
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
SAFETY EVALUATION OF CERTAIN FOOD
ADDITIVES AND CONTAMINANTS
WHO FOOD ADDITIVES SERIES: 44
Prepared by the Fifty-third meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)
World Health Organization, Geneva, 2000
IPCS - International Programme on Chemical Safety
CONTAMINANTS
LEAD
First draft prepared by Dr C. Carrington1, Dr M. Bolger1, Dr J.C.
Larsen2 and Dr B. Peterson3
1US Food and Drug Administration, Washington DC, USA; Department of
Biochemical and Molecular Toxicology, Institute of Toxicology, Danish
Veterinary and Food Administration, Soborg, Denmark; and 5 Novigen
Sciences Inc., Washington DC, USA
Explanation
Biological data
Biochemical aspects
Absorption, distribution and excretion
Biotransformation
Effects on enzymes and other biochemical parameters
Pharmacokinetic models
Biomarkers
Toxicological studies
Acute toxicity
Carcinogenicity
Other effects in laboratory animals
Observations in humans
Death
Haematological effects
Neurological effects
Renal effects
Cardiovascular effects
Developmental effects
Reproductive effects
Carcinogenesis
Exposure to lead
Residue data submitted by national governments
National exposure estimates
Total intake of lead from food
Contribution of foods/food categories to total lead intake
Intake assessments based on the GEMS/Food regional diets and
selected lead concentrations for those foods
Intake estimates based on the proposed CCFAC limits for lead
in selected foodstuffs
Methods
Results
Intake estimates based on estimates of the 'typical'
concentrations of lead in selected foods
Methods
Results
Intake estimates based on estimates of a realistic 'maximum'
concentration of lead in selected foods
Methods
Results
Discussion and conclusions
Exposure from non-dietary sources
Migration from food containers
Soil
Paint
Surveys of blood lead concentrations
Quantitative risk assessment
Exposure assessments
Population estimates of dietary lead exposure
Frequently consumed foods
Infrequently consumed foods
Simulations of the effect on exposure of a commodity
with a high concentration of lead
Relation of dietary lead to blood lead
Drinking-water and blood lead in bottle-fed
infants
Drinking-water and blood lead in adults
Dose-response assessment
Designing an analysis
Dose-response models for blood lead and IQ
A sample diet-response simulation
Comments
Evaluation
References
1. EXPLANATION
The Committee first evaluated lead at its sixteenth meeting
(Annex 1, reference 30), when a provisional tolerable weekly intake
(PTWI) of 3 mg of lead per person, equivalent to 50 µg/kg bw, was
established. This PTWI was reconfirmed by the Committee at its
twenty-second meeting (Annex 1, reference 47). At its thirtieth
meeting (Annex 1, reference 73), the Committee assessed the risk posed
by lead to the health of infants and children and established a PTWI
of 25 µg/kg bw for this population group. The Committee again
evaluated lead at its forty-first meeting (Annex 1, reference 107),
when the previous PTWI of 50 µg/kg bw for adults was withdrawn, and
the existing PTWI of 25 µg/kg bw for infants and children was
reconfirmed and extended to people in all age groups. The review of
the health effects of lead at the forty-first meeting was based on a
recent assessment of inorganic lead performed by an International
Programme on Chemical Safety (IPCS) Task Group and published as an
Environmental Health Criteria monograph (WHO, 1995).
At its present meeting, the Committee was requested to assess the
risk of dietary exposure of infants and children, with special
emphasis on the most critical effect, which was considered to be
impaired neurobehavioural development. The Committee considered
several models that had been developed to define the relationship
between the health effects of current levels of exposure to lead and
the impact on health that might be anticipated from reducing exposure.
The current PTWI was not reconsidered and was retained at its present
value.
The scientific literature on lead is extensive and numerous
reviews have been published. This monograph relies mainly on the
Environmental Health Criteria monograph on inorganic lead (WHO, 1995)
and a document from the Agency for Toxic Substances and Disease
Registry (1997); earlier and later studies of particular significance
are also cited. Only brief summaries of toxicological effects are
given, but studies of the effects critical for the risk assessment are
evaluated in more detail. The main emphasis is laid on studies in
humans.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
The rate of absorption of lead after ingestion can range from 3%
to 80%. It is heavily influenced by food intake, much higher rates of
absorption occurring after fasting than when lead is ingested with a
meal. This effect may be due mainly to competition from other ions,
particularly iron and calcium, for intestinal transport pathways.
Absorption is also affected by age, the typical absorption rates in
adults and infants being 10% and 50%, respectively (O'Flaherty, 1995;
Agency for Toxic Substances and Disease Registry, 1997).
In blood, most lead is found in erythrocytes. The ability of
erythrocytes to sequester lead is attributable to binding by
haemoglobin and other proteins; however, higher plasma:erythrocyte
ratios are seen with increasing blood lead concentrations as a result
of saturation of binding sites (Agency for Toxic Substances and
Disease Registry, 1997).
After its absorption and distribution in blood, lead is initially
distributed to soft tissues throughout the body. Subsequently, lead is
deposited in bone, where it eventual accumulates. The half-life of
lead in blood and other soft tissues is 28-36 days (WHO, 1995). Lead
that is deposited in physiologically inactive cortical bone may
persist for decades without substantially influencing the
concentrations of lead in blood and other tissues (Rabinowitz et al.,
1976), but lead that is accumulated early in life may be released
later, when bone resorption is increased as result of, for example,
calcium deficiency or osteoporosis. Lead that is deposited in
physiologically active trabecular bone is in equilibrium with blood.
The accumulation of high concentrations in blood when exposure is
reduced may be due to the ability of bone to store and release lead.
Dietary lead that is not absorbed in the gastrointestinal tract
is excreted in the faeces. Lead that is not distributed to other
tissues is excreted through the kidney and to a lesser extent by
biliary clearance (Agency for Toxic Substances and Disease Registry,
1997).
2.1.2 Biotransformation
Inorganic lead is not metabolized, although it may be conjugated
with molecules such as glutathione. Organic lead may be metabolized to
inorganic lead.
2.1.3 Effects on enzymes and other biochemical parameters
Lead is known to affect a number of enzymes and physiological
systems, resulting in a wide variety of changes in humans (WHO, 1995).
The best-documented biochemical mechanism is inhibition of
delta-aminolaevulinic acid dehydratase, resulting in binding of lead
to sulfhydryl groups. The concentrations of coproporphyrin in urine
and of 5-aminolaevulinate in blood and urine are increased in
individuals exposed to high concentrations of lead as a result of
inhibition of this enzyme.
Other enzymes have also been reported to be inhibited by lead.
Changes in circulating vitamin D concentrations after exposure to lead
have been attributed to inhibition of 25-hydroxyvitamin D-1
alpha-hydroxylase. The activity of dihydrobiopterin reductase, an
enzyme involved in the synthesis of catechola-mines, is reduced by
lead in rat brain. The activity of nicotinamide adenine dinucleotide
synthetase in erythrocytes may also be inhibited by lead.
2.1.4 Pharmacokinetic models
Several pharmacokinetic models have been developed in an attempt
to relate exposure to lead to concentrations in blood and other
tissues. A model developed by the United States Environmental
Protection Agency (1994) of lead in air, diet, dust, paint, soil, and
drinking-water allows modelling of distribution in a 10-compartment
model that includes plasma, erythrocytes, trabecular bone, and
cortical bone. The parameters in the model were chosen to represent
children, and the model presumes that exposure and pharmaco-kinetics
are in a steady state. Population distributions are derived by using
the model output to estimate the population geometric mean and a fixed
geometric standard deviation.
O'Flaherty (1991, 1993, 1995) developed another pharmacokinetic
model which represents the temporal relationships in the transfer of
lead between blood, bone, and other tissues. It is thus more useful
for predicting physiological lead concentrations when exposure is
inconstant. It also accounts for changes that occur as a function of
age. In particular, the model accounts for differences in
pharmacokinetics attributable to changes in body size and in
age-related changes in absorption, bone growth, or metabolism that
occur during childhood, pregnancy, menopause, and old age. The model
does not attempt to account for population variation, but when it is
used in a simulation designed to represent population variation, the
exposure inputs and some of the biological parameters (such as
absorption rates) can be varied to reflect population differences.
2.1.5 Biomarkers
The concentration of lead in blood is the most widely used
biomarker of exposure, largely because it is the measure that is the
easiest to obtain. Blood lead concentration is typically reported in
micrograms per deciliter. Because blood concentrations are strongly
influenced by recent exposure, they may not reflect long-term
exposure, particularly to intermittent or infrequent, high levels.
A marker that was widely used before techniques for measuring
blood lead became widely available is plasma delta-aminolaevulinic
acid dehydratase activity, which correlates strongly with blood lead
concentration (Agency for Toxic Substances and Disease Registry,
1997). Related markers that have sometimes been used are the urinary
and blood concentrations of delta-amino-laevulinic acid and plasma
d-aminolaevulinic acid synthetase. Both of these markers correlate
positively with exposure to lead.
Other markers that have been employed on occasion include bone
lead concentration, urinary concentration, and dentine lead (Agency
for Toxic Substances and Disease Registry, 1997). Urinary lead is
limited as a marker because the concentrations fluctuate more with
current exposure than in blood, and they tend to be lower and more
difficult to measure. Tooth lead has the advantage of reflecting
chronic exposure to lead and can be collected from children. Bone lead
is also a marker of chronic exposure and can be measured in vivo by
X-ray fluorescence; however, variation in intra-individual
measurements make this technique unreliable for measuring
concentrations of lead in populations that are not heavily exposed.
2.2 Toxicological studies
2.2.1 Acute toxicity
Lead is a classical chronic or cumulative poison. Health effects
are generally not observed after a single exposure, and no LD50
values have been reported in the literature. The lowest observed
lethal doses in animals after short-term oral exposure to lead
acetate, lead chlorate, lead nitrate, lead oleate, lead oxide, and
lead sulfate range from 300 to 4000 mg/kg bw, the doses having been
given in multiple administrations (Lewis, 1992; Agency for Toxic
Substances and Disease Registry, 1997). The wide range is attributable
to differences in absorption of the various lead salts and differences
in exposure.
2.2.2 Carcinogenicity
Studies of carcinogenicity in rodents have been conducted with
lead arsenate and lead phosphate. In several studies, increased
frequencies of renal tumours were seen in both male and female rats,
with a greater increase in males. The doses used in these studies were
quite high, and production of detectable increases in renal tumour
frequency apparently required doses in excess of 10 mg/kg bw per day.
Such concentrations also cause renal toxicity in rodents.
2.2.3 Other effects in laboratory animals
Many of the effects that have been observed in laboratory animals
have also been observed in humans (WHO, 1995). These include effects
on haem synthesis, neurological and behavioural effects, renal
effects, cardiovascular effects, and effects on the reproductive
system. In addition, lead has been shown to have effects on bone and
on the immune system in laboratory animals. Impaired learning ability
has been reported in rats exposed to lead at concentrations of 15-20
µg/dL and in nonhuman primates exposed to < 15 µg/dL. Visual and
auditory impairments have been reported in experimental animals. Renal
toxicity in rats appeared to occur at concentrations exceeding 60
µg/dL, while cardiovascular effects have been noted at 40 µg/dL.
2.3 Observations in humans
2.3.1 Death
Increased death rates have been reported after occupational
exposure to lead resulting in blood lead concentrations in excess of
50 µg/dL. The causes of death most often associated with such exposure
are cardiovascular disease, renal disease, and cancer (Cooper, 1988;
Agency for Toxic Substances and Disease Registry, 1997).
2.3.2 Haematological effects
A primary symptom of chronic lead poisoning, long associated with
exposure to lead, is anaemia. This results from reductions in
erythrocyte life-span and inhibition of haem synthesis by
delta-aminolaevulinic acid dehydratase and ferrochelatase. Inhibition
of delta-aminolaevulinic acid dehydratase can be detected at low blood
lead concentrations and has been considered for use as a biomarker of
exposure, but haemoglobin concentrations are generally not inhibited
sufficiently to result in clinically observable anaemia until a
concentration of at least 80-100 µg/dL is reached (Goyer, 1996).
2.3.3 Neurological effects
The clearest clinical effect that is attributable to very high
levels of exposure to lead is encephalopathy. This effect usually
occurs at blood lead concentra-tions exceeding 300 µg/dL, although
more subtle and infrequent effects on the central nervous system have
been noted at 100 µg/dL (Goyer, 1996; Agency for Toxic Substances and
Disease Registry, 1997). This syndrome has been observed most often in
children, the source of lead usually being paint chips.
Lead-induced encephalopathy may develop within weeks of exposure
and is characterized by irritability, poor attention span, headache,
muscular tremor, loss of memory, and hallucinations. Severe cases are
often fatal. More subtle neurological effects occur after exposure to
lower concentrations. The neurological effects that have been
associated with concentrations in the range of 50-300 µg/dL include
disturbances of motor function, cognitive function, and intelligence
scores and altered emotional states. Effects on the peripheral nervous
system have also been noted. In particular, reductions in nerve
conduction velocity have been associated with blood lead
concentrations in excess of 30 µg/dL.
2.3.4 Renal effects
There are two forms of lead-induced nephropathy. Acute renal
nephropathy, which occurs after short exposure to lead, involves
dysfunction of the proximal tubules and is largely reversible. This
form of renal toxicity is often reported in children after oral
exposure. It is attributable to impairment of energy-dependent
transport functions resulting from inhibition of mitochondrial
respiration and phosphorylation. Detectable effects on renal function
may occur with blood lead concentrations as low as 40 µg/dL (Goyer,
1989; WHO, 1995). Chronic lead-induced nephropathy involves reductions
in glomerular filtration rate and irreversible atrophy of the proximal
and distal tubules. This disease is most often associated with higher
and longer exposure than that required for the acute effect. This
disease is most often associated with occupational exposure to lead.
2.3.5 Cardiovascular effects
Exposure to lead has been associated with increased incidences of
hyper-tension and cardiovascular disease (Pirkle et al., 1985; Agency
for Toxic Substances and Disease Registry, 1997). The relationship
between blood lead and blood pressure appears to be greatest at
concentrations below 50 µg/dL, and increases in blood lead
concentration beyond this level do not appear to correlate with
greater hypertension. The causal relationship between lead and
hypertension is not clear, however: the elevation may be secondary to
effects on other organ systems such as the kidney or the haematopoetic
system.
2.3.6 Developmental effects
The potential developmental effects of maternal exposure to lead
have been studied in several populations. Needleman et al. (1984)
reported a higher incidence of minor congenital anomalies, including
angiomas and papillae, in the offspring of women in Boston (USA) who
had been exposed to lead during pregnancy, but the effects were not
strongly correlated with blood lead concentration. Reduced birth
weight and gestational age have also been associated with exposure to
lead in utero (Baghurst et al., 1987; Dietrich et al., 1987), but
conflicting results were obtained (Graziano et al., 1990; Agency for
Toxic Substances and Disease Registry, 1997).
The most important and best-documented effect of lead at the
concentrations most commonly encountered outside occupational settings
is on the neurobehavioural development of children of mothers exposed
to lead. Early indications of a possible effect were difficult to
disentangle from other factors known to influence development. In a
cross-sectional study, however, Needleman et al. (1979) reported an
association between blood lead concentration and reduced intelligence
quotients (IQs) in a population of middle-class children who were
similar with respect to known confounders. Since that study, a number
of large prospective studies have been conducted, in all of which
cohorts of several hundred children were followed-up from birth to
childhood.
In a study of a cohort of 249 middle-class children in Boston
(USA), blood lead was measured at birth (in cord blood) and at 6
months, 12 months, 18 months, 24 months, 57 months, and 10 years of
age. The mean concentrations in cord blood ranged from < 3 to 25
µg/dL. Mean concentrations of 6-8 µg/dL were found at ages < 57
months, which had decreased to 2.9 µg/dL at 10 years of age.
Behavioural performance tests were administered at the same time as
the blood lead was measured. Prenatal and postnatal blood lead
concentrations were related to performance on the Bayley mental
development index (MDI) and the Bayley psychomotor development index
(PDI) at the age of 6-24 months, to the McCarthy scales of childrens
abilities (MSCA) at 57 months, and to the Weschler intelligence scale
for children (WISC) and the Kaufman test of educational achievement at
10 years of age. The authors reported an inverse correlation between
cord blood lead concentration and MDI scores at 6, 12, 18, and 24
months of age, and inverse relationships were noted between blood lead
concentration at 24 months and MSCA scores at 57 months and WISC and
Kaufman scores at 10 years. There appeared to be a greater effect of
lead on MDI performance at 57 months in infants with a higher
socioeconomic status. No correlation was seen between cord blood lead
concentration and performance on the PDI scale or between postnatal
blood lead concentration and performance on the MDI or PDI scale.
Furthermore, no relationship was noted between cord blood lead
concentration and scores at 57 months or 10 years (Bellinger et al.,
1991; National Research Council, 1993).
In a prospective study conducted in Port Pirie, Australia, 723
children were followed from birth to the age of 7 years. Blood lead
was measured at birth (cord blood) and at 6 months, 15 months, 24
months, 36 months, and 4 years of age. The mean cord blood
concentration was 8.3 µg/dL, whereas the mean concentration in blood
from children up to 48 months was 15-21 µg/dL. Behavioural performance
was evaluated on the Bayley scales at 24 months, as MSCA at 48 months,
and on the WISC at 7 years of age. The authors reported a weak inverse
correlation between cord blood lead concentration and MDI scores at 6
months and an inverse correlation between the integrated average of
postnatal blood lead concentrations and MSCA scores at 48 months and
WISC scores at 7 years of age (Baghurst et al., 1987, 1992; National
Research Council, 1993).
A prospective study conducted in Cincinnati (USA) involved
following 305 children from birth to 78 months of age. Blood lead was
measured at birth (cord blood) and at various times up to 5 years of
age. The mean blood lead concentration was about 6 µg/dL at birth and
up to 3 months, rose to 21 µg/dL at the age of 24 months, and then
fell to a population mean of 12 µg/dL at 5 years of age. Behavioural
performance was evaluated on the Bayley scales at 3, 6, 12, and 24
months, with the Fluarty speech and language screening test at 39
months, with the Kaufman assessment battery for children at 48 and 60
months, with the Bruininks-Osetretsky test of motor proficiency at 72
months, and on the WISC at 78 months. The authors reported an inverse
correlation between cord blood lead concentration and MDI score at 3,
6, and 12 months but a positive association at 24 months. The MDI
scores did not appear to be associated with postnatal measures of
exposure to lead. Inverse associations were found between the Fluarty
test score at 39 months and cord blood lead concentration, the Kaufman
score at the age of 5 and mean blood lead concentration in the
previous year, and the WISC and Bruininks-Osetretsky scores and
integrated measures of postnatal exposure (Dietrich et al., 1987,
1992, 1993; National Research Council, 1993).
A prospective study was conducted in Cleveland (USA) in which a
cohort of 359 children was followed from birth through 10 years of
age. Blood lead was measured at birth (cord blood) and at 6 months, 2
years, and 3 years of age. The mean blood lead concentration was about
6 µg/dL at birth and rose to about 17 µg/dL at 2 and 3 years of age.
Neonatal behavioural performance was evaluated on a neonatal behaviour
assessment scale and in the Gram-Rosenblith behavioural examination.
The Bayley tests were administered at 6, 12, and 24 months and a
sequenced inventory of communication development at 12, 24, and 36
months. Behaviour at the ages of 4-10 was evaluated on the Eschler
preschool and primary scales of intelligence. The authors reported an
inverse correlation between cord blood lead concentration and the
Gram-Rosenblith score at birth and the MDI and PDI scores at 6 months.
No other associations were noted (National Research Council, 1993).
In a prospective study conducted in Sydney, Australia, 318
children were followed from birth through 4 years of age. Blood lead
was measured at birth (cord blood) and every six months until the end
of the study. The mean blood lead concentration was about 8 µg/dL at
birth, rose to about 15 µg/dL at 2 years of age, and then fell to 10
µg/dL at 4 years of age. Behavioural performance was assessed on the
Bayley scales at 6, 12, and 24 months and on the MSCA at 3 and 4 years
of age. No association was noted between any index of exposure to lead
and performance on the tests (Cooney et al., 1989; National Research
Council, 1993).
A prospective study was conducted in two towns in Kosovo,
Yugoslavia, to follow 332 children from birth through 4 years of age.
Behavioural performance was compared for children living in a town in
which a lead smelter was located (Titova Mitrovica) with that of
children living in the other (Pristina). Blood lead was measured every
six months. The geometric mean blood lead concentrations in children
in the two towns were 22 and 5.4 µg/dL at birth and 40 and 9.6 µg/dL
at 4 years of age. Behavioural performance, evaluated on the MSCA at
the age of 4, was associated with all measurements of blood lead,
those taken after 24 months providing stronger associations than
earlier measurements (Graziano et al., 1990; Factor-Litvak et al.,
1991; Wasserman et al., 1992; Agency for Toxic Substances and Disease
Registry, 1997).
A number of cross-sectional and longitudinal studies have been
conducted to study the relationship between lead concentration in
blood or teeth to the outcomes on various tests of behavioural
performance (National Research Council, 1993).
2.3.7 Reproductive effects
Exposure of women to lead before or during pregnancy is
associated with miscarriage and stillbirth (Agency for Toxic
Substances and Disease Registry, 1997), but statistically significant
associations have been observed only in studies in which direct
occupational exposure resulted in blood lead concentrations > 20
µg/dL. Impairment of male reproductive function, in the form of
decreased sperm counts and increased numbers of abnormal sperm, has
been reported in men with heavy occupational exposure to lead (WHO,
1995; Agency for Toxic Substances and Disease Registry, 1997).
2.3.8 Carcinogenesis
Evidence of the carcinogenicity of lead comes from both
epidemiological and experimental studies. The standardized mortality
ratios for cancers of the lung, digestive tract, and kidney were found
to be increased from 1 to 2.5 in studies of workers in lead production
and battery plants who had blood concentrations of 40-100 µg/dL
(Cooper, 1976; Agency for Toxic Substances and Disease Registry,
1997).
3. EXPOSURE TO LEAD
Exposure to lead can occur from many sources but usually arises
from industrial use. Lead and its compounds can enter the environment
at any time during mining, smelting, processing, use, recycling, or
disposal. The main uses of lead are in batteries, cables, pigments,
plumbing, gasoline, solder and steel products, food packaging,
glassware, ceramic products, and pesticides. The main exposure of the
general non-smoking adult population is from food and water. Airborne
lead may contribute significantly to exposure, depending on such
factors as use of tobacco, occupation, and proximity to sources such
as motorways and lead smelters. Food, air, water, and dust or soil are
the main potential sources of exposure of infants and young children,
and milk, formula, and water are significant sources of exposure of
infants up to 4 or 5 months of age (WHO, 1995).
Exposures to lead as a result of its ingestion in food, water, or
air all make important contributions in national assessments. Although
this assessment was limited to food and water, rational
decision-making requires estimates of the major sources of exposure
and the probable impact of proposed controls on exposure.
Estimates of the intake of a contaminant in food are complicated
by the skewed distributions of residues, since food is not
contaminated in a controlled or predictable agricultural or
manufacturing process. This is particularly true for lead, as will be
seen. Nonetheless, it is often possible to control contamination, and
such controls should have the maximum effect on lead intake. For this
assessment, the data that were submitted to the Committee were
reviewed to identify the range and 'typical' concentrations of lead in
the food categories for which limits have been proposed by the Codex
Alimentarius Commission. The objective of the assessment was to
provide information on exposure for use in evaluating the effect of
the proposed limits.
Several countries submitted estimates of the intake of lead by
their populations, and those estimates were reviewed and, when
possible, compared with corresponding data derived from monitoring.
Both bottled and tap water are also potential sources of lead.
