
Pesticide residues in food 2001
First draft prepared by
C. Vleminckx
Scientific Institute of Public Health, Division Toxicology,
Brussels, Belgium.
Prochloraz (N-propyl-N-[2-(2,4,6-trichlorophenoxy)ethyl]-1H-imidazole-1-carboxamide) is a broad-spectrum fungicide. It acts by inhibiting ergosterol biosynthesis. Its toxicology was first evaluated by the Meeting in 1983 (Annex 1, reference 40), when an ADI of 0–0.01 mg/kg bw was established on the basis of a NOAEL of 0.9 mg/kg bw per day in a 2-year study in dogs and a NOAEL of 1.3 mg/kg bw per day in a 2-year study in rats. Since that time, several studies have been conducted: on the absorption, distribution, metabolism and excretion of prochloraz, on its ability to irritate the skin and eye, on developmental toxicity in rabbits, and on the toxicity of plant metabolites. Prochloraz was re-evaluated within the periodic review programme of the Codex Committee on Pesticide Residues.
(a) Absorption, distribution and excretion
Mice
Six male and six female mice were given [14C-phenyl-U]prochloraz (radiochemical purity, > 99%) at a single oral dose of 100 mg kg bw. Urine and faeces were collected 24, 48, 72 and 96 h after dosing. After 96 h, all animals were killed by asphyxiation with CO2. Various tissues were removed and prepared for analysis by liquid scintillation counting. The study was conducted before GLP regulations were issued in the testing facility, but the protocol and results were well reported.
Excretion was virtually complete within 72 h. The overall recovery was 104 ± 15%, urinary excretion (63%) being the major route. There were no significant differences between the sexes in the rate or route of excretion or in the concentrations of residues in tissues. The concentrations were highest in liver (5–7 mg/kg) and lowest in muscle, genitalia, eyes, spleen and fat (0.5–1.0 mg/kg). All other tissues contained mean concentrations > 2.5 mg/kg (Needham, 1982a).
Rats
In a study conducted in compliance with the principles of GLP (with QA certification), five male and five females rats were given [14C-phenyl-U]prochloraz (radiochemical purity, > 98.9%) at a single oral dose of 5 mg/kg bw. Urine, faeces and expired CO2 were collected. After 4 days, all rats were killed by exanguination after ether anaesthesia. Samples of various tissues were analysed by liquid scintillation counting.
Excretion of radiolabelled material in the urine and faeces was rapid and complete (overall recovery, about 98%), faecal excretion predominating. A sex-related difference in the route of excretion was apparent, faecal excretion accounting for 59% of the dose in males and 70% in females. The tissue residue concentrations were very low, often below the limit of detection. Only liver samples contained concentrations consistently > 0.1 mg/kg (0.20 ± 0.04 mg/kg) (Challis & Creedy, 1988).
In another study conducted in compliance with the principles of GLP (with QA certification), five male and five female rats were given [14C-phenyl-U]prochloraz (radiochemical purity, 97%) at a single oral dose of 100 mg/kg bw. Urine, faeces and expired CO2 were collected. After 4 days, all rats were killed by exanguination after ether anaesthesia. Samples of various tissues were analysed by liquid scintillation counting.
Excretion of radiolabelled material in the urine and faeces was rapid and complete (recovery, 100 ± 3.0% in males and 98 ± 6.9% in females), with a half-life in both sexes of < 1 day. A sex difference in the route of excretion was found, urinary excretion accounting for 65% of the activity in male rats and only 41% in females. The pattern of excretion with time also differed between the sexes: peak faecal excretion was at 48 h in females and 24 h in males. There was no significant excretion of radiolabelled material into expired air. The tissue residue concentrations also showed sex differences, some concentrations in female rats being up to twice those in males. The highest concentrations were found in liver (2.8 ± 0.22 mg equivalent per kg tissue in males and 5.1 ± 0.78 mg equivalent per kg tissue in females) and kidneys (1.5 ± 0.18 mg equivalent per kg tissue in males and 2.1 ± 0.18 mg equivalent per kg tissue in females). The concentrations of residues in blood and plasma of both sexes and in lungs and adrenals of females were > 1 mg equivalent per kg tissue. In all other tissues, the residue concentrations were < 1.0 mg equivalent per kg tissue (Dawson, 1989).
In a study of the elimination of prochloraz, five male Sprague-Dawley rats were given [14C-phenyl-U]prochloraz (radiochemical purity, > 98%) at a single oral dose of 50 or 250 mg/kg bw. Urine and faeces were collected daily for 4 days, and radiolabel was measured. For analysis of urinary metabolites, the rats were given [14C]prochloraz at 250 mg/kg bw, urine was collected for 48 h, and radiolabel in excreta was assayed by liquid scintillation spectrometry. The study was reported in detail in a publication and is considered to provide useful additional information.
At both doses, virtually all of the ingested [14C]prochloraz was excreted in the urine or faeces within 96 h, the bulk of excretion occurring between 24 and 48 h after dosing. Urinary elimination accounted for 61% and 68% of the respective initial doses. Prochloraz was completely metabolized, no unchanged compound being detectable in the urine. The main biotransformation products in urine were 2,4,6-trichlorophenoxyacetic acid and its corresponding alcohol, the latter as the glucuronic acid conjugate. Aromatic hydroxylation also occurred, with small amounts of hydroxy-2,4,6-trichlorophenoxyethanol and hydroxy-2,4,6-trichlorophenoxyacetic acid excreted in urine. 2,4,6-Trichlorophenol and unconjugated 2-(2,4,6-trichlorophenoxy)ethanol were identified as minor urinary metabolites (Laignelet et al., 1992).
In a study conducted in compliance with the principles of GLP (with QA certification), the absorption of radiolabelled prochloraz after a single oral dose to rats was determined by characterizing its route and rate of excretion in urine, bile and faeces. Male and female rats with cannulated bile-ducts were given [14C-phenyl-U]prochloraz (radiochemical purity, 98.3%) at a nominal dose of 5 mg/kg bw. The animals were maintained in metabolism cages, and urine, faeces and bile were collected for up to 48 h. After that time, the animals were killed, and the radioactive content of the urine, faeces, bile, the gastrointestinal tract and its contents and residual carcass was determined by liquid scintillation counting.
The overall mean recovery of radiolabel over 48 h was 95 ± 4.5% in males and 91 ± 5.2% in females. No sex differences in the disposition of prochloraz were apparent. Biliary excretion was an important route of elimination and accounted for an overall mean of 48 ± 20% (range, 18–75%) of the dose. Renal elimination (urine plus cage washings) was quantitatively important and accounted for 21 ± 8.9% (range, 15–34%) in males and 23 ± 13% (range, 11–41%) in females. The radiolabel voided in faeces (primarily within 24 h) represented 22 ± 12% in males and 14 ± 9.4% in females, and that found associated with the gastrointestinal tract was minimal (males, 0.16%; females, 1.1%). The residual carcass retained a very small fraction of the dose (mean, 2.6% in males and 3.0% in females). Thus, the recovery of radiolabel was quantitative, and there were no apparent sex differences. [14C]Prochloraz was well absorbed, a mean of 74% of the dose (range, 60–96%) being detected in bile, urine, cage washings and carcass. Biliary excretion was the major route of elimination (D’Souza, 1995).
In a study conducted in compliance with the principles of GLP (with QA certification), the clearances of radiolabelled residues from tissues in rats after a single low or high oral dose of [14C-phenyl-U]prochloraz (radiochemical purity, > 99%) were compared. Groups of 18 males and 18 females were given a dose of either 5 or 100 mg/kg bw. Those at the low dose were necropsied in groups of three males and three females 2, 10, 20, 40, 56 and 72 h after treatment, and those at the high dose were necropsied in groups of three males and three females 10, 20, 40, 56, 72 and 96 h after treatment. These times were set on the basis of the findings of preliminary studies in which the blood Cmax, C3/4, C1/2 and C1/4 were determined at the low and high doses. At necropsy, blood, plasma, liver, kidney, spleen, heart, lung, brain, muscle, gonads, eyes, adrenals, bone, renal fat, gastrointestinal tract and residual carcass were collected, and residual radiolabel was quantified.
At both the low and high doses, radiolabelled residues of prochloraz were cleared rapidly from the tissues, with a marked decrease in concentration by the end of the study. After the low dose, the highest tissue concentrations were seen in the gastrointestinal tract, at 24 ± 1.0 and 28 ± 1.7 mg equivalent per kg, and liver, at 8.5 ± 2.3 and 7.3 ± 0.9 mg equivalent per kg in males and females, respectively, 2 h after dosing. By 72 h after dosing, the residues in all tissues except the liver (0.49 mg equivalent per kg), kidney (0.22 mg equivalent per kg) and gastrointestinal tract (0.22 mg equivalent per kg) of males and females and the plasma (0.13 mg equivalent per kg) of males had fallen to > 0.1 mg equivalent per kg tissue and were below the level of quantification in some tissues. After the high dose, the highest concentrations were seen in the gastrointestinal tract (230 ± 73 and 340 ± 37 mg equivalent per kg), liver (65 ± 19 and 90 ± 12 mg equivalent per kg) and kidney (81 ± 4.6 and 74 ± 10 mg equivalent per kg) in males and females, respectively, 10 h after dosing, with high concentrations also seen in the blood (59 and 38 mg equivalent per kg) and plasma (93 and 57 mg equivalent per kg) in males and females, respectively. By 96 h after dosing, the tissue concentrations were still above the limit of quantification but had decreased markedly, by at least one order of magnitude.
The tissue distribution ratios indicated maximum distribution of radiolabel 2–10 h after the low dose and 10–20 h after the high dose. The highest values were seen in the gastrointestinal tract, carcass, blood, plasma, muscle, liver and renal fat. The calculated terminal half-lives in blood, plasma, liver and kidney were similar in all these tissues in both sexes at both the high and low dose. In blood, the terminal half-life was 11 h in males and 14 h in females at the low dose and 12 h in males and 17 h in females at the high dose. There was no significant difference in the half-lives in blood, plasma and kidney at either dose. For the liver, the terminal half-life was 17 h in males and 18 h in females at the low dose and 22 h in males and 24 h in females at the high dose. These half-lives indicate that the elimination of radiolabelled residues of prochloraz is fairly rapid (Reynolds, 1995, 1996).
Three male and three female rats were given [14C-phenyl]N-formyl-N’-propyl-N’-2-(2,4,6-trichlorophenoxy)ethylurea (M2), a major plant metabolite of prochloraz, at a single oral dose of 89 mg/kg bw, and the excretion of radiolabel was followed for 96 h, after which the rats were killed and dissected. The study was conducted before GLP regulations were issued in the testing facility, but the protocol and results were well reported.
Excretion of radiolabelled material was virtually complete within 72 h. Urinary excretion accounted for 53–63% of the total dose, the remainder being excreted in faeces. The concentration of the compound appeared to decline rapidly in the tissues, the highest concentrations being detected in liver (2.6–3.4 µg/g tissue) and kidney (1.3–5.3 µg/g tissue) and the lowest in muscle and brain (0.05–0.2 µg/g tissue). The remaining tissues had concentrations > 1 ppm, except for the skin. There were no apparent sex differences (Needham, 1981).
Three rats of each sex were given prochloraz at a dose of 90 mg/kg bw, prochloraz manganese chloride complex at 98 mg/kg bw or M2 at 89 mg/kg bw, so that all animals received an equivalent dose, and the amount of 2,4,6-trichlorophenoxyacetic acid (a major urinary metabolite of prochloraz) in urine was determined by gas–liquid chromatography. Excreta were collected at 24-h intervals for 96 h. The study was conducted before GLP regulations were issued in the testing facility, but the protocol and results were well reported.
A sex difference was found in the amount of 2,4,6-trichlorophenoxyacetic acid excreted after administration of all three compounds, male rats excreting approximately twice as much in the urine as did females (36–50% compared with 19–29%). The amounts of 2,4,6-trichlorophenoxyacetic acid excreted by rats given prochloraz or prochloraz manganese chloride complex were similar, but significantly less was excreted by male rats given M2. No significant differences were seen in the excretion of 2,4,6-trichlorophenoxyacetic acid by female rats (Campbell & Needham, 1981).
The pharmacokinetics of [3H-phenyl ring]prochloraz (radiochemical purity, 99%) was studied in rats by measuring radioactivity in plasma and tissues after a single oral dose of 100 mg/kg bw and repeated oral doses of 25 mg/kg bw. Radioactivity was measured for 96 h after the single dose and at specified intervals for 20 days after the repeated doses. The study complied with the general principles of GLP (statement of authenticity from the project manager).
After the single dose, the plasma concentrations were highest at 24 h (males, 166 µg equivalent per ml; females, 82 µg equialent per ml) and declined steadily to consistently low concentrations by 96 h (males, 13 µg equivalent per ml; females, 11 µg equivalent per ml). The tissue concentrations showed a similar pattern, being consistently high up to 24 h after dosing and then declining fairly rapidly to low concentrations at 96 h. In general the plasma concentrations in males were appreciably higher than those in females, but the tissue concentrations were similar in the two sexes, with the exception of the kidney, where the concentrations in females were lower than those in males. Apart from the gastrointestinal tract, the highest concentrations of radiolabel were found in liver and kidneys and the lowest in brain, eyes, male gonads and muscle.
After repeated exposure, the plasma concentrations were again higher in males than in females. The tissue concentrations showed no obvious sex differences, with the exception of the kidney, in which the concentrations in females were lower. In males, the plasma concentration of radiolabel rose irregularly to a peak (72 µg equivalent per ml) at 15 days and then declined at day 20. In females, the peak plasma concentration (35 µg equivalent per ml) was observed after 7 days and was maintained at about this level for the remainder of the treatment. Four days after the last dose, the plasma concentrations in both sexes had fallen considerably (males, 11 µg equivalent per ml; females, 14 µg equivalent per ml) and were similar to that measured 4 days after the single dose. The tissue concentrations in both sexes rose significantly for the first 7 days of dosing and rose only slightly for the remainder of treatment. When selected tissues were analysed for radiolabel during repeated dosing, liver and kidney had the highest concentrations and muscle and fat the lowest. Thus, the phenoxy moiety of prochloraz was rapidly eliminated from the body, leaving no significant concentrations of residues in tissues, and at a comparable rate after repeated and single oral doses. Repeated doses gave rise to residues, the concentration of which reached a plateau after 7–14 days (Boardman, 1979).
Dogs
Three dogs of each sex were given [14C-phenyl-U]prochloraz (radiochemical purity, > 99%) at a single oral dose of 18 mg/kg bw in a gelatine capsule. Blood samples were collected 1, 2, 4, 6, 8, 12, 24, 48, 72 and 96 h after dosing. Urine and faeces were collected daily. The dogs were killed 96 h after dosing by exanguination after barbiturate anaesthesia. An extensive range of tissues were removed and prepared for analysis by liquid scintillation counting. The study was conducted before GLP regulations were issued in the testing facility, but the protocol and results were well reported.
Within the first 24 h, 60 ± 10% of the administered dose was excreted. The overall recovery was 96 ± 4%, and faecal excretion was the major route, accounting for 64 ± 3% of the total dose. There were no significant differences in the pattern or rate of excretion between the two sexes. The high concentrations found in the bile (19 ± 14 mg equivalent per kg in males and 41 ± 36 mg equivalent per kg in females) indicate that biliary excretion is a significant route of elimination for this compound. The plasma concentrations 96 h after dosing (15 ± 6 mg equivalent per l) were significantly greater than those in other tissues. The highest concentrations in tissues were found in liver (7.6 ± 1.7 mg equivalent per kg) and kidney (5.6 ± 2.3 mg equivalent per kg). Most tissues contained < 3 mg equivalent per kg, and the lowest concentrations were found in bone (0.5 ± 0.4 mg/kg), cerebellum (0.7 ± 0.3 mg/kg) and cerebrospinal fluid (0.3 ± 0.6 mg/l). The curve for plasma concentration–time showed a rapid initial absorption phase, followed by a slow final elimination phase with a half-life of approximately 72 h. The peak plasma concentrations (29–31 mg equivalent per l) occurred 8–24 h after dosing (Needham & Campbell, 1982).
Goats
As straw containing residues of prochloraz may be used as fodder for livestock, residues of prochloraz may occur in milk and meat. [14C-phenyl-U]Prochloraz formulated as a 40% emulsifiable concentrate was applied to field-grown wheat at a rate of 0.98 kg/ha. The wheat was harvested 11 weeks after treatment and analysed for residues of prochloraz. The straw, which contained radiolabel equivalent to 19 mg/kg, was fed to a lactating goat for 4 days, and milk and blood samples were collected twice daily. At the end of treatment, the goat was killed by exsanguination after anaesthesia. Various tissues were sampled and analysed by liquid scintillation counting. The maximum concentrations were 0.079 mg/l in plasma and 0.006 mg/l in milk. The concentrations in tissues were highest in liver (0.05 mg/kg), renal fat (0.04 mg/kg) and rumen wall (0.04 mg/kg). All other tissues contained 0.03 mg/kg. Bile contained 0.12 mg/l, and the rumen contents contained 0.13 mg/l (Campbell, 1983).
In another study, a lactating goat was given [14C-phenyl]M2 in two oral doses of 60 mg 8 days apart. The goat was slaughtered 24 h after the second dose. After the first dose, the concentration of residues in milk was highest at 7 h (0.07 ppm equivalent) and declined to 0.01 ppm by 31 h. The concentration in plasma was highest at 7 h (0.33 ppm) and declined to 0.01 ppm by 96 h. After the second dose, the concentration in plasma was highest 2 h after dosing and declined in a manner similar to that after the first dose. Residues were detectable in milk 2 h after dosing (0.01 ppm), and the concentration at 17 h was consistent with that seen after the first dose (0.04 ppm). A sample of urine collected 21 h after dosing contained 1.9 mg equivalents, representing 3.2% of the original dose. By 24 h after the second oral dose, the highest concentration of tissue residues was found in liver (0.59 ppm). The tissues of the digestive tract contained 0.02–0.15 ppm, and the concentration in rumen contents was 0.15 ppm. Bile collected at slaughter contained 3.9 ppm. The concentration of residues in muscle was below the limit of sensitivity of the method (< 0.01 ppm). In general, the tissue concentrations of residues resulting from the ingestion of M2 were lower than those resulting from ingestion of an equivalent dose of prochloraz (Campbell & Needham, 1980).
Pigs
Four sows were treated dermally with radiolabelled prochloraz to determine the extent of absorption and subsequent distribution of radiolabel in the tissues. Two animals were treated with [14C-imidazole ring]prochloraz, while the other two were given [3H-phenyl ring]prochloraz. Both compounds were formulated as 25% emulsifiable concentrates, and each animal received 2 ml of the formulation (about 500 mg of prochloraz). Urine and faeces were collected throughout the study. The animals were killed after 24 h for removal of tissues, while blood samples were taken at intervals throughout the 24-h period.
