
FAO, PL:CP/15
WHO/Food Add./67.32
EVALUATION OF SOME PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party and the WHO Expert Committee on
Pesticide Residues, which met in Geneva, 14-21 November 1966.1
1 Report of a Joint Meeting of the FAO Working Party and the WHO
Expert Committee on Pesticide Residues, FAO Agricultural Studies, in
press; Wld Hlth Org. techn. Rep. Ser., 1967, in press
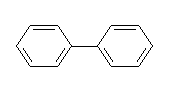
DIPHENYL
IDENTITY
Synonyms
biphenyl, phenylbenzene
Chemical name
diphenyl
Formula
 BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
Metabolic studies following oral administration of diphenyl have been
performed in rats (West, 1940; West et al., 1956), rabbits (Deichmann
et al., 1947; West et al., 1956; Block & Cornish, 1959) and dogs
(Hazleton et al., 1956). In these species a variety of phenolic
compounds were excreted in the urine, mostly as ethereal sulfates and
glucuronides. The main metabolite appeared to be 4-hydroxydiphenyl.
Unchanged diphenyl was excreted in the faeces only after the highest
doses (Hazleton et al., 1956).
In vitro 4-hydroxylation of diphenyl by liver microsomal enzymes has
been demonstrated in several species, including man. In addition,
2-hydroxylation was observed in adult cats, mice, hamsters, coypu and
in young but not in adult rats. The extent of 2-hydroxylation in young
rats is markedly increased in rats pre-treated in vivo with either
3,4 benzpyrene or 20-methyl-cholanthrene and to a much lesser extent
in rats pre-treated with phenobarbitone and nikethamide.
4-hydroxylation was stimulated by pre-treatment with phenobarbitone or
nikethamide but not by benzpyrene or methylcholanthrene (Creaven &
Williams, 1965; Williams, 1965).
Diphenyl was found to be less toxic when given with a diet containing
a supplement of 1-cystine or d1-methionine, but diphenyl is not highly
conjugated with cystine (West, 1940).
Acute toxicity
Animal Route LD50 Reference
mg/kg body-weight
Rat Oral 3300-5000 Deichmann et al., 1947;
Pecchiai & Safflotti, 1957
Rabbit Oral 2400 Deichmann et al., 1947
Cat Oral >2600 McEwen, 1958
Short-term studies
Mouse. Skin applications of a 23 per cent solution in oil were given
twice weekly to a group of 140 mice for 7 months. Local inflammatory
changes were commonly seen. No tumours developed (Selle, 1952; 1953;
1954).
Rat. Groups of 11 young rats were fed for 32 days either a diet low
in casein containing 10 000 ppm diphenyl or a normal diet containing
3000 ppm diphenyl. Growth retardation was observed in both experiments
(West, 1940; West & Jefferson, 1942). Daily oral doses of 0, 2, 20 and
200 mg/kg for 4 weeks and 300 mg/kg for 12 days did not induce growth
retardation (MacIntosh, 1945; Rogliani & Procaccini, 1956).
In another study, groups of young rats were fed diets containing 0,
100 and 1000 ppm diphenyl for 3 months. Growth rate, food efficiency,
organ weights and histology were identical in all groups. At 1000 ppm
only a slight polyuria was noted (Newell, 1953).
A paired feeding test was performed for 89 days on male and female
rats fed 5000 ppm and 10 000 ppm diphenyl in their diet. At both
levels there was no difference in weight gain between the test animals
and pair-fed controls, but the weight gain was slower than in controls
receiving food ad lib. A study of food efficiency over 3 weeks in
groups of 12 male and 12 female rats receiving 0 ppm, 100 ppm, 1000
ppm and 10 000 ppm diphenyl in their diet revealed no significant
differences between the groups (Ambrose et al., 1960).
Small groups of rats were fed diets containing 0, 1000 or 5000 ppm
diphenyl for 11 or 60 days before mating. No effect on reproduction
was noted (Ambrose et al., 1960).
A group of 13 rats was fed 0, 50 or 100 mg diphenyl per animal daily
and killed 2-13 months after the beginning of the treatment.
Regressive changes were noticed in the liver and kidney. Three animals
observed after 7 weeks, 7 months and 1 year had, respectively,
papillomatous hyperplasia of the forestomach, multiple papillomas of
the stomach and a squamous cell carcinoma of the stomach. The
incidence of lesions of the forestomach in untreated rats was not
given (Pecchiai & Saffiotti, 1957).
Rabbit. Five rabbits were given doses of 1000 mg/kg diphenyl by
stomach tube as a 25 per cent solution in olive oil, 2-3 times weekly.
All the animals lost weight and died within 5-20 weeks. Retention of
urea was found in the terminal stages (Deichmann et al., 1947).
Dog. Groups of 3 dogs received diphenyl in corn oil orally at doses
of 0, 2.5 and 25 mg/kg 5 times weekly for 52 weeks. At 3-month
intervals, blood and urine samples were normal. At the end of the
experiment all the animals were killed and no pathological changes
attributable to diphenyl were found (Hazleton et al., 1956).
Monkey. Four groups of 2 female and 1 male rhesus monkeys were given
diets containing 0, 100, 1000 and 10 000 ppm diphenyl for 1 year. No
changes in the body-weight and blood were seen. At the 10 000 ppm
level, a consistent increase of liver weight without histological
change was noticed (Newell, 1953).
Man. A human volunteer was given 35 mg diphenyl weekly per os for
13 weeks without adverse effect (Farkas, 1939)
Long-term studies
Rat. Groups of rats were given diets containing 0, 100, 1000 and
10 000 ppm diphenyl for 2 years. An intercurrent respiratory infection
killed many animals in all groups. Tubular dilatation in the kidney
was noticed at the two highest concentrations; however, 2 animals of
24 of the control group showed similar changes (Newell, 1953).
Eight groups of 15 weanling animals of each sex were given diets
containing 0, 10, 50, 100, 500, 1000, 5000 and 10 000 ppm diphenyl. At
5000 and 10 000 ppm there was growth inhibition, decrease in
longevity, decreased haemoglobin and pathological changes in the
kidneys at autopsy, including scarring, inflammation and tubular
atrophy. These changes were not noticed at concentrations of 1000 ppm
or less. The reversibility of the urinary changes induced by 5000 ppm
diphenyl in the diet for 4 months has been demonstrated (Ambrose et
al., 1960; Booth et al., 1961).
A 4-generation reproduction study was carried out on rats given doses
of 0, 100, 1000 and 10 000 ppm diphenyl. Only at 10 000 ppm fertility
and litter size were affected. This has been attributed to a lowered
food intake rather than to the direct effect of diphenyl (Newell,
1953).
Comments
Diphenyl appears to have been studied mostly in dogs and rats, and
special aspects have been investigated in other species. Long-term
studies have been carried out only in rats. The suspicion of
carcinogenicity raised by a study carried out under inadequate
conditions was not confirmed in later reports. In vivo and in
vitro studies in several species including man, reveal a common
detoxification mechanism.
Long-term feeding studies including recording or tumour incidence,
information on possible chemical changes undergone by diphenyl on the
fruit, and specifications of purity are desired.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 500 ppm in the diet, equivalent to 25 mg/kg/day
Dog: 25 mg/kg/day
Monkey: 50 mg/kg/day
Estimate of acceptable daily intake for man
Since children and ill people may consume a high amount of citrus
fruit and diphenyl exerts a toxic effect on the kidney, a higher
safety factor than usual has been used.
0-0.125 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use Pattern
(a) Pre-harvest treatments
The meeting was unaware of any pre-harvest uses of diphenyl.
(b) Post-harvest treatments
Diphenyl is used exclusively as a fungistatic agent on citrus fruits
during transportation and storage. Its superiority for this purpose
has displaced the use of other compounds in major citrus producing and
exporting countries. These include Australia, Israel, South Africa and
the United States of America. Recently, use in southern Italy has also
been reported. Comprehensive bibliographies covering the history of
development and use in those countries has been prepared by Resnick
(1966) and Rajzman (1965). Treatment of the packaging materials for
citrus is used rather than direct treatment of the fruit. Two methods
are employed.
(1) In Australia, Israel and South Africa, the fruit are wrapped
individually with tissue paper impregnated with diphenyl at from
28 to 40 mg per 100 square inches.
(2) In the USA a collective method is used: paper pads each of about
28 × 44 cm and containing 2.35 gm of diphenyl are placed at the
rate of one pad on the bottom and one on top of a 40 pound
(18 kg) carton of fruit (i.e. about 47 mg diphenyl per individual
180-200 gm fruit).
The fungistatic action depends on providing diphenyl vapour around the
fruit. It is not effective as a fungistat after being absorbed by the
fruit. Hence, the necessity of maintaining a reservoir in packaging
material throughout the period of transport and storage. The
technology of use, in relation to conditions of transport and storage,
distance of shipment and other parameters that control the treatment
have been studied intensively in the USA (Rygg, Wilson & Garber, 1962;
Klotz, L. J., 1957; and Rygg, Wells, Norman & Atrops, 1962), in
Australia (Kiely & Long, 1960), in Israel (Farkas & Aman, 1940;
Littauer & Mintz, 1945; Rajzman, 1961; Rajzman, 1961(a); and Rajzman,
1961(b)) and in South Africa (Christ, 1962) (see also Rajzman, 1965).
The current technology of use has evolved essentially from these
investigations.
(c) Other uses
No other uses have been reported.
National Tolerances
SUMMARY OF LEGAL AND OTHER TOLERANCES FOR CITRUS
Country Tolerance ppm Comment
USA 110
Canada 110
United Kingdom 100
Sweden 100
Belgium 70)
France 70) Subject to review
Federal Republic of Germany 70) in EEC countries
The Netherlands 30)
Residues resulting from supervised trials
The factors controlling the uptake and retention of diphenyl in fruit
have been intensively studied and the literature is reviewed by
Rajzman (1965) and by Resnick (1966). An earlier compilation of the
results of several years of supervised trials was made by Hazleton
(1956). These trials included fruit from the principal citrus
producing areas of the USA, treated and analysed in accordance with
the procedures at that time. Oranges, lemons and grapefruit had a
maximum of 110 ppm, 70 ppm and 30 ppm respectively, but only 10 per
cent of the samples contained more than 60 ppm, 40 ppm and 20 ppm
respectively. In reviewing such data it should be borne in mind that
in the light of later developments in analysis, it is probable that
the residues then reported were probably below the amounts which
actually were present when the analyses were carried out. Rajzman
(1965) reviewed the data obtained between 1945 and 1961 and the
residues found in whole fruit in various countries are summarized as
follows;
Number of More than More than More than
samples 70 ppm 100 ppm 110 ppm
examined
Found % Found % Found %
Oranges 470 32 6.6 9 1.9 6 1.3
Lemons 208 6 3.0 2 1.0 1 0.5
Grapefruit 59 0 0 0 0 0 0
Residues in food moving in commerce
Although much information has been obtained on residues in citrus
moving in commerce, in some cases information on the influence of the
method of sampling or of analysis was lacking. Some of the earlier
results are therefore due to unintentional variations in sampling and
analytical procedures.
Rajzman (1966) compiled a survey of diphenyl residues found in Israel
fruit of different varieties from 1958 to 1965. The fruits were
treated according to current commercial practice and stored under
varying conditions simulating transport and storage of exported fruit.
Residues were determined in the peel, pulp and whole fruit of 2093
samples of oranges, grapefruit and lemons. Fruit variety had no effect
on the quantity or distribution of the diphenyl absorbed. Peel content
varied from 1.0 to 400 ppm. Pulp residues were very low, varying from
0.1 to 0.91 ppm. Whole fruit residues varied from 0.9 ppm to 115.3 ppm
as follows:
More than 30 ppm - 28.77 per cent
" " 50 ppm - 8.0 per cent
" " 70 ppm - 2.01 per cent
" " 100 ppm - 0.10 per cent
" " 110 ppm - 0.05 per cent
Souci & Maier-Haarlander (1961) reported on residues in nine samples
of citrus fruit imported into Germany from USA, Italy and Cyprus in
1961. Only one sample (Italian) contained residues in excess of 100
ppm on a whole fruit basis (103 ppm). Rajzman (1965) gives figures for
citrus imported into Germany in 1959-1965 as follows:
Number of samples % of samples protected
with diphenyl
Year Total Without With 30 70 100 >100
diphenyl diphenyl ppm ppm ppm ppm
1959-61 149 0 149 59 95 97 3
1959-61 100 46 54 36 76 88 12
1961-65 132 83 49 29 68 87 13
1960-65 13 0 13 46 92 92 8
1964-65 17 0 17 29 88 100 0
1965 20 0 20 30 75 85 15
Total 431 129 302 48 88 94 6
1959-65
The meeting was aware that work, on which they have not received
detailed results, had been completed or was in progress in various
countries on the residues which are occurring in fruits received in
the course of commercial practice. From the published information,
however, it was concluded that the majority of samples of citrus fruit
moving in commerce in recent years have contained below 100 ppm; but
in a small per cent of cases residues up to 110 ppm have been
detected.
