
FAO/PL:1968/M/9/1
WHO/FOOD ADD./69.35
1968 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Committee on Pesticide Residues, which met in Geneva, 9-16 December,
1968.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Geneva, 1969
COUMAPHOS
IDENTITY
Chemical names
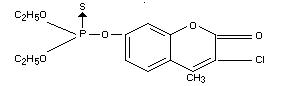
O,O-diethyl O-(3-chloro-4-methyl-coumarin-7-yl)
monothiophosphate
O-(3-chloro-4-methyl-7-coumarinyl) OO-diethyl phosphorothioate
(IUPAC)
O,O-diethyl
O-(3-chloro-4-methyl-2-oxo-2H-1-benzopyran-7-yl)
phosphorothioate
Synonyms
Co-Ral(R), Asuntol(R), Baymix(R), Resistox(R), Agridip(R),
Meldane(R)
Formula
 Other information on identity and properties
Coumaphos is a slightly brownish powder with a weak unpleasant odour.
The compound melts 90-92°C. It is stable to water and moderate heat
but hydrolyzes on refluxing in 1N alkali for two hours. It is soluble
in aromatic solvents, somewhat soluble in alcohols and ketones and
insoluble in water.
Principal formulations are 25 per cent and 50 per cent wettable
powders, 0.5. 1.0 and 5.0 per cent livestock dusts and emulsifiable
concentrates containing 11.6-20 per cent active material. A four per
cent pour-on and two per cent and 50 per cent feed premixes are
also available.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
After oral doses of 20 mg/kg body weight of 32p-labelled coumaphos
were administered to two steers, 38 per cent of the radioactivity was
recorded in the urine and 35 per cent in the faeces during seven days
following dosing. Diethylphosphoric acid and diethylphosphorothioic
acid were the major urinary metabolites. The faeces contained 50 per
cent coumaphos, 32 per cent oxygen analogue and 12 per cent polar
metabolites (Kaplanis et al., 1959).
A similar metabolic pattern was found in rats, goats and cows. A cow
fed 40 mg/kg of 32P-labelled coumaphos had 0.015 ppm equivalents of
radioactive material in the milk after four weeks and a goat fed 30
mg/kg had 0.06 ppm equivalents in the milk after seven days (Krueger
et al., 1959).
Activation of coumaphos to the more potent cholinesterase inhibitor,
the oxygen analogue, was demonstrated in vitro using rat-liver
slices (Vickery and Arthur, 1960).
Acute toxicity
LD50 (mg/kg
Animal Route body-weight) Reference
Mouse Oral 55 Schuleman, 1955
Mouse i.p. 23 Brandenberg, 1956
Rat (M) Oral 35 Bombinski and
DuBois, 1957
Rat (F) Oral 13-30 DuBois and
Schmalegemeier,
1958
Rat (M) i.p. 28-50 DuBois and
Schmalegemeier,
1958
Guinea-pig Oral 160 Bombinski and
(M) DuBois, 1957
Guinea-pig i.p. 140 Bombinski and
(M) DuBois, 1957
Four yearling cattle were given a single dose of 15 mg/kg orally. Very
mild symptoms, principally diarrhoea, were demonstrated by three
cattle. Cholinesterase was depressed 40-75 per cent of normal. At 50
mg/kg two yearling cattle were severely poisoned; one died and the
other recovered after two weeks. Eight out of nine yearling sheep died
when given 40 mg/kg orally (Radeleff et al., 1958). When sheep were
fed 30 mg/kg body weight of coumaphos orally, the mortality was 60 per
cent (Radeleff et al., 1963).
Whole blood cholinesterase was depressed 20 per cent in a horse
poisoned by 25 mg/kg body weight (Jackson et al., 1960).
Administration of coumaphos to rats in combination with 12 other
organo-phosphorus insecticides indicated that significant potentiation
occurred only with malathion. In the case of malathion the LD50 of an
equitoxic mixture with coumaphos was 190 mg/kg as compared with a
value of 455 mg/kg, which would be expected on the basis of strict
additivity. Thus the ratio of observed to expected value is 2.4
(DuBois, 1958a). No potentiation was observed when coumaphos was
administered in combination with three other anticholinesterase
agents, carbaryl, dioxathion and ethion (DuBois, 1960). Simultaneous
administration of piperonyl butoxide clearly increased the dermal
toxicity of coumaphos to rats (DuBois, 1958b).
The joint oral administration to mice of piperonyl butoxide (1:5)
resulted in a four-to six-fold increase in the toxicity of both
coumaphos and its oxygen analogue (Robbins et al., 1959a).
Short-term studies
Rat
Four groups of rats (10 male and 10 female) were fed for 16 weeks on
diets containing 0, 2, 5 or 10 ppm of coumaphos. Growth rate and food
consumption was not significantly different in test and control
groups. None of the dietary levels produced any inhibition of brain
and submaxillary gland cholinesterase. Serum cholinesterase of female
rats fed 10 ppm showed 30 per cent inhibition after eight weeks and
20 per cent after 16 weeks. However, there was no significant change
in that of the males. Erythrocyte cholinesterase of both males and
females fed 10 ppm showed a marked inhibition after eight weeks (40
per cent) but the effect had fallen to 20 per cent inhibition after
16 weeks. No other toxic or pathological lesions were noted in the
test group (Vaughn et al., 1958a).
Groups of five female rats received daily intraperitoneal injections
of coumaphos at levels of 0, 5, 7.5, 12.5 and 25 mg/kg body weight. At
the top dose level all the animals died within eight days,
demonstrating that coumaphos exerts a cumulative lethal effect when
daily doses of one fourth of the acute LD50 are given. No mortalities
occurred during the 60-day experimental period at the lower dosage
levels. At the 12.5 mg/kg level the animals showed typical symptoms
of intoxication, apparent after five injections of the compound. The
effects consisted of extreme irritability, tremors, lacrimation, mild
diarrhoea and rapid loss of weight. After 15 days the symptoms began
to subside and the animals gained weight and appeared nearly normal
throughout the remainder of the 60-day period, with the exception of
the occurrence of mild tremors for about two hours after each
injection. Three out of the five animals in this group developed lens
opacities. At the 5 and 7.5 mg/kg dosage level the animals did not
exhibit any grossly observable symptoms of poisoning or loss of
weight. However, tissue cholinesterase levels were depressed by at
least 75 per cent and remained at that low level throughout the
period of injections (Murphy and DuBois, 1958).
Groups of 12 male and 12 female rats were fed 0, 10, 25 and 100 ppm of
coumaphos in a milk diet for 90 days. Marked weight loss and 100 per
cent mortality after the 90-day period occurred in the 100 ppm group.
Eight of the 24 rats fed 25 ppm died during the 90-day period, whereas
the mortality in the 0 and 10 ppm groups in the milk diet was less
than 10 per cent. Serum and erythrocyte cholinesterase activity of
male and female rats fed 10 ppm was inhibited 60 per cent and 30 per
cent respectively and the effect became more marked at higher dose
levels. Brain and submaxillary gland cholinesterase was inhibited at
the 10 and 25 ppm levels (Doull et al., 1962a).
Dog
Four groups, each of which contained one male and one female dog, were
fed diets containing 2, 5, 10 and 50 ppm of coumaphos for 12 weeks.
None of the animals exhibited any symptoms of cholinesterase
inhibition (parasympathetic stimulation) during the feeding period,
and all dogs appeared normal. Serum and erythrocyte cholinesterase
activity was determined relative to a control value established for
each dog by obtaining samples of blood during an observation period
prior to starting the test diet. At the 50 ppm level, erythrocyte
cholinesterase activity decreased to 65 per cent of control by the
end of four weeks and remained at this low level for the duration of
the experiment. No inhibition of erythrocyte cholinesterase was
observed at the 2, 5 or 10 ppm level. Serum cholinesterase activity
was rapidly decreased to 50 per cent of control at the 10 and 50 ppm
levels after one to two weeks. Return to normal following removal of
coumaphos from the diet at the end of the 12-week period was rapid for
serum (one week) but slower (three weeks) for erythrocyte
cholinesterase. A level of 2 ppm of coumaphos in the diet caused only
slight inhibition of serum cholinesterase (Vaughn et al., 1958b).
Four groups, each of two male and two female dogs, were fed milk diets
containing 0, 10, 25 and 100 ppm for 90 days without any sign of
intoxication except for intermittent periods of diarrhoea in the
female dogs fed 100 ppm. At the 10 ppm level and above, serum
cholinesterase was depressed to greater than 50 per cent of the
control after two weeks. Significant depression of erythrocyte
cholinesterase was evident only at the 100 ppm level and reached a
plateau of 50 per cent of normal after 10 weeks. Sacrifice after 90
days showed no significant depression of brain or liver cholinesterase
except possibly a slight inhibition of brain cholinesterase at 100 ppm
(Doull et al., 1962b).
Four groups of dogs, each of which contained two male and two female
animals, were fed diets containing 0, 2, 10 and 50 ppm of coumaphos.
Growth-rate, food consumption, haematological profile and prothrombin
time was normal in all groups except, possibly, for a slight reduction
in growth-rate of one of the five dogs fed 50 ppm. Inhibition of serum
cholinesterase occurred in the 50 and 10 ppm groups and of erythrocyte
in the 50 ppm group. At sacrifice, after feeding for one year, brain
and liver cholinesterase was depressed in the 50 ppm group and liver
cholinesterase in the 10 ppm group. No inhibition occurred in the 2
ppm group (Doull et al., 1959).
Gross and histological examination of the tissues and organs of
animals used in the latter experiment did not reveal any compound
related effects (Vesselinovitch et al., 1960).
Cattle
Four yearling cattle were fed 5 mg/kg of coumaphos orally daily for
five days. Whole blood cholinesterase was reduced to 50 per cent of
normal (Radeleff et al., 1958).
Long-term studies
Rat
Groups of 50 rats (25 males and 25 females) were fed diets containing
0, 5, 25 or 100 ppm of coumaphos, for two years. Growth and food
consumption was normal for all groups. At the 100 ppm and 25 ppm
level, coumaphos shortened the average life span (25 per cent and 10
per cent respectively). Erythrocyte and serum cholinesterase was
inhibited in the 10 ppm and higher groups, in a dose response
relationship. Inhibition of brain cholinesterase occurred in the 25
ppm and 100 ppm groups. Only animals in the 100 ppm group showed
occasional evidence of toxic effects, mainly irritability and
excitability. The "no effect" level in rats with respect to the
cholinesterase level in tissues assayed is 5 ppm. Kidney weight of
rats in the 10 ppm and higher groups was decreased, this effect being
partly correlated with the dietary levels of coumaphos. At autopsy, no
compound related histologic lesions were found (Doull et al., 1960).
Special studies
(a) Reproduction
Mouse: Reproduction studies have been carried out with groups of 12
male and 24 female mice fed 0, 10, 25 or 100 ppm of coumaphos in their
diet. Male and female mice were able to tolerate coumaphos up to 25
ppm without exhibiting marked changes in fertility, litter size or
ability of offspring to survive for 30 days after birth. At the 100
ppm level, the number of mice that became pregnant was reduced by
about 50 per cent, litter size was reduced by about 50 per cent and
only about 15 per cent of the offsprings survived 30 days. When the
feeding of coumaphos at the 25 ppm level was extended over three
generations of animals, fertility, gestation, viability and lactation
were similar to the controls. Histopathological examination of 12
weanlings of each sex of the third generation did not reveal any
compound-related effects. Only seven per cent of the initial group of
mice fed 100 ppm survived; this incidence of high mortality in the
case of pregnant mice contrasts with a prior study which showed that
when a group of non-pregnant mice were fed 100 ppm of coumaphos for
six weeks, none died. Thus pregnant mice are possibly more susceptible
to the acute toxic effects of coumaphos than are non-pregnant mice.
Cholinesterase inhibition studies were not made in the reproduction
experiment (Doull et al., 1962b).
Chicken: In a three-generation reproduction study, a group of four
male and 20 female chickens were fed diets containing 5, 10 or 25 ppm
coumaphos. Reactions indicative of cholinesterase inhibition were
noted among Fo birds in the 25 ppm group. These reactions occurred at
17 weeks of age and disappeared when levels were reduced to 20 ppm.
The dietary level was returned to 25 ppm for the F1, F2 and F3
birds. No abnormal reactions were noted among any of the F1, F2 and
F3 birds. Body weight, food disappearance, mortality, egg production,
egg weight, egg fertility, egg hatchability and cholinesterase
activity were normal for all test groups in all generations.
Microscopic examination of tissues and organs of F2 birds in the 25
ppm group was also normal (Industrial Bio-test Laboratories, 1966).
(b) Studies of metabolites
The acute oral toxicity of metabolites of coumaphos has been
established. For the oxygen analogue of coumaphos the LD50 for
several species is shown in the following table (DuBois and Plzak,
1959).
Animal Route LD50
mg/kg body weight
Mouse (M) i.p. 4.2
Mouse (F) i.p. 3.8
Rat (M) oral 11.0
Rat (F) oral 8.3
Rat (M) i.p. 2.8
(continued)
Animal Route LD50
mg/kg body weight
Rat (F) i.p. 2.6
Guinea-pig (M) oral 50.0
Guinea-pig (F) i.p. 16.0
At doses approaching the LD50 the animals exhibited symptoms
characteristic of cholinesterase inhibition of the central and
peripheral nervous system. The onset of symptoms occurs more rapidly
with the oxygen analogue than with the parent compound. Inhibition of
cholinesterase activity and its rapid recovery also contrasts with the
prolonged action of coumaphos (DuBois and Plzak, 1959).
The LD50 to rats of the metabolite chlorferron is greater than 1000
mg/kg. It was not possible to kill a rat by either oral or
intraperitoneal administration at this level (DuBois and
Schmalgemeier, 1959).
Groups of rats (10 male and 10 female) were fed diets containing 0, 5,
10 and 50 ppm of chlorferron for 16 weeks. There was no effect on food
consumption, peripheral blood-count and cholinesterase activity of the
brain, submaxillary glands, serum and erythrocytes. Male rats fed
diets containing 10 and 50 ppm showed a slight reduction in growth
rate during the second month, but neither group showed any significant
difference in body weight compared to the controls after 16 weeks.
Studies on organ weight and pathology were omitted (Vaughn et al.,
1958c).
Comments
Early studies on the acute toxicity of coumaphos showed great
variability. The short-term and long-term studies were adequate.
Because cholinesterase inhibition was used as criterion for assessment
the levels studied were too low to detect toxic effects due to
chlorferron. More extensive studies on this metabolite should,
therefore, be carried out. In short-term studies by the
intraperitoneal route in rats, lens opacities were observed at the
highest level tested.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 5 ppm, equivalent to 0.25 mg/kg body weight per day
Dog: 2 ppm, equivalent to 0.05 mg/kg body weight per day
Estimate of temporary acceptable daily intake for man (of parent
compound, oxygen analogue and chlorferron)
0 - 0.0005 mg/kg body weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Coumaphos is used to control pests attacking domestic animals (it is
not used on plants). Numerous studies dealing with various
applications and the efficacy of coumaphos for the control of a
variety of insects have been published. These references are recorded
with FAO. The insect pests against which coumaphos is applied on the
various domestic animals are listed in the following table.
Beef Dairy
Cattle Cattle Sheep Horses Pigs Dogs Poultry Goats
Ticks
(one or
more x x x x x x x
hosts)
Ear
ticks
Mites x x x x x
Fleas x x x
Biting
and x x x x x x x
sucking
lice
Keds x x
Cattle
grubs, x
warbles
Screw worms
and x x x x x x
blowfly
maggots
Stable-,
buffalo and x x x x x x
horn flies
(continued)
Beef Dairy
Cattle Cattle Sheep Horses Pigs Dogs Poultry Goats
Face
flies x x
The compound is administered internally for control of faecal-breeding
flies and of certain endoparasites; in the United States of America up
to 33 ppm of coumaphos is added to the feed of cattle for this purpose
(Anon., 1964-1966, 1968). Enough coumaphos is added to the daily diet
to ensure a dose of 1.2 mg/kg of body weight.
For spray and dip treatments, a suspension of wettable powder with
0.0625 to 0.5 per cent of coumaphos is used and two to four litres of
suspension remain on the animal body. For pour-on treatments, an oil
formulation is applied to the back of cattle in a quantity sufficient
to ensure that the animal receives 10-15 mg of active ingredient per
kg of body weight. Poultry is dusted once weekly with a 0.5 per cent
formulation.
Low dosage treatments (backrubber, up to five per cent dusts, one per
cent mist spray and 1.2 mg/kg/day in feed) may be made to lactating
dairy animals with no time limitation. Lactating animals should not be
given over-all sprays or pour-on treatments. Dry dairy animals should
not be given over-all spray, dip or pour-on treatments within 14 days
of freshening. Baby animals should not be treated before they are
three months old. Three-to six-month-old animals should be sprayed
only lightly. Sheep and goats should not be treated with spray
concentrations greater than 0.25 per cent. Sick animals should not be
treated. Coumaphos should not be used in conjunction with natural and
synthetic pyrethroids or compounds synergizing them (Robbins et al.,
1959a); nor should it be used with other organo-phosphorus compounds
(e.g. malathion) or internal medications, such as phenothiazine (Clark
et al., 1967). Sheep and goats should not be slaughtered within 15
days of treatment.
Residues resulting from supervised trials
A summary of results compiled by Chemagro Corporation and on file with
FAO is given in the following table on page 79. Some of the dose rates
are higher than recommended. The residues consist of coumaphos and its
oxygen analogue.
The residues in cattle, apart from fat samples, ranged up to 0.12 ppm.
The residues in fat fall below 0.05 ppm within three weeks.
Practically no more residues are detectable four weeks after the
treatment (<0.02 ppm); in meat practically no more residues are
detectable after only one week. Following application of low dosages,
no residues larger than 0.01 ppm (limit of detection) occurred in
milk. Following backrubber treatment, only traces of residues
appeared. The milk was free of residues.
Following treatment of pigs, residues were also chiefly found in fat.
The internal organs were free of residues. Curing had no effect.
Generally the results are similar to those obtained for cattle.
For sheep, the highest residues appear in fat and are somewhat higher
than those recorded in cattle and pigs. Following single treatment,
which is the customary method of application, the residues do not
exceed 0.5 ppm.
The residues in poultry are very low. They appear chiefly in skin and
fat.
Practically no residues occurred after administration of coumaphos in
the feed.
Tests from sources other than the manufacturer follow:
No residues (<0.002 ppm) of coumaphos, its oxygen analogue or
O,O-diethyl O-(4-methyl-2-oxo-2H-1-benzopyran-7-yl)
phosphorothioate were found in milk samples from cows receiving up to
44 ppm coumaphos in feed (Bowman et al., 1968). After spray
applications of 0.1 per cent and 0.25 per cent coumaphos to dairy
cows, milk from the first two milkings contained residues of 0.01 to
0.03 ppm; no residues were detected in subsequent milkings (Matthyse
and Lisk, 1968).
Residues in the fat of cattle following a single spray treatment with
0.5 per cent coumaphos reached a maximum of 0.50 ppm within a week
after spraying; the duration of detectable residues was less than two
weeks (Claborn et al., 1960).
When hens were dusted individually with 0.25 or 0.5 per cent
coumaphos at a rate of three to four grams per bird, no detectable
residues (<0.02 ppm) were found in the eggs (Knapp and Krause, 1960).
In another experiment, hens were dusted daily with 0.5 per cent dust
for four weeks, receiving 0.02 grams active coumaphos per treatment.
Twelve days after treatments were discontinued no detectable residues
(<0.02 ppm) were found in five hens and a residue of 0.08 ppm in one
hen. No residues were found in the giblets or in eggs collected
throughout the four-week treatment period and the following 12 days
(Knapp, 1962).
