
FAO Meeting Report No. PL/1965/10/2
WHO/Food Add/28.65
EVALUATION OF THE HAZARDS TO CONSUMERS RESULTING FROM THE USE OF
FUMIGANTS IN THE PROTECTION OF FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met 15-22 March
19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65.
ACRYLONITRILE
Compound
Acrylonitrile
Chemical name
Acrylonitrile
Synonyms
Propene nitrile; vynyl cyanide; cyanoethylene
Empirical formula
CH2CHCN
Structural formula
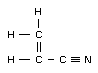 Relevant physical and chemical properties
Physical state (atmospheric pressure 20°C): colourless, volatile
liquid (some technical grades slightly yellow)
Boiling-point: 77°C
Odour: like mustard
Flash Point: 0°C (open cup)
Flammability limits in air: 3 to 17% by volume
Solubility:
Water: 7.5 g/100 ml
Organic solvents: soluble in all common organic solvents
Specific gravity (liquid): 0.800
Specific gravity (gas): 1.83
Uses
Acrylonitrile is a potent, highly effective fumigant; however its
high flammability and cost deter its use. Although it is not widely
employed at present for the fumigation of foodstuffs, acrylonitrile is
occasionally used to treat grain, dried fruit, walnuts and tobacco. It
is also sometimes used as a "spot fumigant" in flour mills and
bakeries.
Residues
Residues are similar to those resulting from the use of hydrogen
cyanide.
Effect of fumigant on treated crop
The effects of acrylonitrile are similar to those of hydrogen
cyanide. Commodities having a high moisture content absorb more
fumigant than commodities with a low moisture content. Acrylonitrile,
used alone or mixed with carbon tetrachloride, does not affect the
germination of a wide range of vegetable, cereal and flower seeds
(Glass and Crosier, 1949; Lindgren et al., 1954). It is, however,
highly toxic to nursery stock and growing plants and seriously damages
fresh fruit.
BIOLOGICAL DATA
Acrylonitrile is highly toxic and the intoxication can be induced
by the oral, percutaneous or inhalation routes (in the form of
vapours).
Biochemical aspects
On the basis of results with acrylonitrile in different species
of animals, its toxicity is considered to be equivalent to that of
hydrogen cyanide which is evolved from acrylonitrile in the organism
(Dudley and Neal, 1942; Dudley et al., 1942; Levina, 1951). Hydrogen
cyanide was demonstrated in the blood of rats after seven hours'
exposure (100 ppm in air) but only in small amounts (1 micromole/100
ml). In dogs the blood concentration of cyanide was ten times greater
after the same exposure. No hydrogen cyanide was found in the blood of
rats after exposure to lower concentrations in the air (Brieger et
al., 1952). From experiments in dogs, it was recommended that
thiocyanate determinations in serum and urine be used to discover
cases of dangerous absorption in workers exposed to acrylonitrile
(Lawton et al., 1943).
On the other hand, a number of authors have not confirmed that
the effects of acrylonitrile are due to hydrogen cyanide (Benes and
Cerna, 1959; Désgrez, 1911; Ghiringhelli, 1954; Lindgren et al., 1954;
Paulet and Desnos, 1961; Sexton, 1950). After the administration of
acrylonitrile to guinea-pigs, rats and rabbits very little cyanide was
recovered from the urine, as thiocyanate, compared with the amounts
excreted in cyanide poisoning (Benes and Cerna, 1959; Ghiringhelli,
1956; Paulet and Desnos, 1961). After subcutaneous injection of
acrylonitrile (130 mg/kg) to guinea-pigs, the hydrogen cyanide
concentration in blood was 0.128 mg % (average). This concentration is
too low to be able to cause death in animals; for comparison the
average HCN concentration in the blood of dogs after intoxication with
inhaled HCN was 1.1 mg % and 0.85 mg % after oral HCN poisoning
(Ghiringhelli, 1954; Gettler and Baine, 1938).
The use of thiosulfate, nitrite and glucose as antidotes to the
acute poisoning of animals with acrylonitrile has not been successful
or convincing (Benes and Cerna, 1959; Ghiringhelli, 1954; Dudley and
Neal, 1942). The combination of thiosulfate and nitrite had a certain
anti-acrylonitrile effect in mice (Benes and Cerna, 1959).
Furthermore, the rate of cyano-methaemoglobin formation in rats
killed by acrylonitrile is lower than that in animals surviving
potassium cyanide and much lower than in animals killed by it (Magos,
1962).
