
PESTICIDE RESIDUES IN FOOD - 1982
Sponsored jointly by FAO and WHO
EVALUATIONS 1982
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 23 November - 2 December 1982
Food and Agriculture Organization of the United Nations
Rome 1983
ETRIMFOS
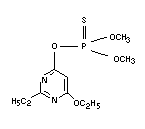 Explanation
Etrimfos was evaluated by the 1980 JMPR (FAO 1981).1 A temporary
ADI for man was recommended, based on no-effect levels observed in
2-year studies in the rat and dog.
The 1980 JMPR Report required disposition and kinetic data on the
whole molecule of etrimfos and its triester metabolites in animals and
considered observations in man as being desirable.
Daily sheet reports on field applicators and comments on the
required information have been provided and are summarized in this
monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Metabolism
Metabolism studies in the rat have shown that the P-O-pyrimidine
ester bond in etrimfos is rapidly cleaved. This is the major
detoxification process. Studies with rat and mouse liver homogenates
(Ioannou and Dauterman 1978) have shown that the P-O-CH3 and the
P-O-pyrimidine ester bonds are rapidly cleaved. These studies clearly
show that the triester molecule has only a transient existence in the
animal system and that the phosphoric acid moiety exists either as
the mono-methyl ester, or possibly as free phosphoric acid.
Re-esterification of the phosphoric acid moiety in the animal system,
if it occurs, would not be something specific to etrimfos, but would
be common to organophosphorus insecticides in general (Karapally
1982).
1 See Annex 2 for WHO and FAO documentation.
TOXICOLOGICAL STUDIES
Observations in Humans
During the developing phase of etrimfos, 167 daily sheet reports
on field trials personnel were completed. The reports cover more than
220 hours of work in the field with different etrimfos-containing
formulations. Applicators worked in different locations under varying
weather conditions for periods of 0.5 to 3 hours/day, wearing in most
cases only boots and protective overcoats.
No noteworthy symptoms or complaints during or at the end of the
working period were recorded.
No clinical investigations were carried out (Sandoz 1976).
COMMENTS
Etrimfos was evaluated for an acceptable daily intake for man by
the 1980 JMPR. The Meeting examined the submitted comments regarding
the metabolism of etrimfos and reports on people engaged in field
applications of etrimfos formulations that were made available.
Studies of the metabolism and distribution of etrimfos in rats
undertaken with 14C in the pyrimidine moiety have provided useful
information and, following clarification by the manufacturers, further
disposition and kinetic data were not considered necessary.
Although the Meeting agreed that the submitted observations in
humans were of little value, primarily because no clinical
investigations were carried out, an ADI was allocated.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 6 ppm in the diet, equivalent to 0.3 mg/kg bw.
Dog : 10 ppm in the diet, equivalent to 0.25 mg/kg bw.
Estimate of Acceptable Daily Intake for Man
0 - 0.003 mg/kg bw
FURTHER WORK OR INFORMATION
Desirable
Further observations in humans.
REFERENCES
Ioannou, Y.M. Dauterman, W.C. In vitro metabolism of etrimfos by rat
1978 and mouse liver. Pest.Biochem. Physiol. 9:190-195.
Karapally, J.C. Personal communication to WHO. Sandroz, Switzerland
1982
Sandoz, Daily sheet reports of field applicators. Agrochemical
1976 Division, Basle, Switzerland. Report from Sandoz submitted
to the WHO by Sandoz. (Unpublished)
Explanation
Etrimfos was evaluated by the 1980 JMPR (FAO 1981).1 A temporary
ADI for man was recommended, based on no-effect levels observed in
2-year studies in the rat and dog.
The 1980 JMPR Report required disposition and kinetic data on the
whole molecule of etrimfos and its triester metabolites in animals and
considered observations in man as being desirable.
Daily sheet reports on field applicators and comments on the
required information have been provided and are summarized in this
monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Metabolism
Metabolism studies in the rat have shown that the P-O-pyrimidine
ester bond in etrimfos is rapidly cleaved. This is the major
detoxification process. Studies with rat and mouse liver homogenates
(Ioannou and Dauterman 1978) have shown that the P-O-CH3 and the
P-O-pyrimidine ester bonds are rapidly cleaved. These studies clearly
show that the triester molecule has only a transient existence in the
animal system and that the phosphoric acid moiety exists either as
the mono-methyl ester, or possibly as free phosphoric acid.
