
PESTICIDE RESIDUES IN FOOD - 1983
Sponsored jointly by FAO and WHO
EVALUATIONS 1983
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Geneva, 5 - 14 December 1983
Food and Agriculture Organization of the United Nations
Rome 1985
PROCHLORAZ
TOXICOLOGY
IDENTITY
Chemical Name(s)
1-(N-propyl-N-(2-(2,4,6-trichlorophenoxy)ethyl))
carbamoylimidazole (IUPAC)
N-propyl-N-[2-(2,4, 6-trichlorophenoxy)ethyl]-1H-imidazole-l-
carboxamide (American Chemical Society)
Synonyms
SPORTAKR, FCB Code No. BTS 40542.
Structural formula
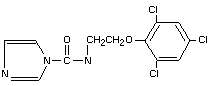 Molecular formula C15 H16 Cl3 N3 O2
Other information on Identity and Properties
Molecular weight: 376.5
State The pure material is a colourless, odourless
crystalline solid; the technical material is a
white to brown solid.
Melting point 38.5 - 41.0°C
Vapour pressure 0.57 × 10-9 Torr at 20°C
Solubility At 25°C 0.055 g/l in water; ca. 16 g/l in
kerosene; ca. 2 500 g/l in chloroform, xylene,
diethyl ether and toluene; ca. 3 500 g/l in
acetone.
Stability The stability in aqueous solution is pH-dependent,
prochloraz being more stable under slightly acidic
conditions than under slightly alkaline
conditions. Hydrolysis under alkaline conditions
leads to the formation of
N-propyl-N-2-(2,4,6-trichlorophenoxy) ethylamine,
the degradation following first order kinetics. At
pH 4.95 and pH 6.98 and 22°C, no degradation of
prochloraz was observed after 30 days. At pH 9.18
and 22°C, the half-life was 78.9 days. Prochloraz
tends to decompose on prolonged heating at high
temperatures (ca. 200°C).
Further information on the stability of prochloraz
in soil, water, during processing and storage and
on photodecomposition can be found under "Fate of
Residues".
Technical Material and Impurities
The Meeting was provided with information on impurities in the
technical material, all of which were said to range from < 0.2
percent to < 1.2 percent. No evidence has been found for the
presence of trichlorodibenzo-p-dioxins or tetrachlorobenzofurans and
all analytical procedures employed have failed to detect (i.e. less
than 1 ppb) any of the notably toxic
2,3,7,8-tetrachlorodibenzo-p-dioxin.
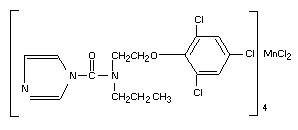
Identity of Prochloraz-Manganese Complex
Chemical Name
tetrakis-{1-[N-propyl-N-[2-(2, 4, 6-trichlorophenoxy)ethyl]]
carbamoylimidazole} manganese (II) chloride complex.
Synonyms
Prochloraz-manganese complex is the trivial name given to the 4:1
manganese coordination complex of prochloraz. FBC Code No. BTS 46828
or FBC 31114.
Structural formula
Molecular formula C15 H16 Cl3 N3 O2
Other information on Identity and Properties
Molecular weight: 376.5
State The pure material is a colourless, odourless
crystalline solid; the technical material is a
white to brown solid.
Melting point 38.5 - 41.0°C
Vapour pressure 0.57 × 10-9 Torr at 20°C
Solubility At 25°C 0.055 g/l in water; ca. 16 g/l in
kerosene; ca. 2 500 g/l in chloroform, xylene,
diethyl ether and toluene; ca. 3 500 g/l in
acetone.
Stability The stability in aqueous solution is pH-dependent,
prochloraz being more stable under slightly acidic
conditions than under slightly alkaline
conditions. Hydrolysis under alkaline conditions
leads to the formation of
N-propyl-N-2-(2,4,6-trichlorophenoxy) ethylamine,
the degradation following first order kinetics. At
pH 4.95 and pH 6.98 and 22°C, no degradation of
prochloraz was observed after 30 days. At pH 9.18
and 22°C, the half-life was 78.9 days. Prochloraz
tends to decompose on prolonged heating at high
temperatures (ca. 200°C).
Further information on the stability of prochloraz
in soil, water, during processing and storage and
on photodecomposition can be found under "Fate of
Residues".
Technical Material and Impurities
The Meeting was provided with information on impurities in the
technical material, all of which were said to range from < 0.2
percent to < 1.2 percent. No evidence has been found for the
presence of trichlorodibenzo-p-dioxins or tetrachlorobenzofurans and
all analytical procedures employed have failed to detect (i.e. less
than 1 ppb) any of the notably toxic
2,3,7,8-tetrachlorodibenzo-p-dioxin.
Identity of Prochloraz-Manganese Complex
Chemical Name
tetrakis-{1-[N-propyl-N-[2-(2, 4, 6-trichlorophenoxy)ethyl]]
carbamoylimidazole} manganese (II) chloride complex.
Synonyms
Prochloraz-manganese complex is the trivial name given to the 4:1
manganese coordination complex of prochloraz. FBC Code No. BTS 46828
or FBC 31114.
Structural formula
 Molecular formula C15 H16 Cl3 N3 O2)4 MnCl2
Other information on Identity and Properties
Molecular weight 1 632
Melting point 141-142.5°C
Solubility At 25°C 4 g/l in water, 7 g/l in acetone.
Stability The manganese complex is quickly dissociated in
water to give prochloraz. A suspension of the 50
percent wettable powder of the manganese complex
in distilled water has been shown to dissociate to
free prochloraz and manganese chloride to the
extent of approximately 55 percent in four hours
at 25°C. This process is unlikely to be affected
greatly at pH 6 to 9 Whiting & Dickson 1979.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
Mouse
After a single oral dose of 14C-phenyl labelled prochloraz at
100 mg/kg b.w. was administered to a group of male and female mice,
the over-all recovery of the given radioactivity was virtually
complete within 72h. Total recovery was complete (104 percent) with
urinary excretion (63 percent) being the major route. There were no
significant sex differences in the rate or route of excretion. Tissue
residues were highest in liver (5-7 mg/kg) and lowest in muscle,
genitals, eyes, spleen and fat (0.5-1.0 mg/kg). In all other tissues
the average value was below 2.5 mg/kg (Needham 1982a).
Rat
The excretion rate of prochloraz was monitored for 96 h in three
male and three female rats administered a single oral dose of
14C-phenyl-labelled prochloraz (91 mg/kg b.w.). The over-all recovery
of the administered radioactivity was 92.7 percent in male and 93.7
percent in female rate, urinary excretion being the predominant route.
The mean radioactivity was 61.7 percent in the urine and 30.8 percent
in the faeces of male rats. It was 54.8 percent in urine and 38.5
percent in the faeces of female rats. Highest residues, 96 h after
dosing, were found in the liver (3.7-6.2 mg/kg) and kidney (1.5-2.5
mg/kg), the tissues of female rats having higher residues (Needham &
Campbell 1980).
The chemokinetics were studied over a 96 h period, using an oral
dose of 100 mg/kg b.w. 14C-imidazole-radiolabelled prochloraz. Male
rats excreted the administered radioactivity almost quantitatively,
with approximately 83 percent in the urine, 9 percent in faeces and
2.4 percent in the expired air. Female rats excreted 56 percent in
urine, 21 percent in faeces and 1.3 percent in expired air. Plasma
radioactivity levels reached a peak in male rate approximately 4 h
after dosing, whereas the levels in female rats remained relatively
uniform but much lower. Liver radioactivity levels were significantly
raised within 15 min. and, in the kidney, 30 min. after dosing. They
generally remained constant for at least 8 h. Significant accumulation
occurred in brain and adipose tissues, which persisted over 1-8 h and
then declined markedly between 8 and 24 h. The levels of radioactivity
in the gastrointestinal tract, 24 h after dosing were significantly
higher in females compared to males. In general, plasma and tissues
showed a decline in radioactivity levels over the 96 h period, after
which time they reached small, though still significant, levels
(Turner & Gilbert 1977).
14C-imidazole ring-prochloraz was administered orally in another
study at 25 mg/kg b.w./day for 24 days. Residues of prochloraz
appeared to reach a plateau at about 15 days in the adrenals, ovaries,
female thyroid and male plasma, while other tissue levels continued to
increase over the entire 24-day period. The highest residues after
administration were found in the liver (20.88 ppm males and 25.03 ppm
females), and the lowest in the fat (2.82 ppm males and 3.72 ppm
females). The radioactivity was eliminated slowly from tissues,
significant residues being found after 96 h. Liver and plasma residues
had a half-life of less than three days and that of muscle was
approximately 17 days in males and 30 days in females (Hamilton 1978a)
3H-phenyl-labelled prochloraz was administered daily in another
study as repeated oral doses of 25 mg/kg for a 20-day period. Plasma
and tissue radioactivity were measured at specified intervals
throughout the period of treatment and at 96 h after the last daily
dose. Plasma levels were higher in males than in females. Tissue
levels for both sexes rose significantly for the first seven days of
dosing and thereafter rose only slightly for the remainder of the
dosing period. Highest levels of radioactivity were found in liver and
kidney and lowest in muscle and fat. Within 96h after treatment, the
radiolabelled phenoxy moiety of prochloraz was rapidly eliminated from
the body without leaving significant tissue residues (Boardman 1979).
Dog
A group of two male and two female dogs was given a single oral
dose of 14C-imidazole-labelled prochloraz at 20 mg/kg b.w., while a
second group received the same amount of 3H-phenyl-labelled
prochloraz. There was a marked difference between the plasma
concentrations of 14C and 3H-labelled prochloraz, suggesting that
the pesticide was metabolized by fission at the chain linking the
imidazole and trichlorophenol moieties. The dogs were killed 24 h
after the administration of the radioactive prochloraz and tissues
subsequently assayed for radioactivity. Both 14C- and 3H-radio-
labelled compounds showed high levels in bile and liver (Hamilton
1978b).
The distribution and excretion were studied in other groups of
male and female dogs following a single oral dose of 18 mg/kg b.w. of
14C-phenyl ring-labelled prochloraz. Peak plasma radioactivity
occurred 8-24 h after dosing and plasma half life was approximately 72
h. Excretion was rapid, 60 percent of the dose being eliminated in the
first 24 h; the over-all recovery after 96 hours was 96 percent with
faecal excretion as the major route (64 percent of the total dose). No
sex differences were noted in the rate of excretion or in the
magnitude of the tissue residues. Highest residues were found in the
liver and kidney (7.6 and 5.6 mg/kg, respectively) and lowest in bone,
cerebellum and CSF (less and 1 mg/kg). High residues were present in
the bile, indicating that biliary excretion represents a significant
route of elimination in dogs. This finding agrees with the high
percentage of radioactivity found in the faeces (Needham & Campbell
1982).
Goat
One lactating goat was treated with two oral doses of 14C-phenyl
prochloraz at 60 mg/kg b.w. given at a 13-day interval. Milk residues
after the first dose were highest 8 h after dosing (0.04 ppm) and
declined to 0.01 ppm within 48 h. Plasma residues were highest at 24 h
(0.33 mg/l), declining to 0.03 mg/l at 13 days. Twenty-four h after
the second dose, the highest tissue residues were found in the liver
(1.67 mg/kg), while residues in muscle were minimal (0.03 mg/kg)
(Campbell 1980).
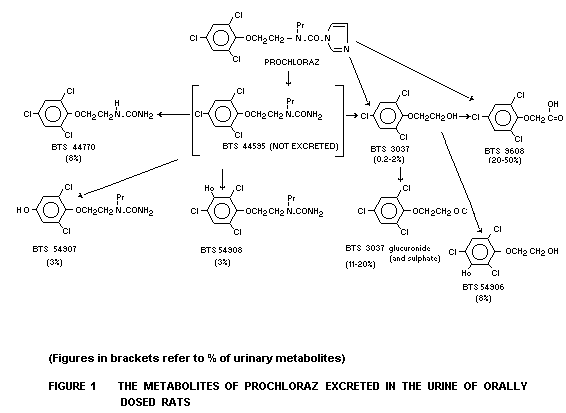
Biotransformation
The biotransformation was studied in a group of male and female
rats given an oral dose of 100 mg/kg b.w. 14C-imidazole ring-or
14C-phenyl ring-labelled prochloraz as a suspension in gum acacia or
dissolved in maize oil. A similar regimen was followed in separate
experiments with mice and dogs, except that only 18 mg/kg b.w. of the
labelled prochloraz was administered to dogs. Prochloraz was
extensively metabolized in the rat, with no unchanged compound being
excreted in the urine. The qualitative and quantitative profiles
depicted in Figure 1 suggest that metabolism proceeds via cleavage of
the imidazole ring into 2-carbon fragments, followed by hydroxylation
of the phenyl ring and/or side chain hydrolysis to form more polar
compounds. Two main metabolites: 2-(2,4, 6, trichlorophenoxy) ethanol
(BTS 3037); 2,4,6, trichlorophenoxyacetic acid (BTS 9608) and five
other minor metabolites were identified. The two main metabolites
accounted for about 80 percent of the urinary metabolites. A similar
metabolic pattern was found in the urine of mice, dogs and the female
goat (Needham 1982b).
Molecular formula C15 H16 Cl3 N3 O2)4 MnCl2
Other information on Identity and Properties
Molecular weight 1 632
Melting point 141-142.5°C
Solubility At 25°C 4 g/l in water, 7 g/l in acetone.
Stability The manganese complex is quickly dissociated in
water to give prochloraz. A suspension of the 50
percent wettable powder of the manganese complex
in distilled water has been shown to dissociate to
free prochloraz and manganese chloride to the
extent of approximately 55 percent in four hours
at 25°C. This process is unlikely to be affected
greatly at pH 6 to 9 Whiting & Dickson 1979.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
Mouse
After a single oral dose of 14C-phenyl labelled prochloraz at
100 mg/kg b.w. was administered to a group of male and female mice,
the over-all recovery of the given radioactivity was virtually
complete within 72h. Total recovery was complete (104 percent) with
urinary excretion (63 percent) being the major route. There were no
significant sex differences in the rate or route of excretion. Tissue
residues were highest in liver (5-7 mg/kg) and lowest in muscle,
genitals, eyes, spleen and fat (0.5-1.0 mg/kg). In all other tissues
the average value was below 2.5 mg/kg (Needham 1982a).
Rat
The excretion rate of prochloraz was monitored for 96 h in three
male and three female rats administered a single oral dose of
14C-phenyl-labelled prochloraz (91 mg/kg b.w.). The over-all recovery
of the administered radioactivity was 92.7 percent in male and 93.7
percent in female rate, urinary excretion being the predominant route.
The mean radioactivity was 61.7 percent in the urine and 30.8 percent
in the faeces of male rats. It was 54.8 percent in urine and 38.5
percent in the faeces of female rats. Highest residues, 96 h after
dosing, were found in the liver (3.7-6.2 mg/kg) and kidney (1.5-2.5
mg/kg), the tissues of female rats having higher residues (Needham &
Campbell 1980).
The chemokinetics were studied over a 96 h period, using an oral
dose of 100 mg/kg b.w. 14C-imidazole-radiolabelled prochloraz. Male
rats excreted the administered radioactivity almost quantitatively,
with approximately 83 percent in the urine, 9 percent in faeces and
2.4 percent in the expired air. Female rats excreted 56 percent in
urine, 21 percent in faeces and 1.3 percent in expired air. Plasma
radioactivity levels reached a peak in male rate approximately 4 h
after dosing, whereas the levels in female rats remained relatively
uniform but much lower. Liver radioactivity levels were significantly
raised within 15 min. and, in the kidney, 30 min. after dosing. They
generally remained constant for at least 8 h. Significant accumulation
occurred in brain and adipose tissues, which persisted over 1-8 h and
then declined markedly between 8 and 24 h. The levels of radioactivity
in the gastrointestinal tract, 24 h after dosing were significantly
higher in females compared to males. In general, plasma and tissues
showed a decline in radioactivity levels over the 96 h period, after
which time they reached small, though still significant, levels
(Turner & Gilbert 1977).
14C-imidazole ring-prochloraz was administered orally in another
study at 25 mg/kg b.w./day for 24 days. Residues of prochloraz
appeared to reach a plateau at about 15 days in the adrenals, ovaries,
female thyroid and male plasma, while other tissue levels continued to
increase over the entire 24-day period. The highest residues after
administration were found in the liver (20.88 ppm males and 25.03 ppm
females), and the lowest in the fat (2.82 ppm males and 3.72 ppm
females). The radioactivity was eliminated slowly from tissues,
significant residues being found after 96 h. Liver and plasma residues
had a half-life of less than three days and that of muscle was
approximately 17 days in males and 30 days in females (Hamilton 1978a)
3H-phenyl-labelled prochloraz was administered daily in another
study as repeated oral doses of 25 mg/kg for a 20-day period. Plasma
and tissue radioactivity were measured at specified intervals
throughout the period of treatment and at 96 h after the last daily
dose. Plasma levels were higher in males than in females. Tissue
levels for both sexes rose significantly for the first seven days of
dosing and thereafter rose only slightly for the remainder of the
dosing period. Highest levels of radioactivity were found in liver and
kidney and lowest in muscle and fat. Within 96h after treatment, the
radiolabelled phenoxy moiety of prochloraz was rapidly eliminated from
the body without leaving significant tissue residues (Boardman 1979).
Dog
A group of two male and two female dogs was given a single oral
dose of 14C-imidazole-labelled prochloraz at 20 mg/kg b.w., while a
second group received the same amount of 3H-phenyl-labelled
prochloraz. There was a marked difference between the plasma
concentrations of 14C and 3H-labelled prochloraz, suggesting that
the pesticide was metabolized by fission at the chain linking the
imidazole and trichlorophenol moieties. The dogs were killed 24 h
after the administration of the radioactive prochloraz and tissues
subsequently assayed for radioactivity. Both 14C- and 3H-radio-
labelled compounds showed high levels in bile and liver (Hamilton
1978b).
The distribution and excretion were studied in other groups of
male and female dogs following a single oral dose of 18 mg/kg b.w. of
14C-phenyl ring-labelled prochloraz. Peak plasma radioactivity
occurred 8-24 h after dosing and plasma half life was approximately 72
h. Excretion was rapid, 60 percent of the dose being eliminated in the
first 24 h; the over-all recovery after 96 hours was 96 percent with
faecal excretion as the major route (64 percent of the total dose). No
sex differences were noted in the rate of excretion or in the
magnitude of the tissue residues. Highest residues were found in the
liver and kidney (7.6 and 5.6 mg/kg, respectively) and lowest in bone,
cerebellum and CSF (less and 1 mg/kg). High residues were present in
the bile, indicating that biliary excretion represents a significant
route of elimination in dogs. This finding agrees with the high
percentage of radioactivity found in the faeces (Needham & Campbell
1982).
Goat
One lactating goat was treated with two oral doses of 14C-phenyl
prochloraz at 60 mg/kg b.w. given at a 13-day interval. Milk residues
after the first dose were highest 8 h after dosing (0.04 ppm) and
declined to 0.01 ppm within 48 h. Plasma residues were highest at 24 h
(0.33 mg/l), declining to 0.03 mg/l at 13 days. Twenty-four h after
the second dose, the highest tissue residues were found in the liver
(1.67 mg/kg), while residues in muscle were minimal (0.03 mg/kg)
(Campbell 1980).
Biotransformation
The biotransformation was studied in a group of male and female
rats given an oral dose of 100 mg/kg b.w. 14C-imidazole ring-or
14C-phenyl ring-labelled prochloraz as a suspension in gum acacia or
dissolved in maize oil. A similar regimen was followed in separate
experiments with mice and dogs, except that only 18 mg/kg b.w. of the
labelled prochloraz was administered to dogs. Prochloraz was
extensively metabolized in the rat, with no unchanged compound being
excreted in the urine. The qualitative and quantitative profiles
depicted in Figure 1 suggest that metabolism proceeds via cleavage of
the imidazole ring into 2-carbon fragments, followed by hydroxylation
of the phenyl ring and/or side chain hydrolysis to form more polar
compounds. Two main metabolites: 2-(2,4, 6, trichlorophenoxy) ethanol
(BTS 3037); 2,4,6, trichlorophenoxyacetic acid (BTS 9608) and five
other minor metabolites were identified. The two main metabolites
accounted for about 80 percent of the urinary metabolites. A similar
metabolic pattern was found in the urine of mice, dogs and the female
goat (Needham 1982b).
 Effects on Enzymes and Other Biochemical Parameters
In a series of investigations in male rats and mice prochloraz
was found to be an inducer of hepatic mixed function oxidases (MFO).
Rats were dosed orally with prochloraz at 10 and 100 mg/kg b.w. twice
daily for four days and killed 18 h after the last dose. Potent
induction of MFO occurred at the higher dose, but only a marginal
effect was seen at the low dose. Relative liver weight and microsomal
protein concentrations were increased at the high dose. The induction
spectrum of MFO was similar to that of phenobarbitone, with the levels
of cytochrome P-450, being increased by both dosages (Needham 1983a).
A similar MFO induction was noted when male rats were fed
prochloraz in their diet at 2 500 ppm for seven days. Rats fed a lower
dose (100 ppm) showed only a small increase in cytochrome P-450
(Riviere 1983).
The same pattern of hepatic MFO induction was observed in mice
after oral administration of doses of 10 and 100 mg/kg b.w. twice
daily for four days (Challis & Campbell 1983). Prochloraz was an
inducer of MFO following dietary administration at 325 and 1 300 mg/kg
in the diet for two weeks. When the compound was administered at 80
mg/kg for periods in excess of two weeks, the effect was marginal,
indicating that this concentration is close to the threshold level for
induction (Needham 1983b).
Effect of Dog Gastric Juice or Plasma on Prochloraz
In vitro studies showed prochloraz to be completely stable
after incubation with dog gastric juice or plasma for 30 min. at 37°C
or for 4 h at 37°C. Less than 2 percent underwent hydrolysis. The
initial step in the metabolism of prochloraz is therefore likely to
occur in the liver (Needham 1980).
TOXICOLOGICAL STUDIES
Special Study on Reproduction
Rat
Groups of rats (30 rats of each sex for the Fo generation and 25
of each sex for the F1 generation) were fed a diet containing 0,
37.5, 150 or 625 ppm prochloraz (nine weeks for Fo and eight weeks for
F1) prior to mating and throughout mating, gestation and lactation.
Two litters per generation were produced for two generations.
Observations included growth, food consumption, mortality and the
usual reproduction indices: mating, fecundity, male and female
fertility, gestation, lactation, pup mortality, litter and mean pup
weights, viability and terminal studies. In the males (Fo), receiving
625 ppm in the diet, a prolonged aggression was observed when rehoused
after both matings; this was not apparent among F1 males. Among Fo
females, the following overt clinical signs were noted in late
gestation and/or in the perinatal period: hunched posture, walking on
toes, piloerection and pallor. Sporadic mortality was recorded at the
different dose levels and was generally related to dystocia. Food
intake during the premating period showed no consistent dosage
relationship for all animals of both generations. However, consumption
was generally lower than that of the controls. Mean body weight gains
were depressed only in the premating period in the Fo animals of both
sexes, given 625 ppm, the same trend being noted during gestation.
There were no significant effects associated with mating performance
and pregnancy rate in the treated animals. Some adverse effects on
gestation periods were noted only at 625 ppm in both generations, with
a small number of females dying or being killed after exhibiting signs
of dystocia. There was also an increase in the proportion of females
with gestation periods in excess of 22 days.
A small number of females dosed at 625 ppm showed a total loss of
litters, a lowered mean litter size at birth, increased pup mortality
at birth and evidence of reduced pup body weight gain in the immediate
perinatal period at both matings of the Fo animals and at the first
mating of the F1a animals. A slight lowering of mean litter size at
both matings was recorded also for the Fo generation fed 150 ppm but
not for the F1 generation. However, none of the findings were
significantly different from the controls. At 37.5 ppm, a reduction in
the mean litter size at birth to day 12 postpartum was noted in the
first mating of the Fo generation. However, the results were again
within the range for controls at the testing laboratory.
No indication of any adverse incidence of structural anomalies
associated with treatment were recorded at terminal macroscopic
examination of the remaining F1a and F2a pups and all F1b and F2b
offspring.
Increased mean liver weight was recorded in weanling and adult
F1a males and F2a weanling females given 625 ppm in the diet. In the
same treated group, mean thymus weight in F1a weanlings of both sexes
and mean brain weight in the F1a female weanlings was lower than in
the controls. Microscopic examination of tissues of both sexes from
weanling F1a and F2a generations and F1a adults selected from
control and treated groups showed no changes that could be considered
attributable to exposure to the test compound; 37.5 ppm represented
the dietary no-effect level (Cozens et al 1982).
Special Studies on Teratogenicity
Rat
Groups of 20 mated Charles River CD rats were given oral doses of
6, 25 or 100 mg/kg daily from day 1 to 20 post coitum (day 1 was the
day when sperm was detected in the vaginal smear). A group of 31 rats
given the vehicle of 10 percent aqueous acacia solution served as
controls. Body weight, food consumption and overt signs of toxicity
were recorded throughout the study. The dams were killed on day 21 of
pregnancy.
The 25 and 100 mg/kg daily dosage elicited maternal toxicity as
evidenced by reduced food consumption, lower body weight and liver
enlargement; in addition, the dams showed increased salivation and
rubbed their noses in the sawdust litter. The only effect noted in the
rats given 6 mg/kg was a marginally lower body weight gain. There were
no macroscopic or microscopic findings attributable to treatment. The
number of corpora lutea was similar for all groups. The highest
dosage level appeared to be embryotoxic, as litter size, implantation
index and viability index were all slightly lower, while the incidence
of dead foetuses was marginally elevated. In addition, mean foetal
weight was lower, one litter containing unusually small foetuses that
appeared to have a gestational age of approximately 19 days. The mean
placental weight for both the male and female foetuses was higher for
the 25 or 100 mg/kg treated groups than for the control. A small
number of foetal abnormalities was noted among the treated groups but
no consistent pattern and no correlation between incidence and dosage
level was noted. The degree of calcification of the sternebrae and
vertebral arches was retarded in foetuses at the 100 mg/kg dose level.
This dosage elicited toxicity in both dams and embryos but produced no
teratogenic effects. The 25 mg/kg dosage was toxic in the dams. The
only findings in the 6 mg/kg group were marginally reduced body weight
gain and a very slight (non-significant) elevation of placental weight
(Beswick 1980).
Rabbit
Groups of 15 rabbits (New Zealand white strain) were administered
daily dosages of 0, 3, 12 and 48 mg/kg b.w. of prochloraz by
intragastric intubation on days 1 to 28 of pregnancy inclusive (day 1
= day of mating). The control group of 15 rabbits was dosed with the
vehicle, i.e. 10 percent acacia solution. All surviving dams were
killed on day 29 of pregnancy for macroscopic examination and the
foetuses removed for external visceral and skeletal examinations. No
treatment-related clinical signs and mortality were noted. Food intake
of the test group remained within 5 percent of the control value, with
a slightly reduced weight gain in all test groups during the first
week of dosing. The pregnancy rate was comparable for all groups. A
significant increase was noted in liver weight, with liver
discolouration in dams given 48 mg/kg day. A single dam from each of
the low-and high-dosage groups aborted completely, while one of the
control dams aborted partially. Litter size, post-implantation loss,
litter weight and mean foetal weight were considered unaffected by
treatment. Embryonic and foetal development, as assessed by incidence
of major malformations, minor anomalies and skeletal variations, were
similarly unaffected by treatment. No evidence of embryonic
teratogenic activity was therefore noted in New Zealand white rabbits
(Palmer et al 1980).
Special Studies on Mutagenicity
Ames test
Purified and technical prochloraz were tested in the Ames test
against Salmonella typhimurium TA 1535, TA 1537, TA 1538, TA 98 and
TA 100. All tests were performed in the presence and absence of a
liver microsome activation system (S-9) derived from
phenobarbitone-induced rats and tested at levels of 62.5, 125, 250,
500 and 1 000 mcg/plate. Positive control mutagens were included in
all tests to demonstrate the mutability of the bacterial strains and
the metabolizing capacity of the S-9 preparation; these comprised
cyclophosphamide, 6-aminochrysene and 2-animofluorene. Dimethyl
sulphoxide served as the negative control throughout. There was no
evidence of mutagenic activity against any of the strains employed but
the antibacterial effects of prochloraz precluded evaluation of
mutagenicity at concentration of 1 000, 500 and, in some cases, 250
mcg/plate (Wilcox 1977).
Micronucleus assay
Groups of five male and five female Charles River CD rats were
given two intraperitoneal injections of 6.25, 25.0 or 100 mg/kg b.w.
of prochloraz 24 h apart. Similar groups were given intraperitoneal
injections of cyclophosphamide 200 mg/kg b.w. to serve as positive
controls and intraperitoneal injections of the vehicle (maize oil) as
the negative controls. Six hours after the second injection all
animals were killed, smears from the bone marrow of the femur were
prepared and examined microscopically to determine the incidence of
micronuclei in the polychromatic erythrocytes. No significant increase
in the incidence of micronuclei was observed in any of the groups of
rats. Prochloraz was without clastogenic activity in rats of both
sexes when tested up to 100 mg/kg b.w. (Everest & Cliffe 1980).
Dominant lethal test
Groups of 20 male CD1 strain pathogen-free male mice (age 5-6
weeks) were given 0, 6, 25 or 100 mg/kg/day prochloraz in the diet for
eight weeks. After this treatment period, they were paired with
untreated females on a 1:1 basis for one week and then with a second
batch of untreated females for a further week. Approximately two weeks
after the discovery of a vaginal plug or 14 days after the midweek of
their pairing, the females were killed and their uterine contents
examined. There were no signs of reaction to treatment and mean weight
gains of all test groups compared favourably with that of the control
group. Intergroup variations in mean food consumption throughout the
entire treatment period were minimal and showed no consistent
dosage-related trend.
Mating performance of treated males and subsequent pregnancy
rates among untreated females were unaffected by treatment at any
dosage, except for one male at 25 mg/kg/day, which failed to induce
pregnancy at either mating. No obvious treatment-related macroscopic
changes were observed at terminal necropsy of the males. Mean weights
of the testes of treated males were slightly higher than the control
value but were not statistically significant. There was no evidence of
a dose-related effect on the pregnancy rate, mean number of
implantations, viable young, embryonic death and post-implantation
losses. Prochloraz showed no evidence of a dominant lethal effect in
male mice (Cozens et al 1980).
Mouse lymphoma mutation assay
Technical prochloraz was tested for mutagenic activity in mouse
lymphoma cells L5178 Y rendered heterozygous at the thymidine kinase
(TK) locus. The tests were carried out in the presence and absence of
a post-mitochondrial supernatant fraction from Arochlor 1254-treated
male rat livers and the co-factors required for mixed-function oxidase
activity. In an initial test carried out over a dose range of 0.16
mcg/ml to 1.6 mg/ml indicated, that prochloraz was toxic to the cells,
causing extensive cytotoxicity at a concentration of 158 mcg/ml. In
two further independent experiments at 50.0 mcg/ml and the second at
70.0 mcg/ml no significant increases in mutation frequency in the
prochloraz-treated cells over the vehicle control values were
observed. The concurrent positive control substances were
ethyl-methanesulphonate, which does not require the presence of S-9
mix, and 2-acetylaminofluorene, which requires the presence of S-9 mix
for metabolism. In both cases, high mutation frequencies were
obtained.
Technical prochloraz was not mutagenic to mouse lymphoma L 5178 Y
cells when tested over a series of dose levels, which extended into
the toxic range, in the presence or absence of S-9 mix (McGregor
et al 1983).
Special Studies on Carcinogenicity
Mouse
Groups of 52 male and 52 female mice CD-1 strain mice were fed
technical prochloraz in the diet at 78, 325 and 1 300 ppm. For each
sex, treatment continued until survival reached 20 percent, which was
106 weeks for males and 121 weeks for females. An additional group of
104 males and 104 females received the normal diet only. Observations
included clinical signs, mortality, body weight, food consumption and,
at 52 weeks and terminally, haematology and weight of selected organs.
Comprehensive histopathological examinations were made of tissues from
all surviving animals. Clinical signs were not evident, except in mice
given 1 300 ppm and in males treated at 325 ppm which were killed
because of distended abdomens. These animals were subsequently found
to have multiple liver tumours. No other overt signs of toxicity
related to treatment were observed. There was no mortality in the
course of the study attributable to prochloraz. Mice given 1 300 ppm
showed slightly reduced body weight gain. No differences were noted,
that were considered to be of toxicological significance, in the
haematological parameters between treated and control mice. Slightly
lower haemoglobin concentrations and lower total white blood cell
counts, due to a lower lymphocyte count, were noted at week 104 in
female mice treated with 1 300 ppm in diet.
At necropsy, an increase of liver weights was recorded in both
sexes given 1 300 ppm prochloraz. There was a dose-related increase in
the incidence of adenomas and adenocarcinomas of the liver in both
males and females. At 1 300 and 325 ppm, the increased incidence was
statistically significant and liver tumours (of hepatocyte origin)
were observed in both sexes, which were associated in a number of mice
with more than one tumour. Some cases at 1 300 ppm showed multiple
tumours as contributory factor to death. At 78 ppm, a slightly higher
incidence of liver tumours in female mice (6 out of 52) compared to
controls (5 out of 104) was noted, though the difference was not
statistically significant. In the males, the incidence of liver
tumours was similar in controls (37 out of 104) and treated mice (21
out of 52). There was no evidence of any other effects of treatment in
terms of either neoplastic or non-neoplastic pathology.
Treatment of mice was associated with a dose-related increase,
evident in both sexes, of liver tumours of all types. The incidence of
liver tumours was statistically significant at 1 300 ppm and 325 ppm
but not at 78 ppm prochloraz. The dietary dosage of 78 ppm was
equivalent to 7.5 mg/kg/day in males and 8.8 mg/kg/day in females
(Colley et al 1983).
Special Studies on Skin and Eye Irritation
A moderate to severe skin irritation produced in the rabbit
suggests that prochloraz is a skin irritant (Kynoch & Liggett 1979a).
Instillation of dilute solution of prochloraz into the everted lower
eyelid of one eye of New Zealand white stream rabbits produced a mild
conjunctival irritation in some animals which persisted up to three
days (Kynoch & Liggett 1979b).
Acute Toxicity
Prochloraz is of low toxicity to rodents when administered
orally. In rats, the LD50 ranged from 1 600 to 2 400 mg/kg b.w. and
in mice it was approximately 2 400 mg/kg b.w. Symptoms of intoxication
became apparent within 30-60 minutes of dosing and included CNS
depression, respiratory disturbance, ataxia, increased salivation,
piloerection, increased lacrimation and distended abdomen with signs
of gastrointestinal irritation. The acute toxicity of prochloraz in
rodents is summarized in Table 1.
Table 1. Acute Toxicity of Prochloraz in Rodents
Species Route LD50 (mg/kg) References
Rat Oral 1 600-2 400 Carter et al. 1978
Mouse Oral 2 400 Shaw & Carter 1976
Rat Dermal 5 000 Carter 1975
Rabbit Dermal 3 000 Kynoch & Liggett 1979a
Rat Inhalation (6h LC50) >420 mg/cu.m. Alexander & Clark 1978
Rat Intraperitoneal 400-800 Smithson & Lancaster 1980
In dogs given a single oral dose of 10, 100 or 250 mg/kg b.w. of
prochloraz, there were no deaths and the only toxic signs were emesis
and diarrhoea at 100 and 250 mg/kg b.w. (Stobart et al. 1978). One
female baboon given prochloraz at 50 mg/kg b.w. showed no toxic effect
while 250 mg/kg b.w. produced only emesis (Morgan et al. 1977).
Following dermal application to rabbits of 3 000 mg/kg for 24 h
there were no toxic effects except moderate to severe erythema and
oedema on the treated skin (Kynoch & Liggett 1979a).
Short-Term Studies
Mouse
Groups of 9 male and 9 female and 15 male and 15 female CD-1
strain mice were fed prochloraz in the diet at 6, 25, 100 and 400
mg/kg/day for 6 weeks and 13 weeks, respectively. Groups of 9 or 24
mice of each sex, given a diet without prochloraz, acted as controls.
Two additional groups of 15 mice of each sex were given a control diet
for 17 weeks and a diet containing prochloraz at a nominal dose level
of 400 mg/kg/day for 13 weeks, followed by a 4-week recovery period.
Overt signs of toxicity were recorded daily and body weight and food
consumption three times weekly; haematological and blood biochemistry
analyses were done at 6 weeks, 13 weeks and 4 weeks off-dose. All mice
were killed at the end of the dosing or recovery period, examined
macroscopically, the main organs were weighed and microscopic
examination of tissues performed.
No deaths occurred that were attributable to treatment with
prochloraz. An increased incidence of piloerection occurred in mice of
both sexes given 400 mg/kg daily. Food consumption of both sexes given
400 mg/kg daily was higher than that of the controls throughout the
dosing period and during the first week of the recovery period, but
was similar to the controls thereafter. A slight weight loss was
observed in the males given 100 mg/kg daily and in the females given
400 mg/kg daily. At week 6, haemoglobin concentration, PCV and red
blood cell counts were increased in both sexes given 400 mg/kg daily;
MCH was also marginally increased in the females and leucocyte counts
were increased in females due to lymphocytosis. At week 13 or after
four weeks recovery, these parameters were no longer affected by
treatment.
Plasma GPT activity was increased at week 6 in some mice of both
sexes given 400 mg/kg daily and some males at 100 mg/kg daily. At week
13, it was increased in the majority of females given 400 mg/kg daily
and in some mice of both sexes given 100 mg/kg daily. After four weeks
recovery, some of the males given 400 mg/kg daily were still affected.
Albumin content and consequently the albumin:globulin ratio, were
slightly reduced in both sexes given 400 mg/kg daily and in the males
given 100 mg/kg daily. At week 13, in both sexes at 400 mg/kg daily,
urea-nitrogen was slightly decreased and glucose levels were
increased.
Liver weight was increased at week 13 in both sexes given 25, 100
or 400 mg/kg daily. The ovaries in the females and the prostate and
seminal vesicles in males were small in the group at 400 mg/kg daily.
After 4 weeks recovery, liver weights were still higher in both sexes
given 400 mg/kg daily, but to a lesser extent than at weeks 6 and 13.
Histopathological examination showed minor liver changes with loss of
glycogen and periportal fat droplets in both male and female mice
receiving 400 mg/kg and in females receiving 100 mg/kg for 13 weeks.
Some male mice receiving 100 or 400 mg/kg also showed centrilobular
hepatocyte enlargement. In both male and female mice killed after four
weeks recovery, none of these changes were present. The no-effect
level in mice was 6 mg/kg/day (Gale 1980; Lancaster 1982).
Rat
Groups of 20 male and 20 female Boots-Wistar rats were given a
daily dose of prochloraz by oral gavage in 10 percent aqueous acacia
suspension at 0, 6, 25 or 100 mg/kg b.w. for 13 weeks. Further groups
of 20 male and 20 female controls given 100 mg/kg daily of prochloraz
remained untreated for four weeks after the end of the dosing period
and, in addition, groups of 10 males and females were given 0, 6, 25
or 100 mg/kg daily of prochloraz for six weeks. Overt signs of
toxicity observed in rats of both sexes were increased salivation at
25 or 100 mg/kg daily and in a few given 6 mg/kg daily. The incidence
of diarrhoea was increased during the first four weeks of dosing in
the males given 100 mg/kg daily and possibly in the first week in the
males given 25 mg/kg daily. In females, a few instances of diarrhoea
were recorded at each dose level.
Food consumption was not affected by treatment. However, test
females gained somewhat more weight than the controls at 25 or 100
mg/kg daily. At week 6, the only consistent haematological change was
a decreased haemoglobin concentration in males at each dose level and
in females given 100 mg/kg daily. A leucocyte count increase was noted
at each dose level in males due to lymphocytosis. At week 13, MCV was
slightly decreased at each dose level in the males and haemoglobin
concentration was slightly decreased in females treated at 25 or 100
mg/kg daily and in males given 100 mg/kg daily after the four weeks
recovery period.
Serum bilirubin was slightly decreased in both sexes at each dose
level at week 6, in the male at each dose level and in the females
given 6 or 100 mg/kg daily at week 13. Serum potassium was slightly
increased at week 6 in the males at 25 or 100 mg/kg and females at 100
mg/kg daily. UGOT excretion was slightly elevated in a few rats of
both sexes at each dose level. Urinary protein content was reduced in
the females at each dose level and in males given 100 mg/kg daily.
Liver weight at week 6 was increased in both sexes given 100
mg/kg daily and in the females given 6 or 25 mg/kg daily; kidney
weight was increased in both sexes given 100 mg/kg daily; spleen
weight was reduced in the males in each dose group; ovary weight was
increased in each dose group and thyroid weight was also increased in
females given 6 or 100 mg/kg daily. After the four-week recovery
period, the weights of the liver, ovaries and thyroids of the females
were slightly higher than the control values. The changes in liver
cell size correlated closely with the increase in liver weight. The
only instance where such correlation was not apparent was at week 13
in the females given 6 mg/kg daily, where the centrilobular cells were
slightly larger but liver weight was unchanged.
No other findings, that could be related to treatment, were
observed at microscopic examination of tissues from the rats given 100
mg/kg daily and killed at week 13. Various treatment-related effects
were noted in the rats of both sexes given 6 mg/kg daily of prochloraz
and a no-effect level was therefore not established. However, all
these effects were marginal (Lancaster & Shaw 1979a).
Dog
Groups of four male and four female beagles were given daily
doses of 1, 2, 5, or 7 mg/kg of prochloraz by gastric intubation for
13 weeks. Groups of eight males and eight females received 20 mg/kg
daily or the vehicle (10 percent aqueous acacia solution)
concurrently. At the end of the dosing period, four males and four
females from each of the 20 mg/kg and control groups were kept for
another four weeks without treatment.
Overt signs of toxicity observed during the dosing period
included isolated bouts of emesis and increased salivation in some
dogs from all groups, including controls, although these were more
prevalent at the high dosage. All dogs at 20 mg/kg daily and one given
7 mg/kg daily occasionally produced yellow mucoid and/or liquid
faeces. No effects of treatment were detected on faecal occult blood
content. During the recovery period, no overt signs of toxicity were
observed. Reduced food consumption was recorded during the dosing
period in two dogs given 20 mg/kg daily. Body weight changes during
the study were comparable in control and treated groups, although
greater fluctuations were recorded in dogs given 20 mg/kg daily. There
was no treatment-related effect detected by ophthalmoscopy or
electrocardiography.
Transient slight decreases in erythrocyte count and/or
haemoglobin concentra- were recorded in a few females given 7 or 20
mg/kg daily. No other treatment-related changes in haematological
parameters were noted. Changes in the activity of serum enzymes were
recorded. The serum alkaline phosphatase was high during the dosing
period in the majority of dogs given 20 mg/kg daily and in some given
7 mg/kg daily. Serum leucine aminopeptidase was slightly elevated at
week 6 in some dogs given 20 and in one given 7 mg/kg and serum GPT
was increased in a dog given 7 mg/kg daily. No change was observed in
urinary parameters.
At necropsy, all dogs given 20 mg/kg daily and three given 7
mg/kg daily had large and heavy livers. Low prostate weights were
recorded at week 14 in three dogs and in another two dogs in the same
group at 17 weeks. One of these dogs, killed at week 10, also had
decreased testes weight. In addition, one dog, given 7 mg/kg daily,
had a low prostate weight relative to body weight. In two females at
the high dose killed at week 14, and one killed at week 18, clear
brown exudate from freshly sectioned mammary glands was observed.
There were no histopathological findings in dogs on the high
prochloraz dosage. Counts of liver nuclei revealed no statistically
significant difference in the cell liver size or in the micronuclei
count between control and treated dogs.
In dogs retained for the recovery period, liver size and weight
returned generally to within normal limits, suggesting that any effect
of treatment on these parameters was reversible. The changes of AP and
LAP also returned to normal during the recovery period. The dose
producing no adverse-effect was 2.27 mg/kg daily (Lancaster et al.
1979b; Lancaster 1980).
Long-Term Studies
Rat
Groups of 150 male and 150 female rats constituted the controls.
Two groups each of 80 males and 80 females were treated with 37.5 and
150 ppm and a further two groups of 90 males and 90 females were given
625 ppm prochloraz in their diet. When the survival approached 20
percent in any group, all animals of that sex group were killed (111
weeks of treatment for females and 115 weeks of treatment in the
males).
There were no overt signs of toxicity recorded during the study
that could be considered related to the treatment. During weeks 20 and
21, the majority of rats from all groups showed signs indicative of
sialodacryo-adenitis infection, with a further relapse of mild
infection in a few rats from each group during the weeks 66 and 67.
Male survival was similar to controls, while treated females survived
longer than controls. A lower food consumption with a reduced body
weight gain was recorded for both sexes given 625 ppm. At 150 ppm,
food consumption was marginally lower throughout in males and until
week 52 in the females. Ophthalmoscopic examination revealed no
abnormalities related to treatment. In a few rats, minor changes were
seen at week 26, which were considered sequelae of
sialodacryo-adenitis infection.
Periodic laboratory measurements of haematological, biochemical
and urinalysis parameters in a few treated rats at 625 ppm showed
changes, but these were minimal and considered of doubtful
toxicological significance. The changes in urinalysis were minor, i.e.
lower total protein and changes in the pH. A minimal higher activity
of serum glutamic oxalacetic transaminase was noted.
No macroscopic findings related to treatment were seen at interim
kill in week 13. However, at the 52-week kill, enlarged or swollen
livers were observed in one male and three females given 150 ppm and
in four males and one female given 625 ppm in the diet. Heavier livers
were noted in males and females given 625 ppm diet at week 52 and
lower pituitary weights were also recorded in a few rats.
Histopathological examination showed hepatic centrilobular enlargement
in females given 625 ppm; periportal glycogen loss and centrilobular
fat deposits were also present in some treated rats of both sexes.
There were no other microscopic findings related to treatment.
Enlarged and swollen livers were observed in a higher proportion
of male rats given 625 ppm than in the controls, and slightly heavier
liver weights were recorded for females given 625 ppm. The incidence
of hyperplastic lesions in the liver was slightly higher than in the
controls in both sexes given 625 ppm, but the differences were not
statistically significant. The incidence of all other non-neoplastic
findings was similar in all groups. There was no evidence of
carcinogenicity from the chronic administration of prochloraz. The
no-effect level for toxicological changes was 37.5 ppm, equivalent to
a mean intake of 1.3 mg/kg/day in males and 1.6 mg/kg/day in females
(Colley et al. 1982).
Dog
Technical prochloraz was fed to groups of five males and five
females at levels of 0, 30, 135 and 600 ppm (increased to 1 000 ppm
from week 57) for 104 weeks. Observations included clinical signs,
mortality, food and water consumption and, at weeks 0, 13, 26, 51, 78
and 104, ophthalmoscopy, electrocardiography and clinical laboratory
studies. These comprised haematology, biochemistry, urinalysis and
faecal occult blood. At necropsy, organs were weighed and
comprehensive macroscopic and microscopic examinations were carried
out.
There were four deaths during the study but none was attributable
to treatment. There were no overt signs of toxicity or effects of
treatment upon body weight, food or water consumption, ophthalmoscopic
or electrocardiographic findings that could be related to treatment.
There were a few changes in the biochemical parameters, including a
higher level of SAP for males receiving 600 ppm in the diet during
week 13 and, during weeks 16 and 50, the elevation was also apparent
in the females. After the dietary level was increased to 1 000 ppm for
this group, there was a marked increase in the SAP levels during week
60, which persisted throughout the rest of the dosing period. The mean
level for males receiving 135 ppm was also higher than the control
mean from week 13 onwards, but the difference was not so marked as at
600 ppm. There was a slight but inconsistent increase in the platelet
count, glucose and cholesterol values in the group receiving 600 to
1 000 ppm, but these could not be related definitely to treatment.
There was no effect of treatment on the urinary parameters or faecal
occult blood content.
Liver weights in five animals that received 600 to 1 000 ppm, one
that received 135 ppm and one control, when expressed as a percentage
of body weight, exceeded the normal upper limit. The mean prostate
weight for animals in the same treatment group was significantly lower
than the control mean weight.
Histopathological examination showed minimal prostatic atrophy,
low grade hepatitis and minimal swelling and rarefaction of
centrilobular hepatocytis at 600 to 1 000 ppm. One male dog given 135
ppm also showed some liver changes. There were no other
histopathological changes related to prochloraz.
The no-effect level was 30 ppm in the diet, equivalent to
approximately 0.92 mg/kg/day (Chesterman et al. 1981).
Observations in Humans
Prochloraz has been produced on a commercial scale since 1980. No
adverse human effects have been detected since then from synthesis
formulation or use of prochloraz or its formulation. No clinical or
laboratory data were included (Bonsall 1982).
COMMENTS
Prochloraz has low oral toxicity. Following oral administration
in the rat, 95 percent of the dose is rapidly absorbed. In rats, the
major excretion is via the urine; in dogs it is via the faeces.
Prochloraz is a demonstrated liver mixed function oxidase inducer.
In rats and rabbits, no evidence of teratogenic effects was noted
at maternally toxic dose levels. A multigeneration reproduction study
in rats did not show any adverse effects at doses up to 37.5 ppm.
Mutagenicity studies, including the Ames test, micronucleus test,
mouse lymphoma assay and a dominant lethal study, were all negative.
In 90-day studies in mice, rats and dogs, liver weight was
increased in all species. No-effect levels of 6 mg/kg/day and of 2.27
mg/kg/day were established for mice and dogs, respectively. However,
in rats 6 mg/kg/day induced increased liver weight and occasional
signs of intoxication (increased salivation, diarrhoea). A two-year
dog study established a no-effect level of 30 ppm.
A long-term rat study did not result in any evidence of oncogenic
potential. A no-effect level of 37.5 ppm in the diet was established.
A mouse carcinogenicity study of 106 weeks duration indicated an
increased incidence of liver adenomas and adenocarcinomas in both
sexes at dose levels of 325 ppm and above. Prochloraz has been shown
to be a hepatocarcinogen in mice.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat (male); 37.5 ppm in the diet, equal to 1.3 mg/kg b.w.
Dog: 30 ppm in he diet, equal to 0.9 mg/kg b.w.
Estimate of Acceptable Daily Intake for Man
0 - 0.01 mg/kg b.w.
FURTHER WORK OR INFORMATION
Desirable
Observations in humans.
REFERENCES-TOXICOLOGY
Alexander, D.J. & Clark, G.C. Preliminary acute inhalation toxicity
1978 study in male and female rats with pure prochloraz (single
exposure). Report TX78053 from Huntingdon Research Centre
and Boots, submitted to WHO by FBC Ltd. (Unpublished)
Boardman, L.E. Plasma and tissue distribution studies in the rat
1979 following single and repeated oral doses of 3H-prochloraz.
Report AX79004 from Hazleton and Boots submitted to WHO by
FBC Ltd. (Unpublished)
Beswick, A.M. The teratogenicity study of technical prochloraz in male
1980 and female rats. Report TX80024 from Boots submitted to WHO
by FBC Ltd. (Unpublished)
Bonsall, J.L. The human exposure to prochloraz since synthesis in 1974
1982 and commercial production in 1980. Report submitted to WHO
by FBC Ltd. (Unpublished)
Campbell, J.K. Residues in milk and tissues of a goat dosed orally
1980 with 14C-prochloraz. Report AX80026 from Boots submitted to
WHO by FBC Ltd. (Unpublished)
Carter, O.A. Acute dermal toxicity of prochloraz to the male Boots
1975 Wistar rat. Report TXM75079 from Boots submitted to WHO by
FBC Ltd. (Unpublished)
Carter, O.A., Smithson, A. & Burnett, R. Acute oral toxicity of
1978 prochloraz, prochloraz pure material, stage 3 and stage 4
technical material and mother liquor concentrate impurities
to male Boots Wistar rats. Report TX78118 from Boots
submitted to WHO by FBC Ltd. (Unpublished)
Challis, I.R. & Campbell, J.K. The effect of prochloraz on the hepatic
1983 mixed function oxidase system of the male mouse after oral
administration. Report METAB/83/6 from FBC submitted to WHO
by FBC Ltd. (Unpublished)
Chesterman, H. et al. Two-year toxicity study in beagle dogs of
1981 technical prochloraz - final report - repeated dietary
administration for 104 weeks. Report TOX/81/173-2 from
Huntingdon Research Centre and FBC submitted to WHO by FBC
Ltd. (Unpublished)
Colley, J. et al. Prochloraz chronic toxicity and carcinogenicity
1982 study in rats by dietary administration - 104 weeks (final
report). Report TOX/82/173-8 from Huntingdon Research
Centre, and FBC submitted to WHO by FBC Ltd. (Unpublished)
Colley, J. et al. Prochloraz tumorigenicity study in mice by dietary
1983 administration (final report). Report TOX/83/173/23 from
Huntingdon Research Centre and FBC submitted to WHO by FBC
Ltd. (Unpublished)
Cozens, D.D., Reid, Y.J., Woodhouse, R.N., Almond, R.H. Anderson, J.
1980 & Ball, S.I. Dominant lethal gene assay of technical
prochloraz in the male mouse. Report TX80077 from the
Huntingdon Research Centre and Boots submitted to WHO by FBC
Ltd. (Unpublished)
Cozens, D.D. et al. The effect of prochloraz on reproductive
function
1982 of multiple generations in the rat. Report TOX/82/173-5 from
Huntingdon Research Centre and FBC submitted to WHO by FBC
Ltd. (Unpublished)
Everest, R.P. & Cliffe, S. Technical prochloraz micronucleus assay in
1980 male and female CD rats of prochloraz. Report TX80003 from
Boots submitted to WHO by FBC Ltd. (Unpublished)
Gale, E.P. 90-day oral toxicity study with prochloraz technical to
1980 male and female CD1 mice. Report TX80040 from Hazleton and
Boots submitted to WHO by FBC Ltd. (Unpublished)
Hamilton, D.Y. The distribution and level of 14C-labelled residues in
1978a rats following repeated oral dosing with 14C-prochloraz at
25 mg/kg/day. Report AX78008 from Boots submitted to WHO by
FBC Ltd. (Unpublished)
Hamilton, D.Y. The distribution and level of radiolabelled residues in
1978b the tissues of the dog following a single oral dose of
prochloraz. Report AX78016 from Boots submitted to WHO by
FBC Ltd. (Unpublished)
Kynoch, S.R. & Liggett, M.P. Primary eye irritancy of prochloraz 40
1979a percent E.C. formulation (bfn 8099). Report TX79050 from
Huntingdon Research Centre and Boots submitted to WHO by FBC
Ltd. (Unpublished.)
Kynoch, S.R. & Liggett, M.P. Primary skin irritancy of prochloraz 40
1979b percent E.C. formulation (bfn 8099). Report TX79074 from
Huntingdon Research Centre and Boots submitted to WHO by FBC
Ltd. (Unpublished)
Lancaster, M.C. & Shaw, J.W. 90-day oral toxicity study with
1979a prochloraz technical in male and female Boots Wistar rats
(4-week off-dose period). Report TX79028 from Boots
submitted to WHO by FBC Ltd. (Unpublished)
Lancaster, M.C., Morgan, H.E. & Stobart, J.E. 90-day oral toxicity
1979b study with prochloraz technical in male and female beagle
dogs (4-week off-dose period). Report TX79010 from Boots
submitted to WHO by FBC Ltd. (Unpublished)
Lancaster, M.C. 13-week oral toxicity study with prochloraz technical
1980 (BX 9/DM 2723) in male and female dogs with a four-week
off-dose period. Histopathological examination of the
remaining tissues. Report TX80034 from Boots submitted to
WHO by FBC Ltd. (Unpublished)
Lancaster, M.C. PRochloraz: 13-week oral toxicity in the mouse -
1982 histopathological examination. Report TOX/82/173/-9 from FBC
Ltd. submitted to WHO by FBC Ltd. (Unpublished)
McGregor, D.B., Riach, C.G. & Brown, A.G. Technical prochloraz
1983 assessment of mutagenic potential in the mouse lymphoma
mutation assay. Report TOX/83/173-22 from Inveresk and FBC
submitted to WHO by FBC Ltd. (Unpublished)
Morgan, G.E., Patton, D.S.G., Shepherd, G.M. & Stobart, J.E. The acute
oral toxicity of bts 40542 (technical batch) to the female
baboon. Report from Boots Laboratories submitted to WHO by
FBC Ltd. (Unpublished)
Needham, D. The effect of dog gastric juice or plasma on prochloraz.
1980 Report AX80011 from Boots submitted to WHO by FBC Ltd.
(Unpublished)
Needham, D. & Campbell, J.K. The excretion of 14C-phenyl-labelled
1980 prochloraz in male and female rats after a single oral dose.
Report AX80037 by Boots submitted to WHO by FBC Ltd.
(Unpublished)
Needham, D. The excretion and tissue residues of 14C-prochloraz in
1982a male and female mice following a single oral dose of 100
mg/kg. Report METAB/82/32 from FBC submitted to WHO by FBC
Ltd. (Unpublished)
Needham, D. & Campbell, J.K. The excretion and tissue residues of 14C
1982 prochloraz in male and female dogs following a single oral
dose of 18 mg/kg. Report METAB/82/30 by FBC submitted to WHO
by FBC Ltd. (Unpublished)
Needham, D. The metabolism of prochloraz in the rat after oral
1982b administration. Report METAB/82/31 from FBC submitted to WHO
by FBC Ltd. (Unpublished)
Needham, D. The effect of prochloraz on the hepatic mixed-function
1983a oxidase system of the male rat after oral administration.
Report METAB/83/5 from FBC submitted to WHO by FBC Ltd.
(Unpublished)
Needham, D. The effect of prochloraz on the hepatic mixed function
1983b oxidase system of the mouse when administered at 80, 325 and
1 300 mg/kg diet for up to 14 weeks. Report METAB/83/7 from
FBC submitted to WHO by FBC Ltd. (Unpublished)
Palmer, A.K., Bottemley, A.M. & Billington, R. The effect of technical
1980 prochloraz on pregnancy of the New Zealand white rabbit.
Report TX80083 from Huntingdon Research Centre and Boots
submitted to WHO by FBC Ltd. (Unpublished)
Rivière, J.L. Prochloraz, a potent inducer of the microsomal
1983 cytochrome P-450 system. Pestic. Biochem. Physiol., 19:
44-52.
Shaw, J.W. & Carter, O.A. Acute oral toxicity to male CD 1 mice of
1976 prochloraz. Report TX76093 from Boots submitted to WHO by
FBC Ltd. (Unpublished)
Smithson, A. & Lancaster, M.C. Acute intraperitoneal toxicity of
1980 prochloraz to male CD rats. Report TX80004 from Boots
submitted to WHO by FBC Ltd. (Unpublished)
Stobart, J.E., Morgan, H.E., Patton, D.S.G. & Shepherd, G.M. Acute
1978 oral toxicity of prochloraz to male and female beagle dogs.
Report TX78049 from Boots submitted to WHO by FBC Ltd.
(Unpublished)
Turner, D.M. & Gilbert, C.M. Pharmacokinetic studies on 14C-labelled
1977 prochloraz in the rat. Report AX77001 from Hazleton and
Boots submitted to WHO by FBC Ltd. (Unpublished)
Wilcox, P. Prochloraz in vitro bacterial mutagenicity testing of
pure
1977 and technical prochloraz. Report TX78002 from Boots
submitted to WHO by FBC Ltd. (Unpublished)
RESIDUES
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Prochloraz is available as 40 percent and 45 percent emulsifiable
concentrate (E.C.) formulations for foliar and postharvest treatments
and is sold under the trademark Sportak. A 50 percent wettable powder
formulation containing a 4:1 prochloraz-manganese coordination complex
active ingredient (a.i.) (3.4 percent manganese) is available and
recommended for foliar application to some broad leaf crops,
ornamentals and mushrooms, which may be susceptible to phytotoxicity
from E.C. formulations. Prochloraz is available in co-formulation with
carbendazim, under the trademarks Sportak PF and Sportak ALPHA, for
foliar application to cereal and oilseed rape. A 200 g/l liquid
formulation is available for cereal seed treatment and a 25 percent
E.C. disinfectant for rice seed.
Prochloraz is used for the control of a variety of fungal
diseases on a variety of crops and is said to be especially active
against Ascomycetes and Fungi Imperfecti. It is currently registered
or approved for use in eight countries and similar measures are
pending or planned in many others. Nationally registered and/or
approved uses are summarized in Table 1.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue trials have been conducted in many countries,
representing a wide range of food crops, climatic conditions,
formulations and foliar, seed and postharvest treatments. Trials data
are summarized in Tables 2-13. Trials data from countries with
approved uses are available only for apples, watermelons, grapes
(Taiwan, province of China), cereal (Belgium, Denmark, France, German
Federal Republic, The Netherlands, United Kingdom) and mushrooms (The
Netherlands).
Sugarbeets
No information was available on good agricultural practices for
prochloraz on sugarbeets. The "recommended" application rate of active
ingredient is said to be 0.5-1 kg/ha for foliar treatment. No
preharvest interval (PHI) is given. Total residues of both free and
conjugated residues of prochloraz (BTS 40 542),
N-formyl-N'-propyl-N'-2(2,4,6-trichlorophenoxy) ethylurea (BTS 44596),
N-propyl-N-2 (2,4,6-trichlorophenoxy) ethylurea (BTS 44595) and
2,4,6-trichlorophenol (BTS 45186), hereafter referred to as total
residues of prochloraz and metabolites, are listed in Table 2. The
values (corrected for 90 percent recoveries) are expressed as
prochloraz equivalents but were determined as 2,4,6-trichlorophenol
after hydrolysis. All residues and the 0.036 mg/kg control are below
the limit of determination, said to be 0.1 mg/kg. Data are
insufficient to support a maximum residue level, even if good
agricultural practices were known.
The "recommended" application rate of active ingredient to
sugarbeet leaves is said to be 0.5-1 kg/ha for foliar treatment. No
PHI was provided. Total residues of prochloraz and metabolites,
expressed as prochloraz equivalents, on sugarbeet leaves were 0.97,
1.3 and 1.2 mg/kg at 5, 21 and 42 days, respectively, after the last
treatment in three trials (Table 2). The control was 0.15 mg/kg.
Lettuce
No information was available on good agricultural practices on
lettuce. Residues of prochloraz only, uncorrected for approximately 72
percent recoveries, ranged from 7.4 mg/kg 18 days after treatment to
0.41 mg/kg after 39 days and <0.07 mg/kg after 71 days with a limit
of sensitivity said to be 0.01 mg/kg, although one of the three
controls has apparent residues of 0.02 mg/kg. Recoveries were 65-76
percent at 0.1-0.5 mg/kg fortification levels.
Watermelons
Residue data reflecting good agricultural practices in Taiwan
Province of China are presented in Table 2. Residues of prochloraz
per se were 0.01 mg/kg in peel and flesh after the approved six-day
interval from last application to harvest. Information was not
provided on the analytical procedure used; a 0.003 mg/kg limit of
detection was claimed.
Citrus Fruits
No information was provided on good agricultural practices on
citrus fruits. Residues from postharvest trials, proposed for uses
(25-70 g/hl for dip treatments or 100-300 g/hl for spray treatments)
where the peels are discarded, are summarized in Table 3. A variety of
treatment and storage conditions are given, representing 23 trials in
five countries. In some cases prochloraz alone is determined, in
others prochloraz and its metabolites and in one case BTS 45 186.
Total residues are generally expressed as prochloraz equivalents,
although they are usually measured as BTS 45 186. Total residues after
storage at ambient of 4°C for up to 70 days and from a variety of
treatments and locations ranged up to 18 mg/kg in peel, 0.44 mg/kg in
flesh and 6 mg/kg on a whole fruit basis. Residue levels of free
prochloraz were not substantially different than those for total
residues, where a comparison was made. More details are reported under
"Fate of Residues, Storage and Processing".
Pome Fruits
Information on good agricultural practice and residue data
(prochloraz only) reflecting such practice on apples are available for
Taiwan Province of China, as well as data for apples and pears from
supervised trials in three additional countries for which good
agricultural practices for prochloraz on pome fruit are not known.
Apples
The most pertinent data were those from Taiwan Province of China,
for which information was available on good agricultural practices on
apples. Data represent recommended and 2X the recommended application
rate. No data were available for the recommended 9-day last treatment
to harvest interval, but they were given for 6 and 12 days. Residues
of prochloraz, per se, from nine applications were 0.07 and 0.01
mg/kg, respectively, at these intervals from the recommended
application rate. No information is available on the analytical method
used other than a claimed 0.003 mg/kg limit of detection. Other apple
residue data (parent compound only) are from application rates three
to eight times that for good agricultural practice known to the
Meeting. Over-all recoveries among studies submitted range from 64-92
percent with claimed sensitivities ranging from 0.001-<0.01 mg/kg.
Apparent residues in untreated controls, where given, are
<0.01-0.02 mg/kg. A reasonable limit of determination would appear
to be about 0.1 mg/kg. One study (Hayto 1977c) indicates a half-life
in apples of 6-7 days.
Pears
No information on good agricultural practice was available for
pears. The application rates are substantially greater than known good
agricultural practice for apples and residues are higher, as would be
expected.
Stone Fruits
No information on good agricultural practice for prochloraz on
stone fruit were available to the Meeting. The "recommended"
application rate for foliar treatment with the prochloraz-50 percent
W.P. manganese complex formulation was reported as 15-30 g a.i./hl. No
preharvest interval was given. Residue data were available on
apricots, nectarines, peaches, cherries and plums from four countries
(Table 2). Total residues of prochloraz and the metabolites were
determined as 2,4,6-trichlorophenol and expressed as prochloraz.
Residues in mature fruit ranged from a maximum of 0.19 mg/kg im plums
at 3 days, 1 mg/kg at 14 days, 0.82 mg/kg at 24 days to <0.05 mg/kg
over 75 days after multiple foliar treatments with either E.C. or W.P.
(manganese complex) formulations. Residues were generally lower for
other stone fruit at comparable intervals after treatment with the
W.P. formulations. At fortification levels of 0.05-1 mg/kg recoveries
are 70-113 percent over-all. Apparent residues in eight untreated
stone fruit controls were 0.009-0.022 mg/kg except one value of 0.06
mg/kg in four controls (plums). The limit of determination for stone
fruit was reported as 0.05 mg/kg.
Grapes
Information on good agricultural practice for the E.C.
formulation and residue data reflecting 1, 1.5, 2 and 4X recommended
application rates were available from one country.
Residues from two applications at recommended rates ranged from
0.39 mg/kg on the day of application to <0.01 mg/kg after 21 days and
0.1 mg/kg at the recommended 9-day preharvest interval. Higher
application rates and numbers of applications, with one or two
exceptions, did not make an appreciable difference in the residue
level at comparable intervals after application. No information was
provided on apparent residues in un-treated controls or on the
analytical procedure; a 0.003 mg/kg limit of detection was reported.
TABLE 1. REGISTRATIONS AND APPROVED USES FOR PROCHLORAZ
Country Product1 Commodity Approved Application Rate Number and timing of Pre-harvest
Product a.i. Applications Interval
(prochloraz)
Belgium SPORTAK 45EC Winter 1 l/ha 0.45 kg/ha One application at earstage in mixture na
wheat with either 0.125 kg/ha carbendazim,
1.2 kg/ha captafol or 1.6 kg/ha maneb.
Winter
barley 1 l/ha 0.45kg/ha One application at stage L or M2
Denmark SPORTAK Barley 100 ml/100 kg 0.2 g/kg na na
BEJOSE (seed
dressing)
SPORTAK 45EC Spring 1 l/ha 0.45 kg/ha One application at stage 4-10.5 or a
barley split dose at half rate with the first
application at stage 4-6 and the second
at stage 8-10.53.
Winter 1 l/ha 0.45 kg/ha One (stage 4-10.5) or two (stage 4-6 cereals
barley and 7-10.5) applications3. 1 month
Winter 1 l/ha 0.45 kg/ha Two applications (stages 5-6 and 9-
wheat 10.53).
Winter 1 l/ha 0.45 kg/ha One application (stages 5-7)3
rye
Eire SPORTAK 40EC Cereals 1 l/ha 0.4 kg/ha Up to three applications between leaf
sheaf erection and complete ear emergence. na
Table 1 (continued)
Country Product1 Commodity Approved Application Rate Number and timing of Pre-harvest
Product a.i. Applications Interval
(prochloraz)
France SPORTAK 40EC Wheat 1.125-1.875 0.45-0.75 Up to two applications (stages 6-7
l/ha kg/ha and 10.3-10.5)3
Barley 1.125 l/ha 0.45 kg/ha Up to two applications (stages 6-7
and 10.1)3 na
SPORTAK 45EC Wheat 1-1.66 l/ha 0.45-0.75 Up to two applications (stages 6-7
kg/ha and 10.3-10.5)3.
Barley 1 l/ha 0.45 kg/ha Up to two applications (stages 6-7
10.1)3
SPORTAK PF Wheat 1.5 l/ha 0.45 kg/ha Up to two applications (stages 6-7
and 10.3-10.5)3.
Barley 1.5 l/ha 0.45 kg/ha Up to two applications (stages 6-7
10.1)3.
Oil seed 1.5 l/ha 0.45 kg/ha Up to two applications, at beginning
rape and end of flowering. na
SPORTAK M Wheat 1.125 l/ha 0.45 kg/ha Up to two applications (stages 6-7
and 10.3-10.5)3.
Barley 1.125 l/ha 0.45 kg/ha Up to two applications (stages 6-7
10.1)3.
Table 1 (continued)
Country Product1 Commodity Approved Application Rate Number and timing of Pre-harvest
Product a.i. Applications Interval
(prochloraz)
German SPORTAK 40EC Wheat 1.2 l/ha 0.48 kg/ha One (stage 29-32) or two
Federal (stages 29-59) applications5.
Republic Cereals -
Barley 1.2 l/ha 0.48 kg/ha One (stage 29-32) or two 35 days6
(stages 29-49) applications5.
Winter 1.2 l/ha 0.48 kg/ha One application at stage 29-325.
rye Restricted to a maximum of three
applications in Winter cereal.
Netherlands SPORTAK 45EC Wheat 1 l/ha 0.45 kg/ha 1-2 treatments after infection.
Barley 1 l/ha 0.45 kg/ha 1-2 treatments after infection. Cereals -
8 weeks7
Winter 1 l/ha 0.45 kg/ha One or two applications.
barley
Ornamentals 0.4% 180 g/hl Flower bulb dip na
SPORGON Mushrooms 3/g/l/m2 1.5g/l/m2 One application of casing soil Mushrooms -
nine days after casing. 10 days
Taiwan SPORTAK 25EC Rice 2000 x 12.5 g/hl 24 hour rice seed soak. na
dilution
Grape 6000 x 4.17 g/hl Two to three applications, Grape -
dilution every 10 days. 9 days
Apple 3000 x 8.33 g/hl Application every 10 days. Apple -
dilution 9 days
Table 1 (continued)
Country Product1 Commodity Approved Application Rate Number and timing of Pre-harvest
Product a.i. Applications Interval
(prochloraz)
Watermelon 4000 x 6.25 g/hl 10 days. 6 days
dilution
United SPORTAK 40EC Cereals 1 /ha 0.4 kg/ha Up to three applications between na
Kingdom leaf sheath erection and complete
ear emergence.
Oil seed 1.25 l/ha 0.5 kg/ha Up to three applications between
stem extension and 90% petal fall.
Ornamentals - 2000 ppm Propagation cutting dip, drench, na
foliar spray or compost incorporation.
SPORTAK 45EC Cereals 1 /ha 0.45 kg/ha Up to three applications between na
leaf sheath erection and complete
ear emergence.
Oil seed 1-1.33 /ha 0.45-0.6 Up to three applications between
rape kg/ha stem extension and 90% petal fall.
Ornamentals - 2000 ppm Propagation cutting dip, drench, na
foliar spray or compost incorporation.
SPORTAK Cereals 1.5 /ha 0.4 kg/ha Up to three applications between
ALPHA leaf sheath erection and complete
ear emergence. na
Oil seed 1.5 /ha 0.4 kg/ha Up to three applications between
rape stem extension and 90% petal fall.
Table 1 (continued)
Country Product1 Commodity Approved Application Rate Number and timing of Pre-harvest
Product a.i. Applications Interval
(prochloraz)
Ornamentals - 2000 ppm Propagation cutting dip, drench, na
foliar spray or compost incorporation.
SPORTAK FE Cereals 4 kg/ha 0.66 kg/ha Up to three applications between
leaf sheath erection and complete
ear emergence. na
Oil seed 3 kg/ha 0.5 kg/ha Up to three applications between
rape stem extension and 90% petal fall.
Ornamentals - 2000 ppm Propagation cutting dip, drench, na
foliar spray or compost incorporation.
SPORGON Mushrooms 3 g/l/m2 1.5 g/l/m2 One application, 1-10 days after Mushrooms -
casing. 10 days
0.6 g/l/m2 0.3 g/l/m2 Three applications, one 1-10 days Mushrooms -
after casing and then after the 2 days
first and second flush
1.2 g/l/m2 0.6 g/l/m2 Two applications, one 1-10 days Mushrooms -
after casing and then between 2 days
second and third flush.
Ornamentals - 2000 ppm Propagation cutting dip, drench, na
foliar spray or compost incorporation.
Table 1 (continued)
Notes: na - not applicable
nd - not defined
ns - not specified
1. The following formulations have been registered:
SPORTAK 40 EC - an emulsifiable concentrate containing 400 g/l prochloraz
SPORTAK 45 EC - an emulsifiable concentrate containing 450 g/l prochloraz
SPORTAK BEJDSE - a liquid seed dressing containing 200 g/l prochloraz
SPORTAK PF - an emulsifiable concentrate containing 300 g/l prochloraz and 80 g/l carbendazim
SPORTAK M - a twin-pack emulsifiable concentrate containing 400 g/l prochloraz and 450 g/l mancozeb
SPORTAK ALPHA - an emulsifiable concentrate containing 266 g/l prochloraz and 100 g/l carbendazim
SPORTAK FE - a wettable powder containing 165 g/kg prochloraz-manganese complex and 533.4 g/kg mancozeb
SPORGON - a wettable powder containing 500 g/kg prochloraz-manganese complex
2. Baggiolini cereal growth stages.
3. Feekes-Large cereal growth stages.
4. Residues of prochloraz alone.
5. Zadoks Decimal cereal growth stages.
6. A proposed MRL of 0.5 mg/kg for prochloraz and metabolites in cereal grain has been submitted.
7. Under review, pending further residue analysis.
8. Residues of prochloraz and metabolites measured as 2,4,6-trichlorophenol and expressed as prochloraz.
Table 2 Prochloraz Residues in Various Crops Following Supervised Trials
Country/ No. of Formulation Application Interval after Residues, Reference
Crop Year trials rate (a.i.) final application (mg/kg)1
(days)
Sugarbeet Italy
root 1981 1 40% E.C. 2 x 1 kg/ha 42-46 0.02 Longland 1983b
leaves 1.17
root 1 40% E.C. 3 x 1 kg/ha 21-25 0.04
leaves 1.32
root 1 40% E.C. 4 x 1 kg/ha 5-6 0.06
leaves 0.97
Lettuce The Netherlands 1 8% dust 8 kg/ha 70-73 0.013 Hayto 1979d
1978 80 0.013
2 8% dust2 8 kg/ha 18-19 7.383
39 0.043
42-46 0.123
70-73 <0.013
80 <0.013
1 8% dust2 6.4 kg/ha 70-73 0.043 Maclaine Pont et al. 1980
1 8% dust2 8 kg/ha 70-73 0.033
8% dust2 9.6 kg/ha4 78 <0.013
Watermelon Taiwan Province
peel of China
1982 1 25% E.C. 3 x 4.17 g/hl 0-1 0.013 Wang 1982
3 <0.013
5-6 <0.013
9 <0.013
12 <0.013
0-1 <0.013
3 <0.013
5-6 <0.013
9 <0.013
12 <0.013
Table 2 (continued)
Country/ No. of Formulation Application Interval after Residues, Reference
Crop Year trials rate (a.i.) final application (mg/kg)1
(days)
Watermelon Taiwan Province 3 x 6.25 0-1 0.023
peel of China 3 <0.013
5-6 <0.013
9 <0.013
flesh 12 <0.013
0-1 <0.013
3 <0.013
5-6 <0.013
9 <0.013
12 <0.013
Apple The Netherlands 2 50% W.P.5 2 x 25 g/hl 63 <0.013 Maclaine Pont et al. 1980
1978 84 <0.013
United Kingdom 2 25% W.P. 7 x 60 g/hl 0-1 0.473 Hayto 1976
1977 14-16 0.083
30-31 0.023
25% E.C. 8 x 51.48 g/hl 0-1 4.183 Hayto 1977c
8 1.763
18-19 0.683
30-31 0.173
50 0.023
Taiwan Province 1 25% E.C. 9 x 8.33 g/hl 0-1 0.243 Wang 1982
of China 3 0.123
1982 5-6 0.073
12 0.013
14-16 0.053
18-19 0.023
Table 2 (continued)
Country/ No. of Formulation Application Interval after Residues, Reference
Crop Year trials rate (a.i.) final application (mg/kg)1
(days)
Apple Taiwan Province 9 x 16.67 g/hl 0-1 0.813
of China 3 0.373
5-6 0.203
12 <0.013
14-16 0.083
18-19 0.073
Pear The Netherlands 1 50% W.P.5 1 x 0.1 kg/hl 21-25 0.033 Maclaine Pont et al. 1980
1978 1 x 0.2 kg/hl 21-25 0.063
2 x 0.1 kg/hl 14-16 0.263
2 x 0.2 kg/hl 14-16 0.243
Apricots Israel 1 50% W.P.5 1 x 60 g/hl 89 <0.05 Snowden 1983
1982
Cherries France 1 50% W.P.5 1 x 10 g/hl 70-73 <0.05
1982 1 x 20 g/hl 70-73 <0.05
4 x 20 g/hl 21-25 0.45
Nectarines France 1 50% W.P.5 5 x 20 g/hl 14-16 0.56
1982
Peaches South Africa 1 50% W.P.5 3 x 10 g/hl 132 <0.05
1982 1 3 x 20 g/hl 132 <0.05
1 8 x 10 g/hl 30-31 0.21
Plums Italy 1 40% E.C. 1 x 30 g/hl 102 <0.05
1982 1 2 x 30 g/hl 77 <0.05
1 3 x 30 g/hl 50 0.82
Table 2 (continued)
Country/ No. of Formulation Application Interval after Residues, Reference
Crop Year trials rate (a.i.) final application (mg/kg)1
(days)
Plums South Africa 1 50% W.P.5 1 x 30 g/hl 50 0.21
1981 1 1 x 40 g/hl 50 0.28
1 2 x 30 g/hl 14-16 0.97
1 2 x 40 g/hl 14-16 0.60
1982 1 50% W.P.5 3 x 10 g/hl 104 <0.05
3 x 20 g/hl 104 <0.05
6 x 10 g/hl 5-6 0.12
4 x 10 g/hl 103 <0.05
4 x 20 g/hl 103 <0.05
7 x 10 g/hl 5-6 0.19
Grapes Taiwan Province 1 25% E.C. 2 x 4.17 g/hl 3 0.393 Wang 1982
of China 5-6 0.193
1982 8 0.203
9 0.103
12 0.073
21-25 0.103
39 0.053
42-46 <0.013
1 25% E.C. 2 x 6.25 g/hl 3 0.493
5-6 0.263
8 0.203
9 0.253
12 0.133
21-25 0.133
39 0.053
42-46 <0.013
Table 2 (continued)
Country/ No. of Formulation Application Interval after Residues, Reference
Crop Year trials rate (a.i.) final application (mg/kg)1
(days)
Grapes Taiwan Province 1 25% E.C. 7 x 10 g/hl 3 0.203
of China 5-6 0.133
8 0.293
9 0.273
12 0.143
21-25 0.223
39 0.083
42-46 0.083
1 25% E.C. 7 x 16.67 g/hl 3 0.423
5-6 0.203
8 0.163
9 0.603
12 0.673
21-25 0.343
39 0.183
42-46 0.103
Strawberries The Netherlands 1 50% W.P.5 2 x 0.1 kg/hl 30-31 0.173 Maclaine Pont et al. 1980
1978 1 2 x 0.15 kg/hl 30-31 0.173
Almonds Israel
shell 1982 1 50% W.P.5 2 x 0.1 kg/hl 0.616 Churchill & Longland 1983d
kernel 0.106
whole nut 0.296
Table 2 (continued)
1 Total residues of prochloraz and major metabolites unless otherwise stated. Total residue measured as 2,4,6-trichlorophenol (BTS 45186)
and converted to prochloraz-derived residues by correcting for the molecular weight factor (1.906).
2 Co-formulation with dicloran.
3 Prochloraz residues only.
4 Preplanting application.
5 Prochloraz-manganese complex 50% wettable powder formulation.
6 Applications made at blossom and 20 days later. Preharvest interval estimated at about 2.5 to 3 months.
Table 3. Supervised Residue Trials With Prochloraz on Citrus Fruits - Post-Harvest Applications
Residues (mg/kg)1
Application
Country/ Crop No. of Formulation rate (a.i.) Peel Flesh Whole Fruit3 Reference
Year Trials and method
Australia Oranges 1 40% E.C. 25g/hl dip3 1.40-3.4 <0.05 n.d. Browne 1981a
1981 (1.45-3.954) (0.3-0.104)
1 40% E.C. 50g/hl dip3 1.58-3.36 <0.05 n.d.
(2.25-3.354) (<0.02-0.054)
Italy Oranges 1 25% W.P. 50g/hl dip5 1.59-1.72 0.04-0.05 0.71-0.80 Browne & Manley
1981 1 25% E.C. 50g/hl dip5 4.57-6.40 0.09-0.16 1.80-2.35 1982b
Lemons 1 25% W.P. 50g/hl dip5 3.61-4.04 0.08-0.14 1.58-1.83
1 25% E.C. 50g/hl dip 5.67-6.95 0.08-0.19 2.36-2.97
South Oranges 1 40% E.C. 100g/hl wax 1.38-2.79 0.03-0.08 0.38-0.72 Manley & Snowden
Africa brush6 (0.34-0.478) 1982b
1982 1 40% E.C. 200g/hl wax 2.45-3.74 0.02-0.07 0.65-0.94
brush6 (0.91-1.198)
1 40% E.C. 200g/hl wax n.d. n.d. 1.36-1.698)
brush7
Oranges 1 45% E.C. 50g/hl brush9 0.34 <0.05 0.08 Snowden & Manley
(0.108) 1983
1 45% E.C. 100g/hl brush9 0.47 <0.05 0.13
(0.16-0.178)
1 45% E.C. 200g/hl brush9 1.84 <0.05 0.39
(0.39-0.438)
1 45% E.C. 400g/hl brush9 3.07 0.07 0.63
(0.70-0.758)
Table 3 (continued)
Residues (mg/kg)1
Application
Country/ Crop No. of Formulation rate (a.i.) Peel Flesh Whole Fruit3 Reference
Year Trials and method
Spain Oranges 1 25% E.C. 50g/hl dip10 1.48-3.224 0.04-0.10 0.67-1.4511 Kelly 1982d
1979 1 25% E.C. 100g/hl dip10 1.40-4.084 0.04-0.11 0.67-1.8011
1980 Oranges 1 40% E.C. 300g/hl wax 3.3-4.74 0.32-0.38 n.d. Richards 1980b
spray12
1 40% E.C. 300g/hl wax n.d. 0.005-0.00813 n.d. Richards 1980e
spray12
1 40% E.C. 200g/hl wax 1.2-6.7 0.04-0.44 n.d. Richards 1980f
spray12
1 40% E.C. 250g/hl wax 3.7-7.9 0.04-0.30 n.d.
spray12
1 40% E.C. 300g/hl wax 5.4-6.4 0.06-0.13 n.d.
spray12
1981 Oranges 1 40% E.C. 300g/hl shower14 4.7-8.4 0.11-0.39 1.50-2.47 Browne 1981b
(1.7-7.34) (0.12-0.394) (0.64-2.434)
1981 Lemons 1 40% E.C. 300g/hl 11.0-17.9 0.17-0.33 3.66-6.13 Browne 1982c
shower15 (9.1-15.64) (0.27-0.304) (3.14-5.554)
United Oranges 1 40% E.C. 70g/hl dip16 5.78-7.81 0.07-0.15 1.37-2.07 Browne 1982i
Kingdom (3.55-5.104) (0.05-0.124) (0.96-1.354)
1981
Table 3 (continued)
n.d. - not determined.
1 Total residues of prochloraz and major metabolites unless otherwise stated (see notes 4 and 13). Total residue measured as
2,4,6-trichlorophenol (BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular weight factor (x 1.906).
2 Residue levels in whole fruit calculated from separate peel and flesh residues unless otherwise stated (see note 8).
3 Residue levels obtained from five sampling intervals at 1,2,4,8 and 16 days storage at ambient temperature after treatment.
4 Determination of free prochloraz levels only.
5 Fruit was stored for 57 days at 7°C after treatment.
6 Residue levels obtained from four sampling intervals under two storage conditions. Treated fruit was sent to the United Kingdom under
normal commercial refrigerated shipment and then stored at either 20° or 4°C, 44 days later. Samples of fruit from each storage
condition were then taken at 0,7,14 and 21-day intervals.
7 Prochloraz applied in mixture with 2,4,-0 and thiabendazole. Storage conditions as in Note 6 except sampled after 44 days refrigerated
shipment and 21 days storage under ambient or cool conditions.
8 Residue analysis of whole fruit.
9 Fruit was treated in July 1982, sent to the United Kingdom under normal refrigerated shipment and stored at ambient temperature until
September 1982.
10 Residue levels following storage of fruit for either 10 days at 20-22°C or 60 days at 3-4°C after treatment.
11 Residue level calculated from determination of free prochloraz residue in peel and total prochloraz plus major metabolites in flesh.
12 Residue levels following storage of fruit for either 7 or 14 days at ambient temperature after treatment.
13 Determination of free 2,4,6-trichlorophenol (BTS 45186) only, expressed as 2,4,6-trichlorophenol.
14 Residue levels following storage of fruit for either 14, 20 or 27 days at ambient temperature after treatment.
15 Residue levels following storage of fruit for either 12 or 16 days at ambient temperature after treatment.
16 Residue levels following storage of fruit for either 1,7, 21, 35 or 70 days in cold storage after treatment.
Strawberries
No information was provided on good agricultural practices for
prochloraz on strawberries. Limited residue data from the use of the
manganese complex W.P. formulation were provided (Table 2) with
residues of 0.2 mg/kg prochloraz found 16 days after treatment.
Recoveries were reported as >80 percent and sensitivity as
0.001-0.005 mg/kg.
Fruit with Inedible Peel
No information on good agricultural practice was available for
the use of prochloraz on fruit with inedible peel. Residue data
resulting from either foliar (Table 4) and/or postharvest applications
(Table 5) were available for several of these crops. The "recommended"
application rates were provided for most of them but no preharvest
intervals were specified.
Avocados
No information on good agricultural practice was provided for
avocados. Residue data at normal harvest and "recommended" application
rates were available for both foliar and postharvest treatments. At 2X
the 10-25 g a.i./hl "recommended" foliar W.P. (manganese complex)
application rate, maximum total residues on whole fruit basis were 2.5
mg/kg seven days after treatment, with approximately 10 percent of the
residue in the flesh (Table 4). No preharvest interval was given.
However, these data are questionable because of conflicting peel
residue levels in the data submission. Data were also available from
multiple applications of an E.C. formulation at 25-50 g a.i./hl,
although not even "recommended" uses were available for this
formulation. In these trials residues continually declined from 14 to
63 days after treatment, with estimated half-lives ranging from 40-98
days (mean 75.4 days). At these longer intervals residues in flesh
account for 20-30 percent of the whole fruit residue. The limit of
determination was said to be 0.15 mg/kg, although control values were
as high as 0.12, 0.16 and 0.39 mg/kg in flesh, peel and whole fruit,
respectively.
For postharvest treatments of avocados 25-50 g a.i./hl
(formulation unspecified) is "recommended", although no good
agricultural practice information was available. Ten trials reflecting
the "recommended" application rates were conducted in one country with
dip or spray E.C. applications (Table 5). Total residues ranged from
0.3 to 3.5 mg/kg on a whole fruit basis, with little difference in
values between stored at 23° for eight days or fruit frozen
immediately. Little difference was reported between 1X and 2X
recommended application rates. These residues levels are generally
comparable to those found from multiple foliar treatments. The limited
data indicate residues in the flesh at levels up to 30 percent of
those in whole fruit, even at relatively short intervals after
treatment.
Table 4 Prochloraz Residues in Fruit Following Supervised Trials - Foliar Application
Country/ Crop No. of
Year trials Formulation Application Interval after Mean residue level (mg/kg)1 Reference
rate (a.i.) final application
(days) Peel Flesh Whole fruit2
Australia Avocado 1 50% W.P. 7x15g/tree3 7 8.1-13.6 0.09-0.28 1.70-2.51 Cron & Longland 1983a
1981/2 (49.5 g/hl) (11)7 (0.16) (2.1)
South Avocado 1 45% E.C. 2x25g/hl4 14 1.52 <0.10 0.44 Churchill &
Africa 48 1.19 <0.10 0.36 Longland 1983b
1982 63 1.01 <0.10 0.30
1 45% E.C. 2x37.5g/hl4 14 1.85 0.12 0.61
48 1.45 <0.10 0.25
63 0.84 <0.10 0.25
1 45% E.C. 2x50g/hl4 14 2.63 0.17 0.83
48 1.94 0.18 0.59
63 2.16 0.14 0.60
Australia Mango 1 50% W.P. 6x100g/hl5 77 0.15-0.30 0.01-0.02 0.05-0.096 Browne & Manley 1982c
1981 (0.22)8 (0.01)8 (0.07)8
Israel Mango 2 50% W.P. 3x50g/hl 15 3.39-4.87 0.03-0.07 0.77-0.956 Longland &
1982 (4.2)8 Churchill 1983
Table 4 (continued)
1 Total residues of prochloraz and major metabolites, measured as 2,4,6-trichlorophenol (BTS 45186) and converted to
prochloraz-derived residue by correcting for the molecular weight factor (x1.906).
2 Residue in whole fruit (without stone) calculated from separate peel and flesh residues.
3 Applications made at approximately monthly intervals from fruit set to fruit full size.
4 Applications made with a seven-week interval between treatments.
5 Applications made from beginning of flowering to Pethenoccupic fruit drop.
6 Residue level in whole fruit including stone (calculated from peel/flesh residues).
7 Mean in parentheses.
8 Four samples.
Table 5 Prochloraz Residues in Fruit Following Supervised Trials - Postharvest Application
Mean residue level (mg/kg)1
Country/ Crop No. of Formulation Application rate
Year trials (a.i.) Peel Flesh Whole fruit2 Reference
Australia Avocado 1 40% E.C. 25 g/hl dip4 n.d. n.d. 0.88-1.313 Browne 1982a
1981 1 40% E.C. 50 g/hl dip4 n.d. n.d. 1.14-1.353
1982 2 45% E.C. 25 g/hl dip4 3.28-5.35 <0.10-0.12 0:30-0.557 Churchill &
2 45% E.C. 25 g/hi dip6 n.d. n.d. 0.16-1.019 Longland 1983e
1 45% E.C. 50 g/hl spray4 2.33-3.90 <0.10-0.11 0.28-0.367
1 45% E.C. 50 g/hl spray6 n.d. n.d. 0.34-0.429
1983 1 45% E.C. 25 g/hl dip6 n.d. n.d. 2.369
1 45% E.C. 50 g/hl dip6 n.d. n.d. 3.499
1981 Banana 1 40% E.C. 25 g/hl dip5 6.06-7.88 0.02-0.04 n.d. Browne 1982b
1 40% E.C. 50 g/hl dip5 5.18-9.97 0.03-0.05 n.d.
South Banana 2 45% E.C. 25 g/hl dip8 6.5-9.1 0.10-0.20 2.78-3.94 Churchill &
Africa 2 45% E.C. 50 g/hl dip8 9.8-16.6 0.14-0.23 4.02-6.75 Longland 1983a
1982
Israel Mango 1 40% E.C. 40 g/hl dip 6.10-8.32 0.02 1.277 Longland &
1982 Churchill 1983
Australia Papaya 1 45% E.C. 25 g/hl dip6 2.13-3.42 0.01-0.18 0.50 Cron & Longland 1983b
1982 1 45% E.C. 50 g/hl dip6 3.86-4.12 0.09-0.15 0.80
Table 5 (continued)
n.d. - not determined.
1 Total residues of prochloraz and major metabolites, measured as 2,4,6-trichlorophenol (BTS 45186) and converted to prochloraz-derived
residue by correcting for the molecular weight factor (x 1.906).
2 Residue in whole fruit calculated from separate peel and flesh residues unless otherwise stated (see notes 3 and 9).
3 Residue analysis of whole fruit (excluding stone).
4 Fruit stored for 7-8 days at 23°C after treatment.
5 Mean residue levels following storage of fruit for either 9,10,12 or 16 days at ambient temperature after treatment.
6 Fruit frozen within one to four hours after treatment.
7 Calculated whole fruit residue including weight of stone.
8 Fruit stored for 7 days at 8°C after treatment.
9 Residue analysis of whole fruit (including stone).
Bananas
No information was provided on goad agricultural practices for
the use of prochloraz on bananas. "Recommended" application rates for
foliar application are 10-25 g a.i./hl WP (manganese complex) for
foliar high volume applications (100-200 g a.i./ha low volume) and
25-50 g a.i./hl for postharvest treatment (formulation not specified).
No trials data were available for foliar applications, but trials
were reported from two countries for postharvest dips that reflected
"recommended" application rates (Table 5). Total prochloraz and
metabolite residues on a whole product basis ranged from 2.8-6.8 mg/kg
when fruit was stored for seven days at 8°C, with less than 2 percent
of the total fruit residue found in the flesh. No significant change
in residues were observed over the storage intervals of 7 to 16 days
or between 8°C and ambient temperatures. No significant difference
between residues resulted from the two application rates.
Analytical recoveries were generally 85 ± 15 percent. The limit
of determination was estimated at 0.05-0.1 mg/kg, although apparent
residues in untreated controls were as much as 0.32 mg/kg in the skin
and <0.2 mg/kg in whole fruit.
Mangoes
No information on good agricultural practice was available for
the use of prochloraz on mangoes. A 50 percent W.P. manganese complex
is "recommended" for foliar treatments at 10-25 g a.i./hl and at 25-50
g a.i./hl for postharvest treatment, although no formulation is
specified for the latter. One trial for foliar application was
conducted in each of two countries with multiple applications at 2X to
4X the "recommended" foliar rate (Table 4). Maximum total residues on
a whole fruit basis were 0.09 mg/kg from the higher application rate
77 days after treatment and up to 1 mg/kg 15 days after application at
the lower rate. In both cases, most of the residue was in the peel,
although flesh residues appeared to increase with time, being
approximately 3 percent of total fruit residue after 15 days to over
10 percent after 77 days (based on peel: flesh:seed ratios from the
77-day study).
One trial was conducted in one country for postharvest treatment
which reflected the "recommended" application rate. At 1.27 mg/kg on a
whole fruit basis, residues were similar to those of the 15-day
preharvest trial, although residues in flesh were only approximately
1 percent of the total for the postharvest treatment. Over-all
analytical recoveries for mangoes were generally 80 percent or better
and apparent residues in untreated controls were <0.02 mg/kg on a
whole fruit basis and up to 0.1 mg/kg on peel. The limit of
determination was estimated at 0.1 mg/kg.
Papaya
No information was available on good agricultural practices for
prochloraz on papaya. A 50 percent W.P. manganese complex is
"recommended" for foliar treatment at 10-25 g a.i./hl and 25-50 g
a.i./hl (formulation not specified) is "recommended" for postharvest
treatments. No preharvest intervals were given.
No residue information was available from foliar treatments, but
two postharvest dip trials with an E.C. formulation were conducted in
one country and reflected the "recommended" application rates (Table
5). Samples were frozen one hour after the dip treatment for analysis
45 days later. On a whole fruit basis, residues of prochloraz and
metabolites (corrected for approximately 80 percent recoveries) were
0.5 and 0.8 mg/kg for the two application rates. Approximately 15
percent of the whole fruit residue was in flesh. Apparent residue in
the untreated control was 0.023 mg/kg on a whole fruit basis. The
limit of determination was estimated as 0.1 mg/kg in flesh and peel.
Cereal Grains
Over 270 residue trials were conducted in 10 European and one
Asian country, which included foliar or seed treatments with six
different E.C. formulations alone or in combination with other
chemicals. Results are reported mostly as total residues of prochloraz
and metabolites, determined as the trichlorophenol but expressed as
prochloraz (Tables 6-9). Some are for residues of prochloraz only.
Good agricultural practice information was available for eight
countries.
Wheat
Over 100 field trials were conducted in nine countries,
representing 270 samples from a variety of formulations and treatment
conditions. Some trials reflected known good agricultural practices
and good agricultural practices could not be determined for others.
Over-all residues of prochloraz and major metabolites in mature grain
from foliar treatment ranged from 0.01-0.3 mg/kg and in straw at
harvest 0.08-16 mg/kg, although most grain residues were <0.1 mg/kg
(Table 6). The high grain value is from a 38-day preharvest interval
in a country whose good agricultural practices are not known, although
the application rate and preharvest interval were compatible with good
agricultural practices in other countries. Apparent residues in
untreated controls were generally <0.02 mg/kg in grain and <0.3
mg/kg in straw, but were occasionally higher in the latter. Residues
of prochloraz alone were lower and ranged from <0.01 to 0.03 mg/kg in
mature grain and <0.01 to 0.19 mg/kg in straw.
Table 6. Supervised Residue Trials with Prochloraz on Wheat at Harvest
Country/ Formulation No. of Application rate2 No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples final application
(days) grain straw
Austria 25% E.C. 2 0.5 2 70-78 <0.013 0.013 Kelly 1979a
1977 2 1.0 2 70-78 <0.01-0.013 <0.013
1978 22.5% E.C. 1 0.45 4 94 <0.02-0.02 3.66-8.39 Kelly 1979f
1 0.9 4 94 0.02-0.04 11.63-12.20
Belgium 45% E.C. 1 0.45 2 (0)5 0.02 n.d. Housden 1982a
1981 1 0.454 2 (0)5 0.01 n.d.
Denmark 25% E.C. 3 0.625 6 95-98 <0.02-0.02 n.d. Kelly 1979g
1979
40% E.C. 1 0.6 2 77 <0.02 2.1-2.5 Richards 1980d
1982 45% E.C. 1 0.45 1 56 0.106 2.01 Housden 1982c
2 2 x 0.23(19)7 2 75-85 <0.02 1.47-1.94
2 0.23 x 0.45(19)7 2 75-85 <0.02 1.87-2.36
2 2 x 0.45(19) 2 75-85 <0.02-0.02 1.56-2.07
2 2 x 0.45(19)7 2 75-85 <0.02 1.72-2.79
Finland 45% E.C. 2 0.45 2 28-50 <0.02 n.d. Heinanen 1983
1982
France 25% E.C. 2 0.5 6 105 <0.01-0.023 <0.01-0.023 Hayto 1978b
1977 2 1.0 6 105 <0.01-0.013 <0.01-0.183
2 2 x 0.5(38-46) 6 67-69 <0.01-0.033 0.01-0.123
2 2 x 1.0(38-46) 6 67-69 <0.01-0.013 0.01-0.0733
1 0.5 3 105 <0.0058 n.d. Kelly 1980b
1 1.0 3 105 <0.0058 n.d.