Thiosulfate with phenobarbitol and commonly used stimulating
preparations protected 70-90% of animals (rats, rabbits, mice) against
doses of acrylonitrile exceeding the LD100 (Paulet and Desnos, 1961).
In guinea-pigs, unmetabolized acrylonitrile was proved by a
chromatographic method 24 and 30 hours after oral and subcutaneous
detected administration (Benes and Cerna, 1959).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse oral 40-46 American Cyanamid, 1951
27 Benes & Cerna, 1959
subcutaneous 35 Benes & Cerna, 1959
Rat oral 78 Benes & Cerna, 1959
93 Smyth & Carpenter, 1948
Guinea-pig subcutaneous 130 Ghiringhelli, 1954
oral 50 Negherbon, 1959
Rabbit intravenous 69 Paulet & Desnos, 1961
Detailed investigation of acute poisoning with acrylonitrile after
intravenous injection in dog and rabbit indicates considerable
differences from cyanide intoxication. In the symptomatology nervous
disorders dominate the picture. Electroencephalographic records show
that the higher nervous centres are affected. Important physiological
differences from cyanide poisoning are absence of polypnoea, increased
oxygen consumption during the first phases of intoxication, and
lowering of the HbO2 level in the mixed venous blood. Hyperglycaemia
and a decrease in the plasma inorganic phosphate concentration were
also found (Paulet and Desnos, 1961).
Man. There are only few reports on the toxic effect of
acrylonitrile on man. Accidental deadly poisonings are described in
two children. In the first case it was an inhalation poisoning, in the
second a percutaneous one (Grunske, 1949, Lorz, 1950).
In industrial workers exposed to acrylonitrile vapours, in
different cases light jaundice, anaemia, leucocytosis, headache or
gastro-intestinal symptoms were observed (Wilson, 1944). In a small
group of men working with a mixture of nitrites for a number of years,
symptoms of liver and kidney irritations were reported; they
disappeared, however, when the exposure was interrupted (Wilson and
McCormick, 1949).
The threshold limit value for acrylonitrile in air is 20 ppm
(about 45 mg/m3) (Anon, 1964).
Comment on experimental studies reported
The acute toxicity and the mechanism of the toxic effect of
acrylonitrile have been studied. Its mode of action is not known but
it can be said that it is not similar to that of hydrogen cyanide.
Evaluation
On the basis of the toxicological investigations done hitherto,
the acceptable daily intake of acrylonitrile for man cannot be
evaluated.
Further work required
Biochemical studies on the effect of the fumigant on food.
Additional toxicological studies are required before an acceptable
daily intake level for man can be considered.
REFERENCES
Anon, (1964) Threshold limit values for 1964. Arch. environm.
Hlth., 9, 545
American Cyanamid Co., New York (1951) The chemistry of acrylonitrile,
Cyanamid's Nitrogen Chemicals Digest
Benes, V. & Cerna, V. (1959) J. Hyg. Epidem. (Praha), 3, 106
Brieger, H., Rieders, F. & Hodes, W. A. (1952) Arch. industr. Hyg.,
6, 128
Désgrez, A. (1911) C.R. Acad. Sci. (Paris), 153, 895
Dudley, H. C. & Neal, P. A. (1942) J. industr. Hyg., 24, 27
Dudley, H. C., Sweeney, T. R. & Miller, J. W. (1942) J. industr.
Hyg., 24, 255
Gettler, A. O. & Baine, J. O. (1938) Amer. J. med. Sci., 195, 182
Ghiringhelli, L. (1954) Med. d. Lavoro, 45, 305
Ghiringhelli, L. (1956) Med. d. Lavoro, 47, 192
Glass, E. H. & Crosier, W. F. (1949) J. econ. Ent., 42, 646
Grunske, F. (1949) Dtsch. med. Wschr., 74, 1081
Lawton, A. H., Sweeney, T. R. & Dudley, H. C. (1943) J. industr.
Hyg., 25, 13
Levina, E. N. (1951) Gig. i Sanit., 2, 34
Lindgren, D. L., Vincent, L. E. & Krohne, H. E. (1954) J. econ.