The concentration of radiolabel recovered from the bandage, treated skin and excreta ranged from 76% to 110% of the activity applied. Less than 1.3% was found in the excreta, and the extent of uptake into the bloodstream and tissues was estimated to have been < 2%. With the exception of treated skin, the highest concentrations of residue were detected in bile ([14C], 13 ppm; [3H], 6.6 ppm). Relatively high concentrations were also found in the iris ([14C], 3.4 ppm; [3H], 2.6 ppm), the liver ([14C], 1.4 ppm; [3H], 2.1 ppm) and adrenals ([14C], 1.6 ppm; [3H], 1.5 ppm). The pattern of uptake of radiolabel into the plasma differed according to the label, suggesting that some degradation of the prochloraz molecule had occurred between application onto the skin and distribution in the blood (Hamilton, 1978).
Rats
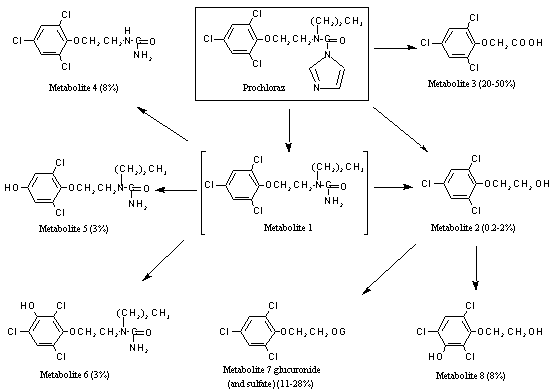
A generalized metabolic pathway for prochloraz in rats is shown in Figure 1.

Figure 1. Metabolites of prochloraz excreted in the urine of rats
The biotransformation of [14C]prochloraz (radiochemical purity, > 99%), uniformly labelled on the phenyl or imidazole ring, was studied in male and female rats given an oral dose of 100 mg/kg bw as a suspension in gum acacia or dissolved in corn oil. A similar dosing regimen was followed in separate experiments with mice and dogs, except that only 18 mg/kg bw was administered to dogs. Urine was collected at 24-h intervals. In another experiment, a female goat was given a single oral dose of 1.5 mg/kg bw of prochloraz, and one urine sample was collected during the first 24 h after dosing. The study was conducted before GLP regulations were issued in the testing facility, but the protocol and results were well reported.
Prochloraz was extensively metabolized in rats, with no unchanged compound detectable in the urine. The main metabolites found were 2,4,6-trichlorophenoxyacetic acid (metabolite 3) and 2-(2,4,6-trichlorophenoxy)ethanol glucuronide conjugate (metabolite 7), which accounted for up to 80% of the urinary radiolabel. Five other metabolites were detected: 2-(2,4,6-trichlorophenoxy)ethanol (metabolite 2), 2-(3-hydroxy-2,4,6-trichlorophenoxy)ethanol (metabolite 8), N-2-(3-hydroxy-2,4,6-trichlorophenoxy)ethyl-N´-propylurea (metabolite 6), N-2-(4-hydroxy-2,6-dichlorophenoxy)ethyl-N’-propylurea (metabolite 5) and N-2-(2,4,6-trichlorophenoxy)ethyl-urea (metabolite 4). The metabolism proceeded via cleavage of the imidazole ring, giving rise to two-carbon fragments which were incorporated into the general metabolic pool, followed by hydroxylation of the phenyl ring and/or side-chain hydrolysis to form more polar compounds. The urinary metabolic profile of prochloraz showed no significant qualitative differences between male and female rats, mice and dogs and the female goat. The metabolism did differ quantitatively according to sex, female rats excreting more of the most polar metabolites than males, but no new metabolites were seen. The major plant metabolites, N-propyl-N-2-(2,4,6-trichlorophenoxy)ethyl-urea (metabolite 1) and 2,4,6-trichlorophenol, were not detected in the urine of dosed rats (Needham, 1982b).
In a study conducted in compliance with the principles of GLP (with QA certification), five male and five female rats were given [14C-U-phenyl]prochloraz (radiochemical purity, > 98.9%) at a dose of 5 mg/kg bw and two rats of each sex were given 100 mg/kg bw, by gavage. Urine and faeces were collected daily. Rats at 100 mg/kg bw were killed by cervical dislocation after 24 h, while those at 5 mg/kg bw were killed 4 days after dosing. The samples were analysed by liquid scintillation counting, thin-layer chromatography and high-performance liquid chromatography.
At both doses, prochloraz and its metabolites were rapidly and completely excreted in the urine and faeces, faecal excretion dominating at the low dose and urinary excretion dominating at the higher dose (Table 1). The main metabolic pathway (Figure 1) at both doses involved opening of the imidazole ring and initial loss of small fragments, which, with a considerable quantity of unchanged prochloraz, were the main compounds found in faeces. Further metabolism of metabolite 1 yielded metabolite 4, which was excreted mainly in faeces or further metabolized to metabolite 2 and then metabolite 3. The latter two compounds were excreted mainly in the urine in free or conjugated form. 2,4,6-Trichlorophenoxyacetic acid (metabolite 3) was the main constituent in urine. A small amount of this acid was further metabolized to the trichlorophenol, which was also excreted in urine. A minor metabolic pathway involved aromatic hydroxylation to give metabolite 5 and metabolite 8, which were excreted in small amounts in urine and faeces. Few differences in the metabolic profiles were seen according to dose, but quantitative differences by sex were apparent with respect to the urinary metabolites in animals at the low dose (Challis & Creedy, 1989; Needham, 1997).
Table 1. Excretion of an oral dose of 5 or 100 mg/kg bw prochloraz in 0–24 h urine and faeces of rats
|
Dose |
Sex |
% excreted in |
||
|
Urine |
Faeces |
Total |
||
|
5 |
Male |
30 ± 4.4 |
53 ± 6.1 |
83 ± 4.6 |
|
|
Female |
23 ± 6.9 |
64 ± 8.6 |
87 ± 1.7 |
|
100 |
Male |
33 ± 10 |
37 ± 15 |
70 ± 5.0 |
|
|
Female |
20 ± 7.0 |
29 ± 10 |
49 ± 17 |
From Challis & Creedy (1989) and Needham (1997)
Male and female rats were given [14C-phenyl-U]prochloraz (radiochemical purity: > 98%) at a single dose of 100 mg/kg bw by gavage. Urine, faeces and expired CO2 were collected. Four days after dosing, the rats were killed, and tissues were removed for analysis by liquid scintillation counting, thin-layer chromatography and high-performance liquid chromatography or gas–liquid chromatography.
Prochloraz underwent extensive metabolism, the primary route being opening of the imidazole ring followed by hydrolysis of the alkyl chain. The main metabolites were 3 and 2, which was present mainly as a glucuronide conjugate. Aromatic hydroxylation occurred to produce several minor metabolites. No unchanged prochloraz was excreted in the urine. The concentrations of tissue residues 96 h after dosing were generally < 1 mg equivalent per kg tissue. The highest concentrations were found in the liver (2.8–5.1 mg equivalent per kg) and kidney (1.5–2.1 mg equivalent per kg). The concentrations of residues in female rats were generally slightly higher than those in males. The metabolites were excreted quantitatively within 96 h, > 50% of the administered radiolabel being found in the 0–24-h excreta. Urinary excretion accounted for 65% of the dose in males and 41% in females (Needham & Challis, 1991).
Cows
In a study conducted in compliance with the principles of GLP (with QA certification), [14C-phenyl-U]prochloraz (radiochemical purity, 98.9%) was administered in gelatin capsules to a cow twice daily for 3 days, at a rate of 1.5 mg/kg bw per day. All milk was collected, and blood samples were taken. The cow was killed 16 h after the final dose, and tissue samples were collected post mortem.
The concentrations of total radiolabel in plasma rose at a constant rate throughout the first 8 h, until administration of the second dose and then continued to rise until they plateaud 72 h after the first dose. The profile of plasma metabolites varied with time. The proportions of products of imidazole ring cleavage (M2, M1 and N-2-(2,4,6-trichlorophenoxy)ethylurea) decreased from 34% of total plasma radiolabel at 8 h to 20% at 72 h (see Figure 2 for structures of M1 and M2). The phenolic metabolites (hydroxy-2,4,6-trichlorophenoxyethanol, N-2-(3-hydroxy-2,4,6-trichlorophenoxy)ethyl-N’-propylurea and 2,4,6-trichlorophenol) and the polar components (2,4,6-trichlorophenoxyacetic acid and baseline material) increased correspondingly. The concentrations of total radiolabel in milk rose to a sustained plateau level of about 0.14 µg equivalent per ml by 24 h, i.e. at the time of administration of the third dose. The metabolites in milk were primarily the phenolic compounds hydroxy-2,4,6-trichlorophenoxyethanol (58%) and N-2-(3-hydroxy-2,4,6-trichlorophenoxy)ethyl-N’-propylurea (8.7%), with 23% attributable to M2. The highest tissue concentration was found in liver (10 µg equivalent per g), with lower concentrations in kidney (1.7 µg equivalent per g), muscle (0.07 µg equivalent per g) and fat (0.2 µg equivalent per g). In the liver, 81% of the residue was characterized, consisting of M1 (13%), M2 (12%), N-2-(2,4,6-trichlorophenoxy)ethylurea (4.9%), hydroxy-2,4,6-trichlorophenoxyethanol (8.3%), N-2-(3-hydroxy-2,4,6-trichlorophenoxy)ethyl-N’-propylurea (6.5%), 2,4,6-trichlorophenol (19%), 2,4,6-trichlorophenoxyacetic acid (10%) and polar material (6.9%). The residues in fat comprised mainly M2 (66%) and M1 (19%), the non-polar products of imidazole-ring opening of prochloraz. No parent prochloraz was observed in any of the samples analysed. Samples of rumen and abomasal contents collected post mortem contained M2, M1, N-2-(2,4,6-trichlorophenoxy)ethylurea and 2,4,6-trichlorophenoxyacetic acid. The pattern of metabolites in bile was the same as that in the gut contents. However, in urine, 37% of the activity was accounted for by the phenolic metabolites (hydroxy-2,4,6-trichlorophenoxyethanol, N-2-(4-hydroxy-2,6-dichlorophenoxy)ethyl-N’-propylurea and N-2-(3-hydroxy-2,4,6-trichlorophenoxy)-ethyl-N’-propylurea), and 22% was polar and unidentified compounds, the remainder comprising the same metabolites as in bile. The dichlorophenyl metabolite N-2-(4-hydroxy-2,6-dichloro-phenoxy)ethyl-N’-propylurea was observed only in urine, none being present in any of the tissues or milk. Therefore, the metabolites in milk and tissues should break down to two common components when analysed by the standard method for residue analysis. The non-phenolic compounds yield trichlorophenol, and the phenolic compounds yield trichlororesorcinol.
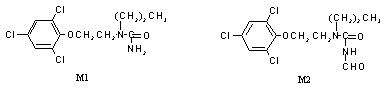
Figure 2. Structures of metabolites M1 and M2 in cows
M1, N-propyl-N-2-(2,4,6-trichlorophenoxy)ethylurea (metabolite 1 in Figure 1); M2, N-formyl-N'-propyl-N'-2-(2,4,6-trichlorophenoxy)ethylurea
The profile of metabolites of prochloraz seen in bovine tissues and milk was very similar to that in rats. No new metabolites were found (Phillips & Swalwell, 1989).
(c) Effects on enzymes and other biochemical parameters
Prochloraz was found to induce hepatic mixed-function oxidases in a series of investigations in rats and mice.
Prochloraz was administered to three groups of mice at a dose of 0, 10 or 100 mg/kg bw, by gavage twice daily for 4 days. A separate group of mice received phenobarbital at 80 mg/kg bw by intraperitoneal injection once daily for 4 days. Twenty-four hours after the last dose, all animals were killed, and a post-mitochondrial supernatant, containing microsomal enzymes, was isolated from each liver, and the content of protein and of cytochromes P450 and b5 was determined. At 100 mg/kg bw, prochloraz was a potent enzyme inducer, liver weight and microsomal protein and cytochrome contents all being significantly increased. At 10 mg/kg bw, only the cytochrome P450 and microsomal protein contents were increased. As the Soret peak of the cytochrome P450 difference spectrum was 450 nm, prochloraz is not a polycyclic aromatic hydrocarbon-type inducer, which have a Soret peak of 448 nm, and therefore similar to phenobarbital (Challis & Campbell, 1983).
Prochloraz was administered in the diet to groups of male and female mice at a concentration of 0, 80 (14 weeks), 320 (6 weeks) or 1300 (2 weeks) ppm. A separate group of male and female mice received four daily intraperitoneal doses of phenobarbital at 80 mg/kg bw. Twenty-four hours after the last treatment, all animals were killed, and a postmitochondrial supernatant, containing microsomal enzymes, was isolated from each liver and assayed for protein and cytochromes P450 and b5 content. Prochloraz was a potent inducer of the hepatic mixed-function oxidase system when given at 320 or 1300 ppm for 2 weeks, but a marginal effect was seen when it was administered at 80 ppm for periods in excess of 2 weeks, indicating that this concentration is at or close to the threshold dose for induction. There was no significant sex difference. The Soret peak of the cyrochrome P450 difference spectrum was 450 nm (Needham, 1983a).
Prochloraz was administered to three groups of six male rats at a dose of 0, 10 or 100 mg/kg bw by gavage twice daily for 4 days. A separate group of six males was given phenobarbital at a dose of 1 g/l in drinking-water for 7 days. Eighteen hours after the last dose, all animals were killed and a post-mitochondrial supernatant, containing microsomal enzymes, was isolated from each liver and assayed for aniline hydroxylase and para-nitroanisole demethylase activities and for the content of cytochromes P450 and b5. The relative weight of the liver and the microsomal protein content were increased at the highest dose. Prochloraz was a potent inducer of the hepatic microsomal enzyme system at 100 mg/kg bw, but a marginal effect was seen at 10 mg/kg bw, indicating that this dose is close to the threshold dose for induction. The spectrum of induction was similar to that caused by phenobarbital, the content of cytochromes P450 being increased at both doses (Needham, 1983b).
Similar induction of mixed-function oxidases was seen when male rats were fed diets containing prochloraz at a concentration of 2500 ppm for 7 days, whereas only a small increase was found with 100 ppm (Rivière, 1983).
The profile of the hepatic mixed-function oxidase system of male rats was examined after treatment with prochloraz and three of its major metabolites: M1, 2,4,6-trichlorophenoxy-ethanol and 2,4,6-trichlorophenoxyacetic acid. All animals were dosed twice daily with a solution or a suspension of a molar equivalent of prochloraz or its metabolites in corn oil for 4 days. A first group of rats were given prochloraz and the first two metabolites at a rate equivalent to 100 mg of prochloraz per kg bw, and 2,4,6-trichlorophenoxyacetic acid at a rate equivalent to 50 mg of prochloraz per kg bw, as the higher dose had been shown to have adverse effects. A second group of rats were dosed with all four compounds at a rate equivalent to 50 mg prochloraz per kg bw. Control animals received corn oil only, and another group received phenobarbital in drinking-water at 0.1% (w/v) for 14 days. A further group received b-naphthoflavone intraperitoneally at 80 mg/kg bw per day for 4 days, and hepatic microsomes were prepared from rats that had been induced with clofibrate by an intraperitoneal dose of 400 mg/kg bw per day for 3 days before the study. At the end of dosing, all animals were killed and a post-mitochondrial supernatant, containing microsomal enzymes, was isolated from each liver and analysed for protein and cytochromes P450 and b5 content and for the activities of 7-ethoxyresorufin-O-deethylase), 7-pentoxyresorufin-O-dealkylase, 7-ethoxycoumarin-O-deethylase, aldrin epoxidase and lauric acid hydroxylase.
The profile of induction caused by prochloraz reflected the contribution of the individual metabolites. The activity of lauric acid hydroxylase was slightly increased, but aldrin epoxidase was induced by sevenfold and 7-pentoxyresorufin-O-dealkylase by 14-fold. M1 was a phenobarbital-type inducer, increasing the activity of aldrin epoxidase by 120% and that of 7-pentoxyresorufin-O-dealkylase by eightfold. 2-(2,4,6-trichlorophenoxy)ethanol and 2,4,6-trichlorophenoxyacetic acid were both inducers of the clofibrate type, increasing the activity of lauric acid 12-hydrolase. The induction profile of prochloraz was mixed, but the predominant characteristics were those of a phenobarbital-type inducer (Needham et al., 1992).
(d) Effects of dog gastric juice and plasma on prochloraz
The stability of prochloraz and its plant metabolites M2 and M1 in the gastric juice and plasma of dogs was investigated in vitro. While both prochloraz and M1 were completely stable in gastric juice, M2 underwent slow hydrolysis to form M1 (< 10% in 2 h). After incubation in plasma, < 2% of the prochloraz present underwent hydrolysis (to yield M2), and about 20% of M2 was converted to M1. No 2,4,6-trichlorophenol was detected as a hydrolysis product in any of these experiments. The initial step in the metabolism of prochloraz is therefore likely to occur in the liver (Needham, 1980).
(i) Lethal doses
The results of studies to establish the LD50 and LC50 of prochloraz are summarized in Table 2.
Table 2. Acute toxicity of prochloraz
|
Species |
Strain |
Sex |
Route |
Vehicle |
LD50 |
LC50 |
GLP or QA |
Reference |
|
Mouse |
CD-1 |
Male |
Oral |
10% acacia solution |
2400 |
|
|
Shaw & Carter (1976) |
|
Rat |
Boots-Wistar |
Male and female |
Oral |
10% aqueous acacia solution |
1600–2400 |
|
GLP |
Shaw et al. (1979a) |
|
Rat |
CFY |
Male and female |
Oral |
10% aqueous acacia solution |
2400 |
|
GLP |
Shaw et al. (1979a) |
|
Dog |
Beagle |
Male and female |
Oral (gelatin capsules) |
|
NOAEL: 10 |
|
|
Morgan et al. (1978) |
|
Baboon |
|
Female |
Oral (gelatin capsules) |
|
NOAEL: 50 |
|
|
Morgan et al. (1977) |
|
Rat |
Wistar |
Male and female |
Inhalation (4 h, whole-body) |
Aerosol |
|
> 2.2a |
GLP & QA |
Jackson & Hardy (1987) |
|
Rat |
Sprague-Dawley |
Male and female |
Dermal |
|
> 2100 |
|
QA |
Hounsell & Ogle (1987) |
|
Rabbit |
New Zealand white |
Male and female |
Dermal |
|
> 3 ml/kg bw |
|
QA |
Kynoch et al. (1979) |
|
Rat |
CD |
Male |
Intraperitoneal |
Corn oil |
400–800 |
|
|
Smithson & Lancaster (1980) |
QA, quality assurance; GLP, good laboratory practice
a Maximum attainable concentration
Groups of 10 male CD-1 mice were given a single oral dose of 0, 400, 800, 1600 or 2400 mg/kg bw by gavage and observed for 7 days. Deaths occurred within 3 days after treatment at doses > 1600 mg/kg bw. No significant abnormality was noted in survivors of the dose of 2400 mg/kg bw, and the alanine aminotransferase activity in all treated mice was within normal limits, indicating that prochloraz was not acutely hepatotoxic at doses up to and including the LD50 (Shaw & Carter, 1976).
