxicity
Rats
Groups of 25 outbred pregnant female Wistar KFM-HAN (SPF) rats
received 0, 1, 3, or 10 mg/kg bw per day cyfluthrin (purity, 93.4%) in
a 1% Cremophor EL solution in drinking-water by gavage on days 6-15 of
gestation. Clinical signs, body weights, and food consumption were
recorded. On day 21 of gestation, females were killed, caesarean
sections performed, and the pups were necropsied. Although the
standard was not specified, the study conduct underwent periodic
inspections by the quality assurance unit of the laboratory.
No effects on mortality were observed. Partial hair loss occurred
in one female at 3 mg/kg bw per day. Body-weight gain was not affected
by the low or middle doses. Rats receiving the high dose had 7.7%
lower corrected mean body-weight gain. Mean food consumption was 14%
lower in rats at the high dose on days 6-11 after mating. Mating,
fertility, and gestation parameters were normal, and no dose-related
difference was seen on examination and necropsy of dams. One dam at
the high dose totally resorbed its litter. Litter size, fetal body
weight, and sex ratio were not affected by treatment. The incidences
of visceral and skeletal anomalies were not increased by treatment in
a dose-dependent manner. No teratogenic effects were seen. As a
maternal maximum tolerated dose was not achieved in this study, the
authors did not report a NOEL (Becker, 1983).
Groups of 25 Bay:FB30 rats received cyfluthrin dissolved in PEG
400 at doses of 0, 3, 10, or 30 mg/kg bw per day by gavage during days
6-15 of gestation. They were mated in a 2:1 ratio with males. The
pregnant females were killed on day 20 of gestation for examination of
their uterine contents. Six rats at 10 or 30 mg/kg bw per day were
reported to have occasional 'high-stepping gait'; at the highest dose,
they showed signs of ataxia and decreased motility. No effects on
maternal weight gain were seen that were attributable to treatment.
Litter size, frequency of resorptions, and placental weights were not
affected by treatment, even at the highest dose. Likewise, no
treatment-related increase in the incidence of stunted growth or bone
anomalies was observed in the fetuses. The author concluded that
cyfluthrin had no specific teratogenic or embryotoxic effect. As 6/25
rats at 10 mg/kg bw per day showed signs of toxicity, the NOEL for
maternal toxicity was 3 mg/kg bw per day; the NOEL for fetal toxicity
was 30 mg/kg bw per day (Schluter, 1982).
Rabbits
In the first part of a two-part study, groups of 15 Himalayan
rabbits received cyfluthrin at 0, 3, 10, or 30 mg/kg bw per day in
Cremophor EL as a solution in drinking-water by gavage on days 6-18 of
gestation. The dams were killed on day 29 of gestation. Maternal
appearance and behaviour were not affected. Lower mean fetal and
placental weights were observed, and a higher incidence of
arthrogryposis (persistent flexure or contracture of a joint) was
observed in the nine fetuses of rabbits receiving 10 or 30 mg/kg bw
per day.
Because an embryotoxic effect was observed, a second study was
conducted. Groups of 15 pregnant Himalayan rabbits received cyfluthrin
at 0, 10, or 30 mg/kg bw per day in Cremophor EL as a solution in
drinking-water by gavage during days 6-18 of gestation. The dams were
killed on day 29 of gestation, and the fetuses removed for
examination. Doses up to and including 30 mg/kg bw per day had no
effect on behaviour or appearance. One dam at the high dose died of an
unknown cause on day 26 of gestation. Mean weight gain was affected by
treatment, and the average weight gain of the animals at 30 mg/kg bw
per day was statistically significantly lower than that of controls.
Treatment had no effect on the fertilization rate or gestation or on
embryonic development, including implantation rate, litter size, and
rate of resorptions. The incidence of skeletal anomalies was not
increased at any dose. One case of arthrogryposis occurred in the
control group. There was no indication of embryotoxicity or
teratogenicity. The NOEL for maternal toxicity was 30 mg/kg bw per day
and that for embryotoxicity was 30 mg/kg bw (Schluter, 1981).
Groups of 15 pregnant female Himalayan CHBB:HM rabbits received
cyfluthrin at 0, 5, 15, or 45 mg/kg bw per day by gavage in 0.5%
aqueous Cremophor EL emulsion during days 6-18 of gestation. Dams were
observed for clinical signs and body weight. On day 29 of gestation,
the dams were killed and their fetuses removed by caesarean section
and examined for anomalies. No effects were observed on mortality,
behaviour, appearance, insemination rate, or maternal weight gain. Two
rabbits at the high dose aborted on gestation days 25 and 28, and one
completely resorbed its implants. The authors concluded that the
effects seen at the high dose were treatment-related. Litter size,
placental weights, and fetal body weights were not affected by
treatment. The rate of resorptions appeared to be dose-related.
Although arthrogryposis was seen in rabbits at the high dose, the
event was considered to be spontaneous. The NOEL for maternal toxicity
was 15 mg/kg bw per day and that for embryotoxicity was 45 mg/kg bw
per day (Roetz, 1983).
In a more recent study, cyfluthrin was tested for its embryonic
and teratogenic potential in pregnant chinchilla rabbits (Chb:CH
hybrids, SPF). Groups of 16 pregnant rabbits received 0, 20, 60, or
180 mg/kg bw per day by gavage during days 6-18 of gestation. They
were killed 28 days after mating, and the fetuses were removed by
caesarean section. The study was conducted in accordance with the
specifications of the US FIFRA GLP (40 CFR Part 160), OECD test
guideline 414, and US EPA test guideline Vol. 43 Series 163.83-3
Subdivision F, Hazard Evaluation.
Clinical observations, appearance, and behaviour were not
affected by treatment. No deaths occurred. Food consumption and
body-weight gain were reduced in a dose-dependent manner in animals at
60 and 180 mg/kg bw per day but were significantly increased 24-28
days after mating. A dose-related, statistically significant increase
in post-implantation losses, due to increased embryonic resorptions,
was observed in rabbits given the two higher doses. Necropsy revealed
no significant alteration due to treatment. The sex ratio and fetal
body weights were unaffected by treatment, and no significant
alterations were found in fetal heads or brains. Skeletal alterations
were seen, including ossification of vertebrae, sternebrae, and ribs,
but these findings were considered to be within the normal spontaneous
range. There was no indication of a teratogenic effect. On the basis
of the increased post-implantation losses, the NOEL for maternal and
embryonic toxicity was 20 mg/kg bw per day (Becker & Biedermann,
1992).
2.2.6 Special studies on neurotoxicity
Rats
Fifty male Sprague-Dawley received technical-grade cyfluthrin
(purity, 95%) as a 1.6% solution in PEG 400 by gavage at a dose of 80
mg/kg bw per day on five consecutive days. The dose was reduced to 40
mg/kg bw per day on days 6-14 owing to the onset of mild to severe
symptoms of toxicity. Twenty-five male rats received PEG 400 alone.
All rats were observed for appearance and behaviour throughout
treatment and a recovery period of three months. Ten treated and five
control rats were killed, and their tissues examined histologically on
the days 1 and 5 of treatment and one, two, and three months after
treatment; tissues from two treated and one control males were also
examined at each time point by electron microscopy. The treated rats
showed symptoms of intoxication throughout the dosing period,
including abnormal, straddled gait, slow leg movements, and excess
salivation, but no symptoms were observed five days after treatment.
The body-weight gain of the treated rats was depressed in comparison
with controls throughout treatment but recovered during the
observation period. Histological examination revealed a minimal degree
of axonal degeneration (swelling and demyelination) in single fibres
of the sciatic nerve in 6/8 rats at the first examination, in 3/8 five
days to one month after treatment, and in 2/9 two months after
treatment. A similar lesion was reported in the femoral nerve of one
treated male examined on the fifth day after treatment but was no
longer seen three months later No alterations were reported in any
other nervous or muscular tissue examined. Electron microscopy
revealed dilatation of neurotubules, accompanied by proliferation of
neurofilaments and degeneration of mitochondria, which was maximal one
month after treatment but was not seen after three months. A NOEL was
not identified (Oikawa et al., 1983).
Groups of 15 male and 15 female Wistar TNO/W74 albino rats
received cyfluthrin (purity, 83.3%) emulsified in PEG 400 by gavage
daily on seven days a week for 35 weeks. The doses administered ranged
from 30 to 80 mg/kg bw per day and were varied intermittently, so that
the rats had signs of acute toxicity after each administration.
Clinical observations were recorded daily, while body weights were
recorded weekly. Clinical chemistry was evaluated at the end of the
study, and liver, kidney, adrenal gland, brain, spinal cord, and
sciatic nerve tissues from five treated and five control rats of each
sex were examined microscopically.
The treated group displayed characteristic signs of acute
toxicity throughout the study, including apathy, ruffled coat, and
respiratory disturbances. Increased salivation, tremor, and
uncoordinated gait were observed in some rats immediately after
dosing. No paralysis of the extremities was observed. Treated males
had a 20-25% reduction in body-weight gain, while no effect was seen
on female body-weight gain. Treatment had no effect on clinical
chemical parameters or induction of cytochrome P450 or N- or
O-demethylase activity. The mortality rate was significantly higher
among treated than control males. Gross pathological and microscopic
examination showed no treatment-related pathological alterations in
any organ examined. The weights of the liver and kidney were lower in
treated males, due mostly to their reduced body weight. Although the
authors reported no histological damage to the nervous tissue, rats
that died during the treatment period were not examined, limiting the
usefulness of this study. A NOEL was not identified (Thyssen & Vogel,
1982).
Groups of five male and five female Wistar (SPF-Cpb) rats
received cyfluthrin at 0 or 60 mg/kg bw per day for two weeks by
gavage, and a supplementary group of male rats received doses of 0 or
50 mg/kg bw per day. Modified preparation techniques, i.e. perfusion
of animals with formalin and fixation of the spinal column with cord
in toto, were used to determine the neurotoxic effect of cyfluthrin.
Symptoms of acute toxicity were observed in all treated animals,
including tremor, altered gait, and increased vocalization. Four males
at 60 mg/kg bw per day died between treatment days 5 and 8, but gross
pathologicam examination revealed no specific alterations. The
body-weight gain of females was not affected by treatment, but
decreases were seen for males at 50 or 60 mg/kg bw per day. Small,
fresh brain haemorrhages were seen in all four males that died on
test. The authors concluded that the 'most likely explanation is that
these are the result of a terminal cardiovascular disorder with
necrosis of the vascular walls'. Since this finding was not seen in
control animals, a treatment-related effect could not be ruled out. A
NOEL was not identified (Heimann & Kaliner, 1983).
In a supplementary study, neuromuscular dysfunction was assessed
in the tilting plane test. Groups of 10 male rats received cyfluthrin
dissolved in Cremophor EL as a single oral administration by stomach
tube, at doses of 0, 0.1, 0.3, or 1.0 mg/kg bw in the first experiment
and 0, 0.01, 0.03, or 0.1 in the second. Diazepam was given at a
single oral dose of 5 mg/kg bw as a positive control. The angle at
which the animal started to slide from a tilting plane was evaluated
for each rat 30 min and 2, 5 and 7 h after administration.
At a dose of 0.01 mg/kg bw, cyfluthrin had no effect at any time
interval. At 0.03 mg/kg bw, the slip angle was marginally
significantly ( p < 0.05) reduced, only at 7 h. At 0.1 mg/kg bw
cyfluthrin, the angle was reduced at 5 h but was normal at other times
(Polacek, 1984). In view of the lack of a standardized protocol for
this study, the use of Cremophor EL as a vehicle (which can
substantially enhance the acute toxicity of cyfluthrin), and the
absence of a dose- or time-dependent reduction in slip angle, the
Committee considered this study inappropriate for assessing
neurotoxicity.
Hens
In a study of delayed neurotoxicity, 10 adult laying hens were
given single doses of cyfluthrin in carbowax at 5000 mg/kg bw by
gavage, and a group of 20 hens received the same dose twice at an
interval of one week. Hens given the single dose were observed for
mortality and symptoms of toxicity and were killed 56 days after
treatment; those given two doses were killed 49 days after treatment.
No treatment-related effects on body-weight gain, mortality, or gross
pathological appearance were observed. No symptoms characteristic of
delayed neurotoxicity were observed in hens given the single dose; one
hen given two doses had abnormal appearance and behaviour but
recovered the next day, while another developed ataxia complicated by
decreased activity and was killed in extremis on day 32. As
histopathological examination revealed no lesions in the brain, spinal
cord, or sciatic nerve tissue, characteristic delayed neurotoxicity
was not indicated in this study (Hixon, 1981).
Groups of white Leghorn hens were treated with cyfluthrin
(purity, 85-94%) in PEG 400 by gavage as either a single dose of 1000,
2500, or 5000 mg/kg bw, two doses of 5000 mg/kg bw at a three-week
interval, or daily administration of 5000 mg/kg bw for five
consecutive days. The single or multiple doses of 5000 mg/kg bw
induced noticeable behavioural alterations, episodes of drowsiness,
weight loss, uncoordinated gait, and some deaths. Several surviving
hens showed signs of excitation before recovery. Gross pathological
examination of hens at the high dose revealed spotty, brittle livers
and pale, slightly mottled kidneys. Histopathological examination
revealed minimal damage to the sciatic nerve, including axonal
swelling and fragmentation, proliferation of Schwann cells, and
increased vacuolization of myelin sheaths (Thyssen et al., 1981).
