
PESTICIDE RESIDUES IN FOOD - 1997
Sponsored jointly by FAO and WHO
with the support of the International Programme
on Chemical Safety (IPCS)
TOXICOLOGICAL AND ENVIRONMENTAL
EVALUATIONS 1994
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Core Assessment Group
Lyon 22 September - 1 October 1997
The summaries and evaluations contained in this book are, in most
cases, based on unpublished proprietary data submitted for the purpose
of the JMPR assessment. A registration authority should not grant a
registration on the basis of an evaluation unless it has first
received authorization for such use from the owner who submitted the
data for JMPR review or has received the data on which the summaries
are based, either from the owner of the data or from a second party
that has obtained permission from the owner of the data for this
purpose.
FIPRONIL
First draft prepared by
K.L. Hamernik
Office of Pesticide Programs, US Environmental Protection Agency
Washington DC, USA
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multigeneration reproductive toxicity
Developmental toxicity
Special studies
Dermal and ocular irritation and dermal
sensitization
Neurotoxicity
Developmental neurotoxicity
Thyroid function
Mode of action of fipronil
Studies on metabolites
Acute toxicity
Fipronil-desulfinyl
M&B 46136: Dermal and ocular irritation
RPA 200766: Short-term toxicity
M&B 45897: Short-term toxicity
Genotoxicity
Comparison of fipronil and its metabolites
Comments
Fipronil
Mammalian metabolites of fipronil
Photodegradation products of fipronil
Toxicological evaluation
References
Explanation
Fipronil, (±)-5-amino-1-(2,6-dichloro-alpha,alpha,alpha-
trifluoro- p-tolyl)-4-trifluoromethylsulfinyl-pyrazole-3-carbonitrile
(IUPAC name), was considered for the first time by the present
Meeting. It has been proposed for indoor and outdoor use in the
control of the mosquito that carries the malaria parasite.
Fipronil is a member of a new class of pesticide chemicals known
as phenylpyrazoles. Its putative mode of insecticidal action is
interference with the passage of chloride ions through the gamma-
aminobutyric acid (GABA)-regulated chloride ion channel, which results
in uncontrolled central nervous system activity and subsequent death
of the insect. Although fipronil is selectively toxic to insects, some
of the toxicity of fipronil observed in mammals also appears to
involve interference with the normal functioning of the GABA receptor.
The toxicological profiles of fipronil, its mammalian
metabolites, and two photodegradation products were considered. The
Meeting concluded that the mammalian metabolites and one of the
photodegradion products have similar toxicological potencies to
fipronil, so they are not considered further in this report. Because
the other photodegradation product, desulfinylated fipronil, appears
to be more toxic than the parent compound, available data on this
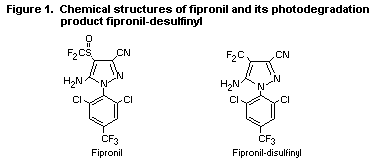
substance are reviewed here. The chemical structures of fipronil and
the photodegradation product of toxicological concern are shown in
Figure 1. The photodegradation product is designated as
fipronil-desulfinyl.
 Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Rats
In a study of the dermal absorption of 14C-fipronil, a
formulation containing 79% fipronil as a suspension in 1% aqueous
carboymethylcellulose was applied to the shaven backs of groups of 24
male Crl:CD BR rats at doses of 0.876 mg/rat (0.07 mg/cm2), 8.35
mg/rat (0.67 mg/cm2), or 48.5 mg/rat (3.9 mg/cm2); two control
animals were treated with 1% carboxymethylcellulose alone. The amounts
of radiolabel absorbed through the skin and left in or on the skin
after washing were determined 0.5, 1, 2, 4, 10, and 24 h after
treatment in four rats in each group: 1.1-2.5% of the applied dose was
found on washed skin after the low dose, 0.6-3.3% after the
intermediate dose, and 0.35-0.8% after the high dose. At all doses and
times up to 24 h, the quantity of 14C-fipronil absorbed was less than
1% of the applied dose, measured as radiolabel recovered in blood,
carcass, cage wash and wipe, urine, and faeces. The percent of the
dose absorbed appeared to decrease with increasing dose, and
absorption was saturated at the highest dose (Cheng, 1995).
The kinetics of fipronil in blood were studied in groups of five
male and five female Charles River CD rats that received a single oral
dose of [14C-phenyl]-fipronil at a dose of 4 or 40 mg/kg bw. Blood
from the tail vein was sampled 0.5, 1, 2, 3, 4, 6, 8, and 24 h after
treatment and at 24-h intervals thereafter up to two weeks. Tissue
distribution was studied in six groups of three males and three
females that received a single oral dose of 4 or 40 mg/kg bw
[14C-phenyl]-fipronil; after treatment, one group at each dose was
killed at times corresponding to one-half the time of maximum
radiolabel in blood after treatment (Tmax; absorption phase), Tmax,
and one-half the Tmax (elimination phase). Up to 20 tissues were
analysed at each time, including fat, gonads, liver, kidney, brain,
adrenals, and thyroid gland. No significant differences between the
sexes were seen in blood kinetics at either dose. At 4 mg/kg bw, the
blood levels reached a maximum at a mean of 5.5 h after treatment and
decreased thereafter, with elimination half-lives of 183 h in males
and 245 h in females. At 40 mg/kg, absorption was slower, with a mean
Tmax in blood of 36 h after treatment; blood radiolabel levels
decreased thereafter with elimination half-lives of 135 h in males and
171 h in females. The author noted that these relatively long
half-lives reflected the slow release of radiolabel from a compartment
such as fat.
Radiolabel was widely distributed in the tissues, with a
predominance in fatty tissues. Aside from the stomach and
gastrointestinal tract, the highest levels of radiolabel were seen
consistently in fat and the adrenals. Intermediate values were seen in
liver, pancreas, thyroid, and ovaries; lower values were seen in
muscle, brain, heart, and cardiac blood. At 38 h after treatment
(Tmax), the tissue concentrations (in fipronil equivalents/g of
tissue) in females at the high dose were: fat, 200 ppm; adrenals, 47
ppm; liver, 32 ppm; pancreas, 32 ppm; thyroid, 16 ppm; ovaries, 44
ppm; cardiac blood, 5 ppm. One week after treatment, the tissue
concentrations were: fat, 39 ppm; adrenals, 14 ppm; thyroid, 13 ppm;
cardiac blood, 0.92 ppm; and all other tissues, < 7.2 ppm. Similar
results were seen in females at the low dose. At 6.2 h after treatment
(Tmax), the tissue concentrations were: fat, 31 ppm; adrenals, 10
ppm; liver, 8 ppm; pancreas, 5 ppm; thyroid, 4 ppm; ovaries, 6 ppm;
cardiac blood, 0.6 ppm. High levels of radiolabel were observed in the
stomach and its contents in rats at the low dose only at the initial
sampling time (one-half Tmax), whereas these values were elevated in
rats at the high dose at the Tmax and the one-half Tmax. The levels
of radiolabel in the stomach and contents were considered to indicate
saturation of the absorption process at the high dose. Metabolites
were not identified in these studies (Totis & Fisher, 1994).
Comparison of humans, rabbits, and rats
Absorption of 14C-fipronil through epidermal membranes of
humans, rabbits, and rats was measured in vitro in horizontal glass
diffusion cells. Rat and rabbit skin was obtained from the dorsal and
flank regions of the animals; female human abdominal skin was obtained
at autopsy and the epidermal membrane separated from the rest of the
tissue. The epidermal membranes were set up as a barrier between the
two halves of the diffusion cells, and the absorption rates of a neat
suspension of fipronil (200 g/L) as a formulation in EP60145A (a
formulation base) and of two aqueous dilutions of the formulation
containing 0.2 and 4 g/L of fipronil suspended in EP 60145A were
determined, together with the absorption rates of testosterone and
hydrocortisone (both at 4 g/L) in an aqueous dilution of EP60145A.
Fipronil at doses of 4 and 200 g/L penetrated rabbit and rat
epidermal membranes to a greater extent than those of humans, whereas
at 0.2 g/L the extent of penetration was similar through human and rat
skin. The extent of penetration increased with time across species.
The percent of the applied dose that had penetrated the different
membranes after 8 h was 0.08% through rat epidermal membranes, 0.07%
through rabbit membranes, and 0.01% through human membranes for the
neat formulation; 0.14, 0.67, and 0.07% of the dose of 4.0 g/L active
ingredient; and 0.9, 13.9, and 0.9% of the dose of 0.2 g/L active
ingredient, respectively. At the dose of 4.0 g/L, fipronil penetrated
the skin of all three species more slowly than either testosterone or
hydrocortisone. These two reference permeants were selected because
their intrinsic rates of dermal penetration differ by two orders of
magnitude, that of testosterone being faster. On the basis of the
results for these two compounds, fipronil was considered to be a slow
penetrant when applied as a formulation in EP 60145A (Walters & Brain,
1990).
(b) Biotransformation
Rats
In a study designed to evaluate the absorption, distribution,
metabolism, excretion, and pharmacokinetics of fipronil in rats,
14C-fipronil (labelled uniformly at the phenyl ring; radiochemical
purity, > 97%) was administered orally in aqueous
carboxymethylcellulose (0.5% w/v) containing Tween 80 (0.01% w/v) to
groups of five male and five female Crl:CD(SD) BR rats as a single
dose of 4 mg/kg bw, in a repeated regimen of unlabelled fipronil for
14 days followed by a single dose of labelled material or as a single
dose of 150 mg/kg bw. In all dosing regimens, radiolabel was
determined in urine and faeces (expired air was found in pilot studies
to be an insignificant route of elimination) collected at various
intervals up to one week after treatment; at the end of the study,
radiolabel was also assayed in the carcass and in selected tissues.
Metabolites of fipronil were analysed in urine, faeces, fat, liver,
kidney, muscle, and uterus and were identified by high-performance
liquid chromatography (HPLC) with reference standards and mass
spectrometry. For the study of pharmacokinetics, groups of five male
and five female rats were given a single oral dose of 14C-fipronil at
either 4 or 150 mg/kg bw; whole-blood samples were obtained from the
lateral tail vein at various intervals up to one week after treatment,
and the concentration of radiolabel in the blood samples determined.
Males and females did not differ appreciably in the absorption,
distribution, metabolism, or elimination of 14C-fipronil after either
a single low or high dose or after pretreatment with unlabelled
compound. Urinary excretion and tissue residues indicated that the
proportion of the dose absorbed depended on treatment, being greatest
after a single dose of 4 mg/kg bw and lowest after the single dose of
150 mg/kg bw, presumably due to saturation of absorption at the high
dose. Urinary excretion and tissue residues also indicated that at
least 50% of the administered dose was absorbed after administration
of the single low dose, 40% after the repeated dose regimen, and about
30% after the single high dose. Once absorbed, the parent compound was
rapidly metabolized. Significant amounts of residual radiolabel were
found in abdominal fat (highest concentration), carcass, adrenal
gland, pancreas, skin, liver, kidney, muscles, ovary, and uterus one
week after treatment in all rats. Repeated treatment with the low dose
or single treatment with a high dose resulted in an overall decrease
in the amount of residual radiolabel in comparison with the single low
dose, but in an increase in the amounts in abdominal fat, carcass, and
adrenals. Faeces appeared to be the main route of excretion for
fipronil-derived radiolabel, accounting for 45-75% of the administered
dose; 5-25% was excreted in urine. The percentages excreted in urine
and faeces increased with repeated low oral treatment or a single high
dose, while the percentage found in all tissues combined decreased.
Several metabolites were identified in urine, faeces, and tissues
of treated rats. The pattern of metabolites found was independent of
sex, dose, and treatment regimen, and only the quantity of each
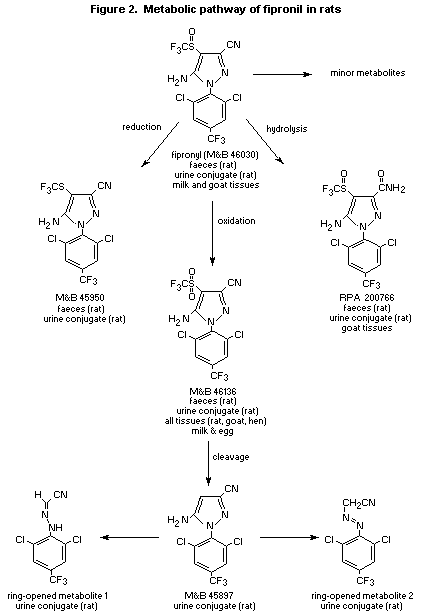
metabolite varied. The main metabolites in urine after deconjugation
(see Figure 2) included the sulfone (M&B 46136) and the amide (RPA
200766) of fipronil, M&B 45897 (a cleavage product of M&B 46136) and
two of its ring-opened products, and a reduction product of fipronil
(M&B 45950). No parent compound was observed in urine before enzymatic
deconjugation of the urine samples. In faeces, parent compound was
detected as a significant fraction of the sample, with M&B 46136, M&B
45950, and some RPA 200766. The main metabolite in tissues, found in
fat (highest concentration), liver, kidney, muscle, and probably
uterus, was the lipophilic M&B 46136. Participation of biliary
excretion in the disposition of fipronil was inferred on the basis of
the presence of metabolites in faeces but was not demonstrated.
Pharmacokinetic investigations showed that the whole-blood
half-life at the single low dose was 149-200 h in male and female
rats; the 0-168-h values for the area under the concentration-time
curve were approximately equal in the two sexes. The prolonged
half-life of radiolabel might suggest bioaccumulation of the metabolic
products of fipronil. At the single high dose, the whole-blood
half-life was noticeably decreased, to 54 h in males and 51 h in
females. The report suggested that the apparently shorter half-life is
in fact the distribution phase half-life and that the true half-life
at the high dose was not fully defined because of the protracted
absorption phase. The area under the concentration-time curve for
blood at the high dose was approximately proportional to the increase
in dose. The results suggest that the bioavailability of fipronil is
similar for each sex and is proportional to dose. Figure 2 presents
the proposed metabolic pathway for the fate of 14C-fipronil in
animals, including rats (Powles, 1992).
Goats
In a study of the absorption, distribution, metabolism, and
excretion of fipronil in ruminants, [phenyl(U)-14C]-fipronil
(19.2 mCi/mmol) was administered orally by capsule twice daily before
feeding to three lactating goats at a dose of 0.05, 2, or 10 ppm for
seven days; assuming a daily intake of 2.0 kg dry matter, these doses
are approximately equivalent to nominal daily doses of 0.1, 4, and 20
mg, respectively. Milk was collected twice daily. The animals were
killed about 24 h after administration of the final dose and tissues
obtained for analysis.
The recovery of radiolabel in urine, milk, and tissues indicated
that the minimum absorption of test material was about 19% at 0.05
ppm, 33% at 2 ppm, and 15% at 10 ppm. Of the administered radiolabel,
18-64% was recovered in faeces, 1-5% in the milk, and 8-25% in the
tissues. Total recovery was similar at the low (83%) and high doses
(77%) but was somewhat lower at the intermediate dose (50%). The
greatest contributor to the difference in recovery between the animals
at the low and high doses and those at the intermediate dose was the
amount of radiolabel excreted in the faeces: 18% of the total
radiolabel administered at 2 ppm, 64% at 0.05 ppm, and 61% at 10 ppm.
The reason for this difference is not clear. The greatest total tissue
residues were observed in omental and renal fat (about 1.9 ppm at the
10 ppm dose), followed by liver (0.86 ppm) and much lower
concentrations in kidney, milk (0.17 ppm), and skeletal muscle.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Rats
In a study of the dermal absorption of 14C-fipronil, a
formulation containing 79% fipronil as a suspension in 1% aqueous
carboymethylcellulose was applied to the shaven backs of groups of 24
male Crl:CD BR rats at doses of 0.876 mg/rat (0.07 mg/cm2), 8.35
mg/rat (0.67 mg/cm2), or 48.5 mg/rat (3.9 mg/cm2); two control
animals were treated with 1% carboxymethylcellulose alone. The amounts
of radiolabel absorbed through the skin and left in or on the skin
after washing were determined 0.5, 1, 2, 4, 10, and 24 h after
treatment in four rats in each group: 1.1-2.5% of the applied dose was
found on washed skin after the low dose, 0.6-3.3% after the
intermediate dose, and 0.35-0.8% after the high dose. At all doses and
times up to 24 h, the quantity of 14C-fipronil absorbed was less than
1% of the applied dose, measured as radiolabel recovered in blood,
carcass, cage wash and wipe, urine, and faeces. The percent of the
dose absorbed appeared to decrease with increasing dose, and
absorption was saturated at the highest dose (Cheng, 1995).
The kinetics of fipronil in blood were studied in groups of five
male and five female Charles River CD rats that received a single oral
dose of [14C-phenyl]-fipronil at a dose of 4 or 40 mg/kg bw. Blood
from the tail vein was sampled 0.5, 1, 2, 3, 4, 6, 8, and 24 h after
treatment and at 24-h intervals thereafter up to two weeks. Tissue
distribution was studied in six groups of three males and three
females that received a single oral dose of 4 or 40 mg/kg bw
[14C-phenyl]-fipronil; after treatment, one group at each dose was
killed at times corresponding to one-half the time of maximum
radiolabel in blood after treatment (Tmax; absorption phase), Tmax,
and one-half the Tmax (elimination phase). Up to 20 tissues were
analysed at each time, including fat, gonads, liver, kidney, brain,
adrenals, and thyroid gland. No significant differences between the
sexes were seen in blood kinetics at either dose. At 4 mg/kg bw, the
blood levels reached a maximum at a mean of 5.5 h after treatment and
decreased thereafter, with elimination half-lives of 183 h in males
and 245 h in females. At 40 mg/kg, absorption was slower, with a mean
Tmax in blood of 36 h after treatment; blood radiolabel levels
decreased thereafter with elimination half-lives of 135 h in males and
171 h in females. The author noted that these relatively long
half-lives reflected the slow release of radiolabel from a compartment
such as fat.
Radiolabel was widely distributed in the tissues, with a
predominance in fatty tissues. Aside from the stomach and
gastrointestinal tract, the highest levels of radiolabel were seen
consistently in fat and the adrenals. Intermediate values were seen in
liver, pancreas, thyroid, and ovaries; lower values were seen in
muscle, brain, heart, and cardiac blood. At 38 h after treatment
(Tmax), the tissue concentrations (in fipronil equivalents/g of
tissue) in females at the high dose were: fat, 200 ppm; adrenals, 47
ppm; liver, 32 ppm; pancreas, 32 ppm; thyroid, 16 ppm; ovaries, 44
ppm; cardiac blood, 5 ppm. One week after treatment, the tissue
concentrations were: fat, 39 ppm; adrenals, 14 ppm; thyroid, 13 ppm;
cardiac blood, 0.92 ppm; and all other tissues, < 7.2 ppm. Similar
results were seen in females at the low dose. At 6.2 h after treatment
(Tmax), the tissue concentrations were: fat, 31 ppm; adrenals, 10
ppm; liver, 8 ppm; pancreas, 5 ppm; thyroid, 4 ppm; ovaries, 6 ppm;
cardiac blood, 0.6 ppm. High levels of radiolabel were observed in the
stomach and its contents in rats at the low dose only at the initial
sampling time (one-half Tmax), whereas these values were elevated in
rats at the high dose at the Tmax and the one-half Tmax. The levels
of radiolabel in the stomach and contents were considered to indicate
saturation of the absorption process at the high dose. Metabolites
were not identified in these studies (Totis & Fisher, 1994).
Comparison of humans, rabbits, and rats
Absorption of 14C-fipronil through epidermal membranes of
humans, rabbits, and rats was measured in vitro in horizontal glass
diffusion cells. Rat and rabbit skin was obtained from the dorsal and
flank regions of the animals; female human abdominal skin was obtained
at autopsy and the epidermal membrane separated from the rest of the
tissue. The epidermal membranes were set up as a barrier between the
two halves of the diffusion cells, and the absorption rates of a neat
suspension of fipronil (200 g/L) as a formulation in EP60145A (a
formulation base) and of two aqueous dilutions of the formulation
containing 0.2 and 4 g/L of fipronil suspended in EP 60145A were
determined, together with the absorption rates of testosterone and
hydrocortisone (both at 4 g/L) in an aqueous dilution of EP60145A.
Fipronil at doses of 4 and 200 g/L penetrated rabbit and rat
epidermal membranes to a greater extent than those of humans, whereas
at 0.2 g/L the extent of penetration was similar through human and rat
skin. The extent of penetration increased with time across species.
The percent of the applied dose that had penetrated the different
membranes after 8 h was 0.08% through rat epidermal membranes, 0.07%
through rabbit membranes, and 0.01% through human membranes for the
neat formulation; 0.14, 0.67, and 0.07% of the dose of 4.0 g/L active
ingredient; and 0.9, 13.9, and 0.9% of the dose of 0.2 g/L active
ingredient, respectively. At the dose of 4.0 g/L, fipronil penetrated
the skin of all three species more slowly than either testosterone or
hydrocortisone. These two reference permeants were selected because
their intrinsic rates of dermal penetration differ by two orders of
magnitude, that of testosterone being faster. On the basis of the
results for these two compounds, fipronil was considered to be a slow
penetrant when applied as a formulation in EP 60145A (Walters & Brain,
1990).
(b) Biotransformation
Rats
In a study designed to evaluate the absorption, distribution,
metabolism, excretion, and pharmacokinetics of fipronil in rats,
14C-fipronil (labelled uniformly at the phenyl ring; radiochemical
purity, > 97%) was administered orally in aqueous
carboxymethylcellulose (0.5% w/v) containing Tween 80 (0.01% w/v) to
groups of five male and five female Crl:CD(SD) BR rats as a single
dose of 4 mg/kg bw, in a repeated regimen of unlabelled fipronil for
14 days followed by a single dose of labelled material or as a single
dose of 150 mg/kg bw. In all dosing regimens, radiolabel was
determined in urine and faeces (expired air was found in pilot studies
to be an insignificant route of elimination) collected at various
intervals up to one week after treatment; at the end of the study,
radiolabel was also assayed in the carcass and in selected tissues.
Metabolites of fipronil were analysed in urine, faeces, fat, liver,
kidney, muscle, and uterus and were identified by high-performance
liquid chromatography (HPLC) with reference standards and mass
spectrometry. For the study of pharmacokinetics, groups of five male
and five female rats were given a single oral dose of 14C-fipronil at
either 4 or 150 mg/kg bw; whole-blood samples were obtained from the
lateral tail vein at various intervals up to one week after treatment,
and the concentration of radiolabel in the blood samples determined.
Males and females did not differ appreciably in the absorption,
distribution, metabolism, or elimination of 14C-fipronil after either
a single low or high dose or after pretreatment with unlabelled
compound. Urinary excretion and tissue residues indicated that the
proportion of the dose absorbed depended on treatment, being greatest
after a single dose of 4 mg/kg bw and lowest after the single dose of
150 mg/kg bw, presumably due to saturation of absorption at the high
dose. Urinary excretion and tissue residues also indicated that at
least 50% of the administered dose was absorbed after administration
of the single low dose, 40% after the repeated dose regimen, and about
30% after the single high dose. Once absorbed, the parent compound was
rapidly metabolized. Significant amounts of residual radiolabel were
found in abdominal fat (highest concentration), carcass, adrenal
gland, pancreas, skin, liver, kidney, muscles, ovary, and uterus one
week after treatment in all rats. Repeated treatment with the low dose
or single treatment with a high dose resulted in an overall decrease
in the amount of residual radiolabel in comparison with the single low
dose, but in an increase in the amounts in abdominal fat, carcass, and
adrenals. Faeces appeared to be the main route of excretion for
fipronil-derived radiolabel, accounting for 45-75% of the administered
dose; 5-25% was excreted in urine. The percentages excreted in urine
and faeces increased with repeated low oral treatment or a single high
dose, while the percentage found in all tissues combined decreased.
Several metabolites were identified in urine, faeces, and tissues
of treated rats. The pattern of metabolites found was independent of
sex, dose, and treatment regimen, and only the quantity of each
metabolite varied. The main metabolites in urine after deconjugation
(see Figure 2) included the sulfone (M&B 46136) and the amide (RPA
200766) of fipronil, M&B 45897 (a cleavage product of M&B 46136) and
two of its ring-opened products, and a reduction product of fipronil
(M&B 45950). No parent compound was observed in urine before enzymatic
deconjugation of the urine samples. In faeces, parent compound was
detected as a significant fraction of the sample, with M&B 46136, M&B
45950, and some RPA 200766. The main metabolite in tissues, found in
fat (highest concentration), liver, kidney, muscle, and probably
uterus, was the lipophilic M&B 46136. Participation of biliary
excretion in the disposition of fipronil was inferred on the basis of
the presence of metabolites in faeces but was not demonstrated.
Pharmacokinetic investigations showed that the whole-blood
half-life at the single low dose was 149-200 h in male and female
rats; the 0-168-h values for the area under the concentration-time
curve were approximately equal in the two sexes. The prolonged
half-life of radiolabel might suggest bioaccumulation of the metabolic
products of fipronil. At the single high dose, the whole-blood
half-life was noticeably decreased, to 54 h in males and 51 h in
females. The report suggested that the apparently shorter half-life is
in fact the distribution phase half-life and that the true half-life
at the high dose was not fully defined because of the protracted
absorption phase. The area under the concentration-time curve for
blood at the high dose was approximately proportional to the increase
in dose. The results suggest that the bioavailability of fipronil is
similar for each sex and is proportional to dose. Figure 2 presents
the proposed metabolic pathway for the fate of 14C-fipronil in
animals, including rats (Powles, 1992).
Goats
In a study of the absorption, distribution, metabolism, and
excretion of fipronil in ruminants, [phenyl(U)-14C]-fipronil
(19.2 mCi/mmol) was administered orally by capsule twice daily before
feeding to three lactating goats at a dose of 0.05, 2, or 10 ppm for
seven days; assuming a daily intake of 2.0 kg dry matter, these doses
are approximately equivalent to nominal daily doses of 0.1, 4, and 20
mg, respectively. Milk was collected twice daily. The animals were
killed about 24 h after administration of the final dose and tissues
obtained for analysis.
The recovery of radiolabel in urine, milk, and tissues indicated
that the minimum absorption of test material was about 19% at 0.05
ppm, 33% at 2 ppm, and 15% at 10 ppm. Of the administered radiolabel,
18-64% was recovered in faeces, 1-5% in the milk, and 8-25% in the
tissues. Total recovery was similar at the low (83%) and high doses
(77%) but was somewhat lower at the intermediate dose (50%). The
greatest contributor to the difference in recovery between the animals
at the low and high doses and those at the intermediate dose was the
amount of radiolabel excreted in the faeces: 18% of the total
radiolabel administered at 2 ppm, 64% at 0.05 ppm, and 61% at 10 ppm.
The reason for this difference is not clear. The greatest total tissue
residues were observed in omental and renal fat (about 1.9 ppm at the
10 ppm dose), followed by liver (0.86 ppm) and much lower
concentrations in kidney, milk (0.17 ppm), and skeletal muscle.
 Metabolites were isolated from various tissues and milk and
identified by HPLC and mass spectrometry. After administration of 10
ppm, the major metabolite in faeces was M&B 46136, with lesser amounts
of fipronil, RPA 200766, and M&B 45950. In fat, milk, and muscle,
fipronil was predominant, with lesser amounts of RPA 200766 (in muscle
and fat), M&B46136, and M&B 45950. In kidney, M&B 46136 predominated,
with lesser amounts of fipronil; while in liver, M&B 46136 was the
major metabolite, with lesser amount of fipronil and RPA 200766. In
all cases, the major metabolite or species represented 44-75% of the
total radiolabelled residues. In urine, the mass of fipronil-derived
material was low, but M&B 46136 was found in small quantities. The
results were similar at 2 ppm, except that more fipronil was
metabolized to M&B 46136 in milk, muscle, and fat. According to the
proposed metabolic pathway for fipronil in ruminants, the sulfoxide
group of fipronil is oxidized to the sulfone (M&B 46136), which is
conjugated and excreted in the urine; the parent sulfoxide group can
also be reduced to the sulfide (M&B 45950), and the nitrile group of
the parent can be hydrolysed to the amide RPA 200766 (see Figure 2)
(Stewart, 1994a).
Laying hens
[phenyl(U)-14C]-Fipronil (19.2 mCi/mmol) was administered orally
in capsules daily before feeding to groups of five laying hens at
doses of 0.05, 2, or 10 ppm for 28 days; assuming a daily intake of
150 g dry matter, these doses were equivalent to nominal daily intakes
of 0.0075, 0.3, and 1.5 mg, respectively. Eggs were collected twice
daily. The animals were killed about 24 h after administration of the
final dose, and tissues were obtained.
Of the administered radiolabel, 28-42% was recovered in excreta,
15-18% in eggs, and 1-5% in tissues; the total recovered was 52-58%.
The greatest total tissue residues after administration of the highest
dose were found in peritoneal fat (56 ppm). The levels in eggs were
also high (30 ppm in yolks) after this dose and had not plateaued by
the end of the study. The levels were lower in skin (17 ppm) and much
lower in liver, egg white, and muscle. Metabolites were isolated from
various tissues and eggs and identified by HPLC and mass spectrometry.
After the 10 ppm dose, the main metabolite in peritoneal fat, egg
yolk, skin, and liver was M&B 46136, representing 96-98% of the total
radiolabelled residues. The remainder of the residue in these tissues
was parent fipronil. In egg white and muscle, M&B 46136 was the only
component of the residue. In excreta, parent fipronil comprised 51% of
the residue, and M&B 46136 accounted for 34%. The results with 0.05
and 2 ppm were similar. According to the proposed metabolic pathway
for fipronil in poultry, the sulfoxide group of the fipronil is
oxidized to the sulfone M&B46136 (see Figure 2) (Stewart, 1994b).
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of fipronil is summarized in Table 1. When
fipronil was administered as a single dose to mice or rats orally or
by inhalation, deaths and signs of toxicity occurred at all or most
doses in animals of each sex. Most or all of the deaths occurred
within several days of treatment. Clinical signs were generally noted
within 24 h of treatment and included tremors and convulsions of
various types, effects on activity or gait, hunched posture, wetness
in various body areas, and seizures (Gardner, 1988a; Cracknell, 1991;
Mondot & Dange, 1995; Nachreiner, 1995).
In studies with female Fischer 344 rats, the oral LD50 of
technical-grade fipronil (purity unspecified) dissolved in
glycerinformal was 175 mg/kg bw. The clinical signs of toxicity did
not reach their peak until two days after treatment in some animals,
and deaths did not occur until four days after treatment. Some signs
of toxicity and body-weight loss were still evident when the
observation period ended at day 7 after treatment. Since these
findings suggested that bioaccumulation of the test material could
occur, a five-day study with cumulative treatment was performed in
which groups of four female Fischer 344 were given fipronil at 75
mg/kg bw per day (one-half the minimum lethal dose determined in the
previous studies) orally for up to five days. Clinical signs of
neurotoxicity were seen after administration of two doses, and three
of four rats died after administration of three or four doses. In the
only rat that survived the study, abnormal behavioural responses
persisted until six days after administration of the final dose, at
which time it had regained most of its pretreatment weight (Ray,
1997).
The dermal LD50 for fipronil applied in distilled water to rats
was > 2000 mg/kg bw in both males and females, while that in rabbits
for test material moistened with corn oil was 354 mg/kg bw for the two
sexes combined. Neither clinical signs of toxicity nor deaths were
seen in rats. In rabbits, fipronil induced deaths and one or more
clinical signs of toxicity including convulsions, sluggishness,
salivation, spasms, tremors, hyperactivity, diarrhoea, emaciation, and
perioral and perinasal red discolouration in all groups except that at
the lowest dose (100 mg/kg bw). Delays in the appearance of signs of
toxicity and death were noted at all doses except the lowest. In
particular, convulsions were not observed until days 3-9 after
treatment, and some animals did not die until days 11-14 (Gardner,
1988b; Myers & Christopher, 1992).
Table 1. Acute toxicity of fipronil
Species Strain Sex Route LD50 or LC50 Reference
(mg/kg bw or
mg/L)
Mousea OF1 M Oralb 98 Mondot & Dange
F 91 (1995)
Rata Crl:CD (SD) BR M Oralc 92 Gardner (1988a)
F 103
Rata Crl:CD (SD BR M Dermald > 2000 Gardner (1988b)
F > 2000
Rat SD albino M,F Inhalation 0.68 Cracknell (1991)
(4-h exposure,
snout only)e
Rat SD albino M Inhalation 0.36 Nachreiner (1995 )
F (4-h exposure, 0.42
nose only)f
Rabbita New Zealand white M Dermalg 445 Myers & Christopher
F 354 (1992)
a Technical-grade fipronil; purity, 93-96.7%
b Administered in aqueous Tween 80 (0.2% w/v)
c Administered in corn oil
d Applied as a 90% w/v concentration in distilled water
e Manufacture-grade dry material; purity, 95.4%; not milled to reduce particle size; mass median
equivalent aerodynamic diameter (stated to be equivalent to mass median aerodynamic diameter),
6.4-8.5 µm
f Milled to meet US Environmental Protection Agency particle size requirements; mass median aerodynamic
diameter, < 2 µm
g Moistened with corn oil before application
(b) Short-term toxicity
Mice
In a preliminary study, groups of 12 male and 12 female CD-1 mice
were fed diets containing technical-grade fipronil (purity, 95.4%) at
levels of 0, 1, 3, 10, or 25 ppm (equal to 0, 0.13, 0.38, 1.3, or 3.2
mg/kg bw per day for males and 0, 0.17, 0.57, 1.7, or 4.5 mg/kg bw per
day for females) for 13 weeks. No clinical chemistry or haematological
measurements were conducted.
There were no deaths, clinical signs of toxicity, or effects on
food consumption. At 25 ppm, the body-weight gain of females was
statistically significantly decreased over the 13-week period to 63%
that of controls; in males, body-weight gains were decreased to 78% of
the control values, but the results were not significant. At necropsy,
there were no treatment-related macroscopic changes. The liver:body
weight ratio was statistically significantly increased in males (by
33%) and females (by 13%) at the high dose. Histopathological
examination revealed a dose-related increase in the incidence of
liver-cell periacinar hypertrophy with cytoplasmic vacuolation in
males (0/12 in controls, 2/12 at 1 ppm, 3/12 at 3 ppm, 6/12 at 10 ppm,
and 10/12 at 25 ppm), which was significant at 10 and 25 ppm.
Additionally, focal necrosis was observed in the liver of one male rat
at the high dose. In females, fatty vacuolation of the liver was
observed in two rats at 10 ppm and one at 25 ppm. There were no
reported effects on the thyroid. No NOAEL was identified because of
the histopathological changes in the livers of males at the lowest
dose (Broadmeadow, 1991).
Rats
Technical-grade fipronil (purity, 93%) was administered in the
diet for four weeks to groups of five Crl:CD (SD) BR rats of each sex
at concentrations of 0, 25, 50, 100, 200, or 400 ppm, equal to 0, 3.4,
6.9, 13, 24, or 45 mg/kg bw per day for males and 0, 3.5, 6.7, 13, 25,
or 55 mg/kg bw per day for females. Although there were no clinical
signs of toxicity, one female at 400 ppm died, however with no
accompanying clinical or pathological findings. Body-weight loss or
decreased body-weight gain seen in animals of each sex at doses >
100 ppm was temporary and possibly due to unpalatability, since food
consumption was also decreased in these groups. The platelet counts of
animals at 200 and 400 ppmwere marginally increased. The results of
urinalysis were negative. Increased total protein and globulin were
seen in all treated animals, and these increases were statistically
significant; however, they were small in comparison with the values in
controls and were poorly correlated with dose. Cholesterol levels were
increased in females at all doses and in males at the high dose.
The target organs were the liver and thyroid. Liver weights were
significantly increased in females at all doses and in males at 200
and 400 ppm. At necropsy, liver enlargement was observed in one or
both sexes starting at 50 ppm, and five males and three females at 400
ppm had enlarged livers. Generalized hepatocyte enlargement was
observed microscopically in one male at 100 ppm, with increasing
incidence in animals of each sex at 200 and 400 ppm. Thyroid
follicular-cell hypertrophy, generally of minimal severity but of
moderate severity in several males at 200 and 400 ppm, was found in
almost all treated animals but not in the controls. No NOAEL was
identified because of changes in blood chemistry in one or both sexes,
increased liver weights in females, and thyroid follicular-cell
hypertrophy in animals of each sex at the lowest dose (Peters et al.,
1990).
Technical-grade fipronil (purity, 95.4%) was administered for 13
weeks in the diet to groups of 10 male and 10 female CD rats at
concentrations of 0, 1, 5, 30, or 300 ppm, equal to 0, 0.07, 0.33,
1.9, or 20 mg/kg bw per day for males and 0, 0.07, 0.37, 2.3, or 24
mg/kg bw per day for females. Standard determinations of toxicity were
made ante and post mortem, and ophthalmological and neurological
examinations were conducted at week 12 on controls and animals at the
high dose. Haematological and clinical chemical evaluations were
performed after week 12.
There were no deaths. A clonic convulsion in one male at the high
dose may have been related to treatment. The ophthalmological and
neurological examinations showed no changes. The body-weight gain and
food consumption of animals at the high dose were decreased during the
first week of the study; by the end of the study, food consumption in
animals of each sex and total body-weight gain in males at 300 ppm
were comparable to those in controls but the total body-weight gain of
females was decreased by 9%. Statistically significant, but generally
minor alterations in comparison with controls were seen in numerous
haematological parameters in females at the high dose (and to a lesser
extent at 30 ppm) and appeared to be related to treatment. The
findings included lower packed cell volume, mean corpuscular volume,
haemoglobin concentration (also in males at the high dose and females
at 30 ppm), and prothrombin time (also in females at 30 ppm) and a
higher platelet count. Overall, minor and sometimes inconsistent
alterations were seen in a number of parameters of blood chemistry,
particularly in animals at 300 ppm and to a lesser extent at 30 ppm
(mostly in females); these were considered to be related to treatment.
The findings included slight but statistically significant increases
in total protein and a1-, a2-, and b-globulins, accompanied by
decreased albumin:globulin ratios at 300 ppm and increases in total
protein and one or more globulins at 30 ppm. At both doses, decreased
alanine and aspartate aminotransferases activities and increased
glucose were seen in females and increased urea in males. Animals at 1
and 5 ppm also showed fluctuations in proteins. These perturbations
may have been related to treatment but were not associated with other
significant findings.
The thyroid and liver were the target organs. The following
statistically significant changes in organ weights were observed at 13
weeks: absolute thyroid weights were increased in males and females at
300 ppm and in females at 30 ppm, and relative thyroid weights were
increased in animals of each sex at 300 ppm; absolute liver weights
were increased in males at 300 ppm and in females at doses > 5 ppm,
and relative liver weights were increased in animals of each sex at 30
and 300 ppm. The results of gross examination were unremarkable.
Histopathologically, a significant increase in the incidence of
hypertrophy of the follicular epithelium of the thyroid was seen in
females at the high dose; a nonsignificant increase was also observed
in males at this dose and to a lesser extent at 30 ppm. The incidence
of follicular-cell hyperplasia was nonsignificantly increased in
animals of each sex at the high dose. Liver sections stained with
haematoxylin and eosin from males and females at the high dose showed
a low incidence of panacinar fatty vacuolation; the incidence in males
at the high dose was more pronounced when Oil-Red-O staining was used.
The changes seen at the two lowest doses -- minor changes in
blood chemistry at 1 and 5 ppm in animals of each sex and increased
absolute (but not relative) liver weight in females at 5 ppm -- did
not appear to be toxicologically significant. At 300 ppm, both
haematological and further blood chemical parameters were altered,
absolute and relative liver weights were increased (p < 0.01) in
females, and significant increases were observed in absolute thyroid
weights in females and relative liver weights in males. Although not
significant, an increased incidence of hypertrophy of thyroid
follicular epithelium that was part of an increasing trend with the
higher dose was observed in males. When these changes are considered
together as part of a continuum towards more severe pathological
effects in the thyroid and liver and in the absence of tests for
thyroid function, the NOAEL was 5 ppm, equal to 0.33 mg/kg bw per day
(Holmes, 1991a).
Rabbits
Technical-grade fipronil (purity 96.7%) was applied in a 0.5%
aqueous solution of carboxymethylcellulose to the intact skin of
groups of six male and six female New Zealand white rabbits at doses
of 0, 0.5, 1, 5, or 10 mg/kg bw per day for 6 h per day for 15 days
within a three-week period. All animals survived. Effects were
observed only in animals at the high dose. Body-weight gains and food
consumption were reduced in animals of each sex over the course of the
study. One male and one female at the high dose showed signs of
extreme hyperactivity near the end of the study, which was possibly
related to treatment. No changes were seen in haematological or
clinical chemical parameters, organ weights, or on gross or
histopathological examination. No skin irritation was observed. The
NOAEL for systemic effects was 5 mg/kg bw per day (Hermansky & Wagner,
1993).
Dogs
Technical-grade fipronil (purity, 95.4%) was administered in
gelatin capsules to groups of four male and four female beagle dogs at
doses of 0, 0.5, 2, or 10 mg/kg bw per day for 13 weeks. Standard
determinations of toxicity were made ante and post mortem.
Neurological examination or testing of cranial nerve reflexes and
nerves, segmental reflexes, postural reactions, and general
observations of behaviour, gait, stance, and the presence of tremor or
other dyskinesia were conducted before treatment and on all surviving
animals after 6 and 12 weeks of treatment.
Mean body-weight gain over the course of the study was reduced by
up to 17% in females at the intermediate and high doses, and mean food
consumption was decreased by up to 9% in animals of each sex at the
high dose and in females at 2 ppm. Some animals at the high dose were
offered meat supplements, diets moistened with water, or an extension
of the feeding period in order to encourage eating. Mean body weight
and food consumption appeared to have recovered by the end of the
study. The results of ophthalmological, urinary, and haematological
examinations were unremarkable.
In animals at 10 mg/kg bw per day, significant clinical signs of
toxicity were seen, which were more prominent during the first two to
three weeks of treatment. These included inappetence, emaciation,
underactivity, weight loss, and hunched posture. Deterioration
progressed in some animals such that one male and three females at the
high dose had to be killed during the second week of treatment. Other
signs in animals at the high dose included dehydration, hypothermia,
subdued behaviour, excessive salivation, irregular heart rate,
convulsions, head nodding, tremors, limb jerk and extension, ataxia,
muscle twitching, abnormal reflexes, and apparent lack of vision. Some
of the last signs in particular were considered indicative of effects
on the central nervous system. The occurrence and frequency of signs
tended to diminish in surviving males after week 4 and in the
surviving female after week 7, although inappetence was seen in this
animal as late as week 12. The only clinical sign of toxicity observed
at 2 mg/kg bw per day was inappetence in two females; no signs were
observed in dogs at 0.5 mg/kg bw per day. Neurological effects were
seen only in animals at the high dose. One male showed head nodding,
facial twitching, and exaggerated blink and gag responses at week 6,
and one female had a depressed tactile placing response at week 12. At
weeks 6 and 12, alkaline phosphatase activity was increased and
cholesterol levels decreased by about 20% in males at the high dose.
The mean absolute and relative organ weights were not affected. No
effects were observed macroscopically, and the only microscopic
findings were follicular and parafollicular atrophy of the mesenteric
lymph nodes and cortical atrophy of the thymus in one male and one
female that were killed during the study; these were considered to be
related to stress. The NOAEL was 0.5 mg/kg bw per day (Holmes, 1991b).
Technical-grade fipronil (purity, 96.8%) was administered in
gelatin capsules to groups of six male and six female beagle dogs at
doses of 0, 0.2, 2, or 5 mg/kg bw per day for one year. For the first
15 days, the chemical was weighed directly into the capsules, but for
the remainder of the study an admixture of fipronil and lactose was
prepared in order to increase the accuracy of the dose. Standard
evaluations of toxicity ante and post mortem were included. In
addition, the brains and spinal cords of one or two animals in each
group that were still alive at the end of the study were examined
after fixation by perfusion with a 4% formaldehyde-saline solution.
Neurological examination or testing of cranial nerve reflexes and
nerves, segmental reflexes, and postural reactions and general
observations of behaviour, gait, and stance and the presence of tremor
or other dyskinesia were conducted before treatment and on all
surviving animals after 12, 24, 38, and 50 weeks of treatment. After
24 and 38 weeks, animals at the high dose were tested for their
proprioceptive positioning reaction ('knuckle' and 'foot sliding'
tests).
Clinical signs, many associated with neurotoxicity, were observed
in most animals at the intermediate dose and all those at the high
dose starting from the second week of treatment. These included
convulsions, twitching and tremors of various muscle beds (frequently
involving the head, pinnae, shoulder, hindlimbs, and sometimes the
whole body), ataxia, unsteady gait and rigidity of limbs (often the
hindlimbs), nervous behaviour, over- or underactivity, vocalization,
head nodding, aggression, resistance to treatment, and inappetence.
One male at 2 mg/kg bw per day and two at 5.0 mg/kg bw per day had to
be killed at weeks 11, 31, and 34, respectively, owing to
treatment-related poor condition. The signs observed in these animals
were similar to those described above and also included weight loss,
apparent loss of vision, and altered respiration. One female at 0.2
mg/kg bw per day showed signs of overactivity in weeks 13-18,
including pacing about the cage, which resulted in lesions on the foot
pad and tail, followed by weight loss and a period of underactivity. A
last incidence of overactivity was reported at about week 36;
adjustments to the diet and cage were used to control the
overactivity. The lack of similar findings in other animals at the low
dose and the overactivity of one control female for several days
around week 41 argued against a treatment-related effect.
The findings in animals at the intermediate and high doses in
routine physical examinations included tenseness, nervous and
excitable behaviour, abnormal stiffness or positioning of hindlimbs,
twitching of facial muscles, and hyperaesthesia. Most animals at the
high dose had exaggerated gag, corneal, blink, and hopping reflexes
and abnormal results in the 'foot sliding' test, and two females at
the intermediate dose showed tenseness. Body-weight gain over the
course of the study was decreased by about 16% only in females at the
high dose, due to effects in only one animal. Overall food consumption
was not affected. All animals were given an additional 200 g/day of
additional basal diet during weeks 16-18. Only minor changes were seen
in haematological and clinical chemical parameters, including slight
increases in packed cell volume, haemoglobin concentration, and
erythrocyte levels and in alanine aminotransferase activity in animals
at the intermediate or high dose, and were not clearly related to
treatment. The results of urinalysis and ophthalmological examinations
were negative. There were no clear-cut effects on organ weights and no
macroscopic or histopathological findings that appeared to be related
to treatment. The only remarkable finding in animals at 0.2 mg/kg bw
per day was overactivity in one female, described above. The NOAEL was
0.2 mg/kg bw per day (Holmes, 1992).
Technical-grade fipronil (purity, 95.4%) was administered in the
diet to groups of five male and five female beagle dogs at doses of 0,
0.075, 0.3, 1, or 3 mg/kg bw day for one year. The diets were given in
two aliquots 3.5-4.5 h apart and were moistened with water before
administration from day 30 onwards to enhance palatability. After the
first 38 days, the dose of 3 mg/kg bw per day was reduced to 2 mg/kg
bw per day because of significant toxicity. Standard evaluations of
toxicity ante and post mortem were included. Neurological
examination or testing of cranial nerve reflexes and nerves, segmental
reflexes, postural reactions, and general observations of behaviour,
gait, and stance, and for the presence of tremor or other dyskinesia
were conducted before treatment and on all surviving animals after 12,
24, 37, and 50 weeks of treatment. Blood samples taken after fasting,
before treatment and after one and 13 weeks of treatment, were
analysed for triiodothyronine (T3) and thyroxine (T4). Plasma from
fasted animals was analysed at weeks 1, 13, 24, 38, and 50 for the
presence of fipronil and a major metabolite, M&B 46136.
There were no overall effects on body-weight gain or food
consumption. The results of routine physical and ophthalmological
examinations and urinalysis were negative. None of the findings in
neurological, haematological, or clinical chemical tests, organ weight
measurements, or macro- or microscopic evaluations could be
definitively attributed to treatment. There were no treatment-related
findings in the animals at 0.3 or 0.075 mg/kg bw per day.
One female at 3 mg/kg bw per day had to be killed on day 32
because of poor health and signs of neurological disturbance. The
clinical signs of toxicity in this animal, which began on day 10,
included convulsions, underactivity, prostration, slow respiration and
tremors. Neurological examination revealed the absence of visual
placing reactions, depressed menace and startle reactions, and
abnormal gait. A blood sample showed increased packed cell volume,
haemoglobin concentration, erythrocyte count, plasma alkaline
phosphatase activity, and total protein and cholesterol
concentrations. Clinical signs of toxicity noted as early as week 1 in
three male and one female survivors at the high dose included
convulsions, head nodding, extensor rigidity, and twitching or tremors
of various muscle beds. One female at 1 mg/kg bw per day showed signs
of toxicity at week 13 (whole-body twitching) and another at week 20
(limb extensor rigidity). After 13 weeks of treatment, the T3 and T4
values were similar in all groups, including controls, the values for
T3 being 0.51-0.63 ng/ml and those for T4, 0.59-0.73 ng/ml.
Dose-related increases in the concentrations of fipronil and
metabolite M&B 46136 were seen in plasma at all measurement times.
Although there were no apparent differences between males and females
in the concentrations of these two compounds, the serum concentrations
of the metabolite were much higher than those of the parent compound
at doses > 0.075 mg/kg bw per day. At the lowest dose, the levels of
parent and metabolite were near or slightly higher than the limit of
quantification. There did not seem to be accumulation of either
chemical over time, and the concentrations of both compounds remained
fairly constant throughout the study. The NOAEL was 0.3 mg/kg bw per
day (Holmes, 1993).
(c) Long-term toxicity and carcinogenicity
Mice
Technical-grade fipronil (purity 95.4%) was administered for 78
weeks in the diet to groups of 52 male and 52 female CD-1 mice at
doses of 0, 0.1, 0.5, 10, 30, or 60 ppm (equal to group mean doses of
0, 0.011, 0.055, 1.2, or 3.4 mg/kg bw per day for males and 0, 0.012,
0.063, 1.2, or 3.6 mg/kg bw per day for females; the doses in mg/kg bw
per day were not determined for the group at 60 ppm) to evaluate
carcinogenicity. Six additional groups of 20 male and 20 female mice
were treated at the same doses for one year to measure toxicity.
Clinical signs of toxicity, body weights and weight gain, food
consumption, food efficiency, haematological parameters, organ weights
(absolute and relative to body weight), and macroscopic and
microscopic pathological appearance were observed.
Owing to excessive treatment-related mortality among animals of
each sex at 60 ppm, the surviving animals in the group were killed
during week 10. The lesions that had contributed to death were not
identified at necropsy, but all of the animals had high relative liver
weights. Several of the males had convulsions near the beginning of
the study. Reduced body-weight gains, food consumption, and food
efficiency were seen in these animals. The survival of the remaining
mice was comparable to or exceeded that of the control group, and no
clinical signs of toxicity were reported. Decreased body-weight gain
was seen in males (74-86% of control value) and females (81-86%) at 30
ppm at most evaluation times; the values for animals at 10 ppm were
also decreased but less consistently. Overall food consumption during
the study was lower than in controls in males (by about 7%) and
females (by 14%) at 30 ppm. Food efficiency was reduced in males and
females at 30 ppm and in males at 10 ppm.
A slightly lower percentage of neutrophils and a slightly higher
percentage of lymphocytes were noted in differential leukocyte counts
among females at 30 ppm after 76 weeks of treatment. Although gross
examination of animals observed for toxicity at week 53 showed no
remarkable effects, males at 30 ppm in the carcinogenicity study
showed liver enlargement and changes on the surface of the liver. The
absolute and/or relative liver weights were statistically
significantly increased in males and females at this dose at weeks 53
and 78. The relative liver weights were increased (p < 0.05) in
males at 10 ppm at both times and were increased (p < 0.05) in
males at 0.5 ppm at week 53 but not week 78. Histopathological
examination revealed a statistically significant increased incidence
of periacinar microvesicular vacuolation in the livers of males at 10
and 30 ppm at the end of 53 and 78 weeks, in females at 0.5 and 30 ppm
at the end of the toxicity study and in those at doses > 0.5 ppm
(significant at 0.5 and 30 ppm) at the end of the carcinogenicity
study; however, the incidence in females at 0.5 and 10 ppm did not
show a clear dose-response relationship, although the increased
incidence at 30 ppm may have been related to treatment. There was an
increased incidence of hepatocellular hyperplasia and chronic
degenerative changes in the livers of males at 30 ppm which died or
were killed during treatment in the carcinogenicity study.
There were no treatment-related neoplastic changes in females,
but males at 30 ppm had an increased incidence of malignant
hepatocellular carcinomas in comparison with concurrent controls: 1/52
in controls, 1/52 at 0.1 ppm, 2/52 at 0.5 ppm, 1/52 at 10 ppm, and
5/52 at 30 ppm. An additional hepatocellular carcinoma was observed in
one male at 30 ppm in the toxicity study. The incidence of
hepatocellular adenomas alone or combined with adenomas (one male at
30 ppm had both an adenoma and a carcinoma) was not significantly
increased. Since the increase in the incidence of carcinomas in males
at 30 ppm was within the range in historical controls in the testing
laboratory and the incidence among male concurrent controls was much
lower than the mean incidence in male historical controls, the
neoplastic findings in males were considered not to be related to
treatment. The NOAEL for systemic effects was 0.5 ppm, equal to 0.055
mg/kg bw per day (Broadmeadow, 1993).
Rats
Technical-grade fipronil (purity, 95.4%) was administered for one
year in the diet to groups of 15 male and 15 female CD rats to assess
its chronic toxicity, and further groups were fed the chemical for one
year and then observed for an additional 13 weeks to observe any
reversal of treatment-related changes. Groups of 50 male and 50 female
rats were originally scheduled to be treated for two years to assess
the carcinogenic potential of the chemical. The doses administered
were 0, 0.5, 1.5, 30, or 300 ppm, equal to 0, 0.019, 0.059, 1.3, or 13
mg/kg bw per day for males and 0, 0.025, 0.078, 1.6, or 17 mg/kg bw
per day for females. Standard evaluations of toxicity ante and
post mortem were included, and thyroid function (T3, T4, and
thyroid-stimulating hormone (TSH)) were measured in fasted animals
after 1, 4, 12, 24, and 50 weeks of treatment and after 2, 4, 7, and
11 weeks of the observation period after cessation of treatment.
The carcinogenicity phase of the study was terminated early owing
to excessive mortality and to ensure that a sufficient number of
animals were available for terminal sacrifices. Males and females were
killed when the number in any group declined to 25% of the original.
Thus, the males were killed after 89 weeks of treatment, when the
number of surviving animals in the group at 300 ppm was 25%, and the
females were killed after 91 weeks of treatment, when survival of
those at 30 ppm was 25%. More than 50% of animals of each sex in all
groups were still alive after 78 weeks. Early in the study, more
females at 300 ppm than controls died or were killed for humane
reasons related to convulsive episodes during this period. A
statistically significant increase in the number of deaths among
females at 30 ppm relative to controls disappeared when humane kills
were included in the mortality count. No significant differences in
mortality were seen among males or between other groups of females and
the control group.
Convulsive episodes, some lasting as long as 25 min and often
fatal, were observed in three males at 1.5 ppm, one male and three
females at 30 ppm, and eight males and 12 females at 300 ppm. The
convulsions tended to occur early in the treatment period but were
also seen later. Other clinical signs of toxicity that occurred
throughout treatment, predominantly in females at the high dose but
also in females given 1.5 and 30 ppm, included irritability,
vocalization, salivation, aggression, overactivity, and bruxism.
During the observation period, aggression, overactivity, irritability,
vocalization, and convulsions were seen in some females at 300 ppm.
Convulsions also occurred in females at 30 ppm and males at 300 ppm
but not in controls.
During the first week of treatment, body-weight gains were
significantly decreased in animals of each sex, by 6-11% at 30 ppm and
by 54-58% at 300 ppm. By one year, a significant 15-18% depression in
body-weight gain was observed only in animals at the high dose. By the
end of treatment, significant depressions were seen in males (18%) and
females (25%) at 300 ppm and in females at 30 ppm (23%). The
body-weight gain of males at 30 ppm was decreased by 7%. This pattern
continued during observation. Food consumption and food conversion
efficiency (calculated through week 14 only) were reduced at the
beginning of the study in animals of each sex at 300 ppm but were
similar to those of controls subsequently. The results of the
ophthalmological examination were negative.
Small but mostly significant decreases in haematological
parameters such as packed cell volume, haemoglobin concentration,
erythrocyte count, mean corpuscular volume, mean corpuscular
haemoglobin, and prothrombin time were seen at various times during
the study, particularly in males and females at the high dose. Slight
decreases in erythrocyte count, haemoglobin concentration, mean
corpuscular volume, and packed cell volume were also noted at 1.5 and
30 ppm.Towards the end of the study, platelet counts were slightly
increased in animals at the high dose and males at 30 ppm. Except for
a continued decrease in prothrombin time in females at 30 and 300 ppm,
the haematological changes did not persist after treatment.
Alterations in clinical chemical parameters, such as increased
cholesterol and calcium values and alterations in protein including
increased total protein, decreased albumin, increases in a1-, a2- and
b-globulins, and decreased albumin:globulin ratio, were seen mostly in
animals at 30 and 300 ppm; protein alterations were also observed in
males at 1.5 ppm towards the end of the study. Increased cholesterol
and calcium levels, total protein, and globulins and a decreased
albumin:globulin ratio persisted after cessation of treatment in
females at the high dose.
The T3 levels did not differ much from those of controls during
treatment but were significantly increased in females at the high dose
from four weeks after treatment and in females at 30 ppm from seven
weeks after treatment. The T4 levels were severely depressed after the
first week of treatment in animals of each sex, such that none was
detectable in animals at 300 ppm and the levels were significantly
depressed in a dose-dependent fashion in animals at 1.5 and 30 ppm.
Subsequently, the levels in animals at the high dose became
detectable, but the pattern of T4 depression observed at doses >
1.5 ppm generally persisted through week 50 of treatment. The T4
levels in animals at 0.5 ppm were occasionally significantly decreased
during treatment but not at the end of treatment. TSH levels were
significantly increased in animals at the high dose at all times and
in males at 30 ppm during the first month of treatment and after 50
weeks. Once treatment had ceased, the alterations in both T4 and TSH
levels were reversed in all groups except males at the high dose, in
which the TSH levels never recovered fully.
Alterations were seen at various times during the study in
urinary parameters (lower pH, higher protein, elevated urine volume,
and decreased specific gravity) in rats at 30 and 300 ppm
(predominately males). The alterations in protein and pH persisted
after treatment. Gross examination of animals in the carcinogenicity
study at termination and animals that were killed or died during the
study revealed increased incidences of large and/or pale kidneys in
animals at the intermediate or high dose and enlarged adrenals in
males at the high dose. Enlarged livers and thyroids were seen in
animals of each sex at the high dose and in males at 30 ppm. At
interim sacrifice (toxicity study), enlarged livers or thyroids were
seen in some animals at 30 or 300 ppm. Absolute thyroid weights were
somewhat increased in males at 0.5 and 1.5 ppm. The absolute and
relative weights of the liver and thyroid were increased in animals of
each sex at 30 or 300 ppm in both the toxicity and the carcinogenicity
study and in animals at the high dose that were killed or died during
the study. Increased absolute and relative kidney weights were also
seen in animals at 30 or 300 ppm, and increased adrenal weights were
seen in males at these doses. Most of the changes in organ weight were
statistically significant. The increases in liver, thyroid, and kidney
weights persisted to some degree after treatment, mostly in animals at
300 ppm. Histopathological examination showed an increased incidence
and severity of progressive senile nephropathy (a non-neoplastic
lesion) in animals of each sex at 300 ppm in the toxicity study and at
30 and 300 ppm in the carcinogenicity study; an increased severity of
the lesion was also reported in rats at 1.5 ppm. These findings
persisted to some extent after treatment.
Benign and malignant neoplastic changes (follicular-cell adenomas
and carcinomas) occurred in the thyroid glands of animals of each sex.
The incidence of these tumours per number of animals examined at 0,
0.5, 1.5, 30, and 300 ppm was, respectively: malignant follicular-cell
carcinomas: 0/49, 0/48, 0/50, 0/50, and 5/50 for males and 0/50, 1/50,
0/50, 1/50, and 2/50 for females; benign follicular-cell adenomas:
0/49, 1/48, 5/50, 3/50, and 12/50 for males and 0/50, 0/50, 0/50,
0/50, and 8/50 for females; total tumours: 0/49, 1/48, 5/50, 3/50, and
17/50 for males and 0/50, 1/50, 0/50, 1/50, and 10/50 for females.
None occurred in concurrent controls. Males in all treated groups and
females in all groups except that receiving 1.5 ppm showed increased
incidences of benign and malignant thyroid tumours combined in the
carcinogenicity study. The increases were significant (at p < 0.05,
< 0.01, or < 0.001 by Fisher's exact one-tailed test for pair-wise
comparisons) for carcinomas alone in males at the high dose and for
adenomas alone and adenomas and carcinomas combined in males and
females at 300 ppm and males at 1.5 ppm. The author concluded that
only the neoplastic changes observed in animals at 300 ppm were
related to treatment, as the increased incidences of benign or
malignant lesions alone or in combination exceeded the historical
control incidences for the testing facility only at the high dose, and
the zero incidences in the concurrent controls were unusually low. The
reported historical control incidence rates for studies of comparable
length (88-95 weeks) were 0-5.5% (males) and 0% (females) for
follicular-cell carcinoma, 1.4-5.7% (males) and 0-1.9% (females) for
follicular-cell adenoma, and 1.9-7.3% (males) and 0-1.9% (females) for
all follicular-cell tumours. The occurrence of these tumours was
attributed to continuous stimulation of the thyroid gland by elevated
TSH levels.
Although the author concluded that there were no significant
intergroup differences in mortality among males, analysis of the data
by the US Environmental Protection Agency using the computer program
of Thomas, Breslow, and Gart showed a significantly increasing trend
in mortality with increasing doses of fipronil in male but not female
rats. This finding does not affect the validity of the study for the
following reasons: firstly, the study was terminated prematurely only
near its end; secondly, the literature indicates that, in general, the
longevity of CD (Charles River) rats has been decreasing and that a
shortened life span is therefore not unique to this study; and
thirdly, the study was long enough for tumours to have developed in
the fipronil-treated animals.
The initial analysis of tumour incidence was based only on the
data from the carcinogenicity phase of the study. Six animals were
reported to have developed thyroid follicular-cell tumours during the
observation phase, with carcinomas in one male at 30 ppm and one at
300 ppm and adenomas in one female at 1.5 ppm and one male and two
females at 300 ppm. No thyroid tumours considered to be related to
treatment were reported to have occurred during the toxicity phase,
although a follicular-cell carcinoma was tabulated for one male at 30
ppm killed after one year of treatment. When these additional data are
included in the analysis and the data are analysed on the basis of the
first appearance of thyroid adenomas or carcinomas in the study, the
incidence is as shown in Table 2. When Peto's prevalence test is used
to analyse the data for male rats (as the first tumour was seen before
interim sacrifice), significantly increasing trends (p < 0.01) are
obtained for thyroid follicular-cell adenomas, carcinomas, and
combined adenomas and carcinomas. Significant differences from
controls in pair-wise comparisons (p < 0.05 or p < 0.01) are
found for adenomas and combined adenomas and carcinomas at doses >
1.5 ppm and for carcinomas at the high dose. When the exact trend test
and Fisher's exact test are used to analyse the data on tumours in
female rats, a significantly increasing trend and a significant
difference in pair-wise comparisons of the group at 300 ppm with the
controls is seen for thyroid follicular-cell adenomas and combined
adenomas and carcinomas (all at p < 0.01).
This analysis suggests that doses > 1.5 ppm are carcinogenic,
since the pair-wise comparisons in males at doses > 1.5 ppm are
significant and there were significant trends for tumour formation.
Since the concurrent controls had an apparently lower incidence than
historical controls at the laboratory where the study was conducted,
however, the apparent inconsistency in the dose-response relationship
at 1.5 and 30 ppm and the fact that TSH levels were significantly and
persistently elevated only at 300 and to a lesser extent at 30 ppm, an
alternative view is that the incidence of thyroid tumours was
toxicologically significant only at the highest dose. In summary, the
study demornstrates that fipronil is clearly carcinogenic to rats at
300 ppm; however, the overall NOAEL is for neurotoxicity and is
0.5 ppm, equal to 0.019 mg/kg bw (Aughton, 1993).
(d) Genotoxicity
The results of assays for genotoxicity with fipronil are
summarized in Table 3.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
In a two-generation study of reproductive toxicity, 30 male and
30 female CD rats received technical-grade fipronil (purity, 95.4%) in
the diet at concentrations of 0, 3, 30, or 300 ppm, equal to 0, 0.25,
2.5, or 26 mg/kg bw per day for males and 0, 0.27, 2.7, or 28 mg/kg bw
per day for females. After two matings of the F0 generation, litters
were culled to four animals of each sex on day 4 post partum, and
physical development was assessed by recording the day of onset and
completion of pinna unfolding, hair growth, tooth eruption, and eye
opening.
Table 2. Incidences of thyroid follicular-cell tumours in rats treated with fipronil
Tumour Dose (ppm)
Males Females
0 0.5 1.5 30 300 0 0.5 1.5 30 300
Adenomas 0/63 1/61 5/63 3/62 12/61 0/48 0/49 0/50 0/45 8/46
% 0 2 8 5 20 0 0 0 0 17
p 0.000** 0.116 0.014* 0.038* 0.000** 0.000** 1.000 1.000 1.000 0.002**
Carcinomas 0/59 0/57 0/62 1/60 5/57 0.48 1/49 0/50 1/45 2/46
% 0 0 0 2 9 0 2 0 2 4
p 0.000** - - 0.186 0.007** 0.084 0.505 1.000 0.484 0.237
Combined 0/63 1/61 5/63 4/62 16/61 0/48 1/49 0/50 1/45 10/46
% 0 2 8 6 26 0 2 0 2 22
p 0.000** 0.116 0.014* 0.024* 0.000** 0.000** 0.505 1.000 0.484 0.001**
For males: no. of tumour-bearing animals/no. of animals examined, excluding those that died before observation of
the first tumour; analysis by Peto's prevalence test. For females: no. of tumour-bearing animals/no. of animals
examined, excluding those that died or were killed before week 54; analysis by exact trend test and Fisher's exact
test. First adenoma observed at 300 ppm in males in week 42 and in females at week 62. First carcinoma observed in
males at 30 ppm in week 53 and in females at 300 ppm in week 79. One male at 300 ppm had both an adenoma and a
carcinoma.
Significance of trend denoted at control; significance of pair-wise comparison with control denoted at dose:
*, p < 0.05; **, p < 0.01
Table 3. Results of assays for genotoxicity with fipronil
End-point Test object Concentration Purity Result Reference
(%)
In vitro
Reverse mutation S. typhimurium 0.8-500 µg/plate 90.6 Negativea,b Clare (1988a)
TA98, TA100, in DMSO
TA1535, TA1537
Gene mutation Chinese hamster 1.13-386 µg/ml 97.2 Negativea,b Lloyd (1990)
cell line V79, in DMSO
hprt locus
Chromosomal Chinese hamster 15-60/µg/ml 6 ha, 98.3 Positiveb,d Wright (1995)
aberration lung cell line 7.5-30 µg/ml 24 hc,
7.5-22.5 µg/ml 48 hc
in DMSO
Chromosomal Human lymphocytes 75-300 µg/ml in 90.6 Negativeb Marshall (1988a)
aberration DMSO
In vivo
Micronucleus CD-1 mice 1-25 mg/kg bw in 97.2 Negativeb,e Edwards (1991)
formation aqueous methyl-
cellulose
Micronucleus CD-1 mice 6.95-48 µg/kg bw 96.2 Negativeb Edwards (1995)
formation in aqueous methyl-
cellulose
DMSO, dimethyl sulfoxide
a With and without metabolic activation
b Appropriate positive controls gave expected positive responses
c Without metabolic activation
d Positive only with 6-h pulse treatment; significant, dose-related effects at 45 and 60 µg/ml without
metabolic activation; non-significant increase at 60 µg/ml with metabolic activation
e Unacceptable; no overt or target-cell toxicity at doses < 25 mg/kg; results at 50 mg/kg were inconclusive.
Systemic toxicity was seen in the parental animals at doses >
30 ppm as increased absolute and relative weights of the thyroid gland
and liver in the F0 and F1 generations, decreased absolute and
relative pituitary gland weights in the F1 females (a decrease in
relative pituitary weight at 3 ppm was minor and was not considered to
be toxicologically significant), and a significantly increased
incidence of follicular epithelial hypertrophy of the thyroid gland in
F1 females and animals of each sex at 300 ppm. Increased mortality
occurred among F0 and F1 animals at this dose, with clinical signs
of toxicity including convulsions; in addition, the F0 generation had
decreased food consumption before mating, and decreased body-weight
gain was seen before mating in the F0 and F1 generations and in F0
females during gestation and lactation. The absolute and relative
weights of the ovaries were decreased in F0 females, and females of
the F0 and F1 generations had a significantly increased incidence of
centriacinar fatty vacuolation in the liver. The increased incidence
of follicular epithelial hypertrophy in adult males at 30 ppm was not
significant but was found in both the F0 generation (in 2/30 animals,
in comparison with 0/30 controls, 0/30 animals at 3 ppm, and 10/29
animals at 300 ppm) and the F1 generation (in 3/30 animals, with 0/30
controls, 2/30 at 3 ppm, and 9/30 at 300 ppm) and was considered to be
related to treatment. The presence of this finding in 2/30 F1 males
at 3 ppm, and not in the F0 generation, was considered plausible in
an organ in rats that is very sensitive to stimuli; it could therefore
not be clearly related to treatment.
Reproductive toxicity observed in animals at 300 ppm consisted of
an increased incidence of clinical signs of toxicity in F1 and F2
offspring (notably convulsions when the offspring first started to eat
the treated diet), decreased litter size and body weights, a decrease
in the percentage of animals that mated, a reduction in the fertility
index of F1 parental animals, reduced postimplantation and postnatal
survival in the F2 litters, and delays in physical development in F1
and F2 litters including slight delays in the onset of tooth eruption
(F1) and pinna unfolding (F2). The NOAEL for parental toxicity was 3
ppm, equal to 0.25 mg/kg bw per day, while that for reproductive
toxicity was 30 ppm, equal to 2.5 mg/kg bw per day (King, 1992).
(ii) Developmental toxicity
Rats
Technical-grade fipronil (purity, 93%) was administered by gavage
as a suspension in 0.5% aqueous methylcellulose to groups of 25
specific pathogen-free female rats of the Crl: CD (SD)BR VAF/Plus
strain (Charles River, France) at doses of 0, 1, 4, or 20 mg/kg bw per
day on days 6-15 of gestation. The study was terminated on day 20.
Adult females were observed for clinical signs, food and water
consumption, and body-weight changes; they underwent a macroscopic
post mortem at study termination. Litters and fetuses were evaluated
with regard to pre- and postimplantation losses, litter size, litter
and mean fetal weights, sex ratios, and malformations or skeletal or
visceral anomalies.
The pregnancy rate was 96-100%. There were no deaths, abortions,
premature deliveries, or clinical signs in the adult females. Maternal
effects associated with treatment occurred only in the animals
receiving the high dose and included reduced body-weight gain,
increased water consumption, and decreased food consumption at various
intervals during gestation. Some of these effects continued after
treatment. The macroscopic examination showed no remarkable effects.
No effects of treatment were seen on developmental parameters. The
NOAEL for maternal toxicity was 4 mg/kg bw day, and that for
developmental toxicity was 20 mg/kg bw day (Brooker & John, 1991).
Rabbits
Technical-grade fipronil (purity, 95.4%) was administered by
gavage as a suspension in 0.5% aqueous methylcellulose mucilage and
0.5% Tween 80 to groups of 22 female New Zealand white rabbits (Ranch
Rabbits, Susse, United Kingdom) at doses of 0, 0.1, 0.2, 0.5, or 1
mg/kg bw per day on days 6-19 of gestation. The study was terminated
on day 29. Adult females were observed for clinical signs, abortion
and total litter loss, food and water consumption, and body-weight
changes; a macroscopic post mortem was performed at study termination.
The litters and fetuses were evaluated with regard to pre- and
postimplantation losses, litter size, litter and mean fetal weights,
sex ratios, and malformations or skeletal or visceral anomalies.
The number of pregnant animals in each group ranged from 18 to
21. There were no treatment-related deaths. At doses of 0.5 and 1 g/kg
bw per day, maternal body-weight gain during treatment was
significantly lower than that of controls by 50 and 70%, respectively,
and the body-weight gain of dams at 0.1 or 0.2 mg/kg bw per day was
reduced by 30%. Animals in all fipronil-treated groups consumed less
food than those in the control group during treatment, and the
decreases in the groups at the two highest doses achieved significance
on gestation days 6-12 and/or 13-19. Other maternal parameters were
not affected. Treatment had no effect on development. No NOAEL for
maternal toxicity was identified; that for developmental toxicity was
1 mg/kg bw per day (King, 1990).
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Technical-grade fipronil (purity, 96.7%) moistened with corn oil
was a slight dermal irritant in male and female New Zealand white
rabbits after a 4-h application to intact skin. The signs of
irritation were slight to well-defined erythema and slight oedema,
which had cleared by day 7 after application. When fipronil (purity,
93%) was moistened with distilled water and applied for 4 h to the
intact skin of male New Zealand white rabbits, no signs of dermal
irritation were seen (Liggett, 1988a; Myers & Christopher, 1993a).
Instillation of technical-grade fipronil (purity, 96.7%) into the
eyes of male and female New Zealand white rabbits produced minor
transient corneal opacity and iritis, which had cleared within 24 h,
and minor-to-moderate conjunctival irritation, which had cleared
within 2-14 days. In a similar study with only male rabbits, fipronil
(purity, 93%) induced transient, minimal conjunctival inflammation,
which cleared within 24-48 h (Liggett, 1988b; Myers & Christopher,
1993b).
Technical-grade fipronil (purity, 95.4%) was tested in male and
female Dunkin-Hartley albino guinea-pigs by a modification of the
Buehler method. Topical application of the highest optimal
concentration for induction and challenge of fipronil (30% w/v) in
paraffin oil to guinea-pig skin produced no sign of dermal
sensitization (Smith, 1990).
In a Magnusson-Kligman maximization test in male and female
Dunkin-Hartley albino guinea-pigs, the optimal induction and challenge
concentrations of technical-grade fipronil (purity, 95.4%) were
determined in advance. In the main study, primary induction
concentrations of 5% (w/v) fipronil in propylene glycol alone or
combined with Freund's complete adjuvant were applied intradermally at
separate sites. In the secondary induction phase, 5% fipronil in
propylene glycol was applied topically to the same sites. A topical 3%
challenge dose produced no significant response, but 24 h after
challenge with 10% fipronil in propylene glycol, four of 20 animals
showed a significant dermal response (eschar formation or slight
erythema). Eschar formation was still evident in two animals 48 h
after the challenge. No significant responses were observed in control
animals challenged with 3 or 10% fipronil in propylene glycol. One
control animal challenged with propylene glycol alone showed slight
erythema at 24 h. By the criteria of the European Economic Community,
the test material did not cause delayed contact hypersensitivity
because the response rate at challenge was < 30%; however, the
positive response rate of 20% in this study could also support the
conclusion that fipronil is a mild or weak skin sensitizer (Johnson,
1993).
(ii) Neurotoxicity
Rats
A single dose of technical-grade fipronil (purity, 96.7%) in corn
oil was administered by gavage to groups of 15 male and 15 female
Sprague-Dawley rats at doses of 0, 0.5, 5, or 50 mg/kg bw. The animals
were about eight weeks of age at the time of treatment. Standard
evaluations of toxicity included observations for mortality and
clinical signs of toxicity and measurements of body weight. The
neurobehavioural evaluations of all surviving animals included a
functional observational battery of tests before treatment and at 7 h,
7 days, and 14 days after treatment, and quantitative assessment of
motor activity before treatment and 1 h after each functional test.
The study was terminated on days 16-19 after treatment. The postmortem
examinations included an abbreviated necropsy of the thoracic and
peritoneal cavities (all groups) and detailed light microscopic
evaluation of the brain, spinal cord, and peripheral nerves of six
male and six female controls and six animals of each sex at the high
dose after intracardiac perfusion in situ with 10% neutral buffered
formalin. Rats that died during the study were necropsied, but their
nervous tissues were not examined microscopically.
Five males and one female at 50 mg/kg bw died during the study,
most within two days of treatment. At necropsy, most had diffuse brain
haemorrhages, which may have caused death or may have been agonal.
Clinical signs of toxicity were seen only at the high dose, and the
incidence of neurological signs was more prevalent in males. On the
day of treatment, males and females at this dose had either clonic or
tonic-clonic convulsions. Other clinical signs of toxicity included
those indicative of cachexia: emaciation, dehydration, unkempt
appearance, urine stains, cold extremities, and/or pallor. Male body
weight was reduced by 6-10% relative to that of controls 7 and 14 days
after treatment.
Treatment-related effects on functional parameters occurred
primarily 7 h after treatment in rats at 50 mg/kg bw, when males were
seen to have drooping or half-shut eyelids. During open-field
evaluations, both stimulation and depression of the nervous system
were seen, which were generally more pronounced in males. Signs
indicative of stimulation of motor systems were convulsions, coarse
and fine tremors, head bobbing, myoclonic movements, and decreased
hindleg splay, which was significantly decreased (p < 0.01) in
animals of each sex. The signs indicative of nervous system depression
were decreased arousal and rearing activity, decreased reflexes such
as response to tail pinching and approach and air righting reflex, and
decreased muscle tone, altered gait, decreased pupil size, and/or
decreased body temperature. Urination was more evident in males at the
high dose. The only treatment-related effect in rats at 5 mg/kg bw 7 h
after treatment was significantly decreased hindleg splay (p <
0.05) in animals of each sex. A stimulatory response in certain
functional tests was seen in some males at the high dose seven days
after treatment, such as increased arousal and rearing activity and
exaggerated touch and sound reflexes. Females at the high dose showed
significantly increased hindleg splay at this time and on day 14 after
treatment. The only finding of note in males at the high dose on day
14 was a higher incidence of urination.
Motor activity 8 h after treatment was decreased by 90% in males
at the high dose and by 93% in females. This was the only effect on
motor activity during the study that was attributed to treatment.
There were no treatment-related gross or microscopic changes on
post-mortem examination of the central and peripheral nervous systems.
The NOAEL was 0.5 mg/kg bw on the basis of decreased hindleg splay 7 h
after treatment (Gill et al., 1993).
Groups of 15 male and 15 female Sprague-Dawley rats were fed
diets containing technical-grade fipronil (purity, 96.7%) at 0, 0.5,
5, or 150 ppm (equivalent to 0, 0.03, 0.3, or 8.9 mg/kg bw per day for
males and 0, 0.03, 0.3, or 11 mg/kg bw per day for females) for 13
weeks. The animals were about eight weeks of age at the start of
treatment. Standard evaluations of toxicity included observations for
mortality and clinical signs of toxicity and measurements of body
weight. Neurobehavioural screening, consisting of a functional
observational battery of tests and motor activity evaluations, was
performed before treatment and during weeks 4, 9, and 13. Post-mortem
examinations at terminal sacrifice, included an abbreviated necropsy
of the thoracic and peritoneal cavities in all groups and a detailed
light microscopic evaluation of the brain, spinal cord, and peripheral
nerves of six males and females of the controls and at the high dose
after intracardiac perfusion in situ with 10% neutral buffered
formalin.
There were no treatment-related deaths or clinical signs of
toxicity. Slight decreases in body weight (6.5-6.9%) and decreased
body-weight gain were seen in males and females at the high dose
during the first few weeks of treatment. These were accompanied by
decreased food consumption, suggesting unpalatability. Functional
changes seen in animals at the high dose in week 4, 9, and/or 13 were
increased urination and an increased incidence of exaggerated response
to tail pinching in males, an increased incidence of exaggerated
startle responses during manipulation in animals of each sex, and
increased forelimb grip strength in females at week 13. No
treatment-related findings were seen at necropsy or during
histopathological examination of nervous tissues. The changes in
animals at the high dose may not have been related to treatment;
however, these findings, although relatively minor when taken
separately, represent a minimal effect of treatment when taken
in toto. In addition, the exaggerated responses to touch and sound,
particularly in males, and the increased urination of males at the
high dose were considered to be related to treatment in the preceding
study. Therefore, the NOAEL for both neurotoxicity and systemic
toxicity was 5 ppm, equal to 0.3 mg/kg bw per day (Driscoll & Hurley,
1993).
Dogs
Technical-grade fipronil (purity, 95.4%) was administered in
capsules to female beagle dogs (26 weeks old at the start of the
study) at doses of 0 (one animal) or 20 mg/kg bw per day (four
animals) until clear signs of neurotoxicity were seen or for a maximum
of 14 days. Immediately after the appearance of neurotoxic signs,
administration was discontinued and a 28-day observation period was
initiated. This phase was begun after five days of treatment in two
animals, seven days of treatment in one animal, and 13 days of
treatment in one animal and the control dog. Animals were monitored
for general health, clinical signs of toxicity, chronic conditions,
food and water consumption, and body-weight changes and were subjected
to a weekly veterinary examination and neurological examinations
before treatment and then at intervals during treatment and
observation. The neurological examination consisted of observation and
testing of cranial nerve reflexes, segmental reflexes, and postural
reactions and general observation of behavioural changes,
abnormalities of gait and stance, and the presence of tremor or other
dyskinesia. An obstacle-avoidance test and a hearing test were
administered at the same time as the neurological examination.
Anaesthetized dogs were killed by perfusion-fixation at the end of the
observation phase. The brain, spinal column, and some peripheral
nerves were examined grossly, but only the brain and spinal column
appear to have been subjected to microscopic examination.
All animals survived to the end of the observation period. The
control animal showed no abnormal signs throughout the treatment and
recovery periods, but all treated animals displayed neurotoxic signs,
which included hypoactivity, salivation, ataxia, convulsions, tremors,
unsteady gait and stiffened body, aggressive behaviour, apparent lack
of vision, muscle twitching, nervous behaviour, and a high-stepping
gait. The time to appearance of signs and the duration of signs after
discontinuation of treatment varied. Some signs, like aggressive
behaviour and visual impairment, first appeared after discontinuation
of treatment. Food consumption was markedly decreased from day 1 or 2
of treatment and for up to seven days during the recovery period. All
treated animals lost weight, but the initial body weight was restored
17 days after cessation of treatment. Veterinary examination showed
similar changes and also emaciation in three treated animals and
hunched posture and peripheral vasodilatation in one animal. The
neurological examinations indicated widely varied responses including
(in addition to the responses noted above) changes in reflex
responses, some exaggerated, including gag and flexor responses, and
some depressed, including blink, pupillary, and consensual light
reflexes and visual and tactile placing responses. One animal still
had slightly exaggerated gag and flexor reflexes at the end of the
recovery period. The results of the obstacle-avoidance and hearing
tests indicated temporary visual impairment and possible hearing
deficiency in one animal. No abnormal findings were reported after
macroscopic or microscopic examination.