Residues at consumption (Effects of processing, cooking, etc.)
(a) Pulp and peel
Residues are mainly localised in the oil glands of citrus immediately
beneath the fruit surface. The peel itself may contain up to several
hundred ppm but the pulp contains amounts up to about 1.0 ppm. In
relation to the original amount of residue present in peel, lemons
contain more residue in pulp than oranges or grapefruit. Even in the
case of lemons, the residue in the pulp does not exceed 2.1 per cent
of the original residue in whole fruit (0.50 ppm). In oranges
containing as high as 400 ppm in the peel only, the pulp content never
exceeded 0.91 ppm (Rajzman, 1966).
(b) Juice
Hazleton (1956) compared residues resulting from extraction of citrus
juice by hand and by mechanical means. (A digest of these data are
also contained in Resnick, 1966). Mechanical extraction of juice
resulted in higher residues, but these did not exceed 4.3 ppm in
orange juice in two samples out of 284 for which the average was 1.15
ppm. This compares with a maximum of 2.5 ppm in one sample of orange
juice pressed by hand, and an average of 0.54 ppm for the 221 samples.
Grapefruit juice contained 0.26 ppm and 0.66 ppm by hand and
mechanical extraction respectively. Mechanically extracted lemon juice
contained the highest residues, ranging from 1.0 to 11.2 ppm,
averaging 3.3 ppm for 71 samples. Newell (1953) sealed diphenyl
protected fruit in wooden boxes for a period of eight weeks to attempt
to produce maximum absorption into fruit. The residues resulting in
orange, grapefruit and lemon juice were 1.3 ppm, 1.1 ppm and 0.4 ppm
respectively. Canadian Food and Drug Directorate (1965) examined
residues in juice from 284 samples of oranges. Forty-five per cent
showed less than 1.0 ppm in the juice, while for the balance only
eight were in excess of 2.5 ppm, with two values grouped at
approximately 4.4 ppm.
(c) Marmalades and jams
Diphenyl residues in citrus peel that may be used for the manufacture
of marmalades and jams are substantially reduced during the mincing of
peel and fruit and the cooking process. Losses of residue by these
processes has been reported by Tomkins & Isherwood (1945), Dickey
(1956), Rajzman (1962) and Souci & Maier-Haarlander (1963). The
elimination of residue is directly related to the degree of chopping
before cooking and whether whole fruit or peel only is used.
Elimination during these processes ranged from 18.6 per cent to 100
per cent depending on the method of preparation. In one case 23.1 ppm
remained in marmalade after cooking, while in most other cases the
remaining residues ranged between 0.0 and 16.8 ppm, with the majority
between 0 and 12 ppm.
Methods of residue analysis
(a) Analysis
Rajzman (1965) reviewed the methods of analysis up until that time.
The two most promising new methods that offered reliability,
reproducibility and speed are: (1) thin layer chromatography followed
by spectrophotometric determination of the TLC spots at 248 mµ
(Norman, Rygg & Wells, 1966) and (2) a gas-liquid chromatography
method based on the methods of Vogel & Deshusses (1964, 1965).
Both methods are currently under study. A collaborative study has been
conducted by five government laboratories in the member countries of
E.E.C. A comparative study of both methods is also in progress at the
Centraal Instituut voor Voedingsonderzoek, TNO, Zeist, the
Netherlands. A progress report from this institute by Vos (1966) was
available to the FAO Working Party on Pesticide Residues. The
conclusion drawn by Vos is as follows:
"The gas chromatographic method is the fastest and most accurate one.
The average value found agrees with that obtained from the thin layer
method. The values of the latter show a larger spreading. It is
possible that the biphenyl zone on the plates still contains some
interfering substances.
Although many other methods for the determination of biphenyl in
citrus fruit have been described in the literature, the G.L.C. - and
thin layer method were selected to be tested in practice. They seemed
to be most suitable for our purposes, viz. routine determination in
large number of samples.
We certainly can recommend the gas chromatographic determination for
this purpose.
If the relatively expensive equipment for gas chromatography should be
a serious objection, we recommend the thin-layer method. Although it
is slightly less accurate, it is also suitable for routine
determinations."
One further refinement is now available in the development of a
totally automated analytical procedure by Gunther & Ott (1966) for
citrus fruit rind employing a continuous flow recording
spectrophotometer.
The F.A.O. Working Party considered that, at the level of the
suggested tolerance, both the thin layer and the gas-liquid
chromatographic methods would be suitable for residue analysis.
Nevertheless it was decided to delay making a firm recommendation
until full reports were available on the investigations currently in
progress in the E.E.C. and T.N.O./Zeist.
(b) Sampling
It is understood that work is also in progress at the TNO, C.I.V.O.
Institute, Zeist, to explore the subject of sampling. In the meantime,
attention is drawn to the ISO Fifth Draft Proposal: Sampling of fresh
fruits and vegetables. ISO/TC 34-Agric. Food Products, Subcommittee 3
- Fruits, Vegetables and Their Derived Products, Working Group 2
- Sampling, October, 1965.
RECOMMENDATION FOR TOLERANCES
As diphenyl is not used on other foods the entire acceptable daily
intake could be assigned to citrus or citrus products. Although no
international agreement has been reached on the amount of citrus and
citrus products in the "high consumption diet", a combined figure of
230 grams per day for fresh citrus plus 60 grams per day for canned
citrus (i.e. a maximum of 290 grams per day) has been suggested. (In
Canada the suggested level of food consumption for these products for
tolerance purposes is 190 grams.)
If the acceptable daily intake level were 0.125 mg/kg for a 60 kg
person the permissible level from all sources becomes 7.5 mg per day.
Evidence has been received to demonstrate the need for the use of
diphenyl in the storage and transport of citrus over long distances.
The residues resulting in the whole fruit only rarely exceed 110 ppm.
In fresh fruit, pulp as eaten by the consumer rarely exceed 1.0 ppm.
Juices in extreme cases may contain residues as high as 4.4 ppm, but
most cases will contain less than 2.0 ppm. Marmalade and jam may also
contain some residues, but these may become insignificant in most
diets. Even in the cases of small children consuming large amounts of
orange juice, the amounts of diphenyl taken would be well below
acceptable daily intake.
Therefore, a tolerance of 110 ppm is recommended to be applied at the
point of entry of the citrus into the country.
Further information
(1) Data are desired on the residues that may result from methods of
preparation of fruit juice which employ the whole of the fruit
including the peel.
(2) A specification for diphenyl for use as a fungistatic agent on
citrus seems desirable. The Working Party would like to receive
further information on the likely impurities in commercial diphenyl.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Ambrose, A. M., Booth, A. N., DeEds, F. & Cox, A. J., jr (1960) Food
Res., 25, 328
Creaven, P. J. & Williams, R. T. (1963) Biochem. J., 87, 19 P
Deichmann, W. B., Kitzmiller, K. V., Dierker, M. & Witherup, S. (1947)
J. Industr. Hyg. Toxicol., 29, 1
Farkas, A. (1939) Hadar, 12, 227
Hazleton, L. W., Kundzins, W., Howard, J. W. & Johnston, C. D. (1956)
Abstract XXth International Physiol. Congress, p. 412
McEwen, M. (1958) Monsanto Chemical Co. Tech. Publ. AT-1
MacIntosh, F. C. (1945) Analyst, 70, 334
Newell, G. W. (1953) Unpublished report of the Stanford Research
Institute
Pecchiai, L. & Saffiotti, U. (1957) Med. Lavoro, 48, 247
Rogliani, E. & Procaccini, S. (1956) Biochim. appl., 3, 193
Selle, W. A. (1952, 1953, 1954) Unpublished reports
West, H. D. (1940) Proc. Soc. exp. Biol. (N.Y.), 43, 573
West, H. D. & Jefferson, N. C. (1942), J. Nutr., 23, 425
West, H. D., Lawson, J. R., Miller, I. H. & Mathura, R. (1956) Arch.
Biochem., 60, 14
Williams, R. T. (1965) Ann. Biol. Clin. (Paris), 23, 7
REFERENCES PERTINENT TO AGRICULTURAL DATA
Canada Food and Drug Directorate (1965), Diphenyl (Biphenyl). Summary
of data dealing with the technological justification of its use in the
food products as an antimicrobial in Canada. Submitted to FAO/WHO
Codex Alimentarius Committee on Food Additives, The Hague, May, 1965
Christ, R. A. (1962) Annual Report 1961. South-African Co-operative
Citrus Exchange. L.T.D., Pretoria No. C 131/1962
Dickey, E. E. (1956) The biphenyl content of citrus marmalade prepared
from consumer-type, biphenyl-treated fruit. Inst. of Paper Chemistry,
Project 1108-7-4, March 22, 1956
Farkas, A. & Aman, J. (1940) The action of diphenyl on Penicillium and
Diplodia molds. Palest. J. Bot. Jerusalem, 2: 38-45
Gunther, F. A. & Ott, D. R. (1966) Rapid automated determination of
biphenyl in citrus fruit rind. Analyst 91:475-481
Hazleton, L. W. (1956) Report of investigations on diphenyl. Part D.
Results of tests on the amount of residue remaining in citrus fruit
after economic use of biphenyl. Hazleton Laboratories Report, Falls
Church, Va.
Kiely, T.B. & Long, J. K. (1960) Market diseases of citrus fruit. Agr.
Gaz. N.S. Wales, 71: 132
Klots, L. J. (1957) Control of decay of citrus fruit in cartons.
Citrus Leaves, 37(4): 6
Littauer, F. S. & Mintz, G. (1945) Citrus wastage investigation,
Report for 1937-1945. Citrus Control Board, Dept. of Hort., Govt. of
Palestine
Norman, S., Rygg, G. L. & Wells A. W. (1966) Improved cleanup method
for determination of biphenyl in citrus fruits and in
biphenyl-impregnated kraft papers by thin layer chromotography.
J.A.O.A.C. 49: 590-595
Rajzman, A. (1961) The diphenyl residue in citrus fruit. National
Univ. Inst. Agr. Rehovot (Israel). Report No. 332
Rajzman, A. (1961a) Diphenyl absorption by citrus fruit wrapped in
plain wraps and stored together with diphenyl-wrapped fruit. Israel J.
Agr. Res. 11: 137
Rajzman, A. (1961b) Effect of washing and Waxing of oranges upon their
diphenyl. absorption. Bull. Res. Council Israel, 10c, 131
Rajzman, A. (1962) Elimination du diphényle des écorces d'agrumes.
Ann. Nutr. Alim. 15: 239-254
Rajzman, A. (1965) Les résidus de biphényle dans les agrumes. Residue
Reviews 8; 1-73
Rajzman, A. (1966) Biphenyl residues found in citrus fruit in Israel
during the citrus season 1958/59 to 1964/65. Volcani Institute of
Agric. Res. National and Univ. Inst. of Agriculture, Rehovot, Pamphlet
105, 29 pp., 24 tables
Resnick, C. (1966) Biphenyl, technological justification of its use as
a citrus protectant. Prepared on behalf of the Government of Israel
for submission to the FAO Working Party on Pesticide Residues.
Rygg, G. L., Wilson, G. W. & Garber, M. J. (1961) Effect of biphenyl
treatment and carton ventilation on decay and spoilage of California
lemons in overseas shipments. U.S. Dept. Agric. Agr. Marketing Service
Rept. No. 500
Rygg, G. L., Wells, A. W., Norman, S. M. & Atrops, E. P. (1962)
Biphenyl control, of lemon spoilage. Influence of time, temperature
and carton venting, U.S.D.A., Agr. Marketing Service Rept. No. 569
Souci, S.W. (1959) Die Behandlung von Citrus-Früchten. In
Lebensmittelforschung und Fremdstoffprobleme in U.S.A. München: Dtsch.
Forschungsanstalt-Für Lebensmittelchem, 5.46
Souci, S.W. & Maier-Haarlander, G. (1961) Work Report, Research on
Diphenyl-content of Citrus Fruit, to Industry Committee on Citrus
Additives, Re German Project No. 50501-31 (copy submitted to FAO and
WHO)
Souci, S.W. & Maier-Haarlander, G. (1963) Untersuchungen zur Analytik
des Diphenyls I. Mitteilung. Lebensm. Untersuch. U. Forsch. 119: 217
Tomkins, P. C. & Isherwood, F. A. (1945) The absorption of diphenyl
and O-phenyl-phenol by oranges. Analyst 70: 330-333.
Vogel, J. & Deshusses, J. (1964) Une methode de dosage du diphényle
dans les agrumes par chromatographie en phase gazeuse. Mitt. Gebiete
Lebensm. Hyg. 553 84-92
Vogel, J. & Deshusses, J. (1965) Modification de la methode de dosage
du diphenyle dans les agrumes par chromtographie en phase gazeuse.