Regional diets were developed by WHO within the Global Environment
Monitoring System-Food Contamination Monitoring and Assessment
Programme (GEMS/Food) and have been used by WHO and others since 1987
to estimate the intake of pesticides and contaminants. The diets were
derived from food balance sheets compiled by FAO and thus provide data
that are comparable across different countries and regions of the
world. The data are based on the countries' annual food production,
imports, and exports. They do not take into account waste at the
household or individual level and are thus expected to be
overestimates of consumption; comparisons with data from detailed
national food consumption surveys indicate that actual food intakes
are overestimated by about 15%. The data do not permit analysis of the
intakes of subgroups such as children and infants consuming the
regional diets.
Data on lead intake in more than 25 countries available from
GEMS/Food (1992) are summarized below. Intake of lead was also
estimated in the GEMS/Food regional diets in combination with the
values for residues that had been submitted, i.e. maximum, 'typical',
or the maximum limits proposed to the Codex Committee on Food
Additives and Contaminants (CCFAC). In view of the toxicity of lead,
the Committee considered the available data and also estimated
children's intakes of lead.
The ideal estimates of the intake of lead from food would be
derived from the entire distribution of residue concentrations in
foodstuffs. This would be particularly desirable, given the skewed
distributions of lead. The data were not, however, submitted in a form
that would permit such an analysis or the numbers of samples were too
limited. The analysis was further complicated by the extremely wide
variety of foods for which data were submitted and the manner in which
the data were reported: for instance, one country would report 'oil,
vegetable' while another would specifiy items such as 'soya bean oil,
pizza, dried tomatoes, and vacuum-packed tofu'.
The data do provide an indication of the range of lead
concentrations in different types of food from different countries and
an indication of national intake levels. These data and the proposed
maximum limits on lead (CCFAC, 1999) were used in combination with
GEMS/Food regional diets to derive estimates of potential lead intakes
around the world. This method provides a measure of the concentrations
of lead in foodstuffs moving in international trade, the likely role
of those concentrations in dietary intake estimates, and the likely
impact of limiting lead concentrations by establishing maximum limits.
These estimates should be combined with expert judgement to establish
appropriate controls on food. Additional data should be collected to
refine the predictions of concentrations in foods before and after
establishing limits.
3.1 Residue data submitted by national governments
Data were submitted to the Committee from more than 30 countries
representing all seven continents. Most of the data were summarized
and the original data were not submitted. The submission from the
United States Total Diet Study was the only one in which values in
individual samples were reported, each of which represented a
composite of samples taken from three supermarkets. Detailed data on
lead residues were also provided for Australia, Slovakia, and France.
Values for lead residues in some beverages in Finland were obtained
from the literature.
The United States Food and Drug Administration collects samples
of more than 200 foods from supermarkets four times each year and
analyses them for contaminants and nutrients. Lead is one of the
contaminants (Food & Drug Administration, 1993-96).
The Australia-New Zealand Food Authority (1998) has monitored
foods for lead as a part of a market basket survey to estimate
potential intake. In this survey, foods are sampled by categories of
core, national, and regional foods. Each sample represents a composite
of three to four samples, and the number of samples analysed varies
depending upon the food, ranging from as few as six up to 28 samples.
The study is repeated each year. All foods are analysed in the form in
which they would be consumed. Samples are taken from 14 major foods
groups: cereal and cereal products, meat and poultry, seafood, eggs,
fats and oils, dairy products, vegetables, fruit, nuts and seeds,
beverages, snack foods, sugar/confectionery, condiments, and foods for
infants.
Lead concentrations in Slovakia were determined in a 'study in
which duplicate samples of meals were consumed and statistically
representative samples were collected four times a year (Ursínyová &
Hladíková,1998). France provided data from its nationwide survey.
A total diet study was conducted in China, and the results from
the 1990 survey (Chen & Gao, 1993) and from a model diet study (Gao,
1998) were available.
3.2 National exposure estimates
3.2.1 Total intake of lead from food
The GEMS/Food (1992) report provides estimates for adults and
children in more than 25 countries. The mean weekly intakes of lead
based on monitoring in the 1980s ranged from less than 1 µg/kg bw to
more than 60 µg/kg bw. When data were available for both children and
adults, the children's intake of lead was found to be two to three
times the adult intake when expressed on the basis of body weight.
The data in GEMS/Food for 1993 represent residue levels in the
1980s. More recent estimates of the intake of lead are available for
populations in Australia, China, Finland, France, New Zealand,
Slovakia, Sweden, and the United States. In some of these countries,
more than one study was conducted to determine lead intake. For
example, Australia provided estimates of lead intake from their market
basket study (Australia-New Zealand Food Authority, 1998) and from
their 'Diamond' model (Baines, 1999), which permits assessment of
individuals such as high consumers because it includes data from the
1995 National Nutrition Survey and also water consumption. The intakes
ranged from 1.6 to 7 µg/kg bw per week, depending on the method used,
the subgroup evaluated, and the residue data used. The inclusion of
estimates of lead concentrations in water further increased the
estimated exposure in Australia to 5.6-12 µg/kg bw per week.
Australia, New Zealand, France, China, Canada, Slovakia and the
United States provided estimates of lead intake for a variety of age
groups including young children (Table 1).
The Ministry of Agriculture, Fisheries and Food in the United
Kingdom conducts a total diet study each year by collecting foods from
retail outlets in 20 towns and preparing them for consumption before
analysis for contaminants. The results for 1982-91 were available for
this evaluation and show that lead intake declined over that period,
from 0.036-0.69 mg/person per day to 0.015-0.028 mg/person per day.
3.2.2 Contribution of foods and food categories to total lead intake
In a recent study in a non-industrialized area of the United
Kingdom, the concentrations of lead ranged from 0.008 to 0.340 µg/g in
plant materials, the value being greater for grasses > herbs >
vegetables > cereals > fruits (Ward & Savage, 1994). Root vegetables
tended to have higher concentrations than other plant stuffs, at
0.02-0.125 µg/g, carrots, leeks, and onions having the largest
amounts.
Table 1. Weekly intake of lead in population groups in various countries
Country or Group Weekly lead intake Assumptions
region (µg/kg bw)
Australia Adults High consumer intakes
Males 2.6-3.4 95th percentile
Females 2.4-3.3
12-year-olds
Boys 1.6-2.5
Girls 1.7-2.7
2-year-olds 3.1-5.0
Infants (9 months) 2.0-5.1
Total 4.9 without water
6.3 including water
Adults 4.2 without water
5.6 including water
Infants 2.5
2-year-olds 7.0 without water
11.9 including water
Canada Children 1-4 years 5.25 Assumed body weight
20 kg bw
Adults 20-33 years 3.3
70 kg bw
Total population 2.4
70 kg bw
China Adults 10.1 From total diet study
60 kg bw Assumed body weight
Children 24.4
16.5 kg bw
Standard man 9.8 From model diet study
58 kg bw
Children 2-7 years 29.7
16.5 kg bw
Table 1. (cont'd)
Country or Group Weekly lead intake Assumptions
region (µg/kg bw)
Children 8-12 years 24.5
29.4 kg
Males 20-50 years 22.0
63 kg bw
Females 20-50 years 19.9
53 kg bw
Finland 1.4
France Adults 8.3 Assumed body weight
60 kg bw
Children 2-8 years 19.4
20 kg bw
New Zealand Males 19-24 years 3.3
Males > 25 years 3.3
Females > 25 years 2.5
Children 4-6 years 5.3
Children 1-3 years 6.3
Slovakia Children
Vegetarian 9.9-48.6 Median-maximum
Non-vegetarian 6.7-57
Sweden 2-6
Taiwan 2.6
United Total population 3.3
Kingdom
United States Infant 6-11 months 0.6 Assumed body weight
10 kg bw
Children 2 years 1.1
15 kg bw
Children 6 years 1.4
18 kg bw
Children 10 years 1.2
22 kg bw
Females 14-16 years 0.4
60 kg bw
Females 25-30 years 0.4
70 kg bw
Females 40-45 years 0.3
70 kg bw
Table 1. (cont'd)
Country or Group Weekly lead intake Assumptions
region (µg/kg bw)
Females 70 years 0.4
70 kg bw
Males 14-16 years 0.4
70 kg bw
Males 25-45 years 0.4
70 kg bw
Males 70 years 0.5
70 kg bw
The commodities with the highest reported concentrations in the
Australian market basket study were samples of lettuce, dried
tomatoes, and psyllium husks. The lead concentrations in most foods in
the United States Total Diet Study were very low, and no categories
had typically high levels. Residue data for Australia were combined
with food consumption data to estimate the contribution of individual
foods or food categories to total intakes across the population. Most
foods contributed less than 1% to total exposure. With one exception,
only heavily consumed foods such as wheat flour and meat contributed
more than 5% to the total intake. The exception was broccoli which
contributed 22% to the total. The foods that contributed to the
exposure of children and infants were milk (16%), pineapple juice
(9%), apple juice (8%), sugar (8%), bread (8%), and tea (3%) for
schoolchildren and milk (24%), juice (21%), and bread (5%) for
toddlers. In Finland, beverages were reported to account for about
one-fifth of the total lead intake from foods (Tahvonen, 1997).
Cereals and vegetables contributed most of the lead intake in the
Chinese total diet study (Chen & Gao, 1998).
Milk and formula are the major components of the diets of most
infants. For infants in Slovakia, the weekly median and range of lead
intake (µg/kg bw per week) was 4.7 (< 0.5-27) from breast milk, 6
(1-8.5) from infants' formula, and 5.6 (2.7-24) from cow's milk
(Ursínyová & Hladikova, 1997). No lead was detected in breast milk in
Australia (Australia/New Zealand Food Authority, 1998) but a range of
concentrations similar to that found in Slovakia was reported in
infant formula (mean, 9 µg/kg; maximum, 18 µg/kg). Cows' milk was not
tested in Australia. The Food and Drug Administration (1993-96)
reported a maximum of 3 µg/kg in cows' milk and did not detect
residues in infant formula. Breast milk was not analysed. As it is not
known whether the data from the three countries are for similar
products or whether the products were analysed in the same form,
comparisons should be considered to be qualitative. In particular, it
is not known whether any of the products had been preserved in
lead-soldered cans. The GEMS/Food (1992) summary reported mean intakes
of 38 µg/kg bw from a diet that included infant formula in
lead-soldered cans and 8 µg/kg bw when unsoldered cans were used.
3.3 Intake assessments based on the GEMS/Food regional diets and
selected lead concentrations in those foods
In order to obtain a global perspective and to permit regional
comparisons of the potential intake of lead, a series of analyses were
conducted of the current GEMS/Food regional diets with various
assumptions about the concentrations of lead in those foods. Three
scenarios were considered: (1) the maximum limits currently proposed
to CCFAC, (2) 'typical' concentrations based on average concentrations
in monitored foodstuffs (the average residue in foods in the United
States between 1993 and 1996 was chosen as the 'typical'
concentration), and (3) 'high' concentrations based on those measured
during monitoring. The Committee noted that these values are not true
mean or maximum international values but considered them to be
measures of the range of residues encountered in a wide variety of
countries.
3.3.1 Intake estimates based on the proposed CCFAC limits for lead in
selected foodstuffs
3.3.1.1 Methods
Estimates of consumption in the GEMS/Food diets were used, and
the intake of each food group with a proposed Codex limit was derived
as the product of the Codex limit and the consumption level. In some
instances, it was necessary to compute total consumption of a specific
food group. For instance, the regional diets do not provide an
estimate of total fruit juice intake. Therefore the estimate was
derived as the sum of all fruit juices identified with the same code.
The total intake was estimated as the sum of all food-specific
intakes. In all these calculation, an average body weight of 60 kg was
used.
3.3.1.2 Results
The estimated weekly intake of lead in each of the regional diets
derived from the proposed Codex limits is 17 µg/kg bw in the Middle
Eastern diet, 15 µg/kg bw in the Far Eastern diet, 13 µg/kg bw in the
African diet, 13 µg/kg bw in the Latin American diet, and 20 µg/kg bw
in the European diet. The contribution of each food category to the
total intake is presented in Table 2.
3.3.2 Intake estimates based on estimates of the 'typical'
concentrations of lead in selected foods
3.3.2.1 Methods
The procedure described above was repeated but substituting a
'typical' observed lead concentration for the proposed limit. The
'typical' value assumed for each food category was derived from United
States Total Diet Study (Food & Drug Administration, 1993-96), and the
estimated 'typical' intakes were derived from the average detectable
concentrations reported in 12 market basket sample collections of 234
foods. The foods were grouped and values selected to represent the
lead concentrations in the WHO regional diets. Samples that contained
no detectable lead residues were assumed to contain residues at the
concentration of the limit of detection. Body weight was assumed to be
60 kg for adults; some data are expressed as milligrams per day.
3.3.2.2 Results
The estimated weekly intake of lead in five regions of the world,
assuming that foods contain a typical concentration of lead as
determined from monitoring in the United States, is 1 µg/kg bw in the
Middle Eastern, Far Eastern, African, and Latin American diets and 2
µg/kg bw in the European diet. The 'typical' values and estimated
intakes of each category of food are shown in Table 3, which shows
that no category contributes the preponderance of the total intake.
3.3.3 Intake estimates based on estimates of a realistic 'maximum'
concentration of lead in selected foods
3.3.3.1 Methods
The procedure described above was repeated but substituting a
likely maximum observed lead concentration for the proposed limit. The
likely maximum concentration was also derived from Total Diet Study of
the United States Food and Drug Administration. The estimated intakes
were derived from average maximum detectable lead concentrations.
Expert judgement was applied in selecting those values, and they
should not be presumed to represent actual concentrations of lead. The
foods were grouped and values selected to represent the lead
concentrations in the WHO regional diets.
3.3.3.2 Results
The resulting intake estimates for individual foods are presented
in Table 4, which shows that no category contributes the preponderance
of the total intake.
Table 2. Estimated intake of lead based on WHO regional diets and proposed CCFAC maximum limits
Food Maximum Intake (mg/person per day)
limit
(mg/kg) Middle Far African Latin European
Eastern Eastern American
Citrus fruits 0.100 0.005 0.001 0.001 0.005 0.0049
Pome fruits 0.100 0.001 0.001 0.000 0.001 0.0051
Stone fruits 0.100 0.001 0.000 0.000 0.000 0.0023
Bulb vegetables 0.100 0.003 0.001 0.001 0.001 0.0031
Fruiting vegetables, 0.100 0.009 0.001 0.002 0.003 0.0078
non-cucurbits
Fruiting vegetables, 0.100 0.008 0.002 0.000 0.003 0.0038
cucurbits
Total roots and tubers 0.100 0.006 0.011 0.032 0.016 0.024
Brassica vegetables 0.300 0.002 0.003 0.000 0.003 0.012
Leafy vegetables 0.300 0.002 0.003 0.000 0.005 0.015
Total cereals 0.200 0.086 0.090 0.064 0.051 0.045
Total pulses 0.200 0.005 0.004 0.004 0.005 0.0024
Legume vegetables 0.200 0.002 0.000 0.000 0.001 0.0052
Meat of cattle, 0.050 0.002 0.002 0.001 0.002 0.0075
pigs, and sheep
Poultry meat 0.050 0.002 0.001 0.000 0.001 0.0026
Fat, mammalian 0.050 0.000 0.000 0.000 0.000 0.00038
Poultry, fats 0.050 0.000 0.000 0.000 0.000 0.00026
Total vegetable 0.050 0.002 0.001 0.001 0.001 0.0019
oils and fats
Edible offal of 0.500 0.002 0.001 0.001 0.003 0.0062
cattle, pigs, and
sheep
Milk of cattle, 0.020 0.002 0.001 0.001 0.003 0.0059
goats, and sheep
Secondary milk 0.020 0.000 0.000 0.000 0.000 0.00094
products
Fish 0.200 0.002 0.005 0.006 0.008 0.0067
Table 2. (continued)
Food Maximum Intake (mg/person per day)
limit
(mg/kg) Middle Far African Latin European
Eastern Eastern American
Crustaceans, fresh 0.050 0.000 0.000 0.000 0.000 0.00015
and frozen
Molluscs, 1.000 0.000 0.004 0.001 0.001 0.0083
excluding
cephalopod frsh
Fruit juices 0.050 0.001 0.000 0.000 0.000 0.0005
Total (mg/person 0.144 0.131 0.114 0.114 0.173
per day)
Weekly potential 17 15 13 13 20
intake (µg/kg bw,
if all foods contain
proposed maximum
CCFAC limit levels)
Table 3. Estimated intake of lead based on WHO regional diets and average residues from
the United States Total Diet Study
Food category Mean Intake (mg/person per day)
(no. of samples)
Middle Far African Latin European
Eastern Eastern American
Total cereals (32) 0.011 0.005 0.005 0.004 0.003 0.003
Citrus fruits (2) 0.007 0.000 0.000 0.000 0.000 0.000
Pome fruits (4) 0.012 0.000 0.000 0.000 0.000 0.001
Stone fruits (3) 0.016 0.000 0.000 0.000 0.000 0.000
Fruit juices 0.006 0.000 0.000 0.000 0.000 0.000
Milk of cattle, goats, 0.008 0.001 0.000 0.000 0.001
and sheep (6) 0.002
Secondary milk 0.013 0.000 0.000 0.000 0.000
products (11) 0.001
Meat of cattle, pigs, 0.013 0.000 0.000 0.000 0.001
and sheep (14) 0.002
Edible offal of cattle, 0.031 0.000 0.000 0.000 0.000
pigs, and sheep (1) 0.000
Total vegetable oils 0.034 0.001 0.000 0.001 0.001
and fats (1) 0.001
Poultry meat (5) 0.010 0.000 0.000 0.000 0.000 0.001
Bulb vegetables (1) 0.008 0.000 0.000 0.000 0.000 0.000
Brassica vegetables (3) 0.009 0.000 0.000 0.000 0.000 0.000
Fruiting vegetables, 0.013 0.001 0.000 0.000 0.000
cucurbits (7) 0.001
Total pulses (5) 0.008 0.000 0.000 0.000 0.000 0.000
Leafy vegetables (6) 0.011 0.000 0.000 0.000 0.000 0.001
Fruiting vegetables, 0.009 0.001 0.000 0.000 0.000
non-cucurbits (9) 0.001
Legume vegetables (2) 0.008 0.000 0.000 0.000 0.000 0.000
Total roots and 0.010 0.001 0.001 0.003 0.002 0.002
tubers (12)
Crustaceans, fresh and 0.039 0.000 0.000 0.000 0.000
frozen (1) 0.000
Fish (3) 0.011 0.000 0.000 0.000 0.000 0.000
Table 3. (continued)
Food category Mean Intake (mg/person per day)
(no. of samples)
Middle Far African Latin European
Eastern Eastern American
Total (mg/person 0.012 0.009 0.009 0.010 0.017
per day)
Total(mg/kg bw 0.001 0.001 0.001 0.001 0.002
per week)
Assuming 60 kg bw
Samples that contained no detectable lead residues were assumed to contain
residues at the concentration of the limit of detection.
Table 4. Estimated intake of lead based on WHO regional diets and the maximum residue for
the food category from the United States Total Diet Study
Food category Maximum Intake (mg/person per day)
(no. of samples) residue
(mg/kg) Middle Far African Latin European
Eastern Eastern American
Total cereals (32) 0.022 0.009 0.010 0.007 0.006 0.005
Citrus fruits (2) 0.013 0.001 0.000 0.000 0.001 0.001
Pome fruits (4) 0.022 0.000 0.000 0.000 0.000 0.001
Stone fruits (3) 0.034 0.000 0.000 0.000 0.000 0.001
Fruit juices 0.019 0.000 0.000 0.000 0.000 0.000
Milk of cattle, goats, 0.015 0.002 0.000 0.001 0.002 0.004
and sheep (6)
Secondary milk 0.014 0.000 0.000 0.000 0.000 0.001
products (11)
Meat of cattle, pigs, 0.022 0.001 0.001 0.000 0.001 0.003
and sheep (14)
Edible offal of cattle, 0.080 0.000 0.000 0.000 0.000 0.001
pigs, and sheep (1)
Total vegetable oils 0.044 0.002 0.001 0.001 0.001 0.002
and fats (1)
Poultry meat (5) 0.018 0.001 0.000 0.000 0.000 0.001
Bulb vegetables (1) 0.018 0.000 0.000 0.000 0.000 0.001
Brassica vegetables (3) 0.032 0.000 0.000 0.000 0.000 0.001
Fruiting vegetables, 0.037 0.003 0.001 0.000 0.001 0.001
cucurbits (7)
Total pulses (5) 0.019 0.000 0.000 0.000 0.000 0.000
Leafy vegetables (6) 0.027 0.000 0.000 0.000 0.000 0.001
Fruiting vegetables, 0.020 0.002 0.000 0.000 0.001 0.002
non-cucurbits (9)
Legume vegetables (2) 0.020 0.000 0.000 0.000 0.000 0.001
Table 4. (continued)
Food category Maximum Intake (mg/person per day)
(no. of samples) residue
(mg/kg) Middle Far African Latin European
Eastern Eastern American
Total roots and 0.017 0.001 0.002 0.006 0.003 0.004
tubers (12)
Crustaceans, fresh 0.210 0.000 0.000 0.000 0.000 0.001
and frozen (1)
Fish 0.015 0.000 0.000 0.000 0.001 0.001
Total (mg/person 0.022 0.015 0.016 0.019 0.032
per day)
Total (mg/kg bw 0.003 0.002 0.002 0.002 0.004
per week)
3.4 Discussion and conclusions
The WHO regional diets have been used in the GEMS/Food programme
since 1987 to estimate exposure to pesticides and were used by the
Committee at its forty-ninth meeting to estimate regional exposure to
aflatoxins. The diets were used in this analysis to estimate potential
exposure to lead under the assumption that all foods contain the
maximum proposed Codex limits for lead. The analyses were repeated
with information derived from national surveys of concentrations of
lead in foods.
The proposed Codex limits are higher than the vast majority of
the concentrations observed during monitoring. If these concentrations
occurred in all foods all of the time, the intake of lead by consumers
would be higher than those found in national monitoring programmes.
The concentrations have declined over the past decade, and high levels
of contamination are not widespread; however, the available data from
monitoring surveys may not capture the extreme values that occur due
to localized pollution or due to the use of containers sealed with
lead-solder.
On the basis of the analyses conducted with the GEMS/Food
regional diets and either 'typical' or maximum lead concentrations,
consumers would not ingest more than the PTWI of 1500 µg/week.
The Committe also evaluated children's intakes. The GEMS/Food
regional diets do not allow international estimates of children's
intake, but national governments in a wide variety of locations have
estimated the intake of lead by children. In the estimates provided by
Australia, China, France, New Zealand, Slovakia, and the United States
for infants and children, the highest estimated children's intakes
were well below the PTWI in all but one country, those for Slovakia
approaching the maximum limit under certain conditions. Five of the
six countries provided estimates for both children and adults. In all
five countries, the intake of children and infants tended to be
somewhat higher than that of the total population when expressed on
the basis of unit body weight. The Committee estimated that the
intakes of children could be two to three times that of adults when
expressed per unit body weight. The data from the national estimates
provide assurance that children's intakes are also below the PTWI.
3.5 Exposure from non-dietary sources
3.5.1 Migration from food containers
Lead may enter food from food containers or serving vessels
containing lead (WHO, 1995). In particular, elevated concentrations of
lead may result from storage in lead-soldered cans, in ceramic vessels
with a lead glaze, or in leaded crystal. Although the use of lead
solder has largely been discontinued, it was a major source of
exposure in many parts of the world. Lead in ceramic-ware is a less
widespread problem, but the most serious cases that have been
documented have arisen from the consumption by children of fruit juice
stored in ceramic pitchers.