1 2 x 0.5(38) 3 67 <0.0058 n.d.
1 2 x 1.0(38) 3 67 0.008-0.0158 n.d.
Table 6 (continued)
Country/ Formulation No. of Application rate2 No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples final application
(days) grain straw
1978 25% E.C. 3 0.45 18 55-113 <0.02-0.06 0.50-9.66 Kelly 1979h
3 0.75 18 55-113 <0.02-0.11 0.88-9.91
1979 40% E.C. 1 0.45 3 80 <0.02 2.42-3.22 Richards 1980a
3 2 x 0.45(23-28) 9 72-77 0.04-0.10 2.40-16.3
1 2 x 0.45(8)9 3 77 0.04-0.08 3.93-4.80
30% E.C.10 1 0.45 3 80 <0.02 2.23-2.55
3 2 x 0.45(22) 9 61-81 <0.02-0.08 2.29-6.16
Federal 25% E.C. 3 0.5 4 81-107 <0.01-0.013 0.01-0.023 Hayto 1979c
Republic of 2 0.75 3 81 <0.013 0.01-0.023
Germany 1 1.0 1 107 <0.013 <0.013
1977
1978 25% E.C. 2 0.5 4 56-61 0.06-0.08 3.6-12.0 Kelly 1979c
1979 40% E.C. 3 2 x 0.48(21-30) 3 54-63 <0.02-0.06 1.0-5.9 Richards 1980g
1980 40% E.C. 3 2 x 0.48(17-23) 3 59-66 0.01-0.04 0.7-2.0 Reary 1981b
3 3 x 0.48(11-23/7-13) 3 59-66 0.03-0.06 1.1-3.6
1981 40% E.C. 5 3 x 0.48(11-29/8-19) 10 57-64 0.02-0.13 1.07-6.40 Browne 1982e
2 2 x 0.48(28-30) 3 43-60 0.06 1.88-3.59 Browne 1982f
3 3 x 0.48(9-22/8-19) 7 43-66 0.05-0.08 4.05-4.55
1 3 x 0.48(17/6) 1 55 0.05 0.4811
1982 30% E.C.10 1 0.45 3 85 <0.05 0.08-0.27 Housden &
Longland 1983
Table 6 (continued)
Country/ Formulation No. of Application rate2 No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples final application
(days) grain straw
Italy 25% E.C. 2 0.07 6 55 <0.01-0.013 0.02-0.093 Hayto 1977b
1977 3 1.0 9 39-55 <0.01-0.013 0.02-0.193,12
1978 25% E.C. 1 0.5 2 38 0.08-0.17 4.82-15.76 Kelly 1979d
1 0.7 38 0.13-0.29 6.29-14.73
The 25% W.P. 1 0.375 3 71 <0.013 <0.013 Hayto 1978a
Netherlands 25% E.C. 1 0.375 3 73 0.01-0.023 <0.01-0.013
1977 1 0.5 3 73 0.01-0.023 0.01-0.023
1978 25% E.C. 1 0.375 3 68 0.04 1.14-1.51 Kelly 1979e
1 0.75 3 68 0.04-0.06 5.66-8.14
1979 40% E.C. 1 0.5 4 71 <0.02 0.88-2.23 Kelly 1980c
1980 45% E.C. 2 0.45 6 69 0.01-0.02 0.41-0.77 Reary 1981c
1 0.675 2 106 0.01 0.21-0.51
1 2 x 0.45 (15) 3 69 0.01-0.02 2.21-3.29
Sweden 45% E.C. 8 0.45 8 67-91 <0.02 0.08-4.08 Longland 1983a
1982 2 2 x 0.45(15-29) 2 58-69 0.03-0.07 1.29-6.34
United 25% E.C. 2 0.4 6 90-115 <0.013 <0.013 Hayto 1977a
Kingdom 2 1.0 6 90-115 <0.013 <0.013
1976
1977 25% E.C. 4 0.5 12 124-126 <0.013 <0.013 Hayto 1979a
1979 40% E.C 2 2 x 0.4(36-37) 6 69-79 <0.02-0.06 4.2-11.2 Richards 1980c
10% S.D.13 1 0.214 2 31015 <0.02 <0.02
Table 6 (continued)
n.d.- not determined.
1/ Total residues of prochloraz and major metabolites, unless otherwise stated (see Notes 3 and 8). Total residue as 2,4,6-trichlorophenol
(BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular weight factor (x 1.906).
2/ Figures in parentheses indicate interval in days between applications.
3/ Determination of free prochloraz residues only.
4/ Prochloraz applied in mixture with carbendazim.
5/ Growth stage at application : 0 - heading.
6/ Residue analysis of whole ears.
7/ Prochloraz applied in mixture with fenpropimorph.
8/ Determination of free 2,4,6-trichlorophenol (BTS 45186) residues only, expressed as BTS 45186.
9/ Prochloraz applied in mixture with mancozeb.
10/ Co-formulation of prochloraz with carbendazim.
11/ Mean residue level in whole ears. A mean residue level of 2.31 mg/kg was found in the whole plant.
12/ Results from two trials.
13/ Seed dressing co-formulation with carboxin.
14/ Rate of application in g a.i./kg seed.
15/ Days after sowing treated seed.
Barley
Information on good agricultural practice for barley was
available from five countries and residue trials reflecting such uses
were reported from those countries and others (Table 7). At-harvest
residues of prochloraz and its major metabolites, from foliar
applications, ranged from <0.01-0.68 mg/kg in mature grain and
<0.01-15 mg/kg in straw (Table 7). Again, the higher values are
generally from the shorter preharvest intervals, which reflect good
agricultural practice, and from trials in which prochloraz was
co-applied with either carbendazim or mancozeb. The rates reflect
usage in the country where the trials were conducted. Most residues in
grain were <0.2 mg/kg. Although grain residues from these shorter
intervals were significantly higher than those from longer intervals,
residues in straw were not.
Analytical recoveries were variable, with means ranging from
70-100 percent. Apparent residues in untreated controls were <0.02
mg/kg in grain and up to 0.2 mg/kg in straw. Residues of prochloraz
alone were substantially lower, ranging from <0.01-0.02 mg/kg in
mature grain and <0.01-0.15 mg/kg in straw, which are comparable to
the values reported in wheat.
Rye
Total prochloraz and its metabolites, from foliar treatments in
mature rye grain or whole ears ranged from <0.02-0.1 mg/kg and in
straw from 0.4-2.5 mg/kg. Application rates reflected good
agricultural practice, although the preharvest interval of 72 to 109
days was significantly greater than the one month permitted (Table 8).
Analytical recoveries were typically >80 percent in grain and
somewhat lower in straw. Apparent residues in untreated controls were
<0.01 mg/kg in grain but as high as 0.04 mg/kg in straw.
Oats
Information on good agricultural practice for oats was not
available for the country in which residue trials were conducted or
from other countries. Residues of prochloraz and its metabolites were
<0.03 mg/kg and <1.6 mg/kg for whole ears and straw, respectively,
62 days after treatment at application rates considered good
agricultural practice for other cereals (Table 8).
Rice
No information on good agricultural practice was available for
rice, except for seed treatments in one country (for which there are
no data). Residues of prochloraz alone in unpolished brown rice 7-21
days after treatment at application rates comparable to good
agricultural practice on other cereals were <0.05 mg/kg (Table 8).
Residues of prochloraz and its metabolites would be expected to be
higher. Residues from seed treatments were lower, but the interval
after treatment was 151-168 days. Analytical recoveries were 86
percent in grain and apparent residues in untreated controls were
<0.005 mg/kg, the limit of detection.
Summary data for residues in cereal grain at harvest (Table 9)
represents foliar applications of all formulations. The data in Table
9 confirm those reported in Tables 6-8, since it is statistically
shown that maximum residues of prochloraz and its metabolites would
normally be less than 0.2 mg/kg at the longer preharvest intervals but
residues up to 0.4 mg/kg can occur. Other trials at shorter intervals
also resulted in higher grain residues than at longer intervals.
Similarly, Table 11 confirms the findings reported in Tables 6-8 for
residues in straw (up to 15 mg/kg). Tables 9 and 11 do not include
more recent studies (Housden 1982c; Longland 1983a; Housden & Longland
1983; Heinanen 1983) reported in Tables 6-8, but these would not
increase expected residues. Tables 9 and 11 also indicate that higher
residues can be expected from multiple or higher doses.
Residues of prochloraz alone were <0.03 mg/kg in cereal grains
at harvest, except for rice where residues were as much as 0.05 mg/kg
7 to 21 days after treatment. From seed treatment alone, residues of
prochloraz and its metabolites in mature grain were <0.02 mg/kg.
Generally, there was no conclusive evidence of increased residues as a
result of co-application of prochloraz and other agricultural
chemicals.
Residue decline studies are summarized in Table 10. Half-lives of
approximately 6 to 17 days could be estimated for ears or whole plants
of wheat or barley on the basis of sampling for at least three
successive intervals. Residues of prochloraz and its metabolites
ranged from 0.15 to 21 mg/kg on green plants or ears on the day of
last treatment to 0.07-3.6 mg/kg at 21 to 48 days, which approximates
typical preharvest intervals.
Almonds
No information was available on good agricultural practice for
prochloraz on almonds. Foliar treatments with the 50 percent W.P.
manganese complex are "recommended" at 15-30 g a.i./hl. No preharvest
interval has been reported. One trial was conducted with foliar
treatments at seven times the maximum recommended application rate
(Table 2) with total residues corrected for recoveries of >86
percent of 0.61, 0.1 and 0.29 mg/kg in shell, kernel and whole nut
(calculated from shell and kernel residues), respectively, about three
months after the last treatment. Apparent residues were 0.06 mg/kg in
un-treated controls on a whole nut basis and up to 0.09 mg/kg in the
shell. The limit of determination was estimated at 0.1 mg/kg.
Table 7 Supervised Residue Trials with Prochloraz on Barley at Harvest
Country/ Formulation No. of Application rate2 No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples final application
(days) grain straw
Austria 25% E.C. 2 0.5 2 61-73 <0.013 <0.01-0.02
1977 1 0.75 1 73 <0.013 0.023
1 1.0 1 61 <0.013 0.033
1 2x0.5(19) 2 54 <0.013 <0.013
1978 22.5% E.C. 2 0.45 69-75 <0.02-0.04 0.65-1.30 Kelly 1979f
1 0.9 75 0.02-0.04 1.0-2.1
Belgium 45% E.C. 3 0.45 6 (M)10 0.01-0.03 n.d. Housden 1982a
1981 7 0.4511 14 (M)10 0.01-0.11 n.d.
2 2x0.4511 (I+M)10 0.02-0.03 n.d.
Denmark 25% E.C. 1 0.75 96 <0.02 n.d. Kelly 1979g
1979 40% E.C. 2 0.6 76-86 <0.02-0.10 1.9-4.6 Richards 1980d
20% E.C. 1 0.6 86 <0.02-0.04 2.5-3.4
20% S.D. 2 0.28 132-1399 <0.02 <0.02
1982 45% E.C. 2 0.234 3 75-87 <0.02-0.03 0.84-1.10 Housden 1982c
4 0.45 4 61-80 <0.02-0.02 1.45-3.24
1 0.45 1 60 0.0514 3.02
2 2x0.45(14) 61-73 <0.02-0.02 1.61-2.59
France 25% E.C. 2 0.5 62-74 <0.01-0.013 <0.01-0.053 Hayto 1978b
1977 2 1.0 62-74 <0.01-0.023 0.01-0.153
1978 25% E.C. 1 0.45 64 0.02-0.06 1.77-8.86 Kelly 1979h
1 0.9 64 0.02-0.08 2.67-10.48
1 2x0.45(19) 62 0.04-0.09 4.23-7.00
1 2x0.9 (19) 62 0.04-0.17 11.19-11.95
Table 7 (continued)
Country/ Formulation No. of Application rate2 No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples final application
(days) grain straw
1979 40% E.C. 2 0.45 4 54-62 <0.02-0.17 5.76-10.81 Richards 1980a
2 2x0.45(21)5 6 38 0.36-0.68 7.20-12.12
30% E.C.6 2 2x0.45(13-21) 38-48 0.10-0.57 6.96-9.47
Fed. Rep. 25% E.C. 1 0.5 1 70 <0.013 <0.013 Hayto 1979c
of Germany 1 0.7 1 70 <0.013 <0.013
1977
1980 40% E.C. 2 0.48 77-79 0.02-0.06 0.06-1.0 Richards 1981b
2 2x0.48(19)12 77-79 0.02-0.06 1.1 -1.3
1981 40% E.C. 2 2x0.48(11-12) 61-62 0.02-0.06 0.95-1.27 Browne 1982f
1982 30% E.C.6 3 0.45 62-76 <0.05 0.23-0.83 Housden & Longland
1983
Netherlands 45% E.C. 1 0.45 3 70 0.04-0.06 0.78-1.21 Reary 1981c
1980
Sweden 45% E.C. 4 0.45 61-72 <0.0213 0.19-1.27 Longland 1983a
1982 2 2x0.225(8-15) 53-55 <0.02 0.24-1.21
2 2x0.45(8-15) 53-55 <0.02 0.34-2.86
United 25% E.C. 3 0.4 54-74 <0.013 <0.01-0.023,7 Hayto 1977a
Kingdom 4 0.5 19-64 <0.013 0.01-0.023,13
1976 2 1.0 64-74 <0.013 <0.013,20
1977 25% E.C. 4 0.5 76-78 <0.013 0.01-0.043 Hayto 1979c
2 1.0 76-78 <0.01-0.01 <0.01-0.02
1979 40% E.C. 3 2x0.4(18-29) 36-63 0.06-0.32 3.0 -14.5 Richards 1980c
Table 7 (continued)
n.d. not determined.
1/ Total residues of prochloraz and major metabolites, unless otherwise stated. Total residue measured as
2,4,6-trichlorophenol (BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular
weight factor (x 1.906).
2/ Figures in parenthesis indicate interval in days between applications.
3/ Determination of free prochloraz residues only.
4/ Prochloraz applies in mixture with fenpropimorph.
5/ Prochloraz applied in mixture with mancozeb.
6/ Co-formulation of prochloraz with carbendazim.
7/ Results from two trials.
8/ Rate of application in g a.i./kg seed.
9/ Days after sowing treated seed.
10/ Growth stages at application: M-heading, I-first joint.
11/ Prochloraz applied in mixture with chlorothalonil.
12/ Interval between treatments not given in one trial.
13/ Results from three trials.
14/ Residue analysis of whole ears.
Table 8 Supervised Residue Trials with Prochloraz on Cereals at Harvest
Crop Country/ Formulation Number of Application rate No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples application
(days) grain straw
Rye Austria 25% E.C. 1 0.5 1 91 0.013 <0.013 Kelly 1979a
1977 1 1.0 1 91 <0.013 <0.013
Denmark 45% E.C. 2 0.45 2 72-77 <0.02-0.10 1.44-2.48 Housden 1982c
1982
Fed. Rep. 40% E.C. 2 0.48 4 89-109 0.03-0.05 0.39-0.53 Browne 1982d
of Germany
1981
Oats Denmark 45% E.C. 2 0.45 2 62 0.02-0.034 1.19-1.64 Housden 1982c
1982
Rice Japan 25% E.C. 2 0.75 12 7-21 <0.01-0.043 n.d. Gato 1980
(brown, 1980 25% E.C. 2 3x0.75 12 7-21 <0.01-0.043 n.d.
unpolished) 25% E.C. 2 25 g/hl2 + 12 7-21 <0.01-0.043 n.d.
3x0.75
25% E.C. 2 25 g/hl2 4 151-168 <0.013 n.d.
n.d. = not determined.
1/ Total residues of prochloraz and major metabolites, unless otherwise stated. Total residues measured as 2,4,6-trichlorophenol
(BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular weight factor (x1.906).
2/ 48-hour seed soak.
3/ Determination of free prochloraz residues only.
4/ Residue analysis of whole ears.
Table 9 Summary of Residues in Mature Grain
Crop Application No. of Total residue (mg/kg)
(kg a.i./ha) results
Mean Std. Dev.
Wheat 1 × 0.375-0.5 36 0.03 0.03
Wheat 2 × 0.375-0.5 36 0.04 0.03
Wheat 3 × 0.375-0.5 12 0.06 0.03
Wheat 1 × 0.6-0.9 31 0.07 0.18
Barley 1 × 0.4-0.5 42 0.03 0.03
Barley 2 × 0.4-0.5 20 0.091 0.091
Barley 1 × 0.6-0.9 14 0.03 0.03
Barley 2 × 0.9 3 0.09 0.05
Rye 1 × 0.48 2 0.04
1/ Excluding a set of 11 results from four replicated applications
of prochloraz alone and with mancozeb, which were exceptionally
high (mean 0.42, std. dev. 0.18 mg/kg), residues in straw were not
exceptionally high. The high grain results may reflect poor
separation of grain and chaff in these samples (Richards 1980a).
Table 10 Supervised Trials on Residue Decline with Prochloraz
Crop Country/ No of Formulation Application rate2 Residues (mg/kg)1 after References
Year trials (kg/a.i./ha) final application (days)
35-36 56 75-85
Wheat Denmark 1 45% E.C. 0.45 whole plant 1.62 Housden 1982c
1982 whole ears 0.10
straw 2.01
2 45% E.C. 2x0.23(19)3 whole plant 0.33-0.94
grain <0.02
straw 1.47-1.94
2 45% E.C. 0.23+0.45(19)3 whole plant 1.11-1.76
grain <0.02
straw 1.87-2.36
2 45% E.C. 2x0.45(19) whole plant 0.70-2.02
grain <0.02-0.02
straw 1.56-2.07
2 45% E.C. 2x0.45(19)3 whole plant 1.38-1.44
grain <0.02
straw 1.72-2.79
Table 10 (Contd.)
Crop Country/ No. of Formulation Application2 After After final application (days) References
Year trials rate penultimate
(kg a.i./ha) application
(days)
20-29 0 15-22 37-42 54-63
Wheat Fed. Rep. 3 40% E.C. 2x0.48 green plant 0.80-1.644 1.62-2.554
of (21-30) ears 0.15-1.114 0.27-1.494 0.23-0.844 0.06-0.084 Richards
Germany grain <0.02-0.06 1980g
1979 straw 1.0-5.9
17-23 0 8-19 39-44 59-66
1980 3 40% E.C. 2x0.48 green plant 0.15-2.7
(17-23) green ears 4.7 -13.6 0.84-2.2 0.28-0.61 Reary
grain 0.01-0.04 1981b
straw 0.7 -2.0
7-13 0 8-19 39-44 59-66
3 40% E.C. 3x0.48 green plant 0.2 -4.7
(11-23/ green ears 5.5 -16.8 0.61-3.3 0.35-0.58
7-13) grain 0.03-0.06
straw 1.1 -3.6
8-19 0 7-14 31-46 58-64
1981 5 40% E.C. 3x0.48 green ears 0.16-0.96 3.87-12.50 2.42-5.85 0.31-1.16 Browne
(11-29/ whole plant 0.51-3.789 1982e
8-19) grain 0.04-0.31 0.02-0.13
straw 1.07-6.40
Table 10 (Contd.)
Crop Country/ No. of Formulation Application2 After After final application (days) References
Year trials rate penultimate
(kg a.i./ha) application
(days)
28-30 0 12-14 21-48 43-60
1981 2 40% E.C. 2x0.48 green ears 0.11-0.84 10.64-13.2 1.14-4.21 0.71-1.18 Browne
(28-30) whole plant 2.078 1982f
grain 0.038 0.06
straw 1.88-3.59
6-19 0 7-14 21-48 43-60
4 40% E.C. 3x0.48 green ears 2.96-8.27 13.90-21.40 3.61-7.25 0.49-2.32
(9-22/ whole plant 1.74-3.614 2.317
6-19) green ears+ 2.318
straw
grain 0.03-0.217 0.05-0.08
straw 4.05-4.55
ears 0.487
0 17 42 57 85
Wheat Fed. 3 30% E.C.5 0.45 whole plant 7.99-13.64 0.74-1.95 0.21-0.94 0.45.0.62 Housden &
Rep. of grain <0.05 Longland
Germany straw 0.08-0.27 1983
1982
34-36 60-87
Barley Denmark 2 45% E.C. 0.233 whole plant 0.64-0.74
1982 grain <0.02-0.03
straw 0.84-1.10
Table 10 (Contd.)
Crop Country/ No. of Formulation Application2 After After final application (days) References
Year trials rate penultimate
(kg a.i./ha) application
(days)
34-36 60-87
5 45% E.C. 0.45 whole plant 0.77-2.32
grain <0.02-0.02
straw 1.45-3.24
whole ears 0.058
2 45% E.C. 2x0.45 whole plant 1.03-3.18
(14) grain <0.02-0.02
straw 1.61-2.59
19 0 13 33-34 77-79
Fed. 2 40% E.C. 0.48 green plant 5.1-10.1 0.4-1.5 0.6-1.7 Richards 1981b
Rep. of grain 0.02-0.06
Germany straw 0.6-1.0
1980 2 40% E.C. 2x0.48 green plant 1.1-3.4 3.2-5.0 1.5-2.5 1.3-2.1
(19)6 grain 0.02-0.06
straw 1.1-1.3
11-12 0 10-18 27-48 61-62
1981 2 40% E.C. 2x0.48 green plant 0.91-7.99 10.40-18.60 Browne 1982f
(11-12) green ears 0.52-3.62 0.07-0.50
ears + straw 0.218
grain 0.088 0.02-0.06
straw 0.95-1.27
Table 10 (Contd.)
Crop Country/ No. of Formulation Application2 After final application (days) References
Year trials rate
(kg a.i./ha)
0 19 38-40 56 62-76
1982 3 30% E.C.10 0.45 whole plant 5.25-10.38 0.21-1.78 0.13-0.80 <0.05-0.78 Housden &
grain <0.05 Longland 1983
straw 0.23-0.83
35 72-77
Rye Denmark 2 45% E.C. 0.45 whole plant 0.85-1.62
1982 whole ears <0.02-0.10
straw 1.44-2.48
0 11 21 34 55-56 69 83 89 93 109
Fed. Rep. 1 40% E.C. 0.48 whole plant 9.6 1.16 0.20 Browne
of green ears 0.03 0.02 1982d
Germany ears 0.03
1981 grain 0.03 0.05
straw 0.39
1 40% E.C. 0.48 whole plant 13.8 1.33 0.05
green ears 0.14 0.05
ears 0.02
grain 0.10 0.03
straw 0.53
36-37 62
Oats Denmark 2 45% E.C. 0.45 whole plant 0.37-0.88 Housden
1982 whole ears 0.02-0.03 1982c
straw 1.19-1.64
Table 10 (Contd.)
1 Total residues of prochloraz and major metabolites, unless otherwise stated. Total residue measured as 2,4,6-trichlorophenol
BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular weight factor (x 1.906).
2 Figures in parentheses indicate interval in days between applications.
3 Prochloraz applied in mixture with fenpropimorph.
4 Results from two trials.
5 Co-formulation of prochloraz with carbendazim.
6 Interval between treatments not given in one trial.
7 Results from three trials.
8 Result from one trial.
9 Results from four trials.
Table 11 Summary of Residues in Mature Straw
Crop Application No. of Total residue (mg/kg)
(kg a.i./ha) results Mean Std. Dev.
Wheat 1 × 0.375-0.5 35 2.83 3.07
Wheat 2 × 0.375-0.5 36 4.85 3.23
Wheat 3 × 0.375-0.5 11 3.20 1.60
Wheat 1 × 0.6-0.9 22 5.03 4.42
Barley 1 × 0.4-0.5 20 3.30 3.05
Barley 2 × 0.4-0.5 26 6.95 3.64
Barley 1 × 0.6-0.9 13 3.36 1.92
Barley 2 × 0.6-0.9 3 11.6 0.4
Barley 1 × 0.48 2 0.46
No information on good agricultural practice was available for
the use of prochloraz on almonds. A 50 percent prochloraz-manganese
complex is "recommended" as a foliar treatment at a 15-30 g a.i./hl
application rate. Two applications at 3X this rate in one trial
resulted in maximum residues up to 0.7 mg/kg (Table 2). The limit of
determination has been estimated at 0.1 mg/kg although the apparent
residues in an untreated control were 0.07 mg/kg. Recoveries from
hulls amended with prochloraz at 1 mg/kg were 96±7 mg/kg.
Oilseed Rape
Information on good agricultural practice was available for
prochloraz on oilseed rape from two countries, from which data on
residue trials were also available. Residue data from 48 trials were
also reported from two countries for which good agricultural practice
was not known to the Meeting (Table 12). At preharvest intervals from
25 to 266 days, residues of prochloraz and its metabolites, reflecting
good agricultural practice, ranged from 0.01 to 0.33 mg/kg. Preharvest
intervals, if applicable, were not provided. The limit of sensitivity
was estimated at 0.05 mg/kg. However, since apparent residues in
untreated controls ranged from <0.01-0.15 mg/kg (but were usually
less than 0.05 mg/kg), a 0.2 mg/kg over-all limit of determination may
be more realistic. Analytical recoveries were > 85 ± 15 percent of
amended levels of 0.2-1 mg/kg.
Mushrooms
Information on good agricultural practice and corresponding
residue trials were available from two countries, as well as data from
residue trials from another country (total of 31 trials, Table 13).
The most common treatment was a single application of the
prochloraz-manganese complex formulation 1-10 days after casing at 1.5
g a.i./l sq m with a 10-day preharvest interval, or three applications
at 0.3 g a.i./l/m2, the first 1-10 days after casing and then after
the first and second flush, with a two-day pre-harvest interval. A
variation of the latter was also used at 2x 0.6 g a.i./l/sq m (Table
1), also with a two-day preharvest interval. Data extracted from Table
13 that reflect good agricultural practice are:
Country/ Applications Residue (mg/kg) Interval after Approved preharvest
and rate last application harvest
(g a.i./sq m) (days) interval (days)
The Netherlands 1 x 1.6 0.11-0.27 total residue 10 10
United Kingdom 2 x 0.6 1.4-1.6 total residue 4 2
0.6-0.8 free prochloraz
1 x 1.25 0.36 total residue 13 10
0.47 free prochloraz 28 10
3 x 0.3 0.24-0.43 total residue 2 2
0.07-0.1 free prochloraz 6 2
0.4-1.4 free prochloraz 3 2
Table 12 Supervised Residue Trials with Prochloraz on Rapeseed
Country/ Formulation No. of Application rate2 No. of Interval after Residues1 References
Year trials (kg a.i./ha) samples final application (mg/kg)
(days)
France 40% E.C. 2 0.45 8 73 0.02-0.04 Cron 1982
1980/1 2 0.453 8 73-76 0.01-0.03
1 0.60 4 73 0.02-0.03
30% E.C.4 2 0.45 8 73-76 0.02-0.03
1982 40% E.C. 4 0.45 12 37-51 0.03-0.15 Peatman & Snowden 1982
4 0.453 12 37-51 0.03-0.13
4 2x0.45 (12-15) 12 25-38 0.04-0.32
30% E.C.4 4 2x0.45 (12-15) 12 25-38 0.06-0.33
Denmark 45% E.C. 3 0.45 6 70-76 <0.05 Peatman & Snowden 1983
1982 3 2x0.45 (18-23) 7 53 0.07-0.19
Sweden 45% E.C. 3 0.45 6 47-72 0.12-0.28 Peatman & Snowden 1983
1982
United 40% E.C. 3 0.45 238-266 <0.05-0.07 Manley & Snowden 1982c
Kingdom 3 0.45 3 119-133 <0.05-0.19
1981/2 3 2x0.4(91-109)5 3 119-133 <0.05
3 3x0.4(91-109/ 3 64-75 <0.05-0.09
51-58)5
2 3x0.4(92-103/ 2 72-73 <0.05-0.06
51-57)
2 3x0.5(92-103/ 1 72-73 0.06
51-57)
Table 12 (continued)
1 Total residues of prochloraz and major metabolites, measured as 2,4,6-trichlorophenol (BTS 45186) and converted to
prochloraz-derived residue by correcting for the molecular weight factor (x 1.906).
2 Figures in parentheses indicate interval in days between applications.
3 Prochloraz applied in mixture with mancozeb.
4 Co-formulation of prochloraz with carbendazim.
5 Prochloraz applied in mixture with tolclofos methyl.
Table 13 Supervised Residue Trials with Prochloraz on Mushrooms
Country/ No. of No. of Application rate (a.i.) Residues (mg/kg)1 days after final application References
Year trials samples and timing2
3 5 10 14 20 21 27 45
Australia 1 1.25g/sq m at casing 0.1 0.1 <0.1 Housden
1981 1 2.50g/sq m at casing 0.23 0.13 0.14 1982
1 1.25g/sq m at casing +
0.63g/sq m after first flush 0.41-0.63 0.22
(24-day interval)
1 2.50g/sq m at casing +
1.25g/sq m after first flush 1.16-1.63
(24-day interval)
1 2.50g/sq m at casing +
2.50g/sq m after first flush <0.1
(24-day interval)
1 0.63g/sq m after first flush 1.06-2.98
1 1.25g/sq m after first flush 4.48-4.61
1 1.25g/sq m after first flush+
0.63g/sq m after second flush 0.29
(7-day interval)
1 2.50g/sq m after first flush+
1.25g/sq m after second flush 0.37
(7-day interval)
The 1 4 1.6g/sq m before inoculation 0.11-0.27 Browne
Netherlands 1 4 0.75g/sq m before inoculation+ 0.15-0.24 1982
1980 0.75g/sq m 14 days later
4 0.75g/sq m before inoculation+
0.75g/sq m after first flush 0.15-1.14
(23-days interval)
Table 13 (Continued)
Country/ No. of Application rate (a.i.) Residues (mg/kg)1 days after References
Year trials and timing2 1st application 2nd application 3rd application
19 7 4
United 1 0.61g/sq m after casing + 0.04 0.10 0.08 Richards 1981c
Kingdom 0.61g/sq m after first flush +
1980 0.61g/sq m after second flush
(19- and 10-day intervals)
1st application 2nd application
21 28 4 12 18
1 0.61g/sq m after casing + 0.04 0.04-0.11 1.37-1.58 <0.02
0.61g/sq m after second flush
(31-day interval)
14 0.61g/sq m after casing + 0.013 0.01-0.33 0.56-0.803 0.08-0.333 0.05-0.183 Whiteoak 1981
0.61g/sq m after second flush
(31-day interval)
Days after application
28 35 43 49
1 1.25g/sq m 7 days after inoculation 0.473 0.043 0.27-0.323 0.203
1 0.625g/sq m 7 days after inoculation 0.133 0.103 0.093 0.07-0.083
2nd application 3rd application
13 6 14 20
1 0.625g/sq m 7 days after inoculation 0.193 0.22-0.243 0.033 0.27-0.323
1 + 2x0.313g/sq m at 14-day intervals
thereafter
1 0.313g/sq m 7 days after inoculation
+ 2x0.313g/sq m at 14-day intervals 0.133 0.07-0.103 0-323 0.053
thereafter
Table 13 (Continued)
Country/ No. of Application rate (a.i.) Residues (mg/kg)1
Year trials and timing2 Days after application
2nd application 3rd application
5 12 3 14 21
1981 1 0.15g/sq m five days after inoculation 0.323 0.123 0.62-0.653 0.193 0.093 Whiteoak 1981
+ 2x0.15g/sq m at 18- and 14-day
intervals thereafter
45 0.3g/sq m + 2x0.3g/sq m. Timing as 0.20-0.373 0.11-0.643 0.39-1.443 0.10-0.313 0.06-0.073
given above
1 0.9g/sq m + 2x0.3g/sq m. Timing as 0.423 0.213 1.293 0.543 0.21-0.243
given above
1 1.5g/sq m + 2x0.3g/sq m. Timing as 0.273 0.523 0.993 n.d. 0.063
given above
1 3.0g/sq m + 2x0.3g/sq m. Timing as 0.623 0.543 0.743 0.343 0.953
given above
Table 13 (Continued)
Country/ No. of Application rate Residues (mg/kg)1 days after References
Year trials (a.i.) and timing2 1st application 2nd application 3rd application
13-14 15-16 18 3 5 7 10 12-13 14-16 2 3-4 7 9-10 12 14 17
United 1 0.3g/sq m 7 days after 0.24 0.20 0.79 0.426 0.30 0.30 0.377 0.23 0.51 0.43 0.33 1.03 0.32 0.23 0.28 0.22 Housden
Kingdom casing + 2x0.3g/sq m &
1981 at 18- and 14-day Whiteoak
intervals thereafter 1982
1982 1 0.3g/sq m 7 days after 0.26 0.14 0.85 0.24 0.24 0.26 0.58 0.24 0.61 0.45 0.61
casing + 2x0.3g/sq m (0.57)8 (1.11)8
at 15- and 17-day
intervals thereafter
Residues (mg/kg)1 days after application
13 15 18 22 25 28 31 34 36 39 42
1982 1 1.25g/sq m 7 days after casing 0.36 0.25 0.33 0.30 0.20 0.38 0.42 0.28 0.54 0.22 0.33
Table 13 (Continued)
n.d. = not determined.
1 Total residues of prochloraz and major metabolites, unless otherwise stated (see note 3). Total residue measured as
2,4,6-trichlorophenol (BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular weight factor (x1.906).
2 All treatments used the prochloraz-manganese complex 50% W.P. formulation.
3 Determination of residues of free prochloraz only.
4 Samples of mushroom also analysed for total residues of prochloraz and metabolites (see Richards 1981c)
5 Mean residue levels from four trials using spray volumes of 100, 200 or 400 l/sq.m.
6 Mean residue level in small and larger mushrooms (0.43 and 0.40 mg/kg respectively).
7 Mean residue level in small and larger mushrooms (0.52 and 0.21 mg/kg respectively).
8 Mushroom emerged before treatment (direct application).
Therefore, residues of prochloraz and its metabolites or of
prochloraz alone may be at least as much as 2 mg/kg from approved
practices. Differences between residues of prochloraz and its
metabolites, compared to free prochloraz, do not appear to be as high
as in cereals. Analytical recoveries of total residues were generally
> 70 percent, with apparent residues of 0.03 ± 0.02 mg/kg in
untreated controls and > 77 percent and 0.01 mg/kg for free
prochloraz.
FATE OF RESIDUES
The fate of prochloraz has been studied in plants and animals. In
plants, it is metabolized to
N-formyl-N'-1-propyl-N-(2-(2,4,6-trichlorophenoxy)ethyl) urea,
also known as BTS 44596 or the "formyl urea"; to
N-propyl-N-(2-(2,4,6-trichlorphenoxy)ethyl) urea, also known as
BTS 44595 or "the urea"; to 2,4,6-trichlorophenoxyacetic acid (BTS
9608), and finally to 2,4,6-trichlorphenol (BTS 45186), all of which
form stable conjugates (Figure 1). Animal metabolism has resulted in
characterization of urinary metabolites (see Figure 1 under
"Toxicology"), information of radioactivity distribution in tissues
from oral or dermal administration and rate and route of excretion.
Qualitatively, urinary metabolites are similar among several test
animals, although the major plant metabolites have not been identified
in the urine. Residues in animal tissues have not been identified.
Metabolism studies have also been conducted on the major plant
metabolite BTS 44596.
In Animals
Metabolism of prochloraz in rats, dogs, rabbits, mice and pigs is
discussed under "Biochemical Aspects".
Goat
A study was conducted to indicate the level of residues that
might occur in tissues and milk of lactating ruminant animals after
ingestion of prochloraz. A lactating goat was given two 60 mg oral
doses of 14C-phenyl labelled prochloraz by gelatin capsules, with 13
days between doses. The dosage was designed to reflect possible
residues resulting from consumption of 3 kg of straw with residues of
20 mg/kg of prochloraz at each dosing (versus maximum straw residues
of 12 mg/kg). Milk and plasma samples were taken at appropriate
intervals after each dose and tissue samples 24 h after the second
dose. After the first dose, residues of prochloraz equivalent in milk
decreased from 0.04 mg/kg after 8 h to 0.01 mg/kg (limit of
determination) after 48 h. Milk residues were 0.01 mg/kg and 0.03
mg/kg at 2.5 and 18.5 h, respectively, after the second dose. In
tissues, residues of prochloraz equivalent were 1.7 mg/kg in liver,
0.2 mg/kg in kidney, 0.36 mg/kg in adrenal, <0.05 mg/kg in fat,
0.03 mg/kg in muscle and <0.2 mg/kg in other tissues. Rumen
contents contained prochloraz equivalent of 0.65 mg/kg and bile 5.4
mg/kg. Residues were not characterized or identified (Campbell 1980).
A second and similar experiment was conducted using the
photodegradant and plant metabolite BTS 44596, with an interval of 8 h
between doses. Results were similar to those for prochloraz, although
the higher maximum milk residues of 0.07 mg/kg BTS-44596 equivalent 7
h after dosing decreased somewhat more rapidly (< 0.01 mg/kg after
31 h). Residues of BTS-44596 equivalent in tissues were somewhat less
than for prochloraz (liver 0.59 mg/kg, kidney 0.12 mg/kg, fat <0.01
mg/kg, muscle <0.01 mg/kg and less in other tissues as well),
Residues were not identified or characterized (Campbell & Needham
1980).
In another study a lactating goat was fed ad libitum for four
days with wheat straw containing 19 mg/kg field-incurred
14C-prochloraz equivalent residues. The straw was harvested at
maturity 11 weeks after treatment at an equivalent rate of 0.94 kg
a.i./ha (twice the recommended rate), Actual straw consumption was
approximately 300 g/day (or approximately 6 mg of prochloraz
equivalent/day), in addition to 1 700 g additional feed made available
daily. After four days, maximum tissue residues of prochloraz
equivalent, as determined by liquid scintillation counting was liver
0.05 mg/kg, kidney fat 0.04 mg/kg and 0.03 mg/kg in other tissues,
with a reported limit of determination of 0.02 mg/kg. Bile and rumen
residues were 0.12 and 0.13 mg/kg, respectively. Maximum residues in
milk were 0.006 mg/l and in plasma 0.079 mg/l. The limit of
determination for milk was 0.001 mg/kg in this experiment. Residues in
milk were relatively consistent over the four-day feeding period. Milk
residues were 0.002 mg/kg prior to the feeding of treated straw
(Campbell 1983).
In Plants
The fate of prochloraz has been studied in wheat (various
stages), citrus and apples. Soil uptake by sugar beet, which
represents a likely rotational crop, was also studied. The metabolic
fate in plants is illustrated in Figure 1.
Wheat
Wheat plants grown under glass were syringe treated at the sixth
leaf stage with a 25 percent a.i. formulation of 3H-labelled
prochloraz (labelled in the 3 and 5 positions of the benzene ring) at
a rate equivalent to 1 kg/ha. After 19 days, the green wheat tissue
was harvested and residues characterized and quantified by a variety
of analytical techniques. Neutral, acid and basic fractions were
analysed, with and without acid hydrolysis. After 19 days, 82.6
percent of the applied radioactivity remained and 96.7 percent of this
was extractable by the analytical procedures used (acetone-water
extraction). The principal residue was free BTS 44596, closely
followed by free and conjugated residues of BTS 44595, with lower
levels of free prochloraz and free and conjugated trichlorphenol,
conjugated trichlorophenoxyacetic acid and seven unknowns. Terminal
prochloraz equivalent was 7 mg/kg. Individual residues were:
% of applied % of terminal
radioactivity residue*
BTS 44596: N-formyl-N'-l-propyl-N-(2-(2,4,6-
trichlorophenoxy) ethyl) urea free 31.6 41
BTS 44595: N-propyl-N-(2-(2,4,6-trichlorophenoxy) free 11 13
ethyl) urea conj. 19.5 23.1
BTS 45186: 2,4,6-trichlorophenol free 1.4 1
conj. 6.8 4.9
BTS 9608 2,4,6-trichlorophenoxy-
acetic acid conj. 0.21 0.2
BTS 40542: Prochloraz (unchanged) free 1.1 1.5
Unknown (seven) 5.5 7.7
Radioactivity undetected 0.93 1.3
Radioactivity unaccounted for 4.56 6.3
82.6 100.0
* Expressed as prochloraz equivalents
None of the seven unknowns exceeded 4.3 percent of the terminal
residue. Analysis of barley plants treated in a similar manner
suggests that over 86 percent of the radioactivity applied to foliage
was absorbed by the leaf within 24 h (McDougall 1979).
In another study, separate plots of wheat at the flag leaf sheath
opening stage were treated with a 25 percent a.i. formulation of
either 3H-prochloraz labelled in the 3 and 5 positions of the phenyl
ring or 14C-prochloraz labelled in the 2 position of the imidazole
ring, at an equivalent rate of 1 kg a.i./ha. Mature wheat was
harvested 13 weeks after treatment and the grain, straw and chaff were
analysed.
Total residues, expressed as prochloraz equivalents, from the
phenyl-labelled prochloraz were grain 0.26 mg/kg, chaff 13.2 mg/kg and
straw 26.5 mg/kg. An almost identical distribution resulted from the
14C-prochloraz treated wheat. From the 3H-prochloraz treated wheat
76-80 percent of the recovered radioactivity was extractable from both
straw and grain. Attempts to hydrolyse polar fractions with HC1 were
unsuccessful, but better results were achieved with pyridine
hydrochloride, which hydrolyses conjugated residues containing the
2,4,6-trichlorophenyl moiety. The procedure liberates
2,4,6-trichlorophenol from both prochloraz and any metabolites
containing the 2,4,6-trichlorophenyl moiety. Results of the analysis
of the 3H-prochloraz treated wheat, expressed as a percentage of
recovered radioactivity in mature tissue, are summarized below:
Effects on Enzymes and Other Biochemical Parameters
In a series of investigations in male rats and mice prochloraz
was found to be an inducer of hepatic mixed function oxidases (MFO).
Rats were dosed orally with prochloraz at 10 and 100 mg/kg b.w. twice
daily for four days and killed 18 h after the last dose. Potent
induction of MFO occurred at the higher dose, but only a marginal
effect was seen at the low dose. Relative liver weight and microsomal
protein concentrations were increased at the high dose. The induction
spectrum of MFO was similar to that of phenobarbitone, with the levels
of cytochrome P-450, being increased by both dosages (Needham 1983a).
A similar MFO induction was noted when male rats were fed
prochloraz in their diet at 2 500 ppm for seven days. Rats fed a lower
dose (100 ppm) showed only a small increase in cytochrome P-450
(Riviere 1983).
The same pattern of hepatic MFO induction was observed in mice
after oral administration of doses of 10 and 100 mg/kg b.w. twice
daily for four days (Challis & Campbell 1983). Prochloraz was an
inducer of MFO following dietary administration at 325 and 1 300 mg/kg
in the diet for two weeks. When the compound was administered at 80
mg/kg for periods in excess of two weeks, the effect was marginal,
indicating that this concentration is close to the threshold level for
induction (Needham 1983b).
Effect of Dog Gastric Juice or Plasma on Prochloraz
In vitro studies showed prochloraz to be completely stable
after incubation with dog gastric juice or plasma for 30 min. at 37°C
or for 4 h at 37°C. Less than 2 percent underwent hydrolysis. The
initial step in the metabolism of prochloraz is therefore likely to
occur in the liver (Needham 1980).
TOXICOLOGICAL STUDIES
Special Study on Reproduction
Rat
Groups of rats (30 rats of each sex for the Fo generation and 25
of each sex for the F1 generation) were fed a diet containing 0,
37.5, 150 or 625 ppm prochloraz (nine weeks for Fo and eight weeks for
F1) prior to mating and throughout mating, gestation and lactation.
Two litters per generation were produced for two generations.
Observations included growth, food consumption, mortality and the
usual reproduction indices: mating, fecundity, male and female
fertility, gestation, lactation, pup mortality, litter and mean pup
weights, viability and terminal studies. In the males (Fo), receiving
625 ppm in the diet, a prolonged aggression was observed when rehoused
after both matings; this was not apparent among F1 males. Among Fo
females, the following overt clinical signs were noted in late
gestation and/or in the perinatal period: hunched posture, walking on
toes, piloerection and pallor. Sporadic mortality was recorded at the
different dose levels and was generally related to dystocia. Food
intake during the premating period showed no consistent dosage
relationship for all animals of both generations. However, consumption
was generally lower than that of the controls. Mean body weight gains
were depressed only in the premating period in the Fo animals of both
sexes, given 625 ppm, the same trend being noted during gestation.
There were no significant effects associated with mating performance
and pregnancy rate in the treated animals. Some adverse effects on
gestation periods were noted only at 625 ppm in both generations, with
a small number of females dying or being killed after exhibiting signs
of dystocia. There was also an increase in the proportion of females
with gestation periods in excess of 22 days.
A small number of females dosed at 625 ppm showed a total loss of
litters, a lowered mean litter size at birth, increased pup mortality
at birth and evidence of reduced pup body weight gain in the immediate
perinatal period at both matings of the Fo animals and at the first
mating of the F1a animals. A slight lowering of mean litter size at
both matings was recorded also for the Fo generation fed 150 ppm but
not for the F1 generation. However, none of the findings were
significantly different from the controls. At 37.5 ppm, a reduction in
the mean litter size at birth to day 12 postpartum was noted in the
first mating of the Fo generation. However, the results were again
within the range for controls at the testing laboratory.
No indication of any adverse incidence of structural anomalies
associated with treatment were recorded at terminal macroscopic
examination of the remaining F1a and F2a pups and all F1b and F2b
offspring.
Increased mean liver weight was recorded in weanling and adult
F1a males and F2a weanling females given 625 ppm in the diet. In the
same treated group, mean thymus weight in F1a weanlings of both sexes
and mean brain weight in the F1a female weanlings was lower than in
the controls. Microscopic examination of tissues of both sexes from
weanling F1a and F2a generations and F1a adults selected from
control and treated groups showed no changes that could be considered
attributable to exposure to the test compound; 37.5 ppm represented
the dietary no-effect level (Cozens et al 1982).
Special Studies on Teratogenicity
Rat
Groups of 20 mated Charles River CD rats were given oral doses of
6, 25 or 100 mg/kg daily from day 1 to 20 post coitum (day 1 was the
day when sperm was detected in the vaginal smear). A group of 31 rats
given the vehicle of 10 percent aqueous acacia solution served as
controls. Body weight, food consumption and overt signs of toxicity
were recorded throughout the study. The dams were killed on day 21 of
pregnancy.
The 25 and 100 mg/kg daily dosage elicited maternal toxicity as
evidenced by reduced food consumption, lower body weight and liver
enlargement; in addition, the dams showed increased salivation and
rubbed their noses in the sawdust litter. The only effect noted in the
rats given 6 mg/kg was a marginally lower body weight gain. There were
no macroscopic or microscopic findings attributable to treatment. The
number of corpora lutea was similar for all groups. The highest
dosage level appeared to be embryotoxic, as litter size, implantation
index and viability index were all slightly lower, while the incidence
of dead foetuses was marginally elevated. In addition, mean foetal
weight was lower, one litter containing unusually small foetuses that
appeared to have a gestational age of approximately 19 days. The mean
placental weight for both the male and female foetuses was higher for
the 25 or 100 mg/kg treated groups than for the control. A small
number of foetal abnormalities was noted among the treated groups but
no consistent pattern and no correlation between incidence and dosage
level was noted. The degree of calcification of the sternebrae and
vertebral arches was retarded in foetuses at the 100 mg/kg dose level.
This dosage elicited toxicity in both dams and embryos but produced no
teratogenic effects. The 25 mg/kg dosage was toxic in the dams. The
only findings in the 6 mg/kg group were marginally reduced body weight
gain and a very slight (non-significant) elevation of placental weight
(Beswick 1980).
Rabbit
Groups of 15 rabbits (New Zealand white strain) were administered
daily dosages of 0, 3, 12 and 48 mg/kg b.w. of prochloraz by
intragastric intubation on days 1 to 28 of pregnancy inclusive (day 1
= day of mating). The control group of 15 rabbits was dosed with the
vehicle, i.e. 10 percent acacia solution. All surviving dams were
killed on day 29 of pregnancy for macroscopic examination and the
foetuses removed for external visceral and skeletal examinations. No
treatment-related clinical signs and mortality were noted. Food intake
of the test group remained within 5 percent of the control value, with
a slightly reduced weight gain in all test groups during the first
week of dosing. The pregnancy rate was comparable for all groups. A
significant increase was noted in liver weight, with liver
discolouration in dams given 48 mg/kg day. A single dam from each of
the low-and high-dosage groups aborted completely, while one of the
control dams aborted partially. Litter size, post-implantation loss,
litter weight and mean foetal weight were considered unaffected by
treatment. Embryonic and foetal development, as assessed by incidence
of major malformations, minor anomalies and skeletal variations, were
similarly unaffected by treatment. No evidence of embryonic
teratogenic activity was therefore noted in New Zealand white rabbits
(Palmer et al 1980).
Special Studies on Mutagenicity
Ames test
Purified and technical prochloraz were tested in the Ames test
against Salmonella typhimurium TA 1535, TA 1537, TA 1538, TA 98 and
TA 100. All tests were performed in the presence and absence of a
liver microsome activation system (S-9) derived from
phenobarbitone-induced rats and tested at levels of 62.5, 125, 250,
500 and 1 000 mcg/plate. Positive control mutagens were included in
all tests to demonstrate the mutability of the bacterial strains and
the metabolizing capacity of the S-9 preparation; these comprised
cyclophosphamide, 6-aminochrysene and 2-animofluorene. Dimethyl
sulphoxide served as the negative control throughout. There was no
evidence of mutagenic activity against any of the strains employed but
the antibacterial effects of prochloraz precluded evaluation of
mutagenicity at concentration of 1 000, 500 and, in some cases, 250
mcg/plate (Wilcox 1977).
Micronucleus assay
Groups of five male and five female Charles River CD rats were
given two intraperitoneal injections of 6.25, 25.0 or 100 mg/kg b.w.
of prochloraz 24 h apart. Similar groups were given intraperitoneal
injections of cyclophosphamide 200 mg/kg b.w. to serve as positive
controls and intraperitoneal injections of the vehicle (maize oil) as
the negative controls. Six hours after the second injection all
animals were killed, smears from the bone marrow of the femur were
prepared and examined microscopically to determine the incidence of
micronuclei in the polychromatic erythrocytes. No significant increase
in the incidence of micronuclei was observed in any of the groups of
rats. Prochloraz was without clastogenic activity in rats of both
sexes when tested up to 100 mg/kg b.w. (Everest & Cliffe 1980).
Dominant lethal test
Groups of 20 male CD1 strain pathogen-free male mice (age 5-6
weeks) were given 0, 6, 25 or 100 mg/kg/day prochloraz in the diet for
eight weeks. After this treatment period, they were paired with
untreated females on a 1:1 basis for one week and then with a second
batch of untreated females for a further week. Approximately two weeks
after the discovery of a vaginal plug or 14 days after the midweek of
their pairing, the females were killed and their uterine contents
examined. There were no signs of reaction to treatment and mean weight
gains of all test groups compared favourably with that of the control
group. Intergroup variations in mean food consumption throughout the
entire treatment period were minimal and showed no consistent
dosage-related trend.
Mating performance of treated males and subsequent pregnancy
rates among untreated females were unaffected by treatment at any
dosage, except for one male at 25 mg/kg/day, which failed to induce
pregnancy at either mating. No obvious treatment-related macroscopic
changes were observed at terminal necropsy of the males. Mean weights
of the testes of treated males were slightly higher than the control
value but were not statistically significant. There was no evidence of
a dose-related effect on the pregnancy rate, mean number of
implantations, viable young, embryonic death and post-implantation
losses. Prochloraz showed no evidence of a dominant lethal effect in
male mice (Cozens et al 1980).
Mouse lymphoma mutation assay
Technical prochloraz was tested for mutagenic activity in mouse
lymphoma cells L5178 Y rendered heterozygous at the thymidine kinase
(TK) locus. The tests were carried out in the presence and absence of
a post-mitochondrial supernatant fraction from Arochlor 1254-treated
male rat livers and the co-factors required for mixed-function oxidase
activity. In an initial test carried out over a dose range of 0.16
mcg/ml to 1.6 mg/ml indicated, that prochloraz was toxic to the cells,
causing extensive cytotoxicity at a concentration of 158 mcg/ml. In
two further independent experiments at 50.0 mcg/ml and the second at
70.0 mcg/ml no significant increases in mutation frequency in the
prochloraz-treated cells over the vehicle control values were
observed. The concurrent positive control substances were
ethyl-methanesulphonate, which does not require the presence of S-9
mix, and 2-acetylaminofluorene, which requires the presence of S-9 mix
for metabolism. In both cases, high mutation frequencies were
obtained.
Technical prochloraz was not mutagenic to mouse lymphoma L 5178 Y
cells when tested over a series of dose levels, which extended into
the toxic range, in the presence or absence of S-9 mix (McGregor
et al 1983).
Special Studies on Carcinogenicity
Mouse
Groups of 52 male and 52 female mice CD-1 strain mice were fed
technical prochloraz in the diet at 78, 325 and 1 300 ppm. For each
sex, treatment continued until survival reached 20 percent, which was
106 weeks for males and 121 weeks for females. An additional group of
104 males and 104 females received the normal diet only. Observations
included clinical signs, mortality, body weight, food consumption and,
at 52 weeks and terminally, haematology and weight of selected organs.
Comprehensive histopathological examinations were made of tissues from
all surviving animals. Clinical signs were not evident, except in mice
given 1 300 ppm and in males treated at 325 ppm which were killed
because of distended abdomens. These animals were subsequently found
to have multiple liver tumours. No other overt signs of toxicity
related to treatment were observed. There was no mortality in the
course of the study attributable to prochloraz. Mice given 1 300 ppm
showed slightly reduced body weight gain. No differences were noted,
that were considered to be of toxicological significance, in the
haematological parameters between treated and control mice. Slightly
lower haemoglobin concentrations and lower total white blood cell
counts, due to a lower lymphocyte count, were noted at week 104 in
female mice treated with 1 300 ppm in diet.
At necropsy, an increase of liver weights was recorded in both
sexes given 1 300 ppm prochloraz. There was a dose-related increase in
the incidence of adenomas and adenocarcinomas of the liver in both
males and females. At 1 300 and 325 ppm, the increased incidence was
statistically significant and liver tumours (of hepatocyte origin)
were observed in both sexes, which were associated in a number of mice
with more than one tumour. Some cases at 1 300 ppm showed multiple
tumours as contributory factor to death. At 78 ppm, a slightly higher
incidence of liver tumours in female mice (6 out of 52) compared to
controls (5 out of 104) was noted, though the difference was not
statistically significant. In the males, the incidence of liver
tumours was similar in controls (37 out of 104) and treated mice (21
out of 52). There was no evidence of any other effects of treatment in
terms of either neoplastic or non-neoplastic pathology.
Treatment of mice was associated with a dose-related increase,
evident in both sexes, of liver tumours of all types. The incidence of
liver tumours was statistically significant at 1 300 ppm and 325 ppm
but not at 78 ppm prochloraz. The dietary dosage of 78 ppm was
equivalent to 7.5 mg/kg/day in males and 8.8 mg/kg/day in females
(Colley et al 1983).
Special Studies on Skin and Eye Irritation
A moderate to severe skin irritation produced in the rabbit
suggests that prochloraz is a skin irritant (Kynoch & Liggett 1979a).
Instillation of dilute solution of prochloraz into the everted lower
eyelid of one eye of New Zealand white stream rabbits produced a mild
conjunctival irritation in some animals which persisted up to three
days (Kynoch & Liggett 1979b).
Acute Toxicity
Prochloraz is of low toxicity to rodents when administered
orally. In rats, the LD50 ranged from 1 600 to 2 400 mg/kg b.w. and
in mice it was approximately 2 400 mg/kg b.w. Symptoms of intoxication
became apparent within 30-60 minutes of dosing and included CNS
depression, respiratory disturbance, ataxia, increased salivation,
piloerection, increased lacrimation and distended abdomen with signs
of gastrointestinal irritation. The acute toxicity of prochloraz in
rodents is summarized in Table 1.
Table 1. Acute Toxicity of Prochloraz in Rodents
Species Route LD50 (mg/kg) References
Rat Oral 1 600-2 400 Carter et al. 1978
Mouse Oral 2 400 Shaw & Carter 1976
Rat Dermal 5 000 Carter 1975
Rabbit Dermal 3 000 Kynoch & Liggett 1979a
Rat Inhalation (6h LC50) >420 mg/cu.m. Alexander & Clark 1978
Rat Intraperitoneal 400-800 Smithson & Lancaster 1980
In dogs given a single oral dose of 10, 100 or 250 mg/kg b.w. of
prochloraz, there were no deaths and the only toxic signs were emesis
and diarrhoea at 100 and 250 mg/kg b.w. (Stobart et al. 1978). One
female baboon given prochloraz at 50 mg/kg b.w. showed no toxic effect
while 250 mg/kg b.w. produced only emesis (Morgan et al. 1977).
Following dermal application to rabbits of 3 000 mg/kg for 24 h
there were no toxic effects except moderate to severe erythema and
oedema on the treated skin (Kynoch & Liggett 1979a).
Short-Term Studies
Mouse
Groups of 9 male and 9 female and 15 male and 15 female CD-1
strain mice were fed prochloraz in the diet at 6, 25, 100 and 400
mg/kg/day for 6 weeks and 13 weeks, respectively. Groups of 9 or 24
mice of each sex, given a diet without prochloraz, acted as controls.
Two additional groups of 15 mice of each sex were given a control diet
for 17 weeks and a diet containing prochloraz at a nominal dose level
of 400 mg/kg/day for 13 weeks, followed by a 4-week recovery period.
Overt signs of toxicity were recorded daily and body weight and food
consumption three times weekly; haematological and blood biochemistry
analyses were done at 6 weeks, 13 weeks and 4 weeks off-dose. All mice
were killed at the end of the dosing or recovery period, examined
macroscopically, the main organs were weighed and microscopic
examination of tissues performed.
No deaths occurred that were attributable to treatment with
prochloraz. An increased incidence of piloerection occurred in mice of
both sexes given 400 mg/kg daily. Food consumption of both sexes given
400 mg/kg daily was higher than that of the controls throughout the
dosing period and during the first week of the recovery period, but
was similar to the controls thereafter. A slight weight loss was
observed in the males given 100 mg/kg daily and in the females given
400 mg/kg daily. At week 6, haemoglobin concentration, PCV and red
blood cell counts were increased in both sexes given 400 mg/kg daily;
MCH was also marginally increased in the females and leucocyte counts
were increased in females due to lymphocytosis. At week 13 or after
four weeks recovery, these parameters were no longer affected by
treatment.
Plasma GPT activity was increased at week 6 in some mice of both
sexes given 400 mg/kg daily and some males at 100 mg/kg daily. At week
13, it was increased in the majority of females given 400 mg/kg daily
and in some mice of both sexes given 100 mg/kg daily. After four weeks
recovery, some of the males given 400 mg/kg daily were still affected.
Albumin content and consequently the albumin:globulin ratio, were
slightly reduced in both sexes given 400 mg/kg daily and in the males
given 100 mg/kg daily. At week 13, in both sexes at 400 mg/kg daily,
urea-nitrogen was slightly decreased and glucose levels were
increased.
Liver weight was increased at week 13 in both sexes given 25, 100
or 400 mg/kg daily. The ovaries in the females and the prostate and
seminal vesicles in males were small in the group at 400 mg/kg daily.
After 4 weeks recovery, liver weights were still higher in both sexes
given 400 mg/kg daily, but to a lesser extent than at weeks 6 and 13.
Histopathological examination showed minor liver changes with loss of
glycogen and periportal fat droplets in both male and female mice
receiving 400 mg/kg and in females receiving 100 mg/kg for 13 weeks.
Some male mice receiving 100 or 400 mg/kg also showed centrilobular
hepatocyte enlargement. In both male and female mice killed after four
weeks recovery, none of these changes were present. The no-effect
level in mice was 6 mg/kg/day (Gale 1980; Lancaster 1982).
Rat
Groups of 20 male and 20 female Boots-Wistar rats were given a
daily dose of prochloraz by oral gavage in 10 percent aqueous acacia
suspension at 0, 6, 25 or 100 mg/kg b.w. for 13 weeks. Further groups
of 20 male and 20 female controls given 100 mg/kg daily of prochloraz
remained untreated for four weeks after the end of the dosing period
and, in addition, groups of 10 males and females were given 0, 6, 25
or 100 mg/kg daily of prochloraz for six weeks. Overt signs of
toxicity observed in rats of both sexes were increased salivation at
25 or 100 mg/kg daily and in a few given 6 mg/kg daily. The incidence
of diarrhoea was increased during the first four weeks of dosing in
the males given 100 mg/kg daily and possibly in the first week in the
males given 25 mg/kg daily. In females, a few instances of diarrhoea
were recorded at each dose level.
Food consumption was not affected by treatment. However, test
females gained somewhat more weight than the controls at 25 or 100
mg/kg daily. At week 6, the only consistent haematological change was
a decreased haemoglobin concentration in males at each dose level and
in females given 100 mg/kg daily. A leucocyte count increase was noted
at each dose level in males due to lymphocytosis. At week 13, MCV was
slightly decreased at each dose level in the males and haemoglobin
concentration was slightly decreased in females treated at 25 or 100
mg/kg daily and in males given 100 mg/kg daily after the four weeks
recovery period.
Serum bilirubin was slightly decreased in both sexes at each dose
level at week 6, in the male at each dose level and in the females
given 6 or 100 mg/kg daily at week 13. Serum potassium was slightly
increased at week 6 in the males at 25 or 100 mg/kg and females at 100
mg/kg daily. UGOT excretion was slightly elevated in a few rats of
both sexes at each dose level. Urinary protein content was reduced in
the females at each dose level and in males given 100 mg/kg daily.
Liver weight at week 6 was increased in both sexes given 100
mg/kg daily and in the females given 6 or 25 mg/kg daily; kidney
weight was increased in both sexes given 100 mg/kg daily; spleen
weight was reduced in the males in each dose group; ovary weight was
increased in each dose group and thyroid weight was also increased in
females given 6 or 100 mg/kg daily. After the four-week recovery
period, the weights of the liver, ovaries and thyroids of the females
were slightly higher than the control values. The changes in liver
cell size correlated closely with the increase in liver weight. The
only instance where such correlation was not apparent was at week 13
in the females given 6 mg/kg daily, where the centrilobular cells were
slightly larger but liver weight was unchanged.
No other findings, that could be related to treatment, were
observed at microscopic examination of tissues from the rats given 100
mg/kg daily and killed at week 13. Various treatment-related effects
were noted in the rats of both sexes given 6 mg/kg daily of prochloraz
and a no-effect level was therefore not established. However, all
these effects were marginal (Lancaster & Shaw 1979a).
Dog
Groups of four male and four female beagles were given daily
doses of 1, 2, 5, or 7 mg/kg of prochloraz by gastric intubation for
13 weeks. Groups of eight males and eight females received 20 mg/kg
daily or the vehicle (10 percent aqueous acacia solution)
concurrently. At the end of the dosing period, four males and four
females from each of the 20 mg/kg and control groups were kept for
another four weeks without treatment.
Overt signs of toxicity observed during the dosing period
included isolated bouts of emesis and increased salivation in some
dogs from all groups, including controls, although these were more
prevalent at the high dosage. All dogs at 20 mg/kg daily and one given
7 mg/kg daily occasionally produced yellow mucoid and/or liquid
faeces. No effects of treatment were detected on faecal occult blood
content. During the recovery period, no overt signs of toxicity were
observed. Reduced food consumption was recorded during the dosing
period in two dogs given 20 mg/kg daily. Body weight changes during
the study were comparable in control and treated groups, although
greater fluctuations were recorded in dogs given 20 mg/kg daily. There
was no treatment-related effect detected by ophthalmoscopy or
electrocardiography.
Transient slight decreases in erythrocyte count and/or
haemoglobin concentra- were recorded in a few females given 7 or 20
mg/kg daily. No other treatment-related changes in haematological
parameters were noted. Changes in the activity of serum enzymes were
recorded. The serum alkaline phosphatase was high during the dosing
period in the majority of dogs given 20 mg/kg daily and in some given
7 mg/kg daily. Serum leucine aminopeptidase was slightly elevated at
week 6 in some dogs given 20 and in one given 7 mg/kg and serum GPT
was increased in a dog given 7 mg/kg daily. No change was observed in
urinary parameters.
At necropsy, all dogs given 20 mg/kg daily and three given 7
mg/kg daily had large and heavy livers. Low prostate weights were
recorded at week 14 in three dogs and in another two dogs in the same
group at 17 weeks. One of these dogs, killed at week 10, also had
decreased testes weight. In addition, one dog, given 7 mg/kg daily,
had a low prostate weight relative to body weight. In two females at
the high dose killed at week 14, and one killed at week 18, clear
brown exudate from freshly sectioned mammary glands was observed.
There were no histopathological findings in dogs on the high
prochloraz dosage. Counts of liver nuclei revealed no statistically
significant difference in the cell liver size or in the micronuclei
count between control and treated dogs.
In dogs retained for the recovery period, liver size and weight
returned generally to within normal limits, suggesting that any effect
of treatment on these parameters was reversible. The changes of AP and
LAP also returned to normal during the recovery period. The dose
producing no adverse-effect was 2.27 mg/kg daily (Lancaster et al.
1979b; Lancaster 1980).
Long-Term Studies
Rat
Groups of 150 male and 150 female rats constituted the controls.
Two groups each of 80 males and 80 females were treated with 37.5 and
150 ppm and a further two groups of 90 males and 90 females were given
625 ppm prochloraz in their diet. When the survival approached 20
percent in any group, all animals of that sex group were killed (111
weeks of treatment for females and 115 weeks of treatment in the
males).
There were no overt signs of toxicity recorded during the study
that could be considered related to the treatment. During weeks 20 and
21, the majority of rats from all groups showed signs indicative of
sialodacryo-adenitis infection, with a further relapse of mild
infection in a few rats from each group during the weeks 66 and 67.
Male survival was similar to controls, while treated females survived
longer than controls. A lower food consumption with a reduced body
weight gain was recorded for both sexes given 625 ppm. At 150 ppm,
food consumption was marginally lower throughout in males and until
week 52 in the females. Ophthalmoscopic examination revealed no
abnormalities related to treatment. In a few rats, minor changes were
seen at week 26, which were considered sequelae of
sialodacryo-adenitis infection.
Periodic laboratory measurements of haematological, biochemical
and urinalysis parameters in a few treated rats at 625 ppm showed
changes, but these were minimal and considered of doubtful
toxicological significance. The changes in urinalysis were minor, i.e.
lower total protein and changes in the pH. A minimal higher activity
of serum glutamic oxalacetic transaminase was noted.
No macroscopic findings related to treatment were seen at interim
kill in week 13. However, at the 52-week kill, enlarged or swollen
livers were observed in one male and three females given 150 ppm and
in four males and one female given 625 ppm in the diet. Heavier livers
were noted in males and females given 625 ppm diet at week 52 and
lower pituitary weights were also recorded in a few rats.
Histopathological examination showed hepatic centrilobular enlargement
in females given 625 ppm; periportal glycogen loss and centrilobular
fat deposits were also present in some treated rats of both sexes.
There were no other microscopic findings related to treatment.
Enlarged and swollen livers were observed in a higher proportion
of male rats given 625 ppm than in the controls, and slightly heavier
liver weights were recorded for females given 625 ppm. The incidence
of hyperplastic lesions in the liver was slightly higher than in the
controls in both sexes given 625 ppm, but the differences were not
statistically significant. The incidence of all other non-neoplastic
findings was similar in all groups. There was no evidence of
carcinogenicity from the chronic administration of prochloraz. The
no-effect level for toxicological changes was 37.5 ppm, equivalent to
a mean intake of 1.3 mg/kg/day in males and 1.6 mg/kg/day in females
(Colley et al. 1982).
Dog
Technical prochloraz was fed to groups of five males and five
females at levels of 0, 30, 135 and 600 ppm (increased to 1 000 ppm
from week 57) for 104 weeks. Observations included clinical signs,
mortality, food and water consumption and, at weeks 0, 13, 26, 51, 78
and 104, ophthalmoscopy, electrocardiography and clinical laboratory
studies. These comprised haematology, biochemistry, urinalysis and
faecal occult blood. At necropsy, organs were weighed and
comprehensive macroscopic and microscopic examinations were carried
out.
There were four deaths during the study but none was attributable
to treatment. There were no overt signs of toxicity or effects of
treatment upon body weight, food or water consumption, ophthalmoscopic
or electrocardiographic findings that could be related to treatment.
There were a few changes in the biochemical parameters, including a
higher level of SAP for males receiving 600 ppm in the diet during
week 13 and, during weeks 16 and 50, the elevation was also apparent
in the females. After the dietary level was increased to 1 000 ppm for
this group, there was a marked increase in the SAP levels during week
60, which persisted throughout the rest of the dosing period. The mean
level for males receiving 135 ppm was also higher than the control
mean from week 13 onwards, but the difference was not so marked as at
600 ppm. There was a slight but inconsistent increase in the platelet
count, glucose and cholesterol values in the group receiving 600 to
1 000 ppm, but these could not be related definitely to treatment.
There was no effect of treatment on the urinary parameters or faecal
occult blood content.
Liver weights in five animals that received 600 to 1 000 ppm, one
that received 135 ppm and one control, when expressed as a percentage
of body weight, exceeded the normal upper limit. The mean prostate
weight for animals in the same treatment group was significantly lower
than the control mean weight.
Histopathological examination showed minimal prostatic atrophy,
low grade hepatitis and minimal swelling and rarefaction of
centrilobular hepatocytis at 600 to 1 000 ppm. One male dog given 135
ppm also showed some liver changes. There were no other
histopathological changes related to prochloraz.
The no-effect level was 30 ppm in the diet, equivalent to
approximately 0.92 mg/kg/day (Chesterman et al. 1981).
Observations in Humans
Prochloraz has been produced on a commercial scale since 1980. No
adverse human effects have been detected since then from synthesis
formulation or use of prochloraz or its formulation. No clinical or
laboratory data were included (Bonsall 1982).
COMMENTS
Prochloraz has low oral toxicity. Following oral administration
in the rat, 95 percent of the dose is rapidly absorbed. In rats, the
major excretion is via the urine; in dogs it is via the faeces.
Prochloraz is a demonstrated liver mixed function oxidase inducer.
In rats and rabbits, no evidence of teratogenic effects was noted
at maternally toxic dose levels. A multigeneration reproduction study
in rats did not show any adverse effects at doses up to 37.5 ppm.
Mutagenicity studies, including the Ames test, micronucleus test,
mouse lymphoma assay and a dominant lethal study, were all negative.
In 90-day studies in mice, rats and dogs, liver weight was
increased in all species. No-effect levels of 6 mg/kg/day and of 2.27
mg/kg/day were established for mice and dogs, respectively. However,
in rats 6 mg/kg/day induced increased liver weight and occasional
signs of intoxication (increased salivation, diarrhoea). A two-year
dog study established a no-effect level of 30 ppm.
A long-term rat study did not result in any evidence of oncogenic
potential. A no-effect level of 37.5 ppm in the diet was established.
A mouse carcinogenicity study of 106 weeks duration indicated an
increased incidence of liver adenomas and adenocarcinomas in both
sexes at dose levels of 325 ppm and above. Prochloraz has been shown
to be a hepatocarcinogen in mice.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat (male); 37.5 ppm in the diet, equal to 1.3 mg/kg b.w.
Dog: 30 ppm in he diet, equal to 0.9 mg/kg b.w.
Estimate of Acceptable Daily Intake for Man
0 - 0.01 mg/kg b.w.
FURTHER WORK OR INFORMATION
Desirable
Observations in humans.
REFERENCES-TOXICOLOGY
Alexander, D.J. & Clark, G.C. Preliminary acute inhalation toxicity
1978 study in male and female rats with pure prochloraz (single
exposure). Report TX78053 from Huntingdon Research Centre
and Boots, submitted to WHO by FBC Ltd. (Unpublished)
Boardman, L.E. Plasma and tissue distribution studies in the rat
1979 following single and repeated oral doses of 3H-prochloraz.
Report AX79004 from Hazleton and Boots submitted to WHO by
FBC Ltd. (Unpublished)
Beswick, A.M. The teratogenicity study of technical prochloraz in male
1980 and female rats. Report TX80024 from Boots submitted to WHO
by FBC Ltd. (Unpublished)
Bonsall, J.L. The human exposure to prochloraz since synthesis in 1974
1982 and commercial production in 1980. Report submitted to WHO
by FBC Ltd. (Unpublished)
Campbell, J.K. Residues in milk and tissues of a goat dosed orally
1980 with 14C-prochloraz. Report AX80026 from Boots submitted to
WHO by FBC Ltd. (Unpublished)
Carter, O.A. Acute dermal toxicity of prochloraz to the male Boots
1975 Wistar rat. Report TXM75079 from Boots submitted to WHO by
FBC Ltd. (Unpublished)
Carter, O.A., Smithson, A. & Burnett, R. Acute oral toxicity of
1978 prochloraz, prochloraz pure material, stage 3 and stage 4
technical material and mother liquor concentrate impurities
to male Boots Wistar rats. Report TX78118 from Boots
submitted to WHO by FBC Ltd. (Unpublished)
Challis, I.R. & Campbell, J.K. The effect of prochloraz on the hepatic
1983 mixed function oxidase system of the male mouse after oral
administration. Report METAB/83/6 from FBC submitted to WHO
by FBC Ltd. (Unpublished)
Chesterman, H. et al. Two-year toxicity study in beagle dogs of
1981 technical prochloraz - final report - repeated dietary
administration for 104 weeks. Report TOX/81/173-2 from
Huntingdon Research Centre and FBC submitted to WHO by FBC
Ltd. (Unpublished)
Colley, J. et al. Prochloraz chronic toxicity and carcinogenicity
1982 study in rats by dietary administration - 104 weeks (final
report). Report TOX/82/173-8 from Huntingdon Research
Centre, and FBC submitted to WHO by FBC Ltd. (Unpublished)
Colley, J. et al. Prochloraz tumorigenicity study in mice by dietary
1983 administration (final report). Report TOX/83/173/23 from
Huntingdon Research Centre and FBC submitted to WHO by FBC
Ltd. (Unpublished)
Cozens, D.D., Reid, Y.J., Woodhouse, R.N., Almond, R.H. Anderson, J.
1980 & Ball, S.I. Dominant lethal gene assay of technical
prochloraz in the male mouse. Report TX80077 from the
Huntingdon Research Centre and Boots submitted to WHO by FBC
Ltd. (Unpublished)
Cozens, D.D. et al. The effect of prochloraz on reproductive
function
1982 of multiple generations in the rat. Report TOX/82/173-5 from
Huntingdon Research Centre and FBC submitted to WHO by FBC
Ltd. (Unpublished)
Everest, R.P. & Cliffe, S. Technical prochloraz micronucleus assay in
1980 male and female CD rats of prochloraz. Report TX80003 from
Boots submitted to WHO by FBC Ltd. (Unpublished)
Gale, E.P. 90-day oral toxicity study with prochloraz technical to
1980 male and female CD1 mice. Report TX80040 from Hazleton and
Boots submitted to WHO by FBC Ltd. (Unpublished)
Hamilton, D.Y. The distribution and level of 14C-labelled residues in
1978a rats following repeated oral dosing with 14C-prochloraz at
25 mg/kg/day. Report AX78008 from Boots submitted to WHO by
FBC Ltd. (Unpublished)
Hamilton, D.Y. The distribution and level of radiolabelled residues in
1978b the tissues of the dog following a single oral dose of
prochloraz. Report AX78016 from Boots submitted to WHO by
FBC Ltd. (Unpublished)
Kynoch, S.R. & Liggett, M.P. Primary eye irritancy of prochloraz 40
1979a percent E.C. formulation (bfn 8099). Report TX79050 from
Huntingdon Research Centre and Boots submitted to WHO by FBC
Ltd. (Unpublished.)
Kynoch, S.R. & Liggett, M.P. Primary skin irritancy of prochloraz 40
1979b percent E.C. formulation (bfn 8099). Report TX79074 from
Huntingdon Research Centre and Boots submitted to WHO by FBC
Ltd. (Unpublished)
Lancaster, M.C. & Shaw, J.W. 90-day oral toxicity study with
1979a prochloraz technical in male and female Boots Wistar rats
(4-week off-dose period). Report TX79028 from Boots
submitted to WHO by FBC Ltd. (Unpublished)
Lancaster, M.C., Morgan, H.E. & Stobart, J.E. 90-day oral toxicity
1979b study with prochloraz technical in male and female beagle
dogs (4-week off-dose period). Report TX79010 from Boots
submitted to WHO by FBC Ltd. (Unpublished)
Lancaster, M.C. 13-week oral toxicity study with prochloraz technical
1980 (BX 9/DM 2723) in male and female dogs with a four-week
off-dose period. Histopathological examination of the
remaining tissues. Report TX80034 from Boots submitted to
WHO by FBC Ltd. (Unpublished)
Lancaster, M.C. PRochloraz: 13-week oral toxicity in the mouse -
1982 histopathological examination. Report TOX/82/173/-9 from FBC
Ltd. submitted to WHO by FBC Ltd. (Unpublished)
McGregor, D.B., Riach, C.G. & Brown, A.G. Technical prochloraz
1983 assessment of mutagenic potential in the mouse lymphoma
mutation assay. Report TOX/83/173-22 from Inveresk and FBC
submitted to WHO by FBC Ltd. (Unpublished)
Morgan, G.E., Patton, D.S.G., Shepherd, G.M. & Stobart, J.E. The acute
oral toxicity of bts 40542 (technical batch) to the female
baboon. Report from Boots Laboratories submitted to WHO by
FBC Ltd. (Unpublished)
Needham, D. The effect of dog gastric juice or plasma on prochloraz.
1980 Report AX80011 from Boots submitted to WHO by FBC Ltd.
(Unpublished)
Needham, D. & Campbell, J.K. The excretion of 14C-phenyl-labelled
1980 prochloraz in male and female rats after a single oral dose.
Report AX80037 by Boots submitted to WHO by FBC Ltd.
(Unpublished)
Needham, D. The excretion and tissue residues of 14C-prochloraz in
1982a male and female mice following a single oral dose of 100
mg/kg. Report METAB/82/32 from FBC submitted to WHO by FBC
Ltd. (Unpublished)
Needham, D. & Campbell, J.K. The excretion and tissue residues of 14C
1982 prochloraz in male and female dogs following a single oral
dose of 18 mg/kg. Report METAB/82/30 by FBC submitted to WHO
by FBC Ltd. (Unpublished)
Needham, D. The metabolism of prochloraz in the rat after oral
1982b administration. Report METAB/82/31 from FBC submitted to WHO
by FBC Ltd. (Unpublished)
Needham, D. The effect of prochloraz on the hepatic mixed-function
1983a oxidase system of the male rat after oral administration.
Report METAB/83/5 from FBC submitted to WHO by FBC Ltd.
(Unpublished)
Needham, D. The effect of prochloraz on the hepatic mixed function
1983b oxidase system of the mouse when administered at 80, 325 and
1 300 mg/kg diet for up to 14 weeks. Report METAB/83/7 from
FBC submitted to WHO by FBC Ltd. (Unpublished)
Palmer, A.K., Bottemley, A.M. & Billington, R. The effect of technical
1980 prochloraz on pregnancy of the New Zealand white rabbit.
Report TX80083 from Huntingdon Research Centre and Boots
submitted to WHO by FBC Ltd. (Unpublished)
Rivière, J.L. Prochloraz, a potent inducer of the microsomal
1983 cytochrome P-450 system. Pestic. Biochem. Physiol., 19:
44-52.
Shaw, J.W. & Carter, O.A. Acute oral toxicity to male CD 1 mice of
1976 prochloraz. Report TX76093 from Boots submitted to WHO by
FBC Ltd. (Unpublished)
Smithson, A. & Lancaster, M.C. Acute intraperitoneal toxicity of
1980 prochloraz to male CD rats. Report TX80004 from Boots
submitted to WHO by FBC Ltd. (Unpublished)
Stobart, J.E., Morgan, H.E., Patton, D.S.G. & Shepherd, G.M. Acute
1978 oral toxicity of prochloraz to male and female beagle dogs.
Report TX78049 from Boots submitted to WHO by FBC Ltd.
(Unpublished)
Turner, D.M. & Gilbert, C.M. Pharmacokinetic studies on 14C-labelled
1977 prochloraz in the rat. Report AX77001 from Hazleton and
Boots submitted to WHO by FBC Ltd. (Unpublished)
Wilcox, P. Prochloraz in vitro bacterial mutagenicity testing of
pure
1977 and technical prochloraz. Report TX78002 from Boots
submitted to WHO by FBC Ltd. (Unpublished)
RESIDUES
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Prochloraz is available as 40 percent and 45 percent emulsifiable
concentrate (E.C.) formulations for foliar and postharvest treatments
and is sold under the trademark Sportak. A 50 percent wettable powder
formulation containing a 4:1 prochloraz-manganese coordination complex
active ingredient (a.i.) (3.4 percent manganese) is available and
recommended for foliar application to some broad leaf crops,
ornamentals and mushrooms, which may be susceptible to phytotoxicity
from E.C. formulations. Prochloraz is available in co-formulation with
carbendazim, under the trademarks Sportak PF and Sportak ALPHA, for
foliar application to cereal and oilseed rape. A 200 g/l liquid
formulation is available for cereal seed treatment and a 25 percent
E.C. disinfectant for rice seed.
Prochloraz is used for the control of a variety of fungal
diseases on a variety of crops and is said to be especially active
against Ascomycetes and Fungi Imperfecti. It is currently registered
or approved for use in eight countries and similar measures are
pending or planned in many others. Nationally registered and/or
approved uses are summarized in Table 1.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue trials have been conducted in many countries,
representing a wide range of food crops, climatic conditions,
formulations and foliar, seed and postharvest treatments. Trials data
are summarized in Tables 2-13. Trials data from countries with
approved uses are available only for apples, watermelons, grapes
(Taiwan, province of China), cereal (Belgium, Denmark, France, German
Federal Republic, The Netherlands, United Kingdom) and mushrooms (The
Netherlands).
Sugarbeets
No information was available on good agricultural practices for
prochloraz on sugarbeets. The "recommended" application rate of active
ingredient is said to be 0.5-1 kg/ha for foliar treatment. No
preharvest interval (PHI) is given. Total residues of both free and
conjugated residues of prochloraz (BTS 40 542),
N-formyl-N'-propyl-N'-2(2,4,6-trichlorophenoxy) ethylurea (BTS 44596),
N-propyl-N-2 (2,4,6-trichlorophenoxy) ethylurea (BTS 44595) and
2,4,6-trichlorophenol (BTS 45186), hereafter referred to as total
residues of prochloraz and metabolites, are listed in Table 2. The
values (corrected for 90 percent recoveries) are expressed as
prochloraz equivalents but were determined as 2,4,6-trichlorophenol
after hydrolysis. All residues and the 0.036 mg/kg control are below
the limit of determination, said to be 0.1 mg/kg. Data are
insufficient to support a maximum residue level, even if good
agricultural practices were known.
The "recommended" application rate of active ingredient to
sugarbeet leaves is said to be 0.5-1 kg/ha for foliar treatment. No
PHI was provided. Total residues of prochloraz and metabolites,
expressed as prochloraz equivalents, on sugarbeet leaves were 0.97,
1.3 and 1.2 mg/kg at 5, 21 and 42 days, respectively, after the last
treatment in three trials (Table 2). The control was 0.15 mg/kg.
Lettuce
No information was available on good agricultural practices on
lettuce. Residues of prochloraz only, uncorrected for approximately 72
percent recoveries, ranged from 7.4 mg/kg 18 days after treatment to
0.41 mg/kg after 39 days and <0.07 mg/kg after 71 days with a limit
of sensitivity said to be 0.01 mg/kg, although one of the three
controls has apparent residues of 0.02 mg/kg. Recoveries were 65-76
percent at 0.1-0.5 mg/kg fortification levels.
Watermelons
Residue data reflecting good agricultural practices in Taiwan
Province of China are presented in Table 2. Residues of prochloraz
per se were 0.01 mg/kg in peel and flesh after the approved six-day
interval from last application to harvest. Information was not
provided on the analytical procedure used; a 0.003 mg/kg limit of
detection was claimed.
Citrus Fruits
No information was provided on good agricultural practices on
citrus fruits. Residues from postharvest trials, proposed for uses
(25-70 g/hl for dip treatments or 100-300 g/hl for spray treatments)
where the peels are discarded, are summarized in Table 3. A variety of
treatment and storage conditions are given, representing 23 trials in
five countries. In some cases prochloraz alone is determined, in
others prochloraz and its metabolites and in one case BTS 45 186.
Total residues are generally expressed as prochloraz equivalents,
although they are usually measured as BTS 45 186. Total residues after
storage at ambient of 4°C for up to 70 days and from a variety of
treatments and locations ranged up to 18 mg/kg in peel, 0.44 mg/kg in
flesh and 6 mg/kg on a whole fruit basis. Residue levels of free
prochloraz were not substantially different than those for total
residues, where a comparison was made. More details are reported under
"Fate of Residues, Storage and Processing".
Pome Fruits
Information on good agricultural practice and residue data
(prochloraz only) reflecting such practice on apples are available for
Taiwan Province of China, as well as data for apples and pears from
supervised trials in three additional countries for which good
agricultural practices for prochloraz on pome fruit are not known.
Apples
The most pertinent data were those from Taiwan Province of China,
for which information was available on good agricultural practices on
apples. Data represent recommended and 2X the recommended application
rate. No data were available for the recommended 9-day last treatment
to harvest interval, but they were given for 6 and 12 days. Residues
of prochloraz, per se, from nine applications were 0.07 and 0.01
mg/kg, respectively, at these intervals from the recommended
application rate. No information is available on the analytical method
used other than a claimed 0.003 mg/kg limit of detection. Other apple
residue data (parent compound only) are from application rates three
to eight times that for good agricultural practice known to the
Meeting. Over-all recoveries among studies submitted range from 64-92
percent with claimed sensitivities ranging from 0.001-<0.01 mg/kg.
Apparent residues in untreated controls, where given, are
<0.01-0.02 mg/kg. A reasonable limit of determination would appear
to be about 0.1 mg/kg. One study (Hayto 1977c) indicates a half-life
in apples of 6-7 days.
Pears
No information on good agricultural practice was available for
pears. The application rates are substantially greater than known good
agricultural practice for apples and residues are higher, as would be
expected.
Stone Fruits
No information on good agricultural practice for prochloraz on
stone fruit were available to the Meeting. The "recommended"
application rate for foliar treatment with the prochloraz-50 percent
W.P. manganese complex formulation was reported as 15-30 g a.i./hl. No
preharvest interval was given. Residue data were available on
apricots, nectarines, peaches, cherries and plums from four countries
(Table 2). Total residues of prochloraz and the metabolites were
determined as 2,4,6-trichlorophenol and expressed as prochloraz.
Residues in mature fruit ranged from a maximum of 0.19 mg/kg im plums
at 3 days, 1 mg/kg at 14 days, 0.82 mg/kg at 24 days to <0.05 mg/kg
over 75 days after multiple foliar treatments with either E.C. or W.P.
(manganese complex) formulations. Residues were generally lower for
other stone fruit at comparable intervals after treatment with the
W.P. formulations. At fortification levels of 0.05-1 mg/kg recoveries
are 70-113 percent over-all. Apparent residues in eight untreated
stone fruit controls were 0.009-0.022 mg/kg except one value of 0.06
mg/kg in four controls (plums). The limit of determination for stone
fruit was reported as 0.05 mg/kg.
Grapes
Information on good agricultural practice for the E.C.
formulation and residue data reflecting 1, 1.5, 2 and 4X recommended
application rates were available from one country.
Residues from two applications at recommended rates ranged from
0.39 mg/kg on the day of application to <0.01 mg/kg after 21 days and
0.1 mg/kg at the recommended 9-day preharvest interval. Higher
application rates and numbers of applications, with one or two
exceptions, did not make an appreciable difference in the residue
level at comparable intervals after application. No information was
provided on apparent residues in un-treated controls or on the
analytical procedure; a 0.003 mg/kg limit of detection was reported.
TABLE 1. REGISTRATIONS AND APPROVED USES FOR PROCHLORAZ
Country Product1 Commodity Approved Application Rate Number and timing of Pre-harvest
Product a.i. Applications Interval
(prochloraz)
Belgium SPORTAK 45EC Winter 1 l/ha 0.45 kg/ha One application at earstage in mixture na
wheat with either 0.125 kg/ha carbendazim,
1.2 kg/ha captafol or 1.6 kg/ha maneb.
Winter
barley 1 l/ha 0.45kg/ha One application at stage L or M2
Denmark SPORTAK Barley 100 ml/100 kg 0.2 g/kg na na
BEJOSE (seed
dressing)
SPORTAK 45EC Spring 1 l/ha 0.45 kg/ha One application at stage 4-10.5 or a
barley split dose at half rate with the first
application at stage 4-6 and the second
at stage 8-10.53.
Winter 1 l/ha 0.45 kg/ha One (stage 4-10.5) or two (stage 4-6 cereals
barley and 7-10.5) applications3. 1 month
Winter 1 l/ha 0.45 kg/ha Two applications (stages 5-6 and 9-
wheat 10.53).
Winter 1 l/ha 0.45 kg/ha One application (stages 5-7)3
rye
Eire SPORTAK 40EC Cereals 1 l/ha 0.4 kg/ha Up to three applications between leaf
sheaf erection and complete ear emergence. na
Table 1 (continued)
Country Product1 Commodity Approved Application Rate Number and timing of Pre-harvest
Product a.i. Applications Interval
(prochloraz)
France SPORTAK 40EC Wheat 1.125-1.875 0.45-0.75 Up to two applications (stages 6-7
l/ha kg/ha and 10.3-10.5)3
Barley 1.125 l/ha 0.45 kg/ha Up to two applications (stages 6-7
and 10.1)3 na
SPORTAK 45EC Wheat 1-1.66 l/ha 0.45-0.75 Up to two applications (stages 6-7
kg/ha and 10.3-10.5)3.
Barley 1 l/ha 0.45 kg/ha Up to two applications (stages 6-7
10.1)3
SPORTAK PF Wheat 1.5 l/ha 0.45 kg/ha Up to two applications (stages 6-7
and 10.3-10.5)3.
Barley 1.5 l/ha 0.45 kg/ha Up to two applications (stages 6-7
10.1)3.
Oil seed 1.5 l/ha 0.45 kg/ha Up to two applications, at beginning
rape and end of flowering. na
SPORTAK M Wheat 1.125 l/ha 0.45 kg/ha Up to two applications (stages 6-7
and 10.3-10.5)3.
Barley 1.125 l/ha 0.45 kg/ha Up to two applications (stages 6-7
10.1)3.
Table 1 (continued)
Country Product1 Commodity Approved Application Rate Number and timing of Pre-harvest
Product a.i. Applications Interval
(prochloraz)
German SPORTAK 40EC Wheat 1.2 l/ha 0.48 kg/ha One (stage 29-32) or two
Federal (stages 29-59) applications5.
Republic Cereals -
Barley 1.2 l/ha 0.48 kg/ha One (stage 29-32) or two 35 days6
(stages 29-49) applications5.
Winter 1.2 l/ha 0.48 kg/ha One application at stage 29-325.
rye Restricted to a maximum of three
applications in Winter cereal.
Netherlands SPORTAK 45EC Wheat 1 l/ha 0.45 kg/ha 1-2 treatments after infection.
Barley 1 l/ha 0.45 kg/ha 1-2 treatments after infection. Cereals -
8 weeks7
Winter 1 l/ha 0.45 kg/ha One or two applications.
barley
Ornamentals 0.4% 180 g/hl Flower bulb dip na
SPORGON Mushrooms 3/g/l/m2 1.5g/l/m2 One application of casing soil Mushrooms -
nine days after casing. 10 days
Taiwan SPORTAK 25EC Rice 2000 x 12.5 g/hl 24 hour rice seed soak. na
dilution
Grape 6000 x 4.17 g/hl Two to three applications, Grape -
dilution every 10 days. 9 days
Apple 3000 x 8.33 g/hl Application every 10 days. Apple -
dilution 9 days
Table 1 (continued)
Country Product1 Commodity Approved Application Rate Number and timing of Pre-harvest
Product a.i. Applications Interval
(prochloraz)
Watermelon 4000 x 6.25 g/hl 10 days. 6 days
dilution
United SPORTAK 40EC Cereals 1 /ha 0.4 kg/ha Up to three applications between na
Kingdom leaf sheath erection and complete
ear emergence.
Oil seed 1.25 l/ha 0.5 kg/ha Up to three applications between
stem extension and 90% petal fall.
Ornamentals - 2000 ppm Propagation cutting dip, drench, na
foliar spray or compost incorporation.
SPORTAK 45EC Cereals 1 /ha 0.45 kg/ha Up to three applications between na
leaf sheath erection and complete
ear emergence.
Oil seed 1-1.33 /ha 0.45-0.6 Up to three applications between
rape kg/ha stem extension and 90% petal fall.
Ornamentals - 2000 ppm Propagation cutting dip, drench, na
foliar spray or compost incorporation.
SPORTAK Cereals 1.5 /ha 0.4 kg/ha Up to three applications between
ALPHA leaf sheath erection and complete
ear emergence. na
Oil seed 1.5 /ha 0.4 kg/ha Up to three applications between
rape stem extension and 90% petal fall.
Table 1 (continued)
Country Product1 Commodity Approved Application Rate Number and timing of Pre-harvest
Product a.i. Applications Interval
(prochloraz)
Ornamentals - 2000 ppm Propagation cutting dip, drench, na
foliar spray or compost incorporation.
SPORTAK FE Cereals 4 kg/ha 0.66 kg/ha Up to three applications between
leaf sheath erection and complete
ear emergence. na
Oil seed 3 kg/ha 0.5 kg/ha Up to three applications between
rape stem extension and 90% petal fall.
Ornamentals - 2000 ppm Propagation cutting dip, drench, na
foliar spray or compost incorporation.
SPORGON Mushrooms 3 g/l/m2 1.5 g/l/m2 One application, 1-10 days after Mushrooms -
casing. 10 days
0.6 g/l/m2 0.3 g/l/m2 Three applications, one 1-10 days Mushrooms -
after casing and then after the 2 days
first and second flush
1.2 g/l/m2 0.6 g/l/m2 Two applications, one 1-10 days Mushrooms -
after casing and then between 2 days
second and third flush.
Ornamentals - 2000 ppm Propagation cutting dip, drench, na
foliar spray or compost incorporation.
Table 1 (continued)
Notes: na - not applicable
nd - not defined
ns - not specified
1. The following formulations have been registered:
SPORTAK 40 EC - an emulsifiable concentrate containing 400 g/l prochloraz
SPORTAK 45 EC - an emulsifiable concentrate containing 450 g/l prochloraz
SPORTAK BEJDSE - a liquid seed dressing containing 200 g/l prochloraz
SPORTAK PF - an emulsifiable concentrate containing 300 g/l prochloraz and 80 g/l carbendazim
SPORTAK M - a twin-pack emulsifiable concentrate containing 400 g/l prochloraz and 450 g/l mancozeb
SPORTAK ALPHA - an emulsifiable concentrate containing 266 g/l prochloraz and 100 g/l carbendazim
SPORTAK FE - a wettable powder containing 165 g/kg prochloraz-manganese complex and 533.4 g/kg mancozeb
SPORGON - a wettable powder containing 500 g/kg prochloraz-manganese complex
2. Baggiolini cereal growth stages.
3. Feekes-Large cereal growth stages.
4. Residues of prochloraz alone.
5. Zadoks Decimal cereal growth stages.
6. A proposed MRL of 0.5 mg/kg for prochloraz and metabolites in cereal grain has been submitted.
7. Under review, pending further residue analysis.
8. Residues of prochloraz and metabolites measured as 2,4,6-trichlorophenol and expressed as prochloraz.
Table 2 Prochloraz Residues in Various Crops Following Supervised Trials
Country/ No. of Formulation Application Interval after Residues, Reference
Crop Year trials rate (a.i.) final application (mg/kg)1
(days)
Sugarbeet Italy
root 1981 1 40% E.C. 2 x 1 kg/ha 42-46 0.02 Longland 1983b
leaves 1.17
root 1 40% E.C. 3 x 1 kg/ha 21-25 0.04
leaves 1.32
root 1 40% E.C. 4 x 1 kg/ha 5-6 0.06
leaves 0.97
Lettuce The Netherlands 1 8% dust 8 kg/ha 70-73 0.013 Hayto 1979d
1978 80 0.013
2 8% dust2 8 kg/ha 18-19 7.383
39 0.043
42-46 0.123
70-73 <0.013
80 <0.013
1 8% dust2 6.4 kg/ha 70-73 0.043 Maclaine Pont et al. 1980
1 8% dust2 8 kg/ha 70-73 0.033
8% dust2 9.6 kg/ha4 78 <0.013
Watermelon Taiwan Province
peel of China
1982 1 25% E.C. 3 x 4.17 g/hl 0-1 0.013 Wang 1982
3 <0.013
5-6 <0.013
9 <0.013
12 <0.013
0-1 <0.013
3 <0.013
5-6 <0.013
9 <0.013
12 <0.013
Table 2 (continued)
Country/ No. of Formulation Application Interval after Residues, Reference
Crop Year trials rate (a.i.) final application (mg/kg)1
(days)
Watermelon Taiwan Province 3 x 6.25 0-1 0.023
peel of China 3 <0.013
5-6 <0.013
9 <0.013
flesh 12 <0.013
0-1 <0.013
3 <0.013
5-6 <0.013
9 <0.013
12 <0.013
Apple The Netherlands 2 50% W.P.5 2 x 25 g/hl 63 <0.013 Maclaine Pont et al. 1980
1978 84 <0.013
United Kingdom 2 25% W.P. 7 x 60 g/hl 0-1 0.473 Hayto 1976
1977 14-16 0.083
30-31 0.023
25% E.C. 8 x 51.48 g/hl 0-1 4.183 Hayto 1977c
8 1.763
18-19 0.683
30-31 0.173
50 0.023
Taiwan Province 1 25% E.C. 9 x 8.33 g/hl 0-1 0.243 Wang 1982
of China 3 0.123
1982 5-6 0.073
12 0.013
14-16 0.053
18-19 0.023
Table 2 (continued)
Country/ No. of Formulation Application Interval after Residues, Reference
Crop Year trials rate (a.i.) final application (mg/kg)1
(days)
Apple Taiwan Province 9 x 16.67 g/hl 0-1 0.813
of China 3 0.373
5-6 0.203
12 <0.013
14-16 0.083
18-19 0.073
Pear The Netherlands 1 50% W.P.5 1 x 0.1 kg/hl 21-25 0.033 Maclaine Pont et al. 1980
1978 1 x 0.2 kg/hl 21-25 0.063
2 x 0.1 kg/hl 14-16 0.263
2 x 0.2 kg/hl 14-16 0.243
Apricots Israel 1 50% W.P.5 1 x 60 g/hl 89 <0.05 Snowden 1983
1982
Cherries France 1 50% W.P.5 1 x 10 g/hl 70-73 <0.05
1982 1 x 20 g/hl 70-73 <0.05
4 x 20 g/hl 21-25 0.45
Nectarines France 1 50% W.P.5 5 x 20 g/hl 14-16 0.56
1982
Peaches South Africa 1 50% W.P.5 3 x 10 g/hl 132 <0.05
1982 1 3 x 20 g/hl 132 <0.05
1 8 x 10 g/hl 30-31 0.21
Plums Italy 1 40% E.C. 1 x 30 g/hl 102 <0.05
1982 1 2 x 30 g/hl 77 <0.05
1 3 x 30 g/hl 50 0.82
Table 2 (continued)
Country/ No. of Formulation Application Interval after Residues, Reference
Crop Year trials rate (a.i.) final application (mg/kg)1
(days)
Plums South Africa 1 50% W.P.5 1 x 30 g/hl 50 0.21
1981 1 1 x 40 g/hl 50 0.28
1 2 x 30 g/hl 14-16 0.97
1 2 x 40 g/hl 14-16 0.60
1982 1 50% W.P.5 3 x 10 g/hl 104 <0.05
3 x 20 g/hl 104 <0.05
6 x 10 g/hl 5-6 0.12
4 x 10 g/hl 103 <0.05
4 x 20 g/hl 103 <0.05
7 x 10 g/hl 5-6 0.19
Grapes Taiwan Province 1 25% E.C. 2 x 4.17 g/hl 3 0.393 Wang 1982
of China 5-6 0.193
1982 8 0.203
9 0.103
12 0.073
21-25 0.103
39 0.053
42-46 <0.013
1 25% E.C. 2 x 6.25 g/hl 3 0.493
5-6 0.263
8 0.203
9 0.253
12 0.133
21-25 0.133
39 0.053
42-46 <0.013
Table 2 (continued)
Country/ No. of Formulation Application Interval after Residues, Reference
Crop Year trials rate (a.i.) final application (mg/kg)1
(days)
Grapes Taiwan Province 1 25% E.C. 7 x 10 g/hl 3 0.203
of China 5-6 0.133
8 0.293
9 0.273
12 0.143
21-25 0.223
39 0.083
42-46 0.083
1 25% E.C. 7 x 16.67 g/hl 3 0.423
5-6 0.203
8 0.163
9 0.603
12 0.673
21-25 0.343
39 0.183
42-46 0.103
Strawberries The Netherlands 1 50% W.P.5 2 x 0.1 kg/hl 30-31 0.173 Maclaine Pont et al. 1980
1978 1 2 x 0.15 kg/hl 30-31 0.173
Almonds Israel
shell 1982 1 50% W.P.5 2 x 0.1 kg/hl 0.616 Churchill & Longland 1983d
kernel 0.106
whole nut 0.296
Table 2 (continued)
1 Total residues of prochloraz and major metabolites unless otherwise stated. Total residue measured as 2,4,6-trichlorophenol (BTS 45186)
and converted to prochloraz-derived residues by correcting for the molecular weight factor (1.906).