The authors of these two studies concluded that a delayed
neurotoxic effect was not apparent or consistent and indicated that
the histopathological findings were attributable to the normal
background neuropathic alterations seen in hens.
A group of 12 hens received a single oral dose of cyfluthrin in
PEG 400 at 4300 mg/kg bw and were observed for three weeks. A second
group of 16 hens received two doses of 4300 mg/kg bw three weeks apart
by gavage and were allowed to recover for eight weeks. A final group
of 12 hens received doses of 1500 mg/kg bw per day by gavage for five
consecutive days and were allowed to recover for eight weeks. All
animals were autopsied after the recovery periods. Neurotoxic esterase
activity was determined in the brains and spinal cords from five hens
of each group 24, 48, and 72 h and seven days after treatment. The
study was carried out in accordance with the specifications of OECD
GLP and US EPA Pesticide Assessment Guidelines, Subdivision F, Hazard
Evaluation, paragraph 81-7.
All treated hens showed signs of drowsiness and emaciation. One
control hen, two receiving 4300 mg/kg bw per day, and one receiving
1500 mg/kg bw per day died during treatment. One hen at the high dose
and one given multiple low doses were killed in extremis. No symptoms
of delayed-type neurotoxicity were seen in any treated birds. Food
consumption and body weights were not affected by treatment, and no
significant inhibition of neurotoxic esterase activity was noted in
any treated bird examined. No histopathological changes were seen in
hens receiving 1500 mg/kg bw per day cyfluthrin. Histopathological
examination of two hens receiving the high dose, one as a single dose
and one as two doses, showed slight axonal degeneration of single
nerve sites in the sciatic nerve and/or spinal cord (Sachsse, 1986).
Groups of 15 white Leghorn Lohmann strain laying hens were given
cyfluthrin formulated in PEG 400 at a dose of 5000 mg/kg bw per day or
tri-ortho-cresylphosphate as a positive control, orally for three
consecutive days. Three hens from each group were killed 24 h after
the first and second administration to determine neurotoxic esterase
activity; the remaining hens were killed 24 h after the third
administration. Hens receiving cyfluthrin displayed increased
salivation, dyspnoea, ruffled coat, and increased vocalization. Two
hens died after the second dose and six after the third dose. No
inhibition of neurotoxic esterase activity and no characteristic
delayed-type neurotoxicity were seen (Flucke & Eben, 1985).
2.2.7 Special studies on tumour promotion in vitro
As inhibition of intercellular communication is a common property
of known or suspected tumour promotors (Trosko et al., 1981; Chen
et al., 1984; Malcolm et al., 1985), inhibition of cellular
communication may be indicative of tumour-promoting potential.
Cyfluthrin at doses of 5-15 µmol/litre did not inhibit intracellular
communication (metabolic cooperation) in V79 fibroblasts and had no
effect on mutant cell recovery (Warngard & Flodstrom, 1989).
2.3 Observations in humans
The symptoms and signs of acute poisoning resulting from exposure
to different pyrethroids are similar. Clinical analysis of 573 cases
of acute pyrethroid poisoning due to occupational or accidental
exposure revealed symptoms including burning, itching, and tingling
sensations of the skin, which resolved after several hours. Washing
was not an effective treatment. The systemic symptoms included
dizziness, headache, nausea, anorexia, and fatigue; vomiting was most
common in cases due to ingestion of pyrethroids. Although less
frequently reported, tightness of the chest, paraesthesia,
palpitation, blurred vision, and increased sweating were observed in
some cases. Coarse muscular fasciculations were observed in more
serious cases. Convulsions and coma can also result from acute
poisoning with pyrethroids (He et al., 1989).
Several cases of acute occupational exposure to cyfluthrin have
been reported. The most significant effect is pronounced irritation
and burning of the skin and especially mucosal areas (lips, prepuce).
The symptoms usually appear after 1-2 h and last for one or two days.
A stinging sensation was observed 12-24 h after facial and mucosal
contact. A greater risk was associated with exposure to powdered
compound than to liquid formulations. The irritating effect was
probably due to a local action on sensitive nerve pathways and sensory
nerve cells (Flucke, 1979) and was reversible. With proper measures,
such as protective gloves and clothing, training, and avoidance of
dermal contact, occupational exposure and its potential effects can be
minimized (Miksche, 1979; Faul, 1984, 1988; Kollert, 1988).
3. COMMENTS
Cyfluthrin was evaluated by the 1987 Joint FAO/WHO Meeting on
Pesticide Residues (JMPR) (WHO/FAO, 1988), which established an ADI of
0-0.02 mg/kg bw. The same data and several new studies were reviewed
by the present Committee. The studies were performed according to
appropriate standards for study protocol and conduct.
In rats, cyfluthrin is readily absorbed and distributed. About
98% of radiolabelled drug was eliminated in urine and faeces within 48
h after oral administration, with similar amounts eliminated after
intravenous administration. In lactating dairy cattle, orally
administered cyfluthrin was also readily absorbed and distributed. The
concentration of cyfluthrin in milk reached a maximum three days after
initial dosing and then declined steadily. In cattle, the liver,
kidney, and fat contained the highest levels of residues, primarily of
unmetabolized parent compound. The major metabolic transformation is
ester hydrolysis to a 3-phenoxy-4-fluorobenzyl alcohol intermediate
and a permethric acid moiety. After ester hydrolysis, the benzyl
alcohol moiety is oxidized to the free 3-phenoxy-4-fluorobenzoic acid
metabolite, which can be either conjugated with glycine or oxidized to
give 4'-hydroxy-3-phenoxy-4-fluorobenzoic acid.
The acute oral LD50 values in rats ranged from 16 to more than
1000 mg/kg bw, depending on the vehicle used. At lethal or near-lethal
doses, signs typical of the toxicity of this class of pyrethroids were
observed. WHO has classified cyfluthrin as 'moderately hazardous'
(WHO, 1996).
Several short-term studies of the toxicity of orally administered
cyfluthrin were available in which it was tested in rats (four weeks;
three months), mice (four weeks), and dogs (six months), at doses
ranging from 5 to 450 mg/kg bw per day. Cyfluthrin reduced body weight
and increased liver and kidney weights. At lethal or near-lethal
doses, signs of neurotoxicity were observed, including ataxia,
abnormal gait, and increased vocalization. Histological evidence of
limited axonal demyelination of the sciatic nerves was observed at
these doses, which was completely reversed within three months of
cessation of treatment. In separate studies, the NOEL in rats ranged
from 5 mg/kg bw per day on the basis of depressed blood glucose levels
to 20 mg/kg bw per day on the basis of mortality and decreased
body-weight gain. The NOEL in mice was 43 mg/kg bw per day on the
basis of swelling of the submaxillary glandular epithelium. The NOEL
in dogs was equivalent to 2 mg/kg bw per day, on the basis of lowered
mean body weights.
In a three-week study of toxicity, rabbits were treated by dermal
application of cyfluthrin (in polyethylene glycol 400) at 0, 50, or
250 mg/kg bw per day for 6 h per day, five days per week for three
weeks. No toxicological effects were observed at any dose.
In a study in which dogs received cyfluthrin at up to 640 mg/kg
of feed, equal to 23 mg/kg bw per day, for one year, two out of six
dogs at the highest dose exhibited slight disturbances in movement and
gait on one occasion. There was no histopathological evidence of
neurotoxicity. Slight increases in the weights of the spleens of
females were observed at the high dose. The NOEL was therefore 160
mg/kg feed, equal to 5.1 mg/kg bw per day.
In long-term studies of toxicity and carcinogenicity in rats and
mice treated at dietary concentrations of cyfluthrin up to 450 and 800
mg/kg feed (equal to 19 mg/kg bw per day in rats and equivalent to 120
mg/kg bw per day in mice), respectively, the toxic effects were
largely non-specific and were essentially restricted to minor
alterations in body weight, organ weights, and blood biochemical
parameters. The NOELs were 50 mg/kg feed in rats, equal to 2 mg/kg bw
per day, and 200 mg/kg feed in mice, equivalent to 30 mg/kg bw per
day, both on the basis of decreased body-weight gain. Cyfluthrin
treatment was not associated with increased tumorigenesis in either
rats or mice.
In a three-generation study of reproductive toxicity in rats,
cyfluthrin at dietary concentrations of 0, 50, 150, or 450 mg/kg feed
had no consistent effect on birth weight or litter size. A decrease in
viability during lactation was noted in animals of the third
generation at the two higher levels, and weight gain was consistently
depressed in these animals. The NOEL was 50 mg/kg feed, equivalent to
2.5 mg/kg bw per day, on the basis of body-weight depression.
In a study of developmental toxicity, cyfluthrin was not
embryotoxic or teratogenic in rats given doses up to 30 mg/kg bw per
day on gestation days 6-15. The NOEL was 3 mg/kg bw per day for
maternal toxicity on the basis of clinical signs of altered gait and
30 mg/kg bw per day, the highest dose tested, for developmental
effects. The developmental toxicity of cyfluthrin has also been
evaluated in rabbits which received doses of 0, 20, 60, or 180 mg/kg
bw per day by gavage on days 6-18 of gestation. Food consumption and
body-weight gain were reduced and a dose-related increase in
post-implantation losses was observed in the groups at 60 and 180
mg/kg bw per day. The NOEL for maternal and embryotoxicity was 20
mg/kg bw per day on the basis of resorptions. The NOEL for
developmental toxicity was 180 mg/kg bw per day, the highest dose
tested.
Cyfluthrin has been tested for its ability to induce DNA damage,
chromosomal aberrations, and gene mutations in vitro and chromosomal
aberrations in vivo. Negative results were obtained in all of these
studies. These results, in conjunction with those of the studies of
carcinogenicity in rodents, indicate that cyfluthrin is neither
genotoxic nor carcinogenic.
In studies of the neurotoxicity of cyfluthrin in rats, daily oral
doses of 30-80 mg/kg bw in polyethylene glycol 400 for up to 35 weeks
resulted in characteristic signs of acute toxicity, including
salivation, tremor, and abnormal gait. Histological evidence of
limited swelling and fragmentation of myelin was observed only
infrequently, and these signs were completely reversible within three
months of cessation of treatment. Rats were also evaluated in a
neurobehavioural test, the inclined plane test, after administration
of single oral doses of up to 0.1 mg/kg bw cyfluthrin in
polyethoxylated castor oil (Cremophor EL). The results were
inconsistent and not dose-dependent. Hence, the Committee considered
that the results of this test were not useful for assessing
neurotoxicity in this case.
Although cyfluthrin is not an organophosphorus compound, it was
also evaluated for its ability to induce delayed-type neurotoxicity in
several studies in adult hens given single or multiple oral treatments
of up to 5000 mg/kg bw. The only neurological effects observed were
acute behavioural disturbances and minor changes in sciatic nerves,
including Schwann-cell proliferation and vacuolization of the myelin
sheath. Cyfluthrin produced no symptoms of delayed-type neurotoxicity
and no inhibition of neurotoxic target esterase in brain, spinal cord,
or the peripheral nervous system.
The Committee concluded that cyfluthrin does not cause
irreversible neurological damage and that the observed effects on the
nervous system occur only at high doses.
4. EVALUATION
The Committee concluded that the effects most relevant for the
toxicological evaluation of cyfluthrin were those observed in the
long-term study in rats fed the compound in the diet. The NOEL was 50
mg/kg in feed, equal to 2 mg/kg bw per day, on the basis of depression
of body-weight gain. Using this NOEL and a safety factor of 100, the
Committee established an ADI for cyfluthrin of 0-20 µg/kg bw. The
Committee noted that the same value had been established by the JMPR
in 1987.
5. REFERENCES
Bayer AG (1995) Chemical structure of cyfluthrin. Toxicological expert
opinion in accordance with Council Regulation (EEC) 2377/90 and
Directive (EEC) 81/852 as amended by Directive 92/18 (EEC).
Unpublished report from Bayer Institute of Toxicology. Submitted to
WHO by Bayer AG, Leverkeusen, Germany.
Becker, H. (1983) Embryotoxicity (including teratogenicity) study with
FCR 1272 in the rat. Unpublished report No. R 2774 from Research and
Consulting Co. AG, Itingen, Switzerland. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Becker, H. & Biedermann, K. (1992) Embryotocity study (including
teratogenicity) with FCR 1272 in the rabbit. Unpublished report No.
5770 from Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Brusick, D.J. (1982a) Mutagenicity evaluation of FCR 1272 in the
reverse mutation induction assay with Saccharomyces cerevisiae strains
S138 and S211, final report. Unpublished report No. 2200 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Brusick, D.J. (1982b) Evaluation of FCR 1272 in the reverse mutation
induction assay with Saccharomyces cerevisiae strains S138 and S211,
addendum to the final report. Unpublished report No. 2248 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Brusick, D.J. (1982c) Evaluation of FCR 1272 in the induced mitotic
crossing over, reverse mutation and gene conversion assay in
Saccharomyces cerevisiae strain D7. Addendum to final report.
Unpublished report No. 2249 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Chen, T.H., Kavanagh, T.J., Chang, C.C. & Trosko, J.E. (1984)
Inhibition of metabolic cooperation in Chinese hamster V79 cells by
various organic solvents and simple compounds. Cell Biol. Toxicol., 1,
155-171. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Curren, R. (1985) Unscheduled DNA synthesis in rat primary
hepatocytes. Unpublished report No. 701 (Mobay) from Microbiological
Associates, Bethesda, MD, USA. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Eben, A. & Thyssen, J. (1981) Thiocyanate excretion in rats' urine
after intraperitoneal administration of FCR 1272 and decamethrin in
comparable doses and after exposure to defined FCR 1272 concentrations
in the inhalation air. Unpublished report No. 10130 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Eben, A., Heimann, K.G. & Machemer, L. (1982) Comparative study of
rats on absorption of FCR 1272 after single oral administration in
polyethylene glycol 400 or Cremophor EL/water as formulation vehicle.
