
PESTICIDE RESIDUES IN FOOD - 1980
Sponsored jointly by FAO and WHO
EVALUATIONS 1980
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Expert Group on Pesticide Residues
Rome, 6-15 October 1980
ETRIMFOS
IDENTITY
Chemical name
O-(6-ethoxy-2-ethyl-4-pyrimidinyl)O,O-dimethyl phosphorothioate
(C.A.)
O-6-ethoxy-2-ethyl-pyrimidin-4-yl O,O-dimethyl phosphorothioate
(IUPAC)
Synonyms
SAN 197 I, SATISFAR(R), EKAMET
Structural formula
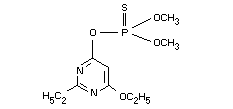 Molecular formula C10H17N2O4PS
Molecular weight 292.3
Specific gravity 1.195 at 20°C
Appearance colourless oil
Odour slight smell, characteristic of
thionophosphoric acid derivatives
Melting Point -3.35°C (pure a.i.)
Vapour pressure 6.5 to 8.7 × 10-2 mbar at 20°C
Refractive index nD20 1.5068
Solubility in water (24°C) 40 mg/l completely miscible
with acetone, ethanol, diethyl ether, chloroform,
dimethylsulphoxide, ethyl-acetate, hexane,
kerosene.
Stability Half-life times in aqueous buffer solution at
25°C and pH 3, 6 and 9 are 0.4, 16 and 14 days
respectively. 1 to 10 mg etrimfos/l acetone,
chloroform, hexane or toluene in the dark at
room temp. were found to be stable for at least
28 days, in methanol and ethylacetate 3% and 12%
degradation were noticed.
Minimum degree of
purity 93%
Impurities in the
technical material Information on the impurities in technical
etrimfos was reported to the meeting.
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, excretion and biotransformation
Female rats were given a single oral dose of 50 mg/kg bw SAN I 197
(14C-labelled at ring-positions 4 and 6) dissolved in 80% aqueous
polyethylene-glycol.
Excretion of radioactivity occurred mainly via the urine, 84%
within 96 hours, of which 68% was within 12 hours; 6.6% was found
in the faeces within 24 hours. One hour after administration
continuous excretion of radioactivity was observed in the bile and
amounted to 6% within 24 hours. Only 0.012% was exhaled within 24
hours, of which 0.011% was within the first 8 hours, with a
peak-value around 1 hour after administration. For most tissues
the peak concentrations were reached at 4 hours after
administration with the exceptions of the liver (2 hours), blood (3
hours) and fat (8 hours). At peak time the fat and kidneys had the
highest concentrations (42 and 62 mg/kg respectively) whereas the
value in the other tissues ranged from 6 to 22 mg/kg with 13 mg/kg
in the blood. The concentrations declined rapidly to values of
0.01 mg/kg at 96 hours except in the fat and skin with 0.3 mg/kg.
In the urine and faeces collected for 96 hours neither etrimfos (I)
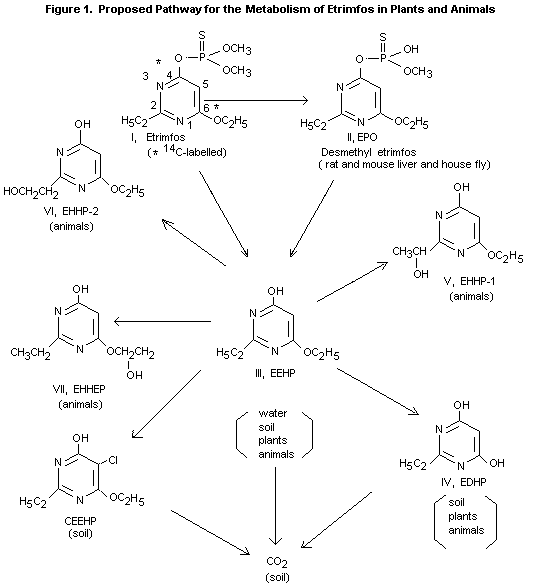
nor its oxygen analogue was detected. The main metabolite in both
excreta was 6-ethoxy-2-ethyl-4-hydroxypyrimidine (III) in amounts
of (% of the dose) 48% in the urine and 3% in the faeces. However
part of this metabolite is possibly formed from I and
desmethyletrimfos (II) during the procedures for analysis (see
below). Other metabolites found in the urine and faeces (see
figure 1) were the hydrolysis product of III (IV, 9% and 0.4%
resp.) and 3 hydroxylation products of III (V, 5% and 0.2% resp.
and VI and VII, together 3% and 0.04% resp.). The metabolites III,
IV and V occurred both free and
conjugated, the metabolites VI and VII mainly as conjugates
(Karapally, 1975 and 1977).
In another study with rats II and III were determined as the main
metabolites in 12-hour urine and faeces; 65% of the
14C-radioactivity found was II and 30% III (Ioannou and Dauterman,
1978).
In vitro
Etrimfos is rapidly degraded to water-soluble metabolites, mainly
desmethyletrimfos (II) and EEHP (III), when incubated with rat or
mouse liver subcellular fractions. The oxygen-analogue of etrimfos
could not be found. Glutathione-transferases and to a lesser
extent mixed-function oxidases are the main groups of enzymes
responsible for etrimfos metabolism (Ioannou and Dauterman, 1978).
Molecular formula C10H17N2O4PS
Molecular weight 292.3
Specific gravity 1.195 at 20°C
Appearance colourless oil
Odour slight smell, characteristic of
thionophosphoric acid derivatives
Melting Point -3.35°C (pure a.i.)
Vapour pressure 6.5 to 8.7 × 10-2 mbar at 20°C
Refractive index nD20 1.5068
Solubility in water (24°C) 40 mg/l completely miscible
with acetone, ethanol, diethyl ether, chloroform,
dimethylsulphoxide, ethyl-acetate, hexane,
kerosene.
Stability Half-life times in aqueous buffer solution at
25°C and pH 3, 6 and 9 are 0.4, 16 and 14 days
respectively. 1 to 10 mg etrimfos/l acetone,
chloroform, hexane or toluene in the dark at
room temp. were found to be stable for at least
28 days, in methanol and ethylacetate 3% and 12%
degradation were noticed.
Minimum degree of
purity 93%
Impurities in the
technical material Information on the impurities in technical
etrimfos was reported to the meeting.
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, excretion and biotransformation
Female rats were given a single oral dose of 50 mg/kg bw SAN I 197
(14C-labelled at ring-positions 4 and 6) dissolved in 80% aqueous
polyethylene-glycol.
Excretion of radioactivity occurred mainly via the urine, 84%
within 96 hours, of which 68% was within 12 hours; 6.6% was found
in the faeces within 24 hours. One hour after administration
continuous excretion of radioactivity was observed in the bile and
amounted to 6% within 24 hours. Only 0.012% was exhaled within 24
hours, of which 0.011% was within the first 8 hours, with a
peak-value around 1 hour after administration. For most tissues
the peak concentrations were reached at 4 hours after
administration with the exceptions of the liver (2 hours), blood (3
hours) and fat (8 hours). At peak time the fat and kidneys had the
highest concentrations (42 and 62 mg/kg respectively) whereas the
value in the other tissues ranged from 6 to 22 mg/kg with 13 mg/kg
in the blood. The concentrations declined rapidly to values of
0.01 mg/kg at 96 hours except in the fat and skin with 0.3 mg/kg.
In the urine and faeces collected for 96 hours neither etrimfos (I)
nor its oxygen analogue was detected. The main metabolite in both
excreta was 6-ethoxy-2-ethyl-4-hydroxypyrimidine (III) in amounts
of (% of the dose) 48% in the urine and 3% in the faeces. However
part of this metabolite is possibly formed from I and
desmethyletrimfos (II) during the procedures for analysis (see
below). Other metabolites found in the urine and faeces (see
figure 1) were the hydrolysis product of III (IV, 9% and 0.4%
resp.) and 3 hydroxylation products of III (V, 5% and 0.2% resp.
and VI and VII, together 3% and 0.04% resp.). The metabolites III,
IV and V occurred both free and
conjugated, the metabolites VI and VII mainly as conjugates
(Karapally, 1975 and 1977).
In another study with rats II and III were determined as the main
metabolites in 12-hour urine and faeces; 65% of the
14C-radioactivity found was II and 30% III (Ioannou and Dauterman,
1978).
In vitro
Etrimfos is rapidly degraded to water-soluble metabolites, mainly
desmethyletrimfos (II) and EEHP (III), when incubated with rat or
mouse liver subcellular fractions. The oxygen-analogue of etrimfos
could not be found. Glutathione-transferases and to a lesser
extent mixed-function oxidases are the main groups of enzymes
responsible for etrimfos metabolism (Ioannou and Dauterman, 1978).
 TOXICOLOGICAL STUDIES
Special studies on antidotes
Treatment of rats with atropine and obidoxime reduced the mortality
after oral administration of 3600 mg etrimfos/kg bw (Hamburger and
Klotzsche, 1978a).
Special studies on mutagenicity
Rat
Groups of 5 male and 5 female rats were administered, by intragastric
intubation 1500, 3000 or 6000 mg etrimfos/kg bw in two equal dosages
separated by an interval of 24 hours. A negative control group
received 1% methylcellulose, whereas a positive control group was
dosed by 2 i.p. injections with 8 mg Mitomycin c/kg bw.
After administration of etrimfos, the aberrant metaphase counts were
comparable with the control values, whereas Mitomycin C produced the
expected increase aberrant counts in the bone marrow cells of the
femurs (Richold and Richardson, 1980).
Hamster
Groups of 6 female Chinese hamsters were given s.c. injections of 30,
100 or 300 mg etrimfos/kg bw twice within 24 hours. MMS (100 mg/kg,
orally) was used as a positive control. Etrimfos did not elicit a
mutagenic response whereas the number of bone marrow micronuclei was
increased in the MMS-treated hamsters (Leuschner, 1978).
Microorganisms
Etrimfos (97%) in dosages from 0.001 up to 5.0 l/plate was not
mutagenic in the Ames test using the Salmonella typhimurium
strains TA-98, TA-100, TA-1535, TA-1537 and TA-1538 and
Saccharomyces cervisiae D-4, both with and without activation by
rat liver homogenate (Brusick and Weir, 1976).
Special studies on reproduction
Rat
Groups of 30 males and 30 females were fed dietary concentrations of
0, 3, 9 and 27 mg/kg in a three-generation, two-litter generation
reproduction study. In the B-generations one day before birth 5
pregnant females were killed and investigated for teratogenic
symptoms. The offspring of 5 other females were x-rayed and their
skeletons examined for abnormalities. After 21 days the tissues of 10
male and 10 female weanlings of the III B generation per dose group
were inspected histopathologically. The body weight gain and food
intake of the females were not affected by the treatment. No effects
were observed on fertility, gestation, viability, lactation, the
number of dead foetuses and the pup weight. Teratogenic events were
not observed over the course of the study. At the highest dose level
cholinesterase activity in plasma and erythrocytes, which was
determined in the weanlings of the B generations of all groups, was
slightly depressed, especially in the female rats. Brain
cholinesterase activity was not affected. Gross and microscopic
examination of tissues of organs of the F III B generation showed no
changes attributable to etrimfos administration (Carpy and Klotzsche,
1979).
Special studies on teratogenicity
Rabbits
Groups of 10 rabbits (12 in the control and in the low dose group)
were given etrimfos orally from day 6 to day 18 of pregnancy at dose
levels of 0, 25, 50 or 100 mg/kg bw. On day 29 of gestation the
animals were sacrificed and a caesarean section was performed. The
uterus was examined and the number, location and distribution of the
foetuses, implantations and resorptions recorded. Corpora lutea were
counted. The foetuses were weighed and examined for external and
skeletal anomalies. In the 50 and 100 mg groups the numbers of
implantations and living foetuses were decreased, whereas the number
of dead foetuses and weight of the placenta were slightly increased.
In the low dose group 2 animals with slightly curved hind legs and in
the highest dose group, in which 43 animals were investigated, one
animal with a rudimental tail and one animal with a skull deformation
were observed. In a historical control group of 286 animals 2 rabbits
with skull deformations were observed (Hamburger and Klotzsche,
1978b).
Special studies on neurotoxicity
Hen
Groups of 5-20 chickens were administered etrimfos in gelatine
capsules at dose levels of 100, 200, 400, 500, 750 or 1000 mg/kg bw.
A positive control group was given 2 × 80 mg tri-o-cresyl phosphate
(TOCP)/kg bw. Before the treatment the animals received atropine or
obidoxime by i.v, or i.m. injection. The observation period varied
from 21-42 days. Mortality was increased in all treated groups.
Doses of 200 mg/kg etrimfos and above caused typical signs of
organophosphate poisoning. A delayed neurotoxic response was not
observed in the chickens treated with etrimfos. TOCP elicited ataxia
and paralysis in all 10 animals. Histopathologically typical
degeneration changes in the ischias nerve were observed in all
TOCP-treated animals. No clear treatment-related alterations were
found in the nerves of the hens given 100 and 200 mg/kg etrimfos
(Hamburger and Klotzsche, 1975d).
Special studies on sensitization
Guinea pig
According to the "Guinea pig maximisation test" 20 guinea pigs were
treated with SAN 197 I as a 5% solution in DMSO and incorporated in
Freund's adjuvant; 10 guinea pigs served as a control group. 5/20 of
the treated and 4/10 of the control animals died before the 22nd day.
A slight development of scales at the application side without any
inflammatory changes or oedema was noticed at 72 hours after the
challenge application; no other effects were noticed (Hamburger and
Klotzsche, 1977b).
Acute toxicity
Symptoms of intoxication in rats and mice after oral, i.p. and s.c.
administration were limpness, lacrimation, salivation, exophthalmus,
laboured respiration, ataxia, tremors and convulsions. After dermal
application to rats and rabbits no toxic symptoms were observed
(Hamburger and Klotzsche, 1975a; Anonymous, 1979a and Anonymous,
1979b).
Rabbit
Etrimfos (0.5 g) applied in the intact or abraded skin of rabbits was
found to be not irritating. Instilled into the eye (100 mg) etrimfos
produced slight redness of the conjunctivae, which lasted 24 hours, in
one of six rabbits (Hamburger and Klotzsche, 1975b).
TABLE 1. Acute toxicity of etrimfos
species sex route LD50 in mg/kg bw purity solvent references
mouse M oral 47O (425-523) 97.2% water Hamburger and Klotzsche, 1975a
F oral 620 ± 28 97.2% water " " "
M oral 1120 (1023-1226) 90.5% corn-oil Anonymous, 1979a
F oral 1100 (930-1289) 90.5% corn-oil " "
M oral 535 (24 hours) - corn-oil Ioannou and Dauterman, 1978
M i.p. 698 (597-817) 90.5% corn-oil Anonymous, 1979a
F i.p. 679 (585-788) 90.5% corn-oil " "
M s.c. 979 (729-1155) 90.5% corn-oil " "
F s.c. 1050 (897-1229) 90.5% corn-oil " "
rat M oral 1800 (765-3733) 97.2% water Hamburger and Klotzsche, 1975a
F oral 2354 (1841-3008)1 97.2% water " " "
M oral 1930 (1664-2239) 90.5% corn-oil Anonymous, 1979b
F oral 1970 (1669-2325) 90.5% corn-oil " "
M oral 2040 (24 hours) - corn-oil Ioannou and Dauterman, 1978
M i.p. 767 (684-873) 97.2% water Hamburger and Klotzsche, 1975a
M i.p. 1310 (1110-1546) 90.5% corn-oil Anonymous, 1979b
F i.p. 1140 (950-1368) 90.5% corn-oil " "
M i.v. 25.5 ± 1.26 97.2% water Hamburger and Klotzsche, 1975a
F i.v. 21.0 ± 1.88 97.2% water " " "
M i.m. 4000 ± 116 94.3% PEG 200 Hamburger and Klotzsche, 1977a
M x.c. 3700 (3710-4563) 90.5% corn-oil Anonymous, 1979a
F s.c. 3690 (3000-4539) 95.5% corn-oil Anonymous, 1979b
dermal >2000 97.2% water Hamburger and Klotzsche, 1975a
M dermal >5000 90.5% corn-oil Anonymous, 1979b
F dermal >5000 90.5% corn-oil " "
rabbit dermal >500 97.2% water Hamburger and Klotzsche, 1975a
1 value corrected by recalculation
TABLE 2. Acute toxicity of metabolites
Species sex route solvent LD50 (mg/kg) references
rat M oral DMSO 1460 ± 52.5 Hamburger and Klotzsche, 1975e
rat F oral DMSO 1170 ± 58.5 " " "
mouse M oral DMSO 1530 ± 87.9 " " "
mouse F oral DMSO 1170 ± 94.8 " " "
P = O analogue of the parent compound
species sex route solvent LD50 (mg/kg) references
rat M oral DMSO 450 ± 35 Hamburger and Klotzsche, 1977c
rat M dermal DMSO 1000 " " "
Short-term studies
Rat
Male and female rats (15/sex/group) were dietary fed 0, 50, 250 or
1250 mg/kg SAN 197 I (97.2%) in the food for 4 weeks; later on in the
test groups (5/sex) on 10 mg/kg with corresponding controls were
added. The animals were examined for general health, body weight,
food consumption, haematology, blood biochemistry, urinalysis, organ
weights and macroscopy. A decreased cholinesterase activity in
erythrocytes was observed at 50 mg/kg and higher doses. Plasma-ChE
was decreased in females from the 50 mg/kg and in males from the 250
mg/kg groups, and brain-ChE in females from the 250 mg/kg and in males
at the 1250 mg/kg groups. Normochromic anaemia was noticed at 250
mg/kg and in females at 1250 mg/kg, changes in blood sugar occurred at
all dosages except 250 mg/kg and changes in reticulocytes at 1250
mg/kg. At these dosage the animals showed slight sedation and
slightly decreased body-weight gain as result of lower food intake
(Carpy and Klotzsche, 1975a).
Rat
Groups of 35 males and 35 females were fed etrimfos at concentrations
of 0, 3, 9 or 27 mg/kg feed for a period of 13 weeks. Body-weight
gain and food intake was recorded throughout the study of 25 females
and 25 males per group. After 4, 8 and 13 weeks haematology clinical
chemistry and urinanalysis was carried out in 10 animals/group. The
same number of rats was studied histopathologically and their organ
weights were recorded. At the end of the study the glucose
concentration in serum was increased in both males and females of the
highest dose level. The weight of the thyroid of the male rats of the
highest dose group was significantly decreased. However microscopic
examination showed no abnormalities attributable to the presence of
etrimfos in the diet. After 8 and 13 weeks the plasma cholinesterase
activity of the female rats in the 9 mg/kg group was slightly (20-24%)
and clearly depressed in the 27 mg/kg group (30-37%). Cholinesterase
activity in erythrocytes and brain was within normal limits (Carpy and
Klotzsche, 1975d).
Rabbit
Male and female rabbits (5/sex/group) received a dermal application of
0, 25 or 100 mg/kg SAN 197 I (97.2%) dissolved in polyethylene-glycol
on patches of intact or abraded skin for 8 hours a day on 5 days a
week during 2 weeks and were examined for general health, body weight,
haematology, blood biochemistry, organ weights and macroscopic changes
(microscopic studies are not yet completed). One male with intact
skin out of the 100 mg/kg group died with an enlarged liver. In the
males the weight of the spleen was decreased and the weight of the
thymus increased while in females a decreased weight of the thymus was
noticed. The animals with abraded skin showed abnormalities only at
the high dosage. Increased organ weights were observed in the female
liver and heart and in the males in the prostate. All these changes
were within the normal range. The animals with intact skin showed, at
both dosages, cholinesterase-inhibition in plasma and in erythrocytes.
In the animals with abraded skin, only in the high dosage, in males as
well as in the females, was cholinesterase inhibited in plasma and
erythrocytes (Carpy and Klotsche, 1975b and c).
Four groups of 4 male and 4 female beagle dogs were fed O, 2.5, 10 or
40 mg/kg etrimfos (97.2% purity) in the food for 26 weeks.
Normal values in haematology, blood chemistry, and urinalysis were
evaluated before starting the study. These determinations were
repeated 6 times during the test period: after 1, 4, 8, 13, 19 and 26
weeks, with exception of BSP which was estimated after 4, 13, and 26
weeks. The physical examination included weekly body weight, food
intake and neurological, oral, behaviourial, and ophthalmoscopic
inspection. The haematological parameters investigated were: RBC,
WBC, differential WBC, MCV, MCH, MCHC, reticulocytes, haemoglobin,
haematocrit, platelets, and prothrombin time, RBC- and
plasma-cholinesterase, FBS, BUN, SGPT, SGOT, LDH, uric acid, total
serum protein, bilirubin, creatinine, serum alkaline phosphatase,
cholesterol, serum sodium, potassium, calcium, chloride, inorganic
phosphate, serum albumin, and serum electrophoresis (A/G ratio). The
activity of cholinesterase in cerebellum and cerebrum, of the liver
cytochrome P-450, of the liver drug metabolising enzymes O-, and
S-demethylases and of the anilin-4-hydroxylase and the amount of
cholesterol, glycogen, lipids, and total protein contained in the
liver were estimated at the end of the study. All animals were
examined for gross pathology, organ weights and histopathology. MCH
and MCHC values were increased in the male animals of the 40 mg/kg
group. From the 11th week on plasma and erythrocyte cholinesterase
activity was depressed in comparison to the pretest and the control
values, with a plateau of about 40% in both males and females. In the
10 mg/kg group a slight depression of cholinesterase activity in
plasma and erythrocytes especially in comparison to the pre-test
values was observed in both sexes. In the cerebrum of the female rat
of the highest dose group a decrease of 22% of the cholinesterase
activity was measured.
From the 13th week on the albumin concentration in serum was
dose-dependant increased. However this was not confirmed by
electrophoresis and had disappeared almost at the end of the study.
The weight of the organs was within normal limits. No gross or
histopathological aberrations were observed (Klotzsche and Carpy,
1975e).
Long-term studies
Rat
Groups of 40 males and 40 females were fed etrimfos (97.8%) in the
diet at concentrations of 0, 6, 12 or 24 mg/kg for a period of 2
years. Body weight, food consumption and water intake of 30 rats per
dose and sex were recorded weekly. After 1, 2 ,3, 6, 9, 12, 15, 18,
21 and 24 months haematology, clinical chemistry and urinalysis were
carried out in 5 male and 5 female animals per group. At the end of
the study BSP a liver function test was carried out in the control and
high-dosed females. In liver homogenates the activity of drug
metabolising enzymes and the total protein and cholesterol
concentration were measured at the end of the study.
All animals dying during the study, or which had to be killed, were
necropsied and tissues were examined microscopically. The organ
weights of 10 rats per dose and sex were recorded.
There was no clear effect of etrimfos on body weight, food and water
intake and mortality. The cholinesterase activity of the plasma and
erythrocytes of male rats was not affected by etrimfos. In the
females the activity of cholinesterase in the plasma of the 24 and 12
mg/kg group was decreased by 35-50 and 20-30% respectively throughout
the study. In the highest dose group a marginal effect was observed
on the activity in the erythrocytes of the female rats. Neither in
the females nor in the males was the cholinesterase activity of the
brain and the liver of the treated animals significantly different
from the control values.
Both in the male and female rats the cytochrome P-450 of the liver of
the animals of the 12 and 24 mg/kg group was significantly decreased
in comparison to the controls; however no dose-response relation was
observed.
The macroscopic and microscopic examination did not reveal organ
lesions that could be attributed to treatment. The tumour rate in
treated animals corresponded with the incidence in control animals.
No-effect level of this study is 6 mg/kg (Carpy and Klotzsche, 1976).
Dog
Groups of 4 male and 4 female beagle dogs received 0, 4, 10 or 25
mg/kg etrimfos in their diet, during 106 weeks. There were no signs
of adverse behaviour and mortality was not noted. Growth and food
consumption were not clearly affected. Eye examinations during the
first, 26th, 52nd, 78th and 104th week did not indicate adverse ocular
changes.
Haematology, clinical chemistry and urinalysis were carried out in
week 1 (pre-test), 4, 8, 13 and quarterly thereafter. Plasma
cholinesterase activity was decreased in the 10 and 25 mg/kg groups by
23% and 24% respectively. The cholinesterase activity of the
erythrocytes was also decreased.
No clear differences or sensitivity were seen between males and
females. In the cerebrum, cerebellum and liver the cholinesterase
activity was not depressed.
Slightly higher activities of N-, O- and S-demethylase activity were
measured in all treated groups. However there was no clear
dose-response relation. Neither the weights of the organs nor the
histopathological evaluation revealed indications for treatment-
related changes in any organ or tissue. No-effect level of this study
is 10 mg/kg (Carpy and Klotzsche, 1977).
RESIDUES IN FOOD
RESIDUES RESULTING FROM SUPERVISED TRIALS
Stored grain products
Wheat grain (variety Probus) was treated in 25 kg lots with
Satisfar(R) (Ekamet 50 EC) at the rate of 5, 10 or 15 mg/kg ai under
conditions as close to normal practice as possible. The grain, which
was stored in fibre-drums at room temperature, was analysed
periodically for etrimfos.
In this small-scale trial the etrimfos content of the wheat grain
immediately after treatment (zero time) was 50 - 60 percent of the
application rate. During the first six months of storage, the amount
of etrimfos in the grain remained fairly constant.
In the next six months its amount was reduced to 34-37% of the
application rate, (or 63-75% of the original concentration in the
grain). At the end of 12 months, the grain treated at the rate of 10
and 15 mg/kg had 100% efficacy against Sitophilus; the grain treated
at the rate of 5 mg/kg had 90% activity. (CBK 3630/79).
Several trials were conducted in different countries on wheat, barley
and corn (table 3).