When hen-houses were treated with five per cent coumaphos dust or
fogged with a suspension of the 25 per cent wettable powder, less
than 0.15 ppm coumaphos was found in the liver and fat of exposed
RESIDUES OF COUMAPHOS IN SUPERVISED TRIALS - CHEMAGRO CORPORATION
Days Residue
Dosage of No. or after in meat and Residue Residuea
Treatment Animal active duration of last internal organs in fat other
ingredient treatments treatment (ppm) (ppm) (ppm)
Sprayb cow 0.5%, 4 litres 1-7 6-28 0.0 - 0.12 0.0 - 0.45
Sprayb sheep 0.25% 6 8-29 0.0 - 0.20 0.05 - 1.73c
Sprayb goat 0.25% 6 8-29 0.0 - 0.05 0.0 - 0.55
Sprayb pig 0.5% 1-6 7-29 0.0 0.0 - 0.16 0.0 - 0.13 (bacon)
Sprayb hen 0.1%, 0.47 litre 1-3 3.21 0.0 - 0.41 0.0 - 0.08
Pour-on calf 2.56ge 1 15-55 0.01 - 0.05 0.01 - 0.11
Pour-on cow 2%d 1 7-42 0.0 - 0.03 0.0 - 0.07
Backrubber cattle 1% emulsion 28 days 0-7 0.0 - 0.03 0.0 - 0.09 0.0 (milk)
Dust hen 0.25 - 1.0% 1-30 1-35 0.0 - 0.09 0.0 - 0.07 0.0 - 0.31 (skin)
Dust (in box) hen 0.075g/hen 1 1-34 - - 0.0 - 0.03 (eggs)
Feed cow 10-66 ppm 8-120 days - 0.0 0.0 0.0 (milk)
Feed pig 40-80 ppm 63-78 days - 0.0 - 0.05 0.0 - 0.41f
Feed hen 40-131 ppm 1-203 days - 0.0 - 0.06 0.0 - 0.05 0.0 - 0.07(?) (eggs)
a Analysis of chlorferron negative in many instances on various tissues and in milk.
b Spray to runoff.
c 1.73 value is from eight-day pre-slaughter interval. Maximum residue at label interval of 15 days was 0.40 ppm.
d in 100 ml of mineral oil.
e in 375 ml of white oil.
f Residue from sample taken on last day of treatment.
hens. The dust application did not result in any detectable residues
in eggs. In one instance a marginal residue (0.03 ppm) was observed in
eggs from hens exposed to fogging (Shaw et al., 1964).
When coumaphos was fed in mash at rates of 0, 5, 10 and 20 ppm for 14
weeks, no residues (<0.02 ppm) were found in the eggs at any time
(Quigley and Harding, 1963).
Fate of residues
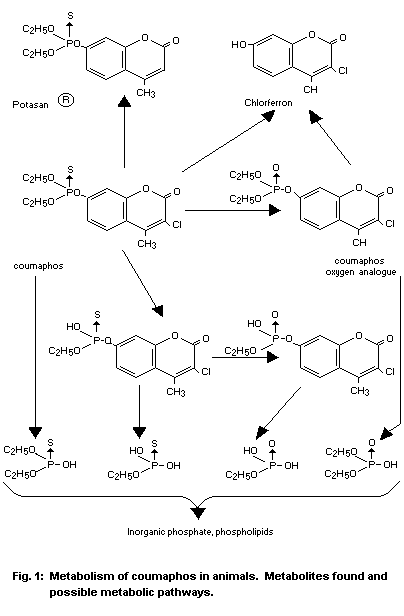
In animals
The metabolism of coumaphos in animals has been extensively studied
following application to cattle, goats, rats and hens; the compound
was administered dermally, orally and by injection of P32-labeled
active ingredient. The diagram in Fig. 1 shows which compounds were
found. The active ingredient and its oxygen analogue undergo the same
metabolic pathway as other diethyl aryl phosphates and thiophosphates
except that the complete degradation of the compounds to phosphoric
acid occurs more rapidly than with most other compounds used for
similar applications. Although coumaphos is more susceptible to
cleavage of the phosphorus-oxygen-ethyl group in vivo than either
diazinon or parathion (O'Brien and Wolfe, 1959; Plapp and Casida,
1958a, 1958b), the resulting desethyl compounds have not been shown to
be present in appreciable amounts as residues and are not likely to
persist in the animal because of their polar nature. A number of
studies, mostly with P32-labeled coumaphos, have shown that the
residues are rapidly eliminated from a variety of animals (Lindquist
et al., 1958; Robbins et al., 1959b; Krueger et al., 1959; Kaplanis et
al., 1959; Vickery and Arthur, 1960; Dorough et al., 1961).
In general, it is expected that the principal components of the
pesticide residue will be the parent compound, its oxygen analogue and
chlorferron. However, a recent study has shown that another metabolite
that may be formed is dechlorinated coumaphos, O,O-diethyl
O-(4-methyl-2-oxo-2H-1-benzopyran-7-yl) phosphorothioate (Potasan
(R)) (Bowman et al., 1968). In the faeces of cows consuming up to 44
ppm coumaphos in their feed, Potasan (R) was found at levels equal to
four to seven per cent of the coumaphos present in the faeces while
the Potasan (R) content of the technical coumaphos used to fortify the
feed was only 0.16 per cent. Potasan (R) has not been found in edible
foods and there is no evidence that it occurs in other than a very
small proportion of the residue.
Evidence of residues in food in commerce or at consumption
In a 1968 survey of slaughter-houses located in five states of the
United States of America, no residues of coumaphos were found in 149
tissue samples (Stewart, 1968).
Other information on identity and properties
Coumaphos is a slightly brownish powder with a weak unpleasant odour.
The compound melts 90-92°C. It is stable to water and moderate heat
but hydrolyzes on refluxing in 1N alkali for two hours. It is soluble
in aromatic solvents, somewhat soluble in alcohols and ketones and
insoluble in water.
Principal formulations are 25 per cent and 50 per cent wettable
powders, 0.5. 1.0 and 5.0 per cent livestock dusts and emulsifiable
concentrates containing 11.6-20 per cent active material. A four per
cent pour-on and two per cent and 50 per cent feed premixes are
also available.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
After oral doses of 20 mg/kg body weight of 32p-labelled coumaphos
were administered to two steers, 38 per cent of the radioactivity was
recorded in the urine and 35 per cent in the faeces during seven days
following dosing. Diethylphosphoric acid and diethylphosphorothioic
acid were the major urinary metabolites. The faeces contained 50 per
cent coumaphos, 32 per cent oxygen analogue and 12 per cent polar
metabolites (Kaplanis et al., 1959).
A similar metabolic pattern was found in rats, goats and cows. A cow
fed 40 mg/kg of 32P-labelled coumaphos had 0.015 ppm equivalents of
radioactive material in the milk after four weeks and a goat fed 30
mg/kg had 0.06 ppm equivalents in the milk after seven days (Krueger
et al., 1959).
Activation of coumaphos to the more potent cholinesterase inhibitor,
the oxygen analogue, was demonstrated in vitro using rat-liver
slices (Vickery and Arthur, 1960).
Acute toxicity
LD50 (mg/kg
Animal Route body-weight) Reference
Mouse Oral 55 Schuleman, 1955
Mouse i.p. 23 Brandenberg, 1956
Rat (M) Oral 35 Bombinski and
DuBois, 1957
Rat (F) Oral 13-30 DuBois and
Schmalegemeier,
1958
Rat (M) i.p. 28-50 DuBois and
Schmalegemeier,
1958
Guinea-pig Oral 160 Bombinski and
(M) DuBois, 1957
Guinea-pig i.p. 140 Bombinski and
(M) DuBois, 1957
Four yearling cattle were given a single dose of 15 mg/kg orally. Very
mild symptoms, principally diarrhoea, were demonstrated by three
cattle. Cholinesterase was depressed 40-75 per cent of normal. At 50
mg/kg two yearling cattle were severely poisoned; one died and the
other recovered after two weeks. Eight out of nine yearling sheep died
when given 40 mg/kg orally (Radeleff et al., 1958). When sheep were
fed 30 mg/kg body weight of coumaphos orally, the mortality was 60 per
cent (Radeleff et al., 1963).
Whole blood cholinesterase was depressed 20 per cent in a horse
poisoned by 25 mg/kg body weight (Jackson et al., 1960).
Administration of coumaphos to rats in combination with 12 other
organo-phosphorus insecticides indicated that significant potentiation
occurred only with malathion. In the case of malathion the LD50 of an
equitoxic mixture with coumaphos was 190 mg/kg as compared with a
value of 455 mg/kg, which would be expected on the basis of strict
additivity. Thus the ratio of observed to expected value is 2.4
(DuBois, 1958a). No potentiation was observed when coumaphos was
administered in combination with three other anticholinesterase
agents, carbaryl, dioxathion and ethion (DuBois, 1960). Simultaneous
administration of piperonyl butoxide clearly increased the dermal
toxicity of coumaphos to rats (DuBois, 1958b).
The joint oral administration to mice of piperonyl butoxide (1:5)
resulted in a four-to six-fold increase in the toxicity of both
coumaphos and its oxygen analogue (Robbins et al., 1959a).
Short-term studies
Rat
Four groups of rats (10 male and 10 female) were fed for 16 weeks on
diets containing 0, 2, 5 or 10 ppm of coumaphos. Growth rate and food
consumption was not significantly different in test and control
groups. None of the dietary levels produced any inhibition of brain
and submaxillary gland cholinesterase. Serum cholinesterase of female
rats fed 10 ppm showed 30 per cent inhibition after eight weeks and
20 per cent after 16 weeks. However, there was no significant change
in that of the males. Erythrocyte cholinesterase of both males and
females fed 10 ppm showed a marked inhibition after eight weeks (40
per cent) but the effect had fallen to 20 per cent inhibition after
16 weeks. No other toxic or pathological lesions were noted in the
test group (Vaughn et al., 1958a).
Groups of five female rats received daily intraperitoneal injections
of coumaphos at levels of 0, 5, 7.5, 12.5 and 25 mg/kg body weight. At
the top dose level all the animals died within eight days,
demonstrating that coumaphos exerts a cumulative lethal effect when
daily doses of one fourth of the acute LD50 are given. No mortalities
occurred during the 60-day experimental period at the lower dosage
levels. At the 12.5 mg/kg level the animals showed typical symptoms
of intoxication, apparent after five injections of the compound. The
effects consisted of extreme irritability, tremors, lacrimation, mild
diarrhoea and rapid loss of weight. After 15 days the symptoms began
to subside and the animals gained weight and appeared nearly normal
throughout the remainder of the 60-day period, with the exception of
the occurrence of mild tremors for about two hours after each
injection. Three out of the five animals in this group developed lens
opacities. At the 5 and 7.5 mg/kg dosage level the animals did not
exhibit any grossly observable symptoms of poisoning or loss of
weight. However, tissue cholinesterase levels were depressed by at
least 75 per cent and remained at that low level throughout the
period of injections (Murphy and DuBois, 1958).
Groups of 12 male and 12 female rats were fed 0, 10, 25 and 100 ppm of
coumaphos in a milk diet for 90 days. Marked weight loss and 100 per
cent mortality after the 90-day period occurred in the 100 ppm group.
Eight of the 24 rats fed 25 ppm died during the 90-day period, whereas
the mortality in the 0 and 10 ppm groups in the milk diet was less
than 10 per cent. Serum and erythrocyte cholinesterase activity of
male and female rats fed 10 ppm was inhibited 60 per cent and 30 per
cent respectively and the effect became more marked at higher dose
levels. Brain and submaxillary gland cholinesterase was inhibited at
the 10 and 25 ppm levels (Doull et al., 1962a).
Dog
Four groups, each of which contained one male and one female dog, were
fed diets containing 2, 5, 10 and 50 ppm of coumaphos for 12 weeks.
None of the animals exhibited any symptoms of cholinesterase
inhibition (parasympathetic stimulation) during the feeding period,
and all dogs appeared normal. Serum and erythrocyte cholinesterase
activity was determined relative to a control value established for
each dog by obtaining samples of blood during an observation period
prior to starting the test diet. At the 50 ppm level, erythrocyte
cholinesterase activity decreased to 65 per cent of control by the
end of four weeks and remained at this low level for the duration of
the experiment. No inhibition of erythrocyte cholinesterase was
observed at the 2, 5 or 10 ppm level. Serum cholinesterase activity
was rapidly decreased to 50 per cent of control at the 10 and 50 ppm
levels after one to two weeks. Return to normal following removal of
coumaphos from the diet at the end of the 12-week period was rapid for
serum (one week) but slower (three weeks) for erythrocyte
cholinesterase. A level of 2 ppm of coumaphos in the diet caused only
slight inhibition of serum cholinesterase (Vaughn et al., 1958b).
Four groups, each of two male and two female dogs, were fed milk diets
containing 0, 10, 25 and 100 ppm for 90 days without any sign of
intoxication except for intermittent periods of diarrhoea in the
female dogs fed 100 ppm. At the 10 ppm level and above, serum
cholinesterase was depressed to greater than 50 per cent of the
control after two weeks. Significant depression of erythrocyte
cholinesterase was evident only at the 100 ppm level and reached a
plateau of 50 per cent of normal after 10 weeks. Sacrifice after 90
days showed no significant depression of brain or liver cholinesterase
except possibly a slight inhibition of brain cholinesterase at 100 ppm
(Doull et al., 1962b).
Four groups of dogs, each of which contained two male and two female
animals, were fed diets containing 0, 2, 10 and 50 ppm of coumaphos.
Growth-rate, food consumption, haematological profile and prothrombin
time was normal in all groups except, possibly, for a slight reduction
in growth-rate of one of the five dogs fed 50 ppm. Inhibition of serum
cholinesterase occurred in the 50 and 10 ppm groups and of erythrocyte
in the 50 ppm group. At sacrifice, after feeding for one year, brain
and liver cholinesterase was depressed in the 50 ppm group and liver
cholinesterase in the 10 ppm group. No inhibition occurred in the 2
ppm group (Doull et al., 1959).
Gross and histological examination of the tissues and organs of
animals used in the latter experiment did not reveal any compound
related effects (Vesselinovitch et al., 1960).
Cattle
Four yearling cattle were fed 5 mg/kg of coumaphos orally daily for
five days. Whole blood cholinesterase was reduced to 50 per cent of
normal (Radeleff et al., 1958).
Long-term studies
Rat
Groups of 50 rats (25 males and 25 females) were fed diets containing
0, 5, 25 or 100 ppm of coumaphos, for two years. Growth and food
consumption was normal for all groups. At the 100 ppm and 25 ppm
level, coumaphos shortened the average life span (25 per cent and 10
per cent respectively). Erythrocyte and serum cholinesterase was
inhibited in the 10 ppm and higher groups, in a dose response
relationship. Inhibition of brain cholinesterase occurred in the 25
ppm and 100 ppm groups. Only animals in the 100 ppm group showed
occasional evidence of toxic effects, mainly irritability and
excitability. The "no effect" level in rats with respect to the
cholinesterase level in tissues assayed is 5 ppm. Kidney weight of
rats in the 10 ppm and higher groups was decreased, this effect being
partly correlated with the dietary levels of coumaphos. At autopsy, no
compound related histologic lesions were found (Doull et al., 1960).
Special studies
(a) Reproduction
Mouse: Reproduction studies have been carried out with groups of 12
male and 24 female mice fed 0, 10, 25 or 100 ppm of coumaphos in their
diet. Male and female mice were able to tolerate coumaphos up to 25
ppm without exhibiting marked changes in fertility, litter size or
ability of offspring to survive for 30 days after birth. At the 100
ppm level, the number of mice that became pregnant was reduced by
about 50 per cent, litter size was reduced by about 50 per cent and
only about 15 per cent of the offsprings survived 30 days. When the
feeding of coumaphos at the 25 ppm level was extended over three
generations of animals, fertility, gestation, viability and lactation
were similar to the controls. Histopathological examination of 12
weanlings of each sex of the third generation did not reveal any
compound-related effects. Only seven per cent of the initial group of
mice fed 100 ppm survived; this incidence of high mortality in the
case of pregnant mice contrasts with a prior study which showed that
when a group of non-pregnant mice were fed 100 ppm of coumaphos for
six weeks, none died. Thus pregnant mice are possibly more susceptible
to the acute toxic effects of coumaphos than are non-pregnant mice.
Cholinesterase inhibition studies were not made in the reproduction
experiment (Doull et al., 1962b).
Chicken: In a three-generation reproduction study, a group of four
male and 20 female chickens were fed diets containing 5, 10 or 25 ppm
coumaphos. Reactions indicative of cholinesterase inhibition were
noted among Fo birds in the 25 ppm group. These reactions occurred at
17 weeks of age and disappeared when levels were reduced to 20 ppm.
The dietary level was returned to 25 ppm for the F1, F2 and F3
birds. No abnormal reactions were noted among any of the F1, F2 and
F3 birds. Body weight, food disappearance, mortality, egg production,
egg weight, egg fertility, egg hatchability and cholinesterase
activity were normal for all test groups in all generations.
Microscopic examination of tissues and organs of F2 birds in the 25
ppm group was also normal (Industrial Bio-test Laboratories, 1966).
(b) Studies of metabolites
The acute oral toxicity of metabolites of coumaphos has been
established. For the oxygen analogue of coumaphos the LD50 for
several species is shown in the following table (DuBois and Plzak,
1959).
Animal Route LD50
mg/kg body weight
Mouse (M) i.p. 4.2
Mouse (F) i.p. 3.8
Rat (M) oral 11.0
Rat (F) oral 8.3
Rat (M) i.p. 2.8
(continued)
Animal Route LD50
mg/kg body weight
Rat (F) i.p. 2.6
Guinea-pig (M) oral 50.0
Guinea-pig (F) i.p. 16.0
At doses approaching the LD50 the animals exhibited symptoms
characteristic of cholinesterase inhibition of the central and
peripheral nervous system. The onset of symptoms occurs more rapidly
with the oxygen analogue than with the parent compound. Inhibition of
cholinesterase activity and its rapid recovery also contrasts with the
prolonged action of coumaphos (DuBois and Plzak, 1959).
The LD50 to rats of the metabolite chlorferron is greater than 1000
mg/kg. It was not possible to kill a rat by either oral or
intraperitoneal administration at this level (DuBois and
Schmalgemeier, 1959).
Groups of rats (10 male and 10 female) were fed diets containing 0, 5,
10 and 50 ppm of chlorferron for 16 weeks. There was no effect on food
consumption, peripheral blood-count and cholinesterase activity of the
brain, submaxillary glands, serum and erythrocytes. Male rats fed
diets containing 10 and 50 ppm showed a slight reduction in growth
rate during the second month, but neither group showed any significant
difference in body weight compared to the controls after 16 weeks.
Studies on organ weight and pathology were omitted (Vaughn et al.,
1958c).
Comments
Early studies on the acute toxicity of coumaphos showed great
variability. The short-term and long-term studies were adequate.
Because cholinesterase inhibition was used as criterion for assessment
the levels studied were too low to detect toxic effects due to
chlorferron. More extensive studies on this metabolite should,
therefore, be carried out. In short-term studies by the
intraperitoneal route in rats, lens opacities were observed at the
highest level tested.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 5 ppm, equivalent to 0.25 mg/kg body weight per day
Dog: 2 ppm, equivalent to 0.05 mg/kg body weight per day
Estimate of temporary acceptable daily intake for man (of parent
compound, oxygen analogue and chlorferron)
0 - 0.0005 mg/kg body weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Coumaphos is used to control pests attacking domestic animals (it is
not used on plants). Numerous studies dealing with various
applications and the efficacy of coumaphos for the control of a
variety of insects have been published. These references are recorded
with FAO. The insect pests against which coumaphos is applied on the
various domestic animals are listed in the following table.
Beef Dairy
Cattle Cattle Sheep Horses Pigs Dogs Poultry Goats
Ticks
(one or
more x x x x x x x
hosts)
Ear
ticks
Mites x x x x x
Fleas x x x
Biting
and x x x x x x x
sucking
lice
Keds x x
Cattle
grubs, x
warbles
Screw worms
and x x x x x x
blowfly
maggots
Stable-,
buffalo and x x x x x x
horn flies
(continued)
Beef Dairy
Cattle Cattle Sheep Horses Pigs Dogs Poultry Goats
Face
flies x x
The compound is administered internally for control of faecal-breeding
flies and of certain endoparasites; in the United States of America up
to 33 ppm of coumaphos is added to the feed of cattle for this purpose
(Anon., 1964-1966, 1968). Enough coumaphos is added to the daily diet
to ensure a dose of 1.2 mg/kg of body weight.
For spray and dip treatments, a suspension of wettable powder with
0.0625 to 0.5 per cent of coumaphos is used and two to four litres of
suspension remain on the animal body. For pour-on treatments, an oil
formulation is applied to the back of cattle in a quantity sufficient
to ensure that the animal receives 10-15 mg of active ingredient per
kg of body weight. Poultry is dusted once weekly with a 0.5 per cent
formulation.
Low dosage treatments (backrubber, up to five per cent dusts, one per
cent mist spray and 1.2 mg/kg/day in feed) may be made to lactating
dairy animals with no time limitation. Lactating animals should not be
given over-all sprays or pour-on treatments. Dry dairy animals should
not be given over-all spray, dip or pour-on treatments within 14 days
of freshening. Baby animals should not be treated before they are
three months old. Three-to six-month-old animals should be sprayed
only lightly. Sheep and goats should not be treated with spray
concentrations greater than 0.25 per cent. Sick animals should not be
treated. Coumaphos should not be used in conjunction with natural and
synthetic pyrethroids or compounds synergizing them (Robbins et al.,
1959a); nor should it be used with other organo-phosphorus compounds
(e.g. malathion) or internal medications, such as phenothiazine (Clark
et al., 1967). Sheep and goats should not be slaughtered within 15
days of treatment.