Virtually complete recovery from what appeared to be functional
changes in the central nervous system was reported by 12 days or less
after cessation of treatment; in the absence of histopathological
changes in the study, the mode of action of the chemical was
considered probably to be pharmacological and temporary. Given the
short duration of treatment (5-13 days), the short observation period
(treatment period plus 28 days), the use of only one dose, one sex,
and only one control animal, however, there is insufficient
information to draw any firm conclusions about the reversibility of
the functional, cellular, and neurotransmitter or receptor effects.
There was no NOAEL (Holmes, 1991c).
(iii) Developmental neurotoxicity
Technical-grade fipronil (purity, 96.1%) was administered to
groups of 30 female Sprague-Dawley (CD) rats at dietary levels of 0,
0.5, 10, or 200 ppm, equal to 0, 0.05, 0.9, or 15 mg/kg bw per day,
from gestation day 6 through lactation day 10, day 0 being defined as
the day mating was confirmed. Clinical observations, body weights, and
food consumption were recorded for all animals at scheduled intervals
throughout gestation and lactation, and litters were examined at
selected intervals throughout lactation. The numbers of live and dead
pups and the sex and body weights of the pups were recorded. Litters
were standardized to eight pups on postnatal day 4. The achievement of
pinna detachment, incisor eruption, eye opening, vaginal patency, and
preputial separation was evaluated for all surviving pups. For one pup
per sex per litter, the following evaluations were conducted on
various postnatal days: motor activity (days 13, 17, 22, 60), auditory
startle response (days 22 and 60), swimming development (days 6, 8,
10, 12, and 14), and learning and memory in a Y-maze (days 24 and 60).
On day 11 of lactation and again on day 60, one pup of each sex per
litter was killed. Brain weights were recorded and neuropathological
evaluation was performed on six pups of each sex from the control and
high-dose groups. Maternal animals were killed and necropsied after
the weaning of the last litter, and all remaining offspring were
killed soon after all the evaluations were completed.
The maternal effects at the dietary level of 200 ppm were reduced
body weight during treatment, reduced body-weight gain during
gestation, and reduced food consumption. No treatment-related findings
were observed at necropsy. Pregnancy status, gestation length, litter
size, and pup sex ratio were not affected by treatment. At 200 ppm,
there was a significant increase in the number of stillborn pups, and
pup and litter survival were reduced. Also at this level, male and
female pup weights were reduced, and upper and lower incisor eruption
and sexual development were delayed. There was a small but significant
increase in the time to preputial separation in animals at 10 or 200
ppm. At 10 ppm, group mean body weights were reduced for female pups
at all recorded intervals and for male pups on days 4 and 17;
postweaning weights were not affected.
With regard to neurodevelopmental effects, at 200 ppm the maximum
auditory startle response time for males and females was significantly
decreased on postnatal day 22; there was no significant difference in
the time to maximum or average response. Swimming development was
delayed on postnatal day 6 for animals of each sex, and motor activity
was increased in females on postnatal day 17. Learning and memory
(Y-maze test) were delayed significantly for females in two trials on
postnatal day 24. Also at 200 ppm, significant decreases in absolute
pup brain weights were noted for animals of each sex on days 11 (-20%
for males and -11% for females) and 60 (-7% for males and females),
but pup body weights were also significantly decreased at these times,
so that the relative brain:body weights were actually increased
relative to those of concurrent controls: +39% for males and +20% for
females on day 11 and +6% for males and females on day 60. Thus, the
lower brain weights may have been due to the overall retarded growth
of the pups associated with maternal toxicity rather than to a
developmental delay in brain size. The statistically significant
increases in motor activity in female pups at 10 ppm observed on day
17 could not be clearly related to treatment when the magnitude of the
response was compared with that of the control group, because the
values for the latter on that day appeared to be unusually low. No
microscopic morphological abnormalities were seen in the brains of
offspring killed on postnatal day 11 or 60. The NOAEL for maternal and
neurodevelopmental effects was 10 ppm, equal to 0.9 mg/kg bw per day.
The NOAEL for developmental effects was 0.5 ppm, equal to 0.05 mg/kg
bw per day, on the basis of reduced pup body weights (Mandella, 1995).
(iv) Thyroid function
Effects on thyroid hormone levels: Groups of 10 Crl:CD (SD) BR
rats of each sex were given technical-grade fipronil (purity, 95.4%)
in the diet at doses of 0, 0.1, 1, 5, or 30 ppm (equal to 0, 0.01,
0.1, 0.49, or 2.9 mg/kg bw per day for males and 0, 0.01, 0.1, 0.48,
or 2.9 mg/kg bw per day for females) for four weeks. The parameters
measured were clinical observations, body weight, food consumption and
efficiency, water consumption, evaluations of T3, T4, and TSH before
treatment and on days 7 and 28, and organ weights and histopathology
of the thyroid and liver at necropsy.
The T3 level was significantly decreased in males at doses > 1
ppm on day 7 and was increased in females at 30 ppm on day 28; T4
levels were significantly decreased in males and females at 30 ppm,
males at 5 ppm on day 7, and males at 30 ppm on day 28; the TSH level
was significantly increased in males at 30 ppm on days 7 and 28 and in
females at 30 ppm on day 28; thyroid weights were nonsignificantly
increased in males and females at 30 ppm; and there was an increased
incidence of higher follicular epithelium in males and females at 30
ppm and in one male at 5 ppm. As re-analysis of the data for T3 in the
controls and rats at 1 ppm showed no significant difference, it was
concluded that this level had no effect.
Increased liver weights were observed in animals at 30 ppm;
however, the change was significant only in females. An increased
incidence of minimal centrilobular hepatocyte enlargement was seen in
males at 30 ppm. Staining of liver sections with Oil Red O
demonstrated an increased incidence of periportal fat deposition in
females at 5 and 30 ppm. The NOAEL was 1 ppm, equal to 0.1 mg/kg bw
per day (Peters et al., 1991a).
Effect on biliary excretion of thyroxine: The objective of this
study was to measure the effects of single and repeated oral doses of
technical-grade fipronil (purity, 96.7%) on the biliary excretion of
intravenously administered 125I-T4 in bile duct-cannulated rats.
Groups of three Sprague-Dawley CD(Crl:CD/BR) VAF plus strain male rats
were given single or repeated daily doses for 14 days of: fipronil (1
or 10 mg/kg bw per day orally at 5 ml/kg bw), phenobarbital (80 mg/kg
bw per day intraperitoneally as a positive control), or 0.5%
methylcellulose (5 ml/kg bw orally as a negative control). Immediately
after treatment, the animals were anaesthetized, the common bile duct
was cannulated, and 1 mg sodium iodide (to block thyroid uptake of
125I) was administered by injection into the stomach, followed 4 h
later by intravenous administration of about 10 µCi of 125I-T4. Bile
samples were collected before treatment and at 15-min intervals for
0-5 h after 125I-T4 administration. Blood samples were taken at 30-min
intervals. Both bile and blood were analysed for radiolabel. After 5
h, the animals were killed and the livers removed. Selected bile
samples were pooled, treated with œ-glucuronidase and analysed by HPLC
for free and conjugated T4.
The single doses of fipronil or phenobarbital did not
significantly increase the amount of bile excreted or the biliary
clearance of 125I-T4. After 14 days of treatment, biliary clearance
was significantly increased with the high dose of fipronil but not
with the low dose. The high dose of fipronil produced a greater
increase than phenobarbital. Bile output was significantly increased
after treatment with the high dose of fipronil and with phenobarbital.
After treatment with œ-glucuronidase, about 50% of the 125I-T4 in bile
was conjugated with either glucuronide or sulfate, but about 30%
remained unidentified. Thus, repeated treatment with fipronil at 10
mg/kg bw per day orally increased the excretion of conjugated T4 in
bile, fipronil being more potent than phenobarbital. A possible
mechanism would be hepatic accumulation of T4 and induction of the
hepatic T4 conjugating enzyme UDP glucuronyltransferase, leading to
increased biliary excretion of T4-glucuronide conjugate (Taylor,
1993).
Effect on thyroxine clearance: Groups of six male Crl:CD(SD)
rats were treated with either technical-grade fipronil (purity, 95.4%)
at 10 mg/kg bw per day by gavage, phenobarbital at 80 mg/kg bw per day
intraperitoneally, or 0.5% methylcellulose (vehicle control) at 5
ml/kg bw by gavage for one or 14 days. Four h after the final dose of
either test substance, each rat received 125I-T4 at a dose of 10
µCi/kg bw. The levels of 125I in whole blood were measured for up to
30 h after T4 administration and were used to calculate the terminal
half-life, clearance, and volume of distribution of this hormone.
Fipronil had no effect on mortality or other parameters ante
mortem. Phenobarbital-treated animals showed collapsed posture,
lethargy, and shallow breathing on the first day of treatment.
Fipronil had no effect on the clearance of T4 after one day of
treatment, but after 14 days a decrease in terminal half-life (52% of
control level) and increases in the clearance (261%) and volume of
distribution (137%) were seen. The effects of phenobarbital were
similar, although quantitatively less severe, and were seen on day 1
of treatment (Peters et al., 1991b).
Effect on thyroid function: The effect of technical-grade
fipronil (purity, 95.4%) on thyroid function was compared with that of
propylthiouracil, a known inhibitor of thyroid organification which
interferes with thyroglobulin iodination, and noxythiolin, a thiourea
compound and putative inhibitor of thyroid iodide organification known
to lower T4 levels in rats. Groups of 27 male Crl:CD(SD)BR rats were
treated for 14 days with either 0.5% methylcellulose (vehicle control)
at 5 ml/kg bw by gavage, fipronil at 10 mg/kg bw per day by gavage,
propylthiouracil at 200 mg/kg per day by gavage, or Noxyflex at 5
mg/kg bw per day intraperitoneally as a saline solution. On day 15,
each animal received 125I-sodium iodide at a dose of 1 µCi. Six hours
later, nine males in each group received 10 or 25 mg/kg bw potassium
perchlorate or a 0.9% saline solution intraperitoneally, and blood was
immediately drawn in order to measure radiolabel. The animals were
then killed, and the thyroids were weighed and analysed for
radioactivity. Treatment with fipronil or Noxyflex appeared to
stimulate the thyroid glands, as evidenced by increased accumulation
of 125I and increased ratios of radiolabel distribution between the
blood and the thyroid. These changes were accompanied by increases in
thyroid weight. Treatment with propylthiouracil decreased the amount
of 125I incorporated in the thyroid and the blood:thyroid ratios, with
elevated levels of 125I in the blood. As the thyroids of these animals
were 2.5 times heavier than those of controls, however, the ratio of
blood 125I to thyroid weight was reduced. Administration of
perchlorate did not further reduce the 125I content of the thyroids or
the blood:thyroid 125I radiolabel ratio. There was no evidence of
inhibition of iodide incorporation (Peters et al., 1991c).
(v) Mode of action of fipronil
GABA is an important inhibitory neurotransmitter in both insects
and mammals. Attempts to demonstrate that the target site for fipronil
at the molecular level is the chloride ion channel of the GABA
receptor include electrophysiological testing, in which fipronil at 10
nmol/L reversed blockage of nervous system activity by GABA at 10
mmol/L, resulting in hyperexcitation in the housefly larva; flux
studies in which fipronil inhibited the passage of radiolabelled
chloride ions through opened GABA receptors in rat brain microsacs;
radioligand binding assays in vitro in which fipronil did not
inhibit binding of 3H-muscimol or 3H-flunitrazepam at, respectively,
the GABA recognition or benzodiazepine sites on the GABA receptor in
rat tissue, but did competitively inhibit binding at sites in the
chloride channel of the GABA receptor of ligands such as
35S- tert-butylbicyclophosphorothionate (TBPS) and
3H-1-[(4-ethyl)phenyl]-4- n-propyl-2,6,7-trioxacicyclo[2.2.2]octane
(EBOB). The latter is known to block the g-amino-butyric acid-gated
chloride channel, and the former binds to the picrotoxin binding site
in the channel. Binding of TBPS was inhibited by fipronil in rat brain
but not in the housefly head, but binding of EBOB was inhibited in
both rat and mouse brain and housefly head. The greater affinity of
fipronil for the EBOB site in insects than for that in mammals has
been postulated to account, at least in part, for the pesticide's
selective toxicity to insects.
In other studies of the role of the GABA receptor in the toxicity
of fipronil, a cloned gene that codes for a GABA receptor subunit in
Drosophila melanogaster was expressed in frog oocytes as a membrane
protein. These receptor-containing oocytes produced a large electrical
current carried by chloride ions in response to treatment with GABA,
which was not seen in control oocytes. The electric response was
reversed in a dose-dependent manner by treatment with fipronil and was
blocked by picrotoxin, TBPS, EBOB, and dieldrin.
Differences in the GABA receptor genome in susceptible and
resistant insects may help to explain resistance to the pesticide
dieldrin (a cyclodiene), which acts at the GABA receptor in insects
but also at the TBPS binding site, at which fipronil is inactive. A
dieldrin-resistant housefly was shown to have a low-affinity EBOB
binding site (see Bushey, 1993; Cole et al., 1993; Gant et al., 1994
for other references).
Although it was reported that fipronil did not affect other
enzyme or receptor systems, such as the acetylcholine receptor,
acetylcholinesterase, the octopamine receptor, and general oxidative
uncouplers, no supporting data were presented (Bushey, 1993).
(g) Studies on metabolites
(i) Acute toxicity
The results of studies on the toxicity of single doses of
fipronil metabolites are summarized in Table 4. The metabolic routes
by which they are formed are shown in Figures 2 and 3. In a study with
M&B 45950, clinical signs of neurotoxicity, including excessive
jumping, increased or reduced motor activity, clonic convulsions,
tremors, curling up, and subdued behaviour, were noted in all animals
at doses > 50 mg/kg bw, and deaths occurred during the first week
of the study, starting from 65 mg/kg bw in males and 90 mg/kg bw in
females (Dange, 1994a).
In a study with M&B 46136, clinical signs observed in animals of
each sex at 64 mg/kg bw within 2 h included hunched posture and
abnormal gait. Most signs such as hunched posture, abnormal gait,
lethargy, pallor of the extremities, diarrhoea, ataxia, clonic
convulsions, increased salivation, and decreased respiratory rate did
not appear until day 2 in animals at doses > 100 mg/kg bw. Deaths
occurred at doses > 100 mg/kg bw in animals of each sex on days 2
and 3 (Gardner, 1988c).
In a study with fipronil-desulfinyl, clinical signs including
reduced motor activity, dyspnoea, bradypnoea, and hyperreaction to
noise were observed in all animals at doses > 10 mg/kg bw. Clonic
and/or tonic convulsions were observed before death in one female at
20 mg/kg bw and in all males at 30 mg/kg bw. Deaths occurred at doses
> 20 mg/kg bw between days 2 and 4 after treatment (Dange, 1993b).
Metabolites were isolated from various tissues and milk and
identified by HPLC and mass spectrometry. After administration of 10
ppm, the major metabolite in faeces was M&B 46136, with lesser amounts
of fipronil, RPA 200766, and M&B 45950. In fat, milk, and muscle,
fipronil was predominant, with lesser amounts of RPA 200766 (in muscle
and fat), M&B46136, and M&B 45950. In kidney, M&B 46136 predominated,
with lesser amounts of fipronil; while in liver, M&B 46136 was the
major metabolite, with lesser amount of fipronil and RPA 200766. In
all cases, the major metabolite or species represented 44-75% of the
total radiolabelled residues. In urine, the mass of fipronil-derived
material was low, but M&B 46136 was found in small quantities. The
results were similar at 2 ppm, except that more fipronil was
metabolized to M&B 46136 in milk, muscle, and fat. According to the
proposed metabolic pathway for fipronil in ruminants, the sulfoxide
group of fipronil is oxidized to the sulfone (M&B 46136), which is
conjugated and excreted in the urine; the parent sulfoxide group can
also be reduced to the sulfide (M&B 45950), and the nitrile group of
the parent can be hydrolysed to the amide RPA 200766 (see Figure 2)
(Stewart, 1994a).
Laying hens
[phenyl(U)-14C]-Fipronil (19.2 mCi/mmol) was administered orally
in capsules daily before feeding to groups of five laying hens at
doses of 0.05, 2, or 10 ppm for 28 days; assuming a daily intake of
150 g dry matter, these doses were equivalent to nominal daily intakes
of 0.0075, 0.3, and 1.5 mg, respectively. Eggs were collected twice
daily. The animals were killed about 24 h after administration of the
final dose, and tissues were obtained.
Of the administered radiolabel, 28-42% was recovered in excreta,
15-18% in eggs, and 1-5% in tissues; the total recovered was 52-58%.
The greatest total tissue residues after administration of the highest
dose were found in peritoneal fat (56 ppm). The levels in eggs were
also high (30 ppm in yolks) after this dose and had not plateaued by
the end of the study. The levels were lower in skin (17 ppm) and much
lower in liver, egg white, and muscle. Metabolites were isolated from
various tissues and eggs and identified by HPLC and mass spectrometry.
After the 10 ppm dose, the main metabolite in peritoneal fat, egg
yolk, skin, and liver was M&B 46136, representing 96-98% of the total
radiolabelled residues. The remainder of the residue in these tissues
was parent fipronil. In egg white and muscle, M&B 46136 was the only
component of the residue. In excreta, parent fipronil comprised 51% of
the residue, and M&B 46136 accounted for 34%. The results with 0.05
and 2 ppm were similar. According to the proposed metabolic pathway
for fipronil in poultry, the sulfoxide group of the fipronil is
oxidized to the sulfone M&B46136 (see Figure 2) (Stewart, 1994b).
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of fipronil is summarized in Table 1. When
fipronil was administered as a single dose to mice or rats orally or
by inhalation, deaths and signs of toxicity occurred at all or most
doses in animals of each sex. Most or all of the deaths occurred
within several days of treatment. Clinical signs were generally noted
within 24 h of treatment and included tremors and convulsions of
various types, effects on activity or gait, hunched posture, wetness
in various body areas, and seizures (Gardner, 1988a; Cracknell, 1991;
Mondot & Dange, 1995; Nachreiner, 1995).
In studies with female Fischer 344 rats, the oral LD50 of
technical-grade fipronil (purity unspecified) dissolved in
glycerinformal was 175 mg/kg bw. The clinical signs of toxicity did
not reach their peak until two days after treatment in some animals,
and deaths did not occur until four days after treatment. Some signs
of toxicity and body-weight loss were still evident when the
observation period ended at day 7 after treatment. Since these
findings suggested that bioaccumulation of the test material could
occur, a five-day study with cumulative treatment was performed in
which groups of four female Fischer 344 were given fipronil at 75
mg/kg bw per day (one-half the minimum lethal dose determined in the
previous studies) orally for up to five days. Clinical signs of
neurotoxicity were seen after administration of two doses, and three
of four rats died after administration of three or four doses. In the
only rat that survived the study, abnormal behavioural responses
persisted until six days after administration of the final dose, at
which time it had regained most of its pretreatment weight (Ray,
1997).
The dermal LD50 for fipronil applied in distilled water to rats
was > 2000 mg/kg bw in both males and females, while that in rabbits
for test material moistened with corn oil was 354 mg/kg bw for the two
sexes combined. Neither clinical signs of toxicity nor deaths were
seen in rats. In rabbits, fipronil induced deaths and one or more
clinical signs of toxicity including convulsions, sluggishness,
salivation, spasms, tremors, hyperactivity, diarrhoea, emaciation, and
perioral and perinasal red discolouration in all groups except that at
the lowest dose (100 mg/kg bw). Delays in the appearance of signs of
toxicity and death were noted at all doses except the lowest. In
particular, convulsions were not observed until days 3-9 after
treatment, and some animals did not die until days 11-14 (Gardner,
1988b; Myers & Christopher, 1992).
Table 1. Acute toxicity of fipronil
Species Strain Sex Route LD50 or LC50 Reference
(mg/kg bw or
mg/L)
Mousea OF1 M Oralb 98 Mondot & Dange
F 91 (1995)
Rata Crl:CD (SD) BR M Oralc 92 Gardner (1988a)
F 103
Rata Crl:CD (SD BR M Dermald > 2000 Gardner (1988b)
F > 2000
Rat SD albino M,F Inhalation 0.68 Cracknell (1991)
(4-h exposure,
snout only)e
Rat SD albino M Inhalation 0.36 Nachreiner (1995 )
F (4-h exposure, 0.42
nose only)f
Rabbita New Zealand white M Dermalg 445 Myers & Christopher
F 354 (1992)
a Technical-grade fipronil; purity, 93-96.7%
b Administered in aqueous Tween 80 (0.2% w/v)
c Administered in corn oil
d Applied as a 90% w/v concentration in distilled water
e Manufacture-grade dry material; purity, 95.4%; not milled to reduce particle size; mass median
equivalent aerodynamic diameter (stated to be equivalent to mass median aerodynamic diameter),
6.4-8.5 µm
f Milled to meet US Environmental Protection Agency particle size requirements; mass median aerodynamic
diameter, < 2 µm
g Moistened with corn oil before application
(b) Short-term toxicity
Mice
In a preliminary study, groups of 12 male and 12 female CD-1 mice
were fed diets containing technical-grade fipronil (purity, 95.4%) at
levels of 0, 1, 3, 10, or 25 ppm (equal to 0, 0.13, 0.38, 1.3, or 3.2
mg/kg bw per day for males and 0, 0.17, 0.57, 1.7, or 4.5 mg/kg bw per
day for females) for 13 weeks. No clinical chemistry or haematological
measurements were conducted.
There were no deaths, clinical signs of toxicity, or effects on
food consumption. At 25 ppm, the body-weight gain of females was
statistically significantly decreased over the 13-week period to 63%
that of controls; in males, body-weight gains were decreased to 78% of
the control values, but the results were not significant. At necropsy,
there were no treatment-related macroscopic changes. The liver:body
weight ratio was statistically significantly increased in males (by
33%) and females (by 13%) at the high dose. Histopathological
examination revealed a dose-related increase in the incidence of
liver-cell periacinar hypertrophy with cytoplasmic vacuolation in
males (0/12 in controls, 2/12 at 1 ppm, 3/12 at 3 ppm, 6/12 at 10 ppm,
and 10/12 at 25 ppm), which was significant at 10 and 25 ppm.
Additionally, focal necrosis was observed in the liver of one male rat
at the high dose. In females, fatty vacuolation of the liver was
observed in two rats at 10 ppm and one at 25 ppm. There were no
reported effects on the thyroid. No NOAEL was identified because of
the histopathological changes in the livers of males at the lowest
dose (Broadmeadow, 1991).
Rats
Technical-grade fipronil (purity, 93%) was administered in the
diet for four weeks to groups of five Crl:CD (SD) BR rats of each sex
at concentrations of 0, 25, 50, 100, 200, or 400 ppm, equal to 0, 3.4,
6.9, 13, 24, or 45 mg/kg bw per day for males and 0, 3.5, 6.7, 13, 25,
or 55 mg/kg bw per day for females. Although there were no clinical
signs of toxicity, one female at 400 ppm died, however with no
accompanying clinical or pathological findings. Body-weight loss or
decreased body-weight gain seen in animals of each sex at doses >
100 ppm was temporary and possibly due to unpalatability, since food
consumption was also decreased in these groups. The platelet counts of
animals at 200 and 400 ppmwere marginally increased. The results of
urinalysis were negative. Increased total protein and globulin were
seen in all treated animals, and these increases were statistically
significant; however, they were small in comparison with the values in
controls and were poorly correlated with dose. Cholesterol levels were
increased in females at all doses and in males at the high dose.
The target organs were the liver and thyroid. Liver weights were
significantly increased in females at all doses and in males at 200
and 400 ppm. At necropsy, liver enlargement was observed in one or
both sexes starting at 50 ppm, and five males and three females at 400
ppm had enlarged livers. Generalized hepatocyte enlargement was
observed microscopically in one male at 100 ppm, with increasing
incidence in animals of each sex at 200 and 400 ppm. Thyroid
follicular-cell hypertrophy, generally of minimal severity but of
moderate severity in several males at 200 and 400 ppm, was found in
almost all treated animals but not in the controls. No NOAEL was
identified because of changes in blood chemistry in one or both sexes,
increased liver weights in females, and thyroid follicular-cell
hypertrophy in animals of each sex at the lowest dose (Peters et al.,
1990).
Technical-grade fipronil (purity, 95.4%) was administered for 13
weeks in the diet to groups of 10 male and 10 female CD rats at
concentrations of 0, 1, 5, 30, or 300 ppm, equal to 0, 0.07, 0.33,
1.9, or 20 mg/kg bw per day for males and 0, 0.07, 0.37, 2.3, or 24
mg/kg bw per day for females. Standard determinations of toxicity were
made ante and post mortem, and ophthalmological and neurological
examinations were conducted at week 12 on controls and animals at the
high dose. Haematological and clinical chemical evaluations were
performed after week 12.
There were no deaths. A clonic convulsion in one male at the high
dose may have been related to treatment. The ophthalmological and
neurological examinations showed no changes. The body-weight gain and
food consumption of animals at the high dose were decreased during the
first week of the study; by the end of the study, food consumption in
animals of each sex and total body-weight gain in males at 300 ppm
were comparable to those in controls but the total body-weight gain of
females was decreased by 9%. Statistically significant, but generally
minor alterations in comparison with controls were seen in numerous
haematological parameters in females at the high dose (and to a lesser
extent at 30 ppm) and appeared to be related to treatment. The
findings included lower packed cell volume, mean corpuscular volume,
haemoglobin concentration (also in males at the high dose and females
at 30 ppm), and prothrombin time (also in females at 30 ppm) and a
higher platelet count. Overall, minor and sometimes inconsistent
alterations were seen in a number of parameters of blood chemistry,
particularly in animals at 300 ppm and to a lesser extent at 30 ppm
(mostly in females); these were considered to be related to treatment.
The findings included slight but statistically significant increases
in total protein and a1-, a2-, and b-globulins, accompanied by
decreased albumin:globulin ratios at 300 ppm and increases in total
protein and one or more globulins at 30 ppm. At both doses, decreased
alanine and aspartate aminotransferases activities and increased
glucose were seen in females and increased urea in males. Animals at 1
and 5 ppm also showed fluctuations in proteins. These perturbations
may have been related to treatment but were not associated with other
significant findings.
The thyroid and liver were the target organs. The following
statistically significant changes in organ weights were observed at 13
weeks: absolute thyroid weights were increased in males and females at
300 ppm and in females at 30 ppm, and relative thyroid weights were
increased in animals of each sex at 300 ppm; absolute liver weights
were increased in males at 300 ppm and in females at doses > 5 ppm,
and relative liver weights were increased in animals of each sex at 30
and 300 ppm. The results of gross examination were unremarkable.
Histopathologically, a significant increase in the incidence of
hypertrophy of the follicular epithelium of the thyroid was seen in
females at the high dose; a nonsignificant increase was also observed
in males at this dose and to a lesser extent at 30 ppm. The incidence
of follicular-cell hyperplasia was nonsignificantly increased in
animals of each sex at the high dose. Liver sections stained with
haematoxylin and eosin from males and females at the high dose showed
a low incidence of panacinar fatty vacuolation; the incidence in males
at the high dose was more pronounced when Oil-Red-O staining was used.
The changes seen at the two lowest doses -- minor changes in
blood chemistry at 1 and 5 ppm in animals of each sex and increased
absolute (but not relative) liver weight in females at 5 ppm -- did
not appear to be toxicologically significant. At 300 ppm, both
haematological and further blood chemical parameters were altered,
absolute and relative liver weights were increased (p < 0.01) in
females, and significant increases were observed in absolute thyroid
weights in females and relative liver weights in males. Although not
significant, an increased incidence of hypertrophy of thyroid
follicular epithelium that was part of an increasing trend with the
higher dose was observed in males. When these changes are considered
together as part of a continuum towards more severe pathological
effects in the thyroid and liver and in the absence of tests for
thyroid function, the NOAEL was 5 ppm, equal to 0.33 mg/kg bw per day
(Holmes, 1991a).
Rabbits
Technical-grade fipronil (purity 96.7%) was applied in a 0.5%
aqueous solution of carboxymethylcellulose to the intact skin of
groups of six male and six female New Zealand white rabbits at doses
of 0, 0.5, 1, 5, or 10 mg/kg bw per day for 6 h per day for 15 days
within a three-week period. All animals survived. Effects were
observed only in animals at the high dose. Body-weight gains and food
consumption were reduced in animals of each sex over the course of the
study. One male and one female at the high dose showed signs of
extreme hyperactivity near the end of the study, which was possibly
related to treatment. No changes were seen in haematological or
clinical chemical parameters, organ weights, or on gross or
histopathological examination. No skin irritation was observed. The
NOAEL for systemic effects was 5 mg/kg bw per day (Hermansky & Wagner,
1993).
Dogs
Technical-grade fipronil (purity, 95.4%) was administered in
gelatin capsules to groups of four male and four female beagle dogs at
doses of 0, 0.5, 2, or 10 mg/kg bw per day for 13 weeks. Standard
determinations of toxicity were made ante and post mortem.
Neurological examination or testing of cranial nerve reflexes and
nerves, segmental reflexes, postural reactions, and general
observations of behaviour, gait, stance, and the presence of tremor or
other dyskinesia were conducted before treatment and on all surviving
animals after 6 and 12 weeks of treatment.
Mean body-weight gain over the course of the study was reduced by
up to 17% in females at the intermediate and high doses, and mean food
consumption was decreased by up to 9% in animals of each sex at the
high dose and in females at 2 ppm. Some animals at the high dose were
offered meat supplements, diets moistened with water, or an extension
of the feeding period in order to encourage eating. Mean body weight
and food consumption appeared to have recovered by the end of the
study. The results of ophthalmological, urinary, and haematological
examinations were unremarkable.
In animals at 10 mg/kg bw per day, significant clinical signs of
toxicity were seen, which were more prominent during the first two to
three weeks of treatment. These included inappetence, emaciation,
underactivity, weight loss, and hunched posture. Deterioration
progressed in some animals such that one male and three females at the
high dose had to be killed during the second week of treatment. Other
signs in animals at the high dose included dehydration, hypothermia,
subdued behaviour, excessive salivation, irregular heart rate,
convulsions, head nodding, tremors, limb jerk and extension, ataxia,
muscle twitching, abnormal reflexes, and apparent lack of vision. Some
of the last signs in particular were considered indicative of effects
on the central nervous system. The occurrence and frequency of signs
tended to diminish in surviving males after week 4 and in the
surviving female after week 7, although inappetence was seen in this
animal as late as week 12. The only clinical sign of toxicity observed
at 2 mg/kg bw per day was inappetence in two females; no signs were
observed in dogs at 0.5 mg/kg bw per day. Neurological effects were
seen only in animals at the high dose. One male showed head nodding,
facial twitching, and exaggerated blink and gag responses at week 6,
and one female had a depressed tactile placing response at week 12. At
weeks 6 and 12, alkaline phosphatase activity was increased and
cholesterol levels decreased by about 20% in males at the high dose.
The mean absolute and relative organ weights were not affected. No
effects were observed macroscopically, and the only microscopic
findings were follicular and parafollicular atrophy of the mesenteric
lymph nodes and cortical atrophy of the thymus in one male and one
female that were killed during the study; these were considered to be
related to stress. The NOAEL was 0.5 mg/kg bw per day (Holmes, 1991b).
Technical-grade fipronil (purity, 96.8%) was administered in
gelatin capsules to groups of six male and six female beagle dogs at
doses of 0, 0.2, 2, or 5 mg/kg bw per day for one year. For the first
15 days, the chemical was weighed directly into the capsules, but for
the remainder of the study an admixture of fipronil and lactose was
prepared in order to increase the accuracy of the dose. Standard
evaluations of toxicity ante and post mortem were included. In
addition, the brains and spinal cords of one or two animals in each
group that were still alive at the end of the study were examined
after fixation by perfusion with a 4% formaldehyde-saline solution.
Neurological examination or testing of cranial nerve reflexes and
nerves, segmental reflexes, and postural reactions and general
observations of behaviour, gait, and stance and the presence of tremor
or other dyskinesia were conducted before treatment and on all
surviving animals after 12, 24, 38, and 50 weeks of treatment. After
24 and 38 weeks, animals at the high dose were tested for their
proprioceptive positioning reaction ('knuckle' and 'foot sliding'
tests).
Clinical signs, many associated with neurotoxicity, were observed
in most animals at the intermediate dose and all those at the high
dose starting from the second week of treatment. These included
convulsions, twitching and tremors of various muscle beds (frequently
involving the head, pinnae, shoulder, hindlimbs, and sometimes the
whole body), ataxia, unsteady gait and rigidity of limbs (often the
hindlimbs), nervous behaviour, over- or underactivity, vocalization,
head nodding, aggression, resistance to treatment, and inappetence.
One male at 2 mg/kg bw per day and two at 5.0 mg/kg bw per day had to
be killed at weeks 11, 31, and 34, respectively, owing to
treatment-related poor condition. The signs observed in these animals
were similar to those described above and also included weight loss,
apparent loss of vision, and altered respiration. One female at 0.2
mg/kg bw per day showed signs of overactivity in weeks 13-18,
including pacing about the cage, which resulted in lesions on the foot
pad and tail, followed by weight loss and a period of underactivity. A
last incidence of overactivity was reported at about week 36;
adjustments to the diet and cage were used to control the
overactivity. The lack of similar findings in other animals at the low
dose and the overactivity of one control female for several days
around week 41 argued against a treatment-related effect.
The findings in animals at the intermediate and high doses in
routine physical examinations included tenseness, nervous and
excitable behaviour, abnormal stiffness or positioning of hindlimbs,
twitching of facial muscles, and hyperaesthesia. Most animals at the
high dose had exaggerated gag, corneal, blink, and hopping reflexes
and abnormal results in the 'foot sliding' test, and two females at
the intermediate dose showed tenseness. Body-weight gain over the
course of the study was decreased by about 16% only in females at the
high dose, due to effects in only one animal. Overall food consumption
was not affected. All animals were given an additional 200 g/day of
additional basal diet during weeks 16-18. Only minor changes were seen
in haematological and clinical chemical parameters, including slight
increases in packed cell volume, haemoglobin concentration, and
erythrocyte levels and in alanine aminotransferase activity in animals
at the intermediate or high dose, and were not clearly related to
treatment. The results of urinalysis and ophthalmological examinations
were negative. There were no clear-cut effects on organ weights and no
macroscopic or histopathological findings that appeared to be related
to treatment. The only remarkable finding in animals at 0.2 mg/kg bw
per day was overactivity in one female, described above. The NOAEL was
0.2 mg/kg bw per day (Holmes, 1992).
Technical-grade fipronil (purity, 95.4%) was administered in the
diet to groups of five male and five female beagle dogs at doses of 0,
0.075, 0.3, 1, or 3 mg/kg bw day for one year. The diets were given in
two aliquots 3.5-4.5 h apart and were moistened with water before
administration from day 30 onwards to enhance palatability. After the
first 38 days, the dose of 3 mg/kg bw per day was reduced to 2 mg/kg
bw per day because of significant toxicity. Standard evaluations of
toxicity ante and post mortem were included. Neurological
examination or testing of cranial nerve reflexes and nerves, segmental
reflexes, postural reactions, and general observations of behaviour,
gait, and stance, and for the presence of tremor or other dyskinesia
were conducted before treatment and on all surviving animals after 12,
24, 37, and 50 weeks of treatment. Blood samples taken after fasting,
before treatment and after one and 13 weeks of treatment, were
analysed for triiodothyronine (T3) and thyroxine (T4). Plasma from
fasted animals was analysed at weeks 1, 13, 24, 38, and 50 for the
presence of fipronil and a major metabolite, M&B 46136.
There were no overall effects on body-weight gain or food
consumption. The results of routine physical and ophthalmological
examinations and urinalysis were negative. None of the findings in
neurological, haematological, or clinical chemical tests, organ weight
measurements, or macro- or microscopic evaluations could be
definitively attributed to treatment. There were no treatment-related
findings in the animals at 0.3 or 0.075 mg/kg bw per day.
One female at 3 mg/kg bw per day had to be killed on day 32
because of poor health and signs of neurological disturbance. The
clinical signs of toxicity in this animal, which began on day 10,
included convulsions, underactivity, prostration, slow respiration and
tremors. Neurological examination revealed the absence of visual
placing reactions, depressed menace and startle reactions, and
abnormal gait. A blood sample showed increased packed cell volume,
haemoglobin concentration, erythrocyte count, plasma alkaline
phosphatase activity, and total protein and cholesterol
concentrations. Clinical signs of toxicity noted as early as week 1 in
three male and one female survivors at the high dose included
convulsions, head nodding, extensor rigidity, and twitching or tremors
of various muscle beds. One female at 1 mg/kg bw per day showed signs
of toxicity at week 13 (whole-body twitching) and another at week 20
(limb extensor rigidity). After 13 weeks of treatment, the T3 and T4
values were similar in all groups, including controls, the values for
T3 being 0.51-0.63 ng/ml and those for T4, 0.59-0.73 ng/ml.
Dose-related increases in the concentrations of fipronil and
metabolite M&B 46136 were seen in plasma at all measurement times.
Although there were no apparent differences between males and females
in the concentrations of these two compounds, the serum concentrations
of the metabolite were much higher than those of the parent compound
at doses > 0.075 mg/kg bw per day. At the lowest dose, the levels of
parent and metabolite were near or slightly higher than the limit of
quantification. There did not seem to be accumulation of either
chemical over time, and the concentrations of both compounds remained
fairly constant throughout the study. The NOAEL was 0.3 mg/kg bw per
day (Holmes, 1993).
(c) Long-term toxicity and carcinogenicity
Mice
Technical-grade fipronil (purity 95.4%) was administered for 78
weeks in the diet to groups of 52 male and 52 female CD-1 mice at
doses of 0, 0.1, 0.5, 10, 30, or 60 ppm (equal to group mean doses of
0, 0.011, 0.055, 1.2, or 3.4 mg/kg bw per day for males and 0, 0.012,
0.063, 1.2, or 3.6 mg/kg bw per day for females; the doses in mg/kg bw
per day were not determined for the group at 60 ppm) to evaluate
carcinogenicity. Six additional groups of 20 male and 20 female mice
were treated at the same doses for one year to measure toxicity.
Clinical signs of toxicity, body weights and weight gain, food
consumption, food efficiency, haematological parameters, organ weights
(absolute and relative to body weight), and macroscopic and
microscopic pathological appearance were observed.
Owing to excessive treatment-related mortality among animals of
each sex at 60 ppm, the surviving animals in the group were killed
during week 10. The lesions that had contributed to death were not
identified at necropsy, but all of the animals had high relative liver
weights. Several of the males had convulsions near the beginning of
the study. Reduced body-weight gains, food consumption, and food
efficiency were seen in these animals. The survival of the remaining
mice was comparable to or exceeded that of the control group, and no
clinical signs of toxicity were reported. Decreased body-weight gain
was seen in males (74-86% of control value) and females (81-86%) at 30
ppm at most evaluation times; the values for animals at 10 ppm were
also decreased but less consistently. Overall food consumption during
the study was lower than in controls in males (by about 7%) and
females (by 14%) at 30 ppm. Food efficiency was reduced in males and
females at 30 ppm and in males at 10 ppm.
A slightly lower percentage of neutrophils and a slightly higher
percentage of lymphocytes were noted in differential leukocyte counts
among females at 30 ppm after 76 weeks of treatment. Although gross
examination of animals observed for toxicity at week 53 showed no
remarkable effects, males at 30 ppm in the carcinogenicity study
showed liver enlargement and changes on the surface of the liver. The
absolute and/or relative liver weights were statistically
significantly increased in males and females at this dose at weeks 53
and 78. The relative liver weights were increased (p < 0.05) in
males at 10 ppm at both times and were increased (p < 0.05) in
males at 0.5 ppm at week 53 but not week 78. Histopathological
examination revealed a statistically significant increased incidence
of periacinar microvesicular vacuolation in the livers of males at 10
and 30 ppm at the end of 53 and 78 weeks, in females at 0.5 and 30 ppm
at the end of the toxicity study and in those at doses > 0.5 ppm
(significant at 0.5 and 30 ppm) at the end of the carcinogenicity
study; however, the incidence in females at 0.5 and 10 ppm did not
show a clear dose-response relationship, although the increased
incidence at 30 ppm may have been related to treatment. There was an
increased incidence of hepatocellular hyperplasia and chronic
degenerative changes in the livers of males at 30 ppm which died or
were killed during treatment in the carcinogenicity study.
There were no treatment-related neoplastic changes in females,
but males at 30 ppm had an increased incidence of malignant
hepatocellular carcinomas in comparison with concurrent controls: 1/52
in controls, 1/52 at 0.1 ppm, 2/52 at 0.5 ppm, 1/52 at 10 ppm, and
5/52 at 30 ppm. An additional hepatocellular carcinoma was observed in
one male at 30 ppm in the toxicity study. The incidence of
hepatocellular adenomas alone or combined with adenomas (one male at
30 ppm had both an adenoma and a carcinoma) was not significantly
increased. Since the increase in the incidence of carcinomas in males
at 30 ppm was within the range in historical controls in the testing
laboratory and the incidence among male concurrent controls was much
lower than the mean incidence in male historical controls, the
neoplastic findings in males were considered not to be related to
treatment. The NOAEL for systemic effects was 0.5 ppm, equal to 0.055
mg/kg bw per day (Broadmeadow, 1993).
Rats
Technical-grade fipronil (purity, 95.4%) was administered for one
year in the diet to groups of 15 male and 15 female CD rats to assess
its chronic toxicity, and further groups were fed the chemical for one
year and then observed for an additional 13 weeks to observe any
reversal of treatment-related changes. Groups of 50 male and 50 female
rats were originally scheduled to be treated for two years to assess
the carcinogenic potential of the chemical. The doses administered
were 0, 0.5, 1.5, 30, or 300 ppm, equal to 0, 0.019, 0.059, 1.3, or 13
mg/kg bw per day for males and 0, 0.025, 0.078, 1.6, or 17 mg/kg bw
per day for females. Standard evaluations of toxicity ante and
post mortem were included, and thyroid function (T3, T4, and
thyroid-stimulating hormone (TSH)) were measured in fasted animals
after 1, 4, 12, 24, and 50 weeks of treatment and after 2, 4, 7, and
11 weeks of the observation period after cessation of treatment.
The carcinogenicity phase of the study was terminated early owing
to excessive mortality and to ensure that a sufficient number of
animals were available for terminal sacrifices. Males and females were
killed when the number in any group declined to 25% of the original.
Thus, the males were killed after 89 weeks of treatment, when the
number of surviving animals in the group at 300 ppm was 25%, and the
females were killed after 91 weeks of treatment, when survival of
those at 30 ppm was 25%. More than 50% of animals of each sex in all
groups were still alive after 78 weeks. Early in the study, more
females at 300 ppm than controls died or were killed for humane
reasons related to convulsive episodes during this period. A
statistically significant increase in the number of deaths among
females at 30 ppm relative to controls disappeared when humane kills
were included in the mortality count. No significant differences in
mortality were seen among males or between other groups of females and
the control group.
Convulsive episodes, some lasting as long as 25 min and often
fatal, were observed in three males at 1.5 ppm, one male and three
females at 30 ppm, and eight males and 12 females at 300 ppm. The
convulsions tended to occur early in the treatment period but were
also seen later. Other clinical signs of toxicity that occurred
throughout treatment, predominantly in females at the high dose but
also in females given 1.5 and 30 ppm, included irritability,
vocalization, salivation, aggression, overactivity, and bruxism.
During the observation period, aggression, overactivity, irritability,
vocalization, and convulsions were seen in some females at 300 ppm.
Convulsions also occurred in females at 30 ppm and males at 300 ppm
but not in controls.
During the first week of treatment, body-weight gains were
significantly decreased in animals of each sex, by 6-11% at 30 ppm and
by 54-58% at 300 ppm. By one year, a significant 15-18% depression in
body-weight gain was observed only in animals at the high dose. By the
end of treatment, significant depressions were seen in males (18%) and
females (25%) at 300 ppm and in females at 30 ppm (23%). The
body-weight gain of males at 30 ppm was decreased by 7%. This pattern
continued during observation. Food consumption and food conversion
efficiency (calculated through week 14 only) were reduced at the
beginning of the study in animals of each sex at 300 ppm but were
similar to those of controls subsequently. The results of the
ophthalmological examination were negative.
Small but mostly significant decreases in haematological
parameters such as packed cell volume, haemoglobin concentration,
erythrocyte count, mean corpuscular volume, mean corpuscular
haemoglobin, and prothrombin time were seen at various times during
the study, particularly in males and females at the high dose. Slight
decreases in erythrocyte count, haemoglobin concentration, mean
corpuscular volume, and packed cell volume were also noted at 1.5 and
30 ppm.Towards the end of the study, platelet counts were slightly
increased in animals at the high dose and males at 30 ppm. Except for
a continued decrease in prothrombin time in females at 30 and 300 ppm,
the haematological changes did not persist after treatment.
Alterations in clinical chemical parameters, such as increased
cholesterol and calcium values and alterations in protein including
increased total protein, decreased albumin, increases in a1-, a2- and
b-globulins, and decreased albumin:globulin ratio, were seen mostly in
animals at 30 and 300 ppm; protein alterations were also observed in
males at 1.5 ppm towards the end of the study. Increased cholesterol
and calcium levels, total protein, and globulins and a decreased
albumin:globulin ratio persisted after cessation of treatment in
females at the high dose.
The T3 levels did not differ much from those of controls during
treatment but were significantly increased in females at the high dose
from four weeks after treatment and in females at 30 ppm from seven
weeks after treatment. The T4 levels were severely depressed after the
first week of treatment in animals of each sex, such that none was
detectable in animals at 300 ppm and the levels were significantly
depressed in a dose-dependent fashion in animals at 1.5 and 30 ppm.
Subsequently, the levels in animals at the high dose became
detectable, but the pattern of T4 depression observed at doses >
1.5 ppm generally persisted through week 50 of treatment. The T4
levels in animals at 0.5 ppm were occasionally significantly decreased
during treatment but not at the end of treatment. TSH levels were
significantly increased in animals at the high dose at all times and
in males at 30 ppm during the first month of treatment and after 50
weeks. Once treatment had ceased, the alterations in both T4 and TSH
levels were reversed in all groups except males at the high dose, in
which the TSH levels never recovered fully.
Alterations were seen at various times during the study in
urinary parameters (lower pH, higher protein, elevated urine volume,
and decreased specific gravity) in rats at 30 and 300 ppm
(predominately males). The alterations in protein and pH persisted
after treatment. Gross examination of animals in the carcinogenicity
study at termination and animals that were killed or died during the
study revealed increased incidences of large and/or pale kidneys in
animals at the intermediate or high dose and enlarged adrenals in
males at the high dose. Enlarged livers and thyroids were seen in
animals of each sex at the high dose and in males at 30 ppm. At
interim sacrifice (toxicity study), enlarged livers or thyroids were
seen in some animals at 30 or 300 ppm. Absolute thyroid weights were
somewhat increased in males at 0.5 and 1.5 ppm. The absolute and
relative weights of the liver and thyroid were increased in animals of
each sex at 30 or 300 ppm in both the toxicity and the carcinogenicity
study and in animals at the high dose that were killed or died during
the study. Increased absolute and relative kidney weights were also
seen in animals at 30 or 300 ppm, and increased adrenal weights were
seen in males at these doses. Most of the changes in organ weight were
statistically significant. The increases in liver, thyroid, and kidney
weights persisted to some degree after treatment, mostly in animals at
300 ppm. Histopathological examination showed an increased incidence
and severity of progressive senile nephropathy (a non-neoplastic
lesion) in animals of each sex at 300 ppm in the toxicity study and at
30 and 300 ppm in the carcinogenicity study; an increased severity of
the lesion was also reported in rats at 1.5 ppm. These findings
persisted to some extent after treatment.
Benign and malignant neoplastic changes (follicular-cell adenomas
and carcinomas) occurred in the thyroid glands of animals of each sex.
The incidence of these tumours per number of animals examined at 0,
0.5, 1.5, 30, and 300 ppm was, respectively: malignant follicular-cell
carcinomas: 0/49, 0/48, 0/50, 0/50, and 5/50 for males and 0/50, 1/50,
0/50, 1/50, and 2/50 for females; benign follicular-cell adenomas:
0/49, 1/48, 5/50, 3/50, and 12/50 for males and 0/50, 0/50, 0/50,
0/50, and 8/50 for females; total tumours: 0/49, 1/48, 5/50, 3/50, and
17/50 for males and 0/50, 1/50, 0/50, 1/50, and 10/50 for females.
None occurred in concurrent controls. Males in all treated groups and
females in all groups except that receiving 1.5 ppm showed increased
incidences of benign and malignant thyroid tumours combined in the
carcinogenicity study. The increases were significant (at p < 0.05,
< 0.01, or < 0.001 by Fisher's exact one-tailed test for pair-wise
comparisons) for carcinomas alone in males at the high dose and for
adenomas alone and adenomas and carcinomas combined in males and
females at 300 ppm and males at 1.5 ppm. The author concluded that
only the neoplastic changes observed in animals at 300 ppm were
related to treatment, as the increased incidences of benign or
malignant lesions alone or in combination exceeded the historical
control incidences for the testing facility only at the high dose, and
the zero incidences in the concurrent controls were unusually low. The
reported historical control incidence rates for studies of comparable
length (88-95 weeks) were 0-5.5% (males) and 0% (females) for
follicular-cell carcinoma, 1.4-5.7% (males) and 0-1.9% (females) for
follicular-cell adenoma, and 1.9-7.3% (males) and 0-1.9% (females) for
all follicular-cell tumours. The occurrence of these tumours was
attributed to continuous stimulation of the thyroid gland by elevated
TSH levels.
Although the author concluded that there were no significant
intergroup differences in mortality among males, analysis of the data
by the US Environmental Protection Agency using the computer program
of Thomas, Breslow, and Gart showed a significantly increasing trend
in mortality with increasing doses of fipronil in male but not female
rats. This finding does not affect the validity of the study for the
following reasons: firstly, the study was terminated prematurely only
near its end; secondly, the literature indicates that, in general, the
longevity of CD (Charles River) rats has been decreasing and that a
shortened life span is therefore not unique to this study; and
thirdly, the study was long enough for tumours to have developed in
the fipronil-treated animals.
The initial analysis of tumour incidence was based only on the
data from the carcinogenicity phase of the study. Six animals were
reported to have developed thyroid follicular-cell tumours during the
observation phase, with carcinomas in one male at 30 ppm and one at
300 ppm and adenomas in one female at 1.5 ppm and one male and two
females at 300 ppm. No thyroid tumours considered to be related to
treatment were reported to have occurred during the toxicity phase,
although a follicular-cell carcinoma was tabulated for one male at 30
ppm killed after one year of treatment. When these additional data are
included in the analysis and the data are analysed on the basis of the
first appearance of thyroid adenomas or carcinomas in the study, the
incidence is as shown in Table 2. When Peto's prevalence test is used
to analyse the data for male rats (as the first tumour was seen before
interim sacrifice), significantly increasing trends (p < 0.01) are
obtained for thyroid follicular-cell adenomas, carcinomas, and
combined adenomas and carcinomas. Significant differences from
controls in pair-wise comparisons (p < 0.05 or p < 0.01) are
found for adenomas and combined adenomas and carcinomas at doses >
1.5 ppm and for carcinomas at the high dose. When the exact trend test
and Fisher's exact test are used to analyse the data on tumours in
female rats, a significantly increasing trend and a significant
difference in pair-wise comparisons of the group at 300 ppm with the
controls is seen for thyroid follicular-cell adenomas and combined
adenomas and carcinomas (all at p < 0.01).
This analysis suggests that doses > 1.5 ppm are carcinogenic,
since the pair-wise comparisons in males at doses > 1.5 ppm are
significant and there were significant trends for tumour formation.
Since the concurrent controls had an apparently lower incidence than
historical controls at the laboratory where the study was conducted,
however, the apparent inconsistency in the dose-response relationship
at 1.5 and 30 ppm and the fact that TSH levels were significantly and
persistently elevated only at 300 and to a lesser extent at 30 ppm, an
alternative view is that the incidence of thyroid tumours was
toxicologically significant only at the highest dose. In summary, the
study demornstrates that fipronil is clearly carcinogenic to rats at
300 ppm; however, the overall NOAEL is for neurotoxicity and is
0.5 ppm, equal to 0.019 mg/kg bw (Aughton, 1993).
(d) Genotoxicity
The results of assays for genotoxicity with fipronil are
summarized in Table 3.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
In a two-generation study of reproductive toxicity, 30 male and
30 female CD rats received technical-grade fipronil (purity, 95.4%) in
the diet at concentrations of 0, 3, 30, or 300 ppm, equal to 0, 0.25,
2.5, or 26 mg/kg bw per day for males and 0, 0.27, 2.7, or 28 mg/kg bw
per day for females. After two matings of the F0 generation, litters
were culled to four animals of each sex on day 4 post partum, and
physical development was assessed by recording the day of onset and
completion of pinna unfolding, hair growth, tooth eruption, and eye
opening.
Table 2. Incidences of thyroid follicular-cell tumours in rats treated with fipronil
Tumour Dose (ppm)
Males Females
0 0.5 1.5 30 300 0 0.5 1.5 30 300
Adenomas 0/63 1/61 5/63 3/62 12/61 0/48 0/49 0/50 0/45 8/46
% 0 2 8 5 20 0 0 0 0 17
p 0.000** 0.116 0.014* 0.038* 0.000** 0.000** 1.000 1.000 1.000 0.002**
Carcinomas 0/59 0/57 0/62 1/60 5/57 0.48 1/49 0/50 1/45 2/46
% 0 0 0 2 9 0 2 0 2 4
p 0.000** - - 0.186 0.007** 0.084 0.505 1.000 0.484 0.237
Combined 0/63 1/61 5/63 4/62 16/61 0/48 1/49 0/50 1/45 10/46
% 0 2 8 6 26 0 2 0 2 22
p 0.000** 0.116 0.014* 0.024* 0.000** 0.000** 0.505 1.000 0.484 0.001**
For males: no. of tumour-bearing animals/no. of animals examined, excluding those that died before observation of
the first tumour; analysis by Peto's prevalence test. For females: no. of tumour-bearing animals/no. of animals
examined, excluding those that died or were killed before week 54; analysis by exact trend test and Fisher's exact
test. First adenoma observed at 300 ppm in males in week 42 and in females at week 62. First carcinoma observed in
males at 30 ppm in week 53 and in females at 300 ppm in week 79. One male at 300 ppm had both an adenoma and a
carcinoma.
Significance of trend denoted at control; significance of pair-wise comparison with control denoted at dose:
*, p < 0.05; **, p < 0.01
Table 3. Results of assays for genotoxicity with fipronil
End-point Test object Concentration Purity Result Reference
(%)
In vitro
Reverse mutation S. typhimurium 0.8-500 µg/plate 90.6 Negativea,b Clare (1988a)
TA98, TA100, in DMSO
TA1535, TA1537
Gene mutation Chinese hamster 1.13-386 µg/ml 97.2 Negativea,b Lloyd (1990)
cell line V79, in DMSO
hprt locus
Chromosomal Chinese hamster 15-60/µg/ml 6 ha, 98.3 Positiveb,d Wright (1995)
aberration lung cell line 7.5-30 µg/ml 24 hc,
7.5-22.5 µg/ml 48 hc
in DMSO
Chromosomal Human lymphocytes 75-300 µg/ml in 90.6 Negativeb Marshall (1988a)
aberration DMSO
In vivo
Micronucleus CD-1 mice 1-25 mg/kg bw in 97.2 Negativeb,e Edwards (1991)
formation aqueous methyl-
cellulose
Micronucleus CD-1 mice 6.95-48 µg/kg bw 96.2 Negativeb Edwards (1995)
formation in aqueous methyl-
cellulose
DMSO, dimethyl sulfoxide
a With and without metabolic activation
b Appropriate positive controls gave expected positive responses
c Without metabolic activation
d Positive only with 6-h pulse treatment; significant, dose-related effects at 45 and 60 µg/ml without
metabolic activation; non-significant increase at 60 µg/ml with metabolic activation
e Unacceptable; no overt or target-cell toxicity at doses < 25 mg/kg; results at 50 mg/kg were inconclusive.
Systemic toxicity was seen in the parental animals at doses >
30 ppm as increased absolute and relative weights of the thyroid gland
and liver in the F0 and F1 generations, decreased absolute and
relative pituitary gland weights in the F1 females (a decrease in
relative pituitary weight at 3 ppm was minor and was not considered to
be toxicologically significant), and a significantly increased
incidence of follicular epithelial hypertrophy of the thyroid gland in
F1 females and animals of each sex at 300 ppm. Increased mortality
occurred among F0 and F1 animals at this dose, with clinical signs
of toxicity including convulsions; in addition, the F0 generation had
decreased food consumption before mating, and decreased body-weight
gain was seen before mating in the F0 and F1 generations and in F0
females during gestation and lactation. The absolute and relative
weights of the ovaries were decreased in F0 females, and females of
the F0 and F1 generations had a significantly increased incidence of
centriacinar fatty vacuolation in the liver. The increased incidence
of follicular epithelial hypertrophy in adult males at 30 ppm was not
significant but was found in both the F0 generation (in 2/30 animals,
in comparison with 0/30 controls, 0/30 animals at 3 ppm, and 10/29
animals at 300 ppm) and the F1 generation (in 3/30 animals, with 0/30
controls, 2/30 at 3 ppm, and 9/30 at 300 ppm) and was considered to be
related to treatment. The presence of this finding in 2/30 F1 males
at 3 ppm, and not in the F0 generation, was considered plausible in
an organ in rats that is very sensitive to stimuli; it could therefore
not be clearly related to treatment.
Reproductive toxicity observed in animals at 300 ppm consisted of
an increased incidence of clinical signs of toxicity in F1 and F2
offspring (notably convulsions when the offspring first started to eat
the treated diet), decreased litter size and body weights, a decrease
in the percentage of animals that mated, a reduction in the fertility
index of F1 parental animals, reduced postimplantation and postnatal
survival in the F2 litters, and delays in physical development in F1
and F2 litters including slight delays in the onset of tooth eruption
(F1) and pinna unfolding (F2). The NOAEL for parental toxicity was 3
ppm, equal to 0.25 mg/kg bw per day, while that for reproductive
toxicity was 30 ppm, equal to 2.5 mg/kg bw per day (King, 1992).
(ii) Developmental toxicity
Rats
Technical-grade fipronil (purity, 93%) was administered by gavage
as a suspension in 0.5% aqueous methylcellulose to groups of 25
specific pathogen-free female rats of the Crl: CD (SD)BR VAF/Plus
strain (Charles River, France) at doses of 0, 1, 4, or 20 mg/kg bw per
day on days 6-15 of gestation. The study was terminated on day 20.
Adult females were observed for clinical signs, food and water
consumption, and body-weight changes; they underwent a macroscopic
post mortem at study termination. Litters and fetuses were evaluated
with regard to pre- and postimplantation losses, litter size, litter
and mean fetal weights, sex ratios, and malformations or skeletal or
visceral anomalies.
The pregnancy rate was 96-100%. There were no deaths, abortions,
premature deliveries, or clinical signs in the adult females. Maternal
effects associated with treatment occurred only in the animals
receiving the high dose and included reduced body-weight gain,
increased water consumption, and decreased food consumption at various
intervals during gestation. Some of these effects continued after
treatment. The macroscopic examination showed no remarkable effects.
No effects of treatment were seen on developmental parameters. The
NOAEL for maternal toxicity was 4 mg/kg bw day, and that for
developmental toxicity was 20 mg/kg bw day (Brooker & John, 1991).
Rabbits
Technical-grade fipronil (purity, 95.4%) was administered by
gavage as a suspension in 0.5% aqueous methylcellulose mucilage and
0.5% Tween 80 to groups of 22 female New Zealand white rabbits (Ranch
Rabbits, Susse, United Kingdom) at doses of 0, 0.1, 0.2, 0.5, or 1
mg/kg bw per day on days 6-19 of gestation. The study was terminated
on day 29. Adult females were observed for clinical signs, abortion
and total litter loss, food and water consumption, and body-weight
changes; a macroscopic post mortem was performed at study termination.
The litters and fetuses were evaluated with regard to pre- and
postimplantation losses, litter size, litter and mean fetal weights,
sex ratios, and malformations or skeletal or visceral anomalies.
The number of pregnant animals in each group ranged from 18 to
21. There were no treatment-related deaths. At doses of 0.5 and 1 g/kg
bw per day, maternal body-weight gain during treatment was
significantly lower than that of controls by 50 and 70%, respectively,
and the body-weight gain of dams at 0.1 or 0.2 mg/kg bw per day was
reduced by 30%. Animals in all fipronil-treated groups consumed less
food than those in the control group during treatment, and the
decreases in the groups at the two highest doses achieved significance
on gestation days 6-12 and/or 13-19. Other maternal parameters were
not affected. Treatment had no effect on development. No NOAEL for
maternal toxicity was identified; that for developmental toxicity was
1 mg/kg bw per day (King, 1990).
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Technical-grade fipronil (purity, 96.7%) moistened with corn oil
was a slight dermal irritant in male and female New Zealand white
rabbits after a 4-h application to intact skin. The signs of
irritation were slight to well-defined erythema and slight oedema,
which had cleared by day 7 after application. When fipronil (purity,
93%) was moistened with distilled water and applied for 4 h to the
intact skin of male New Zealand white rabbits, no signs of dermal
irritation were seen (Liggett, 1988a; Myers & Christopher, 1993a).
Instillation of technical-grade fipronil (purity, 96.7%) into the
eyes of male and female New Zealand white rabbits produced minor
transient corneal opacity and iritis, which had cleared within 24 h,
and minor-to-moderate conjunctival irritation, which had cleared
within 2-14 days. In a similar study with only male rabbits, fipronil
(purity, 93%) induced transient, minimal conjunctival inflammation,
which cleared within 24-48 h (Liggett, 1988b; Myers & Christopher,
1993b).
Technical-grade fipronil (purity, 95.4%) was tested in male and
female Dunkin-Hartley albino guinea-pigs by a modification of the
Buehler method. Topical application of the highest optimal
concentration for induction and challenge of fipronil (30% w/v) in
paraffin oil to guinea-pig skin produced no sign of dermal
sensitization (Smith, 1990).
In a Magnusson-Kligman maximization test in male and female
Dunkin-Hartley albino guinea-pigs, the optimal induction and challenge
concentrations of technical-grade fipronil (purity, 95.4%) were
determined in advance. In the main study, primary induction
concentrations of 5% (w/v) fipronil in propylene glycol alone or
combined with Freund's complete adjuvant were applied intradermally at
separate sites. In the secondary induction phase, 5% fipronil in
propylene glycol was applied topically to the same sites. A topical 3%
challenge dose produced no significant response, but 24 h after
challenge with 10% fipronil in propylene glycol, four of 20 animals
showed a significant dermal response (eschar formation or slight
erythema). Eschar formation was still evident in two animals 48 h
after the challenge. No significant responses were observed in control
animals challenged with 3 or 10% fipronil in propylene glycol. One
control animal challenged with propylene glycol alone showed slight
erythema at 24 h. By the criteria of the European Economic Community,
the test material did not cause delayed contact hypersensitivity
because the response rate at challenge was < 30%; however, the
positive response rate of 20% in this study could also support the
conclusion that fipronil is a mild or weak skin sensitizer (Johnson,
1993).
(ii) Neurotoxicity
Rats
A single dose of technical-grade fipronil (purity, 96.7%) in corn
oil was administered by gavage to groups of 15 male and 15 female
Sprague-Dawley rats at doses of 0, 0.5, 5, or 50 mg/kg bw. The animals
were about eight weeks of age at the time of treatment. Standard
evaluations of toxicity included observations for mortality and
clinical signs of toxicity and measurements of body weight. The
neurobehavioural evaluations of all surviving animals included a
functional observational battery of tests before treatment and at 7 h,
7 days, and 14 days after treatment, and quantitative assessment of
motor activity before treatment and 1 h after each functional test.
The study was terminated on days 16-19 after treatment. The postmortem
examinations included an abbreviated necropsy of the thoracic and
peritoneal cavities (all groups) and detailed light microscopic
evaluation of the brain, spinal cord, and peripheral nerves of six
male and six female controls and six animals of each sex at the high
dose after intracardiac perfusion in situ with 10% neutral buffered
formalin. Rats that died during the study were necropsied, but their
nervous tissues were not examined microscopically.
Five males and one female at 50 mg/kg bw died during the study,
most within two days of treatment. At necropsy, most had diffuse brain
haemorrhages, which may have caused death or may have been agonal.
Clinical signs of toxicity were seen only at the high dose, and the
incidence of neurological signs was more prevalent in males. On the
day of treatment, males and females at this dose had either clonic or
tonic-clonic convulsions. Other clinical signs of toxicity included
those indicative of cachexia: emaciation, dehydration, unkempt
appearance, urine stains, cold extremities, and/or pallor. Male body
weight was reduced by 6-10% relative to that of controls 7 and 14 days
after treatment.
Treatment-related effects on functional parameters occurred
primarily 7 h after treatment in rats at 50 mg/kg bw, when males were
seen to have drooping or half-shut eyelids. During open-field
evaluations, both stimulation and depression of the nervous system
were seen, which were generally more pronounced in males. Signs
indicative of stimulation of motor systems were convulsions, coarse
and fine tremors, head bobbing, myoclonic movements, and decreased
hindleg splay, which was significantly decreased (p < 0.01) in
animals of each sex. The signs indicative of nervous system depression
were decreased arousal and rearing activity, decreased reflexes such
as response to tail pinching and approach and air righting reflex, and
decreased muscle tone, altered gait, decreased pupil size, and/or
decreased body temperature. Urination was more evident in males at the
high dose. The only treatment-related effect in rats at 5 mg/kg bw 7 h
after treatment was significantly decreased hindleg splay (p <
0.05) in animals of each sex. A stimulatory response in certain
functional tests was seen in some males at the high dose seven days
after treatment, such as increased arousal and rearing activity and
exaggerated touch and sound reflexes. Females at the high dose showed
significantly increased hindleg splay at this time and on day 14 after
treatment. The only finding of note in males at the high dose on day
14 was a higher incidence of urination.
Motor activity 8 h after treatment was decreased by 90% in males
at the high dose and by 93% in females. This was the only effect on
motor activity during the study that was attributed to treatment.
There were no treatment-related gross or microscopic changes on
post-mortem examination of the central and peripheral nervous systems.
The NOAEL was 0.5 mg/kg bw on the basis of decreased hindleg splay 7 h
after treatment (Gill et al., 1993).
Groups of 15 male and 15 female Sprague-Dawley rats were fed
diets containing technical-grade fipronil (purity, 96.7%) at 0, 0.5,
5, or 150 ppm (equivalent to 0, 0.03, 0.3, or 8.9 mg/kg bw per day for
males and 0, 0.03, 0.3, or 11 mg/kg bw per day for females) for 13
weeks. The animals were about eight weeks of age at the start of
treatment. Standard evaluations of toxicity included observations for
mortality and clinical signs of toxicity and measurements of body
weight. Neurobehavioural screening, consisting of a functional
observational battery of tests and motor activity evaluations, was
performed before treatment and during weeks 4, 9, and 13. Post-mortem
examinations at terminal sacrifice, included an abbreviated necropsy
of the thoracic and peritoneal cavities in all groups and a detailed
light microscopic evaluation of the brain, spinal cord, and peripheral
nerves of six males and females of the controls and at the high dose
after intracardiac perfusion in situ with 10% neutral buffered
formalin.
There were no treatment-related deaths or clinical signs of
toxicity. Slight decreases in body weight (6.5-6.9%) and decreased
body-weight gain were seen in males and females at the high dose
during the first few weeks of treatment. These were accompanied by
decreased food consumption, suggesting unpalatability. Functional
changes seen in animals at the high dose in week 4, 9, and/or 13 were
increased urination and an increased incidence of exaggerated response
to tail pinching in males, an increased incidence of exaggerated
startle responses during manipulation in animals of each sex, and
increased forelimb grip strength in females at week 13. No
treatment-related findings were seen at necropsy or during
histopathological examination of nervous tissues. The changes in
animals at the high dose may not have been related to treatment;
however, these findings, although relatively minor when taken
separately, represent a minimal effect of treatment when taken
in toto. In addition, the exaggerated responses to touch and sound,
particularly in males, and the increased urination of males at the
high dose were considered to be related to treatment in the preceding
study. Therefore, the NOAEL for both neurotoxicity and systemic
toxicity was 5 ppm, equal to 0.3 mg/kg bw per day (Driscoll & Hurley,
1993).
Dogs
Technical-grade fipronil (purity, 95.4%) was administered in
capsules to female beagle dogs (26 weeks old at the start of the
study) at doses of 0 (one animal) or 20 mg/kg bw per day (four
animals) until clear signs of neurotoxicity were seen or for a maximum
of 14 days. Immediately after the appearance of neurotoxic signs,
administration was discontinued and a 28-day observation period was
initiated. This phase was begun after five days of treatment in two
animals, seven days of treatment in one animal, and 13 days of
treatment in one animal and the control dog. Animals were monitored
for general health, clinical signs of toxicity, chronic conditions,
food and water consumption, and body-weight changes and were subjected
to a weekly veterinary examination and neurological examinations
before treatment and then at intervals during treatment and
observation. The neurological examination consisted of observation and
testing of cranial nerve reflexes, segmental reflexes, and postural
reactions and general observation of behavioural changes,
abnormalities of gait and stance, and the presence of tremor or other
dyskinesia. An obstacle-avoidance test and a hearing test were
administered at the same time as the neurological examination.
Anaesthetized dogs were killed by perfusion-fixation at the end of the
observation phase. The brain, spinal column, and some peripheral
nerves were examined grossly, but only the brain and spinal column
appear to have been subjected to microscopic examination.
All animals survived to the end of the observation period. The
control animal showed no abnormal signs throughout the treatment and
recovery periods, but all treated animals displayed neurotoxic signs,
which included hypoactivity, salivation, ataxia, convulsions, tremors,
unsteady gait and stiffened body, aggressive behaviour, apparent lack
of vision, muscle twitching, nervous behaviour, and a high-stepping
gait. The time to appearance of signs and the duration of signs after
discontinuation of treatment varied. Some signs, like aggressive
behaviour and visual impairment, first appeared after discontinuation
of treatment. Food consumption was markedly decreased from day 1 or 2
of treatment and for up to seven days during the recovery period. All
treated animals lost weight, but the initial body weight was restored
17 days after cessation of treatment. Veterinary examination showed
similar changes and also emaciation in three treated animals and
hunched posture and peripheral vasodilatation in one animal. The
neurological examinations indicated widely varied responses including
(in addition to the responses noted above) changes in reflex
responses, some exaggerated, including gag and flexor responses, and
some depressed, including blink, pupillary, and consensual light
reflexes and visual and tactile placing responses. One animal still
had slightly exaggerated gag and flexor reflexes at the end of the
recovery period. The results of the obstacle-avoidance and hearing
tests indicated temporary visual impairment and possible hearing
deficiency in one animal. No abnormal findings were reported after
macroscopic or microscopic examination.
Virtually complete recovery from what appeared to be functional
changes in the central nervous system was reported by 12 days or less
after cessation of treatment; in the absence of histopathological
changes in the study, the mode of action of the chemical was
considered probably to be pharmacological and temporary. Given the
short duration of treatment (5-13 days), the short observation period
(treatment period plus 28 days), the use of only one dose, one sex,
and only one control animal, however, there is insufficient
information to draw any firm conclusions about the reversibility of
the functional, cellular, and neurotransmitter or receptor effects.
There was no NOAEL (Holmes, 1991c).
(iii) Developmental neurotoxicity
Technical-grade fipronil (purity, 96.1%) was administered to
groups of 30 female Sprague-Dawley (CD) rats at dietary levels of 0,
0.5, 10, or 200 ppm, equal to 0, 0.05, 0.9, or 15 mg/kg bw per day,
from gestation day 6 through lactation day 10, day 0 being defined as
the day mating was confirmed. Clinical observations, body weights, and
food consumption were recorded for all animals at scheduled intervals
throughout gestation and lactation, and litters were examined at
selected intervals throughout lactation. The numbers of live and dead
pups and the sex and body weights of the pups were recorded. Litters
were standardized to eight pups on postnatal day 4. The achievement of
pinna detachment, incisor eruption, eye opening, vaginal patency, and
preputial separation was evaluated for all surviving pups. For one pup
per sex per litter, the following evaluations were conducted on
various postnatal days: motor activity (days 13, 17, 22, 60), auditory
startle response (days 22 and 60), swimming development (days 6, 8,
10, 12, and 14), and learning and memory in a Y-maze (days 24 and 60).
On day 11 of lactation and again on day 60, one pup of each sex per
litter was killed. Brain weights were recorded and neuropathological
evaluation was performed on six pups of each sex from the control and
high-dose groups. Maternal animals were killed and necropsied after
the weaning of the last litter, and all remaining offspring were
killed soon after all the evaluations were completed.
The maternal effects at the dietary level of 200 ppm were reduced
body weight during treatment, reduced body-weight gain during
gestation, and reduced food consumption. No treatment-related findings
were observed at necropsy. Pregnancy status, gestation length, litter
size, and pup sex ratio were not affected by treatment. At 200 ppm,
there was a significant increase in the number of stillborn pups, and
pup and litter survival were reduced. Also at this level, male and
female pup weights were reduced, and upper and lower incisor eruption
and sexual development were delayed. There was a small but significant
increase in the time to preputial separation in animals at 10 or 200
ppm. At 10 ppm, group mean body weights were reduced for female pups
at all recorded intervals and for male pups on days 4 and 17;
postweaning weights were not affected.
With regard to neurodevelopmental effects, at 200 ppm the maximum
auditory startle response time for males and females was significantly
decreased on postnatal day 22; there was no significant difference in
the time to maximum or average response. Swimming development was
delayed on postnatal day 6 for animals of each sex, and motor activity
was increased in females on postnatal day 17. Learning and memory
(Y-maze test) were delayed significantly for females in two trials on
postnatal day 24. Also at 200 ppm, significant decreases in absolute
pup brain weights were noted for animals of each sex on days 11 (-20%
for males and -11% for females) and 60 (-7% for males and females),
but pup body weights were also significantly decreased at these times,
so that the relative brain:body weights were actually increased
relative to those of concurrent controls: +39% for males and +20% for
females on day 11 and +6% for males and females on day 60. Thus, the
lower brain weights may have been due to the overall retarded growth
of the pups associated with maternal toxicity rather than to a
developmental delay in brain size. The statistically significant
increases in motor activity in female pups at 10 ppm observed on day
17 could not be clearly related to treatment when the magnitude of the
response was compared with that of the control group, because the
values for the latter on that day appeared to be unusually low. No
microscopic morphological abnormalities were seen in the brains of
offspring killed on postnatal day 11 or 60. The NOAEL for maternal and
neurodevelopmental effects was 10 ppm, equal to 0.9 mg/kg bw per day.
The NOAEL for developmental effects was 0.5 ppm, equal to 0.05 mg/kg
bw per day, on the basis of reduced pup body weights (Mandella, 1995).
(iv) Thyroid function
Effects on thyroid hormone levels: Groups of 10 Crl:CD (SD) BR
rats of each sex were given technical-grade fipronil (purity, 95.4%)
in the diet at doses of 0, 0.1, 1, 5, or 30 ppm (equal to 0, 0.01,
0.1, 0.49, or 2.9 mg/kg bw per day for males and 0, 0.01, 0.1, 0.48,
or 2.9 mg/kg bw per day for females) for four weeks. The parameters
measured were clinical observations, body weight, food consumption and
efficiency, water consumption, evaluations of T3, T4, and TSH before
treatment and on days 7 and 28, and organ weights and histopathology
of the thyroid and liver at necropsy.
The T3 level was significantly decreased in males at doses > 1
ppm on day 7 and was increased in females at 30 ppm on day 28; T4
levels were significantly decreased in males and females at 30 ppm,
males at 5 ppm on day 7, and males at 30 ppm on day 28; the TSH level
was significantly increased in males at 30 ppm on days 7 and 28 and in
females at 30 ppm on day 28; thyroid weights were nonsignificantly
increased in males and females at 30 ppm; and there was an increased
incidence of higher follicular epithelium in males and females at 30
ppm and in one male at 5 ppm. As re-analysis of the data for T3 in the
controls and rats at 1 ppm showed no significant difference, it was
concluded that this level had no effect.
Increased liver weights were observed in animals at 30 ppm;
however, the change was significant only in females. An increased
incidence of minimal centrilobular hepatocyte enlargement was seen in
males at 30 ppm. Staining of liver sections with Oil Red O
demonstrated an increased incidence of periportal fat deposition in
females at 5 and 30 ppm. The NOAEL was 1 ppm, equal to 0.1 mg/kg bw
per day (Peters et al., 1991a).
Effect on biliary excretion of thyroxine: The objective of this
study was to measure the effects of single and repeated oral doses of
technical-grade fipronil (purity, 96.7%) on the biliary excretion of
intravenously administered 125I-T4 in bile duct-cannulated rats.
Groups of three Sprague-Dawley CD(Crl:CD/BR) VAF plus strain male rats
were given single or repeated daily doses for 14 days of: fipronil (1
or 10 mg/kg bw per day orally at 5 ml/kg bw), phenobarbital (80 mg/kg
bw per day intraperitoneally as a positive control), or 0.5%
methylcellulose (5 ml/kg bw orally as a negative control). Immediately
after treatment, the animals were anaesthetized, the common bile duct
was cannulated, and 1 mg sodium iodide (to block thyroid uptake of
125I) was administered by injection into the stomach, followed 4 h
later by intravenous administration of about 10 µCi of 125I-T4. Bile
samples were collected before treatment and at 15-min intervals for
0-5 h after 125I-T4 administration. Blood samples were taken at 30-min
intervals. Both bile and blood were analysed for radiolabel. After 5
h, the animals were killed and the livers removed. Selected bile
samples were pooled, treated with œ-glucuronidase and analysed by HPLC
for free and conjugated T4.
The single doses of fipronil or phenobarbital did not
significantly increase the amount of bile excreted or the biliary
clearance of 125I-T4. After 14 days of treatment, biliary clearance
was significantly increased with the high dose of fipronil but not
with the low dose. The high dose of fipronil produced a greater
increase than phenobarbital. Bile output was significantly increased
after treatment with the high dose of fipronil and with phenobarbital.
After treatment with œ-glucuronidase, about 50% of the 125I-T4 in bile
was conjugated with either glucuronide or sulfate, but about 30%
remained unidentified. Thus, repeated treatment with fipronil at 10
mg/kg bw per day orally increased the excretion of conjugated T4 in
bile, fipronil being more potent than phenobarbital. A possible
mechanism would be hepatic accumulation of T4 and induction of the
hepatic T4 conjugating enzyme UDP glucuronyltransferase, leading to
increased biliary excretion of T4-glucuronide conjugate (Taylor,
1993).
Effect on thyroxine clearance: Groups of six male Crl:CD(SD)
rats were treated with either technical-grade fipronil (purity, 95.4%)
at 10 mg/kg bw per day by gavage, phenobarbital at 80 mg/kg bw per day
intraperitoneally, or 0.5% methylcellulose (vehicle control) at 5
ml/kg bw by gavage for one or 14 days. Four h after the final dose of
either test substance, each rat received 125I-T4 at a dose of 10
µCi/kg bw. The levels of 125I in whole blood were measured for up to
30 h after T4 administration and were used to calculate the terminal
half-life, clearance, and volume of distribution of this hormone.
Fipronil had no effect on mortality or other parameters ante
mortem. Phenobarbital-treated animals showed collapsed posture,
lethargy, and shallow breathing on the first day of treatment.
Fipronil had no effect on the clearance of T4 after one day of
treatment, but after 14 days a decrease in terminal half-life (52% of
control level) and increases in the clearance (261%) and volume of
distribution (137%) were seen. The effects of phenobarbital were
similar, although quantitatively less severe, and were seen on day 1
of treatment (Peters et al., 1991b).
Effect on thyroid function: The effect of technical-grade
fipronil (purity, 95.4%) on thyroid function was compared with that of
propylthiouracil, a known inhibitor of thyroid organification which
interferes with thyroglobulin iodination, and noxythiolin, a thiourea
compound and putative inhibitor of thyroid iodide organification known
to lower T4 levels in rats. Groups of 27 male Crl:CD(SD)BR rats were
treated for 14 days with either 0.5% methylcellulose (vehicle control)
at 5 ml/kg bw by gavage, fipronil at 10 mg/kg bw per day by gavage,
propylthiouracil at 200 mg/kg per day by gavage, or Noxyflex at 5
mg/kg bw per day intraperitoneally as a saline solution. On day 15,
each animal received 125I-sodium iodide at a dose of 1 µCi. Six hours
later, nine males in each group received 10 or 25 mg/kg bw potassium
perchlorate or a 0.9% saline solution intraperitoneally, and blood was
immediately drawn in order to measure radiolabel. The animals were
then killed, and the thyroids were weighed and analysed for
radioactivity. Treatment with fipronil or Noxyflex appeared to
stimulate the thyroid glands, as evidenced by increased accumulation
of 125I and increased ratios of radiolabel distribution between the
blood and the thyroid. These changes were accompanied by increases in
thyroid weight. Treatment with propylthiouracil decreased the amount
of 125I incorporated in the thyroid and the blood:thyroid ratios, with
elevated levels of 125I in the blood. As the thyroids of these animals
were 2.5 times heavier than those of controls, however, the ratio of
blood 125I to thyroid weight was reduced. Administration of
perchlorate did not further reduce the 125I content of the thyroids or
the blood:thyroid 125I radiolabel ratio. There was no evidence of
inhibition of iodide incorporation (Peters et al., 1991c).
(v) Mode of action of fipronil
GABA is an important inhibitory neurotransmitter in both insects
and mammals. Attempts to demonstrate that the target site for fipronil
at the molecular level is the chloride ion channel of the GABA
receptor include electrophysiological testing, in which fipronil at 10
nmol/L reversed blockage of nervous system activity by GABA at 10
mmol/L, resulting in hyperexcitation in the housefly larva; flux
studies in which fipronil inhibited the passage of radiolabelled
chloride ions through opened GABA receptors in rat brain microsacs;
radioligand binding assays in vitro in which fipronil did not
inhibit binding of 3H-muscimol or 3H-flunitrazepam at, respectively,
the GABA recognition or benzodiazepine sites on the GABA receptor in
rat tissue, but did competitively inhibit binding at sites in the
chloride channel of the GABA receptor of ligands such as
35S- tert-butylbicyclophosphorothionate (TBPS) and
3H-1-[(4-ethyl)phenyl]-4- n-propyl-2,6,7-trioxacicyclo[2.2.2]octane
(EBOB). The latter is known to block the g-amino-butyric acid-gated
chloride channel, and the former binds to the picrotoxin binding site
in the channel. Binding of TBPS was inhibited by fipronil in rat brain
but not in the housefly head, but binding of EBOB was inhibited in
both rat and mouse brain and housefly head. The greater affinity of
fipronil for the EBOB site in insects than for that in mammals has
been postulated to account, at least in part, for the pesticide's
selective toxicity to insects.