Mitt. Gebiete Lebensm. Hyg. 56: 185.
de Vos, R. H. (1966) Determination of biphenyl in citrus fruit,
Progress report No. 1. Report No. R2155a, Centraal Instituut voor
Voedingsonderzoek, T.N.O., Zeist, April, 1966.
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
Metabolic studies following oral administration of diphenyl have been
performed in rats (West, 1940; West et al., 1956), rabbits (Deichmann
et al., 1947; West et al., 1956; Block & Cornish, 1959) and dogs
(Hazleton et al., 1956). In these species a variety of phenolic
compounds were excreted in the urine, mostly as ethereal sulfates and
glucuronides. The main metabolite appeared to be 4-hydroxydiphenyl.
Unchanged diphenyl was excreted in the faeces only after the highest
doses (Hazleton et al., 1956).
In vitro 4-hydroxylation of diphenyl by liver microsomal enzymes has
been demonstrated in several species, including man. In addition,
2-hydroxylation was observed in adult cats, mice, hamsters, coypu and
in young but not in adult rats. The extent of 2-hydroxylation in young
rats is markedly increased in rats pre-treated in vivo with either
3,4 benzpyrene or 20-methyl-cholanthrene and to a much lesser extent
in rats pre-treated with phenobarbitone and nikethamide.
4-hydroxylation was stimulated by pre-treatment with phenobarbitone or
nikethamide but not by benzpyrene or methylcholanthrene (Creaven &
Williams, 1965; Williams, 1965).
Diphenyl was found to be less toxic when given with a diet containing
a supplement of 1-cystine or d1-methionine, but diphenyl is not highly
conjugated with cystine (West, 1940).
Acute toxicity
Animal Route LD50 Reference
mg/kg body-weight
Rat Oral 3300-5000 Deichmann et al., 1947;
Pecchiai & Safflotti, 1957
Rabbit Oral 2400 Deichmann et al., 1947
Cat Oral >2600 McEwen, 1958
Short-term studies
Mouse. Skin applications of a 23 per cent solution in oil were given
twice weekly to a group of 140 mice for 7 months. Local inflammatory
changes were commonly seen. No tumours developed (Selle, 1952; 1953;
1954).
Rat. Groups of 11 young rats were fed for 32 days either a diet low
in casein containing 10 000 ppm diphenyl or a normal diet containing
3000 ppm diphenyl. Growth retardation was observed in both experiments
(West, 1940; West & Jefferson, 1942). Daily oral doses of 0, 2, 20 and
200 mg/kg for 4 weeks and 300 mg/kg for 12 days did not induce growth
retardation (MacIntosh, 1945; Rogliani & Procaccini, 1956).
In another study, groups of young rats were fed diets containing 0,
100 and 1000 ppm diphenyl for 3 months. Growth rate, food efficiency,
organ weights and histology were identical in all groups. At 1000 ppm
only a slight polyuria was noted (Newell, 1953).
A paired feeding test was performed for 89 days on male and female
rats fed 5000 ppm and 10 000 ppm diphenyl in their diet. At both
levels there was no difference in weight gain between the test animals
and pair-fed controls, but the weight gain was slower than in controls
receiving food ad lib. A study of food efficiency over 3 weeks in
groups of 12 male and 12 female rats receiving 0 ppm, 100 ppm, 1000
ppm and 10 000 ppm diphenyl in their diet revealed no significant
differences between the groups (Ambrose et al., 1960).
Small groups of rats were fed diets containing 0, 1000 or 5000 ppm
diphenyl for 11 or 60 days before mating. No effect on reproduction
was noted (Ambrose et al., 1960).
A group of 13 rats was fed 0, 50 or 100 mg diphenyl per animal daily
and killed 2-13 months after the beginning of the treatment.
Regressive changes were noticed in the liver and kidney. Three animals
observed after 7 weeks, 7 months and 1 year had, respectively,
papillomatous hyperplasia of the forestomach, multiple papillomas of
the stomach and a squamous cell carcinoma of the stomach. The
incidence of lesions of the forestomach in untreated rats was not
given (Pecchiai & Saffiotti, 1957).
Rabbit. Five rabbits were given doses of 1000 mg/kg diphenyl by
stomach tube as a 25 per cent solution in olive oil, 2-3 times weekly.
All the animals lost weight and died within 5-20 weeks. Retention of
urea was found in the terminal stages (Deichmann et al., 1947).
Dog. Groups of 3 dogs received diphenyl in corn oil orally at doses
of 0, 2.5 and 25 mg/kg 5 times weekly for 52 weeks. At 3-month
intervals, blood and urine samples were normal. At the end of the
experiment all the animals were killed and no pathological changes
attributable to diphenyl were found (Hazleton et al., 1956).
Monkey. Four groups of 2 female and 1 male rhesus monkeys were given
diets containing 0, 100, 1000 and 10 000 ppm diphenyl for 1 year. No
changes in the body-weight and blood were seen. At the 10 000 ppm
level, a consistent increase of liver weight without histological
change was noticed (Newell, 1953).
Man. A human volunteer was given 35 mg diphenyl weekly per os for
13 weeks without adverse effect (Farkas, 1939)
Long-term studies
Rat. Groups of rats were given diets containing 0, 100, 1000 and
10 000 ppm diphenyl for 2 years. An intercurrent respiratory infection
killed many animals in all groups. Tubular dilatation in the kidney
was noticed at the two highest concentrations; however, 2 animals of
24 of the control group showed similar changes (Newell, 1953).
Eight groups of 15 weanling animals of each sex were given diets
containing 0, 10, 50, 100, 500, 1000, 5000 and 10 000 ppm diphenyl. At
5000 and 10 000 ppm there was growth inhibition, decrease in
longevity, decreased haemoglobin and pathological changes in the
kidneys at autopsy, including scarring, inflammation and tubular
atrophy. These changes were not noticed at concentrations of 1000 ppm
or less. The reversibility of the urinary changes induced by 5000 ppm
diphenyl in the diet for 4 months has been demonstrated (Ambrose et
al., 1960; Booth et al., 1961).
A 4-generation reproduction study was carried out on rats given doses
of 0, 100, 1000 and 10 000 ppm diphenyl. Only at 10 000 ppm fertility
and litter size were affected. This has been attributed to a lowered
food intake rather than to the direct effect of diphenyl (Newell,
1953).
Comments
Diphenyl appears to have been studied mostly in dogs and rats, and
special aspects have been investigated in other species. Long-term
studies have been carried out only in rats. The suspicion of
carcinogenicity raised by a study carried out under inadequate
conditions was not confirmed in later reports. In vivo and in
vitro studies in several species including man, reveal a common
detoxification mechanism.
Long-term feeding studies including recording or tumour incidence,
information on possible chemical changes undergone by diphenyl on the
fruit, and specifications of purity are desired.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 500 ppm in the diet, equivalent to 25 mg/kg/day
Dog: 25 mg/kg/day
Monkey: 50 mg/kg/day
Estimate of acceptable daily intake for man
Since children and ill people may consume a high amount of citrus
fruit and diphenyl exerts a toxic effect on the kidney, a higher
safety factor than usual has been used.
0-0.125 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use Pattern
(a) Pre-harvest treatments
The meeting was unaware of any pre-harvest uses of diphenyl.
(b) Post-harvest treatments
Diphenyl is used exclusively as a fungistatic agent on citrus fruits
during transportation and storage. Its superiority for this purpose
has displaced the use of other compounds in major citrus producing and
exporting countries. These include Australia, Israel, South Africa and
the United States of America. Recently, use in southern Italy has also
been reported. Comprehensive bibliographies covering the history of
development and use in those countries has been prepared by Resnick
(1966) and Rajzman (1965). Treatment of the packaging materials for
citrus is used rather than direct treatment of the fruit. Two methods
are employed.
(1) In Australia, Israel and South Africa, the fruit are wrapped
individually with tissue paper impregnated with diphenyl at from
28 to 40 mg per 100 square inches.
(2) In the USA a collective method is used: paper pads each of about
28 × 44 cm and containing 2.35 gm of diphenyl are placed at the
rate of one pad on the bottom and one on top of a 40 pound
(18 kg) carton of fruit (i.e. about 47 mg diphenyl per individual
180-200 gm fruit).
The fungistatic action depends on providing diphenyl vapour around the
fruit. It is not effective as a fungistat after being absorbed by the
fruit. Hence, the necessity of maintaining a reservoir in packaging
material throughout the period of transport and storage. The
technology of use, in relation to conditions of transport and storage,
distance of shipment and other parameters that control the treatment
have been studied intensively in the USA (Rygg, Wilson & Garber, 1962;
Klotz, L. J., 1957; and Rygg, Wells, Norman & Atrops, 1962), in
Australia (Kiely & Long, 1960), in Israel (Farkas & Aman, 1940;
Littauer & Mintz, 1945; Rajzman, 1961; Rajzman, 1961(a); and Rajzman,
1961(b)) and in South Africa (Christ, 1962) (see also Rajzman, 1965).
The current technology of use has evolved essentially from these
investigations.
(c) Other uses
No other uses have been reported.
National Tolerances
SUMMARY OF LEGAL AND OTHER TOLERANCES FOR CITRUS
Country Tolerance ppm Comment
USA 110
Canada 110
United Kingdom 100
Sweden 100
Belgium 70)
France 70) Subject to review
Federal Republic of Germany 70) in EEC countries
The Netherlands 30)
Residues resulting from supervised trials
The factors controlling the uptake and retention of diphenyl in fruit
have been intensively studied and the literature is reviewed by
Rajzman (1965) and by Resnick (1966). An earlier compilation of the
results of several years of supervised trials was made by Hazleton
(1956). These trials included fruit from the principal citrus
producing areas of the USA, treated and analysed in accordance with
the procedures at that time. Oranges, lemons and grapefruit had a
maximum of 110 ppm, 70 ppm and 30 ppm respectively, but only 10 per
cent of the samples contained more than 60 ppm, 40 ppm and 20 ppm
respectively. In reviewing such data it should be borne in mind that
in the light of later developments in analysis, it is probable that
the residues then reported were probably below the amounts which
actually were present when the analyses were carried out. Rajzman
(1965) reviewed the data obtained between 1945 and 1961 and the
residues found in whole fruit in various countries are summarized as
follows;
Number of More than More than More than
samples 70 ppm 100 ppm 110 ppm
examined
Found % Found % Found %
Oranges 470 32 6.6 9 1.9 6 1.3
Lemons 208 6 3.0 2 1.0 1 0.5
Grapefruit 59 0 0 0 0 0 0
Residues in food moving in commerce
Although much information has been obtained on residues in citrus
moving in commerce, in some cases information on the influence of the
method of sampling or of analysis was lacking. Some of the earlier
results are therefore due to unintentional variations in sampling and
analytical procedures.
Rajzman (1966) compiled a survey of diphenyl residues found in Israel
fruit of different varieties from 1958 to 1965. The fruits were
treated according to current commercial practice and stored under
varying conditions simulating transport and storage of exported fruit.
Residues were determined in the peel, pulp and whole fruit of 2093
samples of oranges, grapefruit and lemons. Fruit variety had no effect
on the quantity or distribution of the diphenyl absorbed. Peel content
varied from 1.0 to 400 ppm. Pulp residues were very low, varying from
0.1 to 0.91 ppm. Whole fruit residues varied from 0.9 ppm to 115.3 ppm
as follows:
More than 30 ppm - 28.77 per cent
" " 50 ppm - 8.0 per cent
" " 70 ppm - 2.01 per cent
" " 100 ppm - 0.10 per cent
" " 110 ppm - 0.05 per cent
Souci & Maier-Haarlander (1961) reported on residues in nine samples
of citrus fruit imported into Germany from USA, Italy and Cyprus in
1961. Only one sample (Italian) contained residues in excess of 100
ppm on a whole fruit basis (103 ppm). Rajzman (1965) gives figures for
citrus imported into Germany in 1959-1965 as follows:
Number of samples % of samples protected
with diphenyl
Year Total Without With 30 70 100 >100
diphenyl diphenyl ppm ppm ppm ppm
1959-61 149 0 149 59 95 97 3
1959-61 100 46 54 36 76 88 12
1961-65 132 83 49 29 68 87 13
1960-65 13 0 13 46 92 92 8
1964-65 17 0 17 29 88 100 0
1965 20 0 20 30 75 85 15
Total 431 129 302 48 88 94 6
1959-65
The meeting was aware that work, on which they have not received
detailed results, had been completed or was in progress in various
countries on the residues which are occurring in fruits received in
the course of commercial practice. From the published information,
however, it was concluded that the majority of samples of citrus fruit
moving in commerce in recent years have contained below 100 ppm; but
in a small per cent of cases residues up to 110 ppm have been
detected.