Residues resulting from supervised trials
A summary of results compiled by Chemagro Corporation and on file with
FAO is given in the following table on page 79. Some of the dose rates
are higher than recommended. The residues consist of coumaphos and its
oxygen analogue.
The residues in cattle, apart from fat samples, ranged up to 0.12 ppm.
The residues in fat fall below 0.05 ppm within three weeks.
Practically no more residues are detectable four weeks after the
treatment (<0.02 ppm); in meat practically no more residues are
detectable after only one week. Following application of low dosages,
no residues larger than 0.01 ppm (limit of detection) occurred in
milk. Following backrubber treatment, only traces of residues
appeared. The milk was free of residues.
Following treatment of pigs, residues were also chiefly found in fat.
The internal organs were free of residues. Curing had no effect.
Generally the results are similar to those obtained for cattle.
For sheep, the highest residues appear in fat and are somewhat higher
than those recorded in cattle and pigs. Following single treatment,
which is the customary method of application, the residues do not
exceed 0.5 ppm.
The residues in poultry are very low. They appear chiefly in skin and
fat.
Practically no residues occurred after administration of coumaphos in
the feed.
Tests from sources other than the manufacturer follow:
No residues (<0.002 ppm) of coumaphos, its oxygen analogue or
O,O-diethyl O-(4-methyl-2-oxo-2H-1-benzopyran-7-yl)
phosphorothioate were found in milk samples from cows receiving up to
44 ppm coumaphos in feed (Bowman et al., 1968). After spray
applications of 0.1 per cent and 0.25 per cent coumaphos to dairy
cows, milk from the first two milkings contained residues of 0.01 to
0.03 ppm; no residues were detected in subsequent milkings (Matthyse
and Lisk, 1968).
Residues in the fat of cattle following a single spray treatment with
0.5 per cent coumaphos reached a maximum of 0.50 ppm within a week
after spraying; the duration of detectable residues was less than two
weeks (Claborn et al., 1960).
When hens were dusted individually with 0.25 or 0.5 per cent
coumaphos at a rate of three to four grams per bird, no detectable
residues (<0.02 ppm) were found in the eggs (Knapp and Krause, 1960).
In another experiment, hens were dusted daily with 0.5 per cent dust
for four weeks, receiving 0.02 grams active coumaphos per treatment.
Twelve days after treatments were discontinued no detectable residues
(<0.02 ppm) were found in five hens and a residue of 0.08 ppm in one
hen. No residues were found in the giblets or in eggs collected
throughout the four-week treatment period and the following 12 days
(Knapp, 1962).
When hen-houses were treated with five per cent coumaphos dust or
fogged with a suspension of the 25 per cent wettable powder, less
than 0.15 ppm coumaphos was found in the liver and fat of exposed
RESIDUES OF COUMAPHOS IN SUPERVISED TRIALS - CHEMAGRO CORPORATION
Days Residue
Dosage of No. or after in meat and Residue Residuea
Treatment Animal active duration of last internal organs in fat other
ingredient treatments treatment (ppm) (ppm) (ppm)
Sprayb cow 0.5%, 4 litres 1-7 6-28 0.0 - 0.12 0.0 - 0.45
Sprayb sheep 0.25% 6 8-29 0.0 - 0.20 0.05 - 1.73c
Sprayb goat 0.25% 6 8-29 0.0 - 0.05 0.0 - 0.55
Sprayb pig 0.5% 1-6 7-29 0.0 0.0 - 0.16 0.0 - 0.13 (bacon)
Sprayb hen 0.1%, 0.47 litre 1-3 3.21 0.0 - 0.41 0.0 - 0.08
Pour-on calf 2.56ge 1 15-55 0.01 - 0.05 0.01 - 0.11
Pour-on cow 2%d 1 7-42 0.0 - 0.03 0.0 - 0.07
Backrubber cattle 1% emulsion 28 days 0-7 0.0 - 0.03 0.0 - 0.09 0.0 (milk)
Dust hen 0.25 - 1.0% 1-30 1-35 0.0 - 0.09 0.0 - 0.07 0.0 - 0.31 (skin)
Dust (in box) hen 0.075g/hen 1 1-34 - - 0.0 - 0.03 (eggs)
Feed cow 10-66 ppm 8-120 days - 0.0 0.0 0.0 (milk)
Feed pig 40-80 ppm 63-78 days - 0.0 - 0.05 0.0 - 0.41f
Feed hen 40-131 ppm 1-203 days - 0.0 - 0.06 0.0 - 0.05 0.0 - 0.07(?) (eggs)
a Analysis of chlorferron negative in many instances on various tissues and in milk.
b Spray to runoff.
c 1.73 value is from eight-day pre-slaughter interval. Maximum residue at label interval of 15 days was 0.40 ppm.
d in 100 ml of mineral oil.
e in 375 ml of white oil.
f Residue from sample taken on last day of treatment.
hens. The dust application did not result in any detectable residues
in eggs. In one instance a marginal residue (0.03 ppm) was observed in
eggs from hens exposed to fogging (Shaw et al., 1964).
When coumaphos was fed in mash at rates of 0, 5, 10 and 20 ppm for 14
weeks, no residues (<0.02 ppm) were found in the eggs at any time
(Quigley and Harding, 1963).
Fate of residues
In animals
The metabolism of coumaphos in animals has been extensively studied
following application to cattle, goats, rats and hens; the compound
was administered dermally, orally and by injection of P32-labeled
active ingredient. The diagram in Fig. 1 shows which compounds were
found. The active ingredient and its oxygen analogue undergo the same
metabolic pathway as other diethyl aryl phosphates and thiophosphates
except that the complete degradation of the compounds to phosphoric
acid occurs more rapidly than with most other compounds used for
similar applications. Although coumaphos is more susceptible to
cleavage of the phosphorus-oxygen-ethyl group in vivo than either
diazinon or parathion (O'Brien and Wolfe, 1959; Plapp and Casida,
1958a, 1958b), the resulting desethyl compounds have not been shown to
be present in appreciable amounts as residues and are not likely to
persist in the animal because of their polar nature. A number of
studies, mostly with P32-labeled coumaphos, have shown that the
residues are rapidly eliminated from a variety of animals (Lindquist
et al., 1958; Robbins et al., 1959b; Krueger et al., 1959; Kaplanis et
al., 1959; Vickery and Arthur, 1960; Dorough et al., 1961).
In general, it is expected that the principal components of the
pesticide residue will be the parent compound, its oxygen analogue and
chlorferron. However, a recent study has shown that another metabolite
that may be formed is dechlorinated coumaphos, O,O-diethyl
O-(4-methyl-2-oxo-2H-1-benzopyran-7-yl) phosphorothioate (Potasan
(R)) (Bowman et al., 1968). In the faeces of cows consuming up to 44
ppm coumaphos in their feed, Potasan (R) was found at levels equal to
four to seven per cent of the coumaphos present in the faeces while
the Potasan (R) content of the technical coumaphos used to fortify the
feed was only 0.16 per cent. Potasan (R) has not been found in edible
foods and there is no evidence that it occurs in other than a very
small proportion of the residue.
Evidence of residues in food in commerce or at consumption
In a 1968 survey of slaughter-houses located in five states of the
United States of America, no residues of coumaphos were found in 149
tissue samples (Stewart, 1968).
 Methods of residue analysis
A fluorescence method has been widely used for determining residues of
coumaphos, its oxygen analogue and the hydrolysis product chlorferron
(Anderson et al., 1959; Adams and Anderson, 1964; MacDougall, (1964).
The recoveries for a dose level of 0.2 ppm are in the range of 90 per
cent, with an error of ± 10 per cent. For coumaphos and/or its oxygen
analogue, the sensitivity of the method is about 0.02 ppm. For
chlorferron, sensitivity is 0.01 to 0.02 ppm according to the nature
and size of the analytical sample. In milk, residues of 0.01 ppm are
detectable for all three compounds.
The gas chromatographic determination of coumaphos itself has been
reported by a number of workers (Bowman and Beroza, 1967; Bonelli et
al., 1964; Bostwick and Giuffrida, 1967; Burke, 1965; Burke and
Holswade, 1964, 1966; Hartmann, 1966; Watts and Storherr, 1968),
usually as part of a general analysis for phosphorus compounds, with
no effort being made to determine the oxygen analogue. Detectors used
in these determinations were electron-capture, microcoulometric,
thermionic and flame-photometric.
The only gas chromatographic determination designed specifically for
coumaphos and some of its metabolites was advanced by Bowman et al.
(1968). They analysed for coumaphos, its oxygen analogue and
Potasan(R) (dechlorinated coumaphos) with the flame-photometric
detector of Brody and Chaney (1966) which is marketed by MicroTek
Instruments Co., Baton Rouge, Louisiana, United States of America. The
method has high specificity, requires little or no clean-up and its
sensitivity is better than 0.003 ppm for the compounds in milk and
0.005 ppm for those in faeces. At the moment, this method appears to
be the most promising one for either regulatory or referee purposes.
However, the fluorescence method may be adequate for regulatory
purposes if a suitable clean-up for the particular product is
available.
Other techniques that have been cited for the analysis of coumaphos
and which may be useful for confirming its presence (usually
qualitatively) are paper chromatography, thin-layer chromatography and
bio-assay. Polarography and colorimetry have also been suggested for
quantitative determination. Methods in addition to those mentioned
that may be used for confirming identity of residues are infra-red,
ultra-violet, mass spectrometry and p-values. Confirmation of the
identity of residues is most desirable.
National tolerances
Country Commodity Tolerance (ppm)
United States of Meat, fat and meat 1
America by-products of cattle,
goats, hogs, horses,
poultry and sheep
Milk fat (reflecting 0.5
negligible residues in
milk)
Eggs zero
Canada Meat of cattle, goats, 0.5
horses, poultry, sheep
and swine
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Coumaphos is used on animals, including poultry, to control insect
pests. It acts both as a contact and systemic insecticide. Application
is made in various ways including dipping, direct spraying, adding to
the feed, pouring over the animals and as dusts in poultry bins. The
insecticide is also added to the feed of livestock to make the faeces
larvicidal (1 mg per kg per day; 33 ppm in the diet) yet no detectable
residues were found in the milk of lactating animals (0.01 ppm
detectable). When the insecticide is used to dust poultry, the eggs
sometimes show residues as high as 0.03 ppm, and residues as high as
0.07 and 0.31 ppm are found in the fat and skin of poultry
respectively.
The compound is widely used in the United States of America and its
use is increasing in such other countries as Canada and Australia.
Several other methods of application are undoubtedly being used in
other countries; however, the meeting had no detailed knowledge of
practices other than those in the United States of America and Canada.
The terminal residues consist of the parent compound plus the oxygen
analogue and certain other degradation products, most of which have
been identified. It was agreed that one of these degradation products,
chlorferron (hydrolysis product), should not be included in tolerance
figures. The tolerance figures should include coumaphos and the oxygen
analogue.
In respect to milk and milk products, the data submitted and reviewed
do not indicate that a tolerance for these products need be
established because no residues were found. In this connexion the
residue data were provided largely by the manufacturer with supporting
data from United States Government experimental stations. There were
no data supplied from other countries.
A method of analysis, recently published, is believed to be suitable
for enforcement purposes. Arrangements should be made for a
collaborative study to evaluate it as a referee method.
Recommendations
Temporary tolerances
The following temporary tolerances (to be in effect until 1972) are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of commodities entering international trade,
the tolerances should be applied by the importing country at the point
of entry or as soon as practicable thereafter. The tolerance figures
include the oxygen analogue.
Meat, including poultry (fat basis) 0.5 ppm (applied at
slaughter)
Eggs (shell-free basis) 0.05 ppm
Further work or information
Required before 30 June 1972
1. Data on the required rates and frequencies of application,
pre-harvest intervals and the resultant residues from countries
other than the United States of America and Canada.
2. Short-term studies of the main metabolites, including
histopathology.
3. Biochemical studies, cholinesterase inhibition studies and
haematological studies, including coagulation effects in man.
Desirable
1. Collaborative studies of the published method of analysis to
evaluate its suitability as a referee method.
2. More extensive studies on the metabolite chlorferron.
3. Further information relating to the observation of lens opacities
in rats.
REFERENCES
Adams, J. M. and Anderson, C. A. (1964) A quantitative method for the
determination of residues of Co-Ral
(0-[3-chloro-4-methyl-umbelliferon] 0,0-diethyl
phosphorothioate) and Chlorferron
(3-chloro-4-methyl-7-hydroxy-coumarin) in animal tissues and milk.
Chemagro Corp. Rep. No. 13:656
Anderson, C. A., Adams, J. M. and MacDougall, D. (1959)
Photofluorometric method for determination of Co-Ral residues in
animal tissues. J. Agr. Food Chem., 7: 256-259
Anon. (1964-1966) U.S. Department of Agriculture Summary of Registered
Agricultural Pesticide Chemical Uses, 2nd Edition and Supplement III.
0,0-diethyl 0-3-chloro-4-methyl-2-oxo-2H-1-benzopyran-7-yl
phosphorothioate, pp. 287-288, issued 10.1.66 and U.S. Department of
Agriculture, Washington, D.C.
Anon. (1968) Suggested guide for the use of insecticides to control
insects affecting crops, livestock, households, stored products,
forests and forest products, 1968. Agriculture Handbook No. 331, U.S.
Department of Agriculture. Superintendent of Documents, U.S.
Government Printing Office, Washington, D.C.
Bombinski, T. J. and DuBois, K. P. (1957) Acute toxicity and
pharmacological effects of
0,0-diethyl-0-(3-chloro-4-methyl-7-courmarinyl) phosphorothioate
(Bayer 21/199). Department of Pharmacology, University of Chicago.
Unpublished report
Bonelli, E. J., Hartmann, H. and Dimick, K. P. (1964) Gas
chromatography retention times and sensitivity data for insecticides
and herbicides. J. Agr. Food Chem., 12: 333-336
Bostwick, D. C. and Giuffrida, L. (1967) Programmed temperature gas
chromatography of pesticides, using electron capture and thermionic
detectors. J. Ass. Offic. Anal. Chem., 50: 577-581
Bowman, M. C. and Beroza, M. (1967) Temperature-programmed gas
chromatography of twenty phosphorus-containing insecticides on four
different columns and its application to the analysis of milk and corn
silage. J. Ass. Offic. Anal. Chem., 50: 1228-1236
Bowman, M. C., Beroza, M., Gordon, C. H., Miller, R. W. and Morgan,
N. O. (1968) A method of analysing the milk and faeces of cows for
coumaphos and its oxygen analogue after feeding coumaphos for control
of house-fly larvae. J. Econ. Entomol., 61: 358-362
Brandenberg, W. (1956) Report of results obtained in the toxicological
examination of Resitox (21/199). Toxicological Industrial Hygiene
Lab., Berlin. Unpublished report
Brody, S. S. and Chaney, J. E. (1966) The application of a specific
detector for phosphorus and for sulfur compounds sensitive to
subnanogram quantities. J. Gas Chromatog., 4: 42-46
Burke, J. A. (1965) Gas chromatography for pesticide residue analysis;
some practical aspects. J. Ass. Offic. Agr. Chem., 48: 1037-1058
Burke, J. and Holswade, W. (1964) Gas chromatography with
microcoulometric detection for pesticide residue analysis. J. Ass.
Offic. Agr. Chem., 47: 845-859
Burke, J. A. and Holswade, W. (1966) A gas chromatographic column for
pesticide residue analysis: retention times and response data. J. Ass.
Offic. Anal. Chem., 49: 374-385
Claborn, H. V., Bushland, R. C., Mann, H. D., Ivey, M. C. and
Radeleff, R. D. (1960) Meat and milk residues from livestock sprays.
J. Agr. Food Chem., 8: 439-442
Clark, D. E., Wright, F. C., Radeleff, R. D., Danz, J. W. and
Lehmann, R. P. (1967) Influence of coumaphos contaminants, vitamin A
and phenothiazine-lead arsenate on certain enzymes and vitamins of
cattle treated with coumaphos. Amer. J. Vet, Res., 28: 89-95
Dorough, H. W., Brady, V. E., jr. Timmerman, J. A., jr and Arthur, B.
W. (1961) Residues in tissues and eggs of poultry receiving Co-Ral
(Bayer 21/199) in the feed. J. Econ. Entomol., 54: 97-100
Doull, J., Root, M. and Cowan, J. (1962a) Ninety-day feeding studies
with Co-Ral in male and female rats and dogs fed a milk diet.
Department of Pharmacology, University of Chicago. Unpublished report
Doull, J., Root, M. and Cowan, J. (1962b) The effect of Co-Ral in the
diet on the reproduction of mice. Department of Pharmacology,
University of Chicago. Unpublished report
Doull, J., Vaughn, G. and Knecht, J. (1959) Chronic toxicity of Co-Ral
(Bayer 21/199) to dogs and rats. Department of Pharmacology,
University of Chicago. Unpublished report
Doull, J., Vesselinovitch, D., Fitch, F., Root, M. and Meskauskas, J.
(1960) Chronic toxicity of Co-Ral fed to rats for a period of two
years. Departments of Pharmacology and Pathology, University of
Chicago. Unpublished report
DuBois, K. P. (1958a) The acute toxicity of Co-Ral in combination with
other organic phosphates. Department of Pharmacology, University of
Chicago. Unpublished report
DuBois, K. P. (1958b) The dermal toxicity of Co-Ral and piperonyl
butoxide given simultaneously to rats. Department of Pharmacology,
University of Chicago. Unpublished report
DuBois, K. P. (1960) The acute toxicity of Co-Ral in combination with
Delnav, Ethion and Sevin to rats. Department of Pharmacology,
University of Chicago. Unpublished report
DuBois, K. P. and Plzak, G. (1959) Studies in the toxicity and anti-
cholinesterase action of the oxygen analogue of Co-Ral. Department of
Pharmacology, University of Chicago. Unpublished report
DuBois, K. P. and Schmalgemeier, D. (1958) Comparison of the acute
toxicity of various Co-Ral preparations to rats. Department of
Pharmacology, University of Chicago. Unpublished report
DuBois, K. P. and Schmalgemeier, D. (1959) The absence of acute
toxicity of chlorferron to rats. Department of Pharmacology,
University of Chicago. Unpublished report
Hartmann, C. H. (1966) Phosphorus detector for pesticides analysis.
Bull. Environ. Contam. Toxicol., 1: 159-168
Hecht, G. (1958) Untitled. Farbenfabriken Bayer, West Germany.
Unpublished report
Industrial Bio-Test Laboratories. (1966) Three generation reproduction
study on Co-Ral. White Leghorn chickens. Unpublished report
Jackson, J. B., Drummond, R. O., Buck, W. B. and Hunt, L. M. (1960)
Toxicity of organic phosphorus insecticides to horses. J. Econ.
Entomol., 53: 602-604
Kaplanis, J. N., Hopkins, D. E. and Treiber, G. H. (1959) Dermal and
oral treatments of cattle with phosphorus-32-labeled Co-Ral. J. Agr.
Food Chem., 7: 483-486
Knapp, F. W. (1962) Poultry tolerance to excessive amounts of Co-Ral
dust. J. Econ. Entomol., 49: 560-561
Knapp, F, W. and Krauss, G. F. (1960) Control of the northern fowl
mite, Ornithonyssus sylviarium (C and F) with ronnel, Bayer L 13/59
and Bayer 21/199. J. Econ. Entomol., 52: 4-5
Krueger, H. R., Casida, J. E. and Niedermeier, R. P. (1959) Bovine
metabolism of organo-phosphorus insecticides. Metabolism and residues
associated with dermal application of Co-Ral to rats, a goat and a
cow. J. Agr. Food Chem., 7: 182-188
Lindquist, D. A., Burns, E. C., Pant, C. P. and Dahm, P. A. (1958)
Fate of P32-labeled Bayer 21/199 in the white rat. J. Econ. Entomol.,
51: 204-206
MacDougall, D. (1964) Co-Ral. In: G. Zweig, ed. Analytical methods
for pesticides, plant growth regulators and food additives. Vol. II,
pp. 83-95. Academic Press, New York and London
Matthysse, J. G. and Lisk, D. (1968) Residues of diazinon, coumaphos,
Ciodrin, methoxychlor and rotenone in cow's milk from treatments
similar to those used for ecto-parasite and fly control on dairy
cattle, with notes on safety of diazinon and Ciodrin to calves.