FATE OF RESIDUES
In plants
The fate of etrimfos in bean and corn plants has been studied (M.
Akram et al., 1978). The primary leaves of bean and corn seedlings
were treated with 14C etrimfos. The treated leaves and the untreated
portions of the plants were sampled at 0, 3, 7, 14 and 21 days after
treatment. The aqueous rinses and the extracts of the treated leaves
were examined for the parent compound and its metabolites. Leaf
remainder, and untreated portions, were analysed for total
radiocarbon.
Etrimfos rapidly volatilised from the leaf surface. Within the first
three days after treatment it is lost with a half-life of approx. 3
days (beans and corn) and during 3 to 15 days with a half-life of
approx. 10 days from beans and approximately 5 days from corn. Three
weeks after treatment the radioactivity, retained by the leaves after
rinsing, ranged from 33% to 42% for bean, and from 25% to 42% for
corn. No translocation to the root system was observed. A small
percentage of the applied radiocarbon was present in the untreated
foliage of bean (0.3-0.6% of the applied radioactivity became
unextractable in bean leaves, and 0.1-0.3% in corn leaves).
Leaf rinses (surface residues) of bean and corn contained mainly
etrimfos and small quantities of 6-ethoxy-2-ethyl-4-hydroxy-pyrimidine
(EEHP). Small amounts of the P=O analogue of etrimfos were also
observed in the corn-rinse.
The leaf extracts (subsurface residues) of bean and corn contained
etrimfos and minor quantities of EEHP and five unknown metabolites, of
which one was tentatively identified as
2-ethyl-4,6-dihydroxy-pyrimidine; 21-day bean leaf contained 33.5% of
the applied radioactivity, of which 11.9% was etrimfos, 2.4% EEHP,
9.7% the unknown metabolites and 9.5% highly polar or conjugated
metabolites (TLC origin). All single metabolites were less than 10%
relative to applied etrimfos; 21-day corn leaf had about 24.3% of the
applied radioactivity, of which 3.8% was etrimfos, 0.4% EEHP, 15.8%
the unknown metabolites and 4.3% polar or conjugated metabolites. All
single metabolites were less than 10% relative to applied etrimfos.
The enzyme and acid-hydrolysed aqueous extracts gave essentially the
same TLC pattern as the unhydrolysed material, with quantitative
differences.
From the described results, we learn that etrimfos is lost from
treated plants in two stages. EEHP was found as a metabolite, but
only in quantities of less than 10% of the applied etrimfos. The P=O
analogue was not found in bean leaves nor in corn leaves. No single
metabolite was present in quantities more than 10% relative to the
applied etrimfos so that the influence of these metabolites on the
toxicity of actual residue levels in practical field samples may be
neglected. Tolerance recommendations may, therefore, be based on
residue data of the etrimfos ai only.
In cereal products
Wheat grain (variety, Probus) was treated in 25 kg lots with
Satisfar(R) (Ekamet 50 EC) at the rate of 5, 10 or 15 mg/kg ai. The
grain was stored at room temperature and at intervals of 2, 4, 6 and
12 months, samples of grain were milled and separated into different
fractions. Also bread was baked from the flour from these samples.
Etrimfos residues were analysed in all these samples.
Etrimfos concentration in the milled fractions of the wheat was the
highest in the bran and the lowest in the white flour. During the
baking process the etrimfos content of the flour was reduced by 58-77%
in the case of white bread, and 43-65% in the case of whole wheat
bread. The etrimfos content of white bread ranged from about 4 to 6%
of the amount applied and that in the whole wheat bread was in the
range of 14 to 30% of the amount applied (CBK 3630/79). Table 4.
In another trial with wheat, the amount of etrimfos in the white flour
ranged from 25 to 33% of that actually present in the grain. Baking
to bread reduced these residues in the flour by about 50% (CBK
4119/79). Table 5.
In a trial with barley, the wort from free grain contained only 4-6%
of the etrimfos originally present in the grain. The wort from malt
grain had no detectable residue of etrimfos (CBK 4120/79). Table 5.
TABLE 3. Residues of etrimfos (mg/kg) in stored products treated after harvest
Application Storage period (months)
Commodity Rate 0 1-2 3-4 6 8-10 12-13 18-24 Remarks Ref.
(country) Type (mg/kg) (24 h)
Cereal grain
Barley
(England) 50 EC 5 2.3 3.2-3.6 2.1 0.8 amount treated:
content: 15%; temp.: CBK
0-15°C; untreated 4121/79
"blank": 0.03-0.04
mg/kg
(Kenya) 0.5 Dust 30 2.25 2.1 0.6 0.3 amount treated:
1 Dust 29 4.5 3.0 1.3 0.8 45 kg each, UK
50 EC 5 1.3 0.5 0.2 stored in bags; 3770/79
2 Dust 22 9 9.7 1.2-2.2 0.8-1.4 conditions 4149/79
50 EC 10 4.1 1.0-2.1 0.5-0.9 not reported
Corn(maize)
(Kenya) 0.5 Dust 30 2.75 0.6-0.8 0.5 0.3-0.4 0.3-0.4 - amount treated:
50 EC 5 1.5-1.6 1.6-1.7 0.8-1.2 1.0-1.1 0.8 45 kg each,
1 Dust 29 5.5 0.9-2.6 0.7-1.5 1.0-1.6 0.8-0.9 0.9-1.3 stored in bags;
50 EC 10 0.8-4.2 1.7-2.9 1.5-2.5 1.9-2.5 2.5-2.6 storage conditions
2 Dust 22 11 1.7-5.5 2.4-3.8 2.2-2.9 1.2-2.4 1.5-2.3 not reported
Wheat
(England) 50 EC 5 6.8 5.6-6.1 5.9 3.9 amount treated:
50 EC 10 10.9 6.7-7.2 5.4 6.4 10 t; 30 t on
conveyer belt; CBK
moisture cont. 11%; 4118/79
0-17°C; untreated
"blank": 0.01-1 mg/kg
TABLE 3. Continued...
Application Storage period (months)
Commodity Rate 0 1-2 3-4 6 8-10 12-13 18-24 Remarks Ref.
(country) Type (mg/kg) (24 h)
(France) 50 EC 5 1.9-2.0 1.4-2.0 1.6 storage conditions not
50 EC 7.5 1.6-2.5 2.1-3.3 2.1-2.9 reported; samples for CBK
50 EC 10 3.3-5.4 4.2 2.6-3.8 analysis: 10 g each 2943/79
(Kenya) 0.5 Dust 30 2.5 1.2 0.7 1.0 0.8 0.4 amount treated:
1 Dust 29 5 2.0 1.8 1.5 1.3 1.0 10 kg each, stored CBK
50 EC 5 1.6 1.0 1.0 0.8 0.5 in bags; storage 3771/79
2 Dust 22 10 3.7 2.8 2.7 2.1 1.8 temp. about 15-25°C; 4117/79
50 EC 10 2.0 2.5 2.6 2.5 1.7 further conditions 4148/79
not reported.
(Switzerland) 50 EC 5 2.9 2.9 2.7 3.0 1.9 1.1 0.6 amount treated: 25 kg
(<0.1) (<0.1) (<0.1) (0.2) (0.4) (0.2) (<0.1) each, stored in air
50 EC 10 5.4 5.8 5.5 5.6 3.4 1.7 1.3 permeable fibre-drums;
(0.1) (0.5) (0.1) (0.3) (1.2) (0.2) (<0.1) moisture content: 12-14%; CBK
50 EC 15 7.4 7.8 7.6 8.1 5.6 temp about 16-20°C; 3630/79
(0.1) (0.2) (0.2) (0.5) (1.5) EEHP residues reported 3707/79
in brackets; EPO 4209/80
residues were always
below 0.002 mg/kg.
Rape seed
(England) EC 10 9-10 8-9 9-10 7-8 :Bin 2 amount treated: Stables
EC 10 11-15 10-15 13-14 13-14 10 t each, stored et al.,
:Bin 3 in metal bins; (1979)
moisture content in seed:
9-11%; temp. 3-15°C; seed
in bin 2 was slightly damper and
more heavily infested by mites
than in bin 3. Etrimfos in rape
seed oil (refined) up to about
0.5 mg/kg/ in spent meal: 0.05 mg/kg.
TABLE 4: Etrimfos1 residues in milled fractions and flour of wheat and in bread
Treatment Residues (mg/kg) 2-12 months after treatment
rate 2 4 6 12
5 mg/kg Etrimfos EEHP2 Etrimfos EEHP Etrimfos EEHP Etrimfos EEHP
Grain 2.92 n.d.3 2.74 n.d. 3.02 0.20 1.89 0.36
Bran 10.47 1.10 9.94 0.39 9.78 0.38 3.50 2.10
Grits 8.67 0.62 8.64 0.62 6.83 0.27 3.48 1.02
White flour 0.82 n.d. 1.02 n.d. 0.73 n.d. 0.70 n.d.
White bread 0.23 0.05 0.24 0.10 0.21 0.11 0.18 n.d.
Whole wheat
flour n.a.4 n.a. 2.66 0.16 2.33 0.11 1.97 0.37
Whole wheat
bread n.a n.a. 1.52 0.22 1.33 0.34 0.69 0.23
10 mg/kg Etrimfos EEHP2 Etrimfos EEHP Etrimfos EEHP Etrimfos EEHP
Grain 5.84 0.54 5.50 0.08 5.59 0.34 3.38 1.20
Bran 15.22 1.92 16.27 0.66 15.83 1.14 6.20 3.34
Grits 41.29 1.27 15.08 0.73 13.14 1.06 7.74 1.97
White flour 1.89 0.14 1.75 n.d. 1.47 0.06 0.96 n.d.
White bread 0.50 0.13 0.61 0.24 0.51 0.20 0.41 n.d.
Whole wheat
flour n.a. n.a. 5.59 0.39 5.07 0.24 3.70 0.69
Whole wheat
bread n.a. n.a. 2.83 0.60 2.38 0.51 1.37 0.51
TABLE 4. Continued...
Treatment Residues (mg/kg) 2-12 months after treatment
rate 2 4 6 12
15 mg/kg Etrimfos EEHP2 Etrimfos EEHP Etrimfos EEHP Etrimfos EEHP
Grain 7.80 0.20 7.61 0.20 8.11 0.46 5.57 1.52
Bran 16.60 1.50 21.65 1.56 19.70 1.51 9.66 4.34
Grits 19.41 1.53 20.84 0.93 19.33 1.06 12.14 1.97
White flour 2.04 0.25 2.62 0.09 2.48 0.10 1.50 n.d.
White bread 0.74 0.18 0.95 0.49 0.78 0.31 0.54 0.23
Whole wheat
flour n.a. n.a. 7.99 0.41 7.84 0.40 5.90 0.71
Whole wheat
bread n.a. n.a. 3.80 0.40 3.77 0.70 2.30 0.46
1 The metabolite P=O etrimfos could not be detected
2 EEHP = 2-ethyl-4-ethoxy-5-hydroxy-pirimidine
3 n.d = not detectable
4 n.a. = not analysed
TABLE 5. Etrimfos residues in cereals processed after treatment (in UK) with SATISFAR(R)
Cereal Formulation Rate of Etrimfos Amount of Method of treatment Reference
treatment residues grain per
(ai/mg/kg) (ppm) treatment
Wheat 50 EC 5 mg/kg grain 0.76 6 kg The required amount of CBK
bran 4.62 Satisfar in 25 ml water 4119/79
offal 3.13 was sprayed onto grain
white flour 0.25 tumbling in a
white bread 0.12 Bluefinsowaway cement mixer.
10 mg/kg grain 2.51 Stored in sealed plastic
bran 4.50 bags.
offal 6.14
white flour 0.63
white bread 0.34
Barley 50 EC 5 mg/kg free grain 1.07 CBK 4120/79
wort 0.07
spent 0.63
malt grain 0.21
wort n.d.
spent gr 0.07
10 mg/kg free grain 3.50
wort 0.16
spent gr 0.52
malt grain -
wort n.d.
spent gr 0.11
In cattle
A three level feeding study in dairy cattle with etrimfos incorporated
in the diet at levels 0.5, 1.5 or 5 mg/kg etrimfos was conducted. The
milk production was not affected. The cows, sacrificed after 28 days
of feeding, had only less than 0.01 mg/kg etrimfos in the muscle,
liver, kidney and fat. Etrimfos residue in the milk throughout the
study was less than 0.01 mg/kg. The residue in the milk solids
separated from the 21st and 28th day samples also had only 0.01 mg/kg
etrimfos (CBK 3011/77).
In soil
Degradation of 14C etrimfos in a sandy loam, silt loam and silty clay
loam soil was studied.
The soils treated with 14C-etrimfos at the rate of 5 mg/kg were
incubated at room temperature for 70 days. At selected time intervals
during the incubation period, the soil samples were analysed for the
parent and degradation products.
Etrimfos was rapidly hydrolysed, in all soils, to
6-ethoxy-2-ethyl-4-hydroxy-pyrimidine (EEHP). Its amount was reduced
to 50% in 3-8 days, and to 10% in 15-30 days.
The formation of EEHP paralleled the dissipation of etrimfos. Its
proportions reached a maximum of 75-85% in 14-28 days, after which
period its concentration began to decrease. The disappearance of EEHP
was fastest in silt, and rather slower in sand, and still more so in
clay. At the end of 70 days, the amounts of EEHP in silt, sand and
clay were 4, 18 and 52% respectively.
A further metabolite, tentatively identified as
5-chloro-6-ethoxy-2-ethyl-4-hydroxy-pyrimidine (CEEHP), was detected
in all soil extracts. Its formation seemed to be related to the
dissipation of EEHP, and its occurrence was highest in silt (24%),
followed by sand and clay (6%).
The soil-"bound" residues became significant only after EEHP began to
be metabolised. A maximum of 12% of these was observed for sand, and
21% for silt and clay. Further extraction with methanol-water 75:25
reduced the bound residue to <10%, the metabolites being mainly EEHP
and CEEHP.
The radiocarbon lost from the soil during the 70 days' incubation
amounted to 60% for sand or silt and 25% for clay. In a separate
experiment, using sandy loom soil treated with 14C-etrimfos, it was
demonstrated that the radioactivity lost from the soil was due to the
14CO2, which began to be generated after a time lag of at least 20
days (CBK 3002/77).
Photodegradation
in water
Under blacklight and daylight, which contain essentially no shorter
wave lengths than natural sunlight, aqueous solution of etrimfos (5
mg/kg) was quite stable: degradations of 10% (blacklight) and 5%
(daylight) occurred within 24 hours of exposure. Under mercury arc
light, approx. 45% degraded during the same time (CBK 1642/75).
on glass plates
2.5 mg of etrimfos, spread over an area of 70 cm2 and covered with
quartz plates, was exposed to the same light sources as in water.
Degradation, after 24 hours under the following conditions, was
daylight <5%, blacklight <5%, mercury light ‰ 25%, (CBK 1642/75).
METHODS OF RESIDUE ANALYSIS
Two methods have been developed - one for determining etrimfos the P=O
analogue and 2-ethyl-4-ethoxy-6-hydroxypyrimidine (EEHP), the
hydrolysis product of etrimfos (CBK 2178/76) and the other for
determining etrimfos only (CBK 3649/79). In both methods grain is
dealt with in the same manner as vegetable crops.
The P=O analogue of etrimfos has been detected in the stored grain
products. The amount of EEHP in the grain is generally quite low and
hence, in these cases, the method CBK 3649/79 is recommended.
In these methods etrimfos residues are extracted with acetone and
partially cleaned up by partitioning between water and methylene
dichloride. If only etrimfos is to be determined, the methylene
dichloride solution is further cleaned up on a silica gel column and
determined by gas chromatography using a flame photometric detector
(P-mode). If the P=O analogue and EEHP are to be included, the
methylene dichloride solution is further cleaned up on a
polyethylene-coated alumina column and determined by gas
chromatography using a nitrogen specific detector.
A series of CBA reports on residues in food and fats in the
environment were submitted by Sandoz Ltd. to FAO for this evaluation.
EVALUATION
Etrimfos is a broad-range non-systemic contact and stomach
organophosphorous insecticide, which was introduced in 1972 and is
registered and/or used in a considerable number of countries. It is
effective against biting and sucking insects such as Lepidoptera,
Coleoptera, Diptera and to a variable extent, Hemiptera at 0.25-0.75
kg ai/ha. It is mainly used on fruit, vegetables, maize and
ornamental plants, but also on alfalfa and paddy rice, as an
emulsifiable concentrate or as granules.
Studies of the metabolism and distribution of etrimfos in rats have
been undertaken with 14C in the pyrimidine moiety. This has provided
useful information on the pyrimidine portion of the molecule, but
disposition and kinetic data on the whole molecule of etrimfos and its
triester metabolites are required. This is especially important since
the compounds containing a fully-esterified phosphoroticate or
phosphate group are the more toxic.
In rats and rabbit no effects on reproduction, including
teratogenicity, were observed. A mutagenic response was negative as
shown by a rat metaphase analysis, a hamster bone marrow micronuclei
count, and an Ames test.
Etrimfos did not induce a delayed neurotoxic response, as generally
noted with TOCP, in hens. A skin-sensitisation study in guinea pigs
was negative.
Etrimfos and the main metabolite
O-6-ethoxy-2-ethyl-4-hydroxypyrimidine (EEHP) were slightly toxic
following acute administration to various animal species. The effects
of etrimfos were typical of those caused by cholinesterase inhibitors.
Several short- and long-term toxicity studies have been carried out
with rats and dogs. The macroscopic and microscopic examination in
these studies did not reveal lesions in organs attributable to
etrimfos. The tumour incidence in treated animals corresponded with
that in control animals. In both species etrimfos produced
cholinesterase inhibition.
Based on the two-year studies with both rat and dog a temporary ADI
was recommended.
Although it is known that data from supervised trials (pre-harvest
use) exist, which show that residues in many fruits and vegetables,
potatoes, maize and rape are in the range 0.05 to 0.5 mg/kg when
etrimfos is used in accordance with good agricultural practice,
information was not made directly available for consideration by the
meeting.
However, in recent tests and trials carried out in England, France,
Kenya and Switzerland etrimfos was also found efficacious against
moths, (malathion-resistant) beetles and (lindane-resistant) mites in
stored crops, especially grain and rape seed. Recommended rates for
dusting or spraying wheat, barley, maize (corn) or rape seed are 5 to
10 mg/kg. Depending on storage conditions (e.g. degree of
infestation, climate, storage period) lower or, exceptionally, higher
dosages up to 15 mg/kg appear appropriate.
Etrimfos in stored rape seed shows no significant loss after 6 months
storage and the estimated half-life in stored grain is about 3 to 8
months. Etrimfos is rapidly lost from treated plants (beans, maize),
about half within the first 3 days, with a half-life of about 5 to 10
days between the 3rd and the 15th day after application, probably
owing to the different rate of volatilisation of the initial deposit
and of the residue after penetration into the surface layer.
In degradation studies with plants treated with 14C-etrimfos,
labelled at carbons 4 and 6 in the pyrimidinyl ring the metabolites
O-6-ethoxy-2-ethyl-4-hydroxypyrimidine (EEHP) and 2-ethyl-4,
6-dihydroxypyrimidine (EDHP) were identified. Small amounts of the
etrimfos P=O analogue (EPO) were also observed, but only on the plant
surface. In stored grain EEHP was the only metabolite found in
significant quantities.
After a single oral does of 50 mg etrimfos/kg, rats excreted more than
50% as EEHP in urine and faeces, with smaller amounts of EDHP,
6-ethoxy-4-hydroxy-2-(1-hydroxyethyl) pyrimidine (EEHP-1), its
2-(2-hydroxyethyl) pyrimidine analogue (EEHP-2) and
2-ethyl-4-hydroxy-6-(2 hydroxyethoxy) pyrimidine (EHHEP). In rat
liver preparations desmethyl-etrimfos was also detected.
In soils the half-life of etrimfos is about 3 to 8 days: degradation
results in EEHP, EDHP and finally carbon dioxide. General degradation
pathways are thus similar in plants, animals and soils, although
proportions of individual metabolites may vary. Hydrolysis of the
phosphorothioate ester seems to be the most significant pathway,
yielding products of lesser toxicological importance.
When dairy cattle were fed with diets containing 5 mg etrimfos/kg for
28 days, etrimfos residues were always below 0.01 mg/kg in meat, milk,
fat and edible offals.
Processing wheat, treated with 10 mg atrimfos/kg and stored for 2 to
12 months, resulted in etrimfos residues up to 16.3 mg/kg in raw bran,
5.6 mg/kg in wholemeal flour, 2.8 mg/kg in wholemeal bread, 1.9 mg/kg
in white flour and 0.6 mg/kg in white bread. Provisional information
on barley treated with 5 to 10 mg etrimfos/kg and used for malting and
brewing showed etrimfos residues up to 0.6 mg/kg in spent grain and
0.2 mg/kg in wort. When stored rape seed containing 10 to 15 mg
etrimfos/kg was used for oil production, preliminary results indicated
that 95-100% of the etrimfos was lost during the refinement of the oil
and that the greatest looses occurred by degradation during bleaching
and steam distillation. No residues were detected in the spent meal.
Analytical methods for determining residues of etrimfos, EEHP and EPO
have been reported. Residues are extracted with acetone and partially
cleaned up by partitioning between water and dichloromethane. For the
determination of etrimfos as such the dichloromethane solution is
further cleaned up on a silica gel column and analysed by gas
chromatography using a flame photometric detector in the P-mode. The
latter method is suitable for adaptation to regulatory purposes.
Level causing no toxicological effect
Rat: 6 mg/kg in the diet equivalent to 0.3 mg/kg bw/day.
Dog: 10 mg/kg in the diet equivalent to 0.25 mg/kg bw/day.
Estimate of acceptable daily intake for man
0-0.003 mg/kg bw/day.
RECOMMENDATIONS OF RESIDUES LIMITS
The Meeting concludes that the residue levels listed below are
suitable for establishing temporary maximum residue limits. The MRLs
should remain as temporary limits, irrespective of the status of the
ADI, until items 1) and 2) of the further work or information,
required by 1982, are provided. The limits refer to the parent
compound etrimfos only.
Commodity (mg/kg) Remarks
Bran of wheat 20
(unprocessed)
Barley, maize, wheat 10
Wheat flour (wholemeal) 10
Wheat flour (white) 2
Rape seed 10
Rape seed oil (refined) 0.5
Carcass meat of cattle 0.011 ) These levels are based
Milk 0.011 ) on animal feeding studies.
Cattle meat byproducts 0.011 )
1 At or about the lower limit of determination
FURTHER WORK OR INFORMATION
Required (by 1982)
1. Adequate additional residue data from large-scale supervised
trials on cereal grains and their products.
2. Further residue data on rape seed and its oil (raw and refined).
3. Information on national use patterns and results of supervised
trials on growing crops destined for human or animal consumption.
4. Data on the amount and nature of the residues to be expected in
eggs and meat of poultry fed on treated grain.
Desirable
1. Data on residues on food in commerce and at consumption.
2. Information on national maximum residue limits.
3. Analytical method for the determination of residues in food of
animal origin.
4. Observations in man.
REFERENCES
Akram M., Ahmad S., and Forgash, A. J. Agric. Food Chem. Vol. 26, No.
4; 925-931.
Anonymous. Etrimfos. Identity. Unpublished report, undated from Sandoz
Ltd. submitted to the World Health Organization by Sandoz Ltd.
Anonymous. Etrimfos. Acute oral, subcutaneous and i.p. LD50 in male
and female mice. (1979a) Unpublished reports ref. Agro Dok CBK
3909/79, 3910/79 and 3911/79, from Sandoz Ltd. Agro Development
Toxicological Department, submitted to WHO by Sandoz Ltd.
Anonymous. Etrimfos. Acute oral, subcutaneous, i.p. and dermal LD50
in male and female rats. (1979b) Unpublished reports ref. Agro Dok CBK
3912/79, 3913/79, 3914/75 and 3915/79, from Sandoz Ltd. Agro
Development Toxicological Department, submitted to WHO by Sandoz Ltd.
Anonymous. The Pesticide Manual (6th edition), p.255. The British Crop
Protection Council. (1979c).
Brusick, D.J. and Weir, R.J. Mutagenicity evaluation of etrimfos.
Unpublished report no. 2683 ref. Agro Dok CBK 2545/77, dd. 30 December
1976 from Litton Bionetics submitted to WHO by Sandoz Ltd.
Carpy, S. and Klotzsche, C. San 197 I. 4-Week feeding study in rats.
Unpublished report no. Agro Dok CBK 754 b/73, dd. 18 March 1975, from
Sandoz Ltd., Agrochemical Research Department, Toxicological Section,
submitted to WHO by Sandoz Ltd.
Carpy, S. and Klotzsche, C. San 197 I. 2-Week dermal study in rabbits
intact skin. Unpublished report no. Agro Dok CBK 1727/75, dd. 15 April
1975, from Sandoz Ltd. Agrochemical Research Department, Toxicological
Section, submitted to WHO by Sandoz Ltd.
Carpy, S. and Klotzsche, C. San 197 I. 2-Week dermal study in rabbits,
abraded skin. Unpublished report no. Agro Dok CBK 1728/75, dd. 30 May
1975, from Sandoz Ltd. Agrochemical Research Department, Toxicological
Section, submitted to WHO by Sandoz Ltd.
Carpy, S. and Klotzsche, C. San 197 I. 3-Month feeding study in rats.
Unpublished report ref. Agro Dok CBK 1720/75, dd. 18 March 1975, from
Sandoz Ltd. Agrochemical Research Department, Toxicological Section,
submitted to WHO by Sandoz Ltd.
Carpy, S. and Klotzsche, C. San 197 I. 6-Month feeding study in dogs.