Residues at consumption (Effects of processing, cooking, etc.)
(a) Pulp and peel
Residues are mainly localised in the oil glands of citrus immediately
beneath the fruit surface. The peel itself may contain up to several
hundred ppm but the pulp contains amounts up to about 1.0 ppm. In
relation to the original amount of residue present in peel, lemons
contain more residue in pulp than oranges or grapefruit. Even in the
case of lemons, the residue in the pulp does not exceed 2.1 per cent
of the original residue in whole fruit (0.50 ppm). In oranges
containing as high as 400 ppm in the peel only, the pulp content never
exceeded 0.91 ppm (Rajzman, 1966).
(b) Juice
Hazleton (1956) compared residues resulting from extraction of citrus
juice by hand and by mechanical means. (A digest of these data are
also contained in Resnick, 1966). Mechanical extraction of juice
resulted in higher residues, but these did not exceed 4.3 ppm in
orange juice in two samples out of 284 for which the average was 1.15
ppm. This compares with a maximum of 2.5 ppm in one sample of orange
juice pressed by hand, and an average of 0.54 ppm for the 221 samples.
Grapefruit juice contained 0.26 ppm and 0.66 ppm by hand and
mechanical extraction respectively. Mechanically extracted lemon juice
contained the highest residues, ranging from 1.0 to 11.2 ppm,
averaging 3.3 ppm for 71 samples. Newell (1953) sealed diphenyl
protected fruit in wooden boxes for a period of eight weeks to attempt
to produce maximum absorption into fruit. The residues resulting in
orange, grapefruit and lemon juice were 1.3 ppm, 1.1 ppm and 0.4 ppm
respectively. Canadian Food and Drug Directorate (1965) examined
residues in juice from 284 samples of oranges. Forty-five per cent
showed less than 1.0 ppm in the juice, while for the balance only
eight were in excess of 2.5 ppm, with two values grouped at
approximately 4.4 ppm.
(c) Marmalades and jams
Diphenyl residues in citrus peel that may be used for the manufacture
of marmalades and jams are substantially reduced during the mincing of
peel and fruit and the cooking process. Losses of residue by these
processes has been reported by Tomkins & Isherwood (1945), Dickey
(1956), Rajzman (1962) and Souci & Maier-Haarlander (1963). The
elimination of residue is directly related to the degree of chopping
before cooking and whether whole fruit or peel only is used.
Elimination during these processes ranged from 18.6 per cent to 100
per cent depending on the method of preparation. In one case 23.1 ppm
remained in marmalade after cooking, while in most other cases the
remaining residues ranged between 0.0 and 16.8 ppm, with the majority
between 0 and 12 ppm.
Methods of residue analysis
(a) Analysis
Rajzman (1965) reviewed the methods of analysis up until that time.
The two most promising new methods that offered reliability,
reproducibility and speed are: (1) thin layer chromatography followed
by spectrophotometric determination of the TLC spots at 248 mµ
(Norman, Rygg & Wells, 1966) and (2) a gas-liquid chromatography
method based on the methods of Vogel & Deshusses (1964, 1965).
Both methods are currently under study. A collaborative study has been
conducted by five government laboratories in the member countries of
E.E.C. A comparative study of both methods is also in progress at the
Centraal Instituut voor Voedingsonderzoek, TNO, Zeist, the
Netherlands. A progress report from this institute by Vos (1966) was
available to the FAO Working Party on Pesticide Residues. The
conclusion drawn by Vos is as follows:
"The gas chromatographic method is the fastest and most accurate one.
The average value found agrees with that obtained from the thin layer
method. The values of the latter show a larger spreading. It is
possible that the biphenyl zone on the plates still contains some
interfering substances.
Although many other methods for the determination of biphenyl in
citrus fruit have been described in the literature, the G.L.C. - and
thin layer method were selected to be tested in practice. They seemed
to be most suitable for our purposes, viz. routine determination in
large number of samples.
We certainly can recommend the gas chromatographic determination for
this purpose.
If the relatively expensive equipment for gas chromatography should be
a serious objection, we recommend the thin-layer method. Although it
is slightly less accurate, it is also suitable for routine
determinations."
One further refinement is now available in the development of a
totally automated analytical procedure by Gunther & Ott (1966) for
citrus fruit rind employing a continuous flow recording
spectrophotometer.
The F.A.O. Working Party considered that, at the level of the
suggested tolerance, both the thin layer and the gas-liquid
chromatographic methods would be suitable for residue analysis.
Nevertheless it was decided to delay making a firm recommendation
until full reports were available on the investigations currently in
progress in the E.E.C. and T.N.O./Zeist.
(b) Sampling
It is understood that work is also in progress at the TNO, C.I.V.O.
Institute, Zeist, to explore the subject of sampling. In the meantime,
attention is drawn to the ISO Fifth Draft Proposal: Sampling of fresh
fruits and vegetables. ISO/TC 34-Agric. Food Products, Subcommittee 3
- Fruits, Vegetables and Their Derived Products, Working Group 2
- Sampling, October, 1965.
RECOMMENDATION FOR TOLERANCES
As diphenyl is not used on other foods the entire acceptable daily
intake could be assigned to citrus or citrus products. Although no
international agreement has been reached on the amount of citrus and
citrus products in the "high consumption diet", a combined figure of
230 grams per day for fresh citrus plus 60 grams per day for canned
citrus (i.e. a maximum of 290 grams per day) has been suggested. (In
Canada the suggested level of food consumption for these products for
tolerance purposes is 190 grams.)
If the acceptable daily intake level were 0.125 mg/kg for a 60 kg
person the permissible level from all sources becomes 7.5 mg per day.
Evidence has been received to demonstrate the need for the use of
diphenyl in the storage and transport of citrus over long distances.
The residues resulting in the whole fruit only rarely exceed 110 ppm.
In fresh fruit, pulp as eaten by the consumer rarely exceed 1.0 ppm.
Juices in extreme cases may contain residues as high as 4.4 ppm, but
most cases will contain less than 2.0 ppm. Marmalade and jam may also
contain some residues, but these may become insignificant in most
diets. Even in the cases of small children consuming large amounts of
orange juice, the amounts of diphenyl taken would be well below
acceptable daily intake.
Therefore, a tolerance of 110 ppm is recommended to be applied at the
point of entry of the citrus into the country.
Further information
(1) Data are desired on the residues that may result from methods of
preparation of fruit juice which employ the whole of the fruit
including the peel.
(2) A specification for diphenyl for use as a fungistatic agent on
citrus seems desirable. The Working Party would like to receive
further information on the likely impurities in commercial diphenyl.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Ambrose, A. M., Booth, A. N., DeEds, F. & Cox, A. J., jr (1960) Food
Res., 25, 328
Creaven, P. J. & Williams, R. T. (1963) Biochem. J., 87, 19 P
Deichmann, W. B., Kitzmiller, K. V., Dierker, M. & Witherup, S. (1947)
J. Industr. Hyg. Toxicol., 29, 1
Farkas, A. (1939) Hadar, 12, 227
Hazleton, L. W., Kundzins, W., Howard, J. W. & Johnston, C. D. (1956)
Abstract XXth International Physiol. Congress, p. 412
McEwen, M. (1958) Monsanto Chemical Co. Tech. Publ. AT-1
MacIntosh, F. C. (1945) Analyst, 70, 334
Newell, G. W. (1953) Unpublished report of the Stanford Research
Institute
Pecchiai, L. & Saffiotti, U. (1957) Med. Lavoro, 48, 247
Rogliani, E. & Procaccini, S. (1956) Biochim. appl., 3, 193
Selle, W. A. (1952, 1953, 1954) Unpublished reports
West, H. D. (1940) Proc. Soc. exp. Biol. (N.Y.), 43, 573
West, H. D. & Jefferson, N. C. (1942), J. Nutr., 23, 425
West, H. D., Lawson, J. R., Miller, I. H. & Mathura, R. (1956) Arch.
Biochem., 60, 14
Williams, R. T. (1965) Ann. Biol. Clin. (Paris), 23, 7
REFERENCES PERTINENT TO AGRICULTURAL DATA
Canada Food and Drug Directorate (1965), Diphenyl (Biphenyl). Summary
of data dealing with the technological justification of its use in the
food products as an antimicrobial in Canada. Submitted to FAO/WHO
Codex Alimentarius Committee on Food Additives, The Hague, May, 1965
Christ, R. A. (1962) Annual Report 1961. South-African Co-operative
Citrus Exchange. L.T.D., Pretoria No. C 131/1962
Dickey, E. E. (1956) The biphenyl content of citrus marmalade prepared
from consumer-type, biphenyl-treated fruit. Inst. of Paper Chemistry,
Project 1108-7-4, March 22, 1956
Farkas, A. & Aman, J. (1940) The action of diphenyl on Penicillium and
Diplodia molds. Palest. J. Bot. Jerusalem, 2: 38-45
Gunther, F. A. & Ott, D. R. (1966) Rapid automated determination of
biphenyl in citrus fruit rind. Analyst 91:475-481
Hazleton, L. W. (1956) Report of investigations on diphenyl. Part D.
Results of tests on the amount of residue remaining in citrus fruit
after economic use of biphenyl. Hazleton Laboratories Report, Falls
Church, Va.
Kiely, T.B. & Long, J. K. (1960) Market diseases of citrus fruit. Agr.
Gaz. N.S. Wales, 71: 132
Klots, L. J. (1957) Control of decay of citrus fruit in cartons.
Citrus Leaves, 37(4): 6
Littauer, F. S. & Mintz, G. (1945) Citrus wastage investigation,
Report for 1937-1945. Citrus Control Board, Dept. of Hort., Govt. of
Palestine
Norman, S., Rygg, G. L. & Wells A. W. (1966) Improved cleanup method
for determination of biphenyl in citrus fruits and in
biphenyl-impregnated kraft papers by thin layer chromotography.
J.A.O.A.C. 49: 590-595
Rajzman, A. (1961) The diphenyl residue in citrus fruit. National
Univ. Inst. Agr. Rehovot (Israel). Report No. 332
Rajzman, A. (1961a) Diphenyl absorption by citrus fruit wrapped in
plain wraps and stored together with diphenyl-wrapped fruit. Israel J.
Agr. Res. 11: 137
Rajzman, A. (1961b) Effect of washing and Waxing of oranges upon their
diphenyl. absorption. Bull. Res. Council Israel, 10c, 131
Rajzman, A. (1962) Elimination du diphényle des écorces d'agrumes.
Ann. Nutr. Alim. 15: 239-254
Rajzman, A. (1965) Les résidus de biphényle dans les agrumes. Residue
Reviews 8; 1-73
Rajzman, A. (1966) Biphenyl residues found in citrus fruit in Israel
during the citrus season 1958/59 to 1964/65. Volcani Institute of
Agric. Res. National and Univ. Inst. of Agriculture, Rehovot, Pamphlet
105, 29 pp., 24 tables
Resnick, C. (1966) Biphenyl, technological justification of its use as
a citrus protectant. Prepared on behalf of the Government of Israel
for submission to the FAO Working Party on Pesticide Residues.
Rygg, G. L., Wilson, G. W. & Garber, M. J. (1961) Effect of biphenyl
treatment and carton ventilation on decay and spoilage of California
lemons in overseas shipments. U.S. Dept. Agric. Agr. Marketing Service
Rept. No. 500
Rygg, G. L., Wells, A. W., Norman, S. M. & Atrops, E. P. (1962)
Biphenyl control, of lemon spoilage. Influence of time, temperature
and carton venting, U.S.D.A., Agr. Marketing Service Rept. No. 569
Souci, S.W. (1959) Die Behandlung von Citrus-Früchten. In
Lebensmittelforschung und Fremdstoffprobleme in U.S.A. München: Dtsch.
Forschungsanstalt-Für Lebensmittelchem, 5.46
Souci, S.W. & Maier-Haarlander, G. (1961) Work Report, Research on
Diphenyl-content of Citrus Fruit, to Industry Committee on Citrus
Additives, Re German Project No. 50501-31 (copy submitted to FAO and
WHO)
Souci, S.W. & Maier-Haarlander, G. (1963) Untersuchungen zur Analytik
des Diphenyls I. Mitteilung. Lebensm. Untersuch. U. Forsch. 119: 217
Tomkins, P. C. & Isherwood, F. A. (1945) The absorption of diphenyl
and O-phenyl-phenol by oranges. Analyst 70: 330-333.
Vogel, J. & Deshusses, J. (1964) Une methode de dosage du diphényle
dans les agrumes par chromatographie en phase gazeuse. Mitt. Gebiete
Lebensm. Hyg. 553 84-92
Vogel, J. & Deshusses, J. (1965) Modification de la methode de dosage
du diphenyle dans les agrumes par chromtographie en phase gazeuse.
