
VAMIDOTHION JMPR 1973
IDENTITY
Chemical name
O,O-dimethyl S-[2-(1-methylcarboylethylthio)ethyl]
phosphorothioate.
Synonyms
10 465 R.P., Kilval(R), Trucidor(R).
Structural formula
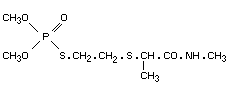 Other information on identity and properties
Physical state: White crystalline solid (pure)
Amber waxy solid (technical)
Melting point: 40°C (pure)
Solubility: Water - about 4 g/ml
Benzene, toluene, methyl ethyl ketone, ethyl
acetate, acetonitrile, methylene chloride,
anisole, cyclohexanone, chloroform - 1 g/ml
[xylene - 0.125 g/ml petroleum ether, cyclohexane
- insoluble
Volatility: Very low. Negligible loss under vacuum (2 mm Hg)
at 20°C.
Stability: The technical solid decomposes slowly at room
temperature but is stable in organic solvents
(e.g. cyclohexanone, methyl ethyl ketone).
Hydrolyzed by alkali
Optical Vamidothion consists of a mixture of optically
isomerism: active isomers. Their systemic activity as
pesticides is similar, but the D form shows higher
contact activity as an araricide.
Formulation: Water-miscible solution containing 400 g/l.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Biodegradation
One NC-5 mouse was administered 30 mg 32P vamidothion orally.
Urine collected over the following 24 hours contained 32P labelled
phosphoric acid, 0-methyl phosphate, 0,0-dimethyl phosphate and an
unknown compound.
Rat liver homogenate incubated for two hours with 32P labelled
vamidothion produced 0,0-dimethyl phosphate, phosphoric acid and an
unknown compound which was possibly dimethyl phosphothionate.
The same metabolites were produced by incubation of 32P labelled
vamidothion with plant leaves. In addition desmethyl vamidothion was
detected; this may not have been detected as a metabolite in animals
because of the low activity of 32p incorporated into the vamidothion
(Morikawa and Saito, 1969).
The oxidation product, vamidothion sulfoxide, has been
demonstrated to be formed in plants (Desmoras et al., 1961).
Effects on enzymes
Vamidothion inhibits cholinesterase in vitro and in vivo. A
concentration of 40 mg/l caused 50% inhibition of enzyme activity of
plasma. Four hours after guinea-pigs had received 40 mg vamidothion/kg
orally, 81% inhibition of plasma and 20% inhibition of cellular enzyme
occurred. With higher dosage levels the plasma cholinesterase level
remained stationary but cellular enzyme activity decreased (Dubost et
al., 1960).
TOXICOLOGICAL STUDIES
Special studies on the metabolites
A summary of the results of acute toxicity studies on
vamidothion sulfoxide is shown in Table 1.
A test for neurotoxicity was carried out in the same manner as
for vamidothion itself. Positive controls showed signs of paralysis in
9-14 days while vamidothion sulfoxide was without effect (Anon.,
1966b).
TABLE 1. THE ACUTE TOXICITY OF VAMIDOTHION SULFOXIDE
Species Sex Route LD50 References
Mouse Oral 80 Desmoras et al., 1961
Rat F Oral 160 Desmoras et al., 1961
Rivett and Corbett, 1966
Guinea-pig Oral 205 Desmoras et al., 1961
Chicken F s.c. 60 Anon., 1966b
A short-term study was carried out on groups of five male and
five female rats which were fed diets containing 0, 5, 50, 100 and 200
ppm vamidothion sulfoxide for three months. The degree of depression
of cholinesterase activity was similar to that in animals receiving
the same dosage levels of vamidothion. The 100 and 200 ppm dosage
levels depressed cholinesterase levels to approximately 20% And 12%
respectively of the control level. Cholinesterase activity returned to
normal within four weeks when vamidothion sulfoxide was withdrawn from
the diet. Histological examination of two male and two female rats
from each group showed no abnormality attributable to ingestion of the
test compound (Rivett and Corbett, 1966).
A three generation reproduction study with each generation
producing two litters, was carried out on groups of 20 male and 40
female rats (generation Fo, test animals), 40 male and 80 female
(generation Fo controls) or 10 male and 30 female (other generations).
Animals were administered 0, 5, 15 or 45 ppm of vamidothion sulfoxide
in the diet for four weeks before the first mating. The study included
tests in which some female test animals were mated with untreated
males and some untreated females were mated with treated male animals.
A small number of females of the F1b and F2b generations were killed
after the thirteenth day of pregnancy and the uterus examined for
implantation sites, viable and resorbed embryos and macroscopically
observable abnormalities. The F1a, F2a and F3a litters were killed
at weaning and autopsied. Rats of the F1b and F2b litters not used to
produce the next generation were killed at weaning and autopsied. The
F3b litters were killed at weaning and the heart, kidneys and livers
weighed and spleen, suprarenals, thyroid and (in the case of the
highest dosage group) brain examined histologically. The results
showed that the dosage levels of vamidothion sulfoxide used had no
untoward effect on treated animals, in particular on fertility or
reproductive activity of rats. There was no indication of teratogenic
activity in this study (Ganter et al., 1969b).
Special studies on neurotoxicity
Groups of five white leghorn chickens were injected s.c. with 60
and 120 mg vamidothion/kg (1X and 2X LD50 dose). Positive control
groups received 20 and 40 mg di-isopropyl-fluorophosphoridono. All
birds were treated with atropine and P2AM. No signs of neurotoxicity
appeared in vamidothiontreated birds while positive control groups
developed paralysis in 9-14 days (Anon., 1966b).
Special studies on the pharmacological effects
The motility of mice was unaffected by 10 mg vamidothion/kg
orally and exploratory activity and conditioned reflexes were normal
in rats treated orally with 30 mg/kg. No effect was found on
neuromuscular transmission, respiratory rate, ECG or heart rate and
the effects of i.v. adrenalin and i.v. acetylcholine were largely
unchanged by treatment with vamidothion, Salivary excretion was
increased, slightly and isolated intestine and uterus preparations
were affected weakly by vamidothion (Julou et al., 1966).
Mice were administered 100 mg vamidothion/kg orally (LD50 dose).
Some also received atropine, pralidoxime or both. Ten to 20 mg
atropine/kg i.p. provided 60-70% and 50 mg pralidoxime/kg i.p. 100%
protection against vamidothion (Dubost et al., 1960; Anon., 1966a).
Groups of 10 rats received 3 x LD50 dose (300mg/kg) of
vamidothion orally. One group received 17.4 mg atropine/kg i.p. when
signs of intoxication showed. Another received atropine plus 50 mg
P2AM/kg i.p. All control animals died within 45 min., 7-10 animals
with atropine died in 7-24 hours and three animals receiving atropine
and P2AM died. All of the latter group appeared normal two hours after
treatment while those on atropine alone appeared ill (Anon., 1966a).
Special studies on reproduction
Rat. A three-generation reproduction study, with each generation
producing two litters, was carried out on groups of 20 male and 40
female rats which were administered 5, 15 and 45 ppm vamidothion in
the diet. The control group consisted of 40 males and 80 females.
Animals of the Fo generation received diets for 11 weeks before being
mated. The study included tests in which some female test animals were
mated with untreated males and untreated females were mated with
treated males. A small number of females of the F1b and F2b
generations were killed after the thirteenth day of pregnancy and the
uterus examined for implantation sites, viable and resorbed embryos
and macroscopically apparent abnormalities. The F1a, F2a and F3a,
litters were killed at weaning and autopsied. Animals of the F1b and
F2b groups not used to produce the next generation were killed at
weaning and autopsied. The F3b litters were killed at weaning and the
heart, kidneys and liver weighed and the spleen, suprarenals, thyroid
and (with the highest dose level only) the brain examined
histologically. Haematological studies and examinations of bone marrow
of one animal of each sex of each group were carried out. Because of a
technical error some animals chosen as parents of the second and third
generations may have been produced from untreated male parents; the
proportion so produced is uncertain. All females were, however,
treated with the correct diets. Results show that at the dosage levels
used vamidothion had no untoward activity on rats, in particular on
their fertility or reproductive functions, There was no indication
that vamidothion is teratogenic (Ganter et al., 1969a).
Acute toxicity
The results of acute toxicity studies on vamidothion are
summarized in Table 2.
Administration of vamidothion in fatal doses produced signs
typical of cholinesterase inhibitors.
Groups of 20 mice were administered half the LD50 dose of
vamidothion plus half the LD50 dose of demeton-methyl, parathion,
phencapton, dimetboate, ethion, malathion, azinphos ethyl, mevinphos
or phosphamidon and observed for five days. No significant
potentiation was observed (Anon., 1966c).
Short-term studies
Rat. Groups of 10 male rats were administered 3 or 6 mg
vamidothion/kg/day orally for one month. It was reported that body
weight was unaffected, no animals died and no clinical signs of
toxicity occurred. Haematological examination including examination of
bone marrow and chemical analysis of urine and blood showed no
abnormalities except for blood cholinesterase activity which was
depressed by 50% (3 mg/kg/day) or 607 (6 mg/kg/day). Brain
cholinesterase levels were not depressed. The weights and histological
appearance of organs were unaffected by treatment (Dubost et al.,
1960).
TABLE 2. SUMMARY OF THE RESULTS OF STUDIES ON ACUTE TOXICITY
Species Sex Route Purity LD50 References
Mouse Oral p 43 Johnston and Rivett, 1966
Mouse Oral p 34 Dubost et al., 1960
Mouse M+F Oral Tech. 64 Pasquet and Mazuret, 1972a
Mouse Oral Tech. 40 Pak, 1970
Mouse s.c. p 34 Johnston and Rivett, 1966
Mouse Dermal p 1450 Johnston and Rivett, 1966
Mouse Dermal Tech. 1060 Pasquet and Mazuret, 1972a
Rat M Oral p 100 Johnston and Rivett, 1966
Rat M Oral p 105 Dubost et al., 1960
Rat M+F Oral p 105 Pasquet and Ma.uret, 1972b
Rat F Oral p 77 Desmoras et al., 1961
Rat M+F Oral Tech. 168 Pasquet and Mazuret, 1972a
Rat Oral Tech. 103 Pak, 1970
Rat F Oral p 64 Johnston and Rivett, 1H6
Rat M s.c. p 48 Johnston and Rivett, 1966
Rat F s.c. p 35 Johnston and Rivett, 1966
Guinea-pig Oral p 85 Dubost et al., 1960
Rabbit Oral Tech. 160 Pak, 1970
Rabbit Dermal p 1160 Quoted in Johnston and
Rivett, 1966
Rabbit M+F Dermal Tech. 3000 Pasquet and Mazuret, 1972a
Dog M+F Oral p 110 Julou and Pasquet, 1967
Mouse Oral p 50 Desmoras and Fournel, 1961
Mouse Oral l-isomer 68 Desmoras and Fournel, 1961
Mouse Oral d-isomer 34 Desmoras and Fournel, 1961
M = male F = female P = pure
Groups of five male and five female rats were fed on diets
containing 0, 0.2, 1 and 5 ppm vamidothion for six weeks after which
the test substance was withdrawn from the diet. Regular observation of
plasma and erythrocyte cholinesterase activity showed that only in the
plasma of female rats on the 5 ppm diet was enzyme activity
consistently depressed to a significant extent. The enzyme level
returned to normal within five weeks. Cholinesterase activity was not
depressed significantly at lower dosage levels (Wheldon et al., 1969).
Groups of five male and five female rats were fed on diets
containing 0, 5 and 50 ppm vamidothion for three months. No ill effect
was seen on growth or general health. Blood cholinesterase levels were
reduced to approximately 75% and 25% respectively of the control level
in the 5 and 50 ppm groups. Cholinesterase levels returned to normal
within four weeks when vamidothion was withdrawn from the diet.
Histopathological examination of two rats of each sex from the 50 ppm
group showed no abnormalities related to ingestion of the compound
(Rivett and Corbett, 1966).
Groups of rats were administered, by gavage, doses of
approximately 2, 5 or 10 mg vamidothion/kg daily for three months. At
the end of the test serum acetylcholinesterase activity was decreased
to 35%, 12% and 8%, respectively, of the normal value (Pak, 1970).
Dog. Groups of three dogs were administered orally 0, 1 and 2
mg/vamidothion/kg/day for one month. Two dogs received 8 mg/kg/day for
one month. No effect was seen on growth except for one animal on the
highest dose level which developed diarrhoea (cause unknown).
Haematological indices and blood coagulation were normal. Slight
differences between groups in urine urobilinogen, glucose and bile
salts, serum protein, PSP clearance and behaviour could not be
attributed to treatment. In particular no neurological abnormalities
were seen although erythrocyte cholinesterase was severely depressed
(100% inhibition at sixteenth day with 8 mg/kg dosage regime). In
six dogs receiving 1 and 2 mg/kg/day no abnormalities were found on
gross and histopathologic examination. (Dubost et al., 1960).
Groups of two male and two female beagle dogs were fed on diets
containing 0, 0.2, 1 and 5 ppm vamidothion for five to six weeks.
Animals on the lowest dosage level were then fed for a further four
weeks on diet containing 20 ppm vamidothion, after which they were
observed for four more weeks on normal diet. No clinical signs of
abnormality were seen in any group. The plasma and erythrocyte
cholinesterase levels were slightly depressed in dogs on the highest
dosage. These levels returned to normal within two weeks of return to
a normal diet. No abnormalities in the appearance or weight of organs
were found in any group (Noel et al., 1969).
Long-term studies
No data are available.
Observations in man
Groups of 6-11 normal healthy volunteers of both sexes were
administered 9.6 or 37.2 µg vamidothion/kg/day orally in aqueous
solution on five days each week for three weeks. Other groups received
78.8 or 122.8 µg vamidothion/kg/day in aqueous solution for five
weeks. A control group was studied for 25 weeks. No clinical signs or
symptoms were found which could be attributed to treatment. Plasma
cholinesterase, estimated weekly, showed no consistent depression in
any group but erythrocyte cholinesterase was depressed in three of six
volunteers receiving 122.8 µg/kg/day. The no-effect level was
considered to be 78.8 µg/kg/day, (calculated to be equivalent to 56.3
µg/kg/day if vamidothion was administered every day without a break)
(Noel et al., 1970).
Serum and erythrocyte cholinesterase levels were determined
periodically in workers who had been involved in the manufacture of
vamidothion over a period of several months to a few years. Enzyme
levels were also determined in experimentalists exposed several times
a year to vamidothion over a seven-year period. The actual exposure is
uncertain. Fluctuations in enzyme levels in both groups were within
the limits of normal (Celice et al., 1966),
Comments
It has been shown that vamidothion is partly absorbed from the
gastric intestinal tract and is excreted in urine. Several metabolic
products have been found in urine; these ewe compounds are also
produced by liver slices. One metabolite (desmethyl vamidothion) was
shown to be produced by plants but has not yet been found as a
metabolite in animals. Vamidothion sulfoxide has been examined
toxicologically but the toxicity of other metabolites has not
apparently been examined.
Vamidothion depresses serum cholinesterase activity at lower
concentrations than it depresses the activity of erythrocyte
cholinesterase, except in dogs. Brain cholinesterase activity is less
affected. The dosage level of vamidothion which is without effect in
man is just over 50 µg/kg/day. The no-effect level with regard to
cholinesterase depression was 1 ppm in the diet of rats and 5 ppm in
the diet of dogs. These dietary levels are approximately equivalent to
50 µg/kg/day in rats and 125 µg/kg/day in dogs.
Studies showed that vamidothion had no adverse effects on
reproduction. Short-term tests were carried out in only small numbers
of dogs and rats and although no ill effects other than that on
cholinesterase activity were detected, the observations were not
sufficiently extensive to eliminate the possibility that the compound
has other significant effects, No long-term tests have been reported.
TOXICOLOGICAL EVALUATION
It is not possible, on the information available, to estimate an
ADI for vamidothion.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Vamidothion is a systemic organophosphorus aphicide and miticide.
Its most important use is on apples and pears against the woolly apple
aphid. It is also used on other pome fruits, sugar beet and brussels
sprouts. and to a lesser extent on grapes, cereal crops, sugar cane
and hops.
Pre-harvest treatments
The recommended uses are as follows:
Orchard fruits. In France, Western Europe, Australia, New Zealand
and South Africa, a single treatment at 40-50 g a.i./100 l. For
low-volume application, a rate of 0.4-0.5 kg a.i./ha is used. In Great
Britain an application at 20 g/100 l before flowering, and one or two
treatments at 40 g/100 l after flowering are recommended for apple and
pear; a single application at 40 g/100 l after flowering for plum and
cherry.
Grapes. A single treatment at 40-50 g/100 l during active sap
movement. A second similar treatment if necessary.
Citrus fruits. 60-80 g/100 l.
Hops. In England, three applications at 700 g/ha, with a minimum
pre-harvest interval of four weeks. In France, Belgium and Germany,
one application at 50 g/100 l when the aphid first appears.
Rice. Three applications at 50 g/100 l (600 g/ha).
Cotton. 40-60 g/100 l high-volume application, repeated, if
necessary.
Post-harvest treatments
None known.
Other possible uses
Trials against aphids of maize and sorghum have been successful.
Aphids and red spider of strawberries, tomatoes and beans have been
successfully controlled. A pre-harvest interval of six weeks is
recommended.