Unpublished report ref. Agro Dok CBK 1715/75, dd. 12 February 1975,
from Sandoz Ltd. Agro chemical Research Department, Toxicological
Section, submitted to WHO by Sandoz Ltd.
Carpy S. and Klotzsche, C. Etrimfos, 2-Year feeding study in rats.
Unpublished report no. 25/76 ref. Agro Dok CBK 1869/76, dd. 19 August
1976, from Sandoz Ltd. Agrochemical Research Department, Toxicological
Section, submitted to WHO by Sandoz Ltd.
Carpy S. and Klotzsche, C. SAN 197 I. 2-Year feeding study in dogs.
Unpublished report no. 41/77 ref. Agro Dok CBK 2568/77, dd. 3 August
1977, from Sandoz Ltd., Agro chemical Research Department,
Toxicological Section, submitted to WHO by Sandoz Ltd.
Carpy S. and Klotzsche C. San 197 I. 3-Generation study in rats.
Reproduction and teratogenicity data. Unpublished report ref. Agro Dok
CBK 3835/79, dd. 26 February 1979, from Sandoz Ltd., Agrochemical
Research Department, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Acute toxicity tests.
Unpublished report ref. Agro Dok CBK 754c/73, dd. 10 March 1975, from
Sandoz Ltd., Agrochemical Research Department, Toxicological Section,
submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Primary skin and eye
irritation tests in rabbits. Unpublished report ref. Agro Dok CBK
1725/75, dd. 18 March 1975, from Sandoz Ltd., Agrochemical Research
Department, Toxicological Section, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Neurotoxicity in chickens.
(With addendum for histopathological investigations for ischias
nerves.) Unpublished report ref. Agro Dok CBK 1852/75, dd. 18 March
1975, from Sandoz Ltd., Agrochemical Research Department,
Toxicological Section, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. Acute oral LD50 in rats and mice
(2-ethyl-4-ethoxy-6-hydroxy-pyrimidine). Unpublished report ref. Agro
Dok CBK 1777/75, dd. 18 March 1975, from Sandoz Ltd., Agrochemical
Research Department, Toxicological Section, submitted to WHO by Sandoz
Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Acute intramuscular LD50
in rats. Unpublished report ref. Agro Dok CBK 2518/77, dd. 21 February
1977, from Sandoz Ltd., Agrochemical Research Department,
Toxicological Section, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Sensitization test in
guinea pigs. Unpublished report ref. Agro Dok CBK 2519/77, dd. 22
February 1977, from Sandoz Ltd., Agrochemical Research Department,
Toxicological Section, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I (P=O analogue). Acute oral
and dermal LD50 in rats. Unpublished report, ref. Agro Dok CBK
2551/77, dd. 24 February 1977, from Sandoz Ltd., Agrochemical Research
Department, Toxicological Section, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Antitode test in rats.
Unpublished report, ref. Agro Dok CBK 3126, dd. 11 April 1978 from
Sandoz Ltd., Agrochemical Research Department, Toxicological Section,
submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Teratogenicity test in
rabbits. Unpublished report, ref. Agro Dok CBK 3132/78, dd. 22 June
1978 from Sandoz Ltd., Agrochemical Research Department, Toxicological
Section, submitted to WHO by Sandoz Ltd.
Ioannou, U.M. and Dauterman, W.C. In vitro metabolism of etrimfos by
rat and mouse liver. Pesticide Biochemistry and Physiology 9, 190-195.
Jucker, O. and Riggenbach, A. Etrimfos. Composition of technical
active ingredient. Unpublished report ref. Agro Dok CBK 3572/79e, dd.
1 November 1979, from Sandoz Ltd., Agro Development, Registration
submitted to WHO by Sandoz Ltd.
Karapally, J.C. Absorption, blood level, distribution and excretion of
SAN I 197 in the rat following a single oral dose. Unpublished report
ref. Agro Dok CBK 1829/75, dd. 21 April 1975, from Sandoz Ltd.,
submitted to WHO by Sandoz Ltd.
Karapally, J.C. Metabolism of 14C-Etrimfos in the rat. Unpublished
report, ref. Agro Dok CBK 3005, dd. 10 May 1977, from Sandoz Ltd.,
submitted to WHO by Sandoz Ltd.
Leuschner, F. Mikronucleus-test am Knockenmark von chinesischen
Hamstern (Test-präparat: Etrimfos). Unpublished report ref. Agro Dok
CBK 3136/78, dd. 4 July 1978, from Laboratorium für Pharmakologie und
Toxikologie, Hamburg submitted to WHO by Sandoz Ltd.
Richold, M. and Richardson, J.C. Motaphase analysis on SAN 197.
Unpublished report ref. CBK I 4798/80, dd. 24 April 1980, from
Huntingdon Research Centre submitted to WHO by Sandoz Ltd.
TOXICOLOGICAL STUDIES
Special studies on antidotes
Treatment of rats with atropine and obidoxime reduced the mortality
after oral administration of 3600 mg etrimfos/kg bw (Hamburger and
Klotzsche, 1978a).
Special studies on mutagenicity
Rat
Groups of 5 male and 5 female rats were administered, by intragastric
intubation 1500, 3000 or 6000 mg etrimfos/kg bw in two equal dosages
separated by an interval of 24 hours. A negative control group
received 1% methylcellulose, whereas a positive control group was
dosed by 2 i.p. injections with 8 mg Mitomycin c/kg bw.
After administration of etrimfos, the aberrant metaphase counts were
comparable with the control values, whereas Mitomycin C produced the
expected increase aberrant counts in the bone marrow cells of the
femurs (Richold and Richardson, 1980).
Hamster
Groups of 6 female Chinese hamsters were given s.c. injections of 30,
100 or 300 mg etrimfos/kg bw twice within 24 hours. MMS (100 mg/kg,
orally) was used as a positive control. Etrimfos did not elicit a
mutagenic response whereas the number of bone marrow micronuclei was
increased in the MMS-treated hamsters (Leuschner, 1978).
Microorganisms
Etrimfos (97%) in dosages from 0.001 up to 5.0 l/plate was not
mutagenic in the Ames test using the Salmonella typhimurium
strains TA-98, TA-100, TA-1535, TA-1537 and TA-1538 and
Saccharomyces cervisiae D-4, both with and without activation by
rat liver homogenate (Brusick and Weir, 1976).
Special studies on reproduction
Rat
Groups of 30 males and 30 females were fed dietary concentrations of
0, 3, 9 and 27 mg/kg in a three-generation, two-litter generation
reproduction study. In the B-generations one day before birth 5
pregnant females were killed and investigated for teratogenic
symptoms. The offspring of 5 other females were x-rayed and their
skeletons examined for abnormalities. After 21 days the tissues of 10
male and 10 female weanlings of the III B generation per dose group
were inspected histopathologically. The body weight gain and food
intake of the females were not affected by the treatment. No effects
were observed on fertility, gestation, viability, lactation, the
number of dead foetuses and the pup weight. Teratogenic events were
not observed over the course of the study. At the highest dose level
cholinesterase activity in plasma and erythrocytes, which was
determined in the weanlings of the B generations of all groups, was
slightly depressed, especially in the female rats. Brain
cholinesterase activity was not affected. Gross and microscopic
examination of tissues of organs of the F III B generation showed no
changes attributable to etrimfos administration (Carpy and Klotzsche,
1979).
Special studies on teratogenicity
Rabbits
Groups of 10 rabbits (12 in the control and in the low dose group)
were given etrimfos orally from day 6 to day 18 of pregnancy at dose
levels of 0, 25, 50 or 100 mg/kg bw. On day 29 of gestation the
animals were sacrificed and a caesarean section was performed. The
uterus was examined and the number, location and distribution of the
foetuses, implantations and resorptions recorded. Corpora lutea were
counted. The foetuses were weighed and examined for external and
skeletal anomalies. In the 50 and 100 mg groups the numbers of
implantations and living foetuses were decreased, whereas the number
of dead foetuses and weight of the placenta were slightly increased.
In the low dose group 2 animals with slightly curved hind legs and in
the highest dose group, in which 43 animals were investigated, one
animal with a rudimental tail and one animal with a skull deformation
were observed. In a historical control group of 286 animals 2 rabbits
with skull deformations were observed (Hamburger and Klotzsche,
1978b).
Special studies on neurotoxicity
Hen
Groups of 5-20 chickens were administered etrimfos in gelatine
capsules at dose levels of 100, 200, 400, 500, 750 or 1000 mg/kg bw.
A positive control group was given 2 × 80 mg tri-o-cresyl phosphate
(TOCP)/kg bw. Before the treatment the animals received atropine or
obidoxime by i.v, or i.m. injection. The observation period varied
from 21-42 days. Mortality was increased in all treated groups.
Doses of 200 mg/kg etrimfos and above caused typical signs of
organophosphate poisoning. A delayed neurotoxic response was not
observed in the chickens treated with etrimfos. TOCP elicited ataxia
and paralysis in all 10 animals. Histopathologically typical
degeneration changes in the ischias nerve were observed in all
TOCP-treated animals. No clear treatment-related alterations were
found in the nerves of the hens given 100 and 200 mg/kg etrimfos
(Hamburger and Klotzsche, 1975d).
Special studies on sensitization
Guinea pig
According to the "Guinea pig maximisation test" 20 guinea pigs were
treated with SAN 197 I as a 5% solution in DMSO and incorporated in
Freund's adjuvant; 10 guinea pigs served as a control group. 5/20 of
the treated and 4/10 of the control animals died before the 22nd day.
A slight development of scales at the application side without any
inflammatory changes or oedema was noticed at 72 hours after the
challenge application; no other effects were noticed (Hamburger and
Klotzsche, 1977b).
Acute toxicity
Symptoms of intoxication in rats and mice after oral, i.p. and s.c.
administration were limpness, lacrimation, salivation, exophthalmus,
laboured respiration, ataxia, tremors and convulsions. After dermal
application to rats and rabbits no toxic symptoms were observed
(Hamburger and Klotzsche, 1975a; Anonymous, 1979a and Anonymous,
1979b).
Rabbit
Etrimfos (0.5 g) applied in the intact or abraded skin of rabbits was
found to be not irritating. Instilled into the eye (100 mg) etrimfos
produced slight redness of the conjunctivae, which lasted 24 hours, in
one of six rabbits (Hamburger and Klotzsche, 1975b).
TABLE 1. Acute toxicity of etrimfos
species sex route LD50 in mg/kg bw purity solvent references
mouse M oral 47O (425-523) 97.2% water Hamburger and Klotzsche, 1975a
F oral 620 ± 28 97.2% water " " "
M oral 1120 (1023-1226) 90.5% corn-oil Anonymous, 1979a
F oral 1100 (930-1289) 90.5% corn-oil " "
M oral 535 (24 hours) - corn-oil Ioannou and Dauterman, 1978
M i.p. 698 (597-817) 90.5% corn-oil Anonymous, 1979a
F i.p. 679 (585-788) 90.5% corn-oil " "
M s.c. 979 (729-1155) 90.5% corn-oil " "
F s.c. 1050 (897-1229) 90.5% corn-oil " "
rat M oral 1800 (765-3733) 97.2% water Hamburger and Klotzsche, 1975a
F oral 2354 (1841-3008)1 97.2% water " " "
M oral 1930 (1664-2239) 90.5% corn-oil Anonymous, 1979b
F oral 1970 (1669-2325) 90.5% corn-oil " "
M oral 2040 (24 hours) - corn-oil Ioannou and Dauterman, 1978
M i.p. 767 (684-873) 97.2% water Hamburger and Klotzsche, 1975a
M i.p. 1310 (1110-1546) 90.5% corn-oil Anonymous, 1979b
F i.p. 1140 (950-1368) 90.5% corn-oil " "
M i.v. 25.5 ± 1.26 97.2% water Hamburger and Klotzsche, 1975a
F i.v. 21.0 ± 1.88 97.2% water " " "
M i.m. 4000 ± 116 94.3% PEG 200 Hamburger and Klotzsche, 1977a
M x.c. 3700 (3710-4563) 90.5% corn-oil Anonymous, 1979a
F s.c. 3690 (3000-4539) 95.5% corn-oil Anonymous, 1979b
dermal >2000 97.2% water Hamburger and Klotzsche, 1975a
M dermal >5000 90.5% corn-oil Anonymous, 1979b
F dermal >5000 90.5% corn-oil " "
rabbit dermal >500 97.2% water Hamburger and Klotzsche, 1975a
1 value corrected by recalculation
TABLE 2. Acute toxicity of metabolites
Species sex route solvent LD50 (mg/kg) references
rat M oral DMSO 1460 ± 52.5 Hamburger and Klotzsche, 1975e
rat F oral DMSO 1170 ± 58.5 " " "
mouse M oral DMSO 1530 ± 87.9 " " "
mouse F oral DMSO 1170 ± 94.8 " " "
P = O analogue of the parent compound
species sex route solvent LD50 (mg/kg) references
rat M oral DMSO 450 ± 35 Hamburger and Klotzsche, 1977c
rat M dermal DMSO 1000 " " "
Short-term studies
Rat
Male and female rats (15/sex/group) were dietary fed 0, 50, 250 or
1250 mg/kg SAN 197 I (97.2%) in the food for 4 weeks; later on in the
test groups (5/sex) on 10 mg/kg with corresponding controls were
added. The animals were examined for general health, body weight,
food consumption, haematology, blood biochemistry, urinalysis, organ
weights and macroscopy. A decreased cholinesterase activity in
erythrocytes was observed at 50 mg/kg and higher doses. Plasma-ChE
was decreased in females from the 50 mg/kg and in males from the 250
mg/kg groups, and brain-ChE in females from the 250 mg/kg and in males
at the 1250 mg/kg groups. Normochromic anaemia was noticed at 250
mg/kg and in females at 1250 mg/kg, changes in blood sugar occurred at
all dosages except 250 mg/kg and changes in reticulocytes at 1250
mg/kg. At these dosage the animals showed slight sedation and
slightly decreased body-weight gain as result of lower food intake
(Carpy and Klotzsche, 1975a).
Rat
Groups of 35 males and 35 females were fed etrimfos at concentrations
of 0, 3, 9 or 27 mg/kg feed for a period of 13 weeks. Body-weight
gain and food intake was recorded throughout the study of 25 females
and 25 males per group. After 4, 8 and 13 weeks haematology clinical
chemistry and urinanalysis was carried out in 10 animals/group. The
same number of rats was studied histopathologically and their organ
weights were recorded. At the end of the study the glucose
concentration in serum was increased in both males and females of the
highest dose level. The weight of the thyroid of the male rats of the
highest dose group was significantly decreased. However microscopic
examination showed no abnormalities attributable to the presence of
etrimfos in the diet. After 8 and 13 weeks the plasma cholinesterase
activity of the female rats in the 9 mg/kg group was slightly (20-24%)
and clearly depressed in the 27 mg/kg group (30-37%). Cholinesterase
activity in erythrocytes and brain was within normal limits (Carpy and
Klotzsche, 1975d).
Rabbit
Male and female rabbits (5/sex/group) received a dermal application of
0, 25 or 100 mg/kg SAN 197 I (97.2%) dissolved in polyethylene-glycol
on patches of intact or abraded skin for 8 hours a day on 5 days a
week during 2 weeks and were examined for general health, body weight,
haematology, blood biochemistry, organ weights and macroscopic changes
(microscopic studies are not yet completed). One male with intact
skin out of the 100 mg/kg group died with an enlarged liver. In the
males the weight of the spleen was decreased and the weight of the
thymus increased while in females a decreased weight of the thymus was
noticed. The animals with abraded skin showed abnormalities only at
the high dosage. Increased organ weights were observed in the female
liver and heart and in the males in the prostate. All these changes
were within the normal range. The animals with intact skin showed, at
both dosages, cholinesterase-inhibition in plasma and in erythrocytes.
In the animals with abraded skin, only in the high dosage, in males as
well as in the females, was cholinesterase inhibited in plasma and
erythrocytes (Carpy and Klotsche, 1975b and c).
Four groups of 4 male and 4 female beagle dogs were fed O, 2.5, 10 or
40 mg/kg etrimfos (97.2% purity) in the food for 26 weeks.
Normal values in haematology, blood chemistry, and urinalysis were
evaluated before starting the study. These determinations were
repeated 6 times during the test period: after 1, 4, 8, 13, 19 and 26
weeks, with exception of BSP which was estimated after 4, 13, and 26
weeks. The physical examination included weekly body weight, food
intake and neurological, oral, behaviourial, and ophthalmoscopic
inspection. The haematological parameters investigated were: RBC,
WBC, differential WBC, MCV, MCH, MCHC, reticulocytes, haemoglobin,
haematocrit, platelets, and prothrombin time, RBC- and
plasma-cholinesterase, FBS, BUN, SGPT, SGOT, LDH, uric acid, total
serum protein, bilirubin, creatinine, serum alkaline phosphatase,
cholesterol, serum sodium, potassium, calcium, chloride, inorganic
phosphate, serum albumin, and serum electrophoresis (A/G ratio). The
activity of cholinesterase in cerebellum and cerebrum, of the liver
cytochrome P-450, of the liver drug metabolising enzymes O-, and
S-demethylases and of the anilin-4-hydroxylase and the amount of
cholesterol, glycogen, lipids, and total protein contained in the
liver were estimated at the end of the study. All animals were
examined for gross pathology, organ weights and histopathology. MCH
and MCHC values were increased in the male animals of the 40 mg/kg
group. From the 11th week on plasma and erythrocyte cholinesterase
activity was depressed in comparison to the pretest and the control
values, with a plateau of about 40% in both males and females. In the
10 mg/kg group a slight depression of cholinesterase activity in
plasma and erythrocytes especially in comparison to the pre-test
values was observed in both sexes. In the cerebrum of the female rat
of the highest dose group a decrease of 22% of the cholinesterase
activity was measured.
From the 13th week on the albumin concentration in serum was
dose-dependant increased. However this was not confirmed by
electrophoresis and had disappeared almost at the end of the study.
The weight of the organs was within normal limits. No gross or
histopathological aberrations were observed (Klotzsche and Carpy,
1975e).
Long-term studies
Rat
Groups of 40 males and 40 females were fed etrimfos (97.8%) in the
diet at concentrations of 0, 6, 12 or 24 mg/kg for a period of 2
years. Body weight, food consumption and water intake of 30 rats per
dose and sex were recorded weekly. After 1, 2 ,3, 6, 9, 12, 15, 18,
21 and 24 months haematology, clinical chemistry and urinalysis were
carried out in 5 male and 5 female animals per group. At the end of
the study BSP a liver function test was carried out in the control and
high-dosed females. In liver homogenates the activity of drug
metabolising enzymes and the total protein and cholesterol
concentration were measured at the end of the study.
All animals dying during the study, or which had to be killed, were
necropsied and tissues were examined microscopically. The organ
weights of 10 rats per dose and sex were recorded.
There was no clear effect of etrimfos on body weight, food and water
intake and mortality. The cholinesterase activity of the plasma and
erythrocytes of male rats was not affected by etrimfos. In the
females the activity of cholinesterase in the plasma of the 24 and 12
mg/kg group was decreased by 35-50 and 20-30% respectively throughout
the study. In the highest dose group a marginal effect was observed
on the activity in the erythrocytes of the female rats. Neither in
the females nor in the males was the cholinesterase activity of the
brain and the liver of the treated animals significantly different
from the control values.
Both in the male and female rats the cytochrome P-450 of the liver of
the animals of the 12 and 24 mg/kg group was significantly decreased
in comparison to the controls; however no dose-response relation was
observed.
The macroscopic and microscopic examination did not reveal organ
lesions that could be attributed to treatment. The tumour rate in
treated animals corresponded with the incidence in control animals.
No-effect level of this study is 6 mg/kg (Carpy and Klotzsche, 1976).
Dog
Groups of 4 male and 4 female beagle dogs received 0, 4, 10 or 25
mg/kg etrimfos in their diet, during 106 weeks. There were no signs
of adverse behaviour and mortality was not noted. Growth and food
consumption were not clearly affected. Eye examinations during the
first, 26th, 52nd, 78th and 104th week did not indicate adverse ocular
changes.
Haematology, clinical chemistry and urinalysis were carried out in
week 1 (pre-test), 4, 8, 13 and quarterly thereafter. Plasma
cholinesterase activity was decreased in the 10 and 25 mg/kg groups by
23% and 24% respectively. The cholinesterase activity of the
erythrocytes was also decreased.
No clear differences or sensitivity were seen between males and
females. In the cerebrum, cerebellum and liver the cholinesterase
activity was not depressed.
Slightly higher activities of N-, O- and S-demethylase activity were
measured in all treated groups. However there was no clear
dose-response relation. Neither the weights of the organs nor the
histopathological evaluation revealed indications for treatment-
related changes in any organ or tissue. No-effect level of this study
is 10 mg/kg (Carpy and Klotzsche, 1977).
RESIDUES IN FOOD
RESIDUES RESULTING FROM SUPERVISED TRIALS
Stored grain products
Wheat grain (variety Probus) was treated in 25 kg lots with
Satisfar(R) (Ekamet 50 EC) at the rate of 5, 10 or 15 mg/kg ai under
conditions as close to normal practice as possible. The grain, which
was stored in fibre-drums at room temperature, was analysed
periodically for etrimfos.
In this small-scale trial the etrimfos content of the wheat grain
immediately after treatment (zero time) was 50 - 60 percent of the
application rate. During the first six months of storage, the amount
of etrimfos in the grain remained fairly constant.
In the next six months its amount was reduced to 34-37% of the
application rate, (or 63-75% of the original concentration in the
grain). At the end of 12 months, the grain treated at the rate of 10
and 15 mg/kg had 100% efficacy against Sitophilus; the grain treated
at the rate of 5 mg/kg had 90% activity. (CBK 3630/79).
Several trials were conducted in different countries on wheat, barley
and corn (table 3).
FATE OF RESIDUES
In plants
The fate of etrimfos in bean and corn plants has been studied (M.
Akram et al., 1978). The primary leaves of bean and corn seedlings
were treated with 14C etrimfos. The treated leaves and the untreated
portions of the plants were sampled at 0, 3, 7, 14 and 21 days after
treatment. The aqueous rinses and the extracts of the treated leaves
were examined for the parent compound and its metabolites. Leaf
remainder, and untreated portions, were analysed for total
radiocarbon.
Etrimfos rapidly volatilised from the leaf surface. Within the first
three days after treatment it is lost with a half-life of approx. 3
days (beans and corn) and during 3 to 15 days with a half-life of
approx. 10 days from beans and approximately 5 days from corn. Three
weeks after treatment the radioactivity, retained by the leaves after
rinsing, ranged from 33% to 42% for bean, and from 25% to 42% for
corn. No translocation to the root system was observed. A small
percentage of the applied radiocarbon was present in the untreated
foliage of bean (0.3-0.6% of the applied radioactivity became
unextractable in bean leaves, and 0.1-0.3% in corn leaves).
Leaf rinses (surface residues) of bean and corn contained mainly
etrimfos and small quantities of 6-ethoxy-2-ethyl-4-hydroxy-pyrimidine
(EEHP). Small amounts of the P=O analogue of etrimfos were also
observed in the corn-rinse.
The leaf extracts (subsurface residues) of bean and corn contained
etrimfos and minor quantities of EEHP and five unknown metabolites, of
which one was tentatively identified as
2-ethyl-4,6-dihydroxy-pyrimidine; 21-day bean leaf contained 33.5% of
the applied radioactivity, of which 11.9% was etrimfos, 2.4% EEHP,
9.7% the unknown metabolites and 9.5% highly polar or conjugated
metabolites (TLC origin). All single metabolites were less than 10%
relative to applied etrimfos; 21-day corn leaf had about 24.3% of the
applied radioactivity, of which 3.8% was etrimfos, 0.4% EEHP, 15.8%
the unknown metabolites and 4.3% polar or conjugated metabolites. All
single metabolites were less than 10% relative to applied etrimfos.
The enzyme and acid-hydrolysed aqueous extracts gave essentially the
same TLC pattern as the unhydrolysed material, with quantitative
differences.
From the described results, we learn that etrimfos is lost from
treated plants in two stages. EEHP was found as a metabolite, but
only in quantities of less than 10% of the applied etrimfos. The P=O
analogue was not found in bean leaves nor in corn leaves. No single
metabolite was present in quantities more than 10% relative to the
applied etrimfos so that the influence of these metabolites on the
toxicity of actual residue levels in practical field samples may be
neglected. Tolerance recommendations may, therefore, be based on
residue data of the etrimfos ai only.
In cereal products
Wheat grain (variety, Probus) was treated in 25 kg lots with
Satisfar(R) (Ekamet 50 EC) at the rate of 5, 10 or 15 mg/kg ai. The
grain was stored at room temperature and at intervals of 2, 4, 6 and
12 months, samples of grain were milled and separated into different
fractions. Also bread was baked from the flour from these samples.
Etrimfos residues were analysed in all these samples.
Etrimfos concentration in the milled fractions of the wheat was the
highest in the bran and the lowest in the white flour. During the
baking process the etrimfos content of the flour was reduced by 58-77%
in the case of white bread, and 43-65% in the case of whole wheat
bread. The etrimfos content of white bread ranged from about 4 to 6%
of the amount applied and that in the whole wheat bread was in the
range of 14 to 30% of the amount applied (CBK 3630/79). Table 4.
In another trial with wheat, the amount of etrimfos in the white flour
ranged from 25 to 33% of that actually present in the grain. Baking
to bread reduced these residues in the flour by about 50% (CBK
4119/79). Table 5.
In a trial with barley, the wort from free grain contained only 4-6%
of the etrimfos originally present in the grain. The wort from malt
grain had no detectable residue of etrimfos (CBK 4120/79). Table 5.