2 Co-formulation with dicloran.
3 Prochloraz residues only.
4 Preplanting application.
5 Prochloraz-manganese complex 50% wettable powder formulation.
6 Applications made at blossom and 20 days later. Preharvest interval estimated at about 2.5 to 3 months.
Table 3. Supervised Residue Trials With Prochloraz on Citrus Fruits - Post-Harvest Applications
Residues (mg/kg)1
Application
Country/ Crop No. of Formulation rate (a.i.) Peel Flesh Whole Fruit3 Reference
Year Trials and method
Australia Oranges 1 40% E.C. 25g/hl dip3 1.40-3.4 <0.05 n.d. Browne 1981a
1981 (1.45-3.954) (0.3-0.104)
1 40% E.C. 50g/hl dip3 1.58-3.36 <0.05 n.d.
(2.25-3.354) (<0.02-0.054)
Italy Oranges 1 25% W.P. 50g/hl dip5 1.59-1.72 0.04-0.05 0.71-0.80 Browne & Manley
1981 1 25% E.C. 50g/hl dip5 4.57-6.40 0.09-0.16 1.80-2.35 1982b
Lemons 1 25% W.P. 50g/hl dip5 3.61-4.04 0.08-0.14 1.58-1.83
1 25% E.C. 50g/hl dip 5.67-6.95 0.08-0.19 2.36-2.97
South Oranges 1 40% E.C. 100g/hl wax 1.38-2.79 0.03-0.08 0.38-0.72 Manley & Snowden
Africa brush6 (0.34-0.478) 1982b
1982 1 40% E.C. 200g/hl wax 2.45-3.74 0.02-0.07 0.65-0.94
brush6 (0.91-1.198)
1 40% E.C. 200g/hl wax n.d. n.d. 1.36-1.698)
brush7
Oranges 1 45% E.C. 50g/hl brush9 0.34 <0.05 0.08 Snowden & Manley
(0.108) 1983
1 45% E.C. 100g/hl brush9 0.47 <0.05 0.13
(0.16-0.178)
1 45% E.C. 200g/hl brush9 1.84 <0.05 0.39
(0.39-0.438)
1 45% E.C. 400g/hl brush9 3.07 0.07 0.63
(0.70-0.758)
Table 3 (continued)
Residues (mg/kg)1
Application
Country/ Crop No. of Formulation rate (a.i.) Peel Flesh Whole Fruit3 Reference
Year Trials and method
Spain Oranges 1 25% E.C. 50g/hl dip10 1.48-3.224 0.04-0.10 0.67-1.4511 Kelly 1982d
1979 1 25% E.C. 100g/hl dip10 1.40-4.084 0.04-0.11 0.67-1.8011
1980 Oranges 1 40% E.C. 300g/hl wax 3.3-4.74 0.32-0.38 n.d. Richards 1980b
spray12
1 40% E.C. 300g/hl wax n.d. 0.005-0.00813 n.d. Richards 1980e
spray12
1 40% E.C. 200g/hl wax 1.2-6.7 0.04-0.44 n.d. Richards 1980f
spray12
1 40% E.C. 250g/hl wax 3.7-7.9 0.04-0.30 n.d.
spray12
1 40% E.C. 300g/hl wax 5.4-6.4 0.06-0.13 n.d.
spray12
1981 Oranges 1 40% E.C. 300g/hl shower14 4.7-8.4 0.11-0.39 1.50-2.47 Browne 1981b
(1.7-7.34) (0.12-0.394) (0.64-2.434)
1981 Lemons 1 40% E.C. 300g/hl 11.0-17.9 0.17-0.33 3.66-6.13 Browne 1982c
shower15 (9.1-15.64) (0.27-0.304) (3.14-5.554)
United Oranges 1 40% E.C. 70g/hl dip16 5.78-7.81 0.07-0.15 1.37-2.07 Browne 1982i
Kingdom (3.55-5.104) (0.05-0.124) (0.96-1.354)
1981
Table 3 (continued)
n.d. - not determined.
1 Total residues of prochloraz and major metabolites unless otherwise stated (see notes 4 and 13). Total residue measured as
2,4,6-trichlorophenol (BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular weight factor (x 1.906).
2 Residue levels in whole fruit calculated from separate peel and flesh residues unless otherwise stated (see note 8).
3 Residue levels obtained from five sampling intervals at 1,2,4,8 and 16 days storage at ambient temperature after treatment.
4 Determination of free prochloraz levels only.
5 Fruit was stored for 57 days at 7°C after treatment.
6 Residue levels obtained from four sampling intervals under two storage conditions. Treated fruit was sent to the United Kingdom under
normal commercial refrigerated shipment and then stored at either 20° or 4°C, 44 days later. Samples of fruit from each storage
condition were then taken at 0,7,14 and 21-day intervals.
7 Prochloraz applied in mixture with 2,4,-0 and thiabendazole. Storage conditions as in Note 6 except sampled after 44 days refrigerated
shipment and 21 days storage under ambient or cool conditions.
8 Residue analysis of whole fruit.
9 Fruit was treated in July 1982, sent to the United Kingdom under normal refrigerated shipment and stored at ambient temperature until
September 1982.
10 Residue levels following storage of fruit for either 10 days at 20-22°C or 60 days at 3-4°C after treatment.
11 Residue level calculated from determination of free prochloraz residue in peel and total prochloraz plus major metabolites in flesh.
12 Residue levels following storage of fruit for either 7 or 14 days at ambient temperature after treatment.
13 Determination of free 2,4,6-trichlorophenol (BTS 45186) only, expressed as 2,4,6-trichlorophenol.
14 Residue levels following storage of fruit for either 14, 20 or 27 days at ambient temperature after treatment.
15 Residue levels following storage of fruit for either 12 or 16 days at ambient temperature after treatment.
16 Residue levels following storage of fruit for either 1,7, 21, 35 or 70 days in cold storage after treatment.
Strawberries
No information was provided on good agricultural practices for
prochloraz on strawberries. Limited residue data from the use of the
manganese complex W.P. formulation were provided (Table 2) with
residues of 0.2 mg/kg prochloraz found 16 days after treatment.
Recoveries were reported as >80 percent and sensitivity as
0.001-0.005 mg/kg.
Fruit with Inedible Peel
No information on good agricultural practice was available for
the use of prochloraz on fruit with inedible peel. Residue data
resulting from either foliar (Table 4) and/or postharvest applications
(Table 5) were available for several of these crops. The "recommended"
application rates were provided for most of them but no preharvest
intervals were specified.
Avocados
No information on good agricultural practice was provided for
avocados. Residue data at normal harvest and "recommended" application
rates were available for both foliar and postharvest treatments. At 2X
the 10-25 g a.i./hl "recommended" foliar W.P. (manganese complex)
application rate, maximum total residues on whole fruit basis were 2.5
mg/kg seven days after treatment, with approximately 10 percent of the
residue in the flesh (Table 4). No preharvest interval was given.
However, these data are questionable because of conflicting peel
residue levels in the data submission. Data were also available from
multiple applications of an E.C. formulation at 25-50 g a.i./hl,
although not even "recommended" uses were available for this
formulation. In these trials residues continually declined from 14 to
63 days after treatment, with estimated half-lives ranging from 40-98
days (mean 75.4 days). At these longer intervals residues in flesh
account for 20-30 percent of the whole fruit residue. The limit of
determination was said to be 0.15 mg/kg, although control values were
as high as 0.12, 0.16 and 0.39 mg/kg in flesh, peel and whole fruit,
respectively.
For postharvest treatments of avocados 25-50 g a.i./hl
(formulation unspecified) is "recommended", although no good
agricultural practice information was available. Ten trials reflecting
the "recommended" application rates were conducted in one country with
dip or spray E.C. applications (Table 5). Total residues ranged from
0.3 to 3.5 mg/kg on a whole fruit basis, with little difference in
values between stored at 23° for eight days or fruit frozen
immediately. Little difference was reported between 1X and 2X
recommended application rates. These residues levels are generally
comparable to those found from multiple foliar treatments. The limited
data indicate residues in the flesh at levels up to 30 percent of
those in whole fruit, even at relatively short intervals after
treatment.
Table 4 Prochloraz Residues in Fruit Following Supervised Trials - Foliar Application
Country/ Crop No. of
Year trials Formulation Application Interval after Mean residue level (mg/kg)1 Reference
rate (a.i.) final application
(days) Peel Flesh Whole fruit2
Australia Avocado 1 50% W.P. 7x15g/tree3 7 8.1-13.6 0.09-0.28 1.70-2.51 Cron & Longland 1983a
1981/2 (49.5 g/hl) (11)7 (0.16) (2.1)
South Avocado 1 45% E.C. 2x25g/hl4 14 1.52 <0.10 0.44 Churchill &
Africa 48 1.19 <0.10 0.36 Longland 1983b
1982 63 1.01 <0.10 0.30
1 45% E.C. 2x37.5g/hl4 14 1.85 0.12 0.61
48 1.45 <0.10 0.25
63 0.84 <0.10 0.25
1 45% E.C. 2x50g/hl4 14 2.63 0.17 0.83
48 1.94 0.18 0.59
63 2.16 0.14 0.60
Australia Mango 1 50% W.P. 6x100g/hl5 77 0.15-0.30 0.01-0.02 0.05-0.096 Browne & Manley 1982c
1981 (0.22)8 (0.01)8 (0.07)8
Israel Mango 2 50% W.P. 3x50g/hl 15 3.39-4.87 0.03-0.07 0.77-0.956 Longland &
1982 (4.2)8 Churchill 1983
Table 4 (continued)
1 Total residues of prochloraz and major metabolites, measured as 2,4,6-trichlorophenol (BTS 45186) and converted to
prochloraz-derived residue by correcting for the molecular weight factor (x1.906).
2 Residue in whole fruit (without stone) calculated from separate peel and flesh residues.
3 Applications made at approximately monthly intervals from fruit set to fruit full size.
4 Applications made with a seven-week interval between treatments.
5 Applications made from beginning of flowering to Pethenoccupic fruit drop.
6 Residue level in whole fruit including stone (calculated from peel/flesh residues).
7 Mean in parentheses.
8 Four samples.
Table 5 Prochloraz Residues in Fruit Following Supervised Trials - Postharvest Application
Mean residue level (mg/kg)1
Country/ Crop No. of Formulation Application rate
Year trials (a.i.) Peel Flesh Whole fruit2 Reference
Australia Avocado 1 40% E.C. 25 g/hl dip4 n.d. n.d. 0.88-1.313 Browne 1982a
1981 1 40% E.C. 50 g/hl dip4 n.d. n.d. 1.14-1.353
1982 2 45% E.C. 25 g/hl dip4 3.28-5.35 <0.10-0.12 0:30-0.557 Churchill &
2 45% E.C. 25 g/hi dip6 n.d. n.d. 0.16-1.019 Longland 1983e
1 45% E.C. 50 g/hl spray4 2.33-3.90 <0.10-0.11 0.28-0.367
1 45% E.C. 50 g/hl spray6 n.d. n.d. 0.34-0.429
1983 1 45% E.C. 25 g/hl dip6 n.d. n.d. 2.369
1 45% E.C. 50 g/hl dip6 n.d. n.d. 3.499
1981 Banana 1 40% E.C. 25 g/hl dip5 6.06-7.88 0.02-0.04 n.d. Browne 1982b
1 40% E.C. 50 g/hl dip5 5.18-9.97 0.03-0.05 n.d.
South Banana 2 45% E.C. 25 g/hl dip8 6.5-9.1 0.10-0.20 2.78-3.94 Churchill &
Africa 2 45% E.C. 50 g/hl dip8 9.8-16.6 0.14-0.23 4.02-6.75 Longland 1983a
1982
Israel Mango 1 40% E.C. 40 g/hl dip 6.10-8.32 0.02 1.277 Longland &
1982 Churchill 1983
Australia Papaya 1 45% E.C. 25 g/hl dip6 2.13-3.42 0.01-0.18 0.50 Cron & Longland 1983b
1982 1 45% E.C. 50 g/hl dip6 3.86-4.12 0.09-0.15 0.80
Table 5 (continued)
n.d. - not determined.
1 Total residues of prochloraz and major metabolites, measured as 2,4,6-trichlorophenol (BTS 45186) and converted to prochloraz-derived
residue by correcting for the molecular weight factor (x 1.906).
2 Residue in whole fruit calculated from separate peel and flesh residues unless otherwise stated (see notes 3 and 9).
3 Residue analysis of whole fruit (excluding stone).
4 Fruit stored for 7-8 days at 23°C after treatment.
5 Mean residue levels following storage of fruit for either 9,10,12 or 16 days at ambient temperature after treatment.
6 Fruit frozen within one to four hours after treatment.
7 Calculated whole fruit residue including weight of stone.
8 Fruit stored for 7 days at 8°C after treatment.
9 Residue analysis of whole fruit (including stone).
Bananas
No information was provided on goad agricultural practices for
the use of prochloraz on bananas. "Recommended" application rates for
foliar application are 10-25 g a.i./hl WP (manganese complex) for
foliar high volume applications (100-200 g a.i./ha low volume) and
25-50 g a.i./hl for postharvest treatment (formulation not specified).
No trials data were available for foliar applications, but trials
were reported from two countries for postharvest dips that reflected
"recommended" application rates (Table 5). Total prochloraz and
metabolite residues on a whole product basis ranged from 2.8-6.8 mg/kg
when fruit was stored for seven days at 8°C, with less than 2 percent
of the total fruit residue found in the flesh. No significant change
in residues were observed over the storage intervals of 7 to 16 days
or between 8°C and ambient temperatures. No significant difference
between residues resulted from the two application rates.
Analytical recoveries were generally 85 ± 15 percent. The limit
of determination was estimated at 0.05-0.1 mg/kg, although apparent
residues in untreated controls were as much as 0.32 mg/kg in the skin
and <0.2 mg/kg in whole fruit.
Mangoes
No information on good agricultural practice was available for
the use of prochloraz on mangoes. A 50 percent W.P. manganese complex
is "recommended" for foliar treatments at 10-25 g a.i./hl and at 25-50
g a.i./hl for postharvest treatment, although no formulation is
specified for the latter. One trial for foliar application was
conducted in each of two countries with multiple applications at 2X to
4X the "recommended" foliar rate (Table 4). Maximum total residues on
a whole fruit basis were 0.09 mg/kg from the higher application rate
77 days after treatment and up to 1 mg/kg 15 days after application at
the lower rate. In both cases, most of the residue was in the peel,
although flesh residues appeared to increase with time, being
approximately 3 percent of total fruit residue after 15 days to over
10 percent after 77 days (based on peel: flesh:seed ratios from the
77-day study).
One trial was conducted in one country for postharvest treatment
which reflected the "recommended" application rate. At 1.27 mg/kg on a
whole fruit basis, residues were similar to those of the 15-day
preharvest trial, although residues in flesh were only approximately
1 percent of the total for the postharvest treatment. Over-all
analytical recoveries for mangoes were generally 80 percent or better
and apparent residues in untreated controls were <0.02 mg/kg on a
whole fruit basis and up to 0.1 mg/kg on peel. The limit of
determination was estimated at 0.1 mg/kg.
Papaya
No information was available on good agricultural practices for
prochloraz on papaya. A 50 percent W.P. manganese complex is
"recommended" for foliar treatment at 10-25 g a.i./hl and 25-50 g
a.i./hl (formulation not specified) is "recommended" for postharvest
treatments. No preharvest intervals were given.
No residue information was available from foliar treatments, but
two postharvest dip trials with an E.C. formulation were conducted in
one country and reflected the "recommended" application rates (Table
5). Samples were frozen one hour after the dip treatment for analysis
45 days later. On a whole fruit basis, residues of prochloraz and
metabolites (corrected for approximately 80 percent recoveries) were
0.5 and 0.8 mg/kg for the two application rates. Approximately 15
percent of the whole fruit residue was in flesh. Apparent residue in
the untreated control was 0.023 mg/kg on a whole fruit basis. The
limit of determination was estimated as 0.1 mg/kg in flesh and peel.
Cereal Grains
Over 270 residue trials were conducted in 10 European and one
Asian country, which included foliar or seed treatments with six
different E.C. formulations alone or in combination with other
chemicals. Results are reported mostly as total residues of prochloraz
and metabolites, determined as the trichlorophenol but expressed as
prochloraz (Tables 6-9). Some are for residues of prochloraz only.
Good agricultural practice information was available for eight
countries.
Wheat
Over 100 field trials were conducted in nine countries,
representing 270 samples from a variety of formulations and treatment
conditions. Some trials reflected known good agricultural practices
and good agricultural practices could not be determined for others.
Over-all residues of prochloraz and major metabolites in mature grain
from foliar treatment ranged from 0.01-0.3 mg/kg and in straw at
harvest 0.08-16 mg/kg, although most grain residues were <0.1 mg/kg
(Table 6). The high grain value is from a 38-day preharvest interval
in a country whose good agricultural practices are not known, although
the application rate and preharvest interval were compatible with good
agricultural practices in other countries. Apparent residues in
untreated controls were generally <0.02 mg/kg in grain and <0.3
mg/kg in straw, but were occasionally higher in the latter. Residues
of prochloraz alone were lower and ranged from <0.01 to 0.03 mg/kg in
mature grain and <0.01 to 0.19 mg/kg in straw.
Table 6. Supervised Residue Trials with Prochloraz on Wheat at Harvest
Country/ Formulation No. of Application rate2 No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples final application
(days) grain straw
Austria 25% E.C. 2 0.5 2 70-78 <0.013 0.013 Kelly 1979a
1977 2 1.0 2 70-78 <0.01-0.013 <0.013
1978 22.5% E.C. 1 0.45 4 94 <0.02-0.02 3.66-8.39 Kelly 1979f
1 0.9 4 94 0.02-0.04 11.63-12.20
Belgium 45% E.C. 1 0.45 2 (0)5 0.02 n.d. Housden 1982a
1981 1 0.454 2 (0)5 0.01 n.d.
Denmark 25% E.C. 3 0.625 6 95-98 <0.02-0.02 n.d. Kelly 1979g
1979
40% E.C. 1 0.6 2 77 <0.02 2.1-2.5 Richards 1980d
1982 45% E.C. 1 0.45 1 56 0.106 2.01 Housden 1982c
2 2 x 0.23(19)7 2 75-85 <0.02 1.47-1.94
2 0.23 x 0.45(19)7 2 75-85 <0.02 1.87-2.36
2 2 x 0.45(19) 2 75-85 <0.02-0.02 1.56-2.07
2 2 x 0.45(19)7 2 75-85 <0.02 1.72-2.79
Finland 45% E.C. 2 0.45 2 28-50 <0.02 n.d. Heinanen 1983
1982
France 25% E.C. 2 0.5 6 105 <0.01-0.023 <0.01-0.023 Hayto 1978b
1977 2 1.0 6 105 <0.01-0.013 <0.01-0.183
2 2 x 0.5(38-46) 6 67-69 <0.01-0.033 0.01-0.123
2 2 x 1.0(38-46) 6 67-69 <0.01-0.013 0.01-0.0733
1 0.5 3 105 <0.0058 n.d. Kelly 1980b
1 1.0 3 105 <0.0058 n.d.
1 2 x 0.5(38) 3 67 <0.0058 n.d.
1 2 x 1.0(38) 3 67 0.008-0.0158 n.d.
Table 6 (continued)
Country/ Formulation No. of Application rate2 No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples final application
(days) grain straw
1978 25% E.C. 3 0.45 18 55-113 <0.02-0.06 0.50-9.66 Kelly 1979h
3 0.75 18 55-113 <0.02-0.11 0.88-9.91
1979 40% E.C. 1 0.45 3 80 <0.02 2.42-3.22 Richards 1980a
3 2 x 0.45(23-28) 9 72-77 0.04-0.10 2.40-16.3
1 2 x 0.45(8)9 3 77 0.04-0.08 3.93-4.80
30% E.C.10 1 0.45 3 80 <0.02 2.23-2.55
3 2 x 0.45(22) 9 61-81 <0.02-0.08 2.29-6.16
Federal 25% E.C. 3 0.5 4 81-107 <0.01-0.013 0.01-0.023 Hayto 1979c
Republic of 2 0.75 3 81 <0.013 0.01-0.023
Germany 1 1.0 1 107 <0.013 <0.013
1977
1978 25% E.C. 2 0.5 4 56-61 0.06-0.08 3.6-12.0 Kelly 1979c
1979 40% E.C. 3 2 x 0.48(21-30) 3 54-63 <0.02-0.06 1.0-5.9 Richards 1980g
1980 40% E.C. 3 2 x 0.48(17-23) 3 59-66 0.01-0.04 0.7-2.0 Reary 1981b
3 3 x 0.48(11-23/7-13) 3 59-66 0.03-0.06 1.1-3.6
1981 40% E.C. 5 3 x 0.48(11-29/8-19) 10 57-64 0.02-0.13 1.07-6.40 Browne 1982e
2 2 x 0.48(28-30) 3 43-60 0.06 1.88-3.59 Browne 1982f
3 3 x 0.48(9-22/8-19) 7 43-66 0.05-0.08 4.05-4.55
1 3 x 0.48(17/6) 1 55 0.05 0.4811
1982 30% E.C.10 1 0.45 3 85 <0.05 0.08-0.27 Housden &
Longland 1983
Table 6 (continued)
Country/ Formulation No. of Application rate2 No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples final application
(days) grain straw
Italy 25% E.C. 2 0.07 6 55 <0.01-0.013 0.02-0.093 Hayto 1977b
1977 3 1.0 9 39-55 <0.01-0.013 0.02-0.193,12
1978 25% E.C. 1 0.5 2 38 0.08-0.17 4.82-15.76 Kelly 1979d
1 0.7 38 0.13-0.29 6.29-14.73
The 25% W.P. 1 0.375 3 71 <0.013 <0.013 Hayto 1978a
Netherlands 25% E.C. 1 0.375 3 73 0.01-0.023 <0.01-0.013
1977 1 0.5 3 73 0.01-0.023 0.01-0.023
1978 25% E.C. 1 0.375 3 68 0.04 1.14-1.51 Kelly 1979e
1 0.75 3 68 0.04-0.06 5.66-8.14
1979 40% E.C. 1 0.5 4 71 <0.02 0.88-2.23 Kelly 1980c
1980 45% E.C. 2 0.45 6 69 0.01-0.02 0.41-0.77 Reary 1981c
1 0.675 2 106 0.01 0.21-0.51
1 2 x 0.45 (15) 3 69 0.01-0.02 2.21-3.29
Sweden 45% E.C. 8 0.45 8 67-91 <0.02 0.08-4.08 Longland 1983a
1982 2 2 x 0.45(15-29) 2 58-69 0.03-0.07 1.29-6.34
United 25% E.C. 2 0.4 6 90-115 <0.013 <0.013 Hayto 1977a
Kingdom 2 1.0 6 90-115 <0.013 <0.013
1976
1977 25% E.C. 4 0.5 12 124-126 <0.013 <0.013 Hayto 1979a
1979 40% E.C 2 2 x 0.4(36-37) 6 69-79 <0.02-0.06 4.2-11.2 Richards 1980c
10% S.D.13 1 0.214 2 31015 <0.02 <0.02
Table 6 (continued)
n.d.- not determined.
1/ Total residues of prochloraz and major metabolites, unless otherwise stated (see Notes 3 and 8). Total residue as 2,4,6-trichlorophenol
(BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular weight factor (x 1.906).
2/ Figures in parentheses indicate interval in days between applications.
3/ Determination of free prochloraz residues only.
4/ Prochloraz applied in mixture with carbendazim.
5/ Growth stage at application : 0 - heading.
6/ Residue analysis of whole ears.
7/ Prochloraz applied in mixture with fenpropimorph.
8/ Determination of free 2,4,6-trichlorophenol (BTS 45186) residues only, expressed as BTS 45186.
9/ Prochloraz applied in mixture with mancozeb.
10/ Co-formulation of prochloraz with carbendazim.
11/ Mean residue level in whole ears. A mean residue level of 2.31 mg/kg was found in the whole plant.
12/ Results from two trials.
13/ Seed dressing co-formulation with carboxin.
14/ Rate of application in g a.i./kg seed.
15/ Days after sowing treated seed.
Barley
Information on good agricultural practice for barley was
available from five countries and residue trials reflecting such uses
were reported from those countries and others (Table 7). At-harvest
residues of prochloraz and its major metabolites, from foliar
applications, ranged from <0.01-0.68 mg/kg in mature grain and
<0.01-15 mg/kg in straw (Table 7). Again, the higher values are
generally from the shorter preharvest intervals, which reflect good
agricultural practice, and from trials in which prochloraz was
co-applied with either carbendazim or mancozeb. The rates reflect
usage in the country where the trials were conducted. Most residues in
grain were <0.2 mg/kg. Although grain residues from these shorter
intervals were significantly higher than those from longer intervals,
residues in straw were not.
Analytical recoveries were variable, with means ranging from
70-100 percent. Apparent residues in untreated controls were <0.02
mg/kg in grain and up to 0.2 mg/kg in straw. Residues of prochloraz
alone were substantially lower, ranging from <0.01-0.02 mg/kg in
mature grain and <0.01-0.15 mg/kg in straw, which are comparable to
the values reported in wheat.
Rye
Total prochloraz and its metabolites, from foliar treatments in
mature rye grain or whole ears ranged from <0.02-0.1 mg/kg and in
straw from 0.4-2.5 mg/kg. Application rates reflected good
agricultural practice, although the preharvest interval of 72 to 109
days was significantly greater than the one month permitted (Table 8).
Analytical recoveries were typically >80 percent in grain and
somewhat lower in straw. Apparent residues in untreated controls were
<0.01 mg/kg in grain but as high as 0.04 mg/kg in straw.
Oats
Information on good agricultural practice for oats was not
available for the country in which residue trials were conducted or
from other countries. Residues of prochloraz and its metabolites were
<0.03 mg/kg and <1.6 mg/kg for whole ears and straw, respectively,
62 days after treatment at application rates considered good
agricultural practice for other cereals (Table 8).
Rice
No information on good agricultural practice was available for
rice, except for seed treatments in one country (for which there are
no data). Residues of prochloraz alone in unpolished brown rice 7-21
days after treatment at application rates comparable to good
agricultural practice on other cereals were <0.05 mg/kg (Table 8).
Residues of prochloraz and its metabolites would be expected to be
higher. Residues from seed treatments were lower, but the interval
after treatment was 151-168 days. Analytical recoveries were 86
percent in grain and apparent residues in untreated controls were
<0.005 mg/kg, the limit of detection.
Summary data for residues in cereal grain at harvest (Table 9)
represents foliar applications of all formulations. The data in Table
9 confirm those reported in Tables 6-8, since it is statistically
shown that maximum residues of prochloraz and its metabolites would
normally be less than 0.2 mg/kg at the longer preharvest intervals but
residues up to 0.4 mg/kg can occur. Other trials at shorter intervals
also resulted in higher grain residues than at longer intervals.
Similarly, Table 11 confirms the findings reported in Tables 6-8 for
residues in straw (up to 15 mg/kg). Tables 9 and 11 do not include
more recent studies (Housden 1982c; Longland 1983a; Housden & Longland
1983; Heinanen 1983) reported in Tables 6-8, but these would not
increase expected residues. Tables 9 and 11 also indicate that higher
residues can be expected from multiple or higher doses.
Residues of prochloraz alone were <0.03 mg/kg in cereal grains
at harvest, except for rice where residues were as much as 0.05 mg/kg
7 to 21 days after treatment. From seed treatment alone, residues of
prochloraz and its metabolites in mature grain were <0.02 mg/kg.
Generally, there was no conclusive evidence of increased residues as a
result of co-application of prochloraz and other agricultural
chemicals.
Residue decline studies are summarized in Table 10. Half-lives of
approximately 6 to 17 days could be estimated for ears or whole plants
of wheat or barley on the basis of sampling for at least three
successive intervals. Residues of prochloraz and its metabolites
ranged from 0.15 to 21 mg/kg on green plants or ears on the day of
last treatment to 0.07-3.6 mg/kg at 21 to 48 days, which approximates
typical preharvest intervals.
Almonds
No information was available on good agricultural practice for
prochloraz on almonds. Foliar treatments with the 50 percent W.P.
manganese complex are "recommended" at 15-30 g a.i./hl. No preharvest
interval has been reported. One trial was conducted with foliar
treatments at seven times the maximum recommended application rate
(Table 2) with total residues corrected for recoveries of >86
percent of 0.61, 0.1 and 0.29 mg/kg in shell, kernel and whole nut
(calculated from shell and kernel residues), respectively, about three
months after the last treatment. Apparent residues were 0.06 mg/kg in
un-treated controls on a whole nut basis and up to 0.09 mg/kg in the
shell. The limit of determination was estimated at 0.1 mg/kg.
Table 7 Supervised Residue Trials with Prochloraz on Barley at Harvest
Country/ Formulation No. of Application rate2 No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples final application
(days) grain straw
Austria 25% E.C. 2 0.5 2 61-73 <0.013 <0.01-0.02
1977 1 0.75 1 73 <0.013 0.023
1 1.0 1 61 <0.013 0.033
1 2x0.5(19) 2 54 <0.013 <0.013
1978 22.5% E.C. 2 0.45 69-75 <0.02-0.04 0.65-1.30 Kelly 1979f
1 0.9 75 0.02-0.04 1.0-2.1
Belgium 45% E.C. 3 0.45 6 (M)10 0.01-0.03 n.d. Housden 1982a
1981 7 0.4511 14 (M)10 0.01-0.11 n.d.
2 2x0.4511 (I+M)10 0.02-0.03 n.d.
Denmark 25% E.C. 1 0.75 96 <0.02 n.d. Kelly 1979g
1979 40% E.C. 2 0.6 76-86 <0.02-0.10 1.9-4.6 Richards 1980d
20% E.C. 1 0.6 86 <0.02-0.04 2.5-3.4
20% S.D. 2 0.28 132-1399 <0.02 <0.02
1982 45% E.C. 2 0.234 3 75-87 <0.02-0.03 0.84-1.10 Housden 1982c
4 0.45 4 61-80 <0.02-0.02 1.45-3.24
1 0.45 1 60 0.0514 3.02
2 2x0.45(14) 61-73 <0.02-0.02 1.61-2.59
France 25% E.C. 2 0.5 62-74 <0.01-0.013 <0.01-0.053 Hayto 1978b
1977 2 1.0 62-74 <0.01-0.023 0.01-0.153
1978 25% E.C. 1 0.45 64 0.02-0.06 1.77-8.86 Kelly 1979h
1 0.9 64 0.02-0.08 2.67-10.48
1 2x0.45(19) 62 0.04-0.09 4.23-7.00
1 2x0.9 (19) 62 0.04-0.17 11.19-11.95
Table 7 (continued)
Country/ Formulation No. of Application rate2 No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples final application
(days) grain straw
1979 40% E.C. 2 0.45 4 54-62 <0.02-0.17 5.76-10.81 Richards 1980a
2 2x0.45(21)5 6 38 0.36-0.68 7.20-12.12
30% E.C.6 2 2x0.45(13-21) 38-48 0.10-0.57 6.96-9.47
Fed. Rep. 25% E.C. 1 0.5 1 70 <0.013 <0.013 Hayto 1979c
of Germany 1 0.7 1 70 <0.013 <0.013
1977
1980 40% E.C. 2 0.48 77-79 0.02-0.06 0.06-1.0 Richards 1981b
2 2x0.48(19)12 77-79 0.02-0.06 1.1 -1.3
1981 40% E.C. 2 2x0.48(11-12) 61-62 0.02-0.06 0.95-1.27 Browne 1982f
1982 30% E.C.6 3 0.45 62-76 <0.05 0.23-0.83 Housden & Longland
1983
Netherlands 45% E.C. 1 0.45 3 70 0.04-0.06 0.78-1.21 Reary 1981c
1980
Sweden 45% E.C. 4 0.45 61-72 <0.0213 0.19-1.27 Longland 1983a
1982 2 2x0.225(8-15) 53-55 <0.02 0.24-1.21
2 2x0.45(8-15) 53-55 <0.02 0.34-2.86
United 25% E.C. 3 0.4 54-74 <0.013 <0.01-0.023,7 Hayto 1977a
Kingdom 4 0.5 19-64 <0.013 0.01-0.023,13
1976 2 1.0 64-74 <0.013 <0.013,20
1977 25% E.C. 4 0.5 76-78 <0.013 0.01-0.043 Hayto 1979c
2 1.0 76-78 <0.01-0.01 <0.01-0.02
1979 40% E.C. 3 2x0.4(18-29) 36-63 0.06-0.32 3.0 -14.5 Richards 1980c
Table 7 (continued)
n.d. not determined.
1/ Total residues of prochloraz and major metabolites, unless otherwise stated. Total residue measured as
2,4,6-trichlorophenol (BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular
weight factor (x 1.906).
2/ Figures in parenthesis indicate interval in days between applications.
3/ Determination of free prochloraz residues only.
4/ Prochloraz applies in mixture with fenpropimorph.
5/ Prochloraz applied in mixture with mancozeb.
6/ Co-formulation of prochloraz with carbendazim.
7/ Results from two trials.
8/ Rate of application in g a.i./kg seed.
9/ Days after sowing treated seed.
10/ Growth stages at application: M-heading, I-first joint.
11/ Prochloraz applied in mixture with chlorothalonil.
12/ Interval between treatments not given in one trial.
13/ Results from three trials.
14/ Residue analysis of whole ears.
Table 8 Supervised Residue Trials with Prochloraz on Cereals at Harvest
Crop Country/ Formulation Number of Application rate No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples application
(days) grain straw
Rye Austria 25% E.C. 1 0.5 1 91 0.013 <0.013 Kelly 1979a
1977 1 1.0 1 91 <0.013 <0.013
Denmark 45% E.C. 2 0.45 2 72-77 <0.02-0.10 1.44-2.48 Housden 1982c
1982
Fed. Rep. 40% E.C. 2 0.48 4 89-109 0.03-0.05 0.39-0.53 Browne 1982d
of Germany
1981
Oats Denmark 45% E.C. 2 0.45 2 62 0.02-0.034 1.19-1.64 Housden 1982c
1982
Rice Japan 25% E.C. 2 0.75 12 7-21 <0.01-0.043 n.d. Gato 1980
(brown, 1980 25% E.C. 2 3x0.75 12 7-21 <0.01-0.043 n.d.
unpolished) 25% E.C. 2 25 g/hl2 + 12 7-21 <0.01-0.043 n.d.
3x0.75
25% E.C. 2 25 g/hl2 4 151-168 <0.013 n.d.
n.d. = not determined.
1/ Total residues of prochloraz and major metabolites, unless otherwise stated. Total residues measured as 2,4,6-trichlorophenol
(BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular weight factor (x1.906).
2/ 48-hour seed soak.
3/ Determination of free prochloraz residues only.
4/ Residue analysis of whole ears.
Table 9 Summary of Residues in Mature Grain
Crop Application No. of Total residue (mg/kg)
(kg a.i./ha) results
Mean Std. Dev.
Wheat 1 × 0.375-0.5 36 0.03 0.03
Wheat 2 × 0.375-0.5 36 0.04 0.03
Wheat 3 × 0.375-0.5 12 0.06 0.03
Wheat 1 × 0.6-0.9 31 0.07 0.18
Barley 1 × 0.4-0.5 42 0.03 0.03
Barley 2 × 0.4-0.5 20 0.091 0.091
Barley 1 × 0.6-0.9 14 0.03 0.03
Barley 2 × 0.9 3 0.09 0.05
Rye 1 × 0.48 2 0.04
1/ Excluding a set of 11 results from four replicated applications
of prochloraz alone and with mancozeb, which were exceptionally
high (mean 0.42, std. dev. 0.18 mg/kg), residues in straw were not
exceptionally high. The high grain results may reflect poor
separation of grain and chaff in these samples (Richards 1980a).
Table 10 Supervised Trials on Residue Decline with Prochloraz
Crop Country/ No of Formulation Application rate2 Residues (mg/kg)1 after References
Year trials (kg/a.i./ha) final application (days)
35-36 56 75-85
Wheat Denmark 1 45% E.C. 0.45 whole plant 1.62 Housden 1982c
1982 whole ears 0.10
straw 2.01
2 45% E.C. 2x0.23(19)3 whole plant 0.33-0.94
grain <0.02
straw 1.47-1.94
2 45% E.C. 0.23+0.45(19)3 whole plant 1.11-1.76
grain <0.02
straw 1.87-2.36
2 45% E.C. 2x0.45(19) whole plant 0.70-2.02
grain <0.02-0.02
straw 1.56-2.07
2 45% E.C. 2x0.45(19)3 whole plant 1.38-1.44
grain <0.02
straw 1.72-2.79
Table 10 (Contd.)
Crop Country/ No. of Formulation Application2 After After final application (days) References
Year trials rate penultimate
(kg a.i./ha) application
(days)
20-29 0 15-22 37-42 54-63
Wheat Fed. Rep. 3 40% E.C. 2x0.48 green plant 0.80-1.644 1.62-2.554
of (21-30) ears 0.15-1.114 0.27-1.494 0.23-0.844 0.06-0.084 Richards
Germany grain <0.02-0.06 1980g
1979 straw 1.0-5.9
17-23 0 8-19 39-44 59-66
1980 3 40% E.C. 2x0.48 green plant 0.15-2.7
(17-23) green ears 4.7 -13.6 0.84-2.2 0.28-0.61 Reary
grain 0.01-0.04 1981b
straw 0.7 -2.0
7-13 0 8-19 39-44 59-66
3 40% E.C. 3x0.48 green plant 0.2 -4.7
(11-23/ green ears 5.5 -16.8 0.61-3.3 0.35-0.58
7-13) grain 0.03-0.06
straw 1.1 -3.6
8-19 0 7-14 31-46 58-64
1981 5 40% E.C. 3x0.48 green ears 0.16-0.96 3.87-12.50 2.42-5.85 0.31-1.16 Browne
(11-29/ whole plant 0.51-3.789 1982e
8-19) grain 0.04-0.31 0.02-0.13
straw 1.07-6.40
Table 10 (Contd.)
Crop Country/ No. of Formulation Application2 After After final application (days) References
Year trials rate penultimate
(kg a.i./ha) application
(days)
28-30 0 12-14 21-48 43-60
1981 2 40% E.C. 2x0.48 green ears 0.11-0.84 10.64-13.2 1.14-4.21 0.71-1.18 Browne
(28-30) whole plant 2.078 1982f
grain 0.038 0.06
straw 1.88-3.59
6-19 0 7-14 21-48 43-60
4 40% E.C. 3x0.48 green ears 2.96-8.27 13.90-21.40 3.61-7.25 0.49-2.32
(9-22/ whole plant 1.74-3.614 2.317
6-19) green ears+ 2.318
straw
grain 0.03-0.217 0.05-0.08
straw 4.05-4.55
ears 0.487
0 17 42 57 85
Wheat Fed. 3 30% E.C.5 0.45 whole plant 7.99-13.64 0.74-1.95 0.21-0.94 0.45.0.62 Housden &
Rep. of grain <0.05 Longland
Germany straw 0.08-0.27 1983
1982
34-36 60-87
Barley Denmark 2 45% E.C. 0.233 whole plant 0.64-0.74
1982 grain <0.02-0.03
straw 0.84-1.10
Table 10 (Contd.)
Crop Country/ No. of Formulation Application2 After After final application (days) References
Year trials rate penultimate
(kg a.i./ha) application
(days)
34-36 60-87
5 45% E.C. 0.45 whole plant 0.77-2.32
grain <0.02-0.02
straw 1.45-3.24
whole ears 0.058
2 45% E.C. 2x0.45 whole plant 1.03-3.18
(14) grain <0.02-0.02
straw 1.61-2.59
19 0 13 33-34 77-79
Fed. 2 40% E.C. 0.48 green plant 5.1-10.1 0.4-1.5 0.6-1.7 Richards 1981b
Rep. of grain 0.02-0.06
Germany straw 0.6-1.0
1980 2 40% E.C. 2x0.48 green plant 1.1-3.4 3.2-5.0 1.5-2.5 1.3-2.1
(19)6 grain 0.02-0.06
straw 1.1-1.3
11-12 0 10-18 27-48 61-62
1981 2 40% E.C. 2x0.48 green plant 0.91-7.99 10.40-18.60 Browne 1982f
(11-12) green ears 0.52-3.62 0.07-0.50
ears + straw 0.218
grain 0.088 0.02-0.06
straw 0.95-1.27
Table 10 (Contd.)
Crop Country/ No. of Formulation Application2 After final application (days) References
Year trials rate
(kg a.i./ha)
0 19 38-40 56 62-76
1982 3 30% E.C.10 0.45 whole plant 5.25-10.38 0.21-1.78 0.13-0.80 <0.05-0.78 Housden &
grain <0.05 Longland 1983
straw 0.23-0.83
35 72-77
Rye Denmark 2 45% E.C. 0.45 whole plant 0.85-1.62
1982 whole ears <0.02-0.10
straw 1.44-2.48
0 11 21 34 55-56 69 83 89 93 109
Fed. Rep. 1 40% E.C. 0.48 whole plant 9.6 1.16 0.20 Browne
of green ears 0.03 0.02 1982d
Germany ears 0.03
1981 grain 0.03 0.05
straw 0.39
1 40% E.C. 0.48 whole plant 13.8 1.33 0.05
green ears 0.14 0.05
ears 0.02
grain 0.10 0.03
straw 0.53
36-37 62
Oats Denmark 2 45% E.C. 0.45 whole plant 0.37-0.88 Housden
1982 whole ears 0.02-0.03 1982c
straw 1.19-1.64
Table 10 (Contd.)
1 Total residues of prochloraz and major metabolites, unless otherwise stated. Total residue measured as 2,4,6-trichlorophenol
BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular weight factor (x 1.906).
2 Figures in parentheses indicate interval in days between applications.
3 Prochloraz applied in mixture with fenpropimorph.
4 Results from two trials.
5 Co-formulation of prochloraz with carbendazim.
6 Interval between treatments not given in one trial.
7 Results from three trials.
8 Result from one trial.
9 Results from four trials.
Table 11 Summary of Residues in Mature Straw
Crop Application No. of Total residue (mg/kg)
(kg a.i./ha) results Mean Std. Dev.
Wheat 1 × 0.375-0.5 35 2.83 3.07
Wheat 2 × 0.375-0.5 36 4.85 3.23
Wheat 3 × 0.375-0.5 11 3.20 1.60
Wheat 1 × 0.6-0.9 22 5.03 4.42
Barley 1 × 0.4-0.5 20 3.30 3.05
Barley 2 × 0.4-0.5 26 6.95 3.64
Barley 1 × 0.6-0.9 13 3.36 1.92
Barley 2 × 0.6-0.9 3 11.6 0.4
Barley 1 × 0.48 2 0.46
No information on good agricultural practice was available for
the use of prochloraz on almonds. A 50 percent prochloraz-manganese
complex is "recommended" as a foliar treatment at a 15-30 g a.i./hl
application rate. Two applications at 3X this rate in one trial
resulted in maximum residues up to 0.7 mg/kg (Table 2). The limit of
determination has been estimated at 0.1 mg/kg although the apparent
residues in an untreated control were 0.07 mg/kg. Recoveries from
hulls amended with prochloraz at 1 mg/kg were 96±7 mg/kg.
Oilseed Rape
Information on good agricultural practice was available for
prochloraz on oilseed rape from two countries, from which data on
residue trials were also available. Residue data from 48 trials were
also reported from two countries for which good agricultural practice
was not known to the Meeting (Table 12). At preharvest intervals from
25 to 266 days, residues of prochloraz and its metabolites, reflecting
good agricultural practice, ranged from 0.01 to 0.33 mg/kg. Preharvest
intervals, if applicable, were not provided. The limit of sensitivity
was estimated at 0.05 mg/kg. However, since apparent residues in
untreated controls ranged from <0.01-0.15 mg/kg (but were usually
less than 0.05 mg/kg), a 0.2 mg/kg over-all limit of determination may
be more realistic. Analytical recoveries were > 85 ± 15 percent of
amended levels of 0.2-1 mg/kg.
Mushrooms
Information on good agricultural practice and corresponding
residue trials were available from two countries, as well as data from
residue trials from another country (total of 31 trials, Table 13).
The most common treatment was a single application of the
prochloraz-manganese complex formulation 1-10 days after casing at 1.5
g a.i./l sq m with a 10-day preharvest interval, or three applications
at 0.3 g a.i./l/m2, the first 1-10 days after casing and then after
the first and second flush, with a two-day pre-harvest interval. A
variation of the latter was also used at 2x 0.6 g a.i./l/sq m (Table
1), also with a two-day preharvest interval. Data extracted from Table
13 that reflect good agricultural practice are:
Country/ Applications Residue (mg/kg) Interval after Approved preharvest
and rate last application harvest
(g a.i./sq m) (days) interval (days)
The Netherlands 1 x 1.6 0.11-0.27 total residue 10 10
United Kingdom 2 x 0.6 1.4-1.6 total residue 4 2
0.6-0.8 free prochloraz
1 x 1.25 0.36 total residue 13 10
0.47 free prochloraz 28 10
3 x 0.3 0.24-0.43 total residue 2 2
0.07-0.1 free prochloraz 6 2
0.4-1.4 free prochloraz 3 2
Table 12 Supervised Residue Trials with Prochloraz on Rapeseed
Country/ Formulation No. of Application rate2 No. of Interval after Residues1 References
Year trials (kg a.i./ha) samples final application (mg/kg)
(days)
France 40% E.C. 2 0.45 8 73 0.02-0.04 Cron 1982
1980/1 2 0.453 8 73-76 0.01-0.03
1 0.60 4 73 0.02-0.03
30% E.C.4 2 0.45 8 73-76 0.02-0.03
1982 40% E.C. 4 0.45 12 37-51 0.03-0.15 Peatman & Snowden 1982
4 0.453 12 37-51 0.03-0.13
4 2x0.45 (12-15) 12 25-38 0.04-0.32
30% E.C.4 4 2x0.45 (12-15) 12 25-38 0.06-0.33
Denmark 45% E.C. 3 0.45 6 70-76 <0.05 Peatman & Snowden 1983
1982 3 2x0.45 (18-23) 7 53 0.07-0.19
Sweden 45% E.C. 3 0.45 6 47-72 0.12-0.28 Peatman & Snowden 1983
1982
United 40% E.C. 3 0.45 238-266 <0.05-0.07 Manley & Snowden 1982c
Kingdom 3 0.45 3 119-133 <0.05-0.19
1981/2 3 2x0.4(91-109)5 3 119-133 <0.05
3 3x0.4(91-109/ 3 64-75 <0.05-0.09
51-58)5
2 3x0.4(92-103/ 2 72-73 <0.05-0.06
51-57)
2 3x0.5(92-103/ 1 72-73 0.06
51-57)
Table 12 (continued)
1 Total residues of prochloraz and major metabolites, measured as 2,4,6-trichlorophenol (BTS 45186) and converted to
prochloraz-derived residue by correcting for the molecular weight factor (x 1.906).
2 Figures in parentheses indicate interval in days between applications.
3 Prochloraz applied in mixture with mancozeb.
4 Co-formulation of prochloraz with carbendazim.
5 Prochloraz applied in mixture with tolclofos methyl.
Table 13 Supervised Residue Trials with Prochloraz on Mushrooms
Country/ No. of No. of Application rate (a.i.) Residues (mg/kg)1 days after final application References
Year trials samples and timing2
3 5 10 14 20 21 27 45
Australia 1 1.25g/sq m at casing 0.1 0.1 <0.1 Housden
1981 1 2.50g/sq m at casing 0.23 0.13 0.14 1982
1 1.25g/sq m at casing +
0.63g/sq m after first flush 0.41-0.63 0.22
(24-day interval)
1 2.50g/sq m at casing +
1.25g/sq m after first flush 1.16-1.63
(24-day interval)
1 2.50g/sq m at casing +
2.50g/sq m after first flush <0.1
(24-day interval)
1 0.63g/sq m after first flush 1.06-2.98
1 1.25g/sq m after first flush 4.48-4.61
1 1.25g/sq m after first flush+
0.63g/sq m after second flush 0.29
(7-day interval)
1 2.50g/sq m after first flush+
1.25g/sq m after second flush 0.37
(7-day interval)
The 1 4 1.6g/sq m before inoculation 0.11-0.27 Browne
Netherlands 1 4 0.75g/sq m before inoculation+ 0.15-0.24 1982
1980 0.75g/sq m 14 days later
4 0.75g/sq m before inoculation+
0.75g/sq m after first flush 0.15-1.14
(23-days interval)
Table 13 (Continued)
Country/ No. of Application rate (a.i.) Residues (mg/kg)1 days after References
Year trials and timing2 1st application 2nd application 3rd application
19 7 4
United 1 0.61g/sq m after casing + 0.04 0.10 0.08 Richards 1981c
Kingdom 0.61g/sq m after first flush +
1980 0.61g/sq m after second flush
(19- and 10-day intervals)
1st application 2nd application
21 28 4 12 18
1 0.61g/sq m after casing + 0.04 0.04-0.11 1.37-1.58 <0.02
0.61g/sq m after second flush
(31-day interval)
14 0.61g/sq m after casing + 0.013 0.01-0.33 0.56-0.803 0.08-0.333 0.05-0.183 Whiteoak 1981
0.61g/sq m after second flush
(31-day interval)
Days after application
28 35 43 49
1 1.25g/sq m 7 days after inoculation 0.473 0.043 0.27-0.323 0.203
1 0.625g/sq m 7 days after inoculation 0.133 0.103 0.093 0.07-0.083
2nd application 3rd application
13 6 14 20
1 0.625g/sq m 7 days after inoculation 0.193 0.22-0.243 0.033 0.27-0.323
1 + 2x0.313g/sq m at 14-day intervals
thereafter
1 0.313g/sq m 7 days after inoculation
+ 2x0.313g/sq m at 14-day intervals 0.133 0.07-0.103 0-323 0.053
thereafter
Table 13 (Continued)
Country/ No. of Application rate (a.i.) Residues (mg/kg)1
Year trials and timing2 Days after application
2nd application 3rd application
5 12 3 14 21
1981 1 0.15g/sq m five days after inoculation 0.323 0.123 0.62-0.653 0.193 0.093 Whiteoak 1981
+ 2x0.15g/sq m at 18- and 14-day
intervals thereafter
45 0.3g/sq m + 2x0.3g/sq m. Timing as 0.20-0.373 0.11-0.643 0.39-1.443 0.10-0.313 0.06-0.073
given above
1 0.9g/sq m + 2x0.3g/sq m. Timing as 0.423 0.213 1.293 0.543 0.21-0.243
given above
1 1.5g/sq m + 2x0.3g/sq m. Timing as 0.273 0.523 0.993 n.d. 0.063
given above
1 3.0g/sq m + 2x0.3g/sq m. Timing as 0.623 0.543 0.743 0.343 0.953
given above
Table 13 (Continued)
Country/ No. of Application rate Residues (mg/kg)1 days after References
Year trials (a.i.) and timing2 1st application 2nd application 3rd application
13-14 15-16 18 3 5 7 10 12-13 14-16 2 3-4 7 9-10 12 14 17
United 1 0.3g/sq m 7 days after 0.24 0.20 0.79 0.426 0.30 0.30 0.377 0.23 0.51 0.43 0.33 1.03 0.32 0.23 0.28 0.22 Housden
Kingdom casing + 2x0.3g/sq m &
1981 at 18- and 14-day Whiteoak
intervals thereafter 1982
1982 1 0.3g/sq m 7 days after 0.26 0.14 0.85 0.24 0.24 0.26 0.58 0.24 0.61 0.45 0.61
casing + 2x0.3g/sq m (0.57)8 (1.11)8
at 15- and 17-day
intervals thereafter
Residues (mg/kg)1 days after application
13 15 18 22 25 28 31 34 36 39 42
1982 1 1.25g/sq m 7 days after casing 0.36 0.25 0.33 0.30 0.20 0.38 0.42 0.28 0.54 0.22 0.33
Table 13 (Continued)
n.d. = not determined.
1 Total residues of prochloraz and major metabolites, unless otherwise stated (see note 3). Total residue measured as
2,4,6-trichlorophenol (BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular weight factor (x1.906).
2 All treatments used the prochloraz-manganese complex 50% W.P. formulation.
3 Determination of residues of free prochloraz only.
4 Samples of mushroom also analysed for total residues of prochloraz and metabolites (see Richards 1981c)
5 Mean residue levels from four trials using spray volumes of 100, 200 or 400 l/sq.m.
6 Mean residue level in small and larger mushrooms (0.43 and 0.40 mg/kg respectively).
7 Mean residue level in small and larger mushrooms (0.52 and 0.21 mg/kg respectively).
8 Mushroom emerged before treatment (direct application).
Therefore, residues of prochloraz and its metabolites or of
prochloraz alone may be at least as much as 2 mg/kg from approved
practices. Differences between residues of prochloraz and its
metabolites, compared to free prochloraz, do not appear to be as high
as in cereals. Analytical recoveries of total residues were generally
> 70 percent, with apparent residues of 0.03 ± 0.02 mg/kg in
untreated controls and > 77 percent and 0.01 mg/kg for free
prochloraz.
FATE OF RESIDUES
The fate of prochloraz has been studied in plants and animals. In
plants, it is metabolized to
N-formyl-N'-1-propyl-N-(2-(2,4,6-trichlorophenoxy)ethyl) urea,
also known as BTS 44596 or the "formyl urea"; to
N-propyl-N-(2-(2,4,6-trichlorphenoxy)ethyl) urea, also known as
BTS 44595 or "the urea"; to 2,4,6-trichlorophenoxyacetic acid (BTS
9608), and finally to 2,4,6-trichlorphenol (BTS 45186), all of which
form stable conjugates (Figure 1). Animal metabolism has resulted in
characterization of urinary metabolites (see Figure 1 under
"Toxicology"), information of radioactivity distribution in tissues
from oral or dermal administration and rate and route of excretion.
Qualitatively, urinary metabolites are similar among several test
animals, although the major plant metabolites have not been identified
in the urine. Residues in animal tissues have not been identified.
Metabolism studies have also been conducted on the major plant
metabolite BTS 44596.
In Animals
Metabolism of prochloraz in rats, dogs, rabbits, mice and pigs is
discussed under "Biochemical Aspects".
Goat
A study was conducted to indicate the level of residues that
might occur in tissues and milk of lactating ruminant animals after
ingestion of prochloraz. A lactating goat was given two 60 mg oral
doses of 14C-phenyl labelled prochloraz by gelatin capsules, with 13
days between doses. The dosage was designed to reflect possible
residues resulting from consumption of 3 kg of straw with residues of
20 mg/kg of prochloraz at each dosing (versus maximum straw residues
of 12 mg/kg). Milk and plasma samples were taken at appropriate
intervals after each dose and tissue samples 24 h after the second
dose. After the first dose, residues of prochloraz equivalent in milk
decreased from 0.04 mg/kg after 8 h to 0.01 mg/kg (limit of
determination) after 48 h. Milk residues were 0.01 mg/kg and 0.03
mg/kg at 2.5 and 18.5 h, respectively, after the second dose. In
tissues, residues of prochloraz equivalent were 1.7 mg/kg in liver,
0.2 mg/kg in kidney, 0.36 mg/kg in adrenal, <0.05 mg/kg in fat,
0.03 mg/kg in muscle and <0.2 mg/kg in other tissues. Rumen
contents contained prochloraz equivalent of 0.65 mg/kg and bile 5.4
mg/kg. Residues were not characterized or identified (Campbell 1980).
A second and similar experiment was conducted using the
photodegradant and plant metabolite BTS 44596, with an interval of 8 h
between doses. Results were similar to those for prochloraz, although
the higher maximum milk residues of 0.07 mg/kg BTS-44596 equivalent 7
h after dosing decreased somewhat more rapidly (< 0.01 mg/kg after
31 h). Residues of BTS-44596 equivalent in tissues were somewhat less
than for prochloraz (liver 0.59 mg/kg, kidney 0.12 mg/kg, fat <0.01
mg/kg, muscle <0.01 mg/kg and less in other tissues as well),
Residues were not identified or characterized (Campbell & Needham
1980).
In another study a lactating goat was fed ad libitum for four
days with wheat straw containing 19 mg/kg field-incurred
14C-prochloraz equivalent residues. The straw was harvested at
maturity 11 weeks after treatment at an equivalent rate of 0.94 kg
a.i./ha (twice the recommended rate), Actual straw consumption was
approximately 300 g/day (or approximately 6 mg of prochloraz
equivalent/day), in addition to 1 700 g additional feed made available
daily. After four days, maximum tissue residues of prochloraz
equivalent, as determined by liquid scintillation counting was liver
0.05 mg/kg, kidney fat 0.04 mg/kg and 0.03 mg/kg in other tissues,
with a reported limit of determination of 0.02 mg/kg. Bile and rumen
residues were 0.12 and 0.13 mg/kg, respectively. Maximum residues in
milk were 0.006 mg/l and in plasma 0.079 mg/l. The limit of
determination for milk was 0.001 mg/kg in this experiment. Residues in
milk were relatively consistent over the four-day feeding period. Milk
residues were 0.002 mg/kg prior to the feeding of treated straw
(Campbell 1983).
In Plants
The fate of prochloraz has been studied in wheat (various
stages), citrus and apples. Soil uptake by sugar beet, which
represents a likely rotational crop, was also studied. The metabolic
fate in plants is illustrated in Figure 1.
Wheat
Wheat plants grown under glass were syringe treated at the sixth
leaf stage with a 25 percent a.i. formulation of 3H-labelled
prochloraz (labelled in the 3 and 5 positions of the benzene ring) at
a rate equivalent to 1 kg/ha. After 19 days, the green wheat tissue
was harvested and residues characterized and quantified by a variety
of analytical techniques. Neutral, acid and basic fractions were
analysed, with and without acid hydrolysis. After 19 days, 82.6
percent of the applied radioactivity remained and 96.7 percent of this
was extractable by the analytical procedures used (acetone-water
extraction). The principal residue was free BTS 44596, closely
followed by free and conjugated residues of BTS 44595, with lower
levels of free prochloraz and free and conjugated trichlorphenol,
conjugated trichlorophenoxyacetic acid and seven unknowns. Terminal
prochloraz equivalent was 7 mg/kg. Individual residues were:
% of applied % of terminal
radioactivity residue*
BTS 44596: N-formyl-N'-l-propyl-N-(2-(2,4,6-
trichlorophenoxy) ethyl) urea free 31.6 41
BTS 44595: N-propyl-N-(2-(2,4,6-trichlorophenoxy) free 11 13
ethyl) urea conj. 19.5 23.1
BTS 45186: 2,4,6-trichlorophenol free 1.4 1
conj. 6.8 4.9
BTS 9608 2,4,6-trichlorophenoxy-
acetic acid conj. 0.21 0.2
BTS 40542: Prochloraz (unchanged) free 1.1 1.5
Unknown (seven) 5.5 7.7
Radioactivity undetected 0.93 1.3
Radioactivity unaccounted for 4.56 6.3
82.6 100.0
* Expressed as prochloraz equivalents
None of the seven unknowns exceeded 4.3 percent of the terminal
residue. Analysis of barley plants treated in a similar manner
suggests that over 86 percent of the radioactivity applied to foliage
was absorbed by the leaf within 24 h (McDougall 1979).
In another study, separate plots of wheat at the flag leaf sheath
opening stage were treated with a 25 percent a.i. formulation of
either 3H-prochloraz labelled in the 3 and 5 positions of the phenyl
ring or 14C-prochloraz labelled in the 2 position of the imidazole
ring, at an equivalent rate of 1 kg a.i./ha. Mature wheat was
harvested 13 weeks after treatment and the grain, straw and chaff were
analysed.
Total residues, expressed as prochloraz equivalents, from the
phenyl-labelled prochloraz were grain 0.26 mg/kg, chaff 13.2 mg/kg and
straw 26.5 mg/kg. An almost identical distribution resulted from the
14C-prochloraz treated wheat. From the 3H-prochloraz treated wheat
76-80 percent of the recovered radioactivity was extractable from both
straw and grain. Attempts to hydrolyse polar fractions with HC1 were
unsuccessful, but better results were achieved with pyridine
hydrochloride, which hydrolyses conjugated residues containing the
2,4,6-trichlorophenyl moiety. The procedure liberates
2,4,6-trichlorophenol from both prochloraz and any metabolites
containing the 2,4,6-trichlorophenyl moiety. Results of the analysis
of the 3H-prochloraz treated wheat, expressed as a percentage of
recovered radioactivity in mature tissue, are summarized below:
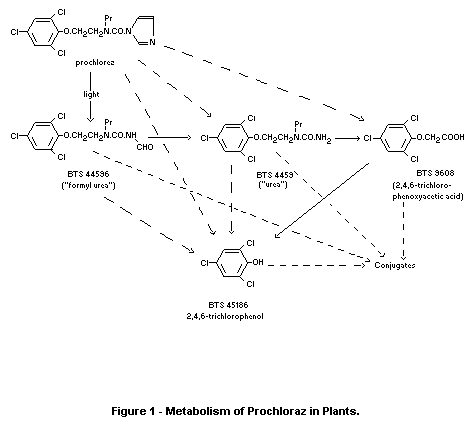 Straw Grain
Free 2,4,6-trichlorophenol (BTPS 45186) 5.2 4.1 3.4 8.4
Residues containing the BTS-45186 moiety 58 37.9 53.9 39.5
Tritiated water 4.8 - 4.2 -
Unidentified extractable residues 9.1 34 15.5 32.1
Unidentified residues in solids 22 24 24.7 20
99.1 100 101.7 100
Of the unidentified extractable residues no one of the grain or
straw acidic, basic, volatile or aqueous fractions analysed contained
more than 6.2 percent of the total residue.
Formation and analysis of the glucosazone after hydrolysing
starch extracted from the 14C-prochloraz treated grain demonstrated
that 32.4 percent of the grain radioactivity was incorporated into the
starch molecules. This is thought to result from the incorporation of
14C-carbon dioxide, resulting from decarboxylation of the metabolite
BTS 44596. The remaining residues were separated into various
fractions but were not identified (Kelly 1980d; Kelly & Krepski 1980).
When wheat seeds were treated at an equivalent rate of 0.4
a.i./kg seed (approximating recommended usage) with a 20 percent a.i.
liquid seed dressing and grown to maturity, residues at maturity (29
weeks) were:
% of total Prochloraz
applied Equivalents (mg/kg)
Treated Controls
Soil 58œ3 0.06 -
Roots 15.1 1.5 -
Chaff 0.1 0.04* 0.004*
Grain 0.04 0.004* 0.002*
Total aerial 6.6 0.16 -
* mean of four determinations.
Translocation into the aerial portion of the plant was complete
after six weeks (Krepski 1982).
Potted wheat seeds were allowed to germinate under glass after
soil surface drench application of a diluted 3H-prochloraz
formulation. Approximately 82 percent of the radioactivity (0.8
percent of applied) was retained in the root system at harvest after
21 days. Residues decreased with increased distance of plant parts
from the root system; they decreased from 8.7 percent of residues
recovered in leaves 1 and 2, 0.62 percent in leaf 3, to 0.08 percent
in leaf 7. Soil contamination was suspected of contributing some of
the residues for leaves 1 and 2 and lower plant parts.
In this same report a 3H-prochloraz formulation was applied with
a syringe at the fifth leaf stage to the third leaf of similarly grown
wheat without the soil drench. Plant parts were harvested and analysed
after 24 days; 99 percent of applied radioactivity was retained in
leaves 3 and 4 (difficulty was encountered in discerning leaves during
applications). Radioactivity decreased from 0.03 percent of that
applied in leaf 5 to 0.01 percent in leaf 9 and increased from 0.23
percent in leaves 1 and 2 to 0.33 percent in the roots (McDougall
1980a).
After spring wheat harvest from soil plots treated at a rate
equivalent to 1 kg/ha with either imidazole ring-labelled
14C-prochloraz or phenyl-labelled 3H-prochloraz, the plots were
re-sown with winter wheat and reharvested 10 months later. Soil
samples at harvest of the winter wheat were 0.11 and 0.43 mg/kg for
14C and 3H treatments, respectively. Residues of 14C or
3H-prochloraz equivalent were < 0.01 mg/kg in grain, chaff and
straw. Grain control values were about half that for grain, but not
statistically different. Low uptake is suggested for 3H-prochloraz
treated chaff and straw where experimental levels are 50 to 100 times
that of the respective controls (Krepski 1981a).
A 1.5 sq m plot was treated with 14C-phenyl labelled prochloraz
formulation by garden sprayer at an application rate equivalent to 395
g a.i./ha, which approximates recommended usage for cereals. After 41
days the soil was turned by hand and sown with sugarbeets as a
representative rotational crop. A control plot was also planted.
Seedlings were sampled 23 days after planting and mature plants (roots
and foliage) at 157 days after planting.
Residues at planting in soil averaged 0.26 mg/kg prochloraz
equivalents. At 23 days after planting, residues of 0.07 mg/kg in
seedlings were significantly greater than the 0.006 mg/kg controls. At
harvest, residues of 0.005 mg/kg in the soils were not significantly
different than in controls, whereas the 0.0052 mg/kg prochloraz
equivalent in foliage was slightly greater than the 0.003 mg/kg
control. This difference is statistically significant, although
residues in the foliage are less than the background level (McGibbon
1982).
Citrus
3H-prochloraz, a 2.5 percent E.C. formulation, was applied by
pipette wash, to simulate a 0.1 percent a.i. postharvest commercial
treatment, to Washington Navel oranges in Spain. Storage was at 4°C in
the dark from 10 to 60 days and at 20-22°C in diffuse light. From 1 to
10 days, surface residues were removed with a 0.1 percent ethylan BCP
solution and those in peel and flesh by extraction with 90 percent
acetone. Surface residues at room temperature decreased from 5.8
percent of applied radioactivity at 1 day to 0.8 percent at 10 days,
and at 4°C from 3.8 mg/kg at 10 days to 1.1 mg/kg at 60 days.
Corresponding residues in the flesh increased from 1.8 percent to 5
percent of the applied dose over the storage period for room
temperature storage, but remained relatively constant at approximately
1.6 percent over the entire 60 day storage period at 4°C.
Radioactivity in the peel remained relatively constant at 92.3-96
percent of the applied dose over the room temperature storage period
and, similarly, 94.7 to 97.2 percent over the storage period at 4°C
(Kelly 1980a). Similar unlabelled studies on citrus residues during
storage are reported under "In Storage and Processing".
Apples
Formulations of phenyl-labelled 14C-prochloraz or its manganese
complex BTS 46828 were applied to individual apples under glass at
rates approximating commercial usage. In samples analysed after 20
days, residue distribution was similar in the surface, peel and flesh
from either chemical. Residues in extracts from the prochloraz-treated
apples (86 percent of the applied dose was recovered) were - wash 7.4
percent, peel 50 percent and flesh 16.9 percent (20 percent of
recovered). BTS 46828 gave almost identical results (Krepski 1981b).
In similar experiments (not carried out under glass) the
metabolism of prochloraz and its manganese complex were compared at
intervals between 0 and 54 days after application by syringe to
individual apples. Radioactivity distribution was again shown to be
similar from use of either chemical, although prochloraz from the E.C.
formulation was initially absorbed into the peel at a faster rate than
the Mn-complex W.P. and was somewhat more persistent. The manganese
complex was also more susceptible to losses from precipitation shortly
after application. As a percent of radioactivity recovered at 0 and 54
days, residues were:
Prochloraz Mn - complex
*day 0 day 54 *day 0 day 54
surface 32.3 1.9 67.6 1.2
flesh 0.0 0.8 0.0 1.2
peel 67.2 81.2 32.4 56.2
Total 100 83.9 100 58.6
* 5 hours (McDougall 1980b).
In another similar study distribution and characterization of
residues were investigated in two apple varieties at intervals ranging
from 16 to 63 days after application by syringe of the Mn-complex to
individual growing apples. As a percent of the applied dose,
representative residues were:
Days after Whole Peel
treatment fruit Flesh Total Extractables
16 72 (83) 15.81 67 (78) 51.3 (61.4)
33 54.8 (76) 3.6 (5.2) 51 (71) 37.2 (55.2)
63 63.5 5.4 60 38
Numbers in parentheses correspond to the Worcester variety and
others to the Cox variety.
Extractable residues in peel were quantified and characterized in
both varieties at 33 and 63 days after treatment. As a percent of
extractable peel residues the metabolic profile at 63 days for the Cox
variety was:
Metabolite Percent
Prochloraz or Unchanged BTS 46 828 5 )
BTS 45186 (free 2,4,6-trichlorophenol) 11.5 )
BTS 44595 15.2 )
BTS 44596 33.1 ) >77.8
Conjugated residues containing )
the BTS 45186 moiety 13 )
Unidentified polar metabolites 6
"Unknowns" 1.6,0.7
Unresolved background 13.9
Total unidentified (excluding conjugates
with BTS 45186) 22.2
100.0
Results were similar at 33 days for both varieties, except that
conjugated residues containing the BTS 45186 moiety were only 3
percent for the Worcester variety, only the first "unknown" was
observed in Cox and the first and a different second "unknown" was
found in Worcester. None of the residues exceeded 2 percent (Kelly
1982a).
In Soil
Prochloraz 25 percent E.C. formulations, labelled with 14C in
the imidazole ring and separately in the phenyl ring with 3H, were
separately applied to acidic sandy loam and silty clay loam soils in
enclosed jars purged with CO2-free air under laboratory conditions
for up to 52 weeks. Application was at 6 mg/g soil, equivalent to 0.7
kg a.i./ha (a recommended crop application rate). Silty clay loam was
similarly treated with prochloraz labelled with 14C in the phenyl
ring and studied over 9 months.
Radioactivity losses as volatiles were about 50 percent of the
applied dose to silty clay for both 14C-imidazole and 3H-phenyl
labelled prochloraz after one year, as was the case for the
14C-phenyl labelled formulation at 9 months. Losses were more rapid
from silty clay loam than from sandy loam, for which losses were half
or less that in silty clay. Experimental evidence indicated, but did
not prove, that volatiles from 14C-phenyl and 14C-imidazole labelled
prochloraz were predominantly CO2. In the case of 3H-phenyl labelled
prochloraz, evidence suggests volatile radioactivity in the form of
tritiated water. The major extracted residue in all cases was
prochloraz per se at 50-68 percent of the applied dose (80-90
percent of extracted) after 8 weeks to 12-30 percent (45-67 percent of
extracted) after 52 weeks. Lower residues of BTS-44596 and BTS 45186
combined were <18 percent of the applied dose (<26 percent of
extracted) after 52 weeks for 3H-labelled treatments. Binding was
more pronounced in sandy loam. The half-life was reported as 3 and 5
months, respectively, in silty clay and sandy loams. In both cases the
acidic pH would increase stability (Kesterton 1981a).
In German standard soils the half-life of unlabelled prochloraz
ranged from 115-135 days, with stability directly related to organic
matter content. For both soils the pH was acidic, which is conducive
to prolonged stability (McGibbon 1982).
14C-imidazole and 3H-phenyl labelled prochloraz, formulated as
a 25 percent E.C., were incubated under anaerobic conditions at a rate
equivalent to 0.7 kg a.i./ha and sampled at 14, 27 and 60 days.
Residues of prochloraz remained relatively constant over the test
period in silty clay loam (ca 68 percent of the applied dose) and
decreased slightly (to ca 54 percent) in silty clay loam. The decrease
in silty clay loam was accompanied by an increase in bound residues.
Anaerobic conditions halted the continuous degradation and formation
of volatiles observed under aerobic conditions. Other components
indicated by TLC from 3H treatments were BTS 44596 and BTS 45186 at
approximately 6 and 3 percent of the applied dose, respectively,
whereas from 14C treatments prochloraz, imidazole and BTS 44596 were
approximately 68, 2 and 1 percent, respectively (Kesterton 1981b).
The absence of significant degradation of a 14C-phenyl labelled
2.5 percent E.C. prochloraz formulation in sandy loam and silty clay
loam under sterile soil conditions for 30 days suggests that
degradation is facilitated primarily by soil microorganisms as opposed
to chemical degradation, at least under the experimental conditions
(acidic pH and 50 percent soil moisture capacity) (Newby 1982).
Sandy loam and silty clay loam soil were treated with a
14C-benzene labelled 25 percent E.C. prochloraz formulation at a rate
equivalent to 0.44 kg a.i./ha, aged 13 days, added to the top of 30 cm
× 7 cm diameter columns containing untreated soil and eluted with an
equivalent of 1.5 cm rain for 18 days. Essentially all radioactivity
was retained in the top 5 cm of soil, with less than 0.3 percent of
the applied amount being detected in leachate (Kesterton & Newby
1980a).
In a similar soil column study, except that the equivalent
application rate was 0.8 kg/ha, the labelled prochloraz was applied to
the same silty clay loam and aged for 30 days, after which it was
eluted for 45 days with a daily equivalent of 0.5 cm rainfall. Results
were similar to the first study in that <0.05 percent of the
applied dose was detected in the eluant. However, even though 88-94
percent was in either the treated portion or the first 5 cm below it,
0.3-0.6 percent was detected in the bottom 25 cm, indicating some
downward mobility other than that detected in the leachate (Kesterton
& Newby 1981).
When an unlabelled prochloraz formulation was applied to West
German standard soil columns at a rate equivalent to 1 kg/ha, <0.05
percent was detected in the leachate (Maier-Bode 1980). In a similar
experiment leachate contained <1.7 percent of the applied dose
(Stockbauer 1983). Details of the preceding two experiments were not
available.
In soil thin-layer mobility tests, the mobility of prochloraz and
soil metabolites BTS 44 596 and BTS 45186 were compared to that of
atrazine and 2,4-D in four soil types. According to the Helling
mobility index, over-all mobility in the four soils were, in order of
increasing mobility, prochloraz, BTS 44596 (low mobility), BTS 45186
(low mobility), atrazine (intermediate mobility) and 2,4-D (mobile).
Mobility of all tested materials was greatest in sand (even BTS 44596
and BTS 45186 would have intermediate mobility). Prochloraz and BTS
44596 were immobile in silt loam, whereas BTS 45186 was of
intermediate mobility. The thin-layer studies confirmed the low, but
positive, mobility detected in soil column experiments (Leake & Lines
1981).
Adsorption/desorption of prochloraz, uniformly labelled in the
phenyl ring and applied as an E.C. formulation to a sandy loam and
silty clay loam, was investigated. The Kd of the sandy and silty
soils were 152 and 256, respectively, indicating slightly more
adsorption by the latter. The K values were 86.8 and 101.7,
respectively, for these soils. Strong adsorption and minimal
desorption is consistent with leaching studies (Kesterton & Newby
1980b).
A trichlorophenol tritiated ring prochloraz E.C. formulation was
applied to one sq m plots of barley and wheat in sandy loam at an
equivalent rate of 1 kg/ha. Core samples from 32.5 cm deep were
analysed after approximately 15 and 30 weeks; > 95 percent of
prochloraz residues were found in the upper 10 cm and in some cases
the top 2.5 cm. Somewhat similar distribution after 15 and 30 weeks
suggests only slight leaching of bound residues as a result of
additional (over 150 mm) rainfall in the latter period (Kesterton
1978).
In Water
No degradation of prochloraz was observed after 30 days in 22°C
buffer solutions at a pH of 4.95 or 6.98. At pH 9.18 degradation does
occur, with 77 percent of the added prochloraz remaining after 30
days. A first order degradation was indicated and a half-life
estimated of approximately 79 days. The only degradant
N-propyl-N-2-(2,4,6-trichlorophenyl) ethylamine (BTS 40348) was
reported to account for more than 2 percent of the total
radioactivity, although the quantitative analyses were not provided
(Kelly 1982b). The 4:1 manganese complex of prochloraz (BTS 46 828),
either as active compound or as a 50 percent W.P. formulation,
disassociated to prochloraz and manganous chloride in the presence of
water (pH 6.1). Approximately 50 percent of the complex was
dissociated within four hours at 25°C (Whiting & Dickinson 1979).
Biodegradability of prochloraz in water was studied using the
closed bottle test according to OECD Guideline 301D. Tests indicate
little biodegradation at 20°C up to 15 days after inoculation, but 13
percent degradation by 28 days, suggesting some initial bacterial
adaptation (Douglas & Pell 1982). When bacteria preadapted to
prochloraz were used as the inoculum, degradation commenced
immediately and reached 47 percent biodegradation by 28 days (Douglas
& Pell 1983).
In Storage and Processing
Processing
Citrus - Oranges were postharvest brush-treated at 1 000 mg/kg with
a 40 percent E.C. formulation and processed into "42% comminuted
orange" and "special whole orange compound". These "whole oranges
juices" (chopped oranges) are used for production of various orange
drinks and utilizes most of the whole orange, except for removal of
some coarse peel. It includes a pasteurization process up to 86°C,
depending on which of two processes were used. Total residues
(converted to 2,4,6-trichlorophenol for analysis) were 0.19 and 0.14
mg/kg expressed as prochloraz for the 42 percent comminuted orange and
special whole orange compound, respectively, approximately 30 percent
of the residues found in whole oranges (Manley & Snowden 1982b).
Cereals - Winter wheat was treated post-emergence at two sites in
the Federal Republic of Germany at growth stage 59 (mid-May to
mid-June) with three applications of a 40 percent E.C. prochloraz
formulation at the 0.48 kg/ha rate recommended in that country. Mature
grain was sampled at 57-58 days after last application and portions
milled and baked into bread. Total residues were determined by
hydrolysis of the trichlorophenol containing moieties followed by EC
analysis and use of a 1.906 factorial conversion to a prochloraz
equivalent basis. Residues in grain and bread were <0.1 mg/kg, the
limit of determination.
Under similar conditions prochloraz was applied twice to barley.
Total residues in barley malt, expressed as prochloraz, were 0.13 and
0.15 mg/kg compared with 0.07 mg/kg in a control. Limit of
determination was 0.1 mg/kg.
Rapeseed Oil - Mean residues of total prochloraz and metabolites
hydrolysable to the trichlorophenol, and expressed as prochloraz, in
mature rapeseed treated in each of four sites in France ranged from
0.06 to 0.2 mg/kg (over-all mean of 0.11 mg/kg). Treatment was with
two applications of an E.C. formulation at a rate of 0.45 kg a.i./ha
and harvest was 25-38 days after the last treatment. Mean residues of
total prochloraz and its metabolites in hexane-extracted oil from
these trials (omitting one individual 0.49 mg/kg outlier from six
replicate results, otherwise ranging from 0.07-0.13 mg/kg) ranged from
0.11 to 0.29 mg/kg (over-all mean 0.10 mg/kg), indicating a residue
concentration factor of approximately 3 times. Residues in the treated
seed and oil only ranged from approximately 1-3 times that in controls
from any given site and were therefore not far removed from the limit
of determination in any one site, with a variation of from 0.05 to 0.2
mg/kg among the sites.
In similar studies at three sites each in Denmark and Sweden, the
concentration factor for prochloraz in the oil ranged from only 1.2 to
2 times that in the seed. Treatment-to-harvest intervals range from 47
to 76 days. In these studies, residues in treated seed and oil derived
therefrom, although comparable to those in France, were significantly
greater than in controls. The limit of determination was estimated at
0.05 and 0.1 mg/kg, respectively, for seed and oil.
Mushrooms - Mushrooms were harvested in France 17 days after
pre-emergence treatment with a 40 percent E.C. prochloraz formulation,
administered at a rate of 40 g a.i./100 sq m. No information on good
agricultural practice is available from France. The mushrooms were
immediately processed by canning and dehydration, after which samples
of canned mushrooms, the liquor and dehydrated mushrooms were analysed
as total residues of prochloraz and its metabolites that were
hydrolysable in the presence of pyridine hydrochloride to the
trichlorophenol moiety. Total residues (apparently uncorrected for
recoveries) and expressed as prochloraz were less than the 0.1 mg/kg
limit of determination for canned mushrooms and the liquor, with
control values of 0.03 and 0.05 mg/kg, respectively, reported. An
apparent residue of 0.14 mg/kg in dehydrated mushrooms was less than
the 0.23 mg/kg control value. Analytical recoveries were 64, 63 and 46
percent, respectively, for dehydrated mushrooms, canned mushrooms and
the liquor (Browne 1982h).
In experiments conducted in The Netherlands, total residues of
prochloraz and its metabolites, expressed as prochloraz, were
determined in canned mushrooms and the liquor after three treatment
schedules with a 50 percent W.P. formulation of the prochloraz
manganese complex, (1) 0.75 a.i./sq m post-casing and 0.94 g a.i./sq m
14 days after casing (2) 1.5 g a.i./sq m 9 days post casing (good
agricultural practice in The Netherlands) and (3) 1.5 g a.i./sq m both
at post-casing and after 14 days. Total residues expressed as
prochloraz were equal to or less than the 0.1 mg/kg limit of
determination in the six liquor samples and five of the six canned
mushroom samples. For treatment (3) above, however, the residue was
0.23 mg/kg in the sixth mushroom sample and 0.1 mg/kg in the control.
No information was available on the interval from last treatment to
harvest. Recoveries were 81 and 94 percent, respectively, for canned
mushrooms and the liquor. No analyses were available for the
unprocessed mushrooms (Churchill & Longland 1983c).
Storage
Citrus - Data are available from a variety of treatment and storage
conditions and are summarized in Table 3. Navel oranges were
commercially treated postharvest in South Africa with a 40 percent
E.C. prochloraz formulation at 1 000 and 2 000 mg/kg a.i. rates then
waxed and shipped by refrigerated vessel to the United Kingdom.
Samples were stored either in the dark at approximately 20°C or at
4°C. Sampling for analysis occurred at 0 (44 days after treatment), 7,
14 and 21 days after storage commenced. Mean (range in parentheses)
residues, corrected for recovery, of total prochloraz and metabolites
expressed as prochloraz at the lower treatment rate were 0.05 mg/kg
(0.030.08 mg/kg) in flesh, 2.1 mg/kg (1.4-2.8 mg/kg) in peel and 0.49
mg/kg (0.3-0.7 mg/kg) on the whole fruit (calculated from peel to
flesh weight ratios) for ambient and 4°C fruit combined over the
entire storage period. Corresponding values for the higher treatment
rate were 0.05 mg/kg (0.02-0.07 mg/kg), 3.3 mg/kg (2.5-3.7 mg/kg) and
0.84 mg/kg (0.7-1.7 mg/kg) respectively.
The list of determination was reported as 0.05 mg/kg, which is
consistent with uncontaminated control values of 0.01-0.02 mg/kg total
residue. No observable trend in lower residues occurred during the
21-day storage period at either temperature and there were no
noteworthy differences in residues between the storage temperatures.
The trials also demonstrated that approximately 94 percent of the
total residue was in the peel (Manley & Snowden 1982b).
In similar postharvest treatments to oranges in Spain, with
0.05-0.1 percent solutions or 3-4 g a.i./tonne of fruit (usually in
wax) with an E.C. formulation as a spray or dip, residues were
determined after storage intervals typically ranging from 1-10 to
10-60 days at 4°C-22°C. Maximum peel residues were as much am 7.3
mg/kg for prochloraz alone and 8.4 mg/kg for total residues of
prochloraz and its metabolites, both expressed as prochloraz. Total
residues in flesh, expressed as prochloraz, were 0.04-0.44 mg/kg and
on a calculated whole fruit basis up to 2.5 mg/kg. As in the South
African study, a definite trend towards decreased residues could not
be determined over the varied storage intervals and temperatures and
little difference was observed in residues where application rates
varied by a factor of two. Peel was repeatedly shown to contain most
of the total whole fruit residue with typically <5 percent being
present in the flesh (Richards 1980b,f; Kelly 1980a; Browne 1981b).
Similar studies were conducted in the United Kingdom where
oranges were dip-treated with a 0.07 percent a.i., 40 percent E.C.
formulation and analysed after 1, 7, 21, 25 and 70 days cold storage
(temperature not provided). Total residues were expressed as
prochloraz. Residues of the parent compound in peel ranged from 3.6 to
5.1 mg/kg compared to 5.8 to 7.8 total residue. In flesh, the residues
were 0.05 to 0.12 mg/kg and 0.07 to 0.15 mg/kg, respectively. No
declining residue trend was observed over the 70-day maximum storage
period, although total residues in flesh increased from about 4
percent of the whole fruit residue at 1-7 days to 11 percent after 70
days (Browne 1982i).
No decline in residues on oranges was observed during storage at
intervals from 1 to 16 days at ambient temperature in Australia after
dip treatment with a 40 percent E.C. prochloraz formulation as a 250
to 500 mg/kg a.i. solution. Total residues determined as the
trichlorophenol expressed as prochloraz were similar from the two
application rates, as were residues of prochloraz alone. Residues of
prochloraz when determined alone ranged from 1.5 to 3.9 mg/kg (2.9
mg/kg) in peel and from <0.02 to 0.1 mg/kg (0.04 mg/kg) in flesh over
the 16-day storage interval for both concentrations. Total residues
were similar 1.4-3.4 kg (2.5 mg/kg) and <0.05 mg/kg, respectively.
Again, most of the residue was in the peel (Browne 1981a).
Residues in Spanish lemons were investigated after a shower
postharvest treatment with a 3 000 a.i. E.C. formulation in wax.
Samples were analysed after 12 and 16 days of storage. The limit of
determination was 0.02 mg/kg and 0.05 mg/kg for prochloraz alone and
total residues expressed as prochloraz, respectively. Residues
expressed as prochloraz were similar after both storage periods, with
approximately 5 percent of the residue in the flesh. Residues of
prochloraz alone and total residues were:
Prochloraz alone (mg/kg) Total residues (mg/kg)
Peel Flesh Whole Peel Flesh Whole*
9.1-15.6 0.27-0.3 3.1-5.0 10.6 0.17-0.33 3.7-6
(12.3 mean) (0.29) (4.6) (14) (0.23) (5.1)
* Calculated from peel/flesh.
Recoveries were 75 percent in peel and 77-100 percent in flesh
(Browne 1982c).
In 1980, postharvest trials on oranges in Spain, using a 40
percent E.C. prochloraz formulation, were made with 200 1 of wax
formulation (3 g a.i./1 wax) to 150 tonnes of fruit (Richards 1980b).
Samples of orange flesh from these trials were analysed for free
2,4,6-trichlorophenol (BTS 45186), since no metabolism study was
available on orange and BTS 45186 has been found in low levels in
wheat (Kelly 1980d). Samples were analysed after 7 and 14 days at
20-22°C. Residues were 0.005-0.01 mg/kg (0.008 mg/kg mean) after 7
days and 0.005 mg/kg after 14 days, as compared to <0.005 mg/kg for
controls. Contrary to the findings in most other citrus postharvest
trials, a slight trend of decreasing residues with time was indicated
(Richards 1980e).
In postharvest dip trials using a 25 percent E.C. or 25 percent
W.P. prochloraz formulation on oranges and lemons in Italy residues
were determined after 57 days storage at 7°C. Significantly higher
residues resulted from E.C. applications in both fruits and lemon
residues were higher than those in oranges (Browne & Manley 1982b).
Oranges treated in South Africa with a postharvest fungicidal
brush at 500-4 000 mg/kg a.i. 45 percent E.C. were analysed after two
months storage at ambient temperature. Again, most of the residue was
in the peel, with total residues, expressed as prochloraz, ranging
from 0.08 mg/kg at the lower application rate to 0.73 mg/kg at the
higher one. There was a good correlation of residue level with
application rate (Snowden & Manley 1983).
Bananas - Bananas were treated by postharvest dipping in Australia
with 250 and 500 mg/kg a.i. solutions of 40 percent E.C. prochloraz
and analysed after ambient storage for 9, 10, 12 and 16 days. Free and
conjugated residues of prochloraz and its metabolites were determined
as the trichlorophenol, expressed as prochloraz, with a 0.05 mg/kg
limit of determination. Residues resulting from both treatment rates
and over the entire storage period ranged from 5.2-10 mg/kg in skin
and 0.02-0.05 mg/kg in flesh. No evidence of decline in residues was
observed in skin or flesh over the storage period and there was no
significant difference in residues between the two application rates
(Browne 1982b).
Photodecomposition
Thin-layer chromatography and nuclear magnetic resonance (NMR)
analysis of a photodecomposition product of an aqueous solution of
prochloraz confirmed the presence of
N-formyl-N-propyl-N',-2-(2,4,6-trichlorophenoxy) ethylurea (BTS 44596)
(Haran 1978). Prochloraz aqueous solutions of 26.1 and 43.3 mg/kg
concentrations in glass-stoppered flasks were exposed to artificial
light (420-760 nm wavelength) at 32°C for 36 and 16 days,
respectively, with periodic analyses by high pressure liquid
chromatography. At the lower concentration residues decreased from
6.93 × 107 moles (26.1 mg/kg) initially to 1.94 × 107 moles after 36
days while BTS 44596 increased from 0.0 to 3.58 × 107 moles. At the
higher concentration, residues decreased from 11.5 × 107 moles (43.3
mg/kg) at zero day to 5.8 × 107 moles after 16 days, while BTS 44596
increased from 0.0 to 35.8 × 107 moles. The first order decay
half-lives were 20.5 and 18.1 days, respectively, for the two
concentrations.
Retention times indicated that a minor unquantified degradation
product was N-propyl-N-2-(2,4,6-trichlorophenoxy) ethylurea (BTS
44595). This appeared only towards the end of the exposure period
(Haran 1980).
METHODS OF RESIDUE ANALYSIS
Analytical methods are available for the analysis of free
prochloraz or total free and conjugated residues of prochloraz and its
major metabolites. Methodology for free prochloraz was first developed
for the analysis of cereal grains and straw (Somerville 1980). Finely
milled straw or ground grain are vigorously extracted with acetone,
filtered, a small portion acidified with HCl and concentrated by
rotary evaporation. The aqueous fraction is partitioned with petroleum
ether, which is discarded, adjusted to pH 6-7 and again partitioned
with petroleum ether, which is concentrated to dryness. The sample is
taken up in ethyl acetate and analysed by electron capture gas
chromatography utilizing a 7 percent OV-101 packed column. Analytical
recoveries of wheat and barley grain, amended with prochloraz at
0.05-0.5 mg/kg, were 78 ± 10.6 percent and 91 ± 10.9 percent,
respectively. For straw, the respective recoveries were 82 ± 12
percent and 80.6 ± 17 percent. The level of "sensitivity" has been
estimated at 0.01 mg/kg for grain and straw, although in the field
trials apparent untreated controls in cereal grain and straw were
occasionally up to 0.02 mg/kg.
With minor modifications, this method has been used for
determining free prochloraz residues in apples, pears, citrus,
mushrooms, strawberries and lettuce (FBC, (Hayto 1979b; Whiteoak 1981;
Kelly 1980a; Browne 1981b, Maclaine Pont et al. 1980) with
analytical recoveries of > 65 percent and usually > 80 percent.
Apparent residues in untreated controls generally ranged from <0.01
to approximately 0.05 mg/kg depending on the commodity.
A method is also available for measuring total free and
conjugated residues of prochloraz and its major plant metabolites,
N-formyl-N'-1-propyl-N'-[2-(2,4,6-trichlorophenoxy) ethyl] urea
(BTS 44596), N-propyl-N-[2-(2,4,6-trichlorophenoxy) ethyl] urea
(BTS 44595) and conjugated 2,4,6-trichlorophenol (BTS-45186), after
converting all of them to the common moiety 2,4,6-trichlorophenol
(Manley & Snowden 1982a). Any other metabolite containing the
2,4,6-trichlorophenol moiety would probably be measured as well.
Although total residues are actually measured as BTS-45186, residues
are usually expressed as prochloraz, after use of a molecular weight
factor of 1.906.
Thoroughly ground, milled, chopped or minced and mixed samples
are Soxhlet extracted with acetone, filtered, dried by passing through
anhydrous sodium sulphate and evaporated to near dryness by a rotary
evaporator. The sample is refluxed with pyridine hydrochloride at
205°c for one hour to hydrolyse to the BTS-45186 moiety, after which
HCl is added to the sample. The sample is liquid-liquid extracted
after addition of petroleum ether. Potassium hydroxide is added, the
sample partitioned and the aqueous phase collected and acidified with
HCl. The sample is partitioned into toluene for analysis by
electron-capture gas chromatography, utilizing a 20 percent carobowax
20M + 1.7 percent phosphoric acid packed column.
The method was validated on fruits and cereal grains with mean
over-all recoveries of 92 ± 15.9 percent when samples were amended at
0.2-10 mg/kg prochloraz. A 0.05 mg/kg limit of determination is
considered attainable for most samples, although apparent residues of
0.2-0.3 mg/kg may be encountered occasionally, especially in cereal
straw.
With minor variations, this method has been successfully used for
the analysis of cereal grain and straw, cereal grain green plant
tissues, apples, citrus fruit (peel, flesh, whole fruit), orange
juice, rapeseed, rapeseed oil, mushrooms, stone fruit and assorted
fruit with inedible peel (Kelly 1979b; Richards 1981a; Reary 1981a;
Browne & Manley 1982a; Manley & Snowden 1982a). Specifics on
individual crops under actual use conditions are considered earlier in
this evaluation under "Residues Resulting From Supervised Trials" or
under "Fate of Residues."
An analytical method is also available for the determination of
free BTS-45186, which may be a minor component of the residue (Kelly
1980b; Richards 1980e).
No analytical method suitable for enforcement purposes for
products of animal origin was made available.
NATIONAL MAXIMUM RESIDUE LIMITS REPORTED TO THE MEETING
Country Commodity MRL (mg/kg)
France cereal grains 0.05 1
rape seed 0.05 2
The Netherlands 3 Mushrooms 0.05
cereal grains 0.1
Milk 0.01
Meat 0.01
Other products 0 (0.01)
German Federal Republic Cereals 0.5 4
1 Residues of prochloraz alone.
2 Residues included not specified.
3 For MRLs of The Netherlands, the residue is prochloraz
and its metabolites, measured as 2,4,6-trichlorophenol
and expressed as prochloraz. Residue on mushrooms and
for most cereal grain trials conducted in The
Netherlands were for total residues of prochloraz and
its major metabolites.
4 A proposed 0.5 mg/kg NRL for prochloraz and metabolites
has been submitted.
APPRAISAL
Prochloraz is a fungicide in use and/or under development for
control of fungal diseases on a variety of commodities in number of
countries. It is used as a foliar spray, for post-harvest or seed
treatments and is available in a variety of formulations for these
uses, including a prochloraz-manganese complex wettable powder
formulation for sensitive plant species.
Residue data are available from field trials on a number of
commodities in several countries, mostly from foliar applications to
fruit and cereal grains, use in mushroom culture and postharvest
applications to citrus and certain other fruit. Some residue data are
also available for vegetables. Residues were determined either as
2,4,6-trichlorophenol, prochloraz alone or prochloraz and its major
metabolites, mostly the latter over-all. In select cases, data are
available for both prochloraz alone and for prochloraz and its major
metabolites. Apparently the earlier studies were for prochloraz alone,
whereas in later studies total residues were emphasized. Data are
insufficient and too variable to draw a general conclusion on the
ratio of parent compound to parent plus metabolites. For example,
there is little difference in residue measured for citrus or possibly
for mushrooms, but for cereals residues appear to be substantially
higher when total residues are determined.
Residue trials on commodities for which there are known
nationally approved or registered uses were available for watermelon,
apple, grape, cereals, mushrooms and oil-seed rape. Therefore, only
for these is consideration given for MRL estimates. However
"recommended" were available for a number of other commodities and
temporary limits are estimated for those with adequate data. Data were
insufficient for estimation of maximum residue levels for sugarbeets,
sugarbeet leaves, strawberries, almonds, almond hulls, lettuce,
watermelon, apples, pears, rice and grapes.