Unpublished report No. 1632 from Bayer Institute of Pharmacokinetics.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Ecker, W. (1982) Biotransformation of (F-phenyl-UL-14C)-cyfluthrin;
characterization and preliminary identification of metabolites.
Unpublished report No. 1632 from Bayer Institute of Pharmacokinetics.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Ecker, W. (1983) [Fluorobenzene ring-UL-14C]FCR 1272;
[fluorobenzene-UL-14C]Cyfluthrin: Metabolism part of the general
metabolism studies in the rat. Unpublished report No. 2059 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Faul, J. (1984) Cyfluthrin (FCR 1272). Unpublished letter report of 2
July 1984 from Bayer Institute of Toxicology. Submitted to WHO by
Bayer AG, Leverkeusen, Germany.
Faul, J. (1988) Medical data on employees in cyfluthrin formulation.
Letter of 28 March 1988 from Bayer Medical Department. Submitted to
WHO by Bayer AG, Leverkeusen, Germany.
Flucke, W. (1979) Irritant effects after work with FCR 1272.
Memorandum dated 8 October 1979 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Flucke, W. (1984a) FCR 1272 (cyfluthrin); BOQ 5812315 (propoxur);
study for combination toxicity. Unpublished report No. 12544 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Flucke, W. (1984b) FCR 1272 (cyfluthrin); DDVP (dichlorvos); study for
combination toxicity. Unpublished report No. 12567 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Flucke, W. (1984c) FCR 1272 (cyfluthrin); NAK 1654 (fenfluthrin);
study for combination toxicity. Unpublished report No. 12572 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Flucke, W. & Eben, A. (1985) FCR 1272. Study for effect on the
neurotoxic target enzyme (NTE) with the chicken (Gallus
domesticus). Unpublished report No. 13821 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Flucke, W. & Schilde, B. (1980) FCR 1272. Subacute oral toxicity study
on rats. Unpublished report No. 9039 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Flucke, W. & Thyssen, J. (1980) FCR 1272. Acute toxicity studies.
Unpublished report No. 8800 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Flucke, W. & Thyssen, J. (1981) FCR 1272 ( cis:trans isomer ratio =
55:45); acute toxicity study. Unpublished report No. 9673 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Flucke, W. & Vogel, O. (1980) FCR 1272. Subacute dermal toxicity study
on rabbits. Unpublished report No. 8928 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
He, F.S., Wang, S.G., Liu, L.H., Liu, L.H., Chen, S.Y., Zhang, Z.X. &
Sun, J.X. (1989) Clinical manifestation and diagnosis of acute
pyrethroid poisoning. Arch. Toxicol., 63, 54-58.
Heimann, K.G. (1982) FCR 1272; comparative tests for acute toxicity
with various formulation aids. Unpublished report No. 10931 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Heimann, K.G. (1983a) Test to determine antidote effect against FCR
1272 toxicity in rats. Unpublished report No. 11854 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Heimann, K.G. (1983b) Cyfluthrin and methamidophos study for
combination toxicity. Unpublished report No. 12003 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Heimann, K.G. (1983c) Determination of acute toxicity (LD50), letter
report of 22 February 1983. Unpublished report from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Heimann, K.G. (1984) Determination of acute toxicity (LD50), letter
report of 5 September 1984. Unpublished report from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Heimann, K.G. (1987) FCR 1272 (c.n. cyfluthrin); study for acute oral
toxicity to rats (formulation acetone and peanut oil). Unpublished
report No. 10931 from Bayer Institute of Toxicology. Submitted to WHO
by Bayer AG, Leverkeusen, Germany.
Heimann, K.G. & Kaliner, G. (1983) FCR 1272. Study for neurotoxic
effect on rats after subacute oral administration (with addendum).
Unpublished reports Nos 12338 and 12338a from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Herbold, B. (1980a) FCR 1272. Salmonella/microsome test for detection
of point-mutagenic effects. Unpublished report No. 9273 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Herbold, B. (1980b) Micronucleus test on mouse to evaluate FCR 1272
for mutagenic potential. Unpublished report No. 9435 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Herbold, B. (1981a) FCR 1272 cyfluthrin. Pol A1-test on E. coli to
evaluate effects for DNA damage. Unpublished report No. 10450 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Herbold, B. (1981b) Dominant lethal test on male mouse to evaluate FCR
1272 for mutagenic potential. Unpublished report No. 9678 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Herbold, B. (1988) FCR 1272 (c.n. cfluthrin). In vitro cytogenetic
study with human lymphocytes for the detection of induced clastogenic
effects. Report No. 17358 from Bayer Institsute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Hixon, E.J. (1981) Investigative neurotoxicity studies in hens.
Unpublished report No. 165 from Mobay Chemical Corporation, Corporate
Toxicology Department. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Hoffmann, K. (1981a) FCR 1272 (Cyfluthrin); acute oral toxicity to
dogs. Unpublished report No. T6010889 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Hoffmann, K. (1981b) FCR 1272. Acute toxicity to sheep after oral
administration. Unpublished report No. 9750 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Hoffmann, K. & Kaliner, G. (1981) FCR 1272. Chronic toxicity study on
dogs (six-month feeding experiment). Unpublished report No. 9991 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Hoffmann, K. & Schilde, B. (1983) FCR 1272 (Cyfluthrin). Chronic
toxicity to dogs on oral administration (12 months feeding study).
Unpublished report No. 11983 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Klein, O., Weber, H. & Suwelak, D. (1983) Biokinetic part of the
general metabolism studies in the rat. Unpublished report No. 11872
from Bayer Institute of Pharmacokinetics. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Kollert, W. (1988) Medical data on workers employed in cyfluthrin
production and formulation/registration of the active ingredient in
Brazil. Letter report of 2 March 1988. Unpublished report from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Krotlinger, F. (1988) E6876 and FCR 1272 (c.n. omethoate, cyfluthrin);
study for combination toxicity on rats. Unpublished report No. 16968
from Bayer Institute of Pharmacokinetics. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Loser, E. & Eiben, R. (1983) FCR 1272. Multigeneration study on rats.
Unpublished report No. 11870 from Bayer Institute of Pharmacokinetics.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Loser, E. & Schilde; B. (1980) FCR 1272. Subchronic toxicity study on
rats. Unpublished report No. 9386 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Malcolm, A.R., Mills, L.J. & Mckenna, E.J. (1985) Effects of phorbol
myristate acetate, phorbol dibutyrate, ethanol, dimethylsulfoxide,
phenol and seven metabolites of phenol on metabolic cooperation
between Chinese hamster V79 lung fibroblasts. Cell Biol. Toxicol., 1,
269-284. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Miksche, L. (1979) Symptoms of irritation when working with FCR 1272.
Unpublished report of 2 August 1979 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Nagane, M., Hatanaka, J. & Iyatomi, A. (1982) FCR 1272. Mutagenicity
test on bacterial system. Unpublished report No. 213 from Nihon
Tokushu Noyaku Seiko K.K. Agricultural Chemicals Institute, Japan.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Ohta, T. & Moriya, M. (1982) FCR 1272; Microbial mutagenicity study.
Unpublished report dated 17 May 1982 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Oikawa, K., Iyatomi, A. & Watanabe, M. (1983) FCR 1272. Special
toxicological study - morphological effects on the nervous system of
rats. Unpublished report dated 30 June 1983 from Nihon Tokushu Noyaku
Seiko K.K. Agricultural Chemicals Institute, Japan, and St Marianna
Medical College, Department of Pathology. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Pauluhn, J. (1987) FCR 1272; study of the acute inhalation toxicity to
rats using OECD guideline No. 403. Unpublished report No. 15612 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Polacek, I. (1984) Study of FCR 1272 on neuromuscular dysfunction in
the tilting plane test on rats. Unpublished report No. R2896 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Putman, D. (1985) Sister chromatid exchange assay in Chinese hamster
ovary (CHO) cells. Test article Baythroid (FCR 1272), technical
cyfluthrin. Unpublished report No. 693 (Mobay) from Microbiological
Associates, Bethesda, MD, USA. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Roetz, R. (1983) FCR 1272 [Cyfluthrin]. Study for embryotoxic effects
on rabbits after oral administration. Unpublished report No. 11855
from Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Sacchse, K. (1986) Acute delayed neurotoxicity study with FCR 1272
(common name cyfluthrin) in the hen. Unpublished report No. 3690 from
Research and Consulting Company AG, Itingen, Switzerland. Submitted to
WHO by Bayer AG, Leverkeusen, Germany.
Sacchse, K. & Zbinden, K. (1985) Acute oral toxicity study with FCR
1272 in the hen. Unpublished report No. R3622 from Research and
Consulting Company AG, Itingen, Switzerland. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Sasaki, Y., Imanishi, H., Watanabe, M. & Ohta, T. (1986) Cyfluthrin:
In vitro cytogenetics test. Unpublished report from Kodaira
Laboratories, Institute of Environmental Toxicology, Japan. Submitted
to WHO by Bayer AG, Leverkeusen, Germany.
Schluter, G. (1981) FCR 1272 [Cyfluthrin]. Study of embryotoxic and/or
teratogenic effects after oral administration to rabbits. Unpublished
report No. 10170 from Bayer Institute of Toxicology. Submitted to WHO
by Bayer AG, Leverkeusen, Germany.
Schluter, G. (1982) FCR 1272 [Cyfluthrin]. Evaluation for embryotoxic
and teratogenic effects on orally dosed rats. Unpublished report No.
10562 from Bayer Institute of Toxicology. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Shaw, H.R., Ayers, J.E. & McCann, S.A. (1983) Metabolism of Baythroid
in a dairy cow. Unpublished report No. 86043 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Suberg, H. & Loser, E. (1983a) FCR 1272 (Cyfluthrin). Chronic toxicity
study on mice (feeding study over 23 months). Unpublished report No.
12035 from Bayer Institute of Toxicology. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Suberg, H. & Loser, E. (1983b) FCR 1272 (Cyfluthrin). Chronic toxicity
study on rats. Unpublished report No. 11949 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Thyssen, J. & Vogel, O. (1982) FCR 1272. Study for nerve damage effect
on the rat after 5-months oral application. Unpublished report No.
10705 from Bayer Institute of Toxicology. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Thyssen, J., Kaliner, G. & Groning, P. (1981) Neurotoxicity studies on
hens. Unpublished report No. 9753 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Trosko, J.E., Yotti, L.P., Dawson, B. & Chang, C.C. (1981) In vitro
assay for tumor promoters. In: Stich, H.F. & San, R.H.C., eds,
Short-term Tests for Chemical Carcinogens, New York, Springer-Verlag,
pp. 420-427. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Warngard, L. & Flodstrom, S. (1989) Effects of tetradecanoyl phorbol
acetate, pyrethroids and DDT in the V79. Cell Biol. Toxicol., 5,
67-75. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Watanabe, M. & Iyatomi, A. (1984) FCR 1272; antidotal test.
Unpublished report No. 271 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Watanabe, M., Hatanaka, J., Uwanuma, Y., Itoh, H. & Iyatomi, A.
(1982a) FCR 1272; short-term toxicity tests on rats (4-week feeding
and 4-week recovery tests). Unpublished report No. 215 from Bayer
Institute of Pharmacokinetics. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Watanabe, M., Hatanaka, J., Uwanuma, Y., Itoh, H. & Iyatomi, A.
(1982b) FCR 1272; short-term toxicity tests on mice (4-week feeding
and 4-week recovery tests). Unpublished report No. 221 from Bayer
Institute of Pharmacokinetics. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.
WHO/FAO (1988) Pesticide Residues in Food - 1987 Report. FAO Plant
Production and Protection Paper 84, Rome.
Yang, L. & Louie, A. (1985) CHO/HGPRT mutation assay in the presence
and absence of exogenous metabolic activation. Test article Baythroid
(FCR 1272), technical cyfluthrin. Unpublished report No. 694 from
(Mobay) Microbiological Associates, Bethesda, MD, USA. Submitted to
WHO by Bayer AG, Leverkeusen, Germany.
tissues was on unmetabolized parent compound. Heart and kidney also
contained a metabolite identified as
4-fluoro-3-phenoxybenzenemethanol, and liver also contained
4-fluoro-3-phenoxybenzaldehyde (Shaw et al., 1983).
2.2 Toxicological studies
2.2.1 Acute toxicity
Cyfluthrin induces signs of toxicity in the species that have
been studied that are typical of cyano-containing type-II pyrethroid
poisoning. After oral administration, the symptoms of toxicity include
increased salivation, uncoordinated movements, increased activity and
vocalization, and reduced, laboured breathing. These signs appeared
within 10-60 min after dosing; the survivors usually recovered within
7-10 days (Flucke & Thyssen, 1980). Apathy, straddle-legged gait
(mostly in the rear legs), and reduced sensitivity to external stimuli
were observed after the acute intoxication phase. Rabbits showed signs
of apathy and suppression of appetite, while dogs vomited for up to
5 h after treatment with 50-100 mg/kg bw.
Anti-inflammatory, analgesic, anti-epileptic and sedative agents
and compounds that regulate neuromuscular transmission failed to
provide adequate protection against the oral toxicity of cyfluthrin.