In other studies of the role of the GABA receptor in the toxicity
of fipronil, a cloned gene that codes for a GABA receptor subunit in
Drosophila melanogaster was expressed in frog oocytes as a membrane
protein. These receptor-containing oocytes produced a large electrical
current carried by chloride ions in response to treatment with GABA,
which was not seen in control oocytes. The electric response was
reversed in a dose-dependent manner by treatment with fipronil and was
blocked by picrotoxin, TBPS, EBOB, and dieldrin.
Differences in the GABA receptor genome in susceptible and
resistant insects may help to explain resistance to the pesticide
dieldrin (a cyclodiene), which acts at the GABA receptor in insects
but also at the TBPS binding site, at which fipronil is inactive. A
dieldrin-resistant housefly was shown to have a low-affinity EBOB
binding site (see Bushey, 1993; Cole et al., 1993; Gant et al., 1994
for other references).
Although it was reported that fipronil did not affect other
enzyme or receptor systems, such as the acetylcholine receptor,
acetylcholinesterase, the octopamine receptor, and general oxidative
uncouplers, no supporting data were presented (Bushey, 1993).
(g) Studies on metabolites
(i) Acute toxicity
The results of studies on the toxicity of single doses of
fipronil metabolites are summarized in Table 4. The metabolic routes
by which they are formed are shown in Figures 2 and 3. In a study with
M&B 45950, clinical signs of neurotoxicity, including excessive
jumping, increased or reduced motor activity, clonic convulsions,
tremors, curling up, and subdued behaviour, were noted in all animals
at doses > 50 mg/kg bw, and deaths occurred during the first week
of the study, starting from 65 mg/kg bw in males and 90 mg/kg bw in
females (Dange, 1994a).
In a study with M&B 46136, clinical signs observed in animals of
each sex at 64 mg/kg bw within 2 h included hunched posture and
abnormal gait. Most signs such as hunched posture, abnormal gait,
lethargy, pallor of the extremities, diarrhoea, ataxia, clonic
convulsions, increased salivation, and decreased respiratory rate did
not appear until day 2 in animals at doses > 100 mg/kg bw. Deaths
occurred at doses > 100 mg/kg bw in animals of each sex on days 2
and 3 (Gardner, 1988c).
In a study with fipronil-desulfinyl, clinical signs including
reduced motor activity, dyspnoea, bradypnoea, and hyperreaction to
noise were observed in all animals at doses > 10 mg/kg bw. Clonic
and/or tonic convulsions were observed before death in one female at
20 mg/kg bw and in all males at 30 mg/kg bw. Deaths occurred at doses
> 20 mg/kg bw between days 2 and 4 after treatment (Dange, 1993b).
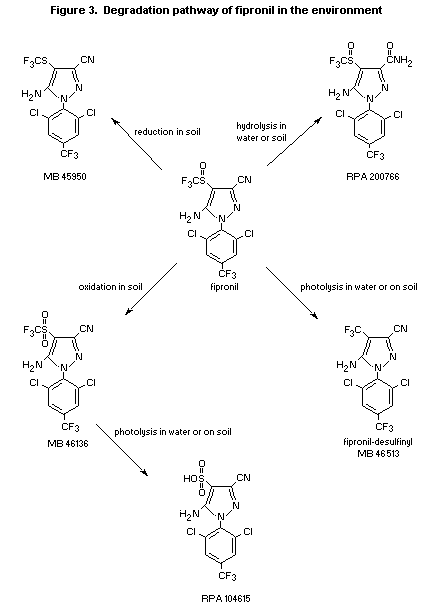 Table 4. Toxicity of single doses of metabolites of fipronil in rats
Metabolite Purity Strain Sex Route LD50 Reference
(%) (mg/kg bw)
M&B 45950 98.9 Sprague-Dawley M Orala 69 Dange (1994a)
F 100
M&B 46136 98 Crl:CD(SD)BR M Orala 184 Gardner (1988c)
F 257
98 Crl:CD(SD)BR M,F Dermalb > 2000 Gardner (1988d)
RPA 200766 > 98 Sprague-Dawley M,F Orala > 2000 Dange (1993a)
Fipronil-desulfinyl 98.6 Sprague-Dawley M Orala 18 Dange (1993b)
F 15
98.6 Sprague-Dawley M,F Dermalc > 2000 Dange (1993c)
RPA 104615 94.7 Sprague-Dawley M,F Orala > 2000 Dange (1993d)
M&B 45897 > 99 Crl:CD(SD)BR M,F Orald > 2000 Haynes (1988a)
> 99 Crl:CD(SD)BR M,F Dermale > 2000 Haynes (1988b)
a In corn oil
b Applied as an 88.2% w/v concentration in distilled water
c Moistened with saline before application
d In sesame oil
e Moistened with distilled water before application
In a study with M&B 45897, transient signs of hypoactivity or
perinasal staining were noted in all or most animals given single oral
or dermal doses, respectively, but no deaths were observed. No deaths
or clinical signs of toxicity were observed in studies of oral
administration of RPA 200766, or RPA 104615 or studies of dermal
application of M&B 46136 or fipronil-desulfinyl (Gardner, 1988d;
Haynes, 1988a,b; Dange, 1993a,c,d).
(ii) Fipronil-desulfinyl
In the presence of sunlight, two photometabolites,
fipronil-desulfinyl and RPA 104615, can form from fipronil. The
available information indicates that fairly high-energy ultraviolet
irradiation is needed for the conversion process (personal
communication from Rhone-Poulenc). Fipronil-desulfinyl could
potentially form in the environment or on surfaces treated during use
against malarial vectors and thus appears to be of toxicological
concern.
Absorption, distribution, metabolism, and excretion: Groups of
24 male Charles River Crl:CD BR rats received a suspension of
14C-fipronil-desulfinyl in 1% aqueous carboxymethylcellulose as a
dermal application at doses of 0.08, 0.88, or 7.2 mg/rat (6.46, 70.5,
or 574 µg/cm2) on an area of about 12.5 cm2 shaved skin, which was
protected with a nonocclusive bandage. Blood, urine, and faeces were
collected to assay radiolabel. Four rats at each dose were killed to
assess dermal absorption after 0.5, 1, 2, 4, 10, and 24 h of exposure.
Before sacrifice, the application site was washed with water and the
washings saved to assay radiolabel. Control rats received only vehicle
and were killed 24 h after treatment.
The mean total recovery of radiolabel was 93-103% of the dose,
most (90-102%) being present in the skin wash. Absorption, measured as
radiolabel in excreta, cage wipe and wash, blood, carcass, and wiped
skin application site, in rats that received 0.08 mg was 0.74% of the
dose at 0.5 h, 2.3% at 10 h, and 6.6% by 24 h; at 0.88 mg/rat, 0.41%
at 0.5 h, 0.95% at 10 h, and 1.4% at 24 h; and at 7.2 mg/rat, 0.27% at
0.5 h, 0.18% at 10 h, and 0.39% at 24 h (Cheng, 1996).
Three groups of five male and five female Sprague Dawley (CD)
rats received either a single oral dose of 14C-fipronil-desulfinyl at
1 or 10 mg/kg bw or 14 daily oral doses of unlabelled
fipronil-desulfinyl, followed by a single oral dose of labelled
compound at 1 mg/kg bw. Urine and faeces were collected at 24-h
intervals until sacrifice at on eweek. At this time, liver, kidney,
brain, fat, and gonads were collected for analysis of residual
radiolabel.
More radiolabel was eliminated in the faeces than the urine. In
single animals at the low dose, elimination of radiolabel in urine
amounted to 6.1% of the dose in males and 4.4% in females, and
elimination in faeces was 60% of the dose in males and 46% in females.
In animals at the high dose, 8.8% was eliminated in the urine of males
and 11% in that of females, and 70% was eliminated in the faeces of
males and 56% in those of females. In animals given the 14 doses,
elimination of radiolabel in urine amounted to 10% in males and 11% in
females and that in faeces to 61% in males and 53% in females.
Residual radiolabel in tissues one week after treatment accounted for
27% in males and 41% in females at the single low dose, for 20% in
males and 30% in females at the high dose, and for 23 and 32% of the
dose in animals given repeated doses. The highest concentrations of
radiolabelled residues were present in fat and residual carcass. The
ratios of radiolabel in fat:plasma reached 12.8 in males and 25.2 in
females at the single low dose and 8.2 in males and 23.2 in females at
the single high dose. The levels of residual radiolabel at this time
were accounted for largely by residues in fat and carcass (also
probably due to fat).
In pharmacokinetic analyses of radiolabel in blood, the area
under the concentration-time curve increased in proportion to dose in
females but not in males. In males, the ratio of the area with the
high dose to that with low dose was 15 instead of the expected 10. The
mean elimination half-life of radiolabel from 14C-fipronil-desulfinyl
was 195 h at the high dose and 183 h at the low dose, with slightly
lower values for males. The increased fat:plasma ratios and the
prolonged elimination half-lives of the radiolabel suggest potential
bioaccumulation of this compound and/or its metabolites.
Up to 17 radiolabelled components were found in urine and up to
13 in faeces. Only untransformed parent compound (fipronil-desulfinyl)
was identified in extracts of liver, fat, skin, and residual carcass.
Biotransformation of fipronil-desulfinyl in rats (Figure 4) largely
involves conjugation and/or biotransformation of the functional groups
attached to the pyrazolyl ring. The main compounds identified included
untransformed parent (0.01-0.9% of the dose in urine and 29-44% in
faeces; UMET/17 and FMET/12 in the Figure), the
4-cyano-5- (N-)cysteine conjugate of fipronil-desulfinyl (up to 1.6%
of the dose in urine and 3.2-14% in faeces; UMET/15 and FMET/10), the
5- (N-)cysteine conjugate (up to 0.69% of the dose in urine and
1.5-3.3% in faeces; UMET/14 and FMET/9), the pyrazole-4-carboxylic
derivative (1.3-5.5% of the dose in urine and 1.7-5.2% in faeces; M&B
46400), the amide derivative (up to 0.62% of the dose in urine; RPA
105048), and the sulfate conjugate (up to 2.4% of the dose in urine;
UMET/3) (Totis,1996).
Short-term toxicity of firponil-deulfinyl
Mice
In a preliminary study, fipronil-desulfinyl (purity, 97.5%) was
administered in the diet for 28 days to groups of 10 male and 10
female OF-1 mice at doses of 0, 0.5, 3, 30, or 60 ppm, equal to 0,
0.08, 0.49, 5.0 mg/kg bw per day, and undetermined, respectively, for
males and 0, 0.1, 0.61, 5.6, and 12 mg/kg bw per day for females. All
of the males and six females at 60 ppm and seven males and two females
Table 4. Toxicity of single doses of metabolites of fipronil in rats
Metabolite Purity Strain Sex Route LD50 Reference
(%) (mg/kg bw)
M&B 45950 98.9 Sprague-Dawley M Orala 69 Dange (1994a)
F 100
M&B 46136 98 Crl:CD(SD)BR M Orala 184 Gardner (1988c)
F 257
98 Crl:CD(SD)BR M,F Dermalb > 2000 Gardner (1988d)
RPA 200766 > 98 Sprague-Dawley M,F Orala > 2000 Dange (1993a)
Fipronil-desulfinyl 98.6 Sprague-Dawley M Orala 18 Dange (1993b)
F 15
98.6 Sprague-Dawley M,F Dermalc > 2000 Dange (1993c)
RPA 104615 94.7 Sprague-Dawley M,F Orala > 2000 Dange (1993d)
M&B 45897 > 99 Crl:CD(SD)BR M,F Orald > 2000 Haynes (1988a)
> 99 Crl:CD(SD)BR M,F Dermale > 2000 Haynes (1988b)
a In corn oil
b Applied as an 88.2% w/v concentration in distilled water
c Moistened with saline before application
d In sesame oil
e Moistened with distilled water before application
In a study with M&B 45897, transient signs of hypoactivity or
perinasal staining were noted in all or most animals given single oral
or dermal doses, respectively, but no deaths were observed. No deaths
or clinical signs of toxicity were observed in studies of oral
administration of RPA 200766, or RPA 104615 or studies of dermal
application of M&B 46136 or fipronil-desulfinyl (Gardner, 1988d;
Haynes, 1988a,b; Dange, 1993a,c,d).
(ii) Fipronil-desulfinyl
In the presence of sunlight, two photometabolites,
fipronil-desulfinyl and RPA 104615, can form from fipronil. The
available information indicates that fairly high-energy ultraviolet
irradiation is needed for the conversion process (personal
communication from Rhone-Poulenc). Fipronil-desulfinyl could
potentially form in the environment or on surfaces treated during use
against malarial vectors and thus appears to be of toxicological
concern.
Absorption, distribution, metabolism, and excretion: Groups of
24 male Charles River Crl:CD BR rats received a suspension of
14C-fipronil-desulfinyl in 1% aqueous carboxymethylcellulose as a
dermal application at doses of 0.08, 0.88, or 7.2 mg/rat (6.46, 70.5,
or 574 µg/cm2) on an area of about 12.5 cm2 shaved skin, which was
protected with a nonocclusive bandage. Blood, urine, and faeces were
collected to assay radiolabel. Four rats at each dose were killed to
assess dermal absorption after 0.5, 1, 2, 4, 10, and 24 h of exposure.
Before sacrifice, the application site was washed with water and the
washings saved to assay radiolabel. Control rats received only vehicle
and were killed 24 h after treatment.
The mean total recovery of radiolabel was 93-103% of the dose,
most (90-102%) being present in the skin wash. Absorption, measured as
radiolabel in excreta, cage wipe and wash, blood, carcass, and wiped
skin application site, in rats that received 0.08 mg was 0.74% of the
dose at 0.5 h, 2.3% at 10 h, and 6.6% by 24 h; at 0.88 mg/rat, 0.41%
at 0.5 h, 0.95% at 10 h, and 1.4% at 24 h; and at 7.2 mg/rat, 0.27% at
0.5 h, 0.18% at 10 h, and 0.39% at 24 h (Cheng, 1996).
Three groups of five male and five female Sprague Dawley (CD)
rats received either a single oral dose of 14C-fipronil-desulfinyl at
1 or 10 mg/kg bw or 14 daily oral doses of unlabelled
fipronil-desulfinyl, followed by a single oral dose of labelled
compound at 1 mg/kg bw. Urine and faeces were collected at 24-h
intervals until sacrifice at on eweek. At this time, liver, kidney,
brain, fat, and gonads were collected for analysis of residual
radiolabel.
More radiolabel was eliminated in the faeces than the urine. In
single animals at the low dose, elimination of radiolabel in urine
amounted to 6.1% of the dose in males and 4.4% in females, and
elimination in faeces was 60% of the dose in males and 46% in females.
In animals at the high dose, 8.8% was eliminated in the urine of males
and 11% in that of females, and 70% was eliminated in the faeces of
males and 56% in those of females. In animals given the 14 doses,
elimination of radiolabel in urine amounted to 10% in males and 11% in
females and that in faeces to 61% in males and 53% in females.
Residual radiolabel in tissues one week after treatment accounted for
27% in males and 41% in females at the single low dose, for 20% in
males and 30% in females at the high dose, and for 23 and 32% of the
dose in animals given repeated doses. The highest concentrations of
radiolabelled residues were present in fat and residual carcass. The
ratios of radiolabel in fat:plasma reached 12.8 in males and 25.2 in
females at the single low dose and 8.2 in males and 23.2 in females at
the single high dose. The levels of residual radiolabel at this time
were accounted for largely by residues in fat and carcass (also
probably due to fat).
In pharmacokinetic analyses of radiolabel in blood, the area
under the concentration-time curve increased in proportion to dose in
females but not in males. In males, the ratio of the area with the
high dose to that with low dose was 15 instead of the expected 10. The
mean elimination half-life of radiolabel from 14C-fipronil-desulfinyl
was 195 h at the high dose and 183 h at the low dose, with slightly
lower values for males. The increased fat:plasma ratios and the
prolonged elimination half-lives of the radiolabel suggest potential
bioaccumulation of this compound and/or its metabolites.
Up to 17 radiolabelled components were found in urine and up to
13 in faeces. Only untransformed parent compound (fipronil-desulfinyl)
was identified in extracts of liver, fat, skin, and residual carcass.
Biotransformation of fipronil-desulfinyl in rats (Figure 4) largely
involves conjugation and/or biotransformation of the functional groups
attached to the pyrazolyl ring. The main compounds identified included
untransformed parent (0.01-0.9% of the dose in urine and 29-44% in
faeces; UMET/17 and FMET/12 in the Figure), the
4-cyano-5- (N-)cysteine conjugate of fipronil-desulfinyl (up to 1.6%
of the dose in urine and 3.2-14% in faeces; UMET/15 and FMET/10), the
5- (N-)cysteine conjugate (up to 0.69% of the dose in urine and
1.5-3.3% in faeces; UMET/14 and FMET/9), the pyrazole-4-carboxylic
derivative (1.3-5.5% of the dose in urine and 1.7-5.2% in faeces; M&B
46400), the amide derivative (up to 0.62% of the dose in urine; RPA
105048), and the sulfate conjugate (up to 2.4% of the dose in urine;
UMET/3) (Totis,1996).
Short-term toxicity of firponil-deulfinyl
Mice
In a preliminary study, fipronil-desulfinyl (purity, 97.5%) was
administered in the diet for 28 days to groups of 10 male and 10
female OF-1 mice at doses of 0, 0.5, 3, 30, or 60 ppm, equal to 0,
0.08, 0.49, 5.0 mg/kg bw per day, and undetermined, respectively, for
males and 0, 0.1, 0.61, 5.6, and 12 mg/kg bw per day for females. All
of the males and six females at 60 ppm and seven males and two females
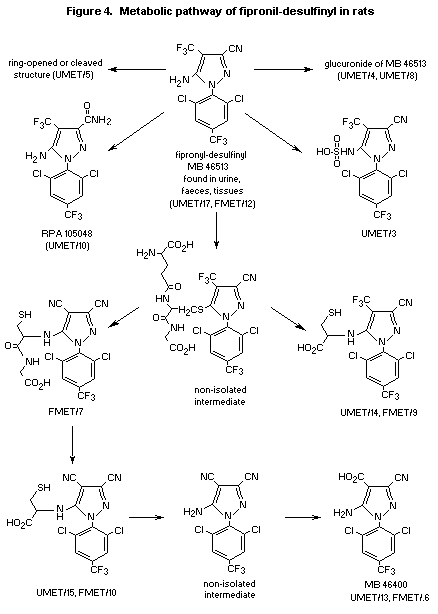 at 30 ppm died during the study. The intake of the test compound was
thus not determined for males at 60 ppm.
Males and females at 30 and 60 ppm had statistically significant
decreases in mean body weight and body-weight gain, and mean food
consumption was significantly decreased. The mean values of chemical
parameters did not differ significantly between treated and control
mice. Changes in the weights of the liver, kidney, and thymus in
animals at 30 and 60 ppm were attributed to the decreased terminal
body weights. The only treatment-related change observed
histopathologically was an increased incidence of centrilobular
hypertrophy in mice at 30 and 60 ppm.
Clinical signs of neurotoxicity -- increased motor activity,
excessive jumping, irritability to touch, compulsive biting, and
convulsions -- were observed in males and females at 30 and 60 ppm.
Two females at 3 ppm showed increased motor activity on one occasion,
accompanied by excessive vocalization in one female. None of these
effects occurred in controls. Increased motor activity has been
observed previously as a clinical sign of the toxicity of
fipronil-desulfinyl and of the parent compound fipronil in several
species. Because of the low incidence of this effect and the lack of
other effects at 3 ppm in this study, however, it cannot be concluded
that this effect is of toxicological concern. The NOAEL was 3 ppm,
equal to 0.49 mg/kg bw per day (Dange, 1994b).
Fipronil-desulfinyl (purity, 96%) was administered to groups of
10 male and 10 female OF1 mice in the diet for 90 days at doses of 0,
0.5, 2, or 10 ppm, equal to 0, 0.08, 0.32, or 1.7 mg/kg bw per day for
males and 0, 0.11, 0.43, or 2.2 mg/kg bw per day for females. Standard
evaluations of toxicity ante and post mortem were included.
Histopathological examinations were conducted on all tissues from
controls and animals at the two higher doses and on the liver, lung,
and kidney and tissues in which there were significant macroscopic
findings from mice at the low dose.
Nine males and one females treated at 10 ppm were found dead
during the study. Significant clinical signs included excessive
jumping in two males at 10 ppm and irritability to touch,
aggressivity, and/or increased motor activity in one male at 10 ppm
and two at 2 ppm on two or more occasions. At 0.5 ppm, aggressivity
was reported in one male on one occasion and in one female on three
occasions. None of these effects was reported in control animals.
There were no significant changes in body weight, food consumption, or
haematological or clinical chemical parameters. Gross pathological
examination of dead animals indicated liver enlargement in three males
and small thymuses in four males at 10 ppm. Microscopy revealed
centrilobular hypertrophy of the liver in six males at this dose. The
only female at 10 ppm that died had marked autolysis, which obscured
the histological details. At scheduled sacrifice, gross and
microscopic examinations revealed no treatment-related findings.
The incidence of aggressivity or irritability to touch was low at
2 ppm and there was no dose-response relationship; however, one male
at this dose showed irritability to touch and agressivity on days
70-72, and the other male at this dose with behavioural changes was
irritable to touch, had increased motor activity on day 29, and showed
aggressivity on day 33. Therefore, two animals of the same sex showed
multiple signs on two or three occasions of effects that have been
recognized as clinical signs of toxicity in other studies with
fipronil-desulfinyl and fipronil. In addition, signs of neurotoxicity
were seen in males at the next highest dose and no signs of toxicity
in concurrent controls. Therefore, the behavioural findings in males
at 2 ppm should not be discounted, despite the lack of other
treatment-related findings at this dose. It is easier to discount the
behavioural effects at 0.5 ppm because of their low incidence and the
lack of a dose-response relationship. Therefore, the NOAEL was
0.5 ppm, equal to 0.08 mg/kg bw per day (Bigot, 1996).
Rats
In an exploratory study, fipronil-desulfinyl (purity, 98.6%) was
administered by gavage to five male and five female Sprague-Dawley
rats at doses of 0, 0.3, 1, 3, or 10 mg/kg bw per day for 14 days. All
of the animals at 10 mg/kg bw per day and one female at 3 mg/kg bw per
day either died or were killed in a moribund condition on days 5-8 of
the study. Clinical signs of toxicity, such as piloerection,
chromodacryorrhoea, prostration, excessive reaction to noise,
convulsions, curling up on handling, hunched posture, nasal discharge,
and few faeces, were observed in animals of each sex at 10 mg/kg bw
per day and in females at 3 mg/kg bw per day. Body-weight gain and
food consumption were markedly decreased in animals at 10 mg/kg bw per
day; males and females at 3 mg/kg bw per day showed decreased
body-weight gain over the course of the study, and food consumption
was decreased at the two times evaluated.
Leukocyte counts and total bilirubin were decreased and total
protein was increased in females at 3 mg/kg bw per day; leukocyte
counts were also decreased in females at 1 mg/kg bw per day. Gross
pathological examination showed pale livers in females at 1, 3, or 10
mg/kg bw per day, and spots in the glandular stomach were seen in a
few animals at 3 and 10 mg/kg bw per day that died during the study.
Histological examination showed an increased number of atrophic
follicles in the thyroids of males and females treated at doses > 3
mg/kg bw per day. The NOAEL was 0.3 mg/kg bw per day (Dange, 1994c).
In a preliminary study, fipronil-desulfinyl (purity, 97.5%) was
administered to groups of 10 male and 10 female Sprague-Dawley rats in
the diet at doses of 0, 0.5, 3, 30, or 100 ppm (equal to 0, 0.04,
0.23, 2.2, or 3.7 mg/kg bw per day for males and 0, 0.04, 0.24, 2.3,
or 3.8 mg/kg bw per day for females) for 28 days. Standard parameters
and T3, T4, and TSH were measured before treatment and on day 7 in
non-fasted animals and on day 23 or 24 in fasted animals.
There were no deaths, clinical signs of toxicity, or effects on
body weight or food consumption at 0.5 and 3 ppm. One male at 30 ppm
was found dead on day 6, and all animals at 100 ppm died within the
first two weeks of the study. Clinical signs at 30 and 100 ppm
included piloerection, curling up on handling, and convulsions (in one
female at 100 ppm). Food consumption and body weights were decreased
at the two highest doses and markedly so at 100 ppm. The only change
observed in urinary, standard haematological, and clinical chemical
parameters were a decrease of about 30% in total bilirubin in animals
of each sex at 30 ppm. Statistically significant decreases (relative
to concurrent controls) were found in the levels of T4 (by 33% in
males at 300 ppm, by 63% in males and 50% in females at 100 ppm on day
7; and by 49% in males and 61% in females at 30 ppm on day 23 [no
animals at the high dose were still alive on that day]) and in T3 (by
46% in females at 100 ppm on day 7 and by 40% in males at 30 ppm on
day 23) were observed, but their toxicological significance was not
clear: the findings at 100 ppm may have been related to the extreme
toxicity of the chemical, and individual values in animals at 30 ppm
were reported to be within the normal range for these parameters. TSH
levels were not affected. At necropsy, the thymus weights were
decreased in females at 30 ppm, but no other findings were clearly
related to treatment. The NOAEL was 3 ppm, equal to 0.23 mg/kg bw
(Dange, 1995a).
Fipronil-desulfinyl (purity, at least 97.5%) was administered in
the diet for 90 days to groups of 10 male and 10 female Sprague-Dawley
rats at doses of 0, 0.5, 3, 10, or 30 ppm (equal to 0, 0.029, 0.18,
0.59, or 1.8 mg/kg bw per day for males and 0, 0.035, 0.21, 0.71, or
2.1 mg/kg bw per day for females). In addition to standard ante- and
post-mortem evaluations of toxicity, T3, T4, and TSH were assayed in
non-fasted animals in weeks 2 and 10. During treatment, one male and
three females at 30 ppm died with clinical signs of distress. Signs of
neurotoxicity (aggressivity, irritability to touch, increased motor
activity, and curling up on handling) were seen in animals at 10 and
30 ppm, and excessive vocalization and increased motor activity were
seen in some animals. None of these effects was observed in controls,
in males at 0.5 ppm, or in females at doses > 3 ppm. One male at 3
ppm showed aggressivity, irritability to touch on several occasions,
and excessive vocalization. These signs were seen mainly in weeks 3-5.
Mean body weights were significantly decreased in males and females at
30 ppm and in males at 10 ppm several times during the study. The
overall mean body-weight gain of males was decreased by 15% in those
at 10 ppm and 13% at 30 ppm. Mean weekly food consumption and food
conversion efficiency of males and females at 30 ppm were lower than
those of controls only during the first two weeks of the study. No
treatment-related changes in haematological, urinary or
ophthalmological parameters were seen. Decreases in total bilirubin
(-43%), total cholesterol (-25%), and triglycerides (-24%) in females
at 30 ppm were considered to be related to treatment but not
toxicologically significant since the individual values were within
the normal range for this age and strain of rat. At 30 ppm,
treatment-related decreases in T4 levels were seen in males (-48%,
p < 0.01, at week 2 and -25% at week 10) and females (-29% at week
10), and T3 levels were decreased (-29%, p < 0.05) in males at week
10. The altered hormone levels were reported to be within the normal
range of values for these parameters in this strain of rat. As no
changes were found in the thyroid gland on macroscopic or microscopic
examination, the toxicological significance of the alterations in
hormone levels is questionable. TSH was not affected. There were no
treatment-related macroscopic changes at necropsy, no changes in organ
weights, and no histopathological findings. The NOAEL was 0.5 ppm,
equal to 0.029 mg/kg bw per day, on the basis of clinical signs of
toxicity in one male (Dange, 1994d).
Dogs
In a preliminary study, fipronil-desulfinyl (purity, 97.5%), was
administered in the diet of groups of two male and two female beagle
dogs at doses of 0, 27, 80, or 270 ppm for 28 days. The group mean
doses achieved were reported to be equal to 1 mg/kg bw per day for
males and females at 27 ppm over the course of the study, 1.9 mg/kg bw
per day for males and 1.7 mg/kg bw per day for females at 80 ppm
during week 1, and 2.3 mg/kg bw per day for animals of each sex at 270
ppm during the same period. During the second week of the study,
intake decreased considerably because of toxicity.
Because of poor health, animals at the two higher doses were
killed early, on days 10 and 15 for those at 80 ppm and day 10 for
those at 270 ppm. The signs of toxicity observed at 80 ppm included
reduced motor activity, staggering, irritability, and increased
salivation. Animals at 80 and 270 ppm had no or few faeces,
emaciation, decreased food consumption, and weight loss. All animals
receiving 27 ppm survived, but one male had a clonic convulsion at the
end of the study. The body weights and food consumption of animals in
this group were comparable to those of controls. No remarkable effects
were seen in ophthalmological, haematological, clinical chemical, or
urinary evaluations. Only animals at 27 and 80 ppm were necropsied.
Reduced thymic weight or small thymuses and pale livers were noted in
both groups, and the livers of those at 80 ppm had multifocal whitish
areas or mottling. Although there were no microscopic findings at 27
ppm, marked thymic atrophy and hepatic changes indicative of toxicity
were seen at 80 ppm, including diffuse sinusoidal leukocytosis,
centrilobular hepatocytic enlargement, mild multifocal hepatocytic
hydropic degeneration, and chronic hepatitis with periportal fibrosis.
No NOAEL was identified (Dange, 1995b).
Fipronil-desulfinyl (purity, 96%) was administered to groups of
five beagle dogs of each sex in the diet for 90 days at doses of 0,
3.5, 9.5, or 35 ppm (equivalent to 0, 0.1, 0.27, or 0.95 mg/kg bw per
day for males and 0, 0.1, 0.29, or 1.05 mg/kg bw per day for females).
General health, clinical signs, food consumption, body weights,
haematology and plasma chemistry, and ophthalmologic and urinary
parameters were evaluated. At necropsy, organ weights and macroscopic
changes were monitored; all tissues from controls and animals at the
high dose and kidney, liver, lung, heart, and gall-bladder from those
at the intermediate and low doses were examined histologically.
One female at 35 ppm was killed on day 28 after signs of
increased salivation, prostration, writhing, tremors, absence of
rotular reflex, noisy breathing, and dyspnoea. These clinical signs
were suggested to be due to coronary arteritis and myocardial necrosis
on the basis of microscopic findings; however, they may have indicated
neurotoxicity, as another female at this dose also exhibited excessive
barking and aggressivity on one occasion and irritability, tremors,
and increased salivation on another. There were no significant changes
in body weight, food consumption, or haematological, clinical
chemical, urinary, or ophthalmological parameters. At scheduled
necropsy, no treatment-related changes were observed, and the
microscopic examination revealed no treatment-related findings. The
NOAEL was 9.5 ppm, equal to 0.29 mg/kg bw per day (Dange, 1996).
Reproductive toxicity: Groups of adult female Sprague-Dawley
Crl:CD(SD)BR rats received 0, 0.5, 1, or 2.5 mg/kg bw per day of
fipronil-desulfinyl (purity, 99.2%) suspended in a 0.5% aqueous
solution of methylcellulose by gavage on days 6-15 of gestation. The
study was terminated on gestation day 20. The animals were evaluated
for clinical signs, food consumption, body-weight changes, and
macroscopic changes post mortem. The litter and fetal parameters
evaluated included pre- and postimplantation losses, litter size,
litter and mean fetal weights, sex ratios, and malformations or
skeletal or visceral anomalies. Hair loss on the paws, limbs, flanks,
abdomen, and/or thorax were seen in dams at the high dose, and these
animals had lower body-weight gain on gestation days 6-9 (28% of
control), 9-12 (27%), 6-16 (58%), and 0-20 corrected for gravid
uterine weight (78%). These animals also consumed less food during
treatment, although an increase was seen after treatment, indicating a
rebound effect. Dams at 1 mg/kg bw per day showed a significant
decrease in body-weight gain (80% of control) on days 9-12 of
gestation. No other effects on body weight, body-weight gain at other
intervals, food consumption, or other parameters were seen at the
intermediate dose.
Developmental toxicity in pups at the high dose was reflected in
a slight increase in the fetal and litter incidence of incomplete or
reduced ossification of several bones, including the hyoid body, fifth
and sixth sternebrae, first thoracic body, pubic bone, and one or two
metatarsi. A slight but significant reduction in fetal body weight
(98% of control weight) at the high dose correlated with the slight
delays in ossification. Although the dams showed reduced body-weight
gain, the change in dams at the intermediate dose occurred over a
small interval and was transient. In the absence of other effects at
this dose, the reduced body-weight gain does not appear to be
toxicologically significant. The NOAEL for maternal toxicity was thus
1 mg/kg bw per day; that for for developmental toxicity was 1 mg/kg bw
per day on the basis of a slight increase in the fetal and litter
incidence of delayed ossification of several bones (Foulon, 1997).
Neurotoxicity: Fipronil-desulfinyl (purity, 99.5%) was
administered by gavage as a single dose in corn oil to groups of 12
male and 12 female Sprague-Dawley Crl:CD(SD)BR rats at doses of 0,
0.5, 2, or 12 mg/kg bw. A functional observation battery of tests and
motor activity testing were conducted at 6 h and seven and 14 days
after treatment; 6 h was chosen as the time of 'peak effect' on the
basis of the results of a range-finding study which included
evaluations at 2, 4, 6, and 24 h. Clinical signs, food consumption,
and body weights were monitored. At study termination on day 15, all
animals were perfused in situ. Brains were weighed and measured, and
tissues from the brain, spinal cord, and peripheral nerves were
collected and processed for microscopic examination. Only tissues from
five male and five female controls and animals at the high dose were
examined histopathologically. Since possibly treatment-related axonal
degeneration was seen in lumbar dorsal root fibres and/or the sciatic
nerve of males at the high dose, lumbar dorsal root fibres and the
sciatic nerve in the notch and mid-thigh from the remaining male
controls and rats at the high dose were prepared for microscopic
evaluation. Slides of all tissues from these areas of concern were
then read or re-read in a 'blinded' manner.
Body-weight gain and food consumption were decreased in males and
females at the high dose only during week 1. The behavioural changes
attributed to treatment were significant decreases in hindlimb foot
splay, rectal temperature, and locomotor activity (assessed
quantitatively in the Coulbourn system) in animals of each sex at 12
mg/kg bw 6 h after treatment. Treatment probably affected the righting
reflex in males at the high dose, as it was significantly slowed on
day 14, with a trend at other times, and possibly affected grip
strength in animals of each sex at the high dose at various times.
Changes in other behavioural and motor activity parameters could not
clearly be related to treatment. The groups did not differ with regard
to brain weights. The incidence and number of tissue sites showing
axonal degeneration were increased slightly over those in controls and
in males at the high dose. Since the effect was of minor severity in
both treated and control animals and since the difference between
these animals was not significantly different by Fisher's exact test,
the changes were considered not to be of biological importance. There
were no significant histopathological changes in female rats. The
NOAEL was 2 mg/kg bw (Hughes, 1996).
(iii) M&B 46136: Dermal and ocular irritation
Three male New Zealand white rabbits were given a single 0.5-g
dose of M&B 46136 (purity, 98%) moistened with distilled water as a
topical application for 4 h. The treated areas were observed for
erythema and oedema for four days after treatment. No signs of dermal
irritation were seen under the conditions of the study (Liggett,
1988c).
A dose of 0.1 ml (60 mg) of M&B 46136 (purity, 98%) was instilled
into the lower eyelid of one eye of three male New Zealand white
rabbits. The other eye served as an untreated control. The eyes were
examined for signs of irritation and scored 1 h and one, two, three,
four, and seven days after instillation. One animal had chemosis after
one day. Redness and any minor or residual irritation present had
cleared by day 3 or 4 in all animals. M&B 46136 was thus a slight
ocular irritant under the conditions of the study (Liggett, 1988d).
(iv) RPA 200766: Short-term toxicity
RPA 200766 (purity, 96.2%) was administered in the diet for 28
days to 10 male and 10 female Sprague-Dawley rats at doses of 0, 50,
500, 5000, or 15 000 ppm (equal to 0, 3.8, 38, 390, or 1100 mg/kg bw
per day for males and 0, 4.4, 44, 390, or 1100 mg/kg bw per day for
females). The animals were examined for general health, clinical signs
of toxicity, body weight, food consumption, urinary and
ophthalmological changes, haematological and clinical chemical
parameters, and macroscopic changes at necropsy. Histopathological
examinations were not conducted on animals at 15 000 ppm, because the
dose was found to be too high, inducing excessive body-weight loss.
Microscopic examinations were carried out on all animals that died,
all those at 5000 ppm, and all controls, on the liver, lungs, and
kidneys of animals in all groups except those at 15 000 ppm, and on
the adrenals and thyroid (the target organs), as considered necessary
to establish a no-effect level.
One female at 15 000 died during the study due to an error in
blood collection. No treatment-related deaths were seen in other
groups, and no clinical or ophthalmological alterations were reported.
The body weights of males and females at 5000 and 15 000 ppm were
significantly decreased on days 8-28. The mean body-weight gain over
the course of the study was decreased by 27% in males at 5000 ppm, 61%
in males at 15 000 ppm, 46% in females at 5000 ppm, and 77% in females
at 15 000 ppm. Mean food consumption over the course of the study was
decreased by 11% in males at 5000 ppm, 25% in males at 15 000 ppm, 22%
in females at 5000 ppm, and 33% in females at 15 000 ppm.
Mean haemoglobin concentrations were decreased in males and
females at doses > 500 ppm and mean haematocrit values were
decreased in animals at doses > 5000 ppm, significantly except in
females at 15 000 ppm. Mean corpuscular haemoglobin values were
decreased in males and females at 15 000 ppm and in males at 5000 ppm.
The mean cholesterol levels were significantly increased in animals at
doses > 500 ppm, mean triglyceride values were increased in animals
at 5000 and 15 000 ppm, urea nitrogen was increased in females at 5000
and 15 000 ppm, and creatinine values were increased in males at doses
> 500 ppm. The results of urinalysis showed no changes.
Dose-related increases in absolute and relative liver weights
were seen in males and females at doses > 500 ppm, and the
liver:brain weights were also increased in these groups. Dark livers
were observed in males at > 500 ppm and in females at 5000 and 15
000 ppm. Significantly increased relative adrenal weights and
adrenal:brain weights were seen in all treated males. The group mean
thyroid weights were increased in males at doses > 50 ppm; however,
the increases were not found consistently, and the individual values
were reported to be generally within the expected range for animals of
this age and strain. As there were also no microscopic changes in the
thyroid, these findings are of questionable toxicological
significance, even though the target organ of the parent compound,
fipronil, is the thyroid.
Microscopic examination showed slight-to-moderate, centrilobular
or diffuse hepatocellular hypertrophy in the livers and
slight-to-extramedullary haematopoiesis in the adrenals of males and
females at 5000 ppm. A dose-related increase in the incidence of fine
or coarse vacuolation of the zona fasciculata of the adrenal gland was
observed in males at doses > 50 ppm, with incidences of 0/10 in
controls, 2/10 at the low dose, 5/10 at the intermediate dose, and
10/10 at the high dose. The severity was slight at 50 and 500 ppm and
mild to marked at 5000 ppm. A similar change was seen in seven females
at 5000 ppm, with slight-to-mild severity; the incidence in the female
controls was 0. Increases in the weights of the adrenals and thyroids
and vacuolation of the adrenal zona fasciculata in males at 50 ppm
were considered to be marginal and of questionable toxicological
significance. The NOAEL was 50 ppm, equal to 3.8 mg/kg bw per day, on
the basis of decreased haemoglobin concentration, increased
cholesterol levels, and increased liver weights in animals of each sex
at the next dose (Berthe, 1996).
(v) M&B 45897: Short-term toxicity
M&B 45897 (purity, 99.7%) was administered to five male and five
female CD rats by gavage at doses of 0, 50, 200, or 1000 mg/kg bw per
day in corn oil for 28 days. Standard evaluations of toxicity ante
and post mortem were included. There were no deaths. Salivation was
observed in all animals from day 2 of treatment with 1000 mg/kg bw per
day, from day 3 at 200 mg/kg bw per day, from day 8 in females at 50
mg/kg bw per day, and on days 8-15 in males at this dose. The control
group was not affected. During the second week, animals at the
intermediate and high doses were also hunched and underactive, and all
males and some females at the high dose showed hair loss from day 3
on. Staggering was observed in all animals at the high dose on day 8.
None of these clinical signs was observed at 50 mg/kg bw per day or in
the controls.
Significant deficits in body-weight gain on days 0-17 were seen
in animals of each sex at 1000 mg/kg bw per day, but food consumption
was not affected by treatment. Animals at this dose also showed
decreased haemoglobin concentration, and females had lower erythrocyte
numbers and packed cell volume in comparison with controls. Slightly
higher plasma protein concentrations were seen in animals of each sex
at the high dose, and higher plasma alanine aminotransferase activity
was seen in females. Increased absolute and relative liver weights
were seen in males and females at 1000 mg/kg bw per day. Macroscopic
examination showed no significant findings, but microscopic
examination revealed periacinar hepatic hypertrophy in the livers of
three male rats at 1000 mg/kg bw per day. The only effects considered
to be related to treatment were the slight decreases in erythrocyte
numbers and packed cell volume and the slightly increased alanine
aminotransferase activity in animals at the high dose. Although an
NOAEL of 200 mg/kg bw per day was proposed, the signs observed at the
intermediate and high doses in all animals were indicative of a
toxicological (perhaps neurotoxic) effect of M&B 45897, as they were
not seen in the controls and animals at the low dose. Similar findings
have been reported in studies with the parent compound fipronil and
some of its other metabolites, which have been shown to be neurotoxic.
Although the salivation could be a treatment-related clinical sign, it
could also be viewed as an indication of irritation by the test
compound or a local reaction to treatment, especially since it was
observed more or less consistently at all doses, except in males at
the low dose, in which it occurred only on days 8-15. This was the
only finding at the low dose. The NOAEL was therefore 50 mg/kg bw per
day on the basis of clinical signs of toxicity at the next highest
dose (Johnson, I.R., 1995).
(vi) Genotoxicity
The results of studies on the genotoxicity of fipronil
metabolites are summarized in Table 5.
(vii) Comparison of fipronil and its metabolites
Table 6 presents a comparison of findings for fipronil and its
metabolites: whether or not the chemical has been found (to a major or
minor extent) in or on plants or in animal (rat, goat, or hen) tissues
or products or can form in the environment or on surfaces, indoors or
outdoors, via reduction, oxidation, hydrolysis, or photolysis (see
Figure 3); the acute oral toxicity of each chemical; and the ability
of each metabolite to compete with the ligands 3H-EBOB and 3H-TBPS
for binding to specific sites in the chloride ion channel of the GABA
receptor. Binding is postulated to serve as an indicator of potential
to disrupt the normal functioning of the GABA receptor by interference
with chloride-ion flux and thus for potential toxicity to the central
nervous system.
Table 5. Results of assays for genotoxicity with metabolites of fipronil metabolites
Metabolite End-point Test object Concentration Purity Results Reference
(%)
In vitro
M&B 45950 Reverse mutation S. typhimurium 0-250 µg/plate 98.9 Negativea,b Percy (1994a)
TA98, TA100, in DMSO
TA1535, TA1537
Chromosomal Human 25-100 µg/ml > 99 Negativea,b Marshall
aberration lymphocytes in DMSO (1988b)
M&B 46136 Reverse mutation S. typhimurium 0.32-200 µg/plate 98.7 Negativea,b Clare (1988b)
TA98, TA100, (-S9), 0.8-500 µg/
TA1535, TA1537 plate (+S9), in
DMSO
Chromosomal Human 75-300 µg/ml 98.7 Negativea,b Marshall
aberration lymphocytes in DMSO (1989)
RPA 200766 Reverse mutation S. typhimurium 250-1000 µg/plate > 98 Negativea,b Percy (1993a)
TA98, TA100, (-S9), 50-2500 µg/
TA1535, TA1537, plate (+S9), in
TA1538 DMSO
Fipronil-desulfinyl Reverse mutation S. typhimurium 10-250 µg/plate 98.6 Negativea,b Percy (1993b)
TA98, TA100, in DMSO
TA1535, TA1537,
TA1538
Gene mutation Chinese hamster 5-125 µg/ml (-S9), 99.5 Negativea,b Adams (1996a)
cell line (CHO- 15-625 µg/ml (+S9)
K1-BH4), hprt locus in DMSO
Table 5. Results of assays for genotoxicity with metabolites of fipronil metabolites
Metabolite End-point Test object Concentration Purity Results Reference
(%)
Chromosomal Human lymphocytes 5-30 µg/ml (-S9), 99.5 Negativea,b Adams (1996b)
aberration 5-60 µg/ml (+S9)
in DMSO
RPA 104615 Reverse mutation S. typhimurium 2250-5000 µg/plate 94.7 Negativea,b Percy (1993c)
TA98, TA100, in DMSO
TA1535, TA1537,
TA1538
M&B 45897 Reverse mutation S. typhimurium 12.5-2500 µg/plate 99.7 Negativea,b Percy (1996)
TA98, TA100, (-S9), 25-2500 µg/
TA1535, TA1537 plate (+S9), in
DMSO
Reverse mutation S. typhimurium 4-2500 µg/plate > 99 Negativea,b Kennelly
TA98, TA100, (-S9), except TA 100; (1988)
TA1535, TA1537 8-5000 µg/plate
(-S9), TA100; 8-5000
µg/plate (+S9), all
strains; in DMSO
Chromosomal Human 50-150 µg/ml (-S9), 99.7 Negativea,b Johnson, A.L.
aberration lymphocytes 100-400 µg/ml (+S9), (1995)
in DMSO; 20- or 44-h
harvest times
Polyploidy Human 50-150 µg/ml (-S9), 99.7 Positivea,c Johnson, A.L.
lymphocytes 100-400 µg/ml (+S9), (1995)
in DMSO; 20- or 44-h
harvest times
RPA 105048 Reverse mutation S. typhimurium 250-5000 µg/plate 98.6 Negativea,c Percy (1994b)
TA98, TA100, in DMSO
TA1535, TA1537
Table 5. Results of assays for genotoxicity with metabolites of fipronil metabolites
Metabolite End-point Test object Concentration Purity Results Reference
(%)
In vivo
Fipronil-desulfinyl Micronucleus CD-1 mice 2-16 mg/kg bw in 99.5 Negativeb Proudlock
formation corn oil (1996)
DMSO, dimethyl sulfoxide, S9, 9000 × g supernatant of rat liver homogenate
a With and without metabolic activation
b Appropriate positive controls gave expected positive responses
c Reproducible increases in polyploid cells with metabolic activation
Table 6. Comparisons of fipronil and its metabolites
Compound In plants In animals Potential environmental Photo- Oral LD50 Binding to
metabolite or surface metabolite (mg/kg bw)a 3H-EBOB &
residue or degradate 3H-TBPS sitesb
Fipronil Yes Rat, goat, hen Water, soil, surfaces No 97
M&B 45950 Yes Rat, goat, hen Soil No 83 * (both sites)
RPA 200766 Yes Rat, goat Water, soil, surfaces No > 2000 @@ (both sites)
Fipronil-desulfinyl Yes No Water, soil, surfaces Yes 16 ** (both sites)
M&B 46136 Yes Rat, goat, hen Soil, surfaces No 218 * (both sites)
RPA 104615 Yes No Water, soil, surfaces Yes > 2000 @@ (both sites)
M&B 45897 Yes Rat Not expected No > 2000 @@ (both sites)
Two ring-opened No Rat No No No data No data
metabolites of
M&B 45897
M&B 105320 Yes No Not expected No > 2000 @@ (both sites)
EBOB, 1-[(4-ethyl)phenyl]-4-n-propyl-2,6,7-trioxacicyclo[2.2.2]octane; TBPS, tert-butylbicyclophosphorothionate
a From references given in Tables 1 and 2
b Increased (*) or decreased (@) binding relative to fipronil; ** or @@ greater differences. Based on IC50 values from
competitive binding assays for fipronil and its metabolites with radiolabelled 1 gands (personal communication from
P. Kwiatkowski, Rhone-Poulenc Worldwide Regulatory Affairs, North Carolina, USA)
The results presented in the table and those of similar studies
indicate that most of the metabolites are of similar or lesser
toxicity than fipronil. Generally, this conclusion reflects the data
on binding, except for M&B 46136 which has greater binding affinity
for sites in the rat brain GABA receptor chloride-ion channel but a
higher acute oral LD50 than fipronil in rats. (No short-term studies
of the toxicity of this metabolite are available.)
Fipronil-desulfinyl and M&B 104615 are photolytic products of
fipronil which could potentially form in the environment or on treated
or exposed surfaces during use to control malarial vectors. The LD50
of fipronil-desulfinyl in rats is much lower than that of fipronil,
and it has much greater binding affinity for GABA receptor
chloride-ion channel sites in rat brain. Both are neurotoxic. The
toxicity of fipronil-desulfinyl is qualitatively similar to that of
fipronil, but the dose-effect curve for neurotoxic effects is steeper
for fipronil-desulfinyl and neurotoic effects occur at lower doses.
The acute oral LD50 for M&B 104615 is much higher and the affinity
for GABA receptor chloride-ion channel sites much less than those for
fipronil.
Comments
Fipronil
In a study of dermal absorption in rats, the quantity of
14C-fipronil absorbed was less than 1% of the applied dose at all
doses tested (0.88-48 mg/rat) and all times up to 24 h. In vitro,
the relative extent of absorption of a formulation of 14C-fipronil
across rat, rabbit, or human epidermal membranes depended on the
concentration of the material used. At the lowest concentration tested
(0.2 g/L), the extent of penetration was greatest for all three
species, and the percentage of the dose absorbed across human and rat
membranes was similar. At higher concentrations (4 and 200 g/L),
penetration was greater through rat and rabbit skin than through human
skin.
There was no appreciable difference between male and female rats
in the absorption, distribution, metabolism, or excretion of fipronil
after oral administration. The proportion of the dose absorbed
appeared to depend on the treatment regimen, being greatest with a
single dose of 4 mg/kg bw of 14C-fipronil (minimum absorption, 50%),
intermediate with a repeated dose regimen of 4 mg/kg bw per day for 14
days followed by a single, oral labelled dose of 4 mg/kg bw (minimum
absorption, 40%), and lowest (minimum absorption, about 30%) with a
single dose of 150 mg/kg bw of 14C-fipronil (presumably due to
saturation of absorption at the high dose). Once absorbed, fipronil
was rapidly metabolized, and the residues widely distributed in
tissues. Significant amounts of residues remained in the tissues,
particularly in fat and fatty tissues, one week after treatment. The
levels of residues in fat and other tissues were greater with repeated
low doses or a single high dose than with a single low dose. The long
half-life (150-245 h in some cases) of fipronil in blood may reflect
slow release of residues from fat and might suggest potential
bioaccumulation of metabolic products of fipronil.
Faeces, followed by urine, were the major routes of elimination
of fipronil in rats. Its biotransformation largely involved changes in
the functional groups attached to the pyrazole ring. The compounds
identified in faeces and urine were the parent compound and the
sulfone, the amide derived from the nitrile group, a reduction
product, and a cleavage product of the sulfone and its derivatives
formed by further cleavage. The sulfone was the major metabolite in
fat and tissues.
Fipronil was moderately hazardous to rats (LD50 = 92 mg/kg bw)
and mice (LD50 = 91 mg/kg bw) after oral administration of single
doses and to rats after single exposure by inhalation (LC50
= 0.36 mg/L). After a single dermal exposure, fipronil was relatively
non-hazardous to rats (LD50 > 2000 mg/kg bw) but was moderately
hazardous to rabbits (LD50 = 354 mg/kg bw). In rats, signs of
toxicity and death were delayed for up to four days after either a
single oral dose or repeated oral doses of 75 mg/kg bw per day for up
to five days. WHO has not yet classified fipronil for acute toxicity.
In a 13-week study of toxicity, mice were fed diets containing
fipronil at doses of 0, 1, 3, 10, or 25 ppm. A dose-related increase
in the incidence of liver-cell periacinar hypertrophy with cytoplasmic
vacuolation was observed in males at doses of 1 ppm (equal to 0.13
mg/kg bw per day) and above. There was no NOAEL.
Rats were fed diets containing 0, 25, 50, 100, 200, or 400 ppm
fipronil for four weeks. At 25 ppm (equal to 3.4 mg/kg bw per day),
liver weights and plasma cholesterol levels were increased in females,
and thyroid follicular-cell hypertrophy of minimal severity was
observed in animals of each sex. The levels of total protein and
globulin were also increased in both males and females, although the
changes at this and higher doses were generally small and poorly
correlated with the dose. There was no NOAEL.
In a 13-week study of toxicity, fipronil was administered in the
diet to rats at doses of 0, 1, 5, 30, or 300 ppm. At 30 ppm and above,
relatively small, sometimes inconsistent changes in haematological
parameters (decreased packed cell volume, mean cell volume,
haemoglobin concentration, and prothrombin time and increased platelet
count) and clinical chemical parameters (increased total protein and
globulins, decreased albumin:globulin ratio and alanine
aminotransferase and aspartate aminotransferase activities) were
observed, mostly in females. Some alterations were seen in plasma
glucose and urea concentrations at 30 ppm; also at 30 ppm, the
absolute and/or relative weights of the liver and thyroid were
increased in either males or females or both, and there was evidence
of thyroid follicular-cell epithelial hypertrophy in males. The NOAEL
was 5 ppm, equal to 0.33 mg/kg bw per day.
Fipronil was administered in gelatin capsules to dogs for 13
weeks in a study of toxicity at doses of 0, 0.5, 2, or 10 mg/kg bw per
day. Inappetence and decreased body-weight gain and food consumption
were noted in females at 2 and 10 mg/kg bw per day. The NOAEL was 0.5
mg/kg bw per day.
In a study of dermal toxicity, fipronil was applied in 0.5%
carboxymethylcellulose to the intact skin of rabbits for 6 h per day
on five days per week for three weeks at doses of 0, 0.5, 1, 5, or 10
mg/kg bw per day. No dermal irritation was observed. At 10 mg/kg bw
per day, body-weight gains and food consumption were reduced in
animals of each sex. Some animals showed hyperactivity. The NOAEL was
5 mg/kg bw per day.
Fipronil was administered to dogs in gelatin capsules for one
year in a study of toxicity at doses of 0, 0.2, 2, or 5 mg/kg bw per
day. At 2 mg/kg bw per day and above, clinical signs of neurotoxicity
(convulsions, twitching, tremors, ataxia, unsteady gait, rigidity of
limbs, nervous behaviour, hyper- or hypoactivity, vocalization,
nodding, aggression, resistance to dosing, inappetence, and abnormal
neurological responses) were observed in animals of each sex. One
animal at 2 mg/kg bw per day was killed because of poor condition
related to treatment. The NOAEL was 0.2 mg/kg bw per day. In a second
one-year study in dogs, fipronil was administered in the diet at doses
of 0, 0.075, 0.3, 1, or 3 mg/kg bw per day. The highest dose was
reduced to 2 mg/kg bw per day after 38 days because of toxicity. At 1
mg/kg bw per day, clinical signs of neurotoxicity (whole body
twitching, and extensor rigidity of limbs) were noted in females.
There were no effects on triiodothyronine or thyroxine levels. The
NOAEL was 0.3 mg/kg bw per day.
In a study of carcinogenicity, fipronil was administered for 78
weeks in the diet to mice at doses of 0, 0.1, 0.5, 10, 30, or 60 ppm.
Additional groups of animals were fed the same doses for 52-53 weeks
and then killed. Survival was greater than or comparable to that of
the control group at doses below 60 ppm. At week 10, all surviving
animals at 60 ppm were killed because of excessive mortality. In
animals at 10 ppm, some decrease in body-weight gain was noted in
males and females, and efficiency of food use was decreased in males.
At 53 and 78 weeks, the absolute and/or relative liver weights of
males were increased, with an increased incidence of liver periacinar
microvesicular vacuolation. There was no evidence of carcinogenicity
at doses considered to be sufficient to measure such potential. The
NOAEL for systemic effects was 0.5 ppm, equal to 0.055 mg/kg bw per
day.
In a study of toxicity and carcinogenicity in rats, fipronil was
administered in the diet at doses of 0, 0.5, 1.5, 30, or 300 ppm. For
the carcinogenicity phase of the study, it was originally planned that
the test material be administered for two years, but excessive
mortality resulted in early termination of this phase at week 89 in
males and week 91 in females. This was not thought to compromise the
study. For the toxicity phase and a reversibility phase of the study,
additional groups of animals were fed the same doses of fipronil for
one year, when some animals were killed and others were allowed to
recover for 13 weeks. Some of the effects noted at the higher doses
persisted into the reversibility phase of the study. During treatment,
convulsive episodes (sometimes fatal) were observed in males at 1.5
ppm and in animals of each sex at higher doses. Animals at 1.5 ppm,
predominantly females, showed irritability, vocalization, salivation,
aggression, hyperactivity, and bruxism. Small decreases were noted in
erythrocyte count, haemoglobin concentration, mean cell volume, and
packed cell volume in either males or females or both, and some
alterations in protein level were observed in males. An apparent
increase in the severity of progressive senile nephropathy was seen in
animals of each sex at this dose. Thyroxine concentrations were
decreased in both males and females. Thyroid-stimulating hormone
levels were increased, notably in males, at doses of 30 ppm and above
and in females at 300 ppm. The levels of triiodothyronine were
elevated in females at 30 ppm, but only during the reversibility
phase. At 300 ppm, fipronil induced follicular-cell adenomas of the
thyroid gland in both males and females; males at this dose also had
an increased incidence of follicular-cell carcinomas. Some thyroid
follicular-cell adenomas were noted in male rats at lower doses, but a
comparison with historical control data indicated no clear
relationship to treatment. The NOAEL for systemic effects was 0.5 ppm,
equal to 0.019 mg/kg bw per day.
Fipronil and its metabolites gave negative results in virtually
all tests for genotoxicity. Equivocal results were seen in assays for
cytogenicity in mammalian cells in vitro with fipronil and for
polyploidy (not clastogenicity) in human lymphocytes with a mammalian
metabolite. The weight of evidence indicates that fipronil and its
metabolites are not genotoxic.
The Meeting concluded that the thyroid tumours observed in the
two-year study in rats occurred by a non-genotoxic, threshold
dose-effect mechanism involving continuous stimulation of the thyroid
gland associated with persistently elevated thyroid-stimulating
hormone levels. It was noted that the levels of this hormone were
clearly elevated only at the two highest doses.
In a two-generation study of reproductive toxicity, rats received
diets containing fipronil at 0, 3, 30 or 300 ppm. F0 parental animals
were mated twice to produce F1a and F1b litters; F1a parents were
mated only once to produce F2 litters. In adult animals at 30 ppm,
the thyroid and liver weights were increased and the pituitary gland
weights were decreased. An increased incidence of thyroid gland
follicular epithelial-cell hypertrophy was seen at this dose in males
of the F0 and F1 generations and F1 females. At 300 ppm,
convulsions were observed in F1 and F2 litters; decreased litter
size, decreased body weights and delays in physical development were
also seen. Postnatal survival was decreased among pups in the F2
litters. Absolute and relative ovarian weights were decreased in F0
females. At 300 ppm, a decreased percentage of animals that mated and
a reduction in the fertility index of F1 parental animals was also
observed. These effects may have been related to the systemic toxicity
of fipronil at this dose. The NOAEL for parental systemic toxicity was
3 ppm, equal to 0.25 mg/kg bw per day, and the NOAEL for reproductive
toxicity was 30 ppm, equal to 2.5 mg/kg bw per day.
Rats were given fipronil by gavage at doses of 0, 1, 4, or 20
mg/kg bw per day on days 6-15 of gestation. Developmental toxicity was
not observed, but there were some signs of maternal toxicity
(decreased body-weight gain and food consumption) at 20 mg/kg bw per
day. The NOAEL for maternal toxicity was 4 mg/kg bw per day, and that
for developmental toxicity was 20 mg/kg bw per day, the highest dose
tested.
Rabbits were given fipronil by gavage at doses of 0, 0.1, 0.2,
0.5, or 1 mg/kg bw per day on days 6-19 of gestation. Developmental
toxicity was not observed, but there were some signs of maternal
toxicity (decreased body-weight gain, decreased food consumption, and
reduced efficiency of food use at all doses. There was no NOAEL for
maternal toxicity; the NOAEL for developmental toxicity was 1 mg/kg bw
per day, the highest dose tested.
Primary dermal irritation in rabbits was examined in two studies.
Fipronil was slightly irritating when moistened with corn oil before
application but was not irritating when moistened with water. Fipronil
was slightly irritating in two studies of primary ocular irritation in
rabbits. It did not sensitize the skin of guinea-pigs when tested by
the Buehler method but was a weak sensitizer in guinea-pigs tested by
the Magnusson-Kligman method.
In a study of neurotoxicity, rats were given single doses of 0,
0.5, 5, or 50 mg/kg bw fipronil by gavage. At 5 mg/kg bw, decreased
hind-leg splay was observed 7 h after treatment in both males and
females. The NOAEL was 0.5 mg/kg bw. In a 13-week study of
neurotoxicity, rats received dietary doses of 0, 0.5, 5, or 150 ppm
fipronil. Body weights, weight gains, and food consumption were
reduced early in the study in animals of each sex at 150 ppm, possibly
owing to problems of palatability. Although the findings in a battery
of functional operational tests at this dose were relatively minor
when taken separately, they appeared to represent a minimal effect of
treatment when taken together. The NOAEL for neurotoxicity and
systemic effects was 5 ppm, equal to 0.3 mg/kg bw per day.
In a study of neurotoxicity in female dogs, fipronil was
administered in capsules at doses of 0 (one animal) or 20 mg/kg bw per
day (four animals) until the appearance of neurotoxic signs in each
animal, after which they were allowed to recover for 28 days. Severe
neurotoxic signs were seen at 20 mg/kg bw per day during the treatment
phase and in some animals only during the recovery phase. Most animals
appeared to recover, although one had exaggerated reflex responses and
was excitable at the end of the recovery period. A limited
histopathological examination showed no change. No firm conclusions
could be drawn about the reversibility of the effects, given the
limitations of the study design. There was no NOAEL.
In a study of developmental neurotoxicity, rats were given
fipronil in the diet from gestation day 6 through lactation day 10 at
doses of 0, 0.5, 10, or 200 ppm. Maternal toxicity manifested as
reduced body weight during the treatment period, reduced body-weight
gain during gestation, and reduced food consumption was observed at
200 ppm. Developmental toxicity (reduced body weights in pups and a
slight increase in the time to preputial separation) was noted at 10
ppm. An increase in motor activity in female pups at 10 ppm only on
day 17 could not be definitively interpreted as an indication of
developmental toxicity. Developmental neurotoxicity was clearly
observed postnatally in pups at 200 ppm, with delayed swimming
development on day 6, increased motor activity on day 17, abnormal
auditory startle response on day 22, and impaired learning and memory
on day 24. The NOAEL for maternal toxicity and developmental
neurotoxicity was 10 ppm (equal to 0.9 mg/kg bw per day) and that for
developmental toxicity was 0.5 ppm (equal to 0.05 mg/kg bw per day).
Mechanistic studies conducted with fipronil in rats suggest that
it does not interfere with the incorporation of iodine into thyroxine
but rather with the biliary clearance of this hormone. This may
trigger an increase in the concentration of thyroid-stimulating
hormone by interference with the feedback mechanism.
Mammalian metabolites of fipronil
Several mammalian metabolites of fipronil were tested for acute
toxicity. Their toxicity was comparable to or substantially less than
that of fipronil.
Photodegradation products of fipronil
Numerous studies were performed with fipronil-desulfinyl, one of
two photodegradation products of fipronil which can be formed in the
presence of sunlight and could potentially be produced in the
environment or on treated surfaces. Neither is a mammalian metabolite
of fipronil. The available information indicates that, of the two,
only fipronil-desulfinyl is highly toxic after exposure to single
doses or over the long term, and is therefore of toxicological
concern.
When 0.08-7.2 mg of 14C-fipronil-desulfinyl were applied
dermally to rats, absorption ranged fron 0.2 to 7% of the applied dose
within 24 h.
The absorption, distribution metabolism, and excretion of
14C-fipronil-desulfinyl were studied in rats which received either a
single oral dose of labelled compound at 1 or 10 mg/kg bw or 14 daily
oral doses of unlabelled compound at 1 mg/kg bw per day followed by a
single oral labelled dose. In animals of each sex, elimination of the
radiolabel was much greater in the faeces (46-70% of the dose) than in
the urine with all dosing regimens. Appreciable residues were found in
the tissues one week after treatment, the highest concentrations being
present in the fat and fatty tissues. The long half-life in blood
(183-195 h) and increased fat:plasma ratios of the radiolabel suggest
potential bioaccumulation of fipronil-desulfinyl and/or its
metabolites. Numerous metabolites or conjugates of fipronil-desulfinyl
were present in the urine and faeces. Biotransformation of
fipronil-desulfinyl involved changes at the functional groups attached
to the pyrazolyl ring. Only unchanged fipronil-desulfinyl was
identified in the liver, fat, skin, and residual carcass.
In a 28-day study of toxicity in which fipronil-desulfinyl was
administered in the diet to mice at doses of 0, 0.5, 3, 30, or 60 ppm,
mortality, neurotoxic signs (increased motor activity, excessive
jumping, irritability to touch, compulsive biting, and evidence of
convulsions), decreased body-weight gain and food consumption, and an
increased incidence of centrilobular hypertrophy of the liver were
observed in animals of each sex at doses of 30 ppm and above. The
NOAEL was 3 ppm, equal to 0.49 mg/kg bw per day. Fipronil-desulfinyl
was administered in the diet for 90 days to mice at doses of 0, 0.5,
2, or 10 ppm. At 2 and 10 ppm, clinical signs of neurotoxicity
(irritability to touch, aggressiveness, and/or increased motor
activity) were noted in males. The NOAEL was 0.5 ppm, equal to
0.08 mg/kg bw per day.
Rats received fipronil-desulfinyl by gavage for two weeks at
doses of 0, 0.3, 1, 3, or 10 mg/kg bw per day. At 1 mg/kg bw per day,
pale livers and reduced leukocyte counts were observed in females.
Some rats at 3 mg/kg bw per day died or were killed because of poor
condition. The NOAEL was 0.3 mg/kg bw per day. Fipronil-desulfinyl was
administered in the diet for 28 days to rats at doses of 0, 0.5, 3, 30
or 100 ppm. One male at 30 ppm died, clinical signs of toxicity
(piloerection and curling up on handling), and decreased body weights,
food consumption, and bilirubin concentration were seen in males and
females at this dose. Thymus weights were lowered in females. The
levels of thyroid-stimulating hormone were measured, but no effects
were noted at any dose. All animals at 100 ppm died. The NOAEL was 3
ppm, equal to 0.23 mg/kg bw per day.
In a 90-day study of toxicity in rats, fipronil-desulfinyl was
administered in the diet at 0, 0.5, 3, 10, or 30 ppm. At 3 ppm and
above, clinical signs of neurotoxicity (aggressiveness, irritability
to touch, and excessive vocalization) were observed in males. The
levels of triiodothyronine and thyroxine were affected at higher
doses, but the toxicological significance of these changes is probably
negligible in the absence of changes in the level of
thyroid-stimulating hormone at any dose. The NOAEL in the study was
0.5 ppm, equal to 0.029 mg/kg bw per day.
Dogs received fipronil-desulfinyl in the diet in a 28-day study
at doses of 0, 27, 80, or 270 ppm. The groups at 80 and 270 ppm were
terminated early because of mortality. One male at 27 ppm had a clonic
convulsion. Reduced thymus weights and pale livers were also reported
at this dose. As effects occurred at the lowest dose, there was no
NOAEL.
In a 90-day study of toxicity, fipronil-desulfinyl was
administered in the diet to dogs at doses of 0, 3.5, 9.5, or 35 ppm.
The clinical findings in one female at 35 ppm (increased salivation,
prostration, writhing, tremors, absence of rotular reflex, noisy
breathing, dyspnoea) were attributed to arteritis and myocardial
necrosis on the basis of microscopic findings; however, they may also
have been indicative (at least in part) of neurotoxicity, because
another female in this group exhibited excessive barking,
aggressiveness, irritability, tremors, and increased salivation. On
this basis, the Meeting concluded that the NOAEL was 9.5 ppm, equal to
0.29 mg/kg bw per day.
In a study of developmental toxicity in rats, fipronil-desulfinyl
was administered by gavage on days 6-15 of gestation at doses of 0,
0.5, 1, or 2.5 mg/kg bw per day. Indications of maternal effects
(decreased body-weight gain and hair loss in various areas) were
observed at 2.5 mg/kg bw per day. Developmental toxicity (increased
incidence of incomplete or reduced ossification of several bones and
slightly reduced fetal body weight in animals of each sex) was also
observed at this dose. The NOAEL for maternal toxicity and
developmental toxicity was 1 mg/kg bw per day.
In a study of neurotoxicity in rats, fipronil-desulfinyl was
administered by gavage as a single dose of 0, 0.5, 2, or 12 mg/kg bw.
At 12 mg/kg bw, decreased body-weight gains and food consumption were
observed during week 1 in animals of each sex. Decreased hind-foot
splay, rectal temperature, and locomotor activity were also seen in
animals of each sex at this dose. There were indications of a slowed
righting reflex in males and decreased grip strength in males and
females at the high dose. The NOAEL was 2 mg/kg bw per day.
In summary, the toxicity of fipronil-desulfinyl is qualitatively
similar to that of fipronil, but the dose-effect curve for neurotoxic
effects appears to be steeper for fipronil-desulfinyl than for
fipronil. Also, fipronil-desulfinyl appears to have a much greater
tendency than fipronil to bind to sites in the chloride ion channel of
the rat brain GABA receptor. This finding appears to be consistent
with the greater toxicity, relative to fipronil, of
fipronil-desulfinyl in the central nervous system of mammals.
The Meeting established an ADI of 0-0.0002 mg/kg bw for fipronil
on the basis of the NOAEL of 0.019 mg/kg bw per day in the two-year
study of toxicity and carcinogenicity in rats and incorporating a
safety factor of 100.
The Meeting considered that a separate ADI should be established
for fipronil-desulfinyl on the basis that it could be a significant
residue and that its toxicity is greater than that of the parent
molecule fipronil. A temporary ADI of 0-0.00003 mg/kg bw for
fipronil-desulfinyl was established on the basis of the NOAEL of 0.029
mg/kg bw per day in the 90-day study in rats and a safety factor of
1000, in view of the lack of a long-term study by oral administration
in rats and a study of neurotoxicity in rats given repeated oral
doses.
Toxicological evaluation
Fipronil
Levels that cause no toxic effect
Mouse: 0.5 ppm, equal to 0.055 mg/kg bw per day (78-week study
of carcinogenicity and toxicity)
Rat: 5 ppm, equal to 0.33 mg/kg bw per day (13-week study of
toxicity)
0.5 ppm, equal to 0.019 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
3 ppm, equal to 0.25 mg/kg bw per day (parental
systemic toxicity in a study of reproductive toxicity)
30 ppm, equal to 2.5 mg/kg bw per day (study of
reproductive toxicity)
4 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity by gavage)
20 mg/kg bw per day (developmental toxicity in a study
of developmental toxicity by gavage; highest dose
tested)
0.5 mg/kg bw (single dose, study of neurotoxicity by
gavage)
5 ppm, equal to 0.3 mg/kg bw per day (repeated doses in
the diet, study of neurotoxicity)
10 ppm, equal to 0.9 mg/kg bw per day (maternal
toxicity and developmental neurotoxicity in a study of
developmental neurotoxicity)
0.5 ppm, equal to 0.05 mg/kg bw per day (developmental
toxicity in a study of developmental neurotoxicity)
Rabbit: 0.1 mg/kg bw per day (LOAEL for maternal toxicity in a
study of developmental toxicity by gavage)
1 mg/kg bw per day (study of developmental toxicity;
highest dose tested by gavage)
Dog: 0.3 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.0002 mg/kg bw
Fipronil-desulfinyl (fipronil photodegradation product)
Levels that cause no toxic effect
Mouse: 3 ppm, equal to 0.49 mg/kg bw per day (28-day study of
toxicity)
0.5 ppm, equal to 0.08 mg/kg bw per day (90-day study
of toxicity)
Rat: 0.3 mg/kg bw per day (two week study of toxicity by
gavage)
3 ppm, equal to 0.23 mg/kg bw per day (28-day study of
toxicity)
0.5 ppm, equal to 0.029 mg/kg bw per day (90-day study
of toxicity)
1 mg/kg bw per day (maternal and developmental toxicity
in a study of developmental toxicity by gavage)
2 mg/kg bw per day (single dose, study of neurotoxicity
by gavage)
Dog: 9.5 ppm, equal to 0.29 mg/kg bw per day (90-day study
of toxicity)
Estimate of temporary acceptable daily intake for humans
0-0.00003 mg/kg bw
Acute reference dose for fipronil
The Meeting allocated an acute reference dose of 0.003 mg/kg bw
for both fipronil and fipronil-desulfinyl on the basis of the NOAEL of
0.3 mg/kg bw per day in a study of neurotoxicity in rats given
repeated doses of fipronil, and a safety factor of 100. The study of
neurotoxicity in rats given single doses was not considered in
allocating the acute reference dose because of concern about the
prolonged toxicokinetics of fipronil. This acute reference dose will
provide a safety factor of about 700 for the NOAEL in the study of
neurotoxicity in rats given single doses of fipronil-desulfinyl.
Studies without which the determination of an ADI is impractable,
to be provided by 2000
1. Short-term study of neurotoxicity in rats with
fipronil-desulfinyl in the diet
2. Developmental neurotoxicity study in rats with
fipronil-desulfinyl in the diet
3. The results of an ongoing long-term study with
fipronil-desulfinyl in rats
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to fipronil and its photodegradation
product fipronil-desulfinyl
Human exposure Relevant route, study type, species Results, remarks
Fipronil
Short-term Skin, irritation, rabbit Slightly imitating
(1-7 days) Eye, irritation, rabbit Minor irritation
Skin, sensitization, guinea-pig Not a sensitizer (Buehler)
Skin, sensitization, guinea-pig Mild sensitizer (Magnusson-Kligman)
Oral, toxicity, rat LD50 = 92 mg/kg bw
Dermal, toxicity, rabbit LD50 = 350 mg/kg bw
Inhalation, toxicity, rat LC50 = 0.36 mg/L
Neurotoxicity, rat NOAEL = 0.5 mg/kg bw per day:
(single dose by gavage) decreased hind-leg splay
Medium-term Repeated dermal, 3 weeks, toxicity, rabbit NOAEL = 5 mg/kg bw per day: reduced body-weight
(1-26 weeks) gains and food consumption; hyperactivity in some
animals; no dermal imitation observed
Repeated oral, reproductive toxicity, rat NOAEL = 0.25 mg/kg bw per day for maternal toxicity.
NOAEL = 2.5 mg/kg bw per day for reproductive
toxicity
Repeated oral, developmental neurotoxicity, rat NOAEL = 0.9 mg/kg bw per day for maternal toxicity.
NOAEL = 0.05 mg/kg bw per day for developmental
toxicity
NOAEL = 0.9 mg/kg bw per day for developmental
neurotoxicity
Long-term Repeated oral, 2 years (terminated at 89-91 NOAEL = 0.019 mg/kg bw per day: convulsions and
(> 1 year) weeks), long-term toxicity and carcinogenicity, rat neurobehavioural clinical signs of toxicity; effects on the
thyroid; thyroid follicular-cell adenomas and carcinomas
Fipronil-desulfinyl
Short-term Oral, toxicity, rat LD50 = 15 mg/kg bw
(1-7 days) Dermal, toxicity, rat LD50 > 2000 mg/kg bw
Neurotoxicity, rat NOAEL = 2 mg/kg bw per day
(single dose by gavage)
(continued)
Human exposure Relevant route, study type, species Results, remarks
Medium-term Repeated oral (diet), 90 days, toxicity, rat NOAEL = 0.029 mg/kg bw per day
(1-26 weeks) Repeated oral (gavage), developmental toxicity, NOAEL = 1.0 mg/kg bw per day: maternal toxicity
rat NOAEL = 1.0 mg/kg bw per day: developmental
toxicity
Long-term Repeated oral toxicity No data
> 1 year)
Studies that would provide information useful for the continued
evaluation of fipronil and fipronil-desulfinyl
1. Additional studies to investigate the reversibility of the
neurotoxic effects of fipronil and its metabolites
(functional, behavioural, learning/memory, cellular, and
neurotransmitter/receptor effects).
2. Observations in humans exposed to fipronil and fipronil-
desulfinyl
References
Adams, K. (1996a) MB 46513: CHO mammalian cell mutation assay.
Unpublished report No. RNP452/950622 from Huntingdon Life Sciences,
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Adams, K. (1996b) MB 46513: Metaphase chromosome analysis of human
lymphocytes cultured in vitro. Unpublished report No. RNP 451/951219
from Huntingdon Life Sciences, Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Aughton, P. (1993) MB 46030: Combined oncogenicity and toxicity study
by dietary administration to CD rats for 104 weeks, including a 13
week reversibility period on completion of 52 weeks of treatment.
Unpublished report No. 93/RHA432/0166 from Pharmaco LSR Ltd. Submitted
to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Berthe, P. (1996) RPA 200766: 28-Day toxicity study in the rat by
dietary administration. Unpublished report No. SA 95273 from
Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Bigot, D. (1996) MB 46513: 90-Day toxicity study in the mouse by
dietary administration. Unpublished report No. SA 95055 from
Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Broadmeadow, A. (1991) M&B 46030: Preliminary toxicity study by
dietary administration to CD-1 mice for 13 weeks. Unpublished report
No. 90/RHA364/0860 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Broadmeadow, A. (1993) M&B 46030: Oncogenicity study by dietary
administration to CD-1 mice for 78 weeks. Unpublished report No.
92/RHA313/0971 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Brooker, A.J. & John, D.M. (1991) The effect of M&B 46,030 on
pregnancy of the rat. Unpublished report No. M&B 335+326/90582 from
Huntingdon Research Centre Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Bushey, D.F. (1993) Fipronil mode of action research summary.
Unpublished memo prepared by Rhone-Poulenc Agrochimie Co., Research
Triangle Park Biochemistry Group. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Cheng, T. (1995) Dermal absorption of 14C-fipronil REGENT 80WDG in
male rats (preliminary and definitive phases). Unpublished report No.
HWI 6224-210 from Hazleton Wisconsin, Inc. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Cheng, T. (1996) Dermal absorption of 14C-MB 46513 in male rats
(preliminary and definitive phases). Unpublished report No.
CHW 6224-230 from Corning Hazleton, Inc. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Clare, C.B. (1988a) Study to determine the ability of M&B 46030 to
induce mutation in four histidine-requiring strains of Salmonella
typhimiurim. Unpublished report No. MAB 20/S from Microtest Research
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Clare, C.B. (1988b) Study to determine the ability of M&B 46136 to
induce mutation in four histidine-requiring strains of Salmonella
typhimiurim. Unpublished report No. MAB 21/S from Microtest Research
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Cole, L.M., Nicholson, R.A. & Casida, J.E. (1993) Action of
phenylpyrazole insecticides at the GABA-gated chloride channel.
Pestic. Biochem. Physiol., 46, 47-54.
Cracknell, S. (1991) M&B 46030: Acute inhalation toxicity study in the
rat. Unpublished report No. 90/RHA358/0791 from Life Science Research
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Dange, M. (1993a) RPA 200766: Acute oral LD50 in the rat. Unpublished
report No. SA 93016 from Rhone-Poulenc Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Dange, M. (1993b) MB 46513. Acute oral LD50 in rats. Unpublished
report No. SA 93074 from Rhone-Poulenc Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Dange, M. (1993c) MB 46513: Acute dermal LD50 in the rat. Unpublished
report No. SA 93095 from Rhone-Poulenc Secteur Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Dange, M. (1993d) RPA 104615 acute oral LD50 in the rat. Unpublished
report No. SA 93046 from Rhone-Poulenc Secteur Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC,USA.
Dange, M. (1994a) MB 45950 acute oral LD50 in the rat. Unpublished
report No. SA 93272 from Rhone-Poulenc Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Dange, M. (1994b) MB 46513: Preliminary 28-day toxicity study in the
mouse by dietary administration. Unpublished report No. SA 93228 from
Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Dange, M. (1994c) MB 46513: Exploratory 14-day toxicity study in the
rat by gavage. Unpublished report No. SA 93063 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Dange, M. (1994d) MB 46513 90-day toxicity study in the rat by dietary
administration. Unpublished report No. 93226 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Dange, M. (1995a) MD 46513: Preliminary 28 day toxicity study in the
rat by dietary administration. Unpublished report No. SA 93138 from
Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Dange, M. (1995b) MB 46513: Preliminary 28-day toxicity study in the
dog by dietary administration. Unpublished report No. SA 94143 from
Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Dange, M. (1996) MB 46513: 90-Day toxicity study in the dog by dietary
administration. Unpublished report No. SA 95100 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Driscoll, C.D. and Hurley, J.M. (1993) MB 46030: Ninety day dietary
neurotoxicity study in Sprague Dawley rats. Unpublished report No.
92N1074 from Union Carbide Bushy Run Research Center. Submitted to WHO
by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Edwards, C.N. (1991) M&B 46030: Assessment of clastogenic action on
bone marrow erythrocytes in the micronucleus test. Unpublished report
No. 90/RHA305/1377 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Edwards, C.N. (1995) M&B 46030: Mouse micronucleus test to comply with
OECD Guideline 474 (1983). Unpublished report No. 95/RHA547/0432 from
Pharmaco LSR Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Foulon, O. (1997) MB 46513 (fipronil photometabolite): Developmental
toxicology study in the rat by gavage. Unpublished report No. SA 96227
from Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Gant, D.B., Chalmers, A.E. & Wolff, M.A. (1994) Fipronil: A novel
insecticide acting at the GABA receptor. Poster presented at the
Eighth International Congress of Pesticide Chemistry, Washington DC by
Rhone-Poulenc Agrochimie Co., Department of
Biochemistry/Biotechnology, Research Triangle Park, NC.
Gardner, J.R. (1988a) Acute oral toxicity to rats of M&B 46,030.
Unpublished report No. 881300D/M&B 290/AC from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Gardner, J.R. (1988b) Acute dermal toxicity to rats of M&B 46,030.
Unpublished report No. 881113 D/M&B 291/AC from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Gardner, J.R. (1988c) Acute oral toxicity to rats of M&B 46136.