Re-esterification of the phosphoric acid moiety in the animal system,
if it occurs, would not be something specific to etrimfos, but would
be common to organophosphorus insecticides in general (Karapally
1982).
1 See Annex 2 for WHO and FAO documentation.
TOXICOLOGICAL STUDIES
Observations in Humans
During the developing phase of etrimfos, 167 daily sheet reports
on field trials personnel were completed. The reports cover more than
220 hours of work in the field with different etrimfos-containing
formulations. Applicators worked in different locations under varying
weather conditions for periods of 0.5 to 3 hours/day, wearing in most
cases only boots and protective overcoats.
No noteworthy symptoms or complaints during or at the end of the
working period were recorded.
No clinical investigations were carried out (Sandoz 1976).
COMMENTS
Etrimfos was evaluated for an acceptable daily intake for man by
the 1980 JMPR. The Meeting examined the submitted comments regarding
the metabolism of etrimfos and reports on people engaged in field
applications of etrimfos formulations that were made available.
Studies of the metabolism and distribution of etrimfos in rats
undertaken with 14C in the pyrimidine moiety have provided useful
information and, following clarification by the manufacturers, further
disposition and kinetic data were not considered necessary.
Although the Meeting agreed that the submitted observations in
humans were of little value, primarily because no clinical
investigations were carried out, an ADI was allocated.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 6 ppm in the diet, equivalent to 0.3 mg/kg bw.
Dog : 10 ppm in the diet, equivalent to 0.25 mg/kg bw.
Estimate of Acceptable Daily Intake for Man
0 - 0.003 mg/kg bw
FURTHER WORK OR INFORMATION
Desirable
Further observations in humans.
REFERENCES
Ioannou, Y.M. Dauterman, W.C. In vitro metabolism of etrimfos by rat
1978 and mouse liver. Pest.Biochem. Physiol. 9:190-195.
Karapally, J.C. Personal communication to WHO. Sandroz, Switzerland
1982
Sandoz, Daily sheet reports of field applicators. Agrochemical
1976 Division, Basle, Switzerland. Report from Sandoz submitted
to the WHO by Sandoz. (Unpublished)
See Also:
Toxicological Abbreviations
Etrimfos (Pesticide residues in food: 1980 evaluations)
 Explanation
Etrimfos was evaluated by the 1980 JMPR (FAO 1981).1 A temporary
ADI for man was recommended, based on no-effect levels observed in
2-year studies in the rat and dog.
The 1980 JMPR Report required disposition and kinetic data on the
whole molecule of etrimfos and its triester metabolites in animals and
considered observations in man as being desirable.
Daily sheet reports on field applicators and comments on the
required information have been provided and are summarized in this
monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Metabolism
Metabolism studies in the rat have shown that the P-O-pyrimidine
ester bond in etrimfos is rapidly cleaved. This is the major
detoxification process. Studies with rat and mouse liver homogenates
(Ioannou and Dauterman 1978) have shown that the P-O-CH3 and the
P-O-pyrimidine ester bonds are rapidly cleaved. These studies clearly
show that the triester molecule has only a transient existence in the
animal system and that the phosphoric acid moiety exists either as
the mono-methyl ester, or possibly as free phosphoric acid.
Re-esterification of the phosphoric acid moiety in the animal system,
if it occurs, would not be something specific to etrimfos, but would
be common to organophosphorus insecticides in general (Karapally
1982).
1 See Annex 2 for WHO and FAO documentation.
TOXICOLOGICAL STUDIES
Observations in Humans
During the developing phase of etrimfos, 167 daily sheet reports
on field trials personnel were completed. The reports cover more than
220 hours of work in the field with different etrimfos-containing
formulations. Applicators worked in different locations under varying
weather conditions for periods of 0.5 to 3 hours/day, wearing in most
cases only boots and protective overcoats.
No noteworthy symptoms or complaints during or at the end of the
working period were recorded.
No clinical investigations were carried out (Sandoz 1976).
COMMENTS
Etrimfos was evaluated for an acceptable daily intake for man by
the 1980 JMPR. The Meeting examined the submitted comments regarding
the metabolism of etrimfos and reports on people engaged in field
applications of etrimfos formulations that were made available.