TABLE 3. Residues of etrimfos (mg/kg) in stored products treated after harvest
Application Storage period (months)
Commodity Rate 0 1-2 3-4 6 8-10 12-13 18-24 Remarks Ref.
(country) Type (mg/kg) (24 h)
Cereal grain
Barley
(England) 50 EC 5 2.3 3.2-3.6 2.1 0.8 amount treated:
content: 15%; temp.: CBK
0-15°C; untreated 4121/79
"blank": 0.03-0.04
mg/kg
(Kenya) 0.5 Dust 30 2.25 2.1 0.6 0.3 amount treated:
1 Dust 29 4.5 3.0 1.3 0.8 45 kg each, UK
50 EC 5 1.3 0.5 0.2 stored in bags; 3770/79
2 Dust 22 9 9.7 1.2-2.2 0.8-1.4 conditions 4149/79
50 EC 10 4.1 1.0-2.1 0.5-0.9 not reported
Corn(maize)
(Kenya) 0.5 Dust 30 2.75 0.6-0.8 0.5 0.3-0.4 0.3-0.4 - amount treated:
50 EC 5 1.5-1.6 1.6-1.7 0.8-1.2 1.0-1.1 0.8 45 kg each,
1 Dust 29 5.5 0.9-2.6 0.7-1.5 1.0-1.6 0.8-0.9 0.9-1.3 stored in bags;
50 EC 10 0.8-4.2 1.7-2.9 1.5-2.5 1.9-2.5 2.5-2.6 storage conditions
2 Dust 22 11 1.7-5.5 2.4-3.8 2.2-2.9 1.2-2.4 1.5-2.3 not reported
Wheat
(England) 50 EC 5 6.8 5.6-6.1 5.9 3.9 amount treated:
50 EC 10 10.9 6.7-7.2 5.4 6.4 10 t; 30 t on
conveyer belt; CBK
moisture cont. 11%; 4118/79
0-17°C; untreated
"blank": 0.01-1 mg/kg
TABLE 3. Continued...
Application Storage period (months)
Commodity Rate 0 1-2 3-4 6 8-10 12-13 18-24 Remarks Ref.
(country) Type (mg/kg) (24 h)
(France) 50 EC 5 1.9-2.0 1.4-2.0 1.6 storage conditions not
50 EC 7.5 1.6-2.5 2.1-3.3 2.1-2.9 reported; samples for CBK
50 EC 10 3.3-5.4 4.2 2.6-3.8 analysis: 10 g each 2943/79
(Kenya) 0.5 Dust 30 2.5 1.2 0.7 1.0 0.8 0.4 amount treated:
1 Dust 29 5 2.0 1.8 1.5 1.3 1.0 10 kg each, stored CBK
50 EC 5 1.6 1.0 1.0 0.8 0.5 in bags; storage 3771/79
2 Dust 22 10 3.7 2.8 2.7 2.1 1.8 temp. about 15-25°C; 4117/79
50 EC 10 2.0 2.5 2.6 2.5 1.7 further conditions 4148/79
not reported.
(Switzerland) 50 EC 5 2.9 2.9 2.7 3.0 1.9 1.1 0.6 amount treated: 25 kg
(<0.1) (<0.1) (<0.1) (0.2) (0.4) (0.2) (<0.1) each, stored in air
50 EC 10 5.4 5.8 5.5 5.6 3.4 1.7 1.3 permeable fibre-drums;
(0.1) (0.5) (0.1) (0.3) (1.2) (0.2) (<0.1) moisture content: 12-14%; CBK
50 EC 15 7.4 7.8 7.6 8.1 5.6 temp about 16-20°C; 3630/79
(0.1) (0.2) (0.2) (0.5) (1.5) EEHP residues reported 3707/79
in brackets; EPO 4209/80
residues were always
below 0.002 mg/kg.
Rape seed
(England) EC 10 9-10 8-9 9-10 7-8 :Bin 2 amount treated: Stables
EC 10 11-15 10-15 13-14 13-14 10 t each, stored et al.,
:Bin 3 in metal bins; (1979)
moisture content in seed:
9-11%; temp. 3-15°C; seed
in bin 2 was slightly damper and
more heavily infested by mites
than in bin 3. Etrimfos in rape
seed oil (refined) up to about
0.5 mg/kg/ in spent meal: 0.05 mg/kg.
TABLE 4: Etrimfos1 residues in milled fractions and flour of wheat and in bread
Treatment Residues (mg/kg) 2-12 months after treatment
rate 2 4 6 12
5 mg/kg Etrimfos EEHP2 Etrimfos EEHP Etrimfos EEHP Etrimfos EEHP
Grain 2.92 n.d.3 2.74 n.d. 3.02 0.20 1.89 0.36
Bran 10.47 1.10 9.94 0.39 9.78 0.38 3.50 2.10
Grits 8.67 0.62 8.64 0.62 6.83 0.27 3.48 1.02
White flour 0.82 n.d. 1.02 n.d. 0.73 n.d. 0.70 n.d.
White bread 0.23 0.05 0.24 0.10 0.21 0.11 0.18 n.d.
Whole wheat
flour n.a.4 n.a. 2.66 0.16 2.33 0.11 1.97 0.37
Whole wheat
bread n.a n.a. 1.52 0.22 1.33 0.34 0.69 0.23
10 mg/kg Etrimfos EEHP2 Etrimfos EEHP Etrimfos EEHP Etrimfos EEHP
Grain 5.84 0.54 5.50 0.08 5.59 0.34 3.38 1.20
Bran 15.22 1.92 16.27 0.66 15.83 1.14 6.20 3.34
Grits 41.29 1.27 15.08 0.73 13.14 1.06 7.74 1.97
White flour 1.89 0.14 1.75 n.d. 1.47 0.06 0.96 n.d.
White bread 0.50 0.13 0.61 0.24 0.51 0.20 0.41 n.d.
Whole wheat
flour n.a. n.a. 5.59 0.39 5.07 0.24 3.70 0.69
Whole wheat
bread n.a. n.a. 2.83 0.60 2.38 0.51 1.37 0.51
TABLE 4. Continued...
Treatment Residues (mg/kg) 2-12 months after treatment
rate 2 4 6 12
15 mg/kg Etrimfos EEHP2 Etrimfos EEHP Etrimfos EEHP Etrimfos EEHP
Grain 7.80 0.20 7.61 0.20 8.11 0.46 5.57 1.52
Bran 16.60 1.50 21.65 1.56 19.70 1.51 9.66 4.34
Grits 19.41 1.53 20.84 0.93 19.33 1.06 12.14 1.97
White flour 2.04 0.25 2.62 0.09 2.48 0.10 1.50 n.d.
White bread 0.74 0.18 0.95 0.49 0.78 0.31 0.54 0.23
Whole wheat
flour n.a. n.a. 7.99 0.41 7.84 0.40 5.90 0.71
Whole wheat
bread n.a. n.a. 3.80 0.40 3.77 0.70 2.30 0.46
1 The metabolite P=O etrimfos could not be detected
2 EEHP = 2-ethyl-4-ethoxy-5-hydroxy-pirimidine
3 n.d = not detectable
4 n.a. = not analysed
TABLE 5. Etrimfos residues in cereals processed after treatment (in UK) with SATISFAR(R)
Cereal Formulation Rate of Etrimfos Amount of Method of treatment Reference
treatment residues grain per
(ai/mg/kg) (ppm) treatment
Wheat 50 EC 5 mg/kg grain 0.76 6 kg The required amount of CBK
bran 4.62 Satisfar in 25 ml water 4119/79
offal 3.13 was sprayed onto grain
white flour 0.25 tumbling in a
white bread 0.12 Bluefinsowaway cement mixer.
10 mg/kg grain 2.51 Stored in sealed plastic
bran 4.50 bags.
offal 6.14
white flour 0.63
white bread 0.34
Barley 50 EC 5 mg/kg free grain 1.07 CBK 4120/79
wort 0.07
spent 0.63
malt grain 0.21
wort n.d.
spent gr 0.07
10 mg/kg free grain 3.50
wort 0.16
spent gr 0.52
malt grain -
wort n.d.
spent gr 0.11
In cattle
A three level feeding study in dairy cattle with etrimfos incorporated
in the diet at levels 0.5, 1.5 or 5 mg/kg etrimfos was conducted. The
milk production was not affected. The cows, sacrificed after 28 days
of feeding, had only less than 0.01 mg/kg etrimfos in the muscle,
liver, kidney and fat. Etrimfos residue in the milk throughout the
study was less than 0.01 mg/kg. The residue in the milk solids
separated from the 21st and 28th day samples also had only 0.01 mg/kg
etrimfos (CBK 3011/77).
In soil
Degradation of 14C etrimfos in a sandy loam, silt loam and silty clay
loam soil was studied.
The soils treated with 14C-etrimfos at the rate of 5 mg/kg were
incubated at room temperature for 70 days. At selected time intervals
during the incubation period, the soil samples were analysed for the
parent and degradation products.
Etrimfos was rapidly hydrolysed, in all soils, to
6-ethoxy-2-ethyl-4-hydroxy-pyrimidine (EEHP). Its amount was reduced
to 50% in 3-8 days, and to 10% in 15-30 days.
The formation of EEHP paralleled the dissipation of etrimfos. Its
proportions reached a maximum of 75-85% in 14-28 days, after which
period its concentration began to decrease. The disappearance of EEHP
was fastest in silt, and rather slower in sand, and still more so in
clay. At the end of 70 days, the amounts of EEHP in silt, sand and
clay were 4, 18 and 52% respectively.
A further metabolite, tentatively identified as
5-chloro-6-ethoxy-2-ethyl-4-hydroxy-pyrimidine (CEEHP), was detected
in all soil extracts. Its formation seemed to be related to the
dissipation of EEHP, and its occurrence was highest in silt (24%),
followed by sand and clay (6%).
The soil-"bound" residues became significant only after EEHP began to
be metabolised. A maximum of 12% of these was observed for sand, and
21% for silt and clay. Further extraction with methanol-water 75:25
reduced the bound residue to <10%, the metabolites being mainly EEHP
and CEEHP.
The radiocarbon lost from the soil during the 70 days' incubation
amounted to 60% for sand or silt and 25% for clay. In a separate
experiment, using sandy loom soil treated with 14C-etrimfos, it was
demonstrated that the radioactivity lost from the soil was due to the
14CO2, which began to be generated after a time lag of at least 20
days (CBK 3002/77).
Photodegradation
in water
Under blacklight and daylight, which contain essentially no shorter
wave lengths than natural sunlight, aqueous solution of etrimfos (5
mg/kg) was quite stable: degradations of 10% (blacklight) and 5%
(daylight) occurred within 24 hours of exposure. Under mercury arc
light, approx. 45% degraded during the same time (CBK 1642/75).
on glass plates
2.5 mg of etrimfos, spread over an area of 70 cm2 and covered with
quartz plates, was exposed to the same light sources as in water.
Degradation, after 24 hours under the following conditions, was
daylight <5%, blacklight <5%, mercury light ‰ 25%, (CBK 1642/75).
METHODS OF RESIDUE ANALYSIS
Two methods have been developed - one for determining etrimfos the P=O
analogue and 2-ethyl-4-ethoxy-6-hydroxypyrimidine (EEHP), the
hydrolysis product of etrimfos (CBK 2178/76) and the other for
determining etrimfos only (CBK 3649/79). In both methods grain is
dealt with in the same manner as vegetable crops.
The P=O analogue of etrimfos has been detected in the stored grain
products. The amount of EEHP in the grain is generally quite low and
hence, in these cases, the method CBK 3649/79 is recommended.
In these methods etrimfos residues are extracted with acetone and
partially cleaned up by partitioning between water and methylene
dichloride. If only etrimfos is to be determined, the methylene
dichloride solution is further cleaned up on a silica gel column and
determined by gas chromatography using a flame photometric detector
(P-mode). If the P=O analogue and EEHP are to be included, the
methylene dichloride solution is further cleaned up on a
polyethylene-coated alumina column and determined by gas
chromatography using a nitrogen specific detector.
A series of CBA reports on residues in food and fats in the
environment were submitted by Sandoz Ltd. to FAO for this evaluation.
EVALUATION
Etrimfos is a broad-range non-systemic contact and stomach
organophosphorous insecticide, which was introduced in 1972 and is
registered and/or used in a considerable number of countries. It is
effective against biting and sucking insects such as Lepidoptera,
Coleoptera, Diptera and to a variable extent, Hemiptera at 0.25-0.75
kg ai/ha. It is mainly used on fruit, vegetables, maize and
ornamental plants, but also on alfalfa and paddy rice, as an
emulsifiable concentrate or as granules.
Studies of the metabolism and distribution of etrimfos in rats have
been undertaken with 14C in the pyrimidine moiety. This has provided
useful information on the pyrimidine portion of the molecule, but
disposition and kinetic data on the whole molecule of etrimfos and its
triester metabolites are required. This is especially important since
the compounds containing a fully-esterified phosphoroticate or
phosphate group are the more toxic.
In rats and rabbit no effects on reproduction, including
teratogenicity, were observed. A mutagenic response was negative as
shown by a rat metaphase analysis, a hamster bone marrow micronuclei
count, and an Ames test.
Etrimfos did not induce a delayed neurotoxic response, as generally
noted with TOCP, in hens. A skin-sensitisation study in guinea pigs
was negative.
Etrimfos and the main metabolite
O-6-ethoxy-2-ethyl-4-hydroxypyrimidine (EEHP) were slightly toxic
following acute administration to various animal species. The effects
of etrimfos were typical of those caused by cholinesterase inhibitors.
Several short- and long-term toxicity studies have been carried out
with rats and dogs. The macroscopic and microscopic examination in
these studies did not reveal lesions in organs attributable to
etrimfos. The tumour incidence in treated animals corresponded with
that in control animals. In both species etrimfos produced
cholinesterase inhibition.
Based on the two-year studies with both rat and dog a temporary ADI
was recommended.
Although it is known that data from supervised trials (pre-harvest
use) exist, which show that residues in many fruits and vegetables,
potatoes, maize and rape are in the range 0.05 to 0.5 mg/kg when
etrimfos is used in accordance with good agricultural practice,
information was not made directly available for consideration by the
meeting.
However, in recent tests and trials carried out in England, France,
Kenya and Switzerland etrimfos was also found efficacious against
moths, (malathion-resistant) beetles and (lindane-resistant) mites in
stored crops, especially grain and rape seed. Recommended rates for
dusting or spraying wheat, barley, maize (corn) or rape seed are 5 to
10 mg/kg. Depending on storage conditions (e.g. degree of
infestation, climate, storage period) lower or, exceptionally, higher
dosages up to 15 mg/kg appear appropriate.
Etrimfos in stored rape seed shows no significant loss after 6 months
storage and the estimated half-life in stored grain is about 3 to 8
months. Etrimfos is rapidly lost from treated plants (beans, maize),
about half within the first 3 days, with a half-life of about 5 to 10
days between the 3rd and the 15th day after application, probably
owing to the different rate of volatilisation of the initial deposit
and of the residue after penetration into the surface layer.
In degradation studies with plants treated with 14C-etrimfos,
labelled at carbons 4 and 6 in the pyrimidinyl ring the metabolites
O-6-ethoxy-2-ethyl-4-hydroxypyrimidine (EEHP) and 2-ethyl-4,
6-dihydroxypyrimidine (EDHP) were identified. Small amounts of the
etrimfos P=O analogue (EPO) were also observed, but only on the plant
surface. In stored grain EEHP was the only metabolite found in
significant quantities.
After a single oral does of 50 mg etrimfos/kg, rats excreted more than
50% as EEHP in urine and faeces, with smaller amounts of EDHP,
6-ethoxy-4-hydroxy-2-(1-hydroxyethyl) pyrimidine (EEHP-1), its
2-(2-hydroxyethyl) pyrimidine analogue (EEHP-2) and
2-ethyl-4-hydroxy-6-(2 hydroxyethoxy) pyrimidine (EHHEP). In rat
liver preparations desmethyl-etrimfos was also detected.
In soils the half-life of etrimfos is about 3 to 8 days: degradation
results in EEHP, EDHP and finally carbon dioxide. General degradation
pathways are thus similar in plants, animals and soils, although
proportions of individual metabolites may vary. Hydrolysis of the
phosphorothioate ester seems to be the most significant pathway,
yielding products of lesser toxicological importance.
When dairy cattle were fed with diets containing 5 mg etrimfos/kg for
28 days, etrimfos residues were always below 0.01 mg/kg in meat, milk,
fat and edible offals.
Processing wheat, treated with 10 mg atrimfos/kg and stored for 2 to
12 months, resulted in etrimfos residues up to 16.3 mg/kg in raw bran,
5.6 mg/kg in wholemeal flour, 2.8 mg/kg in wholemeal bread, 1.9 mg/kg
in white flour and 0.6 mg/kg in white bread. Provisional information
on barley treated with 5 to 10 mg etrimfos/kg and used for malting and
brewing showed etrimfos residues up to 0.6 mg/kg in spent grain and
0.2 mg/kg in wort. When stored rape seed containing 10 to 15 mg
etrimfos/kg was used for oil production, preliminary results indicated
that 95-100% of the etrimfos was lost during the refinement of the oil
and that the greatest looses occurred by degradation during bleaching
and steam distillation. No residues were detected in the spent meal.
Analytical methods for determining residues of etrimfos, EEHP and EPO
have been reported. Residues are extracted with acetone and partially
cleaned up by partitioning between water and dichloromethane. For the
determination of etrimfos as such the dichloromethane solution is
further cleaned up on a silica gel column and analysed by gas
chromatography using a flame photometric detector in the P-mode. The
latter method is suitable for adaptation to regulatory purposes.
Level causing no toxicological effect
Rat: 6 mg/kg in the diet equivalent to 0.3 mg/kg bw/day.
Dog: 10 mg/kg in the diet equivalent to 0.25 mg/kg bw/day.
Estimate of acceptable daily intake for man
0-0.003 mg/kg bw/day.
RECOMMENDATIONS OF RESIDUES LIMITS
The Meeting concludes that the residue levels listed below are
suitable for establishing temporary maximum residue limits. The MRLs
should remain as temporary limits, irrespective of the status of the
ADI, until items 1) and 2) of the further work or information,
required by 1982, are provided. The limits refer to the parent
compound etrimfos only.
Commodity (mg/kg) Remarks
Bran of wheat 20
(unprocessed)
Barley, maize, wheat 10
Wheat flour (wholemeal) 10
Wheat flour (white) 2
Rape seed 10
Rape seed oil (refined) 0.5
Carcass meat of cattle 0.011 ) These levels are based
Milk 0.011 ) on animal feeding studies.
Cattle meat byproducts 0.011 )
1 At or about the lower limit of determination
FURTHER WORK OR INFORMATION
Required (by 1982)
1. Adequate additional residue data from large-scale supervised
trials on cereal grains and their products.
2. Further residue data on rape seed and its oil (raw and refined).
3. Information on national use patterns and results of supervised
trials on growing crops destined for human or animal consumption.
4. Data on the amount and nature of the residues to be expected in
eggs and meat of poultry fed on treated grain.
Desirable
1. Data on residues on food in commerce and at consumption.
2. Information on national maximum residue limits.
3. Analytical method for the determination of residues in food of
animal origin.
4. Observations in man.
REFERENCES
Akram M., Ahmad S., and Forgash, A. J. Agric. Food Chem. Vol. 26, No.
4; 925-931.
Anonymous. Etrimfos. Identity. Unpublished report, undated from Sandoz
Ltd. submitted to the World Health Organization by Sandoz Ltd.
Anonymous. Etrimfos. Acute oral, subcutaneous and i.p. LD50 in male
and female mice. (1979a) Unpublished reports ref. Agro Dok CBK
3909/79, 3910/79 and 3911/79, from Sandoz Ltd. Agro Development
Toxicological Department, submitted to WHO by Sandoz Ltd.
Anonymous. Etrimfos. Acute oral, subcutaneous, i.p. and dermal LD50
in male and female rats. (1979b) Unpublished reports ref. Agro Dok CBK
3912/79, 3913/79, 3914/75 and 3915/79, from Sandoz Ltd. Agro
Development Toxicological Department, submitted to WHO by Sandoz Ltd.
Anonymous. The Pesticide Manual (6th edition), p.255. The British Crop
Protection Council. (1979c).
Brusick, D.J. and Weir, R.J. Mutagenicity evaluation of etrimfos.
Unpublished report no. 2683 ref. Agro Dok CBK 2545/77, dd. 30 December
1976 from Litton Bionetics submitted to WHO by Sandoz Ltd.
Carpy, S. and Klotzsche, C. San 197 I. 4-Week feeding study in rats.
Unpublished report no. Agro Dok CBK 754 b/73, dd. 18 March 1975, from
Sandoz Ltd., Agrochemical Research Department, Toxicological Section,
submitted to WHO by Sandoz Ltd.
Carpy, S. and Klotzsche, C. San 197 I. 2-Week dermal study in rabbits
intact skin. Unpublished report no. Agro Dok CBK 1727/75, dd. 15 April
1975, from Sandoz Ltd. Agrochemical Research Department, Toxicological
Section, submitted to WHO by Sandoz Ltd.
Carpy, S. and Klotzsche, C. San 197 I. 2-Week dermal study in rabbits,
abraded skin. Unpublished report no. Agro Dok CBK 1728/75, dd. 30 May
1975, from Sandoz Ltd. Agrochemical Research Department, Toxicological
Section, submitted to WHO by Sandoz Ltd.
Carpy, S. and Klotzsche, C. San 197 I. 3-Month feeding study in rats.
Unpublished report ref. Agro Dok CBK 1720/75, dd. 18 March 1975, from
Sandoz Ltd. Agrochemical Research Department, Toxicological Section,
submitted to WHO by Sandoz Ltd.
Carpy, S. and Klotzsche, C. San 197 I. 6-Month feeding study in dogs.
Unpublished report ref. Agro Dok CBK 1715/75, dd. 12 February 1975,
from Sandoz Ltd. Agro chemical Research Department, Toxicological
Section, submitted to WHO by Sandoz Ltd.
Carpy S. and Klotzsche, C. Etrimfos, 2-Year feeding study in rats.
Unpublished report no. 25/76 ref. Agro Dok CBK 1869/76, dd. 19 August
1976, from Sandoz Ltd. Agrochemical Research Department, Toxicological
Section, submitted to WHO by Sandoz Ltd.
Carpy S. and Klotzsche, C. SAN 197 I. 2-Year feeding study in dogs.
Unpublished report no. 41/77 ref. Agro Dok CBK 2568/77, dd. 3 August
1977, from Sandoz Ltd., Agro chemical Research Department,
Toxicological Section, submitted to WHO by Sandoz Ltd.
Carpy S. and Klotzsche C. San 197 I. 3-Generation study in rats.
Reproduction and teratogenicity data. Unpublished report ref. Agro Dok
CBK 3835/79, dd. 26 February 1979, from Sandoz Ltd., Agrochemical
Research Department, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Acute toxicity tests.
Unpublished report ref. Agro Dok CBK 754c/73, dd. 10 March 1975, from
Sandoz Ltd., Agrochemical Research Department, Toxicological Section,
submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Primary skin and eye
irritation tests in rabbits. Unpublished report ref. Agro Dok CBK
1725/75, dd. 18 March 1975, from Sandoz Ltd., Agrochemical Research
Department, Toxicological Section, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Neurotoxicity in chickens.
(With addendum for histopathological investigations for ischias
nerves.) Unpublished report ref. Agro Dok CBK 1852/75, dd. 18 March
1975, from Sandoz Ltd., Agrochemical Research Department,
Toxicological Section, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. Acute oral LD50 in rats and mice
(2-ethyl-4-ethoxy-6-hydroxy-pyrimidine). Unpublished report ref. Agro
Dok CBK 1777/75, dd. 18 March 1975, from Sandoz Ltd., Agrochemical
Research Department, Toxicological Section, submitted to WHO by Sandoz
Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Acute intramuscular LD50
in rats. Unpublished report ref. Agro Dok CBK 2518/77, dd. 21 February
1977, from Sandoz Ltd., Agrochemical Research Department,
Toxicological Section, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Sensitization test in
guinea pigs. Unpublished report ref. Agro Dok CBK 2519/77, dd. 22
February 1977, from Sandoz Ltd., Agrochemical Research Department,
Toxicological Section, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I (P=O analogue). Acute oral
and dermal LD50 in rats. Unpublished report, ref. Agro Dok CBK
2551/77, dd. 24 February 1977, from Sandoz Ltd., Agrochemical Research
Department, Toxicological Section, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Antitode test in rats.
Unpublished report, ref. Agro Dok CBK 3126, dd. 11 April 1978 from
Sandoz Ltd., Agrochemical Research Department, Toxicological Section,
submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Teratogenicity test in
rabbits. Unpublished report, ref. Agro Dok CBK 3132/78, dd. 22 June
1978 from Sandoz Ltd., Agrochemical Research Department, Toxicological
Section, submitted to WHO by Sandoz Ltd.
Ioannou, U.M. and Dauterman, W.C. In vitro metabolism of etrimfos by
rat and mouse liver. Pesticide Biochemistry and Physiology 9, 190-195.
Jucker, O. and Riggenbach, A. Etrimfos. Composition of technical
active ingredient. Unpublished report ref. Agro Dok CBK 3572/79e, dd.
1 November 1979, from Sandoz Ltd., Agro Development, Registration
submitted to WHO by Sandoz Ltd.
Karapally, J.C. Absorption, blood level, distribution and excretion of
SAN I 197 in the rat following a single oral dose. Unpublished report
ref. Agro Dok CBK 1829/75, dd. 21 April 1975, from Sandoz Ltd.,
submitted to WHO by Sandoz Ltd.