Groups of five male and five female Boots-Wistar and CFY rats were given a single oral dose of 800, 1600 or 2400 mg/kg bw, and a control group of each strain received the vehicle, 10% aqueous acacia solution. The rats were observed for 14 days. Overt signs of toxicity were apparent within 30 min at all doses and included piloerection and diarrhoea; rats at the two higher doses showed signs of central nervous system depression, adopted a hunched posture, had closed eyes and increased salivation, felt cool to touch, became ataxic and had slowed respiration. In addition, some animals had increased lachrymation, a wasted appearance and were tremorous and limp to touch; a few were excitable and one had convulsions. Generally, the CFY rats appeared to return to normal before the Boots-Wistar rats. At 1600 mg/kg bw, two Boots-Wistar rats of each sex and one male and two female CFY rats died; at 2400 mg/kg bw, three Boots-Wistar rats of each sex and two CFY rats of each sex died. All deaths occurred within 3 days of dosing. At autopsy, macroscopic evidence of gastrointestinal irritation and pale livers were observed. As gastrointestinal irritation was also observed after intraperitoneal administration, this effect may be systemic rather than local (Shaw et al., 1979b).
Five male and five fesmale rats were exposed for 4 h (whole body) to an aerosol containing prochloraz at the maximum attainable concentration of 2.2 mg/l air. The animals were observed for a further 14 days. There were no deaths. The clinical signs observed during exposure were partial closing of eyes, lachrymation, abnormal respiratory rate and/or movements and hunched posture. Brown staining around the snout and jaws, matted body fur and a waxy deposit or accretion on the tail were seen. Other treatment-related effects were transient reductions in body weight and food consumption in males (Jackson & Hardy, 1987).
Technical-grade prochloraz was applied at a dose of 2100 mg/kg bw for 24 h under occlusion to the shaved backs of five male and five female rats, and the animals were observed for 14 days. Five male and five female control rats were treated similarly with no test substance. No clinical signs, no adverse effects on body weight and no signs of irritation were observed. No deaths occurred during the study, and no abnormalities were seen post mortem (Hounsell & Ogle, 1987).
Two male and two female rabbits was treated dermally with prochloraz at a dose of 3 ml/kg bw, the maximum practical dosage. The material was applied to the shaved skin of each animal, and the site was occluded for 24 h. Moderate to severe dermal reactions characterized by erythema and oedema were observed, which generally began to heal from day 12 after treatment (Kynoch et al., 1979).
One male and one female beagle were given a single oral dose of 0, 10, 100 or 250 mg/kg bw. A clinical examination and haematological, biochemical and urinary analyses were conducted twice before dosing and on days 2 and 8 of the study. Body weight, food consumption, faecal appearance and occult blood content were recorded daily for 1 week before dosing and throughout the study. Emesis occurred 2.25–7 h after dosing in both animals given 100 mg/kg bw and in the male given 250 mg/kg bw. During this time, the females in these groups had diarrhoea. The male given 250 mg/kg bw was anorexic on days 1 and 2 and had lost weight by days 2 and 3. Serum alkaline phosphatase activity was elevated on days 2 and 8 in both animals given 250 mg/kg bw, and low potassium concentrations were seen in the male and a high reticulocyte count in the female (Morgan et al., 1978).
One female baboon was given single oral doses of prochloraz at 50 and 250 mg/kg bw in gelatin capsules on days 1 and 17, respectively. A female given empty capsules served as control. Body weight, food and water consumption and faecal appearance were recorded for 1 week before dosing and throughout the study. Haematological, biochemical and urinary analyses were conducted before dosing and on days 2, 8, 18 and 24, and both animals were killed and examined on day 25. Sections of liver and kidney and bone-marrow smears were examined histologically. Emesis and increased salivation were seen in the treated baboon 2–5 h after the higher dose. On day 24, it had a slightly lowered serum potassium concentration. Treatment had no effect on body weight, food or water consumption, faecal appearance or haematological parameters. Examination post mortem revealed no effect on organ weights and no pathological change related to treatment (Morgan et al., 1977).
Prochloraz was more toxic when administered parenterally to rats than when given orally, the LD50 after intraperitoneal injection being 400–800 mg/kg bw. Groups of five male Charles River CD rats were given a single intraperitoneal dose of 50, 100, 200, 400, 800 or 1600 mg/kg bw of a solution of prochloraz in corn oil, and a control group received the vehicle alone. Overt signs of toxicity were apparent within 0.5–1 h after dosing at > 100 mg/kg bw. All these rats showed evidence of central nervous system depression (coma, prostration, lethargy, sedation, inactivity); other signs included ataxia, piloerection, partially closed eyes, irregular respiration, limpness and coolness to touch, cyanosis, adoption of a hunched position, walking on tiptoe, increased nasal exudate, increased lachrymation and salivation, dark staining around the eyes, excitability, urine stains around the genital region, diarrhoea, tremorous and/or convulsive movements and straub tail. No overt signs of toxicity were seen in animals given 50 mg/kg bw. Rats given 100 mg/kg bw recovered overnight after dosing, those given 200 mg/kg bw recovered by day 3, those given 400 mg/kg bw recovered by day 5 and the survivor given 800 mg/kg bw had generally recovered by day 4, although piloerection and excitability persisted throughout the 14-day observation period. Two rats given 400 mg/kg bw, four given 800 mg/kg bw and all given 1600 mg/kg died within 24 h of dosing. The macroscopic findings at autopsy included evidence of gastrointestinal irritation and an oily liquid, probably the injected solution, surrounding some viscera. At autopsy on day 15, white globules were found within the mesentery in the abdomens of most animals (Smithson & Lancaster, 1980).
(ii) Dermal and ocular irritation and dermal sensitization
The dermal irritation potential of technical-grade prochloraz (purity, 96.3%) was tested in three male and three female New Zealand white rabbits to which 0.5 ml of test material was applied under a gauze patch and covered with occlusive tape for 4 h. At the end of this period, the patch was removed, and skin reactions were assessed 48 and 72 h later. No irritation was seen at treated sites at any time during the study. No clinical signs were seen. A quality assurance statement was included (Cuthbert & D’Arcy Burt, 1984a).
The ocular irritation potential of technical-grade prochloraz (purity, 96.3%) was tested in three male and three female New Zealand white rabbits to which 0.1 ml of test material was administered onto the lower eyelid of the right eye. The lids were then gently held together for 1–2 s. The other eye remained untreated to serve as a control. The eyes were examined for irritation under standard illumination, and any ocular reactions were recorded 1, 24, 48 and 72 h after instillation. No corneal or iridal response was seen. Slight conjunctival responses (redness, score 1) were seen only 1 h after instillation. The eyes were normal after 24 h. No clinical signs were seen. A quality assurance statement was included (Cuthbert & D’Arcy Burt, 1984b).
The skin sensitization potential of prochloraz (purity unspecified) was assessed in 20 female guinea-pigs by the method of Magnusson & Kligman (1969, 1970), which consists of induction with intradermal injections (20% dilution in ethanol) of the test material, followed after 1 week by topical application of undiluted test compound and a challenge 3 weeks after induction with undiluted test compound or a 50% dilution in ethanol. None of the animals showed any dermal reaction either 24 or 48 h after challenge. Therefore, there was no evidence that prochloraz acts as a delayed dermal sensitizer in guinea-pigs. The study was conducted before GLP regulations were issued in the testing facility, but both the protocol and test results were well reported (Shaw, 1979).
(b) Short-term studies of toxicity
Mice
Technical-grade prochloraz (purity, 95.29%) was suspended in 10% aqueous acacia and given by gavage to groups of five male and five female CFLP mice at a dose of 96, 240 or 600 mg/kg bw per day for 21 days; a control group received the vehicle alone. Overt signs of toxicity were recorded daily and body weight once weekly throughout the study. All mice were killed at the end of the dosing period, and plasma samples were collected for estimation of alanine aminotransferase activity; the mice were then dissected and examined macroscopically. The livers of all mice, the duodena from mice showing macroscopic abnormalities in the liver and any organ showing macroscopic abnormalities were examined microscopically. The study was conducted before GLP regulations were issued in the testing facility, but the protocol and results were well reported. The study is considered to provide useful additional information.
Overt signs of toxicity occurred in males given 600 mg/kg bw per day; these included loss of condition, evidence of central nervous system depression (sedation or inactivity), piloerection and coolness to touch. Four males and one female given 600 mg/kg bw per day died during the study, but two of these deaths were attributable to administration accidents. The body-weight gain of males surviving 600 mg/kg bw per day was reduced during the first 2 weeks of the study, but that of females given 240 or 600 mg/kg bw per day was increased throughout dosing. Plasma alanine aminotransferase activity was increased in mice of each sex surviving 600 mg/kg bw per day. Hyperkeratinization of the forestomach was seen in three males and all females at 600 mg/kg bw per day, in one male at 240 mg/kg bw per day and in one female control. No microscopic changes that could be related to treatment were observed in the liver, although there was a high incidence of minor background lesions in all groups. The authors concluded that, as the dose of 600 mg/kg bw per day was not tolerated by the males, the appropriate highest dose for the 90-day study would be 400 mg/kg bw per day (Lancaster & Shaw, 1980a).
In a study conducted in compliance with the principles of GLP (with QA certification), groups of 15 male and 15 female CD-1 mice were given diets containing technical-grade prochloraz (purity, 98.2%) at concentrations providing a dose of 6, 25, 100 or 400 mg/kg bw per day for 13 weeks. A control group comprised 24 mice of each sex. Further groups of 15 male and 15 female controls and mice treated at 400 mg/kg bw per day were kept for 4 weeks after the end of dosing, and, in addition, groups of nine males and nine females were treated for 6 weeks. Solutions of the test material were prepared in corn oil. Overt signs of toxicity were recorded daily, and body weight and food consumption were recorded three times weekly throughout the study. Haematological and blood biochemical analyses were conducted at 6 and 13 weeks and after 4 weeks on control diet. All mice were killed at the end of dosing or the recovery period and were dissected and examined macroscopically, and the main organs were weighed. A comprehensive microscopic examination of tissues from 10 male and 10 female controls and all mice at 400 mg/kg bw per day killed at 13 weeks was carried out, and the livers of all remaining animals killed at 13 weeks and all mice killed after 4 weeks on control diet were examined.
No deaths occurred that were attributable to treatment. At 400 mg/kg bw per day, the incidence of piloerection was increased. Weight gain was slightly reduced in both sexes at 400 mg/kg bw per day and in males at 100 mg/kg bw per day. Food consumption was higher in both sexes at the highest dose throughout dosing and during the first week of the recovery period, but thereafter was similar to that of controls.
At week 6, the haemoglobin concentration, packed cell volume and erythrocyte count were increased in both sexes at 400 mg/kg bw per day; mean corpuscular haemoglobin was also marginally increased in males, and the leukocyte count was increased in females due to lymphocytosis. These parameters were no longer affected by treatment at week 13 or after 4 weeks’ recovery. The total leukocyte count was increased in females at the highest dose at weeks 6 and 13. Plasma alanine aminotransferase activity was increased at week 6 in some mice of each sex at 400 mg/kg bw per day and in some males at 100 mg/kg bw per day; at week 13, it was increased in the majority of females at 400 mg/kg bw per day and in some mice of each sex at 100 mg/kg bw per day; after 4 weeks on control diet, some males at 400 mg/kg bw per day were still affected. At weeks 6 and 13, the plasma albumin concentration and, consequently the albumin:globulin ratio were slightly reduced in both sexes at 400 mg/kg bw per day and in males at 100 mg/kg bw per day. At week 13, the plasma glucose concentration were increased and the blood urea nitrogen concentration slightly decreased in both sexes at 400 mg/kg bw per day.
At autopsy at week 6, the weights of the liver was increased in both sexes at 100 or 400 mg/kg bw per day and were slightly increased in females at 25 mg/kg bw per day. The ovaries of some females at the highest dose and in most at 100 mg/kg bw per day were small, and the prostate and seminal vesicles of males at 400 mg/kg bw per day were small. The weight of the spleen was increased in both sexes at 100 mg/kg bw per day and in a few females at 25 mg/kg bw per day, but was low in most females at 6 mg/kg bw per day (this decrease was considered not to be related to treatment). At week 13, the weight of the liver was increased in both sexes at 25, 100 or 400 mg/kg bw per day. The ovaries were small in females and the prostate and seminal vesicles small in males at 400 mg/kg bw per day. After 4 weeks’ recovery, the liver weight was still increased in both sexes at the highest dose, but to a much lesser extent than at weeks 6 and 13. The livers of some mice at 400 mg/kg bw per day appeared to be enlarged, and the lobular pattern was prominent. Histopathological examination showed treatment-related changes only in the liver. Minimal liver changes, with loss of glycogen and periportal fat droplets, were seen in both sexes at the highest dose and in females at 100 mg/kg bw per day after 13 weeks. Some males at the two higher doses also showed centrilobular hepatocyte enlargement. In mice killed after 4 weeks’ recovery, none of these changes was present.
The NOAEL was 6 mg/kg bw per day on the basis of effects on the liver at the next higher dose. The hepatic effects were reversible (Gale, 1980; Lancaster, 1982; Keene, 1988a; Mallyon, 1988a).
Rats
Technical-grade prochloraz (purity, 92.7%) was emulsified in aqueous acacia and given by gavage to groups of five male and five female Boots-Wistar rats at a dose of 25, 100 or 400 mg/kg bw per day for 30 days; a control group of 10 males and 10 females received the vehicle alone. Overt signs of toxicity were recorded daily and body weight and food consumption three times weekly, throughout the study. Haematological, blood biochemical and urinary analyses were conducted during week 4. All rats were killed at the end of the dosing period, and serum samples were collected for estimation of enzyme activity and electrolyte content; the rats were then dissected and examined macroscopically, and the main organs were weighed. A comprehensive histological examination of tissues from half the controls and all the rats at 400 mg/kg bw per day was completed; in addition, the liver, lymph nodes, spleen and bone-marrow smears from the remaining controls and rats given 25 or 100 mg/kg bw per day were examined. The study was conducted before GLP regulations were issued in the testing facility, but the protocol and results were well reported. The study is considered to provide useful additional information.
Increased salivation was seen in both sexes given 100 or 400 mg/kg bw per day, and slight loss of condition was noted in females at 400 mg/kg bw per day. Slight weight loss and decreased food intake were seen at the start of treatment in both sexes at 400 mg/kg bw per day, and food consumption was also marginally reduced in both sexes given 100 mg/kg bw per day. The erythrocyte count was decreased in some rats of each sex at 400 mg/kg bw per day and in some females at 100 mg/kg bw per day; the haemoglobin concentration was also slightly decreased in one rat of each sex at 400 mg/kg bw per day. The mean corpuscular volume was high in some animals at 400 mg/kg bw per day. The leukocyte count was increased due to neutrophilia and lymphocytosis in females at 400 mg/kg bw per day. Urine flow was increased and the specific gravity before water was withheld was decreased in some rats of each sex at 400 mg/kg bw per day and in one male at 100 mg/kg bw per day. Urinary aspartate aminotransferase activity was slightly increased in females, and the urinary pH was reduced in a few rats at 400 mg/kg bw per day. The weight of the liver was increased in both sexes at 100 and 400 mg/kg bw per day, the females tending to be more severely affected. The weight of the adrenals was increased, and the prostate and seminal vesicles were small in the males at 100 and 400 mg/kg bw per day. The only finding possibly related to treatment observed at microscopic examination was slight haematopoiesis in the spleen in most females at 25 mg/kg bw per day and in both sexes at 100 and 400 mg/kg bw per day, although this change was also seen in one male and three female controls. The authors concluded that, because the dose of 100 mg/kg bw per day was tolerated by both sexes, it would be the appropriate highest dose for the 90-day study. The increased salivation may have been related to the route of administration, as similar effects were not seen after dietary administration (Lancaster & Shaw, 1980b).
In a study conducted before GLP regulations were issued in the testing facility but which complied with the general principles of GLP and the final report of which was reviewed for QA, groups of 20 male and 20 female Boots-Wistar rats were given technical-grade prochloraz (purity, 97.5%) at a dose of 6, 25 or 100 mg/kg bw per day by gavage in 10% aqueous acacia suspension for 13 weeks; a control group of 20 males and 20 females received the vehicle alone. Further groups of 20 male and 20 female controls and rats at 100 mg/kg bw per day were kept for 4 weeks after the end of the dosing period. In addition, groups of 10 male and 10 female controls and rats at 6, 25 or 100 mg/kg bw per day were examined after 6 weeks. Overt signs of toxicity, body weight and food consumption were recorded daily throughout the study; haematological, blood biochemical and urinary analyses were conducted at 6 and 13 weeks and after 4 weeks’ recovery. All rats were killed at the end of the dosing or recovery period, and serum samples were collected for estimation of enzyme activity and electrolyte content; the rats were dissected and examined macroscopically, and the main organs were weighed. A comprehensive histological examination of tissues from half the controls and all rats at 100 mg/kg bw per day and killed at 13 weeks was carried out; in addition, the livers from all remaining animals were examined. The investigation was extended to quantify the size of liver cells by counting the number of nuclei in 10 periportal and 10 centrilobular fields of the left lateral lobe of the liver from all rats killed at weeks 6 and 13 and after 4 weeks’ recovery.
Rats of each sex at 25 and 100 mg/kg bw per day and a few at 6 mg/kg bw per day showed increased salivation. The incidence of diarrhoea was increased during the first 4 weeks of dosing in males at 100 mg/kg bw per day and possibly during the first week of dosing in males at 25 mg/kg bw per day. In females, a few instances of diarrhoea were recorded at each dose. Body-weight gain was increased in females at 25 and 100 mg/kg bw per day during dosing. During the first 5 days of the recovery period, females at 100 mg/kg bw per day lost a small amount of weight, the overall weight gain being statistically significantly less than that of the controls. Food consumption was unaffected by treatment except for a slight increase during the recovery period in males at 100 mg/kg bw per day.
At week 6, the haemoglobin concentration was slightly decreased in males at each dose and in females at 100 mg/kg bw per day; the leukocyte count was increased, due to lymphocytosis, in all treated males. At week 13, the mean corpuscular volume was slightly decreased in all treated males and in females at 100 mg/kg bw per day, and the haemoglobin concentration was slightly decreased in females at 25 and 100 mg/kg bw per day and in males at 100 mg/kg bw per day after the 4-week recovery period. The serum bilirubin concentration was slightly decreased in all treated rats at week 6, in males at 25 and 100 mg/kg bw per day and in females at 6 and 100 mg/kg bw per day at week 13, and in a few treated rats of each sex after the 4-week recovery period. The serum potassium concentration was slightly increased at week 6 in males at 25 and 100 mg/kg bw per day and in three females at 100 mg/kg bw per day. At week 6, urinary aspartate aminotransferase activity was slightly increased in a few rats of each sex at each dose. At week 13, the specific gravity or urine before water was withheld and sodium excretion were slightly decreased in both sexes at 100 mg/kg bw per day, urinary aspartate aminotransferase activity was increased in males at 100 mg/kg bw per day, urinary pH was reduced in some females at 25 and 100 mg/kg bw per day and the urinary protein content was reduced in all treated females and in males at 100 mg/kg bw per day. After 4 weeks’ recovery, potassium excretion and urinary pH were slightly reduced in females given 100 mg/kg bw per day.