3.5.2 Soil
The concentrations of lead in soil are typically in the range of
5-100 mg/kg (WHO, 1995), whereas lead from anthropogenic sources may
result in concentrations exceeding 10 000 mg/kg. In particular, soil
in or adjacent to lead smelters, lead mines, houses painted with lead
paint, orchards treated with lead arsenate, and urban areas where
there has been heavy automobile traffic is likely to contain high
concentrations of lead.
Ingestion of soil may therefore result in exposure to lead. This
may occur by direct ingestion, deposition of soil on the hands,
contamination of food, or by inhalation. The relationship between soil
lead and blood lead varies greatly by soil type (WHO, 1995), perhaps
due to behavioural differences among individuals and differences in
the rates of absorption of lead from different sources.
3.5.3 Paint
The paint used in older houses may have very high concentrations
of lead, and very high exposure to lead, sufficient to cause severe
clinical symptoms and death, may result from the direct ingestion of
paint chips, particularly by children (WHO, 1995; Agency for Toxic
Substances and Disease Registry, 1997).
3.6 Surveys of blood lead concentrations
The most extensive surveys of blood lead concentrations that have
been carried out in the United States were the National Health and
Nutrition Examination Surveys (Pirkle et al., 1994). In the second
such survey, conducted between 1976 and 1980, a mean blood lead
concentration of 13 µg/dL was found in persons aged 1-74. In the third
survey, conducted in 1988-91, a mean concentration of 2.8 µg/dL was
found. The decrease can be attributed to the drastic reduction in the
use of lead in gasoline and in soldered cans during the intervening
period.
The concentrations of lead are widely distributed and generally
appear to follow a log normal distribution which is skewed towards
higher concentrations. As a result, many people are exposed to high
concentrations of lead even when the mean concentration is low.
Children of urban and low-income families are particularly likely to
be exposed to high concentrations (Brody et al., 1994).
Surveys of lead concentrations in other parts of the world
suggest that exposure was generally lower than that in the United
States before 1980 (WHO, 1995), and the concentrations have been
lowered further as a a result of programmes to reduce exposure, even
though the reductions were generally more modest than those in the
United States.
4. QUANTITATIVE RISK ASSESSMENT
The brief review presented above of current knowledge about the
presence of lead in the environment and its toxicology is largely
qualitative. Because the health effects associated with exposure to
lead differ in degree, a quantitative description is given below, with
a quantitative statement of the associated uncertainty to convey the
limitations of current knowledge.
As risk assessments are designed to assist in making decisions,
the design of an assessment is influenced by the objectives of the
decision. For a number of reasons, a single risk assessment cannot be
used as the basis for all possible decisions. Many adverse health
effects are associated with exposure to lead, all of which are
potential subjects for a risk assessment. Most of the effects are
associated with the levels of exposure found in occupational settings,
whereas the present exercise is meant to assist decisions about
exposure of the general population, who are exposed to lead mainly at
relatively low concentrations in food. The assessment described below
is focused on the effects of lead on the neurobehavioural development
of children. The carcinogenic effects of lead are also considered
briefly.
An assessment of risk for the world population would be too broad
to allow evaluations of the risks of particular segments of the
population, whereas a risk assessment for a small population group
would be inadequate for another, as exposure to lead varies widely
throughout the world. In order to capture the range, risk assessments
have been constructed for several population groups that vary in their
diet and socioeconomic status.
Two factors limit the scope of a risk assessment. The first is
the availability of data to support particular theories or
predictions, usually because the necessary experiments have not been
or cannot be performed. This limits the level of detail of an
assessment, and model uncertainty becomes an important element. A more
vexing problem arises when the raw data exist but are not available
for use in a risk assessment, for example, when they have not been
published. This makes it difficult for other analysts to draw their
own conclusions and to use the data to answer questions other than
those addressed by the investigators. Many of the analyses of the
relationship between exposure to lead and the neurobehavioural
development of children are based on tests of the statistical
significance of an association. In the absence of the raw data, it is
difficult to use such studies to evaluate mathematical models.
A second consideration is that risk assessments require much time
and effort. Formal, detailed risk assessments are generally worth
undertaking only for serious problems. The fact that one of the
Committee's initial quantitative risk assessments addresses exposure
to lead may be attributed to the fact that lead is generally
considered to be the most serious environmental contaminant.
Because of these limitations, the quantitative risk assessment
presented below is relatively simple; however, a number of ways in
which the assessment could be developed further are discussed.
4.1 Exposure assessment
4.1.1 Population estimates of dietary intake of lead
4.1.1.1 Frequently consumed foods
Since lead is a chronic poison, estimates of long-term exposure
are required. As individuals consume many samples of a particular food
over time, their lead intake is proportional to the arithmetic mean of
the distribution of the concentrations of lead in the commodity. In
such circumstances, the lead intake of the population can be estimated
by multiplying the distribution of consumption of the food by the
arithmetic mean of the lead concentration. The net intake from
multiple studies can be obtained by summing the distributions in a
Monte-Carlo simulation.
The number of food categories included varies according to the
quality of the data available. A simple model and simulation are shown
in Table 5. This simulation is based on estimates of mean intakes in
the United States converted to distributions by assuming that they
follow a standard form: a log normal distribution with a geometric
mean equal to 0.76 times the arithmetic mean and a geometric standard
deviation of 0.76. Since they are based on data for the United States,
they do not reflect any geographic difference in lead concentrations.
Simply summing distributions will not, however, account for
correlations in the consumption of particular foods, in that high
consumption of one food may tend to be accompanied by high consumption
of another. Such correlations can be represented by introducing
statistical correlation models to relate the foods to one another or
by closely tying the simulation to data from consumption surveys.
Either approach will require access to raw data on consumption, which
are not usually published.
Table 5. Estimates of population exposure to lead (µg/day) in different regions
Statistic Middle Eastern Far Eastern African Latin American European
0.05 7.1 7.0 7.1 7.1 6.8
0.5 11.4 11.2 11.5 11.5 11.3
0.95 20.5 20.0 20.3 21.1 21.5
0.99 29.1 25.8 28.8 26.9 32.4
4.1.1.2 Infrequently consumed foods
If a dietary source of very high concentrations of lead is
consumed infrequently, it may be important to simulate long-term
intake. An estimate for an individual can be obtained by averaging the
number of samples of the food expected to be consumed by that
individual over a given time. For example, the intake of an individual
who eats the food at four meals per year would be calculated from four
samples.
4.1.2 Simulations of the effect of a commodity with a high
concentration of lead
To portray the impact of a food commodity with unusually high
concentrations of lead, a simulation was run for a situation in which
the concentrations in grain were 10 times higher than usual (Table 6).
A log normal distribution with a geometric mean of 0.25 and a standard
deviation of 0.6 was used to represent exposure from non-dietary
sources. Although the distribution is represented in terms of dietary
intake, it should be considered to be dietary equivalents since
absorption rates from other media may differ. The values could be
altered to reflect national distributions of blood lead
concentrations. Although they are plausible, these are hypothetical
distributions anddo not apply in any particular case. The effect of
intervention was also simulated for a Middle Eastern diet with two
putative levels of intervention: the first reduced exposure by one
half, while the second resulted in 'normal' concentrations (Table 7).
4.1.3 Relation of dietary lead to blood lead
The blood lead concentrations resulting from a given dietary
intake can be estimated from simple empirical models or from more
complex pharmacokinetic models. The former require fewer assumptions
and are simpler to use. They are reasonably accurate as long as the
data on which they are based are derived under conditions that are
comparable to those for which the predictions are made, but they may
be inadequate in several circumstances.
Table 6. Estimates of population exposure (µg/day) to a commodity that
contains high concentrations of lead and to other sources of lead
Statistic Middle Eastern Far Eastern African Latin American European
0.05 34 31 31 35 25
0.5 73 62 58 62 52
0.95 170 144 124 127 142
0.99 257 238 172 170 247
Table 7. Estimates of the effects on population
exposure (µg/day) of reducing exposure in a
Middle Eastern diet to a commodity with unusually
high concentrations of lead
Statistic Intervention
None Reduction Reduction
by one half to 'normal'
0.05 37.2 29.3 20.4
0.5 74.1 56.1 38.3
0.95 176.1 110.6 80.1
0.99 233.3 155.7 112.0
First, significant exposure to lead may occur from other sources,
and the impact of that source on blood lead concentrations must be
estimated separately. It may also be necessary to evaluate the mutual
effect of several sources of lead on blood concentrations, which may
require a pharmacokinetic model, such as that designed by the United
States Environmental Protection Agency.
Second, if the levels of exposure result in blood concentrations
outside the range of 10-25 µg/dL that has usually been measured, the
model used to represent the relationship between dietary intake and
blood lead (linear or nonlinear) become critical and must be evaluated
critically, perhaps using more than one model to represent the source
of uncertainty.
Third, the exposure may not be in a steady state. Most of the
available data refer to relatively constant exposure to lead, usually
due to contamination of drinking-water from lead plumbing. If exposure
to lead is infrequent or occurs at high concentrations for short
periods of time, use of the O'Flaherty pharmacokinetic model
(O'Flaherty, 1991, 1993, 1995) is clearly warranted.
Fourth, other dietary components or atypical physiological states
may alter the rate of absorption of lead from the intestine into the
blood. In particular, iron and calcium can compete for the active
transport of lead into the blood. As a result lead uptake is greater
when other minerals are not present, for instance when water is drunk
on an empty stomach. Calcium deficiency, which may be triggered by
pregnancy, can lead to up-regulation of the calcium transport
mechanism with a concomitant increase in lead uptake.
The present exercise is limited to simple empirical models.
4.1.3.1 Drinking-water and blood lead concentrations in bottle-fed
infants
The best study for evaluating the relationship between dietary
intake of lead and blood lead values in infants is that of Lacey et
al. (1985), in which the concentrations of lead in water and blood
were measured for a population of Scottish infants. The individual
values were published and were thus available for further analysis.
Since the infants in this study who were exposed to low concentrations
in drinking-water still generally had blood lead concentrations > 10
µg/dL, they would appear to have been exposed other than in the diet.
The data were fit into several models, all of which were based on the
assumption of a linear relationship between dietary intake and blood
lead but with different population models (see Figures 1-3 and Table
8). The models all yielded an intercept of roughly 15 µg/dL which may
reflect exposure from dust or air (leaded gasoline) or maternal
exposure before birth. The models also all yielded a slope of roughly
0.05 µg/dL per µg/L in drinking-water, so that the relation to dietary
intake depends on the amount of water consumed by the infants in the
study. As infants typically consume 500-1000 ml of formula per day
(Ershow & Cantor, 1989), the slope attributable to dietary intake
corresponds roughly to 0.05-0.1 µg/dL per µg/day, or a change of 1
µg/dL per 10-20 µg/day in an infant diet, where the range reflects the
uncertainty in the relationship.
The population variation reflected in the models comes from
several sources, including other exposures to lead, the amount of
formula consumed, and the change in blood attributable to dietary
exposure. In the present exercise, only the latter source of variation
represents true population variation, while the other sources
contribute to the uncertainty of the measure.
4.1.3.2 Drinking-water and blood lead concentrations in adults
The report of a study by Sherlock et al. (1982) does not present
data for individuals but does include a table of the numbers of
persons with blood lead concentrations in specified ranges stratified
by the concentrations of lead in drinking-water. These data are given
in Table 9 after conversion from absolute numbers to percentiles and
are plotted in Figure 4.
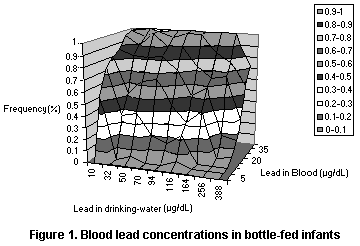
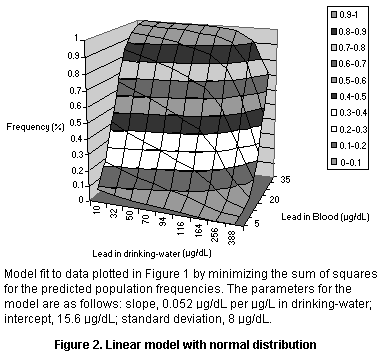
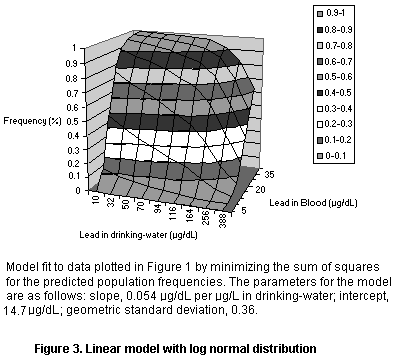
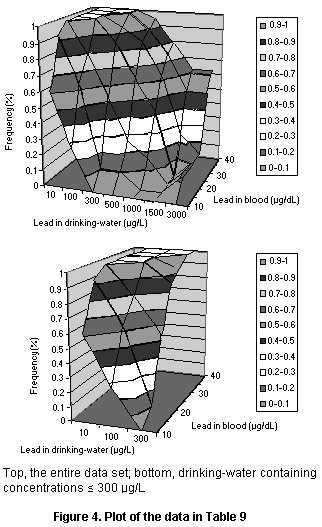 Table 8. Linear models for the relationship between dietary and
blood lead concentrations in bottle-fed infants
Population model Intercept Slope Variance
(µg/dL) (µg/dL per mg/day)
Log normal 14.67 0.054 0.36 (GSD)
Normal 15.59 0.052 8.04 (SD)
Normal, fractional 15.43 0.052 0.37 (FSD)
Parameters for linear models fit to the data of Lacey et al. (1987).
The variance parameters are not directly comparable since they reflect
different assumptions about the shape of the population distribution.
GSD, geometric standard deviation; SD, standard deviation; FSD,
fractional standard deviation (standard deviation as a fraction of
the mean)
Table 9. Relationship between concentrations of lead in drinking-water
and in the blood of adults
Concentration Concentration in drinking-water (µg/L)
in blood
(µg/dL < 10 < 100 < 300 < 500 < 1000 < 1500 > 1500
< 10 0.62 0.26 0.00 0.00 0.00 0.00 0.00
< 15 0.92 0.63 0.11 0.11 0.00 0.00 0.13
< 20 1.00 0.79 0.54 0.26 0.16 0.00 0.13
< 25 1.00 1.00 0.86 0.63 0.42 0.00 0.13
< 30 1.00 1.00 0.93 0.84 0.63 0.25 0.13
< 35 1.00 1.00 1.00 0.89 0.74 0.50 0.50
> 35 1.00 1.00 1.00 0.95 0.79 0.63 0.63
Data from Sherlock et al. (1982)
Cumulative population percentiles from a Scottish village for a
series of blood lead concentrations with varying concentrations
of lead in drinking-water. The percentiles are cumulative with
respect to blood lead, so that each percentile reflects the percentage
of the population in each lead-water grouping with a lower blood
lead concentration.
Several simple three-parameter models were fit to these data
(shown in Figure 5), all of which showed quantitatively similar fits
of the data. Each had consistent deviations from some part of the data
set. The linear model deviated at very high and very low lead
concentrations, whereas the log linear model fit fairly well at low
doses but fit poorly at high doses and predicted higher than observed
blood concentrations at a lead concentration of 100 µg/L. When
concentrations in drinking-water below 300 µg/dL were fit separately,
a linear model with a log normal population distribution produced a
very good fit (see Figure 5). Since this value covers the typical
range of dietary intake, it is suitable for assessing dietary exposure
to lead. The parameters for the model are a slope of 0.035 µg/dL per
µg/L in drinking-water and a geometric standard deviation (reflecting
population variation) of 0.31.
In order to apply this model to general dietary intake, the
amount of tapwater consumed by the individuals in the study must be
estimated. For a range of 0.5-1.5 L/day, the range of slopes
(reflecting uncertainty) from which blood concentrations can be
calculated is 0.023-0.07 µg/dL per µg/day. The slope for conversion
from dietary intake to blood lead concentration is not greatly
different: the conversion factor for infants is only two to four times
greater than that for adults. Blood lead concentrations for a given
level of exposure are therefore not proportional to body weight,
perhaps because of the greater uptake of lead by bone during growth in
children.
4.2 Dose-response assessment
4.2.1 Designing an analysis
Existing analyses of the relationship between exposure to lead
during gestation or childhood and neurobehavioural performance during
childhood have a number of problems, some of which are discussed
below.
First, most of the analyses are of individual studies. Since
there have been many studies with somewhat disparate results, it is
difficult to draw general conclusions. In order to do so, it would be
necessary to obtain the original data from the authors of the studies,
assimilate them into a common format, and design models to accommodate
all the variables included.
Second, the conclusions drawn from large epidemiological studies
with many potential confounding variables often depend on the model
used, and it is difficult to compare studies in which different models
were used. Furthermore, insufficient information may be provided to
select the relevant model. For example, it may be impossible to
ascertain whether an effect is due to lead or to some other variable
whose presence correlates with that of lead. This can be an important
source of uncertainty in a risk assessment.
Table 8. Linear models for the relationship between dietary and
blood lead concentrations in bottle-fed infants
Population model Intercept Slope Variance
(µg/dL) (µg/dL per mg/day)
Log normal 14.67 0.054 0.36 (GSD)
Normal 15.59 0.052 8.04 (SD)
Normal, fractional 15.43 0.052 0.37 (FSD)
Parameters for linear models fit to the data of Lacey et al. (1987).
The variance parameters are not directly comparable since they reflect
different assumptions about the shape of the population distribution.
GSD, geometric standard deviation; SD, standard deviation; FSD,
fractional standard deviation (standard deviation as a fraction of
the mean)
Table 9. Relationship between concentrations of lead in drinking-water
and in the blood of adults
Concentration Concentration in drinking-water (µg/L)
in blood
(µg/dL < 10 < 100 < 300 < 500 < 1000 < 1500 > 1500
< 10 0.62 0.26 0.00 0.00 0.00 0.00 0.00
< 15 0.92 0.63 0.11 0.11 0.00 0.00 0.13
< 20 1.00 0.79 0.54 0.26 0.16 0.00 0.13
< 25 1.00 1.00 0.86 0.63 0.42 0.00 0.13
< 30 1.00 1.00 0.93 0.84 0.63 0.25 0.13
< 35 1.00 1.00 1.00 0.89 0.74 0.50 0.50
> 35 1.00 1.00 1.00 0.95 0.79 0.63 0.63
Data from Sherlock et al. (1982)
Cumulative population percentiles from a Scottish village for a
series of blood lead concentrations with varying concentrations
of lead in drinking-water. The percentiles are cumulative with
respect to blood lead, so that each percentile reflects the percentage
of the population in each lead-water grouping with a lower blood
lead concentration.
Several simple three-parameter models were fit to these data
(shown in Figure 5), all of which showed quantitatively similar fits
of the data. Each had consistent deviations from some part of the data
set. The linear model deviated at very high and very low lead
concentrations, whereas the log linear model fit fairly well at low
doses but fit poorly at high doses and predicted higher than observed
blood concentrations at a lead concentration of 100 µg/L. When
concentrations in drinking-water below 300 µg/dL were fit separately,
a linear model with a log normal population distribution produced a
very good fit (see Figure 5). Since this value covers the typical
range of dietary intake, it is suitable for assessing dietary exposure
to lead. The parameters for the model are a slope of 0.035 µg/dL per
µg/L in drinking-water and a geometric standard deviation (reflecting
population variation) of 0.31.
In order to apply this model to general dietary intake, the
amount of tapwater consumed by the individuals in the study must be
estimated. For a range of 0.5-1.5 L/day, the range of slopes
(reflecting uncertainty) from which blood concentrations can be
calculated is 0.023-0.07 µg/dL per µg/day. The slope for conversion
from dietary intake to blood lead concentration is not greatly
different: the conversion factor for infants is only two to four times
greater than that for adults. Blood lead concentrations for a given
level of exposure are therefore not proportional to body weight,
perhaps because of the greater uptake of lead by bone during growth in
children.
4.2 Dose-response assessment
4.2.1 Designing an analysis
Existing analyses of the relationship between exposure to lead
during gestation or childhood and neurobehavioural performance during
childhood have a number of problems, some of which are discussed
below.
First, most of the analyses are of individual studies. Since
there have been many studies with somewhat disparate results, it is
difficult to draw general conclusions. In order to do so, it would be
necessary to obtain the original data from the authors of the studies,
assimilate them into a common format, and design models to accommodate
all the variables included.
Second, the conclusions drawn from large epidemiological studies
with many potential confounding variables often depend on the model
used, and it is difficult to compare studies in which different models
were used. Furthermore, insufficient information may be provided to
select the relevant model. For example, it may be impossible to
ascertain whether an effect is due to lead or to some other variable
whose presence correlates with that of lead. This can be an important
source of uncertainty in a risk assessment.
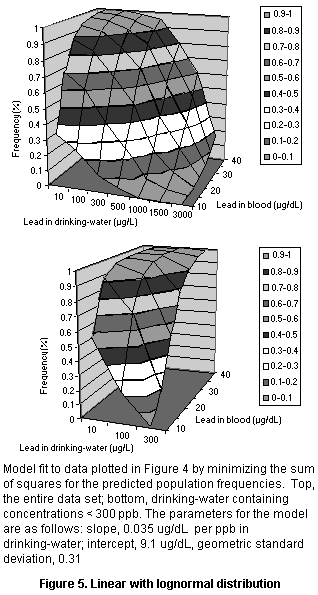 Third, different tests may be used to measure different
end-points. While tests are standardized in terms of normal variation,
they may not be scaled to a common performance standard, and if they
are standardized for different populations at different ages, they may
be standardized in terms of anticipated adult variation. It may be
desirable to recalibrate scales to direct them to particular
performance measures. In particular, measures of cognitive and motor
performance might be preferable to a general measure of IQ.
Fourth, most of the analyses have been performed in order to
establish a relationship between exposure to lead and behavioural
performance rather than to evaluate the quantitative relationship
between exposure to lead and outcome. The analyses are therefore often
based on tests of statistical significance that involve comparisons of
groups with high and low exposure to lead rather than a critical
evaluation of the dose-response relationship.
4.2.2 Dose-response models for blood lead and IQ
Because a comprehensive model is beyond the scope of this
analysis, relatively simple models are used to relate blood lead
concentration to IQ. The analysis with the fewest of the potential
flaws discussed above was conducted by Schwartz (1993). This analysis
relies on the results of Bellinger et al. (1991) and relates blood
lead to IQ as measured by the McCarthy scales (see Figure 6). When 13
confounding variables were modelled, a change of one IQ point was seen
for every 2-4 µg/dL change in blood lead concentration, with a steeper
slope (a greater effect) at higher blood lead concentrations than at
lower ones.
Schwartz (1993) also conducted a meta-analysis of seven studies,
relying on the reported regression coefficients (six positive and one
negative) for the dose-response relationship and t statistics to
estimate the variance. This analysis indicated that an increase in
blood lead concentration from 10 to 20 µg/dL results in a change of
approximately 2.5 IQ points.
The principal shortcoming of this analysis for the purposes of
risk assessment is that there is no statement of the relationship
between dose and response for either population variation or
uncertainty. The same effect may not be seen in all individuals
because of the presence of identified and unidentified confounders.
The analysis also does not indicate the uncertainty in the estimates
that may result from measurement errors, sampling bias, or model
selection. The degree of true variation can be judged to some extent
by comparing the results of different studies, since the variation is
due partly to variation in the magnitude of the effect in the
individuals in each population; however, it is difficult to judge the
extent or shape of the distribution precisely.