Information was available on the fate of residues in animals,
plants, the environment and during storage and processing. In animals,
prochloraz is rapidly metabolized from dermal or oral exposure. Most
of the residue is rapidly excreted, with urinary excretion being the
predominant route in most animals tested, although faeces is the
predominant route in dogs. The principal metabolic products in the
urine were 2,4,6-trichlorophenoxyacetic acid and
2,4,6-trichlorophenoxy-ethanol (mostly conjugated). The major plant
metabolites have not been identified in the urine. Although residues
were qualitatively similar in urine or in tissue distribution among
animals studied, tissue residues have not been identified.
The potential for residues in products of animal origin was
investigated in two studies by feeding a lactating goat with
14C-phenyl-labelled prochloraz either by capsule or ad libitum with
14C-prochloraz field-treated wheat straw. The first study represents
the worst case maximum expected theoretical exposure from wheat straw
and the straw feeding is probably more reflective of maximum exposure
under normal feeding conditions.
These goat metabolism/feeding studies give insight into the
maximum potential for residues, although residues in animal tissues
are not identified. It is not known whether urinary metabolites are
the same as those in milk or tissues.
Although straw was used as a worst-case for potential residues,
foraging would appear to be potentially equally important, if not more
so, if not label-restricted, since it can be fed at higher levels in
the diet, as can citrus pulp. However, citrus uses presumably are
intended where peel is discarded. Although wheat or barley grain
residues were relatively low, grain may comprise up to 50 and 80
percent of the diet of poultry and swine, respectively. No analytical
method suitable for enforcement purposes for animal products is
available, or at least known to be validated for that purpose.
Additional information is needed to support a conclusion that the fate
of residues in animals is adequately understood or to estimate maximum
residue levels for products of animal origin.
The fate of Prochloraz in plants was investigated in wheat
(foliar, pre-emergence and seed treatments), citrus (postharvest),
apples (foliar) and sugarbeet (soil uptake). In wheat plants, major
residues at the vegetative stage from foliar treatment were free
BTS-44596, free and conjugated BTS-44595 and 2,4,6-trichlorophenol in
decreasing order, which together accounted for 83 percent of the
terminal residue after 19 days. Less than 2 percent was unchanged
prochloraz and only 0.2 percent was 2,4,6-trichlorophenoxy acetic
acid. No single unidentified residue accounted for more than 6.3
percent of the remainder. Residues appear to be absorbed primarily
within the first 24 hours.
At the harvest stage, only the free trichlorophenol has been
identified in straw or grain, and it occurs as 3-8 percent of the
terminal residue. This, plus tritiated water and other presumably
conjugated but unidentified residues containing the trichlorophenol
moiety, account for 40-70 percent of the terminal residue. No single
remaining unidentified residue fraction accounts for more than 6
percent of the terminal residue.
When applied to wheat seeds or as a pre-emergence drench, most
residue was in or near the root system, with little upward mobility,
and there is little evidence of upward mobility when prochloraz is
applied to foliage although low levels translocate towards the roots.
Only very low levels of residue were found in chaff and straw when
wheat was grown in soil treated with radioactive 3H-prochloraz ten
months earlier. However, sugarbeet seedlings showed low residues when
planted as a rotational crop, but only trace levels were found in the
foliage at maturity and no significant residue in the roots.
In apples, residues migrated into the peel and by 33-63 days
approximately 60-80 percent of the terminal or applied residue was in
the peel, <2 percent on the surface and generally <7 percent of
the applied dose in the flesh, although it can be twice as much.
Qualitatively extractable peel residues were similar to residues found
in wheat at the vegetative stage with approximately 80 percent of the
residues hydrolysable to 2,4,6-trichlorophenol. This, therefore,
accounts for approximately 50-60 percent of the total fruit residue.
Other residues (primarily peel fiber-bound) were not identified, and
although unresolved background accounted for approximately 10 percent
of the whole fruit residue (14 percent of peel extractable), no one
peel extractable metabolite or fraction exceeded approximately 5
percent of the total fruit residues. The fate of residues in plants is
reasonably well understood for the limited number and type of crops
for which there are approved uses. If uses are expanded significantly
to other food groups, additional metabolism studies would be prudent.
Numerous experiments have been conducted on the fate of
prochloraz residues in soil and water. Prochloraz is degraded under
both aerobic and anaerobic conditions with formation of volatile
compounds, probably CO2 and tritiated water. Residues remaining are
predominantly prochloraz, BTS-44596 and BTS-45186, with binding to
upper soil particles increasing with time. The half-life can range
from 90-155 days, depending on conditions. Formation of volatiles is
decreased under anaerobic conditions. Mobility is low in soils,
although low mobility does occur under some conditions, especially for
the metabolites in sand. Prochloraz is stable in water under acidic
conditions, but does degrade under alkaline conditions.
The fate of residues during processing was investigated for wheat
made into bread, postharvest citrus, rapeseed oil and mushrooms after
processing. Residues in bread made from wheat grain with <0.1 mg/kg
prochloraz also showed <0.1 mg/kg residues. Since the limit of
determination is 0.1 mg/kg, no conclusion could be made on the extent
of residue concentration, if any. No information was available for
residues in flour or other milling fractions. Similarly, low residues
were found in malt from prochloraz-treated barley, but no information
was available on the residue level in grain from which it was made.
Residue levels concentrate from 1 to 3 times in oil processed
from mature oilseed rape that has been field-treated with prochloraz.
Residues in canned or dehydrated mushrooms or canning liquor after
pre-emergence treatment with prochloraz were less than in untreated
controls, except for one sample of canned mushrooms with a residue of
0.23 mg/kg. No information was available on residue levels before
canning or dehydration. No conclusion can be drawn on the extent of
residue concentration, if any. When oranges treated postharvest were
processed into "42 percent comminuted orange" or "whole orange
juices", which are used to manufacture various orange drinks,
approximately 70 percent of the residue in the oranges from which it
was processed was lost.
The fate of residues during storage after various postharvest
prochloraz treatments was investigated for citrus, bananas and
avocadoes. Except for one study where free 2,4,6-trichlorophenol was
determined and where treated citrus was stored under a variety of
conditions (dark or light, cold or room temperature and at intervals
ranging from one to 70 days) total residues were very persistent, with
little evidence of a decreasing trend over the varied treatment or
storage conditions. Modest increases (2X) in application
concentrations had little effect on residue levels, approximately
90-95 percent of which were in the peel. There was evidence of
continued gradual translocation into the flesh as the interval from
treatment increases, especially at room temperature. This was also
found in studies on foliar treatments of mango. Residue levels during
storage of prochloraz alone were similar to those for prochloraz and
its major metabolites, suggesting little degradation or conjugation.
Emulsifiable concentrate formulations gave significantly higher
residues than wettable powder formulations and residues in lemons were
higher than those in oranges.
In bananas treated postharvest with prochloraz, no trend of
decreasing residues was observed over a 16-day storage period at
ambient temperature. Residue levels were similar with modest increases
(2X) in treatment rate and, as in the case of citrus, most of the
residue was in the peel.
In avocadoes treated postharvest with prochloraz, residues were
comparable to those from multiple foliar treatments. Approximately
10-30 percent of the residue could occur in the flesh after relatively
short preharvest intervals. The translocation was therefore somewhat
greater than in citrus, bananas and apples.
Photodecomposition of an aqueous prochloraz solution resulted in
the formation of plant metabolite BTS-44596 and towards the end of a
36-day exposure to artificial light, a trace of plant metabolite
BTS-44595. A half-life of approximately 20 days was observed.
Analytical methods are available for enforcement purposes for a
wide variety of plant commodities. Residues of prochloraz alone or
total residues of prochloraz and its major plant metabolites may be
determined, although more of the field trials data were determined by
the latter. Analytical recoveries were generally better than 70
percent. No analytical methods have been validated for residues in
animal products. The analytical methodology for the parent compound
should be suitable and more appropriate for enforcement purposes and
for estimating maximum residue levels (see Report of this Meeting,
Section 2.3).
RECOMMENDATIONS
The Meeting examined residue data from supervised trials
reflecting established or proposed good agricultural practice. From
the data, the Meeting was able to estimate the maximum residue levels
that are likely to occur when prochloraz is used in practice and when
the reported intervals between last application and harvest or other
restrictions are observed. Maximum residue limits are recommended
where registered or approved uses are known and residues are
determined as free prochloraz. Temporary maximum residue limits are
recommended where only recommended uses are available or where total
residues of prochloraz and its metabolites containing the
2,4,6-trichlorophenol moiety are determined. Regardless of the status
of the ADI, these remain temporary pending receipt of information on
approved or registered uses or data on supervised trials with residues
determined as prochloraz.
All estimates apply to foliar applications unless otherwise
indicated.
Estimated Interval from last
Commodity maximum residue treatment to harvest (days)
limits (mg/kg) on which recommendation
Determined and expressed is based and other
as prochloraz restrictions
Barley 0.05** 40
Oats 0.05** 40
Rye 0.05** 40
Wheat 0.05** 40
Barley straw 0.2 40
Oat straw 0.2 40
Rye straw 0.2 40
Wheat straw 0.2 40
Mushrooms 2 2 3
Citrus 5 (TMRL)1, 4 postharvest
Sum of prochloraz
and metabolites expressed
as prochloraz 4
Bananas 5 (TMRL) 1 postharvest
Stone fruits 1 (TMRL) 1 14
Avocado 5 (TMRL) 1, 2 7 (preharvest)
Mango 2 (TMRL) 1, 2 15 (preharvest)
Papaya 1 (TMRL) 1 postharvest
Rapeseed 0.5 (TMRL) 25
** At or about the limit of determination.
1 No information on approved or registered uses.
2 Limit allows for cumulative residues from both preharvest and postharvest
treatments, both of which are good agricultural practice.
3 Limit accommodates applications after casing or after flushes, but not
direct treatment of mushrooms.
4 Temporary regardless of the status of the ADI.
FURTHER WORK OR INFORMATION
Required (by 1985)
1. In order to establish MRLs for products of animal origin, several
requirements remain:
(a) Metabolism studies in animals, including ruminants and
poultry, at sufficiently high dosing levels to permit
identification and quantification of tissue residues.
(b) If metabolism studies in (a) above indicate any possibility
of residues in animal tissues, feeding trials should be
conducted in animals, including ruminants and poultry, in
which tissues are analysed for residues of concern
identified in metabolism studies. Feeding in an appropriate
number of animals should as nearly as possible reflect
maximum levels of weathered residues likely to occur in
practice and, ideally, at an exaggerated level.
(c) A validated analytical method suitable for enforcement
purposes for products of animal origin.
2. Good agricultural practice information for those commodities for
which temporary maximum residue limits are recommended,
preferably for countries in which the residue trials were
conducted or those in close proximity.
3. Additional data on residue trials reflecting good agricultural
practice for commodities for which temporary maximum residue
limits are recommended (except citrus). The additional data
should be concurrently determined as both prochloraz and total
residues of prochloraz and its metabolites containing the
trichlorophenol moiety on the same samples. Sufficient data
should be provided to permit further utilization of residue data
already provided, therefore permitting recommendation of maximum
residue limits expressed as prochloraz.
Desirable
1. Additional national maximum residue limits.
2. Additional processing studies in cereals to permit a conclusion
on the extent, if any, of residue concentration in milling
fractions. Grain used should have residues sufficiently above the
limit of determination for drawing conclusions.
3. Additional processing studies for mushrooms to permit a
conclusion on the extent, if any, of residue concentration.
Mushrooms should be analysed before and after processing.
4. Information on possible interference in prochloraz analysis in
the presence of other pesticides, especially those containing the
trichlorophenol moiety. If interferences are encountered,
confirmatory analytical procedures for prochloraz and metabolites
may be necessary.
REFERENCES - RESIDUES
Browne, P.M. Residues of prochloraz and major metabolites in oranges
1981a following postharvest dip treatment in Australia, 1981. FBC
report RESID/81/72 submitted to FAO by FBC Ltd.
(Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in oranges
1981b following postharvest shower treatment in Spain, 1981. FBC
report RESID/81/72 submitted to FAO by FBC Ltd.
(Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in avocados
1982a following postharvest dip treatment in Australia, 1981. FBC
report RESID/82/2 submitted to FAO by FBC Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in bananas
1982b following post-harvest dip treatment in Australia, 1981. FBC
report RESID/82/7 submitted to FAO by FBC Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in lemons
1982c following post-harvest shower treatment in Spain. FBC report
RESID/82/8 submitted to FAO by FBC Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in winter
1982d rye treated post-emergence with a 40% E.C. formulation in
West Germany, 1981. FBC report RESID/82/25 submitted to FAO
by FBC Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in winter
1982e wheat treated postemergence with three applications of 40%
E.C. formulation in Fed. Rep. Germany, 1981. FBC report
RESID/82/29 submitted to FAO by FBC Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in spring
1982f wheat and barley postemergence with a 40% E.C. formulation
(two or three applications) in West Germany, 1981. FBC
report RESID/82/31 submitted to FAO by FBC Ltd.
(Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in mushrooms
1982g following application of a 50 W formulation in Holland,
1980. FBC report RESID/82/39 submitted to FAO by FBC Ltd.
(Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in processed
1982h mushrooms following application of a 40 E.C. formulation in
France, 1981. FBC report RESID/82/68 submitted to FAO by FBC
Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in
1982i oranges following post-harvest dip treatment in the U.K.,
1981. FBC report RESID/82/83 submitted to FAO by FBC Ltd.
(Unpublished)
Browne, P.M. & Manley, J.D. Analytical method for residues of
1982a prochloraz and major metabolites in orange juice. FBC report
RESID/82/18 submitted to FAO by FBC Ltd. (Unpublished)
Browne, P.M. & Manley, J.D. Residues of prochloraz and major
1982b metabolites in oranges and lemons following postharvest dip
treatment in Italy, 1979. FBC report RESID/82/32 submitted
to FAO by FBC Ltd. (Unpublished)
Browne, P.M. & Manley, J.D. Residues of prochloraz and major
1982c metabolites in mangoes treated with a 50% W.P. formulation
in Australia, 1981. FBC report RESID/82/34 submitted to FAO
by FBC Ltd. (Unpublished)
Campbell, J.K. Residues in milk and tissues of a goat dosed orally
1980 with 14C-prochloraz. Boots report AX-80026 submitted to FAO
by FBC Ltd. (Unpublished)
Campbell, J.K. Residues of prochloraz in milk and tissues of a
1983 lactating goat fed straw containing residues of radioactive
prochloraz. FBC report METAB/83/8 submitted to FAO by FBC
Ltd. (Unpublished)
Campbell, J.K. & Needham, D. Residues in milk and tissues of a goat
1980 dosed orally with 14C-BTS 44596 (major plant metabolite of
prochloraz). Boots report AX-800333 submitted to FAO by FBC
Ltd. (Unpublished)
Churchill, J.H.M. & Longland, R.C. Residues of prochloraz and major
1983a metabolites in bananas following a single postharvest
application with a 45% E.C. prochloraz formulation in South
Africa, 1982. FBC report RESID/83/5 submitted to FAO by FBC
Ltd. (Unpublished)
Churchill, J.H.M. & Longland, R.C. Residues of prochloraz and major
1983b metabolites in avocadoes following application with a 45%
E.C. formulation in South Africa. FBC report RESID/83/10
submitted to FAO by FBC Ltd. (Unpublished)
Churchill, J.H. & Longland, R.C. Residues of prochloraz and major
1983c metabolites in processed mushrooms following application
with a 50% W.P. formulation of prochloraz-Mn complex in
Holland, 1981. FBC report RESID/83/23 submitted to FAO by
FBC Ltd. (Unpublished)
Churchill, J.H.M. & Longland R.C. Residues of prochloraz and major
1983d metabolites in almonds following foliar application with a
50% W.P. formulation in Israel, 1982. FBC report RESID/83/38
submitted to FAO by FBC Ltd. (Unpublished)
Churchill, J.H.M. & Longland, R.C. Residues of prochloraz and major
1983e metabolites in avocadoes following postharvest treatment
with a 45% E.C. formulation in Australia, 1982/83. FBC
report RESID/83/55 submitted to FAO by FBC Ltd.
(Unpublished)
Cron, J. Residues of prochloraz and major metabolites in rapeseed
1982 following a single postemergence application with a 40 E.C.
prochloraz and a prochloraz/carbendazim E.C. formulation in
France 1980/81. FBC report RESID/82/74 submitted to FAO by
FBC Ltd. (Unpublished)
Cron, J.H. & Longland, R.C. Residues of prochloraz and major
1983a metabolites in avocadoes following multiple foliar
application with a 50% W.P. formulation of prochloraz-Mn
complex in Australia, 1981/82. FBC report RESID/82/120
submitted to FAO by FBC Ltd. (Unpublished)
Cron, J.H. & Longland, R.C. Residues of prochloraz and major
1983b metabolites in papayas treated postharvest with a 45% E.C.
formulation in Australia, 1982. FBC report RESID/83/1
submitted to FAO by FBC Ltd. (Unpublished)
Douglas, M.T. & Pell, I.B. Assessment of the ready degradability of
1982 prochloraz. HRC and FBC report METAB/82/44 submitted to FAO
by FBC Ltd. (Unpublished)
Douglas, M.T. & Pell, I.B. Assessment of biodegradability of
1983 prochloraz by pre-adapted sewage organisms. HRC and FBC
report METAB/83/10 submitted to FAO by FBC Ltd.
(Unpublished)
Goto, H. Residues of prochloraz in rice following seed soak or foliar
1980 application with a 25% E.C. formulation in Japan, 1980.
Inst. Environ. Toxicol., Japan, Nissan and FBC report
submitted to FAO by FBC Ltd. (Unpublished)
Haran, G. Identification of a photodecomposition product of BTS-40
1978 542 (prochloraz). Boots report F-78014 submitted to FAO by
FBC Ltd. (Unpublished)
Haran, G. The effect of simulate& sunlight on aqueous solutions of
1980 prochloraz. Boots report F-80010 submitted to FAO by FBC
Ltd. (Unpublished)
Hayto, E.M. BTS-40542 residues in wheat and barley. Boots report AX-
1977a 77002 submitted to FAO by FBC Ltd. (Unpublished)
Hayto, E.M. BTS-40 542 residues in wheat from Italy, 1977. Boots
1977b report AX-79003 submitted to FAO by FBC Ltd. (Unpublished)
Hayto, E.M. BTS-40 542 residues in apples from Thurgarton, U.K. Boots
1977c report AX-77011 submitted to FAO by FBC Ltd. (Unpublished)
Hayto, E.M. BTS-40542 residues in wheat from Holland, 1977. Boots
1978a report Ax-78006 submitted to FAO by FBC Ltd. (Unpublished)
Hayto, E.M. BTS-40542 residues in wheat and barley from France, 1977.
1978b Boots report AX-78020 submitted to FAO by FBC Ltd.
(Unpublished)
Hayto, E.M. BTS-40542 residues in wheat and barley from the United
1979a Kingdom, 1977. Boots report AX-79001 submitted to FAO by FBC
Ltd. (Unpublished)
Hayto, E.M. BTS-40542 residues in apples from Thurgarton, U.K., 1977.
1979b Boots report AX-79005 submitted to FAO by FBC Ltd.
(Unpublished)
Hayto, E.M. BTS-40542 residues in cereals from Fed. Rep. Germany,
1979c 1977. Boots report AX-79008 submitted to FAO by FBC Ltd.
(Unpublished)
Hayto, E.M. BTS-40542 and dicloran residues in lettuces from Holland,
1979d 1978. Boots report AX-79010 submitted to FAO by FBC Ltd.
(Unpublished)
Heinanen, E. Investigation certification for residues of prochloraz in
1983 spring wheat from Finland, 1982. VML report 2940, 2942/82
submitted to FAO by FBC Ltd. (Unpublished)
Housden, M.C. Residues of prochloraz and major metabolites in grain
1982a treated post-emergence with a 45% E.C. formulation in
Belgium, 1981. FBC report RESID/82/1 submitted to FAO by FBC
Ltd. (Unpublished)
Housden, M.C. Residues of prochloraz and major metabolites in
1982b mushrooms following application of a 50W formulation in
Australia, 1981/82. FBC report RESID/82/48 submitted to FAO
by FBC Ltd. (Unpublished)
Housden, M.C. Residues of prochloraz and major metabolites in cereals
1982c treated post-emergence with a 45% E.C. formulation (single
or double application) in Denmark, 1982. FBC report
RESID/82/111 submitted to FAO by FBC Ltd. (Unpublished)
Housden, M.C. & Longland, R.C. Decline study of residues of prochloraz
1983 and major metabolites in winter barley and wheat treated
once with a prochloraz/carbendazim suspension concentrate
formulation in Fed. Rep. Germany, 1982. FBC report
RESID/83/19 submitted to FAO by FBC Ltd. (Unpublished)
Housden, M.C. & Whiteoak, R.J. Residues of prochloraz and metabolites
1982 in mushrooms following single and multiple applications
(50w formulation) in the U.K. 1981 and 1982. FBC report
RESID/82/63 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. BTS 40 542 residues in cereals from Austria, 1977. Boots
1979a report AX 79006 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of BTS 40
1979b 542, BTS 44 596 and BTS 45 186 in grain. Boots report AX
79007 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979c (BTS 40 542), BTS 44 595, BTS 44 596 and BTS 45 186 in wheat
grain and straw from Fed. Rep. Germany, 1978. Boots report
AX 79015 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979d (BTS 40 542), BTS 44 595, BTS 44 596 and BTS 45 186 in wheat
grain and straw from Italy, 1978. Boots report AX 79017
submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979e (BTS 40 542), BTS 44 595, BTS 44596 and BTS 45 186 in wheat
grain and straw from The Netherlands, 1978. Boots report AX
79 017 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979f (BTS 40 542), BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in cereal grain and straw from
Austria, 1978. Boots report AX 79 020 submitted to FAO by
FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979g (BTS 40 542), BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in wheat and barley grain from
Denmark, 1978. Boots report AX 79021 submitted to FAO by FBC
Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979h (BTS 40 542), BTS 44 595, BTS 44 596 and BTS 45 186 in wheat
and barley grain and straw from France, 1978. Boots report
AX 79023 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. Persistence and uptake of prochloraz in Spanish oranges
1980a following a post-harvest dip. Boots report AX 80003
submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. BTS 45 186 residues in prochloraz-treated wheat grain from
1980b France, 1977. Boots report AX 80010 submitted to FAO by FBC
Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1980c (BTS 40 542) and metabolites containing the
2,4,6-trichlorophenyl moiety in wheat grain and straw from
The Netherlands, 1979. Boots report AX 80025 submitted to
FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The metabolism of 3H-prochloraz in mature wheat grain and
1980d straw. Boots report AX 80028 submitted to FAO by FBC Ltd.
(Unpublished)
Kelly, I.D. The metabolism and distribution of BTS 46828 applied to
1982a the fruit of field-growing apples. FBC report METAB/82/18
submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The hydrolysis of prochloraz in aqueous solution under
1982b acid, neutral and basic conditions. FBC report METAB/82/28
submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. & Krepski, W.J. The metabolism of prochloraz by wheat
1980 grain and straw. Boots report AX 80009 submitted to FAO by
FBC Ltd. (Unpublished)
Kesterton, M.S. Distribution of radio-labelled residues in field soil
1978 after application of 3H-BTS 40542. Boots report AX 78010
submitted to FAO by FBC Ltd. (Unpublished)
Kesterton, M.S. Report on the aerobic metabolism of prochloraz in
1981a soil over a period of 12 months. Boots report AX 81002
submitted to FAO by FBC Ltd. (Unpublished)
Kesterton, M.S. Report on the anaerobic metabolism of prochloraz in
1981b soil. Boots report AX 81004 submitted to FAO by FBC Ltd.
(Unpublished)
Kesterton, M.S. & Newby, S.E. A study of the leaching of prochloraz in
1980a soil. Boots report AX 80008 submitted to FAO by FBC Ltd.
(Unpublished)
Kesterton, M.S. & Newby, S.E. Adsorption and desorption studies of
1980b prochloraz in soil. Boots report AX 80012 submitted to FAO
by FBC Ltd. (Unpublished)
Kesterton, M.S. & Newby, S.E. Leaching study of prochloraz aged in a
1981 silty clay loam soil. Boots report AX 81003 submitted to FAO
by FBC Ltd. (Unpublished)
Krepski, W.J. The uptake of 14C-prochloraz and 3H-prochloraz soil
1981a residues into wheat. Boots report AX 81001 submitted to FAO
by FBC Ltd. (Unpublished)
Krepski, W.J. The metabolism of 14C-prochloraz and 14C-BTS 46828 by
1981b apples grown under glass. Boots and FBC report submitted to
FAO by FBC Ltd. (Unpublished)
Krepski, W.J. Translocation of 14C-prochloraz applied as a liquid
seed
1982 dressing in wheat grown to maturity. FBC report METAB/81/30
submitted to FAO by FBC Ltd. (Unpublished)
Leake, C.R. & Lines, D. The leaching of prochloraz and its major
1981 metabolites in four soil types using TLC. FBC report
METAB/81/35 submitted to FAO by FBC Ltd. (Unpublished)
Longland, R.C. Analysis of residues of prochloraz and major
1983a metabolites in grain and straw from Sweden following
application of 45% E.C., 1982. FBC report RESID/82/114
submitted to FAO by FBC Ltd. (Unpublished)
Longland, R.C. Residues of prochloraz and major metabolites in
1983b sugarbeet following application of a 40% E.C. formulation in
Italy, 1981. FBC report RESID/83/58 submitted to FAO by FBC
Ltd. (Unpublished)
Longland, R.C. & Churchill, J.H. Residues of prochloraz and major
1983 metabolites in mangoes following foliar treatment with 50%
W.P. and postharvest treatment with 40% E.C. formulations in
Israel, 1982. FBC report RESID/83/7 submitted to FAO by FBC
Ltd. (Unpublished)
Maclaine Pont, M.A., Vogelzang, H.P. & Siegmann-Knoester, K.C. The
1980 residue analysis of prochloraz in combination with dicloran.
Asepta-fabrik and Boots report AX 80020/2 submitted to FAO
by FBC Ltd. (Unpublished)
Maier-Bode, H. The leaching of prochloraz in three standard soils from
1980 Fed. Rep. Germany. HMB and FBC report submitted to FAO by
FBC Ltd. (Unpublished)
Manley, J.D. & Snowden, P.J. Analytical method for residues of
1982a prochloraz and major metabolites in miscellaneous fruit,
cereal and vegetable crops (improved method). FBC report
RESID/82/88 submitted to FAO by FBC Ltd. (Unpublished)
Manley, J.D. & Snowden, P.J. Residues of prochloraz and major
1982b metabolites in oranges and processed oranges following
commercial scale postharvest brush treatment with prochloraz
(40 E.C.) in South Africa, 1982. FBC report RESID/82/89
submitted to FAO by FBC Ltd. (Unpublished)
Manley, J.D. & Snowden, P.J. Residues of prochloraz and major
1982c metabolites in rapeseed following treatment with a 40% E.C.
formulation in the U.K., 1981/1982. FBC report RESID/82/90
submitted to FAO by FBC Ltd. (Unpublished)
McDougall, J. The metabolism of 3H-BTS 40 542 in wheat at a
vegetative
1979 growth stage. Boots report AX 79009 submitted to FAO by FBC
Ltd. (Unpublished)
McDougall, J. The uptake and translocation of 3H-prochloraz in wheat.
1980a Boots report AX 80007 submitted to FAO by FBC Ltd.
(Unpublished)
McDougall, J. The relative uptake and persistence of prochloraz and
1980b BTS 46 828 in apples following treatment with 14C-
prochloraz and 14C-BTS 46 828. Boots report AX 80015
submitted to FAO by FBC Ltd. (Unpublished)
McGibbon, A.S. Uptake of prochloraz residues by sugarbeet from
1982 prochloraz-treated soil under field conditions. FBC report
METAB/82/27 submitted to FAO by FBC Ltd. (Unpublished)
Newby, S.E. The laboratory decline of prochloraz in two soils, a sandy
1982 loam and a silty clay loam, under sterile conditions. FBC
report METAB/82/41 submitted to FAO by FBC Ltd.
(Unpublished)
Peatman, M.H. & Snowden, P.J. Residues of prochloraz and major
1982 metabolites in rapeseed treated with the 40% E.C.
formulation in France, 1982. FBC report RESID/82/116
submitted to FAO by FBC Ltd. (Unpublished)
Peatman, M.H. & Snowden, P.J. Residues of prochloraz and major
1983 metabolites in rapeseed treated with a 45% E.C. formulation
in Denmark and Sweden, 1982. FBC report RESID/83/29
submitted to FAO by FBC Ltd. (Unpublished)
Reary, J.B. Analytical method for free and conjugated residues of
1981a prochloraz, BTS 44595, BTS 44 596 and BTS 45 186 in cereals
at different growth stages. FBC report RESID/81/13 submitted
to FAO by FBC Ltd. (Unpublished)
Reary, J.B. Residues of prochloraz and major metabolites in summer
1981b wheat treated post-emergence with a 40% E.C. formulation
(two or three applications) in Fed. Rep. Germany, 1980. FBC
report RESID/81/14 submitted to FAO by FBC Ltd.
(Unpublished)
Reary, J.B. Residues of prochloraz and major metabolites in cereals
1981c treated post-emergence with a 45% E.C. formulation in
Holland, 1980. FBC report RESID/81/46 submitted to FAO by
FBC Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980a prochloraz (BTS 40 542) BTS 44 595 and BTS 44 596 and
conjugated residues of BTS 45 186 in wheat and barley,
France, 1979. Boots report AX 80005 submitted to FAO by FBC
Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980b prochloraz (BTS 40 542), BTS 44 595 and BTS 44 596 and
conjugated residues of BTS 45 186 in Spanish oranges
following a postharvest application, 1980. Boots report AX
80021 submitted to FAO by FBC Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980c prochloraz (BTS 40 542), BTS 44 595, BTS 44 596 and
conjugated residues of BTS 45 186 in wheat and barley, U.K.,
1979. Boots report AX 80022 submitted to FAO by FBC Ltd.
(Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980d prochloraz (BTS 40 542), BTS 44 595, BTS 44 596 and
conjugated residues of BTS 45 186 in wheat and barley,
Denmark, 1979. Boots report AX 80023 submitted to FAO by FBC
Ltd (Unpublished)
Richards, M.E. BTS 45 186 residues in oranges treated with prochloraz,
1980e Spain, 1980. Boots report AX 80027 submitted to FAO by FBC
Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980f prochloraz, BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in Spanish oranges following a
postharvest application, 1980. Report No. 2. Boots report AX
80030 submitted to FAO by FBC Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980g prochloraz, BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in winter wheat, Fed. Rep. Germany,
1979. Boots report AX 80032 submitted to FAO by FBC Ltd.
(Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1981a prochloraz, BTS 44 595 and BTS 44596 and conjugated residues
of BTS 45 186 in grain and straw. Boots and FBC report AX
800 35 submitted to FAO by FBC Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1981b prochloraz, BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in winter barley, Fed. Rep. Germany,
1980. Boots and FBC report AX 81005 submitted to FAO by FBC
Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1981c prochloraz, BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in mushrooms, U.K., 1980. FBC report
METAB/81/17 submitted to FAO by FBC Ltd. (Unpublished)
Snowden, P.J. Residues of prochloraz and major metabolites in stone
1983 fruit following foliar applications of prochloraz (50 E. and
40 E.C.) in Europe (1982) and South Africa (1981/2 and
1982/3). FBC report RESID/83/57 submitted to FAO by FBC Ltd.
(Unpublished)
Snowden, P.J. & Manley, J.D. Residues of prochloraz and major
1983 metabolites in Tambors oranges following commercial scale
postharvest brush treatment with prochloraz (45% E.C.) in
South Africa, 1982. FBC report RESID/83/9 submitted to FAO
by FBC Ltd. (Unpublished)
Somerville, L. The analysis of prochloraz residues in cereals. Boots
1980 report AX 80020/1 submitted to FAO by FBC Ltd. (Unpublished)
Stockbauer, I. Leaching of Sportak PF (prochloraz and carbendazim) in
1983 German soils. ASU and FBC report submitted to FAO by FBC
Ltd. (Unpublished)
Wang, S.S. Residues of prochloraz in grapes, apples and watermelon
1982 following foliar application with a 25% E.C. formulation in
Taiwan, Taiwan Plant Protection Centre report submitted to
FAO by FBC Ltd. (Unpublished)
Whiteoak, R.J. Residues of prochloraz in mushrooms following multiple
1981 applications of a 50% W.P. formulation in the U.K., 1980/81
(ADAS trial results), FBC report RESID/81/41 submitted to
FAO by FBC Ltd. (Unpublished)
Whiting, K.C. & Dickinson, W. The stability of the manganese complex
1979 of prochloraz in water. Boots report F 79005 submitted to
FAO by FBC Ltd. (Unpublished)
Straw Grain
Free 2,4,6-trichlorophenol (BTPS 45186) 5.2 4.1 3.4 8.4
Residues containing the BTS-45186 moiety 58 37.9 53.9 39.5
Tritiated water 4.8 - 4.2 -
Unidentified extractable residues 9.1 34 15.5 32.1
Unidentified residues in solids 22 24 24.7 20
99.1 100 101.7 100
Of the unidentified extractable residues no one of the grain or
straw acidic, basic, volatile or aqueous fractions analysed contained
more than 6.2 percent of the total residue.
Formation and analysis of the glucosazone after hydrolysing
starch extracted from the 14C-prochloraz treated grain demonstrated
that 32.4 percent of the grain radioactivity was incorporated into the
starch molecules. This is thought to result from the incorporation of
14C-carbon dioxide, resulting from decarboxylation of the metabolite
BTS 44596. The remaining residues were separated into various
fractions but were not identified (Kelly 1980d; Kelly & Krepski 1980).
When wheat seeds were treated at an equivalent rate of 0.4
a.i./kg seed (approximating recommended usage) with a 20 percent a.i.
liquid seed dressing and grown to maturity, residues at maturity (29
weeks) were:
% of total Prochloraz
applied Equivalents (mg/kg)
Treated Controls
Soil 58œ3 0.06 -
Roots 15.1 1.5 -
Chaff 0.1 0.04* 0.004*
Grain 0.04 0.004* 0.002*
Total aerial 6.6 0.16 -
* mean of four determinations.
Translocation into the aerial portion of the plant was complete
after six weeks (Krepski 1982).
Potted wheat seeds were allowed to germinate under glass after
soil surface drench application of a diluted 3H-prochloraz
formulation. Approximately 82 percent of the radioactivity (0.8
percent of applied) was retained in the root system at harvest after
21 days. Residues decreased with increased distance of plant parts
from the root system; they decreased from 8.7 percent of residues
recovered in leaves 1 and 2, 0.62 percent in leaf 3, to 0.08 percent
in leaf 7. Soil contamination was suspected of contributing some of
the residues for leaves 1 and 2 and lower plant parts.
In this same report a 3H-prochloraz formulation was applied with
a syringe at the fifth leaf stage to the third leaf of similarly grown
wheat without the soil drench. Plant parts were harvested and analysed
after 24 days; 99 percent of applied radioactivity was retained in
leaves 3 and 4 (difficulty was encountered in discerning leaves during
applications). Radioactivity decreased from 0.03 percent of that
applied in leaf 5 to 0.01 percent in leaf 9 and increased from 0.23
percent in leaves 1 and 2 to 0.33 percent in the roots (McDougall
1980a).
After spring wheat harvest from soil plots treated at a rate
equivalent to 1 kg/ha with either imidazole ring-labelled
14C-prochloraz or phenyl-labelled 3H-prochloraz, the plots were
re-sown with winter wheat and reharvested 10 months later. Soil
samples at harvest of the winter wheat were 0.11 and 0.43 mg/kg for
14C and 3H treatments, respectively. Residues of 14C or
3H-prochloraz equivalent were < 0.01 mg/kg in grain, chaff and
straw. Grain control values were about half that for grain, but not
statistically different. Low uptake is suggested for 3H-prochloraz
treated chaff and straw where experimental levels are 50 to 100 times
that of the respective controls (Krepski 1981a).
A 1.5 sq m plot was treated with 14C-phenyl labelled prochloraz
formulation by garden sprayer at an application rate equivalent to 395
g a.i./ha, which approximates recommended usage for cereals. After 41
days the soil was turned by hand and sown with sugarbeets as a
representative rotational crop. A control plot was also planted.
Seedlings were sampled 23 days after planting and mature plants (roots
and foliage) at 157 days after planting.
Residues at planting in soil averaged 0.26 mg/kg prochloraz
equivalents. At 23 days after planting, residues of 0.07 mg/kg in
seedlings were significantly greater than the 0.006 mg/kg controls. At
harvest, residues of 0.005 mg/kg in the soils were not significantly
different than in controls, whereas the 0.0052 mg/kg prochloraz
equivalent in foliage was slightly greater than the 0.003 mg/kg
control. This difference is statistically significant, although
residues in the foliage are less than the background level (McGibbon
1982).
Citrus
3H-prochloraz, a 2.5 percent E.C. formulation, was applied by
pipette wash, to simulate a 0.1 percent a.i. postharvest commercial
treatment, to Washington Navel oranges in Spain. Storage was at 4°C in
the dark from 10 to 60 days and at 20-22°C in diffuse light. From 1 to
10 days, surface residues were removed with a 0.1 percent ethylan BCP
solution and those in peel and flesh by extraction with 90 percent
acetone. Surface residues at room temperature decreased from 5.8
percent of applied radioactivity at 1 day to 0.8 percent at 10 days,
and at 4°C from 3.8 mg/kg at 10 days to 1.1 mg/kg at 60 days.
Corresponding residues in the flesh increased from 1.8 percent to 5
percent of the applied dose over the storage period for room
temperature storage, but remained relatively constant at approximately
1.6 percent over the entire 60 day storage period at 4°C.
Radioactivity in the peel remained relatively constant at 92.3-96
percent of the applied dose over the room temperature storage period
and, similarly, 94.7 to 97.2 percent over the storage period at 4°C
(Kelly 1980a). Similar unlabelled studies on citrus residues during
storage are reported under "In Storage and Processing".
Apples
Formulations of phenyl-labelled 14C-prochloraz or its manganese
complex BTS 46828 were applied to individual apples under glass at
rates approximating commercial usage. In samples analysed after 20
days, residue distribution was similar in the surface, peel and flesh
from either chemical. Residues in extracts from the prochloraz-treated
apples (86 percent of the applied dose was recovered) were - wash 7.4
percent, peel 50 percent and flesh 16.9 percent (20 percent of
recovered). BTS 46828 gave almost identical results (Krepski 1981b).
In similar experiments (not carried out under glass) the
metabolism of prochloraz and its manganese complex were compared at
intervals between 0 and 54 days after application by syringe to
individual apples. Radioactivity distribution was again shown to be
similar from use of either chemical, although prochloraz from the E.C.
formulation was initially absorbed into the peel at a faster rate than
the Mn-complex W.P. and was somewhat more persistent. The manganese
complex was also more susceptible to losses from precipitation shortly
after application. As a percent of radioactivity recovered at 0 and 54
days, residues were:
Prochloraz Mn - complex
*day 0 day 54 *day 0 day 54
surface 32.3 1.9 67.6 1.2
flesh 0.0 0.8 0.0 1.2
peel 67.2 81.2 32.4 56.2
Total 100 83.9 100 58.6
* 5 hours (McDougall 1980b).
In another similar study distribution and characterization of
residues were investigated in two apple varieties at intervals ranging
from 16 to 63 days after application by syringe of the Mn-complex to
individual growing apples. As a percent of the applied dose,
representative residues were:
Days after Whole Peel
treatment fruit Flesh Total Extractables
16 72 (83) 15.81 67 (78) 51.3 (61.4)
33 54.8 (76) 3.6 (5.2) 51 (71) 37.2 (55.2)
63 63.5 5.4 60 38
Numbers in parentheses correspond to the Worcester variety and
others to the Cox variety.
Extractable residues in peel were quantified and characterized in
both varieties at 33 and 63 days after treatment. As a percent of
extractable peel residues the metabolic profile at 63 days for the Cox
variety was:
Metabolite Percent
Prochloraz or Unchanged BTS 46 828 5 )
BTS 45186 (free 2,4,6-trichlorophenol) 11.5 )
BTS 44595 15.2 )
BTS 44596 33.1 ) >77.8
Conjugated residues containing )
the BTS 45186 moiety 13 )
Unidentified polar metabolites 6
"Unknowns" 1.6,0.7
Unresolved background 13.9
Total unidentified (excluding conjugates
with BTS 45186) 22.2
100.0
Results were similar at 33 days for both varieties, except that
conjugated residues containing the BTS 45186 moiety were only 3
percent for the Worcester variety, only the first "unknown" was
observed in Cox and the first and a different second "unknown" was
found in Worcester. None of the residues exceeded 2 percent (Kelly
1982a).
In Soil
Prochloraz 25 percent E.C. formulations, labelled with 14C in
the imidazole ring and separately in the phenyl ring with 3H, were
separately applied to acidic sandy loam and silty clay loam soils in
enclosed jars purged with CO2-free air under laboratory conditions
for up to 52 weeks. Application was at 6 mg/g soil, equivalent to 0.7
kg a.i./ha (a recommended crop application rate). Silty clay loam was
similarly treated with prochloraz labelled with 14C in the phenyl
ring and studied over 9 months.
Radioactivity losses as volatiles were about 50 percent of the
applied dose to silty clay for both 14C-imidazole and 3H-phenyl
labelled prochloraz after one year, as was the case for the
14C-phenyl labelled formulation at 9 months. Losses were more rapid
from silty clay loam than from sandy loam, for which losses were half
or less that in silty clay. Experimental evidence indicated, but did
not prove, that volatiles from 14C-phenyl and 14C-imidazole labelled
prochloraz were predominantly CO2. In the case of 3H-phenyl labelled
prochloraz, evidence suggests volatile radioactivity in the form of
tritiated water. The major extracted residue in all cases was
prochloraz per se at 50-68 percent of the applied dose (80-90
percent of extracted) after 8 weeks to 12-30 percent (45-67 percent of
extracted) after 52 weeks. Lower residues of BTS-44596 and BTS 45186
combined were <18 percent of the applied dose (<26 percent of
extracted) after 52 weeks for 3H-labelled treatments. Binding was
more pronounced in sandy loam. The half-life was reported as 3 and 5
months, respectively, in silty clay and sandy loams. In both cases the
acidic pH would increase stability (Kesterton 1981a).
In German standard soils the half-life of unlabelled prochloraz
ranged from 115-135 days, with stability directly related to organic
matter content. For both soils the pH was acidic, which is conducive
to prolonged stability (McGibbon 1982).
14C-imidazole and 3H-phenyl labelled prochloraz, formulated as
a 25 percent E.C., were incubated under anaerobic conditions at a rate
equivalent to 0.7 kg a.i./ha and sampled at 14, 27 and 60 days.
Residues of prochloraz remained relatively constant over the test
period in silty clay loam (ca 68 percent of the applied dose) and
decreased slightly (to ca 54 percent) in silty clay loam. The decrease
in silty clay loam was accompanied by an increase in bound residues.
Anaerobic conditions halted the continuous degradation and formation
of volatiles observed under aerobic conditions. Other components
indicated by TLC from 3H treatments were BTS 44596 and BTS 45186 at
approximately 6 and 3 percent of the applied dose, respectively,
whereas from 14C treatments prochloraz, imidazole and BTS 44596 were
approximately 68, 2 and 1 percent, respectively (Kesterton 1981b).
The absence of significant degradation of a 14C-phenyl labelled
2.5 percent E.C. prochloraz formulation in sandy loam and silty clay
loam under sterile soil conditions for 30 days suggests that
degradation is facilitated primarily by soil microorganisms as opposed
to chemical degradation, at least under the experimental conditions
(acidic pH and 50 percent soil moisture capacity) (Newby 1982).
Sandy loam and silty clay loam soil were treated with a
14C-benzene labelled 25 percent E.C. prochloraz formulation at a rate
equivalent to 0.44 kg a.i./ha, aged 13 days, added to the top of 30 cm
× 7 cm diameter columns containing untreated soil and eluted with an
equivalent of 1.5 cm rain for 18 days. Essentially all radioactivity
was retained in the top 5 cm of soil, with less than 0.3 percent of
the applied amount being detected in leachate (Kesterton & Newby
1980a).
In a similar soil column study, except that the equivalent
application rate was 0.8 kg/ha, the labelled prochloraz was applied to
the same silty clay loam and aged for 30 days, after which it was
eluted for 45 days with a daily equivalent of 0.5 cm rainfall. Results
were similar to the first study in that <0.05 percent of the
applied dose was detected in the eluant. However, even though 88-94
percent was in either the treated portion or the first 5 cm below it,
0.3-0.6 percent was detected in the bottom 25 cm, indicating some
downward mobility other than that detected in the leachate (Kesterton
& Newby 1981).
When an unlabelled prochloraz formulation was applied to West
German standard soil columns at a rate equivalent to 1 kg/ha, <0.05
percent was detected in the leachate (Maier-Bode 1980). In a similar
experiment leachate contained <1.7 percent of the applied dose
(Stockbauer 1983). Details of the preceding two experiments were not
available.
In soil thin-layer mobility tests, the mobility of prochloraz and
soil metabolites BTS 44 596 and BTS 45186 were compared to that of
atrazine and 2,4-D in four soil types. According to the Helling
mobility index, over-all mobility in the four soils were, in order of
increasing mobility, prochloraz, BTS 44596 (low mobility), BTS 45186
(low mobility), atrazine (intermediate mobility) and 2,4-D (mobile).
Mobility of all tested materials was greatest in sand (even BTS 44596
and BTS 45186 would have intermediate mobility). Prochloraz and BTS
44596 were immobile in silt loam, whereas BTS 45186 was of
intermediate mobility. The thin-layer studies confirmed the low, but
positive, mobility detected in soil column experiments (Leake & Lines
1981).
Adsorption/desorption of prochloraz, uniformly labelled in the
phenyl ring and applied as an E.C. formulation to a sandy loam and
silty clay loam, was investigated. The Kd of the sandy and silty
soils were 152 and 256, respectively, indicating slightly more
adsorption by the latter. The K values were 86.8 and 101.7,
respectively, for these soils. Strong adsorption and minimal
desorption is consistent with leaching studies (Kesterton & Newby
1980b).
A trichlorophenol tritiated ring prochloraz E.C. formulation was
applied to one sq m plots of barley and wheat in sandy loam at an
equivalent rate of 1 kg/ha. Core samples from 32.5 cm deep were
analysed after approximately 15 and 30 weeks; > 95 percent of
prochloraz residues were found in the upper 10 cm and in some cases
the top 2.5 cm. Somewhat similar distribution after 15 and 30 weeks
suggests only slight leaching of bound residues as a result of
additional (over 150 mm) rainfall in the latter period (Kesterton
1978).
In Water
No degradation of prochloraz was observed after 30 days in 22°C
buffer solutions at a pH of 4.95 or 6.98. At pH 9.18 degradation does
occur, with 77 percent of the added prochloraz remaining after 30
days. A first order degradation was indicated and a half-life
estimated of approximately 79 days. The only degradant
N-propyl-N-2-(2,4,6-trichlorophenyl) ethylamine (BTS 40348) was
reported to account for more than 2 percent of the total
radioactivity, although the quantitative analyses were not provided
(Kelly 1982b). The 4:1 manganese complex of prochloraz (BTS 46 828),
either as active compound or as a 50 percent W.P. formulation,
disassociated to prochloraz and manganous chloride in the presence of
water (pH 6.1). Approximately 50 percent of the complex was
dissociated within four hours at 25°C (Whiting & Dickinson 1979).
Biodegradability of prochloraz in water was studied using the
closed bottle test according to OECD Guideline 301D. Tests indicate
little biodegradation at 20°C up to 15 days after inoculation, but 13
percent degradation by 28 days, suggesting some initial bacterial
adaptation (Douglas & Pell 1982). When bacteria preadapted to
prochloraz were used as the inoculum, degradation commenced
immediately and reached 47 percent biodegradation by 28 days (Douglas
& Pell 1983).
In Storage and Processing
Processing
Citrus - Oranges were postharvest brush-treated at 1 000 mg/kg with
a 40 percent E.C. formulation and processed into "42% comminuted
orange" and "special whole orange compound". These "whole oranges
juices" (chopped oranges) are used for production of various orange
drinks and utilizes most of the whole orange, except for removal of
some coarse peel. It includes a pasteurization process up to 86°C,
depending on which of two processes were used. Total residues
(converted to 2,4,6-trichlorophenol for analysis) were 0.19 and 0.14
mg/kg expressed as prochloraz for the 42 percent comminuted orange and
special whole orange compound, respectively, approximately 30 percent
of the residues found in whole oranges (Manley & Snowden 1982b).
Cereals - Winter wheat was treated post-emergence at two sites in
the Federal Republic of Germany at growth stage 59 (mid-May to
mid-June) with three applications of a 40 percent E.C. prochloraz
formulation at the 0.48 kg/ha rate recommended in that country. Mature
grain was sampled at 57-58 days after last application and portions
milled and baked into bread. Total residues were determined by
hydrolysis of the trichlorophenol containing moieties followed by EC
analysis and use of a 1.906 factorial conversion to a prochloraz
equivalent basis. Residues in grain and bread were <0.1 mg/kg, the
limit of determination.
Under similar conditions prochloraz was applied twice to barley.
Total residues in barley malt, expressed as prochloraz, were 0.13 and
0.15 mg/kg compared with 0.07 mg/kg in a control. Limit of
determination was 0.1 mg/kg.
Rapeseed Oil - Mean residues of total prochloraz and metabolites
hydrolysable to the trichlorophenol, and expressed as prochloraz, in
mature rapeseed treated in each of four sites in France ranged from
0.06 to 0.2 mg/kg (over-all mean of 0.11 mg/kg). Treatment was with
two applications of an E.C. formulation at a rate of 0.45 kg a.i./ha
and harvest was 25-38 days after the last treatment. Mean residues of
total prochloraz and its metabolites in hexane-extracted oil from
these trials (omitting one individual 0.49 mg/kg outlier from six
replicate results, otherwise ranging from 0.07-0.13 mg/kg) ranged from
0.11 to 0.29 mg/kg (over-all mean 0.10 mg/kg), indicating a residue
concentration factor of approximately 3 times. Residues in the treated
seed and oil only ranged from approximately 1-3 times that in controls
from any given site and were therefore not far removed from the limit
of determination in any one site, with a variation of from 0.05 to 0.2
mg/kg among the sites.
In similar studies at three sites each in Denmark and Sweden, the
concentration factor for prochloraz in the oil ranged from only 1.2 to
2 times that in the seed. Treatment-to-harvest intervals range from 47
to 76 days. In these studies, residues in treated seed and oil derived
therefrom, although comparable to those in France, were significantly
greater than in controls. The limit of determination was estimated at
0.05 and 0.1 mg/kg, respectively, for seed and oil.
Mushrooms - Mushrooms were harvested in France 17 days after
pre-emergence treatment with a 40 percent E.C. prochloraz formulation,
administered at a rate of 40 g a.i./100 sq m. No information on good
agricultural practice is available from France. The mushrooms were
immediately processed by canning and dehydration, after which samples
of canned mushrooms, the liquor and dehydrated mushrooms were analysed
as total residues of prochloraz and its metabolites that were
hydrolysable in the presence of pyridine hydrochloride to the
trichlorophenol moiety. Total residues (apparently uncorrected for
recoveries) and expressed as prochloraz were less than the 0.1 mg/kg
limit of determination for canned mushrooms and the liquor, with
control values of 0.03 and 0.05 mg/kg, respectively, reported. An
apparent residue of 0.14 mg/kg in dehydrated mushrooms was less than
the 0.23 mg/kg control value. Analytical recoveries were 64, 63 and 46
percent, respectively, for dehydrated mushrooms, canned mushrooms and
the liquor (Browne 1982h).
In experiments conducted in The Netherlands, total residues of
prochloraz and its metabolites, expressed as prochloraz, were
determined in canned mushrooms and the liquor after three treatment
schedules with a 50 percent W.P. formulation of the prochloraz
manganese complex, (1) 0.75 a.i./sq m post-casing and 0.94 g a.i./sq m
14 days after casing (2) 1.5 g a.i./sq m 9 days post casing (good
agricultural practice in The Netherlands) and (3) 1.5 g a.i./sq m both
at post-casing and after 14 days. Total residues expressed as
prochloraz were equal to or less than the 0.1 mg/kg limit of
determination in the six liquor samples and five of the six canned
mushroom samples. For treatment (3) above, however, the residue was
0.23 mg/kg in the sixth mushroom sample and 0.1 mg/kg in the control.
No information was available on the interval from last treatment to
harvest. Recoveries were 81 and 94 percent, respectively, for canned
mushrooms and the liquor. No analyses were available for the
unprocessed mushrooms (Churchill & Longland 1983c).
Storage
Citrus - Data are available from a variety of treatment and storage
conditions and are summarized in Table 3. Navel oranges were
commercially treated postharvest in South Africa with a 40 percent
E.C. prochloraz formulation at 1 000 and 2 000 mg/kg a.i. rates then
waxed and shipped by refrigerated vessel to the United Kingdom.
Samples were stored either in the dark at approximately 20°C or at
4°C. Sampling for analysis occurred at 0 (44 days after treatment), 7,
14 and 21 days after storage commenced. Mean (range in parentheses)
residues, corrected for recovery, of total prochloraz and metabolites
expressed as prochloraz at the lower treatment rate were 0.05 mg/kg
(0.030.08 mg/kg) in flesh, 2.1 mg/kg (1.4-2.8 mg/kg) in peel and 0.49
mg/kg (0.3-0.7 mg/kg) on the whole fruit (calculated from peel to
flesh weight ratios) for ambient and 4°C fruit combined over the
entire storage period. Corresponding values for the higher treatment
rate were 0.05 mg/kg (0.02-0.07 mg/kg), 3.3 mg/kg (2.5-3.7 mg/kg) and
0.84 mg/kg (0.7-1.7 mg/kg) respectively.
The list of determination was reported as 0.05 mg/kg, which is
consistent with uncontaminated control values of 0.01-0.02 mg/kg total
residue. No observable trend in lower residues occurred during the
21-day storage period at either temperature and there were no
noteworthy differences in residues between the storage temperatures.
The trials also demonstrated that approximately 94 percent of the
total residue was in the peel (Manley & Snowden 1982b).
In similar postharvest treatments to oranges in Spain, with
0.05-0.1 percent solutions or 3-4 g a.i./tonne of fruit (usually in
wax) with an E.C. formulation as a spray or dip, residues were
determined after storage intervals typically ranging from 1-10 to
10-60 days at 4°C-22°C. Maximum peel residues were as much am 7.3
mg/kg for prochloraz alone and 8.4 mg/kg for total residues of
prochloraz and its metabolites, both expressed as prochloraz. Total
residues in flesh, expressed as prochloraz, were 0.04-0.44 mg/kg and
on a calculated whole fruit basis up to 2.5 mg/kg. As in the South
African study, a definite trend towards decreased residues could not
be determined over the varied storage intervals and temperatures and
little difference was observed in residues where application rates
varied by a factor of two. Peel was repeatedly shown to contain most
of the total whole fruit residue with typically <5 percent being
present in the flesh (Richards 1980b,f; Kelly 1980a; Browne 1981b).
Similar studies were conducted in the United Kingdom where
oranges were dip-treated with a 0.07 percent a.i., 40 percent E.C.
formulation and analysed after 1, 7, 21, 25 and 70 days cold storage
(temperature not provided). Total residues were expressed as
prochloraz. Residues of the parent compound in peel ranged from 3.6 to
5.1 mg/kg compared to 5.8 to 7.8 total residue. In flesh, the residues
were 0.05 to 0.12 mg/kg and 0.07 to 0.15 mg/kg, respectively. No
declining residue trend was observed over the 70-day maximum storage
period, although total residues in flesh increased from about 4
percent of the whole fruit residue at 1-7 days to 11 percent after 70
days (Browne 1982i).
No decline in residues on oranges was observed during storage at
intervals from 1 to 16 days at ambient temperature in Australia after
dip treatment with a 40 percent E.C. prochloraz formulation as a 250
to 500 mg/kg a.i. solution. Total residues determined as the
trichlorophenol expressed as prochloraz were similar from the two
application rates, as were residues of prochloraz alone. Residues of
prochloraz when determined alone ranged from 1.5 to 3.9 mg/kg (2.9
mg/kg) in peel and from <0.02 to 0.1 mg/kg (0.04 mg/kg) in flesh over
the 16-day storage interval for both concentrations. Total residues
were similar 1.4-3.4 kg (2.5 mg/kg) and <0.05 mg/kg, respectively.
Again, most of the residue was in the peel (Browne 1981a).
Residues in Spanish lemons were investigated after a shower
postharvest treatment with a 3 000 a.i. E.C. formulation in wax.
Samples were analysed after 12 and 16 days of storage. The limit of
determination was 0.02 mg/kg and 0.05 mg/kg for prochloraz alone and
total residues expressed as prochloraz, respectively. Residues
expressed as prochloraz were similar after both storage periods, with
approximately 5 percent of the residue in the flesh. Residues of
prochloraz alone and total residues were:
Prochloraz alone (mg/kg) Total residues (mg/kg)
Peel Flesh Whole Peel Flesh Whole*
9.1-15.6 0.27-0.3 3.1-5.0 10.6 0.17-0.33 3.7-6
(12.3 mean) (0.29) (4.6) (14) (0.23) (5.1)
* Calculated from peel/flesh.
Recoveries were 75 percent in peel and 77-100 percent in flesh
(Browne 1982c).
In 1980, postharvest trials on oranges in Spain, using a 40
percent E.C. prochloraz formulation, were made with 200 1 of wax
formulation (3 g a.i./1 wax) to 150 tonnes of fruit (Richards 1980b).
Samples of orange flesh from these trials were analysed for free
2,4,6-trichlorophenol (BTS 45186), since no metabolism study was
available on orange and BTS 45186 has been found in low levels in
wheat (Kelly 1980d). Samples were analysed after 7 and 14 days at
20-22°C. Residues were 0.005-0.01 mg/kg (0.008 mg/kg mean) after 7
days and 0.005 mg/kg after 14 days, as compared to <0.005 mg/kg for
controls. Contrary to the findings in most other citrus postharvest
trials, a slight trend of decreasing residues with time was indicated
(Richards 1980e).
In postharvest dip trials using a 25 percent E.C. or 25 percent
W.P. prochloraz formulation on oranges and lemons in Italy residues
were determined after 57 days storage at 7°C. Significantly higher
residues resulted from E.C. applications in both fruits and lemon
residues were higher than those in oranges (Browne & Manley 1982b).
Oranges treated in South Africa with a postharvest fungicidal
brush at 500-4 000 mg/kg a.i. 45 percent E.C. were analysed after two
months storage at ambient temperature. Again, most of the residue was
in the peel, with total residues, expressed as prochloraz, ranging
from 0.08 mg/kg at the lower application rate to 0.73 mg/kg at the
higher one. There was a good correlation of residue level with
application rate (Snowden & Manley 1983).
Bananas - Bananas were treated by postharvest dipping in Australia
with 250 and 500 mg/kg a.i. solutions of 40 percent E.C. prochloraz
and analysed after ambient storage for 9, 10, 12 and 16 days. Free and
conjugated residues of prochloraz and its metabolites were determined
as the trichlorophenol, expressed as prochloraz, with a 0.05 mg/kg
limit of determination. Residues resulting from both treatment rates
and over the entire storage period ranged from 5.2-10 mg/kg in skin
and 0.02-0.05 mg/kg in flesh. No evidence of decline in residues was
observed in skin or flesh over the storage period and there was no
significant difference in residues between the two application rates
(Browne 1982b).
Photodecomposition
Thin-layer chromatography and nuclear magnetic resonance (NMR)
analysis of a photodecomposition product of an aqueous solution of
prochloraz confirmed the presence of
N-formyl-N-propyl-N',-2-(2,4,6-trichlorophenoxy) ethylurea (BTS 44596)
(Haran 1978). Prochloraz aqueous solutions of 26.1 and 43.3 mg/kg
concentrations in glass-stoppered flasks were exposed to artificial
light (420-760 nm wavelength) at 32°C for 36 and 16 days,
respectively, with periodic analyses by high pressure liquid
chromatography. At the lower concentration residues decreased from
6.93 × 107 moles (26.1 mg/kg) initially to 1.94 × 107 moles after 36
days while BTS 44596 increased from 0.0 to 3.58 × 107 moles. At the
higher concentration, residues decreased from 11.5 × 107 moles (43.3
mg/kg) at zero day to 5.8 × 107 moles after 16 days, while BTS 44596
increased from 0.0 to 35.8 × 107 moles. The first order decay
half-lives were 20.5 and 18.1 days, respectively, for the two
concentrations.
Retention times indicated that a minor unquantified degradation
product was N-propyl-N-2-(2,4,6-trichlorophenoxy) ethylurea (BTS
44595). This appeared only towards the end of the exposure period
(Haran 1980).
METHODS OF RESIDUE ANALYSIS
Analytical methods are available for the analysis of free
prochloraz or total free and conjugated residues of prochloraz and its
major metabolites. Methodology for free prochloraz was first developed
for the analysis of cereal grains and straw (Somerville 1980). Finely
milled straw or ground grain are vigorously extracted with acetone,
filtered, a small portion acidified with HCl and concentrated by
rotary evaporation. The aqueous fraction is partitioned with petroleum
ether, which is discarded, adjusted to pH 6-7 and again partitioned
with petroleum ether, which is concentrated to dryness. The sample is
taken up in ethyl acetate and analysed by electron capture gas
chromatography utilizing a 7 percent OV-101 packed column. Analytical
recoveries of wheat and barley grain, amended with prochloraz at
0.05-0.5 mg/kg, were 78 ± 10.6 percent and 91 ± 10.9 percent,
respectively. For straw, the respective recoveries were 82 ± 12
percent and 80.6 ± 17 percent. The level of "sensitivity" has been
estimated at 0.01 mg/kg for grain and straw, although in the field
trials apparent untreated controls in cereal grain and straw were
occasionally up to 0.02 mg/kg.
With minor modifications, this method has been used for
determining free prochloraz residues in apples, pears, citrus,
mushrooms, strawberries and lettuce (FBC, (Hayto 1979b; Whiteoak 1981;
Kelly 1980a; Browne 1981b, Maclaine Pont et al. 1980) with
analytical recoveries of > 65 percent and usually > 80 percent.
Apparent residues in untreated controls generally ranged from <0.01
to approximately 0.05 mg/kg depending on the commodity.
A method is also available for measuring total free and
conjugated residues of prochloraz and its major plant metabolites,
N-formyl-N'-1-propyl-N'-[2-(2,4,6-trichlorophenoxy) ethyl] urea
(BTS 44596), N-propyl-N-[2-(2,4,6-trichlorophenoxy) ethyl] urea
(BTS 44595) and conjugated 2,4,6-trichlorophenol (BTS-45186), after
converting all of them to the common moiety 2,4,6-trichlorophenol
(Manley & Snowden 1982a). Any other metabolite containing the
2,4,6-trichlorophenol moiety would probably be measured as well.
Although total residues are actually measured as BTS-45186, residues
are usually expressed as prochloraz, after use of a molecular weight
factor of 1.906.
Thoroughly ground, milled, chopped or minced and mixed samples
are Soxhlet extracted with acetone, filtered, dried by passing through
anhydrous sodium sulphate and evaporated to near dryness by a rotary
evaporator. The sample is refluxed with pyridine hydrochloride at
205°c for one hour to hydrolyse to the BTS-45186 moiety, after which
HCl is added to the sample. The sample is liquid-liquid extracted
after addition of petroleum ether. Potassium hydroxide is added, the
sample partitioned and the aqueous phase collected and acidified with
HCl. The sample is partitioned into toluene for analysis by
electron-capture gas chromatography, utilizing a 20 percent carobowax
20M + 1.7 percent phosphoric acid packed column.
The method was validated on fruits and cereal grains with mean
over-all recoveries of 92 ± 15.9 percent when samples were amended at
0.2-10 mg/kg prochloraz. A 0.05 mg/kg limit of determination is
considered attainable for most samples, although apparent residues of
0.2-0.3 mg/kg may be encountered occasionally, especially in cereal
straw.
With minor variations, this method has been successfully used for
the analysis of cereal grain and straw, cereal grain green plant
tissues, apples, citrus fruit (peel, flesh, whole fruit), orange
juice, rapeseed, rapeseed oil, mushrooms, stone fruit and assorted
fruit with inedible peel (Kelly 1979b; Richards 1981a; Reary 1981a;
Browne & Manley 1982a; Manley & Snowden 1982a). Specifics on
individual crops under actual use conditions are considered earlier in
this evaluation under "Residues Resulting From Supervised Trials" or
under "Fate of Residues."
An analytical method is also available for the determination of
free BTS-45186, which may be a minor component of the residue (Kelly
1980b; Richards 1980e).
No analytical method suitable for enforcement purposes for
products of animal origin was made available.
NATIONAL MAXIMUM RESIDUE LIMITS REPORTED TO THE MEETING
Country Commodity MRL (mg/kg)
France cereal grains 0.05 1
rape seed 0.05 2
The Netherlands 3 Mushrooms 0.05
cereal grains 0.1
Milk 0.01
Meat 0.01
Other products 0 (0.01)
German Federal Republic Cereals 0.5 4
1 Residues of prochloraz alone.
2 Residues included not specified.
3 For MRLs of The Netherlands, the residue is prochloraz
and its metabolites, measured as 2,4,6-trichlorophenol
and expressed as prochloraz. Residue on mushrooms and
for most cereal grain trials conducted in The
Netherlands were for total residues of prochloraz and
its major metabolites.
4 A proposed 0.5 mg/kg NRL for prochloraz and metabolites
has been submitted.
APPRAISAL
Prochloraz is a fungicide in use and/or under development for
control of fungal diseases on a variety of commodities in number of
countries. It is used as a foliar spray, for post-harvest or seed
treatments and is available in a variety of formulations for these
uses, including a prochloraz-manganese complex wettable powder
formulation for sensitive plant species.
Residue data are available from field trials on a number of
commodities in several countries, mostly from foliar applications to
fruit and cereal grains, use in mushroom culture and postharvest
applications to citrus and certain other fruit. Some residue data are
also available for vegetables. Residues were determined either as
2,4,6-trichlorophenol, prochloraz alone or prochloraz and its major
metabolites, mostly the latter over-all. In select cases, data are
available for both prochloraz alone and for prochloraz and its major
metabolites. Apparently the earlier studies were for prochloraz alone,
whereas in later studies total residues were emphasized. Data are
insufficient and too variable to draw a general conclusion on the
ratio of parent compound to parent plus metabolites. For example,
there is little difference in residue measured for citrus or possibly
for mushrooms, but for cereals residues appear to be substantially
higher when total residues are determined.
Residue trials on commodities for which there are known
nationally approved or registered uses were available for watermelon,
apple, grape, cereals, mushrooms and oil-seed rape. Therefore, only
for these is consideration given for MRL estimates. However
"recommended" were available for a number of other commodities and
temporary limits are estimated for those with adequate data. Data were
insufficient for estimation of maximum residue levels for sugarbeets,
sugarbeet leaves, strawberries, almonds, almond hulls, lettuce,
watermelon, apples, pears, rice and grapes.
Information was available on the fate of residues in animals,
plants, the environment and during storage and processing. In animals,
prochloraz is rapidly metabolized from dermal or oral exposure. Most
of the residue is rapidly excreted, with urinary excretion being the
predominant route in most animals tested, although faeces is the
predominant route in dogs. The principal metabolic products in the
urine were 2,4,6-trichlorophenoxyacetic acid and
2,4,6-trichlorophenoxy-ethanol (mostly conjugated). The major plant
metabolites have not been identified in the urine. Although residues
were qualitatively similar in urine or in tissue distribution among
animals studied, tissue residues have not been identified.
The potential for residues in products of animal origin was
investigated in two studies by feeding a lactating goat with
14C-phenyl-labelled prochloraz either by capsule or ad libitum with
14C-prochloraz field-treated wheat straw. The first study represents
the worst case maximum expected theoretical exposure from wheat straw
and the straw feeding is probably more reflective of maximum exposure
under normal feeding conditions.
These goat metabolism/feeding studies give insight into the
maximum potential for residues, although residues in animal tissues
are not identified. It is not known whether urinary metabolites are
the same as those in milk or tissues.
Although straw was used as a worst-case for potential residues,
foraging would appear to be potentially equally important, if not more
so, if not label-restricted, since it can be fed at higher levels in
the diet, as can citrus pulp. However, citrus uses presumably are
intended where peel is discarded. Although wheat or barley grain
residues were relatively low, grain may comprise up to 50 and 80
percent of the diet of poultry and swine, respectively. No analytical
method suitable for enforcement purposes for animal products is
available, or at least known to be validated for that purpose.
Additional information is needed to support a conclusion that the fate
of residues in animals is adequately understood or to estimate maximum
residue levels for products of animal origin.
The fate of Prochloraz in plants was investigated in wheat
(foliar, pre-emergence and seed treatments), citrus (postharvest),
apples (foliar) and sugarbeet (soil uptake). In wheat plants, major
residues at the vegetative stage from foliar treatment were free
BTS-44596, free and conjugated BTS-44595 and 2,4,6-trichlorophenol in
decreasing order, which together accounted for 83 percent of the
terminal residue after 19 days. Less than 2 percent was unchanged
prochloraz and only 0.2 percent was 2,4,6-trichlorophenoxy acetic
acid. No single unidentified residue accounted for more than 6.3
percent of the remainder. Residues appear to be absorbed primarily
within the first 24 hours.
At the harvest stage, only the free trichlorophenol has been
identified in straw or grain, and it occurs as 3-8 percent of the
terminal residue. This, plus tritiated water and other presumably
conjugated but unidentified residues containing the trichlorophenol
moiety, account for 40-70 percent of the terminal residue. No single
remaining unidentified residue fraction accounts for more than 6
percent of the terminal residue.
When applied to wheat seeds or as a pre-emergence drench, most
residue was in or near the root system, with little upward mobility,
and there is little evidence of upward mobility when prochloraz is
applied to foliage although low levels translocate towards the roots.
Only very low levels of residue were found in chaff and straw when
wheat was grown in soil treated with radioactive 3H-prochloraz ten
months earlier. However, sugarbeet seedlings showed low residues when
planted as a rotational crop, but only trace levels were found in the
foliage at maturity and no significant residue in the roots.
In apples, residues migrated into the peel and by 33-63 days
approximately 60-80 percent of the terminal or applied residue was in
the peel, <2 percent on the surface and generally <7 percent of
the applied dose in the flesh, although it can be twice as much.
Qualitatively extractable peel residues were similar to residues found
in wheat at the vegetative stage with approximately 80 percent of the
residues hydrolysable to 2,4,6-trichlorophenol. This, therefore,
accounts for approximately 50-60 percent of the total fruit residue.
Other residues (primarily peel fiber-bound) were not identified, and
although unresolved background accounted for approximately 10 percent
of the whole fruit residue (14 percent of peel extractable), no one
peel extractable metabolite or fraction exceeded approximately 5
percent of the total fruit residues. The fate of residues in plants is
reasonably well understood for the limited number and type of crops
for which there are approved uses. If uses are expanded significantly
to other food groups, additional metabolism studies would be prudent.
Numerous experiments have been conducted on the fate of
prochloraz residues in soil and water. Prochloraz is degraded under
both aerobic and anaerobic conditions with formation of volatile
compounds, probably CO2 and tritiated water. Residues remaining are
predominantly prochloraz, BTS-44596 and BTS-45186, with binding to
upper soil particles increasing with time. The half-life can range
from 90-155 days, depending on conditions. Formation of volatiles is
decreased under anaerobic conditions. Mobility is low in soils,
although low mobility does occur under some conditions, especially for
the metabolites in sand. Prochloraz is stable in water under acidic
conditions, but does degrade under alkaline conditions.
The fate of residues during processing was investigated for wheat
made into bread, postharvest citrus, rapeseed oil and mushrooms after
processing. Residues in bread made from wheat grain with <0.1 mg/kg
prochloraz also showed <0.1 mg/kg residues. Since the limit of
determination is 0.1 mg/kg, no conclusion could be made on the extent
of residue concentration, if any. No information was available for
residues in flour or other milling fractions. Similarly, low residues
were found in malt from prochloraz-treated barley, but no information
was available on the residue level in grain from which it was made.
Residue levels concentrate from 1 to 3 times in oil processed
from mature oilseed rape that has been field-treated with prochloraz.
Residues in canned or dehydrated mushrooms or canning liquor after
pre-emergence treatment with prochloraz were less than in untreated
controls, except for one sample of canned mushrooms with a residue of
0.23 mg/kg. No information was available on residue levels before
canning or dehydration. No conclusion can be drawn on the extent of
residue concentration, if any. When oranges treated postharvest were
processed into "42 percent comminuted orange" or "whole orange
juices", which are used to manufacture various orange drinks,
approximately 70 percent of the residue in the oranges from which it
was processed was lost.
The fate of residues during storage after various postharvest
prochloraz treatments was investigated for citrus, bananas and
avocadoes. Except for one study where free 2,4,6-trichlorophenol was
determined and where treated citrus was stored under a variety of
conditions (dark or light, cold or room temperature and at intervals
ranging from one to 70 days) total residues were very persistent, with
little evidence of a decreasing trend over the varied treatment or
storage conditions. Modest increases (2X) in application
concentrations had little effect on residue levels, approximately
90-95 percent of which were in the peel. There was evidence of
continued gradual translocation into the flesh as the interval from
treatment increases, especially at room temperature. This was also
found in studies on foliar treatments of mango. Residue levels during
storage of prochloraz alone were similar to those for prochloraz and
its major metabolites, suggesting little degradation or conjugation.
Emulsifiable concentrate formulations gave significantly higher
residues than wettable powder formulations and residues in lemons were
higher than those in oranges.
In bananas treated postharvest with prochloraz, no trend of
decreasing residues was observed over a 16-day storage period at
ambient temperature. Residue levels were similar with modest increases
(2X) in treatment rate and, as in the case of citrus, most of the
residue was in the peel.
In avocadoes treated postharvest with prochloraz, residues were
comparable to those from multiple foliar treatments. Approximately
10-30 percent of the residue could occur in the flesh after relatively
short preharvest intervals. The translocation was therefore somewhat
greater than in citrus, bananas and apples.
Photodecomposition of an aqueous prochloraz solution resulted in
the formation of plant metabolite BTS-44596 and towards the end of a
36-day exposure to artificial light, a trace of plant metabolite
BTS-44595. A half-life of approximately 20 days was observed.
Analytical methods are available for enforcement purposes for a
wide variety of plant commodities. Residues of prochloraz alone or
total residues of prochloraz and its major plant metabolites may be
determined, although more of the field trials data were determined by
the latter. Analytical recoveries were generally better than 70
percent. No analytical methods have been validated for residues in
animal products. The analytical methodology for the parent compound
should be suitable and more appropriate for enforcement purposes and
for estimating maximum residue levels (see Report of this Meeting,
Section 2.3).
RECOMMENDATIONS
The Meeting examined residue data from supervised trials
reflecting established or proposed good agricultural practice. From
the data, the Meeting was able to estimate the maximum residue levels
that are likely to occur when prochloraz is used in practice and when
the reported intervals between last application and harvest or other
restrictions are observed. Maximum residue limits are recommended
where registered or approved uses are known and residues are
determined as free prochloraz. Temporary maximum residue limits are
recommended where only recommended uses are available or where total
residues of prochloraz and its metabolites containing the
2,4,6-trichlorophenol moiety are determined. Regardless of the status
of the ADI, these remain temporary pending receipt of information on
approved or registered uses or data on supervised trials with residues
determined as prochloraz.
All estimates apply to foliar applications unless otherwise
indicated.
Estimated Interval from last
Commodity maximum residue treatment to harvest (days)
limits (mg/kg) on which recommendation
Determined and expressed is based and other
as prochloraz restrictions
Barley 0.05** 40
Oats 0.05** 40
Rye 0.05** 40
Wheat 0.05** 40
Barley straw 0.2 40
Oat straw 0.2 40
Rye straw 0.2 40
Wheat straw 0.2 40
Mushrooms 2 2 3
Citrus 5 (TMRL)1, 4 postharvest
Sum of prochloraz
and metabolites expressed
as prochloraz 4
Bananas 5 (TMRL) 1 postharvest
Stone fruits 1 (TMRL) 1 14
Avocado 5 (TMRL) 1, 2 7 (preharvest)
Mango 2 (TMRL) 1, 2 15 (preharvest)
Papaya 1 (TMRL) 1 postharvest
Rapeseed 0.5 (TMRL) 25
** At or about the limit of determination.
1 No information on approved or registered uses.
2 Limit allows for cumulative residues from both preharvest and postharvest
treatments, both of which are good agricultural practice.
3 Limit accommodates applications after casing or after flushes, but not
direct treatment of mushrooms.
4 Temporary regardless of the status of the ADI.
FURTHER WORK OR INFORMATION
Required (by 1985)
1. In order to establish MRLs for products of animal origin, several
requirements remain:
(a) Metabolism studies in animals, including ruminants and
poultry, at sufficiently high dosing levels to permit
identification and quantification of tissue residues.
(b) If metabolism studies in (a) above indicate any possibility
of residues in animal tissues, feeding trials should be
conducted in animals, including ruminants and poultry, in
which tissues are analysed for residues of concern
identified in metabolism studies. Feeding in an appropriate
number of animals should as nearly as possible reflect
maximum levels of weathered residues likely to occur in
practice and, ideally, at an exaggerated level.
(c) A validated analytical method suitable for enforcement
purposes for products of animal origin.
2. Good agricultural practice information for those commodities for
which temporary maximum residue limits are recommended,
preferably for countries in which the residue trials were
conducted or those in close proximity.
3. Additional data on residue trials reflecting good agricultural
practice for commodities for which temporary maximum residue
limits are recommended (except citrus). The additional data
should be concurrently determined as both prochloraz and total
residues of prochloraz and its metabolites containing the
trichlorophenol moiety on the same samples. Sufficient data
should be provided to permit further utilization of residue data
already provided, therefore permitting recommendation of maximum
residue limits expressed as prochloraz.
Desirable
1. Additional national maximum residue limits.
2. Additional processing studies in cereals to permit a conclusion
on the extent, if any, of residue concentration in milling
fractions. Grain used should have residues sufficiently above the
limit of determination for drawing conclusions.
3. Additional processing studies for mushrooms to permit a
conclusion on the extent, if any, of residue concentration.
Mushrooms should be analysed before and after processing.
4. Information on possible interference in prochloraz analysis in
the presence of other pesticides, especially those containing the
trichlorophenol moiety. If interferences are encountered,
confirmatory analytical procedures for prochloraz and metabolites
may be necessary.
REFERENCES - RESIDUES
Browne, P.M. Residues of prochloraz and major metabolites in oranges
1981a following postharvest dip treatment in Australia, 1981. FBC
report RESID/81/72 submitted to FAO by FBC Ltd.
(Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in oranges
1981b following postharvest shower treatment in Spain, 1981. FBC
report RESID/81/72 submitted to FAO by FBC Ltd.
(Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in avocados
1982a following postharvest dip treatment in Australia, 1981. FBC
report RESID/82/2 submitted to FAO by FBC Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in bananas
1982b following post-harvest dip treatment in Australia, 1981. FBC
report RESID/82/7 submitted to FAO by FBC Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in lemons
1982c following post-harvest shower treatment in Spain. FBC report
RESID/82/8 submitted to FAO by FBC Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in winter
1982d rye treated post-emergence with a 40% E.C. formulation in
West Germany, 1981. FBC report RESID/82/25 submitted to FAO
by FBC Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in winter
1982e wheat treated postemergence with three applications of 40%
E.C. formulation in Fed. Rep. Germany, 1981. FBC report
RESID/82/29 submitted to FAO by FBC Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in spring
1982f wheat and barley postemergence with a 40% E.C. formulation
(two or three applications) in West Germany, 1981. FBC
report RESID/82/31 submitted to FAO by FBC Ltd.
(Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in mushrooms
1982g following application of a 50 W formulation in Holland,
1980. FBC report RESID/82/39 submitted to FAO by FBC Ltd.
(Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in processed
1982h mushrooms following application of a 40 E.C. formulation in
France, 1981. FBC report RESID/82/68 submitted to FAO by FBC
Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in
1982i oranges following post-harvest dip treatment in the U.K.,
1981. FBC report RESID/82/83 submitted to FAO by FBC Ltd.
(Unpublished)
Browne, P.M. & Manley, J.D. Analytical method for residues of
1982a prochloraz and major metabolites in orange juice. FBC report
RESID/82/18 submitted to FAO by FBC Ltd. (Unpublished)
Browne, P.M. & Manley, J.D. Residues of prochloraz and major
1982b metabolites in oranges and lemons following postharvest dip
treatment in Italy, 1979. FBC report RESID/82/32 submitted
to FAO by FBC Ltd. (Unpublished)
Browne, P.M. & Manley, J.D. Residues of prochloraz and major
1982c metabolites in mangoes treated with a 50% W.P. formulation
in Australia, 1981. FBC report RESID/82/34 submitted to FAO
by FBC Ltd. (Unpublished)
Campbell, J.K. Residues in milk and tissues of a goat dosed orally
1980 with 14C-prochloraz. Boots report AX-80026 submitted to FAO
by FBC Ltd. (Unpublished)
Campbell, J.K. Residues of prochloraz in milk and tissues of a
1983 lactating goat fed straw containing residues of radioactive
prochloraz. FBC report METAB/83/8 submitted to FAO by FBC
Ltd. (Unpublished)
Campbell, J.K. & Needham, D. Residues in milk and tissues of a goat
1980 dosed orally with 14C-BTS 44596 (major plant metabolite of
prochloraz). Boots report AX-800333 submitted to FAO by FBC
Ltd. (Unpublished)
Churchill, J.H.M. & Longland, R.C. Residues of prochloraz and major
1983a metabolites in bananas following a single postharvest
application with a 45% E.C. prochloraz formulation in South
Africa, 1982. FBC report RESID/83/5 submitted to FAO by FBC
Ltd. (Unpublished)
Churchill, J.H.M. & Longland, R.C. Residues of prochloraz and major
1983b metabolites in avocadoes following application with a 45%
E.C. formulation in South Africa. FBC report RESID/83/10
submitted to FAO by FBC Ltd. (Unpublished)
Churchill, J.H. & Longland, R.C. Residues of prochloraz and major
1983c metabolites in processed mushrooms following application
with a 50% W.P. formulation of prochloraz-Mn complex in
Holland, 1981. FBC report RESID/83/23 submitted to FAO by
FBC Ltd. (Unpublished)
Churchill, J.H.M. & Longland R.C. Residues of prochloraz and major
1983d metabolites in almonds following foliar application with a
50% W.P. formulation in Israel, 1982. FBC report RESID/83/38
submitted to FAO by FBC Ltd. (Unpublished)
Churchill, J.H.M. & Longland, R.C. Residues of prochloraz and major
1983e metabolites in avocadoes following postharvest treatment
with a 45% E.C. formulation in Australia, 1982/83. FBC
report RESID/83/55 submitted to FAO by FBC Ltd.
(Unpublished)
Cron, J. Residues of prochloraz and major metabolites in rapeseed
1982 following a single postemergence application with a 40 E.C.
prochloraz and a prochloraz/carbendazim E.C. formulation in
France 1980/81. FBC report RESID/82/74 submitted to FAO by
FBC Ltd. (Unpublished)
Cron, J.H. & Longland, R.C. Residues of prochloraz and major
1983a metabolites in avocadoes following multiple foliar
application with a 50% W.P. formulation of prochloraz-Mn
complex in Australia, 1981/82. FBC report RESID/82/120
submitted to FAO by FBC Ltd. (Unpublished)
Cron, J.H. & Longland, R.C. Residues of prochloraz and major
1983b metabolites in papayas treated postharvest with a 45% E.C.
formulation in Australia, 1982. FBC report RESID/83/1
submitted to FAO by FBC Ltd. (Unpublished)
Douglas, M.T. & Pell, I.B. Assessment of the ready degradability of
1982 prochloraz. HRC and FBC report METAB/82/44 submitted to FAO
by FBC Ltd. (Unpublished)
Douglas, M.T. & Pell, I.B. Assessment of biodegradability of
1983 prochloraz by pre-adapted sewage organisms. HRC and FBC
report METAB/83/10 submitted to FAO by FBC Ltd.
(Unpublished)
Goto, H. Residues of prochloraz in rice following seed soak or foliar
1980 application with a 25% E.C. formulation in Japan, 1980.
Inst. Environ. Toxicol., Japan, Nissan and FBC report
submitted to FAO by FBC Ltd. (Unpublished)
Haran, G. Identification of a photodecomposition product of BTS-40
1978 542 (prochloraz). Boots report F-78014 submitted to FAO by
FBC Ltd. (Unpublished)
Haran, G. The effect of simulate& sunlight on aqueous solutions of
1980 prochloraz. Boots report F-80010 submitted to FAO by FBC
Ltd. (Unpublished)
Hayto, E.M. BTS-40542 residues in wheat and barley. Boots report AX-
1977a 77002 submitted to FAO by FBC Ltd. (Unpublished)
Hayto, E.M. BTS-40 542 residues in wheat from Italy, 1977. Boots
1977b report AX-79003 submitted to FAO by FBC Ltd. (Unpublished)
Hayto, E.M. BTS-40 542 residues in apples from Thurgarton, U.K. Boots
1977c report AX-77011 submitted to FAO by FBC Ltd. (Unpublished)
Hayto, E.M. BTS-40542 residues in wheat from Holland, 1977. Boots
1978a report Ax-78006 submitted to FAO by FBC Ltd. (Unpublished)
Hayto, E.M. BTS-40542 residues in wheat and barley from France, 1977.
1978b Boots report AX-78020 submitted to FAO by FBC Ltd.
(Unpublished)
Hayto, E.M. BTS-40542 residues in wheat and barley from the United
1979a Kingdom, 1977. Boots report AX-79001 submitted to FAO by FBC
Ltd. (Unpublished)
Hayto, E.M. BTS-40542 residues in apples from Thurgarton, U.K., 1977.
1979b Boots report AX-79005 submitted to FAO by FBC Ltd.
(Unpublished)
Hayto, E.M. BTS-40542 residues in cereals from Fed. Rep. Germany,
1979c 1977. Boots report AX-79008 submitted to FAO by FBC Ltd.
(Unpublished)
Hayto, E.M. BTS-40542 and dicloran residues in lettuces from Holland,
1979d 1978. Boots report AX-79010 submitted to FAO by FBC Ltd.
(Unpublished)
Heinanen, E. Investigation certification for residues of prochloraz in
1983 spring wheat from Finland, 1982. VML report 2940, 2942/82
submitted to FAO by FBC Ltd. (Unpublished)
Housden, M.C. Residues of prochloraz and major metabolites in grain
1982a treated post-emergence with a 45% E.C. formulation in
Belgium, 1981. FBC report RESID/82/1 submitted to FAO by FBC
Ltd. (Unpublished)
Housden, M.C. Residues of prochloraz and major metabolites in
1982b mushrooms following application of a 50W formulation in
Australia, 1981/82. FBC report RESID/82/48 submitted to FAO
by FBC Ltd. (Unpublished)
Housden, M.C. Residues of prochloraz and major metabolites in cereals
1982c treated post-emergence with a 45% E.C. formulation (single
or double application) in Denmark, 1982. FBC report
RESID/82/111 submitted to FAO by FBC Ltd. (Unpublished)
Housden, M.C. & Longland, R.C. Decline study of residues of prochloraz
1983 and major metabolites in winter barley and wheat treated
once with a prochloraz/carbendazim suspension concentrate
formulation in Fed. Rep. Germany, 1982. FBC report
RESID/83/19 submitted to FAO by FBC Ltd. (Unpublished)
Housden, M.C. & Whiteoak, R.J. Residues of prochloraz and metabolites
1982 in mushrooms following single and multiple applications
(50w formulation) in the U.K. 1981 and 1982. FBC report
RESID/82/63 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. BTS 40 542 residues in cereals from Austria, 1977. Boots
1979a report AX 79006 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of BTS 40
1979b 542, BTS 44 596 and BTS 45 186 in grain. Boots report AX
79007 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979c (BTS 40 542), BTS 44 595, BTS 44 596 and BTS 45 186 in wheat
grain and straw from Fed. Rep. Germany, 1978. Boots report
AX 79015 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979d (BTS 40 542), BTS 44 595, BTS 44 596 and BTS 45 186 in wheat
grain and straw from Italy, 1978. Boots report AX 79017
submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979e (BTS 40 542), BTS 44 595, BTS 44596 and BTS 45 186 in wheat
grain and straw from The Netherlands, 1978. Boots report AX
79 017 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979f (BTS 40 542), BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in cereal grain and straw from
Austria, 1978. Boots report AX 79 020 submitted to FAO by
FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979g (BTS 40 542), BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in wheat and barley grain from
Denmark, 1978. Boots report AX 79021 submitted to FAO by FBC
Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979h (BTS 40 542), BTS 44 595, BTS 44 596 and BTS 45 186 in wheat
and barley grain and straw from France, 1978. Boots report
AX 79023 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. Persistence and uptake of prochloraz in Spanish oranges
1980a following a post-harvest dip. Boots report AX 80003
submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. BTS 45 186 residues in prochloraz-treated wheat grain from
1980b France, 1977. Boots report AX 80010 submitted to FAO by FBC
Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1980c (BTS 40 542) and metabolites containing the
2,4,6-trichlorophenyl moiety in wheat grain and straw from
The Netherlands, 1979. Boots report AX 80025 submitted to
FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The metabolism of 3H-prochloraz in mature wheat grain and
1980d straw. Boots report AX 80028 submitted to FAO by FBC Ltd.
(Unpublished)
Kelly, I.D. The metabolism and distribution of BTS 46828 applied to
1982a the fruit of field-growing apples. FBC report METAB/82/18
submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The hydrolysis of prochloraz in aqueous solution under
1982b acid, neutral and basic conditions. FBC report METAB/82/28
submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. & Krepski, W.J. The metabolism of prochloraz by wheat
1980 grain and straw. Boots report AX 80009 submitted to FAO by
FBC Ltd. (Unpublished)
Kesterton, M.S. Distribution of radio-labelled residues in field soil
1978 after application of 3H-BTS 40542. Boots report AX 78010
submitted to FAO by FBC Ltd. (Unpublished)
Kesterton, M.S. Report on the aerobic metabolism of prochloraz in
1981a soil over a period of 12 months. Boots report AX 81002
submitted to FAO by FBC Ltd. (Unpublished)
Kesterton, M.S. Report on the anaerobic metabolism of prochloraz in
1981b soil. Boots report AX 81004 submitted to FAO by FBC Ltd.
(Unpublished)
Kesterton, M.S. & Newby, S.E. A study of the leaching of prochloraz in
1980a soil. Boots report AX 80008 submitted to FAO by FBC Ltd.
(Unpublished)
Kesterton, M.S. & Newby, S.E. Adsorption and desorption studies of
1980b prochloraz in soil. Boots report AX 80012 submitted to FAO
by FBC Ltd. (Unpublished)
Kesterton, M.S. & Newby, S.E. Leaching study of prochloraz aged in a
1981 silty clay loam soil. Boots report AX 81003 submitted to FAO
by FBC Ltd. (Unpublished)
Krepski, W.J. The uptake of 14C-prochloraz and 3H-prochloraz soil
1981a residues into wheat. Boots report AX 81001 submitted to FAO
by FBC Ltd. (Unpublished)
Krepski, W.J. The metabolism of 14C-prochloraz and 14C-BTS 46828 by
1981b apples grown under glass. Boots and FBC report submitted to
FAO by FBC Ltd. (Unpublished)
Krepski, W.J. Translocation of 14C-prochloraz applied as a liquid
seed
1982 dressing in wheat grown to maturity. FBC report METAB/81/30
submitted to FAO by FBC Ltd. (Unpublished)
Leake, C.R. & Lines, D. The leaching of prochloraz and its major
1981 metabolites in four soil types using TLC. FBC report
METAB/81/35 submitted to FAO by FBC Ltd. (Unpublished)
Longland, R.C. Analysis of residues of prochloraz and major
1983a metabolites in grain and straw from Sweden following
application of 45% E.C., 1982. FBC report RESID/82/114
submitted to FAO by FBC Ltd. (Unpublished)
Longland, R.C. Residues of prochloraz and major metabolites in
1983b sugarbeet following application of a 40% E.C. formulation in
Italy, 1981. FBC report RESID/83/58 submitted to FAO by FBC
Ltd. (Unpublished)
Longland, R.C. & Churchill, J.H. Residues of prochloraz and major
1983 metabolites in mangoes following foliar treatment with 50%
W.P. and postharvest treatment with 40% E.C. formulations in
Israel, 1982. FBC report RESID/83/7 submitted to FAO by FBC
Ltd. (Unpublished)
Maclaine Pont, M.A., Vogelzang, H.P. & Siegmann-Knoester, K.C. The
1980 residue analysis of prochloraz in combination with dicloran.
Asepta-fabrik and Boots report AX 80020/2 submitted to FAO
by FBC Ltd. (Unpublished)
Maier-Bode, H. The leaching of prochloraz in three standard soils from
1980 Fed. Rep. Germany. HMB and FBC report submitted to FAO by
FBC Ltd. (Unpublished)
Manley, J.D. & Snowden, P.J. Analytical method for residues of
1982a prochloraz and major metabolites in miscellaneous fruit,
cereal and vegetable crops (improved method). FBC report
RESID/82/88 submitted to FAO by FBC Ltd. (Unpublished)
Manley, J.D. & Snowden, P.J. Residues of prochloraz and major
1982b metabolites in oranges and processed oranges following
commercial scale postharvest brush treatment with prochloraz
(40 E.C.) in South Africa, 1982. FBC report RESID/82/89
submitted to FAO by FBC Ltd. (Unpublished)
Manley, J.D. & Snowden, P.J. Residues of prochloraz and major
1982c metabolites in rapeseed following treatment with a 40% E.C.
formulation in the U.K., 1981/1982. FBC report RESID/82/90
submitted to FAO by FBC Ltd. (Unpublished)
McDougall, J. The metabolism of 3H-BTS 40 542 in wheat at a
vegetative
1979 growth stage. Boots report AX 79009 submitted to FAO by FBC
Ltd. (Unpublished)
McDougall, J. The uptake and translocation of 3H-prochloraz in wheat.
1980a Boots report AX 80007 submitted to FAO by FBC Ltd.
(Unpublished)
McDougall, J. The relative uptake and persistence of prochloraz and
1980b BTS 46 828 in apples following treatment with 14C-
prochloraz and 14C-BTS 46 828. Boots report AX 80015
submitted to FAO by FBC Ltd. (Unpublished)
McGibbon, A.S. Uptake of prochloraz residues by sugarbeet from
1982 prochloraz-treated soil under field conditions. FBC report
METAB/82/27 submitted to FAO by FBC Ltd. (Unpublished)
Newby, S.E. The laboratory decline of prochloraz in two soils, a sandy
1982 loam and a silty clay loam, under sterile conditions. FBC
report METAB/82/41 submitted to FAO by FBC Ltd.
(Unpublished)
Peatman, M.H. & Snowden, P.J. Residues of prochloraz and major
1982 metabolites in rapeseed treated with the 40% E.C.
formulation in France, 1982. FBC report RESID/82/116
submitted to FAO by FBC Ltd. (Unpublished)
Peatman, M.H. & Snowden, P.J. Residues of prochloraz and major
1983 metabolites in rapeseed treated with a 45% E.C. formulation
in Denmark and Sweden, 1982. FBC report RESID/83/29
submitted to FAO by FBC Ltd. (Unpublished)
Reary, J.B. Analytical method for free and conjugated residues of
1981a prochloraz, BTS 44595, BTS 44 596 and BTS 45 186 in cereals
at different growth stages. FBC report RESID/81/13 submitted
to FAO by FBC Ltd. (Unpublished)
Reary, J.B. Residues of prochloraz and major metabolites in summer
1981b wheat treated post-emergence with a 40% E.C. formulation
(two or three applications) in Fed. Rep. Germany, 1980. FBC
report RESID/81/14 submitted to FAO by FBC Ltd.
(Unpublished)
Reary, J.B. Residues of prochloraz and major metabolites in cereals
1981c treated post-emergence with a 45% E.C. formulation in
Holland, 1980. FBC report RESID/81/46 submitted to FAO by
FBC Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980a prochloraz (BTS 40 542) BTS 44 595 and BTS 44 596 and
conjugated residues of BTS 45 186 in wheat and barley,
France, 1979. Boots report AX 80005 submitted to FAO by FBC
Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980b prochloraz (BTS 40 542), BTS 44 595 and BTS 44 596 and
conjugated residues of BTS 45 186 in Spanish oranges
following a postharvest application, 1980. Boots report AX
80021 submitted to FAO by FBC Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980c prochloraz (BTS 40 542), BTS 44 595, BTS 44 596 and
conjugated residues of BTS 45 186 in wheat and barley, U.K.,
1979. Boots report AX 80022 submitted to FAO by FBC Ltd.
(Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980d prochloraz (BTS 40 542), BTS 44 595, BTS 44 596 and
conjugated residues of BTS 45 186 in wheat and barley,
Denmark, 1979. Boots report AX 80023 submitted to FAO by FBC
Ltd (Unpublished)
Richards, M.E. BTS 45 186 residues in oranges treated with prochloraz,
1980e Spain, 1980. Boots report AX 80027 submitted to FAO by FBC
Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980f prochloraz, BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in Spanish oranges following a
postharvest application, 1980. Report No. 2. Boots report AX
80030 submitted to FAO by FBC Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980g prochloraz, BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in winter wheat, Fed. Rep. Germany,
1979. Boots report AX 80032 submitted to FAO by FBC Ltd.
(Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1981a prochloraz, BTS 44 595 and BTS 44596 and conjugated residues
of BTS 45 186 in grain and straw. Boots and FBC report AX
800 35 submitted to FAO by FBC Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1981b prochloraz, BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in winter barley, Fed. Rep. Germany,
1980. Boots and FBC report AX 81005 submitted to FAO by FBC
Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1981c prochloraz, BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in mushrooms, U.K., 1980. FBC report
METAB/81/17 submitted to FAO by FBC Ltd. (Unpublished)
Snowden, P.J. Residues of prochloraz and major metabolites in stone
1983 fruit following foliar applications of prochloraz (50 E. and
40 E.C.) in Europe (1982) and South Africa (1981/2 and
1982/3). FBC report RESID/83/57 submitted to FAO by FBC Ltd.