At autopsy at week 6, the weight of the liver was increased in both sexes at 100 mg/kg bw per day and in females given 6 or 25 mg/kg bw per day, and the kidney weight was increased in males at 100 mg/kg bw per day and in both sexes at 25 mg/kg bw per day. The prostate and seminal vesicles were smaller than usual in males and the ovaries were larger in females at 100 mg/kg bw per day. The thyroid weight was increased in all treated females. At week 13, the weight of the liver was increased in males at 6 mg/kg bw per day and in both sexes at 25 and 100 mg/kg bw per day; the kidney weight was increased in both sexes at 100 mg/kg bw per day, the spleen weight was slightly decreased in all treated males, the ovary weight was increased in all treated females; and the thyroid weight was increased in females at 6 and 100 mg/kg bw per day. After 4 weeks’ recovery, the weights of the liver, ovaries and thyroids of the females were slightly greater than the control values. At weeks 6 and 13 and after 4 weeks’ recovery, the centrilobular liver cells were larger than the periportal cells in control rats of each sex, reflecting the normal morphology of the liver. This difference was also seen in all treated groups, irrespective of changes in liver weight or cell size. At week 6, the liver cell size was increased in females at 6 and 25 mg/kg bw per day and in both sexes at 100 mg/kg bw per day. At week 13, the liver cell size was increased in both sexes given 6, 25 or 100 mg/kg bw per day. After 4 weeks on control diet, the liver cell size was still increased in males but had returned to normal or was even decreased in comparison with that in controls in females. The changes in liver cell size generally correlated closely with increases in liver weight. The only instance in which such a correlation was not apparent was after 4 weeks’ recovery, when, in treated males, the liver cell size was still slightly increased, even though there was no change in liver weight, and in treated females, the liver cell size was decreased although the liver weight was slightly increased.
Various treatment-related effects were seen in all groups, the most important, apparently reversible, effects being increased liver weight and hepatocyte size. A NOAEL could not be identified (Lancaster & Shaw, 1979; Shaw et al., 1979b; Jackson, 1988a; Markham, 1988a).
Dogs
Groups of two male and two female beagle dogs were given technical-grade prochloraz (purity, 96.6%) suspended in a 5% aqueous acacia solution and administered by gastric intubation at a dose of 10 mg/kg bw per day in a volume of 0.3 ml/kg bw or 40 mg/kg bw per day in a volume of 1.2 ml/kg bw, for 14 days. A group of two males and two females receiving 5% acacia solution at the higher dose volume served as controls. Body weight, food consumption and faecal appearance as well as occult blood content were recorded daily for 1 week before dosing and throughout the study. Overt signs of toxicity were recorded throughout dosing. Comprehensive haematological and blood biochemical analyses were conducted once before dosing and on day 14; additional blood samples were collected on days 3 and 8 for estimation of bilirubin concentration and the activity of certain serum enzymes. The dogs were killed the day after the final dose, dissected and examined macroscopically. The main organs were weighed, and sections of selected organs and tissues were examined histologically. The study was conducted before GLP regulations were issued in the testing facility, but the protocol and results were well reported. The study is considered to provide useful additional information.
Dogs given 40 mg/kg bw per day usually vomited within 1 h after dosing and had diarrhoea within 2 h. Because of the emesis, the daily dose was divided and administered in two equal volumes morning and afternoon to each dog from day 6; thereafter, the incidence of vomiting decreased and, as for the diarrohea, usually occurred only after the morning dose. In addition, increased salivation was seen sporadically in some dogs at both doses. Both males at 40 mg/kg bw per day were intermittently anorexic and lost weight during the study.
On day 14, in animals at 40 mg/kg bw per day, low values were recorded for erythrocyte count, haemoglobin concentration and erythrocyte volume fraction in one female, for haemoglobin concentration in the other female and for erythrocyte count in one male. Serum alkaline phosphatase activity increased progressively throughout the dosing period in both males and one female at the higher dose, and two of these animals also had very slight increases in serum leucine aminopeptidase activity.
At autopsy on day 15, the livers of all dogs at 40 mg/kg bw per day appeared large and bulbous, and the absolute and relative weights in these dogs and the relative weight in one female at 10 mg/kg bw per day were increased. The prostates of both males at 40 mg/kg bw per day and the testes of one of these appeared small and flaccid; the testes had spermatid giant cells. In addition, copious amounts of mucus were seen in the stomachs of one male at the higher dose and both males at the lower dose, and a cream–grey layer was observed on the mucosal surface of the ileum in one male at each dose; furthermore, one female at the higher dose had microscopic evidence of gastritis. Microscopic examination revealed no other change that was considered to be directly related to treatment.
The authors concluded that a single oral dose of 40 mg/kg bw per day would be unacceptable in a long-term study and a single dose of 20 mg/kg bw per day would be well tolerated. This dose was chosen as the highest dose for the 90-day study. The NOAEL was 10 mg/kg bw per day on the basis of the increase in alkaline phosphatase activity. Emesis, which was observed in dogs after both single and short-term intake, was considered not to be a relevant end-point, because it was observed after administration of high doses of prochloraz by capsule or gastric intubation. Dogs are known to be sensitive to nonspecific emetic effects that are not necessarily related to a specific test substance but simply to the stress of administration or direct gastrointestinal discomfort (Morgan et al., 1979).
In a study conducted before GLP regulations were issued in the testing facility but which complied with the general principles of GLP and for which the final report was reveiewed for QA, groups of four male and four female beagle dogs were given technical-grade prochloraz (purity, 97.9%) suspended in a 10% aqueous acacia solution at a dose of 1, 2.5, 7 or 20 mg/kg bw per day by gastric intubation for 13 weeks. A group of four dogs of each sex receiving the vehicle served as controls. Additional groups of four male and four female controls and dogs at 20 mg/kg bw per day were retained for 4 weeks with no treatment at the end of dosing. Overt signs of toxicity, body weights, food consumption and faecal appearance were recorded daily throughout the study. At weeks 6 and 13, a clinical examination, including ophthalmoscopy and electrocardiography, and haematological, blood biochemical, faecal and urinary analyses were conducted; the parameters that were affected at week 13 were measured again at the end of the 4-week recovery period. On each occasion, parameters with values outside normal limits were measured again 1 week later, and serum alkaline phosphatase and leucine aminopeptidase activities were estimated in all dogs at week 9. All dogs were killed at the end of the dosing or recovery period, dissected and examined macroscopically, the main organs were weighed, and a comprehensive histological examination was carried out.
The only overt signs of toxicity were isolated bouts of emesis and increased salivation in some dogs in all groups, including controls, although these were more prevalent at the highest dose. In addition, all dogs at 20 mg/kg bw per day and one at 7 mg/kg bw per day occasionally produced yellow mucoid and/or liquid faeces. During the recovery period, no overt signs of toxicity were observed. Body-weight gain was comparable in control and treated groups. Reduced food consumption was recorded for two dogs at 20 mg/kg bw per day. Clinical examination at week 13 revealed that four dogs at 20 mg/kg bw per day had apparently small and/or flaccid testes; in two of these dogs that were allowed to recover, the change was still apparent at week 17. No treatment-related effects were detected by ophthalmoscopy or electrocardiography.
There was no definite treatment-related effect on haematological parameters. Serum alkaline phosphatase activity was high during dosing in most dogs at 20 mg/kg bw per day and in some at 7 mg/kg bw per day; in those dogs retained for recovery, the activity returned to within normal limits. Serum leucine aminopeptidase activity was very slightly increased at week 6 in four dogs at 20 mg/kg bw per day and in one at 7 mg/kg bw per day. The activity had returned to within normal limits by week 9 or 13. There was no effect of treatment on urinary parameters.
At necropsy, all dogs at 20 mg/kg bw per day and three at 7 mg/kg bw per day had large, heavy livers; this effect was apparent in only one treated dog at the end of the recovery period. Low prostate weight was recorded in three dogs at the highest dose and in two other dogs after the recovery period; one of these killed at week 14 also had a low testes weight. In addition, one dog at 7 mg/kg bw per day had a low relative prostate weight. In two females at the highest dose that were killed at week 14 and in one killed at week 18, a clear brown exudate from freshly sectioned mammary glands was observed. The only histological finding of note was apparent immaturity of the testes of some dogs at 20 mg/kg bw per day. Pre-terminal clinical examination revealed small and/or flaccid testes in four of the eight dogs. Histological changes that were indistinguishable from immaturity were observed in one of two dogs killed after 13 weeks, and comparable findings were made in another dog which had shown no associated clinical signs. At the end of the recovery period, the testes of all dogs appeared normal, indicating that the change was reversible. Counts of liver nuclei revealed no statistically significant differences in liver cell size between controls and animals at the highest dose.
The NOAEL was 2.5 mg/kg bw per day on the basis of effects on alkaline phosphatase and leucine aminopeptidase activity, liver size and weight and prostate and testes weights at the next highest dose. These effects were reversible (Lancaster et al., 1979; Lancaster, 1980; Keene, 1988b; Markham, 1988b; Jackson, 1989).
(c) Long-term studies of toxicity and carcinogenicity
Mice
In a study conducted in compliance with the principles of GLP (with QA certification), groups of 52 male and 52 female CD-1 mice were fed diets containing technical-grade prochloraz (purity, 95.4–96.8%) at a concentration of 78, 325 or 1300 ppm, corresponding to mean achieved intakes of 7.5, 33 and 130 mg/kg bw per day for males and 8.8, 36 and 150 mg/kg bw per day for females. The control group comprised 104 mice of each sex which received a diet containing the vehicle (corn oil) at the same concentration as that admixed with the highest concentration of test compound. Males were treated for 106 weeks and females for 121 weeks. All animals were observed daily, body weight and food consumption were determined weekly, and masses were palpated every 2 weeks. Haematological investigations were carried out at week 52 and at termination and included packed cell volume, haemoglobin and erythrocyte, reticulocyte, total leukocyte and differential leukocyte counts. Treatment was terminated in males when the survival rate of those at 325 ppm was 25% and in females when the survival rate of those at 78 ppm was 27%. All mice were killed by asphyxiation with CO2, and a full post-mortem was carried out. The brain, heart, kidneys, liver, testes and spleen were weighed, and a large number of tissues were selected and preserved in neutral buffered formalin. All tissues were examined microscopically.
Some mice of each sex given 1300 ppm and some males given 325 ppm were killed because their abdomens were distended. These animals were subsequently found to have multiple liver tumours. No other overt signs of toxicity considered to be related to treatment were observed. There were no differences in survival rates on the basis of trend analysis in either sex; however, male mice at 325 ppm showed a pairwise increase in mortality rate. The percentage survival was 52%, 50%, 25% and 48% respectively in control males and those at the low, medium and high dietary concentrations and 30%, 27%, 40% and 29% in females, respectively. Mice receiving 1300 ppm gained less weight than control animals (decrease of 24% in males and 11% in females), but this was statistically significant in males only. Males at 325 ppm also gained less weight (decrease of 12%). Minimally greater food consumption than by controls was recorded for all treated males and females at 78 or 325 ppm.
Haematological investigations at week 52 showed no differences between treated and control mice that were considered to be of toxicological significance. At week 121, slightly lower haemoglobin concentrations were recorded in treated female mice, and lower total leukocyte counts due to lower lymphocyte counts were found in some females in all treated groups, particularly those receiving 1300 ppm.
A dose-related increase in the incidence of macroscopic liver masses was observed in mice of each sex that died during the study or were killed at termination. Livers heavier than those of controls were seen in mice of each sex receiving 1300 ppm; however, most of these livers contained tumours. In males and females at 325 and 1300 ppm, an increased incidence of liver adenomas and carcinomas was observed when compared with controls, and the difference was statistically significant (Tables 3 and 4). The statistical significance, derived from trend tests by the method of Peto et al. (1980) of the number of tumour-bearing animals, was p < 0.001 for carcinomas and for any liver tumour in males at 325 and 1300 ppm and females at 1300 ppm and p < 0.01 for any liver tumours in females at 325 ppm. There was an associated increase in the numbers of males and females with more than one liver tumour. In some mice at 1300 ppm, multiple tumours contributed to death. In males, the first adenoma appeared at 52 weeks and the first carcinoma at 62 weeks, while in females the first tumours were observed at 89 weeks. The approximate time of onset can be assessed from the median time of death. The data (Table 5) show no indication of an effect on liver tumour latency in either sex. Females at 78 ppm had a slightly higher incidence of liver tumours than controls, although this difference was not statistically significant and the incidence was not significantly different from that expected by linear extrapolation from the dose–response relationship at higher doses. In males at 78 ppm, the incidence of liver tumours was similar to that of controls; however, the incidence of liver tumours in male controls (36%) was unusually high, as the incidences in 24-month-old CD-1 male mice at the same laboratory ranged from 0–17% for adenomas and 1.4–14% for carcinomas. The incidence of liver tumours in control females of the same strain in six concurrent studies of 104–108 weeks’ duration was 0–12%. Thus, the number of liver tumours in females given 78 ppm in the present study could occur spontaneously in CD-1 mice. No treatment-related effect was detected on the incidence of tumours at any other site or any of the non-neoplastic changes recorded. Liver-cell tumours contributed to the deaths of more males (20/52) and females (14/52) at 1300 ppm and more males at 325 ppm (13/52) than controls (9/104 males and 1/104 females). Many of these mice were in poor clinical condition when killed. The NOAEL was 78 ppm, equal to 7.5 mg/kg bw per day (Sharp, 1982; Colley et al., 1983, 1988; Offer et al., 1992; Malarkey, 1993a).
Table 3. Incidences of liver tumours in mice that died or were killed at the end of a 2-year study of carcinogenicity
|
Time of kill |
Dietary concentration |
Sex |
No. examined |
No. mice with tumours |
|||||||
|
All tumours |
Malignant tumoursa |
||||||||||
|
1 |
2 |
> 3 |
Total |
1 |
2 |
> 3 |
Total |
||||
|
Interim |
0 |
Male |
50 |
12 |
2 |
0 |
14 |
9 |
2 |
0 |
11 |
|
78 |
26 |
6 |
2 |
1 |
9 |
2 |
0 |
1 |
3 |
||
|
325 |
38 |
12 |
5 |
3 |
20 |
7 |
5 |
2 |
14 (3 M) |
||
|
1300 |
|
27 |
9 |
5 |
9 |
23 |
7 |
4 |
6 |
17 |
|
|
0 |
Female |
73 |
2 |
0 |
0 |
2 |
1 |
0 |
0 |
1 |
|
|
78 |
38 |
3 |
1 |
0 |
4 |
0 |
0 |
0 |
0 |
||
|
325 |
31 |
2 |
1 |
0 |
3 |
0 |
0 |
0 |
0 |
||
|
1300 |
37 |
8 |
2 |
15 |
25 |
0 |
0 |
7 |
7 |
||
|
Termination |
0 |
Male |
54 |
22 |
1 |
0 |
23 |
4 |
1 |
0 |
5 |
|
78 |
26 |
9 |
0 |
3 |
12 |
1 |
0 |
2 |
3 |
||
|
325 |
14 |
5 |
1 |
2 |
8 |
1 |
0 |
2 |
3 (1 M) |
||
|
1300 |
25 |
4 |
4 |
13 |
21 |
0 |
2 |
5 |
7 |
||
|
0 |
Female |
31 |
3 |
0 |
0 |
3 |
0 |
0 |
0 |
0 |
|
|
78 |
14 |
1 |
1 |
0 |
2 |
0 |
0 |
0 |
0 |
||
|
325 |
21 |
2 |
4 |
2 |
8 |
1 |
0 |
0 |
1 |
||
|
1300 |
15 |
5 |
5 |
4 |
14 |
1 |
1 |
0 |
2 |
||
From Sharp (1982), Colley et al. (1983, 1988), Offer et al. (1992) and Malarkey (1993a). M, metastasizing
a Including mice with more than one tumour, at least one of which was malignant
Table 4. Incidences of liver tumours in mice in the 2-year study of carcinogenicity
|
Dietary concentration (ppm) |
Sex |
Incidence of liver tumours |
||||||
|
Adenomas |
Carcinomas |
Total |
||||||
|
Interim kill |
Terminal kill |
Total |
Interim kill |
Terminal kill |
Total |
|
||
|
0 |
Male |
3/50 |
18/54 |
21/104 |
11/50 |
5/54 |
16/104 |
37/104 |
|
78 |
|
6/26* |
9/26 |
15/52 |
3/26 |
3/26 |
6/52 |
21/52 |
|
325 |
|
6/39 |
5+13 |
11/52 |
14/39 |
3/13 |
17/52** |
28/52* |
|
1300 |
|
6/27* |
14/25* |
20/52** |
17/27*** |
7/25* |
24/52*** |
44/52*** |
|
0 |
Female |
1/73 |
3/31 |
4/104 |
1/73 |
0/31 |
1/104 |
5/104 |
|
78 |
|
4/38* |
2/14 |
6/52 |
0/38 |
0/14 |
0/52 |
6/52 |
|
325 |
|
3/31 |
7/21* |
10/52** |
0/31 |
1/21 |
1/52 |
11/52** |
|
1300 |
|
18/37*** |
12/15*** |
30/52*** |
7/37** |
2/15 |
9/52*** |
39/52*** |
From Sharp (1982), Colley et al. (1983, 1988), Offer et al. (1992) and Malarkey (1993a). The incidences of liver tumours in females of the same strain in six concurrent studies of 104–108 weeks’ duration were 1/60, 1/60, 5/100, 5/51, 3/52 and 0/55 adenomas and 0/60, 0/60, 1/100, 1/51, 1/52 and 2/55 carcinomas.
* p < 0.05; ** p < 0.01; p < 0.001, Fisher exact test for comparisons of numbers of tumour-bearing animals in treated groups with those in the control group; one-tailed values
Table 5. Median time to death (weeks) of liver tumour-bearing mice in the 2-year study of carcinogenicity
|
Dietary concentration |
Sex |
Liver tumour |
||
|
Adenoma |
Carcinoma |
Any |
||
|
0 |
Male |
108 |
95 |
107 |
|
78 |
|
107 |
101 |
107 |
|
325 |
|
102 |
93 |
94 |
|
1300 |
|
107 |
102 |
107 |
|
0 |
Female |
123 |
89 |
122 |
|
78 |
|
116 |
– |
116 |
|
325 |
|
123 |
123 |
123 |
|
1300 |
|
116 |
115 |
116 |
From Sharp (1982), Colley et al. (1983, 1988), Offer et al. (1992) and Malarkey (1993a)
Rats
In a study conducted in compliance with the principles of GLP (with QA certification), groups of 60 male and 60 female Sprague-Dawley (CD) rats were fed a diet containing technical-grade prochloraz (purity, 95.1–97.0%) at a concentration of 38, 150 or 625 ppm, corresponding to mean achieved intakes of 1.3, 5.1 and 22 mg/kg bw per day for males and 1.6, 6.4 and 28 mg/kg bw per day for females. The control group comprised 120 rats of each sex; they received a diet containing the vehicle (corn oil) at the same concentration as that admixed with the highest concentration of test compound. Males were treated for 115 weeks and females for 111 weeks. Separate groups of 20 male and female rats were killed after 52 weeks of treatment, and additional groups of controls and rats at the highest concentration, consisting of 10 animals of each sex, were killed after 13 weeks of treatment. All animals were observed daily, body weights and food consumption were recorded weekly, and masses were palpated every 2 weeks. Ophthalmic examinations were performed before treatment and at weeks 6, 13, 26, 51, 78 and 104. Laboratory investigations were carried out during weeks 6–7, 13, 26, 52, 77–78 and 104, and samples were collected for haematology, blood chemistry and urine analysis. Treatment was terminated at week 52 for animals in the interim kill and for males when the survival rate of those at 38 ppm was 25% and for females when the survival rate in the control group was 23%. All rats were killed by asphyxiation with CO2, a full post-mortem was carried out, and a large number of tissues were selected and preserved. All tissues were examined microscopically.