J. Econ. Entomol., 61: 1394-1398
Murphy, S. D. and DuBois, K. P. (1958) The subacute toxicity of Co-Ral
(0,0-diethyl-0-3-chloro-4-methyl-7-courmarinyl-phosphorothioate; Bayer
21/199 to rats). Department of Pharmacology, University of Chicago.
Unpublished report
O'Brien, R. D. and Wolfe, L. S. (1959) The metabolism of Co-Ral (Bayer
21/199) by tissues of the house-fly, cattle grub, ox, rat and mouse.
J. Econ. Entomol., 52: 692-695
Plapp, F. W. and Casida, J. E. (1958a) Bovine metabolism of
organophosphorus insecticides. Metabolic fate of 0,0-dimethyl
0-(2, 4,5-trichlorophenyl) phosphorothioate in rats and a cow.
J. Agr. Food Chem., 6: 662-667
Plapp, F. W. and Casida, J. E. (1958b) Hydrolysis of the
alkyl-phosphate bond in certain diethyl aryl phosphorothioate
insecticides by rats, cockroaches and alkali. J. Econ. Entomol., 51:
800-803
Quigley, G. D. and Harding, W. C., jr. (1963) Use of Co-Ral (R) as a
systemic insecticide for layers. Poultry Sci., 42: 1247-1248
Radeleff, R. D., Jackson, J. B., Buck, W. B., Younger, R. L.,
Weidenbach, C. P. and Roberts, R. H. (1963) Toxicity studies of Bayer
21/199 in livestock. J. Am. Vet. Med. Assoc., 142: 624-631
Radeleff, R. D., Jackson, J. B., Roberts, R. H. and Hunt, L. M. (1958)
Summary of acute toxicity studies of Bayer 21/199 in livestock.
U.S.D.A., Kerrville, Texas. Unpublished report
Robbins, W. E., Hopkins, T. L., Darrow, D. J. and Eddy, G. W. (1959a)
Synergistic action of piperonyl butoxide with Bayer 21/199 and its
corresponding phosphate in mice. J. Econ. Entomol., 52: 660-663
Robbins, W. E., Hopkins, T. L., Darrow, D. I. and Eddy, G. S. (1959b)
Studies with P32-Bayer 21/199 sprayed on cattle. J. Econ. Entomol.,
52: 214-217
Schuleman, W. (1955) Final opinion on the new insecticide Resitox
(21/199). Pharmacological Institute of Rheinische,
Friedrich-Wilhelm-Universität, Bonn. Unpublished report
Shaw, F. R., Smith, C. T., Anderson, D. L., Fischang, W. J., Ziener,
W. H. and Hurny, J. (1964) The effects of coumaphos on poultry and its
residues in tissue and eggs. J. Econ. Entomol., 57: 516-518
Stewart, J. H. (1968) U.S. Department of Agriculture, Washington, D.C.
Private communication
Vaughn, G., Deininger, E. and Doull, J. (1958a) The effects of diets
containing Co-Ral (Bayer 21/199) on rats. Department of Pharmacology,
University of Chicago. Unpublished report
Vaughn, G., Deininger, E. and Doull, J. (1958b) Measurement of the
safe dietary level of Co-Ral for dogs. Department of Pharmacology,
University of Chicago. Unpublished report
Vaughn, G., Deininger, E. and Doull, J. (1958c) The subacute toxicity
of 3-chloro-4-methyl-umbelliferone to rats. Department of
Pharmacology, University of Chicago. Unpublished report
Vessolinovitch, D., Fitch, F. W. and Wissler, R. W. (1960)
Histopathological examination of the tissues of dogs fed Co-Ral for
one year. Departments of Pharmacology and Pathology, University of
Chicago. Unpublished report
Vickery, D. S. and Arthur, B. W. (1960) Animal systemic activity,
metabolism and stability, Bayer 21/199. J. Econ. Entomol., 53:
1037-1043
Watts, R. W. and Storherr, R. W. (1968) GLC of organophosphorus
pesticide residues: Retention times and response data for three
columns. Abstracts 82nd Ann. Mtg. Ass. Offic. Anal. Chem., Washington,
D.C., October 1968. Abstract 4
Methods of residue analysis
A fluorescence method has been widely used for determining residues of
coumaphos, its oxygen analogue and the hydrolysis product chlorferron
(Anderson et al., 1959; Adams and Anderson, 1964; MacDougall, (1964).
The recoveries for a dose level of 0.2 ppm are in the range of 90 per
cent, with an error of ± 10 per cent. For coumaphos and/or its oxygen
analogue, the sensitivity of the method is about 0.02 ppm. For
chlorferron, sensitivity is 0.01 to 0.02 ppm according to the nature
and size of the analytical sample. In milk, residues of 0.01 ppm are
detectable for all three compounds.
The gas chromatographic determination of coumaphos itself has been
reported by a number of workers (Bowman and Beroza, 1967; Bonelli et
al., 1964; Bostwick and Giuffrida, 1967; Burke, 1965; Burke and
Holswade, 1964, 1966; Hartmann, 1966; Watts and Storherr, 1968),
usually as part of a general analysis for phosphorus compounds, with
no effort being made to determine the oxygen analogue. Detectors used
in these determinations were electron-capture, microcoulometric,
thermionic and flame-photometric.
The only gas chromatographic determination designed specifically for
coumaphos and some of its metabolites was advanced by Bowman et al.
(1968). They analysed for coumaphos, its oxygen analogue and
Potasan(R) (dechlorinated coumaphos) with the flame-photometric
detector of Brody and Chaney (1966) which is marketed by MicroTek
Instruments Co., Baton Rouge, Louisiana, United States of America. The
method has high specificity, requires little or no clean-up and its
sensitivity is better than 0.003 ppm for the compounds in milk and
0.005 ppm for those in faeces. At the moment, this method appears to
be the most promising one for either regulatory or referee purposes.
However, the fluorescence method may be adequate for regulatory
purposes if a suitable clean-up for the particular product is
available.
Other techniques that have been cited for the analysis of coumaphos
and which may be useful for confirming its presence (usually
qualitatively) are paper chromatography, thin-layer chromatography and
bio-assay. Polarography and colorimetry have also been suggested for
quantitative determination. Methods in addition to those mentioned
that may be used for confirming identity of residues are infra-red,
ultra-violet, mass spectrometry and p-values. Confirmation of the
identity of residues is most desirable.
National tolerances
Country Commodity Tolerance (ppm)
United States of Meat, fat and meat 1
America by-products of cattle,
goats, hogs, horses,
poultry and sheep
Milk fat (reflecting 0.5
negligible residues in
milk)
Eggs zero
Canada Meat of cattle, goats, 0.5
horses, poultry, sheep
and swine
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Coumaphos is used on animals, including poultry, to control insect
pests. It acts both as a contact and systemic insecticide. Application
is made in various ways including dipping, direct spraying, adding to
the feed, pouring over the animals and as dusts in poultry bins. The
insecticide is also added to the feed of livestock to make the faeces
larvicidal (1 mg per kg per day; 33 ppm in the diet) yet no detectable
residues were found in the milk of lactating animals (0.01 ppm
detectable). When the insecticide is used to dust poultry, the eggs
sometimes show residues as high as 0.03 ppm, and residues as high as
0.07 and 0.31 ppm are found in the fat and skin of poultry
respectively.
The compound is widely used in the United States of America and its
use is increasing in such other countries as Canada and Australia.
Several other methods of application are undoubtedly being used in
other countries; however, the meeting had no detailed knowledge of
practices other than those in the United States of America and Canada.
The terminal residues consist of the parent compound plus the oxygen
analogue and certain other degradation products, most of which have
been identified. It was agreed that one of these degradation products,
chlorferron (hydrolysis product), should not be included in tolerance
figures. The tolerance figures should include coumaphos and the oxygen
analogue.
In respect to milk and milk products, the data submitted and reviewed
do not indicate that a tolerance for these products need be
established because no residues were found. In this connexion the
residue data were provided largely by the manufacturer with supporting
data from United States Government experimental stations. There were
no data supplied from other countries.
A method of analysis, recently published, is believed to be suitable
for enforcement purposes. Arrangements should be made for a
collaborative study to evaluate it as a referee method.
Recommendations
Temporary tolerances
The following temporary tolerances (to be in effect until 1972) are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of commodities entering international trade,
the tolerances should be applied by the importing country at the point
of entry or as soon as practicable thereafter. The tolerance figures
include the oxygen analogue.
Meat, including poultry (fat basis) 0.5 ppm (applied at
slaughter)
Eggs (shell-free basis) 0.05 ppm
Further work or information
Required before 30 June 1972
1. Data on the required rates and frequencies of application,
pre-harvest intervals and the resultant residues from countries
other than the United States of America and Canada.
2. Short-term studies of the main metabolites, including
histopathology.
3. Biochemical studies, cholinesterase inhibition studies and
haematological studies, including coagulation effects in man.
Desirable
1. Collaborative studies of the published method of analysis to
evaluate its suitability as a referee method.
2. More extensive studies on the metabolite chlorferron.
3. Further information relating to the observation of lens opacities
in rats.
REFERENCES
Adams, J. M. and Anderson, C. A. (1964) A quantitative method for the
determination of residues of Co-Ral
(0-[3-chloro-4-methyl-umbelliferon] 0,0-diethyl
phosphorothioate) and Chlorferron
(3-chloro-4-methyl-7-hydroxy-coumarin) in animal tissues and milk.
Chemagro Corp. Rep. No. 13:656
Anderson, C. A., Adams, J. M. and MacDougall, D. (1959)
Photofluorometric method for determination of Co-Ral residues in
animal tissues. J. Agr. Food Chem., 7: 256-259
Anon. (1964-1966) U.S. Department of Agriculture Summary of Registered
Agricultural Pesticide Chemical Uses, 2nd Edition and Supplement III.
0,0-diethyl 0-3-chloro-4-methyl-2-oxo-2H-1-benzopyran-7-yl
phosphorothioate, pp. 287-288, issued 10.1.66 and U.S. Department of
Agriculture, Washington, D.C.
Anon. (1968) Suggested guide for the use of insecticides to control
insects affecting crops, livestock, households, stored products,
forests and forest products, 1968. Agriculture Handbook No. 331, U.S.
Department of Agriculture. Superintendent of Documents, U.S.
Government Printing Office, Washington, D.C.
Bombinski, T. J. and DuBois, K. P. (1957) Acute toxicity and
pharmacological effects of
0,0-diethyl-0-(3-chloro-4-methyl-7-courmarinyl) phosphorothioate
(Bayer 21/199). Department of Pharmacology, University of Chicago.
Unpublished report
Bonelli, E. J., Hartmann, H. and Dimick, K. P. (1964) Gas
chromatography retention times and sensitivity data for insecticides
and herbicides. J. Agr. Food Chem., 12: 333-336
Bostwick, D. C. and Giuffrida, L. (1967) Programmed temperature gas
chromatography of pesticides, using electron capture and thermionic
detectors. J. Ass. Offic. Anal. Chem., 50: 577-581
Bowman, M. C. and Beroza, M. (1967) Temperature-programmed gas
chromatography of twenty phosphorus-containing insecticides on four
different columns and its application to the analysis of milk and corn
silage. J. Ass. Offic. Anal. Chem., 50: 1228-1236
Bowman, M. C., Beroza, M., Gordon, C. H., Miller, R. W. and Morgan,
N. O. (1968) A method of analysing the milk and faeces of cows for
coumaphos and its oxygen analogue after feeding coumaphos for control
of house-fly larvae. J. Econ. Entomol., 61: 358-362
Brandenberg, W. (1956) Report of results obtained in the toxicological
examination of Resitox (21/199). Toxicological Industrial Hygiene
Lab., Berlin. Unpublished report
Brody, S. S. and Chaney, J. E. (1966) The application of a specific
detector for phosphorus and for sulfur compounds sensitive to
subnanogram quantities. J. Gas Chromatog., 4: 42-46
Burke, J. A. (1965) Gas chromatography for pesticide residue analysis;
some practical aspects. J. Ass. Offic. Agr. Chem., 48: 1037-1058
Burke, J. and Holswade, W. (1964) Gas chromatography with
microcoulometric detection for pesticide residue analysis. J. Ass.
Offic. Agr. Chem., 47: 845-859
Burke, J. A. and Holswade, W. (1966) A gas chromatographic column for
pesticide residue analysis: retention times and response data. J. Ass.
Offic. Anal. Chem., 49: 374-385
Claborn, H. V., Bushland, R. C., Mann, H. D., Ivey, M. C. and
Radeleff, R. D. (1960) Meat and milk residues from livestock sprays.
J. Agr. Food Chem., 8: 439-442
Clark, D. E., Wright, F. C., Radeleff, R. D., Danz, J. W. and
Lehmann, R. P. (1967) Influence of coumaphos contaminants, vitamin A
and phenothiazine-lead arsenate on certain enzymes and vitamins of
cattle treated with coumaphos. Amer. J. Vet, Res., 28: 89-95
Dorough, H. W., Brady, V. E., jr. Timmerman, J. A., jr and Arthur, B.
W. (1961) Residues in tissues and eggs of poultry receiving Co-Ral
(Bayer 21/199) in the feed. J. Econ. Entomol., 54: 97-100
Doull, J., Root, M. and Cowan, J. (1962a) Ninety-day feeding studies
with Co-Ral in male and female rats and dogs fed a milk diet.
Department of Pharmacology, University of Chicago. Unpublished report
Doull, J., Root, M. and Cowan, J. (1962b) The effect of Co-Ral in the
diet on the reproduction of mice. Department of Pharmacology,
University of Chicago. Unpublished report
Doull, J., Vaughn, G. and Knecht, J. (1959) Chronic toxicity of Co-Ral
(Bayer 21/199) to dogs and rats. Department of Pharmacology,
University of Chicago. Unpublished report
Doull, J., Vesselinovitch, D., Fitch, F., Root, M. and Meskauskas, J.
(1960) Chronic toxicity of Co-Ral fed to rats for a period of two
years. Departments of Pharmacology and Pathology, University of
Chicago. Unpublished report
DuBois, K. P. (1958a) The acute toxicity of Co-Ral in combination with
other organic phosphates. Department of Pharmacology, University of
Chicago. Unpublished report
DuBois, K. P. (1958b) The dermal toxicity of Co-Ral and piperonyl
butoxide given simultaneously to rats. Department of Pharmacology,
University of Chicago. Unpublished report
DuBois, K. P. (1960) The acute toxicity of Co-Ral in combination with
Delnav, Ethion and Sevin to rats. Department of Pharmacology,
University of Chicago. Unpublished report
DuBois, K. P. and Plzak, G. (1959) Studies in the toxicity and anti-
cholinesterase action of the oxygen analogue of Co-Ral. Department of
Pharmacology, University of Chicago. Unpublished report
DuBois, K. P. and Schmalgemeier, D. (1958) Comparison of the acute
toxicity of various Co-Ral preparations to rats. Department of
Pharmacology, University of Chicago. Unpublished report
DuBois, K. P. and Schmalgemeier, D. (1959) The absence of acute
toxicity of chlorferron to rats. Department of Pharmacology,
University of Chicago. Unpublished report
Hartmann, C. H. (1966) Phosphorus detector for pesticides analysis.
Bull. Environ. Contam. Toxicol., 1: 159-168
Hecht, G. (1958) Untitled. Farbenfabriken Bayer, West Germany.
Unpublished report
Industrial Bio-Test Laboratories. (1966) Three generation reproduction
study on Co-Ral. White Leghorn chickens. Unpublished report
Jackson, J. B., Drummond, R. O., Buck, W. B. and Hunt, L. M. (1960)
Toxicity of organic phosphorus insecticides to horses. J. Econ.
Entomol., 53: 602-604
Kaplanis, J. N., Hopkins, D. E. and Treiber, G. H. (1959) Dermal and
oral treatments of cattle with phosphorus-32-labeled Co-Ral. J. Agr.
Food Chem., 7: 483-486
Knapp, F. W. (1962) Poultry tolerance to excessive amounts of Co-Ral
dust. J. Econ. Entomol., 49: 560-561
Knapp, F, W. and Krauss, G. F. (1960) Control of the northern fowl
mite, Ornithonyssus sylviarium (C and F) with ronnel, Bayer L 13/59
and Bayer 21/199. J. Econ. Entomol., 52: 4-5
Krueger, H. R., Casida, J. E. and Niedermeier, R. P. (1959) Bovine
metabolism of organo-phosphorus insecticides. Metabolism and residues
associated with dermal application of Co-Ral to rats, a goat and a
cow. J. Agr. Food Chem., 7: 182-188
Lindquist, D. A., Burns, E. C., Pant, C. P. and Dahm, P. A. (1958)
Fate of P32-labeled Bayer 21/199 in the white rat. J. Econ. Entomol.,
51: 204-206
MacDougall, D. (1964) Co-Ral. In: G. Zweig, ed. Analytical methods
for pesticides, plant growth regulators and food additives. Vol. II,
pp. 83-95. Academic Press, New York and London
Matthysse, J. G. and Lisk, D. (1968) Residues of diazinon, coumaphos,
Ciodrin, methoxychlor and rotenone in cow's milk from treatments
similar to those used for ecto-parasite and fly control on dairy
cattle, with notes on safety of diazinon and Ciodrin to calves.
J. Econ. Entomol., 61: 1394-1398
Murphy, S. D. and DuBois, K. P. (1958) The subacute toxicity of Co-Ral
(0,0-diethyl-0-3-chloro-4-methyl-7-courmarinyl-phosphorothioate; Bayer
21/199 to rats). Department of Pharmacology, University of Chicago.
Unpublished report
O'Brien, R. D. and Wolfe, L. S. (1959) The metabolism of Co-Ral (Bayer
21/199) by tissues of the house-fly, cattle grub, ox, rat and mouse.
J. Econ. Entomol., 52: 692-695
Plapp, F. W. and Casida, J. E. (1958a) Bovine metabolism of
organophosphorus insecticides. Metabolic fate of 0,0-dimethyl
0-(2, 4,5-trichlorophenyl) phosphorothioate in rats and a cow.
J. Agr. Food Chem., 6: 662-667
Plapp, F. W. and Casida, J. E. (1958b) Hydrolysis of the
alkyl-phosphate bond in certain diethyl aryl phosphorothioate
insecticides by rats, cockroaches and alkali. J. Econ. Entomol., 51:
800-803
Quigley, G. D. and Harding, W. C., jr. (1963) Use of Co-Ral (R) as a
systemic insecticide for layers. Poultry Sci., 42: 1247-1248
Radeleff, R. D., Jackson, J. B., Buck, W. B., Younger, R. L.,
Weidenbach, C. P. and Roberts, R. H. (1963) Toxicity studies of Bayer
21/199 in livestock. J. Am. Vet. Med. Assoc., 142: 624-631
Radeleff, R. D., Jackson, J. B., Roberts, R. H. and Hunt, L. M. (1958)
Summary of acute toxicity studies of Bayer 21/199 in livestock.
U.S.D.A., Kerrville, Texas. Unpublished report
Robbins, W. E., Hopkins, T. L., Darrow, D. J. and Eddy, G. W. (1959a)
Synergistic action of piperonyl butoxide with Bayer 21/199 and its
corresponding phosphate in mice. J. Econ. Entomol., 52: 660-663
Robbins, W. E., Hopkins, T. L., Darrow, D. I. and Eddy, G. S. (1959b)
Studies with P32-Bayer 21/199 sprayed on cattle. J. Econ. Entomol.,
52: 214-217
Schuleman, W. (1955) Final opinion on the new insecticide Resitox
(21/199). Pharmacological Institute of Rheinische,
Friedrich-Wilhelm-Universität, Bonn. Unpublished report
Shaw, F. R., Smith, C. T., Anderson, D. L., Fischang, W. J., Ziener,
W. H. and Hurny, J. (1964) The effects of coumaphos on poultry and its
residues in tissue and eggs. J. Econ. Entomol., 57: 516-518
Stewart, J. H. (1968) U.S. Department of Agriculture, Washington, D.C.