Aphids have been controlled in trials in France, Brazil and Japan
by brushing tree trunks with vamidothion during active sap movement.
Residues resulting from supervised trials
Residue data are available from supervised trials in France,
Germany, Switzerland and the United Kingdom (Rhône-Poulenc, 1966,
1969; May and Baker Ltd. 1963), together with limited data on
application to sugar cane in Trinidad (May and Baker Ltd, 1973). These
data have been deposited with FAO and are summarized in Table 3.
Residues of vamidothion are converted to the sulfoxide in or on the
plant, and residues reported in the table refer to the sum of the
parent compound and the sulfoxide.
The combined residue is unusually persistent. The half-life on
apples and pears is between 35 and 45 days, and more limited results
on peaches, plums, grapes, cherries and straw berries indicate a
similar persistence. Half-life periods on cereals and vegetables are
generally between 6 and 20 days.
Data from supervised trials in Japan (Tomizawa, 1973) show very
low residues, usually below 0.02 ppm in a wide range of crops. As
these data apparently refer only to the parent compound, they are not
quoted in the Table.
Fate of residues
In plants. As mentioned above, the main biologically active product
is the sulfoxide, and this is the main terminal residue. The sulfone
is not found to any significant extent, but it has been detected as a
metabolite in citrus leaves kept at a low temperature. (Results quoted
by Tomizawa, 1973). The other main metabolites are demethyl
vamidothion, phosphoric acid and diethyl phosphate (Morikawa and
Saito, 1969).
In animals. In experiments with 32B-vamidothion (Morikawa and
Saito, 1969), 69% of the activity in the urine of dosed mice appeared
as phosphoric acid and its mono and dimethyl esters. The remaining 31%
was an unknown metabolite. The dimethyl compound was not detectable.
In the same series of experiments, rat liver homogenate metabolized
55% of the vamidothion with which it was incubated to the unknown
metabolite, 31% to phosphoric acid and 14% to diethyl phosphate.
Methods of residue analysis
Residues normally consist essentially of a mixture of vamidothion
and its sulfoxide. Three methods have been developed by the
Rhône-Poulenc Laboratories to determine this mixture after suitable
clean-up: gas chromatography after oxidation of both compounds to the
sulfone, colorimetric determination of total phosphorus after
mineralization, and bioassay (Rhône-Poulenc, 1972). The
gas-chromatographic method should be suitable for regulatory purposes.
The other two are non-specific but are suitable for determining
residues arising from supervised trials: most of the data in Table 3
were obtained by these methods, often by both of them. Identity can be
confirmed, and the parent compound and sulfoxide separately determined
semi-quantitatively if required, by TLC (Rhône-Poulenc, 1972).
Determination by gas chromatography (Desmoras et al., 1972)
The sample is blended with buffered methanol or acetone and the organic
solvent evaporated. The extract is washed with petroleum ether, in
which vamidothion and its sulfoxide are insoluble, and then extracted
continuously with dichloromethane. (Vamidothion is readily extracted,
but continuous extraction is needed for the sulfoxide.) An aliquot of
the solution is evaporated to dryness and oxidized with potassium
permanganate in aqueous acetone. The resulting sulfone is extracted
with dichloromethane and transferred to benzene for determination by
gas chromatography on DEGS (diethylene glycol succinate) stationary
phase with electron capture detection. Recoveries are within a range
of 80-110% and the limit of determination is about 0.05 ppm.
The method described is more sensitive than methods in which
thermionic (Ruzicka et al., 1967) or flame photometric (Mestres, 1973)
detection is used. Separate determination of vamidothion and the
sulfoxide by GLC without oxidation is unsatisfactory because of the
large difference between their retention times. The two compounds can
be differentiated if required by first extracting vamidothion by
shaking with dichloromethane, then obtaining the sulfoxide by
continuous extraction and oxidizing the residues in the two extracts
to the sulfone separately.
Total phosphorus determination. The residue is extracted, and
cleaned-up as in the GLC method and the extract is evaporated to
dryness. The residue is digested with nitric and sulfuric acids and
phosphorus is determined colorimetrically as molybdenum blue. The
limit of determination is about 0.1 ppm.
Biological determination. The solvent is evaporated from the
dichloromethane extract obtained as described above, and some of the
co-extractives are removed by precipitation from acetone solution at
-70°C. The acetone in the filtrate from this step is evaporated and
replaced with water, and residues of vamidothion plus sulfoxide are
determined by bio-assay with Daphnia pulex as the test organism. The
procedure has been described in detail by Desmoras (1963).
Thin-layer chromatography. Thin-layer chromatography, TLC, is useful
as a confirmatory test of identity and to separate the parent compound
from the sulfoxide. It is carried out on the cleaned-up
dichloromethone extract, using silica gel which has been activated by
heating at 120°C for at least one hour. Approximate Rf values of
vamidothion, its sulfoxide and its sulfone on silica gel GF254
(Merek), developed with various solvent systems, are listed in Table
4. Separated spots can be detected by esterase inhibition or by
spraying with iodoplatinate, palladium chloride or
nitrobenzylpyridine. The enzyme inhibition method is the most
sensitive, with detection limits of 30-50 ng for the three compounds.
TLC of the dichloromethane extract does not satisfactorily
indicate the ratio of vamidothion to sulfoxide in the original
residue, because some vamidothion is converted to sulfoxide during the
continuous extraction.
Appraisal
Vamidothion is an organophosphorus compound with pronounced
systemic activity, effective against aphids and mites not resistant to
organophosphorus compounds.
It is applied as water-miscible solution to pome fruits, sugar
beet, brussels sprouts and, to a lesser extent, cereal crops, grapes,
sugar cane and hops. By far the most important use is on apples and
pears against woolly apple aphid.
Vamidothion is particularly persistent. Numerous studies have
been carried out in France, England, Switzerland and Germany and these
indicate the half-life in pome fruit to be between 35 and 45 days. The
half-life on cereals and vegetables is generally between six and 20
days.
The main biologically active metabolite is the sulfoxide which
has a higher systemic insecticidal activity than the parent compound.
The sulfone is not found in plants to any significant extent.
Residues consisting of a mixture of the sulfoxide and the parent
compound, can be determined by bio-assay or estimation of total
phosphorus. The recommended method, which should be suitable :Per
regulatory purposes, is by gas chromatography with electron-capture
detection after oxidation to the sulfone. The limit of determination
is about 0.05 ppm. TLC can be used for confirmation of identity.
The limited data available on sugar cane indicated residues of
about 0.2 ppm soon after application, decreasing to below 0.05 ppm
before harvest. As the available information on wheat and sunflower
was restricted to the whole plant, it was not suitable for judging the
probable residue level in the grain and seed.
National tolerances
In Switzerland a tolerance of 0.6 ppm has been established for
residues arising from applications to fruit trees, except cherry, with
the end of May as the last date of application.
TABLE 3. VAMIDOTHION RESIDUES IN CROPS
Dosage rate, Residue (ppm vamidothion + sulfoxide) after interval (days)
Crop Country a.i. g/100 l
or kg/ha 0-2 5-11 13-18 20-30 34-39 41-49 52-69 72-95 >100
Apple France 50 g/100 l 3.0 2.5 2.0 1.6 1.3 1.1
50 g/100 l 0.1
2 x 50 g/100 l 1.7
2 x 50 g/100 l 0.3 0.3
Germany 60 g/100 l 0.7 < 0.1 0.5 0.5 0.4
1.0 kg/ha 1.1 0.6 0.3
1.8 kg 0.75 0.2
2 x 1.8 kg/ha 0.7 0.2
Switzerland 50 g/100 l 0.85 < 0.65 0.8
50 g/100 l 0.5
England 40+80 g/100 l 3.5*
1.6*
1.7*
2.4*
40+80 g/100 l 3.4
Pear France 50 g/100 l 0.1
2 x 50 g/100 l 0.2 0.2
England 40 g/100 l 1.5* 0.6*
1.9* >0.2*
40+80 g/100 l 2.1*
1.8*
Peach France 50 g/100 l 0.1
2 x 50 g/100 l 0.4 0.3
Cherry England 80 g/100 l >0.4*
>0.4*
>0.4*
Plum England 80 g/100 l 1.9
TABLE 3. (Cont'd.)
Dosage rate, Residue (ppm vamidothion + sulfoxide) after interval (days)
Crop Country a.i. g/100 l
or kg/ha 0-2 5-11 13-18 20-30 34-39 41-49 52-69 72-95 >100
Grape France 50 g/100 l 0.2
2 x 50 g/100 l 0.25 0.15
Hop France 3 x 50 g/100 l <0.1
" (fresh and dry cones) 3 x 100 g/100 l <0.1
" (fresh cones) England 25+40 g/100 l < 0.5
Wheat (whole plant) France 50 g/100 l 9 4.1 1.4 0.4 0.4
50 g/100 l approx. 10 4.2 1.9
" (grain) 50 g/100 l 0.25
50 g/100 l 0.1
Sugar beat (leaves) France 50 g/100 l 1.1*
0.2*
" " (root) 50 g/100 l 0.2
Sunflower (leaves) France 75 g/100 l 60 23 19 10 7 5
Strawberries Scotland 50 g/100 l 0.6*
0.6*
0.6*
50 g/100 l 0.3
Broad beans France 50 g/100 l 81 64 51 40 23 13
(glasshouse,leaves)
French beans France 50 g/100 l 87 35 21 8.5
(glasshouse, leaves)
Sugar cane Trinidad 0.9 kg/ha 0.2 0.1
0.9 kg/ha 0.2 < 0.05
" " (juice) 0.9 kg/ha < 0.05
* Separate crop treatments.
TABLE 4. Rf VALUES OF VAMIDOTHION, ITS SULFOXIDE AND ITS SULFONE
ON SILICA GEL* IN SEVERAL SOLVENT SYSTEMS
Approximate Rf
Developing solvent
Vamidothion Sulfoxide Sulfone
Dichloromethane-methanol (90:10, v/v) 0.65 0.4 0.55
Ethyl acetate-methanol (75:25, v/v) 0.65 0.5 0.65
Acetonitrile-methanol (97:3 v/v) 0.5 0.1 0.65
Acetone-dimethylformanide (99:1, v/v) 0.6 0.35 0.65
* Silica gel GF254 (Merck) activated at 120°C for one hour before use.
RECOMMENDATION
As no acceptable daily intake could be established no tolerances
are recommended. Following officially acceptable use in various
countries residues of vamidothion can occur in the following
commodities up to the levels indicated. The guide-lines indicated
below are unlikely to be exceeded as a result of the recommended use
of vamidothion.
Guide-line levels (based on a pre-harvest interval of six weeks)
Apples and pears 2 ppm
Brussels sprouts 1 ppm
Sugar beet 0.5 ppm
Grapes 0.5 ppm
Hops 0.2 ppm
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake can be established)
1. Long-term studies in at least one animal species.
2. Adequate short-term studies in several species including a
non-rodent species.
3. Studies to identify metabolites and investigate their toxicity.
4. Studies on the nature and level of residues in animal products
from the feeding of residues at levels occurring on food wastes.
5. Information showing the fate of residues in the major crops in
countries with different meteorological and growth conditions.
REFERENCES
Anon. (1966a) Vamidothion. Etude dos Antidotes. Submitted by Société
des Usine Chimiques, Rhône-Poulenc. Unpublished report
Anon. (1966b) Vamidothion. Essai do neurotoxicité, submitted by
Société des Usines Chimiques, Rhône-Poulenc. Unpublished report
Anon. (1966e) Vamidothion. Etude de la potentialisation sur souris
avec des insecticides comerciaux, submitted by Société dos Usines
Chimiques, Rhône-Poulene. Unpublished report
Celice, J., Fournel, J. and Koenig F. (1966) Evaluation des risques
l'intoxication par le vamidothion (10.465 R.P.) au cours de sa
fabrication et do son experimentation plein champ, submitted by
Société dos Usines Chimiques, Rhône-Poulene. Unpublished report
Desmoras, J. (1963) Biological determination of vamidothion in plants.
Mededel. Landbouwhogeschool Opzoekingasta, Staat Gent, 28: 731-742
Desmoras, R. and Fournel, J. (1961) Insecticides. Activités
insecticides ot toxicités des 12.151.R.P. et 12.164 R.P. isomeres
optiques du 10.465 R.P. Submitted by Société des Usines Chimiques,
Rbône-Poulenc. Unpublished report
Desmoras, R., Fournel, J. and Koenig F. (1961) Insecticides. Activités
insecticides et toxicités comparées des 10.465 R.P. et 11.905 R.P.
Submitted by Sociétés des Usines Chimiques, Rhône-Poulene. Unpublished
report
Desmoras, J., Laurent, M. and Buys, M. (1972) Unpublished report No.
15,893 submitted by Rhône-Poulene
Dubost, J.P., Fournel, J., Ganter, P., Julou, L. and Myon, M. (1960)
Produit No. 10.463 R.P. Toxicité ague, tolerance locale, activités
anticholinesterasique et antidotes, toxicité chronique chez le rat et
le chien. Submitted by Société dos Usines Chimiques, Rhône-Poulenc.
Unpublished report
Ganter, P., Julou, L., Pasquet, J., Delesque, M. and Durel, J. (1969a)
Vanidothion (10.465 R.P.) activités sur les fonctions de reproduction
du Rat (Essai sur 3 generations). Submitted by Société des Usines
Chimiques, Rhône-Poulenc. Unpublished report
Ganter, P., Julou, L., Pasquet, J. and Delesque, M. (1969b)
Vamidothion (11.905 R.P.) activité sur les fonctions de reproduction
du rat (Essai sur 3 generations). Submitted by Société des Usines
Chimiques, Rhône-Poulenc. Unpublished report
Johnston, J.P. and Rivett, K.F. (1966) Vamidothion. Acute toxicity to
mammals. Unpublished report of May & Baker, Ltd. submitted by Société
des Usines Chimique, Rhône-Poulenc
Julou, L., Ducrot, R., Gardens, M.C., Detaille, J. Fee, C., Garret, C.
and Pasquet, J. (1966) Etude pharmacologique du vamidothion (10.465
R.P.) comparativement au parathion (3.470 R.P.). Submitted by Société
des Usines Chimiques, Rhône-Poulenc. Unpublished report
Julou, L. and Pasquet, J. (1967) Vamidothion (10.465 R.P.), toxicité
aigue chez le chien par vole orals. Submitted by Société des Usines
Chimiques, Rhône-Poulenc. Unpublished report
May and Baker Ltd. (1963) Unpublished report
May and Baker Ltd. (1973) Unpublished report
Mestres, R. (1973) Personal communication
Morikawa, O. and Saito, T. (1966) Degradations of vamidothion and
dimethoate in plants, insects and mammals. Bochu Kagaku, 31: 130-135
Morikawa, O. and Saito, T. (1969) Degradation of vamidothion and
Dimethoate in plants, insects and mammals. Unpublished report of the
Laboratory of Applied Entomology, Nagoya University, Japan, submitted
by Société des Usines Chimique, Rhône-Poulenc
Noel, P.R.B., Coote, S. and Street, A.E. (1969) Vamidothion (10.465
R.P.). Determination of no-effect level in Beagle dogs. Unpublished
report of Huntingdon Research Centre submitted by Société des Usines
Chimique, Rhône-Poulenc
Noel, P.R.B., Haynes, G. and Street, A.E. (1970) Vamidothion.
Determination of no-effect level in human volunteers. Unpublished
report of Huntingdon Research Centre submitted by Société des Usines
Chimique, Rhône-Poulenc
Pak, L.V. (1970) The toxicity of vamidothion on warm-blooded animals.
Gig. truda i Praftabol, 2: 58-9
Pasquet, J. and Mazuret, A. (1972a) Vamidothion (10.465 R.P.) - Kilval
ou formule S.A.E. 1700 (solution A 400 9/litro) Submitted by Société
des Usines Chimiques, Rhône-Poulenc. Unpublished report
Pasquet, J. and Mazuret, A. (1972b) Vamidothion (10.465 R.P.) -
Toxicité aigue chez le rat par voie orals, Submitted by Société des
Usines Chimiques, Rhône-Poulenc. Unpublished report
Rivett, K.F. and Corbett, K. (1966) Insecticides Vamidothion. The
acute and chronic oral toxicity of vanidothion and its sulphoxide.
Unpublished report of May and Baker Ltd, submitted by Société des
Usines Chimiques, Rhône-Poulenc
Rhône-Poulenc. (1966) Méthodes de dosage et évolution des résidues.
Report R.P. -D.S.Ph./D.S. An. Nord No. 11: 082
Rhône-Poulenc. (1969) Dosage de résidus dans les végétaux. Résumé des
résultats. Report R.P.-D.S.Ph. No. 14, 158. Unpublished. [Results
obtained during the period 1961-1968 are summarized]
Rhône-Poulene. (1972) Report No. R.P.-D.S.Ph./D.S. An. Nord No. 17,
002. Unpublished
Ruzicka, J., Thomson, J. and Wheals, B.B. (1967) The gas
chromatographic examination of organophosphorus pesticides and their
oxidation products. J. Chromatogr., 30: 92-99
Tomizawa, C. (1973) Personal communication
Wheldon, G.H., Odey, R.J. and Street, A.E. (1969) Toxicity of
vamidothion to rats during dietary administration over six weeks.
