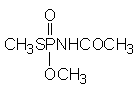

Pesticide residues in food - 2002 - Joint FAO/WHO Meeting on Pesticide Residues
First draft prepared by
Roland Solecki
Pesticides and Biocides Division of the Federal Institute for Health Protection of Consumers and Veterinary Medicine, Berlin, Germany
|
Biochemical aspects: absorption, distribution, excretion and metabolism |
The Joint Meeting previously evaluated the toxicity of acephate (O,S-dimethyl acetylphosphoramidothioate, Figure 1) in 1976, 1982, 1984, 1987, 1988 and 1990 (Annex 1, references 26, 38, 42, 50, 53 and 59). It was re-evaluated by the present Meeting within the periodic review programme of the Codex Committee on Pesticide Residues. The Meeting reviewed new data on acephate that were not previously reviewed and relevant data from the previous evaluations.
Acephate is a racemic organophosphorus insecticide. Inhibition of cholinesterase activity is the basis for its major toxic effects; however, other toxic effects have been observed at higher doses. Methamidophos, the primary metabolite of acephate in plants, birds and mammals, is a significantly more potent inhibitor of cholinesterase activity than acephate.
Acephate is applied as spray in agriculture, horticulture and viticulture for control of insects on a variety of field, fruit and vegetable crops. It is effective against a wide range of Agromyzidae, Aphididae, lepidopterous larvae, Hymenopterae and Thysanopterae. The mechanism of action of acephate is phosphorylation and subsequent inactivation of cholinesterase activity. The selective toxicity of acephate in insects (but not in mammals) is believed to be due to its ready conversion to methamidophos (Mahajna et al., 1997).
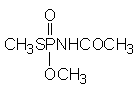
Figure 1. Chemical formula of acephate
The metabolism of acephate was investigated in three male and three female Sprague-Dawley-derived rats in a study that did not conform to good laboratory practice (GLP). An additional male rat was used as the control. Each rat except the control was given acephate (purity not reported) at a dose of 25 mg/kg bw per day for 7 days and then given a single dose of [S-methyl-14C]orthene (purity, > 99.5%) on the eighth day at the same level. All doses were given by intubation of an aqueous solution. Total samples of urine and faeces were collected at intervals of 6, 12, 24, 36 and 48 h after the dose of radioactive material and counted. The CO2 in the exhaled breath was collected and counted, and muscle, fat, liver, kidney, heart, gut, brain and skin were removed for determination of radioactivity. Urine samples were placed directly on silica gel thin-layer chromatography plates.
The pattern of excretion in urine did not differ quantitatively or qualitatively in males and females. Excretion was rapid, most of the radioactive material being recovered within 12 h. Urine contained 8295% of the administered dose, 14% was exhaled, and 1% was found in faeces. Less than 1% was found as residues in tissues and organs 72 h after the last administration. Liver and skin were the tissues with the highest proportions of residues (0.060.27% and 0.050.19%, respectively). The proposed metabolic pathway of acephate in rats is shown in Figure 2. Urinary metabolites were identified as unchanged acephate (7377%), O,S-dimethyl phosphorothioate (36%) and S-methyl acetylphosphoramidothioate (34%). No methamidophos was found (Lee, 1972).
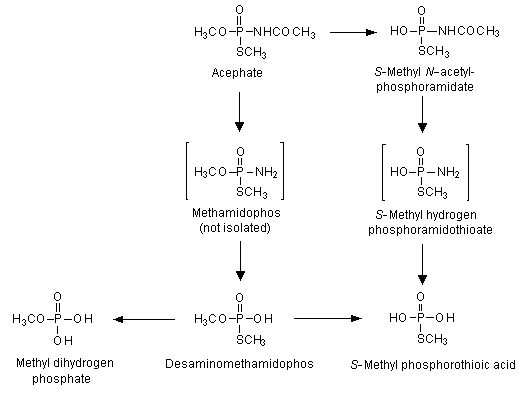
Figure 2. Proposed metabolic pathway of acephate in rats
From Crossley & Tutass (1969), Lee (1972)
In a study conducted to investigate whether methamidophos is formed from acephate in rats, 6-week-old male and female Sprague-Dawley rats were given acephate (purity, 99.94%) by gavage at 100 mg/kg bw per day for 4 days. Two rats were killed 3 h after each dose (except the third), and the whole carcasses were quickly frozen and then analysed for acephate and methamidophos. In addition, three male and three female rats were killed 3 h after the fourth dose of acephate, and methamidophos was determined in tissues. Excreta were collected for analyies during the 24 h following the third dose. The rats were killed 3 h after dosing, when it was estimated that the concentration of methamidophos would be at or near the maximum. The amounts of acephate and methamidophos in the urine were determined by extraction and gasliquid chromatography.
Acephate was rapidly absorbed and rapidly eliminated: the carcasses contained only 1248% and the gastrointestinal tracts 314% of the final dose 3 h after dosing. The excreta (chiefly urine) contained 5456% of the final dose 6 h after dosing. There was no tendency for acephate to concentrate in blood, liver, muscle, fat, heart or brain. Rats converted a portion of acephate to methamidophos, the conversion appearing to take place in the small intestine and, to a lesser extent, in the stomach, apparently by microorganisms. Methamidophos was then absorbed from the stomach and intestines and distributed throughout the body. By 3 h after the last dose, the carcass contained 0.61.6% and the excreta (chiefly urine) 1.11.5% of the final dose of acephate as methamidophos. The concentrations of methamidophos in these tissues varied from 0.2 to 1.1 ppm. The highest concentrations were found in kidney (4.112 ppm), testes (2.43.9 ppm) and brain (2.12.5 ppm). There was no tendency for methamidophos to accumulate in blood, liver, muscle, fat or heart (Warnock, 1973).
A single oral dose of 40 mg/kg of [14C-acetyl]acephate (purity, 98%) was administered on day 18 of gestation to groups of three Sprague-Dawley rats, which were killed after 10 min and 0.5, 1, 3, 6, 12, 24, 48 h. The same dose was administered to the dams immediately after delivery. Nursing and suckling animals were killed at intervals of 1, 3, 6, 12, 24, 36 and 48 h after dosing. Acephate was rapidly absorbed and distributed in the tissues, the concentrations in most tissues reaching a peak within 13 h. The highest concentration of radioactivity was found in maternal stomach, followed by liver. A total of 0.72% of the dose was recovered in the fetuses. More was recovered from the placenta than from the fetuses. The authors concluded that the placenta played an active role in the uptake of acephate. In nursing and suckling animals, the highest concentrations of radioactivity were usually present in the stomach, small intestine, liver, lung and kidney. A total of 0.96% of the dose was recovered in suckling pups. The authors concluded that milk is a relevant pathway for elimination of acephate, and the compound could have adverse effects on suckling pups (Ahmed et al., 1992).
Postmitochondrial supernatant fractions (S9) were prepared from the livers of Sprague-Dawley rats, beagle dogs, rhesus monkeys and humans. Although the study was stated to have been conducted according to GLP, the certifying authority was not reported. The S9 fractions were incubated with 2043 ΅mol/l of [O-methyl-14C]acephate (purity, 99.6%) for 0, 1 and 4 h, and acephate and metabolites were analysed by reversed-phase high-performance liquid chromatography coupled with an on-line radioactivity detector.
Little acephate metabolism by S9 was detected, representing 1314% of the total metabolism for dogs and monkeys, about 9% for rats and about 4% for humans. Five peaks for metabolites were observed, the concentrations increasing in a time-dependent manner. Two primary metabolites accounted for most of the biotransformation of acephate and were formed by S9 from all species. One of these peaks was identified as methamidophos; the other peak, which behaved like an ion and eluted after acephate, was not definitively identified. A radioactive peak eluted in all species with the solvent front, suggesting the presence of a secondary metabolite. A minor metabolite eluting between methamidophos and acephate was detected only in rhesus monkey liver. Another minor metabolite, eluting as a tail of acephate, was detected only in human liver (Green, 1989).
Two male Sprague-Dawley rats each received 1.7 mg of [S-methyl-14C]acephate (purity, 99.5%) in 0.1 ml acetone on a shaved area of the back in a study that did not conform to GLP. The rats were kept in individual metabolism cages which allowed separate collection of urine and faeces, the urine collector beng cooled with dry ice. The animals had free access to food and water. Two control rats were included in the test. Radioactivity in all samples was counted with a liquid scintillation counter equipped with a photon monitor, whereas the total 14C in blood was determined by combustion. The amounts of acephate and methamidophos in the urine were determined by extraction and gasliquid chromatography.
Acephate at 10 mg/kg bw was rapidly absorbed into the blood and excreted in urine, the maximum concentration of residue in urine being 50 ppm 624 h after treatment. By 3 days, approximately 30% of the dermally applied acephate had been excreted in urine as unchanged acephate and about 1% as methamidophos. The maximum concentration of 14C in blood was found 13 h after treatment. Acephate was metabolized mainly to methamidophos, O,S-dimethyl phosphorothioate and S-methyl acetylphosphoramidothioate (Tucker, 1974).
(a) Oral, dermal and inhalation administration
The results of studies on the acute toxicity of acephate after administration orally, dermally or by inhalation are summarized in Table 1. The method used in these studies complied to a certain extent with OECD guidelines. GLP was not compulsory when most of the studies were performed.
Table 1. Acute toxicity and sensitization studies with acephate
|
Species |
Strain |
Sex |
Route |
LD50 |
LC50 |
Purity |
References |
|
|
|
|
|
(mg/kg bw) |
(mg/l air) |
(%) |
|
|
Rat |
Sprague-Dawley |
Male, |
Oral |
1400 |
|
97 |
Rittenhouse et al. (1979a)a |
|
|
|
Female |
|
1000 |
|
|
|
|
Dog |
Beagle |
Male |
Oral |
210b |
|
89.6 |
Mastalski et al. (1970) |
|
|
|
Female |
|
680c |
|
|
|
|
Mouse |
Not specified |
Male, female |
Oral |
360 |
|
88.9 |
Mastri et al. (1970) |
|
Rabbit |
New Zealand |
Male |
Percutaneous |
> 10 000 |
|
98.3 |
Rittenhouse & MacGregor (1977)a |
|
Rabbit |
New Zealand |
Male, female |
Percutaneous |
> 2000 |
|
97.8% |
Rittenhouse et al. (1980) |
|
Rat |
Sprague-Dawley |
Male, female |
Inhalation |
|
> 15 |
97.8% |
Rittenhouse et al. (1979b)a |
a Study not evaluated previously
b Minimum emetic dose
c Minimum lethal dose
The LD50 of acephate administered orally ranged from 360 mg/kg bw in mice to 1400 mg/kg bw in rats. The signs of poisoning were typical of the cholinergic syndrome. These included hypoactivity, tremors, muscular weakness and ruffed fur in mice and lethargy, excess salivation, lachrymation, exophthalmia, urinary incontinence, tremors, ataxia, diarrhoea, depression, collapse, bloody tears and/or decreased food consumption in rats. The toxic signs had disappeared by day 9 in the surviving mice and by day 8 in rats. No gross pathological alterations were seen in mice or rats at necropsy. The minimum emetic dose for beagle dogs was 220 mg/kg bw, and the minimum lethal dose was 680 mg/kg bw. All treated dogs showed excessive salivation, muscular tremors and diarrhoea. Animals at all doses above the lowest dose of 150 mg/kg bw showed emesis and also dyspnoea, ataxia, clonic convulsions and bloody diarrhoea up to 6 h after dosing.
The LD50 of acephate after dermal administration to rabbits was > 10 000 mg/kg bw. All rabbits at this dose showed tremors shortly after dosing, and five had diarrhoea the following day.
The LC50 value for rats treated by inhalation of an aerosol of acephate dissolved in distilled water for 4 h was > 15 mg/l of air. After exposure of the nose and head of Wistar rats to a mean concentration of 6.7 mg/l of air, all animals showed excess salivation and tremors on day 1, two rats showed ataxia, and two rats were lethargic on days 2 and 3. No other signs were recorded from day 4 onwards.
(b) Dermal and ocular irritation
Acephate (purity, 97.8%) caused slight to moderate skin irritation in New Zealand white rabbits. By 24 h, two rabbits had well-defined erythema on the abraded skin; by 48 h, only slight erythema was observed on the intact skin of an additional rabbit. The skin of all rabbits was normal by 72 h. The primary irritation score was 0.1 (Levy et al., 1979a).
Acephate caused conjunctival irritation, slight corneal opacity and iritis in rabbits. Complete reversibility of the ocular irritation was observed after 14 days (Narcisse et al., 1971a,b).
In a study in which the amount of material instilled into the eye was limited to 0.1 ml, as specified in current testing guidelines, acephate caused only slight conjunctival redness and discharge after 1 h in most rabbits. Eyes that were rinsed for 1 min after treatment also showed slight chemosis. All the eyes were normal by 24 h (Levy et al., 1979b).
(c) Dermal sensitization
Acephate was not sensitizing to the skin of guinea-pigs in a modified Buehler test. It also failed to induce sensitization in a maximization test conducted according to GLP, which consisted of a two-phase induction stage, with intradermal injections of test material (purity, 98.2%) and/or Freund complete adjuvant on day 0, topical application of the test material on day 7, and a topical challenge 2 weeks after the induction phase, on day 21 (Silveira et al., 1984).
Groups of 20 male guinea-pigs were given acephate or dinitrochlorobenzene (positive control) or served as controls for these two groups. The acephate-treated guinea-pigs showed no sensitization after the challenge, whereas all those given dinitrochlorobenzene showed sensitization (Thompson et al., 1986).