Private communication
Vaughn, G., Deininger, E. and Doull, J. (1958a) The effects of diets
containing Co-Ral (Bayer 21/199) on rats. Department of Pharmacology,
University of Chicago. Unpublished report
Vaughn, G., Deininger, E. and Doull, J. (1958b) Measurement of the
safe dietary level of Co-Ral for dogs. Department of Pharmacology,
University of Chicago. Unpublished report
Vaughn, G., Deininger, E. and Doull, J. (1958c) The subacute toxicity
of 3-chloro-4-methyl-umbelliferone to rats. Department of
Pharmacology, University of Chicago. Unpublished report
Vessolinovitch, D., Fitch, F. W. and Wissler, R. W. (1960)
Histopathological examination of the tissues of dogs fed Co-Ral for
one year. Departments of Pharmacology and Pathology, University of
Chicago. Unpublished report
Vickery, D. S. and Arthur, B. W. (1960) Animal systemic activity,
metabolism and stability, Bayer 21/199. J. Econ. Entomol., 53:
1037-1043
Watts, R. W. and Storherr, R. W. (1968) GLC of organophosphorus
pesticide residues: Retention times and response data for three
columns. Abstracts 82nd Ann. Mtg. Ass. Offic. Anal. Chem., Washington,
D.C., October 1968. Abstract 4
See Also:
Toxicological Abbreviations
Coumaphos (ICSC)
Coumaphos (WHO Pesticide Residues Series 2)
Coumaphos (WHO Pesticide Residues Series 5)
Coumaphos (Pesticide residues in food: 1978 evaluations)
Coumaphos (Pesticide residues in food: 1980 evaluations)
Coumaphos (Pesticide residues in food: 1983 evaluations)
Coumaphos (Pesticide residues in food: 1990 evaluations Toxicology)
 Other information on identity and properties
Coumaphos is a slightly brownish powder with a weak unpleasant odour.
The compound melts 90-92°C. It is stable to water and moderate heat
but hydrolyzes on refluxing in 1N alkali for two hours. It is soluble
in aromatic solvents, somewhat soluble in alcohols and ketones and
insoluble in water.
Principal formulations are 25 per cent and 50 per cent wettable
powders, 0.5. 1.0 and 5.0 per cent livestock dusts and emulsifiable
concentrates containing 11.6-20 per cent active material. A four per
cent pour-on and two per cent and 50 per cent feed premixes are
also available.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
After oral doses of 20 mg/kg body weight of 32p-labelled coumaphos
were administered to two steers, 38 per cent of the radioactivity was
recorded in the urine and 35 per cent in the faeces during seven days
following dosing. Diethylphosphoric acid and diethylphosphorothioic
acid were the major urinary metabolites. The faeces contained 50 per
cent coumaphos, 32 per cent oxygen analogue and 12 per cent polar
metabolites (Kaplanis et al., 1959).
A similar metabolic pattern was found in rats, goats and cows. A cow
fed 40 mg/kg of 32P-labelled coumaphos had 0.015 ppm equivalents of
radioactive material in the milk after four weeks and a goat fed 30
mg/kg had 0.06 ppm equivalents in the milk after seven days (Krueger
et al., 1959).
Activation of coumaphos to the more potent cholinesterase inhibitor,
the oxygen analogue, was demonstrated in vitro using rat-liver
slices (Vickery and Arthur, 1960).
Acute toxicity
LD50 (mg/kg
Animal Route body-weight) Reference
Mouse Oral 55 Schuleman, 1955
Mouse i.p. 23 Brandenberg, 1956
Rat (M) Oral 35 Bombinski and
DuBois, 1957
Rat (F) Oral 13-30 DuBois and
Schmalegemeier,
1958
Rat (M) i.p. 28-50 DuBois and
Schmalegemeier,
1958
Guinea-pig Oral 160 Bombinski and
(M) DuBois, 1957
Guinea-pig i.p. 140 Bombinski and
(M) DuBois, 1957
Four yearling cattle were given a single dose of 15 mg/kg orally. Very
mild symptoms, principally diarrhoea, were demonstrated by three
cattle. Cholinesterase was depressed 40-75 per cent of normal. At 50
mg/kg two yearling cattle were severely poisoned; one died and the
other recovered after two weeks. Eight out of nine yearling sheep died
when given 40 mg/kg orally (Radeleff et al., 1958). When sheep were
fed 30 mg/kg body weight of coumaphos orally, the mortality was 60 per
cent (Radeleff et al., 1963).
Whole blood cholinesterase was depressed 20 per cent in a horse
poisoned by 25 mg/kg body weight (Jackson et al., 1960).
Administration of coumaphos to rats in combination with 12 other
organo-phosphorus insecticides indicated that significant potentiation
occurred only with malathion. In the case of malathion the LD50 of an
equitoxic mixture with coumaphos was 190 mg/kg as compared with a
value of 455 mg/kg, which would be expected on the basis of strict
additivity. Thus the ratio of observed to expected value is 2.4
(DuBois, 1958a). No potentiation was observed when coumaphos was
administered in combination with three other anticholinesterase
agents, carbaryl, dioxathion and ethion (DuBois, 1960). Simultaneous
administration of piperonyl butoxide clearly increased the dermal
toxicity of coumaphos to rats (DuBois, 1958b).
The joint oral administration to mice of piperonyl butoxide (1:5)
resulted in a four-to six-fold increase in the toxicity of both
coumaphos and its oxygen analogue (Robbins et al., 1959a).
Short-term studies
Rat
Four groups of rats (10 male and 10 female) were fed for 16 weeks on
diets containing 0, 2, 5 or 10 ppm of coumaphos. Growth rate and food
consumption was not significantly different in test and control
groups. None of the dietary levels produced any inhibition of brain
and submaxillary gland cholinesterase. Serum cholinesterase of female
rats fed 10 ppm showed 30 per cent inhibition after eight weeks and
20 per cent after 16 weeks. However, there was no significant change
in that of the males. Erythrocyte cholinesterase of both males and
females fed 10 ppm showed a marked inhibition after eight weeks (40
per cent) but the effect had fallen to 20 per cent inhibition after
16 weeks. No other toxic or pathological lesions were noted in the
test group (Vaughn et al., 1958a).
Groups of five female rats received daily intraperitoneal injections
of coumaphos at levels of 0, 5, 7.5, 12.5 and 25 mg/kg body weight. At
the top dose level all the animals died within eight days,
demonstrating that coumaphos exerts a cumulative lethal effect when
daily doses of one fourth of the acute LD50 are given. No mortalities
occurred during the 60-day experimental period at the lower dosage
levels. At the 12.5 mg/kg level the animals showed typical symptoms
of intoxication, apparent after five injections of the compound. The
effects consisted of extreme irritability, tremors, lacrimation, mild
diarrhoea and rapid loss of weight. After 15 days the symptoms began
to subside and the animals gained weight and appeared nearly normal
throughout the remainder of the 60-day period, with the exception of
the occurrence of mild tremors for about two hours after each
injection. Three out of the five animals in this group developed lens
opacities. At the 5 and 7.5 mg/kg dosage level the animals did not
exhibit any grossly observable symptoms of poisoning or loss of
weight. However, tissue cholinesterase levels were depressed by at
least 75 per cent and remained at that low level throughout the
period of injections (Murphy and DuBois, 1958).
Groups of 12 male and 12 female rats were fed 0, 10, 25 and 100 ppm of
coumaphos in a milk diet for 90 days. Marked weight loss and 100 per
cent mortality after the 90-day period occurred in the 100 ppm group.
Eight of the 24 rats fed 25 ppm died during the 90-day period, whereas
the mortality in the 0 and 10 ppm groups in the milk diet was less
than 10 per cent. Serum and erythrocyte cholinesterase activity of
male and female rats fed 10 ppm was inhibited 60 per cent and 30 per
cent respectively and the effect became more marked at higher dose
levels. Brain and submaxillary gland cholinesterase was inhibited at
the 10 and 25 ppm levels (Doull et al., 1962a).
Dog
Four groups, each of which contained one male and one female dog, were
fed diets containing 2, 5, 10 and 50 ppm of coumaphos for 12 weeks.
None of the animals exhibited any symptoms of cholinesterase
inhibition (parasympathetic stimulation) during the feeding period,
and all dogs appeared normal. Serum and erythrocyte cholinesterase
activity was determined relative to a control value established for
each dog by obtaining samples of blood during an observation period
prior to starting the test diet. At the 50 ppm level, erythrocyte
cholinesterase activity decreased to 65 per cent of control by the
end of four weeks and remained at this low level for the duration of
the experiment. No inhibition of erythrocyte cholinesterase was
observed at the 2, 5 or 10 ppm level. Serum cholinesterase activity
was rapidly decreased to 50 per cent of control at the 10 and 50 ppm
levels after one to two weeks. Return to normal following removal of
coumaphos from the diet at the end of the 12-week period was rapid for
serum (one week) but slower (three weeks) for erythrocyte
cholinesterase. A level of 2 ppm of coumaphos in the diet caused only
slight inhibition of serum cholinesterase (Vaughn et al., 1958b).
Four groups, each of two male and two female dogs, were fed milk diets
containing 0, 10, 25 and 100 ppm for 90 days without any sign of
intoxication except for intermittent periods of diarrhoea in the
female dogs fed 100 ppm. At the 10 ppm level and above, serum
cholinesterase was depressed to greater than 50 per cent of the
control after two weeks. Significant depression of erythrocyte
cholinesterase was evident only at the 100 ppm level and reached a
plateau of 50 per cent of normal after 10 weeks. Sacrifice after 90
days showed no significant depression of brain or liver cholinesterase
except possibly a slight inhibition of brain cholinesterase at 100 ppm
(Doull et al., 1962b).
Four groups of dogs, each of which contained two male and two female
animals, were fed diets containing 0, 2, 10 and 50 ppm of coumaphos.
Growth-rate, food consumption, haematological profile and prothrombin
time was normal in all groups except, possibly, for a slight reduction
in growth-rate of one of the five dogs fed 50 ppm. Inhibition of serum
cholinesterase occurred in the 50 and 10 ppm groups and of erythrocyte
in the 50 ppm group. At sacrifice, after feeding for one year, brain
and liver cholinesterase was depressed in the 50 ppm group and liver
cholinesterase in the 10 ppm group. No inhibition occurred in the 2
ppm group (Doull et al., 1959).
Gross and histological examination of the tissues and organs of
animals used in the latter experiment did not reveal any compound
related effects (Vesselinovitch et al., 1960).
Cattle
Four yearling cattle were fed 5 mg/kg of coumaphos orally daily for
five days. Whole blood cholinesterase was reduced to 50 per cent of
normal (Radeleff et al., 1958).
Long-term studies
Rat
Groups of 50 rats (25 males and 25 females) were fed diets containing
0, 5, 25 or 100 ppm of coumaphos, for two years. Growth and food
consumption was normal for all groups. At the 100 ppm and 25 ppm
level, coumaphos shortened the average life span (25 per cent and 10
per cent respectively). Erythrocyte and serum cholinesterase was
inhibited in the 10 ppm and higher groups, in a dose response
relationship. Inhibition of brain cholinesterase occurred in the 25
ppm and 100 ppm groups. Only animals in the 100 ppm group showed
occasional evidence of toxic effects, mainly irritability and
excitability. The "no effect" level in rats with respect to the
cholinesterase level in tissues assayed is 5 ppm. Kidney weight of
rats in the 10 ppm and higher groups was decreased, this effect being
partly correlated with the dietary levels of coumaphos. At autopsy, no
compound related histologic lesions were found (Doull et al., 1960).
Special studies
(a) Reproduction
Mouse: Reproduction studies have been carried out with groups of 12
male and 24 female mice fed 0, 10, 25 or 100 ppm of coumaphos in their
diet. Male and female mice were able to tolerate coumaphos up to 25
ppm without exhibiting marked changes in fertility, litter size or
ability of offspring to survive for 30 days after birth. At the 100
ppm level, the number of mice that became pregnant was reduced by
about 50 per cent, litter size was reduced by about 50 per cent and
only about 15 per cent of the offsprings survived 30 days. When the
feeding of coumaphos at the 25 ppm level was extended over three
generations of animals, fertility, gestation, viability and lactation
were similar to the controls. Histopathological examination of 12
weanlings of each sex of the third generation did not reveal any
compound-related effects. Only seven per cent of the initial group of
mice fed 100 ppm survived; this incidence of high mortality in the
case of pregnant mice contrasts with a prior study which showed that
when a group of non-pregnant mice were fed 100 ppm of coumaphos for
six weeks, none died. Thus pregnant mice are possibly more susceptible
to the acute toxic effects of coumaphos than are non-pregnant mice.
Cholinesterase inhibition studies were not made in the reproduction
experiment (Doull et al., 1962b).
Chicken: In a three-generation reproduction study, a group of four
male and 20 female chickens were fed diets containing 5, 10 or 25 ppm
coumaphos. Reactions indicative of cholinesterase inhibition were
noted among Fo birds in the 25 ppm group. These reactions occurred at
17 weeks of age and disappeared when levels were reduced to 20 ppm.
The dietary level was returned to 25 ppm for the F1, F2 and F3
birds. No abnormal reactions were noted among any of the F1, F2 and
F3 birds. Body weight, food disappearance, mortality, egg production,
egg weight, egg fertility, egg hatchability and cholinesterase
activity were normal for all test groups in all generations.
Microscopic examination of tissues and organs of F2 birds in the 25
ppm group was also normal (Industrial Bio-test Laboratories, 1966).
(b) Studies of metabolites
The acute oral toxicity of metabolites of coumaphos has been
established. For the oxygen analogue of coumaphos the LD50 for
several species is shown in the following table (DuBois and Plzak,
1959).
Animal Route LD50
mg/kg body weight
Mouse (M) i.p. 4.2
Mouse (F) i.p. 3.8
Rat (M) oral 11.0
Rat (F) oral 8.3
Rat (M) i.p. 2.8
(continued)
Animal Route LD50
mg/kg body weight
Rat (F) i.p. 2.6
Guinea-pig (M) oral 50.0
Guinea-pig (F) i.p. 16.0
At doses approaching the LD50 the animals exhibited symptoms
characteristic of cholinesterase inhibition of the central and
peripheral nervous system. The onset of symptoms occurs more rapidly
with the oxygen analogue than with the parent compound. Inhibition of
cholinesterase activity and its rapid recovery also contrasts with the
prolonged action of coumaphos (DuBois and Plzak, 1959).
The LD50 to rats of the metabolite chlorferron is greater than 1000
mg/kg. It was not possible to kill a rat by either oral or
intraperitoneal administration at this level (DuBois and
Schmalgemeier, 1959).
Groups of rats (10 male and 10 female) were fed diets containing 0, 5,
10 and 50 ppm of chlorferron for 16 weeks. There was no effect on food
consumption, peripheral blood-count and cholinesterase activity of the
brain, submaxillary glands, serum and erythrocytes. Male rats fed
diets containing 10 and 50 ppm showed a slight reduction in growth
rate during the second month, but neither group showed any significant
difference in body weight compared to the controls after 16 weeks.
Studies on organ weight and pathology were omitted (Vaughn et al.,
1958c).
Comments
Early studies on the acute toxicity of coumaphos showed great
variability. The short-term and long-term studies were adequate.
Because cholinesterase inhibition was used as criterion for assessment
the levels studied were too low to detect toxic effects due to
chlorferron. More extensive studies on this metabolite should,
therefore, be carried out. In short-term studies by the
intraperitoneal route in rats, lens opacities were observed at the
highest level tested.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 5 ppm, equivalent to 0.25 mg/kg body weight per day
Dog: 2 ppm, equivalent to 0.05 mg/kg body weight per day
Estimate of temporary acceptable daily intake for man (of parent
compound, oxygen analogue and chlorferron)
0 - 0.0005 mg/kg body weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Coumaphos is used to control pests attacking domestic animals (it is
not used on plants). Numerous studies dealing with various
applications and the efficacy of coumaphos for the control of a
variety of insects have been published. These references are recorded
with FAO. The insect pests against which coumaphos is applied on the
various domestic animals are listed in the following table.
Beef Dairy
Cattle Cattle Sheep Horses Pigs Dogs Poultry Goats
Ticks
(one or
more x x x x x x x
hosts)
Ear
ticks
Mites x x x x x
Fleas x x x
Biting
and x x x x x x x
sucking
lice
Keds x x
Cattle
grubs, x
warbles
Screw worms
and x x x x x x
blowfly
maggots
Stable-,
buffalo and x x x x x x
horn flies
(continued)
Beef Dairy
Cattle Cattle Sheep Horses Pigs Dogs Poultry Goats
Face
flies x x
The compound is administered internally for control of faecal-breeding
flies and of certain endoparasites; in the United States of America up
to 33 ppm of coumaphos is added to the feed of cattle for this purpose
(Anon., 1964-1966, 1968). Enough coumaphos is added to the daily diet
to ensure a dose of 1.2 mg/kg of body weight.
For spray and dip treatments, a suspension of wettable powder with
0.0625 to 0.5 per cent of coumaphos is used and two to four litres of
suspension remain on the animal body. For pour-on treatments, an oil
formulation is applied to the back of cattle in a quantity sufficient
to ensure that the animal receives 10-15 mg of active ingredient per
kg of body weight. Poultry is dusted once weekly with a 0.5 per cent
formulation.
Low dosage treatments (backrubber, up to five per cent dusts, one per
cent mist spray and 1.2 mg/kg/day in feed) may be made to lactating
dairy animals with no time limitation. Lactating animals should not be
given over-all sprays or pour-on treatments. Dry dairy animals should
not be given over-all spray, dip or pour-on treatments within 14 days
of freshening. Baby animals should not be treated before they are
three months old. Three-to six-month-old animals should be sprayed
only lightly. Sheep and goats should not be treated with spray
concentrations greater than 0.25 per cent. Sick animals should not be
treated. Coumaphos should not be used in conjunction with natural and
synthetic pyrethroids or compounds synergizing them (Robbins et al.,
1959a); nor should it be used with other organo-phosphorus compounds
(e.g. malathion) or internal medications, such as phenothiazine (Clark
et al., 1967). Sheep and goats should not be slaughtered within 15
days of treatment.
Residues resulting from supervised trials
A summary of results compiled by Chemagro Corporation and on file with
FAO is given in the following table on page 79. Some of the dose rates
are higher than recommended. The residues consist of coumaphos and its
oxygen analogue.
The residues in cattle, apart from fat samples, ranged up to 0.12 ppm.
The residues in fat fall below 0.05 ppm within three weeks.
Practically no more residues are detectable four weeks after the
treatment (<0.02 ppm); in meat practically no more residues are
detectable after only one week. Following application of low dosages,
no residues larger than 0.01 ppm (limit of detection) occurred in
milk. Following backrubber treatment, only traces of residues
appeared. The milk was free of residues.
Following treatment of pigs, residues were also chiefly found in fat.
The internal organs were free of residues. Curing had no effect.
Generally the results are similar to those obtained for cattle.
For sheep, the highest residues appear in fat and are somewhat higher
than those recorded in cattle and pigs. Following single treatment,
which is the customary method of application, the residues do not
exceed 0.5 ppm.
The residues in poultry are very low. They appear chiefly in skin and
fat.
Practically no residues occurred after administration of coumaphos in
the feed.
Tests from sources other than the manufacturer follow:
No residues (<0.002 ppm) of coumaphos, its oxygen analogue or
O,O-diethyl O-(4-methyl-2-oxo-2H-1-benzopyran-7-yl)
phosphorothioate were found in milk samples from cows receiving up to
44 ppm coumaphos in feed (Bowman et al., 1968). After spray
applications of 0.1 per cent and 0.25 per cent coumaphos to dairy
cows, milk from the first two milkings contained residues of 0.01 to
0.03 ppm; no residues were detected in subsequent milkings (Matthyse
and Lisk, 1968).
Residues in the fat of cattle following a single spray treatment with
0.5 per cent coumaphos reached a maximum of 0.50 ppm within a week
after spraying; the duration of detectable residues was less than two
weeks (Claborn et al., 1960).
When hens were dusted individually with 0.25 or 0.5 per cent
coumaphos at a rate of three to four grams per bird, no detectable
residues (<0.02 ppm) were found in the eggs (Knapp and Krause, 1960).
In another experiment, hens were dusted daily with 0.5 per cent dust
for four weeks, receiving 0.02 grams active coumaphos per treatment.
Twelve days after treatments were discontinued no detectable residues
(<0.02 ppm) were found in five hens and a residue of 0.08 ppm in one
hen. No residues were found in the giblets or in eggs collected
throughout the four-week treatment period and the following 12 days
(Knapp, 1962).