(Unpublished)
Snowden, P.J. & Manley, J.D. Residues of prochloraz and major
1983 metabolites in Tambors oranges following commercial scale
postharvest brush treatment with prochloraz (45% E.C.) in
South Africa, 1982. FBC report RESID/83/9 submitted to FAO
by FBC Ltd. (Unpublished)
Somerville, L. The analysis of prochloraz residues in cereals. Boots
1980 report AX 80020/1 submitted to FAO by FBC Ltd. (Unpublished)
Stockbauer, I. Leaching of Sportak PF (prochloraz and carbendazim) in
1983 German soils. ASU and FBC report submitted to FAO by FBC
Ltd. (Unpublished)
Wang, S.S. Residues of prochloraz in grapes, apples and watermelon
1982 following foliar application with a 25% E.C. formulation in
Taiwan, Taiwan Plant Protection Centre report submitted to
FAO by FBC Ltd. (Unpublished)
Whiteoak, R.J. Residues of prochloraz in mushrooms following multiple
1981 applications of a 50% W.P. formulation in the U.K., 1980/81
(ADAS trial results), FBC report RESID/81/41 submitted to
FAO by FBC Ltd. (Unpublished)
Whiting, K.C. & Dickinson, W. The stability of the manganese complex
1979 of prochloraz in water. Boots report F 79005 submitted to
FAO by FBC Ltd. (Unpublished)
See Also:
Toxicological Abbreviations
Prochloraz (JMPR Evaluations 2001 Part II Toxicological)
 Molecular formula C15 H16 Cl3 N3 O2
Other information on Identity and Properties
Molecular weight: 376.5
State The pure material is a colourless, odourless
crystalline solid; the technical material is a
white to brown solid.
Melting point 38.5 - 41.0°C
Vapour pressure 0.57 × 10-9 Torr at 20°C
Solubility At 25°C 0.055 g/l in water; ca. 16 g/l in
kerosene; ca. 2 500 g/l in chloroform, xylene,
diethyl ether and toluene; ca. 3 500 g/l in
acetone.
Stability The stability in aqueous solution is pH-dependent,
prochloraz being more stable under slightly acidic
conditions than under slightly alkaline
conditions. Hydrolysis under alkaline conditions
leads to the formation of
N-propyl-N-2-(2,4,6-trichlorophenoxy) ethylamine,
the degradation following first order kinetics. At
pH 4.95 and pH 6.98 and 22°C, no degradation of
prochloraz was observed after 30 days. At pH 9.18
and 22°C, the half-life was 78.9 days. Prochloraz
tends to decompose on prolonged heating at high
temperatures (ca. 200°C).
Further information on the stability of prochloraz
in soil, water, during processing and storage and
on photodecomposition can be found under "Fate of
Residues".
Technical Material and Impurities
The Meeting was provided with information on impurities in the
technical material, all of which were said to range from < 0.2
percent to < 1.2 percent. No evidence has been found for the
presence of trichlorodibenzo-p-dioxins or tetrachlorobenzofurans and
all analytical procedures employed have failed to detect (i.e. less
than 1 ppb) any of the notably toxic
2,3,7,8-tetrachlorodibenzo-p-dioxin.
Identity of Prochloraz-Manganese Complex
Chemical Name
tetrakis-{1-[N-propyl-N-[2-(2, 4, 6-trichlorophenoxy)ethyl]]
carbamoylimidazole} manganese (II) chloride complex.
Synonyms
Prochloraz-manganese complex is the trivial name given to the 4:1
manganese coordination complex of prochloraz. FBC Code No. BTS 46828
or FBC 31114.
Structural formula
Molecular formula C15 H16 Cl3 N3 O2
Other information on Identity and Properties
Molecular weight: 376.5
State The pure material is a colourless, odourless
crystalline solid; the technical material is a
white to brown solid.
Melting point 38.5 - 41.0°C
Vapour pressure 0.57 × 10-9 Torr at 20°C
Solubility At 25°C 0.055 g/l in water; ca. 16 g/l in
kerosene; ca. 2 500 g/l in chloroform, xylene,
diethyl ether and toluene; ca. 3 500 g/l in
acetone.
Stability The stability in aqueous solution is pH-dependent,
prochloraz being more stable under slightly acidic
conditions than under slightly alkaline
conditions. Hydrolysis under alkaline conditions
leads to the formation of
N-propyl-N-2-(2,4,6-trichlorophenoxy) ethylamine,
the degradation following first order kinetics. At
pH 4.95 and pH 6.98 and 22°C, no degradation of
prochloraz was observed after 30 days. At pH 9.18
and 22°C, the half-life was 78.9 days. Prochloraz
tends to decompose on prolonged heating at high
temperatures (ca. 200°C).
Further information on the stability of prochloraz
in soil, water, during processing and storage and
on photodecomposition can be found under "Fate of
Residues".
Technical Material and Impurities
The Meeting was provided with information on impurities in the
technical material, all of which were said to range from < 0.2
percent to < 1.2 percent. No evidence has been found for the
presence of trichlorodibenzo-p-dioxins or tetrachlorobenzofurans and
all analytical procedures employed have failed to detect (i.e. less
than 1 ppb) any of the notably toxic
2,3,7,8-tetrachlorodibenzo-p-dioxin.
Identity of Prochloraz-Manganese Complex
Chemical Name
tetrakis-{1-[N-propyl-N-[2-(2, 4, 6-trichlorophenoxy)ethyl]]
carbamoylimidazole} manganese (II) chloride complex.
Synonyms
Prochloraz-manganese complex is the trivial name given to the 4:1
manganese coordination complex of prochloraz. FBC Code No. BTS 46828
or FBC 31114.
Structural formula
 Molecular formula C15 H16 Cl3 N3 O2)4 MnCl2
Other information on Identity and Properties
Molecular weight 1 632
Melting point 141-142.5°C
Solubility At 25°C 4 g/l in water, 7 g/l in acetone.
Stability The manganese complex is quickly dissociated in
water to give prochloraz. A suspension of the 50
percent wettable powder of the manganese complex
in distilled water has been shown to dissociate to
free prochloraz and manganese chloride to the
extent of approximately 55 percent in four hours
at 25°C. This process is unlikely to be affected
greatly at pH 6 to 9 Whiting & Dickson 1979.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
Mouse
After a single oral dose of 14C-phenyl labelled prochloraz at
100 mg/kg b.w. was administered to a group of male and female mice,
the over-all recovery of the given radioactivity was virtually
complete within 72h. Total recovery was complete (104 percent) with
urinary excretion (63 percent) being the major route. There were no
significant sex differences in the rate or route of excretion. Tissue
residues were highest in liver (5-7 mg/kg) and lowest in muscle,
genitals, eyes, spleen and fat (0.5-1.0 mg/kg). In all other tissues
the average value was below 2.5 mg/kg (Needham 1982a).
Rat
The excretion rate of prochloraz was monitored for 96 h in three
male and three female rats administered a single oral dose of
14C-phenyl-labelled prochloraz (91 mg/kg b.w.). The over-all recovery
of the administered radioactivity was 92.7 percent in male and 93.7
percent in female rate, urinary excretion being the predominant route.
The mean radioactivity was 61.7 percent in the urine and 30.8 percent
in the faeces of male rats. It was 54.8 percent in urine and 38.5
percent in the faeces of female rats. Highest residues, 96 h after
dosing, were found in the liver (3.7-6.2 mg/kg) and kidney (1.5-2.5
mg/kg), the tissues of female rats having higher residues (Needham &
Campbell 1980).
The chemokinetics were studied over a 96 h period, using an oral
dose of 100 mg/kg b.w. 14C-imidazole-radiolabelled prochloraz. Male
rats excreted the administered radioactivity almost quantitatively,
with approximately 83 percent in the urine, 9 percent in faeces and
2.4 percent in the expired air. Female rats excreted 56 percent in
urine, 21 percent in faeces and 1.3 percent in expired air. Plasma
radioactivity levels reached a peak in male rate approximately 4 h
after dosing, whereas the levels in female rats remained relatively
uniform but much lower. Liver radioactivity levels were significantly
raised within 15 min. and, in the kidney, 30 min. after dosing. They
generally remained constant for at least 8 h. Significant accumulation
occurred in brain and adipose tissues, which persisted over 1-8 h and
then declined markedly between 8 and 24 h. The levels of radioactivity
in the gastrointestinal tract, 24 h after dosing were significantly
higher in females compared to males. In general, plasma and tissues
showed a decline in radioactivity levels over the 96 h period, after
which time they reached small, though still significant, levels
(Turner & Gilbert 1977).
14C-imidazole ring-prochloraz was administered orally in another
study at 25 mg/kg b.w./day for 24 days. Residues of prochloraz
appeared to reach a plateau at about 15 days in the adrenals, ovaries,
female thyroid and male plasma, while other tissue levels continued to
increase over the entire 24-day period. The highest residues after
administration were found in the liver (20.88 ppm males and 25.03 ppm
females), and the lowest in the fat (2.82 ppm males and 3.72 ppm
females). The radioactivity was eliminated slowly from tissues,
significant residues being found after 96 h. Liver and plasma residues
had a half-life of less than three days and that of muscle was
approximately 17 days in males and 30 days in females (Hamilton 1978a)
3H-phenyl-labelled prochloraz was administered daily in another
study as repeated oral doses of 25 mg/kg for a 20-day period. Plasma
and tissue radioactivity were measured at specified intervals
throughout the period of treatment and at 96 h after the last daily
dose. Plasma levels were higher in males than in females. Tissue
levels for both sexes rose significantly for the first seven days of
dosing and thereafter rose only slightly for the remainder of the
dosing period. Highest levels of radioactivity were found in liver and
kidney and lowest in muscle and fat. Within 96h after treatment, the
radiolabelled phenoxy moiety of prochloraz was rapidly eliminated from
the body without leaving significant tissue residues (Boardman 1979).
Dog
A group of two male and two female dogs was given a single oral
dose of 14C-imidazole-labelled prochloraz at 20 mg/kg b.w., while a
second group received the same amount of 3H-phenyl-labelled
prochloraz. There was a marked difference between the plasma
concentrations of 14C and 3H-labelled prochloraz, suggesting that
the pesticide was metabolized by fission at the chain linking the
imidazole and trichlorophenol moieties. The dogs were killed 24 h
after the administration of the radioactive prochloraz and tissues
subsequently assayed for radioactivity. Both 14C- and 3H-radio-
labelled compounds showed high levels in bile and liver (Hamilton
1978b).
The distribution and excretion were studied in other groups of
male and female dogs following a single oral dose of 18 mg/kg b.w. of
14C-phenyl ring-labelled prochloraz. Peak plasma radioactivity
occurred 8-24 h after dosing and plasma half life was approximately 72
h. Excretion was rapid, 60 percent of the dose being eliminated in the
first 24 h; the over-all recovery after 96 hours was 96 percent with
faecal excretion as the major route (64 percent of the total dose). No
sex differences were noted in the rate of excretion or in the
magnitude of the tissue residues. Highest residues were found in the
liver and kidney (7.6 and 5.6 mg/kg, respectively) and lowest in bone,
cerebellum and CSF (less and 1 mg/kg). High residues were present in
the bile, indicating that biliary excretion represents a significant
route of elimination in dogs. This finding agrees with the high
percentage of radioactivity found in the faeces (Needham & Campbell
1982).
Goat
One lactating goat was treated with two oral doses of 14C-phenyl
prochloraz at 60 mg/kg b.w. given at a 13-day interval. Milk residues
after the first dose were highest 8 h after dosing (0.04 ppm) and
declined to 0.01 ppm within 48 h. Plasma residues were highest at 24 h
(0.33 mg/l), declining to 0.03 mg/l at 13 days. Twenty-four h after
the second dose, the highest tissue residues were found in the liver
(1.67 mg/kg), while residues in muscle were minimal (0.03 mg/kg)
(Campbell 1980).
Biotransformation
The biotransformation was studied in a group of male and female
rats given an oral dose of 100 mg/kg b.w. 14C-imidazole ring-or
14C-phenyl ring-labelled prochloraz as a suspension in gum acacia or
dissolved in maize oil. A similar regimen was followed in separate
experiments with mice and dogs, except that only 18 mg/kg b.w. of the
labelled prochloraz was administered to dogs. Prochloraz was
extensively metabolized in the rat, with no unchanged compound being
excreted in the urine. The qualitative and quantitative profiles
depicted in Figure 1 suggest that metabolism proceeds via cleavage of
the imidazole ring into 2-carbon fragments, followed by hydroxylation
of the phenyl ring and/or side chain hydrolysis to form more polar
compounds. Two main metabolites: 2-(2,4, 6, trichlorophenoxy) ethanol
(BTS 3037); 2,4,6, trichlorophenoxyacetic acid (BTS 9608) and five
other minor metabolites were identified. The two main metabolites
accounted for about 80 percent of the urinary metabolites. A similar
metabolic pattern was found in the urine of mice, dogs and the female
goat (Needham 1982b).
Molecular formula C15 H16 Cl3 N3 O2)4 MnCl2
Other information on Identity and Properties
Molecular weight 1 632
Melting point 141-142.5°C
Solubility At 25°C 4 g/l in water, 7 g/l in acetone.
Stability The manganese complex is quickly dissociated in
water to give prochloraz. A suspension of the 50
percent wettable powder of the manganese complex
in distilled water has been shown to dissociate to
free prochloraz and manganese chloride to the
extent of approximately 55 percent in four hours
at 25°C. This process is unlikely to be affected
greatly at pH 6 to 9 Whiting & Dickson 1979.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
Mouse
After a single oral dose of 14C-phenyl labelled prochloraz at
100 mg/kg b.w. was administered to a group of male and female mice,
the over-all recovery of the given radioactivity was virtually
complete within 72h. Total recovery was complete (104 percent) with
urinary excretion (63 percent) being the major route. There were no
significant sex differences in the rate or route of excretion. Tissue
residues were highest in liver (5-7 mg/kg) and lowest in muscle,
genitals, eyes, spleen and fat (0.5-1.0 mg/kg). In all other tissues
the average value was below 2.5 mg/kg (Needham 1982a).
Rat
The excretion rate of prochloraz was monitored for 96 h in three
male and three female rats administered a single oral dose of
14C-phenyl-labelled prochloraz (91 mg/kg b.w.). The over-all recovery
of the administered radioactivity was 92.7 percent in male and 93.7
percent in female rate, urinary excretion being the predominant route.
The mean radioactivity was 61.7 percent in the urine and 30.8 percent
in the faeces of male rats. It was 54.8 percent in urine and 38.5
percent in the faeces of female rats. Highest residues, 96 h after
dosing, were found in the liver (3.7-6.2 mg/kg) and kidney (1.5-2.5
mg/kg), the tissues of female rats having higher residues (Needham &
Campbell 1980).
The chemokinetics were studied over a 96 h period, using an oral
dose of 100 mg/kg b.w. 14C-imidazole-radiolabelled prochloraz. Male
rats excreted the administered radioactivity almost quantitatively,
with approximately 83 percent in the urine, 9 percent in faeces and
2.4 percent in the expired air. Female rats excreted 56 percent in
urine, 21 percent in faeces and 1.3 percent in expired air. Plasma
radioactivity levels reached a peak in male rate approximately 4 h
after dosing, whereas the levels in female rats remained relatively
uniform but much lower. Liver radioactivity levels were significantly
raised within 15 min. and, in the kidney, 30 min. after dosing. They
generally remained constant for at least 8 h. Significant accumulation
occurred in brain and adipose tissues, which persisted over 1-8 h and
then declined markedly between 8 and 24 h. The levels of radioactivity
in the gastrointestinal tract, 24 h after dosing were significantly
higher in females compared to males. In general, plasma and tissues
showed a decline in radioactivity levels over the 96 h period, after
which time they reached small, though still significant, levels
(Turner & Gilbert 1977).
14C-imidazole ring-prochloraz was administered orally in another
study at 25 mg/kg b.w./day for 24 days. Residues of prochloraz
appeared to reach a plateau at about 15 days in the adrenals, ovaries,
female thyroid and male plasma, while other tissue levels continued to
increase over the entire 24-day period. The highest residues after
administration were found in the liver (20.88 ppm males and 25.03 ppm
females), and the lowest in the fat (2.82 ppm males and 3.72 ppm
females). The radioactivity was eliminated slowly from tissues,
significant residues being found after 96 h. Liver and plasma residues
had a half-life of less than three days and that of muscle was
approximately 17 days in males and 30 days in females (Hamilton 1978a)
3H-phenyl-labelled prochloraz was administered daily in another
study as repeated oral doses of 25 mg/kg for a 20-day period. Plasma
and tissue radioactivity were measured at specified intervals
throughout the period of treatment and at 96 h after the last daily
dose. Plasma levels were higher in males than in females. Tissue
levels for both sexes rose significantly for the first seven days of
dosing and thereafter rose only slightly for the remainder of the
dosing period. Highest levels of radioactivity were found in liver and
kidney and lowest in muscle and fat. Within 96h after treatment, the
radiolabelled phenoxy moiety of prochloraz was rapidly eliminated from
the body without leaving significant tissue residues (Boardman 1979).
Dog
A group of two male and two female dogs was given a single oral
dose of 14C-imidazole-labelled prochloraz at 20 mg/kg b.w., while a
second group received the same amount of 3H-phenyl-labelled
prochloraz. There was a marked difference between the plasma
concentrations of 14C and 3H-labelled prochloraz, suggesting that
the pesticide was metabolized by fission at the chain linking the
imidazole and trichlorophenol moieties. The dogs were killed 24 h
after the administration of the radioactive prochloraz and tissues
subsequently assayed for radioactivity. Both 14C- and 3H-radio-
labelled compounds showed high levels in bile and liver (Hamilton
1978b).
The distribution and excretion were studied in other groups of
male and female dogs following a single oral dose of 18 mg/kg b.w. of
14C-phenyl ring-labelled prochloraz. Peak plasma radioactivity
occurred 8-24 h after dosing and plasma half life was approximately 72
h. Excretion was rapid, 60 percent of the dose being eliminated in the
first 24 h; the over-all recovery after 96 hours was 96 percent with
faecal excretion as the major route (64 percent of the total dose). No
sex differences were noted in the rate of excretion or in the
magnitude of the tissue residues. Highest residues were found in the
liver and kidney (7.6 and 5.6 mg/kg, respectively) and lowest in bone,
cerebellum and CSF (less and 1 mg/kg). High residues were present in
the bile, indicating that biliary excretion represents a significant
route of elimination in dogs. This finding agrees with the high
percentage of radioactivity found in the faeces (Needham & Campbell
1982).
Goat
One lactating goat was treated with two oral doses of 14C-phenyl
prochloraz at 60 mg/kg b.w. given at a 13-day interval. Milk residues
after the first dose were highest 8 h after dosing (0.04 ppm) and
declined to 0.01 ppm within 48 h. Plasma residues were highest at 24 h
(0.33 mg/l), declining to 0.03 mg/l at 13 days. Twenty-four h after
the second dose, the highest tissue residues were found in the liver
(1.67 mg/kg), while residues in muscle were minimal (0.03 mg/kg)
(Campbell 1980).
Biotransformation
The biotransformation was studied in a group of male and female
rats given an oral dose of 100 mg/kg b.w. 14C-imidazole ring-or
14C-phenyl ring-labelled prochloraz as a suspension in gum acacia or
dissolved in maize oil. A similar regimen was followed in separate
experiments with mice and dogs, except that only 18 mg/kg b.w. of the
labelled prochloraz was administered to dogs. Prochloraz was
extensively metabolized in the rat, with no unchanged compound being
excreted in the urine. The qualitative and quantitative profiles
depicted in Figure 1 suggest that metabolism proceeds via cleavage of
the imidazole ring into 2-carbon fragments, followed by hydroxylation
of the phenyl ring and/or side chain hydrolysis to form more polar
compounds. Two main metabolites: 2-(2,4, 6, trichlorophenoxy) ethanol
(BTS 3037); 2,4,6, trichlorophenoxyacetic acid (BTS 9608) and five
other minor metabolites were identified. The two main metabolites
accounted for about 80 percent of the urinary metabolites. A similar
metabolic pattern was found in the urine of mice, dogs and the female
goat (Needham 1982b).
 Effects on Enzymes and Other Biochemical Parameters
In a series of investigations in male rats and mice prochloraz
was found to be an inducer of hepatic mixed function oxidases (MFO).
Rats were dosed orally with prochloraz at 10 and 100 mg/kg b.w. twice
daily for four days and killed 18 h after the last dose. Potent
induction of MFO occurred at the higher dose, but only a marginal
effect was seen at the low dose. Relative liver weight and microsomal
protein concentrations were increased at the high dose. The induction
spectrum of MFO was similar to that of phenobarbitone, with the levels
of cytochrome P-450, being increased by both dosages (Needham 1983a).
A similar MFO induction was noted when male rats were fed
prochloraz in their diet at 2 500 ppm for seven days. Rats fed a lower
dose (100 ppm) showed only a small increase in cytochrome P-450
(Riviere 1983).
The same pattern of hepatic MFO induction was observed in mice
after oral administration of doses of 10 and 100 mg/kg b.w. twice
daily for four days (Challis & Campbell 1983). Prochloraz was an
inducer of MFO following dietary administration at 325 and 1 300 mg/kg
in the diet for two weeks. When the compound was administered at 80
mg/kg for periods in excess of two weeks, the effect was marginal,
indicating that this concentration is close to the threshold level for
induction (Needham 1983b).
Effect of Dog Gastric Juice or Plasma on Prochloraz
In vitro studies showed prochloraz to be completely stable
after incubation with dog gastric juice or plasma for 30 min. at 37°C
or for 4 h at 37°C. Less than 2 percent underwent hydrolysis. The
initial step in the metabolism of prochloraz is therefore likely to
occur in the liver (Needham 1980).
TOXICOLOGICAL STUDIES
Special Study on Reproduction
Rat
Groups of rats (30 rats of each sex for the Fo generation and 25
of each sex for the F1 generation) were fed a diet containing 0,
37.5, 150 or 625 ppm prochloraz (nine weeks for Fo and eight weeks for
F1) prior to mating and throughout mating, gestation and lactation.
Two litters per generation were produced for two generations.
Observations included growth, food consumption, mortality and the
usual reproduction indices: mating, fecundity, male and female
fertility, gestation, lactation, pup mortality, litter and mean pup
weights, viability and terminal studies. In the males (Fo), receiving
625 ppm in the diet, a prolonged aggression was observed when rehoused
after both matings; this was not apparent among F1 males. Among Fo
females, the following overt clinical signs were noted in late
gestation and/or in the perinatal period: hunched posture, walking on
toes, piloerection and pallor. Sporadic mortality was recorded at the
different dose levels and was generally related to dystocia. Food
intake during the premating period showed no consistent dosage
relationship for all animals of both generations. However, consumption
was generally lower than that of the controls. Mean body weight gains
were depressed only in the premating period in the Fo animals of both
sexes, given 625 ppm, the same trend being noted during gestation.
There were no significant effects associated with mating performance
and pregnancy rate in the treated animals. Some adverse effects on
gestation periods were noted only at 625 ppm in both generations, with
a small number of females dying or being killed after exhibiting signs
of dystocia. There was also an increase in the proportion of females
with gestation periods in excess of 22 days.
A small number of females dosed at 625 ppm showed a total loss of
litters, a lowered mean litter size at birth, increased pup mortality
at birth and evidence of reduced pup body weight gain in the immediate
perinatal period at both matings of the Fo animals and at the first
mating of the F1a animals. A slight lowering of mean litter size at
both matings was recorded also for the Fo generation fed 150 ppm but
not for the F1 generation. However, none of the findings were
significantly different from the controls. At 37.5 ppm, a reduction in
the mean litter size at birth to day 12 postpartum was noted in the
first mating of the Fo generation. However, the results were again
within the range for controls at the testing laboratory.
No indication of any adverse incidence of structural anomalies
associated with treatment were recorded at terminal macroscopic
examination of the remaining F1a and F2a pups and all F1b and F2b
offspring.
Increased mean liver weight was recorded in weanling and adult
F1a males and F2a weanling females given 625 ppm in the diet. In the
same treated group, mean thymus weight in F1a weanlings of both sexes
and mean brain weight in the F1a female weanlings was lower than in
the controls. Microscopic examination of tissues of both sexes from
weanling F1a and F2a generations and F1a adults selected from
control and treated groups showed no changes that could be considered
attributable to exposure to the test compound; 37.5 ppm represented
the dietary no-effect level (Cozens et al 1982).
Special Studies on Teratogenicity
Rat
Groups of 20 mated Charles River CD rats were given oral doses of
6, 25 or 100 mg/kg daily from day 1 to 20 post coitum (day 1 was the
day when sperm was detected in the vaginal smear). A group of 31 rats
given the vehicle of 10 percent aqueous acacia solution served as
controls. Body weight, food consumption and overt signs of toxicity
were recorded throughout the study. The dams were killed on day 21 of
pregnancy.
The 25 and 100 mg/kg daily dosage elicited maternal toxicity as
evidenced by reduced food consumption, lower body weight and liver
enlargement; in addition, the dams showed increased salivation and
rubbed their noses in the sawdust litter. The only effect noted in the
rats given 6 mg/kg was a marginally lower body weight gain. There were
no macroscopic or microscopic findings attributable to treatment. The
number of corpora lutea was similar for all groups. The highest
dosage level appeared to be embryotoxic, as litter size, implantation
index and viability index were all slightly lower, while the incidence
of dead foetuses was marginally elevated. In addition, mean foetal
weight was lower, one litter containing unusually small foetuses that
appeared to have a gestational age of approximately 19 days. The mean
placental weight for both the male and female foetuses was higher for
the 25 or 100 mg/kg treated groups than for the control. A small
number of foetal abnormalities was noted among the treated groups but
no consistent pattern and no correlation between incidence and dosage
level was noted. The degree of calcification of the sternebrae and
vertebral arches was retarded in foetuses at the 100 mg/kg dose level.
This dosage elicited toxicity in both dams and embryos but produced no
teratogenic effects. The 25 mg/kg dosage was toxic in the dams. The
only findings in the 6 mg/kg group were marginally reduced body weight
gain and a very slight (non-significant) elevation of placental weight
(Beswick 1980).
Rabbit
Groups of 15 rabbits (New Zealand white strain) were administered
daily dosages of 0, 3, 12 and 48 mg/kg b.w. of prochloraz by
intragastric intubation on days 1 to 28 of pregnancy inclusive (day 1
= day of mating). The control group of 15 rabbits was dosed with the
vehicle, i.e. 10 percent acacia solution. All surviving dams were
killed on day 29 of pregnancy for macroscopic examination and the
foetuses removed for external visceral and skeletal examinations. No
treatment-related clinical signs and mortality were noted. Food intake
of the test group remained within 5 percent of the control value, with
a slightly reduced weight gain in all test groups during the first
week of dosing. The pregnancy rate was comparable for all groups. A
significant increase was noted in liver weight, with liver
discolouration in dams given 48 mg/kg day. A single dam from each of
the low-and high-dosage groups aborted completely, while one of the
control dams aborted partially. Litter size, post-implantation loss,
litter weight and mean foetal weight were considered unaffected by
treatment. Embryonic and foetal development, as assessed by incidence
of major malformations, minor anomalies and skeletal variations, were
similarly unaffected by treatment. No evidence of embryonic
teratogenic activity was therefore noted in New Zealand white rabbits
(Palmer et al 1980).
Special Studies on Mutagenicity
Ames test
Purified and technical prochloraz were tested in the Ames test
against Salmonella typhimurium TA 1535, TA 1537, TA 1538, TA 98 and
TA 100. All tests were performed in the presence and absence of a
liver microsome activation system (S-9) derived from
phenobarbitone-induced rats and tested at levels of 62.5, 125, 250,
500 and 1 000 mcg/plate. Positive control mutagens were included in
all tests to demonstrate the mutability of the bacterial strains and
the metabolizing capacity of the S-9 preparation; these comprised
cyclophosphamide, 6-aminochrysene and 2-animofluorene. Dimethyl
sulphoxide served as the negative control throughout. There was no
evidence of mutagenic activity against any of the strains employed but
the antibacterial effects of prochloraz precluded evaluation of
mutagenicity at concentration of 1 000, 500 and, in some cases, 250
mcg/plate (Wilcox 1977).
Micronucleus assay
Groups of five male and five female Charles River CD rats were
given two intraperitoneal injections of 6.25, 25.0 or 100 mg/kg b.w.
of prochloraz 24 h apart. Similar groups were given intraperitoneal
injections of cyclophosphamide 200 mg/kg b.w. to serve as positive
controls and intraperitoneal injections of the vehicle (maize oil) as
the negative controls. Six hours after the second injection all
animals were killed, smears from the bone marrow of the femur were
prepared and examined microscopically to determine the incidence of
micronuclei in the polychromatic erythrocytes. No significant increase
in the incidence of micronuclei was observed in any of the groups of
rats. Prochloraz was without clastogenic activity in rats of both
sexes when tested up to 100 mg/kg b.w. (Everest & Cliffe 1980).
Dominant lethal test
Groups of 20 male CD1 strain pathogen-free male mice (age 5-6
weeks) were given 0, 6, 25 or 100 mg/kg/day prochloraz in the diet for
eight weeks. After this treatment period, they were paired with
untreated females on a 1:1 basis for one week and then with a second
batch of untreated females for a further week. Approximately two weeks
after the discovery of a vaginal plug or 14 days after the midweek of
their pairing, the females were killed and their uterine contents
examined. There were no signs of reaction to treatment and mean weight
gains of all test groups compared favourably with that of the control
group. Intergroup variations in mean food consumption throughout the
entire treatment period were minimal and showed no consistent
dosage-related trend.
Mating performance of treated males and subsequent pregnancy
rates among untreated females were unaffected by treatment at any
dosage, except for one male at 25 mg/kg/day, which failed to induce
pregnancy at either mating. No obvious treatment-related macroscopic
changes were observed at terminal necropsy of the males. Mean weights
of the testes of treated males were slightly higher than the control
value but were not statistically significant. There was no evidence of
a dose-related effect on the pregnancy rate, mean number of
implantations, viable young, embryonic death and post-implantation
losses. Prochloraz showed no evidence of a dominant lethal effect in
male mice (Cozens et al 1980).
Mouse lymphoma mutation assay
Technical prochloraz was tested for mutagenic activity in mouse
lymphoma cells L5178 Y rendered heterozygous at the thymidine kinase
(TK) locus. The tests were carried out in the presence and absence of
a post-mitochondrial supernatant fraction from Arochlor 1254-treated
male rat livers and the co-factors required for mixed-function oxidase
activity. In an initial test carried out over a dose range of 0.16
mcg/ml to 1.6 mg/ml indicated, that prochloraz was toxic to the cells,
causing extensive cytotoxicity at a concentration of 158 mcg/ml. In
two further independent experiments at 50.0 mcg/ml and the second at
70.0 mcg/ml no significant increases in mutation frequency in the
prochloraz-treated cells over the vehicle control values were
observed. The concurrent positive control substances were
ethyl-methanesulphonate, which does not require the presence of S-9
mix, and 2-acetylaminofluorene, which requires the presence of S-9 mix
for metabolism. In both cases, high mutation frequencies were
obtained.
Technical prochloraz was not mutagenic to mouse lymphoma L 5178 Y
cells when tested over a series of dose levels, which extended into
the toxic range, in the presence or absence of S-9 mix (McGregor
et al 1983).
Special Studies on Carcinogenicity
Mouse
Groups of 52 male and 52 female mice CD-1 strain mice were fed
technical prochloraz in the diet at 78, 325 and 1 300 ppm. For each
sex, treatment continued until survival reached 20 percent, which was
106 weeks for males and 121 weeks for females. An additional group of
104 males and 104 females received the normal diet only. Observations
included clinical signs, mortality, body weight, food consumption and,
at 52 weeks and terminally, haematology and weight of selected organs.
Comprehensive histopathological examinations were made of tissues from
all surviving animals. Clinical signs were not evident, except in mice
given 1 300 ppm and in males treated at 325 ppm which were killed
because of distended abdomens. These animals were subsequently found
to have multiple liver tumours. No other overt signs of toxicity
related to treatment were observed. There was no mortality in the
course of the study attributable to prochloraz. Mice given 1 300 ppm
showed slightly reduced body weight gain. No differences were noted,
that were considered to be of toxicological significance, in the
haematological parameters between treated and control mice. Slightly
lower haemoglobin concentrations and lower total white blood cell
counts, due to a lower lymphocyte count, were noted at week 104 in
female mice treated with 1 300 ppm in diet.
At necropsy, an increase of liver weights was recorded in both
sexes given 1 300 ppm prochloraz. There was a dose-related increase in
the incidence of adenomas and adenocarcinomas of the liver in both
males and females. At 1 300 and 325 ppm, the increased incidence was
statistically significant and liver tumours (of hepatocyte origin)
were observed in both sexes, which were associated in a number of mice
with more than one tumour. Some cases at 1 300 ppm showed multiple
tumours as contributory factor to death. At 78 ppm, a slightly higher
incidence of liver tumours in female mice (6 out of 52) compared to
controls (5 out of 104) was noted, though the difference was not
statistically significant. In the males, the incidence of liver
tumours was similar in controls (37 out of 104) and treated mice (21
out of 52). There was no evidence of any other effects of treatment in
terms of either neoplastic or non-neoplastic pathology.
Treatment of mice was associated with a dose-related increase,
evident in both sexes, of liver tumours of all types. The incidence of
liver tumours was statistically significant at 1 300 ppm and 325 ppm
but not at 78 ppm prochloraz. The dietary dosage of 78 ppm was
equivalent to 7.5 mg/kg/day in males and 8.8 mg/kg/day in females
(Colley et al 1983).
Special Studies on Skin and Eye Irritation
A moderate to severe skin irritation produced in the rabbit
suggests that prochloraz is a skin irritant (Kynoch & Liggett 1979a).
Instillation of dilute solution of prochloraz into the everted lower
eyelid of one eye of New Zealand white stream rabbits produced a mild
conjunctival irritation in some animals which persisted up to three
days (Kynoch & Liggett 1979b).
Acute Toxicity
Prochloraz is of low toxicity to rodents when administered
orally. In rats, the LD50 ranged from 1 600 to 2 400 mg/kg b.w. and
in mice it was approximately 2 400 mg/kg b.w. Symptoms of intoxication
became apparent within 30-60 minutes of dosing and included CNS
depression, respiratory disturbance, ataxia, increased salivation,
piloerection, increased lacrimation and distended abdomen with signs
of gastrointestinal irritation. The acute toxicity of prochloraz in
rodents is summarized in Table 1.
Table 1. Acute Toxicity of Prochloraz in Rodents
Species Route LD50 (mg/kg) References
Rat Oral 1 600-2 400 Carter et al. 1978
Mouse Oral 2 400 Shaw & Carter 1976
Rat Dermal 5 000 Carter 1975
Rabbit Dermal 3 000 Kynoch & Liggett 1979a
Rat Inhalation (6h LC50) >420 mg/cu.m. Alexander & Clark 1978
Rat Intraperitoneal 400-800 Smithson & Lancaster 1980
In dogs given a single oral dose of 10, 100 or 250 mg/kg b.w. of
prochloraz, there were no deaths and the only toxic signs were emesis
and diarrhoea at 100 and 250 mg/kg b.w. (Stobart et al. 1978). One
female baboon given prochloraz at 50 mg/kg b.w. showed no toxic effect
while 250 mg/kg b.w. produced only emesis (Morgan et al. 1977).
Following dermal application to rabbits of 3 000 mg/kg for 24 h
there were no toxic effects except moderate to severe erythema and
oedema on the treated skin (Kynoch & Liggett 1979a).
Short-Term Studies
Mouse
Groups of 9 male and 9 female and 15 male and 15 female CD-1
strain mice were fed prochloraz in the diet at 6, 25, 100 and 400
mg/kg/day for 6 weeks and 13 weeks, respectively. Groups of 9 or 24
mice of each sex, given a diet without prochloraz, acted as controls.
Two additional groups of 15 mice of each sex were given a control diet
for 17 weeks and a diet containing prochloraz at a nominal dose level
of 400 mg/kg/day for 13 weeks, followed by a 4-week recovery period.
Overt signs of toxicity were recorded daily and body weight and food
consumption three times weekly; haematological and blood biochemistry
analyses were done at 6 weeks, 13 weeks and 4 weeks off-dose. All mice
were killed at the end of the dosing or recovery period, examined
macroscopically, the main organs were weighed and microscopic
examination of tissues performed.
No deaths occurred that were attributable to treatment with
prochloraz. An increased incidence of piloerection occurred in mice of
both sexes given 400 mg/kg daily. Food consumption of both sexes given
400 mg/kg daily was higher than that of the controls throughout the
dosing period and during the first week of the recovery period, but
was similar to the controls thereafter. A slight weight loss was
observed in the males given 100 mg/kg daily and in the females given
400 mg/kg daily. At week 6, haemoglobin concentration, PCV and red
blood cell counts were increased in both sexes given 400 mg/kg daily;
MCH was also marginally increased in the females and leucocyte counts
were increased in females due to lymphocytosis. At week 13 or after
four weeks recovery, these parameters were no longer affected by
treatment.
Plasma GPT activity was increased at week 6 in some mice of both
sexes given 400 mg/kg daily and some males at 100 mg/kg daily. At week
13, it was increased in the majority of females given 400 mg/kg daily
and in some mice of both sexes given 100 mg/kg daily. After four weeks
recovery, some of the males given 400 mg/kg daily were still affected.
Albumin content and consequently the albumin:globulin ratio, were
slightly reduced in both sexes given 400 mg/kg daily and in the males
given 100 mg/kg daily. At week 13, in both sexes at 400 mg/kg daily,
urea-nitrogen was slightly decreased and glucose levels were
increased.
Liver weight was increased at week 13 in both sexes given 25, 100
or 400 mg/kg daily. The ovaries in the females and the prostate and
seminal vesicles in males were small in the group at 400 mg/kg daily.
After 4 weeks recovery, liver weights were still higher in both sexes
given 400 mg/kg daily, but to a lesser extent than at weeks 6 and 13.
Histopathological examination showed minor liver changes with loss of
glycogen and periportal fat droplets in both male and female mice
receiving 400 mg/kg and in females receiving 100 mg/kg for 13 weeks.
Some male mice receiving 100 or 400 mg/kg also showed centrilobular
hepatocyte enlargement. In both male and female mice killed after four
weeks recovery, none of these changes were present. The no-effect
level in mice was 6 mg/kg/day (Gale 1980; Lancaster 1982).
Rat
Groups of 20 male and 20 female Boots-Wistar rats were given a
daily dose of prochloraz by oral gavage in 10 percent aqueous acacia
suspension at 0, 6, 25 or 100 mg/kg b.w. for 13 weeks. Further groups
of 20 male and 20 female controls given 100 mg/kg daily of prochloraz
remained untreated for four weeks after the end of the dosing period
and, in addition, groups of 10 males and females were given 0, 6, 25
or 100 mg/kg daily of prochloraz for six weeks. Overt signs of
toxicity observed in rats of both sexes were increased salivation at
25 or 100 mg/kg daily and in a few given 6 mg/kg daily. The incidence
of diarrhoea was increased during the first four weeks of dosing in
the males given 100 mg/kg daily and possibly in the first week in the
males given 25 mg/kg daily. In females, a few instances of diarrhoea
were recorded at each dose level.
Food consumption was not affected by treatment. However, test
females gained somewhat more weight than the controls at 25 or 100
mg/kg daily. At week 6, the only consistent haematological change was
a decreased haemoglobin concentration in males at each dose level and
in females given 100 mg/kg daily. A leucocyte count increase was noted
at each dose level in males due to lymphocytosis. At week 13, MCV was
slightly decreased at each dose level in the males and haemoglobin
concentration was slightly decreased in females treated at 25 or 100
mg/kg daily and in males given 100 mg/kg daily after the four weeks
recovery period.
Serum bilirubin was slightly decreased in both sexes at each dose
level at week 6, in the male at each dose level and in the females
given 6 or 100 mg/kg daily at week 13. Serum potassium was slightly
increased at week 6 in the males at 25 or 100 mg/kg and females at 100
mg/kg daily. UGOT excretion was slightly elevated in a few rats of
both sexes at each dose level. Urinary protein content was reduced in
the females at each dose level and in males given 100 mg/kg daily.
Liver weight at week 6 was increased in both sexes given 100
mg/kg daily and in the females given 6 or 25 mg/kg daily; kidney
weight was increased in both sexes given 100 mg/kg daily; spleen
weight was reduced in the males in each dose group; ovary weight was
increased in each dose group and thyroid weight was also increased in
females given 6 or 100 mg/kg daily. After the four-week recovery
period, the weights of the liver, ovaries and thyroids of the females
were slightly higher than the control values. The changes in liver
cell size correlated closely with the increase in liver weight. The
only instance where such correlation was not apparent was at week 13
in the females given 6 mg/kg daily, where the centrilobular cells were
slightly larger but liver weight was unchanged.
No other findings, that could be related to treatment, were
observed at microscopic examination of tissues from the rats given 100
mg/kg daily and killed at week 13. Various treatment-related effects
were noted in the rats of both sexes given 6 mg/kg daily of prochloraz
and a no-effect level was therefore not established. However, all
these effects were marginal (Lancaster & Shaw 1979a).
Dog
Groups of four male and four female beagles were given daily
doses of 1, 2, 5, or 7 mg/kg of prochloraz by gastric intubation for
13 weeks. Groups of eight males and eight females received 20 mg/kg
daily or the vehicle (10 percent aqueous acacia solution)
concurrently. At the end of the dosing period, four males and four
females from each of the 20 mg/kg and control groups were kept for
another four weeks without treatment.
Overt signs of toxicity observed during the dosing period
included isolated bouts of emesis and increased salivation in some
dogs from all groups, including controls, although these were more
prevalent at the high dosage. All dogs at 20 mg/kg daily and one given
7 mg/kg daily occasionally produced yellow mucoid and/or liquid
faeces. No effects of treatment were detected on faecal occult blood
content. During the recovery period, no overt signs of toxicity were
observed. Reduced food consumption was recorded during the dosing
period in two dogs given 20 mg/kg daily. Body weight changes during
the study were comparable in control and treated groups, although
greater fluctuations were recorded in dogs given 20 mg/kg daily. There
was no treatment-related effect detected by ophthalmoscopy or
electrocardiography.
Transient slight decreases in erythrocyte count and/or
haemoglobin concentra- were recorded in a few females given 7 or 20
mg/kg daily. No other treatment-related changes in haematological
parameters were noted. Changes in the activity of serum enzymes were
recorded. The serum alkaline phosphatase was high during the dosing
period in the majority of dogs given 20 mg/kg daily and in some given
7 mg/kg daily. Serum leucine aminopeptidase was slightly elevated at
week 6 in some dogs given 20 and in one given 7 mg/kg and serum GPT
was increased in a dog given 7 mg/kg daily. No change was observed in
urinary parameters.
At necropsy, all dogs given 20 mg/kg daily and three given 7
mg/kg daily had large and heavy livers. Low prostate weights were
recorded at week 14 in three dogs and in another two dogs in the same
group at 17 weeks. One of these dogs, killed at week 10, also had
decreased testes weight. In addition, one dog, given 7 mg/kg daily,
had a low prostate weight relative to body weight. In two females at
the high dose killed at week 14, and one killed at week 18, clear
brown exudate from freshly sectioned mammary glands was observed.
There were no histopathological findings in dogs on the high
prochloraz dosage. Counts of liver nuclei revealed no statistically
significant difference in the cell liver size or in the micronuclei
count between control and treated dogs.
In dogs retained for the recovery period, liver size and weight
returned generally to within normal limits, suggesting that any effect
of treatment on these parameters was reversible. The changes of AP and
LAP also returned to normal during the recovery period. The dose
producing no adverse-effect was 2.27 mg/kg daily (Lancaster et al.
1979b; Lancaster 1980).
Long-Term Studies
Rat
Groups of 150 male and 150 female rats constituted the controls.
Two groups each of 80 males and 80 females were treated with 37.5 and
150 ppm and a further two groups of 90 males and 90 females were given
625 ppm prochloraz in their diet. When the survival approached 20
percent in any group, all animals of that sex group were killed (111
weeks of treatment for females and 115 weeks of treatment in the
males).
There were no overt signs of toxicity recorded during the study
that could be considered related to the treatment. During weeks 20 and
21, the majority of rats from all groups showed signs indicative of
sialodacryo-adenitis infection, with a further relapse of mild
infection in a few rats from each group during the weeks 66 and 67.
Male survival was similar to controls, while treated females survived
longer than controls. A lower food consumption with a reduced body
weight gain was recorded for both sexes given 625 ppm. At 150 ppm,
food consumption was marginally lower throughout in males and until
week 52 in the females. Ophthalmoscopic examination revealed no
abnormalities related to treatment. In a few rats, minor changes were
seen at week 26, which were considered sequelae of
sialodacryo-adenitis infection.
Periodic laboratory measurements of haematological, biochemical
and urinalysis parameters in a few treated rats at 625 ppm showed
changes, but these were minimal and considered of doubtful
toxicological significance. The changes in urinalysis were minor, i.e.
lower total protein and changes in the pH. A minimal higher activity
of serum glutamic oxalacetic transaminase was noted.
No macroscopic findings related to treatment were seen at interim
kill in week 13. However, at the 52-week kill, enlarged or swollen
livers were observed in one male and three females given 150 ppm and
in four males and one female given 625 ppm in the diet. Heavier livers
were noted in males and females given 625 ppm diet at week 52 and
lower pituitary weights were also recorded in a few rats.
Histopathological examination showed hepatic centrilobular enlargement
in females given 625 ppm; periportal glycogen loss and centrilobular
fat deposits were also present in some treated rats of both sexes.
There were no other microscopic findings related to treatment.
Enlarged and swollen livers were observed in a higher proportion
of male rats given 625 ppm than in the controls, and slightly heavier
liver weights were recorded for females given 625 ppm. The incidence
of hyperplastic lesions in the liver was slightly higher than in the
controls in both sexes given 625 ppm, but the differences were not
statistically significant. The incidence of all other non-neoplastic
findings was similar in all groups. There was no evidence of
carcinogenicity from the chronic administration of prochloraz. The
no-effect level for toxicological changes was 37.5 ppm, equivalent to
a mean intake of 1.3 mg/kg/day in males and 1.6 mg/kg/day in females
(Colley et al. 1982).
Dog
Technical prochloraz was fed to groups of five males and five
females at levels of 0, 30, 135 and 600 ppm (increased to 1 000 ppm
from week 57) for 104 weeks. Observations included clinical signs,
mortality, food and water consumption and, at weeks 0, 13, 26, 51, 78
and 104, ophthalmoscopy, electrocardiography and clinical laboratory
studies. These comprised haematology, biochemistry, urinalysis and
faecal occult blood. At necropsy, organs were weighed and
comprehensive macroscopic and microscopic examinations were carried
out.
There were four deaths during the study but none was attributable
to treatment. There were no overt signs of toxicity or effects of
treatment upon body weight, food or water consumption, ophthalmoscopic
or electrocardiographic findings that could be related to treatment.
There were a few changes in the biochemical parameters, including a
higher level of SAP for males receiving 600 ppm in the diet during
week 13 and, during weeks 16 and 50, the elevation was also apparent
in the females. After the dietary level was increased to 1 000 ppm for
this group, there was a marked increase in the SAP levels during week
60, which persisted throughout the rest of the dosing period. The mean
level for males receiving 135 ppm was also higher than the control
mean from week 13 onwards, but the difference was not so marked as at
600 ppm. There was a slight but inconsistent increase in the platelet
count, glucose and cholesterol values in the group receiving 600 to
1 000 ppm, but these could not be related definitely to treatment.
There was no effect of treatment on the urinary parameters or faecal
occult blood content.
Liver weights in five animals that received 600 to 1 000 ppm, one
that received 135 ppm and one control, when expressed as a percentage
of body weight, exceeded the normal upper limit. The mean prostate
weight for animals in the same treatment group was significantly lower
than the control mean weight.
Histopathological examination showed minimal prostatic atrophy,
low grade hepatitis and minimal swelling and rarefaction of
centrilobular hepatocytis at 600 to 1 000 ppm. One male dog given 135
ppm also showed some liver changes. There were no other
histopathological changes related to prochloraz.
The no-effect level was 30 ppm in the diet, equivalent to
approximately 0.92 mg/kg/day (Chesterman et al. 1981).
Observations in Humans
Prochloraz has been produced on a commercial scale since 1980. No
adverse human effects have been detected since then from synthesis
formulation or use of prochloraz or its formulation. No clinical or
laboratory data were included (Bonsall 1982).
COMMENTS
Prochloraz has low oral toxicity. Following oral administration
in the rat, 95 percent of the dose is rapidly absorbed. In rats, the
major excretion is via the urine; in dogs it is via the faeces.
Prochloraz is a demonstrated liver mixed function oxidase inducer.
In rats and rabbits, no evidence of teratogenic effects was noted
at maternally toxic dose levels. A multigeneration reproduction study
in rats did not show any adverse effects at doses up to 37.5 ppm.
Mutagenicity studies, including the Ames test, micronucleus test,
mouse lymphoma assay and a dominant lethal study, were all negative.
In 90-day studies in mice, rats and dogs, liver weight was
increased in all species. No-effect levels of 6 mg/kg/day and of 2.27
mg/kg/day were established for mice and dogs, respectively. However,
in rats 6 mg/kg/day induced increased liver weight and occasional
signs of intoxication (increased salivation, diarrhoea). A two-year
dog study established a no-effect level of 30 ppm.
A long-term rat study did not result in any evidence of oncogenic
potential. A no-effect level of 37.5 ppm in the diet was established.
A mouse carcinogenicity study of 106 weeks duration indicated an
increased incidence of liver adenomas and adenocarcinomas in both
sexes at dose levels of 325 ppm and above. Prochloraz has been shown
to be a hepatocarcinogen in mice.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat (male); 37.5 ppm in the diet, equal to 1.3 mg/kg b.w.
Dog: 30 ppm in he diet, equal to 0.9 mg/kg b.w.
Estimate of Acceptable Daily Intake for Man
0 - 0.01 mg/kg b.w.
FURTHER WORK OR INFORMATION
Desirable
Observations in humans.
REFERENCES-TOXICOLOGY
Alexander, D.J. & Clark, G.C. Preliminary acute inhalation toxicity
1978 study in male and female rats with pure prochloraz (single
exposure). Report TX78053 from Huntingdon Research Centre
and Boots, submitted to WHO by FBC Ltd. (Unpublished)
Boardman, L.E. Plasma and tissue distribution studies in the rat
1979 following single and repeated oral doses of 3H-prochloraz.
Report AX79004 from Hazleton and Boots submitted to WHO by
FBC Ltd. (Unpublished)
Beswick, A.M. The teratogenicity study of technical prochloraz in male
1980 and female rats. Report TX80024 from Boots submitted to WHO
by FBC Ltd. (Unpublished)
Bonsall, J.L. The human exposure to prochloraz since synthesis in 1974
1982 and commercial production in 1980. Report submitted to WHO
by FBC Ltd. (Unpublished)
Campbell, J.K. Residues in milk and tissues of a goat dosed orally
1980 with 14C-prochloraz. Report AX80026 from Boots submitted to
WHO by FBC Ltd. (Unpublished)
Carter, O.A. Acute dermal toxicity of prochloraz to the male Boots
1975 Wistar rat. Report TXM75079 from Boots submitted to WHO by
FBC Ltd. (Unpublished)
Carter, O.A., Smithson, A. & Burnett, R. Acute oral toxicity of
1978 prochloraz, prochloraz pure material, stage 3 and stage 4
technical material and mother liquor concentrate impurities
to male Boots Wistar rats. Report TX78118 from Boots
submitted to WHO by FBC Ltd. (Unpublished)
Challis, I.R. & Campbell, J.K. The effect of prochloraz on the hepatic
1983 mixed function oxidase system of the male mouse after oral
administration. Report METAB/83/6 from FBC submitted to WHO
by FBC Ltd. (Unpublished)
Chesterman, H. et al. Two-year toxicity study in beagle dogs of
1981 technical prochloraz - final report - repeated dietary
administration for 104 weeks. Report TOX/81/173-2 from
Huntingdon Research Centre and FBC submitted to WHO by FBC
Ltd. (Unpublished)
Colley, J. et al. Prochloraz chronic toxicity and carcinogenicity
1982 study in rats by dietary administration - 104 weeks (final
report). Report TOX/82/173-8 from Huntingdon Research
Centre, and FBC submitted to WHO by FBC Ltd. (Unpublished)
Colley, J. et al. Prochloraz tumorigenicity study in mice by dietary
1983 administration (final report). Report TOX/83/173/23 from
Huntingdon Research Centre and FBC submitted to WHO by FBC
Ltd. (Unpublished)
Cozens, D.D., Reid, Y.J., Woodhouse, R.N., Almond, R.H. Anderson, J.
1980 & Ball, S.I. Dominant lethal gene assay of technical
prochloraz in the male mouse. Report TX80077 from the
Huntingdon Research Centre and Boots submitted to WHO by FBC
Ltd. (Unpublished)
Cozens, D.D. et al. The effect of prochloraz on reproductive
function
1982 of multiple generations in the rat. Report TOX/82/173-5 from
Huntingdon Research Centre and FBC submitted to WHO by FBC
Ltd. (Unpublished)
Everest, R.P. & Cliffe, S. Technical prochloraz micronucleus assay in
1980 male and female CD rats of prochloraz. Report TX80003 from
Boots submitted to WHO by FBC Ltd. (Unpublished)
Gale, E.P. 90-day oral toxicity study with prochloraz technical to
1980 male and female CD1 mice. Report TX80040 from Hazleton and
Boots submitted to WHO by FBC Ltd. (Unpublished)
Hamilton, D.Y. The distribution and level of 14C-labelled residues in
1978a rats following repeated oral dosing with 14C-prochloraz at
25 mg/kg/day. Report AX78008 from Boots submitted to WHO by
FBC Ltd. (Unpublished)
Hamilton, D.Y. The distribution and level of radiolabelled residues in
1978b the tissues of the dog following a single oral dose of
prochloraz. Report AX78016 from Boots submitted to WHO by
FBC Ltd. (Unpublished)
Kynoch, S.R. & Liggett, M.P. Primary eye irritancy of prochloraz 40
1979a percent E.C. formulation (bfn 8099). Report TX79050 from
Huntingdon Research Centre and Boots submitted to WHO by FBC
Ltd. (Unpublished.)
Kynoch, S.R. & Liggett, M.P. Primary skin irritancy of prochloraz 40
1979b percent E.C. formulation (bfn 8099). Report TX79074 from
Huntingdon Research Centre and Boots submitted to WHO by FBC
Ltd. (Unpublished)
Lancaster, M.C. & Shaw, J.W. 90-day oral toxicity study with
1979a prochloraz technical in male and female Boots Wistar rats
(4-week off-dose period). Report TX79028 from Boots
submitted to WHO by FBC Ltd. (Unpublished)
Lancaster, M.C., Morgan, H.E. & Stobart, J.E. 90-day oral toxicity
1979b study with prochloraz technical in male and female beagle
dogs (4-week off-dose period). Report TX79010 from Boots
submitted to WHO by FBC Ltd. (Unpublished)
Lancaster, M.C. 13-week oral toxicity study with prochloraz technical
1980 (BX 9/DM 2723) in male and female dogs with a four-week
off-dose period. Histopathological examination of the
remaining tissues. Report TX80034 from Boots submitted to
WHO by FBC Ltd. (Unpublished)
Lancaster, M.C. PRochloraz: 13-week oral toxicity in the mouse -
1982 histopathological examination. Report TOX/82/173/-9 from FBC
Ltd. submitted to WHO by FBC Ltd. (Unpublished)
McGregor, D.B., Riach, C.G. & Brown, A.G. Technical prochloraz
1983 assessment of mutagenic potential in the mouse lymphoma
mutation assay. Report TOX/83/173-22 from Inveresk and FBC
submitted to WHO by FBC Ltd. (Unpublished)
Morgan, G.E., Patton, D.S.G., Shepherd, G.M. & Stobart, J.E. The acute
oral toxicity of bts 40542 (technical batch) to the female
baboon. Report from Boots Laboratories submitted to WHO by
FBC Ltd. (Unpublished)
Needham, D. The effect of dog gastric juice or plasma on prochloraz.
1980 Report AX80011 from Boots submitted to WHO by FBC Ltd.
(Unpublished)
Needham, D. & Campbell, J.K. The excretion of 14C-phenyl-labelled
1980 prochloraz in male and female rats after a single oral dose.
Report AX80037 by Boots submitted to WHO by FBC Ltd.
(Unpublished)
Needham, D. The excretion and tissue residues of 14C-prochloraz in
1982a male and female mice following a single oral dose of 100
mg/kg. Report METAB/82/32 from FBC submitted to WHO by FBC
Ltd. (Unpublished)
Needham, D. & Campbell, J.K. The excretion and tissue residues of 14C
1982 prochloraz in male and female dogs following a single oral
dose of 18 mg/kg. Report METAB/82/30 by FBC submitted to WHO
by FBC Ltd. (Unpublished)
Needham, D. The metabolism of prochloraz in the rat after oral
1982b administration. Report METAB/82/31 from FBC submitted to WHO
by FBC Ltd. (Unpublished)
Needham, D. The effect of prochloraz on the hepatic mixed-function
1983a oxidase system of the male rat after oral administration.
Report METAB/83/5 from FBC submitted to WHO by FBC Ltd.
(Unpublished)
Needham, D. The effect of prochloraz on the hepatic mixed function
1983b oxidase system of the mouse when administered at 80, 325 and
1 300 mg/kg diet for up to 14 weeks. Report METAB/83/7 from
FBC submitted to WHO by FBC Ltd. (Unpublished)
Palmer, A.K., Bottemley, A.M. & Billington, R. The effect of technical
1980 prochloraz on pregnancy of the New Zealand white rabbit.
Report TX80083 from Huntingdon Research Centre and Boots
submitted to WHO by FBC Ltd. (Unpublished)
Rivière, J.L. Prochloraz, a potent inducer of the microsomal
1983 cytochrome P-450 system. Pestic. Biochem. Physiol., 19:
44-52.
Shaw, J.W. & Carter, O.A. Acute oral toxicity to male CD 1 mice of
1976 prochloraz. Report TX76093 from Boots submitted to WHO by
FBC Ltd. (Unpublished)
Smithson, A. & Lancaster, M.C. Acute intraperitoneal toxicity of
1980 prochloraz to male CD rats. Report TX80004 from Boots
submitted to WHO by FBC Ltd. (Unpublished)
Stobart, J.E., Morgan, H.E., Patton, D.S.G. & Shepherd, G.M. Acute
1978 oral toxicity of prochloraz to male and female beagle dogs.
Report TX78049 from Boots submitted to WHO by FBC Ltd.
(Unpublished)
Turner, D.M. & Gilbert, C.M. Pharmacokinetic studies on 14C-labelled
1977 prochloraz in the rat. Report AX77001 from Hazleton and
Boots submitted to WHO by FBC Ltd. (Unpublished)
Wilcox, P. Prochloraz in vitro bacterial mutagenicity testing of
pure
1977 and technical prochloraz. Report TX78002 from Boots
submitted to WHO by FBC Ltd. (Unpublished)
RESIDUES
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Prochloraz is available as 40 percent and 45 percent emulsifiable
concentrate (E.C.) formulations for foliar and postharvest treatments
and is sold under the trademark Sportak. A 50 percent wettable powder
formulation containing a 4:1 prochloraz-manganese coordination complex
active ingredient (a.i.) (3.4 percent manganese) is available and
recommended for foliar application to some broad leaf crops,
ornamentals and mushrooms, which may be susceptible to phytotoxicity
from E.C. formulations. Prochloraz is available in co-formulation with
carbendazim, under the trademarks Sportak PF and Sportak ALPHA, for
foliar application to cereal and oilseed rape. A 200 g/l liquid
formulation is available for cereal seed treatment and a 25 percent
E.C. disinfectant for rice seed.
Prochloraz is used for the control of a variety of fungal
diseases on a variety of crops and is said to be especially active
against Ascomycetes and Fungi Imperfecti. It is currently registered
or approved for use in eight countries and similar measures are
pending or planned in many others. Nationally registered and/or
approved uses are summarized in Table 1.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue trials have been conducted in many countries,
representing a wide range of food crops, climatic conditions,
formulations and foliar, seed and postharvest treatments. Trials data
are summarized in Tables 2-13. Trials data from countries with
approved uses are available only for apples, watermelons, grapes
(Taiwan, province of China), cereal (Belgium, Denmark, France, German
Federal Republic, The Netherlands, United Kingdom) and mushrooms (The
Netherlands).
Sugarbeets
No information was available on good agricultural practices for
prochloraz on sugarbeets. The "recommended" application rate of active
ingredient is said to be 0.5-1 kg/ha for foliar treatment. No
preharvest interval (PHI) is given. Total residues of both free and
conjugated residues of prochloraz (BTS 40 542),
N-formyl-N'-propyl-N'-2(2,4,6-trichlorophenoxy) ethylurea (BTS 44596),
N-propyl-N-2 (2,4,6-trichlorophenoxy) ethylurea (BTS 44595) and
2,4,6-trichlorophenol (BTS 45186), hereafter referred to as total
residues of prochloraz and metabolites, are listed in Table 2. The
values (corrected for 90 percent recoveries) are expressed as
prochloraz equivalents but were determined as 2,4,6-trichlorophenol
after hydrolysis. All residues and the 0.036 mg/kg control are below
the limit of determination, said to be 0.1 mg/kg. Data are
insufficient to support a maximum residue level, even if good
agricultural practices were known.
The "recommended" application rate of active ingredient to
sugarbeet leaves is said to be 0.5-1 kg/ha for foliar treatment. No
PHI was provided. Total residues of prochloraz and metabolites,
expressed as prochloraz equivalents, on sugarbeet leaves were 0.97,
1.3 and 1.2 mg/kg at 5, 21 and 42 days, respectively, after the last
treatment in three trials (Table 2). The control was 0.15 mg/kg.
Lettuce
No information was available on good agricultural practices on
lettuce. Residues of prochloraz only, uncorrected for approximately 72
percent recoveries, ranged from 7.4 mg/kg 18 days after treatment to
0.41 mg/kg after 39 days and <0.07 mg/kg after 71 days with a limit
of sensitivity said to be 0.01 mg/kg, although one of the three
controls has apparent residues of 0.02 mg/kg. Recoveries were 65-76
percent at 0.1-0.5 mg/kg fortification levels.
Watermelons
Residue data reflecting good agricultural practices in Taiwan
Province of China are presented in Table 2. Residues of prochloraz
per se were 0.01 mg/kg in peel and flesh after the approved six-day
interval from last application to harvest. Information was not
provided on the analytical procedure used; a 0.003 mg/kg limit of
detection was claimed.
Citrus Fruits
No information was provided on good agricultural practices on
citrus fruits. Residues from postharvest trials, proposed for uses
(25-70 g/hl for dip treatments or 100-300 g/hl for spray treatments)
where the peels are discarded, are summarized in Table 3. A variety of
treatment and storage conditions are given, representing 23 trials in
five countries. In some cases prochloraz alone is determined, in
others prochloraz and its metabolites and in one case BTS 45 186.
Total residues are generally expressed as prochloraz equivalents,
although they are usually measured as BTS 45 186. Total residues after
storage at ambient of 4°C for up to 70 days and from a variety of
treatments and locations ranged up to 18 mg/kg in peel, 0.44 mg/kg in
flesh and 6 mg/kg on a whole fruit basis. Residue levels of free
prochloraz were not substantially different than those for total
residues, where a comparison was made. More details are reported under
"Fate of Residues, Storage and Processing".
Pome Fruits
Information on good agricultural practice and residue data
(prochloraz only) reflecting such practice on apples are available for
Taiwan Province of China, as well as data for apples and pears from
supervised trials in three additional countries for which good
agricultural practices for prochloraz on pome fruit are not known.
Apples
The most pertinent data were those from Taiwan Province of China,
for which information was available on good agricultural practices on
apples. Data represent recommended and 2X the recommended application
rate. No data were available for the recommended 9-day last treatment
to harvest interval, but they were given for 6 and 12 days. Residues
of prochloraz, per se, from nine applications were 0.07 and 0.01
mg/kg, respectively, at these intervals from the recommended
application rate. No information is available on the analytical method
used other than a claimed 0.003 mg/kg limit of detection. Other apple
residue data (parent compound only) are from application rates three
to eight times that for good agricultural practice known to the
Meeting. Over-all recoveries among studies submitted range from 64-92
percent with claimed sensitivities ranging from 0.001-<0.01 mg/kg.
Apparent residues in untreated controls, where given, are
<0.01-0.02 mg/kg. A reasonable limit of determination would appear
to be about 0.1 mg/kg. One study (Hayto 1977c) indicates a half-life
in apples of 6-7 days.
Pears
No information on good agricultural practice was available for
pears. The application rates are substantially greater than known good
agricultural practice for apples and residues are higher, as would be
expected.
Stone Fruits
No information on good agricultural practice for prochloraz on
stone fruit were available to the Meeting. The "recommended"
application rate for foliar treatment with the prochloraz-50 percent
W.P. manganese complex formulation was reported as 15-30 g a.i./hl. No
preharvest interval was given. Residue data were available on
apricots, nectarines, peaches, cherries and plums from four countries
(Table 2). Total residues of prochloraz and the metabolites were
determined as 2,4,6-trichlorophenol and expressed as prochloraz.
Residues in mature fruit ranged from a maximum of 0.19 mg/kg im plums
at 3 days, 1 mg/kg at 14 days, 0.82 mg/kg at 24 days to <0.05 mg/kg
over 75 days after multiple foliar treatments with either E.C. or W.P.
(manganese complex) formulations. Residues were generally lower for
other stone fruit at comparable intervals after treatment with the
W.P. formulations. At fortification levels of 0.05-1 mg/kg recoveries
are 70-113 percent over-all. Apparent residues in eight untreated
stone fruit controls were 0.009-0.022 mg/kg except one value of 0.06
mg/kg in four controls (plums). The limit of determination for stone
fruit was reported as 0.05 mg/kg.
Grapes
Information on good agricultural practice for the E.C.
formulation and residue data reflecting 1, 1.5, 2 and 4X recommended
application rates were available from one country.
Residues from two applications at recommended rates ranged from
0.39 mg/kg on the day of application to <0.01 mg/kg after 21 days and
0.1 mg/kg at the recommended 9-day preharvest interval. Higher
application rates and numbers of applications, with one or two
exceptions, did not make an appreciable difference in the residue
level at comparable intervals after application. No information was
provided on apparent residues in un-treated controls or on the
analytical procedure; a 0.003 mg/kg limit of detection was reported.
TABLE 1. REGISTRATIONS AND APPROVED USES FOR PROCHLORAZ
Country Product1 Commodity Approved Application Rate Number and timing of Pre-harvest
Product a.i. Applications Interval
(prochloraz)
Belgium SPORTAK 45EC Winter 1 l/ha 0.45 kg/ha One application at earstage in mixture na
wheat with either 0.125 kg/ha carbendazim,
1.2 kg/ha captafol or 1.6 kg/ha maneb.
Winter
barley 1 l/ha 0.45kg/ha One application at stage L or M2
Denmark SPORTAK Barley 100 ml/100 kg 0.2 g/kg na na
BEJOSE (seed
dressing)
SPORTAK 45EC Spring 1 l/ha 0.45 kg/ha One application at stage 4-10.5 or a
barley split dose at half rate with the first
application at stage 4-6 and the second
at stage 8-10.53.
Winter 1 l/ha 0.45 kg/ha One (stage 4-10.5) or two (stage 4-6 cereals
barley and 7-10.5) applications3. 1 month
Winter 1 l/ha 0.45 kg/ha Two applications (stages 5-6 and 9-
wheat 10.53).
Winter 1 l/ha 0.45 kg/ha One application (stages 5-7)3
rye
Eire SPORTAK 40EC Cereals 1 l/ha 0.4 kg/ha Up to three applications between leaf
sheaf erection and complete ear emergence. na
Table 1 (continued)
Country Product1 Commodity Approved Application Rate Number and timing of Pre-harvest
Product a.i. Applications Interval
(prochloraz)
France SPORTAK 40EC Wheat 1.125-1.875 0.45-0.75 Up to two applications (stages 6-7
l/ha kg/ha and 10.3-10.5)3
Barley 1.125 l/ha 0.45 kg/ha Up to two applications (stages 6-7
and 10.1)3 na
SPORTAK 45EC Wheat 1-1.66 l/ha 0.45-0.75 Up to two applications (stages 6-7
kg/ha and 10.3-10.5)3.
Barley 1 l/ha 0.45 kg/ha Up to two applications (stages 6-7
10.1)3
SPORTAK PF Wheat 1.5 l/ha 0.45 kg/ha Up to two applications (stages 6-7
and 10.3-10.5)3.
Barley 1.5 l/ha 0.45 kg/ha Up to two applications (stages 6-7
10.1)3.
Oil seed 1.5 l/ha 0.45 kg/ha Up to two applications, at beginning
rape and end of flowering. na
SPORTAK M Wheat 1.125 l/ha 0.45 kg/ha Up to two applications (stages 6-7
and 10.3-10.5)3.
Barley 1.125 l/ha 0.45 kg/ha Up to two applications (stages 6-7
10.1)3.
Table 1 (continued)
Country Product1 Commodity Approved Application Rate Number and timing of Pre-harvest
Product a.i. Applications Interval
(prochloraz)
German SPORTAK 40EC Wheat 1.2 l/ha 0.48 kg/ha One (stage 29-32) or two
Federal (stages 29-59) applications5.
Republic Cereals -
Barley 1.2 l/ha 0.48 kg/ha One (stage 29-32) or two 35 days6
(stages 29-49) applications5.
Winter 1.2 l/ha 0.48 kg/ha One application at stage 29-325.
rye Restricted to a maximum of three
applications in Winter cereal.
Netherlands SPORTAK 45EC Wheat 1 l/ha 0.45 kg/ha 1-2 treatments after infection.
Barley 1 l/ha 0.45 kg/ha 1-2 treatments after infection. Cereals -
8 weeks7
Winter 1 l/ha 0.45 kg/ha One or two applications.
barley
Ornamentals 0.4% 180 g/hl Flower bulb dip na
SPORGON Mushrooms 3/g/l/m2 1.5g/l/m2 One application of casing soil Mushrooms -
nine days after casing. 10 days
Taiwan SPORTAK 25EC Rice 2000 x 12.5 g/hl 24 hour rice seed soak. na
dilution
Grape 6000 x 4.17 g/hl Two to three applications, Grape -
dilution every 10 days. 9 days
Apple 3000 x 8.33 g/hl Application every 10 days. Apple -
dilution 9 days
Table 1 (continued)
Country Product1 Commodity Approved Application Rate Number and timing of Pre-harvest
Product a.i. Applications Interval
(prochloraz)
Watermelon 4000 x 6.25 g/hl 10 days. 6 days
dilution
United SPORTAK 40EC Cereals 1 /ha 0.4 kg/ha Up to three applications between na
Kingdom leaf sheath erection and complete
ear emergence.
Oil seed 1.25 l/ha 0.5 kg/ha Up to three applications between
stem extension and 90% petal fall.
Ornamentals - 2000 ppm Propagation cutting dip, drench, na
foliar spray or compost incorporation.
SPORTAK 45EC Cereals 1 /ha 0.45 kg/ha Up to three applications between na
leaf sheath erection and complete
ear emergence.
Oil seed 1-1.33 /ha 0.45-0.6 Up to three applications between
rape kg/ha stem extension and 90% petal fall.
Ornamentals - 2000 ppm Propagation cutting dip, drench, na
foliar spray or compost incorporation.
SPORTAK Cereals 1.5 /ha 0.4 kg/ha Up to three applications between
ALPHA leaf sheath erection and complete
ear emergence. na
Oil seed 1.5 /ha 0.4 kg/ha Up to three applications between
rape stem extension and 90% petal fall.
Table 1 (continued)
Country Product1 Commodity Approved Application Rate Number and timing of Pre-harvest
Product a.i. Applications Interval
(prochloraz)
Ornamentals - 2000 ppm Propagation cutting dip, drench, na
foliar spray or compost incorporation.
SPORTAK FE Cereals 4 kg/ha 0.66 kg/ha Up to three applications between
leaf sheath erection and complete
ear emergence. na
Oil seed 3 kg/ha 0.5 kg/ha Up to three applications between
rape stem extension and 90% petal fall.
Ornamentals - 2000 ppm Propagation cutting dip, drench, na
foliar spray or compost incorporation.
SPORGON Mushrooms 3 g/l/m2 1.5 g/l/m2 One application, 1-10 days after Mushrooms -
casing. 10 days
0.6 g/l/m2 0.3 g/l/m2 Three applications, one 1-10 days Mushrooms -
after casing and then after the 2 days
first and second flush
1.2 g/l/m2 0.6 g/l/m2 Two applications, one 1-10 days Mushrooms -
after casing and then between 2 days
second and third flush.
Ornamentals - 2000 ppm Propagation cutting dip, drench, na
foliar spray or compost incorporation.
Table 1 (continued)
Notes: na - not applicable
nd - not defined
ns - not specified
1. The following formulations have been registered:
SPORTAK 40 EC - an emulsifiable concentrate containing 400 g/l prochloraz
SPORTAK 45 EC - an emulsifiable concentrate containing 450 g/l prochloraz
SPORTAK BEJDSE - a liquid seed dressing containing 200 g/l prochloraz
SPORTAK PF - an emulsifiable concentrate containing 300 g/l prochloraz and 80 g/l carbendazim
SPORTAK M - a twin-pack emulsifiable concentrate containing 400 g/l prochloraz and 450 g/l mancozeb
SPORTAK ALPHA - an emulsifiable concentrate containing 266 g/l prochloraz and 100 g/l carbendazim
SPORTAK FE - a wettable powder containing 165 g/kg prochloraz-manganese complex and 533.4 g/kg mancozeb
SPORGON - a wettable powder containing 500 g/kg prochloraz-manganese complex
2. Baggiolini cereal growth stages.
3. Feekes-Large cereal growth stages.
4. Residues of prochloraz alone.
5. Zadoks Decimal cereal growth stages.
6. A proposed MRL of 0.5 mg/kg for prochloraz and metabolites in cereal grain has been submitted.
7. Under review, pending further residue analysis.
8. Residues of prochloraz and metabolites measured as 2,4,6-trichlorophenol and expressed as prochloraz.
Table 2 Prochloraz Residues in Various Crops Following Supervised Trials
Country/ No. of Formulation Application Interval after Residues, Reference
Crop Year trials rate (a.i.) final application (mg/kg)1
(days)
Sugarbeet Italy
root 1981 1 40% E.C. 2 x 1 kg/ha 42-46 0.02 Longland 1983b
leaves 1.17
root 1 40% E.C. 3 x 1 kg/ha 21-25 0.04
leaves 1.32
root 1 40% E.C. 4 x 1 kg/ha 5-6 0.06
leaves 0.97
Lettuce The Netherlands 1 8% dust 8 kg/ha 70-73 0.013 Hayto 1979d
1978 80 0.013
2 8% dust2 8 kg/ha 18-19 7.383
39 0.043
42-46 0.123
70-73 <0.013
80 <0.013
1 8% dust2 6.4 kg/ha 70-73 0.043 Maclaine Pont et al. 1980
1 8% dust2 8 kg/ha 70-73 0.033
8% dust2 9.6 kg/ha4 78 <0.013
Watermelon Taiwan Province
peel of China
1982 1 25% E.C. 3 x 4.17 g/hl 0-1 0.013 Wang 1982
3 <0.013
5-6 <0.013
9 <0.013
12 <0.013
0-1 <0.013
3 <0.013
5-6 <0.013
9 <0.013
12 <0.013
Table 2 (continued)
Country/ No. of Formulation Application Interval after Residues, Reference
Crop Year trials rate (a.i.) final application (mg/kg)1
(days)
Watermelon Taiwan Province 3 x 6.25 0-1 0.023
peel of China 3 <0.013
5-6 <0.013
9 <0.013
flesh 12 <0.013
0-1 <0.013
3 <0.013
5-6 <0.013
9 <0.013
12 <0.013
Apple The Netherlands 2 50% W.P.5 2 x 25 g/hl 63 <0.013 Maclaine Pont et al. 1980
1978 84 <0.013
United Kingdom 2 25% W.P. 7 x 60 g/hl 0-1 0.473 Hayto 1976
1977 14-16 0.083
30-31 0.023
25% E.C. 8 x 51.48 g/hl 0-1 4.183 Hayto 1977c
8 1.763
18-19 0.683
30-31 0.173
50 0.023
Taiwan Province 1 25% E.C. 9 x 8.33 g/hl 0-1 0.243 Wang 1982
of China 3 0.123
1982 5-6 0.073
12 0.013
14-16 0.053
18-19 0.023
Table 2 (continued)
Country/ No. of Formulation Application Interval after Residues, Reference
Crop Year trials rate (a.i.) final application (mg/kg)1
(days)
Apple Taiwan Province 9 x 16.67 g/hl 0-1 0.813
of China 3 0.373
5-6 0.203
12 <0.013
14-16 0.083
18-19 0.073
Pear The Netherlands 1 50% W.P.5 1 x 0.1 kg/hl 21-25 0.033 Maclaine Pont et al. 1980
1978 1 x 0.2 kg/hl 21-25 0.063
2 x 0.1 kg/hl 14-16 0.263
2 x 0.2 kg/hl 14-16 0.243
Apricots Israel 1 50% W.P.5 1 x 60 g/hl 89 <0.05 Snowden 1983
1982
Cherries France 1 50% W.P.5 1 x 10 g/hl 70-73 <0.05
1982 1 x 20 g/hl 70-73 <0.05
4 x 20 g/hl 21-25 0.45
Nectarines France 1 50% W.P.5 5 x 20 g/hl 14-16 0.56
1982
Peaches South Africa 1 50% W.P.5 3 x 10 g/hl 132 <0.05
1982 1 3 x 20 g/hl 132 <0.05
1 8 x 10 g/hl 30-31 0.21
Plums Italy 1 40% E.C. 1 x 30 g/hl 102 <0.05
1982 1 2 x 30 g/hl 77 <0.05
1 3 x 30 g/hl 50 0.82
Table 2 (continued)
Country/ No. of Formulation Application Interval after Residues, Reference
Crop Year trials rate (a.i.) final application (mg/kg)1
(days)
Plums South Africa 1 50% W.P.5 1 x 30 g/hl 50 0.21
1981 1 1 x 40 g/hl 50 0.28
1 2 x 30 g/hl 14-16 0.97
1 2 x 40 g/hl 14-16 0.60
1982 1 50% W.P.5 3 x 10 g/hl 104 <0.05
3 x 20 g/hl 104 <0.05
6 x 10 g/hl 5-6 0.12
4 x 10 g/hl 103 <0.05
4 x 20 g/hl 103 <0.05
7 x 10 g/hl 5-6 0.19
Grapes Taiwan Province 1 25% E.C. 2 x 4.17 g/hl 3 0.393 Wang 1982
of China 5-6 0.193
1982 8 0.203
9 0.103
12 0.073
21-25 0.103
39 0.053
42-46 <0.013
1 25% E.C. 2 x 6.25 g/hl 3 0.493
5-6 0.263
8 0.203
9 0.253
12 0.133
21-25 0.133
39 0.053
42-46 <0.013
Table 2 (continued)
Country/ No. of Formulation Application Interval after Residues, Reference
Crop Year trials rate (a.i.) final application (mg/kg)1
(days)
Grapes Taiwan Province 1 25% E.C. 7 x 10 g/hl 3 0.203
of China 5-6 0.133
8 0.293
9 0.273
12 0.143
21-25 0.223
39 0.083
42-46 0.083
1 25% E.C. 7 x 16.67 g/hl 3 0.423
5-6 0.203
8 0.163
9 0.603
12 0.673
21-25 0.343
39 0.183
42-46 0.103
Strawberries The Netherlands 1 50% W.P.5 2 x 0.1 kg/hl 30-31 0.173 Maclaine Pont et al. 1980
1978 1 2 x 0.15 kg/hl 30-31 0.173
Almonds Israel
shell 1982 1 50% W.P.5 2 x 0.1 kg/hl 0.616 Churchill & Longland 1983d
kernel 0.106
whole nut 0.296
Table 2 (continued)
1 Total residues of prochloraz and major metabolites unless otherwise stated. Total residue measured as 2,4,6-trichlorophenol (BTS 45186)
and converted to prochloraz-derived residues by correcting for the molecular weight factor (1.906).
2 Co-formulation with dicloran.
3 Prochloraz residues only.
4 Preplanting application.
5 Prochloraz-manganese complex 50% wettable powder formulation.
6 Applications made at blossom and 20 days later. Preharvest interval estimated at about 2.5 to 3 months.
Table 3. Supervised Residue Trials With Prochloraz on Citrus Fruits - Post-Harvest Applications
Residues (mg/kg)1
Application
Country/ Crop No. of Formulation rate (a.i.) Peel Flesh Whole Fruit3 Reference
Year Trials and method
Australia Oranges 1 40% E.C. 25g/hl dip3 1.40-3.4 <0.05 n.d. Browne 1981a
1981 (1.45-3.954) (0.3-0.104)
1 40% E.C. 50g/hl dip3 1.58-3.36 <0.05 n.d.
(2.25-3.354) (<0.02-0.054)
Italy Oranges 1 25% W.P. 50g/hl dip5 1.59-1.72 0.04-0.05 0.71-0.80 Browne & Manley
1981 1 25% E.C. 50g/hl dip5 4.57-6.40 0.09-0.16 1.80-2.35 1982b
Lemons 1 25% W.P. 50g/hl dip5 3.61-4.04 0.08-0.14 1.58-1.83
1 25% E.C. 50g/hl dip 5.67-6.95 0.08-0.19 2.36-2.97
South Oranges 1 40% E.C. 100g/hl wax 1.38-2.79 0.03-0.08 0.38-0.72 Manley & Snowden
Africa brush6 (0.34-0.478) 1982b
1982 1 40% E.C. 200g/hl wax 2.45-3.74 0.02-0.07 0.65-0.94
brush6 (0.91-1.198)
1 40% E.C. 200g/hl wax n.d. n.d. 1.36-1.698)
brush7
Oranges 1 45% E.C. 50g/hl brush9 0.34 <0.05 0.08 Snowden & Manley
(0.108) 1983
1 45% E.C. 100g/hl brush9 0.47 <0.05 0.13
(0.16-0.178)
1 45% E.C. 200g/hl brush9 1.84 <0.05 0.39
(0.39-0.438)
1 45% E.C. 400g/hl brush9 3.07 0.07 0.63
(0.70-0.758)
Table 3 (continued)
Residues (mg/kg)1
Application
Country/ Crop No. of Formulation rate (a.i.) Peel Flesh Whole Fruit3 Reference
Year Trials and method
Spain Oranges 1 25% E.C. 50g/hl dip10 1.48-3.224 0.04-0.10 0.67-1.4511 Kelly 1982d
1979 1 25% E.C. 100g/hl dip10 1.40-4.084 0.04-0.11 0.67-1.8011
1980 Oranges 1 40% E.C. 300g/hl wax 3.3-4.74 0.32-0.38 n.d. Richards 1980b
spray12
1 40% E.C. 300g/hl wax n.d. 0.005-0.00813 n.d. Richards 1980e
spray12
1 40% E.C. 200g/hl wax 1.2-6.7 0.04-0.44 n.d. Richards 1980f
spray12
1 40% E.C. 250g/hl wax 3.7-7.9 0.04-0.30 n.d.
spray12
1 40% E.C. 300g/hl wax 5.4-6.4 0.06-0.13 n.d.
spray12
1981 Oranges 1 40% E.C. 300g/hl shower14 4.7-8.4 0.11-0.39 1.50-2.47 Browne 1981b
(1.7-7.34) (0.12-0.394) (0.64-2.434)
1981 Lemons 1 40% E.C. 300g/hl 11.0-17.9 0.17-0.33 3.66-6.13 Browne 1982c
shower15 (9.1-15.64) (0.27-0.304) (3.14-5.554)
United Oranges 1 40% E.C. 70g/hl dip16 5.78-7.81 0.07-0.15 1.37-2.07 Browne 1982i
Kingdom (3.55-5.104) (0.05-0.124) (0.96-1.354)
1981
Table 3 (continued)
n.d. - not determined.
1 Total residues of prochloraz and major metabolites unless otherwise stated (see notes 4 and 13). Total residue measured as
2,4,6-trichlorophenol (BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular weight factor (x 1.906).
2 Residue levels in whole fruit calculated from separate peel and flesh residues unless otherwise stated (see note 8).
3 Residue levels obtained from five sampling intervals at 1,2,4,8 and 16 days storage at ambient temperature after treatment.
4 Determination of free prochloraz levels only.
5 Fruit was stored for 57 days at 7°C after treatment.
6 Residue levels obtained from four sampling intervals under two storage conditions. Treated fruit was sent to the United Kingdom under
normal commercial refrigerated shipment and then stored at either 20° or 4°C, 44 days later. Samples of fruit from each storage
condition were then taken at 0,7,14 and 21-day intervals.
7 Prochloraz applied in mixture with 2,4,-0 and thiabendazole. Storage conditions as in Note 6 except sampled after 44 days refrigerated
shipment and 21 days storage under ambient or cool conditions.
8 Residue analysis of whole fruit.
9 Fruit was treated in July 1982, sent to the United Kingdom under normal refrigerated shipment and stored at ambient temperature until
September 1982.
10 Residue levels following storage of fruit for either 10 days at 20-22°C or 60 days at 3-4°C after treatment.
11 Residue level calculated from determination of free prochloraz residue in peel and total prochloraz plus major metabolites in flesh.
12 Residue levels following storage of fruit for either 7 or 14 days at ambient temperature after treatment.
13 Determination of free 2,4,6-trichlorophenol (BTS 45186) only, expressed as 2,4,6-trichlorophenol.
14 Residue levels following storage of fruit for either 14, 20 or 27 days at ambient temperature after treatment.
15 Residue levels following storage of fruit for either 12 or 16 days at ambient temperature after treatment.
16 Residue levels following storage of fruit for either 1,7, 21, 35 or 70 days in cold storage after treatment.
Strawberries
No information was provided on good agricultural practices for
prochloraz on strawberries. Limited residue data from the use of the
manganese complex W.P. formulation were provided (Table 2) with
residues of 0.2 mg/kg prochloraz found 16 days after treatment.
Recoveries were reported as >80 percent and sensitivity as
0.001-0.005 mg/kg.
Fruit with Inedible Peel
No information on good agricultural practice was available for
the use of prochloraz on fruit with inedible peel. Residue data
resulting from either foliar (Table 4) and/or postharvest applications
(Table 5) were available for several of these crops. The "recommended"
application rates were provided for most of them but no preharvest
intervals were specified.
Avocados
No information on good agricultural practice was provided for
avocados. Residue data at normal harvest and "recommended" application
rates were available for both foliar and postharvest treatments. At 2X
the 10-25 g a.i./hl "recommended" foliar W.P. (manganese complex)
application rate, maximum total residues on whole fruit basis were 2.5
mg/kg seven days after treatment, with approximately 10 percent of the
residue in the flesh (Table 4). No preharvest interval was given.
However, these data are questionable because of conflicting peel
residue levels in the data submission. Data were also available from
multiple applications of an E.C. formulation at 25-50 g a.i./hl,
although not even "recommended" uses were available for this
formulation. In these trials residues continually declined from 14 to
63 days after treatment, with estimated half-lives ranging from 40-98
days (mean 75.4 days). At these longer intervals residues in flesh
account for 20-30 percent of the whole fruit residue. The limit of
determination was said to be 0.15 mg/kg, although control values were
as high as 0.12, 0.16 and 0.39 mg/kg in flesh, peel and whole fruit,
respectively.
For postharvest treatments of avocados 25-50 g a.i./hl
(formulation unspecified) is "recommended", although no good
agricultural practice information was available. Ten trials reflecting
the "recommended" application rates were conducted in one country with
dip or spray E.C. applications (Table 5). Total residues ranged from
0.3 to 3.5 mg/kg on a whole fruit basis, with little difference in
values between stored at 23° for eight days or fruit frozen
immediately. Little difference was reported between 1X and 2X
recommended application rates. These residues levels are generally
comparable to those found from multiple foliar treatments. The limited
data indicate residues in the flesh at levels up to 30 percent of
those in whole fruit, even at relatively short intervals after
treatment.
Table 4 Prochloraz Residues in Fruit Following Supervised Trials - Foliar Application
Country/ Crop No. of
Year trials Formulation Application Interval after Mean residue level (mg/kg)1 Reference
rate (a.i.) final application
(days) Peel Flesh Whole fruit2
Australia Avocado 1 50% W.P. 7x15g/tree3 7 8.1-13.6 0.09-0.28 1.70-2.51 Cron & Longland 1983a
1981/2 (49.5 g/hl) (11)7 (0.16) (2.1)
South Avocado 1 45% E.C. 2x25g/hl4 14 1.52 <0.10 0.44 Churchill &
Africa 48 1.19 <0.10 0.36 Longland 1983b
1982 63 1.01 <0.10 0.30
1 45% E.C. 2x37.5g/hl4 14 1.85 0.12 0.61
48 1.45 <0.10 0.25
63 0.84 <0.10 0.25
1 45% E.C. 2x50g/hl4 14 2.63 0.17 0.83
48 1.94 0.18 0.59
63 2.16 0.14 0.60
Australia Mango 1 50% W.P. 6x100g/hl5 77 0.15-0.30 0.01-0.02 0.05-0.096 Browne & Manley 1982c
1981 (0.22)8 (0.01)8 (0.07)8
Israel Mango 2 50% W.P. 3x50g/hl 15 3.39-4.87 0.03-0.07 0.77-0.956 Longland &
1982 (4.2)8 Churchill 1983
Table 4 (continued)
1 Total residues of prochloraz and major metabolites, measured as 2,4,6-trichlorophenol (BTS 45186) and converted to
prochloraz-derived residue by correcting for the molecular weight factor (x1.906).
2 Residue in whole fruit (without stone) calculated from separate peel and flesh residues.
3 Applications made at approximately monthly intervals from fruit set to fruit full size.
4 Applications made with a seven-week interval between treatments.
5 Applications made from beginning of flowering to Pethenoccupic fruit drop.
6 Residue level in whole fruit including stone (calculated from peel/flesh residues).
7 Mean in parentheses.
8 Four samples.
Table 5 Prochloraz Residues in Fruit Following Supervised Trials - Postharvest Application
Mean residue level (mg/kg)1
Country/ Crop No. of Formulation Application rate
Year trials (a.i.) Peel Flesh Whole fruit2 Reference
Australia Avocado 1 40% E.C. 25 g/hl dip4 n.d. n.d. 0.88-1.313 Browne 1982a
1981 1 40% E.C. 50 g/hl dip4 n.d. n.d. 1.14-1.353
1982 2 45% E.C. 25 g/hl dip4 3.28-5.35 <0.10-0.12 0:30-0.557 Churchill &
2 45% E.C. 25 g/hi dip6 n.d. n.d. 0.16-1.019 Longland 1983e
1 45% E.C. 50 g/hl spray4 2.33-3.90 <0.10-0.11 0.28-0.367
1 45% E.C. 50 g/hl spray6 n.d. n.d. 0.34-0.429
1983 1 45% E.C. 25 g/hl dip6 n.d. n.d. 2.369
1 45% E.C. 50 g/hl dip6 n.d. n.d. 3.499
1981 Banana 1 40% E.C. 25 g/hl dip5 6.06-7.88 0.02-0.04 n.d. Browne 1982b
1 40% E.C. 50 g/hl dip5 5.18-9.97 0.03-0.05 n.d.
South Banana 2 45% E.C. 25 g/hl dip8 6.5-9.1 0.10-0.20 2.78-3.94 Churchill &
Africa 2 45% E.C. 50 g/hl dip8 9.8-16.6 0.14-0.23 4.02-6.75 Longland 1983a
1982
Israel Mango 1 40% E.C. 40 g/hl dip 6.10-8.32 0.02 1.277 Longland &
1982 Churchill 1983
Australia Papaya 1 45% E.C. 25 g/hl dip6 2.13-3.42 0.01-0.18 0.50 Cron & Longland 1983b
1982 1 45% E.C. 50 g/hl dip6 3.86-4.12 0.09-0.15 0.80
Table 5 (continued)
n.d. - not determined.
1 Total residues of prochloraz and major metabolites, measured as 2,4,6-trichlorophenol (BTS 45186) and converted to prochloraz-derived
residue by correcting for the molecular weight factor (x 1.906).
2 Residue in whole fruit calculated from separate peel and flesh residues unless otherwise stated (see notes 3 and 9).
3 Residue analysis of whole fruit (excluding stone).
4 Fruit stored for 7-8 days at 23°C after treatment.
5 Mean residue levels following storage of fruit for either 9,10,12 or 16 days at ambient temperature after treatment.
6 Fruit frozen within one to four hours after treatment.
7 Calculated whole fruit residue including weight of stone.
8 Fruit stored for 7 days at 8°C after treatment.
9 Residue analysis of whole fruit (including stone).
Bananas
No information was provided on goad agricultural practices for
the use of prochloraz on bananas. "Recommended" application rates for
foliar application are 10-25 g a.i./hl WP (manganese complex) for
foliar high volume applications (100-200 g a.i./ha low volume) and
25-50 g a.i./hl for postharvest treatment (formulation not specified).
No trials data were available for foliar applications, but trials
were reported from two countries for postharvest dips that reflected
"recommended" application rates (Table 5). Total prochloraz and
metabolite residues on a whole product basis ranged from 2.8-6.8 mg/kg
when fruit was stored for seven days at 8°C, with less than 2 percent
of the total fruit residue found in the flesh. No significant change
in residues were observed over the storage intervals of 7 to 16 days
or between 8°C and ambient temperatures. No significant difference
between residues resulted from the two application rates.
Analytical recoveries were generally 85 ± 15 percent. The limit
of determination was estimated at 0.05-0.1 mg/kg, although apparent
residues in untreated controls were as much as 0.32 mg/kg in the skin
and <0.2 mg/kg in whole fruit.
Mangoes
No information on good agricultural practice was available for
the use of prochloraz on mangoes. A 50 percent W.P. manganese complex
is "recommended" for foliar treatments at 10-25 g a.i./hl and at 25-50
g a.i./hl for postharvest treatment, although no formulation is
specified for the latter. One trial for foliar application was
conducted in each of two countries with multiple applications at 2X to
4X the "recommended" foliar rate (Table 4). Maximum total residues on
a whole fruit basis were 0.09 mg/kg from the higher application rate
77 days after treatment and up to 1 mg/kg 15 days after application at
the lower rate. In both cases, most of the residue was in the peel,
although flesh residues appeared to increase with time, being
approximately 3 percent of total fruit residue after 15 days to over
10 percent after 77 days (based on peel: flesh:seed ratios from the
77-day study).
One trial was conducted in one country for postharvest treatment
which reflected the "recommended" application rate. At 1.27 mg/kg on a
whole fruit basis, residues were similar to those of the 15-day
preharvest trial, although residues in flesh were only approximately
1 percent of the total for the postharvest treatment. Over-all
analytical recoveries for mangoes were generally 80 percent or better
and apparent residues in untreated controls were <0.02 mg/kg on a
whole fruit basis and up to 0.1 mg/kg on peel. The limit of
determination was estimated at 0.1 mg/kg.
Papaya
No information was available on good agricultural practices for
prochloraz on papaya. A 50 percent W.P. manganese complex is
"recommended" for foliar treatment at 10-25 g a.i./hl and 25-50 g
a.i./hl (formulation not specified) is "recommended" for postharvest
treatments. No preharvest intervals were given.
No residue information was available from foliar treatments, but
two postharvest dip trials with an E.C. formulation were conducted in
one country and reflected the "recommended" application rates (Table
5). Samples were frozen one hour after the dip treatment for analysis
45 days later. On a whole fruit basis, residues of prochloraz and
metabolites (corrected for approximately 80 percent recoveries) were
0.5 and 0.8 mg/kg for the two application rates. Approximately 15
percent of the whole fruit residue was in flesh. Apparent residue in
the untreated control was 0.023 mg/kg on a whole fruit basis. The
limit of determination was estimated as 0.1 mg/kg in flesh and peel.
Cereal Grains
Over 270 residue trials were conducted in 10 European and one
Asian country, which included foliar or seed treatments with six
different E.C. formulations alone or in combination with other
chemicals. Results are reported mostly as total residues of prochloraz
and metabolites, determined as the trichlorophenol but expressed as
prochloraz (Tables 6-9). Some are for residues of prochloraz only.
Good agricultural practice information was available for eight
countries.
Wheat
Over 100 field trials were conducted in nine countries,
representing 270 samples from a variety of formulations and treatment
conditions. Some trials reflected known good agricultural practices
and good agricultural practices could not be determined for others.
Over-all residues of prochloraz and major metabolites in mature grain
from foliar treatment ranged from 0.01-0.3 mg/kg and in straw at
harvest 0.08-16 mg/kg, although most grain residues were <0.1 mg/kg
(Table 6). The high grain value is from a 38-day preharvest interval
in a country whose good agricultural practices are not known, although
the application rate and preharvest interval were compatible with good
agricultural practices in other countries. Apparent residues in
untreated controls were generally <0.02 mg/kg in grain and <0.3
mg/kg in straw, but were occasionally higher in the latter. Residues
of prochloraz alone were lower and ranged from <0.01 to 0.03 mg/kg in
mature grain and <0.01 to 0.19 mg/kg in straw.
Table 6. Supervised Residue Trials with Prochloraz on Wheat at Harvest
Country/ Formulation No. of Application rate2 No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples final application
(days) grain straw
Austria 25% E.C. 2 0.5 2 70-78 <0.013 0.013 Kelly 1979a
1977 2 1.0 2 70-78 <0.01-0.013 <0.013
1978 22.5% E.C. 1 0.45 4 94 <0.02-0.02 3.66-8.39 Kelly 1979f
1 0.9 4 94 0.02-0.04 11.63-12.20
Belgium 45% E.C. 1 0.45 2 (0)5 0.02 n.d. Housden 1982a
1981 1 0.454 2 (0)5 0.01 n.d.
Denmark 25% E.C. 3 0.625 6 95-98 <0.02-0.02 n.d. Kelly 1979g
1979
40% E.C. 1 0.6 2 77 <0.02 2.1-2.5 Richards 1980d
1982 45% E.C. 1 0.45 1 56 0.106 2.01 Housden 1982c
2 2 x 0.23(19)7 2 75-85 <0.02 1.47-1.94
2 0.23 x 0.45(19)7 2 75-85 <0.02 1.87-2.36
2 2 x 0.45(19) 2 75-85 <0.02-0.02 1.56-2.07
2 2 x 0.45(19)7 2 75-85 <0.02 1.72-2.79
Finland 45% E.C. 2 0.45 2 28-50 <0.02 n.d. Heinanen 1983
1982
France 25% E.C. 2 0.5 6 105 <0.01-0.023 <0.01-0.023 Hayto 1978b
1977 2 1.0 6 105 <0.01-0.013 <0.01-0.183
2 2 x 0.5(38-46) 6 67-69 <0.01-0.033 0.01-0.123
2 2 x 1.0(38-46) 6 67-69 <0.01-0.013 0.01-0.0733
1 0.5 3 105 <0.0058 n.d. Kelly 1980b
1 1.0 3 105 <0.0058 n.d.
1 2 x 0.5(38) 3 67 <0.0058 n.d.
1 2 x 1.0(38) 3 67 0.008-0.0158 n.d.