Ent., 47, 923
Lorz, H. (1950) Dtsch. med. Wschr., 75, 1087
Magos, L. (1962) Brit. J. industr. Med., 19, 283
Negherbon, W. O. (1959) Handbook of Toxicology, vol. 3, Saunders,
Philadelphia
Paulet, G. & Desnos, J. (1961) Arch. int. Pharmacodyn., 131, 54
Sexton, W. A. (1950) Chemical constitution and biological activity,
Von Nostrand Co. Inc., New York
Smyth, H. F. & Carpenter, C. P. (1948) J. industr. Hyg., 30, 63
Wilson, R. H. (1944) J. Amer. med. Ass., 124, 701
Wilson, R. H. & McCormick, W. E. (1949) Industr. Med. Surg., 18,
243
Relevant physical and chemical properties
Physical state (atmospheric pressure 20°C): colourless, volatile
liquid (some technical grades slightly yellow)
Boiling-point: 77°C
Odour: like mustard
Flash Point: 0°C (open cup)
Flammability limits in air: 3 to 17% by volume
Solubility:
Water: 7.5 g/100 ml
Organic solvents: soluble in all common organic solvents
Specific gravity (liquid): 0.800
Specific gravity (gas): 1.83
Uses
Acrylonitrile is a potent, highly effective fumigant; however its
high flammability and cost deter its use. Although it is not widely
employed at present for the fumigation of foodstuffs, acrylonitrile is
occasionally used to treat grain, dried fruit, walnuts and tobacco. It
is also sometimes used as a "spot fumigant" in flour mills and
bakeries.
Residues
Residues are similar to those resulting from the use of hydrogen
cyanide.
Effect of fumigant on treated crop
The effects of acrylonitrile are similar to those of hydrogen
cyanide. Commodities having a high moisture content absorb more
fumigant than commodities with a low moisture content. Acrylonitrile,
used alone or mixed with carbon tetrachloride, does not affect the
germination of a wide range of vegetable, cereal and flower seeds
(Glass and Crosier, 1949; Lindgren et al., 1954). It is, however,
highly toxic to nursery stock and growing plants and seriously damages
fresh fruit.
BIOLOGICAL DATA
Acrylonitrile is highly toxic and the intoxication can be induced
by the oral, percutaneous or inhalation routes (in the form of
vapours).
Biochemical aspects
On the basis of results with acrylonitrile in different species
of animals, its toxicity is considered to be equivalent to that of
hydrogen cyanide which is evolved from acrylonitrile in the organism
(Dudley and Neal, 1942; Dudley et al., 1942; Levina, 1951). Hydrogen
cyanide was demonstrated in the blood of rats after seven hours'
exposure (100 ppm in air) but only in small amounts (1 micromole/100
ml). In dogs the blood concentration of cyanide was ten times greater
after the same exposure. No hydrogen cyanide was found in the blood of
rats after exposure to lower concentrations in the air (Brieger et
al., 1952). From experiments in dogs, it was recommended that
thiocyanate determinations in serum and urine be used to discover
cases of dangerous absorption in workers exposed to acrylonitrile
(Lawton et al., 1943).
On the other hand, a number of authors have not confirmed that
the effects of acrylonitrile are due to hydrogen cyanide (Benes and
Cerna, 1959; Désgrez, 1911; Ghiringhelli, 1954; Lindgren et al., 1954;
Paulet and Desnos, 1961; Sexton, 1950). After the administration of
acrylonitrile to guinea-pigs, rats and rabbits very little cyanide was
recovered from the urine, as thiocyanate, compared with the amounts
excreted in cyanide poisoning (Benes and Cerna, 1959; Ghiringhelli,
1956; Paulet and Desnos, 1961). After subcutaneous injection of
acrylonitrile (130 mg/kg) to guinea-pigs, the hydrogen cyanide
concentration in blood was 0.128 mg % (average). This concentration is
too low to be able to cause death in animals; for comparison the
average HCN concentration in the blood of dogs after intoxication with
inhaled HCN was 1.1 mg % and 0.85 mg % after oral HCN poisoning
(Ghiringhelli, 1954; Gettler and Baine, 1938).
The use of thiosulfate, nitrite and glucose as antidotes to the
acute poisoning of animals with acrylonitrile has not been successful
or convincing (Benes and Cerna, 1959; Ghiringhelli, 1954; Dudley and
Neal, 1942). The combination of thiosulfate and nitrite had a certain
anti-acrylonitrile effect in mice (Benes and Cerna, 1959).
Furthermore, the rate of cyano-methaemoglobin formation in rats
killed by acrylonitrile is lower than that in animals surviving
potassium cyanide and much lower than in animals killed by it (Magos,
1962).
Thiosulfate with phenobarbitol and commonly used stimulating
preparations protected 70-90% of animals (rats, rabbits, mice) against
doses of acrylonitrile exceeding the LD100 (Paulet and Desnos, 1961).