Studies of the metabolism and distribution of etrimfos in rats
undertaken with 14C in the pyrimidine moiety have provided useful
information and, following clarification by the manufacturers, further
disposition and kinetic data were not considered necessary.
Although the Meeting agreed that the submitted observations in
humans were of little value, primarily because no clinical
investigations were carried out, an ADI was allocated.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 6 ppm in the diet, equivalent to 0.3 mg/kg bw.
Dog : 10 ppm in the diet, equivalent to 0.25 mg/kg bw.
Estimate of Acceptable Daily Intake for Man
0 - 0.003 mg/kg bw
FURTHER WORK OR INFORMATION
Desirable
Further observations in humans.
REFERENCES
Ioannou, Y.M. Dauterman, W.C. In vitro metabolism of etrimfos by rat
1978 and mouse liver. Pest.Biochem. Physiol. 9:190-195.
Karapally, J.C. Personal communication to WHO. Sandroz, Switzerland
1982
Sandoz, Daily sheet reports of field applicators. Agrochemical
1976 Division, Basle, Switzerland. Report from Sandoz submitted
to the WHO by Sandoz. (Unpublished)
Explanation
Etrimfos was evaluated by the 1980 JMPR (FAO 1981).1 A temporary
ADI for man was recommended, based on no-effect levels observed in
2-year studies in the rat and dog.
The 1980 JMPR Report required disposition and kinetic data on the
whole molecule of etrimfos and its triester metabolites in animals and
considered observations in man as being desirable.
Daily sheet reports on field applicators and comments on the
required information have been provided and are summarized in this
monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Metabolism
Metabolism studies in the rat have shown that the P-O-pyrimidine
ester bond in etrimfos is rapidly cleaved. This is the major
detoxification process. Studies with rat and mouse liver homogenates
(Ioannou and Dauterman 1978) have shown that the P-O-CH3 and the
P-O-pyrimidine ester bonds are rapidly cleaved. These studies clearly
show that the triester molecule has only a transient existence in the
animal system and that the phosphoric acid moiety exists either as
the mono-methyl ester, or possibly as free phosphoric acid.
Re-esterification of the phosphoric acid moiety in the animal system,
if it occurs, would not be something specific to etrimfos, but would
be common to organophosphorus insecticides in general (Karapally
1982).
1 See Annex 2 for WHO and FAO documentation.
TOXICOLOGICAL STUDIES
Observations in Humans
During the developing phase of etrimfos, 167 daily sheet reports
on field trials personnel were completed. The reports cover more than
220 hours of work in the field with different etrimfos-containing
formulations. Applicators worked in different locations under varying
weather conditions for periods of 0.5 to 3 hours/day, wearing in most
cases only boots and protective overcoats.
No noteworthy symptoms or complaints during or at the end of the
working period were recorded.
No clinical investigations were carried out (Sandoz 1976).
COMMENTS
Etrimfos was evaluated for an acceptable daily intake for man by
the 1980 JMPR. The Meeting examined the submitted comments regarding
the metabolism of etrimfos and reports on people engaged in field
applications of etrimfos formulations that were made available.
Studies of the metabolism and distribution of etrimfos in rats
undertaken with 14C in the pyrimidine moiety have provided useful
information and, following clarification by the manufacturers, further
disposition and kinetic data were not considered necessary.
Although the Meeting agreed that the submitted observations in
humans were of little value, primarily because no clinical
investigations were carried out, an ADI was allocated.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 6 ppm in the diet, equivalent to 0.3 mg/kg bw.
Dog : 10 ppm in the diet, equivalent to 0.25 mg/kg bw.
Estimate of Acceptable Daily Intake for Man
0 - 0.003 mg/kg bw
FURTHER WORK OR INFORMATION
Desirable
Further observations in humans.
REFERENCES
Ioannou, Y.M. Dauterman, W.C. In vitro metabolism of etrimfos by rat
1978 and mouse liver. Pest.Biochem. Physiol. 9:190-195.
Karapally, J.C. Personal communication to WHO. Sandroz, Switzerland
1982
Sandoz, Daily sheet reports of field applicators. Agrochemical
1976 Division, Basle, Switzerland. Report from Sandoz submitted
to the WHO by Sandoz. (Unpublished)