Table 6 (continued)
Country/ Formulation No. of Application rate2 No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples final application
(days) grain straw
1978 25% E.C. 3 0.45 18 55-113 <0.02-0.06 0.50-9.66 Kelly 1979h
3 0.75 18 55-113 <0.02-0.11 0.88-9.91
1979 40% E.C. 1 0.45 3 80 <0.02 2.42-3.22 Richards 1980a
3 2 x 0.45(23-28) 9 72-77 0.04-0.10 2.40-16.3
1 2 x 0.45(8)9 3 77 0.04-0.08 3.93-4.80
30% E.C.10 1 0.45 3 80 <0.02 2.23-2.55
3 2 x 0.45(22) 9 61-81 <0.02-0.08 2.29-6.16
Federal 25% E.C. 3 0.5 4 81-107 <0.01-0.013 0.01-0.023 Hayto 1979c
Republic of 2 0.75 3 81 <0.013 0.01-0.023
Germany 1 1.0 1 107 <0.013 <0.013
1977
1978 25% E.C. 2 0.5 4 56-61 0.06-0.08 3.6-12.0 Kelly 1979c
1979 40% E.C. 3 2 x 0.48(21-30) 3 54-63 <0.02-0.06 1.0-5.9 Richards 1980g
1980 40% E.C. 3 2 x 0.48(17-23) 3 59-66 0.01-0.04 0.7-2.0 Reary 1981b
3 3 x 0.48(11-23/7-13) 3 59-66 0.03-0.06 1.1-3.6
1981 40% E.C. 5 3 x 0.48(11-29/8-19) 10 57-64 0.02-0.13 1.07-6.40 Browne 1982e
2 2 x 0.48(28-30) 3 43-60 0.06 1.88-3.59 Browne 1982f
3 3 x 0.48(9-22/8-19) 7 43-66 0.05-0.08 4.05-4.55
1 3 x 0.48(17/6) 1 55 0.05 0.4811
1982 30% E.C.10 1 0.45 3 85 <0.05 0.08-0.27 Housden &
Longland 1983
Table 6 (continued)
Country/ Formulation No. of Application rate2 No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples final application
(days) grain straw
Italy 25% E.C. 2 0.07 6 55 <0.01-0.013 0.02-0.093 Hayto 1977b
1977 3 1.0 9 39-55 <0.01-0.013 0.02-0.193,12
1978 25% E.C. 1 0.5 2 38 0.08-0.17 4.82-15.76 Kelly 1979d
1 0.7 38 0.13-0.29 6.29-14.73
The 25% W.P. 1 0.375 3 71 <0.013 <0.013 Hayto 1978a
Netherlands 25% E.C. 1 0.375 3 73 0.01-0.023 <0.01-0.013
1977 1 0.5 3 73 0.01-0.023 0.01-0.023
1978 25% E.C. 1 0.375 3 68 0.04 1.14-1.51 Kelly 1979e
1 0.75 3 68 0.04-0.06 5.66-8.14
1979 40% E.C. 1 0.5 4 71 <0.02 0.88-2.23 Kelly 1980c
1980 45% E.C. 2 0.45 6 69 0.01-0.02 0.41-0.77 Reary 1981c
1 0.675 2 106 0.01 0.21-0.51
1 2 x 0.45 (15) 3 69 0.01-0.02 2.21-3.29
Sweden 45% E.C. 8 0.45 8 67-91 <0.02 0.08-4.08 Longland 1983a
1982 2 2 x 0.45(15-29) 2 58-69 0.03-0.07 1.29-6.34
United 25% E.C. 2 0.4 6 90-115 <0.013 <0.013 Hayto 1977a
Kingdom 2 1.0 6 90-115 <0.013 <0.013
1976
1977 25% E.C. 4 0.5 12 124-126 <0.013 <0.013 Hayto 1979a
1979 40% E.C 2 2 x 0.4(36-37) 6 69-79 <0.02-0.06 4.2-11.2 Richards 1980c
10% S.D.13 1 0.214 2 31015 <0.02 <0.02
Table 6 (continued)
n.d.- not determined.
1/ Total residues of prochloraz and major metabolites, unless otherwise stated (see Notes 3 and 8). Total residue as 2,4,6-trichlorophenol
(BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular weight factor (x 1.906).
2/ Figures in parentheses indicate interval in days between applications.
3/ Determination of free prochloraz residues only.
4/ Prochloraz applied in mixture with carbendazim.
5/ Growth stage at application : 0 - heading.
6/ Residue analysis of whole ears.
7/ Prochloraz applied in mixture with fenpropimorph.
8/ Determination of free 2,4,6-trichlorophenol (BTS 45186) residues only, expressed as BTS 45186.
9/ Prochloraz applied in mixture with mancozeb.
10/ Co-formulation of prochloraz with carbendazim.
11/ Mean residue level in whole ears. A mean residue level of 2.31 mg/kg was found in the whole plant.
12/ Results from two trials.
13/ Seed dressing co-formulation with carboxin.
14/ Rate of application in g a.i./kg seed.
15/ Days after sowing treated seed.
Barley
Information on good agricultural practice for barley was
available from five countries and residue trials reflecting such uses
were reported from those countries and others (Table 7). At-harvest
residues of prochloraz and its major metabolites, from foliar
applications, ranged from <0.01-0.68 mg/kg in mature grain and
<0.01-15 mg/kg in straw (Table 7). Again, the higher values are
generally from the shorter preharvest intervals, which reflect good
agricultural practice, and from trials in which prochloraz was
co-applied with either carbendazim or mancozeb. The rates reflect
usage in the country where the trials were conducted. Most residues in
grain were <0.2 mg/kg. Although grain residues from these shorter
intervals were significantly higher than those from longer intervals,
residues in straw were not.
Analytical recoveries were variable, with means ranging from
70-100 percent. Apparent residues in untreated controls were <0.02
mg/kg in grain and up to 0.2 mg/kg in straw. Residues of prochloraz
alone were substantially lower, ranging from <0.01-0.02 mg/kg in
mature grain and <0.01-0.15 mg/kg in straw, which are comparable to
the values reported in wheat.
Rye
Total prochloraz and its metabolites, from foliar treatments in
mature rye grain or whole ears ranged from <0.02-0.1 mg/kg and in
straw from 0.4-2.5 mg/kg. Application rates reflected good
agricultural practice, although the preharvest interval of 72 to 109
days was significantly greater than the one month permitted (Table 8).
Analytical recoveries were typically >80 percent in grain and
somewhat lower in straw. Apparent residues in untreated controls were
<0.01 mg/kg in grain but as high as 0.04 mg/kg in straw.
Oats
Information on good agricultural practice for oats was not
available for the country in which residue trials were conducted or
from other countries. Residues of prochloraz and its metabolites were
<0.03 mg/kg and <1.6 mg/kg for whole ears and straw, respectively,
62 days after treatment at application rates considered good
agricultural practice for other cereals (Table 8).
Rice
No information on good agricultural practice was available for
rice, except for seed treatments in one country (for which there are
no data). Residues of prochloraz alone in unpolished brown rice 7-21
days after treatment at application rates comparable to good
agricultural practice on other cereals were <0.05 mg/kg (Table 8).
Residues of prochloraz and its metabolites would be expected to be
higher. Residues from seed treatments were lower, but the interval
after treatment was 151-168 days. Analytical recoveries were 86
percent in grain and apparent residues in untreated controls were
<0.005 mg/kg, the limit of detection.
Summary data for residues in cereal grain at harvest (Table 9)
represents foliar applications of all formulations. The data in Table
9 confirm those reported in Tables 6-8, since it is statistically
shown that maximum residues of prochloraz and its metabolites would
normally be less than 0.2 mg/kg at the longer preharvest intervals but
residues up to 0.4 mg/kg can occur. Other trials at shorter intervals
also resulted in higher grain residues than at longer intervals.
Similarly, Table 11 confirms the findings reported in Tables 6-8 for
residues in straw (up to 15 mg/kg). Tables 9 and 11 do not include
more recent studies (Housden 1982c; Longland 1983a; Housden & Longland
1983; Heinanen 1983) reported in Tables 6-8, but these would not
increase expected residues. Tables 9 and 11 also indicate that higher
residues can be expected from multiple or higher doses.
Residues of prochloraz alone were <0.03 mg/kg in cereal grains
at harvest, except for rice where residues were as much as 0.05 mg/kg
7 to 21 days after treatment. From seed treatment alone, residues of
prochloraz and its metabolites in mature grain were <0.02 mg/kg.
Generally, there was no conclusive evidence of increased residues as a
result of co-application of prochloraz and other agricultural
chemicals.
Residue decline studies are summarized in Table 10. Half-lives of
approximately 6 to 17 days could be estimated for ears or whole plants
of wheat or barley on the basis of sampling for at least three
successive intervals. Residues of prochloraz and its metabolites
ranged from 0.15 to 21 mg/kg on green plants or ears on the day of
last treatment to 0.07-3.6 mg/kg at 21 to 48 days, which approximates
typical preharvest intervals.
Almonds
No information was available on good agricultural practice for
prochloraz on almonds. Foliar treatments with the 50 percent W.P.
manganese complex are "recommended" at 15-30 g a.i./hl. No preharvest
interval has been reported. One trial was conducted with foliar
treatments at seven times the maximum recommended application rate
(Table 2) with total residues corrected for recoveries of >86
percent of 0.61, 0.1 and 0.29 mg/kg in shell, kernel and whole nut
(calculated from shell and kernel residues), respectively, about three
months after the last treatment. Apparent residues were 0.06 mg/kg in
un-treated controls on a whole nut basis and up to 0.09 mg/kg in the
shell. The limit of determination was estimated at 0.1 mg/kg.
Table 7 Supervised Residue Trials with Prochloraz on Barley at Harvest
Country/ Formulation No. of Application rate2 No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples final application
(days) grain straw
Austria 25% E.C. 2 0.5 2 61-73 <0.013 <0.01-0.02
1977 1 0.75 1 73 <0.013 0.023
1 1.0 1 61 <0.013 0.033
1 2x0.5(19) 2 54 <0.013 <0.013
1978 22.5% E.C. 2 0.45 69-75 <0.02-0.04 0.65-1.30 Kelly 1979f
1 0.9 75 0.02-0.04 1.0-2.1
Belgium 45% E.C. 3 0.45 6 (M)10 0.01-0.03 n.d. Housden 1982a
1981 7 0.4511 14 (M)10 0.01-0.11 n.d.
2 2x0.4511 (I+M)10 0.02-0.03 n.d.
Denmark 25% E.C. 1 0.75 96 <0.02 n.d. Kelly 1979g
1979 40% E.C. 2 0.6 76-86 <0.02-0.10 1.9-4.6 Richards 1980d
20% E.C. 1 0.6 86 <0.02-0.04 2.5-3.4
20% S.D. 2 0.28 132-1399 <0.02 <0.02
1982 45% E.C. 2 0.234 3 75-87 <0.02-0.03 0.84-1.10 Housden 1982c
4 0.45 4 61-80 <0.02-0.02 1.45-3.24
1 0.45 1 60 0.0514 3.02
2 2x0.45(14) 61-73 <0.02-0.02 1.61-2.59
France 25% E.C. 2 0.5 62-74 <0.01-0.013 <0.01-0.053 Hayto 1978b
1977 2 1.0 62-74 <0.01-0.023 0.01-0.153
1978 25% E.C. 1 0.45 64 0.02-0.06 1.77-8.86 Kelly 1979h
1 0.9 64 0.02-0.08 2.67-10.48
1 2x0.45(19) 62 0.04-0.09 4.23-7.00
1 2x0.9 (19) 62 0.04-0.17 11.19-11.95
Table 7 (continued)
Country/ Formulation No. of Application rate2 No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples final application
(days) grain straw
1979 40% E.C. 2 0.45 4 54-62 <0.02-0.17 5.76-10.81 Richards 1980a
2 2x0.45(21)5 6 38 0.36-0.68 7.20-12.12
30% E.C.6 2 2x0.45(13-21) 38-48 0.10-0.57 6.96-9.47
Fed. Rep. 25% E.C. 1 0.5 1 70 <0.013 <0.013 Hayto 1979c
of Germany 1 0.7 1 70 <0.013 <0.013
1977
1980 40% E.C. 2 0.48 77-79 0.02-0.06 0.06-1.0 Richards 1981b
2 2x0.48(19)12 77-79 0.02-0.06 1.1 -1.3
1981 40% E.C. 2 2x0.48(11-12) 61-62 0.02-0.06 0.95-1.27 Browne 1982f
1982 30% E.C.6 3 0.45 62-76 <0.05 0.23-0.83 Housden & Longland
1983
Netherlands 45% E.C. 1 0.45 3 70 0.04-0.06 0.78-1.21 Reary 1981c
1980
Sweden 45% E.C. 4 0.45 61-72 <0.0213 0.19-1.27 Longland 1983a
1982 2 2x0.225(8-15) 53-55 <0.02 0.24-1.21
2 2x0.45(8-15) 53-55 <0.02 0.34-2.86
United 25% E.C. 3 0.4 54-74 <0.013 <0.01-0.023,7 Hayto 1977a
Kingdom 4 0.5 19-64 <0.013 0.01-0.023,13
1976 2 1.0 64-74 <0.013 <0.013,20
1977 25% E.C. 4 0.5 76-78 <0.013 0.01-0.043 Hayto 1979c
2 1.0 76-78 <0.01-0.01 <0.01-0.02
1979 40% E.C. 3 2x0.4(18-29) 36-63 0.06-0.32 3.0 -14.5 Richards 1980c
Table 7 (continued)
n.d. not determined.
1/ Total residues of prochloraz and major metabolites, unless otherwise stated. Total residue measured as
2,4,6-trichlorophenol (BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular
weight factor (x 1.906).
2/ Figures in parenthesis indicate interval in days between applications.
3/ Determination of free prochloraz residues only.
4/ Prochloraz applies in mixture with fenpropimorph.
5/ Prochloraz applied in mixture with mancozeb.
6/ Co-formulation of prochloraz with carbendazim.
7/ Results from two trials.
8/ Rate of application in g a.i./kg seed.
9/ Days after sowing treated seed.
10/ Growth stages at application: M-heading, I-first joint.
11/ Prochloraz applied in mixture with chlorothalonil.
12/ Interval between treatments not given in one trial.
13/ Results from three trials.
14/ Residue analysis of whole ears.
Table 8 Supervised Residue Trials with Prochloraz on Cereals at Harvest
Crop Country/ Formulation Number of Application rate No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples application
(days) grain straw
Rye Austria 25% E.C. 1 0.5 1 91 0.013 <0.013 Kelly 1979a
1977 1 1.0 1 91 <0.013 <0.013
Denmark 45% E.C. 2 0.45 2 72-77 <0.02-0.10 1.44-2.48 Housden 1982c
1982
Fed. Rep. 40% E.C. 2 0.48 4 89-109 0.03-0.05 0.39-0.53 Browne 1982d
of Germany
1981
Oats Denmark 45% E.C. 2 0.45 2 62 0.02-0.034 1.19-1.64 Housden 1982c
1982
Rice Japan 25% E.C. 2 0.75 12 7-21 <0.01-0.043 n.d. Gato 1980
(brown, 1980 25% E.C. 2 3x0.75 12 7-21 <0.01-0.043 n.d.
unpolished) 25% E.C. 2 25 g/hl2 + 12 7-21 <0.01-0.043 n.d.
3x0.75
25% E.C. 2 25 g/hl2 4 151-168 <0.013 n.d.
n.d. = not determined.
1/ Total residues of prochloraz and major metabolites, unless otherwise stated. Total residues measured as 2,4,6-trichlorophenol
(BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular weight factor (x1.906).
2/ 48-hour seed soak.
3/ Determination of free prochloraz residues only.
4/ Residue analysis of whole ears.
Table 9 Summary of Residues in Mature Grain
Crop Application No. of Total residue (mg/kg)
(kg a.i./ha) results
Mean Std. Dev.
Wheat 1 × 0.375-0.5 36 0.03 0.03
Wheat 2 × 0.375-0.5 36 0.04 0.03
Wheat 3 × 0.375-0.5 12 0.06 0.03
Wheat 1 × 0.6-0.9 31 0.07 0.18
Barley 1 × 0.4-0.5 42 0.03 0.03
Barley 2 × 0.4-0.5 20 0.091 0.091
Barley 1 × 0.6-0.9 14 0.03 0.03
Barley 2 × 0.9 3 0.09 0.05
Rye 1 × 0.48 2 0.04
1/ Excluding a set of 11 results from four replicated applications
of prochloraz alone and with mancozeb, which were exceptionally
high (mean 0.42, std. dev. 0.18 mg/kg), residues in straw were not
exceptionally high. The high grain results may reflect poor
separation of grain and chaff in these samples (Richards 1980a).
Table 10 Supervised Trials on Residue Decline with Prochloraz
Crop Country/ No of Formulation Application rate2 Residues (mg/kg)1 after References
Year trials (kg/a.i./ha) final application (days)
35-36 56 75-85
Wheat Denmark 1 45% E.C. 0.45 whole plant 1.62 Housden 1982c
1982 whole ears 0.10
straw 2.01
2 45% E.C. 2x0.23(19)3 whole plant 0.33-0.94
grain <0.02
straw 1.47-1.94
2 45% E.C. 0.23+0.45(19)3 whole plant 1.11-1.76
grain <0.02
straw 1.87-2.36
2 45% E.C. 2x0.45(19) whole plant 0.70-2.02
grain <0.02-0.02
straw 1.56-2.07
2 45% E.C. 2x0.45(19)3 whole plant 1.38-1.44
grain <0.02
straw 1.72-2.79
Table 10 (Contd.)
Crop Country/ No. of Formulation Application2 After After final application (days) References
Year trials rate penultimate
(kg a.i./ha) application
(days)
20-29 0 15-22 37-42 54-63
Wheat Fed. Rep. 3 40% E.C. 2x0.48 green plant 0.80-1.644 1.62-2.554
of (21-30) ears 0.15-1.114 0.27-1.494 0.23-0.844 0.06-0.084 Richards
Germany grain <0.02-0.06 1980g
1979 straw 1.0-5.9
17-23 0 8-19 39-44 59-66
1980 3 40% E.C. 2x0.48 green plant 0.15-2.7
(17-23) green ears 4.7 -13.6 0.84-2.2 0.28-0.61 Reary
grain 0.01-0.04 1981b
straw 0.7 -2.0
7-13 0 8-19 39-44 59-66
3 40% E.C. 3x0.48 green plant 0.2 -4.7
(11-23/ green ears 5.5 -16.8 0.61-3.3 0.35-0.58
7-13) grain 0.03-0.06
straw 1.1 -3.6
8-19 0 7-14 31-46 58-64
1981 5 40% E.C. 3x0.48 green ears 0.16-0.96 3.87-12.50 2.42-5.85 0.31-1.16 Browne
(11-29/ whole plant 0.51-3.789 1982e
8-19) grain 0.04-0.31 0.02-0.13
straw 1.07-6.40
Table 10 (Contd.)
Crop Country/ No. of Formulation Application2 After After final application (days) References
Year trials rate penultimate
(kg a.i./ha) application
(days)
28-30 0 12-14 21-48 43-60
1981 2 40% E.C. 2x0.48 green ears 0.11-0.84 10.64-13.2 1.14-4.21 0.71-1.18 Browne
(28-30) whole plant 2.078 1982f
grain 0.038 0.06
straw 1.88-3.59
6-19 0 7-14 21-48 43-60
4 40% E.C. 3x0.48 green ears 2.96-8.27 13.90-21.40 3.61-7.25 0.49-2.32
(9-22/ whole plant 1.74-3.614 2.317
6-19) green ears+ 2.318
straw
grain 0.03-0.217 0.05-0.08
straw 4.05-4.55
ears 0.487
0 17 42 57 85
Wheat Fed. 3 30% E.C.5 0.45 whole plant 7.99-13.64 0.74-1.95 0.21-0.94 0.45.0.62 Housden &
Rep. of grain <0.05 Longland
Germany straw 0.08-0.27 1983
1982
34-36 60-87
Barley Denmark 2 45% E.C. 0.233 whole plant 0.64-0.74
1982 grain <0.02-0.03
straw 0.84-1.10
Table 10 (Contd.)
Crop Country/ No. of Formulation Application2 After After final application (days) References
Year trials rate penultimate
(kg a.i./ha) application
(days)
34-36 60-87
5 45% E.C. 0.45 whole plant 0.77-2.32
grain <0.02-0.02
straw 1.45-3.24
whole ears 0.058
2 45% E.C. 2x0.45 whole plant 1.03-3.18
(14) grain <0.02-0.02
straw 1.61-2.59
19 0 13 33-34 77-79
Fed. 2 40% E.C. 0.48 green plant 5.1-10.1 0.4-1.5 0.6-1.7 Richards 1981b
Rep. of grain 0.02-0.06
Germany straw 0.6-1.0
1980 2 40% E.C. 2x0.48 green plant 1.1-3.4 3.2-5.0 1.5-2.5 1.3-2.1
(19)6 grain 0.02-0.06
straw 1.1-1.3
11-12 0 10-18 27-48 61-62
1981 2 40% E.C. 2x0.48 green plant 0.91-7.99 10.40-18.60 Browne 1982f
(11-12) green ears 0.52-3.62 0.07-0.50
ears + straw 0.218
grain 0.088 0.02-0.06
straw 0.95-1.27
Table 10 (Contd.)
Crop Country/ No. of Formulation Application2 After final application (days) References
Year trials rate
(kg a.i./ha)
0 19 38-40 56 62-76
1982 3 30% E.C.10 0.45 whole plant 5.25-10.38 0.21-1.78 0.13-0.80 <0.05-0.78 Housden &
grain <0.05 Longland 1983
straw 0.23-0.83
35 72-77
Rye Denmark 2 45% E.C. 0.45 whole plant 0.85-1.62
1982 whole ears <0.02-0.10
straw 1.44-2.48
0 11 21 34 55-56 69 83 89 93 109
Fed. Rep. 1 40% E.C. 0.48 whole plant 9.6 1.16 0.20 Browne
of green ears 0.03 0.02 1982d
Germany ears 0.03
1981 grain 0.03 0.05
straw 0.39
1 40% E.C. 0.48 whole plant 13.8 1.33 0.05
green ears 0.14 0.05
ears 0.02
grain 0.10 0.03
straw 0.53
36-37 62
Oats Denmark 2 45% E.C. 0.45 whole plant 0.37-0.88 Housden
1982 whole ears 0.02-0.03 1982c
straw 1.19-1.64
Table 10 (Contd.)
1 Total residues of prochloraz and major metabolites, unless otherwise stated. Total residue measured as 2,4,6-trichlorophenol
BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular weight factor (x 1.906).
2 Figures in parentheses indicate interval in days between applications.
3 Prochloraz applied in mixture with fenpropimorph.
4 Results from two trials.
5 Co-formulation of prochloraz with carbendazim.
6 Interval between treatments not given in one trial.
7 Results from three trials.
8 Result from one trial.
9 Results from four trials.
Table 11 Summary of Residues in Mature Straw
Crop Application No. of Total residue (mg/kg)
(kg a.i./ha) results Mean Std. Dev.
Wheat 1 × 0.375-0.5 35 2.83 3.07
Wheat 2 × 0.375-0.5 36 4.85 3.23
Wheat 3 × 0.375-0.5 11 3.20 1.60
Wheat 1 × 0.6-0.9 22 5.03 4.42
Barley 1 × 0.4-0.5 20 3.30 3.05
Barley 2 × 0.4-0.5 26 6.95 3.64
Barley 1 × 0.6-0.9 13 3.36 1.92
Barley 2 × 0.6-0.9 3 11.6 0.4
Barley 1 × 0.48 2 0.46
No information on good agricultural practice was available for
the use of prochloraz on almonds. A 50 percent prochloraz-manganese
complex is "recommended" as a foliar treatment at a 15-30 g a.i./hl
application rate. Two applications at 3X this rate in one trial
resulted in maximum residues up to 0.7 mg/kg (Table 2). The limit of
determination has been estimated at 0.1 mg/kg although the apparent
residues in an untreated control were 0.07 mg/kg. Recoveries from
hulls amended with prochloraz at 1 mg/kg were 96±7 mg/kg.
Oilseed Rape
Information on good agricultural practice was available for
prochloraz on oilseed rape from two countries, from which data on
residue trials were also available. Residue data from 48 trials were
also reported from two countries for which good agricultural practice
was not known to the Meeting (Table 12). At preharvest intervals from
25 to 266 days, residues of prochloraz and its metabolites, reflecting
good agricultural practice, ranged from 0.01 to 0.33 mg/kg. Preharvest
intervals, if applicable, were not provided. The limit of sensitivity
was estimated at 0.05 mg/kg. However, since apparent residues in
untreated controls ranged from <0.01-0.15 mg/kg (but were usually
less than 0.05 mg/kg), a 0.2 mg/kg over-all limit of determination may
be more realistic. Analytical recoveries were > 85 ± 15 percent of
amended levels of 0.2-1 mg/kg.
Mushrooms
Information on good agricultural practice and corresponding
residue trials were available from two countries, as well as data from
residue trials from another country (total of 31 trials, Table 13).
The most common treatment was a single application of the
prochloraz-manganese complex formulation 1-10 days after casing at 1.5
g a.i./l sq m with a 10-day preharvest interval, or three applications
at 0.3 g a.i./l/m2, the first 1-10 days after casing and then after
the first and second flush, with a two-day pre-harvest interval. A
variation of the latter was also used at 2x 0.6 g a.i./l/sq m (Table
1), also with a two-day preharvest interval. Data extracted from Table
13 that reflect good agricultural practice are:
Country/ Applications Residue (mg/kg) Interval after Approved preharvest
and rate last application harvest
(g a.i./sq m) (days) interval (days)
The Netherlands 1 x 1.6 0.11-0.27 total residue 10 10
United Kingdom 2 x 0.6 1.4-1.6 total residue 4 2
0.6-0.8 free prochloraz
1 x 1.25 0.36 total residue 13 10
0.47 free prochloraz 28 10
3 x 0.3 0.24-0.43 total residue 2 2
0.07-0.1 free prochloraz 6 2
0.4-1.4 free prochloraz 3 2
Table 12 Supervised Residue Trials with Prochloraz on Rapeseed
Country/ Formulation No. of Application rate2 No. of Interval after Residues1 References
Year trials (kg a.i./ha) samples final application (mg/kg)
(days)
France 40% E.C. 2 0.45 8 73 0.02-0.04 Cron 1982
1980/1 2 0.453 8 73-76 0.01-0.03
1 0.60 4 73 0.02-0.03
30% E.C.4 2 0.45 8 73-76 0.02-0.03
1982 40% E.C. 4 0.45 12 37-51 0.03-0.15 Peatman & Snowden 1982
4 0.453 12 37-51 0.03-0.13
4 2x0.45 (12-15) 12 25-38 0.04-0.32
30% E.C.4 4 2x0.45 (12-15) 12 25-38 0.06-0.33
Denmark 45% E.C. 3 0.45 6 70-76 <0.05 Peatman & Snowden 1983
1982 3 2x0.45 (18-23) 7 53 0.07-0.19
Sweden 45% E.C. 3 0.45 6 47-72 0.12-0.28 Peatman & Snowden 1983
1982
United 40% E.C. 3 0.45 238-266 <0.05-0.07 Manley & Snowden 1982c
Kingdom 3 0.45 3 119-133 <0.05-0.19
1981/2 3 2x0.4(91-109)5 3 119-133 <0.05
3 3x0.4(91-109/ 3 64-75 <0.05-0.09
51-58)5
2 3x0.4(92-103/ 2 72-73 <0.05-0.06
51-57)
2 3x0.5(92-103/ 1 72-73 0.06
51-57)
Table 12 (continued)
1 Total residues of prochloraz and major metabolites, measured as 2,4,6-trichlorophenol (BTS 45186) and converted to
prochloraz-derived residue by correcting for the molecular weight factor (x 1.906).
2 Figures in parentheses indicate interval in days between applications.
3 Prochloraz applied in mixture with mancozeb.
4 Co-formulation of prochloraz with carbendazim.
5 Prochloraz applied in mixture with tolclofos methyl.
Table 13 Supervised Residue Trials with Prochloraz on Mushrooms
Country/ No. of No. of Application rate (a.i.) Residues (mg/kg)1 days after final application References
Year trials samples and timing2
3 5 10 14 20 21 27 45
Australia 1 1.25g/sq m at casing 0.1 0.1 <0.1 Housden
1981 1 2.50g/sq m at casing 0.23 0.13 0.14 1982
1 1.25g/sq m at casing +
0.63g/sq m after first flush 0.41-0.63 0.22
(24-day interval)
1 2.50g/sq m at casing +
1.25g/sq m after first flush 1.16-1.63
(24-day interval)
1 2.50g/sq m at casing +
2.50g/sq m after first flush <0.1
(24-day interval)
1 0.63g/sq m after first flush 1.06-2.98
1 1.25g/sq m after first flush 4.48-4.61
1 1.25g/sq m after first flush+
0.63g/sq m after second flush 0.29
(7-day interval)
1 2.50g/sq m after first flush+
1.25g/sq m after second flush 0.37
(7-day interval)
The 1 4 1.6g/sq m before inoculation 0.11-0.27 Browne
Netherlands 1 4 0.75g/sq m before inoculation+ 0.15-0.24 1982
1980 0.75g/sq m 14 days later
4 0.75g/sq m before inoculation+
0.75g/sq m after first flush 0.15-1.14
(23-days interval)
Table 13 (Continued)
Country/ No. of Application rate (a.i.) Residues (mg/kg)1 days after References
Year trials and timing2 1st application 2nd application 3rd application
19 7 4
United 1 0.61g/sq m after casing + 0.04 0.10 0.08 Richards 1981c
Kingdom 0.61g/sq m after first flush +
1980 0.61g/sq m after second flush
(19- and 10-day intervals)
1st application 2nd application
21 28 4 12 18
1 0.61g/sq m after casing + 0.04 0.04-0.11 1.37-1.58 <0.02
0.61g/sq m after second flush
(31-day interval)
14 0.61g/sq m after casing + 0.013 0.01-0.33 0.56-0.803 0.08-0.333 0.05-0.183 Whiteoak 1981
0.61g/sq m after second flush
(31-day interval)
Days after application
28 35 43 49
1 1.25g/sq m 7 days after inoculation 0.473 0.043 0.27-0.323 0.203
1 0.625g/sq m 7 days after inoculation 0.133 0.103 0.093 0.07-0.083
2nd application 3rd application
13 6 14 20
1 0.625g/sq m 7 days after inoculation 0.193 0.22-0.243 0.033 0.27-0.323
1 + 2x0.313g/sq m at 14-day intervals
thereafter
1 0.313g/sq m 7 days after inoculation
+ 2x0.313g/sq m at 14-day intervals 0.133 0.07-0.103 0-323 0.053
thereafter
Table 13 (Continued)
Country/ No. of Application rate (a.i.) Residues (mg/kg)1
Year trials and timing2 Days after application
2nd application 3rd application
5 12 3 14 21
1981 1 0.15g/sq m five days after inoculation 0.323 0.123 0.62-0.653 0.193 0.093 Whiteoak 1981
+ 2x0.15g/sq m at 18- and 14-day
intervals thereafter
45 0.3g/sq m + 2x0.3g/sq m. Timing as 0.20-0.373 0.11-0.643 0.39-1.443 0.10-0.313 0.06-0.073
given above
1 0.9g/sq m + 2x0.3g/sq m. Timing as 0.423 0.213 1.293 0.543 0.21-0.243
given above
1 1.5g/sq m + 2x0.3g/sq m. Timing as 0.273 0.523 0.993 n.d. 0.063
given above
1 3.0g/sq m + 2x0.3g/sq m. Timing as 0.623 0.543 0.743 0.343 0.953
given above
Table 13 (Continued)
Country/ No. of Application rate Residues (mg/kg)1 days after References
Year trials (a.i.) and timing2 1st application 2nd application 3rd application
13-14 15-16 18 3 5 7 10 12-13 14-16 2 3-4 7 9-10 12 14 17
United 1 0.3g/sq m 7 days after 0.24 0.20 0.79 0.426 0.30 0.30 0.377 0.23 0.51 0.43 0.33 1.03 0.32 0.23 0.28 0.22 Housden
Kingdom casing + 2x0.3g/sq m &
1981 at 18- and 14-day Whiteoak
intervals thereafter 1982
1982 1 0.3g/sq m 7 days after 0.26 0.14 0.85 0.24 0.24 0.26 0.58 0.24 0.61 0.45 0.61
casing + 2x0.3g/sq m (0.57)8 (1.11)8
at 15- and 17-day
intervals thereafter
Residues (mg/kg)1 days after application
13 15 18 22 25 28 31 34 36 39 42
1982 1 1.25g/sq m 7 days after casing 0.36 0.25 0.33 0.30 0.20 0.38 0.42 0.28 0.54 0.22 0.33
Table 13 (Continued)
n.d. = not determined.
1 Total residues of prochloraz and major metabolites, unless otherwise stated (see note 3). Total residue measured as
2,4,6-trichlorophenol (BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular weight factor (x1.906).
2 All treatments used the prochloraz-manganese complex 50% W.P. formulation.
3 Determination of residues of free prochloraz only.
4 Samples of mushroom also analysed for total residues of prochloraz and metabolites (see Richards 1981c)
5 Mean residue levels from four trials using spray volumes of 100, 200 or 400 l/sq.m.
6 Mean residue level in small and larger mushrooms (0.43 and 0.40 mg/kg respectively).
7 Mean residue level in small and larger mushrooms (0.52 and 0.21 mg/kg respectively).
8 Mushroom emerged before treatment (direct application).
Therefore, residues of prochloraz and its metabolites or of
prochloraz alone may be at least as much as 2 mg/kg from approved
practices. Differences between residues of prochloraz and its
metabolites, compared to free prochloraz, do not appear to be as high
as in cereals. Analytical recoveries of total residues were generally
> 70 percent, with apparent residues of 0.03 ± 0.02 mg/kg in
untreated controls and > 77 percent and 0.01 mg/kg for free
prochloraz.
FATE OF RESIDUES
The fate of prochloraz has been studied in plants and animals. In
plants, it is metabolized to
N-formyl-N'-1-propyl-N-(2-(2,4,6-trichlorophenoxy)ethyl) urea,
also known as BTS 44596 or the "formyl urea"; to
N-propyl-N-(2-(2,4,6-trichlorphenoxy)ethyl) urea, also known as
BTS 44595 or "the urea"; to 2,4,6-trichlorophenoxyacetic acid (BTS
9608), and finally to 2,4,6-trichlorphenol (BTS 45186), all of which
form stable conjugates (Figure 1). Animal metabolism has resulted in
characterization of urinary metabolites (see Figure 1 under
"Toxicology"), information of radioactivity distribution in tissues
from oral or dermal administration and rate and route of excretion.
Qualitatively, urinary metabolites are similar among several test
animals, although the major plant metabolites have not been identified
in the urine. Residues in animal tissues have not been identified.
Metabolism studies have also been conducted on the major plant
metabolite BTS 44596.
In Animals
Metabolism of prochloraz in rats, dogs, rabbits, mice and pigs is
discussed under "Biochemical Aspects".
Goat
A study was conducted to indicate the level of residues that
might occur in tissues and milk of lactating ruminant animals after
ingestion of prochloraz. A lactating goat was given two 60 mg oral
doses of 14C-phenyl labelled prochloraz by gelatin capsules, with 13
days between doses. The dosage was designed to reflect possible
residues resulting from consumption of 3 kg of straw with residues of
20 mg/kg of prochloraz at each dosing (versus maximum straw residues
of 12 mg/kg). Milk and plasma samples were taken at appropriate
intervals after each dose and tissue samples 24 h after the second
dose. After the first dose, residues of prochloraz equivalent in milk
decreased from 0.04 mg/kg after 8 h to 0.01 mg/kg (limit of
determination) after 48 h. Milk residues were 0.01 mg/kg and 0.03
mg/kg at 2.5 and 18.5 h, respectively, after the second dose. In
tissues, residues of prochloraz equivalent were 1.7 mg/kg in liver,
0.2 mg/kg in kidney, 0.36 mg/kg in adrenal, <0.05 mg/kg in fat,
0.03 mg/kg in muscle and <0.2 mg/kg in other tissues. Rumen
contents contained prochloraz equivalent of 0.65 mg/kg and bile 5.4
mg/kg. Residues were not characterized or identified (Campbell 1980).
A second and similar experiment was conducted using the
photodegradant and plant metabolite BTS 44596, with an interval of 8 h
between doses. Results were similar to those for prochloraz, although
the higher maximum milk residues of 0.07 mg/kg BTS-44596 equivalent 7
h after dosing decreased somewhat more rapidly (< 0.01 mg/kg after
31 h). Residues of BTS-44596 equivalent in tissues were somewhat less
than for prochloraz (liver 0.59 mg/kg, kidney 0.12 mg/kg, fat <0.01
mg/kg, muscle <0.01 mg/kg and less in other tissues as well),
Residues were not identified or characterized (Campbell & Needham
1980).
In another study a lactating goat was fed ad libitum for four
days with wheat straw containing 19 mg/kg field-incurred
14C-prochloraz equivalent residues. The straw was harvested at
maturity 11 weeks after treatment at an equivalent rate of 0.94 kg
a.i./ha (twice the recommended rate), Actual straw consumption was
approximately 300 g/day (or approximately 6 mg of prochloraz
equivalent/day), in addition to 1 700 g additional feed made available
daily. After four days, maximum tissue residues of prochloraz
equivalent, as determined by liquid scintillation counting was liver
0.05 mg/kg, kidney fat 0.04 mg/kg and 0.03 mg/kg in other tissues,
with a reported limit of determination of 0.02 mg/kg. Bile and rumen
residues were 0.12 and 0.13 mg/kg, respectively. Maximum residues in
milk were 0.006 mg/l and in plasma 0.079 mg/l. The limit of
determination for milk was 0.001 mg/kg in this experiment. Residues in
milk were relatively consistent over the four-day feeding period. Milk
residues were 0.002 mg/kg prior to the feeding of treated straw
(Campbell 1983).
In Plants
The fate of prochloraz has been studied in wheat (various
stages), citrus and apples. Soil uptake by sugar beet, which
represents a likely rotational crop, was also studied. The metabolic
fate in plants is illustrated in Figure 1.
Wheat
Wheat plants grown under glass were syringe treated at the sixth
leaf stage with a 25 percent a.i. formulation of 3H-labelled
prochloraz (labelled in the 3 and 5 positions of the benzene ring) at
a rate equivalent to 1 kg/ha. After 19 days, the green wheat tissue
was harvested and residues characterized and quantified by a variety
of analytical techniques. Neutral, acid and basic fractions were
analysed, with and without acid hydrolysis. After 19 days, 82.6
percent of the applied radioactivity remained and 96.7 percent of this
was extractable by the analytical procedures used (acetone-water
extraction). The principal residue was free BTS 44596, closely
followed by free and conjugated residues of BTS 44595, with lower
levels of free prochloraz and free and conjugated trichlorphenol,
conjugated trichlorophenoxyacetic acid and seven unknowns. Terminal
prochloraz equivalent was 7 mg/kg. Individual residues were:
% of applied % of terminal
radioactivity residue*
BTS 44596: N-formyl-N'-l-propyl-N-(2-(2,4,6-
trichlorophenoxy) ethyl) urea free 31.6 41
BTS 44595: N-propyl-N-(2-(2,4,6-trichlorophenoxy) free 11 13
ethyl) urea conj. 19.5 23.1
BTS 45186: 2,4,6-trichlorophenol free 1.4 1
conj. 6.8 4.9
BTS 9608 2,4,6-trichlorophenoxy-
acetic acid conj. 0.21 0.2
BTS 40542: Prochloraz (unchanged) free 1.1 1.5
Unknown (seven) 5.5 7.7
Radioactivity undetected 0.93 1.3
Radioactivity unaccounted for 4.56 6.3
82.6 100.0
* Expressed as prochloraz equivalents
None of the seven unknowns exceeded 4.3 percent of the terminal
residue. Analysis of barley plants treated in a similar manner
suggests that over 86 percent of the radioactivity applied to foliage
was absorbed by the leaf within 24 h (McDougall 1979).
In another study, separate plots of wheat at the flag leaf sheath
opening stage were treated with a 25 percent a.i. formulation of
either 3H-prochloraz labelled in the 3 and 5 positions of the phenyl
ring or 14C-prochloraz labelled in the 2 position of the imidazole
ring, at an equivalent rate of 1 kg a.i./ha. Mature wheat was
harvested 13 weeks after treatment and the grain, straw and chaff were
analysed.
Total residues, expressed as prochloraz equivalents, from the
phenyl-labelled prochloraz were grain 0.26 mg/kg, chaff 13.2 mg/kg and
straw 26.5 mg/kg. An almost identical distribution resulted from the
14C-prochloraz treated wheat. From the 3H-prochloraz treated wheat
76-80 percent of the recovered radioactivity was extractable from both
straw and grain. Attempts to hydrolyse polar fractions with HC1 were
unsuccessful, but better results were achieved with pyridine
hydrochloride, which hydrolyses conjugated residues containing the
2,4,6-trichlorophenyl moiety. The procedure liberates
2,4,6-trichlorophenol from both prochloraz and any metabolites
containing the 2,4,6-trichlorophenyl moiety. Results of the analysis
of the 3H-prochloraz treated wheat, expressed as a percentage of
recovered radioactivity in mature tissue, are summarized below:
Effects on Enzymes and Other Biochemical Parameters
In a series of investigations in male rats and mice prochloraz
was found to be an inducer of hepatic mixed function oxidases (MFO).
Rats were dosed orally with prochloraz at 10 and 100 mg/kg b.w. twice
daily for four days and killed 18 h after the last dose. Potent
induction of MFO occurred at the higher dose, but only a marginal
effect was seen at the low dose. Relative liver weight and microsomal
protein concentrations were increased at the high dose. The induction
spectrum of MFO was similar to that of phenobarbitone, with the levels
of cytochrome P-450, being increased by both dosages (Needham 1983a).
A similar MFO induction was noted when male rats were fed
prochloraz in their diet at 2 500 ppm for seven days. Rats fed a lower
dose (100 ppm) showed only a small increase in cytochrome P-450
(Riviere 1983).
The same pattern of hepatic MFO induction was observed in mice
after oral administration of doses of 10 and 100 mg/kg b.w. twice
daily for four days (Challis & Campbell 1983). Prochloraz was an
inducer of MFO following dietary administration at 325 and 1 300 mg/kg
in the diet for two weeks. When the compound was administered at 80
mg/kg for periods in excess of two weeks, the effect was marginal,
indicating that this concentration is close to the threshold level for
induction (Needham 1983b).
Effect of Dog Gastric Juice or Plasma on Prochloraz
In vitro studies showed prochloraz to be completely stable
after incubation with dog gastric juice or plasma for 30 min. at 37°C
or for 4 h at 37°C. Less than 2 percent underwent hydrolysis. The
initial step in the metabolism of prochloraz is therefore likely to
occur in the liver (Needham 1980).
TOXICOLOGICAL STUDIES
Special Study on Reproduction
Rat
Groups of rats (30 rats of each sex for the Fo generation and 25
of each sex for the F1 generation) were fed a diet containing 0,
37.5, 150 or 625 ppm prochloraz (nine weeks for Fo and eight weeks for
F1) prior to mating and throughout mating, gestation and lactation.
Two litters per generation were produced for two generations.
Observations included growth, food consumption, mortality and the
usual reproduction indices: mating, fecundity, male and female
fertility, gestation, lactation, pup mortality, litter and mean pup
weights, viability and terminal studies. In the males (Fo), receiving
625 ppm in the diet, a prolonged aggression was observed when rehoused
after both matings; this was not apparent among F1 males. Among Fo
females, the following overt clinical signs were noted in late
gestation and/or in the perinatal period: hunched posture, walking on
toes, piloerection and pallor. Sporadic mortality was recorded at the
different dose levels and was generally related to dystocia. Food
intake during the premating period showed no consistent dosage
relationship for all animals of both generations. However, consumption
was generally lower than that of the controls. Mean body weight gains
were depressed only in the premating period in the Fo animals of both
sexes, given 625 ppm, the same trend being noted during gestation.
There were no significant effects associated with mating performance
and pregnancy rate in the treated animals. Some adverse effects on
gestation periods were noted only at 625 ppm in both generations, with
a small number of females dying or being killed after exhibiting signs
of dystocia. There was also an increase in the proportion of females
with gestation periods in excess of 22 days.
A small number of females dosed at 625 ppm showed a total loss of
litters, a lowered mean litter size at birth, increased pup mortality
at birth and evidence of reduced pup body weight gain in the immediate
perinatal period at both matings of the Fo animals and at the first
mating of the F1a animals. A slight lowering of mean litter size at
both matings was recorded also for the Fo generation fed 150 ppm but
not for the F1 generation. However, none of the findings were
significantly different from the controls. At 37.5 ppm, a reduction in
the mean litter size at birth to day 12 postpartum was noted in the
first mating of the Fo generation. However, the results were again
within the range for controls at the testing laboratory.
No indication of any adverse incidence of structural anomalies
associated with treatment were recorded at terminal macroscopic
examination of the remaining F1a and F2a pups and all F1b and F2b
offspring.
Increased mean liver weight was recorded in weanling and adult
F1a males and F2a weanling females given 625 ppm in the diet. In the
same treated group, mean thymus weight in F1a weanlings of both sexes
and mean brain weight in the F1a female weanlings was lower than in
the controls. Microscopic examination of tissues of both sexes from
weanling F1a and F2a generations and F1a adults selected from
control and treated groups showed no changes that could be considered
attributable to exposure to the test compound; 37.5 ppm represented
the dietary no-effect level (Cozens et al 1982).
Special Studies on Teratogenicity
Rat
Groups of 20 mated Charles River CD rats were given oral doses of
6, 25 or 100 mg/kg daily from day 1 to 20 post coitum (day 1 was the
day when sperm was detected in the vaginal smear). A group of 31 rats
given the vehicle of 10 percent aqueous acacia solution served as
controls. Body weight, food consumption and overt signs of toxicity
were recorded throughout the study. The dams were killed on day 21 of
pregnancy.
The 25 and 100 mg/kg daily dosage elicited maternal toxicity as
evidenced by reduced food consumption, lower body weight and liver
enlargement; in addition, the dams showed increased salivation and
rubbed their noses in the sawdust litter. The only effect noted in the
rats given 6 mg/kg was a marginally lower body weight gain. There were
no macroscopic or microscopic findings attributable to treatment. The
number of corpora lutea was similar for all groups. The highest
dosage level appeared to be embryotoxic, as litter size, implantation
index and viability index were all slightly lower, while the incidence
of dead foetuses was marginally elevated. In addition, mean foetal
weight was lower, one litter containing unusually small foetuses that
appeared to have a gestational age of approximately 19 days. The mean
placental weight for both the male and female foetuses was higher for
the 25 or 100 mg/kg treated groups than for the control. A small
number of foetal abnormalities was noted among the treated groups but
no consistent pattern and no correlation between incidence and dosage
level was noted. The degree of calcification of the sternebrae and
vertebral arches was retarded in foetuses at the 100 mg/kg dose level.
This dosage elicited toxicity in both dams and embryos but produced no
teratogenic effects. The 25 mg/kg dosage was toxic in the dams. The
only findings in the 6 mg/kg group were marginally reduced body weight
gain and a very slight (non-significant) elevation of placental weight
(Beswick 1980).
Rabbit
Groups of 15 rabbits (New Zealand white strain) were administered
daily dosages of 0, 3, 12 and 48 mg/kg b.w. of prochloraz by
intragastric intubation on days 1 to 28 of pregnancy inclusive (day 1
= day of mating). The control group of 15 rabbits was dosed with the
vehicle, i.e. 10 percent acacia solution. All surviving dams were
killed on day 29 of pregnancy for macroscopic examination and the
foetuses removed for external visceral and skeletal examinations. No
treatment-related clinical signs and mortality were noted. Food intake
of the test group remained within 5 percent of the control value, with
a slightly reduced weight gain in all test groups during the first
week of dosing. The pregnancy rate was comparable for all groups. A
significant increase was noted in liver weight, with liver
discolouration in dams given 48 mg/kg day. A single dam from each of
the low-and high-dosage groups aborted completely, while one of the
control dams aborted partially. Litter size, post-implantation loss,
litter weight and mean foetal weight were considered unaffected by
treatment. Embryonic and foetal development, as assessed by incidence
of major malformations, minor anomalies and skeletal variations, were
similarly unaffected by treatment. No evidence of embryonic
teratogenic activity was therefore noted in New Zealand white rabbits
(Palmer et al 1980).
Special Studies on Mutagenicity
Ames test
Purified and technical prochloraz were tested in the Ames test
against Salmonella typhimurium TA 1535, TA 1537, TA 1538, TA 98 and
TA 100. All tests were performed in the presence and absence of a
liver microsome activation system (S-9) derived from
phenobarbitone-induced rats and tested at levels of 62.5, 125, 250,
500 and 1 000 mcg/plate. Positive control mutagens were included in
all tests to demonstrate the mutability of the bacterial strains and
the metabolizing capacity of the S-9 preparation; these comprised
cyclophosphamide, 6-aminochrysene and 2-animofluorene. Dimethyl
sulphoxide served as the negative control throughout. There was no
evidence of mutagenic activity against any of the strains employed but
the antibacterial effects of prochloraz precluded evaluation of
mutagenicity at concentration of 1 000, 500 and, in some cases, 250
mcg/plate (Wilcox 1977).
Micronucleus assay
Groups of five male and five female Charles River CD rats were
given two intraperitoneal injections of 6.25, 25.0 or 100 mg/kg b.w.
of prochloraz 24 h apart. Similar groups were given intraperitoneal
injections of cyclophosphamide 200 mg/kg b.w. to serve as positive
controls and intraperitoneal injections of the vehicle (maize oil) as
the negative controls. Six hours after the second injection all
animals were killed, smears from the bone marrow of the femur were
prepared and examined microscopically to determine the incidence of
micronuclei in the polychromatic erythrocytes. No significant increase
in the incidence of micronuclei was observed in any of the groups of
rats. Prochloraz was without clastogenic activity in rats of both
sexes when tested up to 100 mg/kg b.w. (Everest & Cliffe 1980).
Dominant lethal test
Groups of 20 male CD1 strain pathogen-free male mice (age 5-6
weeks) were given 0, 6, 25 or 100 mg/kg/day prochloraz in the diet for
eight weeks. After this treatment period, they were paired with
untreated females on a 1:1 basis for one week and then with a second
batch of untreated females for a further week. Approximately two weeks
after the discovery of a vaginal plug or 14 days after the midweek of
their pairing, the females were killed and their uterine contents
examined. There were no signs of reaction to treatment and mean weight
gains of all test groups compared favourably with that of the control
group. Intergroup variations in mean food consumption throughout the
entire treatment period were minimal and showed no consistent
dosage-related trend.
Mating performance of treated males and subsequent pregnancy
rates among untreated females were unaffected by treatment at any
dosage, except for one male at 25 mg/kg/day, which failed to induce
pregnancy at either mating. No obvious treatment-related macroscopic
changes were observed at terminal necropsy of the males. Mean weights
of the testes of treated males were slightly higher than the control
value but were not statistically significant. There was no evidence of
a dose-related effect on the pregnancy rate, mean number of
implantations, viable young, embryonic death and post-implantation
losses. Prochloraz showed no evidence of a dominant lethal effect in
male mice (Cozens et al 1980).
Mouse lymphoma mutation assay
Technical prochloraz was tested for mutagenic activity in mouse
lymphoma cells L5178 Y rendered heterozygous at the thymidine kinase
(TK) locus. The tests were carried out in the presence and absence of
a post-mitochondrial supernatant fraction from Arochlor 1254-treated
male rat livers and the co-factors required for mixed-function oxidase
activity. In an initial test carried out over a dose range of 0.16
mcg/ml to 1.6 mg/ml indicated, that prochloraz was toxic to the cells,
causing extensive cytotoxicity at a concentration of 158 mcg/ml. In
two further independent experiments at 50.0 mcg/ml and the second at
70.0 mcg/ml no significant increases in mutation frequency in the
prochloraz-treated cells over the vehicle control values were
observed. The concurrent positive control substances were
ethyl-methanesulphonate, which does not require the presence of S-9
mix, and 2-acetylaminofluorene, which requires the presence of S-9 mix
for metabolism. In both cases, high mutation frequencies were
obtained.
Technical prochloraz was not mutagenic to mouse lymphoma L 5178 Y
cells when tested over a series of dose levels, which extended into
the toxic range, in the presence or absence of S-9 mix (McGregor
et al 1983).
Special Studies on Carcinogenicity
Mouse
Groups of 52 male and 52 female mice CD-1 strain mice were fed
technical prochloraz in the diet at 78, 325 and 1 300 ppm. For each
sex, treatment continued until survival reached 20 percent, which was
106 weeks for males and 121 weeks for females. An additional group of
104 males and 104 females received the normal diet only. Observations
included clinical signs, mortality, body weight, food consumption and,
at 52 weeks and terminally, haematology and weight of selected organs.
Comprehensive histopathological examinations were made of tissues from
all surviving animals. Clinical signs were not evident, except in mice
given 1 300 ppm and in males treated at 325 ppm which were killed
because of distended abdomens. These animals were subsequently found
to have multiple liver tumours. No other overt signs of toxicity
related to treatment were observed. There was no mortality in the
course of the study attributable to prochloraz. Mice given 1 300 ppm
showed slightly reduced body weight gain. No differences were noted,
that were considered to be of toxicological significance, in the
haematological parameters between treated and control mice. Slightly
lower haemoglobin concentrations and lower total white blood cell
counts, due to a lower lymphocyte count, were noted at week 104 in
female mice treated with 1 300 ppm in diet.
At necropsy, an increase of liver weights was recorded in both
sexes given 1 300 ppm prochloraz. There was a dose-related increase in
the incidence of adenomas and adenocarcinomas of the liver in both
males and females. At 1 300 and 325 ppm, the increased incidence was
statistically significant and liver tumours (of hepatocyte origin)
were observed in both sexes, which were associated in a number of mice
with more than one tumour. Some cases at 1 300 ppm showed multiple
tumours as contributory factor to death. At 78 ppm, a slightly higher
incidence of liver tumours in female mice (6 out of 52) compared to
controls (5 out of 104) was noted, though the difference was not
statistically significant. In the males, the incidence of liver
tumours was similar in controls (37 out of 104) and treated mice (21
out of 52). There was no evidence of any other effects of treatment in
terms of either neoplastic or non-neoplastic pathology.
Treatment of mice was associated with a dose-related increase,
evident in both sexes, of liver tumours of all types. The incidence of
liver tumours was statistically significant at 1 300 ppm and 325 ppm
but not at 78 ppm prochloraz. The dietary dosage of 78 ppm was
equivalent to 7.5 mg/kg/day in males and 8.8 mg/kg/day in females
(Colley et al 1983).
Special Studies on Skin and Eye Irritation
A moderate to severe skin irritation produced in the rabbit
suggests that prochloraz is a skin irritant (Kynoch & Liggett 1979a).
Instillation of dilute solution of prochloraz into the everted lower
eyelid of one eye of New Zealand white stream rabbits produced a mild
conjunctival irritation in some animals which persisted up to three
days (Kynoch & Liggett 1979b).
Acute Toxicity
Prochloraz is of low toxicity to rodents when administered
orally. In rats, the LD50 ranged from 1 600 to 2 400 mg/kg b.w. and
in mice it was approximately 2 400 mg/kg b.w. Symptoms of intoxication
became apparent within 30-60 minutes of dosing and included CNS
depression, respiratory disturbance, ataxia, increased salivation,
piloerection, increased lacrimation and distended abdomen with signs
of gastrointestinal irritation. The acute toxicity of prochloraz in
rodents is summarized in Table 1.
Table 1. Acute Toxicity of Prochloraz in Rodents
Species Route LD50 (mg/kg) References
Rat Oral 1 600-2 400 Carter et al. 1978
Mouse Oral 2 400 Shaw & Carter 1976
Rat Dermal 5 000 Carter 1975
Rabbit Dermal 3 000 Kynoch & Liggett 1979a
Rat Inhalation (6h LC50) >420 mg/cu.m. Alexander & Clark 1978
Rat Intraperitoneal 400-800 Smithson & Lancaster 1980
In dogs given a single oral dose of 10, 100 or 250 mg/kg b.w. of
prochloraz, there were no deaths and the only toxic signs were emesis
and diarrhoea at 100 and 250 mg/kg b.w. (Stobart et al. 1978). One
female baboon given prochloraz at 50 mg/kg b.w. showed no toxic effect
while 250 mg/kg b.w. produced only emesis (Morgan et al. 1977).
Following dermal application to rabbits of 3 000 mg/kg for 24 h
there were no toxic effects except moderate to severe erythema and
oedema on the treated skin (Kynoch & Liggett 1979a).
Short-Term Studies
Mouse
Groups of 9 male and 9 female and 15 male and 15 female CD-1
strain mice were fed prochloraz in the diet at 6, 25, 100 and 400
mg/kg/day for 6 weeks and 13 weeks, respectively. Groups of 9 or 24
mice of each sex, given a diet without prochloraz, acted as controls.
Two additional groups of 15 mice of each sex were given a control diet
for 17 weeks and a diet containing prochloraz at a nominal dose level
of 400 mg/kg/day for 13 weeks, followed by a 4-week recovery period.
Overt signs of toxicity were recorded daily and body weight and food
consumption three times weekly; haematological and blood biochemistry
analyses were done at 6 weeks, 13 weeks and 4 weeks off-dose. All mice
were killed at the end of the dosing or recovery period, examined
macroscopically, the main organs were weighed and microscopic
examination of tissues performed.
No deaths occurred that were attributable to treatment with
prochloraz. An increased incidence of piloerection occurred in mice of
both sexes given 400 mg/kg daily. Food consumption of both sexes given
400 mg/kg daily was higher than that of the controls throughout the
dosing period and during the first week of the recovery period, but
was similar to the controls thereafter. A slight weight loss was
observed in the males given 100 mg/kg daily and in the females given
400 mg/kg daily. At week 6, haemoglobin concentration, PCV and red
blood cell counts were increased in both sexes given 400 mg/kg daily;
MCH was also marginally increased in the females and leucocyte counts
were increased in females due to lymphocytosis. At week 13 or after
four weeks recovery, these parameters were no longer affected by
treatment.
Plasma GPT activity was increased at week 6 in some mice of both
sexes given 400 mg/kg daily and some males at 100 mg/kg daily. At week
13, it was increased in the majority of females given 400 mg/kg daily
and in some mice of both sexes given 100 mg/kg daily. After four weeks
recovery, some of the males given 400 mg/kg daily were still affected.
Albumin content and consequently the albumin:globulin ratio, were
slightly reduced in both sexes given 400 mg/kg daily and in the males
given 100 mg/kg daily. At week 13, in both sexes at 400 mg/kg daily,
urea-nitrogen was slightly decreased and glucose levels were
increased.
Liver weight was increased at week 13 in both sexes given 25, 100
or 400 mg/kg daily. The ovaries in the females and the prostate and
seminal vesicles in males were small in the group at 400 mg/kg daily.
After 4 weeks recovery, liver weights were still higher in both sexes
given 400 mg/kg daily, but to a lesser extent than at weeks 6 and 13.
Histopathological examination showed minor liver changes with loss of
glycogen and periportal fat droplets in both male and female mice
receiving 400 mg/kg and in females receiving 100 mg/kg for 13 weeks.
Some male mice receiving 100 or 400 mg/kg also showed centrilobular
hepatocyte enlargement. In both male and female mice killed after four
weeks recovery, none of these changes were present. The no-effect
level in mice was 6 mg/kg/day (Gale 1980; Lancaster 1982).
Rat
Groups of 20 male and 20 female Boots-Wistar rats were given a
daily dose of prochloraz by oral gavage in 10 percent aqueous acacia
suspension at 0, 6, 25 or 100 mg/kg b.w. for 13 weeks. Further groups
of 20 male and 20 female controls given 100 mg/kg daily of prochloraz
remained untreated for four weeks after the end of the dosing period
and, in addition, groups of 10 males and females were given 0, 6, 25
or 100 mg/kg daily of prochloraz for six weeks. Overt signs of
toxicity observed in rats of both sexes were increased salivation at
25 or 100 mg/kg daily and in a few given 6 mg/kg daily. The incidence
of diarrhoea was increased during the first four weeks of dosing in
the males given 100 mg/kg daily and possibly in the first week in the
males given 25 mg/kg daily. In females, a few instances of diarrhoea
were recorded at each dose level.
Food consumption was not affected by treatment. However, test
females gained somewhat more weight than the controls at 25 or 100
mg/kg daily. At week 6, the only consistent haematological change was
a decreased haemoglobin concentration in males at each dose level and
in females given 100 mg/kg daily. A leucocyte count increase was noted
at each dose level in males due to lymphocytosis. At week 13, MCV was
slightly decreased at each dose level in the males and haemoglobin
concentration was slightly decreased in females treated at 25 or 100
mg/kg daily and in males given 100 mg/kg daily after the four weeks
recovery period.
Serum bilirubin was slightly decreased in both sexes at each dose
level at week 6, in the male at each dose level and in the females
given 6 or 100 mg/kg daily at week 13. Serum potassium was slightly
increased at week 6 in the males at 25 or 100 mg/kg and females at 100
mg/kg daily. UGOT excretion was slightly elevated in a few rats of
both sexes at each dose level. Urinary protein content was reduced in
the females at each dose level and in males given 100 mg/kg daily.
Liver weight at week 6 was increased in both sexes given 100
mg/kg daily and in the females given 6 or 25 mg/kg daily; kidney
weight was increased in both sexes given 100 mg/kg daily; spleen
weight was reduced in the males in each dose group; ovary weight was
increased in each dose group and thyroid weight was also increased in
females given 6 or 100 mg/kg daily. After the four-week recovery
period, the weights of the liver, ovaries and thyroids of the females
were slightly higher than the control values. The changes in liver
cell size correlated closely with the increase in liver weight. The
only instance where such correlation was not apparent was at week 13
in the females given 6 mg/kg daily, where the centrilobular cells were
slightly larger but liver weight was unchanged.
No other findings, that could be related to treatment, were
observed at microscopic examination of tissues from the rats given 100
mg/kg daily and killed at week 13. Various treatment-related effects
were noted in the rats of both sexes given 6 mg/kg daily of prochloraz
and a no-effect level was therefore not established. However, all
these effects were marginal (Lancaster & Shaw 1979a).
Dog
Groups of four male and four female beagles were given daily
doses of 1, 2, 5, or 7 mg/kg of prochloraz by gastric intubation for
13 weeks. Groups of eight males and eight females received 20 mg/kg
daily or the vehicle (10 percent aqueous acacia solution)
concurrently. At the end of the dosing period, four males and four
females from each of the 20 mg/kg and control groups were kept for
another four weeks without treatment.
Overt signs of toxicity observed during the dosing period
included isolated bouts of emesis and increased salivation in some
dogs from all groups, including controls, although these were more
prevalent at the high dosage. All dogs at 20 mg/kg daily and one given
7 mg/kg daily occasionally produced yellow mucoid and/or liquid
faeces. No effects of treatment were detected on faecal occult blood
content. During the recovery period, no overt signs of toxicity were
observed. Reduced food consumption was recorded during the dosing
period in two dogs given 20 mg/kg daily. Body weight changes during
the study were comparable in control and treated groups, although
greater fluctuations were recorded in dogs given 20 mg/kg daily. There
was no treatment-related effect detected by ophthalmoscopy or
electrocardiography.
Transient slight decreases in erythrocyte count and/or
haemoglobin concentra- were recorded in a few females given 7 or 20
mg/kg daily. No other treatment-related changes in haematological
parameters were noted. Changes in the activity of serum enzymes were
recorded. The serum alkaline phosphatase was high during the dosing
period in the majority of dogs given 20 mg/kg daily and in some given
7 mg/kg daily. Serum leucine aminopeptidase was slightly elevated at
week 6 in some dogs given 20 and in one given 7 mg/kg and serum GPT
was increased in a dog given 7 mg/kg daily. No change was observed in
urinary parameters.
At necropsy, all dogs given 20 mg/kg daily and three given 7
mg/kg daily had large and heavy livers. Low prostate weights were
recorded at week 14 in three dogs and in another two dogs in the same
group at 17 weeks. One of these dogs, killed at week 10, also had
decreased testes weight. In addition, one dog, given 7 mg/kg daily,
had a low prostate weight relative to body weight. In two females at
the high dose killed at week 14, and one killed at week 18, clear
brown exudate from freshly sectioned mammary glands was observed.
There were no histopathological findings in dogs on the high
prochloraz dosage. Counts of liver nuclei revealed no statistically
significant difference in the cell liver size or in the micronuclei
count between control and treated dogs.
In dogs retained for the recovery period, liver size and weight
returned generally to within normal limits, suggesting that any effect
of treatment on these parameters was reversible. The changes of AP and
LAP also returned to normal during the recovery period. The dose
producing no adverse-effect was 2.27 mg/kg daily (Lancaster et al.
1979b; Lancaster 1980).
Long-Term Studies
Rat
Groups of 150 male and 150 female rats constituted the controls.
Two groups each of 80 males and 80 females were treated with 37.5 and
150 ppm and a further two groups of 90 males and 90 females were given
625 ppm prochloraz in their diet. When the survival approached 20
percent in any group, all animals of that sex group were killed (111
weeks of treatment for females and 115 weeks of treatment in the
males).
There were no overt signs of toxicity recorded during the study
that could be considered related to the treatment. During weeks 20 and
21, the majority of rats from all groups showed signs indicative of
sialodacryo-adenitis infection, with a further relapse of mild
infection in a few rats from each group during the weeks 66 and 67.
Male survival was similar to controls, while treated females survived
longer than controls. A lower food consumption with a reduced body
weight gain was recorded for both sexes given 625 ppm. At 150 ppm,
food consumption was marginally lower throughout in males and until
week 52 in the females. Ophthalmoscopic examination revealed no
abnormalities related to treatment. In a few rats, minor changes were
seen at week 26, which were considered sequelae of
sialodacryo-adenitis infection.
Periodic laboratory measurements of haematological, biochemical
and urinalysis parameters in a few treated rats at 625 ppm showed
changes, but these were minimal and considered of doubtful
toxicological significance. The changes in urinalysis were minor, i.e.
lower total protein and changes in the pH. A minimal higher activity
of serum glutamic oxalacetic transaminase was noted.
No macroscopic findings related to treatment were seen at interim
kill in week 13. However, at the 52-week kill, enlarged or swollen
livers were observed in one male and three females given 150 ppm and
in four males and one female given 625 ppm in the diet. Heavier livers
were noted in males and females given 625 ppm diet at week 52 and
lower pituitary weights were also recorded in a few rats.
Histopathological examination showed hepatic centrilobular enlargement
in females given 625 ppm; periportal glycogen loss and centrilobular
fat deposits were also present in some treated rats of both sexes.
There were no other microscopic findings related to treatment.
Enlarged and swollen livers were observed in a higher proportion
of male rats given 625 ppm than in the controls, and slightly heavier
liver weights were recorded for females given 625 ppm. The incidence
of hyperplastic lesions in the liver was slightly higher than in the
controls in both sexes given 625 ppm, but the differences were not
statistically significant. The incidence of all other non-neoplastic
findings was similar in all groups. There was no evidence of
carcinogenicity from the chronic administration of prochloraz. The
no-effect level for toxicological changes was 37.5 ppm, equivalent to
a mean intake of 1.3 mg/kg/day in males and 1.6 mg/kg/day in females
(Colley et al. 1982).
Dog
Technical prochloraz was fed to groups of five males and five
females at levels of 0, 30, 135 and 600 ppm (increased to 1 000 ppm
from week 57) for 104 weeks. Observations included clinical signs,
mortality, food and water consumption and, at weeks 0, 13, 26, 51, 78
and 104, ophthalmoscopy, electrocardiography and clinical laboratory
studies. These comprised haematology, biochemistry, urinalysis and
faecal occult blood. At necropsy, organs were weighed and
comprehensive macroscopic and microscopic examinations were carried
out.
There were four deaths during the study but none was attributable
to treatment. There were no overt signs of toxicity or effects of
treatment upon body weight, food or water consumption, ophthalmoscopic
or electrocardiographic findings that could be related to treatment.
There were a few changes in the biochemical parameters, including a
higher level of SAP for males receiving 600 ppm in the diet during
week 13 and, during weeks 16 and 50, the elevation was also apparent
in the females. After the dietary level was increased to 1 000 ppm for
this group, there was a marked increase in the SAP levels during week
60, which persisted throughout the rest of the dosing period. The mean
level for males receiving 135 ppm was also higher than the control
mean from week 13 onwards, but the difference was not so marked as at
600 ppm. There was a slight but inconsistent increase in the platelet
count, glucose and cholesterol values in the group receiving 600 to
1 000 ppm, but these could not be related definitely to treatment.
There was no effect of treatment on the urinary parameters or faecal
occult blood content.
Liver weights in five animals that received 600 to 1 000 ppm, one
that received 135 ppm and one control, when expressed as a percentage
of body weight, exceeded the normal upper limit. The mean prostate
weight for animals in the same treatment group was significantly lower
than the control mean weight.
Histopathological examination showed minimal prostatic atrophy,
low grade hepatitis and minimal swelling and rarefaction of
centrilobular hepatocytis at 600 to 1 000 ppm. One male dog given 135
ppm also showed some liver changes. There were no other
histopathological changes related to prochloraz.
The no-effect level was 30 ppm in the diet, equivalent to
approximately 0.92 mg/kg/day (Chesterman et al. 1981).
Observations in Humans
Prochloraz has been produced on a commercial scale since 1980. No
adverse human effects have been detected since then from synthesis
formulation or use of prochloraz or its formulation. No clinical or
laboratory data were included (Bonsall 1982).
COMMENTS
Prochloraz has low oral toxicity. Following oral administration
in the rat, 95 percent of the dose is rapidly absorbed. In rats, the
major excretion is via the urine; in dogs it is via the faeces.
Prochloraz is a demonstrated liver mixed function oxidase inducer.
In rats and rabbits, no evidence of teratogenic effects was noted
at maternally toxic dose levels. A multigeneration reproduction study
in rats did not show any adverse effects at doses up to 37.5 ppm.
Mutagenicity studies, including the Ames test, micronucleus test,
mouse lymphoma assay and a dominant lethal study, were all negative.
In 90-day studies in mice, rats and dogs, liver weight was
increased in all species. No-effect levels of 6 mg/kg/day and of 2.27
mg/kg/day were established for mice and dogs, respectively. However,
in rats 6 mg/kg/day induced increased liver weight and occasional
signs of intoxication (increased salivation, diarrhoea). A two-year
dog study established a no-effect level of 30 ppm.
A long-term rat study did not result in any evidence of oncogenic
potential. A no-effect level of 37.5 ppm in the diet was established.
A mouse carcinogenicity study of 106 weeks duration indicated an
increased incidence of liver adenomas and adenocarcinomas in both
sexes at dose levels of 325 ppm and above. Prochloraz has been shown
to be a hepatocarcinogen in mice.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat (male); 37.5 ppm in the diet, equal to 1.3 mg/kg b.w.
Dog: 30 ppm in he diet, equal to 0.9 mg/kg b.w.
Estimate of Acceptable Daily Intake for Man
0 - 0.01 mg/kg b.w.
FURTHER WORK OR INFORMATION
Desirable
Observations in humans.
REFERENCES-TOXICOLOGY
Alexander, D.J. & Clark, G.C. Preliminary acute inhalation toxicity
1978 study in male and female rats with pure prochloraz (single
exposure). Report TX78053 from Huntingdon Research Centre
and Boots, submitted to WHO by FBC Ltd. (Unpublished)
Boardman, L.E. Plasma and tissue distribution studies in the rat
1979 following single and repeated oral doses of 3H-prochloraz.
Report AX79004 from Hazleton and Boots submitted to WHO by
FBC Ltd. (Unpublished)
Beswick, A.M. The teratogenicity study of technical prochloraz in male
1980 and female rats. Report TX80024 from Boots submitted to WHO
by FBC Ltd. (Unpublished)
Bonsall, J.L. The human exposure to prochloraz since synthesis in 1974
1982 and commercial production in 1980. Report submitted to WHO
by FBC Ltd. (Unpublished)
Campbell, J.K. Residues in milk and tissues of a goat dosed orally
1980 with 14C-prochloraz. Report AX80026 from Boots submitted to
WHO by FBC Ltd. (Unpublished)
Carter, O.A. Acute dermal toxicity of prochloraz to the male Boots
1975 Wistar rat. Report TXM75079 from Boots submitted to WHO by
FBC Ltd. (Unpublished)
Carter, O.A., Smithson, A. & Burnett, R. Acute oral toxicity of
1978 prochloraz, prochloraz pure material, stage 3 and stage 4
technical material and mother liquor concentrate impurities
to male Boots Wistar rats. Report TX78118 from Boots
submitted to WHO by FBC Ltd. (Unpublished)
Challis, I.R. & Campbell, J.K. The effect of prochloraz on the hepatic
1983 mixed function oxidase system of the male mouse after oral
administration. Report METAB/83/6 from FBC submitted to WHO
by FBC Ltd. (Unpublished)
Chesterman, H. et al. Two-year toxicity study in beagle dogs of
1981 technical prochloraz - final report - repeated dietary
administration for 104 weeks. Report TOX/81/173-2 from
Huntingdon Research Centre and FBC submitted to WHO by FBC
Ltd. (Unpublished)
Colley, J. et al. Prochloraz chronic toxicity and carcinogenicity
1982 study in rats by dietary administration - 104 weeks (final
report). Report TOX/82/173-8 from Huntingdon Research
Centre, and FBC submitted to WHO by FBC Ltd. (Unpublished)
Colley, J. et al. Prochloraz tumorigenicity study in mice by dietary
1983 administration (final report). Report TOX/83/173/23 from
Huntingdon Research Centre and FBC submitted to WHO by FBC
Ltd. (Unpublished)
Cozens, D.D., Reid, Y.J., Woodhouse, R.N., Almond, R.H. Anderson, J.
1980 & Ball, S.I. Dominant lethal gene assay of technical
prochloraz in the male mouse. Report TX80077 from the
Huntingdon Research Centre and Boots submitted to WHO by FBC
Ltd. (Unpublished)
Cozens, D.D. et al. The effect of prochloraz on reproductive
function
1982 of multiple generations in the rat. Report TOX/82/173-5 from
Huntingdon Research Centre and FBC submitted to WHO by FBC
Ltd. (Unpublished)
Everest, R.P. & Cliffe, S. Technical prochloraz micronucleus assay in
1980 male and female CD rats of prochloraz. Report TX80003 from
Boots submitted to WHO by FBC Ltd. (Unpublished)
Gale, E.P. 90-day oral toxicity study with prochloraz technical to
1980 male and female CD1 mice. Report TX80040 from Hazleton and
Boots submitted to WHO by FBC Ltd. (Unpublished)
Hamilton, D.Y. The distribution and level of 14C-labelled residues in
1978a rats following repeated oral dosing with 14C-prochloraz at
25 mg/kg/day. Report AX78008 from Boots submitted to WHO by
FBC Ltd. (Unpublished)
Hamilton, D.Y. The distribution and level of radiolabelled residues in
1978b the tissues of the dog following a single oral dose of
prochloraz. Report AX78016 from Boots submitted to WHO by
FBC Ltd. (Unpublished)
Kynoch, S.R. & Liggett, M.P. Primary eye irritancy of prochloraz 40
1979a percent E.C. formulation (bfn 8099). Report TX79050 from
Huntingdon Research Centre and Boots submitted to WHO by FBC
Ltd. (Unpublished.)
Kynoch, S.R. & Liggett, M.P. Primary skin irritancy of prochloraz 40
1979b percent E.C. formulation (bfn 8099). Report TX79074 from
Huntingdon Research Centre and Boots submitted to WHO by FBC
Ltd. (Unpublished)
Lancaster, M.C. & Shaw, J.W. 90-day oral toxicity study with
1979a prochloraz technical in male and female Boots Wistar rats
(4-week off-dose period). Report TX79028 from Boots
submitted to WHO by FBC Ltd. (Unpublished)
Lancaster, M.C., Morgan, H.E. & Stobart, J.E. 90-day oral toxicity
1979b study with prochloraz technical in male and female beagle
dogs (4-week off-dose period). Report TX79010 from Boots
submitted to WHO by FBC Ltd. (Unpublished)
Lancaster, M.C. 13-week oral toxicity study with prochloraz technical
1980 (BX 9/DM 2723) in male and female dogs with a four-week
off-dose period. Histopathological examination of the
remaining tissues. Report TX80034 from Boots submitted to
WHO by FBC Ltd. (Unpublished)
Lancaster, M.C. PRochloraz: 13-week oral toxicity in the mouse -
1982 histopathological examination. Report TOX/82/173/-9 from FBC
Ltd. submitted to WHO by FBC Ltd. (Unpublished)
McGregor, D.B., Riach, C.G. & Brown, A.G. Technical prochloraz
1983 assessment of mutagenic potential in the mouse lymphoma
mutation assay. Report TOX/83/173-22 from Inveresk and FBC
submitted to WHO by FBC Ltd. (Unpublished)
Morgan, G.E., Patton, D.S.G., Shepherd, G.M. & Stobart, J.E. The acute
oral toxicity of bts 40542 (technical batch) to the female
baboon. Report from Boots Laboratories submitted to WHO by
FBC Ltd. (Unpublished)
Needham, D. The effect of dog gastric juice or plasma on prochloraz.
1980 Report AX80011 from Boots submitted to WHO by FBC Ltd.
(Unpublished)
Needham, D. & Campbell, J.K. The excretion of 14C-phenyl-labelled
1980 prochloraz in male and female rats after a single oral dose.
Report AX80037 by Boots submitted to WHO by FBC Ltd.
(Unpublished)
Needham, D. The excretion and tissue residues of 14C-prochloraz in
1982a male and female mice following a single oral dose of 100
mg/kg. Report METAB/82/32 from FBC submitted to WHO by FBC
Ltd. (Unpublished)
Needham, D. & Campbell, J.K. The excretion and tissue residues of 14C
1982 prochloraz in male and female dogs following a single oral
dose of 18 mg/kg. Report METAB/82/30 by FBC submitted to WHO
by FBC Ltd. (Unpublished)
Needham, D. The metabolism of prochloraz in the rat after oral
1982b administration. Report METAB/82/31 from FBC submitted to WHO
by FBC Ltd. (Unpublished)
Needham, D. The effect of prochloraz on the hepatic mixed-function
1983a oxidase system of the male rat after oral administration.
Report METAB/83/5 from FBC submitted to WHO by FBC Ltd.
(Unpublished)
Needham, D. The effect of prochloraz on the hepatic mixed function
1983b oxidase system of the mouse when administered at 80, 325 and
1 300 mg/kg diet for up to 14 weeks. Report METAB/83/7 from
FBC submitted to WHO by FBC Ltd. (Unpublished)
Palmer, A.K., Bottemley, A.M. & Billington, R. The effect of technical
1980 prochloraz on pregnancy of the New Zealand white rabbit.
Report TX80083 from Huntingdon Research Centre and Boots
submitted to WHO by FBC Ltd. (Unpublished)
Rivière, J.L. Prochloraz, a potent inducer of the microsomal
1983 cytochrome P-450 system. Pestic. Biochem. Physiol., 19:
44-52.
Shaw, J.W. & Carter, O.A. Acute oral toxicity to male CD 1 mice of
1976 prochloraz. Report TX76093 from Boots submitted to WHO by
FBC Ltd. (Unpublished)
Smithson, A. & Lancaster, M.C. Acute intraperitoneal toxicity of
1980 prochloraz to male CD rats. Report TX80004 from Boots
submitted to WHO by FBC Ltd. (Unpublished)
Stobart, J.E., Morgan, H.E., Patton, D.S.G. & Shepherd, G.M. Acute
1978 oral toxicity of prochloraz to male and female beagle dogs.
Report TX78049 from Boots submitted to WHO by FBC Ltd.
(Unpublished)
Turner, D.M. & Gilbert, C.M. Pharmacokinetic studies on 14C-labelled
1977 prochloraz in the rat. Report AX77001 from Hazleton and
Boots submitted to WHO by FBC Ltd. (Unpublished)
Wilcox, P. Prochloraz in vitro bacterial mutagenicity testing of
pure
1977 and technical prochloraz. Report TX78002 from Boots
submitted to WHO by FBC Ltd. (Unpublished)
RESIDUES
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Prochloraz is available as 40 percent and 45 percent emulsifiable
concentrate (E.C.) formulations for foliar and postharvest treatments
and is sold under the trademark Sportak. A 50 percent wettable powder
formulation containing a 4:1 prochloraz-manganese coordination complex
active ingredient (a.i.) (3.4 percent manganese) is available and
recommended for foliar application to some broad leaf crops,
ornamentals and mushrooms, which may be susceptible to phytotoxicity
from E.C. formulations. Prochloraz is available in co-formulation with
carbendazim, under the trademarks Sportak PF and Sportak ALPHA, for
foliar application to cereal and oilseed rape. A 200 g/l liquid
formulation is available for cereal seed treatment and a 25 percent
E.C. disinfectant for rice seed.
Prochloraz is used for the control of a variety of fungal
diseases on a variety of crops and is said to be especially active
against Ascomycetes and Fungi Imperfecti. It is currently registered
or approved for use in eight countries and similar measures are
pending or planned in many others. Nationally registered and/or
approved uses are summarized in Table 1.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue trials have been conducted in many countries,
representing a wide range of food crops, climatic conditions,
formulations and foliar, seed and postharvest treatments. Trials data
are summarized in Tables 2-13. Trials data from countries with
approved uses are available only for apples, watermelons, grapes
(Taiwan, province of China), cereal (Belgium, Denmark, France, German
Federal Republic, The Netherlands, United Kingdom) and mushrooms (The
Netherlands).
Sugarbeets
No information was available on good agricultural practices for
prochloraz on sugarbeets. The "recommended" application rate of active
ingredient is said to be 0.5-1 kg/ha for foliar treatment. No
preharvest interval (PHI) is given. Total residues of both free and
conjugated residues of prochloraz (BTS 40 542),
N-formyl-N'-propyl-N'-2(2,4,6-trichlorophenoxy) ethylurea (BTS 44596),
N-propyl-N-2 (2,4,6-trichlorophenoxy) ethylurea (BTS 44595) and
2,4,6-trichlorophenol (BTS 45186), hereafter referred to as total
residues of prochloraz and metabolites, are listed in Table 2. The
values (corrected for 90 percent recoveries) are expressed as
prochloraz equivalents but were determined as 2,4,6-trichlorophenol
after hydrolysis. All residues and the 0.036 mg/kg control are below
the limit of determination, said to be 0.1 mg/kg. Data are
insufficient to support a maximum residue level, even if good
agricultural practices were known.
The "recommended" application rate of active ingredient to
sugarbeet leaves is said to be 0.5-1 kg/ha for foliar treatment. No
PHI was provided. Total residues of prochloraz and metabolites,
expressed as prochloraz equivalents, on sugarbeet leaves were 0.97,
1.3 and 1.2 mg/kg at 5, 21 and 42 days, respectively, after the last
treatment in three trials (Table 2). The control was 0.15 mg/kg.
Lettuce
No information was available on good agricultural practices on
lettuce. Residues of prochloraz only, uncorrected for approximately 72
percent recoveries, ranged from 7.4 mg/kg 18 days after treatment to
0.41 mg/kg after 39 days and <0.07 mg/kg after 71 days with a limit
of sensitivity said to be 0.01 mg/kg, although one of the three
controls has apparent residues of 0.02 mg/kg. Recoveries were 65-76
percent at 0.1-0.5 mg/kg fortification levels.
Watermelons
Residue data reflecting good agricultural practices in Taiwan
Province of China are presented in Table 2. Residues of prochloraz
per se were 0.01 mg/kg in peel and flesh after the approved six-day
interval from last application to harvest. Information was not
provided on the analytical procedure used; a 0.003 mg/kg limit of
detection was claimed.
Citrus Fruits
No information was provided on good agricultural practices on
citrus fruits. Residues from postharvest trials, proposed for uses
(25-70 g/hl for dip treatments or 100-300 g/hl for spray treatments)
where the peels are discarded, are summarized in Table 3. A variety of
treatment and storage conditions are given, representing 23 trials in
five countries. In some cases prochloraz alone is determined, in
others prochloraz and its metabolites and in one case BTS 45 186.
Total residues are generally expressed as prochloraz equivalents,
although they are usually measured as BTS 45 186. Total residues after
storage at ambient of 4°C for up to 70 days and from a variety of
treatments and locations ranged up to 18 mg/kg in peel, 0.44 mg/kg in
flesh and 6 mg/kg on a whole fruit basis. Residue levels of free
prochloraz were not substantially different than those for total
residues, where a comparison was made. More details are reported under
"Fate of Residues, Storage and Processing".
Pome Fruits
Information on good agricultural practice and residue data
(prochloraz only) reflecting such practice on apples are available for
Taiwan Province of China, as well as data for apples and pears from
supervised trials in three additional countries for which good
agricultural practices for prochloraz on pome fruit are not known.
Apples
The most pertinent data were those from Taiwan Province of China,
for which information was available on good agricultural practices on
apples. Data represent recommended and 2X the recommended application
rate. No data were available for the recommended 9-day last treatment
to harvest interval, but they were given for 6 and 12 days. Residues
of prochloraz, per se, from nine applications were 0.07 and 0.01
mg/kg, respectively, at these intervals from the recommended
application rate. No information is available on the analytical method
used other than a claimed 0.003 mg/kg limit of detection. Other apple
residue data (parent compound only) are from application rates three
to eight times that for good agricultural practice known to the
Meeting. Over-all recoveries among studies submitted range from 64-92
percent with claimed sensitivities ranging from 0.001-<0.01 mg/kg.
Apparent residues in untreated controls, where given, are
<0.01-0.02 mg/kg. A reasonable limit of determination would appear
to be about 0.1 mg/kg. One study (Hayto 1977c) indicates a half-life
in apples of 6-7 days.
Pears
No information on good agricultural practice was available for
pears. The application rates are substantially greater than known good
agricultural practice for apples and residues are higher, as would be
expected.
Stone Fruits
No information on good agricultural practice for prochloraz on
stone fruit were available to the Meeting. The "recommended"
application rate for foliar treatment with the prochloraz-50 percent
W.P. manganese complex formulation was reported as 15-30 g a.i./hl. No
preharvest interval was given. Residue data were available on
apricots, nectarines, peaches, cherries and plums from four countries
(Table 2). Total residues of prochloraz and the metabolites were
determined as 2,4,6-trichlorophenol and expressed as prochloraz.
Residues in mature fruit ranged from a maximum of 0.19 mg/kg im plums
at 3 days, 1 mg/kg at 14 days, 0.82 mg/kg at 24 days to <0.05 mg/kg
over 75 days after multiple foliar treatments with either E.C. or W.P.
(manganese complex) formulations. Residues were generally lower for
other stone fruit at comparable intervals after treatment with the
W.P. formulations. At fortification levels of 0.05-1 mg/kg recoveries
are 70-113 percent over-all. Apparent residues in eight untreated
stone fruit controls were 0.009-0.022 mg/kg except one value of 0.06
mg/kg in four controls (plums). The limit of determination for stone
fruit was reported as 0.05 mg/kg.
Grapes
Information on good agricultural practice for the E.C.
formulation and residue data reflecting 1, 1.5, 2 and 4X recommended
application rates were available from one country.
Residues from two applications at recommended rates ranged from
0.39 mg/kg on the day of application to <0.01 mg/kg after 21 days and
0.1 mg/kg at the recommended 9-day preharvest interval. Higher
application rates and numbers of applications, with one or two
exceptions, did not make an appreciable difference in the residue
level at comparable intervals after application. No information was
provided on apparent residues in un-treated controls or on the
analytical procedure; a 0.003 mg/kg limit of detection was reported.
TABLE 1. REGISTRATIONS AND APPROVED USES FOR PROCHLORAZ
Country Product1 Commodity Approved Application Rate Number and timing of Pre-harvest
Product a.i. Applications Interval
(prochloraz)
Belgium SPORTAK 45EC Winter 1 l/ha 0.45 kg/ha One application at earstage in mixture na
wheat with either 0.125 kg/ha carbendazim,
1.2 kg/ha captafol or 1.6 kg/ha maneb.
Winter
barley 1 l/ha 0.45kg/ha One application at stage L or M2
Denmark SPORTAK Barley 100 ml/100 kg 0.2 g/kg na na
BEJOSE (seed
dressing)
SPORTAK 45EC Spring 1 l/ha 0.45 kg/ha One application at stage 4-10.5 or a
barley split dose at half rate with the first
application at stage 4-6 and the second
at stage 8-10.53.
Winter 1 l/ha 0.45 kg/ha One (stage 4-10.5) or two (stage 4-6 cereals
barley and 7-10.5) applications3. 1 month
Winter 1 l/ha 0.45 kg/ha Two applications (stages 5-6 and 9-
wheat 10.53).
Winter 1 l/ha 0.45 kg/ha One application (stages 5-7)3
rye
Eire SPORTAK 40EC Cereals 1 l/ha 0.4 kg/ha Up to three applications between leaf
sheaf erection and complete ear emergence. na
Table 1 (continued)
Country Product1 Commodity Approved Application Rate Number and timing of Pre-harvest
Product a.i. Applications Interval
(prochloraz)
France SPORTAK 40EC Wheat 1.125-1.875 0.45-0.75 Up to two applications (stages 6-7
l/ha kg/ha and 10.3-10.5)3
Barley 1.125 l/ha 0.45 kg/ha Up to two applications (stages 6-7
and 10.1)3 na
SPORTAK 45EC Wheat 1-1.66 l/ha 0.45-0.75 Up to two applications (stages 6-7
kg/ha and 10.3-10.5)3.
Barley 1 l/ha 0.45 kg/ha Up to two applications (stages 6-7
10.1)3
SPORTAK PF Wheat 1.5 l/ha 0.45 kg/ha Up to two applications (stages 6-7
and 10.3-10.5)3.
Barley 1.5 l/ha 0.45 kg/ha Up to two applications (stages 6-7
10.1)3.
Oil seed 1.5 l/ha 0.45 kg/ha Up to two applications, at beginning
rape and end of flowering. na
SPORTAK M Wheat 1.125 l/ha 0.45 kg/ha Up to two applications (stages 6-7
and 10.3-10.5)3.
Barley 1.125 l/ha 0.45 kg/ha Up to two applications (stages 6-7
10.1)3.
Table 1 (continued)
Country Product1 Commodity Approved Application Rate Number and timing of Pre-harvest
Product a.i. Applications Interval
(prochloraz)
German SPORTAK 40EC Wheat 1.2 l/ha 0.48 kg/ha One (stage 29-32) or two
Federal (stages 29-59) applications5.
Republic Cereals -
Barley 1.2 l/ha 0.48 kg/ha One (stage 29-32) or two 35 days6
(stages 29-49) applications5.
Winter 1.2 l/ha 0.48 kg/ha One application at stage 29-325.
rye Restricted to a maximum of three
applications in Winter cereal.
Netherlands SPORTAK 45EC Wheat 1 l/ha 0.45 kg/ha 1-2 treatments after infection.
Barley 1 l/ha 0.45 kg/ha 1-2 treatments after infection. Cereals -
8 weeks7
Winter 1 l/ha 0.45 kg/ha One or two applications.
barley
Ornamentals 0.4% 180 g/hl Flower bulb dip na
SPORGON Mushrooms 3/g/l/m2 1.5g/l/m2 One application of casing soil Mushrooms -
nine days after casing. 10 days
Taiwan SPORTAK 25EC Rice 2000 x 12.5 g/hl 24 hour rice seed soak. na
dilution
Grape 6000 x 4.17 g/hl Two to three applications, Grape -
dilution every 10 days. 9 days
Apple 3000 x 8.33 g/hl Application every 10 days. Apple -
dilution 9 days
Table 1 (continued)
Country Product1 Commodity Approved Application Rate Number and timing of Pre-harvest
Product a.i. Applications Interval
(prochloraz)
Watermelon 4000 x 6.25 g/hl 10 days. 6 days
dilution
United SPORTAK 40EC Cereals 1 /ha 0.4 kg/ha Up to three applications between na
Kingdom leaf sheath erection and complete
ear emergence.
Oil seed 1.25 l/ha 0.5 kg/ha Up to three applications between
stem extension and 90% petal fall.
Ornamentals - 2000 ppm Propagation cutting dip, drench, na
foliar spray or compost incorporation.
SPORTAK 45EC Cereals 1 /ha 0.45 kg/ha Up to three applications between na
leaf sheath erection and complete
ear emergence.
Oil seed 1-1.33 /ha 0.45-0.6 Up to three applications between
rape kg/ha stem extension and 90% petal fall.
Ornamentals - 2000 ppm Propagation cutting dip, drench, na
foliar spray or compost incorporation.
SPORTAK Cereals 1.5 /ha 0.4 kg/ha Up to three applications between
ALPHA leaf sheath erection and complete
ear emergence. na
Oil seed 1.5 /ha 0.4 kg/ha Up to three applications between
rape stem extension and 90% petal fall.
Table 1 (continued)
Country Product1 Commodity Approved Application Rate Number and timing of Pre-harvest
Product a.i. Applications Interval
(prochloraz)
Ornamentals - 2000 ppm Propagation cutting dip, drench, na
foliar spray or compost incorporation.
SPORTAK FE Cereals 4 kg/ha 0.66 kg/ha Up to three applications between
leaf sheath erection and complete
ear emergence. na
Oil seed 3 kg/ha 0.5 kg/ha Up to three applications between
rape stem extension and 90% petal fall.
Ornamentals - 2000 ppm Propagation cutting dip, drench, na
foliar spray or compost incorporation.
SPORGON Mushrooms 3 g/l/m2 1.5 g/l/m2 One application, 1-10 days after Mushrooms -
casing. 10 days
0.6 g/l/m2 0.3 g/l/m2 Three applications, one 1-10 days Mushrooms -
after casing and then after the 2 days
first and second flush
1.2 g/l/m2 0.6 g/l/m2 Two applications, one 1-10 days Mushrooms -
after casing and then between 2 days
second and third flush.
Ornamentals - 2000 ppm Propagation cutting dip, drench, na
foliar spray or compost incorporation.
Table 1 (continued)
Notes: na - not applicable
nd - not defined
ns - not specified
1. The following formulations have been registered:
SPORTAK 40 EC - an emulsifiable concentrate containing 400 g/l prochloraz
SPORTAK 45 EC - an emulsifiable concentrate containing 450 g/l prochloraz
SPORTAK BEJDSE - a liquid seed dressing containing 200 g/l prochloraz
SPORTAK PF - an emulsifiable concentrate containing 300 g/l prochloraz and 80 g/l carbendazim
SPORTAK M - a twin-pack emulsifiable concentrate containing 400 g/l prochloraz and 450 g/l mancozeb
SPORTAK ALPHA - an emulsifiable concentrate containing 266 g/l prochloraz and 100 g/l carbendazim
SPORTAK FE - a wettable powder containing 165 g/kg prochloraz-manganese complex and 533.4 g/kg mancozeb
SPORGON - a wettable powder containing 500 g/kg prochloraz-manganese complex
2. Baggiolini cereal growth stages.
3. Feekes-Large cereal growth stages.
4. Residues of prochloraz alone.
5. Zadoks Decimal cereal growth stages.
6. A proposed MRL of 0.5 mg/kg for prochloraz and metabolites in cereal grain has been submitted.
7. Under review, pending further residue analysis.
8. Residues of prochloraz and metabolites measured as 2,4,6-trichlorophenol and expressed as prochloraz.
Table 2 Prochloraz Residues in Various Crops Following Supervised Trials
Country/ No. of Formulation Application Interval after Residues, Reference
Crop Year trials rate (a.i.) final application (mg/kg)1
(days)
Sugarbeet Italy
root 1981 1 40% E.C. 2 x 1 kg/ha 42-46 0.02 Longland 1983b
leaves 1.17
root 1 40% E.C. 3 x 1 kg/ha 21-25 0.04
leaves 1.32
root 1 40% E.C. 4 x 1 kg/ha 5-6 0.06
leaves 0.97
Lettuce The Netherlands 1 8% dust 8 kg/ha 70-73 0.013 Hayto 1979d
1978 80 0.013
2 8% dust2 8 kg/ha 18-19 7.383
39 0.043
42-46 0.123
70-73 <0.013
80 <0.013
1 8% dust2 6.4 kg/ha 70-73 0.043 Maclaine Pont et al. 1980
1 8% dust2 8 kg/ha 70-73 0.033
8% dust2 9.6 kg/ha4 78 <0.013
Watermelon Taiwan Province
peel of China
1982 1 25% E.C. 3 x 4.17 g/hl 0-1 0.013 Wang 1982
3 <0.013
5-6 <0.013
9 <0.013
12 <0.013
0-1 <0.013
3 <0.013
5-6 <0.013
9 <0.013
12 <0.013
Table 2 (continued)
Country/ No. of Formulation Application Interval after Residues, Reference
Crop Year trials rate (a.i.) final application (mg/kg)1
(days)
Watermelon Taiwan Province 3 x 6.25 0-1 0.023
peel of China 3 <0.013
5-6 <0.013
9 <0.013
flesh 12 <0.013
0-1 <0.013
3 <0.013
5-6 <0.013
9 <0.013
12 <0.013
Apple The Netherlands 2 50% W.P.5 2 x 25 g/hl 63 <0.013 Maclaine Pont et al. 1980
1978 84 <0.013
United Kingdom 2 25% W.P. 7 x 60 g/hl 0-1 0.473 Hayto 1976
1977 14-16 0.083
30-31 0.023
25% E.C. 8 x 51.48 g/hl 0-1 4.183 Hayto 1977c
8 1.763
18-19 0.683
30-31 0.173
50 0.023
Taiwan Province 1 25% E.C. 9 x 8.33 g/hl 0-1 0.243 Wang 1982
of China 3 0.123
1982 5-6 0.073
12 0.013
14-16 0.053
18-19 0.023
Table 2 (continued)
Country/ No. of Formulation Application Interval after Residues, Reference
Crop Year trials rate (a.i.) final application (mg/kg)1
(days)
Apple Taiwan Province 9 x 16.67 g/hl 0-1 0.813
of China 3 0.373
5-6 0.203
12 <0.013
14-16 0.083
18-19 0.073
Pear The Netherlands 1 50% W.P.5 1 x 0.1 kg/hl 21-25 0.033 Maclaine Pont et al. 1980
1978 1 x 0.2 kg/hl 21-25 0.063
2 x 0.1 kg/hl 14-16 0.263
2 x 0.2 kg/hl 14-16 0.243
Apricots Israel 1 50% W.P.5 1 x 60 g/hl 89 <0.05 Snowden 1983
1982
Cherries France 1 50% W.P.5 1 x 10 g/hl 70-73 <0.05
1982 1 x 20 g/hl 70-73 <0.05
4 x 20 g/hl 21-25 0.45
Nectarines France 1 50% W.P.5 5 x 20 g/hl 14-16 0.56
1982
Peaches South Africa 1 50% W.P.5 3 x 10 g/hl 132 <0.05
1982 1 3 x 20 g/hl 132 <0.05
1 8 x 10 g/hl 30-31 0.21
Plums Italy 1 40% E.C. 1 x 30 g/hl 102 <0.05
1982 1 2 x 30 g/hl 77 <0.05
1 3 x 30 g/hl 50 0.82
Table 2 (continued)
Country/ No. of Formulation Application Interval after Residues, Reference
Crop Year trials rate (a.i.) final application (mg/kg)1
(days)
Plums South Africa 1 50% W.P.5 1 x 30 g/hl 50 0.21
1981 1 1 x 40 g/hl 50 0.28
1 2 x 30 g/hl 14-16 0.97
1 2 x 40 g/hl 14-16 0.60
1982 1 50% W.P.5 3 x 10 g/hl 104 <0.05
3 x 20 g/hl 104 <0.05
6 x 10 g/hl 5-6 0.12
4 x 10 g/hl 103 <0.05
4 x 20 g/hl 103 <0.05
7 x 10 g/hl 5-6 0.19
Grapes Taiwan Province 1 25% E.C. 2 x 4.17 g/hl 3 0.393 Wang 1982
of China 5-6 0.193
1982 8 0.203
9 0.103
12 0.073
21-25 0.103
39 0.053
42-46 <0.013
1 25% E.C. 2 x 6.25 g/hl 3 0.493
5-6 0.263
8 0.203
9 0.253
12 0.133
21-25 0.133
39 0.053
42-46 <0.013
Table 2 (continued)
Country/ No. of Formulation Application Interval after Residues, Reference
Crop Year trials rate (a.i.) final application (mg/kg)1
(days)
Grapes Taiwan Province 1 25% E.C. 7 x 10 g/hl 3 0.203
of China 5-6 0.133
8 0.293
9 0.273
12 0.143
21-25 0.223
39 0.083
42-46 0.083
1 25% E.C. 7 x 16.67 g/hl 3 0.423
5-6 0.203
8 0.163
9 0.603
12 0.673
21-25 0.343
39 0.183
42-46 0.103
Strawberries The Netherlands 1 50% W.P.5 2 x 0.1 kg/hl 30-31 0.173 Maclaine Pont et al. 1980
1978 1 2 x 0.15 kg/hl 30-31 0.173
Almonds Israel
shell 1982 1 50% W.P.5 2 x 0.1 kg/hl 0.616 Churchill & Longland 1983d
kernel 0.106
whole nut 0.296
Table 2 (continued)
1 Total residues of prochloraz and major metabolites unless otherwise stated. Total residue measured as 2,4,6-trichlorophenol (BTS 45186)
and converted to prochloraz-derived residues by correcting for the molecular weight factor (1.906).