In order to represent the uncertainty in the dose-response
relationship, the Committee used three models of the relationship
(linear, hockey stick, and Hill) and two models to represent
population variation (normal and log normal). Probabilities were
assigned to the models in relation to the weight of the evidence for
each; thus, the three-parameter Hill model was given greater weight
than the other two because it provides a better fit of the analysis by
Schwartz (1993) of the effects of low doses of lead (see Figure 6)
and, because it is sigmoidal, it can account for the apparently
greater effect of lead at a blood concentration of 20 µg/dL than at
higher concentrations (National Research Council, 1993). It is also a
biologically relevant model which is suitable for modelling saturable
processes in which the effect is the result of reversible binding of
the agent to the target site. The linear model is given some
preference because it is simple, with only one parameter. The hockey
stick model includes two parameters and provides an intermediate fit.
Further uncertainty is represented by using triangular
distributions for the model parameters, which are characterized by
estimates of the minimum, most likely, and maximum values. These are
presented in Tables 10 and 11. The estimates are based largely on the
fits in the analysis of Schwartz (1993; see Figure 7) but were
adjusted downwards by 40% to be more consistent with the other
literature on lead (e.g. Yule et al., 1981; Schroeder et al., 1985;
Hawk et al., 1986; Lansdown et al., 1986; Fulton et al., 1987; Winneke
et al., 1990). The probability assignments in the model also reflect
the analysis of Schwartz (1994), which suggests that a linear model
may be more appropriate than a sigmoidal model. The behaviour of these
models when assembled into a two-dimensional Monte-Carlo simulation is
illustrated in Table 12.
The populations in studies of the relationship between exposure
to lead and performance on behavioural tests have had relatively
constant exposure. In order to gauge the relative importance of
shorter exposures, an equation was used that is based on the
assumption that the net lifetime effect of lead on behavioural
performance is proportional to the average exposure over the first
5-15 years of life. This reflects the notion that sustained exposure
to lead will have a greater effect on adult IQ than shorter exposure.
When the dose received during a shorter exposure is distributed
relative to a 10-year period, the equation for distributing exposure
for the developmental effects of lead is:
actual dose × dose duration (years)
Effective dose =
period of development (years)
where the period of development is an uncertain range of 5-15 years.
Third, different tests may be used to measure different
end-points. While tests are standardized in terms of normal variation,
they may not be scaled to a common performance standard, and if they
are standardized for different populations at different ages, they may
be standardized in terms of anticipated adult variation. It may be
desirable to recalibrate scales to direct them to particular
performance measures. In particular, measures of cognitive and motor
performance might be preferable to a general measure of IQ.
Fourth, most of the analyses have been performed in order to
establish a relationship between exposure to lead and behavioural
performance rather than to evaluate the quantitative relationship
between exposure to lead and outcome. The analyses are therefore often
based on tests of statistical significance that involve comparisons of
groups with high and low exposure to lead rather than a critical
evaluation of the dose-response relationship.
4.2.2 Dose-response models for blood lead and IQ
Because a comprehensive model is beyond the scope of this
analysis, relatively simple models are used to relate blood lead
concentration to IQ. The analysis with the fewest of the potential
flaws discussed above was conducted by Schwartz (1993). This analysis
relies on the results of Bellinger et al. (1991) and relates blood
lead to IQ as measured by the McCarthy scales (see Figure 6). When 13
confounding variables were modelled, a change of one IQ point was seen
for every 2-4 µg/dL change in blood lead concentration, with a steeper
slope (a greater effect) at higher blood lead concentrations than at
lower ones.
Schwartz (1993) also conducted a meta-analysis of seven studies,
relying on the reported regression coefficients (six positive and one
negative) for the dose-response relationship and t statistics to
estimate the variance. This analysis indicated that an increase in
blood lead concentration from 10 to 20 µg/dL results in a change of
approximately 2.5 IQ points.
The principal shortcoming of this analysis for the purposes of
risk assessment is that there is no statement of the relationship
between dose and response for either population variation or
uncertainty. The same effect may not be seen in all individuals
because of the presence of identified and unidentified confounders.
The analysis also does not indicate the uncertainty in the estimates
that may result from measurement errors, sampling bias, or model
selection. The degree of true variation can be judged to some extent
by comparing the results of different studies, since the variation is
due partly to variation in the magnitude of the effect in the
individuals in each population; however, it is difficult to judge the
extent or shape of the distribution precisely.
In order to represent the uncertainty in the dose-response
relationship, the Committee used three models of the relationship
(linear, hockey stick, and Hill) and two models to represent
population variation (normal and log normal). Probabilities were
assigned to the models in relation to the weight of the evidence for
each; thus, the three-parameter Hill model was given greater weight
than the other two because it provides a better fit of the analysis by
Schwartz (1993) of the effects of low doses of lead (see Figure 6)
and, because it is sigmoidal, it can account for the apparently
greater effect of lead at a blood concentration of 20 µg/dL than at
higher concentrations (National Research Council, 1993). It is also a
biologically relevant model which is suitable for modelling saturable
processes in which the effect is the result of reversible binding of
the agent to the target site. The linear model is given some
preference because it is simple, with only one parameter. The hockey
stick model includes two parameters and provides an intermediate fit.
Further uncertainty is represented by using triangular
distributions for the model parameters, which are characterized by
estimates of the minimum, most likely, and maximum values. These are
presented in Tables 10 and 11. The estimates are based largely on the
fits in the analysis of Schwartz (1993; see Figure 7) but were
adjusted downwards by 40% to be more consistent with the other
literature on lead (e.g. Yule et al., 1981; Schroeder et al., 1985;
Hawk et al., 1986; Lansdown et al., 1986; Fulton et al., 1987; Winneke
et al., 1990). The probability assignments in the model also reflect
the analysis of Schwartz (1994), which suggests that a linear model
may be more appropriate than a sigmoidal model. The behaviour of these
models when assembled into a two-dimensional Monte-Carlo simulation is
illustrated in Table 12.
The populations in studies of the relationship between exposure
to lead and performance on behavioural tests have had relatively
constant exposure. In order to gauge the relative importance of
shorter exposures, an equation was used that is based on the
assumption that the net lifetime effect of lead on behavioural
performance is proportional to the average exposure over the first
5-15 years of life. This reflects the notion that sustained exposure
to lead will have a greater effect on adult IQ than shorter exposure.
When the dose received during a shorter exposure is distributed
relative to a 10-year period, the equation for distributing exposure
for the developmental effects of lead is:
actual dose × dose duration (years)
Effective dose =
period of development (years)
where the period of development is an uncertain range of 5-15 years.
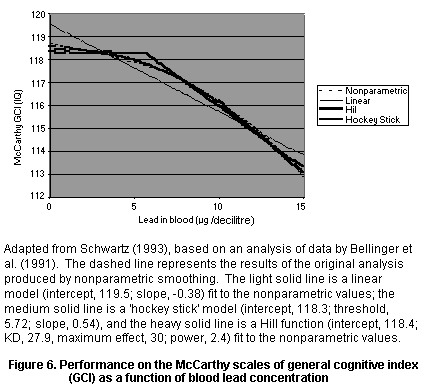
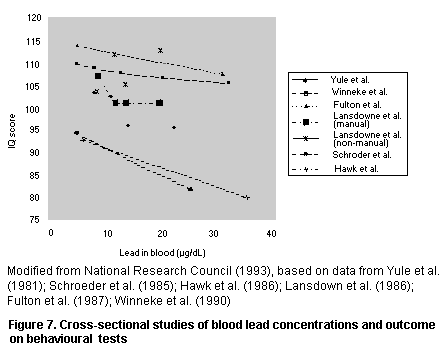 4.2.3 A sample diet-response simulation
The effect of a commodity containing high concentrations of lead
on the IQ of an infant as a result of maternal exposure was simulated
by using the exposure assessment described in Table 6. The functions
described above were used to calculate blood lead concentrations, the
expected change in IQ after lifetime exposure, and the expected effect
on adults after a limited exposure of nine months. These results are
presented in Table 13.
Table 10. Models of the dose-response relationship for exposure
to lead and reduced IQ scores in children
Model Probability Parameter(s) Representation
Linear 0.4 Slope (IQ per µg/dL) Triangular (0, 0.25, 0.4)
Hockey stick 0.2 Slope (IQ per µg/dL) Triangular (0, 0.35, 0.6)
Threshold Triangular (0, 5, 15)
Hill 0.4 KD (µg/dL) Triangular (18,22,33
Maximum Triangular(15,20,40)
Power Uniform (2.0, 2.8)
The median value for the uncertainty distribution is the best fit and is
plotted in Figure 6. The values for the KD (dissociation constant) and
maximum parameters are correlated.
Table 11. Models of the dose-response relationship between
exposure to lead and effects in the population
Model Probability Parameter(s) Representation
Normal 0.4 Fractional Uniform
standard deviation (5-15 % of mean effect)
Log normal 0.6 Geometric Uniform
standard deviation (0.1-0.3)
5. COMMENTS
The most widely used biomarker of exposure to lead is the
concentration in blood (measured in µg/dL). The most critical effect
of lead at low concentrations is reduced cognitive development and
intellectual performance in children. A number of studies in which
various tests of behavioural performance were used have shown an
association between blood lead concentration and reduced intelligence
quotient (IQ) in children exposed pre-and postnatally. At blood
concentrations below 10-15 µg/dL, the effect of confounding variables
and limits to the precision of analytical and psychometric
measurements increase the uncertainty of any estimate of effect. If a
threshold does exist, it is unlikely to be detected because of these
limitations. However, there was some evidence of an association
between cognitive deficits and exposure to concentrations even below
10 µg/dL.
Exposure to lead
Exposure to lead can occur as a result of ingestion in foodstuffs
and water and from other sources, such as air. All three sources make
important contributions. Although the current assessment was limited
to dietary intake, a complete analysis would require addition of
non-food sources of lead to the evaluation.
The Committee reviewed data on lead intake in 25 countries and
conducted several dietary assessments on the assumption that the WHO
GEMS/Food regional diets contain 'typical' levels of lead in the food
categories for which limits have been proposed by the Codex Committee
on Food Additives and Contaminants. The WHO GEMS/Food diets have been
used by the Codex Committee on Pesticide Residues and others to
estimate intakes of pesticides and contaminants since 1987. The
regional diets were used to estimate lead intake under three sets of
assumptions about the concentrations of lead in food: all foods
contain lead (1) at the limits currently proposed by the Codex
Committee on Food Additives and Contaminants, (2) at a typical average
concentration, and (3) at typical high levels found in foods. When the
concentrations at the currently proposed Codex limits were used in the
assessment, the estimated intakes were 13-20 µg/kg bw per week. The
typical average and high levels were derived from monitoring in the
United States and were similar to those reported from other countries.
The weekly intakes ranged from 1 to 2 µg/kg bw per week for 'typical'
lead levels and from 2 to 4 µg/kg bw per week for 'high typical' lead
levels. These narrow ranges in intake estimates reflect the fact that
the monitoring data that were submitted to the Committee did not
include foodstuffs that contained particularly high levels of lead,
and no food group predominated. Virtually no data were submitted on
foods with levels above the currently proposed Codex limit. The Expert
Committee noted that similar intakes were obtained for the five
different GEMS/Food regional diets with these models.
Table 13. Estimated change in IQ after exposure to a commodity
containing high concentrations of lead
Population Intervention
percentile
None Reduction by one half Reduction to 'normal'
0.05 0.008 (0.00, 0.06) 0.005 (0.000, 0.05) 0.003 (0.00, 0.04)
0.5 0.018 (0.00, 0.1) 0.011 (0.00, 0.08) 0.006 (0.00, 0.06)
0.95 0.042 (0.00, 0.16) 0.028 (0.00, 0.13) 0.02 (0.00, 0.10)
0.99 0.051 (0.00, 0.19) 0.034 (0.00, 0.15) 0.02 (0.00, 0.12)
Estimates of the net change in IQ as result of exposure to lead in a commodity
with high concentrations after two levels of intervention. The scenario on which
the estimates are based presumes that exposure occurs as a result of maternal
exposure only.
The Committee reviewed data contained in the WHO GEMS/Food
database and found that foods that were sampled in the 1980s contained
much higher concentrations of lead than those seen presently and
decided to base its conclusions on current data.
Children
The potential intake of lead by children was reported for seven
countries on the basis of monitoring of the general food supply as
well as infant formula and other foods commonly consumed by children.
Several countries specifically provided intake levels of foods
consumed by children. The estimated range of intake levels for
children was 0.6-30 µg/kg bw per week, which was generally two to
three times the adult intake in the same country when evaluated on the
basis of body weight.
Tap water is a significant potential source of lead, particularly
for formula-fed infants; however, the data submitted were inadequate
to permit estimation of the range of levels found in water.
Quantitative risk assessment
Exposure assessment
Several simulation models were developed to estimate the
distributions of dietary lead intake in regional diets. The first
involved a scenario in which the regional populations covered by the
WHO GEMS/Food database consumed food with lead concentrations
corresponding to those found in a survey conducted in the United
States. The second was designed to evaluate the impact of
superimposition of a specific commodity from a source with a much
higher distribution of lead on a background of other regional dietary
and non-dietary exposures. In a third simulation, the impacts of
several putative regulatory interventions on dietary intake were
evaluated.
Estimates of blood lead concentrations from dietary intake levels
In order to predict the biological effects of changes in lead
intake, a relationship must be derived between the concentration of
lead in the diet and changes in the biomarker, the concentration of
lead in blood. To do so, the Committee used simple empirical models to
relate exposure to lead to concentrations in blood and other tissues.
Most of the historical data refer to relatively constant exposure to
lead, usually as a consequence of contamination of drinking-water by
lead plumbing. These data have limited predictive value if the levels
of exposure result in blood lead concentrations higher than 25 mg/dL.
In addition, other dietary components or atypical physiological states
may alter the rate of absorption from the intestine to the blood.
In order to infer a relationship between ingestion of lead and an
increase in the blood lead concentration in infants and young
children, several models were fitted to data from studies of
bottle-fed infants. Reasonable fits required the assumption that a
zero dose in drinking-water corresponds roughly to a blood lead
concentration of 15 µg/dL, perhaps reflecting exposure from the
environment or in utero. The results, attributable to dietary intake
of lead by infants, correspond roughly to a change in blood lead
concentration of 0.05-0.1 µg/dL per µg of lead intake per kg bw per day.
For a 10-kg infant, this corresponds to a range of 0.5-1.0 µg/dL per µg
of lead in the diet per day.
The Committee used data from another study to calculate the
relationship between blood lead concentration and intake of lead from
drinking-water by pregnant women. The sample size was large enough to
allow characterization of population variation. When the raw data were
fitted to several simple models, all resulted in poor fits. When
concentrations in drinking-water below 300 µg/L were fitted
separately, a linear model with a log-normal population distribution
produced a very good fit. A background blood lead concentration of
roughly 9 µg/dL was obtained, probably reflecting exposure from
sources other than drinking-water. The model yielded a blood lead
concentration of 0.035 µg/dL per µg of lead in 1 L of drinking-water.
Because of uncertainty in the amount of water consumed by the
individuals in the study, the variation around this value was wide,
from 0.023 to 0.07 µg/dL per µg of lead intake per kg bw per day.
For a 60-kg person, this corresponds to a range of 1.4-4.2 µg/dL per
µg of lead in drinking-water per day.
Dose-response assessments of neurobehavioural effects of lead in
children
The Committee noted a number of limitations in the available data
on the effects of lead on neurobehavioural development in children.
One problem was that raw data were not available for use in risk
assessments or in evaluating mathematical models of the relationship
between exposure to lead and performance in behavioural tests. Another
problem was the somewhat disparate results obtained. Furthermore, it
was difficult to compare the results of large epidemiological studies
in which many potential confounding variables were analysed in
different models. It is therefore not possible to ascertain whether
any effect is due to lead or to some other variable. A further problem
was that the same tests were not used in all studies, so that
different end-points were measured. (Measures of cognitive and motor
performance might be preferable to a general measure of IQ.) Finally,
most of the analyses were based on tests of statistical significance
between groups with high and low exposure to lead rather than a
critical evaluation of the dose-response relationship.
The analysis that had the fewest of these potential flaws showed
a decrease of one IQ point for every 2-4 µg/dL increase in blood lead
concentration, with a greater effect at higher concentrations than at
lower ones. A meta-analysis of seven studies showed that an increase
in the blood lead concentration from 10 to 20 µg/dL would result in a
decrease of approximately 2.5 IQ points.
This analysis does not include the possibility that the
relationship between blood lead and IQ is nonlinear, in spite of some
evidence that it may be. Furthermore, there is no expression of either
population variation or uncertainty in the dose-response relationship.
In order to address these concerns, probability trees and statistical
distributions were included in the assessment of the dose-response
relationship. Because raw data were not available, the values for the
magnitude of the variation and uncertainty were chosen to be generally
consistent with those available in the literature on the health
effects of lead. As an example of the behaviour of the composite
dose-response function generated, Table 14 gives estimates of the net
decrease in IQ for the population median at four blood lead values,
with a range of uncertainty for each estimate.
Dose-response simulation
A simulation model was developed in which a dose-response
component was added to the exposure model described previously. This
model was used to illustrate the net benefit of imposing limits on the
levels of lead in food with respect to neurobehavioural development of
children, given an anticipated reduction in exposure to lead.
The studies that have related exposure to lead to performance in
behavioural tests have been conducted in populations whose exposure to
lead is relatively constant. In order to gauge the relative importance
of shorter exposures, it was presumed that the net lifetime effect of
lead on intellectual status (i.e. adult IQ) after a limited prenatal
or postnatal exposure may be scaled relative to a period of 5-15
years, the range representing the uncertainty associated with the
adjustment.
The model was based on consumption patterns from a WHO GEMS/Food
Middle Eastern diet, assuming that the lead concentrations in food
were typical of those in the United States, and non-dietary exposures
corresponding roughly to those in the United States. In this
hypothetical scenario, a decrement in IQ of 0.006 points (range,
0-0.06) was estimated for the population mean of children as a result
of maternal exposure for nine months. The procedure described above
was used to adjust for the period of exposure. In a scenario in which
the cereal grains in a regional diet contained 10 times higher lead
levels, the estimated decrement in IQ points attributable to lead was
0.02 (range, 0-0.1). A hypothetical intervention leading to a
reduction in grain lead levels by one-half was estimated to reduce the
decrement in IQ points to 0.011 (range, 0-0.08). Given the similarity
in the estimates of exposure from the different regional diets,
similar results would be anticipated if other WHO GEMS/Food regional
diets were used in the scenario.
Table 14. Net decrease in IQ associated with blood lead
concentration
Concentration of Median IQ decrement
lead in blood (µg/dL) (95% confidence interval)
5 0.4 (0.0-1.5)
10 1.7 (0.5-3.1)
15 3.4 (1.1-5.0)
20 5.5 (1.6-6.9)
The Committee also estimated the effect on blood lead
concentrations of long-term exposure to lead in the three models of
regional diets used to illustrate the ranges of intake of lead under
various assumptions. As a conservative estimate, the Committee assumed
that a dietary intake of 1 µg/kg bw per day (7 µg/kg bw per week)
would result in an increase in the lead concentration of blood of 1
µg/dL, the upper estimate for infants, and that this relationship was
valid during the long-term exposure period (in utero + 10 years). This
would correspond to an increase in blood lead concentration of 0.14
µg/dL per µg lead per kg bw per week. Using this assumption in
combination with the high values for estimated dietary intake, the
Committee calculated that consumption of a diet containing lead at the
currently proposed Codex limits would result in an increase in blood
lead concentration of 3 µg/dL. Consumption of diets with typical
average lead concentrations or with typically high levels would result
in increases in blood lead concentrations of 0.3 and 0.6 µg/dL,
respectively. Table 14, showing the decrements in IQ points predicted
to be associated with various blood lead concentrations, provides
confidence that the current concentrations of lead in foods would have
very little impact on the neurobeha-vioural development of infants and
children. However, the Committee stressed that a full risk assessment
of dietary intake of lead should take other sources of exposures into
account.
6. EVALUATION
The Committee concluded that, overall, current dietary levels of
lead have negligible effects on intellectual development but noted
that foods with high lead contents remain in commerce. The simulation
model presented here can be used to evaluate the effects of any
planned interventions.
7. REFERENCES
Agency for Toxic Substances and Disease Registry (1997)
Toxicological Profile for Lead, Atlanta, GA: Department of Health
and Human Services.
Australia-New Zealand Food Authority (1998) The Australian Market
Basket Survey, Melbourne: Information Australia.
Baghurst, P.A., Robertson, E.F., McMichael, A.J., Vimpani, G.V., Wigg,
N.R. & Roberts, R.R (1987) The Port Pirie cohort study: Lead effects
on pregnancy outcome and early childhood development.
Neurotoxicology, 8, 395-402.
Baghurst, P.A., McMichael, A.L., Wigg, N.R., Vimpani, G.V., Robertson,
E.F., Roberts, R.J. & Tong, S.-L. (1992) Environmental exposure to
lead and children's intelligence at the age of seven years. New
Engl. J. Med., 327, 1279-1284.
Baines, J. (1999) Dietary Exposure Assessment-Lead. Australia and
New Zealand National Assessments. Submitted to JECFA.
Bellinger, D., Sloman, J., Leviton, A., Rabinowitz, M., Needleman,
H.L. & Waternaux, C. (1991) Low-level lead exposure and children's
cognitive function in the preschool years. Pedatrics, 87, 219-227.
Brody, D.J., Pirkle, J.L., Gunter, E.W., Kramer, R.A., Flegal, K.M.,
Matte, T.M. & Paschal, D.C. (1994) Blood lead levels in the US
population. Phase 1 of the National Health and Nutrition Examination
Surveys (NHANES III, 1988 to 1991). J. Am. Med. Assoc., 272,
277-283.
Codex Committee on Food Additives and Contaminants (1999) Report of
the Thirty-first Session of the Codex Committee on Food Additives
and Contaminants, Rome, Food and Agriculture Organization of the
United Nations (document ALINORM 99/12A, Appendix V).
Chen, J. & Gao, J. (1993) The Chinese Total Diet Study in 1990. Part
I. Chemical contaminants. J. AOAC Int., 76, 1193-1205.
Chen, J. & Gao, J. (1998) Personal communication to JECFA.
Cooney, G.H., Bell, A., McBride, W. & Carter, C. (1989)
Neurobehavioral consequences of prenatal low level exposures to lead.
Neurotoxicol. Teratol., 11, 95-104.
Cooper, W.C. (1976) Cancer mortality patterns in the lead industry.
Ann. N>Y> Acad. Sci., 271, 250-259.
Cooper, W.C. (1988) Deaths from chronic renal disease in US battery
and lead production workers. Environ. Health Perspectives, 78,
61-63.
Dietrich, K.N., Krafft, K.M., Bornschein, R.L., Hammond, P.B., Berger,
O., Succop, P.A. & Bier, M. (1987) Low-level fetal lead exposure
effect on neurobehavioral development in early infancy. Pediatrics,
80, 721-730.
Dietrich, K.N., Succop, P.A., Berger, O.G. & Keith, R.W. (1992) Lead
exposure and the central auditory processing abilities and cognitive
development of urban children: The Cincinnati lead study cohort at age
5 years. Neurotoxicol. Teratol., 14, 51-56.
Dietrich, K.N., Berger, O.G., Succop, P.A., Hammond, P.B. &
Bornschein, R.L. (1993) The developmental consequences of low to
moderate prenatal and postnatal lead exposure: Intellectual attainment
in the Cincinnati lead study cohort following school entry.
Neurotoxicol. Teratol., 15, 37-44.
Environmental Protection Agency (1994) Technical Support Document:
Parameters and Equations Used in Integrated Exposure Uptake
Biokinetic Model in Children (v 0.99d) (Publication 9285.7-22,
EPA/540/R-94/040, PB94-963505).