Unpublished report of Huntingdon Research Centre submitted by Société
des Usines Chimiques, Rhône-Poulenc
Other information on identity and properties
Physical state: White crystalline solid (pure)
Amber waxy solid (technical)
Melting point: 40°C (pure)
Solubility: Water - about 4 g/ml
Benzene, toluene, methyl ethyl ketone, ethyl
acetate, acetonitrile, methylene chloride,
anisole, cyclohexanone, chloroform - 1 g/ml
[xylene - 0.125 g/ml petroleum ether, cyclohexane
- insoluble
Volatility: Very low. Negligible loss under vacuum (2 mm Hg)
at 20°C.
Stability: The technical solid decomposes slowly at room
temperature but is stable in organic solvents
(e.g. cyclohexanone, methyl ethyl ketone).
Hydrolyzed by alkali
Optical Vamidothion consists of a mixture of optically
isomerism: active isomers. Their systemic activity as
pesticides is similar, but the D form shows higher
contact activity as an araricide.
Formulation: Water-miscible solution containing 400 g/l.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Biodegradation
One NC-5 mouse was administered 30 mg 32P vamidothion orally.
Urine collected over the following 24 hours contained 32P labelled
phosphoric acid, 0-methyl phosphate, 0,0-dimethyl phosphate and an
unknown compound.
Rat liver homogenate incubated for two hours with 32P labelled
vamidothion produced 0,0-dimethyl phosphate, phosphoric acid and an
unknown compound which was possibly dimethyl phosphothionate.
The same metabolites were produced by incubation of 32P labelled
vamidothion with plant leaves. In addition desmethyl vamidothion was
detected; this may not have been detected as a metabolite in animals
because of the low activity of 32p incorporated into the vamidothion
(Morikawa and Saito, 1969).
The oxidation product, vamidothion sulfoxide, has been
demonstrated to be formed in plants (Desmoras et al., 1961).
Effects on enzymes
Vamidothion inhibits cholinesterase in vitro and in vivo. A
concentration of 40 mg/l caused 50% inhibition of enzyme activity of
plasma. Four hours after guinea-pigs had received 40 mg vamidothion/kg
orally, 81% inhibition of plasma and 20% inhibition of cellular enzyme
occurred. With higher dosage levels the plasma cholinesterase level
remained stationary but cellular enzyme activity decreased (Dubost et
al., 1960).
TOXICOLOGICAL STUDIES
Special studies on the metabolites
A summary of the results of acute toxicity studies on
vamidothion sulfoxide is shown in Table 1.
A test for neurotoxicity was carried out in the same manner as
for vamidothion itself. Positive controls showed signs of paralysis in
9-14 days while vamidothion sulfoxide was without effect (Anon.,
1966b).
TABLE 1. THE ACUTE TOXICITY OF VAMIDOTHION SULFOXIDE
Species Sex Route LD50 References
Mouse Oral 80 Desmoras et al., 1961
Rat F Oral 160 Desmoras et al., 1961
Rivett and Corbett, 1966
Guinea-pig Oral 205 Desmoras et al., 1961
Chicken F s.c. 60 Anon., 1966b
A short-term study was carried out on groups of five male and
five female rats which were fed diets containing 0, 5, 50, 100 and 200
ppm vamidothion sulfoxide for three months. The degree of depression
of cholinesterase activity was similar to that in animals receiving
the same dosage levels of vamidothion. The 100 and 200 ppm dosage
levels depressed cholinesterase levels to approximately 20% And 12%
respectively of the control level. Cholinesterase activity returned to
normal within four weeks when vamidothion sulfoxide was withdrawn from
the diet. Histological examination of two male and two female rats
from each group showed no abnormality attributable to ingestion of the
test compound (Rivett and Corbett, 1966).
A three generation reproduction study with each generation
producing two litters, was carried out on groups of 20 male and 40
female rats (generation Fo, test animals), 40 male and 80 female
(generation Fo controls) or 10 male and 30 female (other generations).
Animals were administered 0, 5, 15 or 45 ppm of vamidothion sulfoxide
in the diet for four weeks before the first mating. The study included
tests in which some female test animals were mated with untreated
males and some untreated females were mated with treated male animals.
A small number of females of the F1b and F2b generations were killed
after the thirteenth day of pregnancy and the uterus examined for
implantation sites, viable and resorbed embryos and macroscopically
observable abnormalities. The F1a, F2a and F3a litters were killed
at weaning and autopsied. Rats of the F1b and F2b litters not used to
produce the next generation were killed at weaning and autopsied. The
F3b litters were killed at weaning and the heart, kidneys and livers
weighed and spleen, suprarenals, thyroid and (in the case of the
highest dosage group) brain examined histologically. The results
showed that the dosage levels of vamidothion sulfoxide used had no
untoward effect on treated animals, in particular on fertility or
reproductive activity of rats. There was no indication of teratogenic
activity in this study (Ganter et al., 1969b).
Special studies on neurotoxicity
Groups of five white leghorn chickens were injected s.c. with 60
and 120 mg vamidothion/kg (1X and 2X LD50 dose). Positive control
groups received 20 and 40 mg di-isopropyl-fluorophosphoridono. All
birds were treated with atropine and P2AM. No signs of neurotoxicity
appeared in vamidothiontreated birds while positive control groups
developed paralysis in 9-14 days (Anon., 1966b).
Special studies on the pharmacological effects
The motility of mice was unaffected by 10 mg vamidothion/kg
orally and exploratory activity and conditioned reflexes were normal
in rats treated orally with 30 mg/kg. No effect was found on
neuromuscular transmission, respiratory rate, ECG or heart rate and
the effects of i.v. adrenalin and i.v. acetylcholine were largely
unchanged by treatment with vamidothion, Salivary excretion was
increased, slightly and isolated intestine and uterus preparations
were affected weakly by vamidothion (Julou et al., 1966).
Mice were administered 100 mg vamidothion/kg orally (LD50 dose).
Some also received atropine, pralidoxime or both. Ten to 20 mg
atropine/kg i.p. provided 60-70% and 50 mg pralidoxime/kg i.p. 100%
protection against vamidothion (Dubost et al., 1960; Anon., 1966a).
Groups of 10 rats received 3 x LD50 dose (300mg/kg) of
vamidothion orally. One group received 17.4 mg atropine/kg i.p. when
signs of intoxication showed. Another received atropine plus 50 mg
P2AM/kg i.p. All control animals died within 45 min., 7-10 animals
with atropine died in 7-24 hours and three animals receiving atropine
and P2AM died. All of the latter group appeared normal two hours after
treatment while those on atropine alone appeared ill (Anon., 1966a).
Special studies on reproduction
Rat. A three-generation reproduction study, with each generation
producing two litters, was carried out on groups of 20 male and 40
female rats which were administered 5, 15 and 45 ppm vamidothion in
the diet. The control group consisted of 40 males and 80 females.
Animals of the Fo generation received diets for 11 weeks before being
mated. The study included tests in which some female test animals were
mated with untreated males and untreated females were mated with
treated males. A small number of females of the F1b and F2b
generations were killed after the thirteenth day of pregnancy and the
uterus examined for implantation sites, viable and resorbed embryos
and macroscopically apparent abnormalities. The F1a, F2a and F3a,
litters were killed at weaning and autopsied. Animals of the F1b and
F2b groups not used to produce the next generation were killed at
weaning and autopsied. The F3b litters were killed at weaning and the
heart, kidneys and liver weighed and the spleen, suprarenals, thyroid
and (with the highest dose level only) the brain examined
histologically. Haematological studies and examinations of bone marrow
of one animal of each sex of each group were carried out. Because of a
technical error some animals chosen as parents of the second and third
generations may have been produced from untreated male parents; the
proportion so produced is uncertain. All females were, however,
treated with the correct diets. Results show that at the dosage levels
used vamidothion had no untoward activity on rats, in particular on
their fertility or reproductive functions, There was no indication
that vamidothion is teratogenic (Ganter et al., 1969a).
Acute toxicity
The results of acute toxicity studies on vamidothion are
summarized in Table 2.
Administration of vamidothion in fatal doses produced signs
typical of cholinesterase inhibitors.
Groups of 20 mice were administered half the LD50 dose of
vamidothion plus half the LD50 dose of demeton-methyl, parathion,
phencapton, dimetboate, ethion, malathion, azinphos ethyl, mevinphos
or phosphamidon and observed for five days. No significant
potentiation was observed (Anon., 1966c).
Short-term studies
Rat. Groups of 10 male rats were administered 3 or 6 mg
vamidothion/kg/day orally for one month. It was reported that body
weight was unaffected, no animals died and no clinical signs of
toxicity occurred. Haematological examination including examination of
bone marrow and chemical analysis of urine and blood showed no
abnormalities except for blood cholinesterase activity which was
depressed by 50% (3 mg/kg/day) or 607 (6 mg/kg/day). Brain
cholinesterase levels were not depressed. The weights and histological
appearance of organs were unaffected by treatment (Dubost et al.,
1960).
TABLE 2. SUMMARY OF THE RESULTS OF STUDIES ON ACUTE TOXICITY
Species Sex Route Purity LD50 References
Mouse Oral p 43 Johnston and Rivett, 1966
Mouse Oral p 34 Dubost et al., 1960
Mouse M+F Oral Tech. 64 Pasquet and Mazuret, 1972a
Mouse Oral Tech. 40 Pak, 1970
Mouse s.c. p 34 Johnston and Rivett, 1966
Mouse Dermal p 1450 Johnston and Rivett, 1966
Mouse Dermal Tech. 1060 Pasquet and Mazuret, 1972a
Rat M Oral p 100 Johnston and Rivett, 1966
Rat M Oral p 105 Dubost et al., 1960
Rat M+F Oral p 105 Pasquet and Ma.uret, 1972b
Rat F Oral p 77 Desmoras et al., 1961
Rat M+F Oral Tech. 168 Pasquet and Mazuret, 1972a
Rat Oral Tech. 103 Pak, 1970
Rat F Oral p 64 Johnston and Rivett, 1H6
Rat M s.c. p 48 Johnston and Rivett, 1966
Rat F s.c. p 35 Johnston and Rivett, 1966
Guinea-pig Oral p 85 Dubost et al., 1960
Rabbit Oral Tech. 160 Pak, 1970
Rabbit Dermal p 1160 Quoted in Johnston and
Rivett, 1966
Rabbit M+F Dermal Tech. 3000 Pasquet and Mazuret, 1972a
Dog M+F Oral p 110 Julou and Pasquet, 1967
Mouse Oral p 50 Desmoras and Fournel, 1961
Mouse Oral l-isomer 68 Desmoras and Fournel, 1961
Mouse Oral d-isomer 34 Desmoras and Fournel, 1961
M = male F = female P = pure
Groups of five male and five female rats were fed on diets
containing 0, 0.2, 1 and 5 ppm vamidothion for six weeks after which
the test substance was withdrawn from the diet. Regular observation of
plasma and erythrocyte cholinesterase activity showed that only in the
plasma of female rats on the 5 ppm diet was enzyme activity
consistently depressed to a significant extent. The enzyme level
returned to normal within five weeks. Cholinesterase activity was not
depressed significantly at lower dosage levels (Wheldon et al., 1969).
Groups of five male and five female rats were fed on diets
containing 0, 5 and 50 ppm vamidothion for three months. No ill effect
was seen on growth or general health. Blood cholinesterase levels were
reduced to approximately 75% and 25% respectively of the control level
in the 5 and 50 ppm groups. Cholinesterase levels returned to normal
within four weeks when vamidothion was withdrawn from the diet.
Histopathological examination of two rats of each sex from the 50 ppm
group showed no abnormalities related to ingestion of the compound
(Rivett and Corbett, 1966).
Groups of rats were administered, by gavage, doses of
approximately 2, 5 or 10 mg vamidothion/kg daily for three months. At
the end of the test serum acetylcholinesterase activity was decreased
to 35%, 12% and 8%, respectively, of the normal value (Pak, 1970).
Dog. Groups of three dogs were administered orally 0, 1 and 2
mg/vamidothion/kg/day for one month. Two dogs received 8 mg/kg/day for
one month. No effect was seen on growth except for one animal on the
highest dose level which developed diarrhoea (cause unknown).
Haematological indices and blood coagulation were normal. Slight
differences between groups in urine urobilinogen, glucose and bile
salts, serum protein, PSP clearance and behaviour could not be
attributed to treatment. In particular no neurological abnormalities
were seen although erythrocyte cholinesterase was severely depressed
(100% inhibition at sixteenth day with 8 mg/kg dosage regime). In
six dogs receiving 1 and 2 mg/kg/day no abnormalities were found on
gross and histopathologic examination. (Dubost et al., 1960).
Groups of two male and two female beagle dogs were fed on diets
containing 0, 0.2, 1 and 5 ppm vamidothion for five to six weeks.
Animals on the lowest dosage level were then fed for a further four
weeks on diet containing 20 ppm vamidothion, after which they were
observed for four more weeks on normal diet. No clinical signs of
abnormality were seen in any group. The plasma and erythrocyte
cholinesterase levels were slightly depressed in dogs on the highest
dosage. These levels returned to normal within two weeks of return to
a normal diet. No abnormalities in the appearance or weight of organs
were found in any group (Noel et al., 1969).
Long-term studies
No data are available.
Observations in man
Groups of 6-11 normal healthy volunteers of both sexes were
administered 9.6 or 37.2 µg vamidothion/kg/day orally in aqueous
solution on five days each week for three weeks. Other groups received
78.8 or 122.8 µg vamidothion/kg/day in aqueous solution for five
weeks. A control group was studied for 25 weeks. No clinical signs or
symptoms were found which could be attributed to treatment. Plasma
cholinesterase, estimated weekly, showed no consistent depression in
any group but erythrocyte cholinesterase was depressed in three of six
volunteers receiving 122.8 µg/kg/day. The no-effect level was
considered to be 78.8 µg/kg/day, (calculated to be equivalent to 56.3
µg/kg/day if vamidothion was administered every day without a break)
(Noel et al., 1970).
Serum and erythrocyte cholinesterase levels were determined
periodically in workers who had been involved in the manufacture of
vamidothion over a period of several months to a few years. Enzyme
levels were also determined in experimentalists exposed several times
a year to vamidothion over a seven-year period. The actual exposure is
uncertain. Fluctuations in enzyme levels in both groups were within
the limits of normal (Celice et al., 1966),
Comments
It has been shown that vamidothion is partly absorbed from the
gastric intestinal tract and is excreted in urine. Several metabolic
products have been found in urine; these ewe compounds are also
produced by liver slices. One metabolite (desmethyl vamidothion) was
shown to be produced by plants but has not yet been found as a
metabolite in animals. Vamidothion sulfoxide has been examined
toxicologically but the toxicity of other metabolites has not
apparently been examined.
Vamidothion depresses serum cholinesterase activity at lower
concentrations than it depresses the activity of erythrocyte
cholinesterase, except in dogs. Brain cholinesterase activity is less
affected. The dosage level of vamidothion which is without effect in
man is just over 50 µg/kg/day. The no-effect level with regard to
cholinesterase depression was 1 ppm in the diet of rats and 5 ppm in
the diet of dogs. These dietary levels are approximately equivalent to
50 µg/kg/day in rats and 125 µg/kg/day in dogs.
Studies showed that vamidothion had no adverse effects on
reproduction. Short-term tests were carried out in only small numbers
of dogs and rats and although no ill effects other than that on
cholinesterase activity were detected, the observations were not
sufficiently extensive to eliminate the possibility that the compound
has other significant effects, No long-term tests have been reported.
TOXICOLOGICAL EVALUATION
It is not possible, on the information available, to estimate an
ADI for vamidothion.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Vamidothion is a systemic organophosphorus aphicide and miticide.
Its most important use is on apples and pears against the woolly apple
aphid. It is also used on other pome fruits, sugar beet and brussels
sprouts. and to a lesser extent on grapes, cereal crops, sugar cane
and hops.
Pre-harvest treatments
The recommended uses are as follows:
Orchard fruits. In France, Western Europe, Australia, New Zealand
and South Africa, a single treatment at 40-50 g a.i./100 l. For
low-volume application, a rate of 0.4-0.5 kg a.i./ha is used. In Great
Britain an application at 20 g/100 l before flowering, and one or two
treatments at 40 g/100 l after flowering are recommended for apple and
pear; a single application at 40 g/100 l after flowering for plum and
cherry.
Grapes. A single treatment at 40-50 g/100 l during active sap
movement. A second similar treatment if necessary.
Citrus fruits. 60-80 g/100 l.
Hops. In England, three applications at 700 g/ha, with a minimum
pre-harvest interval of four weeks. In France, Belgium and Germany,
one application at 50 g/100 l when the aphid first appears.
Rice. Three applications at 50 g/100 l (600 g/ha).
Cotton. 40-60 g/100 l high-volume application, repeated, if
necessary.
Post-harvest treatments
None known.
Other possible uses
Trials against aphids of maize and sorghum have been successful.
Aphids and red spider of strawberries, tomatoes and beans have been
successfully controlled. A pre-harvest interval of six weeks is
recommended.
Aphids have been controlled in trials in France, Brazil and Japan
by brushing tree trunks with vamidothion during active sap movement.