2 Co-formulation with dicloran.
3 Prochloraz residues only.
4 Preplanting application.
5 Prochloraz-manganese complex 50% wettable powder formulation.
6 Applications made at blossom and 20 days later. Preharvest interval estimated at about 2.5 to 3 months.
Table 3. Supervised Residue Trials With Prochloraz on Citrus Fruits - Post-Harvest Applications
Residues (mg/kg)1
Application
Country/ Crop No. of Formulation rate (a.i.) Peel Flesh Whole Fruit3 Reference
Year Trials and method
Australia Oranges 1 40% E.C. 25g/hl dip3 1.40-3.4 <0.05 n.d. Browne 1981a
1981 (1.45-3.954) (0.3-0.104)
1 40% E.C. 50g/hl dip3 1.58-3.36 <0.05 n.d.
(2.25-3.354) (<0.02-0.054)
Italy Oranges 1 25% W.P. 50g/hl dip5 1.59-1.72 0.04-0.05 0.71-0.80 Browne & Manley
1981 1 25% E.C. 50g/hl dip5 4.57-6.40 0.09-0.16 1.80-2.35 1982b
Lemons 1 25% W.P. 50g/hl dip5 3.61-4.04 0.08-0.14 1.58-1.83
1 25% E.C. 50g/hl dip 5.67-6.95 0.08-0.19 2.36-2.97
South Oranges 1 40% E.C. 100g/hl wax 1.38-2.79 0.03-0.08 0.38-0.72 Manley & Snowden
Africa brush6 (0.34-0.478) 1982b
1982 1 40% E.C. 200g/hl wax 2.45-3.74 0.02-0.07 0.65-0.94
brush6 (0.91-1.198)
1 40% E.C. 200g/hl wax n.d. n.d. 1.36-1.698)
brush7
Oranges 1 45% E.C. 50g/hl brush9 0.34 <0.05 0.08 Snowden & Manley
(0.108) 1983
1 45% E.C. 100g/hl brush9 0.47 <0.05 0.13
(0.16-0.178)
1 45% E.C. 200g/hl brush9 1.84 <0.05 0.39
(0.39-0.438)
1 45% E.C. 400g/hl brush9 3.07 0.07 0.63
(0.70-0.758)
Table 3 (continued)
Residues (mg/kg)1
Application
Country/ Crop No. of Formulation rate (a.i.) Peel Flesh Whole Fruit3 Reference
Year Trials and method
Spain Oranges 1 25% E.C. 50g/hl dip10 1.48-3.224 0.04-0.10 0.67-1.4511 Kelly 1982d
1979 1 25% E.C. 100g/hl dip10 1.40-4.084 0.04-0.11 0.67-1.8011
1980 Oranges 1 40% E.C. 300g/hl wax 3.3-4.74 0.32-0.38 n.d. Richards 1980b
spray12
1 40% E.C. 300g/hl wax n.d. 0.005-0.00813 n.d. Richards 1980e
spray12
1 40% E.C. 200g/hl wax 1.2-6.7 0.04-0.44 n.d. Richards 1980f
spray12
1 40% E.C. 250g/hl wax 3.7-7.9 0.04-0.30 n.d.
spray12
1 40% E.C. 300g/hl wax 5.4-6.4 0.06-0.13 n.d.
spray12
1981 Oranges 1 40% E.C. 300g/hl shower14 4.7-8.4 0.11-0.39 1.50-2.47 Browne 1981b
(1.7-7.34) (0.12-0.394) (0.64-2.434)
1981 Lemons 1 40% E.C. 300g/hl 11.0-17.9 0.17-0.33 3.66-6.13 Browne 1982c
shower15 (9.1-15.64) (0.27-0.304) (3.14-5.554)
United Oranges 1 40% E.C. 70g/hl dip16 5.78-7.81 0.07-0.15 1.37-2.07 Browne 1982i
Kingdom (3.55-5.104) (0.05-0.124) (0.96-1.354)
1981
Table 3 (continued)
n.d. - not determined.
1 Total residues of prochloraz and major metabolites unless otherwise stated (see notes 4 and 13). Total residue measured as
2,4,6-trichlorophenol (BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular weight factor (x 1.906).
2 Residue levels in whole fruit calculated from separate peel and flesh residues unless otherwise stated (see note 8).
3 Residue levels obtained from five sampling intervals at 1,2,4,8 and 16 days storage at ambient temperature after treatment.
4 Determination of free prochloraz levels only.
5 Fruit was stored for 57 days at 7°C after treatment.
6 Residue levels obtained from four sampling intervals under two storage conditions. Treated fruit was sent to the United Kingdom under
normal commercial refrigerated shipment and then stored at either 20° or 4°C, 44 days later. Samples of fruit from each storage
condition were then taken at 0,7,14 and 21-day intervals.
7 Prochloraz applied in mixture with 2,4,-0 and thiabendazole. Storage conditions as in Note 6 except sampled after 44 days refrigerated
shipment and 21 days storage under ambient or cool conditions.
8 Residue analysis of whole fruit.
9 Fruit was treated in July 1982, sent to the United Kingdom under normal refrigerated shipment and stored at ambient temperature until
September 1982.
10 Residue levels following storage of fruit for either 10 days at 20-22°C or 60 days at 3-4°C after treatment.
11 Residue level calculated from determination of free prochloraz residue in peel and total prochloraz plus major metabolites in flesh.
12 Residue levels following storage of fruit for either 7 or 14 days at ambient temperature after treatment.
13 Determination of free 2,4,6-trichlorophenol (BTS 45186) only, expressed as 2,4,6-trichlorophenol.
14 Residue levels following storage of fruit for either 14, 20 or 27 days at ambient temperature after treatment.
15 Residue levels following storage of fruit for either 12 or 16 days at ambient temperature after treatment.
16 Residue levels following storage of fruit for either 1,7, 21, 35 or 70 days in cold storage after treatment.
Strawberries
No information was provided on good agricultural practices for
prochloraz on strawberries. Limited residue data from the use of the
manganese complex W.P. formulation were provided (Table 2) with
residues of 0.2 mg/kg prochloraz found 16 days after treatment.
Recoveries were reported as >80 percent and sensitivity as
0.001-0.005 mg/kg.
Fruit with Inedible Peel
No information on good agricultural practice was available for
the use of prochloraz on fruit with inedible peel. Residue data
resulting from either foliar (Table 4) and/or postharvest applications
(Table 5) were available for several of these crops. The "recommended"
application rates were provided for most of them but no preharvest
intervals were specified.
Avocados
No information on good agricultural practice was provided for
avocados. Residue data at normal harvest and "recommended" application
rates were available for both foliar and postharvest treatments. At 2X
the 10-25 g a.i./hl "recommended" foliar W.P. (manganese complex)
application rate, maximum total residues on whole fruit basis were 2.5
mg/kg seven days after treatment, with approximately 10 percent of the
residue in the flesh (Table 4). No preharvest interval was given.
However, these data are questionable because of conflicting peel
residue levels in the data submission. Data were also available from
multiple applications of an E.C. formulation at 25-50 g a.i./hl,
although not even "recommended" uses were available for this
formulation. In these trials residues continually declined from 14 to
63 days after treatment, with estimated half-lives ranging from 40-98
days (mean 75.4 days). At these longer intervals residues in flesh
account for 20-30 percent of the whole fruit residue. The limit of
determination was said to be 0.15 mg/kg, although control values were
as high as 0.12, 0.16 and 0.39 mg/kg in flesh, peel and whole fruit,
respectively.
For postharvest treatments of avocados 25-50 g a.i./hl
(formulation unspecified) is "recommended", although no good
agricultural practice information was available. Ten trials reflecting
the "recommended" application rates were conducted in one country with
dip or spray E.C. applications (Table 5). Total residues ranged from
0.3 to 3.5 mg/kg on a whole fruit basis, with little difference in
values between stored at 23° for eight days or fruit frozen
immediately. Little difference was reported between 1X and 2X
recommended application rates. These residues levels are generally
comparable to those found from multiple foliar treatments. The limited
data indicate residues in the flesh at levels up to 30 percent of
those in whole fruit, even at relatively short intervals after
treatment.
Table 4 Prochloraz Residues in Fruit Following Supervised Trials - Foliar Application
Country/ Crop No. of
Year trials Formulation Application Interval after Mean residue level (mg/kg)1 Reference
rate (a.i.) final application
(days) Peel Flesh Whole fruit2
Australia Avocado 1 50% W.P. 7x15g/tree3 7 8.1-13.6 0.09-0.28 1.70-2.51 Cron & Longland 1983a
1981/2 (49.5 g/hl) (11)7 (0.16) (2.1)
South Avocado 1 45% E.C. 2x25g/hl4 14 1.52 <0.10 0.44 Churchill &
Africa 48 1.19 <0.10 0.36 Longland 1983b
1982 63 1.01 <0.10 0.30
1 45% E.C. 2x37.5g/hl4 14 1.85 0.12 0.61
48 1.45 <0.10 0.25
63 0.84 <0.10 0.25
1 45% E.C. 2x50g/hl4 14 2.63 0.17 0.83
48 1.94 0.18 0.59
63 2.16 0.14 0.60
Australia Mango 1 50% W.P. 6x100g/hl5 77 0.15-0.30 0.01-0.02 0.05-0.096 Browne & Manley 1982c
1981 (0.22)8 (0.01)8 (0.07)8
Israel Mango 2 50% W.P. 3x50g/hl 15 3.39-4.87 0.03-0.07 0.77-0.956 Longland &
1982 (4.2)8 Churchill 1983
Table 4 (continued)
1 Total residues of prochloraz and major metabolites, measured as 2,4,6-trichlorophenol (BTS 45186) and converted to
prochloraz-derived residue by correcting for the molecular weight factor (x1.906).
2 Residue in whole fruit (without stone) calculated from separate peel and flesh residues.
3 Applications made at approximately monthly intervals from fruit set to fruit full size.
4 Applications made with a seven-week interval between treatments.
5 Applications made from beginning of flowering to Pethenoccupic fruit drop.
6 Residue level in whole fruit including stone (calculated from peel/flesh residues).
7 Mean in parentheses.
8 Four samples.
Table 5 Prochloraz Residues in Fruit Following Supervised Trials - Postharvest Application
Mean residue level (mg/kg)1
Country/ Crop No. of Formulation Application rate
Year trials (a.i.) Peel Flesh Whole fruit2 Reference
Australia Avocado 1 40% E.C. 25 g/hl dip4 n.d. n.d. 0.88-1.313 Browne 1982a
1981 1 40% E.C. 50 g/hl dip4 n.d. n.d. 1.14-1.353
1982 2 45% E.C. 25 g/hl dip4 3.28-5.35 <0.10-0.12 0:30-0.557 Churchill &
2 45% E.C. 25 g/hi dip6 n.d. n.d. 0.16-1.019 Longland 1983e
1 45% E.C. 50 g/hl spray4 2.33-3.90 <0.10-0.11 0.28-0.367
1 45% E.C. 50 g/hl spray6 n.d. n.d. 0.34-0.429
1983 1 45% E.C. 25 g/hl dip6 n.d. n.d. 2.369
1 45% E.C. 50 g/hl dip6 n.d. n.d. 3.499
1981 Banana 1 40% E.C. 25 g/hl dip5 6.06-7.88 0.02-0.04 n.d. Browne 1982b
1 40% E.C. 50 g/hl dip5 5.18-9.97 0.03-0.05 n.d.
South Banana 2 45% E.C. 25 g/hl dip8 6.5-9.1 0.10-0.20 2.78-3.94 Churchill &
Africa 2 45% E.C. 50 g/hl dip8 9.8-16.6 0.14-0.23 4.02-6.75 Longland 1983a
1982
Israel Mango 1 40% E.C. 40 g/hl dip 6.10-8.32 0.02 1.277 Longland &
1982 Churchill 1983
Australia Papaya 1 45% E.C. 25 g/hl dip6 2.13-3.42 0.01-0.18 0.50 Cron & Longland 1983b
1982 1 45% E.C. 50 g/hl dip6 3.86-4.12 0.09-0.15 0.80
Table 5 (continued)
n.d. - not determined.
1 Total residues of prochloraz and major metabolites, measured as 2,4,6-trichlorophenol (BTS 45186) and converted to prochloraz-derived
residue by correcting for the molecular weight factor (x 1.906).
2 Residue in whole fruit calculated from separate peel and flesh residues unless otherwise stated (see notes 3 and 9).
3 Residue analysis of whole fruit (excluding stone).
4 Fruit stored for 7-8 days at 23°C after treatment.
5 Mean residue levels following storage of fruit for either 9,10,12 or 16 days at ambient temperature after treatment.
6 Fruit frozen within one to four hours after treatment.
7 Calculated whole fruit residue including weight of stone.
8 Fruit stored for 7 days at 8°C after treatment.
9 Residue analysis of whole fruit (including stone).
Bananas
No information was provided on goad agricultural practices for
the use of prochloraz on bananas. "Recommended" application rates for
foliar application are 10-25 g a.i./hl WP (manganese complex) for
foliar high volume applications (100-200 g a.i./ha low volume) and
25-50 g a.i./hl for postharvest treatment (formulation not specified).
No trials data were available for foliar applications, but trials
were reported from two countries for postharvest dips that reflected
"recommended" application rates (Table 5). Total prochloraz and
metabolite residues on a whole product basis ranged from 2.8-6.8 mg/kg
when fruit was stored for seven days at 8°C, with less than 2 percent
of the total fruit residue found in the flesh. No significant change
in residues were observed over the storage intervals of 7 to 16 days
or between 8°C and ambient temperatures. No significant difference
between residues resulted from the two application rates.
Analytical recoveries were generally 85 ± 15 percent. The limit
of determination was estimated at 0.05-0.1 mg/kg, although apparent
residues in untreated controls were as much as 0.32 mg/kg in the skin
and <0.2 mg/kg in whole fruit.
Mangoes
No information on good agricultural practice was available for
the use of prochloraz on mangoes. A 50 percent W.P. manganese complex
is "recommended" for foliar treatments at 10-25 g a.i./hl and at 25-50
g a.i./hl for postharvest treatment, although no formulation is
specified for the latter. One trial for foliar application was
conducted in each of two countries with multiple applications at 2X to
4X the "recommended" foliar rate (Table 4). Maximum total residues on
a whole fruit basis were 0.09 mg/kg from the higher application rate
77 days after treatment and up to 1 mg/kg 15 days after application at
the lower rate. In both cases, most of the residue was in the peel,
although flesh residues appeared to increase with time, being
approximately 3 percent of total fruit residue after 15 days to over
10 percent after 77 days (based on peel: flesh:seed ratios from the
77-day study).
One trial was conducted in one country for postharvest treatment
which reflected the "recommended" application rate. At 1.27 mg/kg on a
whole fruit basis, residues were similar to those of the 15-day
preharvest trial, although residues in flesh were only approximately
1 percent of the total for the postharvest treatment. Over-all
analytical recoveries for mangoes were generally 80 percent or better
and apparent residues in untreated controls were <0.02 mg/kg on a
whole fruit basis and up to 0.1 mg/kg on peel. The limit of
determination was estimated at 0.1 mg/kg.
Papaya
No information was available on good agricultural practices for
prochloraz on papaya. A 50 percent W.P. manganese complex is
"recommended" for foliar treatment at 10-25 g a.i./hl and 25-50 g
a.i./hl (formulation not specified) is "recommended" for postharvest
treatments. No preharvest intervals were given.
No residue information was available from foliar treatments, but
two postharvest dip trials with an E.C. formulation were conducted in
one country and reflected the "recommended" application rates (Table
5). Samples were frozen one hour after the dip treatment for analysis
45 days later. On a whole fruit basis, residues of prochloraz and
metabolites (corrected for approximately 80 percent recoveries) were
0.5 and 0.8 mg/kg for the two application rates. Approximately 15
percent of the whole fruit residue was in flesh. Apparent residue in
the untreated control was 0.023 mg/kg on a whole fruit basis. The
limit of determination was estimated as 0.1 mg/kg in flesh and peel.
Cereal Grains
Over 270 residue trials were conducted in 10 European and one
Asian country, which included foliar or seed treatments with six
different E.C. formulations alone or in combination with other
chemicals. Results are reported mostly as total residues of prochloraz
and metabolites, determined as the trichlorophenol but expressed as
prochloraz (Tables 6-9). Some are for residues of prochloraz only.
Good agricultural practice information was available for eight
countries.
Wheat
Over 100 field trials were conducted in nine countries,
representing 270 samples from a variety of formulations and treatment
conditions. Some trials reflected known good agricultural practices
and good agricultural practices could not be determined for others.
Over-all residues of prochloraz and major metabolites in mature grain
from foliar treatment ranged from 0.01-0.3 mg/kg and in straw at
harvest 0.08-16 mg/kg, although most grain residues were <0.1 mg/kg
(Table 6). The high grain value is from a 38-day preharvest interval
in a country whose good agricultural practices are not known, although
the application rate and preharvest interval were compatible with good
agricultural practices in other countries. Apparent residues in
untreated controls were generally <0.02 mg/kg in grain and <0.3
mg/kg in straw, but were occasionally higher in the latter. Residues
of prochloraz alone were lower and ranged from <0.01 to 0.03 mg/kg in
mature grain and <0.01 to 0.19 mg/kg in straw.
Table 6. Supervised Residue Trials with Prochloraz on Wheat at Harvest
Country/ Formulation No. of Application rate2 No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples final application
(days) grain straw
Austria 25% E.C. 2 0.5 2 70-78 <0.013 0.013 Kelly 1979a
1977 2 1.0 2 70-78 <0.01-0.013 <0.013
1978 22.5% E.C. 1 0.45 4 94 <0.02-0.02 3.66-8.39 Kelly 1979f
1 0.9 4 94 0.02-0.04 11.63-12.20
Belgium 45% E.C. 1 0.45 2 (0)5 0.02 n.d. Housden 1982a
1981 1 0.454 2 (0)5 0.01 n.d.
Denmark 25% E.C. 3 0.625 6 95-98 <0.02-0.02 n.d. Kelly 1979g
1979
40% E.C. 1 0.6 2 77 <0.02 2.1-2.5 Richards 1980d
1982 45% E.C. 1 0.45 1 56 0.106 2.01 Housden 1982c
2 2 x 0.23(19)7 2 75-85 <0.02 1.47-1.94
2 0.23 x 0.45(19)7 2 75-85 <0.02 1.87-2.36
2 2 x 0.45(19) 2 75-85 <0.02-0.02 1.56-2.07
2 2 x 0.45(19)7 2 75-85 <0.02 1.72-2.79
Finland 45% E.C. 2 0.45 2 28-50 <0.02 n.d. Heinanen 1983
1982
France 25% E.C. 2 0.5 6 105 <0.01-0.023 <0.01-0.023 Hayto 1978b
1977 2 1.0 6 105 <0.01-0.013 <0.01-0.183
2 2 x 0.5(38-46) 6 67-69 <0.01-0.033 0.01-0.123
2 2 x 1.0(38-46) 6 67-69 <0.01-0.013 0.01-0.0733
1 0.5 3 105 <0.0058 n.d. Kelly 1980b
1 1.0 3 105 <0.0058 n.d.
1 2 x 0.5(38) 3 67 <0.0058 n.d.
1 2 x 1.0(38) 3 67 0.008-0.0158 n.d.
Table 6 (continued)
Country/ Formulation No. of Application rate2 No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples final application
(days) grain straw
1978 25% E.C. 3 0.45 18 55-113 <0.02-0.06 0.50-9.66 Kelly 1979h
3 0.75 18 55-113 <0.02-0.11 0.88-9.91
1979 40% E.C. 1 0.45 3 80 <0.02 2.42-3.22 Richards 1980a
3 2 x 0.45(23-28) 9 72-77 0.04-0.10 2.40-16.3
1 2 x 0.45(8)9 3 77 0.04-0.08 3.93-4.80
30% E.C.10 1 0.45 3 80 <0.02 2.23-2.55
3 2 x 0.45(22) 9 61-81 <0.02-0.08 2.29-6.16
Federal 25% E.C. 3 0.5 4 81-107 <0.01-0.013 0.01-0.023 Hayto 1979c
Republic of 2 0.75 3 81 <0.013 0.01-0.023
Germany 1 1.0 1 107 <0.013 <0.013
1977
1978 25% E.C. 2 0.5 4 56-61 0.06-0.08 3.6-12.0 Kelly 1979c
1979 40% E.C. 3 2 x 0.48(21-30) 3 54-63 <0.02-0.06 1.0-5.9 Richards 1980g
1980 40% E.C. 3 2 x 0.48(17-23) 3 59-66 0.01-0.04 0.7-2.0 Reary 1981b
3 3 x 0.48(11-23/7-13) 3 59-66 0.03-0.06 1.1-3.6
1981 40% E.C. 5 3 x 0.48(11-29/8-19) 10 57-64 0.02-0.13 1.07-6.40 Browne 1982e
2 2 x 0.48(28-30) 3 43-60 0.06 1.88-3.59 Browne 1982f
3 3 x 0.48(9-22/8-19) 7 43-66 0.05-0.08 4.05-4.55
1 3 x 0.48(17/6) 1 55 0.05 0.4811
1982 30% E.C.10 1 0.45 3 85 <0.05 0.08-0.27 Housden &
Longland 1983
Table 6 (continued)
Country/ Formulation No. of Application rate2 No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples final application
(days) grain straw
Italy 25% E.C. 2 0.07 6 55 <0.01-0.013 0.02-0.093 Hayto 1977b
1977 3 1.0 9 39-55 <0.01-0.013 0.02-0.193,12
1978 25% E.C. 1 0.5 2 38 0.08-0.17 4.82-15.76 Kelly 1979d
1 0.7 38 0.13-0.29 6.29-14.73
The 25% W.P. 1 0.375 3 71 <0.013 <0.013 Hayto 1978a
Netherlands 25% E.C. 1 0.375 3 73 0.01-0.023 <0.01-0.013
1977 1 0.5 3 73 0.01-0.023 0.01-0.023
1978 25% E.C. 1 0.375 3 68 0.04 1.14-1.51 Kelly 1979e
1 0.75 3 68 0.04-0.06 5.66-8.14
1979 40% E.C. 1 0.5 4 71 <0.02 0.88-2.23 Kelly 1980c
1980 45% E.C. 2 0.45 6 69 0.01-0.02 0.41-0.77 Reary 1981c
1 0.675 2 106 0.01 0.21-0.51
1 2 x 0.45 (15) 3 69 0.01-0.02 2.21-3.29
Sweden 45% E.C. 8 0.45 8 67-91 <0.02 0.08-4.08 Longland 1983a
1982 2 2 x 0.45(15-29) 2 58-69 0.03-0.07 1.29-6.34
United 25% E.C. 2 0.4 6 90-115 <0.013 <0.013 Hayto 1977a
Kingdom 2 1.0 6 90-115 <0.013 <0.013
1976
1977 25% E.C. 4 0.5 12 124-126 <0.013 <0.013 Hayto 1979a
1979 40% E.C 2 2 x 0.4(36-37) 6 69-79 <0.02-0.06 4.2-11.2 Richards 1980c
10% S.D.13 1 0.214 2 31015 <0.02 <0.02
Table 6 (continued)
n.d.- not determined.
1/ Total residues of prochloraz and major metabolites, unless otherwise stated (see Notes 3 and 8). Total residue as 2,4,6-trichlorophenol
(BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular weight factor (x 1.906).
2/ Figures in parentheses indicate interval in days between applications.
3/ Determination of free prochloraz residues only.
4/ Prochloraz applied in mixture with carbendazim.
5/ Growth stage at application : 0 - heading.
6/ Residue analysis of whole ears.
7/ Prochloraz applied in mixture with fenpropimorph.
8/ Determination of free 2,4,6-trichlorophenol (BTS 45186) residues only, expressed as BTS 45186.
9/ Prochloraz applied in mixture with mancozeb.
10/ Co-formulation of prochloraz with carbendazim.
11/ Mean residue level in whole ears. A mean residue level of 2.31 mg/kg was found in the whole plant.
12/ Results from two trials.
13/ Seed dressing co-formulation with carboxin.
14/ Rate of application in g a.i./kg seed.
15/ Days after sowing treated seed.
Barley
Information on good agricultural practice for barley was
available from five countries and residue trials reflecting such uses
were reported from those countries and others (Table 7). At-harvest
residues of prochloraz and its major metabolites, from foliar
applications, ranged from <0.01-0.68 mg/kg in mature grain and
<0.01-15 mg/kg in straw (Table 7). Again, the higher values are
generally from the shorter preharvest intervals, which reflect good
agricultural practice, and from trials in which prochloraz was
co-applied with either carbendazim or mancozeb. The rates reflect
usage in the country where the trials were conducted. Most residues in
grain were <0.2 mg/kg. Although grain residues from these shorter
intervals were significantly higher than those from longer intervals,
residues in straw were not.
Analytical recoveries were variable, with means ranging from
70-100 percent. Apparent residues in untreated controls were <0.02
mg/kg in grain and up to 0.2 mg/kg in straw. Residues of prochloraz
alone were substantially lower, ranging from <0.01-0.02 mg/kg in
mature grain and <0.01-0.15 mg/kg in straw, which are comparable to
the values reported in wheat.
Rye
Total prochloraz and its metabolites, from foliar treatments in
mature rye grain or whole ears ranged from <0.02-0.1 mg/kg and in
straw from 0.4-2.5 mg/kg. Application rates reflected good
agricultural practice, although the preharvest interval of 72 to 109
days was significantly greater than the one month permitted (Table 8).
Analytical recoveries were typically >80 percent in grain and
somewhat lower in straw. Apparent residues in untreated controls were
<0.01 mg/kg in grain but as high as 0.04 mg/kg in straw.
Oats
Information on good agricultural practice for oats was not
available for the country in which residue trials were conducted or
from other countries. Residues of prochloraz and its metabolites were
<0.03 mg/kg and <1.6 mg/kg for whole ears and straw, respectively,
62 days after treatment at application rates considered good
agricultural practice for other cereals (Table 8).
Rice
No information on good agricultural practice was available for
rice, except for seed treatments in one country (for which there are
no data). Residues of prochloraz alone in unpolished brown rice 7-21
days after treatment at application rates comparable to good
agricultural practice on other cereals were <0.05 mg/kg (Table 8).
Residues of prochloraz and its metabolites would be expected to be
higher. Residues from seed treatments were lower, but the interval
after treatment was 151-168 days. Analytical recoveries were 86
percent in grain and apparent residues in untreated controls were
<0.005 mg/kg, the limit of detection.
Summary data for residues in cereal grain at harvest (Table 9)
represents foliar applications of all formulations. The data in Table
9 confirm those reported in Tables 6-8, since it is statistically
shown that maximum residues of prochloraz and its metabolites would
normally be less than 0.2 mg/kg at the longer preharvest intervals but
residues up to 0.4 mg/kg can occur. Other trials at shorter intervals
also resulted in higher grain residues than at longer intervals.
Similarly, Table 11 confirms the findings reported in Tables 6-8 for
residues in straw (up to 15 mg/kg). Tables 9 and 11 do not include
more recent studies (Housden 1982c; Longland 1983a; Housden & Longland
1983; Heinanen 1983) reported in Tables 6-8, but these would not
increase expected residues. Tables 9 and 11 also indicate that higher
residues can be expected from multiple or higher doses.
Residues of prochloraz alone were <0.03 mg/kg in cereal grains
at harvest, except for rice where residues were as much as 0.05 mg/kg
7 to 21 days after treatment. From seed treatment alone, residues of
prochloraz and its metabolites in mature grain were <0.02 mg/kg.
Generally, there was no conclusive evidence of increased residues as a
result of co-application of prochloraz and other agricultural
chemicals.
Residue decline studies are summarized in Table 10. Half-lives of
approximately 6 to 17 days could be estimated for ears or whole plants
of wheat or barley on the basis of sampling for at least three
successive intervals. Residues of prochloraz and its metabolites
ranged from 0.15 to 21 mg/kg on green plants or ears on the day of
last treatment to 0.07-3.6 mg/kg at 21 to 48 days, which approximates
typical preharvest intervals.
Almonds
No information was available on good agricultural practice for
prochloraz on almonds. Foliar treatments with the 50 percent W.P.
manganese complex are "recommended" at 15-30 g a.i./hl. No preharvest
interval has been reported. One trial was conducted with foliar
treatments at seven times the maximum recommended application rate
(Table 2) with total residues corrected for recoveries of >86
percent of 0.61, 0.1 and 0.29 mg/kg in shell, kernel and whole nut
(calculated from shell and kernel residues), respectively, about three
months after the last treatment. Apparent residues were 0.06 mg/kg in
un-treated controls on a whole nut basis and up to 0.09 mg/kg in the
shell. The limit of determination was estimated at 0.1 mg/kg.
Table 7 Supervised Residue Trials with Prochloraz on Barley at Harvest
Country/ Formulation No. of Application rate2 No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples final application
(days) grain straw
Austria 25% E.C. 2 0.5 2 61-73 <0.013 <0.01-0.02
1977 1 0.75 1 73 <0.013 0.023
1 1.0 1 61 <0.013 0.033
1 2x0.5(19) 2 54 <0.013 <0.013
1978 22.5% E.C. 2 0.45 69-75 <0.02-0.04 0.65-1.30 Kelly 1979f
1 0.9 75 0.02-0.04 1.0-2.1
Belgium 45% E.C. 3 0.45 6 (M)10 0.01-0.03 n.d. Housden 1982a
1981 7 0.4511 14 (M)10 0.01-0.11 n.d.
2 2x0.4511 (I+M)10 0.02-0.03 n.d.
Denmark 25% E.C. 1 0.75 96 <0.02 n.d. Kelly 1979g
1979 40% E.C. 2 0.6 76-86 <0.02-0.10 1.9-4.6 Richards 1980d
20% E.C. 1 0.6 86 <0.02-0.04 2.5-3.4
20% S.D. 2 0.28 132-1399 <0.02 <0.02
1982 45% E.C. 2 0.234 3 75-87 <0.02-0.03 0.84-1.10 Housden 1982c
4 0.45 4 61-80 <0.02-0.02 1.45-3.24
1 0.45 1 60 0.0514 3.02
2 2x0.45(14) 61-73 <0.02-0.02 1.61-2.59
France 25% E.C. 2 0.5 62-74 <0.01-0.013 <0.01-0.053 Hayto 1978b
1977 2 1.0 62-74 <0.01-0.023 0.01-0.153
1978 25% E.C. 1 0.45 64 0.02-0.06 1.77-8.86 Kelly 1979h
1 0.9 64 0.02-0.08 2.67-10.48
1 2x0.45(19) 62 0.04-0.09 4.23-7.00
1 2x0.9 (19) 62 0.04-0.17 11.19-11.95
Table 7 (continued)
Country/ Formulation No. of Application rate2 No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples final application
(days) grain straw
1979 40% E.C. 2 0.45 4 54-62 <0.02-0.17 5.76-10.81 Richards 1980a
2 2x0.45(21)5 6 38 0.36-0.68 7.20-12.12
30% E.C.6 2 2x0.45(13-21) 38-48 0.10-0.57 6.96-9.47
Fed. Rep. 25% E.C. 1 0.5 1 70 <0.013 <0.013 Hayto 1979c
of Germany 1 0.7 1 70 <0.013 <0.013
1977
1980 40% E.C. 2 0.48 77-79 0.02-0.06 0.06-1.0 Richards 1981b
2 2x0.48(19)12 77-79 0.02-0.06 1.1 -1.3
1981 40% E.C. 2 2x0.48(11-12) 61-62 0.02-0.06 0.95-1.27 Browne 1982f
1982 30% E.C.6 3 0.45 62-76 <0.05 0.23-0.83 Housden & Longland
1983
Netherlands 45% E.C. 1 0.45 3 70 0.04-0.06 0.78-1.21 Reary 1981c
1980
Sweden 45% E.C. 4 0.45 61-72 <0.0213 0.19-1.27 Longland 1983a
1982 2 2x0.225(8-15) 53-55 <0.02 0.24-1.21
2 2x0.45(8-15) 53-55 <0.02 0.34-2.86
United 25% E.C. 3 0.4 54-74 <0.013 <0.01-0.023,7 Hayto 1977a
Kingdom 4 0.5 19-64 <0.013 0.01-0.023,13
1976 2 1.0 64-74 <0.013 <0.013,20
1977 25% E.C. 4 0.5 76-78 <0.013 0.01-0.043 Hayto 1979c
2 1.0 76-78 <0.01-0.01 <0.01-0.02
1979 40% E.C. 3 2x0.4(18-29) 36-63 0.06-0.32 3.0 -14.5 Richards 1980c
Table 7 (continued)
n.d. not determined.
1/ Total residues of prochloraz and major metabolites, unless otherwise stated. Total residue measured as
2,4,6-trichlorophenol (BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular
weight factor (x 1.906).
2/ Figures in parenthesis indicate interval in days between applications.
3/ Determination of free prochloraz residues only.
4/ Prochloraz applies in mixture with fenpropimorph.
5/ Prochloraz applied in mixture with mancozeb.
6/ Co-formulation of prochloraz with carbendazim.
7/ Results from two trials.
8/ Rate of application in g a.i./kg seed.
9/ Days after sowing treated seed.
10/ Growth stages at application: M-heading, I-first joint.
11/ Prochloraz applied in mixture with chlorothalonil.
12/ Interval between treatments not given in one trial.
13/ Results from three trials.
14/ Residue analysis of whole ears.
Table 8 Supervised Residue Trials with Prochloraz on Cereals at Harvest
Crop Country/ Formulation Number of Application rate No. of Interval after Residue (mg/kg)1 Reference
Year trials (kg a.i./ha) samples application
(days) grain straw
Rye Austria 25% E.C. 1 0.5 1 91 0.013 <0.013 Kelly 1979a
1977 1 1.0 1 91 <0.013 <0.013
Denmark 45% E.C. 2 0.45 2 72-77 <0.02-0.10 1.44-2.48 Housden 1982c
1982
Fed. Rep. 40% E.C. 2 0.48 4 89-109 0.03-0.05 0.39-0.53 Browne 1982d
of Germany
1981
Oats Denmark 45% E.C. 2 0.45 2 62 0.02-0.034 1.19-1.64 Housden 1982c
1982
Rice Japan 25% E.C. 2 0.75 12 7-21 <0.01-0.043 n.d. Gato 1980
(brown, 1980 25% E.C. 2 3x0.75 12 7-21 <0.01-0.043 n.d.
unpolished) 25% E.C. 2 25 g/hl2 + 12 7-21 <0.01-0.043 n.d.
3x0.75
25% E.C. 2 25 g/hl2 4 151-168 <0.013 n.d.
n.d. = not determined.
1/ Total residues of prochloraz and major metabolites, unless otherwise stated. Total residues measured as 2,4,6-trichlorophenol
(BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular weight factor (x1.906).
2/ 48-hour seed soak.
3/ Determination of free prochloraz residues only.
4/ Residue analysis of whole ears.
Table 9 Summary of Residues in Mature Grain
Crop Application No. of Total residue (mg/kg)
(kg a.i./ha) results
Mean Std. Dev.
Wheat 1 × 0.375-0.5 36 0.03 0.03
Wheat 2 × 0.375-0.5 36 0.04 0.03
Wheat 3 × 0.375-0.5 12 0.06 0.03
Wheat 1 × 0.6-0.9 31 0.07 0.18
Barley 1 × 0.4-0.5 42 0.03 0.03
Barley 2 × 0.4-0.5 20 0.091 0.091
Barley 1 × 0.6-0.9 14 0.03 0.03
Barley 2 × 0.9 3 0.09 0.05
Rye 1 × 0.48 2 0.04
1/ Excluding a set of 11 results from four replicated applications
of prochloraz alone and with mancozeb, which were exceptionally
high (mean 0.42, std. dev. 0.18 mg/kg), residues in straw were not
exceptionally high. The high grain results may reflect poor
separation of grain and chaff in these samples (Richards 1980a).
Table 10 Supervised Trials on Residue Decline with Prochloraz
Crop Country/ No of Formulation Application rate2 Residues (mg/kg)1 after References
Year trials (kg/a.i./ha) final application (days)
35-36 56 75-85
Wheat Denmark 1 45% E.C. 0.45 whole plant 1.62 Housden 1982c
1982 whole ears 0.10
straw 2.01
2 45% E.C. 2x0.23(19)3 whole plant 0.33-0.94
grain <0.02
straw 1.47-1.94
2 45% E.C. 0.23+0.45(19)3 whole plant 1.11-1.76
grain <0.02
straw 1.87-2.36
2 45% E.C. 2x0.45(19) whole plant 0.70-2.02
grain <0.02-0.02
straw 1.56-2.07
2 45% E.C. 2x0.45(19)3 whole plant 1.38-1.44
grain <0.02
straw 1.72-2.79
Table 10 (Contd.)
Crop Country/ No. of Formulation Application2 After After final application (days) References
Year trials rate penultimate
(kg a.i./ha) application
(days)
20-29 0 15-22 37-42 54-63
Wheat Fed. Rep. 3 40% E.C. 2x0.48 green plant 0.80-1.644 1.62-2.554
of (21-30) ears 0.15-1.114 0.27-1.494 0.23-0.844 0.06-0.084 Richards
Germany grain <0.02-0.06 1980g
1979 straw 1.0-5.9
17-23 0 8-19 39-44 59-66
1980 3 40% E.C. 2x0.48 green plant 0.15-2.7
(17-23) green ears 4.7 -13.6 0.84-2.2 0.28-0.61 Reary
grain 0.01-0.04 1981b
straw 0.7 -2.0
7-13 0 8-19 39-44 59-66
3 40% E.C. 3x0.48 green plant 0.2 -4.7
(11-23/ green ears 5.5 -16.8 0.61-3.3 0.35-0.58
7-13) grain 0.03-0.06
straw 1.1 -3.6
8-19 0 7-14 31-46 58-64
1981 5 40% E.C. 3x0.48 green ears 0.16-0.96 3.87-12.50 2.42-5.85 0.31-1.16 Browne
(11-29/ whole plant 0.51-3.789 1982e
8-19) grain 0.04-0.31 0.02-0.13
straw 1.07-6.40
Table 10 (Contd.)
Crop Country/ No. of Formulation Application2 After After final application (days) References
Year trials rate penultimate
(kg a.i./ha) application
(days)
28-30 0 12-14 21-48 43-60
1981 2 40% E.C. 2x0.48 green ears 0.11-0.84 10.64-13.2 1.14-4.21 0.71-1.18 Browne
(28-30) whole plant 2.078 1982f
grain 0.038 0.06
straw 1.88-3.59
6-19 0 7-14 21-48 43-60
4 40% E.C. 3x0.48 green ears 2.96-8.27 13.90-21.40 3.61-7.25 0.49-2.32
(9-22/ whole plant 1.74-3.614 2.317
6-19) green ears+ 2.318
straw
grain 0.03-0.217 0.05-0.08
straw 4.05-4.55
ears 0.487
0 17 42 57 85
Wheat Fed. 3 30% E.C.5 0.45 whole plant 7.99-13.64 0.74-1.95 0.21-0.94 0.45.0.62 Housden &
Rep. of grain <0.05 Longland
Germany straw 0.08-0.27 1983
1982
34-36 60-87
Barley Denmark 2 45% E.C. 0.233 whole plant 0.64-0.74
1982 grain <0.02-0.03
straw 0.84-1.10
Table 10 (Contd.)
Crop Country/ No. of Formulation Application2 After After final application (days) References
Year trials rate penultimate
(kg a.i./ha) application
(days)
34-36 60-87
5 45% E.C. 0.45 whole plant 0.77-2.32
grain <0.02-0.02
straw 1.45-3.24
whole ears 0.058
2 45% E.C. 2x0.45 whole plant 1.03-3.18
(14) grain <0.02-0.02
straw 1.61-2.59
19 0 13 33-34 77-79
Fed. 2 40% E.C. 0.48 green plant 5.1-10.1 0.4-1.5 0.6-1.7 Richards 1981b
Rep. of grain 0.02-0.06
Germany straw 0.6-1.0
1980 2 40% E.C. 2x0.48 green plant 1.1-3.4 3.2-5.0 1.5-2.5 1.3-2.1
(19)6 grain 0.02-0.06
straw 1.1-1.3
11-12 0 10-18 27-48 61-62
1981 2 40% E.C. 2x0.48 green plant 0.91-7.99 10.40-18.60 Browne 1982f
(11-12) green ears 0.52-3.62 0.07-0.50
ears + straw 0.218
grain 0.088 0.02-0.06
straw 0.95-1.27
Table 10 (Contd.)
Crop Country/ No. of Formulation Application2 After final application (days) References
Year trials rate
(kg a.i./ha)
0 19 38-40 56 62-76
1982 3 30% E.C.10 0.45 whole plant 5.25-10.38 0.21-1.78 0.13-0.80 <0.05-0.78 Housden &
grain <0.05 Longland 1983
straw 0.23-0.83
35 72-77
Rye Denmark 2 45% E.C. 0.45 whole plant 0.85-1.62
1982 whole ears <0.02-0.10
straw 1.44-2.48
0 11 21 34 55-56 69 83 89 93 109
Fed. Rep. 1 40% E.C. 0.48 whole plant 9.6 1.16 0.20 Browne
of green ears 0.03 0.02 1982d
Germany ears 0.03
1981 grain 0.03 0.05
straw 0.39
1 40% E.C. 0.48 whole plant 13.8 1.33 0.05
green ears 0.14 0.05
ears 0.02
grain 0.10 0.03
straw 0.53
36-37 62
Oats Denmark 2 45% E.C. 0.45 whole plant 0.37-0.88 Housden
1982 whole ears 0.02-0.03 1982c
straw 1.19-1.64
Table 10 (Contd.)
1 Total residues of prochloraz and major metabolites, unless otherwise stated. Total residue measured as 2,4,6-trichlorophenol
BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular weight factor (x 1.906).
2 Figures in parentheses indicate interval in days between applications.
3 Prochloraz applied in mixture with fenpropimorph.
4 Results from two trials.
5 Co-formulation of prochloraz with carbendazim.
6 Interval between treatments not given in one trial.
7 Results from three trials.
8 Result from one trial.
9 Results from four trials.
Table 11 Summary of Residues in Mature Straw
Crop Application No. of Total residue (mg/kg)
(kg a.i./ha) results Mean Std. Dev.
Wheat 1 × 0.375-0.5 35 2.83 3.07
Wheat 2 × 0.375-0.5 36 4.85 3.23
Wheat 3 × 0.375-0.5 11 3.20 1.60
Wheat 1 × 0.6-0.9 22 5.03 4.42
Barley 1 × 0.4-0.5 20 3.30 3.05
Barley 2 × 0.4-0.5 26 6.95 3.64
Barley 1 × 0.6-0.9 13 3.36 1.92
Barley 2 × 0.6-0.9 3 11.6 0.4
Barley 1 × 0.48 2 0.46
No information on good agricultural practice was available for
the use of prochloraz on almonds. A 50 percent prochloraz-manganese
complex is "recommended" as a foliar treatment at a 15-30 g a.i./hl
application rate. Two applications at 3X this rate in one trial
resulted in maximum residues up to 0.7 mg/kg (Table 2). The limit of
determination has been estimated at 0.1 mg/kg although the apparent
residues in an untreated control were 0.07 mg/kg. Recoveries from
hulls amended with prochloraz at 1 mg/kg were 96±7 mg/kg.
Oilseed Rape
Information on good agricultural practice was available for
prochloraz on oilseed rape from two countries, from which data on
residue trials were also available. Residue data from 48 trials were
also reported from two countries for which good agricultural practice
was not known to the Meeting (Table 12). At preharvest intervals from
25 to 266 days, residues of prochloraz and its metabolites, reflecting
good agricultural practice, ranged from 0.01 to 0.33 mg/kg. Preharvest
intervals, if applicable, were not provided. The limit of sensitivity
was estimated at 0.05 mg/kg. However, since apparent residues in
untreated controls ranged from <0.01-0.15 mg/kg (but were usually
less than 0.05 mg/kg), a 0.2 mg/kg over-all limit of determination may
be more realistic. Analytical recoveries were > 85 ± 15 percent of
amended levels of 0.2-1 mg/kg.
Mushrooms
Information on good agricultural practice and corresponding
residue trials were available from two countries, as well as data from
residue trials from another country (total of 31 trials, Table 13).
The most common treatment was a single application of the
prochloraz-manganese complex formulation 1-10 days after casing at 1.5
g a.i./l sq m with a 10-day preharvest interval, or three applications
at 0.3 g a.i./l/m2, the first 1-10 days after casing and then after
the first and second flush, with a two-day pre-harvest interval. A
variation of the latter was also used at 2x 0.6 g a.i./l/sq m (Table
1), also with a two-day preharvest interval. Data extracted from Table
13 that reflect good agricultural practice are:
Country/ Applications Residue (mg/kg) Interval after Approved preharvest
and rate last application harvest
(g a.i./sq m) (days) interval (days)
The Netherlands 1 x 1.6 0.11-0.27 total residue 10 10
United Kingdom 2 x 0.6 1.4-1.6 total residue 4 2
0.6-0.8 free prochloraz
1 x 1.25 0.36 total residue 13 10
0.47 free prochloraz 28 10
3 x 0.3 0.24-0.43 total residue 2 2
0.07-0.1 free prochloraz 6 2
0.4-1.4 free prochloraz 3 2
Table 12 Supervised Residue Trials with Prochloraz on Rapeseed
Country/ Formulation No. of Application rate2 No. of Interval after Residues1 References
Year trials (kg a.i./ha) samples final application (mg/kg)
(days)
France 40% E.C. 2 0.45 8 73 0.02-0.04 Cron 1982
1980/1 2 0.453 8 73-76 0.01-0.03
1 0.60 4 73 0.02-0.03
30% E.C.4 2 0.45 8 73-76 0.02-0.03
1982 40% E.C. 4 0.45 12 37-51 0.03-0.15 Peatman & Snowden 1982
4 0.453 12 37-51 0.03-0.13
4 2x0.45 (12-15) 12 25-38 0.04-0.32
30% E.C.4 4 2x0.45 (12-15) 12 25-38 0.06-0.33
Denmark 45% E.C. 3 0.45 6 70-76 <0.05 Peatman & Snowden 1983
1982 3 2x0.45 (18-23) 7 53 0.07-0.19
Sweden 45% E.C. 3 0.45 6 47-72 0.12-0.28 Peatman & Snowden 1983
1982
United 40% E.C. 3 0.45 238-266 <0.05-0.07 Manley & Snowden 1982c
Kingdom 3 0.45 3 119-133 <0.05-0.19
1981/2 3 2x0.4(91-109)5 3 119-133 <0.05
3 3x0.4(91-109/ 3 64-75 <0.05-0.09
51-58)5
2 3x0.4(92-103/ 2 72-73 <0.05-0.06
51-57)
2 3x0.5(92-103/ 1 72-73 0.06
51-57)
Table 12 (continued)
1 Total residues of prochloraz and major metabolites, measured as 2,4,6-trichlorophenol (BTS 45186) and converted to
prochloraz-derived residue by correcting for the molecular weight factor (x 1.906).
2 Figures in parentheses indicate interval in days between applications.
3 Prochloraz applied in mixture with mancozeb.
4 Co-formulation of prochloraz with carbendazim.
5 Prochloraz applied in mixture with tolclofos methyl.
Table 13 Supervised Residue Trials with Prochloraz on Mushrooms
Country/ No. of No. of Application rate (a.i.) Residues (mg/kg)1 days after final application References
Year trials samples and timing2
3 5 10 14 20 21 27 45
Australia 1 1.25g/sq m at casing 0.1 0.1 <0.1 Housden
1981 1 2.50g/sq m at casing 0.23 0.13 0.14 1982
1 1.25g/sq m at casing +
0.63g/sq m after first flush 0.41-0.63 0.22
(24-day interval)
1 2.50g/sq m at casing +
1.25g/sq m after first flush 1.16-1.63
(24-day interval)
1 2.50g/sq m at casing +
2.50g/sq m after first flush <0.1
(24-day interval)
1 0.63g/sq m after first flush 1.06-2.98
1 1.25g/sq m after first flush 4.48-4.61
1 1.25g/sq m after first flush+
0.63g/sq m after second flush 0.29
(7-day interval)
1 2.50g/sq m after first flush+
1.25g/sq m after second flush 0.37
(7-day interval)
The 1 4 1.6g/sq m before inoculation 0.11-0.27 Browne
Netherlands 1 4 0.75g/sq m before inoculation+ 0.15-0.24 1982
1980 0.75g/sq m 14 days later
4 0.75g/sq m before inoculation+
0.75g/sq m after first flush 0.15-1.14
(23-days interval)
Table 13 (Continued)
Country/ No. of Application rate (a.i.) Residues (mg/kg)1 days after References
Year trials and timing2 1st application 2nd application 3rd application
19 7 4
United 1 0.61g/sq m after casing + 0.04 0.10 0.08 Richards 1981c
Kingdom 0.61g/sq m after first flush +
1980 0.61g/sq m after second flush
(19- and 10-day intervals)
1st application 2nd application
21 28 4 12 18
1 0.61g/sq m after casing + 0.04 0.04-0.11 1.37-1.58 <0.02
0.61g/sq m after second flush
(31-day interval)
14 0.61g/sq m after casing + 0.013 0.01-0.33 0.56-0.803 0.08-0.333 0.05-0.183 Whiteoak 1981
0.61g/sq m after second flush
(31-day interval)
Days after application
28 35 43 49
1 1.25g/sq m 7 days after inoculation 0.473 0.043 0.27-0.323 0.203
1 0.625g/sq m 7 days after inoculation 0.133 0.103 0.093 0.07-0.083
2nd application 3rd application
13 6 14 20
1 0.625g/sq m 7 days after inoculation 0.193 0.22-0.243 0.033 0.27-0.323
1 + 2x0.313g/sq m at 14-day intervals
thereafter
1 0.313g/sq m 7 days after inoculation
+ 2x0.313g/sq m at 14-day intervals 0.133 0.07-0.103 0-323 0.053
thereafter
Table 13 (Continued)
Country/ No. of Application rate (a.i.) Residues (mg/kg)1
Year trials and timing2 Days after application
2nd application 3rd application
5 12 3 14 21
1981 1 0.15g/sq m five days after inoculation 0.323 0.123 0.62-0.653 0.193 0.093 Whiteoak 1981
+ 2x0.15g/sq m at 18- and 14-day
intervals thereafter
45 0.3g/sq m + 2x0.3g/sq m. Timing as 0.20-0.373 0.11-0.643 0.39-1.443 0.10-0.313 0.06-0.073
given above
1 0.9g/sq m + 2x0.3g/sq m. Timing as 0.423 0.213 1.293 0.543 0.21-0.243
given above
1 1.5g/sq m + 2x0.3g/sq m. Timing as 0.273 0.523 0.993 n.d. 0.063
given above
1 3.0g/sq m + 2x0.3g/sq m. Timing as 0.623 0.543 0.743 0.343 0.953
given above
Table 13 (Continued)
Country/ No. of Application rate Residues (mg/kg)1 days after References
Year trials (a.i.) and timing2 1st application 2nd application 3rd application
13-14 15-16 18 3 5 7 10 12-13 14-16 2 3-4 7 9-10 12 14 17
United 1 0.3g/sq m 7 days after 0.24 0.20 0.79 0.426 0.30 0.30 0.377 0.23 0.51 0.43 0.33 1.03 0.32 0.23 0.28 0.22 Housden
Kingdom casing + 2x0.3g/sq m &
1981 at 18- and 14-day Whiteoak
intervals thereafter 1982
1982 1 0.3g/sq m 7 days after 0.26 0.14 0.85 0.24 0.24 0.26 0.58 0.24 0.61 0.45 0.61
casing + 2x0.3g/sq m (0.57)8 (1.11)8
at 15- and 17-day
intervals thereafter
Residues (mg/kg)1 days after application
13 15 18 22 25 28 31 34 36 39 42
1982 1 1.25g/sq m 7 days after casing 0.36 0.25 0.33 0.30 0.20 0.38 0.42 0.28 0.54 0.22 0.33
Table 13 (Continued)
n.d. = not determined.
1 Total residues of prochloraz and major metabolites, unless otherwise stated (see note 3). Total residue measured as
2,4,6-trichlorophenol (BTS 45186) and converted to prochloraz-derived residue by correcting for the molecular weight factor (x1.906).
2 All treatments used the prochloraz-manganese complex 50% W.P. formulation.
3 Determination of residues of free prochloraz only.
4 Samples of mushroom also analysed for total residues of prochloraz and metabolites (see Richards 1981c)
5 Mean residue levels from four trials using spray volumes of 100, 200 or 400 l/sq.m.
6 Mean residue level in small and larger mushrooms (0.43 and 0.40 mg/kg respectively).
7 Mean residue level in small and larger mushrooms (0.52 and 0.21 mg/kg respectively).
8 Mushroom emerged before treatment (direct application).
Therefore, residues of prochloraz and its metabolites or of
prochloraz alone may be at least as much as 2 mg/kg from approved
practices. Differences between residues of prochloraz and its
metabolites, compared to free prochloraz, do not appear to be as high
as in cereals. Analytical recoveries of total residues were generally
> 70 percent, with apparent residues of 0.03 ± 0.02 mg/kg in
untreated controls and > 77 percent and 0.01 mg/kg for free
prochloraz.
FATE OF RESIDUES
The fate of prochloraz has been studied in plants and animals. In
plants, it is metabolized to
N-formyl-N'-1-propyl-N-(2-(2,4,6-trichlorophenoxy)ethyl) urea,
also known as BTS 44596 or the "formyl urea"; to
N-propyl-N-(2-(2,4,6-trichlorphenoxy)ethyl) urea, also known as
BTS 44595 or "the urea"; to 2,4,6-trichlorophenoxyacetic acid (BTS
9608), and finally to 2,4,6-trichlorphenol (BTS 45186), all of which
form stable conjugates (Figure 1). Animal metabolism has resulted in
characterization of urinary metabolites (see Figure 1 under
"Toxicology"), information of radioactivity distribution in tissues
from oral or dermal administration and rate and route of excretion.
Qualitatively, urinary metabolites are similar among several test
animals, although the major plant metabolites have not been identified
in the urine. Residues in animal tissues have not been identified.
Metabolism studies have also been conducted on the major plant
metabolite BTS 44596.
In Animals
Metabolism of prochloraz in rats, dogs, rabbits, mice and pigs is
discussed under "Biochemical Aspects".
Goat
A study was conducted to indicate the level of residues that
might occur in tissues and milk of lactating ruminant animals after
ingestion of prochloraz. A lactating goat was given two 60 mg oral
doses of 14C-phenyl labelled prochloraz by gelatin capsules, with 13
days between doses. The dosage was designed to reflect possible
residues resulting from consumption of 3 kg of straw with residues of
20 mg/kg of prochloraz at each dosing (versus maximum straw residues
of 12 mg/kg). Milk and plasma samples were taken at appropriate
intervals after each dose and tissue samples 24 h after the second
dose. After the first dose, residues of prochloraz equivalent in milk
decreased from 0.04 mg/kg after 8 h to 0.01 mg/kg (limit of
determination) after 48 h. Milk residues were 0.01 mg/kg and 0.03
mg/kg at 2.5 and 18.5 h, respectively, after the second dose. In
tissues, residues of prochloraz equivalent were 1.7 mg/kg in liver,
0.2 mg/kg in kidney, 0.36 mg/kg in adrenal, <0.05 mg/kg in fat,
0.03 mg/kg in muscle and <0.2 mg/kg in other tissues. Rumen
contents contained prochloraz equivalent of 0.65 mg/kg and bile 5.4
mg/kg. Residues were not characterized or identified (Campbell 1980).
A second and similar experiment was conducted using the
photodegradant and plant metabolite BTS 44596, with an interval of 8 h
between doses. Results were similar to those for prochloraz, although
the higher maximum milk residues of 0.07 mg/kg BTS-44596 equivalent 7
h after dosing decreased somewhat more rapidly (< 0.01 mg/kg after
31 h). Residues of BTS-44596 equivalent in tissues were somewhat less
than for prochloraz (liver 0.59 mg/kg, kidney 0.12 mg/kg, fat <0.01
mg/kg, muscle <0.01 mg/kg and less in other tissues as well),
Residues were not identified or characterized (Campbell & Needham
1980).
In another study a lactating goat was fed ad libitum for four
days with wheat straw containing 19 mg/kg field-incurred
14C-prochloraz equivalent residues. The straw was harvested at
maturity 11 weeks after treatment at an equivalent rate of 0.94 kg
a.i./ha (twice the recommended rate), Actual straw consumption was
approximately 300 g/day (or approximately 6 mg of prochloraz
equivalent/day), in addition to 1 700 g additional feed made available
daily. After four days, maximum tissue residues of prochloraz
equivalent, as determined by liquid scintillation counting was liver
0.05 mg/kg, kidney fat 0.04 mg/kg and 0.03 mg/kg in other tissues,
with a reported limit of determination of 0.02 mg/kg. Bile and rumen
residues were 0.12 and 0.13 mg/kg, respectively. Maximum residues in
milk were 0.006 mg/l and in plasma 0.079 mg/l. The limit of
determination for milk was 0.001 mg/kg in this experiment. Residues in
milk were relatively consistent over the four-day feeding period. Milk
residues were 0.002 mg/kg prior to the feeding of treated straw
(Campbell 1983).
In Plants
The fate of prochloraz has been studied in wheat (various
stages), citrus and apples. Soil uptake by sugar beet, which
represents a likely rotational crop, was also studied. The metabolic
fate in plants is illustrated in Figure 1.
Wheat
Wheat plants grown under glass were syringe treated at the sixth
leaf stage with a 25 percent a.i. formulation of 3H-labelled
prochloraz (labelled in the 3 and 5 positions of the benzene ring) at
a rate equivalent to 1 kg/ha. After 19 days, the green wheat tissue
was harvested and residues characterized and quantified by a variety
of analytical techniques. Neutral, acid and basic fractions were
analysed, with and without acid hydrolysis. After 19 days, 82.6
percent of the applied radioactivity remained and 96.7 percent of this
was extractable by the analytical procedures used (acetone-water
extraction). The principal residue was free BTS 44596, closely
followed by free and conjugated residues of BTS 44595, with lower
levels of free prochloraz and free and conjugated trichlorphenol,
conjugated trichlorophenoxyacetic acid and seven unknowns. Terminal
prochloraz equivalent was 7 mg/kg. Individual residues were:
% of applied % of terminal
radioactivity residue*
BTS 44596: N-formyl-N'-l-propyl-N-(2-(2,4,6-
trichlorophenoxy) ethyl) urea free 31.6 41
BTS 44595: N-propyl-N-(2-(2,4,6-trichlorophenoxy) free 11 13
ethyl) urea conj. 19.5 23.1
BTS 45186: 2,4,6-trichlorophenol free 1.4 1
conj. 6.8 4.9
BTS 9608 2,4,6-trichlorophenoxy-
acetic acid conj. 0.21 0.2
BTS 40542: Prochloraz (unchanged) free 1.1 1.5
Unknown (seven) 5.5 7.7
Radioactivity undetected 0.93 1.3
Radioactivity unaccounted for 4.56 6.3
82.6 100.0
* Expressed as prochloraz equivalents
None of the seven unknowns exceeded 4.3 percent of the terminal
residue. Analysis of barley plants treated in a similar manner
suggests that over 86 percent of the radioactivity applied to foliage
was absorbed by the leaf within 24 h (McDougall 1979).
In another study, separate plots of wheat at the flag leaf sheath
opening stage were treated with a 25 percent a.i. formulation of
either 3H-prochloraz labelled in the 3 and 5 positions of the phenyl
ring or 14C-prochloraz labelled in the 2 position of the imidazole
ring, at an equivalent rate of 1 kg a.i./ha. Mature wheat was
harvested 13 weeks after treatment and the grain, straw and chaff were
analysed.
Total residues, expressed as prochloraz equivalents, from the
phenyl-labelled prochloraz were grain 0.26 mg/kg, chaff 13.2 mg/kg and
straw 26.5 mg/kg. An almost identical distribution resulted from the
14C-prochloraz treated wheat. From the 3H-prochloraz treated wheat
76-80 percent of the recovered radioactivity was extractable from both
straw and grain. Attempts to hydrolyse polar fractions with HC1 were
unsuccessful, but better results were achieved with pyridine
hydrochloride, which hydrolyses conjugated residues containing the
2,4,6-trichlorophenyl moiety. The procedure liberates
2,4,6-trichlorophenol from both prochloraz and any metabolites
containing the 2,4,6-trichlorophenyl moiety. Results of the analysis
of the 3H-prochloraz treated wheat, expressed as a percentage of
recovered radioactivity in mature tissue, are summarized below:
 Straw Grain
Free 2,4,6-trichlorophenol (BTPS 45186) 5.2 4.1 3.4 8.4
Residues containing the BTS-45186 moiety 58 37.9 53.9 39.5
Tritiated water 4.8 - 4.2 -
Unidentified extractable residues 9.1 34 15.5 32.1
Unidentified residues in solids 22 24 24.7 20
99.1 100 101.7 100
Of the unidentified extractable residues no one of the grain or
straw acidic, basic, volatile or aqueous fractions analysed contained
more than 6.2 percent of the total residue.
Formation and analysis of the glucosazone after hydrolysing
starch extracted from the 14C-prochloraz treated grain demonstrated
that 32.4 percent of the grain radioactivity was incorporated into the
starch molecules. This is thought to result from the incorporation of
14C-carbon dioxide, resulting from decarboxylation of the metabolite
BTS 44596. The remaining residues were separated into various
fractions but were not identified (Kelly 1980d; Kelly & Krepski 1980).
When wheat seeds were treated at an equivalent rate of 0.4
a.i./kg seed (approximating recommended usage) with a 20 percent a.i.
liquid seed dressing and grown to maturity, residues at maturity (29
weeks) were:
% of total Prochloraz
applied Equivalents (mg/kg)
Treated Controls
Soil 58œ3 0.06 -
Roots 15.1 1.5 -
Chaff 0.1 0.04* 0.004*
Grain 0.04 0.004* 0.002*
Total aerial 6.6 0.16 -
* mean of four determinations.
Translocation into the aerial portion of the plant was complete
after six weeks (Krepski 1982).
Potted wheat seeds were allowed to germinate under glass after
soil surface drench application of a diluted 3H-prochloraz
formulation. Approximately 82 percent of the radioactivity (0.8
percent of applied) was retained in the root system at harvest after
21 days. Residues decreased with increased distance of plant parts
from the root system; they decreased from 8.7 percent of residues
recovered in leaves 1 and 2, 0.62 percent in leaf 3, to 0.08 percent
in leaf 7. Soil contamination was suspected of contributing some of
the residues for leaves 1 and 2 and lower plant parts.
In this same report a 3H-prochloraz formulation was applied with
a syringe at the fifth leaf stage to the third leaf of similarly grown
wheat without the soil drench. Plant parts were harvested and analysed
after 24 days; 99 percent of applied radioactivity was retained in
leaves 3 and 4 (difficulty was encountered in discerning leaves during
applications). Radioactivity decreased from 0.03 percent of that
applied in leaf 5 to 0.01 percent in leaf 9 and increased from 0.23
percent in leaves 1 and 2 to 0.33 percent in the roots (McDougall
1980a).
After spring wheat harvest from soil plots treated at a rate
equivalent to 1 kg/ha with either imidazole ring-labelled
14C-prochloraz or phenyl-labelled 3H-prochloraz, the plots were
re-sown with winter wheat and reharvested 10 months later. Soil
samples at harvest of the winter wheat were 0.11 and 0.43 mg/kg for
14C and 3H treatments, respectively. Residues of 14C or
3H-prochloraz equivalent were < 0.01 mg/kg in grain, chaff and
straw. Grain control values were about half that for grain, but not
statistically different. Low uptake is suggested for 3H-prochloraz
treated chaff and straw where experimental levels are 50 to 100 times
that of the respective controls (Krepski 1981a).
A 1.5 sq m plot was treated with 14C-phenyl labelled prochloraz
formulation by garden sprayer at an application rate equivalent to 395
g a.i./ha, which approximates recommended usage for cereals. After 41
days the soil was turned by hand and sown with sugarbeets as a
representative rotational crop. A control plot was also planted.
Seedlings were sampled 23 days after planting and mature plants (roots
and foliage) at 157 days after planting.
Residues at planting in soil averaged 0.26 mg/kg prochloraz
equivalents. At 23 days after planting, residues of 0.07 mg/kg in
seedlings were significantly greater than the 0.006 mg/kg controls. At
harvest, residues of 0.005 mg/kg in the soils were not significantly
different than in controls, whereas the 0.0052 mg/kg prochloraz
equivalent in foliage was slightly greater than the 0.003 mg/kg
control. This difference is statistically significant, although
residues in the foliage are less than the background level (McGibbon
1982).
Citrus
3H-prochloraz, a 2.5 percent E.C. formulation, was applied by
pipette wash, to simulate a 0.1 percent a.i. postharvest commercial
treatment, to Washington Navel oranges in Spain. Storage was at 4°C in
the dark from 10 to 60 days and at 20-22°C in diffuse light. From 1 to
10 days, surface residues were removed with a 0.1 percent ethylan BCP
solution and those in peel and flesh by extraction with 90 percent
acetone. Surface residues at room temperature decreased from 5.8
percent of applied radioactivity at 1 day to 0.8 percent at 10 days,
and at 4°C from 3.8 mg/kg at 10 days to 1.1 mg/kg at 60 days.
Corresponding residues in the flesh increased from 1.8 percent to 5
percent of the applied dose over the storage period for room
temperature storage, but remained relatively constant at approximately
1.6 percent over the entire 60 day storage period at 4°C.
Radioactivity in the peel remained relatively constant at 92.3-96
percent of the applied dose over the room temperature storage period
and, similarly, 94.7 to 97.2 percent over the storage period at 4°C
(Kelly 1980a). Similar unlabelled studies on citrus residues during
storage are reported under "In Storage and Processing".
Apples
Formulations of phenyl-labelled 14C-prochloraz or its manganese
complex BTS 46828 were applied to individual apples under glass at
rates approximating commercial usage. In samples analysed after 20
days, residue distribution was similar in the surface, peel and flesh
from either chemical. Residues in extracts from the prochloraz-treated
apples (86 percent of the applied dose was recovered) were - wash 7.4
percent, peel 50 percent and flesh 16.9 percent (20 percent of
recovered). BTS 46828 gave almost identical results (Krepski 1981b).
In similar experiments (not carried out under glass) the
metabolism of prochloraz and its manganese complex were compared at
intervals between 0 and 54 days after application by syringe to
individual apples. Radioactivity distribution was again shown to be
similar from use of either chemical, although prochloraz from the E.C.
formulation was initially absorbed into the peel at a faster rate than
the Mn-complex W.P. and was somewhat more persistent. The manganese
complex was also more susceptible to losses from precipitation shortly
after application. As a percent of radioactivity recovered at 0 and 54
days, residues were:
Prochloraz Mn - complex
*day 0 day 54 *day 0 day 54
surface 32.3 1.9 67.6 1.2
flesh 0.0 0.8 0.0 1.2
peel 67.2 81.2 32.4 56.2
Total 100 83.9 100 58.6
* 5 hours (McDougall 1980b).
In another similar study distribution and characterization of
residues were investigated in two apple varieties at intervals ranging
from 16 to 63 days after application by syringe of the Mn-complex to
individual growing apples. As a percent of the applied dose,
representative residues were:
Days after Whole Peel
treatment fruit Flesh Total Extractables
16 72 (83) 15.81 67 (78) 51.3 (61.4)
33 54.8 (76) 3.6 (5.2) 51 (71) 37.2 (55.2)
63 63.5 5.4 60 38
Numbers in parentheses correspond to the Worcester variety and
others to the Cox variety.
Extractable residues in peel were quantified and characterized in
both varieties at 33 and 63 days after treatment. As a percent of
extractable peel residues the metabolic profile at 63 days for the Cox
variety was:
Metabolite Percent
Prochloraz or Unchanged BTS 46 828 5 )
BTS 45186 (free 2,4,6-trichlorophenol) 11.5 )
BTS 44595 15.2 )
BTS 44596 33.1 ) >77.8
Conjugated residues containing )
the BTS 45186 moiety 13 )
Unidentified polar metabolites 6
"Unknowns" 1.6,0.7
Unresolved background 13.9
Total unidentified (excluding conjugates
with BTS 45186) 22.2
100.0
Results were similar at 33 days for both varieties, except that
conjugated residues containing the BTS 45186 moiety were only 3
percent for the Worcester variety, only the first "unknown" was
observed in Cox and the first and a different second "unknown" was
found in Worcester. None of the residues exceeded 2 percent (Kelly
1982a).
In Soil
Prochloraz 25 percent E.C. formulations, labelled with 14C in
the imidazole ring and separately in the phenyl ring with 3H, were
separately applied to acidic sandy loam and silty clay loam soils in
enclosed jars purged with CO2-free air under laboratory conditions
for up to 52 weeks. Application was at 6 mg/g soil, equivalent to 0.7
kg a.i./ha (a recommended crop application rate). Silty clay loam was
similarly treated with prochloraz labelled with 14C in the phenyl
ring and studied over 9 months.
Radioactivity losses as volatiles were about 50 percent of the
applied dose to silty clay for both 14C-imidazole and 3H-phenyl
labelled prochloraz after one year, as was the case for the
14C-phenyl labelled formulation at 9 months. Losses were more rapid
from silty clay loam than from sandy loam, for which losses were half
or less that in silty clay. Experimental evidence indicated, but did
not prove, that volatiles from 14C-phenyl and 14C-imidazole labelled
prochloraz were predominantly CO2. In the case of 3H-phenyl labelled
prochloraz, evidence suggests volatile radioactivity in the form of
tritiated water. The major extracted residue in all cases was
prochloraz per se at 50-68 percent of the applied dose (80-90
percent of extracted) after 8 weeks to 12-30 percent (45-67 percent of
extracted) after 52 weeks. Lower residues of BTS-44596 and BTS 45186
combined were <18 percent of the applied dose (<26 percent of
extracted) after 52 weeks for 3H-labelled treatments. Binding was
more pronounced in sandy loam. The half-life was reported as 3 and 5
months, respectively, in silty clay and sandy loams. In both cases the
acidic pH would increase stability (Kesterton 1981a).
In German standard soils the half-life of unlabelled prochloraz
ranged from 115-135 days, with stability directly related to organic
matter content. For both soils the pH was acidic, which is conducive
to prolonged stability (McGibbon 1982).
14C-imidazole and 3H-phenyl labelled prochloraz, formulated as
a 25 percent E.C., were incubated under anaerobic conditions at a rate
equivalent to 0.7 kg a.i./ha and sampled at 14, 27 and 60 days.
Residues of prochloraz remained relatively constant over the test
period in silty clay loam (ca 68 percent of the applied dose) and
decreased slightly (to ca 54 percent) in silty clay loam. The decrease
in silty clay loam was accompanied by an increase in bound residues.
Anaerobic conditions halted the continuous degradation and formation
of volatiles observed under aerobic conditions. Other components
indicated by TLC from 3H treatments were BTS 44596 and BTS 45186 at
approximately 6 and 3 percent of the applied dose, respectively,
whereas from 14C treatments prochloraz, imidazole and BTS 44596 were
approximately 68, 2 and 1 percent, respectively (Kesterton 1981b).
The absence of significant degradation of a 14C-phenyl labelled
2.5 percent E.C. prochloraz formulation in sandy loam and silty clay
loam under sterile soil conditions for 30 days suggests that
degradation is facilitated primarily by soil microorganisms as opposed
to chemical degradation, at least under the experimental conditions
(acidic pH and 50 percent soil moisture capacity) (Newby 1982).
Sandy loam and silty clay loam soil were treated with a
14C-benzene labelled 25 percent E.C. prochloraz formulation at a rate
equivalent to 0.44 kg a.i./ha, aged 13 days, added to the top of 30 cm
× 7 cm diameter columns containing untreated soil and eluted with an
equivalent of 1.5 cm rain for 18 days. Essentially all radioactivity
was retained in the top 5 cm of soil, with less than 0.3 percent of
the applied amount being detected in leachate (Kesterton & Newby
1980a).
In a similar soil column study, except that the equivalent
application rate was 0.8 kg/ha, the labelled prochloraz was applied to
the same silty clay loam and aged for 30 days, after which it was
eluted for 45 days with a daily equivalent of 0.5 cm rainfall. Results
were similar to the first study in that <0.05 percent of the
applied dose was detected in the eluant. However, even though 88-94
percent was in either the treated portion or the first 5 cm below it,
0.3-0.6 percent was detected in the bottom 25 cm, indicating some
downward mobility other than that detected in the leachate (Kesterton
& Newby 1981).
When an unlabelled prochloraz formulation was applied to West
German standard soil columns at a rate equivalent to 1 kg/ha, <0.05
percent was detected in the leachate (Maier-Bode 1980). In a similar
experiment leachate contained <1.7 percent of the applied dose
(Stockbauer 1983). Details of the preceding two experiments were not
available.
In soil thin-layer mobility tests, the mobility of prochloraz and
soil metabolites BTS 44 596 and BTS 45186 were compared to that of
atrazine and 2,4-D in four soil types. According to the Helling
mobility index, over-all mobility in the four soils were, in order of
increasing mobility, prochloraz, BTS 44596 (low mobility), BTS 45186
(low mobility), atrazine (intermediate mobility) and 2,4-D (mobile).
Mobility of all tested materials was greatest in sand (even BTS 44596
and BTS 45186 would have intermediate mobility). Prochloraz and BTS
44596 were immobile in silt loam, whereas BTS 45186 was of
intermediate mobility. The thin-layer studies confirmed the low, but
positive, mobility detected in soil column experiments (Leake & Lines
1981).
Adsorption/desorption of prochloraz, uniformly labelled in the
phenyl ring and applied as an E.C. formulation to a sandy loam and
silty clay loam, was investigated. The Kd of the sandy and silty
soils were 152 and 256, respectively, indicating slightly more
adsorption by the latter. The K values were 86.8 and 101.7,
respectively, for these soils. Strong adsorption and minimal
desorption is consistent with leaching studies (Kesterton & Newby
1980b).
A trichlorophenol tritiated ring prochloraz E.C. formulation was
applied to one sq m plots of barley and wheat in sandy loam at an
equivalent rate of 1 kg/ha. Core samples from 32.5 cm deep were
analysed after approximately 15 and 30 weeks; > 95 percent of
prochloraz residues were found in the upper 10 cm and in some cases
the top 2.5 cm. Somewhat similar distribution after 15 and 30 weeks
suggests only slight leaching of bound residues as a result of
additional (over 150 mm) rainfall in the latter period (Kesterton
1978).
In Water
No degradation of prochloraz was observed after 30 days in 22°C
buffer solutions at a pH of 4.95 or 6.98. At pH 9.18 degradation does
occur, with 77 percent of the added prochloraz remaining after 30
days. A first order degradation was indicated and a half-life
estimated of approximately 79 days. The only degradant
N-propyl-N-2-(2,4,6-trichlorophenyl) ethylamine (BTS 40348) was
reported to account for more than 2 percent of the total
radioactivity, although the quantitative analyses were not provided
(Kelly 1982b). The 4:1 manganese complex of prochloraz (BTS 46 828),
either as active compound or as a 50 percent W.P. formulation,
disassociated to prochloraz and manganous chloride in the presence of
water (pH 6.1). Approximately 50 percent of the complex was
dissociated within four hours at 25°C (Whiting & Dickinson 1979).
Biodegradability of prochloraz in water was studied using the
closed bottle test according to OECD Guideline 301D. Tests indicate
little biodegradation at 20°C up to 15 days after inoculation, but 13
percent degradation by 28 days, suggesting some initial bacterial
adaptation (Douglas & Pell 1982). When bacteria preadapted to
prochloraz were used as the inoculum, degradation commenced
immediately and reached 47 percent biodegradation by 28 days (Douglas
& Pell 1983).
In Storage and Processing
Processing
Citrus - Oranges were postharvest brush-treated at 1 000 mg/kg with
a 40 percent E.C. formulation and processed into "42% comminuted
orange" and "special whole orange compound". These "whole oranges
juices" (chopped oranges) are used for production of various orange
drinks and utilizes most of the whole orange, except for removal of
some coarse peel. It includes a pasteurization process up to 86°C,
depending on which of two processes were used. Total residues
(converted to 2,4,6-trichlorophenol for analysis) were 0.19 and 0.14
mg/kg expressed as prochloraz for the 42 percent comminuted orange and
special whole orange compound, respectively, approximately 30 percent
of the residues found in whole oranges (Manley & Snowden 1982b).
Cereals - Winter wheat was treated post-emergence at two sites in
the Federal Republic of Germany at growth stage 59 (mid-May to
mid-June) with three applications of a 40 percent E.C. prochloraz
formulation at the 0.48 kg/ha rate recommended in that country. Mature
grain was sampled at 57-58 days after last application and portions
milled and baked into bread. Total residues were determined by
hydrolysis of the trichlorophenol containing moieties followed by EC
analysis and use of a 1.906 factorial conversion to a prochloraz
equivalent basis. Residues in grain and bread were <0.1 mg/kg, the
limit of determination.
Under similar conditions prochloraz was applied twice to barley.
Total residues in barley malt, expressed as prochloraz, were 0.13 and
0.15 mg/kg compared with 0.07 mg/kg in a control. Limit of
determination was 0.1 mg/kg.
Rapeseed Oil - Mean residues of total prochloraz and metabolites
hydrolysable to the trichlorophenol, and expressed as prochloraz, in
mature rapeseed treated in each of four sites in France ranged from
0.06 to 0.2 mg/kg (over-all mean of 0.11 mg/kg). Treatment was with
two applications of an E.C. formulation at a rate of 0.45 kg a.i./ha
and harvest was 25-38 days after the last treatment. Mean residues of
total prochloraz and its metabolites in hexane-extracted oil from
these trials (omitting one individual 0.49 mg/kg outlier from six
replicate results, otherwise ranging from 0.07-0.13 mg/kg) ranged from
0.11 to 0.29 mg/kg (over-all mean 0.10 mg/kg), indicating a residue
concentration factor of approximately 3 times. Residues in the treated
seed and oil only ranged from approximately 1-3 times that in controls
from any given site and were therefore not far removed from the limit
of determination in any one site, with a variation of from 0.05 to 0.2
mg/kg among the sites.
In similar studies at three sites each in Denmark and Sweden, the
concentration factor for prochloraz in the oil ranged from only 1.2 to
2 times that in the seed. Treatment-to-harvest intervals range from 47
to 76 days. In these studies, residues in treated seed and oil derived
therefrom, although comparable to those in France, were significantly
greater than in controls. The limit of determination was estimated at
0.05 and 0.1 mg/kg, respectively, for seed and oil.
Mushrooms - Mushrooms were harvested in France 17 days after
pre-emergence treatment with a 40 percent E.C. prochloraz formulation,
administered at a rate of 40 g a.i./100 sq m. No information on good
agricultural practice is available from France. The mushrooms were
immediately processed by canning and dehydration, after which samples
of canned mushrooms, the liquor and dehydrated mushrooms were analysed
as total residues of prochloraz and its metabolites that were
hydrolysable in the presence of pyridine hydrochloride to the
trichlorophenol moiety. Total residues (apparently uncorrected for
recoveries) and expressed as prochloraz were less than the 0.1 mg/kg
limit of determination for canned mushrooms and the liquor, with
control values of 0.03 and 0.05 mg/kg, respectively, reported. An
apparent residue of 0.14 mg/kg in dehydrated mushrooms was less than
the 0.23 mg/kg control value. Analytical recoveries were 64, 63 and 46
percent, respectively, for dehydrated mushrooms, canned mushrooms and
the liquor (Browne 1982h).
In experiments conducted in The Netherlands, total residues of
prochloraz and its metabolites, expressed as prochloraz, were
determined in canned mushrooms and the liquor after three treatment
schedules with a 50 percent W.P. formulation of the prochloraz
manganese complex, (1) 0.75 a.i./sq m post-casing and 0.94 g a.i./sq m
14 days after casing (2) 1.5 g a.i./sq m 9 days post casing (good
agricultural practice in The Netherlands) and (3) 1.5 g a.i./sq m both
at post-casing and after 14 days. Total residues expressed as
prochloraz were equal to or less than the 0.1 mg/kg limit of
determination in the six liquor samples and five of the six canned
mushroom samples. For treatment (3) above, however, the residue was
0.23 mg/kg in the sixth mushroom sample and 0.1 mg/kg in the control.
No information was available on the interval from last treatment to
harvest. Recoveries were 81 and 94 percent, respectively, for canned
mushrooms and the liquor. No analyses were available for the
unprocessed mushrooms (Churchill & Longland 1983c).
Storage
Citrus - Data are available from a variety of treatment and storage
conditions and are summarized in Table 3. Navel oranges were
commercially treated postharvest in South Africa with a 40 percent
E.C. prochloraz formulation at 1 000 and 2 000 mg/kg a.i. rates then
waxed and shipped by refrigerated vessel to the United Kingdom.
Samples were stored either in the dark at approximately 20°C or at
4°C. Sampling for analysis occurred at 0 (44 days after treatment), 7,
14 and 21 days after storage commenced. Mean (range in parentheses)
residues, corrected for recovery, of total prochloraz and metabolites
expressed as prochloraz at the lower treatment rate were 0.05 mg/kg
(0.030.08 mg/kg) in flesh, 2.1 mg/kg (1.4-2.8 mg/kg) in peel and 0.49
mg/kg (0.3-0.7 mg/kg) on the whole fruit (calculated from peel to
flesh weight ratios) for ambient and 4°C fruit combined over the
entire storage period. Corresponding values for the higher treatment
rate were 0.05 mg/kg (0.02-0.07 mg/kg), 3.3 mg/kg (2.5-3.7 mg/kg) and
0.84 mg/kg (0.7-1.7 mg/kg) respectively.
The list of determination was reported as 0.05 mg/kg, which is
consistent with uncontaminated control values of 0.01-0.02 mg/kg total
residue. No observable trend in lower residues occurred during the
21-day storage period at either temperature and there were no
noteworthy differences in residues between the storage temperatures.
The trials also demonstrated that approximately 94 percent of the
total residue was in the peel (Manley & Snowden 1982b).
In similar postharvest treatments to oranges in Spain, with
0.05-0.1 percent solutions or 3-4 g a.i./tonne of fruit (usually in
wax) with an E.C. formulation as a spray or dip, residues were
determined after storage intervals typically ranging from 1-10 to
10-60 days at 4°C-22°C. Maximum peel residues were as much am 7.3
mg/kg for prochloraz alone and 8.4 mg/kg for total residues of
prochloraz and its metabolites, both expressed as prochloraz. Total
residues in flesh, expressed as prochloraz, were 0.04-0.44 mg/kg and
on a calculated whole fruit basis up to 2.5 mg/kg. As in the South
African study, a definite trend towards decreased residues could not
be determined over the varied storage intervals and temperatures and
little difference was observed in residues where application rates
varied by a factor of two. Peel was repeatedly shown to contain most
of the total whole fruit residue with typically <5 percent being
present in the flesh (Richards 1980b,f; Kelly 1980a; Browne 1981b).
Similar studies were conducted in the United Kingdom where
oranges were dip-treated with a 0.07 percent a.i., 40 percent E.C.
formulation and analysed after 1, 7, 21, 25 and 70 days cold storage
(temperature not provided). Total residues were expressed as
prochloraz. Residues of the parent compound in peel ranged from 3.6 to
5.1 mg/kg compared to 5.8 to 7.8 total residue. In flesh, the residues
were 0.05 to 0.12 mg/kg and 0.07 to 0.15 mg/kg, respectively. No
declining residue trend was observed over the 70-day maximum storage
period, although total residues in flesh increased from about 4
percent of the whole fruit residue at 1-7 days to 11 percent after 70
days (Browne 1982i).
No decline in residues on oranges was observed during storage at
intervals from 1 to 16 days at ambient temperature in Australia after
dip treatment with a 40 percent E.C. prochloraz formulation as a 250
to 500 mg/kg a.i. solution. Total residues determined as the
trichlorophenol expressed as prochloraz were similar from the two
application rates, as were residues of prochloraz alone. Residues of
prochloraz when determined alone ranged from 1.5 to 3.9 mg/kg (2.9
mg/kg) in peel and from <0.02 to 0.1 mg/kg (0.04 mg/kg) in flesh over
the 16-day storage interval for both concentrations. Total residues
were similar 1.4-3.4 kg (2.5 mg/kg) and <0.05 mg/kg, respectively.
Again, most of the residue was in the peel (Browne 1981a).
Residues in Spanish lemons were investigated after a shower
postharvest treatment with a 3 000 a.i. E.C. formulation in wax.
Samples were analysed after 12 and 16 days of storage. The limit of
determination was 0.02 mg/kg and 0.05 mg/kg for prochloraz alone and
total residues expressed as prochloraz, respectively. Residues
expressed as prochloraz were similar after both storage periods, with
approximately 5 percent of the residue in the flesh. Residues of
prochloraz alone and total residues were:
Prochloraz alone (mg/kg) Total residues (mg/kg)
Peel Flesh Whole Peel Flesh Whole*
9.1-15.6 0.27-0.3 3.1-5.0 10.6 0.17-0.33 3.7-6
(12.3 mean) (0.29) (4.6) (14) (0.23) (5.1)
* Calculated from peel/flesh.
Recoveries were 75 percent in peel and 77-100 percent in flesh
(Browne 1982c).
In 1980, postharvest trials on oranges in Spain, using a 40
percent E.C. prochloraz formulation, were made with 200 1 of wax
formulation (3 g a.i./1 wax) to 150 tonnes of fruit (Richards 1980b).
Samples of orange flesh from these trials were analysed for free
2,4,6-trichlorophenol (BTS 45186), since no metabolism study was
available on orange and BTS 45186 has been found in low levels in
wheat (Kelly 1980d). Samples were analysed after 7 and 14 days at
20-22°C. Residues were 0.005-0.01 mg/kg (0.008 mg/kg mean) after 7
days and 0.005 mg/kg after 14 days, as compared to <0.005 mg/kg for
controls. Contrary to the findings in most other citrus postharvest
trials, a slight trend of decreasing residues with time was indicated
(Richards 1980e).
In postharvest dip trials using a 25 percent E.C. or 25 percent
W.P. prochloraz formulation on oranges and lemons in Italy residues
were determined after 57 days storage at 7°C. Significantly higher
residues resulted from E.C. applications in both fruits and lemon
residues were higher than those in oranges (Browne & Manley 1982b).
Oranges treated in South Africa with a postharvest fungicidal
brush at 500-4 000 mg/kg a.i. 45 percent E.C. were analysed after two
months storage at ambient temperature. Again, most of the residue was
in the peel, with total residues, expressed as prochloraz, ranging
from 0.08 mg/kg at the lower application rate to 0.73 mg/kg at the
higher one. There was a good correlation of residue level with
application rate (Snowden & Manley 1983).
Bananas - Bananas were treated by postharvest dipping in Australia
with 250 and 500 mg/kg a.i. solutions of 40 percent E.C. prochloraz
and analysed after ambient storage for 9, 10, 12 and 16 days. Free and
conjugated residues of prochloraz and its metabolites were determined
as the trichlorophenol, expressed as prochloraz, with a 0.05 mg/kg
limit of determination. Residues resulting from both treatment rates
and over the entire storage period ranged from 5.2-10 mg/kg in skin
and 0.02-0.05 mg/kg in flesh. No evidence of decline in residues was
observed in skin or flesh over the storage period and there was no
significant difference in residues between the two application rates
(Browne 1982b).
Photodecomposition
Thin-layer chromatography and nuclear magnetic resonance (NMR)
analysis of a photodecomposition product of an aqueous solution of
prochloraz confirmed the presence of
N-formyl-N-propyl-N',-2-(2,4,6-trichlorophenoxy) ethylurea (BTS 44596)
(Haran 1978). Prochloraz aqueous solutions of 26.1 and 43.3 mg/kg
concentrations in glass-stoppered flasks were exposed to artificial
light (420-760 nm wavelength) at 32°C for 36 and 16 days,
respectively, with periodic analyses by high pressure liquid
chromatography. At the lower concentration residues decreased from
6.93 × 107 moles (26.1 mg/kg) initially to 1.94 × 107 moles after 36
days while BTS 44596 increased from 0.0 to 3.58 × 107 moles. At the
higher concentration, residues decreased from 11.5 × 107 moles (43.3
mg/kg) at zero day to 5.8 × 107 moles after 16 days, while BTS 44596
increased from 0.0 to 35.8 × 107 moles. The first order decay
half-lives were 20.5 and 18.1 days, respectively, for the two
concentrations.
Retention times indicated that a minor unquantified degradation
product was N-propyl-N-2-(2,4,6-trichlorophenoxy) ethylurea (BTS
44595). This appeared only towards the end of the exposure period
(Haran 1980).
METHODS OF RESIDUE ANALYSIS
Analytical methods are available for the analysis of free
prochloraz or total free and conjugated residues of prochloraz and its
major metabolites. Methodology for free prochloraz was first developed
for the analysis of cereal grains and straw (Somerville 1980). Finely
milled straw or ground grain are vigorously extracted with acetone,
filtered, a small portion acidified with HCl and concentrated by
rotary evaporation. The aqueous fraction is partitioned with petroleum
ether, which is discarded, adjusted to pH 6-7 and again partitioned
with petroleum ether, which is concentrated to dryness. The sample is
taken up in ethyl acetate and analysed by electron capture gas
chromatography utilizing a 7 percent OV-101 packed column. Analytical
recoveries of wheat and barley grain, amended with prochloraz at
0.05-0.5 mg/kg, were 78 ± 10.6 percent and 91 ± 10.9 percent,
respectively. For straw, the respective recoveries were 82 ± 12
percent and 80.6 ± 17 percent. The level of "sensitivity" has been
estimated at 0.01 mg/kg for grain and straw, although in the field
trials apparent untreated controls in cereal grain and straw were
occasionally up to 0.02 mg/kg.
With minor modifications, this method has been used for
determining free prochloraz residues in apples, pears, citrus,
mushrooms, strawberries and lettuce (FBC, (Hayto 1979b; Whiteoak 1981;
Kelly 1980a; Browne 1981b, Maclaine Pont et al. 1980) with
analytical recoveries of > 65 percent and usually > 80 percent.
Apparent residues in untreated controls generally ranged from <0.01
to approximately 0.05 mg/kg depending on the commodity.
A method is also available for measuring total free and
conjugated residues of prochloraz and its major plant metabolites,
N-formyl-N'-1-propyl-N'-[2-(2,4,6-trichlorophenoxy) ethyl] urea
(BTS 44596), N-propyl-N-[2-(2,4,6-trichlorophenoxy) ethyl] urea
(BTS 44595) and conjugated 2,4,6-trichlorophenol (BTS-45186), after
converting all of them to the common moiety 2,4,6-trichlorophenol
(Manley & Snowden 1982a). Any other metabolite containing the
2,4,6-trichlorophenol moiety would probably be measured as well.
Although total residues are actually measured as BTS-45186, residues
are usually expressed as prochloraz, after use of a molecular weight
factor of 1.906.
Thoroughly ground, milled, chopped or minced and mixed samples
are Soxhlet extracted with acetone, filtered, dried by passing through
anhydrous sodium sulphate and evaporated to near dryness by a rotary
evaporator. The sample is refluxed with pyridine hydrochloride at
205°c for one hour to hydrolyse to the BTS-45186 moiety, after which
HCl is added to the sample. The sample is liquid-liquid extracted
after addition of petroleum ether. Potassium hydroxide is added, the
sample partitioned and the aqueous phase collected and acidified with
HCl. The sample is partitioned into toluene for analysis by
electron-capture gas chromatography, utilizing a 20 percent carobowax
20M + 1.7 percent phosphoric acid packed column.
The method was validated on fruits and cereal grains with mean
over-all recoveries of 92 ± 15.9 percent when samples were amended at
0.2-10 mg/kg prochloraz. A 0.05 mg/kg limit of determination is
considered attainable for most samples, although apparent residues of
0.2-0.3 mg/kg may be encountered occasionally, especially in cereal
straw.
With minor variations, this method has been successfully used for
the analysis of cereal grain and straw, cereal grain green plant
tissues, apples, citrus fruit (peel, flesh, whole fruit), orange
juice, rapeseed, rapeseed oil, mushrooms, stone fruit and assorted
fruit with inedible peel (Kelly 1979b; Richards 1981a; Reary 1981a;
Browne & Manley 1982a; Manley & Snowden 1982a). Specifics on
individual crops under actual use conditions are considered earlier in
this evaluation under "Residues Resulting From Supervised Trials" or
under "Fate of Residues."
An analytical method is also available for the determination of
free BTS-45186, which may be a minor component of the residue (Kelly
1980b; Richards 1980e).
No analytical method suitable for enforcement purposes for
products of animal origin was made available.
NATIONAL MAXIMUM RESIDUE LIMITS REPORTED TO THE MEETING
Country Commodity MRL (mg/kg)
France cereal grains 0.05 1
rape seed 0.05 2
The Netherlands 3 Mushrooms 0.05
cereal grains 0.1
Milk 0.01
Meat 0.01
Other products 0 (0.01)
German Federal Republic Cereals 0.5 4
1 Residues of prochloraz alone.
2 Residues included not specified.
3 For MRLs of The Netherlands, the residue is prochloraz
and its metabolites, measured as 2,4,6-trichlorophenol
and expressed as prochloraz. Residue on mushrooms and
for most cereal grain trials conducted in The
Netherlands were for total residues of prochloraz and
its major metabolites.
4 A proposed 0.5 mg/kg NRL for prochloraz and metabolites
has been submitted.
APPRAISAL
Prochloraz is a fungicide in use and/or under development for
control of fungal diseases on a variety of commodities in number of
countries. It is used as a foliar spray, for post-harvest or seed
treatments and is available in a variety of formulations for these
uses, including a prochloraz-manganese complex wettable powder
formulation for sensitive plant species.
Residue data are available from field trials on a number of
commodities in several countries, mostly from foliar applications to
fruit and cereal grains, use in mushroom culture and postharvest
applications to citrus and certain other fruit. Some residue data are
also available for vegetables. Residues were determined either as
2,4,6-trichlorophenol, prochloraz alone or prochloraz and its major
metabolites, mostly the latter over-all. In select cases, data are
available for both prochloraz alone and for prochloraz and its major
metabolites. Apparently the earlier studies were for prochloraz alone,
whereas in later studies total residues were emphasized. Data are
insufficient and too variable to draw a general conclusion on the
ratio of parent compound to parent plus metabolites. For example,
there is little difference in residue measured for citrus or possibly
for mushrooms, but for cereals residues appear to be substantially
higher when total residues are determined.
Residue trials on commodities for which there are known
nationally approved or registered uses were available for watermelon,
apple, grape, cereals, mushrooms and oil-seed rape. Therefore, only
for these is consideration given for MRL estimates. However
"recommended" were available for a number of other commodities and
temporary limits are estimated for those with adequate data. Data were
insufficient for estimation of maximum residue levels for sugarbeets,
sugarbeet leaves, strawberries, almonds, almond hulls, lettuce,
watermelon, apples, pears, rice and grapes.
Information was available on the fate of residues in animals,
plants, the environment and during storage and processing. In animals,
prochloraz is rapidly metabolized from dermal or oral exposure. Most
of the residue is rapidly excreted, with urinary excretion being the
predominant route in most animals tested, although faeces is the
predominant route in dogs. The principal metabolic products in the
urine were 2,4,6-trichlorophenoxyacetic acid and
2,4,6-trichlorophenoxy-ethanol (mostly conjugated). The major plant
metabolites have not been identified in the urine. Although residues
were qualitatively similar in urine or in tissue distribution among
animals studied, tissue residues have not been identified.
The potential for residues in products of animal origin was
investigated in two studies by feeding a lactating goat with
14C-phenyl-labelled prochloraz either by capsule or ad libitum with
14C-prochloraz field-treated wheat straw. The first study represents
the worst case maximum expected theoretical exposure from wheat straw
and the straw feeding is probably more reflective of maximum exposure
under normal feeding conditions.
These goat metabolism/feeding studies give insight into the
maximum potential for residues, although residues in animal tissues
are not identified. It is not known whether urinary metabolites are
the same as those in milk or tissues.