Unpublished report No. 881364 D/M&B286/AC from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC,USA.
Gardner, J.R. (1988d) Acute dermal toxicity to rats fo M&B 46,136.
Unpublished report No. 88916 D/M&B287/AC from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Gill, M.W., Wagner, C.L. & Driscoll, C.D. (1993) M&B 46030: Single
exposure peroral (gavage) neurotoxicity study in Sprague Dawley rats.
Unpublished report No. 91N0099 from Union Carbide Bushy Run Research
Center. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Haynes, G. (1988a) M&B 45897 acute oral toxicity study in the rat.
Unpublished report No. A/D/4855 from Toxicol Laboratories Ltd.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Haynes, G. (1988b) M&B 45897 acute dermal toxicity study in the rat.
Unpublished report No. A/D/4856 from Toxicol Laboratories Ltd.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Hermansky, S.J. & Wagner, C.L. (1993) M&B 46030: Twenty-one day
repeated cutaneous dose toxicity study in New Zealand white rabbits
#2. Unpublished report No. 92N1165 from Union Carbide Bushy Run
Research Center. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Holmes, P. (1991a) M&B 46030: Toxicity study by dietary administration
to CD rats for 13 weeks. Unpublished report No. 90/RHA 298/0781 from
Life Science Research Ltd. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Holmes, P. (1991b) M&B 46030: Toxicity study by oral (capsule)
administration to beagle dogs for 13 weeks. Unpublished report No.
90/RHA310/0842 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Holmes, P. (1991c) M&B 46030: Neurotoxicity study by oral (capsule)
administration to female beagle dogs for up to 14 days followed by a
28 day reversibility period. Final report. Unpublished report No.
90/RHA371/0790 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Holmes, P. (1992) M&B 46030: Toxicity study by oral (capsule)
administration to beagle dogs for 52 weeks. Unpublished report No.
92/RHA311/0464 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Holmes, P. (1993) M&B 46030: Toxicity study by dietary administration
to beagle dogs for 52 weeks. Unpublished report No. 93/RHA465/0243
from Pharmaco-LSR Ltd. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Hughes, E.W. (1996) MB 46513: Neurotoxicity to rats by acute oral
administration (including a dose range finding study). Unpublished
report No. RNP 471/951489 from Huntingdon Life Sciences. Submitted to
WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Johnson, A.L. (1995) Pyrazole/MB 45897/RPA 097920: An in vitro test
for induction of chromosome damage: Cytogenetic study in cultured
human peripheral lymphocytes. Unpublished report No. 94/RHA534/1034
from Pharmaco-LSR. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Johnson, I.R. (1993) M&B 46030: Delayed contact hypersensitivity study
in guinea pigs. Unpublished report No. 93/RHA503/0167 from
Pharmaco-LSR Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Johnson, I.R. (1995) Pyrazole/MB 45897/RPA 097920: Intermediate of
fipronil (MB 46030): Four week oral toxicity study in the rat.
Unpublished report No. 95/RHA535/0684 from Pharmaco LSR. Submitted to
WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Kennelly, J.C. (1988) Study to determine the ability of M&B 45897/RPA
97920 to induce mutation in four histidine-requiring strains of
Salmonella typhimurium. Unpublished report No. MAB 19/S from
Microtest Research Ltd. Submitted to WHO by Rhone-Poulenc, Research
Triangle Park, NC, USA.
King, V.C. (1990) M&B 46030: Teratology study in the rabbit.
Unpublished report No. 90/RHA 321/0722 from Life Science Research Ltd.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
King, V.C. (1992) M&B 46030: Reproductive performance study in rats
treated continuously through two successive generations. Unpublished
report No. 92/RHA425/0309 from Life Science Research Ltd. Submitted to
WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Liggett, M.P. (1988a) Irritant effects on rabbit skin of M&B 46,030.
Unpublished report No. 881031 D/M&B 292/SE from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Liggett, M.P. (1988b) Irritant effects on the rabbit eye of M&B
46,030. Unpublished report No. 881032D/M&B 293/SE from Huntingdon
Research Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Liggett, M. P. (1988c) Irritant effects on rabbit skin of M&B 46,136.
Unpublished report No. 88833D/M&B 288/SE from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Liggett, M.P. (1988d) Irritant effects on the rabbit eye of M&B
46,136. Unpublished report No. 881022D/M&B 289/SE from Huntingdon
Research Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Lloyd, J.M. (1990) M&B 46030: Investigation of mutagenic activity at
the HGPRT locus in a Chinese hamster V/79 cell mutation system.
Unpublished report No. 90/RHA304/0418 from Life Science Research Ltd.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Mandella, R.C. (1995) A developmental neurotoxicity study of fipronil
in the rat via dietary administration. Unpublished report No. 93-4508
from Pharmaco-LSR/Huntingdon Life Sciences. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Marshall, R.R. (1988a) Study to evaluate the chromosome damaging
potential of M&B-46030 by its effects on cultured human lymphocytes
using an in vitro cytogenetics assay. Unpublished report No. MAB
20/HLC from Microtest Research Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Marshall, R.R. (1988b) Study to evaluate the chromosome damaging
potential of M&B 45950 by its effects on cultured human lymphocytes
using an in-vitro cytogenetics assay. Unpublished report No. MAB
18/HLC from Microtest Research Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Marshall, R.R. (1989) Study to evaluate the chromosome damaging
potential of M&B 46136 by its effects on cultured human lymphocytes
using an in-vitro cytogenetics assay. Unpublished report No. MAB
21/HLC from Microtest Research Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Mondot, S. & Dange, M. (1995) MB 46030: Acute oral LD50 in the mouse.
Unpublished report No. R&D/CRSA/TO-PHA3 from Rhone-Poulenc Agrochimie
Toxicology. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Myers, R.C. & Christopher, S.M. (1992) MB 46030: Acute percutaneous
toxicity study in the rabbit. Unpublished report No. 92N1009 from
Union Carbide Bushy Run Research Center. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Myers, R.C. & Christopher, S.M. (1993a) MB 46030 (technical):
Cutaneous irritancy study in the rabbit. Unpublished report No.
93N1217A from Union Carbide Bushy Run Research Center. Submitted to
WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Myers, R.C. & Christopher, S.M. (1993b) MB 46030 (technical): Ocular
irritancy study in the rabbit. Unpublished report No. 93N1217B from
Union Carbide Bushy Run Research Center. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Nachreiner, D.J. (1995) Fipronil: Acute nose-only dust inhalation
study in rats. Unpublished report No. 94N1501 from Union Carbide Bushy
Run Research Center. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Percy, A. (1993a) RPA 200766 Salmonella typhimurium reverse mutation
assay (Ames test). Unpublished report No. SA 93174 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Percy, A. (1993b) MB 46513 Salmonella typhimurium reverse mutation
assay (Ames test). Unpublished report No. SA 93135 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Percy, A. (1993c) RPA 104615 Salmonella typhimurium reverse mutation
assay (Ames test). Unpublished report No. SA 93175 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Percy, A. (1994a) MB 45950 Salmonella typhimurium reverse mutation
assay (Ames test). Unpublished report No. SA 93305 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Percy, A. (1994b) RPA 105048 Salmonella typhimurium reverse mutation
assay (Ames test). Unpublished report No. SA 94009 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Percy, A. (1996) M&B 45897/RPA 97920 intermediate of fipronil (MB
46030): Salmonella typhimurium reverse mutation assay. Unpublished
report No. SA 95345 from Rhone-Poulenc Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Peters, D.H., Stuart, V., Crook, D., Gibson, W.A., Gopinath, C. &
Hadley, J. (1990) M&B 46,030: Toxicity to rats by dietary
administration for four weeks. Unpublished report No. M&B 327/891321
from Huntingdon Research Centre Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Peters, D.H., Stuart, V., Hall, M., Chasseaud, L.F. & Chanter, D.O.
(1991a) M&B 46,030: An investigation into the potential effects on
thyroid function in male rats by studying thyroxine clearance.
Unpublished report No. M&B 352/90958 from Huntingdon Research Centre
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Peters, D.H., Stuart, V., Hall, M., Chasseaud, L.F. & Chanter, D.O.
(1991b) M&B 46,030: An investigation into the potential effects on
thyroid function in male rats by using the 'perchlorate discharge
test'. Unpublished report No. M&B 353/90920 from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Peters, D.H., Stuart, V., Crook, D., Chanter, D.O., Colman, K.A. &
Gopinath, C. (1991c) M&B 46,030: 4-Week dietary study to investigate
thyroid hormone levels in the rat. Unpublished report No. M&B
360/901275 from Huntingdon Research Centre Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Powles, P. (1992) (14C)-M&B 46,030: Absorption, distribution,
metabolism and excretion in the rat. Unpublished report No.
7040-68/117 from Hazleton UK. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Proudlock, R.J. (1996) MB46513: Mouse micronucleus test. Unpublished
report No. RNP 453/950649 from Huntingdon Life Sciences Ltd. Submitted
to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Ray, D. (1997) Report on the toxicity of fipronil. Unpublished report
from Medical Research Council. Submitted to WHO by MRC Toxicology
Unit, Leicester, United Kingdom.
Smith, K.D. (1990) M&B 46030: Dermal sensitisation study in guinea
pigs. Unpublished report No. 90/RHA357/0602 from Life Science Research
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Stewart, F.P. (1994a) Revised final report: (14C)-M&B 46030:
Absorption, distribution, metabolism and excretion following repeat
oral administration to the dairy goat. Unpublished report No.
HE68/129R-1011 from Hazleton Europe. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Stewart, F.P. (1994b) Revised final report: (14C)-M&B 46030:
Distribution, metabolism and excretion following multiple oral
administration to the laying hen. Unpublished report No.
HE/68/120R-1011 from Hazleton Europe. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Taylor, T. (1993) The effect of single and repeated oral doses of M&B
46030 (1 mg/kg/day and 10 mg/kg/day) on the biliary excretion of
intravenously administered 125I-Thyroine (T4) from bile duct
cannulated rats. Unpublished report No. HRC/ITT 2/930645 from
Huntingdon Research Centre Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Totis, M. (1996) MB 46513: Absorption, distribution, metabolism, and
excretion in the rat (final report). Unpublished report No. SA 95304
from Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Totis, M. & Fisher, P.J. (1994) Fipronil: Tissue kinetic study in the
rat. Unpublished report No. SA94255 from Rhone-Poulenc Agrochimie
Toxicology. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Walters, K.A. & Brain, K.R. (1990) M&B 46030: In vitro skin
permeability of M&B 46030. Unpublished report No. RD 8 from
Pharmaserve Ltd, and An-eX Analytical Services Ltd. Submitted to WHO
by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Wright, N.P. (1995) Fipronil: Chromosomal aberration test in CHL cells
in vitro. Unpublished report No. 282/456 from Safepharm Laboratories
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
at 30 ppm died during the study. The intake of the test compound was
thus not determined for males at 60 ppm.
Males and females at 30 and 60 ppm had statistically significant
decreases in mean body weight and body-weight gain, and mean food
consumption was significantly decreased. The mean values of chemical
parameters did not differ significantly between treated and control
mice. Changes in the weights of the liver, kidney, and thymus in
animals at 30 and 60 ppm were attributed to the decreased terminal
body weights. The only treatment-related change observed
histopathologically was an increased incidence of centrilobular
hypertrophy in mice at 30 and 60 ppm.
Clinical signs of neurotoxicity -- increased motor activity,
excessive jumping, irritability to touch, compulsive biting, and
convulsions -- were observed in males and females at 30 and 60 ppm.
Two females at 3 ppm showed increased motor activity on one occasion,
accompanied by excessive vocalization in one female. None of these
effects occurred in controls. Increased motor activity has been
observed previously as a clinical sign of the toxicity of
fipronil-desulfinyl and of the parent compound fipronil in several
species. Because of the low incidence of this effect and the lack of
other effects at 3 ppm in this study, however, it cannot be concluded
that this effect is of toxicological concern. The NOAEL was 3 ppm,
equal to 0.49 mg/kg bw per day (Dange, 1994b).
Fipronil-desulfinyl (purity, 96%) was administered to groups of
10 male and 10 female OF1 mice in the diet for 90 days at doses of 0,
0.5, 2, or 10 ppm, equal to 0, 0.08, 0.32, or 1.7 mg/kg bw per day for
males and 0, 0.11, 0.43, or 2.2 mg/kg bw per day for females. Standard
evaluations of toxicity ante and post mortem were included.
Histopathological examinations were conducted on all tissues from
controls and animals at the two higher doses and on the liver, lung,
and kidney and tissues in which there were significant macroscopic
findings from mice at the low dose.
Nine males and one females treated at 10 ppm were found dead
during the study. Significant clinical signs included excessive
jumping in two males at 10 ppm and irritability to touch,
aggressivity, and/or increased motor activity in one male at 10 ppm
and two at 2 ppm on two or more occasions. At 0.5 ppm, aggressivity
was reported in one male on one occasion and in one female on three
occasions. None of these effects was reported in control animals.
There were no significant changes in body weight, food consumption, or
haematological or clinical chemical parameters. Gross pathological
examination of dead animals indicated liver enlargement in three males
and small thymuses in four males at 10 ppm. Microscopy revealed
centrilobular hypertrophy of the liver in six males at this dose. The
only female at 10 ppm that died had marked autolysis, which obscured
the histological details. At scheduled sacrifice, gross and
microscopic examinations revealed no treatment-related findings.
The incidence of aggressivity or irritability to touch was low at
2 ppm and there was no dose-response relationship; however, one male
at this dose showed irritability to touch and agressivity on days
70-72, and the other male at this dose with behavioural changes was
irritable to touch, had increased motor activity on day 29, and showed
aggressivity on day 33. Therefore, two animals of the same sex showed
multiple signs on two or three occasions of effects that have been
recognized as clinical signs of toxicity in other studies with
fipronil-desulfinyl and fipronil. In addition, signs of neurotoxicity
were seen in males at the next highest dose and no signs of toxicity
in concurrent controls. Therefore, the behavioural findings in males
at 2 ppm should not be discounted, despite the lack of other
treatment-related findings at this dose. It is easier to discount the
behavioural effects at 0.5 ppm because of their low incidence and the
lack of a dose-response relationship. Therefore, the NOAEL was
0.5 ppm, equal to 0.08 mg/kg bw per day (Bigot, 1996).
Rats
In an exploratory study, fipronil-desulfinyl (purity, 98.6%) was
administered by gavage to five male and five female Sprague-Dawley
rats at doses of 0, 0.3, 1, 3, or 10 mg/kg bw per day for 14 days. All
of the animals at 10 mg/kg bw per day and one female at 3 mg/kg bw per
day either died or were killed in a moribund condition on days 5-8 of
the study. Clinical signs of toxicity, such as piloerection,
chromodacryorrhoea, prostration, excessive reaction to noise,
convulsions, curling up on handling, hunched posture, nasal discharge,
and few faeces, were observed in animals of each sex at 10 mg/kg bw
per day and in females at 3 mg/kg bw per day. Body-weight gain and
food consumption were markedly decreased in animals at 10 mg/kg bw per
day; males and females at 3 mg/kg bw per day showed decreased
body-weight gain over the course of the study, and food consumption
was decreased at the two times evaluated.
Leukocyte counts and total bilirubin were decreased and total
protein was increased in females at 3 mg/kg bw per day; leukocyte
counts were also decreased in females at 1 mg/kg bw per day. Gross
pathological examination showed pale livers in females at 1, 3, or 10
mg/kg bw per day, and spots in the glandular stomach were seen in a
few animals at 3 and 10 mg/kg bw per day that died during the study.
Histological examination showed an increased number of atrophic
follicles in the thyroids of males and females treated at doses > 3
mg/kg bw per day. The NOAEL was 0.3 mg/kg bw per day (Dange, 1994c).
In a preliminary study, fipronil-desulfinyl (purity, 97.5%) was
administered to groups of 10 male and 10 female Sprague-Dawley rats in
the diet at doses of 0, 0.5, 3, 30, or 100 ppm (equal to 0, 0.04,
0.23, 2.2, or 3.7 mg/kg bw per day for males and 0, 0.04, 0.24, 2.3,
or 3.8 mg/kg bw per day for females) for 28 days. Standard parameters
and T3, T4, and TSH were measured before treatment and on day 7 in
non-fasted animals and on day 23 or 24 in fasted animals.
There were no deaths, clinical signs of toxicity, or effects on
body weight or food consumption at 0.5 and 3 ppm. One male at 30 ppm
was found dead on day 6, and all animals at 100 ppm died within the
first two weeks of the study. Clinical signs at 30 and 100 ppm
included piloerection, curling up on handling, and convulsions (in one
female at 100 ppm). Food consumption and body weights were decreased
at the two highest doses and markedly so at 100 ppm. The only change
observed in urinary, standard haematological, and clinical chemical
parameters were a decrease of about 30% in total bilirubin in animals
of each sex at 30 ppm. Statistically significant decreases (relative
to concurrent controls) were found in the levels of T4 (by 33% in
males at 300 ppm, by 63% in males and 50% in females at 100 ppm on day
7; and by 49% in males and 61% in females at 30 ppm on day 23 [no
animals at the high dose were still alive on that day]) and in T3 (by
46% in females at 100 ppm on day 7 and by 40% in males at 30 ppm on
day 23) were observed, but their toxicological significance was not
clear: the findings at 100 ppm may have been related to the extreme
toxicity of the chemical, and individual values in animals at 30 ppm
were reported to be within the normal range for these parameters. TSH
levels were not affected. At necropsy, the thymus weights were
decreased in females at 30 ppm, but no other findings were clearly
related to treatment. The NOAEL was 3 ppm, equal to 0.23 mg/kg bw
(Dange, 1995a).
Fipronil-desulfinyl (purity, at least 97.5%) was administered in
the diet for 90 days to groups of 10 male and 10 female Sprague-Dawley
rats at doses of 0, 0.5, 3, 10, or 30 ppm (equal to 0, 0.029, 0.18,
0.59, or 1.8 mg/kg bw per day for males and 0, 0.035, 0.21, 0.71, or
2.1 mg/kg bw per day for females). In addition to standard ante- and
post-mortem evaluations of toxicity, T3, T4, and TSH were assayed in
non-fasted animals in weeks 2 and 10. During treatment, one male and
three females at 30 ppm died with clinical signs of distress. Signs of
neurotoxicity (aggressivity, irritability to touch, increased motor
activity, and curling up on handling) were seen in animals at 10 and
30 ppm, and excessive vocalization and increased motor activity were
seen in some animals. None of these effects was observed in controls,
in males at 0.5 ppm, or in females at doses > 3 ppm. One male at 3
ppm showed aggressivity, irritability to touch on several occasions,
and excessive vocalization. These signs were seen mainly in weeks 3-5.
Mean body weights were significantly decreased in males and females at
30 ppm and in males at 10 ppm several times during the study. The
overall mean body-weight gain of males was decreased by 15% in those
at 10 ppm and 13% at 30 ppm. Mean weekly food consumption and food
conversion efficiency of males and females at 30 ppm were lower than
those of controls only during the first two weeks of the study. No
treatment-related changes in haematological, urinary or
ophthalmological parameters were seen. Decreases in total bilirubin
(-43%), total cholesterol (-25%), and triglycerides (-24%) in females
at 30 ppm were considered to be related to treatment but not
toxicologically significant since the individual values were within
the normal range for this age and strain of rat. At 30 ppm,
treatment-related decreases in T4 levels were seen in males (-48%,
p < 0.01, at week 2 and -25% at week 10) and females (-29% at week
10), and T3 levels were decreased (-29%, p < 0.05) in males at week
10. The altered hormone levels were reported to be within the normal
range of values for these parameters in this strain of rat. As no
changes were found in the thyroid gland on macroscopic or microscopic
examination, the toxicological significance of the alterations in
hormone levels is questionable. TSH was not affected. There were no
treatment-related macroscopic changes at necropsy, no changes in organ
weights, and no histopathological findings. The NOAEL was 0.5 ppm,
equal to 0.029 mg/kg bw per day, on the basis of clinical signs of
toxicity in one male (Dange, 1994d).
Dogs
In a preliminary study, fipronil-desulfinyl (purity, 97.5%), was
administered in the diet of groups of two male and two female beagle
dogs at doses of 0, 27, 80, or 270 ppm for 28 days. The group mean
doses achieved were reported to be equal to 1 mg/kg bw per day for
males and females at 27 ppm over the course of the study, 1.9 mg/kg bw
per day for males and 1.7 mg/kg bw per day for females at 80 ppm
during week 1, and 2.3 mg/kg bw per day for animals of each sex at 270
ppm during the same period. During the second week of the study,
intake decreased considerably because of toxicity.
Because of poor health, animals at the two higher doses were
killed early, on days 10 and 15 for those at 80 ppm and day 10 for
those at 270 ppm. The signs of toxicity observed at 80 ppm included
reduced motor activity, staggering, irritability, and increased
salivation. Animals at 80 and 270 ppm had no or few faeces,
emaciation, decreased food consumption, and weight loss. All animals
receiving 27 ppm survived, but one male had a clonic convulsion at the
end of the study. The body weights and food consumption of animals in
this group were comparable to those of controls. No remarkable effects
were seen in ophthalmological, haematological, clinical chemical, or
urinary evaluations. Only animals at 27 and 80 ppm were necropsied.
Reduced thymic weight or small thymuses and pale livers were noted in
both groups, and the livers of those at 80 ppm had multifocal whitish
areas or mottling. Although there were no microscopic findings at 27
ppm, marked thymic atrophy and hepatic changes indicative of toxicity
were seen at 80 ppm, including diffuse sinusoidal leukocytosis,
centrilobular hepatocytic enlargement, mild multifocal hepatocytic
hydropic degeneration, and chronic hepatitis with periportal fibrosis.
No NOAEL was identified (Dange, 1995b).
Fipronil-desulfinyl (purity, 96%) was administered to groups of
five beagle dogs of each sex in the diet for 90 days at doses of 0,
3.5, 9.5, or 35 ppm (equivalent to 0, 0.1, 0.27, or 0.95 mg/kg bw per
day for males and 0, 0.1, 0.29, or 1.05 mg/kg bw per day for females).
General health, clinical signs, food consumption, body weights,
haematology and plasma chemistry, and ophthalmologic and urinary
parameters were evaluated. At necropsy, organ weights and macroscopic
changes were monitored; all tissues from controls and animals at the
high dose and kidney, liver, lung, heart, and gall-bladder from those
at the intermediate and low doses were examined histologically.
One female at 35 ppm was killed on day 28 after signs of
increased salivation, prostration, writhing, tremors, absence of
rotular reflex, noisy breathing, and dyspnoea. These clinical signs
were suggested to be due to coronary arteritis and myocardial necrosis
on the basis of microscopic findings; however, they may have indicated
neurotoxicity, as another female at this dose also exhibited excessive
barking and aggressivity on one occasion and irritability, tremors,
and increased salivation on another. There were no significant changes
in body weight, food consumption, or haematological, clinical
chemical, urinary, or ophthalmological parameters. At scheduled
necropsy, no treatment-related changes were observed, and the
microscopic examination revealed no treatment-related findings. The
NOAEL was 9.5 ppm, equal to 0.29 mg/kg bw per day (Dange, 1996).
Reproductive toxicity: Groups of adult female Sprague-Dawley
Crl:CD(SD)BR rats received 0, 0.5, 1, or 2.5 mg/kg bw per day of
fipronil-desulfinyl (purity, 99.2%) suspended in a 0.5% aqueous
solution of methylcellulose by gavage on days 6-15 of gestation. The
study was terminated on gestation day 20. The animals were evaluated
for clinical signs, food consumption, body-weight changes, and
macroscopic changes post mortem. The litter and fetal parameters
evaluated included pre- and postimplantation losses, litter size,
litter and mean fetal weights, sex ratios, and malformations or
skeletal or visceral anomalies. Hair loss on the paws, limbs, flanks,
abdomen, and/or thorax were seen in dams at the high dose, and these
animals had lower body-weight gain on gestation days 6-9 (28% of
control), 9-12 (27%), 6-16 (58%), and 0-20 corrected for gravid
uterine weight (78%). These animals also consumed less food during
treatment, although an increase was seen after treatment, indicating a
rebound effect. Dams at 1 mg/kg bw per day showed a significant
decrease in body-weight gain (80% of control) on days 9-12 of
gestation. No other effects on body weight, body-weight gain at other
intervals, food consumption, or other parameters were seen at the
intermediate dose.
Developmental toxicity in pups at the high dose was reflected in
a slight increase in the fetal and litter incidence of incomplete or
reduced ossification of several bones, including the hyoid body, fifth
and sixth sternebrae, first thoracic body, pubic bone, and one or two
metatarsi. A slight but significant reduction in fetal body weight
(98% of control weight) at the high dose correlated with the slight
delays in ossification. Although the dams showed reduced body-weight
gain, the change in dams at the intermediate dose occurred over a
small interval and was transient. In the absence of other effects at
this dose, the reduced body-weight gain does not appear to be
toxicologically significant. The NOAEL for maternal toxicity was thus
1 mg/kg bw per day; that for for developmental toxicity was 1 mg/kg bw
per day on the basis of a slight increase in the fetal and litter
incidence of delayed ossification of several bones (Foulon, 1997).
Neurotoxicity: Fipronil-desulfinyl (purity, 99.5%) was
administered by gavage as a single dose in corn oil to groups of 12
male and 12 female Sprague-Dawley Crl:CD(SD)BR rats at doses of 0,
0.5, 2, or 12 mg/kg bw. A functional observation battery of tests and
motor activity testing were conducted at 6 h and seven and 14 days
after treatment; 6 h was chosen as the time of 'peak effect' on the
basis of the results of a range-finding study which included
evaluations at 2, 4, 6, and 24 h. Clinical signs, food consumption,
and body weights were monitored. At study termination on day 15, all
animals were perfused in situ. Brains were weighed and measured, and
tissues from the brain, spinal cord, and peripheral nerves were
collected and processed for microscopic examination. Only tissues from
five male and five female controls and animals at the high dose were
examined histopathologically. Since possibly treatment-related axonal
degeneration was seen in lumbar dorsal root fibres and/or the sciatic
nerve of males at the high dose, lumbar dorsal root fibres and the
sciatic nerve in the notch and mid-thigh from the remaining male
controls and rats at the high dose were prepared for microscopic
evaluation. Slides of all tissues from these areas of concern were
then read or re-read in a 'blinded' manner.
Body-weight gain and food consumption were decreased in males and
females at the high dose only during week 1. The behavioural changes
attributed to treatment were significant decreases in hindlimb foot
splay, rectal temperature, and locomotor activity (assessed
quantitatively in the Coulbourn system) in animals of each sex at 12
mg/kg bw 6 h after treatment. Treatment probably affected the righting
reflex in males at the high dose, as it was significantly slowed on
day 14, with a trend at other times, and possibly affected grip
strength in animals of each sex at the high dose at various times.
Changes in other behavioural and motor activity parameters could not
clearly be related to treatment. The groups did not differ with regard
to brain weights. The incidence and number of tissue sites showing
axonal degeneration were increased slightly over those in controls and
in males at the high dose. Since the effect was of minor severity in
both treated and control animals and since the difference between
these animals was not significantly different by Fisher's exact test,
the changes were considered not to be of biological importance. There
were no significant histopathological changes in female rats. The
NOAEL was 2 mg/kg bw (Hughes, 1996).
(iii) M&B 46136: Dermal and ocular irritation
Three male New Zealand white rabbits were given a single 0.5-g
dose of M&B 46136 (purity, 98%) moistened with distilled water as a
topical application for 4 h. The treated areas were observed for
erythema and oedema for four days after treatment. No signs of dermal
irritation were seen under the conditions of the study (Liggett,
1988c).
A dose of 0.1 ml (60 mg) of M&B 46136 (purity, 98%) was instilled
into the lower eyelid of one eye of three male New Zealand white
rabbits. The other eye served as an untreated control. The eyes were
examined for signs of irritation and scored 1 h and one, two, three,
four, and seven days after instillation. One animal had chemosis after
one day. Redness and any minor or residual irritation present had
cleared by day 3 or 4 in all animals. M&B 46136 was thus a slight
ocular irritant under the conditions of the study (Liggett, 1988d).
(iv) RPA 200766: Short-term toxicity
RPA 200766 (purity, 96.2%) was administered in the diet for 28
days to 10 male and 10 female Sprague-Dawley rats at doses of 0, 50,
500, 5000, or 15 000 ppm (equal to 0, 3.8, 38, 390, or 1100 mg/kg bw
per day for males and 0, 4.4, 44, 390, or 1100 mg/kg bw per day for
females). The animals were examined for general health, clinical signs
of toxicity, body weight, food consumption, urinary and
ophthalmological changes, haematological and clinical chemical
parameters, and macroscopic changes at necropsy. Histopathological
examinations were not conducted on animals at 15 000 ppm, because the
dose was found to be too high, inducing excessive body-weight loss.
Microscopic examinations were carried out on all animals that died,
all those at 5000 ppm, and all controls, on the liver, lungs, and
kidneys of animals in all groups except those at 15 000 ppm, and on
the adrenals and thyroid (the target organs), as considered necessary
to establish a no-effect level.
One female at 15 000 died during the study due to an error in
blood collection. No treatment-related deaths were seen in other
groups, and no clinical or ophthalmological alterations were reported.
The body weights of males and females at 5000 and 15 000 ppm were
significantly decreased on days 8-28. The mean body-weight gain over
the course of the study was decreased by 27% in males at 5000 ppm, 61%
in males at 15 000 ppm, 46% in females at 5000 ppm, and 77% in females
at 15 000 ppm. Mean food consumption over the course of the study was
decreased by 11% in males at 5000 ppm, 25% in males at 15 000 ppm, 22%
in females at 5000 ppm, and 33% in females at 15 000 ppm.
Mean haemoglobin concentrations were decreased in males and
females at doses > 500 ppm and mean haematocrit values were
decreased in animals at doses > 5000 ppm, significantly except in
females at 15 000 ppm. Mean corpuscular haemoglobin values were
decreased in males and females at 15 000 ppm and in males at 5000 ppm.
The mean cholesterol levels were significantly increased in animals at
doses > 500 ppm, mean triglyceride values were increased in animals
at 5000 and 15 000 ppm, urea nitrogen was increased in females at 5000
and 15 000 ppm, and creatinine values were increased in males at doses
> 500 ppm. The results of urinalysis showed no changes.
Dose-related increases in absolute and relative liver weights
were seen in males and females at doses > 500 ppm, and the
liver:brain weights were also increased in these groups. Dark livers
were observed in males at > 500 ppm and in females at 5000 and 15
000 ppm. Significantly increased relative adrenal weights and
adrenal:brain weights were seen in all treated males. The group mean
thyroid weights were increased in males at doses > 50 ppm; however,
the increases were not found consistently, and the individual values
were reported to be generally within the expected range for animals of
this age and strain. As there were also no microscopic changes in the
thyroid, these findings are of questionable toxicological
significance, even though the target organ of the parent compound,
fipronil, is the thyroid.
Microscopic examination showed slight-to-moderate, centrilobular
or diffuse hepatocellular hypertrophy in the livers and
slight-to-extramedullary haematopoiesis in the adrenals of males and
females at 5000 ppm. A dose-related increase in the incidence of fine
or coarse vacuolation of the zona fasciculata of the adrenal gland was
observed in males at doses > 50 ppm, with incidences of 0/10 in
controls, 2/10 at the low dose, 5/10 at the intermediate dose, and
10/10 at the high dose. The severity was slight at 50 and 500 ppm and
mild to marked at 5000 ppm. A similar change was seen in seven females
at 5000 ppm, with slight-to-mild severity; the incidence in the female
controls was 0. Increases in the weights of the adrenals and thyroids
and vacuolation of the adrenal zona fasciculata in males at 50 ppm
were considered to be marginal and of questionable toxicological
significance. The NOAEL was 50 ppm, equal to 3.8 mg/kg bw per day, on
the basis of decreased haemoglobin concentration, increased
cholesterol levels, and increased liver weights in animals of each sex
at the next dose (Berthe, 1996).
(v) M&B 45897: Short-term toxicity
M&B 45897 (purity, 99.7%) was administered to five male and five
female CD rats by gavage at doses of 0, 50, 200, or 1000 mg/kg bw per
day in corn oil for 28 days. Standard evaluations of toxicity ante
and post mortem were included. There were no deaths. Salivation was
observed in all animals from day 2 of treatment with 1000 mg/kg bw per
day, from day 3 at 200 mg/kg bw per day, from day 8 in females at 50
mg/kg bw per day, and on days 8-15 in males at this dose. The control
group was not affected. During the second week, animals at the
intermediate and high doses were also hunched and underactive, and all
males and some females at the high dose showed hair loss from day 3
on. Staggering was observed in all animals at the high dose on day 8.
None of these clinical signs was observed at 50 mg/kg bw per day or in
the controls.
Significant deficits in body-weight gain on days 0-17 were seen
in animals of each sex at 1000 mg/kg bw per day, but food consumption
was not affected by treatment. Animals at this dose also showed
decreased haemoglobin concentration, and females had lower erythrocyte
numbers and packed cell volume in comparison with controls. Slightly
higher plasma protein concentrations were seen in animals of each sex
at the high dose, and higher plasma alanine aminotransferase activity
was seen in females. Increased absolute and relative liver weights
were seen in males and females at 1000 mg/kg bw per day. Macroscopic
examination showed no significant findings, but microscopic
examination revealed periacinar hepatic hypertrophy in the livers of
three male rats at 1000 mg/kg bw per day. The only effects considered
to be related to treatment were the slight decreases in erythrocyte
numbers and packed cell volume and the slightly increased alanine
aminotransferase activity in animals at the high dose. Although an
NOAEL of 200 mg/kg bw per day was proposed, the signs observed at the
intermediate and high doses in all animals were indicative of a
toxicological (perhaps neurotoxic) effect of M&B 45897, as they were
not seen in the controls and animals at the low dose. Similar findings
have been reported in studies with the parent compound fipronil and
some of its other metabolites, which have been shown to be neurotoxic.
Although the salivation could be a treatment-related clinical sign, it
could also be viewed as an indication of irritation by the test
compound or a local reaction to treatment, especially since it was
observed more or less consistently at all doses, except in males at
the low dose, in which it occurred only on days 8-15. This was the
only finding at the low dose. The NOAEL was therefore 50 mg/kg bw per
day on the basis of clinical signs of toxicity at the next highest
dose (Johnson, I.R., 1995).
(vi) Genotoxicity
The results of studies on the genotoxicity of fipronil
metabolites are summarized in Table 5.
(vii) Comparison of fipronil and its metabolites
Table 6 presents a comparison of findings for fipronil and its
metabolites: whether or not the chemical has been found (to a major or
minor extent) in or on plants or in animal (rat, goat, or hen) tissues
or products or can form in the environment or on surfaces, indoors or
outdoors, via reduction, oxidation, hydrolysis, or photolysis (see
Figure 3); the acute oral toxicity of each chemical; and the ability
of each metabolite to compete with the ligands 3H-EBOB and 3H-TBPS
for binding to specific sites in the chloride ion channel of the GABA
receptor. Binding is postulated to serve as an indicator of potential
to disrupt the normal functioning of the GABA receptor by interference
with chloride-ion flux and thus for potential toxicity to the central
nervous system.
Table 5. Results of assays for genotoxicity with metabolites of fipronil metabolites
Metabolite End-point Test object Concentration Purity Results Reference
(%)
In vitro
M&B 45950 Reverse mutation S. typhimurium 0-250 µg/plate 98.9 Negativea,b Percy (1994a)
TA98, TA100, in DMSO
TA1535, TA1537
Chromosomal Human 25-100 µg/ml > 99 Negativea,b Marshall
aberration lymphocytes in DMSO (1988b)
M&B 46136 Reverse mutation S. typhimurium 0.32-200 µg/plate 98.7 Negativea,b Clare (1988b)
TA98, TA100, (-S9), 0.8-500 µg/
TA1535, TA1537 plate (+S9), in
DMSO
Chromosomal Human 75-300 µg/ml 98.7 Negativea,b Marshall
aberration lymphocytes in DMSO (1989)
RPA 200766 Reverse mutation S. typhimurium 250-1000 µg/plate > 98 Negativea,b Percy (1993a)
TA98, TA100, (-S9), 50-2500 µg/
TA1535, TA1537, plate (+S9), in
TA1538 DMSO
Fipronil-desulfinyl Reverse mutation S. typhimurium 10-250 µg/plate 98.6 Negativea,b Percy (1993b)
TA98, TA100, in DMSO
TA1535, TA1537,
TA1538
Gene mutation Chinese hamster 5-125 µg/ml (-S9), 99.5 Negativea,b Adams (1996a)
cell line (CHO- 15-625 µg/ml (+S9)
K1-BH4), hprt locus in DMSO
Table 5. Results of assays for genotoxicity with metabolites of fipronil metabolites
Metabolite End-point Test object Concentration Purity Results Reference
(%)
Chromosomal Human lymphocytes 5-30 µg/ml (-S9), 99.5 Negativea,b Adams (1996b)
aberration 5-60 µg/ml (+S9)
in DMSO
RPA 104615 Reverse mutation S. typhimurium 2250-5000 µg/plate 94.7 Negativea,b Percy (1993c)
TA98, TA100, in DMSO
TA1535, TA1537,
TA1538
M&B 45897 Reverse mutation S. typhimurium 12.5-2500 µg/plate 99.7 Negativea,b Percy (1996)
TA98, TA100, (-S9), 25-2500 µg/
TA1535, TA1537 plate (+S9), in
DMSO
Reverse mutation S. typhimurium 4-2500 µg/plate > 99 Negativea,b Kennelly
TA98, TA100, (-S9), except TA 100; (1988)
TA1535, TA1537 8-5000 µg/plate
(-S9), TA100; 8-5000
µg/plate (+S9), all
strains; in DMSO
Chromosomal Human 50-150 µg/ml (-S9), 99.7 Negativea,b Johnson, A.L.
aberration lymphocytes 100-400 µg/ml (+S9), (1995)
in DMSO; 20- or 44-h
harvest times
Polyploidy Human 50-150 µg/ml (-S9), 99.7 Positivea,c Johnson, A.L.
lymphocytes 100-400 µg/ml (+S9), (1995)
in DMSO; 20- or 44-h
harvest times
RPA 105048 Reverse mutation S. typhimurium 250-5000 µg/plate 98.6 Negativea,c Percy (1994b)
TA98, TA100, in DMSO
TA1535, TA1537
Table 5. Results of assays for genotoxicity with metabolites of fipronil metabolites
Metabolite End-point Test object Concentration Purity Results Reference
(%)
In vivo
Fipronil-desulfinyl Micronucleus CD-1 mice 2-16 mg/kg bw in 99.5 Negativeb Proudlock
formation corn oil (1996)
DMSO, dimethyl sulfoxide, S9, 9000 × g supernatant of rat liver homogenate
a With and without metabolic activation
b Appropriate positive controls gave expected positive responses
c Reproducible increases in polyploid cells with metabolic activation
Table 6. Comparisons of fipronil and its metabolites
Compound In plants In animals Potential environmental Photo- Oral LD50 Binding to
metabolite or surface metabolite (mg/kg bw)a 3H-EBOB &
residue or degradate 3H-TBPS sitesb
Fipronil Yes Rat, goat, hen Water, soil, surfaces No 97
M&B 45950 Yes Rat, goat, hen Soil No 83 * (both sites)
RPA 200766 Yes Rat, goat Water, soil, surfaces No > 2000 @@ (both sites)
Fipronil-desulfinyl Yes No Water, soil, surfaces Yes 16 ** (both sites)
M&B 46136 Yes Rat, goat, hen Soil, surfaces No 218 * (both sites)
RPA 104615 Yes No Water, soil, surfaces Yes > 2000 @@ (both sites)
M&B 45897 Yes Rat Not expected No > 2000 @@ (both sites)
Two ring-opened No Rat No No No data No data
metabolites of
M&B 45897
M&B 105320 Yes No Not expected No > 2000 @@ (both sites)
EBOB, 1-[(4-ethyl)phenyl]-4-n-propyl-2,6,7-trioxacicyclo[2.2.2]octane; TBPS, tert-butylbicyclophosphorothionate
a From references given in Tables 1 and 2
b Increased (*) or decreased (@) binding relative to fipronil; ** or @@ greater differences. Based on IC50 values from
competitive binding assays for fipronil and its metabolites with radiolabelled 1 gands (personal communication from
P. Kwiatkowski, Rhone-Poulenc Worldwide Regulatory Affairs, North Carolina, USA)
The results presented in the table and those of similar studies
indicate that most of the metabolites are of similar or lesser
toxicity than fipronil. Generally, this conclusion reflects the data
on binding, except for M&B 46136 which has greater binding affinity
for sites in the rat brain GABA receptor chloride-ion channel but a
higher acute oral LD50 than fipronil in rats. (No short-term studies
of the toxicity of this metabolite are available.)
Fipronil-desulfinyl and M&B 104615 are photolytic products of
fipronil which could potentially form in the environment or on treated
or exposed surfaces during use to control malarial vectors. The LD50
of fipronil-desulfinyl in rats is much lower than that of fipronil,
and it has much greater binding affinity for GABA receptor
chloride-ion channel sites in rat brain. Both are neurotoxic. The
toxicity of fipronil-desulfinyl is qualitatively similar to that of
fipronil, but the dose-effect curve for neurotoxic effects is steeper
for fipronil-desulfinyl and neurotoic effects occur at lower doses.
The acute oral LD50 for M&B 104615 is much higher and the affinity
for GABA receptor chloride-ion channel sites much less than those for
fipronil.
Comments
Fipronil
In a study of dermal absorption in rats, the quantity of
14C-fipronil absorbed was less than 1% of the applied dose at all
doses tested (0.88-48 mg/rat) and all times up to 24 h. In vitro,
the relative extent of absorption of a formulation of 14C-fipronil
across rat, rabbit, or human epidermal membranes depended on the
concentration of the material used. At the lowest concentration tested
(0.2 g/L), the extent of penetration was greatest for all three
species, and the percentage of the dose absorbed across human and rat
membranes was similar. At higher concentrations (4 and 200 g/L),
penetration was greater through rat and rabbit skin than through human
skin.
There was no appreciable difference between male and female rats
in the absorption, distribution, metabolism, or excretion of fipronil
after oral administration. The proportion of the dose absorbed
appeared to depend on the treatment regimen, being greatest with a
single dose of 4 mg/kg bw of 14C-fipronil (minimum absorption, 50%),
intermediate with a repeated dose regimen of 4 mg/kg bw per day for 14
days followed by a single, oral labelled dose of 4 mg/kg bw (minimum
absorption, 40%), and lowest (minimum absorption, about 30%) with a
single dose of 150 mg/kg bw of 14C-fipronil (presumably due to
saturation of absorption at the high dose). Once absorbed, fipronil
was rapidly metabolized, and the residues widely distributed in
tissues. Significant amounts of residues remained in the tissues,
particularly in fat and fatty tissues, one week after treatment. The
levels of residues in fat and other tissues were greater with repeated
low doses or a single high dose than with a single low dose. The long
half-life (150-245 h in some cases) of fipronil in blood may reflect
slow release of residues from fat and might suggest potential
bioaccumulation of metabolic products of fipronil.
Faeces, followed by urine, were the major routes of elimination
of fipronil in rats. Its biotransformation largely involved changes in
the functional groups attached to the pyrazole ring. The compounds
identified in faeces and urine were the parent compound and the
sulfone, the amide derived from the nitrile group, a reduction
product, and a cleavage product of the sulfone and its derivatives
formed by further cleavage. The sulfone was the major metabolite in
fat and tissues.
Fipronil was moderately hazardous to rats (LD50 = 92 mg/kg bw)
and mice (LD50 = 91 mg/kg bw) after oral administration of single
doses and to rats after single exposure by inhalation (LC50
= 0.36 mg/L). After a single dermal exposure, fipronil was relatively
non-hazardous to rats (LD50 > 2000 mg/kg bw) but was moderately
hazardous to rabbits (LD50 = 354 mg/kg bw). In rats, signs of
toxicity and death were delayed for up to four days after either a
single oral dose or repeated oral doses of 75 mg/kg bw per day for up
to five days. WHO has not yet classified fipronil for acute toxicity.
In a 13-week study of toxicity, mice were fed diets containing
fipronil at doses of 0, 1, 3, 10, or 25 ppm. A dose-related increase
in the incidence of liver-cell periacinar hypertrophy with cytoplasmic
vacuolation was observed in males at doses of 1 ppm (equal to 0.13
mg/kg bw per day) and above. There was no NOAEL.
Rats were fed diets containing 0, 25, 50, 100, 200, or 400 ppm
fipronil for four weeks. At 25 ppm (equal to 3.4 mg/kg bw per day),
liver weights and plasma cholesterol levels were increased in females,
and thyroid follicular-cell hypertrophy of minimal severity was
observed in animals of each sex. The levels of total protein and
globulin were also increased in both males and females, although the
changes at this and higher doses were generally small and poorly
correlated with the dose. There was no NOAEL.
In a 13-week study of toxicity, fipronil was administered in the
diet to rats at doses of 0, 1, 5, 30, or 300 ppm. At 30 ppm and above,
relatively small, sometimes inconsistent changes in haematological
parameters (decreased packed cell volume, mean cell volume,
haemoglobin concentration, and prothrombin time and increased platelet
count) and clinical chemical parameters (increased total protein and
globulins, decreased albumin:globulin ratio and alanine
aminotransferase and aspartate aminotransferase activities) were
observed, mostly in females. Some alterations were seen in plasma
glucose and urea concentrations at 30 ppm; also at 30 ppm, the
absolute and/or relative weights of the liver and thyroid were
increased in either males or females or both, and there was evidence
of thyroid follicular-cell epithelial hypertrophy in males. The NOAEL
was 5 ppm, equal to 0.33 mg/kg bw per day.
Fipronil was administered in gelatin capsules to dogs for 13
weeks in a study of toxicity at doses of 0, 0.5, 2, or 10 mg/kg bw per
day. Inappetence and decreased body-weight gain and food consumption
were noted in females at 2 and 10 mg/kg bw per day. The NOAEL was 0.5
mg/kg bw per day.
In a study of dermal toxicity, fipronil was applied in 0.5%
carboxymethylcellulose to the intact skin of rabbits for 6 h per day
on five days per week for three weeks at doses of 0, 0.5, 1, 5, or 10
mg/kg bw per day. No dermal irritation was observed. At 10 mg/kg bw
per day, body-weight gains and food consumption were reduced in
animals of each sex. Some animals showed hyperactivity. The NOAEL was
5 mg/kg bw per day.
Fipronil was administered to dogs in gelatin capsules for one
year in a study of toxicity at doses of 0, 0.2, 2, or 5 mg/kg bw per
day. At 2 mg/kg bw per day and above, clinical signs of neurotoxicity
(convulsions, twitching, tremors, ataxia, unsteady gait, rigidity of
limbs, nervous behaviour, hyper- or hypoactivity, vocalization,
nodding, aggression, resistance to dosing, inappetence, and abnormal
neurological responses) were observed in animals of each sex. One
animal at 2 mg/kg bw per day was killed because of poor condition
related to treatment. The NOAEL was 0.2 mg/kg bw per day. In a second
one-year study in dogs, fipronil was administered in the diet at doses
of 0, 0.075, 0.3, 1, or 3 mg/kg bw per day. The highest dose was
reduced to 2 mg/kg bw per day after 38 days because of toxicity. At 1
mg/kg bw per day, clinical signs of neurotoxicity (whole body
twitching, and extensor rigidity of limbs) were noted in females.
There were no effects on triiodothyronine or thyroxine levels. The
NOAEL was 0.3 mg/kg bw per day.
In a study of carcinogenicity, fipronil was administered for 78
weeks in the diet to mice at doses of 0, 0.1, 0.5, 10, 30, or 60 ppm.
Additional groups of animals were fed the same doses for 52-53 weeks
and then killed. Survival was greater than or comparable to that of
the control group at doses below 60 ppm. At week 10, all surviving
animals at 60 ppm were killed because of excessive mortality. In
animals at 10 ppm, some decrease in body-weight gain was noted in
males and females, and efficiency of food use was decreased in males.
At 53 and 78 weeks, the absolute and/or relative liver weights of
males were increased, with an increased incidence of liver periacinar
microvesicular vacuolation. There was no evidence of carcinogenicity
at doses considered to be sufficient to measure such potential. The
NOAEL for systemic effects was 0.5 ppm, equal to 0.055 mg/kg bw per
day.
In a study of toxicity and carcinogenicity in rats, fipronil was
administered in the diet at doses of 0, 0.5, 1.5, 30, or 300 ppm. For
the carcinogenicity phase of the study, it was originally planned that
the test material be administered for two years, but excessive
mortality resulted in early termination of this phase at week 89 in
males and week 91 in females. This was not thought to compromise the
study. For the toxicity phase and a reversibility phase of the study,
additional groups of animals were fed the same doses of fipronil for
one year, when some animals were killed and others were allowed to
recover for 13 weeks. Some of the effects noted at the higher doses
persisted into the reversibility phase of the study. During treatment,
convulsive episodes (sometimes fatal) were observed in males at 1.5
ppm and in animals of each sex at higher doses. Animals at 1.5 ppm,
predominantly females, showed irritability, vocalization, salivation,
aggression, hyperactivity, and bruxism. Small decreases were noted in
erythrocyte count, haemoglobin concentration, mean cell volume, and
packed cell volume in either males or females or both, and some
alterations in protein level were observed in males. An apparent
increase in the severity of progressive senile nephropathy was seen in
animals of each sex at this dose. Thyroxine concentrations were
decreased in both males and females. Thyroid-stimulating hormone
levels were increased, notably in males, at doses of 30 ppm and above
and in females at 300 ppm. The levels of triiodothyronine were
elevated in females at 30 ppm, but only during the reversibility
phase. At 300 ppm, fipronil induced follicular-cell adenomas of the
thyroid gland in both males and females; males at this dose also had
an increased incidence of follicular-cell carcinomas. Some thyroid
follicular-cell adenomas were noted in male rats at lower doses, but a
comparison with historical control data indicated no clear
relationship to treatment. The NOAEL for systemic effects was 0.5 ppm,
equal to 0.019 mg/kg bw per day.
Fipronil and its metabolites gave negative results in virtually
all tests for genotoxicity. Equivocal results were seen in assays for
cytogenicity in mammalian cells in vitro with fipronil and for
polyploidy (not clastogenicity) in human lymphocytes with a mammalian
metabolite. The weight of evidence indicates that fipronil and its
metabolites are not genotoxic.
The Meeting concluded that the thyroid tumours observed in the
two-year study in rats occurred by a non-genotoxic, threshold
dose-effect mechanism involving continuous stimulation of the thyroid
gland associated with persistently elevated thyroid-stimulating
hormone levels. It was noted that the levels of this hormone were
clearly elevated only at the two highest doses.
In a two-generation study of reproductive toxicity, rats received
diets containing fipronil at 0, 3, 30 or 300 ppm. F0 parental animals
were mated twice to produce F1a and F1b litters; F1a parents were
mated only once to produce F2 litters. In adult animals at 30 ppm,
the thyroid and liver weights were increased and the pituitary gland
weights were decreased. An increased incidence of thyroid gland
follicular epithelial-cell hypertrophy was seen at this dose in males
of the F0 and F1 generations and F1 females. At 300 ppm,
convulsions were observed in F1 and F2 litters; decreased litter
size, decreased body weights and delays in physical development were
also seen. Postnatal survival was decreased among pups in the F2
litters. Absolute and relative ovarian weights were decreased in F0
females. At 300 ppm, a decreased percentage of animals that mated and
a reduction in the fertility index of F1 parental animals was also
observed. These effects may have been related to the systemic toxicity
of fipronil at this dose. The NOAEL for parental systemic toxicity was
3 ppm, equal to 0.25 mg/kg bw per day, and the NOAEL for reproductive
toxicity was 30 ppm, equal to 2.5 mg/kg bw per day.
Rats were given fipronil by gavage at doses of 0, 1, 4, or 20
mg/kg bw per day on days 6-15 of gestation. Developmental toxicity was
not observed, but there were some signs of maternal toxicity
(decreased body-weight gain and food consumption) at 20 mg/kg bw per
day. The NOAEL for maternal toxicity was 4 mg/kg bw per day, and that
for developmental toxicity was 20 mg/kg bw per day, the highest dose
tested.
Rabbits were given fipronil by gavage at doses of 0, 0.1, 0.2,
0.5, or 1 mg/kg bw per day on days 6-19 of gestation. Developmental
toxicity was not observed, but there were some signs of maternal
toxicity (decreased body-weight gain, decreased food consumption, and
reduced efficiency of food use at all doses. There was no NOAEL for
maternal toxicity; the NOAEL for developmental toxicity was 1 mg/kg bw
per day, the highest dose tested.
Primary dermal irritation in rabbits was examined in two studies.
Fipronil was slightly irritating when moistened with corn oil before
application but was not irritating when moistened with water. Fipronil
was slightly irritating in two studies of primary ocular irritation in
rabbits. It did not sensitize the skin of guinea-pigs when tested by
the Buehler method but was a weak sensitizer in guinea-pigs tested by
the Magnusson-Kligman method.
In a study of neurotoxicity, rats were given single doses of 0,
0.5, 5, or 50 mg/kg bw fipronil by gavage. At 5 mg/kg bw, decreased
hind-leg splay was observed 7 h after treatment in both males and
females. The NOAEL was 0.5 mg/kg bw. In a 13-week study of
neurotoxicity, rats received dietary doses of 0, 0.5, 5, or 150 ppm
fipronil. Body weights, weight gains, and food consumption were
reduced early in the study in animals of each sex at 150 ppm, possibly
owing to problems of palatability. Although the findings in a battery
of functional operational tests at this dose were relatively minor
when taken separately, they appeared to represent a minimal effect of
treatment when taken together. The NOAEL for neurotoxicity and
systemic effects was 5 ppm, equal to 0.3 mg/kg bw per day.
In a study of neurotoxicity in female dogs, fipronil was
administered in capsules at doses of 0 (one animal) or 20 mg/kg bw per
day (four animals) until the appearance of neurotoxic signs in each
animal, after which they were allowed to recover for 28 days. Severe
neurotoxic signs were seen at 20 mg/kg bw per day during the treatment
phase and in some animals only during the recovery phase. Most animals
appeared to recover, although one had exaggerated reflex responses and
was excitable at the end of the recovery period. A limited
histopathological examination showed no change. No firm conclusions
could be drawn about the reversibility of the effects, given the
limitations of the study design. There was no NOAEL.
In a study of developmental neurotoxicity, rats were given
fipronil in the diet from gestation day 6 through lactation day 10 at
doses of 0, 0.5, 10, or 200 ppm. Maternal toxicity manifested as
reduced body weight during the treatment period, reduced body-weight
gain during gestation, and reduced food consumption was observed at
200 ppm. Developmental toxicity (reduced body weights in pups and a
slight increase in the time to preputial separation) was noted at 10
ppm. An increase in motor activity in female pups at 10 ppm only on
day 17 could not be definitively interpreted as an indication of
developmental toxicity. Developmental neurotoxicity was clearly
observed postnatally in pups at 200 ppm, with delayed swimming
development on day 6, increased motor activity on day 17, abnormal
auditory startle response on day 22, and impaired learning and memory
on day 24. The NOAEL for maternal toxicity and developmental
neurotoxicity was 10 ppm (equal to 0.9 mg/kg bw per day) and that for
developmental toxicity was 0.5 ppm (equal to 0.05 mg/kg bw per day).
Mechanistic studies conducted with fipronil in rats suggest that
it does not interfere with the incorporation of iodine into thyroxine
but rather with the biliary clearance of this hormone. This may
trigger an increase in the concentration of thyroid-stimulating
hormone by interference with the feedback mechanism.
Mammalian metabolites of fipronil
Several mammalian metabolites of fipronil were tested for acute
toxicity. Their toxicity was comparable to or substantially less than
that of fipronil.
Photodegradation products of fipronil
Numerous studies were performed with fipronil-desulfinyl, one of
two photodegradation products of fipronil which can be formed in the
presence of sunlight and could potentially be produced in the
environment or on treated surfaces. Neither is a mammalian metabolite
of fipronil. The available information indicates that, of the two,
only fipronil-desulfinyl is highly toxic after exposure to single
doses or over the long term, and is therefore of toxicological
concern.
When 0.08-7.2 mg of 14C-fipronil-desulfinyl were applied
dermally to rats, absorption ranged fron 0.2 to 7% of the applied dose
within 24 h.
The absorption, distribution metabolism, and excretion of
14C-fipronil-desulfinyl were studied in rats which received either a
single oral dose of labelled compound at 1 or 10 mg/kg bw or 14 daily
oral doses of unlabelled compound at 1 mg/kg bw per day followed by a
single oral labelled dose. In animals of each sex, elimination of the
radiolabel was much greater in the faeces (46-70% of the dose) than in
the urine with all dosing regimens. Appreciable residues were found in
the tissues one week after treatment, the highest concentrations being
present in the fat and fatty tissues. The long half-life in blood
(183-195 h) and increased fat:plasma ratios of the radiolabel suggest
potential bioaccumulation of fipronil-desulfinyl and/or its
metabolites. Numerous metabolites or conjugates of fipronil-desulfinyl
were present in the urine and faeces. Biotransformation of
fipronil-desulfinyl involved changes at the functional groups attached
to the pyrazolyl ring. Only unchanged fipronil-desulfinyl was
identified in the liver, fat, skin, and residual carcass.
In a 28-day study of toxicity in which fipronil-desulfinyl was
administered in the diet to mice at doses of 0, 0.5, 3, 30, or 60 ppm,
mortality, neurotoxic signs (increased motor activity, excessive
jumping, irritability to touch, compulsive biting, and evidence of
convulsions), decreased body-weight gain and food consumption, and an
increased incidence of centrilobular hypertrophy of the liver were
observed in animals of each sex at doses of 30 ppm and above. The
NOAEL was 3 ppm, equal to 0.49 mg/kg bw per day. Fipronil-desulfinyl
was administered in the diet for 90 days to mice at doses of 0, 0.5,
2, or 10 ppm. At 2 and 10 ppm, clinical signs of neurotoxicity
(irritability to touch, aggressiveness, and/or increased motor
activity) were noted in males. The NOAEL was 0.5 ppm, equal to
0.08 mg/kg bw per day.
Rats received fipronil-desulfinyl by gavage for two weeks at
doses of 0, 0.3, 1, 3, or 10 mg/kg bw per day. At 1 mg/kg bw per day,
pale livers and reduced leukocyte counts were observed in females.
Some rats at 3 mg/kg bw per day died or were killed because of poor
condition. The NOAEL was 0.3 mg/kg bw per day. Fipronil-desulfinyl was
administered in the diet for 28 days to rats at doses of 0, 0.5, 3, 30
or 100 ppm. One male at 30 ppm died, clinical signs of toxicity
(piloerection and curling up on handling), and decreased body weights,
food consumption, and bilirubin concentration were seen in males and
females at this dose. Thymus weights were lowered in females. The
levels of thyroid-stimulating hormone were measured, but no effects
were noted at any dose. All animals at 100 ppm died. The NOAEL was 3
ppm, equal to 0.23 mg/kg bw per day.
In a 90-day study of toxicity in rats, fipronil-desulfinyl was
administered in the diet at 0, 0.5, 3, 10, or 30 ppm. At 3 ppm and
above, clinical signs of neurotoxicity (aggressiveness, irritability
to touch, and excessive vocalization) were observed in males. The
levels of triiodothyronine and thyroxine were affected at higher
doses, but the toxicological significance of these changes is probably
negligible in the absence of changes in the level of
thyroid-stimulating hormone at any dose. The NOAEL in the study was
0.5 ppm, equal to 0.029 mg/kg bw per day.
Dogs received fipronil-desulfinyl in the diet in a 28-day study
at doses of 0, 27, 80, or 270 ppm. The groups at 80 and 270 ppm were
terminated early because of mortality. One male at 27 ppm had a clonic
convulsion. Reduced thymus weights and pale livers were also reported
at this dose. As effects occurred at the lowest dose, there was no
NOAEL.
In a 90-day study of toxicity, fipronil-desulfinyl was
administered in the diet to dogs at doses of 0, 3.5, 9.5, or 35 ppm.
The clinical findings in one female at 35 ppm (increased salivation,
prostration, writhing, tremors, absence of rotular reflex, noisy
breathing, dyspnoea) were attributed to arteritis and myocardial
necrosis on the basis of microscopic findings; however, they may also
have been indicative (at least in part) of neurotoxicity, because
another female in this group exhibited excessive barking,
aggressiveness, irritability, tremors, and increased salivation. On
this basis, the Meeting concluded that the NOAEL was 9.5 ppm, equal to
0.29 mg/kg bw per day.
In a study of developmental toxicity in rats, fipronil-desulfinyl
was administered by gavage on days 6-15 of gestation at doses of 0,
0.5, 1, or 2.5 mg/kg bw per day. Indications of maternal effects
(decreased body-weight gain and hair loss in various areas) were
observed at 2.5 mg/kg bw per day. Developmental toxicity (increased
incidence of incomplete or reduced ossification of several bones and
slightly reduced fetal body weight in animals of each sex) was also
observed at this dose. The NOAEL for maternal toxicity and
developmental toxicity was 1 mg/kg bw per day.
In a study of neurotoxicity in rats, fipronil-desulfinyl was
administered by gavage as a single dose of 0, 0.5, 2, or 12 mg/kg bw.
At 12 mg/kg bw, decreased body-weight gains and food consumption were
observed during week 1 in animals of each sex. Decreased hind-foot
splay, rectal temperature, and locomotor activity were also seen in
animals of each sex at this dose. There were indications of a slowed
righting reflex in males and decreased grip strength in males and
females at the high dose. The NOAEL was 2 mg/kg bw per day.
In summary, the toxicity of fipronil-desulfinyl is qualitatively
similar to that of fipronil, but the dose-effect curve for neurotoxic
effects appears to be steeper for fipronil-desulfinyl than for
fipronil. Also, fipronil-desulfinyl appears to have a much greater
tendency than fipronil to bind to sites in the chloride ion channel of
the rat brain GABA receptor. This finding appears to be consistent
with the greater toxicity, relative to fipronil, of
fipronil-desulfinyl in the central nervous system of mammals.
The Meeting established an ADI of 0-0.0002 mg/kg bw for fipronil
on the basis of the NOAEL of 0.019 mg/kg bw per day in the two-year
study of toxicity and carcinogenicity in rats and incorporating a
safety factor of 100.
The Meeting considered that a separate ADI should be established
for fipronil-desulfinyl on the basis that it could be a significant
residue and that its toxicity is greater than that of the parent
molecule fipronil. A temporary ADI of 0-0.00003 mg/kg bw for
fipronil-desulfinyl was established on the basis of the NOAEL of 0.029
mg/kg bw per day in the 90-day study in rats and a safety factor of
1000, in view of the lack of a long-term study by oral administration
in rats and a study of neurotoxicity in rats given repeated oral
doses.
Toxicological evaluation
Fipronil
Levels that cause no toxic effect
Mouse: 0.5 ppm, equal to 0.055 mg/kg bw per day (78-week study
of carcinogenicity and toxicity)
Rat: 5 ppm, equal to 0.33 mg/kg bw per day (13-week study of
toxicity)
0.5 ppm, equal to 0.019 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
3 ppm, equal to 0.25 mg/kg bw per day (parental
systemic toxicity in a study of reproductive toxicity)
30 ppm, equal to 2.5 mg/kg bw per day (study of
reproductive toxicity)
4 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity by gavage)
20 mg/kg bw per day (developmental toxicity in a study
of developmental toxicity by gavage; highest dose
tested)
0.5 mg/kg bw (single dose, study of neurotoxicity by
gavage)
5 ppm, equal to 0.3 mg/kg bw per day (repeated doses in
the diet, study of neurotoxicity)
10 ppm, equal to 0.9 mg/kg bw per day (maternal
toxicity and developmental neurotoxicity in a study of
developmental neurotoxicity)
0.5 ppm, equal to 0.05 mg/kg bw per day (developmental
toxicity in a study of developmental neurotoxicity)
Rabbit: 0.1 mg/kg bw per day (LOAEL for maternal toxicity in a
study of developmental toxicity by gavage)
1 mg/kg bw per day (study of developmental toxicity;
highest dose tested by gavage)
Dog: 0.3 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.0002 mg/kg bw
Fipronil-desulfinyl (fipronil photodegradation product)
Levels that cause no toxic effect
Mouse: 3 ppm, equal to 0.49 mg/kg bw per day (28-day study of
toxicity)
0.5 ppm, equal to 0.08 mg/kg bw per day (90-day study
of toxicity)
Rat: 0.3 mg/kg bw per day (two week study of toxicity by
gavage)
3 ppm, equal to 0.23 mg/kg bw per day (28-day study of
toxicity)
0.5 ppm, equal to 0.029 mg/kg bw per day (90-day study
of toxicity)
1 mg/kg bw per day (maternal and developmental toxicity
in a study of developmental toxicity by gavage)
2 mg/kg bw per day (single dose, study of neurotoxicity
by gavage)
Dog: 9.5 ppm, equal to 0.29 mg/kg bw per day (90-day study
of toxicity)
Estimate of temporary acceptable daily intake for humans
0-0.00003 mg/kg bw
Acute reference dose for fipronil
The Meeting allocated an acute reference dose of 0.003 mg/kg bw
for both fipronil and fipronil-desulfinyl on the basis of the NOAEL of
0.3 mg/kg bw per day in a study of neurotoxicity in rats given
repeated doses of fipronil, and a safety factor of 100. The study of
neurotoxicity in rats given single doses was not considered in
allocating the acute reference dose because of concern about the
prolonged toxicokinetics of fipronil. This acute reference dose will
provide a safety factor of about 700 for the NOAEL in the study of
neurotoxicity in rats given single doses of fipronil-desulfinyl.
Studies without which the determination of an ADI is impractable,
to be provided by 2000
1. Short-term study of neurotoxicity in rats with
fipronil-desulfinyl in the diet
2. Developmental neurotoxicity study in rats with
fipronil-desulfinyl in the diet
3. The results of an ongoing long-term study with
fipronil-desulfinyl in rats
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to fipronil and its photodegradation
product fipronil-desulfinyl
Human exposure Relevant route, study type, species Results, remarks
Fipronil
Short-term Skin, irritation, rabbit Slightly imitating
(1-7 days) Eye, irritation, rabbit Minor irritation
Skin, sensitization, guinea-pig Not a sensitizer (Buehler)
Skin, sensitization, guinea-pig Mild sensitizer (Magnusson-Kligman)
Oral, toxicity, rat LD50 = 92 mg/kg bw
Dermal, toxicity, rabbit LD50 = 350 mg/kg bw
Inhalation, toxicity, rat LC50 = 0.36 mg/L
Neurotoxicity, rat NOAEL = 0.5 mg/kg bw per day:
(single dose by gavage) decreased hind-leg splay
Medium-term Repeated dermal, 3 weeks, toxicity, rabbit NOAEL = 5 mg/kg bw per day: reduced body-weight
(1-26 weeks) gains and food consumption; hyperactivity in some
animals; no dermal imitation observed
Repeated oral, reproductive toxicity, rat NOAEL = 0.25 mg/kg bw per day for maternal toxicity.
NOAEL = 2.5 mg/kg bw per day for reproductive
toxicity
Repeated oral, developmental neurotoxicity, rat NOAEL = 0.9 mg/kg bw per day for maternal toxicity.
NOAEL = 0.05 mg/kg bw per day for developmental
toxicity
NOAEL = 0.9 mg/kg bw per day for developmental
neurotoxicity
Long-term Repeated oral, 2 years (terminated at 89-91 NOAEL = 0.019 mg/kg bw per day: convulsions and
(> 1 year) weeks), long-term toxicity and carcinogenicity, rat neurobehavioural clinical signs of toxicity; effects on the
thyroid; thyroid follicular-cell adenomas and carcinomas
Fipronil-desulfinyl
Short-term Oral, toxicity, rat LD50 = 15 mg/kg bw
(1-7 days) Dermal, toxicity, rat LD50 > 2000 mg/kg bw
Neurotoxicity, rat NOAEL = 2 mg/kg bw per day
(single dose by gavage)
(continued)
Human exposure Relevant route, study type, species Results, remarks
Medium-term Repeated oral (diet), 90 days, toxicity, rat NOAEL = 0.029 mg/kg bw per day
(1-26 weeks) Repeated oral (gavage), developmental toxicity, NOAEL = 1.0 mg/kg bw per day: maternal toxicity
rat NOAEL = 1.0 mg/kg bw per day: developmental
toxicity
Long-term Repeated oral toxicity No data
> 1 year)
Studies that would provide information useful for the continued
evaluation of fipronil and fipronil-desulfinyl
1. Additional studies to investigate the reversibility of the
neurotoxic effects of fipronil and its metabolites
(functional, behavioural, learning/memory, cellular, and
neurotransmitter/receptor effects).
2. Observations in humans exposed to fipronil and fipronil-
desulfinyl
References
Adams, K. (1996a) MB 46513: CHO mammalian cell mutation assay.
Unpublished report No. RNP452/950622 from Huntingdon Life Sciences,
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Adams, K. (1996b) MB 46513: Metaphase chromosome analysis of human
lymphocytes cultured in vitro. Unpublished report No. RNP 451/951219
from Huntingdon Life Sciences, Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Aughton, P. (1993) MB 46030: Combined oncogenicity and toxicity study
by dietary administration to CD rats for 104 weeks, including a 13
week reversibility period on completion of 52 weeks of treatment.
Unpublished report No. 93/RHA432/0166 from Pharmaco LSR Ltd. Submitted
to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Berthe, P. (1996) RPA 200766: 28-Day toxicity study in the rat by
dietary administration. Unpublished report No. SA 95273 from
Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Bigot, D. (1996) MB 46513: 90-Day toxicity study in the mouse by
dietary administration. Unpublished report No. SA 95055 from
Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Broadmeadow, A. (1991) M&B 46030: Preliminary toxicity study by
dietary administration to CD-1 mice for 13 weeks. Unpublished report
No. 90/RHA364/0860 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Broadmeadow, A. (1993) M&B 46030: Oncogenicity study by dietary
administration to CD-1 mice for 78 weeks. Unpublished report No.
92/RHA313/0971 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Brooker, A.J. & John, D.M. (1991) The effect of M&B 46,030 on
pregnancy of the rat. Unpublished report No. M&B 335+326/90582 from
Huntingdon Research Centre Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Bushey, D.F. (1993) Fipronil mode of action research summary.
Unpublished memo prepared by Rhone-Poulenc Agrochimie Co., Research
Triangle Park Biochemistry Group. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Cheng, T. (1995) Dermal absorption of 14C-fipronil REGENT 80WDG in
male rats (preliminary and definitive phases). Unpublished report No.
HWI 6224-210 from Hazleton Wisconsin, Inc. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Cheng, T. (1996) Dermal absorption of 14C-MB 46513 in male rats
(preliminary and definitive phases). Unpublished report No.
CHW 6224-230 from Corning Hazleton, Inc. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Clare, C.B. (1988a) Study to determine the ability of M&B 46030 to
induce mutation in four histidine-requiring strains of Salmonella
typhimiurim. Unpublished report No. MAB 20/S from Microtest Research
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Clare, C.B. (1988b) Study to determine the ability of M&B 46136 to
induce mutation in four histidine-requiring strains of Salmonella
typhimiurim. Unpublished report No. MAB 21/S from Microtest Research
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Cole, L.M., Nicholson, R.A. & Casida, J.E. (1993) Action of
phenylpyrazole insecticides at the GABA-gated chloride channel.
Pestic. Biochem. Physiol., 46, 47-54.
Cracknell, S. (1991) M&B 46030: Acute inhalation toxicity study in the
rat. Unpublished report No. 90/RHA358/0791 from Life Science Research
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Dange, M. (1993a) RPA 200766: Acute oral LD50 in the rat. Unpublished
report No. SA 93016 from Rhone-Poulenc Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Dange, M. (1993b) MB 46513. Acute oral LD50 in rats. Unpublished
report No. SA 93074 from Rhone-Poulenc Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Dange, M. (1993c) MB 46513: Acute dermal LD50 in the rat. Unpublished
report No. SA 93095 from Rhone-Poulenc Secteur Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Dange, M. (1993d) RPA 104615 acute oral LD50 in the rat. Unpublished
report No. SA 93046 from Rhone-Poulenc Secteur Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC,USA.
Dange, M. (1994a) MB 45950 acute oral LD50 in the rat. Unpublished
report No. SA 93272 from Rhone-Poulenc Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Dange, M. (1994b) MB 46513: Preliminary 28-day toxicity study in the
mouse by dietary administration. Unpublished report No. SA 93228 from
Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Dange, M. (1994c) MB 46513: Exploratory 14-day toxicity study in the
rat by gavage. Unpublished report No. SA 93063 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Dange, M. (1994d) MB 46513 90-day toxicity study in the rat by dietary
administration. Unpublished report No. 93226 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Dange, M. (1995a) MD 46513: Preliminary 28 day toxicity study in the
rat by dietary administration. Unpublished report No. SA 93138 from
Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Dange, M. (1995b) MB 46513: Preliminary 28-day toxicity study in the
dog by dietary administration. Unpublished report No. SA 94143 from
Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Dange, M. (1996) MB 46513: 90-Day toxicity study in the dog by dietary
administration. Unpublished report No. SA 95100 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Driscoll, C.D. and Hurley, J.M. (1993) MB 46030: Ninety day dietary
neurotoxicity study in Sprague Dawley rats. Unpublished report No.
92N1074 from Union Carbide Bushy Run Research Center. Submitted to WHO
by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Edwards, C.N. (1991) M&B 46030: Assessment of clastogenic action on
bone marrow erythrocytes in the micronucleus test. Unpublished report
No. 90/RHA305/1377 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Edwards, C.N. (1995) M&B 46030: Mouse micronucleus test to comply with
OECD Guideline 474 (1983). Unpublished report No. 95/RHA547/0432 from
Pharmaco LSR Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Foulon, O. (1997) MB 46513 (fipronil photometabolite): Developmental
toxicology study in the rat by gavage. Unpublished report No. SA 96227
from Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Gant, D.B., Chalmers, A.E. & Wolff, M.A. (1994) Fipronil: A novel
insecticide acting at the GABA receptor. Poster presented at the
Eighth International Congress of Pesticide Chemistry, Washington DC by
Rhone-Poulenc Agrochimie Co., Department of
Biochemistry/Biotechnology, Research Triangle Park, NC.
Gardner, J.R. (1988a) Acute oral toxicity to rats of M&B 46,030.
Unpublished report No. 881300D/M&B 290/AC from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Gardner, J.R. (1988b) Acute dermal toxicity to rats of M&B 46,030.
Unpublished report No. 881113 D/M&B 291/AC from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Gardner, J.R. (1988c) Acute oral toxicity to rats of M&B 46136.
Unpublished report No. 881364 D/M&B286/AC from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC,USA.
Gardner, J.R. (1988d) Acute dermal toxicity to rats fo M&B 46,136.
Unpublished report No. 88916 D/M&B287/AC from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Gill, M.W., Wagner, C.L. & Driscoll, C.D. (1993) M&B 46030: Single
exposure peroral (gavage) neurotoxicity study in Sprague Dawley rats.
Unpublished report No. 91N0099 from Union Carbide Bushy Run Research
Center. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Haynes, G. (1988a) M&B 45897 acute oral toxicity study in the rat.
Unpublished report No. A/D/4855 from Toxicol Laboratories Ltd.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Haynes, G. (1988b) M&B 45897 acute dermal toxicity study in the rat.
Unpublished report No. A/D/4856 from Toxicol Laboratories Ltd.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Hermansky, S.J. & Wagner, C.L. (1993) M&B 46030: Twenty-one day
repeated cutaneous dose toxicity study in New Zealand white rabbits
#2. Unpublished report No. 92N1165 from Union Carbide Bushy Run
Research Center. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Holmes, P. (1991a) M&B 46030: Toxicity study by dietary administration
to CD rats for 13 weeks. Unpublished report No. 90/RHA 298/0781 from
Life Science Research Ltd. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Holmes, P. (1991b) M&B 46030: Toxicity study by oral (capsule)
administration to beagle dogs for 13 weeks. Unpublished report No.
90/RHA310/0842 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Holmes, P. (1991c) M&B 46030: Neurotoxicity study by oral (capsule)
administration to female beagle dogs for up to 14 days followed by a
28 day reversibility period. Final report. Unpublished report No.
90/RHA371/0790 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Holmes, P. (1992) M&B 46030: Toxicity study by oral (capsule)
administration to beagle dogs for 52 weeks. Unpublished report No.
92/RHA311/0464 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Holmes, P. (1993) M&B 46030: Toxicity study by dietary administration
to beagle dogs for 52 weeks. Unpublished report No. 93/RHA465/0243
from Pharmaco-LSR Ltd. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Hughes, E.W. (1996) MB 46513: Neurotoxicity to rats by acute oral
administration (including a dose range finding study). Unpublished
report No. RNP 471/951489 from Huntingdon Life Sciences. Submitted to
WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Johnson, A.L. (1995) Pyrazole/MB 45897/RPA 097920: An in vitro test
for induction of chromosome damage: Cytogenetic study in cultured
human peripheral lymphocytes. Unpublished report No. 94/RHA534/1034
from Pharmaco-LSR. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Johnson, I.R. (1993) M&B 46030: Delayed contact hypersensitivity study
in guinea pigs. Unpublished report No. 93/RHA503/0167 from
Pharmaco-LSR Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Johnson, I.R. (1995) Pyrazole/MB 45897/RPA 097920: Intermediate of
fipronil (MB 46030): Four week oral toxicity study in the rat.
Unpublished report No. 95/RHA535/0684 from Pharmaco LSR. Submitted to
WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Kennelly, J.C. (1988) Study to determine the ability of M&B 45897/RPA
97920 to induce mutation in four histidine-requiring strains of
Salmonella typhimurium. Unpublished report No. MAB 19/S from
Microtest Research Ltd. Submitted to WHO by Rhone-Poulenc, Research
Triangle Park, NC, USA.
King, V.C. (1990) M&B 46030: Teratology study in the rabbit.
Unpublished report No. 90/RHA 321/0722 from Life Science Research Ltd.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
King, V.C. (1992) M&B 46030: Reproductive performance study in rats
treated continuously through two successive generations. Unpublished
report No. 92/RHA425/0309 from Life Science Research Ltd. Submitted to
WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Liggett, M.P. (1988a) Irritant effects on rabbit skin of M&B 46,030.
Unpublished report No. 881031 D/M&B 292/SE from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Liggett, M.P. (1988b) Irritant effects on the rabbit eye of M&B
46,030. Unpublished report No. 881032D/M&B 293/SE from Huntingdon
Research Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Liggett, M. P. (1988c) Irritant effects on rabbit skin of M&B 46,136.
Unpublished report No. 88833D/M&B 288/SE from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Liggett, M.P. (1988d) Irritant effects on the rabbit eye of M&B
46,136. Unpublished report No. 881022D/M&B 289/SE from Huntingdon
Research Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Lloyd, J.M. (1990) M&B 46030: Investigation of mutagenic activity at
the HGPRT locus in a Chinese hamster V/79 cell mutation system.
Unpublished report No. 90/RHA304/0418 from Life Science Research Ltd.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Mandella, R.C. (1995) A developmental neurotoxicity study of fipronil
in the rat via dietary administration. Unpublished report No. 93-4508
from Pharmaco-LSR/Huntingdon Life Sciences. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Marshall, R.R. (1988a) Study to evaluate the chromosome damaging
potential of M&B-46030 by its effects on cultured human lymphocytes
using an in vitro cytogenetics assay. Unpublished report No. MAB
20/HLC from Microtest Research Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Marshall, R.R. (1988b) Study to evaluate the chromosome damaging
potential of M&B 45950 by its effects on cultured human lymphocytes
using an in-vitro cytogenetics assay. Unpublished report No. MAB
18/HLC from Microtest Research Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Marshall, R.R. (1989) Study to evaluate the chromosome damaging
potential of M&B 46136 by its effects on cultured human lymphocytes
using an in-vitro cytogenetics assay. Unpublished report No. MAB
21/HLC from Microtest Research Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Mondot, S. & Dange, M. (1995) MB 46030: Acute oral LD50 in the mouse.
Unpublished report No. R&D/CRSA/TO-PHA3 from Rhone-Poulenc Agrochimie
Toxicology. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Myers, R.C. & Christopher, S.M. (1992) MB 46030: Acute percutaneous
toxicity study in the rabbit. Unpublished report No. 92N1009 from
Union Carbide Bushy Run Research Center. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Myers, R.C. & Christopher, S.M. (1993a) MB 46030 (technical):
Cutaneous irritancy study in the rabbit. Unpublished report No.
93N1217A from Union Carbide Bushy Run Research Center. Submitted to
WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Myers, R.C. & Christopher, S.M. (1993b) MB 46030 (technical): Ocular
irritancy study in the rabbit. Unpublished report No. 93N1217B from
Union Carbide Bushy Run Research Center. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Nachreiner, D.J. (1995) Fipronil: Acute nose-only dust inhalation
study in rats. Unpublished report No. 94N1501 from Union Carbide Bushy
Run Research Center. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Percy, A. (1993a) RPA 200766 Salmonella typhimurium reverse mutation
assay (Ames test). Unpublished report No. SA 93174 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Percy, A. (1993b) MB 46513 Salmonella typhimurium reverse mutation
assay (Ames test). Unpublished report No. SA 93135 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Percy, A. (1993c) RPA 104615 Salmonella typhimurium reverse mutation
assay (Ames test). Unpublished report No. SA 93175 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Percy, A. (1994a) MB 45950 Salmonella typhimurium reverse mutation
assay (Ames test). Unpublished report No. SA 93305 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Percy, A. (1994b) RPA 105048 Salmonella typhimurium reverse mutation
assay (Ames test). Unpublished report No. SA 94009 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Percy, A. (1996) M&B 45897/RPA 97920 intermediate of fipronil (MB
46030): Salmonella typhimurium reverse mutation assay. Unpublished
report No. SA 95345 from Rhone-Poulenc Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Peters, D.H., Stuart, V., Crook, D., Gibson, W.A., Gopinath, C. &
Hadley, J. (1990) M&B 46,030: Toxicity to rats by dietary
administration for four weeks. Unpublished report No. M&B 327/891321
from Huntingdon Research Centre Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Peters, D.H., Stuart, V., Hall, M., Chasseaud, L.F. & Chanter, D.O.
(1991a) M&B 46,030: An investigation into the potential effects on
thyroid function in male rats by studying thyroxine clearance.
Unpublished report No. M&B 352/90958 from Huntingdon Research Centre
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Peters, D.H., Stuart, V., Hall, M., Chasseaud, L.F. & Chanter, D.O.
(1991b) M&B 46,030: An investigation into the potential effects on
thyroid function in male rats by using the 'perchlorate discharge
test'. Unpublished report No. M&B 353/90920 from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Peters, D.H., Stuart, V., Crook, D., Chanter, D.O., Colman, K.A. &
Gopinath, C. (1991c) M&B 46,030: 4-Week dietary study to investigate
thyroid hormone levels in the rat. Unpublished report No. M&B
360/901275 from Huntingdon Research Centre Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Powles, P. (1992) (14C)-M&B 46,030: Absorption, distribution,
metabolism and excretion in the rat. Unpublished report No.