Ershow, A.G. & Cantor, K.P. (1989) Total Water and Tapwater Intake
in the United States: Population based Estimates of Quantitities
and Sources, Bethesda, MD: Federation of American Societies for
Experimental Biology.
Factor-Litvak, P., Graziano, J.H., Kline, J.K., Popovac, D., Mehmeti,
A., Rajovic, L., Neneziv, D.U. & Stein, Z.A. (1991) A prospective
study of birthweight and length of gestation in a population
surrounding a lead smelter in Kosovo. Int. J. Epidemiol., 20,
722-728.
Food and Drug Administration (1993-96) Residue monitoring. J. AOAC
Int., 77-80.
Fulton, M., Thomson, G., Hunter, R., Raab, G., Laxen, D. & Hepburn, W.
(1987) Influence of blood lead on the ability and attainment of
children in Edinburgh. Lancet, ii, 1221-1225.
Gao, J. (1998) Personal communication to JECFA.
GEMS/Food (1992) Assessment of Dietary Intake of Chemical
Contaminants (WHO/HPP/FOX/92.6 UNEP/GEMS/92.F2), Geneva: Joint
UNEP/FAO/WHO Food Contamination Monitoring and Assessment Programme.
Goyer, R.A. (1989) Mechanisms of lead and cadmium nephrotoxicity.
Toxicol. Lett., 46, 153-162.
Goyer, R.A. (1996) Toxic effects of metals. In: Klaassen, C., ed.,
Casarett & Doull's Toxicology: The Basic Science of Poisons, New
York: McGraw-Hill, pp. 691-737.
Graziano, J.H., Popova, D., Factor-Litvak, P., Shrout, P., Kline, J.,
Murphy, M.J., Zhao, Y., Mehmeti, A., Ahmedi, X., Rajovic, B., Zvicer,
Z., Nenezic, D.U., Lolacono, N.J. & Stein, Z. (1990) Determinants of
elevated blood lead during pregnancy in a population surrounding a
lead smelter in Kosovo, Yugoslavia. Environ. Health Perspectives,
89, 95-100.
Hawk, B.A., Schroeder, S.R., Robinson, G., Otto, D., Mushal, P.,
Kleinbaum, D. & Dawson, G.(1986) Relation of lead and social factors
to IQ of low-SES chbildren: A partial replication. Am. J. Mental.
Defic., 91, 178-183.
Lacey, R.F., Moore, M.R. & Richards, W.N. (1985) Lead in water, infant
diet and blood. The Glasgow duplicate diet study. Sci. Total
Environ., 41, 235-247.
Lansdown, R., Yale, W., Urbanowicz, M.-A. & Hunter, J. (1986) The
relationship between blood-lead concentrations, intelligence,
attainment and behaviour in a school population: The second London
study. Int. Arch. Occup. Health, 57, 225-235.
Lewis, R.J., ed. (1992) Sax's Dangerous Properties of Industrial
Chemicals, 8th Ed., New York: Van Nostrand Rheinhold.
National Research Council (1993) Measuring Lead Exposure in Infants,
Children, and Other Sensitive Populations, Washington DC: National
Academy Press.
Needleman, H.L., Gunnoe, C., Leviton, A., Reed, R., Peresie, H.,
Maher, C. & Barrett, P. (1979) Deficits in psychologic and classroom
performance of children with elevated dentine levels. New Engl. J.
Med., 300, 689-695.
Needleman, H.L., Rabinowitz, S., Leviton, A., Linn, S. & Schoenbaum,
S. (1984) The relationahip between prenatal exposure to lead and
congenital anomalies. J. Am. Med. Assoc., 251, 2856-2959.
O'Flaherty, E.J. (1991) Physiologically based models for bone-seeking
elements. III. Lead absorption and disposition in childhood.
Toxicol. Appl. Pharmacol., 111, 332-341.
O'Flaherty, E.J. (1993) Physiologically based models for bone-seeking
elements. IV. Kinetic of lead disposition in humans. Toxicol. Appl.
Pharmacol., 118, 16-29.
O'Flaherty, E.J. (1995) Physiologically based models for bone-seeking
elements. V. Lead absorption and disposition in childhood. Toxicol.
Appl. Pharmacol., 131, 297-308.
Pirkle, J.L., Schwartz, J., Landis, J.R. & Harlan, W.R. (1985) The
relationship between blood lead levels and blood pressure and its
cardiovascular risk implications. Am. J. Epidemiol., 121, 246-258.
Pirkle, J.L., Brody, D.J., Gunter, E.W., Kramer, R.A., Paschal, D.C.,
Flegal, K.M.& Matte, T.M. (1994) The decline in blood lead levels in
the United States. The National Health and Nutrition Examination
Surveys (NHANES). J. Am. Med. Assoc., 272, 284-291.
Rabinowitz, M.R., Wetherill, G.W. & Kopple, J.D. (1976) Kinetic
analysis of lead metabolism in healthy humans. J. Clin. Invest., 58,
260-170.
Schroeder, S.R., Hawk, B., Otto, D.A., Mushak, P. & Hicks, R.E. (1985)
Separating the effects of lead and social factors on IQ. Environ.
Res., 38, 144-154.
Schwartz, J. (1993) Beyond LOEL's, p values, and vote counting:
Methods for looking at the shapes and strengths of associations.
Neurotoxicology, 14, 237-246.
Schwartz, J. (1994) Low-level lead exposure and chuldren's IQ: A
meta-analysis and search for a threshold. Environ. Res., 65, 42-55
Sherlock, J.C., Smart, G., Forbes, G.I., Moore, M.R., Patterson, W.J.,
Richards, W.N. & Wilson, T.S. (1982) Assessment of lead intakes and
dose-response for a population in Ayr exposed to a plumbosolvent water
supply. Hum. Toxicol., 1, 115-122.
Tahvonen, R. (1997) Contents of lead in foods and diet in Finland.
Food Rev. Int., 13, 77-90.
Ursínyová, M. & Hladíková, V. (1997) The intake of selected toxic
elements from milk in infants. Fresenius Environ. Bull., 6, 627-632.
Ursínyová, M. & Hladíková, V. (1998) Dietary intake of cadmium, lead
and mercury in vegetarian and non-vegetarian children. Fresenius
Environ. Bull., 7, 585-592.
Ward, N.I. & Savage, J.M. (1994) Metal dispersion and transportational
activities using food crops as biomonitors. Sci. Total Environ.,
146-147, 309-319.
Wasserman, G., Graziano, J.H., Factor-Litvak, P., Popovac, D., Morina,
N., Musabegovic, A., Vrenezi, N., Capuni-Paracka, A., Lekic, V.,
Preteni-Redjepi, E., Hadzialjevic, S., Slavkovich, V., Kline, J.,
Shrout, P. & Stein, Z. (1992) Independent effects of lead exposure and
iron deficiency anemia on developmental outcome at age 2 years. J.
Pediatr., 121, 695-703.
WHO (1995) Enviromental Health Criteria 165: Inorganic Lead, Geneva:
International Programme on Chemical Safety.
Winneke, G., Brockhaus, A., Ewers, U., Krämer, U. & Neuf, M. (1990)
Results from the European multicenter study on lead neurotoxicity in
children: Implications for risk assessment. Neurotoxicol. Teratol.,
12, 553-559.
Yule, W., Lansdown, R., Millar, I.B. & Urbanowicz, M.-A. (1981) The
relationship between blood lead concentrations, intelligence, and
attainment in a school population: A pilot study. Dev. Med. Child.
Neurol., 23, 567-576.
4.2.3 A sample diet-response simulation
The effect of a commodity containing high concentrations of lead
on the IQ of an infant as a result of maternal exposure was simulated
by using the exposure assessment described in Table 6. The functions
described above were used to calculate blood lead concentrations, the
expected change in IQ after lifetime exposure, and the expected effect
on adults after a limited exposure of nine months. These results are
presented in Table 13.
Table 10. Models of the dose-response relationship for exposure
to lead and reduced IQ scores in children
Model Probability Parameter(s) Representation
Linear 0.4 Slope (IQ per µg/dL) Triangular (0, 0.25, 0.4)
Hockey stick 0.2 Slope (IQ per µg/dL) Triangular (0, 0.35, 0.6)
Threshold Triangular (0, 5, 15)
Hill 0.4 KD (µg/dL) Triangular (18,22,33
Maximum Triangular(15,20,40)
Power Uniform (2.0, 2.8)
The median value for the uncertainty distribution is the best fit and is
plotted in Figure 6. The values for the KD (dissociation constant) and
maximum parameters are correlated.
Table 11. Models of the dose-response relationship between
exposure to lead and effects in the population
Model Probability Parameter(s) Representation
Normal 0.4 Fractional Uniform
standard deviation (5-15 % of mean effect)
Log normal 0.6 Geometric Uniform
standard deviation (0.1-0.3)
5. COMMENTS
The most widely used biomarker of exposure to lead is the
concentration in blood (measured in µg/dL). The most critical effect
of lead at low concentrations is reduced cognitive development and
intellectual performance in children. A number of studies in which
various tests of behavioural performance were used have shown an
association between blood lead concentration and reduced intelligence
quotient (IQ) in children exposed pre-and postnatally. At blood
concentrations below 10-15 µg/dL, the effect of confounding variables
and limits to the precision of analytical and psychometric
measurements increase the uncertainty of any estimate of effect. If a
threshold does exist, it is unlikely to be detected because of these
limitations. However, there was some evidence of an association
between cognitive deficits and exposure to concentrations even below
10 µg/dL.
Exposure to lead
Exposure to lead can occur as a result of ingestion in foodstuffs
and water and from other sources, such as air. All three sources make
important contributions. Although the current assessment was limited
to dietary intake, a complete analysis would require addition of
non-food sources of lead to the evaluation.
The Committee reviewed data on lead intake in 25 countries and
conducted several dietary assessments on the assumption that the WHO
GEMS/Food regional diets contain 'typical' levels of lead in the food
categories for which limits have been proposed by the Codex Committee
on Food Additives and Contaminants. The WHO GEMS/Food diets have been
used by the Codex Committee on Pesticide Residues and others to
estimate intakes of pesticides and contaminants since 1987. The
regional diets were used to estimate lead intake under three sets of
assumptions about the concentrations of lead in food: all foods
contain lead (1) at the limits currently proposed by the Codex
Committee on Food Additives and Contaminants, (2) at a typical average
concentration, and (3) at typical high levels found in foods. When the
concentrations at the currently proposed Codex limits were used in the
assessment, the estimated intakes were 13-20 µg/kg bw per week. The
typical average and high levels were derived from monitoring in the
United States and were similar to those reported from other countries.
The weekly intakes ranged from 1 to 2 µg/kg bw per week for 'typical'
lead levels and from 2 to 4 µg/kg bw per week for 'high typical' lead
levels. These narrow ranges in intake estimates reflect the fact that
the monitoring data that were submitted to the Committee did not
include foodstuffs that contained particularly high levels of lead,
and no food group predominated. Virtually no data were submitted on
foods with levels above the currently proposed Codex limit. The Expert
Committee noted that similar intakes were obtained for the five
different GEMS/Food regional diets with these models.
Table 13. Estimated change in IQ after exposure to a commodity
containing high concentrations of lead
Population Intervention
percentile
None Reduction by one half Reduction to 'normal'
0.05 0.008 (0.00, 0.06) 0.005 (0.000, 0.05) 0.003 (0.00, 0.04)
0.5 0.018 (0.00, 0.1) 0.011 (0.00, 0.08) 0.006 (0.00, 0.06)
0.95 0.042 (0.00, 0.16) 0.028 (0.00, 0.13) 0.02 (0.00, 0.10)
0.99 0.051 (0.00, 0.19) 0.034 (0.00, 0.15) 0.02 (0.00, 0.12)
Estimates of the net change in IQ as result of exposure to lead in a commodity
with high concentrations after two levels of intervention. The scenario on which
the estimates are based presumes that exposure occurs as a result of maternal
exposure only.
The Committee reviewed data contained in the WHO GEMS/Food
database and found that foods that were sampled in the 1980s contained
much higher concentrations of lead than those seen presently and
decided to base its conclusions on current data.
Children
The potential intake of lead by children was reported for seven
countries on the basis of monitoring of the general food supply as
well as infant formula and other foods commonly consumed by children.
Several countries specifically provided intake levels of foods
consumed by children. The estimated range of intake levels for
children was 0.6-30 µg/kg bw per week, which was generally two to
three times the adult intake in the same country when evaluated on the
basis of body weight.
Tap water is a significant potential source of lead, particularly
for formula-fed infants; however, the data submitted were inadequate
to permit estimation of the range of levels found in water.
Quantitative risk assessment
Exposure assessment
Several simulation models were developed to estimate the
distributions of dietary lead intake in regional diets. The first
involved a scenario in which the regional populations covered by the
WHO GEMS/Food database consumed food with lead concentrations
corresponding to those found in a survey conducted in the United
States. The second was designed to evaluate the impact of
superimposition of a specific commodity from a source with a much
higher distribution of lead on a background of other regional dietary
and non-dietary exposures. In a third simulation, the impacts of
several putative regulatory interventions on dietary intake were
evaluated.
Estimates of blood lead concentrations from dietary intake levels
In order to predict the biological effects of changes in lead
intake, a relationship must be derived between the concentration of
lead in the diet and changes in the biomarker, the concentration of
lead in blood. To do so, the Committee used simple empirical models to
relate exposure to lead to concentrations in blood and other tissues.
Most of the historical data refer to relatively constant exposure to
lead, usually as a consequence of contamination of drinking-water by
lead plumbing. These data have limited predictive value if the levels
of exposure result in blood lead concentrations higher than 25 mg/dL.
In addition, other dietary components or atypical physiological states
may alter the rate of absorption from the intestine to the blood.
In order to infer a relationship between ingestion of lead and an
increase in the blood lead concentration in infants and young
children, several models were fitted to data from studies of
bottle-fed infants. Reasonable fits required the assumption that a
zero dose in drinking-water corresponds roughly to a blood lead
concentration of 15 µg/dL, perhaps reflecting exposure from the
environment or in utero. The results, attributable to dietary intake
of lead by infants, correspond roughly to a change in blood lead
concentration of 0.05-0.1 µg/dL per µg of lead intake per kg bw per day.
For a 10-kg infant, this corresponds to a range of 0.5-1.0 µg/dL per µg
of lead in the diet per day.
The Committee used data from another study to calculate the
relationship between blood lead concentration and intake of lead from
drinking-water by pregnant women. The sample size was large enough to
allow characterization of population variation. When the raw data were
fitted to several simple models, all resulted in poor fits. When
concentrations in drinking-water below 300 µg/L were fitted
separately, a linear model with a log-normal population distribution
produced a very good fit. A background blood lead concentration of
roughly 9 µg/dL was obtained, probably reflecting exposure from
sources other than drinking-water. The model yielded a blood lead
concentration of 0.035 µg/dL per µg of lead in 1 L of drinking-water.
Because of uncertainty in the amount of water consumed by the
individuals in the study, the variation around this value was wide,
from 0.023 to 0.07 µg/dL per µg of lead intake per kg bw per day.
For a 60-kg person, this corresponds to a range of 1.4-4.2 µg/dL per
µg of lead in drinking-water per day.
Dose-response assessments of neurobehavioural effects of lead in
children
The Committee noted a number of limitations in the available data
on the effects of lead on neurobehavioural development in children.
One problem was that raw data were not available for use in risk
assessments or in evaluating mathematical models of the relationship
between exposure to lead and performance in behavioural tests. Another
problem was the somewhat disparate results obtained. Furthermore, it
was difficult to compare the results of large epidemiological studies
in which many potential confounding variables were analysed in
different models. It is therefore not possible to ascertain whether
any effect is due to lead or to some other variable. A further problem
was that the same tests were not used in all studies, so that
different end-points were measured. (Measures of cognitive and motor
performance might be preferable to a general measure of IQ.) Finally,
most of the analyses were based on tests of statistical significance
between groups with high and low exposure to lead rather than a
critical evaluation of the dose-response relationship.
The analysis that had the fewest of these potential flaws showed
a decrease of one IQ point for every 2-4 µg/dL increase in blood lead
concentration, with a greater effect at higher concentrations than at
lower ones. A meta-analysis of seven studies showed that an increase
in the blood lead concentration from 10 to 20 µg/dL would result in a
decrease of approximately 2.5 IQ points.
This analysis does not include the possibility that the
relationship between blood lead and IQ is nonlinear, in spite of some
evidence that it may be. Furthermore, there is no expression of either
population variation or uncertainty in the dose-response relationship.
In order to address these concerns, probability trees and statistical
distributions were included in the assessment of the dose-response
relationship. Because raw data were not available, the values for the
magnitude of the variation and uncertainty were chosen to be generally
consistent with those available in the literature on the health
effects of lead. As an example of the behaviour of the composite
dose-response function generated, Table 14 gives estimates of the net
decrease in IQ for the population median at four blood lead values,
with a range of uncertainty for each estimate.
Dose-response simulation
A simulation model was developed in which a dose-response
component was added to the exposure model described previously. This
model was used to illustrate the net benefit of imposing limits on the
levels of lead in food with respect to neurobehavioural development of
children, given an anticipated reduction in exposure to lead.
The studies that have related exposure to lead to performance in
behavioural tests have been conducted in populations whose exposure to
lead is relatively constant. In order to gauge the relative importance
of shorter exposures, it was presumed that the net lifetime effect of
lead on intellectual status (i.e. adult IQ) after a limited prenatal
or postnatal exposure may be scaled relative to a period of 5-15
years, the range representing the uncertainty associated with the
adjustment.
The model was based on consumption patterns from a WHO GEMS/Food
Middle Eastern diet, assuming that the lead concentrations in food
were typical of those in the United States, and non-dietary exposures
corresponding roughly to those in the United States. In this
hypothetical scenario, a decrement in IQ of 0.006 points (range,
0-0.06) was estimated for the population mean of children as a result
of maternal exposure for nine months. The procedure described above
was used to adjust for the period of exposure. In a scenario in which
the cereal grains in a regional diet contained 10 times higher lead
levels, the estimated decrement in IQ points attributable to lead was
0.02 (range, 0-0.1). A hypothetical intervention leading to a
reduction in grain lead levels by one-half was estimated to reduce the
decrement in IQ points to 0.011 (range, 0-0.08). Given the similarity
in the estimates of exposure from the different regional diets,
similar results would be anticipated if other WHO GEMS/Food regional
diets were used in the scenario.
Table 14. Net decrease in IQ associated with blood lead
concentration
Concentration of Median IQ decrement
lead in blood (µg/dL) (95% confidence interval)
5 0.4 (0.0-1.5)
10 1.7 (0.5-3.1)
15 3.4 (1.1-5.0)
20 5.5 (1.6-6.9)
The Committee also estimated the effect on blood lead
concentrations of long-term exposure to lead in the three models of
regional diets used to illustrate the ranges of intake of lead under
various assumptions. As a conservative estimate, the Committee assumed
that a dietary intake of 1 µg/kg bw per day (7 µg/kg bw per week)
would result in an increase in the lead concentration of blood of 1
µg/dL, the upper estimate for infants, and that this relationship was
valid during the long-term exposure period (in utero + 10 years). This
would correspond to an increase in blood lead concentration of 0.14
µg/dL per µg lead per kg bw per week. Using this assumption in
combination with the high values for estimated dietary intake, the
Committee calculated that consumption of a diet containing lead at the
currently proposed Codex limits would result in an increase in blood
lead concentration of 3 µg/dL. Consumption of diets with typical
average lead concentrations or with typically high levels would result
in increases in blood lead concentrations of 0.3 and 0.6 µg/dL,
respectively. Table 14, showing the decrements in IQ points predicted
to be associated with various blood lead concentrations, provides
confidence that the current concentrations of lead in foods would have
very little impact on the neurobeha-vioural development of infants and
children. However, the Committee stressed that a full risk assessment
of dietary intake of lead should take other sources of exposures into
account.
6. EVALUATION
The Committee concluded that, overall, current dietary levels of
lead have negligible effects on intellectual development but noted
that foods with high lead contents remain in commerce. The simulation
model presented here can be used to evaluate the effects of any
planned interventions.
7. REFERENCES
Agency for Toxic Substances and Disease Registry (1997)
Toxicological Profile for Lead, Atlanta, GA: Department of Health
and Human Services.
Australia-New Zealand Food Authority (1998) The Australian Market
Basket Survey, Melbourne: Information Australia.
Baghurst, P.A., Robertson, E.F., McMichael, A.J., Vimpani, G.V., Wigg,
N.R. & Roberts, R.R (1987) The Port Pirie cohort study: Lead effects
on pregnancy outcome and early childhood development.
Neurotoxicology, 8, 395-402.
Baghurst, P.A., McMichael, A.L., Wigg, N.R., Vimpani, G.V., Robertson,
E.F., Roberts, R.J. & Tong, S.-L. (1992) Environmental exposure to
lead and children's intelligence at the age of seven years. New
Engl. J. Med., 327, 1279-1284.
Baines, J. (1999) Dietary Exposure Assessment-Lead. Australia and
New Zealand National Assessments. Submitted to JECFA.
Bellinger, D., Sloman, J., Leviton, A., Rabinowitz, M., Needleman,
H.L. & Waternaux, C. (1991) Low-level lead exposure and children's
cognitive function in the preschool years. Pedatrics, 87, 219-227.
Brody, D.J., Pirkle, J.L., Gunter, E.W., Kramer, R.A., Flegal, K.M.,
Matte, T.M. & Paschal, D.C. (1994) Blood lead levels in the US
population. Phase 1 of the National Health and Nutrition Examination
Surveys (NHANES III, 1988 to 1991). J. Am. Med. Assoc., 272,
277-283.
Codex Committee on Food Additives and Contaminants (1999) Report of
the Thirty-first Session of the Codex Committee on Food Additives
and Contaminants, Rome, Food and Agriculture Organization of the
United Nations (document ALINORM 99/12A, Appendix V).
Chen, J. & Gao, J. (1993) The Chinese Total Diet Study in 1990. Part
I. Chemical contaminants. J. AOAC Int., 76, 1193-1205.
Chen, J. & Gao, J. (1998) Personal communication to JECFA.
Cooney, G.H., Bell, A., McBride, W. & Carter, C. (1989)
Neurobehavioral consequences of prenatal low level exposures to lead.
Neurotoxicol. Teratol., 11, 95-104.
Cooper, W.C. (1976) Cancer mortality patterns in the lead industry.
Ann. N>Y> Acad. Sci., 271, 250-259.
Cooper, W.C. (1988) Deaths from chronic renal disease in US battery
and lead production workers. Environ. Health Perspectives, 78,
61-63.
Dietrich, K.N., Krafft, K.M., Bornschein, R.L., Hammond, P.B., Berger,
O., Succop, P.A. & Bier, M. (1987) Low-level fetal lead exposure
effect on neurobehavioral development in early infancy. Pediatrics,
80, 721-730.
Dietrich, K.N., Succop, P.A., Berger, O.G. & Keith, R.W. (1992) Lead
exposure and the central auditory processing abilities and cognitive
development of urban children: The Cincinnati lead study cohort at age
5 years. Neurotoxicol. Teratol., 14, 51-56.
Dietrich, K.N., Berger, O.G., Succop, P.A., Hammond, P.B. &
Bornschein, R.L. (1993) The developmental consequences of low to
moderate prenatal and postnatal lead exposure: Intellectual attainment
in the Cincinnati lead study cohort following school entry.
Neurotoxicol. Teratol., 15, 37-44.
Environmental Protection Agency (1994) Technical Support Document:
Parameters and Equations Used in Integrated Exposure Uptake
Biokinetic Model in Children (v 0.99d) (Publication 9285.7-22,
EPA/540/R-94/040, PB94-963505).
Ershow, A.G. & Cantor, K.P. (1989) Total Water and Tapwater Intake
in the United States: Population based Estimates of Quantitities
and Sources, Bethesda, MD: Federation of American Societies for
Experimental Biology.