When hen-houses were treated with five per cent coumaphos dust or
fogged with a suspension of the 25 per cent wettable powder, less
than 0.15 ppm coumaphos was found in the liver and fat of exposed
RESIDUES OF COUMAPHOS IN SUPERVISED TRIALS - CHEMAGRO CORPORATION
Days Residue
Dosage of No. or after in meat and Residue Residuea
Treatment Animal active duration of last internal organs in fat other
ingredient treatments treatment (ppm) (ppm) (ppm)
Sprayb cow 0.5%, 4 litres 1-7 6-28 0.0 - 0.12 0.0 - 0.45
Sprayb sheep 0.25% 6 8-29 0.0 - 0.20 0.05 - 1.73c
Sprayb goat 0.25% 6 8-29 0.0 - 0.05 0.0 - 0.55
Sprayb pig 0.5% 1-6 7-29 0.0 0.0 - 0.16 0.0 - 0.13 (bacon)
Sprayb hen 0.1%, 0.47 litre 1-3 3.21 0.0 - 0.41 0.0 - 0.08
Pour-on calf 2.56ge 1 15-55 0.01 - 0.05 0.01 - 0.11
Pour-on cow 2%d 1 7-42 0.0 - 0.03 0.0 - 0.07
Backrubber cattle 1% emulsion 28 days 0-7 0.0 - 0.03 0.0 - 0.09 0.0 (milk)
Dust hen 0.25 - 1.0% 1-30 1-35 0.0 - 0.09 0.0 - 0.07 0.0 - 0.31 (skin)
Dust (in box) hen 0.075g/hen 1 1-34 - - 0.0 - 0.03 (eggs)
Feed cow 10-66 ppm 8-120 days - 0.0 0.0 0.0 (milk)
Feed pig 40-80 ppm 63-78 days - 0.0 - 0.05 0.0 - 0.41f
Feed hen 40-131 ppm 1-203 days - 0.0 - 0.06 0.0 - 0.05 0.0 - 0.07(?) (eggs)
a Analysis of chlorferron negative in many instances on various tissues and in milk.
b Spray to runoff.
c 1.73 value is from eight-day pre-slaughter interval. Maximum residue at label interval of 15 days was 0.40 ppm.
d in 100 ml of mineral oil.
e in 375 ml of white oil.
f Residue from sample taken on last day of treatment.
hens. The dust application did not result in any detectable residues
in eggs. In one instance a marginal residue (0.03 ppm) was observed in
eggs from hens exposed to fogging (Shaw et al., 1964).
When coumaphos was fed in mash at rates of 0, 5, 10 and 20 ppm for 14
weeks, no residues (<0.02 ppm) were found in the eggs at any time
(Quigley and Harding, 1963).
Fate of residues
In animals
The metabolism of coumaphos in animals has been extensively studied
following application to cattle, goats, rats and hens; the compound
was administered dermally, orally and by injection of P32-labeled
active ingredient. The diagram in Fig. 1 shows which compounds were
found. The active ingredient and its oxygen analogue undergo the same
metabolic pathway as other diethyl aryl phosphates and thiophosphates
except that the complete degradation of the compounds to phosphoric
acid occurs more rapidly than with most other compounds used for
similar applications. Although coumaphos is more susceptible to
cleavage of the phosphorus-oxygen-ethyl group in vivo than either
diazinon or parathion (O'Brien and Wolfe, 1959; Plapp and Casida,
1958a, 1958b), the resulting desethyl compounds have not been shown to
be present in appreciable amounts as residues and are not likely to
persist in the animal because of their polar nature. A number of
studies, mostly with P32-labeled coumaphos, have shown that the
residues are rapidly eliminated from a variety of animals (Lindquist
et al., 1958; Robbins et al., 1959b; Krueger et al., 1959; Kaplanis et
al., 1959; Vickery and Arthur, 1960; Dorough et al., 1961).
In general, it is expected that the principal components of the
pesticide residue will be the parent compound, its oxygen analogue and
chlorferron. However, a recent study has shown that another metabolite
that may be formed is dechlorinated coumaphos, O,O-diethyl
O-(4-methyl-2-oxo-2H-1-benzopyran-7-yl) phosphorothioate (Potasan
(R)) (Bowman et al., 1968). In the faeces of cows consuming up to 44
ppm coumaphos in their feed, Potasan (R) was found at levels equal to
four to seven per cent of the coumaphos present in the faeces while
the Potasan (R) content of the technical coumaphos used to fortify the
feed was only 0.16 per cent. Potasan (R) has not been found in edible
foods and there is no evidence that it occurs in other than a very
small proportion of the residue.
Evidence of residues in food in commerce or at consumption
In a 1968 survey of slaughter-houses located in five states of the
United States of America, no residues of coumaphos were found in 149
tissue samples (Stewart, 1968).
Other information on identity and properties
Coumaphos is a slightly brownish powder with a weak unpleasant odour.
The compound melts 90-92°C. It is stable to water and moderate heat
but hydrolyzes on refluxing in 1N alkali for two hours. It is soluble
in aromatic solvents, somewhat soluble in alcohols and ketones and
insoluble in water.
Principal formulations are 25 per cent and 50 per cent wettable
powders, 0.5. 1.0 and 5.0 per cent livestock dusts and emulsifiable
concentrates containing 11.6-20 per cent active material. A four per
cent pour-on and two per cent and 50 per cent feed premixes are
also available.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
After oral doses of 20 mg/kg body weight of 32p-labelled coumaphos
were administered to two steers, 38 per cent of the radioactivity was
recorded in the urine and 35 per cent in the faeces during seven days
following dosing. Diethylphosphoric acid and diethylphosphorothioic
acid were the major urinary metabolites. The faeces contained 50 per
cent coumaphos, 32 per cent oxygen analogue and 12 per cent polar
metabolites (Kaplanis et al., 1959).
A similar metabolic pattern was found in rats, goats and cows. A cow
fed 40 mg/kg of 32P-labelled coumaphos had 0.015 ppm equivalents of
radioactive material in the milk after four weeks and a goat fed 30
mg/kg had 0.06 ppm equivalents in the milk after seven days (Krueger
et al., 1959).
Activation of coumaphos to the more potent cholinesterase inhibitor,
the oxygen analogue, was demonstrated in vitro using rat-liver
slices (Vickery and Arthur, 1960).
Acute toxicity
LD50 (mg/kg
Animal Route body-weight) Reference
Mouse Oral 55 Schuleman, 1955
Mouse i.p. 23 Brandenberg, 1956
Rat (M) Oral 35 Bombinski and
DuBois, 1957
Rat (F) Oral 13-30 DuBois and
Schmalegemeier,
1958
Rat (M) i.p. 28-50 DuBois and
Schmalegemeier,
1958
Guinea-pig Oral 160 Bombinski and
(M) DuBois, 1957
Guinea-pig i.p. 140 Bombinski and
(M) DuBois, 1957
Four yearling cattle were given a single dose of 15 mg/kg orally. Very
mild symptoms, principally diarrhoea, were demonstrated by three
cattle. Cholinesterase was depressed 40-75 per cent of normal. At 50
mg/kg two yearling cattle were severely poisoned; one died and the
other recovered after two weeks. Eight out of nine yearling sheep died
when given 40 mg/kg orally (Radeleff et al., 1958). When sheep were
fed 30 mg/kg body weight of coumaphos orally, the mortality was 60 per
cent (Radeleff et al., 1963).
Whole blood cholinesterase was depressed 20 per cent in a horse
poisoned by 25 mg/kg body weight (Jackson et al., 1960).
Administration of coumaphos to rats in combination with 12 other
organo-phosphorus insecticides indicated that significant potentiation
occurred only with malathion. In the case of malathion the LD50 of an
equitoxic mixture with coumaphos was 190 mg/kg as compared with a
value of 455 mg/kg, which would be expected on the basis of strict
additivity. Thus the ratio of observed to expected value is 2.4
(DuBois, 1958a). No potentiation was observed when coumaphos was
administered in combination with three other anticholinesterase
agents, carbaryl, dioxathion and ethion (DuBois, 1960). Simultaneous
administration of piperonyl butoxide clearly increased the dermal
toxicity of coumaphos to rats (DuBois, 1958b).
The joint oral administration to mice of piperonyl butoxide (1:5)
resulted in a four-to six-fold increase in the toxicity of both
coumaphos and its oxygen analogue (Robbins et al., 1959a).
Short-term studies
Rat
Four groups of rats (10 male and 10 female) were fed for 16 weeks on
diets containing 0, 2, 5 or 10 ppm of coumaphos. Growth rate and food
consumption was not significantly different in test and control
groups. None of the dietary levels produced any inhibition of brain
and submaxillary gland cholinesterase. Serum cholinesterase of female
rats fed 10 ppm showed 30 per cent inhibition after eight weeks and
20 per cent after 16 weeks. However, there was no significant change
in that of the males. Erythrocyte cholinesterase of both males and
females fed 10 ppm showed a marked inhibition after eight weeks (40
per cent) but the effect had fallen to 20 per cent inhibition after
16 weeks. No other toxic or pathological lesions were noted in the
test group (Vaughn et al., 1958a).
Groups of five female rats received daily intraperitoneal injections
of coumaphos at levels of 0, 5, 7.5, 12.5 and 25 mg/kg body weight. At
the top dose level all the animals died within eight days,
demonstrating that coumaphos exerts a cumulative lethal effect when
daily doses of one fourth of the acute LD50 are given. No mortalities
occurred during the 60-day experimental period at the lower dosage
levels. At the 12.5 mg/kg level the animals showed typical symptoms
of intoxication, apparent after five injections of the compound. The
effects consisted of extreme irritability, tremors, lacrimation, mild
diarrhoea and rapid loss of weight. After 15 days the symptoms began
to subside and the animals gained weight and appeared nearly normal
throughout the remainder of the 60-day period, with the exception of
the occurrence of mild tremors for about two hours after each
injection. Three out of the five animals in this group developed lens
opacities. At the 5 and 7.5 mg/kg dosage level the animals did not
exhibit any grossly observable symptoms of poisoning or loss of
weight. However, tissue cholinesterase levels were depressed by at
least 75 per cent and remained at that low level throughout the
period of injections (Murphy and DuBois, 1958).
Groups of 12 male and 12 female rats were fed 0, 10, 25 and 100 ppm of
coumaphos in a milk diet for 90 days. Marked weight loss and 100 per
cent mortality after the 90-day period occurred in the 100 ppm group.
Eight of the 24 rats fed 25 ppm died during the 90-day period, whereas
the mortality in the 0 and 10 ppm groups in the milk diet was less
than 10 per cent. Serum and erythrocyte cholinesterase activity of
male and female rats fed 10 ppm was inhibited 60 per cent and 30 per
cent respectively and the effect became more marked at higher dose
levels. Brain and submaxillary gland cholinesterase was inhibited at
the 10 and 25 ppm levels (Doull et al., 1962a).
Dog
Four groups, each of which contained one male and one female dog, were
fed diets containing 2, 5, 10 and 50 ppm of coumaphos for 12 weeks.
None of the animals exhibited any symptoms of cholinesterase
inhibition (parasympathetic stimulation) during the feeding period,
and all dogs appeared normal. Serum and erythrocyte cholinesterase
activity was determined relative to a control value established for
each dog by obtaining samples of blood during an observation period
prior to starting the test diet. At the 50 ppm level, erythrocyte
cholinesterase activity decreased to 65 per cent of control by the
end of four weeks and remained at this low level for the duration of
the experiment. No inhibition of erythrocyte cholinesterase was
observed at the 2, 5 or 10 ppm level. Serum cholinesterase activity
was rapidly decreased to 50 per cent of control at the 10 and 50 ppm
levels after one to two weeks. Return to normal following removal of
coumaphos from the diet at the end of the 12-week period was rapid for
serum (one week) but slower (three weeks) for erythrocyte
cholinesterase. A level of 2 ppm of coumaphos in the diet caused only
slight inhibition of serum cholinesterase (Vaughn et al., 1958b).
Four groups, each of two male and two female dogs, were fed milk diets
containing 0, 10, 25 and 100 ppm for 90 days without any sign of
intoxication except for intermittent periods of diarrhoea in the
female dogs fed 100 ppm. At the 10 ppm level and above, serum
cholinesterase was depressed to greater than 50 per cent of the
control after two weeks. Significant depression of erythrocyte
cholinesterase was evident only at the 100 ppm level and reached a
plateau of 50 per cent of normal after 10 weeks. Sacrifice after 90
days showed no significant depression of brain or liver cholinesterase
except possibly a slight inhibition of brain cholinesterase at 100 ppm
(Doull et al., 1962b).
Four groups of dogs, each of which contained two male and two female
animals, were fed diets containing 0, 2, 10 and 50 ppm of coumaphos.
Growth-rate, food consumption, haematological profile and prothrombin
time was normal in all groups except, possibly, for a slight reduction
in growth-rate of one of the five dogs fed 50 ppm. Inhibition of serum
cholinesterase occurred in the 50 and 10 ppm groups and of erythrocyte
in the 50 ppm group. At sacrifice, after feeding for one year, brain
and liver cholinesterase was depressed in the 50 ppm group and liver
cholinesterase in the 10 ppm group. No inhibition occurred in the 2
ppm group (Doull et al., 1959).
Gross and histological examination of the tissues and organs of
animals used in the latter experiment did not reveal any compound
related effects (Vesselinovitch et al., 1960).
Cattle
Four yearling cattle were fed 5 mg/kg of coumaphos orally daily for
five days. Whole blood cholinesterase was reduced to 50 per cent of
normal (Radeleff et al., 1958).
Long-term studies
Rat
Groups of 50 rats (25 males and 25 females) were fed diets containing
0, 5, 25 or 100 ppm of coumaphos, for two years. Growth and food
consumption was normal for all groups. At the 100 ppm and 25 ppm
level, coumaphos shortened the average life span (25 per cent and 10
per cent respectively). Erythrocyte and serum cholinesterase was
inhibited in the 10 ppm and higher groups, in a dose response
relationship. Inhibition of brain cholinesterase occurred in the 25
ppm and 100 ppm groups. Only animals in the 100 ppm group showed
occasional evidence of toxic effects, mainly irritability and
excitability. The "no effect" level in rats with respect to the
cholinesterase level in tissues assayed is 5 ppm. Kidney weight of
rats in the 10 ppm and higher groups was decreased, this effect being
partly correlated with the dietary levels of coumaphos. At autopsy, no
compound related histologic lesions were found (Doull et al., 1960).
Special studies
(a) Reproduction
Mouse: Reproduction studies have been carried out with groups of 12
male and 24 female mice fed 0, 10, 25 or 100 ppm of coumaphos in their
diet. Male and female mice were able to tolerate coumaphos up to 25
ppm without exhibiting marked changes in fertility, litter size or
ability of offspring to survive for 30 days after birth. At the 100
ppm level, the number of mice that became pregnant was reduced by
about 50 per cent, litter size was reduced by about 50 per cent and
only about 15 per cent of the offsprings survived 30 days. When the
feeding of coumaphos at the 25 ppm level was extended over three
generations of animals, fertility, gestation, viability and lactation
were similar to the controls. Histopathological examination of 12
weanlings of each sex of the third generation did not reveal any
compound-related effects. Only seven per cent of the initial group of
mice fed 100 ppm survived; this incidence of high mortality in the
case of pregnant mice contrasts with a prior study which showed that
when a group of non-pregnant mice were fed 100 ppm of coumaphos for
six weeks, none died. Thus pregnant mice are possibly more susceptible
to the acute toxic effects of coumaphos than are non-pregnant mice.
Cholinesterase inhibition studies were not made in the reproduction
experiment (Doull et al., 1962b).
Chicken: In a three-generation reproduction study, a group of four
male and 20 female chickens were fed diets containing 5, 10 or 25 ppm
coumaphos. Reactions indicative of cholinesterase inhibition were
noted among Fo birds in the 25 ppm group. These reactions occurred at
17 weeks of age and disappeared when levels were reduced to 20 ppm.
The dietary level was returned to 25 ppm for the F1, F2 and F3
birds. No abnormal reactions were noted among any of the F1, F2 and
F3 birds. Body weight, food disappearance, mortality, egg production,
egg weight, egg fertility, egg hatchability and cholinesterase
activity were normal for all test groups in all generations.
Microscopic examination of tissues and organs of F2 birds in the 25
ppm group was also normal (Industrial Bio-test Laboratories, 1966).
(b) Studies of metabolites
The acute oral toxicity of metabolites of coumaphos has been
established. For the oxygen analogue of coumaphos the LD50 for
several species is shown in the following table (DuBois and Plzak,
1959).
Animal Route LD50
mg/kg body weight
Mouse (M) i.p. 4.2
Mouse (F) i.p. 3.8
Rat (M) oral 11.0
Rat (F) oral 8.3
Rat (M) i.p. 2.8
(continued)
Animal Route LD50
mg/kg body weight
Rat (F) i.p. 2.6
Guinea-pig (M) oral 50.0
Guinea-pig (F) i.p. 16.0
At doses approaching the LD50 the animals exhibited symptoms
characteristic of cholinesterase inhibition of the central and
peripheral nervous system. The onset of symptoms occurs more rapidly
with the oxygen analogue than with the parent compound. Inhibition of
cholinesterase activity and its rapid recovery also contrasts with the
prolonged action of coumaphos (DuBois and Plzak, 1959).
The LD50 to rats of the metabolite chlorferron is greater than 1000
mg/kg. It was not possible to kill a rat by either oral or
intraperitoneal administration at this level (DuBois and
Schmalgemeier, 1959).
Groups of rats (10 male and 10 female) were fed diets containing 0, 5,
10 and 50 ppm of chlorferron for 16 weeks. There was no effect on food
consumption, peripheral blood-count and cholinesterase activity of the
brain, submaxillary glands, serum and erythrocytes. Male rats fed
diets containing 10 and 50 ppm showed a slight reduction in growth
rate during the second month, but neither group showed any significant
difference in body weight compared to the controls after 16 weeks.
Studies on organ weight and pathology were omitted (Vaughn et al.,
1958c).
Comments
Early studies on the acute toxicity of coumaphos showed great
variability. The short-term and long-term studies were adequate.
Because cholinesterase inhibition was used as criterion for assessment
the levels studied were too low to detect toxic effects due to
chlorferron. More extensive studies on this metabolite should,
therefore, be carried out. In short-term studies by the
intraperitoneal route in rats, lens opacities were observed at the
highest level tested.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 5 ppm, equivalent to 0.25 mg/kg body weight per day
Dog: 2 ppm, equivalent to 0.05 mg/kg body weight per day
Estimate of temporary acceptable daily intake for man (of parent
compound, oxygen analogue and chlorferron)
0 - 0.0005 mg/kg body weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Coumaphos is used to control pests attacking domestic animals (it is
not used on plants). Numerous studies dealing with various
applications and the efficacy of coumaphos for the control of a
variety of insects have been published. These references are recorded
with FAO. The insect pests against which coumaphos is applied on the
various domestic animals are listed in the following table.
Beef Dairy
Cattle Cattle Sheep Horses Pigs Dogs Poultry Goats
Ticks
(one or
more x x x x x x x
hosts)
Ear
ticks
Mites x x x x x
Fleas x x x
Biting
and x x x x x x x
sucking
lice
Keds x x
Cattle
grubs, x
warbles
Screw worms
and x x x x x x
blowfly
maggots
Stable-,
buffalo and x x x x x x
horn flies
(continued)
Beef Dairy
Cattle Cattle Sheep Horses Pigs Dogs Poultry Goats
Face
flies x x
The compound is administered internally for control of faecal-breeding
flies and of certain endoparasites; in the United States of America up
to 33 ppm of coumaphos is added to the feed of cattle for this purpose
(Anon., 1964-1966, 1968). Enough coumaphos is added to the daily diet
to ensure a dose of 1.2 mg/kg of body weight.
For spray and dip treatments, a suspension of wettable powder with
0.0625 to 0.5 per cent of coumaphos is used and two to four litres of
suspension remain on the animal body. For pour-on treatments, an oil
formulation is applied to the back of cattle in a quantity sufficient
to ensure that the animal receives 10-15 mg of active ingredient per
kg of body weight. Poultry is dusted once weekly with a 0.5 per cent
formulation.
Low dosage treatments (backrubber, up to five per cent dusts, one per
cent mist spray and 1.2 mg/kg/day in feed) may be made to lactating
dairy animals with no time limitation. Lactating animals should not be
given over-all sprays or pour-on treatments. Dry dairy animals should
not be given over-all spray, dip or pour-on treatments within 14 days
of freshening. Baby animals should not be treated before they are
three months old. Three-to six-month-old animals should be sprayed
only lightly. Sheep and goats should not be treated with spray
concentrations greater than 0.25 per cent. Sick animals should not be
treated. Coumaphos should not be used in conjunction with natural and
synthetic pyrethroids or compounds synergizing them (Robbins et al.,
1959a); nor should it be used with other organo-phosphorus compounds
(e.g. malathion) or internal medications, such as phenothiazine (Clark
et al., 1967). Sheep and goats should not be slaughtered within 15
days of treatment.
Residues resulting from supervised trials
A summary of results compiled by Chemagro Corporation and on file with
FAO is given in the following table on page 79. Some of the dose rates
are higher than recommended. The residues consist of coumaphos and its
oxygen analogue.
The residues in cattle, apart from fat samples, ranged up to 0.12 ppm.