In guinea-pigs, unmetabolized acrylonitrile was proved by a
chromatographic method 24 and 30 hours after oral and subcutaneous
detected administration (Benes and Cerna, 1959).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse oral 40-46 American Cyanamid, 1951
27 Benes & Cerna, 1959
subcutaneous 35 Benes & Cerna, 1959
Rat oral 78 Benes & Cerna, 1959
93 Smyth & Carpenter, 1948
Guinea-pig subcutaneous 130 Ghiringhelli, 1954
oral 50 Negherbon, 1959
Rabbit intravenous 69 Paulet & Desnos, 1961
Detailed investigation of acute poisoning with acrylonitrile after
intravenous injection in dog and rabbit indicates considerable
differences from cyanide intoxication. In the symptomatology nervous
disorders dominate the picture. Electroencephalographic records show
that the higher nervous centres are affected. Important physiological
differences from cyanide poisoning are absence of polypnoea, increased
oxygen consumption during the first phases of intoxication, and
lowering of the HbO2 level in the mixed venous blood. Hyperglycaemia
and a decrease in the plasma inorganic phosphate concentration were
also found (Paulet and Desnos, 1961).
Man. There are only few reports on the toxic effect of
acrylonitrile on man. Accidental deadly poisonings are described in
two children. In the first case it was an inhalation poisoning, in the
second a percutaneous one (Grunske, 1949, Lorz, 1950).
In industrial workers exposed to acrylonitrile vapours, in
different cases light jaundice, anaemia, leucocytosis, headache or
gastro-intestinal symptoms were observed (Wilson, 1944). In a small
group of men working with a mixture of nitrites for a number of years,
symptoms of liver and kidney irritations were reported; they
disappeared, however, when the exposure was interrupted (Wilson and
McCormick, 1949).
The threshold limit value for acrylonitrile in air is 20 ppm
(about 45 mg/m3) (Anon, 1964).
Comment on experimental studies reported
The acute toxicity and the mechanism of the toxic effect of
acrylonitrile have been studied. Its mode of action is not known but
it can be said that it is not similar to that of hydrogen cyanide.
Evaluation
On the basis of the toxicological investigations done hitherto,
the acceptable daily intake of acrylonitrile for man cannot be
evaluated.
Further work required
Biochemical studies on the effect of the fumigant on food.
Additional toxicological studies are required before an acceptable
daily intake level for man can be considered.
REFERENCES
Anon, (1964) Threshold limit values for 1964. Arch. environm.
Hlth., 9, 545
American Cyanamid Co., New York (1951) The chemistry of acrylonitrile,
Cyanamid's Nitrogen Chemicals Digest
Benes, V. & Cerna, V. (1959) J. Hyg. Epidem. (Praha), 3, 106
Brieger, H., Rieders, F. & Hodes, W. A. (1952) Arch. industr. Hyg.,
6, 128
Désgrez, A. (1911) C.R. Acad. Sci. (Paris), 153, 895
Dudley, H. C. & Neal, P. A. (1942) J. industr. Hyg., 24, 27
Dudley, H. C., Sweeney, T. R. & Miller, J. W. (1942) J. industr.
Hyg., 24, 255
Gettler, A. O. & Baine, J. O. (1938) Amer. J. med. Sci., 195, 182
Ghiringhelli, L. (1954) Med. d. Lavoro, 45, 305
Ghiringhelli, L. (1956) Med. d. Lavoro, 47, 192
Glass, E. H. & Crosier, W. F. (1949) J. econ. Ent., 42, 646
Grunske, F. (1949) Dtsch. med. Wschr., 74, 1081
Lawton, A. H., Sweeney, T. R. & Dudley, H. C. (1943) J. industr.
Hyg., 25, 13
Levina, E. N. (1951) Gig. i Sanit., 2, 34
Lindgren, D. L., Vincent, L. E. & Krohne, H. E. (1954) J. econ.