7040-68/117 from Hazleton UK. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Proudlock, R.J. (1996) MB46513: Mouse micronucleus test. Unpublished
report No. RNP 453/950649 from Huntingdon Life Sciences Ltd. Submitted
to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Ray, D. (1997) Report on the toxicity of fipronil. Unpublished report
from Medical Research Council. Submitted to WHO by MRC Toxicology
Unit, Leicester, United Kingdom.
Smith, K.D. (1990) M&B 46030: Dermal sensitisation study in guinea
pigs. Unpublished report No. 90/RHA357/0602 from Life Science Research
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Stewart, F.P. (1994a) Revised final report: (14C)-M&B 46030:
Absorption, distribution, metabolism and excretion following repeat
oral administration to the dairy goat. Unpublished report No.
HE68/129R-1011 from Hazleton Europe. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Stewart, F.P. (1994b) Revised final report: (14C)-M&B 46030:
Distribution, metabolism and excretion following multiple oral
administration to the laying hen. Unpublished report No.
HE/68/120R-1011 from Hazleton Europe. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Taylor, T. (1993) The effect of single and repeated oral doses of M&B
46030 (1 mg/kg/day and 10 mg/kg/day) on the biliary excretion of
intravenously administered 125I-Thyroine (T4) from bile duct
cannulated rats. Unpublished report No. HRC/ITT 2/930645 from
Huntingdon Research Centre Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Totis, M. (1996) MB 46513: Absorption, distribution, metabolism, and
excretion in the rat (final report). Unpublished report No. SA 95304
from Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Totis, M. & Fisher, P.J. (1994) Fipronil: Tissue kinetic study in the
rat. Unpublished report No. SA94255 from Rhone-Poulenc Agrochimie
Toxicology. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Walters, K.A. & Brain, K.R. (1990) M&B 46030: In vitro skin
permeability of M&B 46030. Unpublished report No. RD 8 from
Pharmaserve Ltd, and An-eX Analytical Services Ltd. Submitted to WHO
by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Wright, N.P. (1995) Fipronil: Chromosomal aberration test in CHL cells
in vitro. Unpublished report No. 282/456 from Safepharm Laboratories
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
See Also:
Toxicological Abbreviations
Fipronil (ICSC)
Fipronil (JMPR Evaluations 2000 Part II Toxicological)
 Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Rats
In a study of the dermal absorption of 14C-fipronil, a
formulation containing 79% fipronil as a suspension in 1% aqueous
carboymethylcellulose was applied to the shaven backs of groups of 24
male Crl:CD BR rats at doses of 0.876 mg/rat (0.07 mg/cm2), 8.35
mg/rat (0.67 mg/cm2), or 48.5 mg/rat (3.9 mg/cm2); two control
animals were treated with 1% carboxymethylcellulose alone. The amounts
of radiolabel absorbed through the skin and left in or on the skin
after washing were determined 0.5, 1, 2, 4, 10, and 24 h after
treatment in four rats in each group: 1.1-2.5% of the applied dose was
found on washed skin after the low dose, 0.6-3.3% after the
intermediate dose, and 0.35-0.8% after the high dose. At all doses and
times up to 24 h, the quantity of 14C-fipronil absorbed was less than
1% of the applied dose, measured as radiolabel recovered in blood,
carcass, cage wash and wipe, urine, and faeces. The percent of the
dose absorbed appeared to decrease with increasing dose, and
absorption was saturated at the highest dose (Cheng, 1995).
The kinetics of fipronil in blood were studied in groups of five
male and five female Charles River CD rats that received a single oral
dose of [14C-phenyl]-fipronil at a dose of 4 or 40 mg/kg bw. Blood
from the tail vein was sampled 0.5, 1, 2, 3, 4, 6, 8, and 24 h after
treatment and at 24-h intervals thereafter up to two weeks. Tissue
distribution was studied in six groups of three males and three
females that received a single oral dose of 4 or 40 mg/kg bw
[14C-phenyl]-fipronil; after treatment, one group at each dose was
killed at times corresponding to one-half the time of maximum
radiolabel in blood after treatment (Tmax; absorption phase), Tmax,
and one-half the Tmax (elimination phase). Up to 20 tissues were
analysed at each time, including fat, gonads, liver, kidney, brain,
adrenals, and thyroid gland. No significant differences between the
sexes were seen in blood kinetics at either dose. At 4 mg/kg bw, the
blood levels reached a maximum at a mean of 5.5 h after treatment and
decreased thereafter, with elimination half-lives of 183 h in males
and 245 h in females. At 40 mg/kg, absorption was slower, with a mean
Tmax in blood of 36 h after treatment; blood radiolabel levels
decreased thereafter with elimination half-lives of 135 h in males and
171 h in females. The author noted that these relatively long
half-lives reflected the slow release of radiolabel from a compartment
such as fat.
Radiolabel was widely distributed in the tissues, with a
predominance in fatty tissues. Aside from the stomach and
gastrointestinal tract, the highest levels of radiolabel were seen
consistently in fat and the adrenals. Intermediate values were seen in
liver, pancreas, thyroid, and ovaries; lower values were seen in
muscle, brain, heart, and cardiac blood. At 38 h after treatment
(Tmax), the tissue concentrations (in fipronil equivalents/g of
tissue) in females at the high dose were: fat, 200 ppm; adrenals, 47
ppm; liver, 32 ppm; pancreas, 32 ppm; thyroid, 16 ppm; ovaries, 44
ppm; cardiac blood, 5 ppm. One week after treatment, the tissue
concentrations were: fat, 39 ppm; adrenals, 14 ppm; thyroid, 13 ppm;
cardiac blood, 0.92 ppm; and all other tissues, < 7.2 ppm. Similar
results were seen in females at the low dose. At 6.2 h after treatment
(Tmax), the tissue concentrations were: fat, 31 ppm; adrenals, 10
ppm; liver, 8 ppm; pancreas, 5 ppm; thyroid, 4 ppm; ovaries, 6 ppm;
cardiac blood, 0.6 ppm. High levels of radiolabel were observed in the
stomach and its contents in rats at the low dose only at the initial
sampling time (one-half Tmax), whereas these values were elevated in
rats at the high dose at the Tmax and the one-half Tmax. The levels
of radiolabel in the stomach and contents were considered to indicate
saturation of the absorption process at the high dose. Metabolites
were not identified in these studies (Totis & Fisher, 1994).
Comparison of humans, rabbits, and rats
Absorption of 14C-fipronil through epidermal membranes of
humans, rabbits, and rats was measured in vitro in horizontal glass
diffusion cells. Rat and rabbit skin was obtained from the dorsal and
flank regions of the animals; female human abdominal skin was obtained
at autopsy and the epidermal membrane separated from the rest of the
tissue. The epidermal membranes were set up as a barrier between the
two halves of the diffusion cells, and the absorption rates of a neat
suspension of fipronil (200 g/L) as a formulation in EP60145A (a
formulation base) and of two aqueous dilutions of the formulation
containing 0.2 and 4 g/L of fipronil suspended in EP 60145A were
determined, together with the absorption rates of testosterone and
hydrocortisone (both at 4 g/L) in an aqueous dilution of EP60145A.
Fipronil at doses of 4 and 200 g/L penetrated rabbit and rat
epidermal membranes to a greater extent than those of humans, whereas
at 0.2 g/L the extent of penetration was similar through human and rat
skin. The extent of penetration increased with time across species.
The percent of the applied dose that had penetrated the different
membranes after 8 h was 0.08% through rat epidermal membranes, 0.07%
through rabbit membranes, and 0.01% through human membranes for the
neat formulation; 0.14, 0.67, and 0.07% of the dose of 4.0 g/L active
ingredient; and 0.9, 13.9, and 0.9% of the dose of 0.2 g/L active
ingredient, respectively. At the dose of 4.0 g/L, fipronil penetrated
the skin of all three species more slowly than either testosterone or
hydrocortisone. These two reference permeants were selected because
their intrinsic rates of dermal penetration differ by two orders of
magnitude, that of testosterone being faster. On the basis of the
results for these two compounds, fipronil was considered to be a slow
penetrant when applied as a formulation in EP 60145A (Walters & Brain,
1990).
(b) Biotransformation
Rats
In a study designed to evaluate the absorption, distribution,
metabolism, excretion, and pharmacokinetics of fipronil in rats,
14C-fipronil (labelled uniformly at the phenyl ring; radiochemical
purity, > 97%) was administered orally in aqueous
carboxymethylcellulose (0.5% w/v) containing Tween 80 (0.01% w/v) to
groups of five male and five female Crl:CD(SD) BR rats as a single
dose of 4 mg/kg bw, in a repeated regimen of unlabelled fipronil for
14 days followed by a single dose of labelled material or as a single
dose of 150 mg/kg bw. In all dosing regimens, radiolabel was
determined in urine and faeces (expired air was found in pilot studies
to be an insignificant route of elimination) collected at various
intervals up to one week after treatment; at the end of the study,
radiolabel was also assayed in the carcass and in selected tissues.
Metabolites of fipronil were analysed in urine, faeces, fat, liver,
kidney, muscle, and uterus and were identified by high-performance
liquid chromatography (HPLC) with reference standards and mass
spectrometry. For the study of pharmacokinetics, groups of five male
and five female rats were given a single oral dose of 14C-fipronil at
either 4 or 150 mg/kg bw; whole-blood samples were obtained from the
lateral tail vein at various intervals up to one week after treatment,
and the concentration of radiolabel in the blood samples determined.
Males and females did not differ appreciably in the absorption,
distribution, metabolism, or elimination of 14C-fipronil after either
a single low or high dose or after pretreatment with unlabelled
compound. Urinary excretion and tissue residues indicated that the
proportion of the dose absorbed depended on treatment, being greatest
after a single dose of 4 mg/kg bw and lowest after the single dose of
150 mg/kg bw, presumably due to saturation of absorption at the high
dose. Urinary excretion and tissue residues also indicated that at
least 50% of the administered dose was absorbed after administration
of the single low dose, 40% after the repeated dose regimen, and about
30% after the single high dose. Once absorbed, the parent compound was
rapidly metabolized. Significant amounts of residual radiolabel were
found in abdominal fat (highest concentration), carcass, adrenal
gland, pancreas, skin, liver, kidney, muscles, ovary, and uterus one
week after treatment in all rats. Repeated treatment with the low dose
or single treatment with a high dose resulted in an overall decrease
in the amount of residual radiolabel in comparison with the single low
dose, but in an increase in the amounts in abdominal fat, carcass, and
adrenals. Faeces appeared to be the main route of excretion for
fipronil-derived radiolabel, accounting for 45-75% of the administered
dose; 5-25% was excreted in urine. The percentages excreted in urine
and faeces increased with repeated low oral treatment or a single high
dose, while the percentage found in all tissues combined decreased.
Several metabolites were identified in urine, faeces, and tissues
of treated rats. The pattern of metabolites found was independent of
sex, dose, and treatment regimen, and only the quantity of each
metabolite varied. The main metabolites in urine after deconjugation
(see Figure 2) included the sulfone (M&B 46136) and the amide (RPA
200766) of fipronil, M&B 45897 (a cleavage product of M&B 46136) and
two of its ring-opened products, and a reduction product of fipronil
(M&B 45950). No parent compound was observed in urine before enzymatic
deconjugation of the urine samples. In faeces, parent compound was
detected as a significant fraction of the sample, with M&B 46136, M&B
45950, and some RPA 200766. The main metabolite in tissues, found in
fat (highest concentration), liver, kidney, muscle, and probably
uterus, was the lipophilic M&B 46136. Participation of biliary
excretion in the disposition of fipronil was inferred on the basis of
the presence of metabolites in faeces but was not demonstrated.
Pharmacokinetic investigations showed that the whole-blood
half-life at the single low dose was 149-200 h in male and female
rats; the 0-168-h values for the area under the concentration-time
curve were approximately equal in the two sexes. The prolonged
half-life of radiolabel might suggest bioaccumulation of the metabolic
products of fipronil. At the single high dose, the whole-blood
half-life was noticeably decreased, to 54 h in males and 51 h in
females. The report suggested that the apparently shorter half-life is
in fact the distribution phase half-life and that the true half-life
at the high dose was not fully defined because of the protracted
absorption phase. The area under the concentration-time curve for
blood at the high dose was approximately proportional to the increase
in dose. The results suggest that the bioavailability of fipronil is
similar for each sex and is proportional to dose. Figure 2 presents
the proposed metabolic pathway for the fate of 14C-fipronil in
animals, including rats (Powles, 1992).
Goats
In a study of the absorption, distribution, metabolism, and
excretion of fipronil in ruminants, [phenyl(U)-14C]-fipronil
(19.2 mCi/mmol) was administered orally by capsule twice daily before
feeding to three lactating goats at a dose of 0.05, 2, or 10 ppm for
seven days; assuming a daily intake of 2.0 kg dry matter, these doses
are approximately equivalent to nominal daily doses of 0.1, 4, and 20
mg, respectively. Milk was collected twice daily. The animals were
killed about 24 h after administration of the final dose and tissues
obtained for analysis.
The recovery of radiolabel in urine, milk, and tissues indicated
that the minimum absorption of test material was about 19% at 0.05
ppm, 33% at 2 ppm, and 15% at 10 ppm. Of the administered radiolabel,
18-64% was recovered in faeces, 1-5% in the milk, and 8-25% in the
tissues. Total recovery was similar at the low (83%) and high doses
(77%) but was somewhat lower at the intermediate dose (50%). The
greatest contributor to the difference in recovery between the animals
at the low and high doses and those at the intermediate dose was the
amount of radiolabel excreted in the faeces: 18% of the total
radiolabel administered at 2 ppm, 64% at 0.05 ppm, and 61% at 10 ppm.
The reason for this difference is not clear. The greatest total tissue
residues were observed in omental and renal fat (about 1.9 ppm at the
10 ppm dose), followed by liver (0.86 ppm) and much lower
concentrations in kidney, milk (0.17 ppm), and skeletal muscle.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Rats
In a study of the dermal absorption of 14C-fipronil, a
formulation containing 79% fipronil as a suspension in 1% aqueous
carboymethylcellulose was applied to the shaven backs of groups of 24
male Crl:CD BR rats at doses of 0.876 mg/rat (0.07 mg/cm2), 8.35
mg/rat (0.67 mg/cm2), or 48.5 mg/rat (3.9 mg/cm2); two control
animals were treated with 1% carboxymethylcellulose alone. The amounts
of radiolabel absorbed through the skin and left in or on the skin
after washing were determined 0.5, 1, 2, 4, 10, and 24 h after
treatment in four rats in each group: 1.1-2.5% of the applied dose was
found on washed skin after the low dose, 0.6-3.3% after the
intermediate dose, and 0.35-0.8% after the high dose. At all doses and
times up to 24 h, the quantity of 14C-fipronil absorbed was less than
1% of the applied dose, measured as radiolabel recovered in blood,
carcass, cage wash and wipe, urine, and faeces. The percent of the
dose absorbed appeared to decrease with increasing dose, and
absorption was saturated at the highest dose (Cheng, 1995).
The kinetics of fipronil in blood were studied in groups of five
male and five female Charles River CD rats that received a single oral
dose of [14C-phenyl]-fipronil at a dose of 4 or 40 mg/kg bw. Blood
from the tail vein was sampled 0.5, 1, 2, 3, 4, 6, 8, and 24 h after
treatment and at 24-h intervals thereafter up to two weeks. Tissue
distribution was studied in six groups of three males and three
females that received a single oral dose of 4 or 40 mg/kg bw
[14C-phenyl]-fipronil; after treatment, one group at each dose was
killed at times corresponding to one-half the time of maximum
radiolabel in blood after treatment (Tmax; absorption phase), Tmax,
and one-half the Tmax (elimination phase). Up to 20 tissues were
analysed at each time, including fat, gonads, liver, kidney, brain,
adrenals, and thyroid gland. No significant differences between the
sexes were seen in blood kinetics at either dose. At 4 mg/kg bw, the
blood levels reached a maximum at a mean of 5.5 h after treatment and
decreased thereafter, with elimination half-lives of 183 h in males
and 245 h in females. At 40 mg/kg, absorption was slower, with a mean
Tmax in blood of 36 h after treatment; blood radiolabel levels
decreased thereafter with elimination half-lives of 135 h in males and
171 h in females. The author noted that these relatively long
half-lives reflected the slow release of radiolabel from a compartment
such as fat.
Radiolabel was widely distributed in the tissues, with a
predominance in fatty tissues. Aside from the stomach and
gastrointestinal tract, the highest levels of radiolabel were seen
consistently in fat and the adrenals. Intermediate values were seen in
liver, pancreas, thyroid, and ovaries; lower values were seen in
muscle, brain, heart, and cardiac blood. At 38 h after treatment
(Tmax), the tissue concentrations (in fipronil equivalents/g of
tissue) in females at the high dose were: fat, 200 ppm; adrenals, 47
ppm; liver, 32 ppm; pancreas, 32 ppm; thyroid, 16 ppm; ovaries, 44
ppm; cardiac blood, 5 ppm. One week after treatment, the tissue
concentrations were: fat, 39 ppm; adrenals, 14 ppm; thyroid, 13 ppm;
cardiac blood, 0.92 ppm; and all other tissues, < 7.2 ppm. Similar
results were seen in females at the low dose. At 6.2 h after treatment
(Tmax), the tissue concentrations were: fat, 31 ppm; adrenals, 10
ppm; liver, 8 ppm; pancreas, 5 ppm; thyroid, 4 ppm; ovaries, 6 ppm;
cardiac blood, 0.6 ppm. High levels of radiolabel were observed in the
stomach and its contents in rats at the low dose only at the initial
sampling time (one-half Tmax), whereas these values were elevated in
rats at the high dose at the Tmax and the one-half Tmax. The levels
of radiolabel in the stomach and contents were considered to indicate
saturation of the absorption process at the high dose. Metabolites
were not identified in these studies (Totis & Fisher, 1994).
Comparison of humans, rabbits, and rats
Absorption of 14C-fipronil through epidermal membranes of
humans, rabbits, and rats was measured in vitro in horizontal glass
diffusion cells. Rat and rabbit skin was obtained from the dorsal and
flank regions of the animals; female human abdominal skin was obtained
at autopsy and the epidermal membrane separated from the rest of the
tissue. The epidermal membranes were set up as a barrier between the
two halves of the diffusion cells, and the absorption rates of a neat
suspension of fipronil (200 g/L) as a formulation in EP60145A (a
formulation base) and of two aqueous dilutions of the formulation
containing 0.2 and 4 g/L of fipronil suspended in EP 60145A were
determined, together with the absorption rates of testosterone and
hydrocortisone (both at 4 g/L) in an aqueous dilution of EP60145A.
Fipronil at doses of 4 and 200 g/L penetrated rabbit and rat
epidermal membranes to a greater extent than those of humans, whereas
at 0.2 g/L the extent of penetration was similar through human and rat
skin. The extent of penetration increased with time across species.
The percent of the applied dose that had penetrated the different
membranes after 8 h was 0.08% through rat epidermal membranes, 0.07%
through rabbit membranes, and 0.01% through human membranes for the
neat formulation; 0.14, 0.67, and 0.07% of the dose of 4.0 g/L active
ingredient; and 0.9, 13.9, and 0.9% of the dose of 0.2 g/L active
ingredient, respectively. At the dose of 4.0 g/L, fipronil penetrated
the skin of all three species more slowly than either testosterone or
hydrocortisone. These two reference permeants were selected because
their intrinsic rates of dermal penetration differ by two orders of
magnitude, that of testosterone being faster. On the basis of the
results for these two compounds, fipronil was considered to be a slow
penetrant when applied as a formulation in EP 60145A (Walters & Brain,
1990).
(b) Biotransformation
Rats
In a study designed to evaluate the absorption, distribution,
metabolism, excretion, and pharmacokinetics of fipronil in rats,
14C-fipronil (labelled uniformly at the phenyl ring; radiochemical
purity, > 97%) was administered orally in aqueous
carboxymethylcellulose (0.5% w/v) containing Tween 80 (0.01% w/v) to
groups of five male and five female Crl:CD(SD) BR rats as a single
dose of 4 mg/kg bw, in a repeated regimen of unlabelled fipronil for
14 days followed by a single dose of labelled material or as a single
dose of 150 mg/kg bw. In all dosing regimens, radiolabel was
determined in urine and faeces (expired air was found in pilot studies
to be an insignificant route of elimination) collected at various
intervals up to one week after treatment; at the end of the study,
radiolabel was also assayed in the carcass and in selected tissues.
Metabolites of fipronil were analysed in urine, faeces, fat, liver,
kidney, muscle, and uterus and were identified by high-performance
liquid chromatography (HPLC) with reference standards and mass
spectrometry. For the study of pharmacokinetics, groups of five male
and five female rats were given a single oral dose of 14C-fipronil at
either 4 or 150 mg/kg bw; whole-blood samples were obtained from the
lateral tail vein at various intervals up to one week after treatment,
and the concentration of radiolabel in the blood samples determined.
Males and females did not differ appreciably in the absorption,
distribution, metabolism, or elimination of 14C-fipronil after either
a single low or high dose or after pretreatment with unlabelled
compound. Urinary excretion and tissue residues indicated that the
proportion of the dose absorbed depended on treatment, being greatest
after a single dose of 4 mg/kg bw and lowest after the single dose of
150 mg/kg bw, presumably due to saturation of absorption at the high
dose. Urinary excretion and tissue residues also indicated that at
least 50% of the administered dose was absorbed after administration
of the single low dose, 40% after the repeated dose regimen, and about
30% after the single high dose. Once absorbed, the parent compound was
rapidly metabolized. Significant amounts of residual radiolabel were
found in abdominal fat (highest concentration), carcass, adrenal
gland, pancreas, skin, liver, kidney, muscles, ovary, and uterus one
week after treatment in all rats. Repeated treatment with the low dose
or single treatment with a high dose resulted in an overall decrease
in the amount of residual radiolabel in comparison with the single low
dose, but in an increase in the amounts in abdominal fat, carcass, and
adrenals. Faeces appeared to be the main route of excretion for
fipronil-derived radiolabel, accounting for 45-75% of the administered
dose; 5-25% was excreted in urine. The percentages excreted in urine
and faeces increased with repeated low oral treatment or a single high
dose, while the percentage found in all tissues combined decreased.
Several metabolites were identified in urine, faeces, and tissues
of treated rats. The pattern of metabolites found was independent of
sex, dose, and treatment regimen, and only the quantity of each
metabolite varied. The main metabolites in urine after deconjugation
(see Figure 2) included the sulfone (M&B 46136) and the amide (RPA
200766) of fipronil, M&B 45897 (a cleavage product of M&B 46136) and
two of its ring-opened products, and a reduction product of fipronil
(M&B 45950). No parent compound was observed in urine before enzymatic
deconjugation of the urine samples. In faeces, parent compound was
detected as a significant fraction of the sample, with M&B 46136, M&B
45950, and some RPA 200766. The main metabolite in tissues, found in
fat (highest concentration), liver, kidney, muscle, and probably
uterus, was the lipophilic M&B 46136. Participation of biliary
excretion in the disposition of fipronil was inferred on the basis of
the presence of metabolites in faeces but was not demonstrated.
Pharmacokinetic investigations showed that the whole-blood
half-life at the single low dose was 149-200 h in male and female
rats; the 0-168-h values for the area under the concentration-time
curve were approximately equal in the two sexes. The prolonged
half-life of radiolabel might suggest bioaccumulation of the metabolic
products of fipronil. At the single high dose, the whole-blood
half-life was noticeably decreased, to 54 h in males and 51 h in
females. The report suggested that the apparently shorter half-life is
in fact the distribution phase half-life and that the true half-life
at the high dose was not fully defined because of the protracted
absorption phase. The area under the concentration-time curve for
blood at the high dose was approximately proportional to the increase
in dose. The results suggest that the bioavailability of fipronil is
similar for each sex and is proportional to dose. Figure 2 presents
the proposed metabolic pathway for the fate of 14C-fipronil in
animals, including rats (Powles, 1992).
Goats
In a study of the absorption, distribution, metabolism, and
excretion of fipronil in ruminants, [phenyl(U)-14C]-fipronil
(19.2 mCi/mmol) was administered orally by capsule twice daily before
feeding to three lactating goats at a dose of 0.05, 2, or 10 ppm for
seven days; assuming a daily intake of 2.0 kg dry matter, these doses
are approximately equivalent to nominal daily doses of 0.1, 4, and 20
mg, respectively. Milk was collected twice daily. The animals were
killed about 24 h after administration of the final dose and tissues
obtained for analysis.
The recovery of radiolabel in urine, milk, and tissues indicated
that the minimum absorption of test material was about 19% at 0.05
ppm, 33% at 2 ppm, and 15% at 10 ppm. Of the administered radiolabel,
18-64% was recovered in faeces, 1-5% in the milk, and 8-25% in the
tissues. Total recovery was similar at the low (83%) and high doses
(77%) but was somewhat lower at the intermediate dose (50%). The
greatest contributor to the difference in recovery between the animals
at the low and high doses and those at the intermediate dose was the
amount of radiolabel excreted in the faeces: 18% of the total
radiolabel administered at 2 ppm, 64% at 0.05 ppm, and 61% at 10 ppm.
The reason for this difference is not clear. The greatest total tissue
residues were observed in omental and renal fat (about 1.9 ppm at the
10 ppm dose), followed by liver (0.86 ppm) and much lower
concentrations in kidney, milk (0.17 ppm), and skeletal muscle.
 Metabolites were isolated from various tissues and milk and
identified by HPLC and mass spectrometry. After administration of 10
ppm, the major metabolite in faeces was M&B 46136, with lesser amounts
of fipronil, RPA 200766, and M&B 45950. In fat, milk, and muscle,
fipronil was predominant, with lesser amounts of RPA 200766 (in muscle
and fat), M&B46136, and M&B 45950. In kidney, M&B 46136 predominated,
with lesser amounts of fipronil; while in liver, M&B 46136 was the
major metabolite, with lesser amount of fipronil and RPA 200766. In
all cases, the major metabolite or species represented 44-75% of the
total radiolabelled residues. In urine, the mass of fipronil-derived
material was low, but M&B 46136 was found in small quantities. The
results were similar at 2 ppm, except that more fipronil was
metabolized to M&B 46136 in milk, muscle, and fat. According to the
proposed metabolic pathway for fipronil in ruminants, the sulfoxide
group of fipronil is oxidized to the sulfone (M&B 46136), which is
conjugated and excreted in the urine; the parent sulfoxide group can
also be reduced to the sulfide (M&B 45950), and the nitrile group of
the parent can be hydrolysed to the amide RPA 200766 (see Figure 2)
(Stewart, 1994a).
Laying hens
[phenyl(U)-14C]-Fipronil (19.2 mCi/mmol) was administered orally
in capsules daily before feeding to groups of five laying hens at
doses of 0.05, 2, or 10 ppm for 28 days; assuming a daily intake of
150 g dry matter, these doses were equivalent to nominal daily intakes
of 0.0075, 0.3, and 1.5 mg, respectively. Eggs were collected twice
daily. The animals were killed about 24 h after administration of the
final dose, and tissues were obtained.
Of the administered radiolabel, 28-42% was recovered in excreta,
15-18% in eggs, and 1-5% in tissues; the total recovered was 52-58%.
The greatest total tissue residues after administration of the highest
dose were found in peritoneal fat (56 ppm). The levels in eggs were
also high (30 ppm in yolks) after this dose and had not plateaued by
the end of the study. The levels were lower in skin (17 ppm) and much
lower in liver, egg white, and muscle. Metabolites were isolated from
various tissues and eggs and identified by HPLC and mass spectrometry.
After the 10 ppm dose, the main metabolite in peritoneal fat, egg
yolk, skin, and liver was M&B 46136, representing 96-98% of the total
radiolabelled residues. The remainder of the residue in these tissues
was parent fipronil. In egg white and muscle, M&B 46136 was the only
component of the residue. In excreta, parent fipronil comprised 51% of
the residue, and M&B 46136 accounted for 34%. The results with 0.05
and 2 ppm were similar. According to the proposed metabolic pathway
for fipronil in poultry, the sulfoxide group of the fipronil is
oxidized to the sulfone M&B46136 (see Figure 2) (Stewart, 1994b).
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of fipronil is summarized in Table 1. When
fipronil was administered as a single dose to mice or rats orally or
by inhalation, deaths and signs of toxicity occurred at all or most
doses in animals of each sex. Most or all of the deaths occurred
within several days of treatment. Clinical signs were generally noted
within 24 h of treatment and included tremors and convulsions of
various types, effects on activity or gait, hunched posture, wetness
in various body areas, and seizures (Gardner, 1988a; Cracknell, 1991;
Mondot & Dange, 1995; Nachreiner, 1995).
In studies with female Fischer 344 rats, the oral LD50 of
technical-grade fipronil (purity unspecified) dissolved in
glycerinformal was 175 mg/kg bw. The clinical signs of toxicity did
not reach their peak until two days after treatment in some animals,
and deaths did not occur until four days after treatment. Some signs
of toxicity and body-weight loss were still evident when the
observation period ended at day 7 after treatment. Since these
findings suggested that bioaccumulation of the test material could
occur, a five-day study with cumulative treatment was performed in
which groups of four female Fischer 344 were given fipronil at 75
mg/kg bw per day (one-half the minimum lethal dose determined in the
previous studies) orally for up to five days. Clinical signs of
neurotoxicity were seen after administration of two doses, and three
of four rats died after administration of three or four doses. In the
only rat that survived the study, abnormal behavioural responses
persisted until six days after administration of the final dose, at
which time it had regained most of its pretreatment weight (Ray,
1997).
The dermal LD50 for fipronil applied in distilled water to rats
was > 2000 mg/kg bw in both males and females, while that in rabbits
for test material moistened with corn oil was 354 mg/kg bw for the two
sexes combined. Neither clinical signs of toxicity nor deaths were
seen in rats. In rabbits, fipronil induced deaths and one or more
clinical signs of toxicity including convulsions, sluggishness,
salivation, spasms, tremors, hyperactivity, diarrhoea, emaciation, and
perioral and perinasal red discolouration in all groups except that at
the lowest dose (100 mg/kg bw). Delays in the appearance of signs of
toxicity and death were noted at all doses except the lowest. In
particular, convulsions were not observed until days 3-9 after
treatment, and some animals did not die until days 11-14 (Gardner,
1988b; Myers & Christopher, 1992).
Table 1. Acute toxicity of fipronil
Species Strain Sex Route LD50 or LC50 Reference
(mg/kg bw or
mg/L)
Mousea OF1 M Oralb 98 Mondot & Dange
F 91 (1995)
Rata Crl:CD (SD) BR M Oralc 92 Gardner (1988a)
F 103
Rata Crl:CD (SD BR M Dermald > 2000 Gardner (1988b)
F > 2000
Rat SD albino M,F Inhalation 0.68 Cracknell (1991)
(4-h exposure,
snout only)e
Rat SD albino M Inhalation 0.36 Nachreiner (1995 )
F (4-h exposure, 0.42
nose only)f
Rabbita New Zealand white M Dermalg 445 Myers & Christopher
F 354 (1992)
a Technical-grade fipronil; purity, 93-96.7%
b Administered in aqueous Tween 80 (0.2% w/v)
c Administered in corn oil
d Applied as a 90% w/v concentration in distilled water
e Manufacture-grade dry material; purity, 95.4%; not milled to reduce particle size; mass median
equivalent aerodynamic diameter (stated to be equivalent to mass median aerodynamic diameter),
6.4-8.5 µm
f Milled to meet US Environmental Protection Agency particle size requirements; mass median aerodynamic
diameter, < 2 µm
g Moistened with corn oil before application
(b) Short-term toxicity
Mice
In a preliminary study, groups of 12 male and 12 female CD-1 mice
were fed diets containing technical-grade fipronil (purity, 95.4%) at
levels of 0, 1, 3, 10, or 25 ppm (equal to 0, 0.13, 0.38, 1.3, or 3.2
mg/kg bw per day for males and 0, 0.17, 0.57, 1.7, or 4.5 mg/kg bw per
day for females) for 13 weeks. No clinical chemistry or haematological
measurements were conducted.
There were no deaths, clinical signs of toxicity, or effects on
food consumption. At 25 ppm, the body-weight gain of females was
statistically significantly decreased over the 13-week period to 63%
that of controls; in males, body-weight gains were decreased to 78% of
the control values, but the results were not significant. At necropsy,
there were no treatment-related macroscopic changes. The liver:body
weight ratio was statistically significantly increased in males (by
33%) and females (by 13%) at the high dose. Histopathological
examination revealed a dose-related increase in the incidence of
liver-cell periacinar hypertrophy with cytoplasmic vacuolation in
males (0/12 in controls, 2/12 at 1 ppm, 3/12 at 3 ppm, 6/12 at 10 ppm,
and 10/12 at 25 ppm), which was significant at 10 and 25 ppm.
Additionally, focal necrosis was observed in the liver of one male rat
at the high dose. In females, fatty vacuolation of the liver was
observed in two rats at 10 ppm and one at 25 ppm. There were no
reported effects on the thyroid. No NOAEL was identified because of
the histopathological changes in the livers of males at the lowest
dose (Broadmeadow, 1991).
Rats
Technical-grade fipronil (purity, 93%) was administered in the
diet for four weeks to groups of five Crl:CD (SD) BR rats of each sex
at concentrations of 0, 25, 50, 100, 200, or 400 ppm, equal to 0, 3.4,
6.9, 13, 24, or 45 mg/kg bw per day for males and 0, 3.5, 6.7, 13, 25,
or 55 mg/kg bw per day for females. Although there were no clinical
signs of toxicity, one female at 400 ppm died, however with no
accompanying clinical or pathological findings. Body-weight loss or
decreased body-weight gain seen in animals of each sex at doses >
100 ppm was temporary and possibly due to unpalatability, since food
consumption was also decreased in these groups. The platelet counts of
animals at 200 and 400 ppmwere marginally increased. The results of
urinalysis were negative. Increased total protein and globulin were
seen in all treated animals, and these increases were statistically
significant; however, they were small in comparison with the values in
controls and were poorly correlated with dose. Cholesterol levels were
increased in females at all doses and in males at the high dose.
The target organs were the liver and thyroid. Liver weights were
significantly increased in females at all doses and in males at 200
and 400 ppm. At necropsy, liver enlargement was observed in one or
both sexes starting at 50 ppm, and five males and three females at 400
ppm had enlarged livers. Generalized hepatocyte enlargement was
observed microscopically in one male at 100 ppm, with increasing
incidence in animals of each sex at 200 and 400 ppm. Thyroid
follicular-cell hypertrophy, generally of minimal severity but of
moderate severity in several males at 200 and 400 ppm, was found in
almost all treated animals but not in the controls. No NOAEL was
identified because of changes in blood chemistry in one or both sexes,
increased liver weights in females, and thyroid follicular-cell
hypertrophy in animals of each sex at the lowest dose (Peters et al.,
1990).
Technical-grade fipronil (purity, 95.4%) was administered for 13
weeks in the diet to groups of 10 male and 10 female CD rats at
concentrations of 0, 1, 5, 30, or 300 ppm, equal to 0, 0.07, 0.33,
1.9, or 20 mg/kg bw per day for males and 0, 0.07, 0.37, 2.3, or 24
mg/kg bw per day for females. Standard determinations of toxicity were
made ante and post mortem, and ophthalmological and neurological
examinations were conducted at week 12 on controls and animals at the
high dose. Haematological and clinical chemical evaluations were
performed after week 12.
There were no deaths. A clonic convulsion in one male at the high
dose may have been related to treatment. The ophthalmological and
neurological examinations showed no changes. The body-weight gain and
food consumption of animals at the high dose were decreased during the
first week of the study; by the end of the study, food consumption in
animals of each sex and total body-weight gain in males at 300 ppm
were comparable to those in controls but the total body-weight gain of
females was decreased by 9%. Statistically significant, but generally
minor alterations in comparison with controls were seen in numerous
haematological parameters in females at the high dose (and to a lesser
extent at 30 ppm) and appeared to be related to treatment. The
findings included lower packed cell volume, mean corpuscular volume,
haemoglobin concentration (also in males at the high dose and females
at 30 ppm), and prothrombin time (also in females at 30 ppm) and a
higher platelet count. Overall, minor and sometimes inconsistent
alterations were seen in a number of parameters of blood chemistry,
particularly in animals at 300 ppm and to a lesser extent at 30 ppm
(mostly in females); these were considered to be related to treatment.
The findings included slight but statistically significant increases
in total protein and a1-, a2-, and b-globulins, accompanied by
decreased albumin:globulin ratios at 300 ppm and increases in total
protein and one or more globulins at 30 ppm. At both doses, decreased
alanine and aspartate aminotransferases activities and increased
glucose were seen in females and increased urea in males. Animals at 1
and 5 ppm also showed fluctuations in proteins. These perturbations
may have been related to treatment but were not associated with other
significant findings.
The thyroid and liver were the target organs. The following
statistically significant changes in organ weights were observed at 13
weeks: absolute thyroid weights were increased in males and females at
300 ppm and in females at 30 ppm, and relative thyroid weights were
increased in animals of each sex at 300 ppm; absolute liver weights
were increased in males at 300 ppm and in females at doses > 5 ppm,
and relative liver weights were increased in animals of each sex at 30
and 300 ppm. The results of gross examination were unremarkable.
Histopathologically, a significant increase in the incidence of
hypertrophy of the follicular epithelium of the thyroid was seen in
females at the high dose; a nonsignificant increase was also observed
in males at this dose and to a lesser extent at 30 ppm. The incidence
of follicular-cell hyperplasia was nonsignificantly increased in
animals of each sex at the high dose. Liver sections stained with
haematoxylin and eosin from males and females at the high dose showed
a low incidence of panacinar fatty vacuolation; the incidence in males
at the high dose was more pronounced when Oil-Red-O staining was used.
The changes seen at the two lowest doses -- minor changes in
blood chemistry at 1 and 5 ppm in animals of each sex and increased
absolute (but not relative) liver weight in females at 5 ppm -- did
not appear to be toxicologically significant. At 300 ppm, both
haematological and further blood chemical parameters were altered,
absolute and relative liver weights were increased (p < 0.01) in
females, and significant increases were observed in absolute thyroid
weights in females and relative liver weights in males. Although not
significant, an increased incidence of hypertrophy of thyroid
follicular epithelium that was part of an increasing trend with the
higher dose was observed in males. When these changes are considered
together as part of a continuum towards more severe pathological
effects in the thyroid and liver and in the absence of tests for
thyroid function, the NOAEL was 5 ppm, equal to 0.33 mg/kg bw per day
(Holmes, 1991a).
Rabbits
Technical-grade fipronil (purity 96.7%) was applied in a 0.5%
aqueous solution of carboxymethylcellulose to the intact skin of
groups of six male and six female New Zealand white rabbits at doses
of 0, 0.5, 1, 5, or 10 mg/kg bw per day for 6 h per day for 15 days
within a three-week period. All animals survived. Effects were
observed only in animals at the high dose. Body-weight gains and food
consumption were reduced in animals of each sex over the course of the
study. One male and one female at the high dose showed signs of
extreme hyperactivity near the end of the study, which was possibly
related to treatment. No changes were seen in haematological or
clinical chemical parameters, organ weights, or on gross or
histopathological examination. No skin irritation was observed. The
NOAEL for systemic effects was 5 mg/kg bw per day (Hermansky & Wagner,
1993).
Dogs
Technical-grade fipronil (purity, 95.4%) was administered in
gelatin capsules to groups of four male and four female beagle dogs at
doses of 0, 0.5, 2, or 10 mg/kg bw per day for 13 weeks. Standard
determinations of toxicity were made ante and post mortem.
Neurological examination or testing of cranial nerve reflexes and
nerves, segmental reflexes, postural reactions, and general
observations of behaviour, gait, stance, and the presence of tremor or
other dyskinesia were conducted before treatment and on all surviving
animals after 6 and 12 weeks of treatment.
Mean body-weight gain over the course of the study was reduced by
up to 17% in females at the intermediate and high doses, and mean food
consumption was decreased by up to 9% in animals of each sex at the
high dose and in females at 2 ppm. Some animals at the high dose were
offered meat supplements, diets moistened with water, or an extension
of the feeding period in order to encourage eating. Mean body weight
and food consumption appeared to have recovered by the end of the
study. The results of ophthalmological, urinary, and haematological
examinations were unremarkable.
In animals at 10 mg/kg bw per day, significant clinical signs of
toxicity were seen, which were more prominent during the first two to
three weeks of treatment. These included inappetence, emaciation,
underactivity, weight loss, and hunched posture. Deterioration
progressed in some animals such that one male and three females at the
high dose had to be killed during the second week of treatment. Other
signs in animals at the high dose included dehydration, hypothermia,
subdued behaviour, excessive salivation, irregular heart rate,
convulsions, head nodding, tremors, limb jerk and extension, ataxia,
muscle twitching, abnormal reflexes, and apparent lack of vision. Some
of the last signs in particular were considered indicative of effects
on the central nervous system. The occurrence and frequency of signs
tended to diminish in surviving males after week 4 and in the
surviving female after week 7, although inappetence was seen in this
animal as late as week 12. The only clinical sign of toxicity observed
at 2 mg/kg bw per day was inappetence in two females; no signs were
observed in dogs at 0.5 mg/kg bw per day. Neurological effects were
seen only in animals at the high dose. One male showed head nodding,
facial twitching, and exaggerated blink and gag responses at week 6,
and one female had a depressed tactile placing response at week 12. At
weeks 6 and 12, alkaline phosphatase activity was increased and
cholesterol levels decreased by about 20% in males at the high dose.
The mean absolute and relative organ weights were not affected. No
effects were observed macroscopically, and the only microscopic
findings were follicular and parafollicular atrophy of the mesenteric
lymph nodes and cortical atrophy of the thymus in one male and one
female that were killed during the study; these were considered to be
related to stress. The NOAEL was 0.5 mg/kg bw per day (Holmes, 1991b).
Technical-grade fipronil (purity, 96.8%) was administered in
gelatin capsules to groups of six male and six female beagle dogs at
doses of 0, 0.2, 2, or 5 mg/kg bw per day for one year. For the first
15 days, the chemical was weighed directly into the capsules, but for
the remainder of the study an admixture of fipronil and lactose was
prepared in order to increase the accuracy of the dose. Standard
evaluations of toxicity ante and post mortem were included. In
addition, the brains and spinal cords of one or two animals in each
group that were still alive at the end of the study were examined
after fixation by perfusion with a 4% formaldehyde-saline solution.
Neurological examination or testing of cranial nerve reflexes and
nerves, segmental reflexes, and postural reactions and general
observations of behaviour, gait, and stance and the presence of tremor
or other dyskinesia were conducted before treatment and on all
surviving animals after 12, 24, 38, and 50 weeks of treatment. After
24 and 38 weeks, animals at the high dose were tested for their
proprioceptive positioning reaction ('knuckle' and 'foot sliding'
tests).
Clinical signs, many associated with neurotoxicity, were observed
in most animals at the intermediate dose and all those at the high
dose starting from the second week of treatment. These included
convulsions, twitching and tremors of various muscle beds (frequently
involving the head, pinnae, shoulder, hindlimbs, and sometimes the
whole body), ataxia, unsteady gait and rigidity of limbs (often the
hindlimbs), nervous behaviour, over- or underactivity, vocalization,
head nodding, aggression, resistance to treatment, and inappetence.
One male at 2 mg/kg bw per day and two at 5.0 mg/kg bw per day had to
be killed at weeks 11, 31, and 34, respectively, owing to
treatment-related poor condition. The signs observed in these animals
were similar to those described above and also included weight loss,
apparent loss of vision, and altered respiration. One female at 0.2
mg/kg bw per day showed signs of overactivity in weeks 13-18,
including pacing about the cage, which resulted in lesions on the foot
pad and tail, followed by weight loss and a period of underactivity. A
last incidence of overactivity was reported at about week 36;
adjustments to the diet and cage were used to control the
overactivity. The lack of similar findings in other animals at the low
dose and the overactivity of one control female for several days
around week 41 argued against a treatment-related effect.
The findings in animals at the intermediate and high doses in
routine physical examinations included tenseness, nervous and
excitable behaviour, abnormal stiffness or positioning of hindlimbs,
twitching of facial muscles, and hyperaesthesia. Most animals at the
high dose had exaggerated gag, corneal, blink, and hopping reflexes
and abnormal results in the 'foot sliding' test, and two females at
the intermediate dose showed tenseness. Body-weight gain over the
course of the study was decreased by about 16% only in females at the
high dose, due to effects in only one animal. Overall food consumption
was not affected. All animals were given an additional 200 g/day of
additional basal diet during weeks 16-18. Only minor changes were seen
in haematological and clinical chemical parameters, including slight
increases in packed cell volume, haemoglobin concentration, and
erythrocyte levels and in alanine aminotransferase activity in animals
at the intermediate or high dose, and were not clearly related to
treatment. The results of urinalysis and ophthalmological examinations
were negative. There were no clear-cut effects on organ weights and no
macroscopic or histopathological findings that appeared to be related
to treatment. The only remarkable finding in animals at 0.2 mg/kg bw
per day was overactivity in one female, described above. The NOAEL was
0.2 mg/kg bw per day (Holmes, 1992).
Technical-grade fipronil (purity, 95.4%) was administered in the
diet to groups of five male and five female beagle dogs at doses of 0,
0.075, 0.3, 1, or 3 mg/kg bw day for one year. The diets were given in
two aliquots 3.5-4.5 h apart and were moistened with water before
administration from day 30 onwards to enhance palatability. After the
first 38 days, the dose of 3 mg/kg bw per day was reduced to 2 mg/kg
bw per day because of significant toxicity. Standard evaluations of
toxicity ante and post mortem were included. Neurological
examination or testing of cranial nerve reflexes and nerves, segmental
reflexes, postural reactions, and general observations of behaviour,
gait, and stance, and for the presence of tremor or other dyskinesia
were conducted before treatment and on all surviving animals after 12,
24, 37, and 50 weeks of treatment. Blood samples taken after fasting,
before treatment and after one and 13 weeks of treatment, were
analysed for triiodothyronine (T3) and thyroxine (T4). Plasma from
fasted animals was analysed at weeks 1, 13, 24, 38, and 50 for the
presence of fipronil and a major metabolite, M&B 46136.
There were no overall effects on body-weight gain or food
consumption. The results of routine physical and ophthalmological
examinations and urinalysis were negative. None of the findings in
neurological, haematological, or clinical chemical tests, organ weight
measurements, or macro- or microscopic evaluations could be
definitively attributed to treatment. There were no treatment-related
findings in the animals at 0.3 or 0.075 mg/kg bw per day.
One female at 3 mg/kg bw per day had to be killed on day 32
because of poor health and signs of neurological disturbance. The
clinical signs of toxicity in this animal, which began on day 10,
included convulsions, underactivity, prostration, slow respiration and
tremors. Neurological examination revealed the absence of visual
placing reactions, depressed menace and startle reactions, and
abnormal gait. A blood sample showed increased packed cell volume,
haemoglobin concentration, erythrocyte count, plasma alkaline
phosphatase activity, and total protein and cholesterol
concentrations. Clinical signs of toxicity noted as early as week 1 in
three male and one female survivors at the high dose included
convulsions, head nodding, extensor rigidity, and twitching or tremors
of various muscle beds. One female at 1 mg/kg bw per day showed signs
of toxicity at week 13 (whole-body twitching) and another at week 20
(limb extensor rigidity). After 13 weeks of treatment, the T3 and T4
values were similar in all groups, including controls, the values for
T3 being 0.51-0.63 ng/ml and those for T4, 0.59-0.73 ng/ml.
Dose-related increases in the concentrations of fipronil and
metabolite M&B 46136 were seen in plasma at all measurement times.
Although there were no apparent differences between males and females
in the concentrations of these two compounds, the serum concentrations
of the metabolite were much higher than those of the parent compound
at doses > 0.075 mg/kg bw per day. At the lowest dose, the levels of
parent and metabolite were near or slightly higher than the limit of
quantification. There did not seem to be accumulation of either
chemical over time, and the concentrations of both compounds remained
fairly constant throughout the study. The NOAEL was 0.3 mg/kg bw per
day (Holmes, 1993).
(c) Long-term toxicity and carcinogenicity
Mice
Technical-grade fipronil (purity 95.4%) was administered for 78
weeks in the diet to groups of 52 male and 52 female CD-1 mice at
doses of 0, 0.1, 0.5, 10, 30, or 60 ppm (equal to group mean doses of
0, 0.011, 0.055, 1.2, or 3.4 mg/kg bw per day for males and 0, 0.012,
0.063, 1.2, or 3.6 mg/kg bw per day for females; the doses in mg/kg bw
per day were not determined for the group at 60 ppm) to evaluate
carcinogenicity. Six additional groups of 20 male and 20 female mice
were treated at the same doses for one year to measure toxicity.
Clinical signs of toxicity, body weights and weight gain, food
consumption, food efficiency, haematological parameters, organ weights
(absolute and relative to body weight), and macroscopic and
microscopic pathological appearance were observed.
Owing to excessive treatment-related mortality among animals of
each sex at 60 ppm, the surviving animals in the group were killed
during week 10. The lesions that had contributed to death were not
identified at necropsy, but all of the animals had high relative liver
weights. Several of the males had convulsions near the beginning of
the study. Reduced body-weight gains, food consumption, and food
efficiency were seen in these animals. The survival of the remaining
mice was comparable to or exceeded that of the control group, and no
clinical signs of toxicity were reported. Decreased body-weight gain
was seen in males (74-86% of control value) and females (81-86%) at 30
ppm at most evaluation times; the values for animals at 10 ppm were
also decreased but less consistently. Overall food consumption during
the study was lower than in controls in males (by about 7%) and
females (by 14%) at 30 ppm. Food efficiency was reduced in males and
females at 30 ppm and in males at 10 ppm.
A slightly lower percentage of neutrophils and a slightly higher
percentage of lymphocytes were noted in differential leukocyte counts
among females at 30 ppm after 76 weeks of treatment. Although gross
examination of animals observed for toxicity at week 53 showed no
remarkable effects, males at 30 ppm in the carcinogenicity study
showed liver enlargement and changes on the surface of the liver. The
absolute and/or relative liver weights were statistically
significantly increased in males and females at this dose at weeks 53
and 78. The relative liver weights were increased (p < 0.05) in
males at 10 ppm at both times and were increased (p < 0.05) in
males at 0.5 ppm at week 53 but not week 78. Histopathological
examination revealed a statistically significant increased incidence
of periacinar microvesicular vacuolation in the livers of males at 10
and 30 ppm at the end of 53 and 78 weeks, in females at 0.5 and 30 ppm
at the end of the toxicity study and in those at doses > 0.5 ppm
(significant at 0.5 and 30 ppm) at the end of the carcinogenicity
study; however, the incidence in females at 0.5 and 10 ppm did not
show a clear dose-response relationship, although the increased
incidence at 30 ppm may have been related to treatment. There was an
increased incidence of hepatocellular hyperplasia and chronic
degenerative changes in the livers of males at 30 ppm which died or
were killed during treatment in the carcinogenicity study.
There were no treatment-related neoplastic changes in females,
but males at 30 ppm had an increased incidence of malignant
hepatocellular carcinomas in comparison with concurrent controls: 1/52
in controls, 1/52 at 0.1 ppm, 2/52 at 0.5 ppm, 1/52 at 10 ppm, and
5/52 at 30 ppm. An additional hepatocellular carcinoma was observed in
one male at 30 ppm in the toxicity study. The incidence of
hepatocellular adenomas alone or combined with adenomas (one male at
30 ppm had both an adenoma and a carcinoma) was not significantly
increased. Since the increase in the incidence of carcinomas in males
at 30 ppm was within the range in historical controls in the testing
laboratory and the incidence among male concurrent controls was much
lower than the mean incidence in male historical controls, the
neoplastic findings in males were considered not to be related to
treatment. The NOAEL for systemic effects was 0.5 ppm, equal to 0.055
mg/kg bw per day (Broadmeadow, 1993).
Rats
Technical-grade fipronil (purity, 95.4%) was administered for one
year in the diet to groups of 15 male and 15 female CD rats to assess
its chronic toxicity, and further groups were fed the chemical for one
year and then observed for an additional 13 weeks to observe any
reversal of treatment-related changes. Groups of 50 male and 50 female
rats were originally scheduled to be treated for two years to assess
the carcinogenic potential of the chemical. The doses administered
were 0, 0.5, 1.5, 30, or 300 ppm, equal to 0, 0.019, 0.059, 1.3, or 13
mg/kg bw per day for males and 0, 0.025, 0.078, 1.6, or 17 mg/kg bw
per day for females. Standard evaluations of toxicity ante and
post mortem were included, and thyroid function (T3, T4, and
thyroid-stimulating hormone (TSH)) were measured in fasted animals
after 1, 4, 12, 24, and 50 weeks of treatment and after 2, 4, 7, and
11 weeks of the observation period after cessation of treatment.
The carcinogenicity phase of the study was terminated early owing
to excessive mortality and to ensure that a sufficient number of
animals were available for terminal sacrifices. Males and females were
killed when the number in any group declined to 25% of the original.
Thus, the males were killed after 89 weeks of treatment, when the
number of surviving animals in the group at 300 ppm was 25%, and the
females were killed after 91 weeks of treatment, when survival of
those at 30 ppm was 25%. More than 50% of animals of each sex in all
groups were still alive after 78 weeks. Early in the study, more
females at 300 ppm than controls died or were killed for humane
reasons related to convulsive episodes during this period. A
statistically significant increase in the number of deaths among
females at 30 ppm relative to controls disappeared when humane kills
were included in the mortality count. No significant differences in
mortality were seen among males or between other groups of females and
the control group.
Convulsive episodes, some lasting as long as 25 min and often
fatal, were observed in three males at 1.5 ppm, one male and three
females at 30 ppm, and eight males and 12 females at 300 ppm. The
convulsions tended to occur early in the treatment period but were
also seen later. Other clinical signs of toxicity that occurred
throughout treatment, predominantly in females at the high dose but
also in females given 1.5 and 30 ppm, included irritability,
vocalization, salivation, aggression, overactivity, and bruxism.
During the observation period, aggression, overactivity, irritability,
vocalization, and convulsions were seen in some females at 300 ppm.
Convulsions also occurred in females at 30 ppm and males at 300 ppm
but not in controls.
During the first week of treatment, body-weight gains were
significantly decreased in animals of each sex, by 6-11% at 30 ppm and
by 54-58% at 300 ppm. By one year, a significant 15-18% depression in
body-weight gain was observed only in animals at the high dose. By the
end of treatment, significant depressions were seen in males (18%) and
females (25%) at 300 ppm and in females at 30 ppm (23%). The
body-weight gain of males at 30 ppm was decreased by 7%. This pattern
continued during observation. Food consumption and food conversion
efficiency (calculated through week 14 only) were reduced at the
beginning of the study in animals of each sex at 300 ppm but were
similar to those of controls subsequently. The results of the
ophthalmological examination were negative.
Small but mostly significant decreases in haematological
parameters such as packed cell volume, haemoglobin concentration,
erythrocyte count, mean corpuscular volume, mean corpuscular
haemoglobin, and prothrombin time were seen at various times during
the study, particularly in males and females at the high dose. Slight
decreases in erythrocyte count, haemoglobin concentration, mean
corpuscular volume, and packed cell volume were also noted at 1.5 and
30 ppm.Towards the end of the study, platelet counts were slightly
increased in animals at the high dose and males at 30 ppm. Except for
a continued decrease in prothrombin time in females at 30 and 300 ppm,
the haematological changes did not persist after treatment.
Alterations in clinical chemical parameters, such as increased
cholesterol and calcium values and alterations in protein including
increased total protein, decreased albumin, increases in a1-, a2- and
b-globulins, and decreased albumin:globulin ratio, were seen mostly in
animals at 30 and 300 ppm; protein alterations were also observed in
males at 1.5 ppm towards the end of the study. Increased cholesterol
and calcium levels, total protein, and globulins and a decreased
albumin:globulin ratio persisted after cessation of treatment in
females at the high dose.
The T3 levels did not differ much from those of controls during
treatment but were significantly increased in females at the high dose
from four weeks after treatment and in females at 30 ppm from seven
weeks after treatment. The T4 levels were severely depressed after the
first week of treatment in animals of each sex, such that none was
detectable in animals at 300 ppm and the levels were significantly
depressed in a dose-dependent fashion in animals at 1.5 and 30 ppm.
Subsequently, the levels in animals at the high dose became
detectable, but the pattern of T4 depression observed at doses >
1.5 ppm generally persisted through week 50 of treatment. The T4
levels in animals at 0.5 ppm were occasionally significantly decreased
during treatment but not at the end of treatment. TSH levels were
significantly increased in animals at the high dose at all times and
in males at 30 ppm during the first month of treatment and after 50
weeks. Once treatment had ceased, the alterations in both T4 and TSH
levels were reversed in all groups except males at the high dose, in
which the TSH levels never recovered fully.
Alterations were seen at various times during the study in
urinary parameters (lower pH, higher protein, elevated urine volume,
and decreased specific gravity) in rats at 30 and 300 ppm
(predominately males). The alterations in protein and pH persisted
after treatment. Gross examination of animals in the carcinogenicity
study at termination and animals that were killed or died during the
study revealed increased incidences of large and/or pale kidneys in
animals at the intermediate or high dose and enlarged adrenals in
males at the high dose. Enlarged livers and thyroids were seen in
animals of each sex at the high dose and in males at 30 ppm. At
interim sacrifice (toxicity study), enlarged livers or thyroids were
seen in some animals at 30 or 300 ppm. Absolute thyroid weights were
somewhat increased in males at 0.5 and 1.5 ppm. The absolute and
relative weights of the liver and thyroid were increased in animals of
each sex at 30 or 300 ppm in both the toxicity and the carcinogenicity
study and in animals at the high dose that were killed or died during
the study. Increased absolute and relative kidney weights were also
seen in animals at 30 or 300 ppm, and increased adrenal weights were
seen in males at these doses. Most of the changes in organ weight were
statistically significant. The increases in liver, thyroid, and kidney
weights persisted to some degree after treatment, mostly in animals at
300 ppm. Histopathological examination showed an increased incidence
and severity of progressive senile nephropathy (a non-neoplastic
lesion) in animals of each sex at 300 ppm in the toxicity study and at
30 and 300 ppm in the carcinogenicity study; an increased severity of
the lesion was also reported in rats at 1.5 ppm. These findings
persisted to some extent after treatment.
Benign and malignant neoplastic changes (follicular-cell adenomas
and carcinomas) occurred in the thyroid glands of animals of each sex.
The incidence of these tumours per number of animals examined at 0,
0.5, 1.5, 30, and 300 ppm was, respectively: malignant follicular-cell
carcinomas: 0/49, 0/48, 0/50, 0/50, and 5/50 for males and 0/50, 1/50,
0/50, 1/50, and 2/50 for females; benign follicular-cell adenomas:
0/49, 1/48, 5/50, 3/50, and 12/50 for males and 0/50, 0/50, 0/50,
0/50, and 8/50 for females; total tumours: 0/49, 1/48, 5/50, 3/50, and
17/50 for males and 0/50, 1/50, 0/50, 1/50, and 10/50 for females.
None occurred in concurrent controls. Males in all treated groups and
females in all groups except that receiving 1.5 ppm showed increased
incidences of benign and malignant thyroid tumours combined in the
carcinogenicity study. The increases were significant (at p < 0.05,
< 0.01, or < 0.001 by Fisher's exact one-tailed test for pair-wise
comparisons) for carcinomas alone in males at the high dose and for
adenomas alone and adenomas and carcinomas combined in males and
females at 300 ppm and males at 1.5 ppm. The author concluded that
only the neoplastic changes observed in animals at 300 ppm were
related to treatment, as the increased incidences of benign or
malignant lesions alone or in combination exceeded the historical
control incidences for the testing facility only at the high dose, and
the zero incidences in the concurrent controls were unusually low. The
reported historical control incidence rates for studies of comparable
length (88-95 weeks) were 0-5.5% (males) and 0% (females) for
follicular-cell carcinoma, 1.4-5.7% (males) and 0-1.9% (females) for
follicular-cell adenoma, and 1.9-7.3% (males) and 0-1.9% (females) for
all follicular-cell tumours. The occurrence of these tumours was
attributed to continuous stimulation of the thyroid gland by elevated
TSH levels.
Although the author concluded that there were no significant
intergroup differences in mortality among males, analysis of the data
by the US Environmental Protection Agency using the computer program
of Thomas, Breslow, and Gart showed a significantly increasing trend
in mortality with increasing doses of fipronil in male but not female
rats. This finding does not affect the validity of the study for the
following reasons: firstly, the study was terminated prematurely only
near its end; secondly, the literature indicates that, in general, the
longevity of CD (Charles River) rats has been decreasing and that a
shortened life span is therefore not unique to this study; and
thirdly, the study was long enough for tumours to have developed in
the fipronil-treated animals.
The initial analysis of tumour incidence was based only on the
data from the carcinogenicity phase of the study. Six animals were
reported to have developed thyroid follicular-cell tumours during the
observation phase, with carcinomas in one male at 30 ppm and one at
300 ppm and adenomas in one female at 1.5 ppm and one male and two
females at 300 ppm. No thyroid tumours considered to be related to
treatment were reported to have occurred during the toxicity phase,
although a follicular-cell carcinoma was tabulated for one male at 30
ppm killed after one year of treatment. When these additional data are
included in the analysis and the data are analysed on the basis of the
first appearance of thyroid adenomas or carcinomas in the study, the
incidence is as shown in Table 2. When Peto's prevalence test is used
to analyse the data for male rats (as the first tumour was seen before
interim sacrifice), significantly increasing trends (p < 0.01) are
obtained for thyroid follicular-cell adenomas, carcinomas, and
combined adenomas and carcinomas. Significant differences from
controls in pair-wise comparisons (p < 0.05 or p < 0.01) are
found for adenomas and combined adenomas and carcinomas at doses >
1.5 ppm and for carcinomas at the high dose. When the exact trend test
and Fisher's exact test are used to analyse the data on tumours in
female rats, a significantly increasing trend and a significant
difference in pair-wise comparisons of the group at 300 ppm with the
controls is seen for thyroid follicular-cell adenomas and combined
adenomas and carcinomas (all at p < 0.01).
This analysis suggests that doses > 1.5 ppm are carcinogenic,
since the pair-wise comparisons in males at doses > 1.5 ppm are
significant and there were significant trends for tumour formation.
Since the concurrent controls had an apparently lower incidence than
historical controls at the laboratory where the study was conducted,
however, the apparent inconsistency in the dose-response relationship
at 1.5 and 30 ppm and the fact that TSH levels were significantly and
persistently elevated only at 300 and to a lesser extent at 30 ppm, an
alternative view is that the incidence of thyroid tumours was
toxicologically significant only at the highest dose. In summary, the
study demornstrates that fipronil is clearly carcinogenic to rats at
300 ppm; however, the overall NOAEL is for neurotoxicity and is
0.5 ppm, equal to 0.019 mg/kg bw (Aughton, 1993).
(d) Genotoxicity
The results of assays for genotoxicity with fipronil are
summarized in Table 3.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
In a two-generation study of reproductive toxicity, 30 male and
30 female CD rats received technical-grade fipronil (purity, 95.4%) in
the diet at concentrations of 0, 3, 30, or 300 ppm, equal to 0, 0.25,
2.5, or 26 mg/kg bw per day for males and 0, 0.27, 2.7, or 28 mg/kg bw
per day for females. After two matings of the F0 generation, litters
were culled to four animals of each sex on day 4 post partum, and
physical development was assessed by recording the day of onset and
completion of pinna unfolding, hair growth, tooth eruption, and eye
opening.
Table 2. Incidences of thyroid follicular-cell tumours in rats treated with fipronil
Tumour Dose (ppm)
Males Females
0 0.5 1.5 30 300 0 0.5 1.5 30 300
Adenomas 0/63 1/61 5/63 3/62 12/61 0/48 0/49 0/50 0/45 8/46
% 0 2 8 5 20 0 0 0 0 17
p 0.000** 0.116 0.014* 0.038* 0.000** 0.000** 1.000 1.000 1.000 0.002**
Carcinomas 0/59 0/57 0/62 1/60 5/57 0.48 1/49 0/50 1/45 2/46
% 0 0 0 2 9 0 2 0 2 4
p 0.000** - - 0.186 0.007** 0.084 0.505 1.000 0.484 0.237
Combined 0/63 1/61 5/63 4/62 16/61 0/48 1/49 0/50 1/45 10/46
% 0 2 8 6 26 0 2 0 2 22
p 0.000** 0.116 0.014* 0.024* 0.000** 0.000** 0.505 1.000 0.484 0.001**
For males: no. of tumour-bearing animals/no. of animals examined, excluding those that died before observation of
the first tumour; analysis by Peto's prevalence test. For females: no. of tumour-bearing animals/no. of animals
examined, excluding those that died or were killed before week 54; analysis by exact trend test and Fisher's exact
test. First adenoma observed at 300 ppm in males in week 42 and in females at week 62. First carcinoma observed in
males at 30 ppm in week 53 and in females at 300 ppm in week 79. One male at 300 ppm had both an adenoma and a
carcinoma.
Significance of trend denoted at control; significance of pair-wise comparison with control denoted at dose:
*, p < 0.05; **, p < 0.01
Table 3. Results of assays for genotoxicity with fipronil
End-point Test object Concentration Purity Result Reference
(%)
In vitro
Reverse mutation S. typhimurium 0.8-500 µg/plate 90.6 Negativea,b Clare (1988a)
TA98, TA100, in DMSO
TA1535, TA1537
Gene mutation Chinese hamster 1.13-386 µg/ml 97.2 Negativea,b Lloyd (1990)
cell line V79, in DMSO
hprt locus
Chromosomal Chinese hamster 15-60/µg/ml 6 ha, 98.3 Positiveb,d Wright (1995)
aberration lung cell line 7.5-30 µg/ml 24 hc,
7.5-22.5 µg/ml 48 hc
in DMSO
Chromosomal Human lymphocytes 75-300 µg/ml in 90.6 Negativeb Marshall (1988a)
aberration DMSO
In vivo
Micronucleus CD-1 mice 1-25 mg/kg bw in 97.2 Negativeb,e Edwards (1991)
formation aqueous methyl-
cellulose
Micronucleus CD-1 mice 6.95-48 µg/kg bw 96.2 Negativeb Edwards (1995)
formation in aqueous methyl-
cellulose
DMSO, dimethyl sulfoxide
a With and without metabolic activation
b Appropriate positive controls gave expected positive responses
c Without metabolic activation
d Positive only with 6-h pulse treatment; significant, dose-related effects at 45 and 60 µg/ml without
metabolic activation; non-significant increase at 60 µg/ml with metabolic activation
e Unacceptable; no overt or target-cell toxicity at doses < 25 mg/kg; results at 50 mg/kg were inconclusive.
Systemic toxicity was seen in the parental animals at doses >
30 ppm as increased absolute and relative weights of the thyroid gland
and liver in the F0 and F1 generations, decreased absolute and
relative pituitary gland weights in the F1 females (a decrease in
relative pituitary weight at 3 ppm was minor and was not considered to
be toxicologically significant), and a significantly increased
incidence of follicular epithelial hypertrophy of the thyroid gland in
F1 females and animals of each sex at 300 ppm. Increased mortality
occurred among F0 and F1 animals at this dose, with clinical signs
of toxicity including convulsions; in addition, the F0 generation had
decreased food consumption before mating, and decreased body-weight
gain was seen before mating in the F0 and F1 generations and in F0
females during gestation and lactation. The absolute and relative
weights of the ovaries were decreased in F0 females, and females of
the F0 and F1 generations had a significantly increased incidence of
centriacinar fatty vacuolation in the liver. The increased incidence
of follicular epithelial hypertrophy in adult males at 30 ppm was not
significant but was found in both the F0 generation (in 2/30 animals,
in comparison with 0/30 controls, 0/30 animals at 3 ppm, and 10/29
animals at 300 ppm) and the F1 generation (in 3/30 animals, with 0/30
controls, 2/30 at 3 ppm, and 9/30 at 300 ppm) and was considered to be
related to treatment. The presence of this finding in 2/30 F1 males
at 3 ppm, and not in the F0 generation, was considered plausible in
an organ in rats that is very sensitive to stimuli; it could therefore
not be clearly related to treatment.
Reproductive toxicity observed in animals at 300 ppm consisted of
an increased incidence of clinical signs of toxicity in F1 and F2
offspring (notably convulsions when the offspring first started to eat
the treated diet), decreased litter size and body weights, a decrease
in the percentage of animals that mated, a reduction in the fertility
index of F1 parental animals, reduced postimplantation and postnatal
survival in the F2 litters, and delays in physical development in F1
and F2 litters including slight delays in the onset of tooth eruption
(F1) and pinna unfolding (F2). The NOAEL for parental toxicity was 3
ppm, equal to 0.25 mg/kg bw per day, while that for reproductive
toxicity was 30 ppm, equal to 2.5 mg/kg bw per day (King, 1992).
(ii) Developmental toxicity
Rats
Technical-grade fipronil (purity, 93%) was administered by gavage
as a suspension in 0.5% aqueous methylcellulose to groups of 25
specific pathogen-free female rats of the Crl: CD (SD)BR VAF/Plus
strain (Charles River, France) at doses of 0, 1, 4, or 20 mg/kg bw per
day on days 6-15 of gestation. The study was terminated on day 20.
Adult females were observed for clinical signs, food and water
consumption, and body-weight changes; they underwent a macroscopic
post mortem at study termination. Litters and fetuses were evaluated
with regard to pre- and postimplantation losses, litter size, litter
and mean fetal weights, sex ratios, and malformations or skeletal or
visceral anomalies.
The pregnancy rate was 96-100%. There were no deaths, abortions,
premature deliveries, or clinical signs in the adult females. Maternal
effects associated with treatment occurred only in the animals
receiving the high dose and included reduced body-weight gain,
increased water consumption, and decreased food consumption at various
intervals during gestation. Some of these effects continued after
treatment. The macroscopic examination showed no remarkable effects.
No effects of treatment were seen on developmental parameters. The
NOAEL for maternal toxicity was 4 mg/kg bw day, and that for
developmental toxicity was 20 mg/kg bw day (Brooker & John, 1991).
Rabbits
Technical-grade fipronil (purity, 95.4%) was administered by
gavage as a suspension in 0.5% aqueous methylcellulose mucilage and
0.5% Tween 80 to groups of 22 female New Zealand white rabbits (Ranch
Rabbits, Susse, United Kingdom) at doses of 0, 0.1, 0.2, 0.5, or 1
mg/kg bw per day on days 6-19 of gestation. The study was terminated
on day 29. Adult females were observed for clinical signs, abortion
and total litter loss, food and water consumption, and body-weight
changes; a macroscopic post mortem was performed at study termination.
The litters and fetuses were evaluated with regard to pre- and
postimplantation losses, litter size, litter and mean fetal weights,
sex ratios, and malformations or skeletal or visceral anomalies.
The number of pregnant animals in each group ranged from 18 to
21. There were no treatment-related deaths. At doses of 0.5 and 1 g/kg
bw per day, maternal body-weight gain during treatment was
significantly lower than that of controls by 50 and 70%, respectively,
and the body-weight gain of dams at 0.1 or 0.2 mg/kg bw per day was
reduced by 30%. Animals in all fipronil-treated groups consumed less
food than those in the control group during treatment, and the
decreases in the groups at the two highest doses achieved significance
on gestation days 6-12 and/or 13-19. Other maternal parameters were
not affected. Treatment had no effect on development. No NOAEL for
maternal toxicity was identified; that for developmental toxicity was
1 mg/kg bw per day (King, 1990).
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Technical-grade fipronil (purity, 96.7%) moistened with corn oil
was a slight dermal irritant in male and female New Zealand white
rabbits after a 4-h application to intact skin. The signs of
irritation were slight to well-defined erythema and slight oedema,
which had cleared by day 7 after application. When fipronil (purity,
93%) was moistened with distilled water and applied for 4 h to the
intact skin of male New Zealand white rabbits, no signs of dermal
irritation were seen (Liggett, 1988a; Myers & Christopher, 1993a).
Instillation of technical-grade fipronil (purity, 96.7%) into the
eyes of male and female New Zealand white rabbits produced minor
transient corneal opacity and iritis, which had cleared within 24 h,
and minor-to-moderate conjunctival irritation, which had cleared
within 2-14 days. In a similar study with only male rabbits, fipronil
(purity, 93%) induced transient, minimal conjunctival inflammation,
which cleared within 24-48 h (Liggett, 1988b; Myers & Christopher,
1993b).
Technical-grade fipronil (purity, 95.4%) was tested in male and
female Dunkin-Hartley albino guinea-pigs by a modification of the
Buehler method. Topical application of the highest optimal
concentration for induction and challenge of fipronil (30% w/v) in
paraffin oil to guinea-pig skin produced no sign of dermal
sensitization (Smith, 1990).
In a Magnusson-Kligman maximization test in male and female
Dunkin-Hartley albino guinea-pigs, the optimal induction and challenge
concentrations of technical-grade fipronil (purity, 95.4%) were
determined in advance. In the main study, primary induction
concentrations of 5% (w/v) fipronil in propylene glycol alone or
combined with Freund's complete adjuvant were applied intradermally at
separate sites. In the secondary induction phase, 5% fipronil in
propylene glycol was applied topically to the same sites. A topical 3%
challenge dose produced no significant response, but 24 h after
challenge with 10% fipronil in propylene glycol, four of 20 animals
showed a significant dermal response (eschar formation or slight
erythema). Eschar formation was still evident in two animals 48 h
after the challenge. No significant responses were observed in control
animals challenged with 3 or 10% fipronil in propylene glycol. One
control animal challenged with propylene glycol alone showed slight
erythema at 24 h. By the criteria of the European Economic Community,
the test material did not cause delayed contact hypersensitivity
because the response rate at challenge was < 30%; however, the
positive response rate of 20% in this study could also support the
conclusion that fipronil is a mild or weak skin sensitizer (Johnson,
1993).
(ii) Neurotoxicity
Rats
A single dose of technical-grade fipronil (purity, 96.7%) in corn
oil was administered by gavage to groups of 15 male and 15 female
Sprague-Dawley rats at doses of 0, 0.5, 5, or 50 mg/kg bw. The animals
were about eight weeks of age at the time of treatment. Standard
evaluations of toxicity included observations for mortality and
clinical signs of toxicity and measurements of body weight. The
neurobehavioural evaluations of all surviving animals included a
functional observational battery of tests before treatment and at 7 h,
7 days, and 14 days after treatment, and quantitative assessment of
motor activity before treatment and 1 h after each functional test.
The study was terminated on days 16-19 after treatment. The postmortem
examinations included an abbreviated necropsy of the thoracic and
peritoneal cavities (all groups) and detailed light microscopic
evaluation of the brain, spinal cord, and peripheral nerves of six
male and six female controls and six animals of each sex at the high
dose after intracardiac perfusion in situ with 10% neutral buffered
formalin. Rats that died during the study were necropsied, but their
nervous tissues were not examined microscopically.
Five males and one female at 50 mg/kg bw died during the study,
most within two days of treatment. At necropsy, most had diffuse brain
haemorrhages, which may have caused death or may have been agonal.
Clinical signs of toxicity were seen only at the high dose, and the
incidence of neurological signs was more prevalent in males. On the
day of treatment, males and females at this dose had either clonic or
tonic-clonic convulsions. Other clinical signs of toxicity included
those indicative of cachexia: emaciation, dehydration, unkempt
appearance, urine stains, cold extremities, and/or pallor. Male body
weight was reduced by 6-10% relative to that of controls 7 and 14 days
after treatment.
Treatment-related effects on functional parameters occurred
primarily 7 h after treatment in rats at 50 mg/kg bw, when males were
seen to have drooping or half-shut eyelids. During open-field
evaluations, both stimulation and depression of the nervous system
were seen, which were generally more pronounced in males. Signs
indicative of stimulation of motor systems were convulsions, coarse
and fine tremors, head bobbing, myoclonic movements, and decreased
hindleg splay, which was significantly decreased (p < 0.01) in
animals of each sex. The signs indicative of nervous system depression
were decreased arousal and rearing activity, decreased reflexes such
as response to tail pinching and approach and air righting reflex, and
decreased muscle tone, altered gait, decreased pupil size, and/or
decreased body temperature. Urination was more evident in males at the
high dose. The only treatment-related effect in rats at 5 mg/kg bw 7 h
after treatment was significantly decreased hindleg splay (p <
0.05) in animals of each sex. A stimulatory response in certain
functional tests was seen in some males at the high dose seven days
after treatment, such as increased arousal and rearing activity and
exaggerated touch and sound reflexes. Females at the high dose showed
significantly increased hindleg splay at this time and on day 14 after
treatment. The only finding of note in males at the high dose on day
14 was a higher incidence of urination.
Motor activity 8 h after treatment was decreased by 90% in males
at the high dose and by 93% in females. This was the only effect on
motor activity during the study that was attributed to treatment.
There were no treatment-related gross or microscopic changes on
post-mortem examination of the central and peripheral nervous systems.
The NOAEL was 0.5 mg/kg bw on the basis of decreased hindleg splay 7 h
after treatment (Gill et al., 1993).
Groups of 15 male and 15 female Sprague-Dawley rats were fed
diets containing technical-grade fipronil (purity, 96.7%) at 0, 0.5,
5, or 150 ppm (equivalent to 0, 0.03, 0.3, or 8.9 mg/kg bw per day for
males and 0, 0.03, 0.3, or 11 mg/kg bw per day for females) for 13
weeks. The animals were about eight weeks of age at the start of
treatment. Standard evaluations of toxicity included observations for
mortality and clinical signs of toxicity and measurements of body
weight. Neurobehavioural screening, consisting of a functional
observational battery of tests and motor activity evaluations, was
performed before treatment and during weeks 4, 9, and 13. Post-mortem
examinations at terminal sacrifice, included an abbreviated necropsy
of the thoracic and peritoneal cavities in all groups and a detailed
light microscopic evaluation of the brain, spinal cord, and peripheral
nerves of six males and females of the controls and at the high dose
after intracardiac perfusion in situ with 10% neutral buffered
formalin.
There were no treatment-related deaths or clinical signs of
toxicity. Slight decreases in body weight (6.5-6.9%) and decreased
body-weight gain were seen in males and females at the high dose
during the first few weeks of treatment. These were accompanied by
decreased food consumption, suggesting unpalatability. Functional
changes seen in animals at the high dose in week 4, 9, and/or 13 were
increased urination and an increased incidence of exaggerated response
to tail pinching in males, an increased incidence of exaggerated
startle responses during manipulation in animals of each sex, and
increased forelimb grip strength in females at week 13. No
treatment-related findings were seen at necropsy or during
histopathological examination of nervous tissues. The changes in
animals at the high dose may not have been related to treatment;
however, these findings, although relatively minor when taken
separately, represent a minimal effect of treatment when taken
in toto. In addition, the exaggerated responses to touch and sound,
particularly in males, and the increased urination of males at the
high dose were considered to be related to treatment in the preceding
study. Therefore, the NOAEL for both neurotoxicity and systemic
toxicity was 5 ppm, equal to 0.3 mg/kg bw per day (Driscoll & Hurley,
1993).
Dogs
Technical-grade fipronil (purity, 95.4%) was administered in
capsules to female beagle dogs (26 weeks old at the start of the
study) at doses of 0 (one animal) or 20 mg/kg bw per day (four
animals) until clear signs of neurotoxicity were seen or for a maximum
of 14 days. Immediately after the appearance of neurotoxic signs,
administration was discontinued and a 28-day observation period was
initiated. This phase was begun after five days of treatment in two
animals, seven days of treatment in one animal, and 13 days of
treatment in one animal and the control dog. Animals were monitored
for general health, clinical signs of toxicity, chronic conditions,
food and water consumption, and body-weight changes and were subjected
to a weekly veterinary examination and neurological examinations
before treatment and then at intervals during treatment and
observation. The neurological examination consisted of observation and
testing of cranial nerve reflexes, segmental reflexes, and postural
reactions and general observation of behavioural changes,
abnormalities of gait and stance, and the presence of tremor or other
dyskinesia. An obstacle-avoidance test and a hearing test were
administered at the same time as the neurological examination.
Anaesthetized dogs were killed by perfusion-fixation at the end of the
observation phase. The brain, spinal column, and some peripheral
nerves were examined grossly, but only the brain and spinal column
appear to have been subjected to microscopic examination.
All animals survived to the end of the observation period. The
control animal showed no abnormal signs throughout the treatment and
recovery periods, but all treated animals displayed neurotoxic signs,
which included hypoactivity, salivation, ataxia, convulsions, tremors,
unsteady gait and stiffened body, aggressive behaviour, apparent lack
of vision, muscle twitching, nervous behaviour, and a high-stepping
gait. The time to appearance of signs and the duration of signs after
discontinuation of treatment varied. Some signs, like aggressive
behaviour and visual impairment, first appeared after discontinuation
of treatment. Food consumption was markedly decreased from day 1 or 2
of treatment and for up to seven days during the recovery period. All
treated animals lost weight, but the initial body weight was restored
17 days after cessation of treatment. Veterinary examination showed
similar changes and also emaciation in three treated animals and
hunched posture and peripheral vasodilatation in one animal. The
neurological examinations indicated widely varied responses including
(in addition to the responses noted above) changes in reflex
responses, some exaggerated, including gag and flexor responses, and
some depressed, including blink, pupillary, and consensual light
reflexes and visual and tactile placing responses. One animal still
had slightly exaggerated gag and flexor reflexes at the end of the
recovery period. The results of the obstacle-avoidance and hearing
tests indicated temporary visual impairment and possible hearing
deficiency in one animal. No abnormal findings were reported after
macroscopic or microscopic examination.
Virtually complete recovery from what appeared to be functional
changes in the central nervous system was reported by 12 days or less
after cessation of treatment; in the absence of histopathological
changes in the study, the mode of action of the chemical was
considered probably to be pharmacological and temporary. Given the
short duration of treatment (5-13 days), the short observation period
(treatment period plus 28 days), the use of only one dose, one sex,
and only one control animal, however, there is insufficient
information to draw any firm conclusions about the reversibility of
the functional, cellular, and neurotransmitter or receptor effects.
There was no NOAEL (Holmes, 1991c).