Karapally, J.C. Metabolism of 14C-Etrimfos in the rat. Unpublished
report, ref. Agro Dok CBK 3005, dd. 10 May 1977, from Sandoz Ltd.,
submitted to WHO by Sandoz Ltd.
Leuschner, F. Mikronucleus-test am Knockenmark von chinesischen
Hamstern (Test-präparat: Etrimfos). Unpublished report ref. Agro Dok
CBK 3136/78, dd. 4 July 1978, from Laboratorium für Pharmakologie und
Toxikologie, Hamburg submitted to WHO by Sandoz Ltd.
Richold, M. and Richardson, J.C. Motaphase analysis on SAN 197.
Unpublished report ref. CBK I 4798/80, dd. 24 April 1980, from
Huntingdon Research Centre submitted to WHO by Sandoz Ltd.
See Also:
Toxicological Abbreviations
Etrimfos (Pesticide residues in food: 1982 evaluations)
 Molecular formula C10H17N2O4PS
Molecular weight 292.3
Specific gravity 1.195 at 20°C
Appearance colourless oil
Odour slight smell, characteristic of
thionophosphoric acid derivatives
Melting Point -3.35°C (pure a.i.)
Vapour pressure 6.5 to 8.7 × 10-2 mbar at 20°C
Refractive index nD20 1.5068
Solubility in water (24°C) 40 mg/l completely miscible
with acetone, ethanol, diethyl ether, chloroform,
dimethylsulphoxide, ethyl-acetate, hexane,
kerosene.
Stability Half-life times in aqueous buffer solution at
25°C and pH 3, 6 and 9 are 0.4, 16 and 14 days
respectively. 1 to 10 mg etrimfos/l acetone,
chloroform, hexane or toluene in the dark at
room temp. were found to be stable for at least
28 days, in methanol and ethylacetate 3% and 12%
degradation were noticed.
Minimum degree of
purity 93%
Impurities in the
technical material Information on the impurities in technical
etrimfos was reported to the meeting.
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, excretion and biotransformation
Female rats were given a single oral dose of 50 mg/kg bw SAN I 197
(14C-labelled at ring-positions 4 and 6) dissolved in 80% aqueous
polyethylene-glycol.
Excretion of radioactivity occurred mainly via the urine, 84%
within 96 hours, of which 68% was within 12 hours; 6.6% was found
in the faeces within 24 hours. One hour after administration
continuous excretion of radioactivity was observed in the bile and
amounted to 6% within 24 hours. Only 0.012% was exhaled within 24
hours, of which 0.011% was within the first 8 hours, with a
peak-value around 1 hour after administration. For most tissues
the peak concentrations were reached at 4 hours after
administration with the exceptions of the liver (2 hours), blood (3
hours) and fat (8 hours). At peak time the fat and kidneys had the
highest concentrations (42 and 62 mg/kg respectively) whereas the
value in the other tissues ranged from 6 to 22 mg/kg with 13 mg/kg
in the blood. The concentrations declined rapidly to values of
0.01 mg/kg at 96 hours except in the fat and skin with 0.3 mg/kg.
In the urine and faeces collected for 96 hours neither etrimfos (I)
nor its oxygen analogue was detected. The main metabolite in both
excreta was 6-ethoxy-2-ethyl-4-hydroxypyrimidine (III) in amounts
of (% of the dose) 48% in the urine and 3% in the faeces. However
part of this metabolite is possibly formed from I and
desmethyletrimfos (II) during the procedures for analysis (see
below). Other metabolites found in the urine and faeces (see
figure 1) were the hydrolysis product of III (IV, 9% and 0.4%
resp.) and 3 hydroxylation products of III (V, 5% and 0.2% resp.
and VI and VII, together 3% and 0.04% resp.). The metabolites III,
IV and V occurred both free and
conjugated, the metabolites VI and VII mainly as conjugates
(Karapally, 1975 and 1977).
In another study with rats II and III were determined as the main
metabolites in 12-hour urine and faeces; 65% of the
14C-radioactivity found was II and 30% III (Ioannou and Dauterman,
1978).
In vitro
Etrimfos is rapidly degraded to water-soluble metabolites, mainly
desmethyletrimfos (II) and EEHP (III), when incubated with rat or
mouse liver subcellular fractions. The oxygen-analogue of etrimfos
could not be found. Glutathione-transferases and to a lesser
extent mixed-function oxidases are the main groups of enzymes
responsible for etrimfos metabolism (Ioannou and Dauterman, 1978).
Molecular formula C10H17N2O4PS
Molecular weight 292.3
Specific gravity 1.195 at 20°C
Appearance colourless oil
Odour slight smell, characteristic of
thionophosphoric acid derivatives
Melting Point -3.35°C (pure a.i.)
Vapour pressure 6.5 to 8.7 × 10-2 mbar at 20°C
Refractive index nD20 1.5068
Solubility in water (24°C) 40 mg/l completely miscible
with acetone, ethanol, diethyl ether, chloroform,
dimethylsulphoxide, ethyl-acetate, hexane,
kerosene.
Stability Half-life times in aqueous buffer solution at
25°C and pH 3, 6 and 9 are 0.4, 16 and 14 days
respectively. 1 to 10 mg etrimfos/l acetone,
chloroform, hexane or toluene in the dark at
room temp. were found to be stable for at least
28 days, in methanol and ethylacetate 3% and 12%
degradation were noticed.
Minimum degree of
purity 93%
Impurities in the
technical material Information on the impurities in technical
etrimfos was reported to the meeting.
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, excretion and biotransformation
Female rats were given a single oral dose of 50 mg/kg bw SAN I 197
(14C-labelled at ring-positions 4 and 6) dissolved in 80% aqueous
polyethylene-glycol.
Excretion of radioactivity occurred mainly via the urine, 84%
within 96 hours, of which 68% was within 12 hours; 6.6% was found
in the faeces within 24 hours. One hour after administration
continuous excretion of radioactivity was observed in the bile and
amounted to 6% within 24 hours. Only 0.012% was exhaled within 24
hours, of which 0.011% was within the first 8 hours, with a
peak-value around 1 hour after administration. For most tissues
the peak concentrations were reached at 4 hours after
administration with the exceptions of the liver (2 hours), blood (3
hours) and fat (8 hours). At peak time the fat and kidneys had the
highest concentrations (42 and 62 mg/kg respectively) whereas the
value in the other tissues ranged from 6 to 22 mg/kg with 13 mg/kg
in the blood. The concentrations declined rapidly to values of
0.01 mg/kg at 96 hours except in the fat and skin with 0.3 mg/kg.
In the urine and faeces collected for 96 hours neither etrimfos (I)
nor its oxygen analogue was detected. The main metabolite in both
excreta was 6-ethoxy-2-ethyl-4-hydroxypyrimidine (III) in amounts
of (% of the dose) 48% in the urine and 3% in the faeces. However
part of this metabolite is possibly formed from I and
desmethyletrimfos (II) during the procedures for analysis (see
below). Other metabolites found in the urine and faeces (see
figure 1) were the hydrolysis product of III (IV, 9% and 0.4%
resp.) and 3 hydroxylation products of III (V, 5% and 0.2% resp.
and VI and VII, together 3% and 0.04% resp.). The metabolites III,
IV and V occurred both free and
conjugated, the metabolites VI and VII mainly as conjugates
(Karapally, 1975 and 1977).
In another study with rats II and III were determined as the main
metabolites in 12-hour urine and faeces; 65% of the
14C-radioactivity found was II and 30% III (Ioannou and Dauterman,
1978).
In vitro
Etrimfos is rapidly degraded to water-soluble metabolites, mainly
desmethyletrimfos (II) and EEHP (III), when incubated with rat or
mouse liver subcellular fractions. The oxygen-analogue of etrimfos
could not be found. Glutathione-transferases and to a lesser
extent mixed-function oxidases are the main groups of enzymes
responsible for etrimfos metabolism (Ioannou and Dauterman, 1978).
 TOXICOLOGICAL STUDIES
Special studies on antidotes
Treatment of rats with atropine and obidoxime reduced the mortality
after oral administration of 3600 mg etrimfos/kg bw (Hamburger and
Klotzsche, 1978a).
Special studies on mutagenicity
Rat
Groups of 5 male and 5 female rats were administered, by intragastric
intubation 1500, 3000 or 6000 mg etrimfos/kg bw in two equal dosages
separated by an interval of 24 hours. A negative control group
received 1% methylcellulose, whereas a positive control group was
dosed by 2 i.p. injections with 8 mg Mitomycin c/kg bw.
After administration of etrimfos, the aberrant metaphase counts were
comparable with the control values, whereas Mitomycin C produced the
expected increase aberrant counts in the bone marrow cells of the
femurs (Richold and Richardson, 1980).
Hamster
Groups of 6 female Chinese hamsters were given s.c. injections of 30,
100 or 300 mg etrimfos/kg bw twice within 24 hours. MMS (100 mg/kg,
orally) was used as a positive control. Etrimfos did not elicit a
mutagenic response whereas the number of bone marrow micronuclei was
increased in the MMS-treated hamsters (Leuschner, 1978).
Microorganisms
Etrimfos (97%) in dosages from 0.001 up to 5.0 l/plate was not
mutagenic in the Ames test using the Salmonella typhimurium
strains TA-98, TA-100, TA-1535, TA-1537 and TA-1538 and
Saccharomyces cervisiae D-4, both with and without activation by
rat liver homogenate (Brusick and Weir, 1976).
Special studies on reproduction
Rat
Groups of 30 males and 30 females were fed dietary concentrations of
0, 3, 9 and 27 mg/kg in a three-generation, two-litter generation
reproduction study. In the B-generations one day before birth 5
pregnant females were killed and investigated for teratogenic
symptoms. The offspring of 5 other females were x-rayed and their
skeletons examined for abnormalities. After 21 days the tissues of 10
male and 10 female weanlings of the III B generation per dose group
were inspected histopathologically. The body weight gain and food
intake of the females were not affected by the treatment. No effects
were observed on fertility, gestation, viability, lactation, the
number of dead foetuses and the pup weight. Teratogenic events were
not observed over the course of the study. At the highest dose level
cholinesterase activity in plasma and erythrocytes, which was
determined in the weanlings of the B generations of all groups, was
slightly depressed, especially in the female rats. Brain
cholinesterase activity was not affected. Gross and microscopic
examination of tissues of organs of the F III B generation showed no
changes attributable to etrimfos administration (Carpy and Klotzsche,
1979).
Special studies on teratogenicity
Rabbits
Groups of 10 rabbits (12 in the control and in the low dose group)
were given etrimfos orally from day 6 to day 18 of pregnancy at dose
levels of 0, 25, 50 or 100 mg/kg bw. On day 29 of gestation the
animals were sacrificed and a caesarean section was performed. The
uterus was examined and the number, location and distribution of the
foetuses, implantations and resorptions recorded. Corpora lutea were
counted. The foetuses were weighed and examined for external and
skeletal anomalies. In the 50 and 100 mg groups the numbers of
implantations and living foetuses were decreased, whereas the number
of dead foetuses and weight of the placenta were slightly increased.
In the low dose group 2 animals with slightly curved hind legs and in
the highest dose group, in which 43 animals were investigated, one
animal with a rudimental tail and one animal with a skull deformation
were observed. In a historical control group of 286 animals 2 rabbits
with skull deformations were observed (Hamburger and Klotzsche,
1978b).
Special studies on neurotoxicity
Hen
Groups of 5-20 chickens were administered etrimfos in gelatine
capsules at dose levels of 100, 200, 400, 500, 750 or 1000 mg/kg bw.
A positive control group was given 2 × 80 mg tri-o-cresyl phosphate
(TOCP)/kg bw. Before the treatment the animals received atropine or
obidoxime by i.v, or i.m. injection. The observation period varied
from 21-42 days. Mortality was increased in all treated groups.
Doses of 200 mg/kg etrimfos and above caused typical signs of
organophosphate poisoning. A delayed neurotoxic response was not
observed in the chickens treated with etrimfos. TOCP elicited ataxia
and paralysis in all 10 animals. Histopathologically typical
degeneration changes in the ischias nerve were observed in all
TOCP-treated animals. No clear treatment-related alterations were
found in the nerves of the hens given 100 and 200 mg/kg etrimfos
(Hamburger and Klotzsche, 1975d).
Special studies on sensitization
Guinea pig
According to the "Guinea pig maximisation test" 20 guinea pigs were
treated with SAN 197 I as a 5% solution in DMSO and incorporated in
Freund's adjuvant; 10 guinea pigs served as a control group. 5/20 of
the treated and 4/10 of the control animals died before the 22nd day.
A slight development of scales at the application side without any
inflammatory changes or oedema was noticed at 72 hours after the
challenge application; no other effects were noticed (Hamburger and
Klotzsche, 1977b).
Acute toxicity
Symptoms of intoxication in rats and mice after oral, i.p. and s.c.
administration were limpness, lacrimation, salivation, exophthalmus,
laboured respiration, ataxia, tremors and convulsions. After dermal
application to rats and rabbits no toxic symptoms were observed
(Hamburger and Klotzsche, 1975a; Anonymous, 1979a and Anonymous,
1979b).
Rabbit
Etrimfos (0.5 g) applied in the intact or abraded skin of rabbits was
found to be not irritating. Instilled into the eye (100 mg) etrimfos
produced slight redness of the conjunctivae, which lasted 24 hours, in
one of six rabbits (Hamburger and Klotzsche, 1975b).
TABLE 1. Acute toxicity of etrimfos
species sex route LD50 in mg/kg bw purity solvent references
mouse M oral 47O (425-523) 97.2% water Hamburger and Klotzsche, 1975a
F oral 620 ± 28 97.2% water " " "
M oral 1120 (1023-1226) 90.5% corn-oil Anonymous, 1979a
F oral 1100 (930-1289) 90.5% corn-oil " "
M oral 535 (24 hours) - corn-oil Ioannou and Dauterman, 1978
M i.p. 698 (597-817) 90.5% corn-oil Anonymous, 1979a
F i.p. 679 (585-788) 90.5% corn-oil " "
M s.c. 979 (729-1155) 90.5% corn-oil " "
F s.c. 1050 (897-1229) 90.5% corn-oil " "
rat M oral 1800 (765-3733) 97.2% water Hamburger and Klotzsche, 1975a
F oral 2354 (1841-3008)1 97.2% water " " "
M oral 1930 (1664-2239) 90.5% corn-oil Anonymous, 1979b
F oral 1970 (1669-2325) 90.5% corn-oil " "
M oral 2040 (24 hours) - corn-oil Ioannou and Dauterman, 1978
M i.p. 767 (684-873) 97.2% water Hamburger and Klotzsche, 1975a
M i.p. 1310 (1110-1546) 90.5% corn-oil Anonymous, 1979b
F i.p. 1140 (950-1368) 90.5% corn-oil " "
M i.v. 25.5 ± 1.26 97.2% water Hamburger and Klotzsche, 1975a
F i.v. 21.0 ± 1.88 97.2% water " " "
M i.m. 4000 ± 116 94.3% PEG 200 Hamburger and Klotzsche, 1977a
M x.c. 3700 (3710-4563) 90.5% corn-oil Anonymous, 1979a
F s.c. 3690 (3000-4539) 95.5% corn-oil Anonymous, 1979b
dermal >2000 97.2% water Hamburger and Klotzsche, 1975a
M dermal >5000 90.5% corn-oil Anonymous, 1979b
F dermal >5000 90.5% corn-oil " "
rabbit dermal >500 97.2% water Hamburger and Klotzsche, 1975a
1 value corrected by recalculation
TABLE 2. Acute toxicity of metabolites
Species sex route solvent LD50 (mg/kg) references
rat M oral DMSO 1460 ± 52.5 Hamburger and Klotzsche, 1975e
rat F oral DMSO 1170 ± 58.5 " " "
mouse M oral DMSO 1530 ± 87.9 " " "
mouse F oral DMSO 1170 ± 94.8 " " "
P = O analogue of the parent compound
species sex route solvent LD50 (mg/kg) references
rat M oral DMSO 450 ± 35 Hamburger and Klotzsche, 1977c
rat M dermal DMSO 1000 " " "
Short-term studies
Rat
Male and female rats (15/sex/group) were dietary fed 0, 50, 250 or
1250 mg/kg SAN 197 I (97.2%) in the food for 4 weeks; later on in the
test groups (5/sex) on 10 mg/kg with corresponding controls were
added. The animals were examined for general health, body weight,
food consumption, haematology, blood biochemistry, urinalysis, organ
weights and macroscopy. A decreased cholinesterase activity in
erythrocytes was observed at 50 mg/kg and higher doses. Plasma-ChE
was decreased in females from the 50 mg/kg and in males from the 250
mg/kg groups, and brain-ChE in females from the 250 mg/kg and in males
at the 1250 mg/kg groups. Normochromic anaemia was noticed at 250
mg/kg and in females at 1250 mg/kg, changes in blood sugar occurred at
all dosages except 250 mg/kg and changes in reticulocytes at 1250
mg/kg. At these dosage the animals showed slight sedation and
slightly decreased body-weight gain as result of lower food intake
(Carpy and Klotzsche, 1975a).
Rat
Groups of 35 males and 35 females were fed etrimfos at concentrations
of 0, 3, 9 or 27 mg/kg feed for a period of 13 weeks. Body-weight
gain and food intake was recorded throughout the study of 25 females
and 25 males per group. After 4, 8 and 13 weeks haematology clinical
chemistry and urinanalysis was carried out in 10 animals/group. The
same number of rats was studied histopathologically and their organ
weights were recorded. At the end of the study the glucose
concentration in serum was increased in both males and females of the
highest dose level. The weight of the thyroid of the male rats of the
highest dose group was significantly decreased. However microscopic
examination showed no abnormalities attributable to the presence of
etrimfos in the diet. After 8 and 13 weeks the plasma cholinesterase
activity of the female rats in the 9 mg/kg group was slightly (20-24%)
and clearly depressed in the 27 mg/kg group (30-37%). Cholinesterase
activity in erythrocytes and brain was within normal limits (Carpy and
Klotzsche, 1975d).
Rabbit
Male and female rabbits (5/sex/group) received a dermal application of
0, 25 or 100 mg/kg SAN 197 I (97.2%) dissolved in polyethylene-glycol
on patches of intact or abraded skin for 8 hours a day on 5 days a
week during 2 weeks and were examined for general health, body weight,
haematology, blood biochemistry, organ weights and macroscopic changes
(microscopic studies are not yet completed). One male with intact
skin out of the 100 mg/kg group died with an enlarged liver. In the
males the weight of the spleen was decreased and the weight of the
thymus increased while in females a decreased weight of the thymus was
noticed. The animals with abraded skin showed abnormalities only at
the high dosage. Increased organ weights were observed in the female
liver and heart and in the males in the prostate. All these changes
were within the normal range. The animals with intact skin showed, at
both dosages, cholinesterase-inhibition in plasma and in erythrocytes.
In the animals with abraded skin, only in the high dosage, in males as
well as in the females, was cholinesterase inhibited in plasma and
erythrocytes (Carpy and Klotsche, 1975b and c).
Four groups of 4 male and 4 female beagle dogs were fed O, 2.5, 10 or
40 mg/kg etrimfos (97.2% purity) in the food for 26 weeks.
Normal values in haematology, blood chemistry, and urinalysis were
evaluated before starting the study. These determinations were
repeated 6 times during the test period: after 1, 4, 8, 13, 19 and 26
weeks, with exception of BSP which was estimated after 4, 13, and 26
weeks. The physical examination included weekly body weight, food
intake and neurological, oral, behaviourial, and ophthalmoscopic
inspection. The haematological parameters investigated were: RBC,
WBC, differential WBC, MCV, MCH, MCHC, reticulocytes, haemoglobin,
haematocrit, platelets, and prothrombin time, RBC- and
plasma-cholinesterase, FBS, BUN, SGPT, SGOT, LDH, uric acid, total
serum protein, bilirubin, creatinine, serum alkaline phosphatase,
cholesterol, serum sodium, potassium, calcium, chloride, inorganic
phosphate, serum albumin, and serum electrophoresis (A/G ratio). The
activity of cholinesterase in cerebellum and cerebrum, of the liver
cytochrome P-450, of the liver drug metabolising enzymes O-, and
S-demethylases and of the anilin-4-hydroxylase and the amount of
cholesterol, glycogen, lipids, and total protein contained in the
liver were estimated at the end of the study. All animals were
examined for gross pathology, organ weights and histopathology. MCH
and MCHC values were increased in the male animals of the 40 mg/kg
group. From the 11th week on plasma and erythrocyte cholinesterase
activity was depressed in comparison to the pretest and the control
values, with a plateau of about 40% in both males and females. In the
10 mg/kg group a slight depression of cholinesterase activity in
plasma and erythrocytes especially in comparison to the pre-test
values was observed in both sexes. In the cerebrum of the female rat
of the highest dose group a decrease of 22% of the cholinesterase
activity was measured.
From the 13th week on the albumin concentration in serum was
dose-dependant increased. However this was not confirmed by
electrophoresis and had disappeared almost at the end of the study.
The weight of the organs was within normal limits. No gross or
histopathological aberrations were observed (Klotzsche and Carpy,
1975e).
Long-term studies
Rat
Groups of 40 males and 40 females were fed etrimfos (97.8%) in the
diet at concentrations of 0, 6, 12 or 24 mg/kg for a period of 2
years. Body weight, food consumption and water intake of 30 rats per
dose and sex were recorded weekly. After 1, 2 ,3, 6, 9, 12, 15, 18,
21 and 24 months haematology, clinical chemistry and urinalysis were
carried out in 5 male and 5 female animals per group. At the end of
the study BSP a liver function test was carried out in the control and
high-dosed females. In liver homogenates the activity of drug
metabolising enzymes and the total protein and cholesterol
concentration were measured at the end of the study.
All animals dying during the study, or which had to be killed, were
necropsied and tissues were examined microscopically. The organ
weights of 10 rats per dose and sex were recorded.
There was no clear effect of etrimfos on body weight, food and water
intake and mortality. The cholinesterase activity of the plasma and
erythrocytes of male rats was not affected by etrimfos. In the
females the activity of cholinesterase in the plasma of the 24 and 12
mg/kg group was decreased by 35-50 and 20-30% respectively throughout
the study. In the highest dose group a marginal effect was observed
on the activity in the erythrocytes of the female rats. Neither in
the females nor in the males was the cholinesterase activity of the
brain and the liver of the treated animals significantly different
from the control values.
Both in the male and female rats the cytochrome P-450 of the liver of
the animals of the 12 and 24 mg/kg group was significantly decreased
in comparison to the controls; however no dose-response relation was
observed.
The macroscopic and microscopic examination did not reveal organ
lesions that could be attributed to treatment. The tumour rate in
treated animals corresponded with the incidence in control animals.
No-effect level of this study is 6 mg/kg (Carpy and Klotzsche, 1976).
Dog
Groups of 4 male and 4 female beagle dogs received 0, 4, 10 or 25
mg/kg etrimfos in their diet, during 106 weeks. There were no signs
of adverse behaviour and mortality was not noted. Growth and food
consumption were not clearly affected. Eye examinations during the
first, 26th, 52nd, 78th and 104th week did not indicate adverse ocular
changes.
Haematology, clinical chemistry and urinalysis were carried out in
week 1 (pre-test), 4, 8, 13 and quarterly thereafter. Plasma
cholinesterase activity was decreased in the 10 and 25 mg/kg groups by
23% and 24% respectively. The cholinesterase activity of the
erythrocytes was also decreased.
No clear differences or sensitivity were seen between males and
females. In the cerebrum, cerebellum and liver the cholinesterase
activity was not depressed.
Slightly higher activities of N-, O- and S-demethylase activity were
measured in all treated groups. However there was no clear
dose-response relation. Neither the weights of the organs nor the
histopathological evaluation revealed indications for treatment-
related changes in any organ or tissue. No-effect level of this study
is 10 mg/kg (Carpy and Klotzsche, 1977).
RESIDUES IN FOOD
RESIDUES RESULTING FROM SUPERVISED TRIALS
Stored grain products
Wheat grain (variety Probus) was treated in 25 kg lots with
Satisfar(R) (Ekamet 50 EC) at the rate of 5, 10 or 15 mg/kg ai under
conditions as close to normal practice as possible. The grain, which
was stored in fibre-drums at room temperature, was analysed
periodically for etrimfos.
In this small-scale trial the etrimfos content of the wheat grain
immediately after treatment (zero time) was 50 - 60 percent of the
application rate. During the first six months of storage, the amount
of etrimfos in the grain remained fairly constant.
In the next six months its amount was reduced to 34-37% of the
application rate, (or 63-75% of the original concentration in the
grain). At the end of 12 months, the grain treated at the rate of 10
and 15 mg/kg had 100% efficacy against Sitophilus; the grain treated
at the rate of 5 mg/kg had 90% activity. (CBK 3630/79).
Several trials were conducted in different countries on wheat, barley
and corn (table 3).
FATE OF RESIDUES
In plants
The fate of etrimfos in bean and corn plants has been studied (M.
Akram et al., 1978). The primary leaves of bean and corn seedlings
were treated with 14C etrimfos. The treated leaves and the untreated
portions of the plants were sampled at 0, 3, 7, 14 and 21 days after
treatment. The aqueous rinses and the extracts of the treated leaves
were examined for the parent compound and its metabolites. Leaf
remainder, and untreated portions, were analysed for total
radiocarbon.
Etrimfos rapidly volatilised from the leaf surface. Within the first
three days after treatment it is lost with a half-life of approx. 3
days (beans and corn) and during 3 to 15 days with a half-life of
approx. 10 days from beans and approximately 5 days from corn. Three
weeks after treatment the radioactivity, retained by the leaves after
rinsing, ranged from 33% to 42% for bean, and from 25% to 42% for
corn. No translocation to the root system was observed. A small
percentage of the applied radiocarbon was present in the untreated
foliage of bean (0.3-0.6% of the applied radioactivity became
unextractable in bean leaves, and 0.1-0.3% in corn leaves).