Residues resulting from supervised trials
Residue data are available from supervised trials in France,
Germany, Switzerland and the United Kingdom (Rhône-Poulenc, 1966,
1969; May and Baker Ltd. 1963), together with limited data on
application to sugar cane in Trinidad (May and Baker Ltd, 1973). These
data have been deposited with FAO and are summarized in Table 3.
Residues of vamidothion are converted to the sulfoxide in or on the
plant, and residues reported in the table refer to the sum of the
parent compound and the sulfoxide.
The combined residue is unusually persistent. The half-life on
apples and pears is between 35 and 45 days, and more limited results
on peaches, plums, grapes, cherries and straw berries indicate a
similar persistence. Half-life periods on cereals and vegetables are
generally between 6 and 20 days.
Data from supervised trials in Japan (Tomizawa, 1973) show very
low residues, usually below 0.02 ppm in a wide range of crops. As
these data apparently refer only to the parent compound, they are not
quoted in the Table.
Fate of residues
In plants. As mentioned above, the main biologically active product
is the sulfoxide, and this is the main terminal residue. The sulfone
is not found to any significant extent, but it has been detected as a
metabolite in citrus leaves kept at a low temperature. (Results quoted
by Tomizawa, 1973). The other main metabolites are demethyl
vamidothion, phosphoric acid and diethyl phosphate (Morikawa and
Saito, 1969).
In animals. In experiments with 32B-vamidothion (Morikawa and
Saito, 1969), 69% of the activity in the urine of dosed mice appeared
as phosphoric acid and its mono and dimethyl esters. The remaining 31%
was an unknown metabolite. The dimethyl compound was not detectable.
In the same series of experiments, rat liver homogenate metabolized
55% of the vamidothion with which it was incubated to the unknown
metabolite, 31% to phosphoric acid and 14% to diethyl phosphate.
Methods of residue analysis
Residues normally consist essentially of a mixture of vamidothion
and its sulfoxide. Three methods have been developed by the
Rhône-Poulenc Laboratories to determine this mixture after suitable
clean-up: gas chromatography after oxidation of both compounds to the
sulfone, colorimetric determination of total phosphorus after
mineralization, and bioassay (Rhône-Poulenc, 1972). The
gas-chromatographic method should be suitable for regulatory purposes.
The other two are non-specific but are suitable for determining
residues arising from supervised trials: most of the data in Table 3
were obtained by these methods, often by both of them. Identity can be
confirmed, and the parent compound and sulfoxide separately determined
semi-quantitatively if required, by TLC (Rhône-Poulenc, 1972).
Determination by gas chromatography (Desmoras et al., 1972)
The sample is blended with buffered methanol or acetone and the organic
solvent evaporated. The extract is washed with petroleum ether, in
which vamidothion and its sulfoxide are insoluble, and then extracted
continuously with dichloromethane. (Vamidothion is readily extracted,
but continuous extraction is needed for the sulfoxide.) An aliquot of
the solution is evaporated to dryness and oxidized with potassium
permanganate in aqueous acetone. The resulting sulfone is extracted
with dichloromethane and transferred to benzene for determination by
gas chromatography on DEGS (diethylene glycol succinate) stationary
phase with electron capture detection. Recoveries are within a range
of 80-110% and the limit of determination is about 0.05 ppm.
The method described is more sensitive than methods in which
thermionic (Ruzicka et al., 1967) or flame photometric (Mestres, 1973)
detection is used. Separate determination of vamidothion and the
sulfoxide by GLC without oxidation is unsatisfactory because of the
large difference between their retention times. The two compounds can
be differentiated if required by first extracting vamidothion by
shaking with dichloromethane, then obtaining the sulfoxide by
continuous extraction and oxidizing the residues in the two extracts
to the sulfone separately.
Total phosphorus determination. The residue is extracted, and
cleaned-up as in the GLC method and the extract is evaporated to
dryness. The residue is digested with nitric and sulfuric acids and
phosphorus is determined colorimetrically as molybdenum blue. The
limit of determination is about 0.1 ppm.
Biological determination. The solvent is evaporated from the
dichloromethane extract obtained as described above, and some of the
co-extractives are removed by precipitation from acetone solution at
-70°C. The acetone in the filtrate from this step is evaporated and
replaced with water, and residues of vamidothion plus sulfoxide are
determined by bio-assay with Daphnia pulex as the test organism. The
procedure has been described in detail by Desmoras (1963).
Thin-layer chromatography. Thin-layer chromatography, TLC, is useful
as a confirmatory test of identity and to separate the parent compound
from the sulfoxide. It is carried out on the cleaned-up
dichloromethone extract, using silica gel which has been activated by
heating at 120°C for at least one hour. Approximate Rf values of
vamidothion, its sulfoxide and its sulfone on silica gel GF254
(Merek), developed with various solvent systems, are listed in Table
4. Separated spots can be detected by esterase inhibition or by
spraying with iodoplatinate, palladium chloride or
nitrobenzylpyridine. The enzyme inhibition method is the most
sensitive, with detection limits of 30-50 ng for the three compounds.
TLC of the dichloromethane extract does not satisfactorily
indicate the ratio of vamidothion to sulfoxide in the original
residue, because some vamidothion is converted to sulfoxide during the
continuous extraction.
Appraisal
Vamidothion is an organophosphorus compound with pronounced
systemic activity, effective against aphids and mites not resistant to
organophosphorus compounds.
It is applied as water-miscible solution to pome fruits, sugar
beet, brussels sprouts and, to a lesser extent, cereal crops, grapes,
sugar cane and hops. By far the most important use is on apples and
pears against woolly apple aphid.
Vamidothion is particularly persistent. Numerous studies have
been carried out in France, England, Switzerland and Germany and these
indicate the half-life in pome fruit to be between 35 and 45 days. The
half-life on cereals and vegetables is generally between six and 20
days.
The main biologically active metabolite is the sulfoxide which
has a higher systemic insecticidal activity than the parent compound.
The sulfone is not found in plants to any significant extent.
Residues consisting of a mixture of the sulfoxide and the parent
compound, can be determined by bio-assay or estimation of total
phosphorus. The recommended method, which should be suitable :Per
regulatory purposes, is by gas chromatography with electron-capture
detection after oxidation to the sulfone. The limit of determination
is about 0.05 ppm. TLC can be used for confirmation of identity.
The limited data available on sugar cane indicated residues of
about 0.2 ppm soon after application, decreasing to below 0.05 ppm
before harvest. As the available information on wheat and sunflower
was restricted to the whole plant, it was not suitable for judging the
probable residue level in the grain and seed.
National tolerances
In Switzerland a tolerance of 0.6 ppm has been established for
residues arising from applications to fruit trees, except cherry, with
the end of May as the last date of application.
TABLE 3. VAMIDOTHION RESIDUES IN CROPS
Dosage rate, Residue (ppm vamidothion + sulfoxide) after interval (days)
Crop Country a.i. g/100 l
or kg/ha 0-2 5-11 13-18 20-30 34-39 41-49 52-69 72-95 >100
Apple France 50 g/100 l 3.0 2.5 2.0 1.6 1.3 1.1
50 g/100 l 0.1
2 x 50 g/100 l 1.7
2 x 50 g/100 l 0.3 0.3
Germany 60 g/100 l 0.7 < 0.1 0.5 0.5 0.4
1.0 kg/ha 1.1 0.6 0.3
1.8 kg 0.75 0.2
2 x 1.8 kg/ha 0.7 0.2
Switzerland 50 g/100 l 0.85 < 0.65 0.8
50 g/100 l 0.5
England 40+80 g/100 l 3.5*
1.6*
1.7*
2.4*
40+80 g/100 l 3.4
Pear France 50 g/100 l 0.1
2 x 50 g/100 l 0.2 0.2
England 40 g/100 l 1.5* 0.6*
1.9* >0.2*
40+80 g/100 l 2.1*
1.8*
Peach France 50 g/100 l 0.1
2 x 50 g/100 l 0.4 0.3
Cherry England 80 g/100 l >0.4*
>0.4*
>0.4*
Plum England 80 g/100 l 1.9
TABLE 3. (Cont'd.)
Dosage rate, Residue (ppm vamidothion + sulfoxide) after interval (days)
Crop Country a.i. g/100 l
or kg/ha 0-2 5-11 13-18 20-30 34-39 41-49 52-69 72-95 >100
Grape France 50 g/100 l 0.2
2 x 50 g/100 l 0.25 0.15
Hop France 3 x 50 g/100 l <0.1
" (fresh and dry cones) 3 x 100 g/100 l <0.1
" (fresh cones) England 25+40 g/100 l < 0.5
Wheat (whole plant) France 50 g/100 l 9 4.1 1.4 0.4 0.4
50 g/100 l approx. 10 4.2 1.9
" (grain) 50 g/100 l 0.25
50 g/100 l 0.1
Sugar beat (leaves) France 50 g/100 l 1.1*
0.2*
" " (root) 50 g/100 l 0.2
Sunflower (leaves) France 75 g/100 l 60 23 19 10 7 5
Strawberries Scotland 50 g/100 l 0.6*
0.6*
0.6*
50 g/100 l 0.3
Broad beans France 50 g/100 l 81 64 51 40 23 13
(glasshouse,leaves)
French beans France 50 g/100 l 87 35 21 8.5
(glasshouse, leaves)
Sugar cane Trinidad 0.9 kg/ha 0.2 0.1
0.9 kg/ha 0.2 < 0.05
" " (juice) 0.9 kg/ha < 0.05
* Separate crop treatments.
TABLE 4. Rf VALUES OF VAMIDOTHION, ITS SULFOXIDE AND ITS SULFONE
ON SILICA GEL* IN SEVERAL SOLVENT SYSTEMS
Approximate Rf
Developing solvent
Vamidothion Sulfoxide Sulfone
Dichloromethane-methanol (90:10, v/v) 0.65 0.4 0.55
Ethyl acetate-methanol (75:25, v/v) 0.65 0.5 0.65
Acetonitrile-methanol (97:3 v/v) 0.5 0.1 0.65
Acetone-dimethylformanide (99:1, v/v) 0.6 0.35 0.65
* Silica gel GF254 (Merck) activated at 120°C for one hour before use.
RECOMMENDATION
As no acceptable daily intake could be established no tolerances
are recommended. Following officially acceptable use in various
countries residues of vamidothion can occur in the following
commodities up to the levels indicated. The guide-lines indicated
below are unlikely to be exceeded as a result of the recommended use
of vamidothion.
Guide-line levels (based on a pre-harvest interval of six weeks)
Apples and pears 2 ppm
Brussels sprouts 1 ppm
Sugar beet 0.5 ppm
Grapes 0.5 ppm
Hops 0.2 ppm
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake can be established)
1. Long-term studies in at least one animal species.
2. Adequate short-term studies in several species including a
non-rodent species.
3. Studies to identify metabolites and investigate their toxicity.
4. Studies on the nature and level of residues in animal products
from the feeding of residues at levels occurring on food wastes.
5. Information showing the fate of residues in the major crops in
countries with different meteorological and growth conditions.
REFERENCES
Anon. (1966a) Vamidothion. Etude dos Antidotes. Submitted by Société
des Usine Chimiques, Rhône-Poulenc. Unpublished report
Anon. (1966b) Vamidothion. Essai do neurotoxicité, submitted by
Société des Usines Chimiques, Rhône-Poulenc. Unpublished report
Anon. (1966e) Vamidothion. Etude de la potentialisation sur souris
avec des insecticides comerciaux, submitted by Société dos Usines
Chimiques, Rhône-Poulene. Unpublished report
Celice, J., Fournel, J. and Koenig F. (1966) Evaluation des risques
l'intoxication par le vamidothion (10.465 R.P.) au cours de sa
fabrication et do son experimentation plein champ, submitted by
Société dos Usines Chimiques, Rhône-Poulene. Unpublished report
Desmoras, J. (1963) Biological determination of vamidothion in plants.
Mededel. Landbouwhogeschool Opzoekingasta, Staat Gent, 28: 731-742
Desmoras, R. and Fournel, J. (1961) Insecticides. Activités
insecticides ot toxicités des 12.151.R.P. et 12.164 R.P. isomeres
optiques du 10.465 R.P. Submitted by Société des Usines Chimiques,
Rbône-Poulenc. Unpublished report
Desmoras, R., Fournel, J. and Koenig F. (1961) Insecticides. Activités
insecticides et toxicités comparées des 10.465 R.P. et 11.905 R.P.
Submitted by Sociétés des Usines Chimiques, Rhône-Poulene. Unpublished
report
Desmoras, J., Laurent, M. and Buys, M. (1972) Unpublished report No.
15,893 submitted by Rhône-Poulene
Dubost, J.P., Fournel, J., Ganter, P., Julou, L. and Myon, M. (1960)
Produit No. 10.463 R.P. Toxicité ague, tolerance locale, activités
anticholinesterasique et antidotes, toxicité chronique chez le rat et
le chien. Submitted by Société dos Usines Chimiques, Rhône-Poulenc.
Unpublished report
Ganter, P., Julou, L., Pasquet, J., Delesque, M. and Durel, J. (1969a)
Vanidothion (10.465 R.P.) activités sur les fonctions de reproduction
du Rat (Essai sur 3 generations). Submitted by Société des Usines
Chimiques, Rhône-Poulenc. Unpublished report
Ganter, P., Julou, L., Pasquet, J. and Delesque, M. (1969b)
Vamidothion (11.905 R.P.) activité sur les fonctions de reproduction
du rat (Essai sur 3 generations). Submitted by Société des Usines
Chimiques, Rhône-Poulenc. Unpublished report
Johnston, J.P. and Rivett, K.F. (1966) Vamidothion. Acute toxicity to
mammals. Unpublished report of May & Baker, Ltd. submitted by Société
des Usines Chimique, Rhône-Poulenc
Julou, L., Ducrot, R., Gardens, M.C., Detaille, J. Fee, C., Garret, C.
and Pasquet, J. (1966) Etude pharmacologique du vamidothion (10.465
R.P.) comparativement au parathion (3.470 R.P.). Submitted by Société
des Usines Chimiques, Rhône-Poulenc. Unpublished report
Julou, L. and Pasquet, J. (1967) Vamidothion (10.465 R.P.), toxicité
aigue chez le chien par vole orals. Submitted by Société des Usines
Chimiques, Rhône-Poulenc. Unpublished report
May and Baker Ltd. (1963) Unpublished report
May and Baker Ltd. (1973) Unpublished report
Mestres, R. (1973) Personal communication
Morikawa, O. and Saito, T. (1966) Degradations of vamidothion and
dimethoate in plants, insects and mammals. Bochu Kagaku, 31: 130-135
Morikawa, O. and Saito, T. (1969) Degradation of vamidothion and
Dimethoate in plants, insects and mammals. Unpublished report of the
Laboratory of Applied Entomology, Nagoya University, Japan, submitted
by Société des Usines Chimique, Rhône-Poulenc
Noel, P.R.B., Coote, S. and Street, A.E. (1969) Vamidothion (10.465
R.P.). Determination of no-effect level in Beagle dogs. Unpublished
report of Huntingdon Research Centre submitted by Société des Usines
Chimique, Rhône-Poulenc
Noel, P.R.B., Haynes, G. and Street, A.E. (1970) Vamidothion.
Determination of no-effect level in human volunteers. Unpublished
report of Huntingdon Research Centre submitted by Société des Usines
Chimique, Rhône-Poulenc
Pak, L.V. (1970) The toxicity of vamidothion on warm-blooded animals.
Gig. truda i Praftabol, 2: 58-9
Pasquet, J. and Mazuret, A. (1972a) Vamidothion (10.465 R.P.) - Kilval
ou formule S.A.E. 1700 (solution A 400 9/litro) Submitted by Société
des Usines Chimiques, Rhône-Poulenc. Unpublished report
Pasquet, J. and Mazuret, A. (1972b) Vamidothion (10.465 R.P.) -
Toxicité aigue chez le rat par voie orals, Submitted by Société des
Usines Chimiques, Rhône-Poulenc. Unpublished report
Rivett, K.F. and Corbett, K. (1966) Insecticides Vamidothion. The
acute and chronic oral toxicity of vanidothion and its sulphoxide.
Unpublished report of May and Baker Ltd, submitted by Société des
Usines Chimiques, Rhône-Poulenc
Rhône-Poulenc. (1966) Méthodes de dosage et évolution des résidues.
Report R.P. -D.S.Ph./D.S. An. Nord No. 11: 082
Rhône-Poulenc. (1969) Dosage de résidus dans les végétaux. Résumé des
résultats. Report R.P.-D.S.Ph. No. 14, 158. Unpublished. [Results
obtained during the period 1961-1968 are summarized]
Rhône-Poulene. (1972) Report No. R.P.-D.S.Ph./D.S. An. Nord No. 17,
002. Unpublished
Ruzicka, J., Thomson, J. and Wheals, B.B. (1967) The gas
chromatographic examination of organophosphorus pesticides and their
oxidation products. J. Chromatogr., 30: 92-99
Tomizawa, C. (1973) Personal communication
Wheldon, G.H., Odey, R.J. and Street, A.E. (1969) Toxicity of
vamidothion to rats during dietary administration over six weeks.