There were no overt signs of toxicity during the study that were considered to be related to treatment. During weeks 20–21, most rats and during weeks 66 and 67 a few rats in each group showed signs of sialodacryoadenitis infection, which was associated with weight loss and reduced food consumption. The survival rate of males given prochloraz was similar to that of controls, while treated females had better rates than controls (23%, 35%, 37% and 48% of controls and those at the low, medium and high dietary concentrations, respectively). The body-weight gain of both sexes given 625 ppm was reduced (by 9% in males, 13% in females) and was marginally lower in males given 150 ppm (by 6%). The food consumption of males at 625 ppm was lower than that of controls throughout the study, while that of females in this group was initially only marginally lower but became markedly lower as the study progressed. At 150 ppm, food consumption was marginally lower throughout the study for males and up to week 52 for females. Ophthalmoscopy revealed no abnormalities that were considered to be related to treatment. Minor changes considered to be sequelae to sialodacryoadenitis were seen in a few rats at week 26.
Changes in some urinary, blood chemical and haematological parameters were detected in treated rats throughout the study, but these were minimal and inconsistent and of doubtful toxicological significance.
At the interim kill at week 13, the weight of the liver was higher in most females and three males at 625 ppm, and centrilobular hepatocyte enlargement was seen in one male and two females. At the interim kill at week 52, enlarged or swollen livers were observed in one male and three females at 150 ppm and in four males and one female at 625 ppm. Heavier livers and marginally lower pituitary weights were recorded in males and females at the highest dietary concentration. Centrilobular hepatocyte enlargement was seen in 13 females at 625 ppm; periportal glycogen loss and centrilobular fat deposition were also apparent in some treated rats of each sex, particularly at 150 ppm. At the terminal kill and in rats that died during the study, more male rats (18/61) at 625 ppm than controls (24/121) had enlarged or swollen livers. Slightly heavier livers were recorded in females at this concentration.
The incidences and distribution of tumours were unaffected by treatment; a single liver carcinoma observed in one male at 625 ppm was considered incidental. A slightly higher incidence of hyperplastic lesions was seen in the livers of both sexes at 625 ppm (15/61 in males and 23/121 in controls; 21/63 in females and 34/120 in controls), but the difference was not statistically significant. The incidences of other non-neoplastic findings were similar in all groups. The original haematoxylin-and-eosin-stained liver sections from the main group of rats were re-examined by an independent pathologist, who confirmed the original conclusion that there was no treatment-related effect on the incidence of liver tumours (Table 6). A statistically significant trend in the incidence of liver carcinomas was found in males, but there were no statistically significant increase in any individual treated group as compared with concurrent controls. Furthermore, the incidence of liver adenomas or carcinomas in the three treated groups was within or very close to the range of incidences in other CD rats in the same laboratory (0–4% for adenomas in both sexes; 0–4% for adenocarcinomas in males and 0–2% in females). Female rats at 625 ppm showed an increased incidence of foci or areas of altered hepatocytes, mainly of eosinophilic hepatocytes and clear cells, when compared with control females.
Table 6. Hepatic lesions found by an independent pathologist in a 2-year study of carcinogenicity in rats
|
Dietary concentration |
Sex |
No.animals examined |
Hepatic lesioans |
||||
|
Adenoma |
Carcinoma |
Any tumour |
Eosinophilic hepatocytes |
Clear cells |
|||
|
0 |
Male |
120 |
0 |
1 |
1 |
16 |
12 |
|
38 |
|
60 |
0 |
1 |
1 |
6 |
2 |
|
150 |
|
60 |
1 |
2 |
3 |
14 |
1 |
|
625 |
|
60 |
0 |
3 |
3 |
12 |
4 |
|
0 |
Female |
120 |
1 |
0 |
1 |
16 |
4 |
|
38 |
|
60 |
0 |
0 |
0 |
6 |
2 |
|
150 |
|
60 |
0 |
0 |
0 |
12 |
0 |
|
625 |
|
60 |
1 |
0 |
1 |
26 |
9 |
From Colley et al. (1982a,b), Gopinath (1987), Mallyon (1988b) and Malarkey (1993b). The incidences of liver tumours in concurrent control CD rats in the same laboratory were 2/50, 1/100, 0/55, 0/50 and 0/60 adenomas in males and females; 2/50, 0/100, 1/55, 0/50 and 1/60 adenocarcinomas in males and 1/50, 0/100, 0/55, 1/50 and 1/60 adenocarcinomas in females.
The NOAEL was 38 ppm, equal to 1.3 mg/kg bw per day, on the basis of hepatic effects (periportal glycogen loss and centrilobular fat deposition) at the next highest dose (Colley et al., 1982a,b; Gopinath, 1987; Mallyon, 1988b; Malrkey, 1993b).
Dogs
In a study conducted in compliance with the principles of GLP (with QA certification), groups of five male and five female beagle dogs were fed diets containing technical-grade prochloraz (purity, 95.2–97.1%) at a concentration of 30, 135 or 600 ppm (increased to 1000 ppm from week 57) for 104 weeks. These concentrations corresponded to mean achieved intakes of 0.90, 4.1, 18 and 28 mg/kg bw per day for females and 0.94, 4.5, 18 and 29 mg/kg bw per day for males. Control animals received a diet containing the vehicle (corn oil) at the same concentration as that admixed with the highest concentration of test compound. An additional group of two males and two females given 600 ppm prochloraz were treated for 13 weeks (interim kill group). All animals were observed daily, and body weight and food consumption were recorded weekly. Ophthalmoscopy, electrocardiography and laboratory investigations were carried out before treatment and during weeks 13, 26, 50–51, 78 and 104. Samples were taken for haematology, blood chemistry and urine analysis. All dogs were killed by exsanguination under pentobarbital anaesthesia, and a full post mortem was carried out. Bone marrow was obtained by sternal puncture and examined. Various organs were weighed, and a large number of tissues were selected and (except those from interim kill animals) examined microscopically.
Four dogs were killed for humane reasons during the treatment period, but none of the deaths nor the findings post mortem were considered to be related to treatment. There were no overt signs of toxicity and no effect of treatment on body weight or food consumption. Ophthalmoscopy and electrocardiography revealed no effects. The mean serum alkaline phosphatase activity in males and females receiving 600 ppm was higher than that of controls during weeks 13, 26 and 50. When the dietary concentration of this group was increased to 1000 ppm in week 57, a marked increase in alkaline phosphatase activity was seen, which persisted for the remainder of the dosing period. The activity was also higher in males at 135 ppm from week 13, but the difference was not as marked as at the higher concentration. Slight increases in cholesterol concentration were observed at the highest dietary concentration. Slight but inconsistent increases in platelet count and blood glucose concentration were also seen in these animals, but the effects could not be definitely related to treatment. Treatment had no effect on urinary end-points or faecal occult blood content.
Macroscopic examination post mortem revealed no changes that were considered to be related to treatment. At the interim kill, the liver of one animal was heavier than usual, and the liver of one other animal slightly exceeded the normal upper limit when expressed as a percentage of body weight. The prostate of one dog was lighter than expected. At termination, statistical analysis showed that the mean liver weight of males and females at 600/1000 ppm was significantly greater than the corresponding control mean (increase of 42% in males and 30% in females), and the mean prostate weight of animals at these concentrations was significantly lower than the control mean (decrease of 63%). The histopathological treatment-related changes observed after 104 weeks were minimal swelling and rarefaction of centrilobular hepatocytes with associated low-grade hepatitis in all dogs at the highest dietary concentration, minimal swelling and rarefaction of occasional centrilobular hepatocytes and polymorphic leukocyte infiltration in central areas in one dog at the intermediate concentration, and minimal prostatic atrophy or incomplete acinar development with associated stromal, trabecular or capsular fibrosis in four male dogs at the highest dietary concentration. No evidence of neoplasia was found.
The NOAEL was 30 ppm, equal to 0.90 mg/kg bw per day, on the basis of effects on the liver in one dog at the next highest dose (Woodhouse et al., 1979; Chesterman et al., 1981; Mallyon, 1988c; Malarkey, 1993c)
The mutagenic and genotoxic potential of technical-grade prochloraz was investigated in a battery of tests in vitro and in vivo (Table 7). The results of all tests were negative, with the exception of an assay for sister chromatid exchange in vitro, in which a slight increase in frequency was observed in the presence and absence of metabolic activation at doses in the toxic range.
Table 7. Results of studies of the genotoxicity of prochloraz
|
End-point |
Test object |
Concentration |
Purity |
Results |
GLP or QA |
Reference |
|
In vitro |
||||||
|
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
62.5, 125, 250, 500, 1000 µg/plate in dimethyl sulfoxide |
100 and 97.9 |
Negativea,b |
GLP |
Wilcox (1978) |
|
Forward mutation |
Mouse lymphoma L5178Y, Tk locus |
0.5, 1.5, 5, 15, 50 µg/ml30, 40, 50, 60, 70 µg/ml in methanol |
97.8 |
Negativea,c |
QA |
McGregor et al. (1983) |
|
Chromosomal aberration |
Chinese hamster ovary cells |
2.5, 15, 30 µg/ml, 20 h –S97, 35, 50 µg/ml, 2 h +S9, in dimethyl sulfoxide; harvesting 18 h later |
96.1 |
Negativea,d |
GLP & QA |
Allen et al. (1986a) |
|
Sister chromatid exchange |
Chinese hamster ovary cells |
5, 10, 20, 25, 30 µg/ml –S920, 25, 30 µg/ml – S9 |
96.1 |
Weakly positivee |
GLP & QA |
Allen & Brooker (1983) |
|
|
|
7, 20, 28, 35 µg/ml +S9 |
|
Weakly positivef |
|
|
|
|
|
20, 28, 35 µg/ml + S9 in dimethyl sulfoxide |
|
Toxic |
|
|
|
Unscheduled DNA synthesis |
Human embryonic fibroblasts |
10, 40, 70, 100, 130, 160, 190, 220 µg/ml in methanol |
97.8 |
Negativea,g |
QA |
McGregor & Riach (1983) |
|
In vivo |
||||||
|
Micronucleus formation |
Male and female CD-1 mouse bone marrow |
Single oral dose, 270, 540, 1100 mg/kg bw in 1% w/w methylcellulose. Sampling at 24, 48, 72 h |
96.3 |
Negativeh |
GLP & QAs |
Allen et al. (1986b) |
|
Micronucleus formation |
Male and female CD rat bone marrow |
Two intraperitoneal injections of 6.25, 25, 100 mg/kg bw, 24 h apart, in corn oil. Sampling 6 h after second injection |
95.7 |
Negativei |
|
Everest & Cliffe injections of 6.25, 25, |
|
Dominant lethal mutation |
Male CD1 mice |
0, 6, 25, 100 mg/kg bw per day in corn oil for 8 weeks |
NR |
Negativej |
GLP & QA |
Cozens et al. (1980) |
|
|
S9, exogenous metabolic activation system from 9000 x g fraction of rat liver; NR, not reported |
|
|
Positive control substances were used in all assays and gave the expected results. |
|
a |
With and without metabolic activation |
|
b |
Antibacterial effects of the test material precluded evaluation of mutagenicity at 1000, 500 and in some cases 250 µg/plate. |
|
c |
An initial test indicated that prochloraz was toxic to the cells, causing mortality at 158 µg/ml. No meaningful results were obtained at 60 and 70 µg/ml, owing to toxicity. |
|
d |
Reduction of about 50% in mitotic index at highest doses |
|
e |
Dose-related, statistically significant increases in frequency at the highest dose in both studies, at which a reduction of about 40% in mitotic index was observed in a preliminary cytotoxicity test |
|
f |
Dose-related, statistically significant increases in frequency at three highest doses |
|
g |
Some evidence of toxicity at 100 µg/ml and marked toxicity at 1000 µg/ml; precipitation at 1000 µg/ml |
|
h |
At 48 and 72 h, some reduction in ratio of polychromatic:normochromatic erythrocytes in animals at 1088 mg/kg bw, indicating bone-marrow cytotoxicity. At this dose, 7/20 males and 7/19 females died. |
|
i |
In a previous study, a single intraperitoneal dose of 100 mg/kg bw elicited overt signs of toxicity which disappeared within 24 h; at 200 mg/kg bw, the effects persisted but no deaths occurred. The LD50 was 400–800 mg/kg bw. |
|
j |
Mating performance of treated males and subsequent pregnancy rates in untreated females were unaffected by treatment. The mean numbers of implantations, viable young, embryonic deaths and post-implantation losses were unaffected by treatment. At terminal autopsy of males, no obvious treatment-related macroscopic changes were seen, and the mean weights of the testes of treated males compared favourably with that of controls. |
Rats
The effect of prochloraz (purity, 96.2–97%) on reproductive function in two generations (two litters) of CrL:COBS CD (SD) rats was investigated in a study conducted in compliance with the principles of GLP (QA certification). The test compound was mixed into the diet at a concentration of 0, 38, 150 or 625 ppm, corresponding to mean achieved intakes of 3.1, 13 and 57 mg/kg bw per day for F0 males and 3.5, 14 and 58 mg/kg bw per day for F0 females; 3.7, 16 and 70 mg/kg bw per day for F1 males and 4.5, 18 and 81 mg/kg bw per day for F1 females. The parent animals were exposed to the fungicide for 9 weeks before mating, and representative offspring were retained to form a second generation. The initial animals were then re-mated with different males and females, and their offspring were discarded when they were about 3 weeks of age, after macroscopic examination post mortem. Animals forming the second generation were mated about 8 weeks after selection and were also re-mated with different male and female pairings. At autopsy, selected organs from 10 male and 10 female F1a weanlings, F2a weanlings and F1a adults in each group were weighed, and tissues from the 10 male and 10 female F1a adults per group and from five male and five female F1a and F2a weanlings per group were examined histologically.
F0 males at the highest dietary concentration showed a prolongation of aggressive behaviour after re-housing after both matings; this effect was not seen among F1 males. A slight prolongation was also seen in F0 males at 150 ppm, after the first mating only. The clinical signs seen in F0 females at the highest dietary concentration in late gestation and/or the perinatal period were hunched posture, walking on tiptoes, piloerection and pallor. About one-third of the animals were affected in each mating, although not necessarily the same individual each time. These effects were noted less frequently in the F1 generation, the females being least affected at the second mating. Food intake before mating was not consistently related to dose but was generally lower than that of controls. Decreased mean body weight was noted in parental males and females of both generations at 625 ppm. At this dose, there was evidence of extended gestation (> 22 days) and parturition in some females, leading to a small number of deaths associated with dystocia in both generations; there were, however, no effects on pregnancy rates. There were no obvious effects on male mating performance.
A few females at both matings of the F0 animals and at the first mating of the F1a animals lost their litters in the immediate perinatal period. The mean litter size at birth was smaller in both generations at the highest dietary concentration. The mean percentage of pup loss at birth was greater than the control value for both matings of the F0 generation and for the first mating of the F1 generation; however, none of the differences from control values was statistically significant. There was also a higher pup mortality rate at birth. At 625 ppm, impaired growth of the offspring to weaning was noted. The incidence of structural anomalies recorded at terminal macroscopic examination of the remaining F1a and F2a pups and all F1b and F2b offspring provided no indication of any adverse effect of treatment. Increased mean liver weights were recorded in weanling (14%) and adult F1a (13%) males at 625 ppm and in F2a weanling females at all three dietary concentrations (9, 9 and 14%, respectively at the low, intermediate and high concentrations). At the highest concentration, the mean thymus weight was lower than the control value in F1a weanlings of both sexes, and the mean brain weight of F1 female weanlings was also lower than the control value. None of the histopathological changes in the tissues examined was considered to be attributable to treatment.
Reproductive performance was affected only at the highest dietary concentration, which was toxic to the parent animals. The NOAEL was 38 ppm, equal to 3.1 mg/kg bw per day (Cozens et al., 1982).
Rats
In a study conducted in compliance with the principles of GLP (QA audit of final report), groups of 20 mated Charles River CD rats were given technical-grade prochloraz (purity, 96.2%) orally at a dose of 6, 25 or 100 mg/kg bw per day on days 1–20 post coitum, day 1 post coitum being the day on which sperm were detected in a vaginal smear; 31 rats given the vehicle (10% aqueous acacia solution) served as controls. The doses used were determined in a preliminary study in which pregnant rats given the highest dose of 100 mg/kg bw per day showed increased salivation and liver enlargement. Body weight, food consumption and overt signs of toxicity were recorded throughout the study. The dams were killed on day 21 post coitum and necropsied. Their reproductive organs were removed and examined, corpora lutea were counted, and the placenta was weighed. Fetuses were weighed, dissected and examined; half were then decapitated and the heads subsequently sliced and examined. The carcasses of all fetuses were processed and the skeletons stained and examined.
Maternal toxicity was seen at 100 mg/kg bw per day, manifested as increased salivation, reduced food consumption, lower body-weight gain (12%) and liver enlargement. At 25 mg/kg bw per day, increased salivation and a slightly higher liver weight were also observed. Dams gained marginally less body weight at both 6 (decrease of 6%) and 25 mg/kg bw per day (decrease of 5%). There were no macroscopic or microscopic findings attributable to treatment. The number of corpora lutea was similar in all groups.
The highest dose appeared to be embryotoxic, as the litter size, implantation index and viability index were slightly reduced and the incidence of dead fetuses was marginally increased. In addition, the mean fetal weight was lower and the degree of calcification of sternebrae and vertebral arches was retarded. The mean weight of placentae from treated dams was higher than that of controls, and the difference was dose-related. There was no evidence of a teratogenic response.
The NOAEL for maternal toxicity was 6 mg/kg bw per day, as the dose of 25 mg/kg bw per day was slightly toxic to the dams; the NOAEL for developmental toxicity was 25 mg/kg bw per day, on the basis of toxicity to embryos at 100 mg/kg bw per day (Beswick, 1980; Jackson, 1988b).
Rabbits
In a dose range-finding study of embryotoxicity and teratogenicity, groups of five mated female chinchilla rabbits were given technical-grade prochloraz (purity, 95.2%) by gavage on days 6–18 post coitum at a dose of 0, 25, 50, 100 or 200 mg/kg bw per day. The vehicle, distilled water with 8% carboxymethlcellulose, was given to controls. On day 28 post coitum, all females were killed by cervical dislocation, and the fetuses were removed surgically.