Rats
Groups of 10 male Sprague-Dawley rats were given diets containing one of two batches of acephate (SX-992: purity, 93.6% and SX-1102: purity, 98.2%) at a concentration of 0, 10 or 75 ppm, equal to 0.64 and 5.2 mg/kg bw per day of SX-992 and 0.73 and 5.5 mg/kg bw per day of SX-1102, for 4 weeks. No deaths or signs of toxicity related to treatment were observed, there were no treatment-related differences in body weight or food consumption between treated and control animals, and no compound-related macroscopic or microscopic changes were observed. Irrespective of batch, dose-dependent, statistically significant inhibition of brain cholinesterase activity was observed, by 10% and 11% at 10 ppm and 33% and 37% at 75 ppm of SX-992 and SX-1102, respectively. A dose-related but not statistically significant decrease in erythrocyte (by 826%) and plasma cholinesterase activity (by 1225%) was seen in animals exposed to either dose of each test material. The NOAEL was 10 ppm, equal to 0.64 mg/kg bw per day, on the basis of inhibition of brain cholinesterase activity by more than 20% in rats at 75 ppm (Brorby & Beatty, 1987).
Groups of 30 male and 30 female Sprague-Dawley rats were fed diets containing 0, 2, 5, 10 or 150 ppm of acephate (purity, 98.2%) in a study conducted according to GLP. The actual intake of the test material was 0, 0.12, 0.21, 0.58 and 8.9 mg/kg bw per day for males and 0, 0.15, 0.36, 0.76 and 11 mg/kg bw per day for females, respectively. Cholinesterase activity was determined in brain, erythrocytes and plasma from 10 rats of each sex during weeks 4, 9 and 13, and all rats were examined for signs of toxicity. Body weights were measured weekly, and all rats were necropsied at termination.
No deaths or signs of toxicity related to treatment were observed, and there were no compound-related differences in body weights or food consumption between treated and control animals. Dose-dependent inhibition of cholinesterase activity in brain, erythrocytes and plasma was observed in both males and females (Table 2). Brain cholinesterase activity was significantly inhibited in rats at 2 ppm, during week 13 in males (7%) and during weeks 9 and 13 in females (9% each), and was significantly inhibited at all times in rats at 5 ppm (510%), 10 ppm (1016%) and 150 ppm (4453%). The inhibition was similar in males and females. Erythrocyte cholinesterase activity was significantly inhibited (by 3248%) only in rats at 150 ppm, in males during weeks 4 and 9 and in females during weeks 9 and 13. Plasma cholinesterase activity was significantly inhibited (by 43%) only in females at 150 ppm and only during week 13. The NOAEL was 10 ppm, equal to 0.58 mg/kg bw per day, on the basis of inhibition of brain and erythrocyte cholinesterase activity by more than 20% at 150 ppm (Brorby et al., 1987).
Table 2. Per cent inhibition of erythrocyte and brain cholinesterase activity in rats
given diets containing acephate for 13 weeks
|
Sex |
Dietary concentration (ppm) |
Erythrocytes |
Braina |
|
Males |
2 ** |
+5** |
7** |
|
5 ** |
+13*** |
10** |
|
|
10 ** |
+1** |
16** |
|
|
150 ** |
22** |
52** |
|
|
Females |
2 ** |
3** |
9** |
|
5 ** |
9* |
10** |
|
|
10 ** |
10** |
14** |
|
|
150 ** |
44** |
53** |
From Brorby et al. (1987)
a Per mg protein
Significantly lower than controls: * p < 0.05; ** p < 0.01
Groups of male and female rats Fischer 344 rats were exposed to acephate in an aerosol at a concentration of 0, 1, 10 or 100 mg/m3 (purity unknown for days 110; > 99% for days 1321) for 6 h per day, 5 days per week for 4 weeks, in a study that conformed to GLP. The test material was generated as a respirable aqueous aerosol as solutions of 2%, 5% and 15%, corresponding to mean analytical concentrations of 0, 1, 11 and 96 mg/m3. Analysis of particle size distribution showed a mass median aerodynamic diameter of 1.63.6 ΅m. After exposure, 10 animals of each sex from the control and high-dose groups were allowed to recover for 4 weeks.
All animals survived the duration of the study, and no treatment-related changes were noted in food consumption, haematological parameters, gross appearance post mortem or organ weights. Males and females rats at the highest concentration showed tremors and increased secretory responses. Ophthalmic evaluations revealed the formation of miosis in female rats at this concentration, and significant decreases in body-weight gain were observed. Histopathological examination of animals at 96 mg/m3 revealed an exudate in the lumen, suppurative inflammation, individual cell necrosis and regenerative epithelium of the medium and posterior sections of the nasal turbinate. Some resolution of these changes was seen after the 4-week recovery period, but the nasal turbinates had not yet regained normal morphology.
Brain cholinesterase activity was significantly decreased in all treated animals at all times, except for males at the lowest concentration on days 2930 (range, 2936% of control values for animals at 100 mg/m3, 6268% of control values for those at 10 mg/m3 and 6293% of control values for rats at 1 mg/m3; 8384% of control values for rats at 100 mg/m3 after the 4-week recovery period). Plasma and erythrocyte cholinesterase activities were significantly inhibited in males and females at the two higher concentrations during the treatment phase: plasma, 6290% of control values for rats at 10 mg/m3, 2968% of control values for those at 100 mg/m3; erythrocytes, 8085% of control values for those at 10 mg/m3 and 2733% for those at 100 mg/m3. Erythrocyte cholinesterase activity was inhibited in males and females at 1 mg/m3 only on day 16 (8889% of control levels). During the recovery phase, plasma cholinesterase activity was significantly inhibited in males at 100 mg/m3 only on day 44 (89% of control levels); erythrocyte cholinesterase activity was inhibited in males and females at 100 mg/m3 (8284% of control levels) on day 44; neither plasma nor erythrocyte cholinesterase activity was still inhibited on day 59. The NOAEL was < 1 mg/m3, on the basis of dose-dependent, statistically significant inhibition of brain cholinesterase activity at this concentration, the lowest tested. The LOAEL is equivalent to an oral intake of 0.28 mg/kg bw per day, on the basis of the assumption of a respiration rate of 45 l/kg and a default respiratory absorption of 100% (Terrill & Murphy, 1987).
In another 4-week study by whole-body exposure, three groups of 10 male and 10 female Fischer 344 rats were exposed to an aerosol containing acephate (purity, > 99%) at a concentration of 0, 0.2 or 0.5 mg/m3 for 6 h/day, 5 days per week, in a study that conformed to GLP. The test material was generated as a respirable aqueous aerosol in a solution of 2%. The corresponding mean analytical concentrations were 0, 0.19 and 0.51 mg/m3. Analysis of particle size distribution showed a mass median aerodynamic diameter of 2.43.6 ΅m.
All animals survived for the duration of the study. Mean body weights and mean body-weight gain were similar in treated animals and controls, and no treatment-related findings were noted in food consumption, haematological, clinical chemical or ophthalmic parameters, gross appearance post mortem, organ weights or histopathological end-points. There was no evidence of inhibition of plasma, erythrocyte or brain cholinesterase activity in the treated animals. The NOAEL was 0.51 mg/m3, the highest concentration tested, which is equivalent to an oral intake of 0.14 mg/kg bw per day, on the basis of a respiration rate of 45 l/kg and a default respiratory absorption of 100% (Terrill & Murphy, 1988).
In a 4-week study, groups of 10 male and 10 female Sprague-Dawley CD rats were exposed nose only to aerosols containing acephate at a concentration of 0, 1, 3 or 5 mg/m3 for 6 h/day, 5 days per week, for 4 weeks for a total of 20 exposures. The test material (purity, 98.8%) was generated as a respirable aqueous aerosol in solutions of 0.01%, 0.06% and 0.1%, corresponding to mean analytical concentrations of 0, 1.1, 3.1 and 5.6 mg/m3. Analysis of particle size distribution showed an average mass median aerodynamic diameter of 1.1 ΅m and a geometric standard deviation of 1.7.
There were no treatment-related deaths during the study. Laboured breathing was seen in some of the animals at the highest concentration during the last week of exposure. Body weights and feed consumption were comparable in exposed animals and controls given air. Cholinesterase activity in plasma was significantly decreased in male rats after the first exposure to 3.1 or 5.6 mg/m3 (14% of control values each) and was significantly decreased in male rats after the fifth exposure to all concentrations (12%, 17% and 18% of control values, respectively). A similar effect on plasma cholinesterase activity was not seen in male rats after the twentieth exposure or in female rats at any interval during the study. Cholinesterase activity in erythrocytes was significantly decreased in female rats after the fifth exposure to 3.1 and 5.6 mg/m3 (21% and 30% of control values, respectively). A similar effect was not seen in female rats after the first or twentieth exposure or in male rats at any interval during the study. Brain cholinesterase activity was significantly decreased in male rats 1 day after the last exposure to 3.1 and 5.6 mg/m3 (9.9% and 14% of control values, respectively) and in female rats 1 day after the last exposure to 5.6 mg/m3 (13% of control). There were no differences in organ weights or pathological findings that were considered of toxicological significance. The NOAEL was 1.1 mg/m3, on the basis of dose-dependent, statistically significant inhibition of brain cholinesterase activity at 3.1 mg/m3. The NOAEL is equivalent to an oral intake of 0.28 mg/kg bw per day, on the assumption of a respiration rate of 45 l/kg and a default respiratory absorption of 100% (Hoffman, 2000).
Rabbits
Groups of eight male and eight female albino New Zealand white rabbits received a 75% acephate formulation on intact or abraded skin in a study that did not comply with GLP. An aqueous solution of 1 g/ml was applied daily, 6 h/day, 5 days per week, for 3 weeks at a dose of 0, 0.5, 1 or 2 g/kg bw per day. Two animals at the highest dose died, and parasympathomimetic signs of poisoning and weight loss were observed at the two higher doses. No differences in growth or body weight were observed at 0.5 g/kg bw per day. The animals at the two highest doses showed substantial changes in haematological parameters, including reduced erythrocyte count, haemoglobin concentration, erythrocyte volume fraction and blood urea nitrogen. Male rabbits at the lowest dose showed reduced lactate dehydrogenase and aspartate aminotransferase activity. Erythrocyte cholinesterase activity was depressed to about 50% in both sexes at all doses, whereas plasma cholinesterase activity was not affected. Gross examination showed increased organ:body weight ratios for the liver, spleen, kidney and brain in animals at the highest dose. No effects were found on microscopic examination. Slightly deficient erythroid elements were noted in two animals at 0.5 g/kg bw per day and in one animal at 2 g/kg bw per day. Significant inhibition of erythrocyte cholinesterase activity was observed at the lowest dose. Brain cholinesterase was not determined. The NOAEL was < 0.5 g/kg bw per day, on the basis of dose-dependent, statistically significant inhibition of erythrocyte cholinesterase activity at 0.5 g/kg bw per day (Rittenhouse et al., 1972).
Dogs
In a range-finding study conducted according to GLP, groups of four female beagle dogs received diets containing acephate (purity, 99.9%) at a concentration of 0, 8, 20, 250 or 500 ppm (increased to 800 ppm on day 9) for 4 weeks. There were no deaths and no clinical signs that were considered to be related to administration of the test material. All groups showed a steady increase in body-weight gain. Ophthalmology, clinical pathology, gross necropsy and organ weighings showed no treatment-related findings. Erythrocyte cholinesterase activity was most indicative of treatment-related change: in comparison with the mean of the three pre-treatment values, statistically significant inhibition was seen in animals at 250 and 500 ppm (by 49% and 81%, respectively, after 27 days), whereas there was no significant inhibition at 8 or 20 ppm. Plasma cholinesterase activity also showed statistically significant inhibition in animals at 250 and 500 ppm (by 16% and 22%, respectively, after 27 days) but no significant effect at 8 or 20 ppm. Brain cholinesterase activity was significantly lower than in controls only in animals at the highest concentration (by 36%). The NOAEL was 20 ppm, equivalent to 0.5 mg/kg bw per day, on the basis of cholinesterase inhibition in plasma and erythrocytes at 250 ppm (Dalgard, 1984).
Groups of five male and five female beagle dogs were assigned to one of four dietary concentrations of acephate (purity, 99.9%) on the basis of erythrocyte cholinesterase activity. The animals received diets containing a concentration of 0, 10, 120 or 800 ppm (equal to 0, 0.27, 3.1 and 20 mg/kg bw per day) for 52 weeks. One female at 120 ppm died unexpectedly during week 49; the cause of death was not determined. No treatment-related clinical signs, no alterations in body weight or food consumption, no changes in ophthalmic parameters and no findings at gross necropsy were found.
The primary treatment-related effect observed was inhibition of cholinesterase activity in brain and erythrocytes (Table 3). Relative to the control values, brain cholinesterase activity was significantly inhibited in all groups of males (by 17%, 53% and 66%, respectively) and in females at 120 and 800 ppm (by 49% and 66%, respectively); brain cholinesterase activity was not significantly inhibited (by 11%) at 10 ppm. Erythrocyte cholinesterase activity was slightly increased in dogs of each sex at 10 ppm and significantly inhibited in those at 120 ppm (4255%) and 800 ppm (7687%). Despite severe inhibition of brain cholinesterase activity in animals of each sex at the two higher concentrations, the clinical signs usually associated with cholinesterase inhibition were not observed.
Table 3. Per cent inhibition of erythrocyte and brain cholinesterase activity in dogs
given acephate in the diet for 53 weeks
|
Sex |
Dietary concentration (ppm) |
Cholinesterase activity |
|
|
Eythrocytes |
Brain |
||
|
Males |
10 ** |
+8* |
17* |
|
120 ** |
43* |
53* |
|
|
800 ** |
86* |
68* |
|
|
Females |
10 ** |
+16* |
11* |
|
120 ** |
46* |
49* |
|
|
800 ** |
84* |
66* |
|
From Dalgard (1991)
* Significant lower than controls (p < 0.05)
Other treatment-related, statistically significant effects were decreased erythrocyte count (1326%), haemoglobin concentration (1421%) and erythrocyte volume fraction (6-9%), all in males at 800 ppm; increased activated partial thromboplastin time (3496%) in males at 800 ppm; increased absolute weight of the liver in males (29%) and females (17%) at 800 ppm; and perivascular infiltration and pigment in the livers (reticuloendothelial cells) of one male at 120 ppm and most males and females at 800 ppm. The only effect seen at 10 ppm was statistically significant inhibition of brain cholinesterase activity. The NOAEL was 10 ppm, equal to 0.27 mg/kg bw per day, in male dogs, on the basis of inhibition of brain and erythrocyte cholinesterase activity by more than 20% at 120 ppm (Dalgard, 1991).