Unpublished report of Huntingdon Research Centre submitted by Société
des Usines Chimiques, Rhône-Poulenc
See Also:
Toxicological Abbreviations
Vamidothion (ICSC)
Vamidothion (Pesticide residues in food: 1982 evaluations)
Vamidothion (Pesticide residues in food: 1985 evaluations Part II Toxicology)
Vamidothion (Pesticide residues in food: 1988 evaluations Part II Toxicology)
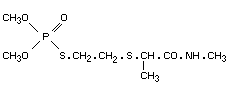 Other information on identity and properties
Physical state: White crystalline solid (pure)
Amber waxy solid (technical)
Melting point: 40°C (pure)
Solubility: Water - about 4 g/ml
Benzene, toluene, methyl ethyl ketone, ethyl
acetate, acetonitrile, methylene chloride,
anisole, cyclohexanone, chloroform - 1 g/ml
[xylene - 0.125 g/ml petroleum ether, cyclohexane
- insoluble
Volatility: Very low. Negligible loss under vacuum (2 mm Hg)
at 20°C.
Stability: The technical solid decomposes slowly at room
temperature but is stable in organic solvents
(e.g. cyclohexanone, methyl ethyl ketone).
Hydrolyzed by alkali
Optical Vamidothion consists of a mixture of optically
isomerism: active isomers. Their systemic activity as
pesticides is similar, but the D form shows higher
contact activity as an araricide.
Formulation: Water-miscible solution containing 400 g/l.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Biodegradation
One NC-5 mouse was administered 30 mg 32P vamidothion orally.
Urine collected over the following 24 hours contained 32P labelled
phosphoric acid, 0-methyl phosphate, 0,0-dimethyl phosphate and an
unknown compound.
Rat liver homogenate incubated for two hours with 32P labelled
vamidothion produced 0,0-dimethyl phosphate, phosphoric acid and an
unknown compound which was possibly dimethyl phosphothionate.
The same metabolites were produced by incubation of 32P labelled
vamidothion with plant leaves. In addition desmethyl vamidothion was
detected; this may not have been detected as a metabolite in animals
because of the low activity of 32p incorporated into the vamidothion
(Morikawa and Saito, 1969).
The oxidation product, vamidothion sulfoxide, has been
demonstrated to be formed in plants (Desmoras et al., 1961).
Effects on enzymes
Vamidothion inhibits cholinesterase in vitro and in vivo. A
concentration of 40 mg/l caused 50% inhibition of enzyme activity of
plasma. Four hours after guinea-pigs had received 40 mg vamidothion/kg
orally, 81% inhibition of plasma and 20% inhibition of cellular enzyme
occurred. With higher dosage levels the plasma cholinesterase level
remained stationary but cellular enzyme activity decreased (Dubost et
al., 1960).
TOXICOLOGICAL STUDIES
Special studies on the metabolites
A summary of the results of acute toxicity studies on
vamidothion sulfoxide is shown in Table 1.
A test for neurotoxicity was carried out in the same manner as
for vamidothion itself. Positive controls showed signs of paralysis in
9-14 days while vamidothion sulfoxide was without effect (Anon.,
1966b).
TABLE 1. THE ACUTE TOXICITY OF VAMIDOTHION SULFOXIDE
Species Sex Route LD50 References
Mouse Oral 80 Desmoras et al., 1961
Rat F Oral 160 Desmoras et al., 1961
Rivett and Corbett, 1966
Guinea-pig Oral 205 Desmoras et al., 1961
Chicken F s.c. 60 Anon., 1966b
A short-term study was carried out on groups of five male and
five female rats which were fed diets containing 0, 5, 50, 100 and 200
ppm vamidothion sulfoxide for three months. The degree of depression
of cholinesterase activity was similar to that in animals receiving
the same dosage levels of vamidothion. The 100 and 200 ppm dosage
levels depressed cholinesterase levels to approximately 20% And 12%
respectively of the control level. Cholinesterase activity returned to
normal within four weeks when vamidothion sulfoxide was withdrawn from
the diet. Histological examination of two male and two female rats
from each group showed no abnormality attributable to ingestion of the
test compound (Rivett and Corbett, 1966).
A three generation reproduction study with each generation
producing two litters, was carried out on groups of 20 male and 40
female rats (generation Fo, test animals), 40 male and 80 female
(generation Fo controls) or 10 male and 30 female (other generations).
Animals were administered 0, 5, 15 or 45 ppm of vamidothion sulfoxide
in the diet for four weeks before the first mating. The study included
tests in which some female test animals were mated with untreated
males and some untreated females were mated with treated male animals.
A small number of females of the F1b and F2b generations were killed
after the thirteenth day of pregnancy and the uterus examined for
implantation sites, viable and resorbed embryos and macroscopically
observable abnormalities. The F1a, F2a and F3a litters were killed
at weaning and autopsied. Rats of the F1b and F2b litters not used to
produce the next generation were killed at weaning and autopsied. The
F3b litters were killed at weaning and the heart, kidneys and livers
weighed and spleen, suprarenals, thyroid and (in the case of the
highest dosage group) brain examined histologically. The results
showed that the dosage levels of vamidothion sulfoxide used had no
untoward effect on treated animals, in particular on fertility or
reproductive activity of rats. There was no indication of teratogenic
activity in this study (Ganter et al., 1969b).
Special studies on neurotoxicity
Groups of five white leghorn chickens were injected s.c. with 60
and 120 mg vamidothion/kg (1X and 2X LD50 dose). Positive control
groups received 20 and 40 mg di-isopropyl-fluorophosphoridono. All
birds were treated with atropine and P2AM. No signs of neurotoxicity
appeared in vamidothiontreated birds while positive control groups
developed paralysis in 9-14 days (Anon., 1966b).
Special studies on the pharmacological effects
The motility of mice was unaffected by 10 mg vamidothion/kg
orally and exploratory activity and conditioned reflexes were normal
in rats treated orally with 30 mg/kg. No effect was found on
neuromuscular transmission, respiratory rate, ECG or heart rate and
the effects of i.v. adrenalin and i.v. acetylcholine were largely
unchanged by treatment with vamidothion, Salivary excretion was
increased, slightly and isolated intestine and uterus preparations
were affected weakly by vamidothion (Julou et al., 1966).
Mice were administered 100 mg vamidothion/kg orally (LD50 dose).
Some also received atropine, pralidoxime or both. Ten to 20 mg
atropine/kg i.p. provided 60-70% and 50 mg pralidoxime/kg i.p. 100%
protection against vamidothion (Dubost et al., 1960; Anon., 1966a).
Groups of 10 rats received 3 x LD50 dose (300mg/kg) of
vamidothion orally. One group received 17.4 mg atropine/kg i.p. when
signs of intoxication showed. Another received atropine plus 50 mg
P2AM/kg i.p. All control animals died within 45 min., 7-10 animals
with atropine died in 7-24 hours and three animals receiving atropine
and P2AM died. All of the latter group appeared normal two hours after
treatment while those on atropine alone appeared ill (Anon., 1966a).
Special studies on reproduction
Rat. A three-generation reproduction study, with each generation
producing two litters, was carried out on groups of 20 male and 40
female rats which were administered 5, 15 and 45 ppm vamidothion in
the diet. The control group consisted of 40 males and 80 females.
Animals of the Fo generation received diets for 11 weeks before being
mated. The study included tests in which some female test animals were
mated with untreated males and untreated females were mated with
treated males. A small number of females of the F1b and F2b
generations were killed after the thirteenth day of pregnancy and the
uterus examined for implantation sites, viable and resorbed embryos
and macroscopically apparent abnormalities. The F1a, F2a and F3a,
litters were killed at weaning and autopsied. Animals of the F1b and
F2b groups not used to produce the next generation were killed at
weaning and autopsied. The F3b litters were killed at weaning and the
heart, kidneys and liver weighed and the spleen, suprarenals, thyroid
and (with the highest dose level only) the brain examined
histologically. Haematological studies and examinations of bone marrow
of one animal of each sex of each group were carried out. Because of a
technical error some animals chosen as parents of the second and third
generations may have been produced from untreated male parents; the
proportion so produced is uncertain. All females were, however,
treated with the correct diets. Results show that at the dosage levels
used vamidothion had no untoward activity on rats, in particular on
their fertility or reproductive functions, There was no indication
that vamidothion is teratogenic (Ganter et al., 1969a).
Acute toxicity
The results of acute toxicity studies on vamidothion are
summarized in Table 2.
Administration of vamidothion in fatal doses produced signs
typical of cholinesterase inhibitors.
Groups of 20 mice were administered half the LD50 dose of
vamidothion plus half the LD50 dose of demeton-methyl, parathion,
phencapton, dimetboate, ethion, malathion, azinphos ethyl, mevinphos
or phosphamidon and observed for five days. No significant
potentiation was observed (Anon., 1966c).
Short-term studies
Rat. Groups of 10 male rats were administered 3 or 6 mg
vamidothion/kg/day orally for one month. It was reported that body
weight was unaffected, no animals died and no clinical signs of
toxicity occurred. Haematological examination including examination of
bone marrow and chemical analysis of urine and blood showed no
abnormalities except for blood cholinesterase activity which was
depressed by 50% (3 mg/kg/day) or 607 (6 mg/kg/day). Brain
cholinesterase levels were not depressed. The weights and histological
appearance of organs were unaffected by treatment (Dubost et al.,
1960).
TABLE 2. SUMMARY OF THE RESULTS OF STUDIES ON ACUTE TOXICITY
Species Sex Route Purity LD50 References
Mouse Oral p 43 Johnston and Rivett, 1966
Mouse Oral p 34 Dubost et al., 1960
Mouse M+F Oral Tech. 64 Pasquet and Mazuret, 1972a
Mouse Oral Tech. 40 Pak, 1970
Mouse s.c. p 34 Johnston and Rivett, 1966
Mouse Dermal p 1450 Johnston and Rivett, 1966
Mouse Dermal Tech. 1060 Pasquet and Mazuret, 1972a
Rat M Oral p 100 Johnston and Rivett, 1966
Rat M Oral p 105 Dubost et al., 1960
Rat M+F Oral p 105 Pasquet and Ma.uret, 1972b
Rat F Oral p 77 Desmoras et al., 1961
Rat M+F Oral Tech. 168 Pasquet and Mazuret, 1972a
Rat Oral Tech. 103 Pak, 1970
Rat F Oral p 64 Johnston and Rivett, 1H6
Rat M s.c. p 48 Johnston and Rivett, 1966
Rat F s.c. p 35 Johnston and Rivett, 1966
Guinea-pig Oral p 85 Dubost et al., 1960
Rabbit Oral Tech. 160 Pak, 1970
Rabbit Dermal p 1160 Quoted in Johnston and
Rivett, 1966
Rabbit M+F Dermal Tech. 3000 Pasquet and Mazuret, 1972a
Dog M+F Oral p 110 Julou and Pasquet, 1967
Mouse Oral p 50 Desmoras and Fournel, 1961
Mouse Oral l-isomer 68 Desmoras and Fournel, 1961
Mouse Oral d-isomer 34 Desmoras and Fournel, 1961
M = male F = female P = pure
Groups of five male and five female rats were fed on diets
containing 0, 0.2, 1 and 5 ppm vamidothion for six weeks after which
the test substance was withdrawn from the diet. Regular observation of
plasma and erythrocyte cholinesterase activity showed that only in the
plasma of female rats on the 5 ppm diet was enzyme activity
consistently depressed to a significant extent. The enzyme level
returned to normal within five weeks. Cholinesterase activity was not
depressed significantly at lower dosage levels (Wheldon et al., 1969).
Groups of five male and five female rats were fed on diets
containing 0, 5 and 50 ppm vamidothion for three months. No ill effect
was seen on growth or general health. Blood cholinesterase levels were
reduced to approximately 75% and 25% respectively of the control level
in the 5 and 50 ppm groups. Cholinesterase levels returned to normal
within four weeks when vamidothion was withdrawn from the diet.
Histopathological examination of two rats of each sex from the 50 ppm
group showed no abnormalities related to ingestion of the compound
(Rivett and Corbett, 1966).
Groups of rats were administered, by gavage, doses of
approximately 2, 5 or 10 mg vamidothion/kg daily for three months. At
the end of the test serum acetylcholinesterase activity was decreased
to 35%, 12% and 8%, respectively, of the normal value (Pak, 1970).
Dog. Groups of three dogs were administered orally 0, 1 and 2
mg/vamidothion/kg/day for one month. Two dogs received 8 mg/kg/day for
one month. No effect was seen on growth except for one animal on the
highest dose level which developed diarrhoea (cause unknown).
Haematological indices and blood coagulation were normal. Slight
differences between groups in urine urobilinogen, glucose and bile
salts, serum protein, PSP clearance and behaviour could not be
attributed to treatment. In particular no neurological abnormalities
were seen although erythrocyte cholinesterase was severely depressed
(100% inhibition at sixteenth day with 8 mg/kg dosage regime). In
six dogs receiving 1 and 2 mg/kg/day no abnormalities were found on
gross and histopathologic examination. (Dubost et al., 1960).
Groups of two male and two female beagle dogs were fed on diets
containing 0, 0.2, 1 and 5 ppm vamidothion for five to six weeks.
Animals on the lowest dosage level were then fed for a further four
weeks on diet containing 20 ppm vamidothion, after which they were
observed for four more weeks on normal diet. No clinical signs of
abnormality were seen in any group. The plasma and erythrocyte
cholinesterase levels were slightly depressed in dogs on the highest
dosage. These levels returned to normal within two weeks of return to
a normal diet. No abnormalities in the appearance or weight of organs
were found in any group (Noel et al., 1969).
Long-term studies
No data are available.
Observations in man
Groups of 6-11 normal healthy volunteers of both sexes were
administered 9.6 or 37.2 µg vamidothion/kg/day orally in aqueous
solution on five days each week for three weeks. Other groups received
78.8 or 122.8 µg vamidothion/kg/day in aqueous solution for five
weeks. A control group was studied for 25 weeks. No clinical signs or
symptoms were found which could be attributed to treatment. Plasma
cholinesterase, estimated weekly, showed no consistent depression in
any group but erythrocyte cholinesterase was depressed in three of six
volunteers receiving 122.8 µg/kg/day. The no-effect level was
considered to be 78.8 µg/kg/day, (calculated to be equivalent to 56.3
µg/kg/day if vamidothion was administered every day without a break)
(Noel et al., 1970).
Serum and erythrocyte cholinesterase levels were determined
periodically in workers who had been involved in the manufacture of
vamidothion over a period of several months to a few years. Enzyme
levels were also determined in experimentalists exposed several times
a year to vamidothion over a seven-year period. The actual exposure is
uncertain. Fluctuations in enzyme levels in both groups were within
the limits of normal (Celice et al., 1966),
Comments
It has been shown that vamidothion is partly absorbed from the
gastric intestinal tract and is excreted in urine. Several metabolic
products have been found in urine; these ewe compounds are also
produced by liver slices. One metabolite (desmethyl vamidothion) was
shown to be produced by plants but has not yet been found as a
metabolite in animals. Vamidothion sulfoxide has been examined
toxicologically but the toxicity of other metabolites has not
apparently been examined.
Vamidothion depresses serum cholinesterase activity at lower
concentrations than it depresses the activity of erythrocyte
cholinesterase, except in dogs. Brain cholinesterase activity is less
affected. The dosage level of vamidothion which is without effect in
man is just over 50 µg/kg/day. The no-effect level with regard to
cholinesterase depression was 1 ppm in the diet of rats and 5 ppm in
the diet of dogs. These dietary levels are approximately equivalent to
50 µg/kg/day in rats and 125 µg/kg/day in dogs.
Studies showed that vamidothion had no adverse effects on
reproduction. Short-term tests were carried out in only small numbers
of dogs and rats and although no ill effects other than that on
cholinesterase activity were detected, the observations were not
sufficiently extensive to eliminate the possibility that the compound
has other significant effects, No long-term tests have been reported.
TOXICOLOGICAL EVALUATION
It is not possible, on the information available, to estimate an
ADI for vamidothion.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Vamidothion is a systemic organophosphorus aphicide and miticide.
Its most important use is on apples and pears against the woolly apple
aphid. It is also used on other pome fruits, sugar beet and brussels
sprouts. and to a lesser extent on grapes, cereal crops, sugar cane
and hops.
Pre-harvest treatments
The recommended uses are as follows:
Orchard fruits. In France, Western Europe, Australia, New Zealand
and South Africa, a single treatment at 40-50 g a.i./100 l. For
low-volume application, a rate of 0.4-0.5 kg a.i./ha is used. In Great
Britain an application at 20 g/100 l before flowering, and one or two
treatments at 40 g/100 l after flowering are recommended for apple and
pear; a single application at 40 g/100 l after flowering for plum and
cherry.
Grapes. A single treatment at 40-50 g/100 l during active sap
movement. A second similar treatment if necessary.
Citrus fruits. 60-80 g/100 l.
Hops. In England, three applications at 700 g/ha, with a minimum
pre-harvest interval of four weeks. In France, Belgium and Germany,
one application at 50 g/100 l when the aphid first appears.
Rice. Three applications at 50 g/100 l (600 g/ha).
Cotton. 40-60 g/100 l high-volume application, repeated, if
necessary.
Post-harvest treatments
None known.
Other possible uses
Trials against aphids of maize and sorghum have been successful.