Mitt. Gebiete Lebensm. Hyg. 56: 185.
de Vos, R. H. (1966) Determination of biphenyl in citrus fruit,
Progress report No. 1. Report No. R2155a, Centraal Instituut voor
Voedingsonderzoek, T.N.O., Zeist, April, 1966.
See Also:
Toxicological Abbreviations
Diphenyl (FAO Nutrition Meetings Report Series 38a)
DIPHENYL (JECFA Evaluation)
Diphenyl (FAO/PL:1967/M/11/1)
 BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
Metabolic studies following oral administration of diphenyl have been
performed in rats (West, 1940; West et al., 1956), rabbits (Deichmann
et al., 1947; West et al., 1956; Block & Cornish, 1959) and dogs
(Hazleton et al., 1956). In these species a variety of phenolic
compounds were excreted in the urine, mostly as ethereal sulfates and
glucuronides. The main metabolite appeared to be 4-hydroxydiphenyl.
Unchanged diphenyl was excreted in the faeces only after the highest
doses (Hazleton et al., 1956).
In vitro 4-hydroxylation of diphenyl by liver microsomal enzymes has
been demonstrated in several species, including man. In addition,
2-hydroxylation was observed in adult cats, mice, hamsters, coypu and
in young but not in adult rats. The extent of 2-hydroxylation in young
rats is markedly increased in rats pre-treated in vivo with either
3,4 benzpyrene or 20-methyl-cholanthrene and to a much lesser extent
in rats pre-treated with phenobarbitone and nikethamide.
4-hydroxylation was stimulated by pre-treatment with phenobarbitone or
nikethamide but not by benzpyrene or methylcholanthrene (Creaven &
Williams, 1965; Williams, 1965).
Diphenyl was found to be less toxic when given with a diet containing
a supplement of 1-cystine or d1-methionine, but diphenyl is not highly
conjugated with cystine (West, 1940).
Acute toxicity
Animal Route LD50 Reference
mg/kg body-weight
Rat Oral 3300-5000 Deichmann et al., 1947;
Pecchiai & Safflotti, 1957
Rabbit Oral 2400 Deichmann et al., 1947
Cat Oral >2600 McEwen, 1958
Short-term studies
Mouse. Skin applications of a 23 per cent solution in oil were given
twice weekly to a group of 140 mice for 7 months. Local inflammatory
changes were commonly seen. No tumours developed (Selle, 1952; 1953;
1954).
Rat. Groups of 11 young rats were fed for 32 days either a diet low
in casein containing 10 000 ppm diphenyl or a normal diet containing
3000 ppm diphenyl. Growth retardation was observed in both experiments
(West, 1940; West & Jefferson, 1942). Daily oral doses of 0, 2, 20 and
200 mg/kg for 4 weeks and 300 mg/kg for 12 days did not induce growth
retardation (MacIntosh, 1945; Rogliani & Procaccini, 1956).
In another study, groups of young rats were fed diets containing 0,
100 and 1000 ppm diphenyl for 3 months. Growth rate, food efficiency,
organ weights and histology were identical in all groups. At 1000 ppm
only a slight polyuria was noted (Newell, 1953).
A paired feeding test was performed for 89 days on male and female
rats fed 5000 ppm and 10 000 ppm diphenyl in their diet. At both
levels there was no difference in weight gain between the test animals
and pair-fed controls, but the weight gain was slower than in controls
receiving food ad lib. A study of food efficiency over 3 weeks in
groups of 12 male and 12 female rats receiving 0 ppm, 100 ppm, 1000
ppm and 10 000 ppm diphenyl in their diet revealed no significant
differences between the groups (Ambrose et al., 1960).
Small groups of rats were fed diets containing 0, 1000 or 5000 ppm
diphenyl for 11 or 60 days before mating. No effect on reproduction
was noted (Ambrose et al., 1960).
A group of 13 rats was fed 0, 50 or 100 mg diphenyl per animal daily
and killed 2-13 months after the beginning of the treatment.
Regressive changes were noticed in the liver and kidney. Three animals
observed after 7 weeks, 7 months and 1 year had, respectively,
papillomatous hyperplasia of the forestomach, multiple papillomas of
the stomach and a squamous cell carcinoma of the stomach. The
incidence of lesions of the forestomach in untreated rats was not
given (Pecchiai & Saffiotti, 1957).
Rabbit. Five rabbits were given doses of 1000 mg/kg diphenyl by
stomach tube as a 25 per cent solution in olive oil, 2-3 times weekly.
All the animals lost weight and died within 5-20 weeks. Retention of
urea was found in the terminal stages (Deichmann et al., 1947).
Dog. Groups of 3 dogs received diphenyl in corn oil orally at doses
of 0, 2.5 and 25 mg/kg 5 times weekly for 52 weeks. At 3-month
intervals, blood and urine samples were normal. At the end of the
experiment all the animals were killed and no pathological changes
attributable to diphenyl were found (Hazleton et al., 1956).
Monkey. Four groups of 2 female and 1 male rhesus monkeys were given
diets containing 0, 100, 1000 and 10 000 ppm diphenyl for 1 year. No
changes in the body-weight and blood were seen. At the 10 000 ppm
level, a consistent increase of liver weight without histological
change was noticed (Newell, 1953).
Man. A human volunteer was given 35 mg diphenyl weekly per os for
13 weeks without adverse effect (Farkas, 1939)
Long-term studies
Rat. Groups of rats were given diets containing 0, 100, 1000 and
10 000 ppm diphenyl for 2 years. An intercurrent respiratory infection
killed many animals in all groups. Tubular dilatation in the kidney
was noticed at the two highest concentrations; however, 2 animals of
24 of the control group showed similar changes (Newell, 1953).
Eight groups of 15 weanling animals of each sex were given diets
containing 0, 10, 50, 100, 500, 1000, 5000 and 10 000 ppm diphenyl. At
5000 and 10 000 ppm there was growth inhibition, decrease in
longevity, decreased haemoglobin and pathological changes in the
kidneys at autopsy, including scarring, inflammation and tubular
atrophy. These changes were not noticed at concentrations of 1000 ppm
or less. The reversibility of the urinary changes induced by 5000 ppm
diphenyl in the diet for 4 months has been demonstrated (Ambrose et
al., 1960; Booth et al., 1961).
A 4-generation reproduction study was carried out on rats given doses
of 0, 100, 1000 and 10 000 ppm diphenyl. Only at 10 000 ppm fertility
and litter size were affected. This has been attributed to a lowered
food intake rather than to the direct effect of diphenyl (Newell,
1953).
Comments
Diphenyl appears to have been studied mostly in dogs and rats, and
special aspects have been investigated in other species. Long-term
studies have been carried out only in rats. The suspicion of
carcinogenicity raised by a study carried out under inadequate
conditions was not confirmed in later reports. In vivo and in
vitro studies in several species including man, reveal a common
detoxification mechanism.
Long-term feeding studies including recording or tumour incidence,
information on possible chemical changes undergone by diphenyl on the
fruit, and specifications of purity are desired.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 500 ppm in the diet, equivalent to 25 mg/kg/day
Dog: 25 mg/kg/day
Monkey: 50 mg/kg/day
Estimate of acceptable daily intake for man
Since children and ill people may consume a high amount of citrus
fruit and diphenyl exerts a toxic effect on the kidney, a higher
safety factor than usual has been used.
0-0.125 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use Pattern
(a) Pre-harvest treatments
The meeting was unaware of any pre-harvest uses of diphenyl.
(b) Post-harvest treatments
Diphenyl is used exclusively as a fungistatic agent on citrus fruits
during transportation and storage. Its superiority for this purpose
has displaced the use of other compounds in major citrus producing and
exporting countries. These include Australia, Israel, South Africa and
the United States of America. Recently, use in southern Italy has also
been reported. Comprehensive bibliographies covering the history of
development and use in those countries has been prepared by Resnick
(1966) and Rajzman (1965). Treatment of the packaging materials for
citrus is used rather than direct treatment of the fruit. Two methods
are employed.
(1) In Australia, Israel and South Africa, the fruit are wrapped
individually with tissue paper impregnated with diphenyl at from
28 to 40 mg per 100 square inches.
(2) In the USA a collective method is used: paper pads each of about
28 × 44 cm and containing 2.35 gm of diphenyl are placed at the
rate of one pad on the bottom and one on top of a 40 pound
(18 kg) carton of fruit (i.e. about 47 mg diphenyl per individual
180-200 gm fruit).
The fungistatic action depends on providing diphenyl vapour around the
fruit. It is not effective as a fungistat after being absorbed by the
fruit. Hence, the necessity of maintaining a reservoir in packaging
material throughout the period of transport and storage. The
technology of use, in relation to conditions of transport and storage,
distance of shipment and other parameters that control the treatment
have been studied intensively in the USA (Rygg, Wilson & Garber, 1962;
Klotz, L. J., 1957; and Rygg, Wells, Norman & Atrops, 1962), in
Australia (Kiely & Long, 1960), in Israel (Farkas & Aman, 1940;
Littauer & Mintz, 1945; Rajzman, 1961; Rajzman, 1961(a); and Rajzman,
1961(b)) and in South Africa (Christ, 1962) (see also Rajzman, 1965).
The current technology of use has evolved essentially from these
investigations.
(c) Other uses
No other uses have been reported.
National Tolerances
SUMMARY OF LEGAL AND OTHER TOLERANCES FOR CITRUS
Country Tolerance ppm Comment
USA 110
Canada 110
United Kingdom 100
Sweden 100
Belgium 70)
France 70) Subject to review
Federal Republic of Germany 70) in EEC countries
The Netherlands 30)
Residues resulting from supervised trials
The factors controlling the uptake and retention of diphenyl in fruit
have been intensively studied and the literature is reviewed by
Rajzman (1965) and by Resnick (1966). An earlier compilation of the
results of several years of supervised trials was made by Hazleton
(1956). These trials included fruit from the principal citrus
producing areas of the USA, treated and analysed in accordance with
the procedures at that time. Oranges, lemons and grapefruit had a
maximum of 110 ppm, 70 ppm and 30 ppm respectively, but only 10 per
cent of the samples contained more than 60 ppm, 40 ppm and 20 ppm
respectively. In reviewing such data it should be borne in mind that
in the light of later developments in analysis, it is probable that
the residues then reported were probably below the amounts which
actually were present when the analyses were carried out. Rajzman
(1965) reviewed the data obtained between 1945 and 1961 and the
residues found in whole fruit in various countries are summarized as
follows;
Number of More than More than More than
samples 70 ppm 100 ppm 110 ppm
examined
Found % Found % Found %
Oranges 470 32 6.6 9 1.9 6 1.3
Lemons 208 6 3.0 2 1.0 1 0.5
Grapefruit 59 0 0 0 0 0 0
Residues in food moving in commerce
Although much information has been obtained on residues in citrus
moving in commerce, in some cases information on the influence of the
method of sampling or of analysis was lacking. Some of the earlier
results are therefore due to unintentional variations in sampling and
analytical procedures.
Rajzman (1966) compiled a survey of diphenyl residues found in Israel
fruit of different varieties from 1958 to 1965. The fruits were
treated according to current commercial practice and stored under
varying conditions simulating transport and storage of exported fruit.
Residues were determined in the peel, pulp and whole fruit of 2093
samples of oranges, grapefruit and lemons. Fruit variety had no effect
on the quantity or distribution of the diphenyl absorbed. Peel content
varied from 1.0 to 400 ppm. Pulp residues were very low, varying from
0.1 to 0.91 ppm. Whole fruit residues varied from 0.9 ppm to 115.3 ppm
as follows:
More than 30 ppm - 28.77 per cent
" " 50 ppm - 8.0 per cent
" " 70 ppm - 2.01 per cent
" " 100 ppm - 0.10 per cent
" " 110 ppm - 0.05 per cent
Souci & Maier-Haarlander (1961) reported on residues in nine samples
of citrus fruit imported into Germany from USA, Italy and Cyprus in
1961. Only one sample (Italian) contained residues in excess of 100
ppm on a whole fruit basis (103 ppm). Rajzman (1965) gives figures for
citrus imported into Germany in 1959-1965 as follows:
Number of samples % of samples protected
with diphenyl
Year Total Without With 30 70 100 >100
diphenyl diphenyl ppm ppm ppm ppm
1959-61 149 0 149 59 95 97 3
1959-61 100 46 54 36 76 88 12
1961-65 132 83 49 29 68 87 13
1960-65 13 0 13 46 92 92 8
1964-65 17 0 17 29 88 100 0
1965 20 0 20 30 75 85 15
Total 431 129 302 48 88 94 6
1959-65
The meeting was aware that work, on which they have not received
detailed results, had been completed or was in progress in various
countries on the residues which are occurring in fruits received in
the course of commercial practice. From the published information,
however, it was concluded that the majority of samples of citrus fruit
moving in commerce in recent years have contained below 100 ppm; but
in a small per cent of cases residues up to 110 ppm have been
detected.