Factor-Litvak, P., Graziano, J.H., Kline, J.K., Popovac, D., Mehmeti,
A., Rajovic, L., Neneziv, D.U. & Stein, Z.A. (1991) A prospective
study of birthweight and length of gestation in a population
surrounding a lead smelter in Kosovo. Int. J. Epidemiol., 20,
722-728.
Food and Drug Administration (1993-96) Residue monitoring. J. AOAC
Int., 77-80.
Fulton, M., Thomson, G., Hunter, R., Raab, G., Laxen, D. & Hepburn, W.
(1987) Influence of blood lead on the ability and attainment of
children in Edinburgh. Lancet, ii, 1221-1225.
Gao, J. (1998) Personal communication to JECFA.
GEMS/Food (1992) Assessment of Dietary Intake of Chemical
Contaminants (WHO/HPP/FOX/92.6 UNEP/GEMS/92.F2), Geneva: Joint
UNEP/FAO/WHO Food Contamination Monitoring and Assessment Programme.
Goyer, R.A. (1989) Mechanisms of lead and cadmium nephrotoxicity.
Toxicol. Lett., 46, 153-162.
Goyer, R.A. (1996) Toxic effects of metals. In: Klaassen, C., ed.,
Casarett & Doull's Toxicology: The Basic Science of Poisons, New
York: McGraw-Hill, pp. 691-737.
Graziano, J.H., Popova, D., Factor-Litvak, P., Shrout, P., Kline, J.,
Murphy, M.J., Zhao, Y., Mehmeti, A., Ahmedi, X., Rajovic, B., Zvicer,
Z., Nenezic, D.U., Lolacono, N.J. & Stein, Z. (1990) Determinants of
elevated blood lead during pregnancy in a population surrounding a
lead smelter in Kosovo, Yugoslavia. Environ. Health Perspectives,
89, 95-100.
Hawk, B.A., Schroeder, S.R., Robinson, G., Otto, D., Mushal, P.,
Kleinbaum, D. & Dawson, G.(1986) Relation of lead and social factors
to IQ of low-SES chbildren: A partial replication. Am. J. Mental.
Defic., 91, 178-183.
Lacey, R.F., Moore, M.R. & Richards, W.N. (1985) Lead in water, infant
diet and blood. The Glasgow duplicate diet study. Sci. Total
Environ., 41, 235-247.
Lansdown, R., Yale, W., Urbanowicz, M.-A. & Hunter, J. (1986) The
relationship between blood-lead concentrations, intelligence,
attainment and behaviour in a school population: The second London
study. Int. Arch. Occup. Health, 57, 225-235.
Lewis, R.J., ed. (1992) Sax's Dangerous Properties of Industrial
Chemicals, 8th Ed., New York: Van Nostrand Rheinhold.
National Research Council (1993) Measuring Lead Exposure in Infants,
Children, and Other Sensitive Populations, Washington DC: National
Academy Press.
Needleman, H.L., Gunnoe, C., Leviton, A., Reed, R., Peresie, H.,
Maher, C. & Barrett, P. (1979) Deficits in psychologic and classroom
performance of children with elevated dentine levels. New Engl. J.
Med., 300, 689-695.
Needleman, H.L., Rabinowitz, S., Leviton, A., Linn, S. & Schoenbaum,
S. (1984) The relationahip between prenatal exposure to lead and
congenital anomalies. J. Am. Med. Assoc., 251, 2856-2959.
O'Flaherty, E.J. (1991) Physiologically based models for bone-seeking
elements. III. Lead absorption and disposition in childhood.
Toxicol. Appl. Pharmacol., 111, 332-341.
O'Flaherty, E.J. (1993) Physiologically based models for bone-seeking
elements. IV. Kinetic of lead disposition in humans. Toxicol. Appl.
Pharmacol., 118, 16-29.
O'Flaherty, E.J. (1995) Physiologically based models for bone-seeking
elements. V. Lead absorption and disposition in childhood. Toxicol.
Appl. Pharmacol., 131, 297-308.
Pirkle, J.L., Schwartz, J., Landis, J.R. & Harlan, W.R. (1985) The
relationship between blood lead levels and blood pressure and its
cardiovascular risk implications. Am. J. Epidemiol., 121, 246-258.
Pirkle, J.L., Brody, D.J., Gunter, E.W., Kramer, R.A., Paschal, D.C.,
Flegal, K.M.& Matte, T.M. (1994) The decline in blood lead levels in
the United States. The National Health and Nutrition Examination
Surveys (NHANES). J. Am. Med. Assoc., 272, 284-291.
Rabinowitz, M.R., Wetherill, G.W. & Kopple, J.D. (1976) Kinetic
analysis of lead metabolism in healthy humans. J. Clin. Invest., 58,
260-170.
Schroeder, S.R., Hawk, B., Otto, D.A., Mushak, P. & Hicks, R.E. (1985)
Separating the effects of lead and social factors on IQ. Environ.
Res., 38, 144-154.
Schwartz, J. (1993) Beyond LOEL's, p values, and vote counting:
Methods for looking at the shapes and strengths of associations.
Neurotoxicology, 14, 237-246.
Schwartz, J. (1994) Low-level lead exposure and chuldren's IQ: A
meta-analysis and search for a threshold. Environ. Res., 65, 42-55
Sherlock, J.C., Smart, G., Forbes, G.I., Moore, M.R., Patterson, W.J.,
Richards, W.N. & Wilson, T.S. (1982) Assessment of lead intakes and
dose-response for a population in Ayr exposed to a plumbosolvent water
supply. Hum. Toxicol., 1, 115-122.
Tahvonen, R. (1997) Contents of lead in foods and diet in Finland.
Food Rev. Int., 13, 77-90.
Ursínyová, M. & Hladíková, V. (1997) The intake of selected toxic
elements from milk in infants. Fresenius Environ. Bull., 6, 627-632.
Ursínyová, M. & Hladíková, V. (1998) Dietary intake of cadmium, lead
and mercury in vegetarian and non-vegetarian children. Fresenius
Environ. Bull., 7, 585-592.
Ward, N.I. & Savage, J.M. (1994) Metal dispersion and transportational
activities using food crops as biomonitors. Sci. Total Environ.,
146-147, 309-319.
Wasserman, G., Graziano, J.H., Factor-Litvak, P., Popovac, D., Morina,
N., Musabegovic, A., Vrenezi, N., Capuni-Paracka, A., Lekic, V.,
Preteni-Redjepi, E., Hadzialjevic, S., Slavkovich, V., Kline, J.,
Shrout, P. & Stein, Z. (1992) Independent effects of lead exposure and
iron deficiency anemia on developmental outcome at age 2 years. J.
Pediatr., 121, 695-703.
WHO (1995) Enviromental Health Criteria 165: Inorganic Lead, Geneva:
International Programme on Chemical Safety.
Winneke, G., Brockhaus, A., Ewers, U., Krämer, U. & Neuf, M. (1990)
Results from the European multicenter study on lead neurotoxicity in
children: Implications for risk assessment. Neurotoxicol. Teratol.,
12, 553-559.
Yule, W., Lansdown, R., Millar, I.B. & Urbanowicz, M.-A. (1981) The
relationship between blood lead concentrations, intelligence, and
attainment in a school population: A pilot study. Dev. Med. Child.
Neurol., 23, 567-576.
See Also:
Toxicological Abbreviations
Lead (EHC 3, 1977)
Lead (ICSC)
Lead (WHO Food Additives Series 4)
Lead (WHO Food Additives Series 13)
Lead (WHO Food Additives Series 21)
LEAD (JECFA Evaluation)
Lead (UKPID)



 Table 8. Linear models for the relationship between dietary and
blood lead concentrations in bottle-fed infants
Population model Intercept Slope Variance
(µg/dL) (µg/dL per mg/day)
Log normal 14.67 0.054 0.36 (GSD)
Normal 15.59 0.052 8.04 (SD)
Normal, fractional 15.43 0.052 0.37 (FSD)
Parameters for linear models fit to the data of Lacey et al. (1987).
The variance parameters are not directly comparable since they reflect
different assumptions about the shape of the population distribution.
GSD, geometric standard deviation; SD, standard deviation; FSD,
fractional standard deviation (standard deviation as a fraction of
the mean)
Table 9. Relationship between concentrations of lead in drinking-water
and in the blood of adults
Concentration Concentration in drinking-water (µg/L)
in blood
(µg/dL < 10 < 100 < 300 < 500 < 1000 < 1500 > 1500
< 10 0.62 0.26 0.00 0.00 0.00 0.00 0.00
< 15 0.92 0.63 0.11 0.11 0.00 0.00 0.13
< 20 1.00 0.79 0.54 0.26 0.16 0.00 0.13
< 25 1.00 1.00 0.86 0.63 0.42 0.00 0.13
< 30 1.00 1.00 0.93 0.84 0.63 0.25 0.13
< 35 1.00 1.00 1.00 0.89 0.74 0.50 0.50
> 35 1.00 1.00 1.00 0.95 0.79 0.63 0.63
Data from Sherlock et al. (1982)
Cumulative population percentiles from a Scottish village for a
series of blood lead concentrations with varying concentrations
of lead in drinking-water. The percentiles are cumulative with
respect to blood lead, so that each percentile reflects the percentage
of the population in each lead-water grouping with a lower blood
lead concentration.
Several simple three-parameter models were fit to these data
(shown in Figure 5), all of which showed quantitatively similar fits
of the data. Each had consistent deviations from some part of the data
set. The linear model deviated at very high and very low lead
concentrations, whereas the log linear model fit fairly well at low
doses but fit poorly at high doses and predicted higher than observed
blood concentrations at a lead concentration of 100 µg/L. When
concentrations in drinking-water below 300 µg/dL were fit separately,
a linear model with a log normal population distribution produced a
very good fit (see Figure 5). Since this value covers the typical
range of dietary intake, it is suitable for assessing dietary exposure
to lead. The parameters for the model are a slope of 0.035 µg/dL per
µg/L in drinking-water and a geometric standard deviation (reflecting
population variation) of 0.31.
In order to apply this model to general dietary intake, the
amount of tapwater consumed by the individuals in the study must be
estimated. For a range of 0.5-1.5 L/day, the range of slopes
(reflecting uncertainty) from which blood concentrations can be
calculated is 0.023-0.07 µg/dL per µg/day. The slope for conversion
from dietary intake to blood lead concentration is not greatly
different: the conversion factor for infants is only two to four times
greater than that for adults. Blood lead concentrations for a given
level of exposure are therefore not proportional to body weight,
perhaps because of the greater uptake of lead by bone during growth in
children.
4.2 Dose-response assessment
4.2.1 Designing an analysis
Existing analyses of the relationship between exposure to lead
during gestation or childhood and neurobehavioural performance during
childhood have a number of problems, some of which are discussed
below.
First, most of the analyses are of individual studies. Since
there have been many studies with somewhat disparate results, it is
difficult to draw general conclusions. In order to do so, it would be
necessary to obtain the original data from the authors of the studies,
assimilate them into a common format, and design models to accommodate
all the variables included.
Second, the conclusions drawn from large epidemiological studies
with many potential confounding variables often depend on the model
used, and it is difficult to compare studies in which different models
were used. Furthermore, insufficient information may be provided to
select the relevant model. For example, it may be impossible to
ascertain whether an effect is due to lead or to some other variable
whose presence correlates with that of lead. This can be an important
source of uncertainty in a risk assessment.
Table 8. Linear models for the relationship between dietary and
blood lead concentrations in bottle-fed infants
Population model Intercept Slope Variance
(µg/dL) (µg/dL per mg/day)
Log normal 14.67 0.054 0.36 (GSD)
Normal 15.59 0.052 8.04 (SD)
Normal, fractional 15.43 0.052 0.37 (FSD)
Parameters for linear models fit to the data of Lacey et al. (1987).
The variance parameters are not directly comparable since they reflect
different assumptions about the shape of the population distribution.
GSD, geometric standard deviation; SD, standard deviation; FSD,
fractional standard deviation (standard deviation as a fraction of
the mean)
Table 9. Relationship between concentrations of lead in drinking-water
and in the blood of adults
Concentration Concentration in drinking-water (µg/L)
in blood
(µg/dL < 10 < 100 < 300 < 500 < 1000 < 1500 > 1500
< 10 0.62 0.26 0.00 0.00 0.00 0.00 0.00
< 15 0.92 0.63 0.11 0.11 0.00 0.00 0.13
< 20 1.00 0.79 0.54 0.26 0.16 0.00 0.13
< 25 1.00 1.00 0.86 0.63 0.42 0.00 0.13
< 30 1.00 1.00 0.93 0.84 0.63 0.25 0.13
< 35 1.00 1.00 1.00 0.89 0.74 0.50 0.50
> 35 1.00 1.00 1.00 0.95 0.79 0.63 0.63
Data from Sherlock et al. (1982)
Cumulative population percentiles from a Scottish village for a
series of blood lead concentrations with varying concentrations
of lead in drinking-water. The percentiles are cumulative with
respect to blood lead, so that each percentile reflects the percentage
of the population in each lead-water grouping with a lower blood
lead concentration.
Several simple three-parameter models were fit to these data
(shown in Figure 5), all of which showed quantitatively similar fits
of the data. Each had consistent deviations from some part of the data
set. The linear model deviated at very high and very low lead
concentrations, whereas the log linear model fit fairly well at low
doses but fit poorly at high doses and predicted higher than observed
blood concentrations at a lead concentration of 100 µg/L. When
concentrations in drinking-water below 300 µg/dL were fit separately,
a linear model with a log normal population distribution produced a
very good fit (see Figure 5). Since this value covers the typical
range of dietary intake, it is suitable for assessing dietary exposure
to lead. The parameters for the model are a slope of 0.035 µg/dL per
µg/L in drinking-water and a geometric standard deviation (reflecting
population variation) of 0.31.
In order to apply this model to general dietary intake, the
amount of tapwater consumed by the individuals in the study must be
estimated. For a range of 0.5-1.5 L/day, the range of slopes
(reflecting uncertainty) from which blood concentrations can be
calculated is 0.023-0.07 µg/dL per µg/day. The slope for conversion
from dietary intake to blood lead concentration is not greatly
different: the conversion factor for infants is only two to four times
greater than that for adults. Blood lead concentrations for a given
level of exposure are therefore not proportional to body weight,
perhaps because of the greater uptake of lead by bone during growth in
children.
4.2 Dose-response assessment
4.2.1 Designing an analysis
Existing analyses of the relationship between exposure to lead
during gestation or childhood and neurobehavioural performance during
childhood have a number of problems, some of which are discussed
below.
First, most of the analyses are of individual studies. Since
there have been many studies with somewhat disparate results, it is
difficult to draw general conclusions. In order to do so, it would be
necessary to obtain the original data from the authors of the studies,
assimilate them into a common format, and design models to accommodate
all the variables included.
Second, the conclusions drawn from large epidemiological studies
with many potential confounding variables often depend on the model
used, and it is difficult to compare studies in which different models
were used. Furthermore, insufficient information may be provided to
select the relevant model. For example, it may be impossible to
ascertain whether an effect is due to lead or to some other variable
whose presence correlates with that of lead. This can be an important
source of uncertainty in a risk assessment.
 Third, different tests may be used to measure different
end-points. While tests are standardized in terms of normal variation,
they may not be scaled to a common performance standard, and if they
are standardized for different populations at different ages, they may
be standardized in terms of anticipated adult variation. It may be
desirable to recalibrate scales to direct them to particular
performance measures. In particular, measures of cognitive and motor
performance might be preferable to a general measure of IQ.
Fourth, most of the analyses have been performed in order to
establish a relationship between exposure to lead and behavioural
performance rather than to evaluate the quantitative relationship
between exposure to lead and outcome. The analyses are therefore often
based on tests of statistical significance that involve comparisons of
groups with high and low exposure to lead rather than a critical
evaluation of the dose-response relationship.
4.2.2 Dose-response models for blood lead and IQ
Because a comprehensive model is beyond the scope of this
analysis, relatively simple models are used to relate blood lead
concentration to IQ. The analysis with the fewest of the potential
flaws discussed above was conducted by Schwartz (1993). This analysis
relies on the results of Bellinger et al. (1991) and relates blood
lead to IQ as measured by the McCarthy scales (see Figure 6). When 13
confounding variables were modelled, a change of one IQ point was seen
for every 2-4 µg/dL change in blood lead concentration, with a steeper
slope (a greater effect) at higher blood lead concentrations than at
lower ones.
Schwartz (1993) also conducted a meta-analysis of seven studies,
relying on the reported regression coefficients (six positive and one
negative) for the dose-response relationship and t statistics to
estimate the variance. This analysis indicated that an increase in
blood lead concentration from 10 to 20 µg/dL results in a change of
approximately 2.5 IQ points.
The principal shortcoming of this analysis for the purposes of
risk assessment is that there is no statement of the relationship
between dose and response for either population variation or
uncertainty. The same effect may not be seen in all individuals
because of the presence of identified and unidentified confounders.
The analysis also does not indicate the uncertainty in the estimates
that may result from measurement errors, sampling bias, or model
selection. The degree of true variation can be judged to some extent
by comparing the results of different studies, since the variation is
due partly to variation in the magnitude of the effect in the
individuals in each population; however, it is difficult to judge the
extent or shape of the distribution precisely.
In order to represent the uncertainty in the dose-response
relationship, the Committee used three models of the relationship
(linear, hockey stick, and Hill) and two models to represent
population variation (normal and log normal). Probabilities were
assigned to the models in relation to the weight of the evidence for
each; thus, the three-parameter Hill model was given greater weight
than the other two because it provides a better fit of the analysis by
Schwartz (1993) of the effects of low doses of lead (see Figure 6)
and, because it is sigmoidal, it can account for the apparently
greater effect of lead at a blood concentration of 20 µg/dL than at
higher concentrations (National Research Council, 1993). It is also a
biologically relevant model which is suitable for modelling saturable
processes in which the effect is the result of reversible binding of
the agent to the target site. The linear model is given some
preference because it is simple, with only one parameter. The hockey
stick model includes two parameters and provides an intermediate fit.
Further uncertainty is represented by using triangular
distributions for the model parameters, which are characterized by
estimates of the minimum, most likely, and maximum values. These are
presented in Tables 10 and 11. The estimates are based largely on the
fits in the analysis of Schwartz (1993; see Figure 7) but were
adjusted downwards by 40% to be more consistent with the other
literature on lead (e.g. Yule et al., 1981; Schroeder et al., 1985;
Hawk et al., 1986; Lansdown et al., 1986; Fulton et al., 1987; Winneke
et al., 1990). The probability assignments in the model also reflect
the analysis of Schwartz (1994), which suggests that a linear model
may be more appropriate than a sigmoidal model. The behaviour of these
models when assembled into a two-dimensional Monte-Carlo simulation is
illustrated in Table 12.
The populations in studies of the relationship between exposure
to lead and performance on behavioural tests have had relatively
constant exposure. In order to gauge the relative importance of
shorter exposures, an equation was used that is based on the
assumption that the net lifetime effect of lead on behavioural
performance is proportional to the average exposure over the first
5-15 years of life. This reflects the notion that sustained exposure
to lead will have a greater effect on adult IQ than shorter exposure.
When the dose received during a shorter exposure is distributed
relative to a 10-year period, the equation for distributing exposure
for the developmental effects of lead is:
actual dose × dose duration (years)
Effective dose =
period of development (years)
where the period of development is an uncertain range of 5-15 years.
Third, different tests may be used to measure different
end-points. While tests are standardized in terms of normal variation,
they may not be scaled to a common performance standard, and if they
are standardized for different populations at different ages, they may
be standardized in terms of anticipated adult variation. It may be
desirable to recalibrate scales to direct them to particular
performance measures. In particular, measures of cognitive and motor
performance might be preferable to a general measure of IQ.
Fourth, most of the analyses have been performed in order to
establish a relationship between exposure to lead and behavioural
performance rather than to evaluate the quantitative relationship
between exposure to lead and outcome. The analyses are therefore often
based on tests of statistical significance that involve comparisons of
groups with high and low exposure to lead rather than a critical
evaluation of the dose-response relationship.
4.2.2 Dose-response models for blood lead and IQ
Because a comprehensive model is beyond the scope of this
analysis, relatively simple models are used to relate blood lead
concentration to IQ. The analysis with the fewest of the potential
flaws discussed above was conducted by Schwartz (1993). This analysis
relies on the results of Bellinger et al. (1991) and relates blood
lead to IQ as measured by the McCarthy scales (see Figure 6). When 13
confounding variables were modelled, a change of one IQ point was seen
for every 2-4 µg/dL change in blood lead concentration, with a steeper
slope (a greater effect) at higher blood lead concentrations than at
lower ones.
Schwartz (1993) also conducted a meta-analysis of seven studies,
relying on the reported regression coefficients (six positive and one
negative) for the dose-response relationship and t statistics to
estimate the variance. This analysis indicated that an increase in
blood lead concentration from 10 to 20 µg/dL results in a change of
approximately 2.5 IQ points.
The principal shortcoming of this analysis for the purposes of
risk assessment is that there is no statement of the relationship
between dose and response for either population variation or
uncertainty. The same effect may not be seen in all individuals
because of the presence of identified and unidentified confounders.
The analysis also does not indicate the uncertainty in the estimates
that may result from measurement errors, sampling bias, or model
selection. The degree of true variation can be judged to some extent
by comparing the results of different studies, since the variation is
due partly to variation in the magnitude of the effect in the
individuals in each population; however, it is difficult to judge the
extent or shape of the distribution precisely.
In order to represent the uncertainty in the dose-response
relationship, the Committee used three models of the relationship
(linear, hockey stick, and Hill) and two models to represent
population variation (normal and log normal). Probabilities were
assigned to the models in relation to the weight of the evidence for
each; thus, the three-parameter Hill model was given greater weight
than the other two because it provides a better fit of the analysis by
Schwartz (1993) of the effects of low doses of lead (see Figure 6)
and, because it is sigmoidal, it can account for the apparently
greater effect of lead at a blood concentration of 20 µg/dL than at
higher concentrations (National Research Council, 1993). It is also a
biologically relevant model which is suitable for modelling saturable
processes in which the effect is the result of reversible binding of
the agent to the target site. The linear model is given some
preference because it is simple, with only one parameter. The hockey
stick model includes two parameters and provides an intermediate fit.
Further uncertainty is represented by using triangular
distributions for the model parameters, which are characterized by
estimates of the minimum, most likely, and maximum values. These are
presented in Tables 10 and 11. The estimates are based largely on the
fits in the analysis of Schwartz (1993; see Figure 7) but were
adjusted downwards by 40% to be more consistent with the other
literature on lead (e.g. Yule et al., 1981; Schroeder et al., 1985;
Hawk et al., 1986; Lansdown et al., 1986; Fulton et al., 1987; Winneke
et al., 1990). The probability assignments in the model also reflect
the analysis of Schwartz (1994), which suggests that a linear model
may be more appropriate than a sigmoidal model. The behaviour of these
models when assembled into a two-dimensional Monte-Carlo simulation is
illustrated in Table 12.
The populations in studies of the relationship between exposure
to lead and performance on behavioural tests have had relatively
constant exposure. In order to gauge the relative importance of
shorter exposures, an equation was used that is based on the
assumption that the net lifetime effect of lead on behavioural
performance is proportional to the average exposure over the first
5-15 years of life. This reflects the notion that sustained exposure
to lead will have a greater effect on adult IQ than shorter exposure.
When the dose received during a shorter exposure is distributed
relative to a 10-year period, the equation for distributing exposure
for the developmental effects of lead is:
actual dose × dose duration (years)
Effective dose =
period of development (years)
where the period of development is an uncertain range of 5-15 years.