Aphids and red spider of strawberries, tomatoes and beans have been
successfully controlled. A pre-harvest interval of six weeks is
recommended.
Aphids have been controlled in trials in France, Brazil and Japan
by brushing tree trunks with vamidothion during active sap movement.
Residues resulting from supervised trials
Residue data are available from supervised trials in France,
Germany, Switzerland and the United Kingdom (Rhône-Poulenc, 1966,
1969; May and Baker Ltd. 1963), together with limited data on
application to sugar cane in Trinidad (May and Baker Ltd, 1973). These
data have been deposited with FAO and are summarized in Table 3.
Residues of vamidothion are converted to the sulfoxide in or on the
plant, and residues reported in the table refer to the sum of the
parent compound and the sulfoxide.
The combined residue is unusually persistent. The half-life on
apples and pears is between 35 and 45 days, and more limited results
on peaches, plums, grapes, cherries and straw berries indicate a
similar persistence. Half-life periods on cereals and vegetables are
generally between 6 and 20 days.
Data from supervised trials in Japan (Tomizawa, 1973) show very
low residues, usually below 0.02 ppm in a wide range of crops. As
these data apparently refer only to the parent compound, they are not
quoted in the Table.
Fate of residues
In plants. As mentioned above, the main biologically active product
is the sulfoxide, and this is the main terminal residue. The sulfone
is not found to any significant extent, but it has been detected as a
metabolite in citrus leaves kept at a low temperature. (Results quoted
by Tomizawa, 1973). The other main metabolites are demethyl
vamidothion, phosphoric acid and diethyl phosphate (Morikawa and
Saito, 1969).
In animals. In experiments with 32B-vamidothion (Morikawa and
Saito, 1969), 69% of the activity in the urine of dosed mice appeared
as phosphoric acid and its mono and dimethyl esters. The remaining 31%
was an unknown metabolite. The dimethyl compound was not detectable.
In the same series of experiments, rat liver homogenate metabolized
55% of the vamidothion with which it was incubated to the unknown
metabolite, 31% to phosphoric acid and 14% to diethyl phosphate.
Methods of residue analysis
Residues normally consist essentially of a mixture of vamidothion
and its sulfoxide. Three methods have been developed by the
Rhône-Poulenc Laboratories to determine this mixture after suitable
clean-up: gas chromatography after oxidation of both compounds to the
sulfone, colorimetric determination of total phosphorus after
mineralization, and bioassay (Rhône-Poulenc, 1972). The
gas-chromatographic method should be suitable for regulatory purposes.
The other two are non-specific but are suitable for determining
residues arising from supervised trials: most of the data in Table 3
were obtained by these methods, often by both of them. Identity can be
confirmed, and the parent compound and sulfoxide separately determined
semi-quantitatively if required, by TLC (Rhône-Poulenc, 1972).
Determination by gas chromatography (Desmoras et al., 1972)
The sample is blended with buffered methanol or acetone and the organic
solvent evaporated. The extract is washed with petroleum ether, in
which vamidothion and its sulfoxide are insoluble, and then extracted
continuously with dichloromethane. (Vamidothion is readily extracted,
but continuous extraction is needed for the sulfoxide.) An aliquot of
the solution is evaporated to dryness and oxidized with potassium
permanganate in aqueous acetone. The resulting sulfone is extracted
with dichloromethane and transferred to benzene for determination by
gas chromatography on DEGS (diethylene glycol succinate) stationary
phase with electron capture detection. Recoveries are within a range
of 80-110% and the limit of determination is about 0.05 ppm.
The method described is more sensitive than methods in which
thermionic (Ruzicka et al., 1967) or flame photometric (Mestres, 1973)
detection is used. Separate determination of vamidothion and the
sulfoxide by GLC without oxidation is unsatisfactory because of the
large difference between their retention times. The two compounds can
be differentiated if required by first extracting vamidothion by
shaking with dichloromethane, then obtaining the sulfoxide by
continuous extraction and oxidizing the residues in the two extracts
to the sulfone separately.
Total phosphorus determination. The residue is extracted, and
cleaned-up as in the GLC method and the extract is evaporated to
dryness. The residue is digested with nitric and sulfuric acids and
phosphorus is determined colorimetrically as molybdenum blue. The
limit of determination is about 0.1 ppm.
Biological determination. The solvent is evaporated from the
dichloromethane extract obtained as described above, and some of the
co-extractives are removed by precipitation from acetone solution at
-70°C. The acetone in the filtrate from this step is evaporated and
replaced with water, and residues of vamidothion plus sulfoxide are
determined by bio-assay with Daphnia pulex as the test organism. The
procedure has been described in detail by Desmoras (1963).
Thin-layer chromatography. Thin-layer chromatography, TLC, is useful
as a confirmatory test of identity and to separate the parent compound
from the sulfoxide. It is carried out on the cleaned-up
dichloromethone extract, using silica gel which has been activated by
heating at 120°C for at least one hour. Approximate Rf values of
vamidothion, its sulfoxide and its sulfone on silica gel GF254
(Merek), developed with various solvent systems, are listed in Table
4. Separated spots can be detected by esterase inhibition or by
spraying with iodoplatinate, palladium chloride or
nitrobenzylpyridine. The enzyme inhibition method is the most
sensitive, with detection limits of 30-50 ng for the three compounds.
TLC of the dichloromethane extract does not satisfactorily
indicate the ratio of vamidothion to sulfoxide in the original
residue, because some vamidothion is converted to sulfoxide during the
continuous extraction.
Appraisal
Vamidothion is an organophosphorus compound with pronounced
systemic activity, effective against aphids and mites not resistant to
organophosphorus compounds.
It is applied as water-miscible solution to pome fruits, sugar
beet, brussels sprouts and, to a lesser extent, cereal crops, grapes,
sugar cane and hops. By far the most important use is on apples and
pears against woolly apple aphid.
Vamidothion is particularly persistent. Numerous studies have
been carried out in France, England, Switzerland and Germany and these
indicate the half-life in pome fruit to be between 35 and 45 days. The
half-life on cereals and vegetables is generally between six and 20
days.
The main biologically active metabolite is the sulfoxide which
has a higher systemic insecticidal activity than the parent compound.
The sulfone is not found in plants to any significant extent.
Residues consisting of a mixture of the sulfoxide and the parent
compound, can be determined by bio-assay or estimation of total
phosphorus. The recommended method, which should be suitable :Per
regulatory purposes, is by gas chromatography with electron-capture
detection after oxidation to the sulfone. The limit of determination
is about 0.05 ppm. TLC can be used for confirmation of identity.
The limited data available on sugar cane indicated residues of
about 0.2 ppm soon after application, decreasing to below 0.05 ppm
before harvest. As the available information on wheat and sunflower
was restricted to the whole plant, it was not suitable for judging the
probable residue level in the grain and seed.
National tolerances
In Switzerland a tolerance of 0.6 ppm has been established for
residues arising from applications to fruit trees, except cherry, with
the end of May as the last date of application.
TABLE 3. VAMIDOTHION RESIDUES IN CROPS
Dosage rate, Residue (ppm vamidothion + sulfoxide) after interval (days)
Crop Country a.i. g/100 l
or kg/ha 0-2 5-11 13-18 20-30 34-39 41-49 52-69 72-95 >100
Apple France 50 g/100 l 3.0 2.5 2.0 1.6 1.3 1.1
50 g/100 l 0.1
2 x 50 g/100 l 1.7
2 x 50 g/100 l 0.3 0.3
Germany 60 g/100 l 0.7 < 0.1 0.5 0.5 0.4
1.0 kg/ha 1.1 0.6 0.3
1.8 kg 0.75 0.2
2 x 1.8 kg/ha 0.7 0.2
Switzerland 50 g/100 l 0.85 < 0.65 0.8
50 g/100 l 0.5
England 40+80 g/100 l 3.5*
1.6*
1.7*
2.4*
40+80 g/100 l 3.4
Pear France 50 g/100 l 0.1
2 x 50 g/100 l 0.2 0.2
England 40 g/100 l 1.5* 0.6*
1.9* >0.2*
40+80 g/100 l 2.1*
1.8*
Peach France 50 g/100 l 0.1
2 x 50 g/100 l 0.4 0.3
Cherry England 80 g/100 l >0.4*
>0.4*
>0.4*
Plum England 80 g/100 l 1.9
TABLE 3. (Cont'd.)
Dosage rate, Residue (ppm vamidothion + sulfoxide) after interval (days)
Crop Country a.i. g/100 l
or kg/ha 0-2 5-11 13-18 20-30 34-39 41-49 52-69 72-95 >100
Grape France 50 g/100 l 0.2
2 x 50 g/100 l 0.25 0.15
Hop France 3 x 50 g/100 l <0.1
" (fresh and dry cones) 3 x 100 g/100 l <0.1
" (fresh cones) England 25+40 g/100 l < 0.5
Wheat (whole plant) France 50 g/100 l 9 4.1 1.4 0.4 0.4
50 g/100 l approx. 10 4.2 1.9
" (grain) 50 g/100 l 0.25
50 g/100 l 0.1
Sugar beat (leaves) France 50 g/100 l 1.1*
0.2*
" " (root) 50 g/100 l 0.2
Sunflower (leaves) France 75 g/100 l 60 23 19 10 7 5
Strawberries Scotland 50 g/100 l 0.6*
0.6*
0.6*
50 g/100 l 0.3
Broad beans France 50 g/100 l 81 64 51 40 23 13
(glasshouse,leaves)
French beans France 50 g/100 l 87 35 21 8.5
(glasshouse, leaves)
Sugar cane Trinidad 0.9 kg/ha 0.2 0.1
0.9 kg/ha 0.2 < 0.05
" " (juice) 0.9 kg/ha < 0.05
* Separate crop treatments.
TABLE 4. Rf VALUES OF VAMIDOTHION, ITS SULFOXIDE AND ITS SULFONE
ON SILICA GEL* IN SEVERAL SOLVENT SYSTEMS
Approximate Rf
Developing solvent
Vamidothion Sulfoxide Sulfone
Dichloromethane-methanol (90:10, v/v) 0.65 0.4 0.55
Ethyl acetate-methanol (75:25, v/v) 0.65 0.5 0.65
Acetonitrile-methanol (97:3 v/v) 0.5 0.1 0.65
Acetone-dimethylformanide (99:1, v/v) 0.6 0.35 0.65
* Silica gel GF254 (Merck) activated at 120°C for one hour before use.
RECOMMENDATION
As no acceptable daily intake could be established no tolerances
are recommended. Following officially acceptable use in various
countries residues of vamidothion can occur in the following
commodities up to the levels indicated. The guide-lines indicated
below are unlikely to be exceeded as a result of the recommended use
of vamidothion.
Guide-line levels (based on a pre-harvest interval of six weeks)
Apples and pears 2 ppm
Brussels sprouts 1 ppm
Sugar beet 0.5 ppm
Grapes 0.5 ppm
Hops 0.2 ppm
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake can be established)
1. Long-term studies in at least one animal species.
2. Adequate short-term studies in several species including a
non-rodent species.
3. Studies to identify metabolites and investigate their toxicity.
4. Studies on the nature and level of residues in animal products
from the feeding of residues at levels occurring on food wastes.
5. Information showing the fate of residues in the major crops in
countries with different meteorological and growth conditions.
REFERENCES
Anon. (1966a) Vamidothion. Etude dos Antidotes. Submitted by Société
des Usine Chimiques, Rhône-Poulenc. Unpublished report
Anon. (1966b) Vamidothion. Essai do neurotoxicité, submitted by
Société des Usines Chimiques, Rhône-Poulenc. Unpublished report
Anon. (1966e) Vamidothion. Etude de la potentialisation sur souris
avec des insecticides comerciaux, submitted by Société dos Usines
Chimiques, Rhône-Poulene. Unpublished report
Celice, J., Fournel, J. and Koenig F. (1966) Evaluation des risques
l'intoxication par le vamidothion (10.465 R.P.) au cours de sa
fabrication et do son experimentation plein champ, submitted by
Société dos Usines Chimiques, Rhône-Poulene. Unpublished report
Desmoras, J. (1963) Biological determination of vamidothion in plants.
Mededel. Landbouwhogeschool Opzoekingasta, Staat Gent, 28: 731-742
Desmoras, R. and Fournel, J. (1961) Insecticides. Activités
insecticides ot toxicités des 12.151.R.P. et 12.164 R.P. isomeres
optiques du 10.465 R.P. Submitted by Société des Usines Chimiques,
Rbône-Poulenc. Unpublished report
Desmoras, R., Fournel, J. and Koenig F. (1961) Insecticides. Activités
insecticides et toxicités comparées des 10.465 R.P. et 11.905 R.P.
Submitted by Sociétés des Usines Chimiques, Rhône-Poulene. Unpublished
report
Desmoras, J., Laurent, M. and Buys, M. (1972) Unpublished report No.
15,893 submitted by Rhône-Poulene
Dubost, J.P., Fournel, J., Ganter, P., Julou, L. and Myon, M. (1960)
Produit No. 10.463 R.P. Toxicité ague, tolerance locale, activités
anticholinesterasique et antidotes, toxicité chronique chez le rat et
le chien. Submitted by Société dos Usines Chimiques, Rhône-Poulenc.
Unpublished report
Ganter, P., Julou, L., Pasquet, J., Delesque, M. and Durel, J. (1969a)
Vanidothion (10.465 R.P.) activités sur les fonctions de reproduction
du Rat (Essai sur 3 generations). Submitted by Société des Usines
Chimiques, Rhône-Poulenc. Unpublished report
Ganter, P., Julou, L., Pasquet, J. and Delesque, M. (1969b)
Vamidothion (11.905 R.P.) activité sur les fonctions de reproduction
du rat (Essai sur 3 generations). Submitted by Société des Usines
Chimiques, Rhône-Poulenc. Unpublished report
Johnston, J.P. and Rivett, K.F. (1966) Vamidothion. Acute toxicity to
mammals. Unpublished report of May & Baker, Ltd. submitted by Société
des Usines Chimique, Rhône-Poulenc
Julou, L., Ducrot, R., Gardens, M.C., Detaille, J. Fee, C., Garret, C.
and Pasquet, J. (1966) Etude pharmacologique du vamidothion (10.465
R.P.) comparativement au parathion (3.470 R.P.). Submitted by Société
des Usines Chimiques, Rhône-Poulenc. Unpublished report
Julou, L. and Pasquet, J. (1967) Vamidothion (10.465 R.P.), toxicité
aigue chez le chien par vole orals. Submitted by Société des Usines
Chimiques, Rhône-Poulenc. Unpublished report
May and Baker Ltd. (1963) Unpublished report
May and Baker Ltd. (1973) Unpublished report
Mestres, R. (1973) Personal communication
Morikawa, O. and Saito, T. (1966) Degradations of vamidothion and
dimethoate in plants, insects and mammals. Bochu Kagaku, 31: 130-135
Morikawa, O. and Saito, T. (1969) Degradation of vamidothion and
Dimethoate in plants, insects and mammals. Unpublished report of the
Laboratory of Applied Entomology, Nagoya University, Japan, submitted
by Société des Usines Chimique, Rhône-Poulenc
Noel, P.R.B., Coote, S. and Street, A.E. (1969) Vamidothion (10.465
R.P.). Determination of no-effect level in Beagle dogs. Unpublished
report of Huntingdon Research Centre submitted by Société des Usines
Chimique, Rhône-Poulenc
Noel, P.R.B., Haynes, G. and Street, A.E. (1970) Vamidothion.
Determination of no-effect level in human volunteers. Unpublished
report of Huntingdon Research Centre submitted by Société des Usines
Chimique, Rhône-Poulenc
Pak, L.V. (1970) The toxicity of vamidothion on warm-blooded animals.
Gig. truda i Praftabol, 2: 58-9
Pasquet, J. and Mazuret, A. (1972a) Vamidothion (10.465 R.P.) - Kilval
ou formule S.A.E. 1700 (solution A 400 9/litro) Submitted by Société
des Usines Chimiques, Rhône-Poulenc. Unpublished report
Pasquet, J. and Mazuret, A. (1972b) Vamidothion (10.465 R.P.) -
Toxicité aigue chez le rat par voie orals, Submitted by Société des
Usines Chimiques, Rhône-Poulenc. Unpublished report
Rivett, K.F. and Corbett, K. (1966) Insecticides Vamidothion. The
acute and chronic oral toxicity of vanidothion and its sulphoxide.
Unpublished report of May and Baker Ltd, submitted by Société des
Usines Chimiques, Rhône-Poulenc
Rhône-Poulenc. (1966) Méthodes de dosage et évolution des résidues.
Report R.P. -D.S.Ph./D.S. An. Nord No. 11: 082
Rhône-Poulenc. (1969) Dosage de résidus dans les végétaux. Résumé des
résultats. Report R.P.-D.S.Ph. No. 14, 158. Unpublished. [Results
obtained during the period 1961-1968 are summarized]
Rhône-Poulene. (1972) Report No. R.P.-D.S.Ph./D.S. An. Nord No. 17,
002. Unpublished
Ruzicka, J., Thomson, J. and Wheals, B.B. (1967) The gas
chromatographic examination of organophosphorus pesticides and their
oxidation products. J. Chromatogr., 30: 92-99
Tomizawa, C. (1973) Personal communication
Wheldon, G.H., Odey, R.J. and Street, A.E. (1969) Toxicity of
vamidothion to rats during dietary administration over six weeks.