Residues at consumption (Effects of processing, cooking, etc.)
(a) Pulp and peel
Residues are mainly localised in the oil glands of citrus immediately
beneath the fruit surface. The peel itself may contain up to several
hundred ppm but the pulp contains amounts up to about 1.0 ppm. In
relation to the original amount of residue present in peel, lemons
contain more residue in pulp than oranges or grapefruit. Even in the
case of lemons, the residue in the pulp does not exceed 2.1 per cent
of the original residue in whole fruit (0.50 ppm). In oranges
containing as high as 400 ppm in the peel only, the pulp content never
exceeded 0.91 ppm (Rajzman, 1966).
(b) Juice
Hazleton (1956) compared residues resulting from extraction of citrus
juice by hand and by mechanical means. (A digest of these data are
also contained in Resnick, 1966). Mechanical extraction of juice
resulted in higher residues, but these did not exceed 4.3 ppm in
orange juice in two samples out of 284 for which the average was 1.15
ppm. This compares with a maximum of 2.5 ppm in one sample of orange
juice pressed by hand, and an average of 0.54 ppm for the 221 samples.
Grapefruit juice contained 0.26 ppm and 0.66 ppm by hand and
mechanical extraction respectively. Mechanically extracted lemon juice
contained the highest residues, ranging from 1.0 to 11.2 ppm,
averaging 3.3 ppm for 71 samples. Newell (1953) sealed diphenyl
protected fruit in wooden boxes for a period of eight weeks to attempt
to produce maximum absorption into fruit. The residues resulting in
orange, grapefruit and lemon juice were 1.3 ppm, 1.1 ppm and 0.4 ppm
respectively. Canadian Food and Drug Directorate (1965) examined
residues in juice from 284 samples of oranges. Forty-five per cent
showed less than 1.0 ppm in the juice, while for the balance only
eight were in excess of 2.5 ppm, with two values grouped at
approximately 4.4 ppm.
(c) Marmalades and jams
Diphenyl residues in citrus peel that may be used for the manufacture
of marmalades and jams are substantially reduced during the mincing of
peel and fruit and the cooking process. Losses of residue by these
processes has been reported by Tomkins & Isherwood (1945), Dickey
(1956), Rajzman (1962) and Souci & Maier-Haarlander (1963). The
elimination of residue is directly related to the degree of chopping
before cooking and whether whole fruit or peel only is used.
Elimination during these processes ranged from 18.6 per cent to 100
per cent depending on the method of preparation. In one case 23.1 ppm
remained in marmalade after cooking, while in most other cases the
remaining residues ranged between 0.0 and 16.8 ppm, with the majority
between 0 and 12 ppm.
Methods of residue analysis
(a) Analysis
Rajzman (1965) reviewed the methods of analysis up until that time.
The two most promising new methods that offered reliability,
reproducibility and speed are: (1) thin layer chromatography followed
by spectrophotometric determination of the TLC spots at 248 mµ
(Norman, Rygg & Wells, 1966) and (2) a gas-liquid chromatography
method based on the methods of Vogel & Deshusses (1964, 1965).
Both methods are currently under study. A collaborative study has been
conducted by five government laboratories in the member countries of
E.E.C. A comparative study of both methods is also in progress at the
Centraal Instituut voor Voedingsonderzoek, TNO, Zeist, the
Netherlands. A progress report from this institute by Vos (1966) was
available to the FAO Working Party on Pesticide Residues. The
conclusion drawn by Vos is as follows:
"The gas chromatographic method is the fastest and most accurate one.
The average value found agrees with that obtained from the thin layer
method. The values of the latter show a larger spreading. It is
possible that the biphenyl zone on the plates still contains some
interfering substances.
Although many other methods for the determination of biphenyl in
citrus fruit have been described in the literature, the G.L.C. - and
thin layer method were selected to be tested in practice. They seemed
to be most suitable for our purposes, viz. routine determination in
large number of samples.
We certainly can recommend the gas chromatographic determination for
this purpose.
If the relatively expensive equipment for gas chromatography should be
a serious objection, we recommend the thin-layer method. Although it
is slightly less accurate, it is also suitable for routine
determinations."
One further refinement is now available in the development of a
totally automated analytical procedure by Gunther & Ott (1966) for
citrus fruit rind employing a continuous flow recording
spectrophotometer.
The F.A.O. Working Party considered that, at the level of the
suggested tolerance, both the thin layer and the gas-liquid
chromatographic methods would be suitable for residue analysis.
Nevertheless it was decided to delay making a firm recommendation
until full reports were available on the investigations currently in
progress in the E.E.C. and T.N.O./Zeist.
(b) Sampling
It is understood that work is also in progress at the TNO, C.I.V.O.
Institute, Zeist, to explore the subject of sampling. In the meantime,
attention is drawn to the ISO Fifth Draft Proposal: Sampling of fresh
fruits and vegetables. ISO/TC 34-Agric. Food Products, Subcommittee 3
- Fruits, Vegetables and Their Derived Products, Working Group 2
- Sampling, October, 1965.
RECOMMENDATION FOR TOLERANCES
As diphenyl is not used on other foods the entire acceptable daily
intake could be assigned to citrus or citrus products. Although no
international agreement has been reached on the amount of citrus and
citrus products in the "high consumption diet", a combined figure of
230 grams per day for fresh citrus plus 60 grams per day for canned
citrus (i.e. a maximum of 290 grams per day) has been suggested. (In
Canada the suggested level of food consumption for these products for
tolerance purposes is 190 grams.)
If the acceptable daily intake level were 0.125 mg/kg for a 60 kg
person the permissible level from all sources becomes 7.5 mg per day.
Evidence has been received to demonstrate the need for the use of
diphenyl in the storage and transport of citrus over long distances.
The residues resulting in the whole fruit only rarely exceed 110 ppm.
In fresh fruit, pulp as eaten by the consumer rarely exceed 1.0 ppm.
Juices in extreme cases may contain residues as high as 4.4 ppm, but
most cases will contain less than 2.0 ppm. Marmalade and jam may also
contain some residues, but these may become insignificant in most
diets. Even in the cases of small children consuming large amounts of
orange juice, the amounts of diphenyl taken would be well below
acceptable daily intake.
Therefore, a tolerance of 110 ppm is recommended to be applied at the
point of entry of the citrus into the country.
Further information
(1) Data are desired on the residues that may result from methods of
preparation of fruit juice which employ the whole of the fruit
including the peel.
(2) A specification for diphenyl for use as a fungistatic agent on
citrus seems desirable. The Working Party would like to receive
further information on the likely impurities in commercial diphenyl.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Ambrose, A. M., Booth, A. N., DeEds, F. & Cox, A. J., jr (1960) Food
Res., 25, 328
Creaven, P. J. & Williams, R. T. (1963) Biochem. J., 87, 19 P
Deichmann, W. B., Kitzmiller, K. V., Dierker, M. & Witherup, S. (1947)
J. Industr. Hyg. Toxicol., 29, 1
Farkas, A. (1939) Hadar, 12, 227
Hazleton, L. W., Kundzins, W., Howard, J. W. & Johnston, C. D. (1956)
Abstract XXth International Physiol. Congress, p. 412
McEwen, M. (1958) Monsanto Chemical Co. Tech. Publ. AT-1
MacIntosh, F. C. (1945) Analyst, 70, 334
Newell, G. W. (1953) Unpublished report of the Stanford Research
Institute
Pecchiai, L. & Saffiotti, U. (1957) Med. Lavoro, 48, 247
Rogliani, E. & Procaccini, S. (1956) Biochim. appl., 3, 193
Selle, W. A. (1952, 1953, 1954) Unpublished reports
West, H. D. (1940) Proc. Soc. exp. Biol. (N.Y.), 43, 573
West, H. D. & Jefferson, N. C. (1942), J. Nutr., 23, 425
West, H. D., Lawson, J. R., Miller, I. H. & Mathura, R. (1956) Arch.
Biochem., 60, 14
Williams, R. T. (1965) Ann. Biol. Clin. (Paris), 23, 7
REFERENCES PERTINENT TO AGRICULTURAL DATA
Canada Food and Drug Directorate (1965), Diphenyl (Biphenyl). Summary
of data dealing with the technological justification of its use in the
food products as an antimicrobial in Canada. Submitted to FAO/WHO
Codex Alimentarius Committee on Food Additives, The Hague, May, 1965
Christ, R. A. (1962) Annual Report 1961. South-African Co-operative
Citrus Exchange. L.T.D., Pretoria No. C 131/1962
Dickey, E. E. (1956) The biphenyl content of citrus marmalade prepared
from consumer-type, biphenyl-treated fruit. Inst. of Paper Chemistry,
Project 1108-7-4, March 22, 1956
Farkas, A. & Aman, J. (1940) The action of diphenyl on Penicillium and
Diplodia molds. Palest. J. Bot. Jerusalem, 2: 38-45
Gunther, F. A. & Ott, D. R. (1966) Rapid automated determination of
biphenyl in citrus fruit rind. Analyst 91:475-481
Hazleton, L. W. (1956) Report of investigations on diphenyl. Part D.
Results of tests on the amount of residue remaining in citrus fruit
after economic use of biphenyl. Hazleton Laboratories Report, Falls
Church, Va.
Kiely, T.B. & Long, J. K. (1960) Market diseases of citrus fruit. Agr.
Gaz. N.S. Wales, 71: 132
Klots, L. J. (1957) Control of decay of citrus fruit in cartons.
Citrus Leaves, 37(4): 6
Littauer, F. S. & Mintz, G. (1945) Citrus wastage investigation,
Report for 1937-1945. Citrus Control Board, Dept. of Hort., Govt. of
Palestine
Norman, S., Rygg, G. L. & Wells A. W. (1966) Improved cleanup method
for determination of biphenyl in citrus fruits and in
biphenyl-impregnated kraft papers by thin layer chromotography.
J.A.O.A.C. 49: 590-595
Rajzman, A. (1961) The diphenyl residue in citrus fruit. National
Univ. Inst. Agr. Rehovot (Israel). Report No. 332
Rajzman, A. (1961a) Diphenyl absorption by citrus fruit wrapped in
plain wraps and stored together with diphenyl-wrapped fruit. Israel J.
Agr. Res. 11: 137
Rajzman, A. (1961b) Effect of washing and Waxing of oranges upon their
diphenyl. absorption. Bull. Res. Council Israel, 10c, 131
Rajzman, A. (1962) Elimination du diphényle des écorces d'agrumes.
Ann. Nutr. Alim. 15: 239-254
Rajzman, A. (1965) Les résidus de biphényle dans les agrumes. Residue
Reviews 8; 1-73
Rajzman, A. (1966) Biphenyl residues found in citrus fruit in Israel
during the citrus season 1958/59 to 1964/65. Volcani Institute of
Agric. Res. National and Univ. Inst. of Agriculture, Rehovot, Pamphlet
105, 29 pp., 24 tables
Resnick, C. (1966) Biphenyl, technological justification of its use as
a citrus protectant. Prepared on behalf of the Government of Israel
for submission to the FAO Working Party on Pesticide Residues.
Rygg, G. L., Wilson, G. W. & Garber, M. J. (1961) Effect of biphenyl
treatment and carton ventilation on decay and spoilage of California
lemons in overseas shipments. U.S. Dept. Agric. Agr. Marketing Service
Rept. No. 500
Rygg, G. L., Wells, A. W., Norman, S. M. & Atrops, E. P. (1962)
Biphenyl control, of lemon spoilage. Influence of time, temperature
and carton venting, U.S.D.A., Agr. Marketing Service Rept. No. 569
Souci, S.W. (1959) Die Behandlung von Citrus-Früchten. In
Lebensmittelforschung und Fremdstoffprobleme in U.S.A. München: Dtsch.
Forschungsanstalt-Für Lebensmittelchem, 5.46
Souci, S.W. & Maier-Haarlander, G. (1961) Work Report, Research on
Diphenyl-content of Citrus Fruit, to Industry Committee on Citrus
Additives, Re German Project No. 50501-31 (copy submitted to FAO and
WHO)
Souci, S.W. & Maier-Haarlander, G. (1963) Untersuchungen zur Analytik
des Diphenyls I. Mitteilung. Lebensm. Untersuch. U. Forsch. 119: 217
Tomkins, P. C. & Isherwood, F. A. (1945) The absorption of diphenyl
and O-phenyl-phenol by oranges. Analyst 70: 330-333.
Vogel, J. & Deshusses, J. (1964) Une methode de dosage du diphényle
dans les agrumes par chromatographie en phase gazeuse. Mitt. Gebiete
Lebensm. Hyg. 553 84-92
Vogel, J. & Deshusses, J. (1965) Modification de la methode de dosage
du diphenyle dans les agrumes par chromtographie en phase gazeuse.