 4.2.3 A sample diet-response simulation
The effect of a commodity containing high concentrations of lead
on the IQ of an infant as a result of maternal exposure was simulated
by using the exposure assessment described in Table 6. The functions
described above were used to calculate blood lead concentrations, the
expected change in IQ after lifetime exposure, and the expected effect
on adults after a limited exposure of nine months. These results are
presented in Table 13.
Table 10. Models of the dose-response relationship for exposure
to lead and reduced IQ scores in children
Model Probability Parameter(s) Representation
Linear 0.4 Slope (IQ per µg/dL) Triangular (0, 0.25, 0.4)
Hockey stick 0.2 Slope (IQ per µg/dL) Triangular (0, 0.35, 0.6)
Threshold Triangular (0, 5, 15)
Hill 0.4 KD (µg/dL) Triangular (18,22,33
Maximum Triangular(15,20,40)
Power Uniform (2.0, 2.8)
The median value for the uncertainty distribution is the best fit and is
plotted in Figure 6. The values for the KD (dissociation constant) and
maximum parameters are correlated.
Table 11. Models of the dose-response relationship between
exposure to lead and effects in the population
Model Probability Parameter(s) Representation
Normal 0.4 Fractional Uniform
standard deviation (5-15 % of mean effect)
Log normal 0.6 Geometric Uniform
standard deviation (0.1-0.3)
5. COMMENTS
The most widely used biomarker of exposure to lead is the
concentration in blood (measured in µg/dL). The most critical effect
of lead at low concentrations is reduced cognitive development and
intellectual performance in children. A number of studies in which
various tests of behavioural performance were used have shown an
association between blood lead concentration and reduced intelligence
quotient (IQ) in children exposed pre-and postnatally. At blood
concentrations below 10-15 µg/dL, the effect of confounding variables
and limits to the precision of analytical and psychometric
measurements increase the uncertainty of any estimate of effect. If a
threshold does exist, it is unlikely to be detected because of these
limitations. However, there was some evidence of an association
between cognitive deficits and exposure to concentrations even below
10 µg/dL.
Exposure to lead
Exposure to lead can occur as a result of ingestion in foodstuffs
and water and from other sources, such as air. All three sources make
important contributions. Although the current assessment was limited
to dietary intake, a complete analysis would require addition of
non-food sources of lead to the evaluation.
The Committee reviewed data on lead intake in 25 countries and
conducted several dietary assessments on the assumption that the WHO
GEMS/Food regional diets contain 'typical' levels of lead in the food
categories for which limits have been proposed by the Codex Committee
on Food Additives and Contaminants. The WHO GEMS/Food diets have been
used by the Codex Committee on Pesticide Residues and others to
estimate intakes of pesticides and contaminants since 1987. The
regional diets were used to estimate lead intake under three sets of
assumptions about the concentrations of lead in food: all foods
contain lead (1) at the limits currently proposed by the Codex
Committee on Food Additives and Contaminants, (2) at a typical average
concentration, and (3) at typical high levels found in foods. When the
concentrations at the currently proposed Codex limits were used in the
assessment, the estimated intakes were 13-20 µg/kg bw per week. The
typical average and high levels were derived from monitoring in the
United States and were similar to those reported from other countries.
The weekly intakes ranged from 1 to 2 µg/kg bw per week for 'typical'
lead levels and from 2 to 4 µg/kg bw per week for 'high typical' lead
levels. These narrow ranges in intake estimates reflect the fact that
the monitoring data that were submitted to the Committee did not
include foodstuffs that contained particularly high levels of lead,
and no food group predominated. Virtually no data were submitted on
foods with levels above the currently proposed Codex limit. The Expert
Committee noted that similar intakes were obtained for the five
different GEMS/Food regional diets with these models.
Table 13. Estimated change in IQ after exposure to a commodity
containing high concentrations of lead
Population Intervention
percentile
None Reduction by one half Reduction to 'normal'
0.05 0.008 (0.00, 0.06) 0.005 (0.000, 0.05) 0.003 (0.00, 0.04)
0.5 0.018 (0.00, 0.1) 0.011 (0.00, 0.08) 0.006 (0.00, 0.06)
0.95 0.042 (0.00, 0.16) 0.028 (0.00, 0.13) 0.02 (0.00, 0.10)
0.99 0.051 (0.00, 0.19) 0.034 (0.00, 0.15) 0.02 (0.00, 0.12)
Estimates of the net change in IQ as result of exposure to lead in a commodity
with high concentrations after two levels of intervention. The scenario on which
the estimates are based presumes that exposure occurs as a result of maternal
exposure only.
The Committee reviewed data contained in the WHO GEMS/Food
database and found that foods that were sampled in the 1980s contained
much higher concentrations of lead than those seen presently and
decided to base its conclusions on current data.
Children
The potential intake of lead by children was reported for seven
countries on the basis of monitoring of the general food supply as
well as infant formula and other foods commonly consumed by children.
Several countries specifically provided intake levels of foods
consumed by children. The estimated range of intake levels for
children was 0.6-30 µg/kg bw per week, which was generally two to
three times the adult intake in the same country when evaluated on the
basis of body weight.
Tap water is a significant potential source of lead, particularly
for formula-fed infants; however, the data submitted were inadequate
to permit estimation of the range of levels found in water.
Quantitative risk assessment
Exposure assessment
Several simulation models were developed to estimate the
distributions of dietary lead intake in regional diets. The first
involved a scenario in which the regional populations covered by the
WHO GEMS/Food database consumed food with lead concentrations
corresponding to those found in a survey conducted in the United
States. The second was designed to evaluate the impact of
superimposition of a specific commodity from a source with a much
higher distribution of lead on a background of other regional dietary
and non-dietary exposures. In a third simulation, the impacts of
several putative regulatory interventions on dietary intake were
evaluated.
Estimates of blood lead concentrations from dietary intake levels
In order to predict the biological effects of changes in lead
intake, a relationship must be derived between the concentration of
lead in the diet and changes in the biomarker, the concentration of
lead in blood. To do so, the Committee used simple empirical models to
relate exposure to lead to concentrations in blood and other tissues.
Most of the historical data refer to relatively constant exposure to
lead, usually as a consequence of contamination of drinking-water by
lead plumbing. These data have limited predictive value if the levels
of exposure result in blood lead concentrations higher than 25 mg/dL.
In addition, other dietary components or atypical physiological states
may alter the rate of absorption from the intestine to the blood.
In order to infer a relationship between ingestion of lead and an
increase in the blood lead concentration in infants and young
children, several models were fitted to data from studies of
bottle-fed infants. Reasonable fits required the assumption that a
zero dose in drinking-water corresponds roughly to a blood lead
concentration of 15 µg/dL, perhaps reflecting exposure from the
environment or in utero. The results, attributable to dietary intake
of lead by infants, correspond roughly to a change in blood lead
concentration of 0.05-0.1 µg/dL per µg of lead intake per kg bw per day.
For a 10-kg infant, this corresponds to a range of 0.5-1.0 µg/dL per µg
of lead in the diet per day.
The Committee used data from another study to calculate the
relationship between blood lead concentration and intake of lead from
drinking-water by pregnant women. The sample size was large enough to
allow characterization of population variation. When the raw data were
fitted to several simple models, all resulted in poor fits. When
concentrations in drinking-water below 300 µg/L were fitted
separately, a linear model with a log-normal population distribution
produced a very good fit. A background blood lead concentration of
roughly 9 µg/dL was obtained, probably reflecting exposure from
sources other than drinking-water. The model yielded a blood lead
concentration of 0.035 µg/dL per µg of lead in 1 L of drinking-water.
Because of uncertainty in the amount of water consumed by the
individuals in the study, the variation around this value was wide,
from 0.023 to 0.07 µg/dL per µg of lead intake per kg bw per day.
For a 60-kg person, this corresponds to a range of 1.4-4.2 µg/dL per
µg of lead in drinking-water per day.
Dose-response assessments of neurobehavioural effects of lead in
children
The Committee noted a number of limitations in the available data
on the effects of lead on neurobehavioural development in children.
One problem was that raw data were not available for use in risk
assessments or in evaluating mathematical models of the relationship
between exposure to lead and performance in behavioural tests. Another
problem was the somewhat disparate results obtained. Furthermore, it
was difficult to compare the results of large epidemiological studies
in which many potential confounding variables were analysed in
different models. It is therefore not possible to ascertain whether
any effect is due to lead or to some other variable. A further problem
was that the same tests were not used in all studies, so that
different end-points were measured. (Measures of cognitive and motor
performance might be preferable to a general measure of IQ.) Finally,
most of the analyses were based on tests of statistical significance
between groups with high and low exposure to lead rather than a
critical evaluation of the dose-response relationship.
The analysis that had the fewest of these potential flaws showed
a decrease of one IQ point for every 2-4 µg/dL increase in blood lead
concentration, with a greater effect at higher concentrations than at
lower ones. A meta-analysis of seven studies showed that an increase
in the blood lead concentration from 10 to 20 µg/dL would result in a
decrease of approximately 2.5 IQ points.
This analysis does not include the possibility that the
relationship between blood lead and IQ is nonlinear, in spite of some
evidence that it may be. Furthermore, there is no expression of either
population variation or uncertainty in the dose-response relationship.
In order to address these concerns, probability trees and statistical
distributions were included in the assessment of the dose-response
relationship. Because raw data were not available, the values for the
magnitude of the variation and uncertainty were chosen to be generally
consistent with those available in the literature on the health
effects of lead. As an example of the behaviour of the composite
dose-response function generated, Table 14 gives estimates of the net
decrease in IQ for the population median at four blood lead values,
with a range of uncertainty for each estimate.
Dose-response simulation
A simulation model was developed in which a dose-response
component was added to the exposure model described previously. This
model was used to illustrate the net benefit of imposing limits on the
levels of lead in food with respect to neurobehavioural development of
children, given an anticipated reduction in exposure to lead.
The studies that have related exposure to lead to performance in
behavioural tests have been conducted in populations whose exposure to
lead is relatively constant. In order to gauge the relative importance
of shorter exposures, it was presumed that the net lifetime effect of
lead on intellectual status (i.e. adult IQ) after a limited prenatal
or postnatal exposure may be scaled relative to a period of 5-15
years, the range representing the uncertainty associated with the
adjustment.
The model was based on consumption patterns from a WHO GEMS/Food
Middle Eastern diet, assuming that the lead concentrations in food
were typical of those in the United States, and non-dietary exposures
corresponding roughly to those in the United States. In this
hypothetical scenario, a decrement in IQ of 0.006 points (range,
0-0.06) was estimated for the population mean of children as a result
of maternal exposure for nine months. The procedure described above
was used to adjust for the period of exposure. In a scenario in which
the cereal grains in a regional diet contained 10 times higher lead
levels, the estimated decrement in IQ points attributable to lead was
0.02 (range, 0-0.1). A hypothetical intervention leading to a
reduction in grain lead levels by one-half was estimated to reduce the
decrement in IQ points to 0.011 (range, 0-0.08). Given the similarity
in the estimates of exposure from the different regional diets,
similar results would be anticipated if other WHO GEMS/Food regional
diets were used in the scenario.
Table 14. Net decrease in IQ associated with blood lead
concentration
Concentration of Median IQ decrement
lead in blood (µg/dL) (95% confidence interval)
5 0.4 (0.0-1.5)
10 1.7 (0.5-3.1)
15 3.4 (1.1-5.0)
20 5.5 (1.6-6.9)
The Committee also estimated the effect on blood lead
concentrations of long-term exposure to lead in the three models of
regional diets used to illustrate the ranges of intake of lead under
various assumptions. As a conservative estimate, the Committee assumed
that a dietary intake of 1 µg/kg bw per day (7 µg/kg bw per week)
would result in an increase in the lead concentration of blood of 1
µg/dL, the upper estimate for infants, and that this relationship was
valid during the long-term exposure period (in utero + 10 years). This
would correspond to an increase in blood lead concentration of 0.14
µg/dL per µg lead per kg bw per week. Using this assumption in
combination with the high values for estimated dietary intake, the
Committee calculated that consumption of a diet containing lead at the
currently proposed Codex limits would result in an increase in blood
lead concentration of 3 µg/dL. Consumption of diets with typical
average lead concentrations or with typically high levels would result
in increases in blood lead concentrations of 0.3 and 0.6 µg/dL,
respectively. Table 14, showing the decrements in IQ points predicted
to be associated with various blood lead concentrations, provides
confidence that the current concentrations of lead in foods would have
very little impact on the neurobeha-vioural development of infants and
children. However, the Committee stressed that a full risk assessment
of dietary intake of lead should take other sources of exposures into
account.
6. EVALUATION
The Committee concluded that, overall, current dietary levels of
lead have negligible effects on intellectual development but noted
that foods with high lead contents remain in commerce. The simulation
model presented here can be used to evaluate the effects of any
planned interventions.
7. REFERENCES
Agency for Toxic Substances and Disease Registry (1997)
Toxicological Profile for Lead, Atlanta, GA: Department of Health
and Human Services.
Australia-New Zealand Food Authority (1998) The Australian Market
Basket Survey, Melbourne: Information Australia.
Baghurst, P.A., Robertson, E.F., McMichael, A.J., Vimpani, G.V., Wigg,
N.R. & Roberts, R.R (1987) The Port Pirie cohort study: Lead effects
on pregnancy outcome and early childhood development.
Neurotoxicology, 8, 395-402.
Baghurst, P.A., McMichael, A.L., Wigg, N.R., Vimpani, G.V., Robertson,
E.F., Roberts, R.J. & Tong, S.-L. (1992) Environmental exposure to
lead and children's intelligence at the age of seven years. New
Engl. J. Med., 327, 1279-1284.
Baines, J. (1999) Dietary Exposure Assessment-Lead. Australia and
New Zealand National Assessments. Submitted to JECFA.
Bellinger, D., Sloman, J., Leviton, A., Rabinowitz, M., Needleman,
H.L. & Waternaux, C. (1991) Low-level lead exposure and children's
cognitive function in the preschool years. Pedatrics, 87, 219-227.
Brody, D.J., Pirkle, J.L., Gunter, E.W., Kramer, R.A., Flegal, K.M.,
Matte, T.M. & Paschal, D.C. (1994) Blood lead levels in the US
population. Phase 1 of the National Health and Nutrition Examination
Surveys (NHANES III, 1988 to 1991). J. Am. Med. Assoc., 272,
277-283.
Codex Committee on Food Additives and Contaminants (1999) Report of
the Thirty-first Session of the Codex Committee on Food Additives
and Contaminants, Rome, Food and Agriculture Organization of the
United Nations (document ALINORM 99/12A, Appendix V).
Chen, J. & Gao, J. (1993) The Chinese Total Diet Study in 1990. Part
I. Chemical contaminants. J. AOAC Int., 76, 1193-1205.
Chen, J. & Gao, J. (1998) Personal communication to JECFA.
Cooney, G.H., Bell, A., McBride, W. & Carter, C. (1989)
Neurobehavioral consequences of prenatal low level exposures to lead.
Neurotoxicol. Teratol., 11, 95-104.
Cooper, W.C. (1976) Cancer mortality patterns in the lead industry.
Ann. N>Y> Acad. Sci., 271, 250-259.
Cooper, W.C. (1988) Deaths from chronic renal disease in US battery
and lead production workers. Environ. Health Perspectives, 78,
61-63.
Dietrich, K.N., Krafft, K.M., Bornschein, R.L., Hammond, P.B., Berger,
O., Succop, P.A. & Bier, M. (1987) Low-level fetal lead exposure
effect on neurobehavioral development in early infancy. Pediatrics,
80, 721-730.
Dietrich, K.N., Succop, P.A., Berger, O.G. & Keith, R.W. (1992) Lead
exposure and the central auditory processing abilities and cognitive
development of urban children: The Cincinnati lead study cohort at age
5 years. Neurotoxicol. Teratol., 14, 51-56.
Dietrich, K.N., Berger, O.G., Succop, P.A., Hammond, P.B. &
Bornschein, R.L. (1993) The developmental consequences of low to
moderate prenatal and postnatal lead exposure: Intellectual attainment
in the Cincinnati lead study cohort following school entry.
Neurotoxicol. Teratol., 15, 37-44.
Environmental Protection Agency (1994) Technical Support Document:
Parameters and Equations Used in Integrated Exposure Uptake
Biokinetic Model in Children (v 0.99d) (Publication 9285.7-22,
EPA/540/R-94/040, PB94-963505).
Ershow, A.G. & Cantor, K.P. (1989) Total Water and Tapwater Intake
in the United States: Population based Estimates of Quantitities
and Sources, Bethesda, MD: Federation of American Societies for
Experimental Biology.
Factor-Litvak, P., Graziano, J.H., Kline, J.K., Popovac, D., Mehmeti,
A., Rajovic, L., Neneziv, D.U. & Stein, Z.A. (1991) A prospective
study of birthweight and length of gestation in a population
surrounding a lead smelter in Kosovo. Int. J. Epidemiol., 20,
722-728.
Food and Drug Administration (1993-96) Residue monitoring. J. AOAC
Int., 77-80.
Fulton, M., Thomson, G., Hunter, R., Raab, G., Laxen, D. & Hepburn, W.
(1987) Influence of blood lead on the ability and attainment of
children in Edinburgh. Lancet, ii, 1221-1225.
Gao, J. (1998) Personal communication to JECFA.
GEMS/Food (1992) Assessment of Dietary Intake of Chemical
Contaminants (WHO/HPP/FOX/92.6 UNEP/GEMS/92.F2), Geneva: Joint
UNEP/FAO/WHO Food Contamination Monitoring and Assessment Programme.
Goyer, R.A. (1989) Mechanisms of lead and cadmium nephrotoxicity.
Toxicol. Lett., 46, 153-162.
Goyer, R.A. (1996) Toxic effects of metals. In: Klaassen, C., ed.,
Casarett & Doull's Toxicology: The Basic Science of Poisons, New
York: McGraw-Hill, pp. 691-737.
Graziano, J.H., Popova, D., Factor-Litvak, P., Shrout, P., Kline, J.,
Murphy, M.J., Zhao, Y., Mehmeti, A., Ahmedi, X., Rajovic, B., Zvicer,
Z., Nenezic, D.U., Lolacono, N.J. & Stein, Z. (1990) Determinants of
elevated blood lead during pregnancy in a population surrounding a
lead smelter in Kosovo, Yugoslavia. Environ. Health Perspectives,
89, 95-100.
Hawk, B.A., Schroeder, S.R., Robinson, G., Otto, D., Mushal, P.,
Kleinbaum, D. & Dawson, G.(1986) Relation of lead and social factors
to IQ of low-SES chbildren: A partial replication. Am. J. Mental.
Defic., 91, 178-183.
Lacey, R.F., Moore, M.R. & Richards, W.N. (1985) Lead in water, infant
diet and blood. The Glasgow duplicate diet study. Sci. Total
Environ., 41, 235-247.
Lansdown, R., Yale, W., Urbanowicz, M.-A. & Hunter, J. (1986) The
relationship between blood-lead concentrations, intelligence,
attainment and behaviour in a school population: The second London
study. Int. Arch. Occup. Health, 57, 225-235.
Lewis, R.J., ed. (1992) Sax's Dangerous Properties of Industrial
Chemicals, 8th Ed., New York: Van Nostrand Rheinhold.
National Research Council (1993) Measuring Lead Exposure in Infants,
Children, and Other Sensitive Populations, Washington DC: National
Academy Press.
Needleman, H.L., Gunnoe, C., Leviton, A., Reed, R., Peresie, H.,
Maher, C. & Barrett, P. (1979) Deficits in psychologic and classroom
performance of children with elevated dentine levels. New Engl. J.
Med., 300, 689-695.
Needleman, H.L., Rabinowitz, S., Leviton, A., Linn, S. & Schoenbaum,
S. (1984) The relationahip between prenatal exposure to lead and
congenital anomalies. J. Am. Med. Assoc., 251, 2856-2959.
O'Flaherty, E.J. (1991) Physiologically based models for bone-seeking
elements. III. Lead absorption and disposition in childhood.
Toxicol. Appl. Pharmacol., 111, 332-341.
O'Flaherty, E.J. (1993) Physiologically based models for bone-seeking
elements. IV. Kinetic of lead disposition in humans. Toxicol. Appl.
Pharmacol., 118, 16-29.
O'Flaherty, E.J. (1995) Physiologically based models for bone-seeking
elements. V. Lead absorption and disposition in childhood. Toxicol.
Appl. Pharmacol., 131, 297-308.
Pirkle, J.L., Schwartz, J., Landis, J.R. & Harlan, W.R. (1985) The
relationship between blood lead levels and blood pressure and its
cardiovascular risk implications. Am. J. Epidemiol., 121, 246-258.
Pirkle, J.L., Brody, D.J., Gunter, E.W., Kramer, R.A., Paschal, D.C.,
Flegal, K.M.& Matte, T.M. (1994) The decline in blood lead levels in
the United States. The National Health and Nutrition Examination
Surveys (NHANES). J. Am. Med. Assoc., 272, 284-291.
Rabinowitz, M.R., Wetherill, G.W. & Kopple, J.D. (1976) Kinetic
analysis of lead metabolism in healthy humans. J. Clin. Invest., 58,
260-170.
Schroeder, S.R., Hawk, B., Otto, D.A., Mushak, P. & Hicks, R.E. (1985)
Separating the effects of lead and social factors on IQ. Environ.
Res., 38, 144-154.
Schwartz, J. (1993) Beyond LOEL's, p values, and vote counting:
Methods for looking at the shapes and strengths of associations.
Neurotoxicology, 14, 237-246.
Schwartz, J. (1994) Low-level lead exposure and chuldren's IQ: A
meta-analysis and search for a threshold. Environ. Res., 65, 42-55
Sherlock, J.C., Smart, G., Forbes, G.I., Moore, M.R., Patterson, W.J.,
Richards, W.N. & Wilson, T.S. (1982) Assessment of lead intakes and
dose-response for a population in Ayr exposed to a plumbosolvent water
supply. Hum. Toxicol., 1, 115-122.
Tahvonen, R. (1997) Contents of lead in foods and diet in Finland.
Food Rev. Int., 13, 77-90.
Ursínyová, M. & Hladíková, V. (1997) The intake of selected toxic
elements from milk in infants. Fresenius Environ. Bull., 6, 627-632.
Ursínyová, M. & Hladíková, V. (1998) Dietary intake of cadmium, lead
and mercury in vegetarian and non-vegetarian children. Fresenius
Environ. Bull., 7, 585-592.
Ward, N.I. & Savage, J.M. (1994) Metal dispersion and transportational
activities using food crops as biomonitors. Sci. Total Environ.,
146-147, 309-319.
Wasserman, G., Graziano, J.H., Factor-Litvak, P., Popovac, D., Morina,
N., Musabegovic, A., Vrenezi, N., Capuni-Paracka, A., Lekic, V.,
Preteni-Redjepi, E., Hadzialjevic, S., Slavkovich, V., Kline, J.,
Shrout, P. & Stein, Z. (1992) Independent effects of lead exposure and
iron deficiency anemia on developmental outcome at age 2 years. J.
Pediatr., 121, 695-703.
WHO (1995) Enviromental Health Criteria 165: Inorganic Lead, Geneva:
International Programme on Chemical Safety.
Winneke, G., Brockhaus, A., Ewers, U., Krämer, U. & Neuf, M. (1990)
Results from the European multicenter study on lead neurotoxicity in
children: Implications for risk assessment. Neurotoxicol. Teratol.,
12, 553-559.
Yule, W., Lansdown, R., Millar, I.B. & Urbanowicz, M.-A. (1981) The
relationship between blood lead concentrations, intelligence, and
attainment in a school population: A pilot study. Dev. Med. Child.
Neurol., 23, 567-576.