Unpublished report of Huntingdon Research Centre submitted by Société
des Usines Chimiques, Rhône-Poulenc
Other information on identity and properties
Physical state: White crystalline solid (pure)
Amber waxy solid (technical)
Melting point: 40°C (pure)
Solubility: Water - about 4 g/ml
Benzene, toluene, methyl ethyl ketone, ethyl
acetate, acetonitrile, methylene chloride,
anisole, cyclohexanone, chloroform - 1 g/ml
[xylene - 0.125 g/ml petroleum ether, cyclohexane
- insoluble
Volatility: Very low. Negligible loss under vacuum (2 mm Hg)
at 20°C.
Stability: The technical solid decomposes slowly at room
temperature but is stable in organic solvents
(e.g. cyclohexanone, methyl ethyl ketone).
Hydrolyzed by alkali
Optical Vamidothion consists of a mixture of optically
isomerism: active isomers. Their systemic activity as
pesticides is similar, but the D form shows higher
contact activity as an araricide.
Formulation: Water-miscible solution containing 400 g/l.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Biodegradation
One NC-5 mouse was administered 30 mg 32P vamidothion orally.
Urine collected over the following 24 hours contained 32P labelled
phosphoric acid, 0-methyl phosphate, 0,0-dimethyl phosphate and an
unknown compound.
Rat liver homogenate incubated for two hours with 32P labelled
vamidothion produced 0,0-dimethyl phosphate, phosphoric acid and an
unknown compound which was possibly dimethyl phosphothionate.
The same metabolites were produced by incubation of 32P labelled
vamidothion with plant leaves. In addition desmethyl vamidothion was
detected; this may not have been detected as a metabolite in animals
because of the low activity of 32p incorporated into the vamidothion
(Morikawa and Saito, 1969).
The oxidation product, vamidothion sulfoxide, has been
demonstrated to be formed in plants (Desmoras et al., 1961).
Effects on enzymes
Vamidothion inhibits cholinesterase in vitro and in vivo. A
concentration of 40 mg/l caused 50% inhibition of enzyme activity of
plasma. Four hours after guinea-pigs had received 40 mg vamidothion/kg
orally, 81% inhibition of plasma and 20% inhibition of cellular enzyme
occurred. With higher dosage levels the plasma cholinesterase level
remained stationary but cellular enzyme activity decreased (Dubost et
al., 1960).
TOXICOLOGICAL STUDIES
Special studies on the metabolites
A summary of the results of acute toxicity studies on
vamidothion sulfoxide is shown in Table 1.
A test for neurotoxicity was carried out in the same manner as
for vamidothion itself. Positive controls showed signs of paralysis in
9-14 days while vamidothion sulfoxide was without effect (Anon.,
1966b).
TABLE 1. THE ACUTE TOXICITY OF VAMIDOTHION SULFOXIDE
Species Sex Route LD50 References
Mouse Oral 80 Desmoras et al., 1961
Rat F Oral 160 Desmoras et al., 1961
Rivett and Corbett, 1966
Guinea-pig Oral 205 Desmoras et al., 1961
Chicken F s.c. 60 Anon., 1966b
A short-term study was carried out on groups of five male and
five female rats which were fed diets containing 0, 5, 50, 100 and 200
ppm vamidothion sulfoxide for three months. The degree of depression
of cholinesterase activity was similar to that in animals receiving
the same dosage levels of vamidothion. The 100 and 200 ppm dosage
levels depressed cholinesterase levels to approximately 20% And 12%
respectively of the control level. Cholinesterase activity returned to
normal within four weeks when vamidothion sulfoxide was withdrawn from
the diet. Histological examination of two male and two female rats
from each group showed no abnormality attributable to ingestion of the
test compound (Rivett and Corbett, 1966).
A three generation reproduction study with each generation
producing two litters, was carried out on groups of 20 male and 40
female rats (generation Fo, test animals), 40 male and 80 female
(generation Fo controls) or 10 male and 30 female (other generations).
Animals were administered 0, 5, 15 or 45 ppm of vamidothion sulfoxide
in the diet for four weeks before the first mating. The study included
tests in which some female test animals were mated with untreated
males and some untreated females were mated with treated male animals.
A small number of females of the F1b and F2b generations were killed
after the thirteenth day of pregnancy and the uterus examined for
implantation sites, viable and resorbed embryos and macroscopically
observable abnormalities. The F1a, F2a and F3a litters were killed
at weaning and autopsied. Rats of the F1b and F2b litters not used to
produce the next generation were killed at weaning and autopsied. The
F3b litters were killed at weaning and the heart, kidneys and livers
weighed and spleen, suprarenals, thyroid and (in the case of the
highest dosage group) brain examined histologically. The results
showed that the dosage levels of vamidothion sulfoxide used had no
untoward effect on treated animals, in particular on fertility or
reproductive activity of rats. There was no indication of teratogenic
activity in this study (Ganter et al., 1969b).
Special studies on neurotoxicity
Groups of five white leghorn chickens were injected s.c. with 60
and 120 mg vamidothion/kg (1X and 2X LD50 dose). Positive control
groups received 20 and 40 mg di-isopropyl-fluorophosphoridono. All
birds were treated with atropine and P2AM. No signs of neurotoxicity
appeared in vamidothiontreated birds while positive control groups
developed paralysis in 9-14 days (Anon., 1966b).
Special studies on the pharmacological effects
The motility of mice was unaffected by 10 mg vamidothion/kg
orally and exploratory activity and conditioned reflexes were normal
in rats treated orally with 30 mg/kg. No effect was found on
neuromuscular transmission, respiratory rate, ECG or heart rate and
the effects of i.v. adrenalin and i.v. acetylcholine were largely
unchanged by treatment with vamidothion, Salivary excretion was
increased, slightly and isolated intestine and uterus preparations
were affected weakly by vamidothion (Julou et al., 1966).
Mice were administered 100 mg vamidothion/kg orally (LD50 dose).
Some also received atropine, pralidoxime or both. Ten to 20 mg
atropine/kg i.p. provided 60-70% and 50 mg pralidoxime/kg i.p. 100%
protection against vamidothion (Dubost et al., 1960; Anon., 1966a).
Groups of 10 rats received 3 x LD50 dose (300mg/kg) of
vamidothion orally. One group received 17.4 mg atropine/kg i.p. when
signs of intoxication showed. Another received atropine plus 50 mg
P2AM/kg i.p. All control animals died within 45 min., 7-10 animals
with atropine died in 7-24 hours and three animals receiving atropine
and P2AM died. All of the latter group appeared normal two hours after
treatment while those on atropine alone appeared ill (Anon., 1966a).
Special studies on reproduction
Rat. A three-generation reproduction study, with each generation
producing two litters, was carried out on groups of 20 male and 40
female rats which were administered 5, 15 and 45 ppm vamidothion in
the diet. The control group consisted of 40 males and 80 females.
Animals of the Fo generation received diets for 11 weeks before being
mated. The study included tests in which some female test animals were
mated with untreated males and untreated females were mated with
treated males. A small number of females of the F1b and F2b
generations were killed after the thirteenth day of pregnancy and the
uterus examined for implantation sites, viable and resorbed embryos
and macroscopically apparent abnormalities. The F1a, F2a and F3a,
litters were killed at weaning and autopsied. Animals of the F1b and
F2b groups not used to produce the next generation were killed at
weaning and autopsied. The F3b litters were killed at weaning and the
heart, kidneys and liver weighed and the spleen, suprarenals, thyroid
and (with the highest dose level only) the brain examined
histologically. Haematological studies and examinations of bone marrow
of one animal of each sex of each group were carried out. Because of a
technical error some animals chosen as parents of the second and third
generations may have been produced from untreated male parents; the
proportion so produced is uncertain. All females were, however,
treated with the correct diets. Results show that at the dosage levels
used vamidothion had no untoward activity on rats, in particular on
their fertility or reproductive functions, There was no indication
that vamidothion is teratogenic (Ganter et al., 1969a).
Acute toxicity
The results of acute toxicity studies on vamidothion are
summarized in Table 2.
Administration of vamidothion in fatal doses produced signs
typical of cholinesterase inhibitors.
Groups of 20 mice were administered half the LD50 dose of
vamidothion plus half the LD50 dose of demeton-methyl, parathion,
phencapton, dimetboate, ethion, malathion, azinphos ethyl, mevinphos
or phosphamidon and observed for five days. No significant
potentiation was observed (Anon., 1966c).
Short-term studies
Rat. Groups of 10 male rats were administered 3 or 6 mg
vamidothion/kg/day orally for one month. It was reported that body
weight was unaffected, no animals died and no clinical signs of
toxicity occurred. Haematological examination including examination of
bone marrow and chemical analysis of urine and blood showed no
abnormalities except for blood cholinesterase activity which was
depressed by 50% (3 mg/kg/day) or 607 (6 mg/kg/day). Brain
cholinesterase levels were not depressed. The weights and histological
appearance of organs were unaffected by treatment (Dubost et al.,
1960).
TABLE 2. SUMMARY OF THE RESULTS OF STUDIES ON ACUTE TOXICITY
Species Sex Route Purity LD50 References
Mouse Oral p 43 Johnston and Rivett, 1966
Mouse Oral p 34 Dubost et al., 1960
Mouse M+F Oral Tech. 64 Pasquet and Mazuret, 1972a
Mouse Oral Tech. 40 Pak, 1970
Mouse s.c. p 34 Johnston and Rivett, 1966
Mouse Dermal p 1450 Johnston and Rivett, 1966
Mouse Dermal Tech. 1060 Pasquet and Mazuret, 1972a
Rat M Oral p 100 Johnston and Rivett, 1966
Rat M Oral p 105 Dubost et al., 1960
Rat M+F Oral p 105 Pasquet and Ma.uret, 1972b
Rat F Oral p 77 Desmoras et al., 1961
Rat M+F Oral Tech. 168 Pasquet and Mazuret, 1972a
Rat Oral Tech. 103 Pak, 1970
Rat F Oral p 64 Johnston and Rivett, 1H6
Rat M s.c. p 48 Johnston and Rivett, 1966
Rat F s.c. p 35 Johnston and Rivett, 1966
Guinea-pig Oral p 85 Dubost et al., 1960
Rabbit Oral Tech. 160 Pak, 1970
Rabbit Dermal p 1160 Quoted in Johnston and
Rivett, 1966
Rabbit M+F Dermal Tech. 3000 Pasquet and Mazuret, 1972a
Dog M+F Oral p 110 Julou and Pasquet, 1967
Mouse Oral p 50 Desmoras and Fournel, 1961
Mouse Oral l-isomer 68 Desmoras and Fournel, 1961
Mouse Oral d-isomer 34 Desmoras and Fournel, 1961
M = male F = female P = pure
Groups of five male and five female rats were fed on diets
containing 0, 0.2, 1 and 5 ppm vamidothion for six weeks after which
the test substance was withdrawn from the diet. Regular observation of
plasma and erythrocyte cholinesterase activity showed that only in the
plasma of female rats on the 5 ppm diet was enzyme activity
consistently depressed to a significant extent. The enzyme level
returned to normal within five weeks. Cholinesterase activity was not
depressed significantly at lower dosage levels (Wheldon et al., 1969).
Groups of five male and five female rats were fed on diets
containing 0, 5 and 50 ppm vamidothion for three months. No ill effect
was seen on growth or general health. Blood cholinesterase levels were
reduced to approximately 75% and 25% respectively of the control level
in the 5 and 50 ppm groups. Cholinesterase levels returned to normal
within four weeks when vamidothion was withdrawn from the diet.
Histopathological examination of two rats of each sex from the 50 ppm
group showed no abnormalities related to ingestion of the compound
(Rivett and Corbett, 1966).
Groups of rats were administered, by gavage, doses of
approximately 2, 5 or 10 mg vamidothion/kg daily for three months. At
the end of the test serum acetylcholinesterase activity was decreased
to 35%, 12% and 8%, respectively, of the normal value (Pak, 1970).
Dog. Groups of three dogs were administered orally 0, 1 and 2
mg/vamidothion/kg/day for one month. Two dogs received 8 mg/kg/day for
one month. No effect was seen on growth except for one animal on the
highest dose level which developed diarrhoea (cause unknown).
Haematological indices and blood coagulation were normal. Slight
differences between groups in urine urobilinogen, glucose and bile
salts, serum protein, PSP clearance and behaviour could not be
attributed to treatment. In particular no neurological abnormalities
were seen although erythrocyte cholinesterase was severely depressed
(100% inhibition at sixteenth day with 8 mg/kg dosage regime). In
six dogs receiving 1 and 2 mg/kg/day no abnormalities were found on
gross and histopathologic examination. (Dubost et al., 1960).
Groups of two male and two female beagle dogs were fed on diets
containing 0, 0.2, 1 and 5 ppm vamidothion for five to six weeks.
Animals on the lowest dosage level were then fed for a further four
weeks on diet containing 20 ppm vamidothion, after which they were
observed for four more weeks on normal diet. No clinical signs of
abnormality were seen in any group. The plasma and erythrocyte
cholinesterase levels were slightly depressed in dogs on the highest
dosage. These levels returned to normal within two weeks of return to
a normal diet. No abnormalities in the appearance or weight of organs
were found in any group (Noel et al., 1969).
Long-term studies
No data are available.
Observations in man
Groups of 6-11 normal healthy volunteers of both sexes were
administered 9.6 or 37.2 µg vamidothion/kg/day orally in aqueous
solution on five days each week for three weeks. Other groups received
78.8 or 122.8 µg vamidothion/kg/day in aqueous solution for five
weeks. A control group was studied for 25 weeks. No clinical signs or
symptoms were found which could be attributed to treatment. Plasma
cholinesterase, estimated weekly, showed no consistent depression in
any group but erythrocyte cholinesterase was depressed in three of six
volunteers receiving 122.8 µg/kg/day. The no-effect level was
considered to be 78.8 µg/kg/day, (calculated to be equivalent to 56.3
µg/kg/day if vamidothion was administered every day without a break)
(Noel et al., 1970).
Serum and erythrocyte cholinesterase levels were determined
periodically in workers who had been involved in the manufacture of
vamidothion over a period of several months to a few years. Enzyme
levels were also determined in experimentalists exposed several times
a year to vamidothion over a seven-year period. The actual exposure is
uncertain. Fluctuations in enzyme levels in both groups were within
the limits of normal (Celice et al., 1966),
Comments
It has been shown that vamidothion is partly absorbed from the
gastric intestinal tract and is excreted in urine. Several metabolic
products have been found in urine; these ewe compounds are also
produced by liver slices. One metabolite (desmethyl vamidothion) was
shown to be produced by plants but has not yet been found as a
metabolite in animals. Vamidothion sulfoxide has been examined
toxicologically but the toxicity of other metabolites has not
apparently been examined.
Vamidothion depresses serum cholinesterase activity at lower
concentrations than it depresses the activity of erythrocyte
cholinesterase, except in dogs. Brain cholinesterase activity is less
affected. The dosage level of vamidothion which is without effect in
man is just over 50 µg/kg/day. The no-effect level with regard to
cholinesterase depression was 1 ppm in the diet of rats and 5 ppm in
the diet of dogs. These dietary levels are approximately equivalent to
50 µg/kg/day in rats and 125 µg/kg/day in dogs.
Studies showed that vamidothion had no adverse effects on
reproduction. Short-term tests were carried out in only small numbers
of dogs and rats and although no ill effects other than that on
cholinesterase activity were detected, the observations were not
sufficiently extensive to eliminate the possibility that the compound
has other significant effects, No long-term tests have been reported.
TOXICOLOGICAL EVALUATION
It is not possible, on the information available, to estimate an
ADI for vamidothion.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Vamidothion is a systemic organophosphorus aphicide and miticide.
Its most important use is on apples and pears against the woolly apple
aphid. It is also used on other pome fruits, sugar beet and brussels
sprouts. and to a lesser extent on grapes, cereal crops, sugar cane
and hops.
Pre-harvest treatments
The recommended uses are as follows:
Orchard fruits. In France, Western Europe, Australia, New Zealand
and South Africa, a single treatment at 40-50 g a.i./100 l. For
low-volume application, a rate of 0.4-0.5 kg a.i./ha is used. In Great
Britain an application at 20 g/100 l before flowering, and one or two
treatments at 40 g/100 l after flowering are recommended for apple and
pear; a single application at 40 g/100 l after flowering for plum and
cherry.
Grapes. A single treatment at 40-50 g/100 l during active sap
movement. A second similar treatment if necessary.
Citrus fruits. 60-80 g/100 l.
Hops. In England, three applications at 700 g/ha, with a minimum
pre-harvest interval of four weeks. In France, Belgium and Germany,
one application at 50 g/100 l when the aphid first appears.
Rice. Three applications at 50 g/100 l (600 g/ha).
Cotton. 40-60 g/100 l high-volume application, repeated, if
necessary.
Post-harvest treatments
None known.
Other possible uses
Trials against aphids of maize and sorghum have been successful.
Aphids and red spider of strawberries, tomatoes and beans have been
successfully controlled. A pre-harvest interval of six weeks is
recommended.
Aphids have been controlled in trials in France, Brazil and Japan
by brushing tree trunks with vamidothion during active sap movement.