Mice
Groups of 75 Charles River CD-1 mice of each sex were fed diets containing acephate (purity, 92.6%) at a nominal concentration of 0, 50, 250 or 1000 ppm for 104 weeks, providing doses of 0, 7, 36 and 150 mg/kg bw per day for males and 0, 8, 42 and 170 mg/kg bw per day for females. The study was conducted according to GLP, although the Food and Drug Administration issued its criteris only after the study was begun.
Female mice fed the diet containing 1000 ppm of acephate had higher incidences of hepatocellular carcinomas and hyperplastic nodules than did the concurrent controls. The incidences of hepatocellular carcinoma were 1.3%, 1.3%, 0 and 16% in females and 5.3%, 2.7%, 4% and 4% in males in the controls and animals at 50, 250 and 1000 ppm, respectively. All these tumours were observed at terminal sacrifice. The incidences of hyperplastic nodules were 2.7%, 1.3%, 0 and 20% in females and 13%, 9.3%, 5.3% and 17% in males in controls and at the three doses, respectively. Most of the nodules (14% in females and 12% in males at 1000 ppm) were observed at terminal sacrifice. The incidence of hepatocellular carcinomas in controls in 22 other studies in the same laboratory involving 1630 CD-1 mice ranged from 0 to 6%.
Other treatment-related findings were liver lesions (hypertrophy of hepatocytes, karyomegaly and intracellular inclusion bodies) in males and females at the two higher doses; lung lesions (dark pigmented alveolar macrophages, eosinophilic foreign bodies and alveolar hyalinosis) and lesions in the nasal cavity (acute rhinitis) in males and females at 250 and 1000 ppm; significantly decreased body-weight gains in males (811%) and females (614%) at 250 ppm during weeks 52104 and in males (1530%) and females (1429%) at 1000 ppm during weeks 13104, when compared with controls; and significant changes in organ weights in males (smaller livers and kidneys) and females (larger livers and smaller kidneys, brains and ovaries) at 1000 ppm when compared with controls. The NOAEL was 50 ppm, equal to 7 mg/kg bw per day, on the basis of morphological changes in the liver, lung and nasal cavity at 250 ppm (Spicer et al., 1982).
Rats
Groups of 75 Sprague-Dawley rats of each sex received diets containing acephate (purity, 92.5%) at a nominal concentration of 0, 5, 50 or 700 ppm, equivalent to 0, 0.25, 2.5 and 35 mg/kg bw per day, for 28 months. The study was conducted according to GLP, but the certifying authority was not stated.
The following treatment-related findings were observed in male rats at 700 ppm: hyperactivity in some during the initial 5 months of the study; increased incidence of aggressive behaviour (31% versus 5% in controls), also during the initial 5 months of the study; decreased body-weight gain (618%) during weeks 8106, when compared with controls; and significantly decreased food efficiency during weeks 18. Aggressive behaviour was also observed in 13% of males at 5 ppm and 13% of those at 50 ppm.
Erythrocyte cholinesterase activity was significantly decreased at all sampling times in males (2167%) and females (2161%) at 700 ppm, whereas the inhibition was 031% in males and 042% in females in animals at 50 ppm and 013% in males and 029% in females at 5 ppm. The inhibition of brain cholinesterase activity relative to control values was 013% at 5 ppm, 3443% at 50 ppm and 6977% at 700 ppm in males and 113%, 3345% and 6683% in females, respectively. Brain cholinesterase activity was significantly decreased in rats at 5 ppm after 7, 12 and 104 weeks in males and after 19 and 104 weeks in females. The inhibition of cholinesterase activity showed a specific rank order, erythrocyte cholinesterase activity being reduced to a lesser extent than that of brain . Thus, after termination at 28 months, the brain and erythrocyte cholinesterase activity in rats at 700 ppm was inhibited by 71% and 57% in males and by 69% and 46% in females, respectively, while the respective cholinesterase activities were inhibited by 50% and 26% in males and by 37% and 21% in females at 50 ppm (Table 4).
Table 4. Per cent inhibition of erythrocyte and brain cholinesterase activity in rats fed diets containing acephate
|
Sex |
Dietary concentration (ppm) |
7 weeks |
12 months |
28 months |
|||
|
Erythrocyte |
Brain |
Erythrocyte |
Brain |
Erythrocyte |
Brain |
||
|
Male |
5 ** |
+6 |
9*** |
22 |
13**** |
+4** |
11** |
|
50 ** |
18 |
21**** |
12 |
41**** |
26** |
40** |
|
|
700 ** |
41 |
77**** |
50** |
70** |
57** |
71** |
|
|
Female |
5 ** |
29 |
13** |
03 |
5** |
4** |
9* |
|
50 ** |
42 |
45**** |
10** |
33** |
21** |
37** |
|
|
700 ** |
58** |
83** |
38** |
70** |
46** |
69** |
|
From Auletta & Hogan (1981)
Significantly lower than controls at *p < 0.05; **p < 0.01
Treated males had a higher incidence of adrenal medullary tumours (phaeochromocytomas) than control males. However, the incidences of 9.7%, 16% and 12% in males at 5, 50 and 700 ppm, respectively, were not dose-related and were within the range for other controls in the same laboratory (0.720%) and for concurrent controls (2.7%). The NOAEL was 5 ppm, equivalent to 0.25 mg/kg bw per day, on the basis of inhibition of brain and erythrocyte cholinesterase activity by more than 20% at 50 ppm (Auletta & Hogan, 1981).
Acephate was investigated in a large number of assays for genotoxicity in vitro and in vivo. These studies were evaluated in 1990, when the Meeting concluded that acephate had genotoxic potential in studies in vitro, but studies in vivo gave negative results for gene mutations and conflicting results for chromosomal aberrations. Six new studies were submitted, which had not been evaluated previously. Table 5 summarizes the results.
A test in vitro for point mutations (bacterial reverse mutation ) gave negative results, as did all tests in vivo for sex-linked recessive lethal mutations, micronuclei in bone marrow, somatic cell mutation in mice and unscheduled DNA synthesis in liver cells. These findings support the conclusion that the weight of evidence indicates that acephate presents little or no genetic hazard to whole animals.
Table 5. Results of studies for genotoxicity with acephate
|
End-point |
Test object |
Concentration/dose |
Purity (%) |
Results |
References |
|
In vitro |
|
|
|
|
|
|
Reverse mutationa |
S. typhimurium TA100 without metabolic activation |
1094 mg/plate |
Not reported |
Mutagenic at all doses |
Machado et al. (1984) |
|
Reverse mutationa |
S. typhimurium TA98, TA100 with and without metabolic activation |
0.1, 0.5, 1, 2.5, 5 mg/plate |
98.5 |
Negative |
Vargas & Bonetti (1993) |
|
In vivo |
|
|
|
|
|
|
Sex-linked recessive lethal mutation |
D. melanogaster, males |
10 ppm |
Not reported |
Negative |
Valencia (1981) |
|
Micronucleus formationb |
Swiss albino male and female mice |
160, 320, 480 mg/kg bw |
95.3 |
Negative |
Vargas & Rodrigues (1993) |
|
Somatic cell mutationb |
T-strain male, C57B1/6 female mice |
50, 200, 600, 800 |
98 ppm |
Negative |
Moore (1986) |
|
Unscheduled DNA synthesisb |
Male CDF Fischer 344 rats |
150, 300, 500 mg/kg bw |
100.6 |
Negative |
Golzio (1999) |
a Test considered not relevant for evaluation of mutagenic potential
b Test performed according to good laboratory practice
(a) In vitro
An assay for reverse mutation was performed with acephate on Salmonella typhimurium TA100 in a study that did not conform to GLP, with no metabolic activation system. The positive control used was sodium azide. Acephate (purity not reported) was applied at a dose of 10, 20 or 94 mg/plate. Increased numbers of revertants were seen at all doses, with an estimated mutagenic activity of 0.001 revertants per nanomole of test material. Although acephate was mutagenic to TA100 at all doses, the study was considered not relevant for evaluating the mutagenic potential of acephate, as the method used was not standard. An Ames test consists of at least four strains of S. typhimurium (TA98, TA100, TA1535 and TA1538) tested with and without a metabolic activation system (Machado et al., 1984).
In another assay for bacterial reverse mutation , conducted according to GLP, acephate (purity, 98.5%) diluted in dimethyl sulfoxide was combined with cultures of S. typhimurium TA98 and TA100 before incubation and tested with and without metabolic activation at concentrations of 0.1, 0.5, 1, 2.5 and 5 mg/plate. 2-Aminofluorene and sodium azide were used as positive controls for the two strains, respectively; dimethyl sulfoxide was used as the negative control. Acephate was not mutagenic (Vargas & Bonetti, 1993).
(b) In vivo
Drosophila melanogaster males were fed (and also exposed by contact and possibly inhalation) to 15 pesticides, including acephate, at adequate concentrations in a study that did not conform to GLP. The purity of the test material was not reported. The flies were then examined for sex-linked recessive lethal point mutations and chromosomal alterations. Acephate gave negative results in this test, whereas the three reference materials (ethyl methanesulfonate, ethylenimine and trimethyl phosphate) gave positive results (Valencia, 1981).
An assay was performed according to GLP to evaluate the potential of acephate (purity, 98%) to induce somatic cell mutations in mice in vivo. The parental animals chosen were T-strain males and C57B1/6 females, the mating of which results in embryos with melanocyte precursor cells that are heterozygous at five specific loci for coat colour. The presence of spots on the neonates 14 and 28 days after birth is taken as evidence of somatic mutations. A total of 854 pregnant female C57B1/6 mice were assigned to six groups: four were fed diets containing acephate at a concentration of 50, 200, 600 or 800 ppm on days 8.512.5 of gestation; a control group received only powdered diet; and the last group received a single treatment with ethylnitrosourea at 50 mg/kg bw on day 10.5 of gestation and was used as a positive control. The 282 litters contained 2186 live pups, of which 1429 and 1385 were scored for recessive coat spots on days 14 and 28. No increase in the number of recessive coat spots was found in treated groups as compared with the negative control. Several signs of toxicity were evident, which were statistically significant in the groups at 200, 600 and 800 ppm. A significant increase in the number of pups with recessive coat spots was found in the positive control group. Thus, treatment with acephate at doses up to 800 ppm orally did not induce somatic cell mutation in mice (Moore, 1986).
A test for micronucleus formation was performed according to GLP in male and female Swiss albino mice given acephate (purity not stated) diluted in corn oil intraperitoneally at a concentration of 160, 320 or 480 mg/kg bw, corresponding to 25%, 50% and 75% of the LD50 for mice, respectively. Negative controls were treated with corn oil and positive controls with cyclophosphamide at 40 mg/kg bw. Femurs were removed and smears of bone-marrow cells were prepared and stained 24 and 48 h after treatment. No increase in the number of polychromatic or normochromatic erythrocytes containing micronuclei and no alteration in the ratio of polychromatic to normochromatic cells was seen in animals treated with acephate when compared with vehicle controls. A statistically significant increase in polychromatic and normochromatic erythrocytes containing micronuclei was observed in animals treated with cyclophosphamide. Under these conditions, acephate did not induce micronuclei in mice (Vargas & Rodrigues, 1993).
An assay was conducted according to GLP in male CDF Fischer 344 rats to evaluate the potential of acephate (purity, 100.6%) to induce reparable DNA damage in liver cells. Acephate was administered orally at a single standard volume of 10 ml/kg bw, providing a dose of 150, 300 or 500 mg/kg bw. Negative controls were given water, the vehicle, and positive controls were given either methylmethane sulfonate at 300 mg/kg bw or 2-acethylaminofluorene at 75 mg/kg bw. Animals were killed 2 and 16 h after dosing, and hepatocytes were isolated, cultured and then treated with 3H-thymidine to assess unscheduled DNA synthesis.
As no significant cytotoxic effects were observed, only cells from the groups at 300 and 500 mg/kg bw were examined. The treated groups showed no statistically significant increase in thymidine incorporation over that in the control groups at either sampling time, whereas the positive control groups showed statistically significant increases. Under the experimental conditions, acephate did not induce unscheduled DNA synthesis in liver cells (Golzio, 1999).
Rats
Groups of 12 male and 24 female Crl:COBS CD(SD)BR rats received diets containing acephate (purity not reported) at a concentration of 0, 50, 150 or 500 ppm through two generations. The study was conducted according to GLP. Administration was initiated at least 100 days before mating of the F0 generation and 120 days before mating of the F1 generation. Each generation produced only one litter. Reduced body-weight gain was observed in animals at 500 ppm, in males in both generations and in females only during gestation and lactation. Increased food consumption was found in both generations and sexes at 150 ppm and in both sexes of the F0 generation and females of the F1 generation at 500 ppm. No deaths occurred that were related to treatment. Reductions in mating performances and numbers of litters born and litters weaned were found in both generations at 150 and 500 ppm and in the F0 generation at 150 ppm. Reduced litter size was reported at birth and at weaning for both generations at 150 and 500 ppm. The NOAEL was 50 ppm, equivalent to 3.3 mg/kg bw per day, on the basis of parental and reproductive effects at 150 ppm (Palmer et al., 1983).