Does receiving 200 mg/kg bw per day lost weight on days 7–13 post coitum (days 2–7 of treatment). Thereafter, slight body-weight gain was noted but the rate was lower than that in the control group. The mean food consumption of does in this group during treatment was distinctly reduced, and this was confirmed by the compensatory increase in food consumption after treatment. The mean liver weights (absolute and relative) were dose-dependently increased (absolute, 5.5%, 10%, 22% and 36%, respectively, and relative, 8.1%, 17%, 24% and 42%, respectively). No treatment-related pathological changes were noted in the liver. The small number of females per group, the wide variations within and between the groups and the insufficient number of pregnant does (two) at the highest dose obviated a definitive conclusion, but no dose-related differences were seen in reproductive parameters. No malformed or anomalous fetuses were found in any group (Becker et al., 1989a).
In another dose range-finding study of embryotoxicity and teratogenicity, groups of six mated female chinchilla rabbits were given technical-grade prochloraz (purity, 95.2%) by gavage on days 6–18 post coitum at a dose of 0, 200 or 250 mg/kg bw per day. The vehicle, distilled water with 1% carboxymethylcellulose, was given to controls. On day 28 post coitum, all females were killed by cervical dislocation, and the fetuses were removed surgically.
Four of the six does at 250 mg/kg bw per day died between days 10 and 13 post coitum. All four lost weight from the start of dosing. Somnolence, ataxia and dyspnoea were observed in one doe, and sedation, catalepsy and dyspnoea were seen in a second before death. At necropsy, macroscopic changes were observed in the livers of two does at the highest dose. The liver:body weight ratios varied from 4.4% to 7.0% (control mean, 2.2%), and histological examination of the livers showed moderate to severe increases in hepatocellular lipid in three of four animals and severe fatty changes with minimal–moderate multifocal necrosis in two of four animals. None of the does at 250 mg/kg bw per day had viable young at termination of the study. Of the two surviving does, one did not become pregnant, and the second lost its litter. Of the does at 200 mg/kg bw per day, three had viable young, two aborted and one did not become pregnant. The overall pregnancy rates (including animals that died) were 100% in controls, 83% at 200 mg/kg bw per day and 33% at 250 mg/kg bw per day. Animals at 200 mg/kg bw per day showed a mean weight loss during the first 5 days of dosing; thereafter, some recovered and there was a slight compensatory increase in weight gain after treatment, in comparison with concurrent control values. At this dose, food and water consumption was reduced throughout the dosing period. No macroscopic changes were observed in any doe surviving to termination on day 28 post coitum. The mean liver weights of all does surviving to termination were increased by 37% and 34% at 200 and 250 mg/kg bw per day, respectively, in comparison with concurrent control values, and the mean relative liver weights were increased by 53% and 46% at the two doses, respectively. No treatment-related histological changes were observed in the livers of does surviving to termination. In the three does at 200 mg/kg bw per day with viable young, there was no evidence of an adverse effect of treatment on reproductive or fetal parameters.
These results indicate a marked maternally toxic effect, which, in some animals, either prevented implantation or caused early post-implantation loss, so that at termination on day 28 post coitum there was no evidence of implantation and the does were categorized as not pregnant. In other animals, pregnancy was maintained slightly longer, but, because of the stress to the maternal system, total resorption or abortion occurred. In those does that maintained pregnancy to term, there did not appear to be an obvious adverse effect on the fetuses. On the basis of the results of these two studies, a dose < 200 mg/kg bw per day was considered the appropriate highest dose for the main study (Becker et al., 1989b).
In a study conducted in compliance with the principles of GLP (QA certification), groups of 16 mated female chinchilla rabbits were given technical-grade prochloraz (purity, 95.2%) at a dose of 10, 40 or 160 mg/kg bw per day on days 6–18 post coitum by gavage. A group of 17 mated rabbits receiving the vehicle alone (1% carboxymethylcellulose in distilled water) served as controls. The does were killed on day 28 post coitum, and the fetuses were removed surgically. Both does and fetuses were examined.
Does at 160 mg/kg bw per day showed significantly reduced food consumption during the dosing period and reduced water consumption during the first 5 days. Weight gain was retarded during the first 5 days of dosing, but the differences from control values did not attain statistical significance. The liver weights (absolute and relative) were significantly increased. There was a slightly increased incidence of non-pregnant animals and animals with total litter loss; these effects were considered treatment-related in view of the results obtained at higher doses in the dose range-finding studies. Does with viable young at termination had a significantly increased incidence of fetal resorption. None of the other reproductive parameters and none of the fetal parameters appeared to be adversely affected by treatment. The NOAEL for both maternal and fetal toxicity was 40 mg/kg bw per day on the basis of maternal toxicity and embryotoxicity at 160 mg/kg bw per day (Becker et al., 1988).
In a study previously evaluated by the Meeting (Annex 1, reference 41), groups of 15 New Zealand white rabbits were given prochloraz at a dose of 0, 3, 12 or 48 mg/kg bw per day by gavage on days 1–28 of gestation. Maternal toxicity was observed at the highest dose, as evidenced by a significant increase in liver weight, with liver discolouration. No evidence of embryotoxicity, fetotoxicity or teratogenicity was found (Palmer et al., 1980).
Rats
Groups of five male and five female Boots-Wistar rats were given prochloraz (purity, 97.9%) at a single oral dose of 100 mg/kg bw, and control groups received the vehicle (10% aqueous acacia solution). A blood sample was obtained from the tail vein of each rat before dosing, and a further sample was collected by heart puncture immediately after the rats were killed 15 min, 90 min or 6 h after dosing. The samples were analysed for erythrocyte and plasma cholinesterase activity. Prochloraz did not affect cholinesterase activity in either sex at any interval after treatment (Smithson, 1979).
Dogs
A single oral dose of 20 mg/kg bw of prochloraz (purity, 97.9%) was given by gavage to three pairs of one male and one female beagle dogs; another pair was given an equivalent volume of the vehicle (10% aqueous acacia solution) and served as controls. A blood sample was taken from each dog immediately before and 0.75, 1.5, 3, 6 and 24 h after dosing and analysed for erythrocyte and plasma cholinesterase activity. There was no consistent difference in cholinesterase activity between treated and control animals (Morgan & Stobart, 1979).
Prochloraz (technical-grade, purity, > 95%; and analytical grade, purity, 99.5%) was investigated for effects on the initiation and promotion stages of hepatocarcinogenesis and for its ability to inhibit gap-junctional intercellular communication in the scrape-loading dye-transfer assay in IAR 20 rat liver epithelial cells. In the test for tumour promotion, female Sprague-Dawley rats were initiated with N-nitrosodiethylamine (30 mg/kg, intraperitoneally) 24 h after partial hepatectomy to maximize any interaction between proliferation and the effects of prochloraz; 2 weeks later, they were given prochloraz on 5 days a week by gavage at 30 or 150 mg/kg bw per day for 10 weeks. Groups of uninitiated and/or non-hepatectomized rats were included. The control group was given the vehicle (corn oil) only. A positive control group was given phenobarbital (500 ppm) in drinking-water for 10 weeks, starting 2 weeks after initiation. In the test for tumour initiation, animals were given prochloraz at 150 mg/kg bw per day by gavage for 12 days, and a control group was given the vehicle according to the same schedule. On day 8 of treatment, all the animals were partially hepatectomized. After 2 weeks’ recovery, promotion was started by giving all groups phenobarbital (500 ppm) in drinking-water for 10 weeks. The rats were killed 1 week after the promotion period. Altered hepatic foci were counted by quantitative stereology in liver sections stained for g-glutamyltranspeptidase and glutathione-S-transferase placental form (GST-P).
Prochloraz inhibited cell–cell communication in the test system used. It had no effect on the initiation of g-glutamyltranspeptidase-positive foci, but significant increases in the percentage of liver tissue occupied by these foci and in the number of GST-P-positive altered hepatic foci per cm3 were recorded at the lower dose. The absence of effects on plasma transaminase activity and on body-weight gain in the prochloraz-treated animals suggests that the doses used in the study were not overtly hepatotoxic. A significantly increased relative liver weight was observed in rats given prochloraz at 150 mg/kg bw per day. The data suggest that prochloraz acts as a weak promoter of hepatocarcinogenesis but does not initiate the process (Kato et al., 1998).
Eight pesticides, including prochloraz, were tested in a bioassay based on the induction of preneoplastic lesions in the liver. Rats were divided into three groups of 15 or 16 animals. Group 1 was given a single intraperitoneal injection of N-nitrosodiethylamine dissolved in 0.9% saline at 200 mg/kg bw to initiate hepatocarcinogenesis; after 2 weeks on a basal diet, they received a diet containing prochloraz (purity, 94.8%) at a concentration of 625 ppm for 6 weeks, being subjected to partial hepatectomy at week 3. Group 2 was given N-nitrosodiethylamine and subjected to partial hepatectomy in the same way but received the basal diet throughout the test period. Group 3 received only 0.9% saline and was then given a diet containing prochloraz from week 2, with partial hepatectomy at week 3. The experiment was terminated at week 8. Tumour-promoting potential was assessed by comparing the number and area (> 0.2 mm) of GST-P-positive foci in the liver with those in controls given N-nitrosodiethylamine alone. Statistical analysis was performed with Student t test. The results were scored on the basis of the difference between the first two groups: a result was considered positive when there was an increase in both the number and area of foci at p < 0.05; a borderline result was an increase in either the number or the area of foci at p < 0.05; and a negative result was no or a non-significant increase in either parameter.
No treatment-related deaths were seen. Body-weight gain at week 8 was impaired in Group 1 (– 12 g; not reported for Group 3). A slight but statistically significant increase (p < 0.01) in relative liver weight was noted, a slight not statistically significant increase in the number of GST-P-positive foci per unit area of liver was found in sections from animals fed prochloraz (8.0 ± 3.3 foci/cm2 in Group 1, 6.5 ± 2.2 foci/cm2 in Group 2), and a slight increase (p < 0.05) was found in the total area of GST-P-positive foci (0.71 ± 0.35 mm2/cm2 in Group 1, 0.44 ± 0.22 mm2/cm2 in Group 2). In Group 3, no GST-P-positive foci > 0.2 mm in diameter were seen. Thus, prochloraz is not an inducer of hepatocarcinogenesis (Cabral et al., 1991).
(iii) Studies on metabolites of prochloraz
A comparison of the acute toxicity of prochloraz and its three main metabolites in plants is shown in Table 8.
Table 8. Studies of the acute toxicity of metabolites of prochloraz administered orally
|
Species |
Strain |
Sex |
Vehicle |
LD50 (mg/kg bw) |
GLP or QA |
Reference |
|
N-Propyl-N-2-(2,4,6-trichlorophenoxy)ethylurea (M1 in Figure 2), major plant metabolite |
||||||
|
Rats |
Boots-Wistar |
Male |
8% aqueous acacia solution |
> 3200 |
|
Carter & Smithson (1979a) |
|
Dogs |
Beagle |
Male and female |
Gelatin capsules |
> 150 |
|
Watson et al. (1980a) |
|
Dogs |
Beagle |
Male and female |
Gelatin capsules |
|
|
Watson et al. (1980b) |
|
N-Formyl-N’-propyl-N’-2-(2,4,6-trichlorophenoxy)ethylurea (M2 in Figure 2), major plant metabolite |
||||||
|
Rats |
Boots-Wistar |
Male |
8% aqueous acacia solution |
> 3200 |
|
Carter & Smithson (1979a) |
|
Dogs |
Beagle |
Male and female |
Gelatin capsules |
> 1000 |
|
Watson et al. (1980a) |
|
Dogs |
Beagle |
Male and female |
Gelatin capsules |
NOAEL: 250 |
|
Watson et al. (1980b) |
|
2,4,6-Trichlorophenol, plant metabolite |
||||||
|
Rats |
Boots-Wistar |
Male |
0.4% aqueous cellosize solution |
> 3200d |
GLP |
Carter & Smithson (1979b) |
|
Dog |
Beagle |
Male and female |
Gelatin capsule |
NOAEL: 250 |
|
Watson et al. (1980b) |
|
2-(2,4,6-Trichlorophenoxy)ethanol, rodent metabolite (metabolite 2 in Figure 1) |
||||||
|
Rats |
Boots-Wistar |
Male |
0.4% aqueous cellosize solution |
800–1600 |
|
Carter et al. (1979a) |
Groups of five male rats were given M1 or M2 (major plant metabolites) at a single oral dose of 3200 mg/kg bw or prochloraz at 1600 mg/kg bw. After dosing with prochloraz or M1, the overt signs of toxicity were similar and were seen within 1 h. Rats in both groups showed signs of central nervous system depression, adopted a hunched posture, had slowed respiration, piloerection and diarrhoea, were cool and limp to touch, tremorous, ataxic and had increased nasal exudate and salivation; in addition, rats given prochloraz had increased lachrymation. Animals had generally recovered within 7 days of dosing with M1 and within 9 days of dosing with prochloraz. Four rats given prochloraz died within 2 days, and one rat given M1 died on the day after dosing. At autopsy, they were found to have gastrointestinal irritation. After dosing with M2, one rat was inactive, adopted a hunched posture and had a wasted appearance, piloerection, staining around the eyes, a nasal exudate and urine staining around the genital region; these signs were seen 2–8 days after dosing. None of the rats died after treatment (Carter & Smithson, 1979a).
Pairs of male and female dogs received M1 at single oral doses (in gelatin capsules) of 50 (day 1) and 150 (day 36) mg/kg bw, M2 at 500 (day 1) and 1000 (day 36) mg/kg bw, or imidazole at 50 (day 1), 50 (day 36) and 10 mg (day 50) mg/kg bw. The control group (one male and one female) received empty gelatin capsules. No effect was seen in dogs given M1 at 50 mg/kg bw or in males at 150 mg/kg bw, but the female at the latter dose appeared subdued and had slightly increased serum alkaline phosphatase activity the following day. M2 at 500 mg/kg elicited neutrophilia and increased serum alkaline phosphatase activity in the male, and M2 at 1000 mg/kg bw caused marginal increases in serum alkaline phosphatase activity and plasma glucose concentration in the female. The female treated with imidazole on day 1 vomited and retched within 1.5 h of dosing, but no toxic effect was seen in the male. On day 36, the male retched but no effect was seen in the female. On day 50, no toxic effect was elicited in either animal (Watson et al., 1980a).
Pairs of male and female dogs were given a single oral dose of 250 mg/kg bw of prochloraz, M1, M2, imidazole or 2,4,6-trichlorophenol on day 1 of the study, while two males and two females received empty gelatin capsules and served as controls. On day 1, emesis and diarrhoea were observed in the pairs given prochloraz or M1, and both dogs given imidazole showed emesis and increased salivation and appeared subdued. On day 2, the male given prochloraz had lost weight, and this dog and the female given M1 had slightly increased serum alkaline phosphatase activity; these parameters were normal by day 5. No effect of treatment with M2 was detected. The toxic signs elicited by imidazole appeared to be different from those induced by prochloraz and were further investigated. The pairs given prochloraz or imidazole were given a second oral dose of the same test article on day 29. The female given prochloraz and both dogs given imidazole salivated during administration of the dose and subsequently vomited. In addition, the female given imidazole had excessive diarrhoea, increased salivation and appeared subdued. On day 30, the dogs given imidazole had increased salivation, and the female had a very slightly increased blood urea nitrogen concentration and increased serum alkaline phosphatase activity. These biochemical parameters were normal on day 36, but the female again showed increased salivation when examined clinically. In order to investigate the different responses to doses of imidazole, one of the pairs of control dogs was given a single oral dose of imidazole at 250 mg/kg bw on day 43, and the other pair received empty gelatin capsules. Emesis and increased salivation were observed on day 43 in the naïve pair given imidazole, but no other effect of treatment was detected. These signs appear to be the only consistent toxic effect of a single dose of imidazole. To investigate whether the instances of increased salivation seen in the first pair during administration of the dose and clinical examination were a conditioned response, these dogs were given a second dose consisting of empty capsules. No effect was elicited. No effect of treatment was seen at autopsy or histopathologically. No effect of treatment with 2,4,6-trichlorophenol was detected. Thus, M2 and 2,4,6-trichlorophenol were less acutely toxic than prochloraz; M1 elicited a toxic response at the same dose, but imidazole appeared to be slightly more toxic (Watson et al., 1980b).
Groups of four or five male rats were given 2,4,6-trichlorophenol at a single oral dose of 3200 mg/kg bw. Ten controls received the vehicle. After treatment, a clear nasal exudate was observed in three rats, and diarrhoea and increased salivation were each seen in one animal; these signs occurred on the day of dosing. The rats had recovered by the following day, except for one rat that had a wasted appearance on day 6 (Carter & Smithson, 1979b).
Groups of five male rats were given 2-(2,4,6-trichlorophenoxy)ethanol (metabolite 2 in Figure 1) at a single oral dose of 800, 1600 or 3200 mg/kg bw, and a group of controls received the vehicle. Overt signs of toxicity were seen within 30 min of dosing. Rats at all doses showed evidence of central nervous system depression, were tremorous and had piloerection, increased nasal exudate and urine staining around the genital region. In addition, some rats at 1600 or 3200 mg/kg bw were ataxic, convulsive and cool to touch, and some given 1600 mg/kg bw showed increased salivation and closed eyes. All rats given 3200 mg/kg bw and four given 1600 mg/kg bw died within 4 days of dosing, and one control lost condition and died 13 days after dosing. The survivors in the treated groups generally recovered within 7 days, although some remained in poor condition throughout the study. Autopsy of the decedents revealed macroscopic and microscopic evidence of gastrointestinal irritation. None of the findings was significant (Carter et al., 1979a).
The mutagenic potential of metabolites of technical-grade prochloraz was assessed in assays for reverse mutation in S. typhimurium in the presence and absence of metabolic activation (Table 9). None of the compounds was mutagenic in this test.
Table 9. Results of assays for reverse mutation with metabolites and impurities of prochloraz
|
Metabolite or impurity |
Test object |
Concentration |
Purity |
Results |
GLP or QA |
Reference |
|
N-Propyl-N-2-(2,4,6-trichloro-phenoxy)ethylurea (M1), major plant metabolite |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
16, 31, 62, 125, 250 µg/plate in dimethyl sulfoxide |
NR |
Negativea,b |
|
Everest & Varley (1979) |
|
N-Formyl-N’-propyl-N’-2-(2,4,6-trichlorophenoxy)ethylurea (M2), major plant metabolite |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
62, 125, 250, 500, 1000 µg/plate in dimethyl sulfoxide |
NR |
Negativea,c |
|
Everest & Varley (1979) |
|
2-(2,4,6-Trichlorophenoxy)-ethanol, rodent metabolite |
S. typhimurium TA98, TA100, TA1535, TA1537; E. coli CM 881 WP2 uvr resistant and CM 891 WP2 uvr A |
5, 15, 50, 150, 500, 1500, 5000 µg/plate in dimethyl sulfoxide |
96% |
Negativea |
GLP& QA |
Jones & Gant (1992a) |
|
|
S9, exogenous metabolic activation system from 9000 × g fraction of rat liver; NR, not reported |
|
|
Positive control substances were used in all assays and gave the expected results. |
|
a |
With and without metabolic activation |
|
b |
No evidence of mutagenic activity when assayed up to a concentration of 125 µl/plate; at higher concentrations, antibacterial activity precluded assessment of mutagenicity. |
|
c |
1000 µg/plate, maximum solubility |
Prochloraz was first synthesized in 1974 and has been produced on a commercial scale since 1980. Few adverse human effects shown to be due to prochloraz have been reported among persons involved in the synthesis, formulation and use (as recommended) of prochloraz and its formulations. The adverse effects comprised two cases of ocular irritation due to splashing of formulation concentrate, one case of cracked and blistered lips after blowing out a blocked spray nozzle, one case of skin rash in a person with multiple allergies who was accidentally soaked in spray and two cases of face and eye irritation after prolonged use of a prochloraz formulation (Davies, 1991).