Ent., 47, 923
Lorz, H. (1950) Dtsch. med. Wschr., 75, 1087
Magos, L. (1962) Brit. J. industr. Med., 19, 283
Negherbon, W. O. (1959) Handbook of Toxicology, vol. 3, Saunders,
Philadelphia
Paulet, G. & Desnos, J. (1961) Arch. int. Pharmacodyn., 131, 54
Sexton, W. A. (1950) Chemical constitution and biological activity,
Von Nostrand Co. Inc., New York
Smyth, H. F. & Carpenter, C. P. (1948) J. industr. Hyg., 30, 63
Wilson, R. H. (1944) J. Amer. med. Ass., 124, 701
Wilson, R. H. & McCormick, W. E. (1949) Industr. Med. Surg., 18,
243
See Also:
Toxicological Abbreviations
Acrylonitrile (EHC 28, 1983)
Acrylonitrile (HSG 1, 1986)
Acrylonitrile (ICSC)
Acrylonitrile (WHO Food Additives Series 19)
ACRYLONITRILE (JECFA Evaluation)
Acrylonitrile (CICADS 39, 2002)
Acrylonitrile (IARC Summary & Evaluation, Volume 71, 1999)
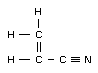 Relevant physical and chemical properties
Physical state (atmospheric pressure 20°C): colourless, volatile
liquid (some technical grades slightly yellow)
Boiling-point: 77°C
Odour: like mustard
Flash Point: 0°C (open cup)
Flammability limits in air: 3 to 17% by volume
Solubility:
Water: 7.5 g/100 ml
Organic solvents: soluble in all common organic solvents
Specific gravity (liquid): 0.800
Specific gravity (gas): 1.83
Uses
Acrylonitrile is a potent, highly effective fumigant; however its
high flammability and cost deter its use. Although it is not widely
employed at present for the fumigation of foodstuffs, acrylonitrile is
occasionally used to treat grain, dried fruit, walnuts and tobacco. It
is also sometimes used as a "spot fumigant" in flour mills and
bakeries.
Residues
Residues are similar to those resulting from the use of hydrogen
cyanide.
Effect of fumigant on treated crop
The effects of acrylonitrile are similar to those of hydrogen
cyanide. Commodities having a high moisture content absorb more
fumigant than commodities with a low moisture content. Acrylonitrile,
used alone or mixed with carbon tetrachloride, does not affect the
germination of a wide range of vegetable, cereal and flower seeds
(Glass and Crosier, 1949; Lindgren et al., 1954). It is, however,
highly toxic to nursery stock and growing plants and seriously damages
fresh fruit.
BIOLOGICAL DATA
Acrylonitrile is highly toxic and the intoxication can be induced
by the oral, percutaneous or inhalation routes (in the form of
vapours).
Biochemical aspects
On the basis of results with acrylonitrile in different species
of animals, its toxicity is considered to be equivalent to that of
hydrogen cyanide which is evolved from acrylonitrile in the organism
(Dudley and Neal, 1942; Dudley et al., 1942; Levina, 1951). Hydrogen
cyanide was demonstrated in the blood of rats after seven hours'
exposure (100 ppm in air) but only in small amounts (1 micromole/100
ml). In dogs the blood concentration of cyanide was ten times greater
after the same exposure. No hydrogen cyanide was found in the blood of
rats after exposure to lower concentrations in the air (Brieger et
al., 1952). From experiments in dogs, it was recommended that
thiocyanate determinations in serum and urine be used to discover
cases of dangerous absorption in workers exposed to acrylonitrile
(Lawton et al., 1943).
On the other hand, a number of authors have not confirmed that
the effects of acrylonitrile are due to hydrogen cyanide (Benes and
Cerna, 1959; Désgrez, 1911; Ghiringhelli, 1954; Lindgren et al., 1954;
Paulet and Desnos, 1961; Sexton, 1950). After the administration of
acrylonitrile to guinea-pigs, rats and rabbits very little cyanide was
recovered from the urine, as thiocyanate, compared with the amounts
excreted in cyanide poisoning (Benes and Cerna, 1959; Ghiringhelli,
1956; Paulet and Desnos, 1961). After subcutaneous injection of
acrylonitrile (130 mg/kg) to guinea-pigs, the hydrogen cyanide
concentration in blood was 0.128 mg % (average). This concentration is
too low to be able to cause death in animals; for comparison the
average HCN concentration in the blood of dogs after intoxication with
inhaled HCN was 1.1 mg % and 0.85 mg % after oral HCN poisoning
(Ghiringhelli, 1954; Gettler and Baine, 1938).
The use of thiosulfate, nitrite and glucose as antidotes to the
acute poisoning of animals with acrylonitrile has not been successful
or convincing (Benes and Cerna, 1959; Ghiringhelli, 1954; Dudley and
Neal, 1942). The combination of thiosulfate and nitrite had a certain
anti-acrylonitrile effect in mice (Benes and Cerna, 1959).
Furthermore, the rate of cyano-methaemoglobin formation in rats
killed by acrylonitrile is lower than that in animals surviving
potassium cyanide and much lower than in animals killed by it (Magos,
1962).