The residues in fat fall below 0.05 ppm within three weeks.
Practically no more residues are detectable four weeks after the
treatment (<0.02 ppm); in meat practically no more residues are
detectable after only one week. Following application of low dosages,
no residues larger than 0.01 ppm (limit of detection) occurred in
milk. Following backrubber treatment, only traces of residues
appeared. The milk was free of residues.
Following treatment of pigs, residues were also chiefly found in fat.
The internal organs were free of residues. Curing had no effect.
Generally the results are similar to those obtained for cattle.
For sheep, the highest residues appear in fat and are somewhat higher
than those recorded in cattle and pigs. Following single treatment,
which is the customary method of application, the residues do not
exceed 0.5 ppm.
The residues in poultry are very low. They appear chiefly in skin and
fat.
Practically no residues occurred after administration of coumaphos in
the feed.
Tests from sources other than the manufacturer follow:
No residues (<0.002 ppm) of coumaphos, its oxygen analogue or
O,O-diethyl O-(4-methyl-2-oxo-2H-1-benzopyran-7-yl)
phosphorothioate were found in milk samples from cows receiving up to
44 ppm coumaphos in feed (Bowman et al., 1968). After spray
applications of 0.1 per cent and 0.25 per cent coumaphos to dairy
cows, milk from the first two milkings contained residues of 0.01 to
0.03 ppm; no residues were detected in subsequent milkings (Matthyse
and Lisk, 1968).
Residues in the fat of cattle following a single spray treatment with
0.5 per cent coumaphos reached a maximum of 0.50 ppm within a week
after spraying; the duration of detectable residues was less than two
weeks (Claborn et al., 1960).
When hens were dusted individually with 0.25 or 0.5 per cent
coumaphos at a rate of three to four grams per bird, no detectable
residues (<0.02 ppm) were found in the eggs (Knapp and Krause, 1960).
In another experiment, hens were dusted daily with 0.5 per cent dust
for four weeks, receiving 0.02 grams active coumaphos per treatment.
Twelve days after treatments were discontinued no detectable residues
(<0.02 ppm) were found in five hens and a residue of 0.08 ppm in one
hen. No residues were found in the giblets or in eggs collected
throughout the four-week treatment period and the following 12 days
(Knapp, 1962).
When hen-houses were treated with five per cent coumaphos dust or
fogged with a suspension of the 25 per cent wettable powder, less
than 0.15 ppm coumaphos was found in the liver and fat of exposed
RESIDUES OF COUMAPHOS IN SUPERVISED TRIALS - CHEMAGRO CORPORATION
Days Residue
Dosage of No. or after in meat and Residue Residuea
Treatment Animal active duration of last internal organs in fat other
ingredient treatments treatment (ppm) (ppm) (ppm)
Sprayb cow 0.5%, 4 litres 1-7 6-28 0.0 - 0.12 0.0 - 0.45
Sprayb sheep 0.25% 6 8-29 0.0 - 0.20 0.05 - 1.73c
Sprayb goat 0.25% 6 8-29 0.0 - 0.05 0.0 - 0.55
Sprayb pig 0.5% 1-6 7-29 0.0 0.0 - 0.16 0.0 - 0.13 (bacon)
Sprayb hen 0.1%, 0.47 litre 1-3 3.21 0.0 - 0.41 0.0 - 0.08
Pour-on calf 2.56ge 1 15-55 0.01 - 0.05 0.01 - 0.11
Pour-on cow 2%d 1 7-42 0.0 - 0.03 0.0 - 0.07
Backrubber cattle 1% emulsion 28 days 0-7 0.0 - 0.03 0.0 - 0.09 0.0 (milk)
Dust hen 0.25 - 1.0% 1-30 1-35 0.0 - 0.09 0.0 - 0.07 0.0 - 0.31 (skin)
Dust (in box) hen 0.075g/hen 1 1-34 - - 0.0 - 0.03 (eggs)
Feed cow 10-66 ppm 8-120 days - 0.0 0.0 0.0 (milk)
Feed pig 40-80 ppm 63-78 days - 0.0 - 0.05 0.0 - 0.41f
Feed hen 40-131 ppm 1-203 days - 0.0 - 0.06 0.0 - 0.05 0.0 - 0.07(?) (eggs)
a Analysis of chlorferron negative in many instances on various tissues and in milk.
b Spray to runoff.
c 1.73 value is from eight-day pre-slaughter interval. Maximum residue at label interval of 15 days was 0.40 ppm.
d in 100 ml of mineral oil.
e in 375 ml of white oil.
f Residue from sample taken on last day of treatment.
hens. The dust application did not result in any detectable residues
in eggs. In one instance a marginal residue (0.03 ppm) was observed in
eggs from hens exposed to fogging (Shaw et al., 1964).
When coumaphos was fed in mash at rates of 0, 5, 10 and 20 ppm for 14
weeks, no residues (<0.02 ppm) were found in the eggs at any time
(Quigley and Harding, 1963).
Fate of residues
In animals
The metabolism of coumaphos in animals has been extensively studied
following application to cattle, goats, rats and hens; the compound
was administered dermally, orally and by injection of P32-labeled
active ingredient. The diagram in Fig. 1 shows which compounds were
found. The active ingredient and its oxygen analogue undergo the same
metabolic pathway as other diethyl aryl phosphates and thiophosphates
except that the complete degradation of the compounds to phosphoric
acid occurs more rapidly than with most other compounds used for
similar applications. Although coumaphos is more susceptible to
cleavage of the phosphorus-oxygen-ethyl group in vivo than either
diazinon or parathion (O'Brien and Wolfe, 1959; Plapp and Casida,
1958a, 1958b), the resulting desethyl compounds have not been shown to
be present in appreciable amounts as residues and are not likely to
persist in the animal because of their polar nature. A number of
studies, mostly with P32-labeled coumaphos, have shown that the
residues are rapidly eliminated from a variety of animals (Lindquist
et al., 1958; Robbins et al., 1959b; Krueger et al., 1959; Kaplanis et
al., 1959; Vickery and Arthur, 1960; Dorough et al., 1961).
In general, it is expected that the principal components of the
pesticide residue will be the parent compound, its oxygen analogue and
chlorferron. However, a recent study has shown that another metabolite
that may be formed is dechlorinated coumaphos, O,O-diethyl
O-(4-methyl-2-oxo-2H-1-benzopyran-7-yl) phosphorothioate (Potasan
(R)) (Bowman et al., 1968). In the faeces of cows consuming up to 44
ppm coumaphos in their feed, Potasan (R) was found at levels equal to
four to seven per cent of the coumaphos present in the faeces while
the Potasan (R) content of the technical coumaphos used to fortify the
feed was only 0.16 per cent. Potasan (R) has not been found in edible
foods and there is no evidence that it occurs in other than a very
small proportion of the residue.
Evidence of residues in food in commerce or at consumption
In a 1968 survey of slaughter-houses located in five states of the
United States of America, no residues of coumaphos were found in 149
tissue samples (Stewart, 1968).
 Methods of residue analysis
A fluorescence method has been widely used for determining residues of
coumaphos, its oxygen analogue and the hydrolysis product chlorferron
(Anderson et al., 1959; Adams and Anderson, 1964; MacDougall, (1964).
The recoveries for a dose level of 0.2 ppm are in the range of 90 per
cent, with an error of ± 10 per cent. For coumaphos and/or its oxygen
analogue, the sensitivity of the method is about 0.02 ppm. For
chlorferron, sensitivity is 0.01 to 0.02 ppm according to the nature
and size of the analytical sample. In milk, residues of 0.01 ppm are
detectable for all three compounds.
The gas chromatographic determination of coumaphos itself has been
reported by a number of workers (Bowman and Beroza, 1967; Bonelli et
al., 1964; Bostwick and Giuffrida, 1967; Burke, 1965; Burke and
Holswade, 1964, 1966; Hartmann, 1966; Watts and Storherr, 1968),
usually as part of a general analysis for phosphorus compounds, with
no effort being made to determine the oxygen analogue. Detectors used
in these determinations were electron-capture, microcoulometric,
thermionic and flame-photometric.
The only gas chromatographic determination designed specifically for
coumaphos and some of its metabolites was advanced by Bowman et al.
(1968). They analysed for coumaphos, its oxygen analogue and
Potasan(R) (dechlorinated coumaphos) with the flame-photometric
detector of Brody and Chaney (1966) which is marketed by MicroTek
Instruments Co., Baton Rouge, Louisiana, United States of America. The
method has high specificity, requires little or no clean-up and its
sensitivity is better than 0.003 ppm for the compounds in milk and
0.005 ppm for those in faeces. At the moment, this method appears to
be the most promising one for either regulatory or referee purposes.
However, the fluorescence method may be adequate for regulatory
purposes if a suitable clean-up for the particular product is
available.
Other techniques that have been cited for the analysis of coumaphos
and which may be useful for confirming its presence (usually
qualitatively) are paper chromatography, thin-layer chromatography and
bio-assay. Polarography and colorimetry have also been suggested for
quantitative determination. Methods in addition to those mentioned
that may be used for confirming identity of residues are infra-red,
ultra-violet, mass spectrometry and p-values. Confirmation of the
identity of residues is most desirable.
National tolerances
Country Commodity Tolerance (ppm)
United States of Meat, fat and meat 1
America by-products of cattle,
goats, hogs, horses,
poultry and sheep
Milk fat (reflecting 0.5
negligible residues in
milk)
Eggs zero
Canada Meat of cattle, goats, 0.5
horses, poultry, sheep
and swine
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Coumaphos is used on animals, including poultry, to control insect
pests. It acts both as a contact and systemic insecticide. Application
is made in various ways including dipping, direct spraying, adding to
the feed, pouring over the animals and as dusts in poultry bins. The
insecticide is also added to the feed of livestock to make the faeces
larvicidal (1 mg per kg per day; 33 ppm in the diet) yet no detectable
residues were found in the milk of lactating animals (0.01 ppm
detectable). When the insecticide is used to dust poultry, the eggs
sometimes show residues as high as 0.03 ppm, and residues as high as
0.07 and 0.31 ppm are found in the fat and skin of poultry
respectively.
The compound is widely used in the United States of America and its
use is increasing in such other countries as Canada and Australia.
Several other methods of application are undoubtedly being used in
other countries; however, the meeting had no detailed knowledge of
practices other than those in the United States of America and Canada.
The terminal residues consist of the parent compound plus the oxygen
analogue and certain other degradation products, most of which have
been identified. It was agreed that one of these degradation products,
chlorferron (hydrolysis product), should not be included in tolerance
figures. The tolerance figures should include coumaphos and the oxygen
analogue.
In respect to milk and milk products, the data submitted and reviewed
do not indicate that a tolerance for these products need be
established because no residues were found. In this connexion the
residue data were provided largely by the manufacturer with supporting
data from United States Government experimental stations. There were
no data supplied from other countries.
A method of analysis, recently published, is believed to be suitable
for enforcement purposes. Arrangements should be made for a
collaborative study to evaluate it as a referee method.
Recommendations
Temporary tolerances
The following temporary tolerances (to be in effect until 1972) are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of commodities entering international trade,
the tolerances should be applied by the importing country at the point
of entry or as soon as practicable thereafter. The tolerance figures
include the oxygen analogue.
Meat, including poultry (fat basis) 0.5 ppm (applied at
slaughter)
Eggs (shell-free basis) 0.05 ppm
Further work or information
Required before 30 June 1972
1. Data on the required rates and frequencies of application,
pre-harvest intervals and the resultant residues from countries
other than the United States of America and Canada.
2. Short-term studies of the main metabolites, including
histopathology.
3. Biochemical studies, cholinesterase inhibition studies and
haematological studies, including coagulation effects in man.
Desirable
1. Collaborative studies of the published method of analysis to
evaluate its suitability as a referee method.
2. More extensive studies on the metabolite chlorferron.
3. Further information relating to the observation of lens opacities
in rats.
REFERENCES
Adams, J. M. and Anderson, C. A. (1964) A quantitative method for the
determination of residues of Co-Ral
(0-[3-chloro-4-methyl-umbelliferon] 0,0-diethyl
phosphorothioate) and Chlorferron
(3-chloro-4-methyl-7-hydroxy-coumarin) in animal tissues and milk.
Chemagro Corp. Rep. No. 13:656
Anderson, C. A., Adams, J. M. and MacDougall, D. (1959)
Photofluorometric method for determination of Co-Ral residues in
animal tissues. J. Agr. Food Chem., 7: 256-259
Anon. (1964-1966) U.S. Department of Agriculture Summary of Registered
Agricultural Pesticide Chemical Uses, 2nd Edition and Supplement III.
0,0-diethyl 0-3-chloro-4-methyl-2-oxo-2H-1-benzopyran-7-yl
phosphorothioate, pp. 287-288, issued 10.1.66 and U.S. Department of
Agriculture, Washington, D.C.
Anon. (1968) Suggested guide for the use of insecticides to control
insects affecting crops, livestock, households, stored products,
forests and forest products, 1968. Agriculture Handbook No. 331, U.S.
Department of Agriculture. Superintendent of Documents, U.S.
Government Printing Office, Washington, D.C.
Bombinski, T. J. and DuBois, K. P. (1957) Acute toxicity and
pharmacological effects of
0,0-diethyl-0-(3-chloro-4-methyl-7-courmarinyl) phosphorothioate
(Bayer 21/199). Department of Pharmacology, University of Chicago.
Unpublished report
Bonelli, E. J., Hartmann, H. and Dimick, K. P. (1964) Gas
chromatography retention times and sensitivity data for insecticides
and herbicides. J. Agr. Food Chem., 12: 333-336
Bostwick, D. C. and Giuffrida, L. (1967) Programmed temperature gas
chromatography of pesticides, using electron capture and thermionic
detectors. J. Ass. Offic. Anal. Chem., 50: 577-581
Bowman, M. C. and Beroza, M. (1967) Temperature-programmed gas
chromatography of twenty phosphorus-containing insecticides on four
different columns and its application to the analysis of milk and corn
silage. J. Ass. Offic. Anal. Chem., 50: 1228-1236
Bowman, M. C., Beroza, M., Gordon, C. H., Miller, R. W. and Morgan,
N. O. (1968) A method of analysing the milk and faeces of cows for
coumaphos and its oxygen analogue after feeding coumaphos for control
of house-fly larvae. J. Econ. Entomol., 61: 358-362
Brandenberg, W. (1956) Report of results obtained in the toxicological
examination of Resitox (21/199). Toxicological Industrial Hygiene
Lab., Berlin. Unpublished report
Brody, S. S. and Chaney, J. E. (1966) The application of a specific
detector for phosphorus and for sulfur compounds sensitive to
subnanogram quantities. J. Gas Chromatog., 4: 42-46
Burke, J. A. (1965) Gas chromatography for pesticide residue analysis;
some practical aspects. J. Ass. Offic. Agr. Chem., 48: 1037-1058
Burke, J. and Holswade, W. (1964) Gas chromatography with
microcoulometric detection for pesticide residue analysis. J. Ass.
Offic. Agr. Chem., 47: 845-859
Burke, J. A. and Holswade, W. (1966) A gas chromatographic column for
pesticide residue analysis: retention times and response data. J. Ass.
Offic. Anal. Chem., 49: 374-385
Claborn, H. V., Bushland, R. C., Mann, H. D., Ivey, M. C. and
Radeleff, R. D. (1960) Meat and milk residues from livestock sprays.
J. Agr. Food Chem., 8: 439-442
Clark, D. E., Wright, F. C., Radeleff, R. D., Danz, J. W. and
Lehmann, R. P. (1967) Influence of coumaphos contaminants, vitamin A
and phenothiazine-lead arsenate on certain enzymes and vitamins of
cattle treated with coumaphos. Amer. J. Vet, Res., 28: 89-95
Dorough, H. W., Brady, V. E., jr. Timmerman, J. A., jr and Arthur, B.
W. (1961) Residues in tissues and eggs of poultry receiving Co-Ral
(Bayer 21/199) in the feed. J. Econ. Entomol., 54: 97-100
Doull, J., Root, M. and Cowan, J. (1962a) Ninety-day feeding studies
with Co-Ral in male and female rats and dogs fed a milk diet.
Department of Pharmacology, University of Chicago. Unpublished report
Doull, J., Root, M. and Cowan, J. (1962b) The effect of Co-Ral in the
diet on the reproduction of mice. Department of Pharmacology,
University of Chicago. Unpublished report
Doull, J., Vaughn, G. and Knecht, J. (1959) Chronic toxicity of Co-Ral
(Bayer 21/199) to dogs and rats. Department of Pharmacology,
University of Chicago. Unpublished report
Doull, J., Vesselinovitch, D., Fitch, F., Root, M. and Meskauskas, J.
(1960) Chronic toxicity of Co-Ral fed to rats for a period of two
years. Departments of Pharmacology and Pathology, University of
Chicago. Unpublished report
DuBois, K. P. (1958a) The acute toxicity of Co-Ral in combination with
other organic phosphates. Department of Pharmacology, University of
Chicago. Unpublished report
DuBois, K. P. (1958b) The dermal toxicity of Co-Ral and piperonyl
butoxide given simultaneously to rats. Department of Pharmacology,
University of Chicago. Unpublished report
DuBois, K. P. (1960) The acute toxicity of Co-Ral in combination with
Delnav, Ethion and Sevin to rats. Department of Pharmacology,
University of Chicago. Unpublished report
DuBois, K. P. and Plzak, G. (1959) Studies in the toxicity and anti-
cholinesterase action of the oxygen analogue of Co-Ral. Department of
Pharmacology, University of Chicago. Unpublished report
DuBois, K. P. and Schmalgemeier, D. (1958) Comparison of the acute
toxicity of various Co-Ral preparations to rats. Department of
Pharmacology, University of Chicago. Unpublished report
DuBois, K. P. and Schmalgemeier, D. (1959) The absence of acute
toxicity of chlorferron to rats. Department of Pharmacology,
University of Chicago. Unpublished report
Hartmann, C. H. (1966) Phosphorus detector for pesticides analysis.
Bull. Environ. Contam. Toxicol., 1: 159-168
Hecht, G. (1958) Untitled. Farbenfabriken Bayer, West Germany.
Unpublished report
Industrial Bio-Test Laboratories. (1966) Three generation reproduction
study on Co-Ral. White Leghorn chickens. Unpublished report
Jackson, J. B., Drummond, R. O., Buck, W. B. and Hunt, L. M. (1960)
Toxicity of organic phosphorus insecticides to horses. J. Econ.
Entomol., 53: 602-604
Kaplanis, J. N., Hopkins, D. E. and Treiber, G. H. (1959) Dermal and
oral treatments of cattle with phosphorus-32-labeled Co-Ral. J. Agr.
Food Chem., 7: 483-486
Knapp, F. W. (1962) Poultry tolerance to excessive amounts of Co-Ral
dust. J. Econ. Entomol., 49: 560-561
Knapp, F, W. and Krauss, G. F. (1960) Control of the northern fowl
mite, Ornithonyssus sylviarium (C and F) with ronnel, Bayer L 13/59
and Bayer 21/199. J. Econ. Entomol., 52: 4-5
Krueger, H. R., Casida, J. E. and Niedermeier, R. P. (1959) Bovine
metabolism of organo-phosphorus insecticides. Metabolism and residues
associated with dermal application of Co-Ral to rats, a goat and a
cow. J. Agr. Food Chem., 7: 182-188
Lindquist, D. A., Burns, E. C., Pant, C. P. and Dahm, P. A. (1958)
Fate of P32-labeled Bayer 21/199 in the white rat. J. Econ. Entomol.,
51: 204-206
MacDougall, D. (1964) Co-Ral. In: G. Zweig, ed. Analytical methods
for pesticides, plant growth regulators and food additives. Vol. II,
pp. 83-95. Academic Press, New York and London
Matthysse, J. G. and Lisk, D. (1968) Residues of diazinon, coumaphos,
Ciodrin, methoxychlor and rotenone in cow's milk from treatments
similar to those used for ecto-parasite and fly control on dairy
cattle, with notes on safety of diazinon and Ciodrin to calves.
J. Econ. Entomol., 61: 1394-1398
Murphy, S. D. and DuBois, K. P. (1958) The subacute toxicity of Co-Ral
(0,0-diethyl-0-3-chloro-4-methyl-7-courmarinyl-phosphorothioate; Bayer
21/199 to rats). Department of Pharmacology, University of Chicago.
Unpublished report
O'Brien, R. D. and Wolfe, L. S. (1959) The metabolism of Co-Ral (Bayer
21/199) by tissues of the house-fly, cattle grub, ox, rat and mouse.