In a three-generation study of reproductive toxicity conducted according to GLP, groups of 30 male and 30 female Charles River rats were fed diets containing acephate (purity, 98.7%) at a nominal concentration of 0, 25, 50 or 500 ppm, equivalent to 1.7, 3.3 and 33 mg/kg bw per day, for 75 days before they were bred to produce F1a, F1b, F2a and F2b litters. Because of low fertility in all groups, including the controls, a third generation (F3a) was produced from the F2b litters. Treatment-related effects were observed only at 500 ppm and included decreased body weight and/or weight gain for adult males (in each generation) and females (in some generations) and for pups in the F2a and F3a generations; increased food consumption for males and females during the pre-mating period and decreased food consumption for females during gestation and lactation; clinical signs involving an increased incidence of alopecia in the first generation and increased incidences of soft or liquid stools in the second and third generations in males; decreased mating performance; decreased mean litter size (by 2530%); and significant decreases in pup survival. The NOAEL was 50 ppm, equivalent to 3.3 mg/kg bw per day, on the basis of parental and reproductive effects at 500 ppm (Hoberman, 1987).
Rats
In a study of developmental toxicity that complied with GLP, virgin female Crl: CD®(SD)BR rats received acephate (purity, 99.7%) in deionized water at a dose of 0, 5, 20 or 75 mg/kg bw per day by gavage on days 615 of gestation and were killed on day 20 of gestation. Animals at the two higher doses had decreased body weights and body-weight gains, and those at the highest dose had a statistically significant increase in the number with tremors and decreased motor activity. Developmental toxicity was seen at the highest dose as slight decreases in the mean numbers of ossified caudal vertebrae, sternal centres, metacarpals and fore-limb and hind-limb phalanges. The NOAELs were 5 mg/kg bw per day for maternal toxicity and 20 mg /kg bw per day for developmental toxicity (Lochry, 1989).
Rabbits
In a study of developmental toxicity that complied with GLP, groups of 16 Dutch belted rabbits were artificially inseminated and then given an injection of chorionic gonadotropin to induce ovulation. They then received acephate (purity, 92.8%) at a dose of 0, 1, 3 or 10 mg/kg bw per day by gavage on days 627 of gestation as an aqueous solution at a constant volume of 1 ml/kg bw.
Two rabbits at 10 mg/kg bw per day aborted and were killed and discarded without examination, one on day 25 and the other on day 27 of gestation. A slight increase in nasal discharge, which was possibly treatment-related, was observed in does at 3 and 10 mg/kg bw per day when compared with controls. The fetal sex distribution was changed at the highest dose, from 49.5:50.5 males:females in controls to 63.6:36.4 at 10 mg/kg bw per day. There were no significant differences between control and treated groups with respect to early or late resorptions, post-implantation loss, mean number of corpora lutea or mean fetal weight. Anasarca was detected in 3/66 fetuses in one litter, and a dome-shaped head was found in an additional fetus in another litter at 10 mg/kg bw per day. Neither of these abnormalities was observed in concurrent controls, in groups at lower dosess or in a total of 741 fetuses from 118 litters in other control groups in the same laboratory. No significant increase in the frequency of any other type of malformation was observed, although a non-dose-related increase in the incidence of total (soft tissue and skeletal) malformations was seen in all treated groups as compared with concurrent controls. No similar increase was evident in comparison with other controls in the same laboratory. The NOAEL was 3 mg/kg bw per day, on the basis of maternal and developmental toxicity at 10 mg/kg bw per day (Rodwell, 1980).
Rats
In a study that conformed to GLP; acephate (purity, 99.0%) admixed with deionized water was administered orally at a single dose of 5, 25, 125 or 500 mg/kg bw to groups of two male and two female Sprague-Dawley Crl:CD® BR rats. A concurrent control group received deionized water on the same regimen. In a second phase, groups of five female rats received a dose of 0.5, 2.5 or 5 mg/kg bw, and an additional five female rats served as the control group.
In phase I, all animals survived to scheduled death 2.5 h after dosing. The main treatment-related findings were tremors of the mouth, fore-limbs, hind-limbs and/or whole body and altered gait in all males and females at 125 and 500 mg/kg bw. In general, these clinical signs were initially observed 12 h after dosing and persisted to the end of the study. Tremors of the mouth were seen only in one male at 25 mg/kg bw 2.5 h after dosing. Other apparently treatment-related clinical signs observed in more than one group were salivation in rats at 125 and 500 mg/kg bw and twitching of both ears in rats at 25, 125 and 500 mg/kg bw; these findings were made in limited numbers of animals 1, 2 and 2.5 h after dosing. Other treatment-related clinical signs were seen only at 500 mg/kg bw and included hypothermia in all animals 2.5 h after dosing and hypoactivity in a single female and a single male 2 and 2.5 h after dosing, respectively. No treatment-related clinical signs were observed at 5 mg/kg bw.
The mean activities of plasma, true erythrocyte and regional brain cholinesterase were reduced in a generally dose-related manner in males and females at 5, 25, 125 and 500 mg/kg bw. The mean values in plasma were reduced by 30%, 5556%, 7577% and 84% for males and by 2526%, 4144%, 6970% and 91% for females at the four doses, respectively; and the mean values for true erythrocyte cholinesterase activity were lower than those of the control group by 1525%, 2932%, 4550% and 4955% for males and by 1314%, 3141%, 4652% and 5058% for females, respectively. Within each group, the decreases in cholinesterase activity in individual brain regions were similar. The mean values in the brain regions examined were lower than in the controls by 1832%, 5059%, 6677% and 7581% for males and by 2531%, 5566%, 7179% and 7985% for females at 5, 25, 125 and 500 mg/kg bw, respectively.
In phase II, all animals survived to scheduled death 2.5 h after dosing, and no significant clinical signs were observed. No treatment-related effects on brain weights were found. The mean activity of cholinesterase in plasma was reduced by 14% at 2.5 mg/kg bw and 10% at 5 mg/kg bw. At 5 mg/kg bw, the true erythrocyte cholinesterase activity was 19% lower than that in controls. The mean values in the brain regions examined were lower than in the control group by 1322% at 2.5 mg/kg bw and 3034% at 5 mg/kg bw. No statistical evaluation was performed in phase II of the study. The decreases in cholinesterase activity did not appear to be associated with any particular brain region. No treatment-related differences in mean plasma, true erythrocyte or regional brain cholinesterase activity were observed in females at 0.5 mg/kg bw. The NOAEL was 2.5 mg/kg bw, on the basis of a toxicologically relevant reduction in cholinesterase activity in all the selected brain regions in males and females at 5 mg/kg bw (Nemec, 1995).
Acephate (purity, 99.0%) was administered to groups of 30 male and 30 female Sprague-Dawley Crl:CD® BR rats by gavage at a single dose of 0, 10, 100 or 500 mg/kg bw. The control group was given deionized water. The study conformed to GLP. The animals were observed daily for neurotoxic clinical signs and for neurobehavioural, physiological and cholinesterase activity. A battery of functional observational tests was administered on the day of dosing and on days 7 and 14 after dosing. All animals survived to scheduled death. Peak effects were seen approximately 2.5 h after dosing. No effects were observed in the control group.
The treatment-related findings observed in males and females at 100 and 500 mg/kg bw were: whole body and/or limb tremors; ataxia, weakness in hind-limbs and repetitive movements of the mouth and jaws; alterations in posture, gait and mobility; low arousal and no approach or touch responses; decreased rearing, motor activities, rotarod performance and body temperature; increased righting reflex and time to first step; lachrymation, salivation and soiled fur; decreased body-weight gain in males only (by 4145% at 500 mg/kg bw and 15% at 100 mg/kg bw); and inhibition of cholinesterase activity in plasma (8688%), erythrocytes (5355%) and six brain regions (8388%) at both doses. The findings observed only at 500 mg/kg bw were: increased time to catalepsy and clonic convulsions; absence of pinch, startle, pupil and olfactory responses; decreased hind-limb foot splay and fore-limb and hind-limb grip strength; chromodacryorrhoea; and clear or coloured (tan, red, brown and/or yellow) staining or matting on various body surfaces. The treatment-related findings at 10 mg/kg bw were: whole body tremors (single occurrences) in one male and one female; inhibition of cholinesterase activity in plasma (3134%), erythrocytes (1819%) and brain regions (3748%); and decreased rotarod performance in males on day 0 (when compared with that of controls).
The toxic signs occurred within 0.52.5 h of dosing and persisted for 48 h or longer, but were not observed the next day. Plasma and erythrocyte cholinesterase activities were inhibited significantly only on the day of dosing, whereas brain cholinesterase activity was inhibited during dosing (all regions), on day 7 after dosing (all regions except olfactory) and on day 14 (midbrain only). Other parameters examined in this study were not affected. The NOAEL was < 10 mg/kg bw, on the basis of dose-dependent, statistically significant inhibition of brain cholinesterase activity and acute neurotoxic effects (e.g. whole body tremors, decreased rotarod performance) and decreased erythrocyte cholinesterase activity at doses >10 mg/kg bw (Nemec, 1996).
Rats
In a study conducted according to GLP, groups of 30 Sprague-Dawley Crl:CD® BR rats of each sex received diets containing acephate (purity, 99.0%) in acetone at a concentration of 0, 5, 50 or 700 ppm, equal to 0, 0.33, 3.3 and 49 mg/kg bw per day for males and 0, 0.41, 4 and 58 mg/kg bw per day for females, for 13 weeks. The control group was fed the basal diet.
All animals survived to scheduled death, and no treatment-related clinical signs were observed. A transient reduction in mean body-weight gain was noted in animals at 700 ppm after the first week of dosing, and decreased mean body weights were seen in these animals during weeks 13. The mean body-weight gains of rats at 5 and 50 ppm were unaffected by treatment. No treatment-related effects on mean food consumption were seen at any concentration, but increased food consumption was found when measured as grams per kilogram body weight per day in animals at 5 and 700 ppm during weeks 12 through 78.
No treatment-related effects were seen in a battery of functional observational tests. Animals at 700 ppm showed increased grooming and increased rearing at week 3, and males showed decreased rotarod time at week 7; however, the means were similar to those before treatment. At week 7, the mean ambulatory and total motor activity counts were significantly decreased by 21% and 24%, respectively, in comparison with control values in females at 700 ppm. In week 12, the mean ambulatory activity count during the last of four 10-min sessions was also decreased in females at 700 ppm. No adverse effects were found on mean ambulatory or total motor activity counts in rats at 5 or 50 ppm or males at 700 ppm at weeks 3, 7 and 12.
Dose-related, statistically significant reductions in mean plasma cholinesterase activity were observed in animals at 50 and 700 ppm in week 3, and the mean values in rats at 700 ppm were still statistically significantly reduced in weeks 7 and 13. The mean erythrocyte activity was statistically significantly reduced in animals at 700 ppm in weeks 3, 7 and 13, except in females during week 13. Regional brain cholinesterase activity was statistically significantly reduced in rats at 50 and 700 ppm (Table 6). Dose-related, statistically significant decreases in brain cholinesterase activity were found in all six brain regions in weeks 3, 7 and 13, except for the brainstem in females at 50 ppm in week 3. The only effects seen at 5 ppm were inhibition by 228% of brain cholinesterase activity, which was significant in at least one sex for all brain regions; the inhibition was > 20% at only one of three measurements in the hippocampus and in the olfactory region of females rats only. Therefore, this dose was not considered to have an adverse effect. No effects on brain weight were observed at any concentration, and no remarkable differences in brain weights or dimensions were observed in animals perfused at scheduled terminal sacrifice. No treatment-related neuropathological lesions were observed on microscopic examination of perfused tissues. The NOAEL was 5 ppm, equal to 0.33 mg/kg bw per day, on the basis of inhibition of cholinesterase activity in all brain regions by more than 20% at all times in rats at 50 ppm (Nemec, 1997).
Table 6. Per cent inhibition of cholinesterase activity in erythrocytes and selected brain
regions in rats given acephate in the diet
|
Substrate |
Sex |
Dietary concentration (ppm) |
Length of treatment (weeks) |
||
|
3 |
7 |
13 |
|||
|
Erythrocytes |
Male |
5** |
18** |
+1** |
1** |
|
50** |
13** |
08** |
11** |
||
|
700** |
46** |
37** |
37** |
||
|
Female |
5** |
08** |
+4** |
+10**** |
|
|
50** |
26** |
08** |
9** |
||
|
700** |
42** |
43** |
25** |
||
|
Brainstem |
Male |
5 ** |
15** |
*9** |
10** |
|
50** |
31** |
34** |
27** |
||
|
700** |
68** |
71** |
68** |
||
|
Female |
5** |
07** |
08** |
04** |
|
|
50** |
18** |
36** |
30** |
||
|
700** |
68** |
72** |
69** |
||
|
Hippocampus |
Male |
5** |
14** |
*7** |
16** |
|
50** |
38** |
47** |
29** |
||
|
700** |
79** |
82** |
81** |
||
|
Female |
5** |
6** |
08** |
28** |
|
|
50** |
48** |
50** |
55** |
||
|
700** |
78** |
82** |
82** |
||
|
Olfactory |
Male |
5** |
05** |
012** |
05** |
|
50** |
45** |
40** |
39** |
||
|
700** |
77** |
82** |
79** |
||
|
Female |
5** |
013*** |
24** |
18** |
|
|
50** |
24** |
54** |
45** |
||
|
700** |
79** |
82** |
79** |
||
|
Cortex |
Male |
5** |
016** |
010** |
018** |
|
50** |
44** |
46** |
49** |
||
|
700** |
80** |
82** |
80** |
||
|
Female |
5** |
07** |
2** |
14** |
|
|
50** |
39** |
44** |
51** |
||
|
700** |
81** |
81** |
81** |
||
From Nemec (1997)
Significantly lower than controls at *p < 0.05;**p < 0.01
Chickens
After determination of an acute oral LD50 of acephate at 300420 mg/kg bw in a preliminary test, 24 white Leghorn hens (about 7 months old) were intubated with a single dose of acephate (purity, 98.9%) at 375 mg/kg bw, followed immediately by atropine sulfate at 5 ml/kg bw intramuscularly. Twelve hens given distilled water were used as negative controls, and 12 positive controls were treated orally with tri-ortho-tolylphosphate at 500 mg/kg bw. Three treated hens were killed in moribund condition within 5 days of dosing. Symptoms of acute poisoning, such as depressed spontaneous activity, were seen up to 11 days, but no delayed neurotoxic symptoms were noted within the 21-day observation period. Histopathological evaluation of the sciatic nerve and several sections of the spinal cord revealed no morphological changes suggestive of delayed neurotoxic activity, whereas the positive controls showed signs and histological changes in the nervous tissue typical of delayed neurotoxicity. Thus, no signs of delayed neurotoxicity were observed in hens treated with acephate at an oral dose equivalent to the median lethal dose (Hayashizaki et al., 1979).