Likewise, calcium, cyanide antidotes and blood pressure regulators had
no antagonistic effects. Tetrazepam, the active ingredient of
Musaril(R), a centrally acting muscle relaxant, increased the oral
LD50 in rats by a factor of 1.6 and delayed the onset of mortality
(Heimann, 1983a); however, the antagonistic effect was weak and did
not provide complete protection. Atropine sulfate at 50 mg/kg bw
combined with methocarbamol at 100 mg/kg bw had a moderate but
incomplete protective effect against the oral toxicity of cyfluthrin.
When one-half of the dose was administered to male rats 20 min, 3 h,
and 24 h after treatment with cyfluthrin, the LD50 increased from
2100 to 3100 mg/kg bw (Watanabe & Iyatomi, 1984).
Sub-additive effects were seen when cyfluthrin was combined in
equitoxic doses with methamidophos, propoxur, dichlorvos, or
fenfluthrin (Heimann, 1983b; Flucke, 1984a,b,c). Krotlinger (1988)
observed a slight super-additive effect after oral administration
simultaneously with the organophosphorous compound omethoate.
The studies on the acute toxicity of cyfluthrin are summarized in
Table 2.
Table 2. Acute toxicity of cyfluthrin to laboratory animals
Species Sex Route Purity LD50 or LC50 Remarks Reference
(%) (mg/kg bw
or mg/m3)
Mouse (NMRI) M Gavage 83.6 291 In PEG 400 Flucke & Thyssen (1980)
Mouse (NMRI) F Gavage 83.6 609 In PEG 400 Flucke & Thyssen (1980)
Rat (Wistar) M Gavage 83.6 1271 Fed, in PEG 400 Flucke & Thyssen (1980)
Rat (Wistar) F Gavage 83.6 869 Fed, in PEG 400 Flucke & Thyssen (1980)
Rat (Wistar) M Gavage 83.6 1189 Fasted, in PEG 400 Flucke & Thyssen (1980)
Rat (Wistar) F Gavage 83.6 590 Fasted, in PEG 400 Flucke & Thyssen (1980)
Rat NR Gavage cis:trans 425 cis:trans isomer Flucke & Thyssen (1981)
55:45 in PEG 400
Rat M Gavage NR 795 Fasted, in PEG 400 Heimann (1984)
Rat M Gavage NR 926 Fasted, in PEG 400 Heimann (1983a) warm)
Rat M Gavage NR 499 Fasted, in xylol Heimann (1983c)
Rat M Gavage NR 505 Fasted, in Heimann (1983c)
isododecan
Rabbit (New M Gavage 83.6 > 1000 In PEG 400 Flucke & Thyssen (1980)
Zealand)
Dog (beagle) M Gavage 83.6 > 100a In PEG 400 Flucke & Thyssen (1980)
Hen (white F Gavage 84-94 5000 In PEG 400 Thyssen et al. (1981)
Leghorn)
Hen (white F Gavage 93.5 > 5000 In Cremophor Sachsse & Zbinden (1985)
Leghorn) EL:dw
Hen (white F Gavage 93.5 4500 In PEG 400 Sachsse & Zbinden (1985)
Leghorn)
Mouse F Oral NR < 100 Fasted, in Heimann (1982)
Cremophor EL:dw
Rat M Oral NR 254 Fasted, in acetone: Heimann (1982)
oil (1:10)
Rat M Oral NR 396 Fasted, in dimethyl Heimann (1982)
sulfoxide
Rat M Oral NR 16.2 Fasted, in Heimann (1982)
Cremophor EL:dw
Rat M Oral NR 500-1000 N-Dimethyl Heimann (1982)
pyrroliodone
Table 2. (continued)
Species Sex Route Purity LD50 or LC50 Remarks Reference
(%) (mg/kg bw
or mg/m3)
Rat M Oral 93 155 Fasted, in acetone: Heimann (1987)
peanut oil
Rat F Oral 93 160 Fasted, in acetone: Heimann (1987)
peanut oil
Dog (beagle) M&F Oral 95 > 100a In Cremophor Hoffmann (1981a)
EL:dw
Sheep M&F Oral NR 1000 50% premix with Hoffmann (1981b)
Wessalon
Rat M Intraperitoneal 83.6 66 In PEG 400 Flucke & Thyssen (1980)
Rat F Intraperitoneal 83.6 104 In PEG 400 Flucke & Thyssen (1980)
Rat M Intraperitoneal NR 20 In Cremophor Heimann (1982)
EL:dw
Rat F Intraperitoneal NR 24 In Cremophor Heimann (1982)
EL:dw
Rat M Intraperitoneal NR 34 In PEG 400 Heimann (1982)
Rats F Intraperitoneal NR 94 In PEG 400 Heimann (1982)
Mouse M Subcutaneous 83.6 > 2500 In PEG 400 Flucke & Thyssen (1980)
Mouse F Subcutaneous 83.6 > 2500 In PEG 400 Flucke & Thyssen (1980)
Rat M Dermal 83.6 > 5000 NR Flucke & Thyssen (1980)
Rat F Dermal 83.6 > 5000 NR Flucke & Thyssen (1980)
Rat M&F Inhalation 83.6 > 1089 In 1:1 ethanol: Flucke & Thyssen (1980)
(1-h) PEG 400
Rat M&F Inhalation 83.6 469-592 In 1:1 ethanol: Flucke & Thyssen (1980)
(4-h) PEG 400
Rat M&F Inhalation cis:trans 180-326 cis:trans isomer in Flucke & Thyssen (1981)
(4-h) 55:45 PEG 400
Rat M&F Inhalation 93 405 PEG 400:ethanol Pauluhn (1987)
(4-h) 1:1
M, male; F, female; NR, not reported; dw, deionized water; PEG, polyethylene glycol
a Vomiting occurred at 50-100 mg/kg, no higher dose used
2.2.2 Short-term toxicity
Mice
Groups of 18 mice of each sex received diets containing 0, 300,
1000, or 3000 mg/kg cyfluthrin in the feed. After one month, 12 rats
of each sex in each group were sacrificed; the remainder were
sacrificed after four weeks of recovery on control diet. Mice at the
highest dose developed ataxia, salivation, and/or emaciation. One
female mouse at 3000 mg/kg feed died. Body-weight gain was depressed
in males at 3000 and in females at 1000 mg/kg feed. Decreased growth
rates were not seen during the recovery period, as all mice recovered
to normal control values. In males at 3000 mg/kg feed, alkaline
phosphatase and blood urea nitrogen levels were slightly increased,
while those of glutamate pyruvate transaminase and cholesterol were
normal throughout. Necropsy after treatment revealed dark-red livers
in some mice at 3000 mg/kg feed; this effect was not observed after
the recovery period. The relative weights of the submaxillary glands
and kidneys were increased in animals at the highest dose, and males
at this dose also had significantly increased relative liver weights.
The weights of both the liver and the submaxillary gland returned to
normal after the four-week recovery period, while those of the kidney
remained elevated. Histopathological examination revealed minimally
increased chromatin in the nuclei of hepatocytes of males receiving
1000 mg/kg feed or more (Watanabe et al., 1982a) and in females at
the high dose. Mice receiving 1000 mg/kg feed or more had cytoplasmic
swelling of the glandular epithelium of the submaxillary glands. These
findings all disappeared after the four-week recovery period. The NOEL
was 300 mg/kg feed, equivalent to 45 mg/kg bw per day (Watanabe
et al., 1982b).
Rats
Groups of 20 SPF Wistar albino rats of each sex received doses of
0, 5, 20, or 80 mg/kg bw per day cyfluthrin (purity, 85%) dissolved in
PEG 400 by gavage once daily for four weeks followed by a six-week
observation period. The high dose was lowered to 40 mg/kg bw per day
in weeks 2 and 4 because of severe intoxication. Physical appearance
and behaviour were assessed daily throughout the study, and body
weights were measured at the start of each week. Blood measurements
and urinalysis were performed on five male and five female rats from
each group at the end of the four-week treatment period and at the end
of the six-week observation period.
The control group and rats given doses of 5 or 20 mg/kg bw per
day behaved normally, but rats at the highest dose developed symptoms
of apathy, ruffled coat, dyspnoea, salivation, hyperkinesis, ataxia,
and uncoordinated movements. The symptoms were more pronounced during
weeks 1 and 3 of treatment at the highest dose. Deaths occurred only
in rats at the high dose, 6/20 males dying between treatment days 3
and 21 and one female on day 26; no deaths were noted during the
recovery period. The body-weight gain of male rats at the high dose
was reduced by 10% during week 4 of treatment, but the weights
returned to normal during the recovery period. The body weights and
body-weight gain of females were not affected by treatment.
Haematological parameters were all within the normal ranges, and no
toxicologically relevant, group-specific or dose-related differences
from controls were seen in serum enzyme activities or electrolyte
concentrations. The plasma activity of glutamate pyruvate transaminase
was slightly higher in rats receiving the highest dose than in
controls. Urinalysis and gross and histopathological examination
revealed no treatment-related variations from normal. Likewise, no
differences in blood chemical parameters were seen at the end of the
observation period. The absolute and relative weights of the adrenal
glands were increased in female rats at the end of treatment with the
highest dose, while only the relative weight of the liver was higher
in males at the high dose in comparison with the control animals. The
increase in relative adrenal weights was not accompanied by
histopathological changes. No deaths occurred during the recovery
period. No gross pathological changes related to administration of
cyfluthrin were seen after treatment or recovery, and there were no
signs of delayed neurotoxicity at the end of the recovery period. The
NOEL was 20 mg/kg bw per day (Flucke & Schilde, 1980).
Groups of 18 rats (strain unspecified) of each sex received diets
containing 0, 100, 300, or 1000 mg/kg cyfluthrin for one month, when
12 rats of each sex per group were sacrificed; the remainder were
killed after one month of recovery on control diet. Rats at the
highest dose developed straddled gait, salivation, and/or nervousness
during treatment, but these symptoms disappeared rapidly during the
recovery period. The body-weight gain, food intake, and water
consumption of animals of each sex at 1000 mg/kg feed were depressed
but returned to normal during the recovery period. Urinalysis
indicated a higher incidence of urobilinogen and ketone bodies in rats
at 1000 mg/kg feed, but, again, these signs returned to normal after
the end of treatment. The haematological effects seen after treatment
with 1000 mg/kg feed included a decrease in erythrocyte count,
haematocrit, and haemoglobin content, which also returned to normal
after the four-week recovery period. No significant effects were seen
on liver microsomal enzyme activity. Total protein and blood glucose
levels were significantly decreased in male rats given 300 mg/kg feed.
No effects were seen on macroscopic examination. Histopathologically,
minimal degeneration of single fibres of the sciatic nerve was noted
occasionally after the treatment period. Cytoplasmic swelling,
increasing the size of the glandular epithelium of the submaxillary
glands was observed at the high dose, but these alterations
disappeared after the recovery period. On the basis of the depressed
blood glucose levels in male rats at 300 mg/kg feed, the NOEL was 100
mg/kg feed, equivalent to 5 mg/kg bw (Watanabe et al., 1982a).
Groups of 30 male and 30 female Wistar rats received diets
containing 0, 30, 100, or 300 mg/kg cyfluthrin (purity, 84.2%) diluted
in an oil vehicle (Wessalon S; equivalent to a 50% premix) for three
months. They were observed daily for alterations in appearance and
behaviour, while body weights and food consumption were recorded
weekly. Five male and five female rats from each group were killed
after one and four weeks for interim examinations, including
microsomal enzyme induction assays ( N- and O-demethylase and
cytochrome P450 content). All rats that survived the treatment were
sacrificed at the end of treatment and examined.
Appearance and behaviour were not modified during treatment, even
at the highest dose. No effects were found on body weight, food
consumption, haematological, clinical chemical or urinary parameters,
or mortality rates. Blood sugar and cholesterol levels were within the
normal range throughout treatment. At week 1, a dose-independent
increase in N-demethylase activity was seen in all treated males and
in O-demethylase activity in females at 100 and 300 mg/kg feed; the
activity of enzymes of the cytochrome P450 system was significantly
increased only in males at 300 mg/kg feed at this time. By three
months, no differences were seen in the activities of the microsomal
enzymes in any treated group. No treatment-related effects were seen
on gross or histopathological examination. No toxic effects were seen
at any dose (Loser & Schilde, 1980).
Groups of 28 male and 28 female Sprague-Dawley rats received
diets containing 0, 100, 300, or 1000 mg/kg cyfluthrin (purity, 95%)
incorporated into the powdered diet on a 0.4% maximum clay carrier,
daily for three months. Body weights were recorded weekly and food
consumption and efficiency were determined. After treatment, 20 rats
at each dose were autopsied immediately, and the remaining eight rats
in each group were allowed to recover on a control diet for a
one-month observation period before autopsy. Clinical chemical and
urinary parameters were investigated before each sacrifice.
Only the rats at the highest dose showed signs of intoxication.