(iii) Developmental neurotoxicity
Technical-grade fipronil (purity, 96.1%) was administered to
groups of 30 female Sprague-Dawley (CD) rats at dietary levels of 0,
0.5, 10, or 200 ppm, equal to 0, 0.05, 0.9, or 15 mg/kg bw per day,
from gestation day 6 through lactation day 10, day 0 being defined as
the day mating was confirmed. Clinical observations, body weights, and
food consumption were recorded for all animals at scheduled intervals
throughout gestation and lactation, and litters were examined at
selected intervals throughout lactation. The numbers of live and dead
pups and the sex and body weights of the pups were recorded. Litters
were standardized to eight pups on postnatal day 4. The achievement of
pinna detachment, incisor eruption, eye opening, vaginal patency, and
preputial separation was evaluated for all surviving pups. For one pup
per sex per litter, the following evaluations were conducted on
various postnatal days: motor activity (days 13, 17, 22, 60), auditory
startle response (days 22 and 60), swimming development (days 6, 8,
10, 12, and 14), and learning and memory in a Y-maze (days 24 and 60).
On day 11 of lactation and again on day 60, one pup of each sex per
litter was killed. Brain weights were recorded and neuropathological
evaluation was performed on six pups of each sex from the control and
high-dose groups. Maternal animals were killed and necropsied after
the weaning of the last litter, and all remaining offspring were
killed soon after all the evaluations were completed.
The maternal effects at the dietary level of 200 ppm were reduced
body weight during treatment, reduced body-weight gain during
gestation, and reduced food consumption. No treatment-related findings
were observed at necropsy. Pregnancy status, gestation length, litter
size, and pup sex ratio were not affected by treatment. At 200 ppm,
there was a significant increase in the number of stillborn pups, and
pup and litter survival were reduced. Also at this level, male and
female pup weights were reduced, and upper and lower incisor eruption
and sexual development were delayed. There was a small but significant
increase in the time to preputial separation in animals at 10 or 200
ppm. At 10 ppm, group mean body weights were reduced for female pups
at all recorded intervals and for male pups on days 4 and 17;
postweaning weights were not affected.
With regard to neurodevelopmental effects, at 200 ppm the maximum
auditory startle response time for males and females was significantly
decreased on postnatal day 22; there was no significant difference in
the time to maximum or average response. Swimming development was
delayed on postnatal day 6 for animals of each sex, and motor activity
was increased in females on postnatal day 17. Learning and memory
(Y-maze test) were delayed significantly for females in two trials on
postnatal day 24. Also at 200 ppm, significant decreases in absolute
pup brain weights were noted for animals of each sex on days 11 (-20%
for males and -11% for females) and 60 (-7% for males and females),
but pup body weights were also significantly decreased at these times,
so that the relative brain:body weights were actually increased
relative to those of concurrent controls: +39% for males and +20% for
females on day 11 and +6% for males and females on day 60. Thus, the
lower brain weights may have been due to the overall retarded growth
of the pups associated with maternal toxicity rather than to a
developmental delay in brain size. The statistically significant
increases in motor activity in female pups at 10 ppm observed on day
17 could not be clearly related to treatment when the magnitude of the
response was compared with that of the control group, because the
values for the latter on that day appeared to be unusually low. No
microscopic morphological abnormalities were seen in the brains of
offspring killed on postnatal day 11 or 60. The NOAEL for maternal and
neurodevelopmental effects was 10 ppm, equal to 0.9 mg/kg bw per day.
The NOAEL for developmental effects was 0.5 ppm, equal to 0.05 mg/kg
bw per day, on the basis of reduced pup body weights (Mandella, 1995).
(iv) Thyroid function
Effects on thyroid hormone levels: Groups of 10 Crl:CD (SD) BR
rats of each sex were given technical-grade fipronil (purity, 95.4%)
in the diet at doses of 0, 0.1, 1, 5, or 30 ppm (equal to 0, 0.01,
0.1, 0.49, or 2.9 mg/kg bw per day for males and 0, 0.01, 0.1, 0.48,
or 2.9 mg/kg bw per day for females) for four weeks. The parameters
measured were clinical observations, body weight, food consumption and
efficiency, water consumption, evaluations of T3, T4, and TSH before
treatment and on days 7 and 28, and organ weights and histopathology
of the thyroid and liver at necropsy.
The T3 level was significantly decreased in males at doses > 1
ppm on day 7 and was increased in females at 30 ppm on day 28; T4
levels were significantly decreased in males and females at 30 ppm,
males at 5 ppm on day 7, and males at 30 ppm on day 28; the TSH level
was significantly increased in males at 30 ppm on days 7 and 28 and in
females at 30 ppm on day 28; thyroid weights were nonsignificantly
increased in males and females at 30 ppm; and there was an increased
incidence of higher follicular epithelium in males and females at 30
ppm and in one male at 5 ppm. As re-analysis of the data for T3 in the
controls and rats at 1 ppm showed no significant difference, it was
concluded that this level had no effect.
Increased liver weights were observed in animals at 30 ppm;
however, the change was significant only in females. An increased
incidence of minimal centrilobular hepatocyte enlargement was seen in
males at 30 ppm. Staining of liver sections with Oil Red O
demonstrated an increased incidence of periportal fat deposition in
females at 5 and 30 ppm. The NOAEL was 1 ppm, equal to 0.1 mg/kg bw
per day (Peters et al., 1991a).
Effect on biliary excretion of thyroxine: The objective of this
study was to measure the effects of single and repeated oral doses of
technical-grade fipronil (purity, 96.7%) on the biliary excretion of
intravenously administered 125I-T4 in bile duct-cannulated rats.
Groups of three Sprague-Dawley CD(Crl:CD/BR) VAF plus strain male rats
were given single or repeated daily doses for 14 days of: fipronil (1
or 10 mg/kg bw per day orally at 5 ml/kg bw), phenobarbital (80 mg/kg
bw per day intraperitoneally as a positive control), or 0.5%
methylcellulose (5 ml/kg bw orally as a negative control). Immediately
after treatment, the animals were anaesthetized, the common bile duct
was cannulated, and 1 mg sodium iodide (to block thyroid uptake of
125I) was administered by injection into the stomach, followed 4 h
later by intravenous administration of about 10 µCi of 125I-T4. Bile
samples were collected before treatment and at 15-min intervals for
0-5 h after 125I-T4 administration. Blood samples were taken at 30-min
intervals. Both bile and blood were analysed for radiolabel. After 5
h, the animals were killed and the livers removed. Selected bile
samples were pooled, treated with œ-glucuronidase and analysed by HPLC
for free and conjugated T4.
The single doses of fipronil or phenobarbital did not
significantly increase the amount of bile excreted or the biliary
clearance of 125I-T4. After 14 days of treatment, biliary clearance
was significantly increased with the high dose of fipronil but not
with the low dose. The high dose of fipronil produced a greater
increase than phenobarbital. Bile output was significantly increased
after treatment with the high dose of fipronil and with phenobarbital.
After treatment with œ-glucuronidase, about 50% of the 125I-T4 in bile
was conjugated with either glucuronide or sulfate, but about 30%
remained unidentified. Thus, repeated treatment with fipronil at 10
mg/kg bw per day orally increased the excretion of conjugated T4 in
bile, fipronil being more potent than phenobarbital. A possible
mechanism would be hepatic accumulation of T4 and induction of the
hepatic T4 conjugating enzyme UDP glucuronyltransferase, leading to
increased biliary excretion of T4-glucuronide conjugate (Taylor,
1993).
Effect on thyroxine clearance: Groups of six male Crl:CD(SD)
rats were treated with either technical-grade fipronil (purity, 95.4%)
at 10 mg/kg bw per day by gavage, phenobarbital at 80 mg/kg bw per day
intraperitoneally, or 0.5% methylcellulose (vehicle control) at 5
ml/kg bw by gavage for one or 14 days. Four h after the final dose of
either test substance, each rat received 125I-T4 at a dose of 10
µCi/kg bw. The levels of 125I in whole blood were measured for up to
30 h after T4 administration and were used to calculate the terminal
half-life, clearance, and volume of distribution of this hormone.
Fipronil had no effect on mortality or other parameters ante
mortem. Phenobarbital-treated animals showed collapsed posture,
lethargy, and shallow breathing on the first day of treatment.
Fipronil had no effect on the clearance of T4 after one day of
treatment, but after 14 days a decrease in terminal half-life (52% of
control level) and increases in the clearance (261%) and volume of
distribution (137%) were seen. The effects of phenobarbital were
similar, although quantitatively less severe, and were seen on day 1
of treatment (Peters et al., 1991b).
Effect on thyroid function: The effect of technical-grade
fipronil (purity, 95.4%) on thyroid function was compared with that of
propylthiouracil, a known inhibitor of thyroid organification which
interferes with thyroglobulin iodination, and noxythiolin, a thiourea
compound and putative inhibitor of thyroid iodide organification known
to lower T4 levels in rats. Groups of 27 male Crl:CD(SD)BR rats were
treated for 14 days with either 0.5% methylcellulose (vehicle control)
at 5 ml/kg bw by gavage, fipronil at 10 mg/kg bw per day by gavage,
propylthiouracil at 200 mg/kg per day by gavage, or Noxyflex at 5
mg/kg bw per day intraperitoneally as a saline solution. On day 15,
each animal received 125I-sodium iodide at a dose of 1 µCi. Six hours
later, nine males in each group received 10 or 25 mg/kg bw potassium
perchlorate or a 0.9% saline solution intraperitoneally, and blood was
immediately drawn in order to measure radiolabel. The animals were
then killed, and the thyroids were weighed and analysed for
radioactivity. Treatment with fipronil or Noxyflex appeared to
stimulate the thyroid glands, as evidenced by increased accumulation
of 125I and increased ratios of radiolabel distribution between the
blood and the thyroid. These changes were accompanied by increases in
thyroid weight. Treatment with propylthiouracil decreased the amount
of 125I incorporated in the thyroid and the blood:thyroid ratios, with
elevated levels of 125I in the blood. As the thyroids of these animals
were 2.5 times heavier than those of controls, however, the ratio of
blood 125I to thyroid weight was reduced. Administration of
perchlorate did not further reduce the 125I content of the thyroids or
the blood:thyroid 125I radiolabel ratio. There was no evidence of
inhibition of iodide incorporation (Peters et al., 1991c).
(v) Mode of action of fipronil
GABA is an important inhibitory neurotransmitter in both insects
and mammals. Attempts to demonstrate that the target site for fipronil
at the molecular level is the chloride ion channel of the GABA
receptor include electrophysiological testing, in which fipronil at 10
nmol/L reversed blockage of nervous system activity by GABA at 10
mmol/L, resulting in hyperexcitation in the housefly larva; flux
studies in which fipronil inhibited the passage of radiolabelled
chloride ions through opened GABA receptors in rat brain microsacs;
radioligand binding assays in vitro in which fipronil did not
inhibit binding of 3H-muscimol or 3H-flunitrazepam at, respectively,
the GABA recognition or benzodiazepine sites on the GABA receptor in
rat tissue, but did competitively inhibit binding at sites in the
chloride channel of the GABA receptor of ligands such as
35S- tert-butylbicyclophosphorothionate (TBPS) and
3H-1-[(4-ethyl)phenyl]-4- n-propyl-2,6,7-trioxacicyclo[2.2.2]octane
(EBOB). The latter is known to block the g-amino-butyric acid-gated
chloride channel, and the former binds to the picrotoxin binding site
in the channel. Binding of TBPS was inhibited by fipronil in rat brain
but not in the housefly head, but binding of EBOB was inhibited in
both rat and mouse brain and housefly head. The greater affinity of
fipronil for the EBOB site in insects than for that in mammals has
been postulated to account, at least in part, for the pesticide's
selective toxicity to insects.
In other studies of the role of the GABA receptor in the toxicity
of fipronil, a cloned gene that codes for a GABA receptor subunit in
Drosophila melanogaster was expressed in frog oocytes as a membrane
protein. These receptor-containing oocytes produced a large electrical
current carried by chloride ions in response to treatment with GABA,
which was not seen in control oocytes. The electric response was
reversed in a dose-dependent manner by treatment with fipronil and was
blocked by picrotoxin, TBPS, EBOB, and dieldrin.
Differences in the GABA receptor genome in susceptible and
resistant insects may help to explain resistance to the pesticide
dieldrin (a cyclodiene), which acts at the GABA receptor in insects
but also at the TBPS binding site, at which fipronil is inactive. A
dieldrin-resistant housefly was shown to have a low-affinity EBOB
binding site (see Bushey, 1993; Cole et al., 1993; Gant et al., 1994
for other references).
Although it was reported that fipronil did not affect other
enzyme or receptor systems, such as the acetylcholine receptor,
acetylcholinesterase, the octopamine receptor, and general oxidative
uncouplers, no supporting data were presented (Bushey, 1993).
(g) Studies on metabolites
(i) Acute toxicity
The results of studies on the toxicity of single doses of
fipronil metabolites are summarized in Table 4. The metabolic routes
by which they are formed are shown in Figures 2 and 3. In a study with
M&B 45950, clinical signs of neurotoxicity, including excessive
jumping, increased or reduced motor activity, clonic convulsions,
tremors, curling up, and subdued behaviour, were noted in all animals
at doses > 50 mg/kg bw, and deaths occurred during the first week
of the study, starting from 65 mg/kg bw in males and 90 mg/kg bw in
females (Dange, 1994a).
In a study with M&B 46136, clinical signs observed in animals of
each sex at 64 mg/kg bw within 2 h included hunched posture and
abnormal gait. Most signs such as hunched posture, abnormal gait,
lethargy, pallor of the extremities, diarrhoea, ataxia, clonic
convulsions, increased salivation, and decreased respiratory rate did
not appear until day 2 in animals at doses > 100 mg/kg bw. Deaths
occurred at doses > 100 mg/kg bw in animals of each sex on days 2
and 3 (Gardner, 1988c).
In a study with fipronil-desulfinyl, clinical signs including
reduced motor activity, dyspnoea, bradypnoea, and hyperreaction to
noise were observed in all animals at doses > 10 mg/kg bw. Clonic
and/or tonic convulsions were observed before death in one female at
20 mg/kg bw and in all males at 30 mg/kg bw. Deaths occurred at doses
> 20 mg/kg bw between days 2 and 4 after treatment (Dange, 1993b).
Metabolites were isolated from various tissues and milk and
identified by HPLC and mass spectrometry. After administration of 10
ppm, the major metabolite in faeces was M&B 46136, with lesser amounts
of fipronil, RPA 200766, and M&B 45950. In fat, milk, and muscle,
fipronil was predominant, with lesser amounts of RPA 200766 (in muscle
and fat), M&B46136, and M&B 45950. In kidney, M&B 46136 predominated,
with lesser amounts of fipronil; while in liver, M&B 46136 was the
major metabolite, with lesser amount of fipronil and RPA 200766. In
all cases, the major metabolite or species represented 44-75% of the
total radiolabelled residues. In urine, the mass of fipronil-derived
material was low, but M&B 46136 was found in small quantities. The
results were similar at 2 ppm, except that more fipronil was
metabolized to M&B 46136 in milk, muscle, and fat. According to the
proposed metabolic pathway for fipronil in ruminants, the sulfoxide
group of fipronil is oxidized to the sulfone (M&B 46136), which is
conjugated and excreted in the urine; the parent sulfoxide group can
also be reduced to the sulfide (M&B 45950), and the nitrile group of
the parent can be hydrolysed to the amide RPA 200766 (see Figure 2)
(Stewart, 1994a).
Laying hens
[phenyl(U)-14C]-Fipronil (19.2 mCi/mmol) was administered orally
in capsules daily before feeding to groups of five laying hens at
doses of 0.05, 2, or 10 ppm for 28 days; assuming a daily intake of
150 g dry matter, these doses were equivalent to nominal daily intakes
of 0.0075, 0.3, and 1.5 mg, respectively. Eggs were collected twice
daily. The animals were killed about 24 h after administration of the
final dose, and tissues were obtained.
Of the administered radiolabel, 28-42% was recovered in excreta,
15-18% in eggs, and 1-5% in tissues; the total recovered was 52-58%.
The greatest total tissue residues after administration of the highest
dose were found in peritoneal fat (56 ppm). The levels in eggs were
also high (30 ppm in yolks) after this dose and had not plateaued by
the end of the study. The levels were lower in skin (17 ppm) and much
lower in liver, egg white, and muscle. Metabolites were isolated from
various tissues and eggs and identified by HPLC and mass spectrometry.
After the 10 ppm dose, the main metabolite in peritoneal fat, egg
yolk, skin, and liver was M&B 46136, representing 96-98% of the total
radiolabelled residues. The remainder of the residue in these tissues
was parent fipronil. In egg white and muscle, M&B 46136 was the only
component of the residue. In excreta, parent fipronil comprised 51% of
the residue, and M&B 46136 accounted for 34%. The results with 0.05
and 2 ppm were similar. According to the proposed metabolic pathway
for fipronil in poultry, the sulfoxide group of the fipronil is
oxidized to the sulfone M&B46136 (see Figure 2) (Stewart, 1994b).
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of fipronil is summarized in Table 1. When
fipronil was administered as a single dose to mice or rats orally or
by inhalation, deaths and signs of toxicity occurred at all or most
doses in animals of each sex. Most or all of the deaths occurred
within several days of treatment. Clinical signs were generally noted
within 24 h of treatment and included tremors and convulsions of
various types, effects on activity or gait, hunched posture, wetness
in various body areas, and seizures (Gardner, 1988a; Cracknell, 1991;
Mondot & Dange, 1995; Nachreiner, 1995).
In studies with female Fischer 344 rats, the oral LD50 of
technical-grade fipronil (purity unspecified) dissolved in
glycerinformal was 175 mg/kg bw. The clinical signs of toxicity did
not reach their peak until two days after treatment in some animals,
and deaths did not occur until four days after treatment. Some signs
of toxicity and body-weight loss were still evident when the
observation period ended at day 7 after treatment. Since these
findings suggested that bioaccumulation of the test material could
occur, a five-day study with cumulative treatment was performed in
which groups of four female Fischer 344 were given fipronil at 75
mg/kg bw per day (one-half the minimum lethal dose determined in the
previous studies) orally for up to five days. Clinical signs of
neurotoxicity were seen after administration of two doses, and three
of four rats died after administration of three or four doses. In the
only rat that survived the study, abnormal behavioural responses
persisted until six days after administration of the final dose, at
which time it had regained most of its pretreatment weight (Ray,
1997).
The dermal LD50 for fipronil applied in distilled water to rats
was > 2000 mg/kg bw in both males and females, while that in rabbits
for test material moistened with corn oil was 354 mg/kg bw for the two
sexes combined. Neither clinical signs of toxicity nor deaths were
seen in rats. In rabbits, fipronil induced deaths and one or more
clinical signs of toxicity including convulsions, sluggishness,
salivation, spasms, tremors, hyperactivity, diarrhoea, emaciation, and
perioral and perinasal red discolouration in all groups except that at
the lowest dose (100 mg/kg bw). Delays in the appearance of signs of
toxicity and death were noted at all doses except the lowest. In
particular, convulsions were not observed until days 3-9 after
treatment, and some animals did not die until days 11-14 (Gardner,
1988b; Myers & Christopher, 1992).
Table 1. Acute toxicity of fipronil
Species Strain Sex Route LD50 or LC50 Reference
(mg/kg bw or
mg/L)
Mousea OF1 M Oralb 98 Mondot & Dange
F 91 (1995)
Rata Crl:CD (SD) BR M Oralc 92 Gardner (1988a)
F 103
Rata Crl:CD (SD BR M Dermald > 2000 Gardner (1988b)
F > 2000
Rat SD albino M,F Inhalation 0.68 Cracknell (1991)
(4-h exposure,
snout only)e
Rat SD albino M Inhalation 0.36 Nachreiner (1995 )
F (4-h exposure, 0.42
nose only)f
Rabbita New Zealand white M Dermalg 445 Myers & Christopher
F 354 (1992)
a Technical-grade fipronil; purity, 93-96.7%
b Administered in aqueous Tween 80 (0.2% w/v)
c Administered in corn oil
d Applied as a 90% w/v concentration in distilled water
e Manufacture-grade dry material; purity, 95.4%; not milled to reduce particle size; mass median
equivalent aerodynamic diameter (stated to be equivalent to mass median aerodynamic diameter),
6.4-8.5 µm
f Milled to meet US Environmental Protection Agency particle size requirements; mass median aerodynamic
diameter, < 2 µm
g Moistened with corn oil before application
(b) Short-term toxicity
Mice
In a preliminary study, groups of 12 male and 12 female CD-1 mice
were fed diets containing technical-grade fipronil (purity, 95.4%) at
levels of 0, 1, 3, 10, or 25 ppm (equal to 0, 0.13, 0.38, 1.3, or 3.2
mg/kg bw per day for males and 0, 0.17, 0.57, 1.7, or 4.5 mg/kg bw per
day for females) for 13 weeks. No clinical chemistry or haematological
measurements were conducted.
There were no deaths, clinical signs of toxicity, or effects on
food consumption. At 25 ppm, the body-weight gain of females was
statistically significantly decreased over the 13-week period to 63%
that of controls; in males, body-weight gains were decreased to 78% of
the control values, but the results were not significant. At necropsy,
there were no treatment-related macroscopic changes. The liver:body
weight ratio was statistically significantly increased in males (by
33%) and females (by 13%) at the high dose. Histopathological
examination revealed a dose-related increase in the incidence of
liver-cell periacinar hypertrophy with cytoplasmic vacuolation in
males (0/12 in controls, 2/12 at 1 ppm, 3/12 at 3 ppm, 6/12 at 10 ppm,
and 10/12 at 25 ppm), which was significant at 10 and 25 ppm.
Additionally, focal necrosis was observed in the liver of one male rat
at the high dose. In females, fatty vacuolation of the liver was
observed in two rats at 10 ppm and one at 25 ppm. There were no
reported effects on the thyroid. No NOAEL was identified because of
the histopathological changes in the livers of males at the lowest
dose (Broadmeadow, 1991).
Rats
Technical-grade fipronil (purity, 93%) was administered in the
diet for four weeks to groups of five Crl:CD (SD) BR rats of each sex
at concentrations of 0, 25, 50, 100, 200, or 400 ppm, equal to 0, 3.4,
6.9, 13, 24, or 45 mg/kg bw per day for males and 0, 3.5, 6.7, 13, 25,
or 55 mg/kg bw per day for females. Although there were no clinical
signs of toxicity, one female at 400 ppm died, however with no
accompanying clinical or pathological findings. Body-weight loss or
decreased body-weight gain seen in animals of each sex at doses >
100 ppm was temporary and possibly due to unpalatability, since food
consumption was also decreased in these groups. The platelet counts of
animals at 200 and 400 ppmwere marginally increased. The results of
urinalysis were negative. Increased total protein and globulin were
seen in all treated animals, and these increases were statistically
significant; however, they were small in comparison with the values in
controls and were poorly correlated with dose. Cholesterol levels were
increased in females at all doses and in males at the high dose.
The target organs were the liver and thyroid. Liver weights were
significantly increased in females at all doses and in males at 200
and 400 ppm. At necropsy, liver enlargement was observed in one or
both sexes starting at 50 ppm, and five males and three females at 400
ppm had enlarged livers. Generalized hepatocyte enlargement was
observed microscopically in one male at 100 ppm, with increasing
incidence in animals of each sex at 200 and 400 ppm. Thyroid
follicular-cell hypertrophy, generally of minimal severity but of
moderate severity in several males at 200 and 400 ppm, was found in
almost all treated animals but not in the controls. No NOAEL was
identified because of changes in blood chemistry in one or both sexes,
increased liver weights in females, and thyroid follicular-cell
hypertrophy in animals of each sex at the lowest dose (Peters et al.,
1990).
Technical-grade fipronil (purity, 95.4%) was administered for 13
weeks in the diet to groups of 10 male and 10 female CD rats at
concentrations of 0, 1, 5, 30, or 300 ppm, equal to 0, 0.07, 0.33,
1.9, or 20 mg/kg bw per day for males and 0, 0.07, 0.37, 2.3, or 24
mg/kg bw per day for females. Standard determinations of toxicity were
made ante and post mortem, and ophthalmological and neurological
examinations were conducted at week 12 on controls and animals at the
high dose. Haematological and clinical chemical evaluations were
performed after week 12.
There were no deaths. A clonic convulsion in one male at the high
dose may have been related to treatment. The ophthalmological and
neurological examinations showed no changes. The body-weight gain and
food consumption of animals at the high dose were decreased during the
first week of the study; by the end of the study, food consumption in
animals of each sex and total body-weight gain in males at 300 ppm
were comparable to those in controls but the total body-weight gain of
females was decreased by 9%. Statistically significant, but generally
minor alterations in comparison with controls were seen in numerous
haematological parameters in females at the high dose (and to a lesser
extent at 30 ppm) and appeared to be related to treatment. The
findings included lower packed cell volume, mean corpuscular volume,
haemoglobin concentration (also in males at the high dose and females
at 30 ppm), and prothrombin time (also in females at 30 ppm) and a
higher platelet count. Overall, minor and sometimes inconsistent
alterations were seen in a number of parameters of blood chemistry,
particularly in animals at 300 ppm and to a lesser extent at 30 ppm
(mostly in females); these were considered to be related to treatment.
The findings included slight but statistically significant increases
in total protein and a1-, a2-, and b-globulins, accompanied by
decreased albumin:globulin ratios at 300 ppm and increases in total
protein and one or more globulins at 30 ppm. At both doses, decreased
alanine and aspartate aminotransferases activities and increased
glucose were seen in females and increased urea in males. Animals at 1
and 5 ppm also showed fluctuations in proteins. These perturbations
may have been related to treatment but were not associated with other
significant findings.
The thyroid and liver were the target organs. The following
statistically significant changes in organ weights were observed at 13
weeks: absolute thyroid weights were increased in males and females at
300 ppm and in females at 30 ppm, and relative thyroid weights were
increased in animals of each sex at 300 ppm; absolute liver weights
were increased in males at 300 ppm and in females at doses > 5 ppm,
and relative liver weights were increased in animals of each sex at 30
and 300 ppm. The results of gross examination were unremarkable.
Histopathologically, a significant increase in the incidence of
hypertrophy of the follicular epithelium of the thyroid was seen in
females at the high dose; a nonsignificant increase was also observed
in males at this dose and to a lesser extent at 30 ppm. The incidence
of follicular-cell hyperplasia was nonsignificantly increased in
animals of each sex at the high dose. Liver sections stained with
haematoxylin and eosin from males and females at the high dose showed
a low incidence of panacinar fatty vacuolation; the incidence in males
at the high dose was more pronounced when Oil-Red-O staining was used.
The changes seen at the two lowest doses -- minor changes in
blood chemistry at 1 and 5 ppm in animals of each sex and increased
absolute (but not relative) liver weight in females at 5 ppm -- did
not appear to be toxicologically significant. At 300 ppm, both
haematological and further blood chemical parameters were altered,
absolute and relative liver weights were increased (p < 0.01) in
females, and significant increases were observed in absolute thyroid
weights in females and relative liver weights in males. Although not
significant, an increased incidence of hypertrophy of thyroid
follicular epithelium that was part of an increasing trend with the
higher dose was observed in males. When these changes are considered
together as part of a continuum towards more severe pathological
effects in the thyroid and liver and in the absence of tests for
thyroid function, the NOAEL was 5 ppm, equal to 0.33 mg/kg bw per day
(Holmes, 1991a).
Rabbits
Technical-grade fipronil (purity 96.7%) was applied in a 0.5%
aqueous solution of carboxymethylcellulose to the intact skin of
groups of six male and six female New Zealand white rabbits at doses
of 0, 0.5, 1, 5, or 10 mg/kg bw per day for 6 h per day for 15 days
within a three-week period. All animals survived. Effects were
observed only in animals at the high dose. Body-weight gains and food
consumption were reduced in animals of each sex over the course of the
study. One male and one female at the high dose showed signs of
extreme hyperactivity near the end of the study, which was possibly
related to treatment. No changes were seen in haematological or
clinical chemical parameters, organ weights, or on gross or
histopathological examination. No skin irritation was observed. The
NOAEL for systemic effects was 5 mg/kg bw per day (Hermansky & Wagner,
1993).
Dogs
Technical-grade fipronil (purity, 95.4%) was administered in
gelatin capsules to groups of four male and four female beagle dogs at
doses of 0, 0.5, 2, or 10 mg/kg bw per day for 13 weeks. Standard
determinations of toxicity were made ante and post mortem.
Neurological examination or testing of cranial nerve reflexes and
nerves, segmental reflexes, postural reactions, and general
observations of behaviour, gait, stance, and the presence of tremor or
other dyskinesia were conducted before treatment and on all surviving
animals after 6 and 12 weeks of treatment.
Mean body-weight gain over the course of the study was reduced by
up to 17% in females at the intermediate and high doses, and mean food
consumption was decreased by up to 9% in animals of each sex at the
high dose and in females at 2 ppm. Some animals at the high dose were
offered meat supplements, diets moistened with water, or an extension
of the feeding period in order to encourage eating. Mean body weight
and food consumption appeared to have recovered by the end of the
study. The results of ophthalmological, urinary, and haematological
examinations were unremarkable.
In animals at 10 mg/kg bw per day, significant clinical signs of
toxicity were seen, which were more prominent during the first two to
three weeks of treatment. These included inappetence, emaciation,
underactivity, weight loss, and hunched posture. Deterioration
progressed in some animals such that one male and three females at the
high dose had to be killed during the second week of treatment. Other
signs in animals at the high dose included dehydration, hypothermia,
subdued behaviour, excessive salivation, irregular heart rate,
convulsions, head nodding, tremors, limb jerk and extension, ataxia,
muscle twitching, abnormal reflexes, and apparent lack of vision. Some
of the last signs in particular were considered indicative of effects
on the central nervous system. The occurrence and frequency of signs
tended to diminish in surviving males after week 4 and in the
surviving female after week 7, although inappetence was seen in this
animal as late as week 12. The only clinical sign of toxicity observed
at 2 mg/kg bw per day was inappetence in two females; no signs were
observed in dogs at 0.5 mg/kg bw per day. Neurological effects were
seen only in animals at the high dose. One male showed head nodding,
facial twitching, and exaggerated blink and gag responses at week 6,
and one female had a depressed tactile placing response at week 12. At
weeks 6 and 12, alkaline phosphatase activity was increased and
cholesterol levels decreased by about 20% in males at the high dose.
The mean absolute and relative organ weights were not affected. No
effects were observed macroscopically, and the only microscopic
findings were follicular and parafollicular atrophy of the mesenteric
lymph nodes and cortical atrophy of the thymus in one male and one
female that were killed during the study; these were considered to be
related to stress. The NOAEL was 0.5 mg/kg bw per day (Holmes, 1991b).
Technical-grade fipronil (purity, 96.8%) was administered in
gelatin capsules to groups of six male and six female beagle dogs at
doses of 0, 0.2, 2, or 5 mg/kg bw per day for one year. For the first
15 days, the chemical was weighed directly into the capsules, but for
the remainder of the study an admixture of fipronil and lactose was
prepared in order to increase the accuracy of the dose. Standard
evaluations of toxicity ante and post mortem were included. In
addition, the brains and spinal cords of one or two animals in each
group that were still alive at the end of the study were examined
after fixation by perfusion with a 4% formaldehyde-saline solution.
Neurological examination or testing of cranial nerve reflexes and
nerves, segmental reflexes, and postural reactions and general
observations of behaviour, gait, and stance and the presence of tremor
or other dyskinesia were conducted before treatment and on all
surviving animals after 12, 24, 38, and 50 weeks of treatment. After
24 and 38 weeks, animals at the high dose were tested for their
proprioceptive positioning reaction ('knuckle' and 'foot sliding'
tests).
Clinical signs, many associated with neurotoxicity, were observed
in most animals at the intermediate dose and all those at the high
dose starting from the second week of treatment. These included
convulsions, twitching and tremors of various muscle beds (frequently
involving the head, pinnae, shoulder, hindlimbs, and sometimes the
whole body), ataxia, unsteady gait and rigidity of limbs (often the
hindlimbs), nervous behaviour, over- or underactivity, vocalization,
head nodding, aggression, resistance to treatment, and inappetence.
One male at 2 mg/kg bw per day and two at 5.0 mg/kg bw per day had to
be killed at weeks 11, 31, and 34, respectively, owing to
treatment-related poor condition. The signs observed in these animals
were similar to those described above and also included weight loss,
apparent loss of vision, and altered respiration. One female at 0.2
mg/kg bw per day showed signs of overactivity in weeks 13-18,
including pacing about the cage, which resulted in lesions on the foot
pad and tail, followed by weight loss and a period of underactivity. A
last incidence of overactivity was reported at about week 36;
adjustments to the diet and cage were used to control the
overactivity. The lack of similar findings in other animals at the low
dose and the overactivity of one control female for several days
around week 41 argued against a treatment-related effect.
The findings in animals at the intermediate and high doses in
routine physical examinations included tenseness, nervous and
excitable behaviour, abnormal stiffness or positioning of hindlimbs,
twitching of facial muscles, and hyperaesthesia. Most animals at the
high dose had exaggerated gag, corneal, blink, and hopping reflexes
and abnormal results in the 'foot sliding' test, and two females at
the intermediate dose showed tenseness. Body-weight gain over the
course of the study was decreased by about 16% only in females at the
high dose, due to effects in only one animal. Overall food consumption
was not affected. All animals were given an additional 200 g/day of
additional basal diet during weeks 16-18. Only minor changes were seen
in haematological and clinical chemical parameters, including slight
increases in packed cell volume, haemoglobin concentration, and
erythrocyte levels and in alanine aminotransferase activity in animals
at the intermediate or high dose, and were not clearly related to
treatment. The results of urinalysis and ophthalmological examinations
were negative. There were no clear-cut effects on organ weights and no
macroscopic or histopathological findings that appeared to be related
to treatment. The only remarkable finding in animals at 0.2 mg/kg bw
per day was overactivity in one female, described above. The NOAEL was
0.2 mg/kg bw per day (Holmes, 1992).
Technical-grade fipronil (purity, 95.4%) was administered in the
diet to groups of five male and five female beagle dogs at doses of 0,
0.075, 0.3, 1, or 3 mg/kg bw day for one year. The diets were given in
two aliquots 3.5-4.5 h apart and were moistened with water before
administration from day 30 onwards to enhance palatability. After the
first 38 days, the dose of 3 mg/kg bw per day was reduced to 2 mg/kg
bw per day because of significant toxicity. Standard evaluations of
toxicity ante and post mortem were included. Neurological
examination or testing of cranial nerve reflexes and nerves, segmental
reflexes, postural reactions, and general observations of behaviour,
gait, and stance, and for the presence of tremor or other dyskinesia
were conducted before treatment and on all surviving animals after 12,
24, 37, and 50 weeks of treatment. Blood samples taken after fasting,
before treatment and after one and 13 weeks of treatment, were
analysed for triiodothyronine (T3) and thyroxine (T4). Plasma from
fasted animals was analysed at weeks 1, 13, 24, 38, and 50 for the
presence of fipronil and a major metabolite, M&B 46136.
There were no overall effects on body-weight gain or food
consumption. The results of routine physical and ophthalmological
examinations and urinalysis were negative. None of the findings in
neurological, haematological, or clinical chemical tests, organ weight
measurements, or macro- or microscopic evaluations could be
definitively attributed to treatment. There were no treatment-related
findings in the animals at 0.3 or 0.075 mg/kg bw per day.
One female at 3 mg/kg bw per day had to be killed on day 32
because of poor health and signs of neurological disturbance. The
clinical signs of toxicity in this animal, which began on day 10,
included convulsions, underactivity, prostration, slow respiration and
tremors. Neurological examination revealed the absence of visual
placing reactions, depressed menace and startle reactions, and
abnormal gait. A blood sample showed increased packed cell volume,
haemoglobin concentration, erythrocyte count, plasma alkaline
phosphatase activity, and total protein and cholesterol
concentrations. Clinical signs of toxicity noted as early as week 1 in
three male and one female survivors at the high dose included
convulsions, head nodding, extensor rigidity, and twitching or tremors
of various muscle beds. One female at 1 mg/kg bw per day showed signs
of toxicity at week 13 (whole-body twitching) and another at week 20
(limb extensor rigidity). After 13 weeks of treatment, the T3 and T4
values were similar in all groups, including controls, the values for
T3 being 0.51-0.63 ng/ml and those for T4, 0.59-0.73 ng/ml.
Dose-related increases in the concentrations of fipronil and
metabolite M&B 46136 were seen in plasma at all measurement times.
Although there were no apparent differences between males and females
in the concentrations of these two compounds, the serum concentrations
of the metabolite were much higher than those of the parent compound
at doses > 0.075 mg/kg bw per day. At the lowest dose, the levels of
parent and metabolite were near or slightly higher than the limit of
quantification. There did not seem to be accumulation of either
chemical over time, and the concentrations of both compounds remained
fairly constant throughout the study. The NOAEL was 0.3 mg/kg bw per
day (Holmes, 1993).
(c) Long-term toxicity and carcinogenicity
Mice
Technical-grade fipronil (purity 95.4%) was administered for 78
weeks in the diet to groups of 52 male and 52 female CD-1 mice at
doses of 0, 0.1, 0.5, 10, 30, or 60 ppm (equal to group mean doses of
0, 0.011, 0.055, 1.2, or 3.4 mg/kg bw per day for males and 0, 0.012,
0.063, 1.2, or 3.6 mg/kg bw per day for females; the doses in mg/kg bw
per day were not determined for the group at 60 ppm) to evaluate
carcinogenicity. Six additional groups of 20 male and 20 female mice
were treated at the same doses for one year to measure toxicity.
Clinical signs of toxicity, body weights and weight gain, food
consumption, food efficiency, haematological parameters, organ weights
(absolute and relative to body weight), and macroscopic and
microscopic pathological appearance were observed.
Owing to excessive treatment-related mortality among animals of
each sex at 60 ppm, the surviving animals in the group were killed
during week 10. The lesions that had contributed to death were not
identified at necropsy, but all of the animals had high relative liver
weights. Several of the males had convulsions near the beginning of
the study. Reduced body-weight gains, food consumption, and food
efficiency were seen in these animals. The survival of the remaining
mice was comparable to or exceeded that of the control group, and no
clinical signs of toxicity were reported. Decreased body-weight gain
was seen in males (74-86% of control value) and females (81-86%) at 30
ppm at most evaluation times; the values for animals at 10 ppm were
also decreased but less consistently. Overall food consumption during
the study was lower than in controls in males (by about 7%) and
females (by 14%) at 30 ppm. Food efficiency was reduced in males and
females at 30 ppm and in males at 10 ppm.
A slightly lower percentage of neutrophils and a slightly higher
percentage of lymphocytes were noted in differential leukocyte counts
among females at 30 ppm after 76 weeks of treatment. Although gross
examination of animals observed for toxicity at week 53 showed no
remarkable effects, males at 30 ppm in the carcinogenicity study
showed liver enlargement and changes on the surface of the liver. The
absolute and/or relative liver weights were statistically
significantly increased in males and females at this dose at weeks 53
and 78. The relative liver weights were increased (p < 0.05) in
males at 10 ppm at both times and were increased (p < 0.05) in
males at 0.5 ppm at week 53 but not week 78. Histopathological
examination revealed a statistically significant increased incidence
of periacinar microvesicular vacuolation in the livers of males at 10
and 30 ppm at the end of 53 and 78 weeks, in females at 0.5 and 30 ppm
at the end of the toxicity study and in those at doses > 0.5 ppm
(significant at 0.5 and 30 ppm) at the end of the carcinogenicity
study; however, the incidence in females at 0.5 and 10 ppm did not
show a clear dose-response relationship, although the increased
incidence at 30 ppm may have been related to treatment. There was an
increased incidence of hepatocellular hyperplasia and chronic
degenerative changes in the livers of males at 30 ppm which died or
were killed during treatment in the carcinogenicity study.
There were no treatment-related neoplastic changes in females,
but males at 30 ppm had an increased incidence of malignant
hepatocellular carcinomas in comparison with concurrent controls: 1/52
in controls, 1/52 at 0.1 ppm, 2/52 at 0.5 ppm, 1/52 at 10 ppm, and
5/52 at 30 ppm. An additional hepatocellular carcinoma was observed in
one male at 30 ppm in the toxicity study. The incidence of
hepatocellular adenomas alone or combined with adenomas (one male at
30 ppm had both an adenoma and a carcinoma) was not significantly
increased. Since the increase in the incidence of carcinomas in males
at 30 ppm was within the range in historical controls in the testing
laboratory and the incidence among male concurrent controls was much
lower than the mean incidence in male historical controls, the
neoplastic findings in males were considered not to be related to
treatment. The NOAEL for systemic effects was 0.5 ppm, equal to 0.055
mg/kg bw per day (Broadmeadow, 1993).
Rats
Technical-grade fipronil (purity, 95.4%) was administered for one
year in the diet to groups of 15 male and 15 female CD rats to assess
its chronic toxicity, and further groups were fed the chemical for one
year and then observed for an additional 13 weeks to observe any
reversal of treatment-related changes. Groups of 50 male and 50 female
rats were originally scheduled to be treated for two years to assess
the carcinogenic potential of the chemical. The doses administered
were 0, 0.5, 1.5, 30, or 300 ppm, equal to 0, 0.019, 0.059, 1.3, or 13
mg/kg bw per day for males and 0, 0.025, 0.078, 1.6, or 17 mg/kg bw
per day for females. Standard evaluations of toxicity ante and
post mortem were included, and thyroid function (T3, T4, and
thyroid-stimulating hormone (TSH)) were measured in fasted animals
after 1, 4, 12, 24, and 50 weeks of treatment and after 2, 4, 7, and
11 weeks of the observation period after cessation of treatment.
The carcinogenicity phase of the study was terminated early owing
to excessive mortality and to ensure that a sufficient number of
animals were available for terminal sacrifices. Males and females were
killed when the number in any group declined to 25% of the original.
Thus, the males were killed after 89 weeks of treatment, when the
number of surviving animals in the group at 300 ppm was 25%, and the
females were killed after 91 weeks of treatment, when survival of
those at 30 ppm was 25%. More than 50% of animals of each sex in all
groups were still alive after 78 weeks. Early in the study, more
females at 300 ppm than controls died or were killed for humane
reasons related to convulsive episodes during this period. A
statistically significant increase in the number of deaths among
females at 30 ppm relative to controls disappeared when humane kills
were included in the mortality count. No significant differences in
mortality were seen among males or between other groups of females and
the control group.
Convulsive episodes, some lasting as long as 25 min and often
fatal, were observed in three males at 1.5 ppm, one male and three
females at 30 ppm, and eight males and 12 females at 300 ppm. The
convulsions tended to occur early in the treatment period but were
also seen later. Other clinical signs of toxicity that occurred
throughout treatment, predominantly in females at the high dose but
also in females given 1.5 and 30 ppm, included irritability,
vocalization, salivation, aggression, overactivity, and bruxism.
During the observation period, aggression, overactivity, irritability,
vocalization, and convulsions were seen in some females at 300 ppm.
Convulsions also occurred in females at 30 ppm and males at 300 ppm
but not in controls.
During the first week of treatment, body-weight gains were
significantly decreased in animals of each sex, by 6-11% at 30 ppm and
by 54-58% at 300 ppm. By one year, a significant 15-18% depression in
body-weight gain was observed only in animals at the high dose. By the
end of treatment, significant depressions were seen in males (18%) and
females (25%) at 300 ppm and in females at 30 ppm (23%). The
body-weight gain of males at 30 ppm was decreased by 7%. This pattern
continued during observation. Food consumption and food conversion
efficiency (calculated through week 14 only) were reduced at the
beginning of the study in animals of each sex at 300 ppm but were
similar to those of controls subsequently. The results of the
ophthalmological examination were negative.
Small but mostly significant decreases in haematological
parameters such as packed cell volume, haemoglobin concentration,
erythrocyte count, mean corpuscular volume, mean corpuscular
haemoglobin, and prothrombin time were seen at various times during
the study, particularly in males and females at the high dose. Slight
decreases in erythrocyte count, haemoglobin concentration, mean
corpuscular volume, and packed cell volume were also noted at 1.5 and
30 ppm.Towards the end of the study, platelet counts were slightly
increased in animals at the high dose and males at 30 ppm. Except for
a continued decrease in prothrombin time in females at 30 and 300 ppm,
the haematological changes did not persist after treatment.
Alterations in clinical chemical parameters, such as increased
cholesterol and calcium values and alterations in protein including
increased total protein, decreased albumin, increases in a1-, a2- and
b-globulins, and decreased albumin:globulin ratio, were seen mostly in
animals at 30 and 300 ppm; protein alterations were also observed in
males at 1.5 ppm towards the end of the study. Increased cholesterol
and calcium levels, total protein, and globulins and a decreased
albumin:globulin ratio persisted after cessation of treatment in
females at the high dose.
The T3 levels did not differ much from those of controls during
treatment but were significantly increased in females at the high dose
from four weeks after treatment and in females at 30 ppm from seven
weeks after treatment. The T4 levels were severely depressed after the
first week of treatment in animals of each sex, such that none was
detectable in animals at 300 ppm and the levels were significantly
depressed in a dose-dependent fashion in animals at 1.5 and 30 ppm.
Subsequently, the levels in animals at the high dose became
detectable, but the pattern of T4 depression observed at doses >
1.5 ppm generally persisted through week 50 of treatment. The T4
levels in animals at 0.5 ppm were occasionally significantly decreased
during treatment but not at the end of treatment. TSH levels were
significantly increased in animals at the high dose at all times and
in males at 30 ppm during the first month of treatment and after 50
weeks. Once treatment had ceased, the alterations in both T4 and TSH
levels were reversed in all groups except males at the high dose, in
which the TSH levels never recovered fully.
Alterations were seen at various times during the study in
urinary parameters (lower pH, higher protein, elevated urine volume,
and decreased specific gravity) in rats at 30 and 300 ppm
(predominately males). The alterations in protein and pH persisted
after treatment. Gross examination of animals in the carcinogenicity
study at termination and animals that were killed or died during the
study revealed increased incidences of large and/or pale kidneys in
animals at the intermediate or high dose and enlarged adrenals in
males at the high dose. Enlarged livers and thyroids were seen in
animals of each sex at the high dose and in males at 30 ppm. At
interim sacrifice (toxicity study), enlarged livers or thyroids were
seen in some animals at 30 or 300 ppm. Absolute thyroid weights were
somewhat increased in males at 0.5 and 1.5 ppm. The absolute and
relative weights of the liver and thyroid were increased in animals of
each sex at 30 or 300 ppm in both the toxicity and the carcinogenicity
study and in animals at the high dose that were killed or died during
the study. Increased absolute and relative kidney weights were also
seen in animals at 30 or 300 ppm, and increased adrenal weights were
seen in males at these doses. Most of the changes in organ weight were
statistically significant. The increases in liver, thyroid, and kidney
weights persisted to some degree after treatment, mostly in animals at
300 ppm. Histopathological examination showed an increased incidence
and severity of progressive senile nephropathy (a non-neoplastic
lesion) in animals of each sex at 300 ppm in the toxicity study and at
30 and 300 ppm in the carcinogenicity study; an increased severity of
the lesion was also reported in rats at 1.5 ppm. These findings
persisted to some extent after treatment.
Benign and malignant neoplastic changes (follicular-cell adenomas
and carcinomas) occurred in the thyroid glands of animals of each sex.
The incidence of these tumours per number of animals examined at 0,
0.5, 1.5, 30, and 300 ppm was, respectively: malignant follicular-cell
carcinomas: 0/49, 0/48, 0/50, 0/50, and 5/50 for males and 0/50, 1/50,
0/50, 1/50, and 2/50 for females; benign follicular-cell adenomas:
0/49, 1/48, 5/50, 3/50, and 12/50 for males and 0/50, 0/50, 0/50,
0/50, and 8/50 for females; total tumours: 0/49, 1/48, 5/50, 3/50, and
17/50 for males and 0/50, 1/50, 0/50, 1/50, and 10/50 for females.
None occurred in concurrent controls. Males in all treated groups and
females in all groups except that receiving 1.5 ppm showed increased
incidences of benign and malignant thyroid tumours combined in the
carcinogenicity study. The increases were significant (at p < 0.05,
< 0.01, or < 0.001 by Fisher's exact one-tailed test for pair-wise
comparisons) for carcinomas alone in males at the high dose and for
adenomas alone and adenomas and carcinomas combined in males and
females at 300 ppm and males at 1.5 ppm. The author concluded that
only the neoplastic changes observed in animals at 300 ppm were
related to treatment, as the increased incidences of benign or
malignant lesions alone or in combination exceeded the historical
control incidences for the testing facility only at the high dose, and
the zero incidences in the concurrent controls were unusually low. The
reported historical control incidence rates for studies of comparable
length (88-95 weeks) were 0-5.5% (males) and 0% (females) for
follicular-cell carcinoma, 1.4-5.7% (males) and 0-1.9% (females) for
follicular-cell adenoma, and 1.9-7.3% (males) and 0-1.9% (females) for
all follicular-cell tumours. The occurrence of these tumours was
attributed to continuous stimulation of the thyroid gland by elevated
TSH levels.
Although the author concluded that there were no significant
intergroup differences in mortality among males, analysis of the data
by the US Environmental Protection Agency using the computer program
of Thomas, Breslow, and Gart showed a significantly increasing trend
in mortality with increasing doses of fipronil in male but not female
rats. This finding does not affect the validity of the study for the
following reasons: firstly, the study was terminated prematurely only
near its end; secondly, the literature indicates that, in general, the
longevity of CD (Charles River) rats has been decreasing and that a
shortened life span is therefore not unique to this study; and
thirdly, the study was long enough for tumours to have developed in
the fipronil-treated animals.
The initial analysis of tumour incidence was based only on the
data from the carcinogenicity phase of the study. Six animals were
reported to have developed thyroid follicular-cell tumours during the
observation phase, with carcinomas in one male at 30 ppm and one at
300 ppm and adenomas in one female at 1.5 ppm and one male and two
females at 300 ppm. No thyroid tumours considered to be related to
treatment were reported to have occurred during the toxicity phase,
although a follicular-cell carcinoma was tabulated for one male at 30
ppm killed after one year of treatment. When these additional data are
included in the analysis and the data are analysed on the basis of the
first appearance of thyroid adenomas or carcinomas in the study, the
incidence is as shown in Table 2. When Peto's prevalence test is used
to analyse the data for male rats (as the first tumour was seen before
interim sacrifice), significantly increasing trends (p < 0.01) are
obtained for thyroid follicular-cell adenomas, carcinomas, and
combined adenomas and carcinomas. Significant differences from
controls in pair-wise comparisons (p < 0.05 or p < 0.01) are
found for adenomas and combined adenomas and carcinomas at doses >
1.5 ppm and for carcinomas at the high dose. When the exact trend test
and Fisher's exact test are used to analyse the data on tumours in
female rats, a significantly increasing trend and a significant
difference in pair-wise comparisons of the group at 300 ppm with the
controls is seen for thyroid follicular-cell adenomas and combined
adenomas and carcinomas (all at p < 0.01).
This analysis suggests that doses > 1.5 ppm are carcinogenic,
since the pair-wise comparisons in males at doses > 1.5 ppm are
significant and there were significant trends for tumour formation.
Since the concurrent controls had an apparently lower incidence than
historical controls at the laboratory where the study was conducted,
however, the apparent inconsistency in the dose-response relationship
at 1.5 and 30 ppm and the fact that TSH levels were significantly and
persistently elevated only at 300 and to a lesser extent at 30 ppm, an
alternative view is that the incidence of thyroid tumours was
toxicologically significant only at the highest dose. In summary, the
study demornstrates that fipronil is clearly carcinogenic to rats at
300 ppm; however, the overall NOAEL is for neurotoxicity and is
0.5 ppm, equal to 0.019 mg/kg bw (Aughton, 1993).
(d) Genotoxicity
The results of assays for genotoxicity with fipronil are
summarized in Table 3.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
In a two-generation study of reproductive toxicity, 30 male and
30 female CD rats received technical-grade fipronil (purity, 95.4%) in
the diet at concentrations of 0, 3, 30, or 300 ppm, equal to 0, 0.25,
2.5, or 26 mg/kg bw per day for males and 0, 0.27, 2.7, or 28 mg/kg bw
per day for females. After two matings of the F0 generation, litters
were culled to four animals of each sex on day 4 post partum, and
physical development was assessed by recording the day of onset and
completion of pinna unfolding, hair growth, tooth eruption, and eye
opening.
Table 2. Incidences of thyroid follicular-cell tumours in rats treated with fipronil
Tumour Dose (ppm)
Males Females
0 0.5 1.5 30 300 0 0.5 1.5 30 300
Adenomas 0/63 1/61 5/63 3/62 12/61 0/48 0/49 0/50 0/45 8/46
% 0 2 8 5 20 0 0 0 0 17
p 0.000** 0.116 0.014* 0.038* 0.000** 0.000** 1.000 1.000 1.000 0.002**
Carcinomas 0/59 0/57 0/62 1/60 5/57 0.48 1/49 0/50 1/45 2/46
% 0 0 0 2 9 0 2 0 2 4
p 0.000** - - 0.186 0.007** 0.084 0.505 1.000 0.484 0.237
Combined 0/63 1/61 5/63 4/62 16/61 0/48 1/49 0/50 1/45 10/46
% 0 2 8 6 26 0 2 0 2 22
p 0.000** 0.116 0.014* 0.024* 0.000** 0.000** 0.505 1.000 0.484 0.001**
For males: no. of tumour-bearing animals/no. of animals examined, excluding those that died before observation of
the first tumour; analysis by Peto's prevalence test. For females: no. of tumour-bearing animals/no. of animals
examined, excluding those that died or were killed before week 54; analysis by exact trend test and Fisher's exact
test. First adenoma observed at 300 ppm in males in week 42 and in females at week 62. First carcinoma observed in
males at 30 ppm in week 53 and in females at 300 ppm in week 79. One male at 300 ppm had both an adenoma and a
carcinoma.
Significance of trend denoted at control; significance of pair-wise comparison with control denoted at dose:
*, p < 0.05; **, p < 0.01
Table 3. Results of assays for genotoxicity with fipronil
End-point Test object Concentration Purity Result Reference
(%)
In vitro
Reverse mutation S. typhimurium 0.8-500 µg/plate 90.6 Negativea,b Clare (1988a)
TA98, TA100, in DMSO
TA1535, TA1537
Gene mutation Chinese hamster 1.13-386 µg/ml 97.2 Negativea,b Lloyd (1990)
cell line V79, in DMSO
hprt locus
Chromosomal Chinese hamster 15-60/µg/ml 6 ha, 98.3 Positiveb,d Wright (1995)
aberration lung cell line 7.5-30 µg/ml 24 hc,
7.5-22.5 µg/ml 48 hc
in DMSO
Chromosomal Human lymphocytes 75-300 µg/ml in 90.6 Negativeb Marshall (1988a)
aberration DMSO
In vivo
Micronucleus CD-1 mice 1-25 mg/kg bw in 97.2 Negativeb,e Edwards (1991)
formation aqueous methyl-
cellulose
Micronucleus CD-1 mice 6.95-48 µg/kg bw 96.2 Negativeb Edwards (1995)
formation in aqueous methyl-
cellulose
DMSO, dimethyl sulfoxide
a With and without metabolic activation
b Appropriate positive controls gave expected positive responses
c Without metabolic activation
d Positive only with 6-h pulse treatment; significant, dose-related effects at 45 and 60 µg/ml without
metabolic activation; non-significant increase at 60 µg/ml with metabolic activation
e Unacceptable; no overt or target-cell toxicity at doses < 25 mg/kg; results at 50 mg/kg were inconclusive.
Systemic toxicity was seen in the parental animals at doses >
30 ppm as increased absolute and relative weights of the thyroid gland
and liver in the F0 and F1 generations, decreased absolute and
relative pituitary gland weights in the F1 females (a decrease in
relative pituitary weight at 3 ppm was minor and was not considered to
be toxicologically significant), and a significantly increased
incidence of follicular epithelial hypertrophy of the thyroid gland in
F1 females and animals of each sex at 300 ppm. Increased mortality
occurred among F0 and F1 animals at this dose, with clinical signs
of toxicity including convulsions; in addition, the F0 generation had
decreased food consumption before mating, and decreased body-weight
gain was seen before mating in the F0 and F1 generations and in F0
females during gestation and lactation. The absolute and relative
weights of the ovaries were decreased in F0 females, and females of
the F0 and F1 generations had a significantly increased incidence of
centriacinar fatty vacuolation in the liver. The increased incidence
of follicular epithelial hypertrophy in adult males at 30 ppm was not
significant but was found in both the F0 generation (in 2/30 animals,
in comparison with 0/30 controls, 0/30 animals at 3 ppm, and 10/29
animals at 300 ppm) and the F1 generation (in 3/30 animals, with 0/30
controls, 2/30 at 3 ppm, and 9/30 at 300 ppm) and was considered to be
related to treatment. The presence of this finding in 2/30 F1 males
at 3 ppm, and not in the F0 generation, was considered plausible in
an organ in rats that is very sensitive to stimuli; it could therefore
not be clearly related to treatment.
Reproductive toxicity observed in animals at 300 ppm consisted of
an increased incidence of clinical signs of toxicity in F1 and F2
offspring (notably convulsions when the offspring first started to eat
the treated diet), decreased litter size and body weights, a decrease
in the percentage of animals that mated, a reduction in the fertility
index of F1 parental animals, reduced postimplantation and postnatal
survival in the F2 litters, and delays in physical development in F1
and F2 litters including slight delays in the onset of tooth eruption
(F1) and pinna unfolding (F2). The NOAEL for parental toxicity was 3
ppm, equal to 0.25 mg/kg bw per day, while that for reproductive
toxicity was 30 ppm, equal to 2.5 mg/kg bw per day (King, 1992).
(ii) Developmental toxicity
Rats
Technical-grade fipronil (purity, 93%) was administered by gavage
as a suspension in 0.5% aqueous methylcellulose to groups of 25
specific pathogen-free female rats of the Crl: CD (SD)BR VAF/Plus
strain (Charles River, France) at doses of 0, 1, 4, or 20 mg/kg bw per
day on days 6-15 of gestation. The study was terminated on day 20.
Adult females were observed for clinical signs, food and water
consumption, and body-weight changes; they underwent a macroscopic
post mortem at study termination. Litters and fetuses were evaluated
with regard to pre- and postimplantation losses, litter size, litter
and mean fetal weights, sex ratios, and malformations or skeletal or
visceral anomalies.
The pregnancy rate was 96-100%. There were no deaths, abortions,
premature deliveries, or clinical signs in the adult females. Maternal
effects associated with treatment occurred only in the animals
receiving the high dose and included reduced body-weight gain,
increased water consumption, and decreased food consumption at various
intervals during gestation. Some of these effects continued after
treatment. The macroscopic examination showed no remarkable effects.
No effects of treatment were seen on developmental parameters. The
NOAEL for maternal toxicity was 4 mg/kg bw day, and that for
developmental toxicity was 20 mg/kg bw day (Brooker & John, 1991).
Rabbits
Technical-grade fipronil (purity, 95.4%) was administered by
gavage as a suspension in 0.5% aqueous methylcellulose mucilage and
0.5% Tween 80 to groups of 22 female New Zealand white rabbits (Ranch
Rabbits, Susse, United Kingdom) at doses of 0, 0.1, 0.2, 0.5, or 1
mg/kg bw per day on days 6-19 of gestation. The study was terminated
on day 29. Adult females were observed for clinical signs, abortion
and total litter loss, food and water consumption, and body-weight
changes; a macroscopic post mortem was performed at study termination.
The litters and fetuses were evaluated with regard to pre- and
postimplantation losses, litter size, litter and mean fetal weights,
sex ratios, and malformations or skeletal or visceral anomalies.
The number of pregnant animals in each group ranged from 18 to
21. There were no treatment-related deaths. At doses of 0.5 and 1 g/kg
bw per day, maternal body-weight gain during treatment was
significantly lower than that of controls by 50 and 70%, respectively,
and the body-weight gain of dams at 0.1 or 0.2 mg/kg bw per day was
reduced by 30%. Animals in all fipronil-treated groups consumed less
food than those in the control group during treatment, and the
decreases in the groups at the two highest doses achieved significance
on gestation days 6-12 and/or 13-19. Other maternal parameters were
not affected. Treatment had no effect on development. No NOAEL for
maternal toxicity was identified; that for developmental toxicity was
1 mg/kg bw per day (King, 1990).
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Technical-grade fipronil (purity, 96.7%) moistened with corn oil
was a slight dermal irritant in male and female New Zealand white
rabbits after a 4-h application to intact skin. The signs of
irritation were slight to well-defined erythema and slight oedema,
which had cleared by day 7 after application. When fipronil (purity,
93%) was moistened with distilled water and applied for 4 h to the
intact skin of male New Zealand white rabbits, no signs of dermal
irritation were seen (Liggett, 1988a; Myers & Christopher, 1993a).
Instillation of technical-grade fipronil (purity, 96.7%) into the
eyes of male and female New Zealand white rabbits produced minor
transient corneal opacity and iritis, which had cleared within 24 h,
and minor-to-moderate conjunctival irritation, which had cleared
within 2-14 days. In a similar study with only male rabbits, fipronil
(purity, 93%) induced transient, minimal conjunctival inflammation,
which cleared within 24-48 h (Liggett, 1988b; Myers & Christopher,
1993b).
Technical-grade fipronil (purity, 95.4%) was tested in male and
female Dunkin-Hartley albino guinea-pigs by a modification of the
Buehler method. Topical application of the highest optimal
concentration for induction and challenge of fipronil (30% w/v) in
paraffin oil to guinea-pig skin produced no sign of dermal
sensitization (Smith, 1990).
In a Magnusson-Kligman maximization test in male and female
Dunkin-Hartley albino guinea-pigs, the optimal induction and challenge
concentrations of technical-grade fipronil (purity, 95.4%) were
determined in advance. In the main study, primary induction
concentrations of 5% (w/v) fipronil in propylene glycol alone or
combined with Freund's complete adjuvant were applied intradermally at
separate sites. In the secondary induction phase, 5% fipronil in
propylene glycol was applied topically to the same sites. A topical 3%
challenge dose produced no significant response, but 24 h after
challenge with 10% fipronil in propylene glycol, four of 20 animals
showed a significant dermal response (eschar formation or slight
erythema). Eschar formation was still evident in two animals 48 h
after the challenge. No significant responses were observed in control
animals challenged with 3 or 10% fipronil in propylene glycol. One
control animal challenged with propylene glycol alone showed slight
erythema at 24 h. By the criteria of the European Economic Community,
the test material did not cause delayed contact hypersensitivity
because the response rate at challenge was < 30%; however, the
positive response rate of 20% in this study could also support the
conclusion that fipronil is a mild or weak skin sensitizer (Johnson,
1993).
(ii) Neurotoxicity
Rats
A single dose of technical-grade fipronil (purity, 96.7%) in corn
oil was administered by gavage to groups of 15 male and 15 female
Sprague-Dawley rats at doses of 0, 0.5, 5, or 50 mg/kg bw. The animals
were about eight weeks of age at the time of treatment. Standard
evaluations of toxicity included observations for mortality and
clinical signs of toxicity and measurements of body weight. The
neurobehavioural evaluations of all surviving animals included a
functional observational battery of tests before treatment and at 7 h,
7 days, and 14 days after treatment, and quantitative assessment of
motor activity before treatment and 1 h after each functional test.
The study was terminated on days 16-19 after treatment. The postmortem
examinations included an abbreviated necropsy of the thoracic and
peritoneal cavities (all groups) and detailed light microscopic
evaluation of the brain, spinal cord, and peripheral nerves of six
male and six female controls and six animals of each sex at the high
dose after intracardiac perfusion in situ with 10% neutral buffered
formalin. Rats that died during the study were necropsied, but their
nervous tissues were not examined microscopically.
Five males and one female at 50 mg/kg bw died during the study,
most within two days of treatment. At necropsy, most had diffuse brain
haemorrhages, which may have caused death or may have been agonal.
Clinical signs of toxicity were seen only at the high dose, and the
incidence of neurological signs was more prevalent in males. On the
day of treatment, males and females at this dose had either clonic or
tonic-clonic convulsions. Other clinical signs of toxicity included
those indicative of cachexia: emaciation, dehydration, unkempt
appearance, urine stains, cold extremities, and/or pallor. Male body
weight was reduced by 6-10% relative to that of controls 7 and 14 days
after treatment.
Treatment-related effects on functional parameters occurred
primarily 7 h after treatment in rats at 50 mg/kg bw, when males were
seen to have drooping or half-shut eyelids. During open-field
evaluations, both stimulation and depression of the nervous system
were seen, which were generally more pronounced in males. Signs
indicative of stimulation of motor systems were convulsions, coarse
and fine tremors, head bobbing, myoclonic movements, and decreased
hindleg splay, which was significantly decreased (p < 0.01) in
animals of each sex. The signs indicative of nervous system depression
were decreased arousal and rearing activity, decreased reflexes such
as response to tail pinching and approach and air righting reflex, and
decreased muscle tone, altered gait, decreased pupil size, and/or
decreased body temperature. Urination was more evident in males at the
high dose. The only treatment-related effect in rats at 5 mg/kg bw 7 h
after treatment was significantly decreased hindleg splay (p <
0.05) in animals of each sex. A stimulatory response in certain
functional tests was seen in some males at the high dose seven days
after treatment, such as increased arousal and rearing activity and
exaggerated touch and sound reflexes. Females at the high dose showed
significantly increased hindleg splay at this time and on day 14 after
treatment. The only finding of note in males at the high dose on day
14 was a higher incidence of urination.
Motor activity 8 h after treatment was decreased by 90% in males
at the high dose and by 93% in females. This was the only effect on
motor activity during the study that was attributed to treatment.
There were no treatment-related gross or microscopic changes on
post-mortem examination of the central and peripheral nervous systems.
The NOAEL was 0.5 mg/kg bw on the basis of decreased hindleg splay 7 h
after treatment (Gill et al., 1993).
Groups of 15 male and 15 female Sprague-Dawley rats were fed
diets containing technical-grade fipronil (purity, 96.7%) at 0, 0.5,
5, or 150 ppm (equivalent to 0, 0.03, 0.3, or 8.9 mg/kg bw per day for
males and 0, 0.03, 0.3, or 11 mg/kg bw per day for females) for 13
weeks. The animals were about eight weeks of age at the start of
treatment. Standard evaluations of toxicity included observations for
mortality and clinical signs of toxicity and measurements of body
weight. Neurobehavioural screening, consisting of a functional
observational battery of tests and motor activity evaluations, was
performed before treatment and during weeks 4, 9, and 13. Post-mortem
examinations at terminal sacrifice, included an abbreviated necropsy
of the thoracic and peritoneal cavities in all groups and a detailed
light microscopic evaluation of the brain, spinal cord, and peripheral
nerves of six males and females of the controls and at the high dose
after intracardiac perfusion in situ with 10% neutral buffered
formalin.
There were no treatment-related deaths or clinical signs of
toxicity. Slight decreases in body weight (6.5-6.9%) and decreased
body-weight gain were seen in males and females at the high dose
during the first few weeks of treatment. These were accompanied by
decreased food consumption, suggesting unpalatability. Functional
changes seen in animals at the high dose in week 4, 9, and/or 13 were
increased urination and an increased incidence of exaggerated response
to tail pinching in males, an increased incidence of exaggerated
startle responses during manipulation in animals of each sex, and
increased forelimb grip strength in females at week 13. No
treatment-related findings were seen at necropsy or during
histopathological examination of nervous tissues. The changes in
animals at the high dose may not have been related to treatment;
however, these findings, although relatively minor when taken
separately, represent a minimal effect of treatment when taken
in toto. In addition, the exaggerated responses to touch and sound,
particularly in males, and the increased urination of males at the
high dose were considered to be related to treatment in the preceding
study. Therefore, the NOAEL for both neurotoxicity and systemic
toxicity was 5 ppm, equal to 0.3 mg/kg bw per day (Driscoll & Hurley,
1993).
Dogs
Technical-grade fipronil (purity, 95.4%) was administered in
capsules to female beagle dogs (26 weeks old at the start of the
study) at doses of 0 (one animal) or 20 mg/kg bw per day (four
animals) until clear signs of neurotoxicity were seen or for a maximum
of 14 days. Immediately after the appearance of neurotoxic signs,
administration was discontinued and a 28-day observation period was
initiated. This phase was begun after five days of treatment in two
animals, seven days of treatment in one animal, and 13 days of
treatment in one animal and the control dog. Animals were monitored
for general health, clinical signs of toxicity, chronic conditions,
food and water consumption, and body-weight changes and were subjected
to a weekly veterinary examination and neurological examinations
before treatment and then at intervals during treatment and
observation. The neurological examination consisted of observation and
testing of cranial nerve reflexes, segmental reflexes, and postural
reactions and general observation of behavioural changes,
abnormalities of gait and stance, and the presence of tremor or other
dyskinesia. An obstacle-avoidance test and a hearing test were
administered at the same time as the neurological examination.
Anaesthetized dogs were killed by perfusion-fixation at the end of the
observation phase. The brain, spinal column, and some peripheral
nerves were examined grossly, but only the brain and spinal column
appear to have been subjected to microscopic examination.
All animals survived to the end of the observation period. The
control animal showed no abnormal signs throughout the treatment and
recovery periods, but all treated animals displayed neurotoxic signs,
which included hypoactivity, salivation, ataxia, convulsions, tremors,
unsteady gait and stiffened body, aggressive behaviour, apparent lack
of vision, muscle twitching, nervous behaviour, and a high-stepping
gait. The time to appearance of signs and the duration of signs after
discontinuation of treatment varied. Some signs, like aggressive
behaviour and visual impairment, first appeared after discontinuation
of treatment. Food consumption was markedly decreased from day 1 or 2
of treatment and for up to seven days during the recovery period. All
treated animals lost weight, but the initial body weight was restored
17 days after cessation of treatment. Veterinary examination showed
similar changes and also emaciation in three treated animals and
hunched posture and peripheral vasodilatation in one animal. The
neurological examinations indicated widely varied responses including
(in addition to the responses noted above) changes in reflex
responses, some exaggerated, including gag and flexor responses, and
some depressed, including blink, pupillary, and consensual light
reflexes and visual and tactile placing responses. One animal still
had slightly exaggerated gag and flexor reflexes at the end of the
recovery period. The results of the obstacle-avoidance and hearing
tests indicated temporary visual impairment and possible hearing
deficiency in one animal. No abnormal findings were reported after
macroscopic or microscopic examination.
Virtually complete recovery from what appeared to be functional
changes in the central nervous system was reported by 12 days or less
after cessation of treatment; in the absence of histopathological
changes in the study, the mode of action of the chemical was
considered probably to be pharmacological and temporary. Given the
short duration of treatment (5-13 days), the short observation period
(treatment period plus 28 days), the use of only one dose, one sex,
and only one control animal, however, there is insufficient
information to draw any firm conclusions about the reversibility of
the functional, cellular, and neurotransmitter or receptor effects.
There was no NOAEL (Holmes, 1991c).
(iii) Developmental neurotoxicity
Technical-grade fipronil (purity, 96.1%) was administered to
groups of 30 female Sprague-Dawley (CD) rats at dietary levels of 0,
0.5, 10, or 200 ppm, equal to 0, 0.05, 0.9, or 15 mg/kg bw per day,
from gestation day 6 through lactation day 10, day 0 being defined as
the day mating was confirmed. Clinical observations, body weights, and
food consumption were recorded for all animals at scheduled intervals
throughout gestation and lactation, and litters were examined at
selected intervals throughout lactation. The numbers of live and dead
pups and the sex and body weights of the pups were recorded. Litters
were standardized to eight pups on postnatal day 4. The achievement of
pinna detachment, incisor eruption, eye opening, vaginal patency, and
preputial separation was evaluated for all surviving pups. For one pup
per sex per litter, the following evaluations were conducted on
various postnatal days: motor activity (days 13, 17, 22, 60), auditory
startle response (days 22 and 60), swimming development (days 6, 8,
10, 12, and 14), and learning and memory in a Y-maze (days 24 and 60).
On day 11 of lactation and again on day 60, one pup of each sex per
litter was killed. Brain weights were recorded and neuropathological
evaluation was performed on six pups of each sex from the control and
high-dose groups. Maternal animals were killed and necropsied after
the weaning of the last litter, and all remaining offspring were
killed soon after all the evaluations were completed.
The maternal effects at the dietary level of 200 ppm were reduced
body weight during treatment, reduced body-weight gain during
gestation, and reduced food consumption. No treatment-related findings
were observed at necropsy. Pregnancy status, gestation length, litter
size, and pup sex ratio were not affected by treatment. At 200 ppm,
there was a significant increase in the number of stillborn pups, and
pup and litter survival were reduced. Also at this level, male and
female pup weights were reduced, and upper and lower incisor eruption
and sexual development were delayed. There was a small but significant
increase in the time to preputial separation in animals at 10 or 200
ppm. At 10 ppm, group mean body weights were reduced for female pups
at all recorded intervals and for male pups on days 4 and 17;
postweaning weights were not affected.
With regard to neurodevelopmental effects, at 200 ppm the maximum
auditory startle response time for males and females was significantly
decreased on postnatal day 22; there was no significant difference in
the time to maximum or average response. Swimming development was
delayed on postnatal day 6 for animals of each sex, and motor activity
was increased in females on postnatal day 17. Learning and memory
(Y-maze test) were delayed significantly for females in two trials on
postnatal day 24. Also at 200 ppm, significant decreases in absolute
pup brain weights were noted for animals of each sex on days 11 (-20%
for males and -11% for females) and 60 (-7% for males and females),
but pup body weights were also significantly decreased at these times,
so that the relative brain:body weights were actually increased
relative to those of concurrent controls: +39% for males and +20% for
females on day 11 and +6% for males and females on day 60. Thus, the
lower brain weights may have been due to the overall retarded growth
of the pups associated with maternal toxicity rather than to a
developmental delay in brain size. The statistically significant
increases in motor activity in female pups at 10 ppm observed on day
17 could not be clearly related to treatment when the magnitude of the
response was compared with that of the control group, because the
values for the latter on that day appeared to be unusually low. No
microscopic morphological abnormalities were seen in the brains of
offspring killed on postnatal day 11 or 60. The NOAEL for maternal and
neurodevelopmental effects was 10 ppm, equal to 0.9 mg/kg bw per day.
The NOAEL for developmental effects was 0.5 ppm, equal to 0.05 mg/kg
bw per day, on the basis of reduced pup body weights (Mandella, 1995).
(iv) Thyroid function
Effects on thyroid hormone levels: Groups of 10 Crl:CD (SD) BR
rats of each sex were given technical-grade fipronil (purity, 95.4%)
in the diet at doses of 0, 0.1, 1, 5, or 30 ppm (equal to 0, 0.01,
0.1, 0.49, or 2.9 mg/kg bw per day for males and 0, 0.01, 0.1, 0.48,
or 2.9 mg/kg bw per day for females) for four weeks. The parameters
measured were clinical observations, body weight, food consumption and
efficiency, water consumption, evaluations of T3, T4, and TSH before
treatment and on days 7 and 28, and organ weights and histopathology
of the thyroid and liver at necropsy.
The T3 level was significantly decreased in males at doses > 1
ppm on day 7 and was increased in females at 30 ppm on day 28; T4
levels were significantly decreased in males and females at 30 ppm,
males at 5 ppm on day 7, and males at 30 ppm on day 28; the TSH level
was significantly increased in males at 30 ppm on days 7 and 28 and in
females at 30 ppm on day 28; thyroid weights were nonsignificantly
increased in males and females at 30 ppm; and there was an increased
incidence of higher follicular epithelium in males and females at 30
ppm and in one male at 5 ppm. As re-analysis of the data for T3 in the
controls and rats at 1 ppm showed no significant difference, it was
concluded that this level had no effect.
Increased liver weights were observed in animals at 30 ppm;
however, the change was significant only in females. An increased
incidence of minimal centrilobular hepatocyte enlargement was seen in
males at 30 ppm. Staining of liver sections with Oil Red O
demonstrated an increased incidence of periportal fat deposition in
females at 5 and 30 ppm. The NOAEL was 1 ppm, equal to 0.1 mg/kg bw
per day (Peters et al., 1991a).
Effect on biliary excretion of thyroxine: The objective of this
study was to measure the effects of single and repeated oral doses of
technical-grade fipronil (purity, 96.7%) on the biliary excretion of
intravenously administered 125I-T4 in bile duct-cannulated rats.
Groups of three Sprague-Dawley CD(Crl:CD/BR) VAF plus strain male rats
were given single or repeated daily doses for 14 days of: fipronil (1
or 10 mg/kg bw per day orally at 5 ml/kg bw), phenobarbital (80 mg/kg
bw per day intraperitoneally as a positive control), or 0.5%
methylcellulose (5 ml/kg bw orally as a negative control). Immediately
after treatment, the animals were anaesthetized, the common bile duct
was cannulated, and 1 mg sodium iodide (to block thyroid uptake of
125I) was administered by injection into the stomach, followed 4 h
later by intravenous administration of about 10 µCi of 125I-T4. Bile
samples were collected before treatment and at 15-min intervals for
0-5 h after 125I-T4 administration. Blood samples were taken at 30-min
intervals. Both bile and blood were analysed for radiolabel. After 5
h, the animals were killed and the livers removed. Selected bile
samples were pooled, treated with œ-glucuronidase and analysed by HPLC
for free and conjugated T4.
The single doses of fipronil or phenobarbital did not
significantly increase the amount of bile excreted or the biliary
clearance of 125I-T4. After 14 days of treatment, biliary clearance
was significantly increased with the high dose of fipronil but not
with the low dose. The high dose of fipronil produced a greater
increase than phenobarbital. Bile output was significantly increased
after treatment with the high dose of fipronil and with phenobarbital.
After treatment with œ-glucuronidase, about 50% of the 125I-T4 in bile
was conjugated with either glucuronide or sulfate, but about 30%
remained unidentified. Thus, repeated treatment with fipronil at 10
mg/kg bw per day orally increased the excretion of conjugated T4 in
bile, fipronil being more potent than phenobarbital. A possible
mechanism would be hepatic accumulation of T4 and induction of the
hepatic T4 conjugating enzyme UDP glucuronyltransferase, leading to
increased biliary excretion of T4-glucuronide conjugate (Taylor,
1993).
Effect on thyroxine clearance: Groups of six male Crl:CD(SD)
rats were treated with either technical-grade fipronil (purity, 95.4%)
at 10 mg/kg bw per day by gavage, phenobarbital at 80 mg/kg bw per day
intraperitoneally, or 0.5% methylcellulose (vehicle control) at 5
ml/kg bw by gavage for one or 14 days. Four h after the final dose of
either test substance, each rat received 125I-T4 at a dose of 10
µCi/kg bw. The levels of 125I in whole blood were measured for up to
30 h after T4 administration and were used to calculate the terminal
half-life, clearance, and volume of distribution of this hormone.
Fipronil had no effect on mortality or other parameters ante
mortem. Phenobarbital-treated animals showed collapsed posture,
lethargy, and shallow breathing on the first day of treatment.
Fipronil had no effect on the clearance of T4 after one day of
treatment, but after 14 days a decrease in terminal half-life (52% of
control level) and increases in the clearance (261%) and volume of
distribution (137%) were seen. The effects of phenobarbital were
similar, although quantitatively less severe, and were seen on day 1
of treatment (Peters et al., 1991b).
Effect on thyroid function: The effect of technical-grade
fipronil (purity, 95.4%) on thyroid function was compared with that of
propylthiouracil, a known inhibitor of thyroid organification which
interferes with thyroglobulin iodination, and noxythiolin, a thiourea
compound and putative inhibitor of thyroid iodide organification known
to lower T4 levels in rats. Groups of 27 male Crl:CD(SD)BR rats were
treated for 14 days with either 0.5% methylcellulose (vehicle control)
at 5 ml/kg bw by gavage, fipronil at 10 mg/kg bw per day by gavage,
propylthiouracil at 200 mg/kg per day by gavage, or Noxyflex at 5
mg/kg bw per day intraperitoneally as a saline solution. On day 15,
each animal received 125I-sodium iodide at a dose of 1 µCi. Six hours
later, nine males in each group received 10 or 25 mg/kg bw potassium
perchlorate or a 0.9% saline solution intraperitoneally, and blood was
immediately drawn in order to measure radiolabel. The animals were
then killed, and the thyroids were weighed and analysed for
radioactivity. Treatment with fipronil or Noxyflex appeared to
stimulate the thyroid glands, as evidenced by increased accumulation
of 125I and increased ratios of radiolabel distribution between the
blood and the thyroid. These changes were accompanied by increases in
thyroid weight. Treatment with propylthiouracil decreased the amount
of 125I incorporated in the thyroid and the blood:thyroid ratios, with
elevated levels of 125I in the blood. As the thyroids of these animals
were 2.5 times heavier than those of controls, however, the ratio of
blood 125I to thyroid weight was reduced. Administration of
perchlorate did not further reduce the 125I content of the thyroids or
the blood:thyroid 125I radiolabel ratio. There was no evidence of
inhibition of iodide incorporation (Peters et al., 1991c).
(v) Mode of action of fipronil
GABA is an important inhibitory neurotransmitter in both insects
and mammals. Attempts to demonstrate that the target site for fipronil
at the molecular level is the chloride ion channel of the GABA
receptor include electrophysiological testing, in which fipronil at 10
nmol/L reversed blockage of nervous system activity by GABA at 10
mmol/L, resulting in hyperexcitation in the housefly larva; flux
studies in which fipronil inhibited the passage of radiolabelled
chloride ions through opened GABA receptors in rat brain microsacs;
radioligand binding assays in vitro in which fipronil did not
inhibit binding of 3H-muscimol or 3H-flunitrazepam at, respectively,
the GABA recognition or benzodiazepine sites on the GABA receptor in
rat tissue, but did competitively inhibit binding at sites in the
chloride channel of the GABA receptor of ligands such as
35S- tert-butylbicyclophosphorothionate (TBPS) and
3H-1-[(4-ethyl)phenyl]-4- n-propyl-2,6,7-trioxacicyclo[2.2.2]octane
(EBOB). The latter is known to block the g-amino-butyric acid-gated
chloride channel, and the former binds to the picrotoxin binding site
in the channel. Binding of TBPS was inhibited by fipronil in rat brain
but not in the housefly head, but binding of EBOB was inhibited in
both rat and mouse brain and housefly head. The greater affinity of
fipronil for the EBOB site in insects than for that in mammals has
been postulated to account, at least in part, for the pesticide's
selective toxicity to insects.
In other studies of the role of the GABA receptor in the toxicity
of fipronil, a cloned gene that codes for a GABA receptor subunit in
Drosophila melanogaster was expressed in frog oocytes as a membrane
protein. These receptor-containing oocytes produced a large electrical
current carried by chloride ions in response to treatment with GABA,
which was not seen in control oocytes. The electric response was
reversed in a dose-dependent manner by treatment with fipronil and was
blocked by picrotoxin, TBPS, EBOB, and dieldrin.
Differences in the GABA receptor genome in susceptible and
resistant insects may help to explain resistance to the pesticide
dieldrin (a cyclodiene), which acts at the GABA receptor in insects
but also at the TBPS binding site, at which fipronil is inactive. A
dieldrin-resistant housefly was shown to have a low-affinity EBOB
binding site (see Bushey, 1993; Cole et al., 1993; Gant et al., 1994
for other references).