Although straw was used as a worst-case for potential residues,
foraging would appear to be potentially equally important, if not more
so, if not label-restricted, since it can be fed at higher levels in
the diet, as can citrus pulp. However, citrus uses presumably are
intended where peel is discarded. Although wheat or barley grain
residues were relatively low, grain may comprise up to 50 and 80
percent of the diet of poultry and swine, respectively. No analytical
method suitable for enforcement purposes for animal products is
available, or at least known to be validated for that purpose.
Additional information is needed to support a conclusion that the fate
of residues in animals is adequately understood or to estimate maximum
residue levels for products of animal origin.
The fate of Prochloraz in plants was investigated in wheat
(foliar, pre-emergence and seed treatments), citrus (postharvest),
apples (foliar) and sugarbeet (soil uptake). In wheat plants, major
residues at the vegetative stage from foliar treatment were free
BTS-44596, free and conjugated BTS-44595 and 2,4,6-trichlorophenol in
decreasing order, which together accounted for 83 percent of the
terminal residue after 19 days. Less than 2 percent was unchanged
prochloraz and only 0.2 percent was 2,4,6-trichlorophenoxy acetic
acid. No single unidentified residue accounted for more than 6.3
percent of the remainder. Residues appear to be absorbed primarily
within the first 24 hours.
At the harvest stage, only the free trichlorophenol has been
identified in straw or grain, and it occurs as 3-8 percent of the
terminal residue. This, plus tritiated water and other presumably
conjugated but unidentified residues containing the trichlorophenol
moiety, account for 40-70 percent of the terminal residue. No single
remaining unidentified residue fraction accounts for more than 6
percent of the terminal residue.
When applied to wheat seeds or as a pre-emergence drench, most
residue was in or near the root system, with little upward mobility,
and there is little evidence of upward mobility when prochloraz is
applied to foliage although low levels translocate towards the roots.
Only very low levels of residue were found in chaff and straw when
wheat was grown in soil treated with radioactive 3H-prochloraz ten
months earlier. However, sugarbeet seedlings showed low residues when
planted as a rotational crop, but only trace levels were found in the
foliage at maturity and no significant residue in the roots.
In apples, residues migrated into the peel and by 33-63 days
approximately 60-80 percent of the terminal or applied residue was in
the peel, <2 percent on the surface and generally <7 percent of
the applied dose in the flesh, although it can be twice as much.
Qualitatively extractable peel residues were similar to residues found
in wheat at the vegetative stage with approximately 80 percent of the
residues hydrolysable to 2,4,6-trichlorophenol. This, therefore,
accounts for approximately 50-60 percent of the total fruit residue.
Other residues (primarily peel fiber-bound) were not identified, and
although unresolved background accounted for approximately 10 percent
of the whole fruit residue (14 percent of peel extractable), no one
peel extractable metabolite or fraction exceeded approximately 5
percent of the total fruit residues. The fate of residues in plants is
reasonably well understood for the limited number and type of crops
for which there are approved uses. If uses are expanded significantly
to other food groups, additional metabolism studies would be prudent.
Numerous experiments have been conducted on the fate of
prochloraz residues in soil and water. Prochloraz is degraded under
both aerobic and anaerobic conditions with formation of volatile
compounds, probably CO2 and tritiated water. Residues remaining are
predominantly prochloraz, BTS-44596 and BTS-45186, with binding to
upper soil particles increasing with time. The half-life can range
from 90-155 days, depending on conditions. Formation of volatiles is
decreased under anaerobic conditions. Mobility is low in soils,
although low mobility does occur under some conditions, especially for
the metabolites in sand. Prochloraz is stable in water under acidic
conditions, but does degrade under alkaline conditions.
The fate of residues during processing was investigated for wheat
made into bread, postharvest citrus, rapeseed oil and mushrooms after
processing. Residues in bread made from wheat grain with <0.1 mg/kg
prochloraz also showed <0.1 mg/kg residues. Since the limit of
determination is 0.1 mg/kg, no conclusion could be made on the extent
of residue concentration, if any. No information was available for
residues in flour or other milling fractions. Similarly, low residues
were found in malt from prochloraz-treated barley, but no information
was available on the residue level in grain from which it was made.
Residue levels concentrate from 1 to 3 times in oil processed
from mature oilseed rape that has been field-treated with prochloraz.
Residues in canned or dehydrated mushrooms or canning liquor after
pre-emergence treatment with prochloraz were less than in untreated
controls, except for one sample of canned mushrooms with a residue of
0.23 mg/kg. No information was available on residue levels before
canning or dehydration. No conclusion can be drawn on the extent of
residue concentration, if any. When oranges treated postharvest were
processed into "42 percent comminuted orange" or "whole orange
juices", which are used to manufacture various orange drinks,
approximately 70 percent of the residue in the oranges from which it
was processed was lost.
The fate of residues during storage after various postharvest
prochloraz treatments was investigated for citrus, bananas and
avocadoes. Except for one study where free 2,4,6-trichlorophenol was
determined and where treated citrus was stored under a variety of
conditions (dark or light, cold or room temperature and at intervals
ranging from one to 70 days) total residues were very persistent, with
little evidence of a decreasing trend over the varied treatment or
storage conditions. Modest increases (2X) in application
concentrations had little effect on residue levels, approximately
90-95 percent of which were in the peel. There was evidence of
continued gradual translocation into the flesh as the interval from
treatment increases, especially at room temperature. This was also
found in studies on foliar treatments of mango. Residue levels during
storage of prochloraz alone were similar to those for prochloraz and
its major metabolites, suggesting little degradation or conjugation.
Emulsifiable concentrate formulations gave significantly higher
residues than wettable powder formulations and residues in lemons were
higher than those in oranges.
In bananas treated postharvest with prochloraz, no trend of
decreasing residues was observed over a 16-day storage period at
ambient temperature. Residue levels were similar with modest increases
(2X) in treatment rate and, as in the case of citrus, most of the
residue was in the peel.
In avocadoes treated postharvest with prochloraz, residues were
comparable to those from multiple foliar treatments. Approximately
10-30 percent of the residue could occur in the flesh after relatively
short preharvest intervals. The translocation was therefore somewhat
greater than in citrus, bananas and apples.
Photodecomposition of an aqueous prochloraz solution resulted in
the formation of plant metabolite BTS-44596 and towards the end of a
36-day exposure to artificial light, a trace of plant metabolite
BTS-44595. A half-life of approximately 20 days was observed.
Analytical methods are available for enforcement purposes for a
wide variety of plant commodities. Residues of prochloraz alone or
total residues of prochloraz and its major plant metabolites may be
determined, although more of the field trials data were determined by
the latter. Analytical recoveries were generally better than 70
percent. No analytical methods have been validated for residues in
animal products. The analytical methodology for the parent compound
should be suitable and more appropriate for enforcement purposes and
for estimating maximum residue levels (see Report of this Meeting,
Section 2.3).
RECOMMENDATIONS
The Meeting examined residue data from supervised trials
reflecting established or proposed good agricultural practice. From
the data, the Meeting was able to estimate the maximum residue levels
that are likely to occur when prochloraz is used in practice and when
the reported intervals between last application and harvest or other
restrictions are observed. Maximum residue limits are recommended
where registered or approved uses are known and residues are
determined as free prochloraz. Temporary maximum residue limits are
recommended where only recommended uses are available or where total
residues of prochloraz and its metabolites containing the
2,4,6-trichlorophenol moiety are determined. Regardless of the status
of the ADI, these remain temporary pending receipt of information on
approved or registered uses or data on supervised trials with residues
determined as prochloraz.
All estimates apply to foliar applications unless otherwise
indicated.
Estimated Interval from last
Commodity maximum residue treatment to harvest (days)
limits (mg/kg) on which recommendation
Determined and expressed is based and other
as prochloraz restrictions
Barley 0.05** 40
Oats 0.05** 40
Rye 0.05** 40
Wheat 0.05** 40
Barley straw 0.2 40
Oat straw 0.2 40
Rye straw 0.2 40
Wheat straw 0.2 40
Mushrooms 2 2 3
Citrus 5 (TMRL)1, 4 postharvest
Sum of prochloraz
and metabolites expressed
as prochloraz 4
Bananas 5 (TMRL) 1 postharvest
Stone fruits 1 (TMRL) 1 14
Avocado 5 (TMRL) 1, 2 7 (preharvest)
Mango 2 (TMRL) 1, 2 15 (preharvest)
Papaya 1 (TMRL) 1 postharvest
Rapeseed 0.5 (TMRL) 25
** At or about the limit of determination.
1 No information on approved or registered uses.
2 Limit allows for cumulative residues from both preharvest and postharvest
treatments, both of which are good agricultural practice.
3 Limit accommodates applications after casing or after flushes, but not
direct treatment of mushrooms.
4 Temporary regardless of the status of the ADI.
FURTHER WORK OR INFORMATION
Required (by 1985)
1. In order to establish MRLs for products of animal origin, several
requirements remain:
(a) Metabolism studies in animals, including ruminants and
poultry, at sufficiently high dosing levels to permit
identification and quantification of tissue residues.
(b) If metabolism studies in (a) above indicate any possibility
of residues in animal tissues, feeding trials should be
conducted in animals, including ruminants and poultry, in
which tissues are analysed for residues of concern
identified in metabolism studies. Feeding in an appropriate
number of animals should as nearly as possible reflect
maximum levels of weathered residues likely to occur in
practice and, ideally, at an exaggerated level.
(c) A validated analytical method suitable for enforcement
purposes for products of animal origin.
2. Good agricultural practice information for those commodities for
which temporary maximum residue limits are recommended,
preferably for countries in which the residue trials were
conducted or those in close proximity.
3. Additional data on residue trials reflecting good agricultural
practice for commodities for which temporary maximum residue
limits are recommended (except citrus). The additional data
should be concurrently determined as both prochloraz and total
residues of prochloraz and its metabolites containing the
trichlorophenol moiety on the same samples. Sufficient data
should be provided to permit further utilization of residue data
already provided, therefore permitting recommendation of maximum
residue limits expressed as prochloraz.
Desirable
1. Additional national maximum residue limits.
2. Additional processing studies in cereals to permit a conclusion
on the extent, if any, of residue concentration in milling
fractions. Grain used should have residues sufficiently above the
limit of determination for drawing conclusions.
3. Additional processing studies for mushrooms to permit a
conclusion on the extent, if any, of residue concentration.
Mushrooms should be analysed before and after processing.
4. Information on possible interference in prochloraz analysis in
the presence of other pesticides, especially those containing the
trichlorophenol moiety. If interferences are encountered,
confirmatory analytical procedures for prochloraz and metabolites
may be necessary.
REFERENCES - RESIDUES
Browne, P.M. Residues of prochloraz and major metabolites in oranges
1981a following postharvest dip treatment in Australia, 1981. FBC
report RESID/81/72 submitted to FAO by FBC Ltd.
(Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in oranges
1981b following postharvest shower treatment in Spain, 1981. FBC
report RESID/81/72 submitted to FAO by FBC Ltd.
(Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in avocados
1982a following postharvest dip treatment in Australia, 1981. FBC
report RESID/82/2 submitted to FAO by FBC Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in bananas
1982b following post-harvest dip treatment in Australia, 1981. FBC
report RESID/82/7 submitted to FAO by FBC Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in lemons
1982c following post-harvest shower treatment in Spain. FBC report
RESID/82/8 submitted to FAO by FBC Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in winter
1982d rye treated post-emergence with a 40% E.C. formulation in
West Germany, 1981. FBC report RESID/82/25 submitted to FAO
by FBC Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in winter
1982e wheat treated postemergence with three applications of 40%
E.C. formulation in Fed. Rep. Germany, 1981. FBC report
RESID/82/29 submitted to FAO by FBC Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in spring
1982f wheat and barley postemergence with a 40% E.C. formulation
(two or three applications) in West Germany, 1981. FBC
report RESID/82/31 submitted to FAO by FBC Ltd.
(Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in mushrooms
1982g following application of a 50 W formulation in Holland,
1980. FBC report RESID/82/39 submitted to FAO by FBC Ltd.
(Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in processed
1982h mushrooms following application of a 40 E.C. formulation in
France, 1981. FBC report RESID/82/68 submitted to FAO by FBC
Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in
1982i oranges following post-harvest dip treatment in the U.K.,
1981. FBC report RESID/82/83 submitted to FAO by FBC Ltd.
(Unpublished)
Browne, P.M. & Manley, J.D. Analytical method for residues of
1982a prochloraz and major metabolites in orange juice. FBC report
RESID/82/18 submitted to FAO by FBC Ltd. (Unpublished)
Browne, P.M. & Manley, J.D. Residues of prochloraz and major
1982b metabolites in oranges and lemons following postharvest dip
treatment in Italy, 1979. FBC report RESID/82/32 submitted
to FAO by FBC Ltd. (Unpublished)
Browne, P.M. & Manley, J.D. Residues of prochloraz and major
1982c metabolites in mangoes treated with a 50% W.P. formulation
in Australia, 1981. FBC report RESID/82/34 submitted to FAO
by FBC Ltd. (Unpublished)
Campbell, J.K. Residues in milk and tissues of a goat dosed orally
1980 with 14C-prochloraz. Boots report AX-80026 submitted to FAO
by FBC Ltd. (Unpublished)
Campbell, J.K. Residues of prochloraz in milk and tissues of a
1983 lactating goat fed straw containing residues of radioactive
prochloraz. FBC report METAB/83/8 submitted to FAO by FBC
Ltd. (Unpublished)
Campbell, J.K. & Needham, D. Residues in milk and tissues of a goat
1980 dosed orally with 14C-BTS 44596 (major plant metabolite of
prochloraz). Boots report AX-800333 submitted to FAO by FBC
Ltd. (Unpublished)
Churchill, J.H.M. & Longland, R.C. Residues of prochloraz and major
1983a metabolites in bananas following a single postharvest
application with a 45% E.C. prochloraz formulation in South
Africa, 1982. FBC report RESID/83/5 submitted to FAO by FBC
Ltd. (Unpublished)
Churchill, J.H.M. & Longland, R.C. Residues of prochloraz and major
1983b metabolites in avocadoes following application with a 45%
E.C. formulation in South Africa. FBC report RESID/83/10
submitted to FAO by FBC Ltd. (Unpublished)
Churchill, J.H. & Longland, R.C. Residues of prochloraz and major
1983c metabolites in processed mushrooms following application
with a 50% W.P. formulation of prochloraz-Mn complex in
Holland, 1981. FBC report RESID/83/23 submitted to FAO by
FBC Ltd. (Unpublished)
Churchill, J.H.M. & Longland R.C. Residues of prochloraz and major
1983d metabolites in almonds following foliar application with a
50% W.P. formulation in Israel, 1982. FBC report RESID/83/38
submitted to FAO by FBC Ltd. (Unpublished)
Churchill, J.H.M. & Longland, R.C. Residues of prochloraz and major
1983e metabolites in avocadoes following postharvest treatment
with a 45% E.C. formulation in Australia, 1982/83. FBC
report RESID/83/55 submitted to FAO by FBC Ltd.
(Unpublished)
Cron, J. Residues of prochloraz and major metabolites in rapeseed
1982 following a single postemergence application with a 40 E.C.
prochloraz and a prochloraz/carbendazim E.C. formulation in
France 1980/81. FBC report RESID/82/74 submitted to FAO by
FBC Ltd. (Unpublished)
Cron, J.H. & Longland, R.C. Residues of prochloraz and major
1983a metabolites in avocadoes following multiple foliar
application with a 50% W.P. formulation of prochloraz-Mn
complex in Australia, 1981/82. FBC report RESID/82/120
submitted to FAO by FBC Ltd. (Unpublished)
Cron, J.H. & Longland, R.C. Residues of prochloraz and major
1983b metabolites in papayas treated postharvest with a 45% E.C.
formulation in Australia, 1982. FBC report RESID/83/1
submitted to FAO by FBC Ltd. (Unpublished)
Douglas, M.T. & Pell, I.B. Assessment of the ready degradability of
1982 prochloraz. HRC and FBC report METAB/82/44 submitted to FAO
by FBC Ltd. (Unpublished)
Douglas, M.T. & Pell, I.B. Assessment of biodegradability of
1983 prochloraz by pre-adapted sewage organisms. HRC and FBC
report METAB/83/10 submitted to FAO by FBC Ltd.
(Unpublished)
Goto, H. Residues of prochloraz in rice following seed soak or foliar
1980 application with a 25% E.C. formulation in Japan, 1980.
Inst. Environ. Toxicol., Japan, Nissan and FBC report
submitted to FAO by FBC Ltd. (Unpublished)
Haran, G. Identification of a photodecomposition product of BTS-40
1978 542 (prochloraz). Boots report F-78014 submitted to FAO by
FBC Ltd. (Unpublished)
Haran, G. The effect of simulate& sunlight on aqueous solutions of
1980 prochloraz. Boots report F-80010 submitted to FAO by FBC
Ltd. (Unpublished)
Hayto, E.M. BTS-40542 residues in wheat and barley. Boots report AX-
1977a 77002 submitted to FAO by FBC Ltd. (Unpublished)
Hayto, E.M. BTS-40 542 residues in wheat from Italy, 1977. Boots
1977b report AX-79003 submitted to FAO by FBC Ltd. (Unpublished)
Hayto, E.M. BTS-40 542 residues in apples from Thurgarton, U.K. Boots
1977c report AX-77011 submitted to FAO by FBC Ltd. (Unpublished)
Hayto, E.M. BTS-40542 residues in wheat from Holland, 1977. Boots
1978a report Ax-78006 submitted to FAO by FBC Ltd. (Unpublished)
Hayto, E.M. BTS-40542 residues in wheat and barley from France, 1977.
1978b Boots report AX-78020 submitted to FAO by FBC Ltd.
(Unpublished)
Hayto, E.M. BTS-40542 residues in wheat and barley from the United
1979a Kingdom, 1977. Boots report AX-79001 submitted to FAO by FBC
Ltd. (Unpublished)
Hayto, E.M. BTS-40542 residues in apples from Thurgarton, U.K., 1977.
1979b Boots report AX-79005 submitted to FAO by FBC Ltd.
(Unpublished)
Hayto, E.M. BTS-40542 residues in cereals from Fed. Rep. Germany,
1979c 1977. Boots report AX-79008 submitted to FAO by FBC Ltd.
(Unpublished)
Hayto, E.M. BTS-40542 and dicloran residues in lettuces from Holland,
1979d 1978. Boots report AX-79010 submitted to FAO by FBC Ltd.
(Unpublished)
Heinanen, E. Investigation certification for residues of prochloraz in
1983 spring wheat from Finland, 1982. VML report 2940, 2942/82
submitted to FAO by FBC Ltd. (Unpublished)
Housden, M.C. Residues of prochloraz and major metabolites in grain
1982a treated post-emergence with a 45% E.C. formulation in
Belgium, 1981. FBC report RESID/82/1 submitted to FAO by FBC
Ltd. (Unpublished)
Housden, M.C. Residues of prochloraz and major metabolites in
1982b mushrooms following application of a 50W formulation in
Australia, 1981/82. FBC report RESID/82/48 submitted to FAO
by FBC Ltd. (Unpublished)
Housden, M.C. Residues of prochloraz and major metabolites in cereals
1982c treated post-emergence with a 45% E.C. formulation (single
or double application) in Denmark, 1982. FBC report
RESID/82/111 submitted to FAO by FBC Ltd. (Unpublished)
Housden, M.C. & Longland, R.C. Decline study of residues of prochloraz
1983 and major metabolites in winter barley and wheat treated
once with a prochloraz/carbendazim suspension concentrate
formulation in Fed. Rep. Germany, 1982. FBC report
RESID/83/19 submitted to FAO by FBC Ltd. (Unpublished)
Housden, M.C. & Whiteoak, R.J. Residues of prochloraz and metabolites
1982 in mushrooms following single and multiple applications
(50w formulation) in the U.K. 1981 and 1982. FBC report
RESID/82/63 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. BTS 40 542 residues in cereals from Austria, 1977. Boots
1979a report AX 79006 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of BTS 40
1979b 542, BTS 44 596 and BTS 45 186 in grain. Boots report AX
79007 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979c (BTS 40 542), BTS 44 595, BTS 44 596 and BTS 45 186 in wheat
grain and straw from Fed. Rep. Germany, 1978. Boots report
AX 79015 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979d (BTS 40 542), BTS 44 595, BTS 44 596 and BTS 45 186 in wheat
grain and straw from Italy, 1978. Boots report AX 79017
submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979e (BTS 40 542), BTS 44 595, BTS 44596 and BTS 45 186 in wheat
grain and straw from The Netherlands, 1978. Boots report AX
79 017 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979f (BTS 40 542), BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in cereal grain and straw from
Austria, 1978. Boots report AX 79 020 submitted to FAO by
FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979g (BTS 40 542), BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in wheat and barley grain from
Denmark, 1978. Boots report AX 79021 submitted to FAO by FBC
Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979h (BTS 40 542), BTS 44 595, BTS 44 596 and BTS 45 186 in wheat
and barley grain and straw from France, 1978. Boots report
AX 79023 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. Persistence and uptake of prochloraz in Spanish oranges
1980a following a post-harvest dip. Boots report AX 80003
submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. BTS 45 186 residues in prochloraz-treated wheat grain from
1980b France, 1977. Boots report AX 80010 submitted to FAO by FBC
Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1980c (BTS 40 542) and metabolites containing the
2,4,6-trichlorophenyl moiety in wheat grain and straw from
The Netherlands, 1979. Boots report AX 80025 submitted to
FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The metabolism of 3H-prochloraz in mature wheat grain and
1980d straw. Boots report AX 80028 submitted to FAO by FBC Ltd.
(Unpublished)
Kelly, I.D. The metabolism and distribution of BTS 46828 applied to
1982a the fruit of field-growing apples. FBC report METAB/82/18
submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The hydrolysis of prochloraz in aqueous solution under
1982b acid, neutral and basic conditions. FBC report METAB/82/28
submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. & Krepski, W.J. The metabolism of prochloraz by wheat
1980 grain and straw. Boots report AX 80009 submitted to FAO by
FBC Ltd. (Unpublished)
Kesterton, M.S. Distribution of radio-labelled residues in field soil
1978 after application of 3H-BTS 40542. Boots report AX 78010
submitted to FAO by FBC Ltd. (Unpublished)
Kesterton, M.S. Report on the aerobic metabolism of prochloraz in
1981a soil over a period of 12 months. Boots report AX 81002
submitted to FAO by FBC Ltd. (Unpublished)
Kesterton, M.S. Report on the anaerobic metabolism of prochloraz in
1981b soil. Boots report AX 81004 submitted to FAO by FBC Ltd.
(Unpublished)
Kesterton, M.S. & Newby, S.E. A study of the leaching of prochloraz in
1980a soil. Boots report AX 80008 submitted to FAO by FBC Ltd.
(Unpublished)
Kesterton, M.S. & Newby, S.E. Adsorption and desorption studies of
1980b prochloraz in soil. Boots report AX 80012 submitted to FAO
by FBC Ltd. (Unpublished)
Kesterton, M.S. & Newby, S.E. Leaching study of prochloraz aged in a
1981 silty clay loam soil. Boots report AX 81003 submitted to FAO
by FBC Ltd. (Unpublished)
Krepski, W.J. The uptake of 14C-prochloraz and 3H-prochloraz soil
1981a residues into wheat. Boots report AX 81001 submitted to FAO
by FBC Ltd. (Unpublished)
Krepski, W.J. The metabolism of 14C-prochloraz and 14C-BTS 46828 by
1981b apples grown under glass. Boots and FBC report submitted to
FAO by FBC Ltd. (Unpublished)
Krepski, W.J. Translocation of 14C-prochloraz applied as a liquid
seed
1982 dressing in wheat grown to maturity. FBC report METAB/81/30
submitted to FAO by FBC Ltd. (Unpublished)
Leake, C.R. & Lines, D. The leaching of prochloraz and its major
1981 metabolites in four soil types using TLC. FBC report
METAB/81/35 submitted to FAO by FBC Ltd. (Unpublished)
Longland, R.C. Analysis of residues of prochloraz and major
1983a metabolites in grain and straw from Sweden following
application of 45% E.C., 1982. FBC report RESID/82/114
submitted to FAO by FBC Ltd. (Unpublished)
Longland, R.C. Residues of prochloraz and major metabolites in
1983b sugarbeet following application of a 40% E.C. formulation in
Italy, 1981. FBC report RESID/83/58 submitted to FAO by FBC
Ltd. (Unpublished)
Longland, R.C. & Churchill, J.H. Residues of prochloraz and major
1983 metabolites in mangoes following foliar treatment with 50%
W.P. and postharvest treatment with 40% E.C. formulations in
Israel, 1982. FBC report RESID/83/7 submitted to FAO by FBC
Ltd. (Unpublished)
Maclaine Pont, M.A., Vogelzang, H.P. & Siegmann-Knoester, K.C. The
1980 residue analysis of prochloraz in combination with dicloran.
Asepta-fabrik and Boots report AX 80020/2 submitted to FAO
by FBC Ltd. (Unpublished)
Maier-Bode, H. The leaching of prochloraz in three standard soils from
1980 Fed. Rep. Germany. HMB and FBC report submitted to FAO by
FBC Ltd. (Unpublished)
Manley, J.D. & Snowden, P.J. Analytical method for residues of
1982a prochloraz and major metabolites in miscellaneous fruit,
cereal and vegetable crops (improved method). FBC report
RESID/82/88 submitted to FAO by FBC Ltd. (Unpublished)
Manley, J.D. & Snowden, P.J. Residues of prochloraz and major
1982b metabolites in oranges and processed oranges following
commercial scale postharvest brush treatment with prochloraz
(40 E.C.) in South Africa, 1982. FBC report RESID/82/89
submitted to FAO by FBC Ltd. (Unpublished)
Manley, J.D. & Snowden, P.J. Residues of prochloraz and major
1982c metabolites in rapeseed following treatment with a 40% E.C.
formulation in the U.K., 1981/1982. FBC report RESID/82/90
submitted to FAO by FBC Ltd. (Unpublished)
McDougall, J. The metabolism of 3H-BTS 40 542 in wheat at a
vegetative
1979 growth stage. Boots report AX 79009 submitted to FAO by FBC
Ltd. (Unpublished)
McDougall, J. The uptake and translocation of 3H-prochloraz in wheat.
1980a Boots report AX 80007 submitted to FAO by FBC Ltd.
(Unpublished)
McDougall, J. The relative uptake and persistence of prochloraz and
1980b BTS 46 828 in apples following treatment with 14C-
prochloraz and 14C-BTS 46 828. Boots report AX 80015
submitted to FAO by FBC Ltd. (Unpublished)
McGibbon, A.S. Uptake of prochloraz residues by sugarbeet from
1982 prochloraz-treated soil under field conditions. FBC report
METAB/82/27 submitted to FAO by FBC Ltd. (Unpublished)
Newby, S.E. The laboratory decline of prochloraz in two soils, a sandy
1982 loam and a silty clay loam, under sterile conditions. FBC
report METAB/82/41 submitted to FAO by FBC Ltd.
(Unpublished)
Peatman, M.H. & Snowden, P.J. Residues of prochloraz and major
1982 metabolites in rapeseed treated with the 40% E.C.
formulation in France, 1982. FBC report RESID/82/116
submitted to FAO by FBC Ltd. (Unpublished)
Peatman, M.H. & Snowden, P.J. Residues of prochloraz and major
1983 metabolites in rapeseed treated with a 45% E.C. formulation
in Denmark and Sweden, 1982. FBC report RESID/83/29
submitted to FAO by FBC Ltd. (Unpublished)
Reary, J.B. Analytical method for free and conjugated residues of
1981a prochloraz, BTS 44595, BTS 44 596 and BTS 45 186 in cereals
at different growth stages. FBC report RESID/81/13 submitted
to FAO by FBC Ltd. (Unpublished)
Reary, J.B. Residues of prochloraz and major metabolites in summer
1981b wheat treated post-emergence with a 40% E.C. formulation
(two or three applications) in Fed. Rep. Germany, 1980. FBC
report RESID/81/14 submitted to FAO by FBC Ltd.
(Unpublished)
Reary, J.B. Residues of prochloraz and major metabolites in cereals
1981c treated post-emergence with a 45% E.C. formulation in
Holland, 1980. FBC report RESID/81/46 submitted to FAO by
FBC Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980a prochloraz (BTS 40 542) BTS 44 595 and BTS 44 596 and
conjugated residues of BTS 45 186 in wheat and barley,
France, 1979. Boots report AX 80005 submitted to FAO by FBC
Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980b prochloraz (BTS 40 542), BTS 44 595 and BTS 44 596 and
conjugated residues of BTS 45 186 in Spanish oranges
following a postharvest application, 1980. Boots report AX
80021 submitted to FAO by FBC Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980c prochloraz (BTS 40 542), BTS 44 595, BTS 44 596 and
conjugated residues of BTS 45 186 in wheat and barley, U.K.,
1979. Boots report AX 80022 submitted to FAO by FBC Ltd.
(Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980d prochloraz (BTS 40 542), BTS 44 595, BTS 44 596 and
conjugated residues of BTS 45 186 in wheat and barley,
Denmark, 1979. Boots report AX 80023 submitted to FAO by FBC
Ltd (Unpublished)
Richards, M.E. BTS 45 186 residues in oranges treated with prochloraz,
1980e Spain, 1980. Boots report AX 80027 submitted to FAO by FBC
Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980f prochloraz, BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in Spanish oranges following a
postharvest application, 1980. Report No. 2. Boots report AX
80030 submitted to FAO by FBC Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980g prochloraz, BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in winter wheat, Fed. Rep. Germany,
1979. Boots report AX 80032 submitted to FAO by FBC Ltd.
(Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1981a prochloraz, BTS 44 595 and BTS 44596 and conjugated residues
of BTS 45 186 in grain and straw. Boots and FBC report AX
800 35 submitted to FAO by FBC Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1981b prochloraz, BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in winter barley, Fed. Rep. Germany,
1980. Boots and FBC report AX 81005 submitted to FAO by FBC
Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1981c prochloraz, BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in mushrooms, U.K., 1980. FBC report
METAB/81/17 submitted to FAO by FBC Ltd. (Unpublished)
Snowden, P.J. Residues of prochloraz and major metabolites in stone
1983 fruit following foliar applications of prochloraz (50 E. and
40 E.C.) in Europe (1982) and South Africa (1981/2 and
1982/3). FBC report RESID/83/57 submitted to FAO by FBC Ltd.
(Unpublished)
Snowden, P.J. & Manley, J.D. Residues of prochloraz and major
1983 metabolites in Tambors oranges following commercial scale
postharvest brush treatment with prochloraz (45% E.C.) in
South Africa, 1982. FBC report RESID/83/9 submitted to FAO
by FBC Ltd. (Unpublished)
Somerville, L. The analysis of prochloraz residues in cereals. Boots
1980 report AX 80020/1 submitted to FAO by FBC Ltd. (Unpublished)
Stockbauer, I. Leaching of Sportak PF (prochloraz and carbendazim) in
1983 German soils. ASU and FBC report submitted to FAO by FBC
Ltd. (Unpublished)
Wang, S.S. Residues of prochloraz in grapes, apples and watermelon
1982 following foliar application with a 25% E.C. formulation in
Taiwan, Taiwan Plant Protection Centre report submitted to
FAO by FBC Ltd. (Unpublished)
Whiteoak, R.J. Residues of prochloraz in mushrooms following multiple
1981 applications of a 50% W.P. formulation in the U.K., 1980/81
(ADAS trial results), FBC report RESID/81/41 submitted to
FAO by FBC Ltd. (Unpublished)
Whiting, K.C. & Dickinson, W. The stability of the manganese complex
1979 of prochloraz in water. Boots report F 79005 submitted to
FAO by FBC Ltd. (Unpublished)
Straw Grain
Free 2,4,6-trichlorophenol (BTPS 45186) 5.2 4.1 3.4 8.4
Residues containing the BTS-45186 moiety 58 37.9 53.9 39.5
Tritiated water 4.8 - 4.2 -
Unidentified extractable residues 9.1 34 15.5 32.1
Unidentified residues in solids 22 24 24.7 20
99.1 100 101.7 100
Of the unidentified extractable residues no one of the grain or
straw acidic, basic, volatile or aqueous fractions analysed contained
more than 6.2 percent of the total residue.
Formation and analysis of the glucosazone after hydrolysing
starch extracted from the 14C-prochloraz treated grain demonstrated
that 32.4 percent of the grain radioactivity was incorporated into the
starch molecules. This is thought to result from the incorporation of
14C-carbon dioxide, resulting from decarboxylation of the metabolite
BTS 44596. The remaining residues were separated into various
fractions but were not identified (Kelly 1980d; Kelly & Krepski 1980).
When wheat seeds were treated at an equivalent rate of 0.4
a.i./kg seed (approximating recommended usage) with a 20 percent a.i.
liquid seed dressing and grown to maturity, residues at maturity (29
weeks) were:
% of total Prochloraz
applied Equivalents (mg/kg)
Treated Controls
Soil 58œ3 0.06 -
Roots 15.1 1.5 -
Chaff 0.1 0.04* 0.004*
Grain 0.04 0.004* 0.002*
Total aerial 6.6 0.16 -
* mean of four determinations.
Translocation into the aerial portion of the plant was complete
after six weeks (Krepski 1982).
Potted wheat seeds were allowed to germinate under glass after
soil surface drench application of a diluted 3H-prochloraz
formulation. Approximately 82 percent of the radioactivity (0.8
percent of applied) was retained in the root system at harvest after
21 days. Residues decreased with increased distance of plant parts
from the root system; they decreased from 8.7 percent of residues
recovered in leaves 1 and 2, 0.62 percent in leaf 3, to 0.08 percent
in leaf 7. Soil contamination was suspected of contributing some of
the residues for leaves 1 and 2 and lower plant parts.
In this same report a 3H-prochloraz formulation was applied with
a syringe at the fifth leaf stage to the third leaf of similarly grown
wheat without the soil drench. Plant parts were harvested and analysed
after 24 days; 99 percent of applied radioactivity was retained in
leaves 3 and 4 (difficulty was encountered in discerning leaves during
applications). Radioactivity decreased from 0.03 percent of that
applied in leaf 5 to 0.01 percent in leaf 9 and increased from 0.23
percent in leaves 1 and 2 to 0.33 percent in the roots (McDougall
1980a).
After spring wheat harvest from soil plots treated at a rate
equivalent to 1 kg/ha with either imidazole ring-labelled
14C-prochloraz or phenyl-labelled 3H-prochloraz, the plots were
re-sown with winter wheat and reharvested 10 months later. Soil
samples at harvest of the winter wheat were 0.11 and 0.43 mg/kg for
14C and 3H treatments, respectively. Residues of 14C or
3H-prochloraz equivalent were < 0.01 mg/kg in grain, chaff and
straw. Grain control values were about half that for grain, but not
statistically different. Low uptake is suggested for 3H-prochloraz
treated chaff and straw where experimental levels are 50 to 100 times
that of the respective controls (Krepski 1981a).
A 1.5 sq m plot was treated with 14C-phenyl labelled prochloraz
formulation by garden sprayer at an application rate equivalent to 395
g a.i./ha, which approximates recommended usage for cereals. After 41
days the soil was turned by hand and sown with sugarbeets as a
representative rotational crop. A control plot was also planted.
Seedlings were sampled 23 days after planting and mature plants (roots
and foliage) at 157 days after planting.
Residues at planting in soil averaged 0.26 mg/kg prochloraz
equivalents. At 23 days after planting, residues of 0.07 mg/kg in
seedlings were significantly greater than the 0.006 mg/kg controls. At
harvest, residues of 0.005 mg/kg in the soils were not significantly
different than in controls, whereas the 0.0052 mg/kg prochloraz
equivalent in foliage was slightly greater than the 0.003 mg/kg
control. This difference is statistically significant, although
residues in the foliage are less than the background level (McGibbon
1982).
Citrus
3H-prochloraz, a 2.5 percent E.C. formulation, was applied by
pipette wash, to simulate a 0.1 percent a.i. postharvest commercial
treatment, to Washington Navel oranges in Spain. Storage was at 4°C in
the dark from 10 to 60 days and at 20-22°C in diffuse light. From 1 to
10 days, surface residues were removed with a 0.1 percent ethylan BCP
solution and those in peel and flesh by extraction with 90 percent
acetone. Surface residues at room temperature decreased from 5.8
percent of applied radioactivity at 1 day to 0.8 percent at 10 days,
and at 4°C from 3.8 mg/kg at 10 days to 1.1 mg/kg at 60 days.
Corresponding residues in the flesh increased from 1.8 percent to 5
percent of the applied dose over the storage period for room
temperature storage, but remained relatively constant at approximately
1.6 percent over the entire 60 day storage period at 4°C.
Radioactivity in the peel remained relatively constant at 92.3-96
percent of the applied dose over the room temperature storage period
and, similarly, 94.7 to 97.2 percent over the storage period at 4°C
(Kelly 1980a). Similar unlabelled studies on citrus residues during
storage are reported under "In Storage and Processing".
Apples
Formulations of phenyl-labelled 14C-prochloraz or its manganese
complex BTS 46828 were applied to individual apples under glass at
rates approximating commercial usage. In samples analysed after 20
days, residue distribution was similar in the surface, peel and flesh
from either chemical. Residues in extracts from the prochloraz-treated
apples (86 percent of the applied dose was recovered) were - wash 7.4
percent, peel 50 percent and flesh 16.9 percent (20 percent of
recovered). BTS 46828 gave almost identical results (Krepski 1981b).
In similar experiments (not carried out under glass) the
metabolism of prochloraz and its manganese complex were compared at
intervals between 0 and 54 days after application by syringe to
individual apples. Radioactivity distribution was again shown to be
similar from use of either chemical, although prochloraz from the E.C.
formulation was initially absorbed into the peel at a faster rate than
the Mn-complex W.P. and was somewhat more persistent. The manganese
complex was also more susceptible to losses from precipitation shortly
after application. As a percent of radioactivity recovered at 0 and 54
days, residues were:
Prochloraz Mn - complex
*day 0 day 54 *day 0 day 54
surface 32.3 1.9 67.6 1.2
flesh 0.0 0.8 0.0 1.2
peel 67.2 81.2 32.4 56.2
Total 100 83.9 100 58.6
* 5 hours (McDougall 1980b).
In another similar study distribution and characterization of
residues were investigated in two apple varieties at intervals ranging
from 16 to 63 days after application by syringe of the Mn-complex to
individual growing apples. As a percent of the applied dose,
representative residues were:
Days after Whole Peel
treatment fruit Flesh Total Extractables
16 72 (83) 15.81 67 (78) 51.3 (61.4)
33 54.8 (76) 3.6 (5.2) 51 (71) 37.2 (55.2)
63 63.5 5.4 60 38
Numbers in parentheses correspond to the Worcester variety and
others to the Cox variety.
Extractable residues in peel were quantified and characterized in
both varieties at 33 and 63 days after treatment. As a percent of
extractable peel residues the metabolic profile at 63 days for the Cox
variety was:
Metabolite Percent
Prochloraz or Unchanged BTS 46 828 5 )
BTS 45186 (free 2,4,6-trichlorophenol) 11.5 )
BTS 44595 15.2 )
BTS 44596 33.1 ) >77.8
Conjugated residues containing )
the BTS 45186 moiety 13 )
Unidentified polar metabolites 6
"Unknowns" 1.6,0.7
Unresolved background 13.9
Total unidentified (excluding conjugates
with BTS 45186) 22.2
100.0
Results were similar at 33 days for both varieties, except that
conjugated residues containing the BTS 45186 moiety were only 3
percent for the Worcester variety, only the first "unknown" was
observed in Cox and the first and a different second "unknown" was
found in Worcester. None of the residues exceeded 2 percent (Kelly
1982a).
In Soil
Prochloraz 25 percent E.C. formulations, labelled with 14C in
the imidazole ring and separately in the phenyl ring with 3H, were
separately applied to acidic sandy loam and silty clay loam soils in
enclosed jars purged with CO2-free air under laboratory conditions
for up to 52 weeks. Application was at 6 mg/g soil, equivalent to 0.7
kg a.i./ha (a recommended crop application rate). Silty clay loam was
similarly treated with prochloraz labelled with 14C in the phenyl
ring and studied over 9 months.
Radioactivity losses as volatiles were about 50 percent of the
applied dose to silty clay for both 14C-imidazole and 3H-phenyl
labelled prochloraz after one year, as was the case for the
14C-phenyl labelled formulation at 9 months. Losses were more rapid
from silty clay loam than from sandy loam, for which losses were half
or less that in silty clay. Experimental evidence indicated, but did
not prove, that volatiles from 14C-phenyl and 14C-imidazole labelled
prochloraz were predominantly CO2. In the case of 3H-phenyl labelled
prochloraz, evidence suggests volatile radioactivity in the form of
tritiated water. The major extracted residue in all cases was
prochloraz per se at 50-68 percent of the applied dose (80-90
percent of extracted) after 8 weeks to 12-30 percent (45-67 percent of
extracted) after 52 weeks. Lower residues of BTS-44596 and BTS 45186
combined were <18 percent of the applied dose (<26 percent of
extracted) after 52 weeks for 3H-labelled treatments. Binding was
more pronounced in sandy loam. The half-life was reported as 3 and 5
months, respectively, in silty clay and sandy loams. In both cases the
acidic pH would increase stability (Kesterton 1981a).
In German standard soils the half-life of unlabelled prochloraz
ranged from 115-135 days, with stability directly related to organic
matter content. For both soils the pH was acidic, which is conducive
to prolonged stability (McGibbon 1982).
14C-imidazole and 3H-phenyl labelled prochloraz, formulated as
a 25 percent E.C., were incubated under anaerobic conditions at a rate
equivalent to 0.7 kg a.i./ha and sampled at 14, 27 and 60 days.
Residues of prochloraz remained relatively constant over the test
period in silty clay loam (ca 68 percent of the applied dose) and
decreased slightly (to ca 54 percent) in silty clay loam. The decrease
in silty clay loam was accompanied by an increase in bound residues.
Anaerobic conditions halted the continuous degradation and formation
of volatiles observed under aerobic conditions. Other components
indicated by TLC from 3H treatments were BTS 44596 and BTS 45186 at
approximately 6 and 3 percent of the applied dose, respectively,
whereas from 14C treatments prochloraz, imidazole and BTS 44596 were
approximately 68, 2 and 1 percent, respectively (Kesterton 1981b).
The absence of significant degradation of a 14C-phenyl labelled
2.5 percent E.C. prochloraz formulation in sandy loam and silty clay
loam under sterile soil conditions for 30 days suggests that
degradation is facilitated primarily by soil microorganisms as opposed
to chemical degradation, at least under the experimental conditions
(acidic pH and 50 percent soil moisture capacity) (Newby 1982).
Sandy loam and silty clay loam soil were treated with a
14C-benzene labelled 25 percent E.C. prochloraz formulation at a rate
equivalent to 0.44 kg a.i./ha, aged 13 days, added to the top of 30 cm
× 7 cm diameter columns containing untreated soil and eluted with an
equivalent of 1.5 cm rain for 18 days. Essentially all radioactivity
was retained in the top 5 cm of soil, with less than 0.3 percent of
the applied amount being detected in leachate (Kesterton & Newby
1980a).
In a similar soil column study, except that the equivalent
application rate was 0.8 kg/ha, the labelled prochloraz was applied to
the same silty clay loam and aged for 30 days, after which it was
eluted for 45 days with a daily equivalent of 0.5 cm rainfall. Results
were similar to the first study in that <0.05 percent of the
applied dose was detected in the eluant. However, even though 88-94
percent was in either the treated portion or the first 5 cm below it,
0.3-0.6 percent was detected in the bottom 25 cm, indicating some
downward mobility other than that detected in the leachate (Kesterton
& Newby 1981).
When an unlabelled prochloraz formulation was applied to West
German standard soil columns at a rate equivalent to 1 kg/ha, <0.05
percent was detected in the leachate (Maier-Bode 1980). In a similar
experiment leachate contained <1.7 percent of the applied dose
(Stockbauer 1983). Details of the preceding two experiments were not
available.
In soil thin-layer mobility tests, the mobility of prochloraz and
soil metabolites BTS 44 596 and BTS 45186 were compared to that of
atrazine and 2,4-D in four soil types. According to the Helling
mobility index, over-all mobility in the four soils were, in order of
increasing mobility, prochloraz, BTS 44596 (low mobility), BTS 45186
(low mobility), atrazine (intermediate mobility) and 2,4-D (mobile).
Mobility of all tested materials was greatest in sand (even BTS 44596
and BTS 45186 would have intermediate mobility). Prochloraz and BTS
44596 were immobile in silt loam, whereas BTS 45186 was of
intermediate mobility. The thin-layer studies confirmed the low, but
positive, mobility detected in soil column experiments (Leake & Lines
1981).
Adsorption/desorption of prochloraz, uniformly labelled in the
phenyl ring and applied as an E.C. formulation to a sandy loam and
silty clay loam, was investigated. The Kd of the sandy and silty
soils were 152 and 256, respectively, indicating slightly more
adsorption by the latter. The K values were 86.8 and 101.7,
respectively, for these soils. Strong adsorption and minimal
desorption is consistent with leaching studies (Kesterton & Newby
1980b).
A trichlorophenol tritiated ring prochloraz E.C. formulation was
applied to one sq m plots of barley and wheat in sandy loam at an
equivalent rate of 1 kg/ha. Core samples from 32.5 cm deep were
analysed after approximately 15 and 30 weeks; > 95 percent of
prochloraz residues were found in the upper 10 cm and in some cases
the top 2.5 cm. Somewhat similar distribution after 15 and 30 weeks
suggests only slight leaching of bound residues as a result of
additional (over 150 mm) rainfall in the latter period (Kesterton
1978).
In Water
No degradation of prochloraz was observed after 30 days in 22°C
buffer solutions at a pH of 4.95 or 6.98. At pH 9.18 degradation does
occur, with 77 percent of the added prochloraz remaining after 30
days. A first order degradation was indicated and a half-life
estimated of approximately 79 days. The only degradant
N-propyl-N-2-(2,4,6-trichlorophenyl) ethylamine (BTS 40348) was
reported to account for more than 2 percent of the total
radioactivity, although the quantitative analyses were not provided
(Kelly 1982b). The 4:1 manganese complex of prochloraz (BTS 46 828),
either as active compound or as a 50 percent W.P. formulation,
disassociated to prochloraz and manganous chloride in the presence of
water (pH 6.1). Approximately 50 percent of the complex was
dissociated within four hours at 25°C (Whiting & Dickinson 1979).
Biodegradability of prochloraz in water was studied using the
closed bottle test according to OECD Guideline 301D. Tests indicate
little biodegradation at 20°C up to 15 days after inoculation, but 13
percent degradation by 28 days, suggesting some initial bacterial
adaptation (Douglas & Pell 1982). When bacteria preadapted to
prochloraz were used as the inoculum, degradation commenced
immediately and reached 47 percent biodegradation by 28 days (Douglas
& Pell 1983).
In Storage and Processing
Processing
Citrus - Oranges were postharvest brush-treated at 1 000 mg/kg with
a 40 percent E.C. formulation and processed into "42% comminuted
orange" and "special whole orange compound". These "whole oranges
juices" (chopped oranges) are used for production of various orange
drinks and utilizes most of the whole orange, except for removal of
some coarse peel. It includes a pasteurization process up to 86°C,
depending on which of two processes were used. Total residues
(converted to 2,4,6-trichlorophenol for analysis) were 0.19 and 0.14
mg/kg expressed as prochloraz for the 42 percent comminuted orange and
special whole orange compound, respectively, approximately 30 percent
of the residues found in whole oranges (Manley & Snowden 1982b).
Cereals - Winter wheat was treated post-emergence at two sites in
the Federal Republic of Germany at growth stage 59 (mid-May to
mid-June) with three applications of a 40 percent E.C. prochloraz
formulation at the 0.48 kg/ha rate recommended in that country. Mature
grain was sampled at 57-58 days after last application and portions
milled and baked into bread. Total residues were determined by
hydrolysis of the trichlorophenol containing moieties followed by EC
analysis and use of a 1.906 factorial conversion to a prochloraz
equivalent basis. Residues in grain and bread were <0.1 mg/kg, the
limit of determination.
Under similar conditions prochloraz was applied twice to barley.
Total residues in barley malt, expressed as prochloraz, were 0.13 and
0.15 mg/kg compared with 0.07 mg/kg in a control. Limit of
determination was 0.1 mg/kg.
Rapeseed Oil - Mean residues of total prochloraz and metabolites
hydrolysable to the trichlorophenol, and expressed as prochloraz, in
mature rapeseed treated in each of four sites in France ranged from
0.06 to 0.2 mg/kg (over-all mean of 0.11 mg/kg). Treatment was with
two applications of an E.C. formulation at a rate of 0.45 kg a.i./ha
and harvest was 25-38 days after the last treatment. Mean residues of
total prochloraz and its metabolites in hexane-extracted oil from
these trials (omitting one individual 0.49 mg/kg outlier from six
replicate results, otherwise ranging from 0.07-0.13 mg/kg) ranged from
0.11 to 0.29 mg/kg (over-all mean 0.10 mg/kg), indicating a residue
concentration factor of approximately 3 times. Residues in the treated
seed and oil only ranged from approximately 1-3 times that in controls
from any given site and were therefore not far removed from the limit
of determination in any one site, with a variation of from 0.05 to 0.2
mg/kg among the sites.
In similar studies at three sites each in Denmark and Sweden, the
concentration factor for prochloraz in the oil ranged from only 1.2 to
2 times that in the seed. Treatment-to-harvest intervals range from 47
to 76 days. In these studies, residues in treated seed and oil derived
therefrom, although comparable to those in France, were significantly
greater than in controls. The limit of determination was estimated at
0.05 and 0.1 mg/kg, respectively, for seed and oil.
Mushrooms - Mushrooms were harvested in France 17 days after
pre-emergence treatment with a 40 percent E.C. prochloraz formulation,
administered at a rate of 40 g a.i./100 sq m. No information on good
agricultural practice is available from France. The mushrooms were
immediately processed by canning and dehydration, after which samples
of canned mushrooms, the liquor and dehydrated mushrooms were analysed
as total residues of prochloraz and its metabolites that were
hydrolysable in the presence of pyridine hydrochloride to the
trichlorophenol moiety. Total residues (apparently uncorrected for
recoveries) and expressed as prochloraz were less than the 0.1 mg/kg
limit of determination for canned mushrooms and the liquor, with
control values of 0.03 and 0.05 mg/kg, respectively, reported. An
apparent residue of 0.14 mg/kg in dehydrated mushrooms was less than
the 0.23 mg/kg control value. Analytical recoveries were 64, 63 and 46
percent, respectively, for dehydrated mushrooms, canned mushrooms and
the liquor (Browne 1982h).
In experiments conducted in The Netherlands, total residues of
prochloraz and its metabolites, expressed as prochloraz, were
determined in canned mushrooms and the liquor after three treatment
schedules with a 50 percent W.P. formulation of the prochloraz
manganese complex, (1) 0.75 a.i./sq m post-casing and 0.94 g a.i./sq m
14 days after casing (2) 1.5 g a.i./sq m 9 days post casing (good
agricultural practice in The Netherlands) and (3) 1.5 g a.i./sq m both
at post-casing and after 14 days. Total residues expressed as
prochloraz were equal to or less than the 0.1 mg/kg limit of
determination in the six liquor samples and five of the six canned
mushroom samples. For treatment (3) above, however, the residue was
0.23 mg/kg in the sixth mushroom sample and 0.1 mg/kg in the control.
No information was available on the interval from last treatment to
harvest. Recoveries were 81 and 94 percent, respectively, for canned
mushrooms and the liquor. No analyses were available for the
unprocessed mushrooms (Churchill & Longland 1983c).
Storage
Citrus - Data are available from a variety of treatment and storage
conditions and are summarized in Table 3. Navel oranges were
commercially treated postharvest in South Africa with a 40 percent
E.C. prochloraz formulation at 1 000 and 2 000 mg/kg a.i. rates then
waxed and shipped by refrigerated vessel to the United Kingdom.
Samples were stored either in the dark at approximately 20°C or at
4°C. Sampling for analysis occurred at 0 (44 days after treatment), 7,
14 and 21 days after storage commenced. Mean (range in parentheses)
residues, corrected for recovery, of total prochloraz and metabolites
expressed as prochloraz at the lower treatment rate were 0.05 mg/kg
(0.030.08 mg/kg) in flesh, 2.1 mg/kg (1.4-2.8 mg/kg) in peel and 0.49
mg/kg (0.3-0.7 mg/kg) on the whole fruit (calculated from peel to
flesh weight ratios) for ambient and 4°C fruit combined over the
entire storage period. Corresponding values for the higher treatment
rate were 0.05 mg/kg (0.02-0.07 mg/kg), 3.3 mg/kg (2.5-3.7 mg/kg) and
0.84 mg/kg (0.7-1.7 mg/kg) respectively.
The list of determination was reported as 0.05 mg/kg, which is
consistent with uncontaminated control values of 0.01-0.02 mg/kg total
residue. No observable trend in lower residues occurred during the
21-day storage period at either temperature and there were no
noteworthy differences in residues between the storage temperatures.
The trials also demonstrated that approximately 94 percent of the
total residue was in the peel (Manley & Snowden 1982b).
In similar postharvest treatments to oranges in Spain, with
0.05-0.1 percent solutions or 3-4 g a.i./tonne of fruit (usually in
wax) with an E.C. formulation as a spray or dip, residues were
determined after storage intervals typically ranging from 1-10 to
10-60 days at 4°C-22°C. Maximum peel residues were as much am 7.3
mg/kg for prochloraz alone and 8.4 mg/kg for total residues of
prochloraz and its metabolites, both expressed as prochloraz. Total
residues in flesh, expressed as prochloraz, were 0.04-0.44 mg/kg and
on a calculated whole fruit basis up to 2.5 mg/kg. As in the South
African study, a definite trend towards decreased residues could not
be determined over the varied storage intervals and temperatures and
little difference was observed in residues where application rates
varied by a factor of two. Peel was repeatedly shown to contain most
of the total whole fruit residue with typically <5 percent being
present in the flesh (Richards 1980b,f; Kelly 1980a; Browne 1981b).
Similar studies were conducted in the United Kingdom where
oranges were dip-treated with a 0.07 percent a.i., 40 percent E.C.
formulation and analysed after 1, 7, 21, 25 and 70 days cold storage
(temperature not provided). Total residues were expressed as
prochloraz. Residues of the parent compound in peel ranged from 3.6 to
5.1 mg/kg compared to 5.8 to 7.8 total residue. In flesh, the residues
were 0.05 to 0.12 mg/kg and 0.07 to 0.15 mg/kg, respectively. No
declining residue trend was observed over the 70-day maximum storage
period, although total residues in flesh increased from about 4
percent of the whole fruit residue at 1-7 days to 11 percent after 70
days (Browne 1982i).
No decline in residues on oranges was observed during storage at
intervals from 1 to 16 days at ambient temperature in Australia after
dip treatment with a 40 percent E.C. prochloraz formulation as a 250
to 500 mg/kg a.i. solution. Total residues determined as the
trichlorophenol expressed as prochloraz were similar from the two
application rates, as were residues of prochloraz alone. Residues of
prochloraz when determined alone ranged from 1.5 to 3.9 mg/kg (2.9
mg/kg) in peel and from <0.02 to 0.1 mg/kg (0.04 mg/kg) in flesh over
the 16-day storage interval for both concentrations. Total residues
were similar 1.4-3.4 kg (2.5 mg/kg) and <0.05 mg/kg, respectively.
Again, most of the residue was in the peel (Browne 1981a).
Residues in Spanish lemons were investigated after a shower
postharvest treatment with a 3 000 a.i. E.C. formulation in wax.
Samples were analysed after 12 and 16 days of storage. The limit of
determination was 0.02 mg/kg and 0.05 mg/kg for prochloraz alone and
total residues expressed as prochloraz, respectively. Residues
expressed as prochloraz were similar after both storage periods, with
approximately 5 percent of the residue in the flesh. Residues of
prochloraz alone and total residues were:
Prochloraz alone (mg/kg) Total residues (mg/kg)
Peel Flesh Whole Peel Flesh Whole*
9.1-15.6 0.27-0.3 3.1-5.0 10.6 0.17-0.33 3.7-6
(12.3 mean) (0.29) (4.6) (14) (0.23) (5.1)
* Calculated from peel/flesh.
Recoveries were 75 percent in peel and 77-100 percent in flesh
(Browne 1982c).
In 1980, postharvest trials on oranges in Spain, using a 40
percent E.C. prochloraz formulation, were made with 200 1 of wax
formulation (3 g a.i./1 wax) to 150 tonnes of fruit (Richards 1980b).
Samples of orange flesh from these trials were analysed for free
2,4,6-trichlorophenol (BTS 45186), since no metabolism study was
available on orange and BTS 45186 has been found in low levels in
wheat (Kelly 1980d). Samples were analysed after 7 and 14 days at
20-22°C. Residues were 0.005-0.01 mg/kg (0.008 mg/kg mean) after 7
days and 0.005 mg/kg after 14 days, as compared to <0.005 mg/kg for
controls. Contrary to the findings in most other citrus postharvest
trials, a slight trend of decreasing residues with time was indicated
(Richards 1980e).
In postharvest dip trials using a 25 percent E.C. or 25 percent
W.P. prochloraz formulation on oranges and lemons in Italy residues
were determined after 57 days storage at 7°C. Significantly higher
residues resulted from E.C. applications in both fruits and lemon
residues were higher than those in oranges (Browne & Manley 1982b).
Oranges treated in South Africa with a postharvest fungicidal
brush at 500-4 000 mg/kg a.i. 45 percent E.C. were analysed after two
months storage at ambient temperature. Again, most of the residue was
in the peel, with total residues, expressed as prochloraz, ranging
from 0.08 mg/kg at the lower application rate to 0.73 mg/kg at the
higher one. There was a good correlation of residue level with
application rate (Snowden & Manley 1983).
Bananas - Bananas were treated by postharvest dipping in Australia
with 250 and 500 mg/kg a.i. solutions of 40 percent E.C. prochloraz
and analysed after ambient storage for 9, 10, 12 and 16 days. Free and
conjugated residues of prochloraz and its metabolites were determined
as the trichlorophenol, expressed as prochloraz, with a 0.05 mg/kg
limit of determination. Residues resulting from both treatment rates
and over the entire storage period ranged from 5.2-10 mg/kg in skin
and 0.02-0.05 mg/kg in flesh. No evidence of decline in residues was
observed in skin or flesh over the storage period and there was no
significant difference in residues between the two application rates
(Browne 1982b).
Photodecomposition
Thin-layer chromatography and nuclear magnetic resonance (NMR)
analysis of a photodecomposition product of an aqueous solution of
prochloraz confirmed the presence of
N-formyl-N-propyl-N',-2-(2,4,6-trichlorophenoxy) ethylurea (BTS 44596)
(Haran 1978). Prochloraz aqueous solutions of 26.1 and 43.3 mg/kg
concentrations in glass-stoppered flasks were exposed to artificial
light (420-760 nm wavelength) at 32°C for 36 and 16 days,
respectively, with periodic analyses by high pressure liquid
chromatography. At the lower concentration residues decreased from
6.93 × 107 moles (26.1 mg/kg) initially to 1.94 × 107 moles after 36
days while BTS 44596 increased from 0.0 to 3.58 × 107 moles. At the
higher concentration, residues decreased from 11.5 × 107 moles (43.3
mg/kg) at zero day to 5.8 × 107 moles after 16 days, while BTS 44596
increased from 0.0 to 35.8 × 107 moles. The first order decay
half-lives were 20.5 and 18.1 days, respectively, for the two
concentrations.
Retention times indicated that a minor unquantified degradation
product was N-propyl-N-2-(2,4,6-trichlorophenoxy) ethylurea (BTS
44595). This appeared only towards the end of the exposure period
(Haran 1980).
METHODS OF RESIDUE ANALYSIS
Analytical methods are available for the analysis of free
prochloraz or total free and conjugated residues of prochloraz and its
major metabolites. Methodology for free prochloraz was first developed
for the analysis of cereal grains and straw (Somerville 1980). Finely
milled straw or ground grain are vigorously extracted with acetone,
filtered, a small portion acidified with HCl and concentrated by
rotary evaporation. The aqueous fraction is partitioned with petroleum
ether, which is discarded, adjusted to pH 6-7 and again partitioned
with petroleum ether, which is concentrated to dryness. The sample is
taken up in ethyl acetate and analysed by electron capture gas
chromatography utilizing a 7 percent OV-101 packed column. Analytical
recoveries of wheat and barley grain, amended with prochloraz at
0.05-0.5 mg/kg, were 78 ± 10.6 percent and 91 ± 10.9 percent,
respectively. For straw, the respective recoveries were 82 ± 12
percent and 80.6 ± 17 percent. The level of "sensitivity" has been
estimated at 0.01 mg/kg for grain and straw, although in the field
trials apparent untreated controls in cereal grain and straw were
occasionally up to 0.02 mg/kg.
With minor modifications, this method has been used for
determining free prochloraz residues in apples, pears, citrus,
mushrooms, strawberries and lettuce (FBC, (Hayto 1979b; Whiteoak 1981;
Kelly 1980a; Browne 1981b, Maclaine Pont et al. 1980) with
analytical recoveries of > 65 percent and usually > 80 percent.
Apparent residues in untreated controls generally ranged from <0.01
to approximately 0.05 mg/kg depending on the commodity.
A method is also available for measuring total free and
conjugated residues of prochloraz and its major plant metabolites,
N-formyl-N'-1-propyl-N'-[2-(2,4,6-trichlorophenoxy) ethyl] urea
(BTS 44596), N-propyl-N-[2-(2,4,6-trichlorophenoxy) ethyl] urea
(BTS 44595) and conjugated 2,4,6-trichlorophenol (BTS-45186), after
converting all of them to the common moiety 2,4,6-trichlorophenol
(Manley & Snowden 1982a). Any other metabolite containing the
2,4,6-trichlorophenol moiety would probably be measured as well.
Although total residues are actually measured as BTS-45186, residues
are usually expressed as prochloraz, after use of a molecular weight
factor of 1.906.
Thoroughly ground, milled, chopped or minced and mixed samples
are Soxhlet extracted with acetone, filtered, dried by passing through
anhydrous sodium sulphate and evaporated to near dryness by a rotary
evaporator. The sample is refluxed with pyridine hydrochloride at
205°c for one hour to hydrolyse to the BTS-45186 moiety, after which
HCl is added to the sample. The sample is liquid-liquid extracted
after addition of petroleum ether. Potassium hydroxide is added, the
sample partitioned and the aqueous phase collected and acidified with
HCl. The sample is partitioned into toluene for analysis by
electron-capture gas chromatography, utilizing a 20 percent carobowax
20M + 1.7 percent phosphoric acid packed column.
The method was validated on fruits and cereal grains with mean
over-all recoveries of 92 ± 15.9 percent when samples were amended at
0.2-10 mg/kg prochloraz. A 0.05 mg/kg limit of determination is
considered attainable for most samples, although apparent residues of
0.2-0.3 mg/kg may be encountered occasionally, especially in cereal
straw.
With minor variations, this method has been successfully used for
the analysis of cereal grain and straw, cereal grain green plant
tissues, apples, citrus fruit (peel, flesh, whole fruit), orange
juice, rapeseed, rapeseed oil, mushrooms, stone fruit and assorted
fruit with inedible peel (Kelly 1979b; Richards 1981a; Reary 1981a;
Browne & Manley 1982a; Manley & Snowden 1982a). Specifics on
individual crops under actual use conditions are considered earlier in
this evaluation under "Residues Resulting From Supervised Trials" or
under "Fate of Residues."
An analytical method is also available for the determination of
free BTS-45186, which may be a minor component of the residue (Kelly
1980b; Richards 1980e).
No analytical method suitable for enforcement purposes for
products of animal origin was made available.
NATIONAL MAXIMUM RESIDUE LIMITS REPORTED TO THE MEETING
Country Commodity MRL (mg/kg)
France cereal grains 0.05 1
rape seed 0.05 2
The Netherlands 3 Mushrooms 0.05
cereal grains 0.1
Milk 0.01
Meat 0.01
Other products 0 (0.01)
German Federal Republic Cereals 0.5 4
1 Residues of prochloraz alone.
2 Residues included not specified.
3 For MRLs of The Netherlands, the residue is prochloraz
and its metabolites, measured as 2,4,6-trichlorophenol
and expressed as prochloraz. Residue on mushrooms and
for most cereal grain trials conducted in The
Netherlands were for total residues of prochloraz and
its major metabolites.
4 A proposed 0.5 mg/kg NRL for prochloraz and metabolites
has been submitted.
APPRAISAL
Prochloraz is a fungicide in use and/or under development for
control of fungal diseases on a variety of commodities in number of
countries. It is used as a foliar spray, for post-harvest or seed
treatments and is available in a variety of formulations for these
uses, including a prochloraz-manganese complex wettable powder
formulation for sensitive plant species.
Residue data are available from field trials on a number of
commodities in several countries, mostly from foliar applications to
fruit and cereal grains, use in mushroom culture and postharvest
applications to citrus and certain other fruit. Some residue data are
also available for vegetables. Residues were determined either as
2,4,6-trichlorophenol, prochloraz alone or prochloraz and its major
metabolites, mostly the latter over-all. In select cases, data are
available for both prochloraz alone and for prochloraz and its major
metabolites. Apparently the earlier studies were for prochloraz alone,
whereas in later studies total residues were emphasized. Data are
insufficient and too variable to draw a general conclusion on the
ratio of parent compound to parent plus metabolites. For example,
there is little difference in residue measured for citrus or possibly
for mushrooms, but for cereals residues appear to be substantially
higher when total residues are determined.
Residue trials on commodities for which there are known
nationally approved or registered uses were available for watermelon,
apple, grape, cereals, mushrooms and oil-seed rape. Therefore, only
for these is consideration given for MRL estimates. However
"recommended" were available for a number of other commodities and
temporary limits are estimated for those with adequate data. Data were
insufficient for estimation of maximum residue levels for sugarbeets,
sugarbeet leaves, strawberries, almonds, almond hulls, lettuce,
watermelon, apples, pears, rice and grapes.
Information was available on the fate of residues in animals,
plants, the environment and during storage and processing. In animals,
prochloraz is rapidly metabolized from dermal or oral exposure. Most
of the residue is rapidly excreted, with urinary excretion being the
predominant route in most animals tested, although faeces is the
predominant route in dogs. The principal metabolic products in the
urine were 2,4,6-trichlorophenoxyacetic acid and
2,4,6-trichlorophenoxy-ethanol (mostly conjugated). The major plant
metabolites have not been identified in the urine. Although residues
were qualitatively similar in urine or in tissue distribution among
animals studied, tissue residues have not been identified.
The potential for residues in products of animal origin was
investigated in two studies by feeding a lactating goat with
14C-phenyl-labelled prochloraz either by capsule or ad libitum with
14C-prochloraz field-treated wheat straw. The first study represents
the worst case maximum expected theoretical exposure from wheat straw
and the straw feeding is probably more reflective of maximum exposure
under normal feeding conditions.
These goat metabolism/feeding studies give insight into the
maximum potential for residues, although residues in animal tissues
are not identified. It is not known whether urinary metabolites are
the same as those in milk or tissues.
Although straw was used as a worst-case for potential residues,
foraging would appear to be potentially equally important, if not more
so, if not label-restricted, since it can be fed at higher levels in
the diet, as can citrus pulp. However, citrus uses presumably are
intended where peel is discarded. Although wheat or barley grain
residues were relatively low, grain may comprise up to 50 and 80
percent of the diet of poultry and swine, respectively. No analytical
method suitable for enforcement purposes for animal products is
available, or at least known to be validated for that purpose.
Additional information is needed to support a conclusion that the fate
of residues in animals is adequately understood or to estimate maximum
residue levels for products of animal origin.
The fate of Prochloraz in plants was investigated in wheat
(foliar, pre-emergence and seed treatments), citrus (postharvest),
apples (foliar) and sugarbeet (soil uptake). In wheat plants, major
residues at the vegetative stage from foliar treatment were free
BTS-44596, free and conjugated BTS-44595 and 2,4,6-trichlorophenol in
decreasing order, which together accounted for 83 percent of the
terminal residue after 19 days. Less than 2 percent was unchanged
prochloraz and only 0.2 percent was 2,4,6-trichlorophenoxy acetic
acid. No single unidentified residue accounted for more than 6.3
percent of the remainder. Residues appear to be absorbed primarily
within the first 24 hours.
At the harvest stage, only the free trichlorophenol has been
identified in straw or grain, and it occurs as 3-8 percent of the
terminal residue. This, plus tritiated water and other presumably
conjugated but unidentified residues containing the trichlorophenol
moiety, account for 40-70 percent of the terminal residue. No single
remaining unidentified residue fraction accounts for more than 6
percent of the terminal residue.
When applied to wheat seeds or as a pre-emergence drench, most
residue was in or near the root system, with little upward mobility,
and there is little evidence of upward mobility when prochloraz is
applied to foliage although low levels translocate towards the roots.
Only very low levels of residue were found in chaff and straw when
wheat was grown in soil treated with radioactive 3H-prochloraz ten
months earlier. However, sugarbeet seedlings showed low residues when
planted as a rotational crop, but only trace levels were found in the
foliage at maturity and no significant residue in the roots.
In apples, residues migrated into the peel and by 33-63 days
approximately 60-80 percent of the terminal or applied residue was in
the peel, <2 percent on the surface and generally <7 percent of
the applied dose in the flesh, although it can be twice as much.
Qualitatively extractable peel residues were similar to residues found
in wheat at the vegetative stage with approximately 80 percent of the
residues hydrolysable to 2,4,6-trichlorophenol. This, therefore,
accounts for approximately 50-60 percent of the total fruit residue.
Other residues (primarily peel fiber-bound) were not identified, and
although unresolved background accounted for approximately 10 percent
of the whole fruit residue (14 percent of peel extractable), no one
peel extractable metabolite or fraction exceeded approximately 5
percent of the total fruit residues. The fate of residues in plants is
reasonably well understood for the limited number and type of crops
for which there are approved uses. If uses are expanded significantly
to other food groups, additional metabolism studies would be prudent.
Numerous experiments have been conducted on the fate of
prochloraz residues in soil and water. Prochloraz is degraded under
both aerobic and anaerobic conditions with formation of volatile
compounds, probably CO2 and tritiated water. Residues remaining are
predominantly prochloraz, BTS-44596 and BTS-45186, with binding to
upper soil particles increasing with time. The half-life can range
from 90-155 days, depending on conditions. Formation of volatiles is
decreased under anaerobic conditions. Mobility is low in soils,
although low mobility does occur under some conditions, especially for
the metabolites in sand. Prochloraz is stable in water under acidic
conditions, but does degrade under alkaline conditions.
The fate of residues during processing was investigated for wheat
made into bread, postharvest citrus, rapeseed oil and mushrooms after
processing. Residues in bread made from wheat grain with <0.1 mg/kg
prochloraz also showed <0.1 mg/kg residues. Since the limit of
determination is 0.1 mg/kg, no conclusion could be made on the extent
of residue concentration, if any. No information was available for
residues in flour or other milling fractions. Similarly, low residues
were found in malt from prochloraz-treated barley, but no information
was available on the residue level in grain from which it was made.
Residue levels concentrate from 1 to 3 times in oil processed
from mature oilseed rape that has been field-treated with prochloraz.
Residues in canned or dehydrated mushrooms or canning liquor after
pre-emergence treatment with prochloraz were less than in untreated
controls, except for one sample of canned mushrooms with a residue of
0.23 mg/kg. No information was available on residue levels before
canning or dehydration. No conclusion can be drawn on the extent of
residue concentration, if any. When oranges treated postharvest were
processed into "42 percent comminuted orange" or "whole orange
juices", which are used to manufacture various orange drinks,
approximately 70 percent of the residue in the oranges from which it
was processed was lost.
The fate of residues during storage after various postharvest
prochloraz treatments was investigated for citrus, bananas and
avocadoes. Except for one study where free 2,4,6-trichlorophenol was
determined and where treated citrus was stored under a variety of
conditions (dark or light, cold or room temperature and at intervals
ranging from one to 70 days) total residues were very persistent, with
little evidence of a decreasing trend over the varied treatment or
storage conditions. Modest increases (2X) in application
concentrations had little effect on residue levels, approximately
90-95 percent of which were in the peel. There was evidence of
continued gradual translocation into the flesh as the interval from
treatment increases, especially at room temperature. This was also
found in studies on foliar treatments of mango. Residue levels during
storage of prochloraz alone were similar to those for prochloraz and
its major metabolites, suggesting little degradation or conjugation.
Emulsifiable concentrate formulations gave significantly higher
residues than wettable powder formulations and residues in lemons were
higher than those in oranges.
In bananas treated postharvest with prochloraz, no trend of
decreasing residues was observed over a 16-day storage period at
ambient temperature. Residue levels were similar with modest increases
(2X) in treatment rate and, as in the case of citrus, most of the
residue was in the peel.
In avocadoes treated postharvest with prochloraz, residues were
comparable to those from multiple foliar treatments. Approximately
10-30 percent of the residue could occur in the flesh after relatively
short preharvest intervals. The translocation was therefore somewhat
greater than in citrus, bananas and apples.
Photodecomposition of an aqueous prochloraz solution resulted in
the formation of plant metabolite BTS-44596 and towards the end of a
36-day exposure to artificial light, a trace of plant metabolite
BTS-44595. A half-life of approximately 20 days was observed.
Analytical methods are available for enforcement purposes for a
wide variety of plant commodities. Residues of prochloraz alone or
total residues of prochloraz and its major plant metabolites may be
determined, although more of the field trials data were determined by
the latter. Analytical recoveries were generally better than 70
percent. No analytical methods have been validated for residues in
animal products. The analytical methodology for the parent compound
should be suitable and more appropriate for enforcement purposes and
for estimating maximum residue levels (see Report of this Meeting,
Section 2.3).
RECOMMENDATIONS
The Meeting examined residue data from supervised trials
reflecting established or proposed good agricultural practice. From
the data, the Meeting was able to estimate the maximum residue levels
that are likely to occur when prochloraz is used in practice and when
the reported intervals between last application and harvest or other
restrictions are observed. Maximum residue limits are recommended
where registered or approved uses are known and residues are
determined as free prochloraz. Temporary maximum residue limits are
recommended where only recommended uses are available or where total
residues of prochloraz and its metabolites containing the
2,4,6-trichlorophenol moiety are determined. Regardless of the status
of the ADI, these remain temporary pending receipt of information on
approved or registered uses or data on supervised trials with residues
determined as prochloraz.
All estimates apply to foliar applications unless otherwise
indicated.
Estimated Interval from last
Commodity maximum residue treatment to harvest (days)
limits (mg/kg) on which recommendation
Determined and expressed is based and other
as prochloraz restrictions
Barley 0.05** 40
Oats 0.05** 40
Rye 0.05** 40
Wheat 0.05** 40
Barley straw 0.2 40
Oat straw 0.2 40
Rye straw 0.2 40
Wheat straw 0.2 40
Mushrooms 2 2 3
Citrus 5 (TMRL)1, 4 postharvest
Sum of prochloraz
and metabolites expressed
as prochloraz 4
Bananas 5 (TMRL) 1 postharvest
Stone fruits 1 (TMRL) 1 14
Avocado 5 (TMRL) 1, 2 7 (preharvest)
Mango 2 (TMRL) 1, 2 15 (preharvest)
Papaya 1 (TMRL) 1 postharvest
Rapeseed 0.5 (TMRL) 25
** At or about the limit of determination.
1 No information on approved or registered uses.
2 Limit allows for cumulative residues from both preharvest and postharvest
treatments, both of which are good agricultural practice.
3 Limit accommodates applications after casing or after flushes, but not
direct treatment of mushrooms.
4 Temporary regardless of the status of the ADI.
FURTHER WORK OR INFORMATION
Required (by 1985)
1. In order to establish MRLs for products of animal origin, several
requirements remain:
(a) Metabolism studies in animals, including ruminants and
poultry, at sufficiently high dosing levels to permit
identification and quantification of tissue residues.
(b) If metabolism studies in (a) above indicate any possibility
of residues in animal tissues, feeding trials should be
conducted in animals, including ruminants and poultry, in
which tissues are analysed for residues of concern
identified in metabolism studies. Feeding in an appropriate
number of animals should as nearly as possible reflect
maximum levels of weathered residues likely to occur in
practice and, ideally, at an exaggerated level.
(c) A validated analytical method suitable for enforcement
purposes for products of animal origin.
2. Good agricultural practice information for those commodities for
which temporary maximum residue limits are recommended,
preferably for countries in which the residue trials were
conducted or those in close proximity.
3. Additional data on residue trials reflecting good agricultural
practice for commodities for which temporary maximum residue
limits are recommended (except citrus). The additional data
should be concurrently determined as both prochloraz and total
residues of prochloraz and its metabolites containing the
trichlorophenol moiety on the same samples. Sufficient data
should be provided to permit further utilization of residue data
already provided, therefore permitting recommendation of maximum
residue limits expressed as prochloraz.
Desirable
1. Additional national maximum residue limits.
2. Additional processing studies in cereals to permit a conclusion
on the extent, if any, of residue concentration in milling
fractions. Grain used should have residues sufficiently above the
limit of determination for drawing conclusions.
3. Additional processing studies for mushrooms to permit a
conclusion on the extent, if any, of residue concentration.
Mushrooms should be analysed before and after processing.
4. Information on possible interference in prochloraz analysis in
the presence of other pesticides, especially those containing the
trichlorophenol moiety. If interferences are encountered,
confirmatory analytical procedures for prochloraz and metabolites
may be necessary.
REFERENCES - RESIDUES
Browne, P.M. Residues of prochloraz and major metabolites in oranges
1981a following postharvest dip treatment in Australia, 1981. FBC
report RESID/81/72 submitted to FAO by FBC Ltd.
(Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in oranges
1981b following postharvest shower treatment in Spain, 1981. FBC
report RESID/81/72 submitted to FAO by FBC Ltd.
(Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in avocados
1982a following postharvest dip treatment in Australia, 1981. FBC
report RESID/82/2 submitted to FAO by FBC Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in bananas
1982b following post-harvest dip treatment in Australia, 1981. FBC
report RESID/82/7 submitted to FAO by FBC Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in lemons
1982c following post-harvest shower treatment in Spain. FBC report
RESID/82/8 submitted to FAO by FBC Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in winter
1982d rye treated post-emergence with a 40% E.C. formulation in
West Germany, 1981. FBC report RESID/82/25 submitted to FAO
by FBC Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in winter
1982e wheat treated postemergence with three applications of 40%
E.C. formulation in Fed. Rep. Germany, 1981. FBC report
RESID/82/29 submitted to FAO by FBC Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in spring
1982f wheat and barley postemergence with a 40% E.C. formulation
(two or three applications) in West Germany, 1981. FBC
report RESID/82/31 submitted to FAO by FBC Ltd.
(Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in mushrooms
1982g following application of a 50 W formulation in Holland,
1980. FBC report RESID/82/39 submitted to FAO by FBC Ltd.
(Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in processed
1982h mushrooms following application of a 40 E.C. formulation in
France, 1981. FBC report RESID/82/68 submitted to FAO by FBC
Ltd. (Unpublished)
Browne, P.M. Residues of prochloraz and major metabolites in
1982i oranges following post-harvest dip treatment in the U.K.,
1981. FBC report RESID/82/83 submitted to FAO by FBC Ltd.
(Unpublished)
Browne, P.M. & Manley, J.D. Analytical method for residues of
1982a prochloraz and major metabolites in orange juice. FBC report
RESID/82/18 submitted to FAO by FBC Ltd. (Unpublished)
Browne, P.M. & Manley, J.D. Residues of prochloraz and major
1982b metabolites in oranges and lemons following postharvest dip
treatment in Italy, 1979. FBC report RESID/82/32 submitted
to FAO by FBC Ltd. (Unpublished)
Browne, P.M. & Manley, J.D. Residues of prochloraz and major
1982c metabolites in mangoes treated with a 50% W.P. formulation
in Australia, 1981. FBC report RESID/82/34 submitted to FAO
by FBC Ltd. (Unpublished)
Campbell, J.K. Residues in milk and tissues of a goat dosed orally
1980 with 14C-prochloraz. Boots report AX-80026 submitted to FAO
by FBC Ltd. (Unpublished)
Campbell, J.K. Residues of prochloraz in milk and tissues of a
1983 lactating goat fed straw containing residues of radioactive
prochloraz. FBC report METAB/83/8 submitted to FAO by FBC
Ltd. (Unpublished)
Campbell, J.K. & Needham, D. Residues in milk and tissues of a goat
1980 dosed orally with 14C-BTS 44596 (major plant metabolite of
prochloraz). Boots report AX-800333 submitted to FAO by FBC
Ltd. (Unpublished)
Churchill, J.H.M. & Longland, R.C. Residues of prochloraz and major
1983a metabolites in bananas following a single postharvest
application with a 45% E.C. prochloraz formulation in South
Africa, 1982. FBC report RESID/83/5 submitted to FAO by FBC
Ltd. (Unpublished)
Churchill, J.H.M. & Longland, R.C. Residues of prochloraz and major
1983b metabolites in avocadoes following application with a 45%
E.C. formulation in South Africa. FBC report RESID/83/10
submitted to FAO by FBC Ltd. (Unpublished)
Churchill, J.H. & Longland, R.C. Residues of prochloraz and major
1983c metabolites in processed mushrooms following application
with a 50% W.P. formulation of prochloraz-Mn complex in
Holland, 1981. FBC report RESID/83/23 submitted to FAO by
FBC Ltd. (Unpublished)
Churchill, J.H.M. & Longland R.C. Residues of prochloraz and major
1983d metabolites in almonds following foliar application with a
50% W.P. formulation in Israel, 1982. FBC report RESID/83/38
submitted to FAO by FBC Ltd. (Unpublished)
Churchill, J.H.M. & Longland, R.C. Residues of prochloraz and major
1983e metabolites in avocadoes following postharvest treatment
with a 45% E.C. formulation in Australia, 1982/83. FBC
report RESID/83/55 submitted to FAO by FBC Ltd.
(Unpublished)
Cron, J. Residues of prochloraz and major metabolites in rapeseed
1982 following a single postemergence application with a 40 E.C.
prochloraz and a prochloraz/carbendazim E.C. formulation in
France 1980/81. FBC report RESID/82/74 submitted to FAO by
FBC Ltd. (Unpublished)
Cron, J.H. & Longland, R.C. Residues of prochloraz and major
1983a metabolites in avocadoes following multiple foliar
application with a 50% W.P. formulation of prochloraz-Mn
complex in Australia, 1981/82. FBC report RESID/82/120
submitted to FAO by FBC Ltd. (Unpublished)
Cron, J.H. & Longland, R.C. Residues of prochloraz and major
1983b metabolites in papayas treated postharvest with a 45% E.C.
formulation in Australia, 1982. FBC report RESID/83/1
submitted to FAO by FBC Ltd. (Unpublished)
Douglas, M.T. & Pell, I.B. Assessment of the ready degradability of
1982 prochloraz. HRC and FBC report METAB/82/44 submitted to FAO
by FBC Ltd. (Unpublished)
Douglas, M.T. & Pell, I.B. Assessment of biodegradability of
1983 prochloraz by pre-adapted sewage organisms. HRC and FBC
report METAB/83/10 submitted to FAO by FBC Ltd.
(Unpublished)
Goto, H. Residues of prochloraz in rice following seed soak or foliar
1980 application with a 25% E.C. formulation in Japan, 1980.
Inst. Environ. Toxicol., Japan, Nissan and FBC report
submitted to FAO by FBC Ltd. (Unpublished)
Haran, G. Identification of a photodecomposition product of BTS-40
1978 542 (prochloraz). Boots report F-78014 submitted to FAO by
FBC Ltd. (Unpublished)
Haran, G. The effect of simulate& sunlight on aqueous solutions of
1980 prochloraz. Boots report F-80010 submitted to FAO by FBC
Ltd. (Unpublished)
Hayto, E.M. BTS-40542 residues in wheat and barley. Boots report AX-
1977a 77002 submitted to FAO by FBC Ltd. (Unpublished)
Hayto, E.M. BTS-40 542 residues in wheat from Italy, 1977. Boots
1977b report AX-79003 submitted to FAO by FBC Ltd. (Unpublished)
Hayto, E.M. BTS-40 542 residues in apples from Thurgarton, U.K. Boots
1977c report AX-77011 submitted to FAO by FBC Ltd. (Unpublished)
Hayto, E.M. BTS-40542 residues in wheat from Holland, 1977. Boots
1978a report Ax-78006 submitted to FAO by FBC Ltd. (Unpublished)
Hayto, E.M. BTS-40542 residues in wheat and barley from France, 1977.
1978b Boots report AX-78020 submitted to FAO by FBC Ltd.
(Unpublished)
Hayto, E.M. BTS-40542 residues in wheat and barley from the United
1979a Kingdom, 1977. Boots report AX-79001 submitted to FAO by FBC
Ltd. (Unpublished)
Hayto, E.M. BTS-40542 residues in apples from Thurgarton, U.K., 1977.
1979b Boots report AX-79005 submitted to FAO by FBC Ltd.
(Unpublished)
Hayto, E.M. BTS-40542 residues in cereals from Fed. Rep. Germany,
1979c 1977. Boots report AX-79008 submitted to FAO by FBC Ltd.
(Unpublished)
Hayto, E.M. BTS-40542 and dicloran residues in lettuces from Holland,
1979d 1978. Boots report AX-79010 submitted to FAO by FBC Ltd.
(Unpublished)
Heinanen, E. Investigation certification for residues of prochloraz in
1983 spring wheat from Finland, 1982. VML report 2940, 2942/82
submitted to FAO by FBC Ltd. (Unpublished)
Housden, M.C. Residues of prochloraz and major metabolites in grain
1982a treated post-emergence with a 45% E.C. formulation in
Belgium, 1981. FBC report RESID/82/1 submitted to FAO by FBC
Ltd. (Unpublished)
Housden, M.C. Residues of prochloraz and major metabolites in
1982b mushrooms following application of a 50W formulation in
Australia, 1981/82. FBC report RESID/82/48 submitted to FAO
by FBC Ltd. (Unpublished)
Housden, M.C. Residues of prochloraz and major metabolites in cereals
1982c treated post-emergence with a 45% E.C. formulation (single
or double application) in Denmark, 1982. FBC report
RESID/82/111 submitted to FAO by FBC Ltd. (Unpublished)
Housden, M.C. & Longland, R.C. Decline study of residues of prochloraz
1983 and major metabolites in winter barley and wheat treated
once with a prochloraz/carbendazim suspension concentrate
formulation in Fed. Rep. Germany, 1982. FBC report
RESID/83/19 submitted to FAO by FBC Ltd. (Unpublished)
Housden, M.C. & Whiteoak, R.J. Residues of prochloraz and metabolites
1982 in mushrooms following single and multiple applications
(50w formulation) in the U.K. 1981 and 1982. FBC report
RESID/82/63 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. BTS 40 542 residues in cereals from Austria, 1977. Boots
1979a report AX 79006 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of BTS 40
1979b 542, BTS 44 596 and BTS 45 186 in grain. Boots report AX
79007 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979c (BTS 40 542), BTS 44 595, BTS 44 596 and BTS 45 186 in wheat
grain and straw from Fed. Rep. Germany, 1978. Boots report
AX 79015 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979d (BTS 40 542), BTS 44 595, BTS 44 596 and BTS 45 186 in wheat
grain and straw from Italy, 1978. Boots report AX 79017
submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979e (BTS 40 542), BTS 44 595, BTS 44596 and BTS 45 186 in wheat
grain and straw from The Netherlands, 1978. Boots report AX
79 017 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979f (BTS 40 542), BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in cereal grain and straw from
Austria, 1978. Boots report AX 79 020 submitted to FAO by
FBC Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979g (BTS 40 542), BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in wheat and barley grain from
Denmark, 1978. Boots report AX 79021 submitted to FAO by FBC
Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1979h (BTS 40 542), BTS 44 595, BTS 44 596 and BTS 45 186 in wheat
and barley grain and straw from France, 1978. Boots report
AX 79023 submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. Persistence and uptake of prochloraz in Spanish oranges
1980a following a post-harvest dip. Boots report AX 80003
submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. BTS 45 186 residues in prochloraz-treated wheat grain from
1980b France, 1977. Boots report AX 80010 submitted to FAO by FBC
Ltd. (Unpublished)
Kelly, I.D. The analysis of free and conjugated residues of prochloraz
1980c (BTS 40 542) and metabolites containing the
2,4,6-trichlorophenyl moiety in wheat grain and straw from
The Netherlands, 1979. Boots report AX 80025 submitted to
FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The metabolism of 3H-prochloraz in mature wheat grain and
1980d straw. Boots report AX 80028 submitted to FAO by FBC Ltd.
(Unpublished)
Kelly, I.D. The metabolism and distribution of BTS 46828 applied to
1982a the fruit of field-growing apples. FBC report METAB/82/18
submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. The hydrolysis of prochloraz in aqueous solution under
1982b acid, neutral and basic conditions. FBC report METAB/82/28
submitted to FAO by FBC Ltd. (Unpublished)
Kelly, I.D. & Krepski, W.J. The metabolism of prochloraz by wheat
1980 grain and straw. Boots report AX 80009 submitted to FAO by
FBC Ltd. (Unpublished)
Kesterton, M.S. Distribution of radio-labelled residues in field soil
1978 after application of 3H-BTS 40542. Boots report AX 78010
submitted to FAO by FBC Ltd. (Unpublished)
Kesterton, M.S. Report on the aerobic metabolism of prochloraz in
1981a soil over a period of 12 months. Boots report AX 81002
submitted to FAO by FBC Ltd. (Unpublished)
Kesterton, M.S. Report on the anaerobic metabolism of prochloraz in
1981b soil. Boots report AX 81004 submitted to FAO by FBC Ltd.
(Unpublished)
Kesterton, M.S. & Newby, S.E. A study of the leaching of prochloraz in
1980a soil. Boots report AX 80008 submitted to FAO by FBC Ltd.
(Unpublished)
Kesterton, M.S. & Newby, S.E. Adsorption and desorption studies of
1980b prochloraz in soil. Boots report AX 80012 submitted to FAO
by FBC Ltd. (Unpublished)
Kesterton, M.S. & Newby, S.E. Leaching study of prochloraz aged in a
1981 silty clay loam soil. Boots report AX 81003 submitted to FAO
by FBC Ltd. (Unpublished)
Krepski, W.J. The uptake of 14C-prochloraz and 3H-prochloraz soil
1981a residues into wheat. Boots report AX 81001 submitted to FAO
by FBC Ltd. (Unpublished)
Krepski, W.J. The metabolism of 14C-prochloraz and 14C-BTS 46828 by
1981b apples grown under glass. Boots and FBC report submitted to
FAO by FBC Ltd. (Unpublished)
Krepski, W.J. Translocation of 14C-prochloraz applied as a liquid
seed
1982 dressing in wheat grown to maturity. FBC report METAB/81/30
submitted to FAO by FBC Ltd. (Unpublished)
Leake, C.R. & Lines, D. The leaching of prochloraz and its major
1981 metabolites in four soil types using TLC. FBC report
METAB/81/35 submitted to FAO by FBC Ltd. (Unpublished)
Longland, R.C. Analysis of residues of prochloraz and major
1983a metabolites in grain and straw from Sweden following
application of 45% E.C., 1982. FBC report RESID/82/114
submitted to FAO by FBC Ltd. (Unpublished)
Longland, R.C. Residues of prochloraz and major metabolites in
1983b sugarbeet following application of a 40% E.C. formulation in
Italy, 1981. FBC report RESID/83/58 submitted to FAO by FBC
Ltd. (Unpublished)
Longland, R.C. & Churchill, J.H. Residues of prochloraz and major
1983 metabolites in mangoes following foliar treatment with 50%
W.P. and postharvest treatment with 40% E.C. formulations in
Israel, 1982. FBC report RESID/83/7 submitted to FAO by FBC
Ltd. (Unpublished)
Maclaine Pont, M.A., Vogelzang, H.P. & Siegmann-Knoester, K.C. The
1980 residue analysis of prochloraz in combination with dicloran.
Asepta-fabrik and Boots report AX 80020/2 submitted to FAO
by FBC Ltd. (Unpublished)
Maier-Bode, H. The leaching of prochloraz in three standard soils from
1980 Fed. Rep. Germany. HMB and FBC report submitted to FAO by
FBC Ltd. (Unpublished)
Manley, J.D. & Snowden, P.J. Analytical method for residues of
1982a prochloraz and major metabolites in miscellaneous fruit,
cereal and vegetable crops (improved method). FBC report
RESID/82/88 submitted to FAO by FBC Ltd. (Unpublished)
Manley, J.D. & Snowden, P.J. Residues of prochloraz and major
1982b metabolites in oranges and processed oranges following
commercial scale postharvest brush treatment with prochloraz
(40 E.C.) in South Africa, 1982. FBC report RESID/82/89
submitted to FAO by FBC Ltd. (Unpublished)
Manley, J.D. & Snowden, P.J. Residues of prochloraz and major
1982c metabolites in rapeseed following treatment with a 40% E.C.
formulation in the U.K., 1981/1982. FBC report RESID/82/90
submitted to FAO by FBC Ltd. (Unpublished)
McDougall, J. The metabolism of 3H-BTS 40 542 in wheat at a
vegetative
1979 growth stage. Boots report AX 79009 submitted to FAO by FBC
Ltd. (Unpublished)
McDougall, J. The uptake and translocation of 3H-prochloraz in wheat.
1980a Boots report AX 80007 submitted to FAO by FBC Ltd.
(Unpublished)
McDougall, J. The relative uptake and persistence of prochloraz and
1980b BTS 46 828 in apples following treatment with 14C-
prochloraz and 14C-BTS 46 828. Boots report AX 80015
submitted to FAO by FBC Ltd. (Unpublished)
McGibbon, A.S. Uptake of prochloraz residues by sugarbeet from
1982 prochloraz-treated soil under field conditions. FBC report
METAB/82/27 submitted to FAO by FBC Ltd. (Unpublished)
Newby, S.E. The laboratory decline of prochloraz in two soils, a sandy
1982 loam and a silty clay loam, under sterile conditions. FBC
report METAB/82/41 submitted to FAO by FBC Ltd.
(Unpublished)
Peatman, M.H. & Snowden, P.J. Residues of prochloraz and major
1982 metabolites in rapeseed treated with the 40% E.C.
formulation in France, 1982. FBC report RESID/82/116
submitted to FAO by FBC Ltd. (Unpublished)
Peatman, M.H. & Snowden, P.J. Residues of prochloraz and major
1983 metabolites in rapeseed treated with a 45% E.C. formulation
in Denmark and Sweden, 1982. FBC report RESID/83/29
submitted to FAO by FBC Ltd. (Unpublished)
Reary, J.B. Analytical method for free and conjugated residues of
1981a prochloraz, BTS 44595, BTS 44 596 and BTS 45 186 in cereals
at different growth stages. FBC report RESID/81/13 submitted
to FAO by FBC Ltd. (Unpublished)
Reary, J.B. Residues of prochloraz and major metabolites in summer
1981b wheat treated post-emergence with a 40% E.C. formulation
(two or three applications) in Fed. Rep. Germany, 1980. FBC
report RESID/81/14 submitted to FAO by FBC Ltd.
(Unpublished)
Reary, J.B. Residues of prochloraz and major metabolites in cereals
1981c treated post-emergence with a 45% E.C. formulation in
Holland, 1980. FBC report RESID/81/46 submitted to FAO by
FBC Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980a prochloraz (BTS 40 542) BTS 44 595 and BTS 44 596 and
conjugated residues of BTS 45 186 in wheat and barley,
France, 1979. Boots report AX 80005 submitted to FAO by FBC
Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980b prochloraz (BTS 40 542), BTS 44 595 and BTS 44 596 and
conjugated residues of BTS 45 186 in Spanish oranges
following a postharvest application, 1980. Boots report AX
80021 submitted to FAO by FBC Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980c prochloraz (BTS 40 542), BTS 44 595, BTS 44 596 and
conjugated residues of BTS 45 186 in wheat and barley, U.K.,
1979. Boots report AX 80022 submitted to FAO by FBC Ltd.
(Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980d prochloraz (BTS 40 542), BTS 44 595, BTS 44 596 and
conjugated residues of BTS 45 186 in wheat and barley,
Denmark, 1979. Boots report AX 80023 submitted to FAO by FBC
Ltd (Unpublished)
Richards, M.E. BTS 45 186 residues in oranges treated with prochloraz,
1980e Spain, 1980. Boots report AX 80027 submitted to FAO by FBC
Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980f prochloraz, BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in Spanish oranges following a
postharvest application, 1980. Report No. 2. Boots report AX
80030 submitted to FAO by FBC Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1980g prochloraz, BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in winter wheat, Fed. Rep. Germany,
1979. Boots report AX 80032 submitted to FAO by FBC Ltd.
(Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1981a prochloraz, BTS 44 595 and BTS 44596 and conjugated residues
of BTS 45 186 in grain and straw. Boots and FBC report AX
800 35 submitted to FAO by FBC Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1981b prochloraz, BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in winter barley, Fed. Rep. Germany,
1980. Boots and FBC report AX 81005 submitted to FAO by FBC
Ltd. (Unpublished)
Richards, M.E. The analysis of free and conjugated residues of
1981c prochloraz, BTS 44 595 and BTS 44 596 and conjugated
residues of BTS 45 186 in mushrooms, U.K., 1980. FBC report
METAB/81/17 submitted to FAO by FBC Ltd. (Unpublished)
Snowden, P.J. Residues of prochloraz and major metabolites in stone
1983 fruit following foliar applications of prochloraz (50 E. and
40 E.C.) in Europe (1982) and South Africa (1981/2 and
1982/3). FBC report RESID/83/57 submitted to FAO by FBC Ltd.
(Unpublished)
Snowden, P.J. & Manley, J.D. Residues of prochloraz and major
1983 metabolites in Tambors oranges following commercial scale
postharvest brush treatment with prochloraz (45% E.C.) in
South Africa, 1982. FBC report RESID/83/9 submitted to FAO
by FBC Ltd. (Unpublished)
Somerville, L. The analysis of prochloraz residues in cereals. Boots
1980 report AX 80020/1 submitted to FAO by FBC Ltd. (Unpublished)
Stockbauer, I. Leaching of Sportak PF (prochloraz and carbendazim) in
1983 German soils. ASU and FBC report submitted to FAO by FBC
Ltd. (Unpublished)
Wang, S.S. Residues of prochloraz in grapes, apples and watermelon
1982 following foliar application with a 25% E.C. formulation in
Taiwan, Taiwan Plant Protection Centre report submitted to
FAO by FBC Ltd. (Unpublished)
Whiteoak, R.J. Residues of prochloraz in mushrooms following multiple
1981 applications of a 50% W.P. formulation in the U.K., 1980/81
(ADAS trial results), FBC report RESID/81/41 submitted to
FAO by FBC Ltd. (Unpublished)
Whiting, K.C. & Dickinson, W. The stability of the manganese complex
1979 of prochloraz in water. Boots report F 79005 submitted to
FAO by FBC Ltd. (Unpublished)