Both males and females of this dose showed abnormal gait and excess
salivation, increasing in incidence until the second week of feeding,
after which a gradual decrease was noted, although recovery from this
effect was somewhat delayed in males. By the third month of treatment,
no such symptoms were seen in any group. Mortality was not affected by
treatment. The food consumption and body-weight gains of females and
males at the highest dose were significantly lower than those of the
control group. The body-weight gain depression in females appeared to
recover after cessation of treatment, while that of the males remained
significantly low. Overall feed efficiency did not vary between
groups. The results of urinalysis and haematological examination were
within the normal physiological range and revealed no
treatment-related effects. Biochemical analysis showed that rats at
300 or 1000 mg/kg feed had significantly increased blood urea nitrogen
levels, and males at 1000 mg/kg feed had significantly increased
aspartate aminotransaminase activity. The blood glucose levels were
depressed in males at 300 or 1000 mg/kg feed and in females at 1000
mg/kg feed. All biochemical parameters were within normal limits after
the recovery period. The absolute weights of the liver, heart, and
lung were decreased in male rats at the high dose, and females at this
dose had depressed liver, kidney, adrenal, and ovarian weights. In
both males and females at the high dose, the relative weight of the
submaxillary gland was increased. No other treatment-related gross
pathological effect was observed. Degeneration of single fibres of the
sciatic nerve was noted in 5/20 males and 3/20 females at the high
dose after the three-month treatment period, and in 1/8 males at the
high dose after the one-month recovery. The authors concluded that the
damage was mild and minimal, corresponding to partial loss of the
myelin sheaths of single nerve fibres. The NOEL was 100 mg/kg feed,
equal to 6.2 mg/kg bw per day (Oikawa & Iyatomi, 1983).
Rabbits
In a three-week study of dermal toxicity, groups of three New
Zealand white rabbits of each sex were given dermal applications of 0,
50, or 250 mg/kg bw cyfluthrin (purity, 85%) dissolved in PEG 400 on
the intact or abraded skin. The treatments were applied five times a
week for a contact time of 6 h/day for three weeks, and then the
rabbits were killed and examined. Tissues were collected for
microscopic examination from controls and rabbits at the high dose;
the testes were collected from animals at 50 mg/kg bw per day. No
effects were observed on appearance, behaviour, or haematological,
clinical chemical, or urinary parameters. All rabbits survived the
treatment period. No toxicologically significant effect on body-weight
gain was observed. Gross and histopathological examination showed no
alterations due to treatment with cyfluthrin. The NOEL was 250 mg/kg
bw per day (Flucke & Vogel, 1980).
Dogs
Groups of six male and six female beagle dogs were fed cyfluthrin
(purity, 84.8%) in the diet at concentrations of 0, 65, 200, or 600
mg/kg for six months. No deaths occurred, and the physical appearance
of the dogs remained normal. Tests of reflexes and measurement of body
temperature showed no significant alterations. Five males and six
females receiving the high dose showed signs of uncoordinated, stiff
gait of the hind limbs, persisting for several hours, on several
occasions after week 21. Vomiting after dosing occurred slightly more
often in animals at the high dose than in controls. Diarrhoea occurred
in all groups including controls, but much more frequently in dogs at
the high dose, from the time of initiation of treatment. No effects
were found on food intake or water consumption in the controls or in
dogs at 65 or 200 mg/kg feed, but males at the high dose had slightly
lower food consumption during the first week of treatment. No group
differences in food consumption were observed by the sixth week. Lower
mean body weights were seen in males and females at 200 or 600 mg/kg
feed from week 2 to the end of treatment. Males at 200 and females at
600 mg/kg feed had lower average weight gains between weeks 1 and 26
of treatment, whereas the average body weights of males at the high
dose were similar to those of controls at the end of the feeding
period. No effects were found on ophthalmoscopic, haematological,
clinical chemical, or urinary parameters or on organ weights; no
treatment-related changes were seen grossly or histologically. The
NOEL was 65 mg/kg feed, equal to 2 mg/kg bw per day (Hoffmann &
Kaliner, 1981).
2.2.3 Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female mice SPF strain CF1/W74 mice
received a diet containing 0, 50, 200, or 800 mg/kg cyfluthrin in the
form of a 50% premix with Wessalon S for 23 months. Clinical
examinations were carried out on 10 mice of each sex at each dose 6,
12, 18, and 23 months after the initial administration. After
treatment, five mice of each sex from each group were selected
randomly for analysis of fluoride accumulation in bone and teeth. The
remaining mice were killed and examined grossly, and tissues were
taken for histopathological examination. Neither appearance nor
behaviour was altered in any group. No effects were observed on
mortality, haematological parameters, fluoridation of bones or teeth,
food intake, or water consumption. The body-weight gain of treated
animals was not affected at doses up to and including 200 mg/kg feed,
but mean body-weight gain was significantly reduced in females at the
high dose and males at this dose had a slightly lower body-weight gain
than controls. Plasma alkaline phosphatase activity was increased in
all treated males at the six-month examination; however, at the end of
the study several controls also showed elevated alkaline phosphatase
activity. Alanine aminotransaminase activity was slightly increased in
males at the high dose after 12 and 18 months of treatment. All other
clinical chemical values were within normal ranges. No
treatment-related alterations were seen at autopsy, and the relative
and absolute organ weights were not affected by treatment. Urinalysis
and microscopic examination revealed no evidence of treatment-related
damage at any dose. The incidence and histological type of
non-neoplastic and neoplastic alterations were all within normal
ranges. The NOEL was 200 mg/kg feed, equivalent to 30 mg/kg bw per day
(Suberg & Loser, 1983a).
Rats
Groups of 65 male and 65 female SPF BOR:WISW strain rats received
a diet containing 50, 150, or 450 mg/kg cyfluthrin premixed with
Wessalon S for two years. The control group consisted of 65 rats of
each sex fed untreated diet. Both the control and the test diets were
given ad libitum. All rats were observed twice a day for alterations
in appearance, behaviour, and activity. Body weights were determined
weekly up to week 4, then every other week to conclusion of treatment.
After seven days of treatment, five rats of each sex at each dose were
killed in order to measure mixed-function enzyme induction and
cytochrome P450 content. Clinical tests were conducted on 10 rats of
each sex per dose 6, 12, 18, and 24 months after the start of
treatment. After one and two years, five rats of each sex per dose
were killed in order to determine the fluoride content of their bones
and teeth. Rats that died during treatment and five rats of each sex
per dose killed at one year were examined grossly. All remaining rats
were autopsied and examined grossly and histologically at the end of
treatment.
No treatment-related effects were seen on appearance, behaviour,
activity, or food consumption. There were no treatment-related effects
on body-weight gain at the low dose, but rats at 150 mg/kg feed
appeared initially to have slightly decreased body-weight gain. The
high dose consistently induced significantly decreased body-weight
gain throughout the experiment. No treatment-related effects were seen
on mortality, and no effects were seen on haematological or urinary
parameters, gross appearance, organ weights, or histopathological
manifestations. N-Demethylase activity was increased by 30% in males
and 20% in females after seven days at the high dose, but there were
no effects on O-demethylase activity or P450 enzyme induction.
Induction of the microsomal enzyme N-demethylase in rats at the high
dose is considered not to be of toxicological relevance as no specific
morphological alteration or damage to the liver was seen at
histopathological examination. No significant differences in fluoride
accumulation in bones and teeth were noted at interim sacrifice.
Significant, dose-related differences were found after two years of
treatment, but the biological relevance of this finding was questioned
by the authors, as minimal changes in fluoride accumulation were seen
and the assay method involved large experimental error. The results of
the final necropsy showed isolated but significant differences in
organ weights, which were attributable to dissimilar body weights and
were not dose-related; they were considered to be of little
toxicological significance. The absolute kidney and liver weights were
decreased in rats at the high dose; however, there was no
dose-dependent effect on the relative weights. The incidence of
tumours was not altered by treatment at any dose, and no other
treatment-related alterations were observed histologically. The NOEL
was 50 mg/kg feed, equal to 2 mg/kg bw per day (Suberg & Loser,
1983b).
Dogs
Groups of six beagle dogs of each sex, 22-30 weeks old, were fed
a diet containing 0, 40, 160, or 640 mg/kg cyfluthrin for one year. No
effects on appearance or behaviour were seen in dogs at 40 or 160
mg/kg feed. Two dogs at the highest dose had slight disturbances in
movement, clumsy gait primarily in the hindquarters, and a reluctance
to move. This observation was made only once during weeks 36-37 of the
study. Reflex tests indicated no deviations in any treated animals.
Body temperature, mortality, and feed and water consumption were not
affected by treatment. The body-weight gain of males at the high dose
was slightly lower than that in the other groups; females at this dose
had somewhat higher weight gains than those at other doses. Males at
the high dose had a higher incidence of vomiting, and soft, pasty
faeces were observed more frequently than in controls and in the other
groups. Ophthalmoloscopy, haematology, clinical chemistry, and
urinalysis revealed no treatment-related alterations. At termination
of treatment, all of the dogs were killed and necropsied, and the
tissues of males at the high dose and of controls were evaluated
histologically. No significant treatment-related effects were found at
autopsy. Treatment had no effect on absolute or relative organ
weights, except that the mean spleen weight of females at the high
dose was absolutely and relatively higher than that of female
controls; however, the histopathological appearance was normal in all
treated animals. Microscopic examination of the sciatic nerve showed
no pathological alterations. The NOEL was 160 mg/kg feed, equal to 5.1
mg/kg bw per day (Hoffmann & Schilde, 1983).
2.2.4 Genotoxicity
Cyfluthrin had no mutagenic potential in vivo or in vitro.
The results of assays for genotoxicity with cyfluthrin are summarized
in Table 3.
2.2.5 Reproductive toxicity
2.2.5.1 Multigeneration reproductive toxicity
Rats
Groups of 10 male and 20 female SPF-Cpb rats, five to six weeks
old, received diets containing 0, 50, 150, or 450 mg/kg cyfluthrin as
a 50% premix with Wessalon S in the feed, given ad libitum 105 days
before and throughout mating, gestation, and rearing of pups. The
parameters measured included behaviour, growth, food consumption,
mortality, fertility, viability during gestation and lactation,
development of the young, parental body-weight gain, and male:female
ratio. The study was conducted for three generations, with two litters
per generation. F0 parents produced F1a and F1b litters; F1b
parents produced F2a and F2b litters; and F2b parents produced
F3a and F3b litters. The parameters assessed included fertility,
litter size, fetal birth weight, fetal variability, and parental
weight gain. Gross and histopathological examinations were also
performed on F2b parental animals and F3b pups.
Treatment did not affect appearance, behaviour, or survival,
although one F2a pup receiving 150 mg/kg feed and another receiving
450 mg/kg feed had convulsions. Treatment did not consistently affect
birth weight or litter size at birth. A decrease in pup survival from
birth through five days of lactation was seen in animals from the
F3a and F3b matings at 150 or 450 mg/kg feed, and decreased
survival of pups on days 5-28 of lactation was noted among pups at the
high dose in all matings. Similarly, body weight was consistently
suppressed in F3a and F3b generation rats at 150 and 450 mg/kg
feed during the 28-day lactation period. Necropsy revealed decreased
absolute liver and kidney weights in rats at 150 and 450 mg/kg feed;
this was probably related to the decreased body-weight gain in these
animals. No effect was observed on relative organ weights. The NOEL
was 50 mg/kg feed, equal to 3 mg/kg bw per day (Loser & Eiben, 1983).
Table 3. Results of assays for genotoxicity with cyfluthrin
End-point Test object Concentration Purity (%) Results S9 QA Reference
In vitro
Reverse mutation Salmonella typhimurium TA100, TA98, 20-24 000 µg/plate 83.6 Negative + No Herbold (1980a)
TA1535, TA1537 Negative -
Reverse mutation Salmonella typhimurium TA100, TA98, 5-5000 µg/plate 95.0 Negative + No Nagane et al.
TA1535, TA1537, TA1538 Negative - (1982)
Reverse mutation Salmonella typhimurium TA100, TA98, 10-25 000 µg/plate 95.0 Negative + No Ohta & Moriya
TA1535, TA1537, TA1538 Negative - (1982)
Reverse mutation Escherichia coli B/r WP2 try- hcr- 5-5000 µg/plate 95.0 Negative + No Nagane et al.
Negative - (1982)
Reverse mutation Escherichia coli WP2 hcr 10-25 000 µg/plate 95.0 Negative + No Ohta & Moriya
Negative - (1982)
Reverse mutation Saccharomyces cerevisiae S138, 312.5-10 000 µg/ml 95.0 Negative + No Brusick (1982a,b)
S211a) Negative -
Reverse mutation Saccharomyces cerevisiae D7 625-10 000 µg/ml 95.0 Negative + No Brusick (1982c)
Negative -
Gene mutation Chinese hamster ovary K1-BH4 cells, 3-10 µl/ml 94.7 Negative + Yes Yang & Louie
hprt locus Negative - (1985)
Chromosomal Chinese hamster lung cells 3.3 × 10-5-3.3 × 10-3 93.7 Negative + Yes Sasaki et al. aber
ration mol/litre Negative - (1986)
Chromosomal Human lymphocytes 500-5000 µg/ml 95.1-95.5 Negative + No Herbold (1988)
aberration Negative -
Sister chromatid Chinese hamster ovary cells 3-20 µg/ml Technical Negative + Yes Putman (1985)
exchange Negative -
DNA damage Escherichia coli pol A+ and pol A1- 62.5-1000 µg/plate 95.0 Negative + No Herbold (1981a)
Negative -
DNA damage Bacillus subtilis H17, M45 rec- 200 µg/disc 95.0 Negative + No Nagane et al.(1982)
DNA damage Bacillus subtillus H17, M45 rec- 100-10 000 µg/disc 95.0 Negative + No Ohta & Moriya
(1982)
Unscheduled DNA Male Sprague-Dawley rat primary 17-5000 µg/ml 94.7 Negative Yes Curren (1985)
synthesis hepatocytes
Table 3. (continued)
End-point Test object Concentration Purity (%) Results S9 QA Reference
Mitotic gene Saccharomyces cerevisiae D7 625-10 000 µg/ml 95.0 Negative + No Brusick (1982c)
conversion Negative -
Mitotic crossing Saccharomyces cerevisiae D7 625-10 000 µg/ml 95.0 Negative + No Brusick (1982c)
over Negative -
In vivo
Micronucleus Male and female NMRI mice, 2 × 7.5, 2 × 15 83.6 Negative No Herbold (1980b)
formation bone-marrow erythroblasts mg/kg bw orally
Dominant lethal Male NMRI/ORIG Kisslegg mice 30, 60 mg/kg bw 83.6 Negative No Herbold (1981b)
mutation orally
a S9, 9000 × g fraction of rat liver
2.2.5.2 Developmental toxicity
Rats
Groups of 25 outbred pregnant female Wistar KFM-HAN (SPF) rats
received 0, 1, 3, or 10 mg/kg bw per day cyfluthrin (purity, 93.4%) in
a 1% Cremophor EL solution in drinking-water by gavage on days 6-15 of
gestation. Clinical signs, body weights, and food consumption were
recorded. On day 21 of gestation, females were killed, caesarean
sections performed, and the pups were necropsied. Although the
standard was not specified, the study conduct underwent periodic
inspections by the quality assurance unit of the laboratory.