After oral administration to rats, prochloraz was rapidly and completely excreted in urine and faeces. There was a noticeable sex difference, faecal excretion predominating in females. After administration of a single oral dose of 5 mg/kg bw [14C]prochloraz to male and female rats with cannulated bile ducts, the radiolabel was recovered quantitatively, with no apparent sex difference. Prochloraz was well absorbed, a mean of 74% of the dose being recovered in the bile, urine, cage washings and carcass. Biliary excretion was the major route of elimination. After an oral dose of 5 mg/kg bw, the tissue concentrations were very low; only the liver contained > 0.1 mg/kg 96 h after dosing. By 96 h after a dose of 100 mg/kg bw, the concentrations in liver, kidneys, blood and plasma of animals of each sex and in the lungs and adrenals of females were > 1 mg/kg.
The main metabolic pathway at both doses involved cleavage of the imidazole ring and initial loss of small fragments, to give M1 and M2, which, together with a considerable quantity of unchanged prochloraz, were the main compounds found in the faeces. Further metabolism yielded the phenoxyethylurea, which was excreted mainly in the faeces or further metabolized to the phenoxyethanol and then to the acid. These latter compounds were excreted mainly in the urine in free or conjugated forms, and trichlorophenoxyacetic acid was the main metabolite in urine. A small amount of this acid may be further metabolized to trichlorophenol, which was also excreted in the urine. A minor metabolic pathway involves aromatic hydroxylation.
Prochloraz has low acute toxicity. The LD50 in rats treated orally was 1600–2400 mg/kg bw, and the main toxic effects were reversible central nervous system depression and gastrointestinal irritation. WHO (1999) has classified prochloraz as ‘slightly hazardous’. The LD50 after dermal application was > 2100 mg/kg bw in rats and > 3000 mg/kg bw in rabbits, and the LC50 in rats exposed by inhalation for 4 h was > 2.2 mg/l of air, the highest achievable concentration. The compound was not irritating to the skin of rabbits after a 4-h exposure, was not irritating to the eyes of rabbits and did not sensitize the guinea-pig skin in a Magnusson and Kligman maximization test.
In short-term studies in mice, rats and dogs, the liver was the principal target organ. Prochloraz is a potent inducer of the hepatic microsomal mixed-function oxidase system of rats and mice after oral administration. The spectrum of induction was similar to that caused by phenobarbital, with increased content and activity of cytochrome P450 enzymes. In a 14-day range-finding study in dogs given 40 mg/kg bw per day, serum alkaline phosphatase activity increased progressively from day 3 throughout treatment. The NOAEL for the increase in alkaline phosphatase activity at day 3 was 10 mg/kg bw per day. In 13-week studies, liver weights were increased in all three species; this response was considered to reflect induction of the hepatic mixed-function oxidase system. In mice and rats, hepatocyte enlargement was observed, with periportal fat infiltration and glycogen loss in mice. No histopathological changes were observed in the liver in dogs. In all studies, the effect was dose-related and showed partial reversal after a 4-week recovery period. Dogs also had decreased weights of the prostate and testis. The NOAELs were 6 mg/kg bw per day in mice and 2.3 mg/kg bw per day in dogs, but no NOAEL could be identified in rats, as at the lowest dose, 6 mg/kg bw per day, increased liver weights and occasional signs of intoxication (increased salivation, diarrhoea) were observed.
In long-term studies in mice and rats and in a 2-year study in dogs, the liver was again the principal target organ. The NOAELs were 1.3 mg/kg bw per day in rats and 0.9 mg/kg bw per day in dogs; no carcinogenic effect was observed in rats. In the study in mice, an increased incidence of liver adenomas and carcinomas was found in both males and females at concentrations > 325 ppm. No significant difference from controls was found in the number of liver tumours in animals of either sex at 78 ppm, equal to 7.5 mg/kg bw per day, which was therefore the NOAEL. Prochloraz was hepatocarcinogenic in mice.
A comprehensive range of studies of genotoxicity gave consistently negative results, except for a weakly positive response in a test for sister chromatid exchange in Chinese hamster ovary cells in vitro in both the presence and the absence of an exogenous metabolic activation system. The Meeting concluded that prochloraz is unlikely to be genotoxic.
Investigation of the effects of prochloraz on the initiation and promotion stages of hepatocarcinogenesis in rats suggested that the substance acts as a weak tumour promoter but does not initiate the process. It was a weak, rodent-specific hepatocarcinogen, with a mode of action similar to that of phenobarbital. The Meeting concluded that the increased incidence of tumours observed in the liver was a threshold phenomenon that was species-specific, and that prochloraz was therefore unlikely to pose a carcinogenic risk to humans.
In a two-generation study of reproductive toxicity in rats, reproductive performance was affected only at a concentration of 625 ppm in the diet, as indicated by total litter loss in a few females, reduced mean litter size at birth, higher pup mortality rates at birth and impaired growth of the offspring; furthermore, parental toxicity was observed. The NOAEL for parental and offspring toxicity was 38 ppm, equal to 3.1 mg/kg bw per day. Prochloraz was not teratogenic in either rats or rabbits. In a study of developmental toxicity in rats, a dose of 100 mg/kg bw per day was toxic in dams, embryos and fetuses. The NOAELs were 6 mg/kg bw per day for maternal toxicity and 25 mg/kg bw per day for developmental toxicity. In a study of developmental toxicity in rabbits, both maternal toxicity and embryotoxicity were seen at 160 mg/kg bw per day; the NOAEL for maternal and fetal toxicity was 40 mg/kg bw per day.
Prochloraz did not affect plasma or erythrocyte cholinesterase activity in rats or dogs.
In a comparative study of the acute toxicity of prochloraz and the plant metabolites M1 and M2 in rats treated orally, the signs of toxicity were qualitatively similar, but prochloraz was more acutely toxic than either of the metabolites. Trichlorophenol was also less acutely toxic than prochloraz. In dogs, M2 and trichlorophenol were less acutely toxic than prochloraz, and M1 elicited a similar toxic response at the same dose. Neither M1 nor M2 induced reverse mutation in S. typhimurium.
Since the initial synthesis of prochloraz in 1974 and its commercial introduction in 1980, only a few cases of skin and eye irritation have been reported in humans heavily exposed to products containing prochloraz.
The Meeting concluded that the existing database was adequate to characterize the potential hazards of prochloraz to fetuses, infants and children.
The Meeting confirmed the ADI of 0–0.01 mg/kg bw established in 1983, on the basis of a NOAEL for effects on the liver of 0.9 mg/kg bw per day in a 2-year study in dogs, a NOAEL of 1.3 mg/kg bw per day in a 2-year study in rats and a safety factor of 100.
The Meeting established an acute reference dose of 0.1 mg/kg bw, on the basis of a NOAEL of 10 mg/kg bw per day for effects on the liver at day 3 (increased serum alkaline phosphatase activity) in a 14-day study in dogs, and a safety factor of 100.
Levels relevant to risk assessment
|
Species |
Study |
Effect |
NOAEL |
LOAEL |
|
Mouse |
2-year study of toxicity and carcinogenicitya |
Toxicity |
78 ppm, equal to 7.5 mg/kg bw per day |
325 ppm, equal to 33 mg/kg bw per day |
|
Carcinogenicity |
78 ppm, equal to 7.5 mg/kg bw per day |
325 ppm, equal to 33 mg/kg bw per day |
||
|
Rat |
2-year study of toxicity and carcinogenicitya |
Toxicity |
38 ppm, equal to 1.3 mg/kg bw per day |
150 ppm, equal to 5.1 mg/kg bw per day |
|
Carcinogenicity |
625 ppm, equal to 28 mg/kg bw per dayc |
– |
||
|
Multigeneration reproductive toxicitya |
Parental toxicity |
38 ppm, equal to 3.1 mg/kg bw per day |
150 ppm, equal to 13 mg/kg bw per day |
|
|
Offspring toxicity |
38 ppm, equal to 3.7 mg/kg bw per day |
150 ppm, equal to 16 mg/kg bw per day |
||
|
Developmental toxicityb |
Maternal toxicity |
6 mg/kg bw per day |
25 mg/kg bw per day |
|
|
Embryo- and fetotoxicity |
25 mg/kg bw per day |
100 mg/kg bw per day |
||
|
Rabbit |
Developmental toxicityb |
Maternal toxicity |
40 mg/kg bw per day |
160 mg/kg bw per day |
|
Embryo- and fetotoxicity |
40 mg/kg bw per day |
160 mg/kg bw per day |
|
|
|
Dog |
14-day study of toxicityb,d |
Toxicity |
10 mg/kg bw per daye |
40 mg/kg bw per daye |
|
2-year study of toxicitya |
Toxicity |
30 ppm, equal to 0.90 mg/kg bw per day |
135 ppm, equal to 4.1 mg/kg bw per day |
a Diet
b Gavage
c Highest dose tested
d This study was used to establish the acute reference dose.
e Based on an increase in alkaline phosphatase activity at day 3
Estimate of acceptable daily intake for humans
0–0.01 mg/kg bw
Estimate of acute reference dose
0.1 mg/kg bw
Studies that would provide information useful for continued evaluation of the compound
Further observations in humans
Summary of critical end-points for prochloraz
|
Absorption, distribution, excretion and metabolism in mammals |
|
|
Rate and extent of oral absorption |
Rapidly and well absorbed; mean of 74% at low dose (bile duct-cannulated rats) |
|
Dermal absorption |
Poor; < 2% in pigs |
|
Distribution |
Rapid and extensive. At high dose, liver, kidneys, blood, and plasma of males and females and lungs and adrenals of females had concentrations > 1 mg/kg 96 h after dosing. |
|
Potential for accumulation |
None |
|
Rate and extent of excretion |
Rapid and complete, significant sex difference, faecal excretion predominating in females (70% vs 59% in males at low dose). Urinary excretion: 65% in males, 41% in females at high dose. |
|
Metabolism in animals |
Major pathway: cleavage of imidazole ring and initial loss of small fragments. Further metabolism yields the urea, which is excreted in faeces or further metabolized to the phenoxyethanol and then to the acid, which is excreted in urine in free and conjugated forms. A small amount may be further metabolized to the trichlorophenol. |
|
Minor metabolic pathway involves aromatic hydroxylation. |
|
|
Toxicologically significant compounds |
Prochloraz |
|
Acute toxicity |
|
|
Rat, LD50, oral |
1600 mg/kg bw |
|
Rat, LD50 , dermal |
> 2100 mg/kg bw |
|
Rat, LC50, inhalation |
> 2.2 mg/l of air (4 h) |
|
Rabbit, skin irritation |
Not irritating (4 h) |
|
Rabbit, eye irritation |
Not irritating |
|
Guinea-pig, skin sensitization (test method) |
Not sensitizing (Magnusson and Kligman) |
|
Short-term studies of toxicity |
|
|
Target/critical effect |
Liver: increased weight (rats, mice, dogs), hepatocyte enlargement (rats, mice), periportal fat infiltration and glycogen loss (mice) |
|
Prostate and testis: decreased weight (dogs) |
|
|
Lowest relevant oral NOAEL |
10 mg/kg bw per day (dogs, 14 days, NOAEL based on increase in alkaline phosphatase activity at day 3) |
|
2.3 mg/kg bw per day (dogs, 90 days) |
|
|
Lowest relevant dermal NOAEL |
No data |
|
Lowest relevant inhalation NOAEL |
No data |
|
Long-term studies of toxicity and carcinogenicity |
|
|
Target/critical effect |
Liver (mice, rats, dogs), prostate (dogs) |
|
Lowest relevant NOAEL |
0.90 mg/kg bw per day (dogs), 1.3 mg/kg bw per day (rats) |
|
Carcinogenicity |
Not carcinogenic in rats |
|
Increased incidence of liver adenomas and carcinomas in mice |
|
|
Genotoxicity |
No genotoxic potential |
|
Reproductive toxicity |
|
|
Reproduction target/critical effect |
Decreased litter size, increased pup mortality rate, and impaired growth of pups at parentally toxic dose |
|
Lowest relevant reproductive NOAEL |
3.1 mg/kg bw per day |
|
Developmental target/critical effect |
Embryo and fetotoxicity at maternally toxic dose |
|
Lowest relevant developmental NOAEL |
Maternal toxicity: 6 mg/kg bw per day |
|
Developmental toxicity: 25 mg/kg bw per day (rats) |
|
|
Neurotoxicity/delayed neurotoxicity |
No concern from other studies |
|
Mechanistic studies |
Potent inducer of hepatic microsomal monooxygenase system in rats and mice after oral administration, with spectrum of induction similar to that caused by phenobarbital |
|
|
Weak tumour promoter but not an initiator |
|
Medical data |
A few cases of skin and eye irritation after heavy exposure to products containing prochloraz |
|
Summary |
Value |
Study |
Safety factor |
|
ADI |
0–0.01 mg/kg bw |
2 years, dogs and rats, toxicity |
100 |
|
Acute RfD |
0.1 mg/kg bw |
14 days, dogs, toxicity |
100 |
Allen, J.A. & Brooker, P.C. (1983) Technical prochloraz: Frequency of sister chromatid exchange in Chinese hamster ovary cells cultured in vitro. Unpublished report No. TOX/86/173-135 from Huntingdon Research Centre & FBC. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Allen, J.A., Brooker, P.C., Birt, D.M. & McCaffrey, K.J. (1986a) Technical prochloraz: Metaphase chromosome analysis of CHO cells cultured in vitro. Unpublished report No. TOX/86/173-136 from Huntingdon Research Centre & FBC. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Allen, J.A., Proudlock, R.J. & Pugh, L.C. (1986b) Technical prochloraz: Mouse micronucleus test. Unpublished report No. TOX/86/173-132 from Huntingdon Research Centre & FBC. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Becker, H., Mueller, E., Vogel, W., Vogel, O. & Terrier, C. (1988) Embryotoxicity study (including teratogenicity) with prochloraz technical in the rabbit. Unpublished report No. PF-86.814 from Schering & RCC. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Becker, H., Muller, E. & Vogel, W. (1989a) First dose range-finding embryotoxicity study (including teratogenicity) with prochloraz technical in the rabbit. Unpublished report No. RCC 067511 from Schering & RCC—Addendum 1 to Becker H., Muller E. & Vogel W. (1989), unpublished report No. PF-86.814. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Becker, H., Muller, E. & Vogel, W. (1989b) Second dose range-finding embryotoxicity study (including teratogenicity) with prochloraz technical in the rabbit. Unpublished report No. RCC 074891 from Schering & RCC—Addendum 2 to Becker H., Muller E. & Vogel W. (1989), unpublished report No. PF-86.814. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Beswick, A.M. (1980) The teratogenicity study of technical prochloraz in male and female rats. Unpublished report No. TX 80024 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Boardman, L.E. (1979) Plasma and tissue distribution studies in the rat following single and repeated oral doses of (3H)-BTS 40542. Unpublished report No. AX 79004 from Corning Hazleton & Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Cabral, R., Hoshiya, T., Hakoi, K., Hasegawa, R., Fukushima, S. & Nobuyuki, I. (1991) A rapid in vivo bioassay for the carcinogenicity of pesticides. Tumori, 77, 185–188.
Campbell, J.K. (1983) Residues of prochloraz in milk and tissues of a lacting goat fed straw containing residues of radiactive prochloraz. Unpublished report No. METAB/83/8 from FBC. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Campbell, J.K. & Needham, D. (1980) Residues in milk and tissues of a goat dosed orally with 14C-BTS 44596 (major plant metabolite of prochloraz). Unpublished report No. AX 80033 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Campbell, J.K. & Needham, D. (1981) The quantitative excretion of BTS 9608 in the urine of rats after oral administration of prochloraz, BTS 46828 or BTS 44596. Unpublished report No. METAB/81/29 from FBC. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Carter, O.A. & Smithson, A. (1979a) Acute oral toxicity of the metabolites BTS 44595 and BTS 44596 to male Boots Wistar rats. Unpublished report No. TX 79031 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Carter, O.A. & Smithson, A. (1979b) Acute oral toxicity to male Boots Wistar rats of the impurities BTS 42825 and BTS 45186. Unpublished report No. TX 79008 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Carter, O.A. & Smithson, A. (1979c) BTS 40542 impurity BTS 43026 acute oral toxicity to male Boots Wistar rats. Unpublished report No. TX 79022 (2nd edition) from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Carter, O.A., Lancaster, M.C. & Smithson, A. (1979a) Acute oral toxicity in male Wistar rats of the impurity BTS 3037. Unpublished report No. TX 79040 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Carter, O.A., Lancaster, M.C. & Smithson, A. (1979b) Acute oral toxicity in male Boots Wistar rats of the impurity BTS 39883. Unpublished report No. TX 79039 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Carter, O.A., Lancaster, M.C. & Smithson, A. (1979c) BTS 40542 impurity BTS 42140 acute oral toxicity study in male rats. Unpublished report No. TX 79038 (2nd edition) from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Challis, I.R. & Campbell, J.M. (1983) The effect of prochloraz on the hepatic mixed function oxidase system of the male mouse after oral administration. Unpublished report No. METAB/83/6 from FBC. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Challis, I.R. & Creedy, C.L. (1988) The excretion and distribution of tissue residues in rats dosed orally with prochloraz at 5 mg/kg bodyweight. Unpublished report No. ENVIR/88/39 from Schering. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Challis, I.R. & Creedy, C.L. (1989) The metabolism of prochloraz in the rat following oral dosing at 5 and 100 mg/kg bodyweight. Unpublished report No. ENVIR/88/42 from Schering. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Chesterman, H., Perkin, C.J., Heywood, R., Street, A.E., Prentice, D.E., Woodhouse, R.N. & Majeed, S.K. (1981) Two-year toxicity study in beagle dogs of technical BTS 40542—Final report—Repeated dietary administration for 104 weeks. Unpublished report No. TOX/83/173-2 from Huntingdon Research Centre & FBC. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Colley, J., Wood, J.D., Heywood, R., Street, A.E., Prentice, D.E., Gibson, W.A. & Almond, R.H. (1982a) BTS 40542 (prochloraz) chronic toxicity and carcinogenicity study in rats by dietary administration—104 weeks (final report). Unpublished report No. TOX/82/173-8 from Huntingdon Research Centre & FBC. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Colley, J., Wood, J.D., Heywood, R., Street, A.E., Cherry, C.P., Gibson, W.A. & Almond, R.H. (1982b) BTS 40542 chronic toxicity and carcinogenicity study in rats by dietary administration (addendum 1 to final unpublished report No. TOX/82/173-8). Unpublished report No. TOX/82/173-20 from Huntingdon Research Centre & FBC. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Colley, J., Nunn, G., Heywood, R., Prentice, D.E., Buckley, P., Offer, J.M., Gibson, W.A., Almond, R.H. & Chauter, D.O. (1983) Prochloraz (BTS 40542) tumorigenicity study in mice by dietary administration (final report). Unpublished report No. TOX/83/173-23 from Huntingdon Research Centre & FBC. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Colley, J., Gopinath, C. & Offer, J.M. (1988) BTS 40542 tumorigenicity study in mice by dietary administration. Unpublished report No TOX/83/173-23 from Huntingdon Research Centre & Schering, Addendum 1 to Colley J., Gopinath C. & Offer J.M. (1988), unpublished report No. TOX/83/173-23. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Cozens, D.D., Reid, Y.J., Woodhouse, R.N., Almond, R.H., Anderson, J. & Ball, S.I. (1980) Dominant lethal gene assay of BTS 40542 (prochloraz technical) in the male mouse. Unpublished report No. TX 80077 from Huntingdon Research Centre & Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Cozens, D.D., Bottomley, A.M., Smith, J.A., Offer, J.M., Greyson, R.L., Gibson, W.A., Almond, R.H., Anderson, J. & Ball, I.S. (1982) The effect of BTS 40542 (prochloraz) on reproductive function of multiple generations in the rat. Unpublished report No. TOX/82/173 from Huntingdon Research Centre & FBC. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Cuthbert, J.A. & D’Arcy-Burt, K.J. (1984a) Technical prochloraz: Primary skin irritancy study in rabbits. Unpublished report No. TOX/84/173-124 from Inveresk Research International & FBC. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Cuthbert, J.A. & D’Arcy-Burt, K.J. (1984b) Technical prochloraz: Primary eye irritancy study in rabbits. Unpublished report No. TOX/84/173-125 from Inveresk Research International & FBC. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Davies, W.W. (1991) Medical statement on the human exposure to prochloraz. Schering (Unpublished, 3rd edition). Submitted to WHO by Aventis CropScience SA, Lyon, France.