Thiosulfate with phenobarbitol and commonly used stimulating
preparations protected 70-90% of animals (rats, rabbits, mice) against
doses of acrylonitrile exceeding the LD100 (Paulet and Desnos, 1961).
In guinea-pigs, unmetabolized acrylonitrile was proved by a
chromatographic method 24 and 30 hours after oral and subcutaneous
detected administration (Benes and Cerna, 1959).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse oral 40-46 American Cyanamid, 1951
27 Benes & Cerna, 1959
subcutaneous 35 Benes & Cerna, 1959
Rat oral 78 Benes & Cerna, 1959
93 Smyth & Carpenter, 1948
Guinea-pig subcutaneous 130 Ghiringhelli, 1954
oral 50 Negherbon, 1959
Rabbit intravenous 69 Paulet & Desnos, 1961
Detailed investigation of acute poisoning with acrylonitrile after
intravenous injection in dog and rabbit indicates considerable
differences from cyanide intoxication. In the symptomatology nervous
disorders dominate the picture. Electroencephalographic records show
that the higher nervous centres are affected. Important physiological
differences from cyanide poisoning are absence of polypnoea, increased
oxygen consumption during the first phases of intoxication, and
lowering of the HbO2 level in the mixed venous blood. Hyperglycaemia
and a decrease in the plasma inorganic phosphate concentration were
also found (Paulet and Desnos, 1961).
Man. There are only few reports on the toxic effect of
acrylonitrile on man. Accidental deadly poisonings are described in
two children. In the first case it was an inhalation poisoning, in the
second a percutaneous one (Grunske, 1949, Lorz, 1950).
In industrial workers exposed to acrylonitrile vapours, in
different cases light jaundice, anaemia, leucocytosis, headache or
gastro-intestinal symptoms were observed (Wilson, 1944). In a small
group of men working with a mixture of nitrites for a number of years,
symptoms of liver and kidney irritations were reported; they
disappeared, however, when the exposure was interrupted (Wilson and
McCormick, 1949).
The threshold limit value for acrylonitrile in air is 20 ppm
(about 45 mg/m3) (Anon, 1964).
Comment on experimental studies reported
The acute toxicity and the mechanism of the toxic effect of
acrylonitrile have been studied. Its mode of action is not known but
it can be said that it is not similar to that of hydrogen cyanide.
Evaluation
On the basis of the toxicological investigations done hitherto,
the acceptable daily intake of acrylonitrile for man cannot be
evaluated.
Further work required
Biochemical studies on the effect of the fumigant on food.
Additional toxicological studies are required before an acceptable
daily intake level for man can be considered.
REFERENCES
Anon, (1964) Threshold limit values for 1964. Arch. environm.
Hlth., 9, 545
American Cyanamid Co., New York (1951) The chemistry of acrylonitrile,
Cyanamid's Nitrogen Chemicals Digest
Benes, V. & Cerna, V. (1959) J. Hyg. Epidem. (Praha), 3, 106
Brieger, H., Rieders, F. & Hodes, W. A. (1952) Arch. industr. Hyg.,
6, 128
Désgrez, A. (1911) C.R. Acad. Sci. (Paris), 153, 895
Dudley, H. C. & Neal, P. A. (1942) J. industr. Hyg., 24, 27
Dudley, H. C., Sweeney, T. R. & Miller, J. W. (1942) J. industr.
Hyg., 24, 255
Gettler, A. O. & Baine, J. O. (1938) Amer. J. med. Sci., 195, 182
Ghiringhelli, L. (1954) Med. d. Lavoro, 45, 305
Ghiringhelli, L. (1956) Med. d. Lavoro, 47, 192
Glass, E. H. & Crosier, W. F. (1949) J. econ. Ent., 42, 646
Grunske, F. (1949) Dtsch. med. Wschr., 74, 1081
Lawton, A. H., Sweeney, T. R. & Dudley, H. C. (1943) J. industr.
Hyg., 25, 13
Levina, E. N. (1951) Gig. i Sanit., 2, 34
Lindgren, D. L., Vincent, L. E. & Krohne, H. E. (1954) J. econ.