In a study conducted according to GLP, acephate (purity, 98.8%) was administered to 16 white Leghorn hens by cannula into the crop or proventriculus at a dose of 760 mg/kg bw, which was the LD50 determined in a previous study. Eight hens given distilled water only were used as controls, and six hens received tri-ortho-tolylphosphate at 600 mg/kg bw as a positive control. All birds were fasted for at least 15 h before dosing. After 21 days, the birds in the treated and negative control groups were dosed again and observed for a further 21 days. The birds given tri-ortho-tolylphosphate were killed at 21 days. Locomotor impairment was assessed twice weekly. Hens were dropped from a height of approximately 1 m in order to evaluate their landing ability, and they were then made to walk about 8 m and to hop onto a surface 10 cm above the floor, followed by a further hop of the same height. The birds were scored on a points system for ataxia.
There were no deaths and no abnormalities in mobility in the negative controls. The positive control birds had diarrhoea during the first day, and three birds showed ataxia at day 14. Neurotoxicity was more obvious from days 1421, when two hens showed loss of coordination, three more showed this change together with limb weakness, and one was severely incapacitiated. Four treated hens died 47 days after dosing, and five died 34 days after the second dose. Symptoms of acute poisoning (diarrhoea and relative immobility) were noted but at no time was there evidence of neurotoxicity, as assessed clinically. Histopathological assessment of the brain, sciatic nerve and upper cervical, mid-thoracic and lumbosacral sections of the spinal cord from acephate-treated birds showed no significant changes, whereas the positive controls showed clinical and pathological changes typical of delayed neurotoxicity. Thus, no signs of delayed neuropathy were found in hens given acephate at an oral dose equivalent to the median lethal dose (Beavers & Jaber, 1985).
Groups of six white Leghorn hens received acephate (purity, 98%) by gavage at a dose of 0, 5, 25, 100, 200 or 700 mg/kg bw, and groups of three hens received diisopropyl fluorophosphate at 50, 100 or 200 ΅g/kg bw. All were killed 24 h later, and brain homogenates were assayed for cholinesterase activity as a measure of acute toxicity, for neuropathy target esterase to indicate the potential to cause organophosphate-induced delayed neuropathy and for residues of acephate and its metabolite methamidophos. A range-finding study confirmed the LD50 of approximately 800 mg/kg bw. Regression analysis indicated an ID50 (a dose that inhibits 50% of activity) for inhibition of cholinesterase activity of 10 mg/kg bw and an extrapolated ID50 for inhibition of neuropathy target esterase of 1300 mg/kg, almost twice the LD50. In contrast, the ID50 values for diisopropyl fluorophosphate were similar for cholinesterase (150 ΅g/kg) and neuropathy target esterase (130 ΅g/kg). The concentration of methamidophos in brain was 1016% that of total acephate plus methamidophos; the lower the dose of acephate, the higher the relative percentage of methamidophos (Wilson et al., 1990).
Mice
Adult male ICR (CD-1) mice were treated by gavage with acephate (purity, 98.0%) at a dose of 0, 7, 14 or 28 mg/kg bw per day for 4 weeks before mating with untreated females. Brain and muscle cholinesterase activity was significantly inhibited only at the highest dose, by 68% and 79%, respectively. Acephate decreased the number of implantations and live fetuses and increased the number of early resorptions in females mated with males at highest dose. Testis weights, fertility index, spermatozoa count and spermatid count were significantly decreased at the two higher doses. Histological changes in the testis, including exfoliation of the germinal cells into the lumen with ill-defined spermatogenesis, were observed at these doses. The NOAEL was 7 mg/kg bw per day, on the basis of significantly decreased testis weight, fertility index and spermatozoa and spermatid counts as well as histological changes in the testis (Farag et al., 2000)
Cholinesterase activity was assessed in vitro after incubation of preparations of brain and erythrocytes derived from monkeys (species not stated) and Sprague-Dawley rats with various concentrations of acephate (purity, 99.3%), methamidophos (purity, 99.6%) and technical-grade acephate (purity, 93.6%). Acephate with 1 or 3% methamidophos was also incubated with rat brain and erythrocyte cholinesterase. Inhibition was measured as the per cent decrease in cholinesterase activity relative to control activity.
Acephate was a weak cholinesterase inhibitor but inhibited brain cholinesterase nine times more it did than erythrocyte cholinesterase. Technical-grade acephate was 218 times more potent than acephate and had comparable inhibitory activity on brain and erythrocyte cholinesterase. Methamidophos was about 1000 times more potent than acephate in inhibiting either erythrocyte or brain cholinesterase, and it was approximately five times more potent in inhibiting erythrocyte than brain cholinesterase in rat tissues. This is the reverse of the pattern with acephate. Methamidophos was 10 times less potent in inhibiting erythrocyte cholinesterase in monkeys than rats. The relative inhibitory potencies of acephate, technical-grade acephate and methamidophos were similar in rats and monkeys (Wong et al., 1979).
The kinetics of recovery was followed after inhibition of plasma, erythrocyte and brain cholinesterase activity in groups of male Sprague-Dawley rats fed a diet containing 75 ppm of acephate. The study was conducted in two parts: in the first, groups of five rats were treated for 20 days and cholinesterase was determined on days 6, 11, 15 and 20; in the second, groups of 10 rats were treated for 7 days, followed by a 6-week recovery period with determinations of cholinesterase activity after 1, 2, 4 and 6 weeks.
Plasma and erythrocyte cholinesterase activity was slightly inhibited (by 818%) during the 20 days of dietary feeding, but normal cholinesterase activity was restored by day 7 of the recovery period. Brain cholinesterase activity was moderately inhibited (by 3042%) throughout the 20 days of treatment. Rapid, but partial, recovery of normal activity was observed within 7 days and complete recovery within 1428 days. Other parameters evaluated (mortality, body weight, food consumption, physical observations) indicated no effect (Daley & Hogan, 1980).
Cholinesterase from plasma, erythrocytes and brain of CD:SD Crl rats, cynomolgus monkeys and humans was incubated with acephate (purity, 98.7%) at concentrations varying from < 1 Χ 104 to 5 Χ 102 mol/l. Acephate was a weak inhibitor of cholinesterase from all three species, the median inhibitory concentration (IC50) being between 1 Χ 103 and 5 Χ 103 mol/l for all enzyme sources. Acephate was approximately 100 000 times less effective than eserine (positive control) as an inhibitor of cholinesterase and 10 000 times less effective as an inhibitor of plasma cholinesterase. Acephate was more effective against rat brain and erythrocyte cholinesterase (1.6 Χ 103 and 1.3 Χ 103 mol/l) than against cynomolgus monkey (3.4 Χ 103 and 2.7 Χ 103 mol/l) and human (5.4 Χ 103 and 2.7 Χ 103 mol/l) brain and erythrocyte cholinesterase, respectively. For plasma cholinesterase activity, the IC50 value was lower for human (1.8 Χ 103 mol/l) and cynomolgus monkey (2.3 Χ 103 mol/l) than for rat enzyme (4.5 Χ 103 mol/l). The authors concluded that the species sensitivity for inhibition of brain cholinesterase by acephate was rat > cynomolgus monkey > human, that for inhibition of erythrocyte cholinesterase was rat > cynomolgus monkey = human, while that for inhibition of plasma cholinesterase was the reverse, human > cynomolgus monkey > rat (Bennett & Morimoto, 1982).
See periodic review on methamidophos in this volume.
Five employees who were occupationally exposed to acephate (composition not specified) were evaluated clinically over 10 weeks. The employees had worked in an acephate pilot plant or formulation laboratory for periods of 336 months (average, 26 months). Detailed histories were obtained, and physical examinations were performed at the beginning and end of the study. Each employee was evaluated periodically for evidence of adverse health effects due to exposure. Appropriate laboratory studies were performed at regular intervals, including an audiogram, vision and grip tests, chest X-ray, electrocardiogram, plasma and erythrocyte cholinesterase determinations and a standard, 12-panel blood chemistry analysis. At no time during the study were any clinical manifestations of organic phosphate toxicity noted. The results of the laboratory studies were within normal limits (Swencki & Hartz, 1972).
Seven male and seven female volunteers were randomly divided into a control group consisting of four subjects and two test groups, in which four subjects received acephate (purity, 100%) and methamidophos (purity, 99.9%) in a 4:1 ratio and six subjects received these compounds in a 9:1 ratio. The test material was administered in corn oil at a concentration of 1%, 2%, 3% or 4%, corresponding to doses of 0.1, 0.2, 0.3 and 0.4 mg/kg bw per day, in gelatine capsule in three equally divided doses. The group receiving the 9:1 ratio was given the combination for consecutive periods of 21 days at graded doses of 0.1, 0.2 and 0.3 mg/kg bw per day, followed by a 7-day recovery period; after that time the dose was increased to 0.4 mg/kg bw per day for 10 days for three women only, with measurements at only two times. The group given the 4:1 ratio received the combination at a dose of 0.1 or 0.2 mg/kg bw per day for 21 consecutive days. Plasma and erythrocyte cholinesterase activity was examined periodically.
No symptoms of systemic toxicity were observed, and no effect on erythrocyte cholinesterase activity was noted at any time. No inhibition of plasma cholinesterase was observed in men at 0.1 and 0.2 mg/kg bw per day or in women at 0.1, 0.2 and 0.3 mg/kg bw per day. Inhibition of plasma cholinesterase activity by >20% was attained only at 0.3 and 0.4 mg/kg bw per day of the 9:1 ratio in men and women, respectively. Even with the 4:1 mixture, containing a higher concentration of methamidophos, a dose of 0.2 mg/kg bw per day was required to inhibit plasma cholinesterase activity significantly in both sexes. The values returned to normal within 7 days of cessation of dosing. The dose of 0.2 mg/kg bw per day of 4 parts of acephate and 1 part of methamidophos was thus the minimum that induced significant depression of plasma cholinesterase activity in four of four subjects after 16 days of dosing. In all subjects given this ratio of acephate:methamidophos, the inhibition of plasma cholinesterase activity was > 2 standard deviations below the mean pre-test activity at two consecutive bleedings. All men given the 9:1 ratio of acephate:methamidophos at 0.3 mg/kg bw per day showed depression of cholinesterase activity > 2 standard deviations below the mean pre-test activity. At 0.4 mg/kg bw per day of the 9:1 ratio, two of the three women showed cholinesterase depression > 2 standard deviations below the mean pre-test activity after 10 days of dosing. Plasma cholinesterase activity returned to normal within 7 days of cessation of dosing (Garofalo et al., 1973).
Although the design of this study does not meet current criteria for a biomedical test involving human subjects, the results indicate that erythrocyte cholinesterase activity was not inhibited at a dose of 0.3 mg/kg bw per day of the 9:1 mixture, equivalent to a dose of acephate of 0.27 mg/kg bw per day. For these reasons and because of the lower sensitivity of erythrocyte than brain cholinesterase activity to inhibition by acephate observed in experimental animals, this study was considered to be only supportive for establishment of reference values.
Four workers engaged in 8-day spraying campaigns with a formulation of technical-grade acephate (purity, 97%) were monitored before, during and after exposure for their urinary content of acephate and methamidophos and for erythrocyte and plasma cholinesterase activity. The median air concentrations ranged from 0.3 to 2.1 mg/m3, and the median total deposition on body skin ranged from 26 to 42 mg/day. Urinary excretion of unchanged acephate followed a pattern consistent with exposure, showing peak values during the work shift or during the 8 h after the end of the shift. The urinary concentration of unchanged acephate was 110 mg/l. Methamidophos was not detected in any urine sample (detection limit, 30 ΅g/l). A high correlation (r2 = 0.78) was found between the dermal exposure level and elimination of acephate in urine.
No changes with respect to pre-exposure values were found in erythrocyte or plasma cholinesterase activity for workers whose urinary concentration of acephate was 1 or 2 mg/l. On the basis of the air concentrations and the dermal deposition, the highest median daily total concentration of acephate that did not affect cholinesterase activity was estimated to be 41 mg. One subject with a total estimated exposure to acephate of 66 mg/day and a urinary excretion of 38 mg/l showed decreased plasma cholinesterase activity during exposure (by 20% compared with pre-exposure values) and decreased erythrocyte cholinesterase activity after exposure (by 18% compared with pre-exposure values). These values were considered to be outside the limits of analytical and biological variability (Maroni et al., 1990).
Healthy volunteers were recruited through a generic advertisement and screened for eligibility in a medical examination at the clinical research facility 3 weeks before the beginning of the study. Forty male and 10 female volunteers aged 1850 who met the inclusion criteria were enrolled in a double-blind, placebo-controlled study with a single ascending oral dose, conducted according to GLP. The protocol was reviewed and approved by an independent research ethics committee, and all study participants gave informed consent. All the volunteers underwent a screening physical examination, including assays for plasma and erythrocyte cholinesterase, to establish baseline conditions. This biomedical test involving human subjects was properly designed and meets the appropriate ethical guidelines required by the 1998 JMPR (Annex 1, reference 83).