No effects on mortality were observed. Partial hair loss occurred
in one female at 3 mg/kg bw per day. Body-weight gain was not affected
by the low or middle doses. Rats receiving the high dose had 7.7%
lower corrected mean body-weight gain. Mean food consumption was 14%
lower in rats at the high dose on days 6-11 after mating. Mating,
fertility, and gestation parameters were normal, and no dose-related
difference was seen on examination and necropsy of dams. One dam at
the high dose totally resorbed its litter. Litter size, fetal body
weight, and sex ratio were not affected by treatment. The incidences
of visceral and skeletal anomalies were not increased by treatment in
a dose-dependent manner. No teratogenic effects were seen. As a
maternal maximum tolerated dose was not achieved in this study, the
authors did not report a NOEL (Becker, 1983).
Groups of 25 Bay:FB30 rats received cyfluthrin dissolved in PEG
400 at doses of 0, 3, 10, or 30 mg/kg bw per day by gavage during days
6-15 of gestation. They were mated in a 2:1 ratio with males. The
pregnant females were killed on day 20 of gestation for examination of
their uterine contents. Six rats at 10 or 30 mg/kg bw per day were
reported to have occasional 'high-stepping gait'; at the highest dose,
they showed signs of ataxia and decreased motility. No effects on
maternal weight gain were seen that were attributable to treatment.
Litter size, frequency of resorptions, and placental weights were not
affected by treatment, even at the highest dose. Likewise, no
treatment-related increase in the incidence of stunted growth or bone
anomalies was observed in the fetuses. The author concluded that
cyfluthrin had no specific teratogenic or embryotoxic effect. As 6/25
rats at 10 mg/kg bw per day showed signs of toxicity, the NOEL for
maternal toxicity was 3 mg/kg bw per day; the NOEL for fetal toxicity
was 30 mg/kg bw per day (Schluter, 1982).
Rabbits
In the first part of a two-part study, groups of 15 Himalayan
rabbits received cyfluthrin at 0, 3, 10, or 30 mg/kg bw per day in
Cremophor EL as a solution in drinking-water by gavage on days 6-18 of
gestation. The dams were killed on day 29 of gestation. Maternal
appearance and behaviour were not affected. Lower mean fetal and
placental weights were observed, and a higher incidence of
arthrogryposis (persistent flexure or contracture of a joint) was
observed in the nine fetuses of rabbits receiving 10 or 30 mg/kg bw
per day.
Because an embryotoxic effect was observed, a second study was
conducted. Groups of 15 pregnant Himalayan rabbits received cyfluthrin
at 0, 10, or 30 mg/kg bw per day in Cremophor EL as a solution in
drinking-water by gavage during days 6-18 of gestation. The dams were
killed on day 29 of gestation, and the fetuses removed for
examination. Doses up to and including 30 mg/kg bw per day had no
effect on behaviour or appearance. One dam at the high dose died of an
unknown cause on day 26 of gestation. Mean weight gain was affected by
treatment, and the average weight gain of the animals at 30 mg/kg bw
per day was statistically significantly lower than that of controls.
Treatment had no effect on the fertilization rate or gestation or on
embryonic development, including implantation rate, litter size, and
rate of resorptions. The incidence of skeletal anomalies was not
increased at any dose. One case of arthrogryposis occurred in the
control group. There was no indication of embryotoxicity or
teratogenicity. The NOEL for maternal toxicity was 30 mg/kg bw per day
and that for embryotoxicity was 30 mg/kg bw (Schluter, 1981).
Groups of 15 pregnant female Himalayan CHBB:HM rabbits received
cyfluthrin at 0, 5, 15, or 45 mg/kg bw per day by gavage in 0.5%
aqueous Cremophor EL emulsion during days 6-18 of gestation. Dams were
observed for clinical signs and body weight. On day 29 of gestation,
the dams were killed and their fetuses removed by caesarean section
and examined for anomalies. No effects were observed on mortality,
behaviour, appearance, insemination rate, or maternal weight gain. Two
rabbits at the high dose aborted on gestation days 25 and 28, and one
completely resorbed its implants. The authors concluded that the
effects seen at the high dose were treatment-related. Litter size,
placental weights, and fetal body weights were not affected by
treatment. The rate of resorptions appeared to be dose-related.
Although arthrogryposis was seen in rabbits at the high dose, the
event was considered to be spontaneous. The NOEL for maternal toxicity
was 15 mg/kg bw per day and that for embryotoxicity was 45 mg/kg bw
per day (Roetz, 1983).
In a more recent study, cyfluthrin was tested for its embryonic
and teratogenic potential in pregnant chinchilla rabbits (Chb:CH
hybrids, SPF). Groups of 16 pregnant rabbits received 0, 20, 60, or
180 mg/kg bw per day by gavage during days 6-18 of gestation. They
were killed 28 days after mating, and the fetuses were removed by
caesarean section. The study was conducted in accordance with the
specifications of the US FIFRA GLP (40 CFR Part 160), OECD test
guideline 414, and US EPA test guideline Vol. 43 Series 163.83-3
Subdivision F, Hazard Evaluation.
Clinical observations, appearance, and behaviour were not
affected by treatment. No deaths occurred. Food consumption and
body-weight gain were reduced in a dose-dependent manner in animals at
60 and 180 mg/kg bw per day but were significantly increased 24-28
days after mating. A dose-related, statistically significant increase
in post-implantation losses, due to increased embryonic resorptions,
was observed in rabbits given the two higher doses. Necropsy revealed
no significant alteration due to treatment. The sex ratio and fetal
body weights were unaffected by treatment, and no significant
alterations were found in fetal heads or brains. Skeletal alterations
were seen, including ossification of vertebrae, sternebrae, and ribs,
but these findings were considered to be within the normal spontaneous
range. There was no indication of a teratogenic effect. On the basis
of the increased post-implantation losses, the NOEL for maternal and
embryonic toxicity was 20 mg/kg bw per day (Becker & Biedermann,
1992).
2.2.6 Special studies on neurotoxicity
Rats
Fifty male Sprague-Dawley received technical-grade cyfluthrin
(purity, 95%) as a 1.6% solution in PEG 400 by gavage at a dose of 80
mg/kg bw per day on five consecutive days. The dose was reduced to 40
mg/kg bw per day on days 6-14 owing to the onset of mild to severe
symptoms of toxicity. Twenty-five male rats received PEG 400 alone.
All rats were observed for appearance and behaviour throughout
treatment and a recovery period of three months. Ten treated and five
control rats were killed, and their tissues examined histologically on
the days 1 and 5 of treatment and one, two, and three months after
treatment; tissues from two treated and one control males were also
examined at each time point by electron microscopy. The treated rats
showed symptoms of intoxication throughout the dosing period,
including abnormal, straddled gait, slow leg movements, and excess
salivation, but no symptoms were observed five days after treatment.
The body-weight gain of the treated rats was depressed in comparison
with controls throughout treatment but recovered during the
observation period. Histological examination revealed a minimal degree
of axonal degeneration (swelling and demyelination) in single fibres
of the sciatic nerve in 6/8 rats at the first examination, in 3/8 five
days to one month after treatment, and in 2/9 two months after
treatment. A similar lesion was reported in the femoral nerve of one
treated male examined on the fifth day after treatment but was no
longer seen three months later No alterations were reported in any
other nervous or muscular tissue examined. Electron microscopy
revealed dilatation of neurotubules, accompanied by proliferation of
neurofilaments and degeneration of mitochondria, which was maximal one
month after treatment but was not seen after three months. A NOEL was
not identified (Oikawa et al., 1983).
Groups of 15 male and 15 female Wistar TNO/W74 albino rats
received cyfluthrin (purity, 83.3%) emulsified in PEG 400 by gavage
daily on seven days a week for 35 weeks. The doses administered ranged
from 30 to 80 mg/kg bw per day and were varied intermittently, so that
the rats had signs of acute toxicity after each administration.
Clinical observations were recorded daily, while body weights were
recorded weekly. Clinical chemistry was evaluated at the end of the
study, and liver, kidney, adrenal gland, brain, spinal cord, and
sciatic nerve tissues from five treated and five control rats of each
sex were examined microscopically.
The treated group displayed characteristic signs of acute
toxicity throughout the study, including apathy, ruffled coat, and
respiratory disturbances. Increased salivation, tremor, and
uncoordinated gait were observed in some rats immediately after
dosing. No paralysis of the extremities was observed. Treated males
had a 20-25% reduction in body-weight gain, while no effect was seen
on female body-weight gain. Treatment had no effect on clinical
chemical parameters or induction of cytochrome P450 or N- or
O-demethylase activity. The mortality rate was significantly higher
among treated than control males. Gross pathological and microscopic
examination showed no treatment-related pathological alterations in
any organ examined. The weights of the liver and kidney were lower in
treated males, due mostly to their reduced body weight. Although the
authors reported no histological damage to the nervous tissue, rats
that died during the treatment period were not examined, limiting the
usefulness of this study. A NOEL was not identified (Thyssen & Vogel,
1982).
Groups of five male and five female Wistar (SPF-Cpb) rats
received cyfluthrin at 0 or 60 mg/kg bw per day for two weeks by
gavage, and a supplementary group of male rats received doses of 0 or
50 mg/kg bw per day. Modified preparation techniques, i.e. perfusion
of animals with formalin and fixation of the spinal column with cord
in toto, were used to determine the neurotoxic effect of cyfluthrin.
Symptoms of acute toxicity were observed in all treated animals,
including tremor, altered gait, and increased vocalization. Four males
at 60 mg/kg bw per day died between treatment days 5 and 8, but gross
pathologicam examination revealed no specific alterations. The
body-weight gain of females was not affected by treatment, but
decreases were seen for males at 50 or 60 mg/kg bw per day. Small,
fresh brain haemorrhages were seen in all four males that died on
test. The authors concluded that the 'most likely explanation is that
these are the result of a terminal cardiovascular disorder with
necrosis of the vascular walls'. Since this finding was not seen in
control animals, a treatment-related effect could not be ruled out. A
NOEL was not identified (Heimann & Kaliner, 1983).
In a supplementary study, neuromuscular dysfunction was assessed
in the tilting plane test. Groups of 10 male rats received cyfluthrin
dissolved in Cremophor EL as a single oral administration by stomach
tube, at doses of 0, 0.1, 0.3, or 1.0 mg/kg bw in the first experiment
and 0, 0.01, 0.03, or 0.1 in the second. Diazepam was given at a
single oral dose of 5 mg/kg bw as a positive control. The angle at
which the animal started to slide from a tilting plane was evaluated
for each rat 30 min and 2, 5 and 7 h after administration.
At a dose of 0.01 mg/kg bw, cyfluthrin had no effect at any time
interval. At 0.03 mg/kg bw, the slip angle was marginally
significantly ( p < 0.05) reduced, only at 7 h. At 0.1 mg/kg bw
cyfluthrin, the angle was reduced at 5 h but was normal at other times
(Polacek, 1984). In view of the lack of a standardized protocol for
this study, the use of Cremophor EL as a vehicle (which can
substantially enhance the acute toxicity of cyfluthrin), and the
absence of a dose- or time-dependent reduction in slip angle, the
Committee considered this study inappropriate for assessing
neurotoxicity.
Hens
In a study of delayed neurotoxicity, 10 adult laying hens were
given single doses of cyfluthrin in carbowax at 5000 mg/kg bw by
gavage, and a group of 20 hens received the same dose twice at an
interval of one week. Hens given the single dose were observed for
mortality and symptoms of toxicity and were killed 56 days after
treatment; those given two doses were killed 49 days after treatment.
No treatment-related effects on body-weight gain, mortality, or gross
pathological appearance were observed. No symptoms characteristic of
delayed neurotoxicity were observed in hens given the single dose; one
hen given two doses had abnormal appearance and behaviour but
recovered the next day, while another developed ataxia complicated by
decreased activity and was killed in extremis on day 32. As
histopathological examination revealed no lesions in the brain, spinal
cord, or sciatic nerve tissue, characteristic delayed neurotoxicity
was not indicated in this study (Hixon, 1981).