Leaf rinses (surface residues) of bean and corn contained mainly
etrimfos and small quantities of 6-ethoxy-2-ethyl-4-hydroxy-pyrimidine
(EEHP). Small amounts of the P=O analogue of etrimfos were also
observed in the corn-rinse.
The leaf extracts (subsurface residues) of bean and corn contained
etrimfos and minor quantities of EEHP and five unknown metabolites, of
which one was tentatively identified as
2-ethyl-4,6-dihydroxy-pyrimidine; 21-day bean leaf contained 33.5% of
the applied radioactivity, of which 11.9% was etrimfos, 2.4% EEHP,
9.7% the unknown metabolites and 9.5% highly polar or conjugated
metabolites (TLC origin). All single metabolites were less than 10%
relative to applied etrimfos; 21-day corn leaf had about 24.3% of the
applied radioactivity, of which 3.8% was etrimfos, 0.4% EEHP, 15.8%
the unknown metabolites and 4.3% polar or conjugated metabolites. All
single metabolites were less than 10% relative to applied etrimfos.
The enzyme and acid-hydrolysed aqueous extracts gave essentially the
same TLC pattern as the unhydrolysed material, with quantitative
differences.
From the described results, we learn that etrimfos is lost from
treated plants in two stages. EEHP was found as a metabolite, but
only in quantities of less than 10% of the applied etrimfos. The P=O
analogue was not found in bean leaves nor in corn leaves. No single
metabolite was present in quantities more than 10% relative to the
applied etrimfos so that the influence of these metabolites on the
toxicity of actual residue levels in practical field samples may be
neglected. Tolerance recommendations may, therefore, be based on
residue data of the etrimfos ai only.
In cereal products
Wheat grain (variety, Probus) was treated in 25 kg lots with
Satisfar(R) (Ekamet 50 EC) at the rate of 5, 10 or 15 mg/kg ai. The
grain was stored at room temperature and at intervals of 2, 4, 6 and
12 months, samples of grain were milled and separated into different
fractions. Also bread was baked from the flour from these samples.
Etrimfos residues were analysed in all these samples.
Etrimfos concentration in the milled fractions of the wheat was the
highest in the bran and the lowest in the white flour. During the
baking process the etrimfos content of the flour was reduced by 58-77%
in the case of white bread, and 43-65% in the case of whole wheat
bread. The etrimfos content of white bread ranged from about 4 to 6%
of the amount applied and that in the whole wheat bread was in the
range of 14 to 30% of the amount applied (CBK 3630/79). Table 4.
In another trial with wheat, the amount of etrimfos in the white flour
ranged from 25 to 33% of that actually present in the grain. Baking
to bread reduced these residues in the flour by about 50% (CBK
4119/79). Table 5.
In a trial with barley, the wort from free grain contained only 4-6%
of the etrimfos originally present in the grain. The wort from malt
grain had no detectable residue of etrimfos (CBK 4120/79). Table 5.
TABLE 3. Residues of etrimfos (mg/kg) in stored products treated after harvest
Application Storage period (months)
Commodity Rate 0 1-2 3-4 6 8-10 12-13 18-24 Remarks Ref.
(country) Type (mg/kg) (24 h)
Cereal grain
Barley
(England) 50 EC 5 2.3 3.2-3.6 2.1 0.8 amount treated:
content: 15%; temp.: CBK
0-15°C; untreated 4121/79
"blank": 0.03-0.04
mg/kg
(Kenya) 0.5 Dust 30 2.25 2.1 0.6 0.3 amount treated:
1 Dust 29 4.5 3.0 1.3 0.8 45 kg each, UK
50 EC 5 1.3 0.5 0.2 stored in bags; 3770/79
2 Dust 22 9 9.7 1.2-2.2 0.8-1.4 conditions 4149/79
50 EC 10 4.1 1.0-2.1 0.5-0.9 not reported
Corn(maize)
(Kenya) 0.5 Dust 30 2.75 0.6-0.8 0.5 0.3-0.4 0.3-0.4 - amount treated:
50 EC 5 1.5-1.6 1.6-1.7 0.8-1.2 1.0-1.1 0.8 45 kg each,
1 Dust 29 5.5 0.9-2.6 0.7-1.5 1.0-1.6 0.8-0.9 0.9-1.3 stored in bags;
50 EC 10 0.8-4.2 1.7-2.9 1.5-2.5 1.9-2.5 2.5-2.6 storage conditions
2 Dust 22 11 1.7-5.5 2.4-3.8 2.2-2.9 1.2-2.4 1.5-2.3 not reported
Wheat
(England) 50 EC 5 6.8 5.6-6.1 5.9 3.9 amount treated:
50 EC 10 10.9 6.7-7.2 5.4 6.4 10 t; 30 t on
conveyer belt; CBK
moisture cont. 11%; 4118/79
0-17°C; untreated
"blank": 0.01-1 mg/kg
TABLE 3. Continued...
Application Storage period (months)
Commodity Rate 0 1-2 3-4 6 8-10 12-13 18-24 Remarks Ref.
(country) Type (mg/kg) (24 h)
(France) 50 EC 5 1.9-2.0 1.4-2.0 1.6 storage conditions not
50 EC 7.5 1.6-2.5 2.1-3.3 2.1-2.9 reported; samples for CBK
50 EC 10 3.3-5.4 4.2 2.6-3.8 analysis: 10 g each 2943/79
(Kenya) 0.5 Dust 30 2.5 1.2 0.7 1.0 0.8 0.4 amount treated:
1 Dust 29 5 2.0 1.8 1.5 1.3 1.0 10 kg each, stored CBK
50 EC 5 1.6 1.0 1.0 0.8 0.5 in bags; storage 3771/79
2 Dust 22 10 3.7 2.8 2.7 2.1 1.8 temp. about 15-25°C; 4117/79
50 EC 10 2.0 2.5 2.6 2.5 1.7 further conditions 4148/79
not reported.
(Switzerland) 50 EC 5 2.9 2.9 2.7 3.0 1.9 1.1 0.6 amount treated: 25 kg
(<0.1) (<0.1) (<0.1) (0.2) (0.4) (0.2) (<0.1) each, stored in air
50 EC 10 5.4 5.8 5.5 5.6 3.4 1.7 1.3 permeable fibre-drums;
(0.1) (0.5) (0.1) (0.3) (1.2) (0.2) (<0.1) moisture content: 12-14%; CBK
50 EC 15 7.4 7.8 7.6 8.1 5.6 temp about 16-20°C; 3630/79
(0.1) (0.2) (0.2) (0.5) (1.5) EEHP residues reported 3707/79
in brackets; EPO 4209/80
residues were always
below 0.002 mg/kg.
Rape seed
(England) EC 10 9-10 8-9 9-10 7-8 :Bin 2 amount treated: Stables
EC 10 11-15 10-15 13-14 13-14 10 t each, stored et al.,
:Bin 3 in metal bins; (1979)
moisture content in seed:
9-11%; temp. 3-15°C; seed
in bin 2 was slightly damper and
more heavily infested by mites
than in bin 3. Etrimfos in rape
seed oil (refined) up to about
0.5 mg/kg/ in spent meal: 0.05 mg/kg.
TABLE 4: Etrimfos1 residues in milled fractions and flour of wheat and in bread
Treatment Residues (mg/kg) 2-12 months after treatment
rate 2 4 6 12
5 mg/kg Etrimfos EEHP2 Etrimfos EEHP Etrimfos EEHP Etrimfos EEHP
Grain 2.92 n.d.3 2.74 n.d. 3.02 0.20 1.89 0.36
Bran 10.47 1.10 9.94 0.39 9.78 0.38 3.50 2.10
Grits 8.67 0.62 8.64 0.62 6.83 0.27 3.48 1.02
White flour 0.82 n.d. 1.02 n.d. 0.73 n.d. 0.70 n.d.
White bread 0.23 0.05 0.24 0.10 0.21 0.11 0.18 n.d.
Whole wheat
flour n.a.4 n.a. 2.66 0.16 2.33 0.11 1.97 0.37
Whole wheat
bread n.a n.a. 1.52 0.22 1.33 0.34 0.69 0.23
10 mg/kg Etrimfos EEHP2 Etrimfos EEHP Etrimfos EEHP Etrimfos EEHP
Grain 5.84 0.54 5.50 0.08 5.59 0.34 3.38 1.20
Bran 15.22 1.92 16.27 0.66 15.83 1.14 6.20 3.34
Grits 41.29 1.27 15.08 0.73 13.14 1.06 7.74 1.97
White flour 1.89 0.14 1.75 n.d. 1.47 0.06 0.96 n.d.
White bread 0.50 0.13 0.61 0.24 0.51 0.20 0.41 n.d.
Whole wheat
flour n.a. n.a. 5.59 0.39 5.07 0.24 3.70 0.69
Whole wheat
bread n.a. n.a. 2.83 0.60 2.38 0.51 1.37 0.51
TABLE 4. Continued...
Treatment Residues (mg/kg) 2-12 months after treatment
rate 2 4 6 12
15 mg/kg Etrimfos EEHP2 Etrimfos EEHP Etrimfos EEHP Etrimfos EEHP
Grain 7.80 0.20 7.61 0.20 8.11 0.46 5.57 1.52
Bran 16.60 1.50 21.65 1.56 19.70 1.51 9.66 4.34
Grits 19.41 1.53 20.84 0.93 19.33 1.06 12.14 1.97
White flour 2.04 0.25 2.62 0.09 2.48 0.10 1.50 n.d.
White bread 0.74 0.18 0.95 0.49 0.78 0.31 0.54 0.23
Whole wheat
flour n.a. n.a. 7.99 0.41 7.84 0.40 5.90 0.71
Whole wheat
bread n.a. n.a. 3.80 0.40 3.77 0.70 2.30 0.46
1 The metabolite P=O etrimfos could not be detected
2 EEHP = 2-ethyl-4-ethoxy-5-hydroxy-pirimidine
3 n.d = not detectable
4 n.a. = not analysed
TABLE 5. Etrimfos residues in cereals processed after treatment (in UK) with SATISFAR(R)
Cereal Formulation Rate of Etrimfos Amount of Method of treatment Reference
treatment residues grain per
(ai/mg/kg) (ppm) treatment
Wheat 50 EC 5 mg/kg grain 0.76 6 kg The required amount of CBK
bran 4.62 Satisfar in 25 ml water 4119/79
offal 3.13 was sprayed onto grain
white flour 0.25 tumbling in a
white bread 0.12 Bluefinsowaway cement mixer.
10 mg/kg grain 2.51 Stored in sealed plastic
bran 4.50 bags.
offal 6.14
white flour 0.63
white bread 0.34
Barley 50 EC 5 mg/kg free grain 1.07 CBK 4120/79
wort 0.07
spent 0.63
malt grain 0.21
wort n.d.
spent gr 0.07
10 mg/kg free grain 3.50
wort 0.16
spent gr 0.52
malt grain -
wort n.d.
spent gr 0.11
In cattle
A three level feeding study in dairy cattle with etrimfos incorporated
in the diet at levels 0.5, 1.5 or 5 mg/kg etrimfos was conducted. The
milk production was not affected. The cows, sacrificed after 28 days
of feeding, had only less than 0.01 mg/kg etrimfos in the muscle,
liver, kidney and fat. Etrimfos residue in the milk throughout the
study was less than 0.01 mg/kg. The residue in the milk solids
separated from the 21st and 28th day samples also had only 0.01 mg/kg
etrimfos (CBK 3011/77).
In soil
Degradation of 14C etrimfos in a sandy loam, silt loam and silty clay
loam soil was studied.
The soils treated with 14C-etrimfos at the rate of 5 mg/kg were
incubated at room temperature for 70 days. At selected time intervals
during the incubation period, the soil samples were analysed for the
parent and degradation products.
Etrimfos was rapidly hydrolysed, in all soils, to
6-ethoxy-2-ethyl-4-hydroxy-pyrimidine (EEHP). Its amount was reduced
to 50% in 3-8 days, and to 10% in 15-30 days.
The formation of EEHP paralleled the dissipation of etrimfos. Its
proportions reached a maximum of 75-85% in 14-28 days, after which
period its concentration began to decrease. The disappearance of EEHP
was fastest in silt, and rather slower in sand, and still more so in
clay. At the end of 70 days, the amounts of EEHP in silt, sand and
clay were 4, 18 and 52% respectively.
A further metabolite, tentatively identified as
5-chloro-6-ethoxy-2-ethyl-4-hydroxy-pyrimidine (CEEHP), was detected
in all soil extracts. Its formation seemed to be related to the
dissipation of EEHP, and its occurrence was highest in silt (24%),
followed by sand and clay (6%).
The soil-"bound" residues became significant only after EEHP began to
be metabolised. A maximum of 12% of these was observed for sand, and
21% for silt and clay. Further extraction with methanol-water 75:25
reduced the bound residue to <10%, the metabolites being mainly EEHP
and CEEHP.
The radiocarbon lost from the soil during the 70 days' incubation
amounted to 60% for sand or silt and 25% for clay. In a separate
experiment, using sandy loom soil treated with 14C-etrimfos, it was
demonstrated that the radioactivity lost from the soil was due to the
14CO2, which began to be generated after a time lag of at least 20
days (CBK 3002/77).
Photodegradation
in water
Under blacklight and daylight, which contain essentially no shorter
wave lengths than natural sunlight, aqueous solution of etrimfos (5
mg/kg) was quite stable: degradations of 10% (blacklight) and 5%
(daylight) occurred within 24 hours of exposure. Under mercury arc
light, approx. 45% degraded during the same time (CBK 1642/75).
on glass plates
2.5 mg of etrimfos, spread over an area of 70 cm2 and covered with
quartz plates, was exposed to the same light sources as in water.
Degradation, after 24 hours under the following conditions, was
daylight <5%, blacklight <5%, mercury light ‰ 25%, (CBK 1642/75).
METHODS OF RESIDUE ANALYSIS
Two methods have been developed - one for determining etrimfos the P=O
analogue and 2-ethyl-4-ethoxy-6-hydroxypyrimidine (EEHP), the
hydrolysis product of etrimfos (CBK 2178/76) and the other for
determining etrimfos only (CBK 3649/79). In both methods grain is
dealt with in the same manner as vegetable crops.
The P=O analogue of etrimfos has been detected in the stored grain
products. The amount of EEHP in the grain is generally quite low and
hence, in these cases, the method CBK 3649/79 is recommended.
In these methods etrimfos residues are extracted with acetone and
partially cleaned up by partitioning between water and methylene
dichloride. If only etrimfos is to be determined, the methylene
dichloride solution is further cleaned up on a silica gel column and
determined by gas chromatography using a flame photometric detector
(P-mode). If the P=O analogue and EEHP are to be included, the
methylene dichloride solution is further cleaned up on a
polyethylene-coated alumina column and determined by gas
chromatography using a nitrogen specific detector.
A series of CBA reports on residues in food and fats in the
environment were submitted by Sandoz Ltd. to FAO for this evaluation.
EVALUATION
Etrimfos is a broad-range non-systemic contact and stomach
organophosphorous insecticide, which was introduced in 1972 and is
registered and/or used in a considerable number of countries. It is
effective against biting and sucking insects such as Lepidoptera,
Coleoptera, Diptera and to a variable extent, Hemiptera at 0.25-0.75
kg ai/ha. It is mainly used on fruit, vegetables, maize and
ornamental plants, but also on alfalfa and paddy rice, as an
emulsifiable concentrate or as granules.
Studies of the metabolism and distribution of etrimfos in rats have
been undertaken with 14C in the pyrimidine moiety. This has provided
useful information on the pyrimidine portion of the molecule, but
disposition and kinetic data on the whole molecule of etrimfos and its
triester metabolites are required. This is especially important since
the compounds containing a fully-esterified phosphoroticate or
phosphate group are the more toxic.
In rats and rabbit no effects on reproduction, including
teratogenicity, were observed. A mutagenic response was negative as
shown by a rat metaphase analysis, a hamster bone marrow micronuclei
count, and an Ames test.
Etrimfos did not induce a delayed neurotoxic response, as generally
noted with TOCP, in hens. A skin-sensitisation study in guinea pigs
was negative.
Etrimfos and the main metabolite
O-6-ethoxy-2-ethyl-4-hydroxypyrimidine (EEHP) were slightly toxic
following acute administration to various animal species. The effects
of etrimfos were typical of those caused by cholinesterase inhibitors.
Several short- and long-term toxicity studies have been carried out
with rats and dogs. The macroscopic and microscopic examination in
these studies did not reveal lesions in organs attributable to
etrimfos. The tumour incidence in treated animals corresponded with
that in control animals. In both species etrimfos produced
cholinesterase inhibition.
Based on the two-year studies with both rat and dog a temporary ADI
was recommended.
Although it is known that data from supervised trials (pre-harvest
use) exist, which show that residues in many fruits and vegetables,
potatoes, maize and rape are in the range 0.05 to 0.5 mg/kg when
etrimfos is used in accordance with good agricultural practice,
information was not made directly available for consideration by the
meeting.
However, in recent tests and trials carried out in England, France,
Kenya and Switzerland etrimfos was also found efficacious against
moths, (malathion-resistant) beetles and (lindane-resistant) mites in
stored crops, especially grain and rape seed. Recommended rates for
dusting or spraying wheat, barley, maize (corn) or rape seed are 5 to
10 mg/kg. Depending on storage conditions (e.g. degree of
infestation, climate, storage period) lower or, exceptionally, higher
dosages up to 15 mg/kg appear appropriate.
Etrimfos in stored rape seed shows no significant loss after 6 months
storage and the estimated half-life in stored grain is about 3 to 8
months. Etrimfos is rapidly lost from treated plants (beans, maize),
about half within the first 3 days, with a half-life of about 5 to 10
days between the 3rd and the 15th day after application, probably
owing to the different rate of volatilisation of the initial deposit
and of the residue after penetration into the surface layer.
In degradation studies with plants treated with 14C-etrimfos,
labelled at carbons 4 and 6 in the pyrimidinyl ring the metabolites
O-6-ethoxy-2-ethyl-4-hydroxypyrimidine (EEHP) and 2-ethyl-4,
6-dihydroxypyrimidine (EDHP) were identified. Small amounts of the
etrimfos P=O analogue (EPO) were also observed, but only on the plant
surface. In stored grain EEHP was the only metabolite found in
significant quantities.
After a single oral does of 50 mg etrimfos/kg, rats excreted more than
50% as EEHP in urine and faeces, with smaller amounts of EDHP,
6-ethoxy-4-hydroxy-2-(1-hydroxyethyl) pyrimidine (EEHP-1), its
2-(2-hydroxyethyl) pyrimidine analogue (EEHP-2) and
2-ethyl-4-hydroxy-6-(2 hydroxyethoxy) pyrimidine (EHHEP). In rat
liver preparations desmethyl-etrimfos was also detected.
In soils the half-life of etrimfos is about 3 to 8 days: degradation
results in EEHP, EDHP and finally carbon dioxide. General degradation
pathways are thus similar in plants, animals and soils, although
proportions of individual metabolites may vary. Hydrolysis of the
phosphorothioate ester seems to be the most significant pathway,
yielding products of lesser toxicological importance.
When dairy cattle were fed with diets containing 5 mg etrimfos/kg for
28 days, etrimfos residues were always below 0.01 mg/kg in meat, milk,
fat and edible offals.
Processing wheat, treated with 10 mg atrimfos/kg and stored for 2 to
12 months, resulted in etrimfos residues up to 16.3 mg/kg in raw bran,
5.6 mg/kg in wholemeal flour, 2.8 mg/kg in wholemeal bread, 1.9 mg/kg
in white flour and 0.6 mg/kg in white bread. Provisional information
on barley treated with 5 to 10 mg etrimfos/kg and used for malting and
brewing showed etrimfos residues up to 0.6 mg/kg in spent grain and
0.2 mg/kg in wort. When stored rape seed containing 10 to 15 mg
etrimfos/kg was used for oil production, preliminary results indicated
that 95-100% of the etrimfos was lost during the refinement of the oil
and that the greatest looses occurred by degradation during bleaching
and steam distillation. No residues were detected in the spent meal.
Analytical methods for determining residues of etrimfos, EEHP and EPO
have been reported. Residues are extracted with acetone and partially
cleaned up by partitioning between water and dichloromethane. For the
determination of etrimfos as such the dichloromethane solution is
further cleaned up on a silica gel column and analysed by gas
chromatography using a flame photometric detector in the P-mode. The
latter method is suitable for adaptation to regulatory purposes.
Level causing no toxicological effect
Rat: 6 mg/kg in the diet equivalent to 0.3 mg/kg bw/day.
Dog: 10 mg/kg in the diet equivalent to 0.25 mg/kg bw/day.
Estimate of acceptable daily intake for man
0-0.003 mg/kg bw/day.
RECOMMENDATIONS OF RESIDUES LIMITS
The Meeting concludes that the residue levels listed below are
suitable for establishing temporary maximum residue limits. The MRLs
should remain as temporary limits, irrespective of the status of the
ADI, until items 1) and 2) of the further work or information,
required by 1982, are provided. The limits refer to the parent
compound etrimfos only.
Commodity (mg/kg) Remarks
Bran of wheat 20
(unprocessed)
Barley, maize, wheat 10
Wheat flour (wholemeal) 10
Wheat flour (white) 2
Rape seed 10
Rape seed oil (refined) 0.5
Carcass meat of cattle 0.011 ) These levels are based
Milk 0.011 ) on animal feeding studies.
Cattle meat byproducts 0.011 )
1 At or about the lower limit of determination
FURTHER WORK OR INFORMATION
Required (by 1982)
1. Adequate additional residue data from large-scale supervised
trials on cereal grains and their products.
2. Further residue data on rape seed and its oil (raw and refined).
3. Information on national use patterns and results of supervised
trials on growing crops destined for human or animal consumption.
4. Data on the amount and nature of the residues to be expected in
eggs and meat of poultry fed on treated grain.
Desirable
1. Data on residues on food in commerce and at consumption.
2. Information on national maximum residue limits.
3. Analytical method for the determination of residues in food of
animal origin.
4. Observations in man.
REFERENCES
Akram M., Ahmad S., and Forgash, A. J. Agric. Food Chem. Vol. 26, No.
4; 925-931.
Anonymous. Etrimfos. Identity. Unpublished report, undated from Sandoz
Ltd. submitted to the World Health Organization by Sandoz Ltd.
Anonymous. Etrimfos. Acute oral, subcutaneous and i.p. LD50 in male
and female mice. (1979a) Unpublished reports ref. Agro Dok CBK
3909/79, 3910/79 and 3911/79, from Sandoz Ltd. Agro Development
Toxicological Department, submitted to WHO by Sandoz Ltd.
Anonymous. Etrimfos. Acute oral, subcutaneous, i.p. and dermal LD50
in male and female rats. (1979b) Unpublished reports ref. Agro Dok CBK
3912/79, 3913/79, 3914/75 and 3915/79, from Sandoz Ltd. Agro
Development Toxicological Department, submitted to WHO by Sandoz Ltd.
Anonymous. The Pesticide Manual (6th edition), p.255. The British Crop
Protection Council. (1979c).
Brusick, D.J. and Weir, R.J. Mutagenicity evaluation of etrimfos.
Unpublished report no. 2683 ref. Agro Dok CBK 2545/77, dd. 30 December
1976 from Litton Bionetics submitted to WHO by Sandoz Ltd.
Carpy, S. and Klotzsche, C. San 197 I. 4-Week feeding study in rats.
Unpublished report no. Agro Dok CBK 754 b/73, dd. 18 March 1975, from
Sandoz Ltd., Agrochemical Research Department, Toxicological Section,
submitted to WHO by Sandoz Ltd.
Carpy, S. and Klotzsche, C. San 197 I. 2-Week dermal study in rabbits
intact skin. Unpublished report no. Agro Dok CBK 1727/75, dd. 15 April
1975, from Sandoz Ltd. Agrochemical Research Department, Toxicological
Section, submitted to WHO by Sandoz Ltd.
Carpy, S. and Klotzsche, C. San 197 I. 2-Week dermal study in rabbits,
abraded skin. Unpublished report no. Agro Dok CBK 1728/75, dd. 30 May
1975, from Sandoz Ltd. Agrochemical Research Department, Toxicological
Section, submitted to WHO by Sandoz Ltd.
Carpy, S. and Klotzsche, C. San 197 I. 3-Month feeding study in rats.
Unpublished report ref. Agro Dok CBK 1720/75, dd. 18 March 1975, from
Sandoz Ltd. Agrochemical Research Department, Toxicological Section,
submitted to WHO by Sandoz Ltd.
Carpy, S. and Klotzsche, C. San 197 I. 6-Month feeding study in dogs.
Unpublished report ref. Agro Dok CBK 1715/75, dd. 12 February 1975,
from Sandoz Ltd. Agro chemical Research Department, Toxicological
Section, submitted to WHO by Sandoz Ltd.
Carpy S. and Klotzsche, C. Etrimfos, 2-Year feeding study in rats.
Unpublished report no. 25/76 ref. Agro Dok CBK 1869/76, dd. 19 August
1976, from Sandoz Ltd. Agrochemical Research Department, Toxicological
Section, submitted to WHO by Sandoz Ltd.
Carpy S. and Klotzsche, C. SAN 197 I. 2-Year feeding study in dogs.
Unpublished report no. 41/77 ref. Agro Dok CBK 2568/77, dd. 3 August
1977, from Sandoz Ltd., Agro chemical Research Department,
Toxicological Section, submitted to WHO by Sandoz Ltd.