Ent., 47, 923
Lorz, H. (1950) Dtsch. med. Wschr., 75, 1087
Magos, L. (1962) Brit. J. industr. Med., 19, 283
Negherbon, W. O. (1959) Handbook of Toxicology, vol. 3, Saunders,
Philadelphia
Paulet, G. & Desnos, J. (1961) Arch. int. Pharmacodyn., 131, 54
Sexton, W. A. (1950) Chemical constitution and biological activity,
Von Nostrand Co. Inc., New York
Smyth, H. F. & Carpenter, C. P. (1948) J. industr. Hyg., 30, 63
Wilson, R. H. (1944) J. Amer. med. Ass., 124, 701
Wilson, R. H. & McCormick, W. E. (1949) Industr. Med. Surg., 18,
243
Relevant physical and chemical properties
Physical state (atmospheric pressure 20°C): colourless, volatile
liquid (some technical grades slightly yellow)
Boiling-point: 77°C
Odour: like mustard
Flash Point: 0°C (open cup)
Flammability limits in air: 3 to 17% by volume
Solubility:
Water: 7.5 g/100 ml
Organic solvents: soluble in all common organic solvents
Specific gravity (liquid): 0.800
Specific gravity (gas): 1.83
Uses
Acrylonitrile is a potent, highly effective fumigant; however its
high flammability and cost deter its use. Although it is not widely
employed at present for the fumigation of foodstuffs, acrylonitrile is
occasionally used to treat grain, dried fruit, walnuts and tobacco. It
is also sometimes used as a "spot fumigant" in flour mills and
bakeries.
Residues
Residues are similar to those resulting from the use of hydrogen
cyanide.
Effect of fumigant on treated crop
The effects of acrylonitrile are similar to those of hydrogen
cyanide. Commodities having a high moisture content absorb more
fumigant than commodities with a low moisture content. Acrylonitrile,
used alone or mixed with carbon tetrachloride, does not affect the
germination of a wide range of vegetable, cereal and flower seeds
(Glass and Crosier, 1949; Lindgren et al., 1954). It is, however,
highly toxic to nursery stock and growing plants and seriously damages
fresh fruit.
BIOLOGICAL DATA
Acrylonitrile is highly toxic and the intoxication can be induced
by the oral, percutaneous or inhalation routes (in the form of
vapours).
Biochemical aspects
On the basis of results with acrylonitrile in different species
of animals, its toxicity is considered to be equivalent to that of
hydrogen cyanide which is evolved from acrylonitrile in the organism
(Dudley and Neal, 1942; Dudley et al., 1942; Levina, 1951). Hydrogen
cyanide was demonstrated in the blood of rats after seven hours'
exposure (100 ppm in air) but only in small amounts (1 micromole/100
ml). In dogs the blood concentration of cyanide was ten times greater
after the same exposure. No hydrogen cyanide was found in the blood of
rats after exposure to lower concentrations in the air (Brieger et
al., 1952). From experiments in dogs, it was recommended that
thiocyanate determinations in serum and urine be used to discover
cases of dangerous absorption in workers exposed to acrylonitrile
(Lawton et al., 1943).
On the other hand, a number of authors have not confirmed that
the effects of acrylonitrile are due to hydrogen cyanide (Benes and
Cerna, 1959; Désgrez, 1911; Ghiringhelli, 1954; Lindgren et al., 1954;
Paulet and Desnos, 1961; Sexton, 1950). After the administration of
acrylonitrile to guinea-pigs, rats and rabbits very little cyanide was
recovered from the urine, as thiocyanate, compared with the amounts
excreted in cyanide poisoning (Benes and Cerna, 1959; Ghiringhelli,
1956; Paulet and Desnos, 1961). After subcutaneous injection of
acrylonitrile (130 mg/kg) to guinea-pigs, the hydrogen cyanide
concentration in blood was 0.128 mg % (average). This concentration is
too low to be able to cause death in animals; for comparison the
average HCN concentration in the blood of dogs after intoxication with
inhaled HCN was 1.1 mg % and 0.85 mg % after oral HCN poisoning
(Ghiringhelli, 1954; Gettler and Baine, 1938).
The use of thiosulfate, nitrite and glucose as antidotes to the
acute poisoning of animals with acrylonitrile has not been successful
or convincing (Benes and Cerna, 1959; Ghiringhelli, 1954; Dudley and
Neal, 1942). The combination of thiosulfate and nitrite had a certain
anti-acrylonitrile effect in mice (Benes and Cerna, 1959).
Furthermore, the rate of cyano-methaemoglobin formation in rats
killed by acrylonitrile is lower than that in animals surviving
potassium cyanide and much lower than in animals killed by it (Magos,
1962).
Thiosulfate with phenobarbitol and commonly used stimulating
preparations protected 70-90% of animals (rats, rabbits, mice) against
doses of acrylonitrile exceeding the LD100 (Paulet and Desnos, 1961).