Groups of white Leghorn hens were treated with cyfluthrin
(purity, 85-94%) in PEG 400 by gavage as either a single dose of 1000,
2500, or 5000 mg/kg bw, two doses of 5000 mg/kg bw at a three-week
interval, or daily administration of 5000 mg/kg bw for five
consecutive days. The single or multiple doses of 5000 mg/kg bw
induced noticeable behavioural alterations, episodes of drowsiness,
weight loss, uncoordinated gait, and some deaths. Several surviving
hens showed signs of excitation before recovery. Gross pathological
examination of hens at the high dose revealed spotty, brittle livers
and pale, slightly mottled kidneys. Histopathological examination
revealed minimal damage to the sciatic nerve, including axonal
swelling and fragmentation, proliferation of Schwann cells, and
increased vacuolization of myelin sheaths (Thyssen et al., 1981).
The authors of these two studies concluded that a delayed
neurotoxic effect was not apparent or consistent and indicated that
the histopathological findings were attributable to the normal
background neuropathic alterations seen in hens.
A group of 12 hens received a single oral dose of cyfluthrin in
PEG 400 at 4300 mg/kg bw and were observed for three weeks. A second
group of 16 hens received two doses of 4300 mg/kg bw three weeks apart
by gavage and were allowed to recover for eight weeks. A final group
of 12 hens received doses of 1500 mg/kg bw per day by gavage for five
consecutive days and were allowed to recover for eight weeks. All
animals were autopsied after the recovery periods. Neurotoxic esterase
activity was determined in the brains and spinal cords from five hens
of each group 24, 48, and 72 h and seven days after treatment. The
study was carried out in accordance with the specifications of OECD
GLP and US EPA Pesticide Assessment Guidelines, Subdivision F, Hazard
Evaluation, paragraph 81-7.
All treated hens showed signs of drowsiness and emaciation. One
control hen, two receiving 4300 mg/kg bw per day, and one receiving
1500 mg/kg bw per day died during treatment. One hen at the high dose
and one given multiple low doses were killed in extremis. No symptoms
of delayed-type neurotoxicity were seen in any treated birds. Food
consumption and body weights were not affected by treatment, and no
significant inhibition of neurotoxic esterase activity was noted in
any treated bird examined. No histopathological changes were seen in
hens receiving 1500 mg/kg bw per day cyfluthrin. Histopathological
examination of two hens receiving the high dose, one as a single dose
and one as two doses, showed slight axonal degeneration of single
nerve sites in the sciatic nerve and/or spinal cord (Sachsse, 1986).
Groups of 15 white Leghorn Lohmann strain laying hens were given
cyfluthrin formulated in PEG 400 at a dose of 5000 mg/kg bw per day or
tri-ortho-cresylphosphate as a positive control, orally for three
consecutive days. Three hens from each group were killed 24 h after
the first and second administration to determine neurotoxic esterase
activity; the remaining hens were killed 24 h after the third
administration. Hens receiving cyfluthrin displayed increased
salivation, dyspnoea, ruffled coat, and increased vocalization. Two
hens died after the second dose and six after the third dose. No
inhibition of neurotoxic esterase activity and no characteristic
delayed-type neurotoxicity were seen (Flucke & Eben, 1985).
2.2.7 Special studies on tumour promotion in vitro
As inhibition of intercellular communication is a common property
of known or suspected tumour promotors (Trosko et al., 1981; Chen
et al., 1984; Malcolm et al., 1985), inhibition of cellular
communication may be indicative of tumour-promoting potential.
Cyfluthrin at doses of 5-15 µmol/litre did not inhibit intracellular
communication (metabolic cooperation) in V79 fibroblasts and had no
effect on mutant cell recovery (Warngard & Flodstrom, 1989).
2.3 Observations in humans
The symptoms and signs of acute poisoning resulting from exposure
to different pyrethroids are similar. Clinical analysis of 573 cases
of acute pyrethroid poisoning due to occupational or accidental
exposure revealed symptoms including burning, itching, and tingling
sensations of the skin, which resolved after several hours. Washing
was not an effective treatment. The systemic symptoms included
dizziness, headache, nausea, anorexia, and fatigue; vomiting was most
common in cases due to ingestion of pyrethroids. Although less
frequently reported, tightness of the chest, paraesthesia,
palpitation, blurred vision, and increased sweating were observed in
some cases. Coarse muscular fasciculations were observed in more
serious cases. Convulsions and coma can also result from acute
poisoning with pyrethroids (He et al., 1989).
Several cases of acute occupational exposure to cyfluthrin have
been reported. The most significant effect is pronounced irritation
and burning of the skin and especially mucosal areas (lips, prepuce).
The symptoms usually appear after 1-2 h and last for one or two days.
A stinging sensation was observed 12-24 h after facial and mucosal
contact. A greater risk was associated with exposure to powdered
compound than to liquid formulations. The irritating effect was
probably due to a local action on sensitive nerve pathways and sensory
nerve cells (Flucke, 1979) and was reversible. With proper measures,
such as protective gloves and clothing, training, and avoidance of
dermal contact, occupational exposure and its potential effects can be
minimized (Miksche, 1979; Faul, 1984, 1988; Kollert, 1988).
3. COMMENTS
Cyfluthrin was evaluated by the 1987 Joint FAO/WHO Meeting on
Pesticide Residues (JMPR) (WHO/FAO, 1988), which established an ADI of
0-0.02 mg/kg bw. The same data and several new studies were reviewed
by the present Committee. The studies were performed according to
appropriate standards for study protocol and conduct.
In rats, cyfluthrin is readily absorbed and distributed. About
98% of radiolabelled drug was eliminated in urine and faeces within 48
h after oral administration, with similar amounts eliminated after
intravenous administration. In lactating dairy cattle, orally
administered cyfluthrin was also readily absorbed and distributed. The
concentration of cyfluthrin in milk reached a maximum three days after
initial dosing and then declined steadily. In cattle, the liver,
kidney, and fat contained the highest levels of residues, primarily of
unmetabolized parent compound. The major metabolic transformation is
ester hydrolysis to a 3-phenoxy-4-fluorobenzyl alcohol intermediate
and a permethric acid moiety. After ester hydrolysis, the benzyl
alcohol moiety is oxidized to the free 3-phenoxy-4-fluorobenzoic acid
metabolite, which can be either conjugated with glycine or oxidized to
give 4'-hydroxy-3-phenoxy-4-fluorobenzoic acid.
The acute oral LD50 values in rats ranged from 16 to more than
1000 mg/kg bw, depending on the vehicle used. At lethal or near-lethal
doses, signs typical of the toxicity of this class of pyrethroids were
observed. WHO has classified cyfluthrin as 'moderately hazardous'
(WHO, 1996).
Several short-term studies of the toxicity of orally administered
cyfluthrin were available in which it was tested in rats (four weeks;
three months), mice (four weeks), and dogs (six months), at doses
ranging from 5 to 450 mg/kg bw per day. Cyfluthrin reduced body weight
and increased liver and kidney weights. At lethal or near-lethal
doses, signs of neurotoxicity were observed, including ataxia,
abnormal gait, and increased vocalization. Histological evidence of
limited axonal demyelination of the sciatic nerves was observed at
these doses, which was completely reversed within three months of
cessation of treatment. In separate studies, the NOEL in rats ranged
from 5 mg/kg bw per day on the basis of depressed blood glucose levels
to 20 mg/kg bw per day on the basis of mortality and decreased
body-weight gain. The NOEL in mice was 43 mg/kg bw per day on the
basis of swelling of the submaxillary glandular epithelium. The NOEL
in dogs was equivalent to 2 mg/kg bw per day, on the basis of lowered
mean body weights.
In a three-week study of toxicity, rabbits were treated by dermal
application of cyfluthrin (in polyethylene glycol 400) at 0, 50, or
250 mg/kg bw per day for 6 h per day, five days per week for three
weeks. No toxicological effects were observed at any dose.
In a study in which dogs received cyfluthrin at up to 640 mg/kg
of feed, equal to 23 mg/kg bw per day, for one year, two out of six
dogs at the highest dose exhibited slight disturbances in movement and
gait on one occasion. There was no histopathological evidence of
neurotoxicity. Slight increases in the weights of the spleens of
females were observed at the high dose. The NOEL was therefore 160
mg/kg feed, equal to 5.1 mg/kg bw per day.
In long-term studies of toxicity and carcinogenicity in rats and
mice treated at dietary concentrations of cyfluthrin up to 450 and 800
mg/kg feed (equal to 19 mg/kg bw per day in rats and equivalent to 120
mg/kg bw per day in mice), respectively, the toxic effects were
largely non-specific and were essentially restricted to minor
alterations in body weight, organ weights, and blood biochemical
parameters. The NOELs were 50 mg/kg feed in rats, equal to 2 mg/kg bw
per day, and 200 mg/kg feed in mice, equivalent to 30 mg/kg bw per
day, both on the basis of decreased body-weight gain. Cyfluthrin
treatment was not associated with increased tumorigenesis in either
rats or mice.
In a three-generation study of reproductive toxicity in rats,
cyfluthrin at dietary concentrations of 0, 50, 150, or 450 mg/kg feed
had no consistent effect on birth weight or litter size. A decrease in
viability during lactation was noted in animals of the third
generation at the two higher levels, and weight gain was consistently
depressed in these animals. The NOEL was 50 mg/kg feed, equivalent to
2.5 mg/kg bw per day, on the basis of body-weight depression.
In a study of developmental toxicity, cyfluthrin was not
embryotoxic or teratogenic in rats given doses up to 30 mg/kg bw per
day on gestation days 6-15. The NOEL was 3 mg/kg bw per day for
maternal toxicity on the basis of clinical signs of altered gait and
30 mg/kg bw per day, the highest dose tested, for developmental
effects. The developmental toxicity of cyfluthrin has also been
evaluated in rabbits which received doses of 0, 20, 60, or 180 mg/kg
bw per day by gavage on days 6-18 of gestation. Food consumption and
body-weight gain were reduced and a dose-related increase in
post-implantation losses was observed in the groups at 60 and 180
mg/kg bw per day. The NOEL for maternal and embryotoxicity was 20
mg/kg bw per day on the basis of resorptions. The NOEL for
developmental toxicity was 180 mg/kg bw per day, the highest dose
tested.
Cyfluthrin has been tested for its ability to induce DNA damage,
chromosomal aberrations, and gene mutations in vitro and chromosomal
aberrations in vivo. Negative results were obtained in all of these
studies. These results, in conjunction with those of the studies of
carcinogenicity in rodents, indicate that cyfluthrin is neither
genotoxic nor carcinogenic.
In studies of the neurotoxicity of cyfluthrin in rats, daily oral
doses of 30-80 mg/kg bw in polyethylene glycol 400 for up to 35 weeks
resulted in characteristic signs of acute toxicity, including
salivation, tremor, and abnormal gait. Histological evidence of
limited swelling and fragmentation of myelin was observed only
infrequently, and these signs were completely reversible within three
months of cessation of treatment. Rats were also evaluated in a
neurobehavioural test, the inclined plane test, after administration
of single oral doses of up to 0.1 mg/kg bw cyfluthrin in
polyethoxylated castor oil (Cremophor EL). The results were
inconsistent and not dose-dependent. Hence, the Committee considered
that the results of this test were not useful for assessing
neurotoxicity in this case.
Although cyfluthrin is not an organophosphorus compound, it was
also evaluated for its ability to induce delayed-type neurotoxicity in
several studies in adult hens given single or multiple oral treatments
of up to 5000 mg/kg bw. The only neurological effects observed were
acute behavioural disturbances and minor changes in sciatic nerves,
including Schwann-cell proliferation and vacuolization of the myelin
sheath. Cyfluthrin produced no symptoms of delayed-type neurotoxicity
and no inhibition of neurotoxic target esterase in brain, spinal cord,
or the peripheral nervous system.
The Committee concluded that cyfluthrin does not cause
irreversible neurological damage and that the observed effects on the
nervous system occur only at high doses.
4. EVALUATION
The Committee concluded that the effects most relevant for the
toxicological evaluation of cyfluthrin were those observed in the
long-term study in rats fed the compound in the diet. The NOEL was 50
mg/kg in feed, equal to 2 mg/kg bw per day, on the basis of depression
of body-weight gain. Using this NOEL and a safety factor of 100, the
Committee established an ADI for cyfluthrin of 0-20 µg/kg bw. The
Committee noted that the same value had been established by the JMPR
in 1987.
5. REFERENCES
Bayer AG (1995) Chemical structure of cyfluthrin. Toxicological expert
opinion in accordance with Council Regulation (EEC) 2377/90 and
Directive (EEC) 81/852 as amended by Directive 92/18 (EEC).
Unpublished report from Bayer Institute of Toxicology. Submitted to
WHO by Bayer AG, Leverkeusen, Germany.
Becker, H. (1983) Embryotoxicity (including teratogenicity) study with
FCR 1272 in the rat. Unpublished report No. R 2774 from Research and
Consulting Co. AG, Itingen, Switzerland. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Becker, H. & Biedermann, K. (1992) Embryotocity study (including
teratogenicity) with FCR 1272 in the rabbit. Unpublished report No.
5770 from Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Brusick, D.J. (1982a) Mutagenicity evaluation of FCR 1272 in the
reverse mutation induction assay with Saccharomyces cerevisiae strains
S138 and S211, final report. Unpublished report No. 2200 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Brusick, D.J. (1982b) Evaluation of FCR 1272 in the reverse mutation
induction assay with Saccharomyces cerevisiae strains S138 and S211,
addendum to the final report. Unpublished report No. 2248 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Brusick, D.J. (1982c) Evaluation of FCR 1272 in the induced mitotic
crossing over, reverse mutation and gene conversion assay in
Saccharomyces cerevisiae strain D7. Addendum to final report.