Groups of seven subjects received a single oral dose of acephate (purity, 99.0%) and three received lactose (placebo) in a hard gelatine capsule daily for 14 days. Four groups of men received a dose of 0.35, 0.7, 1 or 1.2 mg/kg bw, and the one group of women received 1 mg/kg bw. The volunteers lived at the clinic for 72 h after dosing. Vital signs were monitored at various times after dosing, and electrocardiograms, haematology, clinical chemistry and urine analysis were performed. The pharmacokinetics of acephate and its primary metabolite, methamidophos, was determined at several times. Blood samples were collected 1, 2, 4, 8, 12, 24, 48 and 72 h and 7 and 14 days after dosing. Urine samples were collected for 12 h before dosing and for 012 h, 1224 h and 2448 h after dosing. The subjects were monitored for adverse events throughout the study.
Systemic exposure to acephate appeared to increase with dose. No difference in pharmacokinetics was seen between men and women, the Tmax being 14 h. The concentration of acephate in plasma of all treated groups was < 6% of the Cmax by 24 h, and no acephate was detectable in plasma by 48 h (Figure 3). The terminal elimination half-life for acephate was between 3.5 and 6.6 h for all subjects. Methamidophos was not quantifiable in the plasma of any subject given 0.35 mg/kg bw but the concentration increased in a dose-related fashion in the other treated groups. As for acephate, the Tmax was 14 h. The concentration of methamidophos was greatly reduced in plasma by 24 h after administration, and the terminal elimination half-life was between 3.5 and 12 h for all subjects. The recovery of test material (acephate plus acephate equivalents from methamidophos) in urine, measured during the 48 h after dosing, represented 2662% of the administered dose in males and 1253% in females. Most of the recovered acephate and methamidophos was found in urine during the first 12 h after dosing. Methamidophos accounted for about 1.3% of the amount recovered in urine, independently of the dose administered. During the first 12 h after dosing with 1 mg/kg bw, 35 000 and 31 000 ng/l of acephate and 300 and 280 ng/l of methamidophos were found in the urine of men and women, respectively. The fate of the other 50% or more of the dose administered is unknown, but may be accounted for mainly by incomplete gastrointestinal absorption.
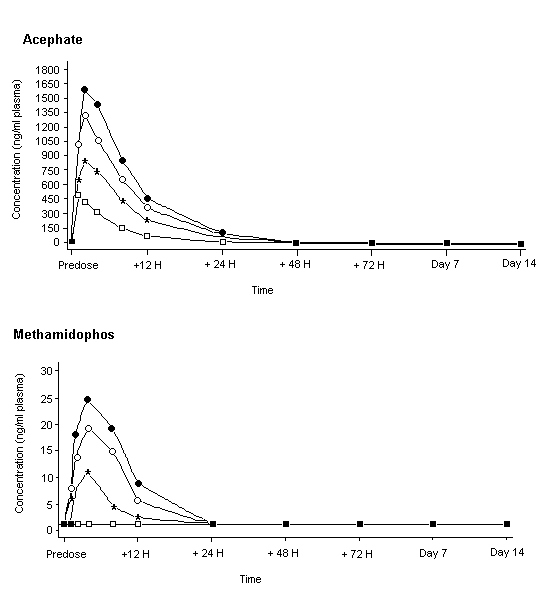
Figure 3. Mean concentrations of acephate and methamidophos in plasma of male volunteers given oral doses
From Freestone & McFarlane (2000)
Squares, treatment at 0.35 mg/kg bw; stars, 0.7 mg/kg bw; circles, 1 mg/kg bw; crosses, 1.25 mg/kg bw
The times on the x axes are not shown to scale; concentrations below the limit of quantification were set to 0.
Administration of up to 1.2 mg/kg bw to men and 1.0 mg/kg bw to women did not cause any clinically significant changes in vital signs, electrocardiograms or haematological, clinical chemical or urinary parameters or the results of physical examinations. Six adverse effects were seen after administration of acephate or placebo, none of which was serious or exceeded grade 2 in severity. There was no increase in the frequency of adverse events with increasing dose of acephate. The highest dose resulted in a statistically significant reduction in mean plasma cholinesterase activity in men, with decreases of 13%, 8.9%, 9.1% and 8.6% from the baseline value 12, 24, 48 and 72 h after dosing, respectively. A statistically significant reduction in mean erythrocyte cholinesterase activity was found 12 h after dosing in men (6.7% change from baseline, Figure 4), the only dose and time at which both plasma and erythrocyte cholinesterase activity was similarly affected in men. No significant effects on cholinesterase activity were found at lower doses. Statistical analysis of the results for women given 1 mg/kg bw showed a significant reduction in mean plasma cholinesterase activity at 8, 12 and 24 h (by 13%, 12% and 10% from baseline) and a significant reduction in mean erythrocyte cholinesterase activity 12 h after dosing only after exclusion of outliers (11% change from baseline). None of the other comparisons was statistically significant (Table 7).
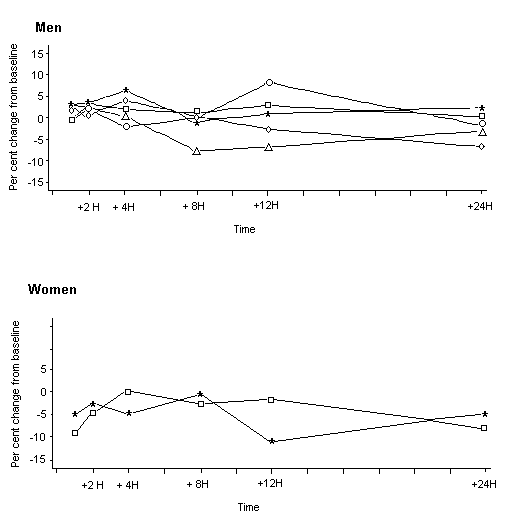
Figure 4. Inhibition of erythrocyte cholinesterase activity in male and female volunteers given acephate orally
From Freestone & McFarlane (2000)
Squares, placebo; crosses, treatment at 0.35 mg/kg bw; circles, 0.7 mg/kg bw; diamonds, 1 mg/kg bw; triangles,
1.25 mg/kg bw. Baseline defined as mean of all assessments before dosing, except screening
Table 7. Mean per cent change in plasma and erythrocyte cholinesterase activity from baseline values in male and female volunteers given acephate orally
|
Sampling time (h) |
Sex |
Substrate |
Dose (mg/kg bw) |
||||
|
|
|
|
0 |
0.35 |
0.7 |
1 |
1.25 |
|
1 |
Male |
Plasma |
7.4 |
8.8 |
10 |
5.6 |
6.2 |
|
|
Female |
Plasma |
4.6 |
|
|
9.0 |
|
|
|
Male |
Erythrocytes |
0.45 |
2.8 |
0.33 |
2.3 |
3.1 |
|
|
Female |
Erythrocytes |
8.8 |
|
|
4.6 |
|
|
12 |
Male |
Plasma |
4.9 |
11 |
10 |
2.9 |
13 |
|
|
Female |
Plasma |
2.3 |
|
|
12a |
|
|
|
Male |
Erythrocytes |
2.9 |
0.95 |
8.2 |
2.5 |
6.8* |
|
|
Female |
Erythrocytes |
1.6 |
|
|
11 |
|
|
48 |
Male |
Plasma |
0.41 |
1.1 |
4.7 |
4.2 |
9.1* |
|
|
Female |
Plasma |
6.7 |
|
|
0.37 |
|
|
|
Male |
Erythrocytes |
0.13 |
8.0 |
3.3 |
1.5 |
3.1 |
|
|
Female |
Erythrocytes |
1.9 |
|
|
5.6 |
|
From Freestone & McFarlane (2000)
* Statistically significant decrease in linear trend analysis
a Statistically significant after exclusion of outliers
The results indicated that erythrocyte cholinesterase activity is not inhibited at 1 mg/kg bw in women and 1.2 mg/kg bw in men, the highest doses tested (Freestone & McFarlane, 2000). In view of the lower sensitivity of erythrocyte than brain cholinesterase activity to inhibition by acephate observed in experimental animals, this study was considered to be only supportive for establishment of reference values.
Systemic exposure to acephate and methamidophos in humans was evaluated from the results of the above study, in which a single oral dose of acephate was given to volunteers (Freestone & McFarlane, 2000). The results for pharmacokinetics indicated that the plasma concentrations of acephate and its primary metabolite methamidophos after repeated daily intake of acephate could be predicted. The underlying concept is that the exposuretime profiles for repeated doses with a fixed interval between doses and plasma concentrationtime profiles based on complete elimination after each single dose should be additive. As the plasma half-life determined within 024 h was 4.36.6 h, accumulation of acephate was negligible. Thus, the predicted plasma concentrations after infinite doses of acephate are comparable with that observed after a single dose, owing to the relatively short half-life of acephate. The situation for methamidophos is similar.
In conclusion, the predicted systemic concentrations of both acephate and methamidophos after infinite, repeated daily doses of acephate might be comparable to the actual measured systemic concentration after a single oral dose. If it is assumed that adverse effects are a monotonic function of systemic exposure to acephate and methamidophos and that their concentrationresponse relationships do not change with time, the NOAELs after a single oral dose might also apply to repeated daily intake of acephate (Tozer, 2000).
The active ingredient acephate has been formulated since 1988 at Cheste, Valencia (Spain), without interruption. Since that time, approximately 450 tonnes of acephate have been formulated. The work involved approximately 700 manyears. Exposure of operators is minimized by routine use of local extract ventilation and personal protective equipment, including respiratory protection in the form of a dust mask. All potentially exposed workers are examined medically before starting work and annually thereafter. The medical examination includes a general clinical examination, eyesight test, audiometry, lung function measurement, urine analysis and blood tests for haematological parameters, glucose, cholesterol, triglycerides, liver and renal function, uric acid and blood cholinesterase activity (periodically when organophosphorus compounds are being formulated). No adverse work-related health problems have been reported in relation to acephate formulation (Davies, 2000).
After oral administration at a dose of 25 mg/kg bw per day for 8 days to rats, [S-methyl-14C]acephate was rapidly absorbed and uniformly distributed. The highest concentrations of radiolabelled residues were found in liver and skin. Most of the radioactive material recovered was excreted within 12 h. Urine contained 8295% of the administered dose, 14% was exhaled, and 1% was found in faeces. Less than 1% was found as a residue in tissues and organs 72 h after the last dose. Unchanged acephate (7377%), O,S-dimethyl phosphorothioate (36%) and S-methyl acetylphosphoramidothioate (34%) were identified in urine, but no methamidophos was found.
After oral administration of acephate at a dose of 100 mg/kg bw per day for 4 days, rats converted a portion to methamidophos. Both acephate and methamidophos are highly water-soluble and are rapidly metabolized and excreted. There was no tendency for acephate or methamidophos to accumulate. Three hours after the last dose, the carcass contained 0.61.6% and the excreta (chiefly urine) 1.11.5% of the final dose of acephate as methamidophos.
The pharmacokinetics of acephate were similar in men and women given a single oral dose. The time to maximum concentration in plasma (Tmax) was 14 h for both acephate and methamidophos. The terminal elimination half-life was between 3.5 and 6.6 h for acephate and between 3.5 and 12 h for methamidophos. Most of the recovered acephate and methamidophos were found in urine during the first 12 h after dosing. Methamidophos accounted for about 1.3% of the amount recovered in urine, independently of the dose administered.
A single oral dose of 40 mg/kg bw of [14C-acetyl]acephate was administered on day 18 of gestation to rats and to dams immediately after delivery. Fetuses contained a total of 0.72% of the radioactivity, and more was recovered from the placenta than from the fetuses. A total of 0.96% of the administered dose was recovered in suckling pups after administration to lactating dams.
The LD50 values were 10001400 mg/kg bw after oral administration in rats and > 10 000 mg/kg bw after dermal administration in rabbits. The LC50 value was > 15 mg/l of air (4 h, nose-only) in rats. The clinical signs of toxicity corresponded to those typical of cholinergic poisoning. Acephate was not irritating to the eyes or skin of rabbits, and was not a dermal sensitizer in the maximization test in guinea-pigs. WHO has classified acephate as slightly hazardous (WHO, 2000).
The inhibitory effects of acephate and its main metabolite on cholinesterase activity have been investigated extensively both in vivo and in vitro in several species, including humans. It is likely that the inhibitory effect of acephate is due to its conversion to methamidophos. No significant sex or species difference in cholinesterase inhibition was observed in vivo.
The NOAEL for acephate given as a single dose by gavage to rats was 2.5 mg/kg bw, on the basis of a 3034% reduction in brain cholinesterase activity in females at 5 mg/kg bw. The Meeting considered that the 1322% reduction in cholinesterase activity at 2.5 mg/kg bw in various regions of the brain was not a toxicologically significant effect. No treatment-related clinical signs were observed at doses up to 5 mg/kg bw. Behavioural effects and decreased erythrocyte cholinesterase activity were found at doses of 10 mg/kg bw and above.
Several studies of toxicity in rats and dogs given repeated doses provided useful information for assessing the effects of acephate on brain cholinesterase activity. These are discussed below.
In a 90-day study of neurotoxicity in rats given 0, 5, 50 or 700 ppm, equal to 0, 0.33, 3.3 and 49 mg/kg bw per day, regional brain cholinesterase activity was reduced in a dose-related, statistically significant manner in all treated groups, with inhibition of 928% at 5 ppm, 2455% at 50 ppm and 6382% at 700 ppm. Brain cholinesterase activity was inhibited by more than 20% in only one of three measurements in the hippocampus and the olfactory region of female rats at 5 ppm. This dose, equal to 0.33 mg/kg bw per day, was therefore considered not to have an adverse effect. Females at 700 ppm showed significantly decreased mean ambulatory and total motor activity counts and decreased cholinesterase activity in erythrocytes.