4.2.3 A sample diet-response simulation
The effect of a commodity containing high concentrations of lead
on the IQ of an infant as a result of maternal exposure was simulated
by using the exposure assessment described in Table 6. The functions
described above were used to calculate blood lead concentrations, the
expected change in IQ after lifetime exposure, and the expected effect
on adults after a limited exposure of nine months. These results are
presented in Table 13.
Table 10. Models of the dose-response relationship for exposure
to lead and reduced IQ scores in children
Model Probability Parameter(s) Representation
Linear 0.4 Slope (IQ per µg/dL) Triangular (0, 0.25, 0.4)
Hockey stick 0.2 Slope (IQ per µg/dL) Triangular (0, 0.35, 0.6)
Threshold Triangular (0, 5, 15)
Hill 0.4 KD (µg/dL) Triangular (18,22,33
Maximum Triangular(15,20,40)
Power Uniform (2.0, 2.8)
The median value for the uncertainty distribution is the best fit and is
plotted in Figure 6. The values for the KD (dissociation constant) and
maximum parameters are correlated.
Table 11. Models of the dose-response relationship between
exposure to lead and effects in the population
Model Probability Parameter(s) Representation
Normal 0.4 Fractional Uniform
standard deviation (5-15 % of mean effect)
Log normal 0.6 Geometric Uniform
standard deviation (0.1-0.3)
5. COMMENTS
The most widely used biomarker of exposure to lead is the
concentration in blood (measured in µg/dL). The most critical effect
of lead at low concentrations is reduced cognitive development and
intellectual performance in children. A number of studies in which
various tests of behavioural performance were used have shown an
association between blood lead concentration and reduced intelligence
quotient (IQ) in children exposed pre-and postnatally. At blood
concentrations below 10-15 µg/dL, the effect of confounding variables
and limits to the precision of analytical and psychometric
measurements increase the uncertainty of any estimate of effect. If a
threshold does exist, it is unlikely to be detected because of these
limitations. However, there was some evidence of an association
between cognitive deficits and exposure to concentrations even below
10 µg/dL.
Exposure to lead
Exposure to lead can occur as a result of ingestion in foodstuffs
and water and from other sources, such as air. All three sources make
important contributions. Although the current assessment was limited
to dietary intake, a complete analysis would require addition of
non-food sources of lead to the evaluation.
The Committee reviewed data on lead intake in 25 countries and
conducted several dietary assessments on the assumption that the WHO
GEMS/Food regional diets contain 'typical' levels of lead in the food
categories for which limits have been proposed by the Codex Committee
on Food Additives and Contaminants. The WHO GEMS/Food diets have been
used by the Codex Committee on Pesticide Residues and others to
estimate intakes of pesticides and contaminants since 1987. The
regional diets were used to estimate lead intake under three sets of
assumptions about the concentrations of lead in food: all foods
contain lead (1) at the limits currently proposed by the Codex
Committee on Food Additives and Contaminants, (2) at a typical average
concentration, and (3) at typical high levels found in foods. When the
concentrations at the currently proposed Codex limits were used in the
assessment, the estimated intakes were 13-20 µg/kg bw per week. The
typical average and high levels were derived from monitoring in the
United States and were similar to those reported from other countries.
The weekly intakes ranged from 1 to 2 µg/kg bw per week for 'typical'
lead levels and from 2 to 4 µg/kg bw per week for 'high typical' lead
levels. These narrow ranges in intake estimates reflect the fact that
the monitoring data that were submitted to the Committee did not
include foodstuffs that contained particularly high levels of lead,
and no food group predominated. Virtually no data were submitted on
foods with levels above the currently proposed Codex limit. The Expert
Committee noted that similar intakes were obtained for the five
different GEMS/Food regional diets with these models.
Table 13. Estimated change in IQ after exposure to a commodity
containing high concentrations of lead
Population Intervention
percentile
None Reduction by one half Reduction to 'normal'
0.05 0.008 (0.00, 0.06) 0.005 (0.000, 0.05) 0.003 (0.00, 0.04)
0.5 0.018 (0.00, 0.1) 0.011 (0.00, 0.08) 0.006 (0.00, 0.06)
0.95 0.042 (0.00, 0.16) 0.028 (0.00, 0.13) 0.02 (0.00, 0.10)
0.99 0.051 (0.00, 0.19) 0.034 (0.00, 0.15) 0.02 (0.00, 0.12)
Estimates of the net change in IQ as result of exposure to lead in a commodity
with high concentrations after two levels of intervention. The scenario on which
the estimates are based presumes that exposure occurs as a result of maternal
exposure only.
The Committee reviewed data contained in the WHO GEMS/Food
database and found that foods that were sampled in the 1980s contained
much higher concentrations of lead than those seen presently and
decided to base its conclusions on current data.
Children
The potential intake of lead by children was reported for seven
countries on the basis of monitoring of the general food supply as
well as infant formula and other foods commonly consumed by children.
Several countries specifically provided intake levels of foods
consumed by children. The estimated range of intake levels for
children was 0.6-30 µg/kg bw per week, which was generally two to
three times the adult intake in the same country when evaluated on the
basis of body weight.
Tap water is a significant potential source of lead, particularly
for formula-fed infants; however, the data submitted were inadequate
to permit estimation of the range of levels found in water.
Quantitative risk assessment
Exposure assessment
Several simulation models were developed to estimate the
distributions of dietary lead intake in regional diets. The first
involved a scenario in which the regional populations covered by the
WHO GEMS/Food database consumed food with lead concentrations
corresponding to those found in a survey conducted in the United
States. The second was designed to evaluate the impact of
superimposition of a specific commodity from a source with a much
higher distribution of lead on a background of other regional dietary
and non-dietary exposures. In a third simulation, the impacts of
several putative regulatory interventions on dietary intake were
evaluated.
Estimates of blood lead concentrations from dietary intake levels
In order to predict the biological effects of changes in lead
intake, a relationship must be derived between the concentration of
lead in the diet and changes in the biomarker, the concentration of
lead in blood. To do so, the Committee used simple empirical models to
relate exposure to lead to concentrations in blood and other tissues.
Most of the historical data refer to relatively constant exposure to
lead, usually as a consequence of contamination of drinking-water by
lead plumbing. These data have limited predictive value if the levels
of exposure result in blood lead concentrations higher than 25 mg/dL.
In addition, other dietary components or atypical physiological states
may alter the rate of absorption from the intestine to the blood.
In order to infer a relationship between ingestion of lead and an
increase in the blood lead concentration in infants and young
children, several models were fitted to data from studies of
bottle-fed infants. Reasonable fits required the assumption that a
zero dose in drinking-water corresponds roughly to a blood lead
concentration of 15 µg/dL, perhaps reflecting exposure from the
environment or in utero. The results, attributable to dietary intake
of lead by infants, correspond roughly to a change in blood lead
concentration of 0.05-0.1 µg/dL per µg of lead intake per kg bw per day.
For a 10-kg infant, this corresponds to a range of 0.5-1.0 µg/dL per µg
of lead in the diet per day.
The Committee used data from another study to calculate the
relationship between blood lead concentration and intake of lead from
drinking-water by pregnant women. The sample size was large enough to
allow characterization of population variation. When the raw data were
fitted to several simple models, all resulted in poor fits. When
concentrations in drinking-water below 300 µg/L were fitted
separately, a linear model with a log-normal population distribution
produced a very good fit. A background blood lead concentration of
roughly 9 µg/dL was obtained, probably reflecting exposure from
sources other than drinking-water. The model yielded a blood lead
concentration of 0.035 µg/dL per µg of lead in 1 L of drinking-water.
Because of uncertainty in the amount of water consumed by the
individuals in the study, the variation around this value was wide,
from 0.023 to 0.07 µg/dL per µg of lead intake per kg bw per day.
For a 60-kg person, this corresponds to a range of 1.4-4.2 µg/dL per
µg of lead in drinking-water per day.
Dose-response assessments of neurobehavioural effects of lead in
children
The Committee noted a number of limitations in the available data
on the effects of lead on neurobehavioural development in children.
One problem was that raw data were not available for use in risk
assessments or in evaluating mathematical models of the relationship
between exposure to lead and performance in behavioural tests. Another
problem was the somewhat disparate results obtained. Furthermore, it
was difficult to compare the results of large epidemiological studies
in which many potential confounding variables were analysed in
different models. It is therefore not possible to ascertain whether
any effect is due to lead or to some other variable. A further problem
was that the same tests were not used in all studies, so that
different end-points were measured. (Measures of cognitive and motor
performance might be preferable to a general measure of IQ.) Finally,
most of the analyses were based on tests of statistical significance
between groups with high and low exposure to lead rather than a
critical evaluation of the dose-response relationship.
The analysis that had the fewest of these potential flaws showed
a decrease of one IQ point for every 2-4 µg/dL increase in blood lead
concentration, with a greater effect at higher concentrations than at
lower ones. A meta-analysis of seven studies showed that an increase
in the blood lead concentration from 10 to 20 µg/dL would result in a
decrease of approximately 2.5 IQ points.
This analysis does not include the possibility that the
relationship between blood lead and IQ is nonlinear, in spite of some
evidence that it may be. Furthermore, there is no expression of either
population variation or uncertainty in the dose-response relationship.
In order to address these concerns, probability trees and statistical
distributions were included in the assessment of the dose-response
relationship. Because raw data were not available, the values for the
magnitude of the variation and uncertainty were chosen to be generally
consistent with those available in the literature on the health
effects of lead. As an example of the behaviour of the composite
dose-response function generated, Table 14 gives estimates of the net
decrease in IQ for the population median at four blood lead values,
with a range of uncertainty for each estimate.
Dose-response simulation
A simulation model was developed in which a dose-response
component was added to the exposure model described previously. This
model was used to illustrate the net benefit of imposing limits on the
levels of lead in food with respect to neurobehavioural development of
children, given an anticipated reduction in exposure to lead.
The studies that have related exposure to lead to performance in
behavioural tests have been conducted in populations whose exposure to
lead is relatively constant. In order to gauge the relative importance
of shorter exposures, it was presumed that the net lifetime effect of
lead on intellectual status (i.e. adult IQ) after a limited prenatal
or postnatal exposure may be scaled relative to a period of 5-15
years, the range representing the uncertainty associated with the
adjustment.
The model was based on consumption patterns from a WHO GEMS/Food
Middle Eastern diet, assuming that the lead concentrations in food
were typical of those in the United States, and non-dietary exposures
corresponding roughly to those in the United States. In this
hypothetical scenario, a decrement in IQ of 0.006 points (range,
0-0.06) was estimated for the population mean of children as a result
of maternal exposure for nine months. The procedure described above
was used to adjust for the period of exposure. In a scenario in which
the cereal grains in a regional diet contained 10 times higher lead
levels, the estimated decrement in IQ points attributable to lead was
0.02 (range, 0-0.1). A hypothetical intervention leading to a
reduction in grain lead levels by one-half was estimated to reduce the
decrement in IQ points to 0.011 (range, 0-0.08). Given the similarity
in the estimates of exposure from the different regional diets,
similar results would be anticipated if other WHO GEMS/Food regional
diets were used in the scenario.
Table 14. Net decrease in IQ associated with blood lead
concentration
Concentration of Median IQ decrement
lead in blood (µg/dL) (95% confidence interval)
5 0.4 (0.0-1.5)
10 1.7 (0.5-3.1)
15 3.4 (1.1-5.0)
20 5.5 (1.6-6.9)
The Committee also estimated the effect on blood lead
concentrations of long-term exposure to lead in the three models of
regional diets used to illustrate the ranges of intake of lead under
various assumptions. As a conservative estimate, the Committee assumed
that a dietary intake of 1 µg/kg bw per day (7 µg/kg bw per week)
would result in an increase in the lead concentration of blood of 1
µg/dL, the upper estimate for infants, and that this relationship was
valid during the long-term exposure period (in utero + 10 years). This
would correspond to an increase in blood lead concentration of 0.14
µg/dL per µg lead per kg bw per week. Using this assumption in
combination with the high values for estimated dietary intake, the
Committee calculated that consumption of a diet containing lead at the
currently proposed Codex limits would result in an increase in blood
lead concentration of 3 µg/dL. Consumption of diets with typical
average lead concentrations or with typically high levels would result
in increases in blood lead concentrations of 0.3 and 0.6 µg/dL,
respectively. Table 14, showing the decrements in IQ points predicted
to be associated with various blood lead concentrations, provides
confidence that the current concentrations of lead in foods would have
very little impact on the neurobeha-vioural development of infants and
children. However, the Committee stressed that a full risk assessment
of dietary intake of lead should take other sources of exposures into
account.
6. EVALUATION
The Committee concluded that, overall, current dietary levels of
lead have negligible effects on intellectual development but noted
that foods with high lead contents remain in commerce. The simulation
model presented here can be used to evaluate the effects of any
planned interventions.
7. REFERENCES
Agency for Toxic Substances and Disease Registry (1997)
Toxicological Profile for Lead, Atlanta, GA: Department of Health
and Human Services.
Australia-New Zealand Food Authority (1998) The Australian Market
Basket Survey, Melbourne: Information Australia.
Baghurst, P.A., Robertson, E.F., McMichael, A.J., Vimpani, G.V., Wigg,
N.R. & Roberts, R.R (1987) The Port Pirie cohort study: Lead effects
on pregnancy outcome and early childhood development.
Neurotoxicology, 8, 395-402.
Baghurst, P.A., McMichael, A.L., Wigg, N.R., Vimpani, G.V., Robertson,
E.F., Roberts, R.J. & Tong, S.-L. (1992) Environmental exposure to
lead and children's intelligence at the age of seven years. New
Engl. J. Med., 327, 1279-1284.
Baines, J. (1999) Dietary Exposure Assessment-Lead. Australia and
New Zealand National Assessments. Submitted to JECFA.
Bellinger, D., Sloman, J., Leviton, A., Rabinowitz, M., Needleman,
H.L. & Waternaux, C. (1991) Low-level lead exposure and children's
cognitive function in the preschool years. Pedatrics, 87, 219-227.
Brody, D.J., Pirkle, J.L., Gunter, E.W., Kramer, R.A., Flegal, K.M.,
Matte, T.M. & Paschal, D.C. (1994) Blood lead levels in the US
population. Phase 1 of the National Health and Nutrition Examination
Surveys (NHANES III, 1988 to 1991). J. Am. Med. Assoc., 272,
277-283.
Codex Committee on Food Additives and Contaminants (1999) Report of
the Thirty-first Session of the Codex Committee on Food Additives
and Contaminants, Rome, Food and Agriculture Organization of the
United Nations (document ALINORM 99/12A, Appendix V).
Chen, J. & Gao, J. (1993) The Chinese Total Diet Study in 1990. Part
I. Chemical contaminants. J. AOAC Int., 76, 1193-1205.
Chen, J. & Gao, J. (1998) Personal communication to JECFA.
Cooney, G.H., Bell, A., McBride, W. & Carter, C. (1989)
Neurobehavioral consequences of prenatal low level exposures to lead.
Neurotoxicol. Teratol., 11, 95-104.
Cooper, W.C. (1976) Cancer mortality patterns in the lead industry.
Ann. N>Y> Acad. Sci., 271, 250-259.
Cooper, W.C. (1988) Deaths from chronic renal disease in US battery
and lead production workers. Environ. Health Perspectives, 78,
61-63.
Dietrich, K.N., Krafft, K.M., Bornschein, R.L., Hammond, P.B., Berger,
O., Succop, P.A. & Bier, M. (1987) Low-level fetal lead exposure
effect on neurobehavioral development in early infancy. Pediatrics,
80, 721-730.
Dietrich, K.N., Succop, P.A., Berger, O.G. & Keith, R.W. (1992) Lead
exposure and the central auditory processing abilities and cognitive
development of urban children: The Cincinnati lead study cohort at age
5 years. Neurotoxicol. Teratol., 14, 51-56.
Dietrich, K.N., Berger, O.G., Succop, P.A., Hammond, P.B. &
Bornschein, R.L. (1993) The developmental consequences of low to
moderate prenatal and postnatal lead exposure: Intellectual attainment
in the Cincinnati lead study cohort following school entry.
Neurotoxicol. Teratol., 15, 37-44.
Environmental Protection Agency (1994) Technical Support Document:
Parameters and Equations Used in Integrated Exposure Uptake
Biokinetic Model in Children (v 0.99d) (Publication 9285.7-22,
EPA/540/R-94/040, PB94-963505).
Ershow, A.G. & Cantor, K.P. (1989) Total Water and Tapwater Intake
in the United States: Population based Estimates of Quantitities
and Sources, Bethesda, MD: Federation of American Societies for
Experimental Biology.
Factor-Litvak, P., Graziano, J.H., Kline, J.K., Popovac, D., Mehmeti,
A., Rajovic, L., Neneziv, D.U. & Stein, Z.A. (1991) A prospective
study of birthweight and length of gestation in a population
surrounding a lead smelter in Kosovo. Int. J. Epidemiol., 20,
722-728.
Food and Drug Administration (1993-96) Residue monitoring. J. AOAC
Int., 77-80.
Fulton, M., Thomson, G., Hunter, R., Raab, G., Laxen, D. & Hepburn, W.
(1987) Influence of blood lead on the ability and attainment of
children in Edinburgh. Lancet, ii, 1221-1225.
Gao, J. (1998) Personal communication to JECFA.
GEMS/Food (1992) Assessment of Dietary Intake of Chemical
Contaminants (WHO/HPP/FOX/92.6 UNEP/GEMS/92.F2), Geneva: Joint
UNEP/FAO/WHO Food Contamination Monitoring and Assessment Programme.
Goyer, R.A. (1989) Mechanisms of lead and cadmium nephrotoxicity.
Toxicol. Lett., 46, 153-162.
Goyer, R.A. (1996) Toxic effects of metals. In: Klaassen, C., ed.,
Casarett & Doull's Toxicology: The Basic Science of Poisons, New
York: McGraw-Hill, pp. 691-737.
Graziano, J.H., Popova, D., Factor-Litvak, P., Shrout, P., Kline, J.,
Murphy, M.J., Zhao, Y., Mehmeti, A., Ahmedi, X., Rajovic, B., Zvicer,
Z., Nenezic, D.U., Lolacono, N.J. & Stein, Z. (1990) Determinants of
elevated blood lead during pregnancy in a population surrounding a
lead smelter in Kosovo, Yugoslavia. Environ. Health Perspectives,
89, 95-100.
Hawk, B.A., Schroeder, S.R., Robinson, G., Otto, D., Mushal, P.,
Kleinbaum, D. & Dawson, G.(1986) Relation of lead and social factors
to IQ of low-SES chbildren: A partial replication. Am. J. Mental.
Defic., 91, 178-183.
Lacey, R.F., Moore, M.R. & Richards, W.N. (1985) Lead in water, infant
diet and blood. The Glasgow duplicate diet study. Sci. Total
Environ., 41, 235-247.
Lansdown, R., Yale, W., Urbanowicz, M.-A. & Hunter, J. (1986) The
relationship between blood-lead concentrations, intelligence,
attainment and behaviour in a school population: The second London
study. Int. Arch. Occup. Health, 57, 225-235.
Lewis, R.J., ed. (1992) Sax's Dangerous Properties of Industrial
Chemicals, 8th Ed., New York: Van Nostrand Rheinhold.
National Research Council (1993) Measuring Lead Exposure in Infants,
Children, and Other Sensitive Populations, Washington DC: National
Academy Press.
Needleman, H.L., Gunnoe, C., Leviton, A., Reed, R., Peresie, H.,
Maher, C. & Barrett, P. (1979) Deficits in psychologic and classroom
performance of children with elevated dentine levels. New Engl. J.
Med., 300, 689-695.
Needleman, H.L., Rabinowitz, S., Leviton, A., Linn, S. & Schoenbaum,
S. (1984) The relationahip between prenatal exposure to lead and
congenital anomalies. J. Am. Med. Assoc., 251, 2856-2959.
O'Flaherty, E.J. (1991) Physiologically based models for bone-seeking
elements. III. Lead absorption and disposition in childhood.
Toxicol. Appl. Pharmacol., 111, 332-341.
O'Flaherty, E.J. (1993) Physiologically based models for bone-seeking
elements. IV. Kinetic of lead disposition in humans. Toxicol. Appl.
Pharmacol., 118, 16-29.
O'Flaherty, E.J. (1995) Physiologically based models for bone-seeking
elements. V. Lead absorption and disposition in childhood. Toxicol.
Appl. Pharmacol., 131, 297-308.
Pirkle, J.L., Schwartz, J., Landis, J.R. & Harlan, W.R. (1985) The
relationship between blood lead levels and blood pressure and its
cardiovascular risk implications. Am. J. Epidemiol., 121, 246-258.
Pirkle, J.L., Brody, D.J., Gunter, E.W., Kramer, R.A., Paschal, D.C.,
Flegal, K.M.& Matte, T.M. (1994) The decline in blood lead levels in
the United States. The National Health and Nutrition Examination
Surveys (NHANES). J. Am. Med. Assoc., 272, 284-291.
Rabinowitz, M.R., Wetherill, G.W. & Kopple, J.D. (1976) Kinetic
analysis of lead metabolism in healthy humans. J. Clin. Invest., 58,
260-170.
Schroeder, S.R., Hawk, B., Otto, D.A., Mushak, P. & Hicks, R.E. (1985)
Separating the effects of lead and social factors on IQ. Environ.
Res., 38, 144-154.
Schwartz, J. (1993) Beyond LOEL's, p values, and vote counting:
Methods for looking at the shapes and strengths of associations.
Neurotoxicology, 14, 237-246.
Schwartz, J. (1994) Low-level lead exposure and chuldren's IQ: A
meta-analysis and search for a threshold. Environ. Res., 65, 42-55
Sherlock, J.C., Smart, G., Forbes, G.I., Moore, M.R., Patterson, W.J.,
Richards, W.N. & Wilson, T.S. (1982) Assessment of lead intakes and
dose-response for a population in Ayr exposed to a plumbosolvent water
supply. Hum. Toxicol., 1, 115-122.
Tahvonen, R. (1997) Contents of lead in foods and diet in Finland.
Food Rev. Int., 13, 77-90.
Ursínyová, M. & Hladíková, V. (1997) The intake of selected toxic
elements from milk in infants. Fresenius Environ. Bull., 6, 627-632.
Ursínyová, M. & Hladíková, V. (1998) Dietary intake of cadmium, lead
and mercury in vegetarian and non-vegetarian children. Fresenius
Environ. Bull., 7, 585-592.
Ward, N.I. & Savage, J.M. (1994) Metal dispersion and transportational
activities using food crops as biomonitors. Sci. Total Environ.,
146-147, 309-319.
Wasserman, G., Graziano, J.H., Factor-Litvak, P., Popovac, D., Morina,
N., Musabegovic, A., Vrenezi, N., Capuni-Paracka, A., Lekic, V.,
Preteni-Redjepi, E., Hadzialjevic, S., Slavkovich, V., Kline, J.,
Shrout, P. & Stein, Z. (1992) Independent effects of lead exposure and
iron deficiency anemia on developmental outcome at age 2 years. J.
Pediatr., 121, 695-703.
WHO (1995) Enviromental Health Criteria 165: Inorganic Lead, Geneva:
International Programme on Chemical Safety.
Winneke, G., Brockhaus, A., Ewers, U., Krämer, U. & Neuf, M. (1990)
Results from the European multicenter study on lead neurotoxicity in
children: Implications for risk assessment. Neurotoxicol. Teratol.,
12, 553-559.
Yule, W., Lansdown, R., Millar, I.B. & Urbanowicz, M.-A. (1981) The
relationship between blood lead concentrations, intelligence, and
attainment in a school population: A pilot study. Dev. Med. Child.
Neurol., 23, 567-576.