Unpublished report No. 2249 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Chen, T.H., Kavanagh, T.J., Chang, C.C. & Trosko, J.E. (1984)
Inhibition of metabolic cooperation in Chinese hamster V79 cells by
various organic solvents and simple compounds. Cell Biol. Toxicol., 1,
155-171. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Curren, R. (1985) Unscheduled DNA synthesis in rat primary
hepatocytes. Unpublished report No. 701 (Mobay) from Microbiological
Associates, Bethesda, MD, USA. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Eben, A. & Thyssen, J. (1981) Thiocyanate excretion in rats' urine
after intraperitoneal administration of FCR 1272 and decamethrin in
comparable doses and after exposure to defined FCR 1272 concentrations
in the inhalation air. Unpublished report No. 10130 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Eben, A., Heimann, K.G. & Machemer, L. (1982) Comparative study of
rats on absorption of FCR 1272 after single oral administration in
polyethylene glycol 400 or Cremophor EL/water as formulation vehicle.
Unpublished report No. 1632 from Bayer Institute of Pharmacokinetics.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Ecker, W. (1982) Biotransformation of (F-phenyl-UL-14C)-cyfluthrin;
characterization and preliminary identification of metabolites.
Unpublished report No. 1632 from Bayer Institute of Pharmacokinetics.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Ecker, W. (1983) [Fluorobenzene ring-UL-14C]FCR 1272;
[fluorobenzene-UL-14C]Cyfluthrin: Metabolism part of the general
metabolism studies in the rat. Unpublished report No. 2059 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Faul, J. (1984) Cyfluthrin (FCR 1272). Unpublished letter report of 2
July 1984 from Bayer Institute of Toxicology. Submitted to WHO by
Bayer AG, Leverkeusen, Germany.
Faul, J. (1988) Medical data on employees in cyfluthrin formulation.
Letter of 28 March 1988 from Bayer Medical Department. Submitted to
WHO by Bayer AG, Leverkeusen, Germany.
Flucke, W. (1979) Irritant effects after work with FCR 1272.
Memorandum dated 8 October 1979 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Flucke, W. (1984a) FCR 1272 (cyfluthrin); BOQ 5812315 (propoxur);
study for combination toxicity. Unpublished report No. 12544 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Flucke, W. (1984b) FCR 1272 (cyfluthrin); DDVP (dichlorvos); study for
combination toxicity. Unpublished report No. 12567 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Flucke, W. (1984c) FCR 1272 (cyfluthrin); NAK 1654 (fenfluthrin);
study for combination toxicity. Unpublished report No. 12572 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Flucke, W. & Eben, A. (1985) FCR 1272. Study for effect on the
neurotoxic target enzyme (NTE) with the chicken (Gallus
domesticus). Unpublished report No. 13821 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Flucke, W. & Schilde, B. (1980) FCR 1272. Subacute oral toxicity study
on rats. Unpublished report No. 9039 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Flucke, W. & Thyssen, J. (1980) FCR 1272. Acute toxicity studies.
Unpublished report No. 8800 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Flucke, W. & Thyssen, J. (1981) FCR 1272 ( cis:trans isomer ratio =
55:45); acute toxicity study. Unpublished report No. 9673 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Flucke, W. & Vogel, O. (1980) FCR 1272. Subacute dermal toxicity study
on rabbits. Unpublished report No. 8928 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
He, F.S., Wang, S.G., Liu, L.H., Liu, L.H., Chen, S.Y., Zhang, Z.X. &
Sun, J.X. (1989) Clinical manifestation and diagnosis of acute
pyrethroid poisoning. Arch. Toxicol., 63, 54-58.
Heimann, K.G. (1982) FCR 1272; comparative tests for acute toxicity
with various formulation aids. Unpublished report No. 10931 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Heimann, K.G. (1983a) Test to determine antidote effect against FCR
1272 toxicity in rats. Unpublished report No. 11854 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Heimann, K.G. (1983b) Cyfluthrin and methamidophos study for
combination toxicity. Unpublished report No. 12003 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Heimann, K.G. (1983c) Determination of acute toxicity (LD50), letter
report of 22 February 1983. Unpublished report from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Heimann, K.G. (1984) Determination of acute toxicity (LD50), letter
report of 5 September 1984. Unpublished report from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Heimann, K.G. (1987) FCR 1272 (c.n. cyfluthrin); study for acute oral
toxicity to rats (formulation acetone and peanut oil). Unpublished
report No. 10931 from Bayer Institute of Toxicology. Submitted to WHO
by Bayer AG, Leverkeusen, Germany.
Heimann, K.G. & Kaliner, G. (1983) FCR 1272. Study for neurotoxic
effect on rats after subacute oral administration (with addendum).
Unpublished reports Nos 12338 and 12338a from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Herbold, B. (1980a) FCR 1272. Salmonella/microsome test for detection
of point-mutagenic effects. Unpublished report No. 9273 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Herbold, B. (1980b) Micronucleus test on mouse to evaluate FCR 1272
for mutagenic potential. Unpublished report No. 9435 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Herbold, B. (1981a) FCR 1272 cyfluthrin. Pol A1-test on E. coli to
evaluate effects for DNA damage. Unpublished report No. 10450 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Herbold, B. (1981b) Dominant lethal test on male mouse to evaluate FCR
1272 for mutagenic potential. Unpublished report No. 9678 from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Herbold, B. (1988) FCR 1272 (c.n. cfluthrin). In vitro cytogenetic
study with human lymphocytes for the detection of induced clastogenic
effects. Report No. 17358 from Bayer Institsute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Hixon, E.J. (1981) Investigative neurotoxicity studies in hens.
Unpublished report No. 165 from Mobay Chemical Corporation, Corporate
Toxicology Department. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Hoffmann, K. (1981a) FCR 1272 (Cyfluthrin); acute oral toxicity to
dogs. Unpublished report No. T6010889 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Hoffmann, K. (1981b) FCR 1272. Acute toxicity to sheep after oral
administration. Unpublished report No. 9750 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Hoffmann, K. & Kaliner, G. (1981) FCR 1272. Chronic toxicity study on
dogs (six-month feeding experiment). Unpublished report No. 9991 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Hoffmann, K. & Schilde, B. (1983) FCR 1272 (Cyfluthrin). Chronic
toxicity to dogs on oral administration (12 months feeding study).
Unpublished report No. 11983 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Klein, O., Weber, H. & Suwelak, D. (1983) Biokinetic part of the
general metabolism studies in the rat. Unpublished report No. 11872
from Bayer Institute of Pharmacokinetics. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Kollert, W. (1988) Medical data on workers employed in cyfluthrin
production and formulation/registration of the active ingredient in
Brazil. Letter report of 2 March 1988. Unpublished report from Bayer
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkeusen,
Germany.
Krotlinger, F. (1988) E6876 and FCR 1272 (c.n. omethoate, cyfluthrin);
study for combination toxicity on rats. Unpublished report No. 16968
from Bayer Institute of Pharmacokinetics. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Loser, E. & Eiben, R. (1983) FCR 1272. Multigeneration study on rats.
Unpublished report No. 11870 from Bayer Institute of Pharmacokinetics.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Loser, E. & Schilde; B. (1980) FCR 1272. Subchronic toxicity study on
rats. Unpublished report No. 9386 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Malcolm, A.R., Mills, L.J. & Mckenna, E.J. (1985) Effects of phorbol
myristate acetate, phorbol dibutyrate, ethanol, dimethylsulfoxide,
phenol and seven metabolites of phenol on metabolic cooperation
between Chinese hamster V79 lung fibroblasts. Cell Biol. Toxicol., 1,
269-284. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Miksche, L. (1979) Symptoms of irritation when working with FCR 1272.
Unpublished report of 2 August 1979 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Nagane, M., Hatanaka, J. & Iyatomi, A. (1982) FCR 1272. Mutagenicity
test on bacterial system. Unpublished report No. 213 from Nihon
Tokushu Noyaku Seiko K.K. Agricultural Chemicals Institute, Japan.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Ohta, T. & Moriya, M. (1982) FCR 1272; Microbial mutagenicity study.
Unpublished report dated 17 May 1982 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Oikawa, K., Iyatomi, A. & Watanabe, M. (1983) FCR 1272. Special
toxicological study - morphological effects on the nervous system of
rats. Unpublished report dated 30 June 1983 from Nihon Tokushu Noyaku
Seiko K.K. Agricultural Chemicals Institute, Japan, and St Marianna
Medical College, Department of Pathology. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Pauluhn, J. (1987) FCR 1272; study of the acute inhalation toxicity to
rats using OECD guideline No. 403. Unpublished report No. 15612 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Polacek, I. (1984) Study of FCR 1272 on neuromuscular dysfunction in
the tilting plane test on rats. Unpublished report No. R2896 from
Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Putman, D. (1985) Sister chromatid exchange assay in Chinese hamster
ovary (CHO) cells. Test article Baythroid (FCR 1272), technical
cyfluthrin. Unpublished report No. 693 (Mobay) from Microbiological
Associates, Bethesda, MD, USA. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Roetz, R. (1983) FCR 1272 [Cyfluthrin]. Study for embryotoxic effects
on rabbits after oral administration. Unpublished report No. 11855
from Bayer Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Sacchse, K. (1986) Acute delayed neurotoxicity study with FCR 1272
(common name cyfluthrin) in the hen. Unpublished report No. 3690 from
Research and Consulting Company AG, Itingen, Switzerland. Submitted to
WHO by Bayer AG, Leverkeusen, Germany.
Sacchse, K. & Zbinden, K. (1985) Acute oral toxicity study with FCR
1272 in the hen. Unpublished report No. R3622 from Research and
Consulting Company AG, Itingen, Switzerland. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Sasaki, Y., Imanishi, H., Watanabe, M. & Ohta, T. (1986) Cyfluthrin:
In vitro cytogenetics test. Unpublished report from Kodaira
Laboratories, Institute of Environmental Toxicology, Japan. Submitted
to WHO by Bayer AG, Leverkeusen, Germany.
Schluter, G. (1981) FCR 1272 [Cyfluthrin]. Study of embryotoxic and/or
teratogenic effects after oral administration to rabbits. Unpublished
report No. 10170 from Bayer Institute of Toxicology. Submitted to WHO
by Bayer AG, Leverkeusen, Germany.
Schluter, G. (1982) FCR 1272 [Cyfluthrin]. Evaluation for embryotoxic
and teratogenic effects on orally dosed rats. Unpublished report No.
10562 from Bayer Institute of Toxicology. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Shaw, H.R., Ayers, J.E. & McCann, S.A. (1983) Metabolism of Baythroid
in a dairy cow. Unpublished report No. 86043 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Suberg, H. & Loser, E. (1983a) FCR 1272 (Cyfluthrin). Chronic toxicity
study on mice (feeding study over 23 months). Unpublished report No.
12035 from Bayer Institute of Toxicology. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Suberg, H. & Loser, E. (1983b) FCR 1272 (Cyfluthrin). Chronic toxicity
study on rats. Unpublished report No. 11949 from Bayer Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Thyssen, J. & Vogel, O. (1982) FCR 1272. Study for nerve damage effect
on the rat after 5-months oral application. Unpublished report No.
10705 from Bayer Institute of Toxicology. Submitted to WHO by Bayer
AG, Leverkeusen, Germany.
Thyssen, J., Kaliner, G. & Groning, P. (1981) Neurotoxicity studies on
hens. Unpublished report No. 9753 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Trosko, J.E., Yotti, L.P., Dawson, B. & Chang, C.C. (1981) In vitro
assay for tumor promoters. In: Stich, H.F. & San, R.H.C., eds,
Short-term Tests for Chemical Carcinogens, New York, Springer-Verlag,
pp. 420-427. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Warngard, L. & Flodstrom, S. (1989) Effects of tetradecanoyl phorbol
acetate, pyrethroids and DDT in the V79. Cell Biol. Toxicol., 5,
67-75. Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Watanabe, M. & Iyatomi, A. (1984) FCR 1272; antidotal test.
Unpublished report No. 271 from Bayer Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkeusen, Germany.
Watanabe, M., Hatanaka, J., Uwanuma, Y., Itoh, H. & Iyatomi, A.
(1982a) FCR 1272; short-term toxicity tests on rats (4-week feeding
and 4-week recovery tests). Unpublished report No. 215 from Bayer
Institute of Pharmacokinetics. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
Watanabe, M., Hatanaka, J., Uwanuma, Y., Itoh, H. & Iyatomi, A.
(1982b) FCR 1272; short-term toxicity tests on mice (4-week feeding
and 4-week recovery tests). Unpublished report No. 221 from Bayer
Institute of Pharmacokinetics. Submitted to WHO by Bayer AG,
Leverkeusen, Germany.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.
WHO/FAO (1988) Pesticide Residues in Food - 1987 Report. FAO Plant
Production and Protection Paper 84, Rome.
Yang, L. & Louie, A. (1985) CHO/HGPRT mutation assay in the presence
and absence of exogenous metabolic activation. Test article Baythroid
(FCR 1272), technical cyfluthrin. Unpublished report No. 694 from
(Mobay) Microbiological Associates, Bethesda, MD, USA. Submitted to
WHO by Bayer AG, Leverkeusen, Germany.