In a 13-week study of toxicity in rats given 0, 2, 5, 10 or 150 ppm in the diet, equal to 0.12, 0.21, 0.58 and 8.9 mg/kg bw per day, statistically significant inhibition of brain cholinesterase activity was observed in all treated groups. The inhibition was similar in males and females. Erythrocyte cholinesterase activity was inhibited (by 3248%) only in rats at 150 ppm. No other signs of toxicity related to treatment were observed. The NOAEL was 10 ppm, equal to 0.58 mg/kg bw per day, on the basis of more than 20% inhibition of brain and erythrocyte cholinesterase activity in rats at 150 ppm.
In a 1-year study of toxicity in beagle dogs given 0, 10, 120 or 800 ppm of acephate in the diet, equal to 0.27, 3.1 and 20 mg/kg bw per day, statistically significant inhibition of brain cholinesterase activity was observed in males, by 17% in those at 10 ppm, 53% at 120 ppm and 68% at 800 ppm, and in females, by 49% at 120 ppm and 66% at 800 ppm. Erythrocyte cholinesterase activity was significantly inhibited at the two higher doses in animals of each sex. Despite severe inhibition of brain cholinesterase activity at the two higher doses in all treated animals, the signs usually associated with inhibition of cholinesterase activity were not observed.
In a 28-month study in rats given 0, 5, 50 or 700 ppm in the diet, equivalent to 0, 0.25, 2.5 and 35 mg/kg bw per day, erythrocyte cholinesterase activity was reduced to a lesser extent than that in brain. At termination at 28 months, brain and erythrocyte cholinesterase activity in the rats given 700 ppm was inhibited by 71% and 57% in males and by 69% and 46% in females, respectively, while in rats fed 50 ppm, the respective cholinesterase activities were inhibited by 50% and 26% in males and by 37% and 21% in females. Males at the highest dose showed hyperactivity, increased incidence of aggressive behaviour, decreased body-weight gain and significantly decreased food use efficiency.
As marginal, but statistically significant, changes in brain cholinesterase activity were observed at 5 and 10 ppm in these studies in rats and dogs, a more detailed analysis was undertaken. The doseresponse curve was found to be flat at these dietary concentrations, while clinical signs occurred at much higher doses. These marginal effects on brain cholinesterase activity were therefore considered to be of equivocal toxicological relevance. The Meeting concluded that the overall NOAEL was 10 ppm, equal to 0.58 mg/kg bw per day identified in the 13-week study in rats.
Acephate was not carcinogenic in a 28-month study in rats given 0, 5, 50 or 700 ppm in the diet. In a 104-week study in mice given 0, 50, 250 or 1000 ppm in the diet, acephate induced tumours at the highest dose, which was clearly toxic, causing lesions in the liver, lung and nasal cavity, significantly decreased body-weight gain and significant changes in organ weights. As noted by the 1978 Joint Meeting, the apparent increase in the incidence of liver tumours may have been the result of excessively high doses and is therefore of minimal concern.
An extensive range of studies of genotoxicity both in vitro and in vivo has been performed with acephate. The Meeting concluded that the existing database was adequate to characterize the genotoxic potential of acephate and concluded that it is unlikely to be genotoxic in vivo.
In view of the lack of genotoxicity in vivo and the finding of liver tumours only in female mice and only at concentrations at which severe toxicity was observed, the Meeting concluded that acephate is not likely to pose a carcinogenic risk to humans.
In two multigeneration studies of reproductive toxicity in rats given diets containing acephate at 0, 50, 150 or 500 ppm or 0, 25, 50 or 500 ppm, reproductive toxicity was observed only at parentally toxic doses, with reductions in live litter size and number of litters born at 150 ppm and 500 ppm, equivalent to 10 and 33 mg/kg bw per day, respectively. Postnatal survival and postnatal growth were reduced at 500 ppm. Body weight and/or body-weight gain were affected at this dose in both pups and parental animals.
Studies of developmental toxicity were conducted in rats given a dose of 0, 5, 20 or 75 mg/kg bw per day and in rabbits at 0, 3, 10, 30 or 100 mg/kg bw per day, with NOAELs of 20 and 3 mg/kg bw per day, respectively. In rats, growth retardation (considered to be a developmental effect) occurred at 75 mg/kg bw per day, a dose at which maternal food consumption and body-weight gain were also affected. In rabbits, slight developmental effects occurred at low incidence at 10 mg/kg bw per day, a dose that also caused maternal toxicity. The Meeting concluded that acephate is not teratogenic.
The Meeting concluded that the existing database was adequate to characterize the potential hazard of acephate to fetuses, infants and children.
When tested in hens, acephate did not induce delayed polyneuropathy after single or repeated doses.
Volunteers received single oral doses of acephate (purity, 99%) of 0, 0.35, 0.7, 1 or 1.2 mg/kg bw for men and 0 or 1 mg/kg bw for women. No inhibition of erythrocyte cholinesterase activity was reported in either sex, even at the highest doses. No clinically significant changes were seen in vital signs or on electrocardiography, haematology, clinical chemistry, urine analysis or physical examination. In view of the lower sensitivity of erythrocyte than brain cholinesterase activity to inhibition by acephate observed in experimental animals, the Meeting considered that this study was only supportive for establishing an acute reference dose (acute RfD).
In a study in volunteers given repeated doses, a mixture containing acephate and methamidophos (4:1 or 9:1 ratio) was administered, and plasma and erythrocyte cholinesterase activities were measured throughout the 21-day test period. Although this study was not conducted according to current standards, erythrocyte cholinesterase activity was not inhibited at 0.3 mg/kg bw per day of the 9:1 mixture, equivalent to a dose of acephate of 0.27 mg/kg bw per day, in either sex. In view of the lower sensitivity of erythrocyte than brain cholinesterase activity to inhibition by acephate observed in experimental animals, and as this study was not conducted according to current standards, the Meeting considered that it was only supportive for establishing reference values.
The Meeting established an ADI of 00.01 mg/kg bw on the basis of the NOAEL of 10 ppm, equal to 0.58 mg/kg bw per day, in the 13-week study in rats and a safety factor of 50. As marginal but statistically significant inhibition of brain cholinesterase activity was observed in rats and dogs at 5 and 10 ppm, the Meeting considered an additional safety factor of 2 to be appropriate. Since there were no relevant sex or species (including human) differences in inhibition of cholinesterase activity or in kinetics and the effect was dependent on the Cmax, a fourfold reduction in the safety factor was considered to be appropriate (see report of 2000 JMPR, Annex 5). On this basis, the Meeting used an overall safety factor of 50 (100 x 2/4). The Meeting noted that the ADI provides a margin of safety of 30 for inhibition of erythrocyte cholinesterase activity after repeated doses of a mixture containing acephate and methamidophos (9:1 ratio) to humans. This was considered to be adequate to cover the difference in sensitivity between erythrocyte and brain cholinesterase activity to inhibition by acephate observed in vivo in experimental animals.
The Meeting established an acute RfD of 0.05 mg/kg bw on the basis of the NOAEL of 2.5 mg/kg bw in female rats in the study of acute neurotoxicity (considered to be appropriate, since no sex differences were observed in other studies) and a safety factor of 50, considered to be appropriate for the reasons given above for the ADI. The Meeting noted that the acute RfD provides a margin of safety of 20 for inhibition of erythrocyte cholinesterase activity after administration of a single dose of acephate to women.
Levels relevant to risk assessment
|
Species |
Study |
Effect |
NOAEL |
LOAEL |
|
Mouse |
104-week study of toxicity and carcinogenicitya |
Toxicity |
50 ppm, equal to 7 mg/kg bw per day |
250 ppm, equal to 36 mg/kg bw per day |
|
|
|
Carcinogenicity |
250 ppm, equal to36 mg/kg bw per day |
1000 ppm, equal to 140 mg/kg bw per day |
|
Rat |
13-week study of toxicitya |
Toxicity |
10 ppm , equal to 0.58 mg/kg bw per dayb |
50 ppm, equal to 2.5 mg/kg bw per day |
|
|
28-month study of toxicity and carcinogenicitya |
Toxicity |
5 ppm, equivalent to 0.25 mg/kg bw per dayb |
50 ppm, equivalent to 2.5 mg/kg bw per day |
|
|
|
Carcinogenicity |
700 ppm, equivalent to 35 mg/kg bw per dayc |
|
|
|
Two-generation study of reproductive toxicitya |
Parental toxicity |
50 ppm, equivalent to 3.3 mg/kg bw per day |
150 ppm, equivalent to 10 mg/kg bw per day |
|
|
|
Pup toxicity |
50 ppm, equivalent to 3.3 mg/kg bw per day |
150 ppm, equivalent to 10 mg/kg bw per day |
|
|
Developmental toxicityd |
Maternal toxicity |
5 mg/kg bw per day |
20 mg/kg bw per day |
|
|
|
Embryo- and feto-toxicity |
20 mg/kg bw per day |
75 mg/kg bw per day |
|
|
Acute neurotoxicityd,e |
|
2.5 mg/kg bw |
5 mg/kg bw |
|
Rabbit |
Developmental toxicityd |
Maternal toxicity |
3 mg/kg bw per day |
10 mg/kg bw per day |
|
|
|
Embryo- and feto-toxicity |
3 mg/kg bw per day |
10 mg/kg bw per day |
|
Dog |
52-week study of toxicitya |
Toxicity |
10 ppm, equal to 0.27 mg/kg bw per dayb |
120 ppm, equal to 3.1 mg/kg bw per day |
|
Human |
Single-dose studyf |
Toxicity |
1 mg/kg bw per dayc |
|
|
|
21-day studyf |
Toxicity |
0.27 mg/kg bw per dayc |
|
|
a |
Dietary administration |
|
b |
Marginal effects of equivocal toxicological relevance on brain cholinesterase activity |
|
c |
Highest dose tested |
|
d |
Administration by gavage |
|
e |
Tested only in females |
|
f |
Only supportive for establishing reference values |
Estimate of acceptable daily intake for humans
00.01 mg/kg bw
Estimate of acute reference dose
0.05 mg/kg bw
Studies that would provide information useful for continued evaluation of the compound
List of end-points relevant for setting guidance values for dietary and non-dietary exposure
|
Absorption, distribution, excretion and metabolism in mammals |
|
|
Rate and extent of oral absorption |
Extensive and rapid |
|
Distribution |
Widely distributed |
|
Potential for accumulation |
None |
|
Rate and extent of excretion |
Rapid and nearly completely, mainly via urine |
|
Metabolism in animals |
Limited |
|
Toxicologically significant compounds (animals, plants and environment) |
Acephate and methamidophos |
|
|
|
|
Acute toxicity |
|
|
Rat, LD50, oral |
10001400 mg/kg bw |
|
Rabbit, LD50, dermal |
> 2000 mg/kg bw |
|
Rat, LC50, inhalation |
> 15 mg/l air (4 h, nose-only) |
|
Skin irritation |
Not irritating |
|
Eye irritation |
Not irritating |
|
Skin sensitization |
Not sensitizing (Magnusson & Kligman) |
|
|
|
|
Short-term studies of toxicity |
|
|
Target / critical effect |
Nervous system/inhibition of cholinesterase activity |
|
Lowest relevant oral NOAELa |
13-week study in rats: 10 ppm (equal to 0.58 mg/kg bw per day) |
|
Genotoxicity |
Unlikely to be genotoxic in vivo |
|
|
|
|
Long-term studies of toxicity and carcinogenicity |
|
|
Target/critical effect |
Nervous system/inhibition of cholinesterase activity |
|
Lowest relevant NOAELa |
28-month study in rats: 5 ppm (equivalent to 0.25 mg/kg bw per day) |
|
Carcinogenicity |
Not likely to pose a carcinogenic risk to humans |
|
|
|
|
Reproductive toxicity |
|
|
Target for reproductive toxicity / critical effect |
Number of pups and postnatal survival decreased at parentally toxic doses |
|
Lowest relevant NOAEL for reproductive toxicity |
50 ppm (equivalent to 3.3 mg/kg bw per day) |
|
Target for developmental toxicity / critical effect |
Decreased fetal body weight and reduced ossification (rat) and slight developmental effects (rabbit) at maternally toxic doses; not teratogenic |
|
Lowest relevant NOAEL for developmental toxicity |
Rabbit: 3 mg/kg bw per day |
|
|
|
|
Neurotoxicity |
No signs of delayed polyneuropathy (hens) |
|
NOAEL for acute neurotoxicity, rata |
2.5 mg/kg bw |
|
NOAEL for short-term neurotoxicity, rata |
5 ppm (equivalent to 0.33 mg/kg bw per day) |
|
|
|
|
Other toxicological studies |
Brain cholinesterase activity in rats and dogs more sensitive to acephate than plasma or erythrocyte cholinesterase activity in vivo; studies on the metabolite methamidophos are reported separately. |
|
Human data b |
In a study with single oral doses in volunteers, no inhibition of erythrocyte cholinesterase activity was seen at 1 mg/kg bw; in an older study with repeated oral doses, no inhibition of erythrocyte cholinesterase activity was seen at 0.27 mg/kg bw per day. |
|
Summary |
Value |
Study |
Safety factor |
|
ADI |
0.01 mg/kg bw |
13-week study in rats |
50 |
|
Acute reference dose |
0.05 mg/kg bw |
Acute study of neurotoxicity in rats |
50 |
|
a |
Marginal effects of equivocal toxicological relevance on brain cholinesterase activity |
|
b |
Used only for establishment of reference values |
Ahmed, K.S., Nabila, M.B., Hassan, A.A. & Mohamed, B.A.D. (1992) Placental and milk transfer, disposition, and elimination of a single oral dose of [14C-acetyl]acephate in Sprague Dawley rats. J. Occup. Med. Toxicol., 1, 265274.