Carpy S. and Klotzsche C. San 197 I. 3-Generation study in rats.
Reproduction and teratogenicity data. Unpublished report ref. Agro Dok
CBK 3835/79, dd. 26 February 1979, from Sandoz Ltd., Agrochemical
Research Department, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Acute toxicity tests.
Unpublished report ref. Agro Dok CBK 754c/73, dd. 10 March 1975, from
Sandoz Ltd., Agrochemical Research Department, Toxicological Section,
submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Primary skin and eye
irritation tests in rabbits. Unpublished report ref. Agro Dok CBK
1725/75, dd. 18 March 1975, from Sandoz Ltd., Agrochemical Research
Department, Toxicological Section, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Neurotoxicity in chickens.
(With addendum for histopathological investigations for ischias
nerves.) Unpublished report ref. Agro Dok CBK 1852/75, dd. 18 March
1975, from Sandoz Ltd., Agrochemical Research Department,
Toxicological Section, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. Acute oral LD50 in rats and mice
(2-ethyl-4-ethoxy-6-hydroxy-pyrimidine). Unpublished report ref. Agro
Dok CBK 1777/75, dd. 18 March 1975, from Sandoz Ltd., Agrochemical
Research Department, Toxicological Section, submitted to WHO by Sandoz
Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Acute intramuscular LD50
in rats. Unpublished report ref. Agro Dok CBK 2518/77, dd. 21 February
1977, from Sandoz Ltd., Agrochemical Research Department,
Toxicological Section, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Sensitization test in
guinea pigs. Unpublished report ref. Agro Dok CBK 2519/77, dd. 22
February 1977, from Sandoz Ltd., Agrochemical Research Department,
Toxicological Section, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I (P=O analogue). Acute oral
and dermal LD50 in rats. Unpublished report, ref. Agro Dok CBK
2551/77, dd. 24 February 1977, from Sandoz Ltd., Agrochemical Research
Department, Toxicological Section, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Antitode test in rats.
Unpublished report, ref. Agro Dok CBK 3126, dd. 11 April 1978 from
Sandoz Ltd., Agrochemical Research Department, Toxicological Section,
submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Teratogenicity test in
rabbits. Unpublished report, ref. Agro Dok CBK 3132/78, dd. 22 June
1978 from Sandoz Ltd., Agrochemical Research Department, Toxicological
Section, submitted to WHO by Sandoz Ltd.
Ioannou, U.M. and Dauterman, W.C. In vitro metabolism of etrimfos by
rat and mouse liver. Pesticide Biochemistry and Physiology 9, 190-195.
Jucker, O. and Riggenbach, A. Etrimfos. Composition of technical
active ingredient. Unpublished report ref. Agro Dok CBK 3572/79e, dd.
1 November 1979, from Sandoz Ltd., Agro Development, Registration
submitted to WHO by Sandoz Ltd.
Karapally, J.C. Absorption, blood level, distribution and excretion of
SAN I 197 in the rat following a single oral dose. Unpublished report
ref. Agro Dok CBK 1829/75, dd. 21 April 1975, from Sandoz Ltd.,
submitted to WHO by Sandoz Ltd.
Karapally, J.C. Metabolism of 14C-Etrimfos in the rat. Unpublished
report, ref. Agro Dok CBK 3005, dd. 10 May 1977, from Sandoz Ltd.,
submitted to WHO by Sandoz Ltd.
Leuschner, F. Mikronucleus-test am Knockenmark von chinesischen
Hamstern (Test-präparat: Etrimfos). Unpublished report ref. Agro Dok
CBK 3136/78, dd. 4 July 1978, from Laboratorium für Pharmakologie und
Toxikologie, Hamburg submitted to WHO by Sandoz Ltd.
Richold, M. and Richardson, J.C. Motaphase analysis on SAN 197.
Unpublished report ref. CBK I 4798/80, dd. 24 April 1980, from
Huntingdon Research Centre submitted to WHO by Sandoz Ltd.
TOXICOLOGICAL STUDIES
Special studies on antidotes
Treatment of rats with atropine and obidoxime reduced the mortality
after oral administration of 3600 mg etrimfos/kg bw (Hamburger and
Klotzsche, 1978a).
Special studies on mutagenicity
Rat
Groups of 5 male and 5 female rats were administered, by intragastric
intubation 1500, 3000 or 6000 mg etrimfos/kg bw in two equal dosages
separated by an interval of 24 hours. A negative control group
received 1% methylcellulose, whereas a positive control group was
dosed by 2 i.p. injections with 8 mg Mitomycin c/kg bw.
After administration of etrimfos, the aberrant metaphase counts were
comparable with the control values, whereas Mitomycin C produced the
expected increase aberrant counts in the bone marrow cells of the
femurs (Richold and Richardson, 1980).
Hamster
Groups of 6 female Chinese hamsters were given s.c. injections of 30,
100 or 300 mg etrimfos/kg bw twice within 24 hours. MMS (100 mg/kg,
orally) was used as a positive control. Etrimfos did not elicit a
mutagenic response whereas the number of bone marrow micronuclei was
increased in the MMS-treated hamsters (Leuschner, 1978).
Microorganisms
Etrimfos (97%) in dosages from 0.001 up to 5.0 l/plate was not
mutagenic in the Ames test using the Salmonella typhimurium
strains TA-98, TA-100, TA-1535, TA-1537 and TA-1538 and
Saccharomyces cervisiae D-4, both with and without activation by
rat liver homogenate (Brusick and Weir, 1976).
Special studies on reproduction
Rat
Groups of 30 males and 30 females were fed dietary concentrations of
0, 3, 9 and 27 mg/kg in a three-generation, two-litter generation
reproduction study. In the B-generations one day before birth 5
pregnant females were killed and investigated for teratogenic
symptoms. The offspring of 5 other females were x-rayed and their
skeletons examined for abnormalities. After 21 days the tissues of 10
male and 10 female weanlings of the III B generation per dose group
were inspected histopathologically. The body weight gain and food
intake of the females were not affected by the treatment. No effects
were observed on fertility, gestation, viability, lactation, the
number of dead foetuses and the pup weight. Teratogenic events were
not observed over the course of the study. At the highest dose level
cholinesterase activity in plasma and erythrocytes, which was
determined in the weanlings of the B generations of all groups, was
slightly depressed, especially in the female rats. Brain
cholinesterase activity was not affected. Gross and microscopic
examination of tissues of organs of the F III B generation showed no
changes attributable to etrimfos administration (Carpy and Klotzsche,
1979).
Special studies on teratogenicity
Rabbits
Groups of 10 rabbits (12 in the control and in the low dose group)
were given etrimfos orally from day 6 to day 18 of pregnancy at dose
levels of 0, 25, 50 or 100 mg/kg bw. On day 29 of gestation the
animals were sacrificed and a caesarean section was performed. The
uterus was examined and the number, location and distribution of the
foetuses, implantations and resorptions recorded. Corpora lutea were
counted. The foetuses were weighed and examined for external and
skeletal anomalies. In the 50 and 100 mg groups the numbers of
implantations and living foetuses were decreased, whereas the number
of dead foetuses and weight of the placenta were slightly increased.
In the low dose group 2 animals with slightly curved hind legs and in
the highest dose group, in which 43 animals were investigated, one
animal with a rudimental tail and one animal with a skull deformation
were observed. In a historical control group of 286 animals 2 rabbits
with skull deformations were observed (Hamburger and Klotzsche,
1978b).
Special studies on neurotoxicity
Hen
Groups of 5-20 chickens were administered etrimfos in gelatine
capsules at dose levels of 100, 200, 400, 500, 750 or 1000 mg/kg bw.
A positive control group was given 2 × 80 mg tri-o-cresyl phosphate
(TOCP)/kg bw. Before the treatment the animals received atropine or
obidoxime by i.v, or i.m. injection. The observation period varied
from 21-42 days. Mortality was increased in all treated groups.
Doses of 200 mg/kg etrimfos and above caused typical signs of
organophosphate poisoning. A delayed neurotoxic response was not
observed in the chickens treated with etrimfos. TOCP elicited ataxia
and paralysis in all 10 animals. Histopathologically typical
degeneration changes in the ischias nerve were observed in all
TOCP-treated animals. No clear treatment-related alterations were
found in the nerves of the hens given 100 and 200 mg/kg etrimfos
(Hamburger and Klotzsche, 1975d).
Special studies on sensitization
Guinea pig
According to the "Guinea pig maximisation test" 20 guinea pigs were
treated with SAN 197 I as a 5% solution in DMSO and incorporated in
Freund's adjuvant; 10 guinea pigs served as a control group. 5/20 of
the treated and 4/10 of the control animals died before the 22nd day.
A slight development of scales at the application side without any
inflammatory changes or oedema was noticed at 72 hours after the
challenge application; no other effects were noticed (Hamburger and
Klotzsche, 1977b).
Acute toxicity
Symptoms of intoxication in rats and mice after oral, i.p. and s.c.
administration were limpness, lacrimation, salivation, exophthalmus,
laboured respiration, ataxia, tremors and convulsions. After dermal
application to rats and rabbits no toxic symptoms were observed
(Hamburger and Klotzsche, 1975a; Anonymous, 1979a and Anonymous,
1979b).
Rabbit
Etrimfos (0.5 g) applied in the intact or abraded skin of rabbits was
found to be not irritating. Instilled into the eye (100 mg) etrimfos
produced slight redness of the conjunctivae, which lasted 24 hours, in
one of six rabbits (Hamburger and Klotzsche, 1975b).
TABLE 1. Acute toxicity of etrimfos
species sex route LD50 in mg/kg bw purity solvent references
mouse M oral 47O (425-523) 97.2% water Hamburger and Klotzsche, 1975a
F oral 620 ± 28 97.2% water " " "
M oral 1120 (1023-1226) 90.5% corn-oil Anonymous, 1979a
F oral 1100 (930-1289) 90.5% corn-oil " "
M oral 535 (24 hours) - corn-oil Ioannou and Dauterman, 1978
M i.p. 698 (597-817) 90.5% corn-oil Anonymous, 1979a
F i.p. 679 (585-788) 90.5% corn-oil " "
M s.c. 979 (729-1155) 90.5% corn-oil " "
F s.c. 1050 (897-1229) 90.5% corn-oil " "
rat M oral 1800 (765-3733) 97.2% water Hamburger and Klotzsche, 1975a
F oral 2354 (1841-3008)1 97.2% water " " "
M oral 1930 (1664-2239) 90.5% corn-oil Anonymous, 1979b
F oral 1970 (1669-2325) 90.5% corn-oil " "
M oral 2040 (24 hours) - corn-oil Ioannou and Dauterman, 1978
M i.p. 767 (684-873) 97.2% water Hamburger and Klotzsche, 1975a
M i.p. 1310 (1110-1546) 90.5% corn-oil Anonymous, 1979b
F i.p. 1140 (950-1368) 90.5% corn-oil " "
M i.v. 25.5 ± 1.26 97.2% water Hamburger and Klotzsche, 1975a
F i.v. 21.0 ± 1.88 97.2% water " " "
M i.m. 4000 ± 116 94.3% PEG 200 Hamburger and Klotzsche, 1977a
M x.c. 3700 (3710-4563) 90.5% corn-oil Anonymous, 1979a
F s.c. 3690 (3000-4539) 95.5% corn-oil Anonymous, 1979b
dermal >2000 97.2% water Hamburger and Klotzsche, 1975a
M dermal >5000 90.5% corn-oil Anonymous, 1979b
F dermal >5000 90.5% corn-oil " "
rabbit dermal >500 97.2% water Hamburger and Klotzsche, 1975a
1 value corrected by recalculation
TABLE 2. Acute toxicity of metabolites
Species sex route solvent LD50 (mg/kg) references
rat M oral DMSO 1460 ± 52.5 Hamburger and Klotzsche, 1975e
rat F oral DMSO 1170 ± 58.5 " " "
mouse M oral DMSO 1530 ± 87.9 " " "
mouse F oral DMSO 1170 ± 94.8 " " "
P = O analogue of the parent compound
species sex route solvent LD50 (mg/kg) references
rat M oral DMSO 450 ± 35 Hamburger and Klotzsche, 1977c
rat M dermal DMSO 1000 " " "
Short-term studies
Rat
Male and female rats (15/sex/group) were dietary fed 0, 50, 250 or
1250 mg/kg SAN 197 I (97.2%) in the food for 4 weeks; later on in the
test groups (5/sex) on 10 mg/kg with corresponding controls were
added. The animals were examined for general health, body weight,
food consumption, haematology, blood biochemistry, urinalysis, organ
weights and macroscopy. A decreased cholinesterase activity in
erythrocytes was observed at 50 mg/kg and higher doses. Plasma-ChE
was decreased in females from the 50 mg/kg and in males from the 250
mg/kg groups, and brain-ChE in females from the 250 mg/kg and in males
at the 1250 mg/kg groups. Normochromic anaemia was noticed at 250
mg/kg and in females at 1250 mg/kg, changes in blood sugar occurred at
all dosages except 250 mg/kg and changes in reticulocytes at 1250
mg/kg. At these dosage the animals showed slight sedation and
slightly decreased body-weight gain as result of lower food intake
(Carpy and Klotzsche, 1975a).
Rat
Groups of 35 males and 35 females were fed etrimfos at concentrations
of 0, 3, 9 or 27 mg/kg feed for a period of 13 weeks. Body-weight
gain and food intake was recorded throughout the study of 25 females
and 25 males per group. After 4, 8 and 13 weeks haematology clinical
chemistry and urinanalysis was carried out in 10 animals/group. The
same number of rats was studied histopathologically and their organ
weights were recorded. At the end of the study the glucose
concentration in serum was increased in both males and females of the
highest dose level. The weight of the thyroid of the male rats of the
highest dose group was significantly decreased. However microscopic
examination showed no abnormalities attributable to the presence of
etrimfos in the diet. After 8 and 13 weeks the plasma cholinesterase
activity of the female rats in the 9 mg/kg group was slightly (20-24%)
and clearly depressed in the 27 mg/kg group (30-37%). Cholinesterase
activity in erythrocytes and brain was within normal limits (Carpy and
Klotzsche, 1975d).
Rabbit
Male and female rabbits (5/sex/group) received a dermal application of
0, 25 or 100 mg/kg SAN 197 I (97.2%) dissolved in polyethylene-glycol
on patches of intact or abraded skin for 8 hours a day on 5 days a
week during 2 weeks and were examined for general health, body weight,
haematology, blood biochemistry, organ weights and macroscopic changes
(microscopic studies are not yet completed). One male with intact
skin out of the 100 mg/kg group died with an enlarged liver. In the
males the weight of the spleen was decreased and the weight of the
thymus increased while in females a decreased weight of the thymus was
noticed. The animals with abraded skin showed abnormalities only at
the high dosage. Increased organ weights were observed in the female
liver and heart and in the males in the prostate. All these changes
were within the normal range. The animals with intact skin showed, at
both dosages, cholinesterase-inhibition in plasma and in erythrocytes.
In the animals with abraded skin, only in the high dosage, in males as
well as in the females, was cholinesterase inhibited in plasma and
erythrocytes (Carpy and Klotsche, 1975b and c).
Four groups of 4 male and 4 female beagle dogs were fed O, 2.5, 10 or
40 mg/kg etrimfos (97.2% purity) in the food for 26 weeks.
Normal values in haematology, blood chemistry, and urinalysis were
evaluated before starting the study. These determinations were
repeated 6 times during the test period: after 1, 4, 8, 13, 19 and 26
weeks, with exception of BSP which was estimated after 4, 13, and 26
weeks. The physical examination included weekly body weight, food
intake and neurological, oral, behaviourial, and ophthalmoscopic
inspection. The haematological parameters investigated were: RBC,
WBC, differential WBC, MCV, MCH, MCHC, reticulocytes, haemoglobin,
haematocrit, platelets, and prothrombin time, RBC- and
plasma-cholinesterase, FBS, BUN, SGPT, SGOT, LDH, uric acid, total
serum protein, bilirubin, creatinine, serum alkaline phosphatase,
cholesterol, serum sodium, potassium, calcium, chloride, inorganic
phosphate, serum albumin, and serum electrophoresis (A/G ratio). The
activity of cholinesterase in cerebellum and cerebrum, of the liver
cytochrome P-450, of the liver drug metabolising enzymes O-, and
S-demethylases and of the anilin-4-hydroxylase and the amount of
cholesterol, glycogen, lipids, and total protein contained in the
liver were estimated at the end of the study. All animals were
examined for gross pathology, organ weights and histopathology. MCH
and MCHC values were increased in the male animals of the 40 mg/kg
group. From the 11th week on plasma and erythrocyte cholinesterase
activity was depressed in comparison to the pretest and the control
values, with a plateau of about 40% in both males and females. In the
10 mg/kg group a slight depression of cholinesterase activity in
plasma and erythrocytes especially in comparison to the pre-test
values was observed in both sexes. In the cerebrum of the female rat
of the highest dose group a decrease of 22% of the cholinesterase
activity was measured.
From the 13th week on the albumin concentration in serum was
dose-dependant increased. However this was not confirmed by
electrophoresis and had disappeared almost at the end of the study.
The weight of the organs was within normal limits. No gross or
histopathological aberrations were observed (Klotzsche and Carpy,
1975e).
Long-term studies
Rat
Groups of 40 males and 40 females were fed etrimfos (97.8%) in the
diet at concentrations of 0, 6, 12 or 24 mg/kg for a period of 2
years. Body weight, food consumption and water intake of 30 rats per
dose and sex were recorded weekly. After 1, 2 ,3, 6, 9, 12, 15, 18,
21 and 24 months haematology, clinical chemistry and urinalysis were
carried out in 5 male and 5 female animals per group. At the end of
the study BSP a liver function test was carried out in the control and
high-dosed females. In liver homogenates the activity of drug
metabolising enzymes and the total protein and cholesterol
concentration were measured at the end of the study.
All animals dying during the study, or which had to be killed, were
necropsied and tissues were examined microscopically. The organ
weights of 10 rats per dose and sex were recorded.
There was no clear effect of etrimfos on body weight, food and water
intake and mortality. The cholinesterase activity of the plasma and
erythrocytes of male rats was not affected by etrimfos. In the
females the activity of cholinesterase in the plasma of the 24 and 12
mg/kg group was decreased by 35-50 and 20-30% respectively throughout
the study. In the highest dose group a marginal effect was observed
on the activity in the erythrocytes of the female rats. Neither in
the females nor in the males was the cholinesterase activity of the
brain and the liver of the treated animals significantly different
from the control values.
Both in the male and female rats the cytochrome P-450 of the liver of
the animals of the 12 and 24 mg/kg group was significantly decreased
in comparison to the controls; however no dose-response relation was
observed.
The macroscopic and microscopic examination did not reveal organ
lesions that could be attributed to treatment. The tumour rate in
treated animals corresponded with the incidence in control animals.
No-effect level of this study is 6 mg/kg (Carpy and Klotzsche, 1976).
Dog
Groups of 4 male and 4 female beagle dogs received 0, 4, 10 or 25
mg/kg etrimfos in their diet, during 106 weeks. There were no signs
of adverse behaviour and mortality was not noted. Growth and food
consumption were not clearly affected. Eye examinations during the
first, 26th, 52nd, 78th and 104th week did not indicate adverse ocular
changes.
Haematology, clinical chemistry and urinalysis were carried out in
week 1 (pre-test), 4, 8, 13 and quarterly thereafter. Plasma
cholinesterase activity was decreased in the 10 and 25 mg/kg groups by
23% and 24% respectively. The cholinesterase activity of the
erythrocytes was also decreased.
No clear differences or sensitivity were seen between males and
females. In the cerebrum, cerebellum and liver the cholinesterase
activity was not depressed.
Slightly higher activities of N-, O- and S-demethylase activity were
measured in all treated groups. However there was no clear
dose-response relation. Neither the weights of the organs nor the
histopathological evaluation revealed indications for treatment-
related changes in any organ or tissue. No-effect level of this study
is 10 mg/kg (Carpy and Klotzsche, 1977).
RESIDUES IN FOOD
RESIDUES RESULTING FROM SUPERVISED TRIALS
Stored grain products
Wheat grain (variety Probus) was treated in 25 kg lots with
Satisfar(R) (Ekamet 50 EC) at the rate of 5, 10 or 15 mg/kg ai under
conditions as close to normal practice as possible. The grain, which
was stored in fibre-drums at room temperature, was analysed
periodically for etrimfos.
In this small-scale trial the etrimfos content of the wheat grain
immediately after treatment (zero time) was 50 - 60 percent of the
application rate. During the first six months of storage, the amount
of etrimfos in the grain remained fairly constant.
In the next six months its amount was reduced to 34-37% of the
application rate, (or 63-75% of the original concentration in the
grain). At the end of 12 months, the grain treated at the rate of 10
and 15 mg/kg had 100% efficacy against Sitophilus; the grain treated
at the rate of 5 mg/kg had 90% activity. (CBK 3630/79).
Several trials were conducted in different countries on wheat, barley
and corn (table 3).
FATE OF RESIDUES
In plants
The fate of etrimfos in bean and corn plants has been studied (M.
Akram et al., 1978). The primary leaves of bean and corn seedlings
were treated with 14C etrimfos. The treated leaves and the untreated
portions of the plants were sampled at 0, 3, 7, 14 and 21 days after
treatment. The aqueous rinses and the extracts of the treated leaves
were examined for the parent compound and its metabolites. Leaf
remainder, and untreated portions, were analysed for total
radiocarbon.
Etrimfos rapidly volatilised from the leaf surface. Within the first
three days after treatment it is lost with a half-life of approx. 3
days (beans and corn) and during 3 to 15 days with a half-life of
approx. 10 days from beans and approximately 5 days from corn. Three
weeks after treatment the radioactivity, retained by the leaves after
rinsing, ranged from 33% to 42% for bean, and from 25% to 42% for
corn. No translocation to the root system was observed. A small
percentage of the applied radiocarbon was present in the untreated
foliage of bean (0.3-0.6% of the applied radioactivity became
unextractable in bean leaves, and 0.1-0.3% in corn leaves).
Leaf rinses (surface residues) of bean and corn contained mainly
etrimfos and small quantities of 6-ethoxy-2-ethyl-4-hydroxy-pyrimidine
(EEHP). Small amounts of the P=O analogue of etrimfos were also
observed in the corn-rinse.
The leaf extracts (subsurface residues) of bean and corn contained
etrimfos and minor quantities of EEHP and five unknown metabolites, of
which one was tentatively identified as
2-ethyl-4,6-dihydroxy-pyrimidine; 21-day bean leaf contained 33.5% of
the applied radioactivity, of which 11.9% was etrimfos, 2.4% EEHP,
9.7% the unknown metabolites and 9.5% highly polar or conjugated
metabolites (TLC origin). All single metabolites were less than 10%
relative to applied etrimfos; 21-day corn leaf had about 24.3% of the
applied radioactivity, of which 3.8% was etrimfos, 0.4% EEHP, 15.8%
the unknown metabolites and 4.3% polar or conjugated metabolites. All
single metabolites were less than 10% relative to applied etrimfos.
The enzyme and acid-hydrolysed aqueous extracts gave essentially the
same TLC pattern as the unhydrolysed material, with quantitative
differences.
From the described results, we learn that etrimfos is lost from
treated plants in two stages. EEHP was found as a metabolite, but
only in quantities of less than 10% of the applied etrimfos. The P=O
analogue was not found in bean leaves nor in corn leaves. No single
metabolite was present in quantities more than 10% relative to the
applied etrimfos so that the influence of these metabolites on the
toxicity of actual residue levels in practical field samples may be
neglected. Tolerance recommendations may, therefore, be based on
residue data of the etrimfos ai only.
In cereal products
Wheat grain (variety, Probus) was treated in 25 kg lots with
Satisfar(R) (Ekamet 50 EC) at the rate of 5, 10 or 15 mg/kg ai. The
grain was stored at room temperature and at intervals of 2, 4, 6 and
12 months, samples of grain were milled and separated into different
fractions. Also bread was baked from the flour from these samples.
Etrimfos residues were analysed in all these samples.
Etrimfos concentration in the milled fractions of the wheat was the
highest in the bran and the lowest in the white flour. During the
baking process the etrimfos content of the flour was reduced by 58-77%
in the case of white bread, and 43-65% in the case of whole wheat
bread. The etrimfos content of white bread ranged from about 4 to 6%
of the amount applied and that in the whole wheat bread was in the
range of 14 to 30% of the amount applied (CBK 3630/79). Table 4.
In another trial with wheat, the amount of etrimfos in the white flour
ranged from 25 to 33% of that actually present in the grain. Baking
to bread reduced these residues in the flour by about 50% (CBK
4119/79). Table 5.
In a trial with barley, the wort from free grain contained only 4-6%
of the etrimfos originally present in the grain. The wort from malt
grain had no detectable residue of etrimfos (CBK 4120/79). Table 5.
TABLE 3. Residues of etrimfos (mg/kg) in stored products treated after harvest
Application Storage period (months)
Commodity Rate 0 1-2 3-4 6 8-10 12-13 18-24 Remarks Ref.