In guinea-pigs, unmetabolized acrylonitrile was proved by a
chromatographic method 24 and 30 hours after oral and subcutaneous
detected administration (Benes and Cerna, 1959).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse oral 40-46 American Cyanamid, 1951
27 Benes & Cerna, 1959
subcutaneous 35 Benes & Cerna, 1959
Rat oral 78 Benes & Cerna, 1959
93 Smyth & Carpenter, 1948
Guinea-pig subcutaneous 130 Ghiringhelli, 1954
oral 50 Negherbon, 1959
Rabbit intravenous 69 Paulet & Desnos, 1961
Detailed investigation of acute poisoning with acrylonitrile after
intravenous injection in dog and rabbit indicates considerable
differences from cyanide intoxication. In the symptomatology nervous
disorders dominate the picture. Electroencephalographic records show
that the higher nervous centres are affected. Important physiological
differences from cyanide poisoning are absence of polypnoea, increased
oxygen consumption during the first phases of intoxication, and
lowering of the HbO2 level in the mixed venous blood. Hyperglycaemia
and a decrease in the plasma inorganic phosphate concentration were
also found (Paulet and Desnos, 1961).
Man. There are only few reports on the toxic effect of
acrylonitrile on man. Accidental deadly poisonings are described in
two children. In the first case it was an inhalation poisoning, in the
second a percutaneous one (Grunske, 1949, Lorz, 1950).
In industrial workers exposed to acrylonitrile vapours, in
different cases light jaundice, anaemia, leucocytosis, headache or
gastro-intestinal symptoms were observed (Wilson, 1944). In a small
group of men working with a mixture of nitrites for a number of years,
symptoms of liver and kidney irritations were reported; they
disappeared, however, when the exposure was interrupted (Wilson and
McCormick, 1949).
The threshold limit value for acrylonitrile in air is 20 ppm
(about 45 mg/m3) (Anon, 1964).
Comment on experimental studies reported
The acute toxicity and the mechanism of the toxic effect of
acrylonitrile have been studied. Its mode of action is not known but
it can be said that it is not similar to that of hydrogen cyanide.
Evaluation
On the basis of the toxicological investigations done hitherto,
the acceptable daily intake of acrylonitrile for man cannot be
evaluated.
Further work required
Biochemical studies on the effect of the fumigant on food.
Additional toxicological studies are required before an acceptable
daily intake level for man can be considered.
REFERENCES
Anon, (1964) Threshold limit values for 1964. Arch. environm.
Hlth., 9, 545
American Cyanamid Co., New York (1951) The chemistry of acrylonitrile,
Cyanamid's Nitrogen Chemicals Digest
Benes, V. & Cerna, V. (1959) J. Hyg. Epidem. (Praha), 3, 106
Brieger, H., Rieders, F. & Hodes, W. A. (1952) Arch. industr. Hyg.,
6, 128
Désgrez, A. (1911) C.R. Acad. Sci. (Paris), 153, 895
Dudley, H. C. & Neal, P. A. (1942) J. industr. Hyg., 24, 27
Dudley, H. C., Sweeney, T. R. & Miller, J. W. (1942) J. industr.
Hyg., 24, 255
Gettler, A. O. & Baine, J. O. (1938) Amer. J. med. Sci., 195, 182
Ghiringhelli, L. (1954) Med. d. Lavoro, 45, 305
Ghiringhelli, L. (1956) Med. d. Lavoro, 47, 192
Glass, E. H. & Crosier, W. F. (1949) J. econ. Ent., 42, 646
Grunske, F. (1949) Dtsch. med. Wschr., 74, 1081
Lawton, A. H., Sweeney, T. R. & Dudley, H. C. (1943) J. industr.
Hyg., 25, 13
Levina, E. N. (1951) Gig. i Sanit., 2, 34
Lindgren, D. L., Vincent, L. E. & Krohne, H. E. (1954) J. econ.
Ent., 47, 923
Lorz, H. (1950) Dtsch. med. Wschr., 75, 1087
Magos, L. (1962) Brit. J. industr. Med., 19, 283
Negherbon, W. O. (1959) Handbook of Toxicology, vol. 3, Saunders,
Philadelphia
Paulet, G. & Desnos, J. (1961) Arch. int. Pharmacodyn., 131, 54
Sexton, W. A. (1950) Chemical constitution and biological activity,
Von Nostrand Co. Inc., New York
Smyth, H. F. & Carpenter, C. P. (1948) J. industr. Hyg., 30, 63
Wilson, R. H. (1944) J. Amer. med. Ass., 124, 701
Wilson, R. H. & McCormick, W. E. (1949) Industr. Med. Surg., 18,
243