Residues resulting from supervised trials
Residue data are available from supervised trials in France,
Germany, Switzerland and the United Kingdom (Rhône-Poulenc, 1966,
1969; May and Baker Ltd. 1963), together with limited data on
application to sugar cane in Trinidad (May and Baker Ltd, 1973). These
data have been deposited with FAO and are summarized in Table 3.
Residues of vamidothion are converted to the sulfoxide in or on the
plant, and residues reported in the table refer to the sum of the
parent compound and the sulfoxide.
The combined residue is unusually persistent. The half-life on
apples and pears is between 35 and 45 days, and more limited results
on peaches, plums, grapes, cherries and straw berries indicate a
similar persistence. Half-life periods on cereals and vegetables are
generally between 6 and 20 days.
Data from supervised trials in Japan (Tomizawa, 1973) show very
low residues, usually below 0.02 ppm in a wide range of crops. As
these data apparently refer only to the parent compound, they are not
quoted in the Table.
Fate of residues
In plants. As mentioned above, the main biologically active product
is the sulfoxide, and this is the main terminal residue. The sulfone
is not found to any significant extent, but it has been detected as a
metabolite in citrus leaves kept at a low temperature. (Results quoted
by Tomizawa, 1973). The other main metabolites are demethyl
vamidothion, phosphoric acid and diethyl phosphate (Morikawa and
Saito, 1969).
In animals. In experiments with 32B-vamidothion (Morikawa and
Saito, 1969), 69% of the activity in the urine of dosed mice appeared
as phosphoric acid and its mono and dimethyl esters. The remaining 31%
was an unknown metabolite. The dimethyl compound was not detectable.
In the same series of experiments, rat liver homogenate metabolized
55% of the vamidothion with which it was incubated to the unknown
metabolite, 31% to phosphoric acid and 14% to diethyl phosphate.
Methods of residue analysis
Residues normally consist essentially of a mixture of vamidothion
and its sulfoxide. Three methods have been developed by the
Rhône-Poulenc Laboratories to determine this mixture after suitable
clean-up: gas chromatography after oxidation of both compounds to the
sulfone, colorimetric determination of total phosphorus after
mineralization, and bioassay (Rhône-Poulenc, 1972). The
gas-chromatographic method should be suitable for regulatory purposes.
The other two are non-specific but are suitable for determining
residues arising from supervised trials: most of the data in Table 3
were obtained by these methods, often by both of them. Identity can be
confirmed, and the parent compound and sulfoxide separately determined
semi-quantitatively if required, by TLC (Rhône-Poulenc, 1972).
Determination by gas chromatography (Desmoras et al., 1972)
The sample is blended with buffered methanol or acetone and the organic
solvent evaporated. The extract is washed with petroleum ether, in
which vamidothion and its sulfoxide are insoluble, and then extracted
continuously with dichloromethane. (Vamidothion is readily extracted,
but continuous extraction is needed for the sulfoxide.) An aliquot of
the solution is evaporated to dryness and oxidized with potassium
permanganate in aqueous acetone. The resulting sulfone is extracted
with dichloromethane and transferred to benzene for determination by
gas chromatography on DEGS (diethylene glycol succinate) stationary
phase with electron capture detection. Recoveries are within a range
of 80-110% and the limit of determination is about 0.05 ppm.
The method described is more sensitive than methods in which
thermionic (Ruzicka et al., 1967) or flame photometric (Mestres, 1973)
detection is used. Separate determination of vamidothion and the
sulfoxide by GLC without oxidation is unsatisfactory because of the
large difference between their retention times. The two compounds can
be differentiated if required by first extracting vamidothion by
shaking with dichloromethane, then obtaining the sulfoxide by
continuous extraction and oxidizing the residues in the two extracts
to the sulfone separately.
Total phosphorus determination. The residue is extracted, and
cleaned-up as in the GLC method and the extract is evaporated to
dryness. The residue is digested with nitric and sulfuric acids and
phosphorus is determined colorimetrically as molybdenum blue. The
limit of determination is about 0.1 ppm.
Biological determination. The solvent is evaporated from the
dichloromethane extract obtained as described above, and some of the
co-extractives are removed by precipitation from acetone solution at
-70°C. The acetone in the filtrate from this step is evaporated and
replaced with water, and residues of vamidothion plus sulfoxide are
determined by bio-assay with Daphnia pulex as the test organism. The
procedure has been described in detail by Desmoras (1963).
Thin-layer chromatography. Thin-layer chromatography, TLC, is useful
as a confirmatory test of identity and to separate the parent compound
from the sulfoxide. It is carried out on the cleaned-up
dichloromethone extract, using silica gel which has been activated by
heating at 120°C for at least one hour. Approximate Rf values of
vamidothion, its sulfoxide and its sulfone on silica gel GF254
(Merek), developed with various solvent systems, are listed in Table
4. Separated spots can be detected by esterase inhibition or by
spraying with iodoplatinate, palladium chloride or
nitrobenzylpyridine. The enzyme inhibition method is the most
sensitive, with detection limits of 30-50 ng for the three compounds.
TLC of the dichloromethane extract does not satisfactorily
indicate the ratio of vamidothion to sulfoxide in the original
residue, because some vamidothion is converted to sulfoxide during the
continuous extraction.
Appraisal
Vamidothion is an organophosphorus compound with pronounced
systemic activity, effective against aphids and mites not resistant to
organophosphorus compounds.
It is applied as water-miscible solution to pome fruits, sugar
beet, brussels sprouts and, to a lesser extent, cereal crops, grapes,
sugar cane and hops. By far the most important use is on apples and
pears against woolly apple aphid.
Vamidothion is particularly persistent. Numerous studies have
been carried out in France, England, Switzerland and Germany and these
indicate the half-life in pome fruit to be between 35 and 45 days. The
half-life on cereals and vegetables is generally between six and 20
days.
The main biologically active metabolite is the sulfoxide which
has a higher systemic insecticidal activity than the parent compound.
The sulfone is not found in plants to any significant extent.
Residues consisting of a mixture of the sulfoxide and the parent
compound, can be determined by bio-assay or estimation of total
phosphorus. The recommended method, which should be suitable :Per
regulatory purposes, is by gas chromatography with electron-capture
detection after oxidation to the sulfone. The limit of determination
is about 0.05 ppm. TLC can be used for confirmation of identity.
The limited data available on sugar cane indicated residues of
about 0.2 ppm soon after application, decreasing to below 0.05 ppm
before harvest. As the available information on wheat and sunflower
was restricted to the whole plant, it was not suitable for judging the
probable residue level in the grain and seed.
National tolerances
In Switzerland a tolerance of 0.6 ppm has been established for
residues arising from applications to fruit trees, except cherry, with
the end of May as the last date of application.
TABLE 3. VAMIDOTHION RESIDUES IN CROPS
Dosage rate, Residue (ppm vamidothion + sulfoxide) after interval (days)
Crop Country a.i. g/100 l
or kg/ha 0-2 5-11 13-18 20-30 34-39 41-49 52-69 72-95 >100
Apple France 50 g/100 l 3.0 2.5 2.0 1.6 1.3 1.1
50 g/100 l 0.1
2 x 50 g/100 l 1.7
2 x 50 g/100 l 0.3 0.3
Germany 60 g/100 l 0.7 < 0.1 0.5 0.5 0.4
1.0 kg/ha 1.1 0.6 0.3
1.8 kg 0.75 0.2
2 x 1.8 kg/ha 0.7 0.2
Switzerland 50 g/100 l 0.85 < 0.65 0.8
50 g/100 l 0.5
England 40+80 g/100 l 3.5*
1.6*
1.7*
2.4*
40+80 g/100 l 3.4
Pear France 50 g/100 l 0.1
2 x 50 g/100 l 0.2 0.2
England 40 g/100 l 1.5* 0.6*
1.9* >0.2*
40+80 g/100 l 2.1*
1.8*
Peach France 50 g/100 l 0.1
2 x 50 g/100 l 0.4 0.3
Cherry England 80 g/100 l >0.4*
>0.4*
>0.4*
Plum England 80 g/100 l 1.9
TABLE 3. (Cont'd.)
Dosage rate, Residue (ppm vamidothion + sulfoxide) after interval (days)
Crop Country a.i. g/100 l
or kg/ha 0-2 5-11 13-18 20-30 34-39 41-49 52-69 72-95 >100
Grape France 50 g/100 l 0.2
2 x 50 g/100 l 0.25 0.15
Hop France 3 x 50 g/100 l <0.1
" (fresh and dry cones) 3 x 100 g/100 l <0.1
" (fresh cones) England 25+40 g/100 l < 0.5
Wheat (whole plant) France 50 g/100 l 9 4.1 1.4 0.4 0.4
50 g/100 l approx. 10 4.2 1.9
" (grain) 50 g/100 l 0.25
50 g/100 l 0.1
Sugar beat (leaves) France 50 g/100 l 1.1*
0.2*
" " (root) 50 g/100 l 0.2
Sunflower (leaves) France 75 g/100 l 60 23 19 10 7 5
Strawberries Scotland 50 g/100 l 0.6*
0.6*
0.6*
50 g/100 l 0.3
Broad beans France 50 g/100 l 81 64 51 40 23 13
(glasshouse,leaves)
French beans France 50 g/100 l 87 35 21 8.5
(glasshouse, leaves)
Sugar cane Trinidad 0.9 kg/ha 0.2 0.1
0.9 kg/ha 0.2 < 0.05
" " (juice) 0.9 kg/ha < 0.05
* Separate crop treatments.
TABLE 4. Rf VALUES OF VAMIDOTHION, ITS SULFOXIDE AND ITS SULFONE
ON SILICA GEL* IN SEVERAL SOLVENT SYSTEMS
Approximate Rf
Developing solvent
Vamidothion Sulfoxide Sulfone
Dichloromethane-methanol (90:10, v/v) 0.65 0.4 0.55
Ethyl acetate-methanol (75:25, v/v) 0.65 0.5 0.65
Acetonitrile-methanol (97:3 v/v) 0.5 0.1 0.65
Acetone-dimethylformanide (99:1, v/v) 0.6 0.35 0.65
* Silica gel GF254 (Merck) activated at 120°C for one hour before use.
RECOMMENDATION
As no acceptable daily intake could be established no tolerances
are recommended. Following officially acceptable use in various
countries residues of vamidothion can occur in the following
commodities up to the levels indicated. The guide-lines indicated
below are unlikely to be exceeded as a result of the recommended use
of vamidothion.
Guide-line levels (based on a pre-harvest interval of six weeks)
Apples and pears 2 ppm
Brussels sprouts 1 ppm
Sugar beet 0.5 ppm
Grapes 0.5 ppm
Hops 0.2 ppm
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake can be established)
1. Long-term studies in at least one animal species.
2. Adequate short-term studies in several species including a
non-rodent species.
3. Studies to identify metabolites and investigate their toxicity.
4. Studies on the nature and level of residues in animal products
from the feeding of residues at levels occurring on food wastes.
5. Information showing the fate of residues in the major crops in
countries with different meteorological and growth conditions.
REFERENCES
Anon. (1966a) Vamidothion. Etude dos Antidotes. Submitted by Société
des Usine Chimiques, Rhône-Poulenc. Unpublished report
Anon. (1966b) Vamidothion. Essai do neurotoxicité, submitted by
Société des Usines Chimiques, Rhône-Poulenc. Unpublished report
Anon. (1966e) Vamidothion. Etude de la potentialisation sur souris
avec des insecticides comerciaux, submitted by Société dos Usines
Chimiques, Rhône-Poulene. Unpublished report
Celice, J., Fournel, J. and Koenig F. (1966) Evaluation des risques
l'intoxication par le vamidothion (10.465 R.P.) au cours de sa
fabrication et do son experimentation plein champ, submitted by
Société dos Usines Chimiques, Rhône-Poulene. Unpublished report
Desmoras, J. (1963) Biological determination of vamidothion in plants.
Mededel. Landbouwhogeschool Opzoekingasta, Staat Gent, 28: 731-742
Desmoras, R. and Fournel, J. (1961) Insecticides. Activités
insecticides ot toxicités des 12.151.R.P. et 12.164 R.P. isomeres
optiques du 10.465 R.P. Submitted by Société des Usines Chimiques,
Rbône-Poulenc. Unpublished report
Desmoras, R., Fournel, J. and Koenig F. (1961) Insecticides. Activités
insecticides et toxicités comparées des 10.465 R.P. et 11.905 R.P.
Submitted by Sociétés des Usines Chimiques, Rhône-Poulene. Unpublished
report
Desmoras, J., Laurent, M. and Buys, M. (1972) Unpublished report No.
15,893 submitted by Rhône-Poulene
Dubost, J.P., Fournel, J., Ganter, P., Julou, L. and Myon, M. (1960)
Produit No. 10.463 R.P. Toxicité ague, tolerance locale, activités
anticholinesterasique et antidotes, toxicité chronique chez le rat et
le chien. Submitted by Société dos Usines Chimiques, Rhône-Poulenc.
Unpublished report
Ganter, P., Julou, L., Pasquet, J., Delesque, M. and Durel, J. (1969a)
Vanidothion (10.465 R.P.) activités sur les fonctions de reproduction
du Rat (Essai sur 3 generations). Submitted by Société des Usines
Chimiques, Rhône-Poulenc. Unpublished report
Ganter, P., Julou, L., Pasquet, J. and Delesque, M. (1969b)
Vamidothion (11.905 R.P.) activité sur les fonctions de reproduction
du rat (Essai sur 3 generations). Submitted by Société des Usines
Chimiques, Rhône-Poulenc. Unpublished report
Johnston, J.P. and Rivett, K.F. (1966) Vamidothion. Acute toxicity to
mammals. Unpublished report of May & Baker, Ltd. submitted by Société
des Usines Chimique, Rhône-Poulenc
Julou, L., Ducrot, R., Gardens, M.C., Detaille, J. Fee, C., Garret, C.
and Pasquet, J. (1966) Etude pharmacologique du vamidothion (10.465
R.P.) comparativement au parathion (3.470 R.P.). Submitted by Société
des Usines Chimiques, Rhône-Poulenc. Unpublished report
Julou, L. and Pasquet, J. (1967) Vamidothion (10.465 R.P.), toxicité
aigue chez le chien par vole orals. Submitted by Société des Usines
Chimiques, Rhône-Poulenc. Unpublished report
May and Baker Ltd. (1963) Unpublished report
May and Baker Ltd. (1973) Unpublished report
Mestres, R. (1973) Personal communication
Morikawa, O. and Saito, T. (1966) Degradations of vamidothion and
dimethoate in plants, insects and mammals. Bochu Kagaku, 31: 130-135
Morikawa, O. and Saito, T. (1969) Degradation of vamidothion and
Dimethoate in plants, insects and mammals. Unpublished report of the
Laboratory of Applied Entomology, Nagoya University, Japan, submitted
by Société des Usines Chimique, Rhône-Poulenc
Noel, P.R.B., Coote, S. and Street, A.E. (1969) Vamidothion (10.465
R.P.). Determination of no-effect level in Beagle dogs. Unpublished
report of Huntingdon Research Centre submitted by Société des Usines
Chimique, Rhône-Poulenc
Noel, P.R.B., Haynes, G. and Street, A.E. (1970) Vamidothion.
Determination of no-effect level in human volunteers. Unpublished
report of Huntingdon Research Centre submitted by Société des Usines
Chimique, Rhône-Poulenc
Pak, L.V. (1970) The toxicity of vamidothion on warm-blooded animals.
Gig. truda i Praftabol, 2: 58-9
Pasquet, J. and Mazuret, A. (1972a) Vamidothion (10.465 R.P.) - Kilval
ou formule S.A.E. 1700 (solution A 400 9/litro) Submitted by Société
des Usines Chimiques, Rhône-Poulenc. Unpublished report
Pasquet, J. and Mazuret, A. (1972b) Vamidothion (10.465 R.P.) -
Toxicité aigue chez le rat par voie orals, Submitted by Société des
Usines Chimiques, Rhône-Poulenc. Unpublished report
Rivett, K.F. and Corbett, K. (1966) Insecticides Vamidothion. The
acute and chronic oral toxicity of vanidothion and its sulphoxide.
Unpublished report of May and Baker Ltd, submitted by Société des
Usines Chimiques, Rhône-Poulenc
Rhône-Poulenc. (1966) Méthodes de dosage et évolution des résidues.
Report R.P. -D.S.Ph./D.S. An. Nord No. 11: 082
Rhône-Poulenc. (1969) Dosage de résidus dans les végétaux. Résumé des
résultats. Report R.P.-D.S.Ph. No. 14, 158. Unpublished. [Results
obtained during the period 1961-1968 are summarized]
Rhône-Poulene. (1972) Report No. R.P.-D.S.Ph./D.S. An. Nord No. 17,
002. Unpublished
Ruzicka, J., Thomson, J. and Wheals, B.B. (1967) The gas
chromatographic examination of organophosphorus pesticides and their
oxidation products. J. Chromatogr., 30: 92-99
Tomizawa, C. (1973) Personal communication
Wheldon, G.H., Odey, R.J. and Street, A.E. (1969) Toxicity of
vamidothion to rats during dietary administration over six weeks.
Unpublished report of Huntingdon Research Centre submitted by Société
des Usines Chimiques, Rhône-Poulenc