(country) Type (mg/kg) (24 h)
Cereal grain
Barley
(England) 50 EC 5 2.3 3.2-3.6 2.1 0.8 amount treated:
content: 15%; temp.: CBK
0-15°C; untreated 4121/79
"blank": 0.03-0.04
mg/kg
(Kenya) 0.5 Dust 30 2.25 2.1 0.6 0.3 amount treated:
1 Dust 29 4.5 3.0 1.3 0.8 45 kg each, UK
50 EC 5 1.3 0.5 0.2 stored in bags; 3770/79
2 Dust 22 9 9.7 1.2-2.2 0.8-1.4 conditions 4149/79
50 EC 10 4.1 1.0-2.1 0.5-0.9 not reported
Corn(maize)
(Kenya) 0.5 Dust 30 2.75 0.6-0.8 0.5 0.3-0.4 0.3-0.4 - amount treated:
50 EC 5 1.5-1.6 1.6-1.7 0.8-1.2 1.0-1.1 0.8 45 kg each,
1 Dust 29 5.5 0.9-2.6 0.7-1.5 1.0-1.6 0.8-0.9 0.9-1.3 stored in bags;
50 EC 10 0.8-4.2 1.7-2.9 1.5-2.5 1.9-2.5 2.5-2.6 storage conditions
2 Dust 22 11 1.7-5.5 2.4-3.8 2.2-2.9 1.2-2.4 1.5-2.3 not reported
Wheat
(England) 50 EC 5 6.8 5.6-6.1 5.9 3.9 amount treated:
50 EC 10 10.9 6.7-7.2 5.4 6.4 10 t; 30 t on
conveyer belt; CBK
moisture cont. 11%; 4118/79
0-17°C; untreated
"blank": 0.01-1 mg/kg
TABLE 3. Continued...
Application Storage period (months)
Commodity Rate 0 1-2 3-4 6 8-10 12-13 18-24 Remarks Ref.
(country) Type (mg/kg) (24 h)
(France) 50 EC 5 1.9-2.0 1.4-2.0 1.6 storage conditions not
50 EC 7.5 1.6-2.5 2.1-3.3 2.1-2.9 reported; samples for CBK
50 EC 10 3.3-5.4 4.2 2.6-3.8 analysis: 10 g each 2943/79
(Kenya) 0.5 Dust 30 2.5 1.2 0.7 1.0 0.8 0.4 amount treated:
1 Dust 29 5 2.0 1.8 1.5 1.3 1.0 10 kg each, stored CBK
50 EC 5 1.6 1.0 1.0 0.8 0.5 in bags; storage 3771/79
2 Dust 22 10 3.7 2.8 2.7 2.1 1.8 temp. about 15-25°C; 4117/79
50 EC 10 2.0 2.5 2.6 2.5 1.7 further conditions 4148/79
not reported.
(Switzerland) 50 EC 5 2.9 2.9 2.7 3.0 1.9 1.1 0.6 amount treated: 25 kg
(<0.1) (<0.1) (<0.1) (0.2) (0.4) (0.2) (<0.1) each, stored in air
50 EC 10 5.4 5.8 5.5 5.6 3.4 1.7 1.3 permeable fibre-drums;
(0.1) (0.5) (0.1) (0.3) (1.2) (0.2) (<0.1) moisture content: 12-14%; CBK
50 EC 15 7.4 7.8 7.6 8.1 5.6 temp about 16-20°C; 3630/79
(0.1) (0.2) (0.2) (0.5) (1.5) EEHP residues reported 3707/79
in brackets; EPO 4209/80
residues were always
below 0.002 mg/kg.
Rape seed
(England) EC 10 9-10 8-9 9-10 7-8 :Bin 2 amount treated: Stables
EC 10 11-15 10-15 13-14 13-14 10 t each, stored et al.,
:Bin 3 in metal bins; (1979)
moisture content in seed:
9-11%; temp. 3-15°C; seed
in bin 2 was slightly damper and
more heavily infested by mites
than in bin 3. Etrimfos in rape
seed oil (refined) up to about
0.5 mg/kg/ in spent meal: 0.05 mg/kg.
TABLE 4: Etrimfos1 residues in milled fractions and flour of wheat and in bread
Treatment Residues (mg/kg) 2-12 months after treatment
rate 2 4 6 12
5 mg/kg Etrimfos EEHP2 Etrimfos EEHP Etrimfos EEHP Etrimfos EEHP
Grain 2.92 n.d.3 2.74 n.d. 3.02 0.20 1.89 0.36
Bran 10.47 1.10 9.94 0.39 9.78 0.38 3.50 2.10
Grits 8.67 0.62 8.64 0.62 6.83 0.27 3.48 1.02
White flour 0.82 n.d. 1.02 n.d. 0.73 n.d. 0.70 n.d.
White bread 0.23 0.05 0.24 0.10 0.21 0.11 0.18 n.d.
Whole wheat
flour n.a.4 n.a. 2.66 0.16 2.33 0.11 1.97 0.37
Whole wheat
bread n.a n.a. 1.52 0.22 1.33 0.34 0.69 0.23
10 mg/kg Etrimfos EEHP2 Etrimfos EEHP Etrimfos EEHP Etrimfos EEHP
Grain 5.84 0.54 5.50 0.08 5.59 0.34 3.38 1.20
Bran 15.22 1.92 16.27 0.66 15.83 1.14 6.20 3.34
Grits 41.29 1.27 15.08 0.73 13.14 1.06 7.74 1.97
White flour 1.89 0.14 1.75 n.d. 1.47 0.06 0.96 n.d.
White bread 0.50 0.13 0.61 0.24 0.51 0.20 0.41 n.d.
Whole wheat
flour n.a. n.a. 5.59 0.39 5.07 0.24 3.70 0.69
Whole wheat
bread n.a. n.a. 2.83 0.60 2.38 0.51 1.37 0.51
TABLE 4. Continued...
Treatment Residues (mg/kg) 2-12 months after treatment
rate 2 4 6 12
15 mg/kg Etrimfos EEHP2 Etrimfos EEHP Etrimfos EEHP Etrimfos EEHP
Grain 7.80 0.20 7.61 0.20 8.11 0.46 5.57 1.52
Bran 16.60 1.50 21.65 1.56 19.70 1.51 9.66 4.34
Grits 19.41 1.53 20.84 0.93 19.33 1.06 12.14 1.97
White flour 2.04 0.25 2.62 0.09 2.48 0.10 1.50 n.d.
White bread 0.74 0.18 0.95 0.49 0.78 0.31 0.54 0.23
Whole wheat
flour n.a. n.a. 7.99 0.41 7.84 0.40 5.90 0.71
Whole wheat
bread n.a. n.a. 3.80 0.40 3.77 0.70 2.30 0.46
1 The metabolite P=O etrimfos could not be detected
2 EEHP = 2-ethyl-4-ethoxy-5-hydroxy-pirimidine
3 n.d = not detectable
4 n.a. = not analysed
TABLE 5. Etrimfos residues in cereals processed after treatment (in UK) with SATISFAR(R)
Cereal Formulation Rate of Etrimfos Amount of Method of treatment Reference
treatment residues grain per
(ai/mg/kg) (ppm) treatment
Wheat 50 EC 5 mg/kg grain 0.76 6 kg The required amount of CBK
bran 4.62 Satisfar in 25 ml water 4119/79
offal 3.13 was sprayed onto grain
white flour 0.25 tumbling in a
white bread 0.12 Bluefinsowaway cement mixer.
10 mg/kg grain 2.51 Stored in sealed plastic
bran 4.50 bags.
offal 6.14
white flour 0.63
white bread 0.34
Barley 50 EC 5 mg/kg free grain 1.07 CBK 4120/79
wort 0.07
spent 0.63
malt grain 0.21
wort n.d.
spent gr 0.07
10 mg/kg free grain 3.50
wort 0.16
spent gr 0.52
malt grain -
wort n.d.
spent gr 0.11
In cattle
A three level feeding study in dairy cattle with etrimfos incorporated
in the diet at levels 0.5, 1.5 or 5 mg/kg etrimfos was conducted. The
milk production was not affected. The cows, sacrificed after 28 days
of feeding, had only less than 0.01 mg/kg etrimfos in the muscle,
liver, kidney and fat. Etrimfos residue in the milk throughout the
study was less than 0.01 mg/kg. The residue in the milk solids
separated from the 21st and 28th day samples also had only 0.01 mg/kg
etrimfos (CBK 3011/77).
In soil
Degradation of 14C etrimfos in a sandy loam, silt loam and silty clay
loam soil was studied.
The soils treated with 14C-etrimfos at the rate of 5 mg/kg were
incubated at room temperature for 70 days. At selected time intervals
during the incubation period, the soil samples were analysed for the
parent and degradation products.
Etrimfos was rapidly hydrolysed, in all soils, to
6-ethoxy-2-ethyl-4-hydroxy-pyrimidine (EEHP). Its amount was reduced
to 50% in 3-8 days, and to 10% in 15-30 days.
The formation of EEHP paralleled the dissipation of etrimfos. Its
proportions reached a maximum of 75-85% in 14-28 days, after which
period its concentration began to decrease. The disappearance of EEHP
was fastest in silt, and rather slower in sand, and still more so in
clay. At the end of 70 days, the amounts of EEHP in silt, sand and
clay were 4, 18 and 52% respectively.
A further metabolite, tentatively identified as
5-chloro-6-ethoxy-2-ethyl-4-hydroxy-pyrimidine (CEEHP), was detected
in all soil extracts. Its formation seemed to be related to the
dissipation of EEHP, and its occurrence was highest in silt (24%),
followed by sand and clay (6%).
The soil-"bound" residues became significant only after EEHP began to
be metabolised. A maximum of 12% of these was observed for sand, and
21% for silt and clay. Further extraction with methanol-water 75:25
reduced the bound residue to <10%, the metabolites being mainly EEHP
and CEEHP.
The radiocarbon lost from the soil during the 70 days' incubation
amounted to 60% for sand or silt and 25% for clay. In a separate
experiment, using sandy loom soil treated with 14C-etrimfos, it was
demonstrated that the radioactivity lost from the soil was due to the
14CO2, which began to be generated after a time lag of at least 20
days (CBK 3002/77).
Photodegradation
in water
Under blacklight and daylight, which contain essentially no shorter
wave lengths than natural sunlight, aqueous solution of etrimfos (5
mg/kg) was quite stable: degradations of 10% (blacklight) and 5%
(daylight) occurred within 24 hours of exposure. Under mercury arc
light, approx. 45% degraded during the same time (CBK 1642/75).
on glass plates
2.5 mg of etrimfos, spread over an area of 70 cm2 and covered with
quartz plates, was exposed to the same light sources as in water.
Degradation, after 24 hours under the following conditions, was
daylight <5%, blacklight <5%, mercury light ‰ 25%, (CBK 1642/75).
METHODS OF RESIDUE ANALYSIS
Two methods have been developed - one for determining etrimfos the P=O
analogue and 2-ethyl-4-ethoxy-6-hydroxypyrimidine (EEHP), the
hydrolysis product of etrimfos (CBK 2178/76) and the other for
determining etrimfos only (CBK 3649/79). In both methods grain is
dealt with in the same manner as vegetable crops.
The P=O analogue of etrimfos has been detected in the stored grain
products. The amount of EEHP in the grain is generally quite low and
hence, in these cases, the method CBK 3649/79 is recommended.
In these methods etrimfos residues are extracted with acetone and
partially cleaned up by partitioning between water and methylene
dichloride. If only etrimfos is to be determined, the methylene
dichloride solution is further cleaned up on a silica gel column and
determined by gas chromatography using a flame photometric detector
(P-mode). If the P=O analogue and EEHP are to be included, the
methylene dichloride solution is further cleaned up on a
polyethylene-coated alumina column and determined by gas
chromatography using a nitrogen specific detector.
A series of CBA reports on residues in food and fats in the
environment were submitted by Sandoz Ltd. to FAO for this evaluation.
EVALUATION
Etrimfos is a broad-range non-systemic contact and stomach
organophosphorous insecticide, which was introduced in 1972 and is
registered and/or used in a considerable number of countries. It is
effective against biting and sucking insects such as Lepidoptera,
Coleoptera, Diptera and to a variable extent, Hemiptera at 0.25-0.75
kg ai/ha. It is mainly used on fruit, vegetables, maize and
ornamental plants, but also on alfalfa and paddy rice, as an
emulsifiable concentrate or as granules.
Studies of the metabolism and distribution of etrimfos in rats have
been undertaken with 14C in the pyrimidine moiety. This has provided
useful information on the pyrimidine portion of the molecule, but
disposition and kinetic data on the whole molecule of etrimfos and its
triester metabolites are required. This is especially important since
the compounds containing a fully-esterified phosphoroticate or
phosphate group are the more toxic.
In rats and rabbit no effects on reproduction, including
teratogenicity, were observed. A mutagenic response was negative as
shown by a rat metaphase analysis, a hamster bone marrow micronuclei
count, and an Ames test.
Etrimfos did not induce a delayed neurotoxic response, as generally
noted with TOCP, in hens. A skin-sensitisation study in guinea pigs
was negative.
Etrimfos and the main metabolite
O-6-ethoxy-2-ethyl-4-hydroxypyrimidine (EEHP) were slightly toxic
following acute administration to various animal species. The effects
of etrimfos were typical of those caused by cholinesterase inhibitors.
Several short- and long-term toxicity studies have been carried out
with rats and dogs. The macroscopic and microscopic examination in
these studies did not reveal lesions in organs attributable to
etrimfos. The tumour incidence in treated animals corresponded with
that in control animals. In both species etrimfos produced
cholinesterase inhibition.
Based on the two-year studies with both rat and dog a temporary ADI
was recommended.
Although it is known that data from supervised trials (pre-harvest
use) exist, which show that residues in many fruits and vegetables,
potatoes, maize and rape are in the range 0.05 to 0.5 mg/kg when
etrimfos is used in accordance with good agricultural practice,
information was not made directly available for consideration by the
meeting.
However, in recent tests and trials carried out in England, France,
Kenya and Switzerland etrimfos was also found efficacious against
moths, (malathion-resistant) beetles and (lindane-resistant) mites in
stored crops, especially grain and rape seed. Recommended rates for
dusting or spraying wheat, barley, maize (corn) or rape seed are 5 to
10 mg/kg. Depending on storage conditions (e.g. degree of
infestation, climate, storage period) lower or, exceptionally, higher
dosages up to 15 mg/kg appear appropriate.
Etrimfos in stored rape seed shows no significant loss after 6 months
storage and the estimated half-life in stored grain is about 3 to 8
months. Etrimfos is rapidly lost from treated plants (beans, maize),
about half within the first 3 days, with a half-life of about 5 to 10
days between the 3rd and the 15th day after application, probably
owing to the different rate of volatilisation of the initial deposit
and of the residue after penetration into the surface layer.
In degradation studies with plants treated with 14C-etrimfos,
labelled at carbons 4 and 6 in the pyrimidinyl ring the metabolites
O-6-ethoxy-2-ethyl-4-hydroxypyrimidine (EEHP) and 2-ethyl-4,
6-dihydroxypyrimidine (EDHP) were identified. Small amounts of the
etrimfos P=O analogue (EPO) were also observed, but only on the plant
surface. In stored grain EEHP was the only metabolite found in
significant quantities.
After a single oral does of 50 mg etrimfos/kg, rats excreted more than
50% as EEHP in urine and faeces, with smaller amounts of EDHP,
6-ethoxy-4-hydroxy-2-(1-hydroxyethyl) pyrimidine (EEHP-1), its
2-(2-hydroxyethyl) pyrimidine analogue (EEHP-2) and
2-ethyl-4-hydroxy-6-(2 hydroxyethoxy) pyrimidine (EHHEP). In rat
liver preparations desmethyl-etrimfos was also detected.
In soils the half-life of etrimfos is about 3 to 8 days: degradation
results in EEHP, EDHP and finally carbon dioxide. General degradation
pathways are thus similar in plants, animals and soils, although
proportions of individual metabolites may vary. Hydrolysis of the
phosphorothioate ester seems to be the most significant pathway,
yielding products of lesser toxicological importance.
When dairy cattle were fed with diets containing 5 mg etrimfos/kg for
28 days, etrimfos residues were always below 0.01 mg/kg in meat, milk,
fat and edible offals.
Processing wheat, treated with 10 mg atrimfos/kg and stored for 2 to
12 months, resulted in etrimfos residues up to 16.3 mg/kg in raw bran,
5.6 mg/kg in wholemeal flour, 2.8 mg/kg in wholemeal bread, 1.9 mg/kg
in white flour and 0.6 mg/kg in white bread. Provisional information
on barley treated with 5 to 10 mg etrimfos/kg and used for malting and
brewing showed etrimfos residues up to 0.6 mg/kg in spent grain and
0.2 mg/kg in wort. When stored rape seed containing 10 to 15 mg
etrimfos/kg was used for oil production, preliminary results indicated
that 95-100% of the etrimfos was lost during the refinement of the oil
and that the greatest looses occurred by degradation during bleaching
and steam distillation. No residues were detected in the spent meal.
Analytical methods for determining residues of etrimfos, EEHP and EPO
have been reported. Residues are extracted with acetone and partially
cleaned up by partitioning between water and dichloromethane. For the
determination of etrimfos as such the dichloromethane solution is
further cleaned up on a silica gel column and analysed by gas
chromatography using a flame photometric detector in the P-mode. The
latter method is suitable for adaptation to regulatory purposes.
Level causing no toxicological effect
Rat: 6 mg/kg in the diet equivalent to 0.3 mg/kg bw/day.
Dog: 10 mg/kg in the diet equivalent to 0.25 mg/kg bw/day.
Estimate of acceptable daily intake for man
0-0.003 mg/kg bw/day.
RECOMMENDATIONS OF RESIDUES LIMITS
The Meeting concludes that the residue levels listed below are
suitable for establishing temporary maximum residue limits. The MRLs
should remain as temporary limits, irrespective of the status of the
ADI, until items 1) and 2) of the further work or information,
required by 1982, are provided. The limits refer to the parent
compound etrimfos only.
Commodity (mg/kg) Remarks
Bran of wheat 20
(unprocessed)
Barley, maize, wheat 10
Wheat flour (wholemeal) 10
Wheat flour (white) 2
Rape seed 10
Rape seed oil (refined) 0.5
Carcass meat of cattle 0.011 ) These levels are based
Milk 0.011 ) on animal feeding studies.
Cattle meat byproducts 0.011 )
1 At or about the lower limit of determination
FURTHER WORK OR INFORMATION
Required (by 1982)
1. Adequate additional residue data from large-scale supervised
trials on cereal grains and their products.
2. Further residue data on rape seed and its oil (raw and refined).
3. Information on national use patterns and results of supervised
trials on growing crops destined for human or animal consumption.
4. Data on the amount and nature of the residues to be expected in
eggs and meat of poultry fed on treated grain.
Desirable
1. Data on residues on food in commerce and at consumption.
2. Information on national maximum residue limits.
3. Analytical method for the determination of residues in food of
animal origin.
4. Observations in man.
REFERENCES
Akram M., Ahmad S., and Forgash, A. J. Agric. Food Chem. Vol. 26, No.
4; 925-931.
Anonymous. Etrimfos. Identity. Unpublished report, undated from Sandoz
Ltd. submitted to the World Health Organization by Sandoz Ltd.
Anonymous. Etrimfos. Acute oral, subcutaneous and i.p. LD50 in male
and female mice. (1979a) Unpublished reports ref. Agro Dok CBK
3909/79, 3910/79 and 3911/79, from Sandoz Ltd. Agro Development
Toxicological Department, submitted to WHO by Sandoz Ltd.
Anonymous. Etrimfos. Acute oral, subcutaneous, i.p. and dermal LD50
in male and female rats. (1979b) Unpublished reports ref. Agro Dok CBK
3912/79, 3913/79, 3914/75 and 3915/79, from Sandoz Ltd. Agro
Development Toxicological Department, submitted to WHO by Sandoz Ltd.
Anonymous. The Pesticide Manual (6th edition), p.255. The British Crop
Protection Council. (1979c).
Brusick, D.J. and Weir, R.J. Mutagenicity evaluation of etrimfos.
Unpublished report no. 2683 ref. Agro Dok CBK 2545/77, dd. 30 December
1976 from Litton Bionetics submitted to WHO by Sandoz Ltd.
Carpy, S. and Klotzsche, C. San 197 I. 4-Week feeding study in rats.
Unpublished report no. Agro Dok CBK 754 b/73, dd. 18 March 1975, from
Sandoz Ltd., Agrochemical Research Department, Toxicological Section,
submitted to WHO by Sandoz Ltd.
Carpy, S. and Klotzsche, C. San 197 I. 2-Week dermal study in rabbits
intact skin. Unpublished report no. Agro Dok CBK 1727/75, dd. 15 April
1975, from Sandoz Ltd. Agrochemical Research Department, Toxicological
Section, submitted to WHO by Sandoz Ltd.
Carpy, S. and Klotzsche, C. San 197 I. 2-Week dermal study in rabbits,
abraded skin. Unpublished report no. Agro Dok CBK 1728/75, dd. 30 May
1975, from Sandoz Ltd. Agrochemical Research Department, Toxicological
Section, submitted to WHO by Sandoz Ltd.
Carpy, S. and Klotzsche, C. San 197 I. 3-Month feeding study in rats.
Unpublished report ref. Agro Dok CBK 1720/75, dd. 18 March 1975, from
Sandoz Ltd. Agrochemical Research Department, Toxicological Section,
submitted to WHO by Sandoz Ltd.
Carpy, S. and Klotzsche, C. San 197 I. 6-Month feeding study in dogs.
Unpublished report ref. Agro Dok CBK 1715/75, dd. 12 February 1975,
from Sandoz Ltd. Agro chemical Research Department, Toxicological
Section, submitted to WHO by Sandoz Ltd.
Carpy S. and Klotzsche, C. Etrimfos, 2-Year feeding study in rats.
Unpublished report no. 25/76 ref. Agro Dok CBK 1869/76, dd. 19 August
1976, from Sandoz Ltd. Agrochemical Research Department, Toxicological
Section, submitted to WHO by Sandoz Ltd.
Carpy S. and Klotzsche, C. SAN 197 I. 2-Year feeding study in dogs.
Unpublished report no. 41/77 ref. Agro Dok CBK 2568/77, dd. 3 August
1977, from Sandoz Ltd., Agro chemical Research Department,
Toxicological Section, submitted to WHO by Sandoz Ltd.
Carpy S. and Klotzsche C. San 197 I. 3-Generation study in rats.
Reproduction and teratogenicity data. Unpublished report ref. Agro Dok
CBK 3835/79, dd. 26 February 1979, from Sandoz Ltd., Agrochemical
Research Department, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Acute toxicity tests.
Unpublished report ref. Agro Dok CBK 754c/73, dd. 10 March 1975, from
Sandoz Ltd., Agrochemical Research Department, Toxicological Section,
submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Primary skin and eye
irritation tests in rabbits. Unpublished report ref. Agro Dok CBK
1725/75, dd. 18 March 1975, from Sandoz Ltd., Agrochemical Research
Department, Toxicological Section, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Neurotoxicity in chickens.
(With addendum for histopathological investigations for ischias
nerves.) Unpublished report ref. Agro Dok CBK 1852/75, dd. 18 March
1975, from Sandoz Ltd., Agrochemical Research Department,
Toxicological Section, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. Acute oral LD50 in rats and mice
(2-ethyl-4-ethoxy-6-hydroxy-pyrimidine). Unpublished report ref. Agro
Dok CBK 1777/75, dd. 18 March 1975, from Sandoz Ltd., Agrochemical
Research Department, Toxicological Section, submitted to WHO by Sandoz
Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Acute intramuscular LD50
in rats. Unpublished report ref. Agro Dok CBK 2518/77, dd. 21 February
1977, from Sandoz Ltd., Agrochemical Research Department,
Toxicological Section, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Sensitization test in
guinea pigs. Unpublished report ref. Agro Dok CBK 2519/77, dd. 22
February 1977, from Sandoz Ltd., Agrochemical Research Department,
Toxicological Section, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I (P=O analogue). Acute oral
and dermal LD50 in rats. Unpublished report, ref. Agro Dok CBK
2551/77, dd. 24 February 1977, from Sandoz Ltd., Agrochemical Research
Department, Toxicological Section, submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Antitode test in rats.
Unpublished report, ref. Agro Dok CBK 3126, dd. 11 April 1978 from
Sandoz Ltd., Agrochemical Research Department, Toxicological Section,
submitted to WHO by Sandoz Ltd.
Hamburger, F. and Klotzsche, C. San 197 I. Teratogenicity test in
rabbits. Unpublished report, ref. Agro Dok CBK 3132/78, dd. 22 June
1978 from Sandoz Ltd., Agrochemical Research Department, Toxicological
Section, submitted to WHO by Sandoz Ltd.
Ioannou, U.M. and Dauterman, W.C. In vitro metabolism of etrimfos by
rat and mouse liver. Pesticide Biochemistry and Physiology 9, 190-195.
Jucker, O. and Riggenbach, A. Etrimfos. Composition of technical
active ingredient. Unpublished report ref. Agro Dok CBK 3572/79e, dd.
1 November 1979, from Sandoz Ltd., Agro Development, Registration
submitted to WHO by Sandoz Ltd.
Karapally, J.C. Absorption, blood level, distribution and excretion of
SAN I 197 in the rat following a single oral dose. Unpublished report
ref. Agro Dok CBK 1829/75, dd. 21 April 1975, from Sandoz Ltd.,
submitted to WHO by Sandoz Ltd.
Karapally, J.C. Metabolism of 14C-Etrimfos in the rat. Unpublished
report, ref. Agro Dok CBK 3005, dd. 10 May 1977, from Sandoz Ltd.,
submitted to WHO by Sandoz Ltd.
Leuschner, F. Mikronucleus-test am Knockenmark von chinesischen
Hamstern (Test-präparat: Etrimfos). Unpublished report ref. Agro Dok
CBK 3136/78, dd. 4 July 1978, from Laboratorium für Pharmakologie und
Toxikologie, Hamburg submitted to WHO by Sandoz Ltd.
Richold, M. and Richardson, J.C. Motaphase analysis on SAN 197.
Unpublished report ref. CBK I 4798/80, dd. 24 April 1980, from
Huntingdon Research Centre submitted to WHO by Sandoz Ltd.