Although it was reported that fipronil did not affect other
enzyme or receptor systems, such as the acetylcholine receptor,
acetylcholinesterase, the octopamine receptor, and general oxidative
uncouplers, no supporting data were presented (Bushey, 1993).
(g) Studies on metabolites
(i) Acute toxicity
The results of studies on the toxicity of single doses of
fipronil metabolites are summarized in Table 4. The metabolic routes
by which they are formed are shown in Figures 2 and 3. In a study with
M&B 45950, clinical signs of neurotoxicity, including excessive
jumping, increased or reduced motor activity, clonic convulsions,
tremors, curling up, and subdued behaviour, were noted in all animals
at doses > 50 mg/kg bw, and deaths occurred during the first week
of the study, starting from 65 mg/kg bw in males and 90 mg/kg bw in
females (Dange, 1994a).
In a study with M&B 46136, clinical signs observed in animals of
each sex at 64 mg/kg bw within 2 h included hunched posture and
abnormal gait. Most signs such as hunched posture, abnormal gait,
lethargy, pallor of the extremities, diarrhoea, ataxia, clonic
convulsions, increased salivation, and decreased respiratory rate did
not appear until day 2 in animals at doses > 100 mg/kg bw. Deaths
occurred at doses > 100 mg/kg bw in animals of each sex on days 2
and 3 (Gardner, 1988c).
In a study with fipronil-desulfinyl, clinical signs including
reduced motor activity, dyspnoea, bradypnoea, and hyperreaction to
noise were observed in all animals at doses > 10 mg/kg bw. Clonic
and/or tonic convulsions were observed before death in one female at
20 mg/kg bw and in all males at 30 mg/kg bw. Deaths occurred at doses
> 20 mg/kg bw between days 2 and 4 after treatment (Dange, 1993b).
 Table 4. Toxicity of single doses of metabolites of fipronil in rats
Metabolite Purity Strain Sex Route LD50 Reference
(%) (mg/kg bw)
M&B 45950 98.9 Sprague-Dawley M Orala 69 Dange (1994a)
F 100
M&B 46136 98 Crl:CD(SD)BR M Orala 184 Gardner (1988c)
F 257
98 Crl:CD(SD)BR M,F Dermalb > 2000 Gardner (1988d)
RPA 200766 > 98 Sprague-Dawley M,F Orala > 2000 Dange (1993a)
Fipronil-desulfinyl 98.6 Sprague-Dawley M Orala 18 Dange (1993b)
F 15
98.6 Sprague-Dawley M,F Dermalc > 2000 Dange (1993c)
RPA 104615 94.7 Sprague-Dawley M,F Orala > 2000 Dange (1993d)
M&B 45897 > 99 Crl:CD(SD)BR M,F Orald > 2000 Haynes (1988a)
> 99 Crl:CD(SD)BR M,F Dermale > 2000 Haynes (1988b)
a In corn oil
b Applied as an 88.2% w/v concentration in distilled water
c Moistened with saline before application
d In sesame oil
e Moistened with distilled water before application
In a study with M&B 45897, transient signs of hypoactivity or
perinasal staining were noted in all or most animals given single oral
or dermal doses, respectively, but no deaths were observed. No deaths
or clinical signs of toxicity were observed in studies of oral
administration of RPA 200766, or RPA 104615 or studies of dermal
application of M&B 46136 or fipronil-desulfinyl (Gardner, 1988d;
Haynes, 1988a,b; Dange, 1993a,c,d).
(ii) Fipronil-desulfinyl
In the presence of sunlight, two photometabolites,
fipronil-desulfinyl and RPA 104615, can form from fipronil. The
available information indicates that fairly high-energy ultraviolet
irradiation is needed for the conversion process (personal
communication from Rhone-Poulenc). Fipronil-desulfinyl could
potentially form in the environment or on surfaces treated during use
against malarial vectors and thus appears to be of toxicological
concern.
Absorption, distribution, metabolism, and excretion: Groups of
24 male Charles River Crl:CD BR rats received a suspension of
14C-fipronil-desulfinyl in 1% aqueous carboxymethylcellulose as a
dermal application at doses of 0.08, 0.88, or 7.2 mg/rat (6.46, 70.5,
or 574 µg/cm2) on an area of about 12.5 cm2 shaved skin, which was
protected with a nonocclusive bandage. Blood, urine, and faeces were
collected to assay radiolabel. Four rats at each dose were killed to
assess dermal absorption after 0.5, 1, 2, 4, 10, and 24 h of exposure.
Before sacrifice, the application site was washed with water and the
washings saved to assay radiolabel. Control rats received only vehicle
and were killed 24 h after treatment.
The mean total recovery of radiolabel was 93-103% of the dose,
most (90-102%) being present in the skin wash. Absorption, measured as
radiolabel in excreta, cage wipe and wash, blood, carcass, and wiped
skin application site, in rats that received 0.08 mg was 0.74% of the
dose at 0.5 h, 2.3% at 10 h, and 6.6% by 24 h; at 0.88 mg/rat, 0.41%
at 0.5 h, 0.95% at 10 h, and 1.4% at 24 h; and at 7.2 mg/rat, 0.27% at
0.5 h, 0.18% at 10 h, and 0.39% at 24 h (Cheng, 1996).
Three groups of five male and five female Sprague Dawley (CD)
rats received either a single oral dose of 14C-fipronil-desulfinyl at
1 or 10 mg/kg bw or 14 daily oral doses of unlabelled
fipronil-desulfinyl, followed by a single oral dose of labelled
compound at 1 mg/kg bw. Urine and faeces were collected at 24-h
intervals until sacrifice at on eweek. At this time, liver, kidney,
brain, fat, and gonads were collected for analysis of residual
radiolabel.
More radiolabel was eliminated in the faeces than the urine. In
single animals at the low dose, elimination of radiolabel in urine
amounted to 6.1% of the dose in males and 4.4% in females, and
elimination in faeces was 60% of the dose in males and 46% in females.
In animals at the high dose, 8.8% was eliminated in the urine of males
and 11% in that of females, and 70% was eliminated in the faeces of
males and 56% in those of females. In animals given the 14 doses,
elimination of radiolabel in urine amounted to 10% in males and 11% in
females and that in faeces to 61% in males and 53% in females.
Residual radiolabel in tissues one week after treatment accounted for
27% in males and 41% in females at the single low dose, for 20% in
males and 30% in females at the high dose, and for 23 and 32% of the
dose in animals given repeated doses. The highest concentrations of
radiolabelled residues were present in fat and residual carcass. The
ratios of radiolabel in fat:plasma reached 12.8 in males and 25.2 in
females at the single low dose and 8.2 in males and 23.2 in females at
the single high dose. The levels of residual radiolabel at this time
were accounted for largely by residues in fat and carcass (also
probably due to fat).
In pharmacokinetic analyses of radiolabel in blood, the area
under the concentration-time curve increased in proportion to dose in
females but not in males. In males, the ratio of the area with the
high dose to that with low dose was 15 instead of the expected 10. The
mean elimination half-life of radiolabel from 14C-fipronil-desulfinyl
was 195 h at the high dose and 183 h at the low dose, with slightly
lower values for males. The increased fat:plasma ratios and the
prolonged elimination half-lives of the radiolabel suggest potential
bioaccumulation of this compound and/or its metabolites.
Up to 17 radiolabelled components were found in urine and up to
13 in faeces. Only untransformed parent compound (fipronil-desulfinyl)
was identified in extracts of liver, fat, skin, and residual carcass.
Biotransformation of fipronil-desulfinyl in rats (Figure 4) largely
involves conjugation and/or biotransformation of the functional groups
attached to the pyrazolyl ring. The main compounds identified included
untransformed parent (0.01-0.9% of the dose in urine and 29-44% in
faeces; UMET/17 and FMET/12 in the Figure), the
4-cyano-5- (N-)cysteine conjugate of fipronil-desulfinyl (up to 1.6%
of the dose in urine and 3.2-14% in faeces; UMET/15 and FMET/10), the
5- (N-)cysteine conjugate (up to 0.69% of the dose in urine and
1.5-3.3% in faeces; UMET/14 and FMET/9), the pyrazole-4-carboxylic
derivative (1.3-5.5% of the dose in urine and 1.7-5.2% in faeces; M&B
46400), the amide derivative (up to 0.62% of the dose in urine; RPA
105048), and the sulfate conjugate (up to 2.4% of the dose in urine;
UMET/3) (Totis,1996).
Short-term toxicity of firponil-deulfinyl
Mice
In a preliminary study, fipronil-desulfinyl (purity, 97.5%) was
administered in the diet for 28 days to groups of 10 male and 10
female OF-1 mice at doses of 0, 0.5, 3, 30, or 60 ppm, equal to 0,
0.08, 0.49, 5.0 mg/kg bw per day, and undetermined, respectively, for
males and 0, 0.1, 0.61, 5.6, and 12 mg/kg bw per day for females. All
of the males and six females at 60 ppm and seven males and two females
Table 4. Toxicity of single doses of metabolites of fipronil in rats
Metabolite Purity Strain Sex Route LD50 Reference
(%) (mg/kg bw)
M&B 45950 98.9 Sprague-Dawley M Orala 69 Dange (1994a)
F 100
M&B 46136 98 Crl:CD(SD)BR M Orala 184 Gardner (1988c)
F 257
98 Crl:CD(SD)BR M,F Dermalb > 2000 Gardner (1988d)
RPA 200766 > 98 Sprague-Dawley M,F Orala > 2000 Dange (1993a)
Fipronil-desulfinyl 98.6 Sprague-Dawley M Orala 18 Dange (1993b)
F 15
98.6 Sprague-Dawley M,F Dermalc > 2000 Dange (1993c)
RPA 104615 94.7 Sprague-Dawley M,F Orala > 2000 Dange (1993d)
M&B 45897 > 99 Crl:CD(SD)BR M,F Orald > 2000 Haynes (1988a)
> 99 Crl:CD(SD)BR M,F Dermale > 2000 Haynes (1988b)
a In corn oil
b Applied as an 88.2% w/v concentration in distilled water
c Moistened with saline before application
d In sesame oil
e Moistened with distilled water before application
In a study with M&B 45897, transient signs of hypoactivity or
perinasal staining were noted in all or most animals given single oral
or dermal doses, respectively, but no deaths were observed. No deaths
or clinical signs of toxicity were observed in studies of oral
administration of RPA 200766, or RPA 104615 or studies of dermal
application of M&B 46136 or fipronil-desulfinyl (Gardner, 1988d;
Haynes, 1988a,b; Dange, 1993a,c,d).
(ii) Fipronil-desulfinyl
In the presence of sunlight, two photometabolites,
fipronil-desulfinyl and RPA 104615, can form from fipronil. The
available information indicates that fairly high-energy ultraviolet
irradiation is needed for the conversion process (personal
communication from Rhone-Poulenc). Fipronil-desulfinyl could
potentially form in the environment or on surfaces treated during use
against malarial vectors and thus appears to be of toxicological
concern.
Absorption, distribution, metabolism, and excretion: Groups of
24 male Charles River Crl:CD BR rats received a suspension of
14C-fipronil-desulfinyl in 1% aqueous carboxymethylcellulose as a
dermal application at doses of 0.08, 0.88, or 7.2 mg/rat (6.46, 70.5,
or 574 µg/cm2) on an area of about 12.5 cm2 shaved skin, which was
protected with a nonocclusive bandage. Blood, urine, and faeces were
collected to assay radiolabel. Four rats at each dose were killed to
assess dermal absorption after 0.5, 1, 2, 4, 10, and 24 h of exposure.
Before sacrifice, the application site was washed with water and the
washings saved to assay radiolabel. Control rats received only vehicle
and were killed 24 h after treatment.
The mean total recovery of radiolabel was 93-103% of the dose,
most (90-102%) being present in the skin wash. Absorption, measured as
radiolabel in excreta, cage wipe and wash, blood, carcass, and wiped
skin application site, in rats that received 0.08 mg was 0.74% of the
dose at 0.5 h, 2.3% at 10 h, and 6.6% by 24 h; at 0.88 mg/rat, 0.41%
at 0.5 h, 0.95% at 10 h, and 1.4% at 24 h; and at 7.2 mg/rat, 0.27% at
0.5 h, 0.18% at 10 h, and 0.39% at 24 h (Cheng, 1996).
Three groups of five male and five female Sprague Dawley (CD)
rats received either a single oral dose of 14C-fipronil-desulfinyl at
1 or 10 mg/kg bw or 14 daily oral doses of unlabelled
fipronil-desulfinyl, followed by a single oral dose of labelled
compound at 1 mg/kg bw. Urine and faeces were collected at 24-h
intervals until sacrifice at on eweek. At this time, liver, kidney,
brain, fat, and gonads were collected for analysis of residual
radiolabel.
More radiolabel was eliminated in the faeces than the urine. In
single animals at the low dose, elimination of radiolabel in urine
amounted to 6.1% of the dose in males and 4.4% in females, and
elimination in faeces was 60% of the dose in males and 46% in females.
In animals at the high dose, 8.8% was eliminated in the urine of males
and 11% in that of females, and 70% was eliminated in the faeces of
males and 56% in those of females. In animals given the 14 doses,
elimination of radiolabel in urine amounted to 10% in males and 11% in
females and that in faeces to 61% in males and 53% in females.
Residual radiolabel in tissues one week after treatment accounted for
27% in males and 41% in females at the single low dose, for 20% in
males and 30% in females at the high dose, and for 23 and 32% of the
dose in animals given repeated doses. The highest concentrations of
radiolabelled residues were present in fat and residual carcass. The
ratios of radiolabel in fat:plasma reached 12.8 in males and 25.2 in
females at the single low dose and 8.2 in males and 23.2 in females at
the single high dose. The levels of residual radiolabel at this time
were accounted for largely by residues in fat and carcass (also
probably due to fat).
In pharmacokinetic analyses of radiolabel in blood, the area
under the concentration-time curve increased in proportion to dose in
females but not in males. In males, the ratio of the area with the
high dose to that with low dose was 15 instead of the expected 10. The
mean elimination half-life of radiolabel from 14C-fipronil-desulfinyl
was 195 h at the high dose and 183 h at the low dose, with slightly
lower values for males. The increased fat:plasma ratios and the
prolonged elimination half-lives of the radiolabel suggest potential
bioaccumulation of this compound and/or its metabolites.
Up to 17 radiolabelled components were found in urine and up to
13 in faeces. Only untransformed parent compound (fipronil-desulfinyl)
was identified in extracts of liver, fat, skin, and residual carcass.
Biotransformation of fipronil-desulfinyl in rats (Figure 4) largely
involves conjugation and/or biotransformation of the functional groups
attached to the pyrazolyl ring. The main compounds identified included
untransformed parent (0.01-0.9% of the dose in urine and 29-44% in
faeces; UMET/17 and FMET/12 in the Figure), the
4-cyano-5- (N-)cysteine conjugate of fipronil-desulfinyl (up to 1.6%
of the dose in urine and 3.2-14% in faeces; UMET/15 and FMET/10), the
5- (N-)cysteine conjugate (up to 0.69% of the dose in urine and
1.5-3.3% in faeces; UMET/14 and FMET/9), the pyrazole-4-carboxylic
derivative (1.3-5.5% of the dose in urine and 1.7-5.2% in faeces; M&B
46400), the amide derivative (up to 0.62% of the dose in urine; RPA
105048), and the sulfate conjugate (up to 2.4% of the dose in urine;
UMET/3) (Totis,1996).
Short-term toxicity of firponil-deulfinyl
Mice
In a preliminary study, fipronil-desulfinyl (purity, 97.5%) was
administered in the diet for 28 days to groups of 10 male and 10
female OF-1 mice at doses of 0, 0.5, 3, 30, or 60 ppm, equal to 0,
0.08, 0.49, 5.0 mg/kg bw per day, and undetermined, respectively, for
males and 0, 0.1, 0.61, 5.6, and 12 mg/kg bw per day for females. All
of the males and six females at 60 ppm and seven males and two females
 at 30 ppm died during the study. The intake of the test compound was
thus not determined for males at 60 ppm.
Males and females at 30 and 60 ppm had statistically significant
decreases in mean body weight and body-weight gain, and mean food
consumption was significantly decreased. The mean values of chemical
parameters did not differ significantly between treated and control
mice. Changes in the weights of the liver, kidney, and thymus in
animals at 30 and 60 ppm were attributed to the decreased terminal
body weights. The only treatment-related change observed
histopathologically was an increased incidence of centrilobular
hypertrophy in mice at 30 and 60 ppm.
Clinical signs of neurotoxicity -- increased motor activity,
excessive jumping, irritability to touch, compulsive biting, and
convulsions -- were observed in males and females at 30 and 60 ppm.
Two females at 3 ppm showed increased motor activity on one occasion,
accompanied by excessive vocalization in one female. None of these
effects occurred in controls. Increased motor activity has been
observed previously as a clinical sign of the toxicity of
fipronil-desulfinyl and of the parent compound fipronil in several
species. Because of the low incidence of this effect and the lack of
other effects at 3 ppm in this study, however, it cannot be concluded
that this effect is of toxicological concern. The NOAEL was 3 ppm,
equal to 0.49 mg/kg bw per day (Dange, 1994b).
Fipronil-desulfinyl (purity, 96%) was administered to groups of
10 male and 10 female OF1 mice in the diet for 90 days at doses of 0,
0.5, 2, or 10 ppm, equal to 0, 0.08, 0.32, or 1.7 mg/kg bw per day for
males and 0, 0.11, 0.43, or 2.2 mg/kg bw per day for females. Standard
evaluations of toxicity ante and post mortem were included.
Histopathological examinations were conducted on all tissues from
controls and animals at the two higher doses and on the liver, lung,
and kidney and tissues in which there were significant macroscopic
findings from mice at the low dose.
Nine males and one females treated at 10 ppm were found dead
during the study. Significant clinical signs included excessive
jumping in two males at 10 ppm and irritability to touch,
aggressivity, and/or increased motor activity in one male at 10 ppm
and two at 2 ppm on two or more occasions. At 0.5 ppm, aggressivity
was reported in one male on one occasion and in one female on three
occasions. None of these effects was reported in control animals.
There were no significant changes in body weight, food consumption, or
haematological or clinical chemical parameters. Gross pathological
examination of dead animals indicated liver enlargement in three males
and small thymuses in four males at 10 ppm. Microscopy revealed
centrilobular hypertrophy of the liver in six males at this dose. The
only female at 10 ppm that died had marked autolysis, which obscured
the histological details. At scheduled sacrifice, gross and
microscopic examinations revealed no treatment-related findings.
The incidence of aggressivity or irritability to touch was low at
2 ppm and there was no dose-response relationship; however, one male
at this dose showed irritability to touch and agressivity on days
70-72, and the other male at this dose with behavioural changes was
irritable to touch, had increased motor activity on day 29, and showed
aggressivity on day 33. Therefore, two animals of the same sex showed
multiple signs on two or three occasions of effects that have been
recognized as clinical signs of toxicity in other studies with
fipronil-desulfinyl and fipronil. In addition, signs of neurotoxicity
were seen in males at the next highest dose and no signs of toxicity
in concurrent controls. Therefore, the behavioural findings in males
at 2 ppm should not be discounted, despite the lack of other
treatment-related findings at this dose. It is easier to discount the
behavioural effects at 0.5 ppm because of their low incidence and the
lack of a dose-response relationship. Therefore, the NOAEL was
0.5 ppm, equal to 0.08 mg/kg bw per day (Bigot, 1996).
Rats
In an exploratory study, fipronil-desulfinyl (purity, 98.6%) was
administered by gavage to five male and five female Sprague-Dawley
rats at doses of 0, 0.3, 1, 3, or 10 mg/kg bw per day for 14 days. All
of the animals at 10 mg/kg bw per day and one female at 3 mg/kg bw per
day either died or were killed in a moribund condition on days 5-8 of
the study. Clinical signs of toxicity, such as piloerection,
chromodacryorrhoea, prostration, excessive reaction to noise,
convulsions, curling up on handling, hunched posture, nasal discharge,
and few faeces, were observed in animals of each sex at 10 mg/kg bw
per day and in females at 3 mg/kg bw per day. Body-weight gain and
food consumption were markedly decreased in animals at 10 mg/kg bw per
day; males and females at 3 mg/kg bw per day showed decreased
body-weight gain over the course of the study, and food consumption
was decreased at the two times evaluated.
Leukocyte counts and total bilirubin were decreased and total
protein was increased in females at 3 mg/kg bw per day; leukocyte
counts were also decreased in females at 1 mg/kg bw per day. Gross
pathological examination showed pale livers in females at 1, 3, or 10
mg/kg bw per day, and spots in the glandular stomach were seen in a
few animals at 3 and 10 mg/kg bw per day that died during the study.
Histological examination showed an increased number of atrophic
follicles in the thyroids of males and females treated at doses > 3
mg/kg bw per day. The NOAEL was 0.3 mg/kg bw per day (Dange, 1994c).
In a preliminary study, fipronil-desulfinyl (purity, 97.5%) was
administered to groups of 10 male and 10 female Sprague-Dawley rats in
the diet at doses of 0, 0.5, 3, 30, or 100 ppm (equal to 0, 0.04,
0.23, 2.2, or 3.7 mg/kg bw per day for males and 0, 0.04, 0.24, 2.3,
or 3.8 mg/kg bw per day for females) for 28 days. Standard parameters
and T3, T4, and TSH were measured before treatment and on day 7 in
non-fasted animals and on day 23 or 24 in fasted animals.
There were no deaths, clinical signs of toxicity, or effects on
body weight or food consumption at 0.5 and 3 ppm. One male at 30 ppm
was found dead on day 6, and all animals at 100 ppm died within the
first two weeks of the study. Clinical signs at 30 and 100 ppm
included piloerection, curling up on handling, and convulsions (in one
female at 100 ppm). Food consumption and body weights were decreased
at the two highest doses and markedly so at 100 ppm. The only change
observed in urinary, standard haematological, and clinical chemical
parameters were a decrease of about 30% in total bilirubin in animals
of each sex at 30 ppm. Statistically significant decreases (relative
to concurrent controls) were found in the levels of T4 (by 33% in
males at 300 ppm, by 63% in males and 50% in females at 100 ppm on day
7; and by 49% in males and 61% in females at 30 ppm on day 23 [no
animals at the high dose were still alive on that day]) and in T3 (by
46% in females at 100 ppm on day 7 and by 40% in males at 30 ppm on
day 23) were observed, but their toxicological significance was not
clear: the findings at 100 ppm may have been related to the extreme
toxicity of the chemical, and individual values in animals at 30 ppm
were reported to be within the normal range for these parameters. TSH
levels were not affected. At necropsy, the thymus weights were
decreased in females at 30 ppm, but no other findings were clearly
related to treatment. The NOAEL was 3 ppm, equal to 0.23 mg/kg bw
(Dange, 1995a).
Fipronil-desulfinyl (purity, at least 97.5%) was administered in
the diet for 90 days to groups of 10 male and 10 female Sprague-Dawley
rats at doses of 0, 0.5, 3, 10, or 30 ppm (equal to 0, 0.029, 0.18,
0.59, or 1.8 mg/kg bw per day for males and 0, 0.035, 0.21, 0.71, or
2.1 mg/kg bw per day for females). In addition to standard ante- and
post-mortem evaluations of toxicity, T3, T4, and TSH were assayed in
non-fasted animals in weeks 2 and 10. During treatment, one male and
three females at 30 ppm died with clinical signs of distress. Signs of
neurotoxicity (aggressivity, irritability to touch, increased motor
activity, and curling up on handling) were seen in animals at 10 and
30 ppm, and excessive vocalization and increased motor activity were
seen in some animals. None of these effects was observed in controls,
in males at 0.5 ppm, or in females at doses > 3 ppm. One male at 3
ppm showed aggressivity, irritability to touch on several occasions,
and excessive vocalization. These signs were seen mainly in weeks 3-5.
Mean body weights were significantly decreased in males and females at
30 ppm and in males at 10 ppm several times during the study. The
overall mean body-weight gain of males was decreased by 15% in those
at 10 ppm and 13% at 30 ppm. Mean weekly food consumption and food
conversion efficiency of males and females at 30 ppm were lower than
those of controls only during the first two weeks of the study. No
treatment-related changes in haematological, urinary or
ophthalmological parameters were seen. Decreases in total bilirubin
(-43%), total cholesterol (-25%), and triglycerides (-24%) in females
at 30 ppm were considered to be related to treatment but not
toxicologically significant since the individual values were within
the normal range for this age and strain of rat. At 30 ppm,
treatment-related decreases in T4 levels were seen in males (-48%,
p < 0.01, at week 2 and -25% at week 10) and females (-29% at week
10), and T3 levels were decreased (-29%, p < 0.05) in males at week
10. The altered hormone levels were reported to be within the normal
range of values for these parameters in this strain of rat. As no
changes were found in the thyroid gland on macroscopic or microscopic
examination, the toxicological significance of the alterations in
hormone levels is questionable. TSH was not affected. There were no
treatment-related macroscopic changes at necropsy, no changes in organ
weights, and no histopathological findings. The NOAEL was 0.5 ppm,
equal to 0.029 mg/kg bw per day, on the basis of clinical signs of
toxicity in one male (Dange, 1994d).
Dogs
In a preliminary study, fipronil-desulfinyl (purity, 97.5%), was
administered in the diet of groups of two male and two female beagle
dogs at doses of 0, 27, 80, or 270 ppm for 28 days. The group mean
doses achieved were reported to be equal to 1 mg/kg bw per day for
males and females at 27 ppm over the course of the study, 1.9 mg/kg bw
per day for males and 1.7 mg/kg bw per day for females at 80 ppm
during week 1, and 2.3 mg/kg bw per day for animals of each sex at 270
ppm during the same period. During the second week of the study,
intake decreased considerably because of toxicity.
Because of poor health, animals at the two higher doses were
killed early, on days 10 and 15 for those at 80 ppm and day 10 for
those at 270 ppm. The signs of toxicity observed at 80 ppm included
reduced motor activity, staggering, irritability, and increased
salivation. Animals at 80 and 270 ppm had no or few faeces,
emaciation, decreased food consumption, and weight loss. All animals
receiving 27 ppm survived, but one male had a clonic convulsion at the
end of the study. The body weights and food consumption of animals in
this group were comparable to those of controls. No remarkable effects
were seen in ophthalmological, haematological, clinical chemical, or
urinary evaluations. Only animals at 27 and 80 ppm were necropsied.
Reduced thymic weight or small thymuses and pale livers were noted in
both groups, and the livers of those at 80 ppm had multifocal whitish
areas or mottling. Although there were no microscopic findings at 27
ppm, marked thymic atrophy and hepatic changes indicative of toxicity
were seen at 80 ppm, including diffuse sinusoidal leukocytosis,
centrilobular hepatocytic enlargement, mild multifocal hepatocytic
hydropic degeneration, and chronic hepatitis with periportal fibrosis.
No NOAEL was identified (Dange, 1995b).
Fipronil-desulfinyl (purity, 96%) was administered to groups of
five beagle dogs of each sex in the diet for 90 days at doses of 0,
3.5, 9.5, or 35 ppm (equivalent to 0, 0.1, 0.27, or 0.95 mg/kg bw per
day for males and 0, 0.1, 0.29, or 1.05 mg/kg bw per day for females).
General health, clinical signs, food consumption, body weights,
haematology and plasma chemistry, and ophthalmologic and urinary
parameters were evaluated. At necropsy, organ weights and macroscopic
changes were monitored; all tissues from controls and animals at the
high dose and kidney, liver, lung, heart, and gall-bladder from those
at the intermediate and low doses were examined histologically.
One female at 35 ppm was killed on day 28 after signs of
increased salivation, prostration, writhing, tremors, absence of
rotular reflex, noisy breathing, and dyspnoea. These clinical signs
were suggested to be due to coronary arteritis and myocardial necrosis
on the basis of microscopic findings; however, they may have indicated
neurotoxicity, as another female at this dose also exhibited excessive
barking and aggressivity on one occasion and irritability, tremors,
and increased salivation on another. There were no significant changes
in body weight, food consumption, or haematological, clinical
chemical, urinary, or ophthalmological parameters. At scheduled
necropsy, no treatment-related changes were observed, and the
microscopic examination revealed no treatment-related findings. The
NOAEL was 9.5 ppm, equal to 0.29 mg/kg bw per day (Dange, 1996).
Reproductive toxicity: Groups of adult female Sprague-Dawley
Crl:CD(SD)BR rats received 0, 0.5, 1, or 2.5 mg/kg bw per day of
fipronil-desulfinyl (purity, 99.2%) suspended in a 0.5% aqueous
solution of methylcellulose by gavage on days 6-15 of gestation. The
study was terminated on gestation day 20. The animals were evaluated
for clinical signs, food consumption, body-weight changes, and
macroscopic changes post mortem. The litter and fetal parameters
evaluated included pre- and postimplantation losses, litter size,
litter and mean fetal weights, sex ratios, and malformations or
skeletal or visceral anomalies. Hair loss on the paws, limbs, flanks,
abdomen, and/or thorax were seen in dams at the high dose, and these
animals had lower body-weight gain on gestation days 6-9 (28% of
control), 9-12 (27%), 6-16 (58%), and 0-20 corrected for gravid
uterine weight (78%). These animals also consumed less food during
treatment, although an increase was seen after treatment, indicating a
rebound effect. Dams at 1 mg/kg bw per day showed a significant
decrease in body-weight gain (80% of control) on days 9-12 of
gestation. No other effects on body weight, body-weight gain at other
intervals, food consumption, or other parameters were seen at the
intermediate dose.
Developmental toxicity in pups at the high dose was reflected in
a slight increase in the fetal and litter incidence of incomplete or
reduced ossification of several bones, including the hyoid body, fifth
and sixth sternebrae, first thoracic body, pubic bone, and one or two
metatarsi. A slight but significant reduction in fetal body weight
(98% of control weight) at the high dose correlated with the slight
delays in ossification. Although the dams showed reduced body-weight
gain, the change in dams at the intermediate dose occurred over a
small interval and was transient. In the absence of other effects at
this dose, the reduced body-weight gain does not appear to be
toxicologically significant. The NOAEL for maternal toxicity was thus
1 mg/kg bw per day; that for for developmental toxicity was 1 mg/kg bw
per day on the basis of a slight increase in the fetal and litter
incidence of delayed ossification of several bones (Foulon, 1997).
Neurotoxicity: Fipronil-desulfinyl (purity, 99.5%) was
administered by gavage as a single dose in corn oil to groups of 12
male and 12 female Sprague-Dawley Crl:CD(SD)BR rats at doses of 0,
0.5, 2, or 12 mg/kg bw. A functional observation battery of tests and
motor activity testing were conducted at 6 h and seven and 14 days
after treatment; 6 h was chosen as the time of 'peak effect' on the
basis of the results of a range-finding study which included
evaluations at 2, 4, 6, and 24 h. Clinical signs, food consumption,
and body weights were monitored. At study termination on day 15, all
animals were perfused in situ. Brains were weighed and measured, and
tissues from the brain, spinal cord, and peripheral nerves were
collected and processed for microscopic examination. Only tissues from
five male and five female controls and animals at the high dose were
examined histopathologically. Since possibly treatment-related axonal
degeneration was seen in lumbar dorsal root fibres and/or the sciatic
nerve of males at the high dose, lumbar dorsal root fibres and the
sciatic nerve in the notch and mid-thigh from the remaining male
controls and rats at the high dose were prepared for microscopic
evaluation. Slides of all tissues from these areas of concern were
then read or re-read in a 'blinded' manner.
Body-weight gain and food consumption were decreased in males and
females at the high dose only during week 1. The behavioural changes
attributed to treatment were significant decreases in hindlimb foot
splay, rectal temperature, and locomotor activity (assessed
quantitatively in the Coulbourn system) in animals of each sex at 12
mg/kg bw 6 h after treatment. Treatment probably affected the righting
reflex in males at the high dose, as it was significantly slowed on
day 14, with a trend at other times, and possibly affected grip
strength in animals of each sex at the high dose at various times.
Changes in other behavioural and motor activity parameters could not
clearly be related to treatment. The groups did not differ with regard
to brain weights. The incidence and number of tissue sites showing
axonal degeneration were increased slightly over those in controls and
in males at the high dose. Since the effect was of minor severity in
both treated and control animals and since the difference between
these animals was not significantly different by Fisher's exact test,
the changes were considered not to be of biological importance. There
were no significant histopathological changes in female rats. The
NOAEL was 2 mg/kg bw (Hughes, 1996).
(iii) M&B 46136: Dermal and ocular irritation
Three male New Zealand white rabbits were given a single 0.5-g
dose of M&B 46136 (purity, 98%) moistened with distilled water as a
topical application for 4 h. The treated areas were observed for
erythema and oedema for four days after treatment. No signs of dermal
irritation were seen under the conditions of the study (Liggett,
1988c).
A dose of 0.1 ml (60 mg) of M&B 46136 (purity, 98%) was instilled
into the lower eyelid of one eye of three male New Zealand white
rabbits. The other eye served as an untreated control. The eyes were
examined for signs of irritation and scored 1 h and one, two, three,
four, and seven days after instillation. One animal had chemosis after
one day. Redness and any minor or residual irritation present had
cleared by day 3 or 4 in all animals. M&B 46136 was thus a slight
ocular irritant under the conditions of the study (Liggett, 1988d).
(iv) RPA 200766: Short-term toxicity
RPA 200766 (purity, 96.2%) was administered in the diet for 28
days to 10 male and 10 female Sprague-Dawley rats at doses of 0, 50,
500, 5000, or 15 000 ppm (equal to 0, 3.8, 38, 390, or 1100 mg/kg bw
per day for males and 0, 4.4, 44, 390, or 1100 mg/kg bw per day for
females). The animals were examined for general health, clinical signs
of toxicity, body weight, food consumption, urinary and
ophthalmological changes, haematological and clinical chemical
parameters, and macroscopic changes at necropsy. Histopathological
examinations were not conducted on animals at 15 000 ppm, because the
dose was found to be too high, inducing excessive body-weight loss.
Microscopic examinations were carried out on all animals that died,
all those at 5000 ppm, and all controls, on the liver, lungs, and
kidneys of animals in all groups except those at 15 000 ppm, and on
the adrenals and thyroid (the target organs), as considered necessary
to establish a no-effect level.
One female at 15 000 died during the study due to an error in
blood collection. No treatment-related deaths were seen in other
groups, and no clinical or ophthalmological alterations were reported.
The body weights of males and females at 5000 and 15 000 ppm were
significantly decreased on days 8-28. The mean body-weight gain over
the course of the study was decreased by 27% in males at 5000 ppm, 61%
in males at 15 000 ppm, 46% in females at 5000 ppm, and 77% in females
at 15 000 ppm. Mean food consumption over the course of the study was
decreased by 11% in males at 5000 ppm, 25% in males at 15 000 ppm, 22%
in females at 5000 ppm, and 33% in females at 15 000 ppm.
Mean haemoglobin concentrations were decreased in males and
females at doses > 500 ppm and mean haematocrit values were
decreased in animals at doses > 5000 ppm, significantly except in
females at 15 000 ppm. Mean corpuscular haemoglobin values were
decreased in males and females at 15 000 ppm and in males at 5000 ppm.
The mean cholesterol levels were significantly increased in animals at
doses > 500 ppm, mean triglyceride values were increased in animals
at 5000 and 15 000 ppm, urea nitrogen was increased in females at 5000
and 15 000 ppm, and creatinine values were increased in males at doses
> 500 ppm. The results of urinalysis showed no changes.
Dose-related increases in absolute and relative liver weights
were seen in males and females at doses > 500 ppm, and the
liver:brain weights were also increased in these groups. Dark livers
were observed in males at > 500 ppm and in females at 5000 and 15
000 ppm. Significantly increased relative adrenal weights and
adrenal:brain weights were seen in all treated males. The group mean
thyroid weights were increased in males at doses > 50 ppm; however,
the increases were not found consistently, and the individual values
were reported to be generally within the expected range for animals of
this age and strain. As there were also no microscopic changes in the
thyroid, these findings are of questionable toxicological
significance, even though the target organ of the parent compound,
fipronil, is the thyroid.
Microscopic examination showed slight-to-moderate, centrilobular
or diffuse hepatocellular hypertrophy in the livers and
slight-to-extramedullary haematopoiesis in the adrenals of males and
females at 5000 ppm. A dose-related increase in the incidence of fine
or coarse vacuolation of the zona fasciculata of the adrenal gland was
observed in males at doses > 50 ppm, with incidences of 0/10 in
controls, 2/10 at the low dose, 5/10 at the intermediate dose, and
10/10 at the high dose. The severity was slight at 50 and 500 ppm and
mild to marked at 5000 ppm. A similar change was seen in seven females
at 5000 ppm, with slight-to-mild severity; the incidence in the female
controls was 0. Increases in the weights of the adrenals and thyroids
and vacuolation of the adrenal zona fasciculata in males at 50 ppm
were considered to be marginal and of questionable toxicological
significance. The NOAEL was 50 ppm, equal to 3.8 mg/kg bw per day, on
the basis of decreased haemoglobin concentration, increased
cholesterol levels, and increased liver weights in animals of each sex
at the next dose (Berthe, 1996).
(v) M&B 45897: Short-term toxicity
M&B 45897 (purity, 99.7%) was administered to five male and five
female CD rats by gavage at doses of 0, 50, 200, or 1000 mg/kg bw per
day in corn oil for 28 days. Standard evaluations of toxicity ante
and post mortem were included. There were no deaths. Salivation was
observed in all animals from day 2 of treatment with 1000 mg/kg bw per
day, from day 3 at 200 mg/kg bw per day, from day 8 in females at 50
mg/kg bw per day, and on days 8-15 in males at this dose. The control
group was not affected. During the second week, animals at the
intermediate and high doses were also hunched and underactive, and all
males and some females at the high dose showed hair loss from day 3
on. Staggering was observed in all animals at the high dose on day 8.
None of these clinical signs was observed at 50 mg/kg bw per day or in
the controls.
Significant deficits in body-weight gain on days 0-17 were seen
in animals of each sex at 1000 mg/kg bw per day, but food consumption
was not affected by treatment. Animals at this dose also showed
decreased haemoglobin concentration, and females had lower erythrocyte
numbers and packed cell volume in comparison with controls. Slightly
higher plasma protein concentrations were seen in animals of each sex
at the high dose, and higher plasma alanine aminotransferase activity
was seen in females. Increased absolute and relative liver weights
were seen in males and females at 1000 mg/kg bw per day. Macroscopic
examination showed no significant findings, but microscopic
examination revealed periacinar hepatic hypertrophy in the livers of
three male rats at 1000 mg/kg bw per day. The only effects considered
to be related to treatment were the slight decreases in erythrocyte
numbers and packed cell volume and the slightly increased alanine
aminotransferase activity in animals at the high dose. Although an
NOAEL of 200 mg/kg bw per day was proposed, the signs observed at the
intermediate and high doses in all animals were indicative of a
toxicological (perhaps neurotoxic) effect of M&B 45897, as they were
not seen in the controls and animals at the low dose. Similar findings
have been reported in studies with the parent compound fipronil and
some of its other metabolites, which have been shown to be neurotoxic.
Although the salivation could be a treatment-related clinical sign, it
could also be viewed as an indication of irritation by the test
compound or a local reaction to treatment, especially since it was
observed more or less consistently at all doses, except in males at
the low dose, in which it occurred only on days 8-15. This was the
only finding at the low dose. The NOAEL was therefore 50 mg/kg bw per
day on the basis of clinical signs of toxicity at the next highest
dose (Johnson, I.R., 1995).
(vi) Genotoxicity
The results of studies on the genotoxicity of fipronil
metabolites are summarized in Table 5.
(vii) Comparison of fipronil and its metabolites
Table 6 presents a comparison of findings for fipronil and its
metabolites: whether or not the chemical has been found (to a major or
minor extent) in or on plants or in animal (rat, goat, or hen) tissues
or products or can form in the environment or on surfaces, indoors or
outdoors, via reduction, oxidation, hydrolysis, or photolysis (see
Figure 3); the acute oral toxicity of each chemical; and the ability
of each metabolite to compete with the ligands 3H-EBOB and 3H-TBPS
for binding to specific sites in the chloride ion channel of the GABA
receptor. Binding is postulated to serve as an indicator of potential
to disrupt the normal functioning of the GABA receptor by interference
with chloride-ion flux and thus for potential toxicity to the central
nervous system.
Table 5. Results of assays for genotoxicity with metabolites of fipronil metabolites
Metabolite End-point Test object Concentration Purity Results Reference
(%)
In vitro
M&B 45950 Reverse mutation S. typhimurium 0-250 µg/plate 98.9 Negativea,b Percy (1994a)
TA98, TA100, in DMSO
TA1535, TA1537
Chromosomal Human 25-100 µg/ml > 99 Negativea,b Marshall
aberration lymphocytes in DMSO (1988b)
M&B 46136 Reverse mutation S. typhimurium 0.32-200 µg/plate 98.7 Negativea,b Clare (1988b)
TA98, TA100, (-S9), 0.8-500 µg/
TA1535, TA1537 plate (+S9), in
DMSO
Chromosomal Human 75-300 µg/ml 98.7 Negativea,b Marshall
aberration lymphocytes in DMSO (1989)
RPA 200766 Reverse mutation S. typhimurium 250-1000 µg/plate > 98 Negativea,b Percy (1993a)
TA98, TA100, (-S9), 50-2500 µg/
TA1535, TA1537, plate (+S9), in
TA1538 DMSO
Fipronil-desulfinyl Reverse mutation S. typhimurium 10-250 µg/plate 98.6 Negativea,b Percy (1993b)
TA98, TA100, in DMSO
TA1535, TA1537,
TA1538
Gene mutation Chinese hamster 5-125 µg/ml (-S9), 99.5 Negativea,b Adams (1996a)
cell line (CHO- 15-625 µg/ml (+S9)
K1-BH4), hprt locus in DMSO
Table 5. Results of assays for genotoxicity with metabolites of fipronil metabolites
Metabolite End-point Test object Concentration Purity Results Reference
(%)
Chromosomal Human lymphocytes 5-30 µg/ml (-S9), 99.5 Negativea,b Adams (1996b)
aberration 5-60 µg/ml (+S9)
in DMSO
RPA 104615 Reverse mutation S. typhimurium 2250-5000 µg/plate 94.7 Negativea,b Percy (1993c)
TA98, TA100, in DMSO
TA1535, TA1537,
TA1538
M&B 45897 Reverse mutation S. typhimurium 12.5-2500 µg/plate 99.7 Negativea,b Percy (1996)
TA98, TA100, (-S9), 25-2500 µg/
TA1535, TA1537 plate (+S9), in
DMSO
Reverse mutation S. typhimurium 4-2500 µg/plate > 99 Negativea,b Kennelly
TA98, TA100, (-S9), except TA 100; (1988)
TA1535, TA1537 8-5000 µg/plate
(-S9), TA100; 8-5000
µg/plate (+S9), all
strains; in DMSO
Chromosomal Human 50-150 µg/ml (-S9), 99.7 Negativea,b Johnson, A.L.
aberration lymphocytes 100-400 µg/ml (+S9), (1995)
in DMSO; 20- or 44-h
harvest times
Polyploidy Human 50-150 µg/ml (-S9), 99.7 Positivea,c Johnson, A.L.
lymphocytes 100-400 µg/ml (+S9), (1995)
in DMSO; 20- or 44-h
harvest times
RPA 105048 Reverse mutation S. typhimurium 250-5000 µg/plate 98.6 Negativea,c Percy (1994b)
TA98, TA100, in DMSO
TA1535, TA1537
Table 5. Results of assays for genotoxicity with metabolites of fipronil metabolites
Metabolite End-point Test object Concentration Purity Results Reference
(%)
In vivo
Fipronil-desulfinyl Micronucleus CD-1 mice 2-16 mg/kg bw in 99.5 Negativeb Proudlock
formation corn oil (1996)
DMSO, dimethyl sulfoxide, S9, 9000 × g supernatant of rat liver homogenate
a With and without metabolic activation
b Appropriate positive controls gave expected positive responses
c Reproducible increases in polyploid cells with metabolic activation
Table 6. Comparisons of fipronil and its metabolites
Compound In plants In animals Potential environmental Photo- Oral LD50 Binding to
metabolite or surface metabolite (mg/kg bw)a 3H-EBOB &
residue or degradate 3H-TBPS sitesb
Fipronil Yes Rat, goat, hen Water, soil, surfaces No 97
M&B 45950 Yes Rat, goat, hen Soil No 83 * (both sites)
RPA 200766 Yes Rat, goat Water, soil, surfaces No > 2000 @@ (both sites)
Fipronil-desulfinyl Yes No Water, soil, surfaces Yes 16 ** (both sites)
M&B 46136 Yes Rat, goat, hen Soil, surfaces No 218 * (both sites)
RPA 104615 Yes No Water, soil, surfaces Yes > 2000 @@ (both sites)
M&B 45897 Yes Rat Not expected No > 2000 @@ (both sites)
Two ring-opened No Rat No No No data No data
metabolites of
M&B 45897
M&B 105320 Yes No Not expected No > 2000 @@ (both sites)
EBOB, 1-[(4-ethyl)phenyl]-4-n-propyl-2,6,7-trioxacicyclo[2.2.2]octane; TBPS, tert-butylbicyclophosphorothionate
a From references given in Tables 1 and 2
b Increased (*) or decreased (@) binding relative to fipronil; ** or @@ greater differences. Based on IC50 values from
competitive binding assays for fipronil and its metabolites with radiolabelled 1 gands (personal communication from
P. Kwiatkowski, Rhone-Poulenc Worldwide Regulatory Affairs, North Carolina, USA)
The results presented in the table and those of similar studies
indicate that most of the metabolites are of similar or lesser
toxicity than fipronil. Generally, this conclusion reflects the data
on binding, except for M&B 46136 which has greater binding affinity
for sites in the rat brain GABA receptor chloride-ion channel but a
higher acute oral LD50 than fipronil in rats. (No short-term studies
of the toxicity of this metabolite are available.)
Fipronil-desulfinyl and M&B 104615 are photolytic products of
fipronil which could potentially form in the environment or on treated
or exposed surfaces during use to control malarial vectors. The LD50
of fipronil-desulfinyl in rats is much lower than that of fipronil,
and it has much greater binding affinity for GABA receptor
chloride-ion channel sites in rat brain. Both are neurotoxic. The
toxicity of fipronil-desulfinyl is qualitatively similar to that of
fipronil, but the dose-effect curve for neurotoxic effects is steeper
for fipronil-desulfinyl and neurotoic effects occur at lower doses.
The acute oral LD50 for M&B 104615 is much higher and the affinity
for GABA receptor chloride-ion channel sites much less than those for
fipronil.
Comments
Fipronil
In a study of dermal absorption in rats, the quantity of
14C-fipronil absorbed was less than 1% of the applied dose at all
doses tested (0.88-48 mg/rat) and all times up to 24 h. In vitro,
the relative extent of absorption of a formulation of 14C-fipronil
across rat, rabbit, or human epidermal membranes depended on the
concentration of the material used. At the lowest concentration tested
(0.2 g/L), the extent of penetration was greatest for all three
species, and the percentage of the dose absorbed across human and rat
membranes was similar. At higher concentrations (4 and 200 g/L),
penetration was greater through rat and rabbit skin than through human
skin.
There was no appreciable difference between male and female rats
in the absorption, distribution, metabolism, or excretion of fipronil
after oral administration. The proportion of the dose absorbed
appeared to depend on the treatment regimen, being greatest with a
single dose of 4 mg/kg bw of 14C-fipronil (minimum absorption, 50%),
intermediate with a repeated dose regimen of 4 mg/kg bw per day for 14
days followed by a single, oral labelled dose of 4 mg/kg bw (minimum
absorption, 40%), and lowest (minimum absorption, about 30%) with a
single dose of 150 mg/kg bw of 14C-fipronil (presumably due to
saturation of absorption at the high dose). Once absorbed, fipronil
was rapidly metabolized, and the residues widely distributed in
tissues. Significant amounts of residues remained in the tissues,
particularly in fat and fatty tissues, one week after treatment. The
levels of residues in fat and other tissues were greater with repeated
low doses or a single high dose than with a single low dose. The long
half-life (150-245 h in some cases) of fipronil in blood may reflect
slow release of residues from fat and might suggest potential
bioaccumulation of metabolic products of fipronil.
Faeces, followed by urine, were the major routes of elimination
of fipronil in rats. Its biotransformation largely involved changes in
the functional groups attached to the pyrazole ring. The compounds
identified in faeces and urine were the parent compound and the
sulfone, the amide derived from the nitrile group, a reduction
product, and a cleavage product of the sulfone and its derivatives
formed by further cleavage. The sulfone was the major metabolite in
fat and tissues.
Fipronil was moderately hazardous to rats (LD50 = 92 mg/kg bw)
and mice (LD50 = 91 mg/kg bw) after oral administration of single
doses and to rats after single exposure by inhalation (LC50
= 0.36 mg/L). After a single dermal exposure, fipronil was relatively
non-hazardous to rats (LD50 > 2000 mg/kg bw) but was moderately
hazardous to rabbits (LD50 = 354 mg/kg bw). In rats, signs of
toxicity and death were delayed for up to four days after either a
single oral dose or repeated oral doses of 75 mg/kg bw per day for up
to five days. WHO has not yet classified fipronil for acute toxicity.
In a 13-week study of toxicity, mice were fed diets containing
fipronil at doses of 0, 1, 3, 10, or 25 ppm. A dose-related increase
in the incidence of liver-cell periacinar hypertrophy with cytoplasmic
vacuolation was observed in males at doses of 1 ppm (equal to 0.13
mg/kg bw per day) and above. There was no NOAEL.
Rats were fed diets containing 0, 25, 50, 100, 200, or 400 ppm
fipronil for four weeks. At 25 ppm (equal to 3.4 mg/kg bw per day),
liver weights and plasma cholesterol levels were increased in females,
and thyroid follicular-cell hypertrophy of minimal severity was
observed in animals of each sex. The levels of total protein and
globulin were also increased in both males and females, although the
changes at this and higher doses were generally small and poorly
correlated with the dose. There was no NOAEL.
In a 13-week study of toxicity, fipronil was administered in the
diet to rats at doses of 0, 1, 5, 30, or 300 ppm. At 30 ppm and above,
relatively small, sometimes inconsistent changes in haematological
parameters (decreased packed cell volume, mean cell volume,
haemoglobin concentration, and prothrombin time and increased platelet
count) and clinical chemical parameters (increased total protein and
globulins, decreased albumin:globulin ratio and alanine
aminotransferase and aspartate aminotransferase activities) were
observed, mostly in females. Some alterations were seen in plasma
glucose and urea concentrations at 30 ppm; also at 30 ppm, the
absolute and/or relative weights of the liver and thyroid were
increased in either males or females or both, and there was evidence
of thyroid follicular-cell epithelial hypertrophy in males. The NOAEL
was 5 ppm, equal to 0.33 mg/kg bw per day.
Fipronil was administered in gelatin capsules to dogs for 13
weeks in a study of toxicity at doses of 0, 0.5, 2, or 10 mg/kg bw per
day. Inappetence and decreased body-weight gain and food consumption
were noted in females at 2 and 10 mg/kg bw per day. The NOAEL was 0.5
mg/kg bw per day.
In a study of dermal toxicity, fipronil was applied in 0.5%
carboxymethylcellulose to the intact skin of rabbits for 6 h per day
on five days per week for three weeks at doses of 0, 0.5, 1, 5, or 10
mg/kg bw per day. No dermal irritation was observed. At 10 mg/kg bw
per day, body-weight gains and food consumption were reduced in
animals of each sex. Some animals showed hyperactivity. The NOAEL was
5 mg/kg bw per day.
Fipronil was administered to dogs in gelatin capsules for one
year in a study of toxicity at doses of 0, 0.2, 2, or 5 mg/kg bw per
day. At 2 mg/kg bw per day and above, clinical signs of neurotoxicity
(convulsions, twitching, tremors, ataxia, unsteady gait, rigidity of
limbs, nervous behaviour, hyper- or hypoactivity, vocalization,
nodding, aggression, resistance to dosing, inappetence, and abnormal
neurological responses) were observed in animals of each sex. One
animal at 2 mg/kg bw per day was killed because of poor condition
related to treatment. The NOAEL was 0.2 mg/kg bw per day. In a second
one-year study in dogs, fipronil was administered in the diet at doses
of 0, 0.075, 0.3, 1, or 3 mg/kg bw per day. The highest dose was
reduced to 2 mg/kg bw per day after 38 days because of toxicity. At 1
mg/kg bw per day, clinical signs of neurotoxicity (whole body
twitching, and extensor rigidity of limbs) were noted in females.
There were no effects on triiodothyronine or thyroxine levels. The
NOAEL was 0.3 mg/kg bw per day.
In a study of carcinogenicity, fipronil was administered for 78
weeks in the diet to mice at doses of 0, 0.1, 0.5, 10, 30, or 60 ppm.
Additional groups of animals were fed the same doses for 52-53 weeks
and then killed. Survival was greater than or comparable to that of
the control group at doses below 60 ppm. At week 10, all surviving
animals at 60 ppm were killed because of excessive mortality. In
animals at 10 ppm, some decrease in body-weight gain was noted in
males and females, and efficiency of food use was decreased in males.
At 53 and 78 weeks, the absolute and/or relative liver weights of
males were increased, with an increased incidence of liver periacinar
microvesicular vacuolation. There was no evidence of carcinogenicity
at doses considered to be sufficient to measure such potential. The
NOAEL for systemic effects was 0.5 ppm, equal to 0.055 mg/kg bw per
day.
In a study of toxicity and carcinogenicity in rats, fipronil was
administered in the diet at doses of 0, 0.5, 1.5, 30, or 300 ppm. For
the carcinogenicity phase of the study, it was originally planned that
the test material be administered for two years, but excessive
mortality resulted in early termination of this phase at week 89 in
males and week 91 in females. This was not thought to compromise the
study. For the toxicity phase and a reversibility phase of the study,
additional groups of animals were fed the same doses of fipronil for
one year, when some animals were killed and others were allowed to
recover for 13 weeks. Some of the effects noted at the higher doses
persisted into the reversibility phase of the study. During treatment,
convulsive episodes (sometimes fatal) were observed in males at 1.5
ppm and in animals of each sex at higher doses. Animals at 1.5 ppm,
predominantly females, showed irritability, vocalization, salivation,
aggression, hyperactivity, and bruxism. Small decreases were noted in
erythrocyte count, haemoglobin concentration, mean cell volume, and
packed cell volume in either males or females or both, and some
alterations in protein level were observed in males. An apparent
increase in the severity of progressive senile nephropathy was seen in
animals of each sex at this dose. Thyroxine concentrations were
decreased in both males and females. Thyroid-stimulating hormone
levels were increased, notably in males, at doses of 30 ppm and above
and in females at 300 ppm. The levels of triiodothyronine were
elevated in females at 30 ppm, but only during the reversibility
phase. At 300 ppm, fipronil induced follicular-cell adenomas of the
thyroid gland in both males and females; males at this dose also had
an increased incidence of follicular-cell carcinomas. Some thyroid
follicular-cell adenomas were noted in male rats at lower doses, but a
comparison with historical control data indicated no clear
relationship to treatment. The NOAEL for systemic effects was 0.5 ppm,
equal to 0.019 mg/kg bw per day.
Fipronil and its metabolites gave negative results in virtually
all tests for genotoxicity. Equivocal results were seen in assays for
cytogenicity in mammalian cells in vitro with fipronil and for
polyploidy (not clastogenicity) in human lymphocytes with a mammalian
metabolite. The weight of evidence indicates that fipronil and its
metabolites are not genotoxic.
The Meeting concluded that the thyroid tumours observed in the
two-year study in rats occurred by a non-genotoxic, threshold
dose-effect mechanism involving continuous stimulation of the thyroid
gland associated with persistently elevated thyroid-stimulating
hormone levels. It was noted that the levels of this hormone were
clearly elevated only at the two highest doses.
In a two-generation study of reproductive toxicity, rats received
diets containing fipronil at 0, 3, 30 or 300 ppm. F0 parental animals
were mated twice to produce F1a and F1b litters; F1a parents were
mated only once to produce F2 litters. In adult animals at 30 ppm,
the thyroid and liver weights were increased and the pituitary gland
weights were decreased. An increased incidence of thyroid gland
follicular epithelial-cell hypertrophy was seen at this dose in males
of the F0 and F1 generations and F1 females. At 300 ppm,
convulsions were observed in F1 and F2 litters; decreased litter
size, decreased body weights and delays in physical development were
also seen. Postnatal survival was decreased among pups in the F2
litters. Absolute and relative ovarian weights were decreased in F0
females. At 300 ppm, a decreased percentage of animals that mated and
a reduction in the fertility index of F1 parental animals was also
observed. These effects may have been related to the systemic toxicity
of fipronil at this dose. The NOAEL for parental systemic toxicity was
3 ppm, equal to 0.25 mg/kg bw per day, and the NOAEL for reproductive
toxicity was 30 ppm, equal to 2.5 mg/kg bw per day.
Rats were given fipronil by gavage at doses of 0, 1, 4, or 20
mg/kg bw per day on days 6-15 of gestation. Developmental toxicity was
not observed, but there were some signs of maternal toxicity
(decreased body-weight gain and food consumption) at 20 mg/kg bw per
day. The NOAEL for maternal toxicity was 4 mg/kg bw per day, and that
for developmental toxicity was 20 mg/kg bw per day, the highest dose
tested.
Rabbits were given fipronil by gavage at doses of 0, 0.1, 0.2,
0.5, or 1 mg/kg bw per day on days 6-19 of gestation. Developmental
toxicity was not observed, but there were some signs of maternal
toxicity (decreased body-weight gain, decreased food consumption, and
reduced efficiency of food use at all doses. There was no NOAEL for
maternal toxicity; the NOAEL for developmental toxicity was 1 mg/kg bw
per day, the highest dose tested.
Primary dermal irritation in rabbits was examined in two studies.
Fipronil was slightly irritating when moistened with corn oil before
application but was not irritating when moistened with water. Fipronil
was slightly irritating in two studies of primary ocular irritation in
rabbits. It did not sensitize the skin of guinea-pigs when tested by
the Buehler method but was a weak sensitizer in guinea-pigs tested by
the Magnusson-Kligman method.
In a study of neurotoxicity, rats were given single doses of 0,
0.5, 5, or 50 mg/kg bw fipronil by gavage. At 5 mg/kg bw, decreased
hind-leg splay was observed 7 h after treatment in both males and
females. The NOAEL was 0.5 mg/kg bw. In a 13-week study of
neurotoxicity, rats received dietary doses of 0, 0.5, 5, or 150 ppm
fipronil. Body weights, weight gains, and food consumption were
reduced early in the study in animals of each sex at 150 ppm, possibly
owing to problems of palatability. Although the findings in a battery
of functional operational tests at this dose were relatively minor
when taken separately, they appeared to represent a minimal effect of
treatment when taken together. The NOAEL for neurotoxicity and
systemic effects was 5 ppm, equal to 0.3 mg/kg bw per day.
In a study of neurotoxicity in female dogs, fipronil was
administered in capsules at doses of 0 (one animal) or 20 mg/kg bw per
day (four animals) until the appearance of neurotoxic signs in each
animal, after which they were allowed to recover for 28 days. Severe
neurotoxic signs were seen at 20 mg/kg bw per day during the treatment
phase and in some animals only during the recovery phase. Most animals
appeared to recover, although one had exaggerated reflex responses and
was excitable at the end of the recovery period. A limited
histopathological examination showed no change. No firm conclusions
could be drawn about the reversibility of the effects, given the
limitations of the study design. There was no NOAEL.
In a study of developmental neurotoxicity, rats were given
fipronil in the diet from gestation day 6 through lactation day 10 at
doses of 0, 0.5, 10, or 200 ppm. Maternal toxicity manifested as
reduced body weight during the treatment period, reduced body-weight
gain during gestation, and reduced food consumption was observed at
200 ppm. Developmental toxicity (reduced body weights in pups and a
slight increase in the time to preputial separation) was noted at 10
ppm. An increase in motor activity in female pups at 10 ppm only on
day 17 could not be definitively interpreted as an indication of
developmental toxicity. Developmental neurotoxicity was clearly
observed postnatally in pups at 200 ppm, with delayed swimming
development on day 6, increased motor activity on day 17, abnormal
auditory startle response on day 22, and impaired learning and memory
on day 24. The NOAEL for maternal toxicity and developmental
neurotoxicity was 10 ppm (equal to 0.9 mg/kg bw per day) and that for
developmental toxicity was 0.5 ppm (equal to 0.05 mg/kg bw per day).
Mechanistic studies conducted with fipronil in rats suggest that
it does not interfere with the incorporation of iodine into thyroxine
but rather with the biliary clearance of this hormone. This may
trigger an increase in the concentration of thyroid-stimulating
hormone by interference with the feedback mechanism.
Mammalian metabolites of fipronil
Several mammalian metabolites of fipronil were tested for acute
toxicity. Their toxicity was comparable to or substantially less than
that of fipronil.
Photodegradation products of fipronil
Numerous studies were performed with fipronil-desulfinyl, one of
two photodegradation products of fipronil which can be formed in the
presence of sunlight and could potentially be produced in the
environment or on treated surfaces. Neither is a mammalian metabolite
of fipronil. The available information indicates that, of the two,
only fipronil-desulfinyl is highly toxic after exposure to single
doses or over the long term, and is therefore of toxicological
concern.
When 0.08-7.2 mg of 14C-fipronil-desulfinyl were applied
dermally to rats, absorption ranged fron 0.2 to 7% of the applied dose
within 24 h.
The absorption, distribution metabolism, and excretion of
14C-fipronil-desulfinyl were studied in rats which received either a
single oral dose of labelled compound at 1 or 10 mg/kg bw or 14 daily
oral doses of unlabelled compound at 1 mg/kg bw per day followed by a
single oral labelled dose. In animals of each sex, elimination of the
radiolabel was much greater in the faeces (46-70% of the dose) than in
the urine with all dosing regimens. Appreciable residues were found in
the tissues one week after treatment, the highest concentrations being
present in the fat and fatty tissues. The long half-life in blood
(183-195 h) and increased fat:plasma ratios of the radiolabel suggest
potential bioaccumulation of fipronil-desulfinyl and/or its
metabolites. Numerous metabolites or conjugates of fipronil-desulfinyl
were present in the urine and faeces. Biotransformation of
fipronil-desulfinyl involved changes at the functional groups attached
to the pyrazolyl ring. Only unchanged fipronil-desulfinyl was
identified in the liver, fat, skin, and residual carcass.
In a 28-day study of toxicity in which fipronil-desulfinyl was
administered in the diet to mice at doses of 0, 0.5, 3, 30, or 60 ppm,
mortality, neurotoxic signs (increased motor activity, excessive
jumping, irritability to touch, compulsive biting, and evidence of
convulsions), decreased body-weight gain and food consumption, and an
increased incidence of centrilobular hypertrophy of the liver were
observed in animals of each sex at doses of 30 ppm and above. The
NOAEL was 3 ppm, equal to 0.49 mg/kg bw per day. Fipronil-desulfinyl
was administered in the diet for 90 days to mice at doses of 0, 0.5,
2, or 10 ppm. At 2 and 10 ppm, clinical signs of neurotoxicity
(irritability to touch, aggressiveness, and/or increased motor
activity) were noted in males. The NOAEL was 0.5 ppm, equal to
0.08 mg/kg bw per day.
Rats received fipronil-desulfinyl by gavage for two weeks at
doses of 0, 0.3, 1, 3, or 10 mg/kg bw per day. At 1 mg/kg bw per day,
pale livers and reduced leukocyte counts were observed in females.
Some rats at 3 mg/kg bw per day died or were killed because of poor
condition. The NOAEL was 0.3 mg/kg bw per day. Fipronil-desulfinyl was
administered in the diet for 28 days to rats at doses of 0, 0.5, 3, 30
or 100 ppm. One male at 30 ppm died, clinical signs of toxicity
(piloerection and curling up on handling), and decreased body weights,
food consumption, and bilirubin concentration were seen in males and
females at this dose. Thymus weights were lowered in females. The
levels of thyroid-stimulating hormone were measured, but no effects
were noted at any dose. All animals at 100 ppm died. The NOAEL was 3
ppm, equal to 0.23 mg/kg bw per day.
In a 90-day study of toxicity in rats, fipronil-desulfinyl was
administered in the diet at 0, 0.5, 3, 10, or 30 ppm. At 3 ppm and
above, clinical signs of neurotoxicity (aggressiveness, irritability
to touch, and excessive vocalization) were observed in males. The
levels of triiodothyronine and thyroxine were affected at higher
doses, but the toxicological significance of these changes is probably
negligible in the absence of changes in the level of
thyroid-stimulating hormone at any dose. The NOAEL in the study was
0.5 ppm, equal to 0.029 mg/kg bw per day.
Dogs received fipronil-desulfinyl in the diet in a 28-day study
at doses of 0, 27, 80, or 270 ppm. The groups at 80 and 270 ppm were
terminated early because of mortality. One male at 27 ppm had a clonic
convulsion. Reduced thymus weights and pale livers were also reported
at this dose. As effects occurred at the lowest dose, there was no
NOAEL.
In a 90-day study of toxicity, fipronil-desulfinyl was
administered in the diet to dogs at doses of 0, 3.5, 9.5, or 35 ppm.
The clinical findings in one female at 35 ppm (increased salivation,
prostration, writhing, tremors, absence of rotular reflex, noisy
breathing, dyspnoea) were attributed to arteritis and myocardial
necrosis on the basis of microscopic findings; however, they may also
have been indicative (at least in part) of neurotoxicity, because
another female in this group exhibited excessive barking,
aggressiveness, irritability, tremors, and increased salivation. On
this basis, the Meeting concluded that the NOAEL was 9.5 ppm, equal to
0.29 mg/kg bw per day.
In a study of developmental toxicity in rats, fipronil-desulfinyl
was administered by gavage on days 6-15 of gestation at doses of 0,
0.5, 1, or 2.5 mg/kg bw per day. Indications of maternal effects
(decreased body-weight gain and hair loss in various areas) were
observed at 2.5 mg/kg bw per day. Developmental toxicity (increased
incidence of incomplete or reduced ossification of several bones and
slightly reduced fetal body weight in animals of each sex) was also
observed at this dose. The NOAEL for maternal toxicity and
developmental toxicity was 1 mg/kg bw per day.
In a study of neurotoxicity in rats, fipronil-desulfinyl was
administered by gavage as a single dose of 0, 0.5, 2, or 12 mg/kg bw.
At 12 mg/kg bw, decreased body-weight gains and food consumption were
observed during week 1 in animals of each sex. Decreased hind-foot
splay, rectal temperature, and locomotor activity were also seen in
animals of each sex at this dose. There were indications of a slowed
righting reflex in males and decreased grip strength in males and
females at the high dose. The NOAEL was 2 mg/kg bw per day.
In summary, the toxicity of fipronil-desulfinyl is qualitatively
similar to that of fipronil, but the dose-effect curve for neurotoxic
effects appears to be steeper for fipronil-desulfinyl than for
fipronil. Also, fipronil-desulfinyl appears to have a much greater
tendency than fipronil to bind to sites in the chloride ion channel of
the rat brain GABA receptor. This finding appears to be consistent
with the greater toxicity, relative to fipronil, of
fipronil-desulfinyl in the central nervous system of mammals.
The Meeting established an ADI of 0-0.0002 mg/kg bw for fipronil
on the basis of the NOAEL of 0.019 mg/kg bw per day in the two-year
study of toxicity and carcinogenicity in rats and incorporating a
safety factor of 100.
The Meeting considered that a separate ADI should be established
for fipronil-desulfinyl on the basis that it could be a significant
residue and that its toxicity is greater than that of the parent
molecule fipronil. A temporary ADI of 0-0.00003 mg/kg bw for
fipronil-desulfinyl was established on the basis of the NOAEL of 0.029
mg/kg bw per day in the 90-day study in rats and a safety factor of
1000, in view of the lack of a long-term study by oral administration
in rats and a study of neurotoxicity in rats given repeated oral
doses.
Toxicological evaluation
Fipronil
Levels that cause no toxic effect
Mouse: 0.5 ppm, equal to 0.055 mg/kg bw per day (78-week study
of carcinogenicity and toxicity)
Rat: 5 ppm, equal to 0.33 mg/kg bw per day (13-week study of
toxicity)
0.5 ppm, equal to 0.019 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
3 ppm, equal to 0.25 mg/kg bw per day (parental
systemic toxicity in a study of reproductive toxicity)
30 ppm, equal to 2.5 mg/kg bw per day (study of
reproductive toxicity)
4 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity by gavage)
20 mg/kg bw per day (developmental toxicity in a study
of developmental toxicity by gavage; highest dose
tested)
0.5 mg/kg bw (single dose, study of neurotoxicity by
gavage)
5 ppm, equal to 0.3 mg/kg bw per day (repeated doses in
the diet, study of neurotoxicity)
10 ppm, equal to 0.9 mg/kg bw per day (maternal
toxicity and developmental neurotoxicity in a study of
developmental neurotoxicity)
0.5 ppm, equal to 0.05 mg/kg bw per day (developmental
toxicity in a study of developmental neurotoxicity)
Rabbit: 0.1 mg/kg bw per day (LOAEL for maternal toxicity in a
study of developmental toxicity by gavage)
1 mg/kg bw per day (study of developmental toxicity;
highest dose tested by gavage)
Dog: 0.3 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.0002 mg/kg bw
Fipronil-desulfinyl (fipronil photodegradation product)
Levels that cause no toxic effect
Mouse: 3 ppm, equal to 0.49 mg/kg bw per day (28-day study of
toxicity)
0.5 ppm, equal to 0.08 mg/kg bw per day (90-day study
of toxicity)
Rat: 0.3 mg/kg bw per day (two week study of toxicity by
gavage)
3 ppm, equal to 0.23 mg/kg bw per day (28-day study of
toxicity)
0.5 ppm, equal to 0.029 mg/kg bw per day (90-day study
of toxicity)
1 mg/kg bw per day (maternal and developmental toxicity
in a study of developmental toxicity by gavage)
2 mg/kg bw per day (single dose, study of neurotoxicity
by gavage)
Dog: 9.5 ppm, equal to 0.29 mg/kg bw per day (90-day study
of toxicity)
Estimate of temporary acceptable daily intake for humans
0-0.00003 mg/kg bw
Acute reference dose for fipronil
The Meeting allocated an acute reference dose of 0.003 mg/kg bw
for both fipronil and fipronil-desulfinyl on the basis of the NOAEL of
0.3 mg/kg bw per day in a study of neurotoxicity in rats given
repeated doses of fipronil, and a safety factor of 100. The study of
neurotoxicity in rats given single doses was not considered in
allocating the acute reference dose because of concern about the
prolonged toxicokinetics of fipronil. This acute reference dose will
provide a safety factor of about 700 for the NOAEL in the study of
neurotoxicity in rats given single doses of fipronil-desulfinyl.
Studies without which the determination of an ADI is impractable,
to be provided by 2000
1. Short-term study of neurotoxicity in rats with
fipronil-desulfinyl in the diet
2. Developmental neurotoxicity study in rats with
fipronil-desulfinyl in the diet
3. The results of an ongoing long-term study with
fipronil-desulfinyl in rats
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to fipronil and its photodegradation
product fipronil-desulfinyl
Human exposure Relevant route, study type, species Results, remarks
Fipronil
Short-term Skin, irritation, rabbit Slightly imitating
(1-7 days) Eye, irritation, rabbit Minor irritation
Skin, sensitization, guinea-pig Not a sensitizer (Buehler)
Skin, sensitization, guinea-pig Mild sensitizer (Magnusson-Kligman)
Oral, toxicity, rat LD50 = 92 mg/kg bw
Dermal, toxicity, rabbit LD50 = 350 mg/kg bw
Inhalation, toxicity, rat LC50 = 0.36 mg/L
Neurotoxicity, rat NOAEL = 0.5 mg/kg bw per day:
(single dose by gavage) decreased hind-leg splay
Medium-term Repeated dermal, 3 weeks, toxicity, rabbit NOAEL = 5 mg/kg bw per day: reduced body-weight
(1-26 weeks) gains and food consumption; hyperactivity in some
animals; no dermal imitation observed
Repeated oral, reproductive toxicity, rat NOAEL = 0.25 mg/kg bw per day for maternal toxicity.
NOAEL = 2.5 mg/kg bw per day for reproductive
toxicity
Repeated oral, developmental neurotoxicity, rat NOAEL = 0.9 mg/kg bw per day for maternal toxicity.
NOAEL = 0.05 mg/kg bw per day for developmental
toxicity
NOAEL = 0.9 mg/kg bw per day for developmental
neurotoxicity
Long-term Repeated oral, 2 years (terminated at 89-91 NOAEL = 0.019 mg/kg bw per day: convulsions and
(> 1 year) weeks), long-term toxicity and carcinogenicity, rat neurobehavioural clinical signs of toxicity; effects on the
thyroid; thyroid follicular-cell adenomas and carcinomas
Fipronil-desulfinyl
Short-term Oral, toxicity, rat LD50 = 15 mg/kg bw
(1-7 days) Dermal, toxicity, rat LD50 > 2000 mg/kg bw
Neurotoxicity, rat NOAEL = 2 mg/kg bw per day
(single dose by gavage)
(continued)
Human exposure Relevant route, study type, species Results, remarks
Medium-term Repeated oral (diet), 90 days, toxicity, rat NOAEL = 0.029 mg/kg bw per day
(1-26 weeks) Repeated oral (gavage), developmental toxicity, NOAEL = 1.0 mg/kg bw per day: maternal toxicity
rat NOAEL = 1.0 mg/kg bw per day: developmental
toxicity
Long-term Repeated oral toxicity No data
> 1 year)
Studies that would provide information useful for the continued
evaluation of fipronil and fipronil-desulfinyl
1. Additional studies to investigate the reversibility of the
neurotoxic effects of fipronil and its metabolites
(functional, behavioural, learning/memory, cellular, and
neurotransmitter/receptor effects).
2. Observations in humans exposed to fipronil and fipronil-
desulfinyl
References
Adams, K. (1996a) MB 46513: CHO mammalian cell mutation assay.
Unpublished report No. RNP452/950622 from Huntingdon Life Sciences,
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Adams, K. (1996b) MB 46513: Metaphase chromosome analysis of human
lymphocytes cultured in vitro. Unpublished report No. RNP 451/951219
from Huntingdon Life Sciences, Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Aughton, P. (1993) MB 46030: Combined oncogenicity and toxicity study
by dietary administration to CD rats for 104 weeks, including a 13
week reversibility period on completion of 52 weeks of treatment.
Unpublished report No. 93/RHA432/0166 from Pharmaco LSR Ltd. Submitted
to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Berthe, P. (1996) RPA 200766: 28-Day toxicity study in the rat by
dietary administration. Unpublished report No. SA 95273 from
Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Bigot, D. (1996) MB 46513: 90-Day toxicity study in the mouse by
dietary administration. Unpublished report No. SA 95055 from
Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Broadmeadow, A. (1991) M&B 46030: Preliminary toxicity study by
dietary administration to CD-1 mice for 13 weeks. Unpublished report
No. 90/RHA364/0860 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Broadmeadow, A. (1993) M&B 46030: Oncogenicity study by dietary
administration to CD-1 mice for 78 weeks. Unpublished report No.
92/RHA313/0971 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Brooker, A.J. & John, D.M. (1991) The effect of M&B 46,030 on
pregnancy of the rat. Unpublished report No. M&B 335+326/90582 from
Huntingdon Research Centre Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Bushey, D.F. (1993) Fipronil mode of action research summary.
Unpublished memo prepared by Rhone-Poulenc Agrochimie Co., Research
Triangle Park Biochemistry Group. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Cheng, T. (1995) Dermal absorption of 14C-fipronil REGENT 80WDG in
male rats (preliminary and definitive phases). Unpublished report No.
HWI 6224-210 from Hazleton Wisconsin, Inc. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Cheng, T. (1996) Dermal absorption of 14C-MB 46513 in male rats
(preliminary and definitive phases). Unpublished report No.
CHW 6224-230 from Corning Hazleton, Inc. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Clare, C.B. (1988a) Study to determine the ability of M&B 46030 to
induce mutation in four histidine-requiring strains of Salmonella
typhimiurim. Unpublished report No. MAB 20/S from Microtest Research
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Clare, C.B. (1988b) Study to determine the ability of M&B 46136 to
induce mutation in four histidine-requiring strains of Salmonella
typhimiurim. Unpublished report No. MAB 21/S from Microtest Research
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Cole, L.M., Nicholson, R.A. & Casida, J.E. (1993) Action of
phenylpyrazole insecticides at the GABA-gated chloride channel.
Pestic. Biochem. Physiol., 46, 47-54.
Cracknell, S. (1991) M&B 46030: Acute inhalation toxicity study in the
rat. Unpublished report No. 90/RHA358/0791 from Life Science Research
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Dange, M. (1993a) RPA 200766: Acute oral LD50 in the rat. Unpublished
report No. SA 93016 from Rhone-Poulenc Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Dange, M. (1993b) MB 46513. Acute oral LD50 in rats. Unpublished
report No. SA 93074 from Rhone-Poulenc Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Dange, M. (1993c) MB 46513: Acute dermal LD50 in the rat. Unpublished
report No. SA 93095 from Rhone-Poulenc Secteur Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Dange, M. (1993d) RPA 104615 acute oral LD50 in the rat. Unpublished
report No. SA 93046 from Rhone-Poulenc Secteur Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC,USA.
Dange, M. (1994a) MB 45950 acute oral LD50 in the rat. Unpublished
report No. SA 93272 from Rhone-Poulenc Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Dange, M. (1994b) MB 46513: Preliminary 28-day toxicity study in the
mouse by dietary administration. Unpublished report No. SA 93228 from
Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Dange, M. (1994c) MB 46513: Exploratory 14-day toxicity study in the
rat by gavage. Unpublished report No. SA 93063 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Dange, M. (1994d) MB 46513 90-day toxicity study in the rat by dietary
administration. Unpublished report No. 93226 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Dange, M. (1995a) MD 46513: Preliminary 28 day toxicity study in the
rat by dietary administration. Unpublished report No. SA 93138 from
Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Dange, M. (1995b) MB 46513: Preliminary 28-day toxicity study in the
dog by dietary administration. Unpublished report No. SA 94143 from
Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Dange, M. (1996) MB 46513: 90-Day toxicity study in the dog by dietary
administration. Unpublished report No. SA 95100 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Driscoll, C.D. and Hurley, J.M. (1993) MB 46030: Ninety day dietary
neurotoxicity study in Sprague Dawley rats. Unpublished report No.
92N1074 from Union Carbide Bushy Run Research Center. Submitted to WHO
by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Edwards, C.N. (1991) M&B 46030: Assessment of clastogenic action on
bone marrow erythrocytes in the micronucleus test. Unpublished report
No. 90/RHA305/1377 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Edwards, C.N. (1995) M&B 46030: Mouse micronucleus test to comply with
OECD Guideline 474 (1983). Unpublished report No. 95/RHA547/0432 from
Pharmaco LSR Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Foulon, O. (1997) MB 46513 (fipronil photometabolite): Developmental
toxicology study in the rat by gavage. Unpublished report No. SA 96227
from Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Gant, D.B., Chalmers, A.E. & Wolff, M.A. (1994) Fipronil: A novel
insecticide acting at the GABA receptor. Poster presented at the
Eighth International Congress of Pesticide Chemistry, Washington DC by
Rhone-Poulenc Agrochimie Co., Department of
Biochemistry/Biotechnology, Research Triangle Park, NC.
Gardner, J.R. (1988a) Acute oral toxicity to rats of M&B 46,030.
Unpublished report No. 881300D/M&B 290/AC from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Gardner, J.R. (1988b) Acute dermal toxicity to rats of M&B 46,030.
Unpublished report No. 881113 D/M&B 291/AC from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Gardner, J.R. (1988c) Acute oral toxicity to rats of M&B 46136.
Unpublished report No. 881364 D/M&B286/AC from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC,USA.
Gardner, J.R. (1988d) Acute dermal toxicity to rats fo M&B 46,136.
Unpublished report No. 88916 D/M&B287/AC from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Gill, M.W., Wagner, C.L. & Driscoll, C.D. (1993) M&B 46030: Single
exposure peroral (gavage) neurotoxicity study in Sprague Dawley rats.
Unpublished report No. 91N0099 from Union Carbide Bushy Run Research
Center. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Haynes, G. (1988a) M&B 45897 acute oral toxicity study in the rat.
Unpublished report No. A/D/4855 from Toxicol Laboratories Ltd.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Haynes, G. (1988b) M&B 45897 acute dermal toxicity study in the rat.
Unpublished report No. A/D/4856 from Toxicol Laboratories Ltd.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Hermansky, S.J. & Wagner, C.L. (1993) M&B 46030: Twenty-one day
repeated cutaneous dose toxicity study in New Zealand white rabbits
#2. Unpublished report No. 92N1165 from Union Carbide Bushy Run
Research Center. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Holmes, P. (1991a) M&B 46030: Toxicity study by dietary administration
to CD rats for 13 weeks. Unpublished report No. 90/RHA 298/0781 from
Life Science Research Ltd. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Holmes, P. (1991b) M&B 46030: Toxicity study by oral (capsule)
administration to beagle dogs for 13 weeks. Unpublished report No.
90/RHA310/0842 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Holmes, P. (1991c) M&B 46030: Neurotoxicity study by oral (capsule)
administration to female beagle dogs for up to 14 days followed by a
28 day reversibility period. Final report. Unpublished report No.
90/RHA371/0790 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Holmes, P. (1992) M&B 46030: Toxicity study by oral (capsule)
administration to beagle dogs for 52 weeks. Unpublished report No.
92/RHA311/0464 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Holmes, P. (1993) M&B 46030: Toxicity study by dietary administration
to beagle dogs for 52 weeks. Unpublished report No. 93/RHA465/0243
from Pharmaco-LSR Ltd. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Hughes, E.W. (1996) MB 46513: Neurotoxicity to rats by acute oral
administration (including a dose range finding study). Unpublished
report No. RNP 471/951489 from Huntingdon Life Sciences. Submitted to
WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Johnson, A.L. (1995) Pyrazole/MB 45897/RPA 097920: An in vitro test
for induction of chromosome damage: Cytogenetic study in cultured
human peripheral lymphocytes. Unpublished report No. 94/RHA534/1034
from Pharmaco-LSR. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Johnson, I.R. (1993) M&B 46030: Delayed contact hypersensitivity study
in guinea pigs. Unpublished report No. 93/RHA503/0167 from
Pharmaco-LSR Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Johnson, I.R. (1995) Pyrazole/MB 45897/RPA 097920: Intermediate of
fipronil (MB 46030): Four week oral toxicity study in the rat.