Mitt. Gebiete Lebensm. Hyg. 56: 185.
de Vos, R. H. (1966) Determination of biphenyl in citrus fruit,
Progress report No. 1. Report No. R2155a, Centraal Instituut voor
Voedingsonderzoek, T.N.O., Zeist, April, 1966.
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
Metabolic studies following oral administration of diphenyl have been
performed in rats (West, 1940; West et al., 1956), rabbits (Deichmann
et al., 1947; West et al., 1956; Block & Cornish, 1959) and dogs
(Hazleton et al., 1956). In these species a variety of phenolic
compounds were excreted in the urine, mostly as ethereal sulfates and
glucuronides. The main metabolite appeared to be 4-hydroxydiphenyl.
Unchanged diphenyl was excreted in the faeces only after the highest
doses (Hazleton et al., 1956).
In vitro 4-hydroxylation of diphenyl by liver microsomal enzymes has
been demonstrated in several species, including man. In addition,
2-hydroxylation was observed in adult cats, mice, hamsters, coypu and
in young but not in adult rats. The extent of 2-hydroxylation in young
rats is markedly increased in rats pre-treated in vivo with either
3,4 benzpyrene or 20-methyl-cholanthrene and to a much lesser extent
in rats pre-treated with phenobarbitone and nikethamide.
4-hydroxylation was stimulated by pre-treatment with phenobarbitone or
nikethamide but not by benzpyrene or methylcholanthrene (Creaven &
Williams, 1965; Williams, 1965).
Diphenyl was found to be less toxic when given with a diet containing
a supplement of 1-cystine or d1-methionine, but diphenyl is not highly
conjugated with cystine (West, 1940).
Acute toxicity
Animal Route LD50 Reference
mg/kg body-weight
Rat Oral 3300-5000 Deichmann et al., 1947;
Pecchiai & Safflotti, 1957
Rabbit Oral 2400 Deichmann et al., 1947
Cat Oral >2600 McEwen, 1958
Short-term studies
Mouse. Skin applications of a 23 per cent solution in oil were given
twice weekly to a group of 140 mice for 7 months. Local inflammatory
changes were commonly seen. No tumours developed (Selle, 1952; 1953;
1954).
Rat. Groups of 11 young rats were fed for 32 days either a diet low
in casein containing 10 000 ppm diphenyl or a normal diet containing
3000 ppm diphenyl. Growth retardation was observed in both experiments
(West, 1940; West & Jefferson, 1942). Daily oral doses of 0, 2, 20 and
200 mg/kg for 4 weeks and 300 mg/kg for 12 days did not induce growth
retardation (MacIntosh, 1945; Rogliani & Procaccini, 1956).
In another study, groups of young rats were fed diets containing 0,
100 and 1000 ppm diphenyl for 3 months. Growth rate, food efficiency,
organ weights and histology were identical in all groups. At 1000 ppm
only a slight polyuria was noted (Newell, 1953).
A paired feeding test was performed for 89 days on male and female
rats fed 5000 ppm and 10 000 ppm diphenyl in their diet. At both
levels there was no difference in weight gain between the test animals
and pair-fed controls, but the weight gain was slower than in controls
receiving food ad lib. A study of food efficiency over 3 weeks in
groups of 12 male and 12 female rats receiving 0 ppm, 100 ppm, 1000
ppm and 10 000 ppm diphenyl in their diet revealed no significant
differences between the groups (Ambrose et al., 1960).
Small groups of rats were fed diets containing 0, 1000 or 5000 ppm
diphenyl for 11 or 60 days before mating. No effect on reproduction
was noted (Ambrose et al., 1960).
A group of 13 rats was fed 0, 50 or 100 mg diphenyl per animal daily
and killed 2-13 months after the beginning of the treatment.
Regressive changes were noticed in the liver and kidney. Three animals
observed after 7 weeks, 7 months and 1 year had, respectively,
papillomatous hyperplasia of the forestomach, multiple papillomas of
the stomach and a squamous cell carcinoma of the stomach. The
incidence of lesions of the forestomach in untreated rats was not
given (Pecchiai & Saffiotti, 1957).
Rabbit. Five rabbits were given doses of 1000 mg/kg diphenyl by
stomach tube as a 25 per cent solution in olive oil, 2-3 times weekly.
All the animals lost weight and died within 5-20 weeks. Retention of
urea was found in the terminal stages (Deichmann et al., 1947).
Dog. Groups of 3 dogs received diphenyl in corn oil orally at doses
of 0, 2.5 and 25 mg/kg 5 times weekly for 52 weeks. At 3-month
intervals, blood and urine samples were normal. At the end of the
experiment all the animals were killed and no pathological changes
attributable to diphenyl were found (Hazleton et al., 1956).
Monkey. Four groups of 2 female and 1 male rhesus monkeys were given
diets containing 0, 100, 1000 and 10 000 ppm diphenyl for 1 year. No
changes in the body-weight and blood were seen. At the 10 000 ppm
level, a consistent increase of liver weight without histological
change was noticed (Newell, 1953).
Man. A human volunteer was given 35 mg diphenyl weekly per os for
13 weeks without adverse effect (Farkas, 1939)
Long-term studies
Rat. Groups of rats were given diets containing 0, 100, 1000 and
10 000 ppm diphenyl for 2 years. An intercurrent respiratory infection
killed many animals in all groups. Tubular dilatation in the kidney
was noticed at the two highest concentrations; however, 2 animals of
24 of the control group showed similar changes (Newell, 1953).
Eight groups of 15 weanling animals of each sex were given diets
containing 0, 10, 50, 100, 500, 1000, 5000 and 10 000 ppm diphenyl. At
5000 and 10 000 ppm there was growth inhibition, decrease in
longevity, decreased haemoglobin and pathological changes in the
kidneys at autopsy, including scarring, inflammation and tubular
atrophy. These changes were not noticed at concentrations of 1000 ppm
or less. The reversibility of the urinary changes induced by 5000 ppm
diphenyl in the diet for 4 months has been demonstrated (Ambrose et
al., 1960; Booth et al., 1961).
A 4-generation reproduction study was carried out on rats given doses
of 0, 100, 1000 and 10 000 ppm diphenyl. Only at 10 000 ppm fertility
and litter size were affected. This has been attributed to a lowered
food intake rather than to the direct effect of diphenyl (Newell,
1953).
Comments
Diphenyl appears to have been studied mostly in dogs and rats, and
special aspects have been investigated in other species. Long-term
studies have been carried out only in rats. The suspicion of
carcinogenicity raised by a study carried out under inadequate
conditions was not confirmed in later reports. In vivo and in
vitro studies in several species including man, reveal a common
detoxification mechanism.
Long-term feeding studies including recording or tumour incidence,
information on possible chemical changes undergone by diphenyl on the
fruit, and specifications of purity are desired.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 500 ppm in the diet, equivalent to 25 mg/kg/day
Dog: 25 mg/kg/day
Monkey: 50 mg/kg/day
Estimate of acceptable daily intake for man
Since children and ill people may consume a high amount of citrus
fruit and diphenyl exerts a toxic effect on the kidney, a higher
safety factor than usual has been used.
0-0.125 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use Pattern
(a) Pre-harvest treatments
The meeting was unaware of any pre-harvest uses of diphenyl.
(b) Post-harvest treatments
Diphenyl is used exclusively as a fungistatic agent on citrus fruits
during transportation and storage. Its superiority for this purpose
has displaced the use of other compounds in major citrus producing and
exporting countries. These include Australia, Israel, South Africa and
the United States of America. Recently, use in southern Italy has also
been reported. Comprehensive bibliographies covering the history of
development and use in those countries has been prepared by Resnick
(1966) and Rajzman (1965). Treatment of the packaging materials for
citrus is used rather than direct treatment of the fruit. Two methods
are employed.
(1) In Australia, Israel and South Africa, the fruit are wrapped
individually with tissue paper impregnated with diphenyl at from
28 to 40 mg per 100 square inches.
(2) In the USA a collective method is used: paper pads each of about
28 × 44 cm and containing 2.35 gm of diphenyl are placed at the
rate of one pad on the bottom and one on top of a 40 pound
(18 kg) carton of fruit (i.e. about 47 mg diphenyl per individual
180-200 gm fruit).
The fungistatic action depends on providing diphenyl vapour around the
fruit. It is not effective as a fungistat after being absorbed by the
fruit. Hence, the necessity of maintaining a reservoir in packaging
material throughout the period of transport and storage. The
technology of use, in relation to conditions of transport and storage,
distance of shipment and other parameters that control the treatment
have been studied intensively in the USA (Rygg, Wilson & Garber, 1962;
Klotz, L. J., 1957; and Rygg, Wells, Norman & Atrops, 1962), in
Australia (Kiely & Long, 1960), in Israel (Farkas & Aman, 1940;
Littauer & Mintz, 1945; Rajzman, 1961; Rajzman, 1961(a); and Rajzman,
1961(b)) and in South Africa (Christ, 1962) (see also Rajzman, 1965).
The current technology of use has evolved essentially from these
investigations.
(c) Other uses
No other uses have been reported.
National Tolerances
SUMMARY OF LEGAL AND OTHER TOLERANCES FOR CITRUS
Country Tolerance ppm Comment
USA 110
Canada 110
United Kingdom 100
Sweden 100
Belgium 70)
France 70) Subject to review
Federal Republic of Germany 70) in EEC countries
The Netherlands 30)
Residues resulting from supervised trials
The factors controlling the uptake and retention of diphenyl in fruit
have been intensively studied and the literature is reviewed by
Rajzman (1965) and by Resnick (1966). An earlier compilation of the
results of several years of supervised trials was made by Hazleton
(1956). These trials included fruit from the principal citrus
producing areas of the USA, treated and analysed in accordance with
the procedures at that time. Oranges, lemons and grapefruit had a
maximum of 110 ppm, 70 ppm and 30 ppm respectively, but only 10 per
cent of the samples contained more than 60 ppm, 40 ppm and 20 ppm
respectively. In reviewing such data it should be borne in mind that
in the light of later developments in analysis, it is probable that
the residues then reported were probably below the amounts which
actually were present when the analyses were carried out. Rajzman
(1965) reviewed the data obtained between 1945 and 1961 and the
residues found in whole fruit in various countries are summarized as
follows;
Number of More than More than More than
samples 70 ppm 100 ppm 110 ppm
examined
Found % Found % Found %
Oranges 470 32 6.6 9 1.9 6 1.3
Lemons 208 6 3.0 2 1.0 1 0.5
Grapefruit 59 0 0 0 0 0 0
Residues in food moving in commerce
Although much information has been obtained on residues in citrus
moving in commerce, in some cases information on the influence of the
method of sampling or of analysis was lacking. Some of the earlier
results are therefore due to unintentional variations in sampling and
analytical procedures.
Rajzman (1966) compiled a survey of diphenyl residues found in Israel
fruit of different varieties from 1958 to 1965. The fruits were
treated according to current commercial practice and stored under
varying conditions simulating transport and storage of exported fruit.
Residues were determined in the peel, pulp and whole fruit of 2093
samples of oranges, grapefruit and lemons. Fruit variety had no effect
on the quantity or distribution of the diphenyl absorbed. Peel content
varied from 1.0 to 400 ppm. Pulp residues were very low, varying from
0.1 to 0.91 ppm. Whole fruit residues varied from 0.9 ppm to 115.3 ppm
as follows:
More than 30 ppm - 28.77 per cent
" " 50 ppm - 8.0 per cent
" " 70 ppm - 2.01 per cent
" " 100 ppm - 0.10 per cent
" " 110 ppm - 0.05 per cent
Souci & Maier-Haarlander (1961) reported on residues in nine samples
of citrus fruit imported into Germany from USA, Italy and Cyprus in
1961. Only one sample (Italian) contained residues in excess of 100
ppm on a whole fruit basis (103 ppm). Rajzman (1965) gives figures for
citrus imported into Germany in 1959-1965 as follows:
Number of samples % of samples protected
with diphenyl
Year Total Without With 30 70 100 >100
diphenyl diphenyl ppm ppm ppm ppm
1959-61 149 0 149 59 95 97 3
1959-61 100 46 54 36 76 88 12
1961-65 132 83 49 29 68 87 13
1960-65 13 0 13 46 92 92 8
1964-65 17 0 17 29 88 100 0
1965 20 0 20 30 75 85 15
Total 431 129 302 48 88 94 6
1959-65
The meeting was aware that work, on which they have not received
detailed results, had been completed or was in progress in various
countries on the residues which are occurring in fruits received in
the course of commercial practice. From the published information,
however, it was concluded that the majority of samples of citrus fruit
moving in commerce in recent years have contained below 100 ppm; but
in a small per cent of cases residues up to 110 ppm have been
detected.
Residues at consumption (Effects of processing, cooking, etc.)