Auletta, C.S. & Hogan, G.K. (1981) Lifetime oral toxicity/carcinogenicity study with technical RE12420 in rats. Unpublished report No. 78-2135 from Bio/dynamics, Inc., East Millstone, New Jersey, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Beavers, J.B. & Jaber, M. (1985) Acute delayed neurotoxicity study in chickens with Chevron acephate technical. Unpublished report No. 162-151 from Wildlife International Ltd, Easton, Maryland, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Bennett, E.L. & Morimoto, H. (1982) The comparative in vitro activity of acephate technical on brain, erythrocyte and plasma ChE from the human, monkey and rat. Unpublished report No. 2150 from University of California, Berkeley, California, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Brorby, G.P. & Beatty, P.W. (1987) The cholinesterase inhibition potential of acephate technical (SX-992, SX-1102) following four-week dietary administration in male rats. Unpublished report No. CEHC S-3054 from Chevron Environmental Health Center, Richmond, California, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Brorby, G.P., Rosenberg, D.W. & Wong, Z.A. (1987) The cholinesterase inhibition potential of acephate technical (SX-1102) following 4-, 9-, or 13-week dietary administration in male and female rats. Unpublished report No. CECH 2821 from Chevron Environmental Health Center, Richmond, California, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Daley, I.W. & Hogan, G.K. (1980) Recovery from cholinesterase inhibition following dietary exposure to Orthene in the rat. Unpublished report No. 80-2454 from Bio/dynamics, Inc., East Millstone, New Jersey, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Dalgard, D.W. (1984) A 4-week pilot oral toxicity study in dogs with Chevron acephate technical. Unpublished report No. HWA 2107-164 from Hazleton Washington, Vienna, Virginia, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA; Chevron Environmental Health Center, Inc., Richmond, California, USA.
Dalgard DW (1991) One-year oral toxicity study in dogs with Chevron acephate technical. Unpublished report No. 2107-165 from Hazleton Washington, Vienna, Virginia, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA; Chevron Environmental Health Center, Inc., Richmond, California, USA.
Davies, W.W. (2000) Medical surveillance on manufacturing plant personnel. Unpublished report No. ks-09-08-2000. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA; Global Environment, Health & Safety Management, Aventis Crop Science UK Ltd, Hauxton, Cambridge.
Farag, A.T., Eweidah, M.H., Tayel, S.M. & El-Sebae, A.H. (2000) Developmental toxicity of acephate by gavage in mice. Reprod. Toxicol., 14, 241245.
Freestone, S. & McFarlane, P. (2000) A single oral dose study with acephate technical in humans. Unpublished report No. ICR 013072 from Inveresk Research, Tranent, Scotland. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA; Valent USA Corp., Walnut Creek, California, USA.
Garofalo, M.J., Palazzolo, R.J. & Sanders, R.G. (1973) A study of the effects of Orthene and Monitor on plasma, erythrocyte cholinesterase activity in human subjects during subacute oral administration. Unpublished report No. 636-02498 from Industrial Bio-Test Laboratories, Inc., Northbrook, Illinois, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Golzio, L. (1999) Acephate: Unscheduled DNA synthesis in rat liver cells in vivo. Unpublished report No. 990173 from Istituto di Ricerche Biomediche Antoine Marxer RBM SpA, Colleretto Giacosa, Italy. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Green, C.E. (1989) Comparative metabolism of [14C] acephate by in vitro preparations from rat, dog, monkey and human liver tissue. Unpublished report No. LSC-6402 from Chevron Chemical Co., Richmond, California, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Hayashizaki, A., Kawai, M. & Sunomata, M. (1979) Studies on acute delayed neurotoxicity. Unpublished report from Bozo Research Center, Inc., Mishima, Shizuoka, Japan. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Hoberman, A.M. (1987) Two-generation (two litter) reproduction study in rats with Chevron acephate technical. Unpublished report No. 303005 from Argus Research Laboratories, Inc., Horsham, Pennsylvania, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Hoffman, G.M. (2000) A 4-week nose-only inhalation toxicity study in rats with acephate technical. Unpublished report No. 99-6124 from Huntingdon Life Sciences, East Millstone, New Jersey, USA. Submitted to WHO by Valent USA Corp., Walnut Creek, California, USA.
Lee, H. (1972) Metabolism of Orthene in rats. Unpublished report No. 721-14 from Chevron Chemical Co., Richmond, California, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Levy, J.E., Wong, Z.A. & MacGregor, J.A. (1979a) The skin irritation potential of Orthene specialty concentration. Unpublished report No. SOCAL 1418 from Chevron Environmental Health Center, Richmond, California, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Levy, J.E., Wong, Z.A. & MacGregor, J.A. (1979b) The eye irritation potential of Orthene specialty concentration. Unpublished report No. SOCAL 1419 from Chevron Environmental Health Center, Richmond, California, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Lochry, E.A. (1989) Oral teratogenicity and developmental toxicity study in rats with Chevron acephate technical. Unpublished report No. 303-008 from Argus Research Laboratories, Inc., Horsham, Pennsylvania, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Machado, M.L., Carver, J.H. & Kodama, J.K. (1984) Microbial/mammalian microsome mutagenicity plate incorporation assay with Orthene technical (SX-1102). Unpublished report No. SOCAL 2313 from Chevron Environmental Health Center, Inc., Richmond, California, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Mahajna, M., Quistad, B.G. & Casida, J.E. (1997) Acephate insecticide toxicity: Safety conferred by inhibition of bioactivating carboxyamidase by the metabolite methamidophos. Chem. Res. Toxicol., 10, 6469.
Maroni, M., Catenacci, G., Galli, D., Cavallo, D. & Ravazzani, G. (1990) Biological monitoring of human exposure to acephate. Arch. Environ. Contam. Toxicol., 19, 782788.
Mastalski, K., Jenkins, D.H., Keplinger, M.L. & Fancher, O.E. (1970) Acute oral toxicity study on RE 12,420 technical in beagle dogs. Unpublished report No. J8888 from Industrial Bio-Test Laboratories, Inc., Northbrook, Illinois, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Mastri, C., Keplinger, M.L. & Fancher, O.E. (1970) Acute oral toxicity study on RE 12,420 technical in white mice. Unpublished report No. A8889 from Industrial Bio-Test Laboratories, Inc., Northbrook, Illinois, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Moore, M.R. (1986) Evaluation of Chevron acephate technical in the mouse somatic cell mutation assay. Unpublished report No. 2107-141 from Hazleton Laboratories America, Inc., Rockville, Maryland, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Narcisse, J.K., Cavalli, R.D. & Spence, J.A. (1971a) Eye irritation potential of Orthene 75% soluble powder. Unpublished report No. SOCAL 218 from Chevron Environmental Health Center, Richmond, California, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Narcisse, J.K., Cavalli, R.D. & Spence, J.A. (1971b) Eye irritation potential of Orthene technical, Orthene 75S, (CC-2153) and Orthene 7S (CC-2152). Unpublished report No. SOCAL 273 from Standard Oil Company of California. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Nemec, M.D. (1995) A range-finding acute study of Orthene technical in rats. Project No. WIL-194015: 9700023. Unpublished study from WIL Research Labs., Inc., Ashland, Ohio, USA, Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Nemec, M.D. (1996) An acute neurotoxicity study of Orthene technical in rats. Unpublished report No. WIL-194013 from WIL Research Laboratories, Inc., Ashland, Ohio, USA, Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Nemec, M.D. (1997) A subchronic (13-week) neurotoxicity study of Orthene technical in rats. Unpublished report No. WIL-194014 from WIL Research Laboratories, Inc., Ashland, Ohio, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA; Valent USA Corp., Walnut Creek, California, USA.
Palmer, A.K., Offer, J.M., Barton, S.J., Gregson, R.L., Gibson, W.A. & Almond, R.H.. (1983) Effect of technical RE-12420 on reproductive function of multiple generations in the rat. Unpublished report No. CHR 11/81957 from Huntingdon Research Centre, Huntingdon, Cambridgeshire, England. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Rittenhouse, J.R. & MacGregor, J.A. (1977) The acute dermal toxicity of Orthene technical. Unpublished report No. SOCAL 1110 from Standard Oil Company, San Francisco, California, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Rittenhouse, J.R., Narcisse, J.R., Cavalli, R.D. & Spence, J.A. (1972) 21-day sub-acute dermal study in rabbits with Orthene 75S. Unpublished report No. SOCAL 278 from Standard Oil Company of California, San Francisco, California, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Rittenhouse, J.R., Wong, Z.A. & MacGregor, J.A. (1979a) The acute oral toxicity of Orthene specialty concentrate. Unpublished report No. SOCAL 1416 from Chevron Chemical Co., Richmond, California, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Rittenhouse, J.R., Wong, Z.A. & MacGregor, J.A. (1979b) The acute inhalation toxicity of Orthene specialty concentration. Unpublished report No. SOCAL 1420 from Chevron Environmental Health Center, Richmond, California, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Rittenhouse, J.R., Wong, Z.A. & MacGregor, J.A. (1980) The acute dermal toxicity of Orthene specialty concentrate. Unpublished report No. SOCAL 1417 from Chevron Environmental Health Center, Richmond, California, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Rodwell, D.E. (1980) Teratology study in rabbits. Unpublished report No. 415-024 from International Research & Development Corp., Mattawan, Michigan, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Silveira, R.F., Cushman, J.R. & Wong, Z.A. (1984) Modified Buehler test for the skin sensitization potential of Chevron acephate technical (SX-1102). Unpublished report No. SOCAL 1840 from Chevron Environmental Health Center, Richmond, California, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Spicer, E.J.F., Phillips, L.M., Jefferson, M.D. & Blair, M. (1982) Lifetime oral carcinogenicity study in mice with Orthene technical (RE12420). Unpublished report No. 415-006 from International Research & Development Corp., Mattawan, Michigan, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Swencki, M.J. & Hartz, W.P. (1972) Orthene human exposure study clinical evaluation. Unpublished report from Standard Oil Company of California, Inc., Richmond, California, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Terrill, J.B. & Murphy, C.L. (1987) Four-week inhalation study in rats with acephate technical. Unpublished report No. 2107-155 from Hazleton Laboratories America, Inc., Vienna, Virginia, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Terrill, J.B. & Murphy, C.L. (1988) Four-week satellite inhalation study in rats with acephate technical. Unpublished report No. 2107-156 from Hazleton Laboratories America, Inc., Vienna, Virginia, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Thompson, J.D., Cushman, J.R. & Wong, Z.A. (1986) Guinea pig maximization test for the skin sensitization potential of technical (SX-1102). Unpublished report No. SOCAL 1839 from Chevron Environmental Health Center, Richmond, California, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Tozer, T.N. (2000) Systemic exposure to acephate and methamidophos in humans on repeated daily oral doses to acephate technical: Predictive analysis using single dose data. Unpublished report from University of California, San Francisco, California, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Tucker, B.V. (1974) S-Methyl-14C-Orthene dermal treatment of rats. Unpublished report No. 721-11 from Chevron Chemical Co., Richmond, California, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
Valencia, R. (1981) Mutagenesis screening of pesticides using Drosophila. US Department of Commerce, National Technical Information Service, Springfield, Virginia, USA. Report No. EPA-600/1-81-017, Research Triangle Park, North Carolina, USA.
Vargas, A.A.T. & Bonetti, R. (1993) The Salmonella typhimurium reverse mutation by Orthene Tecnico Hokko. Unpublished report No. 51/92 from Bioagri Biotecnologia Agricola, Piracicaba, SP, Brazil. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA; Hokko do Brazil Industria Quimica e Agropecuaria Ltda, Brazil.
Vargas, A.A.T. & Rodrigues, J.L.M. (1993) A micronucleus study in mice for the product Orthene Tecnico Hokko. Unpublished report,No. 10/93 from Bioagri Biotecnologia Agricola, Piracicaba, SP, Brazil. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA; Hokko do Brazil Industria Quimica e Agropecuaria Ltda, Brazil.
Warnock, R.E. (1973) Metabolism of Orthene to Ortho 9006 in rats. Unpublished report No. 721-11/Orthene from Chevron Chemical Co., Richmond, California, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
WHO (2000) The WHO recommended classification of pesticides by hazard and guidelines to classification 20002202 (WHO/PCS/01.5). Available from the International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland.
Wilson, B.W., Henderson, J.D., Kellner, T.P., McEuen, S.F., Griffis, L.C. & Lai, J.C. (1990) Acetylcholinesterase and neuropathy target esterase in chickens treated with acephate. Neurotoxicology, 11, 483491.
Wong, Z.A., Kodama, J.K. & MacGregor, J.A. (1979) Characterization of the in vitro inhibition of rat and monkey red blood cell and brain cholinesterase by acephate technical, acephate and methamidophos and their mixtures. Unpublished report No. SOCAL 1628 from Chevron Environmental Health Center, Richmond, California, USA. Submitted to WHO by Tomen Agro, Inc., San Francisco, California, USA.
See Also: Toxicological Abbreviations Acephate (ICSC) Acephate (Pesticide residues in food: 1976 evaluations) Acephate (Pesticide residues in food: 1979 evaluations) Acephate (Pesticide residues in food: 1981 evaluations) Acephate (Pesticide residues in food: 1982 evaluations) Acephate (Pesticide residues in food: 1984 evaluations) Acephate (Pesticide residues in food: 1984 evaluations) Acephate (Pesticide residues in food: 1987 evaluations Part II Toxicology) Acephate (Pesticide residues in food: 1988 evaluations Part II Toxicology) Acephate (Pesticide residues in food: 1990 evaluations Toxicology) Acephate (JMPR Evaluations 2005 Part II Toxicological)