Dawson, J.R. (1989) The excretion and distribution of tissue residues of prochloraz in rats dosed orally with prochloraz at 100 mg/kg. Unpublished report No. ENVIR/88/51 from Schering. Submitted to WHO by Aventis CropScience SA, Lyon, France.
D’Souza, G.A. (1995) Prochloraz (14C)-prochloraz BTS 40542 a biliary cannulation study in rats following a single oral dose of 5 mg/kg bodyweight. Unpublished report No. 194/122 from Corning Hazleton. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Everest, R.P & Cliffe, S. (1980) BTS 40542 (technical) Micronucleus assay in male and female CD rats of prochloraz. Unpublished report No. TX 80003 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Everest, R.P. & Varley, R. (1979) The in vitro bacterial mutagenicity testing of the plant metabolites BTS 44595 and BTS 44596. Unpublished report No. TX 79123 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Gale, E.P. (1980) 90 day oral toxicity study with prochloraz technical to male and female CD 1 mice. Unpublished report No. TX 80040 from Corning Hazleton & Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Gopinath, C. (1987) BTS 40542 chronic toxicity and carcinogenicity study in rats by dietary administration, histopathological reexamination of H & E stained sections of liver from all main group rats. Unpublished report No. TOX/82/173-8 from Huntingdon Research Centre & Schering, Addendum 2 to Colley J. et al. (1982), unpublished report No. TOX/82/173-8. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Hamilton, D.Y. (1978) The distribution of radiolabelled residues in the tissues of the pig following a single dermal application of BTS 40542. Unpublished report No. AX 78007 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Hounsell, I.A. & Ogle, A. (1987) Technical prochloraz: Acute dermal toxicity in the rat. Unpublished report No. TOX/86/173-142 from Schering. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Jackson, M. (1988a) BTS 40542: 13 week oral toxicity study with a 4 week off dose period. Unpublished report No. TOX/83/173-75 from Schering, Addendum 2 to Lancaster M.C. & Shaw J.W. (1979) unpublished report No. TX 79028. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Jackson, C.M. (1988b) The teratogenicity study of technical prochloraz in male and female rats. Unpublished report No. TOX/83/173-97 from Schering, Addendum 1 to Beswick A.M. (1980), unpublished report No. TX 80024. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Jackson, C.M. (1989) BTS 40542: 13 week oral toxicity study in dogs with a four week off dose period. Unpublished report from Schering, Addendum 5 to Lancaster, M.C., Morgan, H.E. & Stobart, J.E. (1979) unpublished report No. TX 79010. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Jackson, G.C. & Hardy, C.J. (1987) Technical prochloraz: acute inhalation toxicity (4-hour exposure) in rats. Unpublished report No. TOX/87/173-167 from Huntingdon Research Centre & Schering. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Jones, E. & Gant, R.A. (1991) SN 604904: Bacterial mutation assay. Unpublished report No. TOX/91/173-267 from Huntingdon Research Centre & Schering. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Jones, E. & Gant, R.A. (1992a) BTS 3037: Bacterial mutation assay. Unpublished report No. TOX/92/173-279 from Huntingdon Research Centre & Schering. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Jones, E. & Gant, R.A. (1992b) BTS 39883: Bacterial mutation assay. Unpublished report No. TOX/92/173-278 from Huntingdon Research Centre & Schering. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Jones, E. & Wilson, L.A. (1988a) Analytical BTS 43298: Ames bacterial mutagenicity test. Unpublished report No. TOX/88/173-191 from Huntingdon Research Centre & Schering. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Jones, E. & Wilson, L.A. (1988b) Analytical BTS 42140: Ames bacterial mutagenicity test. Unpublished report No. TOX/88/173-193 from Huntingdon Research Centre & Schering. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Jones, E. & Wilson, L.A. (1988c) Analytical BTS 43026: Ames bacterial mutagenicity test. Unpublished report No. TOX/88/173-192 from Huntingdon Research Centre & Schering. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Jones, E. & Wilson, L.A. (1988d) Analytical BTS 40348: Ames bacterial mutagenicity test. Unpublished report No. TOX/88/173-196 from Huntingdon Research Centre & Schering. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Kato, Y., Flodström, S. & Wärngard, L. (1998) Initiation and promotion of altered hepatic foci in female rats and inhibition of cell–cell communication by the imidazole fungicide prochloraz. Chemosphere, 37, 393–403.
Keene, A.T. (1988a) BTS 40542: 13 week oral toxicity in the mouse (macroscopic and microscopic observations). Unpublished report No. TOX/82/173-9 from Schering, Addendum 3 to Gale, E.P. (1980) unpublished report No. TX 80040. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Keene, A.T. (1988b) 90 day oral toxicity study with prochloraz technical in male and female beagle dogs 4 week off dose period (summary tables and statistical analysis). Unpublished report No. TOX/83/173-74 from Schering, Addendum 1 to Lancaster, M.C., Morgan, H.E. & Stobart, J.E. (1979) unpublished report No. TX 79010. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Kynoch, S.R., Lloyd, G.K. & Mallard, J.R. (1979) Acute dermal toxicity of BTS 40542 (prochloraz) to male and female rabbits. Unpublished report No. TX 79076 from Huntingdon Research Centre & Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Laignelet, L., Rivière, J.-L. & Lhuguenot, J.-C. (1992) Metabolism of an imidazole fungicide (prochloraz) in the rat after oral administration. Food Chem. Toxicol., 30, 575–583.
Lancaster, M.C. (1980) 13-week oral toxicity study with prochloraz technical (BX 9/DM 2723) in male and female dogs with a four week off dose period, histopathological examination of the remaining tissues. Unpublished report No. TX 80034 from Boots, Supplement 1 to Lancaster, M.C., Morgan, H.E. & Stobart, J.E. (1979) unpublished report No. TX 79010. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Lancaster, M.C. (1982) BTS 40542: 13 week oral toxicity in the mouse: Histopathological examination. Unpublished report No. TOX/82/173-9 from Boots, Supplement 1 to Gale E.P. (1980) unpublished report No. TX 80040. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Lancaster M.C. & Shaw J.W. (1979) 90 day oral toxicity study with prochloraz technical in male and female Boots Wistar rats (4 week off dose period). Unpublished report No. TX 79028 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Lancaster M.C. & Shaw J.W. (1980a) 21 day cumulative oral toxicity study with technical prochloraz in male and female mice. Unpublished report No. TX 79127 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Lancaster M.C. & Shaw J.W. (1980b) 30 day oral toxicity study with technical BTS 40542 in male and female rats. Unpublished report No. TX 79126 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Lancaster M.C., Morgan H.E. & Stobart J.E. (1979) 90 day oral toxicity study with prochloraz technical in male and female Beagle dogs (4 week off dose period). Unpublished report No. TX 79010 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Magnusson B. & Kligman A.M. (1969) J. Invest. Dermatol., 52: 268-276.
Magnusson B. & Kligman A.M. (1970) in: Allergic Contact Dermatitis in the Guinea Pig, ed. Magnusson B. & Kligman A.M., pp 102-123.
Malarkey, P. (1993a) BTS 40542 tumorigenicity study in mice by dietary administration. Unpublished report No TOX/83/173-23 from Schering, Addendum 5 to Colley, J., Gopinath, C. & Offer, J.M. (1988), unpublished report No. TOX/83/173-23. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Malarkey, P. (1993b) BTS 40542 chronic toxicity and carcinogenicity study in rats by dietary administration. Unpublished report from Schering, Addendum 4 to Colley, J. et al. (1982), unpublished report No. TOX/82/173-8. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Malarkey, P. (1993c) BTS 40542: 2 year toxicity study in dogs. Unpublished report No. TOX/81/173-2 from Schering, Addendum 3 to Chesterman, H. et al. (1981), unpublished report No. TOX/83/173-2. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Mallyon, B. (1988a) BTS 40542: 13 week oral toxicity in the mouse. Unpublished report No. TOX/82/173-9 from Schering, Addendum 1 to Gale, E.P. (1980) unpublished report No. TX 80040. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Mallyon, B. (1988b) BTS 40542 chronic toxicity and carcinogenicity study in rats by dietary administration. Unpublished report No. TOX/88/173-8 from Schering, Addendum 3 to Colley, J. et al. (1982), unpublished report No. TOX/82/173-8. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Mallyon, B.A. (1988c) BTS 40542: 2 year toxicity study in dogs. Unpublished report No. TOX/81/173-2 from Schering, Addendum 2 to Chesterman, H. et al. (1981), unpublished report No. TOX/83/173-2. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Markham, L.P. (1988a) BTS 40542: 13 week oral toxicity study in the rat (individual macroscopic and microscopic pathology data). Unpublished report No. TOX/83/173-75 from Schering, Addendum I to Lancaster, M.C. & Shaw, J.W. (1979) unpublished report No. TX 79028. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Markham, L.P. (1988b) BTS 40542: 13 week oral toxicity study in dogs. Unpublished report No. TOX/93/173-74 from Schering, Addendum 4 to Lancaster, M.C., Morgan, H.E. & Stobart, J.E. (1979) unpublished report No. TX 79010. Submitted to WHO by Aventis CropScience SA, Lyon, France.
McGregor, D.B. & Riach, C.G. (1983) Technical prochloraz: Unscheduled DNA synthesis in human embryonic fibroblasts. Unpublished report No. TOX/83/173-117 from Inveresk Research International & FBC. Submitted to WHO by Aventis CropScience SA, Lyon, France.
McGregor, D.B., Riach, C.G. & Brown, A.G. (1983) Technical prochloraz assessment of mutagenic potential in the mouse lymphoma mutation assay. Unpublished report No. TOX/83/173-22 from Inveresk Research International & FBC. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Morgan, H.E. & Stobart, J.E. (1979) The effect of a single oral dose of prochloraz technical on the cholinesterase activity in Beagle dogs. Unpublished report No. TX 79029 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Morgan, H.E., Patton, D.S.G., Shepherd, G.M. & Stobart, J.E. (1977) Acute oral toxicity of prochloraz to female baboon. Unpublished report No. TX 78060 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Morgan, H.E., Patton, D.S.G., Shepherd, G.M. & Stobart, J.E. (1978) Acute oral toxicity of BTS 40542 to male and female beagle dogs. Unpublished report No. TX 78049 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Morgan, H.E., Lancaster, M.C., Patton, D.S.G. & Stobart, J.E. (1979) 14 day cumulative oral toxicity study with prochloraz technical in male and female beagle dogs. Unpublished report No. TX 79030 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Needham, D. (1980) The effect of dog gastric juice or plasma on prochloraz. Unpublished report No. AX 80011 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Needham, D. (1981) The excretion of (14C)-phenyl labelled BTS 44596 by male and female rats after a single oral dose. Unpublished report No. METAB/81/10 from FBC. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Needham, D. (1982a) The excretion and tissue residues of (14C)-prochloraz in male and female mice following a single oral dose of 100 mg/kg. Unpublished report No. METAB/82/32 from FBC. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Needham, D. (1982b) The metabolism of prochloraz in the rat after oral administration. Unpublished report No. METAB/82/31 from FBC. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Needham, D. (1983a) The effect of prochloraz on the hepatic mixed function oxidase system of the mouse when administered at 80, 325 and 1300 mg/kg diet for up to 14 weeks. Unpublished report No. METAB/83/7 from FBC. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Needham, D. (1983b) The effect of prochloraz on the hepatic mixed-function oxidase system of the male rat after oral administration. Unpublished report No. METAB/83/5 from FBC. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Needham, D. (1997) The metabolism of prochloraz in the rat following oral dosing at 5 and 100 mg/kg bodyweight. Unpublished report No. ENVIR/88/42-Amendment to report from Challis, I.R. & Creedy, C.L. (1989) from AgrEvo. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Needham, D. & Campbell J.K. (1982) The excretion and tissue residues of (14C)-prochloraz in male and female dogs following a single oral dose of 18 mg/kg. Unpublished report No. METAB/82/30 from FBC. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Needham, D. & Challis, I.R. (1991) The metabolism and excretion of prochloraz, an imidazole-based fungicide, in the rat. Xenobiotica, 21, 1473–1482.
Needham, D., Creedy, C.L. & Dawson, J.R. (1992) The profile of rat liver enzyme induction produced by prochloraz and its major metabolites. Xenobiotica, 22, 283–291.
O’Donovan, M.R. & Smithson, A. (1978) Acute oral toxicity to male and female Boots Wistar rats of BTS 40348 (stages 1b intermediate). Unpublished report No. TX 78114 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Offer, J.M., Gopinath, C., Colley, J.C. & Cannon, M.W.J. (1992) Photomicrographic addendum to histopathology report BTS 145 BTS 40542 tumorigenicity study in mice by dietary administration. Unpublished report No. TOX/83/173-23 from Huntingdon Research Centre & Schering, Addendum 4 to Colley, J., Gopinath, C. & Offer, J.M. (1988), unpublished report No. TOX/83/173-23. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Palmer, A.K., Bottomley, A.M. & Billington, R. (1980) The effect of technical prochloraz on pregnancy of the New Zealand white rabbit. Unpublished report No. TX 80083 from Huntingdon Research Centre & Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Peto, R., Pike, M.C., Day, N.E., Gray, R.G., Lee, P.N., Parish, S., Peto, J., Richards, S. & Wahrendorf, J. (1980) Guidelines for simple sensitive significance tests for carcinogenic effects in long-term animal experiments. In: IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Suppl. 2, Long-term and Short-term Screening Assays for Carcinogens: A Critical Appraisal, Lyon, IARCPress, pp 311–346.
Phillips, M.W.A. & Swalwell, L.M. (1989) The residues of prochloraz in the edible tissues of a cow following oral administration of prochloraz for 3 days at 1.5 mg prochloraz/kg bodyweight/day. Unpublished report No. ENVIR/89/24 from Schering. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Reynolds, C.M.M. (1995) Prochloraz clearance of a single oral dose from rat tissue. Unpublished report No. TOX/94/173-321 from AgrEvo. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Reynolds, C.M.M. (1996) Prochloraz clearance of a single oral dose from rat tissue. Unpublished report No. TOX/94/173-321- 1st addendum from AgrEvo. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Rivière, J.-L. (1983) Prochloraz, a potent inducer of the microsomal cytochrome P450 system. Pestic. Biochem. Physiol., 19, 44–52.
Sharp, D.W. (1982) Summary of prochloraz 2-year tumorigenicity study in mice. From FBC (unpublished) . Submitted to WHO by Aventis CropScience SA, Lyon, France.
Shaw, J.W. (1979) The delayed dermal sensitisation study of BTS 40542 in the female guinea pig. Unpublished report No. TX 79058 from Boots & Quintiles Toxicol. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Shaw, J.W. & Carter, O.A. (1976) Acute oral toxicity to male CD1 mice of BTS 40542 prochloraz. Unpublished report No. TX 76093 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Shaw, J.W., Lancaster, M.C. & Smithson, A. (1979a) Acute oral toxicity study in Boots Wistar and CFY rats with BTS 40542 unformulated material. Unpublished report No. TX 79051 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Shaw, J.W., Crowley, J., Wilcox, K. & Lancaster, M.C. (1979b) BTS 40542: 13 week oral toxicity study in rats with a four week off dose period, quantitative assessment of liver cell size. Unpublished report No. TX 79128 from Boots, Supplement I to Lancaster, M.C. & Shaw, J.W. (1979) unpublished report No. TX 79028. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Shepherd, G.M., Smithson, A. & Stobart, J.E. (1996) Prochloraz technical impurities BTS 40348, BTS 41995, BTS 43298 Acute oral toxicity to male Boots Wistar rats. Unpublished report No. TX 78093 (3rd edition, original report 1978) from AgrEvo. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Smithson, A. (1979) BTS 40542 (prochloraz technical): the effect of a single dose on cholinesterase activity in male and female Boots Wistar rats. Unpublished report No. TX 79086 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Smithson, A. & Lancaster M.C. (1980) Acute intraperitoneal toxicity of BTS 40542 prochloraz to male CD rats. Unpublished report No. TX 80004 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Wason, S.M. (1991) SN 604904 (R001041): Rat acute oral toxicity study. Unpublished report No. TOX/91/173-265 from Schering. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Watson, J.E., Lancaster, M.C. & Robinson, A.J. (1980a) Acute oral toxicity in male and female beagle dogs of the plant metabolites BTS 19036, BTS 44595 and BTS 44596. Unpublished report No. TX 80021 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Watson, J.E., Lancaster, M.C. & Robinson, A.J., (1980b) Acute oral toxicity in male and female beagle dogs of the plant metabolites BTS 19036, BTS 44595, BTS 44596 and BTS 45186. Unpublished report No. TX 80010 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
WHO (1999) Recommended Classification of Pesticides by Hazard and Guidelines to Classification 1998–1999 (WHO/PCS/98.21/Rev. 1), Geneva, International Programme on Chemical Safety.Wilcox, P. (1978) BTS 40542 In vitro bacterial mutagenicity testing of pure and technical prochloraz. Unpublished report No. TX 78002 from Boots. Submitted to WHO by Aventis CropScience SA, Lyon, France.
Woodhouse, R.N., Almond, R.H. & Ball, M.G. (1979) Validation of the method of analysis and determination of homogenicity and stability of BTS 40542 in rodent and dog diets. Unpublished report No. TX 79059 from Boots, Addendum 1 to Chesterman, H. et al. (1981), unpublished report No. TOX/83/173-2. Submitted to WHO by Aventis CropScience SA, Lyon, France.
See Also:
Toxicological Abbreviations
Prochloraz (Pesticide residues in food: 1983 evaluations)