(a) Pulp and peel
Residues are mainly localised in the oil glands of citrus immediately
beneath the fruit surface. The peel itself may contain up to several
hundred ppm but the pulp contains amounts up to about 1.0 ppm. In
relation to the original amount of residue present in peel, lemons
contain more residue in pulp than oranges or grapefruit. Even in the
case of lemons, the residue in the pulp does not exceed 2.1 per cent
of the original residue in whole fruit (0.50 ppm). In oranges
containing as high as 400 ppm in the peel only, the pulp content never
exceeded 0.91 ppm (Rajzman, 1966).
(b) Juice
Hazleton (1956) compared residues resulting from extraction of citrus
juice by hand and by mechanical means. (A digest of these data are
also contained in Resnick, 1966). Mechanical extraction of juice
resulted in higher residues, but these did not exceed 4.3 ppm in
orange juice in two samples out of 284 for which the average was 1.15
ppm. This compares with a maximum of 2.5 ppm in one sample of orange
juice pressed by hand, and an average of 0.54 ppm for the 221 samples.
Grapefruit juice contained 0.26 ppm and 0.66 ppm by hand and
mechanical extraction respectively. Mechanically extracted lemon juice
contained the highest residues, ranging from 1.0 to 11.2 ppm,
averaging 3.3 ppm for 71 samples. Newell (1953) sealed diphenyl
protected fruit in wooden boxes for a period of eight weeks to attempt
to produce maximum absorption into fruit. The residues resulting in
orange, grapefruit and lemon juice were 1.3 ppm, 1.1 ppm and 0.4 ppm
respectively. Canadian Food and Drug Directorate (1965) examined
residues in juice from 284 samples of oranges. Forty-five per cent
showed less than 1.0 ppm in the juice, while for the balance only
eight were in excess of 2.5 ppm, with two values grouped at
approximately 4.4 ppm.
(c) Marmalades and jams
Diphenyl residues in citrus peel that may be used for the manufacture
of marmalades and jams are substantially reduced during the mincing of
peel and fruit and the cooking process. Losses of residue by these
processes has been reported by Tomkins & Isherwood (1945), Dickey
(1956), Rajzman (1962) and Souci & Maier-Haarlander (1963). The
elimination of residue is directly related to the degree of chopping
before cooking and whether whole fruit or peel only is used.
Elimination during these processes ranged from 18.6 per cent to 100
per cent depending on the method of preparation. In one case 23.1 ppm
remained in marmalade after cooking, while in most other cases the
remaining residues ranged between 0.0 and 16.8 ppm, with the majority
between 0 and 12 ppm.
Methods of residue analysis
(a) Analysis
Rajzman (1965) reviewed the methods of analysis up until that time.
The two most promising new methods that offered reliability,
reproducibility and speed are: (1) thin layer chromatography followed
by spectrophotometric determination of the TLC spots at 248 mµ
(Norman, Rygg & Wells, 1966) and (2) a gas-liquid chromatography
method based on the methods of Vogel & Deshusses (1964, 1965).
Both methods are currently under study. A collaborative study has been
conducted by five government laboratories in the member countries of
E.E.C. A comparative study of both methods is also in progress at the
Centraal Instituut voor Voedingsonderzoek, TNO, Zeist, the
Netherlands. A progress report from this institute by Vos (1966) was
available to the FAO Working Party on Pesticide Residues. The
conclusion drawn by Vos is as follows:
"The gas chromatographic method is the fastest and most accurate one.
The average value found agrees with that obtained from the thin layer
method. The values of the latter show a larger spreading. It is
possible that the biphenyl zone on the plates still contains some
interfering substances.
Although many other methods for the determination of biphenyl in
citrus fruit have been described in the literature, the G.L.C. - and
thin layer method were selected to be tested in practice. They seemed
to be most suitable for our purposes, viz. routine determination in
large number of samples.
We certainly can recommend the gas chromatographic determination for
this purpose.
If the relatively expensive equipment for gas chromatography should be
a serious objection, we recommend the thin-layer method. Although it
is slightly less accurate, it is also suitable for routine
determinations."
One further refinement is now available in the development of a
totally automated analytical procedure by Gunther & Ott (1966) for
citrus fruit rind employing a continuous flow recording
spectrophotometer.
The F.A.O. Working Party considered that, at the level of the
suggested tolerance, both the thin layer and the gas-liquid
chromatographic methods would be suitable for residue analysis.
Nevertheless it was decided to delay making a firm recommendation
until full reports were available on the investigations currently in
progress in the E.E.C. and T.N.O./Zeist.
(b) Sampling
It is understood that work is also in progress at the TNO, C.I.V.O.
Institute, Zeist, to explore the subject of sampling. In the meantime,
attention is drawn to the ISO Fifth Draft Proposal: Sampling of fresh
fruits and vegetables. ISO/TC 34-Agric. Food Products, Subcommittee 3
- Fruits, Vegetables and Their Derived Products, Working Group 2
- Sampling, October, 1965.
RECOMMENDATION FOR TOLERANCES
As diphenyl is not used on other foods the entire acceptable daily
intake could be assigned to citrus or citrus products. Although no
international agreement has been reached on the amount of citrus and
citrus products in the "high consumption diet", a combined figure of
230 grams per day for fresh citrus plus 60 grams per day for canned
citrus (i.e. a maximum of 290 grams per day) has been suggested. (In
Canada the suggested level of food consumption for these products for
tolerance purposes is 190 grams.)
If the acceptable daily intake level were 0.125 mg/kg for a 60 kg
person the permissible level from all sources becomes 7.5 mg per day.
Evidence has been received to demonstrate the need for the use of
diphenyl in the storage and transport of citrus over long distances.
The residues resulting in the whole fruit only rarely exceed 110 ppm.
In fresh fruit, pulp as eaten by the consumer rarely exceed 1.0 ppm.
Juices in extreme cases may contain residues as high as 4.4 ppm, but
most cases will contain less than 2.0 ppm. Marmalade and jam may also
contain some residues, but these may become insignificant in most
diets. Even in the cases of small children consuming large amounts of
orange juice, the amounts of diphenyl taken would be well below
acceptable daily intake.
Therefore, a tolerance of 110 ppm is recommended to be applied at the
point of entry of the citrus into the country.
Further information
(1) Data are desired on the residues that may result from methods of
preparation of fruit juice which employ the whole of the fruit
including the peel.
(2) A specification for diphenyl for use as a fungistatic agent on
citrus seems desirable. The Working Party would like to receive
further information on the likely impurities in commercial diphenyl.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Ambrose, A. M., Booth, A. N., DeEds, F. & Cox, A. J., jr (1960) Food
Res., 25, 328
Creaven, P. J. & Williams, R. T. (1963) Biochem. J., 87, 19 P
Deichmann, W. B., Kitzmiller, K. V., Dierker, M. & Witherup, S. (1947)
J. Industr. Hyg. Toxicol., 29, 1
Farkas, A. (1939) Hadar, 12, 227
Hazleton, L. W., Kundzins, W., Howard, J. W. & Johnston, C. D. (1956)
Abstract XXth International Physiol. Congress, p. 412
McEwen, M. (1958) Monsanto Chemical Co. Tech. Publ. AT-1
MacIntosh, F. C. (1945) Analyst, 70, 334
Newell, G. W. (1953) Unpublished report of the Stanford Research
Institute
Pecchiai, L. & Saffiotti, U. (1957) Med. Lavoro, 48, 247
Rogliani, E. & Procaccini, S. (1956) Biochim. appl., 3, 193
Selle, W. A. (1952, 1953, 1954) Unpublished reports
West, H. D. (1940) Proc. Soc. exp. Biol. (N.Y.), 43, 573
West, H. D. & Jefferson, N. C. (1942), J. Nutr., 23, 425
West, H. D., Lawson, J. R., Miller, I. H. & Mathura, R. (1956) Arch.
Biochem., 60, 14
Williams, R. T. (1965) Ann. Biol. Clin. (Paris), 23, 7
REFERENCES PERTINENT TO AGRICULTURAL DATA
Canada Food and Drug Directorate (1965), Diphenyl (Biphenyl). Summary
of data dealing with the technological justification of its use in the
food products as an antimicrobial in Canada. Submitted to FAO/WHO
Codex Alimentarius Committee on Food Additives, The Hague, May, 1965
Christ, R. A. (1962) Annual Report 1961. South-African Co-operative
Citrus Exchange. L.T.D., Pretoria No. C 131/1962
Dickey, E. E. (1956) The biphenyl content of citrus marmalade prepared
from consumer-type, biphenyl-treated fruit. Inst. of Paper Chemistry,
Project 1108-7-4, March 22, 1956
Farkas, A. & Aman, J. (1940) The action of diphenyl on Penicillium and
Diplodia molds. Palest. J. Bot. Jerusalem, 2: 38-45
Gunther, F. A. & Ott, D. R. (1966) Rapid automated determination of
biphenyl in citrus fruit rind. Analyst 91:475-481
Hazleton, L. W. (1956) Report of investigations on diphenyl. Part D.
Results of tests on the amount of residue remaining in citrus fruit
after economic use of biphenyl. Hazleton Laboratories Report, Falls
Church, Va.
Kiely, T.B. & Long, J. K. (1960) Market diseases of citrus fruit. Agr.
Gaz. N.S. Wales, 71: 132
Klots, L. J. (1957) Control of decay of citrus fruit in cartons.
Citrus Leaves, 37(4): 6
Littauer, F. S. & Mintz, G. (1945) Citrus wastage investigation,
Report for 1937-1945. Citrus Control Board, Dept. of Hort., Govt. of
Palestine
Norman, S., Rygg, G. L. & Wells A. W. (1966) Improved cleanup method
for determination of biphenyl in citrus fruits and in
biphenyl-impregnated kraft papers by thin layer chromotography.
J.A.O.A.C. 49: 590-595
Rajzman, A. (1961) The diphenyl residue in citrus fruit. National
Univ. Inst. Agr. Rehovot (Israel). Report No. 332
Rajzman, A. (1961a) Diphenyl absorption by citrus fruit wrapped in
plain wraps and stored together with diphenyl-wrapped fruit. Israel J.
Agr. Res. 11: 137
Rajzman, A. (1961b) Effect of washing and Waxing of oranges upon their
diphenyl. absorption. Bull. Res. Council Israel, 10c, 131
Rajzman, A. (1962) Elimination du diphényle des écorces d'agrumes.
Ann. Nutr. Alim. 15: 239-254
Rajzman, A. (1965) Les résidus de biphényle dans les agrumes. Residue
Reviews 8; 1-73
Rajzman, A. (1966) Biphenyl residues found in citrus fruit in Israel
during the citrus season 1958/59 to 1964/65. Volcani Institute of
Agric. Res. National and Univ. Inst. of Agriculture, Rehovot, Pamphlet
105, 29 pp., 24 tables
Resnick, C. (1966) Biphenyl, technological justification of its use as
a citrus protectant. Prepared on behalf of the Government of Israel
for submission to the FAO Working Party on Pesticide Residues.
Rygg, G. L., Wilson, G. W. & Garber, M. J. (1961) Effect of biphenyl
treatment and carton ventilation on decay and spoilage of California
lemons in overseas shipments. U.S. Dept. Agric. Agr. Marketing Service
Rept. No. 500
Rygg, G. L., Wells, A. W., Norman, S. M. & Atrops, E. P. (1962)
Biphenyl control, of lemon spoilage. Influence of time, temperature
and carton venting, U.S.D.A., Agr. Marketing Service Rept. No. 569
Souci, S.W. (1959) Die Behandlung von Citrus-Früchten. In
Lebensmittelforschung und Fremdstoffprobleme in U.S.A. München: Dtsch.
Forschungsanstalt-Für Lebensmittelchem, 5.46
Souci, S.W. & Maier-Haarlander, G. (1961) Work Report, Research on
Diphenyl-content of Citrus Fruit, to Industry Committee on Citrus
Additives, Re German Project No. 50501-31 (copy submitted to FAO and
WHO)
Souci, S.W. & Maier-Haarlander, G. (1963) Untersuchungen zur Analytik
des Diphenyls I. Mitteilung. Lebensm. Untersuch. U. Forsch. 119: 217
Tomkins, P. C. & Isherwood, F. A. (1945) The absorption of diphenyl
and O-phenyl-phenol by oranges. Analyst 70: 330-333.
Vogel, J. & Deshusses, J. (1964) Une methode de dosage du diphényle
dans les agrumes par chromatographie en phase gazeuse. Mitt. Gebiete
Lebensm. Hyg. 553 84-92
Vogel, J. & Deshusses, J. (1965) Modification de la methode de dosage
du diphenyle dans les agrumes par chromtographie en phase gazeuse.
Mitt. Gebiete Lebensm. Hyg. 56: 185.
de Vos, R. H. (1966) Determination of biphenyl in citrus fruit,
Progress report No. 1. Report No. R2155a, Centraal Instituut voor
Voedingsonderzoek, T.N.O., Zeist, April, 1966.