J. Econ. Entomol., 52: 692-695
Plapp, F. W. and Casida, J. E. (1958a) Bovine metabolism of
organophosphorus insecticides. Metabolic fate of 0,0-dimethyl
0-(2, 4,5-trichlorophenyl) phosphorothioate in rats and a cow.
J. Agr. Food Chem., 6: 662-667
Plapp, F. W. and Casida, J. E. (1958b) Hydrolysis of the
alkyl-phosphate bond in certain diethyl aryl phosphorothioate
insecticides by rats, cockroaches and alkali. J. Econ. Entomol., 51:
800-803
Quigley, G. D. and Harding, W. C., jr. (1963) Use of Co-Ral (R) as a
systemic insecticide for layers. Poultry Sci., 42: 1247-1248
Radeleff, R. D., Jackson, J. B., Buck, W. B., Younger, R. L.,
Weidenbach, C. P. and Roberts, R. H. (1963) Toxicity studies of Bayer
21/199 in livestock. J. Am. Vet. Med. Assoc., 142: 624-631
Radeleff, R. D., Jackson, J. B., Roberts, R. H. and Hunt, L. M. (1958)
Summary of acute toxicity studies of Bayer 21/199 in livestock.
U.S.D.A., Kerrville, Texas. Unpublished report
Robbins, W. E., Hopkins, T. L., Darrow, D. J. and Eddy, G. W. (1959a)
Synergistic action of piperonyl butoxide with Bayer 21/199 and its
corresponding phosphate in mice. J. Econ. Entomol., 52: 660-663
Robbins, W. E., Hopkins, T. L., Darrow, D. I. and Eddy, G. S. (1959b)
Studies with P32-Bayer 21/199 sprayed on cattle. J. Econ. Entomol.,
52: 214-217
Schuleman, W. (1955) Final opinion on the new insecticide Resitox
(21/199). Pharmacological Institute of Rheinische,
Friedrich-Wilhelm-Universität, Bonn. Unpublished report
Shaw, F. R., Smith, C. T., Anderson, D. L., Fischang, W. J., Ziener,
W. H. and Hurny, J. (1964) The effects of coumaphos on poultry and its
residues in tissue and eggs. J. Econ. Entomol., 57: 516-518
Stewart, J. H. (1968) U.S. Department of Agriculture, Washington, D.C.
Private communication
Vaughn, G., Deininger, E. and Doull, J. (1958a) The effects of diets
containing Co-Ral (Bayer 21/199) on rats. Department of Pharmacology,
University of Chicago. Unpublished report
Vaughn, G., Deininger, E. and Doull, J. (1958b) Measurement of the
safe dietary level of Co-Ral for dogs. Department of Pharmacology,
University of Chicago. Unpublished report
Vaughn, G., Deininger, E. and Doull, J. (1958c) The subacute toxicity
of 3-chloro-4-methyl-umbelliferone to rats. Department of
Pharmacology, University of Chicago. Unpublished report
Vessolinovitch, D., Fitch, F. W. and Wissler, R. W. (1960)
Histopathological examination of the tissues of dogs fed Co-Ral for
one year. Departments of Pharmacology and Pathology, University of
Chicago. Unpublished report
Vickery, D. S. and Arthur, B. W. (1960) Animal systemic activity,
metabolism and stability, Bayer 21/199. J. Econ. Entomol., 53:
1037-1043
Watts, R. W. and Storherr, R. W. (1968) GLC of organophosphorus
pesticide residues: Retention times and response data for three
columns. Abstracts 82nd Ann. Mtg. Ass. Offic. Anal. Chem., Washington,
D.C., October 1968. Abstract 4
Methods of residue analysis
A fluorescence method has been widely used for determining residues of
coumaphos, its oxygen analogue and the hydrolysis product chlorferron
(Anderson et al., 1959; Adams and Anderson, 1964; MacDougall, (1964).
The recoveries for a dose level of 0.2 ppm are in the range of 90 per
cent, with an error of ± 10 per cent. For coumaphos and/or its oxygen
analogue, the sensitivity of the method is about 0.02 ppm. For
chlorferron, sensitivity is 0.01 to 0.02 ppm according to the nature
and size of the analytical sample. In milk, residues of 0.01 ppm are
detectable for all three compounds.
The gas chromatographic determination of coumaphos itself has been
reported by a number of workers (Bowman and Beroza, 1967; Bonelli et
al., 1964; Bostwick and Giuffrida, 1967; Burke, 1965; Burke and
Holswade, 1964, 1966; Hartmann, 1966; Watts and Storherr, 1968),
usually as part of a general analysis for phosphorus compounds, with
no effort being made to determine the oxygen analogue. Detectors used
in these determinations were electron-capture, microcoulometric,
thermionic and flame-photometric.
The only gas chromatographic determination designed specifically for
coumaphos and some of its metabolites was advanced by Bowman et al.
(1968). They analysed for coumaphos, its oxygen analogue and
Potasan(R) (dechlorinated coumaphos) with the flame-photometric
detector of Brody and Chaney (1966) which is marketed by MicroTek
Instruments Co., Baton Rouge, Louisiana, United States of America. The
method has high specificity, requires little or no clean-up and its
sensitivity is better than 0.003 ppm for the compounds in milk and
0.005 ppm for those in faeces. At the moment, this method appears to
be the most promising one for either regulatory or referee purposes.
However, the fluorescence method may be adequate for regulatory
purposes if a suitable clean-up for the particular product is
available.
Other techniques that have been cited for the analysis of coumaphos
and which may be useful for confirming its presence (usually
qualitatively) are paper chromatography, thin-layer chromatography and
bio-assay. Polarography and colorimetry have also been suggested for
quantitative determination. Methods in addition to those mentioned
that may be used for confirming identity of residues are infra-red,
ultra-violet, mass spectrometry and p-values. Confirmation of the
identity of residues is most desirable.
National tolerances
Country Commodity Tolerance (ppm)
United States of Meat, fat and meat 1
America by-products of cattle,
goats, hogs, horses,
poultry and sheep
Milk fat (reflecting 0.5
negligible residues in
milk)
Eggs zero
Canada Meat of cattle, goats, 0.5
horses, poultry, sheep
and swine
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Coumaphos is used on animals, including poultry, to control insect
pests. It acts both as a contact and systemic insecticide. Application
is made in various ways including dipping, direct spraying, adding to
the feed, pouring over the animals and as dusts in poultry bins. The
insecticide is also added to the feed of livestock to make the faeces
larvicidal (1 mg per kg per day; 33 ppm in the diet) yet no detectable
residues were found in the milk of lactating animals (0.01 ppm
detectable). When the insecticide is used to dust poultry, the eggs
sometimes show residues as high as 0.03 ppm, and residues as high as
0.07 and 0.31 ppm are found in the fat and skin of poultry
respectively.
The compound is widely used in the United States of America and its
use is increasing in such other countries as Canada and Australia.
Several other methods of application are undoubtedly being used in
other countries; however, the meeting had no detailed knowledge of
practices other than those in the United States of America and Canada.
The terminal residues consist of the parent compound plus the oxygen
analogue and certain other degradation products, most of which have
been identified. It was agreed that one of these degradation products,
chlorferron (hydrolysis product), should not be included in tolerance
figures. The tolerance figures should include coumaphos and the oxygen
analogue.
In respect to milk and milk products, the data submitted and reviewed
do not indicate that a tolerance for these products need be
established because no residues were found. In this connexion the
residue data were provided largely by the manufacturer with supporting
data from United States Government experimental stations. There were
no data supplied from other countries.
A method of analysis, recently published, is believed to be suitable
for enforcement purposes. Arrangements should be made for a
collaborative study to evaluate it as a referee method.
Recommendations
Temporary tolerances
The following temporary tolerances (to be in effect until 1972) are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of commodities entering international trade,
the tolerances should be applied by the importing country at the point
of entry or as soon as practicable thereafter. The tolerance figures
include the oxygen analogue.
Meat, including poultry (fat basis) 0.5 ppm (applied at
slaughter)
Eggs (shell-free basis) 0.05 ppm
Further work or information
Required before 30 June 1972
1. Data on the required rates and frequencies of application,
pre-harvest intervals and the resultant residues from countries
other than the United States of America and Canada.
2. Short-term studies of the main metabolites, including
histopathology.
3. Biochemical studies, cholinesterase inhibition studies and
haematological studies, including coagulation effects in man.
Desirable
1. Collaborative studies of the published method of analysis to
evaluate its suitability as a referee method.
2. More extensive studies on the metabolite chlorferron.
3. Further information relating to the observation of lens opacities
in rats.
REFERENCES
Adams, J. M. and Anderson, C. A. (1964) A quantitative method for the
determination of residues of Co-Ral
(0-[3-chloro-4-methyl-umbelliferon] 0,0-diethyl
phosphorothioate) and Chlorferron
(3-chloro-4-methyl-7-hydroxy-coumarin) in animal tissues and milk.
Chemagro Corp. Rep. No. 13:656
Anderson, C. A., Adams, J. M. and MacDougall, D. (1959)
Photofluorometric method for determination of Co-Ral residues in
animal tissues. J. Agr. Food Chem., 7: 256-259
Anon. (1964-1966) U.S. Department of Agriculture Summary of Registered
Agricultural Pesticide Chemical Uses, 2nd Edition and Supplement III.
0,0-diethyl 0-3-chloro-4-methyl-2-oxo-2H-1-benzopyran-7-yl
phosphorothioate, pp. 287-288, issued 10.1.66 and U.S. Department of
Agriculture, Washington, D.C.
Anon. (1968) Suggested guide for the use of insecticides to control
insects affecting crops, livestock, households, stored products,
forests and forest products, 1968. Agriculture Handbook No. 331, U.S.
Department of Agriculture. Superintendent of Documents, U.S.
Government Printing Office, Washington, D.C.
Bombinski, T. J. and DuBois, K. P. (1957) Acute toxicity and
pharmacological effects of
0,0-diethyl-0-(3-chloro-4-methyl-7-courmarinyl) phosphorothioate
(Bayer 21/199). Department of Pharmacology, University of Chicago.
Unpublished report
Bonelli, E. J., Hartmann, H. and Dimick, K. P. (1964) Gas
chromatography retention times and sensitivity data for insecticides
and herbicides. J. Agr. Food Chem., 12: 333-336
Bostwick, D. C. and Giuffrida, L. (1967) Programmed temperature gas
chromatography of pesticides, using electron capture and thermionic
detectors. J. Ass. Offic. Anal. Chem., 50: 577-581
Bowman, M. C. and Beroza, M. (1967) Temperature-programmed gas
chromatography of twenty phosphorus-containing insecticides on four
different columns and its application to the analysis of milk and corn
silage. J. Ass. Offic. Anal. Chem., 50: 1228-1236
Bowman, M. C., Beroza, M., Gordon, C. H., Miller, R. W. and Morgan,
N. O. (1968) A method of analysing the milk and faeces of cows for
coumaphos and its oxygen analogue after feeding coumaphos for control
of house-fly larvae. J. Econ. Entomol., 61: 358-362
Brandenberg, W. (1956) Report of results obtained in the toxicological
examination of Resitox (21/199). Toxicological Industrial Hygiene
Lab., Berlin. Unpublished report
Brody, S. S. and Chaney, J. E. (1966) The application of a specific
detector for phosphorus and for sulfur compounds sensitive to
subnanogram quantities. J. Gas Chromatog., 4: 42-46
Burke, J. A. (1965) Gas chromatography for pesticide residue analysis;
some practical aspects. J. Ass. Offic. Agr. Chem., 48: 1037-1058
Burke, J. and Holswade, W. (1964) Gas chromatography with
microcoulometric detection for pesticide residue analysis. J. Ass.
Offic. Agr. Chem., 47: 845-859
Burke, J. A. and Holswade, W. (1966) A gas chromatographic column for
pesticide residue analysis: retention times and response data. J. Ass.
Offic. Anal. Chem., 49: 374-385
Claborn, H. V., Bushland, R. C., Mann, H. D., Ivey, M. C. and
Radeleff, R. D. (1960) Meat and milk residues from livestock sprays.
J. Agr. Food Chem., 8: 439-442
Clark, D. E., Wright, F. C., Radeleff, R. D., Danz, J. W. and
Lehmann, R. P. (1967) Influence of coumaphos contaminants, vitamin A
and phenothiazine-lead arsenate on certain enzymes and vitamins of
cattle treated with coumaphos. Amer. J. Vet, Res., 28: 89-95
Dorough, H. W., Brady, V. E., jr. Timmerman, J. A., jr and Arthur, B.
W. (1961) Residues in tissues and eggs of poultry receiving Co-Ral
(Bayer 21/199) in the feed. J. Econ. Entomol., 54: 97-100
Doull, J., Root, M. and Cowan, J. (1962a) Ninety-day feeding studies
with Co-Ral in male and female rats and dogs fed a milk diet.
Department of Pharmacology, University of Chicago. Unpublished report
Doull, J., Root, M. and Cowan, J. (1962b) The effect of Co-Ral in the
diet on the reproduction of mice. Department of Pharmacology,
University of Chicago. Unpublished report
Doull, J., Vaughn, G. and Knecht, J. (1959) Chronic toxicity of Co-Ral
(Bayer 21/199) to dogs and rats. Department of Pharmacology,
University of Chicago. Unpublished report
Doull, J., Vesselinovitch, D., Fitch, F., Root, M. and Meskauskas, J.
(1960) Chronic toxicity of Co-Ral fed to rats for a period of two
years. Departments of Pharmacology and Pathology, University of
Chicago. Unpublished report
DuBois, K. P. (1958a) The acute toxicity of Co-Ral in combination with
other organic phosphates. Department of Pharmacology, University of
Chicago. Unpublished report
DuBois, K. P. (1958b) The dermal toxicity of Co-Ral and piperonyl
butoxide given simultaneously to rats. Department of Pharmacology,
University of Chicago. Unpublished report
DuBois, K. P. (1960) The acute toxicity of Co-Ral in combination with
Delnav, Ethion and Sevin to rats. Department of Pharmacology,
University of Chicago. Unpublished report
DuBois, K. P. and Plzak, G. (1959) Studies in the toxicity and anti-
cholinesterase action of the oxygen analogue of Co-Ral. Department of
Pharmacology, University of Chicago. Unpublished report
DuBois, K. P. and Schmalgemeier, D. (1958) Comparison of the acute
toxicity of various Co-Ral preparations to rats. Department of
Pharmacology, University of Chicago. Unpublished report
DuBois, K. P. and Schmalgemeier, D. (1959) The absence of acute
toxicity of chlorferron to rats. Department of Pharmacology,
University of Chicago. Unpublished report
Hartmann, C. H. (1966) Phosphorus detector for pesticides analysis.
Bull. Environ. Contam. Toxicol., 1: 159-168
Hecht, G. (1958) Untitled. Farbenfabriken Bayer, West Germany.
Unpublished report
Industrial Bio-Test Laboratories. (1966) Three generation reproduction
study on Co-Ral. White Leghorn chickens. Unpublished report
Jackson, J. B., Drummond, R. O., Buck, W. B. and Hunt, L. M. (1960)
Toxicity of organic phosphorus insecticides to horses. J. Econ.
Entomol., 53: 602-604
Kaplanis, J. N., Hopkins, D. E. and Treiber, G. H. (1959) Dermal and
oral treatments of cattle with phosphorus-32-labeled Co-Ral. J. Agr.
Food Chem., 7: 483-486
Knapp, F. W. (1962) Poultry tolerance to excessive amounts of Co-Ral
dust. J. Econ. Entomol., 49: 560-561
Knapp, F, W. and Krauss, G. F. (1960) Control of the northern fowl
mite, Ornithonyssus sylviarium (C and F) with ronnel, Bayer L 13/59
and Bayer 21/199. J. Econ. Entomol., 52: 4-5
Krueger, H. R., Casida, J. E. and Niedermeier, R. P. (1959) Bovine
metabolism of organo-phosphorus insecticides. Metabolism and residues
associated with dermal application of Co-Ral to rats, a goat and a
cow. J. Agr. Food Chem., 7: 182-188
Lindquist, D. A., Burns, E. C., Pant, C. P. and Dahm, P. A. (1958)
Fate of P32-labeled Bayer 21/199 in the white rat. J. Econ. Entomol.,
51: 204-206
MacDougall, D. (1964) Co-Ral. In: G. Zweig, ed. Analytical methods
for pesticides, plant growth regulators and food additives. Vol. II,
pp. 83-95. Academic Press, New York and London
Matthysse, J. G. and Lisk, D. (1968) Residues of diazinon, coumaphos,
Ciodrin, methoxychlor and rotenone in cow's milk from treatments
similar to those used for ecto-parasite and fly control on dairy
cattle, with notes on safety of diazinon and Ciodrin to calves.
J. Econ. Entomol., 61: 1394-1398
Murphy, S. D. and DuBois, K. P. (1958) The subacute toxicity of Co-Ral
(0,0-diethyl-0-3-chloro-4-methyl-7-courmarinyl-phosphorothioate; Bayer
21/199 to rats). Department of Pharmacology, University of Chicago.
Unpublished report
O'Brien, R. D. and Wolfe, L. S. (1959) The metabolism of Co-Ral (Bayer
21/199) by tissues of the house-fly, cattle grub, ox, rat and mouse.
J. Econ. Entomol., 52: 692-695
Plapp, F. W. and Casida, J. E. (1958a) Bovine metabolism of
organophosphorus insecticides. Metabolic fate of 0,0-dimethyl
0-(2, 4,5-trichlorophenyl) phosphorothioate in rats and a cow.
J. Agr. Food Chem., 6: 662-667
Plapp, F. W. and Casida, J. E. (1958b) Hydrolysis of the
alkyl-phosphate bond in certain diethyl aryl phosphorothioate
insecticides by rats, cockroaches and alkali. J. Econ. Entomol., 51:
800-803
Quigley, G. D. and Harding, W. C., jr. (1963) Use of Co-Ral (R) as a
systemic insecticide for layers. Poultry Sci., 42: 1247-1248
Radeleff, R. D., Jackson, J. B., Buck, W. B., Younger, R. L.,
Weidenbach, C. P. and Roberts, R. H. (1963) Toxicity studies of Bayer
21/199 in livestock. J. Am. Vet. Med. Assoc., 142: 624-631
Radeleff, R. D., Jackson, J. B., Roberts, R. H. and Hunt, L. M. (1958)
Summary of acute toxicity studies of Bayer 21/199 in livestock.
U.S.D.A., Kerrville, Texas. Unpublished report
Robbins, W. E., Hopkins, T. L., Darrow, D. J. and Eddy, G. W. (1959a)
Synergistic action of piperonyl butoxide with Bayer 21/199 and its
corresponding phosphate in mice. J. Econ. Entomol., 52: 660-663
Robbins, W. E., Hopkins, T. L., Darrow, D. I. and Eddy, G. S. (1959b)
Studies with P32-Bayer 21/199 sprayed on cattle. J. Econ. Entomol.,
52: 214-217
Schuleman, W. (1955) Final opinion on the new insecticide Resitox
(21/199). Pharmacological Institute of Rheinische,
Friedrich-Wilhelm-Universität, Bonn. Unpublished report
Shaw, F. R., Smith, C. T., Anderson, D. L., Fischang, W. J., Ziener,
W. H. and Hurny, J. (1964) The effects of coumaphos on poultry and its
residues in tissue and eggs. J. Econ. Entomol., 57: 516-518
Stewart, J. H. (1968) U.S. Department of Agriculture, Washington, D.C.
Private communication
Vaughn, G., Deininger, E. and Doull, J. (1958a) The effects of diets
containing Co-Ral (Bayer 21/199) on rats. Department of Pharmacology,
University of Chicago. Unpublished report
Vaughn, G., Deininger, E. and Doull, J. (1958b) Measurement of the
safe dietary level of Co-Ral for dogs. Department of Pharmacology,
University of Chicago. Unpublished report
Vaughn, G., Deininger, E. and Doull, J. (1958c) The subacute toxicity
of 3-chloro-4-methyl-umbelliferone to rats. Department of
Pharmacology, University of Chicago. Unpublished report
Vessolinovitch, D., Fitch, F. W. and Wissler, R. W. (1960)
Histopathological examination of the tissues of dogs fed Co-Ral for
one year. Departments of Pharmacology and Pathology, University of
Chicago. Unpublished report
Vickery, D. S. and Arthur, B. W. (1960) Animal systemic activity,
metabolism and stability, Bayer 21/199. J. Econ. Entomol., 53:
1037-1043
Watts, R. W. and Storherr, R. W. (1968) GLC of organophosphorus
pesticide residues: Retention times and response data for three
columns. Abstracts 82nd Ann. Mtg. Ass. Offic. Anal. Chem., Washington,
D.C., October 1968. Abstract 4