Unpublished report No. 95/RHA535/0684 from Pharmaco LSR. Submitted to
WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Kennelly, J.C. (1988) Study to determine the ability of M&B 45897/RPA
97920 to induce mutation in four histidine-requiring strains of
Salmonella typhimurium. Unpublished report No. MAB 19/S from
Microtest Research Ltd. Submitted to WHO by Rhone-Poulenc, Research
Triangle Park, NC, USA.
King, V.C. (1990) M&B 46030: Teratology study in the rabbit.
Unpublished report No. 90/RHA 321/0722 from Life Science Research Ltd.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
King, V.C. (1992) M&B 46030: Reproductive performance study in rats
treated continuously through two successive generations. Unpublished
report No. 92/RHA425/0309 from Life Science Research Ltd. Submitted to
WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Liggett, M.P. (1988a) Irritant effects on rabbit skin of M&B 46,030.
Unpublished report No. 881031 D/M&B 292/SE from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Liggett, M.P. (1988b) Irritant effects on the rabbit eye of M&B
46,030. Unpublished report No. 881032D/M&B 293/SE from Huntingdon
Research Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Liggett, M. P. (1988c) Irritant effects on rabbit skin of M&B 46,136.
Unpublished report No. 88833D/M&B 288/SE from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Liggett, M.P. (1988d) Irritant effects on the rabbit eye of M&B
46,136. Unpublished report No. 881022D/M&B 289/SE from Huntingdon
Research Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Lloyd, J.M. (1990) M&B 46030: Investigation of mutagenic activity at
the HGPRT locus in a Chinese hamster V/79 cell mutation system.
Unpublished report No. 90/RHA304/0418 from Life Science Research Ltd.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Mandella, R.C. (1995) A developmental neurotoxicity study of fipronil
in the rat via dietary administration. Unpublished report No. 93-4508
from Pharmaco-LSR/Huntingdon Life Sciences. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Marshall, R.R. (1988a) Study to evaluate the chromosome damaging
potential of M&B-46030 by its effects on cultured human lymphocytes
using an in vitro cytogenetics assay. Unpublished report No. MAB
20/HLC from Microtest Research Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Marshall, R.R. (1988b) Study to evaluate the chromosome damaging
potential of M&B 45950 by its effects on cultured human lymphocytes
using an in-vitro cytogenetics assay. Unpublished report No. MAB
18/HLC from Microtest Research Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Marshall, R.R. (1989) Study to evaluate the chromosome damaging
potential of M&B 46136 by its effects on cultured human lymphocytes
using an in-vitro cytogenetics assay. Unpublished report No. MAB
21/HLC from Microtest Research Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Mondot, S. & Dange, M. (1995) MB 46030: Acute oral LD50 in the mouse.
Unpublished report No. R&D/CRSA/TO-PHA3 from Rhone-Poulenc Agrochimie
Toxicology. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Myers, R.C. & Christopher, S.M. (1992) MB 46030: Acute percutaneous
toxicity study in the rabbit. Unpublished report No. 92N1009 from
Union Carbide Bushy Run Research Center. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Myers, R.C. & Christopher, S.M. (1993a) MB 46030 (technical):
Cutaneous irritancy study in the rabbit. Unpublished report No.
93N1217A from Union Carbide Bushy Run Research Center. Submitted to
WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Myers, R.C. & Christopher, S.M. (1993b) MB 46030 (technical): Ocular
irritancy study in the rabbit. Unpublished report No. 93N1217B from
Union Carbide Bushy Run Research Center. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Nachreiner, D.J. (1995) Fipronil: Acute nose-only dust inhalation
study in rats. Unpublished report No. 94N1501 from Union Carbide Bushy
Run Research Center. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Percy, A. (1993a) RPA 200766 Salmonella typhimurium reverse mutation
assay (Ames test). Unpublished report No. SA 93174 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Percy, A. (1993b) MB 46513 Salmonella typhimurium reverse mutation
assay (Ames test). Unpublished report No. SA 93135 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Percy, A. (1993c) RPA 104615 Salmonella typhimurium reverse mutation
assay (Ames test). Unpublished report No. SA 93175 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Percy, A. (1994a) MB 45950 Salmonella typhimurium reverse mutation
assay (Ames test). Unpublished report No. SA 93305 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Percy, A. (1994b) RPA 105048 Salmonella typhimurium reverse mutation
assay (Ames test). Unpublished report No. SA 94009 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Percy, A. (1996) M&B 45897/RPA 97920 intermediate of fipronil (MB
46030): Salmonella typhimurium reverse mutation assay. Unpublished
report No. SA 95345 from Rhone-Poulenc Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Peters, D.H., Stuart, V., Crook, D., Gibson, W.A., Gopinath, C. &
Hadley, J. (1990) M&B 46,030: Toxicity to rats by dietary
administration for four weeks. Unpublished report No. M&B 327/891321
from Huntingdon Research Centre Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Peters, D.H., Stuart, V., Hall, M., Chasseaud, L.F. & Chanter, D.O.
(1991a) M&B 46,030: An investigation into the potential effects on
thyroid function in male rats by studying thyroxine clearance.
Unpublished report No. M&B 352/90958 from Huntingdon Research Centre
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Peters, D.H., Stuart, V., Hall, M., Chasseaud, L.F. & Chanter, D.O.
(1991b) M&B 46,030: An investigation into the potential effects on
thyroid function in male rats by using the 'perchlorate discharge
test'. Unpublished report No. M&B 353/90920 from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Peters, D.H., Stuart, V., Crook, D., Chanter, D.O., Colman, K.A. &
Gopinath, C. (1991c) M&B 46,030: 4-Week dietary study to investigate
thyroid hormone levels in the rat. Unpublished report No. M&B
360/901275 from Huntingdon Research Centre Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Powles, P. (1992) (14C)-M&B 46,030: Absorption, distribution,
metabolism and excretion in the rat. Unpublished report No.
7040-68/117 from Hazleton UK. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Proudlock, R.J. (1996) MB46513: Mouse micronucleus test. Unpublished
report No. RNP 453/950649 from Huntingdon Life Sciences Ltd. Submitted
to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Ray, D. (1997) Report on the toxicity of fipronil. Unpublished report
from Medical Research Council. Submitted to WHO by MRC Toxicology
Unit, Leicester, United Kingdom.
Smith, K.D. (1990) M&B 46030: Dermal sensitisation study in guinea
pigs. Unpublished report No. 90/RHA357/0602 from Life Science Research
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Stewart, F.P. (1994a) Revised final report: (14C)-M&B 46030:
Absorption, distribution, metabolism and excretion following repeat
oral administration to the dairy goat. Unpublished report No.
HE68/129R-1011 from Hazleton Europe. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Stewart, F.P. (1994b) Revised final report: (14C)-M&B 46030:
Distribution, metabolism and excretion following multiple oral
administration to the laying hen. Unpublished report No.
HE/68/120R-1011 from Hazleton Europe. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Taylor, T. (1993) The effect of single and repeated oral doses of M&B
46030 (1 mg/kg/day and 10 mg/kg/day) on the biliary excretion of
intravenously administered 125I-Thyroine (T4) from bile duct
cannulated rats. Unpublished report No. HRC/ITT 2/930645 from
Huntingdon Research Centre Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Totis, M. (1996) MB 46513: Absorption, distribution, metabolism, and
excretion in the rat (final report). Unpublished report No. SA 95304
from Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Totis, M. & Fisher, P.J. (1994) Fipronil: Tissue kinetic study in the
rat. Unpublished report No. SA94255 from Rhone-Poulenc Agrochimie
Toxicology. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Walters, K.A. & Brain, K.R. (1990) M&B 46030: In vitro skin
permeability of M&B 46030. Unpublished report No. RD 8 from
Pharmaserve Ltd, and An-eX Analytical Services Ltd. Submitted to WHO
by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Wright, N.P. (1995) Fipronil: Chromosomal aberration test in CHL cells
in vitro. Unpublished report No. 282/456 from Safepharm Laboratories
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
at 30 ppm died during the study. The intake of the test compound was
thus not determined for males at 60 ppm.
Males and females at 30 and 60 ppm had statistically significant
decreases in mean body weight and body-weight gain, and mean food
consumption was significantly decreased. The mean values of chemical
parameters did not differ significantly between treated and control
mice. Changes in the weights of the liver, kidney, and thymus in
animals at 30 and 60 ppm were attributed to the decreased terminal
body weights. The only treatment-related change observed
histopathologically was an increased incidence of centrilobular
hypertrophy in mice at 30 and 60 ppm.
Clinical signs of neurotoxicity -- increased motor activity,
excessive jumping, irritability to touch, compulsive biting, and
convulsions -- were observed in males and females at 30 and 60 ppm.
Two females at 3 ppm showed increased motor activity on one occasion,
accompanied by excessive vocalization in one female. None of these
effects occurred in controls. Increased motor activity has been
observed previously as a clinical sign of the toxicity of
fipronil-desulfinyl and of the parent compound fipronil in several
species. Because of the low incidence of this effect and the lack of
other effects at 3 ppm in this study, however, it cannot be concluded
that this effect is of toxicological concern. The NOAEL was 3 ppm,
equal to 0.49 mg/kg bw per day (Dange, 1994b).
Fipronil-desulfinyl (purity, 96%) was administered to groups of
10 male and 10 female OF1 mice in the diet for 90 days at doses of 0,
0.5, 2, or 10 ppm, equal to 0, 0.08, 0.32, or 1.7 mg/kg bw per day for
males and 0, 0.11, 0.43, or 2.2 mg/kg bw per day for females. Standard
evaluations of toxicity ante and post mortem were included.
Histopathological examinations were conducted on all tissues from
controls and animals at the two higher doses and on the liver, lung,
and kidney and tissues in which there were significant macroscopic
findings from mice at the low dose.
Nine males and one females treated at 10 ppm were found dead
during the study. Significant clinical signs included excessive
jumping in two males at 10 ppm and irritability to touch,
aggressivity, and/or increased motor activity in one male at 10 ppm
and two at 2 ppm on two or more occasions. At 0.5 ppm, aggressivity
was reported in one male on one occasion and in one female on three
occasions. None of these effects was reported in control animals.
There were no significant changes in body weight, food consumption, or
haematological or clinical chemical parameters. Gross pathological
examination of dead animals indicated liver enlargement in three males
and small thymuses in four males at 10 ppm. Microscopy revealed
centrilobular hypertrophy of the liver in six males at this dose. The
only female at 10 ppm that died had marked autolysis, which obscured
the histological details. At scheduled sacrifice, gross and
microscopic examinations revealed no treatment-related findings.
The incidence of aggressivity or irritability to touch was low at
2 ppm and there was no dose-response relationship; however, one male
at this dose showed irritability to touch and agressivity on days
70-72, and the other male at this dose with behavioural changes was
irritable to touch, had increased motor activity on day 29, and showed
aggressivity on day 33. Therefore, two animals of the same sex showed
multiple signs on two or three occasions of effects that have been
recognized as clinical signs of toxicity in other studies with
fipronil-desulfinyl and fipronil. In addition, signs of neurotoxicity
were seen in males at the next highest dose and no signs of toxicity
in concurrent controls. Therefore, the behavioural findings in males
at 2 ppm should not be discounted, despite the lack of other
treatment-related findings at this dose. It is easier to discount the
behavioural effects at 0.5 ppm because of their low incidence and the
lack of a dose-response relationship. Therefore, the NOAEL was
0.5 ppm, equal to 0.08 mg/kg bw per day (Bigot, 1996).
Rats
In an exploratory study, fipronil-desulfinyl (purity, 98.6%) was
administered by gavage to five male and five female Sprague-Dawley
rats at doses of 0, 0.3, 1, 3, or 10 mg/kg bw per day for 14 days. All
of the animals at 10 mg/kg bw per day and one female at 3 mg/kg bw per
day either died or were killed in a moribund condition on days 5-8 of
the study. Clinical signs of toxicity, such as piloerection,
chromodacryorrhoea, prostration, excessive reaction to noise,
convulsions, curling up on handling, hunched posture, nasal discharge,
and few faeces, were observed in animals of each sex at 10 mg/kg bw
per day and in females at 3 mg/kg bw per day. Body-weight gain and
food consumption were markedly decreased in animals at 10 mg/kg bw per
day; males and females at 3 mg/kg bw per day showed decreased
body-weight gain over the course of the study, and food consumption
was decreased at the two times evaluated.
Leukocyte counts and total bilirubin were decreased and total
protein was increased in females at 3 mg/kg bw per day; leukocyte
counts were also decreased in females at 1 mg/kg bw per day. Gross
pathological examination showed pale livers in females at 1, 3, or 10
mg/kg bw per day, and spots in the glandular stomach were seen in a
few animals at 3 and 10 mg/kg bw per day that died during the study.
Histological examination showed an increased number of atrophic
follicles in the thyroids of males and females treated at doses > 3
mg/kg bw per day. The NOAEL was 0.3 mg/kg bw per day (Dange, 1994c).
In a preliminary study, fipronil-desulfinyl (purity, 97.5%) was
administered to groups of 10 male and 10 female Sprague-Dawley rats in
the diet at doses of 0, 0.5, 3, 30, or 100 ppm (equal to 0, 0.04,
0.23, 2.2, or 3.7 mg/kg bw per day for males and 0, 0.04, 0.24, 2.3,
or 3.8 mg/kg bw per day for females) for 28 days. Standard parameters
and T3, T4, and TSH were measured before treatment and on day 7 in
non-fasted animals and on day 23 or 24 in fasted animals.
There were no deaths, clinical signs of toxicity, or effects on
body weight or food consumption at 0.5 and 3 ppm. One male at 30 ppm
was found dead on day 6, and all animals at 100 ppm died within the
first two weeks of the study. Clinical signs at 30 and 100 ppm
included piloerection, curling up on handling, and convulsions (in one
female at 100 ppm). Food consumption and body weights were decreased
at the two highest doses and markedly so at 100 ppm. The only change
observed in urinary, standard haematological, and clinical chemical
parameters were a decrease of about 30% in total bilirubin in animals
of each sex at 30 ppm. Statistically significant decreases (relative
to concurrent controls) were found in the levels of T4 (by 33% in
males at 300 ppm, by 63% in males and 50% in females at 100 ppm on day
7; and by 49% in males and 61% in females at 30 ppm on day 23 [no
animals at the high dose were still alive on that day]) and in T3 (by
46% in females at 100 ppm on day 7 and by 40% in males at 30 ppm on
day 23) were observed, but their toxicological significance was not
clear: the findings at 100 ppm may have been related to the extreme
toxicity of the chemical, and individual values in animals at 30 ppm
were reported to be within the normal range for these parameters. TSH
levels were not affected. At necropsy, the thymus weights were
decreased in females at 30 ppm, but no other findings were clearly
related to treatment. The NOAEL was 3 ppm, equal to 0.23 mg/kg bw
(Dange, 1995a).
Fipronil-desulfinyl (purity, at least 97.5%) was administered in
the diet for 90 days to groups of 10 male and 10 female Sprague-Dawley
rats at doses of 0, 0.5, 3, 10, or 30 ppm (equal to 0, 0.029, 0.18,
0.59, or 1.8 mg/kg bw per day for males and 0, 0.035, 0.21, 0.71, or
2.1 mg/kg bw per day for females). In addition to standard ante- and
post-mortem evaluations of toxicity, T3, T4, and TSH were assayed in
non-fasted animals in weeks 2 and 10. During treatment, one male and
three females at 30 ppm died with clinical signs of distress. Signs of
neurotoxicity (aggressivity, irritability to touch, increased motor
activity, and curling up on handling) were seen in animals at 10 and
30 ppm, and excessive vocalization and increased motor activity were
seen in some animals. None of these effects was observed in controls,
in males at 0.5 ppm, or in females at doses > 3 ppm. One male at 3
ppm showed aggressivity, irritability to touch on several occasions,
and excessive vocalization. These signs were seen mainly in weeks 3-5.
Mean body weights were significantly decreased in males and females at
30 ppm and in males at 10 ppm several times during the study. The
overall mean body-weight gain of males was decreased by 15% in those
at 10 ppm and 13% at 30 ppm. Mean weekly food consumption and food
conversion efficiency of males and females at 30 ppm were lower than
those of controls only during the first two weeks of the study. No
treatment-related changes in haematological, urinary or
ophthalmological parameters were seen. Decreases in total bilirubin
(-43%), total cholesterol (-25%), and triglycerides (-24%) in females
at 30 ppm were considered to be related to treatment but not
toxicologically significant since the individual values were within
the normal range for this age and strain of rat. At 30 ppm,
treatment-related decreases in T4 levels were seen in males (-48%,
p < 0.01, at week 2 and -25% at week 10) and females (-29% at week
10), and T3 levels were decreased (-29%, p < 0.05) in males at week
10. The altered hormone levels were reported to be within the normal
range of values for these parameters in this strain of rat. As no
changes were found in the thyroid gland on macroscopic or microscopic
examination, the toxicological significance of the alterations in
hormone levels is questionable. TSH was not affected. There were no
treatment-related macroscopic changes at necropsy, no changes in organ
weights, and no histopathological findings. The NOAEL was 0.5 ppm,
equal to 0.029 mg/kg bw per day, on the basis of clinical signs of
toxicity in one male (Dange, 1994d).
Dogs
In a preliminary study, fipronil-desulfinyl (purity, 97.5%), was
administered in the diet of groups of two male and two female beagle
dogs at doses of 0, 27, 80, or 270 ppm for 28 days. The group mean
doses achieved were reported to be equal to 1 mg/kg bw per day for
males and females at 27 ppm over the course of the study, 1.9 mg/kg bw
per day for males and 1.7 mg/kg bw per day for females at 80 ppm
during week 1, and 2.3 mg/kg bw per day for animals of each sex at 270
ppm during the same period. During the second week of the study,
intake decreased considerably because of toxicity.
Because of poor health, animals at the two higher doses were
killed early, on days 10 and 15 for those at 80 ppm and day 10 for
those at 270 ppm. The signs of toxicity observed at 80 ppm included
reduced motor activity, staggering, irritability, and increased
salivation. Animals at 80 and 270 ppm had no or few faeces,
emaciation, decreased food consumption, and weight loss. All animals
receiving 27 ppm survived, but one male had a clonic convulsion at the
end of the study. The body weights and food consumption of animals in
this group were comparable to those of controls. No remarkable effects
were seen in ophthalmological, haematological, clinical chemical, or
urinary evaluations. Only animals at 27 and 80 ppm were necropsied.
Reduced thymic weight or small thymuses and pale livers were noted in
both groups, and the livers of those at 80 ppm had multifocal whitish
areas or mottling. Although there were no microscopic findings at 27
ppm, marked thymic atrophy and hepatic changes indicative of toxicity
were seen at 80 ppm, including diffuse sinusoidal leukocytosis,
centrilobular hepatocytic enlargement, mild multifocal hepatocytic
hydropic degeneration, and chronic hepatitis with periportal fibrosis.
No NOAEL was identified (Dange, 1995b).
Fipronil-desulfinyl (purity, 96%) was administered to groups of
five beagle dogs of each sex in the diet for 90 days at doses of 0,
3.5, 9.5, or 35 ppm (equivalent to 0, 0.1, 0.27, or 0.95 mg/kg bw per
day for males and 0, 0.1, 0.29, or 1.05 mg/kg bw per day for females).
General health, clinical signs, food consumption, body weights,
haematology and plasma chemistry, and ophthalmologic and urinary
parameters were evaluated. At necropsy, organ weights and macroscopic
changes were monitored; all tissues from controls and animals at the
high dose and kidney, liver, lung, heart, and gall-bladder from those
at the intermediate and low doses were examined histologically.
One female at 35 ppm was killed on day 28 after signs of
increased salivation, prostration, writhing, tremors, absence of
rotular reflex, noisy breathing, and dyspnoea. These clinical signs
were suggested to be due to coronary arteritis and myocardial necrosis
on the basis of microscopic findings; however, they may have indicated
neurotoxicity, as another female at this dose also exhibited excessive
barking and aggressivity on one occasion and irritability, tremors,
and increased salivation on another. There were no significant changes
in body weight, food consumption, or haematological, clinical
chemical, urinary, or ophthalmological parameters. At scheduled
necropsy, no treatment-related changes were observed, and the
microscopic examination revealed no treatment-related findings. The
NOAEL was 9.5 ppm, equal to 0.29 mg/kg bw per day (Dange, 1996).
Reproductive toxicity: Groups of adult female Sprague-Dawley
Crl:CD(SD)BR rats received 0, 0.5, 1, or 2.5 mg/kg bw per day of
fipronil-desulfinyl (purity, 99.2%) suspended in a 0.5% aqueous
solution of methylcellulose by gavage on days 6-15 of gestation. The
study was terminated on gestation day 20. The animals were evaluated
for clinical signs, food consumption, body-weight changes, and
macroscopic changes post mortem. The litter and fetal parameters
evaluated included pre- and postimplantation losses, litter size,
litter and mean fetal weights, sex ratios, and malformations or
skeletal or visceral anomalies. Hair loss on the paws, limbs, flanks,
abdomen, and/or thorax were seen in dams at the high dose, and these
animals had lower body-weight gain on gestation days 6-9 (28% of
control), 9-12 (27%), 6-16 (58%), and 0-20 corrected for gravid
uterine weight (78%). These animals also consumed less food during
treatment, although an increase was seen after treatment, indicating a
rebound effect. Dams at 1 mg/kg bw per day showed a significant
decrease in body-weight gain (80% of control) on days 9-12 of
gestation. No other effects on body weight, body-weight gain at other
intervals, food consumption, or other parameters were seen at the
intermediate dose.
Developmental toxicity in pups at the high dose was reflected in
a slight increase in the fetal and litter incidence of incomplete or
reduced ossification of several bones, including the hyoid body, fifth
and sixth sternebrae, first thoracic body, pubic bone, and one or two
metatarsi. A slight but significant reduction in fetal body weight
(98% of control weight) at the high dose correlated with the slight
delays in ossification. Although the dams showed reduced body-weight
gain, the change in dams at the intermediate dose occurred over a
small interval and was transient. In the absence of other effects at
this dose, the reduced body-weight gain does not appear to be
toxicologically significant. The NOAEL for maternal toxicity was thus
1 mg/kg bw per day; that for for developmental toxicity was 1 mg/kg bw
per day on the basis of a slight increase in the fetal and litter
incidence of delayed ossification of several bones (Foulon, 1997).
Neurotoxicity: Fipronil-desulfinyl (purity, 99.5%) was
administered by gavage as a single dose in corn oil to groups of 12
male and 12 female Sprague-Dawley Crl:CD(SD)BR rats at doses of 0,
0.5, 2, or 12 mg/kg bw. A functional observation battery of tests and
motor activity testing were conducted at 6 h and seven and 14 days
after treatment; 6 h was chosen as the time of 'peak effect' on the
basis of the results of a range-finding study which included
evaluations at 2, 4, 6, and 24 h. Clinical signs, food consumption,
and body weights were monitored. At study termination on day 15, all
animals were perfused in situ. Brains were weighed and measured, and
tissues from the brain, spinal cord, and peripheral nerves were
collected and processed for microscopic examination. Only tissues from
five male and five female controls and animals at the high dose were
examined histopathologically. Since possibly treatment-related axonal
degeneration was seen in lumbar dorsal root fibres and/or the sciatic
nerve of males at the high dose, lumbar dorsal root fibres and the
sciatic nerve in the notch and mid-thigh from the remaining male
controls and rats at the high dose were prepared for microscopic
evaluation. Slides of all tissues from these areas of concern were
then read or re-read in a 'blinded' manner.
Body-weight gain and food consumption were decreased in males and
females at the high dose only during week 1. The behavioural changes
attributed to treatment were significant decreases in hindlimb foot
splay, rectal temperature, and locomotor activity (assessed
quantitatively in the Coulbourn system) in animals of each sex at 12
mg/kg bw 6 h after treatment. Treatment probably affected the righting
reflex in males at the high dose, as it was significantly slowed on
day 14, with a trend at other times, and possibly affected grip
strength in animals of each sex at the high dose at various times.
Changes in other behavioural and motor activity parameters could not
clearly be related to treatment. The groups did not differ with regard
to brain weights. The incidence and number of tissue sites showing
axonal degeneration were increased slightly over those in controls and
in males at the high dose. Since the effect was of minor severity in
both treated and control animals and since the difference between
these animals was not significantly different by Fisher's exact test,
the changes were considered not to be of biological importance. There
were no significant histopathological changes in female rats. The
NOAEL was 2 mg/kg bw (Hughes, 1996).
(iii) M&B 46136: Dermal and ocular irritation
Three male New Zealand white rabbits were given a single 0.5-g
dose of M&B 46136 (purity, 98%) moistened with distilled water as a
topical application for 4 h. The treated areas were observed for
erythema and oedema for four days after treatment. No signs of dermal
irritation were seen under the conditions of the study (Liggett,
1988c).
A dose of 0.1 ml (60 mg) of M&B 46136 (purity, 98%) was instilled
into the lower eyelid of one eye of three male New Zealand white
rabbits. The other eye served as an untreated control. The eyes were
examined for signs of irritation and scored 1 h and one, two, three,
four, and seven days after instillation. One animal had chemosis after
one day. Redness and any minor or residual irritation present had
cleared by day 3 or 4 in all animals. M&B 46136 was thus a slight
ocular irritant under the conditions of the study (Liggett, 1988d).
(iv) RPA 200766: Short-term toxicity
RPA 200766 (purity, 96.2%) was administered in the diet for 28
days to 10 male and 10 female Sprague-Dawley rats at doses of 0, 50,
500, 5000, or 15 000 ppm (equal to 0, 3.8, 38, 390, or 1100 mg/kg bw
per day for males and 0, 4.4, 44, 390, or 1100 mg/kg bw per day for
females). The animals were examined for general health, clinical signs
of toxicity, body weight, food consumption, urinary and
ophthalmological changes, haematological and clinical chemical
parameters, and macroscopic changes at necropsy. Histopathological
examinations were not conducted on animals at 15 000 ppm, because the
dose was found to be too high, inducing excessive body-weight loss.
Microscopic examinations were carried out on all animals that died,
all those at 5000 ppm, and all controls, on the liver, lungs, and
kidneys of animals in all groups except those at 15 000 ppm, and on
the adrenals and thyroid (the target organs), as considered necessary
to establish a no-effect level.
One female at 15 000 died during the study due to an error in
blood collection. No treatment-related deaths were seen in other
groups, and no clinical or ophthalmological alterations were reported.
The body weights of males and females at 5000 and 15 000 ppm were
significantly decreased on days 8-28. The mean body-weight gain over
the course of the study was decreased by 27% in males at 5000 ppm, 61%
in males at 15 000 ppm, 46% in females at 5000 ppm, and 77% in females
at 15 000 ppm. Mean food consumption over the course of the study was
decreased by 11% in males at 5000 ppm, 25% in males at 15 000 ppm, 22%
in females at 5000 ppm, and 33% in females at 15 000 ppm.
Mean haemoglobin concentrations were decreased in males and
females at doses > 500 ppm and mean haematocrit values were
decreased in animals at doses > 5000 ppm, significantly except in
females at 15 000 ppm. Mean corpuscular haemoglobin values were
decreased in males and females at 15 000 ppm and in males at 5000 ppm.
The mean cholesterol levels were significantly increased in animals at
doses > 500 ppm, mean triglyceride values were increased in animals
at 5000 and 15 000 ppm, urea nitrogen was increased in females at 5000
and 15 000 ppm, and creatinine values were increased in males at doses
> 500 ppm. The results of urinalysis showed no changes.
Dose-related increases in absolute and relative liver weights
were seen in males and females at doses > 500 ppm, and the
liver:brain weights were also increased in these groups. Dark livers
were observed in males at > 500 ppm and in females at 5000 and 15
000 ppm. Significantly increased relative adrenal weights and
adrenal:brain weights were seen in all treated males. The group mean
thyroid weights were increased in males at doses > 50 ppm; however,
the increases were not found consistently, and the individual values
were reported to be generally within the expected range for animals of
this age and strain. As there were also no microscopic changes in the
thyroid, these findings are of questionable toxicological
significance, even though the target organ of the parent compound,
fipronil, is the thyroid.
Microscopic examination showed slight-to-moderate, centrilobular
or diffuse hepatocellular hypertrophy in the livers and
slight-to-extramedullary haematopoiesis in the adrenals of males and
females at 5000 ppm. A dose-related increase in the incidence of fine
or coarse vacuolation of the zona fasciculata of the adrenal gland was
observed in males at doses > 50 ppm, with incidences of 0/10 in
controls, 2/10 at the low dose, 5/10 at the intermediate dose, and
10/10 at the high dose. The severity was slight at 50 and 500 ppm and
mild to marked at 5000 ppm. A similar change was seen in seven females
at 5000 ppm, with slight-to-mild severity; the incidence in the female
controls was 0. Increases in the weights of the adrenals and thyroids
and vacuolation of the adrenal zona fasciculata in males at 50 ppm
were considered to be marginal and of questionable toxicological
significance. The NOAEL was 50 ppm, equal to 3.8 mg/kg bw per day, on
the basis of decreased haemoglobin concentration, increased
cholesterol levels, and increased liver weights in animals of each sex
at the next dose (Berthe, 1996).
(v) M&B 45897: Short-term toxicity
M&B 45897 (purity, 99.7%) was administered to five male and five
female CD rats by gavage at doses of 0, 50, 200, or 1000 mg/kg bw per
day in corn oil for 28 days. Standard evaluations of toxicity ante
and post mortem were included. There were no deaths. Salivation was
observed in all animals from day 2 of treatment with 1000 mg/kg bw per
day, from day 3 at 200 mg/kg bw per day, from day 8 in females at 50
mg/kg bw per day, and on days 8-15 in males at this dose. The control
group was not affected. During the second week, animals at the
intermediate and high doses were also hunched and underactive, and all
males and some females at the high dose showed hair loss from day 3
on. Staggering was observed in all animals at the high dose on day 8.
None of these clinical signs was observed at 50 mg/kg bw per day or in
the controls.
Significant deficits in body-weight gain on days 0-17 were seen
in animals of each sex at 1000 mg/kg bw per day, but food consumption
was not affected by treatment. Animals at this dose also showed
decreased haemoglobin concentration, and females had lower erythrocyte
numbers and packed cell volume in comparison with controls. Slightly
higher plasma protein concentrations were seen in animals of each sex
at the high dose, and higher plasma alanine aminotransferase activity
was seen in females. Increased absolute and relative liver weights
were seen in males and females at 1000 mg/kg bw per day. Macroscopic
examination showed no significant findings, but microscopic
examination revealed periacinar hepatic hypertrophy in the livers of
three male rats at 1000 mg/kg bw per day. The only effects considered
to be related to treatment were the slight decreases in erythrocyte
numbers and packed cell volume and the slightly increased alanine
aminotransferase activity in animals at the high dose. Although an
NOAEL of 200 mg/kg bw per day was proposed, the signs observed at the
intermediate and high doses in all animals were indicative of a
toxicological (perhaps neurotoxic) effect of M&B 45897, as they were
not seen in the controls and animals at the low dose. Similar findings
have been reported in studies with the parent compound fipronil and
some of its other metabolites, which have been shown to be neurotoxic.
Although the salivation could be a treatment-related clinical sign, it
could also be viewed as an indication of irritation by the test
compound or a local reaction to treatment, especially since it was
observed more or less consistently at all doses, except in males at
the low dose, in which it occurred only on days 8-15. This was the
only finding at the low dose. The NOAEL was therefore 50 mg/kg bw per
day on the basis of clinical signs of toxicity at the next highest
dose (Johnson, I.R., 1995).
(vi) Genotoxicity
The results of studies on the genotoxicity of fipronil
metabolites are summarized in Table 5.
(vii) Comparison of fipronil and its metabolites
Table 6 presents a comparison of findings for fipronil and its
metabolites: whether or not the chemical has been found (to a major or
minor extent) in or on plants or in animal (rat, goat, or hen) tissues
or products or can form in the environment or on surfaces, indoors or
outdoors, via reduction, oxidation, hydrolysis, or photolysis (see
Figure 3); the acute oral toxicity of each chemical; and the ability
of each metabolite to compete with the ligands 3H-EBOB and 3H-TBPS
for binding to specific sites in the chloride ion channel of the GABA
receptor. Binding is postulated to serve as an indicator of potential
to disrupt the normal functioning of the GABA receptor by interference
with chloride-ion flux and thus for potential toxicity to the central
nervous system.
Table 5. Results of assays for genotoxicity with metabolites of fipronil metabolites
Metabolite End-point Test object Concentration Purity Results Reference
(%)
In vitro
M&B 45950 Reverse mutation S. typhimurium 0-250 µg/plate 98.9 Negativea,b Percy (1994a)
TA98, TA100, in DMSO
TA1535, TA1537
Chromosomal Human 25-100 µg/ml > 99 Negativea,b Marshall
aberration lymphocytes in DMSO (1988b)
M&B 46136 Reverse mutation S. typhimurium 0.32-200 µg/plate 98.7 Negativea,b Clare (1988b)
TA98, TA100, (-S9), 0.8-500 µg/
TA1535, TA1537 plate (+S9), in
DMSO
Chromosomal Human 75-300 µg/ml 98.7 Negativea,b Marshall
aberration lymphocytes in DMSO (1989)
RPA 200766 Reverse mutation S. typhimurium 250-1000 µg/plate > 98 Negativea,b Percy (1993a)
TA98, TA100, (-S9), 50-2500 µg/
TA1535, TA1537, plate (+S9), in
TA1538 DMSO
Fipronil-desulfinyl Reverse mutation S. typhimurium 10-250 µg/plate 98.6 Negativea,b Percy (1993b)
TA98, TA100, in DMSO
TA1535, TA1537,
TA1538
Gene mutation Chinese hamster 5-125 µg/ml (-S9), 99.5 Negativea,b Adams (1996a)
cell line (CHO- 15-625 µg/ml (+S9)
K1-BH4), hprt locus in DMSO
Table 5. Results of assays for genotoxicity with metabolites of fipronil metabolites
Metabolite End-point Test object Concentration Purity Results Reference
(%)
Chromosomal Human lymphocytes 5-30 µg/ml (-S9), 99.5 Negativea,b Adams (1996b)
aberration 5-60 µg/ml (+S9)
in DMSO
RPA 104615 Reverse mutation S. typhimurium 2250-5000 µg/plate 94.7 Negativea,b Percy (1993c)
TA98, TA100, in DMSO
TA1535, TA1537,
TA1538
M&B 45897 Reverse mutation S. typhimurium 12.5-2500 µg/plate 99.7 Negativea,b Percy (1996)
TA98, TA100, (-S9), 25-2500 µg/
TA1535, TA1537 plate (+S9), in
DMSO
Reverse mutation S. typhimurium 4-2500 µg/plate > 99 Negativea,b Kennelly
TA98, TA100, (-S9), except TA 100; (1988)
TA1535, TA1537 8-5000 µg/plate
(-S9), TA100; 8-5000
µg/plate (+S9), all
strains; in DMSO
Chromosomal Human 50-150 µg/ml (-S9), 99.7 Negativea,b Johnson, A.L.
aberration lymphocytes 100-400 µg/ml (+S9), (1995)
in DMSO; 20- or 44-h
harvest times
Polyploidy Human 50-150 µg/ml (-S9), 99.7 Positivea,c Johnson, A.L.
lymphocytes 100-400 µg/ml (+S9), (1995)
in DMSO; 20- or 44-h
harvest times
RPA 105048 Reverse mutation S. typhimurium 250-5000 µg/plate 98.6 Negativea,c Percy (1994b)
TA98, TA100, in DMSO
TA1535, TA1537
Table 5. Results of assays for genotoxicity with metabolites of fipronil metabolites
Metabolite End-point Test object Concentration Purity Results Reference
(%)
In vivo
Fipronil-desulfinyl Micronucleus CD-1 mice 2-16 mg/kg bw in 99.5 Negativeb Proudlock
formation corn oil (1996)
DMSO, dimethyl sulfoxide, S9, 9000 × g supernatant of rat liver homogenate
a With and without metabolic activation
b Appropriate positive controls gave expected positive responses
c Reproducible increases in polyploid cells with metabolic activation
Table 6. Comparisons of fipronil and its metabolites
Compound In plants In animals Potential environmental Photo- Oral LD50 Binding to
metabolite or surface metabolite (mg/kg bw)a 3H-EBOB &
residue or degradate 3H-TBPS sitesb
Fipronil Yes Rat, goat, hen Water, soil, surfaces No 97
M&B 45950 Yes Rat, goat, hen Soil No 83 * (both sites)
RPA 200766 Yes Rat, goat Water, soil, surfaces No > 2000 @@ (both sites)
Fipronil-desulfinyl Yes No Water, soil, surfaces Yes 16 ** (both sites)
M&B 46136 Yes Rat, goat, hen Soil, surfaces No 218 * (both sites)
RPA 104615 Yes No Water, soil, surfaces Yes > 2000 @@ (both sites)
M&B 45897 Yes Rat Not expected No > 2000 @@ (both sites)
Two ring-opened No Rat No No No data No data
metabolites of
M&B 45897
M&B 105320 Yes No Not expected No > 2000 @@ (both sites)
EBOB, 1-[(4-ethyl)phenyl]-4-n-propyl-2,6,7-trioxacicyclo[2.2.2]octane; TBPS, tert-butylbicyclophosphorothionate
a From references given in Tables 1 and 2
b Increased (*) or decreased (@) binding relative to fipronil; ** or @@ greater differences. Based on IC50 values from
competitive binding assays for fipronil and its metabolites with radiolabelled 1 gands (personal communication from
P. Kwiatkowski, Rhone-Poulenc Worldwide Regulatory Affairs, North Carolina, USA)
The results presented in the table and those of similar studies
indicate that most of the metabolites are of similar or lesser
toxicity than fipronil. Generally, this conclusion reflects the data
on binding, except for M&B 46136 which has greater binding affinity
for sites in the rat brain GABA receptor chloride-ion channel but a
higher acute oral LD50 than fipronil in rats. (No short-term studies
of the toxicity of this metabolite are available.)
Fipronil-desulfinyl and M&B 104615 are photolytic products of
fipronil which could potentially form in the environment or on treated
or exposed surfaces during use to control malarial vectors. The LD50
of fipronil-desulfinyl in rats is much lower than that of fipronil,
and it has much greater binding affinity for GABA receptor
chloride-ion channel sites in rat brain. Both are neurotoxic. The
toxicity of fipronil-desulfinyl is qualitatively similar to that of
fipronil, but the dose-effect curve for neurotoxic effects is steeper
for fipronil-desulfinyl and neurotoic effects occur at lower doses.
The acute oral LD50 for M&B 104615 is much higher and the affinity
for GABA receptor chloride-ion channel sites much less than those for
fipronil.
Comments
Fipronil
In a study of dermal absorption in rats, the quantity of
14C-fipronil absorbed was less than 1% of the applied dose at all
doses tested (0.88-48 mg/rat) and all times up to 24 h. In vitro,
the relative extent of absorption of a formulation of 14C-fipronil
across rat, rabbit, or human epidermal membranes depended on the
concentration of the material used. At the lowest concentration tested
(0.2 g/L), the extent of penetration was greatest for all three
species, and the percentage of the dose absorbed across human and rat
membranes was similar. At higher concentrations (4 and 200 g/L),
penetration was greater through rat and rabbit skin than through human
skin.
There was no appreciable difference between male and female rats
in the absorption, distribution, metabolism, or excretion of fipronil
after oral administration. The proportion of the dose absorbed
appeared to depend on the treatment regimen, being greatest with a
single dose of 4 mg/kg bw of 14C-fipronil (minimum absorption, 50%),
intermediate with a repeated dose regimen of 4 mg/kg bw per day for 14
days followed by a single, oral labelled dose of 4 mg/kg bw (minimum
absorption, 40%), and lowest (minimum absorption, about 30%) with a
single dose of 150 mg/kg bw of 14C-fipronil (presumably due to
saturation of absorption at the high dose). Once absorbed, fipronil
was rapidly metabolized, and the residues widely distributed in
tissues. Significant amounts of residues remained in the tissues,
particularly in fat and fatty tissues, one week after treatment. The
levels of residues in fat and other tissues were greater with repeated
low doses or a single high dose than with a single low dose. The long
half-life (150-245 h in some cases) of fipronil in blood may reflect
slow release of residues from fat and might suggest potential
bioaccumulation of metabolic products of fipronil.
Faeces, followed by urine, were the major routes of elimination
of fipronil in rats. Its biotransformation largely involved changes in
the functional groups attached to the pyrazole ring. The compounds
identified in faeces and urine were the parent compound and the
sulfone, the amide derived from the nitrile group, a reduction
product, and a cleavage product of the sulfone and its derivatives
formed by further cleavage. The sulfone was the major metabolite in
fat and tissues.
Fipronil was moderately hazardous to rats (LD50 = 92 mg/kg bw)
and mice (LD50 = 91 mg/kg bw) after oral administration of single
doses and to rats after single exposure by inhalation (LC50
= 0.36 mg/L). After a single dermal exposure, fipronil was relatively
non-hazardous to rats (LD50 > 2000 mg/kg bw) but was moderately
hazardous to rabbits (LD50 = 354 mg/kg bw). In rats, signs of
toxicity and death were delayed for up to four days after either a
single oral dose or repeated oral doses of 75 mg/kg bw per day for up
to five days. WHO has not yet classified fipronil for acute toxicity.
In a 13-week study of toxicity, mice were fed diets containing
fipronil at doses of 0, 1, 3, 10, or 25 ppm. A dose-related increase
in the incidence of liver-cell periacinar hypertrophy with cytoplasmic
vacuolation was observed in males at doses of 1 ppm (equal to 0.13
mg/kg bw per day) and above. There was no NOAEL.
Rats were fed diets containing 0, 25, 50, 100, 200, or 400 ppm
fipronil for four weeks. At 25 ppm (equal to 3.4 mg/kg bw per day),
liver weights and plasma cholesterol levels were increased in females,
and thyroid follicular-cell hypertrophy of minimal severity was
observed in animals of each sex. The levels of total protein and
globulin were also increased in both males and females, although the
changes at this and higher doses were generally small and poorly
correlated with the dose. There was no NOAEL.
In a 13-week study of toxicity, fipronil was administered in the
diet to rats at doses of 0, 1, 5, 30, or 300 ppm. At 30 ppm and above,
relatively small, sometimes inconsistent changes in haematological
parameters (decreased packed cell volume, mean cell volume,
haemoglobin concentration, and prothrombin time and increased platelet
count) and clinical chemical parameters (increased total protein and
globulins, decreased albumin:globulin ratio and alanine
aminotransferase and aspartate aminotransferase activities) were
observed, mostly in females. Some alterations were seen in plasma
glucose and urea concentrations at 30 ppm; also at 30 ppm, the
absolute and/or relative weights of the liver and thyroid were
increased in either males or females or both, and there was evidence
of thyroid follicular-cell epithelial hypertrophy in males. The NOAEL
was 5 ppm, equal to 0.33 mg/kg bw per day.
Fipronil was administered in gelatin capsules to dogs for 13
weeks in a study of toxicity at doses of 0, 0.5, 2, or 10 mg/kg bw per
day. Inappetence and decreased body-weight gain and food consumption
were noted in females at 2 and 10 mg/kg bw per day. The NOAEL was 0.5
mg/kg bw per day.
In a study of dermal toxicity, fipronil was applied in 0.5%
carboxymethylcellulose to the intact skin of rabbits for 6 h per day
on five days per week for three weeks at doses of 0, 0.5, 1, 5, or 10
mg/kg bw per day. No dermal irritation was observed. At 10 mg/kg bw
per day, body-weight gains and food consumption were reduced in
animals of each sex. Some animals showed hyperactivity. The NOAEL was
5 mg/kg bw per day.
Fipronil was administered to dogs in gelatin capsules for one
year in a study of toxicity at doses of 0, 0.2, 2, or 5 mg/kg bw per
day. At 2 mg/kg bw per day and above, clinical signs of neurotoxicity
(convulsions, twitching, tremors, ataxia, unsteady gait, rigidity of
limbs, nervous behaviour, hyper- or hypoactivity, vocalization,
nodding, aggression, resistance to dosing, inappetence, and abnormal
neurological responses) were observed in animals of each sex. One
animal at 2 mg/kg bw per day was killed because of poor condition
related to treatment. The NOAEL was 0.2 mg/kg bw per day. In a second
one-year study in dogs, fipronil was administered in the diet at doses
of 0, 0.075, 0.3, 1, or 3 mg/kg bw per day. The highest dose was
reduced to 2 mg/kg bw per day after 38 days because of toxicity. At 1
mg/kg bw per day, clinical signs of neurotoxicity (whole body
twitching, and extensor rigidity of limbs) were noted in females.
There were no effects on triiodothyronine or thyroxine levels. The
NOAEL was 0.3 mg/kg bw per day.
In a study of carcinogenicity, fipronil was administered for 78
weeks in the diet to mice at doses of 0, 0.1, 0.5, 10, 30, or 60 ppm.
Additional groups of animals were fed the same doses for 52-53 weeks
and then killed. Survival was greater than or comparable to that of
the control group at doses below 60 ppm. At week 10, all surviving
animals at 60 ppm were killed because of excessive mortality. In
animals at 10 ppm, some decrease in body-weight gain was noted in
males and females, and efficiency of food use was decreased in males.
At 53 and 78 weeks, the absolute and/or relative liver weights of
males were increased, with an increased incidence of liver periacinar
microvesicular vacuolation. There was no evidence of carcinogenicity
at doses considered to be sufficient to measure such potential. The
NOAEL for systemic effects was 0.5 ppm, equal to 0.055 mg/kg bw per
day.
In a study of toxicity and carcinogenicity in rats, fipronil was
administered in the diet at doses of 0, 0.5, 1.5, 30, or 300 ppm. For
the carcinogenicity phase of the study, it was originally planned that
the test material be administered for two years, but excessive
mortality resulted in early termination of this phase at week 89 in
males and week 91 in females. This was not thought to compromise the
study. For the toxicity phase and a reversibility phase of the study,
additional groups of animals were fed the same doses of fipronil for
one year, when some animals were killed and others were allowed to
recover for 13 weeks. Some of the effects noted at the higher doses
persisted into the reversibility phase of the study. During treatment,
convulsive episodes (sometimes fatal) were observed in males at 1.5
ppm and in animals of each sex at higher doses. Animals at 1.5 ppm,
predominantly females, showed irritability, vocalization, salivation,
aggression, hyperactivity, and bruxism. Small decreases were noted in
erythrocyte count, haemoglobin concentration, mean cell volume, and
packed cell volume in either males or females or both, and some
alterations in protein level were observed in males. An apparent
increase in the severity of progressive senile nephropathy was seen in
animals of each sex at this dose. Thyroxine concentrations were
decreased in both males and females. Thyroid-stimulating hormone
levels were increased, notably in males, at doses of 30 ppm and above
and in females at 300 ppm. The levels of triiodothyronine were
elevated in females at 30 ppm, but only during the reversibility
phase. At 300 ppm, fipronil induced follicular-cell adenomas of the
thyroid gland in both males and females; males at this dose also had
an increased incidence of follicular-cell carcinomas. Some thyroid
follicular-cell adenomas were noted in male rats at lower doses, but a
comparison with historical control data indicated no clear
relationship to treatment. The NOAEL for systemic effects was 0.5 ppm,
equal to 0.019 mg/kg bw per day.
Fipronil and its metabolites gave negative results in virtually
all tests for genotoxicity. Equivocal results were seen in assays for
cytogenicity in mammalian cells in vitro with fipronil and for
polyploidy (not clastogenicity) in human lymphocytes with a mammalian
metabolite. The weight of evidence indicates that fipronil and its
metabolites are not genotoxic.
The Meeting concluded that the thyroid tumours observed in the
two-year study in rats occurred by a non-genotoxic, threshold
dose-effect mechanism involving continuous stimulation of the thyroid
gland associated with persistently elevated thyroid-stimulating
hormone levels. It was noted that the levels of this hormone were
clearly elevated only at the two highest doses.
In a two-generation study of reproductive toxicity, rats received
diets containing fipronil at 0, 3, 30 or 300 ppm. F0 parental animals
were mated twice to produce F1a and F1b litters; F1a parents were
mated only once to produce F2 litters. In adult animals at 30 ppm,
the thyroid and liver weights were increased and the pituitary gland
weights were decreased. An increased incidence of thyroid gland
follicular epithelial-cell hypertrophy was seen at this dose in males
of the F0 and F1 generations and F1 females. At 300 ppm,
convulsions were observed in F1 and F2 litters; decreased litter
size, decreased body weights and delays in physical development were
also seen. Postnatal survival was decreased among pups in the F2
litters. Absolute and relative ovarian weights were decreased in F0
females. At 300 ppm, a decreased percentage of animals that mated and
a reduction in the fertility index of F1 parental animals was also
observed. These effects may have been related to the systemic toxicity
of fipronil at this dose. The NOAEL for parental systemic toxicity was
3 ppm, equal to 0.25 mg/kg bw per day, and the NOAEL for reproductive
toxicity was 30 ppm, equal to 2.5 mg/kg bw per day.
Rats were given fipronil by gavage at doses of 0, 1, 4, or 20
mg/kg bw per day on days 6-15 of gestation. Developmental toxicity was
not observed, but there were some signs of maternal toxicity
(decreased body-weight gain and food consumption) at 20 mg/kg bw per
day. The NOAEL for maternal toxicity was 4 mg/kg bw per day, and that
for developmental toxicity was 20 mg/kg bw per day, the highest dose
tested.
Rabbits were given fipronil by gavage at doses of 0, 0.1, 0.2,
0.5, or 1 mg/kg bw per day on days 6-19 of gestation. Developmental
toxicity was not observed, but there were some signs of maternal
toxicity (decreased body-weight gain, decreased food consumption, and
reduced efficiency of food use at all doses. There was no NOAEL for
maternal toxicity; the NOAEL for developmental toxicity was 1 mg/kg bw
per day, the highest dose tested.
Primary dermal irritation in rabbits was examined in two studies.
Fipronil was slightly irritating when moistened with corn oil before
application but was not irritating when moistened with water. Fipronil
was slightly irritating in two studies of primary ocular irritation in
rabbits. It did not sensitize the skin of guinea-pigs when tested by
the Buehler method but was a weak sensitizer in guinea-pigs tested by
the Magnusson-Kligman method.
In a study of neurotoxicity, rats were given single doses of 0,
0.5, 5, or 50 mg/kg bw fipronil by gavage. At 5 mg/kg bw, decreased
hind-leg splay was observed 7 h after treatment in both males and
females. The NOAEL was 0.5 mg/kg bw. In a 13-week study of
neurotoxicity, rats received dietary doses of 0, 0.5, 5, or 150 ppm
fipronil. Body weights, weight gains, and food consumption were
reduced early in the study in animals of each sex at 150 ppm, possibly
owing to problems of palatability. Although the findings in a battery
of functional operational tests at this dose were relatively minor
when taken separately, they appeared to represent a minimal effect of
treatment when taken together. The NOAEL for neurotoxicity and
systemic effects was 5 ppm, equal to 0.3 mg/kg bw per day.
In a study of neurotoxicity in female dogs, fipronil was
administered in capsules at doses of 0 (one animal) or 20 mg/kg bw per
day (four animals) until the appearance of neurotoxic signs in each
animal, after which they were allowed to recover for 28 days. Severe
neurotoxic signs were seen at 20 mg/kg bw per day during the treatment
phase and in some animals only during the recovery phase. Most animals
appeared to recover, although one had exaggerated reflex responses and
was excitable at the end of the recovery period. A limited
histopathological examination showed no change. No firm conclusions
could be drawn about the reversibility of the effects, given the
limitations of the study design. There was no NOAEL.
In a study of developmental neurotoxicity, rats were given
fipronil in the diet from gestation day 6 through lactation day 10 at
doses of 0, 0.5, 10, or 200 ppm. Maternal toxicity manifested as
reduced body weight during the treatment period, reduced body-weight
gain during gestation, and reduced food consumption was observed at
200 ppm. Developmental toxicity (reduced body weights in pups and a
slight increase in the time to preputial separation) was noted at 10
ppm. An increase in motor activity in female pups at 10 ppm only on
day 17 could not be definitively interpreted as an indication of
developmental toxicity. Developmental neurotoxicity was clearly
observed postnatally in pups at 200 ppm, with delayed swimming
development on day 6, increased motor activity on day 17, abnormal
auditory startle response on day 22, and impaired learning and memory
on day 24. The NOAEL for maternal toxicity and developmental
neurotoxicity was 10 ppm (equal to 0.9 mg/kg bw per day) and that for
developmental toxicity was 0.5 ppm (equal to 0.05 mg/kg bw per day).
Mechanistic studies conducted with fipronil in rats suggest that
it does not interfere with the incorporation of iodine into thyroxine
but rather with the biliary clearance of this hormone. This may
trigger an increase in the concentration of thyroid-stimulating
hormone by interference with the feedback mechanism.
Mammalian metabolites of fipronil
Several mammalian metabolites of fipronil were tested for acute
toxicity. Their toxicity was comparable to or substantially less than
that of fipronil.
Photodegradation products of fipronil
Numerous studies were performed with fipronil-desulfinyl, one of
two photodegradation products of fipronil which can be formed in the
presence of sunlight and could potentially be produced in the
environment or on treated surfaces. Neither is a mammalian metabolite
of fipronil. The available information indicates that, of the two,
only fipronil-desulfinyl is highly toxic after exposure to single
doses or over the long term, and is therefore of toxicological
concern.
When 0.08-7.2 mg of 14C-fipronil-desulfinyl were applied
dermally to rats, absorption ranged fron 0.2 to 7% of the applied dose
within 24 h.
The absorption, distribution metabolism, and excretion of
14C-fipronil-desulfinyl were studied in rats which received either a
single oral dose of labelled compound at 1 or 10 mg/kg bw or 14 daily
oral doses of unlabelled compound at 1 mg/kg bw per day followed by a
single oral labelled dose. In animals of each sex, elimination of the
radiolabel was much greater in the faeces (46-70% of the dose) than in
the urine with all dosing regimens. Appreciable residues were found in
the tissues one week after treatment, the highest concentrations being
present in the fat and fatty tissues. The long half-life in blood
(183-195 h) and increased fat:plasma ratios of the radiolabel suggest
potential bioaccumulation of fipronil-desulfinyl and/or its
metabolites. Numerous metabolites or conjugates of fipronil-desulfinyl
were present in the urine and faeces. Biotransformation of
fipronil-desulfinyl involved changes at the functional groups attached
to the pyrazolyl ring. Only unchanged fipronil-desulfinyl was
identified in the liver, fat, skin, and residual carcass.
In a 28-day study of toxicity in which fipronil-desulfinyl was
administered in the diet to mice at doses of 0, 0.5, 3, 30, or 60 ppm,
mortality, neurotoxic signs (increased motor activity, excessive
jumping, irritability to touch, compulsive biting, and evidence of
convulsions), decreased body-weight gain and food consumption, and an
increased incidence of centrilobular hypertrophy of the liver were
observed in animals of each sex at doses of 30 ppm and above. The
NOAEL was 3 ppm, equal to 0.49 mg/kg bw per day. Fipronil-desulfinyl
was administered in the diet for 90 days to mice at doses of 0, 0.5,
2, or 10 ppm. At 2 and 10 ppm, clinical signs of neurotoxicity
(irritability to touch, aggressiveness, and/or increased motor
activity) were noted in males. The NOAEL was 0.5 ppm, equal to
0.08 mg/kg bw per day.
Rats received fipronil-desulfinyl by gavage for two weeks at
doses of 0, 0.3, 1, 3, or 10 mg/kg bw per day. At 1 mg/kg bw per day,
pale livers and reduced leukocyte counts were observed in females.
Some rats at 3 mg/kg bw per day died or were killed because of poor
condition. The NOAEL was 0.3 mg/kg bw per day. Fipronil-desulfinyl was
administered in the diet for 28 days to rats at doses of 0, 0.5, 3, 30
or 100 ppm. One male at 30 ppm died, clinical signs of toxicity
(piloerection and curling up on handling), and decreased body weights,
food consumption, and bilirubin concentration were seen in males and
females at this dose. Thymus weights were lowered in females. The
levels of thyroid-stimulating hormone were measured, but no effects
were noted at any dose. All animals at 100 ppm died. The NOAEL was 3
ppm, equal to 0.23 mg/kg bw per day.
In a 90-day study of toxicity in rats, fipronil-desulfinyl was
administered in the diet at 0, 0.5, 3, 10, or 30 ppm. At 3 ppm and
above, clinical signs of neurotoxicity (aggressiveness, irritability
to touch, and excessive vocalization) were observed in males. The
levels of triiodothyronine and thyroxine were affected at higher
doses, but the toxicological significance of these changes is probably
negligible in the absence of changes in the level of
thyroid-stimulating hormone at any dose. The NOAEL in the study was
0.5 ppm, equal to 0.029 mg/kg bw per day.
Dogs received fipronil-desulfinyl in the diet in a 28-day study
at doses of 0, 27, 80, or 270 ppm. The groups at 80 and 270 ppm were
terminated early because of mortality. One male at 27 ppm had a clonic
convulsion. Reduced thymus weights and pale livers were also reported
at this dose. As effects occurred at the lowest dose, there was no
NOAEL.
In a 90-day study of toxicity, fipronil-desulfinyl was
administered in the diet to dogs at doses of 0, 3.5, 9.5, or 35 ppm.
The clinical findings in one female at 35 ppm (increased salivation,
prostration, writhing, tremors, absence of rotular reflex, noisy
breathing, dyspnoea) were attributed to arteritis and myocardial
necrosis on the basis of microscopic findings; however, they may also
have been indicative (at least in part) of neurotoxicity, because
another female in this group exhibited excessive barking,
aggressiveness, irritability, tremors, and increased salivation. On
this basis, the Meeting concluded that the NOAEL was 9.5 ppm, equal to
0.29 mg/kg bw per day.
In a study of developmental toxicity in rats, fipronil-desulfinyl
was administered by gavage on days 6-15 of gestation at doses of 0,
0.5, 1, or 2.5 mg/kg bw per day. Indications of maternal effects
(decreased body-weight gain and hair loss in various areas) were
observed at 2.5 mg/kg bw per day. Developmental toxicity (increased
incidence of incomplete or reduced ossification of several bones and
slightly reduced fetal body weight in animals of each sex) was also
observed at this dose. The NOAEL for maternal toxicity and
developmental toxicity was 1 mg/kg bw per day.
In a study of neurotoxicity in rats, fipronil-desulfinyl was
administered by gavage as a single dose of 0, 0.5, 2, or 12 mg/kg bw.
At 12 mg/kg bw, decreased body-weight gains and food consumption were
observed during week 1 in animals of each sex. Decreased hind-foot
splay, rectal temperature, and locomotor activity were also seen in
animals of each sex at this dose. There were indications of a slowed
righting reflex in males and decreased grip strength in males and
females at the high dose. The NOAEL was 2 mg/kg bw per day.
In summary, the toxicity of fipronil-desulfinyl is qualitatively
similar to that of fipronil, but the dose-effect curve for neurotoxic
effects appears to be steeper for fipronil-desulfinyl than for
fipronil. Also, fipronil-desulfinyl appears to have a much greater
tendency than fipronil to bind to sites in the chloride ion channel of
the rat brain GABA receptor. This finding appears to be consistent
with the greater toxicity, relative to fipronil, of
fipronil-desulfinyl in the central nervous system of mammals.
The Meeting established an ADI of 0-0.0002 mg/kg bw for fipronil
on the basis of the NOAEL of 0.019 mg/kg bw per day in the two-year
study of toxicity and carcinogenicity in rats and incorporating a
safety factor of 100.
The Meeting considered that a separate ADI should be established
for fipronil-desulfinyl on the basis that it could be a significant
residue and that its toxicity is greater than that of the parent
molecule fipronil. A temporary ADI of 0-0.00003 mg/kg bw for
fipronil-desulfinyl was established on the basis of the NOAEL of 0.029
mg/kg bw per day in the 90-day study in rats and a safety factor of
1000, in view of the lack of a long-term study by oral administration
in rats and a study of neurotoxicity in rats given repeated oral
doses.
Toxicological evaluation
Fipronil
Levels that cause no toxic effect
Mouse: 0.5 ppm, equal to 0.055 mg/kg bw per day (78-week study
of carcinogenicity and toxicity)
Rat: 5 ppm, equal to 0.33 mg/kg bw per day (13-week study of
toxicity)
0.5 ppm, equal to 0.019 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
3 ppm, equal to 0.25 mg/kg bw per day (parental
systemic toxicity in a study of reproductive toxicity)
30 ppm, equal to 2.5 mg/kg bw per day (study of
reproductive toxicity)
4 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity by gavage)
20 mg/kg bw per day (developmental toxicity in a study
of developmental toxicity by gavage; highest dose
tested)
0.5 mg/kg bw (single dose, study of neurotoxicity by
gavage)
5 ppm, equal to 0.3 mg/kg bw per day (repeated doses in
the diet, study of neurotoxicity)
10 ppm, equal to 0.9 mg/kg bw per day (maternal
toxicity and developmental neurotoxicity in a study of
developmental neurotoxicity)
0.5 ppm, equal to 0.05 mg/kg bw per day (developmental
toxicity in a study of developmental neurotoxicity)
Rabbit: 0.1 mg/kg bw per day (LOAEL for maternal toxicity in a
study of developmental toxicity by gavage)
1 mg/kg bw per day (study of developmental toxicity;
highest dose tested by gavage)
Dog: 0.3 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.0002 mg/kg bw
Fipronil-desulfinyl (fipronil photodegradation product)
Levels that cause no toxic effect
Mouse: 3 ppm, equal to 0.49 mg/kg bw per day (28-day study of
toxicity)
0.5 ppm, equal to 0.08 mg/kg bw per day (90-day study
of toxicity)
Rat: 0.3 mg/kg bw per day (two week study of toxicity by
gavage)
3 ppm, equal to 0.23 mg/kg bw per day (28-day study of
toxicity)
0.5 ppm, equal to 0.029 mg/kg bw per day (90-day study
of toxicity)
1 mg/kg bw per day (maternal and developmental toxicity
in a study of developmental toxicity by gavage)
2 mg/kg bw per day (single dose, study of neurotoxicity
by gavage)
Dog: 9.5 ppm, equal to 0.29 mg/kg bw per day (90-day study
of toxicity)
Estimate of temporary acceptable daily intake for humans
0-0.00003 mg/kg bw
Acute reference dose for fipronil
The Meeting allocated an acute reference dose of 0.003 mg/kg bw
for both fipronil and fipronil-desulfinyl on the basis of the NOAEL of
0.3 mg/kg bw per day in a study of neurotoxicity in rats given
repeated doses of fipronil, and a safety factor of 100. The study of
neurotoxicity in rats given single doses was not considered in
allocating the acute reference dose because of concern about the
prolonged toxicokinetics of fipronil. This acute reference dose will
provide a safety factor of about 700 for the NOAEL in the study of
neurotoxicity in rats given single doses of fipronil-desulfinyl.
Studies without which the determination of an ADI is impractable,
to be provided by 2000
1. Short-term study of neurotoxicity in rats with
fipronil-desulfinyl in the diet
2. Developmental neurotoxicity study in rats with
fipronil-desulfinyl in the diet
3. The results of an ongoing long-term study with
fipronil-desulfinyl in rats
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to fipronil and its photodegradation
product fipronil-desulfinyl
Human exposure Relevant route, study type, species Results, remarks
Fipronil
Short-term Skin, irritation, rabbit Slightly imitating
(1-7 days) Eye, irritation, rabbit Minor irritation
Skin, sensitization, guinea-pig Not a sensitizer (Buehler)
Skin, sensitization, guinea-pig Mild sensitizer (Magnusson-Kligman)
Oral, toxicity, rat LD50 = 92 mg/kg bw
Dermal, toxicity, rabbit LD50 = 350 mg/kg bw
Inhalation, toxicity, rat LC50 = 0.36 mg/L
Neurotoxicity, rat NOAEL = 0.5 mg/kg bw per day:
(single dose by gavage) decreased hind-leg splay
Medium-term Repeated dermal, 3 weeks, toxicity, rabbit NOAEL = 5 mg/kg bw per day: reduced body-weight
(1-26 weeks) gains and food consumption; hyperactivity in some
animals; no dermal imitation observed
Repeated oral, reproductive toxicity, rat NOAEL = 0.25 mg/kg bw per day for maternal toxicity.
NOAEL = 2.5 mg/kg bw per day for reproductive
toxicity
Repeated oral, developmental neurotoxicity, rat NOAEL = 0.9 mg/kg bw per day for maternal toxicity.
NOAEL = 0.05 mg/kg bw per day for developmental
toxicity
NOAEL = 0.9 mg/kg bw per day for developmental
neurotoxicity
Long-term Repeated oral, 2 years (terminated at 89-91 NOAEL = 0.019 mg/kg bw per day: convulsions and
(> 1 year) weeks), long-term toxicity and carcinogenicity, rat neurobehavioural clinical signs of toxicity; effects on the
thyroid; thyroid follicular-cell adenomas and carcinomas
Fipronil-desulfinyl
Short-term Oral, toxicity, rat LD50 = 15 mg/kg bw
(1-7 days) Dermal, toxicity, rat LD50 > 2000 mg/kg bw
Neurotoxicity, rat NOAEL = 2 mg/kg bw per day
(single dose by gavage)
(continued)
Human exposure Relevant route, study type, species Results, remarks
Medium-term Repeated oral (diet), 90 days, toxicity, rat NOAEL = 0.029 mg/kg bw per day
(1-26 weeks) Repeated oral (gavage), developmental toxicity, NOAEL = 1.0 mg/kg bw per day: maternal toxicity
rat NOAEL = 1.0 mg/kg bw per day: developmental
toxicity
Long-term Repeated oral toxicity No data
> 1 year)
Studies that would provide information useful for the continued
evaluation of fipronil and fipronil-desulfinyl
1. Additional studies to investigate the reversibility of the
neurotoxic effects of fipronil and its metabolites
(functional, behavioural, learning/memory, cellular, and
neurotransmitter/receptor effects).
2. Observations in humans exposed to fipronil and fipronil-
desulfinyl
References
Adams, K. (1996a) MB 46513: CHO mammalian cell mutation assay.
Unpublished report No. RNP452/950622 from Huntingdon Life Sciences,
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Adams, K. (1996b) MB 46513: Metaphase chromosome analysis of human
lymphocytes cultured in vitro. Unpublished report No. RNP 451/951219
from Huntingdon Life Sciences, Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Aughton, P. (1993) MB 46030: Combined oncogenicity and toxicity study
by dietary administration to CD rats for 104 weeks, including a 13
week reversibility period on completion of 52 weeks of treatment.
Unpublished report No. 93/RHA432/0166 from Pharmaco LSR Ltd. Submitted
to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Berthe, P. (1996) RPA 200766: 28-Day toxicity study in the rat by
dietary administration. Unpublished report No. SA 95273 from
Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Bigot, D. (1996) MB 46513: 90-Day toxicity study in the mouse by
dietary administration. Unpublished report No. SA 95055 from
Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Broadmeadow, A. (1991) M&B 46030: Preliminary toxicity study by
dietary administration to CD-1 mice for 13 weeks. Unpublished report
No. 90/RHA364/0860 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Broadmeadow, A. (1993) M&B 46030: Oncogenicity study by dietary
administration to CD-1 mice for 78 weeks. Unpublished report No.
92/RHA313/0971 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Brooker, A.J. & John, D.M. (1991) The effect of M&B 46,030 on
pregnancy of the rat. Unpublished report No. M&B 335+326/90582 from
Huntingdon Research Centre Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Bushey, D.F. (1993) Fipronil mode of action research summary.
Unpublished memo prepared by Rhone-Poulenc Agrochimie Co., Research
Triangle Park Biochemistry Group. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Cheng, T. (1995) Dermal absorption of 14C-fipronil REGENT 80WDG in
male rats (preliminary and definitive phases). Unpublished report No.
HWI 6224-210 from Hazleton Wisconsin, Inc. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Cheng, T. (1996) Dermal absorption of 14C-MB 46513 in male rats
(preliminary and definitive phases). Unpublished report No.
CHW 6224-230 from Corning Hazleton, Inc. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Clare, C.B. (1988a) Study to determine the ability of M&B 46030 to
induce mutation in four histidine-requiring strains of Salmonella
typhimiurim. Unpublished report No. MAB 20/S from Microtest Research
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Clare, C.B. (1988b) Study to determine the ability of M&B 46136 to
induce mutation in four histidine-requiring strains of Salmonella
typhimiurim. Unpublished report No. MAB 21/S from Microtest Research
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Cole, L.M., Nicholson, R.A. & Casida, J.E. (1993) Action of
phenylpyrazole insecticides at the GABA-gated chloride channel.
Pestic. Biochem. Physiol., 46, 47-54.
Cracknell, S. (1991) M&B 46030: Acute inhalation toxicity study in the
rat. Unpublished report No. 90/RHA358/0791 from Life Science Research
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Dange, M. (1993a) RPA 200766: Acute oral LD50 in the rat. Unpublished
report No. SA 93016 from Rhone-Poulenc Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Dange, M. (1993b) MB 46513. Acute oral LD50 in rats. Unpublished
report No. SA 93074 from Rhone-Poulenc Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Dange, M. (1993c) MB 46513: Acute dermal LD50 in the rat. Unpublished
report No. SA 93095 from Rhone-Poulenc Secteur Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Dange, M. (1993d) RPA 104615 acute oral LD50 in the rat. Unpublished
report No. SA 93046 from Rhone-Poulenc Secteur Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC,USA.
Dange, M. (1994a) MB 45950 acute oral LD50 in the rat. Unpublished
report No. SA 93272 from Rhone-Poulenc Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Dange, M. (1994b) MB 46513: Preliminary 28-day toxicity study in the
mouse by dietary administration. Unpublished report No. SA 93228 from
Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Dange, M. (1994c) MB 46513: Exploratory 14-day toxicity study in the
rat by gavage. Unpublished report No. SA 93063 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Dange, M. (1994d) MB 46513 90-day toxicity study in the rat by dietary
administration. Unpublished report No. 93226 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Dange, M. (1995a) MD 46513: Preliminary 28 day toxicity study in the
rat by dietary administration. Unpublished report No. SA 93138 from
Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Dange, M. (1995b) MB 46513: Preliminary 28-day toxicity study in the
dog by dietary administration. Unpublished report No. SA 94143 from
Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Dange, M. (1996) MB 46513: 90-Day toxicity study in the dog by dietary
administration. Unpublished report No. SA 95100 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Driscoll, C.D. and Hurley, J.M. (1993) MB 46030: Ninety day dietary
neurotoxicity study in Sprague Dawley rats. Unpublished report No.
92N1074 from Union Carbide Bushy Run Research Center. Submitted to WHO
by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Edwards, C.N. (1991) M&B 46030: Assessment of clastogenic action on
bone marrow erythrocytes in the micronucleus test. Unpublished report
No. 90/RHA305/1377 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Edwards, C.N. (1995) M&B 46030: Mouse micronucleus test to comply with
OECD Guideline 474 (1983). Unpublished report No. 95/RHA547/0432 from
Pharmaco LSR Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Foulon, O. (1997) MB 46513 (fipronil photometabolite): Developmental
toxicology study in the rat by gavage. Unpublished report No. SA 96227
from Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Gant, D.B., Chalmers, A.E. & Wolff, M.A. (1994) Fipronil: A novel
insecticide acting at the GABA receptor. Poster presented at the
Eighth International Congress of Pesticide Chemistry, Washington DC by
Rhone-Poulenc Agrochimie Co., Department of
Biochemistry/Biotechnology, Research Triangle Park, NC.
Gardner, J.R. (1988a) Acute oral toxicity to rats of M&B 46,030.
Unpublished report No. 881300D/M&B 290/AC from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Gardner, J.R. (1988b) Acute dermal toxicity to rats of M&B 46,030.
Unpublished report No. 881113 D/M&B 291/AC from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Gardner, J.R. (1988c) Acute oral toxicity to rats of M&B 46136.
Unpublished report No. 881364 D/M&B286/AC from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC,USA.
Gardner, J.R. (1988d) Acute dermal toxicity to rats fo M&B 46,136.
Unpublished report No. 88916 D/M&B287/AC from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Gill, M.W., Wagner, C.L. & Driscoll, C.D. (1993) M&B 46030: Single
exposure peroral (gavage) neurotoxicity study in Sprague Dawley rats.
Unpublished report No. 91N0099 from Union Carbide Bushy Run Research
Center. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Haynes, G. (1988a) M&B 45897 acute oral toxicity study in the rat.
Unpublished report No. A/D/4855 from Toxicol Laboratories Ltd.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Haynes, G. (1988b) M&B 45897 acute dermal toxicity study in the rat.
Unpublished report No. A/D/4856 from Toxicol Laboratories Ltd.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Hermansky, S.J. & Wagner, C.L. (1993) M&B 46030: Twenty-one day
repeated cutaneous dose toxicity study in New Zealand white rabbits
#2. Unpublished report No. 92N1165 from Union Carbide Bushy Run
Research Center. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Holmes, P. (1991a) M&B 46030: Toxicity study by dietary administration
to CD rats for 13 weeks. Unpublished report No. 90/RHA 298/0781 from
Life Science Research Ltd. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Holmes, P. (1991b) M&B 46030: Toxicity study by oral (capsule)
administration to beagle dogs for 13 weeks. Unpublished report No.
90/RHA310/0842 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Holmes, P. (1991c) M&B 46030: Neurotoxicity study by oral (capsule)
administration to female beagle dogs for up to 14 days followed by a
28 day reversibility period. Final report. Unpublished report No.
90/RHA371/0790 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Holmes, P. (1992) M&B 46030: Toxicity study by oral (capsule)
administration to beagle dogs for 52 weeks. Unpublished report No.
92/RHA311/0464 from Life Science Research Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Holmes, P. (1993) M&B 46030: Toxicity study by dietary administration
to beagle dogs for 52 weeks. Unpublished report No. 93/RHA465/0243
from Pharmaco-LSR Ltd. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Hughes, E.W. (1996) MB 46513: Neurotoxicity to rats by acute oral
administration (including a dose range finding study). Unpublished
report No. RNP 471/951489 from Huntingdon Life Sciences. Submitted to
WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Johnson, A.L. (1995) Pyrazole/MB 45897/RPA 097920: An in vitro test
for induction of chromosome damage: Cytogenetic study in cultured
human peripheral lymphocytes. Unpublished report No. 94/RHA534/1034
from Pharmaco-LSR. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Johnson, I.R. (1993) M&B 46030: Delayed contact hypersensitivity study
in guinea pigs. Unpublished report No. 93/RHA503/0167 from
Pharmaco-LSR Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Johnson, I.R. (1995) Pyrazole/MB 45897/RPA 097920: Intermediate of
fipronil (MB 46030): Four week oral toxicity study in the rat.
Unpublished report No. 95/RHA535/0684 from Pharmaco LSR. Submitted to
WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Kennelly, J.C. (1988) Study to determine the ability of M&B 45897/RPA
97920 to induce mutation in four histidine-requiring strains of
Salmonella typhimurium. Unpublished report No. MAB 19/S from
Microtest Research Ltd. Submitted to WHO by Rhone-Poulenc, Research
Triangle Park, NC, USA.
King, V.C. (1990) M&B 46030: Teratology study in the rabbit.
Unpublished report No. 90/RHA 321/0722 from Life Science Research Ltd.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
King, V.C. (1992) M&B 46030: Reproductive performance study in rats
treated continuously through two successive generations. Unpublished
report No. 92/RHA425/0309 from Life Science Research Ltd. Submitted to
WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Liggett, M.P. (1988a) Irritant effects on rabbit skin of M&B 46,030.
Unpublished report No. 881031 D/M&B 292/SE from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Liggett, M.P. (1988b) Irritant effects on the rabbit eye of M&B
46,030. Unpublished report No. 881032D/M&B 293/SE from Huntingdon
Research Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Liggett, M. P. (1988c) Irritant effects on rabbit skin of M&B 46,136.
Unpublished report No. 88833D/M&B 288/SE from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Liggett, M.P. (1988d) Irritant effects on the rabbit eye of M&B
46,136. Unpublished report No. 881022D/M&B 289/SE from Huntingdon
Research Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Lloyd, J.M. (1990) M&B 46030: Investigation of mutagenic activity at
the HGPRT locus in a Chinese hamster V/79 cell mutation system.
Unpublished report No. 90/RHA304/0418 from Life Science Research Ltd.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Mandella, R.C. (1995) A developmental neurotoxicity study of fipronil
in the rat via dietary administration. Unpublished report No. 93-4508
from Pharmaco-LSR/Huntingdon Life Sciences. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Marshall, R.R. (1988a) Study to evaluate the chromosome damaging
potential of M&B-46030 by its effects on cultured human lymphocytes
using an in vitro cytogenetics assay. Unpublished report No. MAB
20/HLC from Microtest Research Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Marshall, R.R. (1988b) Study to evaluate the chromosome damaging
potential of M&B 45950 by its effects on cultured human lymphocytes
using an in-vitro cytogenetics assay. Unpublished report No. MAB
18/HLC from Microtest Research Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Marshall, R.R. (1989) Study to evaluate the chromosome damaging
potential of M&B 46136 by its effects on cultured human lymphocytes
using an in-vitro cytogenetics assay. Unpublished report No. MAB
21/HLC from Microtest Research Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Mondot, S. & Dange, M. (1995) MB 46030: Acute oral LD50 in the mouse.
Unpublished report No. R&D/CRSA/TO-PHA3 from Rhone-Poulenc Agrochimie
Toxicology. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Myers, R.C. & Christopher, S.M. (1992) MB 46030: Acute percutaneous
toxicity study in the rabbit. Unpublished report No. 92N1009 from
Union Carbide Bushy Run Research Center. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Myers, R.C. & Christopher, S.M. (1993a) MB 46030 (technical):
Cutaneous irritancy study in the rabbit. Unpublished report No.
93N1217A from Union Carbide Bushy Run Research Center. Submitted to
WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Myers, R.C. & Christopher, S.M. (1993b) MB 46030 (technical): Ocular
irritancy study in the rabbit. Unpublished report No. 93N1217B from
Union Carbide Bushy Run Research Center. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Nachreiner, D.J. (1995) Fipronil: Acute nose-only dust inhalation
study in rats. Unpublished report No. 94N1501 from Union Carbide Bushy
Run Research Center. Submitted to WHO by Rhone-Poulenc, Inc., Research
Triangle Park, NC, USA.
Percy, A. (1993a) RPA 200766 Salmonella typhimurium reverse mutation
assay (Ames test). Unpublished report No. SA 93174 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Percy, A. (1993b) MB 46513 Salmonella typhimurium reverse mutation
assay (Ames test). Unpublished report No. SA 93135 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Percy, A. (1993c) RPA 104615 Salmonella typhimurium reverse mutation
assay (Ames test). Unpublished report No. SA 93175 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Percy, A. (1994a) MB 45950 Salmonella typhimurium reverse mutation
assay (Ames test). Unpublished report No. SA 93305 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Percy, A. (1994b) RPA 105048 Salmonella typhimurium reverse mutation
assay (Ames test). Unpublished report No. SA 94009 from Rhone-Poulenc
Agrochimie Toxicology. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Percy, A. (1996) M&B 45897/RPA 97920 intermediate of fipronil (MB
46030): Salmonella typhimurium reverse mutation assay. Unpublished
report No. SA 95345 from Rhone-Poulenc Agrochimie Toxicology.
Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC,
USA.
Peters, D.H., Stuart, V., Crook, D., Gibson, W.A., Gopinath, C. &
Hadley, J. (1990) M&B 46,030: Toxicity to rats by dietary
administration for four weeks. Unpublished report No. M&B 327/891321
from Huntingdon Research Centre Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Peters, D.H., Stuart, V., Hall, M., Chasseaud, L.F. & Chanter, D.O.
(1991a) M&B 46,030: An investigation into the potential effects on
thyroid function in male rats by studying thyroxine clearance.
Unpublished report No. M&B 352/90958 from Huntingdon Research Centre
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Peters, D.H., Stuart, V., Hall, M., Chasseaud, L.F. & Chanter, D.O.
(1991b) M&B 46,030: An investigation into the potential effects on
thyroid function in male rats by using the 'perchlorate discharge
test'. Unpublished report No. M&B 353/90920 from Huntingdon Research
Centre Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Peters, D.H., Stuart, V., Crook, D., Chanter, D.O., Colman, K.A. &
Gopinath, C. (1991c) M&B 46,030: 4-Week dietary study to investigate
thyroid hormone levels in the rat. Unpublished report No. M&B
360/901275 from Huntingdon Research Centre Ltd. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Powles, P. (1992) (14C)-M&B 46,030: Absorption, distribution,
metabolism and excretion in the rat. Unpublished report No.
7040-68/117 from Hazleton UK. Submitted to WHO by Rhone-Poulenc, Inc.,
Research Triangle Park, NC, USA.
Proudlock, R.J. (1996) MB46513: Mouse micronucleus test. Unpublished
report No. RNP 453/950649 from Huntingdon Life Sciences Ltd. Submitted
to WHO by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Ray, D. (1997) Report on the toxicity of fipronil. Unpublished report
from Medical Research Council. Submitted to WHO by MRC Toxicology
Unit, Leicester, United Kingdom.
Smith, K.D. (1990) M&B 46030: Dermal sensitisation study in guinea
pigs. Unpublished report No. 90/RHA357/0602 from Life Science Research
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
Stewart, F.P. (1994a) Revised final report: (14C)-M&B 46030:
Absorption, distribution, metabolism and excretion following repeat
oral administration to the dairy goat. Unpublished report No.
HE68/129R-1011 from Hazleton Europe. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Stewart, F.P. (1994b) Revised final report: (14C)-M&B 46030:
Distribution, metabolism and excretion following multiple oral
administration to the laying hen. Unpublished report No.
HE/68/120R-1011 from Hazleton Europe. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Taylor, T. (1993) The effect of single and repeated oral doses of M&B
46030 (1 mg/kg/day and 10 mg/kg/day) on the biliary excretion of
intravenously administered 125I-Thyroine (T4) from bile duct
cannulated rats. Unpublished report No. HRC/ITT 2/930645 from
Huntingdon Research Centre Ltd. Submitted to WHO by Rhone-Poulenc,
Inc., Research Triangle Park, NC, USA.
Totis, M. (1996) MB 46513: Absorption, distribution, metabolism, and
excretion in the rat (final report). Unpublished report No. SA 95304
from Rhone-Poulenc Agrochimie Toxicology. Submitted to WHO by
Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Totis, M. & Fisher, P.J. (1994) Fipronil: Tissue kinetic study in the
rat. Unpublished report No. SA94255 from Rhone-Poulenc Agrochimie
Toxicology. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle
Park, NC, USA.
Walters, K.A. & Brain, K.R. (1990) M&B 46030: In vitro skin
permeability of M&B 46030. Unpublished report No. RD 8 from
Pharmaserve Ltd, and An-eX Analytical Services Ltd. Submitted to WHO
by Rhone-Poulenc, Inc., Research Triangle Park, NC, USA.
Wright, N.P. (1995) Fipronil: Chromosomal aberration test in CHL cells
in vitro. Unpublished report No. 282/456 from Safepharm Laboratories
Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park,
NC, USA.
